
- General Chemistry
- Chemical and Environmental Health and Safety


Human and Ecological Risk Assessment: Theory and Practice - Set, 3rd Edition
ISBN: 978-1-119-55110-2
Digital Evaluation Copy

Dennis J. Paustenbach
Understand the fundamentals of human risk assessment with this introduction and reference
Human risk assessments are a precondition for virtually all industrial action or environmental regulation, all the more essential in a world where chemical and environmental hazards are becoming more abundant. These documents catalog potential environmental, toxicological, ecological, or other harms resulting from a particular hazard, from chemical spills to construction projects to dangerous workplaces. They turn on a number of variables, of which the most significant is the degree of human exposure to the hazardous agent or process.
Human and Ecological Risk Assessment combines the virtues of a textbook and reference work to introduce and analyze these vital documents. Beginning with the foundational theory of human health risk assessment, it then supplies case studies and detailed analysis illustrating the practice of producing risk assessment documents. Fully updated and authored by leading authorities in the field, the result is an indispensable work.
Readers of the second edition of Human and Ecological Risk Assessment will also find:
- Over 40 entirely new case studies reflecting the latest in risk assessment practice
- Detailed discussion of hazards including air emissions, contaminated food and soil, hazardous waste sites, and many more
- Case studies from multiple countries to reflect diverse international standards
Human and Ecological Risk Assessment is ideal for professionals and advanced graduate students in toxicology, industrial hygiene, occupational medicine, environmental science, and all related subjects.
Dennis J. Paustenbach, PhD, has more than 35 years experience in the field of risk assessment and has conducted as many as 1000 risk assessments personally. He was the founder of ChemRisk and has published extensively on risk assessment and related subjects.

NGFS Occasional Papers Case Studies of Environmental Risk Analysis Methodologies
Share links.
- Send by email
- Download PDF
- Alert by email
Download the PDF version of this document

- Published on 09/10/2020
- PDF (21.45 MB)
Updated on: 09/10/2020 16:01
- Download the PDF version
- Open access
- Published: 15 November 2021
Role of epidemiology in risk assessment: a case study of five ortho-phthalates
- Maricel V. Maffini ORCID: orcid.org/0000-0002-3853-9461 1 ,
- Birgit Geueke ORCID: orcid.org/0000-0002-0749-3982 2 ,
- Ksenia Groh 3 ,
- Bethanie Carney Almroth ORCID: orcid.org/0000-0002-5037-4612 4 &
- Jane Muncke ORCID: orcid.org/0000-0002-6942-0594 2
Environmental Health volume 20 , Article number: 114 ( 2021 ) Cite this article
8793 Accesses
17 Citations
83 Altmetric
Metrics details
The association between environmental chemical exposures and chronic diseases is of increasing concern. Chemical risk assessment relies heavily on pre-market toxicity testing to identify safe levels of exposure, often known as reference doses (RfD), expected to be protective of human health. Although some RfDs have been reassessed in light of new hazard information, it is not a common practice. Continuous surveillance of animal and human data, both in terms of exposures and associated health outcomes, could provide valuable information to risk assessors and regulators. Using ortho-phthalates as case study, we asked whether RfDs deduced from male reproductive toxicity studies and set by traditional regulatory toxicology approaches sufficiently protect the population for other health outcomes.
We searched for epidemiological studies on benzyl butyl phthalate (BBP), diisobutyl phthalate (DIBP), dibutyl phthalate (DBP), dicyclohexyl phthalate (DCHP), and bis(2-ethylhexyl) phthalate (DEHP). Data were extracted from studies where any of the five chemicals or their metabolites were measured and showed a statistically significant association with a health outcome; 38 studies met the criteria. We estimated intake for each phthalate from urinary metabolite concentration and compared estimated intake ranges associated with health endpoints to each phthalate’s RfD.
For DBP, DIBP, and BBP, the estimated intake ranges significantly associated with health endpoints were all below their individual RfDs. For DEHP, the intake range included associations at levels both below and above its RfD. For DCHP, no relevant studies could be identified. The significantly affected endpoints revealed by our analysis include metabolic, neurodevelopmental and behavioral disorders, obesity, and changes in hormone levels. Most of these conditions are not routinely evaluated in animal testing employed in regulatory toxicology.
We conclude that for DBP, DIBP, BBP, and DEHP current RfDs estimated based on male reproductive toxicity may not be sufficiently protective of other health effects. Thus, a new approach is needed where post-market exposures, epidemiological and clinical data are systematically reviewed to ensure adequate health protection.
Peer Review reports
Non-communicable diseases (NCD) are a global burden to public health [ 1 ]. Nutritional shortcomings and lifestyle factors have been associated with increased incidence of diabetes and obesity, but current evidence indicates that exposures to environmental chemical contaminants also play a role in the development of NCDs [ 2 ]. In the US, cardiovascular diseases and mental health conditions impose the highest economic burden followed by cancer, diabetes, and chronic respiratory diseases [ 3 ]. Of particular concern are exposures during gestation and early childhood [ 4 ] . A recent review [ 5 ] proposed incorporating environmental health risk factors when estimating global burden of disease, including air pollutants, neurotoxicants, endocrine disrupting chemicals, and climate-related factors. To do this successfully, the components of risk assessment such as exposure sources and levels, as well as data about chemical effects and associated health outcomes, are required [ 6 ].
One source of chemical exposure is plastic. With a global production of almost 360 million metric tons in 2018 [ 7 ], manufacturing, use, and disposal of plastic materials pose major safety concerns. Leachate from landfills, migration from consumer products (e.g., food packaging, toys, flooring, textiles), and air pollution from burning plastic materials are just some of the sources of chemical contamination affecting humans and the environment [ 8 , 9 , 10 ] . Because information on chemicals present in plastics is difficult to obtain and their hazards often remain unknown, Groh and colleagues [ 11 ] published a comprehensive database with more than 900 chemicals likely associated with plastic packaging as part of the Hazardous Chemicals in Plastic Packaging (HCPP) project. The authors also ranked the chemicals based on hazards to human and environmental health according to the United Nations’ Globally Harmonized System of Classification and Labelling of Chemicals [ 12 ] The 63 chemicals that ranked highest for human health concerns underwent a tiered prioritization [ 13 ] based on biomonitoring data, endocrine disrupting properties, and their regulatory status under the European Chemicals Regulation REACH. This prioritization approach identified five ortho-phthalates (referred to as phthalates in this article) for which the risk to human health was considered the highest: benzyl butyl phthalate (BBP, CAS 85-68-7); dibutyl phthalate (DBP, CAS 84-74-2); diisobutyl phthalate (DIBP, CAS 84-69-5); bis(2-ethylhexyl) phthalate (DEHP, CAS 117-81-7); dicyclohexyl phthalate (DCHP, CAS 84-61-7).
Phthalates are highly abundant plastic additives used primarily as plasticizers to soften materials and make them flexible [ 14 ]. Human biomonitoring shows widespread exposure to phthalates [ 15 ] from diverse sources including food which could be contaminated from its packaging as well as other food contact materials such as conveyor belts and tubing used in food processing [ 16 , 17 , 18 , 19 , 20 ]. Personal care products and building materials also contribute to human exposure to phthalates [ 21 , 22 ].
Several regulatory authorities have assessed the toxicity of BBP, DBP, DIBP, DEHP, and DCHP [ 23 , 24 , 25 ] and established the amount of each chemical above which the risk to human health increases. Regulatory agencies give different names to these so-called ‘safe’ levels including derived no-effect level (DNEL) used by the European Chemical Agency (ECHA) [ 26 ], acceptable daily intake (ADI) used by International Programme on Chemical Safety [ 27 ], total dietary intake (TDI) used by the European Food Safety Authority (EFSA) [ 28 ] and reference dose (RfD) used by the U.S. Environmental Protection Agency (EPA) [ 29 ]. Although the nomenclature is different, the meaning is similar, namely, exposures above established amounts of chemicals are not safe. For simplicity, we use the term RfD throughout the article.
The established RfDs for the five phthalates are the result of risk assessments of mostly animal studies showing adverse effects on male reproductive development due to the anti-androgenic properties of these chemicals. These risk assessments’ results have led to the restriction of some uses of these phthalates. In 2008, the Congress of the United States banned the use of DEHP, BBP and DBP in children’s toys and childcare articles [ 30 ] and in 2017, the Consumer Protection Safety Commission increased the list of prohibited phthalates to eight [ 23 ]. Similarly, the European Union has also listed DEHP and DBP in its authorization list under REACH and more than a dozen phthalates are included in the candidate list for authorization [ 31 ]. The observed decline in human exposure to restricted phthalates in industrialized countries over the years [ 15 , 32 , 33 ] have been attributed to these regulatory measures. Notably there are yet no major restrictions to uses in food contact materials (e.g., packaging, processing equipment), pharmaceuticals, and medical devices.
Epidemiological data published in the last 15 years indicate that in some cases exposure to phthalates is still a cause of concern to human health. For example, recent publications by the U.S. Environmental Protection Agency (EPA) show a strong association between exposure to low concentrations of DEHP, DBP, and DIBP and increased risk of diabetes [ 34 ], and between exposure to DEHP and DBP and male reproductive effects such as reduced semen quality and testosterone levels [ 35 ]. Several small- and large-scale human studies have also shown phthalates to associate in a dose-dependent manner with negative effects on neurodevelopment [ 36 , 37 ], metabolic function [ 38 ] and female reproduction [ 39 ]. Therefore, we aimed to investigate whether regulatory safe levels of phthalates are protective of the public for other relevant health outcomes in addition to male reproductive development. We conducted a targeted literature search of human studies showing association between any of the five phthalates, BBP, DBP, DIBP, DEHP, and DCHP and health effects. Furthermore, we back-estimated daily intake for each phthalate that showed a statistically significant association with health effects, and compared these estimated intake values to the individual RfD.
Targeted literature search
We searched the Public Library of Medicine for human studies on phthalates published between 2003 and 2019. Search terms included compounds’ full name, abbreviation, and chemical abstracts service (CAS) numbers in combination with human exposure, epidemiological studies and metabolites among others. See Supplemental Materials for additional information. This targeted search aimed at obtaining information on the five phthalates including concentration of parent phthalates or metabolites in any bodily fluid, description of measured endpoint, and statistical significance of the association between health endpoint and concentration measured. When a study met these criteria, we extracted the following data: 1) population sampled and population in which the endpoints were measured (e.g., men; pregnant women/children; children, etc.); age; gestational age where appropriate; 2) metabolite or parent compound concentration as percentile, geometric mean or other available concentration measure; 3) concentration at which metabolite(s) or parent compound had a statistically significant correlation with an endpoint; 4) statistically significant endpoint and outcome (e.g., increase/decrease; positive/negative association). We used the studies that met the criteria described above to perform the analysis and controlled for quality, specifically, whether the studies included controls for covariates and confounders such as race, maternal/paternal age, child’s sex, IQ, socioeconomic status, smoking, physical activity, caloric intake, etc.; however, we did not control for potential bias.
Intake estimation from urinary concentration
From the studies that met the inclusion criteria, we identified the lowest phthalate metabolite concentration that was associated with a statistically significant endpoint. Concentration data were expressed in various ways including geometric means of a population, percentiles, and average of urine collections per individual visits. We established the following assumptions: 1) the 25th percentile concentration was considered equivalent to a no-observed-adverse-effect level when concentrations were expressed as quartiles, meaning that only concentrations at or greater than the 25th percentile were included; 2) unless specified in the studies, logistic regressions were considered linear.
For each phthalate, we estimated intake using urinary concentration of its metabolite(s), daily urine volume, body weight (bw), and creatinine correction values for the different populations assessed [ 40 , 41 ]. In the case of DEHP, we considered the individual excretion of its four metabolites over time and expressed it as percent of the parent phthalate’s intake as described previously [ 42 , 43 ]. We used the following mean percentage excretion values for DEHP metabolites: 6% for monoethylhexyl phthalate (MEHP), 11% for mono-(2-ethyl-5-oxohexyl phthalate (5oxo MEHP), 15% for mono(2-ethyl-5-hydroxyhexyl) phthalate (5OH MEHP) and 14% for mono-(2-ethyl-5-carboxypentyl) phthalate (5cx MEHP). For DBP, DIBP and BBP, we followed the European Chemical Agency (ECHA) assumption of 100% elimination of the parent compound as phthalate monoesters [ 25 ]. We used the following formula:
Intake (μg/ bw (kg)/d) = Metabolite concentration (μg/L) x (Vol (L)/day) x (1/bw (kg)) x (1/% elimination)
In cases when creatinine correction was needed, concentration of urinary metabolite in microgram per gram (μg/g) creatinine was multiplied by the urinary concentration of creatinine in gram per liter (g/L). The Supplemental Materials include an example of the intake calculations and the assumptions made for each population (children, pregnant women, non-pregnant women, men) regarding body weight, daily urine volume, and creatinine excretion.
Regulation of priority phthalates
The uses of and exposure to phthalates are regulated in the European Union and the United States [ 23 , 24 , 25 , 44 , 45 ]. We chose the regulatory limits set by ECHA and the US Consumer Protection Safety Commission (CPSC) to compare against the estimated intakes associated with health endpoints because these safe levels have been reaffirmed or established in the last 5 years using current scientific evidence. In addition, both assessments target products that are commonly used by children, a susceptible population as highlighted by government regulatory agencies [ 23 , 25 , 46 ]. Regulatory RfDs are commonly expressed as the amount of chemical a person is safe to consume per kilogram of body weight per day, over their expected lifetime. Table 1 summarizes the RfD for BBP, DBP, DIBP and DEHP and the health endpoint selected by ECHA to establish each reference dose. Because ECHA did not establish an RfD for DCHP, we used a regulatory limit set by the US CPSC, i.e., less than 0.1% DCHP per weight of the final product for children’s toys and articles [ 23 ]. This assessment was also based on DCHP’s anti-androgenic effects (i.e., reduced anogenital distance) observed in male rodents [ 54 ].
We identified 38 out of 64 publications that met our selection criteria (Table 2 ). The studies included longitudinal and cross-sectional studies; small cohorts (e.g., patients at fertility clinics; under-represented urban populations) and nationally representative cohorts such as the National Health and Nutrition Examination Survey (NHANES) of the U.S. Center for Disease Control and Prevention; and prenatal exposure studies where phthalates were measured in the mothers but the health outcomes were assessed in their children months or years after birth. Supplemental Materials Table S1 lists the 26 publications that did not meet our criteria and therefore were not included in this case study.
All 38 studies reported phthalate metabolites measured in urine. DEHP was the phthalate most frequently assessed. There were 12 studies on mother-child pairs evaluating prenatal exposure effects, 12 women-only studies, six men-only studies, eight children studies evaluating postnatal exposure effects, and two studies including both men and women. A few studies included more than one population (e.g., children and adults) and only one study was a prospective mother-child study. It is worth noting that none of the studies included evaluation of DCHP, neither as a parent compound nor its metabolite. The lack of epidemiological studies on DCHP is likely due to the fact that the urinary concentration of DCHP metabolite has been found to be consistently below the limit of detection at the 75th percentile in the NHANES 1999–2010 period [ 83 ] and, when measured, the frequency of detection has been low (e.g., less than 10% of the population tested) [ 33 , 83 ].
Table 1 lists the range of exposure for each phthalate and their association with significant endpoints. All phthalates measured in urine as metabolites of DEHP, DBP, BBP and DIBP showed significant associations with reproductive (male and female), neurodevelopmental, behavioral, hormonal, and metabolic endpoints at estimated intake values well below their respective RfDs.
Figure 1 shows the estimated intake distribution per phthalate compared to the respective RfD. DEHP had the widest range of estimated intakes associated with statistically significant endpoints: 0.03–242.5 μg/kg-bw/d (Table 1 , Fig. 1 ). The highest estimate was almost seven times greater than the RfD (35 μg/kg-bw/d) which is an indication that some individuals could already be exposed to unsafe levels of the chemical as judged by the current regulatory limits. As shown in Table 1 , the highest DEHP intake was associated with decreased semen quality [ 47 ]. On the lower end, DEHP was associated with significantly lower number of ovarian antral follicles (a measure of remaining oocytes supply) [ 39 ] at an estimated intake three-orders of magnitude lower than the RfD (0.03 and 35 μg/kg-bw/d, respectively).
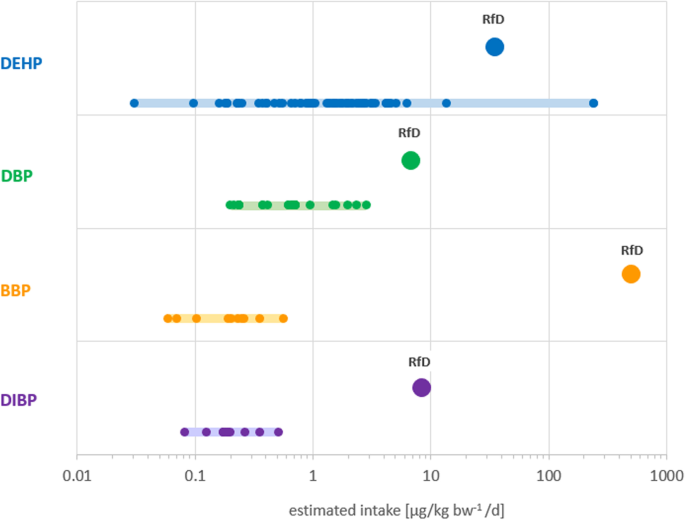
Schematic representation of the range of estimated intake for individual phthalates (solid light-colored bars) associated with statistically significant endpoints (small circles) in relation to their respective reference doses (RfD; large circles). Each small circle corresponds to an endpoint significantly associated with an estimated intake. The lowest metabolite concentrations measured in urine that were found to be associated with statistically significant endpoints were 0.03, 0.19, 0.06 and 0.08 μg/L for DEHP, DBP, BBP and DIBP, respectively. See Supplemental Table S2 for additional data. DEHP: diethylhexyl phthalate; DBP: dibutyl phthalate; BBP: butylbenzyl phthalate; DIBP: diisobutyl phthalate
For DBP, DIBP and BBP, the ranges of intake associated with statistically significant endpoints were all below their respective RfDs. (Fig. 1 ). The lowest estimated intake for DBP (0.19 μg/kg-bw/d) was associated with decreased sperm motility and semen concentration [ 48 ] while the highest intake (2.86 μg/kg-bw/d) was associated with decreased concentration of total thyroid hormone thyroxine (T4) and free T4 (fT4) in women [ 49 ]. The lowest DIBP intake measured in pregnant women (0.08 μg/kg-bw/d) was associated with decrease in masculine play behavior in boys [ 52 ] and the highest intake (0.51 μg/kg-bw/d) also measured in pregnant women, was significantly associated with increased occurrence of eczema in children [ 53 ]. The range of estimated intake for BBP associated with significant endpoints showed the greatest difference with the RfD. The lowest intake of 0.06 μg/kg-bw/d was associated with increased levels of steroid hormone binding globulin (SHBG) in children [ 50 ]. SHBG is a protein that transports estrogen and testosterone in the blood and regulates their access to tissues [ 84 ]. The highest estimated intake for BBP (0.6 μg/kg-bw/d) was associated with increased body mass index and waist circumference in men and women [ 51 ]. These intakes are eight-thousand to five-thousand times lower than BBP’s RfD of 500 μg/kg-bw/d.
The four phthalates for which we found data are known to affect male reproductive development due to their anti-androgenic properties which are the basis of their regulation. However, other systems are also affected at exposure levels similar to those associated with anti-androgenicity as seen in Table 2 . Our analysis shows the 10 lowest estimated intakes were significantly associated with endpoints measured in women and children. Many of these endpoints relate to endocrine function and neurobehavioral development in children as well as female reproductive system (Table 3 ).
Prenatal exposures to DEHP, DBP, BBP and DIBP were significantly associated with a diverse set of negative outcomes in the neurological system, and all endpoints were associated with intakes well below the RfD for each phthalate. Supplemental Table S2 shows that children born to mothers exposed to phthalates during pregnancy display delayed psychomotor and mental development [ 65 , 66 ]; decreased intellectual, memory and executive function development [ 36 ]; and behavioral changes associated with both delinquency and externalization [ 64 ] as well as withdrawn personalities and internalization of problems [ 65 , 68 ]. Increased odds of attention deficit hyperactivity disorder [ 69 ] and decreased masculine behavior in boys [ 52 ] were also observed.
We identified three major systems associated with metabolic function that were affected by phthalates: thyroid, pancreas, and fat tissue (Supplemental Table S3 ). DEHP, DBP and BBP were associated with decreased levels of triiodothyronine (T3) in men and children as young as 4 years of age. DEHP was also associated with decreased levels of free T4 in women [ 56 ] and men [ 82 ] and decreased thyroid stimulating hormone (TSH) in men [ 47 ].
DIBP and DEHP intakes were positively associated with insulin resistance in children [ 72 , 78 ] and men and women [ 60 , 77 ]. The effect of DEHP on fat tissue was more diverse. For instance, in adults, body mass index (BMI) was negatively associated with DEHP levels in men and women [ 59 ], while Hatch et al. [ 51 ] reported a positive correlation in women). Maternal DEHP levels were inversely associated with their daughters’ BMI at a young age (4–7 years) [ 67 ] and Zang and colleagues also observed a negative association between DEHP levels and obesity in 8–10-year-old girls [ 74 ]. DBP and BBP showed a positive correlation with obesity in boys [ 74 ], BMI and waist circumference in women and men [ 51 ].
All the estimated intakes were below their respective RfDs, except for the reduction in TSH level in men that was associated with the highest DEHP intake of 242.55 μg/kg-bw/d [ 47 ].
Both, the male and female reproductive systems and their associated hormones, were negatively affected by the four phthalates (Supplemental Table S4 ). DEHP, DBP and DIBP intakes were associated with reduced number of antral follicles in women [ 39 ] and DEHP, DBP and BBP with delayed puberty in girls [ 73 ]. DEHP and DBP were associated with decreased number of fertilized eggs and total oocytes, and lower quality of oocytes [ 57 ]. DEHP and DIBP showed a negative association with trophoblast differentiation genes [ 58 ]. DEHP was also associated with decreased levels of inhibin [ 61 ], a critical hormone in reproductive functions [ 85 ], and showed inconsistent association with gestational length [ 62 , 63 ].
In adult men, DEHP, DBP and BBP all had a negative association with semen quality including concentration and sperm motility [ 47 , 48 , 79 ]. DEHP was associated with decreased total and free testosterone and estradiol, as well as increased levels of SHBG [ 80 ]. DEHP also had a positive association with testosterone/estradiol ratio [ 81 ]. In boys gestationally exposed to known levels of phthalates, DEHP and DIBP were negatively associated with free and total testosterone and estradiol [ 50 ]. DEHP, DBP and BBP were associated with increased SHBG. DBP was associated with decreased levels of dehydroepiandrosterone [ 50 ]. Finally, DEHP was also associated with reduced anogenital distance in boys [ 70 ].
This case study shows that low dose exposures to BBP, DBP, DIDP and DEHP are associated with health endpoints in organs and systems not usually assessed in regulatory toxicology studies. These endpoints differ markedly from the well-studied effects of phthalates on male reproductive development. Furthermore, there are significant physiological effects (i.e., early biological perturbations that may lead to overt effects) and disorders that may require clinical interventions later in life associated with estimated intake levels lower than the current RfD. We also observed that some individuals appear to be exposed to levels of DEHP higher than its RfD. This may be the case if there are yet to be identified exposure routes and sources, or if the metabolism or excretion of DEHP is altered. Overall, these data, although with limitations, show weaknesses in a chemical regulation framework that is in need of improvement.
Some of the limitations are, first, this study is similar to a mapping of evidence; it is not a systematic review that must follow stricter protocols and methods. Second, our approach aimed to capture as many publications as possible. However, although we used broad search terms, we may still have missed relevant publications. Third, we trust the integrity and quality of the journal peer-reviewed conducted for each of the studies we included. However, we understand the peer review process is not perfect. An example of this less-than-perfect process is the lack of clarity or data that prevented us to include an additional 26 human studies as shown in Table S1. Importantly, only six studies were excluded because of the lack of statistical significance, hence, the body of evidence is consistent with the associations. Fourth, in some cases, data interpretation had to be based on information that was available. Although we contacted authors from some of the studies that did not meet our criteria to obtain additional data, only a few responded to our request and were willing to share additional data. Fifth, the number of subjects in the studies varied from less than 100 to thousands of people; although the population size as such could be a limitation, strong and weak statistical significance was observed in all cases. As all but one study was cross-sectional, we are mindful about implying that they show causality. Lastly, some assumptions made in our calculations may have been outdated. For example, the EPA handbook on exposure is from 2011. Although it is our understanding that the agency and others continue to use this handbook in their analysis, we cannot rule out that parameters such as body weight by age range may have changed in the last decade and could have affected our estimates.
Overall, the case study we present here specifically aimed to use strong human data to perform a first examination of a hypothesis, namely that the current animal-based testing methods to estimate “safe” exposure levels of chemicals could be significantly underestimating actual human health risk if epidemiological data are not considered. Following the initial confirmatory findings presented here, this hypothesis will serve as a basis to guide further testing and more detailed assessments in a follow-up work.
The protection of public health from detrimental effects of environmental chemical exposures should ideally incorporate the expertise from two sides: the risk assessors and the healthcare community, including epidemiologists. On the one hand, risk assessment relies on evaluating exposure to a chemical and using animal models to identify which organ(s) would be affected, in order to find a dose that would cause no harm. On the other hand, the medical community is confronted with a wide range of health outcomes in the human population—from acute to chronic and from subtle to clinically defined—and tries to identify what caused them, whether environmental chemical exposure or otherwise, in order to support prevention. But there is a disconnect between these bookends of environmental health which hinders effective protection of the public from chemical exposures. In 2017, the US National Academy of Sciences [ 86 ] recommended that for evaluating evidence of low dose effects, regulators should surveil for signals indicating an adverse outcome in a human population or evidence that a particular low dose effect may not be detectable with traditional toxicity testing. The authors stated that one way to seek out information is by conducting regular surveys of the scientific literature. Our limited case study of five phthalates shows that many of the health effects observed to occur in humans at very low exposure levels are not traditionally evaluated in animal toxicology testing. Metabolic, neurodevelopmental and behavioral disorders, obesity, levels of hormones and transport proteins are just a few examples of endpoints not commonly included in toxicity testing guidelines despite their relevance to human health. It is also important to point out that traditional toxicology studies only infrequently evaluate a dose-effect relationship using chemical levels relevant to human exposures occurring at different life-stages. Rather, assumptions of safe levels are commonly made based on adult non-pregnant animal data. These omissions thus result in significant gaps in chemicals regulation that may put human health at risk [ 87 ].
The current chemical risk assessment approach to establish an RfD used by most regulatory agencies around the world combines a dose that did not cause an adverse effect in animal studies using high exposure doses and safety factors (also known as uncertainty factors) to account for incomplete data and variability between and within species. Although not routinely, regulatory ‘safe’ levels have been reviewed. For example, ECHA lowered the derived no effect level for DIBP from 420 to 8.3 μg/kg bw/d in 2016 [ 25 ]; similarly, the European Food Safety Authority (EFSA) lowered the tolerable daily intake of bisphenol A from 50 to 4 μg/kg bw/d in 2015 [ 88 ]. In both cases, new scientific information was available at the time the agencies were responding to requests for reassessment of those chemicals. However, we would argue that, in addition to specific requests made to regulatory agencies, a more systematic reevaluation of RfDs could be incorporated into the risk assessment and management processes. For example, a post-market RfD reassessment could be triggered by 1) human studies showing associations between exposure and endpoints previously not measured; 2) information on reported uses or biomonitoring indicating increased exposures due to chemical production volumes or reduced exposure due to abandoned uses; or 3) new hazard information. Lastly, this information surveillance should not be the exclusive responsibility of the regulatory agencies; rather, companies with approved chemical uses should submit new available information that could potentially raise questions about the safety of their product and agencies should establish a mechanism to enforce this requirement.
Both, scientific information and market behavior, are dynamic. Advances in science and technology allow scientists to develop new methods to measure chemicals in humans and gain new knowledge and understanding of chemicals’ interactions with physiological systems at different life stages. To account for these developments, epidemiological and clinical studies together with chemical biomonitoring data should be evaluated at regular intervals as recommended by the NAS [ 86 ] in order to check whether an RfD review is warranted to better protect public health. We are cognizant that this approach, although promising, is not without shortcomings. For instance, biomonitoring data alone cannot account for all sources of exposure. For chemicals like phthalates, with many sources ranging from the diet to personal care products and house dust, it may be challenging to design mitigating strategies to reduce the most significant sources of exposure. However, well designed surveys and a better understanding of materials’ composition may help identifying the major exposure sources for various populations as it was described by Lioy and colleagues [ 89 ].
As implied earlier, the RfD represents a concept of ‘safety’, a bright line between ‘no risk’ or ‘safe’ when the exposure estimate is below the established number and ‘risk’ or ‘unsafe’ when the value is greater than the RfD. In reality, it is far more complicated, namely, chemical hazard information and populations’ background exposures from multiple chemicals, health conditions and life-stages change with time. In its 2009 Science and Decisions report [ 90 ], the NAS recognized this complexity and recommended a progression away from the current concept of ‘safety’ and towards dose-response methods that quantify risk at doses used in animal experiments as well as lower doses representing human exposures. As much as two-thirds of the human population suffers from chronic diseases that cannot be explained by genetic causes alone [ 91 ] and it is becoming increasingly apparent that life-long chemical exposures can contribute to this burden [ 5 ]. Yet, for the great majority, chemicals are not evaluated for their contribution to common chronic ailments in the human population [ 92 , 93 ]. As a consequence, the current work on toxicology and epidemiology is inundated with disconnected data that misses the bigger picture: better protection of the entire human population’s health. Perhaps it is time to reconsider the status quo to ensure adequate population health protection. Issues to be interrogated may include, among others, strategies for proper assessment of the risk of developmental exposures; use of early biomarkers of health effects; integration of evidence from different data streams including predictive modeling, in vitro, animals and humans; development of new and redesign of old testing protocols; optimization of in vitro testing to minimize the use of laboratory animals; design of protocols to more efficiently monitor human exposures.
Conclusions
Phthalates have been used in many products for many decades. There are substantial animal and human data available which allowed us to use these substances in case studies such as this one. However, a similar question could be raised for many other chemicals with a growing body of human biomonitoring data and evidence of human health effects [ 94 ].
To set the course for a better, more efficient and health protective risk assessment of chemicals, a dialogue should be established between risk assessors, the medical community, and academic researchers. Until a profound modernization of the risk assessment and management of chemicals occurs, human studies should be taken into account to identify whether the health risk of chemicals already in the marketplace, such as phthalates, should be reassessed.
Abbreviations
First trimester
Second trimester
Third trimester
Acceptable daily intake
Benzyl butyl phthalate
Body mass index
Body weight
Chemical abstract service
Consumer products safety commission
Dibutyl phthalate
Dicyclohexyl phthalate
Bis(2-ethylhexyl) phthalate
Diisobutyl phthalate
Derived no-effect level
European chemical agency
European food safety authority
Environmental protection agency
Homeostatic model assessment of insulin resistance
Intelligence quotient
Non-communicable disease
National health and nutrition examination survey
Registration, evaluation, authorization and restriction of chemicals
- Reference dose
Steroid hormone binding globulin
Triiodothyronine
Tolerable daily intake
Thyroid stimulating hormone
United States
World Health Organization: Global status report on noncommunicable diseases. 2014.
Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu N, et al. The lancet commission on pollution and health. Lancet. 2017;391:462–512.
Chen S, Kuhn M, Prettner K, Bloom DE. The macroeconomic burden of noncommunicable diseases in the United States: estimates and projections. PLoS One. 2018;13(11):e0206702.
Article Google Scholar
Balbus JM, Barouki R, Birnbaum LS, Etzel RA, Gluckman PD, Grandjean P, et al. Early-life prevention of non-communicable diseases. Lancet. 2013;381(9860):3–4.
Shaffer RM, Sellers SP, Baker MG, de Buen KR, Frostad J, Suter MK, et al. Improving and expanding estimates of the global burden of disease due to environmental health risk factors. Environ Health Perspect. 2019;127(10):105001.
Muncke J, Backhaus T, Geueke B, Maffini MV, Martin OV, Myers JP, et al. Scientific challenges in the risk assessment of Food contact materials. Environ Health Perspect. 2017;125(9):095001.
PlasticsEurope: Plastics - the Facts 2019. In.; 2019.
Kawagoshi Y, Tsukagoshi Y, Fukunaga I. Determination of estrogenic activity in landfill leachate by simplified yeast two-hybrid assay. JEM. 2002;4(6):1040–6.
CAS Google Scholar
Biedermann-Brem S, Biedermann M, Pfenninger S, Bauer M, Altkofer W, Rieger K, et al. Plasticizers in PVC toys and childcare products: what succeeds the phthalates? Market survey 2007. Chromatographia. 2008;68(3):227–34.
Article CAS Google Scholar
Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, et al. Plastic waste inputs from land into the ocean. Science. 2015;347(6223):768–71.
Groh KJ, Backhaus T, Carney-Almroth B, Geueke B, Inostroza PA, Lennquist A, et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci Total Environ. 2019;651(Pt 2):3253–68.
United Nations Economic Commission for Europe: Globally harmonized system of classification and labelling of chemicals (GHS). 2015. https://unece.org/about-ghs .
Geueke B, Inostroza PA, Maffini M, Backhaus T, Carney-Almroth B, Groh KJ, et al. Prioritization approaches for hazardous chemicals associated with plastic packaging. Food Packaging Forum Zurich. 2018:1–14.
Nerin C, Canellas E, Vera P. Plasticizer migration into foods. In: Reference Module in Food Science. Amsterdam: Elsevier; 2018.
Frederiksen H, Nielsen O, Koch HM, Skakkebaek NE, Juul A, Jørgensen N, et al. Changes in urinary excretion of phthalates, phthalate substitutes, bisphenols and other polychlorinated and phenolic substances in young Danish men; 2009–2017. Int J Hyg Environ Health. 2020;223(1):93–105.
Husøy T, Martínez MA, Sharma RP, Kumar V, Andreassen M, Sakhi AK, et al. Comparison of aggregated exposure to di(2-ethylhexyl) phthalate from diet and personal care products with urinary concentrations of metabolites using a PBPK model - results from the Norwegian biomonitoring study in EuroMix. Food Chem Toxicol. 2020;143:111510.
Geueke B, Muncke J. Substances of very high concern in food contact materials: migration and regulatory background. Packag Technol Sci. 2018;31(12):757–69.
Van Holderbeke M, Geerts L, Vanermen G, Servaes K, Sioen I, De Henauw S, et al. Determination of contamination pathways of phthalates in food products sold on the Belgian market. Environ Res. 2014;134:345–52.
Schecter A, Lorber M, Guo Y, Wu Q, Yun SH, Kannan K, et al. Phthalate concentrations and dietary exposure from food purchased in New York state. Environ Health Perspect. 2013;121(4):473–94.
Guart A, Bono-Blay F, Borrell A, Lacorte S. Effect of bottling and storage on the migration of plastic constituents in Spanish bottled waters. Food Chem. 2014;156:73–80.
Koch HM, Lorber M, Christensen KL, Pälmke C, Koslitz S, Brüning T. Identifying sources of phthalate exposure with human biomonitoring: results of a 48 h fasting study with urine collection and personal activity patterns. Int J Hyg Environ. 2013;216(6):672–81.
Hammel SC, Levasseur JL, Hoffman K, Phillips AL, Lorenzo AM, Calafat AM, et al. Children's exposure to phthalates and non-phthalate plasticizers in the home: the TESIE study. Environ Int. 2019;132:105061.
Consumer Protection Safety Commission. Prohibition of Children's Toys and Child Care Articles Containing Specified Phthalates. In: 16 CFR 1307. United States: Federal Register; 2017. p. 49938–82.
EFSA Panel on Food contact materials E, aids P, Silano V, Barat Baviera JM, Bolognesi C, Chesson A, Cocconcelli PS, et al. Update of the risk assessment of di-butylphthalate (DBP), butyl-benzyl-phthalate (BBP), bis (2-ethylhexyl) phthalate (DEHP), di-isononylphthalate (DINP) and di-isodecylphthalate (DIDP) for use in food contact materials. EFSA J. 2019;17(12):e05838.
Google Scholar
European Chemical Agency Committee for Risk Assessment: Opinion on an Annex XV dossier proposing restrictions on FOUR PHTHALATES (DEHP, BBP, DBP, DIBP). ECHA/RAC/RES-O-0000001412-86-140/F. 2017.
European Commision: Registration, evaluation, authorisation and restriction of chemicals (REACH). EC 1907/2006; 2006.
Joint FAO/WHO Expert Committee on Food Additives: Principles and methods for the risk assessment of chemicals in food. Environmental Health Criteria 2009.
European Food Safety Authority. Glossary [ https://www.efsa.europa.eu/en/glossary-taxonomy-terms ].
US Environmental Protection Agency Integrated Risk Information System (IRIS). Glossary [ https://sor.epa.gov/sor_internet/registry/termreg/searchandretrieve/glossariesandkeywordlists/search.do?details=&glossaryName=IRIS%20Glossary ].
Consumer Product Safety Improvement Act of 2008. 122 STAT 3016 2008.
ECHA Authorization List [ https://echa.europa.eu/authorisation-list ].
Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: findings from the National Health and nutrition examination survey, 2001-2010. Environ Health Perspect. 2014;122(3):235–41.
Schwedler G, Rucic E, Lange R, Conrad A, Koch HM, Pälmke C, et al. Phthalate metabolites in urine of children and adolescents in Germany. Human biomonitoring results of the German Environmental Survey GerES V, 2014–2017. Int J Hyg Environ Health. 2020;225:113444.
Radke EG, Galizia A, Thayer KA, Cooper GS. Phthalate exposure and metabolic effects: a systematic review of the human epidemiological evidence. Environ Int. 2019;132:104768.
Radke EG, Braun JM, Meeker JD, Cooper GS. Phthalate exposure and male reproductive outcomes: a systematic review of the human epidemiological evidence. Environ Int. 2018;121(Pt 1):764–93.
Factor-Litvak P, Insel B, Calafat AM, Liu X, Perera F, Rauh VA, et al. Persistent associations between maternal prenatal exposure to phthalates on child IQ at age 7 years. PLoS One. 2014;9(12):e114003.
Radke EG, Braun JM, Nachman RM, Cooper GS. Phthalate exposure and neurodevelopment: a systematic review and meta-analysis of human epidemiological evidence. Environ Int. 2020;137:105408.
James-Todd TM, Chiu YH, Messerlian C, Mínguez-Alarcón L, Ford JB, Keller M, et al. Trimester-specific phthalate concentrations and glucose levels among women from a fertility clinic. Environ Health. 2018;17(1):55.
Messerlian C, Souter I, Gaskins AJ, Williams PL, Ford JB, Chiu YH, et al. Urinary phthalate metabolites and ovarian reserve among women seeking infertility care. Hum Reprod. 2016;31(1):75–83.
US Environmental Protection Agency: Exposure factors handbook 2011 edition (final report). Washington, DC 2011.
Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113(2):192–200.
Koch HM, Bolt HM, Preuss R, Angerer J. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch Toxicol. 2005;79(7):367–76.
Anderson WA, Castle L, Hird S, Jeffery J, Scotter MJ. A twenty-volunteer study using deuterium labelling to determine the kinetics and fractional excretion of primary and secondary urinary metabolites of di-2-ethylhexylphthalate and di-iso-nonylphthalate. Food Chem Toxicol. 2011;49(9):2022–9.
US Food and Drug Administration. Cosmetic Ingredients: Phthalates [ https://www.fda.gov/cosmetics/cosmetic-ingredients/phthalates ].
US Environmental Protection Agency. Assessing and Managing Chemicals under TSCA: Risk Management for Phthalates [ https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/risk-management-phthalates ].
Neal-Kluever A, Aungst J, Gu Y, Hatwell K, Muldoon-Jacobs K, Liem A, et al. Infant toxicology: state of the science and considerations in evaluation of safety. Food Chem Toxicol. 2014;70:68–83.
Wang YX, Zhou B, Chen YJ, Liu C, Huang LL, Liao JQ, et al. Thyroid function, phthalate exposure and semen quality: exploring associations and mediation effects in reproductive-aged men. Environ Int. 2018;116:278–85.
Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology. 2006;17(6):682–91.
Huang PC, Kuo PL, Guo YL, Liao PC, Lee CC. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum Reprod. 2007;22(10):2715–22.
Ferguson KK, Peterson KE, Lee JM, Mercado-García A, Blank-Goldenberg C, Téllez-Rojo MM, et al. Prenatal and peripubertal phthalates and bisphenol a in relation to sex hormones and puberty in boys. Reprod Toxicol. 2014;47:70–6.
Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, et al. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999-2002. Environ Health. 2008;7:27.
Swan SH, Liu F, Hines M, Kruse RL, Wang C, Redmon JB, et al. Prenatal phthalate exposure and reduced masculine play in boys. Int J Androl. 2010;33(2):259–69.
Soomro MH, Baiz N, Philippat C, Vernet C, Siroux V, Nichole Maesano C, et al. Prenatal exposure to phthalates and the development of eczema phenotypes in male children: results from the EDEN mother-child cohort study. Environ Health Perspect. 2018;126(2):027002.
US Consumer Product Safety Commission: Chronic Hazard Advisory Panel on Phthalates and Phthalates Alternatives. cpsc.gov/chap ; 2014.
Huang PC, Tsai CH, Liang WY, Li SS, Huang HB, Kuo PL. Early phthalates exposure in pregnant women is associated with alteration of thyroid hormones. PLoS One. 2016;11(7):e0159398.
Johns LE, Ferguson KK, Soldin OP, Cantonwine DE, Rivera-González LO, Del Toro LV, et al. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis. Reprod Biol Endocrinol. 2015;13:4.
Machtinger R, Gaskins AJ, Racowsky C, Mansur A, Adir M, Baccarelli AA, et al. Urinary concentrations of biomarkers of phthalates and phthalate alternatives and IVF outcomes. Environ Int. 2018;111:23–31.
Adibi JJ, Whyatt RM, Hauser R, Bhat HK, Davis BJ, Calafat AM, et al. Transcriptional biomarkers of steroidogenesis and trophoblast differentiation in the placenta in relation to prenatal phthalate exposure. Environ Health Perspect. 2010;118(2):291–6.
Yaghjyan L, Sites S, Ruan Y, Chang SH. Associations of urinary phthalates with body mass index, waist circumference and serum lipids among females: National Health and nutrition examination survey 1999-2004. Int J Obes. 2015;39(6):994–1000.
Kim JH, Park HY, Bae S, Lim YH, Hong YC. Diethylhexyl phthalates is associated with insulin resistance via oxidative stress in the elderly: a panel study. PLoS One. 2013;8(8):e71392.
Du YY, Guo N, Wang YX, Hua X, Deng TR, Teng XM, et al. Urinary phthalate metabolites in relation to serum anti-Müllerian hormone and inhibin B levels among women from a fertility center: a retrospective analysis. Reprod Health. 2018;15(1):33.
Boss J, Zhai J, Aung MT, Ferguson KK, Johns LE, McElrath TF, et al. Associations between mixtures of urinary phthalate metabolites with gestational age at delivery: a time to event analysis using summative phthalate risk scores. Environ Health. 2018;17(1):56.
Adibi JJ, Hauser R, Williams PL, Whyatt RM, Calafat AM, Nelson H, et al. Maternal urinary metabolites of Di-(2-Ethylhexyl) phthalate in relation to the timing of labor in a US multicenter pregnancy cohort study. Am J Epidemiol. 2009;169(8):1015–24.
Huang HB, Kuo PH, Su PH, Sun CW, Chen WJ, Wang SL. Prenatal and childhood exposure to phthalate diesters and neurobehavioral development in a 15-year follow-up birth cohort study. Environ Res. 2019;172:569–77.
Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, et al. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ Health Perspect. 2012;120(2):290–5.
Kim Y, Ha EH, Kim EJ, Park H, Ha M, Kim JH, et al. Prenatal exposure to phthalates and infant development at 6 months: prospective mothers and Children's environmental health (MOCEH) study. Environ Health Perspect. 2011;119(10):1495–500.
Buckley JP, Engel SM, Braun JM, Whyatt RM, Daniels JL, Mendez MA, et al. Prenatal phthalate exposures and body mass index among 4- to 7-year-old children: a pooled analysis. Epidemiology. 2016;27(3):449–58.
Philippat C, Nakiwala D, Calafat AM, Botton J, De Agostini M, Heude B, et al. Prenatal exposure to nonpersistent endocrine disruptors and behavior in boys at 3 and 5 years. Environ Health Perspect. 2017;125(9):097014.
Engel SM, Villanger GD, Nethery RC, Thomsen C, Sakhi AK, Drover SSM, et al. Prenatal phthalates, maternal thyroid function, and risk of attention-deficit hyperactivity disorder in the Norwegian mother and child cohort. Environ Health Perspect. 2018;126(5):057004.
Swan SH, Sathyanarayana S, Barrett ES, Janssen S, Liu F, Nguyen RH, et al. First trimester phthalate exposure and anogenital distance in newborns. Hum Reprod. 2015;30(4):963–72.
Boas M, Frederiksen H, Feldt-Rasmussen U, Skakkebæk NE, Hegedüs L, Hilsted L, et al. Childhood exposure to phthalates: associations with thyroid function, insulin-like growth factor I, and growth. Environ Health Perspect. 2010;118(10):1458–64.
Smerieri A, Testa C, Lazzeroni P, Nuti F, Grossi E, Cesari S, et al. Di-(2-ethylhexyl) phthalate metabolites in urine show age-related changes and associations with adiposity and parameters of insulin sensitivity in childhood. PLoS One. 2015;10(2):e0117831.
Kasper-Sonnenberg M, Wittsiepe J, Wald K, Koch HM, Wilhelm M. Pre-pubertal exposure with phthalates and bisphenol a and pubertal development. PLoS One. 2017;12(11):e0187922.
Zhang Y, Meng X, Chen L, Li D, Zhao L, Zhao Y, et al. Age and sex-specific relationships between phthalate exposures and obesity in Chinese children at puberty. PLoS One. 2014;9(8):e104852.
Trasande L, Sathyanarayana S, Trachtman H. Dietary phthalates and low-grade albuminuria in US children and adolescents. Clin J Am Soc Nephrol. 2014;9(1):100–9.
Trasande L, Sathyanarayana S, Spanier AJ, Trachtman H, Attina TM, Urbina EM. Urinary phthalates are associated with higher blood pressure in childhood. J Pediatr. 2013;163(3):747–753.e741.
Meeker JD, Ferguson KK. Relationship between urinary phthalate and bisphenol a concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and nutrition examination survey (NHANES) 2007-2008. Environ Health Perspect. 2011;119(10):1396–402.
Trasande L, Spanier AJ, Sathyanarayana S, Attina TM, Blustein J. Urinary phthalates and increased insulin resistance in adolescents. Pediatrics. 2013;132(3):e646–55.
Duty SM, Silva MJ, Barr DB, Brock JW, Ryan L, Chen Z, et al. Phthalate exposure and human semen parameters. Epidemiology. 2003;14(3):269–77.
Mendiola J, Meeker JD, Jørgensen N, Andersson AM, Liu F, Calafat AM, et al. Urinary concentrations of di(2-ethylhexyl) phthalate metabolites and serum reproductive hormones: pooled analysis of fertile and infertile men. J Androl. 2012;33(3):488–98.
Meeker JD, Calafat AM, Hauser R. Urinary metabolites of di(2-ethylhexyl) phthalate are associated with decreased steroid hormone levels in adult men. J Androl. 2009;30(3):287–97.
Meeker JD, Calafat AM, Hauser R. Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ Health Perspect. 2007;115(7):1029–34.
Center for Disease Control and Prevention: Fourth National Report on Human Exposure to Environmental Chemicals. 2019.
Hammond GL. Diverse roles for sex hormone-binding globulin in reproduction. Biol Reprod. 2011;85(3):431–41.
Luisi S, Florio P, Reis FM, Petraglia F. Inhibins in female and male reproductive physiology: role in gametogenesis, conception, implantation and early pregnancy. Hum Reprod Update. 2005;11(2):123–35.
National Academies of Sciences E, Medicine: Application of systematic review methods in an overall strategy for evaluating low-dose toxicity from endocrine active chemicals. 2017.
Maffini M, Vandenberg L. Closing the gap: improving additives safety evaluation to reflect human health concerns. Environ Risk Assess Remediat. 2017;1(3):26–33.
EFSA Panel on Food Contact Materials E, Flavourings, Aids P. Scientific opinion on the risks to public health related to the presence of bisphenol a (BPA) in foodstuffs. EFSA J. 2015;13(1):3978.
Lioy PJ, Hauser R, Gennings C, Koch HM, Mirkes PE, Schwetz BA, et al. Assessment of phthalates/phthalate alternatives in children's toys and childcare articles: review of the report including conclusions and recommendation of the chronic Hazard advisory panel of the consumer product safety commission. J Expo Sci Environ Epidemiol. 2015;25(4):343–53.
National Academy of Sciences: Science and Decisions - Advancing Risk Assessment. In. National Academies of Science, Engineering, and Medicine; 2009.
Rappaport SM, Smith MT. Epidemiology. Environment and disease risks. Science. 2010;330(6003):460–1.
US Food and Drug Administration: Guidance for Industry and Other Stakeholders: Redbook 2000. Toxicological Principles for the Safety Assessment of Food Ingredients 2007.
European Chemical Agency: Framework, Read-Across Assessment. 2017.
Madia F, Worth A, Whelan M, Corvi R. Carcinogenicity assessment: addressing the challenges of cancer and chemicals in the environment. Environ Int. 2019;128:417–29.
Download references
Acknowledgments
The authors are grateful to Dr. Leonardo Trasande for his expert advice and guidance and to those investigators that shared detailed data not included in the public versions of their publications.
This work was funded in part by a grant from MAVA Foundation and by the Food Packaging Forum (FPF). BG and JM are employees of FPF. FPF receives unconditional donations for unrestricted funding, as well as project-related grants, and all funding sources are listed.
https://www.foodpackagingforum.org/about-us/funding . Neither the board of FPF, nor MAVA Foundation interfered with the authors’ freedom to design, conduct, interpret and publish this information.
Author information
Authors and affiliations.
Independent Consultant, Frederick, MD, USA
Maricel V. Maffini
Food Packaging Forum Foundation, Zurich, Switzerland
Birgit Geueke & Jane Muncke
Eawag, Swiss Federal Institute of Aquatic Science and Technology, Dübendorf, Switzerland
Ksenia Groh
Department of Biological and Environmental Sciences, University of Gothenburg, Gothenburg, Sweden
Bethanie Carney Almroth
You can also search for this author in PubMed Google Scholar
Contributions
MVM: Conceptualization, Methodology, Investigation and Original draft. BG: Validation, Visualization, Review and Editing. KG: Validation, Review and Editing. BCA: Review and Editing. JM: Conceptualization, Review and Editing, Project administration, Funding acquisition. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Maricel V. Maffini .
Ethics declarations
Competing interests.
MVM and KG are members of FPF science advisory board. MVM is a co-author on a petition to the US Food and Drug Administration to revoke the authorizations to use phthalates in food packaging and processing equipment.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Maffini, M.V., Geueke, B., Groh, K. et al. Role of epidemiology in risk assessment: a case study of five ortho-phthalates. Environ Health 20 , 114 (2021). https://doi.org/10.1186/s12940-021-00799-8
Download citation
Received : 09 November 2020
Accepted : 18 October 2021
Published : 15 November 2021
DOI : https://doi.org/10.1186/s12940-021-00799-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Risk assessment
- Epidemiology
- Human health
Environmental Health
ISSN: 1476-069X
- General enquiries: [email protected]
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
An environmental risk assessment of filling stations using the principles of security management. a case study in the slovak republic.

1. Introduction
2. materials and methods, 2.1. approaches to an environmental risk assessment, 2.1.1. the netherlands, 2.1.2. sweden.
- Preventive planning;
- Examination of environmental risks;
- Identification and classification of risk areas.
- Tox—acute toxicity to aquatic organisms [mg/L];
- Am—stored/transported quantity [t];
- Con—viscosity of the substance [cSt];
- Sol—solubility [wt.%];
- Sur—properties of the environment (distance to the nearest water flow, groundwater depth, groundwater gradient, soil thickness above groundwater).
2.1.3. The Czech Republic
2.1.4. the slovak republic, 2.1.5. united kingdom.
- Identifying the hazard(s);
- Assessing the potential consequences;
- Assessing the probability of the consequences;
- Characterizing the risk and uncertainty.
2.1.6. The USA
- Problem formulation;
- Risk characterization.
2.1.7. Evaluation of Methodologies and Approaches to Environmental Risk Assessment
2.2. design of a methodological procedure for environmental risk assessment.
- Determination of the type of liability for the environmental damage (in concordance with the directive).
- Identification of sources of risk and determination of emergency scenarios.
- Initial assessment of emergency scenarios (by index methods).
- Detailed assessment of major emergency scenarios (based on the results of the initial assessment).
2.3. Characteristics of the Filling Station Facility
2.3.1. an identification of the subject and its activity.
- Unloading of fuels from tanks into storage tanks;
- Dispensing of fuels into the tanks of customers’ vehicles alone;
- Control of fuel dispensing from the service room of the filling station;
- Additional functions (washing vehicles in the washing line, inflating tires for passenger cars, dry cleaning of vehicle interiors—vacuuming).
2.3.2. An Identification of the Sources of Risk and Their Location
- A large amount of hazardous substances is unloaded from the tank vehicle into the storage tanks and, at the same time, recovered gases are discharged from the storage tanks into the tanks;
- The handling area for the unloading of fuels is not fenced, i.e., it is accessible to unauthorized persons who may interfere with the unloading of fuels;
- There is a risk of fuel spillage;
- There is a risk of the storage tanks being overfilled.
2.4. An Identification of Emergency Scenarios
Results of the assessment of emergency scenarios.
- Distance to the nearest watercourse (DNW): approx. 60 m;
- Groundwater depth (DGS): 2–5 m;
- Slope of the groundwater level: the groundwater level tends to have water flow;
- Strength and character of the soil above groundwater: concrete area.
4. Discussion
Author contributions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest.
- Bonvicini, S.; Antonioni, G.; Cozzani, V. Assessment of the risk related to environmental damage following major accidents in onshore pipelines. J. Loss Prev. Process. Ind. 2018 , 56 , 505–516. [ Google Scholar ] [ CrossRef ]
- Khahro, S.H.; Matori, A.N.; Chandio, I.A.; Talpur, M.H. Land Suitability Analysis for Installing New Petrol Filling Stations Using GIS. Procedia Eng. 2018 , 23 , 28–36. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Liu, D.; Xu, Z.; Fan, C.; Zhou, Y. Regional evaluation of fire apparatus requirements for petrol stations based on travel times. Proc. Saf. Environ. Prot. 2020 , 135 , 350–363. [ Google Scholar ] [ CrossRef ]
- Terrés, I.M.M.; Miñarro, M.D.; Ferradas, E.G.; Caracena, A.B.; Rico, J.B. Assessing the impact of petrol stations on their immediate surroundings. J. Environ. Manag. 2010 , 91 , 2754–2762. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Maksoud, H.A.A.; Elharrif, M.G.; Mahfouz, M.K.; Omnia, M.A.; Abdullah, M.H.; Eltabey, M.E. Biochemical study on occupational inhalation of benzene vapours in petrol station. Resp. Med. Case Rep. 2019 , 27 , 100836. [ Google Scholar ]
- Makka, K.; Kampova, K.; Lovecek, T.; Bernatik, A.; Rehak, D.; Ondrejka, R. Prevention and mitigation of injuries and damages arising from the activity of subliminal enterprises: A case study in Slovakia. J. Loss Prev. Process. Ind. 2021 , 70 , 104410. [ Google Scholar ] [ CrossRef ]
- Geraldino, B.R.; Nunes, R.F.N.; Gomes, J.B.; Giardini, I.; da Silva, P.V.B.; Campos, É.; da Poça, K.S.; Hassan, R.; Otero, U.B.; Sarpa, M. Analysis of Benzene Exposure in Gas Station Workers Using Trans, Trans-Muconic Acid. Int. J. Environ. Res. Public Health 2020 , 17 , 5295. [ Google Scholar ] [ CrossRef ]
- Tongsantia, U.; Chaiklieng, S.; Suggaravetsiri, P.; Andajani, S.; Autrup, H. Factors Affecting Adverse Health Effects of Gasoline Station Workers. Int. J. Environ. Res. Public Health 2021 , 18 , 10014. [ Google Scholar ] [ CrossRef ]
- Ma, G.; Huang, Y. Safety assessment of explosions during gas stations refilling process. J. Loss Prev. Process. Ind. 2019 , 60 , 133–144. [ Google Scholar ] [ CrossRef ]
- Sigut, M.; Alayón, S.; Hernández, E. Applying pattern classification techniques to the early detection of fuel leaks in petrol stations. J. Clean. Prod. 2014 , 80 , 262–270. [ Google Scholar ] [ CrossRef ]
- Alayón, S.; Sigut, M.; Arnay, R.; Toledo, P. Time windows: The key to improving the early detection of fuel leaks in petrol stations. Saf. Sci. 2020 , 130 , 104874. [ Google Scholar ] [ CrossRef ]
- Sikorova, A.K.; Bernatik, A.; Lunghi, E.; Fabiano, B. Lessons learned from environmental risk assessment within the framework of Seveso Directive in Czech Republic and Italy. J. Loss Prev. Process. Ind. 2017 , 49 , 47–60. [ Google Scholar ] [ CrossRef ]
- Lee, M. New’ Environmental Liabilities: The Purpose and Scope of the Contaminated Land Regime and the Environmental Liability Directive. Environ. Law Rev. 2009 , 11 , 264–278. [ Google Scholar ] [ CrossRef ]
- Pouikli, K.C. Overview of the implementation of the directive 2004/35/EC on environmental liability with regard to the prevention and remedying of environmental damage at European level. Desalination Water Treat. 2016 , 57 , 11520–11527. [ Google Scholar ] [ CrossRef ]
- Staccione, A.; Mysiak, J.; Ostoich, M.; Marcomini, A. Financial liability for environmental damage: Insurance market in Italy, focus on Veneto region experience. Environ. Sci. Pollut. Res. 2019 , 26 , 25749–25761. [ Google Scholar ] [ CrossRef ]
- Nigar, M. Environmental liability and global commons: A critical study. Int. J. Law Manag. 2018 , 60 , 435–451. [ Google Scholar ] [ CrossRef ]
- Descamps, H. Natural resource damage assessment (NRDA) under the European Directive on Environmental Liability: A comparative legal point of view. Oceanis 2008 , 32 , 439–461. [ Google Scholar ]
- Filentas, F.; Paralikas, A. Legal tools of environmental liability in Greece: Application in the broader region of Asopos. Int. J. Environ. Sustain. Dev. 2014 , 13 , 224–238. [ Google Scholar ] [ CrossRef ]
- Czech, E.K. Liability for Environmental Damage According to Directive 2004/35/EC. Pol. J. Environ. Stud. 2007 , 16 , 321–324. [ Google Scholar ]
- Cassota, S. Environmental Damage and Liability Problems in a Multilevel Context: The Case of the Environmental Liability Directive ; Kluwer Law International: Bedfordshire, UK, 2012. [ Google Scholar ]
- Ortega, J.M.; Brouwer, R.; Aiking, H. Application of a value-based equivalency method to assess environmental damage compensation under the European Environmental Liability Directive. J. Environ. Manag. 2011 , 92 , 1461–1470. [ Google Scholar ] [ CrossRef ]
- Goisis, F.; Stefani, L. The polluter-pays principle and site ownership: The European jurisprudential developments and the Italian experience. J. Eur. Environ. Plan. Law 2016 , 13 , 18–237. [ Google Scholar ] [ CrossRef ]
- Brans, E.H.P. The Environmental Liability Directive: Legal Background and Requirements. Equivalency Methods for Environmental Liability ; Springer: Dordrecht, The Netherlands, 2018. [ Google Scholar ]
- Directive 2004/35/CE of the European Parliament and of the Council of 21 April 2004 on Environmental Liability with Regard to the Prevention and Remedying of Environmental Damage. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32004L0035 (accessed on 22 November 2020).
- Ajman, N.N.; Zainun, N.Y.; Sulaiman, N.; Khahro, S.H.; Ghazali, F.E.M.; Ahmad, M.H. Environmental Impact Assessment (EIA) Using Geographical Information System (GIS): An Integrated Land Suitability Analysis of Filling Stations. Sustainability 2021 , 13 , 9859. [ Google Scholar ] [ CrossRef ]
- Bernatík, A. Hazard and Risk Analysis. Available online: https://www.fbi.vsb.cz/export/sites/fbi/U3V/cs/materialy/U3V_AnalyzaRizik.pdf (accessed on 13 May 2020).
- Polorecka, M.; Svetlik, J.; Mitrengova, J. Professional education and preparation for the performance of professional fire services in the field of environmental interventions. In Proceedings of the 14th International Technology, Education and Development Conference, Valencia, Spain, 2–4 March 2020. [ Google Scholar ]
- Smith, K. Environmental Hazards: Assessing and Reducing Disasters , 6th ed.; Routledge: London, UK; New York, NY, USA, 2013. [ Google Scholar ]
- Stam, G.J.; Bottelberghs, P.H.; Post, J.; Bos, G.; Proteus, H. A technical and management model for aquatic risk assessment of industrial spills. J. Hazard. Mater. 2000 , 71 , 439–448. [ Google Scholar ] [ CrossRef ]
- Andersson, A.S. Environment-Accident Index. A Planning Tool to Protect the Environment in Case of a Chemical Accident. Available online: https://www.diva-portal.org/smash/get/diva2:141970/FULLTEXT01.pdf (accessed on 15 April 2021).
- Methodical Instruction No. 2 of the Department of Environmental Risks for the Determination of Environmental Vulnerability EN-VITech03 and the Procedure for the Analysis of the Impacts of Accidents Involving a Dangerous Substance on the Environment Using the H&V Index Method. Available online: https://www.mzp.cz/web/edice.nsf/F5964CAB7EF17D95C12572590045E61A/ $ file/vestnik_03-2007_web.pdf (accessed on 8 December 2020).
- Bernatík, A.; Nevrlá, P. Impact of Accidents on the Environment. Available online: https://www.fbi.vsb.cz/export/sites/fbi/040/.content/galerie-souboru/studijni-materialy/skripta_VHZP-2005 (accessed on 24 May 2020).
- Zelenáková, M.; Labant, S.; Zvijáková, L.; Weiss, E.; Cepelová, H.; Weiss, R.; Fialová, J.; Mindas, J. Methodology for environmental assessment of proposed activity using risk analysis. Environ. Impact Assess. Rev. 2020 , 80 , 106333. [ Google Scholar ] [ CrossRef ]
- Guidelines for Environmental Risk Assessment and Management. Available online: http://www.iehconsulting.co.uk/IEH_Consulting/IEHCPubs/HumExpRiskAssess/guidelinesforenvironmental.pdf (accessed on 14 April 2021).
- Gormley, Á.; Pollard, S.; Rocks, S. Guidelines for Environmental Risk Assessment and Management: Green Leaves III , 1st ed.; Cranfield University: Cranfield, UK, 2011. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/69450/pb13670-green-leaves-iii-1111071.pdf (accessed on 11 January 2021).
- U.S. Environmental Protection Agency. Guidelines for Ecological Risk Assessment ; EPA/630/R095/002F; Risk Assessment Forum: Washington, DC, USA, 1998. Available online: https://www.epa.gov/sites/default/files/2014-11/documents/eco_risk_assessment1998.pdf (accessed on 11 January 2021).
- U.S. Environmental Protection Agency. Conducting an Ecological Risk Assessment. Available online: https://www.epa.gov/risk/conducting-ecological-risk-assessment#tab-2 (accessed on 15 March 2021).
- Tixier, J.; Dusserre, G.; Salvi, O.; Gaston, D. Review of 62 risk analysis methodologies of industrial plants. Loss Prev. Process. Ind. 2002 , 15 , 291–303. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Bernatík, A. Prevention of Major Accidents I. Available online: /http://www.portalbozp.cz/wp-content/uploads/2015/01/Bernatik_skripta-PZH-I.pdf (accessed on 25 November 2020).
- Bernatík, A. Prevention of Major Accidents II. Available online: https://www.fbi.vsb.cz/export/sites/fbi/040/.content/galerie-souboru/studijni-materialy/skripta-PZH-II.pdf (accessed on 25 November 2020).
- United Kingdom’s Health and Safety Authority (UKHSA) 2018. Petrol Station Safety. Available online: http://www.hsa.ie/eng/Your_Industry/Petrol_Stations (accessed on 11 March 2021).
- Mäkká, K.; Kampová, K.; (University of Žilina, Žilina, Slovakia). Personal communication, 2020.
- Andersson, A.S.; Tysklind, M.; Fangmark, I. A method to relate chemical accident properties and expert judgements in order to derive useful information for the development of Environment-Accident Index. J. Hazard. Mater. 2007 , 147 , 524–533. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Good, D.; Tynan, C.; Cleary, K. Environmental Liability Risk Assessment ; Verde Environmental Consultants Ltd.: Wicklow, Ireland, 2016. [ Google Scholar ]
- Mirae, Y.; Robby, C.; Bo, G.K.; Belal, A.; Jaehyun, H.; Sanghoon, L.; Hyun, G. A software tool for integrated risk assessment of spent fuel transportation and storage. Nucl. Eng. Technol. 2017 , 49 , 721–733. [ Google Scholar ]
- Tahmid, M.; Dey, S.; Syeda, S.R. Mapping human vulnerability and risk due to chemical accidents. J. Loss Prev. Process. Ind. 2020 , 68 , 104289. [ Google Scholar ] [ CrossRef ]
- Tang, Y.; Jing, J.J.; Zhang, Z.D.; Yang, Y. A Quantitative Risk Analysis Method for the High Hazard Mechanical System in Petroleum and Petrochemical Industry. Energies 2018 , 11 , 14. [ Google Scholar ] [ CrossRef ] [ Green Version ]
Click here to enlarge figure
| EAI Value | Proposed Procedure | Comment |
|---|---|---|
| 1–100 | Input hazard analysis (HA) | The study covers the properties of substances related to mobility, degradation, toxicity, classification, etc. |
| 100–500 | Initial hazard analysis (HA) + initial environmental risk assessment (ERA) | The study includes an extended form of HA (data on the site where the schematic substance is handled) and the determination of the affinity for a particular environmental compartment. |
| >500 | Extended environmental risk assessment (ERA) | The study includes a comparison of the predicted watercourse concentration (PEC) with concentrations without a negative effect on the watercourse (PNEC), the determination of emergency scenarios, and the modeling of the impact of the watercourse. This part of the evaluation also includes an evaluation of the impact of a hazardous substance on groundwater. |
| Hazardous Substance | Equipment | Quantity (kg) | Physical Form of the Substance | Classification H-Phrases |
|---|---|---|---|---|
| Diesel fuel | Storage tank | 39,100 | Liquid | H226 (Cat. 3); H351 (Cat. 2); H332 (Cat. 4); H304 (Cat. 1); H315 (Cat. 2); H373 (Cat. 2); H411 (Cat.2) |
| Tank | 30,505 | Liquid | ||
| Petrol “Natural 95” | Storage tank | 36,800 | Liquid | H224 (Cat. 1); H304 (Cat. 1); H315 (Cat.2); H361 (Cat. 2); H340 (muta 1B); H350 (carc. 1B); H336 STOT Single exp. 3; H411 (Cat.2) |
| Tank | 27,978 | Liquid |
| Emergency Scenario | Type of Spill | Cause of Spill | Consequences of the Spill | |
|---|---|---|---|---|
| 1. | Fuel spill during unloading | One-time leakage of the entire transported quantity or only one chamber | Damage to the integrity of the tank shell | Leaked fuel will get outside the paved area. |
| Continuous leakage of unloaded fuels | Rupture of the unloading hose, or its incorrect installation on the connections with the unloading pipe or the tank; overfilling of tanks | Leaked fuel will not spill outside the paved area; it will be caught in the emergency tank. | ||
| 2. | Fuel spill from the storage tanks | Continuous | Tank leak | Leaked fuel will not spill outside the paved area; it will be caught in the emergency tank. |
| 3. | Fuel spill from the storage tanks | Continuous | Rupture or leakage of the transport line | Leaked fuel will not spill outside the paved area; it will be caught in the emergency tank. |
| 4. | Fuel spill from dispensers during refueling | Continuous | Damage to the dispenser or dispensing guns when refueling customers’ vehicles | Leaked fuel will not spill outside the paved area; it will be caught in the emergency tank. |
| Hazardous Substance | Equipment | Quantity (kg) | Tox | Am | Con | Sol | Sur | EAI |
|---|---|---|---|---|---|---|---|---|
| Diesel fuel | Tank (the entire volume) | 30,505 | 4 | 5 | 4 | 1 | 5 | 300 |
| Tank (1 chamber) | 4136 | 4 | 3 | 4 | 1 | 5 | 120 | |
| Petrol | Tank (the entire volume) | 27,978 | 6 | 5 | 4 | 1 | 5 | 200 |
| Tank 1 (chamber) | 3525 | 6 | 3 | 4 | 1 | 5 | 180 |
| MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
Share and Cite
Mäkká, K.; Kampová, K.; Loveček, T.; Petrlová, K. An Environmental Risk Assessment of Filling Stations Using the Principles of Security Management. A Case Study in the Slovak Republic. Sustainability 2021 , 13 , 12452. https://doi.org/10.3390/su132212452
Mäkká K, Kampová K, Loveček T, Petrlová K. An Environmental Risk Assessment of Filling Stations Using the Principles of Security Management. A Case Study in the Slovak Republic. Sustainability . 2021; 13(22):12452. https://doi.org/10.3390/su132212452
Mäkká, Katarína, Katarína Kampová, Tomáš Loveček, and Katarína Petrlová. 2021. "An Environmental Risk Assessment of Filling Stations Using the Principles of Security Management. A Case Study in the Slovak Republic" Sustainability 13, no. 22: 12452. https://doi.org/10.3390/su132212452

Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Environmental risk assessment of ivermectin: A case study
Affiliation.
- 1 ECT Oekotoxikologie GmbH, Böttgerstrasse 2-14, D-65439 Flörsheim/Main, Germany. [email protected]
- PMID: 20821718
- DOI: 10.1002/ieam.96
- Integr Environ Assess Manag. 2010 Oct;6(4):790
The veterinary parasiticide ivermectin was selected as a case study compound within the project ERAPharm (Environmental Risk Assessment of Pharmaceuticals). Based on experimental data generated within ERAPharm and additional literature data, an environmental risk assessment (ERA) was performed mainly according to international and European guidelines. For the environmental compartments surface water, sediment, and dung, a risk was indicated at all levels of the tiered assessment approach. Only for soil was no risk indicated after the lower tier assessment. However, the use of effects data from additional 2-species and multispecies studies resulted in a risk indication for collembolans. Although previously performed ERAs for ivermectin revealed no concern for the aquatic compartment, and transient effects on dung-insect populations were not considered as relevant, the present ERA clearly demonstrates unacceptable risks for all investigated environmental compartments and hence suggests the necessity of reassessing ivermectin-containing products. Based on this case study, several gaps in the existing guidelines for ERA of pharmaceuticals were shown and improvements have been suggested. The action limit at the start of the ERA, for example, is not protective for substances such as ivermectin when used on intensively reared animals. Furthermore, initial predicted environmental concentrations (PECs) of ivermectin in soil were estimated to be lower than refined PECs, indicating that the currently used tiered approach for exposure assessment is not appropriate for substances with potential for accumulation in soil. In addition, guidance is lacking for the assessment of effects at higher tiers of the ERA, e.g., for field studies or a tiered effects assessment in the dung compartment.
PubMed Disclaimer
Similar articles
- A ranking of European veterinary medicines based on environmental risks. Kools SA, Boxall A, Moltmann JF, Bryning G, Koschorreck J, Knacker T. Kools SA, et al. Integr Environ Assess Manag. 2008 Oct;4(4):399-408. doi: 10.1897/IEAM_2008-002.1. Integr Environ Assess Manag. 2008. PMID: 18563959
- Effects of the veterinary pharmaceutical ivermectin on soil invertebrates in laboratory tests. Römbke J, Krogh KA, Moser T, Scheffczyk A, Liebig M. Römbke J, et al. Arch Environ Contam Toxicol. 2010 Feb;58(2):332-40. doi: 10.1007/s00244-009-9414-8. Epub 2009 Nov 1. Arch Environ Contam Toxicol. 2010. PMID: 19882295
- Effects of ivermectin-spiked cattle dung on a water-sediment system with the aquatic invertebrates Daphnia magna and Chironomus riparius. Schweitzer N, Fink G, Ternes TA, Duis K. Schweitzer N, et al. Aquat Toxicol. 2010 May 10;97(4):304-13. doi: 10.1016/j.aquatox.2009.12.017. Epub 2009 Dec 22. Aquat Toxicol. 2010. PMID: 20060604
- Human pharmaceuticals in surface waters. Implementation of a prioritization methodology and application to the French situation. Besse JP, Garric J. Besse JP, et al. Toxicol Lett. 2008 Jan 30;176(2):104-23. doi: 10.1016/j.toxlet.2007.10.012. Epub 2007 Oct 30. Toxicol Lett. 2008. PMID: 18077113 Review.
- Chemicals of emerging concern in the Great Lakes Basin: an analysis of environmental exposures. Klecka G, Persoon C, Currie R. Klecka G, et al. Rev Environ Contam Toxicol. 2010;207:1-93. doi: 10.1007/978-1-4419-6406-9_1. Rev Environ Contam Toxicol. 2010. PMID: 20652664 Review.
- Ivermectin toxicokinetics in rainbow trout (Oncorhynchus mykiss) following P-glycoprotein inhibition. Johnston CU, Azevedo VC, Kennedy CJ. Johnston CU, et al. Vet Res Commun. 2024 Aug 6. doi: 10.1007/s11259-024-10480-3. Online ahead of print. Vet Res Commun. 2024. PMID: 39106005
- Co-Processed Crystalline Solids of Ivermectin with Span ® 60 as Solubility Enhancers of Ivermectin in Natural Oils. de Vos L, Gerber M, Liebenberg W, Wessels JC, Lemmer HJR. de Vos L, et al. AAPS PharmSciTech. 2024 Mar 22;25(4):67. doi: 10.1208/s12249-024-02783-0. AAPS PharmSciTech. 2024. PMID: 38519767
- Isolation of soil bacteria able to degrade the anthelminthic compound albendazole. Lagos S, Koutroutsiou K, Karpouzas DG. Lagos S, et al. PeerJ. 2023 Nov 6;11:e16127. doi: 10.7717/peerj.16127. eCollection 2023. PeerJ. 2023. PMID: 37953781 Free PMC article.
- Effects of larval exposure to sublethal doses of ivermectin on adult fitness and susceptibility to ivermectin in Anopheles gambiae s.s. Kiuru C, Ominde K, Muturi M, Babu L, Wanjiku C, Chaccour C, Maia MF. Kiuru C, et al. Parasit Vectors. 2023 Aug 21;16(1):293. doi: 10.1186/s13071-023-05888-w. Parasit Vectors. 2023. PMID: 37605264 Free PMC article.
- Veterinary drug albendazole inhibits root colonization and symbiotic function of the arbuscular mycorrhizal fungus Rhizophagus irregularis. Gkimprixi E, Lagos S, Nikolaou CN, Karpouzas DG, Tsikou D. Gkimprixi E, et al. FEMS Microbiol Ecol. 2023 May 31;99(6):fiad048. doi: 10.1093/femsec/fiad048. FEMS Microbiol Ecol. 2023. PMID: 37156498 Free PMC article.
Publication types
- Search in MeSH
Related information
- PubChem Compound (MeSH Keyword)
LinkOut - more resources
Full text sources.

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
JavaScript appears to be disabled on this computer. Please click here to see any active alerts .
Risk Assessment Forum White Paper: Probabilistic Risk Assessment Methods and Case Studies
Probabilistic Risk Assessment White Paper and Supporting Documents
The goal of the white paper on probabilistic risk assessment (PRA) is to examine the potential and actual uses of probabilistic tools in the risk assessment process, and to encourage their use in EPA decision making.
- Risk Assessment Forum White Paper: Probabilistic Risk Assessment Methods and Case Studies (pdf) (5.24 MB, July 2014, 100-R-14-004)
- Scientific Leadership Home
- Risk Assessment Support
- Human Subjects Research
- Peer Review
- Public Access
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Environmental risk assessment of ivermectin: A case study

2010, Integrated Environmental Assessment and Management
Related Papers
Integrated Environmental Assessment and Management
Jörg Römbke , Jeanne Garric
Environmental Science and Pollution Research
Carlos Fernandez
theodoros arampatzis
Trac Trends in Analytical Chemistry
Martin Hansen
Aquatic Toxicology
Andrew Peregrine , Hans Sanderson
Applied Soil Ecology
The Science of the total environment
Jan Linders
The proposed methodology for the assessment of the environmental risks of the use of veterinary medicinal products is the hazard quotient approach. This article will elaborate on the exposure assessment with exposure models adapted to the Dutch agricultural situation. Based on the exposure assessment, decisions are made on the acceptability of the environmental risk of a product. Therefore, regulatory authorities have to be careful in choosing exposure scenarios. It will be shown that simple alterations of general models with an eye for common agricultural practice change predicted exposure concentrations by a factor of 2-40. In addition, the current proposals for effect assessment are too diverse, and the triggers that lead to further extensive testing are either useless, or at the least inconsistent with each other. An interdisciplinary approach using knowledge of veterinary and agricultural practice, risk management, and environmental toxicology and chemistry, is needed to determ...
Soil & Sediment Contamination
Ivermectin is a worldwide used antiparasitic compound acting against both endo- and ecto parasites of livestock. Ivermectin can reach the environment through the direct emission of dung from livestock on pasture and via manure application on agricultural lands. Due to its very high acute toxicity to many invertebrates, especially to D. magna, the excretion profile of ivermectin in dung after
ATUL PRATAP SINGH
Integrated …
Nika Galic , Udo Hommen , hans baveco
Several European directives and regulations address the environmental risk assessment of chemicals. We used the protection of freshwater ecosystems against plant protection products, biocidal products, human and veterinary pharmaceuticals, and other chemicals and priority substances under the Water Framework Directive as examples to explore the potential of ecological effect models for a refined risk assessment. Our analysis of the directives, regulations, and related guidance documents lead us to distinguish the following 5 areas for the application of ecological models in chemical risk assessment: 1) Extrapolation of organism-level effects to the population level: The protection goals are formulated in general terms, e.g., avoiding “unacceptable effects” or “adverse impact” on the environment or the “viability of exposed species.” In contrast, most of the standard ecotoxicological tests provide data only on organism-level endpoints and are thus not directly linked to the protection goals which focus on populations and communities. 2) Extrapolation of effects between different exposure profiles: Especially for plant protection products, exposure profiles can be very variable and impossible to cover in toxicological tests. 3) Extrapolation of recovery processes: As a consequence of the often short-term exposures to plant protection products, the risk assessment is based on the community recovery principle. On the other hand, assessments under the other directives assume a more or less constant exposure and are based on the ecosystem threshold principle. 4) Analysis and prediction of indirect effects: Because effects on 1 or a few taxa might have consequences on other taxa that are not directly affected by the chemical, such indirect effects on communities have to be considered. 5) Prediction of bioaccumulation within food chains: All directives take the possibility of bioaccumulation, and thus secondary poisoning within the food chain, into account. Integr Environ Assess Manag 2010;6:325–337. © 2010 SETAC
Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
RELATED PAPERS
Aquaculture Research
Chemosphere
Soeren Nors Nielsen
Jeanne Garric
Sara Monteiro
Ecotoxicology and Environmental Safety
Svetlana Skurtveit
Environmental Toxicology and Chemistry
Jean-Pierre Lumaret
Integrated environmental assessment and management
alistair boxall
Andrea Waichman , Marcos Garcia
Andrea Waichman , Jörg Römbke
hemantha kumara
Ajit Sarmah
Journal of Soils and Sediments
Carsten Prasse , Jörg Oehlmann
Piotr Stepnowski
Handbook of experimental pharmacology
Toxicological & Environmental Chemistry
Paola Bottoni
Environmental science & technology
Magnus Breitholtz , B. Brunstrom
Handbook of Experimental Pharmacology
Martinez Martinez , Peter Silley
Ecotoxicology
Milan Pogačnik , Vesna Flajs
Journal of Hazardous Materials
Andrzej Sobczak
Water, Air, & Soil Pollution
Stephan Hannappel , Beate Müller
Environmental Pollution
Johannes Lijzen
Trends in Biotechnology
Oliver A H Jones
Aquaculture
junghyup lee
Stefania Mattana , Xavier Domene
Science of The Total Environment
Trang Trinh
Amadeu Soares
TrAC Trends in Analytical Chemistry
Sandra Babić
Joaquim Macedo de Sousa
marcos pereira
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
A new approach for risk assessment of pulmonary toxicants: a preliminary study using didecyldimethylammonium chloride
- Research Article
- Published: 13 September 2024
Cite this article

- Jun Woo Kim ORCID: orcid.org/0009-0009-7661-3157 1 ,
- Yu Bin Han ORCID: orcid.org/0009-0008-7654-1109 2 ,
- Kyu Hyuck Chung ORCID: orcid.org/0000-0003-4283-0742 3 &
- Yong Joo Park ORCID: orcid.org/0000-0002-0528-6197 3
Current human risk assessments often rely on animal toxicity data to establish point of departure (POD) values, followed by the application of uncertainty factors. Consequently, there is growing interest in alternative toxicity testing methods that reduce reliance on animal models. In this study, we propose a novel approach for inhalation toxicity risk assessment that integrates in silico and in vitro methods. Human primary alveolar epithelial cells were exposed to aerosolized didecyldimethylammonium chloride (DDAC) to assess cytotoxicity. This was followed by transcriptome analysis and biological pathway investigation, utilizing adverse outcome pathway (AOP), to calculate the POD. Additionally, human DDAC exposure was simulated using a multiple-path particle dosimetry (MPPD) model to predict exposure levels in the human alveolar region via inhalation. The results from in silico and in vitro studies were then compared, and a comprehensive risk assessment was performed. The POD for AOP 452 key events—oxidative stress, inflammation, epithelial-mesenchymal transition (EMT), apoptosis, and autophagy—was found to range between 19.0 and 23.89 ng/cm 2 , according to benchmark dose calculation tools. The estimated human exposure to DDAC in the alveolar region under actual exposure conditions was 0.164 ng/cm 2 /day, resulting in a margin of exposure (MOE) ranging from 121 to 145, suggesting caution regarding DDAC inhalation exposure. This study presents a novel risk assessment method that compares estimated human inhalation exposure values to in vitro results, applying the human equivalent concentration concept. Our findings demonstrate the potential for conducting human risk assessments using both in silico and in vitro methods as alternatives to traditional in vivo studies.
Graphical abstract

This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others
Predicting the in vivo pulmonary toxicity induced by acute exposure to poorly soluble nanomaterials by using advanced in vitro methods
Development and evaluation of a high throughput inhalation model for organic chemicals
Nano-risk science: application of toxicogenomics in an adverse outcome pathway framework for risk assessment of multi-walled carbon nanotubes, explore related subjects.
- Environmental Chemistry
Data availability
Source of data collected have been mentioned in the text. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ambade B, Kumar A, Sahu LK (2021) Characterization and health risk assessment of particulate bound polycyclic aromatic hydrocarbons (PAHs) in indoor and outdoor atmosphere of Central East India. Environ Sci Pollut Res Int 28:56269–56280
Article Google Scholar
Ambade B, Sethi SS, Giri B, Biswas JK, Bauddh K (2022) Characterization, behavior, and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in the estuary sediments. Bull Environ Contam Toxicol 108:243–252
Article CAS Google Scholar
Borisova A, Rudus K, Pavlovska I, Martinsone Ž, Mārtiņsone I (2023) Multiple path particle dosimetry model concept and its application to determine respiratory tract hazards in the 3D printing. Environ Technol Resour Proc Int Sci Pract Conf 2:23–27
Google Scholar
Brown JS, Diamond GL (2023) Derivation of first-order dissolution rates to estimate particle clearance and burden in the human respiratory tract. Part Fibre Toxicol 20:17
Dejobert Y, Martin P, Piette F, Thomas P, Bergoend H (1997) Contact dermatitis from didecyldimethylammonium chloride and bis-(aminopropyl)-lauryl amine in a detergent-disinfectant used in hospital. Contact Dermatitis 37:95–96
Dumas O, Varraso R, Boggs KM, Quinot C, Zock JP, Henneberger PK, Speizer FE, Le Moual N, Camargo CA Jr (2019) Association of occupational exposure to disinfectants with incidence of chronic obstructive pulmonary disease among US female nurses. JAMA Netw Open 2:e1913563
Farmahin R, Williams A, Kuo B, Chepelev NL, Thomas RS, Barton-Maclaren TS, Curran IH, Nong A, Wade MG, Yauk CL (2017) Recommended approaches in the application of toxicogenomics to derive points of departure for chemical risk assessment. Arch Toxicol 91:2045–2065
Gonzalez M, Jégu J, Kopferschmitt M-C, Donnay C, Hedelin G, Matzinger F, Velten M, Guilloux L, Cantineau A, de Blay F (2014) Asthma among workers in healthcare settings: role of disinfection with quaternary ammonium compounds. Clin Exp Allergy 44:393–406
Hadzic S, Wu C-Y, Avdeev S, Weissmann N, Schermuly RT, Kosanovic D (2020) Lung epithelium damage in COPD – an unstoppable pathological event? Cell Signal 68:109540
Hansen SF, Sørensen SN, Skjolding LM, Hartmann NB, Baun A (2017) Revising REACH guidance on information requirements and chemical safety assessment for engineered nanomaterials for aquatic ecotoxicity endpoints: recommendations from the EnvNano project. Env Sci Europe 29:14
Karmaus AL, Bialk H, Fitzpatrick S, Krishan M (2020) State of the science on alternatives to animal testing and integration of testing strategies for food safety assessments: workshop proceedings. Regul Toxicol Pharmacol 110:104515
Kim YS, Lee SB, Lim CH (2017) Effects of didecyldimethylammonium chloride (DDAC) on Sprague-Dawley rats after 13 weeks of inhalation exposure. Toxicol Res 33:7–14
Kim JW, Kim HS, Kim HR, Chung KH (2024) Next generation risk assessment of biocides (PHMG-p and CMIT/MIT)-induced pulmonary fibrosis using adverse outcome pathway-based transcriptome analysis. J Hazard Mater 476:134986
Kinaret PAS, Serra A, Federico A et al (2020) Transcriptomics in toxicogenomics, part i: experimental design, technologies, publicly available data, and regulatory aspects. Nanomaterials (Basel) 10:750
Kuempel ED, Sweeney LM, Morris JB, Jarabek AM (2015) Advances in inhalation dosimetry models and methods for occupational risk assessment and exposure limit derivation. J Occup Environ Hyg 12(Suppl 1):S18-40
Lim CH, Chung YH (2014) Effects of didecyldimethylammonium chloride on Sprague-Dawley rats after two weeks of inhalation exposure. Toxicol Res 30:205–210
Lin C-R, Bahmed K, Kosmider B (2022) Impaired alveolar re-epithelialization in pulmonary emphysema. Cells 11:2055
Lippmann M, Yeates DB, Albert RE (1980) Deposition, retention, and clearance of inhaled particles. Br J Ind Med 37:337–362
CAS Google Scholar
Löndahl J, Möller W, Pagels JH, Kreyling WG, Swietlicki E, Schmid O (2014) Measurement techniques for respiratory tract deposition of airborne nanoparticles: a critical review. J Aerosol Med Pulm Drug Deliv 27:229–254
Lovén K, Isaxon C, Wierzbicka A, Gudmundsson A (2019) Characterization of airborne particles from cleaning sprays and their corresponding respiratory deposition fractions. J Occup Environ Hyg 16:656–667
Manojkumar N, Srimuruganandam B, Shiva Nagendra SM (2019) Application of multiple-path particle dosimetry model for quantifying age specified deposition of particulate matter in human airway. Ecotoxicol Environ Saf 168:241–248
McCarrick S, Karlsson HL, Carlander U (2022) Modelled lung deposition and retention of welding fume particles in occupational scenarios: a comparison to doses used in vitro. Arch Toxicol 96:969–985
Miller FJ, Asgharian B, Schroeter JD, Price O (2016) Improvements and additions to the multiple path particle dosimetry model. J Aerosol Sci 99:14–26
Nault R, Fader KA, Zacharewski T (2015) RNA-Seq versus oligonucleotide array assessment of dose-dependent TCDD-elicited hepatic gene expression in mice. BMC Genomics 16:373
Pack EC, Lee HG, Jeong H-j, Lee J, Jang DY, Kim HS, Lee SH, Lim KM, Choi D (2023) Tiered human health risk assessment of antibacterial quaternary ammonium compounds (QACs) in dishwashing detergents. Regul Toxicol Pharmacol 137:105306
Pallocca G, Moné MJ, Kamp H, Luijten M, van de Water B, Leist M (2022) Next-generation risk assessment of chemicals – rolling out a human-centric testing strategy to drive 3R implementation: The RISK-HUNT3R project perspective. ALTEX 39:419–426
Phillips JR, Svoboda DL, Tandon A, Patel S, Sedykh A, Mav D, Kuo B, Yauk CL, Yang L, Thomas RS, Gift JS, Davis JA, Olszyk L, Merrick BA, Paules RS, Parham F, Saddler T, Shah RR, Auerbach SS (2018) BMDExpress 2: enhanced transcriptomic dose-response analysis workflow. Bioinformatics 35:1780–1782
Ramanarayanan T, Szarka A, Flack S, Hinderliter P, Corley R, Charlton A, Pyles S, Wolf D (2022) Application of a new approach method (NAM) for inhalation risk assessment. Regul Toxicol Pharmacol 133:105216
Reale E, Zare Jeddi M, Paini A et al (2024) Human biomonitoring and toxicokinetics as key building blocks for next generation risk assessment. Environ Int 184:108474
Silva S, Bicker J, Falcão A, Fortuna A (2023) Air-liquid interface (ALI) impact on different respiratory cell cultures. Eur J Pharm Biopharm 184:62–82
Vincent G, Kopferschmitt-Kubler MC, Mirabel P, Pauli G, Millet M (2007) Sampling and analysis of quaternary ammonium compounds (QACs) traces in indoor atmosphere. Environ Monit Assess 133:25–30
Webster AF, Chepelev N, Gagné R, Kuo B, Recio L, Williams A, Yauk CL (2015) Impact of genomics platform and statistical filtering on transcriptional benchmark doses (BMD) and multiple approaches for selection of chemical point of departure (PoD). PLoS One 10:e0136764
Download references
This research was supported by the Korea Environment Industry & Technology Institute (KEITI) through the Technology Development Project for Safety Management of Household Chemical Products, funded by the Korea Ministry of Environment (MOE) (2022002960003, NTIS-1485018900).
Author information
Authors and affiliations.
Inhalation Toxicology Center for Airborne Risk Factors, Korea Institute of Toxicology, Jeongeup, 56212, Republic of Korea
Jun Woo Kim
College of Pharmacy, Korea University, Sejong, 30019, Republic of Korea
College of Pharmacy, Kyungsung University, Busan, 48434, Republic of Korea
Kyu Hyuck Chung & Yong Joo Park
You can also search for this author in PubMed Google Scholar
Contributions
JW Kim: Investigation, writing—original draft, writing—review editing
YB Han: Data curation, methodology
KH Chung: Investigation, writing—review editing
YJ Park: Investigation, writing—original draft, writing—review editing, supervision
Corresponding author
Correspondence to Yong Joo Park .
Ethics declarations
Ethical approval.
This research did not involve human participants and/or animals and informed consent was not required.
Consent to participate
Not applicable here.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- Inhalation toxicity of didecyldimethylammonium chloride (DDAC) was assessed using a next-generation risk assessment approach.
- Point of departure was determined by analyzing cellular transcriptome based on the adverse outcome pathway.
- Human equivalent concentration was compared with a review of human DDAC exposure estimated by multiple-path particle dosimetry model and aerosol exposure of DDAC in vitro assay.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 67 KB)
Supplementary file2 (docx 19 kb), rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Kim, J.W., Han, Y.B., Chung, K.H. et al. A new approach for risk assessment of pulmonary toxicants: a preliminary study using didecyldimethylammonium chloride. Environ Sci Pollut Res (2024). https://doi.org/10.1007/s11356-024-34905-3
Download citation
Received : 03 June 2024
Accepted : 30 August 2024
Published : 13 September 2024
DOI : https://doi.org/10.1007/s11356-024-34905-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Inhalation toxicity
- Next-generation risk assessment
- Didecyldimethylammonium chloride
- Aerosol exposure
- Multiple-path particle dosimetry
- Adverse outcome pathway
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
These fourteen (14) case studies illustrate how assessors have developed and interpreted evidence to determine causes of biological impairments. They provide examples of how to organize an assessment report, analyze data, and present results. Most of the cases assess rivers and streams, but a few assess terrestrial ecosystems.
Risk assessment (RA) and life cycle assessment (LCA) are two analytical tools used to support decision making in environmental management. This study reviewed 30 environmental assessment case studies that claimed an integration, combination, hybridization, or complementary use of RA and LCA. The focus of the analysis was on how the respective case studies evaluated emissions of chemical ...
case studies showing risk assessments for chemical mixtures. The aim was to find ... of human health and environmental risk assessment. Mostly concentration addition (CA) based methods are used for predicting mixture effects. In contrast, several
Environmental risk assessment (ERA) is a systematic process of evaluating the potential adverse effects of human activities and natural phenomena on the environment, ecosystems, and human health. ... Mapping suitable areas for crop cultivation under climate change scenarios: A case study of maize in China. Journal of Geographical Sciences, 27(8 ...
Risk assessment (RA) and life cycle assessment (LCA) are two analytical tools used to support decision making in environmental management. This study reviewed 30 environmental assessment case ...
A REVIEW OF ECOLOGICAL ASSESSMENT CASE STUDIES FROM A RISK ASSESSMENT PERSPECTIVE VOLUME II Risk Assessment Forum U.S. Environmental Protection Agency Washington, DC 20460. ... (Environmental Monitoring and Assessment Program—EMAP) is described. # Regional scale assessments (EMAP, wetlands) are included. ...
The scientific community recommends that the environmental risk assessment ... (RIAM) analysis as decision tool to select new site for municipal solid waste disposal: A case study of Dehradun city, India. Sustainable Cities and Society, 13 (2014), pp. 12-19, 10.1016/j.scs.2014.03.007. View PDF View article View in Scopus Google Scholar.
A small-scale organic crop producing the protected geographical indication (PGI) cultivar "Faba Asturiana", located in northern Spain, was considered to be a case study for analyzing the environmental impacts associated with the production of this legume (Phaseolus vulgaris L.). The life cycle assessment (LCA) methodology was employed for the analysis with a "cradle-to-gate ...
Risk assessment (RA) and life cycle assessment (LCA) are two analytical tools used to support decision making in environmental management. This study reviewed 30 environmental assessment case studies that claimed an integration, combination, hybridization, or complementary use of RA and LCA. The focus of the analysis was on how the respective ...
Fully updated and authored by leading authorities in the field, the result is an indispensable work. Readers of the second edition of Human and Ecological Risk Assessment will also find: Over 40 entirely new case studies reflecting the latest in risk assessment practice Detailed discussion of hazards including air emissions, contaminated food ...
Probabilistic Risk Assessment Methods and Case Studies. EPA/100/R‐09/001A. Washington, D.C.: Risk Assessment Forum, Office of the Science Advisor, USEPA. ... Case Study 7: Environmental Monitoring and Assessment Program: Using Probabilistic Sampling to Evaluate the Condition of the Nation's Aquatic
Untreated hexamethylenetetramine (HMT) was discharged into the Tone River in the central area of Japan, and the risk management plan in the watershed area has been strengthened because HMT is the precursor of formaldehyde (FA) regulated by Japanese water supply law. The release of HMT could occur not only in steady but also in unsteady environmental conditions. In this context, no quantitative ...
NGFS Occasional Papers Case Studies of Environmental Risk Analysis Methodologies. Published on 09/10/2020. 616 pages. EN.
This paper presents a standardised approach to environmental risk assessment for extraction of sand (used in small scale construction projects) in a data-limited situation. ... The assessment of the case study was carried out by UK marine scientists with expert knowledge of regulatory processes, environmental assessment of marine activities ...
protection good practice and the environmental risk tolerability guidelines for Great Britain (GB). This framework for risk assessment and control is illustrated using Major Accident to the Environment (MATTE) case studies that have been selected to illustrate a range of issues relevant to environmental protection.
The study area is in southwest Western Australia (WA), roughly between 31°S∼35°S and 115°E∼120°E (Fig.1). WA is a significant bushfire risk area, with 90% of its landscape prone to bushfires (DFES, 2022). It has distinctly dry and hot summers (BOM, 2022), with the fire season spreading from October to March (Russell-Smith et al., 2007).
The association between environmental chemical exposures and chronic diseases is of increasing concern. Chemical risk assessment relies heavily on pre-market toxicity testing to identify safe levels of exposure, often known as reference doses (RfD), expected to be protective of human health. Although some RfDs have been reassessed in light of new hazard information, it is not a common practice.
The case study's writers carried out an environmental risk assessment associated with the operation of the filling station at a selected filling station facility. In conjunction with the request of the operator to maintain the anonymity of the filling station, the paper will neither publish data such as the name of the company nor mention a ...
Taking China as a case study, this study calculated the environmental risk value of every sub-unit. Through the clustering function of Statistical Package for Social Sciences and the visual representation of a geographic information system, the study area was divided into nine zones that were visualized on maps according to their different risk ...
In this research, a scientific nature and accuracy the coastal petrochemical projects' environmental risk system of Quanzhou petrochemical project is constructed, as well as the assessment method through the instance analysis.This system contains 3 citerion layer indexes, 2 expanding layer indexes and 22 factor layer indexes, forming a multi ...
Divergence between general public's risk perception and environmental risk assessment: A case study of p-xylene projects in China. ... In a case study in Fujian Province of China, a questionnaire was designed to investigate the public perception of risks of a proposed PX project, interviews were further conducted in Zhangzhou City and Xiamen ...
ENVIRONMENTAL RISK - CASE STUDIES. February 2018. Publisher: CZECH-POL TRADE. ISBN: 978-80-907124--9. Authors: Adam Pawełczyk. Wroclaw University of Science and Technology. František Božek ...
Because ecotoxicological data on β-blockers are scarce, it was decided to choose the β-blocker atenolol as a case study pharmaceutical within the project ERAPharm. A starting point for the assessment of potential environmental risks was the European guideline on the environmental risk assessment of medicinal products for human use.
assessment, but also impr oves the r elevance of risk assessment thr ough case studies that demonstrate good practice. The assessment and management of envir onmental risk is central to the envir ...
EPA Guidance. In order to conduct respectable risk assessments, based on sound science, that can respond to the needs of our nation, EPA has developed guidance, handbooks, framework and general standard operating procedures. In some cases, these resources are broad enough to be relevant across all statutes that EPA administers while in other ...
For the environmental compartments surface water, sediment, and dung, a risk was indicated at all levels of the tiered assessment approach. Only for soil was no risk indicated after the lower tier assessment. However, the use of effects data from additional 2-species and multispecies studies resulted in a risk indication for collembolans.
Ivermectin is unlikely to present a risk for freshwater algae. For fish, a PNEC of 3 ng/L was derived based on the lowest LC50. This value is within the range of the initial PECsw. The RQ using the worst-case PECsw for the IR scenario is above the threshold of 1, indicating a risk for freshwater fish.
The goal of the white paper on probabilistic risk assessment (PRA) is to examine the potential and actual uses of probabilistic tools in the risk assessment process, and to encourage their use in EPA decision making. Risk Assessment Forum White Paper: Probabilistic Risk Assessment Methods and Case Studies (pdf) (5.24 MB, July 2014, 100-R-14-004 ...
Environmental Risk Assessment of Ivermectin—Integr Environ Assess Manag 6, 2010 cores, these data support laboratory results indicating a low degradation of this compound in soil. The fate of ivermectin was also assessed in an aquatic semifield mescocosm study (Sanderson et al. 2007).
Current human risk assessments often rely on animal toxicity data to establish point of departure (POD) values, followed by the application of uncertainty factors. Consequently, there is growing interest in alternative toxicity testing methods that reduce reliance on animal models. In this study, we propose a novel approach for inhalation toxicity risk assessment that integrates in silico and ...