Substance Use Disorders and Addiction: Mechanisms, Trends, and Treatment Implications
Information & authors, metrics & citations, view options, insights into mechanisms related to cocaine addiction using a novel imaging method for dopamine neurons, treatment implications of understanding brain function during early abstinence in patients with alcohol use disorder, relatively low amounts of alcohol intake during pregnancy are associated with subtle neurodevelopmental effects in preadolescent offspring, increased comorbidity between substance use and psychiatric disorders in sexual identity minorities, trends in nicotine use and dependence from 2001–2002 to 2012–2013, conclusions, information, published in.

- Substance-Related and Addictive Disorders
- Addiction Psychiatry
- Transgender (LGBT) Issues

Competing Interests
Export citations.
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download. For more information or tips please see 'Downloading to a citation manager' in the Help menu .
| Format | |
|---|---|
| Citation style | |
| Style | |
To download the citation to this article, select your reference manager software.
There are no citations for this item
View options
Login options.
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Not a subscriber?
Subscribe Now / Learn More
PsychiatryOnline subscription options offer access to the DSM-5-TR ® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).
Share article link
Copying failed.
NEXT ARTICLE
Request username.
Can't sign in? Forgot your username? Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username
Create a new account
Change password, password changed successfully.
Your password has been changed
Reset password
Can't sign in? Forgot your password?
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password.
Your Phone has been verified
As described within the American Psychiatric Association (APA)'s Privacy Policy and Terms of Use , this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences. Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Masks Strongly Recommended but Not Required in Maryland, Starting Immediately
Due to the downward trend in respiratory viruses in Maryland, masking is no longer required but remains strongly recommended in Johns Hopkins Medicine clinical locations in Maryland. Read more .
- Vaccines
- Masking Guidelines
- Visitor Guidelines
Institute for Basic Biomedical Sciences
Drug abuse and addiction research at johns hopkins institute of basic biomedical sciences.
IBBS researchers are studying how chronic drug use causes lasting changes in the brain that can lead to addiction. Their findings may aid in the development of more effective treatments for addiction.
Current addiction treatments use a combination of counseling and complete abstinence, slow weaning, or drug replacement that either substitutes for the drug or blocks withdrawal symptoms. Although these therapies control physical cravings, they don’t seem to reverse the lasting changes in the brain caused by drug abuse, and therefore may only provide a temporary fix.
During learning and memory formation, the brain’s neurons create new connections to strengthen or weaken communication routes between neighboring neurons. Similarly, chronic drug use modifies neuron connections, leading to permanent alterations in the brain’s circuitry. Taking drugs creates memories of objects, places or people that users associate with doing drugs, which triggers cravings and drug-seeking behavior when the user re-encounters those situations. Several IBBS neuroscientists study these molecular changes as they occur during learning, memory and chronic drug use.
Jay Baraban of the Solomon H. Snyder Department of Neuroscience studies how exposure to drugs such as cocaine or morphine triggers long-term adaptations in the brain that underlie addiction. Persistent changes in the strength of nerve connections encode memory and drug cravings. These adaptations are mediated by rapid synthesis of plasticity proteins that modify the strength of nerve connections. Baraban and colleagues have identified a pair of proteins that play a key role in driving rapid synthesis of synaptic proteins that change the efficacy of neuronal contacts and encode long-term memory. These researchers have engineered mice that lack these proteins in selected neuronal populations and are using these valuable tools to learn more about how this novel signaling pathway contributes to drug addiction.
Paul Worley , also from the neuroscience department, studies the molecular basis of specific forms of long-term learning and memory. His laboratory focuses on a class of proteins found at the interface between connecting neurons—synapses—that ramp up as the neurons engage in information processing and storage. These proteins directly modify the strength of the signals sent between neurons and are essential for information storage. Recent work reveals how molecules that regulate neuronal responses that signal reward, such as dopamine, can selectively strengthen communication across synapses, and implicates this process in addiction.
Mollie Meffert , a faculty member in the Department of Biological Chemistry and in the neuroscience department, investigates the formation of lasting memories. She focuses on growth factors in the hippocampus that turn on or off the particular genes involved in the growth of neurons and in establishing memories. Levels of these growth factors elevate during activity in the normal brain, and mice with lower-than-usual levels perform poorly on spatial memory tests such as navigating mazes. In addiction studies, researchers showed chronic drug use causes the release of brain-derived growth factors in rat brain areas involved in sensing the drug-associated “reward.” Meffert’s group studies how the brain-derived growth factors turn genes on or off to control long-lasting brain responses, such as those occurring in learning and memory, or addiction. By investigating the regulation of these genes in healthy and diseased neurons, the Meffert lab uncovered the mechanism by which brain-derived growth factors rapidly and specifically alter these genes. These findings may one day help us understand and develop therapeutic targets for failures in memory and brain processing as they pertain to addiction.

addictionresearch.nih.gov
CRAN: A trans-NIH initiative to promote collaborative research on addiction.
The mission of the National Institutes of Health (NIH) partnership, Collaborative Research on Addiction at NIH (CRAN) , is to provide a strong collaborative framework to enable the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Drug Abuse (NIDA), and the National Cancer Institute (NCI) to integrate resources and expertise to advance substance use, misuse, and addiction research and public health outcomes.

Adolescent Brain Cognitive Development Study
The ABCD Study is a landmark study on brain development and child health supported by the National Institutes of Health (NIH).
Find out more about the ABCD Study »
Funding Opportunities
CRAN IC’s solicit extramural grant applications through a variety of Notices of Funding Opportunities (NOFOS) to promote research in cross-cutting areas of substance use, misuse, addiction, and their related health consequences.
For more information, visit:
https://www.niaaa.nih.gov/grants-funding/funding-opportunities
https://nida.nih.gov/funding/nida-funding-opportunities
https://cancercontrol.cancer.gov/brp/funding
- Request Info
- Browse Degrees
- Life at SLU
- Give to SLU
- Search & Directory
Research in Focus: Addiction and Opioids
The opioid crisis continues to touch far too many lives. At Saint Louis University, an interdisciplinary group of researchers is working toward compassionate and innovative solutions.
Four of these researchers discuss their work and its connection to the University’s Catholic, Jesuit Mission.
The opioid crisis is one of the most devastating public health crises of the 21st century, with the number of opioid-related deaths quadrupling in the last 20 years. Researchers at SLU are mobilizing to provide relief in the midst of this.
“This issue of addiction has touched everyone. Everyone has had a friend, a loved one, whose life has deteriorated because of addiction,” said Fred Rottnek, M.D., professor of family and community medicine at SLU. “We’re in a unique position at Saint Louis University as a research institution and as a Catholic Jesuit institution to look very broadly at… all the things that have come together to create a perfect storm with the opioid epidemic and with the problems associated with that.”
Novel Ideas
A number of the researchers working on this issue today have been studying it for some time now. Their work continues to provide novel insights into addiction, criminal justice, pain management, and a number of other related areas.
Jeffrey Scherrer, Ph.D., is one of these researchers. Scherrer, who is a professor of family and community medicine at SLU, has conducted a number of insightful studies at the University, including a first-of-its-kind study into prescription opioid use and depression. The study discovered a dangerous, self-perpetuating cycle of abuse.
“The longer you use, the greater your risk of developing new onset depression,” Scherrer said. “And what it’s going to do is make it more difficult to manage pain, creating a cycle of increasing exposure to opioids, higher doses, and this cycle that kind of feeds on itself.”
Scherrer’s research highlights the critical importance of addressing upstream risk factors like mental health as part of pain management.
Daniela Salvemini, Ph.D., is another researcher whose work is breaking new ground. Salvemini is a professor of pharmacology and physiology at SLU. She studies neuropathic pain, one of the most common pain states and one of the most difficult to manage.
Salvemini’s recent work has focused on two research projects: the development of novel, non-opiate medication and the development of adjuncts so that opioids can be used at very low doses, thus greatly mitigating adverse side effects. A number of these are going into clinical trials very soon.
It’s all about helping people,” said Salvemini. “Helping come up with something that will alleviate human suffering. We’re getting there slowly, but surely we’re getting there.”
National Recognition
These researchers have been the recipients of both prestigious accolades and significant federal funding, establishing SLU as a national leader in the fight against addiction.
In April 2019, Scherrer was awarded over $3 million from the National Institute on Drug Abuse of the National Institutes of Health (NIH ) to study the pathways from chronic opioid use to new onset mood disorder.
In October 2018, Salvemini was awarded $4.5 million from the NIH to help find solutions to, among other things, opioid addiction, and in August 2019, she was awarded $2.1 million from the NIH to investigate a pain signaling pathway that could open up a new avenue for pain medication research. She is also a Fellow of the Saint Louis Academy of Science.
Liz Chiarello, Ph.D., associate professor of sociology at SLU, studies strategies to address the opioid crisis and was recently selected for an elite fellowship at the Radcliffe Institute for Advanced Study at Harvard University for this work.
Chiarello’s current project is determining how the health care and criminal justice systems are managing the crisis. Specifically, Chiarello is interested in what resources can be provided to health care providers and law enforcement officials to curb the effects of the crisis. She believes that a prescription drug monitoring program is one resource that could help; Missouri is the only state without one.
“We have to figure out how to make more meaningful entry points into the health care system instead of meaningful entry points into the criminal justice system,” said Chiarello.
The Center for Substance Use Disorder and Pain Management
Rottnek, Scherrer, Salvemini, and Chiarello are all among the researchers working in the Center for Substance Use Disorders and Pain Management at SLU. The Center supports and advances excellence in research and clinical care pertaining to substance use disorder and pain management.
“We have so many tools in our tool belt here at SLU to create this very broad, ambitious initiative which not only reflects the values of our university, but allows us to engage more fully as a regional leader in this work,” said Rottnek. "I have not seen any other institution be this bold in terms of an idea."
The Center has catalyzed existing research ambitions and strengthened connections with researchers across the university.
“The energy and commitment and investment in research at SLU in the past two years has probably been the greatest since I’ve been involved at SLU, and that goes back 25 years,” Scherrer said.
The work of the Center is informed by SLU's Catholic, Jesuit Mission and the institution's commitment to the local community in St. Louis. Much of this research informs the primary care that is being given to the local community.
“SLU has really positioned itself as a community ally. So many people across campus are doing work that has a meaningful impact,” Chiarello said.
“So much of what we do well here at SLU is grounded in the community in St. Louis,” Rottnek said. “These issues touch all of our lives. We can learn together, and we can find ways to support each other.”
In May 2019, the Center joined the National Academy of Medicine and more than 100 other organizations across the nation in declaring a commitment to countering the opioid epidemic .
About the Faculty
Learn more about the four faculty members featured in this article and video:

Liz Chiarello, Ph.D.
Associate Professor, Sociology
In May 2019, Chiarello joined 50 scholars, scientists, and artists from 10 countries in receiving a prestigious fellowship from the Radcliffe Institute for Advanced Study at Harvard University. The fellowship has an acceptance rate of 3.7% and will allow Chiarello to write a book that examines the impact of the U.S. opioid crisis on how health care providers and law enforcement officials do their job. Chiarello is also in the second year of a five-year NSF Faculty Early Career Development grant . These grants are given to early-career faculty who demonstrate potential to serve as leaders within their institutions and as academic role models in research and education.
Fred Rottnek, M.D., MAHCM
Professor, Family and Community Medicine
Rottnek is the Director of Community Medicine in the department of Family and Community Medicine and the medical director of the Physician Assistant program at SLU. Rottnek was among the first individuals in the region to be Board-Certified in Addiction Medicine and founded the Addiction Medicine Fellowship program at SLU, for which he currently serves as program director.
Daniela Salvemini, Ph.D.
Salvemini is a national leader in the study of neuropathic pain. In October 2018, she was awarded $4.5 million from the NIH to study “chemo brain” and opioid addiction , and in August 2019, she was awarded $2.1 million from the NIH to investigate a pain signaling pathway that could open up a new avenue for pain medication research . She is also the director of the Henry and Amelia Nasrallah Center for Neuroscience and a fellow of the Saint Louis Academy of Science. She is also chief scientific advisor and chair of the research advisory board of BioIntervene Inc., a SLU based start-up which she founded in 2014.
Jeffrey Scherrer, Ph.D.
Scherrer is the research division director in the department of Family and Community Medicine and the associate director for research at the Advanced HEAlth Data (AHEAD) Institute . He has been awarded a number of grants to study a variety of complex issues; in September 2016, he was awarded $2.3 million from the NIH to study PTSD and, in April 2019, he was awarded $3 million from the National Institute on Drug Abuse of the NIH to study the pathways from chronic opioid use to new onset mood disorder . He is also a research leader on campus; in January 2019, he became one of the first 15 faculty members to receive a total of $1.8 million in research growth funding from the SLU Research Institute .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 22 February 2021
Addiction as a brain disease revised: why it still matters, and the need for consilience
- Markus Heilig 1 ,
- James MacKillop ORCID: orcid.org/0000-0003-4118-9500 2 , 3 ,
- Diana Martinez 4 ,
- Jürgen Rehm ORCID: orcid.org/0000-0001-5665-0385 5 , 6 , 7 , 8 ,
- Lorenzo Leggio ORCID: orcid.org/0000-0001-7284-8754 9 &
- Louk J. M. J. Vanderschuren ORCID: orcid.org/0000-0002-5379-0363 10
Neuropsychopharmacology volume 46 , pages 1715–1723 ( 2021 ) Cite this article
93k Accesses
115 Citations
333 Altmetric
Metrics details
A Correspondence to this article was published on 03 May 2021
The view that substance addiction is a brain disease, although widely accepted in the neuroscience community, has become subject to acerbic criticism in recent years. These criticisms state that the brain disease view is deterministic, fails to account for heterogeneity in remission and recovery, places too much emphasis on a compulsive dimension of addiction, and that a specific neural signature of addiction has not been identified. We acknowledge that some of these criticisms have merit, but assert that the foundational premise that addiction has a neurobiological basis is fundamentally sound. We also emphasize that denying that addiction is a brain disease is a harmful standpoint since it contributes to reducing access to healthcare and treatment, the consequences of which are catastrophic. Here, we therefore address these criticisms, and in doing so provide a contemporary update of the brain disease view of addiction. We provide arguments to support this view, discuss why apparently spontaneous remission does not negate it, and how seemingly compulsive behaviors can co-exist with the sensitivity to alternative reinforcement in addiction. Most importantly, we argue that the brain is the biological substrate from which both addiction and the capacity for behavior change arise, arguing for an intensified neuroscientific study of recovery. More broadly, we propose that these disagreements reveal the need for multidisciplinary research that integrates neuroscientific, behavioral, clinical, and sociocultural perspectives.
Similar content being viewed by others
Subtypes in addiction and their neurobehavioral profiles across three functional domains
Drug addiction: from bench to bedside
The neurobiology of drug addiction: cross-species insights into the dysfunction and recovery of the prefrontal cortex
Introduction.
Close to a quarter of a century ago, then director of the US National Institute on Drug Abuse Alan Leshner famously asserted that “addiction is a brain disease”, articulated a set of implications of this position, and outlined an agenda for realizing its promise [ 1 ]. The paper, now cited almost 2000 times, put forward a position that has been highly influential in guiding the efforts of researchers, and resource allocation by funding agencies. A subsequent 2000 paper by McLellan et al. [ 2 ] examined whether data justify distinguishing addiction from other conditions for which a disease label is rarely questioned, such as diabetes, hypertension or asthma. It concluded that neither genetic risk, the role of personal choices, nor the influence of environmental factors differentiated addiction in a manner that would warrant viewing it differently; neither did relapse rates, nor compliance with treatment. The authors outlined an agenda closely related to that put forward by Leshner, but with a more clinical focus. Their conclusion was that addiction should be insured, treated, and evaluated like other diseases. This paper, too, has been exceptionally influential by academic standards, as witnessed by its ~3000 citations to date. What may be less appreciated among scientists is that its impact in the real world of addiction treatment has remained more limited, with large numbers of patients still not receiving evidence-based treatments.
In recent years, the conceptualization of addiction as a brain disease has come under increasing criticism. When first put forward, the brain disease view was mainly an attempt to articulate an effective response to prevailing nonscientific, moralizing, and stigmatizing attitudes to addiction. According to these attitudes, addiction was simply the result of a person’s moral failing or weakness of character, rather than a “real” disease [ 3 ]. These attitudes created barriers for people with substance use problems to access evidence-based treatments, both those available at the time, such as opioid agonist maintenance, cognitive behavioral therapy-based relapse prevention, community reinforcement or contingency management, and those that could result from research. To promote patient access to treatments, scientists needed to argue that there is a biological basis beneath the challenging behaviors of individuals suffering from addiction. This argument was particularly targeted to the public, policymakers and health care professionals, many of whom held that since addiction was a misery people brought upon themselves, it fell beyond the scope of medicine, and was neither amenable to treatment, nor warranted the use of taxpayer money.
Present-day criticism directed at the conceptualization of addiction as a brain disease is of a very different nature. It originates from within the scientific community itself, and asserts that this conceptualization is neither supported by data, nor helpful for people with substance use problems [ 4 , 5 , 6 , 7 , 8 ]. Addressing these critiques requires a very different perspective, and is the objective of our paper. We readily acknowledge that in some cases, recent critiques of the notion of addiction as a brain disease as postulated originally have merit, and that those critiques require the postulates to be re-assessed and refined. In other cases, we believe the arguments have less validity, but still provide an opportunity to update the position of addiction as a brain disease. Our overarching concern is that questionable arguments against the notion of addiction as a brain disease may harm patients, by impeding access to care, and slowing development of novel treatments.
A premise of our argument is that any useful conceptualization of addiction requires an understanding both of the brains involved, and of environmental factors that interact with those brains [ 9 ]. These environmental factors critically include availability of drugs, but also of healthy alternative rewards and opportunities. As we will show, stating that brain mechanisms are critical for understanding and treating addiction in no way negates the role of psychological, social and socioeconomic processes as both causes and consequences of substance use. To reflect this complex nature of addiction, we have assembled a team with expertise that spans from molecular neuroscience, through animal models of addiction, human brain imaging, clinical addiction medicine, to epidemiology. What brings us together is a passionate commitment to improving the lives of people with substance use problems through science and science-based treatments, with empirical evidence as the guiding principle.
To achieve this goal, we first discuss the nature of the disease concept itself, and why we believe it is important for the science and treatment of addiction. This is followed by a discussion of the main points raised when the notion of addiction as a brain disease has come under criticism. Key among those are claims that spontaneous remission rates are high; that a specific brain pathology is lacking; and that people suffering from addiction, rather than behaving “compulsively”, in fact show a preserved ability to make informed and advantageous choices. In the process of discussing these issues, we also address the common criticism that viewing addiction as a brain disease is a fully deterministic theory of addiction. For our argument, we use the term “addiction” as originally used by Leshner [ 1 ]; in Box 1 , we map out and discuss how this construct may relate to the current diagnostic categories, such as Substance Use Disorder (SUD) and its different levels of severity (Fig. 1) .
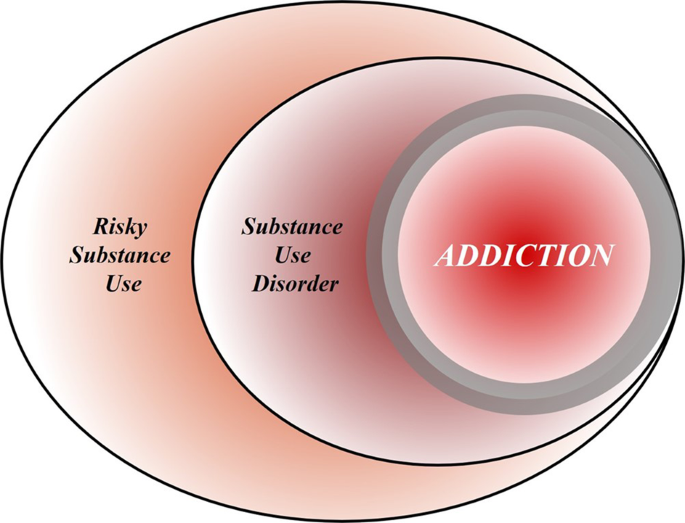
Risky (hazardous) substance use refers to quantity/frequency indicators of consumption; SUD refers to individuals who meet criteria for a DSM-5 diagnosis (mild, moderate, or severe); and addiction refers to individuals who exhibit persistent difficulties with self-regulation of drug consumption. Among high-risk individuals, a subgroup will meet criteria for SUD and, among those who have an SUD, a further subgroup would be considered to be addicted to the drug. However, the boundary for addiction is intentionally blurred to reflect that the dividing line for defining addiction within the category of SUD remains an open empirical question.
Box 1 What’s in a name? Differentiating hazardous use, substance use disorder, and addiction
Although our principal focus is on the brain disease model of addiction, the definition of addiction itself is a source of ambiguity. Here, we provide a perspective on the major forms of terminology in the field.
Hazardous Substance Use
Hazardous (risky) substance use refers to quantitative levels of consumption that increase an individual’s risk for adverse health consequences. In practice, this pertains to alcohol use [ 110 , 111 ]. Clinically, alcohol consumption that exceeds guidelines for moderate drinking has been used to prompt brief interventions or referral for specialist care [ 112 ]. More recently, a reduction in these quantitative levels has been validated as treatment endpoints [ 113 ].
Substance Use Disorder
SUD refers to the DSM-5 diagnosis category that encompasses significant impairment or distress resulting from specific categories of psychoactive drug use. The diagnosis of SUD is operationalized as 2 or more of 11 symptoms over the past year. As a result, the diagnosis is heterogenous, with more than 1100 symptom permutations possible. The diagnosis in DSM-5 is the result of combining two diagnoses from the DSM-IV, abuse and dependence, which proved to be less valid than a single dimensional approach [ 114 ]. Critically, SUD includes three levels of severity: mild (2–3 symptoms), moderate (4–5 symptoms), and severe (6+ symptoms). The International Classification of Diseases (ICD) system retains two diagnoses, harmful use (lower severity) and substance dependence (higher severity).
Addiction is a natural language concept, etymologically meaning enslavement, with the contemporary meaning traceable to the Middle and Late Roman Republic periods [ 115 ]. As a scientific construct, drug addiction can be defined as a state in which an individual exhibits an inability to self-regulate consumption of a substance, although it does not have an operational definition. Regarding clinical diagnosis, as it is typically used in scientific and clinical parlance, addiction is not synonymous with the simple presence of SUD. Nowhere in DSM-5 is it articulated that the diagnostic threshold (or any specific number/type of symptoms) should be interpreted as reflecting addiction, which inherently connotes a high degree of severity. Indeed, concerns were raised about setting the diagnostic standard too low because of the issue of potentially conflating a low-severity SUD with addiction [ 116 ]. In scientific and clinical usage, addiction typically refers to individuals at a moderate or high severity of SUD. This is consistent with the fact that moderate-to-severe SUD has the closest correspondence with the more severe diagnosis in ICD [ 117 , 118 , 119 ]. Nonetheless, akin to the undefined overlap between hazardous use and SUD, the field has not identified the exact thresholds of SUD symptoms above which addiction would be definitively present.
Integration
The ambiguous relationships among these terms contribute to misunderstandings and disagreements. Figure 1 provides a simple working model of how these terms overlap. Fundamentally, we consider that these terms represent successive dimensions of severity, clinical “nesting dolls”. Not all individuals consuming substances at hazardous levels have an SUD, but a subgroup do. Not all individuals with a SUD are addicted to the drug in question, but a subgroup are. At the severe end of the spectrum, these domains converge (heavy consumption, numerous symptoms, the unambiguous presence of addiction), but at low severity, the overlap is more modest. The exact mapping of addiction onto SUD is an open empirical question, warranting systematic study among scientists, clinicians, and patients with lived experience. No less important will be future research situating our definition of SUD using more objective indicators (e.g., [ 55 , 120 ]), brain-based and otherwise, and more precisely in relation to clinical needs [ 121 ]. Finally, such work should ultimately be codified in both the DSM and ICD systems to demarcate clearly where the attribution of addiction belongs within the clinical nosology, and to foster greater clarity and specificity in scientific discourse.
What is a disease?
In his classic 1960 book “The Disease Concept of Alcoholism”, Jellinek noted that in the alcohol field, the debate over the disease concept was plagued by too many definitions of “alcoholism” and too few definitions of “disease” [ 10 ]. He suggested that the addiction field needed to follow the rest of medicine in moving away from viewing disease as an “entity”, i.e., something that has “its own independent existence, apart from other things” [ 11 ]. To modern medicine, he pointed out, a disease is simply a label that is agreed upon to describe a cluster of substantial, deteriorating changes in the structure or function of the human body, and the accompanying deterioration in biopsychosocial functioning. Thus, he concluded that alcoholism can simply be defined as changes in structure or function of the body due to drinking that cause disability or death. A disease label is useful to identify groups of people with commonly co-occurring constellations of problems—syndromes—that significantly impair function, and that lead to clinically significant distress, harm, or both. This convention allows a systematic study of the condition, and of whether group members benefit from a specific intervention.
It is not trivial to delineate the exact category of harmful substance use for which a label such as addiction is warranted (See Box 1 ). Challenges to diagnostic categorization are not unique to addiction, however. Throughout clinical medicine, diagnostic cut-offs are set by consensus, commonly based on an evolving understanding of thresholds above which people tend to benefit from available interventions. Because assessing benefits in large patient groups over time is difficult, diagnostic thresholds are always subject to debate and adjustments. It can be debated whether diagnostic thresholds “merely” capture the extreme of a single underlying population, or actually identify a subpopulation that is at some level distinct. Resolving this issue remains challenging in addiction, but once again, this is not different from other areas of medicine [see e.g., [ 12 ] for type 2 diabetes]. Longitudinal studies that track patient trajectories over time may have a better ability to identify subpopulations than cross-sectional assessments [ 13 ].
By this pragmatic, clinical understanding of the disease concept, it is difficult to argue that “addiction” is unjustified as a disease label. Among people who use drugs or alcohol, some progress to using with a quantity and frequency that results in impaired function and often death, making substance use a major cause of global disease burden [ 14 ]. In these people, use occurs with a pattern that in milder forms may be challenging to capture by current diagnostic criteria (See Box 1 ), but is readily recognized by patients, their families and treatment providers when it reaches a severity that is clinically significant [see [ 15 ] for a classical discussion]. In some cases, such as opioid addiction, those who receive the diagnosis stand to obtain some of the greatest benefits from medical treatments in all of clinical medicine [ 16 , 17 ]. Although effect sizes of available treatments are more modest in nicotine [ 18 ] and alcohol addiction [ 19 ], the evidence supporting their efficacy is also indisputable. A view of addiction as a disease is justified, because it is beneficial: a failure to diagnose addiction drastically increases the risk of a failure to treat it [ 20 ].
Of course, establishing a diagnosis is not a requirement for interventions to be meaningful. People with hazardous or harmful substance use who have not (yet) developed addiction should also be identified, and interventions should be initiated to address their substance-related risks. This is particularly relevant for alcohol, where even in the absence of addiction, use is frequently associated with risks or harm to self, e.g., through cardiovascular disease, liver disease or cancer, and to others, e.g., through accidents or violence [ 21 ]. Interventions to reduce hazardous or harmful substance use in people who have not developed addiction are in fact particularly appealing. In these individuals, limited interventions are able to achieve robust and meaningful benefits [ 22 ], presumably because patterns of misuse have not yet become entrenched.
Thus, as originally pointed out by McLellan and colleagues, most of the criticisms of addiction as a disease could equally be applied to other medical conditions [ 2 ]. This type of criticism could also be applied to other psychiatric disorders, and that has indeed been the case historically [ 23 , 24 ]. Today, there is broad consensus that those criticisms were misguided. Few, if any healthcare professionals continue to maintain that schizophrenia, rather than being a disease, is a normal response to societal conditions. Why, then, do people continue to question if addiction is a disease, but not whether schizophrenia, major depressive disorder or post-traumatic stress disorder are diseases? This is particularly troubling given the decades of data showing high co-morbidity of addiction with these conditions [ 25 , 26 ]. We argue that it comes down to stigma. Dysregulated substance use continues to be perceived as a self-inflicted condition characterized by a lack of willpower, thus falling outside the scope of medicine and into that of morality [ 3 ].
Chronic and relapsing, developmentally-limited, or spontaneously remitting?
Much of the critique targeted at the conceptualization of addiction as a brain disease focuses on its original assertion that addiction is a chronic and relapsing condition. Epidemiological data are cited in support of the notion that large proportions of individuals achieve remission [ 27 ], frequently without any formal treatment [ 28 , 29 ] and in some cases resuming low risk substance use [ 30 ]. For instance, based on data from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) study [ 27 ], it has been pointed out that a significant proportion of people with an addictive disorder quit each year, and that most afflicted individuals ultimately remit. These spontaneous remission rates are argued to invalidate the concept of a chronic, relapsing disease [ 4 ].
Interpreting these and similar data is complicated by several methodological and conceptual issues. First, people may appear to remit spontaneously because they actually do, but also because of limited test–retest reliability of the diagnosis [ 31 ]. For instance, using a validated diagnostic interview and trained interviewers, the Collaborative Studies on Genetics of Alcoholism examined the likelihood that an individual diagnosed with a lifetime history of substance dependence would retain this classification after 5 years. This is obviously a diagnosis that, once met, by definition cannot truly remit. Lifetime alcohol dependence was indeed stable in individuals recruited from addiction treatment units, ~90% for women, and 95% for men. In contrast, in a community-based sample similar to that used in the NESARC [ 27 ], stability was only ~30% and 65% for women and men, respectively. The most important characteristic that determined diagnostic stability was severity. Diagnosis was stable in severe, treatment-seeking cases, but not in general population cases of alcohol dependence.
These data suggest that commonly used diagnostic criteria alone are simply over-inclusive for a reliable, clinically meaningful diagnosis of addiction. They do identify a core group of treatment seeking individuals with a reliable diagnosis, but, if applied to nonclinical populations, also flag as “cases” a considerable halo of individuals for whom the diagnostic categorization is unreliable. Any meaningful discussion of remission rates needs to take this into account, and specify which of these two populations that is being discussed. Unfortunately, the DSM-5 has not made this task easier. With only 2 out of 11 symptoms being sufficient for a diagnosis of SUD, it captures under a single diagnostic label individuals in a “mild” category, whose diagnosis is likely to have very low test–retest reliability, and who are unlikely to exhibit a chronic relapsing course, together with people at the severe end of the spectrum, whose diagnosis is reliable, many of whom do show a chronic relapsing course.
The NESARC data nevertheless show that close to 10% of people in the general population who are diagnosed with alcohol addiction (here equated with DSM-IV “dependence” used in the NESARC study) never remitted throughout their participation in the survey. The base life-time prevalence of alcohol dependence in NESARC was 12.5% [ 32 ]. Thus, the data cited against the concept of addiction as a chronic relapsing disease in fact indicate that over 1% of the US population develops an alcohol-related condition that is associated with high morbidity and mortality, and whose chronic and/or relapsing nature cannot be disputed, since it does not remit.
Secondly, the analysis of NESARC data [ 4 , 27 ] omits opioid addiction, which, together with alcohol and tobacco, is the largest addiction-related public health problem in the US [ 33 ]. This is probably the addictive condition where an analysis of cumulative evidence most strikingly supports the notion of a chronic disorder with frequent relapses in a large proportion of people affected [ 34 ]. Of course, a large number of people with opioid addiction are unable to express the chronic, relapsing course of their disease, because over the long term, their mortality rate is about 15 times greater than that of the general population [ 35 ]. However, even among those who remain alive, the prevalence of stable abstinence from opioid use after 10–30 years of observation is <30%. Remission may not always require abstinence, for instance in the case of alcohol addiction, but is a reasonable proxy for remission with opioids, where return to controlled use is rare. Embedded in these data is a message of literally vital importance: when opioid addiction is diagnosed and treated as a chronic relapsing disease, outcomes are markedly improved, and retention in treatment is associated with a greater likelihood of abstinence.
The fact that significant numbers of individuals exhibit a chronic relapsing course does not negate that even larger numbers of individuals with SUD according to current diagnostic criteria do not. For instance, in many countries, the highest prevalence of substance use problems is found among young adults, aged 18–25 [ 36 ], and a majority of these ‘age out’ of excessive substance use [ 37 ]. It is also well documented that many individuals with SUD achieve longstanding remission, in many cases without any formal treatment (see e.g., [ 27 , 30 , 38 ]).
Collectively, the data show that the course of SUD, as defined by current diagnostic criteria, is highly heterogeneous. Accordingly, we do not maintain that a chronic relapsing course is a defining feature of SUD. When present in a patient, however, such as course is of clinical significance, because it identifies a need for long-term disease management [ 2 ], rather than expectations of a recovery that may not be within the individual’s reach [ 39 ]. From a conceptual standpoint, however, a chronic relapsing course is neither necessary nor implied in a view that addiction is a brain disease. This view also does not mean that it is irreversible and hopeless. Human neuroscience documents restoration of functioning after abstinence [ 40 , 41 ] and reveals predictors of clinical success [ 42 ]. If anything, this evidence suggests a need to increase efforts devoted to neuroscientific research on addiction recovery [ 40 , 43 ].
Lessons from genetics
For alcohol addiction, meta-analysis of twin and adoption studies has estimated heritability at ~50%, while estimates for opioid addiction are even higher [ 44 , 45 ]. Genetic risk factors are to a large extent shared across substances [ 46 ]. It has been argued that a genetic contribution cannot support a disease view of a behavior, because most behavioral traits, including religious and political inclinations, have a genetic contribution [ 4 ]. This statement, while correct in pointing out broad heritability of behavioral traits, misses a fundamental point. Genetic architecture is much like organ structure. The fact that normal anatomy shapes healthy organ function does not negate that an altered structure can contribute to pathophysiology of disease. The structure of the genetic landscape is no different. Critics further state that a “genetic predisposition is not a recipe for compulsion”, but no neuroscientist or geneticist would claim that genetic risk is “a recipe for compulsion”. Genetic risk is probabilistic, not deterministic. However, as we will see below, in the case of addiction, it contributes to large, consistent probability shifts towards maladaptive behavior.
In dismissing the relevance of genetic risk for addiction, Hall writes that “a large number of alleles are involved in the genetic susceptibility to addiction and individually these alleles might very weakly predict a risk of addiction”. He goes on to conclude that “generally, genetic prediction of the risk of disease (even with whole-genome sequencing data) is unlikely to be informative for most people who have a so-called average risk of developing an addiction disorder” [ 7 ]. This reflects a fundamental misunderstanding of polygenic risk. It is true that a large number of risk alleles are involved, and that the explanatory power of currently available polygenic risk scores for addictive disorders lags behind those for e.g., schizophrenia or major depression [ 47 , 48 ]. The only implication of this, however, is that low average effect sizes of risk alleles in addiction necessitate larger study samples to construct polygenic scores that account for a large proportion of the known heritability.
However, a heritability of addiction of ~50% indicates that DNA sequence variation accounts for 50% of the risk for this condition. Once whole genome sequencing is readily available, it is likely that it will be possible to identify most of that DNA variation. For clinical purposes, those polygenic scores will of course not replace an understanding of the intricate web of biological and social factors that promote or prevent expression of addiction in an individual case; rather, they will add to it [ 49 ]. Meanwhile, however, genome-wide association studies in addiction have already provided important information. For instance, they have established that the genetic underpinnings of alcohol addiction only partially overlap with those for alcohol consumption, underscoring the genetic distinction between pathological and nonpathological drinking behaviors [ 50 ].
It thus seems that, rather than negating a rationale for a disease view of addiction, the important implication of the polygenic nature of addiction risk is a very different one. Genome-wide association studies of complex traits have largely confirmed the century old “infinitisemal model” in which Fisher reconciled Mendelian and polygenic traits [ 51 ]. A key implication of this model is that genetic susceptibility for a complex, polygenic trait is continuously distributed in the population. This may seem antithetical to a view of addiction as a distinct disease category, but the contradiction is only apparent, and one that has long been familiar to quantitative genetics. Viewing addiction susceptibility as a polygenic quantitative trait, and addiction as a disease category is entirely in line with Falconer’s theorem, according to which, in a given set of environmental conditions, a certain level of genetic susceptibility will determine a threshold above which disease will arise.
A brain disease? Then show me the brain lesion!
The notion of addiction as a brain disease is commonly criticized with the argument that a specific pathognomonic brain lesion has not been identified. Indeed, brain imaging findings in addiction (perhaps with the exception of extensive neurotoxic gray matter loss in advanced alcohol addiction) are nowhere near the level of specificity and sensitivity required of clinical diagnostic tests. However, this criticism neglects the fact that neuroimaging is not used to diagnose many neurologic and psychiatric disorders, including epilepsy, ALS, migraine, Huntington’s disease, bipolar disorder, or schizophrenia. Even among conditions where signs of disease can be detected using brain imaging, such as Alzheimer’s and Parkinson’s disease, a scan is best used in conjunction with clinical acumen when making the diagnosis. Thus, the requirement that addiction be detectable with a brain scan in order to be classified as a disease does not recognize the role of neuroimaging in the clinic.
For the foreseeable future, the main objective of imaging in addiction research is not to diagnose addiction, but rather to improve our understanding of mechanisms that underlie it. The hope is that mechanistic insights will help bring forward new treatments, by identifying candidate targets for them, by pointing to treatment-responsive biomarkers, or both [ 52 ]. Developing innovative treatments is essential to address unmet treatment needs, in particular in stimulant and cannabis addiction, where no approved medications are currently available. Although the task to develop novel treatments is challenging, promising candidates await evaluation [ 53 ]. A particular opportunity for imaging-based research is related to the complex and heterogeneous nature of addictive disorders. Imaging-based biomarkers hold the promise of allowing this complexity to be deconstructed into specific functional domains, as proposed by the RDoC initiative [ 54 ] and its application to addiction [ 55 , 56 ]. This can ultimately guide the development of personalized medicine strategies to addiction treatment.
Countless imaging studies have reported differences in brain structure and function between people with addictive disorders and those without them. Meta-analyses of structural data show that alcohol addiction is associated with gray matter losses in the prefrontal cortex, dorsal striatum, insula, and posterior cingulate cortex [ 57 ], and similar results have been obtained in stimulant-addicted individuals [ 58 ]. Meta-analysis of functional imaging studies has demonstrated common alterations in dorsal striatal, and frontal circuits engaged in reward and salience processing, habit formation, and executive control, across different substances and task-paradigms [ 59 ]. Molecular imaging studies have shown that large and fast increases in dopamine are associated with the reinforcing effects of drugs of abuse, but that after chronic drug use and during withdrawal, brain dopamine function is markedly decreased and that these decreases are associated with dysfunction of prefrontal regions [ 60 ]. Collectively, these findings have given rise to a widely held view of addiction as a disorder of fronto-striatal circuitry that mediates top-down regulation of behavior [ 61 ].
Critics reply that none of the brain imaging findings are sufficiently specific to distinguish between addiction and its absence, and that they are typically obtained in cross-sectional studies that can at best establish correlative rather than causal links. In this, they are largely right, and an updated version of a conceptualization of addiction as a brain disease needs to acknowledge this. Many of the structural brain findings reported are not specific for addiction, but rather shared across psychiatric disorders [ 62 ]. Also, for now, the most sophisticated tools of human brain imaging remain crude in face of complex neural circuit function. Importantly however, a vast literature from animal studies also documents functional changes in fronto-striatal circuits, as well their limbic and midbrain inputs, associated with addictive behaviors [ 63 , 64 , 65 , 66 , 67 , 68 ]. These are circuits akin to those identified by neuroimaging studies in humans, implicated in positive and negative emotions, learning processes and executive functions, altered function of which is thought to underlie addiction. These animal studies, by virtue of their cellular and molecular level resolution, and their ability to establish causality under experimental control, are therefore an important complement to human neuroimaging work.
Nevertheless, factors that seem remote from the activity of brain circuits, such as policies, substance availability and cost, as well as socioeconomic factors, also are critically important determinants of substance use. In this complex landscape, is the brain really a defensible focal point for research and treatment? The answer is “yes”. As powerfully articulated by Francis Crick [ 69 ], “You, your joys and your sorrows, your memories and your ambitions, your sense of personal identity and free will, are in fact no more than the behavior of a vast assembly of nerve cells and their associated molecules”. Social and interpersonal factors are critically important in addiction, but they can only exert their influences by impacting neural processes. They must be encoded as sensory data, represented together with memories of the past and predictions about the future, and combined with representations of interoceptive and other influences to provide inputs to the valuation machinery of the brain. Collectively, these inputs drive action selection and execution of behavior—say, to drink or not to drink, and then, within an episode, to stop drinking or keep drinking. Stating that the pathophysiology of addiction is largely about the brain does not ignore the role of other influences. It is just the opposite: it is attempting to understand how those important influences contribute to drug seeking and taking in the context of the brain, and vice versa.
But if the criticism is one of emphasis rather than of principle—i.e., too much brain, too little social and environmental factors – then neuroscientists need to acknowledge that they are in part guilty as charged. Brain-centric accounts of addiction have for a long time failed to pay enough attention to the inputs that social factors provide to neural processing behind drug seeking and taking [ 9 ]. This landscape is, however, rapidly changing. For instance, using animal models, scientists are finding that lack of social play early in life increases the motivation to take addictive substances in adulthood [ 70 ]. Others find that the opportunity to interact with a fellow rat is protective against addiction-like behaviors [ 71 ]. In humans, a relationship has been found between perceived social support, socioeconomic status, and the availability of dopamine D2 receptors [ 72 , 73 ], a biological marker of addiction vulnerability. Those findings in turn provided translation of data from nonhuman primates, which showed that D2 receptor availability can be altered by changes in social hierarchy, and that these changes are associated with the motivation to obtain cocaine [ 74 ].
Epidemiologically, it is well established that social determinants of health, including major racial and ethnic disparities, play a significant role in the risk for addiction [ 75 , 76 ]. Contemporary neuroscience is illuminating how those factors penetrate the brain [ 77 ] and, in some cases, reveals pathways of resilience [ 78 ] and how evidence-based prevention can interrupt those adverse consequences [ 79 , 80 ]. In other words, from our perspective, viewing addiction as a brain disease in no way negates the importance of social determinants of health or societal inequalities as critical influences. In fact, as shown by the studies correlating dopamine receptors with social experience, imaging is capable of capturing the impact of the social environment on brain function. This provides a platform for understanding how those influences become embedded in the biology of the brain, which provides a biological roadmap for prevention and intervention.
We therefore argue that a contemporary view of addiction as a brain disease does not deny the influence of social, environmental, developmental, or socioeconomic processes, but rather proposes that the brain is the underlying material substrate upon which those factors impinge and from which the responses originate. Because of this, neurobiology is a critical level of analysis for understanding addiction, although certainly not the only one. It is recognized throughout modern medicine that a host of biological and non-biological factors give rise to disease; understanding the biological pathophysiology is critical for understanding etiology and informing treatment.
Is a view of addiction as a brain disease deterministic?
A common criticism of the notion that addiction is a brain disease is that it is reductionist and in the end therefore deterministic [ 81 , 82 ]. This is a fundamental misrepresentation. As indicated above, viewing addiction as a brain disease simply states that neurobiology is an undeniable component of addiction. A reason for deterministic interpretations may be that modern neuroscience emphasizes an understanding of proximal causality within research designs (e.g., whether an observed link between biological processes is mediated by a specific mechanism). That does not in any way reflect a superordinate assumption that neuroscience will achieve global causality. On the contrary, since we realize that addiction involves interactions between biology, environment and society, ultimate (complete) prediction of behavior based on an understanding of neural processes alone is neither expected, nor a goal.
A fairer representation of a contemporary neuroscience view is that it believes insights from neurobiology allow useful probabilistic models to be developed of the inherently stochastic processes involved in behavior [see [ 83 ] for an elegant recent example]. Changes in brain function and structure in addiction exert a powerful probabilistic influence over a person’s behavior, but one that is highly multifactorial, variable, and thus stochastic. Philosophically, this is best understood as being aligned with indeterminism, a perspective that has a deep history in philosophy and psychology [ 84 ]. In modern neuroscience, it refers to the position that the dynamic complexity of the brain, given the probabilistic threshold-gated nature of its biology (e.g., action potential depolarization, ion channel gating), means that behavior cannot be definitively predicted in any individual instance [ 85 , 86 ].
Driven by compulsion, or free to choose?
A major criticism of the brain disease view of addiction, and one that is related to the issue of determinism vs indeterminism, centers around the term “compulsivity” [ 6 , 87 , 88 , 89 , 90 ] and the different meanings it is given. Prominent addiction theories state that addiction is characterized by a transition from controlled to “compulsive” drug seeking and taking [ 91 , 92 , 93 , 94 , 95 ], but allocate somewhat different meanings to “compulsivity”. By some accounts, compulsive substance use is habitual and insensitive to its outcomes [ 92 , 94 , 96 ]. Others refer to compulsive use as a result of increasing incentive value of drug associated cues [ 97 ], while others view it as driven by a recruitment of systems that encode negative affective states [ 95 , 98 ].
The prototype for compulsive behavior is provided by obsessive-compulsive disorder (OCD), where compulsion refers to repeatedly and stereotypically carrying out actions that in themselves may be meaningful, but lose their purpose and become harmful when performed in excess, such as persistent handwashing until skin injuries result. Crucially, this happens despite a conscious desire to do otherwise. Attempts to resist these compulsions result in increasing and ultimately intractable anxiety [ 99 ]. This is in important ways different from the meaning of compulsivity as commonly used in addiction theories. In the addiction field, compulsive drug use typically refers to inflexible, drug-centered behavior in which substance use is insensitive to adverse consequences [ 100 ]. Although this phenomenon is not necessarily present in every patient, it reflects important symptoms of clinical addiction, and is captured by several DSM-5 criteria for SUD [ 101 ]. Examples are needle-sharing despite knowledge of a risk to contract HIV or Hepatitis C, drinking despite a knowledge of having liver cirrhosis, but also the neglect of social and professional activities that previously were more important than substance use. While these behaviors do show similarities with the compulsions of OCD, there are also important differences. For example, “compulsive” substance use is not necessarily accompanied by a conscious desire to withhold the behavior, nor is addictive behavior consistently impervious to change.
Critics question the existence of compulsivity in addiction altogether [ 5 , 6 , 7 , 89 ], typically using a literal interpretation, i.e., that a person who uses alcohol or drugs simply can not do otherwise. Were that the intended meaning in theories of addiction—which it is not—it would clearly be invalidated by observations of preserved sensitivity of behavior to contingencies in addiction. Indeed, substance use is influenced both by the availability of alternative reinforcers, and the state of the organism. The roots of this insight date back to 1940, when Spragg found that chimpanzees would normally choose a banana over morphine. However, when physically dependent and in a state of withdrawal, their choice preference would reverse [ 102 ]. The critical role of alternative reinforcers was elegantly brought into modern neuroscience by Ahmed et al., who showed that rats extensively trained to self-administer cocaine would readily forego the drug if offered a sweet solution as an alternative [ 103 ]. This was later also found to be the case for heroin [ 103 ], methamphetamine [ 104 ] and alcohol [ 105 ]. Early residential laboratory studies on alcohol use disorder indeed revealed orderly operant control over alcohol consumption [ 106 ]. Furthermore, efficacy of treatment approaches such as contingency management, which provides systematic incentives for abstinence [ 107 ], supports the notion that behavioral choices in patients with addictions remain sensitive to reward contingencies.
Evidence that a capacity for choosing advantageously is preserved in addiction provides a valid argument against a narrow concept of “compulsivity” as rigid, immutable behavior that applies to all patients. It does not, however, provide an argument against addiction as a brain disease. If not from the brain, from where do the healthy and unhealthy choices people make originate? The critical question is whether addictive behaviors—for the most part—result from healthy brains responding normally to externally determined contingencies; or rather from a pathology of brain circuits that, through probabilistic shifts, promotes the likelihood of maladaptive choices even when reward contingencies are within a normal range. To resolve this question, it is critical to understand that the ability to choose advantageously is not an all-or-nothing phenomenon, but rather is about probabilities and their shifts, multiple faculties within human cognition, and their interaction. Yes, it is clear that most people whom we would consider to suffer from addiction remain able to choose advantageously much, if not most, of the time. However, it is also clear that the probability of them choosing to their own disadvantage, even when more salutary options are available and sometimes at the expense of losing their life, is systematically and quantifiably increased. There is a freedom of choice, yet there is a shift of prevailing choices that nevertheless can kill.
Synthesized, the notion of addiction as a disease of choice and addiction as a brain disease can be understood as two sides of the same coin. Both of these perspectives are informative, and they are complementary. Viewed this way, addiction is a brain disease in which a person’s choice faculties become profoundly compromised. To articulate it more specifically, embedded in and principally executed by the central nervous system, addiction can be understood as a disorder of choice preferences, preferences that overvalue immediate reinforcement (both positive and negative), preferences for drug-reinforcement in spite of costs, and preferences that are unstable ( “I’ll never drink like that again;” “this will be my last cigarette” ), prone to reversals in the form of lapses and relapse. From a contemporary neuroscience perspective, pre-existing vulnerabilities and persistent drug use lead to a vicious circle of substantive disruptions in the brain that impair and undermine choice capacities for adaptive behavior, but do not annihilate them. Evidence of generally intact decision making does not fundamentally contradict addiction as a brain disease.
Conclusions
The present paper is a response to the increasing number of criticisms of the view that addiction is a chronic relapsing brain disease. In many cases, we show that those criticisms target tenets that are neither needed nor held by a contemporary version of this view. Common themes are that viewing addiction as a brain disease is criticized for being both too narrow (addiction is only a brain disease; no other perspectives or factors are important) or too far reaching (it purports to discover the final causes of addiction). With regard to disease course, we propose that viewing addiction as a chronic relapsing disease is appropriate for some populations, and much less so for others, simply necessitating better ways of delineating the populations being discussed. We argue that when considering addiction as a disease, the lens of neurobiology is valuable to use. It is not the only lens, and it does not have supremacy over other scientific approaches. We agree that critiques of neuroscience are warranted [ 108 ] and that critical thinking is essential to avoid deterministic language and scientific overreach.
Beyond making the case for a view of addiction as a brain disease, perhaps the more important question is when a specific level of analysis is most useful. For understanding the biology of addiction and designing biological interventions, a neurobiological view is almost certainly the most appropriate level of analysis, in particular when informed by an understanding of the behavioral manifestations. In contrast, for understanding the psychology of addiction and designing psychological interventions, behavioral science is the natural realm, but one that can often benefit from an understanding of the underlying neurobiology. For designing policies, such as taxation and regulation of access, economics and public administration provide the most pertinent perspectives, but these also benefit from biological and behavioral science insights.
Finally, we argue that progress would come from integration of these scientific perspectives and traditions. E.O. Wilson has argued more broadly for greater consilience [ 109 ], unity of knowledge, in science. We believe that addiction is among the areas where consilience is most needed. A plurality of disciplines brings important and trenchant insights to bear on this condition; it is the exclusive remit of no single perspective or field. Addiction inherently and necessarily requires multidisciplinary examination. Moreover, those who suffer from addiction will benefit most from the application of the full armamentarium of scientific perspectives.
Funding and disclosures
Supported by the Swedish Research Council grants 2013-07434, 2019-01138 (MH); Netherlands Organisation for Health Research and Development (ZonMw) under project number 912.14.093 (LJMJV); NIDA and NIAAA intramural research programs (LL; the content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health); the Peter Boris Chair in Addictions Research, Homewood Research Institute, and the National Institute on Alcohol Abuse and Alcoholism grants AA025911, AA024930, AA025849, AA027679 (JM; the content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health).
MH has received consulting fees, research support or other compensation from Indivior, Camurus, BrainsWay, Aelis Farma, and Janssen Pharmaceuticals. JM is a Principal and Senior Scientist at BEAM Diagnostics, Inc. DM, JR, LL, and LJMJV declare no conflict of interest.
Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–7.
Article CAS PubMed Google Scholar
McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–95.
Schomerus G, Lucht M, Holzinger A, Matschinger H, Carta MG, Angermeyer MC. The stigma of alcohol dependence compared with other mental disorders: a review of population studies. Alcohol Alcohol. 2011;46:105–12.
Heyman GM. Addiction and choice: theory and new data. Front Psychiatry. 2013;4:31.
Article PubMed PubMed Central Google Scholar
Heather N, Best D, Kawalek A, Field M, Lewis M, Rotgers F, et al. Challenging the brain disease model of addiction: European launch of the addiction theory network. Addict Res Theory. 2018;26:249–55.
Article Google Scholar
Pickard H, Ahmed SH, Foddy B. Alternative models of addiction. Front Psychiatr.y 2015;6:20.
Google Scholar
Hall W, Carter A, Forlini C. The brain disease model of addiction: is it supported by the evidence and has it delivered on its promises? Lancet Psychiatr. 2015;2:105–10.
Hart CL. Viewing addiction as a brain disease promotes social injustice. Nat Hum Behav. 2017;1:0055.
Heilig M, Epstein DH, Nader MA, Shaham Y. Time to connect: bringing social context into addiction neuroscience. Nat Rev Neurosc.i 2016;17:592–9.
Article CAS Google Scholar
Jellinek EM. The disease concept of alcoholism. Hillhouse Press on behalf of the Christopher J. Smithers Foundation: New Haven, CT; 1960.
Stevenson A. Oxford dictionary of English. 3 ed. New York, NY: Oxford University Press; 2010.
Fan J, May SJ, Zhou Y, Barrett-Connor E. Bimodality of 2-h plasma glucose distributions in whites: the Rancho Bernardo study. Diabetes Care 2005;28:1451–6.
King AC, Vena A, Hasin D, De Wit D, O’Connor CJ, Cao D. Subjective responses to alcohol in the development and maintenance of alcohol use disorder (AUD). Am J Psychiatry. 2021. https://doi.org/10.1176/appi.ajp.2020.20030247 .
GBD. 2016 Alcohol and Drug Use Collaborators. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. 2018;5:987–1012.
Edwards G, Gross MM. Alcohol dependence: provisional description of a clinical syndrome. Br Med J. 1976;1:1058–61.
Article CAS PubMed PubMed Central Google Scholar
Epstein DH, Heilig M, Shaham Y. Science-based actions can help address the opioid crisis. Trends Pharm Sci. 2018;39:911–16.
Amato L, Davoli M, Perucci CA, Ferri M, Faggiano F, Mattick RP. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research. J Subst Abus Treat. 2005;28:321–9.
Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Datab System Rev. 2013;5:CD009329.
Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings a systematic review and meta-analysis. JAMA. 2014;311:1889–900.
Article PubMed CAS Google Scholar
Mark TL, Kranzler HR, Song X. Understanding US addiction physicians’ low rate of naltrexone prescription. Drug Alcohol Depend. 2003;71:219–28.
Article PubMed Google Scholar
Nutt DJ, King LA, Phillips LD. Drug harms in the UK: a multicriteria decision analysis. Lancet. 2010;376:1558–65.
Wilk AI, Jensen NM, Havighurst TC. Meta-analysis of randomized control trials addressing brief interventions in heavy alcohol drinkers. J Gen Intern Med. 1997;12:274–83.
Laing RD. The divided self; a study of sanity and madness. London: Tavistock Publications; 1960.
Foucault M, Khalfa J. History of madness. New York: Routledge; 2006.
Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL. et al.Comorbidity of mental disorders with alcohol and other drug abuse. Results Epidemiologic Catchment Area (ECA) study.JAMA. 1990;264:2511–8.
Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807–16.
Lopez-Quintero C, Hasin DS, de Los Cobos JP, Pines A, Wang S, Grant BF, et al. Probability and predictors of remission from life-time nicotine, alcohol, cannabis or cocaine dependence: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Addiction. 2011;106:657–69.
Humphreys K. Addiction treatment professionals are not the gatekeepers of recovery. Subst Use Misuse. 2015;50:1024–7.
Cohen E, Feinn R, Arias A, Kranzler HR. Alcohol treatment utilization: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2007;86:214–21.
Sobell LC, Cunningham JA, Sobell MB. Recovery from alcohol problems with and without treatment: prevalence in two population surveys. Am J Public Health. 1996;86:966–72.
Culverhouse R, Bucholz KK, Crowe RR, Hesselbrock V, Nurnberger JI Jr, Porjesz B, et al. Long-term stability of alcohol and other substance dependence diagnoses and habitual smoking: an evaluation after 5 years. Arch Gen Psychiatry. 2005;62:753–60.
Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–42.
Skolnick P. The opioid epidemic: crisis and solutions. Annu Rev Pharm Toxicol. 2018;58:143–59.
Hser YI, Evans E, Grella C, Ling W, Anglin D. Long-term course of opioid addiction. Harv Rev Psychiatry. 2015;23:76–89.
Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman M. Mortality among people who inject drugs: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:102–23.
Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757–66.
Article PubMed PubMed Central CAS Google Scholar
Lee MR, Sher KJ. “Maturing Out” of binge and problem drinking. Alcohol Res: Curr Rev. 2018;39:31–42.
Dawson DA, Grant BF, Stinson FS, Chou PS, Huang B, Ruan WJ. Recovery from DSM-IV alcohol dependence: United States, 2001–2002. Addiction. 2005;100:281–92.
Berridge V. The rise, fall, and revival of recovery in drug policy. Lancet. 2012;379:22–23.
Parvaz MA, Moeller SJ, d’Oleire Uquillas F, Pflumm A, Maloney T, Alia-Klein N, et al. Prefrontal gray matter volume recovery in treatment-seeking cocaine-addicted individuals: a longitudinal study. Addict Biol. 2017;22:1391–401.
Korponay C, Kosson DS, Decety J, Kiehl KA, Koenigs M. Brain volume correlates with duration of abstinence from substance abuse in a region-specific and substance-specific manner. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:626–35.
PubMed PubMed Central Google Scholar
Janes AC, Datko M, Roy A, Barton B, Druker S, Neal C, et al. Quitting starts in the brain: a randomized controlled trial of app-based mindfulness shows decreases in neural responses to smoking cues that predict reductions in smoking. Neuropsychopharmacology. 2019;44:1631–38.
Humphreys K, Bickel WK. Toward a neuroscience of long-term recovery from addiction. JAMA Psychiatry. 2018;75:875–76.
Verhulst B, Neale MC, Kendler KS. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychol Med. 2015;45:1061–72.
Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–32.
Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. AJ Psychiatry. 2003;160:687–95.
Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7.
Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50:668–81.
Wray NR, Lin T, Austin J, McGrath JJ, Hickie IB, Murray GK, et al. From basic science to clinical application of polygenic risk scores: a primer. JAMA Psychiatry. 2021;78:101–9.
Walters RK, Polimanti R, Johnson EC, McClintick JN, Adams MJ, Adkins AE, et al. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat Neurosci. 2018;21:1656–69.
Visscher PM, Wray NR. Concepts and misconceptions about the polygenic additive model applied to disease. Hum Hered. 2015;80:165–70.
Heilig M, Leggio L. What the alcohol doctor ordered from the neuroscientist: theragnostic biomarkers for personalized treatments. Prog Brain Res. 2016;224:401–18.
Rasmussen K, White DA, Acri JB. NIDA’s medication development priorities in response to the Opioid Crisis: ten most wanted. Neuropsychopharmacology. 2019;44:657–59.
Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. AJ Psychiatry. 2010;167:748–51.
Kwako LE, Schwandt ML, Ramchandani VA, Diazgranados N, Koob GF, Volkow ND, et al. Neurofunctional domains derived from deep behavioral phenotyping in alcohol use disorder. AJ Psychiatry. 2019;176:744–53.
Kwako LE, Bickel WK, Goldman D. Addiction biomarkers: dimensional approaches to understanding addiction. Trends Mol Med. 2018;24:121–28.
Xiao P, Dai Z, Zhong J, Zhu Y, Shi H, Pan P. Regional gray matter deficits in alcohol dependence: a meta-analysis of voxel-based morphometry studies. Drug Alcohol Depend. 2015;153:22–8.
Ersche KD, Williams GB, Robbins TW, Bullmore ET. Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Curr Opin Neurobiol. 2013;23:615–24.
Klugah-Brown B, Di X, Zweerings J, Mathiak K, Becker B, Biswal B. Common and separable neural alterations in substance use disorders: a coordinate-based meta-analyses of functional neuroimaging studies in humans. Hum Brain Mapp. 2020;41:4459–77.
Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Investig. 2003;111:1444–51.
Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–69.
Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–15.
Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, et al. The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharm Rev. 2016;68:816–71.
Korpi ER, den Hollander B, Farooq U, Vashchinkina E, Rajkumar R, Nutt DJ, et al. Mechanisms of action and persistent neuroplasticity by drugs of abuse. Pharm Rev. 2015;67:872–1004.
Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–63.
Everitt BJ. Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories–indications for novel treatments of addiction. Eur J Neurosci. 2014;40:2163–82.
Lesscher HM, Vanderschuren LJ. Compulsive drug use and its neural substrates. Rev Neurosci. 2012;23:731–45.
Cruz FC, Koya E, Guez-Barber DH, Bossert JM, Lupica CR, Shaham Y, et al. New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat Rev Neurosci. 2013;14:743–54.
Crick F. The astonishing hypothesis: the scientific search for the soul. Scribner; Maxwell Macmillan International: New York, NY; 1994.
Vanderschuren LJ, Achterberg EJ, Trezza V. The neurobiology of social play and its rewarding value in rats. Neurosci Biobehav Rev. 2016;70:86–105.
Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, et al. Volitional social interaction prevents drug addiction in rat models. Nat Neurosci. 2018;21:1520–29.
Martinez D, Orlowska D, Narendran R, Slifstein M, Liu F, Kumar D, et al. Dopamine type 2/3 receptor availability in the striatum and social status in human volunteers. Biol Psychiatry. 2010;67:275–8.
Wiers CE, Shokri-Kojori E, Cabrera E, Cunningham S, Wong C, Tomasi D, et al. Socioeconomic status is associated with striatal dopamine D2/D3 receptors in healthy volunteers but not in cocaine abusers. Neurosci Lett. 2016;617:27–31.
Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–74.
Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health. 2017;2:e356–e66.
Gilbert PA, Zemore SE. Discrimination and drinking: a systematic review of the evidence. Soc Sci Med 2016;161:178–94.
Oshri A, Gray JC, Owens MM, Liu S, Duprey EB, Sweet LH, et al. Adverse childhood experiences and amygdalar reduction: high-resolution segmentation reveals associations with subnuclei and psychiatric outcomes. Child Maltreat. 2019;24:400–10.
Holmes CJ, Barton AW, MacKillop J, Galván A, Owens MM, McCormick MJ, et al. Parenting and salience network connectivity among African Americans: a protective pathway for health-risk behaviors. Biol Psychiatry. 2018;84:365–71.
Brody GH, Gray JC, Yu T, Barton AW, Beach SR, Galván A, et al. Protective prevention effects on the association of poverty with brain development. JAMA Pediatr. 2017;171:46–52.
Hanson JL, Gillmore AD, Yu T, Holmes CJ, Hallowell ES, Barton AW, et al. A family focused intervention influences hippocampal-prefrontal connectivity through gains in self-regulation. Child Dev. 2019;90:1389–401.
Borsboom D, Cramer A, Kalis A. Brain disorders? Not really… why network structures block reductionism in psychopathology research. Behav Brain Sci. 2018;42:1–54.
Field M, Heather N, Wiers RW. Indeed, not really a brain disorder: Implications for reductionist accounts of addiction. Behav Brain Sci. 2019;42:e9.
Pascoli V, Hiver A, Van Zessen R, Loureiro M, Achargui R, Harada M, et al. Stochastic synaptic plasticity underlying compulsion in a model of addiction. Nature. 2018;564:366–71.
James W. The dilemma of determinism. Whitefish, MT: Kessinger Publishing; 2005.
Gessell B. Indeterminism in the brain. Biol Philos. 2017;32:1205–23.
Jedlicka P. Revisiting the quantum brain hypothesis: toward quantum (neuro)biology? Front Mol Neurosci. 2017;10:366.
Heyman GM. Addiction: a disorder of choice. Cambridge, MA: Harvard University Press; 2010.
Heather NQ. Is addiction a brain disease or a moral failing? A: Neither. Neuroethics. 2017;10:115–24.
Ahmed SH, Lenoir M, Guillem K. Neurobiology of addiction versus drug use driven by lack of choice. Curr Opin Neurobiol. 2013;23:581–7.
Hogarth L, Lam-Cassettari C, Pacitti H, Currah T, Mahlberg J, Hartley L, et al. Intact goal-directed control in treatment-seeking drug users indexed by outcome-devaluation and Pavlovian to instrumental transfer: critique of habit theory. Eur J Neurosci. 2019;50:2513–25.
Mathis V, Kenny PJ. From controlled to compulsive drug-taking: the role of the habenula in addiction. Neurosci Biobehav Rev. 2019;106:102–11.
Luscher C, Robbins TW, Everitt BJ. The transition to compulsion in addiction. Nat Rev Neurosci. 2020;21:247–63.
Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53.
Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–89.
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38.
Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–68.
Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–91.
Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–4.
Stein DJ, Costa DLC, Lochner C, Miguel EC, Reddy YCJ, Shavitt RG, et al. Obsessive-compulsive disorder. Nat Rev Dis Prim. 2019;5:52.
Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 2004;305:1017–9.
American_Psychiatric_Association. Diagnostic and statistical manual of mental disorders: DSM-5™. 5th ed. Arlington, VA, US: American Psychiatric Publishing, Inc; 2013.
Book Google Scholar
Spragg SDS. Morphine addiction in chimpanzees. Comp Psychol Monogr. 1940;15:132–32.
Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology. 2013;38:1209–20.
Caprioli D, Venniro M, Zeric T, Li X, Adhikary S, Madangopal R, et al. Effect of the novel positive allosteric modulator of metabotropic glutamate receptor 2 AZD8529 on incubation of methamphetamine craving after prolonged voluntary abstinence in a rat model. Biol Psychiatry. 2015;78:463–73.
Augier E, Barbier E, Dulman RS, Licheri V, Augier G, Domi E, et al. A molecular mechanism for choosing alcohol over an alternative reward. Science. 2018;360:1321–26.
Bigelow GE. An operant behavioral perspective on alcohol abuse and dependence. In: Heather N, Peters TJ, Stockwell T, editors. International handbook of alcohol dependence and problems. John Wiley & Sons Ltd; 2001. p. 299–315.
Higgins ST, Heil SH, Lussier JP. Clinical implications of reinforcement as a determinant of substance use disorders. Annu Rev Psychol. 2004;55:431–61.
Satel S, Lilienfeld SO. Brainwashed: the seductive appeal of mindless neuroscience. New York, NY: Basic Books; 2015.
Wilson EO. Consilience: the unity of knowledge. New York, NY: Vintage Books; 1999.
Saunders JB, Degenhardt L, Reed GM, Poznyak V. Alcohol use disorders in ICD-11: past, present, and future. Alcohol Clin Exp Res 2019;43:1617–31.
Organization. WH. ICD-11 for mortality and morbidity statistics. 2018. https://icd.who.int/browse11/l-m/en . Accessed 21 Oct 2020.
Babor TF, McRee BG, Kassebaum PA, Grimaldi PL, Ahmed K, Bray J. Screening, brief intervention, and referral to treatment (SBIRT): toward a public health approach to the management of substance abuse. Subst Abus. 2007;28:7–30.
Witkiewitz K, Hallgren KA, Kranzler HR, Mann KF, Hasin DS, Falk DE, et al. Clinical validation of reduced alcohol consumption after treatment for alcohol dependence using the World Health Organization risk drinking levels. Alcohol Clin Exp Res 2017;41:179–86.
Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, et al. DSM-5 criteria for substance use disorders: recommendations and rationale. AJ Psychiatry. 2013;170:834–51.
Rosenthal RJ, Faris SB. The etymology and early history of ‘addiction’. Addict Res Theory. 2019;27:437–49.
Martin CS, Steinley DL, Verges A, Sher KJ. The proposed 2/11 symptom algorithm for DSM-5 substance-use disorders is too lenient. Psychol Med. 2011;41:2008–10.
Degenhardt L, Bharat C, Bruno R, Glantz MD, Sampson NA, Lago L, et al. Concordance between the diagnostic guidelines for alcohol and cannabis use disorders in the draft ICD-11 and other classification systems: analysis of data from the WHO’s World Mental Health Surveys. Addiction. 2019;114:534–52.
PubMed Google Scholar
Lago L, Bruno R, Degenhardt L. Concordance of ICD-11 and DSM-5 definitions of alcohol and cannabis use disorders: a population survey. Lancet Psychiatry. 2016;3:673–84.
Lundin A, Hallgren M, Forsman M, Forsell Y. Comparison of DSM-5 classifications of alcohol use disorders with those of DSM-IV, DSM-III-R, and ICD-10 in a general population sample in Sweden. J Stud Alcohol Drugs. 2015;76:773–80.
Kwako LE, Momenan R, Litten RZ, Koob GF, Goldman D. Addictions neuroclinical assessment: a neuroscience-based framework for addictive disorders. Biol Psychiatry. 2016;80:179–89.
Rehm J, Heilig M, Gual A. ICD-11 for alcohol use disorders: not a convincing answer to the challenges. Alcohol Clin Exp Res. 2019;43:2296–300.
Download references
Acknowledgements
The authors want to acknowledge comments by Drs. David Epstein, Kenneth Kendler and Naomi Wray.
Author information
Authors and affiliations.
Center for Social and Affective Neuroscience, Department of Biomedical and Clinical Sciences, Linköping University, Linköping, Sweden
Markus Heilig
Peter Boris Centre for Addictions Research, McMaster University and St. Joseph’s Healthcare Hamilton, Hamilton, ON, Canada
James MacKillop
Homewood Research Institute, Guelph, ON, Canada
New York State Psychiatric Institute and Columbia University Irving Medical Center, New York, NY, USA
Diana Martinez
Institute for Mental Health Policy Research & Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health (CAMH), Toronto, ON, Canada
Jürgen Rehm
Dalla Lana School of Public Health and Department of Psychiatry, University of Toronto (UofT), Toronto, ON, Canada
Klinische Psychologie & Psychotherapie, Technische Universität Dresden, Dresden, Germany
Department of International Health Projects, Institute for Leadership and Health Management, I.M. Sechenov First Moscow State Medical University, Moscow, Russia
Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section, Translational Addiction Medicine Branch, National Institute on Drug Abuse Intramural Research Program and National Institute on Alcohol Abuse and Alcoholism Division of Intramural Clinical and Biological Research, National Institutes of Health, Baltimore and Bethesda, MD, USA
Lorenzo Leggio
Department of Population Health Sciences, Unit Animals in Science and Society, Faculty of Veterinary Medicine, Utrecht University, Utrecht, the Netherlands
Louk J. M. J. Vanderschuren
You can also search for this author in PubMed Google Scholar
Contributions
All authors jointly drafted the paper.
Corresponding author
Correspondence to Markus Heilig .
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Heilig, M., MacKillop, J., Martinez, D. et al. Addiction as a brain disease revised: why it still matters, and the need for consilience. Neuropsychopharmacol. 46 , 1715–1723 (2021). https://doi.org/10.1038/s41386-020-00950-y
Download citation
Received : 10 November 2020
Revised : 11 December 2020
Accepted : 14 December 2020
Published : 22 February 2021
Issue Date : September 2021
DOI : https://doi.org/10.1038/s41386-020-00950-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
The role of attachment and personality traits in choosing opiate addiction replacement therapy.
- Alena Gizdic
- Vesna Antičević
- Igna Brajević-Gizdić
Scientific Reports (2024)

Punishment-resistant alcohol intake is mediated by the nucleus accumbens shell in female rats
- Allison J. McDonald
- Panthea Nemat
- Nathan J. Marchant
Neuropsychopharmacology (2024)
The importance of choice and agency in animal models of addiction
- Serge H. Ahmed
Journal of Neural Transmission (2024)
Heterogeneity in choice models of addiction: the role of context
- Samuel F. Acuff
- Justin C. Strickland
Psychopharmacology (2024)
Neuromarkers in addiction: definitions, development strategies, and recent advances
- Nicholas R. Harp
- Tor D. Wager
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Perceived Need for Substance Abuse Treatment Among Illicit Stimulant Drug Users in Rural Areas of Ohio, Arkansas, and Kentucky
- Population and Public Health Sciences
Research output : Contribution to journal › Article › peer-review
Non-medical drug use in rural communities in the United States is a significant and growing public health threat. Understanding what motivates drug users in rural areas to seek substance abuse treatment may help in addressing the problem. Perceived need for treatment, a construct indicative of problem recognition and belief in problem solution, has been identified as an important predictor of help-seeking behavior. This cross-sectional study used data collected through face-to-face interviews to examine factors associated with perceived need for drug abuse treatment among not-in-treatment, adult, illicit stimulant drug users ( n = 710) in rural areas of Ohio, Kentucky, and Arkansas. More than one-quarter of the sample perceived a need for treatment. Results from a stepwise multiple regression analysis showed that white users, users with better physical and mental health status, and occasional users of methamphetamine were significantly less likely to see a need for treatment. Users with higher Addiction Severity Index composite scores for family/social problems or legal problems, and users with prior drug abuse treatment experience were significantly more likely to perceive a need for treatment. These findings have practical implications for efforts addressing substance abuse in rural areas.
| Original language | American English |
|---|---|
| Journal | |
| Volume | 91 |
| DOIs | |
| State | Published - Dec 1 2007 |
- perceived need for treatment
- substance abuse
- methamphetamine
Disciplines
- Health Services Administration
- Health Services Research
- Medicine and Health Sciences
- Mental and Social Health
- Public Health
- Substance Abuse and Addiction
Access to Document
- 10.1016/j.drugalcdep.2007.05.015 License: CC BY
- http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2679091/pdf/nihms-33183.pdf
Other files and links
- Link to repository
- Link to publication
T1 - Perceived Need for Substance Abuse Treatment Among Illicit Stimulant Drug Users in Rural Areas of Ohio, Arkansas, and Kentucky
AU - Falck, Russel S.
AU - Wang, Jichuan
AU - Carlson, Robert G.
AU - Krishnan, Laura L.
AU - Leukefeld, Carl G.
AU - Booth, Brenda M.
PY - 2007/12/1
Y1 - 2007/12/1
N2 - Non-medical drug use in rural communities in the United States is a significant and growing public health threat. Understanding what motivates drug users in rural areas to seek substance abuse treatment may help in addressing the problem. Perceived need for treatment, a construct indicative of problem recognition and belief in problem solution, has been identified as an important predictor of help-seeking behavior. This cross-sectional study used data collected through face-to-face interviews to examine factors associated with perceived need for drug abuse treatment among not-in-treatment, adult, illicit stimulant drug users ( n = 710) in rural areas of Ohio, Kentucky, and Arkansas. More than one-quarter of the sample perceived a need for treatment. Results from a stepwise multiple regression analysis showed that white users, users with better physical and mental health status, and occasional users of methamphetamine were significantly less likely to see a need for treatment. Users with higher Addiction Severity Index composite scores for family/social problems or legal problems, and users with prior drug abuse treatment experience were significantly more likely to perceive a need for treatment. These findings have practical implications for efforts addressing substance abuse in rural areas.
AB - Non-medical drug use in rural communities in the United States is a significant and growing public health threat. Understanding what motivates drug users in rural areas to seek substance abuse treatment may help in addressing the problem. Perceived need for treatment, a construct indicative of problem recognition and belief in problem solution, has been identified as an important predictor of help-seeking behavior. This cross-sectional study used data collected through face-to-face interviews to examine factors associated with perceived need for drug abuse treatment among not-in-treatment, adult, illicit stimulant drug users ( n = 710) in rural areas of Ohio, Kentucky, and Arkansas. More than one-quarter of the sample perceived a need for treatment. Results from a stepwise multiple regression analysis showed that white users, users with better physical and mental health status, and occasional users of methamphetamine were significantly less likely to see a need for treatment. Users with higher Addiction Severity Index composite scores for family/social problems or legal problems, and users with prior drug abuse treatment experience were significantly more likely to perceive a need for treatment. These findings have practical implications for efforts addressing substance abuse in rural areas.
KW - perceived need for treatment
KW - substance abuse
KW - treatment
KW - methamphetamine
KW - cocaine
UR - https://corescholar.libraries.wright.edu/ruralstim/3
UR - http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2679091/pdf/nihms-33183.pdf
U2 - 10.1016/j.drugalcdep.2007.05.015
DO - 10.1016/j.drugalcdep.2007.05.015
M3 - Article
C2 - 17604917
JO - Drug and Alcohol Dependence
JF - Drug and Alcohol Dependence

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
Drugs, Brains, and Behavior: The Science of Addiction Drug Misuse and Addiction
What is drug addiction.
Addiction is defined as a chronic, relapsing disorder characterized by compulsive drug seeking and use despite adverse consequences. † It is considered a brain disorder, because it involves functional changes to brain circuits involved in reward, stress, and self-control. Those changes may last a long time after a person has stopped taking drugs. 11
Addiction is a lot like other diseases, such as heart disease. Both disrupt the normal, healthy functioning of an organ in the body, both have serious harmful effects, and both are, in many cases, preventable and treatable. If left untreated, they can last a lifetime and may lead to death.
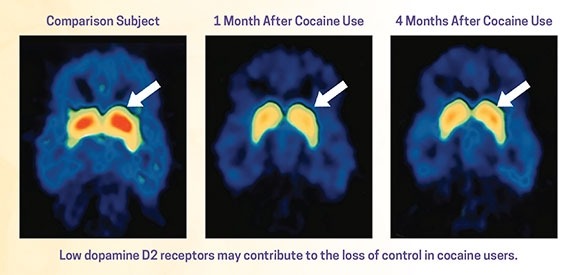
Why do people take drugs?
In general, people take drugs for a few reasons:
- To feel good. Drugs can produce intense feelings of pleasure. This initial euphoria is followed by other effects, which differ with the type of drug used. For example, with stimulants such as cocaine, the high is followed by feelings of power, self-confidence, and increased energy. In contrast, the euphoria caused by opioids such as heroin is followed by feelings of relaxation and satisfaction.
- To feel better. Some people who suffer from social anxiety, stress, and depression start using drugs to try to feel less anxious. Stress can play a major role in starting and continuing drug use as well as relapse (return to drug use) in patients recovering from addiction.
- To do better. Some people feel pressure to improve their focus in school or at work or their abilities in sports. This can play a role in trying or continuing to use drugs, such as prescription stimulants or cocaine.
- Curiosity and social pressure. In this respect, teens are particularly at risk because peer pressure can be very strong. Adolescence is a developmental period during which the presence of risk factors, such as peers who use drugs, may lead to substance use.
If taking drugs makes people feel good or better, what's the problem?

When they first use a drug, people may perceive what seem to be positive effects. They also may believe they can control their use. But drugs can quickly take over a person's life. Over time, if drug use continues, other pleasurable activities become less pleasurable, and the person has to take the drug just to feel “normal.” They have a hard time controlling their need to take drugs even though it causes many problems for themselves and their loved ones. Some people may start to feel the need to take more of a drug or take it more often, even in the early stages of their drug use. These are the signs of an addiction.
Even relatively moderate drug use poses dangers. Consider how a social drinker can become intoxicated, get behind the wheel of a car, and quickly turn a pleasurable activity into a tragedy that affects many lives. Occasional drug use, such as misusing an opioid to get high, can have similarly disastrous effects, including impaired driving and overdose.
Do people choose to keep using drugs?
The initial decision to take drugs is typically voluntary. But with continued use, a person's ability to exert self-control can become seriously impaired. This impairment in self-control is the hallmark of addiction.
Brain imaging studies of people with addiction show physical changes in areas of the brain that are critical to judgment, decision-making, learning and memory, and behavior control. 12 These changes help explain the compulsive nature of addiction.
Why do some people become addicted to drugs, while others do not?
As with other diseases and disorders, the likelihood of developing an addiction differs from person to person, and no single factor determines whether a person will become addicted to drugs. In general, the more risk factors a person has, the greater the chance that taking drugs will lead to drug use and addiction. Protective factors, on the other hand, reduce a person's risk. Risk and protective factors may be either environmental or biological.
| Risk Factors | Protective Factors |
|---|---|
| Aggressive behavior in childhood | Self-efficacy (belief in self-control) |
| Lack of parental supervision | Parental monitoring and support |
| Low peer refusal skills | Positive relationships |
| Drug experimentation | Good grades |
| Availability of drugs at school | School anti-drug policies |
| Community poverty | Neighborhood resources |
What biological factors increase risk of addiction?
Biological factors that can affect a person's risk of addiction include their genes, stage of development, and even gender or ethnicity. Scientists estimate that genes, including the effects environmental factors have on a person's gene expression, called epigenetics, account for between 40 and 60 percent of a person's risk of addiction. 27 Also, teens and people with mental disorders are at greater risk of drug use and addiction than others. 28
What environmental factors increase the risk of addiction?
Environmental factors are those related to the family, school, and neighborhood. Factors that can increase a person's risk include the following:
- Home and Family. The home environment, especially during childhood, is a very important factor. Parents or older family members who use drugs or misuse alcohol, or who break the law, can increase children's risk of future drug problems. 29
- Peer and School. Friends and other peers can have an increasingly strong influence during the teen years. Teens who use drugs can sway even those without risk factors to try drugs for the first time. Struggling in school or having poor social skills can put a child at further risk for using or becoming addicted to drugs. 30
What other factors increase the risk of addiction?
- Early use. Although taking drugs at any age can lead to addiction, research shows that the earlier people begin to use drugs, the more likely they are to develop serious problems. 31 This may be due to the harmful effect that drugs can have on the developing brain. 32 It also may result from a mix of early social and biological risk factors, including lack of a stable home or family, exposure to physical or sexual abuse, genes, or mental illness. Still, the fact remains that early use is a strong indicator of problems ahead, including addiction.
- How the drug is taken. Smoking a drug or injecting it into a vein increases its addictive potential. 33,34 Both smoked and injected drugs enter the brain within seconds, producing a powerful rush of pleasure. However, this intense high can fade within a few minutes. Scientists believe this powerful contrast drives some people to repeatedly use drugs to recapture the fleeting pleasurable state.
Images of Brain Development in Healthy Children and Teens (Ages 5-20)
The brain continues to develop into adulthood and undergoes dramatic changes during adolescence.
One of the brain areas still maturing during adolescence is the prefrontal cortex—the part of the brain that allows people to assess situations, make sound decisions, and keep emotions and desires under control. The fact that this critical part of a teen’s brain is still a work in progress puts them at increased risk for trying drugs or continuing to take them. Introducing drugs during this period of development may cause brain changes that have profound and long-lasting consequences.
† The term addiction as used in this booklet is equivalent to a severe substance use disorder as defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5, 2013).
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.5(9); 2019 Sep
Advances in the science and treatment of alcohol use disorder
K. witkiewitz.
1 Department of Psychology and Center on Alcoholism, Substance Abuse, and Addictions, University of New Mexico, 2650 Yale Blvd. SE, Albuquerque, NM 87106, USA.
R. Z. Litten
2 Division of Medications Development and Division of Treatment and Recovery Research, National Institute on Alcohol Abuse and Alcoholism, 6700B Rockledge Drive, Bethesda, MD 20892-6902, USA.
3 Section on Clinical Psychoneuroendocrinology and Neuropsychopharmacology, National Institute on Alcohol Abuse and Alcoholism Division of Intramural Clinical and Biological Research, and National Institute on Drug Abuse Intramural Research Program, National Institutes of Health, 10 Center Drive (10CRC/15330), Bethesda, MD 21224, USA.
4 Medication Development Program, National Institute on Drug Abuse Intramural Research Program, 251 Bayview Blvd., Baltimore, MD 21224, USA.
5 Center for Alcohol and Addiction Studies, Brown University, Providence, RI 02912, USA.
Pharmacological and behavioral treatments exist for alcohol use disorder, but more are needed, and several are under development.
Alcohol is a major contributor to global disease and a leading cause of preventable death, causing approximately 88,000 deaths annually in the United States alone. Alcohol use disorder is one of the most common psychiatric disorders, with nearly one-third of U.S. adults experiencing alcohol use disorder at some point during their lives. Alcohol use disorder also has economic consequences, costing the United States at least $249 billion annually. Current pharmaceutical and behavioral treatments may assist patients in reducing alcohol use or facilitating alcohol abstinence. Although recent research has expanded understanding of alcohol use disorder, more research is needed to identify the neurobiological, genetic and epigenetic, psychological, social, and environmental factors most critical in the etiology and treatment of this disease. Implementation of this knowledge in clinical practice and training of health care providers is also needed to ensure appropriate diagnosis and treatment of individuals suffering from alcohol use disorder.
INTRODUCTION
In most regions of the world, most adults consume alcohol at least occasionally ( 1 ). Alcohol is among the leading causes of preventable death worldwide, with 3 million deaths per year attributable to alcohol. In the United States, more than 55% of those aged 26 and older consumed alcohol in a given month, and one in four adults in this age group engaged in binge drinking (defined as more than four drinks for women and five drinks for men on a single drinking occasion) ( 2 ). Excessive alcohol use costs U.S. society more than $249 billion annually and is the fifth leading risk factor for premature death and disability ( 3 ).
The morbidity and mortality associated with alcohol are largely due to the high rates of alcohol use disorder in the population. Alcohol use disorder is defined in the Diagnostic and Statistical Manual for Mental Disorders , 5th edition (DSM-5) ( 4 ) as a pattern of alcohol consumption, leading to problems associated with 2 or more of 11 potential symptoms of alcohol use disorder (see Table 1 for criteria). In the United States, approximately one-third of all adults will meet criteria for alcohol use disorder at some point during their lives ( 5 ), and approximately 15.1 million of U.S. adults meet criteria for alcohol use disorder in the previous 12 months ( 6 ). The public health impacts of alcohol use extend far beyond those individuals who drink alcohol, engage in heavy alcohol use, and/or meet criteria for an alcohol use disorder. Alcohol use is associated with increased risk of accidents, workplace productivity losses, increased medical and mental health costs, and greater rates of crime and violence ( 1 ). Analyses that take into account the overall harm due to drugs (harm to both users and others) show that alcohol is the most harmful drug ( 7 ).
| | |
| Tolerance | Tolerance |
| Withdrawal | Withdrawal |
| Difficulties controlling drinking (unsuccessful in cutting down or stopping drinking) | Difficulties controlling drinking (unsuccessful in cutting down or stopping drinking) |
| Neglect of activities | Neglect of activities |
| Time spent drinking or recovering from effects of alcohol | Time spent drinking or recovering from effects of alcohol |
| Drinking despite physical/ psychological problems | Drinking despite physical/ psychological problems |
| Craving | Craving |
| Alcohol consumed in larger amounts or over longer periods than was intended | |
| Failure to fulfill major role obligations | |
| Recurrent alcohol use in hazardous situations | |
| Drinking despite social/ interpersonal problems | |
| | |
Only a small percent of individuals with alcohol use disorder contribute to the greatest societal and economic costs ( 8 ). For example, in the 2015 National Survey on Drug Use and Health survey (total n = 43,561), a household survey conducted across the United States, 11.8% met criteria for an alcohol use disorder ( n = 5124) ( 6 ). Of these 5124 individuals, 67.4% ( n = 3455) met criteria for a mild disorder (two or three symptoms, based on DSM-5), 18.8% ( n = 964) met criteria for a moderate disorder (four or five symptoms, based on DSM-5), and only 13.8% ( n = 705) met criteria for a severe disorder (six or more symptoms) ( 6 ). There is a large treatment gap for alcohol use disorder, arising from the fact that many individuals with alcohol use disorder do not seek treatment. Those with a mild or moderate alcohol use disorder may be able to reduce their drinking in the absence of treatment ( 9 ) and have a favorable course; but it is those with more severe alcohol use disorder who most often seek treatment and who may experience a chronic relapsing course ( 10 ).
HISTORY OF TREATMENT FOR ALCOHOL USE DISORDER
Near the end of the 18th century, the Pennsylvania physician Benjamin Rush described the loss of control of alcohol and its potential treatments ( 11 ). His recommendations for remedies and case examples included practicing the Christian religion, experiencing guilt and shame, pairing alcohol with aversive stimuli, developing other passions in life, following a vegetarian diet, taking an oath to not drink alcohol, and sudden and absolute abstinence from alcohol. Through the 1800s and early 1900s, the temperance movement laid the groundwork for mutual help organizations, and the notion of excessive alcohol use as a moral failing. During the same period, inebriate asylums emerged as a residential treatment option for excessive alcohol use, although the only treatment offered was forced abstinence from alcohol ( 12 ). The founding of Alcoholics Anonymous (A.A.) in the 1930s ( 13 ) and the introduction of the modern disease concept of alcohol use disorder (previously called “alcoholism”) in the 1940s ( 14 ) laid the groundwork for many of the existing treatment programs that remain widely available today. Over the past 80 years, empirical studies have provided support for both mutual support [A.A. and other support groups, such as SMART (Self-Management and Recovery Training)] and medical models of treatment for alcohol use disorder, as well as the development of new pharmacological and behavioral treatment options. In addition, there are several public health policy initiatives (e.g., taxation, restrictions on advertising, and outlet density) and brief intervention programs (e.g., social norms interventions) that can be effective in reducing prevalence of alcohol use disorder and alcohol-related harms ( 1 ).
NEUROBIOLOGY OF ALCOHOL USE DISORDER
Alcohol use disorder is characterized by loss of control over alcohol drinking that is accompanied by changes in brain regions related to the execution of motivated behaviors and to the control of stress and emotionality (e.g., the midbrain, the limbic system, the prefrontal cortex, and the amygdala). Mechanisms of positive and negative reinforcement both play important roles with individual drinking behavior being maintained by positive reinforcement (rewarding and desirable effects of alcohol) and/or negative reinforcement mechanisms (negative affective and physiological states that are relieved by alcohol consumption) ( 15 , 16 ). At the neurotransmitter level, the positive reinforcing effects of alcohol are primarily mediated by dopamine, opioid peptides, serotonin, γ-aminobutyric acid (GABA), and endocannabinoids, while negative reinforcement involves increased recruitment of corticotropin-releasing factor and glutamatergic systems and down-regulation of GABA transmission ( 16 ). Long-term exposure to alcohol causes adaptive changes in several neurotransmitters, including GABA, glutamate, and norepinephrine, among many others. Discontinuation of alcohol ingestion results in the nervous system hyperactivity and dysfunction that characterizes alcohol withdrawal ( 15 , 16 ). Acting on several types of brain receptors, glutamate represents one of the most common excitatory neurotransmitters. As one of the major inhibitory neurotransmitters, GABA plays a key role in the neurochemical mechanisms involved in intoxication, tolerance, and withdrawal. This brief review can offer only a very simplified overview of the complex neurobiological basis of alcohol use disorder. For deeper, more detailed analysis of this specific topic, the reader is encouraged to consult other reviews ( 15 , 16 ).
CLINICAL MANAGEMENT OF ALCOHOL WITHDRAWAL SYNDROME
Alcohol withdrawal symptoms may include anxiety, tremors, nausea, insomnia, and, in severe cases, seizures and delirium tremens. Although up to 50% of individuals with alcohol use disorder present with some withdrawal symptoms after stopping drinking, only a small percentage requires medical treatment for detoxification, and some individuals may be able to reduce their drinking spontaneously. Medical treatment may take place either in an outpatient or, when clinically indicated, inpatient setting. In some cases, clinical monitoring may suffice, typically accompanied by supportive care for hydration and electrolytes and thiamine supplementation. For those patients in need of pharmacological treatment, benzodiazepines (e.g., diazepam, chlordiazepoxide, lorazepam, oxazepam, and midazolam) are the most commonly used medications to treat alcohol withdrawal syndrome. Benzodiazepines work by enhancing the effect of the GABA neurotransmitter at the GABA A receptor. Notably, benzodiazepines represent the gold standard treatment, as they are the only class of medications that not only reduces the severity of the alcohol withdrawal syndrome but also reduces the risk of withdrawal seizures and/or delirium tremens. Because of the potential for benzodiazepine abuse and the risk of overdose, if benzodiazepine treatment for alcohol withdrawal syndrome is managed in an outpatient setting, careful monitoring is required, particularly when combined with alcohol and/or opioid medications ( 17 ).
a-2 agonists (e.g., clonidine) and β-blockers (atenolol) are sometimes used as an adjunct treatment to benzodiazepines to control neuro-autonomic manifestations of alcohol withdrawal not fully controlled by benzodiazepine administration ( 18 ). However, because of the lack of efficacy of a-2 agonists and β-blockers in preventing severe alcohol withdrawal syndrome and the risk of masking withdrawal symptoms, these drugs are recommended not as monotherapy, but only as a possible adjunctive treatment.
Of critical importance to a successful outcome is the fact that alcohol withdrawal treatment provides an opportunity for the patient and the health care provider to engage the patient in a treatment program aimed at achieving and maintaining long-term abstinence from alcohol or reductions in drinking. Such a treatment may include pharmacological and/or psychosocial tools, as summarized in the next sections.
PHARMACOLOGICAL APPROACHES TO THE TREATMENT OF ALCOHOL USE DISORDER
U.s. food and drug administration–approved pharmacological treatments.
Development of novel pharmaceutical reagents is a lengthy, costly, and expensive process. Once a new compound is ready to be tested for human research use, it is typically tested for safety first via phase 0 and phase 1 clinical studies in a very limited number of individuals. Efficacy and side effects may then be further tested in larger phase 2 clinical studies, which may be followed by larger phase 3 clinical studies, typically conducted in several centers and are focused on efficacy, effectiveness, and safety. If approved for use in clinical practice, this medication is still monitored from a safety standpoint, via phase 4 postmarketing surveillance.
Only three drugs are currently approved by the U.S. Food and Drug Administration (FDA) for use in alcohol use disorder. The acetaldehyde dehydrogenase inhibitor disulfiram was the first medication approved for the treatment of alcohol use disorder by the FDA, in 1951. The most common pathway in alcohol metabolism is the oxidation of alcohol via alcohol dehydrogenase, which metabolizes alcohol to acetaldehyde, and aldehyde dehydrogenase, which converts acetaldehyde into acetate. Disulfiram leads to an irreversible inhibition of aldehyde dehydrogenase and accumulation of acetaldehyde, a highly toxic substance. Although additional mechanisms (e.g., inhibition of dopamine β-hydroxylase) may also play a role in disulfiram’s actions, the blockade of aldehyde dehydrogenase activity represents its main mechanism of action. Therefore, alcohol ingestion in the presence of disulfiram leads to the accumulation of acetaldehyde, resulting in numerous related unpleasant symptoms, including tachycardia, headache, nausea, and vomiting. In this way, disulfiram administration paired with alcohol causes the aversive reaction, initially proposed as a remedy for alcohol use disorder by Rush ( 11 ) in 1784. One challenge in conducting a double-blind, placebo-controlled alcohol trial of disulfiram is that it is easy to break the blind unless the “placebo” medication also creates an aversive reaction when consumed with alcohol, which would then provide the same mechanism of action as the medication (e.g., the placebo and disulfiram would both have the threat of an aversive reaction). Open-label studies of disulfiram do provide support for its efficacy, as compared to controls, with a medium effect size ( 19 ), as defined by Cohen’s d effect size ranges of small d = 0.2, medium d = 0.5, and large d = 0.8 ( 20 ). The efficacy of disulfiram largely depends on patient motivation to take the medication and/or supervised administration, given that the medication is primarily effective by the potential threat of an aversive reaction when paired with alcohol ( 21 ).
The next drug approved for treatment of alcohol use disorder was acamprosate; first approved as a treatment for alcohol dependence in Europe in 1989, acamprosate has subsequently been approved for use in the United States, Canada, and Japan. Although the exact mechanisms of acamprosate action are still not fully understood, there is evidence that it targets the glutamate system by modulating hyperactive glutamatergic states, possibly acting as an N -methyl- d -aspartate receptor agonist ( 22 ). The efficacy of acamprosate has been evaluated in numerous double-blind, randomized controlled trials and meta-analyses, with somewhat mixed conclusions ( 23 – 26 ). Although a meta-analysis conducted in 2013 ( 25 ) indicated small to medium effect sizes in favor of acamprosate over placebo in supporting abstinence, recent large-scale trials conducted in the United States ( 27 ) and Germany ( 28 ) failed to find effects of acamprosate distinguishable from those of a placebo. Overall, there is evidence that acamprosate may be more effective in promoting abstinence and preventing relapse in already detoxified patients than in helping individuals reduce drinking ( 25 ), therefore suggesting its use as an important pharmacological aid in treatment of abstinent patients with alcohol use disorder. The most common side effect with acamprosate is diarrhea. Other less common side effects may include nausea, vomiting, stomachache, headache, and dizziness, although the causal role of acamprosate in giving these side effects is unclear.
A third drug, the opioid receptor antagonist naltrexone, was approved for the treatment of alcohol dependence by the FDA in 1994. Later, a monthly extended-release injectable formulation of naltrexone, developed with the goal of improving patient adherence, was also approved by the FDA in 2006. Naltrexone reduces craving for alcohol and has been found to be most effective in reducing heavy drinking ( 25 ). The efficacy of naltrexone in reducing relapse to heavy drinking, in comparison to placebo, has been supported in numerous meta-analyses ( 23 – 25 ), although there is less evidence for its efficacy in supporting abstinence ( 25 ). Fewer studies have been conducted with the extended-release formulation, but its effects on heavy drinking, craving, and quality of life are promising ( 29 , 30 ). Common side effects of naltrexone may include nausea, headache, dizziness, and sleep problems. Historically, naltrexone’s package insert has been accompanied by a risk of hepatotoxicity, a precaution primarily due to observed liver toxicity in an early clinical trial with administrating a naltrexone dosage of 300 mg per day to obese men ( 31 ). However, there is no published evidence of severe liver toxicity at the lower FDA-approved dosage of naltrexone for alcohol use disorder (50 mg per day). Nonetheless, transient, asymptomatic hepatic transaminase elevations have also been observed in some clinical trials and in the postmarketing period; therefore, naltrexone should be used with caution in patients with active liver disease and should not be used in patients with acute hepatitis or liver failure.
Additional pharmacological treatments approved for alcohol use disorder in Europe
Disulfiram, acamprosate, and naltrexone have been approved for use in Europe and in the United States. Pharmacologically similar to naltrexone, nalmefene was also approved for the treatment of alcohol dependence in Europe in 2013. Nalmefene is a m- and d-opioid receptor antagonist and a partial agonist of the k-opioid receptor ( 32 ). Side effects of nalmefene are similar to naltrexone; compared to naltrexone, nalmefene has a longer half-life. Meta-analyses have indicated that nalmefene is effective in reducing heavy drinking days ( 32 ). An indirect meta-analysis of these two drugs concluded that nalmefene may be more effective than naltrexone ( 33 ), although whether a clinically relevant difference between the two medications really exists is still an open question ( 34 ). Network meta-analysis and microsimulation studies suggest that nalmefene may have some benefits over placebo for reducing total alcohol consumption ( 35 , 36 ). The approval of nalmefene in Europe was accompanied by some controversy ( 37 ); a prospective head-to-head trial of nalmefene and naltrexone could help clarify whether nalmefene has added benefits to the existing medications available for alcohol use disorder. Last, nalmefene was approved in Europe as a medication that can be taken “as needed” (i.e., on days when drinking was going to occur). Prior work has also demonstrated the efficacy of taking naltrexone only on days that drinking was potentially going to occur ( 38 ).
In addition to these drugs, a GABA B receptor agonist used to treat muscle spasms, baclofen, was approved for treatment of alcohol use disorder in France in 2018 and has been used off label for alcohol use disorder for over a decade in other countries, especially in other European countries and in Australia ( 39 , 40 ). Recent human laboratory work suggests that baclofen may disrupt the effects of an initial priming dose of alcohol on subsequent craving and heavy drinking ( 41 ). Meta-analyses and systematic reviews examining the efficacy of baclofen have yielded mixed results ( 35 , 39 , 42 ); however, there is some evidence that baclofen might be useful in treatment of alcohol use disorder among individuals with liver disease ( 43 , 44 ). Evidence of substantial heterogeneity in baclofen pharmacokinetics among different individuals with alcohol use disorder ( 41 ) could explain the variability in the efficacy of baclofen across studies. The appropriate dose of baclofen for use in treatment of alcohol use disorder remains a controversial topic, and a recent international consensus statement highlighted the importance of tailoring doses based on safety, tolerability, and efficacy ( 40 ).
Promising pharmacological treatments
Numerous other medications have been used off label in the treatment of alcohol use disorder, and many of these have been shown to be modestly effective in meta-analyses and systematic reviews ( 23 , 24 , 26 , 35 ). Systematic studies of these medications suggest promising findings for topiramate, ondansetron, gabapentin, and varenicline. The anticonvulsant drug topiramate represents one of the most promising medications in terms of efficacy, based on its medium effect size from several clinical trials [for a review, see ( 45 )], including a multisite clinical study ( 46 ). One strength of topiramate is the possibility of starting treatment while people are still drinking alcohol, therefore serving as a potentially effective treatment to initiate abstinence (or to reduce harm) rather than to prevent relapse in already detoxified patients ( 45 ). Although not approved by the FDA, it is worth noticing that topiramate is a recommended treatment for alcohol use disorder in the U.S. Department of Veterans Affairs ( 47 ). A concern with topiramate is the potential for significant side effects, especially those affecting cognition and memory, warranting a slow titration of its dose and monitoring for side effects. Furthermore, recent attention has been paid on zonisamide, another anticonvulsant medication, whose pharmacological mechanisms of actions are similar to topiramate but with a better tolerability and safety profile ( 48 ). Recently published and ongoing research focuses on a potential pharmacogenetic approach to treatment in the use of topiramate to treat alcohol use disorder, based on the possibility that both efficacy and tolerability and safety of topiramate may be moderated by a functional single-nucleotide polymorphism (rs2832407) in GRIK1, encoding the kainate GluK1 receptor subunit ( 49 ). Human laboratory studies ( 50 ) and treatment clinical trials ( 51 ) have also used a primarily pharmacogenetic approach to testing the efficacy of the antinausea drug ondansetron, a 5HT 3 antagonist, in alcohol use disorder. Overall, these studies suggest a potential role for ondansetron in alcohol use disorder, but only in those individuals with certain variants of the genes encoding the serotonin transporter 5-HTT and the 5-HT 3 receptor. The anticonvulsant gabapentin has shown promising results in human laboratory studies and clinical trials ( 52 – 54 ), although a more recent multisite trial with an extended-release formulation of the medication did not have an effect of gabapentin superior to that of a placebo ( 55 ). Although the latter findings might be related to potential pharmacokinetic issues secondary to the specific formulation used, it is nonetheless possible that gabapentin may be more effective in patients with more clinically relevant alcohol withdrawal symptoms ( 52 ). Several human laboratory studies support a role for varenicline, a nicotinic acetylcholine receptor partial agonist approved for smoking cessation, in alcohol use disorder [for a review, see ( 56 )], and two of three clinical trials also support its efficacy on alcohol outcomes ( 57 – 59 ), especially in heavy drinkers who are males ( 59 ) and in male and female alcohol-dependent individuals who are also smokers ( 60 ). Additional details on the FDA-approved medications and other medications tested in clinical research settings for the treatment of alcohol use disorder are summarized in Table 2 .
FDA, U.S. Food and Drug Administration; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; NMDA, N -methyl- d -aspartate; PO, per os (oral); IM, intramuscular; HT, serotonin.
| | ||
| Acamprosate (PO) | 1998 mg per day | Unclear—it has been suggested that acamprosate is a modulator of hyperactive glutamatergic states, possibly as an NMDA receptor agonist |
| Disulfiram (PO) | 250–500 mg per day | Inhibition of acetaldehyde dehydrogenase |
| Naltrexone (PO) | 50 mg per day | m-opioid receptor antagonist |
| Naltrexone (IM) | 380 mg once a month | m-opioid receptor antagonist |
| Baclofen (PO) | 30–80 mg per day | GABA receptor agonist |
| Approved in France by the National Agency for the Safety of Medicines and Healthcare Products | ||
| Gabapentin (PO) | 900–1800 mg per day | Unclear—the most likely mechanism is blockade of voltage-dependent Ca channels. Although it is a GABA analog, gabapentin does not seem to act on the GABA receptors |
| Nalmefene (PO) | 18 mg per day | m- and d-opioid receptor antagonist and k-opioid receptor partial agonist |
| Approved in Europe by the European Medicines Agency | ||
| Ondansetron (PO) | 0.5 mg per day (fixed dose) or up to 36 mcg/kg per day | 5HT antagonist |
| Prazosin/doxazosin (PO) | Up to 16 mg per day | a-1 receptor antagonists |
| Topiramate (PO) | Up to 300 mg per day | Topiramate is an anticonvulsant with multiple targets. It increases GABA -facilitated neuronal activity and simultaneously antagonizes AMPA and kainate glutamate receptors. It also inhibits l-type calcium channels, limits the activity of voltage-dependent sodium channels and facilitates potassium conductance. Furthermore, it is a weak inhibition of the carbonic anhydrase isoenzymes, CA-II and CA-IV |
| Varenicline (PO) | 2 mg per day | Nicotinic acetylcholine receptor partial agonist |
The medications and targets described above have shown promising results in phase 2 or phase 3 medication trials. However, owing to the development of novel neuroscience techniques, a growing and exciting body of data is expanding the armamentarium of targets currently under investigation in animal models and/or in early-phase clinical studies. Pharmacological approaches with particular promise for future drug development include, but are not limited to the following [for recent reviews, see, e.g., ( 56 , 61 – 68 )]: the antipsychotic drug aripiprazole, which has multiple pharmacological actions (mainly on dopamine and serotonin receptors), the antihypertensive alpha-1 blocker drugs prazosin and doxazosin, neurokinin-1 antagonism, the glucocorticoid receptor blocker mifepristone, vasopressin receptor 1b antagonism, oxytocin, ghrelin receptor antagonism, glucagon-like peptide-1 agonism, and pharmacological manipulations of the nociception receptor (We are intentionally using a general pharmacological terminology for the nociceptin receptor, given that it is unclear whether agonism, antagonism, or both may represent the best approach.). New medications development is particularly important for the treatment of comorbid disorders that commonly co-occur among individuals with alcohol use disorder, particularly affective disorders, anxiety disorders, suicidality, and other substance use disorders. This aspect of alcohol use disorder is relevant to the fact that addictive disorders often present with significantly more severe symptoms when they coexist with other mental health disorders ( 69 ). Likewise, there is evidence that pharmacotherapy is most effective when implemented in conjunction with behavioral interventions ( 70 ), and all phase 2 and phase 3 medication trials, mentioned above, have included a brief psychosocial behavioral treatment in combination with medication.
BEHAVIORAL/PSYCHOLOGICAL TREATMENTS FOR ALCOHOL USE DISORDER
Evidence-based treatments.
A wide range of behavioral and psychological treatments are available for alcohol use disorder, and many treatments are equally effective in supporting abstinence or drinking reduction goals ( 71 – 74 ). Treatments with the greatest evidence of efficacy range from brief interventions, including motivational interviewing approaches, to operant conditioning approaches, including contingency management and the community reinforcement approach, to cognitive behavioral treatments, including coping skills training and relapse prevention, and to acceptance- and mindfulness-based approaches. Twelve-step facilitation, which was designed specifically to connect individuals with mutual support groups, has also been shown to be effective ( 75 ). In addition, harm reduction treatments, including guided self-control training and controlled drinking interventions, have been successful in supporting drinking reduction goals ( 70 ).
Meta-analyses and systematic reviews have found that brief interventions, especially those based on the principles of motivational interviewing, are effective in the treatment of alcohol use disorder. These interventions can include self-monitoring of alcohol use, increasing awareness of high-risk situations, and training in cognitive and behavioral techniques to help clients cope with potential drinking situations, as well as life skills training, communication training, and coping skills training. Cognitive behavioral treatments can be delivered in individual or group settings and can also be extended to the treatment of families and couples ( 72 , 73 ).
Acceptance- and mindfulness-based interventions are increasingly being used to target alcohol use disorder and show evidence of efficacy in a variety of settings and formats, including brief intervention formats ( 76 ). Active ingredients include raising present moment awareness, developing a nonjudgmental approach to self and others, and increasing acceptance of present moment experiences. Acceptance- and mindfulness-based interventions are commonly delivered in group settings and can also be delivered in individual therapy contexts.
Computerized, web-based, and mobile interventions have also been developed, incorporating the principles of brief interventions, behavioral and cognitive behavioral approaches, as well as mindfulness and mutual support group engagement; many of these approaches have demonstrated efficacy in initial trials ( 77 – 79 ). For example, the National Institute on Alcohol Abuse and Alcoholism (NIAAA) has developed the Take Control computerized intervention that includes aspects of motivational interviewing and coping skills training and was designed to provide psychosocial support (particularly among those assigned to the placebo medication) and also to increase adherence and retention among individuals enrolled in pharmacotherapy trials ( 80 ).
Mutual support group (e.g., A.A. and SMART) attendance and engagement have been shown to be associated with recovery from alcohol use disorder, even in the absence of formal treatment ( 81 ). However, selection biases (e.g., people selecting to attend these groups) raise difficulties in assessing whether other factors that are associated with treatment effectiveness may be the active ingredients for improving outcomes among those who attend mutual support groups. For example, individuals who are highly motivated to change might be more likely to attend mutual support groups. Likewise, mutual support groups often provide individuals with increased social network support for abstinence ( 82 ). Motivation to change and having a social network that supports abstinence (or reductions in drinking) are both factors that are associated with greater treatment effectiveness ( 83 ).
As noted above, most behavioral and psychological treatments are equally effective with small effect size differences [Cohen’s d = 2.0 to 0.3 ( 20 )] between active treatments ( 84 – 88 ). Behavioral interventions have also been shown to be as effective as pharmacotherapy options, with a 16-week cognitive behavioral intervention shown to be statistically equivalent to naltrexone in reducing heavy drinking days in a large randomized trial ( 27 ). One of the challenges of examining behavioral interventions in randomized trials is that intervention blinding and placebo controls cannot be implemented in most contexts, other than in computerized interventions. Furthermore, the general therapeutic factors common to most behavioral interventions (e.g., therapist empathy and supportive therapeutic relationship) in treatment of alcohol use disorder are as powerful as the specific therapeutic targets of specific behavioral interventions (e.g., teaching skills in a cognitive behavioral treatment) in facilitating behavioral change ( 89 ).
Promising future behavioral treatments and neuromodulation treatments
With respect to behavioral treatments, there are numerous opportunities for the development of novel mobile interventions that could provide treatment and recovery support in near real time. This mobile technology may also extend the reach of treatments to individuals with alcohol use disorder, particularly in rural areas. On the basis of a contextual self-regulation model of alcohol use ( 90 ), it is critical to address the immediate situational context alongside the broader social, environmental, and familial context in which an individual experiences the world and engages in momentary decision-making. Ambulatory assessment, particularly tools that require only passive monitoring (e.g., GPS, heart rate, and skin conductance) and real-time support via mobile health, could provide immediate environmental supports and could extend the reach of medications and behavioral treatments for alcohol use disorder. For example, a mobile device could potentially signal a high-risk situation by indicating the geographic location (near a favorite drinking establishment) and the heart rate (increased heart rate when approaching the establishment). The device could provide a warning either to the individual under treatment and/or to a person supporting that individual’s recovery. In addition, developments in alcohol sensing technology (e.g., transdermal alcohol sensors) could greatly increase rigor of research on alcohol use disorder and also provide real-time feedback on alcohol consumption levels to individuals who are attempting to moderate and/or reduce their alcohol use.
Recent advances in neuromodulation techniques may also hold promise for the development of novel treatments for alcohol use disorder. Deep brain stimulation, transcranial magnetic stimulation, transcranial electrical stimulation (including transcranial direct current stimulation and transcranial alternating current stimulation), and real-time neurofeedback have recently been tested as potential treatments for addiction, although evidence in favor of these treatments is currently uncertain and focused mostly on intermediate targets (e.g., alcohol craving) ( 91 ). These techniques attempt to directly target specific brain regions and addiction-related cognitive processes via surgically implanted electrodes (deep brain stimulation), electrical currents or magnetic fields applied to the scalp (transcranial electrical and magnetic stimulation, respectively), or individual self-generated modulation via feedback (neurofeedback). Although robust large scale trials with double-blind, sham controls, and long-term follow-ups of alcohol behavior change and relapse have not been conducted ( 91 ), the heterogeneity of alcohol use disorder suggests that targeting one specific neural region may be insufficient to treat such a complex disorder, with its multiple etiologies and diverse clinical courses ( 92 ).
Factors contributing to the effectiveness of treatments
Numerous models have examined factors that predict treatment readiness, treatment engagement, and treatment outcomes for alcohol use disorder. The transtheoretical model of change proposes that an individual’s own readiness to change his or her drinking behavior may have an impact on treatment engagement and effectiveness ( 93 ). The dynamic model of relapse proposes the involvement of multiple interacting biological, psychological, cognitive, emotional, social, and situational risk factors that are static and dynamic in their association with treatment outcomes ( 83 ). Neurobiological models of addiction focus on the brain reward and stress system dysfunction that contributes to the development and maintenance of alcohol use disorder, that is, the “addiction cycle” ( 15 , 16 ). The alcohol and addiction research domain criteria (AARDoC) ( 92 ), which have been operationalized in the addictions neuroclinical assessment ( 94 ), focus on the following three domains that correspond to particular phases in the addiction cycle: incentive salience in the binge/intoxication phase, negative emotionality in the withdrawal/negative affect phase, and executive function in the preoccupation/anticipation phase. Within each domain of the AARDoC, the addictions neuroclinical assessment proposes constructs that can be measured at multiple levels of analysis, such as craving in the incentive salience domain, negative affect and emotion dysregulation in the negative emotionality domain, and cognitive impairment and impulsivity in the executive function domain. The AARDoC acknowledge that environmental and contextual factors play a role in alcohol use disorder and treatment outcomes. Moreover, because of the heterogeneity of alcohol use disorder, the significance of these domains in causing alcohol use disorder and alcohol-related problems will vary among individuals.
Each of the abovementioned theoretical models proposes factors that may affect treatment effectiveness; however, many of the constructs proposed in each of these models are overlapping and likely contribute to the effectiveness of alcohol use disorder treatment across a range of populations and settings. A heuristic model combining components from each of these models is shown in Fig. 1 . Specifically, this model highlights the precipitants of alcohol use that are influenced by the neurobiological adaptations proposed in the addiction cycle (indicated by bold font) and additional contextual factors (regular font) that decrease or increase the likelihood of drinking in context, depending on whether an individual uses effective coping regulation in the moment. The domains supporting alcohol use/coping regulation (negative emotionality, executive function, incentive salience, and social environment) may interact to predict alcohol use or coping regulation in the moment. For example, network support for abstinence could improve decision-making and decrease likelihood of drinking. Conversely, experiences of physical pain are associated with increases in negative affect and poorer executive function, which could both increase likelihood of drinking. Both of these examples require environmental access to alcohol and a desire to drink alcohol. Treatment effectiveness will depend on the extent to which a particular treatment targets those risk factors that are most likely to increase or decrease the likelihood of drinking for each individual, as well as the personal resources that each individual brings to treatment and/or that could be enhanced in treatment. A functional analysis of contextual risk and protective factors can be critically important in guiding treatment.

Risk factors proposed in the AARDoC, including incentive salience, negative emotionality, executive function, and social environmental factors, are shown in black bold font encircling alcohol use. Contextual risk factors, including decision-making, self-efficacy, pain, craving, etc., are shown in black font in colored boxes. Risk and protective factors overlap with alcohol use and interact in predicting coping regulation and alcohol use among individual patients.
For example, there is considerable heterogeneity in treatment response to naltrexone, which may vary in efficacy in some individuals. Recent studies conducted to determine whether certain patients may benefit more from naltrexone have yielded mixed findings ( 95 ). Promising evidence suggests that individuals with the OPRM1 A118G G (Asp40) allele may have a better response to naltrexone ( 96 – 98 ); however, a prospective study of medication response among individuals stratified by presence of the Asp40 allele did not provide support for the genotype by treatment interaction ( 99 ), and recent human laboratory studies have not confirmed the hypothesized mechanisms underlying the pharmacogenomic effect ( 100 ). Initial evidence suggests that naltrexone may be more effective in reducing heavy drinking among smokers ( 101 ) and among those with a larger number of heavy drinkers in their social networks ( 102 ). With respect to reinforcement typologies, recent work has found that naltrexone may be more effective among those who tend to drink alcohol for rewarding effects ( 103 ), and acamprosate may also be more effective for individuals who drink to relieve negative affect ( 104 ).
GAPS IN SCIENTIFIC KNOWLEDGE AND NEW RESEARCH DIRECTIONS
Heterogeneity of individuals with alcohol use disorder.
This review has briefly summarized the treatments currently available for alcohol use disorder that are relatively effective, at least in some patients. Many new treatments are also being developed, and some of them seem promising. Nevertheless, numerous gaps in scientific knowledge remain. Notably, most people who drink alcohol do not develop an alcohol use disorder, most people with alcohol use disorder do not seek treatment, and most of those who do not seek treatment “recover” from alcohol use disorder without treatment ( 2 ). Very little is known about factors, particularly neurobiological, genetic, and epigenetic factors, that predict the transition from alcohol use to alcohol use disorder, although basic science models suggest that a cycle of neuroadaptations could be at play ( 15 , 16 ). We also lack a basic understanding of how individuals recover from alcohol use disorder in the absence of treatment and what neurobiological, psychological, social, and environmental factors are most important for supporting recovery from alcohol use disorder. Gaining a better understanding of recovery in the absence of treatment, particularly modifiable psychological, neurobiological, and epigenetic factors, could provide novel insights for medications and behavioral treatment development. Among many other factors, special attention is needed in future studies to shed light on the role of sex and gender in the development and maintenance of alcohol use disorder and on the response to pharmacological, behavioral, and other treatments.
The heterogeneity of alcohol use disorder presents a major challenge to scientific understanding and to the development of effective treatments for prevention and intervention ( 92 ). For example, a DSM-5 diagnosis of alcohol use disorder requires 2 or more symptoms, out of 11, over the past year. That requirement equates to exactly 2048 potential symptom combinations that would meet the criteria of alcohol use disorder. An individual who only meets criteria for tolerance and withdrawal (i.e., physiological dependence) likely requires a very different course of treatment from an individual who only meets the criteria for failure to fulfill role obligations and use of alcohol in hazardous situations. Gaining a better understanding of the etiology and course of alcohol use disorder, as well as identifying whether different subtypes of drinkers may respond better to certain treatments ( 103 , 104 ), is critical for advancing the science of alcohol use disorder prevention and treatment. Alternative conceptualizations of alcohol use disorder may also aid in improving our understanding of the disorder and reducing heterogeneity. For example, the pending International Classification of Diseases , 11th edition, will simplify the diagnosis of alcohol dependence to requiring only two of three criteria in the past 12 months: (i) impaired control over alcohol use; (ii) alcohol use that dominates over other life activities; and (iii) persistence of alcohol use despite consequences. The diagnosis will be made with or without physiological dependence, as characterized by tolerance, withdrawal, or repeated use to prevent or alleviate withdrawal ( 105 ). It remains to be seen whether simplification of the criteria set will narrow our conceptualization or potentially increase heterogeneity of this disorder among those diagnosed with alcohol dependence.
Placebo effect
An additional challenge to development of pharmacological treatments for alcohol use disorder is the high placebo response rates seen in drug trials ( 106 ). The tendency for individuals to have a good treatment response when assigned to placebo medication reflects both the high probability of recovery without treatment and the heterogeneity in the disorder itself. Many people who enter treatment are already motivated to change behavior, and receiving a placebo medication can help these individuals continue the process of change. Gaining a better understanding of which kinds of individuals respond to placebo and of the overall physiological and behavioral complexities in the placebo response is critical to identifying those individuals who will benefit the most from active medication. More generally, very little is understood about how motivation to change drinking behavior may influence the efficacy of active medications, particularly via adherence mechanisms. Additional research on targeted (i.e., as needed) dosing of medications, such as nalmefene and naltrexone ( 32 , 38 ), would be promising from the perspective of increasing adherence to medications and also raising awareness of potentially heavy drinking occasions.
Recent developments in pharmacological and behavioral approaches
In addition to gaining a better understanding of the disorder and who benefits from existing treatments, the examination of molecular targets for alcohol use disorder could open up multiple innovative directions for future translational research on the treatment of alcohol use disorder. Recent research has identified many targets that might be important for future medication trials ( 67 ). For example, most of the medication development efforts in past decades have focused on pathways and targets typically related to reward processing and positive reinforcement. While important, this approach ignores the important role of stress-related pathways (e.g., corticotropin release factor and other related pathways) in negative reinforcement and in the later stages of alcohol use disorder, which is often characterized by physical dependence, anxiety, and relief drinking [for reviews, see ( 15 , 16 )]. Furthermore, it is also becoming more and more apparent that other promising targets may be identified by looking at the brain not as an isolated system but rather as an organ with bidirectional interactions with peripheral systems. Examples of the latter approach include the growing evidence suggesting a potential role of inflammation and neuroinflammation and of the gut-liver-brain axis in the neurobiological mechanisms that regulate the development and/or maintenance of alcohol use disorder ( 107 – 109 ). Moving medications development from phase 1 to phase 2 and 3 trials has also been a difficulty in the field. Future directions that might improve translation of basic science into clinical practice include the broader use of human laboratory models and pilot clinical trials ( 110 ), as well as expanding the outcomes that might be targeted in phase 2 and phase 3 trials to include drinking reduction outcomes ( 111 , 112 ).
New directions for behavioral treatment development include a greater focus on identifying effective elements of behavioral treatments and on the components of treatment that are most critical for successful behavior change ( 89 , 113 ). Studies investigating the effects of specific treatment components are critical for refining treatment protocols to more efficiently target the symptoms of alcohol use disorder. Continued development of mobile health interventions will also help with disseminating treatment to a wider range of individuals struggling with alcohol use disorder.
Translation of addiction science to clinical practice
Last, but not the least, there is also a critical need for more research on dissemination and implementation, given the fact that many treatment programs still do not incorporate evidence-based practices, such as cognitive behavioral skills training, mindfulness-based interventions, and medications. Both pharmacological and behavioral treatments for alcohol use disorder are markedly underused; the recent Surgeon General’s report Facing Addiction in America ( 114 ) highlights the fact that only about 1 in 10 people with a substance use disorder receives any type of specialty treatment. Therefore, basic science and human research efforts will need to be accompanied by translational approaches, where effective novel medications and precision medicine strategies are effectively translated from research settings to clinical practice. Greater integration of alcohol screening and medication in primary care and other clinical settings, as well as research on best methods for implementation, has great potential for expanding access to effective treatment options ( 115 ). Because the heterogeneity of alcohol use disorder makes it highly unlikely that one single treatment will work for all individuals, it is important to provide a menu of options for pharmacological and behavioral therapies to both clinicians and patients. Reducing the stigma of alcohol use disorder and moving toward a public health approach to addressing this problem may further increase the range of acceptable treatment options.
Acknowledgment
Funding: This research was supported by a grant from NIAAA (R01 AA022328) awarded to K.W. (principal investigator). R.Z.L. is funded by NIAAA. L.L. is jointly funded by NIAAA and the National Institute on Drug Abuse (NIDA) (ZIA-AA000218). The content of this review does not necessarily represent the official views of the funders. Author contributions: K.W. wrote the first draft of the manuscript. K.W., R.Z.L., and L.L. provided additional text and edits. All authors approved the final draft. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or in the materials cited herein. Additional data related to this paper may be requested from the authors.
REFERENCES AND NOTES
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
News releases.
News Release
Thursday, August 15, 2024
NIH launches program to advance research led by Native American communities on substance use and pain
Effort aims to elevate Indigenous Knowledge and culture in research, to respond to the overdose crisis and address related health disparities.

The National Institutes of Health (NIH) has launched a program that will support Native American communities to lead public health research to address overdose, substance use, and pain, including related factors such as mental health and wellness. Despite the inherent strengths in Tribal communities, and driven in part by social determinants of health, Native American communities face unique health disparities related to the opioid crisis. For instance, in recent years, overdose death rates have been highest among American Indian and Alaska Native people . Research prioritized by Native communities is essential for enhancing effective, culturally grounded public health interventions and promoting positive health outcomes.
“Elevating the knowledge, expertise, and inherent strengths of Native people in research is crucial for creating sustainable solutions that can effectively promote public health and health equity,” said Nora D. Volkow, M.D., director of NIH’s National Institute on Drug Abuse (NIDA). “As we look for ways to best respond to the overdose crisis across the country, it is crucial to recognize that Native American communities have the best perspective for developing prevention and therapeutic interventions consistent with their traditions and needs. This program will facilitate research that is led by Native American communities, for Native American communities.”
Totaling approximately $268 million over seven years, pending the availability of funds, the Native Collective Research Effort to Enhance Wellness (N CREW) Program will support research projects that are led directly by Tribes and organizations that serve Native American communities, and was established in direct response to priorities identified by Tribes and Native American communities.
Many Tribal Nations have developed and continue to develop innovative approaches and systems of care for community members with substance use and pain disorders. During NIH Tribal Consultations in 2018 and 2022 , Tribal leaders categorized the opioid overdose crisis as one of their highest priority issues and called for research and support to respond. They shared that Native communities must lead the science and highlighted the need for research capacity building, useful real-time data, and approaches that rely on Indigenous Knowledge and community strengths to meet the needs of Native people.
The N CREW Program focuses on:
- Supporting research prioritized by Native communities, including research elevating and integrating Indigenous Knowledge and culture
- Enhancing capacity for research led by Tribes and Native American Serving Organizations by developing and providing novel, accessible, and culturally grounded technical assistance and training, resources, and tools
- Improving access to, and quality of, data on substance use, pain, and related factors to maximize the potential for use of these data in local decision-making.
“Native American communities have been treating pain in their communities for centuries, and this program will uplift that knowledge to support research that is built around cultural strengths and priorities,” said Walter Koroshetz, M.D., director of NIH’s National Institute of Neurological Disorders and Stroke (NINDS). “These projects will further our collective understanding of key programs and initiatives that can effectively improve chronic pain management for Native American and other communities.”
The first phase of the program will support projects to plan, develop, and pilot community-driven research and/or data improvement projects to address substance use and pain. In this phase, NIH will also support the development of a Native Research Resource Network to provide comprehensive training, resources, and real-time support to N CREW participants.
The second phase of the program, anticipated to begin in fall 2026, will build on the work conducted in the initial phase of the program to further capacity building efforts and implement community-driven research and/or data improvements projects. Additional activities that support the overarching goals of the N CREW Program may also be identified as the program develops.
The N CREW Program is led by the NIH’s NIDA, NINDS, and National Center for Advancing Translational Sciences (NCATS), with participation from numerous other NIH Institutes, Centers, and Offices. The N CREW Program is funded through the NIH Helping to End Addiction Long-term Initiative (or NIH HEAL Initiative) , which is jointly managed by NIDA and NINDS. For the purposes of the N CREW Program, Native Americans include American Indians, Alaska Natives, and Native Hawaiians. Projects will be awarded on a rolling basis and publicly listed .
This new program is part of work to advance the Biden/Harris Administration’s Unity Agenda and the HHS Overdose Prevention Strategy .
Helping to End Addiction Long-term® and NIH HEAL Initiative® are registered service marks of the Department of Health and Human Services.
About the National Institute on Drug Abuse (NIDA): NIDA is a component of the National Institutes of Health, U.S. Department of Health and Human Services. NIDA supports most of the world’s research on the health aspects of drug use and addiction. The Institute carries out a large variety of programs to inform policy, improve practice, and advance addiction science. For more information about NIDA and its programs, visit www.nida.nih.gov .
About the National Institute of Neurological Disorders and Stroke (NINDS) : NINDS is the nation’s leading funder of research on the brain and nervous system. The mission of NINDS is to seek fundamental knowledge about the brain and nervous system and to use that knowledge to reduce the burden of neurological disease. For more information about NINDS and its programs, visit www.ninds.nih.gov .
About substance use disorders: Substance use disorders are chronic, treatable conditions from which people can recover. In 2023, nearly 49 million people in the United States had at least one substance use disorder. Substance use disorders are defined in part by continued use of substances despite negative consequences. They are also relapsing conditions, in which periods of abstinence (not using substances) can be followed by a return to use. Stigma can make individuals with substance use disorders less likely to seek treatment. Using preferred language can help accurately report on substance use and addiction. View NIDA’s online guide .
About chronic pain: Chronic pain affects more than 50 million adults in the U.S. It may last for months, years, or a lifetime after its onset from trauma or another chronic health disorder. Multidisciplinary approaches and access to safe, effective, and quality care are essential for reducing pain and improving quality of life.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
NIH…Turning Discovery Into Health ®
Connect with Us
- More Social Media from NIH
People in impoverished neighborhoods struggle to access drug addiction medication, study finds
National study highlights racial disparities in opioid overdoses and the lack of treatment access.
FILE - Tabs of buprenorphine, a drug which controls heroin and opioid cravings, are photographed in Greenfield, Mass., on July 23, 2018.
Elise Amendola / AP
Patients battling addiction may struggle to fill their prescription for an opioid use disorder at their local pharmacy if they live in a low-income neighborhood or minority community, according to a new national study led by an Oregon State University researcher.
The study, also led by a researcher from John Hopkins University, underscores disparities in the health care system that dispenses life-saving medication amid a national epidemic of overdoses. In Oregon, nearly 1,400 people died of opioid overdoses in 2023, up from 280 in 2019, according to Oregon Health Authority data.
Nationwide, more than 100,000 people died of overdoses, three-quarters of which involved opioids. The fatalities disproportionately impact communities of color, including Black and Hispanic people. Their overdose fatality rates have nearly tripled in the last decade, compared to a 58% increase among white people, said the study , which was published in journal “Drug and Alcohol and Dependence Reports.”
“While there have been notable policy changes over the past decade that have improved access to meds used for opioid use disorder and made headway against racial disparities, those efforts haven’t taken into consideration the issue of whether patients can actually get their prescription filled,” said study co-author Dan Hartung, a researcher and professor who teaches at the OSU College of Pharmacy and Oregon Health & Science University.
Related: Oregon health officials approve $13 million from opioid settlement for treatment options
Fentanyl is a synthetic opioid that can be legally prescribed to relieve severe pain. But fentanyl, which is highly addictive and lethal, also is illegally manufactured and sold on the streets, often after it is mixed with other drugs to make them more addictive.
The prescription drug buprenorphine offers people a chance to recover. The drug helps reduce pain and cravings during withdrawal from opioids, and it reduces the risk of death from overdose.
But to access that care, people need treatment — including a prescription and a pharmacy that will dispense it.
“It’s a life-saving medication,” Hartung said. “It’s difficult enough to find prescribers of these therapies. But then you have to find a pharmacy, and then you have to make sure that pharmacy is going to dispense it. So there’s multiple barriers facing patients with addiction.”
Related: Oregon board allocates nearly $14 million for addiction prevention
The researchers looked at data from telephone calls to 858 pharmacies in 473 counties across the United States. In each instance, the caller contacted a pharmacy and asked about getting a buprenorphine prescription filled.
Overall, about 20% of pharmacies were not able to provide the medication, Hartung said. But pharmacies in low-income neighborhoods were more than two times as likely to restrict access compared to privileged areas, he said.
Oregon pharmacies played a small role in the study’s findings. Just 6% of polled pharmacies — about 54 — were located in the West, including Oregon. But the findings still underscore the need for access to the medication in Oregon, especially as small pharmacies shutter in rural areas, Hartung said.
There are multiple potential reasons for the pharmacies’ inability to dispense. Buprenorphine distribution is regulated by the Drug Enforcement Agency, and pharmacists may be cautious about how much of the drug they purchase from wholesalers to avoid triggering an investigation. Pharmacies, especially independent ones with fewer resources, may stock less because of the costs.
The history of medication for opioid addiction treatment has racial underpinnings. In the 1960s, methadone — also a medication for treatment — came into use in urban areas seeking to combat crime, the study said. Amid that era of heightened civil unrest, those programs commonly were started in neighborhoods with people of color, the study said.
Related: Portland’s first responders will give immediate opiate treatment after overdoses
Buprenorphine, however, developed during the opioid overdose epidemic tied to prescription drugs. The Food and Drug Administration in 2002 approved its use to treat opioid addiction. With that action, physicians in offices could write prescriptions for buprenorphine and it reached white middle-class patients, the study said. The result: White patients are about four times more likely to receive buprenorphine as Black patients.
“These pharmacy dispensing barriers have the potential to exacerbate inequities in access to treatment,” Kyle Moon, a researcher at John Hopkins University, said in a statement. “And it shows that future policy interventions aimed at improving health care equity need to target dispensing capacity to augment the ones already put in place that make it easier for providers to prescribe buprenorphine.”
In Oregon, lawmakers passed House Bill 4002 , which allows counties to set up programs to help people in addiction get help rather than face criminal drug possession charges and jail time. The bill also makes changes intended to help people access medication to treat addiction.
For example, the bill blocks insurers from asking for time to review the claim for a prescription before approving treatment, a process called prior authorization that can delay the dispensing of medication. The bill also gives pharmacists more flexibility to dispense early refills.
Oregon Capital Chronicle is part of States Newsroom, a network of news bureaus supported by grants and a coalition of donors as a 501(c)(3) public charity. Oregon Capital Chronicle maintains editorial independence. Contact Editor Lynne Terry for questions: [email protected] . Follow Oregon Capital Chronicle on Facebook and X .
OPB’s First Look newsletter
Streaming Now
All Things Considered

IMAGES
COMMENTS
How Science Has Revolutionized the Understanding of Drug Addiction For much of the past century, scientists studying drugs and drug use labored in the shadows of powerful myths and misconceptions about the nature of addiction. When scientists began to study addictive behavior in the 1930s, people with an addiction were thought to be morally flawed and lacking in willpower. Those views shaped ...
Addiction is the key process that underlies substance use disorders, and research using animal models and humans has revealed important insights into the neural circuits and molecules that mediate addiction.
The view that substance addiction is a brain disease, although widely accepted in the neuroscience community, has become subject to acerbic criticism in recent years. These criticisms state that the brain disease view is deterministic, fails to account ...
Advances in understanding addiction treatment and recovery. This Special Collection on Addiction focuses on scientific advances in the treatment and recovery mechanisms of addiction related to four widely misused substances: alcohol, nicotine, cocaine, and opioids. Although the opioid crisis has taken center stage across public policy and ...
Provides an overview of drug use and addiction, including what happens in the brain during drug use, why some people become addicted while others don't, and the importance of prevention.
Drug addiction is a complex medical condition. Learn more about the science of and research into addiction, health, and the brain.
Research guided by the brain disease model of addiction has led to the development of more effective methods of prevention and treatment and to more informed public health policies.
In this review, we synthetise the contribution of fundamental research to the understanding drug addiction and its contribution to potential novel therapeutics.
Drug experimentation is commonly initiated during adolescence and the risk to addiction is increased with early drug use. The greater vulnerability of adolescents to drug use and experimentation is driven by multiple factors including genetics that are associated with the developmental trajectories of the human brain.
Observational designs may provide the best evidence to evaluate interventions to address chronic conditions such as substance use and addiction. Unfortunately, assuming epistemic superiority of RCTs has unnecessarily slowed the uptake of substance use research and harm reduction services for people who use drugs.
Because drugs of abuse act on the brain's dopaminergic system, much of the focus in early drug addiction research has sought to investigate drugs' rewarding effects via the limbic systems.
The National Institute on Drug Abuse (NIDA) is the lead federal agency supporting scientific research on drug use and addiction. NIDA's mission is to advance science on drug use and addiction and to apply that knowledge to improve individual and public health.
Addiction, the most severe form of substance use disorder, is a chronic brain disorder molded by strong biosocial factors that has devastating consequences to individuals and to society. Our understanding of substance use disorder has advanced significantly over the last 3 decades in part due to major progress in genetics and neuroscience research and to the development of new technologies ...
Current addiction treatments use a combination of counseling and complete abstinence, slow weaning, or drug replacement that either substitutes for the drug or blocks withdrawal symptoms. Although these therapies control physical cravings, they don't seem to reverse the lasting changes in the brain caused by drug abuse, and therefore may only provide a temporary fix.
Research Topics. The National Institute on Drug Abuse (NIDA) is the largest supporter of the world's research on substance use and addiction. Part of the National Institutes of Health, NIDA conducts and supports biomedical research to advance the science on substance use and addiction and improve individual and public health.
Treatment interventions intended to reverse these neuroadaptations show promise as therapeutic approaches for addiction. Neuroscience research has revealed that addiction is a chronic, relapsing disease of the brain triggered by repeated exposure to drugs in those who are vulnerable because of genetics and developmental or adverse social exposures.
The mission of the National Institutes of Health (NIH) partnership, Collaborative Research on Addiction at NIH (CRAN), is to provide a strong collaborative framework to enable the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Drug Abuse (NIDA), and the National Cancer Institute (NCI) to integrate resources and expertise to advance substance use, misuse ...
Research at the MBI. Over the last decade, UF has exponentially expanded its number of faculty members devoted to the understanding of addiction and the pursuit of new treatments, with funding from the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism on a parallel rise.
In April 2019, Scherrer was awarded over $3 million from the National Institute on Drug Abuse of the National Institutes of Health (NIH) to study the pathways from chronic opioid use to new onset mood disorder.
The definition of addiction among the general population is "the condition of being addicted to a particular substance, thing, or activity." Medically, it may be defined as "a chronic, relapsing condition characterized by a compulsive drug-seeking behavior, continued use despite harmful consequence, and long-lasting changes in the brain." This current Oxford English dictionary definition is ...
Dec. 15, 2021 — A molecular switch influences addiction behavior and determines how strong the response to addictive drugs is. A research team made the discovery in mice treated with cocaine.
Addiction articles from across Nature Portfolio. Addiction involves loss of control over use of a substance, often in the presence of physiological and psychological dependence on a substance and ...
The view that substance addiction is a brain disease, although widely accepted in the neuroscience community, has become subject to acerbic criticism in recent years.
Users with higher Addiction Severity Index composite scores for family/social problems or legal problems, and users with prior drug abuse treatment experience were significantly more likely to perceive a need for treatment. These findings have practical implications for efforts addressing substance abuse in rural areas.
How does science provide solutions for drug use and addiction? Scientists study the effects drugs have on the brain and behavior. They use this information to develop programs for preventing drug use and for helping people recover from addiction. Further research helps transfer these ideas into practice in the community.
Research reveals a new brain region's role in opioid addiction, paving the way for non-addictive pain relief and innovative treatments.
Addiction is defined as a chronic, relapsing disorder characterized by compulsive drug seeking and use despite adverse consequences. † It is considered a brain disorder, because it involves functional changes to brain circuits involved in reward, stress, and self-control. Those changes may last a long time after a person has stopped taking ...
The alcohol and addiction research domain criteria (AARDoC) ( 92 ), which have been operationalized in the addictions neuroclinical assessment ( 94 ), focus on the following three domains that correspond to particular phases in the addiction cycle: incentive salience in the binge/intoxication phase, negative emotionality in the withdrawal ...
The National Institutes of Health (NIH) has launched a program that will support Native American communities to lead public health research to address overdose, substance use, and pain, including related factors such as mental health and wellness.
In Oregon, lawmakers passed House Bill 4002, which allows counties to set up programs to help people in addiction get help rather than face criminal drug possession charges and jail time. The bill ...