- Open access
- Published: 07 May 2019

Knowledge translation in health: how implementation science could contribute more
- Michel Wensing ORCID: orcid.org/0000-0001-6569-8137 1 &
- Richard Grol 2 , 3
BMC Medicine volume 17 , Article number: 88 ( 2019 ) Cite this article
43k Accesses
204 Citations
50 Altmetric
Metrics details
Despite increasing interest in research on how to translate knowledge into practice and improve healthcare, the accumulation of scientific knowledge in this field is slow. Few substantial new insights have become available in the last decade.
Various problems hinder development in this field. There is a frequent misfit between problems and approaches to implementation, resulting in the use of implementation strategies that do not match with the targeted problems. The proliferation of concepts, theories and frameworks for knowledge transfer – many of which are untested – has not advanced the field. Stakeholder involvement is regarded as crucial for successful knowledge implementation, but many approaches are poorly specified and unvalidated. Despite the apparent decreased appreciation of rigorous designs for effect evaluation, such as randomized trials, these should remain within the portfolio of implementation research. Outcome measures for knowledge implementation tend to be crude, but it is important to integrate patient preferences and the increased precision of knowledge.
Conclusions
We suggest that the research enterprise be redesigned in several ways to address these problems and enhance scientific progress in the interests of patients and populations. It is crucially important to establish substantial programmes of research on implementation and improvement in healthcare, and better recognize the societal and practical benefits of research.
Peer Review reports
Across the world, decision makers in healthcare struggle with the uptake of rapidly evolving scientific knowledge into healthcare practice, organisation, and policy. Rapid uptake of high-value clinical procedures, technologies, and organisational models is needed to achieve the best possible healthcare outcomes. Perhaps an even bigger struggle is that of stopping practices that do not or no longer have high value, such as the use of antibiotics for mild respiratory infections. Targeted interventions to improve healthcare practice exist in nearly all countries, and include, for instance, financial incentive programs to enhance the performance of healthcare providers, continuing professional education, and tools to involve patients more actively in their care and enhance shared decision-making. Evaluations of such implementation interventions in realistic settings found mixed, and overall moderate effects [ 1 , 2 ]. As a consequence, there have been calls to harness research and development on this topic [ 3 ]. We believe, however, that in recent years there has been little progress in our understanding of how healthcare practice can be improved.
The growing field of research on how to improve healthcare is known under various names, such as quality improvement, (dissemination and) implementation research, and knowledge transfer or translation [ 4 ]. Implementation science has been defined as the “scientific study of methods to promote the systematic uptake of research findings and other evidence-based practices into routine practice, and, hence, to improve the quality and effectiveness of health services and care” [ 3 ]. Knowledge translation is a related field that aims to enhance the use and usefulness of research. It covers the design and conduct of studies, as well as the dissemination and implementation of findings [ 4 ]. In healthcare, quality and safety management comprises a set of activities that aim to improve healthcare quality and safety, using measurement, feedback to decision-makers and organizational change [ 5 ]. In this article, we consider these and related fields as largely overlapping, because, through better uptake of putting knowledge in practice, they all aim to improve healthcare practice and thus provide better outcomes for patients and populations [ 6 ]. Knowledge may take various forms, such as evidence-based practice guidelines, technologies, or healthcare delivery models with proven value. The field is usually associated with change of practice and behaviors, but resisting change may occasionally serve the same purpose (e.g. in the case of promoted changes for which the evidence is limited or negative).
An illustrative example of research on knowledge transfer in health concerns pay-for-performance in general practice, which was introduced more than a decade ago in the UK. Practice performance was operationalized in terms of performance indicators, for which many had strong underlying evidence. Higher scores were associated with higher financial payments. For instance, an interrupted time series analysis of changes in performance scores for diabetes, asthma, and coronary heart diseases between 1998 and 2007 showed substantial improvements. These improvements had started before 2004; the year in which the contract was introduced. In diabetes and asthma, but not for coronary heart disease, a significant acceleration of improvement has been found since 2004 [ 7 ]. Other research in the field has focused, for instance, on continuing education, organizational change, health system reforms, and patient involvement for improving healthcare and implementation of recommended practice.
Looking at the infrastructure for research on how to improve healthcare practice, many positive developments can be noted in recent decades. Major funders, such as the National Institute for Health Research in the UK, the National Institutes of Health in the USA, the Canadian Institutes of Health Research, and Innovationsfond in Germany, have made substantial funding available. Several large research programs (funded with many millions of euros, pounds or dollars) have been established, such as the Collaborations for Leadership in Applied Health Research and Care (CLAHRCs; soon to be ARCs) in the UK. Scientific journals dedicated to the field have emerged, such as BMJ Quality & Safety , and Implementation Science , and many medical and health science journals publish research on healthcare improvement. Professorships and training programs for implementation science and quality improvement have been created, most notably in North America, but also in other countries. A variety of yearly or biannual scientific conferences focus on healthcare improvement, implementation science, and related fields.
Despite this emerging infrastructure and growing interest in the field, it seems to lag behind the high expectations. We are still far from understanding how healthcare practice can be improved rapidly, comprehensively, at large scale, and sustainably. In fact, this observation has been made several times in previous decades. For instance, in the year 2000, reflecting on 20 years of implementation research in healthcare, Grol and Jones argued that many questions had remained unanswered [ 8 ]. Seven years later, Grol, Wensing and Berwick argued that research on quality and safety in healthcare must be strengthened to develop the science in this field [ 9 ]. In 2014, Ivers and colleagues suggested that science is stagnating, based on an analysis of audit and feedback strategies [ 10 ]. We feel that progress in the field has been limited in the previous decade. This Opinion article focuses on what may be wrong in research and practice, and what could help to address the problems. It is informed by our research projects, lectures, teaching at graduate and postgraduate levels, participation in academic review committees, roles as journal editors, and repeated updates of our books on improving healthcare practice [ 11 , 12 ] in the previous 25 years.
Problems and possible strategies
Table 1 provides an overview of our analysis of problems in research and practice, and possible ways to address these.
Misfit between problems and approaches
Healthcare challenges vary widely. For instance, implementing the centralization of surgical procedures or emergency services in specific centers is very different from a change in a medication protocol, or the introduction of nurses for counseling on lifestyle changes. All of these changes may be backed up by strong research evidence on their value, but analysis of the implementation challenges in each of these examples would reveal very different determinants of implementation and outcomes. For instance, health system factors may be crucial for the effective centralization of services, while individual factors related to knowledge or behavioral routines are probably most relevant in changing medication protocols. However, there is often little association between the type of problem and the approach to change taken. More particularly, organizational and system-related problems tend to be ignored, even when these were detected, favoring individual educational and psychological approaches [ 13 ]. Organizational and system change may be difficult to achieve within the typical course of a research project.
Probably even more relevant is that many researchers and consultants tend to be stubbornly consistent in their approach, and stick, for instance, either to a psychological approach (arguing that all implementation requires individual behavior change), an organizational systems approach (arguing that organizations rather than individuals need to change), or an economic perspective (arguing that financial factors override everything else). We believe, however, that the chosen approach to change should match the problems to achieve change, rather than the favored discipline of the researcher or consultant [ 14 ]. To remedy the current situation, options include training researchers and consultants and providing them with a multi-faceted perspective on the issue of knowledge translation and with theoretical frameworks that cover multiple approaches, as well as working in multidisciplinary improvement teams. These approaches require multidisciplinary academic training to be better appreciated in universities, which tend to facilitate academic careers within traditional scientific disciplines.
Proliferation of concepts, theories and frameworks
In the past [ 15 ], research on the translation of knowledge into practice was dominated by a few theories, such as the ‘Diffusion of Innovations’ theory [ 16 ]. Nowadays, research on improving healthcare practice is characterized by a proliferation of concepts, theories and frameworks for knowledge translation and implementation [ 17 ]. Most are essentially structured lists of disconnected items, which are not explicitly linked to higher-level scientific theory [ 18 ]. They distinguish, for instance, between individual, organizational, system-related and innovation-related factors. Many of these proposed frameworks have not been applied and tested in more than one study. New frameworks typically ignore published work – particularly if it is older than a decade – so the reinvention of existing concepts and frameworks is common. The field has also suffered from many fashions and hypes, which often present high-level concepts (e.g. the ‘breakthrough’ approach to improvement) and were popular for a while, but then disappeared without contributing much to scientific progress.
Research that applies and tests concepts, theories or frameworks is largely organized in silos – bubbles of like-minded academics (e.g. epidemiologists, psychologists, sociologists, or economists). Among researchers, there is mixed interest in testing conceptual ideas in rigorous empirical research. Some are close to healthcare practice (e.g., as clinicians), but are not aware of the full range of available concepts, theories and frameworks. Others know and apply specific theories or frameworks, but may be insufficiently familiar with healthcare practice or policy to assess their usefulness. Furthermore, head-to-head comparisons and integrations of proposals from different silos are rare, because many researchers are inclined to stick to their favored approach. We suggest, however, that these would advance science far more than the continuous development of new theories within disconnected worlds. Rather than adding new frameworks, the focus should be on the testing, refinement and integration of theories. Furthermore, we think that these should be sufficiently concrete and specific. To be sufficiently informative, they may be related to a specific topic or field of application – although it remains open for debate as to what would be the appropriate aggregation level (e.g., antibiotics prescribing in primary care, medication prescribing generally, or ambulatory medical care).
Non-validated methods for stakeholder involvement
The involvement of stakeholders (e.g., patients, providers, payers) in the design and conduct of interventions to improve healthcare practice has been emphasized to the extent that it is now seen as the ‘holy grail’ of improvement. For instance, ‘mode 2’ knowledge generation has been characterized as socially distributed, organizationally diverse, application-oriented, and transdisciplinary, as opposed to the traditional, ‘ivory tower’ mode 1 [ 19 ]. Involvement usually takes the form of consultation of stakeholders through interviews or surveys, or the participation of stakeholders in boards. However, the evidence for this belief is – so far – largely anecdotal, and many methods for stakeholder involvement are poorly specified, so replication is difficult. For instance, integrated knowledge translation (collaboration between researchers and decision-makers) uses a variety of methods, but its outcomes are unknown [ 20 ]. Stakeholders are often heterogeneous with respect to knowledge, needs and preferences regarding a particular change. Attempts to develop and validate stakeholder-based, tailored interventions have met with various difficulties and uncertainties [ 21 ]. For example, it is unclear how available research evidence and theory is combined with stakeholder involvement, if stakeholders have suggestions that contradict existing knowledge. Stakeholder involvement also implies the use of resources, particularly health professionals’ time, which must be considered when planning implementation programs. We suggest that methods for stakeholder involvement must be better specified and validated in empirical research.
Decreased appreciation of rigorous designs for effect evaluation
In some circles of researchers, practitioners, and policy makers, there seems to have emerged a decreased appreciation of rigorous designs for effect evaluation in healthcare, such as randomized trials. Arguments for the criticism of randomized trials as a preferred study design are manifold, and include, for instance, the belief that many interventions are changed during application in practice, outcomes of interventions are largely context-dependent, rigorous evaluation is time-consuming and expensive, and biomedical knowledge is evolving too quickly to allow rigorous outcomes evaluation [ 22 ]. Implementation science needs a variety of study designs and methods, including systematic intervention development, observational pilot tests, qualitative studies, and quantitative simulation modeling. We believe that rigorous designs for effect evaluation, including randomized trials, should remain on the menu.
Several researchers appreciate randomized trials and other rigorous evaluation designs, but they focus on the effectiveness of interventions with respect to patients’ health. Implementation researchers can indeed make useful contributions to process evaluation and feasibility testing of interventions in studies of intervention effectiveness. While such research is important, it is unlikely to advance implementation science much, because this is not its primary objective, and, as a consequence, the methods are not fully aligned. For instance, clinical trials and trials in public health often benefit from optimized intervention fidelity and strict inclusion criteria for participants, which does not match with the requirements of research on quality improvement, knowledge translation and implementation science.
There are clearly various issues that must be carefully considered when designing rigorous evaluations of improvement and implementation interventions, such as the choice of outcome measures, the duration of follow-up, and the approach in control groups. Several trial designs, such as stepped wedge trials, provide options beyond the classic two-armed randomized trial – albeit often at the price of more complex statistical analysis. Advanced designs for rigorous evaluation can only be considered if the people involved understand and appreciate outcome evaluation in the first place. It cannot be assumed that this is the case. We therefore argue that the appreciation of outcome evaluation must be nurtured among healthcare providers, managers and policy-makers. This appreciation should extend to interventions for the improvement of healthcare practice.
Suboptimal outcomes measures
Strategies for improvement or implementation must be evaluated with respect to their effectiveness in changing healthcare practices as a primary outcome of interest. Many studies of interventions to improve healthcare practice use relatively simple outcomes measures, such as clinical behaviors, which have been documented in patient records or procedures for which reimbursement was requested. While such measures can be informative, they are often only crude indicators of the actual use of knowledge in healthcare practice. Recent and continuing work on the pragmatic use of outcomes in implementation research has emphasized the importance of acceptability, feasibility, compatibility with routines, and perceptions of usefulness [ 23 ]. In some studies, however, health outcomes are primary outcomes – although these may not be responsive for improvements in the quality of care. The number of steps from interventions to health outcomes is relatively great, so that interpretation of causality is difficult if intermediate factors are not measured – particularly if health outcomes do not show intervention effects.
Furthermore, a fundamental problem is that the use of knowledge in decision-making rarely has simple, predictable associations with the decisions made. Ultimately, the key outcome is not crude frequencies of behaviors or health outcomes, but whether the available knowledge was taken into consideration in decision-making and healthcare practice. This knowledge is increasingly individualized on the basis of patients’ biological and psychological features, and comes from computerized decision support systems rather than clinical guidelines for patient populations. Patients’ preferences must also be taken into account when assessing the quality of decision-making. A knowledge-informed conversation between a patient and a clinician, resulting in a decision that is not coherent with some recommendation, should not automatically be documented as non-use of knowledge. We believe there is a need for new outcome measures of knowledge implementation, which take the changing nature of knowledge and the impact of patient preferences into account.
A fundamental challenge is to overcome the misconceptions, silo-thinking and self-interests among stakeholders. These stakeholders include politicians and managers who prefer to act on the basis of conviction rather than research evidence, healthcare providers who deny that research findings apply to them, and researchers who prefer to focus on concepts and approaches that fit their particular academic background. Global investment in the research infrastructure of knowledge implementation and quality improvement in healthcare provides major opportunities, but also a high responsibility for all involved.
After participating in many grant review panels, we conclude that the assessment of project applications is often dominated by the perceived relevance of the health issue or healthcare problem on which it focuses. The project’s contribution to the agenda of research on how to improve healthcare practice is usually of secondary importance, at best. This practice of healthcare research funding has resulted in many researchers in the field who do one or two projects on how to improve healthcare practice, and then leave the field, because they see no perspective for a career, or lack real interest. As a result, research on healthcare improvement and knowledge implementation is currently fragmented in different, largely disconnected, communities [ 24 ].
We think that coordinated and longer lasting research programs are needed to enhance the continuity of researchers in the field, which is crucial for knowledge accumulation. Knowledge translation and healthcare improvement in healthcare needs research centers or networks that bring together scientists with different backgrounds who can work on sequential projects over a longer period of time. Examples exist, and include an international research network to examine and optimize feedback interventions for implementation [ 25 ], and a large center for the study of healthcare improvement [ 26 ]. Effective programmatic research requires, among other things, institutional funding for core staff, multidisciplinary composition of groups, realistic and continuous funding opportunities for research projects, career opportunities for young and mid-career researchers, and integration in locally relevant infrastructures (e.g., routine quality improvement in hospitals). It also requires focused education. For instance, some graduation programs for implementation science in healthcare have been established [ 24 ], but their long-term success depends on graduated students’ opportunities in the labor market.
The field would also be enhanced by the revision of procedures for accountability and recognition of performance in academic institutions. Research on healthcare improvement and implementation is unlikely to provide ‘discoveries’, but studies can add substantially to the body of knowledge and thus support users in practice, management and policy. Citations in scientific journals are problematic as a sole criterion for review of performance because users (e.g. clinicians, managers, policy-makers) rarely cite publications, as most do not publish scientific papers. Several complementary methods for performance review are available, but their validity and feasibility remain challenging [ 27 ]. Perhaps most importantly, research on improving healthcare and knowledge implementation requires a higher appreciation of the field in the academic and health community, and alignment of resources and power in institutions accordingly.
The ultimate aim of research on knowledge implementation in healthcare is for interventions to improve healthcare practice to become more effective, thus leading to better care and outcomes for patients and populations. We believe that the research enterprise in this field must be redesigned in several ways to make this happen.
Grol R, Grimshaw J. From best evidence to best practice. Lancet. 2003;361:1225–30.
Article Google Scholar
Grimshaw JM, Eccles MP, Lavis JN, Hill SJ, Squires JE. Knowledge translation of research findings. Implem Sci. 2012;7:50.
Eccles MP, Mittmann BS. Welcome to Implementation Science. Implem Sci. 2006;1:1.
Graham ID, Logan J, Harrison MB, Straus SE, Tetroe J, Caswell W, et al. Lost in knowledge translation: time for a map? J Cont Educ Health Prof. 2006;26:13–24.
Dixon-Woods M. Harveian oration 2018: improving quality and safety in healthcare. Clin Med. 2019;19:47–56.
Theobald S, Brandes N, Gyapong M, El-Saharty S, Proctor E, Diaz T, et al. Implementation research: new imperatives and opportunities in global health. Lancet. 2018;392:S0140–6736.
Campbell SM, Reeves D, Kontopantelis E, Sibbald B, Roland R. Effects of pay for performance on the quality of primary care in England. N Engl J Med. 2009;361:368–78.
Article CAS Google Scholar
Grol R, Jones R. Twenty years of implementation research. Fam Pract. 2000;17(Suppl 1):S32–5.
Grol R, Berwick D, Wensing M. On the trail of quality and safety in healthcare. BMJ. 2008;338:74–6.
Ivers NM, Grimshaw JM, Jamvedt G, Flottorp S, O’Brien MA, French SD, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative meta-analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29:1534–41.
Wensing M, Grol R. Implementatie: Effectieve verandering in de patiëntenzorg. Zevende Druk. [effective change in patient care. 7th edition.] Houten: Boom Stafleu Van Loghum, 2017.
Grol R, Wensing M, Eccles M, Davies. Improving patient care. The implementation of change in clinical practice. 2nd ed. Chichester: Wiley, BMJ Books; 2013.
Book Google Scholar
Bosch M, Van der Weijden T, Wensing M, Grol R. Tailoring quality improvement interventions to identified barriers: a multiple case analysis. J Eval Clin Pract. 2007;13:161–8.
Grol R. Beliefs and evidence in changing clinical practice. BMJ. 1997;315:418–21.
Estabrooks CA, Derksen L, Winther C, Lavis JN, Scott SD, Wallin L, et al. The intellectual structure and substance of the knowledge utilization field: alongitudinal author co-citation analysis, 1945 to 2004. Implement Sci. 2008;3:49.
Rogers EM. Diffusion of innovations. 4th ed. New York: The Free Press; 1995.
Google Scholar
Strifler L, Cardosa R, McGowan J, Cogo E, Nincic V, Khan PA, et al. Scoping review identifies significant number of knowledge translation theories, models, and frameworks with limited use. J Clin Epidemiol. 2018;100:92–102.
Nilsen P. Making sense of implementation theories, models and frameworks. Implem Sci. 2015;10:53.
Gibbons M, Limoges C, Nowotny H. The new production of knowledge: the dynamics of science and research in contemporary societies. London: Sage; 1997.
Gagliardi AR, Berta W, Kothari A, Boyko J, Urquhart R. Integrated knowledge translation (IKT) in health care: a scoping review. Implem Sci. 2016;11:38.
Wensing M. The tailored implementation in chronic diseases project: introduction and main findings. Implem Sci. 2017;12:5.
Greenhalgh T, Howick J, Maskrey N. Evidence-based medicine renaissance group. Evidence based medicine: a movement in crisis? BMJ. 2014;348:g3725.
Powell BJ, Stanick CF, Halko HM, Dorsey CN, Weiner BJ, Barwick M, et al. Toward criteria for pragmatic measurement in implementation research and practice: a stakeholder-driven approach using concept mapping. Implem Sci. 2017;12:118.
Wensing M, Grimshaw JM, Eccles MP. Does the world need a scientific society for research on how to improve healthcare? Implem Sci. 2012;7:10.
Ivers NM, Grimshaw JM. Reducing waste with implementation laboratories. Lancet. 2016;388:547–8.
Ullrich C, Mahler C, Forstner J, Szecsenyi J, Wensing M. Teaching implementation science in a new master of science program in Germany: a survey of stakeholder expectations. Implem Sci. 2017;12:55.
Greenhalgh T, Raftery J, Hanney S, Glover M. Research impact: a narrative review. BMC Med. 2016;14:78.
Download references
Acknowledgements
Not applicable.
There was no specific funding for this manuscript.
Availability of data and materials
Author information, authors and affiliations.
Department of General Practice and Health Services Research, Heidelberg University Hospital, Im Neuenheimer Feld, 130.3, 69120, Heidelberg, Germany
Michel Wensing
Radboud Institute for Health Sciences, Radboud University Medical Center, Geert Grooteplein Zuid 10, 6525, Nijmegen, GA, Netherlands
Richard Grol
Maastricht University, Minderbroedersberg 4-6, 6211, Maastricht, LK, Netherlands
You can also search for this author in PubMed Google Scholar
Contributions
MW and RG collaboratively conceived the idea for this manuscript. MW wrote draft versions and RG provided substantial comments. Both authors read and approved the final version of the manuscript. MW guarantees the scientific integrity of the manuscript.
Corresponding author
Correspondence to Michel Wensing .
Ethics declarations
Authors’ information.
Michel Wensing is Professor of Health Services Research and Implementation Science at Heidelberg University and Editor-in-Chief of the journal Implementation Science . Richard Grol is a retired professor of Quality of Care with an extensive scientific and societal track record.
Ethics approval and consent to participate
Consent for publication, competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Wensing, M., Grol, R. Knowledge translation in health: how implementation science could contribute more. BMC Med 17 , 88 (2019). https://doi.org/10.1186/s12916-019-1322-9
Download citation
Received : 10 February 2019
Accepted : 10 April 2019
Published : 07 May 2019
DOI : https://doi.org/10.1186/s12916-019-1322-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Knowledge transfer
- Implementation science
- Quality improvement
- Research policy
BMC Medicine
ISSN: 1741-7015
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Systematic review
- Open access
- Published: 04 March 2020
Knowledge translation strategies for dissemination with a focus on healthcare recipients: an overview of systematic reviews
- Evelina Chapman 1 ,
- Michelle M. Haby ORCID: orcid.org/0000-0001-6203-9195 2 , 3 ,
- Tereza Setsuko Toma 4 ,
- Maritsa Carla de Bortoli 4 ,
- Eduardo Illanes 5 ,
- Maria Jose Oliveros 6 &
- Jorge O. Maia Barreto 1
Implementation Science volume 15 , Article number: 14 ( 2020 ) Cite this article
28k Accesses
59 Citations
27 Altmetric
Metrics details
While there is an ample literature on the evaluation of knowledge translation interventions aimed at healthcare providers, managers, and policy-makers, there has been less focus on patients and their informal caregivers. Further, no overview of the literature on dissemination strategies aimed at healthcare users and their caregivers has been conducted. The overview has two specific research questions: (1) to determine the most effective strategies that have been used to disseminate knowledge to healthcare recipients, and (2) to determine the barriers (and facilitators) to dissemination of knowledge to this group.
This overview used systematic review methods and was conducted according to a pre-defined protocol. A comprehensive search of ten databases and five websites was conducted. Both published and unpublished reviews in English, Spanish, or Portuguese were included. A methodological quality assessment was conducted; low-quality reviews were excluded. A narrative synthesis was undertaken, informed by a matrix of strategy by outcome measure. The Health System Evidence taxonomy for “consumer targeted strategies” was used to separate strategies into one of six categories.
We identified 44 systematic reviews that describe the effective strategies to disseminate health knowledge to the public, patients, and caregivers. Some of these reviews also describe the most important barriers to the uptake of these effective strategies. When analyzing those strategies with the greatest potential to achieve behavioral changes, the majority of strategies with sufficient evidence of effectiveness were combined, frequent, and/or intense over time. Further, strategies focused on the patient, with tailored interventions, and those that seek to acquire skills and competencies were more effective in achieving these changes. In relation to barriers and facilitators, while the lack of health literacy or e-literacy could increase inequities, the benefits of social media were also emphasized, for example by widening access to health information for ethnic minorities and lower socioeconomic groups.
Conclusions
Those interventions that have been shown to be effective in improving knowledge uptake or health behaviors should be implemented in practice, programs, and policies—if not already implemented. When implementing strategies, decision-makers should consider the barriers and facilitators identified by this overview to ensure maximum effectiveness.
Protocol registration
PROSPERO: CRD42018093245 .
Peer Review reports
Contributions to the literature
Much evidence has been developed to ensure that the results of research are used by health policy-makers and practitioners. However, the challenges of research use for patients and the public are greater and there is less research in this area.
This review is the first synthesis of systematic review evidence that can help ensure that research results are used by patients and the public and that is not limited to specific diseases.
The use of Information and Communication Technologies is the new great challenge to increase access and to achieve greater equity in health, especially in low-middle income countries.
Knowledge translation (KT) is “the synthesis, exchange, and application of knowledge by relevant stakeholders to accelerate the benefits of global and local innovation in strengthening health systems and improving people’s health” [ 1 ]. The process of KT ensures that evidence from research is used by relevant stakeholders, including healthcare providers, managers, policy-makers, informal caregivers, patients, and the public in the improvement of health [ 2 ]. While there is an ample literature on the evaluation of interventions aimed at healthcare providers, managers, and policy-makers, there has been less focus on patients and their informal caregivers.
“Patient-mediated” KT interventions are those strategies that involve patients in their own healthcare and have the aim to improve patient knowledge, relationship with the provider, the appropriateness of health service use, satisfaction with the provision of care experience, adherence to the recommended treatment, and other health behaviors and outcomes [ 3 ].
The Canadian Institutes for Health Research (CIHR), a leader in the science and practice of knowledge translation, have recognized four key elements in the process of KT: synthesis, dissemination, exchange, and ethically sound application of knowledge. For this overview, we will be focusing on dissemination as a core strategy in KT. Dissemination involves identifying the appropriate audience and tailoring the message and medium to the audience [ 4 ]. Dissemination of health-related information is the active, tailored, and targeted distribution of information or interventions via determined channels using planned strategies to a specific public health or clinical practice audience, and has been characterized as a necessary but not sufficient antecedent of knowledge adoption and implementation [ 5 ]. According to CIHR, dissemination can include elements such as summaries for/briefings to stakeholders, educational sessions with patients, practitioners and/or policy makers, engaging knowledge users in developing and executing dissemination/implementation plans, tools creation, and media engagement. Dissemination can be done through different information and communication technologies (ICT) based or not on the internet, i.e., videos, websites, brochures, decision aids, or art pieces.
There are many models and theories to explain what makes KT for healthcare recipients (and providers) effective [ 6 , 7 , 8 , 9 ]. These theories have varying objectives, which range from information provision individually or to large audiences (e.g., mass media) to achieving behavior change through education or skills acquisition. When focusing on behavior change, the aim is to increase the capacity to use and apply evidence effectively, thus achieving better health outcomes including quality of life. Desired outcomes of these models include shared decision-making between patients, their families, and providers; patient-provider communication; self-efficacy; adherence; improved access; and cure or survival. Intermediate outcomes could include healthcare users’ improved health knowledge, health behaviors, and physiologic measures; patient satisfaction; and reduced costs [ 10 ].
Further, in KT processes addressed to patients and informal caregivers, it is important to consider determinants or barriers at the level of healthcare recipients, i.e., knowledge, language, and cultural differences, skills deficits, attitudes, access to care and motivation to change, among others [ 7 , 10 , 11 , 12 ]. Also, it is usual practice to combine multicomponent dissemination strategies such as a combination of reach, motivation, or ability goals.
For the purpose of this overview, we have focused on dissemination strategies aimed at healthcare users and their caregivers in order to improve health and wellbeing. We used the taxonomy developed by Lavis et al. to organize the results, which includes six groups of strategies that are explained later [ 13 ].
This overview addressed two specific research questions:
How effective are the strategies that have been used to disseminate knowledge to healthcare recipients (both for the general public and patients)?
What are the barriers (and facilitators) to disseminate knowledge to healthcare recipients (both for the general public and patients)?
This overview used systematic review methodology and adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [ 14 ]. A systematic review protocol was written and registered prior to undertaking the searches [ 15 ]. Deviations from the protocol are noted.
Inclusion criteria for studies
Studies were selected based on the following inclusion criteria.
Types of studies
Systematic reviews (SRs) that included quantitative studies of any design that provided information on the effectiveness of dissemination strategies. SRs of qualitative studies that describe barriers and facilitators to uptake of research evidence were also included.
Types of participants
We included studies that involved healthcare recipients as the main focus, such as the general public, patients, caregivers, or patient groups. We excluded studies where other users, such as practitioners, policy-makers, educators, decision-makers, health care administrators, and community leaders, were the main focus. We also excluded studies where the dissemination strategy was directed to participants with a single health issue, e.g., multimedia interventions to promote HIV testing. This was to ensure a more general approach to strategies for dissemination of knowledge to healthcare recipients.
Types of interventions
SRs that evaluated KT dissemination strategies aimed at healthcare recipients or caregivers were included. The dissemination strategies were defined based on the Health System Evidence taxonomy [ 13 ] for “consumer targeted strategies” as follow:
Information or education provision: strategies to enable consumers to know about their treatment and their health.
Behavior change support: interventions which focus on the adoption or promotion of health and treatment behaviors at an individual level, such as adherence to medicines.
Skills and competencies development: strategies that focus on the acquisition of skills relevant to self-management.
(Personal) Support: interventions which provide assistance and encouragement to help patients cope with and manage their health and ongoing medical issues, such as counseling and follow up on treatment efficacy.
Communication and decision-making facilitation: strategies to involve consumers in decision-making about healthcare.
System participation: interventions to involve patients and/or caregivers in decision-making processes at a system level.
The dissemination element could be written on paper (i.e., pamphlets, flyers, booklets), verbal (i.e., using telephone), or written or verbal using ICT (i.e., e-health, m-health, websites, multimedia, telemedicine, patient reminder, etc.). The dissemination could be done individually, in groups, or massively.
Types of comparisons
There were no restrictions on types of comparisons.
Types of outcome measures
We included outcomes related to the effectiveness of dissemination strategies addressed to health-care recipients, caregivers, or the general public, including change in knowledge, understanding, perception, attitudes, adherence to health recommendations, and behavior changes. Other proposed results were health status, access, use of services, social outcomes, user satisfaction, costs, and cost-effectiveness. Additionally, we considered barriers to uptake of research evidence through dissemination strategies at the level of knowledge, competency, health literacy, attitudes, access to care, and motivation to change.
Publications in English, Spanish, or Portuguese were included and there were no restrictions on the year of publication. Both published and gray literature were included.
Search strategy and sources of systematic reviews
A comprehensive search of ten databases and five websites was conducted. The databases searched for SRs were MEDLINE (Ovid); Embase (Ovid); ERIC (EBSCOHost); CINAHL (EBSCOHost); PsycINFO (Ovid); LILACS (BVSalud); and World Wide Science. The specialized sources of SRs were the Cochrane Library (including Cochrane Reviews, the Database of Abstracts of Reviews of Effects and Health Technology Assessment); Epistemonikos; and Health Systems Evidence.
Manual searches were conducted in Google and Google Scholar; EPPI-Center Systematic Reviews; Rx for Change ( https://www.cadth.ca/rx-change ); and 3ie–International Initiative for Impact Evaluation. In addition to the above sources that included gray literature, we manually searched the System for Information on Grey Literature in Europe (Open Grey— http://www.opengrey.eu ).
Electronic searches were conducted between 21 and 23 May 2018 and supplementary searches (reference lists, contact with authors, and gray literature) were conducted in January 2019. Databases were searched using keywords from keyword areas related to the participants, the intervention, outcomes, and study type—combined using “AND.” Keywords were searched for in the title and abstract fields and using Medical Subject Headings (MeSH) terms where available (search terms and strategies for the electronic searches are in Additional file 1 ). Results were downloaded into the EndNote reference management program (version X8.2) and duplicates were removed.
Screening and selection of studies
Titles and abstracts were screened independently according to the selection criteria by pairs of review authors (EC, JB, and MO). The full text of any potentially relevant papers was retrieved for closer examination. The inclusion criteria were then applied against the full text version of the papers independently by two reviewers (EC and MO). Disagreements regarding eligibility of studies were resolved by discussion, and a third reviewer (JB) consulted when necessary. All studies which initially appeared to meet the inclusion criteria but on inspection of the full text paper did not meet the inclusion criteria are listed in a table “Characteristics of excluded studies” together with reasons for their exclusion.
Data extraction
Information extracted from included SRs were objectives, study designs and number of studies included, date of last search, intervention/strategy, participants, settings, country of studies, and financing source, as well as outcome measures, findings, barriers, research gaps, and theories or frameworks. Data extraction was shared between six reviewers (EC, MB, TT, EI, MH, and MO) and checked by a second reviewer (EC). Disagreements were resolved through discussion and consensus.
After extracting data from the included SRs, reviewers completed a matrix previously designed using the Health Systems Evidence taxonomy [ 13 ] for each of the six strategies down the left-hand side with the different outcome measures across the top. While we started with the list of outcome measures specified in the protocol we had to expand the matrix because we found more types of outcome measures than originally proposed. Classification was done by each reviewer (EC, MB, TT, EI, MH, and MO) and checked by a second reviewer (EC).
Assessment of methodological quality
The methodological quality of included SRs was assessed independently by pairs of reviewers using A MeaSurement Tool to Assess systematic Reviews (AMSTAR) [ 16 ]. Disagreements in scoring were resolved by discussion and consensus. For this overview, SRs that achieved AMSTAR scores of 8 to 11 were considered high quality, scores of 4 to 7 medium quality, and scores of 0 to 3 low quality. SRs of low quality were excluded. We did not find any SRs of exclusively qualitative studies to inform barriers and facilitators so could not use the Confidence in the Evidence from Reviews of Qualitative research approach to assess the confidence of findings from qualitative syntheses [ 17 ] as proposed in the protocol. Instead, we found SRs with a mix of study designs that included qualitative research so the overall quality of these studies was evaluated with AMSTAR tool.
Data analysis
Findings from the included publications were synthesized using tables and a narrative summary informed by the matrix of strategy by outcome measure. Meta-analysis was not possible because the included studies were heterogeneous in terms of the populations, strategies/interventions tested, and outcomes measured. Further, few studies informed effectiveness measures. Thus, to inform the main results, we developed effectiveness statements using four categories and standardized language as proposed by Ryan et al. [ 9 ]. The decision rules took into account the results, their statistical significance, and the quality and number of studies that support the result. The four categories are (1) sufficient evidence, (2) some evidence, (3) insufficient evidence, and (4) insufficient evidence to determine effectiveness (Additional file 2 ). Category 4 was used to inform research gaps.
Search results
Forty-four SRs met the inclusion criteria for the overview [ 3 , 5 , 7 , 9 , 10 , 11 , 12 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 ]. The selection process for SRs and the number of papers found at each stage are shown in Fig. 1 . The reasons for exclusion of the 47 papers at full text stage are shown in Additional file 3 .
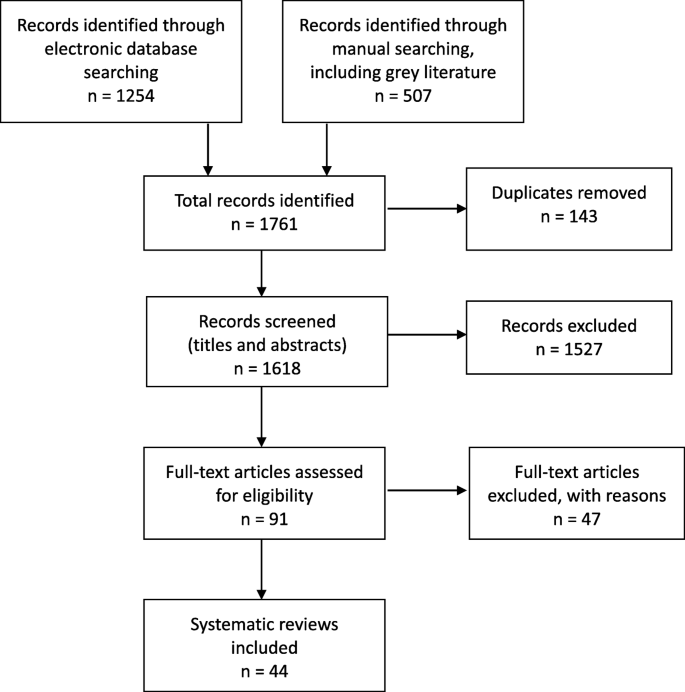
Study selection flow chart
Characteristics of included studies and quality assessment
Details of the characteristics of the included SRs and AMSTAR scores are in Additional file 4 (Tables 1 and 2 ). Of the 44 SRs, 19 had AMSTAR scores of high quality [ 7 , 9 , 10 , 12 , 20 , 21 , 24 , 31 , 35 , 36 , 38 , 43 , 44 , 45 , 47 , 48 , 49 , 50 , 54 ] and 25 were of medium quality [ 3 , 5 , 11 , 18 , 19 , 22 , 23 , 25 , 26 , 27 , 28 , 29 , 30 , 32 , 33 , 34 , 37 , 39 , 40 , 41 , 42 , 46 , 51 , 52 , 53 ]. Of the 44 SRs, 24 included both experimental and quasi-experimental designs [ 3 , 5 , 7 , 9 , 11 , 12 , 20 , 21 , 25 , 27 , 28 , 29 , 30 , 32 , 33 , 34 , 36 , 38 , 41 , 42 , 49 , 51 , 53 , 54 ], ten only included randomized controlled trials [ 18 , 23 , 24 , 26 , 35 , 43 , 47 , 48 , 50 , 52 ], and ten included both quantitative and qualitative research [ 10 , 19 , 22 , 31 , 37 , 39 , 40 , 44 , 45 , 46 ]. Seventeen SRs included only patients or caregivers [ 7 , 9 , 10 , 20 , 24 , 31 , 32 , 34 , 35 , 37 , 39 , 40 , 43 , 44 , 49 , 50 , 53 ], and the remaining 27 also included providers. Twenty SRs were informed by, or based on, a theory or framework [ 3 , 7 , 9 , 11 , 12 , 18 , 21 , 25 , 27 , 29 , 31 , 33 , 36 , 38 , 41 , 42 , 46 , 49 , 51 , 54 ].
The different strategies tested, and types of communication or dissemination tested in each of the SRs are shown in Additional file 5 . When reviewing the included SRs, we found outcome measures that were not included in the protocol (or in the “ Methods ” section of this review). These included shared decision-making between patients, their families, and providers, patient-provider communication, self-efficacy and/or self-management, awareness, beliefs, clinical results, coverage, use of services, empowerment, less suffering or anxiety, persuasion, safety, social support and influence, quality of life, health status and wellbeing, hospitalizations, length of consultation, participation in health, sustainability, choice, addiction to media, and readability. More details are in Additional file 6 .
Effectiveness statements
The effects of interventions are presented below by strategy according to the adopted taxonomy [ 13 ]. The SRs were divided into those testing the specific strategy alone (single) or in combination with other strategies (combined). Many reviews evaluated interventions involving multiple strategies and so contributed evidence to more than one category. More details are in Additional file 5 , Table 1 . The effectiveness statements are presented in Tables 1 , 2 , 3 , 4 , and 5 for those with “sufficient” or “some evidence” (categories 1 and 2 in Additional file 2 ). Those with “insufficient evidence” (category 3) are in Additional file 7 and those with “insufficient evidence to determine” (category 4) were used to inform the research gaps (Additional file 8 ).
Providing information or education
Forty-one reviews included this strategy (Additional file 5 , Table 1 ) but only 17 provided evidence that was useful for the development of the effectiveness statements [ 7 , 9 , 11 , 12 , 21 , 26 , 28 , 29 , 33 , 34 , 35 , 37 , 39 , 40 , 44 , 49 , 52 ]. Seven of these 17 reviews were of high quality [ 7 , 9 , 12 , 21 , 35 , 44 , 49 ]. The remaining 24 SRs were used to inform the research gaps (Additional file 8 ). The effectiveness statements are presented in Table 1 and Additional file 7 .
Communication and decision-making facilitation
Twenty-seven reviews included this strategy (Additional file 5 , Table 1 ) but only 11 provided evidence that was useful for the development of the effectiveness statements [ 9 , 10 , 24 , 28 , 30 , 31 , 46 , 47 , 48 , 49 , 50 ]. Eight of these 11 reviews were of high quality [ 9 , 10 , 24 , 31 , 47 , 48 , 49 , 50 ]. The effectiveness statements are presented in Table 2 and Additional file 7 .
Acquiring skills and competencies
Twenty-six reviews included this strategy but only five provided evidence that was useful for the development of the effectiveness statements [ 5 , 9 , 11 , 22 , 53 ]. One of these five reviews was of high quality [ 9 ]. See Table 3 and Additional file 7 for the effectiveness statements.
Behavior change support
Thirty-nine reviews included this strategy but only 19 provided evidence that was useful for the development of the effectiveness statements [ 3 , 9 , 11 , 18 , 19 , 20 , 23 , 24 , 25 , 27 , 30 , 36 , 38 , 41 , 42 , 43 , 50 , 51 , 54 ]. Seven of these reviews were of high quality [ 9 , 20 , 21 , 24 , 36 , 38 , 43 , 50 ]. See Table 4 and Additional file 7 for the effectiveness statements.
Personal support
Thirty reviews included this strategy but only two provided evidence that was useful for the development of the effectiveness statements [ 9 , 49 ]—both were of high quality. See Table 5 and Additional file 7 for the effectiveness statements.
Consumer system participation
Twenty-eight reviews included this strategy but only six provided evidence that was useful for the development of the effectiveness statements [ 10 , 19 , 23 , 32 , 42 , 45 ]—two of these were of high quality [ 10 , 45 ]. In relation to consumer system participation, no single strategies were identified. For the combined strategies, none had sufficient evidence and only one had some evidence of effectiveness, with the resulting effectiveness statement:
The use of social media and telemonitoring (ICT platforms) for promoting patient engagement and delivering behavior change interventions may improve health outcomes [ 42 ].
The combined strategies with insufficient evidence are listed in Additional file 7 .
We did not find any SRs of exclusively qualitative studies. Of the 44 included SRs, ten included qualitative research among other study designs. For the synthesis of barriers (and facilitators) to KT to healthcare participants, 31 SRs contributed information. The barriers identified were grouped following the type of communication used for the intervention or strategy (Additional file 5 , Table 2 ). While this method of grouping barriers was not originally stated in the protocol, we found it to be the most logical way to group them due to the way in which barriers were reported in the included systematic reviews.
None of the SRs identified barriers to verbal communication specifically. However, in relation to patient advisory councils (which may use both verbal and electronic communication), the main barrier described was that the implementation takes a significant amount of time and resources for recruitment, holding meetings, and providing follow up [ 45 ].
For written information that does not require the internet, concerns were raised about motivation and awareness [ 29 , 49 ], health literacy [ 40 , 53 ], and comprehension and understanding [ 29 , 40 ]. Other possible barriers that should be considered are the reliability and trustworthiness of the information [ 29 ], personal needs [ 49 ], and text complexity and design [ 40 ].
For information technology interventions in general, barriers raised include health literacy, privacy and information quality concerns, access to technology, and information design [ 42 ].
Computer-based strategies, whether internet-based or not, present as barriers difficulties in the management of technologies, mainly for the elderly [ 19 ], e-literacy [ 25 , 31 , 51 ], privacy concerns, consumer’s personal feelings, socioeconomic factors [ 31 , 41 ], and health literacy [ 31 , 44 ]. Other barriers include reliability and trustworthiness in the information, lack of time and personal impairment [ 31 ], motivation and awareness, and information that does not meet personal needs [ 49 ], text complexity and lack of access to information [ 44 ].
Strategies that use multimedia not based on the internet bring difficulties like motivation, awareness, information that does not respond to personal need or without sufficient detail [ 49 ], problems of communication [ 35 ], and health literacy [ 18 , 52 ] for their implementation.
Internet-based multimedia strategies face barriers such as e-literacy [ 12 , 25 , 51 ], health literacy [ 11 , 37 , 44 ], motivation, awareness [ 12 , 29 ], concerns about reliability and trustworthiness [ 29 , 37 ], complexity of the text [ 37 , 44 ], consumer’s personal feelings, information overload, and information that does not match personal needs [ 37 ]. Other barriers were lack of internet access and personal skills [ 12 , 24 , 44 , 51 ], and comprehension, understanding, and self-management when self-management interventions packaged with guidelines are used [ 5 ].
Most of the studies did not discuss issues such as ethnicity, income level, or homelessness, which are important when considering the use of an internet-based technology to deliver an outpatient intervention. The long-term effects on individual persistence with chosen therapies and cost-effectiveness of the use of internet-based therapies and hardware and software development require continued evaluation [ 51 ]. Recent SRs have mentioned inequities such as lack of access to technology [ 42 ]. However, one review noted that a benefit of social media is that it can widen access to those who may not easily access health information via traditional methods, such as younger people, ethnic minorities, and lower socioeconomic groups [ 39 ].
For social media interventions, barriers on the individual level include health literacy [ 7 , 42 ] and the risk of a deterioration in the relationship between health professionals and patients [ 7 , 39 ], including the inability to meet the patients’ emotional and information needs [ 46 ]. Other concerns include how the information is presented [ 7 , 21 ], privacy, information quality, lack of internet access, trustworthiness in the information, information overload, and stigma about certain conditions [ 39 ]. Another highlighted barrier was the fact that the social content is used more than the educational content, i.e., participants use the social media to interact with other users more than as a means for self-education [ 38 ].
m-health (with mobile phone) strategies raise as the main barriers to its implementation issues such as e-literacy and lack of internet access [ 10 , 27 ], health literacy [ 34 , 44 ], socioeconomic factors [ 27 , 50 ], privacy concerns, lack of personal skills [ 10 ], text complexity [ 44 ], and the time-consuming nature of the technology [ 54 ]. Another potential limitation of m-health could be that the delivery of interventions can be interrupted if the mobile phone is stolen or lost. However, the same limitations exist with many other forms of communication (e.g., postal mail may be delivered to the wrong address, email boxes may be too full to receive messages) [ 27 ].
Barriers to implementation of telemedicine are also related to e-literacy, privacy concerns, lack of internet access, and personal skills [ 10 ].
For the implementation of patient decision aids, which can include pamphlets, videos, or web-based tools, barriers detected include decision aids that do not meet the needs of the population, clinicians unwilling to use them, and clinicians and healthcare consumers without skills for shared decision-making [ 48 ].
For this overview, we identified 44 SRs that describe the effective strategies to disseminate health knowledge to the public, patients, and caregivers. Some of these SRs also describe the most important barriers to the uptake of these effective strategies. The reviews that tested more general strategies were selected instead of those directed to a particular condition or setting. To our knowledge, this is the first overview of SRs addressing this objective.
While we reported the strategies and results according to the taxonomy adapted from the Health System Evidence database [ 13 ], we found that many strategies overlapped for both the type of intervention and the outcome measures. For example, interventions providing information or education could report outcomes related to behavior change or self-efficacy, and the primary intention could have been to increase knowledge. Situations like these were frequent and could be due to the use of combined strategies or to characteristics of the intervention itself, its intensity, frequency, or duration. The strategies reported in the included SRs could be directed to individuals or groups, in print or verbally, face to face, or remotely. In addition, interventions could range from single (e.g., a written information leaflet) to combined strategies. We considered a strategy to be combined when it used two or more verbal, print, or remote health information strategies (e.g., video, computer, and slide show presentations [ 11 ]), or different electronic communication types (based or not on the internet), such as telemedicine, ICT applications or ICT platforms [ 10 , 42 ], or social networking like Facebook or Twitter [ 39 , 46 ].
We found few SRs with a meta-analysis that could inform the magnitude of effects. Thus, an overall meta-analysis for each of the strategies could not be conducted, which is why we chose to adopt the approach proposed by Ryan et al. [ 9 ] and have presented the findings as evidence statements.
A key objective of the included interventions was to inform, improve knowledge, or to change health behaviors. To achieve behavioral changes, different strategies were used, such as training, coaching, or text messages. Factors that affected the effectiveness of the intervention included its frequency, intensity, and follow-up time. These factors are important to consider when implementing the chosen intervention strategies, including the applicability of the intervention in different modes of implementation and contexts.
When analyzing those strategies with the greatest potential to achieve behavioral changes, the majority of strategies with sufficient evidence of effectiveness were combined, frequent, and/or intense over time. Further, strategies focused on the patient, with tailored interventions, and those that seek to acquire skills and competencies were more effective in achieving these changes. Many of these strategies used toolkits or different platforms, based or not on the internet. Examples of strategies based on the internet include social networks, specific portals, tailored text messaging, or email [ 23 , 27 , 30 , 36 , 42 , 43 , 50 , 54 ]. Examples of strategies that are not always based on the internet are the use of videos, telephone calls, telemedicine, and telemonitoring [ 9 , 10 , 41 , 48 , 49 , 53 ].
Other examples of effective tailored interventions, such as those designed to improve communication or participation in decision-making between patients and healthcare providers, were the use of patient decision aids and patient information leaflets, provided electronically or not [ 10 , 48 , 49 ]. Interestingly, when coaching was added to patient decision aids, we found some evidence for improvements in knowledge and participation. Also, coaching, when compared to patient decision aids alone, increased values-choice agreement and improved satisfaction with the decision-making process [ 47 ]. In relation to satisfaction, we also found some evidence for improvement in patient satisfaction for interventions through multimedia before consultations designed to help patients with their information needs [ 35 ].
With regard to caregivers, in particular of patients with Alzheimer’s disease, we found good evidence for the effect of a home safety toolkit for improvements in home safety, risky behavior, and caregiver self-efficacy [ 53 ]. For interventions that involved patients and/or caregivers in decision-making processes at a system level, we did not find sufficient evidence to make any statements. Further, few studies included a follow-up period longer than 1 year or reported retention rate, thus it is not known if behavior change results are sustained over time [ 32 , 36 , 42 , 51 ].
Our second research question focused on barriers to the dissemination of knowledge to healthcare recipients, which are important to consider when implementing chosen intervention strategies. The barriers most frequently mentioned were related to ICT or to the information itself. For ICT, the main concerns were access to the technologies, including availability of the internet. On a personal level, the lack of skills for managing new technologies, privacy issues, lack of time, and deterioration of the doctor-patient relationship were also mentioned, especially when using social media or websites. As for the information itself, the lack of understanding or comprehension, the volume of information, text complexity and its design, information that did not meet the needs of the patients, and trustworthiness were the key barriers mentioned. While inequities were mentioned and were often related to the lack of health literacy or e-literacy, the benefits of social media were also emphasized, for example by widening access to health information, particularly for ethnic minorities and lower socioeconomic groups.
Strengths and limitations of the overview
Strengths of our overview were that only reviews of medium or high quality were included, as well as our focus on strategies that translated health information to patients and caregivers through different strategies and types of dissemination. Further, we focused on more general interventions rather than specific interventions, which are already abundant in the scientific literature and could be among the list of SRs that were excluded. While we did not find SRs of qualitative studies to analyze barriers to a better implementation of dissemination interventions, we did find considerable information and analysis of barriers in many of the included SRs. These included good quality studies on health literacy. Further, we were able to identify many research gaps that are detailed in Additional file 8 .
Limitations of our overview include limitations in the included SRs, such as the lack of clear description of the interventions, setting or samples, and outcomes in some reviews. Further, not all of the included SRs used theories or frameworks to inform the strategies. Finally, due to the heterogeneity in the interventions and outcomes, a meta-analysis was not possible.
Achieving improvements in knowledge uptake or health behaviors is difficult and the literature of effectiveness for the different strategies in the clinical field has been presented using a range of frameworks, theories, or taxonomies. While work is underway to develop consistent taxonomies for the design and reporting of behavior change and dissemination and implementation interventions, such as the behavior change wheel [ 55 ], the theoretical domains framework [ 56 , 57 ], and other taxonomies [ 58 ], these are not consistently applied in the existing literature. Further, few have been developed for patients or their caregivers, and there is more of a focus on implementation rather than dissemination. None of the developed frameworks were suitable for our context ( https://dissemination-implementation.org/viewAll_di.aspx ). Thus, given that this overview was aimed at healthcare decision-makers, we chose to use the Health Systems Evidence taxonomy of Lavis and colleagues [ 13 ]. The advantage of using this taxonomy is that it makes it easier for healthcare decision-makers to find, understand, and use the evidence contained in the overview. Further, while there is debate about how best to measure the effectiveness of complex behavior change interventions [ 59 , 60 ], these authors acknowledge that further work is needed. Until that work is conducted and consensus achieved, systematic reviews of randomized controlled trials (and other designs), as used in this overview, are the currently accepted best method.
This overview of systematic reviews has shown that a variety of dissemination strategies aimed at healthcare users and their caregivers can improve health and wellbeing in different ways. However, implementation of our findings will need to consider the particular context in which a strategy is to be implemented. This overview will help decision-makers choose the most effective dissemination strategies and will also inform them as to the factors that they should consider when implementing those strategies.
Implications for practice and policy
Those interventions that have been shown to be effective in improving knowledge uptake or health behaviors should be implemented in practice, programs, and policies—if not already implemented. The benefits of strategies such as e-health and m-health, including telemedicine, should be considered for knowledge dissemination and to improve health behaviors—especially in populations with lack of access to traditional sources of healthcare, including in remote or rural areas. The application of distance technology may strengthen the continuity of care between patient and clinician by improving access and supporting the coordination of healthcare activities from a single source. When designing KT strategies, not only the effectiveness of the strategy but also the characteristics of the interventions should be taken into account, such as the type of dissemination (electronic or not), frequency, intensity, and follow-up time. It is also important to ensure that the content of the messages is addressed to people with low literacy, low numeracy, and low e-literacy. The knowledge disseminated should be readable, comprehensible, relevant, consistent, unambiguous, and credible for patients. Moreover, patients should be invited to participate in its design. All of these strategies are likely to increase the success of the dissemination.
Implications for research
Future research should focus on the areas identified as research gaps in Additional file 8 . In addition, researchers should ensure that the interventions tested are well described in their papers. Likewise, systematic reviewers should also ensure that they include a clear description of the interventions, settings, samples, and outcomes included in their reviews to facilitate their evaluation and implementation by decision-makers.
Availability of data and materials
All files supporting the conclusions of this article are included within the article or in Additional files 1 , 2 , 3 , 4 , 5 , and 6 .
Abbreviations
A MeaSurement Tool to Assess systematic Reviews
Absolute risk reduction
Canadian Institutes for Health Research
Information and Communication Technologies
- Knowledge translation
Medical Subject Headings
Number needed to treat
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Relative risk reduction
Short message service
Pablos-Mendez A, Shademani R. Knowledge translation in global health. J Contin Educ Heal Prof. 2006;26(1):81–6.
Article Google Scholar
WHO. Knowledge translation framework for Ageing and health. Geneva: Department of Ageing and Life-Course, World Health Organization; 2012.
Google Scholar
Gagliardi AR, Legare F, Brouwers MC, Webster F, Badley E, Straus S. Patient-mediated knowledge translation (PKT) interventions for clinical encounters: a systematic review. Implement Sci. 2016;11(1):26.
Article PubMed PubMed Central Google Scholar
Graham ID, Tetroe JM. Getting evidence into policy and practice: perspective of a health research funder. J Can Acad Child Adolesc Psychiatry. 2009;18(1):46–50.
PubMed PubMed Central Google Scholar
Vernooij RW, Willson M, Gagliardi AR, members of the Guidelines International Network Implementation Working G. Characterizing patient-oriented tools that could be packaged with guidelines to promote self-management and guideline adoption: a meta-review. Implement Sci. 2016;11:52.
Albrecht L, Archibald M, Arseneau D, Scott SD. Development of a checklist to assess the quality of reporting of knowledge translation interventions using the workgroup for intervention development and evaluation research (WIDER) recommendations. Implement Sci. 2013;8(1):52.
McCormack L, Sheridan S, Lewis M, Boudewyns V, Melvin CL, Kistler C, et al. Communication and dissemination strategies to facilitate the use of health-related evidence. Evidence Report/Technology Assessment No. 213. AHRQ Publication No. 13(14)-E003-EF. Rockville: Agency for Healthcare Research and Quality; 2013.
Pantoja T, Opiyo N, Lewin S, Paulsen E, Ciapponi A, Wiysonge CS, et al. Implementation strategies for health systems in low-income countries: an overview of systematic reviews. Cochrane Database Syst Rev. 2017;9:CD011086.
PubMed Google Scholar
Ryan R, Santesso N, Lowe D, Hill S, Grimshaw J, Prictor M, et al. Interventions to improve safe and effective medicines use by consumers: an overview of systematic reviews. Cochrane Database Syst Rev. 2014;4:CD007768.
Finkelstein J, Knight A, Marinopoulos S, Gibbons MC, Berger Z, Aboumatar H, et al. Enabling patient-centered care through health information technology. Evidence Report/Technology Assessment No. 206. AHRQ Publication No. 12-E005-EF. Rockville: Agency for Healthcare Research and Quality. 2012.
Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Viera A, Crotty K, et al. Health literacy interventions and outcomes: an updated systematic review. Evidence Report/Technology Assesment No. 199. AHRQ Publication Number 11-E006. Rockville: Agency for Healthcare Research and Quality. 2011.
Car J, Lang B, Colledge A, Ung C, Majeed A. Interventions for enhancing consumers’ online health literacy. Cochrane Database Syst Rev. 2011;(6). Art. No.: CD007092.
Lavis JN, Wilson MG, Moat KA, Hammill AC, Boyko JA, Grimshaw JM, et al. Developing and refining the methods for a ‘one-stop shop’ for research evidence about health systems. Health Res Policy Syst. 2015;13(1):10.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Chapman E, Barreto JOM. Knowledge translation overview: strategies for dissemination with a focus on recipient health care. PROSPERO 2018 CRD42018093245. Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018093245 .
Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10.
Lewin S, Glenton C, Munthe-Kaas H, Carlsen B, Colvin CJ, Gulmezoglu M, et al. Using qualitative evidence in decision making for health and social interventions: an approach to assess confidence in findings from qualitative evidence syntheses (GRADE-CERQual). PLoS Med. 2015;12(10):e1001895.
Abu Abed M, Himmel W, Vormfelde S, Koschack J. Video-assisted patient education to modify behavior: a systematic review. Patient Educ Couns. 2014;97(1):16–22.
Article PubMed Google Scholar
Akesson KM, Saveman BI, Nilsson G. Health care consumers’ experiences of information communication technology--a summary of literature. Int J Med Inform. 2007;76(9):633–45.
Akl EA, Oxman AD, Herrin J, Vist GE, Terrenato I, Sperati F, et al. Framing of health information messages. Cochrane Database Syst Rev. 2011;(12). Art. No.: CD006777.
Akl EA, Oxman AD, Herrin J, Vist GE, Terrenato I, Sperati F, et al. Using alternative statistical formats for presenting risks and risk reductions. Cochrane Database Syst Rev. 2011;3:1–90.
Ammentorp J, Uhrenfeldt L, Angel F, Ehrensvard M, Carlsen EB, Kofoed PE. Can life coaching improve health outcomes?—a systematic review of intervention studies. BMC Health Serv Res. 2013;13:428.
Ammenwerth E, Schnell-Inderst P, Hoerbst A. The impact of electronic patient portals on patient care: a systematic review of controlled trials. J Med Internet Res. 2012;14(6):e162.
Atherton H, Sawmynaden P, Sheikh A, Majeed A, Car J. Email for clinical communication between patients/caregivers and healthcare professionals. Cochrane Database Syst Rev. 2012;11:CD007978.
Bekker HL, Winterbottom AE, Butow P, Dillard AJ, Feldman-Stewart D, Fowler FJ, et al. Do personal stories make patient decision aids more effective? A critical review of theory and evidence. BMC Med Inform Decis Mak. 2013;13(Suppl 2):S9.
Buchter R, Fechtelpeter D, Knelangen M, Ehrlich M, Waltering A. Words or numbers? Communicating risk of adverse effects in written consumer health information: a systematic review and meta-analysis. BMC Med Inform Decis Mak. 2014;14:76.
Cole-Lewis H, Kershaw T. Text messaging as a tool for behavior change in disease prevention and management. Epidemiol Rev. 2010;32:56–69.
Edwards A, Hood K, Matthews E, Russell D, Russell I, Barker J, et al. The effectiveness of one-to-one risk communication interventions in health care: a systematic review. Med Decis Mak. 2000;20(3):290–7.
Article CAS Google Scholar
Faber M, Bosch M, Wollersheim H, Leatherman S, Grol R. Public reporting in health care: how do consumers use quality-of-care information? A systematic review. Med Care. 2009;47(1):1–8.
Fjeldsoe BS, Marshall AL, Miller YD. Behavior change interventions delivered by mobile telephone short-message service. Am J Prev Med. 2009;36(2):165–73.
Gibbons MC, Wilson RF, Samal L, Lehman CU, Dickersin K, Lehmann HP, et al. Impact of consumer health informatics applications. Evidence Report/Technology Assessment No. 188. AHRQ Publication No. 09(10)-E019. Rockville: Agency for Healthcare Research and Quality. 2009.
Health Quality Ontario. Electronic tools for health information exchange: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13(11):1–76. Available from: http://www.hqontario.ca/en/documents/eds/2013/full-report-OCDM-etools.pdf .
PubMed Central Google Scholar
Hoffman BL, Shensa A, Wessel C, Hoffman R, Primack BA. Exposure to fictional medical television and health: a systematic review. Health Educ Res. 2017;32(2):107–23.
Ketelaar NA, Faber MJ, Flottorp S, Rygh LH, Deane KH, Eccles MP. Public release of performance data in changing the behaviour of healthcare consumers, professionals or organisations. Cochrane Database Syst Rev. 2011;(11). Art. No.: CD004538.
Kinnersley P, Edwards A, Hood K, Cadbury N, Ryan R, Prout H, et al. Interventions before consultations for helping patients address their information needs. Cochrane Database Syst Rev. 2007;(3). Art. No.: CD004565.
Laranjo L, Arguel A, Neves AL, Gallagher AM, Kaplan R, Mortimer N, et al. The influence of social networking sites on health behavior change: a systematic review and meta-analysis. J Am Med Inform Assoc. 2015;22(1):243–56.
Loudon K, Santesso N, Callaghan M, Thornton J, Harbour J, Graham K, et al. Patient and public attitudes to and awareness of clinical practice guidelines: a systematic review with thematic and narrative syntheses. BMC Health Serv Res. 2014;14:321.
Maher CA, Lewis LK, Ferrar K, Marshall S, De Bourdeaudhuij I, Vandelanotte C. Are health behavior change interventions that use online social networks effective? A systematic review. J Med Internet Res. 2014;16(2):e40.
Moorhead SA, Hazlett DE, Harrison L, Carroll JK, Irwin A, Hoving C. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. J Med Internet Res. 2013;15(4):e85.
Pires C, Vigário M, Cavaco A. Readability of medicinal package leaflets: a systematic review. Rev Saude Publica. 2015;49:1–13.
Revere D, Dunbar PJ. Review of computer-generated outpatient health behavior interventions: clinical encounters “in absentia”. J Am Med Inform Assoc. 2001;8(1):62–79.
Article CAS PubMed PubMed Central Google Scholar
Sawesi S, Rashrash M, Phalakornkule K, Carpenter JS, Jones JF. The impact of information technology on patient engagement and health behavior change: a systematic review of the literature. JMIR Med Inform. 2016;4(1):e1.
Sawmynaden P, Atherton H, Majeed A, Car J. Email for the provision of information on disease prevention and health promotion. Cochrane Database Syst Rev. 2012;11:CD007982.
Schipper K, Bakker M, De Wit M, Ket JC, Abma TA. Strategies for disseminating recommendations or guidelines to patients: a systematic review. Implement Sci. 2016;11(1):82.
Sharma AE, Knox M, Mleczko VL, Olayiwola JN. The impact of patient advisors on healthcare outcomes: a systematic review. BMC Health Serv Res. 2017;17(1):693.
Smailhodzic E, Hooijsma W, Boonstra A, Langley DJ. Social media use in healthcare: a systematic review of effects on patients and on their relationship with healthcare professionals. BMC Health Serv Res. 2016;16:442.
Stacey D, Kryworuchko J, Bennett C, Murray MA, Mullan S, Legare F. Decision coaching to prepare patients for making health decisions: a systematic review of decision coaching in trials of patient decision AIDS. Med Decis Mak. 2012;32(3):E22–33.
Stacey D, Legare F, Lewis K, Barry MJ, Bennett CL, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431.
Sustersic M, Gauchet A, Foote A, Bosson JL. How best to use and evaluate patient information leaflets given during a consultation: a systematic review of literature reviews. Health Expect. 2017;20(4):531–42.
Vodopivec-Jamsek V, de Jongh T, Gurol-Urganci I, Atun R, Car J. Mobile phone messaging for preventive health care. Cochrane Database Syst Rev. 2012;12:CD007457.
Wantland DJ, Portillo CJ, Holzemer WL, Slaughter R, McGhee EM. The effectiveness of web-based vs. non-web-based interventions: a meta-analysis of behavioral change outcomes. J Med Internet Res. 2004;6(4):e40.
Wilson EA, Makoul G, Bojarski EA, Bailey SC, Waite KR, Rapp DN, et al. Comparative analysis of print and multimedia health materials: a review of the literature. Patient Educ Couns. 2012;89(1):7–14.
Yamada J, Shorkey A, Barwick M, Widger K, Stevens BJ. The effectiveness of toolkits as knowledge translation strategies for integrating evidence into clinical care: a systematic review. BMJ Open. 2015;5(4):e006808.
Zhao J, Freeman B, Li M. Can Mobile phone apps influence People’s health behavior change? An evidence review. J Med Internet Res. 2016;18(11):e287.
Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6(1):42.
Atkins L, Francis J, Islam R, O'Connor D, Patey A, Ivers N, et al. A guide to using the theoretical domains framework of behaviour change to investigate implementation problems. Implement Sci. 2017;12(1):77.
Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci. 2012;7(1):37.
Michie S, Wood CE, Johnston M, Abraham C, Francis JJ, Hardeman W. Behaviour change techniques: the development and evaluation of a taxonomic method for reporting and describing behaviour change interventions (a suite of five studies involving consensus methods, randomised controlled trials and analysis of qualitative data). Health Technol Assess. 2015;19(99):1–188.
Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ. 2000;321(7262):694–6.
Michie S, West R, Sheals K, Godinho CA. Evaluating the effectiveness of behavior change techniques in health-related behavior: a scoping review of methods used. Transl Behav Med. 2018;8(2):212–24.
Download references
Acknowledgments
Not applicable.
This study was supported by a grant from the Ministry of Health of Brazil (TED MS/SCTIE-Fiocruz #43/2016). The funder had no role in the design of the study, collection, analysis or interpretation of data or in the writing of the manuscript.
Author information
Authors and affiliations.
Fundação Oswaldo Cruz, Fiocruz, Brasília, Brazil
Evelina Chapman & Jorge O. Maia Barreto
Departamento de Ciencias Químico Biológicas, Universidad de Sonora, Hermosillo, Sonora, Mexico
Michelle M. Haby
Centre for Health Policy, Melbourne School of Population and Global Health, The University of Melbourne, Melbourne, VIC, Australia
Instituto de Saúde, Secretaria de Estado da Saúde de São Paulo, São Paulo, Brazil
Tereza Setsuko Toma & Maritsa Carla de Bortoli
School of Psychology Universidad Mayor, Santiago, Chile
Eduardo Illanes
Departamento de Medicina Interna, Facultad de Medicina, Universidad de La Frontera, Temuco, Chile
Maria Jose Oliveros
You can also search for this author in PubMed Google Scholar
Contributions
EC and JB conceived the study and wrote the protocol. MH and EC conducted the searching. EC, JB and MO selected studies for inclusion. All authors contributed to data extraction and quality assessment. EC, JB, and MH contributed to the analysis and interpretation of results. EC drafted the first version of the manuscript, with input from JB and MH. All authors read and approved the final manuscript.
Authors’ information
Corresponding author.
Correspondence to Michelle M. Haby .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1..
Search terms and results.
Additional file 2.
Evidence rating scheme (based on Ryan et al. 2014 [ 9 ]).
Additional file 3
Excluded studies ( N = 47).
Additional file 4.
Characteristics of included studies and AMSTAR quality assessment. Table 1 Characteristics of included systematic reviews. Table 2 Quality of included systematic reviews (AMSTAR).
Additional file 5.
Strategies and types of communication or dissemination. Table 1 Strategies/Interventions (adapted from Lavis et al. 2015 [ 13 ]). Table 2 Types of communication or dissemination. Table 3 Characteristics of interventions (details).
Additional file 6.
Types of outcome measures.
Additional file 7.
Strategies categorized as having insufficient evidence.
Additional file 8.
Research gaps.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Chapman, E., Haby, M.M., Toma, T.S. et al. Knowledge translation strategies for dissemination with a focus on healthcare recipients: an overview of systematic reviews. Implementation Sci 15 , 14 (2020). https://doi.org/10.1186/s13012-020-0974-3
Download citation
Received : 17 July 2019
Accepted : 17 February 2020
Published : 04 March 2020
DOI : https://doi.org/10.1186/s13012-020-0974-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Research uptake
Implementation Science
ISSN: 1748-5908
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Search Menu
Sign in through your institution
- Browse content in Arts and Humanities
- Browse content in Archaeology
- Anglo-Saxon and Medieval Archaeology
- Archaeological Methodology and Techniques
- Archaeology by Region
- Archaeology of Religion
- Archaeology of Trade and Exchange
- Biblical Archaeology
- Contemporary and Public Archaeology
- Environmental Archaeology
- Historical Archaeology
- History and Theory of Archaeology
- Industrial Archaeology
- Landscape Archaeology
- Mortuary Archaeology
- Prehistoric Archaeology
- Underwater Archaeology
- Zooarchaeology
- Browse content in Architecture
- Architectural Structure and Design
- History of Architecture
- Residential and Domestic Buildings
- Theory of Architecture
- Browse content in Art
- Art Subjects and Themes
- History of Art
- Industrial and Commercial Art
- Theory of Art
- Biographical Studies
- Byzantine Studies
- Browse content in Classical Studies
- Classical History
- Classical Philosophy
- Classical Mythology
- Classical Numismatics
- Classical Literature
- Classical Reception
- Classical Art and Architecture
- Classical Oratory and Rhetoric
- Greek and Roman Epigraphy
- Greek and Roman Law
- Greek and Roman Archaeology
- Greek and Roman Papyrology
- Late Antiquity
- Religion in the Ancient World
- Social History
- Digital Humanities
- Browse content in History
- Colonialism and Imperialism
- Diplomatic History
- Environmental History
- Genealogy, Heraldry, Names, and Honours
- Genocide and Ethnic Cleansing
- Historical Geography
- History by Period
- History of Agriculture
- History of Education
- History of Emotions
- History of Gender and Sexuality
- Industrial History
- Intellectual History
- International History
- Labour History
- Legal and Constitutional History
- Local and Family History
- Maritime History
- Military History
- National Liberation and Post-Colonialism
- Oral History
- Political History
- Public History
- Regional and National History
- Revolutions and Rebellions
- Slavery and Abolition of Slavery
- Social and Cultural History
- Theory, Methods, and Historiography
- Urban History
- World History
- Browse content in Language Teaching and Learning
- Language Learning (Specific Skills)
- Language Teaching Theory and Methods
- Browse content in Linguistics
- Applied Linguistics
- Cognitive Linguistics
- Computational Linguistics
- Forensic Linguistics
- Grammar, Syntax and Morphology
- Historical and Diachronic Linguistics
- History of English
- Language Acquisition
- Language Variation
- Language Families
- Language Evolution
- Language Reference
- Lexicography
- Linguistic Theories
- Linguistic Typology
- Linguistic Anthropology
- Phonetics and Phonology
- Psycholinguistics
- Sociolinguistics
- Translation and Interpretation
- Writing Systems
- Browse content in Literature
- Bibliography
- Children's Literature Studies
- Literary Studies (Asian)
- Literary Studies (European)
- Literary Studies (Eco-criticism)
- Literary Studies (Modernism)
- Literary Studies (Romanticism)
- Literary Studies (American)
- Literary Studies - World
- Literary Studies (1500 to 1800)
- Literary Studies (19th Century)
- Literary Studies (20th Century onwards)
- Literary Studies (African American Literature)
- Literary Studies (British and Irish)
- Literary Studies (Early and Medieval)
- Literary Studies (Fiction, Novelists, and Prose Writers)
- Literary Studies (Gender Studies)
- Literary Studies (Graphic Novels)
- Literary Studies (History of the Book)
- Literary Studies (Plays and Playwrights)
- Literary Studies (Poetry and Poets)
- Literary Studies (Postcolonial Literature)
- Literary Studies (Queer Studies)
- Literary Studies (Science Fiction)
- Literary Studies (Travel Literature)
- Literary Studies (War Literature)
- Literary Studies (Women's Writing)
- Literary Theory and Cultural Studies
- Mythology and Folklore
- Shakespeare Studies and Criticism
- Browse content in Media Studies
- Browse content in Music
- Applied Music
- Dance and Music
- Ethics in Music
- Ethnomusicology
- Gender and Sexuality in Music
- Medicine and Music
- Music Cultures
- Music and Religion
- Music and Culture
- Music and Media
- Music Education and Pedagogy
- Music Theory and Analysis
- Musical Scores, Lyrics, and Libretti
- Musical Structures, Styles, and Techniques
- Musicology and Music History
- Performance Practice and Studies
- Race and Ethnicity in Music
- Sound Studies
- Browse content in Performing Arts
- Browse content in Philosophy
- Aesthetics and Philosophy of Art
- Epistemology
- Feminist Philosophy
- History of Western Philosophy
- Metaphysics
- Moral Philosophy
- Non-Western Philosophy
- Philosophy of Science
- Philosophy of Action
- Philosophy of Law
- Philosophy of Religion
- Philosophy of Language
- Philosophy of Mind
- Philosophy of Perception
- Philosophy of Mathematics and Logic
- Practical Ethics
- Social and Political Philosophy
- Browse content in Religion
- Biblical Studies
- Christianity
- East Asian Religions
- History of Religion
- Judaism and Jewish Studies
- Qumran Studies
- Religion and Education
- Religion and Health
- Religion and Politics
- Religion and Science
- Religion and Law
- Religion and Art, Literature, and Music
- Religious Studies
- Browse content in Society and Culture
- Cookery, Food, and Drink
- Cultural Studies
- Customs and Traditions
- Ethical Issues and Debates
- Hobbies, Games, Arts and Crafts
- Natural world, Country Life, and Pets
- Popular Beliefs and Controversial Knowledge
- Sports and Outdoor Recreation
- Technology and Society
- Travel and Holiday
- Visual Culture
- Browse content in Law
- Arbitration
- Browse content in Company and Commercial Law
- Commercial Law
- Company Law
- Browse content in Comparative Law
- Systems of Law
- Competition Law
- Browse content in Constitutional and Administrative Law
- Government Powers
- Judicial Review
- Local Government Law
- Military and Defence Law
- Parliamentary and Legislative Practice
- Construction Law
- Contract Law
- Browse content in Criminal Law
- Criminal Procedure
- Criminal Evidence Law
- Sentencing and Punishment
- Employment and Labour Law
- Environment and Energy Law
- Browse content in Financial Law
- Banking Law
- Insolvency Law
- History of Law
- Human Rights and Immigration
- Intellectual Property Law
- Browse content in International Law
- Private International Law and Conflict of Laws
- Public International Law
- IT and Communications Law
- Jurisprudence and Philosophy of Law
- Law and Politics
- Law and Society
- Browse content in Legal System and Practice
- Courts and Procedure
- Legal Skills and Practice
- Legal System - Costs and Funding
- Primary Sources of Law
- Regulation of Legal Profession
- Medical and Healthcare Law
- Browse content in Policing
- Criminal Investigation and Detection
- Police and Security Services
- Police Procedure and Law
- Police Regional Planning
- Browse content in Property Law
- Personal Property Law
- Restitution
- Study and Revision
- Terrorism and National Security Law
- Browse content in Trusts Law
- Wills and Probate or Succession
- Browse content in Medicine and Health
- Browse content in Allied Health Professions
- Arts Therapies
- Clinical Science
- Dietetics and Nutrition
- Occupational Therapy
- Operating Department Practice
- Physiotherapy
- Radiography
- Speech and Language Therapy
- Browse content in Anaesthetics
- General Anaesthesia
- Browse content in Clinical Medicine
- Acute Medicine
- Cardiovascular Medicine
- Clinical Genetics
- Clinical Pharmacology and Therapeutics
- Dermatology
- Endocrinology and Diabetes
- Gastroenterology
- Genito-urinary Medicine
- Geriatric Medicine
- Infectious Diseases
- Medical Oncology
- Medical Toxicology
- Pain Medicine
- Palliative Medicine
- Rehabilitation Medicine
- Respiratory Medicine and Pulmonology
- Rheumatology
- Sleep Medicine
- Sports and Exercise Medicine
- Clinical Neuroscience
- Community Medical Services
- Critical Care
- Emergency Medicine
- Forensic Medicine
- Haematology
- History of Medicine
- Browse content in Medical Dentistry
- Oral and Maxillofacial Surgery
- Paediatric Dentistry
- Restorative Dentistry and Orthodontics
- Surgical Dentistry
- Medical Ethics
- Browse content in Medical Skills
- Clinical Skills
- Communication Skills
- Nursing Skills
- Surgical Skills
- Medical Statistics and Methodology
- Browse content in Neurology
- Clinical Neurophysiology
- Neuropathology
- Nursing Studies
- Browse content in Obstetrics and Gynaecology
- Gynaecology
- Occupational Medicine
- Ophthalmology
- Otolaryngology (ENT)
- Browse content in Paediatrics
- Neonatology
- Browse content in Pathology
- Chemical Pathology
- Clinical Cytogenetics and Molecular Genetics
- Histopathology
- Medical Microbiology and Virology
- Patient Education and Information
- Browse content in Pharmacology
- Psychopharmacology
- Browse content in Popular Health
- Caring for Others
- Complementary and Alternative Medicine
- Self-help and Personal Development
- Browse content in Preclinical Medicine
- Cell Biology
- Molecular Biology and Genetics
- Reproduction, Growth and Development
- Primary Care
- Professional Development in Medicine
- Browse content in Psychiatry
- Addiction Medicine
- Child and Adolescent Psychiatry
- Forensic Psychiatry
- Learning Disabilities
- Old Age Psychiatry
- Psychotherapy
- Browse content in Public Health and Epidemiology
- Epidemiology
- Public Health
- Browse content in Radiology
- Clinical Radiology
- Interventional Radiology
- Nuclear Medicine
- Radiation Oncology
- Reproductive Medicine
- Browse content in Surgery
- Cardiothoracic Surgery
- Gastro-intestinal and Colorectal Surgery
- General Surgery
- Neurosurgery
- Paediatric Surgery
- Peri-operative Care
- Plastic and Reconstructive Surgery
- Surgical Oncology
- Transplant Surgery
- Trauma and Orthopaedic Surgery
- Vascular Surgery
- Browse content in Science and Mathematics
- Browse content in Biological Sciences
- Aquatic Biology
- Biochemistry
- Bioinformatics and Computational Biology
- Developmental Biology
- Ecology and Conservation
- Evolutionary Biology
- Genetics and Genomics
- Microbiology
- Molecular and Cell Biology
- Natural History
- Plant Sciences and Forestry
- Research Methods in Life Sciences
- Structural Biology
- Systems Biology
- Zoology and Animal Sciences
- Browse content in Chemistry
- Analytical Chemistry
- Computational Chemistry
- Crystallography
- Environmental Chemistry
- Industrial Chemistry
- Inorganic Chemistry
- Materials Chemistry
- Medicinal Chemistry
- Mineralogy and Gems
- Organic Chemistry
- Physical Chemistry
- Polymer Chemistry
- Study and Communication Skills in Chemistry
- Theoretical Chemistry
- Browse content in Computer Science
- Artificial Intelligence
- Computer Architecture and Logic Design
- Game Studies
- Human-Computer Interaction
- Mathematical Theory of Computation
- Programming Languages
- Software Engineering
- Systems Analysis and Design
- Virtual Reality
- Browse content in Computing
- Business Applications
- Computer Security
- Computer Games
- Computer Networking and Communications
- Digital Lifestyle
- Graphical and Digital Media Applications
- Operating Systems
- Browse content in Earth Sciences and Geography
- Atmospheric Sciences
- Environmental Geography
- Geology and the Lithosphere
- Maps and Map-making
- Meteorology and Climatology
- Oceanography and Hydrology
- Palaeontology
- Physical Geography and Topography
- Regional Geography
- Soil Science
- Urban Geography
- Browse content in Engineering and Technology
- Agriculture and Farming
- Biological Engineering
- Civil Engineering, Surveying, and Building
- Electronics and Communications Engineering
- Energy Technology
- Engineering (General)
- Environmental Science, Engineering, and Technology
- History of Engineering and Technology
- Mechanical Engineering and Materials
- Technology of Industrial Chemistry
- Transport Technology and Trades
- Browse content in Environmental Science
- Applied Ecology (Environmental Science)
- Conservation of the Environment (Environmental Science)
- Environmental Sustainability
- Environmentalist Thought and Ideology (Environmental Science)
- Management of Land and Natural Resources (Environmental Science)
- Natural Disasters (Environmental Science)
- Nuclear Issues (Environmental Science)
- Pollution and Threats to the Environment (Environmental Science)
- Social Impact of Environmental Issues (Environmental Science)
- History of Science and Technology
- Browse content in Materials Science
- Ceramics and Glasses
- Composite Materials
- Metals, Alloying, and Corrosion
- Nanotechnology
- Browse content in Mathematics
- Applied Mathematics
- Biomathematics and Statistics
- History of Mathematics
- Mathematical Education
- Mathematical Finance
- Mathematical Analysis
- Numerical and Computational Mathematics
- Probability and Statistics
- Pure Mathematics
- Browse content in Neuroscience
- Cognition and Behavioural Neuroscience
- Development of the Nervous System
- Disorders of the Nervous System
- History of Neuroscience
- Invertebrate Neurobiology
- Molecular and Cellular Systems
- Neuroendocrinology and Autonomic Nervous System
- Neuroscientific Techniques
- Sensory and Motor Systems
- Browse content in Physics
- Astronomy and Astrophysics
- Atomic, Molecular, and Optical Physics
- Biological and Medical Physics
- Classical Mechanics
- Computational Physics
- Condensed Matter Physics
- Electromagnetism, Optics, and Acoustics
- History of Physics
- Mathematical and Statistical Physics
- Measurement Science
- Nuclear Physics
- Particles and Fields
- Plasma Physics
- Quantum Physics
- Relativity and Gravitation
- Semiconductor and Mesoscopic Physics
- Browse content in Psychology
- Affective Sciences
- Clinical Psychology
- Cognitive Neuroscience
- Cognitive Psychology
- Criminal and Forensic Psychology
- Developmental Psychology
- Educational Psychology
- Evolutionary Psychology
- Health Psychology
- History and Systems in Psychology
- Music Psychology
- Neuropsychology
- Organizational Psychology
- Psychological Assessment and Testing
- Psychology of Human-Technology Interaction
- Psychology Professional Development and Training
- Research Methods in Psychology
- Social Psychology
- Browse content in Social Sciences
- Browse content in Anthropology
- Anthropology of Religion
- Human Evolution
- Medical Anthropology
- Physical Anthropology
- Regional Anthropology
- Social and Cultural Anthropology
- Theory and Practice of Anthropology
- Browse content in Business and Management
- Business Strategy
- Business History
- Business Ethics
- Business and Government
- Business and Technology
- Business and the Environment
- Comparative Management
- Corporate Governance
- Corporate Social Responsibility
- Entrepreneurship
- Health Management
- Human Resource Management
- Industrial and Employment Relations
- Industry Studies
- Information and Communication Technologies
- International Business
- Knowledge Management
- Management and Management Techniques
- Operations Management
- Organizational Theory and Behaviour
- Pensions and Pension Management
- Public and Nonprofit Management
- Social Issues in Business and Management
- Strategic Management
- Supply Chain Management
- Browse content in Criminology and Criminal Justice
- Criminal Justice
- Criminology
- Forms of Crime
- International and Comparative Criminology
- Youth Violence and Juvenile Justice
- Development Studies
- Browse content in Economics
- Agricultural, Environmental, and Natural Resource Economics
- Asian Economics
- Behavioural Finance
- Behavioural Economics and Neuroeconomics
- Econometrics and Mathematical Economics
- Economic Systems
- Economic Methodology
- Economic History
- Economic Development and Growth
- Financial Markets
- Financial Institutions and Services
- General Economics and Teaching
- Health, Education, and Welfare
- History of Economic Thought
- International Economics
- Labour and Demographic Economics
- Law and Economics
- Macroeconomics and Monetary Economics
- Microeconomics
- Public Economics
- Urban, Rural, and Regional Economics
- Welfare Economics
- Browse content in Education
- Adult Education and Continuous Learning
- Care and Counselling of Students
- Early Childhood and Elementary Education
- Educational Equipment and Technology
- Educational Strategies and Policy
- Higher and Further Education
- Organization and Management of Education
- Philosophy and Theory of Education
- Schools Studies
- Secondary Education
- Teaching of a Specific Subject
- Teaching of Specific Groups and Special Educational Needs
- Teaching Skills and Techniques
- Browse content in Environment
- Applied Ecology (Social Science)
- Climate Change
- Conservation of the Environment (Social Science)
- Environmentalist Thought and Ideology (Social Science)
- Management of Land and Natural Resources (Social Science)
- Natural Disasters (Environment)
- Pollution and Threats to the Environment (Social Science)
- Social Impact of Environmental Issues (Social Science)
- Sustainability
- Browse content in Human Geography
- Cultural Geography
- Economic Geography
- Political Geography
- Browse content in Interdisciplinary Studies
- Communication Studies
- Museums, Libraries, and Information Sciences
- Browse content in Politics
- African Politics
- Asian Politics
- Chinese Politics
- Comparative Politics
- Conflict Politics
- Elections and Electoral Studies
- Environmental Politics
- Ethnic Politics
- European Union
- Foreign Policy
- Gender and Politics
- Human Rights and Politics
- Indian Politics
- International Relations
- International Organization (Politics)
- Irish Politics
- Latin American Politics
- Middle Eastern Politics
- Political Methodology
- Political Communication
- Political Philosophy
- Political Sociology
- Political Theory
- Political Behaviour
- Political Economy
- Political Institutions
- Politics and Law
- Politics of Development
- Public Administration
- Public Policy
- Qualitative Political Methodology
- Quantitative Political Methodology
- Regional Political Studies
- Russian Politics
- Security Studies
- State and Local Government
- UK Politics
- US Politics
- Browse content in Regional and Area Studies
- African Studies
- Asian Studies
- East Asian Studies
- Japanese Studies
- Latin American Studies
- Middle Eastern Studies
- Native American Studies
- Scottish Studies
- Browse content in Research and Information
- Research Methods
- Browse content in Social Work
- Addictions and Substance Misuse
- Adoption and Fostering
- Care of the Elderly
- Child and Adolescent Social Work
- Couple and Family Social Work
- Direct Practice and Clinical Social Work
- Emergency Services
- Human Behaviour and the Social Environment
- International and Global Issues in Social Work
- Mental and Behavioural Health
- Social Justice and Human Rights
- Social Policy and Advocacy
- Social Work and Crime and Justice
- Social Work Macro Practice
- Social Work Practice Settings
- Social Work Research and Evidence-based Practice
- Welfare and Benefit Systems
- Browse content in Sociology
- Childhood Studies
- Community Development
- Comparative and Historical Sociology
- Disability Studies
- Economic Sociology
- Gender and Sexuality
- Gerontology and Ageing
- Health, Illness, and Medicine
- Marriage and the Family
- Migration Studies
- Occupations, Professions, and Work
- Organizations
- Population and Demography
- Race and Ethnicity
- Social Theory
- Social Movements and Social Change
- Social Research and Statistics
- Social Stratification, Inequality, and Mobility
- Sociology of Religion
- Sociology of Education
- Sport and Leisure
- Urban and Rural Studies
- Browse content in Warfare and Defence
- Defence Strategy, Planning, and Research
- Land Forces and Warfare
- Military Administration
- Military Life and Institutions
- Naval Forces and Warfare
- Other Warfare and Defence Issues
- Peace Studies and Conflict Resolution
- Weapons and Equipment

A newer edition of this book is available.
- < Previous chapter
- Next chapter >

7.5 Knowledge transfer
- Published: February 2013
- Cite Icon Cite
- Permissions Icon Permissions
Effective research transfer will ensure patients and populations benefit from evidence-based best practice. While there is an increasing rigor with which to approach research transfer in health care settings, greater demand among those responsible for research transfer for a more scientifically sound knowledge base will accelerate development of the discipline. There is greater recognition that research transfer requires sophisticated, theoretically informed and phased designs. Practitioners who seek to transfer evidence into practice must work with these epistemological deficits as best they can. Reading this chapter will help you to identify and respond to situations that require research transfer, apply a systematic approach to research transfer, learning from the work of others and planning locally in context, and contribute to a growing body of evidence about research transfer itself.
Personal account
- Sign in with email/username & password
- Get email alerts
- Save searches
- Purchase content
- Activate your purchase/trial code
- Add your ORCID iD
Institutional access
Sign in with a library card.
- Sign in with username/password
- Recommend to your librarian
- Institutional account management
- Get help with access
Access to content on Oxford Academic is often provided through institutional subscriptions and purchases. If you are a member of an institution with an active account, you may be able to access content in one of the following ways:
IP based access
Typically, access is provided across an institutional network to a range of IP addresses. This authentication occurs automatically, and it is not possible to sign out of an IP authenticated account.
Choose this option to get remote access when outside your institution. Shibboleth/Open Athens technology is used to provide single sign-on between your institution’s website and Oxford Academic.
- Click Sign in through your institution.
- Select your institution from the list provided, which will take you to your institution's website to sign in.
- When on the institution site, please use the credentials provided by your institution. Do not use an Oxford Academic personal account.
- Following successful sign in, you will be returned to Oxford Academic.
If your institution is not listed or you cannot sign in to your institution’s website, please contact your librarian or administrator.
Enter your library card number to sign in. If you cannot sign in, please contact your librarian.
Society Members
Society member access to a journal is achieved in one of the following ways:
Sign in through society site
Many societies offer single sign-on between the society website and Oxford Academic. If you see ‘Sign in through society site’ in the sign in pane within a journal:
- Click Sign in through society site.
- When on the society site, please use the credentials provided by that society. Do not use an Oxford Academic personal account.
If you do not have a society account or have forgotten your username or password, please contact your society.
Sign in using a personal account
Some societies use Oxford Academic personal accounts to provide access to their members. See below.
A personal account can be used to get email alerts, save searches, purchase content, and activate subscriptions.
Some societies use Oxford Academic personal accounts to provide access to their members.
Viewing your signed in accounts
Click the account icon in the top right to:
- View your signed in personal account and access account management features.
- View the institutional accounts that are providing access.
Signed in but can't access content
Oxford Academic is home to a wide variety of products. The institutional subscription may not cover the content that you are trying to access. If you believe you should have access to that content, please contact your librarian.
For librarians and administrators, your personal account also provides access to institutional account management. Here you will find options to view and activate subscriptions, manage institutional settings and access options, access usage statistics, and more.
Our books are available by subscription or purchase to libraries and institutions.
| Month: | Total Views: |
|---|---|
| October 2022 | 3 |
| November 2022 | 1 |
| December 2022 | 3 |
| January 2023 | 2 |
| February 2023 | 4 |
| March 2023 | 4 |
| April 2023 | 1 |
| May 2023 | 1 |
| June 2023 | 2 |
| July 2023 | 2 |
| August 2023 | 2 |
| September 2023 | 2 |
| October 2023 | 2 |
| November 2023 | 2 |
| December 2023 | 2 |
| January 2024 | 1 |
| February 2024 | 1 |
| March 2024 | 2 |
| April 2024 | 1 |
| May 2024 | 7 |
| June 2024 | 4 |
| July 2024 | 3 |
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Rights and permissions
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Knowledge transfer between physicians from different geographical regions in China’s online health communities
- Published: 19 May 2023
Cite this article

- Zihao Deng 1 ,
- Zhaohua Deng ORCID: orcid.org/0000-0002-7744-7818 1 ,
- Shan Liu 2 &
- Richard Evans 3
2141 Accesses
7 Citations
Explore all metrics
Online Health Communities (OHCs) are a type of self-organizing platform that provide users with access to social support, information, and knowledge transfer opportunities. The medical expertise of registered physicians in OHCs plays a crucial role in maintaining the quality of online medical services. However, few studies have examined the effectiveness of OHCs in transferring knowledge between physicians and most do not distinguish between the explicit and tacit knowledge transferred between physicians. This study aims to demonstrate the cross-regional transfer characteristics of medical knowledge, especially tacit and explicit knowledge. Based on data collected from 4716 registered physicians on Lilac Garden (DXY.cn), a leading Chinese OHC, Exponential Random Graph Models are used to (1) examine the overall network and two subnets of tacit and explicit knowledge (i.e., clinical skills and medical information), and (2) identify patterns in the knowledge transferred between physicians, based on regional variations. Analysis of the network shows that physicians located in economically developed regions or regions with sufficient workforces are more likely to transfer medical knowledge to those from poorer regions. Analysis of the subnets demonstrate that only Gross Domestic Product (GDP) flows are supported in the clinical skill network since discussions around tacit knowledge are a direct manifestation of physicians’ professional abilities. These findings extend current understanding about social value creation in OHCs by examining the medical knowledge flows generated by physicians between regions with different health resources. Moreover, this study demonstrates the cross-regional transfer characteristics of explicit and tacit knowledge to complement the literature on the effectiveness of OHCs to transfer different types of knowledge.
Similar content being viewed by others
Knowledge structure and theme trends analysis on general practitioner research: a co-word perspective.

Knowledge Networks in Nursing

Faculty members as academic knowledge brokers in Iran's health sector: a social network analysis study
Explore related subjects.
- Artificial Intelligence
Avoid common mistakes on your manuscript.
1 Introduction
Advancements in online collaboration tools have given rise to online communities across various sectors. Among these, online health communities have emerged as self-organizing platforms where diverse users (e.g., patients, physicians, and healthcare workers) can freely create, share, disseminate, and exchange information beyond the constraints of time and space [ 22 ]. Patients use OHCs to communicate with others, share information, and extend emotional support, while physicians use them to exchange knowledge and ideas related to treatment methods and diagnostic experiences. Notably, developments in OHCs have helped address the imbalance in hygiene procedures to a certain extent [ 67 , 70 ], which is considered a social value resulting from the creation of OHCs [ 19 ].
OHCs provide value to users through knowledge transfer opportunities [ 11 , 72 ]. Like Communities of Practice (CoP), OHCs allow for collaboration and knowledge exchange between physicians, transcending offline boundaries [ 34 ]. Physician-physician collaboration is a key feature of OHCs, which aims to address patients’ needs, reducing their uncertainty and anxiety by combining the experiences and knowledge of physicians from around the world [ 66 ]. Within OHCs, physicians learn from each other by observing their behaviors, asking questions, and contributing to discussions [ 47 ]. Additionally, they share their medical routines [ 65 ], which helps to transfer and enrich health knowledge and improve the quality of healthcare services delivered to patients [ 52 ].
Physicians are a special group of knowledge workers in the healthcare industry with a narrow scope of work who possess the ability to use knowledge to solve practical problems [ 14 ]. As such, the knowledge gap in this group reflects the gap in physicians’ professional competence. First, the transfer of knowledge between physicians is crucial in improving the quality of online healthcare services [ 53 ]. Second, in the face of emerging diseases, such as COVID-19, knowledge transfer between physicians is essential for diagnosis and the identification of treatment options [ 63 ]. Third, the cross-regional flow of medical knowledge offers the potential to bridge the knowledge gap among physicians working in different regions and alleviate geographical disparities. These findings underscore the importance of physicians’ knowledge transfer in the medical profession.
Extant research has mainly investigated the antecedents and consequences of knowledge transfer among physicians in online and offline contexts [ 53 , 60 ]. Within the online transfer model, technologies that provide collaboration between remote users has received the greatest attention [ 68 ]. OHCs represent a novel information exchange platform that allow physicians to seek and share knowledge, surpassing the limitations of telemedicine. As such, they have become an important platform for physicians to exchange and transfer experiences and medical knowledge, while alleviating the constraints of time and space. Despite the increasing prevalence of OHCs, few studies have empirically examined their role in transferring medical knowledge among physicians. We, therefore, ask our first research question: Can OHCs support the transfer of medical knowledge between physicians across different regions in China?
In healthcare, knowledge can be classified as explicit or tacit, both of which play important, but distinct roles [ 49 ]. Explicit knowledge, represented by clinical guidelines, is easily accessible through the Internet, while tacit knowledge, represented by personal experiences, is more challenging to transfer but offers greater value [ 22 , 58 ]. In the healthcare context, physicians can often recall knowledge about diagnosis and treatment from their previous experiences and rely on pre-existing experiences to consider them comprehensively and systematically [ 25 ]. Newly qualified physicians entering the workforce may lack experience compared to clinical guidelines while traditional medical settings offer limited access to fellow physicians for gaining such clinical experience. OHCs help in this regard by visualizing and recording tacit medical knowledge, enabling the large-scale transfer of explicit and tacit knowledge [ 74 ].
As mentioned earlier, knowledge transfer is critical for physicians. This study, therefore, considers the transfer of tacit knowledge an important part of achieving knowledge transfer in the healthcare industry. First, the quality of medical services delivered by physicians and other healthcare workers largely depends on the physicians’ tacit knowledge. Second, tacit knowledge is considered an excellent source of innovation [ 18 ]. Third, offline physicians have limited access to tacit knowledge compared to explicit knowledge and, therefore, OHCs offer the possibility of making tacit knowledge explicit, thus enhancing the exchange of tacit knowledge across regions to balance the differences in physicians’ competencies. While the first research question may only detect the overall knowledge flow between regions in OHCs, its usefulness for transferring specific types of knowledge has not been tested [ 8 , 10 , 44 ]. This study, therefore, poses the second research question: What role do OHCs play in tacit and explicit knowledge transfer between physicians from different regions?
To answer these research questions, we categorize the different types of knowledge transferred by physicians in OHCs based on their geographical regions. Data was collected from 4,716 registered physicians in the Cardiology community on Lilac Garden ( www.dxy.cn/bbs/ ), a leading OHC in China used by physicians and other healthcare professionals. The data collected included physicians’ interactions (i.e., posts and replies), department(s), geographical location, and other community characteristics. First, concerning the categorization of geographical regions, prior studies show that health outcomes are strongly linked to human resources and the high density and quality of physicians have a positive effect on outcomes [ 51 , 56 , 57 , 59 ]. The quality of physicians in a region is related to the economic level of the region [ 35 ] and, therefore, two indicators are used to divide the regions where physicians are located, namely, the healthcare workforce and GDP. Second, we examine the content of physicians’ posts to identify whether the knowledge conveyed is explicit or tacit. Specifically, physicians discuss cases in detail and express their diagnostic and treatment opinions in OHCs, and this information about their practice experience is considered tacit knowledge, while the rest of the objective information shared, such as textbooks, pictures, videos, and clinical guidelines, is considered explicit knowledge.
This study models and analyzes the OHC social networks using exponential random graph models with findings revealing a net surplus of total knowledge from regions with sufficient workforces to insufficient ones and developed to less developed regions. To identify tacit and explicit knowledge flows in the network, we focused on two subnets: clinical skills and medical information. Analysis of the two subnets demonstrates that medical information is the same across the overall network while only developed to less developed flows are supported in the clinical skills network; in other words, tacit knowledge is only transferred between regions from high GDP to low, while explicit knowledge can be transferred between regions from high GDP and sufficient resources to low and insufficient. These findings demonstrate that tacit knowledge is more difficult to transfer than explicit knowledge. With regards the clinical skills network, the process of physicians colliding with each other can be reflected. Physicians who have interacted previously will continue to cooperate using a follow-up process, while the general medical information network is mainly used for temporary one-sided help; this finding suggests that tacit knowledge requires richer media for successful knowledge transfer.
This study provides three key contributions to current understanding. First, it contributes to the existing literature on knowledge transfer between physicians in OHCs and demonstrates, through empirical analysis, that medical knowledge flows from superior regions to less superior regions in online platforms. Second, this study shows the interaction network between physicians in OHCs, especially regarding the exchange of tacit and explicit knowledge, and discusses the characteristics of two types of knowledge transfer networks separately to enrich the literature on physicians’ online interactions. Third, it examines the effectiveness of OHCs in transferring tacit and explicit knowledge between regions with different human resources, and the features of two different types of medical knowledge in cross-regional transfer to complement the literature on the effectiveness of OHCs in transferring different knowledge.
2 Literature review
2.1 definitions of medical knowledge types.
Nonaka [ 45 ] argued that knowledge can be categorized into two types: explicit and tacit. Explicit knowledge is a kind of objective knowledge in formal and systematic language, usually shared in the form of raw data, formulas, specifications, and manuals [ 28 ], while tacit knowledge is the result of an individuals’ experiences, senses or intuition, and it is highly dependent on context [ 9 ]. The classification of explicit and tacit knowledge is based mainly on its economic value and ease of delivery [ 33 ]. Generally, tacit knowledge is that which is not written down or coded, often obtained through deep processing of information. Therefore, it has greater value and is particularly difficult to transfer [ 64 ].
Medical knowledge, like most other fields of knowledge, can be divided into two types: tacit and explicit [ 49 , 69 ]. Explicit medical knowledge is written or codified as “fact,” “scientific evidence,” and other official documents generated by research and policy, such as clinical guidelines [ 58 ]. In contrast, tacit knowledge is generally less concrete, deeply rooted in practice, and is comprised of skills, ideas, and experience. This knowledge is more difficult to transfer than explicit knowledge [ 16 ]. Previous studies suggest that both explicit and tacit knowledge are important [ 49 ], however personal experiences appear to play a greater role in healthcare settings where the expertise of healthcare professionals is usually dominated by practice experience [ 22 , 58 ]. Subsequently, tacit knowledge should receive more attention.
Existing research on explicit and tacit medical knowledge has mainly focused on distinguishing between explicit and tacit knowledge in healthcare settings [ 12 , 17 , 61 ], and on exploring the role played by these two types of knowledge [ 18 , 24 , 31 , 58 ]. Such studies highlight the importance of tacit knowledge in delivering traditional medical care, but it is unclear whether tacit knowledge can still be useful in online contexts, as it is difficult to materialize. Therefore, it is necessary to explore the explicit and tacit knowledge generated in online medical environments, such as in OHCs.
2.2 Medical knowledge transfer in OHCs
Knowledge transfer is the process of sharing or disseminating knowledge between two or more parties through a medium [ 39 ]. Extant research classifies this knowledge transfer into explicit and tacit knowledge transfer and validates it on a variety of information system media [ 36 ]. Nonaka and Takeuchi [ 46 ] explained how explicit and tacit knowledge can be transferred from experts to novices in groups and organizations, which has been widely explored in online CoPs [ 4 , 40 ]. Online communities are viewed as virtual platforms where knowledge transfer takes place. Faraj et al. [ 15 ] distinguished the flows of tacit and explicit knowledge in online communities and suggested that it can give rise to tacit knowledge transfer between participants. Previous studies demonstrate that online communities can effectively enhance and support the different phases of the knowledge transfer model [ 5 ].
As healthcare is a knowledge-intensive industry, it is crucial to establish a knowledge-exchange platform through which healthcare professionals can share, acquire, and use medical knowledge [ 2 ]. The emergence of OHCs has broken the limits of time and space, providing the possibility for knowledge transfer between remote physicians. In OHCs, the transfer of tacit knowledge is often in the form of discussions among physicians, which is aimed at exchanging ideas and experiences that are essential to one’s ability to practice [ 64 ], while the transfer of explicit medical knowledge is often reflected in the sharing of clinical guidelines, relevant literature, and other materials by physicians, which are usually objectively accessible knowledge. The development of such knowledge platforms has, to some extent, enhanced the transfer of medical knowledge, especially tacit knowledge, among physicians [ 13 , 47 , 62 ].
Existing studies have explored the antecedents and consequences of knowledge transfer. Antecedents can be divided into individual levels, knowledge levels, and organizational levels [ 23 , 42 ]. Among them, the importance of individual-level factors has attracted greatest attention, including intrinsic and extrinsic factors [ 73 ], such as attitude towards innovation [ 71 ], experience [ 29 ], and social capital [ 74 ], etc. Research on consequences has predominantly focused on innovative behavior [ 18 ] and service quality [ 53 ], etc., while few studies have discussed the impact of knowledge transfer from a cross-regional perspective. Goh et al. [ 19 ] found that knowledge flows in OHCs can reduce health gaps by developing the health capability of rural patients. Similarly, Cao and Wang [ 7 ] applied OHCs to urban–rural health inequality to empirically examine whether knowledge transfer in OHCs reduces health disparities between different regions in mainland China and drew the same conclusion.
However, to date, a lack of empirical research exists on knowledge transfer between physicians, especially since physicians’ expertise plays a crucial role in healthcare delivery [ 47 ]. Furthermore, although the role of certain information technologies in facilitating the transfer of explicit and tacit knowledge has been explored [ 6 , 62 ], few empirical studies have examined the process of cross-regional transfer of the two types of knowledge with the effectiveness of OHCs between different healthcare resource areas. Therefore, this study aims to explore the process of knowledge transfer between physicians in OHCs and identify whether OHCs can achieve an effective transfer of both explicit and tacit knowledge between different regions.
3 Methodology
3.1 data collection.
Data were collected from the original posts and replies to posts submitted to the Cardiology community on Lilac Garden from January 2017 to May 2020. In total, data were obtained from 4716 registered physicians. Lilac Garden is one of the largest professional OHCs in the world and the largest online community for physicians in China. The platform aims to provide a professional online community for medical and healthcare practitioners. In 2018, the platform had more than 2 million registered physicians. Many diseases discussed in the department of Cardiology are chronic diseases. The reason for choosing chronic diseases is that patients with this type of disease generally require long-term treatment and physicians must, therefore, have a strong understanding of patients’ diagnosis and treatment plans at each stage, which has higher requirements for their professional ability. The data collected were divided into two parts for analysis. The first part contained users’ posts and replies in the Cardiology community, representing physicians’ interactions. The second part comprised the personal information of the physicians’ departments and community characteristics from users’ profiles.
A Python-based program was used to download data and classify them by province. Based on the China Statistical Yearbook [ 43 ], published in 2020, the 31 provinces in mainland China were divided into two parts on a ratio of 3 to 1 based on the 75% rule [ 50 ]. One part included a higher number of practicing physicians per 1000 population and another with a lower number. In this study, we describe the two parts as sufficient for the first 75 percent and insufficient for the remaining 25 percent. Before analysis, all invalid data was removed, such as special community managers and physicians whose geographic location was outside of the 31 provinces (e.g., Taiwan and Hong Kong). We finally obtained data from 3997 physicians from sufficient regions and 719 from insufficient regions. Table 1 shows the distribution of data by province. Furthermore, prior research demonstrates that physicians from regions with a higher GDP generally have better medical expertise than those from regions with a lower GDP [ 37 ], which means that a physician from a developed region is likely to be more professional. Therefore, we examined the effect of GDP on physicians’ knowledge transfer in OHCs, which is the same as health technical personnel based on gross regional product per capita [ 43 ]. The two parts were identified as developed regions and less developed regions (see Table 1 , column 3), with 4021 and 695 physicians, respectively. In summary, this study aims to examine the characteristics of knowledge transfer between different regions under two division methods.
3.2 Research methods
A directed unweighted social network was developed using the interactive relationship between posters and repliers. According to existing research [ 7 , 19 ], posts can be divided into two types: information sharing and help seeking. For information sharing, the posters are regarded as suppliers, while repliers act as recipients, which means a flow from A to B if A replies to B’s post at least once. Conversely, for help seeking, posters ask questions and wait for respondents to answer, with the flow being reversed [ 7 ]. The dxy forum divides its posts into different categories, taking cardiovascular as an example, such as academic frontiers, case studies, Electrocardiography (ECG), etc. Based on the platform criteria, we obtained the post and reply information separately. We consider image diagnosis, case discussion, and clinical experience as clinical skills, while the academic frontiers section is classified separately, which is the reflection of the tacit and explicit knowledge transfer in the OHC. With regards clinical skills, physicians express their views on a case and provide their diagnoses and suggested treatments for others to use as reference. In this scenario, intense discussion occurs where each physician contributes their tacit knowledge related to their treatment experience; this includes the sharing of typical cases or questions about a difficult-to-treat disease. In the medical information part, most physicians ask for help or share some textbooks, images or videos, medical knowledge, cutting-edge research results, etc. Medical information offers a summary of existing literature, which is explicit knowledge and easier to obtain. However, clinical skills are considered physicians’ personal experiences and directly manifest physicians’ professional abilities, which belong to tacit knowledge, and are more difficult to transfer. Figures 1 and 2 provide two examples of these scenarios. The division of the sample by post type revealed that 2393 physicians were involved in tacit knowledge transfer and 736 physicians were involved in explicit knowledge transfer.

Transfer of Clinical skills

Transfer of Medical information
Finally, an overall network and two subnets were created. Each node in the network represents a physician, while the arrows represent an information flow via a directed dyadic tie. Prior studies have proven that a binary graph does not influence the interpretation of the information flow because it focuses on the region effect [ 1 ]. The summary statistics for various indicators of centrality for information support networks are provided in Table 2 .
3.3 Exponential random graph models
Exponential random graph models were used to analyze the created network. ERGMs are a stochastic network modeling method based on exponential-family theory for analyzing the probability distribution of a set of networks [ 55 ]. This study aimed to explain how and why connections occur in the network; its explained variable is the probability of a network appearing. In fact, any network is a special case of the possible concentration of all the networks formed by the network nodes. This method seeks to analyze the special structural effects commonly observed in social networks, including reciprocity, transitivity, homophily, and attribute-based activity [ 54 ]. In this study, the mechanisms of reciprocity and individual-level attributes are tested interdependent of each other, where ERGMs are considered an appropriate approach for estimating how those mechanisms predict a network’s ties formation [ 20 ]. The model is specified as follows:
where \(z\left(y\right)\) represents a set of network structure statistics that may influence the formation of relationships and organization in the network, \({z}_{\alpha }\left(y,x\right)\) is some network statistics about node attributes, \({z}_{\beta }(y,g)\) is a series of statistics related to other external relationship networks, and accordingly, \(\theta , {\theta }_{\alpha }, {\theta }_{\beta }\) are the estimated parameter vectors of the corresponding network statistics. If these parameter estimates pass the significance test, then it indicates that the structure has an important influence on the formation of network relationships and organization construction. The positive estimated value of this parameter indicates that the structure in the network is higher than the randomly expected value when other conditions are controlled. Finally, \(k(\theta )\) is a normalizing constant that ensures that the sum of probability equals one.
ERGMs were used for two main reasons. First, they allow dependent ties in the network, while most traditional network modeling approaches assume that network ties are independent [ 38 ]. This relaxed assumption is closer to the true network. Second, ERGMs use a bottom-up approach to model social networks, which is in line with the self-organizing nature of OHCs. The most common example of self-organizing network property is reciprocity [ 26 ], which is the structure of interest in this study. Robins et al. [ 55 ] found that ERGMs tend to produce more conservative estimates results than regressions.
3.4 Measures
This study examines the variables of network self-organization and actor attributes. Actor attributes include department , reputation reward (i.e., number of followers and likes), and online rating . Several variables were also incorporated to control other factors that may influence the knowledge flows between regions (i.e., the number of posts and community points, which reflects users’ activity). The definitions and interpretation of the variables are discussed below. Table 3 shows the descriptive statistics. Table 4 provides the configurations and network statistic definitions. The statnet package in R was used to estimate the built ERGM [ 21 ].
3.4.1 Network self-organization and actor attributes
The characteristics of individuals in the network is paramount to the formation of network connections [ 30 , 48 ]. In considering OHCs, attributes of the community level are critical. In this study, reputation reward (i.e., number of followers and likes) was included to reflect how popular someone is in the OHC and online rating was used to reflect status capital. Physicians’ department was added to reflect the variability of expertise among physicians in the OHC. Moreover, activity (i.e., post and point) was included for control purposes. In processing these attributes, we controlled for homophily, which suggests that two nodes with the same property form a connection.
3.4.2 Location measures
This study focused on the impact of geographical location (i.e., sufficient and insufficient, developed and less developed) on the features of knowledge networks, with the direct connections between the two kinds of regions being tested. Taking health technical personnel, as an example, they are divided into four types: sufficient→sufficient, sufficient→insufficient, insufficient→sufficient, and insufficient→insufficient. To avoid multicollinearity [ 19 ], sufficient→insufficient, and insufficient→sufficient, were respectively considered as a base group in the two models. On this basis, we calculated the value of the remaining three variables. The GDP group is approached in the same way.
4.1 ERGM model results
The results of the overall network are shown in Table 5 . Model 1 is a baseline model with two terms of edges and mutual. The first term indicates a baseline tendency of a node forming a tie with another, while mutual is about the reciprocity of two nodes in the network. A negative effect for edge suggests that the network density is lower than others that accidentally occur [ 75 ], while a positive coefficient for mutual implies that the relations for reciprocity of two nodes are more likely to appear in this network.
The other models include node attributes, location variables, and other control variables. First, with regard to location variables, Models 3 and 4 are used to analyze the directed knowledge flow between sufficient and insufficient regions. To better illustrate the possibility of the occurrence among different knowledge flows, we set sufficient → insufficient as a control group in Model 3, and insufficient → sufficient in Model 4. Our results show that insufficient → sufficient is negative in Model 3, while sufficient → insufficient is positive in Model 4, and they are all significant. This finding suggests that the sufficient → insufficient tie has a greater probability of existence, while insufficient→sufficient has a lower probability. By combining the results of the two models, the likelihood of physicians from sufficient regions replying to physicians from insufficient regions is higher than the likelihood of physicians from insufficient regions replying to ones from sufficient regions. As for Models 5 and 6, they examine the knowledge flow among developed and less developed regions. The negative and significant coefficient for less developed → developed implies that the flow from less developed regions to developed ones is less likely to occur in the network than from developed to less developed regions. A positive and significant value of developed → less developed shows that, in comparison with the flow from less developed to developed regions, the possibility of this occurring in the community network is higher. Existing studies prove the role of online health platforms in knowledge transfer from the patient perspective only [ 7 , 19 ], and the results of this study validate it again from the physician perspective.
Secondly, node attributes were examined with results remaining consistent across all models, which shows that apart from the number of followers, other variables are all significant.
Reputation reward There is no evidence that the number of followers of physicians influences the tendency for ties to form. However, a positive and significant coefficient for Absdiff (like) indicates that the greater the difference in the numbers of likes in OHCs between the nodes, the higher the possibility for a tie between them. Like can be regarded as the online reputation of physicians in OHCs, which represents how well they are recognized by others. Someone who receives more likes will possibly act as a knowledge supplier to maintain their reputation [ 41 , 69 ]; therefore, knowledge transferred between physicians possessing varying amounts of likes is more likely.
Physicians’ professional departments The coefficient is positive and significant, which suggests that physicians in the same department are more likely to form ties (n.b., the same department refers to similar diseases, work, diagnosis, and treatment experience). Due to the higher clinical professionalism of physicians in the same department, more interactions occur [ 27 ].
Status capital The negative coefficient of Nodematch (level) suggests that physicians with different online ratings are connected in OHCs. This finding is consistent with other studies on social groups and online communities where tenure is often shown to play an important part in predicting individual contribution behavior [ 3 , 32 ]. Goh et al. [ 19 ], however, found that small differences in tenure, such as online ratings, created more supportive ties between patients. The reason for this may be that physicians belong to a highly specialized group, compared to patients. A physician with a higher rating has greater credibility and some young doctors turn to their senior counterparts for advice due to their limited professional experience, while the communication between physicians at the same level is relatively small.
Lastly, from the results of the control variables, we were able to draw an interesting finding. The nodes with the greater difference in the number of posts are more likely to generate connections, while the smaller the difference in the points, the easier it is to form a tie; this shows that users with more posts generally act as senders in the community and tend to pass on knowledge, while users with fewer posts tend to be recipients and absorb more knowledge from others. Point is another measure of a user’s activity in the community. In general, the more points, the more active the user is in the community, and most of the knowledge transfer and reception in the community occur between active users, not the lurkers.
Table 6 shows the results of the knowledge subnets. The two types of knowledge results are described as follows. First, concerning location variables, the results of the clinical skills net show that the knowledge flow from developed to less developed regions is more likely to appear, compared to less developed→developed regions. However, the flow of sufficient→insufficient is not supported, while the knowledge flow of medical information net is supported in both classification methods. One possible reason for this is that physicians often work on cases that are described by someone and they combine this with their tacit knowledge (i.e., clinical experience) to provide a diagnosis and treatment plan in the clinical skill network. To transfer tacit knowledge in this network, physicians’ professional ability must be strong, and, for medical information, physicians’ explicit knowledge integration ability is more tested. Therefore, the regions’ variable GDP, representing the quality of physicians, plays a significant role, while the variable, healthcare workforces, which represents the quantity of physicians, is not supported in the clinical skills net. In the medical information net, the professionalism of physicians in the process of explicit knowledge transfer is relatively low, so it is also supported in the type of regional divisions by the quantity of physicians.
Secondly, the results of node attribute of the two subnets with the overall net were compared. Our findings are as follows.
Reputation reward The effect of Absdiff (like) in the two subnets is the same as the overall net. However, Absdiff (follower) is positive and significant in the medical information net. Similar to likes , the variable of followers is a reflection of physicians’ reputation in OHCs and its role is supported in the subnet of medical information.
Physicians’ professional departments In the subnets, knowledge transfer is more likely to occur between physicians in the same department, which is consistent with the overall network finding.
Status capital About the knowledge flows at different levels, the results of the clinical skill net are the same as the overall net. However, the medical information net is the opposite. A possible reason for this is that the online rating of the community also reflects, to some extent, the social status of physicians. In general, physicians with high community rankings are required to share their knowledge with others. Physicians who communicate clinical skills are often cross-hierarchical, as the disease discussion is often extremely specialized, with some high-level physicians generally providing tacit knowledge to other physicians. Conversely, medical knowledge is considered explicit knowledge (i.e., disease guidelines, literature, etc.) and the transfer of this knowledge usually occurs between low-level physicians.
Interestingly, we find that reciprocal relationships do not occur in the medical information network, whose coefficient is negative infinity. There are, however, differences between medical information and clinical skills. The former is more explicit, while the latter is more tacit, which requires more rich media exchange. Therefore, after an interaction occurs, the two physicians who exchange clinical skills usually form an invisible connection, which encourages them to continue communication and help each other when encountering similar problems in the future. General medical information acts as temporary support, only provided to physicians who need the knowledge urgently, and usually without further communication.
4.2 Robustness check
First, we tested the robustness of the results by randomly reducing the samples. The variables were retested by randomly reducing the number of physicians, 50 samples at a time. Moreover, to identify if the findings were sensitive to special network structures [ 19 ], such as physician community status, we performed ERGM by eliminating users who had the top likes (the top 10, 15, and 20). Third, to avoid the influence of two types of threads called an exogenous contextual factor, we tested these posts separately. Fourth, we found that physicians in Zhejiang province accounted for a large proportion of the network, so we replicated our analysis after excluding physicians from Zhejiang to check whether the results were purely driven by physicians from Zhejiang [ 26 ]. Additionally, in considering the difference between the two kinds of region physician sizes, which may influence our conclusions, we readjusted the regional division method to ensure that the two types of regions have a similar number of physicians (i.e., 50.42%, 51.86%, 52.72% in sufficient regions and 60.24%, 59.84%, 62.64% in developed regions, respectively in the overall net, clinical skills, and medical information net) for further test results. The results are presented in Table 7 .
From our findings, it is suggested that physicians in insufficient and less developed regions receive a net surplus in every network built. It is, therefore, concluded that the study’s findings are not sensitive to the number of physicians and the special characteristics. However, for the type of thread, the results seem to be partly unstable.
5 Discussion and implications
5.1 discussion.
This study used ERGMs to analyze the effect of variables, including network self-organization, control variables, and node attributions, on physicians’ community network formation on OHCs. The study’s results demonstrate that physicians with different reputation rewards (e.g., number of likes), status capital (i.e., community level), and who work in the same department, are more likely to transfer knowledge in OHCs.
Moreover, we examined the total effect of the quantity and quality distribution of physicians and the results are all supportive. The number of certified physicians per thousand population was used to measure the regional differences in quantity distribution. Physicians from sufficient regions were found to be more likely to provide online support to those working in insufficient regions. Meanwhile, developed regions seem to have more professional physicians [ 37 ] and, therefore, we separate the different regions based on GDP to explore the effect of the OHCs. The study's results show that physicians from developed regions are net suppliers of online information support to others from less developed regions. RQ1 is, therefore, answered and the OHCs do support the transfer of medical information between physicians across regions in China, especially since there is a significant flow of information from developed/sufficient to less developed/insufficient regions.
We subdivided the overall network into explicit and tacit knowledge subnets to answer RQ2. The study’s results demonstrate that reciprocal relationships do not appear to exist in medical information networks, but do in clinical skills networks, suggesting that tacit knowledge transfer requires more continuous discussion among physicians and is more difficult to transfer, while explicit knowledge transfer is easier. Furthermore, our results show that connections appear between physicians with the same status capital in the medical information net, which indicates that explicit medical knowledge flows within one level and it is a cross-level flow in the tacit knowledge net. These results demonstrate that OHCs differ in transferring explicit versus tacit knowledge from a micro level and that these differences arise from the characteristics of the knowledge itself.
In the explicit network, our results are the same as the overall net. Since professional ability is demonstrated more in the transfer of tacit knowledge, physicians from developed regions are more likely to transfer tacit knowledge to others in less developed regions, and the flow from sufficient to insufficient is not proven. This result answers RQ1 at a fine-grained level while answering RQ2 for the differences in cross-regional transfer between the two types of knowledge. OHCs enable the cross-regional transfer of tacit knowledge on the GDP dimension, while the transfer of explicit knowledge exists in both the GDP and human resource dimensions. It also reveals the differences in cross-regional transfer between the two knowledge types from a macro perspective.
5.2 Theoretical implications
This study explores the effectiveness of OHCs in allowing physicians to transfer knowledge, especially tacit and explicit knowledge, and examines the knowledge flows from sufficient to insufficient and developed to less developed regions. The findings reveal the differences between tacit and explicit medical knowledge flows and, hence, this study offers several theoretical implications.
First, this study provides evidence showing which physicians transfer knowledge through OHCs and finds which scenarios this knowledge transfer primarily occurs in to extend our understanding of the social value created by physicians in OHCs. By discussing the interaction between physicians in different health resource allocation regions, overall knowledge flows are found to occur from areas of superior quantity and quality to areas of lesser superiority. OHCs are a type of digital platform where a high level of information asymmetry exists for both physicians and patients [ 70 ]. For example, highly skilled physicians from developed regions know more about medical dynamics or diagnosis and have greater treatment experience. Others can provide more suitable services based on the information found in OHCs to enhance their capability. To date, prior research has mainly focused on online patient communities to explore the direct impact of OHCs in reducing the gap through enhanced patient health capability [ 7 , 19 ]. However, the role of physicians is often neglected. This study, therefore, focuses on the effect of online physician communities in improving the problem of health resource allocation via knowledge transfer, thus reducing health service disparities among regions. Moreover, we demonstrate the social value created by OHCs.
Second, this study shows the networks of interactions between physicians in OHCs regarding explicit and tacit knowledge and discusses the characteristics of each of the two types of knowledge transfer networks to enrich the literature on physician online interactions. Although previous studies have identified the existence of explicit and tacit knowledge exchange in online CoPs, the characteristics of these two types of knowledge transfer networks have not been explored [ 8 , 10 , 44 ]. Moreover, OHCs, an important type of online community, have received less attention than other types of communities. Tacit and explicit knowledge play different roles in the medical field, with some studies suggesting that tacit medical knowledge is more important for physicians as it helps guide their practice [ 22 , 58 ]. We found that due to the difficulty in transferring medical tacit knowledge, users usually form a hidden connection during discussions with others, and this connection makes them continue to help each other in the next discussion while, explicit knowledge, due to its multiple access channels and content that has been coded and processed several times, or confirmed, does not require additional discussions to achieve transmission. Additionally, unlike explicit knowledge, tacit knowledge is usually transferred across physician levels.
Third, this study demonstrates the cross-regional transfer characteristics of explicit and tacit knowledge to complement studies on the effectiveness of OHCs to transfer different types of knowledge. We found that the transfer of explicit knowledge among physicians can occur between regions with large differences in the quantity and quality of human resources, which may be related to the ease of access to explicit knowledge. In contrast, the amount of tacit knowledge reflects the professional competence of a particular physician, so the transfer of tacit knowledge is only significant between developed and less-developed regions, which is more indicative of the quality of human resources.
5.3 Practical implications
This study provides important practical implications to improve current understanding about how tacit and explicit knowledge transfer between physicians in networks are organized in the specific context of China and reveals the connections and relationships between the nodes in such a network. The study’s results are relevant to developers and users of OHCs and should help in the future creation and deployment of OHCs.
First, the importance and value of OHCs are increasingly being appreciated by the research community. However, compared with patient-to-patient and physician–patient communities, the importance of physician-to-physician communities appears somewhat neglected. Healthcare policymakers should, therefore, pay greater attention to platforms that encourage physician communication as the benefits to physicians of online social networks have been proven. To address and improve the health inequities and narrow the gap between different regions, governments should strongly support the participation of not only patients but also physicians in OHCs; they must be introduced to them from the perspective of both physicians and patients. For example, governments can increase investment in online physician communities, while healthcare policymakers can create policies that encourage physicians to engage with them.
Second, tacit knowledge is extremely difficult to transfer as it requires continuous mutual discussion between physicians. Therefore, this study suggests that OHCs can introduce friend recommendations so that the invisible connection formed by physicians who have exchanged clinical experiences once can be visualized. This would allow them to hold further discussions when they encounter similar problems. In addition, with regards the transfer of explicit medical knowledge, OHCs can help users integrate knowledge so that physicians in need can more easily retrieve the information they require, ultimately enhancing the efficiency of knowledge transferred in OHCs.
Third, this study provides an auxiliary means for physicians, with relatively junior qualifications and poor ability, to learn clinical expertise, acquire practical experience and, thus, accumulate tacit medical knowledge, which is of vital importance to their clinical practice. Therefore, it is strongly recommended that physicians working in less developed regions who have relatively limited learning opportunities in the real environment attend the parts of case discussions or medical information in OHCs to obtain more chances to learn from others, especially experts in the field. Meanwhile, they can raise their problems in the community to find the most appropriate solutions and improve their service delivery.
5.4 Limitations and future research
This study has several limitations that can be addressed in future research. First, only physicians’ online characteristics and those of their departments were considered, while other unobserved factors, such as physicians’ titles, hospital level, etc. may have affected the level of knowledge transfer in OHCs. Second, the ERGM models reported in this study were created using a simple directional binary network. Although this can conservatively account for the physician relationship network, future studies should develop weighted networks or two-mode networks to provide more valuable insights. Third, the study’s results seem to be unstable in the type of help-seeking network. This may be attributed to the subjectivity of manual classification. Future studies can use supervised machine learning methods to classify the content of posts found in OHCs. Finally, this study only included physicians who had posted or replied to posts in the OHC, but it is argued that any physician who browses the threads in OHCs are likely to benefit as a receiver, even though they do not post. Therefore, the evidence presented in this study should be considered as a conservative estimate, which reflects a subset of the knowledge transferred by physicians in OHCs.
Ackland R, O’Neil M (2011) Online collective identity: the case of the environmental movement. Soc Netw 33(3):177–190. https://doi.org/10.1016/j.socnet.2011.03.001
Article Google Scholar
Ali N, Tretiakov A, Whiddett D, Hunter I (2017) Knowledge management systems success in healthcare: leadership matters. Int J Med Inform 97:331–340. https://doi.org/10.1016/j.ijmedinf.2016.11.004
Ancona DG, Caldwell DF (1992) Demography and design: predictors of new product team performance. Organ Sci 3(3):321–341. https://doi.org/10.1287/orsc.3.3.321
Assegaff S, Hussin ARC, Dahlan HM (2013) Knowledge management system as enabler for knowledge management practices in virtual communities. Int J Comput Sci Issues 10(1):685–688
Google Scholar
Bartolacci C, Cristalli C, Isidori D, Niccolini F (2016) Ba virtual and inter-organizational evolution: a case study from a EU research project. J Knowl Manag 20(4):793–811. https://doi.org/10.1108/JKM-09-2015-0342
Bloodgood JM, Salisbury WD (2001) Understanding the influence of organizational change strategies on information technology and knowledge management strategies. Decis Support Syst 31(1):55–69. https://doi.org/10.1016/S0167-9236(00)00119-6
Cao X, Wang D (2018) The role of online communities in reducing urban-rural health disparities in China. J Am Soc Inf Sci 69(7):890–899. https://doi.org/10.1002/asi.24013
Chen H, Baptista Nunes M, Ragsdell G, An X (2018) Extrinsic and intrinsic motivation for experience grounded tacit knowledge sharing in Chinese software organisations. J Knowl Manag 22(2):478–498. https://doi.org/10.1108/JKM-03-2017-0101
Chen L, Fong PSW (2012) Revealing performance heterogeneity through knowledge management maturity evaluation: a capability-based approach. Expert Syst Appl 39(18):13523–13539. https://doi.org/10.1016/j.eswa.2012.07.005
Chen W, Wei X, Zhu K (2017) Engaging voluntary contributions in online communities: a hidden markov model. MIS Q 42(1):83–100
Chiu C-M, Hsu M-H, Wang ETG (2006) Understanding knowledge sharing in virtual communities: an integration of social capital and social cognitive theories. Decis Support Syst 42(3):1872–1888. https://doi.org/10.1016/j.dss.2006.04.001
Connell NAD, Klein JH, Powell PL (2003) It’s tacit knowledge but not as we know it: redirecting the search for knowledge. J Oper Res Soc 54(2):140–152. https://doi.org/10.1057/palgrave.jors.2601444
Del Giudice M, Della Peruta MR (2016) The impact of IT-based knowledge management systems on internal venturing and innovation: a structural equation modeling approach to corporate performance. J Knowl Manag 20(3):484–498. https://doi.org/10.1108/JKM-07-2015-0257
Dove R (1998) The knowledge worker. Automot Manuf Prod 110(6):26–28
Faraj S, von Krogh G, Monteiro E, Lakhani KR (2016) Special Section introduction—online community as space for knowledge flows. Inf Syst Res 27(4):668–684. https://doi.org/10.1287/isre.2016.0682
Foster D (2016) ‘Keep complaining til someone listens’: exchanges of tacit healthcare knowledge in online illness communities. Soc Sci Med 166:25–32. https://doi.org/10.1016/j.socscimed.2016.08.007
Gabbay J, le May A (2004) Evidence based guidelines or collectively constructed ‘mindlines?’ Ethnographic study of knowledge management in primary care. BMJ Br Med J 329(7473):1013. https://doi.org/10.1136/bmj.329.7473.1013
Ganguly A, Talukdar A, Chatterjee D (2019) Evaluating the role of social capital, tacit knowledge sharing, knowledge quality and reciprocity in determining innovation capability of an organization. J Knowl Manag 23(6):1105–1135. https://doi.org/10.1108/JKM-03-2018-0190
Goh JM, Gao G, Agarwal R (2016) The creation of social value: Can an online health community reduce rural-urban health disparities? MIS Q 40(1):247–263. https://doi.org/10.25300/MISQ/2016/40.1.11
Goodreau SM (2007) Advances in exponential random graph (p*) models applied to a large social network. Soc Netw 29(2):231–248. https://doi.org/10.1016/j.socnet.2006.08.001
Goodreau SM, Handcock MS, Hunter DR, Butts CT, Morris M (2008) A statnet tutorial. J Stat Softw 24(9):1–27
Guo S, Guo X, Fang Y, Vogel D (2017) How doctors gain social and economic returns in online health-care communities: a professional capital perspective. J Manag Inf Syst 34(2):487–519. https://doi.org/10.1080/07421222.2017.1334480
Harb Y, Zahrawi A, Shehabat I, Zhang Z (2021) Managing knowledge workers in healthcare context: role of individual and knowledge characteristics in physicians’ knowledge sharing. Ind Manag Data Syst 121(2):381–408. https://doi.org/10.1108/IMDS-02-2020-0097
Heiberg Engel PJ (2008) Tacit knowledge and visual expertise in medical diagnostic reasoning: implications for medical education. Med Teach 30(7):e184–e188. https://doi.org/10.1080/01421590802144260
Henry SG (2010) Polanyi’s tacit knowing and the relevance of epistemology to clinical medicine. J Eval Clin Pract 16(2):292–297. https://doi.org/10.1111/j.1365-2753.2010.01387.x
Hwang EH, Guo X, Tan Y, Dang Y (2022) Delivering healthcare through teleconsultations: implications for offline healthcare disparity. Inf Syst Res. https://doi.org/10.1287/isre.2021.1055
Jabr NH (2007) Physicians’ attitudes towards knowledge transfer and sharing. Compet Rev Int Bus J 17(4):248–260. https://doi.org/10.1108/10595420710844334
Ju TL, Li C, Lee T (2006) A contingency model for knowledge management capability and innovation. Ind Manag Data Syst 106(6):855–877. https://doi.org/10.1108/02635570610671524
Kessel M, Kratzer J, Schultz C (2012) Psychological safety, knowledge sharing, and creative performance in healthcare teams. Creat Innov Manag 21(2):147–157. https://doi.org/10.1111/j.1467-8691.2012.00635.x
Kilduff M, Krackhardt D (2008) Interpersonal networks in organizations: cognition, personality, dynamics, and culture. Cambridge University Press, Cambridge
Book Google Scholar
Kothari A, Rudman D, Dobbins M, Rouse M, Sibbald S, Edwards N (2012) The use of tacit and explicit knowledge in public health: a qualitative study. Implement Sci 7(1):20. https://doi.org/10.1186/1748-5908-7-20
Kraut R, Kiesler S, Boneva B, Cummings J, Helgeson V, Crawford A (2002) Internet paradox revisited. J Soc Issues 58(1):49–74. https://doi.org/10.1111/1540-4560.00248
Law KK, Chan A, Ozer M (2017) Towards an integrated framework of intrinsic motivators, extrinsic motivators and knowledge sharing. J Knowl Manag 21(6):1486–1502. https://doi.org/10.1108/JKM-03-2016-0119
Lee S, Hong J (2019) Analyzing the change in knowledge sharing efficiency of knowledge networks: a case study. Inf Technol Manage 20(1):41–53. https://doi.org/10.1007/s10799-018-0292-5
Li D, Zhou Z, Si Y, Xu Y, Shen C, Wang Y, Wang X (2018) Unequal distribution of health human resource in mainland China: What are the determinants from a comprehensive perspective? Int J Equity Health 17:29. https://doi.org/10.1186/s12939-018-0742-z
Li W (2010) Virtual knowledge sharing in a cross-cultural context. J Knowl Manag 14(1):38–50. https://doi.org/10.1108/13673271011015552
Liu QB, Liu X, Guo X (2020) The effects of participating in a physician-driven online health community in managing chronic disease: evidence from two natural experiments. MIS Q 44(1):391–419. https://doi.org/10.25300/MISQ/2020/15102
Lusher D, Koskinen J, Robins G (2013) Exponential random graph models for social networks: theory. Methods Appl. https://doi.org/10.1017/CBO9780511894701
Ma Z, Huang Y, Wu J, Dong W, Qi L (2014) What matters for knowledge sharing in collectivistic cultures? Empirical evidence from China. J Knowl Manag 18(5):1004–1019. https://doi.org/10.1108/JKM-06-2014-0252
Maras M-H, Arsovska J, Wandt AS, Knieps M, Logie K (2022) The SECI model and darknet markets: knowledge creation in criminal organizations and communities of practice. Eur J Criminol. https://doi.org/10.1177/14773708221115167
Meng F, Zhang X, Liu L, Ren C (2021) Converting readers to patients? From free to paid knowledge-sharing in online health communities. Inf Process Manag 58(3):102490. https://doi.org/10.1016/j.ipm.2021.102490
Min J, Lee J, Ryu S, Lee H (2022) The effects of interaction between team climates and KMS value perception on knowledge activities: a multilevel socio-technical systems approach. Inf Technol Manage 23(1):1–21. https://doi.org/10.1007/s10799-021-00337-5
National Bureau of Statistics (2020) China statistical yearbook
Nguyen T-M, Malik A (2020) Cognitive processes, rewards and online knowledge sharing behaviour: the moderating effect of organisational innovation. J Knowl Manag 24(6):1241–1261. https://doi.org/10.1108/JKM-12-2019-0742
Nonaka I (1994) A dynamic theory of organizational knowledge creation. Organ Sci 5(1):14–37
Nonaka I, Takeuchi H (1995) The knowledge-creating company: how japanese companies create the dynamics of innovation. Oxford University Press, New York
Panahi S, Watson J, Partridge H (2016) Conceptualising social media support for tacit knowledge sharing: physicians’ perspectives and experiences. J Knowl Manag 20(2):344–363. https://doi.org/10.1108/JKM-06-2015-0229
Parkhe A, Wasserman S, Ralston DA (2006) New frontiers in network theory development. Acad Manag Rev 31(3):560–568. https://doi.org/10.5465/amr.2006.21318917
Paul DL (2006) Collaborative activities in virtual settings: a knowledge management perspective of telemedicine. J Manag Inf Syst 22(4):143–176. https://doi.org/10.2753/MIS0742-1222220406
Pellegrini G, Terribile F, Tarola O, Muccigrosso T, Busillo F (2013) Measuring the effects of European Regional Policy on economic growth: a regression discontinuity approach. Pap Reg Sci 92(1):217-+. https://doi.org/10.1111/j.1435-5957.2012.00459.x
Prada G, Grimes K, Sklokin I (2014) Defining health and health care sustainability. In: The Conference Board of Canada. Ottawa
Qiao W, Yan Z, Wang X (2021) Join or not: the impact of physicians’ group joining behavior on their online demand and reputation in online health communities. Inf Process Manag 58(5):102634. https://doi.org/10.1016/j.ipm.2021.102634
Radević I, Dimovski V, Lojpur A, Colnar S (2021) Quality of healthcare services in focus: the role of knowledge transfer, hierarchical organizational structure and trust. Knowl Manag Res Pract. https://doi.org/10.1080/14778238.2021.1932623
Robins G (2011) Exponential random graph models for social networks. Handbook of social network analysis. Sage
Robins G, Pattison P, Kalish Y, Lusher D (2007) An introduction to exponential random graph (p*) models for social networks. Soc Netw 29(2):173–191. https://doi.org/10.1016/j.socnet.2006.08.002
Roj J (2020) Inequality in the distribution of healthcare human resources in Poland. Sustain 12(5):2043. https://doi.org/10.3390/su12052043
Romanelli M (2017) Towards sustainable health care organizations. Manag Dyn Knowl Econ 5(3):377–394
Sanford S, Schwartz B, Khan Y (2020) The role of tacit knowledge in communication and decision-making during emerging public health incidents. Int J Disaster Risk Reduct 50:101681. https://doi.org/10.1016/j.ijdrr.2020.101681
Scrutton J, Holley-Moore G, Bamford SM (2015) Creating a sustainable 21st century healthcare system. The International Longevity Centre-UK (ILC-UK), London
Secundo G, Toma A, Schiuma G, Passiante G (2018) Knowledge transfer in open innovation: a classification framework for healthcare ecosystems. Bus Process Manag J 25(1):144–163. https://doi.org/10.1108/BPMJ-06-2017-0173
Smith A, Goodwin D, Mort M, Pope C (2003) Expertise in practice: an ethnographic study exploring acquisition and use of knowledge in anaesthesia. BJA Br J Anaesth 91(3):319–328. https://doi.org/10.1093/bja/aeg180
Turulja L, Cinjarevic M, Veselinovic L (2020) Information technology and knowledge sharing for better health care: an emerging economy context. J Knowl Manag 25(3):559–572. https://doi.org/10.1108/JKM-09-2019-0514
Wang C, Dong Y, Ye Z, Feng J (2022) Linking online and offline intergenerational knowledge transfer to younger employees’ innovative work behaviors: evidence from Chinese hospitals. J Knowl Manag 27(3):762–784. https://doi.org/10.1108/JKM-11-2021-0839
Wang N, Yin J, Ma Z, Liao M (2021) The influence mechanism of rewards on knowledge sharing behaviors in virtual communities. J Knowl Manag 26(3):485–505. https://doi.org/10.1108/JKM-07-2020-0530
Wieringa S, Engebretsen E, Heggen K, Greenhalgh T (2018) How knowledge is constructed and exchanged in virtual communities of physicians: qualitative study of mindlines online. J Med Internet Res 20(2):e8325. https://doi.org/10.2196/jmir.8325
Wu H, Deng Z (2019) Knowledge collaboration among physicians in online health communities: a transactive memory perspective. Int J Inf Manage 49:13–33. https://doi.org/10.1016/j.ijinfomgt.2019.01.003
Wu H, Deng Z, Evans R (2022) Building patients’ trust in psychologists in online mental health communities. Data Sci Manag 5(1):21–27. https://doi.org/10.1016/j.dsm.2022.03.001
Yan A, Zou Y, Mirchandani DA (2020) How hospitals in mainland China responded to the outbreak of COVID-19 using information technology–enabled services: an analysis of hospital news webpages. J Am Med Inform Assoc 27(7):991–999. https://doi.org/10.1093/jamia/ocaa064
Yan Z, Wang T, Chen Y, Zhang H (2016) Knowledge sharing in online health communities: a social exchange theory perspective. Inf Manag 53(5):643–653. https://doi.org/10.1016/j.im.2016.02.001
Yang H, Yan Z, Jia L, Liang H (2021) The impact of team diversity on physician teams’ performance in online health communities. Inf Process Manag 58(1):102421. https://doi.org/10.1016/j.ipm.2020.102421
Zappa P (2011) The network structure of knowledge sharing among physicians. Qual Quant 45(5):1109–1126. https://doi.org/10.1007/s11135-011-9494-1
Zhang X, Liu S (2021) Understanding relationship commitment and continuous knowledge sharing in online health communities: a social exchange perspective. J Knowl Manag 26(3):592–614. https://doi.org/10.1108/JKM-12-2020-0883
Zhang X, Liu S, Deng Z, Chen X (2017) Knowledge sharing motivations in online health communities: a comparative study of health professionals and normal users. Comput Hum Behav 75:797–810. https://doi.org/10.1016/j.chb.2017.06.028
Zhao J, Ha S, Widdows R (2016) The influence of social capital on knowledge creation in online health communities. Inf Technol Manage 17(4):311–321. https://doi.org/10.1007/s10799-014-0211-3
Zhu M, Bergner Y, Zhang Y, Baker R, Wang Y, Paquette L (2016) Longitudinal engagement, performance, and social connectivity: a MOOC case study using exponential random graph models. In: Proceedings of the sixth international conference on learning analytics & knowledge. Association for Computing Machinery, Edinburgh, United Kingdom, pp 223–230. https://doi.org/10.1145/2883851.2883934
Download references
Acknowledgements
This research was funded by the National Natural Science Foundation of China (NO. 71971092 and NO.72271102).
Author information
Authors and affiliations.
School of Management, Huazhong University of Science and Technology, Wuhan, 430074, China
Zihao Deng & Zhaohua Deng
School of Management, Xi’an Jiaotong University, Xi’an, 710049, China
Faculty of Computer Science, Dalhousie University, Halifax, NS, B3H 4R2, Canada
Richard Evans
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Zhaohua Deng .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Deng, Z., Deng, Z., Liu, S. et al. Knowledge transfer between physicians from different geographical regions in China’s online health communities. Inf Technol Manag (2023). https://doi.org/10.1007/s10799-023-00400-3
Download citation
Accepted : 28 April 2023
Published : 19 May 2023
DOI : https://doi.org/10.1007/s10799-023-00400-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Online health communities
- Knowledge transfer
- Tacit knowledge
- Explicit knowledge
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Characteristics and determinants of knowledge transfer policies at universities and public institutions in medical research--protocol for a systematic review of the qualitative research literature
Affiliations.
- 1 Institute for Public Health, Im Neuenheimer Feld 324, Heidelberg, Germany. [email protected].
- 2 Institute for Public Health, Im Neuenheimer Feld 324, Heidelberg, Germany. [email protected].
- 3 Department of General Practice and Health Services Research, University Hospital Heidelberg, Voßstr.2, Heidelberg, 69115, Germany. [email protected].
- PMID: 26286398
- PMCID: PMC4545361
- DOI: 10.1186/s13643-015-0094-3
Background: Universities, public institutions, and the transfer of knowledge to the private sector play a major role in the development of medical technologies. The decisions of universities and public institutions regarding the transfer of knowledge impact the accessibility of the final product, making it easier or more difficult for consumers to access these products. In the case of medical research, these products are pharmaceuticals, diagnostics, or medical procedures. The ethical dimension of access to these potentially lifesaving products is apparent and distinguishes the transfer of medical knowledge from the transfer of knowledge in other areas. While the general field of technology transfer from academic and public to private actors is attracting an increasing amount of scholarly attention, the specifications of knowledge transfer in the medical field are not as well explored. This review seeks to provide a systematic overview and analysis of the qualitative literature on the characteristics and determinants of knowledge transfer in medical research and development.
Methods: The review systematically searches the literature for qualitative studies that focus on knowledge transfer characteristics and determinants at medical academic and public research institutions. It aims at identifying and analyzing the literature on the content and context of knowledge transfer policies, decision-making processes, and actors at academic and public institutions. The search strategy includes the databases PubMed, Web of Science, ProQuest, and DiVa. These databases will be searched based on pre-specified search terms. The studies selected for inclusion in the review will be critically assessed for their quality utilizing the Qualitative Research Checklist developed by the Clinical Appraisal Skills Programme. Data extraction and synthesis will be based on the meta-ethnographic approach.
Discussion: This review seeks to further the understanding of the kinds of transfer pathways that exist in medical knowledge transfer as well as what factors lead to the adoption of one pathway over another. The aim is to provide evidence for political and academic actors designing policies for the translation of medical knowledge and public-private cooperation.
Systematic review registration: PROSPERO CRD42015014241 .
PubMed Disclaimer
Similar articles
- Public-private knowledge transfer and access to medicines: a systematic review and qualitative study of perceptions and roles of scientists involved in HPV vaccine research. Jahn R, Müller O, Nöst S, Bozorgmehr K. Jahn R, et al. Global Health. 2020 Mar 5;16(1):22. doi: 10.1186/s12992-020-00552-9. Global Health. 2020. PMID: 32138789 Free PMC article.
- Policies on faculty conflicts of interest at US universities. Cho MK, Shohara R, Schissel A, Rennie D. Cho MK, et al. JAMA. 2000 Nov 1;284(17):2203-8. doi: 10.1001/jama.284.17.2203. JAMA. 2000. PMID: 11056591
- Student and educator experiences of maternal-child simulation-based learning: a systematic review of qualitative evidence protocol. MacKinnon K, Marcellus L, Rivers J, Gordon C, Ryan M, Butcher D. MacKinnon K, et al. JBI Database System Rev Implement Rep. 2015 Jan;13(1):14-26. doi: 10.11124/jbisrir-2015-1694. JBI Database System Rev Implement Rep. 2015. PMID: 26447004
- Health professionals' experience of teamwork education in acute hospital settings: a systematic review of qualitative literature. Eddy K, Jordan Z, Stephenson M. Eddy K, et al. JBI Database System Rev Implement Rep. 2016 Apr;14(4):96-137. doi: 10.11124/JBISRIR-2016-1843. JBI Database System Rev Implement Rep. 2016. PMID: 27532314 Review.
- The experience of adults who choose watchful waiting or active surveillance as an approach to medical treatment: a qualitative systematic review. Rittenmeyer L, Huffman D, Alagna M, Moore E. Rittenmeyer L, et al. JBI Database System Rev Implement Rep. 2016 Feb;14(2):174-255. doi: 10.11124/jbisrir-2016-2270. JBI Database System Rev Implement Rep. 2016. PMID: 27536798 Review.
- Kneller R. The importance of new companies for drug discovery: origins of a decade of new drugs. Nat Rev Drug Discov. 2010;9(11):867–882. doi: 10.1038/nrd3251. - DOI - PubMed
- Crager S, Guillen E, Price M. University contributions to the HPV vaccine and implications for access to vaccines in developing countries: addressing materials and know-how in university technology transfer policy. Am J Law Med. 2009;35(2–3):253–279. - PubMed
- Clark A. Biomedical Innovation and the Politics of Scientific Knowledge: A case study of Gardasil. University of Maryland. 2008.
- Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(5):F12–F23. doi: 10.1016/j.vaccine.2012.07.055. - DOI - PubMed
- Bozeman B. Technology transfer and public policy: a review of research and theory. Res Policy. 2000;29:627–655. doi: 10.1016/S0048-7333(99)00093-1. - DOI
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- BioMed Central
- Europe PubMed Central
- PubMed Central
Other Literature Sources
- scite Smart Citations

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

- Academia & Careers , Conduct
Knowledge Translation: The Missing Link between Research and Practice
Introduction.
Knowledge translation is a fundamental scientific paradigm, aiming to close the gap between research and practice.
Although health researchers and pharmaceutical companies do not hesitate to invest a fortune in research areas, such as biomedical research, clinical trials, risk management, and professional development; data shows that healthcare systems worldwide fail to provide affordable services and effective treatment to patients.
In fact, it can take up to 20 years for research knowledge collected via randomized controlled trials to be implemented in practice. The underutilization of evidence-based knowledge across medical settings and the growing complexity of today’s healthcare systems can result in unnecessary risks and skyrocketing costs.
For instance, patients in the US receive only 55% of recommended care, and approximately 20%-30% of patients across the globe receive care that is not needed ( Grimshaw et al., 2012 ).
The know-do gap in research has been recognized as a major obstacle to improved health outcomes and drug development.
Consequently, numerous initiatives have been created to reduce this gap and tackle processes, such as quality assurance, technology transfer, innovation diffusion, evidence-informed policy, and implementation research. Although such terms are overlapping, the definition of “knowledge translation” has gained currency across Canada and the rest of the world.
Knowledge translation – also known as research utilization in Europe and dissemination or uptake in the US – refers to the process of transferring scientific knowledge, or what we’ve learned through research, into practice ( Straus, Tetroe & Graham, 2011 ).
To be more precise, the Canadian Institutes of Health Research defines this process as “a dynamic and iterative process that includes the synthesis, dissemination, exchange and ethically sound application of knowledge to improve health, provide more effective health services and products and strengthen the healthcare system.”
Knowledge translation is not only about the passive transfer of evidence for clinical research but the optimal utilization of evidence-based data across different levels of the healthcare system (e.g., consumers, healthcare professionals, researchers, and policymakers).
The Importance of Knowledge Translation
The importance of knowledge translation in healthcare is immense. Despite the relatively new terminology, knowledge translation can be traced back to the beginning of the last century when sociologist Gabriel Tarde attempted to explain the nature of innovation diffusion ( Grimshaw et al., 2012 ).
Evidence proves that conducting research and publishing the results is not enough for an innovation or a medical intervention to be implemented into practice.
To provide an example, although clinical trials show that statins can benefit post-stroke patients, statins are rarely prescribed in practice. Moreover, policymaking processes seldom include citable research or evidence from systematic reviews.
Knowledge translation research can enhance knowledge uptake and target numerous barriers to behavior and policy change at different levels of the healthcare system (e.g., lack of integrated information, poor standards of care, patients’ attitudes).
Note that one of the main challenges is the lack of knowledge management skills, such as insufficient time to read and the inability to appraise the huge volume of research publications.
To overcome barriers to behavior change, knowledge translation researchers must determine the know-do gap and explore the context of knowledge uptake in order to implement and assess effective research strategies in practice ( Straus, Tetroe & Graham, 2011 ).
As stated above, effective models should target all those involved in healthcare decision-making (e.g., patients, clinicians, policymakers, researchers, the public) and should consider the knowledge being disseminated (e.g., clinical, biomedical).
Understanding the barriers and the context of knowledge translation is one of the first steps towards closing the know-do gap in healthcare.
Knowledge Translation: Factors to Consider
What should be transferred .
In the vast labyrinths of scientific research, the numbers of research publications and contradicting data are increasing.
As clinicians, consumers, and policymakers often lack the time and skills needed to appraise research publications and individual studies; systematic reviews become the most effective methods to transfer knowledge and evidence-based data.
Note that programs, such as the Cochrane Collaboration, aim to help healthcare practitioners and consumers access easy-to-comprehend knowledge syntheses.
Knowledge translation should reach all levels of the healthcare system, with target audiences depending on the context of research.
For example, regulatory bodies and pharmaceutical companies might benefit from research sufficient to warrant a drug’s withdrawal from the market, while patients and providers might benefit from data demonstrating advantages from a treatment.
The key messages should be easy to comprehend by different audiences (e.g., policy briefs for policymakers).
Messenger credibility is another vital fact to consider in knowledge translation, which can be supported via an authoritative endorsement by a respected figure or a research group. Messages can be transferred by an individual (e.g., researcher), organization, or healthcare system.
Knowledge infrastructures also play a crucial role; they can be divided into technological (e.g., search engines) and organizational components (e.g., data analysts). ( Grimshaw et al., 2012 ).
Knowledge Translation: Barriers and Interventions
After identifying the know-do gap, researchers must assess barriers to knowledge translation and adapt knowledge to the local context. Barriers, which often obstruct different levels of the healthcare system, can be identified via surveys, focus groups, interviews, or observations.
Common barriers impact different healthcare levels: structural (e.g., financial restraints), organizational (e.g., lack of equipment), peer-group (e.g., poor standards of care), professionals (e.g., skills), professional-patients interaction (e.g., communication).
To provide an example, as professionals often overestimate their performance by approximately 20%-30%, individual capacities, training, and motivations should be explored further ( Llopis et al., 2018 ).
For the successful utilization of research knowledge, behavior change interventions should be selected and tailored according to the local context. Grimshaw et al. (2012 ) systematized the following interventions divided into three categories:
- professional behavior change strategies;
- knowledge translation interventions focusing on consumers;
- knowledge translation initiatives focusing on policymakers:
1) Professional behavior change strategies encompass a large number of tools and models:
- Printed educational materials : Such materials can include scientific publications, audio-visual materials, and practice guidelines. They can be distributed at a relatively low cost and across a wide range of settings.
- Educational meetings : From didactic meetings and lectures to interactive workshops and traineeships, educational meetings can target different barriers to knowledge translation (e.g., professional-peer levels and attitudes). Such approaches are feasible in most medical settings.
- Educational outreach : Educational outreach is another effective method, which can be conducted by a trained person in order to change a simple physician’s behavior (e.g., drug prescribing).
- Local opinion leaders: With a unique and innovative approach and strong social networks, opinion leaders play a crucial role in knowledge translation. Leaders can easily influence people and change their behavior in medical settings.
- Audit and feedback : Audit and feedback obtained from patients, databases, and medical records are essential in transparent medical care.
- Computerized reminders : Computerized reminders are also integrated into care. Although such tools provide mixed data, reminders can help professionals determine aspects such as drug dosage and side effects.
- Tailored interventions : Tailored interventions can be used to change aspects, such as poor information management, clinical uncertainty, lack of competence, perceptions of liability, patient expectations, financial disincentives, administrative constraints, and standards of practice.
- Multifaceted interventions : Multifaceted interventions can include two or more of the behavior change strategies indicated above.
2) Knowledge translation interventions focusing on consumers can be divided into three models:
Interventions to facilitate communication and decision-making; strategies to support behavior change; and techniques to educate consumers:
- Decision aids : Decision aids are used to facilitate communication and decision-making. Moreover, data shows that such tools can reduce decision conflict.
- Personalized-risk communication : Written, spoken, or visual personalized-risk communication can be implemented to enhance doctor-patient relationships.
- Communication before consultation : Communication before the consultation is also used to facilitate interactions and informed decision-making. Evidence shows that such strategies increase question asking and satisfaction.
- Interactive apps : Applications are used to support behavior change. Additionally, they show an increase in knowledge acquisition and social support.
- Contracts : Such behavior change interventions can be utilized to increase adherence to medical procedures and regimes. They can also benefit from prevention initiatives.
- Medication adherence strategies : Such behavior change strategies can include calls or written material and improve short-term medication adherence treatments.
- New behavior change tools : Novel methods, such as feedback on imaging and DNA-based risk assessment, can be effective behavior change interventions, specifically in smoking cessation programs.
- Written material : Written information is an effective method to educate consumers and increase knowledge of medicines and interventions.
- Self-management interventions : Such models can also educate consumers and improve pain and fatigue self-management.
3) Strategies focusing on policymakers and senior health service managers seem to receive relatively low attention in research:
- Push strategies : Push strategies aim to educate policy-makers and provide clear and easy-to-understand syntheses of scientific publications and statistical data.
- Tailored interventions : Tailored messages, access to online registries, and seminars can improve knowledge translation and decision-making.
- Pull activities and training: Such strategies can create an appetite for medical research and knowledge uptake.
- Exchange activities , such as co-funding, can help researchers and policymakers build new relationships and promote knowledge translation.
With numerous barriers and a variety of interventions, knowledge translation becomes a complex process that should be monitored effectively. Outcomes and practical impact should be assessed on a regular basis to sustain continued knowledge use and exchange.
Closing the know-do gap in medical research is essential as failure to transfer research findings into practice contributes to more than $200 billion of wasted funding and poor health outcomes ( Graham, Kothari & McCutcheon, 2018 ).
Enhancing Dissemination | Types of Knowledge Translation
To enhance dissemination and improve health outcomes, Web 2.0 technologies are becoming intergraded part of knowledge translation ( Bernhardt, Mays & Kreuter, 2011 ). Web 2.0 technologies refer to apps and Internet-based tools with user-centered designs that facilitate interactive information sharing.
One of the main features of such technologies is that users can create content and exercise control through such digital tools. In contrast to passive Web 1.0 technologies, Web 2.0 tools empower users and their role in digital healthcare ( Valdez et al., 2016 ).
Such tools have been implemented by numerous federal agencies, such as the Centers for Disease Control and Prevention, and engage prospective end-users from different directions. Users can either share one-to-one communication or engage in interactive communication (e.g., online forums).
Web 2.0 tools can overcome geographical barriers and allow users to form partnerships through conferences, virtual meetings, and calls. Success stories and influencers can also reshape the uptake of knowledge and highlight the demand for evidence-based medicine.
Note that to promote research products and increase the accessibility of services, smart tagging and search engine optimization are common strategies to create content based on search engine indexing.
Buttons or badges (defined as graphics with embedded links for information), widgets or gadgets (defined as applications that can be displayed from one site to another), and RSS feeds (defined as updated content of interest) are also used to engage users and promote knowledge translation.
Some of the most prominent types of new media and Web 2.0 technologies for knowledge translation include:
- Social networking platforms (e.g., Facebook): Facebook is one of the most influential social networking websites, which can increase user engagement, patient recruitment, and knowledge uptake. As the platform is easily accessible and engaging, it’s no surprise Facebook has 1.44 billion active monthly users. Additional tools, such as targeted advertising ( Isaacson et al., 2018 ), can help family caregivers and patients access high-quality resources and educational tools.
- Microblogs (e.g., Twitter): Twitter is another influential network consisting of 140-character tweets, which can foster eHealth communication and dialog-based strategies. More than 53% of the Internet population report having Twitter accounts; there are more than 500 million tweets a day in more than 33 different languages. In Saudi Arabia, for instance, out of 18 million Internet users, 60% report having Twitter accounts ( Albalawi & Sixsmith, 2015 ). Interestingly, 77% of life scientists use social media, and 85% of them admit that digital communication can influence their decisions.
- Blogs (e.g., WordPress): WordPress is perhaps the most popular open-source blogging platform, which can foster community-engaged research as it allows users to create web pages. Data shows there are more than 60.1 million new posts each month and more than 409 million views. In fact, blogs on research topics can have higher traffic than digital journal articles ( Lord et al., 2019 ).
- Sharing websites (e.g., YouTube): Audio and video tools (e.g., podcasts) can capture talks and training sessions and be successfully disseminated through sharing platforms, such as YouTube. Note that short YouTube videos with engaging key messages seem to be highly beneficial in knowledge translation and decision-making. Harrison et al. (2016) employed a brief consumer-targeted YouTube video on vaccination pain treatments for babies, which showed to have an extensive reach over 12 months.
- Wikis (e.g., Wikipedia): Wikis allow stakeholders to raise awareness of evidence-based practices and make research findings more relevant beyond borders and settings. Digital dissemination channels also allow access to updated information and success stories.
Knowledge Translation: Closing the Gap between Research and Practice
Knowledge translation is paramount to closing the gap between research and practice. The underutilization of evidence-based data results in poor health outcomes and unnecessary expenditures.
For instance, despite the high level of funding in the US, Americans experience poorer health outcomes compared to other developed nations.
Knowledge translation research aims to foster the optimal utilization of evidence-based information (e.g., systematic reviews) at different levels of the healthcare system (e.g., professionals, consumers, and policymakers).
To enhance knowledge uptake, knowledge translation researchers should understand the context of knowledge translation and target a wide variety of barriers to behavior change (e.g., lack of knowledge management skills), which will help them close the know-do gap in medical research.
Successful interventions include printed materials, audits, interactive apps, contracts, pull activities, push strategies, and much more.
One of the most popular strategies to enhance knowledge dissemination is the use of new media and Web 2.0 tools, such as social networking platforms, blogs, and sharing websites.
In the end, knowledge translation is an ongoing process that involves the frequent assessment of interventions, outcomes, and practical impact.
Continued knowledge use and exchange are essential in closing the know-do gap in research. Because moving research knowledge into practice settings can improve health outcomes and reduce waste in research!
- Albalawi, Y., & Sixsmith, J. (2015). Agenda Setting for Health Promotion: Exploring an Adapted Model for the Social Media Era . JMIR Public Health and Surveillance , 1 (2).
- Bernhardt, J., Mays, D., & Kreuter, M. (2011). Dissemination 2.0: closing the gap between knowledge and practice with new media and marketing. Journal of Health Communication , p. 32-44.
- Graham, I., Kothari, A., & McCutcheon, C. (2018). Moving knowledge into action for more effective practice, programmes and policy: protocol for a research programme on integrated knowledge translation . Implementation Science , 13.
- Grimshaw, J., Eccles, M., Lavis, J., Hill, S., & Squires, J. (2012). Knowledge translation of research findings . Implementation Science.
- Harrison, D., Wilding, J., Bowman, A., Fuller, A., Nicholls, S., Pound, C., Reszel, J., & Sampson, M. (2016). Using YouTube to Disseminate Effective Vaccination Pain Treatment for Babies. PLoS One , 11 (10).
- Isaacson, R., Seifan, A., Haddox, C., Mureb, M., Rahman, A., Scheyer, O., Hackett, K., Caesar, E., et al. (2018). Using social media to disseminate education about Alzheimer’s prevention & treatment: a pilot study on Alzheimer’s universe (www.AlzU.org) . J Commun Healthc , 11 (2), p. 106-113.
- Llopis, O., Sanchez-Barrioluengo, M., Olmos-Penuela, J., & Castro-Martinez, E. (2018). Scientists’ engagement in knowledge transfer and exchange: Individual factors, variety of mechanisms and users. Science and Public Policy , 45 (6), p. 790-803.
- Lord, S., Seavey, K., Oren, S., Budney, A., & Marsch, L. (2019). Digital Presence of a Research Center as a Research Dissemination Platform: Reach and Resources. JMIR Mental Health , 6 (4).
- Straus, S., Tetroe, J., & Graham, I. (2011). Knowledge translation is the use of knowledge in health care decision making. Journal of Clinical Epidemiology , 64 (1).
- Valdez Soto, M., Balls-Berry, J., Bishop, S., Aase, L., Timimi, F., Montori, V., & Patten, C. (2016). Use of Web 2.0 Social Media Platforms to Promote Community-Engaged Research Dialogs: A Preliminary Program Evaluation. JMIR Research Protocols , 5 (3).
We’ve collected the items for you to purchase for your convenience.
Get the entire package for up to 50% discount with our Replication program.

Our Location
Conduct science.
- Become a Partner
- Social Media
- Career /Academia
- Privacy Policy
- Shipping & Returns
- Request a quote
Customer service
- Account details
- Lost password
DISCLAIMER: ConductScience and affiliate products are NOT designed for human consumption, testing, or clinical utilization. They are designed for pre-clinical utilization only. Customers purchasing apparatus for the purposes of scientific research or veterinary care affirm adherence to applicable regulatory bodies for the country in which their research or care is conducted.
This paper is in the following e-collection/theme issue:
Published on 8.8.2024 in Vol 26 (2024)
Mapping Knowledge Landscapes and Emerging Trends in AI for Dementia Biomarkers: Bibliometric and Visualization Analysis
Authors of this article:

Original Paper
- Wenhao Qi 1 , BSc ;
- Xiaohong Zhu 1 , BSc ;
- Danni He 1, 2 , MSc ;
- Bin Wang 1 , BSc ;
- Shihua Cao 1 , PhD ;
- Chaoqun Dong 1 , BSc ;
- Yunhua Li 3 , MSc ;
- Yanfei Chen 1, 4 , MSc ;
- Bingsheng Wang 1 , BSc ;
- Yankai Shi 1 , BSc ;
- Guowei Jiang 5 , MSc ;
- Fang Liu 6 , PhD ;
- Lizzy M M Boots 5 , PhD ;
- Jiaqi Li 1 , BSc ;
- Xiajing Lou 1 , BSc ;
- Jiani Yao 1 , BSc ;
- Xiaodong Lu 7 , PhD ;
- Junling Kang 8 , PhD
1 School of Nursing, Hangzhou Normal University, Hangzhou, China
2 Nursing Department, Zhejiang Provincial People's Hospital, Hangzhou, China
3 College of Education, Chengdu College of Arts and Sciences, Sichuan, China
4 Nursing Department, Affiliated Hospital of Hangzhou Normal University, Hangzhou, China
5 Department of Psychiatry and Neuropsychology and Alzheimer Center Limburg, School for Mental Health and Neuroscience (MHeNS), Maastricht University, Maastricht, Netherlands
6 College of Electronics and Information Engineering, Shenzhen University, Shenzhen, China
7 Department of Neurology, Affiliated Hospital of Hangzhou Normal University, Hangzhou, China
8 Department of Neurology, The Third Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, China
Corresponding Author:
Shihua Cao, PhD
School of Nursing
Hangzhou Normal University
No. 2318, Yuhangtang Road, Yuhang District
Hangzhou, 310021
Phone: 86 13777861361
Email: [email protected]
Background: With the rise of artificial intelligence (AI) in the field of dementia biomarker research, exploring its current developmental trends and research focuses has become increasingly important. This study, using literature data mining, analyzes and assesses the key contributions and development scale of AI in dementia biomarker research.
Objective: The aim of this study was to comprehensively evaluate the current state, hot topics, and future trends of AI in dementia biomarker research globally.
Methods: This study thoroughly analyzed the literature in the application of AI to dementia biomarkers across various dimensions, such as publication volume, authors, institutions, journals, and countries, based on the Web of Science Core Collection. In addition, scales, trends, and potential connections between AI and biomarkers were extracted and deeply analyzed through multiple expert panels.
Results: To date, the field includes 1070 publications across 362 journals, involving 74 countries and 1793 major research institutions, with a total of 6455 researchers. Notably, 69.41% (994/1432) of the researchers ceased their studies before 2019. The most prevalent algorithms used are support vector machines, random forests, and neural networks. Current research frequently focuses on biomarkers such as imaging biomarkers, cerebrospinal fluid biomarkers, genetic biomarkers, and blood biomarkers. Recent advances have highlighted significant discoveries in biomarkers related to imaging, genetics, and blood, with growth in studies on digital and ophthalmic biomarkers.
Conclusions: The field is currently in a phase of stable development, receiving widespread attention from numerous countries, institutions, and researchers worldwide. Despite this, stable clusters of collaborative research have yet to be established, and there is a pressing need to enhance interdisciplinary collaboration. Algorithm development has shown prominence, especially the application of support vector machines and neural networks in imaging studies. Looking forward, newly discovered biomarkers are expected to undergo further validation, and new types, such as digital biomarkers, will garner increased research interest and attention.
Introduction
As the global population ages and life expectancy increases, the number of individuals with dementia is rising at an alarming rate. It is estimated that >55 million people are currently affected by dementia, and this number is expected to continue to grow [ 1 ]. The 4 most common subtypes of dementia are Alzheimer disease (AD), vascular dementia (VaD), dementia with Lewy bodies (DLB), and frontotemporal dementia (FTD). Their typical symptoms include cognitive dysfunction, memory loss, and mood fluctuations [ 2 ], significantly impacting patients’ quality of life and social function. Currently, there is no complete cure for these diseases, posing a substantial burden on patients and their families [ 3 ]. Therefore, early diagnosis is crucial for the intervention and management of these diseases [ 4 ]. At present, the diagnosis of these conditions largely relies on manual assessments by neurologists or other medical experts, which can be challenging to access in economically disadvantaged areas, leading to many cases of dementia going undiagnosed or misdiagnosed [ 5 ]. In addition, neurologists may take a considerable amount of time to make a final diagnosis for a single patient [ 6 ].
Biomarkers, as measurable biological indicators that can reflect normal physiological processes, disease progression, or responses to treatment [ 7 ], are crucial for the clinical diagnosis, management, and treatment of dementia. The National Institute on Aging and Alzheimer’s Association in the United States have recognized the use of biomarkers for diagnosing AD and monitoring its progression [ 8 ]. These markers aid clinicians in identifying high-risk groups, making early diagnoses [ 9 ], determining subtypes [ 10 ], predicting prognosis [ 11 ], and assessing drug responses or adverse events. However, with the exponential growth of multiomics and multimodal data, traditional statistical methods are no longer sufficient to meet the needs of discovering new biomarkers [ 12 ]. Artificial intelligence (AI), a widely used tool in the health care sector, offers a new perspective for accelerating the discovery of more reliable and clinically applicable biomarkers for dementia [ 13 ].
AI, an interdisciplinary field merging computer and data sciences, aims to simulate and extend human intelligence through machines [ 14 ]. Core technologies in AI, such as machine learning (ML), natural language processing, and computer vision [ 15 , 16 ], allow researchers to analyze and mine vast amounts of clinical and biomarker data. Through techniques such as ML and deep learning, more accurate and personalized predictions and diagnoses for dementia are made possible [ 12 ]; for instance, deep learning and ML as well as using diverse biomarker data types such as imaging, genetic information, and proteomics have been highly accurate in early diagnosis and classification of dementia [ 17 - 19 ]. Genetic and neurobiological data reveal the neuroglial activation and inflammatory states in dementia, identifying pathological stages of the disease [ 20 , 21 ], thereby deepening the understanding of its onset and progression. Similarly, AI identifies patterns and features in these data sets, analyzing potential disease biomarkers. This helps researchers save significant time and resources as well as identify more diagnostic biomarkers for earlier interventions and treatments, ultimately leading to better therapeutic outcomes.
To assess effective diagnostic biomarkers, the Alzheimer’s Disease Neuroimaging Initiative (ADNI) has used a multifaceted approach, including imaging and cerebrospinal fluid (CSF) tests, aimed at identifying the most predictive biomarkers for dementia [ 22 ]. Yang and Qu [ 23 ] analyzed AD biomarker research from 2000 to 2023, using network analysis to highlight CSF and beta amyloid (Aβ) protein as research hot spots and cutting-edge areas. Noda et al [ 24 ] identified the research dynamics involving the emerging biomarker neurofilament light (NFL) through keyword trend analysis. Similarly, Wu et al [ 25 ] emphasized the significance of AI in dementia research using bibliometrics. In review studies, Aberathne et al [ 26 ] highlighted the effectiveness of AI and ML in processing magnetic resonance imaging (MRI) and positron emission tomography (PET) imaging data. Blanco et al [ 27 ] and Falahati et al [ 28 ] demonstrated the application of algorithms in fluid biomarker research and imaging biomarker performance, respectively, while Chang et al [ 13 ] emphasized that ML combined with novel biomarkers and multivariate data could enhance the sensitivity and specificity of AD diagnosis. In addition, Li et al [ 29 ] reviewed the use of AI in digital biomarkers. Tzimourta et al [ 30 ] reviewed the application of various AI algorithms in 49 experimental studies analyzing electroencephalography (EEG) recordings, summarizing EEG features associated with AD.
However, the existing reviews summarizing the latest findings on AI algorithms and biomarkers often focus solely on 1 type of biomarker, failing to conduct multicategory induction and identify specific patterns. Current bibliometric studies have not yet explored the specific applications of AI in the field of dementia biomarkers. Therefore, this study combines bibliometric and content mining analysis to provide a comprehensive overview of research hot spots and developmental trends, offering valuable insights for future research directions.
Research Problem and Aim
Bibliometrics, as a method for analyzing quantitative information in scholarly literature [ 31 ], plays a crucial role in the evaluation of scientific advances within research areas [ 32 ]. Through bibliometric analysis as well as content mining and analysis, our study aims to achieve the following objectives:
- Thoroughly analyze the current status and various stage applications of AI in dementia biomarkers
- Highlight the research hot spots and future trends in this field
- Identify and emphasize the contributions of prolific authors, leading countries, and the most productive academic institutions in this field
- Explore potential future collaborative opportunities
- Examine the connections and application scale between biomarkers and AI methods
Through this research, we aim to comprehensively understand and evaluate the application of AI in the field of dementia biomarkers and make substantive contributions to the future research development in this area.
Leveraging the Web of Science Core Collection database and various bibliometric tools, we conducted a detailed collaborative analysis of annual publication volume and trends, author publication dynamics and collaboration networks, institutional publications and collaboration networks, national publications, collaboration networks, distribution of disciplines and interdisciplinary activities, and keyword clustering. By using literature mining and content analysis, we captured the prevalence, trends, connections, newly discovered biomarkers associated with AI algorithms, and various types of dementia biomarkers, distinguishing and analyzing them according to the classification of dementia subtypes.
Data Sources and Search Strategy
Following the suggestion by Donthu et al [ 33 ] to minimize potential human errors during format conversion among different databases (manual calibration is required to standardize different database formats, including manually establishing and entering profiles for funds, authors, etc; in addition, discrepancies in citation statistics from different databases and the untraceability of local citations have been noted), we decided to collect bibliometric data from only 1 database. This study selected the Web of Science Core Collection as the platform for the literature search. To ensure comprehensive coverage, all editions of the citation index database were chosen to avoid any omission of relevant literature. This database is widely recognized as a core resource for interdisciplinary academic research and has received high acclaim in numerous bibliometric studies [ 25 , 34 , 35 ]. Before conducting the search, all team members underwent professional training based on the Medical Literature Information Retrieval textbook [ 36 ], and a web-based search of the Web of Science Core Collection was conducted on November 2, 2023. The search used keywords such as “artificial intelligence,” “dementia,” and “biomarker,” along with their derivatives, synonyms, and Boolean operators, to construct the search formula ( Multimedia Appendix 1 ). The scope of the search extended from the database’s inception to the date of the search. A total of 2315 relevant documents were retrieved, exported with full records and complete citations, and saved in plain-text format. To avoid bias due to daily updates of the Web of Science Core Collection database, all searching and exporting tasks were completed within the same day.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) document types restricted to “articles” or “reviews,” (2) papers written in “English,” and (3) research topics related to “artificial intelligence” and “dementia biomarkers.” The exclusion criteria were as follows: (1) duplicate publications; (2) nonjournal literature such as conference papers, books, and comments; (3) documents with missing abstract, keywords, or main text; and (4) studies unrelated to “artificial intelligence” and “dementia biomarkers.”
Screening Strategy
After establishing the inclusion and exclusion criteria, to ensure the reliability of the material selection process, 2 evaluators (WQ and XZ) conducted a preliminary screening trial of 50 papers based on the titles, abstracts, and keywords [ 37 ]. The Cohen κ coefficient was calculated to be approximately 0.88, indicating a high level of agreement between the evaluators (the Cohen κ coefficient ranges from –1 to 1, with higher values denoting better consistency [ 38 , 39 ]; the specific formulas and methods are provided in Multimedia Appendix 2 ).
Therefore, we decided not to make any changes to the inclusion and exclusion criteria or to the evaluators. In case of any disagreements during the official selection process, 3 authors (WQ, XZ, and SC) would discuss the matter until a consensus was reached in a team meeting. The literature screening and verification were successfully completed on November 25, 2023. Of the 2315 papers identified, 1070 (46.22%) were included, while 1245 (53.78%) were excluded (type mismatch: n=60, 4.82%; irrelevant to the topic: n=1184, 95.1%; missing abstract: n=1, 0.08%). The detailed search and selection process is recorded in Figure 1 .

Data Cleaning
In the author analysis process, we conducted further reviews for authors with similar names to determine whether they were the same individual and decide whether further merging was necessary. The review was facilitated by examining the consistency of their Open Researcher and Contributor ID records, publication history, affiliation with the same institution, and information on professional sites such as ResearchGate. During the institutional analysis, we adopted the institutional affiliation standardization model developed by Nam et al [ 40 ], selecting the first-listed institution, usually the primary affiliation, for authors associated with multiple institutions. In addition, we consolidated various institutions’ full names and abbreviations. In analyzing international collaborations, we acknowledged authors affiliated with multiple international institutions because this could indicate potential transnational visiting scholarships or other forms of international cooperation. For funding analysis, we reviewed and appropriately merged various forms of sponsor names, including full names and abbreviations. Before the keyword analysis, to ensure the uniformity and accuracy of author keywords, we used the Bibliometrix package in R to merge synonyms; for instance, “Alzheimer disease” and “AD” were unified under “Alzheimer’s disease” (specific merged keywords are detailed in Multimedia Appendix 3 ).
Data Analysis
Currently, single bibliometric tools still have limitations in information extraction and content analysis [ 41 ]. To avoid bias and ensure the completeness and detail of information, we adopted a joint analysis strategy based on the strengths of various tools, as detailed in Multimedia Appendix 4 . Brief introductions to the tools used are presented in Textbox 1 .
- CiteSpace (version 5.7.R5; Drexel University): a Java scientometric tool developed by Chen [ 42 ], used for visualizing trends and patterns in scientific literature as well as revealing hot spots and the evolution of knowledge structures
- VOSviewer (version 1.6.19; Leiden University): free Java document-mapping software developed by the Centre for Science and Technology Studies at Leiden University, Leiden, Netherlands, assists in building various visualization networks [ 43 ]
- Bibliometrix : an R-based tool for extracting, processing, and analyzing literature data from the Web of Science database [ 44 ]
- gCLUTO (version 1.0; Kerapis Lab): focuses on data clustering, offering various clustering methods and visualization options [ 45 ]
- Publish or Perish (Harzing.com): used for assessing the publication and citation records of scholars, providing multiple metrics for comprehensive and fair academic research evaluation
- Gephi (version 0.10.1; Gephi.org): software for visualizing social and citation networks, providing significant flexibility in graph rendering
- Joinpoint (version 5.0.2; National Cancer Institute, United States): software designed for identifying and analyzing trend change points in time series data, allowing for the detection of points where there is a significant shift in the slope of the trend [ 46 ]
- Scimago Graphica (version 1.0.16; Scimago Lab) [ 35 ] and Pajek (64-bit version) Portable (version 5.18; University of Ljubljana) [ 47 ]: for enhanced readability of knowledge maps, Scimago Graphica and Pajek (64-bit version) Portable were incorporated for layout purposes
The analysis for each section adopted the bibliometrics analysis scheme proposed by Cobo et al [ 48 ].
Statistical Analysis
Extraction and classification of biomarkers and ai algorithms.
We specifically established an interdisciplinary professional team responsible for reading the full texts of research papers to extract and classify specific biomarkers and AI algorithms and to handle discussions and disputes that arose. The team consisted of 2 neurology experts, 2 AI domain experts, and 1 medical informatics expert. The classification process for biomarkers and algorithms was conducted independently by the neurology experts and the AI domain experts, without interference from each other. In addition, each expert conducted evaluations independently, and in cases of dispute, the medical informatics expert intervened to discuss the issue and take a decision. We referred to the classification of ML algorithms by Gutierrez [ 15 ] and Silva-Spínola et al [ 49 ], classified the biomarkers based on their nature and acquisition methods, and ultimately used Gephi (version 0.10.1) to construct a co-occurrence network between them. The specific classification process and network construction are shown in Figure 2 . The detailed classification methods of biomarkers are presented in Multimedia Appendix 5 .

Publication Output and Growth of Research Interest
We used CiteSpace to analyze the annual publication trends of the literature and applied polynomial fitting using the least squares method in OriginPro 2021 (OriginLab Corporation) [ 50 ]. The R ² value is an indicator of the fit of a trend line, reflecting the degree of fit between the estimated values of the trend line and the corresponding actual data. The closer the R ² value is to 1, the higher the degree of fit and the greater the reliability of the trend line [ 51 ]. The annual growth rate of publications was calculated using the following compound formula [ 52 , 53 ]:
Growth rate = ([number of publications in the last year / number of publications in the first year]1 / (last year − first year) − 1) × 100
Joinpoint software was used to evaluate time trends in a structured manner and to test which trends between junction points were statistically significant [ 54 ]. The software applies recommended schemes for the number of turning points in the model. To indicate the direction and magnitude of trends, this study calculated the changes in the trend slope. The slope represents the rate of change of the dependent variable over a specific period. When the difference in slopes between 2 line segments is significantly different from 0, it indicates a significant change in the trend at the corresponding time point (ie, the node). P <.05 was considered statistically significant.
Author Analysis
We used VOSviewer and Bibliometrix to analyze key information of the top 10 authors with the highest publication volume. Considering the differences in interdisciplinary citation habits, we used Publish or Perish software to calculate the h-index [ 55 ], g-index [ 56 ], and e-index [ 56 , 57 ] scores, thus avoiding assessment biases that might arise from relying on a single metric [ 58 ]. A higher e-index score indicates that an author has produced a series of high-quality, high-impact research works in their field, rather than just a few widely cited papers. Detailed methods and formulas for calculating the e-index score are provided in Multimedia Appendix 2 .
We used Microsoft Excel 2019 to compile the annual output of all authors, analyzing their publication dynamics to identify new researchers and terminators [ 59 ]. New researchers are defined as those who started publishing in a specific year without any prior related publications, while terminators are those who published articles before a specific year but did not publish any article after that year [ 59 ]. The Price law formula [ 60 ] was applied to identify the core group of authors and calculate their productivity. The specific formulas and methods are provided in Multimedia Appendix 2 .
Journal Analysis
To identify core journals in the field, we applied the Bradford law [ 61 , 62 ]. We conducted a fair and comprehensive evaluation of the journals’ academic impact, integrating metrics such as CiteScore 2022 [ 63 , 64 ], Scimago Journal Rank [ 63 , 65 ], Journal Citation Reports Quartile rankings [ 66 , 67 ], and Impact Factor [ 67 , 68 ]. These measures help in assessing the journals’ influence and relevance in the field accurately [ 58 , 63 ].
Country Analysis
A detailed analysis of the countries leading in global publication volume was performed using VOSviewer. The Scimago Graphica tool was used to create a world map illustrating publication volumes and regional densities. The gross domestic product of these countries was estimated and analyzed, taking into account data from the International Monetary Fund’s World Economic Outlook report [ 69 ]. In addition, the prevalence and mortality rates of dementia in these countries were examined by consulting reports from the World Health Organization’s Global Dementia Observatory [ 70 ] and age-standardized dementia mortality rates [ 71 ].
Analysis of Highly Cited Papers
On the basis of the local citation index, the top 10 highly cited papers were identified, and their standardized citation indices were calculated. The normalized citation score is derived by dividing the number of citations of a key paper by the average number of citations for comparable papers in the same field or subfield and publication year. A final impact score (normalized citation score) of >1 indicates that the paper’s citation rate is above the average for that field or subfield, while a score of <1 indicates that it is below average [ 72 ].
Author Keywords
High-frequency keywords were then clustered using gCLUTO based on their proximity, using hierarchical clustering with repeated bisection, and using the cosine function to calculate similarity. The clustering criterion function was set to I 2 , and the results were selected for display based on high internal similarity and low external similarity, with the results displayed using matrix and mound visualization techniques [ 73 ]. The selection of high-frequency keywords for clustering is based on the method described by Bai et al [ 74 ], which involves extracting keywords that cumulatively account for >30% of the total frequency. If the number of included keywords is <30, the threshold is adjusted to include high-frequency words that cumulatively account for >40% until the number exceeds 30. Building on this approach, we observed the importance of subsequent keywords and incorporated them appropriately.
Disciplinary Analysis
Through disciplinary analysis, we can gain a comprehensive understanding of the research content within a field and interdisciplinary collaborations. The fields of study form the subject classification scheme shared across all Web of Science product databases. Each document indexed in the Web of Science Core Collection is assigned to at least 1 subject category, which maps to a research field. Using VOSviewer, we constructed a disciplinary collaboration network to understand the distribution of disciplines within the field and the nature of interdisciplinary collaborations, where each node represents a discipline, and the connections between nodes represent collaborations among disciplines [ 75 ].
Ethical Considerations
Ethics committee approval was not required because this study was a retrospective bibliometric analysis of existing published studies.
The Annual Trends of Publications
Our study incorporated 1070 research papers, of which 993 (92.8%) were articles and 77 (7.2%) were reviews, indicating that the research in the field of dementia biomarkers using AI is primarily driven by original articles.
The change in publication volume reflects the dynamic development of this field. The earliest study on this topic dates back to 2007. In 2020, of the 1070 included papers, 131 (12.24%) were published (the 100-paper mark was crossed for the first time), and publication peaked at 229 (21.4%) papers in 2022. To visually represent the change in publication volume, we used a cubic trendline model. As shown in Figure 3 A, the red dashed line represents the fitted trendline, with an R ² value of 0.95760 and an adjusted R ² value of 0.94783, indicating a good model fit and accurately reflecting the growth trend in publication volume. On the basis of the trend analysis using Joinpoint software, 2 potential turning points were identified in the years 2018 and 2021. The slopes calculated for these periods are as follows: slope 1 (from 2007 to 2018)=4.02, slope 2 (from 2018 to 2021)=48.58, and slope 3 (from 2021 to 2023)=16.33. The differences in slopes between slope 1 and slope 2 as well as those between slope 2 and slope 3 have P values <.05, indicating significant changes in the growth trends, as illustrated in Figure 3 B.

On the basis of the changes in publication volume and slope, the development of this field can be preliminarily divided into 3 stages. The first stage (2007-2017) is the initiation stage, with 183 (17.1%) of the 1070 papers published during this period, and an annual publication volume not exceeding 50 papers (growth rate of 34.2%). The second stage (2018-2020) is marked by rapid growth, with 271 (25.33%) of the 1070 papers published during this period, and an annual publication volume not exceeding 100 papers (growth rate of 58.7%). The third stage (2021-2023) is characterized as a stable development phase, influenced by a larger publication base, with 616 (57.57%) of the 1070 papers published during this period (growth rate of 7.2%).
The participation of researchers in the field reflects the level of interest in it. A total of 6455 authors have been involved in publishing papers. The top 10 authors have collectively contributed 125 (1.35%) of the 9246 studies. Among them, Morris, JC, is the most prolific author (16/9246, 0.17%). Shen, DG, has the highest h-index and e-index scores among these prolific authors. The majority of these prolific authors (8/10, 80%) published their works between 2018 and 2023, while the publications of Shen, DG, and Zhang, DQ, are mainly concentrated between 2007 and 2017, as shown in Table 1 .
Adhering to the Price law, the minimum publication threshold for core authors is approximately 3 papers. Using VOSviewer for analysis, 663 (10.27%) of the 6455 core authors were identified, contributing a total of 2635 (28.5%) of the 9246 papers, which does not meet the standard of the Price law (>50%) [ 60 ]. In the collaboration network diagram, the co-occurrence network among core authors is relatively independent with fewer connections, indicating a pattern of high cohesion and low coupling. Networks centered around the top 10 most prolific authors are more developed compared to those of others, as illustrated in Figure 4 .
Figure 5 illustrates the annual influx of researchers into the field of AI in dementia biomarkers. Of the 6455 authors involved in publishing papers in the field, there were only 14 (0.22%) in 2007, while in 2023, the number of new researchers entering the field soared to 1208 (18.71%). The trend line indicates that there will be an increasing number of new researchers joining this field in the future. On the basis of the influx of new authors, the year 2019 was selected as a specific point in time [ 59 ] to identify new researchers and those who ceased their research in this area at the current stage. Among them, 5023 (77.81%) of the 6455 researchers are new to this field since 2019, and of the 1432 researchers who were active before 2019, a total of 994 (69.41%) ceased publishing after 2019. In addition, in exploring the demographics of new researchers, it was found that 372 (56.1%) of the 663 core authors identified by the Price law are newcomers to the field.
| Rank | Author | Output (n=9246), n (%) | h-index score | e-index score | g-index score | Period 1: 2007-2017, n (%) | Period 2: 2018-2020, n (%) | Period 3: 2021-2023, n (%) |
| 1 | Morris, JC | 16 (0.2) | 10 | 19.4 | 16 | 2 (12.5) | 3 (18.7) | 11 (68.7) |
| 2 | Jack, CR | 13 (0.1) | 9 | 20.1 | 13 | 3 (23.1) | 3 (23.1) | 7 (53.8) |
| 3 | Liu, Y | 13 (0.1) | 9 | 11.6 | 13 | 0 (0) | 5 (38.5) | 8 (61.5) |
| 4 | Saykin, AJ | 13 (0.1) | 9 | 30.4 | 13 | 4 (30.8) | 7 (53.8) | 2 (15.4) |
| 5 | Shen, DG | 13 (0.1) | 13 | 42.5 | 13 | 7 (53.8) | 6 (46.1) | 0 (0) |
| 6 | O’Bryant, SE | 12 (0.1) | 9 | 22.2 | 12 | 4 (33.3) | 3 (25) | 5 (41.7) |
| 7 | Zetterberg, H | 12 (0.1) | 9 | 17.6 | 12 | 0 (0) | 6 (50) | 6 (50) |
| 8 | Han, Y | 11 (0.1) | 8 | 14.3 | 11 | 1 (9.09) | 7 (63.6) | 3 (27.3) |
| 9 | Wang, L | 11 (0.1) | 6 | 13.4 | 11 | 2 (18.2) | 3 (27.3) | 6 (54.5) |
| 10 | Zhang, DQ | 11 (0.1) | 9 | 40.3 | 11 | 7 (63.6) | 2 (18.2) | 2 (18.2) |
a At least h papers have been cited h times each.
b The supplementary measure of the h-index score.
c The total citation count of the first g papers is ≥ g 2 .
d The denominator is the n value in “Output” column.

The journal analysis showcases the structure and characteristics of the field. A total of 362 journals have published relevant articles. Following the Bradford law [ 61 , 62 ], we identified 12 core journals in this field that collectively contributed 360 (33.6%) of the 1070 studies. Of these, the Journal of Alzheimer’s Disease (Netherlands) had the highest output with 22.7% (78/344) of the published papers. In terms of citation frequency, NeuroImage (United States) leads, with a citation percentage of 13.8% (3293/23,842), averaging 122 citations per paper. The journal with the highest impact factor is Alzheimer’s and Dementia (United States). These journals are all ranked in the top 2 quartiles of the Journal Citation Reports Quartile rankings and have achieved notable CiteScore 2022 and Scimago Journal Rank rankings, as shown in Table 2 .
The dual map overlay of the journals reveals the thematic distribution across academic journals ( Figure 6 ). Figure 6 A shows the citing journals, while Figure 6 B shows the cited journals; the colored paths indicate citation relationships. There are 5 cited paths: 2 yellow, 2 pink, and 1 green. The analysis indicates that papers in psychology, education, or sociology journals are often cited by journals from fields such as molecular biology, immunology, medicine, clinical studies, ophthalmology, kinesiology, and neurology. Similarly, papers from molecular biology, genetics, or genomics journals are often cited by journals from fields such as medicine, clinical studies, and neurology, highlighting the importance of interdisciplinary research.
| Rank | Journal | Output (n=1070), n (%) | Citations (n=23,842), n (%) | CiteScore 2022 | Impact Factor 2022 | JCR | SJR | Country |
| 1 | 78 (7.3) | 1445 (6.1) | 6.4 | 4.0 | Q 2 | 1.146 | Netherlands | |
| 2 | 58 (5.4) | 887 (3.7) | 5.2 | 4.8 | Q2 | 1.211 | Switzerland | |
| 3 | 34 (3.2) | 741 (3.1) | 7.5 | 4.6 | Q2 | 0.973 | United Kingdom | |
| 4 | 27 (2.5) | 3293 (13.8) | 11.6 | 5.7 | Q1 | 2.512 | United States | |
| 5 | 26 (2.4) | 826 (3.5) | 14.7 | 14.0 | Q1 | 3.288 | United States | |
| 6 | 26 (2.4) | 1140 (4.8) | 6.0 | 3.7 | Q2 | 0.885 | United States | |
| 7 | 21 (2.0) | 374 (1.6) | 12.0 | 9.0 | Q1 | 2.650 | United Kingdom | |
| 8 | 20 (1.9) | 369 (1.5) | 6.8 | 5.2 | Q2 | 1.161 | Switzerland | |
| 9 | 19 (1.8) | 652 (2.7) | 8.1 | 4.2 | Q2 | 1.395 | Netherlands | |
| 10 | 18 (1.7) | 670 (2.8) | 9.1 | 4.8 | Q1 | 1.688 | United States | |
| 11 | 17 (1.6) | 166 (0.7) | 9.0 | 3.9 | Q2 | 0.926 | United States | |
| 12 | 16 (1.5) | 192 (0.8) | 4.8 | 3.4 | Q2 | 0.978 | Switzerland |
a Impact factor based on Clarivate Analytics Journal Citation Report (2022).
b JCR: Journal Citation Reports.
c SJR: Scimago Journal Rank.
d Q: quartile ranking position.

Institutional Analysis
The institutional analysis reveals the organizational structure characteristics of research in the field of dementia biomarkers. A total of 1793 institutions have conducted research on AI in dementia biomarkers and published papers. The highest publishing volumes come from the University of Pennsylvania in Philadelphia, United States, which contributed 31 (0.9%) of the 3442 papers. The University of North Carolina Chapel Hill in North Carolina, United States, has the highest citation index, with 2235 (2.73%) of the 81,952 citations, averaging 111.8 citations per paper. Among the top 10 institutions in terms of publication volume, 5 (50%) are located in the United States, 3 (30%) in the United Kingdom, 2 (20%) in China, and 1 (10%) is the globally renowned Mayo Clinic in the United States, as shown in Table 3 .
To further explore the collaboration patterns among these institutions, we selected the top 100 institutions by publication volume (the list includes 102 institutions due to institutional ties, collectively publishing 992/3442, 28.82% of the papers, with a minimum publication count of 6) to construct a collaboration network map. The map reveals that most of these institutions (94/102, 92.2%) are research-intensive universities. Notably, institutions from China, the United States, and the United Kingdom form 3 major collaborative networks, with specific network relationships detailed in Figure 7 .
| Rank | Organization | Output (n=3442), n (%) | Citations (n=81,952), n (%) | PPC | Country |
| 1 | University of Pennsylvania | 31 (0.9) | 790 (0.96) | 25.5 | United States |
| 2 | University College London | 26 (0.76) | 682 (0.83) | 26.2 | United Kingdom |
| 3 | King’s College London | 25 (0.73) | 1931 (2.36) | 77.2 | United Kingdom |
| 4 | Mayo Clinic | 22 (0.64) | 640 (0.78) | 29.1 | United States |
| 5 | Capital Medical University | 21 (0.61) | 338 (0.41) | 16.1 | China |
| 6 | University of Cambridge | 21 (0.61) | 582 (0.71) | 27.7 | United Kingdom |
| 7 | University of California San Francisco | 20 (0.58) | 814 (0.99) | 40.7 | United States |
| 8 | University of North Carolina Chapel Hill | 20 (0.58) | 2235 (2.72) | 111.8 | United States |
| 9 | Chinese Academy of Sciences | 19 (0.55) | 467 (0.57) | 24.6 | China |
| 10 | Washington University | 17 (0.49) | 623 (0.76) | 36.6 | United States |
a PPC: per-paper citations.

The participation of 74 countries in dementia biomarker research highlights the global interest in the topic. The 10 most productive countries contributed 1216 (69.64%) of the 1746 papers and 1110 (61.91%) of the 1793 research institutions. The United States led in publication and citation counts with 346 (19.82%) of the 1746 papers and 10,745 (25.28%) of the 42,496 citations. China had the most research institutions (281/1793, 15.67%). South Korea had a dementia prevalence rate of 7%, China 4.5%, and India 3.7%. The standardized dementia mortality rates in the United States and the United Kingdom were higher than in other countries, as detailed in Table 4 .
A visualization map was created using Scimago Graphica software to display the level of attention different regions pay to the field. In the map, the size of the circles and the color of the circles represent the publication volume of each country. The European region shows a higher interest in this field than other continents, with 30 countries participating in publishing research, as seen in Figure 8 .
A chord diagram of international collaboration based on the number of joint papers was produced. The lines represent collaborative relationships between countries, with the width indicating the strength of collaboration. Each country’s end point on its own axis represents its total number of collaborations with other countries. Among the top 10 productive countries, the United States is at the core of a network covering 69% (51/74) of the countries, with 377 collaborations; the United Kingdom covers 66% (49/74) of the countries, with 366 collaborations; and China covers 42% (31/74) of the countries, with 191 collaborations, as illustrated in Figure 9 .
| Rank | Country | Output (n=1746), n (%) | Citations (n=42,496), n (%) | Organizations (n=1793), n (%) | 2023 GDP rank | Partner countries (n=74), n (%) | Prevalence rate (%) | Mortality rate , n (‱) |
| 1 | United States | 346 (19.82) | 10,745 (25.28) | 241 (13.44) | 1 | 51 (68.9) | 6.4 | 3.33 |
| 2 | China | 282 (16.15) | 3782 (8.9) | 281 (15.67) | 2 | 31 (41.9) | 4.5 | 1.74 |
| 3 | United Kingdom | 143 (8.19) | 5079 (11.95) | 82 (4.57) | 6 | 49 (66.2) | — | 4.27 |
| 4 | Italy | 79 (4.52) | 2687 (6.32) | 103 (5.74) | 8 | 36 (48.6) | 6.9 | 1.49 |
| 5 | South Korea | 70 (4.01) | 1355 (3.19) | 52 (2.9) | 13 | 31 (41.9) | 7 | 1.63 |
| 6 | India | 70 (4.01) | 920 (2.16) | 95 (5.3) | 5 | 15 (20.3) | 3.7 | 1.46 |
| 7 | Germany | 65 (3.72) | 2376 (5.59) | 79 (4.41) | 3 | 34 (45.9) | 6.9 | 1.55 |
| 8 | Spain | 58 (3.32) | 912 (2.15) | 87 (4.85) | 15 | 32 (43.2) | — | 2.15 |
| 9 | Canada | 57 (3.26) | 1170 (2.75) | 43 (2.4) | 10 | 28 (37.8) | 6.4 | 2.79 |
| 10 | Australia | 46 (2.63) | 1786 (4.2) | 47 (2.62) | 14 | 40 (54.1) | 6.7 | 2.26 |
a GDP: gross domestic product.
b The World Health Organization’s Global Dementia Observatory’s estimate of the unstandardized prevalence rate of dementia in the Global Burden of Disease region report for the year 2017.
c The World Health Organization’s age-standardized dementia mortality rates per 100,000 population in 2019 by country.
d Not available.

Fund Analysis
The funding situation for projects in this field is a key indicator of the level of investment and government emphasis in each country. The study identified 450 funding projects providing 1604 instances of support for such research. Upon reviewing the top 10 funding projects with the most contributions, it was found that 5 (50%) are from the United States, 2 (20%) from China, 1 (10%) each from South Korea and the United Kingdom, and 2 (20%) from international organizations. Notably, of the 1604 studies in this area, the National Institutes of Health in the United States provided funding for 161 (10.04%), and the ADNI funded 135 (8.42%). Detailed information can be found in Table 5 .
| Rank | Funders | Studies, n (%) | Country |
| 1 | National Institutes of Health | 161 (10.03) | United States |
| 2 | Alzheimer’s Disease Neuroimaging Initiative | 135 (8.42) | United States |
| 3 | National Natural Science Foundation of China | 119 (7.41) | China |
| 4 | Department of Defense–Alzheimer’s Disease Neuroimaging Initiative | 118 (7.36) | United States |
| 5 | National Institute on Aging | 61 (3.8) | United States |
| 6 | National Research Foundation of Korea | 28 (1.75) | South Korea |
| 7 | Medical Research Council | 26 (1.62) | United Kingdom |
| 8 | National Key Research and Development Program of China | 23 (1.43) | China |
| 9 | Alzheimer’s Association | 21 (1.31) | United States |
| 10 | European Union | 16 (1) | — |
a Not applicable.
Compared to global citations, local citations, or peer citations, more accurately reflect the academic community’s recognition and importance of specific articles locally, as well as the influence, quality, and collaboration status of the literature in local academic research [ 75 , 76 ]. The top 10 high-value publications, based on local citations, accumulated a total of 392 (18.2%) of the 2157 local peer citations, averaging 39.2 citations per year for each publication. The local and global normalized citation indices for these studies are both >1, indicating that their citation rates exceed the average level for research published in the same year. Of the 10 highly cited papers, 8 (80%) were published between 2007 and 2017 (for detailed information, refer to Table 6 ).
A deeper analysis of these 10 highly cited papers revealed valuable information regarding their specific tasks and research outcomes. Of the 10 papers, 1 (10%) is a review paper [ 11 ], and 9 (90%) are research papers [ 77 - 85 ]. These studies predominantly conducted binary classification analyses using the ADNI data set, with 9 (90%) of the 10 papers using multimodal biomarkers. Of the 10 papers, 8 (80%) applied ML methods, and 2 (20%) used deep learning techniques. These studies detailed their methods for classifying specific diseases; the types of biomarkers used; and the accuracy, sensitivity, specificity, and fitting of their classification tasks. However, not all studies reported these specific values in detail. More details about these studies can be found in Table 7 and Multimedia Appendix 6 . The top 10 locally normalized cited documents can be found in Multimedia Appendix 7 .
| Rank | Article title | Study | LCS (n=2157), n (%) | GCS (n=23,842), n (%) | NLCS | NGCS | PY |
| 1 | Multimodal classification of Alzheimer’s disease and mild cognitive impairment | Zhang et al [ ] | 109 (5.05) | 883 (3.7) | 7.6 | 6.0 | 2011 |
| 2 | Machine learning framework for early MRI-based Alzheimer’s conversion prediction in MCI subjects | Moradi et al [ ] | 52 (2.41) | 421 (1.77) | 7.7 | 5.2 | 2015 |
| 3 | Multi-modal multi-task learning for joint prediction of multiple regression and classification variables in Alzheimer’s disease | Zhang and Shen [ ] | 40 (1.85) | 453 (1.9) | 6.2 | 4.0 | 2012 |
| 4 | Deep learning in Alzheimer’s disease: diagnostic classification and prognostic prediction using neuroimaging data | Jo et al [ ] | 35 (1.62) | 229 (0.96) | 9.3 | 6.9 | 2019 |
| 5 | Accurate multimodal probabilistic prediction of conversion to Alzheimer’s disease in patients with mild cognitive impairment | Young et al [ ] | 31 (1.44) | 183 (0.77) | 6.4 | 3.2 | 2013 |
| 6 | Predicting Alzheimer’s disease progression using multi-modal deep learning approach | Lee et al [ ] | 29 (1.34) | 158 (0.66) | 7.7 | 4.8 | 2019 |
| 7 | Random forest–based similarity measures for multi-modal classification of Alzheimer’s disease | Gray et al [ ] | 25 (1.16) | 306 (1.28) | 5.1 | 5.4 | 2013 |
| 8 | Multimodal neuroimaging feature learning for multiclass diagnosis of Alzheimer’s disease | Liu et al [ ] | 25 (1.16) | 323 (1.35) | 3.7 | 4.0 | 2015 |
| 9 | Spatially augmented LPboosting for AD classification with evaluations on the ADNI dataset | Hinrichs et al [ ] | 23 (1.07) | 157 (0.66) | 2.0 | 1.7 | 2009 |
| 10 | Early detection of Alzheimer’s disease using MRI hippocampal texture | Sorensen et al [ ] | 23 (1.07) | 120 (0.5) | 6.4 | 2.9 | 2016 |
a LCS: local citation score.
b GCS: global citation score.
c NLCS: normalized local citation score.
d NGCS: normalized global citation score.
e PY: publication year.
| Study | PY | Database | Classifier | Input features |
| Hinrichs et al [ ] | 2009 | ADNI | Spatially augmented LPboosting | MRI +FDG-PET |
| Zhang et al [ ] | 2011 | ADNI | Multiple-kernel SVM | MRI+PET+CSF |
| Zhang and Shen [ ] | 2012 | ADNI | M3T | MRI+PET+CSF |
| Young et al [ ] | 2013 | ADNI | SVM+GP | MRI+FDG-PET+CSF+APOE |
| Gray et al [ ] | 2013 | ADNI | Random forest | MRI+FDG-PET+CSF+APOE |
| Moradi et al [ ] | 2015 | ADNI | LDS +random forest | MRI+aggregate biomarker |
| Liu et al [ ] | 2015 | ADNI | SAE +softmax regression+SVM | MRI+FDG-PET |
| Sorensen et al [ ] | 2016 | ADNI+AIBL +Metropolit | SVM+logistic regression | MRI+CSF |
| Lee et al [ ] | 2019 | ADNI | CNN | MRI+CSF+APOE |
a PY: publication year.
b ADNI: Alzheimer’s Disease Neuroimaging Initiative.
c LPboosting: linear programming boosting.
d MRI: magnetic resonance imaging.
e FDG-PET: fluorodeoxyglucose positron emission tomography.
f SVM: support vector machine.
g CSF: cerebrospinal fluid.
h M3T: multimodal multitask.
i GP: Gaussian process.
j APOE: apolipoprotein E.
k LDS: low density separation.
l SAE: stacked autoencoder.
m AIBL: Australian Imaging, Biomarker & Lifestyle.
n CNN: convolutional neural network.
Analysis of Author Keywords
By analyzing keywords in a specific field, we can gain insights into its research directions and trends. In this study, the most frequent keywords identified were “Alzheimer’s disease” (603/5467, 11.03%), “machine learning” (302/5467, 5.52%), “mild cognitive impairment” (166/5467, 3.04%), “biomarker” (152/5467, 2.78%), and “deep learning” (127/5467, 2.32%). Notably, “Alzheimer’s disease,” “mild cognitive impairment,” “biomarker,” and “magnetic resonance imaging” were high-frequency keywords used consistently throughout all 3 stages (2007-2023), while “deep learning” emerged in the first stage (2007-2017) and increased in the third stage (2021-2023), as shown in Table 8 . A detailed time-segmented analysis of the 20 high-frequency keywords was conducted, resulting in a heat map where lighter blue indicates lower frequency in a given year and deep red indicates higher frequency; for instance, “artificial neural networks” appeared as early as 2007, decreased in frequency, and then consistently appeared at a high frequency in recent years. The keyword “Alzheimer’s disease” shows a progressive increase in occurrences each year. Nearly all keywords shifted toward orange and red in 2021 and through the third phase (2021-2023). However, the keyword “support vector machine” changed from orange-red to light blue in 2023. In addition, as classification is one of the primary tasks of AI, its frequency of appearance has remained stable annually, as seen in Figure 10 .
| Rank | Keyword | Occurrences (N=5467), n (%) | Period 1 (2007-2017), n (%) | Period 2 (2018-2020), n (%) | Period 3 (2021-2023), n (%) |
| 1 | Alzheimer’s disease | 603 (11.03) | 102 (16.92) | 143 (23.71) | 358 (59.37) |
| 2 | Machine learning | 302 (5.52) | 24 (7.95) | 77 (25.5) | 201 (66.56) |
| 3 | Mild cognitive impairment | 166 (3.04) | 43 (25.9) | 40 (24.1) | 83 (50) |
| 4 | Biomarker | 153 (2.8) | 34 (22.22) | 33 (21.57) | 86 (56.21) |
| 5 | Deep learning | 128 (2.34) | 3 (2.34) | 25 (19.53) | 100 (78.13) |
| 6 | Magnetic resonance imaging | 126 (2.3) | 31 (24.6) | 29 (23.02) | 66 (52.38) |
| 7 | Dementia | 83 (1.52) | 10 (12.05) | 22 (26.51) | 51 (61.45) |
| 8 | Support vector machine | 78 (1.43) | 23 (29.49) | 22 (28.21) | 33 (42.31) |
| 9 | Classification | 57 (1.04) | 22 (38.6) | 17 (29.82) | 18 (31.58) |
| 10 | Artificial Intelligence | 48 (0.88) | 1 (2.08) | 9 (18.75) | 38 (79.17) |
| 11 | Convolutional neural network | 43 (0.79) | 0 (0) | 10 (23.26) | 33 (76.74) |
| 12 | Neuroimaging | 39 (0.71) | 8 (20.51) | 12 (30.77) | 19 (48.72) |
| 13 | Random forest | 39 (0.71) | 4 (10.26) | 10 (25.64) | 25 (64.1) |
| 14 | Diagnosis | 31 (0.57) | 7 (22.58) | 5 (16.13) | 19 (61.29) |
| 15 | Feature selection | 31 (0.57) | 10 (32.26) | 4 (12.9) | 17 (54.84) |
| 16 | Amyloid-Beta | 28 (0.51) | 7 (25) | 3 (10.71) | 18 (64.29) |
| 17 | Cerebrospinal fluid biomarker | 25 (0.46) | 7 (28) | 5 (20) | 13 (52) |
| 18 | Amyloid | 24 (0.44) | 5 (20.83) | 5 (20.83) | 14 (58.33) |
| 19 | Artificial neural network | 24 (0.44) | 4 (16.67) | 8 (33.33) | 12 (50) |
| 20 | Structural magnetic resonance imaging | 24 (0.44) | 8 (33.33) | 9 (37.5) | 7 (29.17) |
a The denominator is the n value in “Occurrences” column.

Analysis of Keyword Clusters
Identifying keyword clusters allows for an intuitive understanding of subfields within specific research areas. A total of 36 high-frequency keywords were included for clustering. These keywords accounted for 41.92% (2292/5467) of the occurrences, meeting the requirements for clustering. High-frequency keywords were analyzed using gCLUTO software to generate dendrograms and mound maps, revealing 6 distinct clusters. Each mound represents a unique cluster, with its height and volume indicating the similarity and number of documents, respectively. The colors on the mound tops signify different levels of internal SDs, with red indicating low internal SD and blue high internal SD [ 73 ]. The tops of these 6 mounds are not blue, indicating no high internal SD, especially in clusters 0 and 4, where the peaks are red and the internal SDs are lower, as shown in Figure 11 .

In the dendrogram, the depth of the color blocks indicates the strength of the association between the keywords on the vertical axis and those on the horizontal axis. Deep red signifies a high association strength, while white indicates a lower association strength. The dendrogram shows that AI research hot spots in dementia biomarkers primarily focus on diseases such as “Alzheimer’s disease,” “Dementia with Lewy bodies,” “mild cognitive impairment,” and “frontotemporal dementia.” Cluster 4 is the largest cluster, containing 10 keywords that can be categorized into 3 aspects: AI (“artificial neural network,” “machine learning,” “diagnosis,” and “feature extraction”), diseases (“Alzheimer’s disease,” “Parkinson’s disease,” “disease,” and “Dementia with Lewy bodies”), and biomarkers (“Electroencephalogram” and “Electroencephalography”). The theme reflected here is the application of neural networks in neurodegenerative diseases, with EEG features used for diagnosing such diseases. Cluster 5 includes 8 keywords, divided into 2 aspects: algorithms (“random forests,” “support vector machines,” “classification,” and “feature selection”) and biomarkers (“structural magnetic resonance imaging” “ADNI,” “mild cognitive impairment,” and “radiomics”). This cluster reflects the theme of traditional ML algorithms classifying biomarkers in neuroimaging. Cluster 0, the smallest cluster, contains just 3 keywords, succinctly summarizing the application of AI in FTD. Cluster 2 consists of 6 keywords mainly related to CSF biomarkers: “tau,” “beta-amyloid,” and “proteomics.” This cluster highlights the primary protein markers in CSF. Cluster 1 contains 5 keywords, divided into deep learning and imaging biomarkers. Deep learning (“deep learning,” “transfer learning” and “Convolutional Neural Network”) and imaging markers (“magnetic resonance imaging” and “hippocampus”) reflect the application of nontraditional ML methods in imaging biomarkers. Cluster 3 contains 4 keywords related to imaging markers, as shown in Figure 12 .

We identified cross-disciplinary connections among 46 subjects, finding that each paper involved an average of 1.55 disciplines. Neuroscience and neurology (524/1661, 31.55%) were the most frequently involved disciplines, significantly more than other subjects. Engineering (128/1661, 7.71%) and computer science (126/1661, 7.59%) followed, highlighting the central role of neuroscience in this research area. Network analysis revealed 117 interdisciplinary connections, most of which were weak, indicating that direct collaboration between different disciplines is relatively limited. By contrast, collaborations within the same disciplinary group were more frequent. Specifically, the connections between neurology and geriatric medicine were the closest, followed by radiology, nuclear medicine, and medical imaging. Computer science was most closely connected to engineering. However, the connection strength between the neurosciences representing AD and the engineering and computer sciences representing AD appeared to be weak, suggesting that interdisciplinary research between these 2 fields has potential for growth, as shown in Figure 13 .

Biomarker and AI Method Analysis
Given that review articles often cover algorithms and biomarkers that overlap with those discussed in research literature, we focused on the content of 993 articles to classify biomarkers into 9 major categories based on their sources and characteristics: imaging biomarkers, CSF biomarkers, genetic markers, blood biomarkers, digital biomarkers, ophthalmic and retinal markers, neurophysiological markers, fecal and other bodily fluid markers, and other types of markers. Among the 993 studies, 973 (98%) addressed AD, 32 (3.2%) discussed FTD, 17 (1.7%) referenced DLB, and 10 (1%) focused on VaD. Overall, the main biomarkers across these subtypes were imaging, genetic, CSF, and blood biomarkers, each mentioned >100 times. Specifically, of the 1060 citations, imaging biomarkers were cited 473 (44.62%) times, genetic biomarkers 187 (17.64%) times, CSF biomarkers 148 (13.96%) times, and blood biomarkers 111 (10.47%) times.
In terms of trends, the use of AD biomarkers has been notably increasing year by year, with imaging biomarkers consistently being the most used annually. The use of genetic biomarkers surged in 2021, surpassing both CSF and blood biomarkers. CSF biomarkers have shown a fluctuating upward trend, while the use of blood biomarkers has gradually increased, recently approaching the use levels of CSF biomarkers. In addition, after 2018, various types of biomarkers have shown some intermittent growth trends. Among the other 3 subtypes, only the imaging biomarkers for FTD and the CSF biomarkers for DLB exhibited brief spikes in growth in 2022 and 2020, respectively. The trends for the other subtypes are not as pronounced, as shown in Figure 14 .
The AI methods extracted from the literature were categorized into 2 main classes: supervised learning and unsupervised learning, further subdivided according to the tasks performed. In this field, classification tasks predominate. Among the algorithms used for the 4 subtypes of dementia, support vector machines (SVMs; 302/1581, 19.1%) were the most frequently applied. Various neural network algorithms (229/1581, 14.48%) ranked second overall, followed by random forests (221/1581, 13.98%). However, it is noteworthy that in 2023, SVMs were used 52 times, a stark contrast to their mere 2 mentions in keyword heat map analyses.
Regarding trends in algorithm use for AD, there has been a noticeable increase over time. Neural networks started to become popular after 2018 and surpassed SVMs by 2022. Since 2016, random forests have been used nearly as frequently as SVMs. In addition, after 2018, various types of algorithms have demonstrated a clear growth trend. In the other 3 subtypes, although there is a slight growth trend in algorithm use for FTD, the use of algorithms in DLB and VaD has not shown a significant growth trend, as depicted in Figure 15 .
In the co-occurrence network of biomarkers and the 20 most commonly used AI methods, the thickness of the lines and the depth of their colors intuitively reflect the frequency and strength of their associations: thicker lines and darker colors indicate higher co-occurrence frequency and tighter connections ( Figure 16 ). Overall, clustering, regression, and dimension reduction algorithms are significantly less used in various types of biomarkers than classification algorithms. In AD, only 2 clustering algorithms appear among the top 20 most frequently used, with no use in other subtypes.

In each dementia subtype, the connections between classification algorithms and biomarkers are generally thicker and darker, especially the link between SVMs and imaging biomarkers in AD, followed by the connection between neural networks and imaging biomarkers. The thickest line in blood biomarkers is associated with random forests. In the other 3 subtypes, the connections between algorithms and biomarkers are weaker, particularly in VaD. The variety of algorithms used in FTD is second only to those used in AD, with the most notable associations being between imaging biomarkers and SVMs, which is also observed in VaD. In DLB, random forests appear to be more frequently used with imaging and CSF biomarkers, as illustrated in Figure 16 .
Discoveries of New Biomarkers
Overall, there have been significant new findings in dementia biomarkers. A total of 244 research reports have identified new biomarkers: 231 (94.7%) for AD, 3 (1.2%) for FTD, 5 (2%) for DLB, and 5 (2%) for VaD. Of these, 211 (86.5%) new biomarkers were discovered after 2018. Among these 211 biomarkers, imaging biomarkers and genetic biomarkers have been found most frequently, with 68 (32.2%) and 70 (33.2%) new findings, respectively, followed by blood biomarkers with 34 (16.1%) new findings. New biomarkers in emerging areas such as ophthalmology and retinal studies as well as digital biomarkers have also been identified in recent years, as shown in Figure 17 .

Compared to other bibliometric studies on dementia biomarkers [ 23 , 24 ], our research not only reveals basic data, such as publication volumes, institutions, and national trends, but also delves deeply into the phenomena of author turnover and collaboration network flaws and more specifically highlights the contributions of prolific authors and key national efforts. In addition, we have successfully captured and quantified the developmental trends and dynamics of various biomarkers. In contrast to another study [ 12 ], we have detailed the contributions of various algorithms in this domain and followed the latest advances in biomarkers. Our analysis supports earlier research [ 31 , 33 ] regarding the prevalence of SVMs in imaging biomarkers and further augments the significance of other algorithms in biomarker research. Specifically, through mining analyses of high-frequency author keywords, keyword clustering, and literature content, we identified research hot spots, including the diagnosis and classification of dementia subtypes and neurodegenerative diseases, an exploration of CSF proteomic markers, and the application of traditional algorithms and neural networks in imaging biomarkers. SVMs, neural networks, and random forests are widely used as popular algorithms. Random forests are most frequently used in blood and genetic biomarkers. Newly discovered biomarkers primarily focus on imaging, genetics, and blood domains. We discuss these key findings in detail in the following subsections.
In dividing the development stages of research on AI in dementia biomarkers, the analysis went beyond just publication volume and annual growth rates. It also considered key factors such as changes in publication numbers of prolific authors, fluctuations in high-frequency keywords each year, and the evolution of algorithms observed in 973 research papers. This comprehensive analysis supported the definition of 3 development stages, outlined in the following subsections.
Initial Exploration and Methodological Advances (2007-2017)
This stage is characterized by limited publications and growing interest in AI in dementia biomarkers. Key reasons included nascent AI technology in the field, limited availability of data sets [ 12 , 49 , 86 - 88 ], and immature development of biomarkers; for example, early PET radioactive tracers were not yet capable of specifically measuring the burden of neurofibrillary tangles and other tau protein abnormalities [ 89 ].
Rapid Development Period (2018-2020)
This stage marked a turning point with a surge in high-quality research methods. This was driven by the rise of deep learning [ 90 ], multimodal biomarker use [ 91 ], and expansion of public data sets (eg, ADNI) [ 92 , 93 ].

Stable Development Period (2021-2023)
This stage is characterized by a substantial increase in research volume, indicating a period of fast growth. Advances in image segmentation [ 94 ], deep learning algorithms [ 95 - 97 ], large public data sets [ 12 ], and digital biomarkers [ 98 ] contributed to this growth.
Enhance Collaboration Among Authors and Maintain Their Interest in Research
The field in question has attracted considerable attention from researchers, with the majority being newcomers who entered after 2019 in particular. This influx of new researchers indicates a strong interest within the scientific community toward this field. According to the Price law, the current output from core authors has not yet reached 50% of the total output, suggesting that a core group of authors has not been fully established. More than half of the current core authors (372/663, 56.1%) are new researchers from recent years, an indication perhaps that more researchers will emerge as leading figures in this domain. However, an important observation is that 69.41% (994/1432) of the researchers active before 2019 have not continued to produce related research, potentially indicating a decline in interest or a shift in research focus. While the contributions of most authors may be transient, a small number of researchers, such as the 10 highly productive researchers identified, have maintained consistent research output. Sustained knowledge accumulation in a research field greatly relies on ongoing studies and the establishment of a core group of authors [ 59 ].
Furthermore, establishing collaboration networks is a critical issue. Although most researchers (451/663, 68%) have formed collaborative groups, the majority of these networks (39/57, 68%) are still underdeveloped. Given the potential of AI in processing and analyzing large-scale biomedical data, as well as the need for the validation and correct use of new biomarkers, close collaboration among computer scientists, neuroscientists, and biostatisticians becomes particularly important [ 99 ]. The Brookings Institution in the United States also highlights the critical role of interdisciplinary collaboration in research innovation and standard setting within the AI field [ 100 ]. Therefore, both core authors and new researchers need to strengthen collaborations, especially interdisciplinary ones. New researchers, in particular, face challenges such as geography and costs in the process of interdisciplinary collaboration [ 101 , 102 ], and they often lack a deep understanding of other disciplines, which hinders the smooth progress of collaboration.
Interdisciplinary Collaborative Innovation
In the construction of cross-industry innovation systems between AI and medicine, AI often plays the role of outbound innovation, introducing AI technologies into the medical field. Conversely, the medical sector tends to embrace inbound innovation, adopting AI to address medical issues. This division primarily stems from the medical sector’s needs for diagnosis and treatment [ 102 ]. However, the ultimate goal is to achieve a close integration of both domains, advancing the integration of science and technology by developing new knowledge through collaboration with partners from various industries [ 103 ].
In the medical field, leadership teams proactively seek external knowledge based on their experience and standards to build interdisciplinary collaborations; for example, radiomics research teams can seek collaboration with partners skilled in image segmentation techniques. In addition, the shift from a closed to an open team model is crucial and involves adopting analogical thinking. This approach can draw from successful interdisciplinary collaborations already established in the medical field; for instance, the field of cardiology has set a commendable example with its multi-institutional interdisciplinary collaborations on AI [ 104 ]. For the AI sector, the main challenges lie in technological support and innovation, necessitating enhancements to algorithms and the development of new technological frameworks in response to medical needs. This not only requires medical knowledge but also entails the acquisition, assimilation, transformation, and development of knowledge within interdisciplinary teams. These learning processes demand active participation from team members and standardized sharing of information and knowledge, thereby facilitating advances in AI and its commercialization. Establishing connections between different disciplinary teams and building bridges for communication across fields are essential starting points. Cross-disciplinary academic conferences and web-based public courses serve as effective means to construct initial cooperative bridges. In addition, the establishment of cross-departmental digital platforms enables researchers to access and collaboratively analyze existing research data, exemplified by several searchable professional websites related to AI medical devices [ 105 ], fostering the development of tacit cooperation. Furthermore, several forward-thinking higher education institutions have already begun to informally incorporate the principles of AI into undergraduate courses through lectures. A new graduate module on radiology AI has also been established [ 106 ]. At Stanford University in Stanford, California, United States, leaders across various disciplines have formed interdisciplinary teams dedicated to teaching and researching AI to address health care issues [ 107 ].
Despite these measures aiding in the establishment of initial collaborative networks, the involvement of government and social enterprises as intermediaries is necessary to overcome informational disparities and promote deeper exchanges. Forming multidisciplinary societies, such as dedicated biomarker research associations, and enhancing interdisciplinary integration through research funding and incentive mechanisms are crucial measures to foster cooperation. The participation of diverse organizations, including universities, medical institutions, and corporations, will provide a broader scope and vision for the development of these associations. Finally, we also advocate for interdisciplinary information exchange within the respective fields of medicine and AI. Although this may provoke some potential internal competition, the convenience of this communication method and the potential for innovative benefits significantly outweigh the challenges it presents.
Regional Proximity Collaboration
Regional proximity has long been recognized as a crucial objective factor influencing innovation activities. Participants concentrated in a specific area benefit from the knowledge externalities produced by short distances, facilitating the exchange of knowledge between proximate entities and thereby fostering the development of innovation and the flow of tacit knowledge [ 108 ]. The convenience of such networks, coupled with cultural and institutional similarities, helps to keep cooperative networks vibrant [ 109 ]. For newcomers to the field, considering the advantages brought by regional proximity is key to building a stable foundational cooperative network. As the importance of complementary capabilities in partners continues to increase [ 110 ], seeking technological complementarity has become essential for maintaining active and robust cooperative networks. Particularly in the field of dementia research, the high heterogeneity of the disease requires us to construct knowledge networks from a global perspective, making full use of the differences in AI technologies across different countries. Relying solely on cooperation networks within a single country may overlook the value of global and nonlocalized knowledge networks, hindering the further integration of technology; for example, constructing diversified data sets will benefit from the inclusion of different regions and ethnicities. For transnational collaboration, the successful cases across multiple European countries serve as instructive examples. These nations have demonstrated the advantages of collaboration facilitated by regional proximity. Moreover, collaborating with high-output countries in the field is also a wise choice because these countries typically possess advanced technology and extensive resources. These nations are distributed across various continents, playing a significant radiative role, thus providing a more diversified array of options for establishing cooperative networks. Therefore, we recommend building foundational cooperative networks based on the principle of regional proximity and actively seeking partnerships with technologically leading countries to stimulate sustained network activity. In addition, governments and research institutions should support the construction of these transnational cooperative networks by increasing research funding and establishing incentive mechanisms to ensure the continuity and development of research.
Preferred Journals
In the field of dementia biomarkers, AI-related research has identified 12 core journals. These journals rank well across multiple platforms, reflecting the favorability of AI research in dementia biomarkers among numerous prestigious publications, including well-known journals such as Alzheimer’s & Dementia and NeuroImage . Dual-map overlays of the journals indicate extensive coverage of topics such as psychology, education, molecular biology, medicine, genetics, and immunology in this field. Therefore, scholars eager to delve into AI in dementia biomarkers should follow these high-output, influential journals. Simultaneously, they should explore interdisciplinary reports aligned with their research interests and content. This approach will help them comprehensively understand the latest developments and trends in the field.
Leading Countries and International Collaboration
Currently, dementia biomarker research involving AI has seen participation from 74 countries worldwide, demonstrating widespread international interest. In particular, the European region exhibits a higher level of attention toward this type of research, which correlates with its dementia incidence rates exceeding the global average at 1123 cases per 10,000 individuals [ 111 ], underscoring the urgent need to address this challenging issue. Similarly, the higher rates of dementia incidence and mortality in the majority of high-producing countries reflect how research is influenced by the dementia situation in each country. However, the concentration of research activities is closely related to the scientific capabilities and resource allocation of specific countries. The leading positions of the United States, China, and the United Kingdom in this field not only reflect these countries’ strong capabilities in research infrastructure, funding support, and technological innovation but also highlight their proactive roles in addressing global health challenges. This situation also suggests a potential issue of uneven resource distribution globally and the challenges other countries and regions may face in enhancing their research capabilities.
Therefore, to further enhance the contribution and impact of global research on dementia biomarkers, it is necessary to take measures to strengthen international cooperation, promote resource sharing, and encourage countries to increase research investment, especially in countries and regions with fewer resources. Fortunately, in terms of international collaboration, most high-producing countries have >100 instances of cross-border cooperation, indicating a strong willingness for international collaboration, particularly the United States and the United Kingdom, which lead not only in the number of countries they collaborate with but also in the frequency of such collaborations. Their implementation of AI in health care provides guidance for development and regulation for other countries; for example, the National Institutes of Health in the United States, in collaboration with multiple countries, has established one of the largest public AD data set in the world (ADNI) [ 112 ], offering data support for numerous studies. The United Kingdom’s Code of Conduct for Data-Driven Health and Care Technology provides funding, research opportunities, and tools for researchers in low- and middle-income countries, encouraging their participation in AI research and fostering connections [ 113 ]. By contrast, although China is the second largest producer of research outputs globally, it has fewer instances of international collaboration. This is related to China’s later start in AI compared to the United States and the United Kingdom, with its current AI strategy focusing more on the localization and training of AI talents [ 114 ], and international cooperation has not yet fully taken off. However, it cannot be denied that China possesses many research institutions and leading funding support, harboring significant potential for international collaboration that will play a substantial role in future international efforts.
For researchers, this information is valuable for considering international collaborations, applying for visiting scholar positions, or participating in educational projects. For nations, actively engaging with leading countries in this field and establishing collaborations can foster development in this area, particularly for low- and middle-income countries that have high dementia rates but lack AI technology.
Highly Cited Papers
A substantial body of ML research has focused on integrating brain imaging with both structured and unstructured clinical data to predict disease progression. The integration of multimodal data, such as imaging, CSF biomarkers, and demographic data, is considered one of the best approaches to address data heterogeneity [ 115 ]. This study identified 10 highly cited papers that provide significant insights into the analysis and application of various multimodal biomarkers, especially in terms of feature selection and the construction of new multimodal data sets. Among them, Zhang et al [ 77 ] adopted a multimodal classification strategy and a multikernel SVM in 2011 to enhance the classification performance for AD and mild cognitive impairment, demonstrating higher accuracy and sensitivity. This approach, based on the construction and kernel combination of multimodal heterogeneous biological data, overcomes the limitations of traditional studies that rely on a single biomarker, offering a more comprehensive and precise analytical framework. Subsequently, Zhang and Shen [ 79 ] introduced a method that combines multimodal data and multitask learning to jointly predict multivariate regression and classification variables from baseline multimodal data, providing new perspectives and tools for subsequent research. Gray et al [ 82 ] used the random forest algorithm to extract paired similarity measures, constructing a manifold-based representation that integrates information from multiple modalities. This approach enhances the accuracy and efficiency of classification. For newcomers to this field, a thorough examination of these high-value publications will facilitate a deeper understanding and inspiration.
Research Trends in Clusters of Highly Productive Authors
Since 2018, research on various biomarkers has been gradually increasing. Among them, 10 prolific authors are noteworthy. The research themes of Shen, DG, and Zhang, DQ, are highly similar. Their research teams mainly focus on imaging biomarkers, particularly the multimodal fusion of brain structural data, and they lean toward algorithmic improvements. Their research, initially centered on multikernel SVM studies, has progressively shifted toward studies using deep learning architectures [ 116 ]. Similarly, the research team led by Zetterberg [ 117 ] also shows interest in imaging biomarkers but tends toward newer biomarkers. In recent research, a deep learning–based model developed by them used computed tomography imaging biomarkers to distinguish people with dementia from healthy individuals with performance similar to MRI [ 117 ]. In addition, in brain age difference studies, the use of algorithms such as extreme gradient boosting has revealed a positive correlation between brain age difference and NFL [ 118 ]. Jack, CR, also exhibits a certain interest in imaging biomarkers; he used SVMs to achieve multimodal fusion of imaging data as early as 2010 [ 119 ]. However, in recent years, his research interests have diversified: he is not only using deep learning for predicting brain age [ 120 ] but also exploring blood biomarkers [ 121 ] and genetic biomarkers [ 122 ], which have become increasingly popular in recent years.
Furthermore, the collaborative group led by Morris, JC, is the most prolific in recent years, with their research primarily focused after 2021. Moreover, they seem to have a broader interest in emerging biomarkers such as imaging-based brain age difference [ 123 ], digital biomarkers based on driving behavior [ 124 ], gut microbiota [ 125 ], and MTBR-tau243 in CSF [ 126 ]. The research team led by Saykin, AJ, has been more focused on genetic biomarkers in recent years. They have used deep learning methods to identify potential AD-risk single nucleotide polymorphisms, discovering that rs561311966 (located in the APOC1 gene) and rs2229918 (located in the ERCC1 / CD3EAP genes) are significant factors influencing AD risk [ 127 ]. Similarly, the research team led by O’Bryant, SE, tends to focus on blood biomarkers, initiating the search for dementia-related blood biomarkers using random forests in 2011 [ 128 ]. Their recent research has found that a combination of serum and plasma biomarkers has higher predictive performance than serum or plasma biomarkers alone, providing a new approach to diagnosis using blood biomarkers [ 129 ]. Different prolific research groups seem to have certain differences in research interests and trends, but they generally converge on the study of imaging, blood, genetic, and some emerging biomarkers. Regarding algorithm use, besides traditional algorithms, there is also a growing trend toward the use of deep learning algorithms. Keeping tabs on the latest research trends concerning these highly prolific authors will aid in grasping the cutting-edge developments in various types of biomarkers.
Research Hot Spots
Research on dementia subtypes.
In the context of AI, the research focus on different dementia subtypes varies significantly, with AD dominating the field. This predominance is primarily due to the high prevalence of AD and its significant societal impact, which have attracted more resources and efforts. By contrast, research on other dementia subtypes started later, and most studies are either based on AD or aimed at differentiating from AD. Independent research on other subtypes such as FTD has shown some modest increases in the number of studies and algorithm use recently, but no similar trend is evident for VaD and DLB. Therefore, for VaD and DLB, we only discuss their latest biomarker findings based on AI.
New Biomarkers for FTD
Distinct from AD, FTD represents the second most common subtype of dementia. Since 2015, research on FTD has shown a growing trend. However, the diagnosis of FTD remains challenging due to the high symptom overlap with AD. Studies have shown that imaging biomarkers can distinctly differentiate AD from FTD [ 130 , 131 ]. This success is partly due to FTD subtypes affecting different brain regions; for example, behavioral variant FTD is typically associated with atrophy in the frontal and anterior temporal lobes; progressive nonfluent aphasia mainly impacts the left inferior frontal gyrus, leading to motor speech disorders; and semantic dementia primarily affects the left anterior temporal region [ 132 ]. This also explains why the use of imaging biomarkers is more widespread in FTD than in other biomarkers. Recently, significant white matter (WM) damage revealed by ML has been validated as an effective imaging biomarker for FTD, with WM degeneration in behavioral variant FTD being more pronounced than in AD, supporting the hypothesis that neurodegenerative changes in FTD start in the WM [ 133 , 134 ].
In addition, several CSF cobiomarkers have been proposed for FTD, such as NFL chain and TDP-43 [ 135 ]. In recent research, Bergström et al [ 136 ] used the least absolute shrinkage and selection operator (LASSO) and random forest methods to analyze protein data obtained from CSF samples, identifying NFM, aquaporin-4, neuronal pentraxin 2, and the neurosecretory protein VGF as potential diagnostic tools for FTD. In the genetic domain, Magen et al [ 137 ] developed a nonlinear predictive model based on gradient boosting trees, successfully identifying 13 microRNA (miRNA) features, offering new possibilities for early diagnosis and treatment of FTD. In other biomarker research, EEG features have achieved an accuracy rate of 73% in distinguishing FTD from AD using decision tree algorithms [ 138 ]. However, the current number of biomarkers available for FTD is still limited, necessitating more research to develop novel biomarkers to aid in distinguishing FTD from other types of dementia, particularly AD [ 139 ]. In this process, the application of AI will undoubtedly play an increasingly vital role.
New Biomarkers for DLB
The pathological hallmark of DLB is the presence of Lewy bodies containing alpha-synuclein in the neocortex and limbic areas [ 140 ]. Research has identified several potential biomarkers for the diagnosis of DLB, such as alpha-synuclein, Aβ42, and phosphorylated tau (p-tau) [ 141 ]. However, autopsy results indicate that 50% to 80% of DLB cases show cortical Aβ deposits similar to those in patients with AD [ 142 ]. In addition, the early cognitive symptoms of DLB highly overlap with those of AD, posing a challenge for clinical diagnosis. van Steenoven et al [ 143 ] used a random forest algorithm to identify 6 proteins in CSF—VGF, SCG2, neuronal pentraxin 2, NPTXR, PDYN, and PCSK1N—as candidate biomarkers for DLB. Moreover, EEG has become a research focus due to its accuracy in reflecting brain electrophysiological activity, surpassing neuroimaging and CSF biomarkers [ 144 ]. EEG has revealed specific electrophysiological patterns associated with DLB, particularly a dominant frequency of <8 Hz, which helps differentiate DLB from AD in 85% to 100% of patients [ 145 ]. Suzuki et al [ 146 ] used an EEG-based ML algorithm, MC-004, to distinguish DLB from AD with an accuracy rate of 79.5%. Recently, changes in miRNA expression have also been linked to various neurodegenerative diseases, providing new hope for diagnosing and differentiating DLB; for example, the pathological link between the genes BCL2L1 and PIK3R2 has been further supported [ 147 ]. The latest research by Soto et al [ 148 ] using ML has revealed 12 miRNAs with continuous expression dysregulation throughout the development of DLB. Zhou et al [ 141 ] used logistic regression and SVMs to build a predictive model and identified 5 potential DLB hub genes— SRF , MAPK1 , YWHAE , RPS6KA3 , and KDM7A —that may provide new biomarkers for the diagnosis and treatment of DLB.
New Biomarkers for VaD
Research on VaD is the least extensive, primarily because its pathological mechanisms involve complex issues related to cerebral vascular health, unlike specific intracellular pathogenic protein accumulation seen in other dementias, such as AD. VaD is mainly associated with cerebrovascular disease, and its onset and progression are often abrupt. This makes the development of biomarkers for VaD more challenging than for other types of dementia. Some researchers believe that VaD may be linked to systemic autoimmune diseases, and through bioinformatics and ML methods, genes such as C1QA , CD163 , LY96 , and MS4A4A have been identified as potential biomarkers for the link between VaD and systemic lupus erythematosus [ 149 ]. In addition, other studies have identified potential biomarkers for VaD, including digital clock drawing tests [ 150 ], lipids [ 151 ], the REPS1 gene [ 152 ], and brain tissue volume [ 153 ]. While these findings have opened new research avenues, no class of biomarkers has been widely applied in clinical settings to date. Future research needs to further validate these potential biomarkers and explore more from a multiomics perspective. This could help establish reliable biomarkers, thereby enhancing the diagnostic accuracy and treatment efficacy for VaD.
New Biomarkers for AD
Hippocampal atrophy, cortical thinning, and ventricular enlargement are classic manifestations of AD in MRI scans. The use of brain PET scans to detect tau and Aβ proteins has been extensively applied in ML models, with their effectiveness continually validated. With advances in imaging technology and AI, we can now process high-dimensional data, identify relevant patterns in complex data sets, and decipher the brain’s intricate network structures. In the hippocampal region, multivariate morphometry statistics [ 154 ], feature sets [ 155 ], and principal curvature ratios [ 24 ] provide new perspectives for analyzing structural changes in the AD brain. Compared to studies on physical structural changes, those on brain functional connectivity have revealed insights into the brain’s functional organization and operational mechanisms, becoming a vital resource for exploring new biomarkers; for instance, dynamic functional connectivity obtained from functional MRI [ 156 ] and correlated transfer function connectivity strength [ 157 ] have demonstrated potential as biomarkers. Zhao et al [ 158 ] have confirmed the excellent feature selection performance of dynamic functional connectivity by analyzing the functional connections between gray matter and WM and using SVMs for feature evaluation. Recent studies, such as that by Zhu et al [ 159 ], have combined SVMs with the apolipoprotein E ( APOE ) genotype, CSF biomarkers (Aβ, tau, and p-tau), and neuroimaging markers, finding that connections between the left insula and the left posterior middle temporal gyrus, the left medial superior frontal gyrus, and the right lingual gyrus are significant for cognitive functions. Sadiq et al [ 160 ] demonstrated the potential value of these signals in diagnosing AD by using SVMs to process nonfractal connectivity features extracted from resting-state functional MRI data through wavelet-based fractal analysis. In addition, dynamic connectivity anomalies between the hippocampus and the default mode network [ 156 ], as well as functional connectivity abnormalities in the posterior brain regions [ 28 ] and corticosubcortical circuits [ 161 ], have been identified as newly discovered key biomarkers.
Brain age discrepancies, evaluated by comparing the deviation of predicted brain functional connectivity age from actual age, have been shown to correlate with genetic markers such as APOE ε4 alleles across multiple study cohorts [ 118 ]. Lee et al [ 120 ] used a deep learning model based on structural MRI and fluorodeoxyglucose-PET to predict brain age, demonstrating that brain age differences can effectively predict the transition from no cognitive impairment to mild cognitive impairment or AD. Zhang et al [ 162 ] used SVMs and arterial spin labeling to reveal significant declines in blood flow in the posterior cingulate cortex and precuneus, providing evidence for regional cerebral blood flow as a new biomarker. Moreover, changes in the microstructure and integrity of brain WM fiber tracts captured via diffusion tensor imaging, such as changes in the parietal WM, limbic and high-order association areas WM, medial temporal WM, posterior cingulate and precuneal WM [ 163 ], and whole-brain WM fiber connectivity [ 164 ], also show great potential for predicting AD precursors. These imaging biomarkers discovered through AI offer significant research prospects, and their application could aid in the early diagnosis and development of treatment strategies for AD.
In genetic biomarker research, recent years have seen the identification of multiple genes associated with AD using large training data sets and complex analyses of genetic relationships. Zhuang et al [ 165 ] used methods such as random forest and LASSO to identify, for the first time in AD research, 10 biomarkers related to immune infiltration. Similarly, Zhou et al [ 166 ] successfully identified 5 potential AD predictive biomarkers— FAM71E1 , DDB2 , AP4M1 , GPR4 , and DOC2A —using transcriptome-wide association studies and weighted gene coexpression network analysis, combined with random forest and SVM algorithms. In addition, recent research has shown that genes such as BAG2 , HSC70 , STUB1 , and MAPT are closely related to the occurrence and progression of AD [ 167 ]. Small noncoding RNA molecules, or miRNAs, have also garnered significant attention in recent years for their multifaceted roles in AD development, including regulating the formation of Aβ plaques, phosphorylation of the tau protein, and involvement in inflammatory processes [ 168 ]. Tan et al [ 169 ] used an integrated framework of statistical and ML methods to perform differential expression analysis of miRNA, identifying 3 highly significant and relevant miRNA candidates: has-miR-6501-5p, has-miR-4433b-5p, and has-miR-143-3p. Likewise, Alamro et al [ 170 ] identified 6 AD-related miRNAs using ML and deep learning models. In addition, ferroptosis has been implicated in the pathogenesis of AD [ 171 ], and Deng et al [ 172 ] used various ML methods to build models and identify 5 genes related to ferroptosis ( RAF1 , NFKBIA , MOV10L1 , IQGAP1 , and FOXO1 ). Wang et al [ 173 ] used a random forest classifier to screen 12 differentially expressed genes associated with ferroptosis.
Although these discoveries are significant for understanding the genetic foundation of AD, the new gene biomarkers identified are often limited to specific gene data sets and lack validation across broader data sets. Furthermore, additional comprehensive studies are needed to elucidate the specific mechanisms of these genes and their impact on the pathological progression of AD.
In AD diagnostic research, biomarkers such as the ratio of Aβ42/Aβ40 in plasma and p-tau proteins (p-tau181, p-tau231, and p-tau217) have demonstrated high diagnostic accuracy, further supporting their potential as noninvasive diagnostic tools. In addition to these biomarkers, which are also present in CSF, changes in the expression of the RTN1 protein in the blood, related to the production of Aβ and BACE1 enzyme activity, may affect the pathological process of AD [ 174 ]. Yu et al [ 175 ] achieved a diagnostic accuracy rate of 99% using a random forest model constructed with 8 different serum proteins, providing potential new biomarkers for a noninvasive serum diagnostic platform for AD. Moreover, discoveries of more related blood biomarkers, such as tumor necrosis factor-alpha and monocyte chemoattractant protein-1 [ 176 ], plasma levels of D-glutamate [ 177 ], changes in platelet proteins [ 178 ], and expression changes in immune cells [ 179 , 180 ], are continually increasing, but the specific mechanisms behind them still require further investigation.
As the association between metabolic abnormalities and the onset of AD is increasingly confirmed, blood-based metabolic biomarkers are receiving more attention [ 181 ]. Recent studies have shown that cystatin C and carboxypeptidase B2 have potential as blood biomarkers, with a diagnostic model based on logistic regression algorithms showing a high accuracy rate of 93.8% [ 182 ]. In addition, lipids [ 183 - 185 ], arginine, and pentanoylcarnitine [ 84 ] as blood metabolic biomarkers also show diagnostic potential.
The development of new biomarkers in CSF has been slower than anticipated due to challenges in sample collection, high costs, and analytical complexities. Besides traditional biomarkers such as Aβ and p-tau, new CSF proteins such as NFL, soluble triggering receptor expressed on myeloid cells 2, and YKL-40 have been identified as indicators of neuronal damage. In recent research, Gaetani et al [ 186 ] performed a quantitative analysis of multiple biomarkers in CSF and used ML models, including penalized logistic regression, to identify biomarkers indicative of neuroinflammation’s role in AD, such as SIRT2, HGF, MMP-10, and CXCL5. In addition, the study by Horie et al [ 126 ] on MTBR-tau243 in CSF demonstrated that its association with tau tangles and cognitive impairment in AD exceeds that of traditional p-tau biomarkers [ 126 ]. This discovery provides new insights for updating the amyloid, tau, and neurodegeneration diagnostic framework for AD.
In recent years, a series of emerging biomarkers for dementia have been continually identified and validated. These biomarkers are at the initial stages of research. In the realm of digital biomarkers, Bayat et al [ 124 ] achieved an accuracy rate of 89% in predicting preclinical AD by analyzing natural driving GPS data and building a random forest model. Thompson et al [ 187 ] used ML to analyze the graphics and features during the digital clock drawing test, finding a potential correlation between lower scores and a higher presence of APOE ε4 alleles. In ophthalmology, Cheung et al [ 188 ] discovered new biomarkers related to dementia risk through the diameters of retinal blood vessels using a deep learning model. Recent confirmations also show that macular thickness and volume obtained from optical coherence tomography measurements [ 189 ] and the thickness of the retinal nerve fiber layer [ 190 ] have potential as AD biomarkers. In addition, metabolites in urine [ 191 ] and EEG features [ 192 ] have also demonstrated new research outcomes with the aid of AI.
Overall, many of the newly discovered biomarkers are still in the initial stages of discovery and validation. For these biomarkers to be translated into clinical applications, they must undergo thorough validation in broader data sets and larger population cohorts. In addition, assessing their diagnostic efficacy and reliability through longitudinal studies is a prerequisite for their future integration into clinical practice, a process that may take considerable time. However, it is encouraging that with the assistance of AI, researchers have discovered more biomarkers, significantly aiding in the refinement of dementia’s pathological mechanisms and the exploration of potential therapeutic avenues.
Application of Popular Algorithms in Imaging Biomarkers
On the basis of our research, biomarkers obtained through various imaging techniques, such as MRI, offer detailed information about brain structure and are among the most widely used biomarkers today. Incorporating imaging biomarkers in multimodal data fusion strategies often significantly enhances classification accuracy [ 115 ]. SVMs are not only the most commonly used classification algorithms to date but also the most prevalent method for processing imaging data. SVMs excel in handling high-dimensional neuroimaging data, leveraging their kernel trick to handle nonlinear data in high-dimensional spaces, which is crucial for capturing complex biomarkers [ 193 ].
However, we have observed an interesting phenomenon: despite the low frequency of the keyword “SVM” in 2023, the actual use of SVMs in research as classifiers and as part of ensemble learning architectures has not shown a significant downward trend, with only a slight decrease of 2 instances compared to 2022.
This discrepancy is linked to a reduction in studies focusing solely on SVMs as independent algorithms, shifting toward comparative studies of various ML models, multivariate classification research, and an increase in ensemble learning approaches; for instance, Zubrikhina et al [ 99 ] found that SVMs showed the best performance among various ML models when classifying MRI data. Similarly, Tan et al [ 194 ] demonstrated that an ensemble model comprising gradient boosting, logistic regression, and SVMs outperformed single classifiers in multiple performance aspects [ 194 ]. Shukla et al [ 195 ] achieved an accuracy rate of 96% in a ternary classification of individuals with AD versus individuals with MCI versus cognitively normal individuals using multimodal imaging data combined with gradient boosting and SVMs. These studies indicate that SVMs remain a key component in many research projects. However, the use of “SVM” as a keyword may have declined due to a tendency to highlight emerging or innovative methods, leading to a reduced frequency of “SVM” in keyword use.
In recent years, neural networks have gained unprecedented popularity in the imaging biomarker domain, surpassing SVMs, primarily due to their reduced dependency on manual feature engineering and their proficiency in automatically identifying and learning the most significant features within data. Specifically, convolutional neural networks (CNNs) have demonstrated exceptional performance in the realm of image processing. A notable instance is the work of Lee et al [ 120 ], who used a deep learning model (3D-DenseNet) to process fluorodeoxyglucose-PET and MRI images. This model’s architecture includes multiple dense blocks and convolutional layers capable of autonomously extracting complex features from imaging data. The feedforward connections within each dense block aid in acquiring a rich feature representation. Using the discrepancy between actual age and estimated brain age (brain age gap), they conducted classification diagnostics for dementia. Furthermore, a CNN model introduced by Ahmed et al [ 196 ] achieved an accuracy rate of 94% in distinguishing between patients with AD and healthy individuals through the analysis of imaging data from the ADNI data set.
Moreover, the application of transfer learning has significantly reduced the need for extensive data and computational resources required for training new models, thereby allowing researchers to forgo the necessity of developing CNN models from scratch. Hence, the synergy of imaging biomarkers and neural networks holds considerable potential and prospects for future research, particularly in terms of processing complex imaging data with greater precision and efficiency.
CSF Proteomics Biomarkers
CSF biomarkers have been among the earliest studied markers in dementia research due to their direct link with the brain and spinal cord, serving as a vital source of biochemical information. Specifically, tau proteins and Aβ in the CSF are core markers for dementia diagnosis. The pursuit of new proteomic biomarkers in CSF has been a continual area of interest; for instance, Gogishvili et al [ 197 ] used a random forest classification model to analyze proteomics data from CSF, successfully identifying new biomarkers such as CLEC1B, TNFRSF4, and TGF-β-1. However, obtaining CSF samples requires an invasive procedure known as lumbar puncture, and the analysis is costly, which somewhat limits the feasibility of large-scale data collection.
In more recent studies, increasing evidence has shown that tau proteins and Aβ, as well as their derivative forms, such as p-tau and total tau [ 198 , 199 ], can be obtained through multiple pathways [ 200 ]. This not only adds dimensions and richness to the data but also allows more research institutions access to these biomarkers. For regions with limited resources or underdeveloped technology, this accessibility helps reduce the costs of diagnosis and monitoring and provides more strategies to construct diverse cohorts and data sets for a more comprehensive understanding of neuropathological diversity. This shift in accessibility might explain why CSF biomarkers were more prevalently used in early research than other types of biomarkers but are now gradually being surpassed by other types of markers.
The Use of Random Forests in Blood and Genetic Markers
Compared to CSF and imaging biomarkers, genetic and blood markers have garnered considerable attention from researchers in recent years due to their minimally invasive collection process and ease of acquisition. Our research indicates that random forests have become more popular than neural networks and SVMs in the application of blood and genetic markers. By integrating multiple decision trees, random forests can effectively capture the complex nonlinear relationships in data and handle various types of data. They not only possess robust predictive capabilities but also prevent overfitting [ 201 ], offering an intuitive understanding of the most critical features (such as specific biomarkers) in model predictions [ 202 ].
In genome-wide association studies, random forests can capture complex epistatic interactions and select key genetic variations [ 203 ], which is invaluable for identifying potential biomarkers in genes and blood; for instance, Kelly et al [ 204 ] used various ML models and gene expression profiles in their study of blood-based biomarkers, finding that random forests performed best in AD diagnostic models with an accuracy rate of 81%, identifying 159 gene markers. Beltrán et al [ 205 ] compared 4 ML methods, noting that random forests could achieve competitive results with costly medical imaging techniques when applied to readily available measurements (such as cognitive scores, genetic risk, and plasma biomarkers), identifying APOE and plasma C-reactive protein as the most significant features. However, each of these prevalent ML methods has its shortcomings; for example, neural networks have issues with interpretability and training costs; SVMs are highly sensitive to parameter selection, where inappropriate use of the kernel function or regularization parameters can lead to poor model performance; and, by contrast, random forests require extensive experimentation to adjust the number of trees, depth, and other parameters.
The Relationship Between Other Algorithms and Biomarkers
The connection between other algorithms and biomarkers is not as prominent or popular as that between the aforementioned algorithms and biomarkers. However, LASSO is observed to be frequently used in genetic biomarkers due to its efficiency in selecting disease-related feature genes from high-dimensional data [ 206 ]. By contrast, linear discriminant analysis and principal component analysis are more often applied in imaging biomarkers for feature reduction in MRI and PET modalities and fusion analysis of multimodal data [ 207 ]. Gradient boosting seems to be more inclined toward imaging and genetic markers, the k-nearest neighbors algorithm leans more toward imaging and neurophysiological markers, and logistic regression is more favored for imaging markers. Currently, many ML models lack standard settings and guidelines, making a robust comparison of these experiments difficult. Moreover, the specific combinations of ML methods and biomarkers may be influenced by various factors, such as the accessibility of variables, cost-effectiveness, and the adaptability of the model to the application context (eg, clinical and research environments) [ 208 ]. The diversity and complexity of these factors mean that the same algorithm might show different effectiveness and applicability in different studies. Nonetheless, by conducting an in-depth analysis of numerous studies to explore the relationship between different ML models and biomarker research, valuable insights and references can be provided for the field.
The Progress of AI
From the transition of traditional ML algorithms to the widespread application of deep learning and neural networks, significant progress has been marked in the field of medical AI [ 209 ]. Notably, since 2018, neural networks have increasingly dominated the research of dementia biomarkers, showcasing the potential to become the leading algorithms. A similar trend has been observed in other medical disciplines, such as cardiology, which has broadly implemented neural networks and deep learning technologies since 2015 [ 210 ]. In gastric cancer research, Shichijo et al [ 211 ] first used CNNs in 2017 to evaluate their effectiveness in diagnosing Helicobacter pylori infection. By 2020, deep learning technologies were extensively applied in the study of biomarkers for gastric cancer [ 212 ].
In addition, oncology is at the forefront of using multiomics data for patient stratification and personalized treatment [ 213 ]. In the imaging of brain tumors, neural networks have significantly enhanced the accuracy of detection and classification; for instance, Özkaraca et al [ 214 ] successfully applied a dense CNN architecture, using MRI images to precisely classify different brain tumors, thus supporting the development of accurate treatment plans. In the research of genetic and hematologic biomarkers, neural networks have opened new pathways for the early detection and classification of various cancer types. The studies by Liu et al [ 215 ] and Almarzouki [ 216 ] have demonstrated the potential of neural networks, with their capability to identify biomarkers with high specificity and sensitivity, in processing complex biological data. Advanced algorithms are also extensively used in specific tumor subtyping, grading, and staging [ 209 ], as well as predicting treatment outcomes [ 217 ]. These are directions that dementia research needs to learn from and emulate. Currently, dementia research mainly revolves around diagnosing and classifying AD, and there is a need to strengthen the study of other subtypes and expand the scope and objectives of the research.
Commercialization of AI in Dementia
Although the potential for AI technology in the medical field is immense, the use of commercial AI products for dementia in clinical settings remains relatively limited. This is partly due to significant unresolved limitations associated with ML applications. Furthermore, obtaining regulatory approval for AI products in the tightly regulated health care sector is a major challenge and a prerequisite for their practical application.
However, in recent years, AI-based methods have made significant strides. Particularly following the release of the US Food and Drug Administration Action Plan , which classifies AI- and ML-based software as a medical device [ 218 ], the market has begun to see approvals for such products. While no AI devices specifically targeted at dementia have been approved yet, in the field of radiology, AI software such as SubtlePET and SubtleMR, which process imaging data, have been approved [ 105 , 219 ], indirectly advancing AI in the clinical diagnosis of dementia. In addition, Cheung et al [ 188 ] recently developed the Singapore I Vessel Analyzer deep learning system for automatic measurement of retinal vessel calibers in dementia, and the commercially available Idx software for diagnosing retinal diseases through retinal examination [ 105 ] may further promote the application of ophthalmic biomarkers in the clinical diagnosis of dementia. The use of computer-aided diagnosis systems [ 220 ], which provide radiologists with areas of interest or risk assessments, also aids in better guiding clinicians in diagnosing dementia.
Development of Emerging Technologies
The application of emerging technologies has provided more opportunities for the use of AI and the discovery of new biomarkers. Specifically, advanced imaging techniques such as structural MRI, functional MRI, Pittsburgh compound B PET, and diffusion tensor imaging have significantly enhanced our ability to capture detailed information about the brain. Single molecule array technology, genome-wide association studies, and high-throughput sequencing techniques also play a crucial role in identifying blood and genetic biomarkers.
The introduction of a series of emerging digital and wearable devices has created new opportunities for the diagnosis and assessment of dementia. Zhang et al [ 221 ] recorded participants’ trail making test hand-drawn strokes using an electromagnetic tablet and used random forest analysis to examine the drawing features, discovering that models combining paper-based and electronic trail making tests improved the accuracy of assessing cognitive impairments. Ghosh et al [ 222 ] used GPS tracking to measure ecological outdoor behavior and differentiated individuals with AD from healthy individuals using data-driven ML methods. In addition, gait data obtained from accelerometers and inertial measurement units, eye movement variations captured by eye trackers, voice data recorded by microphones, and a range of digital biomarkers captured by other devices show promising applications [ 208 ].
Furthermore, digital biomarkers are significant due to their close connection with daily life. The deployment of Internet of Things devices based on environmental sensors and monitoring software in homes can enable long-term monitoring and assessment of the behaviors of patients with dementia. Khodabandehloo and Riboni [ 223 ] used environmental sensors to monitor real-life activities to detect wandering behavior, combined with ML methods to detect cognitive decline. Lotfi et al [ 224 ] conducted studies using various standard home automation sensors to monitor activities and movements at home, using neural networks for data analysis to detect abnormal behaviors in dementia. All these studies provide new insights into the exploration of dementia biomarkers. As the Internet of Things, particularly wearable devices, becomes more prevalent, it will further drive the development and commercialization of software as a medical device.
Potential Biases in AI
Currently, the application of AI in dementia biomarkers faces multiple challenges. First, many studies rely heavily on specific data sets, particularly the ADNI, which, although they provide high-quality data, may limit the universality of the research due to overreliance. These data sets may not adequately represent all races, cultures, or geographic locations, potentially leading to algorithmic bias and affecting the broad applicability and clinical translation of the research findings.
Second, although many AI studies show promise in the preliminary stages, they often lack external validation on independent data sets during the validation phase. External validation is a crucial step to assess the model’s generalizability, ensuring the effectiveness of research outcomes across different populations and clinical settings.
Moreover, although AI technologies such as deep learning excel in identifying and predicting dementia biomarkers, the black box nature of these models poses challenges in enhancing transparency and gaining trust from medical professionals. The limited interpretability of deep learning models restricts their practical application in clinical decision-making. Therefore, using techniques such as feature importance analysis and model visualization tools to help medical professionals and patients understand the logic behind AI decisions—explainable AI—is becoming an important research area, aiming to make the ML process more transparent and comprehensible [ 27 ].
Finally, the imbalance in the number of samples for each category label within training data sets also imposes additional constraints on the model’s robustness and clinical applicability. Addressing these challenges requires broader sample collection; more rigorous model design and testing, such as using synthetic minority oversampling technique, adjusting class weights, or using specific loss functions to minimize the impact of minority categories; and other new methods to enhance model interpretability.
Ethical and Privacy Challenges
Data collection involves handling a significant amount of sensitive personal information. Without appropriate data protection measures, this could infringe on the participants’ privacy rights. In addition, during the data storage process, it is essential to ensure data encryption and anonymization. It is also necessary to clearly define the ownership and use rights of the data, ensuring that only authorized personnel can access this information to prevent misuse. When sharing these data as data sets or in other forms publicly, patient consent is also required.
From an ethical standpoint, researchers have the responsibility to ensure that participants fully understand the significance of their involvement in the research, which should be based on voluntary principles and clear consent, especially for the collection of novel biomarkers such as digital biomarkers. This is particularly important for patients with AD who may not fully comprehend the research content. Ensuring the reasonableness and fairness of the consent process is essential. When errors occur in the diagnostic process using AI systems, a clear accountability mechanism should be in place. This involves how to handle medical errors caused by AI decisions and how to correct these errors.
Moreover, it is necessary to establish relevant policies and regulations to regulate the use of AI in the medical field, ensuring that it complies with medical ethical standards. In facing these challenges, researchers, technology developers, and policy makers need to work together to ensure that the development of AI technology proceeds under the premise of respecting patient rights and ensuring data security. By establishing strict industry standards and ethical guidelines, the responsible use of AI in the medical field can be facilitated.
Application and Development Trend of Research
Strengthening interdisciplinary collaboration.
As more new researchers join the field, the demand for external disciplinary knowledge continues to expand, making it especially important to establish a stable and continually active collaboration network, particularly an interdisciplinary one. In the future, exploring this model of interdisciplinary collaboration will become a focus of research.
Exploration of New Biomarkers
AI is widely used in the research of various dementia biomarkers, including imaging, CSF, and genetic markers, which remain the primary subjects of current research. However, there is a growing demand for economically efficient and noninvasive biomarkers [ 89 , 225 ]. Digital biomarkers and ophthalmic biomarkers hold significant research prospects for the future. Currently, Alzheimer’s Research UK is studying combinations of various digital biomarkers and exploring the application of ML algorithms [ 208 ]. Once these new biomarkers are validated through neuroimaging and CSF tests [ 226 ], they may become a more cost-effective tool for the early detection of dementia, especially in resource-limited areas [ 227 ].
Validation of Newly Discovered Biomarkers
Researchers have used AI to successfully identify patterns and correlations that may have gone unnoticed previously, uncovering a cohort of new candidate biomarkers, particularly in imaging and genetic biomarkers. However, these novel biomarkers generally lack external validation. Hence, future research trends will focus on further validation and comparison of these biomarkers in larger data sets or cohorts to confirm their effectiveness.
Enhancing Interpretability
In the medical domain, the interpretability and transparency of algorithms are paramount. With the increasing popularity of neural networks, researchers must carefully select or design algorithms, focusing on interpretable ML and AI use. This emphasis will drive further innovation and development in the fields of neuroscience and medical research.
Increasing Research on Dementia Subtypes
AI has demonstrated the potential to differentiate between subtypes of dementia and identify new biomarkers for these conditions. However, research into subtypes other than AD remains scarce. Increasing the number of studies on these specific subtypes and expanding the diversity of research will help to enhance our comprehensive understanding of dementia.
Strengths and Limitations
To our knowledge, this study is the first to conduct a comprehensive analysis of AI in the field of dementia biomarkers using bibliometric methods. By integrating a strategy of multiple tools, we not only improved the accuracy of the analysis but also expanded the dimensions of the comprehensive analysis. We presented the current status and research hot spots of the field from multiple aspects (eg, keywords, countries or regions, authors, and funding) and, for the first time, used text mining methods to specifically quantify the scale and relationship of biomarker and algorithm use.
Nevertheless, this study has limitations. To mitigate potential human errors associated with manual database management, only 1 database was included, which may have resulted in the omission of a small number of relevant studies. Furthermore, our investigation was restricted to studies published in English, potentially overlooking high-quality research in other languages, and did not account for potential self-citation bias, although its impact on the trends displayed is likely minimal. Therefore, future studies should leverage programming languages such as Python or R to expand database inclusion and analyze research across multiple languages. In addition, the quality and potential biases of the included studies were not assessed, which might affect the depicted trends due to the influence of low-quality and biased research. Future efforts should include a detailed quality evaluation of the studies.
Conclusions
In this study, we conducted a comprehensive analysis of research on AI in dementia biomarkers, using the Web of Science Core Collection. The objective was to summarize the latest advances and trends in this field. Our findings reveal significant progress since 2018, with numerous biomarkers identified in the areas of imaging and genetics. The United States, China, and the United Kingdom have been instrumental in driving progress in this domain. In addition, we noted a trend of author turnover and a need for stronger collaboration, suggesting that governments and researchers should develop strategies to facilitate the involvement and initiation of research by new scholars. Furthermore, through content mining and analysis, this study explored the popularity trends of various algorithms and biomarkers and delved into the pivotal applications of AI technologies across different types of dementia biomarkers. It also summarized newly discovered biomarkers identified through AI. In conclusion, as new biomarkers continue to be developed, and new algorithmic architectures are constructed, the application of AI in the field of dementia biomarkers is emerging as a promising area of research.
Acknowledgments
This study was supported by the Key Project of University Laboratory Research Work of Zhejiang Province (ZD202202); the Zhejiang Province Traditional Chinese Medicine, Science, and Technology Project (2023ZF134); the Medical and Health Technology Plan of Zhejiang Province (2022507615); the Zhejiang Provincial Medical and Health Science and Technology Program Project (2022KY1052); and the First-Class Course of Zhejiang Province (2022-1133).
Data Availability
The data sets generated and analyzed during this study are available from the corresponding author on reasonable request. The code required for software operation is also available from the corresponding author on reasonable request.
Authors' Contributions
WQ conceptualized the study, set the research methodology, performed data visualization, and edited the manuscript. XZ was responsible for the methodology and wrote the original draft. DH provided software and other resources and wrote and reviewed the original draft. BW, CD, YL, YC, and BW organized the data and set the research methodology. SC conceptualized the study, reviewed and edited the manuscript, acquired funding, managed the project, and performed formal analysis. YS provided software and other resources and set the research methodology. FL, LMMB, XL, JK, and GJ conducted formal analysis and supervision. XL, JY, and JL were responsible for data visualization. All authors reviewed the draft, provided comments and revisions, and approved the final version of the manuscript.
Conflicts of Interest
None declared.
Search query.
Calculation formulas used for data statistics and analysis.
Keyword merging.
Use strategies for each tool.
Specific classification methods for various types of biomarkers.
Artificial intelligence classification of diseases and performance of highly cited local literature.
Top 10 research studies of artificial intelligence in dementia biomarkers, ranked by a standardized local citation index.
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. Aug 08, 2020;396(10248):413-446. [ FREE Full text ] [ CrossRef ] [ Medline ]
- -. 2023 Alzheimer's disease facts and figures. Alzheimers Dement. Apr 2023;19(4):1598-1695. [ CrossRef ] [ Medline ]
- -. 2021 Alzheimer's disease facts and figures. Alzheimers Dement. Mar 2021;17(3):327-406. [ CrossRef ] [ Medline ]
- Mirkin S, Albensi BC. Should artificial intelligence be used in conjunction with neuroimaging in the diagnosis of Alzheimer's disease? Front Aging Neurosci. Apr 18, 2023;15:1094233. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Qiu S, Miller MI, Joshi PS, Lee JC, Xue C, Ni Y, et al. Multimodal deep learning for Alzheimer's disease dementia assessment. Nat Commun. Jun 20, 2022;13(1):3404. [ FREE Full text ] [ CrossRef ] [ Medline ]
- AlSharabi K, Salamah YB, Aljalal M, Abdurraqeeb AM, Alturki FA. EEG-based clinical decision support system for Alzheimer's disorders diagnosis using EMD and deep learning techniques. Front Hum Neurosci. Aug 31, 2023;17:1190203. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. Mar 2001;69(3):89-95. [ CrossRef ] [ Medline ]
- Jack CRJ, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. Apr 2018;14(4):535-562. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Simrén J, Leuzy A, Karikari TK, Hye A, Benedet AL, Lantero-Rodriguez J, AddNeuroMed consortium, et al. The diagnostic and prognostic capabilities of plasma biomarkers in Alzheimer's disease. Alzheimers Dement. Jul 2021;17(7):1145-1156. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. Aug 2017;134(2):171-186. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Jo T, Nho K, Saykin AJ. Deep learning in Alzheimer's disease: diagnostic classification and prognostic prediction using neuroimaging data. Front Aging Neurosci. Aug 20, 2019;11:220. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Winchester LM, Harshfield EL, Shi L, Badhwar A, Khleifat AA, Clarke N, et al. Artificial intelligence for biomarker discovery in Alzheimer's disease and dementia. Alzheimers Dement. Dec 2023;19(12):5860-5871. [ CrossRef ] [ Medline ]
- Chang CH, Lin CH, Lane HY. Machine learning and novel biomarkers for the diagnosis of Alzheimer's disease. Int J Mol Sci. Mar 09, 2021;22(5):2761. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zhang C, Lu Y. Study on artificial intelligence: the state of the art and future prospects. J Ind Inf Integr. Sep 2021;23:100224. [ CrossRef ]
- Gutierrez G. Artificial intelligence in the intensive care unit. Crit Care. Mar 24, 2020;24(1):101. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kameyama M, Umeda-Kameyama Y. Applications of artificial intelligence in dementia. Geriatr Gerontol Int. Mar 2024;24 Suppl 1:25-30. [ CrossRef ] [ Medline ]
- Aschenbrenner AJ, Gordon BA, Benzinger TL, Morris JC, Hassenstab JJ. Influence of tau PET, amyloid PET, and hippocampal volume on cognition in Alzheimer disease. Neurology. Aug 28, 2018;91(9):e859-e866. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Koychev I, Gunn RN, Firouzian A, Lawson J, Zamboni G, Ridha B, et al. PET Tau and Amyloid-β burden in mild Alzheimer's disease: divergent relationship with age, cognition, and cerebrospinal fluid biomarkers. J Alzheimers Dis. 2017;60(1):283-293. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Li CC, Wang ZY, Wang LJ, Zhang CY. Biosensors for epigenetic biomarkers detection: a review. Biosens Bioelectron. Nov 01, 2019;144:111695. [ CrossRef ] [ Medline ]
- Nordengen K, Kirsebom BE, Henjum K, Selnes P, Gísladóttir B, Wettergreen M, et al. Glial activation and inflammation along the Alzheimer's disease continuum. J Neuroinflammation. Feb 21, 2019;16(1):46. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Gordon BA, Blazey TM, Su Y, Hari-Raj A, Dincer A, Flores S, et al. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer's disease: a longitudinal study. Lancet Neurol. Mar 2018;17(3):241-250. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer's disease neuroimaging initiative (ADNI): clinical characterization. Neurology. Jan 19, 2010;74(3):201-209. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Yang X, Qu H. Bibliometric review on biomarkers for Alzheimer's disease between 2000 and 2023. Medicine (Baltimore). Sep 08, 2023;102(36):e34982. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Noda K, Lim Y, Sengoku S, Kodama K. Global biomarker trends in Alzheimer's research: a bibliometric analysis. Drug Discov Today. Aug 2023;28(8):103677. [ CrossRef ] [ Medline ]
- Wu CC, Su CH, Islam MM, Liao MH. Artificial intelligence in dementia: a bibliometric study. Diagnostics (Basel). Jun 19, 2023;13(12):2109. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Aberathne I, Kulasiri D, Samarasinghe S. Detection of Alzheimer's disease onset using MRI and PET neuroimaging: longitudinal data analysis and machine learning. Neural Regen Res. Oct 2023;18(10):2134-2140. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Blanco K, Salcidua S, Orellana P, Sauma-Pérez T, León T, Steinmetz LC, et al. Systematic review: fluid biomarkers and machine learning methods to improve the diagnosis from mild cognitive impairment to Alzheimer's disease. Alzheimers Res Ther. Oct 14, 2023;15(1):176. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Falahati F, Westman E, Simmons A. Multivariate data analysis and machine learning in Alzheimer's disease with a focus on structural magnetic resonance imaging. J Alzheimers Dis. 2014;41(3):685-708. [ CrossRef ] [ Medline ]
- Li R, Wang X, Lawler K, Garg S, Bai Q, Alty J. Applications of artificial intelligence to aid early detection of dementia: a scoping review on current capabilities and future directions. J Biomed Inform. Mar 2022;127:104030. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Tzimourta KD, Christou V, Tzallas AT, Giannakeas N, Astrakas LG, Angelidis P, et al. Machine learning algorithms and statistical approaches for Alzheimer's disease analysis based on resting-state EEG recordings: a systematic review. Int J Neural Syst. May 2021;31(5):2130002. [ CrossRef ] [ Medline ]
- Ninkov A, Frank JR, Maggio LA. Bibliometrics: methods for studying academic publishing. Perspect Med Educ. Jun 2022;11(3):173-176. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Bordons M, Zulueta MA. [Evaluation of the scientific activity through bibliometric indices]. Rev Esp Cardiol. Oct 1999;52(10):790-800. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Donthu N, Kumar S, Mukherjee D, Pandey N, Lim WM. How to conduct a bibliometric analysis: an overview and guidelines. J Bus Res. Sep 2021;133:285-296. [ CrossRef ]
- Wu CC, Huang CW, Wang YC, Islam MM, Kung WM, Weng YC, et al. mHealth research for weight loss, physical activity, and sedentary behavior: bibliometric analysis. J Med Internet Res. Jun 08, 2022;24(6):e35747. [ FREE Full text ] [ CrossRef ] [ Medline ]
- He D, Cao S, Le Y, Wang M, Chen Y, Qian B. Virtual reality technology in cognitive rehabilitation application: bibliometric analysis. JMIR Serious Games. Oct 19, 2022;10(4):e38315. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Aijing L, Shuangcheng Y, Lu M. Medical Literature Information Retrieval. Third Edition. Beijing, China. People's Medical Publishing House; 2005.
- Guo Y, Hao Z, Zhao S, Gong J, Yang F. Artificial intelligence in health care: bibliometric analysis. J Med Internet Res. Jul 29, 2020;22(7):e18228. [ FREE Full text ] [ CrossRef ] [ Medline ]
- McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22(3):276-282. [ FREE Full text ] [ Medline ]
- Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20(1):37-46. [ FREE Full text ] [ CrossRef ]
- Nam S, Kim D, Jung W, Zhu Y. Understanding the research landscape of deep learning in biomedical science: scientometric analysis. J Med Internet Res. Apr 22, 2022;24(4):e28114. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Osinska V, Klimas R. Mapping science: tools for bibliometric and altmetric studies. Inf Res. Dec 2021;26(4). [ CrossRef ]
- Chen C. CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol. Dec 14, 2005;57(3):359-377. [ CrossRef ]
- van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. Aug 2010;84(2):523-538. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Aria M, Cuccurullo C. bibliometrix : an R-tool for comprehensive science mapping analysis. J Informetr. Nov 2017;11(4):959-975. [ CrossRef ]
- Yin S, Zhu F, Li Z, Che D, Li L, Zhang L, et al. Research hotspots and trends in music therapy intervention for patients with dementia: a bibliometrics and visual analysis of papers published from 2010 to 2021. Front Psychiatry. Apr 28, 2022;13:860758. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Joinpoint trend analysis software. National Cancer Institute Division of Cancer Control and Population Sciences. URL: https://surveillance.cancer.gov/joinpoint/ [accessed 2024-04-05]
- Niazi MA, Vasilakos A, Temkin A. Review of “Exploratory social network analysis with Pajek” by Wouter de Nooy, Andrej Mrvar and Vladimir Batageli. Complex Adapt Syst Model. Jun 17, 2019;7:1. [ CrossRef ]
- Cobo MJ, López-Herrera AG, Herrera-Viedma E, Herrera F. Science mapping software tools: review, analysis, and cooperative study among tools. J Am Soc Inf Sci Technol. May 02, 2011;62(7):1382-1402. [ CrossRef ]
- Silva-Spínola A, Baldeiras I, Arrais JP, Santana I. The road to personalized medicine in Alzheimer's disease: the use of artificial intelligence. Biomedicines. Jan 29, 2022;10(2):315. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Golub GH, van Loan CF. An analysis of the total least squares problem. SIAM J Numer Anal. 1980;17(6):883-893. [ CrossRef ]
- Yao J, Cao S, Le Y, He D, Chen Y, Huang C, et al. Mapping knowledge landscapes and emerging trends of non-contact vital signs monitoring: a bibliometric and visualization analysis from 2002 to 2023. Alex Eng J. Feb 2024;88:197-209. [ CrossRef ]
- Tang R, Zhang S, Ding C, Zhu M, Gao Y. Artificial intelligence in intensive care medicine: bibliometric analysis. J Med Internet Res. Nov 30, 2022;24(11):e42185. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Garrett S. An Introduction to the Mathematics of Finance: A Deterministic Approach. Amsterdam, The Netherlands. Elsevier Science; 2013.
- Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond). Oct 2021;41(10):1037-1048. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Shah FA, Jawaid SA. The h-index: an indicator of research and publication output. Pak J Med Sci. 2023;39(2):315-316. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ali M. Understanding the 'g-index' and the 'e-index'. Semin Ophthalmol. 2021;36(4):139. [ CrossRef ]
- Zhang CT. The e-index, complementing the h-index for excess citations. PLoS One. May 5, 2009;4(5):e5429. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Choudhri AF, Siddiqui A, Khan NR, Cohen HL. Understanding bibliometric parameters and analysis. Radiographics. 2015;35(3):736-746. [ CrossRef ] [ Medline ]
- Gordon A. Transient and continuant authors in a research field: the case of terrorism. Scientometrics. Jun 17, 2007;72(2):213-224. [ CrossRef ]
- Price DJ. Networks of scientific papers. Science. Jul 30, 1965;149(3683):510-515. [ CrossRef ] [ Medline ]
- Weinstock M. Bradford's Law. Nature. Oct 08, 1971;233(5319):434. [ CrossRef ] [ Medline ]
- Venable GT, Shepherd BA, Loftis CM, McClatchy SG, Roberts ML, Fillinger ME, et al. Bradford’s law: identification of the core journals for neurosurgery and its subspecialties. J Neurosurg. Feb 2016;124(2):569-579. [ CrossRef ]
- Roldan-Valadez E, Salazar-Ruiz SY, Ibarra-Contreras R, Rios C. Current concepts on bibliometrics: a brief review about impact factor, Eigenfactor score, CiteScore, SCImago Journal Rank, Source-Normalised Impact per Paper, H-index, and alternative metrics. Ir J Med Sci. Aug 3, 2019;188(3):939-951. [ CrossRef ] [ Medline ]
- Sources. Scopus Preview. URL: https://www.scopus.com/sources.uri?RN_AG_Sourced_400000654 [accessed 2023-12-10]
- Scimago journal and country rank. Scimago Lab. URL: https://www.scimagojr.com/ [accessed 2023-12-12]
- Garfield E. How can impact factors be improved? BMJ. Aug 17, 1996;313(7054):411-413. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Sign in to continue with Journal Citation Reports. Clarivate. URL: https://jcr.clarivate.com/jcr/browse-journals[accessed [accessed 2024-07-17]
- Garfield E. The history and meaning of the journal impact factor. JAMA. Jan 04, 2006;295(1):90-93. [ CrossRef ] [ Medline ]
- List of countries by GDP (nominal). Wikipedia: The Free Encyclopedia. URL: https://en.wikipedia.org/wiki/List_of_countries_by_GDP_(nominal) [accessed 2023-12-12]
- Global Dementia Observatory (GDO). World Health Organization. URL: https://who.int/data/gho/data/themes/global-dementia-observatory-gdo [accessed 2024-01-20]
- Global health estimates: leading causes of death. World Health Organization. URL: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death [accessed 2024-01-20]
- Bornmann L, Wohlrabe K. Normalisation of citation impact in economics. Scientometrics. Jun 14, 2019;120(2):841-884. [ CrossRef ]
- Rasmussen M, Karypis G. gCLUTO -- an interactive clustering, visualization, and analysis system. University of Minnesota. May 25, 2004. URL: https://conservancy.umn.edu/items/5deba1b8-6c14-4dee-9f82-da3b2210e7ed [accessed 2024-04-12]
- Bai J, Li W, Huang YM, Guo Y. Bibliometric study of research and development for neglected diseases in the BRICS. Infect Dis Poverty. Sep 06, 2016;5(1):89. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Shen L, Wang S, Dai W, Zhang Z. Detecting the interdisciplinary nature and topic hotspots of robotics in surgery: social network analysis and bibliometric study. J Med Internet Res. Mar 26, 2019;21(3):e12625. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Wang J, Liang Y, Cao S, Cai P, Fan Y. Application of artificial intelligence in geriatric care: bibliometric analysis. J Med Internet Res. Jun 23, 2023;25:e46014. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zhang D, Wang Y, Zhou L, Yuan H, Shen D, Alzheimer's Disease Neuroimaging Initiative. Multimodal classification of Alzheimer's disease and mild cognitive impairment. Neuroimage. Apr 01, 2011;55(3):856-867. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Moradi E, Pepe A, Gaser C, Huttunen H, Tohka J, Alzheimer's Disease Neuroimaging Initiative. Machine learning framework for early MRI-based Alzheimer's conversion prediction in MCI subjects. Neuroimage. Jan 01, 2015;104:398-412. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zhang D, Shen D, Alzheimer's Disease Neuroimaging Initiative. Multi-modal multi-task learning for joint prediction of multiple regression and classification variables in Alzheimer's disease. Neuroimage. Jan 16, 2012;59(2):895-907. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Young J, Modat M, Cardoso MJ, Mendelson A, Cash D, Ourselin S. Accurate multimodal probabilistic prediction of conversion to Alzheimer's disease in patients with mild cognitive impairment. Neuroimage Clin. 2013;2:735-745. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lee G, Nho K, Kang B, Sohn KA, Kim D, for Alzheimer’s Disease Neuroimaging Initiative. Predicting Alzheimer's disease progression using multi-modal deep learning approach. Sci Rep. Feb 13, 2019;9(1):1952. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Gray KR, Aljabar P, Heckemann RA, Hammers A, Rueckert D, Alzheimer's Disease Neuroimaging Initiative. Random forest-based similarity measures for multi-modal classification of Alzheimer's disease. Neuroimage. Jan 15, 2013;65:167-175. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Liu S, Liu S, Cai W, Che H, Pujol S, Kikinis R, et al. Multimodal neuroimaging feature learning for multiclass diagnosis of Alzheimer's disease. IEEE Trans Biomed Eng. Apr 2015;62(4):1132-1140. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Hinrichs C, Singh V, Mukherjee L, Xu G, Chung MK, Johnson SC. Spatially augmented LPboosting for AD classification with evaluations on the ADNI dataset. Neuroimage. Oct 15, 2009;48(1):138-149. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Sørensen L, Igel C, Liv Hansen N, Osler M, Lauritzen M, Rostrup E, et al. Early detection of Alzheimer's disease using MRI hippocampal texture. Hum Brain Mapp. Mar 21, 2016;37(3):1148-1161. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, et al. Recent publications from the Alzheimer's disease neuroimaging initiative: reviewing progress toward improved AD clinical trials. Alzheimers Dement. Apr 2017;13(4):e1-85. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Marcus DS, Wang TH, Parker J, Csernansky JG, Morris JC, Buckner RL. Open Access Series of Imaging Studies (OASIS): cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. J Cogn Neurosci. Sep 2007;19(9):1498-1507. [ CrossRef ] [ Medline ]
- Ellis KA, Bush AI, Darby D, de Fazio D, Foster J, Hudson P, et al. The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer's disease. Int Psychogeriatr. Aug 2009;21(4):672-687. [ CrossRef ] [ Medline ]
- Jack CRJ, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. Jan 2010;9(1):119-128. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Cavedoni S, Chirico A, Pedroli E, Cipresso P, Riva G. Digital biomarkers for the early detection of mild cognitive impairment: artificial intelligence meets virtual reality. Front Hum Neurosci. Jul 24, 2020;14:245. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Teipel SJ, Sabri O, Grothe M, Barthel H, Prvulovic D, Buerger K, et al. Perspectives for multimodal neurochemical and imaging biomarkers in Alzheimer's disease. J Alzheimers Dis. 2013;33 Suppl 1:S329-S347. [ CrossRef ] [ Medline ]
- Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Cedarbaum J, et al. Impact of the Alzheimer's disease neuroimaging initiative, 2004 to 2014. Alzheimers Dement. Jul 2015;11(7):865-884. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, et al. The Alzheimer's Disease Neuroimaging Initiative 3: continued innovation for clinical trial improvement. Alzheimers Dement. May 2017;13(5):561-571. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Xu M, Zhang T, Li Z, Liu M, Zhang D. Towards evaluating the robustness of deep diagnostic models by adversarial attack. Med Image Anal. Apr 2021;69:101977. [ CrossRef ] [ Medline ]
- Khojaste-Sarakhsi M, Haghighi SS, Ghomi SM, Marchiori E. Deep learning for Alzheimer's disease diagnosis: a survey. Artif Intell Med. Aug 2022;130:102332. [ CrossRef ] [ Medline ]
- Gautam R, Sharma M. Prevalence and diagnosis of neurological disorders using different deep learning techniques: a meta-analysis. J Med Syst. Jan 04, 2020;44(2):49. [ CrossRef ] [ Medline ]
- Segato A, Marzullo A, Calimeri F, de Momi E. Artificial intelligence for brain diseases: a systematic review. APL Bioeng. Dec 2020;4(4):041503. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ding Z, Lee TL, Chan AS. Digital cognitive biomarker for mild cognitive impairments and dementia: a systematic review. J Clin Med. Jul 19, 2022;11(14):4191. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zubrikhina MO, Abramova OV, Yarkin VE, Ushakov VL, Ochneva AG, Bernstein AV, et al. Machine learning approaches to mild cognitive impairment detection based on structural MRI data and morphometric features. Cognit Syst Res. Mar 2023;78:87-95. [ CrossRef ]
- Meltzer JP, Kerry CF. Strengthening international cooperation on artificial intelligence. Policy Commons. Feb 17, 2021. URL: https://policycommons.net/artifacts/4143996/strengthening-international-cooperation-on-artificial-intelligence/4951885/ [accessed 2023-12-20]
- Gaebel W, Jessen F, Kanba S. Neurocognitive disorders in ICD-11: the debate and its outcome. World Psychiatry. Jun 2018;17(2):229-230. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kusters R, Misevic D, Berry H, Cully A, Le Cunff Y, Dandoy L, et al. Interdisciplinary research in artificial intelligence: challenges and opportunities. Front Big Data. Nov 23, 2020;3:577974. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Carmona-Lavado A, Gimenez-Fernandez EM, Vlaisavljevic V, Cabello-Medina C. Cross-industry innovation: a systematic literature review. Technovation. Jun 2023;124:102743. [ CrossRef ]
- Brown SA, Sparapani R, Osinski K, Zhang J, Blessing J, Cheng F, et al. Establishing an interdisciplinary research team for cardio-oncology artificial intelligence informatics precision and health equity. Am Heart J Plus. Jan 2022;13:100094. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Benjamens S, Dhunnoo P, Meskó B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digit Med. Sep 11, 2020;3(1):118. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Malamateniou C, Knapp KM, Pergola M, Woznitza N, Hardy M. Artificial intelligence in radiography: where are we now and what does the future hold? Radiography (Lond). Oct 2021;27 Suppl 1:S58-S62. [ CrossRef ] [ Medline ]
- Liu DS, Abu-Shaban K, Halabi SS, Cook TS. Changes in radiology due to artificial intelligence that can attract medical students to the specialty. JMIR Med Educ. Mar 20, 2023;9:e43415. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lazzeretti L, Capone F. How proximity matters in innovation networks dynamics along the cluster evolution. A study of the high technology applied to cultural goods. J Bus Res. Dec 2016;69(12):5855-5865. [ CrossRef ]
- Geldes C, Felzensztein C, Turkina E, Durand A. How does proximity affect interfirm marketing cooperation? a study of an agribusiness cluster. J Bus Res. Feb 2015;68(2):263-272. [ CrossRef ]
- Xu G, Dong C, Meng L. Research on the collaborative innovation relationship of artificial intelligence technology in Yangtze river delta of China: a complex network perspective. Sustainability. Oct 27, 2022;14(21):14002. [ CrossRef ]
- Cao Q, Tan CC, Xu W, Hu H, Cao XP, Dong Q, et al. The prevalence of dementia: a systematic review and meta-analysis. J Alzheimers Dis. 2020;73(3):1157-1166. [ CrossRef ] [ Medline ]
- Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack C, Jagust W, et al. The Alzheimer's disease neuroimaging initiative. Neuroimaging Clin N Am. Nov 2005;15(4):869-77, xi. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Morley J, Floridi L. NHS AI lab: why we need to be ethically mindful about AI for healthcare. SSRN. Sep 6, 2019. URL: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3445421 [accessed 2023-12-21]
- Wu F, Lu C, Zhu M, Chen H, Zhu J, Yu K, et al. Towards a new generation of artificial intelligence in China. Nat Mach Intell. Jun 16, 2020;2(6):312-316. [ CrossRef ]
- Li Z, Jiang X, Wang Y, Kim Y. Applied machine learning in Alzheimer's disease research: omics, imaging, and clinical data. Emerg Top Life Sci. Dec 21, 2021;5(6):765-777. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zhu Q, Xu B, Huang J, Wang H, Xu R, Shao W, et al. Deep multi-modal discriminative and interpretability network for Alzheimer's disease diagnosis. IEEE Trans Med Imaging. May 2023;42(5):1472-1483. [ CrossRef ] [ Medline ]
- Srikrishna M, Ashton NJ, Moscoso A, Pereira JB, Heckemann RA, van Westen D, et al. CT-based volumetric measures obtained through deep learning: association with biomarkers of neurodegeneration. Alzheimers Dement. Jan 2024;20(1):629-640. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Cumplido-Mayoral I, García-Prat M, Operto G, Falcon C, Shekari M, Cacciaglia R, et al. Biological brain age prediction using machine learning on structural neuroimaging data: multi-cohort validation against biomarkers of Alzheimer's disease and neurodegeneration stratified by sex. Elife. Apr 17, 2023;12:e81067. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kohannim O, Hua X, Hibar DP, Lee S, Chou YY, Toga AW, et al. Boosting power for clinical trials using classifiers based on multiple biomarkers. Neurobiol Aging. Aug 2010;31(8):1429-1442. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lee J, Burkett BJ, Min HK, Senjem ML, Lundt ES, Botha H, et al. Deep learning-based brain age prediction in normal aging and dementia. Nat Aging. May 2022;2(5):412-424. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ramanan VK, Gebre RK, Graff-Radford J, Hofrenning E, Algeciras-Schimnich A, Figdore DJ, et al. Genetic risk scores enhance the diagnostic value of plasma biomarkers of brain amyloidosis. Brain. Nov 02, 2023;146(11):4508-4519. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Luckett PH, Chen C, Gordon BA, Wisch J, Berman SB, Chhatwal JP, et al. Biomarker clustering in autosomal dominant Alzheimer's disease. Alzheimers Dement. Jan 2023;19(1):274-284. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Millar PR, Gordon BA, Luckett PH, Benzinger TL, Cruchaga C, Fagan AM, Dominantly Inherited Alzheimer Network, et al. Multimodal brain age estimates relate to Alzheimer disease biomarkers and cognition in early stages: a cross-sectional observational study. Elife. Jan 06, 2023;12:e81869. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Bayat S, Babulal GM, Schindler SE, Fagan AM, Morris JC, Mihailidis A, et al. GPS driving: a digital biomarker for preclinical Alzheimer disease. Alzheimers Res Ther. Jun 14, 2021;13(1):115. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ferreiro AL, Choi J, Ryou J, Newcomer EP, Thompson R, Bollinger RM, et al. Gut microbiome composition may be an indicator of preclinical Alzheimer's disease. Sci Transl Med. Jun 14, 2023;15(700):eabo2984. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Horie K, Salvadó G, Barthélemy NR, Janelidze S, Li Y, He Y, et al. CSF MTBR-tau243 is a specific biomarker of tau tangle pathology in Alzheimer's disease. Nat Med. Aug 2023;29(8):1954-1963. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Bae J, Logan PE, Acri DJ, Bharthur A, Nho K, Saykin AJ, et al. A simulative deep learning model of SNP interactions on chromosome 19 for predicting Alzheimer's disease risk and rates of disease progression. Alzheimers Dement. Dec 2023;19(12):5690-5699. [ CrossRef ] [ Medline ]
- O'Bryant SE, Xiao G, Barber R, Huebinger R, Wilhelmsen K, Edwards M, et al. A blood-based screening tool for Alzheimer's disease that spans serum and plasma: findings from TARC and ADNI. PLoS One. 2011;6(12):e28092. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zhang F, Petersen M, Johnson L, Hall J, O'Bryant SE. Combination of serum and plasma biomarkers could improve prediction performance for Alzheimer's disease. Genes (Basel). Sep 27, 2022;13(10):1738. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Nguyen HD, Clément M, Planche V, Mansencal B, Coupé P. Deep grading for MRI-based differential diagnosis of Alzheimer's disease and frontotemporal dementia. Artif Intell Med. Oct 2023;144:102636. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Pérez-Millan A, Contador J, Juncà-Parella J, Bosch B, Borrell L, Tort-Merino A, et al. Classifying Alzheimer's disease and frontotemporal dementia using machine learning with cross-sectional and longitudinal magnetic resonance imaging data. Hum Brain Mapp. Apr 15, 2023;44(6):2234-2244. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Planche V, Mansencal B, Manjon JV, Tourdias T, Catheline G, Coupé P. Anatomical MRI staging of frontotemporal dementia variants. Alzheimers Dement. Aug 2023;19(8):3283-3294. [ CrossRef ] [ Medline ]
- Canu E, Agosta F, Mandic-Stojmenovic G, Stojković T, Stefanova E, Inuggi A, et al. Multiparametric MRI to distinguish early onset Alzheimer's disease and behavioural variant of frontotemporal dementia. Neuroimage Clin. 2017;15:428-438. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Feis RA, Bouts MJ, Panman JL, Jiskoot LC, Dopper EG, Schouten TM, et al. Single-subject classification of presymptomatic frontotemporal dementia mutation carriers using multimodal MRI. Neuroimage Clin. 2019;22:101718. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Teunissen CE, Elias N, Koel-Simmelink MJ, Durieux-Lu S, Malekzadeh A, Pham TV, et al. Novel diagnostic cerebrospinal fluid biomarkers for pathologic subtypes of frontotemporal dementia identified by proteomics. Alzheimers Dement (Amst). 2016;2:86-94. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Bergström S, Öijerstedt L, Remnestål J, Olofsson J, Ullgren A, Seelaar H, et al. A panel of CSF proteins separates genetic frontotemporal dementia from presymptomatic mutation carriers: a GENFI study. Mol Neurodegener. Nov 27, 2021;16(1):79. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Magen I, Yacovzada NS, Warren JD, Heller C, Swift I, Bobeva Y, et al. microRNA-based predictor for diagnosis of frontotemporal dementia. Neuropathol Appl Neurobiol. Aug 2023;49(4):e12916. [ CrossRef ] [ Medline ]
- Miltiadous A, Tzimourta KD, Giannakeas N, Tsipouras MG, Afrantou T, Ioannidis P, et al. Alzheimer's disease and frontotemporal dementia: a robust classification method of EEG signals and a comparison of validation methods. Diagnostics (Basel). Aug 09, 2021;11(8):1437. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Swift IJ, Sogorb-Esteve A, Heller C, Synofzik M, Otto M, Graff C, et al. Fluid biomarkers in frontotemporal dementia: past, present and future. J Neurol Neurosurg Psychiatry. Feb 13, 2021;92(2):204-215. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Bonanni L, Thomas A, Onofrj M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. May 09, 2006;66(9):1455; author reply 1455. [ CrossRef ] [ Medline ]
- Zhou S, Meng Q, Li L, Hai L, Wang Z, Li Z, et al. Identification of a qualitative signature for the diagnosis of dementia with Lewy bodies. Front Genet. 2021;12:758103. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Villemagne VL, Chételat G. Neuroimaging biomarkers in Alzheimer's disease and other dementias. Ageing Res Rev. Sep 2016;30:4-16. [ CrossRef ] [ Medline ]
- van Steenoven I, Koel-Simmelink MJ, Vergouw LJ, Tijms BM, Piersma SR, Pham TV, et al. Identification of novel cerebrospinal fluid biomarker candidates for dementia with Lewy bodies: a proteomic approach. Mol Neurodegener. Jun 18, 2020;15(1):36. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ferreira D, Jelic V, Cavallin L, Oeksengaard AR, Snaedal J, Høgh P, et al. Electroencephalography is a good complement to currently established dementia biomarkers. Dement Geriatr Cogn Disord. 2016;42(1-2):80-92. [ CrossRef ] [ Medline ]
- Law ZK, Todd C, Mehraram R, Schumacher J, Baker MR, LeBeau FE, et al. The role of EEG in the diagnosis, prognosis and clinical correlations of dementia with Lewy bodies-a systematic review. Diagnostics (Basel). Aug 20, 2020;10(9):616. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Suzuki Y, Suzuki M, Shigenobu K, Shinosaki K, Aoki Y, Kikuchi H, et al. A prospective multicenter validation study of a machine learning algorithm classifier on quantitative electroencephalogram for differentiating between dementia with Lewy bodies and Alzheimer's dementia. PLoS One. 2022;17(3):e0265484. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Shigemizu D, Akiyama S, Asanomi Y, Boroevich KA, Sharma A, Tsunoda T, et al. A comparison of machine learning classifiers for dementia with Lewy bodies using miRNA expression data. BMC Med Genomics. Oct 30, 2019;12(1):150. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Soto M, Iranzo A, Lahoz S, Fernández M, Serradell M, Gaig C, et al. Serum MicroRNAs predict isolated rapid eye movement sleep behavior disorder and Lewy body diseases. Mov Disord. Oct 2022;37(10):2086-2098. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Chen J, Zhao X, Huang C, Lin J. Novel insights into molecular signatures and pathogenic cell populations shared by systemic lupus erythematosus and vascular dementia. Funct Integr Genomics. Nov 16, 2023;23(4):337. [ CrossRef ] [ Medline ]
- Davoudi A, Dion C, Amini S, Tighe PJ, Price CC, Libon DJ, et al. Classifying non-dementia and Alzheimer’s disease/vascular dementia patients using kinematic, time-based, and visuospatial parameters: the digital clock drawing test. J Alzheimers Dis. Jun 29, 2021;82(1):47-57. [ CrossRef ]
- Liu Y, Chan DK, Thalamuthu A, Wen W, Jiang J, Paradise M, et al. Plasma lipidomic biomarker analysis reveals distinct lipid changes in vascular dementia. Comput Struct Biotechnol J. Jun 9, 2020;18:1613-1624. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Luo J, Chen L, Huang X, Xie J, Zou C, Pan M, et al. REPS1 as a potential biomarker in Alzheimer's disease and vascular dementia. Front Aging Neurosci. 2022;14:894824. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zheng Y, Guo H, Zhang L, Wu J, Li Q, Lv F. Machine learning-based framework for differential diagnosis between vascular dementia and Alzheimer's disease using structural MRI features. Front Neurol. Oct 25, 2019;10:1097. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zheng W, Liu H, Li Z, Li K, Wang Y, Hu B, et al. Classification of Alzheimer's disease based on hippocampal multivariate morphometry statistics. CNS Neurosci Ther. Sep 2023;29(9):2457-2468. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zhang Y, Li H, Zheng Q. A comprehensive characterization of hippocampal feature ensemble serves as individualized brain signature for Alzheimer's disease: deep learning analysis in 3238 participants worldwide. Eur Radiol. Aug 2023;33(8):5385-5397. [ CrossRef ] [ Medline ]
- Qiao J, Wang R, Liu H, Xu G, Wang Z. Brain disorder prediction with dynamic multivariate spatio-temporal features: application to Alzheimer's disease and autism spectrum disorder. Front Aging Neurosci. 2022;14:912895. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Mousa D, Zayed N, Yassine IA. Alzheimer disease stages identification based on correlation transfer function system using resting-state functional magnetic resonance imaging. PLoS One. Apr 12, 2022;17(4):e0264710. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zhao J, Ding X, Du Y, Wang X, Men G. Functional connectivity between white matter and gray matter based on fMRI for Alzheimer's disease classification. Brain Behav. Oct 2019;9(10):e01407. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zhu Y, Wu Y, Lv X, Wu J, Shen C, Tang Q, et al. The relationship between APOE genotype, CSF Tau and cognition across the Alzheimer's disease spectrum, moderation and mediation role of insula network connectivity. CNS Neurosci Ther. Jan 2024;30(1):e14401. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Sadiq A, Yahya N, Tang TB, Hashim H, Naseem I. Wavelet-based fractal analysis of rs-fMRI for classification of Alzheimer's disease. Sensors (Basel). Apr 19, 2022;22(9):3102. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Xu X, Wang T, Li W, Li H, Xu B, Zhang M, et al. Morphological, structural, and functional networks highlight the role of the cortical-subcortical circuit in individuals with subjective cognitive decline. Front Aging Neurosci. Jul 9, 2021;13:688113. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zhang Q, Wang Q, He C, Fan D, Zhu Y, Zang F, et al. Altered regional cerebral blood flow and brain function across the Alzheimer's disease spectrum: a potential biomarker. Front Aging Neurosci. Feb 22, 2021;13:630382. [ CrossRef ] [ Medline ]
- Zhou Y, Song Z, Han X, Li H, Tang X. Prediction of Alzheimer's disease progression based on magnetic resonance imaging. ACS Chem Neurosci. Nov 17, 2021;12(22):4209-4223. [ CrossRef ] [ Medline ]
- He F, Li Y, Li C, Zhao J, Liu T, Fan L, et al. Changes in the connection network of whole-brain fiber tracts in patients with Alzheimer's disease have a tendency of lateralization. Neuroreport. Oct 06, 2021;32(14):1175-1182. [ CrossRef ] [ Medline ]
- Zhuang X, Zhang G, Bao M, Jiang G, Wang H, Li S, et al. Development of a novel immune infiltration-related diagnostic model for Alzheimer's disease using bioinformatic strategies. Front Immunol. Jul 20, 2023;14:1147501. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zhou S, Ma G, Luo H, Shan S, Xiong J, Cheng G. Identification of 5 potential predictive biomarkers for Alzheimer's disease by integrating the unified test for molecular signatures and weighted gene coexpression network analysis. J Gerontol A Biol Sci Med Sci. Mar 30, 2023;78(4):653-658. [ CrossRef ] [ Medline ]
- Yang X, Guo W, Yang L, Li X, Zhang Z, Pang X, et al. The relationship between protein modified folding molecular network and Alzheimer's disease pathogenesis based on BAG2-HSC70-STUB1-MAPT expression patterns analysis. Front Aging Neurosci. 2023;15:1090400. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Wei W, Wang ZY, Ma LN, Zhang TT, Cao Y, Li H. MicroRNAs in Alzheimer's disease: function and potential applications as diagnostic biomarkers. Front Mol Neurosci. 2020;13:160. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Tan MS, Cheah PL, Chin AV, Looi LM, Chang SW. Differential expression analysis of blood MicroRNA in identifying potential genes relevant to Alzheimer’s disease pathogenesis, using an integrated bioinformatics and machine learning approach. Appl Sci. Feb 27, 2023;13(5):3071. [ CrossRef ]
- Alamro H, Thafar MA, Albaradei S, Gojobori T, Essack M, Gao X. Exploiting machine learning models to identify novel Alzheimer's disease biomarkers and potential targets. Sci Rep. Mar 27, 2023;13(1):4979. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zhang C, Rodriguez C, Spaulding J, Aw TY, Feng J. Age-dependent and tissue-related glutathione redox status in a mouse model of Alzheimer's disease. J Alzheimers Dis. 2012;28(3):655-666. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Deng Y, Feng Y, Lv Z, He J, Chen X, Wang C, et al. Machine learning models identify ferroptosis-related genes as potential diagnostic biomarkers for Alzheimer's disease. Front Aging Neurosci. Sep 28, 2022;14:994130. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Wang X, Tian Y, Li C, Chen M. Exploring the key ferroptosis-related gene in the peripheral blood of patients with Alzheimer's disease and its clinical significance. Front Aging Neurosci. 2022;14:970796. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Yu H, Li M, Pan Q, Liu Y, Zhang Y, He T, et al. Integrated analyses of brain and platelet omics reveal their common altered and driven molecules in Alzheimer's disease. MedComm (2020). Dec 2022;3(4):e180. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Yu S, Liu YP, Liu HL, Li J, Xiang Y, Liu YH, et al. Serum protein-based profiles as novel biomarkers for the diagnosis of Alzheimer's disease. Mol Neurobiol. May 2018;55(5):3999-4008. [ CrossRef ] [ Medline ]
- Lin H, Himali JJ, Satizabal CL, Beiser AS, Levy D, Benjamin EJ, et al. Identifying blood biomarkers for dementia using machine learning methods in the Framingham Heart Study. Cells. Apr 30, 2022;11(9):1506. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Chang CH, Lin CH, Liu CY, Huang CS, Chen SJ, Lin WC, et al. Plasma d-glutamate levels for detecting mild cognitive impairment and Alzheimer's disease: machine learning approaches. J Psychopharmacol. Mar 2021;35(3):265-272. [ CrossRef ] [ Medline ]
- Yu H, Liu Y, He B, He T, Chen C, He J, et al. Platelet biomarkers for a descending cognitive function: a proteomic approach. Aging Cell. May 2021;20(5):e13358. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Gericke C, Kirabali T, Flury R, Mallone A, Rickenbach C, Kulic L, et al. Early β-amyloid accumulation in the brain is associated with peripheral T cell alterations. Alzheimers Dement. Dec 2023;19(12):5642-5662. [ CrossRef ] [ Medline ]
- Wu CT, Chu CI, Wang FY, Yang HY, Tseng WS, Chang CR, et al. A change of PD-1/PD-L1 expression on peripheral T cell subsets correlates with the different stages of Alzheimer's disease. Cell Biosci. Sep 30, 2022;12(1):162. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Feng Y, Chen X, Zhang XD, Huang C. Metabolic pathway pairwise-based signature as a potential non-invasive diagnostic marker in Alzheimer's disease patients. Genes (Basel). Jun 17, 2023;14(6):1285. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Yang L, Xuan C, Yu C, Zheng P, Yan J. Diagnostic model of Alzheimer’s disease in the elderly based on protein and metabolic biomarkers. J Alzheimers Dis. Feb 01, 2022;85(3):1163-1174. [ CrossRef ]
- Wang Q, Zang F, He C, Zhang Z, Xie C, Alzheimer’s Disease Neuroimaging Initiative. Dyslipidemia induced large-scale network connectivity abnormality facilitates cognitive decline in the Alzheimer's disease. J Transl Med. Dec 06, 2022;20(1):567. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Gurke R, Etyemez S, Prvulovic D, Thomas D, Fleck SC, Reif A, et al. A data science-based analysis points at distinct patterns of lipid mediator plasma concentrations in patients with dementia. Front Psychiatry. Feb 11, 2019;10:41. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Varma VR, Oommen AM, Varma S, Casanova R, An Y, Andrews RM, et al. Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: a targeted metabolomics study. PLoS Med. Jan 2018;15(1):e1002482. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Gaetani L, Bellomo G, Parnetti L, Blennow K, Zetterberg H, Di Filippo M. Neuroinflammation and Alzheimer's disease: a machine learning approach to CSF proteomics. Cells. Jul 29, 2021;10(8):1930. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Thompson LI, Cummings M, Emrani S, Libon DJ, Ang A, Karjadi C, et al. Digital clock drawing as an Alzheimer's disease susceptibility biomarker: associations with genetic risk score and APOE in older adults. J Prev Alzheimers Dis. 2024;11(1):79-87. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Cheung CY, Wong WL, Hilal S, Kan CN, Gyanwali B, Tham YC, et al. Deep-learning retinal vessel calibre measurements and risk of cognitive decline and dementia. Brain Commun. Aug 17, 2022;4(4):fcac212. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Wang X, Jiao B, Liu H, Wang Y, Hao X, Zhu Y, et al. Machine learning based on optical coherence tomography images as a diagnostic tool for Alzheimer's disease. CNS Neurosci Ther. Dec 2022;28(12):2206-2217. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lemmens S, van Craenendonck T, van Eijgen J, de Groef L, Bruffaerts R, de Jesus DA, et al. Combination of snapshot hyperspectral retinal imaging and optical coherence tomography to identify Alzheimer's disease patients. Alzheimers Res Ther. Nov 10, 2020;12(1):144. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Yilmaz A, Ugur Z, Bisgin H, Akyol S, Bahado-Singh R, Wilson G, et al. Targeted metabolic profiling of urine highlights a potential biomarker panel for the diagnosis of Alzheimer's disease and mild cognitive impairment: a pilot study. Metabolites. Aug 31, 2020;10(9):357. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Simfukwe C, Youn YC, Kim MJ, Paik J, Han SH. CNN for a regression machine learning algorithm for predicting cognitive impairment using qEEG. Neuropsychiatr Dis Treat. Apr 12, 2023;19:851-863. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Gomes TA, Prudêncio RB, Soares C, Rossi AL, Carvalho A. Combining meta-learning and search techniques to select parameters for support vector machines. Neurocomputing. Jan 2012;75(1):3-13. [ CrossRef ]
- Tan WY, Hargreaves C, Chen C, Hilal S. A machine learning approach for early diagnosis of cognitive impairment using population-based data. J Alzheimers Dis. 2023;91(1):449-461. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Shukla A, Tiwari R, Tiwari S. Alzheimer’s disease detection from fused PET and MRI modalities using an ensemble classifier. MAKE. May 18, 2023;5(2):512-538. [ CrossRef ]
- Ahmed S, Kim BC, Lee KH, Jung HY, Alzheimer’s Disease Neuroimaging Initiative. Ensemble of ROI-based convolutional neural network classifiers for staging the Alzheimer disease spectrum from magnetic resonance imaging. PLoS One. 2020;15(12):e0242712. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Gogishvili D, Vromen EM, Koppes-den Hertog S, Lemstra AW, Pijnenburg YA, Visser PJ, et al. Discovery of novel CSF biomarkers to predict progression in dementia using machine learning. Sci Rep. Apr 21, 2023;13(1):6531. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lee WJ, Brown JA, Kim HR, La Joie R, Cho H, Lyoo CH, et al. Regional Aβ-tau interactions promote onset and acceleration of Alzheimer's disease tau spreading. Neuron. Jun 15, 2022;110(12):1932-43.e5. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Mahaman YA, Embaye KS, Huang F, Li L, Zhu F, Wang JZ, et al. Biomarkers used in Alzheimer's disease diagnosis, treatment, and prevention. Ageing Res Rev. Feb 2022;74:101544. [ CrossRef ] [ Medline ]
- Chandra A, Dervenoulas G, Politis M, Alzheimer’s Disease Neuroimaging Initiative. Magnetic resonance imaging in Alzheimer's disease and mild cognitive impairment. J Neurol. Jun 2019;266(6):1293-1302. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Byeon H. Can the random forests model improve the power to predict the intention of the elderly in a community to participate in a cognitive health promotion program? Iran J Public Health. Feb 2021;50(2):315-324. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Wu X, Peng C, Nelson PT, Cheng Q. Random forest-integrated analysis in AD and LATE brain transcriptome-wide data to identify disease-specific gene expression. PLoS One. Sep 7, 2021;16(9):e0256648. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Bettencourt C, Skene N, Bandres-Ciga S, Anderson E, Winchester LM, Foote IF, Deep Dementia Phenotyping (DEMON) Network, et al. Artificial intelligence for dementia genetics and omics. Alzheimers Dement. Dec 2023;19(12):5905-5921. [ CrossRef ] [ Medline ]
- Kelly J, Moyeed R, Carroll C, Luo S, Li X. Blood biomarker-based classification study for neurodegenerative diseases. Sci Rep. Oct 11, 2023;13(1):17191. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Beltrán JF, Wahba BM, Hose N, Shasha D, Kline RP, Alzheimer’s Disease Neuroimaging Initiative. Inexpensive, non-invasive biomarkers predict Alzheimer transition using machine learning analysis of the Alzheimer's Disease Neuroimaging (ADNI) database. PLoS One. Jun 27, 2020;15(7):e0235663. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zou C, Su L, Pan M, Chen L, Li H, Zou C, et al. Exploration of novel biomarkers in Alzheimer's disease based on four diagnostic models. Front Aging Neurosci. Feb 16, 2023;15:1079433. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lin W, Gao Q, Du M, Chen W, Tong T. Multiclass diagnosis of stages of Alzheimer's disease using linear discriminant analysis scoring for multimodal data. Comput Biol Med. Jul 2021;134:104478. [ CrossRef ] [ Medline ]
- Lyall DM, Kormilitzin A, Lancaster C, Sousa J, Petermann-Rocha F, Buckley C, Deep Dementia Phenotyping (DEMON) Network, et al. Artificial intelligence for dementia-applied models and digital health. Alzheimers Dement. Dec 2023;19(12):5872-5884. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Jiang Y, Yang M, Wang S, Li X, Sun Y. Emerging role of deep learning-based artificial intelligence in tumor pathology. Cancer Commun (Lond). Apr 2020;40(4):154-166. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Suero-Abreu GA, Hamid A, Akbilgic O, Brown SA. Trends in cardiology and oncology artificial intelligence publications. Am Heart J Plus. May 2022;17:100162. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Shichijo S, Nomura S, Aoyama K, Nishikawa Y, Miura M, Shinagawa T, et al. Application of convolutional neural networks in the diagnosis of helicobacter pylori infection based on endoscopic images. EBioMedicine. Nov 2017;25:106-111. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zhang G, Song J, Feng Z, Zhao W, Huang P, Liu L, et al. Artificial intelligence applicated in gastric cancer: a bibliometric and visual analysis via CiteSpace. Front Oncol. 2022;12:1075974. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kourou K, Exarchos TP, Exarchos KP, Karamouzis MV, Fotiadis DI. Machine learning applications in cancer prognosis and prediction. Comput Struct Biotechnol J. 2015;13:8-17. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Özkaraca O, Bağrıaçık OI, Gürüler H, Khan F, Hussain J, Khan J, et al. Multiple brain tumor classification with dense CNN architecture using brain MRI images. Life (Basel). Jan 28, 2023;13(2):349. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Liu B, Liu Y, Pan X, Li M, Yang S, Li SC. DNA methylation markers for pan-cancer prediction by deep learning. Genes (Basel). Oct 04, 2019;10(10):778. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Almarzouki HZ. Deep-learning-based cancer profiles classification using gene expression data profile. J Healthc Eng. Jan 7, 2022;2022:4715998. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Jiang Y, Wang H, Wu J, Chen C, Yuan Q, Huang W, et al. Corrigendum to "noninvasive imaging evaluation of tumor immune microenvironment to predict outcomes in gastric cancer": annals of oncology 2020; 31: 760-768. Ann Oncol. Apr 2021;32(4):578. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Merkin A, Krishnamurthi R, Medvedev ON. Machine learning, artificial intelligence and the prediction of dementia. Curr Opin Psychiatry. Mar 01, 2022;35(2):123-129. [ CrossRef ] [ Medline ]
- Pemberton HG, Zaki LA, Goodkin O, Das RK, Steketee RM, Barkhof F, et al. Technical and clinical validation of commercial automated volumetric MRI tools for dementia diagnosis-a systematic review. Neuroradiology. Nov 2021;63(11):1773-1789. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Carnevale L, Lembo G. Innovative MRI techniques in neuroimaging approaches for cerebrovascular diseases and vascular cognitive impairment. Int J Mol Sci. May 30, 2019;20(11):2656. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zhang W, Zheng X, Tang Z, Wang H, Li R, Xie Z, et al. Combination of paper and electronic trail making tests for automatic analysis of cognitive impairment: development and validation study. J Med Internet Res. Jun 09, 2023;25:e42637. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ghosh A, Puthusseryppady V, Chan D, Mascolo C, Hornberger M. Machine learning detects altered spatial navigation features in outdoor behaviour of Alzheimer's disease patients. Sci Rep. Feb 24, 2022;12(1):3160. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Khodabandehloo E, Riboni D. Collaborative trajectory mining in smart-homes to support early diagnosis of cognitive decline. IEEE Trans Emerg Topics Comput. Jul 1, 2021;9(3):1194-1205. [ CrossRef ]
- Lotfi A, Langensiepen C, Mahmoud SM, Akhlaghinia MJ. Smart homes for the elderly dementia sufferers: identification and prediction of abnormal behaviour. J Ambient Intell Human Comput. Jan 7, 2011;3(3):205-218. [ CrossRef ]
- Petermann-Rocha F, Lyall DM, Quinn TJ, Ho FK, Pell JP, Celis-Morales C. Associations between physical frailty and dementia incidence: a prospective study from UK Biobank - authors' reply. Lancet Healthy Longev. Feb 2021;2(2):e68. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Korolev IO, Symonds LL, Bozoki AC, Alzheimer's Disease Neuroimaging Initiative. Predicting progression from mild cognitive impairment to Alzheimer's dementia using clinical, MRI, and plasma biomarkers via probabilistic pattern classification. PLoS One. 2016;11(2):e0138866. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Godfrey A, Brodie M, van Schooten KS, Nouredanesh M, Stuart S, Robinson L. Inertial wearables as pragmatic tools in dementia. Maturitas. Sep 2019;127:12-17. [ CrossRef ] [ Medline ]
Abbreviations
| Alzheimer disease |
| Alzheimer’s Disease Neuroimaging Initiative |
| artificial intelligence |
| apolipoprotein E |
| beta amyloid |
| convolutional neural network |
| cerebrospinal fluid |
| dementia with Lewy bodies |
| electroencephalography |
| frontotemporal dementia |
| least absolute shrinkage and selection operator |
| microRNA |
| machine learning |
| magnetic resonance imaging |
| neurofilament light |
| positron emission tomography |
| phosphorylated tau |
| support vector machine |
| vascular dementia |
| white matter |
Edited by G Eysenbach, T de Azevedo Cardoso; submitted 27.02.24; peer-reviewed by X Ouyang, Y Zhang, J Liang, K Kodama, A Azizan, S Sengoku; comments to author 22.03.24; revised version received 04.05.24; accepted 25.06.24; published 08.08.24.
©Wenhao Qi, Xiaohong Zhu, Danni He, Bin Wang, Shihua Cao, Chaoqun Dong, Yunhua Li, Yanfei Chen, Bingsheng Wang, Yankai Shi, Guowei Jiang, Fang Liu, Lizzy M M Boots, Jiaqi Li, Xiajing Lou, Jiani Yao, Xiaodong Lu, Junling Kang. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 08.08.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research (ISSN 1438-8871), is properly cited. The complete bibliographic information, a link to the original publication on https://www.jmir.org/, as well as this copyright and license information must be included.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 06 August 2024
Breaking down barriers: exploring the impact of social capital on knowledge sharing and transfer in the workplace
- Michael Yao-Ping Peng 1 , 2
Humanities and Social Sciences Communications volume 11 , Article number: 1007 ( 2024 ) Cite this article
303 Accesses
Metrics details
- Business and management
The COVID-19 pandemic has posed formidable challenges to economic mobility and corporate expansion. Among these challenges is its pronounced effect on knowledge innovation, a cornerstone upon which many organizations depend. To re-establish the flow of internal knowledge, organizations are compelled to refine their knowledge management strategies and amplify employees’ motivation and eagerness to share and transfer information. This study delves into the influence of knowledge management processes on employees’ knowledge-sharing and transfer behaviors, viewed through the lens of the social exchange theory. It also probes the role of social capital in fostering and augmenting employees’ involvement in refining these processes. Data was gleaned from 30 information service firms in mainland China, resulting in 483 valid responses. Our findings highlight that both relational and structural forms of social capital positively influence the knowledge management processes, subsequently enhancing employees’ knowledge-sharing and transfer behaviors.
Similar content being viewed by others
The effects of knowledge management processes on service sector performance: evidence from Saudi Arabia
The impact of perceived organizational support on employees’ knowledge transfer and innovative behavior: comparisons between Taiwan and mainland China

Knowledge sharing and innovation performance: a case study on the impact of organizational culture, structural capital, human resource management practices, and relational capital of real estate agents
Introduction.
Over the past two decades, the topic of knowledge governance has garnered significant interest within the academic realm, resulting in a plethora of studies and diverse conclusions (Hu et al., 2019 ). The economic implications of the COVID-19 pandemic have hindered the propensity for effective information exchange among employees, thereby attenuating the momentum toward knowledge innovation and management in various firms (Pemsel et al., 2016 ; Hu et al., 2019 ). Hence, it becomes imperative to adopt a robust knowledge management strategy to invigorate knowledge innovation within the workforce. The knowledge management process serves as a structural mechanism, designed to streamline, invigorate, steer, and oversee knowledge management initiatives and other pertinent activities within an entity (Ye et al., 2021 ; Wang et al., 2019 ). The Knowledge Management Process (KMP) is a foundational framework within organizations aimed at creating, sharing, utilizing, and managing the knowledge and information possessed by the entities (Zaim et al., 2019 ). KMP encompasses several core activities, including but not limited to, knowledge creation (Syed et al., 2021 ), storage/retrieval (Al-Emran et al., 2018 ), transfer (Borges et al., 2019 ; Cao et al., 2022 ), and application (Farooq, 2019 ). This process facilitates the efficient and effective management of organizational knowledge resources, supporting the achievement of goals such as innovation (Ahmed et al., 2019 ; Fang et al., 2013 ), competitive advantage (Peng et al., 2023 ), and continuous improvement of practices and processes (Shahzad et al., 2020 ). While evidence underscores the profound influence of this process on knowledge sharing (Al-Emran et al., 2018 ; Hu et al., 2019 ), the nuances of informal knowledge governance remain somewhat underexplored, leaving interrelationships between associated variables ambiguous (Chuang et al., 2019 ). Acknowledging the paramount importance of information for enterprises, a growing contingent of scholars posits that knowledge stands as a pivotal asset, ushering in benefits related to customer satisfaction and competitive edge (Mothe et al., 2018 ; Wang et al., 2019 ). Moreover, the knowledge management framework offers advantages like operational continuity and agile adaptability to environmental shifts—including the economic turbulence instigated by the COVID-19 pandemic—which can engender trust amongst stakeholders and clientele (Albort-Morant et al., 2018 ; Hu et al., 2019 ). This study aspires to illuminate the merits of knowledge management processes, extending novel insights into the discourse (Chuang et al., 2019 ).
This study postulates that through social exchange mechanisms, employees can bolster their positive affective orientations towards their peers, consequently aligning their behaviors more closely with the tenets of the knowledge management process (Hu et al., 2019 ). Social exchange mechanisms refer to the interpersonal interactions and social behaviors within an organization that facilitate the sharing of knowledge, grounded in mutual trust and reciprocal benefits. These mechanisms may include but are not limited to, mentoring relationships (McFadyen and Cannella Jr, 2004 ), team-based collaborative projects (Janowicz-Panjaitan and Noorderhaven, 2009 ), and systems that reward contributions to collective knowledge pools (Gagné et al., 2019 ). Such practices foster a culture where trust is paramount, and knowledge is exchanged as part of reciprocal social interactions, creating an environment conducive to innovation and collaboration (Adler and Kwon, 2002 ). To achieve more notable results in knowledge exchange, management must nurture these social exchange mechanisms. This involves instituting policies that promote reciprocal knowledge sharing, such as establishing mentorship programs that pair less experienced employees with more seasoned colleagues (Bolino et al., 2002 ), deploying collaborative platforms that encourage cross-functional dialog and idea exchange (Reagans and McEvily, 2003 ), and adopting leadership approaches that prioritize open communication and mutual support within the team dynamic (Le and Lei, 2018 ). Additionally, management should work towards breaking down silos within the organization to enhance cross-functional collaboration and ensure that knowledge flows seamlessly across departments and teams (Cao and Xiang, 2012 ). This assumption is grounded in the theoretical framework of the social exchange relationship that exists between organizations and their constituents (Chuang et al., 2019 ). The established codes and relational dynamics are anticipated to catalyze the dissemination of knowledge within employee cohorts (Wang et al., 2019 ). Those beneficiaries of such shared expertise are theorized to reciprocate emotionally with the knowledge sharer, invoking a virtuous cycle of interaction consistent with Bearman’s ( 1997 ) conceptualization of social exchange. It is noteworthy that certain scholars have identified persisting lacunae in the literature concerning knowledge management processes (Chuang et al., 2019 ). Empirical findings delineating the motivations and modalities of information propagation and transference remain nebulous, notwithstanding the considerable scholarly attention directed toward the intricacies of the knowledge management process (Al-Emran et al., 2018 ; Hu et al., 2019 ).
Historically, academic discourse has gravitated more toward the availability of information-sharing platforms than the intrinsic motivational drivers underpinning knowledge sharing (Wang et al., 2019 ). However, numerous empirical inquiries have corroborated that the knowledge management architecture intrinsically augments knowledge dissemination and transference (Shraah et al., 2022 ; Hu et al., 2019 ; Peng and Shao, 2021 ). To elucidate, both the infrastructural avenues facilitating knowledge sharing and individual’s intrinsic motivations critically influence knowledge exchange behaviors (Chuang et al., 2019 ). The multifaceted construct of knowledge sharing encompasses aspects of information interchange, archival, retrieval, and the codification of systematic organizational procedures (Hassan et al., 2016 ). As articulated by Lailin and Gang ( 2016 ), knowledge transfer transcends mere information relay and encapsulates the holistic process of selection, assimilation, integration, and application of knowledge (Zhao et al., 2021 ). It is therefore incumbent upon the knowledge management framework to engender conducive environments for knowledge disseminators, while concurrently proffering appropriate incentives (Harzing et al., 2016 ; Shraah et al., 2022 ). A conspicuous gap in empirical literature persists regarding the functionality, relevance, and potency of knowledge-sharing and transfer mechanisms, with a marked dearth of insights specific to the information service sector (Chuang et al., 2019 ). This investigation endeavors to elucidate the ramifications of the knowledge management paradigm on employee’s propensities towards knowledge sharing and transference (Hu et al., 2019 ).
Organizations institute knowledge management processes with the intent of enhancing the exchange and transfer of knowledge among employees (Zhao et al., 2021 ). However, existing literature suggests that a rigid management framework might engender feelings of exclusion among employees, possibly diminishing their propensity to disseminate information and prompting opportunistic tendencies (Hassan et al., 2016 ). Areed et al. ( 2021 ) posited that intra-organizational social dynamics influence the appraisal of an individual’s social capital. This, in turn, can facilitate knowledge transfer at an individualistic level, thereby augmenting organizational value. In this context, social capital is understood as the sum of actual and potential resources embedded within, available through, and derived from the network of relationships possessed by an individual or organization (Adler and Kwon, 2002 ; Bolino et al., 2002 ). It is broadly categorized into structural social capital, which refers to the impersonal configuration of linkages between individuals or units (Burt, 2000 ; Coleman, 1988 ), and relational social capital, which emphasizes the personal relationships developed through a history of interactions, characterized by trust, reciprocity, and mutual respect (Putnam, 1995 ; Nahapiet & Ghoshal, 1998 ). These dimensions of social capital facilitate cooperative behaviors and knowledge exchange, enhancing organizational and individual performance (Le and Lei, 2018 ; Reagans and McEvily, 2003 ). The knowledge management process shapes individuals’ perceptions regarding governance protocols and is demonstrably influenced by social capital (Al-Emran et al., 2018 ). Interpersonal affiliations can bolster both formal and informal communication channels among staff, thereby promoting resource and expertise exchange (Shraah et al., 2022 ). Recent empirical evidence from Bhatti et al. ( 2021 ) underscores the profound influence of social capital on members’ tendencies towards knowledge sharing. Furthermore, Swanson et al. ( 2020 ) asserted that social capital positively modulates information dissemination across structural, relational, and cognitive dimensions. Nevertheless, the nexus between social capital and the knowledge management process remains underexplored (Lailin and Gang, 2016 ). Consequently, this study contends that delving into the ramifications of social capital on the knowledge management process holds significant academic and practical implications (Harzing et al., 2016 ; Shraah et al., 2022 ).
Literature review
Social exchange theory.
The social exchange theory offers an analytical framework to interpret behaviors manifesting in social transactions. The rewards derived from social exchanges, both intrinsic and extrinsic, are often nebulous and defy precise quantification (Blau, 1964 ). Consequently, the emphasis on social exchanges gravitates toward the cultivation of enduring relationships rather than transient transactions (Shariq et al., 2019 ; Bolino et al., 2002 ; Bourdieu, 1986 ). Within this paradigm, knowledge dissemination can be conceptualized as a variant of social exchange where participants engage in oblique transactions (Shariq et al., 2019 ; Al-Emran et al., 2018 ; Farooq, 2019 ). The individual proffering knowledge prioritizes relationship cultivation over immediate gains, with the knowledge management system serving as an intermediary bridge connecting knowledge donors and recipients (Kankanhalli et al., 2005 ; Foss et al., 2010 ; Hansen, 1999 ). Leveraging the social exchange theory can yield insights into the merits of the knowledge management process, thereby optimizing returns for employees and fostering a robust culture of knowledge exchange and transfer (Ganguly et al., 2019 ; Ghahtarani et al., 2020 ; Mohajan, 2019 ).
The knowledge management process can significantly enhance the efficacy of knowledge transfer within organizational frameworks, as articulated by Ye et al. ( 2021 ). “Knowledge transfer” is defined as the dissemination of expertise from one entity to another via experienced conduits, and it is instrumental in amplifying organizational performance (Hamdoun et al., 2018 ; Lombardi, 2019 ; Al-Emran et al., 2018 ). As delineated by Farooq ( 2019 ), knowledge transfer is inherently unidirectional, signified by the transmission of information from the donor to the recipient. This encompasses the donor’s act of proffering information and the recipient’s subsequent assimilation and application of said information (Hansen, 1999 ; Foss and Pedersen, 2019 ). Moreover, the process of knowledge transfer is punctuated by stages of translation and transformation, as expounded by Krylova et al. ( 2016 ). Through these stages, knowledge is rendered more comprehensible and actionable (Lombardi, 2019 ; Ferraris et al., 2020 ), underscoring the applicability of the transferred knowledge within the recipient’s domain and illustrating the continuity of information flow (Lilleoere and Holme, 2011 ). Within the realm of academia, knowledge governance should pivot on the methodologies employed by educators in disseminating their knowledge and the pivotal role of leadership in orchestrating knowledge governance (Fabiano et al., 2020 ; Foss et al., 2010 ).
Information dissemination is a critical facet of knowledge-oriented endeavors and is imperative for transmuting individualized knowledge into an organizational asset. This practice augments capabilities pertaining to innovation, knowledge synthesis, and generative knowledge, whilst facilitating integration and application at the organizational level (Foss and Pedersen, 2019 ; Ritala and Stefan, 2021 ; Farooq, 2019 ). Hansen ( 1999 ) postulates that knowledge dissemination encompasses the mutual exchange of expertise, acumen, insights, and advisories amongst team constituents. The propensity to support peers is intrinsically linked with knowledge-sharing behavior, and both extrinsic and intrinsic inducements exert a profound influence on the predisposition toward knowledge dissemination (Foss and Pedersen, 2019 ; Ganguly et al., 2019 ; Borges et al., 2019 ; Yong et al., 2020 ; Peng, 2022 ). Amplified motivation heightens members’ discernment of the merits inherent in knowledge contribution, thereby catalyzing the sharing dynamic. The volition for information exchange, coupled with the presence of conducive platforms, predicates the volume and quality of expertise disseminated (Foss and Pedersen, 2019 ; Ganguly et al., 2019 ; Zhao et al., 2021 ; Abbas et al., 2020 ). Lilleoere and Hansen ( 2011 ) assert that members’ perception of available dissemination avenues critically influences intra-organizational knowledge sharing. The attendant risks and overheads associated with sharing diminish when individuals can leverage platforms underpinned by their social affiliations, thus nurturing a pro-sharing disposition (Anwar et al., 2019 ; Bhatti et al., 2021 ). A predisposition to share is fortified when individuals perceive unencumbered access to sharing conduits (Gagné et al., 2019 ; Saleh and Bista, 2017 ).
Knowledge management process
The Knowledge Management Process (KMP) serves as an instrumental framework within the ambit of the knowledge-based economy. In an era characterized by rapid shifts in consumer expectations and relentless market competition, organizations are increasingly reliant on KMP for the procurement and operationalization of innovative knowledge (Ahmed et al., 2019 ; Xie et al., 2019 ). KMP is delineated as a structured endeavor aimed at either enhancing organizational performance or offering value-added services to the community through the strategic deployment of extant expertise (Zaim et al., 2019 ; Farooq, 2019 ). It acts as a foundational nexus for the acquisition, dissemination, and efficacious utilization of knowledge assets, which in turn catalyze organizational innovation (Migdadi, 2021 ; Al-Emran et al., 2018 ). Empirical studies underscore the pivotal role of KMP’s triadic components: knowledge acquisition (KA), knowledge dissemination (KD), and knowledge application (KAP), in augmenting the processes of information sharing and transfer (Qasrawi et al., 2017 ; Shahzad et al., 2020 ; Han et al., 2019 ). These components serve as the foundational mechanisms through which knowledge is managed within organizations. Specifically, KA involves the identification and absorption of new knowledge (Al-Emran et al., 2018 ); KD refers to the distribution of knowledge within the organization (Borges et al., 2019 ); and KAP pertains to the effective utilization of knowledge in decision-making and organizational practices (Farooq, 2019 ). To clarify, the impact of KMP extends beyond the mere facilitation of these components. The successful implementation of KMP leads to tangible outcomes, including enhanced organizational innovation (Ahmed et al., 2019 ), improved employee performance (Abbas et al., 2020 ), and increased competitive advantage (Zaim et al., 2019 ). Therefore, when it is stated that KMP fosters knowledge sharing, it implies that the systematic and structured approach to managing knowledge—encompassing acquisition, dissemination, and application—enables a culture and practice of sharing, which in turn contributes to these broader organizational outcomes. This delineation ensures that the discussion of KMP’s role in fostering knowledge sharing is not circular but indicative of its comprehensive impact on organizational knowledge dynamics. Engaging with stakeholders through this structured paradigm enables organizations to assimilate novel information and gain nuanced insights into consumer predilections within evolving market landscapes (Shahzad et al., 2020 ; Borges et al., 2019 ).
Furthermore, the assimilated knowledge is harnessed to enhance both the final products and internal processes of the enterprise (Migdadi, 2021 ; Farooq, 2019 ). Institutions that prioritize knowledge often motivate their personnel to actively engage in organizational activities, thereby offering pragmatic solutions (Abbas et al., 2020 ). Environmental specialists and behavioral scientists posit that the consumption of non-sustainable products significantly contributes to environmental degradation, manifested in pollution, deteriorated air quality, and climate perturbations (Li et al., 2019; Hamdoun et al., 2018 ). The social exchange theory postulates that organizations fortified with robust KMP and nimble competencies are better positioned to innovate and manufacture sustainable commodities, thereby mitigating adverse impacts on both society and the environment (Foss and Pedersen, 2019 ; Ganguly et al., 2019 ).
Research has underscored the advantages of KMP in facilitating knowledge exchange (Al-Emran et al., 2018 ; Olaisen & Revang, 2017 ; Shahzad et al., 2020 ). Han et al. ( 2019 ) contend that there exists a lacuna in understanding the influence of structured knowledge governance on knowledge dissemination. A recent investigation by Syed et al. ( 2021 ) assessed the ramifications of both structured and unstructured KMP on knowledge dissemination, revealing a dichotomy between organizational expectations and individual employee motivations (Migdadi, 2021 ). While enterprises anticipate that employees will disseminate knowledge for collective advantage, individuals often retain specialized knowledge to safeguard their personal vested interests and organizational stature (Farooq, 2019 ). Consequently, a socio-organizational paradox emerges between firms and their staff. Cao and Xiang ( 2012 ) posit that KMP is pivotal in augmenting knowledge dissemination, serving as a catalyst for collaborative knowledge sharing among personnel (Ali et al., 2018 ; Qi and Chau, 2018 ; Xie et al., 2019 ). Given these considerations, this study propounds the ensuing hypothesis:
H1: Knowledge management process has a positive impact on knowledge sharing behavior .
The efficacy of a knowledge management approach, as delineated by Syed et al. ( 2021 ), holds promise not merely for bolstering information dissemination but also for enhancing the cognitive capacities of employees, paving the way for sustained knowledge transmission (Migdadi, 2021 ). An integral knowledge management framework is quintessential for cultivating a sharing ethos, institutionalizing methodologies, and judicious resource allocation within enterprises (Shahzad et al., 2020 ). Cultivating a sharing ethos stimulates employees to disseminate their acquired insights with colleagues (Al-Emran et al., 2018 ). Standardizing methodologies, encompassing operational protocols, documentation architectures, and reward-sanction mechanisms, lays the groundwork for facilitating knowledge exchange among staff (Fabiano et al., 2020 ; Olaisen & Revang, 2017 ). In scenarios of constrained resources, prudent resource stewardship becomes pivotal to amplifying knowledge transmission efficiency (Xie et al., 2019 ). Concurrently, elements such as trust, socio-professional networks, and personal identification exert significant influence on knowledge propagation (Han et al., 2019 ; Zhao et al., 2021 ; Qi and Chau, 2018 ). Interpersonal network affiliations and trust modulate the extent of tacit knowledge dissemination, while personal identification gauges the intrinsic worth of knowledge (Ali et al., 2018 ). Given the intricacies inherent in knowledge transmission, the salience of knowledge governance in elevating the efficacy of knowledge asset dissemination is accentuated (Zaim et al., 2019 ). In light of these insights, this study advances the subsequent hypothesis:
H2: Knowledge management process has a positive impact on knowledge transfer behavior .
Social capital
While earlier research acknowledged the role of knowledge management in integrating various organizational processes, it often overlooked the critical influence of social connections (Syed et al., 2021 ). Recently, however, there has been a noticeable shift towards examining the social aspects of knowledge management, particularly the role of social capital among organizational members, as highlighted in the studies by Ghahtarani et al. ( 2020 ) and Pemsel et al. ( 2016 ). The exploration of social capital has evolved significantly, tracing back to the pioneering works of Coleman ( 1988 ) and Putnam ( 1995 ), while also acknowledging the contributions of Pierre Bourdieu (Han et al., 2019 ). Coleman ( 1988 ) introduced social capital within a broader sociological framework, emphasizing its role in enabling specific actions through leveraging norms, networks, and social trust within social structures. Putnam ( 1995 ) further elaborated on social capital, elucidating its capacity to strengthen communities and organizations through networks of civic engagement, trust, and reciprocity, thus underlining the vital contribution of social capital to societal betterment and organizational innovation (Akram et al., 2017 ; Peng, 2022 ).
Simultaneously, Bourdieu’s examination offers a comprehensive perspective on social capital as the accumulation of real or potential resources stemming from one’s network, characterized by various degrees of institutionalized relationships, mutual familiarity, and recognition. His insights underscore the importance of networking and its benefits within a sociological context (Zhao et al., 2021 ). Social capital, as articulated by these scholars, comprises both hidden and overt resources that are accessible through networks of affiliation. According to Ganguly et al. ( 2019 ), Edinger & Edinger ( 2018 ), and as reinforced by Qi and Chau ( 2018 ), social capital within a team or organization facilitates the achievement of collective goals through enhanced cooperation and trust. Teams or collectives that effectively leverage their social capital, described by Al-Emran et al. ( 2018 ) as possessing a more readily mobilizable form, demonstrate greater efficiency in accessing, sharing knowledge, and mutual support, thus significantly elevating organizational performance.
Therefore, assessing a team’s social capital necessitates a comprehensive understanding of its broader organizational context, focusing on how social structures, networks, and the nature of interpersonal relationships contribute to achieving organizational goals (Coleman, 1988 ; Putnam, 1995 ; Alghababsheh & Gallear, 2020 ). Research efforts, as noted by Ganguly et al. ( 2019 ), Lucas et al. ( 2018 ), and Pinho & Prange ( 2016 ), commonly employ both relational and structural measures to quantify social capital. The relational dimension of social capital refers to the quality of personal relationships that exist within networks, characterized by trust, mutual respect, and an obligation to reciprocate, which facilitate cooperative behaviors and information sharing among individuals (Akram et al., 2017 ; Chen et al., 2020 ; Zhou et al., 2021 ). This dimension emphasizes the importance of strong, trust-based relationships in enabling effective communication and collaboration (Zhou et al., 2022 ). Conversely, the structural dimension of social capital pertains to the overall configuration of connections within a network, including the density and connectivity of social ties that enable individuals to access resources and information (Akram et al., 2017 ; Chen et al., 2020 ; Zhou et al., 2021 ). This dimension focuses on how the structure of networks, rather than the quality of individual relationships, facilitates or impedes the flow of information and resources across an organization. This study posits that an encompassing assessment that incorporates both relational and structural dimensions is crucial to fully appreciate the extensive benefits that social capital brings to information and knowledge management (Shahzad et al., 2020 ). Recent studies further underscore this point, showing that the interplay between relational and structural dimensions of social capital significantly impacts organizational innovation and performance (Reagans and McEvily, 2003 ; Hu and Randel, 2014 ). Solely focusing on one dimension might obscure the depth of insights and information employees derive from their social networks (Olaisen & Revang, 2017 ). This integrated perspective combines the foundational theories of social capital with current research, highlighting its pivotal role in enhancing knowledge management within organizations.
The construct of social capital encapsulates the inherent attributes of mutualistic network relationships, exerting influence not solely on knowledge dissemination but also bolstering the aptitude of employees to assimilate and deploy novel insights (Syed et al., 2021 ). Fundamental social dynamics, epitomized by intra-organizational cohesion and trust, stand as linchpins in sculpting a vibrant social network (Han et al., 2019 ). Such a network serves as a conduit for the seamless transition of organizational assets, acumen, and proficiencies (Danilov and Mihailova, 2021 ; Ahmed et al., 2019 ). The intricacies of social processes are instrumental in the genesis and operationalization of knowledge, underscoring the indispensable nature of social capital (Ghahtarani et al., 2020 ; Pemsel et al., 2016 ). In light of these considerations, this study delineates the ensuing hypotheses:
H3a: Relational social capital has a positive impact on knowledge management process .
H3b: Structural social capital has a positive impact on knowledge management process .
Prior empirical investigations, as highlighted by Swanson et al. ( 2020 ) and Rezaei et al. ( 2020 ), have meticulously probed the nexus between structural social capital and knowledge management. Their collective inference suggests that knowledge management acts as a catalyst for enhancing structural social capital (Liu & Meyer, 2020 ). Structural social capital inherently mirrors the intensity and regularity of affiliations among colleagues (Sheng & Hartmann, 2019 ). An amplified frequency of engagements, underpinned by social capital, bestows upon employees augmented avenues to disseminate explicit data and assimilate tacit wisdom (Foss and Pedersen, 2019 ). Such dynamics inevitably bolster the propensity to disseminate information, magnifying its periodicity, depth, and breadth within the confines of social exchanges (Khan and Khan, 2019 ). Bolino et al. ( 2002 ) accentuated that reciprocal trust stands as the cornerstone in the edifice of social capital connections. The magnitude of mutual trust and collaboration epitomizes the essence of social capital affiliations (Ganguly et al., 2019 ; Le and Lei, 2018 ). An elevated echelon of trust within the workforce invariably catalyzes a heightened inclination to unveil tacit insights and privileged intelligence (Ferraris et al., 2020 ). Intimate synergies among employees expedite the conveyance of tacit understanding, whereas the prevailing norms, trust, and collaboration within the social capital framework magnify the prospects for personnel to reciprocate and promulgate explicit knowledge (Gubbins and Dooley, 2021 ). Given these precepts, the subsequent hypothesis is posited:
H4a: Relational social capital has a positive impact on knowledge sharing behavior .
H4b: Structural social capital has a positive impact on knowledge sharing behavior .
H5a: Relational social capital has a positive impact on knowledge transfer behavior .
H5b: Structural social capital has a positive impact on knowledge transfer behavior .
According to the above hypotheses, the research framework is shown in Fig. 1 :
Research framework.
Methodology
This study aims to explore knowledge management practices within the research and development (R&D) sector of the information service industry, with a keen focus on companies operating within the People’s Republic of China. Recognizing the importance of ethical research practices, particularly in safeguarding the confidentiality of participants’ identities during the survey process, this study employs a purposive sampling method. This approach facilitates a targeted examination of specific characteristics within a select population group, ensuring that the identity of respondents is meticulously protected from any potential threats or breaches of confidentiality. The research was conducted among companies characterized by a unique amalgamation of state-driven and market-driven economic practices, a hallmark of the Chinese business environment. This environment, distinct from traditional capitalist market economies due to significant state intervention, impacts various management processes and practices. The companies surveyed span multiple sectors—manufacturing, technology, and services—and are situated in major economic hubs such as Shanghai, Shenzhen, and Guangzhou. Engaging with companies that, despite being rooted in a Chinese context, often participate in global markets, provides a relevant and rich field for examining knowledge management practices. In conducting this study, special emphasis was placed on research ethics to protect the identities of participants and prevent any inadvertent threats. The unique blend of influences in the Chinese context—ranging from state policies and cultural nuances to the historical evolution of the Chinese economic system—contributes significantly to the shaping of management processes. This backdrop allows for an intriguing exploration of knowledge management practices in an environment that diverges from the purely capitalist model, underpinned by a strong commitment to maintaining the highest standards of confidentiality and ethical rigor in the research process.
The study population was comprised of R&D workers from high-tech companies, excluding administrative personnel, to ensure a representative sample. Before commencing the sampling process, all research procedures, including the methods of data collection and analysis, were reviewed and approved. This was to ensure that the study adhered to the highest ethical standards and that the rights and privacy of participants were protected. All participants were informed about the purpose of the study, and their informed consent was obtained. They were also assured of the confidentiality of their responses and were informed that they could withdraw from the study at any time without any repercussions. The study utilized an electronic questionnaire to gather data from the participants. Out of 490 individuals approached, we received 483 valid responses after removing 7 invalid ones, yielding a response rate of approximately 98.6%. Such a high response rate is in line with Saleh and Bista’s ( 2017 ) emphasis on the importance of response rates in determining the reliability and validity of survey findings. The results showed that the majority of the participants were male (63.1%), highly educated with a master’s degree or above (61.4%), and between the ages of 30 and 40 years old (72.1%). The average work experience of the participants was 5.2 years. These demographic characteristics provide a clear picture of the study participants and offer important insights into the impact of identity threats on information-sharing behaviors among R&D workers in the information service industry.
The study employed a bespoke questionnaire to evaluate various factors within the realm of industrial practice. A five-point Likert scale was used to gauge the magnitude of each factor, where 1 signifies “strongly disagree” and 5 represents “strongly agree.” The instrument for assessing social capital was informed by the model proposed by Tsai et al. ( 2014 ) and underwent modifications building on scales developed by Lin and Huang ( 2010 ), Yilmaz and Hunt ( 2001 ), and Croteau and Raymond ( 2004 ). This questionnaire encompasses seven items probing both relational and structural dimensions of social capital, with the verbiage tailored to the industrial milieu.
The knowledge management process is gauged using a questionnaire grounded in the scales formulated by Shahzad et al. ( 2020 ) and refined by Migdadi ( 2021 ) to suit the information service industrial setting. The tripartite components of the knowledge management process—knowledge acquisition, knowledge dissemination, and knowledge application—are delineated into 6, 5, and 5 items correspondingly. The metric for assessing knowledge-sharing is derived from Al-Emran et al. ( 2018 ), encompassing 11 items that scrutinize three facets of knowledge-sharing practices: motivation, opportunities, and behavior.
The knowledge transfer scale is adapted from Reagans and McEvily ( 2003 ), and the five questions in the questionnaire evaluate employees’ knowledge transfer situations. The terminology is altered to fit the context, and the questions aim to assess the ease of transferring knowledge and information. The scale of constructs is shown in Table 1 .
Analysis strategy
In this study, we employed Structural Equation Modeling (SEM) as our primary analytical tool. SEM was chosen due to its capability to assess complex relationships between observed and latent variables, allowing for a comprehensive understanding of the underlying constructs in our research model. SEM is particularly beneficial for research like ours, where multiple relationships are hypothesized simultaneously, and it provides a more nuanced understanding of the direct and indirect effects between variables (Dash and Paul, 2021 ; Savalei, 2020 ). Moreover, SEM’s flexibility in handling both measurement and structural models makes it an apt choice for our study, ensuring robustness in our findings (Hallgren et al., 2019 ).
Measurement
In line with the recommendations of Hair et al. ( 2017 ), our first step was to evaluate the measurement model. This involved assessing the reliability and validity of the constructs used in the study. The validity of the postulated factor structure was appraised using confirmatory factor analysis (CFA). Adhering to the two-step CFA approach recommended by Anderson and Gerbing ( 1988 ), the construct validity of the model was ascertained. Initially, individual item reliability was evaluated by analyzing the direct loadings or correlations between the measures (or indicators) and their pertinent constructs. It was deemed imperative to verify that the factor loadings of these indicators surpassed 0.7, denoting a robust linkage (Hair et al., 2014 ). Subsequently, the model’s reliability was affirmed by scrutinizing Cronbach’s alpha and composite reliability (CR) metrics, both of which exceeded the benchmark value of 0.7 as posited by Hair et al. ( 2017 ). In the third step, the average variance extracted (AVE) metrics were observed to exceed the threshold of 0.50 (Hair et al., 2017 ), indicating satisfactory convergent validity, as shown in Table 2 .
Discriminant validity is a crucial aspect of construct validity, ensuring that a construct is distinctly different from other constructs within the model (Hair et al., 2016 ). In essence, it assesses the extent to which a construct is truly unique and not just a reflection of other constructs in the model. For our study, discriminant validity was tested to ensure that the measures of our constructs were not highly correlated with measures of other constructs, thereby confirming that each construct captures a unique phenomenon. In Table 3 , the results for discriminant validity are presented. The diagonal values represent the square root of the average variance extracted (AVE) for each construct, while the off-diagonal values are the correlations between constructs. For adequate discriminant validity, the diagonal values (square root of AVE) should be greater than the off-diagonal values in the corresponding rows and columns (Fornell & Larcker, 1981 ). As can be observed in Table 3 , our model meets this criterion, indicating satisfactory discriminant validity. It’s worth noting that discriminant validity is not just a statistical requirement but also a theoretical one. Ensuring distinct constructs allows for clearer interpretations of the relationships among constructs and enhances the robustness of the theoretical framework (Henseler et al., 2015 ).
Hypothesis testing
In this study, the structural model was assessed utilizing SmartPLS 3.0, with the linkages and foundational assumptions of the conceptual framework validated via PLS-SEM. Subsequent to the evaluation of the measurement model, we advanced to the structural model examination, adhering to the guidelines proposed by Hair et al. ( 2017 ). This phase entailed scrutinizing the interrelations among the constructs and appraising the posited hypotheses. When deploying PLS-SEM, it is imperative to assess both the model’s quality and the variances of the dependent variables, with pertinent metrics encompassing SRMR, NFI, Q2, and R2. Prior to delving into hypothesis testing, collinearity’s potential influence must be ascertained within the structural model. This entails examining whether variance inflation factors (VIFs) exceed the conventional threshold of 3. The results elucidate that collinearity is not a concern in this study, given that all VIF metrics fall below 3. Additionally, a bootstrapping method with 5000 subsamples was employed for the structural models in this investigation.
The findings of this investigation are shown in Fig. 2 and Table 4 . Regarding H1 and H2, the findings show that the knowledge management process has a favorable and substantial impact on workers’ knowledge-sharing and transfer behaviors ( β = 0.634, p = 0.000) and ( β = 0.587, p = 0.000). H1 and H2 are thus supported. Additionally, the findings indicate that structural social capital ( β = 0.525, p = 0.000) and relational social capital ( β = 0.464, p = 0.000) both have a favorable and substantial impact on the knowledge management process, supporting H3a and H3b. H4a and H4b are verified because relational social capital ( β = 0.532, p = 0.000) and structural social capital ( β = 0.214, p = 0.000) have a favorable effect on workers’ knowledge-sharing behavior. Additionally, H5a and H5b are supported by our results, which show that relational social capital ( β = 0.324, p = 0.000) and structural social capital ( β = 0.413, p = 0.000) positively influence workers’ knowledge transfer behavior.
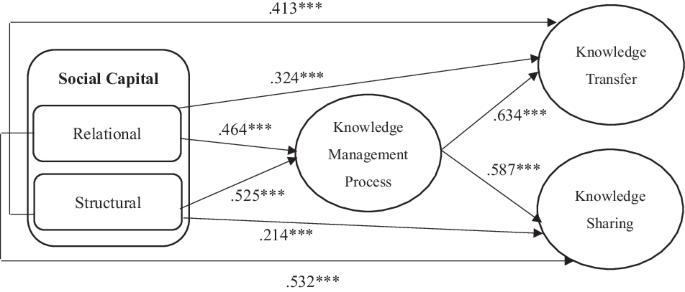
Structural model.
Conclusions
The findings from this investigation underscore that KMP, defined as the systematic approach to acquiring, disseminating, and effectively using knowledge within organizations, exerts a positive influence on the dissemination and sharing of knowledge among staff members (Foss and Pedersen, 2019 ; McFadyen and Cannella Jr, 2004 ). This observation is consonant with perspectives delineated by Abbas et al. ( 2020 ), Olaisen and Revang ( 2017 ), and Areed et al. ( 2021 ). Furthermore, it is posited that an exhaustive knowledge management regimen—encompassing the key components of knowledge acquisition, dissemination, and application—significantly enhances the flow of information by fostering an environment that encourages employees to engage in knowledge transfer activities. This is largely due to the synergistic effect these components have when effectively integrated within an organization’s practices (Zaim et al., 2019 ; Al-Emran et al., 2018 ). Specifically, by establishing a methodical and formalized framework for KMP, organizations can significantly optimize the efficacy of knowledge exchange and transmission across individuals and entities (Ferraris et al., 2020 ; Han et al., 2019 ). This approach not only streamlines the process of sharing critical information but also ensures that knowledge is accurately and efficiently circulated among employees, thereby facilitating improved decision-making and innovation (Farooq, 2019 ; Borges et al., 2019 ). The organizational socialization paradigm, as expounded by Ali et al. ( 2018 ), Qi and Chau ( 2018 ), and Han et al. ( 2019 ), modulates the employees’ inclination toward fortifying knowledge dissemination and sharing. A strong organizational affiliation and a cohesive group identity serve to enhance interpersonal communication and collaboration among staff, creating a fertile ground for the effective implementation of KMP (Ahmed et al., 2019 ; Bhatti et al., 2021 ). Consequently, the leadership’s strategic approach to knowledge management plays a crucial role in shaping the dynamics of knowledge-sharing and transfer among employees, highlighting KMP’s significant contribution to promoting a culture of open information exchange and continuous improvement (Anwar et al., 2019 ; Hamdoun et al., 2018 ; Adler and Kwon, 2002 ; Bolino et al., 2002 ).
This study ascertains that, when enriched with substantial structural and relational social capital, employees exhibit an increased propensity to engage in knowledge-sharing and transfer endeavors. In this context, social capital is defined by the structural dimension, which refers to the objective and quantifiable connections among individuals or groups, such as network ties and configurations, and the relational dimension, which pertains to the subjective and qualitative aspects of relationships, such as trust, norms, and obligations (Putnam, 1995 ; Chen et al., 2020 ; Zhou et al., 2021 ; Adler and Kwon, 2002 ; Borges et al., 2019 ). The findings underscore that both structural and relational dimensions of social capital bolster the knowledge management procedure, consequently amplifying knowledge transfer and sharing proclivities (Ganguly et al., 2019 ; Ferraris et al., 2020 ). Accordingly, the structural dimension of social capital contributes to knowledge exchange by providing a framework of connections through which information can flow, while the relational dimension enhances the quality and effectiveness of these exchanges through interpersonal rapport and mutual understanding (Adler and Kwon, 2002 ; Borges et al., 2019 ; Chen et al., 2020 ; Zhou et al., 2021 ). An elevated reservoir of social capital, as articulated by Han et al. ( 2019 ), Zhao et al. ( 2021 ), and Bhatti et al. ( 2021 ), galvanizes employee participation in knowledge management undertakings and augments knowledge-centric collaborations. Moreover, components of social capital such as trust and shared language, which facilitate mutual understanding, are identified as critical factors that yield better results in information exchange (Coleman, 1988 ; McFadyen and Cannella Jr, 2004 ; Peng et al., 2021 ). Intimate social affiliations, coupled with a multifaceted social network matrix, incentivize employees to assimilate more nuanced and invaluable insights (Borges et al., 2019 ; Hu and Randel, 2014 ). These insights lead to deeper comprehension and utilization of knowledge within the organization, as individuals feel more confident and committed to sharing information in a trustworthy environment (McFadyen and Cannella Jr, 2004 ; Hau et al., 2013 ). Consequently, this equips them with an enhanced eagerness to acquire, disseminate, and implement knowledge, thereby refining their capabilities in knowledge innovation (Foss and Pedersen, 2019 ; Gubbins and Dooley, 2021 ).
The findings of this study corroborate the theoretical perspectives delineated by Alghababsheh and Gallear ( 2020 ), Edinger and Edinger ( 2018 ), and Ganguly et al. ( 2019 ). These theories underscore that an employee’s social capital acts as a pivotal conduit for external knowledge acquisition and as a barometer for interpersonal dynamics (Bolino et al., 2002 ; Bourdieu, 1986 ). The concept of ‘bridging’ and ‘bonding’ social capital further refines this understanding by distinguishing between the types of network connections that facilitate the flow of new information (bridging) and those that strengthen existing relationships (bonding), respectively (Putnam, 1995 ; Adler and Kwon, 2002 ). Enhanced social capital can fortify the nexus between employees and their organization, thereby smoothing the channels for information dissemination (Ahmed et al., 2019 ; Han et al., 2019 ). This fortification of relationships within the organization creates a conducive environment for the free flow of innovative ideas and critical information, integral to the adaptive and competitive capabilities of firms (Peng et al., 2021 ; Zhou et al., 2021 ). As evidenced by Crompton et al. ( 2020 ) and supported by Zhao et al. ( 2021 ), social interactions wield a positive influence on the magnitude of information exchanged. Through the creation of virtual social collectives—platforms where insights, expertise, and experiences are pooled—employees can foster mutual connections, thereby facilitating knowledge assimilation and dissemination amongst themselves (Gubbins and Dooley, 2021 ; Reagans and McEvily, 2003 ).
Implications
The findings of this study illuminate that employees’ perception of the institutionalization of knowledge management augments information-sharing behaviors, especially in the context of the economic repercussions stemming from the COVID-19 pandemic (Abbas et al., 2020 ; Olaisen & Revang, 2017 ). There is a discernible positive association between the employment of a social capital-centric knowledge management strategy and the knowledge transfer and sharing behaviors of employees (Han et al., 2019 ; Zhao et al., 2021 ). Primarily, the knowledge management strategy notably bolsters employees’ proclivities towards knowledge dissemination and transfer (Alghababsheh & Gallear, 2020 ; Edinger & Edinger, 2018 ; Ganguly et al., 2019 ). For organizations aspiring to stimulate knowledge-sharing behaviors among their workforce and elevate the efficacy of intra-organizational knowledge circulation, championing a comprehensive and structured knowledge management initiative is paramount (Ali et al., 2018 ; Qi and Chau, 2018 ). By harnessing interactive knowledge management infrastructures, organizations can stimulate knowledge creation endeavors, steward knowledge transfer and sharing both intrinsically and extrinsically, and solidify synergies between macro and micro organizational tiers (Crompton et al., 2020 ). This not only augments employees’ knowledge-sharing and transfer behaviors but also nurtures a culture imbued with sharing ethos. Moreover, it anchors a robust knowledge management framework, judiciously allocates resources and precipitates favorable organizational outcomes.
The augmentation of employees’ human capital is intrinsically linked to their knowledge acquisition. Furthermore, it is their inherent social capital that catalyzes knowledge dissemination and sharing behaviors (Foss and Pedersen, 2019 ). Effective management of social capital within the workforce empowers organizations to foster their human capital, which subsequently reciprocates by amplifying their social capital (Sheng & Hartmann, 2019 ). Consequently, employees endowed with elevated social capital are more predisposed to engage in regular interpersonal exchanges, cultivating a more conducive work environment (Ghahtarani et al., 2020 ). This dynamic facilitates rapid access to and sharing of both explicit and tacit knowledge, streamlining organizational information flow and optimizing the efficacy of knowledge transfer processes (Mohajan, 2019 ).
Limitations
Despite its insights, this study is not without limitations. Firstly, this study primarily investigates the knowledge management process through the lens of social interaction (Ganguly et al., 2019 ; Le and Lei, 2018 ). While this perspective offers valuable insights, theoretical models encompassing a broader spectrum, such as embeddedness theory (Crompton et al., 2020 ) and absorptive capacity (Abbas et al., 2020 ), exist and can enrich our understanding when aligned with diverse theoretical orientations. To further enrich the conceptual depth of knowledge management theories, it is posited that scholars develop management frameworks that more effectively promote employees’ knowledge dissemination and transfer practices (Olaisen & Revang, 2017 ).
Historically, social capital has been identified as a pivotal precursor in discussions centered on its influence on knowledge transfer and sharing behaviors (Foss and Pedersen, 2019 ; Sheng & Hartmann, 2019 ). However, contemporary research positions social capital as a salient mediating variable, asserting that robust social capital can amplify the effectiveness of knowledge management strategies (Han et al., 2019 ; Zhao et al., 2021 ). Consequently, future inquiries should delve into the intermediary role of social capital to furnish a more nuanced understanding (Ghahtarani et al., 2020 ).
Lastly, this study did not undertake a comparative analysis of high-tech employees across different countries (Ali et al., 2018 ; Qi and Chau, 2018 ). Given the intricate tapestry of societal and cultural nuances, discernible disparities might exist in employees’ information-sharing tendencies across national boundaries (Borges et al., 2019 ). Therefore, it is prudent for subsequent research to evaluate the mediating effects of regional characteristics on employees’ knowledge-sharing proclivities (Valk and Planojevic, 2021 ).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. The data comprises questionnaire survey responses, which were collected and curated specifically for this research. Due to the nature of the data, it cannot be directly deposited in a public repository, but the corresponding author is willing to share the anonymized dataset with interested researchers upon request.
Abbas J, Zhang Q, Hussain I, Akram S, Afaq A, Shad MA (2020) Sustainable innovation in small medium enterprises: the impact of knowledge management on organizational innovation through a mediation analysis by using SEM approach. Sustainability 12(6):2407
Article Google Scholar
Adler PS, Kwon SW (2002) Social capital: prospects for a new concept. Acad Manag Rev 27(1):17–40
Ahmed YA, Ahmad MN, Ahmad N, Zakaria NH (2019) Social media for knowledge-sharing: a systematic literature review. Telemat Inform 37:72–112
Akram T, Lei S, Haider MJ, Akram MW (2017) What impact do structural, relational and cognitive organisational social capital have on employee innovative work behaviour? A study from China. Int J Innov Manag 21(02):1750012
Albort-Morant G, Leal-Rodríguez AL, De Marchi V (2018) Absorptive capacity and relationship learning mechanisms as complementary drivers of green innovation performance. J Knowl Manag 22(2):432–452
Al-Emran M, Mezhuyev V, Kamaludin A, Shaalan K (2018) The impact of knowledge management processes on information systems: a systematic review. Int J Inf Manag 43:173–187
Alghababsheh M, Gallear D (2020) Social capital in buyer-supplier relationships: A review of antecedents, benefits, risks, and boundary conditions. Ind Mark Manag 91:338–361
Ali I, Musawir AU, Ali M (2018) Impact of knowledge sharing and absorptive capacity on project performance: the moderating role of social processes. J Knowl Manag 22(2):453–477
Anderson JC, Gerbing DW (1988) Structural equation modeling in practice: A review and recommended two-step approach. Psychol Bull 103(3):411
Anwar R, Rehman M, Wang KS, Hashmani MA (2019) Systematic literature review of knowledge sharing barriers and facilitators in global software development organizations using concept maps. IEEE Access 7:24231–24247
Areed S, Salloum SA, Shaalan K (2021) The role of knowledge management processes for enhancing and supporting innovative organizations: a systematic review. In: Al-Emran M, Shaalan K, Hassanien A (eds) Recent Advances in Intelligent Systems and Smart Applications. Studies in Systems, Decision and Control, Vol. 295. Springer, Cham. https://doi.org/10.1007/978-3-030-47411-9_8
Bearman P (1997) Generalized exchange. Am J Sociol 102(5):1383–1415
Bhatti SH, Vorobyev D, Zakariya R, Christofi M (2021) Social capital, knowledge sharing, work meaningfulness and creativity: evidence from the Pakistani pharmaceutical industry. J Intellect Cap 22(2):243–259
Blau PM (1964) Justice in social exchange. Sociol Inq 34(2)
Bolino MC, Turnley WH, Bloodgood JM (2002) Citizenship behavior and the creation of social capital in organizations. Acad Manag Rev 27(4):505–522
Borges R, Bernardi M, Petrin R (2019) Cross-country findings on tacit knowledge sharing: evidence from the Brazilian and Indonesian IT workers. J Knowl Manag 23(4):742–762
Bourdieu P (1986) The forms of capital. In: Richardson J (Ed.) Handbook of theory and research for the sociology of education. Greenwood, New York, pp. 241–258
Google Scholar
Burt RS (2000) The network structure of social capital. Res Organ Behav 22:345–423
Cao C, Peng MYP, Xu Y (2022) How determinants of employee innovation behavior matter during the COVID-19 pandemic: investigating cross-regional role via multi-group partial least squares structural equation modeling analysis. Front Psychol 13:739898
Article PubMed PubMed Central Google Scholar
Cao Y, Xiang Y (2012) The impact of knowledge governance on knowledge sharing. Manag Decis 50(4):591–610
Chen Z, Peng MYP (2020) Impact of organizational support and social capital on university faculties’ working performance. Front Psychol 11:571559
Chen Z, Chen D, Peng MYP, Li Q, Shi Y, Li J (2020) Impact of organizational support and social capital on university faculties’ working performance. Front Psychol 11:571559
Chuang FH, Weng HC, Hsieh PN (2019) A qualitative study of barriers to innovation in academic libraries in Taiwan. Libr Manag 40(6/7):402–415
Coleman JS (1988) Social capital in the creation of human capital. Am J Sociol 94:S95–S120
Crompton CJ, Ropar D, Evans-Williams CV, Flynn EG, Fletcher-Watson S (2020) Autistic peer-to-peer information transfer is highly effective. Autism 24(7):1704–1712
Croteau AM, Raymond L (2004) Performance outcomes of strategic and IT competencies Alignment1. J Inf Technol 19(3):178–190
Danilov IV, Mihailova S (2021) Knowledge sharing in social interaction: towards the problem of primary data entry. In: 11th Eurasian Conference on Language & Social Sciences, Gjakova (virtual), Kosovo
Dash G, Paul J (2021) CB-SEM vs PLS-SEM methods for research in social sciences and technology forecasting. Technol Forecast Soc Change 173:121092
Denieffe S (2020) Commentary: purposive sampling: complex or simple? Research case examples. J Res Nurs 25:662–663
Edinger SK, Edinger MJ (2018) Improving teacher job satisfaction: The roles of social capital, teacher efficacy, and support. J Psychol 152(8):573–593
Article PubMed Google Scholar
Fabiano G, Marcellusi A, Favato G (2020) Channels and processes of knowledge transfer: How does knowledge move between university and industry? Sci Public Policy 47(2):256–270
Fang SC, Yang CW, Hsu WY (2013) Inter-organizational knowledge transfer: the perspective of knowledge governance. J Knowl Manag 17(6):943–957
Farooq R (2019) Developing a conceptual framework of knowledge management. Int J Innov Sci 11(1):139–160
Ferraris A, Santoro G, Scuotto V (2020) Dual relational embeddedness and knowledge transfer in European multinational corporations and subsidiaries. J Knowl Manag 24(3):519–533
Fornell C, Larcker DF (1981) Evaluating structural equation models with unobservable variables and measurement error. J Mark Res 18(1):39–50
Foss NJ, Pedersen T (2019) Microfoundations in international management research: the case of knowledge sharing in multinational corporations. J Int Bus Stud 50(9):1594–1621
Foss NJ, Husted K, Michailova S (2010) Governing knowledge sharing in organizations: levels of analysis, governance mechanisms, and research directions. J Manag Stud 47(3):455–482
Gagné M, Tian AW, Soo C, Zhang B, Ho KSB, Hosszu K (2019) Different motivations for knowledge sharing and hiding: the role of motivating work design. J Organ Behav 40(7):783–799
Ganguly A, Talukdar A, Chatterjee D (2019) Evaluating the role of social capital, tacit knowledge sharing, knowledge quality and reciprocity in determining innovation capability of an organization. J Knowl Manag 23(6):1105–1135
Ghahtarani A, Sheikhmohammady M, Rostami M (2020) The impact of social capital and social interaction on customers’ purchase intention, considering knowledge sharing in social commerce context. J Innov Knowl 5(3):191–199
Gubbins C, Dooley L (2021) Delineating the tacit knowledge‐seeking phase of knowledge sharing: the influence of relational social capital components. Hum Resour Dev Q 32(3):319–348
Hair JF, Black WC, Babin BJ, Anderson RE (2016) Multivariate data analysis (7th edn.). Cengage
Hair Jr J, Hair Jr JF, Hult GTM, Ringle CM, Sarstedt, M (2017) A primer on partial least squares structural equation modeling (PLS-SEM). Sage publications
Hair Jr JF, Sarstedt M, Hopkins LG, Kuppelwieser V (2014) Partial least squares structural equation modeling (PLS-SEM) An emerging tool in business research. Eur Bus Rev 26(2):106–121
Hallgren KA, McCabe CJ, King K, Atkins DC (2019) Beyond path diagrams: Enhancing applied structural equation modeling research through data visualization. Addict Behav. 94:74–82
Hamdoun M, Jabbour CJC, Othman HB (2018) Knowledge transfer and organizational innovation: Impacts of quality and environmental management. J Clean Prod 193:759–770
Han SH, Yoon DY, Suh B, Li B, Chae C (2019) Organizational support on knowledge sharing: a moderated mediation model of job characteristics and organizational citizenship behavior. J Knowl Manag 23(4):687–704
Hansen MT (1999) The search-transfer problem: the role of weak ties in sharing knowledge across organization subunits. Adm Sci Q 44(1):82–111
Harzing AW, Pudelko M, Sebastian Reiche B (2016) The bridging role of expatriates and inpatriates in knowledge transfer in multinational corporations. Hum Resour Manag 55(4):679–695
Hassan M, Aksel I, Nawaz MS, Shaukat S (2016) Knowledge sharing behavior of business teachers of Pakistani universities: an empirical testing of theory of planned behavior. Eur Sci J 12(13):29–40
Hau YS, Kim B, Lee H, Kim YG (2013) The effects of individual motivations and social capital on employees’ tacit and explicit knowledge sharing intentions. Int J Inf Manag 33(2):356–366
Henseler J, Ringle CM, Sarstedt M (2015) A new criterion for assessing discriminant validity in variance-based structural equation modeling. J Acad Mark Sci 43(1):115–135
Hu L, Randel AE (2014) Knowledge sharing in teams: social capital, extrinsic incentives, and team innovation. Group Organ Manag 39(2):213–243
Hu YF, Hou JL, Chien CF (2019) A UNISON framework for knowledge management of university–industry collaboration and an illustration. Comput Ind Eng 129:31–43
Islam T, Ahmad S, Kaleem A, Mahmood K (2020) Abusive supervision and knowledge sharing: moderating roles of Islamic work ethic and learning goal orientation. Management Decision
Janowicz-Panjaitan M, Noorderhaven NG (2009) Trust, calculation, and interorganizational learning of tacit knowledge: an organizational roles perspective. Organ Stud 30(10):1021–1044
Kankanhalli A, Tan BC, Wei KK (2005) Contributing knowledge to electronic knowledge repositories: An empirical investigation. MIS Quarterly 113–143
Khan NA, Khan AN (2019) What followers are saying about transformational leaders fostering employee innovation via organisational learning, knowledge sharing and social media use in public organisations? Gov Inf Q 36(4):101391
Krylova KO, Vera D, Crossan M (2016) Knowledge transfer in knowledge-intensive organizations: the crucial role of improvisation in transferring and protecting knowledge. J Knowl Manag 20(5):1045–1064
Lailin H, Gang Y (2016) An empirical study of teacher knowledge transfer based on e-learning. Mod Distance Educ Res 4:80–90
Le PB, Lei H (2018) The mediating role of trust in stimulating the relationship between transformational leadership and knowledge sharing processes. J Knowl Manag 22(3):521–537
Lee TC, Yao-Ping Peng M, Wang L, Hung HK (2021) Factors influencing employees’ subjective wellbeing and job performance during the COVID-19 global pandemic: the perspective of social cognitive career theory. Front Psychol 12:577028
Lilleoere AM, Holme Hansen E (2011) Knowledge‐sharing enablers and barriers in pharmaceutical research and development. J Knowl Manag 15(1):53–70
Lilleoere AM, Hansen EH (2011) Knowledge‐sharing practices in pharmaceutical research and development—A case study. Knowl Process Manag 18(3):121–132
Lin K, Peng MYP, Anser MK, Yousaf Z, Sharif A (2021) Bright harmony of environmental management initiatives for achieving corporate social responsibility authenticity and legitimacy: glimpse of hotel and tourism industry. Corp Soc Responsib Environ Manag 28(2):640–647
Lin TC, Huang CC (2010) Withholding effort in knowledge contribution: The role of social exchange and social cognitive on project teams. Inf Manag 47(3):188–196
Liu Y, Meyer KE (2020) Boundary spanners, HRM practices, and reverse knowledge transfer: The case of Chinese cross-border acquisitions. J World Bus 55(2):100958
Lombardi R (2019) Knowledge transfer and organizational performance and business process: past, present and future researches. Bus Process Manag J 25(1):2–9
Lucas K, Philips I, Mulley C, Ma L (2018) Is transport poverty socially or environmentally driven? Comparing the travel behaviours of two low-income populations living in central and peripheral locations in the same city. Transportation Res Part A: Policy Pract 116:622–634
McFadyen MA, Cannella Jr AA (2004) Social capital and knowledge creation: diminishing returns of the number and strength of exchange relationships. Acad Manag J 47(5):735–746
Migdadi MM (2021) Knowledge management, customer relationship management and innovation capabilities. J Bus Ind Mark 36(1):111–124
Mohajan HK (2019) Knowledge sharing among employees in organizations. J Econ Dev Environ People 8(1):52–61
Mothe C, Nguyen-Thi UT, Triguero Á (2018) Innovative products and services with environmental benefits: design of search strategies for external knowledge and absorptive capacity. J Environ Plan Manag 61(11):1934–1954
Moysidou K, Hausberg JP (2020) In crowdfunding we trust: a trust-building model in lending crowdfunding. J Small Bus Manag 58(3):511–543
Nahapiet J, Ghoshal S (1998) Social capital, intellectual capital, and the organizational advantage. Acad Manag Rev 23(2):242–266
Olaisen J, Revang O (2017) Working smarter and greener: Collaborative knowledge sharing in virtual global project teams. Int J Inf Manag 37(1):1441–1448
Pemsel S, Müller R, Söderlund J (2016) Knowledge governance strategies in project-based organizations. Long Range Plan 49(6):648–660
Peng MYP (2022) Future time orientation and learning engagement through the lens of self-determination theory for freshman: evidence from cross-lagged analysis. Front Psychol 12:760212
Peng MYP (2022) The roles of dual networks and ties on absorptive capacity in SMEs: the complementary perspective. Total Qual Manag Bus Excell 33(5-6):566–589
Peng MYP, Shao L (2021) How do the determinants of new product development matter in the international context? The moderating role of learning orientation. J Competitiveness 13(3):129
Peng MYP, Feng Y, Zhao X, Chong W (2021) Use of knowledge transfer theory to improve learning outcomes of cognitive and non-cognitive skills of university students: evidence from Taiwan. Front Psychol 12:583722
Peng MYP, Xu C, Zheng R, He Y (2023) The impact of perceived organizational support on employees’ knowledge transfer and innovative behavior: comparisons between Taiwan and mainland China. Humanit Soc Sci Commun 10(1):1–13
Pinho JC, Prange C (2016) The effect of social networks and dynamic internationalization capabilities on international performance. J World Bus 51(3):391–403
Putnam RD (1995) Tuning in, tuning out: the strange disappearance of social capital in America. Polit Sci Polit 28(4):664–683
Qasrawi BT, Almahamid SM, Qasrawi ST (2017) The impact of TQM practices and KM processes on organisational performance: An empirical investigation. Int J Qual Reliab Manag 34(7):1034–1055
Qi C, Chau PYK (2018) Will enterprise social networking systems promote knowledge management and organizational learning? An empirical study. J Organ Comput Electron Commer 28(1):31–57
Reagans R, McEvily B (2003) Network structure and knowledge transfer: the effects of cohesion and range. Adm Sci Q 48(2):240–267
Rezaei M, Jafari-Sadeghi V, Bresciani S (2020) What drives the process of knowledge management in a cross-cultural setting: the impact of social capital. Eur Bus Rev 32(3):485–511
Ritala P, Stefan I (2021) A paradox within the paradox of openness: the knowledge leveraging conundrum in open innovation. Ind Mark Manag 93:281–292
Saleh A, Bista K(2017) Examining factors impacting online survey response rates in educational research: perceptions of graduate students. Online Submission 13(2):63–74
Savalei V (2020) Improving fit indices in structural equation modeling with categorical data. Multivar Behav Res 56:390–407
Setia M (2016) Methodology series module 5: sampling strategies. Indian J Dermatol 61:505–509
Shahzad M, Qu Y, Zafar AU, Rehman SU, Islam T (2020) Exploring the influence of knowledge management process on corporate sustainable performance through green innovation. J Knowl Manag 24(9):2079–2106
Shariq SM, Mukhtar U, Anwar S (2019) Mediating and moderating impact of goal orientation and emotional intelligence on the relationship of knowledge oriented leadership and knowledge sharing. J Knowl Manag 23(2):332–350
Sheng ML, Hartmann NN (2019) Impact of subsidiaries’ cross-border knowledge tacitness shared and social capital on MNCs' explorative and exploitative innovation capability. J Int Manag 25(4):100705
Shraah A, Abu-Rumman A, Alqhaiwi L, AlShaar H (2022) The impact of sourcing strategies and logistics capabilities on organizational performance during the COVID-19 pandemic: Evidence from Jordanian pharmaceutical industries. Uncertain Supply Chain Manag 10(3):1077–1090
Swanson E, Kim S, Lee SM, Yang JJ, Lee YK (2020) The effect of leader competencies on knowledge sharing and job performance: Social capital theory. J Hospit Tour Manag 42:88–96
Syed A, Gul N, Khan HH, Danish M, Ul Haq SM, Sarwar B, Ahmed W (2021) The impact of knowledge management processes on knowledge sharing attitude: the role of subjective norms. J Asian Financ Econ Bus 8(1):1017–1030
Tsai YH, Ma HC, Lin CP, Chiu CK, Chen SC (2014) Group social capital in virtual teaming contexts: a moderating role of positive affective tone in knowledge sharing. Technol Forecast Soc Change 86:13–20
Valk R, Planojevic G (2021) Addressing the knowledge divide: Digital knowledge sharing and social learning of geographically dispersed employees during the COVID-19 pandemic. J Glob Mobil Home Expatriate Manag Res 9(4):591–621
Wang L, Huo D, Motohashi K (2019) Coordination mechanisms and overseas knowledge acquisition for Chinese suppliers: the contingent impact of production mode and contractual governance. J Int Manag 25(2):100653
Xie L, Guan X, Huan TC (2019) A case study of hotel frontline employees’ customer need knowledge relating to value co-creation. J Hospitality Tour Manag 39:76–86
Ye X, Wang Z, Zhang Y, Li H (2021) How do knowledge governance mechanisms impact on repatriate knowledge transfer intention? The moderating role of perceived career and repatriation support and person-organization fit. Manag Decisi 59(2):324–340
Yilmaz C, Hunt SD (2001) Salesperson cooperation: the influence of relational, task, organizational, and personal factors. J Acad Mark Sci 29(4):335–357
Yong JY, Yusliza MY, Ramayah T, Chiappetta Jabbour CJ, Sehnem S, Mani V (2020) Pathways towards sustainability in manufacturing organizations: empirical evidence on the role of green human resource management. Bus Strategy Environ 29(1):212–228
Zaim H, Muhammed S, Tarim M (2019) Relationship between knowledge management processes and performance: critical role of knowledge utilization in organizations. Knowl Manag Res Pract 17(1):24–38
Zhao G, Hormazabal JH, Elgueta S, Manzur JP, Liu S, Chen H, Chen X (2021) The impact of knowledge governance mechanisms on supply chain performance: empirical evidence from the agri-food industry. Prod Plan Control 32(15):1313–1336
Zhao WX, Peng MYP, Liu F (2021) Cross-cultural differences in adopting social cognitive career theory at student employability in PLS-SEM: the mediating roles of self-efficacy and deep approach to learning. Front Psychol 12:586839
Zhou L, Chen Z, Peng MYP (2022) The role of relational embeddedness in enhancing absorptive capacity and relational performance of internationalized SMEs: evidence from mainland China. Front Psychol 13:896521
Zhou L, Peng MYP, Shao L, Yen HY, Lin KH, Anser MK (2021) Ambidexterity in social capital, dynamic capability, and SMEs’ performance: quadratic effect of dynamic capability and moderating role of market orientation. Front Psychol 11:584969
Download references
Acknowledgements
This study is supported by Philosophical and Social Science Planning Project of Guangdong Province (GD23CJY15).
Author information
Authors and affiliations.
School of Economics and Management, Foshan University, Foshan, China
Michael Yao-Ping Peng
School of Economics and Trade, Foshan University, Foshan, China
You can also search for this author in PubMed Google Scholar
Contributions
Michael Yao-Ping Peng composed the conception and design and drafted the article; interpreted data and revised it critically for important intellectual content; collaborated with the writing of the study; provided data methodology and analysis help.
Corresponding author
Correspondence to Michael Yao-Ping Peng .
Ethics declarations
Competing interests.
The author declares no competing interests.
Ethical approval
Approval for this study was obtained from the Ethics Committee of Foshan University. The procedures used in this study adhere to the tenets of the Declaration of Helsinki. The ethical approval was requested and granted in August 2023, prior to the commencement of data collection.
Informed consent
Informed consent was obtained from all participants and/or their legal guardians prior to their participation in the study. Participants were informed about the nature and purpose of the study, as well as their rights to withdraw at any time without any consequences. The informed consent process was conducted from September to October 2023, concurrently with the distribution of the questionnaires.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Peng, M.YP. Breaking down barriers: exploring the impact of social capital on knowledge sharing and transfer in the workplace. Humanit Soc Sci Commun 11 , 1007 (2024). https://doi.org/10.1057/s41599-024-03384-9
Download citation
Received : 04 January 2024
Accepted : 24 June 2024
Published : 06 August 2024
DOI : https://doi.org/10.1057/s41599-024-03384-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Open access
- Published: 07 August 2024
Towards inclusive healthcare: evaluating knowledge, confidence and awareness of LGBTQ + health among Internal Medicine Trainees in London
- Andrew Crowe 1 ,
- Patrick Hogan 2 ,
- Christoper Morrison 3 ,
- Catherine Meads 1 &
- Daniel Bailey 4
BMC Medical Education volume 24 , Article number: 851 ( 2024 ) Cite this article
174 Accesses
Metrics details
Patients from the lesbian, gay, bisexual, transgender, queer plus (LGBTQ +) community face various health inequalities and report poor healthcare experiences. Little is known about how knowledgeable and confident UK doctors are around LGBTQ + health, and previous research demonstrates that UK medical schools rarely deliver teaching in this area. This research evaluated the level of knowledge, awareness and confidence of LGBTQ + health among Internal Medical Trainees (IMTs) in London.
London IMTs were invited to complete an online questionnaire evaluating knowledge, awareness and confidence in LGBTQ + health. Stratified analysis of results by demographics was performed.
Three hundred and fifteen surveys were analysed from 796 eligible trainees (40%). Confidence in caring for LGBTQ + patients was variable. Confidence in discussing gender identity was lower than for sexual orientation. Knowledge of health issues affecting LGBTQ + patients varied. Most participants had never received training on LGBTQ + health at undergraduate ( n = 201, 64%) or postgraduate level ( n = 252, 80%), but the majority of participants felt that training would be useful ( n = 233, 74%). Stratified analysis revealed that IMTs who received previous LGBTQ + teaching at undergraduate or postgraduate level were considerably more confident discussing sexual orientation with patients, compared to those who received no previous teaching.
Conclusions
There is a clear need for education on LGBTQ + health, given the varied levels of knowledge and confidence identified. A significant majority of IMTs in London have never received teaching on LGBTQ + health, although there exists a strong desire for this. LGBTQ + health topics should be integrated into undergraduate and postgraduate training and examinations for IMTs. This would support IMTs in delivering high quality and inclusive care for all patients, particularly those of sexual orientation and gender identity minorities. There are relatively few published studies exploring competency in LGBTQ + health among doctors, and this is the first among UK Internal Medicine Trainees.
Peer Review reports
In recent times, the spotlight on healthcare disparities faced by marginalised communities has grown stronger, and the voices of these communities have grown louder [ 1 ]. LGBTQ + communities are one such marginalised group, composed of people who are Lesbian, Gay, Bisexual, Transgender, or Queer. The “plus” denotes people who are part of the community, but for whom LGBTQ + neither accurately captures, nor reflects their identity. They frequently report negative encounters in the healthcare setting and experience unique health inequalities in areas such as physical health, sexual health, and mental health [ 2 ].
Cancer burden is greater in the LGBTQ + communities, with higher rates of anal cancer among men who have sex with men [ 3 ] and higher rates of cervical intraepithelial neoplasia among women who have sex exclusively with women [ 4 ]. In addition, lesbian and bisexual women in the UK have higher rates of asthma and obesity compared to heterosexual women [ 5 , 6 ]. Transgender individuals are significantly more likely to be living with chronic medical and psychiatric conditions (including dementia) and have suicide rates at least 5 times higher than their cisgender peers [ 7 , 8 ]. LGBTQ + patients of nearly all age groups are more likely to avoid seeing their GP, contributing to late diagnosis and poor outcomes [ 9 ].
One potential factor contributing to these health inequalities is the ‘Minority stress theory’, which suggests that LGBTQ + people experience chronic stress from both “distal” sources (e.g. discrimination, victimisation, bullying, stigmatisation, violence, and social injustice), and “proximal” sources (e.g. internalised homophobia and perceived prejudice) [ 10 ]. This chronic stress response may lead to increased risk of various physical health conditions, mental health conditions including suicidality, and increases the likelihood of engaging in high-risk and harmful behaviours [ 11 , 12 ]. Among transgender people, negative physical health outcomes were actually more common in those with past experiences of significant harassment or violence, compared to those without [ 13 ]. Through education, clinicians can become aware of the Minority stress theory, and actions that can potentially contribute to this either overtly (e.g. through expression of prejudicial opinions), or inadvertently (e.g. by using heteronormative and cisnormative language, by providing services that do not appear inclusive).
Another factor potentially contributing to health inequalities is engagement with healthcare. LGBTQ + people may have concerns about disclosing sexual orientation/gender identity to healthcare providers, based on previous experience of discrimination, the perception or fear of it, and concerns that services will neither understand, nor support their needs [ 14 ]. As a result, they may not engage with screening programmes or seek help for concerning symptoms, leading to missed opportunities for cancer detection, or primary/secondary prevention of disease [ 2 ]. For example, women who have sex exclusively with women are less likely to attend for cervical cancer screening [ 4 ], and as previously stated, they have higher rates of cervical intraepithelial neoplasia (a precursor to cervical cancer). Transgender people report higher rates of negative experiences in healthcare, and are more likely to avoid seeking care than their cisgender counterparts [ 15 ]. Studies demonstrate they have increased rates of chronic medical conditions, and poor mental health, particularly suicidal ideation [ 7 , 8 ].
LGBTQ + communities have contrasting experiences of health and healthcare compared to the general population. Current and future clinicians should be cognisant of these differences and their role in addressing them.
The area of LGBTQ + health remains understudied and under-researched; it is not widely covered in curricula of UK medical schools. For many medical schools, there is little or no exposure to LGBTQ + teaching during the undergraduate programmes [ 16 , 17 ]. Medical students feel unprepared for encounters with LGBTQ + patients, which could translate into poor quality of care [ 18 , 19 ]. Inclusion and cultural competence are increasingly recognised to be important in healthcare, and this knowledge gap may contribute to suboptimal care, and worsen health disparities experienced by LGBTQ + individuals. With increasing numbers of people identifying as LGBTQ + , doctors must be competent to provide care to patients from these communities [ 20 ].
There is a dearth of literature describing LGBTQ + health in medical education and little is known about the knowledge and confidence of UK clinicians around these issues. The vast majority of published literature in this area focuses on the undergraduate setting and explores how confident and knowledgeable medical students are, or evaluates the amount of LGBTQ + teaching in undergraduate curricula [ 16 , 17 ]. In relation to medical graduates (i.e. qualified doctors), there are very few published studies and only one other in the British setting which focuses solely on Oncologists [ 21 ], making this the first study of its kind among IMTs in the United Kingdom.
The core aim of this study was to evaluate the levels of knowledge, confidence, and awareness that Internal Medicine Trainees (IMTs) in London have around the health needs of patients from the LGBTQ + community. Our objectives included: assessing how confident IMTs feel when caring for patients from this community, examining how knowledgeable IMTs are in LGBTQ + health, determining how much prior teaching IMTs have received on LGBTQ + health and how useful they feel specialist teaching would be, and investigating the demographics of participants in a stratified analysis.
In this article, we use the terms MSM (men who have sex with men) and WSW (women who have sex with women). These terms describe sexual activities without assuming identities like gay, lesbian, or bisexual, recognising that not everyone who engages in same-sex activities identifies with these labels.
Through this research, we identify areas for improvement, and consequently, provide the evidence needed to design targeted interventions and implement curricular changes that could equip future doctors with the skills to confidently care for this marginalised and vulnerable population group.
Study design
We designed and conducted an observational cross-sectional study with mixed quantitative/qualitative methods. Our core research question was: What is the level of awareness, confidence and knowledge in LGBTQ + health among IMTs in London?
We included all 796 IMTs (years 1–3) currently training in a London Deanery. IMTs are qualified doctors who have completed Foundation Training and have chosen to train in Internal Medicine (they are at least 2 years after graduation). After completion of Internal Medicine training, the majority will enter specialist medical training (in Cardiology, Gastroenterology, Neurology, etc.).
We identified IMTs for inclusion as they form a large and accessible cohort of doctors, thus providing a suitable sample size. In addition, they interact with patients on a daily basis and are likely to encounter members of LGBTQ + community in a professional context. We focused on London as it has the largest proportion of LGBTQ + residents in the United Kingdom [ 22 ].
The online questionnaire was designed using Jisc software, a program for designing and distributing online surveys. The surveys were emailed to participants four times over a 2-month period via the London School of Medicine. These questionnaires were self-administered by participants, and participation was voluntary. Consent was compulsory in order to complete the questionnaire and participants were asked to read the Participation Information Leaflet and tick the consent box if in agreement. The participants were not asked for personally identifiable information such as name, date of birth or address, but were asked to provide some demographic details. There was a "prefer not to say" option for each demographic question.
There were 33 questions, in 5 sections. The majority were closed questions with true/false or yes/no answers. Other question formats included multiple choice questions, Likert scale questions and free text boxes for comments or feedback.
The first section assessed demographics, the second section explored levels of awareness and confidence in caring for LGBTQ + patients, the third section assessed prior teaching on LGBTQ + health received by participants, the fourth section examined knowledge of LGBTQ + health and the fifth section asked for comments and feedback. The correct answers to each question in the knowledge section, along with an explanation and reference to the literature, were provided upon completion of the survey to promote learning for all participants.
These questions were designed to focus on scenarios encountered by IMTs, thus making the survey directly relevant to their practice. A pilot questionnaire was completed by a small group of IMTs, and questions were refined based on their feedback. We concentrated on general internal medicine, an area often neglected in LGBTQ + health research, rather than other areas such as sexual health. We designed 3 separate question stems to individually test knowledge on gay male health, gay female health, and trans health. Due to limited survey space, we were unable to include as many identities as we wished (bisexual, non binary etc.).
Data analysis
Every survey answered was used in data analysis, which was done with SPSS software and descriptive analysis of the data. Data was presented in graphs and charts made using Microsoft Excel. In certain demographic questions and other parts of the results where fewer than five respondents answered, the results are reported in text and tables as < 5 in order to promote confidentiality and reduce risk of participant identification.
Ethical Approval was granted by the School Research Ethics Panel (SREP) of the Health, Education, Medicine and Social Care (HEMS) faculty of Anglia Ruskin University.
There were 315 responses (40% of the total eligible population). All surveys were fully completed. Most respondents were aged 26-30yrs. ( n = 198, 62.9%), and slightly more participants were female, with 160 female participants (50.8%), 140 male participants (44.4%), and the rest indicating 'prefer not to say' ( n = 15, 4.8%). 23.1% of participants identified as LGBTQ + , with 6.7% ticking "prefer not to say" for sexual orientation, and 5.7% for gender identity. For demographics—See Table 1 .
Confidence/awareness
When asked about confidence in discussing issues of sexual orientation and gender identity with patients (See Table 2 ), responses varied, but confidence levels around gender identity were lower than sexual orientation. Just over half of participants (54.3%) felt confident asking a patient about sexual orientation, while 27.6% did not feel confident, and 18.1% felt somewhat confident. Regarding gender identity, 45.1% of participants felt confident asking patients about gender identity, 33.3% did not feel confident, and 21.6% felt somewhat confident. Less than half (46.0%) felt confident using terms related to gender identity (pronouns, transgender, non-binary etc.), while 30.8% did not feel confident, and 23.2% felt somewhat confident. When asked if participants had ever treated patients who identified as LGBTQ + , 289 respondents (91.7%) replied Yes, 12 participants (3.8%) replied No, and 14 (4.4%) were not sure.
Most participants reported having no prior exposure to training on LGBTQ + health, (See Table 3 ), a slightly greater proportion of participants received LGBTQ + training during their undergraduate training than during postgraduate training (36.1% during undergraduate vs 20.0% during postgraduate). A large proportion felt that LGBTQ + teaching was useful: 233 participants (73.9%) felt it was "very useful", 79 participants (25.1%) felt it was "somewhat useful", and 3 participants (0.9%) felt it was "not useful". Participants were keen for teaching on various areas of LGBTQ + health but particularly on the topics of general medicine in LGBTQ + patients (85.4%) and transgender healthcare (66.7%).
Distribution of knowledge scores was varied (See Table 4 and Fig. 1 ). Below are some pertinent results from the knowledge section:
When asked about rates of asthma and average BMI in lesbian women, most answers were incorrect (90.8% incorrect and 72.1% incorrect respectively)
64.8% of respondents correctly identified that lesbian women in the UK do not have higher rates of cardiovascular disease compared to the general populations, and 60.6% correctly recognised that nulliparity is a risk factor for breast cancer in lesbian women (as for all nulliparous women)
72.1% correctly identified that men who have sex with men (MSM) are more likely to develop anal cancer than heterosexual men. However, over one third (34.0%) incorrectly believed they are more likely to develop colon cancer, compared to heterosexual men.
65.0% of respondents correctly answered than older gay men are twice as likely to be living alone compared to older heterosexual men.
67.9% correctly answered that older LGBTQ + individuals are less likely to attend their GP than non-LGBTQ + individuals.
A minority of respondents (41.0%) correctly answered that rates of Subjective Cognitive Decline (SCD) are higher among LGBTQ + individuals.
Distribution of knowledge scores
Stratified analysis
Stratified analysis (See Table 5 ) revealed that the participants who received previous LGBTQ + teaching at undergraduate or postgraduate level were considerably more confident discussing sexual orientation with patients, compared to those who received no previous teaching (statistically significant) These participants were also more confident in discussing gender identity with patients – this was statistically significant for participants who received teaching at undergraduate level, but not for those who received teaching at postgraduate level. Males felt slightly more confident discussing sexual orientation and gender identity with patients compared to females (not statistically significant). IMTs with prior teaching were more likely to feel that knowing a patient’s sexual orientation or gender identity is important when caring for them, compared to those who with no prior training (statistically significant).
Participants were invited to give feedback in two free text boxes (See Table 6 ). The first box asked if LGBTQ + teaching was worthwhile and how should it be done. The second box asked for any further comments or feedback. There were 113 responses in total. Some commonly occurring themes were;
Desire for teaching, particularly on trans healthcare and general internal medical issues in LGBTQ + patients:
“I have looked after patients who identify as LGBTQ+ and have felt ill-equipped to manage this well. Since these experiences I have tried to look up the correct terminology and language to use but it is still not an area of confidence for me, and I do not know much about the impact of this on general medicine for this patient cohort. Any teaching would be gratefully received”
Authenticity of teaching,
“Ideally teaching should be delivered in-person by people of the LGBTQ+ community so that they are not misrepresented and we can hear patient’s perspectives”
Negative experiences while working.
“Have seen some really transphobic and homophobic stuff working in the NHS and so we definitely need more education and open dialogues about LGBTQ health”
Summary of findings
Overall, this study reveals that knowledge levels around LGBTQ + health among IMTs in London are varied. They are moderately confident discussing sexual orientation with patients, but less confident discussing gender identity and its related terminology (transgender, non-binary, pronouns etc.). Most participants have never received any formal teaching on LGBTQ + health, which is consistent with the literature showing these topics are rarely covered at undergraduate or postgraduate level [ 16 , 17 ]. However, it is encouraging to see there is a strong demand for this, particularly teaching on general medicine for LGBTQ + patients and transgender healthcare.
Our results compare similarly to findings from two American studies [ 23 , 24 ]. In both studies, IMTs felt that LGBTQ + health was important, but they reported minimal prior teaching in this area and assessment of their knowledge revealed numerous deficits. Confidence levels were varied but increased after teaching.
A significant proportion of the surveyed IMTs felt under-confident discussing sexual orientation and gender identity with patients. Of note, participants were less confident discussing gender identity (and related terms such as transgender, non-binary and pronouns) than sexual orientation. One third of participants were not confident asking patients about gender identity. Stratified analysis revealed that participants who had received previous formal LGBTQ + training (at undergraduate or postgraduate level) reported higher levels of confidence in these areas compared to those who never received teaching, demonstrating the benefits of teaching, and reinforcing the need for formal education. Of note, participants who received training during university reported feeling more confident than those who did not. Although causation cannot be assumed, these findings suggest the effect of training in improving confidence may last for several years (at least 3 years in the case of this cohort of IMTs).
The proportion of surveyed participants identifying as gay (12.1%), bisexual (8.3%) or other (1.6%) was higher than the proportion in the general population. In the 2021 UK Census [ 17 ], 4.3% of London residents identified as lesbian, gay, bisexual, or other. Our figures could be explained by the younger age group of IMT participants (93.4% of participants were in the 26–35 age bracket) who are statistically more likely to identify as LGBTQ + than older age groups [ 22 ]. Additionally, these figures could reflect the potential responder bias associated with voluntary participation in surveys – for example, people identifying as gay, or bisexual are more likely to voluntarily take surveys about LGBTQ + issues. Regarding gender identity, just 0.3% of participants identified as transgender, and 1.6% as non-binary, which compares slightly differently to the general population of London residents where 0.78% identify as transgender/gender different from that assigned at birth, and 0.8% identify as non-binary [ 20 ].
Many feedback comments expressed a strong desire for LGBTQ + health teaching, with some calling for it to be mandatory during the IMT programme, and others calling for it to be integrated into the IMT curriculum. Some participants were enthusiastic for teaching to be partly delivered by members of the LGBTQ + community as they felt it was important to hear “first hand patient experiences”.
While most of the feedback was positive, it is important to acknowledge the criticisms. One participant felt that LGBTQ + training is important during IMT, but "should not be priority". Another participant called for LGBTQ + training to be "carefully balanced against other learning needs" and that it should be implemented and "governed according to clinical need only".
Strengths and limitations
Strengths of our study include the large sample size, and the fact that participants came from a diverse range of areas, both north and south London. Our research separately evaluated lesbian, gay, bisexual, and transgender health in certain questions, giving us a deeper insight into participants' understanding of these specific areas, something which is often omitted from studies in LGBTQ + health. The knowledge section presented three separate scenarios (lesbian woman, gay man, transgender man) while the confidence section examined sexual orientation and gender identity independently. Lastly, the knowledge section focused on areas of general medicine other than sexual health or mental health, which are often neglected in LGBTQ + medical education.
In terms of limitations, the generalisability of these results is restricted given the 40% response rate and the specific geographic location of this study. Participants were IMTs based in London, and consequently, one cannot draw accurate conclusions about levels of knowledge, confidence, and awareness among other groups of doctors, or doctors in other locations around the UK. Two potential explanations for the low response rate include the voluntary participation of the survey, and the fact that people may be reluctant to take surveys on “sensitive” topics (such as sexual orientation and gender identity). 23% of doctors in this survey identified as LGBTQ + , a higher proportion than expected in the general population, which could skew results. In the interests of time, and to avoid a lengthy survey, certain parameters were omitted, such as ethnicity (black, hispanic etc.), political affiliations (liberal, conservative, etc.), stage of Internal Medicine Training (IMT1, IMT2, IMT3), and attitudes towards LGBTQ + individuals.
Implications for practice
Educational programs.
Dedicated LGBTQ + educational programs are central in raising awareness among medical students and doctors about the healthcare disparities faced by LGBTQ + individuals and equipping them with the skills and knowledge to provide quality care. These programs should be designed by clinicians in conjunction with members of the LGTBQ + community. Constructivist educational activities should be prioritised, such as case-based discussions, patient interactions and role-play scenarios, as these promote active participation of learners which is key for cultural change [ 25 ]. Teaching should take place within a comfortable learning environment so that students feel safe to express opinions and critically examine various approaches to LGBTQ + healthcare, without feeling their views may be perceived as wrong or inappropriate. Educational programmes may be further enriched by embracing validated clinician self assessment tools; such as the Lesbian, Gay, Bisexual and Transgender Development of Clinical Skills assessment (LGBT-DOCSS); which allow trainees and clinicians to reflect upon their own knowledge and self-efficacy [ 26 ].
In designing education, we should avoid focusing solely on topics that are traditionally associated with LGBTQ + patients, such as sexual health. Links between the LGBTQ + community and general medical conditions such as cancer, cardiovascular disease, asthma and cognitive problems are less recognised, as evidenced by the results and feedback comments in our study. For example, the classic exam question of a gay male presenting with a new diagnosis of HIV or a sexually transmitted infection is useful to some degree, but it can lead to healthcare stereotyping [ 27 ] and fails to consider other associated medical conditions to which he is at risk. Our results show that doctors were particularly interested in teaching on transgender healthcare, especially terminology and relevant hormones. This is important to acknowledge, as we know that some clinicians feel under confident treating this group, and are not comfortable prescribing hormonal treatments [ 28 ].
Educators can be assisted in developing teaching materials by accessing support from partner organisations such as GLADD (The Association of LGBTQ + Doctors and Dentists) and the Fenway Health National LGBTQIA + Health Education Centre who can sign post to resources that providers might use, and support the building of networks that can share best practice in education [ 29 , 30 ].
Integration into examinations
Integration of LGBTQ + health topics into formal assessments, both at undergraduate and postgraduate level, is important to promote an inclusive healthcare environment. Integration can be achieved by weaving LGBTQ + health topics into examinations, for example multiple choice questions and essay questions. Integration can also be achieved by swapping heterosexual or cisgender patients for LGBTQ + patients in clinical scenarios. For example, a traditional examination of an elderly patient with Parkinson’s disease can be swapped for an elderly transgender man with Parkinson’s disease. Most of the marks are still awarded for taking an appropriate neurological history and eliciting the correct signs on physical examination, but a small number of marks go towards appropriate communication, using correct pronouns and inclusive language. This encourages normalisation of these encounters and helps build confidence for doctors caring for these communities. For IMTs, LGBTQ + health topics could be integrated into the MRCPUK (Membership of Royal College of Physicians of the United Kingdom) examinations, required for successful progression to higher medical training. These topics should feature in the written sections, as well as the clinical sections (PACES) as suggested by participants in the feedback.
Curricular change
One of the most practical ways to ensure a topic is covered effectively during training is through integration into a curriculum. Currently, LGBTQ + health is not mandatory in British medical undergraduate curricula and studies demonstrate that coverage of LGBTQ + health topics at university level is very limited and extremely dependent on the staff in each university [ 16 ]. Growing voices are calling for this to be mandated with regulation from the General Medical Council [ 31 ]. Looking to the postgraduate setting, the situation is relatively similar with no mandatory coverage of LGBTQ + health topics for Foundation level or IMT doctors. The curriculum of the UK Foundation Programme asks for doctors to develop an understanding of "equality and diversity in health" but it fails to elaborate and does not specifically mention the LGBTQ + community, or other marginalised groups [ 32 ]. Likewise, the curriculum of Internal Medicine Training in the UK vaguely asks that "training bodies comply with equality and diversity standards", but again, fails to mention anything specific to the LGBTQ + communities [ 33 ]. LGBTQ + health training needs to be integrated into curricula, both undergraduate and postgraduate, with direct reference to sexuality and gender identity minorities, and their health inequalities. Furthermore, framework resources for reforming undergraduate curricula have already been published [ 34 , 35 ], and these could be adapted for postgraduate curricula with relative ease.
Implications for research
Further studies are needed to evaluate levels of confidence and knowledge among other groups of clinicians. A comparative analysis could be done according to speciality (Psychiatrist, GP etc.), grade (registrar, consultant etc.) demographics, or geographic location, in an effort to identify factors associated with greater LGBTQ + health competency and disparities across various groups. Ideally, this would be carried out at a national level given that communities of LGBTQ + individuals are found throughout the country. Research should examine effective teaching methodologies to determine how best to integrate LGBTQ + topics into education and examinations. Longitudinal studies would help track changes in doctors' attitudes and behaviour over time, and examine competency before and after teaching interventions. In addition to targeting clinicians, future projects should explore the perspectives of LGBTQ + patients and their experiences in hospitals and clinics to determine the best ways of delivering high quality and healthcare.
The results show there is a clear need for education on LGBTQ + health, given the variable levels of knowledge and confidence identified among Internal Medicine Trainees in London. A significant majority of participants have never received teaching on LGBTQ + health, although there exists a strong desire for this, particularly teaching on general medical issues facing LGBTQ + patients and transgender healthcare. Recommendations from our research include the creation of LGBTQ + educational programs, curricular change to include LGBTQ + topics, and the integration of LGBTQ + cases in postgraduate training and examinations for IMTs. There are very few published studies exploring competency in LGBTQ + health among doctors, with only one other in the United Kingdom, but none among British Internal Medicine doctors, making this study the first of its kind.
Our research highlights the necessity to address the educational needs of Internal Medicine Trainees in London in relation to LGBTQ + health, to improve patient experiences and outcomes, and to promote an inclusive healthcare environment for all.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Lavizzo-Mourey, Besser, Williams, Understanding and Mitigating Health Inequities — Past, Current, and Future Directions. N Engl J Med. 2021; https://doi.org/10.1056/NEJMp2008628 .
Bailey D, Calasanti T, Crowe A, di Lorito C, Hogan P, de Vries B. Equal but different! Improving care for older LGBT+ adults. Age Ageing. 2022;51(6): https://doi.org/10.1093/ageing/afac142 . PMID: 35751872.
Quinn GP, Sanchez JA, Sutton SK, et al. Cancer and lesbian, gay, bisexual, transgender/transsexual, and queer/questioning (LGBTQ) populations. CA Cancer J Clin. 2015;65:384–400. https://doi.org/10.3322/caac.21288 .
Article Google Scholar
Saunders C, Massou E, Waller J, Meads C, Marlow LA, Usher-Smith J. Cervical screening attendance and cervical cancer risk among women who have sex with women. J Med Screen. 2021;28(3):349–56. https://doi.org/10.1177/0969141320987271 . Epub 2021 Jan 21. PMID: 33476213; PMCID: PMC8366122.
Meads C, Martin A, Grierson J, et al. Systematic review and meta-analysis of diabetes mellitus, cardiovascular and respiratory condition epidemiology in sexual minority women. BMJ Open. 2018;8(4):e020776. https://doi.org/10.1136/bmjopen-2017-020776 . PMID:29666136;PMCID:PMC5905763.
Semlyen J, Curtis TJ, Varney J. Sexual orientation identity in relation to unhealthy body mass index: individual participant data meta-analysis of 93 429 individuals from 12 UK health surveys. J Public Health (Oxf). 2020;42(1):98–106. https://doi.org/10.1093/pubmed/fdy224 .
Saunders, CL, Berner, A, Lund, J, et al. Demographic characteristics, long-term health conditions, and healthcare experiences of 6,333 trans and non-binary adults in England: nationally representative evidence from the 2021 GP Patient Survey. BMJ Open. 2023; https://doi.org/10.1136/bmjopen-2022-068099
Biggs M. Suicide by clinic-referred transgender adolescents in the United Kingdom. Arch Sex Behav. 2022;51:685–90. https://doi.org/10.1007/s10508-022-02287-7 .
National GP Patient survey 2022, NHS England, Available at: https://www.england.nhs.uk/statistics/statistical-work-areas/gp-patient-survey/
Kneale D, Thomas J, French R. Inequalities in Health and Care Among Lesbian, Gay, and Bisexual People Aged 50 and Older in the United Kingdom: a systematic review and meta-analysis of sources of individual participant data. J Gerontology B Psychol Sci Soc Sci. 2020;75(8):1758–71. https://doi.org/10.1093/geronb/gbaa071 .
Frost DM, Ilan H, et al. Minority stress theory: Application, critique, and continued relevance. Curr Opin Psychol. 2023;51:101579. https://doi.org/10.1016/j.copsyc.2023.101579 . ISSN 2352-250X.
Li H, Liu X, Zheng Q, et al. Minority stress, social support and mental health among lesbian, gay, and bisexual college students in China: a moderated mediation analysis. BMC Psychiatry. 2023;23:746. https://doi.org/10.1186/s12888-023-05202-z .
Veale JF. Transgender-related stigma and gender minority stress related health disparities in Aotearoa New Zealand: hypercholesterolemia, hypertension, myocardial infarction, stroke, diabetes, and general health. Lancet Reg Health West Pac. 2023;39:100816. https://doi.org/10.1016/j.lanwpc.2023.100816 .
Alencar Albuquerque G, de Lima GC, da Silva QG, et al. Access to health services by lesbian, gay, bisexual, and transgender persons: systematic literature review. BMC Int Health Hum Rights. 2016;14(16):2. https://doi.org/10.1186/s12914-015-0072-9 . PMID:26769484;PMCID:PMC4714514.
National LGBT Survey, United Kingdom, Published 2018. Available at: https://www.gov.uk/government/publications/national-lgbt-survey-summary-report .
Tollemache N, Shrewsbury D, Llewellyn C. Que(e) rying undergraduate medical curricula: a cross-sectional online survey of lesbian, gay, bisexual, transgender, and queer content inclusion in UK undergraduate medical education. BMC Med Educ. 2021;21:100. https://doi.org/10.1186/s12909-021-02532-y .
Obedin-Maliver J, Goldsmith ES, Stewart L, et al. Lesbian, gay, bisexual, and transgender-related content in undergraduate medical education. JAMA. 2011;306(9):971–7.
Greene MZ, France K, Kreider EF, et al. Comparing medical, dental, and nursing students’ preparedness to address lesbian, gay, bisexual, transgender, and queer health. PLoS One. 2018;13(9):e0204104. https://doi.org/10.1371/journal.pone.0204104 .
Barber A, Flach A, Bonnington J, et al. LGBTQ+ Healthcare Teaching in UK Medical Schools: An Investigation into Medical Students’ Understanding and Preparedness for Practice. J Med Educ Curric Dev. 2023;10. https://doi.org/10.1177/23821205231164893
Office of National Statistics, United Kingdom. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/sexuality/bulletins/sexualidentityuk/2021and2022#:~:text=1.-,Main%20points,increase%20from%202.1%25%20in%202017 . Accessed 3 Jan 2024.
Berner AM, Hughes DJ, Tharmalingam H, et al. An evaluation of self-perceived knowledge, attitudes and behaviours of UK oncologists about LGBTQ+ patients with cancer. ESMO open. 2020;5(6): e000906. https://doi.org/10.1136/esmoopen-2020-000906 .
UK Census 2021, Office of National Statistics. Available at : https://census.gov.uk/census-2021-results
Carrie D. Johnston, Lee S. Shearer. Internal Medicine Resident Attitudes, Prior Education, Comfort, and Knowledge Regarding Delivering Comprehensive Primary Care to Transgender Patients., Transgend Health. 2017 91-95. https://doi.org/10.1089/trgh.2017.0007
Streed CG Jr, Hedian HF, Bertram A, et al. Assessment of Internal Medicine Resident Preparedness to Care for Lesbian, Gay, Bisexual, Transgender, and Queer/Questioning Patients. J Gen Intern Med. 2019;34(6):893–8. https://doi.org/10.1007/s11606-019-04855-5 . Epub 2019 Mar 7. PMID: 30847829; PMCID: PMC6544682.
Narayan R, Rodriguez C, Araujo J, et al. Constructivism—Constructivist learning theory. In: Irby BJ, Brown G, Lara-Alecio R, Jackson S, editors., et al., The handbook of educational theories. IAP Information Age Publishing; 2013. p. 169–83.
Google Scholar
Bidell MP. The Lesbian, Gay, Bisexual, and Transgender Development of Clinical Skills Scale (LGBT-DOCSS): Establishing a New Interdisciplinary Self-Assessment for Health Providers. J Homosex. 2017;64(10):1432–60. https://doi.org/10.1080/00918369.2017.1321389 .
Singleton M, Green D, Enguidanos S. Identifying Healthcare Stereotype Threat in Older Gay Men Living with HIV. J Appl Gerontol. 2023; 7334648231167944–7334648231167944.
Shires DA, Stroumsa D, Jaffee KD, Woodford MR. Primary care providers’ willingness to continue gender-affirming hormone therapy for transgender patients. Fam Pract. 2018;35(5):576–81. https://doi.org/10.1093/fampra/cmx119 .
GLAAD United Kingdom Education and Training. Available at: https://gladd.co.uk/#education-and-activism . Accessed 11 May 2024.
Education and Training at Fenway Health Institute. Available at : https://fenwayhealth.org/the-fenway-institute/education/ . Accessed 11 May 2024.
LGBT in Britain report - Health. Stonewall; 2018. Available from: https://www.stonewall.org.uk/lgbt-britain-health
UK Foundation Programmes Curriculum 2021, UK General Medical Council, UKFP Curriculum 2021 (gmc-uk.org), Available at: https://www.gmc-uk.org/-/media/documents/fp-curr-oct22-v7_pdf-101343583.pdf
JRCPTB IMT curriculum 2019, Internal Medicine stage 1 curriculum FINAL 111217.pdf (jrcptb.org.uk). Available at: https://www.jrcptb.org.uk/internal-medicine
Cooper MB, Chacko M, Christner J. Incorporating LGBT Health in an Undergraduate Medical Education Curriculum Through the Construct of Social Determinants of Health. MedEdPORTAL. 2018;7(14):10781. https://doi.org/10.15766/mep_2374-8265.10781 . PMID:30800981;PMCID:PMC6342423.
Burcheri A, Coutin A, Bigham, et al. Exploring a case for education about sexual and gender minorities in postgraduate emergency medicine training: forming recommendations for change. Postgrad Med. 2023;135(6):623–32. https://doi.org/10.1080/00325481.2023.2225329 .
Download references
Acknowledgements
Rachel McDonnell, Rahul Pathak, Gerald Edgbury, Michael Brady, Catherine Bryant, Ban Haider, Margot Turner, Muhammad Hyder Junejo
No funding was obtained for this research project.
Author information
Authors and affiliations.
Faculty of Health, Medicine and Social Care, Anglia Ruskin University, Cambridge, UK
Andrew Crowe & Catherine Meads
St Pancras Hospital, Central and North West London NHS Foundation Trust, London, UK
Patrick Hogan
Public Health Directorate, NHS Grampian, Aberdeen, UK
Christoper Morrison
Kings College Hospital NHS Foundation Trust, London, UK
Daniel Bailey
You can also search for this author in PubMed Google Scholar
Contributions
All authors were involved in the design of this study. AC was involved in acquisition, analysis, and interpretation of data, along with drafting the manuscript. All authors contributed equally to the critical review, editing and final approval of the manuscript and were responsible for the decision to submit the manuscript.
Corresponding author
Correspondence to Andrew Crowe .
Ethics declarations
Ethics approval and consent to participate.
Ethical Approval was granted by the School Research Ethics Panel (SREP) of the Health, Education, Medicine, and Social Care (HEMS) faculty of Anglia Ruskin University . All participants consented to participate before taking the online survey by reading the participant information leaflet and ticking a box. This was informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Crowe, A., Hogan, P., Morrison, C. et al. Towards inclusive healthcare: evaluating knowledge, confidence and awareness of LGBTQ + health among Internal Medicine Trainees in London. BMC Med Educ 24 , 851 (2024). https://doi.org/10.1186/s12909-024-05827-y
Download citation
Received : 16 February 2024
Accepted : 26 July 2024
Published : 07 August 2024
DOI : https://doi.org/10.1186/s12909-024-05827-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Medical education
- Postgraduate
- Sexual orientation
- Gender identity
- Internal medicine
BMC Medical Education
ISSN: 1472-6920
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Knowledge transfer within enterprises from the perspective of innovation quality management: a decision analysis based on the stackelberg game.

Share and Cite
Wang, S.; Sun, M.; Xu, Y. Knowledge Transfer within Enterprises from the Perspective of Innovation Quality Management: A Decision Analysis Based on the Stackelberg Game. Sustainability 2024 , 16 , 7018. https://doi.org/10.3390/su16167018
Wang S, Sun M, Xu Y. Knowledge Transfer within Enterprises from the Perspective of Innovation Quality Management: A Decision Analysis Based on the Stackelberg Game. Sustainability . 2024; 16(16):7018. https://doi.org/10.3390/su16167018
Wang, Shumei, Ming Sun, and Yaoqun Xu. 2024. "Knowledge Transfer within Enterprises from the Perspective of Innovation Quality Management: A Decision Analysis Based on the Stackelberg Game" Sustainability 16, no. 16: 7018. https://doi.org/10.3390/su16167018
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
- Open access
- Published: 19 August 2015
Characteristics and determinants of knowledge transfer policies at universities and public institutions in medical research—protocol for a systematic review of the qualitative research literature
- Rosa Jahn 1 ,
- Olaf Müller 1 &
- Kayvan Bozorgmehr 2
Systematic Reviews volume 4 , Article number: 110 ( 2015 ) Cite this article
6028 Accesses
3 Citations
1 Altmetric
Metrics details
Universities, public institutions, and the transfer of knowledge to the private sector play a major role in the development of medical technologies. The decisions of universities and public institutions regarding the transfer of knowledge impact the accessibility of the final product, making it easier or more difficult for consumers to access these products. In the case of medical research, these products are pharmaceuticals, diagnostics, or medical procedures. The ethical dimension of access to these potentially lifesaving products is apparent and distinguishes the transfer of medical knowledge from the transfer of knowledge in other areas. While the general field of technology transfer from academic and public to private actors is attracting an increasing amount of scholarly attention, the specifications of knowledge transfer in the medical field are not as well explored. This review seeks to provide a systematic overview and analysis of the qualitative literature on the characteristics and determinants of knowledge transfer in medical research and development.
The review systematically searches the literature for qualitative studies that focus on knowledge transfer characteristics and determinants at medical academic and public research institutions. It aims at identifying and analyzing the literature on the content and context of knowledge transfer policies, decision-making processes, and actors at academic and public institutions. The search strategy includes the databases PubMed, Web of Science, ProQuest, and DiVa. These databases will be searched based on pre-specified search terms. The studies selected for inclusion in the review will be critically assessed for their quality utilizing the Qualitative Research Checklist developed by the Clinical Appraisal Skills Programme. Data extraction and synthesis will be based on the meta-ethnographic approach.
This review seeks to further the understanding of the kinds of transfer pathways that exist in medical knowledge transfer as well as what factors lead to the adoption of one pathway over another. The aim is to provide evidence for political and academic actors designing policies for the translation of medical knowledge and public-private cooperation.
Systematic review registration
PROSPERO CRD42015014241 .
Peer Review reports
Universities and public institutions play an important role in the development of new technologies in medicine. Footnote 1 A 2010 study examining all pharmaceuticals developed between 1998 to 2007 found that of those pharmaceuticals classified as scientifically novel, more than 30 % originated from universities and were later developed by pharmaceutical or biotech companies [ 1 ]. This illustrates the role the public sector and the interaction between private and public actors play in the development of new medicines and medical technologies. The nature of this interaction has a great impact not only on the kinds of medicines that are developed but also on how well they are accessible. For example, the first candidates for the HPV vaccines preventing cervical cancer, Gardasil, and Cervarix, were developed by public institutions and universities [ 2 ]. These were later licensed to Glaxo Smith Kline and Merck Sharpe and Dohme. As the licenses were exclusive, only these two companies had the right to develop and sell the resulting vaccines when they came to market in 2006 and 2009 [ 3 ]. The resulting lack of competition has led to high prices, making it difficult for poorer populations to afford vaccination. However, HPV disproportionately affects the world’s poor, with over 80 % of the cases occurring in developing countries [ 4 ].
As the case of the HPV vaccine illustrates, furthering the understanding of how universities and public institutions transfer their technologies is of great interest to public health professionals. However, while university technology transfer in general has attracted an increasing amount of scholarly attention, the transfer of knowledge in the development of new medical technologies specifically has rarely been addressed.
However, “anyone studying technology transfer understands just how complicated it can be” [ 5 ]. Defining the terms “technology” and “transfer” is the first challenge.
According to the “system’s view of technology” [ 6 ], technology can be described as a system of processes and products and the knowledge of their use and production. The process of innovation is an evolution of this system and its configuration. Basic principles serve as a guide for further development, and breakthroughs result from the culmination of prior, smaller changes to it. These then in turn form the basis for new developments in a continuous evolutionary process of innovation. This evolutionary process of innovation makes it difficult to demarcate individual technologies [ 6 ].
In addition to “technology”, the term “knowledge” has also been used, albeit without a clear distinction between the two terms. Sahal argues that whenever a technology is transferred, the knowledge of its use and its production process has to be transferred with it. Thus, “the knowledge base is inherent, not ancillary” [ 5 ]. For the purpose of this review, the broader term “knowledge” is used and defined to include “technology”. According to the “system’s view of technology”, any new knowledge, or minor modification of existing knowledge, is part of the innovation process, making it unnecessary to demarcate specific technologies [ 6 ]. Therefore, this review involves studies that address the exchange of any scientific knowledge generated by a researcher at a public institution or university.
The scope of the word “transfer” has been the subject of discussions as well. One definition commonly used in empirical studies is “transfer of physical devices, technological processes, or ‘know how’ from your organization to another” [ 7 ]. In medical research, two types of knowledge transfer can be discerned. The first consists of the utilization of basic medical research for the “development of new methods for diagnosis, therapy, and prevention and their first testing in humans”, while the second describes the “translation of results from clinical studies into everyday clinical practice and health decision-making”. [ 8 – 10 ] In this study, we address the first type of knowledge transfer. However, as the two processes are closely related, our conceptual findings might be relevant for the study of knowledge translation into practice as well.
A suggested model of “transfer” in the context of university research was developed by Bradley, Hayter, and Link [ 11 ]. Their “new model of university technology transfer” distinguishes two main types of technology transfer—formal transfer (through the technology transfer office) and informal transfer (informal exchange with colleagues, conferences, etc.). They argue that the scientist, as the originator of the knowledge, is central to the transfer process. He/she decides whether or not to declare an invention to the institution’s technology transfer office (TTO). If the researcher declares the invention, the TTO can decide whether to patent the discovery or not. If it chooses to patent, it markets the innovation to a third party or founds a new company to commercialize the invention, a so-called spin-off; if it does not patent the invention, the knowledge enters the public domain or is claimed by the scientist. However, new knowledge that is not declared by the scientist as an invention can be transferred informally, at conferences, through joint publications or informal meetings. According to Bradley, Hayter, and Link, these different transfer pathways can happen in various ways and often simultaneously [ 11 ]. However, this concept is limited in scope, as it only includes transfer processes that arise from a, usually patentable, discovery and ends with the adoption of a product. It does not take into account collaborative research, especially if it precedes discovery, and transfer processes that do not have the goal of commercializing a product. This gap is filled by the concept of academic engagement, which includes all “knowledge related collaboration by academic researchers with non-academic organisations” [ 12 ]. Academic engagement differs from the alternative view of technology transfer in so far as the focus is less on “transfer” and more on “collaboration” between institutions, often based on individual interaction. Its focus is broader, it does not stipulate an immediate financial objective but acknowledges that some collaborations aim at generating a more vague kind of utility. Combined, academic engagement and the alternative view of university technology transfer provide a comprehensive basis for analyzing knowledge exchange between public and private research entities. This study seeks to review the qualitative literature on the formal or informal transfer of medical knowledge from public and academic research institutions to private entities. We aim to improve the understanding of public-private knowledge transfer by addressing four key questions: What is the context in which knowledge transfer occurs? What are possible transfer pathways? What is the process by which a pathway is chosen? Who are the actors involved in the decision-making and what power do they have?
Methods/design
This review will include studies that address any kind of formal or informal method to transfer knowledge created at public institutions or universities to the private sector; and the factors that determine which of the possible policies is adopted. However, the scope of this literature review is limited to qualitative studies. Qualitative research “is most revealing when the variables of greatest concern are unclear” [ 13 ]. The questions of what the possible knowledge transfer methods are and what determines which strategy scientists and university staff apply are therefore properly addressed through qualitative research.
Search strategy
The search strategy aims at finding both published and unpublished studies. A three-step search strategy will be utilized. In the first step, keywords for the search have been developed based on the PICo approach. The PICo mnemonic has been developed for systematic reviews of qualitative literature, its components are population (P), phenomenon of interest (I), and context (Co). In comparison to quantitative reviews, it does not include an outcome, as “the expression of the phenomena of interest is the outcome.” [ 14 ] In the case of this review, scientists at universities and public research institutions represent the population, knowledge transfer, the phenomenon of interest, and medical research and development the context.
The databases to be searched are PubMed and Web of Science. The search for unpublished studies will include ProQuest and DiVA. Initial keywords included: research, development, medical, pharmaceutical, biomedical, university, academia, publicly funded, technology, innovation, results, discovery, knowledge, patent, transfer, translation, commercialization, transfer method, transfer pathway, transfer process, license, formal, informal. For full search terms, see Table 1 . The keywords were used in a cursory search of PubMed, Web of Science, ProQuest, and DiVA. The first results of this limited search were evaluated for relevance and the search terms were refined accordingly and adapted to the respective database. In the second step, a full search of the databases was undertaken using the refined and prespecified search terms.
A pre-selection based on title and abstract will be identified by applying the inclusion and exclusion criteria. A random set of 10 % of the studies will be pre-selected in duplicate (RJ, KB) to test the criteria for inclusion and exclusion and establish a consensus on the selection procedure. The remainder of the studies will be pre-selected by one researcher (RJ), unclear cases will be discussed in the review team.
The research team then scans the full text articles, again applying the inclusion and exclusion criteria.
Studies published in German and English and between 1995 and 2014 will be considered for inclusion in this review. This is because the major international agreement regarding the protection of intellectual property, “Trade Related Aspects of Intellectual Property” (TRIPS), including medical inventions, entered into force on 1 January 1995 and led to a restructuring of technology transfer nationally and internationally [ 15 ].
The resulting studies will be critically appraised for their quality using the qualitative research checklist developed by the Critical Appraisal Skills Programme (CASP) [ 16 ], and data will be extracted. The data will be analyzed using the meta-ethnographic approach. To ensure relevance to policy makers, the synthesis will be framed in accordance with the health policy triangle, a policy analysis framework developed by Gill Walt and Lucy Gilson (1994).
Inclusion criteria
The systematic review will include primary research studies that focus on qualitative data, cover technology transfer policies at universities or public institutions and address at least one of the following: a) content – possible technology transfer policies (spin-off, types of licenses, informal transfer, etc.), b) context – individual or institutional determinants of the adoption of technology transfer policies, c) process – procedural characteristics of decision-making regarding technology transfer, and d) actors – information about parties involved in the decision-making regarding technology transfer. The studies must also cover medical research as a single study subject or within a range of study subjects, (medical meaning pharmaceuticals, diagnostics, medical devices and procedures), have an empirical or systematic approach and be peer reviewed or dissertations.
Exclusion criteria
The systematic review will exclude works that are commentaries, theoretical texts, books, and meeting reports that do not specifically address medical research and development or focus on an industry perspective or on effectiveness and performance of technology transfer.
Critical quality appraisal
Qualitative papers selected for retrieval will be assessed by two independent reviewers for methodological rigour using the qualitative research checklist developed by CASP in 2012 [ 16 ]. It evaluates theoretical approach, study design, data collection, data analysis, and ethics on the basis of ten questions. Any disagreements in grading that arise between the reviewers will be resolved through discussion. The score (1–10) of each included study will be indicated in the final report, and the possible influence of quality issues on the overall synthesis will be discussed.
Data extraction and synthesis
Data extraction and synthesis will follow the meta-ethnography approach first developed by Noblit and Hare. It describes an approach whereby major themes, “metaphors” are identified and then compared across studies. They describe three major strategies, of which we will follow the “lines-of-argument synthesis” (LOA). This approach is appropriate in cases where “many studies suggest a lines-of-argument or inference about some larger issue or phenomenon” [ 17 ]. It allows for the synthesis of studies that are diverse and offer an illustration of different aspects of a larger “whole”. This is likely to be the case in this study because the included studies are going to cover various aspects of knowledge transfer. Synthesis “involves building a general interpretation grounded in the findings of the separate studies” [ 18 ]. The findings, or key metaphors, will be identified using open coding in MAXQDA to allow for systematic analysis of a larger number of studies and facilitate oversight. After extracting the data of the first two studies, the review team will evaluate and if necessary revise the extraction strategy. The coded metaphors will be grouped according to their content so related metaphors can be analyzed and translated into each other across studies. These translations and their relationships will be reported in the synthesis. Data extraction and synthesis will be done in duplicate. The synthesis of the systematic review should not just be well-grounded in theory, but it should also be relevant and appropriate for policy makers. Embrett and Randall have examined the health and health equity policy literature. They have stated that issues raised by scholars in this field rarely make it to the policy agenda, identifying the common misuse and nonuse of political analysis theory as one of the reasons [ 19 ]. Therefore, this study uses the health policy triangle as a policy analysis tool to ensure the synthesized evidence will be relevant and useful to policy makers.
The health policy triangle is a policy analysis tool developed by Gill Walt and Lucy Gilson. It states that the adoption of policies depends on four aspects: context, content, process and actors [ 20 ]. In the context of this review, these aspects are:
Context: What is the context in which knowledge transfer occurs?
Content: What are possible technology transfer policies?
Process: How is the transfer policy negotiated within the institution and with external partners?
Actors: Who is involved in the decision-making at the university / public institution?
The health policy triangle will be used to frame the synthesis in a meaningful way. Its categories are broad enough to accommodate all findings and categories that might emerge from the data and can therefore be applied in conjunction with the meta-ethnographic approach.
This review seeks to further the understanding of the kinds of transfer pathways that exist in medical knowledge transfer as well as what factors lead to the adoption of one pathway over another. The aim is to provide evidence for political and academic actors designing policies for the translation of medical knowledge and public-private cooperation. Understanding the importance of technology transfer and its effect on access to medicines and equality in health, in conjunction with improved knowledge of how this transfer comes about and why might aid individual, institutional and political actors in shaping a research environment that is conducive to global health.
For the purpose of this study, the term “public research institution” includes all medical research entities primarily funded from public sources.
Abbreviations
Human papilloma virus
Clinical Appraisal Skills Programme
Kneller R. The importance of new companies for drug discovery: origins of a decade of new drugs. Nat Rev Drug Discov. 2010;9(11):867–82.
Article CAS PubMed Google Scholar
Crager S, Guillen E, Price M. University contributions to the HPV vaccine and implications for access to vaccines in developing countries: addressing materials and know-how in university technology transfer policy. Am J Law Med. 2009;35(2–3):253–79.
PubMed Google Scholar
Clark A. Biomedical Innovation and the Politics of Scientific Knowledge: A case study of Gardasil. University of Maryland. 2008.
Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(5):F12–23.
Article PubMed Google Scholar
Bozeman B. Technology transfer and public policy: a review of research and theory. Res Policy. 2000;29:627–55.
Article Google Scholar
Sahal D. Alternative conceptions of technology. Res Policy. 1981;10:2–24
Bozeman B. Technology transfer research and evaluation: implications for federal laboratory practice. Final report to VNS group, Inc. and the U.S. national institute of standards. http://www.nist.gov/tpo/publications/other-economic-impact-related-studies.cfm. Accessed 28 April 15. 2013.
Sung NS, Crowley WF, Genel M, Salber P, Sandy L, Sherwood LM, et al. Central challenges facing the national clinical research enterprise. JAMA. 2003;280(10):1278–87.
Grimshaw JM, Eccles MP, Lavis JN, Hill SJ, Squires JE. Knowledge translation of research findings. Implement sci. 2012;7(50). doi: 10.1186/1748-5908-7-50
Straus SE, Tetroe J, Graham I. Defining knowledge translation. Can Med Assoc J. 2009;181(3–4):165–8.
Bradley S, Hayter C, Link A. Models and methods of university technology transfer. Department of economics working paper series: University of North Carolina; 2013.
Google Scholar
Perkman M, Tartari V, McKelvey M, Autio E, Broström A, D’Este P, et al. Academic engagement and commercialization: a review of the literature on university-industry relations. Res Policy. 2013;42:423–42.
Black N. Why we need qualitative research. J Epidemiol Commun H. 1994;48(5):425–6.
Article CAS Google Scholar
Joanna Briggs Institute. Joanna Briggs Institute Reviewer’s Manual 2014. http://joannabriggs.org/assets/docs/sumari/ReviewersManual-2014.pdf . Accessed 14 Oct 2014.
Smith RD, Correa C, Oh C. Trade, TRIPS, and pharmaceuticals. Lancet. 2009;373(9664):684–91.
The Clinical Appraisal Skill Programme. Qualitative Research Checklist. http://www.caspinternational.org/mod_product/uploads/CASP%20Qualitative%20Research%20Checklist%2031.05.13.pdf . Accessed 14 Oct 2014
Campbell R, Pound P, Morgan M, Daker-White G, Britten N, Pill R, et al. Evaluating meta-ethnography: systematic analysis and synthesis of qualitative research. Health Technol Assess. 2011;15(43):1–164.
Dixon-Woods M, Agarwal S, Jones D, Young B, Sutton A. Synthesizing qualitative and quantitative evidence: a review of possible methods. J Health Serv Res Po. 2005;10(1):45–53.
Embrett MG, Randall GE. Social determinants of health and health equity policy research: exploring the use, misuse, and nonuse of policy analysis theory. Soc Sci Med. 2014;108:147155.
Buse K, Mays N, Walt G. Making Health policy. 1st ed. Ottawa: Open University Press; 2012.
Download references
Acknowledgements
We acknowledge financial support by Deutsche Forschungsgemeinschaft and Ruprecht Karls-Universität Heidelberg within the funding programme Open Access Publishing.
Author information
Authors and affiliations.
Institute for Public Health, Im Neuenheimer Feld 324, Heidelberg, Germany
Rosa Jahn & Olaf Müller
Department of General Practice and Health Services Research, University Hospital Heidelberg, Voßstr.2, Heidelberg, 69115, Germany
Kayvan Bozorgmehr
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Rosa Jahn .
Additional information
Competing interests.
RJ is a longstanding and active member of the worldwide student organization “Universities Allied for Essential Medicines” (UAEM). This organisation works in the area of access to medicines, with a special focus on technology transfer policies that ensure global access to the resulting products.
Authors’ contributions
RJ conceived of the study, developed the protocol and drafted the first and final manuscript. KB conceived of the study, developed the protocol, and revised the manuscript for important content. OM: conceived of the study, developed the protocol, and revised the manuscript for important content. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Jahn, R., Müller, O. & Bozorgmehr, K. Characteristics and determinants of knowledge transfer policies at universities and public institutions in medical research—protocol for a systematic review of the qualitative research literature. Syst Rev 4 , 110 (2015). https://doi.org/10.1186/s13643-015-0094-3
Download citation
Received : 10 February 2015
Accepted : 16 July 2015
Published : 19 August 2015
DOI : https://doi.org/10.1186/s13643-015-0094-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Medical research policy
- Technology transfer
- Knowledge transfer
- University-industry collaboration
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

Patient-specific therapeutic benefit of MuSK agonist antibody ARGX-119 in MuSK myasthenia gravis passive transfer models
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- ORCID record for Jaap J. Plomp
- ORCID record for Silvère M. van der Maarel
- ORCID record for Jan. J. Verschuuren
- For correspondence: [email protected]
- Info/History
- Supplementary material
- Preview PDF
Muscle-specific kinase (MuSK) orchestrates establishment and maintenance of neuromuscular synapses, which enable muscle contraction. Autoantibodies targeting MuSK cause myasthenia gravis (MG), a disease characterized by fatigable skeletal muscle weakness which requires chronic immunosuppressive treatment and ventilatory support at some point in ∼30% of patients. MuSK autoantibodies are predominantly IgG4 and are bispecific, functionally monovalent antibodies due to Fab-arm exchange. Through monovalent binding, MuSK IgG4 autoantibodies act as antagonists on the MuSK signalling pathway, impairing neuromuscular synaptic function. In contrast, bivalent MuSK antibodies act as agonists of the MuSK signalling pathway. Since symptoms in MuSK MG are largely caused by antagonistic monovalent MuSK antibodies, we hypothesized that a bivalent MuSK agonist could rescue MuSK MG, bypassing the need for generalized immunosuppression. In this study, we investigated whether an agonist antibody targeting the Frizzled-like domain of MuSK, ARGX-119, can ameliorate disease in MuSK MG models induced by passive transfer of polyclonal IgG4 from unrelated patients. For each patient material we first established the minimal dose for a progressive MG phenotype based on muscle function tests. ARGX-119 significantly improved survival and muscle weakness in a mouse model induced by one patient material, but not by three others. Mechanistically, this patient-specific efficacy could not be explained by autoantibody epitope specificity, titer or competition for ARGX-119 binding, but rather correlated to the presence of MuSK activating antibodies in some patients. We further provide evidence that an in vitro assay may predict which patients potentially benefit from ARGX-119 and that this treatment, when effective in MuSK MG mice, follows a bell-shaped dose-effect curve. These results provide first proof of concept of a MuSK agonist in a clinically relevant model for MuSK MG. We anticipate this to be a starting point for investigating the therapeutic benefit of ARGX-119 in MuSK MG and other neuromuscular diseases hallmarked by neuromuscular synaptic dysfunction.
- Download figure
- Open in new tab
MuSK agonist ARGX-119 can rescue MuSK MG in a patient-specific manner
MuSK agonism follows a bell-shaped efficacy curve in this MuSK MG mouse model
Variation in ARGX-119 efficacy between patient models is not explained by competition for binding on MuSK, but rather appears related to an agonistic fraction of patient antibodies
An in vitro assay is potentially predictive for treatment efficacy of the MuSK agonist
Competing Interest Statement
J.J.V., S.M.v.d.M., M.G.H. and J.J.P. are co-inventors on MuSK-related pending patents and receive royalties. LUMC receives royalties on a MuSK ELISA. J.J.V. and M.G.H. are consultant for argenx, and J.J.V. is also consultant for Alexion and NMD Pharma. M.R.T. reports consultancies for argenx, UCB Pharma, Johnson and Johnson, Peervoice and Medtalks, and research funding from NWO, argenx and NMD Pharma. All reimbursements were received by the Leiden University Medical Center. B.V. J.L.L., C.K., R.C., C.S., K.M., L.D.C., P.U., K.S. and R.V. are employees / consultants of argenx B.V. and are holders of employee equity in argenx. The remaining authors declare no interests.
↵ * Shared first authorship
View the discussion thread.
Supplementary Material
Thank you for your interest in spreading the word about bioRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
Subject Area
- Neuroscience
- Animal Behavior and Cognition (5522)
- Biochemistry (12563)
- Bioengineering (9421)
- Bioinformatics (30801)
- Biophysics (15839)
- Cancer Biology (12908)
- Cell Biology (18505)
- Clinical Trials (138)
- Developmental Biology (9995)
- Ecology (14966)
- Epidemiology (2067)
- Evolutionary Biology (19148)
- Genetics (12729)
- Genomics (17527)
- Immunology (12669)
- Microbiology (29696)
- Molecular Biology (12360)
- Neuroscience (64682)
- Paleontology (479)
- Pathology (2000)
- Pharmacology and Toxicology (3449)
- Physiology (5324)
- Plant Biology (11084)
- Scientific Communication and Education (1728)
- Synthetic Biology (3063)
- Systems Biology (7682)
- Zoology (1728)
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Implement Sci

Knowledge translation of research findings
Jeremy m grimshaw.
1 Department of Medicine, Clinical Epidemiology Program, Ottawa Hospital Research Institute, University of Ottawa, 501 Smyth Road, Box 711, Ottawa, ON, K1H 8L6, Canada
Martin P Eccles
2 Newcastle University, Institute of Health and Society, Baddiley-Clark Building, Richardson Road, Newcastle upon Tyne, NE2 4AX, UK
John N Lavis
3 Department of Clinical Epidemiology and Biostatistics; and Department of Political Science, McMaster Health Forum, Centre for Health Economics and Policy Analysis, McMaster University, Hamilton, ON, Canada
Sophie J Hill
4 Centre for Health Communication and Participation, Australian Institute for Primary Care & Ageing, La Trobe University, Bundoora, VIC, 3086, Australia
Janet E Squires
5 Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, ON, Canada
One of the most consistent findings from clinical and health services research is the failure to translate research into practice and policy. As a result of these evidence-practice and policy gaps, patients fail to benefit optimally from advances in healthcare and are exposed to unnecessary risks of iatrogenic harms, and healthcare systems are exposed to unnecessary expenditure resulting in significant opportunity costs. Over the last decade, there has been increasing international policy and research attention on how to reduce the evidence-practice and policy gap. In this paper, we summarise the current concepts and evidence to guide knowledge translation activities, defined as T2 research (the translation of new clinical knowledge into improved health). We structure the article around five key questions: what should be transferred; to whom should research knowledge be transferred; by whom should research knowledge be transferred; how should research knowledge be transferred; and, with what effect should research knowledge be transferred?
We suggest that the basic unit of knowledge translation should usually be up-to-date systematic reviews or other syntheses of research findings. Knowledge translators need to identify the key messages for different target audiences and to fashion these in language and knowledge translation products that are easily assimilated by different audiences. The relative importance of knowledge translation to different target audiences will vary by the type of research and appropriate endpoints of knowledge translation may vary across different stakeholder groups. There are a large number of planned knowledge translation models, derived from different disciplinary, contextual ( i.e. , setting), and target audience viewpoints. Most of these suggest that planned knowledge translation for healthcare professionals and consumers is more likely to be successful if the choice of knowledge translation strategy is informed by an assessment of the likely barriers and facilitators. Although our evidence on the likely effectiveness of different strategies to overcome specific barriers remains incomplete, there is a range of informative systematic reviews of interventions aimed at healthcare professionals and consumers ( i.e. , patients, family members, and informal carers) and of factors important to research use by policy makers.
There is a substantial (if incomplete) evidence base to guide choice of knowledge translation activities targeting healthcare professionals and consumers. The evidence base on the effects of different knowledge translation approaches targeting healthcare policy makers and senior managers is much weaker but there are a profusion of innovative approaches that warrant further evaluation.
Globally we spend billions of dollars each year in both the public and private sectors on biomedical, clinical, and health services research, undergraduate healthcare professional training and continuing professional development, quality improvement, patient safety, and risk management. Despite this, healthcare systems fail to ensure that effective and cost-effective programs, services, and drugs get to all of those who need them; and healthcare professionals fail to provide the level of care to which they aspire. One of the most consistent findings from clinical and health services research is the failure to translate research into practice and policy. For example, McGlynn and colleagues observed that patients in the USA received 55% of recommended care, and that quality varied by medical condition ranging from 79% of recommended care for senile cataract to 11% of recommended care for alcohol dependence [ 1 ]. Similar findings have been reported globally in both developed and developing settings, in both primary care and specialty-provided care and in care provided by all disciplines [ 2 ]. As a result of these evidence-practice gaps, patients fail to benefit optimally from advances in healthcare resulting in poorer quality of life and loss of productivity both personally and at the societal level.
In addition to the limited use of effective treatments, there is also evidence that around 20% to 30% of patients may get care that is not needed or care that could be potentially harmful [ 3 ]. Because of these evidence-practice gaps, patients are exposed to unnecessary risks of iatrogenic harms and healthcare systems are exposed to unnecessary expenditure resulting in significant opportunity costs.
Over the last 10 to 15 years, there has been increasing international policy and research attention on how to reduce the evidence-practice and policy gap. Across different healthcare systems, different terms describe these efforts including quality assurance, quality improvement, knowledge translation, knowledge utilisation, knowledge transfer and exchange, innovation diffusion, implementation research, research utilisation, evidence-informed policy, and evidence-informed health systems [ 4 , 5 ]. These different terms often cover related and overlapping constructs. Commenting on the terminology of quality assurance in 1982, Donabedian noted that ‘we have used these words in so many different ways that we no longer clearly understand each other when we say them’ [ 6 ]. Throughout this paper, we use the term ‘knowledge translation’ which has gained currency in Canada and globally over the last decade. There are two main types of translational research. T1 research refers to the translation of basic biomedical research into clinical science and knowledge, while T2 research refers to the translation of this new clinical science and knowledge into improved health [ 7 ]. In this paper, we refer to T2 research. We define knowledge translation as ‘ensuring that stakeholders are aware of and use research evidence to inform their health and healthcare decision-making.’ This definition recognizes that there are a wide range of stakeholders or target audiences for knowledge translation, including policy makers, professionals (practitioners), consumers ( i.e. , patients, family members, and informal carers), researchers, and industry.
While knowledge translation is a relatively new term, the notion of moving research findings into practice is not new. It can be traced back to the investigations of French sociologist Gabriel Tarde at the beginning of the 20th century who attempted to explain why some innovations are adopted and spread throughout a society, while others are ignored [ 8 ]. The current conceptualization of knowledge translation evolved out of several diverse disciplinary perspectives, including knowledge utilisation, diffusion of innovations, technology transfer, evidence-based medicine, and quality improvement [ 9 ]. Interest in knowledge translation has increased dramatically in recent years due to recognition that traditional approaches to moving research into practice, which were predominantly based on education ( e.g. , continuing professional development CPD), did not lead to optimal care. In this paper, based on a previously published monograph chapter [ 10 ], we summarise the current concepts and evidence to guide knowledge translation activities. We structure the article around five key questions identified by Lavis and colleagues [ 11 ]:
1. What should be transferred?
2. To whom should research knowledge be transferred?
3. By whom should research knowledge be transferred?
4. How should research knowledge be transferred?
5. With what effect should research knowledge be transferred?
What should be transferred?
The increased focus on knowledge translation has frequently emphasised individual studies as the unit for knowledge translation. While this may be appropriate when the targets for knowledge translation are other researchers or research funders (who need to be aware of primary research results), we argue that this is inappropriate when the targets for knowledge translation are consumers, healthcare professionals, and/or policy makers. This is because individual studies rarely, by themselves, provide sufficient evidence for practice and policy changes. In fact, individual studies may be misleading due to bias in their conduct or random variations in their findings, although some exceptionally large randomised trials may be sufficiently persuasive by themselves to warrant practice or policy change, e.g. , the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) [ 12 ] and the International Study of Infarct Survival 2 (ISIS-2) Trial [ 13 ].
Ioannidis and colleagues undertook a series of landmark studies of research exploring the evolution of evidence in healthcare (summarized in [ 14 ]). In both basic and clinical sciences, they observed the ‘Proteus phenomenon’—that the first published study on a scientific question may find the most extravagant effect size and that as further evidence is gathered, effect sizes tend to diminish [ 14 ]. They observed that thousands of observations were required before estimates of gene disease association became stable [ 15 ]. They also noted that the results of even highly cited clinical research studies published in major medical and specialty journals were likely to be contraindicated or found to be exaggerated with further accumulation of evidence [ 16 ]. As a result, Ioannidis and colleagues argued that replication and evidence synthesis is needed before knowledge translation [ 14 ].
We suggest that the results of individual studies need to be interpreted within the context of global evidence before deciding whether it is ready for knowledge translation. In other words, the basic unit of knowledge translation should be up-to-date systematic reviews or other syntheses of the global evidence. Greater emphasis on the results of systematic reviews would increase the ‘signal to noise’ of knowledge translation activities and may increase the likelihood of their success. Over the last twenty years, healthcare research funders and healthcare systems have made considerable investments in knowledge syntheses, especially those targeting the needs of healthcare practitioners and patients. Examples include the substantial number of publicly funded national guideline and health technology programs, The Cochrane Collaboration [ 17 ], and the US funded Evidence-based Practice Centers [ 18 ].
The question ‘What should be transferred?’ also challenges knowledge translators to identify the key messages for different target audiences and to fashion these in language and knowledge translation products that are easily assimilated by different audiences. Over the past decade, a variety of different products have been developed targeting different audiences (for example, decision aids for patients [ 19 ], clinical practice guidelines for healthcare professionals [ 20 ], and actionable messages [ 11 ] and policy briefs [ 21 ] for policy makers).
To whom should research knowledge be transferred?
The relative importance of knowledge translation to different target audiences will vary by the type of research being translated. For example, primary target audiences for knowledge translation of the results of basic science include other researchers and industry; whereas primary target audiences for knowledge translation of the results of population health research include other researchers, administrators, and policy makers (See Table 1 ).
Stakeholders for different types of research
Table Legend:
- Not Relevant.
+ Low Relevance to +++ High Relevance.
The relative importance of different target audiences will also vary by the results of the research [ 22 ]. For example, the primary target audiences for clinical research demonstrating lack of benefit or harms from a drug sufficient to warrant its withdrawal might be national policy makers (including regulatory bodies) and industry. Whereas, the primary target audiences for clinical research demonstrating benefits from a drug to suggest its widespread use might be patients, healthcare practitioners, local administrators as well as national policy makers, and industry (See Table 2 ).
Potential target audiences for clinical research about a drug (adapted from Mowatt et al. , 1998 [ 22 ])
| S | S | P | S | P | |
| S | S | P | P | P | |
| S | S | P | P | P | |
| P | P | P | P | P | |
| P | P | P | P | P | |
| P | P | P | P | P | |
| P | |||||
| P |
P = Primary Target Audience.
S = Secondary Target Audience.
By whom should research knowledge be transferred?
The messenger in knowledge translation efforts may be an individual ( e.g. , healthcare practitioner, researcher, or consumer), group, organization, or even healthcare system. The most appropriate messenger will vary according to the target audience and research knowledge being transferred. Shonkoff suggests that in determining ‘who’ should be the messenger credibility is important [ 23 ]. Research supports this view; Hayward and colleagues found that an authoritative endorsement by a respected physician organization or physician colleague influenced physicians’ use of clinical practice guidelines in practice [ 24 ]. With public policy makers, Lavis and colleagues suggest that the most credible messengers might include organizations of government officials [ 11 ].
Building credibility and acting as a messenger for the transfer of research knowledge is a time-consuming and skill-intensive process, making it impossible to use a ‘one size fits all’ approach to deciding ‘by whom should research knowledge be transferred’ [ 11 ]. Researchers typically carry the responsibility for conducting knowledge translation. They should, however, only be the messenger when they have credibility with the target audience, possess the skills and experience needed to transfer the research knowledge at hand, and have time and resources to do so. A more appropriate approach to effective and sustainable knowledge translation may be the development of research knowledge infrastructures by healthcare systems that address the needs of their various stakeholders ( e.g. , consumers, practitioners, managers, and policy makers). Ellen and colleagues define research knowledge infrastructure as any instrument ( i.e. , programs, tools, devices) implemented in a healthcare system in order to facilitate access, dissemination, exchange, and/or use of evidence [ 25 ]. Components of research knowledge infrastructures are classified into two broad categories: technological and organizational. Technological components include electronic databases and search engines. Organizational components include documentation specialists, data analysts, knowledge brokers ( i.e. , individuals who manage the collaboration between an organization, external information, and knowledge producers and users), and training programs (to assist with activities such as searching for information, quality appraisal, adaption and use of the research findings) [ 25 , 26 ].
In Canada, some knowledge translation researchers have invested significant time and financial resources into building technological (online databases with built-in search engines) resources that can be used by healthcare systems as part of a research knowledge infrastructure. Rx for Change is an online database that houses syntheses of the global evidence from systematic reviews: on the effectiveness of interventions for improving prescribing by healthcare professionals and medicines use by consumers; of professional interventions that impact the delivery of care; and of organizational, financial, and regulatory interventions that influence professional behaviour. The methods used to populate the database parallel systematic-review methodology. Rx for Change is publicly accessible and contains research information relevant to healthcare professionals, consumers, policy makers, and researchers [ 27 ].
Health Systems Evidence is also an online database, but primarily targets policy makers and senior managers (and other individuals responsible for assisting or making public policy decisions). Common criticisms of systematic reviews by policy makers include the absence of relevant reviews, and difficulty accessing and understanding reviews. Health Systems Evidence addresses these criticisms in order to facilitate the use of systematic reviews in health systems and policy decision making. Health Systems Evidence is a repository of syntheses of research evidence about governance, financial, and delivery arrangements within health systems, and about implementation strategies that can support change in health systems. The database contains policy briefs, overviews of systematic reviews, systematic reviews, and soon will contain a range of other types of documents needed in the policymaking process, such as economic evaluations.
Both databases ( Rx for Change and Health Systems Evidence ) provide improved access to research information for consumers, practitioners, and/or policy makers. However, this access is necessary but not sufficient to ensure knowledge translation. Effective and sustainable knowledge translation also requires organizational knowledge infrastructure components. Ellen and colleagues developed a research knowledge infrastructure framework that identified potential organizational components that a healthcare system could have in its research knowledge infrastructure. This framework is based on an environmental scan and scoping review of existing literature. The broad organizational domains included in the framework are: climate for research use, research production, activities used to link research to action including push efforts ( i.e. , efforts undertaken by researchers to disseminate research evidence to knowledge users), pull efforts ( i.e. , efforts by knowledge users to access and use research evidence), and exchange efforts ( i.e. , efforts focused on building and maintaining relationships between researchers and knowledge users), and evaluation of efforts [ 25 ]. This framework is currently being evaluated in a study examining knowledge-translation platforms in 41 countries [ 25 , 28 ].
How should research knowledge be transferred?
Planning for knowledge translation.
There are a large number of planned knowledge translation models, derived from different disciplinary and contextual viewpoints [ 29 , 30 ]. Most of these suggest that planned knowledge translation is more likely to be successful if an assessment of the likely barriers and facilitators informs the choice of knowledge translation strategy. In this section, we briefly discuss types of barriers, potential approaches for identifying barriers, and factors influencing the choice of knowledge translation intervention.
Identifying barriers to knowledge translation
Common barriers across target groups include issues relating to knowledge management, such as the sheer volume of research evidence currently produced, access to research evidence sources, time to read evidence sources and skills to appraise and understand research evidence. Over the past twenty years, there has been substantial investment by many healthcare systems to address these knowledge management barriers. For example, the conduct of systematic reviews and development of clinical practice guidelines to reduce the volume of research evidence and the time needed to read evidence sources; investment in electronic libraries of health and public access evidence sources to improve access to research evidence; and the development of critical appraisal skills tools and training to improve research literacy skills.
However while better knowledge management is necessary, it is unlikely by itself to be sufficient to ensure knowledge translation because of barriers working at different levels of healthcare systems, many of which operate at levels beyond the control of an individual practitioner. For example, barriers may operate at other levels of a healthcare system including: structural barriers ( e.g. financial disincentives), organizational barriers ( e.g. inappropriate skill mix, lack of facilities or equipment), peer group barriers ( e.g. local standards of care not in line with desired practice), professional ( e.g. knowledge, attitudes and skills) and professional-patient interaction barriers ( e.g. communication and information processing issues). Evidence in support of this can be found in a structured review of healthcare professionals’ views on engagement in quality improvement activities [ 31 ]. In this review, Davies and colleagues concluded that many of the barriers to participating in quality improvement activities identified by professionals arise from problems related to working effectively between and across health professions. This means that although knowledge management resources ( e.g. , more time and more resources) may be necessary and even helpful, they are unlikely to be sufficient to overcome the other ‘organizational’ barriers professionals face to engage in quality improvement (and knowledge translation) activities [ 31 ].
There are diverse methods for identifying potential barriers including qualitative approaches (individual interviews, focus groups), surveys and direct observation [ 32 ]. However, there are no standard approaches available yet. As a result, those involved with knowledge translation activities need to use their judgement about how best to elicit barriers given their understanding of the context and potential barriers and resources available to them.
Choosing interventions
Unfortunately, our evidence on the likely effectiveness of different strategies to overcome specific barriers to knowledge translation remains incomplete. Individuals involved in knowledge translation need to: identify modifiable and non-modifiable barriers relating to behavior; identify potential adopters and practice environments; and prioritise which barriers to target based upon consideration of ‘mission critical’ barriers. Furthermore, the potential for addressing these barriers through knowledge translation activities (based upon consideration of the likely mechanisms of action of interventions) and the resources available for knowledge translation activities also needs to be addressed.
Effectiveness of professional behaviour change strategies
The Cochrane Effective Practice and Organisation of Care (EPOC) group supports reviews of interventions to improve healthcare systems and healthcare delivery [ 33 ]. It has identified over 7,000 randomised and quasi-experimental studies and conducted 80 systematic reviews of professional, organisational, financial, and regulatory interventions within its scope by August 2011.
EPOC has prepared two overviews of systematic reviews and is currently updating these [ 34 , 35 ]. It has identified over 300 systematic reviews of professional behaviour change strategies. In this section, we summarise the results of key Cochrane EPOC reviews, selected because they are in general of higher quality and more up-to-date than non-Cochrane systematic reviews of similar focus [ 36 ]. We provide a definition of each intervention, the likely mechanism of action of the intervention, and any comments relating to the practical delivery of the intervention (including the resources required). The details and findings of the reviews of the interventions, including the median and range of effect sizes observed, are presented in Table 3 .
Effectiveness of professional behaviour change strategies from selected EPOC systematic reviews
| 12 randomised trials | Median absolute improvement of care on categorical process outcomes ( , x-ray requests, prescribing and smoking cessation activities) of 4.3% (range −8.0% to +9.6%) across studies. | |
| 11 nonrandomised studies | ||
| Farmer [ ] | ||
| 81 randomised trials (involving more than 11,000 health professionals) | Median absolute improvement in care of 6.0% (interquartile range +1.8% to 15.3%). | |
| Forsetlund [ ] | ||
| Larger effects were associated with higher attendance rates, mixed interactive and didactic meetings and interactive meetings. | ||
| Smaller effects were observed for complex behaviours and for less serious outcomes. | ||
| 69 randomised trials (involving more than 15,000 health professionals) | Median absolute improvements in: | |
| ·prescribing behaviours (17 comparisons) of 4.8% (interquartile range +3.0% to + 6.5%); | ||
| O’Brien [ ] | ||
| ·other behaviours (17 comparisons) of 6.0% (interquartile range +3.6% to +16.0%). | ||
| The effects of educational outreach for changing more complex behaviours are less certain. | ||
| 18 randomised trials (involving more than 296 hospitals and 318 primary care physicians) | Median absolute improvement of care of 12.0% across studies (interquartile range +6.0% to +14.5%). | |
| Flodgren [ ] | ||
| 118 randomised trials | Median absolute improvement of care of 5.0% (interquartile range +3% to +11%). | |
| Jamtvedt [ ] | ||
| In general, larger effects were seen if baseline compliance was low. | ||
| 28 randomised trials | Median absolute improvement of care of 4.2% (interquartile range +0.8% to +18.8%). | |
| Shojania [ ] | ||
| Comment: Most studies have examined the effects of relatively simple reminders; the results of more complex decision support systems, especially for chronic disease management, have been less successful. | ||
| 26 randomised trials | Meta-regression using 12 randomised trials. Pooled odds ratio of 1.52 (95% CI, 1.27 to 1.82, p < .001) | |
| Baker [ ] |
Generally, similar median absolute effect sizes are reported across the interventions. While one interpretation might be that the choice of intervention is less important than doing something/anything ( i.e. , that the observed effects are largely non specific (Hawthorne-like) effects), we do not believe this to be the case. The interquartile range of absolute effect sizes covers almost 30 percentage points and varies by intervention (see Table 3 ). Furthermore, the variation in observed effects within intervention category (for example the interquartile range of observed effects in trials of audit and feedback was +3% to +11% absolute improvements in performance) suggest that the effects of interventions vary presumably related to the degree to which the mechanism of action of the intervention addresses the underlying barriers in a study. The interventions also have very different mechanisms of action, and there is likely to be confounding within and across reviews. In other words, researchers are likely to have tested interventions that they believed likely effective given the particular behaviours and likely barriers within the context of their study. Finally, because we are reporting absolute effects some broad commonality of effect sizes is to be expected. In general, interventions are not tested in the expectation of producing large absolute effect sizes. Most cluster trials are powered to detect effects in the range of 10 to 20 percent absolute improvement. Under these circumstances similarity of observed effects is not surprising.
Printed educational materials
EPOC defines printed educational materials as the ‘distribution of published or printed recommendations for clinical care, including clinical practice guidelines, audio-visual materials and electronic publications. The materials may have been delivered personally or through mass mailings’ [ 37 ]. In general, printed educational materials target knowledge and potential skill gaps of individual healthcare professionals. While they could also be used to target motivation when written as a ‘persuasive communication’ there is little evidence of them being used in this way. Printed educational materials are commonly used, have a relatively low cost and are generally feasible in most settings.
Educational meetings
EPOC defines educational meetings as the ‘participation of healthcare providers in conferences, lectures, workshops or traineeships’ [ 38 ]. An important distinction is between didactic meetings (that largely target knowledge barriers at the individual healthcare professional/peer group level) and interactive workshops (that can target knowledge, attitudes, and skills at the individual healthcare professional/peer group level). Educational meetings are commonly used, with the main cost related to the release time for healthcare professionals, and are generally feasible in most settings.
Educational outreach
EPOC defines educational outreach or academic detailing as ‘use of a trained person who meets with providers in their practice settings to give information with the intent of changing the providers’ practice. The information given may have included feedback on the performance of the provider(s)’ [ 39 ]. Soumerai and Avorn suggest that educational outreach derives from social marketing approaches that target an individual’s knowledge and attitudes [ 44 ]. Typically, the detailer aims to get a maximum of three messages across during a 10 to 15 minute meeting with a healthcare provider. The detailer will tailor their approach to the characteristics of the individual healthcare provider, and typically use additional provider behaviour change strategies to reinforce their message. Most studies of educational outreach have focused on changing relatively simple behaviours in the control of individual physician behaviors such as the choice of drugs to prescribe.
Educational outreach programs have been used across a wide range of healthcare settings especially to target prescribing behaviours. They require considerable resources including the costs of detailers and preparation of materials. Nevertheless, Mason and colleagues observed that educational outreach may still be efficient to change prescribing patterns [ 45 ].
Local opinion leaders
EPOC defines local opinion leaders as ‘use of providers nominated by their colleagues as ‘educationally influential’ [ 40 ]. The investigators must have explicitly stated that their colleagues identified the opinion leaders.’ Opinion leadership is the degree to which an individual is able to influence other individuals’ attitudes or overt behaviour informally in a desired way with relative frequency. This informal leadership is not a function of the individual’s formal position or status in the system; it is earned and maintained by the individual’s technical competence, social accessibility, and conformity to the systems norms. When compared to their peers, opinion leaders have greater exposure to all forms of external communication, have somewhat higher social status and are more innovative. However, the most striking feature of opinion leaders is their unique and influential position in their system’s communication structure; they are at the centre of interpersonal communication networks (interconnected individuals who are linked by patterned flows of information). Opinion leaders target the knowledge, attitudes, and social norms of their peer group. The potential success of opinion leaders is dependent upon the existence of intact social networks within professional communities. Grimshaw and colleagues observed that the existence of such networks varied across communities and settings within the UK [ 46 ]. They also observed that opinion leaders were condition-specific; in other words, colleagues identified different opinion leaders for different clinical problems. Doumit also observed that opinion leaders where not stable over time [ 47 ]. The resources required for opinion leaders include costs of the identification method, training of opinion leaders and additional service costs.
Audit and feedback
EPOC defines audit and feedback as ‘any summary of clinical performance of healthcare over a specified period of time’ to change health professional behaviour, as indexed by ‘objectively measured professional practice in a healthcare setting or healthcare outcomes.’ The summary may also have included recommendations for clinical action. The information may have been obtained from medical records, computerised databases, or observations from patients. The subsequent feedback of and resulting action planning based on the audit summary are also important elements of an audit and feedback intervention [ 41 , 48 ]. Adams and colleagues observed that healthcare professionals often over estimated their performance by around 20% to 30% [ 49 ]. Audit and feedback target healthcare provider/peer groups’ perceptions of current performance levels and is useful to create cognitive dissonance within healthcare professionals as a stimulus for behaviour change. The resources required to deliver audit and feedback include data abstraction and analysis costs and dissemination costs. The feasibility of audit and feedback may depend on the availability of meaningful routine administrative data for feedback.
EPOC defines reminders as ‘patient or encounter specific information, provided verbally, on paper or on a computer screen, which is designed or intended to prompt a health professional to recall information [ 42 ]. This would usually be encountered through their general education, in the medical records or through interactions with peers, and so remind them to perform or avoid some action to aid individual patient care. Computer aided decision support and drugs dosage are included.’ Reminders prompt healthcare professionals to remember to do important items during professional-patient interactions [ 50 ]. The majority of early studies on computerized reminders were undertaken in highly computerized US academic health science centres, and their generalisability to other settings is less certain [ 51 ]. The resources required vary across the delivery mechanism. Additionally, there is insufficient knowledge at present about how to prioritise and optimize reminders.
Tailored interventions
Tailored interventions are ‘strategies to improve professional practice that are planned taking account of prospectively identified barriers to change’ [ 43 ]. Barriers to change refer to factors that have the potential to impair the effectiveness of interventions designed to improve professional behaviour/practice. EPOC classifies barriers to change into nine categories (information management, clinical uncertainty, sense of competence, perceptions of liability, patient expectations, standards of practice, financial disincentives, administrative constraints, and other) [ 52 ]. In a recent review, Baker and colleagues assessed the effectiveness of interventions tailored to address identified barriers to change on professional practice or patient outcomes and found that tailored interventions are more likely to improve professional practice ( e.g. , prescribing and adherence to guideline recommendations) than is no intervention or the dissemination of guidelines or educational materials. Further research is needed to determine the effectiveness of tailored interventions in comparison with other interventions [ 43 ].
Multifaceted interventions
EPOC defines multifaceted interventions as ‘any intervention including two or more components.’ Multifaceted interventions potentially target different barriers in the system. Grimshaw and colleagues explored whether there was a dose response curve for multifaceted interventions and observed that effect sizes did not necessarily increase with increasing number of components (Figure 1 ) [ 20 ]. They also observed that few studies provided any explicit rationale or theoretical base for the choice of intervention. As a result, it was unclear whether researchers had an a priori rationale for the choice of components in multifaceted interventions based upon possible causal mechanisms or whether a ‘kitchen sink’ approach formed the basis for the choice. It is plausible that multifaceted interventions built upon a careful assessment of barriers and coherent theoretical base may be more effective than single interventions. Multifaceted interventions are likely to be more costly than single interventions. When planning multifaceted interventions, it is important to carefully consider how components are likely to interact to maximise benefits.

Effect sizes of multifaceted interventions by number of interventions.
Effectiveness of knowledge translation strategies focusing on consumers
The Cochrane Consumers and Communication Review Group supports systematic reviews of the effects of interventions (particularly those which focus on information and communication) which affect consumers’ interactions with healthcare professionals, healthcare services and healthcare researchers [ 53 ]. Outcomes of interest include effects on people’s knowledge and decision-making, healthcare use, experience of healthcare, and health and wellbeing. They have identified over 7,000 randomised studies and conducted 35 systematic reviews of interventions and one overview of systematic reviews [ 54 ] within their scope to August 2011.
The Cochrane Consumers and Communication Review Group have developed a taxonomy for organising interventions. Categories relevant to knowledge translation include interventions: to facilitate communication and/or decision making; to support behaviour change; and to inform and educate. In this section, we summarize the range of intervention types relevant to knowledge translation by consumers. Drawing from the Cochrane reviews, we present the authors’ definition of each intervention; the details and findings of the reviews are presented in Table 4 .
Effectiveness of knowledge translation strategies focusing on consumers from selected systematic reviews
| 86 randomised trials (involving more than 20,209 participants) | Compared with usual care, decision aids: | |
| Stacey [ ] | ·improved knowledge and accuracy of risk perceptions; | |
| ·reduced the proportion of people who were passive in decision-making; | ||
| ·resulted in a higher proportion of patients achieving decisions informed and consistent with their values (when decision aids included an explicit values clarification component); | ||
| ·reduced the number of people remaining undecided; | ||
| ·reduced decisional conflict; | ||
| ·decreased the choice of major elective surgery in favour of conservative options. | ||
| Decision aids have no adverse effects on satisfaction but further research is needed to clarify their effect on adherence to chosen option, patient-practitioner communication, cost-effectiveness and use with developing or lower literacy populations. | ||
| 22 randomised trials | There was weak evidence, consistent with a small effect, that personalised risk communication (whether written, spoken or visually presented) increases uptake of screening tests. | |
| Edwards [ ] | ||
| 33 randomised trials (involving 8244 participants) | Compared with a control, communication before consultations increased question asking during consultations. They may also increase patient participation in consultation and improve patient satisfaction. | |
| Kinnersley [ ] | ||
| Both coaching and written material interventions produced similar effects on question asking, but coaching produced a larger increase in patient satisfaction. | ||
| Overall the benefits of ‘communication before consultations’ interventions were minor. | ||
| (2 reviews) | ||
| Murray [ ] | 24 randomised trials (involving 3739 participants) | IHCAs had a significant positive effect on knowledge, social support and clinical outcomes. |
| Bailey [ ] | 15 randomised trials (involving 3917 participants) | Positive effects of IHCAs on knowledge, safer sex self-efficacy and intentions and sexual health behavior were found. |
| Comment: Data were insufficient for meta-analysis of biological outcomes or analysis of cost- effectiveness and thus, the effects on these outcome categories remain unknown. | ||
| 78 randomised trials | Mixed effects were observed for short term and long- term medication adherence. | |
| Haynes [ ] | Some, but not all, of the simple interventions, such as counselling, written information and personal phone calls, were effective with people on short-term medication treatments. | |
| The picture for the effectiveness of interventions for longer-term treatments was mixed; few interventions showed promise and those that were effective were complex and multifaceted in nature. | ||
| 30 randomised trials (involving 4691 participants) | Contracts were shown to ‘potentially’ improve patient adherence (as applied to diagnostic procedures, therapeutic regimens, and/or a health promotion or illness prevention initiative). | |
| Bosch-Capblanch [ ] | ||
| Comment: The result above is based on only half of the included studies; the effects were not detected over longer periods. | ||
| (2 reviews) | ||
| Marteau [ ] | 13 randomised trials | Little or no effect was shown with respect to smoking cessation or increasing physical activity. A small effect was shown for changing diet. |
| (on communicating DNA-based disease risk estimates) | ||
| The intervention showed potential for altering intentions to change behaviour (in six non-clinical analogue studies). | ||
| Comment: The authors concluded that given the small number of trials in this area, more research involving ‘better-quality RCTs’ is needed before recommending application in practice. | ||
| Hollands [ ] | 9 randomised trials (involving 1371 participants) | Overall, results were mixed: |
| ·a positive effect was found for smoking cessation (three trials); | ||
| ·a positive effect was found for skin examination behaviour (one trial); | ||
| (providing visual feedback on medical imaging results) | ·no effect was found for change in physical activity (one trial). | |
| Comment: The authors concluded that due to the small number of trials and the mixed results found, the effectiveness of communicating medical imaging results to change health behaviour is largely unknown and thus, its application in practice is not yet recommended. | ||
| Nicolson [ ] | 25 randomised trials (involving 4788 participants) | Written material significantly improved knowledge of medicines in six of twelve trials. In three of these six trials recall of side effects also improved, but medicines recall significantly improved in only a minority of trials (one of four). |
| The results for attitudinal and behavioural outcomes were mixed. | ||
| Comment: Overall, the authors concluded the combined evidence from this review is not sufficient to say whether written medicines information is effective in changing behaviours related to medicine taking. | ||
| Foster [ ] | 17 randomised trials (involving 7442 participants) | Small (clinically insignificant) short-term improvements in pain, disability, fatigue and depression were found. |
| (Self management programmes run by lay people) | Positive effects on confidence to manage and self- rated health were also found. | |
| There was no effect on quality of life or use of health services. |
Interventions to facilitate communication and/or decision-making
Three interventions to facilitate communication and/or decision making that have been the focus of Cochrane systematic reviews are decision aids, personalised risk communication, and communication before consultations. Decision aids are a type of decision support intervention designed to help people make choices about health treatment options. Stacey (following O’Connor, who prepared the first Cochrane review), defines them as interventions containing ‘detailed, specific, and personalized information to help people focus on options and outcomes for the purpose of decision making’ [ 55 ]. They are important for decisions where there is uncertainty about a specific course of action. Personalised risk communication refers to the provision of information to consumers that is personally relevant to them. It is sometimes used to present and discuss the risks and benefits of healthcare in general, and of screening in particular, to consumers. As Edwards and colleagues outline, it can be based on a consumer’s own risk factors for a condition ( e.g. , their age) or calculated from their risk factors using epidemiological formulas. In the latter, the information is often presented as an absolute risk or as a risk score, or categorised into, for example, high-, medium-, or low-risk groups. Personalised risk communication may also be less detailed, for example, a listing of a consumer’s risk factors to guide discussion and intervention [ 56 ]. In their Cochrane review, Kinnersley and colleagues operationalise communication before consultations to include any intervention delivered before consultations, and which has been designed to help consumers (and/or their representatives) address their information needs within consultations [ 57 ].
Interventions to support behaviour change
One area that continues to challenge the Cochrane Consumers and Communication Review Group is the identification of effective interventions that support behaviour change. Four interventions which have been the focus of Cochrane reviews in this area are: interactive health communication applications; interventions to enhance medication adherence; contracts; and new methods of communication. Interactive health communication applications, defined by Murray and colleagues, are computer-based (usually web-based) information packages for patients that combine health information with at least one of: social support, decision support, or behaviour change support [ 58 ]. Interventions to enhance medication adherence include a wide range of single and multifaceted interventions; Haynes and colleagues identified: instruction, counseling, automated telephone monitoring and counseling, manual telephone follow-up, family intervention, increasing the convenience of care, simplified dosing, self-monitoring, reminders, special ‘reminder’ pill packaging, dose-dispensing units and medication charts, appointment and prescription refill reminders, reinforcement/rewards, medication formulations, crisis intervention, direct observation of treatments, lay health mentoring, comprehensive pharmaceutical care services, and psychological therapy in their Cochrane review [ 60 ]. Contracts refer to formalised (written or verbal) mutual agreements between two or more parties [ 61 ]. New methods of communication to date have included communicating DNA-based disease risk estimates to change health behaviours on lifestyle ( e.g. , smoking, physical activity, diet) [ 62 ] and providing consumers with a visual presentation ( i.e. , the source images) of their medical imaging ( i.e. , of magnetic resonance imaging, tomography, radiography, and/or ultrasonography) results to increase consumers’ engagement in health-related behaviours [ 63 ].
Interventions to inform and educate
Two interventions which have been the focus of Cochrane reviews to ‘inform and educate’ consumers are written information and self-management programmes. Written information is one of the most ubiquitous interventions targeting consumers [ 64 ].
Self management programmes have become a major initiative of government and community organizations in the area of chronic illness [ 65 ]. They promote various strategies for people to take an active approach to managing their health.
Effectiveness of knowledge translation strategies focusing on policy makers and senior health service managers
In contrast to the substantial evidence base on the effectiveness of knowledge translation strategies targeting healthcare professionals and consumers, few systematic reviews exist of interventions evaluating the effects of knowledge translation strategies for policy makers or senior health service managers. One review, conducted by Perrier and colleagues, evaluated interventions to increase the use of systematic reviews by health policy makers and managers [ 66 ]. Two studies were included in the review. The first study utilized a non-experimental design to report an intervention where public health policy makers were offered the opportunity to receive five relevant reviews. At three months and two years, respectively, 23% and 63% of respondents reported using at least one of the systematic reviews to make a policy decision. The second study was a randomised trial where health departments received one of three interventions: access to an online registry of systematic reviews, tailored messages plus access to the online registry of systematic reviews, or tailored messages plus access to the registry along with a knowledge broker who worked one-on-one with decision makers over a period of one year. While none of the interventions showed a significant effect on global evidence-informed decision making, tailored messages plus access to the online registry of systematic reviews showed a positive significant effect on public health policies and programs [ 66 ].
Lavis and colleagues conducted a systematic review of factors that influence the use of research evidence in public policy making [ 67 ]. Five criteria were used to assess validity of the included studies: the use of two or more data collection methods; a random or purposive sampling strategy; response rate >60%; two or more types of research use are examined; and two or more competing variables are examined.
A total of 16 studies met the criteria of using two or more data collection methods. These studies were conducted across a variety of jurisdictions, policy domains, content areas, and time periods. There was relatively little consistency in findings. However, two factors emerged with some frequency as being important to policy makers’ use of research evidence: interactions between researchers and policy makers in the context of policy networks such as formal advisory committees and in the context of informal relationships; and research that matched the beliefs, values, interests, or political goals and strategies of elected officials, social interest groups, and others. Both factors increased the prospects for research use by policy makers [ 67 ].
The findings from these reviews and other findings have led to the development of a number of knowledge translation approaches targeting policy makers and senior health services managers [ 28 , 68 , 69 ]. For example, a series of tools called SUPPORT Tools for evidence-informed health policy making (STP) were developed to assist policy makers in using research evidence. These tools were developed by members of the SUPporting POlicy relevant Reviews and Trials (SUPPORT) project, an international collaboration funded by the European Commission’s 6th Framework [ 70 ] ( http://www.supportcollaboration.org ). The SUPPORT tools describe a series of processes to help ensure that relevant research is identified, appraised and used appropriately by policy makers. The tools address four broad areas of interest related to policymaking: supporting evidence-informed policymaking [ 71 - 73 ]; identifying needs for research evidence in relation to clarifying problems, framing options, and planning implementation [ 74 - 76 ]; finding and assessing evidence from systematic reviews [ 77 - 79 ] and other kinds of evidence [ 80 , 81 ]; and moving from research evidence to decisions. The focus in this final area is on engaging stakeholders in evidence-informed policymaking [ 21 , 82 , 83 ] and on addressing how to use research evidence in decisions [ 84 - 86 ]. By focusing on how to ‘support’ the use of research evidence in health policymaking, the SUPPORT tools should increase the use of research evidence by policy makers [ 87 ] .
The SUPPORT tools describe a variety of packaging and push, facilitating pull, and exchange activities. Packaging and push activities focus on the activities of researchers to disseminate their research findings to a broad audience above and beyond traditional routes of dissemination such as publication in peer reviewed journals [ 11 ]. Examples of packaging and push activities include: increased emphasis on knowledge syntheses as the unit for knowledge translation; actionable messages; graded entry formats to allow the research user to access the level of detail that he or she requires (for example, the Canadian Health Services Research Foundation requires research reports to have one page of main messages, a three-page executive summary, and then no more than 25 pages for the complete project); using multiple communication channels tailored to the target audience; targeted electronic push of information relevant to the specific needs of research users—examples include the Contacts, Help, Advice and Information Network (C.H.A.I.N.) ([ 88 ], http://chain.ulcc.ac.uk/chain/ accessed 5 July 2011) and E-watch bulletin on Innovation in Health Services ( http://www.ohpe.ca/node/2740 accessed 5 July 2011); workshops and seminars with target audiences; and development of tools to help research users apply research findings in their own settings.
Facilitating pull activities focus on the needs of users, and creating an appetite for research results [ 11 ]. Pull activities include various training activities to improve policy makers’ and senior managers’ research literacy and interest. For example, the Canadian Health Services Research Foundation provides the EXTRA program to train senior healthcare executives in research application ( http://www.chsrf.ca/Programs/EXTRA.aspx accessed 5 July 2011). ‘One stop’ initiatives such as Health Systems Evidence also facilitate pull.
Exchange activities focus on building and maintaining new relationships between researchers and policy makers and senior managers to exchange knowledge and ideas [ 69 , 89 ]. For example, several research-funding programs require active participation of decision makers (sometimes including co-funding by healthcare organisations) in research teams. The rationale is that decision makers are more likely to consider research findings if they are actively involved in the research conducted in their settings to answer specific contextualized questions. These approaches legitimately focus on local knowledge translation of individual studies. However, the results of these studies should still be incorporated into systematic reviews to judge whether additional knowledge translation activities should be undertaken outside the context and relationships of the original study. Other exchange approaches include deliberative dialogues and the use of knowledge brokers to act as ‘human intermediaries’ between the world of research and action [ 69 , 82 , 90 ].
This profusion of approaches to improving knowledge translation to policy makers and senior healthcare managers highlights the increased recognition of the failure of traditional diffusion approaches to knowledge translation for this target group ( e.g. , [ 90 ]). Most of these approaches have a strong theoretical basis and face validity. However, it will be important to evaluate their benefits, harms and costs fully.
With what effect should research knowledge be transferred?
Appropriate endpoints of knowledge translation may vary across different stakeholder groups. For example, knowledge translation targeting policy makers and consumers should ensure that consideration of research evidence is a key component of their decision making, but recognize that there are other legitimate factors (for example, the policy context for policy makers, values and preferences of individual patients) that need to considered [ 91 - 93 ]. Thus, the resulting decision is likely to be evidence-informed but may not be particularly evidence-based. However, knowledge translation targeting professionals should result in practice that is more evidence-based and is likely to be observable as reflected in changes in professional behaviours and quality indicators.
In this paper, we have attempted to briefly summarise some of the key concepts and evidence about the effectiveness of knowledge translation activities targeting different stakeholder groups. We particularly recommend the five key questions developed by Lavis and colleagues as an aide for researchers and others involved in knowledge translation when developing knowledge translation activities [ 11 ]. There is a substantial (if incomplete) evidence base to guide choice of knowledge translation activities targeting healthcare professionals and patients. The evidence base on the effects of different knowledge translation approaches targeting healthcare policy makers and senior managers is much weaker but there are a profusion of innovative approaches that warrant further evaluation.
Grol observed that many current knowledge translation activities are based on participants’ beliefs, rather than evidence about the likely effectiveness of different approaches [ 94 ]. Grol challenged healthcare systems to develop and use a robust evidence base to support the choice of knowledge translation strategies, arguing, ‘evidence-based medicine should be complemented by evidence-based implementation.’ While we are some way from achieving this goal, there are grounds for optimism. Over the past twenty-five years, healthcare systems have invested heavily in knowledge synthesis activities that facilitate timely access of evidence. Further, it is possible to achieve clinically important practice changes by healthcare professionals and improved patient decision making with current knowledge translation activities.
Competing interests
MPE is Co-Editor in Chief and JMG and JNL are Editorial Board members of Implementation Science. All editorial decisions on this manuscript were made by another editor.
Authors’ contributions
JMG conceived of the idea of the paper. All authors contributed to writing the manuscript and approved the final version.
Acknowledgements
We are grateful to Egon Jonson for agreeing that we could use the chapter Grimshaw J, Eccles MP (2008). Knowledge Translation of Research Findings. In IHE Report June 2008: Effective Dissemination of Findings from Research. Institute of Health Economics, Alberta, Canada (2008) as the basis for this paper. JMG holds a Canada Research Chair. JES is a Postdoctoral Fellow funded by Canadian Institutes for Health Research. JMG and JNL are members and MPE is a member of the Scientific Advisory Board of KT Canada. Sophie Hill’s role as Coordinating Editor of Cochrane Consumers and Communication Review Group is supported by funding from the Quality, Safety and Patient Experience Branch, Department of Health Victoria, Australia and the National Health and Medical Research Council Funding for Australian-based Cochrane Collaboration Activities.
- McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, Kerr EA. The quality of health care delivered to adults in the United States. N Engl J Med. 2003; 348 :2635–2645. doi: 10.1056/NEJMsa022615. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001; 39 :II46–II54. [ PubMed ] [ Google Scholar ]
- Schuster MA, McGlynn EA, Brook RH. How good is the quality of health care in the United States? 1998. Milbank Q. 2005; 83 :843–895. doi: 10.1111/j.1468-0009.2005.00403.x. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McKibbon KA, Lokker C, Wilczynski NL, Ciliska D, Dobbins M, Davis DA, Haynes RB, Straus SE. A cross-sectional study of the number and frequency of terms used to refer to knowledge translation in a body of health literature in 2006: a Tower of Babel? Implement Sci. 2010; 5 :16. doi: 10.1186/1748-5908-5-16. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tetroe JM, Graham ID, Foy R, Robinson N, Eccles MP, Wensing M, Durieux P, Legare F, Nielson CP, Adily A, Ward JE, Porter C, Shea B, Grimshaw JM. Health research funding agencies' support and promotion of knowledge translation: an international study. Milbank Q. 2008; 86 :125–155. doi: 10.1111/j.1468-0009.2007.00515.x. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Donabedian A. Explorations in quality assessment and monitoring: II - the criteria and standards of quality. Health Administration Press, Ann Arbor, Michigan; 1982. [ Google Scholar ]
- Sung NS, Crowley WF, Genel M, Salber P, Sandy L, Sherwood L, Johnson S, Catanese V, Tilson H, Getz K, Larson E, Scheinberg D, Reece E, Slavkin H, Dobs A, Grebb J, Martinez R, Korn A, Rimoin D. Central challenges facing national clinical research enterprise. JAMA. 2003; 289 :1278–1287. doi: 10.1001/jama.289.10.1278. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tarde G. The law of imitiation. HOLT, New York; 1903. [ Google Scholar ]
- Estabrooks CA, Derksen L, Winther C, Lavis JN, Scott SD, Wallin L, Profetto-McGrath J. The intellectual structure and substance of the knowledge utilization field: A longitudinal author co-citation analysis, 1945 to 2004. Implement Sci. 2008; 3 :49. doi: 10.1186/1748-5908-3-49. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Grimshaw J, Eccles MP. In: Effective Dissemination of Findings from Research. Jonson E, editor. Institute of Health Economics, Edmonton; 2008. Knowledge Translation Of Research Findings. [ Google Scholar ]
- Lavis JN, Robertson D, Woodside JM, McLeod CB, Abelson J. How can research organizations more effectively transfer research knowledge to decision makers? Milbank Q. 2003; 81 :221–222. doi: 10.1111/1468-0009.t01-1-00052. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium chanel blocker vs diuretic. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002; 288 :2981–2997. doi: 10.1001/jama.288.23.2981. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- International Study of Infarct Survival Collaborative Group. Randomised trial of intravenous streptokinase, oral asprin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet. 1988; 2 :349–360. [ PubMed ] [ Google Scholar ]
- Ioannidis JP. Evolution and translation of research findings: from bench to where? PLoS Clin Trials. 2006; 1 :e36. doi: 10.1371/journal.pctr.0010036. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet. 2001; 29 :306–309. doi: 10.1038/ng749. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ioannidis JP. Contradicted and initially stronger effects in highly cited clinical research. JAMA. 2005; 294 :218–228. doi: 10.1001/jama.294.2.218. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Grimshaw JM, Santesso N, Cumpston M, Mayhew A, McGowan J. Knowledge for knowledge translation: the role of the Cochrane Collaboration. J Contin Educ Health Prof. 2006; 26 :55–62. doi: 10.1002/chp.51. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Helfand M, Morton SC, Guallar E, Mulrow C. Challenges of Summarizing Better Information for Better Health: The Evidence-based Practice Center Experience. Ann Intern Med. 2005; 142 :1033–1126. [ Google Scholar ]
- O'Connor AM, Bennett C, Stacey D, Barry MJ, Col NF, Eden KB, Entwistle V, Fiset V, Holmes-Rovner M, Khangura S, Llewellyn-Thomas H, Rovner D. Do patient decision aids meet effectiveness criteria of the International Patient Decision Aid Standards Collaboration? A systematic review and meta-analysis. Med Decis Making. 2007; 27 [ PubMed ] [ Google Scholar ]
- Grimshaw JM, Thomas RE, Maclennan G, Fraser C, Ramsay CR, Vale L, Whitty P, Eccles MP, Matowe L, Shirran L, Wensing M, Dijkstra R, Donaldson C. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004; 8 :iii-72. [ PubMed ] [ Google Scholar ]
- Lavis JN, Permanand G, Oxman AD, Lewin S, Fretheim A. SUPPORT Tools for evidence-informed health Policymaking (STP) 13: Preparing and using policy briefs to support evidence-informed policymaking. Health Res Policy Syst. 2009; 7 (Suppl 1):S13. doi: 10.1186/1478-4505-7-S1-S13. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mowatt G, Thomson MA, Grimshaw J, Grant A. Implementing early warning messages on emerging health technologies. Int J Technol Assess Health Care. 1998; 14 :663–670. doi: 10.1017/S0266462300011971. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shonkoff JP. Science, policy, and practice: Three cultures in search of a shared mission. Child Development. 2000; 71 :181–187. doi: 10.1111/1467-8624.00132. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hayward RSA, Guyatt G, Moore KA, McKibbon KA, Carter AO. Canadian physicians' attitudes about and preferences regarding clinical practice guidelines. Canadian Medical Association Journal. 1997; 156 :1715–1723. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ellen ME, Lavis J, Ouimet M, Grimshaw JM, Bedard PO. Determining research knowledge infrastructure for healthcare systems: A qualitative study. Implement Sci. 2011; 6 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Meso P, Smith R. A resource-based view of organizational knowledge management systems. J Knowledge Management. 2000; 4 :224–234. doi: 10.1108/13673270010350020. [ CrossRef ] [ Google Scholar ]
- Weir M, Ryan R, Mayhew A, Worswick J, Santesso N, Lowe D, Leslie B, Stevens A, Hill S, Grimshaw JM. The Rx for Change database: A first-in-class tool for optimal prescribing and medicines use. Implement Sci. 2010; 5 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lavis JN, Lomas J, Hamid M, Sewankambo NK. Assessing country-level efforts to link research to action. Bull World Health Organ. 2006; 84 :620–628. doi: 10.2471/BLT.06.030312. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003; 362 :1225–1230. doi: 10.1016/S0140-6736(03)14546-1. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Graham ID, Logan J, Harrison MB, Straus SE, Tetroe J, Caswell W, Robinson N. Lost in knowledge translation: time for a map? J Contin Educ Health Prof. 2006; 26 :13–24. doi: 10.1002/chp.47. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Davies H, Powell A, Rushmer R. Healthcare professionals' views on clinician engagement in quality improvement: A literature review. Health Foundation. 2007.
- Grol R, Wensing M, Eccles MP. Improving patient care: implementing change in clinical practice. Elsevier, Oxford; 2004. [ Google Scholar ]
- Ballini L, Bero L, Eccles MP, Grimshaw J, Gruen RL, Lewin S, Effective Practice and Organisation of Care Group. The Cochrane Library About The Cochrane Collaboration (Cochrane Review Groups (CRGs) 2011.
- Bero LA, Grilli R, Grimshaw JM, Harvey E, Oxman AD, Thomson MA. Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. The Cochrane Effective Practice and Organization of Care Review Group. BMJ. 1998; 317 :465–468. doi: 10.1136/bmj.317.7156.465. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Grimshaw JM, Shirran L, Thomas R, Mowatt G, Fraser C, Bero L, Grilli R, Harvey E, Oxman A, O'Brien MA. Changing provider behavior: an overview of systematic reviews of interventions. Med Care. 2001; 39 :II2–45. [ PubMed ] [ Google Scholar ]
- Moher D, Tetzlaff J, Tricco AC, Sampson M, Altman DG. Epidemiology and reporting characteristics of systematic reviews. PLoS Med. 2007; 4 :e78. doi: 10.1371/journal.pmed.0040078. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Farmer AP, Legare F, Turcot L, Grimshaw J, Harvey E, McGowan JL, Wolf F. Printed educational materials: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2011. p. CD004398. [ PubMed ]
- Forsetlund L, Bjorndal A, Rashidian A, Jamtvedt G, O'Brien MA, Wolf F, Davis D, Odgaard-Jensen J, Oxman AD. Continuing education meetings and workshops: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2009. p. CD003030. [ PMC free article ] [ PubMed ]
- O'Brien MA, Rogers S, Jamtvedt G, Oxman A, Odgaard-Jensen J, Kristoffersen D, Forsetlund L, Bainbridge D, Freemantle N, Davis DA, Haynes R, Harvey E. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2008. p. CD000409. [ PMC free article ] [ PubMed ]
- Flodgren G, Parmelli E, Doumit G, Gattellari M, O'Brien MA, Grimshaw J, Eccles MP. Local opinion leaders: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2010. p. CD000125. [ PMC free article ] [ PubMed ]
- Jamtvedt G, Young JM, Kristoffersen DT, O'Brien MA, Oxman AD. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2010. p. CD000259. [ PubMed ]
- Shojania KG, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on-screen, point of care computer reminders on processes and outcomes of care. Cochrane Database Syst Rev. 2011. p. CD001096. [ PMC free article ] [ PubMed ]
- Baker R, Camosso-Stefanovic J, Gilliss CL, Shaw EJ, Cheater F, Flottorp S, Robertson N. Tailored interventions to overcome identified barriers to change: Effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2010. p. CD005470. [ PMC free article ] [ PubMed ]
- Soumerai SB, Avorn J. Principles of educational outreach ('academic detailing') to improve clinical decision making. JAMA. 1990; 263 :549–556. doi: 10.1001/jama.1990.03440040088034. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mason J, Freemantle N, Nazareth I, Eccles M, Haines A, Drummond M. When is it cost-effective to change the behavior of health professionals? JAMA. 2001; 286 :2988–2992. doi: 10.1001/jama.286.23.2988. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Grimshaw JM, Eccles MP, Greener J, Maclennan G, Ibbotson T, Kahan JP, Sullivan F. Is the involvement of opinion leaders in the implementation of research findings a feasible strategy? Implement Sci. 2006; 1 :3. doi: 10.1186/1748-5908-1-3. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Doumit G. Opinion leaders: Effectiveness, Identification, Stability, Specificity, and Mechanism of Action. PhD Thesis. University Of Ottawa; 2006. [ Google Scholar ]
- Gardner B, Whittington C, McAteer J, Eccles M, Michie S. Using theory to synthesize evidence from behaviour change interventions: The example of audit and feedback. Social Science & Medicine. 2010; 70 :1618–1625. doi: 10.1016/j.socscimed.2010.01.039. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Adams AS, Soumerai SB, Lomas J, Ross-Degnan D. Evidence of self-report bias in assessing adherence to guidelines. Int J Qual Health Care. 1999; 11 :187–192. doi: 10.1093/intqhc/11.3.187. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McDonald C. Protocol-based computer reminders, the quality of care and the non-prefectability of man. N Engl J Med. 1976; 295 :1351–1355. doi: 10.1056/NEJM197612092952405. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chaudhry B, Wang J, Wu S, Maglione M, Mojica W, Roth E, Morton SC, Shekelle PG. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006; 144 :742–752. [ PubMed ] [ Google Scholar ]
- Cochrane Effective Practice and Organisation of Care group. Data collection checklist. EPOC measures for review authors. 2002.
- Prictor M, Hill S, Car J, Edwards A, Glenton C. Horey D et al. Cochrane Consumers and Communications Group, About The Cochrane Collaboration (Cochrane Review Groups (CRGs)); 2010. [ Google Scholar ]
- Ryan R, Santesso N, Hill S, Lowe D, Kaufman C, Grimshaw JM. Consumer-oriented interventions for evidence-based prescribing and medicines use: An overview of systematic reviews. Cochrane Database Syst Rev. 2011. [ PubMed ]
- Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes-Rovner M, Llewellyn-Thomas H, Lyddiatt A, Legare F, Thomson R. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011. [ PubMed ]
- Edwards AG, Evans R, Dundon J, Haigh S, Hood K, Elwyn GJ. Personalised risk communication for informed decision making about taking screening tests. Cochrane Database Syst Rev. 2006. p. CD001865. [ PubMed ]
- Kinnersley P, Edwards A, Hood K, Cadbury N, Ryan R, Prout H, Owen D, Macbeth F, Butow P, Butler C. Interventions before consultations for helping patients address their information needs. Cochrane Database Syst Rev. 2007. p. CD004565. [ PMC free article ] [ PubMed ]
- Murray E, Burns J, See TS, Lai R, Nazareth I. Interactive Health Communication Applications for people with chronic disease. Cochrane Database Syst Rev. 2005. p. CD004274. [ PubMed ]
- Bailey JV, Murray E, Rait G, Mercer CH, Morris RW, Peacock R, Cassell J, Nazareth I. Interactive computer-based interventions for sexual health promotion. Cochrane Database Syst Rev. 2010. p. CD006483. [ PubMed ]
- Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008. p. CD000011. [ PubMed ]
- Bosch-Capblanch X, Abba K, Prictor M, Garner P. Contracts between patients and healthcare practitioners for improving patients' adherence to treatment, prevention and health promotion activities. Cochrane Database Syst Rev. 2009. [ PMC free article ] [ PubMed ]
- Marteau TM, French DP, Griffin SJ, Prevost AT, Sutton S, Watkinson C, Attwood S, Hollonds GJ. Effects of communicating DNA-based disease risk estimates on risk-reducing behaviours. Cochrane Database Syst Rev. 2010. [ PubMed ]
- Hollands GJ, Hankins M, Marteau TM. Visual feedback of individuals' medical imaging results for changing health behaviour. Cochrane Database Syst Rev. 2010. [ PubMed ]
- Nicolson D, Knapp P, Raynor DK, Spoor P. Written information about individual medicines for consumers. Cochrane Database Syst Rev. 2009. [ PMC free article ] [ PubMed ]
- Foster G, Taylor SJ, Eldridge SE, Ramsay J, Griffiths CJ. Self-management education programmes by lay leaders for people with chronic conditions. Cochrane Database Syst Rev. 2007. p. CD005108. [ PubMed ]
- Perrier L, Mrklas K, Lavis J, Straus S. Interventions encouraging the use of systematic reviews by health policymakers and managers: A systematic review. Implement Sci. 2011; 6 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lavis J, Hammill AC, Gildiner A, McDonagh RJ, Wilson MG, Ross SE, Ouimet M, Stoddart GL. A systematic review of the factors that influence the use of research evidence by public policymakers: Report submitted to the Canadian Population Health Initiative. Hamilton, Canada; 2005. [ Google Scholar ]
- Lavis JN. Research, public policymaking, and knowledge-translation processes: Canadian efforts to build bridges. J Contin Educ Health Prof. 2006; 26 :37–45. doi: 10.1002/chp.49. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lomas J. The in-between world of knowledge brokering. BMJ. 2007; 334 :129–132. doi: 10.1136/bmj.39038.593380.AE. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lavis JN, Oxman AD, Lewin S, Fretheim A. SUPPORT Tools for evidence-informed health Policymaking (STP) Health Res Policy Syst. 2009; 7 (Suppl 1):I1. doi: 10.1186/1478-4505-7-S1-I1. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oxman AD, Lavis JN, Lewin S, Fretheim A. SUPPORT Tools for evidence-informed health Policymaking (STP) 1: What is evidence-informed policymaking? Health Res Policy Syst. 2009; 7 (Suppl 1):S1. doi: 10.1186/1478-4505-7-S1-S1. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oxman AD, Vandvik PO, Lavis JN, Fretheim A, Lewin S. SUPPORT Tools for evidence-informed health Policymaking (STP) 2: Improving how your organisation supports the use of research evidence to inform policymaking. Health Res Policy Syst. 2009; 7 (Suppl 1):S2. doi: 10.1186/1478-4505-7-S1-S2. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lavis JN, Oxman AD, Lewin S, Fretheim A. SUPPORT Tools for evidence-informed health Policymaking (STP) 3: Setting priorities for supporting evidence-informed policymaking. Health Res Policy Syst. 2009; 7 (Suppl 1):S3. doi: 10.1186/1478-4505-7-S1-S3. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lavis JN, Wilson MG, Oxman AD, Lewin S, Fretheim A. SUPPORT Tools for evidence-informed health Policymaking (STP) 4: Using research evidence to clarify a problem. Health Res Policy Syst. 2009; 7 (Suppl 1):S4. doi: 10.1186/1478-4505-7-S1-S4. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lavis JN, Wilson MG, Oxman AD, Grimshaw J, Lewin S, Fretheim A. SUPPORT Tools for evidence-informed health Policymaking (STP) 5: Using research evidence to frame options to address a problem. Health Res Policy Syst. 2009; 7 (Suppl 1):S5. doi: 10.1186/1478-4505-7-S1-S5. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fretheim A, Munabi-Babigumira S, Oxman AD, Lavis JN, Lewin S. SUPPORT Tools for Evidence-informed policymaking in health 6: Using research evidence to address how an option will be implemented. Health Res Policy Syst. 2009; 7 (Suppl 1):S6. doi: 10.1186/1478-4505-7-S1-S6. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lavis JN, Oxman AD, Grimshaw J, Johansen M, Boyko JA, Lewin S, Fretheim A. SUPPORT Tools for evidence-informed health Policymaking (STP) 7: Finding systematic reviews. Health Res Policy Syst. 2009; 7 (Suppl 1):S7. doi: 10.1186/1478-4505-7-S1-S7. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lewin S, Oxman AD, Lavis JN, Fretheim A. SUPPORT Tools for evidence-informed health Policymaking (STP) 8: Deciding how much confidence to place in a systematic review. Health Res Policy Syst. 2009; 7 (Suppl 1):S8. doi: 10.1186/1478-4505-7-S1-S8. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lavis JN, Oxman AD, Souza NM, Lewin S, Gruen RL, Fretheim A. SUPPORT Tools for evidence-informed health Policymaking (STP) 9: Assessing the applicability of the findings of a systematic review. Health Res Policy Syst. 2009; 7 (Suppl 1):S9. doi: 10.1186/1478-4505-7-S1-S9. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lewin S, Oxman AD, Lavis JN, Fretheim A, Marti SG, Munabi-Babigumira S. SUPPORT Tools for evidence-informed Policymaking in health 11: Finding and using evidence about local conditions. Health Res Policy Syst. 2009; 7 (Suppl 1):S11. doi: 10.1186/1478-4505-7-S1-S11. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oxman AD, Fretheim A, Lavis JN, Lewin S. SUPPORT Tools for evidence-informed health Policymaking (STP) 12: Finding and using research evidence about resource use and costs. Health Res Policy Syst. 2009; 7 (Suppl 1):S12. doi: 10.1186/1478-4505-7-S1-S12. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lavis JN, Boyko JA, Oxman AD, Lewin S, Fretheim A. SUPPORT Tools for evidence-informed health Policymaking (STP) 14: Organising and using policy dialogues to support evidence-informed policymaking. Health Res Policy Syst. 2009; 7 (Suppl 1):S14. doi: 10.1186/1478-4505-7-S1-S14. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oxman AD, Lewin S, Lavis JN, Fretheim A. SUPPORT Tools for evidence-informed health Policymaking (STP) 15: Engaging the public in evidence-informed policymaking. Health Res Policy Syst. 2009; 7 (Suppl 1):S15. doi: 10.1186/1478-4505-7-S1-S15. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oxman AD, Lavis JN, Fretheim A, Lewin S. SUPPORT Tools for evidence-informed health Policymaking (STP) 16: Using research evidence in balancing the pros and cons of policies. Health Res Policy Syst. 2009; 7 (Suppl 1):S16. doi: 10.1186/1478-4505-7-S1-S16. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oxman AD, Lavis JN, Fretheim A, Lewin S. SUPPORT Tools for evidence-informed health Policymaking (STP) 17: Dealing with insufficient research evidence. Health Res Policy Syst. 2009; 7 (Suppl 1):S17. doi: 10.1186/1478-4505-7-S1-S17. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fretheim A, Oxman AD, Lavis JN, Lewin S. SUPPORT Tools for Evidence-informed policymaking in health 18: Planning monitoring and evaluation of policies. Health Res Policy Syst. 2009; 7 (Suppl 1):S18. doi: 10.1186/1478-4505-7-S1-S18. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lavis J, Oxman A, Lewin S, Fretheim A. SUPPORT Tools for evidence-informed health Policymaking (STP) Health Research Policy and Systems. 2009; 7 :I1. doi: 10.1186/1478-4505-7-S1-I1. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Evans D, McAuley L, Santesso N, McGowan J, Grimshaw JM. In: Innovations in Health Care: A Reality Check. Casebeer AH, Mark AL, editor. Palgrave Macmillan, Houndmills; 2006. Effective Health Care CHAIN (Contacts, Help, Advice and Information Network): Breaking Down Barriers between Professions, Organizations, Researchers and Practitioners in the UK and Canada; pp. 220–231. [ Google Scholar ]
- Canadian Health Services Research Foundation. Issues in Linkage and Exchange between Researchers and Decision Makers. 1999.
- Lomas J. Improving research dissemination and uptake in the health sector: Beyond the sound of one hand clapping. ONT, Mcmaster University, Hamilton; 1997. 4-8-2011. [ Google Scholar ]
- Lavis J, Ross S, McLeod C, Glidiner A. Measuring the impact of health research. Journal of Health Services Research and Policy. 2003; 8 :165–170. doi: 10.1258/135581903322029520. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lavis JN, Ross SE, Hurley JE, Hohenadel JM, Stoddart GL, Woodward CA, Abelson J. Examining the role of health services research in public policymaking. Milbank Q. 2002; 80 :125–154. doi: 10.1111/1468-0009.00005. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Squires J, Estabrooks C, O'Rourke H, Gustavsson P, Newburn-Cook C, Wallin L. A systematic review of the psychometric properties of self-report research utilization measures used in healthcare. Implement Sci. 2011; 6 :83. doi: 10.1186/1748-5908-6-83. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Grol R. Beliefs and evidence in changing clinical practice. British Medical Journal. 1997; 315 :418–421. doi: 10.1136/bmj.315.7105.418. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
This device is too small
If you're on a Galaxy Fold, consider unfolding your phone or viewing it in full screen to best optimize your experience.
- Personal Finance
This Biden Administration Proposal Just Might Salvage Your Credit Report
Published on Aug. 13, 2024
By: Dana George
- The Biden administration wants medical bills removed from credit reports to prevent lenders from making decisions based on medical information.
- Medical bills do not serve as a fair indicator of how likely someone is to pay their bills.
- Medical debt is the top reason for bankruptcies in the U.S.
Medical debt is no joke. Not only does the U.S. have the most expensive healthcare of any country, but medical debt is the No. 1 reason Americans file for bankruptcy, according to the American Medical Association (AMA).
Medical debt can also play havoc on a person's budget and personal finances, causing their credit score to plummet and making it harder for them to get credit when it's needed.
A step in the right direction
Two years ago, Experian, Equifax, and TransUnion -- the big three national credit reporting agencies -- announced that they were removing medical debts under $500 from credit reports. Removing relatively small medical debts certainly represented a step in the right direction for many U.S. consumers. Still, 15 million Americans were left with $49 billion worth of medical bills on their credit reports.
The Biden administration plan would force credit reporting agencies to go much further.
What this could mean for you
What matters is how erasing medical debt from credit reports will impact the everyday American. If your credit report currently lists medical expenses among your unpaid debts, here's how this change may benefit you.
Your credit score will get a boost
The Consumer Financial Protection Bureau (CFPB) estimates that Americans with medical debt on their credit reports will see their credit scores rise by an average of 20 points once medical debt is removed. A sudden 20-point boost may be just what some households need to qualify for a loan or open a credit card.
Your medical issues won't be public knowledge
While credit reports only show that you have outstanding medical debt and don't spell out the specifics, anyone who sees a copy of your report can tell that you (or someone in your household) have been ill.
That may not matter if you're applying for a car loan, but it could impact whether a potential employer checking your credit report decides to take a chance on you or a landlord believes you can pay your rent every month. In other words, it raises questions that should never be raised.
Your medical equipment cannot be repossessed
The proposed rule would prevent lenders from repossessing medical equipment like wheelchairs if you can't repay a loan.
Bill collectors can no longer use medical debt as a weapon
According to the CFPB, under the current system, medical debt collectors use the credit reporting system to force people to pay debts -- some of which they may not owe. CFPB reports that many debt collectors use a practice known as "debt parking."
Here's how it works: Debt collectors purchase medical debt at a discount. They then place the debt on your credit report, typically without your knowledge. It's only when you apply for credit that you discover that medical debt is holding you back from loan approval.
If you really need that loan, you may feel forced to pay the medical bill just to get it off your credit report and improve your credit score. Once medical debt no longer shows up on credit reports, bill collectors can no longer use this manipulative tactic.
This doesn't mean debt will be erased
There are undoubtedly benefits associated with having medical debts removed from your credit report, but you will still be responsible for repaying the debt . The point of removing the debt from your report is to make it easier for you to carry on with your financial responsibilities and get back on your financial feet.
The CFPB will continue to accept comments and feedback on the Biden administration proposal through Aug. 12, 2024. If all goes well, the rule will be finalized early next year.
Alert: our top-rated cash back card now has 0% intro APR until 2025
This credit card is not just good – it’s so exceptional that our experts use it personally. It features a lengthy 0% intro APR period, a cash back rate of up to 5%, and all somehow for no annual fee! Click here to read our full review for free and apply in just 2 minutes.
Our Research Expert
Dana is a full-time personal finance writer, with more than two decades of experience. Her focus is on helping readers feel less alone as they navigate their personal finances and offering actionable insights.
Share this page
We're firm believers in the Golden Rule, which is why editorial opinions are ours alone and have not been previously reviewed, approved, or endorsed by included advertisers. The Ascent, a Motley Fool service, does not cover all offers on the market. The Ascent has a dedicated team of editors and analysts focused on personal finance, and they follow the same set of publishing standards and editorial integrity while maintaining professional separation from the analysts and editors on other Motley Fool brands.
Related Articles
By: Cole Tretheway | Published on June 7, 2024
By: Lyle Daly | Published on June 5, 2024
By: Christy Bieber | Published on June 5, 2024
By: Lyle Daly | Published on June 4, 2024
The Ascent is a Motley Fool service that rates and reviews essential products for your everyday money matters.
Copyright © 2018 - 2024 The Ascent. All rights reserved.

IMAGES
VIDEO
COMMENTS
This suggests a relatively recent increase in interest in the issue of knowledge transfer in health research. In the 79 articles were references to 88 models or frameworks (including multiple occurrences across articles), with 49 unique models/frameworks named and 13 models not explicitly named. ... BMC Medical Research Methodology, 6, 35 10. ...
Despite increasing interest in research on how to translate knowledge into practice and improve healthcare, the accumulation of scientific knowledge in this field is slow. Few substantial new insights have become available in the last decade. Various problems hinder development in this field. There is a frequent misfit between problems and approaches to implementation, resulting in the use of ...
Introduction. Failing to translate research knowledge into action in health care contributes to health inequities and wastes costly and time-consuming research 1-3.The gap between what is known and what is done leads not only to the under-use of effective treatments, but also to the incorrect use of treatments and the over-use of unhelpful or unproven treatments, all of which lead to negative ...
In this context, different terms are mainly used interchangeably. However, some studies used the terms knowledge transfer and knowledge diffusion to refer to the passive, untargeted, and unplanned spread of research findings, and the term knowledge dissemination for a more active spread, based on planned strategies (Graham et al. 2006; Nilsen ...
Some other commonly used terms are: knowledge translation, knowledge exchange, knowledge sharing, research utilization, implementation, dissemination, diffusion, continuing education, or continuing professional development (Graham et al. 2006). In the case of healthcare the term knowledge transfer is preferred because it indicates that the ...
One of the most consistent findings from clinical and health services research is the failure to translate research into practice and policy. For example, McGlynn and colleagues observed that patients in the USA received 55% of recommended care, and that quality varied by medical condition ranging from 79% of recommended care for senile ...
Knowledge translation (KT) is "the synthesis, exchange, and application of knowledge by relevant stakeholders to accelerate the benefits of global and local innovation in strengthening health systems and improving people's health" [].The process of KT ensures that evidence from research is used by relevant stakeholders, including healthcare providers, managers, policy-makers, informal ...
Empowering Knowledge Transfer in Healthcare. A Framework of Knowledge Transfer M ethods. Paul Kruse, Christian Kummer, Anja Jannack. 1 Knowledge Research Center e.V. 2 TU Dresden, Chair of ...
This collection aims to challenge so-called knowledge translation (hereinafter KT) in medicine and healthcare. The abbreviation 'KT' refers to a variety of scientific practices and research ...
Knowledge translation (KT) is a concept first used in 2000 by the Canadian Institute of Health Research (CIHR) ( 1 - 7) to address the gap between research knowledge and its application in clinical practice in health ( 1, 2, 4, 5 ). Since that time, use of the term has grown dramatically, with a tenfold increase revealed by a search of Medline ...
From Research to Practice: A Knowledge Transfer Planning Guide (2006) About this report: Authors: Rhoda Reardon, John Lavis, Jane Gibson. If you would like to receive a copy of this or any other of our reports, please contact us at: Institute for Work & Health 481 University Avenue, 8th Floor Toronto, Ontario M5G 2E9 416-927-2027 extension 2173.
Effective research transfer will ensure patients and populations benefit from evidence-based best practice. While there is an increasing rigor with which to approach research transfer in health care settings, greater demand among those responsible for research transfer for a more scientifically sound knowledge base will accelerate development of the discipline.
2.2 Medical knowledge transfer in OHCs. Knowledge transfer is the process of sharing or disseminating knowledge between two or more parties through a medium . Extant research classifies this knowledge transfer into explicit and tacit knowledge transfer and validates it on a variety of information system media .
explore possibilities to study knowledge transfer between disciplines by analysing cross-disciplinary citations in research literature. Part of this project was a comparison of age distributions of mono- and cross disciplinary citations, in which field specific differences were found between the speed of knowledge transfer within a discipline and
Background: Universities, public institutions, and the transfer of knowledge to the private sector play a major role in the development of medical technologies. The decisions of universities and public institutions regarding the transfer of knowledge impact the accessibility of the final product, making it easier or more difficult for consumers to access these products.
There is recognition in the CIHR definition that KT stages must consider coordinated communication, marketing, and training to facilitate KT. The seven stages are the following: Research priority setting. Research. Knowledge priority setting. Knowledge synthesis. Knowledge distribution and application. Use. Evaluation of uptake.
Knowledge transfer and exchange (KTE) is an interactive interchange of knowledge between research users and researcher producers (Kiefer et al. 2005).The primary purposes of KTE are to increase the likelihood that research evidence will be used in policy and practice decisions and to enable researchers to identify practice and policy-relevant research questions.
Pull activities and training: Such strategies can create an appetite for medical research and knowledge uptake. ... Closing the know-do gap in medical research is essential as failure to transfer research findings into practice contributes to more than $200 billion of wasted funding and poor health outcomes (Graham, Kothari & McCutcheon, 2018).
EVALUATING KNOWLEDGE TRANSFER IN NEURAL NETWORK FOR MEDICAL IMAGES Sina Akbarian, Laleh Seyyed-kalantari, Farzad Khalvati, and Elham Dolatabadi Abstract—Deep learning and knowledge transfer techniques have permeated the field of medical imaging and are consid-ered as key approaches for revolutionizing diagnostic imaging practices.
Background: With the rise of artificial intelligence (AI) in the field of dementia biomarker research, exploring its current developmental trends and research focuses has become increasingly important. This study, using literature data mining, analyzes and assesses the key contributions and development scale of AI in dementia biomarker research.
Sampling. This study aims to explore knowledge management practices within the research and development (R&D) sector of the information service industry, with a keen focus on companies operating ...
The search strategy included four search terms and their variations (knowledge (evidence, research, information, data), transfer (exchange, generation, translation, uptake, mobilization, dissemination, implementation), framework (model, concept) and health care (health system, health service, healthcare provider)) and was designed to be as ...
Background Patients from the lesbian, gay, bisexual, transgender, queer plus (LGBTQ +) community face various health inequalities and report poor healthcare experiences. Little is known about how knowledgeable and confident UK doctors are around LGBTQ + health, and previous research demonstrates that UK medical schools rarely deliver teaching in this area. This research evaluated the level of ...
In medical research, two types of knowledge transfer can be discerned. The first consists of the utilization of basic medical research for the "development of new methods for diagnosis, therapy, and prevention and their first testing in humans", while the second describes the "translation of results from clinical studies into everyday ...
The research methodology employed in this paper was limited to a case study, and the data utilized are not empirical data. ... Since the amount of knowledge transfer is an important aspect of the effectiveness of knowledge transfer, this paper constructs a Stackelberg game model with an innovation-quality-oriented threshold of the knowledge ...
Background Universities, public institutions, and the transfer of knowledge to the private sector play a major role in the development of medical technologies. The decisions of universities and public institutions regarding the transfer of knowledge impact the accessibility of the final product, making it easier or more difficult for consumers to access these products. In the case of medical ...
Muscle-specific kinase (MuSK) orchestrates establishment and maintenance of neuromuscular synapses, which enable muscle contraction. Autoantibodies targeting MuSK cause myasthenia gravis (MG), a disease characterized by fatigable skeletal muscle weakness which requires chronic immunosuppressive treatment and ventilatory support at some point in ~30% of patients. MuSK autoantibodies are ...
One of the most consistent findings from clinical and health services research is the failure to translate research into practice and policy. For example, McGlynn and colleagues observed that patients in the USA received 55% of recommended care, and that quality varied by medical condition ranging from 79% of recommended care for senile ...
Medical debt is no joke. Not only does the U.S. have the most expensive healthcare of any country, but medical debt is the No. 1 reason Americans file for bankruptcy, according to the American ...
To assess frequency, knowledge, attitude health behavior toward the risks and complication of cigarettes smoking and hookah in particular of Al-Kindy medical students. Materials and methods A cross-sectional study was conducted from December 2022 to April 2023 to assess frequency of Hookah smoking among 507 medical students at Al-Kindy Medical ...