- Frontiers in Anesthesiology
- Perioperative Medicine
- Research Topics

Patient Blood Management and Transfusion Strategies in Perioperative Settings
Total Downloads
Total Views and Downloads
About this Research Topic
Recent years have witnessed an outstanding and impressive contribution of patient blood management strategies to global perioperative patient care, particularly with the implementation of pre-, per-, and post-operative strategies. The Management of blood transfusion in the perioperative period is of particular importance due to several reasons, including possible complications related to the use of blood products, not limited to immunizations, the risk of infection, Transfusion Acute Lung Injury (TRALI), electrolyte disturbances, and coagulopathy. Several years ago, the three pillars matrix for patient perioperative blood management was defined and included optimizing red cell mass, minimizing blood loss, and mobilizing and optimizing the physiological reserve of anaemia. (Isbister). Thus, different techniques and strategies were proposed in each pillar to be implemented either in the pre-, per, or postoperative period, aiming either to reduce blood loss, avoid the use of blood products, or use alternatives to allogeneic blood transfusion. These techniques include (and are not limited to) normovolemic haemodilution, autologous blood transfusion, pre-operative optimization using Iron with or without erythropoietin, the use of cell saver, and many more. In these management strategies, drugs such as Tranexamic acid are also useful tools. Among the remaining questions in the process were: to define the right product needed for the replacement therapy, the specific lab test needed to help such determination, and the ideal timing and protocol to decide on this replacement therapy. Whenever surgery would result in significant blood loss (typically 500-1000 ml or more), a discussion involving the surgical team and haemostasis expert is needed to design a targeted patient blood management strategy. Major surgeries have been associated with higher blood loss. Depending on the pathophysiology of the patient's disease and comorbidities, the surgery type, the risk of significant blood loss, and the case complexity, many techniques have been proposed in different settings including all pediatric populations’ surgeries, cardiac, trauma-based, orthopedic, digestive surgeries, and obstetrics. The previous works have also led to the diversification of techniques and strategies, using techniques such as thromboelastography (TEG) and rotational thromboelastography (ROTEM). advantages of this latter technique include a shorter turnaround time, rapid decision-making, and guidance on the specific blood product, factor concentrate, or adjuvant needed. These techniques could be used alone or with specific decision algorithms targeting patient needs, procedures, hemostatic objectives, and possible alternatives to allogeneic blood transfusion. These technics and strategies can be implemented for all ages and might be used in various applications. An example of these management strategies was recently published in a review article on the advantages of Prothrombin complex concentrate (PCC) in the pediatric population in the failure of blood transfusion to achieve hemostasis and coagulation based on experiences in cardiac, trauma, and other coagulopathy scenarios, emphasizing particularly on Pediatric patients with congenital heart disease. At this time, researchers are studying various algorithms and bundle strategies adapted to specific patients, and postoperative coagulopathy management strategies that could be used to optimize patient care. Others research areas are in perioperative blood transfusion management. In this research topic, we are seeking original papers from authors with articles focusing on the domain of perioperative blood management strategies and associated domains such as hemostasis and perioperative coagulation.
Keywords : Patient Blood Management, Blood Transfusion, Perioperative, Blood Sparing Strategies, Transfusion Medicine, Bleeding
Important Note : All contributions to this Research Topic must be within the scope of the section and journal to which they are submitted, as defined in their mission statements. Frontiers reserves the right to guide an out-of-scope manuscript to a more suitable section or journal at any stage of peer review.
Topic Editors
Topic coordinators, submission deadlines.
| Manuscript | |
| Manuscript Extension |
Participating Journals
Manuscripts can be submitted to this Research Topic via the following journals:
total views
- Demographics
No records found
total views article views downloads topic views
Top countries
Top referring sites, about frontiers research topics.
With their unique mixes of varied contributions from Original Research to Review Articles, Research Topics unify the most influential researchers, the latest key findings and historical advances in a hot research area! Find out more on how to host your own Frontiers Research Topic or contribute to one as an author.
On Medicine

Topics in blood transfusion
Today marks World Blood Donor Day and so we asked Deputy Editor for Journal of Intensive Care , Hiroshi Morisaki, to explain more about the importance of blood transfusion, and how research in this area is progressing.
Hiroshi Morisaki 14 Jun 2016
To date, humans have uncovered a number of natural principles and issues such as the origin and mysteries of the universe, the earth and even life. We have simultaneously developed innumerable non-natural products for human use.
However, we have not yet succeeded in creating a man-made, cost-effective alternative to red blood cells (RBCs) despite the performance of extensive research and numerous clinical trials.
A life-saving intervention
The cellular health of the host requires an oxygen (O 2 ) supply that matches the O 2 requirements of its tissue. An insufficient O 2 supply results in ischemia, subsequently inducing tissue and/or organ injury, frequently observed in critically ill patients.
RBC transfusion, first performed over 300 years ago, remains a fundamental life-saving intervention in medicine.
Hemoglobin, which is enclosed in RBCs as an O 2 carrier, plays the most important role in supplying O 2 to the tissues. Accordingly, RBC transfusion, first performed over 300 years ago, remains a fundamental life-saving intervention in medicine.
Until the early 1980s, RBC transfusion was considered to be practically risk-free and a truly effective intervention in patients with active bleeding or anemia due to a variety of reasons in the intensive care field.
However, the threat of potentially-fatal transfusion-related infections, i.e., human immunodeficiency virus, has led physicians throughout the world to obviate this conventional intervention to the extent that is possible.
Research in the literature
In 1999, Canadian investigators examined the effects of a restrictive RBC transfusion strategy in comparison to a liberal strategy in critically ill patients. They indicated that a restrictive strategy was at least as effective as and possibly superior to a liberal transfusion strategy in critically ill patients with some exceptions.
Although several debates are currently ongoing, most physicians now agree that a restrictive strategy to limit RBC transfusion is a valuable approach in the treatment of stable patients with anemia.
However, we need to be cautious when interpreting the results of the Canadian study as it indicated some exceptions.
Even though we have seen extraordinary advances in medical science and related technology over the last several decades, clinical practices have been determined based on the balance between the benefits and related risks of intervention.
They found that a restrictive transfusion strategy was significantly associated with reduced mortality in younger patients and in those with less severe conditions. In other words, a liberal RBC transfusion strategy might be more effective for older patients and patients with more severe conditions.
RBC transfusion by itself is not an exception. The level of hemoglobin that works in some patients may not work in others. Indeed, a previous cohort study of intensive care unit patients suggests that restrictive RBC transfusion policies may not be uniformly applicable in the clinical setting.
World Blood Donor Day
In 2012, the World Health Organization (WHO) released a document entitled, “ Blood donor selection – Guideline on assessing donor suitability for blood donation ”. In this guideline, the authors noted that a careful process to assess the suitability of donors is essential for protecting the safety and sufficiency of the blood supply, and safeguarding the health of both ‘recipients’ and ‘donors’. We should therefore understand that blood transfusion not only improves the recipients’ conditions but also affects the donors’ health.
Away from the discussion of whether restrictive or liberal RBC transfusion strategies should be applied, natural human blood is needed to save the lives in emergency and long-term treatment settings, even in the 21 st century.
If you believe yourself to be in good health, you should donate your blood to prove it and to save lives at the same time.
View the latest posts on the On Medicine homepage
- Latest Posts
Hiroshi Morisaki
Latest posts by hiroshi morisaki ( see all ).
- Topics in blood transfusion - 14th June 2016
Recommended posts
Popular on medicine tags.
- BMC Medicine
- clinical trials
- medical evidence
- Genome Medicine
- ISRCTN Registry
- Alzheimer's
- Alzheimer's Research & Therapy
- breast cancer
Popular posts
- Most shared
Sorry. No data so far.
Most Shared Posts
- Teaching recovery techniques plus parenting: a cluster randomized controlled trial in Ukrainian schools in Ternopil (TRUST)
- Winners of the 2022 Cardio-Oncology Journal Prize
- A unique approach. A unique population. Investing in nurses’ mental health
- Does the midwife-led continuity of carer model improve birth outcomes and maternal mental health in vulnerable women?
- A proactive diabetes review model: from concept to nurse-led research
- March 2024 (1)
- February 2024 (2)
- January 2024 (1)
- November 2023 (1)
- October 2023 (1)
- September 2023 (7)
- July 2023 (1)
- June 2023 (1)
- May 2023 (4)
- April 2023 (1)
- March 2023 (2)
- February 2023 (7)
Be a Part of an AABB Committee - Apply by Feb. 15
AABB to Present on Global Transfusion Projects During Bloodsafe Symposium
June 18, 2024
AABB will present on the Association’s global transfusion projects during the 2024 Bloodsafe Symposium, taking place Friday in Accra, Ghana. In her presentation, Christine Bales, BS, MLS (ASCP) I, CQA (ASQ), AABB’s vice president, global impact, will highlight AABB’s international accreditation for blood banks and transfusion services, cellular therapy and relationship testing. Bales will also highlight the AABB Quality Certificate Program , available in both English and Spanish, and version 2 of the Fundamental Standards for Blood Collection . The presentation will also address AABB’s Global Standards Committee, the Global Transfusion Forum, the AABB Leadership Certificate and the CABP credential .
The Bloodsafe Program supports research to enhance the availability of safe blood for patients in low or lower-middle-income countries in Sub-Saharan Africa. Additional information about the initiative and the 2024 Bloodsafe Symposium is available online .

AABB is now the Association for the Advancement of Blood & Biotherapies. Learn more about our new name and brand – and watch as we evolve throughout 2022.
Privacy Policy | Terms of Use

4550 Montgomery Avenue Suite 700, North Tower Bethesda, MD 20814 301.907.6977
www.aabb.org
- Our Social Media
AABB (Association for the Advancement of Blood & Biotherapies) is an international, not-for-profit organization representing individuals and institutions involved in the fields of transfusion medicine and biotherapies. The Association works collaboratively to advance the field through the development and delivery of standards, accreditation and education programs. AABB is dedicated to its mission of improving lives by making transfusion medicine and biotherapies safe, available and effective worldwide.
© 2022 All Rights Reserved. AABB - Association for the Advancement of Blood & Biotherapies
Web Design and Development by Matrix Group International, Inc .
- Open access
- Published: 15 June 2024
Cultural competences among future nurses and midwives: a case of attitudes toward Jehovah’s witnesses’ stance on blood transfusion
- Jan Domaradzki ORCID: orcid.org/0000-0002-9710-832X 1 na1 ,
- Katarzyna Głodowska ORCID: orcid.org/0000-0001-8887-3364 1 ,
- Einat Doron ORCID: orcid.org/0009-0002-4807-3471 2 ,
- Natalia Markwitz-Grzyb ORCID: orcid.org/0009-0004-8126-838X 1 &
- Piotr Jabkowski ORCID: orcid.org/0000-0002-8650-9558 3 na1
BMC Medical Education volume 24 , Article number: 663 ( 2024 ) Cite this article
171 Accesses
Metrics details
Transcultural nursing recognises the significance of cultural backgrounds in providing patients with quality care. This study investigates the opinions of master’s students in nursing and midwifery regarding the attitudes of Jehovah’s Witnesses towards refusing blood transfusions.
349 master’s students in nursing and midwifery participated in a quantitative study and were surveyed via the Web to evaluate their awareness of the stance of Jehovah’s Witnesses on blood transfusions and the ethical and legal dilemmas associated with caring for Jehovah’s Witness (JW) patients.
The study yielded three significant findings. It unequivocally demonstrates that nursing and midwifery students possess inadequate knowledge regarding Jehovah’s Witnesses’ stance on blood transfusions and their acceptance of specific blood products and medical procedures. Despite being cognisant of the ethical and legal dilemmas of caring for JW patients, students lack an understanding of patients’ autonomy to reject blood transfusions and their need for bloodless medicine. Students also articulated educational needs regarding cultural competencies regarding the Jehovah’s Witnesses’ beliefs on blood transfusions and non-blood management techniques.
Conclusions
Healthcare professionals need the knowledge and skills necessary to provide holistic, patient-centred and culturally sensitive care. This study emphasises the urgent need for university curricula and nursing postgraduate training to include modules on transcultural nursing and strategies for minimising blood loss.
Peer Review reports
Transcultural nursing entails nurses’ ability to approach each patient in a culturally sensitive and inclusive manner. It emphasises the need to consider patients’ cultural backgrounds, including values and norms, religious beliefs, traditional customs and lifestyles, as an essential part of quality care [ 1 , 2 ], and has a central role in the healthcare domain, requiring nurses to embody cultural competence as an integral aspect of their daily practice [ 3 ]. The concept of cultural competence itself, which originated in social work, was developed in the 1970s by Madeleine Leininger, who emphasised that healthcare should include multiple aspects of culture, as they influence the way a person or a group perceives health and disease, approaches healthcare and copes with illness or death [ 4 , 5 , 6 ]. Cultural competence therefore entails a process that involves a heightened self-awareness, an appreciation of diversity and the acquisition of knowledge concerning cultural strengths [ 7 ]. Nurses conceptualise cultural competence as the capacity to understand cultural distinctions and the continuous process of effectively engaging with diverse individuals, helping them deliver quality care to a culturally diverse population [ 5 , 6 ]. Culturally competent nurses show sensitivity to issues of culture, religion, race, ethnicity, gender and sexual orientation, highlighting their ability to communicate, perform cultural assessments and acquire knowledge related to diverse health practices.
Culturally competent nurses display a nuanced understanding of diverse cultural practices, enabling them to discern distinct patterns and formulate individualised care plans tailored to meet both healthcare goals and the individual needs of every patient [ 8 ]. While the overarching goal of transcultural nursing is to foster the values, knowledge and skills required for the provision of culturally different and sensitive care within a culturally diverse environment [ 2 , 9 ], it is an integral part of holistic nursing which aims at addressing patients’ physical, psychological, emotional, spiritual and social needs, and underscores the imperative of individualised care [ 10 ]. In pursuing holistic care, nurses must meticulously consider cultural variations in their care plans, ensuring a thorough and culturally competent approach [ 8 ]. Appreciating patients’ cultural perspectives is paramount in delivering effective care and navigating intricate ethical scenarios, particularly within diverse cultural backgrounds [ 5 , 6 ]. A detailed understanding of patients’ cultural backgrounds ensures a holistic and culturally competent approach to nursing care.
Given their prominence as the largest group of healthcare professionals, nurses are crucial in addressing global health challenges and disparities. The evolving landscape of global healthcare needs adjustments in nursing practice, positioning nurses at the forefront of addressing cultural backgrounds and global events that affect patients’ needs [ 11 ]. Nurses must be prepared to discern global healthcare issues and cultivate skills to attain cultural competences [ 12 , 13 ].
While there are many groups of patients whose cultural background, religious beliefs or traditional customs are an essential part of their identity and may therefore influence their health and medical behaviour, psychological reaction to illness, treatment preferences and communication with the healthcare team, one notable example is Jehovah’s Witnesses (JWs), a Christian denomination founded in 1872 in the United States by Charles Taze Russell. Although JWs represent a religious minority in Poland, they have been enormously successful and, according to the Central Statistical Office, there are currently more than 114,000 JWs in Poland, making them the third largest religious group in Poland after Roman Catholics and Orthodox Christians [ 14 ].
One of the central beliefs adopted by JWs is their refusal to accept allogenic blood transfusions, even in cases in which the outcome may be death [ 15 , 16 , 17 , 18 , 19 , 20 , 21 ]. While JWs argue that there are also some medical grounds for refusing blood, this refusal is based on religious grounds and is the result of their interpretation of several verses in the Bible ( Genesis 9:4; Leviticus 17:10; Deuteronomy 12:23; Acts 15:28–29) [ 22 ]. JWs therefore refuse transfusions of whole blood (including pre-operative autologous donation, i.e. auto-transfusion) and its four primary components (red cells, white cells, platelets and unfractionated plasma). In 2000, however, JWs were informed that ambiguity in the Bible meant that the use of blood fractions is not absolutely prohibited and that they may accept them as a matter of personal choice. JWs may consequently accept such derivatives of primary blood components as albumin solutions, cryoprecipitate, clotting factor concentrates and immunoglobulins [ 23 , 24 ]. At the same time, although JWs reject allogeneic blood transfusions and pre-operative autologous transfusions, many other medical procedures are permitted and are left to the discretion of individual members, including blood donation, autologous transfusions, intra-operative blood salvage, dialysis, aphaeresis and cardiac bypass or organ transplants (on condition it is performed on a bloodless basis) [ 23 , 24 ].
Many clinicians, including physicians (e.g. cardiac surgeons, obstetricians and anaesthesiologists), nurses and midwives who treat their patients with blood products [ 23 , 24 , 25 , 26 , 27 , 28 ], either whole blood transfusions or blood component therapy (e.g. red cell concentrates, fresh frozen plasma, platelet concentrates or cryoprecipitate) [ 29 , 30 ] therefore face a challenging ethical and medico-legal dilemma: whether to respect patients’ autonomy and right to follow their religious beliefs, albeit this may result in death, or to remain faithful to the doctor’s duty to preserve life even against patients’ own wishes [ 16 , 17 , 18 , 19 , 26 , 31 ]. Although there is no official data, according to some estimates, up to one thousand JWs die in the United States each year due to their refusal of blood transfusions [ 32 , 33 , 34 ].
Since JWs carry a ready-made document regarding health care, a No Blood card, i.e. a declaration of the person’s resolution against blood transfusions, signed by the person and confirmed by two witnesses, which is compatible with the requirements of the Civil Code and is binding on an attending doctor. According to Polish law, any JW patients who have procedures performed on them that involve a blood transfusion against their will have the right to initiate any of three types of proceedings: criminal, civil or disciplinary [ 35 , 36 , 37 , 38 ].
If a JW patient refuses a blood transfusion, healthcare professionals must provide comprehensive information regarding treatment methods available with blood substitutes and other alternative methods used during surgical procedures. Since JWs reject pre-operative autologous blood donation, they may, for instance, be offered bloodless medical care, i.e. transfusion-free health care that uses neither allogeneic blood transfusion nor blood products during medical procedures and surgeries but instead uses blood conservation techniques and various blood transfusion alternatives, such as extra-corporeal circulation combined with recovery of patients’ blood from the surgical field in a closed circuit [ 39 , 40 , 41 ].
While earlier studies in Poland have focused on the moral (ethical) dilemmas and legal aspects of providing care to JW patients [ 35 , 36 , 37 , 38 ], there remains a shortage of research on the awareness among (future) healthcare professionals of JWs’ refusal of blood transfusions. This study, therefore, seeks to explore nursing and midwifery master’s students’ views on JWs’ attitudes towards blood transfusions, including (1) their awareness regarding JWs’ stance on blood transfusion, (2) students’ opinions on the ethical and legal dilemmas related to caring for JW patients, (3) students’ educational needs for non-blood management techniques, and (4) factors associated with future nurses’ and midwifes’ perception of JWs’ refusal of blood transfusion.
Study design
This research was part of a larger project aimed at assessing healthcare professionals’ attitudes towards JWs’ refusal of blood transfusions [ 42 ], but it was designed to explore the views of nursing and midwifery master’s students. It includes data from a self-administered, anonymised Web survey about future healthcare professionals’ awareness of JWs’ stance and the ethical and legal dilemmas related to their refusal of blood transfusions.
Research tool
A modified version of a previously developed questionnaire that assessed the knowledge and attitudes of Polish nurses towards JWs’ stance in refusing blood transfusions was used [ 42 ]. The development of the questionnaire followed the guidelines of the European Statistical System [ 43 ]. It was constructed after the published literature had been reviewed [ 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 31 , 35 , 36 , 37 , 38 ], and a focus group discussion with four research experts (a nurse, a medical sociologist and two Jehovah’s Witnesses) was carried out. They discussed the list of questions regarding critical issues related to JWs’ stance on refusing blood transfusions and decided which issues to address. A preliminary questionnaire was pre-tested on ten nursing students via a communication platform used at the Poznan University of Medical Sciences for educational purposes (Microsoft Teams), which resulted in reformulating the three questions. It was then re-evaluated by the same experts: a nurse, a sociologist and two JWS.
The final version of the questionnaire consisted of 25 questions divided into four sections. The first dealt with students’ demographic data. The second section addressed students’ knowledge and awareness of JWs’ stance in refusing blood transfusions. The third section included questions about students’ opinions on the bioethical and legal dilemmas related to JWs’ stance in refusing blood transfusions. The last section referred to students’ educational needs regarding bloodless medicine , i.e., non-blood management strategies to minimise blood loss during surgery and obviate the need for blood transfusions (Supplementary material).
Participants and setting
Nursing and midwifery master’s students were targeted for recruitment. The rationale behind choosing such students was two-fold: firstly, after completing the first stage of studies (3 years), which ends with a Bachelor’s Degree in Nursing or Midwifery, they are already qualified healthcare professionals and during the second stage (2 years), i.e. master’s studies, the vast majority already worked professionally in a variety of healthcare facilities; secondly, as qualified nurses and midwives who already worked in the profession, they were liable to face bioethical and legal dilemmas related to caring for a JW patient who refuses a blood transfusion in a life-threatening situation.
The inclusion criteria were: (1) being a nursing or midwifery master’s student, (2) being enrolled in the Poznań University of Medical Science (PUMS), (3) being willing to participate in the study, and (4) providing written informed consent before completing the survey.
Data collection
The study was conducted between October and November 2023 among master’s students of nursing and midwifery at PUMS. Students were recruited during regular classes.
Before completing the survey, all students were informed by two members of the research team (JD and KG) about the study’s aim, as well as its voluntary, anonymous, confidential and non-compensatory character. They were also instructed about their right to abandon the survey without consequences. After informed consent was obtained from all students who agreed to complete the survey, all participants received a QR code and, once they had scanned it with their smartphones, they received access to the questionnaire posted on a Web platform. Completing the questionnaire took between 8 and 10 min.
Ethical issues
This study followed the principles of the Declaration of Helsinki [ 44 ]. Ethics and research governance approval were also obtained from the Poznan University of Medical Sciences Bioethics Committee (KB – 760/22). All participants provided written informed consent before completing the survey.
Data analysis
All analyses were conducted using the R Project for Statistical Computing [ 45 ], where we utilised various open-source R packages such as tidyverse [ 46 ], flextable [ 47 ] and ggplot [ 48 ] for tasks including data manipulation, statistical analysis and data visualisation.
We conducted a comprehensive analysis to examine potential statistical differences among the socio-demographic categories of students participating in the survey. Firstly, we implemented a descriptive analysis, offering insights into the variability and tendencies of the data. We also employed graphical representations of data, such as density curves, histograms and correlation plots, to depict the observed patterns visually. Finally, to rigorously assess the differences between various categories of survey participants, we applied formal statistical tests. A two-tail t-test for the mean and a chi-square test were employed to scrutinise the differences in variable distributions, ensuring a robust evaluation of the statistical significance of variations observed. Comparisons of 95% confidence intervals for the mean values were also undertaken to bolster the reliability of the findings. The analytical procedures chosen were paramount in providing a thorough and systematic exploration of the data, enabling a nuanced comprehension of potential distinctions between student groups and augmenting the scientific rigour of our study.
The main goal of our analysis was to assess the students’ knowledge of JWs’ stance in refusing blood transfusions. Respondents were presented with dozens of statements, some intentionally false, describing reasons for refusing an allogenic blood transfusion and medical procedures and the blood products accepted and those JWs would refuse. In total each respondent determined the truth of 51 sentences, based on which we built three indices of knowledge covering distinct aspects of JWs’ stance in refusing blood transfusions. While Index 1 measured the general knowledge of JWs’ stance in refusing blood transfusions; Index 2 measured knowledge regarding blood products approved by JWs; and Index 3 measured knowledge regarding medical procedures accepted by JWs. Note that each index consists of 17 statements formulated as a priori in a questionnaire to measure the students’ knowledge (consult Supplementary Materials for details). For each respondent the value of each index ranged from 0 (if none of the sentences were indicated correctly) to 17 (if the respondent indicated all the sentences correctly).
Of the 349 students approached, 302 (86.5%) participated in the study by completing the questionnaire (Table 1 ). Forty-seven students who refused to participate did so because they were either absent during the classes, lacked interest in the study or were unwilling to discuss their opinions. The feedback on surveys from the nursing students (NSs) was 145/188 (77.12%), and from the midwifery students (MSs) 157/161 (97.51%).
The sample comprised 145 NSs (48%) and 157 MSs (52%), all of Polish origin. While women predominated over men in the student body (95.7% vs. 4.3%), this disproportion results from the fact that both courses are strongly gendered in Poland. In 2021 women accounted for 73.76% of all medical and healthcare students in the country and this disproportion was even higher among nursing and midwifery students (89% and 99.54% respectively) [ 49 ].
Less than one-third (32.8%) of students claimed religion played any significant role in their life (32.4% NSs and 33.2% MSs) and 67.2% declared it was of little or no importance (67.6% NSs and 66.9% MSs).
A considerable number of respondents were professionally active (63.9%). The proportion of NSs working in their profession, however, was double that of MSs (86.2% vs. 43.3%, p < 0.001). 18.9% of respondents said that they had prior professional experience with patients who refused allogeneic blood transfusions because of their religious beliefs (17.2% and 20.4% MS).
Our analysis began by assessing the student’s knowledge of JWs’ stance in refusing blood transfusions. Figure 1 presents the distribution of the scores and correlation plots between the indices of students’ knowledge of JWs’ stance on blood transfusions. The results showed that the students scored highest on Index 1, with a mean score of 12.4, indicating that they had the greatest knowledge of JWs’ position on blood transfusion, on average correctly answering over 12 out of 17 statements. The highest mean score for Index 1 was followed by Index 2, with a mean score of 10.9, and Index 3, with the lowest mean score of 8.4, reflecting less knowledge about specific blood products and accepted medical procedures respectively. The correlations between all three indices were also generally low, highlighting the distinct nature of the domains of knowledge. In fact, Pearson’s linear correlation between Index 1 and Index 2 was − 0.14 ( p = 0.019), indicating a significant, albeit only slightly negative relationship. The correlation between Index 1 and Index 3 was 0.26 ( p < 0.001), indicating a significant but moderately positive relationship. The weakest Pearson correlation of 0.06 was between Index 2 and Index 3 ( p = 0.28), indicating almost no relationship. In conclusion, while students have a good knowledge of the JW position on blood transfusion, their knowledge of specific blood products and accepted medical procedures is limited, suggesting the need for increased educational efforts to improve students’ overall understanding of medical practices accepted by the JW.

Histograms and correlation plots for indexes of students’ knowledge on JWs’ stand toward blood transfusions
While the overall mean scores provide a general overview, specific group comparisons highlight nuanced differences. Table 2 compares the mean values of the indices measuring students’ knowledge of JWs’ stand on blood transfusions in groups delimited by selected socio-demographic characteristics.
The results show that midwifery students have greater knowledge regarding JWs’ concerns about blood transfusion but poorer knowledge of blood products and medical procedures accepted by JWs. The differences between the two categories of students are only significant, however, for the third index (the mean for nurses is 9.3, while for midwifery students it is 7.6, with p < 0.001), possibly indicating nurses’ deeper understanding of the issue of medical procedures. The participants’ employment status also plays a role, as those not currently working tended to have slightly lower mean scores (7.6 vs. 8.8, p < 0.001) in knowledge related to blood products and medical procedures accepted by JWs. Participants who attached little or no importance to religion and those who had never experienced a refusal also tended to have slightly lower knowledge scores in their awareness of medical the procedures accepted by JWs. The differences, however, remain negligible at p < 0.05.
Figure 2 outlines students’ perception of the bioethical and legal dilemmas surrounding the refusal of blood transfusion in JW patients in total and broken down into two groups of students, i.e., MSs and NSs. The majority of students experience bioethical dilemmas as most of them disagree with the right of JW parents to refuse blood transfusion for JW children (73.2% overall, with nurses tending to agree more often than midwifery students: 77.9% vs. 68.8%) and showed a limited understanding of JWs’ position on their choice of treatment methods (63.9% in total: 66.2 for nursing students and 61.8 for midwifery students), as well as agreeing that JWs should have the right to refuse blood transfusions on religious grounds in life-threatening circumstances (45.7% in general, with nursing students more likely to agree than midwifery students: 46.9 vs. 44.6). Regarding legal dilemmas, most students (83.4%) agreed that adult JW patients should have access to medical care using non-blood management techniques (midwifery students were more likely to agree than nursing students: 88.5% vs. 77.9%). Respondents also felt that the guardianship court should authorise blood transfusions for JW children in cases where parental consent is withheld (62.6%, with 88.5% of midwifery students and 77.9% of nursing students agreeing). A clear majority (74.8%) of participants also agreed that an individual’s decision to refuse treatment should be subject to legal regulation, with midwifery students (78.3%) more likely to agree than nurses (71.0%).

Students’ dilemmas related to JW’s stance toward blood transfusions
Table 3 illustrates the variations in students’ views regarding bioethical and legal dilemmas across categories delineated by socio-demographic characteristics. Although there is no discernible trend in the influence of specific socio-demographics, some interesting differences were observed. The results demonstrate that the differences between survey participant groups are negligible in almost all cases, so we will briefly describe the differences between midwifery and nursing students. MSs agree more strongly than their nursing counterparts with JWs’ stance on treatment methods in which they refuse allogeneic blood transfusion in adults (32.5% vs. 26.2%). While a slightly higher proportion of MSs support the right of JWs to refuse blood transfusions in life-threatening circumstances, NSs display a marginally higher inclination toward disagreement (48.4% vs. 41.4%). A discrepancy exists in accepting legal regulations describing the way to express informed consent for medical treatment, with MSs registering a notably higher agreement percentage than their nursing counterparts (78.3% vs. 71.0%). The only significant discrepancy between students of nursing and midwifery surfaces in their acceptance of the right to medical care from doctors specialised in non-blood management techniques, with MSs registering a notably higher agreement percentage than their nursing counterparts (88.5% vs. 77.9%, p = 0.040). The results also show that the distribution of opinions on whether JWs should have the right to refuse blood transfusions on religious grounds, even in life-and-death situations, is firmly based on the perceived role of religion in their lives ( p = 0.020).
Among students for whom religion plays a very or fairly important role 34.3% agree that JWs should have this right. Conversely, among those who see religion as playing little or no role in their lives, 50.2% agree that JWs should have the right to refuse blood transfusions. Those who see religion as very important in their lives are therefore more likely to oppose the right to refuse transfusions, while those who see religion as less important are more likely to support this right.
Finally, Table 4 presents students’ educational needs regarding non-blood management techniques. The results indicate that many nurses and midwives have had no courses on non-blood management techniques (overall 61.3%, with significantly ( p < 0.001) more midwifery students (75.2%) reporting having had no such courses during their studies, compared to nursing students (46.2%)). There was also a significant consensus in favour of the inclusion of mandatory courses on strategies to minimise blood loss in medical curricula (overall support above 80%, with midwifery students significantly more likely to agree (85.4%) than their nursing counterparts (75.2%), p < 0.001. A relatively small percentage of participants (11.3%) felt adequately prepared to care for patients who require non-blood management techniques despite this inclination, but nursing students were significantly ( p < 0.001) more likely to report being prepared than midwifery students (17.9% vs. 5.1%; p < 0.01). The findings underscore the need for targeted educational interventions and training programmes to bridge gaps in healthcare professionals’ preparedness for non-blood management, especially given the apparent positive disposition toward such training courses. Note that the differences between the nursing and midwifery students are statistically significant, except for their willingness to expand their knowledge about non-blood management techniques. In both groups, the vast majority of students declared the intention to expand their knowledge. Note that the differences for other socio-demographic categories are insignificant.
Poland remains one of Europe’s most ethnically and culturally homogeneous and religious countries. It has an extremely low rate of people of non-Polish descent, and Polish society is predominantly Christian. Most Poles identify as Roman Catholics (71.3%) [ 14 ]. Over the past few decades, however, Polish society has become more diverse. Demographic changes in Europe require that all healthcare professionals, including nurses and midwives, develop the knowledge and skills needed to provide holistic, patient-centred, culturally sensitive care [ 5 , 6 ] and the growing body of literature in Poland stresses the importance of cultural competency in healthcare [ 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 ]. While many educational programmes that seek to develop nurses’ cultural competence have been implemented in Europe and elsewhere [ 58 , 59 , 60 , 61 , 62 , 63 , 64 ], this has only recently begun in Poland [ 65 , 66 ].
Earlier studies have shown that the Polish public is relatively poorly informed about other cultures and religions. On the other hand, although JWs are more familiar than other faith groups, such as Muslims, Jews, Hindus and Buddhists, and 60% of people claim to know a JW personally, many Poles are still critical of JWs [ 67 , 68 ]. More importantly, research has demonstrated that 61.3% of nurses in Poland have prior experiences with a patient with a distinct cultural background, less than half had heard the term cultural competences (47.2%), and 92.5% felt unprepared to care for patients from different cultures. 91.5% of nurses also declared that all nurses should know other cultures, including their impact on healthcare and disease (57.5%), be able to identify problems arising from cultural differences (59.4%) and have the skills required to overcome ethnocentrism, stereotypes and prejudices (59.4%) [ 54 ].
In another study 86.8% of nurses claimed to have had little or no contact with patients from a different culture or religion and 62.3% experienced difficulties interacting with such patients due to a lack of knowledge or communication skills. Finally, 74.3% of nurses admitted to having various stereotypes of Muslims, JWs, the Roma or Hindus and 55.7% had an unfavourable image of such patients [ 68 ]. A recent study by Zalewska-Puchała et al. showed that, since many Polish nurses revealed varying levels of social distance towards followers of various religions, there is a need to train nurses in transcultural nursing [ 69 ]. Walkowska et al., however, demonstrated that cross-cultural education increases future healthcare professionals’ levels of cultural competence and professional confidence [ 66 ].
This research therefore reports three significant findings. Firstly, it shows that future nurses and midwives have limited knowledge regarding JWs’ stance in refusing blood transfusions. Nursing students taking part in this study showed some general knowledge regarding JWs’ refusal of blood transfusion, but their awareness of blood products and medical procedures approved by JWs was relatively low. This result aligns with a previous study, indicating that while many nurses in Poland lack the cultural competences required to care for JW patients and, even though they tend to support adult JWs’ right to refuse a blood transfusion, they show little understanding of such a decision and expressed resentment towards JWs’ stance [ 42 ]. More than 83% of nurses in Lublin, Poland, claimed to have had contact with JW patients and more than half (50.02%) rejected JWs’ position concerning blood treatment, 44.23% admitting to having tried to persuade JW parents to change their minds and accept blood transfusions [ 70 ]. While 83% of anaesthesiologists, physicians and surgeons in France did not oppose the medical care of JWs, they remained committed to their primary focus: to save the patient, as long as it is not an end-of-life situation, and 67% admitted that in life and death situations, where there is a lack of alternative procedures, blood products should be administered [ 71 ]. Although German doctors stressed the importance of personal autonomy, they also referred to doctors’ consciences and their ethical professional obligations [ 25 ].
Secondly, this research also found that future nurses and midwives are aware of the bioethical and legal dilemmas healthcare professionals face when caring for JW patients. The majority, however, showed limited support for both JWs’ stance in their refusal of blood transfusions and their preferences for bloodless medicine. Less than half of respondents supported JWs’ right to refuse blood transfusions for religious reasons in life-threatening situations and the majority stressed JWs’ right to alternative, non-blood management techniques. Even fewer supported JW parents’ right to refuse blood transfusion for their children. Similar results were found in other studies, suggesting that in the case of infant or juvenile patients, blood transfusions should be performed even against parents’ will [ 69 , 70 ].
Thirdly, these findings underscore the educational needs regarding cultural competences in nursing, both in terms of general knowledge regarding JWs’ stance in refusing blood transfusions and non-blood management techniques. Since nursing and midwifery students felt unprepared to care for JW patients, this study shows an urgent need to include transcultural nursing and strategies to minimise blood loss modules in university curricula and postgraduate nursing training [ 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 ].
Limitations
This study has some limitations that should be acknowledged. Firstly, although the response rate was high (86.5%), the sample size was still small. Secondly, since 47 students decided against participating, this survey study solely represents the opinions of students who completed the questionnaire. Thirdly, nursing and midwifery students from only one Polish medical university participated in this study. For all these reasons, our results cannot be extrapolated to include the entire population of nursing and midwifery students, either in Poznan or Poland and further in-depth studies are required. Fourthly, it would be desirable to compare our findings with students of other departments and those in contact with patients who refuse blood transfusion, i.e. medicine or medical rescue. The questionnaire used in this survey was also ad hoc and, though we consulted four specialists in nursing, sociology and the culture of Jehovah’s Witnesses, it was not validated. Finally, this study is based exclusively on the quantitative method. Further in-depth studies based on qualitative methods are recommended in order better to understand students’ attitudes towards and experiences in providing medical care for JW patients.
Despite these limitations, there are some advantages to this study. Most importantly, as there is a scarcity of previous work on the topic, this research helps bridge the gap in research on the knowledge of future healthcare professionals on JWs’ stance toward blood transfusion. This study compares the knowledge of nursing and midwifery students and may also stimulate further discussion on the need for better education and increasing cross-cultural competences among future nurses and midwives, whose roles in caring for JW patients is vital.
This study demonstrates that nursing and midwifery students possess inadequate knowledge regarding JWs’ stance on blood transfusions and their acceptance of specific blood products and medical procedures. It also shows that, despite being cognisant of the ethical and legal dilemmas of caring for JW patients, future nurses and midwives show limited support for patients’ autonomy to reject blood transfusions and their preferences for bloodless medicine. Finally, students articulated educational needs regarding cultural competencies on the JWs’ beliefs regarding blood transfusions and non-blood management techniques. Since culturally competent nurses and midwives must establish trust and approach all patients with respect for their cultural identity and values, this study reveals an urgent need to train future nurses and midwives in transcultural nursing and increase their cultural competencies. To achieve this goal, all medical curricula should include a transcultural nursing module akin to those in other European countries. Students should also be trained in the ways cultural norms and healthcare professionals’ personal beliefs may affect their decision-making, hinder patient communication and prevent individuals from receiving patient-centred and culturally sensitive care. Finally, future nurses and midwives must be taught and trained about the challenges of caring for JW patients, including ethical and legal dilemmas.
Data availability
Data generated as part of this study with replication codes for all analyses are available from the corresponding author upon reasonable request.
Abbreviations
Jehovah’s Witnesses
- Midwifery students
Nursing students
Maier-Lorentz MM. Transcultural nursing: its importance in nursing practice. J Cult Divers. 2008;15(1):37–43.
Google Scholar
Sharifi N, Adib-Hajbaghery M, Najafi M. Cultural competence in nursing: a concept analysis. Int J Nurs Stud. 2019;99:103386. https://doi.org/10.1016/j.ijnurstu.2019.103386 .
Article Google Scholar
Ličen S, Prosen M. The development of cultural competences in nursing students and their significance in shaping the future work environment: a pilot study. BMC Med Educ. 2023;23:819. https://doi.org/10.1186/s12909-023-04800-5 .
Leininger M. Transcultural nursing. Thorofare, NJ: Slack; 1978.
Leininger M. Culture care theory: a major contribution to advance transcultural nursing knowledge and practices. J Transcult Nurs. 2002;13(3):189–92. https://doi.org/10.1177/10459602013003005 .
Leininger M, McFarland RM, editors. Transcultural nursing: concepts, theories, Research and Practice. New York: McGraw-Hill; 2002.
Bonecutter RJ, Gleeson JP. Broadening our view: lessons from kinship foster care. J Multicult Soc Work. 1997;5(1):99–119. https://doi.org/10.1300/J285v05n01_08 .
Gustafson DL. Transcultural nursing theory from a critical cultural perspective. Adv Nurs Sci. 2005;28(1):2–16. https://doi.org/10.1097/00012272-200501000-00002 .
Andrews MM, Boyle JS. Transcultural concepts in nursing care. Seventh Edition. Wolters Kluwer; 2016.
Locsin RC. The culture corner: culture-centrism and holistic care in nursing practice. Holist Nurs Pract. 2001;15(4):1–3. https://doi.org/10.1097/00004650-200107000-00003 .
Bradbury-Jones C, Clark M. Globalisation and global health: issues for nursing. Nurs Stand. 2017;31(39):54–63. https://doi.org/10.7748/ns.2017.e10797 .
Prosen M. Introducing transcultural nursing education: implementation of transcultural nursing in the postgraduate nursing curriculum. Procedia - Soc Behav Sci. 2015;174:149–55. https://doi.org/10.1016/j.sbspro.2015.01.640 .
Prosen M, Karnjuš I, Ličen S. Developing cross-cultural competences among nursing students. In: Rutar S, Čotar Konrad S, Štemberger T, Bratož S, editors. Perspectives on Internationalisation and Quality in Higher Education. University of Primorska; 2017. pp. 199–213.
Główny Urząd Statystyczny. Wyznania Religijne W Polsce 2019–2021. (2021). https://stat.gov.pl/obszary-tematyczne/inne-opracowania/wyznania-religijne/wyznania-religijne-w-polsce-2019-2021,5,3.html . Accessed 16 Jan 2024.
Bodnaruk ZM, Wong CJ, Thomas MJ. Meeting the clinical challenge of care for Jehovah’s witnesses. Transfus Med Rev. 2004;18(2):105–16. https://doi.org/10.1016/j.tmrv.2003.12.004 .
McCormick TR. Ethical issues inherent to Jehovah’s witnesses. Perioper Nurs Clin. 2008;3(3):253–8. https://doi.org/10.1016/j.cpen.2008.04.007 .
Bock GL. Jehovah’s witnesses and autonomy: honouring the refusal of blood transfusions. J Med Ethics. 2012;38(11):652–6. https://doi.org/10.1136/medethics-2012-100802 .
van Knapp D. Ethics and medicine: Jehovah’s witnesses and the new blood transfusion rules. S Afr Fam Pract. 2013;55(1):S6–9. https://doi.org/10.1080/20786204.2013.10874313 .
West JM. Ethical issues in the care of Jehovah’s witnesses. Curr Opin Anaesthesiol. 2014;7(2):170–6. https://doi.org/10.1097/ACO.0000000000000053 .
Mason CL, Tran CK. Caring for the Jehovah’s Witness parturient. Anesth Analg. 2015;121(6):1564–9. https://doi.org/10.1213/ANE.0000000000000933 .
Rashid M, Kromah F, Cooper C. Blood transfusion and alternatives in Jehovah’s Witness patients. Curr Opin Anaesthesiol. 2021;34(2):125–30. https://doi.org/10.1097/ACO.0000000000000961 .
Spencer JR. A point of contention: the scriptural basis for the Jehovah’s witnesses’ refusal of blood transfusions. Christ Bioeth. 2002;8(1):63–90. https://doi.org/10.1076/chbi.8.1.63.8761 .
Trzciński R, Kujawski R, Mik M, Berut M, Dziki Ł, Dziki A. Surgery in Jehovah’s witnesses – our experience. Prz Gastroenterol. 2015;10(1):33–40. https://doi.org/10.5114/pg.2014.47496 .
Rajewska A, Mikołajek-Bedner W, Sokołowska M, Lebdowicz J, Kwiatkowski S, Torbè A. The Jehovah’s Witness obstetric patient – a literature review. Anaesthesiol Intensive Ther. 2019;51(5):390–403. https://doi.org/10.5114/ait.2019.90991 .
Rajtar M. Bioethics and religious bodies: refusal of blood transfusions in Germany. Soc Sci Med. 2013;98:271–7. https://doi.org/10.1016/j.socscimed.2013.02.043 .
Wong DSY. Blood transfusion and Jehovah’s witnesses revisited: implications for surgeons. Surg Pract. 2012;16(4):128–32. https://doi.org/10.1111/j.1744-1633.2012.00612.x .
Zeybek B, Childress A, Kilic GS, et al. Management of the Jehovah’s Witness in obstetrics and gynaecology: a comprehensive medical, ethical and legal approach. Obstet Gynecol Surv. 2016;71(8):488–500. https://doi.org/10.1097/OGX.0000000000000343 .
Scharman CD, Burger D, Shatzel JJ, Kim E, DeLoughery TG. Treatment of individuals who cannot receive blood products for religious or other reasons. Am J Hematol. 2017;92(12):1370–81. https://doi.org/10.1002/ajh.24889 .
Arya RC, Wander G, Gupta P. Blood component therapy: which, when and how much. J Anaesthesiol Clin Pharmacol. 2011;27(2):278–84. https://doi.org/10.4103/0970-9185.81849 .
Davis W, Frantz A, Brennan M, Scher CS. Blood component therapy: the history, efficacy, and adverse effects in clinical practice. In: Liu H, Kaye AD, Jahr JS, editors. Blood substitutes and Oxygen Biotherapeutics. Cham: Springer; 2022. https://doi.org/10.1007/978-3-030-95975-3_6 .
Chapter Google Scholar
Petrini C. Ethical and legal aspects of refusal of blood transfusions by Jehovah’s witnesses, with particular reference to Italy. Blood Transfus. 2014;12(Suppl 1):s395–401. https://doi.org/10.2450/2013.0017-13 .
Wilson P. Jehovah’s Witness children: when religion and the law collide. Paediatr Nurs. 2005;17(3):34–7. https://doi.org/10.7748/paed2005.04.17.3.34.c978 .
Chua R, Tham KF. Will no blood kill Jehovah witnesses? Singap Med J. 2006;47(11):994–1001.
Habler O, Thörner M, Schmidt C, Hofmann P, Döbert U, Höhler M, Klingler S, Moog S, Oehme A, Schäufele M, Wege C, Voß B. Letalität Nach Operativen Risikoeingriffen Bei Zeugen Jehovas. Anaesthesist. 2019;68(7):444–55. https://doi.org/10.1007/s00101-019-0617-8 .
Żaba C, Świderski P, Żaba Z, Klimberg A, Przybylski Z. Zgoda Świadków Jehowy na leczenie preparatami krwi – aspekty prawne i etyczne. Arch Med Sadowej Kryminol. 2007;57(1):138–43.
Bujny J. Prawne aspekty oświadczeń składanych przez Świadków Jehowy na Wypadek Utraty przytomności. Anestezjol Ratown. 2008;2:195–200.
Zając P. Odpowiedzialność Lekarza Za przeprowadzenie zabiegu leczniczego związanego z transfuzją krwi bez uzyskania zgody Świadka Jehowy. Biuletyn Stowarzyszenia Absolwentów i Przyjaciół Wydziału . Prawa Katolickiego Uniwersytetu Lubelskiego. 2015;10(12/1):81–101. https://doi.org/10.32084/bsawp.5027 .
Krzysztofek K. Stanowisko Świadków Jehowy Wobec Wybranych współczesnych procedur medycznych w świetle prawa polskiego. Studia z Prawa Wyznaniowego. 2015;18:287–310. https://doi.org/10.31743/spw.5093 .
Waters JH, Ness PM. Patient blood management: a growing challenge and opportunity. Transfusion. 2011;51(5):902–3. https://doi.org/10.1111/j.1537-2995.2011.03122.x .
Resar LM, Frank SM. Bloodless medicine: what to do when you can’t transfuse. Hematol Am Soc Hematol Educ Program. 2014;2014(1):553–8. https://doi.org/10.1182/asheducation-2014.1.553 .
Resar LM, Wick EC, Almasri TN, Dackiw EA, Ness PM, Frank SM. Bloodless medicine: current strategies and emerging treatment paradigms. Transfusion. 2016;56(10):2637–47. https://doi.org/10.1111/trf.13736 .
Domaradzki J, Głodowska K, Jabkowski P. Between autonomy and paternalism: attitudes of nursing personnel towards Jehovah’s witnesses’ refusal of blood transfusion. Int J Public Health. 2023;68:1606291. https://doi.org/10.3389/ijph.2023 .
Eurostat, Brancato G, Macchia S, Murgia M, Signore M, Simeoni G, Blanke K, Körner T, Nimmergut A, Lima P, Paulino R, Hoffmeyer-Zlotnik JHP. The handbook of recommended practices for questionnaire development and testing in the european statistical system. 2005. https://ec.europa.eu/eurostat/documents/3859598/13925930/KS-GQ-21-021-EN-N.pdf . Accessed 8 Jan 2024.
Sawicka-Gutaj N, Gruszczyński D, Guzik P, Mostowska A, Walkowiak J. Publication ethics of human studies in the light of the declaration of Helsinki – a mini-review. J Med Sci. 2022;91(e700). https://doi.org/10.20883/medical.e700 .
R Core Team. R: A language and environment for statistical computing. R Foundation for statistical computing, Vienna. 2021. https://www.R-project.org/ . Accessed 8 Jan 2024.
Wickham H, Averick M, Bryan J, Chang W, McGowan LDA, François R, Grolemund G, Hayes A, Henry L, Hester J. Welcome to the Tidyverse. J Open Source Soft. 2019;4(43):1686. https://doi.org/10.21105/joss.01686 .
Gohel D, flextable. Functions for Tabular Reporting. R package version 0.6.9., 2021. https://CRAN.R-project.org/package=flextable . Accessed 8 Jan 2024.
Wickham H. ggplot2: Elegant Graphics for Data Analysis . Use R! Springer Cham, 2016. https://doi.org/10.1007/978-3-319-24277-4 . Accessed 8 Jan 2024.
Fundacja Polki w Medycynie. 2022. Szklany sufit czy ruchome schody? Pozycja kobiet na uczelni medycznej. Retrived at: https://polkiwmedycynie.pl/szklany-sufit-czy-ruchome-schody-pozycja-kobiet-na-uczelni-medycznej-raport/ . Accessed 8 Jan 2024.
Majda A, Zalewska-Puchała J, Ogórek-Tęcza B, editors. Pielęgniarstwo transkulturowe. Warszawa: Wyd. PZWL; 2009.
Krajewska-Kułak E, Wrońska I, Kędziora-Kornatowska K. Problemy wielokulturowości w medycynie. Warszawa: PZWL; 2010.
Majda A, Zalewska-Puchała J. Wrażliwość międzykulturowa w opiece pielęgniarskiej. Probl Pielęg. 2011;19(2):253–8.
Zalewska-Puchała J, Majda A. Wrażliwość międzykulturowa w opiece położniczej. Probl Pielęg. 2012;20(3):416–22.
Zdziebło K, Nowak-Starz G, Makieła E, Stępień. R,Wiraszka G. Kompetencje międzykulturowe w pielęgniarstwie. Probl Pielęg. 2014;22(2):367–72.
Ślusarska B, Zarzycka D, Majda A, Dobrowolska B. Kompetencje kulturowe w pielęgniarstwie– podstawy konceptualizacji i narzędzia Pomiaru Naukowego. Pielęgniarstwo XXI Wieku. 2017;17(4):40–5. https://doi.org/10.1515/pielxxiw-2017-0033 .
Bernaciak E, Farbicka P, Jaworska-Czerwińska A, Szotkiewicz R. Intercultural competences in health care: Jehovah’s witnesses. J Educ Health Sport. 2019;9(3):301–20.
Głodowska KB, Baum E, Staszewski R, Murawska E, editors. Kulturowe uwarunkowania opieki nad pacjentem. Poznań: Wydawnictwo Naukowe Uniwersytetu Medycznego im. Karola Marcinkowskiego, Wydawnictwo Miejskie Posnania; 2019.
El-Messoudi Y, Lillo-Crespo M, Leyva-Moral J. Exploring the education in cultural competence and transcultural care in Spanish for nurses and future nurses: a scoping review and gap analysis. BMC Nurs. 2023;16(1):320. https://doi.org/10.1186/s12912-023-01483-7 .
Osmancevic S, Großschädl F, Lohrmann C. Cultural competence among nursing students and nurses working in acute care settings: a cross-sectional study. BMC Health Serv Res. 2023;23:105. https://doi.org/10.1186/s12913-023-09103-5 .
Repo H, Vahlberg T, Salminen L, Papadopoulos I, Leino-Kilpi H. The Cultural competence of graduating nursing students. J Transcult Nurs. 2017;28(1):98–107. https://doi.org/10.1177/1043659616632046 .
Ličen S, Karnjuš I, Prosen M. Measuring cultural awareness among Slovene nursing student: a cross-sectional study. J Transcult Nurs. 2021;32(1):77–85. https://doi.org/10.1177/1043659620941585 .
Liu TT, Chen MY, Chang YM, Lin MH. A preliminary study on the cultural competence of nurse practitioners and its affecting factors. Healthcare. 2023;10(4):678. https://doi.org/10.3390/healthcare10040678 .
Castro A, Ruiz E. The effects of nurse practitioner cultural competence on Latina patient satisfaction. J Am Acad Nurse Pract. 2009;21(5):278–86. https://doi.org/10.1111/j.1745-7599.2009.00406.x .
Cruz JP, Alquwez N, Cruz CP, Felicilda-Reynaldo RFD, Vitorino LM, Islam SMS. Cultural competence among nursing students in Saudi Arabia: a cross-sectional study. Int Nurs Rev. 2017;64(2):215–23. https://doi.org/10.1111/inr.12370 .
Majda A, Zalewska-Puchała J, Bodys-Cupak I, Kurowska A, Barzykowski K. Evaluating the effectiveness of cultural education training: cultural competence and cultural intelligence development among nursing students. Int J Environ Res Public Health. 2021;18(8):4002. https://doi.org/10.3390/ijerph18084002 .
Walkowska A, Przymuszała P, Marciniak-Stępak P, Nowosadko M, Baum E. Enhancing cross-cultural competence of medical and healthcare students with the use of simulated patients – A systematic review. Int J Environ Res Public Health. 2023;20(3):2505. https://doi.org/10.3390/ijerph20032505 .
Centrum Badania Opinii Społecznej. Społeczne Postawy Wobec Wyznawców Różnych Religii. 2012. https://cbos.pl/SPISKOM.POL/2012/K_130_12.PDF . Accessed 8 Jan 2024.
Cieślar-Greń M, Forysiak I, Kolasa K. „Świadkowie Jehowy mordują dzieci – stereotypy na temat członków Towarzystwa Biblijnego i Traktatowego „Strażnica. Świat i Słowo. 2014;12(2):23:343–52.
Ogórek-Tęcza B, Kamińska A, Matusiak M, Skupnik R. Wpływ Poziomu Empatii na postrzeganie relacji pielęgniarka–pacjent z innego obszaru kulturowego. Pielęgniarstwo XXI Wieku. 2012;4:61–5.
Zalewska-Puchała J, Bodys-Cupak I, Majda A. Attitudes of Polish nurses towards representatives of certain religions. BMC Nurs. 2022;21(1):28. https://doi.org/10.1186/s12912-021-00798-7 .
Jakubowska K, Kuczek B, Wiśniewska A, Pilewska-Kozak A, Dobrowolska B. Opinions of pediatric nurses about Jehovah’s witnesses’ refusal of blood transfusion for their child. Piel XXI Wieku. 2018;17(3):46–53.
Gouezec H, Lerenard I, Jan S, Bajeux E, Renaudier P, Mertes PM. Groupe Des Hémobiologistes et correspondants d’Hémovigilance (GHCOH) de la Société française de vigilance et de thérapeutique transfusionnelle (SFVTT). Perception par les médecins des conditions de prise en charge D’un Témoin De Jéhovah. Transfus Clin Biol. 2016;23(4):196–201. https://doi.org/10.1016/j.tracli.2016.08.002 .
Download references
Acknowledgements
We are grateful to all the students who completed the survey. We also thank the Jehovah’s Witness Hospital Liaison Committee for their support in designing the research questionnaire. We are also indebted to Mr Bob France for his assistance with the language editing of the manuscript.
The authors received no financial support for this research.
Author information
Jan Domaradzki and Piotr Jabkowski contributed equally to this work.
Authors and Affiliations
Department of Social Sciences and Humanities, Poznan University of Medical Sciences, Rokietnicka 7, Poznań, 60-806, Poland
Jan Domaradzki, Katarzyna Głodowska & Natalia Markwitz-Grzyb
Independent researcher, Binyamina, Israel
Einat Doron
Faculty of Sociology, Adam Mickiewicz University, Poznań, Poland
Piotr Jabkowski
You can also search for this author in PubMed Google Scholar
Contributions
JD and KG conceptualised the study, designed the questionnaire and collected data. JD administrated and supervised the study. PJ performed the statistical analyses and prepared the tables and figures. JD and PJ discussed the study results and assisted in interpreting the data. JD, ED, NMG, and PJ conducted the literature study and drafted the original manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Correspondence to Jan Domaradzki .
Ethics declarations
Ethics approval and consent to participate.
This study was carried out in line with the principles of the Declaration of Helsinki. Ethics approval and research governance approval were obtained from the Poznan University of Medical Sciences Bioethics Committee (KB – 760/22). Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Domaradzki, J., Głodowska, K., Doron, E. et al. Cultural competences among future nurses and midwives: a case of attitudes toward Jehovah’s witnesses’ stance on blood transfusion. BMC Med Educ 24 , 663 (2024). https://doi.org/10.1186/s12909-024-05646-1
Download citation
Received : 31 January 2024
Accepted : 10 June 2024
Published : 15 June 2024
DOI : https://doi.org/10.1186/s12909-024-05646-1

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Blood transfusion
- Cultural competences
- Jehovah’s witnesses
- Knowledge and attitudes
- Nursing students; transcultural nursing
BMC Medical Education
ISSN: 1472-6920
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Transfusion medicine: A research agenda for the coming years
Affiliations.
- 1 Department of Pathology and Laboratory Medicine (Transfusion Medicine), University of Rochester Medical Center, Rochester, NY, USA. Electronic address: [email protected].
- 2 Department of Pediatrics, Division of Critical Care, University of Rochester Medical Center, Rochester, NY, USA; Department of Pediatrics, Division of Cardiology, University of Rochester Medical Center, Rochester, NY, USA.
- 3 Department of Pathology and Laboratory Medicine (Transfusion Medicine), University of Rochester Medical Center, Rochester, NY, USA.
- 4 Department of Medicine, Division of Critical Care and Pulmonary, University of Rochester Medical Center, Rochester, NY, USA.
- 5 Department of Pathology and Laboratory Medicine (Transfusion Medicine), University of Rochester Medical Center, Rochester, NY, USA; Department of Medicine, Division of Critical Care and Pulmonary, University of Rochester Medical Center, Rochester, NY, USA.
- 6 Department of Pediatrics, Division of Hematology-Oncology, University of Rochester Medical Center, Rochester, NY, USA.
- 7 Department of Pediatrics, Division of Cardiology, University of Rochester Medical Center, Rochester, NY, USA; Department of Medicine, Hematology-Oncology Division,Rochester, NY, USA.
- 8 Department of Pathology and Laboratory Medicine (Transfusion Medicine), University of Rochester Medical Center, Rochester, NY, USA; Department of Medicine, Hematology-Oncology Division,Rochester, NY, USA.
- PMID: 31402101
- DOI: 10.1016/j.transci.2019.08.015
The important scientific and clinical advances of the last century in transfusion medicine include methods for avoiding hemolytic transfusion reactions and preventing transmission of viral infectious diseases. The next great clinical advances will require improving the efficacy and safety of transfusions, as well as acknowledgement of the now proven serious complications of transfusion, including nosocomial infection, thrombosis, inflammation and multi-organ failure. Possible strategies include (1) universal leukoreduction to mitigate transfusion immunomodulation effects and improve storage conditions, (2) minimizing transfusion of ABO incompatible antibodies and cellular/soluble antigens, (3) substituting use of safer solutions for normal saline during apheresis, component infusion and washing (4) new techniques to improve the efficacy and safety of blood components, including improved storage solutions/conditions, supernatant removal by washing, and rejuvenation and (5) maximizing the risk to benefit ratio of transfusions by employing more restrictive and physiologic indications for transfusion (including patient blood management) and improving clinical decision making through novel laboratory and bedside tests such as thromboelastography.
Keywords: Cellular therapies; Hemostasis; Inflammation; Thrombosis; Transfusion.
Copyright © 2019. Published by Elsevier Ltd.
PubMed Disclaimer
Similar articles
- How do we forecast tomorrow's transfusion? - Next generation transfusion practices to improve recipient safety. Blumberg N, Heal JM. Blumberg N, et al. Transfus Clin Biol. 2023 Feb;30(1):31-34. doi: 10.1016/j.tracli.2022.09.005. Epub 2022 Sep 9. Transfus Clin Biol. 2023. PMID: 36096445
- Reflections on current status of blood transfusion transplant viral safety in UK/Europe and on novel strategies for enhancing donors/recipients healthcare in promising era of advanced cell therapy/regenerative medicine. Seghatchian J. Seghatchian J. Transfus Apher Sci. 2019 Aug;58(4):532-537. doi: 10.1016/j.transci.2019.06.007. Epub 2019 Jun 22. Transfus Apher Sci. 2019. PMID: 31248735 Review.
- Noninfectious transfusion-associated adverse events and their mitigation strategies. Goel R, Tobian AAR, Shaz BH. Goel R, et al. Blood. 2019 Apr 25;133(17):1831-1839. doi: 10.1182/blood-2018-10-833988. Epub 2019 Feb 26. Blood. 2019. PMID: 30808635 Review.
- Blood product transfusions and reactions. Osterman JL, Arora S. Osterman JL, et al. Emerg Med Clin North Am. 2014 Aug;32(3):727-38. doi: 10.1016/j.emc.2014.04.012. Epub 2014 Jun 12. Emerg Med Clin North Am. 2014. PMID: 25060259 Review.
- The transfusion dilemma--weighing the known and newly proposed risks of blood transfusions against the uncertain benefits. Refaai MA, Blumberg N. Refaai MA, et al. Best Pract Res Clin Anaesthesiol. 2013 Mar;27(1):17-35. doi: 10.1016/j.bpa.2012.12.006. Best Pract Res Clin Anaesthesiol. 2013. PMID: 23590913 Review.
- Dynamics of shape recovery by stored red blood cells during washing at the single cell level. Lu M, Shevkoplyas SS. Lu M, et al. Transfusion. 2020 Oct;60(10):2370-2378. doi: 10.1111/trf.15979. Epub 2020 Aug 4. Transfusion. 2020. PMID: 32748970 Free PMC article.
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- Elsevier Science
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Volume 30, Number 7—July 2024
Research Letter
Serosurvey of blood donors to assess west nile virus exposure, south-central spain.
Suggested citation for this article
We analyzed West Nile Virus (WNV) exposure from 1,222 blood donors during 2017–2018 from an area of south-central Spain. Results revealed WNV seroprevalence of 0.08% (95% CI 0.004%–0.4%) in this population. Our findings underscore the need for continued surveillance and research to manage WNV infection in this region.
West Nile virus (WNV), a member of the family Flaviviridae, genus Orthoflavivirus , is classified within the Japanese encephalitis virus (JEV) serocomplex ( 1 ). It is the most widespread arbovirus globally, primarily because of the abundance and broad distribution of its main competent vector, mosquitoes belonging to the genus Culex ( 2 ). During the past 2 decades, WNV has led to epidemic outbreaks with a substantial proportion of severe cases in Europe, emerging as a considerable threat to public and animal health in these regions. Nonetheless, very limited information exists on seroprevalence in the general population, hindering a comprehensive understanding of the virus’ epidemiologic landscape.
In Spain, WNV is considered endemic because of conducive conditions for virus maintenance and circulation, including diverse bird reservoirs, geographic characteristics such as migratory bird routes, and specific climatic conditions. Since a notable outbreak reported in 2020, the virus has produced human cases annually ( 3 ), demonstrating the spread of the virus in the country ( 4 ). Therefore, vigilant surveillance in new risk areas is imperative to anticipate potential human health emergencies. Studies in vectors and animal hosts in south-central Spain have underscored the region’s potential as a hotspot zone ( 5 – 7 ). Within this area, the province of Ciudad Real, where no human WNV cases have been reported to date, serves as an ideal scenario for assessing circulation of the virus in the general population. We conducted a serosurvey in blood donors to investigate WNV exposure in the general population of this region in Spain, shedding light on the transmission dynamics of this emergent virus.
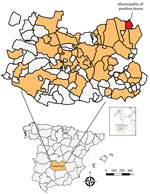
Figure . Locations sampled in serosurvey of blood donors to assess West Nile virus exposure in the general population, Spain, 2017–2018. Inset maps show location of study area in Spain and of...
We conducted a retrospective cross-sectional study to analyze the seroprevalence of WNV in serum samples collected from blood donors at the Transfusion Center of the Hospital General Universitario de Ciudad Real (south-central Spain) ( Figure ) during 2017–2018 ( Appendix ). We selected and analyzed blood from 1,222 donors ( Appendix Table 1). Sex and age data were not available for 129 (10.5%) donors. Of the 1,093 donors for whom information was available, 571 (52.2%) were men and 522 (47.8%) women. The age of the donors was categorized into 3 classes: <30 years (21.8% of samples), 30–50 years (34.8%), and >50 years (32.7%). Nineteen (1.6%) of the samples reacted positively to the IgG WNV ELISA. We administered an epidemiologic survey to the 19 ELISA-positive donors; 16 donors responded ( Appendix Table 2).
We analyzed all ELISA-positive samples by using a virus neutralization test (VNT) ( Appendix Table 2). Regarding WNV, ELISA reactivity was only confirmed by VNT in 1 donor who showed a titer of 1/256, which indicated a seroprevalence of 0.08% (95% CI 0.04%–0.4%) for WNV. This donor declared that he had not traveled outside of Spain and therefore did not receive any vaccine against yellow fever virus, tick-borne encephalitis virus, or Japanese encephalitis virus.
In Europe, no seroepidemiologic studies have been conducted since 2013; therefore, our study would provide valuable insights into the current status of WNV exposure. Our study encompasses a vast region of south-central Spain and marks initial identification of seropositivity in humans in this specific region of Spain, indicating a broad spread of the virus. In Spain, recent serosurveys are lacking; 2 studies were conducted in Catalonia in 2001 (0.2%) ( 8 ) and 2011 (0.12%) ( 9 ), and another was conducted in the province of Sevilla in 2006 (0.6%) ( 10 ). In the past 3 years, the regions of those studies have experienced large WNV outbreaks, similar to that which occurred in summer of 2020 ( 3 ) or the first description of clinical cases in Catalonia in 2022 and 2023 ( 4 ). This development suggests greater exposure to the virus than in the previous decade and highlights the need to carry out new serosurveys in the general population that enable collection of updated data.
The observed seroprevalence among blood donors from south-central Spain in our study suggests a low exposure (0.08%) to WNV in the general population within this spatiotemporal context. Of note, the number of WNV cases in Spain has been on the rise in recent years, being detected even in areas where previously no evidence of WNV circulation existed, suggesting that WNV has been expanding during recent years and that outbreaks can be expected in areas not currently considered endemic for WNV.
In our study, and in line with other studies ( 9 ), a high percentage (94.7%) of ELISA-positive WNV samples could not be confirmed as positive for specific antibodies. This finding highlights the need to perform additional neutralization tests against other flaviviruses in the serosurvey studies. The absence of an ELISA test with high sensitivity and, more crucially, specificity for WNV, limits the design of large-scale population serosurvey studies. Urgent efforts are required to address this limitation.
In conclusion, our study indicated seropositivity in the south-central region of Spain. In this way, reporting cases in Spain may be plausible even in areas not at high risk, highlighting the importance of ongoing surveillance and research to manage WNV infection in this region.
Dr. Frías is a postdoctoral researcher at the Animal Health and Zoonosis Research Group at the University of Cordoba and the Clinical Virology and Zoonoses Group at the Maimonides Biomedical Research Institute of Cordoba. His primary research interests are emerging zoonotic diseases.
Acknowledgments
We gratefully acknowledge Laura Ruiz Torres and Ismael Zafra Soto for their technical support in sample processing.
This work was supported by Secretaría General de Investigación, Desarrollo e Innovación en Salud (grant no. PI-0287-2019) for grants for the financing of Investigación, Desarrollo e Innovación Biomédica y en Ciencias de la Salud en Andalucía; the Ministerio de Sanidad (grant no. RD12/0017/0012) integrated into the Plan Nacional de I+D+I and co-financed by the ISCIII-Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional. A.R.J. is supported by a contract from the Spanish Junta de Andalucia (Nicolas Monardes program, grant no. C1-0001-2023). J.C.G. is supported by the CIBER Consorcio Centro de Investigación Biomédica en Red (grant no. CB21/13/00083), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación and Unión Europea–NextGenerationEU.
- Kramer LD , Li J , Shi PY . West Nile virus. Lancet Neurol . 2007 ; 6 : 171 – 81 . DOI PubMed Google Scholar
- Kramer LD , Styer LM , Ebel GD . A global perspective on the epidemiology of West Nile virus. Annu Rev Entomol . 2008 ; 53 : 61 – 81 . DOI PubMed Google Scholar
- García San Miguel Rodríguez-Alarcón L , Fernández-Martínez B , Sierra Moros MJ , Vázquez A , Julián Pachés P , García Villacieros E , et al. Unprecedented increase of West Nile virus neuroinvasive disease, Spain, summer 2020. Euro Surveill . 2021 ; 26 : 19 . DOI PubMed Google Scholar
- Ministerio de Agricultura . Pesca y Alimentación. Update on the epidemiological situation of West Nile [ cited 2024 May 14 ]. https://www.mapa.gob.es/es/ganaderia/temas/sanidad-animal-higiene-ganadera/informefno_tcm30-435293.pdf
- Durán-Martínez M . Distribución, Abundancia y Composición de la Comunidad de Dípteros Hematófagos Vectores de Enfermedades en Castilla-La Mancha: Riesgos para la Salud Pública y la Sanidad Animal. 2012 [ cited 2024 Jan 25 ]. https://digital.csic.es/handle/10261/147173 .
- García-Carrasco JM , Muñoz AR , Olivero J , Segura M , García-Bocanegra I , Real R . West Nile virus in the Iberian Peninsula: using equine cases to identify high-risk areas for humans. Euro Surveill . 2023 ; 28 : 2200844 . DOI PubMed Google Scholar
- Casades-Martí L , Cuadrado-Matías R , Peralbo-Moreno A , Baz-Flores S , Fierro Y , Ruiz-Fons F . Insights into the spatiotemporal dynamics of West Nile virus transmission in emerging scenarios. One Health . 2023 ; 16 : 100557 . DOI PubMed Google Scholar
- Bofill D , Domingo C , Cardeñosa N , Zaragoza J , de Ory F , Minguell S , et al. Human West Nile virus infection, Catalonia, Spain. Emerg Infect Dis . 2006 ; 12 : 1163 – 4 . DOI PubMed Google Scholar
- Piron M , Plasencia A , Fleta-Soriano E , Martinez A , Martinez JP , Torner N , et al. Low seroprevalence of West Nile virus in blood donors from Catalonia, Spain. Vector Borne Zoonotic Dis . 2015 ; 15 : 782 – 4 . DOI PubMed Google Scholar
- Bernabeu-Wittel M , Ruiz-Pérez M , del Toro MD , Aznar J , Muniain A , de Ory F , et al. West Nile virus past infections in the general population of Southern Spain. Enferm Infecc Microbiol Clin . 2007 ; 25 : 561 – 5 . DOI PubMed Google Scholar
- Figure . Locations sampled in serosurvey of blood donors to assess West Nile virus exposure in the general population, Spain, 2017–2018. Inset maps show location of study area in Spain and...
Suggested citation for this article : Frías M, Caballero-Gómez J, Vázquez A, Madrigal E, Ruiz-Fons F, Gallo M, et al. Serosurvey of blood donors to assess West Nile virus exposure, south-central Spain. Emerg Infect Dis. 2024 Jul [ date cited ]. https://doi.org/10.3201/eid3007.240450
DOI: 10.3201/eid3007.240450
Original Publication Date: June 18, 2024
Table of Contents – Volume 30, Number 7—July 2024
| EID Search Options |
|---|
| – Search articles by author and/or keyword. |
| – Search articles by the topic country. |
| – Search articles by article type and issue. |
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Metric Details
Article views: 10.
Data is collected weekly and does not include downloads and attachments. View data is from .
What is the Altmetric Attention Score?
The Altmetric Attention Score for a research output provides an indicator of the amount of attention that it has received. The score is derived from an automated algorithm, and represents a weighted count of the amount of attention Altmetric picked up for a research output.
- Scope of the Problem
- Major Advances
- Current Scientific Foundation
- Current Cutting-Edge Research
- Critical Needs
- Forecast of Major Advances
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Silberstein LE , Toy P. Research Opportunities in Transfusion Medicine. JAMA. 2001;285(5):577–580. doi:10.1001/jama.285.5.577
Manage citations:
© 2024
- Permissions
Research Opportunities in Transfusion Medicine
Author Affiliations: Harvard Medical School, Boston, Mass (Dr Silberstein), and the Department of Laboratory Medicine, University of California, San Francisco (Dr Toy).
In recent years, the translation of basic research in transfusion medicine has led to development of novel cellular therapies using well-characterized cell populations isolated from either bone marrow or blood (eg, hematopoietic stem and progenitor cells, T lymphocytes, dendritic cells). Refinements in cell therapies will make possible optimal stem cell engraftment, gene therapy, immunotherapy of cancer and infectious disease, and even solid organ regeneration. Moreover, the immune consequences of transfusion therapy are better appreciated and opportunities are at hand to prevent or blunt unwanted immune responses, such as platelet refractoriness and graft-vs-host disease. Transfusion medicine has become a broad, multidisciplinary field that has evolved beyond issues related to blood procurement and storage. The next series of advances in transfusion medicine will complement the current approaches of donor blood screening and viral/bacterial inactivation steps to ensure a safe and adequate blood supply.
The field of transfusion medicine began 100 years ago, in 1900, with the discovery by Landsteiner 1 of the ABO blood group system. This discovery demonstrated that plasma proteins have defined specificities. These plasma proteins, later termed antibodies, recognize epitopes on red blood cells. These discoveries constituted a starting point for blood banking—collection and storage of blood—and for immunohematology, the serological investigation of blood group antigens ( Figure 1 ). During the past 3 to 4 decades, significant advances have been achieved in improving the blood supply with respect to availability, safety, and fractionation into components, such as red blood cells, platelet concentrates, and plasma proteins.
Donors currently donate approximately 12 million units of blood annually in the United States. 2 Without these donations, many procedures and treatments, such as hematopoietic stem cell transplantation, complex cardiac and orthopedic surgery, and organ transplantation, would not be possible. A safe and adequate blood supply is a fundamental necessity to support state-of-the-art medical and surgical therapies. However, resources are now needed to translate advances in the biology of hematopoietic cells into newer cellular therapies and to investigate the unique immunological effects that result from transfusion of blood cells. This article discusses current status and progress in several aspects of transfusion medicine, including adequacy and safety of the blood supply, appropriate use of transfusion therapy, development of novel cellular therapies, and manipulation and prevention of immune responses.
Advances in transfusion medicine that have occurred during the past 25 years include reduction in risk of virally transmitted disease, pharmaceutical production of recombinant clotting factors, isolation and storage of stem and progenitor cell populations for transplantation, and genetic characterization of blood group antigens.
Reduction in transfusion-transmitted viral disease was achieved by a conversion from paid to volunteer donors, by improvement in donor screening, and by improvement of assays that detect viruses in donor blood. Since implementation of nucleic acid testing of donor blood, the estimated risks of hepatitis B (1 per 63 000 units), hepatitis C (1-3 per 1 million units), and human immunodeficiency virus (HIV) (1-2 per 1 million units), are now minuscule ( Figure 2 ). 3 At the same time, pasteurization and solvent-detergent treatment have virtually eliminated risk of HIV and hepatitis transmission through clotting factor concentrates and other plasma derivatives.
Advances have also been made in hematopoietic stem and progenitor cell transplantation for both hematologic and nonhematologic malignancies. The realization that stem and progenitor cells circulate in peripheral blood and that these cells can be mobilized from bone marrow using cytokines has led to outpatient cytapheresis procedures. 4 Transfusion medicine specialists are now involved in collection, in vitro manipulation, and storage of hematopoietic stem and progenitor cells for both allotransplantation and autologous transplantation.
Historically, blood group antigens were defined only by serological means, and their expression was thought to be limited to red blood cells. During the past 2 decades, establishment of the molecular basis for the majority of these blood group antigens has led to the realization that many of these antigens are expressed on other tissue cells. In addition, the biological functions of some of these antigens have been defined (eg, as receptors for pathogens or as ion transporters in the cell membrane 5 ). It is now possible to develop blood group antigen testing by genetic approaches. 6 Genotyping may lend itself to screening larger numbers of units in the blood supply and may be more amenable to laboratory automation. 7 This development could therefore enhance compatibility testing by selecting donor red blood cell units, which genetically match the red blood cells of the recipient. As a result, alloimmunization would be less likely.
The indications for transfusion of blood products continue to evolve. The hemoglobin concentration alone is an inadequate indication for red blood cell transfusion but improved clinical guidelines have not yet been defined. The importance of this issue is underscored by evidence that suggests that more liberal use of red blood cell transfusion may possibly harm younger, less severely ill patients in the intensive care unit. 8 Similar questions pertain to the indications for platelet concentrates, plasma, and specialized blood products (eg, leukoreduced cellular products, cytomegalovirus seronegative blood, washed red blood cells, fresh blood).
Alloimmunization is a major clinical problem in transfusion medicine, particularly in the setting of patients with multiple transfusions who become refractory to both red blood cells and platelet concentrates. For example, patients with hemoglobinopathies, such as sickle cell disease, rely heavily on transfusions for prevention and treatment of stroke, treatment for acute pulmonary disease, and preparation for surgery. 9 Unfortunately, a large percentage of sickle cell disease patients (25%-30%) develop multiple alloantibodies and autoantibody syndromes and become refractory to transfusion. Similarly, patients undergoing stem cell transplantation and aggressive chemotherapy often require frequent and sustained platelet transfusion support. A significant proportion of patients (30%) develop anti-HLA antibodies, resulting in platelet refractoriness. 10 A different aspect of alloimmunization pertains to graft-vs-host disease, in which transfused allogeneic T cells react with antigens on host tissues. These allogeneic T cells are transfused via traditional blood components (eg, packed red blood cells and platelet concentrates) or via stem cell products for hematopoietic stem cell transplantation.
There is also a deliberate use of alloimmunity in transfusion medicine, which involves the transfusion of allogeneic T cells to induce a graft vs leukemia/tumor effect for treatment of residual or recurrent tumor. 11 In addition, transfusion of viable donor leukocytes may induce ill-defined immunosuppression, referred to as the immunomodulatory effect, leading to tumor recurrence and postoperative infections. 12 It has been argued that more research is needed to understand the biology and clinical implications of the immunomodulatory effect before expensive strategies, such as universal leukoreduction of blood products, are instituted. 13
Current research is aimed at reducing viral transmission of blood products. Several viral/bacterial inactivation methods are being investigated for cellular products that are not amenable to solvent-detergent treatment or pasteurization. 14 Also, artificial blood substitutes (now in phase 3 trials) ultimately may have clinical utility, but they are unlikely to replace the volunteer donor blood supply. 15
With respect to cell therapies, attempts are under way to define hematopoietic stem cells and to understand stem and progenitor cell replication and differentiation. 16 These studies may lead to better approaches for ex vivo stem and progenitor cell expansion. Also, stem cell homing (eg, chemokines) and engraftment (eg, facilitator cells) are critical for hematopoietic stem cell transplantation. 17 Other cell therapies in development include T-cell therapies, for their graft vs tumor effect (ie, residual/recurrent chronic myelogenous leukemia), and the ex vivo generation of dendritic cells, for use in tumor vaccines. 18 , 19 Progress also is being made in differentiating the cells (and surface molecules) of the immune system that generate immune responses to foreign and host antigens. 20 , 21 As a result, experimental research and clinical trials are ongoing to find ways to either prevent or blunt autoimmune or alloimmune responses by costimulatory molecules (eg, using anti-CD40, CTLA4-Ig). 22 - 24
Basic principles of quality and risk management support establishment of evidence-based guidelines. A network of clinical research centers in transfusion medicine is essential to improve the effectiveness of the research that this discipline requires. Basic research analyzing the control of hematopoiesis is critical to expansion of hematopoietic cells and important to development of novel cellular therapies. Several issues must be resolved to advance hematopoietic stem cell transplantation. For example, which cells or factors are necessary for long-term engraftment? Which cells exist in the bone marrow environment and how do they influence hematopoietic growth and differentiation? Which factors influence the retention and trafficking of hematopoietic progenitor cells to and from the bone marrow environment?
Alloimmunity is a common consequence of blood cell transfusion. Further studies are needed to elucidate the mechanisms involved in generation of these immune responses. Such studies may focus on definition of target antigens (eg, tumor antigens, antigens in graft-vs-host disease, immune cell types, and the membrane molecules involved), cell origin (eg, host vs donor), the role of cytokines in establishing and perpetuating an alloimmune response, and the role of chemokines and chemokine receptors in homing immune cells. To date, few animal models exist that can be used to study alloimmune responses to blood cell elements. A special need exists to develop murine models to take advantage of existing reagents; the ability to inactivate murine background genes and knock-in human genes may be useful to study the induction, prevention, and modulation of immune-hematological responses. Such models may help define the role of specific immune cells (eg, T cells, dendritic cells), the role of donor vs host antigen-presenting cells, and the role of certain receptors (eg, Fcγ).
Current problems with blood shortages can be solved by finding ways to increase blood donations, by establishing donor criteria that maintain a safe blood supply without turning away safe donors, and by more accurately defining the indications for transfusions. Blood group genotyping will improve compatibility testing and selection of appropriate donor blood. In addition, genotyping will more precisely define fetal risk for neonatal hemolytic disease. Transfusion safety will be further enhanced by improved genetic testing of donated blood for known pathogens and by use of viral/bacterial inactivation steps suitable for cellular products. While reduction in the transmission risk of hepatitis B, hepatitis C, and HIV infection during the past 15 years is a major advantage, it is important to continue surveillance for newly emerging viruses and other pathogens (eg, prions, associated with variant mad cow disease) ( Figure 2 ). 25
Advances in cell and molecular biology of immune and hematopoietic cells will make it possible to isolate and grow cells in vitro for specific cell therapies. 26 Such therapies will include hematopoietic stem and progenitor cell populations with optimal engraftment and minimal graft-vs-host disease, 27 dendritic cells for tumor vaccines, T-cell populations defined for their antitumor/antiviral effects, 28 and stem and progenitor cells that are rendered resistant to infectious disease or genetically modified to correct genetic disorders. Since hematopoietic stem cells are capable of differentiating into cells of different lineage (eg, liver cells), it may possible to generate human tissue and blood cells in vitro for therapeutic applications. 29 ( Figure 3 )
Further understanding of alloimmune responses after transfusion may lead to ways to mitigate or prevent unwanted consequences of transfusion, such as graft-vs-host disease and refractoriness to platelet transfusion. Collectively, this interdisciplinary research effort will translate into safer and more effective transfusion therapy with a wide range of applications.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Springer Nature - PMC COVID-19 Collection

Blood Transfusion-Associated Infections in the Twenty-First Century: New Challenges
St. Michael’s Hospital, University of Toronto, Toronto, ON Canada
Blood transfusions are vital components of modern medical treatment to which there is no viable alternative despite efforts to create artificial blood. Each year thousands of lives are saved by blood transfusions in every country of the world. However, blood and blood products can result in significant adverse events including immunologic reactions, infections, inefficacy, and others which can sometimes result in death and severe disability. Thus, the sustainability of safe blood systems and costs are considered to be at crisis level. In industrialized countries, the risk of transfusion-transmitted infections such as HIV, syphilis, hepatitis viruses B and C are very low [generally [<1 in a million units], but in developing countries [especially in Africa] blood safety is still not assured. Compounding the problem of blood/product safety with respect to infectious agents are new emerging infectious microbes that are not being routinely tested for in blood that are donated. This chapter reviews the infectious risk of blood transfusions, types, mode and geographic variation, and the methods being used by blood services to attenuate and prevent these risks.
History of Blood Transfusion
Research into blood transfusion began in the seventeenth century after William Harvey experiments on the circulation of blood and Richard Lower pioneered the first blood transfusion between animals in 1665 [Royal Society]; but the first blood transfusion from animal to human was carried out by Jean-Baptiste Denys in France [Blood transfusion—Wikipedia]. The first successful blood transfusion was performed using a syringe by the British obstetrician James Blundell to treat postpartum hemorrhage in 1818. Subsequently, the first successful whole blood transfusion was performed to treat a patient with hemophilia in 1840 by Samuel Lane in London. Blood transfusions were avoided in the late nineteenth century because of severe reactions and high mortality.
It was not until 1901, after discovery of the three blood groups [O, A, and B] by the Austrian Karl Landsteiner, that blood transfusion became safer and led to the acceptance for modern treatment in emergency blood loss and surgeries [Wikipedia]. The first blood transfusion for surgery was performed in 1906 at Case Western Reserve University in Cleveland. The First World War was the stimulus for the rapid development of blood banks and transfusion techniques, and the world’s first blood donor service was established in 1921 by the British Red Cross. The first blood bank was established in a Leningrad hospital in 1932 and the US government established a nationwide program for blood collection in 1940 [Highlights of transfusion medicine history; http://www.aabb.org/tm/Pages/highlights.aspx ].
Transfusion was recognized as a source of infection before 1941 with description of transfusion-transmitted [TT] syphilis and screening for syphilis in blood donors was instituted before blood banks became common. In 1943, Paul Beeson published the classic description of TT–hepatitis [Highlights of transfusion medicine history], but the malarial parasite may be the first microbe known to be transmitted by transfusion. Globally, it is estimated that 85 million units of red blood cells are transfused every year.
Adverse Effects
Transfusions of blood products are associated with several complications and most of these are due to immunological reactions or infections. The immunological reactions are more frequent than infections and include (1) acute hemolytic anemia [most often due to human error in cross matching of mismatch blood types]; (2) delayed hemolytic reaction >24 h to 28 days [usually due to low or undetectable anti-Rh and anti- Kid antibodies]; (3) febrile nonhemolytic reactions, one of the most common transfusion reaction that occurs in about 7% [due to release of inflammatory chemical mediators from stored white blood cells]; (4) allergic reactions caused by IgE anti-allergen antibodies from the donor or recipient [more common in patients with hay fever/allergies]; (5) rarely anaphylactic reactions, caused by IgA anti-plasma protein antibodies; (6) extremely rare post-transfusion purpura, associated with the presence of antibodies directed against both the donor and recipient platelets [human platelet antigen]; (7) transfusion-associated acute lung injury [similar to acute respiratory distress syndrome [ARDS]] within 6 h of transfusion, related to donor antibodies interacting with recipient tissue antigen with release of inflammatory cytokines resulting in capillary leakage; (8) and transfusion-associated graft versus host disease which occurs in immunodeficient patients whose body failed to eliminate the donor’s T cells [Blood transfusion—Wikipedia]. A common non-immunological complication is transfusion-related circulatory overload within 6 h and acute respiratory distress with signs of heart failure.
Infections Associated with Blood Products
Blood products transfusion can cause infectious complications through three mechanisms: (a) transfusion of microbes present in asymptomatic donor blood [mainly viruses]; (b) contamination of stored blood products [primarily bacteria in platelets]; and (c) transfusion-related immunosuppression predisposing to postoperative infections and others. The risk of infection increases with the amount of red blood cell units or blood products transfused and patients requiring chronic blood or products are most vulnerable .
Transfusion-Transmitted Infectious Diseases
Despite the remarkable progress made in blood or blood products safety achieved in the last 30 years since the identification of the human immunodeficiency virus [ HIV] and hepatitis C virus [ HCV] , concerns still abound with the risk of transmission from emerging infectious agents. For microbes to be transmitted by transfusion certain attributes are considered necessary: presence of the agent in blood during the donor’s asymptomatic phase, the agent’s survival/persistence in blood during processing/storage, and the agent must be recognized as responsible for a clinically illness/outcome in a proportion of the infected recipients [ 1 ]. A group of experts in 2009 [members of the AABB’s Transfusion Transmitted Diseases Committee] identified 68 infectious disease agents capable of being transmitted by blood transfusion [ 1 ]. However, the list now is even larger and will keep expanding as the rate of emergence of new agents from 1940 to 2004 was 3–5 new viruses discovered every year, 60–70% from animal origin that can infect humans [ 2 , 3 ]. The infectious agents were classified and prioritized on risk level based on combination of scientific/epidemiological assessment, public perception, and regulatory concern into red, orange, yellow, and white categories [ 1 ]. The list did not include the well-acknowledged transfusion-transmitted agents—HIV, HCV, hepatitis B virus [HBV], and Treponema pallidum.
Red agents have low to high scientific evidence of blood safety risk with the potential for severe clinical outcomes, including human variant of Creutzfeldt-Jakob disease [ vCJD] , dengue viruses [ DENV] , and Babseia s pecies. vCJD has a low to very low risk of transmission in North America [NA] but higher risk of transmission in the United Kingdom [UK] where it was first described. DENV has a low to very low [almost absent] risk of transmission in NA but moderate risk in endemic countries. However, has moderate risk for transmission in the USA but very low risk in Canada and Europe or countries where the parasite is not endemic. Orange category agents have sufficient scientific/epidemiological evidence of blood transmission risk that may support higher priority in the future. These include Chikungunya virus [ CHIKV] , potential risk as not proven transfusion transmitted; St. Louis encephalitis virus [ SLEV] , potential risk but not proven; Leishmania species low risk with blood transmission proven in possibly 10 cases mainly in endemic areas [ 4 ]; Plasmodium species are well documented with blood transmission, low in non-endemic countries and high in hyperendemic regions; Trypanosoma cruzi [ Chagas disease] is well documented to be blood transmitted, low in the USA and Europe but moderate in South and Central America. Yellow category agents have low to absent risk of blood transmission, but there is public and regulatory concern. These agents include chronic wasting disease [ CWD] prion agent, never detected in humans or donated blood; human herpesvirus-8 [HHV-8], transmitted by transfusion in Africa [ 5 ] and possible in the USA but not proven [ 6 ], and resulted in no clinical disease; HIV variants are potentially blood transmissible but never proven; human parvovirus B19 which has been proven to be transmitted by blood [four documented cases by 2009], but very low risk except for hemophilia and conditions requiring recurrent chronic transfusion and immunosuppression; avian influenza A virus subtype H5N1 unlikely to be blood transmitted, but high profile for possible pandemic spread; simian foamy virus can be transmitted by blood transfusion in nonhuman primates, but never demonstrated in humans and is theoretically possible; Borrelia burgdorferi [Lyme disease agent] potentially possible but no proven cases of transfusion transmitted; and hepatitis A virus [ HAV] is very rarely transmitted by transfusion in neonatal intensive care units. White category agents represent a watch list, subject to modifications with change according to circumstances. These agents include hepatitis E virus [ HEV] which is documented to be blood transfusion transmitted in endemic regions and industrialized countries, mainly the zoonotic subtypes [ 7 , 8 ]; and Anaplasma phagocytocytophilum which has been documented to be transfusion transmitted in the USA [8 cases by 2014] with potential for greater blood transmission due to high seroprevalence in some regions of the USA, unknown period of bacteremia, survival in refrigerated stored blood, and shown in animal models to be transfusion transmittable [ 1 ]. Since the AABB group publication in 2009, the list of agents was updated in 2014 with six new additions [ 9 ]. These included yellow fever viruses, miscellaneous arboviruses, XMRV, human parvoviruses other than B19, bocaviruses, measles virus, and MERS-CoV. Tables 8.1 and 8.2 lists the microorganisms recommended for screening donated blood.
Screening for transfusion-transmissible infections
| Mandatory screening | |||
|---|---|---|---|
| Agent | Screening marker | Assay | Comments |
| 1. HIV | Anti-HIV, p24 Ag, RNA | EIA, CLIA, NAT | Ag-antibody assays for all some countries NAT |
| 2. HBV | HBsAg, anti-HBc, DNA | EIA, CLIA, NAT | HBsAg—all, anti-HBc—few NAT—some countries |
| 3. HCV | Anti-HCV, HCV-Ag, RNA | EIA, CLIA, NAT | Anti-HCV—all, ant-HCV/-Ag-limited; NAT—some areas |
| 4. | Anti-TP, aniti-reagin | TPHA, EIA, VDRL/RPR | EIA preferred; VDRL in high incidence countries |
Data obtained from the World Health Organization [WHO], Geneva; 2009. https://www.ncbi.nim.nih.gov/books/NKB142989/
Ag antigen, CLIA/EIA enzyme immunoassays, NAT nucleic acid technique, HBc hepatitis B core
Selective screening for specific blood-transmissible infections
| Agents | Screening marker | Assay | Regions | Comments |
|---|---|---|---|---|
| 1. CMV | Anti-CMV | EIA | None | Blood for immunosupp |
| 2. Malaria | Parasite or Ag | Thick smear, EIA | Endemic, risk | Donor screening |
| 3. | Anti- | EIA | Endemic | Migrants from endemic areas—screening |
| 4. HTLV I/II | Anti-HTLV-I/II | EIA | Endemic | Some non-endemic areas |
| 5. WNV | Anti-WNV, RNA | EIA, NAT | US, parts of Europe | Seasonal |
| 6. HEV | RNA | NAT | Parts of Europe | Controversial |
| 7. B19V | DNA | NAT | US, parts of Europe | Pooled plasma prod. |
Data obtained from [ 126 ]
Ag antigen, EIA enzyme immunoassay, NAT nucleic acid technique, immunosupp. immunosuppressed, prod. products
Recent Trends in Transmittable Agents in Blood Donors
There is a strong correlation between the risk of transfusion-transmitted agents and the prevalence of endemic rate in the local population of the country or region. Hence epidemiological data on the prevalence of high-risk infectious microbes in blood donors can be used as a guide to assess blood transfusion risk in conjunction with other preventative measures. There is a marked regional variation in prevalence of these agents and the data in recent years will be presented by country, but is incomplete from lack of recent studies from some regions.
In China recent data from 2008 to 2015 for blood donors screened for HIV, HBV, HCV, and syphilis from the southwest region showed a decreasing trend over the time period, from 2.39 to 1.98% [combination of the four agents], slightly lower than other regions [ 10 ]. Syphilis was the most prevalent, especially in females and farmers in rural regions. Since establishing the blood services, China had experienced several catastrophes with transfusion-transmitted diseases in the past, but since 1998 has undergone transformative changes in donor screening and donor testing. Donor selection is now voluntary donation with fixed groups and donated blood undergoes two rounds of testing with different equipment or reagents by different personnel [ 11 ]. Since 2010, nucleic acid test [NAT] was established in several blood centers and in 2015 the government invested for nationwide expansion of NAT. However, China’s blood services do not screen for other agents, which are regionally endemic and can be transmitted by transfusion. A nationwide distribution of nine potential agents that could be targeted was recently reviewed. These infectious agents include Plasmodium spp. , human parvovirus B19 [B19V], DENV, Brucella spp., severe fever with thrombocytopenia syndrome virus [SFTSV], Leishmania spp., HTLV, and Coxiella burnetii [Q fever] [ 12 ].
Despite malaria being endemic in some regions of China and previous reports of at least 87 cases transmitted by transfusion from 1992 to 2015 [ 12 ], the prevalence of malarial parasites in blood donors is unknown and screening of blood is not performed. DENV is also endemic in parts of China and blood donors in 2014 from endemic areas in Guangdong province had IgM prevalence rate of 2.4% with one donor with RNA load of 944 copies/mL. Brucellosis is endemic in the farming communities of North China and the prevalence rate among blood donors from an endemic area [Xinjiang Province] was reported in 2015 to be 1% and Brucella DNA was detected in 0.39% [ 13 ]. SFTSV, a tick-borne bunyavirus, was first described in China concentrated in the mountainous rural areas in central-eastern China with episodic outbreaks from spring to autumn. No transfusion-transmitted cases has been described and the seroprevalence rates in blood donors were 0.54% from endemic area and 0.28% from non-endemic area, and two low-grade suspected viremic samples were detected by RNA testing [ 12 ]. HEV is found worldwide and many cases have been transmitted by blood transfusion all over the world [ 14 ]. Screening of blood donors from six urban blood centers in China was reported in 2010 as showing prevalence of anti-HEV IgG of 32.6%, anti-HEV IgM of 0.94%, and HEV RNA in 0.07% among 44,816 donations [ 15 ]. HTLV prevalence in China is low and the latest nationwide surveillance in blood donors reported a rate of 0.03% in 2014 [ 16 ]. Leishmania spp. can be transmitted by transfusion and the parasite can survive in human red blood cells [RBC] in storage conditions up to 15 days [ 17 ]. Up to 2010, there were 300 cases of kala-azar reported yearly from China, mainly from Xinjiang Province and other western provinces [ 12 ]. However, there are no surveillance studies in blood donors and no documented transfusion-transmitted cases in China. B19V has been documented to be transmitted by blood transfusion and can cause RBC aplasia in the immunosuppressed [ 18 ]. Among Chinese blood donors, the B19V DNA was detected in 0.58% but was 4.8% in the Tibetan population. However, B19V DNA was detected in 54.2% of plasma pools used to manufacture intravenous immunoglobulin, factor VIII, fibrinogen, etc. with viral loads of 1 × 10 2 to 1 × 10 7 gEq/mL, which is higher than recommended by the US FDA [1 × 10 4 gEq/mL] [ 12 ]. C. burnetii DNA has been detected in blood donated [0.3%] of seropositive donors of 12.2% during a large outbreak of Q-fever in the Netherlands [ 19 ], but blood donor screening has not been initiated in China. Q-fever is mainly endemic in Tibet, inner Mongolia, and Western China.
The threat of blood-borne pathogens is disproportionately high in Sub-Saharan Africa , but there is variation among countries. In Eritrea, 60,236 blood donors were screened between 2010 and 2016, and at least 3.6% of donated blood was positive for one of the acknowledged transfusion-transmittable infections [TTI], HBV, HIV, HCV and syphilis, and 0.1% for multiple infections [ 20 ]. The seroprevalence of HBV, HCV, HIV, syphilis, and co-infections were 2.0, 0.7, 0.3, and 0.6%, respectively. These rates are relatively low compared to other countries in Sub-Saharan Africa.
In Ethiopia, similar data was collected from 11,382 blood donors from 2008 to 2015 with overall seroprevalence of 6.6% of the TTI, and prevalence of HBV, HIV, HCV, and syphilis were 4.4, 0.6, 0.8, and 1.1%, respectively [ 21 ]. Higher prevalence of any TTI was reported in Eastern Ethiopia [11.5%] with the majority [94.5%] due to HBV [ 22 ]. In Western Kenya, the seroprevalence of the four TTI in voluntary blood donors was even higher at 9.4%, distributed among HIV, HBV, HCV, and syphilis at 1.15, 3.46, 3.21, and 1.56%, respectively [ 23 ]. Prospectively screened donors from 2005 to 2016 in Nigeria showed 14. 96% were infected with at least one of the four TTI with overall prevalence of HBV, HCV, Syphilis, and HIV of 4.1, 3.6, 3.1, and 4.2%, respectively [ 24 ]. However, the rate of all TTI declined significantly over the years with a remarkable decline in HIV. The seroprevalence of the four main TTI among blood donors reported from 17 different and reported between 2009 and 2016 was recently reviewed [ 25 ]. This study found that West African countries had the highest seroprevalence of the TTI than other countries, especially for HBV [10.0–14.96%] and HCV [1.5–8.69%], but HIV prevalence demonstrated declining pattern throughout the years. Blood transfusion-transmitted-associated HIV infection in Nigeria was previously reported to be responsible for 14 [2.3%] of 597 children infection between 2004 and 2011 [ 26 ]. The Western Cape Province of South Africa’s blood supply appears to be exceptionally safe with the introduction of NAT since 2005 [ 27 ].
Middle East
The prevalence rate of the main TTI in blood donors appears to be lower in Middle Eastern countries than in Africa and Asian countries, with declining rates from 2004 to 2014 in Iran [ 28 ]. The overall seroprevalence rates of HBV, HCV, and HIV were 0.15, 0.1, and 0.004%, respectively. Similarly, low rates of TTI have been reported among blood donors from Jordan [ 29 ]. Data from Egypt in 2013 indicate that the prevalence of HIV and syphilis were extremely low [0%] but HCV and HBV were 7.2% and 2.3%, respectively, still posed a significant risk of blood transmission [ 30 ]. Recent prevalence data from Saudi Arabia among blood donors used serological testing and NAT from relatively small sample [3028] found: HBV sAg 0.33%, HCV 0.40%, HIV 0.13%, HTLV 0.20%, and HBV cAbs 9.81%; with additional detection by NAT.
Southeast Asia
Among blood donors from Pakistan [2014–2015] TTI was detected in 5.46% and 0.38% had multiple infections by rapid immunochromatographic technique [ 31 ]. The overall frequency of HCV, syphilis, HBV, malaria, and HIV was 2.62, 1.55, 1.10, 0.10, and 0.02, respectively. The prevalence rate of the TTI appears to be lower in India with overall positive for any TTI among the donors 2.79% in Kolkata [ 32 ] and the cumulative seroprevalence in Darjeeling of HIV, HBV, HCV, and syphilis of 0.42, 1.24, 0.62, and 0.65%, respectively [ 33 ]. Another study from India reported that the prevalence of TTI was higher in replacement donors than voluntary donors, but overall decreased from 2008 to 2012 [ 34 ]. The prevalence of infection in donated blood has been decreasing in Thailand from 2010 to 2012 compared to 2007–2009, and rates up to 2012 for HIV, HBV, HCV, and syphilis were 0.26–0.28, 0.97–1.42, 0.26–0.42, and 0.35–0.53%, respectively [ 35 ].
South America
Seroprevalence data of the usual TTI in blood donors in South American countries are incomplete and only partially present for few countries within the last 5 years. In Brazil, NAT was introduced for HIV and HCV in 2012 and the prevalence of these two viruses was recently estimated to be 209.9 and 66.3 per 100,000 donations, respectively [ 36 ]. Argentina introduced NAT screening in blood banks for >10 years and reported positive donors for HIV at 0.075%, HCV 0.05%, and HBV 0.045% [ 37 ], which may represent intermediate risk in comparison between African countries and developed nations. Limited data from Colombia of 41,575 donors over a year note prevalence rates of: Chagas disease 0.49%, HBV 0.21%, HCV 0.45%, HIV 0.12%, and syphilis 1.68%, total prevalence of 2.95% [ 38 ].
Developed Countries
Most high-income industrialized countries have very low rates of the usual TTI in blood donors but recent updates of seroprevalence are only present for a few. Data from 14.8 million donations were collected from 2011 to 2012 in the USA, representing 50% of the blood supply [ 39 ]. Surveillance-positive rates per 10,000 donations were: HBV, 0.76 [0.00076%]; HCV, 2.0 [0.002%]; HIV, 0.28 [0.00028%]; and HTLV 0.34 [0.00034%]. The Dutch experience from 1995 to 2014 was recently updated and the prevalence of TTIs among blood donors was 6 to 60-fold lower than the general population [ 40 ]. New donors had higher rates of TTIs compared to repeat donors, and the prevalence rates of the TTIs from 2009 to 2014 per 100,000 donors were: HBV, 39 [0.00039%]; HCV, 16 [0.00016%], HIV, 2.4 [0.00024%], HTLV, 4.2 [0.00042%], and syphilis, 28 [0.00028%]. The prevalence of TTIs in the Dutch donor population was typically lower than in other industrialized countries where the rates varied from 32 to 136 for HBV, 31 to 82 for HCV, 1 to 4 for HIV and 1 to 10 for HTLV per 100,000 donors [ 40 ].
Risk of Transfusion-Transmitted Infections
The risk of TTIs is variable in different regions of the world and is dependent on several factors: prevalence of TTIs in the donor population, type of donors routinely used [voluntary, replacement, or paid], screening or deferral of donors, method of testing blood donated [serology, antigen detection, and NAT], and pathogen reduction techniques for treating donated blood. In general, industrialized countries use multiple methods, including NAT, plus with a low prevalence of the major TTIs have the lowest risk of TTIs. Poor or low-income countries, especially in Africa, with a higher prevalence of TTIs, less dependent on voluntary donation and lacking facilities for NAT have the greatest risk of TTIs. Whereas, middle-income countries [Brazil, China, etc.] have intermediate risks of the usual TTIs, see Table 8.3 for comparative rates of TTIs [ 41 – 44 ].
Residual risk of TTI in various regions of the world
| Agents | High income | Middle income | Low income | Comments |
|---|---|---|---|---|
| 1. HIV | ≤0.33 × 10 | ≤11 × 10 | ≤64 × 10 | Depends on NAT |
| 2. HBV | ≥0.16 × 10 ≥0.16 × 10 | ≥289 × 10 | ≥534 × 10 | Depends on NAT |
| 3. HCV | ≥0.03 × 10 | ≥191 × 10 | ≥207 × 10 | Depends on NAT |
Data obtained from [ 43 , 127 , 128 ]
NAT nucleic acid technique
Note: High-income countries as exemplified by France; middle-income countries as exemplified by Brazil; low-income countries as in Sub-Saharan Africa, i.e., Gabon
Risk of Blood Transmission of Specific Viruses
Cytomegalovirus.
Cytomegalovirus [ CMV] latent infection is very common in the adult population of industrialized countries [about 60%] and developing nations [>80%] and can be transmitted by blood, as it resides in leukocytes, once a person is infected indefinitely. Transmission of CMV does not pose a significant health hazard to the healthy adult or older child, but it can result in severe disease in the immunocompromised CMV-seronegative patients, i.e., stem cell transplantation and for premature neonates. Measures to reduce TT-CMV for high-risk groups include depletion of cellular blood products [leukoreduction] and selection of CMV-negative donations. Studies indicate that newly positive CMV-IgG donors pose the highest risk of transmitting CMV as their blood contains the highest levels of CMV DNA [ 45 ]. However, there is no scientific evidence according to a recent review that leukoreduction or any single strategy reduces the risk of TT-CMV infection in high-risk patients [ 46 ].
Occult Hepatitis B
Transfusion-transmission of HBV is extremely low in developed and middle-income countries that screen blood for HBsAg and NAT, but a residual risk still remains from blood donors with extremely low viral DNA in the blood with occult HBV infection, that are intermittently shed from the liver or not detectable by even highly sensitive NAT. Models estimate a residual transmission risk of 3–14% with occult HBV donation after HBsAg and NAT non-reactivity [ 47 ]. Despite NAT testing for HBV, up to 2013 4–13 cases of TT-HBV infection occurred annually from occult or recent infection in the window period in Japan [ 48 ]. Individual NAT revealed that 1.94% of donations with low anti-HBc and anti-HBs titers were viremic. Since then the Japanese blood services had elected to discard all units with low anti-HBc and anti-HBs that accounted for only 1.3% of total donations [ 48 ]. A study from Australia [without universal anti-HBc testing] estimated that occult HBV residual risk was 1 in 982,000 units transfused which represented 55% of the total HBV risk, and was decreasing with individual NAT identifying repeat blood donors with occult infection [ 49 ]. Data from Brazil indicate that the presence of high anti-HBs titers [>100 mIU/mL] did preclude the presence of HBV DNA in the donor blood [ 50 ].
A recent study reported that 3 Slovenian blood donors with occult HBV infection infected 9 of 31 [29%] recipients with extremely low viral loads [ 51 ]. The study suggested that the minimal infectious dose should be revised from 100 to 16 copies [or 3 IU] of HBV DNA and that further prevention could be achieved by universal anti-HBc screening [performed by a few centers] or highly sensitive NAT able to detect 0.8 copies [0.15 IU/mL] or pathogen reduction methods .
Hepatitis E
Hepatitis E virus [ HEV] is of worldwide distribution with >20 million cases each year in tropical/subtropical countries causing more than 56,000 deaths each year [ 52 ]. Endemic and epidemic diseases in these countries [Asia, Africa, Central America, etc.] are caused by genotypes 1 and 2 by oral–fecal route of transmission. But in Europe, North America, and parts of Asia [i.e., Japan] genotypes 3 and 4 are zoonoses present in many animals [especially domestic pigs] that cause sporadic infections by consumption of raw or undercooked pork but also by blood transfusion [ 53 ]. HEV viremic blood donors are usually asymptomatic with normal transaminase and donor screening interviews are not beneficial. Moreover, the asymptomatic HEV-viremia can be present for up to 68 days [ 54 ]. Although most infection with HEV causes asymptomatic or mild hepatitis, fulminant disease is seen in pregnancy and patients with preexisting cirrhosis and chronic hepatitis progressing to cirrhosis can occur in the immunosuppressed [ 53 ]. This is also of concern as organ transplant recipients and patients with hematological malignancy more commonly receive blood or blood products transfusion, and immunocompromised patients develop chronic HEV in about 60% with infection [ 55 ].
HEV [genotype 1] transmission by blood transfusion was first described in an endemic area in 2004 [ 56 ], but since then most cases have been reported in industrialized countries with genotype 3. Cases have been reported from Europe [France, Germany, Spain, the UK], Australia, Canada, and Japan [ 57 ]. TT-HEV can occur with transfusion of RBCs, platelet concentrate, fresh frozen plasma, and pooled granulocytes. Presently, there are about 40 cases of TT-HEV with 21 from Japan and at least 17 cases of transfusion of HEV blood products not resulting in HEV infection [ 57 ]. Universal screening of blood products for HEV RNA is a very controversial topic in Europe and policies vary among countries. In 2012–2013 in England, 225,000 blood donations were retrospectively screened and 79 had detectable HEV RNA [1:2850], and follow up of 43 recipients showed 18 [43%] had evidence of HEV infection [ 58 ]. The prevalence of HEV viremia in blood donors vary from 1:762 in the Netherlands to 1:9500 in the USA, and the risk of viremic blood leading to infection was estimated to be 40–50% [ 59 ]. The risk of developing clinical infection in the recipient of viremic blood products may depend on the presence of antibodies, viral load, and volume of transfused blood. The minimal infective dose is unknown but low viral load <100 IU/mL has not been associated with HEV infection and the lowest inoculum known to lead to infection in the recipient is 2 × 10 4 IU [ 58 ]. HEV RNA screening of blood donations is now routinely performed in Ireland, the UK, and the Netherlands, but selective screening for use in high-risk patients is performed in some blood centers in Germany, France, and Switzerland [ 57 ].
Arboviruses
Arboviruses are of worldwide distribution with regional variation depending on the species. There is a significant risk of transmission by transfusion during the short period of asymptomatic viremia, especially during peak season with a high incidence of infection. However, it is often difficult to prove TT-arbovirus infection from vector-borne transmission in endemic regions. Although transmission by blood products had been proven only for a few arboviruses, there is a major concern since the Zika virus epidemic in the Americas 2 years ago. Infection with Zika virus is the most commonly asymptomatic and viremic donors could be easily missed. Moreover, TT-Zika to pregnant women could result in severe neurological fetal abnormalities [ 60 ].
Although Colorado tick fever virus was the first arbovirus reported to be transmitted by blood transfusion in 1975 [ 61 ], concerns of TT-arboviruses became a blood safety issue, not until the West Nile virus outbreak in the USA in 2002. West Nile virus causes asymptomatic infection in the majority of patients [about 80%], but the severe neurological disease can occur in the elderly and immunocompromised subjects. In the US outbreak, 16 blood donors were linked to 23 infected recipients and all donors were negative for West Nile-specific IgM antibody at the time of donation [ 62 ]. The estimated risk of TT-West Nile virus during the epidemic period was 1.46–12.33 per 10,000 donations [ 63 ]. Since then yearly seasonal outbreaks [summer to fall] have occurred in North America but with decreased intensity. National screening by NAT was instituted in 2003, initially by minipool but after 2 years switched to individual donation, as one-third of RNA-positive donations were missed by minipool screening due to low-level viremia which can cause infection [ 64 ]. The estimated cost–benefit of West Nile virus screening in the USA in 2003 was $483,000 per quality-adjusted life year [ 65 ].
Other arboviruses shown to be transmitted by transfusion are dengue virus [DENV] and tick-borne encephalitis virus [ 66 ]. Despite high incidence of dengue fever in many tropical countries, annually at least 50 million globally, DENV has rarely been reported to be transmitted by transfusion. Up to 2016, there were only 5 well-documented clusters of TT-DENV infection [ 67 ]. However, a retrospective analysis of a large 2012 epidemic in Brazil was able to identify the 6th cluster of TT-DENV [ 68 ]. DENV-4 viremia was confirmed in 0.5–0.8% of donations during the epidemic and 42 DENV RNA-positive units were transfused to 35 recipients. However, 6 infections occurred in 16 susceptible recipients [37.5%]. Analysis revealed no significant association with transmission and viral load and 90% of donors and recipients had evidence of past DENV infection of one or more serotypes.
Chikungunya virus [CHIKV] is another arbovirus that results in clinical illness mimicking dengue fever, but results in more severe and persistent arthralgia and arthritis, and is widely distributed in the tropics with large outbreaks in the Americas and Caribbean in 2013–2014 [ 60 ]. CHIKV potentially can be transmitted by transfusion but there is no report of this occurring. CHIKV infection differs from DENV, Zika virus, and West Nile virus infections as most infected subjects are symptomatic and, thus, there is a lower risk of asymptomatic viremic donations. The risk of TT-CHIKV was recently assessed in a study from Thailand. The mean and maximal risks of viremic donations during an epidemic period was estimated to be 0.9% and 4.8%, but with only 10% asymptomatic cases, screening of donors could reduce the risk by 88.4% [ 69 ].
The rapid pandemic spread of Zika virus [ZIKV] since 2015 with reported cases in 85 countries and territories has posed the greatest risk for TT-arbovirus. Most viremic patients infected with ZIKV are asymptomatic and pose a threat to the blood supply in outbreaks and low endemic spread. Moreover, ZIKV produces severe teratogenic effects, can persist in whole blood up to 2 months [ 70 ] and four possible cases of TT-ZIKV have been reported from Brazil [ 71 , 72 ]. During the ZIKV outbreak in the French Polynesia of 2013–2014, 42 of 1505 blood donors [2.8%] were positive for ZIKV RNA and only 11 subsequently became symptomatic [ 73 ]. Puerto Rico introduced NAT of donated blood in 2016 during an outbreak and ZIKV RNA was detected in up to 1.1% [ 74 ]. Similarly, NAT of asymptomatic blood donors in Martinique in 2016 detected ZIKV RNA in 1.8% and 54% reported symptoms 1–6 days post-donation [ 75 ]. In the mainland USA, more than 200 locally acquired cases of mosquito-borne ZIKV infection and >5300 cases of travel-associated infection have been reported [ 76 ]. As a consequence since August 2016, all donated blood in the USA has been screened for ZIKV RNA. Over four million donations were screened with 9 confirmed positive [only on individual tested samples] for a rate of 1:480,654 donations [ 77 ]. ZIKV RNA levels in RBC varied from 40 to 800,000 copies/mL and detection up to 154 days after donation, but in plasma detected levels ranged from 12 to 20,000 copies/mL and detection up to 80 days after donation. The present plan of NAT of individual donors is projected to cost $137 million annually [ 78 ] and the cost-effectiveness of the blood donation screening exceed $1 million/quality-adjusted life-year [QALY] gained, which is about 10 times as high as costs considered appropriate in clinical medicine [ 79 ]. The current US strategy for individual NAT for ZIKV was more recently estimated to cost $341 million per QALY and screening was cost-effective only in the high mosquito season in Puerto Rico [ 80 ].
Ross River virus [RRV] is an arbovirus unique to the Australian region with confirmed cases of 5000 per year with the largest outbreak affecting 50,000 people in Australia, Papua New Guinea, and the Solomon Island and with recognized risk to the blood supply [ 81 ]. Like CHKV, RRV can cause epidemics of debilitating polyarthritis. The first case of TT-RRV was recently described, which prompted a comprehensive risk review. Modeling estimated the risk of infection in donors in Australia as 1:14,943 to 1: 95,039 and predicted 8–11 RRV-infected blood components issued in Australia during a 1-year period [ 82 ].
Other Viruses
Parvovirus B19 [ B19V] infection is common in childhood and adulthood with seroprevalence of 30–40% in adolescents and 40–60% in adults, and more than 85% in the elderly population [ 83 ]. Many infected subjects are asymptomatic [approximately 25% of adults and 50% of children in outbreaks] or experience mild nonspecific viral-like illness [ 84 ]. Thus, blood may be donated during the period of viremia which occurs 1 week after exposure and lasts for about 5 days. An important pathogenic feature of B19V is the bone marrow cell tropism, especially erythroid progenitor cells, with increased susceptibility for infection with differentiation [ 85 ]. Transmission of B19V through blood products is feasible as high-level viremia regularly occurs during primary infection with >10 12 geq/mL in the early phase of acute infection of asymptomatic individuals [ 86 ]. B19V is frequently found in blood and plasma donations and is more commonly transmitted by pool plasma-derived products than RBC. Transmission via plasma-derived products can occur due to incomplete clearance of the virus from the blood, high-level viremia in acute infection, and the resistance of the B19V to most inactivation procedures used in preparing blood-derived products [ 87 ].
B19V is a frequent contaminant of blood and plasma donations and the virus DNA is most commonly found in blood products from multiple donors. B19V DNA is detectable in 50–80% of non-inactivated factor VIII concentrates and in 30–50% of solvent/detergent-inactivated IX concentrates [ 88 ]. High rates of B19V DNA have been found in albumin [25%], immunoglobulin [IgG] preparations [20–75%], factor IX, and pooled plasma [>60%] with viral loads of 1 × 10 2 to 1 × 10 8 geq/mL [ 87 ]. Since no B19V transmission has not been documented from pooled-plasma products with less than 10 3 to 10 4 IU/mL B19V DNA, the US Food and Drug Administration [FDA] imposed a limit of 10 4 geq/mL B19V DNA from pooled plasma [ 87 ]. Cellular blood products are found to have B19V DNA in about 1% and RBC transfusion has been associated with the transmission of B19V primarily with high-level titers of >10 7 IU/mL [ 89 ]. However, a recent report from Japan described persistent symptomatic B19V infection with severe thrombocytopenia transmitted by RBC transfusion low levels of B19V DNA [1.0 × 10 4 IU/mL] [ 90 ]. Fortunately, most patients with TT-B19V are asymptomatic but the extent of clinical disease from transfusion transmission is unknown. The development of disease is influenced by the presence of hematological and immunocompromised disorders and the immune status of the host. Three groups of patients are at particular risk for serious disease with infection. Patients with chronic hemolytic disorders [i.e., thalassemia major, sickle cell disease] may develop transient aplastic crisis with acute infection; subjects with combined immunodeficiency syndrome can develop chronic severe anemia from bone marrow failure; patients with AIDS can develop pure red cell aplasia; and fetal abnormalities [hydrops fetalis] can occur in pregnant women [ 87 ]. Methods used to ensure safety of plasma-derived products include NAT of plasma minipools and individual donations and multiple steps of viral inactivation and removal with solvent/detergent, superheating at 80° C for 3 days, pasteurization, and nano-filtration [ 87 ].
Human T-lymphocytic virus types-1 and -2 [HTLV-1 and -2] are retroviruses that chronically infect lymphocytes that can be transmitted by transfusions, but only a small proportion of infected individuals will develop clinical diseases after many years. HTLV-1 infects five to ten million people worldwide from Africa, Asia, Caribbean, Central and South America and can cause debilitating spastic myelopathy, HTLV-associated myelopathy [HAM], and adult T-cell leukemia/lymphoma [ATL] [ 91 ]. HTLV-2 has not been linked with any specific disease entity but there is limited evidence that some affected patients may develop chronic neurological problems [sensory neuropathies, gait disturbance, bladder dysfunction, motor abnormalities, and mild cognitive impairment] and chronic lung infections and dermatitis [ 92 ]. HTLV-2 primarily occurs in the Americas, especially in Amerindians in North, Central, and South Americas [5–30% seropositivity] and in pygmy tribes of Africa; but the virus has been found in intravenous drug abusers [IVDA] in the USA and southern Europe [10–15%] [ 93 ]. Both HTLV-1 and -2 [primarily HTLV-1] have been shown to be transmitted by cellular blood products in Asia [first reported in Japan], Caribbean [Jamaica], and North America, but rarely recognized clinically [ 93 ]. A heart transplant recipient in France was reported to develop early signs of HAM within 4–5 months of TT-HTLV-1, the rapid onset most likely related to immunosuppression [ 94 ]. Two cases have been reported of ATL after TT-HTLV-1 in Taiwan in patients with pre-existing lymphoma and promyelocytic leukemia, 6 months and 11 years after the transmission [ 95 ]. Currently, many countries test for HTLV-1/2 antibodies in blood donors which may be cost-effective in high prevalence regions, but its value in high-income low-prevalence countries that perform universal screening is controversial and debatable [ 93 ]. Using a mathematical cost-effective model, it has been estimated that testing all new blood donors for HTLV costs US $9.2 million per life saved, or $420,000 per quality-adjusted life-year gained, when the HTLV prevalence is 1 per 100,000 [ 96 ]. When the prevalence among donors is 10 per 100,000 the cost is estimated to be US $0.9 million per life saved, or $41,000 per quality-adjusted life-year gained. In many developed countries in North America, Europe, and Australia where the prevalence of HTLV-1 is less than 1 per million universal testing of donors does not appear to be cost-effective; yet in many low- and middle-income countries with much higher prevalence antibody screening is not performed [ 93 ]. Further investigation of filter leukoreduction and pathogen inactivation methods and their cost–benefit compared to antibody screening are needed to guide national blood collection systems .
Transfusion Transmission of Parasites
The malarial protozoa , Plasmodium species, appear to be one of the first, if not the first, TT-infection described in 1911 [ 97 ]. The major four Plasmodium species [ P. falciparum, P. vivax, P. malariae, and P. ovale ] can cause TT-malaria, as they can survive in stored blood even when frozen [ 98 ]. The longest interval between exposure and transmission by donation was estimated to be variable by species: 8 years for P. falciparum, 5 years for P. vivax , 7 years for P. ovale, and 44 years for P. malariae [ 97 , 98 ]. TT-malaria has been described in both endemic and non-endemic countries. The risk of TT-malaria is extremely rare in industrialized countries but still a major challenge in resource-limited endemic countries, especially in sub-Saharan Africa. In endemic countries, it is challenging to differentiate between mosquito-transmitted from TT-malaria and, thus, transmission by blood transfusion is frequently unrecognized and underestimated. Recent estimates indicate that TT-malaria is <0.2 cases per million in non-endemic countries to >50 cases per million in endemic countries [ 98 ]. Donors are asymptomatic and invariably semi-immune with low levels of parasitemia that can be missed on microscopy. Transmission is usually through whole blood or packed RBC but platelets and leukocytes seldom transmit malaria from RBC carry over.
Recent studies within the past 10 years demonstrated a high-risk TT-malaria in sub-Saharan countries with median prevalence of malaria parasites in donated blood by thick smears of 10.2% [range 0.7% in Kenya to 55% in Nigeria] [ 99 ]. Blood donors are not routinely tested for malaria in most malaria-endemic countries in Africa including Nigeria. In Pakistan, blood smear detected malarial parasites in 0.57% of healthy blood donors and in India the rate was 0.03% with rapid diagnostic tests confirmed with microscopy [ 100 , 101 ]. Although non-endemic regions are at very low risk for TT-malaria, this may depend on the proximity to endemic areas. In Brazil, malaria is endemic in the Amazon River basin and non-endemic in the extra-Amazon regions, i.e., Sao Paulo state. However, a recent study found that 7.4% of blood donors from Sao Paulo were positive for P. falciparum [5.14%] or P. vivax [2.26%] [ 102 ].
A recent review of TT-malaria in non-endemic regions reported 100 cases from 1911 to 2015 with only a few cases in the twenty-first century, the two most recent cases in 2015 were from the USA [ 103 ]. Fifty-four of these cases occurred in the American continent, 38 in Europe, 3 in the Mediterranean area, 1 in India, and 4 in Southeast Asia. The frequency of the different Plasmodium species was: P. falciparum 45%, P. malariae 30%, P. vivax 16%, P. ovale 4%, P. knowlesi 2%, 1 mixed infection with P. falciparum/P. malariae , and 1 case of an avian species [Plasmodium praecox ] acquired in Greece. Fatal outcomes occurred mainly with P. falciparum [11/45] and rarely from P. malariae [2/30] or P. ovale [1/4] but the fatalities were not attributable to malaria [ 103 ].
Preventative measures taken by blood banks to avoid TT-malaria varies widely even in endemic regions and only a few countries in sub-Saharan Africa [Malawi, Sao Tome, Principe, and Sierra Leone] screen donated blood for malaria as of 2010 [ 98 ]. In non-endemic countries also varies with some countries [i.e., USA] rely on predonation questionnaire for potential screening and others [France, UK, and Australia] use antibody testing on donors considered at risk from preliminary questionnaire [ 103 ]. Screening of donated blood is most commonly by microscopy of blood smear or rapid diagnostic tests which are insensitive for low parasitemia, and serological tests cannot differentiate between remote and current infection. PCR is the most sensitive method but most endemic resource countries cannot afford this method for widespread use. Pathogen inactivation method using a combination of riboflavin as a photosensitizer with UV light device [Mirasol System for Whole Blood, Terumo BCT, Lakewood, Colorado] can reduces TT- malaria without damaging RBC [ 104 ].
Chagas Disease
Trypanosoma cruzi, the cause of Chagas disease , is widespread throughout rural Central and South America where it is transmitted by the triatomine bugs among the poor living in substandard houses. Severe cardiac disease occurs in 30–40% of chronically infected untreated individuals. TT-Chagas disease [CD] has been recognized in endemic areas for many years where screening of blood donors has been instituted [ 105 ]. With increased migration of Latin Americans to North America and Europe, TT-CD in non-endemic countries has become a concern. Transmission of CD was first recognized to be transmitted by transfusion in 1952 and the total number of TT-CD is estimated to be 300–800 in the last decades [ 106 ]. TT-CD in non-endemic countries has been reported from the USA [ n = 7], Spain [ n = 5], Canada [ n = 2], and Australia [ 106 ] and recently Switzerland [ 107 ].
Low-level parasitemia may be detected several years after infection in asymptomatic individuals in up to 50% of those infected and the parasite can survive blood storage at 4–22 °C and even freezing and thawing [ 106 ]. Cellular components of blood can transmit the disease but not plasma. Platelet transfusion is the most commonly reported blood products associated with TT-CD probably because of the higher parasitic load than other blood products [ 108 ]. Prevention of TT-Chagas includes universal or selective donor screening [questionnaire] and testing for T. cruzi antibodies. Blood donor screening in the USA was first instituted in 2007 for Chagas disease and as of December 2017, at least 2300 infected blood donors were reported from blood banks in the USA [CDC. Chagas disease surveillance activities—seven states, 2017. Weekly/July 6, 2018/67[26]:738–41]. Donor screening for Chagas disease in non-endemic countries includes: USA, Canada, Spain, UK, France, Switzerland, and Australia [ 106 ].
Babesiosis is a zoonosis caused by an intraerythrocytic parasite, Babesia spp., most commonly Babesia microti , usually transmitted by Ixodes ticks and resembles the malarial parasite on the blood smear, but smaller. Babesiosis is most commonly reported from the Northeast and upper Midwestern USA, Europe and Asia Pacific including China. In immunocompetent hosts, it causes mild febrile illness, but severe disease with significant mortality occurs in immunocompromised, asplenic, and elderly patients. TT-babesiosis was first described in 1979 in the USA and since then there have been over 200 cases related to transfusion described with mortality of about 18–19% [ 109 – 111 ]. Over 95% of the cases were due to B. microti but at least 3 cases were from Babesia duncani [ 109 ] and recently a case from Arkansas secondary to Babesia divergens from multiple RBC transfusion was described [ 112 ]. Babesiosis has been transmitted by RBC stored for up to 35 days and by previous frozen RBC and rarely by platelets [ 113 ].
TT-babesiosis in endemic regions of the USA is increasing and of a public health concern as screening donors for B. microti is not yet mandated or routinely performed. Babesia seroprevalence in blood donors in foci of New York has been found to be up to 4.3% and 3.0% along the coastal Connecticut [ 111 ]. In a study on screening donated blood from Connecticut, Massachusetts, Minnesota, and Wisconsin with serology and PCR for B. microti , 335 [0.38%] of 89,153 blood samples were confirmed positive with 67 [20%] PCR-positive [ 114 ]. Thus, screening of donated blood in endemic regions of the USA would decrease the risk of TT- babesiosis .
Bacterial Infection from Blood Transfusion
Bacterial infections represent the foremost infectious risk from transfusion of blood products. This is most commonly due to bacterial contamination during the processing or storage of blood products [direct effect], but there is increasing recognition of an indirect effect. Blood transfusion is associated with immunomodulation which may result in increased risk of infection. Leukocyte reduction of blood has been shown to reduce the risk of health care-associated infections [ 115 ]. In a recent review of health care-associated infection after RBC transfusion, restrictive transfusion compared to liberal transfusion strategy did not reduce the overall health care-associated infections, but reduced the risk of serious infections [ 116 ]. This was particularly significant for patients undergoing hip and knee arthroplasty as well for those with sepsis.
Bacterial contamination of blood products can be from the donor’s skin [i.e., Propionibacterium acnes or staphylococci] or from the environment with a variety of bacteria: Yersinia, Pseudomonas, Proteus, Escherichia coli, Klebsiella, Acinetobacter , and Serratia [ 117 ]. Some investigations found Yersinia enterocolitica as being most common as the organism is capable of growing and multiplying at low temperatures. Septic transfusion reaction is most commonly from platelet rather than RBC transfusion. Estimated risk of blood products contamination with bacteria is 1 in 5000 for platelets and 1 in 30,000 for RBC [ 117 ]. There is recent evidence from the Netherlands that platelet concentrate stored in platelet additive solution is associated with fourfold increased risk of bacterial infections [ 118 ]. In the USA, approximately 2.2 million units of platelets are transfused yearly [2011 data] and over a 5-year period from 2009 to 2013, 13 fatalities from bacterial contamination of platelet products were recorded, 2.6 per year or ≈1.3 per million platelet transfusion [ 119 ]. Since then there does not appear to be any improvement, as 5 fatalities were recorded from a bacterial infection in 2015 [ 120 ]. Staphylococcus aureus accounted for the greatest number of deaths due to contamination in the preceding 5 years [5/18] and other bacteria associated with fatalities included: Serratia marcescens, Klebsiella pneumoniae, Morganella morganii, Pseudomonas fluorescens, Acinetobacter species , and Enterococcus faecium.
Studies on active and passive surveillance for bacterial contamination of platelets have been reported with the culture of platelet samples. In a study over a 7-year period [2007–2013], 20 of 51,440 platelet units transfused were bacterially contaminated [0.004%; 389 per million] and only resulted in 5 septic transfusion reaction [ 121 ]. In high-income countries bacterial contamination of platelets, though the most common transfusion-transmitted infections, ranging from 0.01 to 0.07% of platelet units, but the rates are much higher in resource-poor countries such as in Africa. The rate of bacterial contamination in whole blood or RBC concentrate in 7 studies from sub-Sahara Africa average 8.8% and platelet contamination is likely much higher [ 122 ]. To prevent bacterial contamination of platelets the US FDA recommends enhanced bacterial testing or pathogen reduction/inactivation strategies or both. One system which combines ultraviolet A and amotosalen for broad-spectrum pathogen inactivation is approved in the USA and Europe [ 123 ].
Summary and Future Directions
Although the blood supply is safer than ever before, there are still major concerns with respect to transfusion-transmitted infections, especially with the advent of emerging infectious agents. Moreover, the situation in resource-poor countries, especially in sub-Saharan Africa, still remains a challenge to provide safe blood supply comparable to developed nations. Blood use has declined significantly in the past decade in the USA, between 2009 and 2016 the number of blood units collected and distributed by the American Red Cross decreased by 26% and predictions for 40% decrease by 2020, raises the issue of a crisis in the US blood system [ 124 ]. Blood is an essential medicine with no replacement likely in the foreseeable future and safer blood supply is paramount for public health planning.
Prevention of multiple infectious agents being transmitted by blood transfusion is very expensive, time consuming and cumbersome. Many of the blood donation screening measures exceed US $1 million per quality-adjusted life-year gained, which are 10 times as high as deemed appropriate in clinical medicine [ 125 ]. The key to a safe and affordable blood system is a universally applied pathogen-reduction system that can inactivate all or most viruses, parasites, bacteria, and prions that can be implemented by resource-poor and resource-rich countries alike. This would obviate the need for expensive screening by serology, NAT, and others. Several methods of pathogen reduction are already in use including Mirasol [TerumoBCT, Lakewood, Co, USA] using a combination of riboflavin and UVB light can be applied to RBC and platelets to reduce most TT-viruses including HIV, HCV, and HBV by 2.3–5.19 log reduction as well parasites and bacteria; INTERCEPT [Cerus Corporation, Concord, CA, USA] utilize amotosalen and UVA light has shown similar properties against viruses, bacteria, and parasites; THERAFLEX [MacoPharma, Lille, France] uses photochemical inactivation with different methods for plasma and platelets, has demonstrated efficacy against viruses and bacteria, but 2 cases of HIV transmission have occurred after treatment of plasma; solvents/detergents for treatment of plasma is very effective against a wide array of enveloped and intracellular viruses, bacteria, and protozoa and can be combined with filtration to improve efficacy; chemical alkylating agents are also under investigation [ 126 ]. Larger comparative trials are needed to find the most suitable technique that can be used for whole blood, RBC, platelets, and plasma, to prevent transfusion-transmitted infection in the future.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Development & Approval Process | Drugs
- Drug Approvals and Databases
- Resources for Information | Approved Drugs
FDA approves imetelstat for low- to intermediate-1 risk myelodysplastic syndromes with transfusion-dependent anemia
On June 6, 2024, the Food and Drug Administration approved imetelstat (Rytelo, Geron Corporation), an oligonucleotide telomerase inhibitor, for adults with low- to intermediate-1 risk myelodysplastic syndromes (MDS) with transfusion-dependent anemia requiring four or more red blood cell units over 8 weeks who have not responded to or have lost response to or are ineligible for erythropoiesis-stimulating agents (ESAs).
Full prescribing information for Rytelo will be posted here .
Efficacy was evaluated in IMerge (NCT02598661), a randomized (2:1), double-blind, placebo-controlled multicenter trial in 178 patients with MDS. Patients received an intravenous infusion of imetelstat 7.1 mg/kg or placebo in 28-day treatment cycles until disease progression or unacceptable toxicity. Randomization was stratified by prior red blood cell (RBC) transfusion burden and by International Prognostic Scoring System (IPSS) risk group. All patients received supportive care, which included RBC transfusions.
Efficacy was established after a median follow up time of 19.5 months (range: 1.4 to 36.2) in the imetelstat group and 17.5 months (range: 0.7 to 34.3) in the placebo group based upon the proportion of patients who achieved ≥ 8-week and ≥ 24-week RBC transfusion independence (RBC-TI), defined as the absence of RBC transfusion(s) during any consecutive 8 week period, and during any consecutive 24 week period, respectively, from randomization until the start of subsequent anti-cancer therapy (if any). The rate of ≥ 8-week RBC-TI was 39.8% (95% CI: 30.9, 49.3) in the imetelstat group and 15% (95% CI: 7.1, 26.6) in the placebo group (p-value < 0.001). The rate of ≥ 24-week RBC-TI was 28% (95% CI: 20.1, 37) in the imetelstat group and 3.3% (95% CI: 0.4, 11.5) in the placebo group (p-value < 0.001).
The most common adverse reactions (≥ 10% with a difference between arms of > 5% compared to placebo), including laboratory abnormalities, were decreased platelets, decreased white blood cells, decreased neutrophils, increased aspartate aminotransferase, increased alkaline phosphatase, increased alanine aminotransferase, fatigue, prolonged partial thromboplastin time, arthralgia/myalgia, COVID-19 infections, and headache.
The recommended imetelstat dosage is 7.1 mg/kg administered as an intravenous infusion over 2 hours every 4 weeks.
This review used the Assessment Aid , a voluntary submission from the applicant to facilitate the FDA’s assessment.
This product was granted orphan drug designation.
Healthcare professionals should report all serious adverse events suspected to be associated with the use of any medicine and device to FDA’s MedWatch Reporting System or by calling 1-800-FDA-1088.
For assistance with single-patient INDs for investigational oncology products, healthcare professionals may contact OCE’s Project Facilitate at 240-402-0004 or email [email protected] .
Follow the Oncology Center of Excellence on X (formerly Twitter) @FDAOncology .

COMMENTS
Numerous blood donor centers in the US implemented SARS-CoV-2 IgG antibody testing of blood donors in spring/summer 2020 to assist in the recruitment and collection of CCP. 11,14-20 The FDA requires CCP to contain anti-SARS-CoV-2 antibodies; to ensure that the donors have sufficient antibodies, they must have had symptoms of COVID-19 and a ...
The Association for the Advancement of Blood and Biotherapies Clinical Transfusion Medicine Committee (CTMC) composes a summary of new and important advances in transfusion medicine (TM) on an annual basis. Since 2018, this has been assembled into a manuscript and published in Transfusion. Study design and methods
Background: The Association for the Advancement of Blood and Biotherapies Clinical Transfusion Medicine Committee (CTMC) composes a summary of new and important advances in transfusion medicine (TM) on an annual basis. Since 2018, this has been assembled into a manuscript and published in Transfusion. Study design and methods: CTMC members selected original manuscripts relevant to TM that were ...
Blood transfusion in the ICU is primarily done to increase the oxygen-carrying capacity that has been reduced by ... Section and Topic Item # Checklist item Location where item is reported; ... This study has been approved by the Ethics Committee of High Institute for Education and Research in Transfusion on May 20, 2020 (MedicineIR.TMI.REC ...
Abstract. Transfusion Medicine is a dynamically evolving field. Recent high-quality research has reshaped the paradigms guiding blood transfusion. As increasing evidence supports the benefit of limiting transfusion, guidelines have been developed and disseminated into clinical practice governing optimal transfusion of red cells, platelets ...
Background: The AABB Clinical Transfusion Medicine Committee (CTMC) compiles an annual synopsis of the published literature covering important developments in the field of transfusion medicine (TM), which has been made available as a manuscript published in Transfusion since 2018. Methods: CTMC committee members reviewed original manuscripts including TM-related topics published electronically ...
The science of blood transfusion is at the heart of medicine.The present research topic will cover all the advancements in transfusion medicine and blood, and will provide a whole picture of the current knowledge and future challenges. Moreover, the content will be useful both for undergrad or postgrad students, as well as advanced scientists ...
The following topics are included: infectious risks to the blood supply, iron donor studies, pre-transfusion testing interference and genotyping, cold agglutinin disease (CAD), HLA alloimmunization in platelet transfusions, patient blood management, updates to TACO and TRALI definitions, pediatric TM, and advances in apheresis medicine. CONCLUSION
This has been made available as a publication in Transfusion since 2018. Methods CTMC members reviewed and identified original manuscripts covering TM-related topics published electronically (ahead-of-print) or in print from December 2020 to December 2021.
The recognition that HIV and HCV are transmissible by blood significantly contributed to making transfusion medicine increasingly focused on patient care. 2 Research in this field has provided reliable tests for detecting virus-specific antibodies, antigens, and nucleic acid sequences, so that risks per blood transfusion unit are now <1 in 1 ...
From Product to Patient—Transfusion and Patient Blood Management. Blood transfusion is firmly established in contemporary medical practice. More than 16 million units are administered each year in the US, 1 cementing its status as one of the most commonly used medical procedures. Over the past century, pioneers in transfusion medicine have ...
Blood transfusions are some of the most common therapeutic procedures performed in hospitalized patients. There are robust randomized clinical trial data that have guided transfusion practices, including trials demonstrating the safety and efficacy of restrictive red blood cell (RBC) transfusion strategies in diverse patient populations. 1-3 Implementation of blood management programs based on ...
Blood transfusion is a very common procedure in hospitals and represents an example of an intervention where patients face potentially significant ... The topic guides were developed in collaboration between psychologists, a patient representative and Consultant Haematologist, and piloted prior to data collection. ... This research adds ...
Importance Red blood cell (RBC) transfusion is a common medical intervention to treat anemia in very preterm neonates; however, best transfusion practices, such as thresholds, remain uncertain.. Objective To develop recommendations for clinicians on the use of RBC transfusions in very preterm neonates.. Evidence Review An international steering committee reviewed evidence from a systematic ...
Others research areas are in perioperative blood transfusion management. In this research topic, we are seeking original papers from authors with articles focusing on the domain of perioperative blood management strategies and associated domains such as hemostasis and perioperative coagulation.
The first successful human blood transfusion was nearly 250 years ago, now used in the treatment of life-threatening hemorrhage, surgical bleeding, and countless other indications. Blood is also a scare resource and should not be wasted. ... Research Ethics Topics and Collections Visual Abstracts War and Health Women's Health ...
This synopsis provides easy access to relevant topics and may be useful as an educational tool. ... a 2019 review of selected topics from the AABB Clinical Transfusion Medicine Committee Transfusion. 2020 Jul;60(7):1614-1623. doi: 10.1111/trf.15848 ... 17 Office of Blood Research and Review, Food and Drug Administration, Silver ...
The seeming duality of transfusion medicine as both a remarkably consistent and a notably dynamic clinical field is fascinating. Over a century ago, Karl Landsteiner discovered the ABO blood group by mixing red blood cells and sera from different individuals and observing agglutination, and this method, albeit with refinements, to characterize both red blood cells and antibodies against them ...
A total of 742 patients underwent randomization, with 371 assigned to each group. The analysis of the primary outcome included 722 patients. The median hemoglobin level in the intensive care unit ...
Topics in blood transfusion. Today marks World Blood Donor Day and so we asked Deputy Editor for Journal of Intensive Care, Hiroshi Morisaki, to explain more about the importance of blood transfusion, and how research in this area is progressing. Hiroshi Morisaki 14 Jun 2016
Dr. Philip Syng Physick carried out the first human blood transfusion in 1795, and the first transfusion of human blood for treating hemorrhage happened in England in 1818 by Dr. James Blundell. [1] Rapid strides have been made in understanding blood typing, blood components, and storage since the early 1900s.
The Ontario Regional Blood Coordinating Network (ORBCoN) and Canadian Blood Services co-hosted the 18th Annual ORBCoN/Canadian Blood Services Transfusion Education Workshop and Symposium in April 2023. Tracy Cameron, ORBCoN's North and East Ontario regional manager, summarizes highlights from the event.
The presentation will also address AABB's Global Standards Committee, the Global Transfusion Forum, the AABB Leadership Certificate and the CABP credential. The Bloodsafe Program supports research to enhance the availability of safe blood for patients in low or lower-middle-income countries in Sub-Saharan Africa.
Most importantly, as there is a scarcity of previous work on the topic, this research helps bridge the gap in research on the knowledge of future healthcare professionals on JWs' stance toward blood transfusion. This study compares the knowledge of nursing and midwifery students and may also stimulate further discussion on the need for better ...
Transfusion-transmissible infection monitoring system: a tool to monitor changes in blood safety Brian Custer PhD, MPH , Susan L. Stramer PhD , Simone Glynn MD, MSc, MPH , Alan E. Williams PhD , Steven A. Anderson PhD ,
Abstract. The important scientific and clinical advances of the last century in transfusion medicine include methods for avoiding hemolytic transfusion reactions and preventing transmission of viral infectious diseases. The next great clinical advances will require improving the efficacy and safety of transfusions, as well as acknowledgement of ...
We conducted a retrospective cross-sectional study to analyze the seroprevalence of WNV in serum samples collected from blood donors at the Transfusion Center of the Hospital General Universitario de Ciudad Real (south-central Spain) during 2017-2018 ().We selected and analyzed blood from 1,222 donors (Appendix Table 1).Sex and age data were not available for 129 (10.5%) donors.
Abstract. In recent years, the translation of basic research in transfusion medicine has led to development of novel cellular therapies using well-characterized cell populations isolated from either bone marrow or blood (eg, hematopoietic stem and progenitor cells, T lymphocytes, dendritic cells). Refinements in cell therapies will make ...
History of Blood Transfusion. Research into blood transfusion began in the seventeenth century after William Harvey experiments on the circulation of blood and Richard Lower pioneered the first blood transfusion between animals in 1665 [Royal Society]; but the first blood transfusion from animal to human was carried out by Jean-Baptiste Denys in France [Blood transfusion—Wikipedia].
Efficacy was established after a median follow up time of 19.5 months (range: 1.4 to 36.2) in the imetelstat group and 17.5 months (range: 0.7 to 34.3) in the placebo group based upon the ...