Effects of nicotine on homeostatic and hedonic components of food intake
Affiliations.
- 1 Department of Pharmaceutical SciencesCollege of Pharmacy, Western University of Health Sciences, Pomona, California, USA.
- 2 Mitochondrial Neurobiology and Therapeutics LaboratoryMayo Clinic, Rochester, Minnesota, USA.
- 3 Faculty of MedicineSchool of Clinica Biochemistry, Pontifical Catholic University of Ecuador (PUCE), Quito, Ecuador.
- 4 Department of Pharmaceutical SciencesCollege of Pharmacy, Western University of Health Sciences, Pomona, California, USA [email protected].
- PMID: 28814527
- PMCID: PMC5578410
- DOI: 10.1530/JOE-17-0166
Chronic tobacco use leads to nicotine addiction that is characterized by exaggerated urges to use the drug despite the accompanying negative health and socioeconomic burdens. Interestingly, nicotine users are found to be leaner than the general population. Review of the existing literature revealed that nicotine affects energy homeostasis and food consumption via altering the activity of neurons containing orexigenic and anorexigenic peptides in the brain. Hypothalamus is one of the critical brain areas that regulates energy balance via the action of these neuropeptides. The equilibrium between these two groups of peptides can be shifted by nicotine leading to decreased food intake and weight loss. The aim of this article is to review the existing literature on the effect of nicotine on food intake and energy homeostasis and report on the changes that nicotine brings about in the level of these peptides and their receptors that may explain changes in food intake and body weight induced by nicotine. Furthermore, we review the effect of nicotine on the hedonic aspect of food intake. Finally, we discuss the involvement of different subtypes of nicotinic acetylcholine receptors in the regulatory action of nicotine on food intake and energy homeostasis.
Keywords: anorexigenic peptides; food intake; nicotine; obesity; orexigenic peptides.
© 2017 Society for Endocrinology.

Publication types
- Appetite Regulation / drug effects
- Eating / drug effects*
- Energy Metabolism / drug effects
- Homeostasis / drug effects*
- Nicotine / pharmacology*
Grants and funding
- HHSN275201300005C/HD/NICHD NIH HHS/United States
- HHSN275201500005C/HD/NICHD NIH HHS/United States
REVIEW article
Cigarette smoking and brain regulation of energy homeostasis.

- 1 Faculty of Science, School of Medical and Molecular Biosciences, University of Technology, Sydney, NSW, Australia
- 2 Faculty of Medicine, Department of Pharmacology, School of Medical Sciences, University of New South Wales, Sydney, NSW, Australia
- 3 Renal Research Group, Kolling Institute, University of Sydney, Sydney, NSW, Australia
- 4 Faculty of Medicine, Department of Physiology, School of Medical Sciences, University of New South Wales, Sydney, NSW, Australia
Cigarette smoking is an addictive behavior, and is the primary cause of cardiovascular and pulmonary disease, and cancer (among other diseases). Cigarette smoke contains thousands of components that may affect caloric intake and energy expenditure, although nicotine is the major addictive substance present, and has the best described actions. Nicotine exposure from cigarette smoke can change brain feeding regulation to reduce appetite via both energy homeostatic and reward mechanisms, causing a negative energy state which is characterized by reduced energy intake and increased energy expenditure that are linked to low body weight. These findings have led to the public perception that smoking is associated with weight loss. However, its effects at reducing abdominal fat mass (a predisposing factor for glucose intolerance and insulin resistance) are marginal, and its promotion of lean body mass loss in animal studies suggests a limited potential for treatment in obesity. Smoking during pregnancy puts pressure on the mother’s metabolic system and is a significant contributor to adverse pregnancy outcomes. Smoking is a predictor of future risk for respiratory dysfunction, social behavioral problems, cardiovascular disease, obesity, and type-2 diabetes. Catch-up growth is normally observed in children exposed to intrauterine smoke, which has been linked to subsequent childhood obesity. Nicotine can have a profound impact on the developing fetal brain, via its ability to rapidly and fully pass the placenta. In animal studies this has been linked with abnormal hypothalamic gene expression of appetite regulators such as downregulation of NPY and POMC in the arcuate nucleus of the hypothalamus. Maternal smoking or nicotine replacement leads to unhealthy eating habits (such as junk food addiction) and other behavioral disorders in the offspring.
Introduction
Cigarette smoking is the leading preventable cause of death and disability from respiratory disease. Smoking causes addiction and is negatively correlated with body weight and caloric intake; an effect which appears to be nicotine-mediated ( Hajek et al., 1988 ). It is this action of nicotine on energy homeostasis that is attracting attention as a potential weight loss treatment during the current global obesity pandemic. However, the fat loss associated with nicotine has not been confirmed in human subjects under well-controlled experimental conditions. This review will decipher the neurophysiological mechanisms that underlie the regulation of cigarette smoking/nicotine on energy homeostasis based on both animal and human studies. The impact of maternal smoking on fetal energy homeostatic regulation will also been discussed, as there is a relatively high rate of smoking during pregnancy. Finally, whether or not nicotine is a good candidate as a weight loss treatment will be discussed.
Cigarette Smoking and Weight Control
Cigarette smoking is an addictive behavior with the consequences being the leading preventable cause of death and disability worldwide. It is a primary cause of cancer and cardiovascular and pulmonary disease. There are >1 billion people who smoke around the world ( DeMarini, 2004 ), with ~6 million deaths each year being due to tobacco/cigarette smoking-related disease; resulting in significant social and economic cost to Society ( World Health Organization, 2011 ). It has been estimated that in less than 40 years, deaths due to smoking-related illness will rise to ~10 million per year ( DeMarini, 2004 ; Hussein et al., 2007 ).
Smoking induces a negative energy state, characterized by reduced energy intake and body weight, which has been well documented across species ( Perkins, 1992 ; Strauss and Mir, 2001 ; Bellinger et al., 2003 ; Fulkerson and French, 2003 ; Chen et al., 2006 , 2007 , 2008 ). The lowered body weight has been shown to be independent of diet type, with a similar proportion of weight loss displayed in mice consuming a diet with either low or high-fat concentrations after 7 weeks of cigarette smoke exposure ( Chen et al., 2007 ). Unfortunately, these and similar observations have led to the public perception that smoking is associated with weight loss, and it is commonly used as a weight control strategy, especially among the young, and females ( Camp et al., 1993 ; Wiseman, 1998 ; Fulkerson and French, 2003 ). Weight gain and increased craving for high caloric junk food on cessation of smoking without nicotine supplementation is one of the reasons given by people that prevents them from ceasing smoking ( Stamford et al., 1986 ; Grunberg et al., 1988 ; Filozof et al., 2004 ), and this is also supported by the literature, with >75% of former smokers gaining weight after cessation ( Williamson et al., 1991 ; Leischow et al., 1992 ).
Cigarette smoke contains at least 6000 components that may directly or indirectly affect caloric intake and energy expenditure. Nicotine, the major addictive substance within cigarette smoke, is the best described for its suppressive effects on body weight and appetite in both humans and animal models ( Wager-Srdar et al., 1984 ; Grunberg et al., 1986 ; Bellinger et al., 2003 ). Furthermore, cigarette smoke stimulates the inflammatory response associated with elevated circulating levels of inflammatory cytokines, such as tumor necrosis factor α and interleukin 6, which are associated with the development of disease states related to smoking ( Fernandez-Real et al., 2003 ). These cytokines have been shown to inhibit appetite and affect lipid metabolism ( Langhans and Hrupka, 1999 ; Jansson et al., 2003 ). Overall, studies using cigarette smoke exposure have improved insight into the effects of cigarette smoking-related anorexia and weight loss.
An important question that arises from such studies is whether lower caloric intake is the main contributor to the generally lower body weight in smokers. This question can be answered by the use of pair-fed animals, which receive the same amount of food as that consumed by smoke-exposed litter-mates. According to the results of such studies, the weight loss effects of cigarette smoke exposure were not only due to the predicted reduction in energy intake, but also to an enhanced capacity for energy expenditure ( Chen et al., 2006 , 2008 ). Increased energy expenditure and thermogenesis can occur when the proton gradient of the inner mitochondrial membrane dissipates; a state which occurs via the action of mitochondrial carrier proteins termed uncoupling proteins (UCPs; Dalgaard and Pedersen, 2001 ). Uncoupling of the mitochondrial proton gradient is thought to be important for the maintenance of cellular respiration, activation of substrate oxidation, and prevention of the generation of reactive oxygen species ( Lee et al., 1999 ). There are several homologs of UCPs including UCP1, which, when active in brown fat is responsible for non-shivering thermogenesis in newborn humans, in cold acclimatization, and hibernating mammals ( Cannon and Nedergaard, 2004 ). In contrast, UCP3 is implicated in the regulation of shivering and other forms of thermogenesis, mitochondrial fatty acid transport, and basal metabolic rate ( Samec et al., 1998 ; Argyropoulos and Harper, 2002 ; Schrauwen and Hesselink, 2003 ). Fasting or chronic food restriction normally results in the downregulation of UCP1 expression in brown fat ( Champigny and Ricquier, 1990 ) while nicotine induces UCP1 mRNA expression, which likely leads to enhanced energy expenditure ( Yoshida et al., 1999 ; Arai et al., 2001 ). In mice directly exposed to cigarette smoke, both UCP1 and three mRNA expression was increased compared with pair-fed animals ( Chen et al., 2006 , 2008 ), suggesting that increased energy expenditure occurred despite their reduced energy intake. This theory has also been supported by data from humans, where energy expenditure was increased by nicotine administration ( Perkins et al., 1989 ).
Cigarette Smoking and Adiposity
Although smokers are generally thought to weigh less than non-smokers, smoking is actually a predisposing factor for abdominal obesity, glucose intolerance, and insulin resistance ( Canoy et al., 2005 ; Chen et al., 2007 ), which is a situation not well recognized by the general public. In a rodent model, we have shown that the reduction in fat mass after cigarette smoke exposure occurred only if the mice consumed a low-fat balanced diet. In addition, this weight loss was accompanied by lean body mass wasting, including that associated with some major organs such as liver, kidney, and skeletal muscle ( Chen et al., 2005 , 2006 , 2008 ). Cigarette smoke exposure failed to cause fat loss when the mice consumed a high-fat cafeteria style diet consisting of foods such as fried potatoes, cakes, and sweet biscuits; whereas lean body mass loss became the prominent cause of weight loss in these mice ( Chen et al., 2007 ). We speculate that this observation was due to a change of food preference induced by cigarette smoke exposure or, perhaps that the nature of the high-fat diet to induce over accumulation of fat mass, even with restricted caloric intake. In both human and animal studies, food high in refined sugar and fat is more preferred when they are exposed to cigarette smoke ( Marangon et al., 1998 ; Chen et al., 2007 ). Consuming such food can increase fat mass, blood lipid levels, and glucose intolerance even when the total calorie intake does not exceed the daily requirement ( Shiraev et al., 2009 ). In contrast, when smoke-exposed mice consume a high-fat diet, they consume twice the energy of the recommended daily requirement ( Chen et al., 2007 ). Thus, we can speculate that adiposity induced by consumption of a high-fat diet, together with the loss of lean body mass found exclusively after cigarette smoke exposure may increase the risk of metabolic disorders.
In fact, both active and passive smoking contribute to glucose intolerance and insulin resistance, leading to type-2 diabetes; and smoking cessation has been demonstrated to improve insulin sensitivity ( Facchini et al., 1992 ; Eliasson et al., 1997 ). It has been suggested that insulin resistance among smokers may be due to the direct impact of nicotine, carbon monoxide, or other agents in the tobacco smoke ( Facchini et al., 1992 ). Nicotine infusion stimulates lipolysis to increase triglyceride levels in both human and animal studies ( Sztalryd et al., 1996 ; Andersson and Arner, 2001 ), while hyperlipidemia is strongly associated with the onset of insulin resistance ( Stannard and Johnson, 2004 ). Anorexia developed in long-term smokers also contributes to muscle wasting, especially in those with chronic obstructive pulmonary disease ( Morrison et al., 1988 ; Jagoe and Engelen, 2003 ). Skeletal muscle is one of the major sites for insulin-dependent glucose deposition when blood glucose rises. Thus, in smokers, the reduction in muscle mass can directly impair systemic glucose uptake, contributing to postprandial hyperglycemia, and an elevated risk of developing type-2 diabetes. Vascular changes associated with prolonged smoking may also lead to reduced blood flow to skeletal muscle and decreased insulin-mediated glucose uptake ( Facchini et al., 1992 ).
Neurological Mechanisms Underlying Suppressed Appetite
Classical feeding regulators.
The reduction in energy intake associated with smoking shows a relationship to the effects of several brain appetite regulators, and indeed, nicotinic receptors have been demonstrated in the appetite regulating area of the hypothalamus ( Jo et al., 2002 ). The most widely studied appetite regulator is neuropeptide Y (NPY), a 36 amino acid peptide. NPY is a member of the pancreatic polypeptide family, and is abundant throughout the central nervous system and the periphery ( Tatemoto et al., 1982 ; Allen et al., 1983 ). NPY is a powerful neurochemical stimulator of feeding in many species ( Vettor et al., 1994 ; Raposinho et al., 2001 ), with its levels reflecting the nutritional status of the body, and contributing to the long-term regulation of energy homeostasis. Administration of NPY into different brain regions, including the hypothalamus, frontal cortex, hindbrain, and hippocampus, induces hyperphagia (even in a satiated state), decreased sympathetic activity and thermogenesis, increased fat deposition, and promotion of weight gain and obesity ( Clark et al., 1984 ; Billington et al., 1991 ; Egawa et al., 1991 ; Raposinho et al., 2001 ).
In studies of a mouse model of cigarette smoke exposure, the hypothalamic NPY concentration was significantly suppressed by smoke exposure, compared with food restriction (pair-feeding; Chen et al., 2006 , 2008 ). This effect appears to be predominately nicotine-mediated, as a similar suppression of NPY has been observed in nicotine-treated animals ( Jo et al., 2002 ). Physiologically, the decreased hypothalamic NPY levels can upregulate the expression of orexigenic NPY receptors. However, the hypothalamic density of the NPY Y 1 receptor is reduced by chronic nicotine treatment ( Kane et al., 2001 ). Thus, it is possible that a voluntary reduction in energy intake in smokers can be attributed to suppressed NPY signaling in both the presynaptic production of the peptide and at the postsynaptic receptor level. This inhibitory effect of nicotine on appetite may be an important clue for therapy development for the treatment of obesity. This is of significant relevance, as clinical trials targeting NPY pathways have failed in obese patients due to redundancy in the mechanisms regulating energy homeostasis.
Neuropeptide Y is not the only neuropeptide in the central nerve system that can regulate appetite and energy balance. Agouti-related protein (AgRP) is another potent orexigenic molecule, which co-localizes with NPY in hypothalamic neurons ( Hahn et al., 1998 ). In addition, there are also melanocortins, including adrenocorticotropin and melanocyte-stimulating hormones (MSH), which are peptide cleavage products of proopiomelanocortin (POMC) and exert their effects by binding to the melanocortin receptors (MCRs). The melanocortin system is thought to be one of the most important pathways involved in food intake and energy regulation, with mutations contributing to ~4% of genetic obesity in humans ( Horvath et al., 2004 ). Neurons expressing orexigenic NPY and AgRP cooperate with neurons expressing anorexigenic POMC and cocaine-amphetamine-regulated transcript (CART). In the diet-induced obese mouse, when hypothalamic NPY mRNA expression was reduced, AgRP and POMC mRNA were also downregulated ( Lin et al., 2000 ; Wang et al., 2002 ). This suggests that the anorexigenic neurons containing POMC respond synchronously with orexigenic neurons to maintain the balance between orexigenic and anorexigenic neuropeptides. However, in nicotine-treated mice, the hypothalamic level of CART and POMC derived α-MSH has been shown to be increased ( Marty et al., 1985 ; Kramer et al., 2007 ), in the face of suppression of NPY and AgRP levels ( Chen et al., 2006 ; Martínez de Morentin et al., 2012 ). In addition, it has been shown that nicotine withdrawal is linked to increased hypothalamic NPY and AgRP, although with reduced UCP3 expression ( Fornari et al., 2006 ) resulting in an increased drive to eat, and reduced capacity for energy expenditure.
Psychological Regulators
Feeding is not only controlled by homeostatic mechanisms, which theoretically would allow an individual to maintain an ideal body weight in the long term. Feeding is also controlled by brain reward systems and psychological states, which reinforce the motives for excessive eating without homeostatic value ( Saper et al., 2002 ); namely, those independent of energy expenditure. The consumption of highly palatable foods is now considered to be an addictive behavior ( Heilig et al., 1989 ). In this respect, food and nicotine addiction may share the same central pathways. Addictive eating behavior has been suggested to be predominantly controlled by the interactions between the classical “feeding center” in the lateral hypothalamus and the nucleus accumbens within the mesolimbic system, and coordination between the neurotransmitters, such as dopamine, serotonin, and the opioid system ( Saper et al., 2002 ). Nicotine administration releases dopamine in many brain regions involved in reward, such as the mesolimbic area, the corpus striatum, the frontal cortex, and ventral tegmental area in the brain stem ( Gilbert et al., 1989 ; Benowitz, 2010 ). Increased brain release of serotonin and endogenous opioid peptides, as well as the upregulation of opioid receptors, have also been reported in various animals models following nicotine administration ( Marty et al., 1985 ; Martínez de Morentin et al., 2012 ). Eating, especially binge eating, is considered to be a physiological reaction to counteract stress in some individuals ( Polivy et al., 1994 ). Nicotine has been shown to reduce anxiety in a dose-dependent manner ( Gilbert et al., 1989 ; Pomerleau and Pomerleau, 2007 ), which may also overpower the desire to eat, in addition to its suppressive ability of central orexigenic pathways. Nicotine withdrawal can cause anxiety and stress ( Picciotto et al., 2002 ), and both can serve as powerful incentives for former smokers to either overeat or smoke again.
Tolerance due to chronic nicotine use may potentially affect its activation of the brain reward pathway. To date, only the impact of nicotine tolerance on brain dopamine release is well studied, which is also site dependent ( Damsma et al., 1989 ; Izenwasser and Cox, 1992 ). Nicotine tolerance is only seen in subjective mood effects, such as dizziness and confusion as reviewed by Perkins (2002) . However, this tolerance may still lead to an increased demand for nicotine if it is used as an appetite suppressant.
Smoking during Pregnancy and the Impact on Offspring
Smoking during pregnancy puts physiological pressure on the mother’s metabolic system and is a significant contributor to adverse pregnancy outcomes, including miscarriage, low birth weight, preterm birth, and perinatal death ( Ng et al., 2006 ; Nielsen et al., 2006 ; Raatikainen et al., 2007 ). Moreover, it significantly interrupts fetal development and predicts the future risks for respiratory dysfunction, social behavioral problems, cardiovascular disease, obesity, and type-2 diabetes ( Whincup et al., 1989 ; Orlebeke et al., 1999 ; Stocks and Dezateux, 2003 ; Burke et al., 2004 ; Al Mamun et al., 2006 ; Bruin et al., 2008b ). Despite the disadvantages of maternal smoking, reports still show that ~25–29% pregnant women smoke during pregnancy ( Contal et al., 2005 ). Some of these processes along with the underlying neurophysiological changes are shown diagrammatically in Figure 1 .
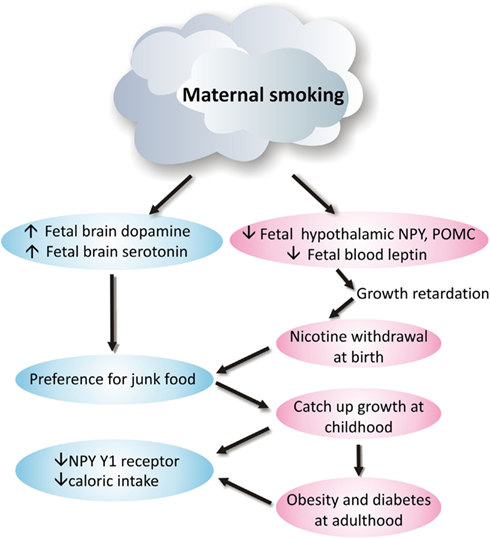
Figure 1. Neurophysiological mechanism of how maternal smoking programs metabolic disorders in offspring .
Effects on Body Weight and Eating Behavior in Offspring
In Western countries, it is maternal smoking during pregnancy rather than poverty that is the major cause of low birth weight ( Power and Jefferis, 2002 ). Even maternal obesity cannot counteract the infant growth retardation due to smoking during pregnancy ( Haworth et al., 1980 ). Studies in humans and other primates suggest that lower birth weight associated with maternal smoking is mainly nicotine-mediated ( Haworth et al., 1980 ; Grove et al., 2001 ; Collet and Beillard, 2005 ). However, brain weight does not appear to be affected by intrauterine nicotine exposure ( Grove et al., 2001 ); an observation that may be due to the redistribution of nutrients to preserve brain growth, at the cost of the development of other organs such as the liver and pancreas ( Ernst et al., 2001 ).
Catch-up growth is normally observed in children exposed to intrauterine maternal smoking, and there is evidence linking maternal smoking and childhood obesity in offspring, especially those from the mothers who smoke during early pregnancy ( Power and Jefferis, 2002 ; Al Mamun et al., 2006 ). It has been reported that children of mothers who smoked during pregnancy started to display an increased risk of being overweight at 5 years of age ( Wideroe et al., 2003 ). Adolescents who are the offspring of mothers who smoked had an increased risk of being among the highest percentile for body mass index ( Power and Jefferis, 2002 ; Al Mamun et al., 2006 ). Interestingly, smoking cessation after the first trimester does not appear to reduce this risk to the offspring ( Toschke et al., 2003 ), suggesting that the first 3 months of pregnancy are critical for long-term impacts on the wellbeing of the offspring. However, children from former smoking mothers did not show increased risk of obesity ( Oken et al., 2005 ).
Smoking mothers tend to have a shorter breastfeeding period, which deprives the offspring of the protection provided by breast milk against future eating disorders ( Gilchrist et al., 2004 ; Mayer-Davis et al., 2006 ). On this basis, it can be suggested that the rapid weight gain during the early postnatal period may be due to the effect of nicotine withdrawal, in a similar manner to the increased craving for food and subsequent weight gain seen in smokers after smoking cessation ( Lerman et al., 2004 ). Furthermore, as children also tend to copy the eating habits of their parents, this will be detrimental in the children of smokers, as smokers are more likely to choose foods low in fiber, vitamins and minerals, and high in monounsaturated fatty acids, starch, as well as sugar-sweetened soft drinks ( Crawley and While, 1996 ; Rogers et al., 2003 ). Indeed, the children of smokers are more likely to be exposed to passive smoking, with ongoing detrimental effects of the chemicals in the cigarette smoke.
Effects on Brain Energy Homeostatic Regulators
Nicotine can have a profound impact on the developing fetal brain, via its ability to rapidly and fully pass across the placenta, with fetal concentrations ~115% of maternal levels ( Walker et al., 1999 ). When the fetus leaves the womb, the supply of nicotine is removed, and the impact of nicotine withdrawal can be observed in these newborns, as they show increased signs of stress and dysregulation of the hypothalamic-pituitary-adrenal axis ( Huizink and Mulder, 2006 ). Studies in humans, other primates, and mice have observed some neuronal abnormalities relevant to feeding regulation that result from maternal smoking or exposure to nicotine ( Mantzoros et al., 1997 ; Grove et al., 2001 ; Bruin et al., 2008a ). However, the impact of maternal smoking during gestation on brain energy homeostatic pathways in the offspring requires further study.
Maternal smoking is clearly linked to abnormal hypothalamic gene expression of appetite regulators, with NPY and POMC gene expression in the arcuate nucleus of the hypothalamus being significantly downregulated in the newborn primate following intrauterine nicotine exposure ( Grove et al., 2001 ); a state that may reflect an under-developed brain. This state is similar to observations in adult animals with nicotine or cigarette smoke exposure, as clarified above. Indeed, it can be suggested that without the continuing inhibition of nicotine, NPY, and POMC gene expression can rebound to that equal to an early postnatal age, leading to hyperphagia and future obesity. As yet there is no direct data to date to support this hypothesis. However, studies of mouse models have examined the adult offspring from mothers exposed to cigarette smoke and/or those consuming a high-fat diet during the pregnancy ( Chen et al., 2011 ). Surprisingly, despite increased adiposity in offspring from smoke-exposed mothers, their daily caloric intake was actually lower than the offspring from control mothers, regardless of postnatal diet type. Although the levels of POMC were not different between groups, NPY gene expression was only suppressed by maternal consumption of a high-fat diet, and not intrauterine smoke exposure per se . However, NPY Y1 receptor gene expression was significantly downregulated by both maternal smoke exposure and a high-fat diet, with this being reflected by reduced food intake in those offspring ( Chen et al., 2011 ). In addition, other components of cigarette smoke, such as carbon monoxide and ingredients in tobacco tar, can also directly affect the fetal brain, and thereby contribute to the above changes in the fetal brain ( Ernst et al., 2001 ). It can be suggested that at adulthood, the changes in brain appetite regulators may be an adaptation to increased adiposity, rather than a prolonged impact of intrauterine smoke exposure.
Another important appetite regulator is the adipocyte-derived hormone leptin, which is critical for the development of neurons and neural projections between hypothalamic nuclei involved in appetite control in early life ( Bouret et al., 2004 ). In mice, a lack of leptin during the early postnatal period results in sparse neuronal projections in the hypothalamus, and later in life, an obese phenotype ( Zhang et al., 1994 ; Chua et al., 1996 ; Bouret et al., 2004 ). Leptin supplementation during this early postnatal period can partially restore the reduced hypothalamic neural projections in the leptin-deficient ob / ob mouse, and partially reverse the hyperphagic phenotype ( Bouret et al., 2004 ). In humans, cord blood leptin concentrations in both full-term and preterm newborns from smoking mothers are reported to be significantly decreased compared to those from non-smoking mothers ( Mantzoros et al., 1997 ). It has been suggested that smoking might increase the production of catecholamines in the infants leading to lipolysis and fat loss, which can be associated with decreased leptin levels ( Mantzoros et al., 1997 ; Ozkan et al., 2005 ), as circulating leptin levels are in relative proportion to fat mass. In a similar manner, in primates serum leptin levels are reduced by ~50% in newborns from nicotine-treated mothers compared with those from control mothers ( Grove et al., 2001 ). One hypothesis that may account for this observation is that reduced leptin in newborns from smoking mothers may interrupt the development of the neurons controlling energy homeostasis, contributing to unhealthy eating behavior at adulthood. As with smokers, it may be that the reward pathways override the energy homeostatic control in such offspring, resulting in a preference for junk foods. Studies of offspring from nicotine-treated animals show that dopamine receptor binding affinity is increased, despite reduced receptor density; while brain serotonin turnover was reduced, whilst its transporter was increased in such offspring ( Fung and Lau, 1989 ; Muneoka et al., 1997 , 2001 ). In the original studies of this topic, this finding was used to explain the abnormal social behavioral problems, such as attention deficit hyperactivity disorder or addiction, as found in offspring with intrauterine nicotine exposure. However, changes in the reward pathway may also underlie the unhealthy eating behavior.
Nicotine can change brain feeding regulation to reduce appetite via both energy homeostatic and reward mechanisms. In animal models, the effects of cigarette smoke exposure on energy homeostasis are clearly both time and dose dependent. As such, the higher the dose, the greater the reduction in caloric intake and body weight. However, the marginal effect of nicotine at reducing abdominal fat in high-fat diet fed animals may shed light on its potential application in the treatment of obesity. Maternal smoking or nicotine replacement can clearly lead to unhealthy eating habits (such as junk food addiction) and other behavioral disorders in the offspring. Thus, smoking cessation without nicotine replacement during pregnancy is recommended. Although the direct use of nicotine for fat loss in the obese is not plausible, the appetite suppressive and energy expenditure promoting effects of nicotine may still be useful. The development of nicotine analogs should be encouraged which avoid addiction, but retain the fat burning-obesity reduction effect.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Al Mamun, A., Lawlor, D. A., Alati, R., O’Callaghan, M. J., Williams, G. M., and Najman, J. M. (2006). Does maternal smoking during pregnancy have a direct effect on future offspring obesity? Evidence from a prospective birth cohort study. Am. J. Epidemiol. 164, 317–325.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Allen, Y. S., Adrian, T. E., Allen, J. M., Tatemoto, K., Crow, T. J., Bloom, S. R., and Polak, J. M. (1983). Neuropeptide Y distribution in the rat brain. Science 221, 877–879.
Andersson, K., and Arner, P. (2001). Systemic nicotine stimulates human adipose tissue lipolysis through local cholinergic and catecholaminergic receptors. Int. J. Obes. Relat. Metab. Disord. 25, 1225–1232.
Arai, K., Kim, K., Kaneko, K., Iketani, M., Otagiri, A., Yamauchi, N., and Shibasaki, T. (2001). Nicotine infusion alters leptin and uncoupling protein 1 mRNA expression in adipose tissues of rats. Am. J. Physiol. Endocrinol. Metab. 280, E867–E876.
Pubmed Abstract | Pubmed Full Text
Argyropoulos, G., and Harper, M. E. (2002). Uncoupling proteins and thermoregulation. J. Appl. Physiol. 92, 2187–2198.
Bellinger, L., Cepeda-Benito, A., and Wellman, P. J. (2003). Meal patterns in male rats during and after intermittent nicotine administration. Pharmacol. Biochem. Behav. 74, 495–504.
Benowitz, N. L. (2010). Nicotine addiction. N. Engl. J. Med. 362, 2295–2303.
Billington, C. J., Briggs, J. E., Grace, M., and Levine, A. S. (1991). Effects of intracerebroventricular injection of neuropeptide Y on energy metabolism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 260, R321–R327.
Bouret, S. G., Draper, S. J., and Simerly, R. B. (2004). Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304, 108–110.
Bruin, J. E., Petre, M. A., Lehman, M. A., Raha, S., Gerstein, H. C., Morrison, K. M., and Holloway, A. C. (2008a). Maternal nicotine exposure increases oxidative stress in the offspring. Free Radic. Biol. Med. 44, 1919–1925.
CrossRef Full Text
Bruin, J. E., Petre, M. A., Raha, S., Morrison, K. M., Gerstein, H. C., and Holloway, A. C. (2008b). Fetal and neonatal nicotine exposure in Wistar rats causes progressive pancreatic mitochondrial damage and beta cell dysfunction. PLoS ONE 3, e3371. doi:10.1371/journal.pone.0003371
Burke, V., Beilin, L. J., Simmer, K., Oddy, W. H., Blake, K. V., Doherty, D., Kendall, G. E., Newnham, J. P., Landau, L. I., and Stanley, F. J. (2004). Predictors of body mass index and associations with cardiovascular risk factors in Australian children: a prospective cohort study. Int. J. Obes. 29, 15–23.
Camp, D. E., Klesges, R. C., and Relyea, G. (1993). The relationship between body weight concerns and adolescent smoking. Health Psychol. 12, 24–32.
Cannon, B., and Nedergaard, J. (2004). Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359.
Canoy, D., Wareham, N., Luben, R., Welch, A., Bingham, S., Day, N., and Khaw, K. T. (2005). Cigarette smoking and fat distribution in 21,828 British men and women: a population-based study. Obes. Res. 13, 1466–1475.
Champigny, O., and Ricquier, D. (1990). Effects of fasting and refeeding on the level of uncoupling protein mRNA in rat brown adipose tissue: evidence for diet-induced and cold-induced responses. J. Nutr. 120, 1730–1736.
Chen, H., Hansen, M. J., Jones, J. E., Vlahos, R., Anderson, G., and Morris, M. J. (2007). Detrimental metabolic effects of combining long term cigarette smoke exposure and high-fat diet in mice. Am. J. Physiol. Endocrinol. Metab. 293, E1564–E1571.
Chen, H., Hansen, M. J., Jones, J. E., Vlahos, R., Anderson, G. P., and Morris, M. J. (2008). Long-term cigarette smoke exposure increases uncoupling protein expression but reduces energy intake. Brain Res. 1228, 81–88.
Chen, H., Hansen, M. J., Jones, J. E., Vlahos, R., Bozinovski, S., Anderson, G. P., and Morris, M. J. (2006). Cigarette smoke exposure reprograms the hypothalamic neuropeptide Y axis to promote weight loss. Am. J. Respir. Crit. Care Med. 173, 1248–1254.
Chen, H., Iglesias, M. A., Caruso, V., and Morris, M. J. (2011). Maternal cigarette smoke exposure contributes to glucose intolerance and decreased brain insulin action in mice offspring independent of maternal diet. PLoS ONE 6, e27260. doi:10.1371/journal.pone.0027260
Chen, H., Vlahos, R., Bozinovski, S., Jones, J., Anderson, G. P., and Morris, M. J. (2005). Effect of short-term cigarette smoke exposure on body weight, appetite and brain neuropeptide y in mice. Neuropsychopharmacology 30, 713–719.
Chua, S. C. Jr., Chung, W. K., Wu-Peng, X. S., Zhang, Y., Liu, S. M., Tartaglia, L., and Leibel, R. L. (1996). Phenotypes of mouse diabetes and rat fatty due to mutations in the ob (leptin) receptor. Science 271, 994–996.
Clark, J. T., Kalra, P. S., Crowley, W. R., and Kalra, S. P. (1984). Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology 115, 427–429.
Collet, M., and Beillard, C. (2005). Consequences of smoking on fetal development and risk of intra-uterine growth retardation or in utero fetal death. J. Gynecol. Obstet. Biol. Reprod. 34 3S135–S145.
Contal, M., Masson, G., Boyer, C., Cazevielle, C., and Mares, P. (2005). Neonatal consequences of maternal smoking during pregnancy. J. Gynecol. Obstet. Biol. Reprod. 34 3S215–S222.
Crawley, H. F., and While, D. (1996). Parental smoking and the nutrient intake and food choice of British teenagers aged 16–17 years. J. Epidemiol. Community Health 50, 306–312.
Dalgaard, L. T., and Pedersen, O. (2001). Uncoupling proteins: functional characteristics and role in the pathogenesis of obesity and Type II diabetes. Diabetologia 44, 946–965.
Damsma, G., Day, J., and Fibiger, H. C. (1989). Lack of tolerance to nicotine-induced dopamine release in the nucleus accumbens. Eur. J. Pharmacol. 168, 363–368.
DeMarini, D. M. (2004). Genotoxicity of tobacco smoke and tobacco smoke condensate: a review. Mutat. Res. 567, 447–474.
Egawa, M., Yoshimatsu, H., and Bray, G. A. (1991). Neuropeptide Y suppresses sympathetic activity to interscapular brown adipose tissue in rats. Am. J. Physiol. 260, R328–R334.
Eliasson, B., Attvall, S., Taskinen, M. R., and Smith, U. (1997). Smoking cessation improves insulin sensitivity in healthy middle-aged men. Eur. J. Clin. Invest. 27, 450–456.
Ernst, M., Moolchan, E. T., and Robinson, M. L. (2001). Behavioral and neural consequences of prenatal exposure to nicotine. J. Am. Acad. Child Adolesc. Psychiatry 40, 630–641.
Facchini, F. S., Hollenbeck, C. B., Jeppesen, J., Chen, Y.-D., and Reaven, G. M. (1992). Insulin resistance and cigarette smoking. Lancet 339, 1128–1130.
Fernandez-Real, J. M., Broch, M., Vendrell, J., and Ricart, W. (2003). Smoking, fat mass and activation of the tumor necrosis factor-alpha pathway. Int. J. Obes. Relat. Metab. Disord. 27, 1552–1556.
Filozof, C., Fernandez Pinilla, M. C., and Fernandez-Cruz, A. (2004). Smoking cessation and weight gain. Obes. Rev. 5, 95–103.
Fornari, A., Pedrazzi, P., Lippi, G., Picciotto, M. R., Zoli, M., and Zini, I. (2006). Nicotine withdrawal increases body weight, neuropeptide Y and Agouti-related protein expression in the hypothalamus and decreases uncoupling protein-3 expression in the brown adipose tissue in high-fat fed mice. Neurosci. Lett. 411, 72–76.
Fulkerson, J. A., and French, S. A. (2003). Cigarette smoking for weight loss or control among adolescents: gender and racial/ethnic differences. J. Adolesc. Health 32, 306–313.
Fung, Y. K., and Lau, Y.-S. (1989). Effects of prenatal nicotine exposure on rat striatal dopaminergic and nicotinic systems. Pharmacol. Biochem. Behav. 33, 1–6.
Gilbert, D. G., Robinson, J. H., Chamberlin, C. L., and Spielberger, C. D. (1989). Effects of smoking/nicotine on anxiety, heart rate, and lateralization of EEG during a stressful movie. Psychophysiology 26, 311–320.
Gilchrist, D., Woods, B., Binns, C. W., Scott, J. A., Gracey, M., and Smith, H. (2004). Aboriginal mothers, breastfeeding and smoking. Aust. N. Z. J. Public Health 28, 225–228.
Grove, K. L., Sekhon, H. S., Brogan, R. S., Keller, J. A., Smith, M. S., and Spindel, E. R. (2001). Chronic maternal nicotine exposure alters neuronal systems in the arcuate nucleus that regulate feeding behavior in the newborn rhesus macaque. J. Clin. Endocrinol. Metab. 86, 5420–5426.
Grunberg, N. E., Bowen, D. J., and Winders, S. E. (1986). Effects of nicotine on body weight and food consumption in female rats. Psychopharmacology (Berl.) 90, 101–105.
Grunberg, N. E., Popp, K. A., and Winders, S. E. (1988). Effects of nicotine on body weight in rats with access to “junk” foods. Psychopharmacology (Berl.) 94, 536–539.
Hahn, T. M., Breininger, J. F., Baskin, D. G., and Schwartz, M. W. (1998). Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat. Neurosci. 1, 271–272.
Hajek, P., Jackson, P., and Belcher, M. (1988). Long-term use of nicotine chewing gum. Occurrence, determinants, and effect on weight gain. JAMA 260, 1593–1596.
Haworth, J. C., Ellestad-Sayed, J. J., King, J., and Dilling, L. A. (1980). Relation of maternal cigarette smoking, obesity, and energy consumption to infant size. Am. J. Obstet. Gynecol. 138, 1185–1189.
Heilig, M., Soderpalm, B., Engel, J. A., and Widerlov, E. (1989). Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacology (Berl.) 98, 524–529.
Horvath, T. L., Diano, S., and Tschop, M. (2004). Brain circuits regulating energy homeostasis. Neuroscientist 10, 235–246.
Huizink, A. C., and Mulder, E. J. (2006). Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci. Biobehav. Rev. 30, 24–41.
Hussein, J., Farkas, S., Mackinnon, Y., Ariano, R. E., Sitar, D. S., and Hasan, S. U. (2007). Nicotine dose–concentration relationship and pregnancy outcomes in rat: biologic plausibility and implications for future research. Toxicol. Appl. Pharmacol. 218, 1–10.
Izenwasser, S., and Cox, B. M. (1992). Inhibition of dopamine uptake by cocaine and nicotine: tolerance to chronic treatments. Brain Res. 573, 119–125.
Jagoe, R. T., and Engelen, M. P. (2003). Muscle wasting and changes in muscle protein metabolism in chronic obstructive pulmonary disease. Eur. Respir. J. Suppl. 46, 52s–63s.
Jansson, J. O., Wallenius, K., Wernstedt, I., Ohlsson, C., Dickson, S. L., and Wallenius, V. (2003). On the site and mechanism of action of the anti-obesity effects of interleukin-6. Growth Horm. IGF Res. 13(Suppl A), S28–S32.
Jo, Y. H., Talmage, D. A., and Role, L. W. (2002). Nicotinic receptor-mediated effects on appetite and food intake. J. Neurobiol. 53, 618–632.
Kane, J. K., Parker, S. L., and Li, M. D. (2001). Hypothalamic orexin-A binding sites are downregulated by chronic nicotine treatment in the rat. Neurosci. Lett. 298, 1–4.
Kramer, P. R., Kramer, S. F., Marr, K., Guan, G., Wellman, P. J., and Bellinger, L. L. (2007). Nicotine administration effects on feeding and cocaine–amphetamine-regulated transcript (CART) expression in the hypothalamus. Regul. Pept. 138, 66–73.
Langhans, W., and Hrupka, B. (1999). Interleukins and tumor necrosis factor as inhibitors of food intake. Neuropeptides 33, 415–424.
Lee, F. Y., Li, Y., Zhu, H., Yang, S., Lin, H. Z., Trush, M., and Diehl, A. M. (1999). Tumor necrosis factor increases mitochondrial oxidant production and induces expression of uncoupling protein-2 in the regenerating mice [correction of rat] liver. Hepatology 29, 677–687.
Leischow, S. J., Sachs, D. P., Bostrom, A. G., and Hansen, M. D. (1992). Effects of differing nicotine-replacement doses on weight gain after smoking cessation. Arch. Fam. Med. 1, 233–237.
Lerman, C., Berrettini, W., Pinto, A., Patterson, F., Crystal-Mansour, S., Wileyto, E. P., Restine, S. L., Leonard, D. G., Shields, P. G., and Epstein, L. H. (2004). Changes in food reward following smoking cessation: a pharmacogenetic investigation. Psychopharmacology (Berl.) 174, 571–577.
Lin, S., Storlien, L. H., and Huang, X. F. (2000). Leptin receptor, NPY, POMC mRNA expression in the diet-induced obese mouse brain. Brain Res. 875, 89–95.
Mantzoros, C. S., Varvarigou, A., Kaklamani, V. G., Beratis, N. G., and Flier, J. S. (1997). Effect of birth weight and maternal smoking on cord blood leptin concentrations of full-term and preterm newborns. J. Clin. Endocrinol. Metab. 82, 2856–2861.
Marangon, K., Herbeth, B., Lecomte, E., Paul-Dauphin, A., Grolier, P., Chancerelle, Y., Artur, Y., and Siest, G. (1998). Diet, antioxidant status, and smoking habits in French men. Am. J. Clin. Nutr. 67, 231–239.
Martínez de Morentin, P. B., Whittle, A. J., Fernø, J., Nogueiras, R., Diéguez, C., Vidal-Puig, A., and López, M. (2012). Nicotine induces negative energy balance through hypothalamic AMP-activated protein kinase. Diabetes 61, 807–817.
Marty, M. A., Erwin, V. G., Cornell, K., and Zgombick, J. M. (1985). Effects of nicotine on beta-endorphin, alpha MSH, and ACTH secretion by isolated perfused mouse brains and pituitary glands, in vitro. Pharmacol. Biochem. Behav. 22, 317–325.
Mayer-Davis, E. J., Rifas-Shiman, S. L., Zhou, L., Hu, F. B., Colditz, G. A., and Gillman, M. W. (2006). Breast-feeding and risk for childhood obesity: does maternal diabetes or obesity status matter? Diab. Care 29, 2231–2237.
Morrison, W. L., Gibson, J. N., Scrimgeour, C., and Rennie, M. J. (1988). Muscle wasting in emphysema. Clin. Sci. 75, 415–420.
Muneoka, K., Ogawa, T., Kamei, K., Mimura, Y., Kato, H., and Takigawa, M. (2001). Nicotine exposure during pregnancy is a factor which influences serotonin transporter density in the rat brain. Eur. J. Pharmacol. 411, 279–282.
Muneoka, K., Ogawa, T., Kamei, K., Muraoka, S.-I., Tomiyoshi, R., Mimura, Y., Kato, H., Suzuki, M. R., and Takigawa, M. (1997). Prenatal nicotine exposure affects the development of the central serotonergic system as well as the dopaminergic system in rat offspring: involvement of route of drug administrations. Dev. Brain Res. 102, 117–126.
Ng, S. P., Steinetz, B. G., Lasano, S. G., and Zelikoff, J. T. (2006). Hormonal changes accompanying cigarette smoke-induced preterm births in a mouse model. Exp. Biol. Med. 231, 1403–1409.
Nielsen, A., Hannibal, C. G., Lindekilde, B. E., Tolstrup, J., Frederiksen, K., Munk, C., Bergholt, T., Buss, L., Ottesen, B., Gronbaek, M., and Kjaer, S. K. (2006). Maternal smoking predicts the risk of spontaneous abortion. Acta Obstet. Gynecol. Scand. 85, 1057–1065.
Oken, E., Huh, S. Y., Taveras, E. M., Rich-Edwards, J. W., and Gillman, M. W. (2005). Associations of maternal penatal smoking with child adiposity and blood pressure. Obes. Res. 13, 2021–2028.
Orlebeke, J. F., Knol, D. L., and Verhulst, F. C. (1999). Child behavior problems increased by maternal smoking during pregnancy. Arch. Environ. Health 54, 15–19.
Ozkan, B., Ermis, B., Tastekin, A., Doneray, H., Yildirim, A., and Ors, R. (2005). Effect of smoking on neonatal and maternal serum and breast milk leptin levels. Endocr. Res. 31, 177–183.
Perkins, K. A. (1992). Effects of tobacco smoking on caloric intake. Br. J. Addict. 87, 193–205.
Perkins, K. A. (2002). Chronic tolerance to nicotine in humans and its relationship to tobacco dependence. Nicotine Tob. Res. 4, 405–422.
Perkins, K. A., Epstein, L. H., Stiller, R. L., Marks, B. L., and Jacob, R. G. (1989). Acute effects of nicotine on resting metabolic rate in cigarette smokers. Am. J. Clin. Nutr. 50, 545–550.
Picciotto, M. R., Brunzell, D. H., and Caldarone, B. J. (2002). Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport 13, 1097–1106.
Polivy, J., Herman, C. P., and Mcfarlane, T. (1994). Effects of anxiety on eating: does palatability moderate distress-induced overeating in dieters? J. Abnorm. Psychol. 103, 505–510.
Pomerleau, O. F., and Pomerleau, C. S. (2007). “Behavioural studies in humans: anxiety, stress and smoking,” in Ciba Foundation Symposium 152 – The Biology of Nicotine Dependence , eds G. Bock and J. Marsh (Chichester: John Wiley & Sons, Ltd.), 225–254.
Power, C., and Jefferis, B. J. (2002). Fetal environment and subsequent obesity: a study of maternal smoking. Int. J. Epidemiol. 31, 413–419.
Raatikainen, K., Huurinainen, P., and Heinonen, S. (2007). Smoking in early gestation or through pregnancy: a decision crucial to pregnancy outcome. Prev. Med. 44, 59–63.
Raposinho, P., Pierroz, D. D., Broqua, P., White, R. B., Pedrazzini, T., and Aubert, M. L. (2001). Chronic administration of neuropeptide Y into the lateral ventricle of C57BL/6J male mice produces an obesity syndrome including hyperphagia, hyperleptinemia, insulin resistance, and hypogonadism. Mol. Cell. Endocrinol. 185, 195–204.
Rogers, I., and Emmett, P. Alspac Study Team (2003). The effect of maternal smoking status, educational level and age on food and nutrient intakes in preschool children: results from the Avon Longitudinal Study of Parents and Children. Eur. J. Clin. Nutr. 57, 854–864.
Samec, S., Seydoux, J., and Dulloo, A. G. (1998). Role of UCP homologues in skeletal muscles and brown adipose tissue: mediators of thermogenesis or regulators of lipids as fuel substrate? FASEB J. 12, 715–724.
Saper, C. B., Chou, T. C., and Elmquist, J. K. (2002). The need to feed: homeostatic and hedonic control of eating. Neuron 36, 199–211.
Schrauwen, P., and Hesselink, M. (2003). Uncoupling protein 3 and physical activity: the role of uncoupling protein 3 in energy metabolism revisited. Proc. Nutr. Soc. 62, 635–643.
Shiraev, T., Chen, H., and Morris, M. J. (2009). Differential effects of restricted versus unlimited high-fat feeding in rats on fat mass, plasma hormones and brain appetite regulators. J. Neuroendocrinol. 21, 602–609.
Stamford, B. A., Matter, S., Fell, R. D., and Papanek, P. (1986). Effects of smoking cessation on weight gain, metabolic rate, caloric consumption, and blood lipids. Am. J. Clin. Nutr. 43, 486–494.
Stannard, S. R., and Johnson, N. A. (2004). Insulin resistance and elevated triglyceride in muscle: more important for survival than ‘thrifty’ genes? J. Physiol. 554, 595–607.
Stocks, J., and Dezateux, C. (2003). The effect of parental smoking on lung function and development during infancy. Respirology 8, 266–285.
Strauss, R. S., and Mir, H. M. (2001). Smoking and weight loss attempts in overweight and normal-weight adolescents. Int. J. Obes. Relat. Metab. Disord. 25, 1381–1385.
Sztalryd, C., Hamilton, J., Horwitz, B. A., Johnson, P., and Kraemer, F. B. (1996). Alterations of lipolysis and lipoprotein lipase in chronically nicotine-treated rats. Am. J. Physiol. Endocrinol. Metab. 270, E215–E223.
Tatemoto, K., Carlquist, M., and Mutt, V. (1982). Neuropeptide Y – a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature 296, 659–660.
Toschke, A. M., Montgomery, S. M., Pfeiffer, U., and Von Kries, R. (2003). Early intrauterine exposure to tobacco-inhaled products and obesity. Am. J. Epidemiol. 158, 1068–1074.
Vettor, R., Zarjevski, N., Cusin, I., Rohner-Jeanrenaud, F., and Jeanrenaud, B. (1994). Induction and reversibility of an obesity syndrome by intracerebroventricular neuropeptide Y administration to normal rats. Diabetologia 37, 1202–1208.
Wager-Srdar, S. A., Levine, A. S., Morley, J. E., Hoidal, J. R., and Niewoehner, D. E. (1984). Effects of cigarette smoke and nicotine on feeding and energy. Physiol. Behav. 32, 389–395.
Walker, A., Rosenberg, M., and Balaban-Gil, K. (1999). Neurodevelopmental and neurobehavioral sequelae of selected substances of abuse and psychiatric medications in utero. Child Adolesc. Psychiatr. Clin. N. Am. 8, 845–867.
Wang, H., Storlien, L. H., and Huang, X. F. (2002). Effects of dietary fat types on body fatness, leptin, and ARC leptin receptor, NPY, and AgRP mRNA expression. Am. J. Physiol. Endocrinol. Metab. 282, E1352–E1359.
Whincup, P. H., Cook, D. G., and Shaper, A. G. (1989). Early influences on blood pressure: a study of children aged 5–7 years. BMJ 299, 587–591.
Wideroe, M., Vik, T., Jacobsen, G., and Bakketeig, L. S. (2003). Does maternal smoking during pregnancy cause childhood overweight? Paediatr. Perinat. Epidemiol. 17, 171–179.
Williamson, D. F., Madans, J., Anda, R. F., Kleinman, J. C., Giovino, G. A., and Byers, T. (1991). Smoking cessation and severity of weight gain in a national cohort. N. Engl. J. Med. 324, 739–745.
Wiseman, C. V. (1998). Smoking and body image concerns in adolescent girls. Int. J. Eat. Disord. 24, 429–433.
World Health Organization. (2011). WHO Report on the Global Tobacco Epidemic, 2011: Warning about the Dangers of Tobacco . Available at: http://whqlibdoc.who.int/publications/2011/9789240687813_eng.pdf
Yoshida, T., Sakane, N., Umekawa, T., Kogure, A., Kondo, M., Kumamoto, K., Kawada, T., Nagase, I., and Saito, M. (1999). Nicotine induces uncoupling protein 1 in white adipose tissue of obese mice. Int. J. Obes. Relat. Metab. Disord. 23, 570–575.
Zhang, Y., Proenca, R., Maffei, M., Barone, M., Leopold, L., and Friedman, J. M. (1994). Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432.
Keywords: smoking, nicotine, appetite regulation, reward, programming
Citation: Chen H, Saad S, Sandow SL and Bertrand PP (2012) Cigarette smoking and brain regulation of energy homeostasis. Front. Pharmacol. 3 :147. doi: 10.3389/fphar.2012.00147
Received: 14 May 2012; Accepted: 09 July 2012; Published online: 25 July 2012.
Reviewed by:
Copyright: © 2012 Chen, Saad, Sandow and Bertrand. This is an open-access article distributed under the terms of the Creative Commons Attribution License , which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and subject to any copyright notices concerning any third-party graphics etc.
*Correspondence: Hui Chen, School of Medical and Molecular Biosciences, University of Technology, PO Box 123, Broadway, Sydney, NSW 2007, Australia. e-mail: hui.chen-1@uts.edu.au
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Mol Sci
- PMC10572882

Nicotine: From Discovery to Biological Effects
Luigi sansone.
1 Department of Human Sciences and Quality, Life Promotion San Raffaele University, Via di Val Cannuta 247, 00166 Rome, Italy; [email protected] (L.S.); [email protected] (M.B.); [email protected] (M.C.); [email protected] (A.d.I.); [email protected] (S.B.)
2 MEBIC Consortium, San Raffaele University, 00166 Rome, Italy
Francesca Milani
3 Clinical and Molecular Epidemiology, IRCCS San Raffaele Roma, Via di Val Cannuta 247, 00166 Rome, Italy; [email protected] (F.M.); [email protected] (R.F.); [email protected] (L.C.)
Riccardo Fabrizi
Manuel belli, mario cristina.
4 Department of Molecular Medicine, University La Sapienza, Viale del Policlinico 155, 00161 Rome, Italy
Vincenzo Zagà
5 Italian Society of Tabaccology (SITAB), 00136 Bologna, Italy; [email protected]
Antonio de Iure
6 Experimental Neurophysiology IRCCS San Raffaele Roma, Via di Val Cannuta 247, 00166 Rome, Italy
Luca Cicconi
Stefano bonassi, patrizia russo.
Nicotine, the primary psychoactive agent in tobacco leaves, has led to the widespread use of tobacco, with over one billion smokers globally. This article provides a historical overview of tobacco and discusses tobacco dependence, as well as the biological effects induced by nicotine on mammalian cells. Nicotine induces various biological effects, such as neoangiogenesis, cell division, and proliferation, and it affects neural and non-neural cells through specific pathways downstream of nicotinic receptors (nAChRs). Specific effects mediated by α7 nAChRs are highlighted. Nicotine is highly addictive and hazardous. Public health initiatives should prioritize combating smoking and its associated risks. Understanding nicotine’s complex biological effects is essential for comprehensive research and informed health policies. While potential links between nicotine and COVID-19 severity warrant further investigation, smoking remains a significant cause of morbidity and mortality globally. Effective public health strategies are vital to promote healthier lifestyles.
1. Introduction
To introduce nicotine and discuss its biological effects, it is necessary to mention tobacco, since nicotine is the psychoactive agent found in tobacco leaves. Currently, there are over one billion smokers worldwide, making tobacco the second most commonly used psychoactive substance [ 1 ]. Smokers become addicted to nicotine through the consumption of cigarettes or cigars. This addiction is referred to as tobacco dependence in the International Classification of Diseases, Tenth Revision (ICD-10), or tobacco use disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [ 2 ]. Besides nicotine, other components found in cigarettes, such as flavorings and non-nicotine compounds, can influence the addictive potential of tobacco [ 3 ].
This review is organized into several sections, beginning with the history of tobacco, followed by a discussion of the characteristics of nicotine, including a botanical overview of the tobacco plant, an examination of nicotine as a secondary metabolite, and concluding with an exploration of the chemical, physical, and biological properties of nicotine. Nicotine is intricate and multifaceted, encompassing historical, pharmacological, biological, and behavioral dimensions. The novelty of this review lies precisely in providing a comprehensive description of nicotine within a single piece of work. The purpose is to evaluate the impact of nicotine as a negative biological agent on human health by examining all its aspects to understand the reasons for its usage and spread. While much is known of the role of nicotine as a psychotropic agent and its impact on neurocircuits [ 2 , 4 , 5 , 6 , 7 , 8 , 9 ], its role in human carcinogenesis remains controversial. The availability of data regarding the genotoxic effects of nicotine, including sister chromatid exchange, chromosome aberration, and induction of DNA double-strand breaks in mammalian cells, is not yet sufficient [ 10 , 11 , 12 , 13 ]. On the other hand, as outlined in Section 2.4 , the role of nicotine in cell proliferation, especially in tumor cells, is well documented, supporting the hypothesis of its role as a promoting agent in the process of human carcinogenesis [ 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 ].
Studies have demonstrated that nicotine alters the expression of microRNAs (miRNAs) in various smoking-related disorders and exerts its effects through miRNA-related pathways [ 32 , 33 ]. In a recent review [ 32 ], a comprehensive summary was provided of all the miRNAs influenced by nicotine and the activity of nAChRs. This influence leads to subsequent changes in the expression of target genes. Importantly, alterations in miRNA expression can have both protective effects, such as the activation of anti-inflammatory processes, and detrimental effects, including those associated with conditions like atherosclerosis and Alzheimer’s disease.
Perinatal exposure to tobacco smoke and nicotine has been linked to a multitude of epigenetic changes, such as modified DNA methylation of genes in offspring. These changes are associated with various conditions, including cancer, Alzheimer’s disease, addiction, diabetes, and neural development. This suggests that perinatal nicotine exposure could potentially influence development and increase the risk of disease development through altered DNA methylation patterns. Additionally, prenatal exposure to nicotine has been associated with changes in miRNA signaling linked to inflammatory responses, which are correlated with lower birth weights and disrupted lung development. Both of these factors are connected to developmental exposure to nicotine and tobacco [ 33 ].
Developmental exposure to nicotine also leads to changes in histone methylation in the brain and alterations in dendritic complexity, contributing to mental health issues such as depression, addiction, and ADHD. The negative impacts of prenatal nicotine exposure can extend into adulthood, implying that developmental exposure to nicotine can have enduring implications for one’s health.
1.1. History of Tobacco
The history of tobacco has its roots in various ancient civilizations. Officially, the recorded history of tobacco is considered to have commenced with the encounter between Christopher Columbus and the indigenous people of the New World in 1492. During their interactions in the Bahamas, the Lucayan, Taíno, and Arawak people presented Columbus and his crew with dried tobacco leaves [ 34 ]. However, there are indications suggesting the existence of tobacco even prior to this encounter. In 1976, Michèle (Layer-)Lescot discovered fragments of tobacco leaves in the remains of Ramses II (1279-1213 BCE), the Egyptian pharaoh [ 35 ]. Similarly, a German research team reported the identification of psychoactive substances, including nicotine, in Egyptian mummies dating from 1070 BCE to 395 CE [ 36 ]. The explanations regarding the presence of tobacco in Egyptian mummies do not account for their post-excavation histories. In fact, the intricate story of the discovery of Ramses II’s mummy involves its movement to various tombs over the course of millennia, introducing the possibility of contamination and intervention.
The consensus among researchers is that tobacco (genus Nicotiana ) originated in the Andes of South America around 6000 BCE [ 37 ]. Cultivated varieties of tobacco, including Nicotiana rustica and Nicotiana tabacum , characterized by larger leaves and higher nicotine content, spread to regions like Mesoamerica, the Caribbean, and parts of what is now the southeastern and southwestern United States. However, the earliest archaeological evidence comes from a terracotta tobacco pipe discovered in the Banda region of West Africa, dating back to the 19th century BC. This dating is supported by gas chromatography/mass spectrometry (GC–MS) analysis of pipe residues, which identified peaks identical to those found in pure nicotine samples [ 38 , 39 ].
During the first millennium CE, Native Americans began incorporating tobacco into religious ceremonies and for medicinal purposes. The Maya civilization, for instance, utilized tobacco in recreational, ceremonial, and medicinal contexts. They even depicted individuals of high rank smoking cigars, and priests employing tobacco smoke in human sacrifices [ 40 ].
The Toltecs, responsible for the establishment of the Aztec empire, adopted the smoking tradition from the Mayans. The Mayans, who inhabited the Mississippi Valley region, introduced the use of tobacco to neighboring tribes, leading to the incorporation of tobacco smoking into their religious rituals. These tribes believed that their deity, Manitou, manifested through the ascending smoke [ 40 ].
A Native American myth recounts the tale of a woman dispatched by the Great Spirit to rescue humanity. As she journeyed, wherever her right hand touched the ground, potatoes sprouted, and wherever her left hand touched, corn grew. When she paused to rest, tobacco plants began to flourish, symbolizing the earth’s abundance and fertility [ 40 ].
These historical narratives highlight the enduring cultural and ritual importance of tobacco across various civilizations throughout history [ 41 ]. In Central America, an intricate system of religious and political practices evolved around tobacco. Over countless years, tobacco has held a revered role within numerous Native American tribes, serving as a conduit for prayer, a symbol of reverence, a source of healing, and a means of protection. The use of tobacco was never meant for misuse and has never been employed for recreational pursuits.
Table 1 presents the history of tobacco.
History of tobacco.
1.2. Tobacco Plant
Tobacco is derived from various species of Nicotiana , belonging to the botanical family Solanaceae (the nightshade family). Among these, Nicotiana tabacum stands as the most extensively cultivated species. This plant is identifiable by its short visco-glandular hairs and the release of a yellow secretion containing nicotine [ 44 ].
The Solanaceae family constitutes a monophyletic group encompassing approximately 99 genera and around 3000 species. This family displays a wide array of diversity in terms of habitats, morphology, and ecology. Although its distribution spans the globe, the highest biodiversity is concentrated in the Americas [ 45 ].
The genus Nicotiana was named by Linnaeus in tribute to Jean Nicot, a French diplomat who introduced tobacco seeds from Portugal to France in the 16th century [ 46 ]. Initially, Linnaeus classified four Nicotiana species, all indigenous to the Americas. Later, Lehmann incorporated 21 species originating from Australia. George Don further categorized the family into four sections based on flower shape and color. The taxonomic details of the genus, encompassing the distribution, morphology, and cytology of known species, were meticulously documented in the Goodspeed monograph [ 47 , 48 ]. Goodspeed divided the genus into three subgenera and identified 60 species, including several novel species from Australia, Africa, and South America.
Nicotiana tabacum is a perennial or robust annual herbaceous plant that can reach heights of 1–2 m. It features ovate to lanceolate leaves arranged spirally along its stem. Nicotiana tabacum is an allotetraploid, likely arising from the hybridization of Nicotiana sylvestris, Nicotiana tomentosiformis , and possibly Nicotiana otophora [ 48 ].
1.3. Nicotine as Secondary Metabolite
The general metabolism in an organism includes all metabolic pathways essential for its growth and development. In contrast, specialized metabolites or secondary metabolites (SM) are low-molecular-weight natural products with a narrow taxonomic distribution. They are often synthesized in cells or tissues after active growth has ceased. SMs are typically non-essential for normal growth, development, or reproduction. Their functions, such as pigments and perfumes, include attracting pollinators. SMs encompass a diverse group of natural products synthesized by plants, fungi, bacteria, algae, and animals. They are generally classified into three main groups: terpenes (including plant volatiles, cardiac glycosides, carotenoids, and sterols), phenolic compounds (such as phenolic acids, coumarins, lignans, stilbenes, flavonoids, tannins, and lignins), and nitrogen- or sulfur-containing compounds (such as alkaloids and glucosinolates). SMs play key roles in functions including defense against herbivores and microbial pathogens, UV protection, pollinator attraction, and fertility. They are produced at the highest levels during the transition from active growth to differentiation [ 49 , 50 ].
Nicotine is produced as a defense against predatory insects. Its biosynthesis and aerial accumulation typically increase after herbivore or insect attack, wounding, or jasmonate treatment of the leaf. Experimental evidence supports the hypothesis that tobacco alkaloids, including nicotine, are synthesized in the roots and then transported to the leaves (the site of herbivore or insect attack) through the xylem stream, where they accumulate significantly. In the past, nicotine was used as a pesticide worldwide, including in the United States, until its ban in the mid-1960s [ 49 , 50 ].
During evolution, herbivores and insects develop mechanisms of resistance to nicotine. Notably, the tobacco hornworm ( Manduca sexta ) from the Sphingidae family is the only insect that is unaffected by nicotine’s negative effects. Its defense system against nicotine involves carrying an altered amino acid sequence of the receptor, limiting nicotine’s affinity for its receptors, and possessing a functional equivalent of a blood–brain barrier. Astrocytes enveloping neurons express nicotine-binding proteins, acting as scavengers and releasing nicotine into the surrounding hemolymph, protecting the neurons [ 51 ]. Manduca sexta converts nicotine into metabolites via cytochrome P450 6B46 (CYP6B46), which is known for its unique role in perceiving signaling molecules of plant defense responses [ 52 ]. These metabolites are then transported from the gut to the hemolymph, reconverted to nicotine, and released into the air as a deterrent to spiders, termed “toxic halitosis”. However, the braconid wasp Cotesia congregata can lay its eggs in the bodies of hornworms, and its larvae feed internally on them, despite Manduca sexta ’s ability to metabolize nicotine and use it as a defense against predators.
2. Nicotine
Nicotine is classified as a tertiary amine consisting of a pyridine and a pyrrolidine ring. It is primarily present in the (S)-nicotine form, and can occur in concentrations as high as 3% in dried leaves of the tobacco plant ( Nicotiana tabacum ). In the lesser-known “Aztec tobacco” ( Nicotiana rustica ), nicotine concentrations can be notably higher, extending to levels as high as 14% [ 53 ]. Table 2 provides an overview of the chemical, physical, and toxicological information pertinent to nicotine [ 54 , 55 , 56 , 57 , 58 ].
Nicotine: chemical, physical, and toxicological data. Adapted from [ 54 ].
2.1. Nicotinic Acetylcholine Receptors (nAChRs)
Nicotinic acetylcholine receptors (nAChRs) are members of the superfamily of pentameric ligand-gated ion channels, also known as Cys-loop receptors. This name is derived from the presence of conserved residues flanked by linked cysteines at the N-terminal domain of each subunit. These receptors are well conserved from plants to mammals [ 59 , 60 , 61 ].
Each pentamer of nAChRs consists of an extracellular domain (ECD), a transmembrane domain (TMD) with a central ion channel, and an intracellular domain (ICD). Cys-loop receptors can form both homo- (composed of five identical subunits) and heteropentameric (composed of at least one α and one β subunit) configurations, with the five subunits arranged symmetrically around a central channel axis. Based on their subunit composition and physiological function, nAChRs can be divided into two main classes: muscle type and neuronal type [ 62 ].
The International Union of Basic and Clinical Pharmacology Committee on Receptor Nomenclature and Drug Classification (NC-IUPHAR [ 61 ]) provides a nomenclature and classification scheme for nAChRs based on the subunit composition of known receptor subtypes [ 63 , 64 ]. Human neuronal nAChRs consist of 11 subunits (eight α subunits: α2–α7, α9–α10; and three β subunits: β2–β4), which generate a limited number of distinct pentameric subtypes. However, the α7 and α9 subunits typically form homopentamers, although α9 may interact with α10 subunits to form heteropentamers (α9–α10). Specifically, in tissues such as the human basal forebrain, α7–β2 heteromers are expressed. The α2–α6 and β2–β4 subunits exclusively form heteromers. All α subunits are involved in forming the ligand-binding site, and at least two α subunits are required for the receptor to be functional [ 65 ].
Despite their diversity, all mammalian neuronal nAChR subtypes are permeable to Na + , K + , and Ca 2+ ions. nAChR can exist in different conformational states, including closed, open and conducting (activated by ligand binding), and desensitized (closed and unresponsive to ligand binding). The physiological ligand for nAChRs is acetylcholine (ACh). When ACh or nicotine (receptor agonist) binds to the receptor, the ion channel briefly opens, allowing cation flow and altering the membrane potential, typically resulting in depolarization. The channel can then return to its resting state (closed and responsive to activation) or enter a desensitized state, where it is unresponsive to ACh, nicotine, or other agonists [ 66 , 67 , 68 ].
Although nAChRs are expressed throughout the body, we will focus on their presence in neural and non-neuronal tissues. Neuronal nAChRs are found in nearly every region of the brain, both pre- and post-synaptically, and can be located on axon terminals, axons, dendrites, and somata. On the other hand, non-neuronal nAChRs are expressed on epithelial, endothelial, and immunological cells [ 69 , 70 , 71 , 72 , 73 ].
nAChRs play diverse roles, depending on their tissue location. In neural tissues, they are involved in cognition, addiction, and cell growth. For example, they have been implicated in cognitive processes, addiction-related mechanisms, and cellular growth regulation. In non-neuronal tissues, nAChRs contribute to various functions including inflammation, immunity, and cell growth regulation [ 69 , 70 , 71 , 72 , 73 ].
Furthermore, recent studies have also explored the potential involvement of nAChRs in COVID-19 severity. While the specific mechanisms and implications are still being investigated, there is emerging evidence linking nAChRs to COVID-19 pathophysiology [ 54 , 74 , 75 , 76 , 77 , 78 , 79 ].
2.2. Nicotine and nAChRs
nAChRs can exist in different conformational states: (i) closed and able to be activated by ligands such as Ach or nicotine; (ii) open and conducting to small cations; and (iii) desensitized, closed, and unresponsive to ligand activation. When ACh or nicotine binds to an nAChR in the open channel state, it rapidly evokes depolarization, allowing cation flux within milliseconds. Subsequently, a gradual decrease in agonist-evoked current indicates channel closure. Prolonged exposure to agonists leads to the desensitization of nAChR, rendering them non-functional [ 80 ]. The subunit composition of nAChRs determines the kinetics of these conformational states, the selective cationic permeability of the ion channel pore, and the pharmacological affinities for various agonists. Different nAChR subtypes exhibit distinct functional responses to nicotine. For instance, (α4β2) 2 β2 receptors are considered to be high-affinity receptors, while (α4β2) 2 α4 receptors are classified as low-affinity receptors. Activation of nAChRs can mediate long-term modifications of cellular functions through specific signaling pathways [ 80 , 81 ]. One prominent signaling pathway involving nAChRs, particularly α7 nAChRs, is the generation of complex Ca 2+ -mediated signals. These signals can involve various enzymes and kinases, such as adenylyl cyclase, protein kinase A and/or C, Ca 2+ -calmodulin-dependent kinase, and phosphatidylinositol 3-kinase (PI3K) [ 82 ]. In brief, nicotine binding to homomeric (α7 or α9) or heteromeric (α4β2) nAChRs in the concentration range of 10 −8 to 10 −6 M leads to the opening of receptor gates, enabling the influx of ions into the cytoplasm. This ion flow induces subsequent membrane depolarization, which then triggers the opening of voltage-gated Ca 2+ channels. As a result, there is a further elevation in intracellular Ca 2+ levels. The influx of Ca 2+ activates downstream signal transduction pathways. In the central nervous system (CNS), both homomeric and heteromeric receptors, when stimulated by nicotine, release DA, contributing to the onset of addiction [ 80 , 81 , 82 ]. In the case of α7nAChR activation by nicotine, it prompts the release of serotonin, mammalian bombesin, as well as stress neurotransmitters like adrenaline and noradrenaline. However, in non-neuronal cells, these neurotransmitters play a role in fostering the growth of various cancer types. This can occur through direct activation of intracellular signaling pathways (PKC, AKT, ERK) or indirect release of factors that influence proliferation, migration, and angiogenesis (such as epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF)) [ 80 , 81 , 82 ]. On the other hand, the activation of heteromeric α4β2nAChRs by nicotine in non-neuronal cells prompts the release of the neurotransmitter γ-aminobutyric acid (GABA). Importantly, GABA exhibits a tumor suppressor function for several types of cancer. Interestingly, in neuronal cells, the activation of α4β2nAChRs by nicotine contributes to the development of addiction [ 8 ]. This passage highlights the complex and diverse effects of nicotine binding to different nAChRs and the subsequent outcomes on neurotransmitter release, signaling pathways, and cellular responses in both neuronal and non-neuronal cells.
In summary, as reported in the previous paragraphs, AChRs mediate the effects of their physiological agonist, acetylcholine, as well as those of the external agonist nicotine. Dysregulation of AChRs and their downstream signaling pathways can contribute to the development of various diseases.

2.3. Nicotine and Biological Effects
Nicotine induces various biological effects, as summarized in Table 3 .
Principal biological effects induced by nicotine.
The biological effects of nicotine are diverse, and include both negative effects on the cardiovascular system and addiction (now classified as Substance Use Disorders) [ 2 ], as well as positive effects such as enhancing cognitive function in individuals with Alzheimer’s disease [ 86 ]. A significant portion of the clinical phenotype observed in Alzheimer’s disease (AD) occurs through nAChRs. Degeneration of cholinergic neurons, combined with aberrant nAChR expression and activation partially through amyloid-beta peptide (Aβ)-nAChR leads to the upregulation of pro-inflammatory pathways and subsequently progressive cognitive decline in AD. Interestingly, the cholinergic anti-inflammatory pathway is also mediated through α7-nAChR, in particular. Thus, agonists of these receptors will likely exert pro-cognitive benefits through multiple mechanisms, including stimulating the cholinergic pathway, modulating inflammation, and buffering the effects of amyloid. Despite this promising theoretical use, trials thus far have been complicated by adverse effects or minimal improvement [ 14 , 87 , 88 , 89 ].
The most well-known aspect is the involvement of nicotine in addiction phenomena, craving, and reward processes, as discussed in various reviews [ 4 , 5 , 6 , 7 , 8 , 9 ]. Benowitz, in his seminal review [ 7 ] and subsequent works [ 9 , 90 ], provides a comprehensive explanations of these phenomena. In summary, nicotine interacts with nAChR, initiating the release of neurotransmitters—predominantly dopamine (DA), but also norepinephrine, acetylcholine, serotonin, GABA, glutamate, and endorphins—which subsequently induce sensations of pleasure, stimulation, and mood modulation. Activation of these receptors also leads to the establishment of new neural pathways (neural plasticity) and, in conjunction with environmental cues, behavioral conditioning. Following nicotine activation, nAChRs ultimately undergo desensitization, resulting in short-term tolerance to nicotine and diminished satisfaction from smoking. During periods between cigarette consumption or after discontinuing tobacco use, brain nicotine levels decline, causing reductions in DA and other neurotransmitters, accompanied by withdrawal symptoms like cravings. In the absence of nicotine, nAChRs regain their sensitivity to nicotine and are reactivated in response to a new dose.
A new emerging role of nicotine is being observed in relation to human diseases, particularly in the context of COVID-19. Previous studies have demonstrated that nicotine, when present alongside SARS-CoV-2 [ 54 , 79 ], intensifies the cytopathic effects of SARS-CoV-2. This leads to an escalation in the levels of inflammatory cytokines such as TNF-α, IL6, IL8, and IL10, causing significant cellular damage and even cell death. These detrimental outcomes exhibit characteristics akin to pyroptosis and necroptosis. It is worth noting that these severe consequences are notably linked to nicotine’s capacity to activate α7-nicotinic receptors (α7-nAChR), consequently heightening ACE2 activity [ 78 ].
Importantly, these effects did not manifest in the presence of an α7-nAChR antagonist (e.g., bungarotoxin) or in cells where α7-nAChR expression was suppressed [ 54 , 91 ]. A systematic review and meta-analysis were conducted to investigate the association between current smoking and the progression of coronavirus disease 2019 (COVID-19). The study analyzed the impact of cigarette smoking on various COVID-19 outcomes, including hospitalization, severity, and mortality. The analysis was conducted up until 23 February 2022. The results of the study suggest that the risk of COVID-19 progressing to more severe conditions and leading to mortality is 30–50% higher for both current and former smokers compared to individuals who have never smoked [ 77 ].
In a separate study, Williamson et al. [ 92 ] analyzed data from 17,278,392 adults in the UK. They identified a significant risk of death in patients with severe asthma (defined as asthma requiring recent use of oral corticosteroids) and those with respiratory diseases. Patients with chronic obstructive pulmonary disease (COPD) also exhibited worse outcomes upon contracting COVID-19, despite potentially not having a higher risk of contracting the virus initially [ 92 ]. Notably, the expression of ACE-2 (the potential receptor for SARS-CoV-2) was notably elevated in COPD patients when compared to control subjects. Additionally, among current smokers, ACE-2 expression was higher than in former and never smokers [ 78 ]. These findings strongly indicate that the upregulation of ACE2 resulting from nicotine exposure is contingent upon the activation of α7-nAChR.
Finally, in an Italian population, a borderline significant elevated risk of higher COVID-19 severity was observed among individuals who had ever used e-cigarettes containing nicotine, as compared to those who had never used e-cigarettes (adjusted odds ratio 1.60; 95% confidence interval, 0.96–2.67) [ 76 ].
Several natural compounds have been analyzed for their ability to counteract the effects induced by nicotine [ 93 , 94 , 95 ]. The following compounds appear to be of potential interest:
- Proanthocyanidins (PCs) and anthocyanins (ACNs) are the predominant flavonoid pigments that are widely distributed in plants, and are known for their therapeutic potential in addressing certain chronic diseases. Treatment with non-toxic concentrations of PCs and ACNs exhibits diverse effects against nicotine-induced non-small-cell lung cancer (NSCLC), encompassing anti-proliferative, anti-migratory, anti-metastatic, anti-invasive, and anti-angiogenic effects, as well as induction of apoptosis and autophagy. The utilization of PC-rich extracts derived from grape seeds and/or Cinnamomi Cortex, in conjunction with radiation or chemotherapy, holds promise for yielding anti-proliferative, anti-inflammatory, and apoptotic benefits against nicotine-induced NSCLC. Moreover, compounds such as delphinidin and cyanidin exhibit the potential to enhance apoptotic and autophagic activity by augmenting the chemosensitivity and/or radiosensitivity of NSCLC cells [ 93 ].
- Quercetin stands as a safe and natural compound with substantial potential to address cigarette-smoking-induced chronic obstructive pulmonary disease (CS-COPD). Quercetin prevents CS-COPD and mitigates airway remodeling through a range of mechanisms, including its antioxidant, anti-inflammatory, and immunomodulatory properties, as well as anti-cellular senescence, modulation of mitochondrial autophagy, and regulation of gut microbiota. Quercetin exhibits potential synergistic effects when combined with beta-agonists and M-receptor antagonists, corticosteroids and roflumilast, antibiotics, and N-acetylcysteine (NAC). This collaboration enhances bronchodilatory, anti-inflammatory, antibacterial, and antiviral effects [ 94 ].
- Scutellaria baicalensis and its flavone compounds exhibit therapeutic effects in nicotine-induced NSCLC. These therapeutic effects against NSCLC cells, activated by nicotine via α7nAChR, stem from their capacity to impede proliferation, invasion, migration, metastasis, and angiogenesis. Furthermore, they induce apoptosis, halt cell cycle progression, and trigger autophagy by inhibiting the signaling pathways implicated in NSCLC development. Consequently, targeting α7nAChR and its downstream signaling pathways using flavone compounds holds promise for the development of drugs to counter nicotine-induced NSCLC cells and the treatment of NSCLC in smokers. Combining flavone compounds with chemotherapeutic agents such as cisplatin, which can modulate NSCLC-related signaling pathways, presents a potential strategy for enhancing the anti-NSCLC efficacy of these agents. As such, flavone compounds alone or in synergy with chemotherapeutics could emerge as approved medicinal interventions for NSCLC in smokers [ 95 ].
In this review, we will primarily focus on the effects mediated by nicotine binding to α7 nAChR. The α7 nAChR subtype possesses distinctive properties, as outlined in Table 4 .
α7nAChR properties.
The specific effects induced by nicotine following α7 nAChR activation are diverse when considering human airway epithelial cells, whether tumoral or non-affected. These effects include:
- Increase in α7 nAChR expression at both mRNA and protein levels [ 14 ].
- Elevation of intracellular calcium ions (Ca 2+ ) [ 14 , 103 ].
- Upregulation of ACE2 expression at both mRNA and protein levels [ 54 , 78 , 91 ].
- Activation of signaling cascades such as ERK/MAPK and Phospho-p38 [ 14 , 54 ].
- Augmentation of proliferation markers like Ki67 and EGFR/EGFR [ 14 , 54 ].
- Reduction in markers of senescence, such as SA-β-Gal activity and induction of apoptosis markers, including p53/phospho-p53 [ 54 ].
- Induction of EMT (epithelial–mesenchymal transition): decrease in E-Cadherin, increase in Fibronectin (FN), increase in Vimentin [ 25 , 54 ].
- Increase in markers of neo-angiogenesis, such as VEGF [ 30 , 54 ]. The downstream pathways activated by nicotine, promoting the proliferation, migration, and invasion of airway epithelial cancer cells, as well as of other cancer cell type (i.e., pancreatic [ 31 ]). ultimately resulting in a transition toward a more severe neoplastic phenotype.
2.4. Ultrastructure of Human Adenocarcinoma Cell Line A549 Treated with Nicotine
This section offers a comprehensive insight into the ultrastructural effects brought about by nicotine, describing the observations made using transmission electron microscopy (TEM) in recent studies. Several studies have explored the ultrastructural alterations induced by nicotine in various types of cell. For instance, in human periodontal ligament stem cells, nicotine was observed to activate α7-nAChR. This activation led to the upregulation of nuclear paraspeckle assembly transcript 1 ( NEAT1 ), a nuclear-enriched long non-coding RNA (lncRNA) that plays a critical role as a scaffolding factor for nuclear paraspeckles. Nicotine-induced NEAT1 upregulation results in the suppression of its functional target gene, STX17 . This phenomenon contributes to the blockage of autophagy flux and the production of inflammation factors within human periodontal ligament stem cells (PDLSCs) [ 125 ]. Given the role played by nicotine in the proliferation of human lung tumor cells, a thorough ultrastructural analysis of the effects caused by nicotine in these cells is important. As a prototype, human adenocarcinoma cells A549 were chosen.
The ultrastructure of untreated human adenocarcinoma cells A549 cells revealed the presence of two distinct cell subpopulations as reported in Sansone et al. [ 79 ]. The first subpopulation consisted primarily of well-organized monolayers resembling type 1 pneumocytes. The second subpopulation mainly consisted of cells resembling type 2 pneumocytes, characterized by the presence of basal and apical poles and large osmiophilic lipid granules. These granules contained unsaturated lipid precursors of surfactant and were primarily associated with the Golgi apparatus and endoplasmic reticulum. The mitochondria in the cells appeared well preserved and exhibited a typical orthodox form, with a higher abundance observed in more differentiated cells. The osmiophilic lipid bodies were often closely associated with the apical pole of the plasma membrane, suggesting their involvement in secretion or release into the extracellular space within membrane-bound vesicles [ 79 ].
Following nicotine exposure at 0.1 μM for 48 h, A549 cells underwent three main modifications while maintaining their overall preservation and organization [ 79 ]. Firstly, the cell area appeared to be substantially increased, with enlarged nuclei and cytoplasm, indicating a hypertrophic variation. Secondly, the cytoplasm contained a higher number of osmiophilic lipid bodies compared to untreated cells. Lastly, the occurrence of cells lacking osmiophilic granules, similar to type 1 pneumocytes, was relatively rare or diminished. Three types of granules were identified: very dense and homogeneous granules, granules with reduced density and homogeneity, and vacuoles containing myelin-like osmiophilic membranes, indicating the final stages of cell differentiation [ 79 ]. The majority of cells showed a cytoplasm with a large nucleus. The mitochondria appeared numerous and well preserved. The cytoplasm contained a larger number of osmiophilic lipid bodies. Three types of granules were found: very dense and homogeneous; low density and homogeneous; and vacuoles containing myelin-like osmiophilic membranes [ 79 ].
3. Discussion
Nicotine is widely acknowledged as the psychoactive substance found in the tobacco plant, which sustains tobacco addiction by binding to nicotinic acetylcholine receptors (nAChRs). This binding process facilitates the release of neurotransmitters such as dopamine, glutamate, and gamma-aminobutyric acid (GABA), thereby mediating the intricate effects of nicotine in individuals who use tobacco [ 5 , 6 , 7 , 8 ]. Nicotine has a long history of use, dating back thousands of years before the Common Era (CE). Initially, its use can be traced back to religious rituals and ceremonies. It was often employed in spiritual practices and cultural traditions by indigenous peoples in various parts of the world. Over time, the recreational use of nicotine emerged, as people began to discover its stimulating and mood-altering effects. The consumption of tobacco, which contains nicotine, became popular for its recreational and social aspects (see Table 1 ). In the years following World War I, a growing body of scientific evidence began to link tobacco use with various health issues, including cancer. As this evidence accumulated, awareness of the harmful effects of tobacco grew, and public health concerns led to increased regulation of tobacco use.
More recently, extensive research has implicated nicotine in a wide range of biological processes, as summarized in Table 4 . Figure 1 presents a schematic representation of nicotine’s effects, including its metabolism.

Schematic representation of nicotine’s effects. Nicotine is a tertiary amine consisting of a pyridine and a pyrrolidine ring. (S)-nicotine, which is found in tobacco, selectively binds to nAChRs in a stereoselective manner. The nAChR complex comprises five subunits and is present in both neuronal and non-neuronal cells. There are up to nine α subunits (α2 to α10) and three β subunits (β2 to β4). The most prevalent receptor subtypes in the human brain are α4β2, α3β4, and α7 (homomeric). α4β2* (the asterisk indicates the possible presence of other subunits in the receptor) is the predominant subtype in the human brain, and is believed to be the primary receptor responsible for nicotine dependence. The α3β4 nAChR is thought to mediate the cardiovascular effects of nicotine, while α7 is the most abundant in non-neuronal cells and mediates nicotine’s biological effects, such as cell proliferation, neo-angiogenesis, and resistance to drug-induced apoptosis. Despite their diversity, all mammalian nAChR subtypes are permeable to Na + , K + , and Ca 2+ ions. nAChRs can exist in different conformational states, including closed, open, and conducting states (activated by ligand binding such as Ach or nicotine), as well as desensitized states (closed and unresponsive to ligand binding). The physiological ligand for nAChRs is ACh. When ACh or nicotine (a receptor agonist) binds to the receptor, the ion channel briefly opens, allowing the flow of cations and altering the membrane potential, typically resulting in depolarization. The channel can then return to its resting state (closed and responsive to activation) or enter a desensitized state, where it is unresponsive to ACh, nicotine, or other agonists. Nicotine is rapidly and extensively metabolized by the liver, primarily by the liver enzyme CYP2A6 (and to a lesser extent by CYP2B6 and CYP2E1), to form cotinine. Cotinine is subsequently metabolized exclusively or nearly exclusively to trans-3′-hydroxycotinine (3HC) by CYP2A6. The half-life of nicotine averages approximately 2 h, while the half-life of cotinine averages around 16 h. For more comprehensive explanations and references, please refer to the accompanying test.
The discovery of nicotine receptors on non-neuronal epithelial cells present in different organs has shed light on the diverse biological effects of nicotine, particularly its role in cell division and proliferation. Moreover, nicotine has been found to stimulate neo-angiogenesis, which refers to the formation of new blood vessels. This process is crucial for tissue growth and repair, but has also been implicated in various pathological conditions, including cancer.
Recent findings suggest a potential link between nicotine and the severity of COVID-19. It has been observed that nicotine, through the activation of τ7-nAChR, can increase the expression of ACE2 (angiotensin-converting enzyme 2). ACE2 is the receptor that the SARS-CoV-2 virus uses to enter human cells. This finding has raised concerns about the potential implications for individuals who smoke tobacco or use nicotine products. If nicotine increases the expression of ACE2, it could theoretically enhance the susceptibility to SARS-CoV-2 infection and potentially worsen the severity of COVID-19 symptoms in smokers [ 75 , 76 , 77 , 78 ].
Many smoking individuals suffer from respiratory or cardiovascular diseases. The presence of COPD is common in smokers with frequent exacerbations. These patients, after the acute phase, require rehabilitation programs. These programs should be supported by thorough education about the damage caused by tobacco smoking. Additionally, the effects of nicotine must be well understood in order to achieve an effective rehabilitation process.
Ongoing research is crucial for gaining a comprehensive understanding of the intricate biological effects of nicotine and its potential involvement in various cellular processes and diseases. Further investigation is necessary to fully elucidate the mechanisms and consequences of nicotine’s actions on human health. However, it is important to emphasize that smoking habits are highly dangerous for individuals and public health. With smoking being associated with significant morbidity and mortality worldwide.
Implementing and enforcing effective public health policies is essential for addressing the harmful effects of smoking. This can include the adoption of laws or regulations, the creation of smoking prevention and cessation programs, or the implementation of awareness campaigns.
The enforcement aspect involves the rigorous application of these policies and measures. This means ensuring that smoking-related laws and rules are adhered to and that there are consequences for those who violate them, for example, the enforcement of smoking bans in public places through penalties for anyone who disregards them.
Effective public health policies: These are public health policies that are effective at reducing smoking and its related harms. These can include policies that encourage people to quit smoking, reduce access to tobacco products, or promote a healthy lifestyle.
Harmful effects of smoking: The harmful effects of smoking include the negative health consequences associated with smoking, including lung diseases, heart diseases, cancer, and many other health conditions. These harmful effects represent a significant public health threat.
In essence, this study emphasizes that in order to reduce the harmful effects of smoking on people’s health and on society as a whole, it is necessary to have effective public health policies that are rigorously implemented and enforced. These policies can help prevent smoking, promote cessation, and protect people’s health. Furthermore, providing accessible support and resources for individuals looking to quit smoking is crucial for improving public health outcomes.
4. Materials and Methods
Major databases, including Medline, Scopus, and Web of Science, were used to discuss:
- The biosynthesis and accumulation of nicotine in tobacco plants with a focus on its role as a defense against predatory insects.
- The interaction of nicotine with nicotinic acetylcholine receptors (nAChRs) and its downstream effects.
5. Conclusions
Nicotine is a highly addictive and dangerous substance. Governments, healthcare organizations, and society at large should prioritize public health initiatives to combat smoking and reduce the associated risks. By implementing comprehensive strategies, it is possible to protect individuals from the harmful effects of smoking and strive towards a healthier future for everyone.
Acknowledgments
The work was supported by current research funding from the Ministry of Health (Ricerca Corrente), Rome, Italy, to Patrizia Russo.
Funding Statement
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
- Search Menu
- Sign in through your institution
- Advance Articles
- Case of the Year
- Competition Winners
- Grand Rounds
- ESC Content Collections
- Author Guidelines
- Submission Site
- Open Access Options
- Self-Archiving Policy
- About European Heart Journal - Case Reports
- About European Society of Cardiology
- ESC Publications
- Editorial Board
- Editor and Reviewer Programmes
- Advertising & Corporate Services
- Journals Career Network
- Code of Conduct
- Publons: Recognising Review
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, case presentation, lead author biography, supplementary material.
- < Previous
Nicotine e-vaping and cardiovascular consequences: a case series and literature review
Maryam Jessri and Ahmed S. Sultan contributed equally to the manuscript.
- Article contents
- Figures & tables
- Supplementary Data
Maryam Jessri, Ahmed S Sultan, Emad Magdy, Niamh Hynes, Sherif Sultan, Nicotine e-vaping and cardiovascular consequences: a case series and literature review, European Heart Journal - Case Reports , Volume 4, Issue 6, December 2020, Pages 1–7, https://doi.org/10.1093/ehjcr/ytaa330
- Permissions Icon Permissions
Cardiovascular toxicity as a consequence of nicotine from conventional tobacco cigarette smoking is well documented. However, little is known about the cardiovascular consequences of nicotine e-vaping. We review the literature and report a case series of three cases of major adverse cardiovascular clinical effects post nicotine e-vaping.
Three patients with known peripheral arterial disease who switched from heavy cigarette smoking consumption to a high-intensity dose of nicotine e-vaping all developed further arterial complications within 6–30 months.
With the recent epidemic of e-vaping in young individuals and the national outbreak of e-vaping use-associated lung injury (EVALI), the dangers of e-vaping are now coming to light. The pulmonary effects are now well described, and this paper highlights three new cases of cardiovascular toxicity associated with e-vaping. The potential role of nicotine e-vaping and the risk of coronavirus disease-2019 (COVID-19) will also be discussed.
In this case series, three patients with known peripheral arterial disease who switched from heavy cigarette smoking consumption to a high-intensity dose of nicotine e-vaping all developed further arterial complications.
Nicotine e-vaping is associated with severe cardiovascular toxicity and should be discouraged as a smoking cessation tool.
Detrimental health consequences of tobacco cigarette (TC) smoking have long been established and, consequently, significant public health resources have been allocated to tobacco cessation efforts. In the past two decades, electronic nicotine delivery systems (ENDs) and, most commonly, electronic cigarettes (E-cigs) have emerged as an alternative to tobacco consumption. While END companies and smokers claim that ENDs are an effective means for smoking cessation, studies have shown no real-life evidence for this presumed benefit. Additionally, a 2018 systematic review of 29 original articles focusing on the efficacy of ENDs as smoking cessation alternatives found only a modest behavioural and sensory gratification rate in a setting of continued use of E-cigs instead of quitting. 1
Historically, TC smoking is considered the primary cause of preventable cardiovascular disease (CVD) in the USA. The tobacco/END industry has been citing the lower level of nicotine and particulates in END vapours for advocating ENDs as a ‘safe’ alternative to smoking. While this may be true, END particulates are different from conventional cigarettes and, given their novelty, their toxicity is unknown.
With the recent epidemic of e-vaping in young individuals and the national outbreak of e-vaping use-associated lung injury (EVALI), the dangers of e-vaping are now coming to light. The pulmonary effects are now well described, and this case series highlights three new cases of cardiovascular toxicity associated with e-vaping. A comprehensive literature review is also presented, and the potential role of nicotine e-vaping and the risk of coronavirus disease-2019 (COVID-19) will be discussed.
A 78-year old-female with a past medical history of peripheral vascular disease, atrial fibrillation, ischaemic heart disease, ulcerative colitis, and arthritis presented with a 1-year history of bilateral intermittent claudication (more on the right side) at ∼50 yards.
The patient was a former heavy TC smoker (100 pack-year). On examination there was no tissue loss or pain at rest. Absent femoral pulsation was observed on the right leg and there was weak femoral pulsation on the left leg. The ABPI (ankle–brachial pulse index) at this visit was 0.7 (right leg; toe index of 0.42) and 0.9 (left leg; toe index of 0.5). The patient was treated with best medical therapy.
The patient switched to E-cigs after she was gifted a vaping device by her daughter in January 2019. She vaped three 10 mg cartridges per week (30 mg/week) and the approximate nicotine concentration was 12 mg/cartridge. In August 2019, the patient presented to the emergency department with confusion, left-sided weakness, abdominal pain, and right leg pain. A brain computed tomography (CT) and brain magnetic resonance imaging (MRI) showed no evidence of stroke. A CT thorax abdomen pelvis (TAP) was performed which showed complete right common iliac occlusion, bilateral renal infarction, and portal vein thrombosis ( Figure 1A ). Axial cuts showing bilateral renal infarction ( Figure 1B ). The patient was admitted to the intensive care unit (ICU). She desaturated (SO 2 : 89 on room air) and she was disoriented to time, place, and person (she could not recognize her family members). On examination, her left leg was warm with normal capillary refill time. No pulses could be palpated in her right leg. There was no motor or sensory deficit. She was treated conservatively in the ICU with anticoagulation and antibiotics for sepsis. Thrombophilia screen was negative.

(A) CT thorax abdomen pelvis (TAP) showed complete right common iliac occlusion, bilateral renal infarction, and portal vein thrombosis. ( B ) Axial cuts showed bilateral renal infarction. (C) Retrograde on-table angiography for the right leg and stenting of the right common iliac with a covered stent were performed.
After the patient stabilized in October 2019, she had a retrograde on-table angiography for the right leg and stenting of the right common iliac with a covered stent ( Figure 1C ). The patient was discharged the day following surgery. Leg digital pressures were 35 mmHg for her right leg (ABPI: 0.48) and 73 mmHg for the left leg (ABPI: 0.7). She was able to walk for ∼1 mile without claudication. Her examination showed normal pulses and she ceased vaping.
A 55-year-old male with ischaemic heart disease, managed conservatively with medical therapy and an exercise programme for the past 3 years (no stent placement), presented with short distance claudication of the left leg. Of note, the patient was a former TC smoker (40 pack-year) who had switched to E-cigs 2 years ago. He vaped three 10 mg cartridges per week (30 mg/week) and the approximate nicotine concentration was 12 mg/cartridge.
He had a past medical history of ischaemic heart disease for which a coronoray angiogram was performed in September 2015 due to ongoing chest pain that radiated to his left arm. In addition, he had dyspnoea during exertion. No flow-limiting lesion was found and no stents were inserted. Thus, he was only given best medical therapy (aspirin, clopidogrel, and atorvastatin).
On his most recent presentation to our clinic in 2019, he had symptoms present at ∼50 m, worse on going uphill, and interfering with daily life. Upon examination, there was normal capillary refill, no left femoral pulsation, and the left leg was colder than the right leg. No ulcers or tissue loss were present. Bilateral ABPI was reduced at 0.8, and his toe pressures were 73 mmHg on the right and 65 mmHg on the left. CT angiogram revealed complete left iliac occlusion ( Figure 2A and B ), and chest CT revealed a popcorn appearance (bronchiolitis obliterans) of the lungs ( Figure 2C ).

(A) CT angio periphery axial section showing complete left iliac occlusion. (B) CT angio periphery coronal section showing complete left common iliac occlusion. (C) CT of thorax showing popcorn appearance of the lung.
The patient was advised to stop vaping and successfully stopped for 6 months. Following this, his haemodynamic studies improved, with increase in his toe pressure to 100 mmHg on the right and 88 mmHg on the left. However, he subsequently resumed vaping and continues to vape. Consequently his most recent haemodynamic studies revealed a drop in toe pressures again to 81 mmHg bilaterally.
A 55-year-old female with a past medical history of type 2 diabetes mellitus (controlled with oral hypoglycaemics) and ischaemic heart disease (cardiac stent placement 14 years previously) presented with a 2-year history of bilateral intermittent claudication of <50 yards (increasing on going uphill and more severe in the left leg), interfering with daily activities. She is a former TC smoker (30 pack-year). She switched to E-cigs and vaped three 10 mg cartridges per week (30 mg/week), and the approximate nicotine concentration was 12 mg/cartridge. Of note, the patient’s symptoms developed ∼6 months after she switched to E-cigs.
Upon examination, there was normal capillary refill in a setting of bilateral weak femoral pulsation, and bilateral cold legs. In addition, no pulsation could be felt at the popliteal or more distally, bilaterally. Bilateral ABPI was reduced at 0.5. Right leg digital pressure was 80 mmHg and the leg digital pressure was 75 mmHg. A three-dimensional (3D) reconstruction of a CT angiography showed aortoiliac occlusive disease, and thoracic CT was significant for bronchiolitis obliterans ( Figure 3A–C ).

(A) 3D reconstruction showing aortoiliac occlusive disease. (B) CT angio showing aortoiliac occlusive disease. (C) Axial section of thoracic CT showing popcorn appearance.
The patient was treated with antiplatelet medications with no improvement and is currently awaiting aortoiliac endarterectomy. She was asked to stop vaping and, during a recent visit (August 2019), she demonstrated significant improvement and was able to walk for 150 m. Her improvement continued and at her most recent visit (October 2019) her digital pressure improved to 80 mmHg bilaterally.
In response to a congressional mandate, the National Academies of Science, Engineering and Medicine (NASEM) convened an ad-hoc committee of experts that appraised >800 studies to report on public health consequences of E-cigs. In January 2018, NASEM released a report which, above all else, elucidated the gaps in our current knowledge and identified research priorities as they pertain to the benefits and harms of E-cigs. 2
ENDs are highly variable in design and delivery, and consequently their health effects are heatedly debated. The potential cardiovascular side effects of ENDs have been generally attributed to (i) nicotine and (ii) oxidizing chemicals, particulates, and acrolein. While the former activates the sympathetic nervous system, and causes vasoconstriction and arrythmogenesis, the latter influence CVD through inducing inflammation in endothelial cells and platelet activation. 3 Despite different pathways, the unfortunate end result of both pathways may be an increased risk of developing myocardial infarction and sudden death. 1
In pre-clinical studies of ENDs, cell culture or animal models are exposed to high concentrations of END aerosols which do not reflect dose or duration of real-life exposure. Sassano et al. developed a high-throughput screening assay to evaluate the toxicity of e-liquids, and found the presence of vanillin and a higher number of chemicals in e-liquids to be positively associated with higher toxicity. 4 Depending on make and model, ENDs produce variable amounts of toxic aldehydes, namely acetaldehyde, acrolein, and formaldehyde, which are also present in cigarette smoke. Exposure to these low molecular weight aldehydes may result in acute lung injury, chronic obstructive pulmonary disease (COPD), asthma exacerbation, and lung cancer, as well as CVD. 5 Although it is difficult to prove direct causation, it is suspected that exposure to aldehydes may have contributed to significant cardiovascular toxicity in already predisposed individuals at high risk for cardiovascular disease, as exemplified in the above three cases.
Studies have shown that the vapour produced by E-cigs reduces immune and alveolar function, with a decrease in surfactant within the air sacs. This leads to failure of gas exchange within the lung tissue. Some flavourings in E-cigs have been associated with depression of respiratory cilia. 6 Impaired ciliary function may in turn predispose the individual to an increased risk of viral infection such as SARS-CoV-2. Chronic exposure to ENDs has also been reported to down-regulate the innate immunity against viral pathogens. Furthermore, independent of nicotine, END-exposed mice infected with influenza virus demonstrated enhanced lung inflammation and tissue damage. 7 Chronic exposure to E-cig vapour aberrantly alters the physiology of lung epithelial cells and resident immune cells, and promotes poor response to infectious challenge. 7 As of yet, there is no direct evidence of the link between ENDs and COVID-19; however, the aforementioned negative effects of ENDs on immune and alveolar function raise serious concerns about the potential increased risk of developing COVID-19. Moreover, it is known that TC smoke can up-regulate the angiotensin-converting enzyme 2 (ACE2) receptor which is the receptor involved in SARS-CoV-2 viral uptake into host cells. 8 Importantly, second-hand vapour generated by ENDs could enhance the dissemination of SARS-CoV-2 among non-infected individuals in close proximity to SARS-CoV-2-infected vapers. 9
Given the higher concentration of formaldehyde in high-voltage E-cigs, Jensen et al . considered high-voltage E-cig users to be 15 times more at risk of developing upper aerodigestive tract cancer. 10 However, others argue that the tested devices may have overheated, a phenomenon commonly referred to as ‘dry puff’. 11 Sultan et al. reviewed the existing ENDs and cautioned practitioners against considering and promoting nicotine e-vaping as safe devices for smoking cessation until further evidence regarding their long-term use and health complications is available. 12
Although replacement of conventional TC with E-cigs has been associated with reduction in central and brachial systolic blood pressure, arterial wave reflection, and oxidative stress, both conventional TC smoking and E-cigs negatively disturb arterial elasticity and increase oxidative stress. 13 An important consideration in replacing conventional TC with E-cigs is the fact that the role of nicotine in CVD development is non-linear, and small amounts of nicotine may suffice to cause CVD and accelerated atherogenesis. 3 , 14 E-cig use is correlated with 2- to 3-fold higher odds of stroke, myocardial infarction, angina, and coronary heart disease, and induces atherosclerotic states in otherwise normal healthy individuals with an increased risk of subsequent CVD. 15–17 Additionally, greater concentrations of biomarkers of nicotine, tobacco-specific nitrosamines, volatile organic compounds, and metals compared with never TC users have been reported in sole E-cig users. 18 Therefore, it is likely that new exposure to these various components of E-cigs in the three cases described above contributed to disruptions in arterial elasticity, an increase in oxidative stress, and ultimately worsening CVD. Furthermore, the significant amelioration of physical findings after cessation of E-cigs provides indirect supporting evidence that E-cigs have damaging effects on the cardiovascular system and their removal promotes cardiovascular recovery.
The most recent clinical practice guidelines on primary prevention from the American College of Cardiology/American Heart Association Task Force state that ENDs can increase the risk of arrhythmias and hypertension, and can also increase oxidative stress and sympathetic stimulation in young healthy individuals. 19 A randomized crossover study by Franzen et al. in 2018 demonstrated the impact of ENDs on worsening peripheral arterial function. 20 In particular, they recorded an increase in peripheral systolic pressure which was sustained for three times longer in those using ENDs compared with TCs. The adverse impact of ENDs on arterial stiffnes was further demonstrated by an increase in pulse wave velocities which were independent of mean arterial pressure. In 2019, Osei et al. analysed 449 092 participants from the Behavioral Risk Factor Surveillance System (BRFSS) and found that there was significantly higher odds of CVD among dual users of E-cigs and TCs compared with TC users. 21
While one hopes that all research is conducted in good faith, the influence of the tobacco/END industry cannot be ignored. Pisinger et al. analysed the contradictory outcomes of studies on potential side effects of E-cigs due to investigator’s financial conflict of interest (COI). 22 While 95% of published work without COI found potentially harmful effects and substances, only 8% of tobacco industry-funded studies found potential harm. 22 This equated to a 66-fold increase in odds of finding of no harm in industry-funded studies.
A limitation of our study was the small sample size and that no objective quantification of nicotine levels was measured for any of the three cases. Importantly, the Centers for Disease Control and Prevention (CDC) reported >2800 hospitalized EVALI cases in the USA as of February 2020. The recent exponential rise in EVALI cases and their potential negative effects on the cardiovascular system, coupled with the data in the published literature and the case series described above, all add to the evidence that ENDs as smoking cessation alternatives should not be recommended.

Professor Sherif Sultan obtained his medical degree from the Ain Shams University in 1987. Following completion of a master degree in surgery in 1991, he then finished his MD degree, and moved to Ireland and was awarded his FRCS in Dublin 1995. He completed a fellowship from Arizona Heart Institute in 1997, followed by a Diploma in Endovascular Surgery from University of Paris XII in 1998. He attained his Intercollegiate FRCS in vascular surgery in March 2001 in London and was certified with the European Board of Vascular Surgery in September 2001 in Lucerne Switzerland. Professor Sultan was awarded an honorary PhD from the University of Sibiu, in 2015. He is a senior vascular surgeon at West Northwest Hospital group of the National HSE, Ireland.
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing these case and suitable for local presentation is available online as Supplementary data .
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patients in line with COPE guidance.
Conflict of interest : none declared.
Rehan HS , Maini J , Hungin APS. Vaping versus smoking: a quest for efficacy and safety of E-cigarette . Curr Drug Saf 2018 ; 13 : 92 – 101 .
Google Scholar
Rigotti NA. Balancing the benefits and harms of E-cigarettes: a National Academies of Science, Engineering, and Medicine Report . Ann Intern Med 2018 ; 168 : 666 – 667 .
Benowitz NL , Fraiman JB. Cardiovascular effects of electronic cigarettes . Nat Rev Cardiol 2017 ; 14 : 447 – 456 .
Sassano MF , Davis ES , Keating JE , Zorn BT, , Kochar TK , Wolfgang MC , Glish GL , Tarran R. Evaluation of e-liquid toxicity using an open-source high-throughput screening assay . PLoS Biol 2018 ; 16 : e2003904 .
Ogunwale MA , Li M , Ramakrishnam Raju MV , Chen Y , Nantz MH , Conklin DJ , Fu X-A. Aldehyde detection in electronic cigarette aerosols . ACS Omega 2017 ; 2 : 1207 – 1214 .
Park HR , O’Sullivan M , Vallarino J , Shumyatcher M , Himes BE , Park JA , Christiani DC , Allen J , Lu Q. Transcriptomic response of primary human airway epithelial cells to flavoring chemicals in electronic cigarettes . Sci Rep 2019 ; 9 : 1400 .
Madison MC , Landers CT , Gu BH , Chang CY , Tung HY , You R , Hong MJ , Baghaei N , Song LZ , Porter P , Putluri N , Salas R , Gilbert BE , Levental I , Campen MJ , Corry DB , Kheradmand F. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine . J Clin Invest 2019 ; 129 : 4290 – 4304 .
Brake SJ , Barnsley K , Lu W , McAlinden KD , Eapen MS , Sohal SS. Smoking upregulates angiotensin-converting enzyme-2 receptor: a potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19) . J Clin Med 2020 ; 9 : 841 .
Javelle E. Electronic cigarette and vaping should be discouraged during the new coronavirus SARS-CoV-2 pandemic . Arch Toxicol 2020 ; 94 : 2261 – 2262 .
Jensen RP , Luo W , Pankow JF , Strongin RM , Peyton DH. Hidden formaldehyde in e-cigarette aerosols . N Engl J Med 2015 ; 372 : 392 – 394 .
Thomson RH , Lewis PM. More on hidden formaldehyde in e-cigarette aerosols . N Engl J Med 2015 ; 372 : 1575 – 1576 .
Sultan AS , Jessri M , Farah CS. Electronic nicotine delivery systems: oral health implications and oral cancer risk . J Oral Pathol Med 2018 ;doi: 10.1111/jop. 12810 .
Ikonomidis I , Vlastos D , Kourea K , Kostelli G , Varoudi M , Pavlidis G , Ikonomidis I , Vlastos D , Kourea K , Kostelli G , Varoudi M , Pavlidis G. Electronic cigarette smoking increases arterial stiffness and oxidative stress to a lesser extent than a single conventional cigarette: an acute and chronic study . Circulation 2018 ; 137 : 303 – 306 .
Pope CA 3rd , Burnett RT , Krewski D , Jerrett M , Shi Y , Calle EE , Thun MJ. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure–response relationship . Circulation 2009 ; 120 : 941 – 948 .
Ndunda M. Electronic cigarette use is associated with a higher risk of stroke . Stroke 2019 ; 20(Suppl_1 ): A9 .
Darville A , Hahn EJ. E-cigarettes and atherosclerotic cardiovascular disease: what clinicians and researchers need to know . Curr Atheroscler Rep 2019 ; 21 : 15 .
Alzahrani T , Pena I , Temesgen N , Glantz SA. Association between electronic cigarette use and myocardial infarction . Am J Prev Med 2018 ; 55 : 455 – 461 .
Goniewicz ML , Smith DM , Edwards KC , Blount BC , Caldwell KL , Feng J , Wang L , Christensen C, , Ambrose B , Borek N , van Bemmel D , Konkel K , Erives G , Stanton CA , Lambert E , Kimmel HL , Hatsukami D , Hecht SS , Niaura RS , Travers M, , Lawrence C , Hyland AJ. Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes . JAMA Netw Open 2018 ; 1 : e185937 .
Arnett DK , Blumenthal RS , Albert MA, , Buroker AB , Goldberger ZD , Hahn EJ , Himmelfarb CD, , Khera A , Lloyd-Jones D , McEvoy JW , Michos ED , Miedema MD , Muñoz D , Smith SC Jr , Virani SS , Williams KA Sr , Yeboah J , Ziaeian B. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines . Circulation 2019 ; 140 : e596 – e646 .
Franzen KF , Willig J , Cayo Talavera S , Meusel M , Sayk F , Reppel M , Dalhoff K , Mortensen K , Droemann D. E- cigarettes and cigarettes worsen peripheral and central hemodynamics as well as arterial stiffness: a randomized, double-blinded pilot study . Vasc Med 2018 ; 23 : 419 – 425 .
Osei AD , Mirbolouk M , Orimoloye OA, , Dzaye O , Uddin SMI , Benjamin EJ , Hall ME , DeFilippis AP , Stokes A , Bhatnagar A , Nasir K , Blaha MJ. Association between e-cigarette use and cardiovascular disease among never and current combustible-cigarette smokers . Am J Med 2019 ; 132 : 949 – 54 .
Pisinger C , Godtfredsen N , Bender AM. A conflict of interest is strongly associated with tobacco industry-favourable results, indicating no harm of e-cigarettes . Prev Med 2019 ; 119 : 124 – 131 .
Author notes
- cardiovascular system
- electronic cigarettes
- electronic nicotine delivery system
Supplementary data
Email alerts, more on this topic, related articles in pubmed, citing articles via.
- General Instructions
- Advertising and Corporate Services
Affiliations
- Online ISSN 2514-2119
- Copyright © 2024 European Society of Cardiology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- About Health Effects of Cigarette Smoking
- Secondhand Smoke
- E-cigarettes (Vapes)
- Menthol Tobacco Products
- Morbidity and Mortality Weekly Reports (MMWR)
- About Surveys
- Other Tobacco Products
- Smoking and Tobacco Use Features
- Patient Care Settings and Smoking Cessation
- Patient Care
- Funding Opportunity Announcements
- State and Community Work
- National and State Tobacco Control Program
- Multimedia and Tools
- Tobacco - Health Equity
- Tobacco - Surgeon General's Reports
- State Tobacco Activities Tracking and Evaluation (STATE) System
- Global Tobacco Control
E-Cigarette Use Among Youth
What to know.
E-cigarettes are the most commonly used tobacco product among U.S. youth. No tobacco products, including e-cigarettes, are safe, especially for children, teens, and young adults. Learn more about e-cigarette use among youth.
- In the United States, youth use e-cigarettes, or vapes, more than any other tobacco product. 1
- No tobacco products, including e-cigarettes, are safe, especially for children, teens, and young adults. 2
- Most e-cigarettes contain nicotine, which is highly addictive. Nicotine can harm the parts of an adolescent's brain that control attention, learning, mood, and impulse control. 2
- E-cigarette marketing, the availability of flavored products, social influences, and the effects of nicotine can influence youth to start or continue vaping. 3 4
- Most middle and high school students who vape want to quit. 5
- Many people have an important role in protecting youth from vaping including parents and caregivers, educators and school administrators, health care providers, and community partners.
- States and local communities can implement evidence-based policies, programs, and services to reduce youth vaping.
E-cigarette use among U.S. youth
In 2023, e-cigarettes were the most commonly used tobacco product among middle and high school students in the United States. In 2023: 6
- 550,000 (4.6%) middle school students.
- 1.56 million (10.0%) high school students.
- Among students who had ever used e-cigarettes, 46.7% reported current e-cigarette use.
- 1 in 4 (25.2%) used an e-cigarette every day.
- 1 in 3 (34.7%) used an e-cigarette on at least 20 of the last 30 days.
- 9 in 10 (89.4%) used flavored e-cigarettes.
- Most often used disposable e-cigarettes (60.7%) followed by e-cigarettes with prefilled or refillable pods or cartridges (16.1%).
- Most commonly reported using the following brands: Elf Bar, Esco Bars, Vuse, JUUL, and Mr. Fog.
Most middle and high school students who vape want to quit and have tried to quit. 5 In 2020:
- 63.9% of students who currently used e-cigarettes reported wanting to quit.
- 67.4% of students who currently used e-cigarettes reported trying to quit in the last year.
Most tobacco use, including vaping, starts and is established during adolescence. There are many factors associated with youth tobacco product use . These include:
- Tobacco advertising that targets youth.
- Product accessibility.
- Availability of flavored products.
- Social influences.
- Adolescent brain sensitivity to nicotine.
Some groups of middle and high school students use e-cigarettes at a higher percentage than others. For example, in 2023: 6
- More females than males reported current e-cigarette use.
- Non-Hispanic multiracial students: 20.8%.
- Non-Hispanic White students: 18.4%.
- Hispanic or Latino students: 18.2%.
- Non-Hispanic American Indian and Alaska Native students: 15.4%.
- Non-Hispanic Black or African American students: 12.9%.
Many young people who vape also use other tobacco products, including cigarettes and cigars. 7 This is called dual use. In 2020: 8
- About one in three high school students (36.8%) who vaped also used other tobacco products.
- One in two middle school students (49.0%) who vaped also used other tobacco products.
E-cigarettes can also be used to deliver other substances, including cannabis. In 2016, nearly one in three (30.6%) of U.S. middle and high school students who had ever used an e-cigarette reported using marijuana in the device. 9
- Park-Lee E, Ren C, Cooper M, Cornelius M, Jamal A, Cullen KA. Tobacco product use among middle and high school students—United States, 2022 . MMWR Morb Mortal Wkly Rep. 2022;71:1429–1435.
- U.S. Department of Health and Human Services. E-cigarette Use Among Youth and Young Adults: A Report of the Surgeon General . Centers for Disease Control and Prevention; 2016. Accessed Feb 14, 2024.
- Apelberg BJ, Corey CG, Hoffman AC, et al. Symptoms of tobacco dependence among middle and high school tobacco users: results from the 2012 National Youth Tobacco Survey . Am J Prev Med. 2014;47(Suppl 1):S4–14.
- Gentzke AS, Wang TW, Cornelius M, et al. Tobacco product use and associated factors among middle and high school students—National Youth Tobacco Survey, United States, 2021 . MMWR Surveill Summ. 2022;71(No. SS-5):1–29.
- Zhang L, Gentzke A, Trivers KF, VanFrank B. Tobacco cessation behaviors among U.S. middle and high school students, 2020 . J Adolesc Health. 2022;70(1):147–154.
- Birdsey J, Cornelius M, Jamal A, et al. Tobacco product use among U.S. middle and high school students—National Youth Tobacco Survey, 2023 . MMWR Morb Mortal Wkly Rep. 2023;72:1173–1182.
- Wang TW, Gentzke AS, Creamer MR, et al. Tobacco product use and associated factors among middle and high school students—United States, 2019 . MMWR Surveill Summ. 2019;68(No. SS-12):1–22.
- Wang TW, Gentzke AS, Neff LJ, et al. Characteristics of e-cigarette use behaviors among US youth, 2020 . JAMA Netw Open. 2021;4(6):e2111336.
- Trivers KF, Phillips E, Gentzke AS, Tynan MA, Neff LJ. Prevalence of cannabis use in electronic cigarettes among U.S. youth . JAMA Pediatr. 2018;172(11):1097–1099.
Smoking and Tobacco Use
Commercial tobacco use is the leading cause of preventable disease, disability, and death in the United States.
For Everyone
Health care providers, public health.
We've detected unusual activity from your computer network
To continue, please click the box below to let us know you're not a robot.
Why did this happen?
Please make sure your browser supports JavaScript and cookies and that you are not blocking them from loading. For more information you can review our Terms of Service and Cookie Policy .
For inquiries related to this message please contact our support team and provide the reference ID below.
- Getting Results.
- Newsletters
Vape-detecting dog sniffs out nicotine, THC in Lake County schools
School district purchased vape-detecting dog in 2023.
Emily McLeod , Anchor/Reporter
LAKE COUNTY, Fla. – The Lake County School District is getting results when it comes to lowering the number of kids bringing vapes to school.
Back in November, we told you about Samba, the vape-detecting K-9 purchased by the Lake County School District. Since our first report, Samba has completed her training and has been working at Lake County middle and high schools since March 2024.
“It really is a problem, and I’m just glad we’re getting them out,” said School Resource Officer and K-9 Handler Erica Stamborski.
Stamborski said Samba has found more than 40 nicotine vapes and five THC vapes since March, but Samba’s efforts are leading to other items being found in addition to the vapes.
“They’ll also have, like, marijuana, or in one circumstance the student had alcohol in his backpack,” Stamborski said. “I was just shocked when they started pulling it out of the backpack.”
Stamborski said it didn’t take Samba long to find something in a student’s backpack.
“Our very first school walk, we walked a locker room where the students had left their backpacks (...) I put her down on the floor, gave her her command, and within seconds she was over on a backpack, sat down and alerted on it,” Stamborski said. “As they’re searching the student’s backpack, they found a nicotine vape and a THC vape.”
Abby Crosby, principal of Tavares Middle School, said she’s happy with the results Samba has been able to provide and prior to her arrival, Crosby said she was frequently dealing with vape-related issues on campus.
“We would sometimes hear about it on a daily basis and that has greatly decreased since Samba and Erica have been around,” Crosby said.
Crosby said they want to educate students while keeping them safe.
“We want to make sure that they are making good choices and creating healthy habits because especially at the middle school age, they are just finding themselves,” Crosby said.
Kristen Lamoreaux is a potential specialist at Tavares Middle School, but she also has two children who attend classes in the Lake County School District.
“As a teacher, very glad it’s on campus, taken care of off the teacher’s responsibility, and then as a parent, I feel that extra sense of relief that there’s backup,” Lamoreaux said. “I mean, it’s hard to do it alone as a parent, so it’s great that the schools are there.”
Sgt. Yancy Isaacs with the Lake County Sheriff’s Office is head of the agency’s K-9 unit. Isaacs not only picked out Samba, but trained her, too.
“We have not had a dog like her yet that just specifically does vapes,” Isaacs said. “So far it has worked out really well. I mean, she’s finding a lot of vapes in the schools and it’s also a deterrent because if the kids know she’s coming to the school, it should act as a deterrent.”
Isaacs also tells us there is a possible discussion on a second vape-detecting dog coming to the district.
Get today’s headlines in minutes with Your Florida Daily :
Copyright 2024 by WKMG ClickOrlando - All rights reserved.
About the Author
Emily mcleod.
Emily joined WKMG-TV in November 2022, returning home to Central Florida.
RELATED STORIES
Boater found dead after jumping into lake minneola id’d as retired orlando police lieutenant, have you seen her lake county deputies seek missing teen, lake county schools consider shelling out $60k for vape-sniffing dog.
Recommended Videos
Zyn, America's favorite nicotine pouch, is running out of stock in some states
- Zyn shortages have been spotted in New York, New Jersey, and Florida.
- Philip Morris International's CFO acknowledged supply chain issues on a recent company call.
- Zyn's popularity has surged among white-collar workers, including on Wall Street.

Zyn , a popular brand of nicotine pouches, may be harder to find in several states, including New York, New Jersey, and Florida.
Some smoke shops in New York said they are out of the pouches, which retail for about $5, and wholesalers in New Jersey and Florida said they've been hard to get, Bloomberg reported. One worker in the industry told the outlet that the shortage has been ongoing for several weeks.
The pouches are produced by Philip Morris International, the tobacco products maker that distributes Marlboro cigarettes outside the US. In an earnings call last month, chief financial officer Emmanuel Babeau said that Zyn's growth "is indeed creating some tensions on the supply chain, without any doubt."
Related stories
PMI, which bought the company that makes Zyn in 2022, did not immediately respond to Business Insider's request for comment.
Zyn has been available in the US since 2014, but has boomed in popularity recently. The colorful, flavored gum-like pouches have become a common "pick-me-up" among office employees looking to get work done faster. They have also become routine with high-powered Wall Street traders and Republican lawmakers . Loyal users of these pouches have said that Zyn has helped them lose weight , comparing them to the viral weight-loss drug Ozempic.
In February, the company reported that nearly 385 million cans of the flavored nicotine pouches shipped in the US in 2023, up 62% year-over-year. The firm expects to do even better business in 2024, forecasting US shipments of around 520 million cans this year, its February earnings report said.
The company has been pushing to create more smoke-free products, as cigarette smoking declines worldwide .
Medical experts and research studies warn nicotine can be addictive and can have harmful effects on the body, including its cardiovascular and respiratory systems, BI previously reported.
The Food and Drug Administration has been cracking down on underage Zyn sales. Last month, the agency said it sent 119 warning letters to retailers and filed 41 civil complaints for sales of Zyn to underage buyers last year and this year.
Watch: FDA Bans Juul, Here's How We Got Here
- Main content

IMAGES
VIDEO
COMMENTS
1.1.1 Effects of nicotine on central regulatory mechanisms of energy homeostasis . Food intake is a process controlled by the CNS and it is stimulated by sensations such as hunger, craving, pleasure and reward (Schwartz, Woods et al. 2000).The hypothalamus is the main brain region responsible for the control of food intake via the actions of certain neuropeptides that are secreted from two ...
Imbalances in NAD+ homeostasis have been linked to aging and various diseases. Nicotine, a metabolite of the NAD+ metabolic pathway, has been found to possess anti-inflammatory and neuroprotective ...
Interestingly, nicotine users are found to be leaner than the general population. Review of the existing literature revealed that nicotine affects energy homeostasis and food consumption via altering the activity of neurons containing orexigenic and anorexigenic peptides in the brain. Hypothalamus is one of the critical brain areas that ...
In a human study, intravenous (IV) infusion of a relatively low dose of nicotine (0.5 µg/kg/min for 30 min) did not alter plasma insulin levels in healthy nonsmokers [48].In another study, short-term nicotine treatment with a transdermal nicotine patch similarly did not alter glucose or insulin levels or insulin sensitivity in healthy volunteers who were smokers [49].
Introduction. Cigarette smoking is the leading preventable cause of death and disability from respiratory disease. Smoking causes addiction and is negatively correlated with body weight and caloric intake; an effect which appears to be nicotine-mediated (Hajek et al., 1988).It is this action of nicotine on energy homeostasis that is attracting attention as a potential weight loss treatment ...
A sensitization-homeostasis model of nicotine craving, withdrawal, and tolerance: Integrating the clinical and basic science literature Joseph R. DiFranza, Robert J. Wellman [Received 23 November 2003; accepted 3 August 2004] Recent reports suggest that nicotine withdrawal symptoms are common among adolescents after a few weeks of
Some of the actions of nicotine on energy expenditure take place in the VMH. The VMH is involved in the regulation of many homeostatic and behavioral functions, such as regulation of sexual behavior, fear response, cardiovascular function, and satiety (Choi, Fujikawa, Lee, Reuter, & Kim, 2013).Specifically, the VMH establishes multiple connections with other areas related to energy homeostasis ...
This review was presented at the Physiology 2019 symposium "A nasty case of the vapours - E-cigarettes friend or foe?", which took place at the Aberdeen Exhibition and Conference Centre, Aberdeen, UK, 8-10 July 2019. ... Nicotine intake alters lung homeostasis and acts in the brain to promote addiction ... Studies of freshly isolated ...
Studies have demonstrated that nicotine alters the expression of microRNAs ... In the case of α7nAChR activation by nicotine, it prompts the release of serotonin, mammalian bombesin, as well as stress neurotransmitters like adrenaline and noradrenaline. ... α7 choline-activated current may play an important role in Ca 2+ homeostasis ...
The role of neuronal plasticity in supporting the addictive state has generated much research and some conceptual theories. One such theory, the sensitization-homeostasis (SH) model, postulates that nicotine suppresses craving circuits, and this triggers the development of homeostatic adaptations that autonomously support craving. Based on clinical studies, the SH model predicts the existence ...
Nicotine stimulates increases in blood levels of glucose by acting on neurons within the medial habenula, according to a new study published in Nature.The authors show that repeated exposure to ...
An integrated depiction of the results from the current study that show the role of nicotine in the regulation of cell homeostasis. α7nAChR stimulation by nicotine induces a rapid increase in cytosolic Ca 2+ uptake and Ca 2+ release from ER. This signaling is associated with mitochondrial ROS production and induction of mitochondrial and ER ...
While various modalities of chronic nicotine use have been associated with numerous negative consequences to human health, one possible benefit of nicotine exposure has been uncovered. The discovery of an inverse correlation between smoking and Parkinson's disease, and later Alzheimer's disease as well, motivated investigation of nicotine as a neuroprotective agent. Some studies have ...
The ability of nicotine, the primary psychoactive substance in tobacco smoke, to regulate appetite and body weight is one of the factors cited by smokers that prevents them from quitting and is the primary reason for smoking initiation in teenage girls. The regulation of feeding and metabolism by nicotine is complex, and recent studies have ...
The first known E-cigarette (EC), described as a tobacco-free nicotine aerosol device, was invented in 1963 by a man named Herbert A. Gilbert. ... a detailed case study of 53 affected EVALI patients in Wisconsin and Illinois revealed that ... can be detected in human serum and demonstrates a baseline level of cell turnover and homeostasis. A ...
The results of the present study might be, at least in part, due to indirect effects of nicotine and/or cotinine exposure on maternal drinking behavior 42 and pup care 65 rather than to direct ...
Haemodynamic studies improved, with increase in his toe pressure to 100 mmHg on the right and 88 mmHg on the left: Final visit, 24 months after initial presentation: He subsequently resumed vaping and continues to vape. Consequently, most recent haemodynamic studies showed a drop in toe pressures again to 81 mmHg bilaterally. Case 3: Init ial visit
Overview. In the United States, youth use e-cigarettes, or vapes, more than any other tobacco product. 1. No tobacco products, including e-cigarettes, are safe, especially for children, teens, and young adults. 2. Most e-cigarettes contain nicotine, which is highly addictive. Nicotine can harm the parts of an adolescent's brain that control ...
Out of 160 adult e-cigarette users who were part of a new study, those who took cytisinicline over a 12-week period were more than two times as likely to successfully quit vaping in weeks 9 to 12 ...
Andrea Hernández, the author of the consumer-trend newsletter Snaxshot, pointed out this craze this week, sharing a collection of social-media posts about Zyn and weight loss. "Your boy's down ...
Get four FREE subscriptions included with Chegg Study or Chegg Study Pack, and keep your school days running smoothly. 1. ^ Chegg survey fielded between Sept. 24-Oct 12, 2023 among a random sample of U.S. customers who used Chegg Study or Chegg Study Pack in Q2 2023 and Q3 2023. Respondent base (n=611) among approximately 837K invites.
2:21. Zyn, a hit nicotine pouch made by Philip Morris International Inc., is out of stock with with multiple retailers who ship nationwide, and some wholesalers are also reporting they're having ...
Zyn (stylized in all caps as "ZYN") is a brand of nicotine pouches originating in Sweden.Zyn pouches are designed to be placed between the gum and upper lip and are available in several variants with different nicotine strengths and flavors. Unlike snus, these pouches contain no tobacco.. The brand was created by Swedish Match, a subsidiary of Philip Morris International since 2022.
LAKE COUNTY, Fla. - The Lake County School District is getting results when it comes to lowering the number of kids bringing vapes to school. Back in November, we told you about Samba, the vape ...
Zyn, America's favorite nicotine pouch, is running out of stock in some states. Shubhangi Goel. May 19, 2024, 8:21 PM PDT. Containers of Zyn's nicotine pouches. Michael M. Santiago/Getty Images ...