9 Deep Vein Thrombosis Nursing Care Plans

Deep vein thrombosis ( DVT ) is a common and potentially life-threatening condition that requires prompt medical attention. As a nurse, understanding the nursing care plans and nursing diagnosis for DVT is essential to providing the best care for clients. This guide provides a comprehensive overview of DVT nursing care plans and nursing diagnoses , including common symptoms, nursing interventions , nursing management, and treatment options.

Table of Contents
What is deep vein thrombosis, nursing problem priorities, nursing assessment, nursing diagnosis, nursing goals, 1. promoting effective gas exchange, 2. enhancing peripheral tissue perfusion, 3. managing acute pain, 4. preventing bleeding risk and injury, 5. initiating health teaching and patient education, 6. assessing and monitoring for potential complications, 7. administering medications and pharmacologic support, 8. monitoring laboratory and diagnostic procedures, 9. providing perioperative care, recommended resources, references and sources.
Thrombophlebitis is the inflammation of the vein wall resulting in the formation of a thrombosis ( blood clot) that may interfere with the normal blood flow through the vessel.
Typically, venous thrombophlebitis occurs in the lower extremities. It may also occur in superficial veins such as cephalic, basilic, and greater saphenous veins, which usually is not life-threatening and does not necessitate hospitalization, or it may happen in a deep vein, which can be life-threatening because clots may travel to the bloodstream and cause a pulmonary embolism .
Three contributing factors (known as Virchow’s triad) can lead to the development of deep vein thrombosis (DVT), which includes venous stasis, hypercoagulability, and vessel wall injury .
Venous stasis occurs when blood flow is decreased, as in immobility, medication therapies, and in heart failure. Hypercoagulability occurs most commonly in clients with deficient fluid volume , pregnancy, oral contraceptive use, smoking, and some blood dyscrasias. Venous wall damage may occur secondary to venipuncture, certain medications, trauma , and surgery . The objective of treatment of DVT involves preventing the clot from dislodgement (risking pulmonary embolism) and reducing the risk of post-thrombotic syndrome.
DVT is a common venous thromboembolic (VTE) disorder with an incidence of 1.6 per 1000 annually. Even in clients who do not get pulmonary emboli , recurrent thrombosis and “post-thrombotic syndrome” are major causes of morbidity. DVT is a major medical problem accounting for most cases of pulmonary embolism. Only through early diagnosis and treatment can the morbidity be reduced (Schick, 2023).
Nursing Care Plans and Management
Nursing care management for patients with deep vein thrombosis (DVT) involves thorough assessment of the patient’s history and symptoms, administering anticoagulant medications, managing pain , promoting circulation through compression therapy and activity, educating the patient about DVT and self-care measures, providing psychosocial support, collaborating with the healthcare team, and closely monitoring the patient’s condition.
The following are the nursing priorities for patients with deep vein thrombosis:
- Preventing pulmonary embolism.
- Management of pain .
- Promotion of circulation and prevention of complications.
- Patient education and health teachings.
- Anticoagulant therapy.
Assess for the following subjective and objective data :
- Apprehension
- Hypercapnia
- Restlessness
- Asymptomatic
- Increased leg warmth
- Edema (Unilateral)
- Pain during palpation of a calf muscle
Following a thorough assessment , a nursing diagnosis is formulated to specifically address the challenges associated with deep vein thrombosis based on the nurse’s clinical judgement and understanding of the patient’s unique health condition. While nursing diagnoses serve as a framework for organizing care, their usefulness may vary in different clinical situations. In real-life clinical settings, it is important to note that the use of specific nursing diagnostic labels may not be as prominent or commonly utilized as other components of the care plan. It is ultimately the nurse’s clinical expertise and judgment that shape the care plan to meet the unique needs of each patient, prioritizing their health concerns and priorities.
Goals and expected outcomes may include:
- The client will demonstrate adequate ventilation and oxygenation , as evidenced by ABGs within the normal range.
- The client will report or display resolution or absence of symptoms of respiratory distress.
- The client will maintain optimal peripheral tissue perfusion in the affected extremity, as evidenced by strong palpable pulses, reduction in and/or absence of pain , warm and dry extremities, and adequate capillary refill.
- The client will not experience pulmonary embolism, as evidenced by normal breathing, heart rate , and absence of dyspnea and chest pain .
- The client will report that pain or discomfort is alleviated or controlled, and verbalize methods that provide relief.
- The client will display a relaxed manner, be able to sleep or rest, and engage in desired activities.
Nursing Interventions and Actions
Therapeutic interventions and nursing actions for patients with deep vein thrombosis may include:
Clients with deep vein thrombosis (DVT) can experience impaired gas exchange due to altered blood flow to the alveoli and changes in the alveolar-capillary membrane. DVT can obstruct blood flow to the lungs , reducing the amount of blood that reaches the alveoli, where gas exchange takes place. Additionally, changes in the alveolar-capillary membrane, such as inflammation and increased permeability, can further impair gas exchange by reducing the diffusion of oxygen and carbon dioxide between the lungs and the bloodstream. To ensure optimal gas exchange, healthcare providers focus on interventions to improve lung function. Regular monitoring of respiratory status allows for early detection of complications.
1. Assess the level of consciousness and changes in mentation. Initial signs of systemic hypoxemia include restlessness and irritability, followed by progressively decreased mentation. Reduced oxygenation is a risk factor for thrombosis since the incidence of thrombosis is increased under systemic or local hypoxia. Hypoxia occurs when oxygen demand is greater than oxygen supply, for example, when blood flow is reduced by immobility or reduced by trauma (Gupta et al., 2019).
2. Auscultate lungs for areas of decreased and absent breath sounds and the presence of adventitious sounds (crackles). Non-ventilated areas may be identified by the absence of breath sounds. Crackles may be seen in fluid-filled tissues and the airway or may indicate cardiac decompensation. A client with a developing pulmonary embolism (PE) may exhibit dyspnea. Dyspnea may be acute and severe in central PE, whereas it is often mild and transient in small peripheral PE (Vyas & Goyal, 2022).
3. Monitor vital signs. Observe changes in cardiac rhythm . Tachycardia, tachypnea , and BP changes are associated with progressing hypoxemia and acidosis. Alterations in heart rhythm and extra heart sounds may indicate increased cardiac workload related to worsening ventilation imbalance. PE may become apparent when the client exhibits hypotension (systolic blood pressure less than 90 mm Hg or a drop in SBP of 40 mm Hg or more from baseline) (Vyas & Goyal, 2022).
4. Assess respiratory rate and rhythm. Observe for use of accessory muscles, nasal flaring, and pursed lip breathing. Tachypnea and dyspnea are indicative of pulmonary obstruction. Dyspnea and increased work of breathing may be the first or only signs of subacute pulmonary embolism. Severe respiratory distress and failure accompany moderate to a severe loss of functional lung units. Shock and right ventricular dysfunction confer a poor prognosis and predict mortality. Clients with PE and a coexisting DVT are also at an increased risk for death (Vyas & Goyal, 2022).
5. Observe for generalized duskiness and cyanosis in the earlobes, lips, tongue, and buccal membranes. This is suggestive of systemic hypoxemia. Late signs of hypoxia include bluish discoloration of the skin and mucous membranes, where vasoconstriction of the peripheral vessels causes cyanosis. Cyanosis is most easily seen around the lips and in the oral mucosa. However, the nurse should never assume the absence of cyanosis means adequate oxygenation (Doyle & McCutcheon, 2015).
6. Assess activity tolerance , such as reports of weakness and fatigue , vital sign changes, or increased dyspnea during exertion. Encourage rest periods, and limit activities to client tolerance. These guidelines help in determining the response of the client to resume activities and the ability to engage in self-care. The nurse may use the six-minute walk test to assess the response of oxyhemoglobin saturation to exercise or activities, as well as the total distance the client can walk in six minutes on a ground level (Bhutta et al., 2022).
7. Monitor ABGs or pulse oximetry. Hypoxemia is present in varying degrees, depending on the degree of airway obstruction , cardiopulmonary status, and presence and degree of shock. Respiratory alkalosis and metabolic acidosis may also be present. Arterial oxygen saturation refers to the amount of oxygen bound to hemoglobin in arterial blood. ABGs are useful tools to evaluate hypoxia because they can also shed light on the etiology of the disease process (Bhutta et al., 2022).
8. Evaluate sleep patterns, noting reports of difficulties and whether the client feels well-rested. The client may have difficulty sleeping due to the feeling of dyspnea. Nocturnal trend oximetry provides information about oxyhemoglobin saturation over a period (usually overnight). This test is primarily used to assess the adequacy or need for oxygen supplementation at night. The use of overnight trend oximetry as a surrogate for a diagnostic sleep study is possible, however, a formal sleep study should be used whenever possible (Bhutta et al., 2022).
9. Check the client frequently and arrange for someone to stay with the client, as indicated. This assures that changes in condition will be noted and that assistance is readily available. The client may manifest neurological symptoms such as restlessness, headache, and confusion with moderate hypoxia, therefore, the client must be assessed and checked as frequently as possible to avoid further deterioration of the client’s condition (Bhutta et al., 2022).
10. Assist with frequent changes of position, and encourage ambulation as tolerated. Turning and ambulation enhance the aeration of different lung segments, thereby improving oxygenation. The ACCP Consensus Conference on Antithrombotic and Thrombolytic Therapy for venous thromboembolism recommended ambulation as tolerated for clients with DVT. Therefore, early ambulation on day 2 after initiation of outpatient anticoagulant therapy, in addition to effective compression, is strongly recommended. Early ambulation without ECS is not recommended (Patel, 2019).
11. Encourage coughing , deep breathing exercises, and suctioning as indicated. Increases oxygen delivery to the lungs by mobilizing secretions and enhancing ventilation. Deep breathing exercises are used to decrease the incidence and severity of pulmonary complications such as pneumonia , atelectasis , and hypoxemia. During exercise education, the nurse explains and demonstrates how to take a deep, slow breath, and how to exhale slowly, three to five times every one to two hours. Clients who performed deep breathing exercises had better pulmonary function compared to the performing no exercise group (Unver et al., 2018).
12. Keep the head of the bed elevated. This promotes maximal chest expansion, making it easier to breathe and enhancing physiological and psychological comfort . A prone position should be avoided. In COVID-19 acute respiratory distress syndrome (ARDS), a prone position is frequently applied. During prone ventilation, the client’s position remains nearly unchanged, with minimal movements limited to the head and limbs. Therefore, the prone position can be a potential contributor to blood flow changes in these clients (Gebhard et al., 2021).
See also: Patient Positioning: Complete Guide and Cheat Sheet for Nurses
13. Assist with chest physiotherapies, such as postural drainage and percussion of the non-affected area, and with an incentive spirometer . This facilitates deeper respiratory effort and promotes drainage of secretions from lung segments into bronchi , where they may more readily be removed by coughing or suctioning. Pulmonary rehabilitation can significantly improve dyspnea, overall health, and exercise endurance in clients with PE. The existing evidence suggests that pulmonary rehabilitation is a potential treatment for alleviating post-PE syndrome, which improves the quality of life and prognosis of clients with PE (Yu et al., 2022).
14. Provide supplemental humidification, such as ultrasonic nebulizers. Nebulization gives moisture to mucous membranes and helps liquefy secretions to facilitate airway clearance. Dry nasal mucosa occurs when the flow is greater than or equal to 4 L/minute, therefore humidification is necessary for clients using low-flow oxygen devices (Bhutta et al., 2022).
15. Provide oxygen therapy with an appropriate method as ordered. Oxygen therapy can help increase oxygen levels and enhance tissue perfusion, decreasing the risk of hypoxia and other related complications. Oxygen therapy may be indicated for clients with low PaO2 that is less than 60 or SaO2 less than 90, and this can be achieved by increasing the percentage of oxygen in the inspired air that reaches the alveoli (Bhutta et al., 2022).
16. Provide adequate hydration, either oral (PO) or IV, as indicated. Increased fluids may be given to decrease the hyperviscosity of blood, which can potentiate thrombus formation, or support circulating volume and tissue perfusion. A low fluid volume state can lead to hemoconcentration and low venous flow. Clients who experienced a VTE were found to have elevated biochemical indices of dehydration , in comparison to clients who had not (Keiter et al., 2015).
17. Administer medications, as indicated . See Pharmacologic Management
18. Prepare the client for a lung scan. This may reveal the pattern of abnormal perfusion in areas of ventilation, reflecting ventilation and perfusion mismatch, confirming the diagnosis of pulmonary embolism and the degree of obstruction. The absence of both ventilation and perfusion reflects alveolar congestion or airway obstruction. The planar ventilation/perfusion scan is an established diagnostic test for suspected PE. V/Q scanning is mostly performed for clients in whom computed tomographic pulmonary angiography (CTPA) is contraindicated or inconclusive, or when additional testing is needed (Vyas & Goyal, 2022).
19. Prepare for and assist with bronchoscopy . The purpose of this procedure is to remove blood clots and clear the airway. During flexible bronchoscopy, clots can be removed piecemeal by biopsy forceps, dislodged using a Fogarty catheter, or removed en bloc using either suctioning or a cryoprobe (Sehgal et al., 2017).
20. Prepare for surgical intervention , if indicated. Vena caval ligation or insertion of an intracaval umbrella is intended for clients with recurrent emboli despite adequate anticoagulation, when anticoagulation is contraindicated, or when septic emboli arising from below the renal veins unresponsive to treatment; Pulmonary embolectomy is often done as a last resort treatment of PE. Traditional venous thrombectomy is performed by surgically exposing the common femoral vein and saphenofemoral junction through a longitudinal skin incision. Care must be taken to avoid dislodging the clot or breaking it into small fragments because pulmonary embolus will result (Patel, 2019).
21. Assist the client to deal with fear and anxiety that may be present. Feelings of fear and severe anxiety are associated with the inability to breathe and may actually increase oxygen consumption and demand. Encourage the client to express their feelings so that the client may regain some sense of control over emotions. Provide the client with brief explanations of what is happening and the expected effects of outcomes. This may allay anxiety related to the unknown and help reduce fears concerning personal safety.
Deep vein thrombosis (DVT) can lead to ineffective tissue perfusion due to several factors. The increased blood coagulability makes it more likely for a blood clot to form in the deep veins, leading to restricted blood flow. Venous stasis, caused by reduced blood flow and muscle contractions, can also contribute to DVT, while vessel wall injury can cause inflammation and clot formation, further impeding blood flow. All these factors combined can result in ineffective tissue perfusion, which can lead to a range of complications. Healthcare providers employ various interventions to improve tissue perfusion in these patients.
1. Assess for contributing factors such as a family history of blood clots or inherited blood clotting disorders, prolonged immobility, trauma to the veins, such as from surgery , injury, or infection , use of hormonal birth control or hormone replacement therapy, obesity, sedentary lifestyle , smoking, and alcohol consumption. Most clients with DVT are asymptomatic. Knowledge of high-risk situations helps in early detection. Genetic mutations within the blood’s coagulation cascade represent those at the highest risk for the development of venous thrombosis. Genetic thrombophilia is identified in 30% of clients with idiopathic venous thrombosis. Immobility can be as transient as that occurring during a transcontinental airplane flight or an operation under general anesthesia (Patel, 2019).
2. Assess for the signs and symptoms of deep vein thrombosis (DVT). The signs and symptoms that occur in the leg affected by the deep vein clot include swelling , pain or tenderness, increased warmth, and changes in skin color (redness). Edema is the most specific symptom of DVT. Thrombus that involves the iliac bifurcation, the pelvic veins, or the vena cava produces leg edema that is usually bilateral rather than unilateral. Superficial thrombophlebitis is characterized by the finding of a palpable, indurated, cordlike, tender, subcutaneous venous segment (Patel, 2019).
3. Measure the circumference of the affected leg with a tape measure. Unilateral leg and thigh swelling can be assessed by measuring the circumference of the affected leg 10 cm below the tibial tuberosity and 10 cm to 15 cm above the upper edge of the patella. Deep vein thrombosis is suspected if there is a difference of >3 cm between the extremities. High partial obstruction often produces mild bilateral edema that is mistaken for the dependent edema of right-sided heart failure, fluid overload , or hepatic or renal insufficiency (Patel, 2019).
4. Monitor the results of diagnostic tests . See Laboratory and Diagnostic Procedures
5. Monitor the following coagulation profile: international normalized ratio (INR), prothrombin time (PT), and partial thromboplastin time (PTT) results . These are used to measure the effectiveness of anticoagulant therapy. The PT/INR is used for clients receiving warfarin . Baseline values are obtained before the first dose of anticoagulant is administered. Repeated tests are done at prescribed intervals to adjust drug dosages to achieve desired changes in coagulation. A prolonged prothrombin time or activated partial thromboplastin time does not imply a lower risk of new thrombosis. Progression of DVT and PE can occur despite full therapeutic anticoagulation in 13% of clients (Patel, 2019).
6. Assess the client’s level of pain. Leg pain occurs in 50% of clients, but this is entirely nonspecific. Pain can occur on dorsiflexion of the foot (Homan sign). Tenderness occurs in 75% of clients but is also found in 50% of clients without objectively confirmed DVT. When tenderness is present, it is usually confined to the calf muscles or along the course of the deep veins in the medial thigh (Patel, 2019).
7. Maintain adequate hydration. Hydration prevents an increased viscosity of blood, which contributes to venous stasis and clotting. A low fluid volume state can lead to hemoconcentration and low venous flow. In a prospective study, dehydration was independently linked to VTE in those clients who had previously had an acute ischemic stroke (Keiter et al., 2015).
8. Encourage bed rest and keep the affected leg elevated (depending on the size and location of the clot) as indicated. Clients usually require bed rest until symptoms are relieved. The affected leg should be elevated to a position above the heart to decrease swelling. Leg elevation is a simple intraoperative and postoperative technique for improving venous drainage from the lower extremities, which minimizes venous stasis (Keiter et al., 2015).
9. Provide warm, moist heat to the affected site. Heat promotes comfort and reduces inflammation. Vascular boot warming, also known as Rooke boot warming, helps to vasodilate the distal arterial bed, improves perfusion, raises tissue pressure, and increases venous blood return from the lower extremities, thus improving clinical outcomes for DVT. Compared to other mechanical methods, such as intermittent compression stockings, the vascular boot is relatively more comfortable to wear as it has less pressure on the heel and other bony areas (Zhang et al., 2021).
10. Apply below-knee compression stockings as prescribed. Ensure that the stockings are the correct size and are applied correctly. Compression stockings enhance circulation by providing graduated pressure on the affected leg to help return the venous blood to the heart. Inaccurately applied stockings can serve as a tourniquet and can promote clot formation. Below-the-knee elastic compression stockings (ECS) assist the calf muscle pump and reduce venous hypertension and venous valvular reflux. This reduces leg edema, aids in microcirculation, and prevents venous ischemia . Graduated compression stockings, on the other hand, with ankle pressures of 30 to 40 mm Hg reduced the incidence of PTS by 50% (Patel, 2019).
11. Administer analgesics as prescribed. Analgesics relieve pain and promote comfort . Acetaminophen is the safest pain reliever while taking an anticoagulant, but the daily dose recommended must not be exceeded. NSAIDs should be avoided for clients taking anticoagulants because they are associated with an increased risk of bleeding (The North American Thrombosis Forum, 2022).
12. Administer anticoagulants ( heparin / warfarin ) as prescribed. Treatment with anticoagulant is used primarily to prevent the formation of new clots by decreasing the normal activity of the clotting mechanism. Heparin IV or subcutaneous low-molecular-weight heparin is started initially. Oral anticoagulant therapy ( warfarin ) will be initiated while the client is still receiving heparin because the onset of action for warfarin can be up to 72 hours. Heparin will be discontinued once the warfarin reaches therapeutic levels.
13. With a massive DVT severely comprising tissue perfusion, anticipate thrombolytic therapy. Thrombolytic therapy is used only in severe embolism that significantly comprises blood flow to the tissues since they can cause sudden bleeding. For maximum effectiveness, therapy must be started soon after the onset of symptoms (within 5 days). Accordingly, careful assessment of the indications for lysis against the possibility of bleeding must be carried out before pharmacologic thrombolysis is attempted (Patel, 2019).
14. For clients who are unresponsive to anticoagulant therapy, anticipate the following surgical treatment . See Preoperative Care
15. Encourage the client to ambulate as tolerated. In Europe, early ambulation and compression have been the mainstay of adjunctive therapy for DVT. In North America, the unsubstantiated fear of dislodging clots by ambulation led clinicians to recommend bed rest and leg elevation to their clients. The authors explained that bed rest promotes venous stasis, which is a major risk factor for DVT, and therefore, may actually enhance thrombus propagation and the risk of subsequent PE (Patel, 2019).
Clients with deep vein thrombosis (DVT) can experience acute pain due to several factors. The presence of a blood clot in the affected vein can lead to diminished arterial circulation and oxygenation of tissues, causing the accumulation of lactic acid and triggering pain receptors. Additionally, the inflammation response in the affected vein can further exacerbate pain by sensitizing pain receptors and causing tissue damage. Healthcare providers employ various strategies to manage acute pain in these patients.
1. Assess the degree and characteristics of discomfort and pain. The degree of pain depends on the extent of circulatory deficit, the inflammatory process, the degree of tissue ischemia , and the extent of edema associated with thrombus development. Changes in the characteristics of pain may indicate the development of complications. Leg pain can occur in 50% of clients, but it can be entirely unspecific. Pain can occur on dorsiflexion of the foot (Homan sign) (Patel, 2019).
2. Investigate reports of sudden or sharp chest pain , accompanied by dyspnea, tachycardia, and apprehension, or the development of new pain with signs of another site of vascular involvement. These signs and symptoms suggest the presence of pulmonary embolism as a complication of DVT or peripheral arterial occlusion associated with heparin‐induced thrombocytopenia with thrombosis syndrome (HITT). Both conditions require immediate medical treatment. Pleuritic chest pain without other symptoms or risk factors may be a presentation of pulmonary embolism. Pleuritic or respirophasic chest pain is a particularly worrisome symptom. Its presence suggests that the embolus is located more peripherally and thus may be smaller (Ouellette & Mosenifar, 2020).
3. Monitor vital signs, noting increased temperature. Elevations in heart rate may indicate increased discomfort or may occur in response to fever and inflammatory processes. Fever can also increase the client’s discomfort. Fever of less than 39℃ (102.2℉) may be present in 14% of clients; however, a temperature higher than 39.5℃ (103.1℉) is not from pulmonary embolism (Ouellette & Mosenifar, 2020).
4. Maintain bed rest during the acute phase, then start early ambulation gradually as tolerated. This decreases discomfort associated with muscle contraction and movement. However, the concept of immobilizing DVT clients is outdated, since early mobilization has been demonstrated not to increase the risk of embolization, and large cohort studies have clearly proven that outpatient DVT treatment is feasible and safe for the majority of clients being diagnosed with DVT outside of hospitals. Admitted clients with DVT may be safely mobilized, provided that adequate anticoagulation is immediately initiated (Endig et al., 2016).
5. Encourage the client to change position frequently. This reduces muscle fatigue, helps minimize muscle spasms, and maximizes circulation to tissues. Position changes can help reduce pain in DVT by improving blood flow and reducing pressure on the affected vein. When the client changes their position, this helps prevent blood from pooling in the affected area and decreases the risk of further developing blood clots.
6. Provide a foot cradle. The cradle keeps the pressure of bedclothes off the affected leg, thereby reducing pressure discomfort. Additionally, a foot cradle supports the foot and the ankle in a neutral position, which can help improve blood flow and reduce the risk of further blood clots. However, this measure should be used in conjunction with other strategies to prevent DVT, such as regular exercise, position changes, and the use of compression stockings.
7. Elevate affected extremity. This encourages a venous return to facilitate circulation, reducing stasis and edema formation. Virchow’s triad states that venous stasis is a predisposing factor for DVT. Leg elevation is a simple intraoperative and postoperative technique for improving venous drainage from the lower extremities, which minimizes venous stasis (Keiter et al., 2015).
8. Apply a warm compress to the affected leg using a 2-hour-on, 2-hour-off schedule around the clock. Moist heat may be applied to the affected region to relieve pain and improve circulation through vasodilation. Heat therapy produces increased collagen extensibility, increased blood flow, metabolic rate, and inflammation resolution. Decreased joint stiffness, muscle spasm, and pain are also positive effects of heat therapy. Heat raises the pain threshold and acts directly on the muscle spindle, decreasing spindle excitability (El-Tallawy et al., 2021).
9. Teach the client non-pharmacological pain management techniques, such as deep breathing exercises, guided imagery, or relaxation techniques. These techniques can help alleviate pain and decrease the need for opioids or other pain medications, which may have adverse effects. These therapies may also help clients to feel more in control of pain management , improve overall well-being, and promote better compliance with the prescribed treatment plan.
10. Administer medications, as indicated . See Pharmacologic Management
11. Recommend the use of a vascular warming boot or Rooke boot. A study evaluated the safety, efficacy, and impact of vascular boot warming on post-thrombotic syndrome in DVT. Pain and swelling are two critical concerns for clients and are closely correlated to their quality of life. Results suggest that vascular boot warming can reduce pain and swell much faster than the standard of care, and it does not increase bleeding (Zhang et al., 2021).
12. Encourage therapeutic exercise as tolerated. When the pain decreases, mobilization should be regained gradually. The best treatment in such cases is combined gradual stretching and strengthening exercises. Client education is mandatory about a therapeutic exercise regimen at home once therapeutic sessions have ceased. Therapeutic exercise consists of passive movements, active-assistive exercises, active exercises, stretching, and relaxation exercises (El-Tallawy et al., 2021).
13. Ensure proper wearing of compression stockings. The more a DVT client is affected by pain and leg swelling, the more likely they will benefit from compression therapy (stockings or bandages), provided that the compression device is fitted correctly. Clients receiving compression therapy need to be educated on proper use and the risk of developing pressure injuries , which is a significant risk. Daily skin inspections are necessary during compression therapy (Endig et al., 2016).
Clients with deep vein thrombosis (DVT) are at risk for bleeding due to several factors. Abnormal blood profile, such as low platelet counts or coagulopathy, can increase the risk of bleeding. Additionally, anticoagulation therapy, which is commonly used to prevent new blood clots in DVT, can also increase the risk of bleeding by reducing the blood’s ability to clot. This can result in bleeding or hemorrhage , especially in patients who are also taking other medications that can further increase the risk of bleeding, such as aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs). Healthcare providers utilize several strategies to prevent bleeding and injury in these patients.
1. Assess for the signs and symptoms of bleeding. Bruises, epistaxis, and gum bleeding are early signs of spontaneous bleeding. Significant bleeding, such as hematemesis, hematuria , or GI hemorrhage should be thoroughly investigated because anticoagulant therapy may unmask a pre-existing disease like cancer , peptic ulcer disease , or an arteriovenous malformation (Patel, 2019).
2. Monitor platelet counts and coagulation test results (INR, PT, PTT). The effects of anticoagulation therapy must be closely monitored to reduce the risk of bleeding. The clotting time is the time it takes for plasma to clot after the addition of different substrates in vitro under standard conditions using the capillary method. PT/INR is the initial test used to identify defects in secondary hemostasis . It is the time taken for blood to clot and generates thrombin. A delay in the PT or aPTT indicates the presence of either a deficiency or inhibitor of the clotting factor (Umerah & Momodu, 2022).
3. Monitor platelets and the heparin-induced platelet aggregation (HIPA) status. A sudden decrease in the platelet count can occur with heparin use and is known as heparin-induced thrombocytopenia (HIT). HIT is less commonly seen with the use of low-molecular-weight heparin. HIT typically presents as a steady drop in platelet counts (no fluctuations), while hemoglobin and hematocrit counts remain relatively stable. The most common symptom of HIT is the enlargement or extension of a blood clot or the development of a new blood clot. In clients receiving IV heparin, they may experience chills, fever, hypertension , tachycardia, shortness of breath, and chest pain (Nicolas et al., 2023).
4. Administer anticoagulant therapy as prescribed (continuous IV heparin/subcutaneous low-molecular-weight heparin; oral warfarin). Anticoagulants are given to prevent further clot formation. The type of medication varies per protocol and severity of the clot. First-line therapy for non-high-risk VTE or PE consists of direct oral anticoagulants over vitamin K antagonists. There are also possibilities for advances in anticoagulant delivery systems including the expansion of new oral agents and their antidotes, reducing the size of heparins, developing oral or topical heparins, and modifying physical or chemical formulations. Ita suggests that transdermal delivery may potentially bypass known issues with heparin use (Patel, 2019).
5. If bleeding occurs while on IV heparin: terminate the infusion then recheck the PTT level stat, and reevaluate the dose of heparin based on the PTT result. Laboratory data guide further treatment. The guide for the PTT level is 1.5 to 2 times normal. Anticoagulation-related major bleeding is associated with an increased risk of death and thrombotic events, independent of the class of anticoagulant used. With the increasing use of non-vitamin K antagonists or oral anticoagulants, the number of clients who require reversal of their anticoagulant effects can be expected to rise (Patel, 2019).
6. Avoid the use of invasive procedures, such as injections or venipuncture, if possible, and use caution during any necessary procedures to minimize the risk of bleeding. If an invasive procedure is necessary, nurses should take appropriate precautions, such as using a smaller gauge needle or applying pressure to the puncture site, to reduce the risk of bleeding. The management of anticoagulation in clients undergoing surgical procedures is challenging since interrupting anticoagulation for a procedure transiently increases the risk of thromboembolism. At the same time, surgery and invasive procedures have associated bleeding risks that are increased by anticoagulants. A balance between reducing the risk of thromboembolism and preventing excessive bleeding must be reached for each client (Douketis, 2023).
7. Teach the client the importance of complying with the prescribed medication regimen and report any signs of bleeding, such as unusual bruising, nosebleeds, or blood in the stool or urine . Adhering to the medication regimen prevents the formation of new blood clots and decreases the risk of serious complications, such as pulmonary embolism. Anticoagulants need to be taken regularly and as prescribed by the physician to ensure that the blood’s clotting parameters are within the therapeutic range. Long-term anticoagulation is necessary to prevent the high frequency of recurrent venous thrombosis or thromboembolic events. Interruption of anticoagulation within the first 12 weeks of therapy appears to result in a 25% incidence of recurrent thrombosis (Schreiber & Brenner, 2020).
8. Convert from IV anticoagulation to oral anticoagulation after the appropriate length of therapy. Monitor INR, PT, and PTT levels. PT or INR levels should be in a therapeutic range for anticoagulation before discontinuing heparin. Oral vitamin K antagonists (VKAs) remain the preferred approach for long-term treatment, which allows for single-dosing oral therapy that can be continued on an outpatient basis. The American College of Chest Physicians (ACCP) recommends cessation of anticoagulant therapy after 3 months of treatment in those with surgery -associated acute proximal DVT, an acute proximal DVT or PE provoked by a nonsurgical transient risk factor, and a first unprovoked VTE and a high risk of bleeding (Schreiber & Brenner, 2020).
9. If HIPA is positive, stop all heparin products and anticipate a hematology consult. The continuation of heparin products further complicates the situation. Specialty expertise is needed. Laboratory confirmation of HIT is of crucial importance and remains challenging and relies on platelet functional assays highlighting the presence of heparin-dependent platelet-activating antibodies in the client’s serum or plasma. Platelet functional assays using washed platelets include the C-serotonin release assay (SRA), usually described as the gold standard, and HIPA (Gonthier et al., 2021).
10. Keep reversal agents for different anticoagulants within easy access. The initial step for any condition requiring urgent reversal of anticoagulation is always to discontinue the anticoagulant. Protamine sulfate counteracts the activity of unfractionated heparin. It is also indicated for bleeding in clients on LMWH, although it is not as effective as with bleeding associated with UFH. Idarucizumab is an anti-dabigatran monoclonal antibody fragment used in clients treated with dabigatran presenting with life-threatening bleeding. Andexanet alfa can be given to clients receiving apixaban, betrixaban, edoxaban, and rivaroxaban (Umerah & Momodu, 2022).
11. Avoid administering platelet transfusions to clients with confirmed HIT in the acute phase. Platelet transfusions are contraindicated during the acute phase, as transfused platelets can bind to IgG and become activated and release PF4, thus worsening the hypercoagulable state. Activated platelets release prothrombotic substances and PF4, creating a continuous cycle that can only be broken when heparin is discontinued and appropriate treatment is initiated (Nicolas et al., 2023).
Lack of knowledge can be a common issue for clients with deep vein thrombosis (DVT) due to unfamiliarity with the disease and its management. This can result in a lack of understanding about the importance of adherence to treatment and lifestyle modifications that can help prevent future occurrences of DVT. Initiating health teaching and patient education is an essential component of caring for patients with deep vein thrombosis (DVT). Education plays a vital role in empowering patients to actively participate in their own care and make informed decisions regarding their health. Healthcare providers provide comprehensive information to patients about DVT, its risk factors, signs and symptoms, and the importance of adherence to the prescribed treatment plan. Patients are educated about the rationale and potential side effects of anticoagulant medications, as well as the importance of regular monitoring and follow-up appointments.
1. Assess the client’s understanding of the causes, treatment, and prevention plan for deep vein thrombosis. This information gives an important starting point in education. DVT requires preventive effort to reduce the risk of reoccurrence. DVT is one of the most prevalent medical problems today, with an annual incidence of 80 cases per 100,000. Early recognition and appropriate treatment of DVT and its complications can save many lives (Patel, 2019).
2. Assess the following signs of pulmonary embolus such as shortness of breath, chest pain that worsens with deep breathing or coughing, palpitations, clammy skin, lightheadedness, and cough . These symptoms can be caused by a blood clot that breaks off from the original clot in the leg and travels to the lung. The challenge in dealing with pulmonary embolism is that clients rarely display the classic presentation of this problem, that is, the abrupt onset of pleuritic chest pain, shortness of breath, and hypoxia. The presentation of PE may vary from sudden catastrophic hemodynamic collapse to gradually progressive dyspnea (Ouellette & Mosenifar, 2020).
3. Instruct the client to take medications as indicated, explaining their actions, dosages, and side effects. Correct knowledge decreases future complications. Analgesics and anti-inflammatory medications are indicated for short-term symptom relief. Clients may require anticoagulation for weeks or long term, depending on the risks. The immediate symptoms of DVT often resolve with anticoagulation alone, and the rationale for intervention is often the reduction of the 75% long-term risk of PTS. Systemic IV thrombolysis is no longer recommended because of an elevated incidence of bleeding complications, a slightly increased risk of death, and insignificant improvement in PTS (Patel, 2019).
4. Inform the client of the need for regular laboratory testing while on oral anticoagulation. Routine coagulation monitoring is necessary to ensure that a therapeutic response is obtained and prevent reoccurrences of clots. For admitted clients with unfractionated heparin (UFH), the aPTT or heparin activity level must be monitored every six hours while the client is taking IV heparin until the dose is stabilized in the therapeutic range. Clients treated with LMWH or fondaparinux do not require monitoring of the aPTT (Patel, 2019).
5. Discuss and give the client a list of signs and symptoms of excessive anticoagulation. Clients need to self-manage their condition. The early assessment facilitates prompt treatment. Hemorrhagic complications are the most common adverse effects of anticoagulant therapy. Anticoagulation therapy for three to six months results in major bleeding complications. Significant bleeding, such as hematemesis, hematuria, or GI hemorrhage, should be thoroughly investigated because anticoagulant therapy may unmask a preexisting disease (Patel, 2019).
6. Provide teaching regarding the safety measures while on anticoagulant therapy such as the use of an electric razor, and the use of a soft toothbrush. These precautionary measures help reduce the risk of bleeding. The use of a soft-bristled toothbrush prevents trauma to the oral mucous membranes and the risk of bleeding from the gums. Toothpicks and dental floss should also be avoided, as they can injure the gums when used vigorously (Wayne, 2023).
7. Instruct the client to avoid rubbing or massaging the calf. This will prevent breaking off the clot, which may travel into the circulation as an embolus. Massaging lower extremities using forceful techniques and for prolonged periods where a client has been diagnosed with DVT in the leg veins is associated with complications. Massage can dislodge an already established thrombus, embolus, or blood clot and predispose the client to develop PE and cause sudden death (Behera et al., 2017).
8. Instruct the client on the correct application of compression stockings. Stockings applied inaccurately can serve as a tourniquet and promote clot formation. The incorrect use of compression stockings can be unsafe; thigh-length stockings that are fitted incorrectly or that roll down the leg can create a tourniquet effect, which can potentially damage the skin and reduce venous outflow. Additionally, one length of stocking may be more appropriate than the other in certain clients; knee-length stockings may be more likely to induce wound complications in clients undergoing knee replacement surgery as the elastic support lies over the wound, creating unwanted localized pressure (Wade et al., 2016).
9. Educate the client about the following measures to prevent reoccurrence:
- 9.1. Avoid constricting garters or socks with tight bands Wearing constricting clothing decreases normal blood flow and promotes clotting. Constricting socks or garters work by applying pressure to the lower extremities which helps to improve blood flow and prevent blood clots from forming. However, if the pressure is too high or the socks are too tight, it can restrict blood flow, increasing the risk of blood clots.
- 9.2. Avoid staying in one position for long periods; get up and move around every hour or so on a long flight. This will avoid the occurrence of venous stasis. The concept of immobilizing DVT clients is outdated, since early mobilization has been demonstrated not to increase the risk of embolization, and large cohort studies have proven that outpatient DVT treatment is feasible and safe for the majority of clients being diagnosed with DVT outside of hospitals (Endig et al., 2016).
- 9.3. Maintain adequate hydration. Sufficient hydration prevents hypercoagulability. A low-volume state can lead to hemoconcentration and low venous flow. A study found that clients who experienced VTE have elevated biochemical indices of dehydration , in comparison to clients who had not (Keiter et al., 2015).
- 9.4. Maintaining a healthy body weight Obesity contributes to venous insufficiency and venous hypertension through the compression of the main veins in the pelvic region. It has been shown that the risk of VTE increases with increasing BMI . the risk is higher when obesity interacts with other thrombotic risk factors (Hotoleanu, 2020).
- 9.5. Not sitting with the legs crossed The client should refrain from any position that promotes vein compression. When the client crosses their legs or thighs, they obstruct some of the veins in the legs, slowing down blood flow. As a result, blood can pool in the veins, which may slightly increase the risk of blood clots in the legs (Triffin & Ketchum, 2020).
- 9.6. Participating in an exercise program Walking, swimming, and cycling help promote venous return through the contraction of the calf and thigh muscles. These muscles act as a pump to compress veins and support the column of blood returning to the heart. A narrative summary of the included studies related to DVT shows that exercise sessions with a target intensity of 70% peak heart rate significantly improved cardiorespiratory fitness. Additionally, exercise training also improved leg strength and flexibility, as well as calf pump function (Xu et al., 2021).
- 9.7. Quitting smoking Cigarettes contain nicotine which is a vasoconstrictor that affects blood clotting and circulation. Researchers described through their research that the mechanism of smoking impacts on blood is very dangerous because in smokers the activities of platelet predispose them to blood clots. Platelets regulate clot formation, which is a primary cause of heart attacks (Aslam et al., 2017).
- 9.8. Wearing properly sized, correctly applied compression stockings as indicated. Clients with DVT are at high risk for redevelopment and may need to wear stockings over the long term. The regular use of graduated elastic compression stockings reduced the incidence of PTS by 50%. Authors strongly recommend the early use and widespread implementation of graduated elastic stockings with adequate anticoagulant therapy for symptomatic proximal DVT to prevent the development of PTS (Patel, 2019).
10. Explain the purpose of activity restrictions and the need for balance between activity and rest. Rest reduces the oxygen and nutrient needs of compromised tissues and decreases the risk of fragmentation of thrombosis. Balancing rest with activity prevents exhaustion and further impairment of cellular perfusion. A systematic review found that in clients with acute DVT, early walking exercise is safe and may help to reduce acute symptoms and that in clients with previous DVT, exercise training does not increase leg symptoms acutely and may help to prevent or improve the postthrombotic syndrome (Patel, 2019).
11. Educate women about the possible effect of hormonal contraceptives on the risk of developing DVT. Hormonal contraceptives are widely used throughout the world and have been associated with blood clots in the legs and lungs. Users of hormonal contraceptives have a significantly increased risk of DVT compared to non-users. Women should be informed of these risks and offered education in fertility-awareness-based methods with comparable efficacy for family planning (Keenan et al., 2019).
12. Instruct in meticulous skin care of the lower extremities. Instruct the client to prevent or promptly treat breaks in the skin and report the development of ulcers or changes in skin color. Chronic venous congestion and post-phlebitis syndrome may develop, especially in the presence of severe vascular involvement and recurrent DVT, potentiating the risk of stasis ulcers.
13. Review the client’s usual medications and foods when on oral anticoagulant therapy. Warfarin interacts with many foods and drugs either increasing or decreasing the anticoagulant effect. Salicylates and excess alcohol decrease prothrombin activity, whereas vitamin K (multivitamins, bananas, leafy green vegetables) increases prothrombin activity and can cause a higher or lower INR, possibly outside the therapeutic range. Barbiturates increase the metabolism of coumadin drugs; antibiotics alter intestinal flora and may interfere with vitamin K synthesis.
Assessing and monitoring for potential complications is an integral part of the care provided to patients with deep vein thrombosis (DVT). Healthcare providers play a crucial role in assessing and monitoring patients for these potential complications to ensure timely intervention and optimal outcomes. Through vigilant assessment, including regular evaluation of vital signs, monitoring of coagulation profiles, and examination for signs and symptoms of complications such as pulmonary embolism or post-thrombotic syndrome, healthcare providers can identify any changes or developments that may require immediate attention.
1. Assess vital signs frequently. Vital signs provide baseline data and can indicate changes in the patient’s condition. Elevated temperature, increased heart rate, and decreased blood pressure may suggest infection or other complications associated with DVT.
2. Assess and document the location, size, and characteristics of the thrombus. This information helps evaluate the severity of the clot, the potential for migration or obstruction, and the effectiveness of treatment. It also serves as a baseline for comparison in subsequent assessments.
3. Monitor and document peripheral pulses. Diminished or absent pulses distal to the clot site may indicate compromised circulation due to clot extension or obstruction. Prompt recognition of reduced peripheral pulses is crucial for early intervention.
4. Assess for signs of pulmonary embolism (PE). DVT can lead to PE, a potentially life-threatening complication. Monitor for signs such as sudden dyspnea, tachypnea , chest pain, hemoptysis, anxiety, or changes in mental status. Early detection and treatment of PE are essential.
5. Observe for signs of compartment syndrome . Extensive or massive DVT can cause compartment syndrome due to increased pressure within the affected limb. Monitor for signs such as severe pain, swelling, paresthesia, pallor, and loss of pulse distal to the clot. Timely intervention can prevent tissue damage.
6. Perform regular neurological assessments. Neurological changes may indicate complications associated with DVT, such as stroke or cerebral venous thrombosis. Assess for changes in level of consciousness, motor or sensory deficits, visual disturbances, or sudden severe headaches.
7. Monitor laboratory values. Regularly assess coagulation studies, including prothrombin time (PT), activated partial thromboplastin time (aPTT), international normalized ratio (INR), and D-dimer levels. Abnormal results may indicate the progression or resolution of DVT or the effectiveness of anticoagulant therapy.
8. Evaluate for signs of bleeding or hemorrhage. Anticoagulant therapy increases the risk of bleeding. Monitor for signs such as tachycardia, hypotension , melena, hematemesis, hematuria, ecchymosis, or bleeding from invasive lines or puncture sites. Early recognition and intervention can prevent complications.
9. Assess for skin changes and potential ulceration. Chronic DVT can cause venous stasis ulcers. Monitor for skin discoloration, edema, increased warmth, or breakdown in the affected limb. Prompt identification and management of ulcers can prevent infection and further tissue damage.
10. Educate the patient on signs and symptoms to report. Providing education empowers the patient to recognize and report any changes or symptoms promptly. Reinforce the importance of reporting pain, swelling, redness, warmth, shortness of breath, chest pain, or any other concerning symptoms.
Administering medications and providing pharmacologic support are essential components of the comprehensive management of patients with deep vein thrombosis (DVT). Pharmacologic support in patients with DVT requires close monitoring of medication effectiveness, adverse effects, and potential drug interactions. Regular laboratory assessments, including coagulation studies and complete blood counts, are essential to ensure appropriate dosing and therapeutic efficacy while minimizing the risk of bleeding complications. Nursing professionals play a crucial role in administering medications, monitoring patient response , and educating patients about the importance of adherence and potential side effects.
1. Thrombolytic agents , such as alteplase, anistreplase, reteplase, streptokinase, tenecteplase, and urokinase These agents are intended to bring about clot lysis (breakdown of the clot) and immediate normalization of venous blood flow. The use of thrombolytic medications to lyse DVT can cause intracranial bleeding, though this is infrequent, and death or impairment can result. The need should be compelling when thrombolysis is considered in a setting of known contraindications (Patel, 2019).
2. Morphine sulfate and anti-anxiety agents These are given to decrease pain or anxiety and improve the work of breathing, maximizing gas exchange. However, caution should be practiced when giving morphine because current morphine use is associated with PE in DVT clients. The risk of PE increased with augmented morphine dosage only in clients treated with morphine within the past 30 days, according to a study (Lee et al., 2014).
3. Anticoagulants The mainstay of medical therapy has been anticoagulation since the introduction of heparin in the 1930s. Other anticoagulation drugs have subsequently been added to the treatment armamentarium over the years, such as vitamin K antagonists and low-molecular-weight heparin (LMWH). Long-term coagulation is necessary to prevent the high frequency of recurrent venous thrombosis or thromboembolic events (Patel, 2019).
4. Opioid and nonopioid analgesics This relieves pain and decreases muscle tension. Opioids produce their effect by acting as agonists at opioid receptors, which are found in the brain , spinal cord , and sites outside the CNS. Most opioids have a similar spectrum of adverse effects, such as respiratory depression , sedation, nausea / vomiting , and constipation (El-Tallawy et al., 2021).
5. Antipyretics (Acetaminophen) This reduces fever and inflammation. Since NSAIDs are not recommended for clients with DVT, acetaminophen may be appropriate for short-term use. Regular monitoring for hepatotoxicity is required for clients who receive acetaminophen regularly and beyond the maximum dosage of 3 g daily (El-Tallawy et al., 2021).
Laboratory and diagnostic tests provide valuable information about the patient’s clotting status, the severity of the thrombus, and the effectiveness of treatment interventions. Monitoring laboratory and diagnostic procedures in patients with DVT allows healthcare providers to assess the effectiveness of treatment, detect potential complications, and adjust the management plan accordingly. Close collaboration between nursing professionals, laboratory personnel, and radiologists is crucial to ensure timely and accurate testing, interpretation of results, and appropriate interventions.
1. Ultrasonography Ultrasonography is currently the first-line imaging examination for DVT because of its relative ease of use, absence of irradiation or contrast material, and high sensitivity and specificity in institutions with experienced sonographers. Compression ultrasonography entails imaging the calf to the groin in the axial plane. Some protocols use gray-scale ultrasonography alone, whereas others include Doppler interrogation (Hoffer & Cho, 2022).
2. D-dimer assay D-dimer is a marker for clot lysis. This test can also be used to check the effectiveness of the treatment. D-dimer levels remain elevated in DVT for about seven days. Clients presenting late in the course, after clot organization and adherence have occurred, may have low levels of D-dimer. Current evidence strongly supports using a D-dimer assay in the setting of suspected DVT (Patel, 2019).
3. Impedance plethysmography (IPG) This test uses an inflated cuff for blocking the venous flow and monitoring the blood volume increase in the limb. In some countries, IPG has been the initial non-invasive diagnostic test of choice and is sensitive and specific for proximal vein thrombosis. However, IPG also has several limitations; among them is insensitivity for calf vein thrombosis, non-occluding proximal vein thrombus, and iliofemoral vein thrombosis above the inguinal ligament (Patel, 2019).
4. Contrast venography This test uses radiopaque contrast media injected through a foot vein to localize thrombi in the deep venous system. The criterion standard for diagnostic imaging for DVT remains venography with pedal vein cannulation, intravenous contrast injection, and serial limb radiographs (Patel, 2019).
Providing perioperative care for patients with deep vein thrombosis (DVT) requires a comprehensive and tailored approach to ensure optimal outcomes. Individualized treatment plans are essential when providing perioperative care for patients with DVT. The management approach should be based on a thorough assessment of the patient’s medical history , clot characteristics, and overall risk profile.
1. Placement of a vena cava filter The filter is inserted inside the vena cava. The filter catches blood clots before they travel to the lungs, which prevents pulmonary embolism. Inferior vena cava filters are not recommended in clients with acute VTE on anticoagulant therapy. An inferior vena cava filter is a mechanical barrier to the flow of emboli larger than 4 mm (Patel, 2019).
2. Thrombectomy The most severe cases of DVT may require the surgical removal of the blood clot from the vein (thrombectomy). Surgical thrombus removal has traditionally been used in clients with massive swelling and phlegmasia cerulea dolens. When thrombosis is extensive, fibrinolysis alone may be inadequate to dissolve the volume of the thrombus present (Patel, 2019).
3. Replacement of venous valves Percutaneously placed bioprosthetic venous valves are under development and may provide a minimally invasive therapy for the long-term complication of postthrombotic syndrome (PTS) due to valve destruction. Effective therapy should diminish one of the primary indications for aggressive thrombolytic therapy for acute DVT (Patel, 2019).
Recommended nursing diagnosis and nursing care plan books and resources.
Disclosure: Included below are affiliate links from Amazon at no additional cost from you. We may earn a small commission from your purchase. For more information, check out our privacy policy .
Ackley and Ladwig’s Nursing Diagnosis Handbook: An Evidence-Based Guide to Planning Care We love this book because of its evidence-based approach to nursing interventions. This care plan handbook uses an easy, three-step system to guide you through client assessment, nursing diagnosis, and care planning. Includes step-by-step instructions showing how to implement care and evaluate outcomes, and help you build skills in diagnostic reasoning and critical thinking.

Nursing Care Plans – Nursing Diagnosis & Intervention (10th Edition) Includes over two hundred care plans that reflect the most recent evidence-based guidelines. New to this edition are ICNP diagnoses, care plans on LGBTQ health issues, and on electrolytes and acid-base balance.

Nurse’s Pocket Guide: Diagnoses, Prioritized Interventions, and Rationales Quick-reference tool includes all you need to identify the correct diagnoses for efficient patient care planning. The sixteenth edition includes the most recent nursing diagnoses and interventions and an alphabetized listing of nursing diagnoses covering more than 400 disorders.

Nursing Diagnosis Manual: Planning, Individualizing, and Documenting Client Care Identify interventions to plan, individualize, and document care for more than 800 diseases and disorders. Only in the Nursing Diagnosis Manual will you find for each diagnosis subjectively and objectively – sample clinical applications, prioritized action/interventions with rationales – a documentation section, and much more!

All-in-One Nursing Care Planning Resource – E-Book: Medical-Surgical, Pediatric, Maternity, and Psychiatric-Mental Health Includes over 100 care plans for medical-surgical, maternity/OB, pediatrics, and psychiatric and mental health. Interprofessional “patient problems” focus familiarizes you with how to speak to patients.

Other recommended site resources for this nursing care plan:
- Nursing Care Plans (NCP): Ultimate Guide and Database MUST READ! Over 150+ nursing care plans for different diseases and conditions. Includes our easy-to-follow guide on how to create nursing care plans from scratch.
- Nursing Diagnosis Guide and List: All You Need to Know to Master Diagnosing Our comprehensive guide on how to create and write diagnostic labels. Includes detailed nursing care plan guides for common nursing diagnostic labels.
Other care plans for hematologic and lymphatic system disorders:
- Anaphylactic Shock
- Aortic Aneurysm
- Bleeding Risk & Hemophilia
- Deep Vein Thrombosis
- Disseminated Intravascular Coagulation
- Sepsis and Septicemia
- Sickle Cell Anemia Crisis
Recommended journals, books, and other interesting materials to help you learn more about deep vein thrombosis nursing care plans and nursing diagnosis:
- Aslam, S., Mirza, R., & Shaukat, H. (2017). Obesity and Smoking are Risk Factors for Deep Vein Thrombosis in general population – a comparative clinical study. Pakistan Journal of Medical & Health Sciences , 11 (4).
- Behera, C., Devassy, S., & Gupta, S. K. (2017). Leg massage by mother resulting in fatal pulmonary thromboembolism. Medico-Legal Journal , 86 (3).
- Bhutta, B. S., Alghoula, F., & Berim, I. (2022). Hypoxia – StatPearls . NCBI. Retrieved March 27, 2023.
- Douketis, J. D. (2023). Perioperative management of patients receiving anticoagulants. UpToDate .
- Doyle, G. R., & McCutcheon, J. A. (2015). Clinical Procedures for Safer Patient Care . BC Open Textbook Project.
- El-Tallawy, S. N., Nalamasu, R., Salem, G. I., LeQuang, J. A. K., Pergolizzi, J. V., & Christo, P. J. (2021). Management of Musculoskeletal Pain: An Update with Emphasis on Chronic Musculoskeletal Pain. Pain and Therapy , 10 .
- Endig, H., Michalski, F., & Beyer-Westendorf, J. (2016). Deep Vein Thrombosis – Current Management Strategies. Clinical Medicine Insights: Therapeutics .
- Gebhard, C. E., Zellweger, N., Gebhard, C., Hollinger, A., Chrobok, L., Stahli, D., Schönenberger, C. M., Todorov, A., Aschwanden, M., & Siegemund, M. (2021, December 25). Prone Positioning as a Potential Risk Factor for Deep Vein Thrombosis in COVID-19 Patients: A Hypothesis Generating Observation . NCBI. Retrieved March 27, 2023.
- Gonthier, M.-C., Gendron, N., Eloy, P., Bourrienne, M.-C., Alhenc-Gelas, M., Pouplard, C., Tardy, B., Szymezak, J., Burdet, C., Gkalea, V., Faille, D., & Ajzenberg, N. (2021, September 24). Heparin-induced Thrombocytopenia Diagnosis: A Retrospective Study Comparing Heparin-induced Platelet Activation Test to 14 C-serotonin Release Assay . NCBI. Retrieved March 29, 2023.
- Gupta, N., Zhao, Y.-Y., & Evans, C. E. (2019, September). The stimulation of thrombosis by hypoxia. Thrombosis Research , 181 .
- Hoffer, E. K., & Cho, K. J. (2022, August 15). Imaging in Deep Venous Thrombosis of the Lower Extremity: Practice Essentials, Computed Tomography, Magnetic Resonance Imaging . Medscape Reference. Retrieved March 27, 2023.
- Hotoleanu, C. (2020, April). Association between obesity and venous thromboembolism. Medicine Pharmacy Reports , 93 (2).
- Keenan, L., Kerr, T., Duane, M., & Van Gundy, K. (2019, January). Systematic Review of Hormonal Contraception and Risk of Venous Thrombosis. The Linacre Quarterly , 85 (4).
- Keiter, J. E., Johns, D., & Rockwell, W. B. (2015). Importance of Postoperative Hydration and Lower Extremity Elevation in Preventing Deep Venous Thrombosis in Full Abdominoplasty: A Report on 450 Consecutive Cases Over a 37-Year Period. Aesthetic Surgery Journal , 35 (7).
- Klaiber, U., Stephan-Paulsen, L. M., Bruckner, T., Müller, G., Auer, S., Farrenkopf, I., Fink, C., Dörr-Harim, C., Diener, M. K., Büchler, M. W., & Knebel, P. (2018). Impact of preoperative patient education on the prevention of postoperative complications after major visceral surgery: the cluster randomized controlled PEDUCAT trial. Trials , 19 (288).
- Lee, C. W.-S., Muo, C.-H., Liang, J.-A., Sung, F.-C., Kao, C.-H., & Yeh, J.-J. (2014, March). Pulmonary embolism is associated with current morphine treatment in patients with deep vein thrombosis. The Clinical Respiratory Journal , 9 (2).
- Nicolas, D., Nicolas, S., Hodgens, A., & Reed, M. (2023, March). Heparin Induced Thrombocytopenia – StatPearls . NCBI. Retrieved March 29, 2023, from
- The North American Thrombosis Forum. (2022, February 17). Patient Pulse: Pain Relievers and Anticoagulation – What’s the Story? North American Thrombosis Forum. Retrieved March 27, 2023.
- Ouellette, D. R., & Mosenifar, Z. (2020, September 18). Pulmonary Embolism (PE) Clinical Presentation: History, Physical Examination, Complications . Medscape Reference. Retrieved March 28, 2023.
- Patel, K. (2019, June 5). Deep Venous Thrombosis (DVT): Practice Essentials, Background, Anatomy . Medscape Reference. Retrieved March 27, 2023.
- Schick, M. (2023, January 19). Deep Vein Thrombosis – StatPearls . NCBI. Retrieved March 27, 2023.
- Schreiber, D., & Brenner, B. E. (2020, October 30). Anticoagulation in Deep Venous Thrombosis: Advantages of Anticoagulant Therapy, Initial Anticoagulation Therapy, Long-Term Anticoagulation . Medscape Reference. Retrieved March 29, 2023.
- Sehgal, I. S., Dhooria, S., Agarwal, R., & Behera, D. (2017, December 30). Use of a Flexible Cryoprobe for Removal of Tracheobronchial Blood Clots . Respiratory Care. Retrieved March 27, 2023.
- Triffin, M., & Ketchum, D. (2020, May 18). Is Sitting With Your Legs Crossed Bad for You? Here’s What to Know | livestrong. Livestrong.com .
- Umerah, C. O., & Momodu, I. I. (2022, July). Anticoagulation – StatPearls . NCBI. Retrieved March 29, 2023.
- Unver, S., Kivanc, G., & Aiptekin, H. M. (2018). Deep breathing exercise education receiving and performing status of patients undergoing abdominal surgery . NCBI. Retrieved March 27, 2023.
- Vyas, V., & Goyal, A. (2022). Acute Pulmonary Embolism – StatPearls . NCBI. Retrieved March 27, 2023.
- Wade, R., Paton, F., & Woolacott, N. (2016). Systematic review of patient preference and adherence to the correct use of graduated compression stockings to prevent deep vein thrombosis in surgical patients. Journal of Advanced Nursing , 73 (2).
- Wayne, G. (2023, March 18). Risk for Bleeding – Nursing Diagnosis & Care Plan . Nurseslabs. Retrieved March 28, 2023.
- Xu, L., Fu, C., Zhang, Q., He, C., & Wei, Q. (2021). The effectiveness of exercise training in treating venous thromboembolism: a systematic review. The Physician and Sportsmedicine , 49 (1).
- Yu, A., Ding, W., Lin, W., Cai, J., & Huang, W. (2022, January). Application of pulmonary rehabilitation in patients with pulmonary embolism (Review). Experimental and Therapeutic Medicine , 23 (1).
- Zhang, Y., Jin, J., Song, B., Wang, Y., & Liang, M. (2021, April 15). Vascular boot warming improves clinical outcomes of patients with deep vein thrombosis in lower extremities . NCBI. Retrieved March 27, 2023.
Leave a Comment Cancel reply
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
Deep vein thrombosis
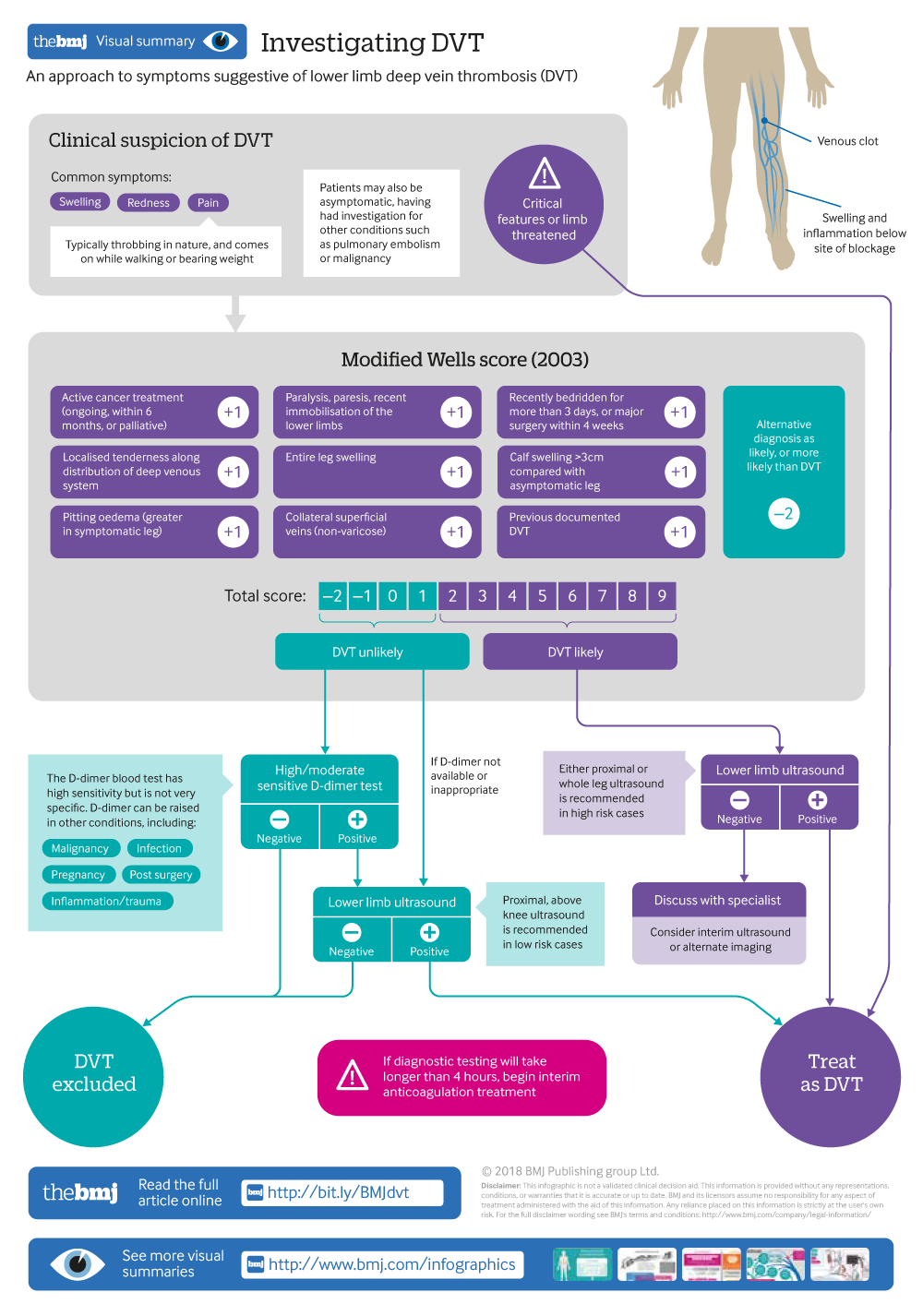
Investigating DVT
An approach to symptoms suggestive of lower deep vein thrombosis (DVT)
- Related content
- Peer review
This article has a correction. Please see:
- Deep vein thrombosis - March 21, 2018
- M J Stubbs , clinical research fellow and haematology registrar 1 ,
- Maria Mouyis , consultant rheumatologist 2 ,
- Mari Thomas , consultant haematologist 1
- 1 University College London Hospital, London, UK
- 2 North West London Hospitals NHS Trust, London, UK
- Correspondence to M Stubbs m.stubbs{at}doctors.org.uk
What you need to know
Pain, swelling, and redness of the affected limb are common symptoms of deep vein thrombosis (DVT)
Assess patients’ clinical risk of DVT using the Wells score
Refer urgently patients with suspected DVT for D-dimer test and/or proximal leg ultrasound
Anticoagulation to prevent clot extension and embolisation is initiated in secondary care, ideally within four hours of presentation
A direct oral anticoagulant is now first line for anticoagulation in patients with DVT not associated with cancer
Deep vein thrombosis (DVT) commonly affects the lower limb, with clot formation beginning in a deep calf vein and propagating proximally. 1 It is a common venous thromboembolic (VTE) disorder with an incidence of nearly 1.6 per 1000 inhabitants a year. 2 3 4 The rate of involvement of particular sites varies: distal veins 40%, popliteal 16%, femoral 20%, common femoral 20%, and iliac veins 4%. 1 Certain medical conditions listed in box 1 increase the likelihood of clot formation in the deep veins. Upper limb DVT represents less than 10% of all DVT, and central venous catheters are the main risk factor. 7 Venocaval thromboses are rare and are associated with malignancy, compression, and vascular abnormalities. 8 This article provides an overview for non-specialists on initial approach to patients with suspected DVT.
DVT risk factors 5 6
Transient risk factors.
Surgery with general anaesthetic (increased if >30 minutes)*
Hospitalisation (increased if >3 days with “bed rest”)*
Caesarean section*
Oestrogen therapy
Pregnancy or puerperium
Leg injury with reduced mobility for at least three days
Persistent risk factors
Active cancer
Medical condition with increased risk of recurrent VTE (inflammatory bowel disease, systemic lupus erythematosus)
Unprovoked VTE
If the above “Transient” and “Persistent” criteria are not met
*10 fold increase in VTE risk
Sources and selection criteria
We searched Medline and Cochrane databases for clinical trials, systematic reviews, and meta-analyses relevant to the diagnosis and management of DVT. Search terms included “deep vein thrombosis,” …
Log in using your username and password
BMA Member Log In
If you have a subscription to The BMJ, log in:
- Need to activate
- Log in via institution
- Log in via OpenAthens
Log in through your institution
Subscribe from £184 *.
Subscribe and get access to all BMJ articles, and much more.
* For online subscription
Access this article for 1 day for: £50 / $60/ €56 ( excludes VAT )
You can download a PDF version for your personal record.
Buy this article

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
Case Study #8: Deep Vein Thrombosis (DVT)
Health Case Studies Copyright © 2017 by BCIT is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License , except where otherwise noted.
Share This Book
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Risk factors associated with deep venous thrombosis in patients with different bed-rest durations: A multi-institutional case-control study
Affiliations.
- 1 Chinese Academy of Medical Sciences, Peking Union Medical College, Peking Union Medical College Hospital, 1 Shuaifuyuan, Dongcheng District, Beijing, China. Electronic address: [email protected].
- 2 Chinese Academy of Medical Sciences, Peking Union Medical College, Peking Union Medical College Hospital, 1 Shuaifuyuan, Dongcheng District, Beijing, China. Electronic address: [email protected].
- 3 Chinese Academy of Medical Sciences, Peking Union Medical College, Peking Union Medical College Hospital, 1 Shuaifuyuan, Dongcheng District, Beijing, China.
- 4 Department of Nursing, Henan Provincial People's Hospital, Zhengzhou, Henan, China.
- 5 Department of Nursing, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China.
- 6 Department of Nursing, Wuhan Union Hospital, Wuhan, Hubei, China.
- 7 Department of Nursing, Sichuan Provincial People's Hospital, Chengdu, Sichuan, China.
- 8 Department of Nursing, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China.
- 9 Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences and School of Basic Medicine, Beijing, China.
- 10 Chinese Academy of Medical Sciences, Peking Union Medical College, Peking Union Medical College Hospital, 1 Shuaifuyuan, Dongcheng District, Beijing, China. Electronic address: [email protected].
- PMID: 33352438
- DOI: 10.1016/j.ijnurstu.2020.103825
Background: Deep vein thrombosis represents a threat to public health and a heavy economic burden to society, and often occurs as a complication or cause of death in bedridden patients. How to prevent deep vein thrombosis is a general concern in clinical practice. However, it remains uncertain whether the risk factors for deep vein thrombosis would be affected by different bed-rest durations. Solving this issue will be invaluable for the provision of more rational medical care to prevent deep vein thrombosis.
Objective: To explore whether risk factors for deep vein thrombosis are affected by bed-rest durations and to identify different risk factors in groups with different bed-rest durations.
Design: A retrospective multicenter case-control study.
Settings and participants: This multicenter study was conducted in wards with high rates of bed rest in 25 general hospitals in China. Participants were bedridden patients from these wards.
Methods: Bedridden patients were identified from the research database of bedridden patients' major immobility complications. These data were collected from prospective descriptive studies by a standardized web-based online case report form. Cases were defined as bedridden patients who suffered deep vein thrombosis during hospitalization (n=186). Each case was matched with three controls, bedridden patients who did not suffer deep vein thrombosis in the same center with the same bed-rest duration (n=558). Descriptive statistics, univariate analysis, and multivariate conditional logistic regression models were employed.
Results: Among 23,985 patients, the overall incidence of deep vein thrombosis during hospitalization was 1.0%. Multivariate analysis showed that for patients with bed-rest duration of 4 weeks or less, older age (odds ratio [OR] =1.027, 95% confidence interval [CI] 1.013-1.041) and being in a surgical department (OR=2.527, 95% CI 1.541-4.144) were significantly associated with increased risk of deep vein thrombosis. Female sex (OR=4.270, 95% CI 1.227-14.862), smoking (OR=10.860, 95% CI 2.130-55.370), and special treatment (OR=3.455, 95% CI 1.006-11.869) were independent factors predicting deep vein thrombosis for patients with bed-rest durations from 5 to 8 weeks. For those with bed-rest durations from 9 to 13 weeks, Charlson Comorbidity Index (OR=1.612, 95% CI 1.090-2.385) was the only independent risk factor for deep vein thrombosis.
Conclusions: Risk factors for deep vein thrombosis varied among patients with different bed-rest durations. This finding is helpful for nurses to increase their awareness of prevention of deep vein thrombosis in patients with different bed-rest durations, and lays a more solid foundation for clinical decision making.
Keywords: Bed rest; Case-control study; Deep vein thrombosis; Lower limb; Risk factor.
Copyright © 2020. Published by Elsevier Ltd.
PubMed Disclaimer
Conflict of interest statement
Declaration of Competing Interest On behalf of all authors, the corresponding author states that there is no conflict of interest. No prior presentation.
- Comment on Cao J et al. (2021) 'Managing multiple variables relating deep venous thrombosis'. Ito H. Ito H. Int J Nurs Stud. 2022 Apr;128:104113. doi: 10.1016/j.ijnurstu.2021.104113. Epub 2021 Oct 16. Int J Nurs Stud. 2022. PMID: 34774332 No abstract available.
Similar articles
- Nursing resources and major immobility complications among bedridden patients: A multicenter descriptive study in China. Li J, Wu X, Li Z, Zhou X, Cao J, Jia Z, Wan X, Jiao J, Liu G, Liu Y, Li F, Song B, Jin J, Liu Y, Wen X, Cheng S. Li J, et al. J Nurs Manag. 2019 Jul;27(5):930-938. doi: 10.1111/jonm.12731. Epub 2019 May 31. J Nurs Manag. 2019. PMID: 30422361
- [Incidence and risk factor analysis of deep venous thrombosis in patients with severe traumatic brain injury]. Yang T, Wei G, Zhu C, Pan A. Yang T, et al. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019 Feb;31(2):182-186. doi: 10.3760/cma.j.issn.2095-4352.2019.02.012. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019. PMID: 30827306 Chinese.
- Nurses' knowledge and attitudes regarding major immobility complications among bedridden patients: A prospective multicentre study. Li Z, Zhou X, Cao J, Li Z, Wan X, Li J, Jiao J, Liu G, Liu Y, Li F, Song B, Jin J, Liu Y, Wen X, Cheng S, Wu X. Li Z, et al. J Clin Nurs. 2018 May;27(9-10):1969-1980. doi: 10.1111/jocn.14339. J Clin Nurs. 2018. PMID: 29546731
- Deep vein thrombosis in hospitalized patients: a review of evidence-based guidelines for prevention. Kehl-Pruett W. Kehl-Pruett W. Dimens Crit Care Nurs. 2006 Mar-Apr;25(2):53-9, quiz 60-1. doi: 10.1097/00003465-200603000-00001. Dimens Crit Care Nurs. 2006. PMID: 16552270 Review.
- [Therapy and prevention of deep venous thrombosis and pulmonary embolism in gynecology and obstetrics]. Vucić N, Cala K, Rancić I, Pticar R. Vucić N, et al. Acta Med Croatica. 2003;57(2):123-30. Acta Med Croatica. 2003. PMID: 12879692 Review. Croatian.
- Prevalence, severity, and risk factors of cancer-related fatigue among working cancer survivors: a systematic review and meta-analysis. Matsunaga M, He Y, Khine MT, Shi X, Okegawa R, Li Y, Yatsuya H, Ota A. Matsunaga M, et al. J Cancer Surviv. 2024 Feb 28. doi: 10.1007/s11764-024-01557-8. Online ahead of print. J Cancer Surviv. 2024. PMID: 38418754 Review.
- Nomogram for predicting postoperative deep vein thrombosis in patients with spinal fractures caused by high-energy injuries. Lv B, Wang H, Zhang Z, Li W, Han G, Liu X, Zhang C. Lv B, et al. Arch Orthop Trauma Surg. 2024 Jan;144(1):171-177. doi: 10.1007/s00402-023-05085-5. Epub 2023 Oct 4. Arch Orthop Trauma Surg. 2024. PMID: 37792059
- A Case Report: Multifocal Necrotizing Encephalitis and Myocarditis after BNT162b2 mRNA Vaccination against COVID-19. Mörz M. Mörz M. Vaccines (Basel). 2022 Oct 1;10(10):1651. doi: 10.3390/vaccines10101651. Vaccines (Basel). 2022. PMID: 36298516 Free PMC article.
- Effective prediction model for preventing postoperative deep vein thrombosis during bladder cancer treatment. Liu X, Xu A, Huang J, Shen H, Liu Y. Liu X, et al. J Int Med Res. 2022 Jan;50(1):3000605211067688. doi: 10.1177/03000605211067688. J Int Med Res. 2022. PMID: 34986677 Free PMC article.
- The burden of boundedness and the implication for nursing: A scoping review. Schirghuber J, Schrems B. Schirghuber J, et al. Nurs Forum. 2021 Oct;56(4):950-970. doi: 10.1111/nuf.12637. Epub 2021 Jul 26. Nurs Forum. 2021. PMID: 34312866 Free PMC article. Review.
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Elsevier Science
Other Literature Sources
- scite Smart Citations
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Subscribe to journal Subscribe
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Case Report
Lower extremity deep vein thrombosis following an intense calf workout.
Yim, Eugene S. MD, MPH 1 ; Friedberg, Ryan P. MD 2,3
1 Harvard Affiliated Emergency Medicine Residency, Beth Israel Deaconess Medical Center, Boston, MA; 2 Department of Emergency Medicine, Beth Israel Deaconess Medical Center, Boston, MA; and 3 Department of Orthopedic Surgery, Harvard Medical School, Boston, MA
Address for correspondence: Eugene S. Yim, MD, MPH, Emergency Medicine, Beth Israel Deaconess Medical Center, W-CC 2330, Brookline Ave, Boston, MA 02215; E-mail: [email protected] .
We report a case of a high-performance athlete with hemoglobin SC who presented with asymmetric calf soreness after an intense calf workout. By ultrasonography, he was diagnosed with a deep vein thrombosis (DVT) of his right calf. Subsequently he presented with a number of sequelae of sickle cell disease: acute chest syndrome, avascular necrosis of the hips, and chronic kidney disease. The case is instructive as an example of DVT after exercise of the lower extremities, which has not been documented well. The case also illustrates a number of health sequelae of sickle cell disease that mimic more common musculoskeletal complaints. Sports medicine providers will have to consider these uncommon but profound diagnostic entities when caring for athletes with sickle cell disease. The case further highlights how research can inform the clinical decisions and policies aimed at reducing the risk of life-threatening and lifelong sequelae of sickle cell disease in athletes.
Introduction
In 2010, the National Collegiate Athletic Association mandated testing for sickle cell trait in all athletes planning to participate in division I sports ( 18 ). Concerned with the implications of such a policy on the health, safety, and privacy of competitive athletes, the Sickle Cell Disease Association of America and the National Athletic Trainers’ Association put forth consensus statements to direct the implementation of these guidelines ( 17,23 ).
The origin of these efforts can be traced back to a handful of tragic health outcomes and resultant medicolegal disputes revolving around athletes with sickle cell trait, the carrier state of sickle cell disease ( 3,19,22 ). A number of cases of sudden death have been documented in athletes with sickle cell trait. In a recent case report, a 19-year-old football player with sickle cell trait developed a fatal case of rhabdomyolysis minutes after running 16 successive 100-yd sprints ( 1 ). Seven cases of fatal exertional rhabdomyolysis associated with sickle cell trait were documented in a review of nontraumatic causes of sports-related death in athletes ( 26 ). In such cases, sickle cell trait increases the risk of rhabdomyolysis, which may be triggered by periods of intense dehydration or hyperthermia produced during exercise ( 5 ). As a result of such tragic cases and the resultant policy changes, the sports medicine community has redirected its attention to the relevant health sequelae of sickle cell trait and sickle cell disease in athletes.
Approximately 5.2% of the global population has at least one abnormal variant of hemoglobin, with an especially high prevalence (18.2%) among African populations ( 14 ). The aforementioned policies most directly are related to athletes with sickle cell trait, a carrier state in which an individual possesses only one allele for an abnormal form of hemoglobin — HgS. The HgS allele produces an abnormal beta-globin chain of hemoglobin that is poorly soluble in environments with low oxygen content ( 4 ). Having one abnormal HgS allele and one normal hemoglobin allele leads to the carrier state, sickle cell trait. In contrast, sickle cell disease is composed of different permutations of abnormal forms of hemoglobin, such as HgS. Having two HgS alleles, for example, leads to one form of sickle cell disease — sickle cell SS. There is yet another abnormal form of hemoglobin (HgC) that can be combined in various permutations with normal hemoglobin and HgS to produce even more abnormal phenotypes. In sickle cell SC, a form of sickle cell disease, the abnormal variant HgC is combined with HgS to produce a disease phenotype. In total, there are roughly 16 different variations of sickle cell disease that span a spectrum of disease severity ( 21 ).
The spectrum of disease ranges from asymptomatic to life-threatening complications, such as myocardial infarction and stroke ( 11,27 ). These manifestations are the result of vaso-occlusive crises that ensue when abnormal variants of hemoglobin sickle in blood are exposed to low oxygen levels and inflammation ( 9 ). Individuals with sickle cell disease are prone to painful crises when sickling of abnormal cells causes entrapment and occlusion of microvasculature in the chest (acute chest syndrome) or other body parts ( 10 ). Recent evidence suggests that even with the most benign genotype, sickle cell trait, there is increased risk of exercise-related rhabdomyolysis, deep venous thrombosis (DVT), and kidney disease ( 6,8,12 ).
The evidence for life-threatening sequelae and exercise-related adverse events highlights the relevance of sickle cell trait and disease to the sports medicine community and lends credence to the screening and testing programs that have been implemented in athletes. Understandably, the life-threatening sequelae have received most of the attention in the literature thus far, while the subacute and chronic sequelae have received less. These complications pose a challenge to sports medicine practitioners, since they are composed of clinically significant disease processes that can mimic more benign pathology seen commonly in athletes. This case study illustrates many of the relevant disease processes in sickle cell disease and also documents a possible connection between exercise and one of these clinical entities in a high-performance athlete.
Case Presentation
A 50-year-old African American athlete, who was a former college football player, presented to our emergency room with asymmetric lower leg pain after an intense calf workout. The athlete engaged in competitive sports since the age of 15 years and played as a safety for the football team at the University of Southern California. He also played football competitively through his 30s, as a member of a semipro team. He continued his active lifestyle thereafter with weight lifting, giving special attention to strength conditioning of his lower body musculature. At his peak, he was able to lift greater than 1,000 lb in multiple repetitions with seated leg press.
At time of presentation to our emergency department, the athlete was known to have a history of type 2 diabetes, hypertension, chronic kidney disease, and gout. The patient also recalled a history of what he thought was “sickle cell trait,” reportedly diagnosed when he immigrated to the States at the age of 5 years. He was screened for sickle cell trait at that time because his mother and sister both had sickle cell disease. From his recollection, he was only a carrier and did not have the full disease genotype.
As an athlete with sickle cell trait, the patient did not have any medical limitations to his physical activity throughout his career as a football player. He did not suffer from any hospitalizations or medical complications as a result of his high performance activity. At no point did the athlete feel that his genetic condition limited his ability to perform.
A couple of weeks prior to presenting to our emergency room with asymmetric calf pain, the athlete had more than doubled the weight used in his calf raise exercises. After this intense work out, the athlete immediately felt sore in his calves and thought he had overworked his muscles. When his symptoms had not resolved after a couple of weeks, he decided to seek care in our emergency room. He particularly was concerned because his soreness was mainly in the right calf rather than in both calves.
In the emergency room, the athlete was noted to have tenderness along the right calf with no palpable cords. He had good strength and range of motion of his lower leg but demonstrated pain when flexing his right calf muscle with plantarflexion of the foot. He denied long distance travel, immobilization, surgery, smoking, or prior malignancy. His vitals signs were unremarkable, with no tachycardia or respiratory distress. Routine laboratory tests were not drawn for the patient at that time. A lower extremity venous ultrasound examination was performed and revealed a clot in his right peroneal and posterior tibial veins. To thin his blood and prevent further clotting, the patient was started on enoxaparin and bridged to warfarin, which was continued for 6 months.
In the ensuing months after presenting with DVT, the patient returned to the emergency room six times for a variety of complaints, including chest pain, hip pain, and shoulder pain. In working up the patient’s chest pain, pulmonary embolism was a primary concern given the patient’s history of DVT. Due to the patient’s impaired renal function from chronic kidney disease, ventilation-perfusion (V-Q) lung scans were performed to evaluate for pulmonary embolism rather than spiral computed tomography (CT) with intravenous contrast. On three separate occasions, the patient received V-Q lung scans that showed very low likelihood of pulmonary embolism. To evaluate for a possible cardiogenic cause of his chest pain, the patient also received a cardiac stress test. This test showed no evidence of cardiac ischemia.
The patient’s chest pain was evaluated further with noncontrast chest CT, which showed puzzling parenchymal opacities on the side of the chest corresponding to his pain. These abnormalities appeared first in the right lower and middle lobes and subsequently in the middle and upper lobes of the right lung ( Fig. 1 ). Although these radiological findings were read initially as likely infectious or inflammatory in etiology, upon retrospective review with a staff radiologist, they were interpreted as being consistent with acute chest syndrome.

In evaluating the patient’s hip pain, recurrent DVT was a primary concern given the patient’s history. To evaluate this, repeat lower extremity ultrasounds were performed to evaluate for thrombosis in the proximal veins of the lower extremities. These ultrasound studies did not show evidence of recurrent thrombosis. The source of the patient’s hip pain was revealed eventually with magnetic resonance imaging (MRI), which demonstrated avascular necrosis of both femoral heads ( Fig. 2 ).

While off warfarin, the athlete also presented on one occasion with left-sided shoulder and upper arm pain that occurred 14 h after heavy bench pressing exercise. A rotator cuff tear was suspected in the emergency room, and the patient was referred to a sports medicine specialist for further evaluation. The patient did not follow up, however, since the pain resolved spontaneously before he saw a specialist.
During the athlete’s most recent admission, our clinical team investigated a possible unifying diagnosis to bring together the multiple complaints the patient had experienced. We honed in on the patient’s purported status as a carrier for sickle cell. A previous hemoglobin electrophoresis test performed decades earlier demonstrated 52% HgS and 48% HgC . This was consistent with a more severe form of sickle cell disease called hemoglobin SC rather than simple sickle cell trait.
After this hospitalization, the athlete finally was referred to a hematologist for ongoing care for his hematologic condition. He was started recently on folic acid supplementation to support the high level of red blood cell production (erythropoiesis) required to replace cells lost in his sickling episodes. He was instructed also to discontinue the use of glucocorticoids, which previously had been used to treat his gout, but were likely accelerating the progression of his hip avascular necrosis.
This athlete with hemoglobin SC presented with many characteristic sequelae of sickle cell disease that have been documented in the literature: DVT, acute chest syndrome, avascular necrosis of the hip, and chronic kidney disease. This case illustrates relevant clinical sequelae that sports medicine practitioners need to keep in mind when caring for athletes with sickle cell disease. This is especially important since these clinical entities specific to sickle cell disease can mimic common clinical complaints with more benign etiologies ( Table ). Chest pain, calf pain, shoulder pain, and hip pain can all be symptoms associated with acute chest syndrome, DVT, and avascular necrosis.

These clinical sequelae develop over a subacute to chronic time course, and sports medicine practitioners have the opportunity to catch these problems before they lead to significant morbidity. This can occur in the form of chronic pulmonary, vascular, and musculoskeletal complications in this population of athletes. Repetitive episodes of acute chest syndrome, for example, can lead to significant scarring and reduction in pulmonary function. Recurrent thromboses require lifelong anticoagulation, which comes with the risk of life threatening hemorrhage from minor trauma. Avascular necrosis causes destruction of the hip joints and can lead to functional impairment with chronic pain. With a better awareness of these sequelae of sickle cell in athletes, sports medicine providers will have an appropriately broad differential when caring for their athletes with sickle cell.
This case also reveals some of the unanswered questions that remain with regard to athletes with sickle cell. One specific question that arises from the case is whether there are relevant exercise-related risk factors for DVT in athletes with sickle cell. In this report, an athlete presented with a DVT associated with antecedent exercise of the muscles in the area affected by clot. Other risk factors for DVT — such as surgery, long travel, smoking, malignancy, hemoconcentration, and polycythemia — were absent.
Although sickle cell alone affords an increased risk of clot, exercise could possibly increase that risk through a mechanism associated with microvascular injury to the endothelial wall through sheer forces generated through flexion and stretching of muscle, consistent with the traditional association of vessel injury to thrombosis, through Virchow’s triad ( 2 ). Alternatively, external compression of the affected veins from hypertrophic musculofascial bands or anatomic variants could confer the risk. Also possible is a mechanism whereby deoxygenation of tissue during exercise increases risk of thrombosis through molecular changes to plasma adhesions molecules ( 15 ). These molecular changes to plasma adhesion molecules could increase risk of thrombosis and clot in the setting of exercise.
Dehydration also may play a role. In addition to examining the broader relationship between exercise and DVT formation, the more specific role of dehydration also demands further investigation. Hemoconcentration and polycythemia vera are well-known risk factors for the formation of DVT in athletes ( 16 ). The hemoconcentrated state of the dehydrated athlete thus may confer an increased risk of DVT formation during and after intense exercise. Furthermore, medications causing increased urine output (including commonly prescribed diuretics such as furosemide and hydrochlorothiazide) may increase the risk of clot formation by depleting intravascular volume. High urine output, as would occur in the diabetic athlete with polyuria, also similarly would increase risk. This line of research underscores the importance of proper hydration and fluid balance in athletes with sickle cell disease.
The possibility of exercise-induced DVT as a clinical entity in patients with sickle cell disease is plausible considering previous documentation of exercise-induced thrombosis in patients without clotting disorders. In the so-called subclavian vein effort-induced thrombosis (Paget-Schroetter syndrome), patients without underlying coagulopathies have presented with thrombosis of upper extremity veins with exercise ( 7,13,24,25 ). In our case, the athlete presented on one occasion with upper extremity pain after intense bench pressing exercise. This could have been due to an undetected episode of exercise-induced upper extremity thrombosis.
Further investigation and research will be helpful in elucidating the connection between exercise and DVT formation in athletes with sickle cell disease in order to confirm this relationship and to guide practitioners on how they can reduce risk of DVT in this population. Establishing a causal relationship between exercise and DVT in athletes with sickle cell disease would be the first step. This report is limited because it represents a single case; further research with case series, retrospective analyses, and ultimately prospective studies would be instructive.
Investigation of the causal pathophysiologic mechanism connecting increased risk of DVT with exercise in patients with sickle cell disease is also necessary to better delineate whether vascular injury, external compression, deoxygenation, dehydration, or another mechanism is responsible for increased risk of thrombosis. This will be especially helpful in later translating this research into practical recommendations that can inform existing guidelines regarding how sports medicine practitioners design exercise regimens for athletes with sickle cell trait and disease.
Of course, these guidelines should be individualized depending on the disease severity of each athlete. Current guidelines stipulate that “athletes need individual assessment” and that “if illness permits, all but high exertion, collision, and contact sports may be played.” They further state that “overheating, dehydration, and chilling must be avoided” ( 20 ). Confirmatory research will be required to substantiate these recommendations and will inform also the individual recommendations that are made for each athlete based on more general guidelines. In the case presented, for example, the athlete may have ramped up his weight lifting too quickly. The patient also may have not been hydrated adequately or may have been on medications, such as diuretics, which were depleting his intravascular volume. Based on confirmatory research, athletic trainers justifiably would be able to recommend conservatively graded weight increases and optimal hydration in such athletes.
This case also touches upon relevant questions surrounding the recently implemented screening programs that have drawn attention in the sports medicine community. On the one hand, the case demonstrates how athletes with sickle cell disease can participate safely in competitive sports with no serious health outcomes or perceived limitations to performance. The athlete in this case successfully played in division I football without any adverse health events or subjective limitations to physical performance. Moreover, the athlete had a more severe form of sickle cell than the athletes that would be identified through present screening programs. This may support the view of those who claim that athletes with sickle cell disease are safe to participate in competitive sports and that screening programs are nonessential. However, in counterargument, the rare but life-threatening complications that can occur in this population support the need for such screening programs.
Disagreement regarding the need for such screening programs will continue invariably, but in the meantime, research can contribute to the understanding of the health sequelae of sickle cell trait and disease in athletes. By doing so, research can inform how sports medicine practitioners care for and respond to athletes who are identified through screening. Although initial concern has focused on the life-threatening complications that drove the inception of these screening programs in the first place, this case illustrates how the long-term health sequelae in these athletes are just as important to investigate and better understand.
Sickle cell disease is a condition that has both immediate life-threatening health threats as well as longer-term, subacute, and chronic medical sequelae that are relevant to athletes. Screening programs have been implemented in college athletics to help identify individuals with this condition, primarily in hopes of reducing the risk of life-threatening adverse health events. These programs have helped focus the attention of sports medicine providers on the life-threatening health sequelae of this genetic condition. However, equal attention should be directed toward the longer-term chronic medical complications of sickle cell that pose a lifelong threat to these athletes. These clinical entities pose a diagnostic challenge to sports medicine practitioners, because they mimic common clinical complaints with more benign etiologies in the general population. Adequate understanding of the sequelae of sickle cell is thus essential for sports medicine practitioners to appropriately care for athletes with sickle cell. A better understanding through research of the disease as it relates to sports and exercise will be instrumental in reducing the risk of both immediate and lifelong health sequelae that threaten the athlete with sickle cell.
The authors would like to thank Drs. Jonathan Edlow and Leon Adelman from the Department of Emergency Medicine as well as Drs. Roger Yu and Rebecca Karp from the Department of Internal Medicine for their efforts providing clinical care to the patient while in the hospital.
The authors declare no conflict of interest and do not have any financial disclosures.
- + Favorites
- View in Gallery
Readers Of this Article Also Read
Exercise collapse associated with sickle cell trait (ecast): case report and..., abdominal pain in a marathon runner, thyroid disorders in athletes, glutamine: the nonessential amino acid for performance enhancement, sports medicine pearls and pitfalls: pearls on the power of placebo.
Deep vein thrombosis
- Overview
- Theory
- Diagnosis
- Management
- Follow up
- Resources
When viewing this topic in a different language, you may notice some differences in the way the content is structured, but it still reflects the latest evidence-based guidance.
Deep vein thrombosis (DVT) is the development of a blood clot within a vein deep to the muscular tissue planes.
Patients who develop DVT commonly have risk factors, such as active cancer, trauma, major surgery, hospitalisation, immobilisation, pregnancy, or oral contraceptive use. DVT may also be unprovoked (idiopathic) and occur in the absence of any identifiable extrinsic risk factors.
DVTs commonly cause asymmetrical leg swelling, unilateral leg pain, dilation or distension of superficial veins, and red or discolored skin, but can also be asymptomatic.
Assessment of pre-test probability (using a validated score such as Wells) is key if DVT is suspected, and should be used in combination with an algorithmic diagnostic approach to avoid unnecessary imaging when the likelihood of DVT is low.
Diagnosis requires confirmation of a blood clot in a deep vein in the leg, pelvis, or vena cava by venous ultrasound imaging (or other imaging techniques such as computed tomography scan).
DVT is usually treated with anticoagulants such as unfractionated heparin, low molecular weight heparin, fondaparinux, rivaroxaban, apixaban, edoxaban, dabigatran, and/or warfarin. Interventional therapies, including thrombolysis, are rarely indicated.
Generally, anticoagulant therapy for at least 3 months is required for patients with DVT. Thereafter, continued anticoagulant therapy for secondary prevention is indicated in selected patients to reduce the risk of recurrent events.
Post-thrombotic syndrome may occur with symptoms of chronic pain, swelling, skin discoloration, or venous ulcers following chronic obstruction of venous outflow and/or incompetence of venous valves.
DVT is the development of a blood clot in a major deep vein in the leg, thigh, pelvis, or abdomen. It may also occur in less common locations such as the arm veins; the portal, mesenteric, ovarian, or retinal veins; or the veins and venous sinuses of the brain. DVT can result in impaired venous blood flow. DVT is rarely life-threatening on its own, but has the potential to cause pulmonary embolism (PE), which can be fatal. Venous thromboembolism is the broad term that includes DVT and PE. Superficial vein thrombophlebitis (also known as superficial vein thrombosis), a common related condition, affects veins superficial to the musculature. This topic focuses on the diagnosis and management of lower-extremity DVT.
History and exam
Key diagnostic factors.
- calf swelling
- localised pain along deep venous system
- presence of risk factors
- positive Wells score
- redness and warmth
Other diagnostic factors
- asymmetric oedema
- prominent superficial veins
- swelling of the entire leg
Risk factors
- recently bedridden for 3 days or more
- major surgery within the preceding 12 weeks
- medical hospitalisation within the preceding 2 months
- active cancer
- previous venous thromboembolic event
- recent trauma or fracture
- increasing age
- pregnancy and the postnatal period
- paralysis, paresis, or recent plaster immobilisation of the lower extremities
- factor V Leiden
- prothrombin gene G20210A mutation
- protein C or protein S deficiency
- antithrombin deficiency
- antiphospholipid antibody syndrome
- medical comorbidity
- use of specific drugs
- cigarette smoking
- recent long-distance air travel
- family history
Diagnostic investigations
1st investigations to order.
- quantitative D-dimer level
- venous ultrasound
- full blood count
- urea and creatinine
- liver function tests
- clotting screen
Investigations to consider
- CT/MRI venography
- further investigation for unprovoked DVT
Treatment algorithm
Suspected dvt of the leg, initiation-phase therapy: confirmed proximal dvt of the leg, initiation phase therapy: confirmed distal dvt of the leg, treatment-phase therapy: confirmed dvt of the leg, extended-phase therapy: confirmed dvt of the leg, recurrent venous thromboembolism, contributors, expert advisers, ian chetter, mbchb, frcs (eng), md, frcs (gen surg), pgcert medical ultrasound, pgdip clinical education.
Chair of Surgery
University of Hull
Honorary Consultant Vascular Surgeon
Hull University Teaching Hospitals NHS Trust
Disclosures
IC declares he has no competing interests
Acknowledgements
BMJ Best Practice would like to gratefully acknowledge the previous expert contributors, whose work has been retained in parts of the content:
Scott M. Stevens, MD
Thrombosis Clinic
Intermountain Medical Center
Professor of Medicine
Department of Medicine
Intermountain Healthcare and University of Utah
Salt Lake City
Scott C. Woller, MD
Gabriel V. Fontaine, PharmD, MBA, BCPS
Clinical Pharmacy Manager
Critical Care and Emergency Medicine
Advanced Clinical Pharmacist
Neuroscience Critical Care
Disclosures: SMS declares that he has no competing interests. SCW serves as co-chair of the American College of Chest Physicians (CHEST) guideline on the treatment of venous thrombotic disease. GVF has received consulting fees and honoraria from Alexion Pharmaceuticals.
Peer reviewers
Lara roberts, mbbs, md(res), mrcp, frcpath.
Consultant Haematologist
King's College Hospital
LR declares that she has no competing interests.
Steve Goodacre, MBChB, MRCP, DipIMC, FRCEM, MSc, PhD
Professor of Emergency Medicine
University of Sheffield
Consultant in Emergency Medicine
Sheffield Teaching Hospitals NHS Foundation Trust
SG was Chief Investigator or joint Chief Investigator for the NIHR-funded DiPEP, TiLLI, and VTEAM studies. He has also undertaken consultancy work on behalf of the University of Sheffield for ThinkSono.
Annabel Sidwell
Section Editor and Comorbidities Editor, BMJ Best Practice
AS declares that she has no competing interests.
Tannaz Aliabadi-Oglesby
Lead Section Editor, BMJ Best Practice
TAO declares that she has no competing interests.
Adam Mitchell
Drug Editor, BMJ Best Practice
AM declares that he has no competing interests.

Differentials
- Calf muscle tear/Achilles' tendon tear
- Calf muscle haematoma
- Venous thromboembolic diseases: diagnosis, management and thrombophilia testing
- European Society for Vascular Surgery (ESVS) 2021 clinical practice guidelines on the management of venous thrombosis
Calculators
Modified Wells score for deep vein thrombosis (DVT)
Pretest Probability of Heparin Induced Thrombocytopenia (4-T's score)
Patient information
DVT and long-distance travel
Use of this content is subject to our disclaimer
Log in or subscribe to access all of BMJ Best Practice
Log in to access all of bmj best practice, help us improve bmj best practice.
Please complete all fields.
I have some feedback on:
We will respond to all feedback.
For any urgent enquiries please contact our customer services team who are ready to help with any problems.
Phone: +44 (0) 207 111 1105
Email: [email protected]
Your feedback has been submitted successfully.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Semin Intervent Radiol
- v.29(1); 2012 Mar


Acute Deep Vein Thrombosis Cases in the Real World
Seth j. klein.
1 The Interventional Radiology Section, Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, Missouri
Suresh Vedantham
Practicing interventional radiologists (IRs) are routinely faced with challenging decisions that pertain to the management of patients with acute deep vein thrombosis (DVT). In this article, we describe five questions that are commonly posed by interventionalists and discuss both the indirect published evidence as well as our own experience in dealing with these issues. Our aim is to address procedural and, perhaps more importantly, nonprocedural-related knowledge domains with which the IR physician is less familiar and are often not directly addressed by published data or evidence-based clinical practice guidelines. This discussion is meant to facilitate a stronger understanding of the published literature as it pertains to the justifiable indications for endovascular thrombolytic therapy, the optimal use of anticoagulant therapy, and the reasonable use of adjuncts such as inferior vena cava filters and elastic compression stockings. Our goal is to provide a framework for practicing IRs to help them make the best clinical decisions for their individual patients and, ultimately, achieve optimal DVT treatment outcomes.
Objectives: Upon completion of this article, the reader should be able to list the current evidence, published literature, and the on-going challenges that pertain to the management of patients with acute deep vein thrombosis (DVT).
Accreditation: Tufts University School of Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™ . Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Practicing interventional radiologists are routinely faced with challenging decisions pertaining to the management of patients with acute deep vein thrombosis (DVT). These questions may encompass both procedural and nonprocedural knowledge domains with which the physician is less familiar and often are not directly addressed by published data or evidence-based clinical practice guidelines. In this article, we describe five questions that are commonly posed by interventionalists who care for patients with DVT. We describe the indirect published evidence that may bear on these issues, with the goal of providing the interventional radiologist with a stronger framework from which to make the best possible decisions for individual patients.
Clinical Question 1
“I was recently referred a 25-year-old man with a first-episode left lower extremity DVT that involved only the femoral vein and popliteal vein. The lower extremity pain and swelling began approximately 8 days previously. The common femoral vein and iliac vein were not involved. Is this a good indication for catheter-directed thrombolytic therapy?”
Answer and Discussion
For any DVT patient, the decision to use endovascular therapy hinges on a balanced assessment of several key factors to determine the patient's likelihood of treatment success. The anatomical extent of thrombosis, the clinical severity of DVT manifestations, and the patient's projected risk of experiencing a bleeding complication must be assessed and weighed. 1 , 2 The presence of comorbidities and the patient's baseline activity level, ambulatory capacity, and life expectancy should all be considered in determining a patient's suitability for aggressive DVT therapy.
A first observation to be made in this scenario is that this patient's DVT is limited to the femoropopliteal venous segments. Although any patient with proximal DVT is at risk for developing the postthrombotic syndrome (PTS), the available literature suggests that most patients with femoropopliteal DVT do well with a combination of anticoagulant therapy and elastic compression stockings. 3 , 4 , 5 , 6 Ultrasound studies have shown that most anticoagulated patients with femoropopliteal DVT ultimately experience venous recanalization, although valvular reflux may develop. 7 Also, the studies 8 , 9 comparing endovascular thrombolysis plus anticoagulation versus anticoagulation alone had methodological limitations and largely focused on patients with acute iliofemoral DVT (IFDVT, defined as DVT involving the iliac vein and/or common femoral vein). 10 , 11
Therefore, given the small risk of bleeding complications with thrombolytic therapy and the lack of published data confirming the presence of a patient benefit, the clinical threshold for intervening in this scenario should be high. Specifically, assuming the patient does not have acute limb-threatening circulatory compromise, three questions should be asked: (1) Is the patient entirely free of identifiable risk factors for bleeding and other comorbidities that would increase the risk of procedure-related complications? (2) Is the patient experiencing severe lower extremity pain, swelling, and/or inability to ambulate despite the use of therapeutic-level anticoagulant therapy for at least 3 to 5 days? (3) Is the patient actively requesting that a more aggressive approach be taken? In general, only if the answer to all three questions is yes should endovascular thrombolytic therapy be offered to the patient. If it is offered, the informed consent process should include a balanced discussion of the possible benefits, the possible lack of benefits, the risks, and alternatives including that of simply switching to another anticoagulant regimen. For example, in some patients, the use of low molecular weight heparin (LMWH) monotherapy for a prolonged period may be effective in relieving persistent symptoms of acute DVT, presumably due to the anti-inflammatory properties of these agents and the more consistent anticoagulant effect they provide compared with oral warfarin therapy.
It is important to note that patients with asymptomatic DVT, isolated calf DVT, or chronic femoropopliteal DVT are poor thrombolysis candidates. The first two categories of patients do not develop life-limiting PTS at a rate that outweighs the risks of an aggressive approach, and catheter-directed thrombolysis is ineffective for removal of organized thrombus. 12 , 13
Finally, it is important to remember that the equation is likely to be substantially different for patients with acute IFDVT. These patients have been shown to experience a higher rate of recurrent DVT, PTS, and late disability than patients with DVT of lesser anatomical extent. 14 , 15 , 16 Although there is as yet no adequately designed study that has definitively shown endovascular thrombolysis to be of benefit, the preponderance of the available data supports its judicious use in this population. In particular, four separate studies (each with key methodological limitations) have been concordant in finding reduced PTS and/or improved quality of life (QOL) with the use of endovascular thrombolysis. 8 , 9 , 17 , 18 Therefore, the guidelines of the American College of Chest Physicians, 19 the American Heart Association (AHA), 11 and the Society of Interventional Radiology (SIR) 1 , 20 all suggest that it may be reasonable to offer selected patients with acute IFDVT endovascular thrombolysis as first-line adjunctive therapy.
Of note, both patients with IFDVT and those with DVT limited to the femoropopliteal venous segments are included in two ongoing multicenter randomized trials that are evaluating the use of catheter-directed thrombolysis (CDT) and pharmacomechanical CDT (PCDT) for PTS prevention in patients with proximal DVT. 21 , 22 Therefore, within several years, the risk-benefit ratio of CDT/PCDT in these two different cohorts should be better understood.
Clinical Question 2
“I just completed catheter-directed thrombolysis in a patient with proximal DVT. For how long should I continue my patient on anticoagulant therapy?”
The optimal duration of anticoagulation for DVT has been the subject of extensive study in contemporary randomized trials. In practice, this decision is made by balancing the estimated risks of recurrent venous thromboembolism (VTE) against those of treatment-induced bleeding complications in the individual patient. 19 It is accepted that nearly a third of patients with symptomatic DVT will present with recurrent VTE episodes at some future date. 3 A key concept for interventional radiologists to understand is the fact that patients who develop a DVT due to a major provocation such as recent major surgery or major trauma are much less prone to develop recurrent VTE than patients who develop DVT in the course of normal daily living (“unprovoked DVT”). 23 , 24 , 25 , 26 This distinction carries important implications regarding disease prognosis and treatment duration. When considering the optimal treatment duration for anticoagulant therapy for DVT, most patients may be grouped into one of three main categories 1 : first-episode DVT related to a major reversible risk factor (i.e., recent surgery or trauma), 2 recurrent DVT or unprovoked DVT, 3 and DVT in patients with cancer.
In most patients with first-episode DVT related to a major reversible risk factor, anticoagulation may be safely stopped after 3 months. 19 , 27 , 28 , 29 , 30 However, in actual clinical practice, one finds that treatment is often extended to 6 months for proximal leg or pelvic DVT. Reassessment of the transient, provoking risk factor can then be made and treatment altered accordingly.
In the absence of known reversible risk factors, patients with recurrent DVT or unprovoked DVT should be considered for treatment of indefinite duration, with periodic reassessment of the risk and benefit of anticoagulation. 31 , 32 , 33 , 34 , 35 This is largely based on data from the randomized PREVENT (Prevention of Recurrent Venous Thromboembolism) Trial, in which indefinite-duration warfarin was compared with placebo in patients with idiopathic VTE who had received 6 months of anticoagulation before randomization. 32 This trial was terminated early because of a highly significant (64%) reduction in recurrent VTE among the patients receiving long-term warfarin.
For most cancer patients with DVT, first-line therapy should be weight-based LMWH for at least 3 to 6 months, or for as long as the cancer or its treatment (e.g., chemotherapy) is ongoing. This is based on randomized trial data that clearly demonstrate LMWH to be superior to oral warfarin in preventing recurrent VTE. 36 , 37 , 38
The use of direct thrombin inhibitors for the initial and long-term treatment of DVT has also shown significant promise, with one study suggesting that dabigatran may provide comparable outcomes to warfarin therapy. 39 Although as of this writing no direct thrombin inhibitors are approved by the U.S. Food and Drug Administration for the treatment of DVT, several clinical trials are in progress.
Recent studies suggest using ultrasound findings of residual thrombus to determine whether to continue anticoagulation beyond 3 to 6 months may reduce recurrent VTE in patients with proximal DVT. 40 , 41 , 42 Specifically, Palareti et al reported a reduction (12% versus 17%) in recurrent VTE events in one trial testing such an approach. Another tool that may aid the decision process (and that is less costly than ultrasound) is the D-dimer test. Data from the multicenter PROLONG Study show that patients with an abnormal D-dimer level 1 month following discontinuation of anticoagulation have a significantly increased risk of recurrent VTE, which is reduced by the resumption of anticoagulation. 43
The preceding information is given to provide the interventional radiologist with a context within which to place decisions on anticoagulant therapy in patients who have undergone endovascular thrombolytic therapy for DVT. It should be noted that observational studies have noted high rates of early rethrombosis following CDT. In one pooled analysis of 19 published peer-reviewed CDT studies, the mean frequency of rethrombosis at 30 days follow-up was 20% (range: 6 to 25%). 1 However, in prospective studies, the rate of recurrent DVT after CDT/PCDT has been quite low. For example, a single-center randomized trial found recurrent DVT to be reduced with use of CDT/PCDT plus anticoagulation compared with anticoagulation alone (2.3% versus 14.8%; p = 0.003). 18 This apparent discrepancy may reflect the fact that patients in randomized trials are more likely to have garden-variety first-episode DVT, whereas patients who receive CDT/PCDT in clinical practice (and who are reported in observational studies) tend to be referred for aggressive therapy only when there is physician concern about greater thrombus extent (IFDVT) or lack of response to initial anticoagulant therapy.
It must be presumed that catheter manipulations create venous endothelial trauma that is a potent procoagulant factor. There currently exist no data comparing different anticoagulant strategies in the early weeks and months after CDT. However, the authors often leave CDT recipients on LMWH for a longer period of time (several weeks) prior to converting to warfarin. This approach may be influenced by other factors including the quality of the anatomical result obtained. Whatever approach is chosen, it is imperative for the interventional radiologist to ensure that the patient remains fully therapeutic on some form of anticoagulation during the early weeks after CDT when his or her care is being transitioned back to a primary medical physician.
Clinical Question 3
“Should I use fully therapeutic heparin or “subtherapeutic” heparin dosing during CDT/PCDT?”
Before and after CDT/PCDT, patients should receive therapeutic-level anticoagulation with similar dosing, monitoring, and duration as DVT patients who are not undergoing thrombolysis. 19
During CDT infusions, however, many physicians have evolved toward the use of reduced-dose unfractionated heparin (UFH) over therapeutic-level UFH. We believe that this practice is reasonably supported by indirect findings from clinical studies. First, in arterial thrombolysis trials, a supra-therapeutic UFH level has been shown to correlate with thrombolysis-related bleeding. 44 , 45 In one study in which recombinant urokinase was the thrombolytic drug, the target UFH level was reduced to a subtherapeutic level midstudy, and a reduction in major bleeding events was subsequently observed. 45 In contrast, a relatively low rate of major bleeding was observed in an article reporting findings from the first 100 patients randomized in the CaVenT Trial, in which reduced-dose UFH was used along with recombinant tissue plasminogen activator (rt-PA) CDT for proximal DVT. 21 The use of subtherapeutic UFH during DVT thrombolysis is supported by current guidelines of the AHA and the SIR. 1 , 11 The optimal UFH dosing during CDT may also differ among the different thrombolytic agents due to their individual biological properties. That said, there are no scientific studies that specifically address the question of UFH dosing for any particular thrombolytic agent or technique.
It should be noted that the optimal degree of anticoagulant effect may differ substantially for patients who are being treated with a strategy of single-session PCDT. In this situation, the catheter and device manipulations tend to be more robust and the procedure itself may last for anywhere between 1 and 3 hours. Because greater endothelial damage may occur, and as the potential for thrombus migration and embolization may be slightly higher, we generally use fully therapeutic UFH during single-session PCDT and, for that matter, at follow-up sessions after infusion CDT in which adjunctive techniques are used. If all of the thrombus is not removed at the session and an overnight infusion is needed, the UFH dose is reduced to subtherapeutic levels. It may be particularly important to fully anticoagulate the patient in low-inflow situations, such as when the popliteal vein and/or its major tributaries are thrombosed.
The use of LMWHs and antiplatelet therapies during or after DVT thrombolysis has not been directly studied. We and others have used LMWH as a substitute for UFH during PCDT and anecdotally have not noticed any reduction in anticoagulant efficacy. We believe this practice is safe even during continuous rt-PA infusions because even though LMWH will result in fully therapeutic anticoagulation its use is not associated with the broader and unpredictable swings in partial thromboplastin times that are often observed with the use of UFH.
Clinical Question 4
“Should I place a retrievable inferior vena cava filter (IVC) filter for periprocedure pulmonary embolism (PE) prevention prior to performing CDT or PCDT?”
In a large prospective registry of 473 proximal DVT patients undergoing infusion CDT (without percutaneous mechanical thrombectomy), symptomatic PE occurred in only six patients (1.3%), of whom one died (0.2%). 13 The relative rarity of major PE in CDT recipients may partly arise from the ability of the thrombolytic drug to quickly dissolve any circulating thrombus fragments. When one considers that two thirds of the patients in the registry just described had IFDVT, the 1.3% PE rate is not likely to be substantially higher than the PE rate that would have been observed in a similar cohort of patients who received anticoagulation alone. Therefore, given that IVC filters impose costs and risks such as migration and late recurrent DVT, we do not advocate IVC filters for most patients undergoing traditional infusion CDT. 46 , 47 , 48
However, for patients being treated with single-session PCDT methods (which can involve more upfront clot manipulation), there are sparse data to support or refute the use of IVC filters; limited evidence suggests that major PE can occur as a PCDT complication. 49 , 50 , 51 Therefore, it may be reasonable to use a retrievable IVC filter prior to initiation of single-session PCDT, especially when iliocaval thrombus is present. This decision may be further individualized by considering the patient's overall clinical status, with a lower threshold for IVC filter utilization used in patients with pulmonary hypertension or other cardiopulmonary comorbidities. When an IVC filter is placed, it is imperative that the interventional radiologist share in the responsibility for ensuring that it is removed at the first clinically appropriate opportunity. 11
Clinical Question 5
“After performing CDT for a 30-year-old woman with iliofemoral DVT, I saw her in follow-up at 1 month. She says that she bought a pair of compression hose at the local medical supply store. She is asking how often she should wear the stockings. How should I advise her?”
Graduated elastic compression stockings (ECS) are considered a mainstay treatment in the management of acute DVT and prevention of PTS. 19 By augmenting the calf muscle pump, ECS effectively reduce the cross-sectional area of the limb, leading to increased linear velocity of venous blood flow, decreased vein wall distention, and improved valve function. This reduction in venous hypertension and reflux leads to decreased edema and better tissue microcirculation.
Three European single-center RCTs found that the daily use of sized-to-fit, 30 to 40 mm Hg knee-high ECS for 2 years after the diagnosis of first-episode proximal DVT reduces the risk of PTS by ~50%. 4 , 5 , 6 It should be noted that these studies were limited by the lack of a placebo control, blinding, and/or separate delineation of outcomes for patients with IFDVT. For this reason, a double-blind, placebo-controlled, multicenter randomized trial (the SOX trial) is being performed to definitively address the issue of whether ECS truly prevent PTS. 52
Nevertheless, given the strength of the currently available evidence and the recommendations of current practice guidelines, 11 , 19 interventional radiologists should strongly encourage the use of ECS along with anticoagulant therapy after CDT/PCDT. It is important to understand that the hose purchased by patients in their local store may not be properly sized, and that they typically will not provide a compressive force (most such hose fall into the 3 to 15 mm Hg range) that is comparable with prescription ECS. Therefore, the patient described here should have his or her legs carefully measured and should be prescribed 30 to 40 mm Hg ECS for routine daytime use. The patient should be educated that the stockings are not merely provided to reduce ongoing symptoms, but that they are an important element in the long-term prevention of PTS, which, once established, often produces irreversible impairment of quality of life.
With specific regard to IFDVT patients following CDT, there are no studies that directly address the comparative efficacy of knee-high versus thigh-high compression stockings. In these patients, we usually start by encouraging the use of thigh-high stockings if the patient can tolerate them. If not, the use of knee-high stockings may be recommended.
The main contraindication to the use of ECS is lower extremity arterial insufficiency. In patients with documented significant peripheral arterial disease, ECS should be avoided. Because many elderly patients with atherosclerotic risk factors may have mild arterial insufficiency, the elderly patient should be reminded only to wear them in the daytime when the leg is dependent.
The interventional radiologist is most likely to achieve optimal DVT treatment outcomes with a strong understanding of the published literature as it pertains to the justifiable indications for endovascular thrombolytic therapy, the optimal use of anticoagulant therapy, and the reasonable use of adjuncts such as IVC filters and elastic compression stockings.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 07 September 2024
Integrated landscape of plasma metabolism and proteome of patients with post-traumatic deep vein thrombosis
- Kun Zhang 1 , 2 , 3 na1 ,
- Pengfei Wang 1 na1 ,
- Wei Huang 1 , 2 , 3 na1 ,
- Shi-Hao Tang 2 , 3 ,
- Hanzhong Xue 1 ,
- Hao Wu 2 , 3 ,
- Ying Zhang 4 ,
- Yu Rong 2 , 3 ,
- Shan-Shan Dong ORCID: orcid.org/0000-0001-6976-4576 2 , 3 ,
- Jia-Bin Chen 1 ,
- Yan Zou 2 , 3 ,
- Ding Tian 1 ,
- Na Yang 1 ,
- Yifan Liang 1 ,
- Chungui Liu 1 ,
- Dongyang Li 1 ,
- Kun Zhang 1 ,
- Tie-Lin Yang ORCID: orcid.org/0000-0001-7062-3025 2 , 3 &
- Yan Guo ORCID: orcid.org/0000-0002-7364-2392 1 , 2 , 3
Nature Communications volume 15 , Article number: 7831 ( 2024 ) Cite this article
1 Altmetric
Metrics details
- Metabolomics
Deep vein thrombosis (DVT) is a leading cause of morbidity and mortality after trauma. Here, we integrate plasma metabolomics and proteomics to evaluate the metabolic alterations and their function in up to 680 individuals with and without DVT after trauma (pt-DVT). We identify 28 metabolites and 2 clinical parameter clusters associated with pt-DVT. Then, we develop a panel of 9 metabolites (hexadecanedioic acid, pyruvic acid, L-Carnitine, serotonin, PE(P-18:1(11Z)/18:2(9Z,12Z)), 3-Hydroxycapric acid, 5,6-DHET, 3-Methoxybenzenepropanoic acid and pentanenitrile) that can predict pt-DVT with high performance, which can be verified in an independent cohort. Furthermore, the integration analysis of metabolomics and proteomics data indicates that the upregulation of glycolysis/gluconeogenesis-TCA cycle may promote thrombosis by regulating ROS levels in red blood cells, suggesting that interfering with this process might be potential therapeutic strategies for pt-DVT. Together, our study comprehensively delineates the metabolic and hematological dysregulations for pt-DVT, and provides potential biomarkers for early detection.
Similar content being viewed by others
Serum metabolome associated with severity of acute traumatic brain injury
Plasma metabolites with mechanistic and clinical links to the neurovascular disease cavernous angioma
Lipidomic signatures align with inflammatory patterns and outcomes in critical illness
Introduction.
Deep vein thrombosis (DVT) is a major health problem that can lead to a variety of complications, such as post-thrombotic syndrome, recurrent DVT, and life-threatening pulmonary embolism (PE) 1 , 2 . DVT and PE are collectively known as venous thromboembolism (VTE). Previous studies have identified multiple risk factors of DVT, such as advanced age, immobility, surgery, and hospitalization 1 , 3 . Particularly, the risk of DVT increased in trauma individuals secondary to injury patterns and immobility 3 , 4 . Post-traumatic DVT (pt-DVT) has long been recognized as one of the most relevant clinical problems in the adult population 5 since failure to recognize this common morbidity could lead to an unacceptable rate of PE. It is also a leading cause of morbidity and mortality after trauma 6 , 7 . Furthermore, traumatic bone fractures are a major public health issue in China 8 , and the incidence of pt-DVT can reach up to 40% 9 , 10 , 11 , 12 , which makes pt-DVT a serious threat to public health in China.
Currently, the diagnosis and treatment of pt-DVT follow the VTE management approach. The diagnosis relies on laboratory measures of D-dimer and specific imaging features of ultrasound (US) 1 , 13 , 14 , 15 , while the treatment and prevention include widespread employment of anticoagulants, mechanical prophylaxis, or inferior vena cava filter (IVCF) placement 16 . Although D-dimer has been validated as a risk tool in hospitalized adult patients, the application in patients at risk of DVT after surgery or a traumatic event is unclear. In addition, surgery may cause secondary trauma, and prevention and early diagnosis of thrombosis in trauma patients are challenging during hospitalization and perioperative nursing. Therefore, the identification of biomarkers for pt-DVT is necessary and important to make an early diagnosis and provide appropriate DVT management.
Metabolomics is a promising approach for biomarker discovery, which could provide insights into pathology, treatment, and early diagnosis of diseases 17 , 18 , 19 . Although previous studies 20 , 21 , 22 have found several metabolic alterations associated with VTE, including carnitines, carnitine species, glucose, phenylalanine, 3-hydroxybutarate, lactic acid, tryptophan, and some monounsaturated and polyunsaturated fatty acids, our understanding on the global metabolic alterations in pt-DVT is still limited.
Therefore, to investigate the potential metabolic mechanisms of pt-DVT and recognize DVT patients among individuals with traumatic fractures, we used untargeted metabolomics with liquid chromatography-mass spectrometry (LC-MS) to systematically characterize plasma metabolites profiles between pt-DVT patients and controls and screened the hematological alterations associated with pt-DVT. Our study identified multiple metabolites, clinical parameters (CPs), and metabolic pathways for pt-DVT. Leveraging the multidimensional datasets, we further developed a panel of biomarkers using a machine learning method to discriminate post-traumatic DVT patients. It will be valuable for the design of an early diagnostic test for pt-DVT. Finally, by proteomics and metabolomics integrative analysis, we get insight into the altered metabolic pathways and provide potential therapeutic strategies for pt-DVT.
Clinical characteristics of the studied cohort
The study design and analysis workflow is illustrated in Fig. 1 . Based on the strict inclusion/exclusion criteria, a total of 680 patients were enrolled in the discovery ( N = 580) and the validation ( N = 100) cohorts. Briefly, for the discovery cohort, 252 patients diagnosed with incident pt-DVT were selected as cases, and 328 patients without DVT were selected as controls, which were enrolled from October 2018 to December 2020. Subsequently, from March 2021 to July 2021, 50 pt-DVT patients and 50 controls were selected with the same recruitment criteria as a separate validation cohort. The clinical characteristics of all the participants are shown in Supplementary Data 1 , including 4 basic characteristics and 34 cardiovascular and hematological characteristics. The basic characteristics were approximately balanced between the pt-DVT group and controls.
a The design of the current study. b The analysis workflow of the current study. OPLS-DA, orthogonal partial least squares discriminant analysis; DSPC, debiased sparse partial correlation; ROC, receiver operating characteristic.
Global plasma metabolic profiles
After MS/MS identification and data filtering, 326 metabolites that could be reproducibly detected in all batches were considered stable and reserved for subsequent analyses (Supplementary Data 2 and Supplementary Data 3 ). The identified metabolites were categorized into 8 functional groups and an unknown set according to the metabolism pathways in the Kyoto Encyclopedia of Genes and Genomes (KEGG) (Fig. 2a ), including lipid, amino acid, xenobiotics, carbohydrate, nucleotide, peptide, cofactors, and vitamins, energy, and others. Principal components analysis (PCA) was performed to evaluate the global metabolic variations and data quality in metabolic analysis. The optimal separation of groups was obtained in PC 1 and 2, which accounted for 8.6% and 4.7% of the whole variance of the dataset, respectively (Supplementary Fig. 1 ).

a Metabolite distribution over pathway-based classes. b Volcano plot of differential metabolites. The Benjamin-Hochberg false discovery rate (FDR) method was used to address multiple comparisons. Metabolites with a fold change of < 3/4 or > 4/3 and adjusted P value of two-tailed unpaired Student’s t test/Mann-Whitney U-test less than 0.05 (FDR < 0.05) are considered significantly decreased (blue) or increased (pink). Changes in other metabolites are not significant. The top 10 increased and decreased metabolites are labeled. c Plot of orthogonal partial least squares discriminant analysis (OPLS-DA) score. d Variable importance in projection (VIP) score of OPLS-DA model. Red dots represent the metabolites that significantly (based on fold change and two-tailed unpaired Student’s t test/Mann-Whitney U-test) altered in pt-DVT patients. e Heatmap of 28 differential metabolites throughout individuals. Red indicates metabolites that are increased, and blue indicates metabolites that are decreased in pt-DVT patients compared to controls. Source data are provided as a Source Data file.
Metabolite groups altered in pt-DVT
The volcano plot visualizes the significantly increased/decreased metabolites between the two groups by the univariate analysis (Fig. 2b ). For multivariate analysis, we implemented orthogonal partial least squares discriminant analysis (OPLS-DA) (Fig. 2c ) and identified 96 metabolites with a significant contribution to the variation (Fig. 2d ). The permutation test was performed to evaluate the validity of the discriminant model to avoid overfitting (Supplementary Fig. 2 ). Together, a total of 28 metabolites (Supplementary Data 4 ) identified both by univariate and multivariate analyses were considered as significantly changed metabolites, including 11 lipids, 5 amino acids, 3 carbohydrates, 2 peptides, 2 nucleotides, 2 xenobiotics, 1 energy metabolic group, and 2 other groups. All 28 metabolites were used for hierarchical clustering in a heatmap separating the 2 groups (pt-DVT or Controls; Fig. 2e ).
The debiased sparse partial correlation (DSPC) network (Fig. 3a ) shows the relationships in which the partial correlation coefficients were significant (Supplementary Data 5 ). The topology of the network demonstrates dense interactions occurred between both inter- and intra-functional metabolite groups with the densest interactions between lipid and amino acid metabolism. Besides, metabolites of unknown function mainly occupied the position near these two metabolite groups, N2,N2-Dimethylguanosine, and pyruvic acid, suggesting that they may be involved in lipid, amino acid, nucleotide, and carbohydrate metabolism. Together, these findings indicate that a highly coordinated metabolite regulatory network underlies thrombosis.

a Debiased sparse partial correlation (DSPC) network of 28 significantly altered metabolites. Here, each node represents a metabolite, and each edge represents the strength of partial correlation between two metabolites. Edge weights represent the partial correlation coefficient. b Metabolic pathway undergoing significant changes in pt-DVT patients. The Benjamin-Hochberg false discovery rate (FDR) method was used to address multiple comparisons. Red dots mean pt-DVT related pathways with an adjusted P value of two-tailed Global test less than 0.05 (FDR < 0.05). c A pathway-based analysis of metabolic changes for pt-DVT. The differential abundance (DA) score captures the average gross change for all metabolite measures in a pathway. A score of 1 indicates that all annotated metabolites in the pathway increase in pt-DVT patients compared to controls, and a score of − 1 indicates that all annotated metabolites in the pathway decrease. The size of the dot represents the number of annotated metabolites in the pathway. Source data are provided as a Source Data file.
Dysregulation of multiple metabolic pathways related to pt-DVT
For pathway analysis, a total of 307 metabolites could be mapped to 52 KEGG pathways. We identified 17 significantly dysregulated pathways involved in amino acid metabolism, carbohydrate metabolism, and lipid metabolism (Fig. 3b and Supplementary Data 6 ), suggesting a large-scale metabolic dysregulation in the pt-DVT group. The differential abundance (DA) score of 17 significantly altered pathways (Fig. 3c and Supplementary Data 6 ) showed that 6 pathways involved in amino acid metabolism were elevated activities. The activities of all 5 pathways associated with carbohydrate metabolism were notably upregulated, including the well-studied citrate cycle (TCA cycle) and glycolysis/gluconeogenesis. While the activities of all the 3 pathways associated with lipid metabolism were downgraded. Moreover, we identified several pathways for amino acid metabolism which were less studied with pt-DVT, such as Cysteine and methionine metabolism, Glycine, serine and threonine metabolism, Arginine and proline metabolism, Alanine, aspartate, and glutamate metabolism, and Histidine metabolism.
CP Clusters altered in pt-DVT
We identified 14 significantly changed CPs, 5 of which were increased in pt-DVT patients, and the others were decreased (Fig. 4a and Supplementary Data 7 ). The Pearson correlation analysis showed that the CPs were significantly increased/decreased and tended to cluster together (Fig. 4b ). Interestingly, the clinical features with functional similarity were grouped within a cluster, such as the top increased CP cluster, including fibrinogen (Fbg), platelet hematocrit (PCT), and platelet count (PLT), while the most decreased cluster included the number of red blood cell count (RBC), hematocrit percentage (HCT), and hemoglobin concentration (HGB). Based on these, we revealed two typical clinical features for pt-DVT, one is increased PLT-PCT-Fbg cluster (named PLT cluster), and another one is decreased RBC-HCT-HGB cluster (named RBC cluster).

a Volcano plot of differential clinical parameters (CPs). The Benjamin-Hochberg false discovery rate (FDR) method was used to address multiple comparisons. CPs with a fold change of < 3/4 or > 4/3 and adjusted P value of two-tailed unpaired Student’s t test/Mann-Whitney U-test less than 0.05 (FDR < 0.05) are considered significantly decreased (blue) or increased (pink). Changes in other CPs are not significant. All significant increased and decreased CPs are labeled. b Correlation matrix colored by the two-tailed Pearson correlation coefficient of each pair of pt-DVT-related CPs across samples. The Benjamin-Hochberg false discovery rate (FDR) method was used to address multiple comparisons. The asterisk (*) represents that each pair is significantly correlated (FDR < 0.05), and the P value < 0.0001 are marked in white. c , d Associations between pt-DVT related metabolites and PLT cluster ( c ) and RBC cluster ( d ) using linear regression model in 580 participants from the discovery cohort. The Benjamin-Hochberg false discovery rate (FDR) method was used to address multiple comparisons. Metabolites with adjusted two-tailed P value less than 0.05 (FDR < 0.05) are considered significant. Data are presented as coefficients ± SE. e , f Metabolic dysregulation associated with PLT cluster ( e ) and RBC cluster ( f ). Diamond represents the metabolite that was significantly (FDR < 0.05) associated with CP clusters, while ellipse is the pathway associated with the metabolites. Ellipse size represents the enrichment ratio of the pathway. Each edge represents that the metabolites can be annotated in the pathway, and the dotted edge suggests a close relationship between the pathways. Source data are provided as a Source Data file.
We further evaluated the diagnosis effects of these 6 CPs in PLT and RBC clusters for the diagnosis of pt-DVT (Supplementary Data 8 ). The results showed that PLT and PCT had excellent specificity for pt-DVT (Discovery cohort, PLT: specificity = 0.991; PCT: specificity = 0.994, Validation cohort, PLT: specificity = 0.96; PCT: specificity = 1) and might be the promising diagnostic markers for clinical use.
Association between metabolic alteration and pt-DVT-related blood characteristics
The correlation matrix by Pearson coefficient presents the distinctive metabolic patterns for the PLT and RBC cluster (Supplementary Fig. 3 ). In total, 25 metabolites showed significant correlations with at least one of 6 CPs, of which 18 metabolites were related to the PLT cluster, while 17 metabolites were associated with the RBC cluster, indicating that the CPs within a cluster had similar metabolic patterns.
Next, using the linear regression model, we found 16 metabolites significantly associated with the PLT cluster (Fig. 4c and Supplementary Data 9 ) and 15 metabolites associated with the RBC cluster (Fig. 4d and Supplementary Data 9 ). We further conducted pathway-based quantitative enrichment using the metabolites associated with two clusters, respectively (Supplementary Data 10 ). As shown in Fig. 4e , 8 metabolic pathways that may regulate the alteration of the PLT cluster are mainly involved in lipid metabolism, especially with the oxidation of fatty acids. We identified 4 key metabolites in these pathways, including propionylcarnitine, l-carnitine, 5,6-DHET, and serotonin. Elevated plasma propionylcarnitine, l-carnitine, and serotonin levels were positively associated with PLT number, PCT, and Fbg level in the blood. Moreover, 26 metabolic pathways are related to the RBC cluster (Fig. 4f ), including the pathways involved in amino acid metabolism, carbohydrate metabolism, and lipid metabolism. Apart from fatty acid oxidation and other pathways associated with lipid metabolism, increased imidazoleacetic acid and pyruvic acid levels were negatively associated with RBC number, HCT, and HGB levels in blood. It may contribute to pt-DVT pathogenesis through carbohydrate and amino acid metabolism, such as two additional energy metabolisms of the TCA cycle and glycolysis/gluconeogenesis.
Machine learning identified hematal-metabolic block to discriminate pt-DVT
To verify the generalization ability of the predictive model, we performed metabolomic analysis on 100 participants from an independent validation cohort (Supplementary Datas 11 , 12 ). Firstly, we evaluated the performance of the three models using all significantly altered CPs ( N = 14) and metabolites ( N = 28) identified above and found that model 2 with 28 metabolites and model 3 with 42 predictors showed excellent predictability both in the discovery and validation cohort (Supplementary Fig. 4 ).
For potential clinical use, we next tested whether we could use fewer features to distinguish pt-DVT and choose P value ranking for model reduction due to better predictive performance (Supplementary Fig. 5a–c ). Based on the results of model reduction, we, therefore, selected the top 9 predictors for each model. The prediction effects of the three models were rapidly reduced with the decreasing of features (Supplementary Fig. 5d–f ). As shown in Fig. 5 a, b , the performance of model 1 (Discovery cohort: AUC = 0.873, Validation cohort: AUC = 0.857), which predicted pt-DVT using only CPs was the worst, indicating that CPs alone might be not enough to diagnose pt-DVT at present. Correspondingly, model 2 (Discovery cohort: AUC = 0.956, Validation cohort: AUC = 0.935) and model 3 (Discovery cohort: AUC = 0.935, Validation cohort: AUC = 0.910) were well able to discriminate DVT from trauma patients, which suggested that plasma metabolites had a great contribution to pt-DVT early diagnosis and prediction in clinical use. In particular, the performance of model 2 comprising 9 metabolites (hexadecanedioic acid, pyruvic acid, L-Carnitine, serotonin, PE(P−18:1(11Z)/18:2(9Z,12Z)), 3-Hydroxycapric acid, 5,6-DHET, 3-Methoxybenzenepropanoic acid and pentanenitrile) (Fig. 5c ) was superior to other models both in the discovery cohort and the validation cohort, indicating a better predictability and generalization ability.

a Area under the receiver operating characteristic curve (AUROC) of model 1 (CPs only), model 2 (metabolites only), and model 3 (CPs and metabolites) in the discovery cohort. b AUROC of model 1, model 2, and model 3 in the validation cohort. c Box and violin plot shows the relative abundance of 9 features in model 2 across 580 samples in the discovery cohort. Statistical analyses were performed by two-tailed unpaired Student’s t test/Mann-Whitney U-test, and data were presented as mean ± SD. The effect size of t test was presented as Cohen’s D value and 95% confidence interval (CI). Box-plot, center line, median; box limits, upper and lower quartiles; whiskers, 1.5x interquartile range. Source data are provided as a Source Data file.
Metabolomics and proteomics analyses reveal potential therapeutic strategies for pt-DVT
Considering that proteomics can provide an insightful metabolic perspective by profiling the metabolic proteins and contribute to the understanding of pt-DVT metabolism, we performed a global proteomics study on 96 pt-DVT patients and 87 control participants derived from our cohort. In total, 524 plasma proteins were quantified (Supplementary Datas 13, 14 ), and 214 proteins were identified as differential between the two groups (FDR < 0.05 and FC < 3/4 or > 4/3), with 153 upregulated and 61 downregulated in patients with pt-DVT (Fig. 6a and Supplementary Data 15 ). KEGG pathway enrichment of differential proteins found that 15 pathways were significantly altered (FDR < 0.05) in pt-DVT patients (Fig. 6b ). Notably, complement and coagulation cascades were identified as the most significantly altered pathway, which is also the regulatory pathway that directly delivers to DVT.

a Volcano plot of differential proteins. The Benjamin-Hochberg false discovery rate (FDR) method was used to address multiple comparisons. Proteins with a fold change of < 3/4 or > 4/3 and adjusted P value of two-tailed unpaired Student’s t test/Mann-Whitney U-test less than 0.05 (FDR < 0.05) are considered significantly decreased or increased. Changes in other proteins are not significant. The top 40 changed proteins are labeled. b KEGG pathway enrichment of differential proteins identified 15 significant pathways associated with pt-DVT. The Benjamin-Hochberg false discovery rate (FDR) method was used to address multiple comparisons. Pathways with an adjusted P value of one-tailed Fisher Exact test less than 0.05 (FDR < 0.05) are considered significant enrichment. c Schema of metabolic pathways (glycolysis/gluconeogenesis and TCA cycles) with select metabolites and proteins. Metabolites and proteins with upregulated, downregulated, and unchanged were colored in red, blue, and black, respectively. Gray nodes represent proteins that were not detected. Statistical analyses were performed by two-tailed unpaired Student’s t test/Mann-Whitney U-test, and data were presented as mean ± SD. The effect size of t test was presented as Cohen’s D value and 95% confidence interval (CI). Box-plot, center line, median; box limits, upper and lower quartiles; whiskers, 1.5x interquartile range. Source data are provided as a Source Data file.
Combining metabolomics and proteomics findings, we found that glycolysis/gluconeogenesis was changed both in metabolite and protein levels. Glycolysis/gluconeogenesis produces pyruvic acid for the TCA cycle, and our study revealed that pyruvic acid, citric acid, and several proteins in the glycolysis/gluconeogenesis pathway were significantly increased in pt-DVT patients. Therefore, it is reasonable to speculate that the upregulation of glycolysis/gluconeogenesis may result in the pathogenesis of thrombosis. This promoted us to further explore the roles of glycolysis/gluconeogenesis by integrating the metabolomics and proteomics data. As shown in Fig. 6c , we found 8 proteins (LDHA, LDHB, GAPDH, GPI, PKM, MINPP1, ENO1, and TPI1) upstream of pyruvic acid were significantly upregulated (FDR < 0.05, P value ranged from 2.88 × 10 −10 to 1.87 × 10 −2 ) in pt-DVT patients. The glycolysis/gluconeogenesis-TCA cycle cascaded by pyruvic acid is a major source generating nicotinamide adenine dinucleotide (NADH), which plays a crucial role in the cellular redox status 23 . Previous studies have demonstrated that reactive oxygen species (ROS) in RBCs will alter erythrocyte membrane structure and be enhanced in thrombosis 24 , 25 , 26 . Consistent with these reports, our data indicated that ROS-related peroxiredoxins were slightly disturbed, with PRDX5 upregulated (FC = 1.558, P value = 2.71 × 10 − 2 ) and PRDX2 downregulated (FC = 0.599, P value = 3.96 × 10 − 4 ) in pt-DVT. The correlation analysis found that most differential proteins in the glycolysis/gluconeogenesis pathway (7/8) were significantly correlated with PRDX5 (Supplementary Fig. 6 ). In addition, lactate dehydrogenases (LDHA/LDHB), the most significantly changed proteins in glycolysis/gluconeogenesis, were reported to be involved in ROS production in a variety of cells 27 , 28 . It can be proposed that the upregulation of glycolysis/gluconeogenesis-TCA cycle cascaded by pyruvic acid may be associated with the accumulation of ROS in RBCs, thereby enhancing thrombosis. Together, our metabolomics and proteomics data highlight that intervening with glycolysis/gluconeogenesis and redox homeostasis might serve as potential therapeutic targets for pt-DVT. Based on these results, we performed pharmacological analysis with the related proteins associated with glycolysis/gluconeogenesis and redox homeostasis in our data and identified 50 potential compounds targeting glycolysis/gluconeogenesis and redox homeostasis (Supplementary Data 16 ), which facilitates subsequent functional studies and drug development.
Since inflammation appears to be central to the thrombosis 29 , 30 , 31 , 32 , we also focused on the inflammation markers and found that C-reactive protein (CRP) was significantly elevated ( P value = 2.39 × 10 − 4 ) in the pt-DVT patients (Supplementary Data 15 ). To further assess the relationship between inflammation and pt-DVT-related metabolites and CPs, we performed linear regression in 156 participants, both measured proteomics and metabolomics. The results showed that CRP was significantly associated with Fbg, RBC, HCT, HGB, and PE(P−18:1(11Z)/18:2(9Z,12Z)) (FDR < 0.05) (Supplementary Data 17 ), suggesting that the dysregulated lipid metabolism and hematological characteristics related to pt-DVT may be associated with the inflammatory response triggered after trauma.
In two independent trauma cohorts, we identified a set of 28 metabolites and 14 CPs as potential biomarkers of pt-DVT, revealing the metabolic and hematological alterations in pt-DVT. Beyond that, we developed a panel of biomarkers including 9 features to distinguish pt-DVT patients efficiently using a machine learning algorithm, suggesting the potential clinical use of an early diagnostic test in pt-DVT. Finally, data resulting from integrative metabolomics and proteomic analyses indicated that the upregulation of glycolysis/gluconeogenesis-TCA cycle cascaded by pyruvic acid may be related to ROS in RBCs, thus enhancing thrombosis.
Based on our study design, the metabolites and pathways we identified may be potentially suggestive of DVT or involved in the mechanism of thrombosis. Among the changing metabolites and pathways, the major class that increased was carbohydrates, including pyruvic acid and citric acid, which participate in the TCA cycle and glycolysis. Pyruvic acid originated from pyruvate metabolism and glycolysis/gluconeogenesis is transported to the mitochondria where it is converted to acetyl coenzyme A (acetyl-CoA) and further produced as citric acid for the TCA cycle. The disturbance of the TCA cycle and glycolysis/gluconeogenesis have been reported to be closely related to venous thrombosis 20 , 33 , but the role of pyruvate and citric acid in the disease remains unclear. Besides, another class that significantly changed in pt-DVT was lipids, particularly l-carnitine and fatty acids metabolism, which have been validated in previous studies 33 , 34 , 35 . It should be noted that some of the altered metabolites showed different trends in diverse studies due to discrepant detection platforms and study participants. For instance, Sung et al 33 . studied metabolic alterations in serum and vein wall extracts of the mouse model of DVT and found that citric acid was decreased in the DVT group, which was inconsistent with our finding. In contrast, l-carnitine was found to be of greater abundance in the serum of DVT animals 33 , which was consistent with our study.
To get insight into the metabolic perspective and enhance the understanding of pt-DVT metabolism, we combined the proteomics data to profile the proteins related to pt-DVT metabolism. The integration of metabolomics and proteomics data suggested that the upregulation of pyruvic acid, citric acid, and several proteins in glycolysis/gluconeogenesis may produce more ROS and enhance thrombosis. ROS accumulate within RBCs due to endogenous hemoglobin autoxidation and uptake of extracellular ROS released by other cells. Previous studies 24 , 26 have found that elevated ROS in RBC affects the structure and function of RBC membranes, leading to loss of membrane integrity and reduced deformability. These alterations impair the function of RBCs in hemostasis and thrombosis by enhancing RBC aggregation, RBC binding to endothelial cells, RBC-induced platelet activation, RBC interaction with and activation of coagulation factors, and favoring a hypercoagulable state 24 , 26 . Consistent with these reports, ROS-related peroxiredoxins (PRDX5 and PRDX2) were also disturbed in our data. In addition, our study observed that lactate dehydrogenases (LDHA/LDHB) were drastically increased in pt-DVT, which have been reported to be involved in ROS production in a variety of cells 27 , 28 . A recent study 27 for cholangiocarcinoma found that LDHA and LDHB both exhibited hydrogen peroxide-producing activity or promoted oxidative stress in cancer cells in vitro and in vivo. Another study 28 in chondrocytes found that LDHA can promote ROS and may be a potential therapeutic target for osteoarthritis treatment. In general, our findings identified that intervening with glycolysis/gluconeogenesis and redox homeostasis might serve as potential therapeutic targets for pt-DVT in a relatively large population, while this hypothesis needs to be verified by further functional studies.
Among the changed CPs, we identified two major features, including the increased PLT-PCT-Fbg cluster and the decreased RBC-HGB-HCT cluster in pt-DVT patients. Accumulating evidence indicates that platelets contribute to thrombosis and might regulate effector functions of innate immune cells recruited to the thrombus 1 , 36 . Besides, mechanistic studies indicate that RBCs can promote thrombus formation and enhance thrombus stability 37 . When venous thrombosis is formed, a large number of RBCs will be recruited, which may result in a decrease in the number of RBCs in circulating blood. In addition to PLT and RBC, we also identified multiple pt-DVT-related CPs that have not been reported in previous studies, such as PCT, HGB, and HCT, of which PCT has the same excellent specificity for pt-DVT as PLT. It should be addressed that D-dimer (D-D), the laboratory measure for VTE or DVT diagnosis, did not significantly change in our study. Our data showed that D-D had the lowest false negative rates (FNR) (Supplementary Data 8 ), indicating that although D-D had poor specificity, it had the highest sensitivity and could be used as a preliminary indication for pt-DVT.
Assessment of the relationship between CPs and metabolites shows that the elevation of the PLT cluster is associated with lipid and amino acid metabolism, while the decrease of the RBC cluster is associated with energy metabolic processes, such as TCA cycle and glycolysis/gluconeogenesis, in addition to lipid and amino acid metabolism. The role of lipid metabolism, particularly fatty acids oxidation and arachidonic acid metabolism, in platelet promotion of thrombosis has not been well elucidated. A study 38 in diabetic patients found that l-carnitine might aggravate platelet hyperactivity by increasing the provision of surplus acetyl-CoA to the cytoplasmic compartment, which may explain the mediation of fatty acid oxidation in platelet promotion of thrombosis. In addition, l-carnitine has been shown to promote HGB elevation 39 . For RBC cluster-related metabolic dysregulation, the most interest is pyruvic acid involved in glycolysis/gluconeogenesis and TCA cycle, which was consistent with our findings that the upregulation of glycolysis/gluconeogenesis-TCA cycle cascaded by pyruvic acid may be associated with accumulation of ROS in RBCs, thus enhancing thrombosis.
It should be noted that the results that have been reported by other studies 20 , 22 , 33 , 40 , 41 mainly in patients without trauma, such as l-carnitine, pyruvic acid, citric acid, PLT, and RBC, which are not specific to post-traumatic DVT. Some of our findings that have not been reported might be specific to DVT after trauma, such as hexadecanedioic acid, D-Xylulose, 5,6-DHET, HGB, HCT, and PCT. However, the role of these metabolites and CPs in thrombosis for trauma patients is unclear. The traditional Virchow Triad pathogenetic mechanism of VTE indicates that venous injury, slow blood flow, and hypercoagulability of the blood are three important factors in thrombosis 1 , 42 . Trauma may trigger vascular injury and slow blood flow caused by immobility, leading to specific metabolic and hematologic features of DVT. For example, immobility in patients with bone fractures can disrupt venous flow in venous valves, thereby promoting platelet retention and contributing to thrombosis 43 . In addition, the inflammatory response triggered by vascular injury may also contribute to specific metabolic and hematologic patterns that favor thrombosis. Previous studies showed that CRP was not only a marker of inflammation but also had significant biological effects in regulating many of the aspects central to the pathogenesis of VTE 29 . The significant association results between CRP and Fbg, RBC cluster, and PE(P-18:1(11Z)/18:2(9Z,12Z)) suggest that the dysregulated lipid metabolism and hematological characteristics of pt-DVT may be related to the inflammatory response triggered by vascular injury. The detailed mechanisms of PE(P-18:1(11Z)/18:2(9Z,12Z)), RBC cluster, and Fbg in DVT are currently unknown, but observational studies have found that PE levels were prognostic for worse outcomes in trauma 32 , while lipid levels are associated with favorable changes in coagulation and inflammatory biomarkers in causal models 32 . Although there are no definitive studies elucidating how our findings play a role in the mechanism of inflammatory response-mediated thrombosis, observational studies have identified PE and lipids associated with trauma, inflammatory response, and coagulation, which may facilitate the traditional Virchow Triad pathogenetic mechanism of VTE.
An important result of this study is that we constructed a predictive model for pt-DVT using a machine learning algorithm. Using a similar approach, a recent study 22 identified plasma biomarkers to characterize venous thromboembolism. Nevertheless, the participants in the study were diagnosed with VTE rather than pt-DVT, and the plasma was collected 3 months after an incident VTE, which could not present the metabolic changes at disease states. Our design allowed us to sensitively capture the metabolic and hematological alterations in pt-DVT patients during thrombogenesis and to ensure good generalization ability of the prediction model through an independent cohort. We hypothesized that this information might help us to screen the more effective biomarkers and develop a more accurate diagnostic model to improve current trauma care through early diagnosis or prediction of thrombosis formation.
An apparent advantage of this study is the large sample size in pt-DVT metabolomics and proteomics analyses till now. This may give our study higher statistical power to guarantee reliable results. Meanwhile, the limitations of our study should also be addressed. First, due to the different medical treatment times, the trauma time before blood sampling in this study could not be unified. To eliminate this interference, we implemented multiple linear models fitted with ‘sex’, ‘age’, and ‘time from trauma to sampling’ as independent variables. Second, to obtain the metabolite that can be consistently detected for diagnostic biomarkers screening, we kept metabolites that were identified in all three batches for analysis, which may lead to the missing of some potentially relevant metabolites. Third, the severity of trauma is related to hemorrhage as such, the hematological alterations may be a surrogate for the severity of trauma, which could be associated with pt-DVT and thus confounding the results. Due to the lack of information on hemorrhage at the time of patient admission, we were unable to correct this problem during analyses. However, the participants in our study are only patients with mild trauma severity (ISS < 16) and with a similar trauma mode (trauma cause: 73.8% participants are falling during physical activity and biking; fracture location; 70.29% participants are femur, tibia or fibula), which may minimize the potential impact of trauma severity and hemorrhage. Fourth, our study is only suggestive of the metabolic changes and hematological characteristics associated with pt-DVT, and the in-depth mechanisms of thrombosis in trauma patients need more functional experiments to investigate.
In conclusion, this cohort study identified 28 metabolites and 14 CPs significantly associated with pt-DVT and comprehensively demonstrated the metabolic and hematological alterations in pt-DVT patients. Based on these significantly altered metabolites and CPs, we developed a panel of 9 metabolites to effectively distinguish pt-DVT patients. More importantly, combined with proteomics data, we found that the upregulation of the glycolysis/gluconeogenesis-TCA cycle cascaded by pyruvic acid may promote thrombosis regulating ROS levels in RBCs. It suggests that interfering with glycolysis/gluconeogenesis and redox homeostasis might be potential therapeutic strategies for pt-DVT treatment. In general, our study characterizes the metabolic dysregulation in pt-DVT and identifies plasma biomarkers with a large-scale cohort. We believe that our findings can facilitate functional research of pt-DVT, and contribute to early diagnosis for clinical use.
Ethics statement
The study was approved by the Ethics Committee of Xi’an Jiaotong University Honghui Hospital. All patients were provided written, informed consent before participating in the study. The study protocol can be available from the corresponding author upon reasonable request.
Study design and participants
The patients in our study were recruited as part of the study which was registered in the Chinese Clinical Trial Registry (ChiCTR) (Registration number: ChiCTR1800017754). It can be available in the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) ( https://trialsearch.who.int/ ). The study design is illustrated in Fig. 1a . We carried out a nested case-control study design using a prospective cohort, which enrolled ~ 4000 Chinese Han participants diagnosed with acute traumatic fractures at the Department of Trauma Surgery, Honghui Hospital (Xi’an, China) from October 2018 to October 2022 (Fig. 1a ). All participants were adult inpatients (over 18 years of age). All patients were not on anticoagulants or antiplatelet drugs before sampling, and fasting venous blood samples were obtained from each patient less than 12 hours after hospitalization. Follow-up assessments to determine DVT status were confirmed by US screening after blood sampling and prior to surgery, usually within 72 hours of admission. We didn’t consider the sex of participants before the study design, and there were no sex-based analyses in our study because we corrected all data for sex as a covariate.
Based on this prospective cohort, we adopted strict inclusion/exclusion criteria to select samples for our study: (i) participants with major diseases or known metabolic diseases were excluded, including type 2 diabetes, coronary artery disease, hypertension, obesity, kidney or liver diseases, hyperthyroidism, dyslipidemia, and cancers; (ii) participants with chronic use of medications affecting metabolism (hormone replacement therapy and corticosteroid therapy) were excluded; (iii) we mainly focused on traumatic fractures (falling during physical activity and biking: 73.8%; traffic accident: 12.9%; falling down from a high altitude: 9.4%; and crushing by the heavy object: 3.9%), and participants with osteoporotic fractures or anti-osteoporosis drug intake were excluded to minimize potential influence on metabolic backgrounds; (iv) we focused on lower extremity fractures (femur: 50%; tibia or fibula: 20.29%; pelvis: 6.62%; knee: 9.85%; ankle: 6.03%; multiple bone fractures: 7.21%) and included only patients with mild trauma severity based on the Injury Severity Score (ISS) (definition of mild injury: ISS ≤ 16) to reduce the potential impact of trauma severity or mode; (v) all cases were patients with distal DVT of lower extremity (DVT involved the gastroc-soleal veins: 63.89%; DVT involved the axial calf veins: 10.32%; DVT involved both the axial calf veins and the gastroc-soleal veins: 25.79%) and the thrombi in all cases were relatively large in size (> 10 millimeters × 2 millimeters, length × width). Based on the strict inclusion/exclusion criteria, 20.1% of patients were diagnosed as pt-DVT cases.
Clinical parameters characterization
The participants were required to fast overnight before blood collection and tested for three cardiovascular characteristics, including heart rate, systolic blood pressure (SBP), and diastolic blood pressure (DBP) in the meantime. Coagulation tests and complete blood count (CBC) were performed in the clinical laboratory of Honghui Hospital using the reagents purchased from SUNBIO (Shanghai, China) coupled with an Automatic Coagulation Analyzer (Sysmex, Japan) and an Automatic Hematology Analyzer (Sysmex, Japan).
Plasma metabolomics analysis
We performed untargeted metabolomics analyses on the discovery and validation cohorts, respectively. The discovery cohort was used to identify pt-DVT-related metabolic features and explore their relationships with CPs. Based on the identified metabolic and clinical features, we constructed a prediction model for pt-DVT using a machine learning algorithm. To further evaluate the generalization ability of the model, we assessed the predictive performance of the corresponding metabolic and clinical features in the validation sample. All the plasma samples from both cohorts were separated from whole blood and extracted using chemical reagents for metabolomics analysis. Samples from the discovery cohort were analyzed in 2 batches at different time points, and the samples from the validation cohort were analyzed in a separate batch. The quality control (QC) sample was prepared by mixing an equal aliquot of the supernatants from all of the samples. We used the same QC (mixed in batch 1) for all batches. LC-MS/MS analyses were performed using a UHPLC system (Vanquish, Thermo Fisher Scientific) with a UPLC BEH Amide column (2.1 mm × 100 mm, 1.7 μm) coupled to Q Exactive HFX mass spectrometer (Orbitrap MS, Thermo). For each data batch, the acquisition order of all samples was randomly distributed and the QC injections were inserted every 10 samples injections. The raw data were converted to the mzXML format and processed with an in-house program, which was developed using R and based on XCMS 44 . Then, an in-house MS/MS database (BiotreeDB) was applied in metabolite annotation and the cutoff of similarity score was set at 0.4. The in-house software package and database have been widely used in many metabolic studies 45 , 46 , 47 , 48 . For the features detected in both positive and negative modes, we kept the one with a higher annotation score. Within a different analytical batch, all metabolic peaks were filtered to remove noise. After data filtering, the abundances of remaining peaks were normalized by dividing the intensity of internal standard (IS), and the data were log-transformed and scaled by median centered within a batch 17 . Metabolites measured in all batches were retained for study. Additional details about plasma metabolomics analysis are available in Supplementary Methods.
Plasma proteomics analysis
Sample preparation, including protein denaturation, reduction, alkylation, digestion, and peptide cleaning, was performed according to the iST kit (PreOmics, Germany) protocol. All samples were re-dissovled and added 11 tryptic iRT peptides (Biognosys, KI-3002-1) for LC-MS analysis. The peptide mixture was fractionated by high pH separation using the Ultimate 3000 system (ThermoFisher scientific, MA, USA) connected to a reverse phase column (XBridge C18 column, 4.6 mm × 250 mm, 5 μm, Waters Corporation, MA, USA). A total of 14 fractions were collected and analyzed by Q Exactive™ Plus coupled to a U3000 system (Thermo Fisher Scientific, MA, USA). The mass spectrometer was run under data-dependent acquisition (DDA) mode, and automatically switched between MS and MS/MS mode. Raw Data of DDA were processed and analyzed by Spectronaut 16.0 (Biognosys AG, Switzerland) with default settings to generate an initial target list. Furthermore, the mass spectrometer was run under data-independent acquisition (DIA) mode with a hybrid data strategy 49 . Raw Data of DIA were processed by Spectronaut 17.0 (Biognosys AG, Switzerland) software with default settings. The summation of the top 3 filtered peptides that passed the 1% false discovery rate (FDR) cutoff was used to calculate the major group quantities. Based on processed protein profiles, we also conducted data imputation consistent with metabolomics data. After data filtering, the abundances of remaining proteins were log-transformed and scaled by median centered. Additional details about plasma proteomics analysis are available in Supplementary Methods.
Statistical analyses
The Benjamin-Hochberg false discovery rate (FDR) method was used to address multiple comparisons issue, and the significance threshold of all statistical analyses in our study is corrected P value less than 0.05. All statistical analyses were performed using R software (version 3.6.1).
Covariate adjustment
The whole analyses workflow is illustrated in Fig. 1b . To minimize the influence of confounding factors, the metabolites, proteomics and clinical parameters profile data were first corrected with “sex”, “age”, and “trauma time” as covariates. In short, a multiple linear model was fitted with ‘sex’, ‘age’, and ‘trauma time’ as independent variables and the relative abundance of each metabolite, protein and CP as the dependent variable. The correction for metabolites and CPs was performed within the discovery and the validation cohorts separately. The correct data were residuals transformed and were scaled by median centered for subsequent analysis. After data pre-processing, we evaluated whether all metabolites, proteins, and CPs conformed to a normal distribution ( P value > 0.05) using the Kolmogorov-Smirnov (KS) Test. The two-tailed Student’s t tests were performed for normally distributed features, and the two-tailed Mann-Whitney U-tests were used for non-normally distributed features. The effect size of the statistical test was calculated using the Computation of Effect Sizes ( http://www.psychometrica.de/effect_size.html ) website, with Cohen’s D value used for t test and Eta squared (η 2 ) used for the U-test.
Identification of differential metabolites for pt-DVT
Identification of significant alteration metabolites was performed using a two-step approach comprising univariate analysis and multivariate analysis in the discovery cohort. For univariate analysis, we performed a two-tailed unpaired Student’s t test/Mann-Whitney U-test and fold change (FC) to select differential metabolites. FDR < 0.05, and FC > 4/3 or < 3/4 was considered significant 18 . In multivariate analysis, unsupervised PCA was performed to explore the global metabolic variations between cases and controls. The supervised OPLS-DA 50 was used to maximize the global metabolic variations between two groups, and the metabolites with the threshold of variable importance in projection (VIP) score > 1 were supposed to significantly change. The OPLS-DA model was validated by 1000 permutation tests to avoid overfitting. The metabolites that up to all significant criteria were used for hierarchical clustering and DSPC network analysis (R package MetaboAnalystR) 51 , which can capture the association between two metabolites after conditioning on all other variables in the network. The partial correlation coefficients were significant at FDR < 0.05, and the visualization of the DSPC network was realized by Cytoscape software 52 .
Metabolic pathway analysis
The metabolic pathway analysis was performed with all identified metabolites utilizing MetaboAnalystR 5.0 ( https://www.metaboanalyst.ca/MetaboAnalyst/home.xhtml ) 53 , 54 . The metabolites were mapped into the KEGG database, and the statistical significance of the changes in a pathway’s activity between two groups was evaluated by global testing (FDR < 0.05). The topological pathway impacts were calculated according to relative-betweenness centrality. To quantify pathway activity, we calculated the DA score to demonstrate the tendency for a pathway with increased/decreased levels of metabolites compared to the control group 48 . The DA score was calculated by applying a differential abundance test (FDR corrected Student’s t tests) to all metabolites in a pathway. After determining which metabolites were significantly altered, the DA score was defined as:
DA score = (No. of metabolites increased -No. of metabolites decreased)/No. of measured metabolites in the pathway.
In short, the DA score varies from − 1 to 1 which indicates all metabolites in a pathway decreased or increased in abundance.
Identification of differential CPs and the related metabolic characterization
For clinical profiling, we used logistic regression (R function glm), two-tailed unpaired Student’s t test/Mann-Whitney U-test, and FC to select significantly altered CPs in the discovery cohort. FDR < 0.05, and FC > 4/3 or < 3/4 was considered significant. To get insight into the metabolic dysregulation of pt-DVT-related blood characteristics above, Pearson correlation analysis was applied to evaluate the association between a pair of CPs and pt-DVT-related metabolites. FDR < 0.05 was considered a significant correlation. In addition, a linear regression model was carried out to identify the metabolites associated with altered CP clusters (FDR < 0.05), which used the relative abundances of metabolites as the independent variables and the average level of CPs in a cluster as the dependent variables. The metabolites identified above were further employed to conduct pathway-based quantitative enrichment based on the small molecule pathway database (SMBPD) 55 using MetaboAnalystR 5.0 56 .
Identification of differential proteins for pt-DVT and pharmacological evaluation
For proteomics data, we also used two-tailed unpaired Student’s t test/Mann-Whitney U-test and FC to select significantly altered proteins associated with pt-DVT. FDR < 0.05, and FC > 4/3 or < 3/4 was considered significant. KEGG pathway analysis was performed with differential proteins using The Database for Annotation, Visualization, and Integrated Discovery (DAVID) website, and FDR < 0.05 was considered significantly enriched. For the potential target proteins screened in conjunction with metabolomics, we used the Connectivity Map (CMAP) ( https://clue.io/ ) 57 to identify compounds with pt-DVT-related proteins 58 . Based on their algorithm, compounds with lower scores showed better overall inhibition of all input genes, and we show results with scores less than -99, considered as potential compounds for targeting pathways.
Machine learning for pt-DVT prediction
To predict pt-DVT from bone-fractured patients, we built the prediction models developed with support vector machine (SVM; R package e1071) algorithms. We tried three kernel parameters of the SVM algorithm (Linear, Radial Basis Function, Polynomial) and chose the best-performing polynomial for model construction. Based on different feature groups, we developed 3 models, including CPs only (model 1), metabolites only (model 2), and the full model comprising CPs and metabolites (model 3). The prediction ability was assessed by the area under the curve (AUC) of the receiver operating characteristic (ROC) curve (R package pROC). In addition, the bootstrap method was applied 1000 times in the discovery cohort to develop the training sets and the test sets (8:2) to dilute the selection bias. The discovery cohort was divided into training sets ( N = 465, pt-DVT: controls = 202: 263) and test sets ( N = 115, pt-DVT: controls = 50: 65) by bootstrap method, and the validation cohort ( N = 100, pt-DVT: controls = 50: 50) was used to further evaluate the generalization ability of the model. We selected features by model reduction method according to the P value ( t test/U-test, in all 580 samples from the discovery cohort) and the importance (SVM model, mean value of 1000 training sets) of potential variates. Then, the performance of the prediction model was evaluated in test sets and independent validation cohorts. Based on the bootstrap method, we obtained the AUC 1000 times in test sets and calculated the 95% confidence interval (95% CI). Besides, we chose the data division that corresponded to the average value of 1000 AUC as the final statistical model.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All relevant data support the key findings of this study are available within the article and its Supplementary Information files. Source data are provided in this paper. The raw intensity values and processed data matrix of metabolomics and proteomics are available in Supplementary Data. The mass spectrometry metabolomics data generated in this study have been deposited in the OMIX, China National Center for Bioinformation / Beijing Institute of Genomics, Chinese Academy of Sciences ( https://ngdc.cncb.ac.cn/omix ) 59 , 60 under accession code OMIX005819. The mass spectrometry proteomics data generated in this study have been deposited in the ProteomeXchange ( https://proteomecentral.proteomexchange.org ) Consortium via iProX repository 61 , 62 with the dataset identifier PXD054131. Raw data for clinical information are available from the corresponding author upon request. All data in this study is only allowed for academic use. Source data are provided in this paper.
Code availability
No custom code or mathematical algorithm was used in the methods. All statistical analyses were conducted in R using published libraries and functions.
Wolberg, A. S., et al. Venous thrombosis. Nat. Rev. Dis. Primers 1 , 15006 (2015).
Kafeza, M. et al. A systematic review of clinical prediction scores for deep vein thrombosis. Phlebology 32 , 516–531 (2017).
Article PubMed Google Scholar
Beckman, M. G., Hooper, W. C., Critchley, S. E. & Ortel, T. L. Venous thromboembolism a public health concern. Am. J. Prev. Med. 38 , S495–S501 (2010).
Strandvik, G., El-Menyar, A., Asim, M., Galwankar, S. & Al-Thani, H. Clinical characteristics, management practices, and in-hospital outcomes among trauma patients with venous thromboembolism. J. Emerg. Trauma Shock 13 , 124–130 (2020).
Article PubMed PubMed Central Google Scholar
Geerts, W. H., Code, K. I., Jay, R. M., Chen, E. L. & Szalai, J. P. A prospective-study of venous thromboembolism after major trauma. N. Engl. J. Med. 331 , 1601–1606 (1994).
Rogers, F. B. Venous thromboembolism in trauma patients: A review. Surgery 130 , 1–12 (2001).
Knudson, M. M., Gomez, D., Haas, B., Cohen, M. J. & Nathens, A. B. Three thousand seven hundred thirty-eight posttraumatic pulmonary emboli a new look at an old disease. Ann. Surg. 254 , 625–632 (2011).
Chen, W. et al. National incidence of traumatic fractures in China: a retrospective survey of 512 187 individuals. Lancet Glob. Health 5 , E807–E817 (2017).
Dwyer, K. M. et al. Predictors of posttraumatic deep vein thrombosis (DVT): Hospital practice versus patient factors-An analysis of the National Trauma Data Bank (NTDB) DISCUSSION. J. Trauma 66 , 999–1001 (2009).
Google Scholar
Zhang, B. F. et al. Deep vein thrombosis in bilateral lower extremities after hip fracture: a retrospective study of 463 patients. Clin. Inter. Aging 13 , 681–689 (2018).
Article Google Scholar
Zee, A. A. G., van Lieshout, K., van der Heide, M., Janssen, L. & Janzing, H. M. J. Low molecular weight heparin for prevention of venous thromboembolism in patients with lower-limb immobilization. Cochrane. Db. Syst. Rev. https://doi.org/10.1002/14651858.CD006681.pub4 (2017).
Cerbasi, S. et al. Assessment of deep vein thrombosis using routine pre- and postoperative duplex Doppler ultrasound in patients with pelvic trauma. Bone Jt. J. 104-B , 283–289 (2022).
Palareti, G. et al. D-dimer testing to determine the duration of anticoagulation therapy. N. Engl. J. Med. 355 , 1780–1789 (2006).
Tritschler, T., Kraaijpoel, N., Le Gal, G. & Wells, P. S. Venous thromboembolism advances in diagnosis and treatment. JAMA 320 , 1583–1594 (2018).
Chopard, R., Albertsen, I. E. & Piazza, G. Diagnosis and treatment of lower extremity venous thromboembolism: A review. JAMA 324 , 1765–1776 (2020).
Toker, S., Hak, D. J. & Morgan, S. J. Deep vein thrombosis prophylaxis in trauma patients. Thrombosis 2011 , 505373–505373 (2011).
Liang, L. et al. Metabolic dynamics and prediction of gestational age and time to delivery in pregnant women. Cell 181 , 1680–1692 (2020).
Shen, X. et al. Serum metabolomics identifies dysregulated pathways and potential metabolic biomarkers for hyperuricemia and gout. Arthritis Rheumatol. 73 , 1738–1748 (2021).
Liu, J. et al. Integrative metabolomic characterisation identifies altered portal vein serum metabolome contributing to human hepatocellular carcinoma. Gut 71 , 1203–1213 (2022).
Franczyk, B., Gluba-Brzozka, A., Lawinski, J., Rysz-Gorzynska, M. & Rysz, J. Metabolomic Profile in Venous Thromboembolism (VTE). Metabolites 11 , 495 (2021).
Escobar, M. Q., et al. Serum metabolic profiles based on nuclear magnetic resonance spectroscopy among patients with deep vein thrombosis and healthy controls. Metabolites 11 , 874 (2021).
Fraser, K. et al. Plasma biomarkers and identification of resilient metabolic disruptions in patients with venous thromboembolism using a metabolic systems approach. Arterioscler. Thromb. Vasc. Biol. 40 , 2527–2538 (2020).
Chini, C. C. S., Zeidler, J. D., Kashyap, S., Warner, G. & Chini, E. N. Evolving concepts in NAD(+) metabolism. Cell Metab. 33 , 1076–1087 (2021).
Bettiol, A. et al. Erythrocyte oxidative stress and thrombosis. Expert Rev. Mol. Med. 24 , e31 (2022).
Lichota, A., Szewczyk, E. M. & Gwozdzinski, K. Factors affecting the formation and treatment of thrombosis by natural and synthetic compounds. Int. J. Mol. Sci. 21 , 7975 (2020).
Wang, Q. H. & Zennadi, R. Oxidative stress and thrombosis during aging: The roles of oxidative stress in RBCs in venous thrombosis. Int. J. Mol. Sci. 21 , 4259 (2020).
Wu, H. et al. Lactate dehydrogenases amplify reactive oxygen species in cancer cells in response to oxidative stimuli. Signal Transduct. Tar. 6 , 242 (2021).
Arra, M. et al. LDHA-mediated ROS generation in chondrocytes is a potential therapeutic target for osteoarthritis. Nat. Commun. 11 , 3427 (2020).
Article ADS PubMed PubMed Central Google Scholar
Dix, C. et al. C-reactive protein, immunothrombosis and venous thromboembolism. Front. Immunol. 13 , 1002652 (2022).
Branchford, B. R. & Carpenter, S. L. The role of inflammation in venous thromboembolism. Front. Pediatr. 6 , 142 (2018).
Gupta, L. et al. Inflammation in cardiovascular disease: A comprehensive review of biomarkers and therapeutic targets. Cureus 15 , e45483 (2023).
PubMed PubMed Central Google Scholar
Wu, J. et al. Lipidomic signatures align with inflammatory patterns and outcomes in critical illness. Nat. Commun. 13 , 6789 (2022).
Sung, Y. J. et al. Deep vein thrombosis exhibits characteristic serum and vein wall metabolic phenotypes in the inferior vena cava ligation mouse model. Eur. J. Vasc. Endovasc. 55 , 703–713 (2018).
Ma, N., et al. Plasma metabonomics and proteomics studies on the anti-thrombosis mechanism of aspirin eugenol ester in rat tail thrombosis model. J. Proteom. 215 , 103631 (2020).
Lee, T. Y. et al. Platelet autophagic machinery involved in thrombosis through a novel linkage of AMPK-MTOR to sphingolipid metabolism. Autophagy 17 , 4141–4158 (2021).
Engelmann, B. & Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 13 , 34–45 (2013).
Byrnes, J. R. & Wolberg, A. S. Red blood cells in thrombosis. Blood 130 , 1795–1799 (2017).
Michno, A., Raszeja-Specht, A., Jankowska-Kulawy, A., Pawelczyk, T. & Szutowicz, A. Effect of L-carnitine on acetyl-CoA content and activity of blood platelets in healthy and diabetic persons. Clin. Chem. 51 , 1673–1682 (2005).
Hurot, J. M., Cucherat, M., Haugh, M. & Fouque, D. Effects of L-carnitine supplementation in maintenance Hemodialysis patients: A systematic review. J. Am. Soc. Nephrol. 13 , 708–714 (2002).
Maekawa, K. et al. Higher lactate and purine metabolite levels in erythrocyte-rich fresh venous thrombus: Potential markers for early deep vein thrombosis. Thromb. Res. 177 , 136–144 (2019).
Jiang, X. et al. Metabolites associated with the risk of incident venous thromboembolism: A metabolomic analysis. J. Am. Heart Assoc. 7 , e010317 (2018).
White, R. H. The epidemiology of venous thromboembolism. Circulation 107 , I4–I8 (2003).
Navarrete, S., et al. Pathophysiology of deep vein thrombosis. Clin. Exp. Med. 23 , 645–654 (2022).
Smith, C. A., Want, E. J., O’Maille, G., Abagyan, R. & Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using Nonlinear peak alignment, matching, and identification. Anal. Chem. 78 , 779–787 (2006).
Gong, Y. et al. Metabolic-pathway-based subtyping of triple-negative breast cancer reveals potential therapeutic targets. Cell Metab. 33 , 51–64 (2021).
Liu, Q. et al. Altered faecal microbiome and metabolome in IgG4-related sclerosing cholangitis and primary sclerosing cholangitis. Gut 71 , 899–909 (2022).
Zhang, X. et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 70 , 761–774 (2021).
Xiao, Y., et al. Comprehensive metabolomics expands precision medicine for triple-negative breast cancer. Cell Res. 32 , 477–490 (2022).
Prakash, A. et al. Hybrid data acquisition and processing strategies with increased throughput and selectivity: pSMART Analysis for global qualitative and quantitative analysis. J. Proteome Res. 13 , 5415–5430 (2014).
Thevenot, E. A., Roux, A., Xu, Y., Ezan, E. & Junot, C. Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS statistical analyses. J. Proteome Res. 14 , 3322–3335 (2015).
Basu, S. et al. Sparse network modeling and metscape-based visualization methods for the analysis of large-scale metabolomics data. Bioinformatics 33 , 1545–1553 (2017).
Shannon, P. et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 13 , 2498–2504 (2003).
Pang, Z. Q. et al. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 49 , W388–W396 (2021).
Xia, J. G. & Wishart, D. S. MetPA: a web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 26 , 2342–2344 (2010).
Jewison, T. et al. SMPDB 2.0: Big Improvements to the Small Molecule Pathway Database. Nucleic Acids Res. 42 , D478–D484 (2014).
Xia, J. G. & Wishart, D. S. MSEA: a web-based tool to identify biologically meaningful patterns in quantitative metabolomic data. Nucleic Acids Res. 38 , W71–W77 (2010).
Subramanian, A. et al. A next generation connectivity Map: L1000 platform and the first 1,000,000. Profiles Cell 171 , 1437–1452 e1417 (2017).
PubMed Google Scholar
Franciosa, G. et al. Proteomics of resistance to Notch1 inhibition in acute lymphoblastic leukemia reveals targetable kinase signatures. Nat. Commun. 12 , 2507 (2021).
Members, C.-N. & Partners Database resources of the National Genomics Data Center, China National Center for Bioinformation in 2022. Nucleic Acids Res. 50 , D27–D38 (2022).
Chen, T. et al. The genome sequence archive family: Toward explosive data growth and diverse data types. Genom. Proteom. Bioinform. 19 , 578–583 (2021).
Ma, J. et al. iProX: an integrated proteome resource. Nucleic Acids Res. 47 , D1211–D1217 (2019).
Chen, T. et al. iProX in 2021: connecting proteomics data sharing with big data. Nucleic Acids Res. 50 , D1522–D1527 (2022).
Download references
Acknowledgements
We thank the dedicated participants who made this study possible. We thank the assistance from Shanghai Biotree Biotech Co.Ltd for plasma metabolomics analysis. We also thank the High-Performance Computing Platform and Instrument Analysis Center of Xi’an Jiaotong University for supporting this study. This study is supported by grants from the National Natural Science Foundation of China (32170616 (T.L.Y), 32370653 (Y.G), and 82170896 (Y.G)), Key Research and Development Project of Shaanxi Province (2019ZDLSF01-09 (H.Z.X) and 2022GXLH-01-22 (T.L.Y)), Social Development Foundation of Shaanxi Province (2022SF-394 (P.F.W)), Science Fund for Distinguished Young Scholars of Shaanxi Province (2021JC-02 (T.L.Y)), Innovation Capability Support Program of Shaanxi Province (2022TD-44 (T.L.Y)), Science and Technology Planning Project of Xi’an (2019115713YX012SF052 (K.Z)), and the Fundamental Research Funds for the Central Universities.
Author information
These authors contributed equally: Kun Zhang, Pengfei Wang, Wei Huang.
Authors and Affiliations
Department of Trauma Surgery, Honghui Hospital, Xi’an Jiaotong University, Xi’an, Shaanxi, P. R. China
Kun Zhang, Pengfei Wang, Wei Huang, Hanzhong Xue, Jia-Bin Chen, Ding Tian, Na Yang, Yifan Liang, Chungui Liu, Dongyang Li, Kun Zhang & Yan Guo
Key Laboratory of Biomedical Information Engineering of Ministry of Education, Biomedical Informatics & Genomics Center, School of Life Science and Technology, Xi’an Jiaotong University, Xi’an, Shaanxi, P. R. China
Kun Zhang, Wei Huang, Shi-Hao Tang, Hao Wu, Yu Rong, Shan-Shan Dong, Yan Zou, Tie-Lin Yang & Yan Guo
Key Laboratory of Biology Multiomics and Diseases in Shaanxi Province Higher Education Institutions, Xi’an Jiaotong University, Xi’an, Shaanxi, P. R. China
Instrument Analysis Center, Xi’an Jiaotong University, Xi’an, Shaanxi, P. R. China
You can also search for this author in PubMed Google Scholar
Contributions
K.Z. performed the data analyses and wrote the manuscript. W. H., H.Z.X. and P.F.W. evaluated the clinical images and supervised sampling. S.H.T., H.W., J.B.C., Y.Z. (Yan Zou), Y.F.L., C.G.L. and D.Y.L. recruited samples and collected clinical data; Y.Z. (Ying Zhang) performed proteomics analyses. Y.R. generated figures for the manuscript. Y.G., K.Z. and T.L.Y. designed, coordinated, and supervised the project. Y.G., T.L.Y. and S.S.D. revised the manuscript. D.T. and N.Y. performed administrative assistance.
Corresponding authors
Correspondence to Kun Zhang , Tie-Lin Yang or Yan Guo .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks Ahsan Khandoker, Antonino Tuttolomondo, Belinda Willard, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, description of additional supplementary files, supplementary data 1, supplementary data 2, supplementary data 3, supplementary data 4, supplementary data 5, supplementary data 6, supplementary data 7, supplementary data 8, supplementary data 9, supplementary data 10, supplementary data 11, supplementary data 12, supplementary data 13, supplementary data 14, supplementary data 15, supplementary data 16, supplementary data 17, reporting summary, source data, source data, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Zhang, K., Wang, P., Huang, W. et al. Integrated landscape of plasma metabolism and proteome of patients with post-traumatic deep vein thrombosis. Nat Commun 15 , 7831 (2024). https://doi.org/10.1038/s41467-024-52262-0
Download citation
Received : 14 July 2023
Accepted : 02 September 2024
Published : 07 September 2024
DOI : https://doi.org/10.1038/s41467-024-52262-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.

IMAGES
VIDEO
COMMENTS
Deep Vein Thrombosis Nursing Care Management and ...
Nurse Hakeem works on a Medical-Surgical unit and is caring for Lucille, a 72-year-old female who's being admitted for a deep vein thrombosis, or DVT in her left iliofemoral vein. After settling Lucille in her room, Nurse Hakeem goes through the steps of the Clinical Judgment Measurement Model to make clinical decisions about Lucille's care ...
This page titled 9: Case Study #8- Deep Vein Thrombosis (DVT) is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Glynda Rees, Rob Kruger, and Janet Morrison via source content that was edited to the style and standards of the LibreTexts platform.
9 Deep Vein Thrombosis Nursing Care Plans
Deep Vein Thrombosis (DVT) nursing NCLEX case study and review on the d symptoms, treatment, pathophysiology, and key nursing interventions.A DVT is the form...
Deep Vein Thrombosis - StatPearls
Deep vein thrombosis: pathogenesis, diagnosis, and ...
Deep vein thrombosis (DVT) is the formation of blood clots (thrombi) in the deep veins. It commonly affects the deep leg veins (such as the calf veins, femoral vein, or popliteal vein) or the deep veins of the pelvis. ... Meade TW. Recent respiratory infection and risk of venous thromboembolism: case-control study through a general practice ...
Jamie Douglas is a 35 year old female. She was recently diagnosed with laryngeal cancer, for which she had a laryngectomy 12 days ago. She now has a permanent tracheostomy and communicates by writing on a white board. Her cancer was caught in the early stages and she is expected to make a full recovery. Jamie has a supportive husband of three ...
One, some, or all options may be correct.) Perform a focused assessment on the lower right extremity. Implement a numeric pain assessment on a scale of 1 to 10. Document a baseline of vital signs including a pulse oximetry. Use at least two client identifiers before administering the medication.
Case Study: Deep Vein Thrombosis. The nurse is assigned to care for a 70-year-old retired bus driver who has just been admitted to the medical floor with R leg deep vein thrombosis (DVT). The patient has a 48 pack-year smoking history, although he states he quit 2 years ago.
Deep vein thrombosis (DVT) commonly affects the lower limb, with clot formation beginning in a deep calf vein and propagating proximally.1 It is a common venous thromboembolic (VTE) disorder with an incidence of nearly 1.6 per 1000 inhabitants a year.2 3 4 The rate of involvement of particular sites varies: distal veins 40%, popliteal 16%, femoral 20%, common femoral 20%, and iliac veins 4%.1 ...
PNW College of Nursing NUR 28201 Deep Vein Thrombosis Case study: Patient Profile D. is a 74-year-old obese Hispanic woman who is in the third postoperative day after an open reduction internal fixation (ORIF) for repair of a left femoral neck fracture after a fall at home.
Trixie, finally leashed, is lifted down and out they go through the back door into the cold winter air. Erin gets down the steps and leans against the house to catch her breath. Meanwhile, Trixie relieves herself against a flower pot. After about a minute, Erin begins to walk very slowly, with Trixie pulling on the leash.
Background: Deep vein thrombosis represents a threat to public health and a heavy economic burden to society, and often occurs as a complication or cause of death in bedridden patients. How to prevent deep vein thrombosis is a general concern in clinical practice. However, it remains uncertain whether the risk factors for deep vein thrombosis would be affected by different bed-rest durations.
disease: acute chest syndrome, avascular necrosis of the hips, and chronic kidney disease. The case is instructive as an example of DVT after exercise of the lower extremities, which has not been documented well. The case also illustrates a number of health sequelae of sickle cell disease that mimic more common musculoskeletal complaints. Sports medicine providers will have to consider these ...
Introduction. Venous Thromboembolism (VTE) includes Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE), which occur from a blood clot formed in the venous circulation. 1 VTE has become the third most significant common cardiovascular disease after stroke and myocardial infarction and is considered the main problem among medical and surgical patients. 2, 3
Case Study - Deep Vein Thrombosis (DVT) Ron L. is a 75-year-old man just admitted to your medical surgical orthopedic floor after undergoing a right knee arthroplasty surgery. He arrives awake and alert with no pain. The surgical dressing is clean and dry. Ron has anti-embolic stockings and Sequential Compression Devices to both legs.
The practice of nurses towards prevention of deep vein thrombosis. From this study majority of the respondents, 230 (57.5%) had poor practice and only 170 (42.5%) had a good practice on the prevention of DVT among staff nurses. ... The study was tried to include study participants in all nursing disciplines. ... A population-based case-control ...
Case 8 describes a patient's experience with Deep Vein Thrombosis (DVT) that develops as a complication of her hospitalization. Learners reviewing this case have an opportunity to explore how DVT develops, treatment options and prevention.
IN-CLASS/ONLINE CASE STUDY. Deep Vein Thrombosis. Paient Proile D. is a 74-year-old obese Hispanic woman who is in the third postoperaive day ater an open reducion internal ixaion (ORIF), for repair of a let femoral neck fracture ater a fall at home.. Subjecive Data States pain in her let hip is a 4 to 5 on a 1-to-10 scale States pain in her let calf area is a 3 on a 1-to-10 scale
DVT is the development of a blood clot in a major deep vein in the leg, thigh, pelvis, or abdomen. It may also occur in less common locations such as the arm veins; the portal, mesenteric, ovarian, or retinal veins; or the veins and venous sinuses of the brain. DVT can result in impaired venous blood flow. DVT is rarely life-threatening on its ...
Abstract. Practicing interventional radiologists (IRs) are routinely faced with challenging decisions that pertain to the management of patients with acute deep vein thrombosis (DVT). In this article, we describe five questions that are commonly posed by interventionalists and discuss both the indirect published evidence as well as our own ...
Deep vein thrombosis (DVT) is a major health problem that can lead to a variety of complications, such as post-thrombotic syndrome, recurrent DVT, and life-threatening pulmonary embolism (PE) 1,2 ...