Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Browse by collection
- BMJ Journals More You are viewing from: Google Indexer

You are here
- Volume 11, Issue 5
- Exploring patient safety outcomes for people with learning disabilities in acute hospital settings: a scoping review
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0001-6946-3693 Gemma Louch 1 , 2 ,
- Abigail Albutt 1 , 2 ,
- Joanna Harlow-Trigg 3 ,
- Sally Moore 1 ,
- Kate Smyth 2 , 4 ,
- Lauren Ramsey 1 , 2 ,
- Jane K O'Hara 2 , 5
- 1 Bradford Institute for Health Research , Bradford Teaching Hospitals NHS Foundation Trust , Bradford , UK
- 2 NIHR Yorkshire and Humber Patient Safety Translational Research Centre , Bradford , UK
- 3 School of Psychology , University of Leeds , Leeds , UK
- 4 Lancashire Teaching Hospitals NHS Foundation Trust , Preston , UK
- 5 School of Healthcare , University of Leeds , Leeds , UK
- Correspondence to Dr Gemma Louch; Gemma.Louch{at}bthft.nhs.uk
Objectives To produce a narrative synthesis of published academic and grey literature focusing on patient safety outcomes for people with learning disabilities in an acute hospital setting.
Design Scoping review with narrative synthesis.
Methods The review followed the six stages of the Arksey and O’Malley framework. We searched four research databases from January 2000 to March 2021, in addition to handsearching and backwards searching using terms relating to our eligibility criteria—patient safety and adverse events, learning disability and hospital setting. Following stakeholder input, we searched grey literature databases and specific websites of known organisations until March 2020. Potentially relevant articles and grey literature materials were screened against the eligibility criteria. Findings were extracted and collated in data charting forms.
Results 45 academic articles and 33 grey literature materials were included, and we organised the findings around six concepts: (1) adverse events, patient safety and quality of care; (2) maternal and infant outcomes; (3) postoperative outcomes; (4) role of family and carers; (5) understanding needs in hospital and (6) supporting initiatives, recommendations and good practice examples. The findings suggest inequalities and inequities for a range of specific patient safety outcomes including adverse events, quality of care, maternal and infant outcomes and postoperative outcomes, in addition to potential protective factors, such as the roles of family and carers and the extent to which health professionals are able to understand the needs of people with learning disabilities.
Conclusion People with learning disabilities appear to experience poorer patient safety outcomes in hospital. The involvement of family and carers, and understanding and effectively meeting the needs of people with learning disabilities may play a protective role. Promising interventions and examples of good practice exist, however many of these have not been implemented consistently and warrant further robust evaluation.
- quality in health care
- organisation of health services
- health services administration & management
Data availability statement
No additional data available.
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/ .
https://doi.org/10.1136/bmjopen-2020-047102
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Strengths and limitations
A key strength is the synthesis of both academic and grey literature materials.
A further strength is our approach to patient and public involvement and engagement throughout the review process.
We did not conduct formal quality assessments and are therefore unable to make reflections and comparisons of article quality.
Introduction
Inequalities in health and inequities in access to healthcare and technologies are a persistent and significant problem. 1–3 It is clear from previous research that certain demographic factors are associated with increased likelihood of poorer health, and variation in the use of and access to healthcare services. 4 5
One population that may experience greater vulnerabilities in terms of health and healthcare inequalities are people with learning disabilities. These vulnerabilities might arise as a result of barriers to accessing services and challenges associated with service organisation and delivery. 6 Learning disabilities are defined as ‘the presence of a significantly reduced ability to understand new or complex information, to learn new skills (impaired intelligence), with a reduced ability to cope independently (impaired social functioning) which started before adulthood, with a lasting effect on development’( https://www.datadictionary.nhs.uk/data_dictionary/nhs_business_definitions/l/learning_disability_de.asp?shownav=1 ). In this review, we have also drawn from the definition presented in the White Paper Valuing People , 7 which states that learning disability includes the presence of:
A significantly reduced ability to understand new or complex information, to learn new skills (impaired intelligence), with.
A reduced ability to cope independently (impaired social functioning).
Which started before adulthood, with a lasting effect on development.
This broad definition includes adults with autism who also have learning disabilities, but not those with a higher-level autistic spectrum disorder, such as some people with Asperger’s syndrome. Learning disability is the term most commonly used in the UK, although it is recognised as being synonymous with intellectual disability. 8
In 2013, the final report of a Confidential Inquiry into Premature Deaths of People with Learning Disabilities (CIPOLD) in England was published. 9 The report found that people with learning disabilities have higher rates of avoidable death compared with the general population, and that avoidable deaths arising from causes relating to poorer quality healthcare were more common in this population. On average, the life expectancy of people with learning disabilities is shorter than the general population. 10 The 2019 Learning Disabilities Mortality Review (LeDeR) report highlighted that people with learning disabilities died from an avoidable medical cause of death twice as frequently as people in the general population, and that the greatest difference between people with learning disabilities and the general population was in relation to medical causes of death which are treatable with access to timely and effective healthcare. 11
In the UK, the need for accessible healthcare environments for people with autism is recognised, 12 and in 2019, the government announced plans to pilot and then roll out learning disability and autism mandatory training for health and care staff in England ( https://www.gov.uk/government/consultations/learning-disability-and-autism-training-for-health-and-care-staff ). Furthermore, national projects such as Stopping Over-Medication of People with a Learning Disability, Autism or Both ( https://www.england.nhs.uk/learning-disabilities/improving-health/stomp/ ) have addressed issues around medicines practices.
Although there is increasing interest in this important issue from academics, healthcare staff, managers and policy-makers, much of this has focused on health inequalities and healthcare access more generally. What has been lacking to date is a critical examination of this issue as a patient safety phenomenon. This is important, as it opens up new avenues for conceptualising this problem, along with different framings for potential improvement and service development.
There is clear evidence that people with learning disabilities may be more at risk in terms of patient safety in hospital as well as known challenges around recognising and reporting patient safety incidents in this population. 13–15 Therefore, the need to bring together what is known about the safety of people with learning disabilities receiving healthcare, is clear.
In this review, we aimed to produce a narrative synthesis of published academic and grey literature focusing on people with learning disabilities in an acute hospital setting. We limited this review to the hospital setting because we were particularly interested in the care people with learning disabilities receive in a setting that may be predominantly related to physical health. We aimed to generate evidence that may facilitate the development of more tailored patient safety interventions for people with learning disabilities in an acute hospital setting. Our specific objectives were to:
Understand patient safety and adverse events in this population.
Explore protective factors and potential explanatory mechanisms.
Identify patient safety interventions, improvement initiatives, recommendations and examples of good practice.
A scoping review was considered the most suitable approach to produce a comprehensive, yet broad overview of the topic area. 16 17 We used Arksey and O’Malley’s 18 six stage framework and subsequent amendments to guide the review. 16 19 The stages include: (1) identifying the research question(s); (2) identifying relevant research studies; (3) selecting relevant research studies; (4) charting the data; (5) collating, summarising and reporting the study findings and (6) consulting with key stakeholders throughout the process. The review has been drafted in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Extension for Scoping Reviews. 20 We developed a broad search strategy, informed by the PRISMA Extension for Systematic Reviews with a Focus on Health Equity (2012). 21
Patient and public involvement and engagement
Our review team includes a lay representative (coauthor) who provided input into the protocol, reviewed the search strategy and helped develop materials for the wider patient and public involvement and engagement approach. We invited stakeholders to contribute search terms and assist in identifying grey literature. Stakeholders included representatives from the Yorkshire Quality and Safety Research Group patient panel, representatives from the NIHR Yorkshire and Humber Patient Safety Translational Research Centre Citizen Participation Group, and healthcare staff.
Eligibility criteria
The ‘Population-Concept-Context’ approach was used to specify study characteristics. 16 Inclusion and exclusion criteria were developed and iteratively refined as the review progressed. Studies reporting on patient safety, adverse events, protective factors, potential explanatory mechanisms, intervention and improvement initiatives, recommendations and good practice examples related to these topic areas were eligible. There was no restriction of study design, quantitative and qualitative methodologies were eligible for inclusion, and we limited the search to English language only.
Inclusion criteria
Articles that report on people with learning disabilities as the core focus (population). Articles may use terms synonymous with learning disability such as intellectual disability or refer to a condition related to learning disability, for example, autism (autism and learning disabilities are often coassociated 22 23 ), attention deficit hyperactivity disorder (high comorbidity for learning disabilities and attention deficit hyperactivity disorder 24 ), or Down’s syndrome.
Articles that investigate adverse events, patient safety, protective factors, potential explanatory mechanisms, patient safety interventions and improvement initiatives, recommendations and good practice examples (concept).
Articles relating to patients receiving care in an acute hospital setting (context). No restriction on age.
Articles relating to any country (context).
Study type: No restriction—qualitative, quantitative, mixed methods, case studies, primary research, retrospective review, systematic or scoping reviews/integrative reviews/meta-synthesis.
Language: Only articles published in the English Language due to lack of resources for an interpreter.
Exclusion criteria
Articles relating to primary care settings and inpatient mental health settings.
Articles focusing on patient experience/satisfaction.
Articles focusing on a specific drug treatment or procedure without a non-learning disability comparison group.
Information sources and search strategy
Academic literature search.
The search terms built on terms used in prior reviews framed around the eligibility criteria. 15 25–28 An initial limited search of MEDLINE was conducted ( online supplemental appendix 1 ). The search strategy was peer reviewed by a Knowledge and Information Librarian reviewer using the Peer Review of Electronic Search Strategies (PRESS), 29 and reviewed by academic researchers (patient safety), lay representatives and learning disability healthcare professionals. Following the initial search, all four included databases were searched: MEDLINE, CINAHL, PsycINFO and Web of Science from 2000 to 12 March 2021. The time period searched from was 2000 in line with the seminal publication of ‘To Err is Human: Building a Safer Health System’ as this publication arguably launched the modern patient safety movement. 30 The search was organised in three blocks: block 1—terms relating to learning disability (combined with OR); block 2—terms relating to adverse events and patient safety (combined with OR); block 3—terms relating to acute hospital setting (combined with OR). Blocks 1–3 were combined with the AND function. The reference lists of included articles were assessed, and we handsearched targeted journals including: the British Journal of Learning Disabilities, Journal of Learning Disabilities, Journal of Intellectual Disability Research, BMJ Quality and Safety, Journal of Patient Safety, Health Expectations, BMC Health Services Research, BMJ Open.
Supplemental material
Grey literature search.
The grey literature search included suggestions made via stakeholder input, such as terms to search, known publicly available materials and specific organisations to search online ( online supplemental appendix 2 ). We searched using the same combinations of terms relating to our eligibility criteria (eg, ‘patient safety and learning disability’, ‘learning disability and hospital’, ‘learning disability and adverse events’). All the online materials returned were initially screened according to title/summary information. In addition, the first 100 pages of Google, Google Scholar and all materials returned from OpenGrey and Royal College of Nursing Database were screened. The latest date for grey literature searches was 10 March 2020.
Study selection
Identified articles were collated in reference software (EndNote) and duplicates removed. Study selection involved two levels of screening: (1) title and abstract (2) full text. Three reviewers (GL, AA and JH-T) screened at title and abstract level according to the eligibility criteria, and 10% were independently checked to assess agreement. Articles that appeared to be eligible were screened at full-text level. When a full text was unavailable, authors were contacted directly. We were unable to obtain two full texts. Two independent reviewers assessed the full-text articles (GL and AA) and at this stage the reasons for exclusion were recorded. There were no discrepancies between reviewers regarding the eligibility of articles. Two authors carried out the grey literature search (GL and AA), and one author independently screened the potential grey literature for inclusion (SM), and 10% were independently checked to assess agreement.
Charting the data
Standardised data collection forms were developed and information from academic articles and grey literature material were collated into separate data collection forms, which were piloted prior to full data extraction. 19 For academic articles, key data were extracted including: publication year, publication type, country, study design, population and summary information relating to adverse events, patient safety, protective factors, potential explanatory mechanisms, intervention or improvement initiatives, recommendations and good practice examples. Following piloting, two reviewers (AA and JH-T) independently extracted the data from all included articles, and one reviewer checked 10% of the data extracted for consistency (JOH).
Study quality was not assessed as the aim of the review was to synthesise the emerging evidence rather than assess quality of individual articles. The grey literature data collection form was amended from the research article data collection form. Three reviewers (SM, AA and LR) independently extracted the data from all included publications using the adapted data collection form, and one reviewer checked 10% of the data extracted for consistency (JOH).
Data synthesis
Data were collated in two spreadsheets, one for academic articles and one for grey literature. A narrative synthesis followed to develop a narrative description of the findings and to highlight concepts that key findings could be organised around. 31 32 Authors (GL, AA, SM, LR and JOH) held meetings to discuss the key findings and generate concepts.
Title and abstract screening identified 140 articles eligible for full-text screening. Where studies appeared in review articles that met the eligibility criteria, these were not analysed separately and excluded (n=7). Thirty-four articles were eligible for inclusion in the review. A further 11 articles were included via backward and handsearching. In total, 45 articles were included (see figure 1 ). The grey literature search identified 92 potentially eligible materials, and 33 were included.
- Download figure
- Open in new tab
- Download powerpoint
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Summary characteristics
Characteristics of included articles and grey literature materials are displayed in online supplemental appendices 3 and 4 .
Of the academic articles, 19 related to paediatric patients, 5 to pregnant women/infant outcomes, 4 to adult patients, 2 to healthcare staff, 1 to healthcare staff and carers, 1 to parents or guardians and 13 articles related to hospital patients/setting more generally or did not specify the participants in more detail. All studies and reviews were conducted in high-income countries. Eighteen articles were from the USA, 13 were from the UK, 8 were from Australia, 2 were from Canada, 2 were from Taiwan and 1 each was from Hungary and The Netherlands. Twenty-one articles were retrospective and/or cohort studies, 6 were a type of literature review, 4 were discussion/opinion pieces, 3 articles used mixed methods, 3 improvement projects, 2 were qualitative, 2 were featured/special interest articles, 1 commentary, 1 case study, 1 short report and 1 secondary analysis. Fourteen articles referred specifically to intellectual disability, 10 to Down syndrome, 8 to learning disability, 5 to autism, 3 to intellectual and developmental disability, 2 to communication disability, 1 to developmental delay, 1 to cognitive impairment and 1 to attention deficit hyperactivity disorder. Throughout the results section we use the same terms as those used in the original articles and grey literature materials.
Key concepts
Our data synthesis generated six concepts: (1) adverse events, patient safety and quality of care; (2) maternal and infant outcomes; (3) postoperative outcomes; (4) role of family and carers; (5) understanding needs in hospital and (6) supporting initiatives, recommendations and good practice. We present these concepts below and specify how they map onto the three review objectives.
Objective 1: understand patient safety and adverse events in this population
Adverse events, patient safety and quality of care.
Six articles concentrated on either specific types of adverse events, quality of care or had a patient safety focus (see table 1 ). A systematic review of the experience of iatrogenic harm during hospitalisation for children with intellectual disability found that there are specific aspects of hospitalisation that expose children with intellectual disability to harms that are preventable, avoidable and not experienced to the same extent by children without intellectual disability. 15 Also focused on children, a further study indicated that children with pre-existing cognitive impairment received lower doses of analgesia and sedation medication, although the authors acknowledged it was not clear whether this was due to lower requirements or inadequate assessment. 33
- View inline
An overview of articles relating to adverse events, patient safety and quality of care
An integrative review investigated the care and safety of adults with communication disabilities in hospital and included a significant amount of studies specifically focused on intellectual disability. 34 The review concluded that patient safety incident and adverse event reporting lacked detail, and that successful advocacy affected outcomes, suggesting that when advocacy was ignored outcomes were worse. The review reported adverse event themes, including isolation due to limited methods to communicate with nurses, and that carers had a protective role in uncovering or preventing adverse events. Two primary studies reported within the aforementioned integrative review warrant further attention. 14 35 First, a mixed-methods study concluded that hospitals often lack effective systems for identifying patients which makes monitoring safety incidents difficult. This study also highlighted that staff do not always readily identify patient safety issues or report them, with incident reports commonly focused on events causing immediate or potential physical harm, and that safety issues were mostly related to delays and omissions of care. 14 Second, a study underpinned by a conceptual framework on patient safety aimed to identify factors that promote and compromise the implementation of reasonably adjusted healthcare services for patients with intellectual disabilities. This study emphasised the importance of ward culture, staff attitudes and staff knowledge in ensuring that hospital services are accessible to vulnerable patients. 35
A study assessing readmission found no significant difference in 30-day readmission rates for people with and without learning disabilities, but that 69% of readmissions of people with learning disabilities were potentially preventable, 36 and a study examining outcomes and toxicity of chemotherapy for acute lymphoblastic leukaemia in children with Down syndrome found that these patients spent more days in hospital particularly during the induction phase of treatment. 37
In a mixed-methods study, staff survey respondents reported feeling less confident about managing challenging behaviour and always delivering safe care to children and young people with learning disabilities, compared with children and young people without learning disabilities, as well as reporting that the environment was less safe for meeting the needs of children and young people with learning disabilities compared with those without. 38
A wealth of grey literature further evidenced vulnerabilities in terms of adverse events, quality of care and patient safety for people with learning disabilities. 9 11 39–52 This included influential reports such as the 2013 CIPOLD 9 and the subsequent LeDer programme annual reports, which evaluate the LeDer programme. 11 45–47
Maternal and infant outcomes
Five articles examined maternal and infant outcomes utilising a retrospective and/or cohort design, either focusing on women with intellectual and developmental disabilities, 53–55 intellectual disability and/or self reported learning difficulties, 56 and attention deficit hyperactivity disorder. 57 Higher rates of complications such as pre-eclampsia, 53 55–57 preterm birth, 55 57 low birth weight, 55 56 and labour interventions including induction and caesarean 53 55 57 were reported. One study reported higher prevalence rates for hospital admission and emergency department visits during all critical postpartum periods for those with intellectual and developmental disabilities, and higher risk of repeated hospitalisations. 54
A survey led by Patient Experience Network (not-for-profit organisation) and CHANGE (national human rights organisation) supported by NHS England, aimed to capture the experience of parents with learning disabilities. 58 Training for health professionals to better support parents with learning disability and improving accessibility to services were highlighted as essential.
Postoperative outcomes
The postoperative experience featured significantly in the systematic review of the experience of iatrogenic harm during hospitalisation for children with intellectual disability included within this review (referred to in adverse events, patient safety and quality of care findings). 15 Thirteen further articles reported on postoperative outcomes. 59–71 The majority of articles included data relating to Down syndrome, 59–61 63–65 68 69 followed by intellectual disability, 62 67 70 developmental delay 66 and autism spectrum disorder. 71 Increased rates of complications 60 62 63 66 69 70 were reported in a number of studies. However, in one study comorbidities rather than Down syndrome were a greater risk factor for complications when adjusting for other covariates, 59 and after propensity matching, another study also focusing on patients with and without Down syndrome, found no significant variation regarding rates of postoperative complications. 64 Furthermore, one study focusing on risk factors for major complications related to percutaneous endoscopic gastrostomy placement in children concluded that when adjusting for other variables, intellectual disability was not a significant risk factor. 67
A longer length of stay was reported in four studies 60 62 63 70 with one study reporting a similar length of stay for those with Down syndrome compared to those without, 65 and one study reporting that patients with autism spectrum disorder had a shorter length of stay and were less likely to experience complications. 71 In one study mortality and major complication rates were lower for patients with Down syndrome. 65 Similarly, further studies also focusing on Down syndrome found mortality and medical complications to be significantly lower for patients with Down syndrome with no significant differences in terms of surgical complications, 68 and lower odds of in-hospital death for patients with Down syndrome when controlling for other factors such as risk category and premature birth. 61 In four studies no differences in mortality were reported, 62–64 66 and in one study children with intellectual disability had a higher risk of 30-day mortality compared with children with no intellectual disability. 70
Objective 2: explore protective factors and potential explanatory mechanisms
Role of family and carers.
Reliance on parental presence as a protective factor from poor care quality was emphasised in the systematic review of the experience of iatrogenic harm during hospitalisation for children with intellectual disability included within this review (referred to in objective 1 findings). 15 Furthermore, a primary study included within an already included literature review 34 (referred to in objective 1 findings) warranted further attention within this concept. The qualitative interview study explored paid carers’ roles in supporting adults with developmental disability and complex communication needs and described how paid carers are often motivated by perceived responsibility for safety, well-being and communication, but that their role can sometimes be blurred with nursing and family carer roles. 72
Five further articles highlighted the significant role of families and carers. A meta-narrative approach to understand the experience for the parent of a child with intellectual disability in hospital resulted in a synthesis of 11 studies. A working model for professional parent partnership was developed which reinforced the importance of hospital/multidisciplinary approaches to care centring on the child, understanding previous negative experiences and negotiating care and shared learning to lessen reliance on parental presence. 73 A further review evaluated how hospital systems respond to adults with intellectual disability, their families and carers. Key themes included: individual fear of hospital encounters, reliance on paid family carers for basic needs and advocacy, responsibilities and staff knowledge, skills and attitudes. 25
A key finding from a qualitative study with medical practitioners concluded that practitioners make limited use of ‘reasonable adjustments’ and turned to caregivers to facilitate communication and manage behaviours likely to upset hospital routines. 74 A mixed-methods study aiming to identify factors that affect carer involvement for people with intellectual disabilities in acute hospitals presented a model for clarifying carer involvement that sought to highlight the degree to which carers are ‘workers’ contributing to basic nursing care, and the degree to which carers are experts or non-experts. 75 The authors suggested that making these two aspects explicit might facilitate staff to understand carer contributions more comprehensively. Finally, a quantitative case note audit demonstrated poor performance across a range of elements of hospital care for people with learning disability. 6 One notable positive finding of the audit was that in most cases family or carers were involved in discharge planning. 6 However, the thoroughness of this was questioned as many carers were not signposted to an assessment of their needs prior to discharge.
In terms of grey literature, a doctoral thesis which investigated emergency healthcare from the perspective of the carers of people with learning disabilities, highlighted the relationship staff had with both service users and carers as fundamental to a high-quality service. 76
Understanding needs in hospital
Six articles had content relating to the needs of people with learning disabilities in hospital. 77–82 One article concluded that to ensure nurses do as much as possible to identify risk they must recognise prejudices and overcome them, develop further understanding of learning disabilities and acknowledge the rights of people with learning disabilities and collaborate with carers and professionals. 78 Similarly, a literature review around communication, recognised the importance of collaborating effectively with carers, as well as access to personally held written health information, inter-agency communication, devoting time to communication and access to communication tools and aids. 79 A literature review assessing evidence around the promotion of health, safety and welfare of adults with learning disabilities in acute care emphasised the importance of care provision, communication, staff attitudes, staff knowledge, supporters and carers and the physical environment. 77 Crucially, communication was highlighted as a fundamental issue, such that people with learning disabilities often have difficulty communicating their needs. The literature review presented strategies and resources that may support this such as videos, accessible booklets, augmentative and alternative communication and pictures/symbols.
To help improve the inpatient experience of hospital patients with autism, a survey of parents and guardians with qualitative and quantitative items highlighted the need for an individualised approach to assess and accommodate needs. 81 This approach was taken in a case study that described the plan of care for a patient with moderate level of learning disability scheduled for a tonsillectomy. The report gave a specific example of how investing time to understand a patient’s need can improve experience. 82 When the patient’s details were being checked, the door knocked into the patient’s chair as staff entered the room for equipment, and this exacerbated the patient’s anxiety. This was acknowledged quickly and a do not disturb sign was placed on the door.
An article aiming to familiarise the paediatric nurse with autism and create a resource for successful inpatient treatment put forward key themes such as change is a challenge, consistent caregivers, safe environment, encouraging family involvement, ways of communicating, emotional triggers and reward systems and multidisciplinary team from admission. 80 Indeed, the NHS long-term plan published in 2019, 83 emphasised that the whole NHS will improve its understanding of the needs of people with learning disabilities and autism, with plans in place for staff to receive training on supporting people with a learning disability and/or autism alongside the implementation of national learning disability improvement standards. Furthermore, the government response to the consultation on learning disability and autism training for health and care staff also published in 2019, underlined the importance of gaining a better understanding of how to ensure that patients and service users receive safe, effective and dignified care, and the need to equip those providing care with the necessary skills, knowledge and behaviours. 84
The importance of staff being knowledgeable about the children they care for and their intellectual disability also featured in the systematic review of the experience of iatrogenic harm during hospitalisation for children with intellectual disability included within this review (referred to in objective 1 and role of family and carers findings). 15
Objective 3: identify patient safety interventions, improvement initiatives, recommendations and examples of good practice
Supporting initiatives, recommendations and good practice.
Ten articles using diverse designs (including commentary/opinion pieces, qualitative methods, service improvement, discussion/special interest/featured articles and short reports), reported either examples of initiatives to support safe care for people with learning disabilities in hospital, or recommendations to support good practice (see table 2 ). 85–94 A qualitative content analysis of 60 documents mapped the content of existing hospital passports for people with intellectual disability and concluded that this approach can enhance safety and person-centred care, but acknowledged there is much variation between current hospital passports which may limit effectiveness. 89 Six articles provided specific examples of how to enhance good practice. 85 88 91–94 These included a commentary highlighting how hospital pharmacists can contribute to safety when supporting people with intellectual disability in hospital, 85 a special interest/review article focusing on the presurgical needs of those with Down syndrome and how patient safety can be optimised, 88 and an opinion piece/review presenting recommendations for the perioperative management of children with autism. 91 Additionally, a featured article presented how simulations can educate nurses to maintain safety when caring for patients with autism spectrum disorder, 92 and a short report highlighted the importance of: reliable identification of children with intellectual disability; exploring indirect indicators of poor quality care and consumer engagement and the voice of the child with intellectual disability. 93 Finally, a research/discussion article explored key issues in working with people with intellectual disabilities and provided methods to improve the care provided. 94
An overview of articles relating to supporting initiatives, recommendations and good practice
Three articles described improvement work. 86 87 90 One project identified areas of risk for people with intellectual disability while in hospital, and developed and successfully implemented a rapid risk assessment tool to assess immediate and potential risk, identify risk reduction actions and develop appropriate care bundles. 90 The second project identified core tasks of a specialist learning disability team to improve patient care for those with learning disabilities, examples included: educating acute staff, developing training materials for staff and trainees, considering consent issues and facilitating community support before discharge. 87 A mixed-methods study comprising literature review and improvement work, developed care plans and an educational module. After completing the module, there was an increase in nurses’ confidence when caring for people with learning disabilities. 86
Further initiatives, recommendations and good practice examples were identified in the grey literature. 95–107 For brevity, we provide further information and signpost to these resources in online supplemental appendix 4 .
To the authors’ knowledge, this is the first scoping review to synthesise both the academic and grey literature focusing on hospital patient safety outcomes for people with learning disabilities. While, as a narrative synthesis we are unable to state unequivocally the relationship between having learning disabilities and safety outcomes, our findings do suggest that there are multiple ways in which people with learning disabilities might experience poorer outcomes compared with people without. Our review demonstrates that there are inequalities and inequities for a range of specific patient safety outcomes including adverse events, quality of care, maternal and infant outcomes and postoperative outcomes. This disparity needs urgent attention. Nonetheless, we did identify a range of potential protective factors, such as the roles of family and carers and the extent to which health professionals are able to understand the needs of people with learning disabilities. Research has focused on developing interventions and good practice guidance, yet this is predominantly accounted for within the grey literature, meaning that robust evidence is still needed.
Some poorer outcomes are likely through the ‘direct effects’ of having a learning disability, for example, the increased incidence of comorbidities in children with learning disabilities accounted for the increased likelihood of postoperative complications in one study. 59 However, it is also abundantly clear that there are multiple ‘indirect effects’ of having learning disabilities that may amplify problems. The review highlighted the prevailing potential risk of inadequate systems to identify and flag people with a learning disability when they enter an acute hospital setting, and the knock on effect this can have on the ability to effectively monitor patient safety incidents for these patients. 14 93 Crucially, if patient identification and flagging and therefore patient safety incident monitoring is not fit for purpose, this creates a significant knowledge gap which greatly limits the development of much needed solutions to address patient safety issues.
Further principal issues likely to manifest in differential outcomes included problems with communication (eg, patients to staff, staff to patients, intra-agency and interagency), staff attitudes, the role of family and carers, staff awareness and knowledge/training and variation in the quality and level of healthcare received. These indirect effects fall squarely in the realm of quality and safety efforts, modifiable potentially through service redesign, increased resources, training, professional specialisation and appropriate adaptation of practice. Promising interventions and good practice examples were identified such as risk assessment tools, 90 preoperative and perioperative management recommendations, 88 91 hospital passports 89 94 95 and education modules. 86
We explore these issues through a patient safety ‘lens’, and what is perhaps most striking about our findings, is their lack of novelty. One of the earliest national reports within the UK—‘Healthcare for all’ 41 —found similar issues, and made a series of recommendations. It is clear from our review that since this report, very little has changed in terms of the experience of people with learning disabilities and their families within acute care settings, either nationally or internationally. The exploration of this issue as a ‘patient safety problem ’ allows us to understand how, through the design of our healthcare system we create—and seek to solve—safety problems from the perspective of those moving through and navigating the system.
In an unrelated study, Fylan et al examined the medicines management system for heart failure patients discharged from hospital into the community, and developed a framework called ‘Gaps, Traps, Bridges and Props’ which may be useful when thinking about our review findings. 108 ‘Gaps ’ occur in our systems at points of discontinuity or transition, and evidence from across patient safety literature indicates that gaps in the structure and design of services create ‘safety gaps’ that present opportunities for problems for patients, especially when care is suboptimal or fails. 109 110 It is arguable that those patients with complex needs or specific vulnerabilities that require greater continuity of care, are more at risk when crossing these ‘safety gaps’—in effect, their vulnerability amplifies the risk of experiencing a patient safety problem. In our review, it is evident that people with learning disabilities may disproportionately suffer due to these gaps in healthcare systems. Examples of this would include poor inter-agency communication, 79 and hospitals lacking effective systems for identifying and flagging patients. 14 Sometimes, the design of services/organisations goes beyond creating a ‘gap ’ —which may or may not result in a safety problem for patients. ‘Traps ’ are here defined as features of system design that actively make problems more likely. An example of a ‘trap ’ from our review is the need for training on learning disabilities for healthcare staff. 49 83 84 Without specific knowledge of, and training in caring for those with a range of learning disabilities, it is perhaps understandable that staff regularly fail to make reasonable adjustments to accommodate specific needs. 74
This framing provides the possibility to ameliorate the issues that result, either through formalised ‘bridges ’ , or further supporting the range of informal ‘props ’ that serve to reduce problems when care is suboptimal, or fails. ‘Bridges ’ are viewed as formalised features of a system, designed to span service gaps, and support continuity of care. 108 We found a number examples—from patient-held passports, 89 94 95 to specialist learning disability teams. 87 However, our review also found that these ‘bridges ’ are often inconsistently available or applied, a position that could further amplify problems if staff have come to rely on them for support when needed. The most prevalent mechanism for supporting patients with learning disabilities came through the role of families and carers. Although the need to reduce ambiguity about the role of the parent 73 and the importance of clarifying what carer involvement includes 75 were emphasised, we found a range of evidence that suggested families and carers regularly ‘prop ’ up services—from helping with feeding and personal care, 25 to facilitating communication 74 and being involved in discharge planning 6 —and that without this ‘prop ’ , the outcomes for patients with learning disabilities may well be poorer.
Implications
Our review demonstrates the piecemeal and wide-ranging nature of the extant evidence, in terms of specific learning disabilities and outcomes of interest, and with a range of methodologies used. Therefore, we propose that research is needed to establish the burden of harm for people with learning disabilities as a result of patient safety incidents and poor quality of care, in hospital settings. This goes beyond learning from deaths—we need to understand what happens with care for people with learning disabilities more generally. Second, research needs to understand the mechanisms through which these effects might be seen. It is this approach that holds significant promise from the point of view of service improvement and redesign, as well as training and curriculum development. Put simply, we cannot change what we do not yet fully understand. Finally, attention must be given to the existing recommendations from the range of reports already published. For example, common recommendations across many previous reports include: the need for better systems to identify people with learning disabilities in healthcare settings 9 39 41 46 ; the need for improved communication and information sharing between agencies and providers 9 46 47 ; and the need for education and training in caring for people with learning disabilities. 11 39 41 46 47 49 83 84 There is already a wealth of learning about the problems that exist for people with learning disabilities and their families, what is needed now is policy level action.
Limitations
Despite an inclusive search strategy, relevant articles may not have been identified if they were not available in the sources searched. Additionally, due to the nature of the review, we did not conduct formal quality assessments and were therefore unable to make reflections and comparisons of article quality.
The academic and grey literature indicates that while in hospital, people with learning disabilities might experience poorer patient safety outcomes. The involvement of family and carers, and understanding the needs of people with learning disabilities in hospital were highlighted as potential protective factors. Many promising interventions and examples of good practice exist, however, these may not be widely available or have been applied inconsistently.
Acknowledgments
We are extremely grateful for the contributions from a number of people and groups. Thank you to the Yorkshire Quality and Safety Research (YQSR) Group patient panel and NIHR YH PSTRC Citizen Participation Group members for their contributions to the search strategy. Second, thank you to Amanda Mckie, Beth Fylan, Jonathan Benn, Kate Pickett, Steph Prady and Federica Bianchini for their contributions to the protocol draft and search strategy. Finally, thank you to Sara Ryan and John Baker for their feedback on the draft manuscript.
- Arcaya MC ,
- Arcaya AL ,
- Subramanian SV
- Cookson R ,
- Propper C ,
- Sheehan R ,
- Gandesha A ,
- Hassiotis A , et al
- Department of Health
- Fleming P , et al
- Primary Care Domain
- NHS Digital
- University of Bristol Norah Fry Centre for Disability Studies
- National Patient Safety Agency
- Tuffrey-Wijne I ,
- Goulding L ,
- Gordon V , et al
- Harrison R ,
- Hinchcliff R
- Godfrey C ,
- McInerney P
- Stewart L ,
- Colquhoun H ,
- Tricco AC ,
- Zarin W , et al
- Petticrew M ,
- Tugwell P , et al
- Rydzewska E ,
- MacIntyre C , et al
- O'Brien G ,
- Calhoun SL ,
- Unsworth C , et al
- Keen D , et al
- Panagioti M ,
- Geraghty K ,
- Johnson J , et al
- Hesselink G ,
- Geense W , et al
- McGowan J ,
- Sampson M ,
- Salzwedel DM , et al
- Donaldson MS ,
- Corrigan JM ,
- Roberts H ,
- Campbell M ,
- Katikireddi SV ,
- Sowden A , et al
- Curley MAQ , et al
- Hemsley B ,
- Georgiou A ,
- Hill S , et al
- Giatras N , et al
- Thomson K ,
- Wagner AP , et al
- Al-Ahmari A ,
- Al-Yamani A , et al
- Carr L , et al
- Royal Society for Mentally Handicapped Children and Adults
- Michael J ,
- Richardson A
- Local Government Ombudsman
- NHS England
- NHS Improvement
- ↵ Interim BULLETIN undiagnosed cardiomyopathy in a young person with autism. healthcare safety investigation branch , 2019 . Available: https://www.hsib.org.uk/documents/139/hsib_interim_bulletin_undiagnosed_cardiomyopathy_autism.pdf
- Care Quality Commission
- National Institute for Health Research
- Kirkham YA ,
- Cobigo V , et al
- Parish SL ,
- Akobirshoev I , et al
- Son E , et al
- McConnell D ,
- Llewellyn G
- Poulton AS ,
- Armstrong B ,
- Change and PEN
- Bartz-Kurycki MA ,
- Anderson KT ,
- Austin MT , et al
- Boylan MR ,
- Kapadia BH ,
- Issa K , et al
- Dharmar M ,
- Meierhenry E , et al
- Chang C-C , et al
- Purifoy ET ,
- Riley JS , et al
- Prodán Z , et al
- St Louis JD ,
- Jacobs JP , et al
- Pugely AJ ,
- Martin CT ,
- Gao Y , et al
- Vervloessem D ,
- van Leersum F ,
- Boer D , et al
- Zeinali LI ,
- Berkelhamer SK , et al
- Graber TJ ,
- Baskin PL ,
- Soria C , et al
- Huang S-Y ,
- Chang C-C ,
- Huang Shih‐Yu ,
- Chang Chuen‐Chau ,
- Lin Chao‐Shun , et al
- Printz JN ,
- Mirkin KA ,
- Hollenbeak CS , et al
- Balandin S ,
- Woolfenden S ,
- Travaglia J , et al
- Lancaster I ,
- Pitt A , et al
- Abraham E ,
- Goulding L , et al
- Bradbury-Jones C ,
- Rattray J ,
- Jones M , et al
- Kopecky K ,
- Broder-Fingert S ,
- Iannuzzi D , et al
- Wilkinson S
- Department of Health and Social Care
- Lewanda AF ,
- Matisoff A ,
- Revenis M , et al
- Northway R ,
- Davies M , et al
- Vlassakova BG ,
- Emmanouil DE
- McIntosh CE ,
- Royal College of Nursing
- Guidelines and Audit Implementation Network
- The Hillingdon Hospitals NHS Foundation Trust
- Public Health Nursing Division
- Department of Health in partnership with the Modernising Learning Disabilities Nursing UK Implementation Group
- National Quality Board
- Mencap and The National Autistic Society
- The Westminster Commission on Autism
- Marques I ,
- Ismail H , et al
- O'Hara JK ,
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
- Data supplement 2
- Data supplement 3
- Data supplement 4
Twitter @Gemma_Louch, @janekohara
Contributors GL conceived the idea for the scoping review, led all stages of the scoping review and drafted the manuscript. AA contributed to all stages of the scoping review. KS reviewed the protocol, contributed to the search strategy and worked with the research team to develop materials for the wider patient and public involvement and engagement (PPIE) approach. JH-T, SM and LR contributed to the literature searching, screening and data extraction. JOH contributed to the consistency checks and wrote the first draft of the discussion. All authors provided comments and approved the final version.
Funding The scoping review was funded by the National Institute for Health Research Yorkshire and Humber Patient Safety Translational Research Centre (NIHR YH PSTRC).
Disclaimer The views expressed in this article are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
- Open access
- Published: 22 March 2021
Patient safety from the perspective of quality management frameworks: a review
- Amrita Shenoy ORCID: orcid.org/0000-0001-8355-7792 1
Patient Safety in Surgery volume 15 , Article number: 12 ( 2021 ) Cite this article
21k Accesses
3 Citations
1 Altmetric
Metrics details
Patient safety is one of the overarching goals of patient care and quality management. Of the many quality management frameworks, Beauchamp and Childress’s four principles of biomedical ethics presents aspects of patient centeredness in clinical care. The Institute of Medicine’s six aims for improvement encapsulates elements of high-quality patient care. The Institute of Healthcare Improvement’s Triple Aim focuses on three aspects of care, cost, and health. Given the above frameworks, the present review was designed to emphasize the initiatives the system has taken to address various efforts of improving quality and patient safety. We, hereby, present a contemplative review of the concepts of informed consent, informed refusal, healthcare laws, policy programs, and regulations. The present review, furthermore, outlines measures and policies that management and administration implement and enforce, respectively, to ensure patient centered care. We, conclusively, explore prototype policies such as the Delivery System Reform Incentive Payment Program that imbues the elements of quality management frameworks, Hospital-Acquired Conditions Reduction Program that supports patient safety, and Hospital Readmissions Reduction Program that focuses on curbing readmissions.
The logistics of patient care and healthcare management revolve around many aspects of optimized high-quality care. The Joint Commission (TJC), Malcolm Baldrige National Quality Award (MBNQA), and The Magnet Recognition Program signify healthcare accreditation, performance excellence, and nursing excellence, respectively [ 1 , 2 , 3 ]. TJC is the recognized global leader of healthcare accreditation [ 4 ]. It is an independent not-for-profit organization that offers an unbiased assessment of quality achievement in patient care and safety [ 4 ]. MBNQA is the nation’s highest presidential honor for performance excellence [ 5 ]. The Magnet Recognition Program designates organizations worldwide where nursing leaders successfully align their nursing strategic goals to improve the organization’s patient outcomes [ 6 ]. In addition to the above healthcare recognition, the Institute of Medicine (IOM) categorizes aspects of care delivery with its six aims for improvement [ 7 ]. The Institute of Healthcare Improvement’s (IHI's) Triple Aim comprises of three aspects: improving the experience of care, improving the health of populations, and reducing per capita costs of healthcare.
We, hereby, present a synthesis of how the perspectives of biomedical ethics, six aims for improvement, and the Triple Aim converge into a focal point of preserving patient safety and promoting improvement in care delivery. The present review elaborates and explains the clinical and managerial roles inherent in the logistics of patient safety in emergencies and non-emergencies. The impetus here is to exemplify existing policies supporting patient centeredness while preserving the parameters that improve patient care, preserve quality, and promote patient safety.
As one of the cornerstones of high-quality healthcare, patient safety is intrinsic to all healthcare professionals. Clinicians are involved in direct patient care. However, does that imply that policymakers, leadership, and managers are separate and distinct components not involved in patient safety? The answer to the above question is not likely because these entities devise and enforce policies to preserve and augment patient safety in communities, institutions, and departments. At the macro-level, policymakers devise and recommend healthcare policies that at the micro-level, leadership, management, and clinicians enforce, adopt, and practice, respectively, at the point of patient care.
Research questions and objectives
Past literature establishes quality management frameworks such as Beauchamp and Childress’s Principles of Biomedical Ethics, six aims for improvement and the Triple Aim. The above frameworks, broadly, capture the patient’s needs/preferences while aligning with improvement in care delivery. However, there are instances in which patients when presented in an unconscious or inebriated state cannot communicate their treatment preferences. Given the above case, the first research question is: what are some recourses that providers can choose to adopt as safe harbors while treating such patients? The second research question is: what are the practices that clinicians could potentially adhere when the patient consents or refuses to consent? As a close follow-up, the third research question is: what is the role of administration in implementing policies that fall outside the purview of already enforced laws? The objective of the present review is threefold. First, we aim to propose answers to the dos and don’ts that clinicians could potentially adopt in emergency and non-emergency cases, given the concepts of informed consent and informed refusal. Second, we attempt to explain how hospital leadership can best facilitate patient safety and manage risk while facilitating high-quality patient care. Finally, we explore prototype policies such as the Delivery System Reform Incentive Payment program, Quadruple Aim, Hospital-Acquired Conditions Reduction Program, and Hospital Readmissions Reduction Program which have been implemented more recently as systemic initiatives to preserve patient safety and promote measures in care delivery.
Literature review
Quality management frameworks preserving patient safety: an overview of three established frameworks, beauchamp and childress’s principles of biomedical ethics.
Faculty in medicine and surgery have a substantial role in ethically creating a culture of safety via medical and surgical treatments for patients. In this context, four principles of biomedical ethics come into the picture. Those principles are autonomy, non-maleficence, beneficence, and justice [ 9 ]. The above four principles are the four pillars of medical ethics and form the basis of ethical practice in medicine and surgery. Some more aspects of biomedical ethics stemming from the above four principles are considered in ethical medical and surgical decision making [ 10 ]. A list of those additional aspects are as follows: [ 10 ].
Truthfulness, Full Disclosure, and Confidentiality: On the one hand, truthfulness is not distorting facts while presenting information to the patient; full disclosure is accurately and completely informing the details of the patient’s medical condition. On the other hand, confidentiality is the principle of not revealing information about the patient’s medical condition to third parties [ 10 ].
Autonomy and Freedom: Autonomy is the principle of providing the patient discretion, freedom, and independence to choose treatment preferences. This concept particularly comes into the spotlight in end-of-life hospice treatments and medical terminations of pregnancies [ 10 ].
Beneficence is the principle of doing good and inflicting the least harm to the patient.
The Institute of medicine’s six aims for improvement model
The Agency for Healthcare Research and Quality (AHRQ) Patient Safety Network expands upon the definition of prevention of harm as, “freedom from accidental or preventable injuries produced by medical care” [ 11 ]. Furthermore, the IOM introduced six aims for improvement in healthcare to meet the patient’s healthcare needs and preserve patient safety. Those six aims are as follows: [ 7 ].
Safe: avoiding injuries to patients from the care that is intended to help them. Patient safety can be a system-wide approach when patients see measures adopted and practiced that create a safe environment [ 7 ].
Efficient: avoiding waste including waste from equipment, supplies, ideas, and energy. Healthcare wastes are also in the form of defensive medicine, malpractice litigation, systemic complexities, and administrative fraud and abuse. Cost-effective care potentially supports efficiency in healthcare [ 7 ].
Effective: providing services based on scientific knowledge to all those who could benefit. In this context, Evidence Based Medicine is incorporating scientific knowledge into treatment and procedure options [ 7 ].
Patient-centered: providing care that is respectful of and responsive to the patient’s needs, preferences, and values. Delivery of care is considered to be patient-centered when the patient can choose certain aspects of care. This approach towards patient care prospectively ingrains elements of cooperation and collaboration [ 7 ].
Timely: reducing waiting times and detrimental delays for both, recipients and providers of care. Waits and harmful delays potentially produce life threatening illnesses worsening quality outcomes throughout the continuum of a patient care [ 7 ].
Equitable: providing care that is consistent and does not vary in quality based on personal aspects such as gender, ethnicity, geographic location, and socioeconomic status, etc. [ 7 ].
As per the IOM’s six aims for improvement, first, healthcare processes need to be safe which implies the provider makes an active attempt to ensure patient safety. Second, patient care prospectively needs to be aligned with recent developments to be potentially effective. Third, patient-centered care takes into consideration the patient’s culture, dietary and personal preferences incorporated into care delivery methods. The above concept plays an important role in end-of-life or hospice care provided to the elderly. Fourth, timeliness is providing and receiving care in a manner that reduces waiting times and delays. On the one hand, unforeseen wait periods may delay care and result in serious unintended harm to patients. On the other hand, the provision of timely care is essential to patient safety. Fifth, focusing on eliminating wastes and redundant processes could potentially help conserving resources and making care more affordable. Finally, providing equitable care is that which does not vary in terms of race, ethnicity, socioeconomic status, and income [ 7 ].
The Institute of healthcare improvement’s triple aim model
The Institute of Healthcare Improvement’s (IHI’s) Triple Aim model synthesizes and incorporates aspects of care, cost, and health [ 8 ]. The IHI’s Triple Aim model involves the following three components: [ 8 ].
Improving the experience of care: Implementing Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) and Consumer Assessment of Healthcare Providers and Systems (CAHPS) surveys are few of the many ways of recording patient experience of care [ 12 , 13 ]. The National Practitioner Data Bank (NPDB), additionally, assists in promoting quality health care and deterring fraud and abuse within health care delivery systems [ 14 ].
Reducing per capita costs of care: Cost of care could be reduced with the help of using generic drugs instead of brand name drugs for prescriptions, as an example [ 8 ].
Improving the health of populations [ 8 ].
The IHI's Triple Aim is a framework that describes an approach with a threefold purpose. First, improving the experience of care regarding healthcare quality, second, decreasing per capita costs of care that aims at reducing wastes and variation in healthcare, and third, improving the health of populations. The IHI’s Triple Aim model has universal applications that cover medical treatment, surgical care, therefore, opening avenues to solve administrative complexities for preserving health and wellness in populations.
The first component of the Triple Aim, improving the experience of care applies to advances in medical technology making a positive impact in the patient experience of care [ 8 ]. The second component of the Triple Aim, reducing per capita costs of care, applies to implementing telemedicine and telehealth projects, as an example. Telemedicine brings to fruition, efficient and timely care when physicians may not be in the vicinity of the patient [ 8 ]. On the one hand, one of pros of telemedicine is the potential to enhance access to care. On the other hand, it introduces this concept to some practitioners and patients who have little to no experience with e-health. The third component of the Triple Aim, improving the overall health of the population applies to facilitating a combination of the above two aims. The IHI’s Triple Aim model, therefore, is a three-pointed framework in which the first two aims are intrinsic to the third aim, improving population health [ 8 ].
The roles of clinical faculty and administration in patient safety: adoption and implementation of best practices in emergency and non-emergency cases
Emergency Medical Treatment and Active Labor Act (EMTALA) is a federal law that requires anyone coming to an emergency department to be stabilized and treated, regardless of their insurance status or ability to pay [ 15 ]. As per EMTALA, the patient has a right to be treated and clinicians are bound to provide treatment [ 15 ]. In this context, let us consider an example of an unconscious patient in the emergency department that does not culturally prefer receiving blood transfusions. In the above case, hypothetically, if the treating provider is not knowledgeable of the cultural preference of the unconscious patient and proceeds to revive the patient via a blood transfusion, then, was patient centered care provided? The answer likely lies in the provider’s assessment in the context of EMTALA. The assessment, first and foremost, relates to the binding duty of the clinician to provide care to every patient, especially in times of emergencies.
The dynamics of the above hypothetical scenario entirely changes in non-emergency situations in which patients can choose a provider to treat them; and reciprocally, even providers can choose whom to treat. The rationale behind this is the physician-patient relationship that specifies the terms and conditions of a physician-patient contract [ 16 ]. This legal relationship is based on contract principles because the physician agrees to provide treatment in return for payment in the presence of the contract [ 16 ]. The law usually imposes no duty on the physician to treat the patient in the absence of a physician-patient contract [ 16 ].
In the process of providing treatment, obtaining informed consent is the concept in which the clinician explains the proposed line of treatment, duration, benefits, risks of opting in as well as opting out of the treatment, alternatives to the proposed treatment with an opportunity to answer patient questions [ 17 ]. In 1914, an American judge Benjamin Cardozo composed the foundational principle of informed consent as, “Every human being of adult years and sound mind has a right to determine what shall be done with his own body; and a surgeon who performs an operation without his patient’s consent commits an assault for which he is liable in damages” [ 18 ]. An interesting aspect of treatment in non-emergency cases is when the patient does not agree to informed consent which brings forth the concept of “Informed Refusal” [ 19 , 20 ]. A living will is an example of an informed refusal document in which the patient states his or her end of life preferences [ 21 ]. In the above case, the provider honors the patient’s end of life preferences and/or withholds treatment for the patient as specified in the living will.
The role of leadership is to enforce EMTALA and help clinicians' awareness of informed consent and informed refusal processes in organizations. Moreover, they ensure that providers implement the above policies regarding patient preferences. In medical cases that fall outside the purview of the already enforced laws, leadership can prospectively make rules but with caution that those rules are not against public policy.
Macro-level healthcare programs focusing on patient safety: prototype policies
Delivery system reform incentive payment program: focusing on alignment with quality management frameworks.
The Delivery System Reform Incentive Payment (DSRIP) program is one prototype policy that incorporates six aims for improvement and the Triple Aim model. DSRIP has multiple healthcare projects that improve health statuses incorporating numerous metrics and milestones in primary care, specialty care, chronic care, navigation and case management, disease prevention and wellness, and general categories [ 23 , 24 ]. These projects are reimbursed by the State Department of Health in a systematic manner when adopted by healthcare institutions [ 22 , 23 , 24 , 25 , 26 ].
DSRIP’s framework involves four components: (1) Infrastructure Development, (2) Program Innovation and Redesign, (3) Quality Improvement, and (4) Improvement in Population Health in states where its projects are implemented [ 22 , 23 , 24 , 25 , 26 ]. In its third year of implementation, the Texas DSRIP program in the southeastern county region had about 172 projects in eight cohorts those being, primary care, emergency care, chronic care, navigation/case management, disease prevention and wellness, behavioral health/substance abuse prevention, and general.[ 22 , 23 , 25 ] Each cohort had a set number of projects that involve meeting patient care milestones and metrics, simultaneously incorporating IOM’s six patient care aims of medical care being safe, efficient, effective, patient centered, timely, and equitable [ 22 , 23 , 24 , 25 ].
DSRIP, with all its projects implemented in the adopted regions and counties has been measured to improve population health [ 25 ]. A metric of measuring improvement in population health within the DSRIP program was preventable hospitalization rate [ 24 ]. The decrease in preventable hospitalization rates may have been attributed to the inherent design and dynamics of the DSRIP policy [ 23 , 24 ]. Those dynamics comprised of factors such as physician-administrator collaboration, mechanisms of incentive payments, types of measures for reporting outcomes in quality, and interplaying healthcare externalities [ 24 ]. In the adopted regions and counties, a statistically significant decrease in preventable hospitalization rates was observed when tested with an interrupted time series method [ 25 ].
There were two phases of the Texas DSRIP program, DSRIP 1.0 and 2.0. It was in DSRIP 2.0 that comprehensive Diabetes Care: eye exam metric improved by 16 % while Influenza immunization improved by 12 % in the latter [ 27 ]. Researchers Revere et al. have identified that in DSRIP 2.0, the metrics for Central Line Associated Bloodstream Infection (CLABSI) rates, Catheter Associated Urinary Tract Infections (CAUTI), and Surgical Site Infection (SSI) rates improved by 26 %, 10 %, and 9 %, respectively [ 27 ].
Quadruple aim framework: focusing on the evolution of the triple aim
The Triple Aim, formulated in 2008, drew focus on three aims which were based on care, cost, and health. Sikka and colleagues, in 2015, constructed a fourth aim, improving the experience of providing care. This was made to acknowledge the importance of physicians, nurses, and all employees in “finding joy and meaning in their work and in doing so improving the experience of providing care” [ 28 ]. At the core of the fourth aim is the experience of joy and meaning in providing care making it synonymous with acquiring accomplishment and meaning in their contributions. The Quadruple Aim has broad implications in theory and practice factoring inclusiveness in terms of all members in the healthcare workforce [ 28 ].
Hospital-Acquired conditions reduction program: focusing on patient safety
The Hospital-Acquired Conditions Reduction Program (HACRP) is a Medicare pay-for-performance program that supports the CMS’ long-standing effort to link Medicare payments to healthcare quality in the inpatient hospital setting [ 29 ]. HACRP focuses on specific conditions that the Centers for Disease Control and Prevention (CDC) National Healthcare Safety Network (NHSN) healthcare- associated infection (HAI) measures which are: [ 30 ] (1) Central Line Associated Blood Stream Infection (CLABSI), (2) Catheter Associated Urinary Tract Infection (CAUTI), (3) Surgical Site Infection (SSI) for colon and hysterectomy, (4) Methicillin-Resistant Staphylococcus Aureus (MRSA) bacteremia, (5) Clostridium Difficile Infection (CDI).
Additionally, eight Patient Safety Indicators (PSIs) included in the program comprise of: [ 31 ] (1) PSI 03 - Pressure Ulcer Rate, (2) PSI 06 - Iatrogenic Pneumothorax Rate (3) PSI 07 - Central Venous Catheter-Related Bloodstream Infection Rate, (4) PSI 08 - Postoperative Hip Fracture Rate, (5) PSI 12 - Perioperative Pulmonary Embolism or Deep Vein Thrombosis Rate, (6) PSI 13 - Postoperative Sepsis Rate, (7) PSI 14 - Postoperative Wound Dehiscence Rate, (8) PSI 15 - Accidental Puncture or Laceration Rate.
Hospital readmissions reduction program: focusing on patient safety
The Hospital Readmissions Reduction Program (HRRP) is a Medicare value-based purchasing program that reduces payments to hospitals with excess readmissions. The program supports the national goal of improving healthcare by linking payment to the quality of hospital care [ 32 ]. HRRP has a specific focus on the following conditions to reduce readmissions that in turn improve patient safety [ 32 ]. Those conditions are as follows: [ 32 ] (1) Acute Myocardial Infarction (AMI), (2) Chronic Obstructive Pulmonary Disease (COPD), (3) Heart Failure (HF), (4) Pneumonia (5) Coronary Artery Bypass Graft (CABG) surgery, and (6) Elective Primary Total Hip Arthroplasty and/or Total Knee Arthroplasty (THA/TKA) [ 32 ].
The purpose of the present review was to analyze patient safety through the lens of the above quality management frameworks. We, specifically, illuminated policies and laws such as EMTALA, informed consent, informed refusal, and living will as examples. In emergency cases, the rules of EMTALA apply whereas in non-emergency cases, the same applies to obtaining informed consent from the patient. In the event the patient refuses treatment, documenting the informed refusal would be ideal. We underscored selective new prototype policies percolating from national policymaking to institutional levels with a focus on the initiatives the system has actively taken to preserve patient safety and promote improvement in care delivery.
Facts about The Joint Commission. Retrieved from https://www.jointcommission.org/about_us/about_the_joint_commission_main.aspx . Accessed 16 Feb 2021
Malcolm Baldrige Award. Retrieved from https://baldrigefoundation.org/ . Accessed 16 Feb 2021
Magnet Recognition Criteria. Retrieved from https://www.mghpcs.org/PCS/Magnet/Documents/Education_Toolbox/01_Intro-Ovrvw/Magnet-Overview-2017.pdf . Accessed 16 Feb 2021.
The Joint Commission is the recognized global leader of healthcare accreditation and offers an unbiased assessment of quality achievement in patient care and safety Retrieved from: https://www.jointcommission.org/accreditation-and-certification/why-the-joint-commission/ . Accessed 16 Feb 2021
The Malcolm Baldrige National Quality Award being the nation's highest presidential honor for performance excellence. Retrieved from: https://asq.org/quality-resources/malcolm-baldrige-national-quality-award . Accessed 16 Feb 2021
The Magnet Recognition Program’s alignment of nursing strategic goals to improve the organization’s patient outcomes. Retrieved from: https://www.nursingworld.org/organizational-programs/magnet/ . Accessed 16 Feb 2021
The Institute of Medicine Committee on Quality of Health Care in America. Crossing the quality chasm: A new health system for the 21st century. Washington, DC: National Academies Press (US); 2001.
Google Scholar
Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff. 2008;27(3):759–69.
Article Google Scholar
Page K. The four principles: Can they be measured and do they predict ethical decision making? BMC Med Ethics. 2012;13(1):10.
Landau R, Osmo R. Professional and personal hierarchies of ethical principles. Int J Soc Welfare. 2003;12(1):42–9.
Mitchell PH. Defining patient safety and quality care. In: Hughes RG, editor Patient safety and quality: An evidence-based handbook for nurses. Rockville: Agency for Healthcare Research and Quality (US); 2008. Chapter 1. NBK2681.
Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) Survey of Patient Perspectives. Retrieved from https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/HospitalHCAHPS . Accessed 16 Feb 2021.
Consumer Assessment of Healthcare Providers and Systems (CAHPS) Survey assessing patient experience. Retrieved from https://www.ahrq.gov/cahps/about-cahps/cahps-program/index.html . Accessed 16 Feb 2021
National Practitioner Data Bank Web site. Retrieved from: https://www.npdb.hrsa.gov/topNavigation/aboutUs.jsp . Accessed 16 Feb 2021.
Emergency Medical Treatment and Labor Act (EMTALA). Retrieved from https://www.acep.org/life-as-a-physician/ethics--legal/emtala/emtala-fact-sheet/ . Accessed 16 Feb 2021.
Showalter JS. The Law of Healthcare Administration. Eighth Edition. Chicago, Washington, DC: Health Administration Press; 2017.
Boland GL. The doctrines of lack of consent and lack of informed consent in medical procedures in Louisiana. La L Rev. 1984;45:1.
Alexis O, Caldwell J. Administration of medicines–the nurse’s role in ensuring patient safety. Brit J Nurs. 2013;22(1):32–5.
Wagner RF Jr, Torres A, Proper S. Informed consent and informed refusal. Dermatol Surg. 1995;21(6):555–9.
Ridley DT. Informed consent, informed refusal, informed choice–what is it that makes a patient’s medical treatment decisions informed? Med Law. 2001;20(2):205–14.
CAS PubMed Google Scholar
Emanuel L. Living wills can help doctors and patients talk about dying. West J Med. 2000;173(6):368–9. https://doi.org/10.1136/ewjm.173.6.368 .
Article CAS PubMed PubMed Central Google Scholar
Shenoy A, Revere L, Begley C, Linder S, Daiger S. The Texas DSRIP program: An exploratory evaluation of its alignment with quality assessment models in healthcare. Int J Healthcare Manage. 2017;12(2):165–72. https://doi.org/10.1080/20479700.2017.1397339 .
Begley C, Hall J, Shenoy A, Hanke J, Wells R, Revere L, Lievsay N. Design and implementation of the Texas Medicaid DSRIP program. Popul Health Manag. 2017;20(2):139–45.
Shenoy A, Begley C, Revere L, Linder S, Daiger SP. Delivery system innovation and collaboration: A case study on influencers of preventable hospitalizations. Int J Healthcare Manage. 2017:1–8. DOI: https://doi.org/10.1080/20479700.2017.14057772017.1405777
Shenoy AG, Begley CE, Revere L, Linder SH, Daiger SP. Innovating patient care delivery: DSRIP’s interrupted time series analysis paradigm. Healthcare. 2019;7(1):44–50. https://doi.org/10.1016/j.hjdsi.2017.11.004 .
Article PubMed Google Scholar
Shenoy AG. DSRIP’s innovation and collaboration in population health management: A cross-sectional segmented time series model. Health Serv Manage Res. 2020;33(1):2–12. https://doi.org/10.1177/0951484819868679 .
Revere L, Kavarthapu N, Hall J, Begley C. Achieving triple aim outcomes: An evaluation of the Texas medicaid waiver. Inquiry. 2020;57:46958020923547. https://doi.org/10.1177/0046958020923547 .
Sikka R, Morath JM, Leape L. The Quadruple aim: care, health, cost and meaning in work. BMJ Qual Safety. 2015;24:608–10.
Hospital Acquired Conditions Reduction Program. Retrieved from https://www.cms.gov/Medicare/Medicare-Fee-for-ServicePayment/AcuteInpatientPPS/HAC-Reduction-Program . Accessed 16 Feb 2021.
HACRP List of Conditions. Retrieved from https://qualitynet.cms.gov/inpatient/hac Accessed 16 Feb 2021.
List of PSI 90. Retrieved from https://www.qualityindicators.ahrq.gov/Modules/PSI_TechSpec_ICD10_v2020.aspx . Accessed 16 Feb 2021
Hospital Readmissions Reduction Program. Retrieved from https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program . Accessed 16 Feb 2021
Download references
Acknowledgements
Not applicable.
Conflict of interest and financial disclosure statement
The authors collectively declare that there is no conflict of interest and have no financial interests with any sponsoring organization, for-profit or not-for-profit.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
College of Public Affairs, School of Health and Human Services, Healthcare Administration Program, University of Baltimore, 1420 N. Charles Street, MD, 21201, Baltimore, USA
Amrita Shenoy
You can also search for this author in PubMed Google Scholar
Contributions
The author read and approved the final manuscript. AS conducted the literature search and review, drafted the manuscript and responded the reviewer's comments.
Authors' information
Dr. Amrita Shenoy is an Assistant Professor of Healthcare Admin-istration at the University of Baltimore and the Winner of the 2011 McGraw-Hill/Irwin Distinguished Paper Award. She leverageseconometrics to quantify policy impact and qualitatively exploreshealthcare laws and policies for a deeper comprehension of its ana-lytical spectra. Dr. Shenoy received her PhD from the University ofTexas Health Science Center at Houston School of Public Health,MHA/MBA from the University of Houston — Clear Lake and MScfrom Nottingham Trent University, United Kingdom. Her researchareas spotlight topics in healthcare law, policy, and quality with abroad emphasis on public health and healthcare management.
Corresponding author
Correspondence to Amrita Shenoy .
Ethics declarations
Ethics approval and consent to participate.
Ethics approval and consent to participate on data involving the use of any animal/human tissue Not applicable
Consent for publication
Not applicable
Competing interests
The authors have no competing interests to declare.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Shenoy, A. Patient safety from the perspective of quality management frameworks: a review. Patient Saf Surg 15 , 12 (2021). https://doi.org/10.1186/s13037-021-00286-6
Download citation
Received : 04 January 2021
Accepted : 23 February 2021
Published : 22 March 2021
DOI : https://doi.org/10.1186/s13037-021-00286-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Quality management frameworks
- IOM’s six aims for improvement
- IHI’s Triple Aim
- Patient safety
- Patient centeredness
- High‐quality clinical care
Patient Safety in Surgery
ISSN: 1754-9493
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Search Menu
- Advance articles
- Editor's Choice
- Supplements
- French Abstracts
- Portuguese Abstracts
- Spanish Abstracts
- Author Guidelines
- Submission Site
- Open Access
- About International Journal for Quality in Health Care
- About the International Society for Quality in Health Care
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Contact ISQua
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, conclusions, data availability statement, appendix a. medline search strategy.
- < Previous
Clinical handover and handoff in healthcare: a systematic review of systematic reviews
- Article contents
- Figures & tables
- Supplementary Data
Melissa Desmedt, Dorien Ulenaers, Joep Grosemans, Johan Hellings, Jochen Bergs, Clinical handover and handoff in healthcare: a systematic review of systematic reviews, International Journal for Quality in Health Care , Volume 33, Issue 1, 2021, mzaa170, https://doi.org/10.1093/intqhc/mzaa170
- Permissions Icon Permissions
The purpose of this systematic review is to appraise and summarize existing literature on clinical handover.
We searched EMBASE, MEDLINE, Database of Abstracts of Reviews of Effects and Cochrane Database of Systematic Reviews.
Included articles were reviewed independently by the review team.
The review team extracted data under the following headers: author(s), year of publication, journal, scope, search strategy, number of studies included, type of studies included, study quality assessment, used definition of handover, healthcare setting, outcomes measured, findings and finally some comments or remarks.
First, research indicates that poor handover is associated with multiple potential hazards such as lack of availability of required equipment for patients, information omissions, diagnosis errors, treatment errors, disposition errors and treatment delays. Second, our systematic review indicates that no single tool arises as best for any particular specialty or use to evaluate the handover process. Third, there is little evidence delineating what constitutes best handoff practices. Most efforts facilitated the coordination of care and communication between healthcare professionals using electronic tools or a standardized form. Fourth, our review indicates that the principal teaching methods are role-playing and simulation, which may result in better knowledge transfer to the work environment, better health and patients’ well-being.
This review emphasizes the importance of staff education (including simulation-based and team training), non-technical skills and the implementation process of clinical handover in healthcare settings.
In recent years, patient safety has picked up momentum. Within the field of patient safety research, there is a focus on the incidence of adverse events. Patient harm is not only estimated to be the 14th leading cause of the global disease burden, but it is also associated with negative repercussions for healthcare professionals and increasing healthcare costs for the hospital and society [ 1 ]. Handover is a process central to the delivery of high-quality and safe care. It is defined as ‘the transfer of professional responsibility and liability for some or all aspects of care for a patient or patient group, to another person or a professional group on a temporary or permanent basis’ [ 2 ]. The transfer of essential information from one healthcare provider to another is an integral component of healthcare [ 3 ].
Handover has been recognized internationally as a high-risk area for patient safety, and the call for interventions to improve the handover process has increased. For example, patient handover is one of the World Health Organization’s top five priorities [ 4 ], and it is furthermore included as a patient safety parameter by the Joint Commission on Accreditation of Healthcare [ 5 ] and the Australian Medical Association [ 2 ]. Consequently, several interventions to improve the handover process have been launched. These interventions usually target process standardization (e.g. SBAR, P-VITAL, SURPASS and SHARED), training or education, changes to the physical environment and technology [ 6 ].
The importance of patient handover becomes evident from the number of preventable incidents and adverse events resulting from poor communication between healthcare professionals [ 7 , 8 ]. Moreover, suboptimal handover quality is a persisting deficit in safety culture evaluations [ 9 , 10 ]. Several factors contribute to ineffective handover, including an increase in workload [ 11 ], lack of policies and standard protocols [ 12 ], team awareness [ 13 ], communication breakdowns [ 14 ], lack of training [ 15 ], infrastructure [ 13 ], attitude and organizational culture [ 16 ].
Despite the importance of the handover process and the clear need for improvement, the availability of effective interventions is limited [ 17 , 18 ]. This systematic review aims to appraise and summarize existing literature on this topic and give a comprehensive, thorough and objective picture of ‘clinical handover’.
We performed a systematic review of systematic reviews on clinical handover in healthcare settings. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines to identify, screen, select and include papers [ 19 ]. We also carefully read Smith et al .’s (2011) article on methods in conducting a systematic review of systematic reviews of healthcare interventions [ 20 ].
Search strategy
We searched EMBASE, MEDLINE, Cochrane Database of Systematic Reviews and the Database of Abstracts of Reviews of Effects, using a search strategy (see Appendix A ). The search strategy was initially created for MEDLINE (see Appendix A ) and subsequently adapted for the other databases. No review protocol exists. We decided on the eligibility criteria a priori. We only included systematic literature reviews regarding the handover process. We considered studies of handover in all healthcare settings. There were no restrictions based on publication date or language. We only included articles with English abstracts.
Study selection
The identified studies in the searches were de-duplicated. The first author (M.D.) reviewed the abstracts for compliance with the eligibility criteria and their relevance for inclusion. In consultation with a third reviewer, the review team (M.D. and D.U.) independently reviewed the resulting full-text articles. The review team included one person with methodological experience and one person with expertise on the topic. If there were differences in opinion about eligibility, these were resolved by mutual agreement, and if this was not reached, an external opinion was sought (J.B.). We also conducted a manual search of the reference lists of reviews captured by the initial inquiry to minimize the risk of missing relevant reviews.
Data extraction
We extracted data out of the included articles using a predefined form in Microsoft Excel under the following headers: author(s), year of publication, journal, scope of review, search strategy, number of studies included, type of studies included, study quality assessment, used definition of handover, healthcare setting/context, outcomes measured, findings and finally some comments or remarks. To conceptualize and evaluate the success of the various implementation strategies to improve handover, we used the model of Proctor et al . (2011) to categorize the type of outcomes by implementation (e.g. acceptability), service (e.g. effectiveness) or client (e.g. satisfaction) outcomes [ 21 ]. The second author (D.U.) then cross-checked and confirmed all abstracted data after an independent review. In case of disagreement, we reached a consensus through discussion. We descriptively analysed all data extracted from the reviews. We could not conduct a meta-analysis due to the heterogeneity of the reviews and their included studies.
Quality assessment
Two reviewers (M.D. and D.U.) independently assessed the quality of included reviews using the (Assessing the Methodological Quality of Systematic Reviews (AMSTAR 2) Checklist.
We retained 168 potentially relevant articles. After a critical assessment of title and abstract, we selected 22 papers for full-text evaluation, of which 6 were excluded. A hand search of the reference lists resulted in the addition of three publications. Finally, we considered 19 papers for further analysis [ 22–40 ]. See PRISMA flow chart ( Figure 1 ) for a detailed overview of the study selection. The majority of excluded studies were narrative reviews or had no relevance to this systematic review’s scope (see Table 1 ).

PRISMA flow chart.
Article characteristics
Table 2 represents the results of the critical appraisal using the AMSTAR 2 tool. The high risk of bias rating was due to no adequate investigation of publication bias [ 17 , 22 , 24–30 , 32–39 , 41 ], no satisfactory technique for assessing the risk of bias in individual studies [ 17 , 22 , 24–30 , 32–39 , 41 ], no list of excluded studies and justification of exclusion [ 17 , 22 , 24–30 , 32–39 , 41 ], no explanation for the selection of the study designs [ 17 , 22 , 24–30 , 32 , 34–38 , 41 ] and, finally, no protocol establishing the review method prior to the conduct [ 17 , 22 , 24–30 , 33 , 34 , 37–39 , 41 ]. All reviews indicated a relatively poor methodological quality of their included studies.
Results on the critical appraisal of the included reviews using AMSTAR 2
Review characteristics
We present the review characteristics in Table 3 . The reviews were published between 2009 [ 25 ] and 2018 [ 30 , 34 ]. The total number of studies included in the reviews ranged from 10 [ 25 , 29 ] to 95 [ 39 ]. One review did not include any studies [ 31 ]. Most reviews included various study designs such as pre-/post-test interventions [ 17 , 25 , 27 , 29 , 30 , 32 , 34–37 ], Randomized controlled trials RCTs [ 17 , 25 , 30 , 32 , 34–37 ] and cross-sectional designs [ 17 , 33 , 39 ]. Only one review included only RCTs [ 41 ].
We included various healthcare settings: primary care and/or the interface with secondary care [ 34 , 38 , 39 , 41 ] and hospital-based care [ 17 , 25–37 , 39 ], including postoperative handovers [ 28 , 33 ], surgical handovers [ 27 ], handover between clinical departments [ 37 ], handover between paramedics and the emergency department [ 17 ], and handover between the intensive care unit and general wards [ 32 ]. Only one review included a variety of healthcare environments [ 39 ]. Four studies focused on trainees to evaluate specific educational interventions targeting the handover process [ 22 , 24 , 29 , 30 ].
The reviews included various outcomes (see Table 4 ), which we organized—according to the model of Proctor et al . (2011)—in implementation, service and patient outcomes [ 21 ]. Two articles included implementation outcomes and, more specifically, the perception among stakeholders that a given intervention is satisfactory [ 26 , 39 ]. Several patient outcomes were also measured: satisfaction, empowerment, adverse events, length of stay, unplanned readmissions and mortality [ 17 , 25–32 , 34 , 35 , 39 , 41 ]. However, most outcomes referred to the service level of the improvement interventions (e.g. information transfer, technical errors, teamwork, handover duration, hospital use and continuity of care).
Outcomes organized according to the model of Proctor et al . (2011)
Characteristics of handover and potential hazards
The most commonly used definition of ‘clinical handover’ was the one of the British Medical Association (2004): ‘the transfer of professional responsibility and accountability for some or all aspects of care for a patient, or group of patients, to another person or professional group on a temporary or permanent basis’ [ 2 ].
Lack of active listening, lack of attention or divided attention of the receiving healthcare professional(s), workload and lack of time were the leading causes of poor handover [ 38 ]. Handover effectiveness was associated with the availability of appropriate personnel to receive the handover and a positive relation between healthcare professionals [ 38 ]. Most frequently cited barriers to effective handovers were communication barriers, problems associated with standardization, environmental issues, equipment issues, a lack or misuse of time, lack of training or education, human factors and difficulties related to the complexity of cases or high caseloads [ 39 ].
Poor handover is associated with multiple potential hazards such as lack of availability required equipment for patients, information omissions, diagnosis errors, treatment errors, disposition errors, treatment delays, delays in pending tests and diagnostic results, and failure to verify that the correct patient was transported [ 26 ].
Feedback or assessment tools
Research indicated that feedback or assessment improves the content or organization measures of handover, while process and professionalism measures are less reliably improved [ 36 ]. The most commonly described method to provide feedback on the handover process was the one-on-one approach, often accompanied by significant reductions in medical errors and better documentation of diagnostic tests/results [ 36 ].
All existing evaluation tools on handover quality were used in an inpatient setting [ 36 , 37 ] and mainly focused on information transfer. Some also assessed clinical task performance, non-technical skills and nursing satisfaction with handover quality [ 33 ]. Most tools were observational, and the extent to which their validity and reliability have been evaluated was variable [ 33 ]. No single tool arises as best for any particular specialty or use, but the Handoff CEX—or tools based on it—was the most widely studied tool [ 36 ]. The most frequently used handover activity-related outcome measures were information gaps, handover duration, number of patients handed off, interruptions, care quality, frequency of tool use, handoff efficiency, user satisfaction metrics and length of shift-report [ 37 ].
Interventions to improve handover
If possible, we detailed the effects of interventions in Table 5 by linking specific improvement strategies to specific results. Often-cited strategies to improve the handover process were liaison nurses [ 26 , 32 ], standardization strategies [ 17 , 25–27 , 32 , 34 , 35 , 38 , 39 ], technological solutions [ 27 , 35 , 41 ], training and education [ 35 ] and staff involvement [ 41 ]. Improvements in information transfer were the most commonly reported successes, being found in more than half of the studies. Staff satisfaction was the next most commonly improved [ 35 ]. Additionally, interventions such as handover protocols, checklists and team training improved metrics of effectiveness (i.e. decreased technical errors and information omissions), efficiency (i.e. reduced handover duration, or time to complete specific tasks) and perceived teamwork [ 33 ].
Results of possible handover interventions implemented
Mnemonics—an alphabetical listing technique that aids information retention—were mostly used to standardize the handover process [ 28 ]. In a review of handover mnemonics, 14 different mnemonics were identified, of which SBAR was most frequently described [ 39 ]. In general, the use of standardized written reports resulted in greater accuracy and consistency of information transfer, increased necessary elements communicated during handover, decreased numbers of dropped tasks, saved nursing time, increased healthcare professionals’ and patients’ satisfaction, improved teamwork and nursing knowledge and increased patient empowerment [ 17 , 25–28 , 32 , 38 , 39 ]. Although Müller et al . (2018) concluded some evidence of the effectiveness of SBAR implementation on patient outcomes, this evidence is limited to specific circumstances such as communication over the phone. Only half of the studies found significant improvements in eight patient outcomes: international normalized ratio values, critical incident reporting system events (communication errors), unexpected death and ICU admission, patient falls and 30-day readmissions, transfers to hospital and avoidable hospitalizations [ 34 ].
The included reviews evaluated several other strategies to improve handover. The implementation of a liaison nurse was found to improve communication and coordination of care [ 26 , 32 ], and computerized tools were associated with a reduction of pre-rounding time and frequency of missed patients [ 27 ]. The Six Sigma methodology and TeamSTEPPS resulted in reduced handover time and time required to access lab study results. The ‘pit-stop’ model was associated with a significant decrease in handover omissions [ 26 , 27 ]. Precisely at the interface between primary and secondary care, medication reconciliation, electronic tools, discharge planning, shared involvement in follow-up by hospital and community care providers, use of electronic discharge notifications and web-based access to discharge information for general practitioners had a significant effect in reducing hospital use, in improving continuity of care (e.g. accurate discharge information) and in enhancing patient status after discharge (e.g. satisfaction) [ 41 ].
Only a few studies found positive effects of handover interventions on patient outcomes. Robertson et al . (2014) found only one study (out of 29) that reported a 12% decrease in adverse events due to standardization. Another study reported a significant reduction in the length of stay due to implementation of an electronic handover system [ 35 ]. Moller et al . (2013) also found one study (out of 28) that demonstrated a significant reduction in the length of stay, in-hospital mortality, and unplanned admission as a result of a structured handover form included in the clinical pathway [ 28 ]. Pucher et al. (2015) found only one study (out of 19) that reported a reduction in the length of stay from 5 to 4 days after the implementation of a computerized template [ 27 ]. Arora et al . (2009) found that a structured format or a technology solution resulted in a drop in preventable adverse events by 1.2% [ 25 ]. Finally, van Sluisveld et al . (2015) found that liaison nurses and handover forms effectively reduced preventable adverse events [ 32 ].
Educational interventions to improve handover
The principal teaching methods were role-play and simulation, which were better received by learners than in didactic sessions [ 24 , 29 , 30 ]. Popular content themes were facilitating information management and communication skills (i.e. specific handover techniques such as mnemonics and electronic tools), reducing the potential for errors (i.e. identifying the components of effective and ineffective handovers) and improving provider confidence (i.e. ensuring that participants felt comfortable challenging or requesting additional information from others) [ 22 , 29 , 30 ].
Results on educational interventions’ outcomes indicated that learners’ knowledge and skills had transferred to the work environment. The health and well-being of patients had improved, and residents felt more confident in their handover. The latter often resulted in improved reporting of patient information and reduced errors of omission [ 29 , 30 ].
Effective communication is essential to ensure patient safety. Research linked poor handover to inaccurate diagnoses, delayed treatments, medical incidents, patient falls, more extended hospital stays, lower satisfaction rates for patients and healthcare staff and increased costs [ 42–45 ]. We aimed to appraise and summarize existing literature on ‘clinical handover’.
First, research indicated that poor handover is associated with multiple potential hazards such as lack of availability of required equipment for patients, information omissions, diagnosis errors, treatment errors, disposition errors, treatment delays, delays in pending tests and diagnostic results and failure in verifying that the correct patient was transported. Identified problems for handover were lack of active listening, lack of attention, divided attention of the receiving healthcare professional(s), workload and lack of time. A significant challenge that healthcare systems face is an increase in patients with chronic conditions, which leads to more healthcare transitions. A review of 21 clinical handover studies from Wood et al . (2015) raised concerns about the quality of communication and information exchange between prehospital and hospital staff [ 38 ]. Handover outside the hospital is becoming increasingly important due to an increase in number of chronic patients, a decrease of the length of hospital stay and an increase in proportion of outpatient care. As a result, more and more patients experience multiple transitions during their trajectory, and discontinuity in their care may threaten patient safety. An additional challenge is the different perspectives among healthcare providers in differing settings regarding the most crucial information to be exchanged in handover [ 46 ].
Second, most evaluation tools measured handoff activity-related outcomes such as information gaps, handover duration, number of patients handed off, interruptions, care quality, frequency of tool use, handoff efficiency, user satisfaction metrics and length of shift-report. Our systematic review indicates that no single tool arises as best to evaluate the handover process. In their review of the handoff literature, Patterson and Wears (2010) were also not able to make recommendations for using any particular standardized, reliable measurement tool suitable for all clinical areas [ 43 ].
Third, there is little evidence delineating what constitutes the best handover practices. Most efforts primarily aim to facilitate care coordination and communication between healthcare professionals by using electronic tools or a standardized form. Reported success in handover improvements seems to be limited to specific projects and is mostly limited to efficiency outcomes or surrogate patient safety measures such as saved nursing time, increased healthcare professionals’ and patients’ satisfaction, improved teamwork, improved nursing knowledge and greater patient empowerment. Only a few individual studies found positive effects on patient outcomes such as adverse events, readmissions and mortality. One hopeful prospective intervention study of a resident handoff-improvement program in nine hospitals (including a mnemonic to standardize verbal and written handovers, handoff and communication training, faculty development and observation program and a sustainability campaign) showed a decrease of 23% in medical-error rate and a 30% reduction in the number of preventable adverse events in 10.740 patient admissions [ 47 ].
The SBAR model and its adaptations remain the only model employed across various specialties. While many reviews continue to advocate using mnemonics in the handover process, the evidence for their usefulness is inconclusive. Local modifications to improve acceptance and adoption by healthcare professionals are always required. One additional challenge of standardizing handoff information is the variation in clinical practice between disciplines and settings. Therefore, a common ground between disciplines is necessary to improve interdisciplinary communication, including practical information (e.g. bed location), background information (e.g. the reason for admission), planning information (e.g. discharge plan) and safety information (e.g. allergies) in an electronic health record [ 48 ].
Although research is inconclusive about interventions to improve handover, studies showed that good handover is essential to reduce potential hazards. Therefore, all healthcare professionals must learn and maintain excellent handover skills as poor handover is associated with multiple potential risks. Our systematic review indicates that the principal teaching methods are role-playing and simulation, which are better received by learners than didactic sessions and which may result in better knowledge transfer to the work environment, better health and well-being of patients, increased confidence of healthcare professionals in their handover, which again results in improved reporting of patient information and reduced errors of omission. Handover education is often non-existent or inadequate, and frameworks often lack or vary greatly [ 49 , 50 ].
Based on the results of our systematic review, we formulate several recommendations. We recommend harmonizing terminology and definitions for describing handover, improvement methods or types of outcomes. Second, our review has demonstrated that there is a limited amount of good-quality research on handover. Failure to show effects may be that the included studies are of little quality and yield heterogeneous results. We suggest implementing more feasible study designs such as pragmatic trials and high-fidelity simulation studies, large-scale studies with comparison groups and no simultaneous implementation of multiple interventions for outcome measures. Finally, assessment, ongoing feedback and training or education are essential components for improving handover [ 36 ]. We recommend curricular interventions designed for mixed audiences as handover is a rather heterogeneous activity and requires a multidisciplinary approach. We emphasize on a combination of lectures, simulation-based workshops and case-based discussions, with a move towards more competency-based training.
Our review has a few limitations. First, there may be primary studies in the field that are not included by existing reviews. Second, as with all systematic reviews, publication bias may be present. Finally, studies’ heterogeneity—both in methodology and interventions—limits the conclusions drawn.
Clinical handover is an essential element of healthcare, ensuring continuity of care for the patient. Our systematic review found that poor handover is associated with multiple potential hazards. No single tool arises as best to evaluate the handover process. There is little empirical evidence delineating what constitutes best handover practices. Finally, our review found that healthcare professionals receive little formal training in this critical responsibility. The interpretation of our findings is tempered by a profound lack of methodological quality in current published evidence. We recommend emphasizing staff education (including simulation-based and team training), non-technical skills and the implementation process.
This work was supported by the SafePAT-project and was being executed within the context of Interreg V-A Euregion Meuse-Rhine, with funding from the European Regional Development Fund, Province of Limburg and Hasselt University.
Data and research materials supporting the results in the article are available.
(hand over? or handing over or handover? or hand-over?)
(shift to shift?)
(inter shift?)
(shift report? or shift-report?)
(handoff? or handing off or hand off?)
(signout? or sign out? or sign off?)
(patient transfer?)
(systematic review or literature review)
Slawomirski L , Auraaen A , Klazinga NS . The economics of patient safety . OECD 2017 ; 26 :96.
Google Scholar
British Medical Association . Handoffs: implications for nurses . In: Mary Ann Friesen , Susan V. White , Jacqueline F. Byers , Ronda G Hughes (eds). Patient Safety and Quality: An Evidence-Based Handbook for Nurses . Rockville (MD) : Agency for Healthcare Research and Quality (US) , 2008 April Chapter 34.
Google Preview
Hughes RG , Friesen MA , White SV et al. Handoffs: Implications for Nurses . Rockville, MD : Agency for Healthcare Research and Quality (US) , 2008 .
Lawton R , McEachan RRC , Giles SJ et al. Development of an evidence-based framework of factors contributing to patient safety incidents in hospital settings: a systematic review . Qual Saf Health Care 2012 ; 21 : 369 – 80 .
Kluger MT , Bullock MFM . Recovery room incidents: a review of 419 reports from the Anaesthetic Incident Monitoring Study (AIMS) . Anaesthesia 2002 ; 57 : 1060 – 6 .
Vlayen A , Hellings J , Claes N et al. A nationwide hospital survey on patient safety culture in Belgian hospitals: setting priorities at the launch of a 5-year patient safety plan . Qual Saf Health Care 2012 ; 21 : 760 – 7 .
Desmedt M , Bergs J , Willaert B et al. ( February 1 , 2018 ) Exploring and evaluating patient safety culture in a community-based primary care setting . J Patient Saf . Publish ahead of print:1. doi: 10.1097/PTS.0000000000000458.
Barach P , Philibert I . The July effect: fertile ground for systems improvement . Ann Int Med Am Coll Phys 2011 ; 155 : 331 – 2 .
Iedema R , Merrick ET , Kerridge R et al. Handover—Enabling Learning in Communication for Safety (HELiCS): a report on achievements at two hospital sites . Med J Aust 2009 ; 190 : S133 – 6 .
Siemsen IMD , Madsen MD , Pedersen LF et al. Factors that impact on the safety of patient handovers: an interview study . Scand J Public Health 2012 ; 40 : 439 – 48 .
Berkenstadt H , Haviv Y , Tuval A et al. Improving handoff communications in critical care: utilizing simulation-based training toward process improvement in managing patient risk . Chest 2008 ; 134 : 158 – 62 .
Horwitz LI , Moin T , Green ML . Development and implementation of an oral sign-out skills curriculum . J Gen Intern Med 2007 ; 22 : 1470 – 4 .
Arora VM , Johnson JK , Meltzer DO et al. A theoretical framework and competency-based approach to improving handoffs . Qual Saf Health Care 2008 ; 17 : 11 – 14 .
Research Priority Setting Working Group . Global Priorities for Research in Patient Safety . Geneva : World Health Organization , 2008 .
Harris J , Schmitt L . National patient safety goals guide safe care . J Nurs Care Qual 2004 ; 19 :88.
Riesenberg LA , Berg K , Berg D et al. Resident and attending physician perception of maladaptive response to stress in residents . Med Educ Online 2014 ; 19 :25041.
Manser T , Foster S . Effective handover communication: an overview of research and improvement efforts . Best Pract Res Clin Anaesthesiol 2011 ; 25 : 181 – 91 .
Cohen MD , Hilligoss PB . The published literature on handoffs in hospitals: deficiencies identified in an extensive review . Qual Saf Health Care 2010 ; 19 : 493 – 7 .
Moher D , Liberati A , Tetzlaff J et al. For the PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement . BMJ 2009 ; 339 : b2535 – 5 .
Smith V , Devane D , Begley CM et al. Methodology in conducting a systematic review of systematic reviews of healthcare interventions . BMC Med Res Methodol BioMed Cent 2011 ; 11 : 1 – 6 .
Proctor EK , Landsverk J , Aarons G et al. Implementation research in mental health services: an emerging science with conceptual, methodological, and training challenges . Adm Policy Ment Health 2008 ; 36 : 24 – 34 .
Buchanan IM , Besdine RW . A systematic review of curricular interventions teaching transitional care to physicians-in-training and physicians . Acad Med 2011 ; 86 : 628 – 39 .
Foster S , Manser T . The effects of patient handoff characteristics on subsequent care: a systematic review and areas for future research . Acad Med 2012 ; 87 : 1105 – 24 .
Masterson MF , Gill RS , Turner SR et al. A systematic review of educational resources for teaching patient handover skills to resident physicians and other healthcare professionals . Can Med Educ J 2013 ; 4 : e96 – 110 .
Arora VM , Manjarrez E , Dressler DD et al. Hospitalist handoffs: a systematic review and task force recommendations . J Hosp Med 2009 ; 4 : 433 – 40 .
Ong M-S , Coiera E . A systematic review of failures in handoff communication during intrahospital transfers . Jt Comm J Qual Patient Saf 2011 ; 37 : 274 – 84 .
Pucher PH , Johnston MJ , Aggarwal R et al. Effectiveness of interventions to improve patient handover in surgery: a systematic review . Surgery 2015 ; 158 : 85 – 95 .
Møller TP , Madsen MD , Fuhrmann L et al. Postoperative handover: characteristics and considerations on improvement: a systematic review . Eur J Anaesthesiol 2013 ; 30 : 229 – 42 .
Gordon M , Findley R . Educational interventions to improve handover in health care: a systematic review . Med Edu 2011 ; 45 : 1081 – 9 .
Gordon M , Hill E , Stojan JN et al. Educational interventions to improve handover in health care: an updated systematic review . Acad Med 2018 ; 93 : 1234 – 44 .
Smeulers M , Lucas C , Vermeulen H . Effectiveness of different nursing handover styles for ensuring continuity of information in hospitalised patients. Cochrane Effective Practice and Organisation of Care Group . Cochrane Database Syst Rev 2014 ; 43 :CD009979.
van Sluisveld N , Hesselink G , van der Hoeven JG et al. Improving clinical handover between intensive care unit and general ward professionals at intensive care unit discharge . Intensive Care Med 2015 ; 41 : 589 – 604 .
Segall N , Bonifacio AS , Schroeder RA et al. Can we make postoperative patient handovers safer? A systematic review of the literature . Anesth Analg 2012 ; 115 : 102 – 15 .
Müller M , Jürgens J , Redaèlli M et al. Impact of the communication and patient hand-off tool SBAR on patient safety: a systematic review . BMJ Open 2018 ; 8 :e022202.
Robertson ER , Morgan L , Bird S et al. Interventions employed to improve intrahospital handover: a systematic review . Qual Saf Health Care 2014 ; 23 : 600 – 7 .
Davis J , Roach C , Elliott C et al. Feedback and assessment tools for handoffs: a systematic review . J Grad Med Educ 2017 ; 9 : 18 – 32 .
Abraham J , Kannampallil T , Patel VL . A systematic review of the literature on the evaluation of handoff tools: implications for research and practice . J Am Med Inform Assoc 2014 ; 21 : 154 – 62 .
Wood K , Crouch R , Rowland E et al. Clinical handovers between prehospital and hospital staff: literature review . Emerg Med J 2015 ; 32 : 577 – 81 .
Riesenberg LA , Leitzsch J , Cunningham JM . Nursing handoffs: a systematic review of the literature . Am J Nurs 2010 ; 110 :24–34–quiz35–6.
Hesselink G , Zegers M , Vernooij-Dassen M et al. Improving patient discharge and reducing hospital readmissions by using Intervention Mapping . BMC Health Serv Res 2014 ; 14 :389.
Hesselink G , Schoonhoven L , Barach P et al. Improving patient handovers from hospital to primary care: a systematic review . Ann Int Med Am Coll Phys 2012 ; 157 : 417 – 28 .
Jeffcott SA , Evans SM , Cameron PA et al. Improving measurement in clinical handover . Qual Saf Health Care 2009 ; 18 : 272 – 7 .
Patterson ES , Wears RL . Patient handoffs: standardized and reliable measurement tools remain elusive . Jt Comm J Qual Patient Saf 2010 ; 36 : 52 – 61 .
Mardis T , Mardis M , Davis J et al. Bedside shift-to-shift handoffs: a systematic review of the literature . J Nurs Care Qual 2016 ; 31 : 54 – 60 .
Gordon M . Non-technical skills training to enhance patient safety . Clin Teach 2013 ; 10 : 170 – 5 .
Hellesø R , Lorensen M , Sorensen L . Challenging the information gap—the patients transfer from hospital to home health care . Int J Med Inform 2004 ; 73 : 569 – 80 .
Starmer AJ , Spector ND , Srivastava R et al. Changes in medical errors after implementation of a handoff program . N Engl J Med 2014 ; 371 : 1803 – 12 .
Collins SA , Stein DM , Vawdrey DK et al. Content overlap in nurse and physician handoff artifacts and the potential role of electronic health records: a systematic review . J Biomed Inform 2011 ; 44 : 704 – 12 .
Lee J , Mast M , Humbert J et al. Teaching handoff communication to nursing students: a teaching intervention and lessons learned . Nurse Educ 2016 ; 41 : 189 – 93 .
Gaffney S , Farnan JM , Hirsch K et al. The Modified, Multi-patient Observed Simulated Handoff Experience (M-OSHE): assessment and feedback for entering residents on handoff performance . J Gen Intern Med 2016 ; 31 : 438 – 41 .
Author notes
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
- transition of care
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1464-3677
- Print ISSN 1353-4505
- Copyright © 2024 International Society for Quality in Health Care and Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Open access
- Published: 05 December 2019
Networked information technologies and patient safety: a protocol for a realist synthesis
- Justin Keen 1 ,
- Joanne Greenhalgh 2 ,
- Rebecca Randell 3 ,
- Peter Gardner 4 ,
- Justin Waring 5 ,
- Roberta Longo 1 ,
- Jon Fistein 1 ,
- Maysam Abdulwahid 1 ,
- Natalie King 1 &
- Judy Wright 1
Systematic Reviews volume 8 , Article number: 307 ( 2019 ) Cite this article
1782 Accesses
3 Citations
1 Altmetric
Metrics details
There is a widespread belief that information technologies will improve diagnosis, treatment and care. Evidence about their effectiveness in health care is, however, mixed. It is not clear why this is the case, given the remarkable advances in hardware and software over the last 20 years. This review focuses on interoperable information technologies, which governments are currently advocating and funding. These link organisations across a health economy, with a view to enabling health and care professionals to coordinate their work with one another and to access patient data wherever it is stored. Given the mixed evidence about information technologies in general, and current policies and funding, there is a need to establish the value of investments in this class of system. The aim of this review is to establish how, why and in what circumstances interoperable systems affect patient safety.
A realist synthesis will be undertaken, to understand how and why inter-organisational systems reduce patients’ clinical risks, or fail to do so. The review will follow the steps in most published realist syntheses, including (1) clarifying the scope of the review and identifying candidate programme and mid-range theories to evaluate, (2) searching for evidence, (3) appraising primary studies in terms of their rigour and relevance and extracting evidence, (4) synthesising evidence, (5) identifying recommendations, based on assessment of the extent to which findings can be generalised to other settings.
The findings of this realist synthesis will shed light on how and why an important class of systems, that span organisations in a health economy, will contribute to changes in patients’ clinical risks. We anticipate that the findings will be generalizable, in two ways. First, a refined mid-range theory will contribute to our understanding of the underlying mechanisms that, for a range of information technologies, lead to changes in clinical practices and hence patients’ risks (or not). Second, many governments are funding and implementing cross-organisational IT networks. The findings can inform policies on their design and implementation.
Systematic review registration
PROSPERO CRD42017073004
Peer Review reports
There is a widespread belief, particularly among policy makers, that information technologies will improve diagnosis, treatment and care [ 1 ]. Evidence about the effectiveness of a range of IT applications is, however, mixed [ 2 , 3 , 4 , 5 ]. A comprehensive review by Brenner and colleagues covered a number of technologies and focused on safety-related end-points including mortality, infection rates and medication error rates [ 2 ]. Twenty-five out of 69 studies included in their review reported a statistically significant positive effect. The other 44 studies reported no or negative effects. More recent systematic reviews report broadly similar findings [ 6 , 7 ]. Authors stress that evidence is uneven in both coverage and quality, and care needs to be taken in interpreting the findings, but the general picture is clear.
There is, therefore, a need to understand why the evidence has, to date, fallen short of expectations. We seek to shed light on the question in this systematic review. It focuses on the effects of a particular class of IT systems, interoperable systems, on patient safety. The term ‘system’ here refers to the combination of technologies and the people who used them. In a well-designed system, technologies are seamlessly integrated into users’ working practices. In poorly designed systems, in contrast, the technologies do not fit easily into users’ working practices, and in the worst cases can make it more difficult to deliver safe treatment and care. The term ‘interoperable’ refers to the ability of any two or more IT systems to exchange data, and for the receiving system to make use of the data. The IT systems of interest in this protocol allow professionals to access patients’ records held in other organisations.
Policy makers’ assumptions
Many people who live in their own homes, and who are frail or have chronic health problems, need support from a range of professionals. These include general (or family) practitioners, community nurses, therapists, social workers, and planned and emergency hospital services. There is good evidence that treatment and care is often fragmented [ 8 , 9 , 10 ]. Linking IT systems across a health economy can, policy makers reason, help to improve the coordination of services. Professionals can, the thinking goes, use the IT networks to communicate with one another, and hence effectively coordinate a patient’s treatment and care. The value of this function might be particularly evident at transition points, for example in a health emergency or at the point of returning from hospital to home. The IT systems can also be designed to enable access to all parts of a patient’s record, so that professionals can search for and locate information wherever it is held. The Obama administration in the USA committed considerable sums to linking hospital, family physician, pharmacy and other IT systems. Similarly, and more recently, the National Health Service in England has emphasised the importance of linking hitherto fragmented IT systems across organisations in local health economies [ 11 , 12 ].
- Realist synthesis
This protocol describes a realist synthesis, which seeks to understand how and why inter-organisational IT systems reduce patients’ clinical risks, or fail to do so. The realist synthesis method involves opening up the ‘black box’ of events that lie between an intervention and its effects [ 13 , 14 ]. It does so by identifying programme theories: these are sequences of decisions and actions that capture the intended effects of an intervention, and the underlying logic that links them together. A number of initial theories are typically identified.
Literature searches are then designed. It is not usually feasible to identify evidence about all of the sequences in all of the theories. Searches therefore focus on two or three theories, or on key sequences within those theories. Empirical evidence is then identified, assessed and synthesised, in order to evaluate the actual steps in the sequences. The evidence can, in addition, lead teams to realise that a programme is effective, but not in the way originally envisaged. A programme theory therefore needs to be reformulated. More searches may then be needed, to evaluate elements of the revised theory. That is, the cycle of programme theory formulation and evaluation can be iterative and—resources permitting—pursued until a settled, evidence-based account has been identified [ 15 ].
There are literatures that will enable us to develop and evaluate programme theories [ 16 ]. For example, the computer-supported cooperative work literature sits at the intersection of computer science and psychology, and is a source of evidence about the inter-relationships of IT systems and peoples’ working practices. A comprehensive review suggests that we have a reasonable understanding of the design features and organisational settings that are associated with the effective integration of systems into clinical working practices [ 17 ]. Similarly, there are literatures on health professionals’ decisions and actions, and their consequences for patients’ clinical risks. In particular, there is a substantial literature based on retrospective analysis of adverse events, both in health care and other sectors [ 18 ].
This protocol has been written in accordance with PRISMA-P guidelines [ 19 ].
We will undertake a realist synthesis. The aim of the study is to establish how, why and in what circumstances networked, inter-organisational IT systems affect patient safety. The objectives of the study are to:
Identify initial programme theories and prioritise theories to review;
Search systematically for evidence to test and refine the theories;
Undertake quality appraisal and use included texts to support, refine or reject programme theories;
Synthesise the findings;
Disseminate the findings to a range of audiences.
The following sections focus on the first four objectives.
Stage 1: Identification of programme and mid-range theories
This stage is essentially developmental in nature. The first step in this review will be to construct one or more programme theories, concerning the use of networked IT systems and their effects on patient safety. Mid-range theories, which are usefully thought of as a broader class of theory than programme theories, and which will be used to generalise findings at the end of the review, will also be identified. We will supplement our existing knowledge of candidate theories with literature searches to define and develop them. The programme theories will determine the scope (inclusion and exclusion criteria) for the searches and synthesis in the following stages, which will be used to support, refine or reject each theory examined.
Search strategy and information resources
Theories can be explicitly mentioned in research articles and policy documents, or they can be implied in the introductory or discussion sections of documents. They can also be found in commentaries and opinion pieces. We will use conventional literature searching using free text words, synonyms and subject index terms, and some CLUSTER searching techniques (identifying a few key relevant studies and finding further relevant studies via forwards and backwards citation searches, author searches, searching for reports of a particular project) [ 20 ]. Using this combined approach, we aim to identify literature that leads us to theories or fragments of theories that can be used to construct programme and mid-range theories.
We anticipate that three searches will supplement the policy documents and academic literature already known to the project team. MEDLINE (1946–present) and EMBASE (1947–present) will be searched as a core set of databases for all of the theory generating searches.
Background search of systematic reviews . We will search for systematic reviews that link IT systems and patient safety. This will identify any reviews on this topic that have been published since our scoping review, undertaken before the start of the project. The reviews may describe programme theories or fragments in their introduction or discussion sections, providing insights into the sequences of events linking the intervention and outcomes. We will search the core databases plus the Cochrane Database of Systematic Reviews, the Epistemonikos database and Health Systems Evidence (McMaster University). An example MEDLINE search strategy containing a full set of search terms is available in the Additional file 1 (Search 1).
Policies, opinion pieces and research reports . We will search the core databases plus Health Management Information Consortium (1983–present) and Web of Science - Science Citation Index (1990–present) for policy documents, opinion pieces (e.g. editorials) and reports describing leading theories about the relationships between IT systems and patient safety (Additional file 1 Search 2). We will also undertake Google searches to locate reports on key policies, e.g. about the Health Information Technology for Economic and Clinical Health (HITECH) Act 2009, a major US initiative promoting the implementation of cross-organisational IT networks.
Author search and Citation search . We will search for reports and articles authored by influential commentators in the core databases plus Health Management Information Consortium (1983–present), Web of Science - Science Citation Index (1990–present), Google Scholar and Scopus (1823–present). Literature by David Bates, the most cited author in the health informatics literature, and Robert Wachter, the author of an influential report on IT in the NHS in England, will be searched (Additional file 1 Search 3). Citation searching may be required due to the iterative nature of developing searches for a realist synthesis.
Inclusion, analysis and synthesis
The records identified in the searches will be saved and managed in an EndNote library. Details of all search activities (databases, websites, date of search, number of records found, search strategies) will be recorded in a timeline spreadsheet. The inclusion criteria for the three searches will be:
IT networks that link two or more organisations outside (but possibly including) hospitals;
IT networks that support direct treatment and care;
Arguments that identify relationships between IT networks and patient safety;
Published in the English language between 2000 and 2018.
Non-English language articles will not be included. The project does not have sufficient resources to translate the full text of articles that may be relevant. In conventional systematic reviews, it is not necessary to translate whole papers, as it is only necessary to identify defined data. In this review, however, we will be looking for data that might occur anywhere in a paper, and a full translation would be needed.
The exclusion criteria will be for studies that:
Describe hospital-only IT systems;
Describe systems that do not link two or more distinct services;
Focus on IT systems that support secondary uses of data, e.g for service planning, research;
are published before 2000;
are published in languages other than English.
Two reviewers (MA and JK) will first independently screen the titles and abstracts of the records for relevance and then assess full-text reports. Any discrepancies will be solved by discussion, and if needed consultation with a third author (JG or RR). Data extraction forms will be developed to capture basic details of studies—authors, publication year, etc.—and passages on theories and theory fragments.
The selected studies will be used to develop visual representations of programme theories, with accompanying text that explains the reasoning that underpins those theories. Experience gained in earlier reviews suggests that there are likely to be several programme theories at this point, and that some of them will be partial, in the sense that the chains of reasoning are ‘high level’ and not fully articulated, or only cover some of the steps linking networked information technologies and patient safety. Where available, claims about the reasons why programmes succeed or fail in practice will be used to annotate the representations.
The programme theories will be used as the basis for consultation with three groups of stakeholders—policy makers, senior IT managers and frontline clinicians. We will use the nominal group technique, which has been used in a previous realist study [ 21 ]. At the nominal group meetings participants will be asked to comment critically on the programme theories, on the basis of their knowledge and experience. They will also be asked to develop and then prioritise theories, or particular chains of reasoning within theories, for further study. The prioritisation will take into account the potential to provide learning for the NHS, and the types of networked systems that NHS organisations are implementing. The groups will be re-convened for consultation by email at the end of stage 4 (see below).
The outputs of the three groups will be further reviewed by a patient and public involvement (PPI) group, and discussed with our project steering group. Following these meetings, we will decide on the programme theories, and key elements of those theories, that we will explore in depth in stages 2–4.
Stage 2: Systematic search for evidence
The next stage of the review is a search for empirical studies to test and refine the leading programme theories identified in stage 1. The initial searches will be designed to identify evidence about the steps in the chains of reasoning in each theory. Literatures often focus on one or other section in a chain of reasoning, and as a result, individual searches will often focus on sections rather than a whole programme theory [ 22 ]. We will undertake searches using resources which span health and computing literatures including—but not limited to—MEDLINE (1946–present), EMBASE (1947–present), Web of Science Core Collection (1900–present) and INSPEC (1896–present).
We anticipate hand searching papers from leading conferences which are not indexed, for example Software Engineering in Healthcare workshops papers, from the International Conference on Software Engineering. The search strategies for identifying empirical evidence for programme theories can only be fully developed once the programme theories are agreed. However, we anticipate they will contain search concepts for an aspect of patient safety such as medication reconciliation, inter-organisational IT networks and evaluative studies.
The search results will be reviewed in stage 3 (evidence review, see below) and further searches will develop iteratively to follow lines of enquiry. Initial searches may not identify empirical evidence that supports or rejects a programme theory. If that happens, the search will be re-designed to capture empirical evidence that may be found in a different discipline or information resource. For example, we could extend the scope of our searches to look for evidence about other IT applications, including hospital-based systems, and/or extend the scope of the populations of interest. As in stage 1, these searches may use CLUSTER search techniques as an efficient method for finding relevant papers [ 20 ].
The results of the electronic searches and all references that are retrieved for stage 2 will be kept in the same EndNote library as those found during stage 1. Details of all search activities during stage 2 will be recorded in the timeline spreadsheet.
Stage 3: Evidence review and quality appraisal
Titles and abstracts of records identified, and the full-text papers selected in stage 2, will be independently screened by two reviewers (MA and JK) to identify those which contain evidence that sheds light on one or more elements of the programme theories identified in stage 1. The RAMESES I guidance states that:
An appraisal of the contribution of any section of data (within a document) should be made on two criteria: • Relevance – whether it can contribute to theory building and/or testing; and • Rigour – whether the method used to generate that particular piece of data is credible and trustworthy [ 14 ].
We will follow Rycroft-Malone and colleagues in developing criteria for judging relevance [ 23 ]. We will, further, use the mid-range theory developed in stage 1 to refine the criteria. Rigour refers to the requirement for an investigation to be of sufficient standard within type , whether that is a process evaluation, an ethnography or other type of study [ 14 ].
The empirical data for supporting and/or refuting programme theories will be extracted from the included studies. It is anticipated that a significant proportion of the evidence will be in the form of narrative data and will accordingly be copied into Word files. To maximise accuracy and transparency, a proportion of data extraction will be performed independently by two members of the research team.
Stage 4: Synthesis
Synthesis involves two distinct, but linked, activities. In the first, the empirical evidence identified in stages 2 and 3 will be used to evaluate the programme theories developed in stage 1. In the most straightforward case, the evidence will support the chains of reasoning in one programme theory and serve to reject alternative or competing theories. Less straightforwardly, the evidence might provide support for one part of a programme theory and adverse evidence for another part of the same theory. Or, it might not ‘fit’ a programme theory, neither supporting nor undermining it. Both instances suggest that there may be a problem with the programme theory itself and will lead us to refine it, to achieve a better fit between evidence and theory. On the basis of experience of earlier realist syntheses, we expect that at least one of the selected theories—or theory fragments—will be reasonably well supported by empirical evidence, and at least one will not be not supported.
Second, when a settled programme theory or theories have been produced, they will be interpreted in the broader context of the mid-range theory. This involves abduction, where inferences that lead to the best available explanation are identified. The details of the abductive reasoning processes vary from review to review, but a key point is that it involves inter-play between situation-specific programme theories and broader mid-range theory [ 24 ]. In the simplest case, the (now evidence-based) programme theories will be consistent with the mid-range theory. If we find that a programme theory holds across a number of settings (e.g. different combinations of health services and/or different patient groups), this will increase our confidence in it. Alternatively, evidence or argument (or both) may point in different directions, and the wider project team will use the mid-range theory to ‘adjudicate’ between contending programme theories.
Nominal group email consultation
The nominal groups will be re-convened for email consultation. We will summarise our findings to this point, including our provisional syntheses, and present them to the groups. They will be asked to comment on the findings, including whether any of the theories can be rejected, and whether any further searches are merited. The PPI group will also meet at the end of this stage and review the findings and interpretations of the three nominal groups. We will refine our interpretations on the basis of the comments of all four groups.
Policy makers in many countries believe that IT systems can contribute to safer patient care. Interoperable systems are currently being promoted by governments, and funding made available for their development, in many countries. As noted above, though, the empirical evidence about this belief is mixed, and it is not clear why this is the case. We are using the realist synthesis method because we believe that it will shed light on how and why interoperable systems contribute to reductions in patients’ clinical risks, and hence help to explain why the evidence is so mixed.
As with any evidence synthesis, there are risks and limitations associated with the method. The most obvious risk, in common with other review methods that focus on effectiveness, is that we are not able to identify high-quality effectiveness evidence. This will necessarily limit the extent to which we are able to explain ‘what works’ when interoperable systems are deployed. Another significant risk is the flip side of a potential strength. The explicit inclusion of theory in the method means that the basis for interpreting the available evidence is clear. But, there must be a risk that—however much care is taken—a sub-optimal theoretical framework will be used, and valuable insights foregone. If we are able to mitigate these risks in the course of the review, though, it should produce two main outcomes. First, the refined mid-range theory will contribute to our understanding of the underlying mechanisms that lead to changes in clinical practices and hence patients’ risks (or not). Second, many governments are funding and implementing cross-organisational IT networks. The findings can be used to inform policies on their design and implementation.
Availability of data and materials
This is a protocol for a systematic review. We will make all searches, and the results of those searches, available publicly when the review is completed. This will, again, require clearance by NIHR—but we do not anticipate any problems, given the nature of the study.
Agboola SO, Bates DW, Kvedar JC. Digital health and patient safety. JAMA. 2016;315(16):1697–8.
Article CAS Google Scholar
Brenner SK, et al. Effects of health information technology on patient outcomes: a systematic review. J Am Med Inform Assoc. 2016;23(5):1016–36.
Article Google Scholar
Black AD, et al. The impact of eHealth on the quality and safety of health care: a systematic overview. PLoS Med. 2011;8(1):e1000387.
Kruse CS, Beane A. Health information technology continues to show positive effect on medical outcomes: systematic review. J Med Internet Res. 2018;20(2):e41.
Chaudhry B, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144(10):742–52.
Feldman SS, Buchalter S, Hayes LW. Health information technology in healthcare quality and patient safety: literature review. JMIR Med Inform. 2018;6(2):e10264.
Garavand A, et al. Factors influencing the adoption of health information technologies: a systematic review. Electron Physician. 2016;8(8):2713–8.
O’Hara JK, Aase K, Waring J. Scaffolding our systems? Patients and families ‘reaching in’ as a source of healthcare resilience. BMJ Qual Saf. 2019;28(1):3–6.
Care Quality Commission, Building bridges, breaking barriers. Newcastle upon Tyne: CQC, 2017.
Leigh Johnston DR, Dorrans S, Dussin L. A.T. Barker, Integration 2020: Scoping research. p. 2017.
Jacob JA. On the road to interoperability, public and private organizations work to connect health care data. JAMA. 2015;314(12):1213–5.
Bates DW. Health information technology and care coordination: the next big opportunity for informatics? Yearb Med Inform. 2015;10(1):11–4.
Pawson R. Evidence Based Policy: A Realist Perspective. London: Sage; 2006.
Book Google Scholar
Wong G, et al. Development of methodological guidance, publication standards and training materials for realist and meta-narrative reviews: the RAMESES (Realist And Meta-narrative Evidence Syntheses – Evolving Standards) project. Health Serv Del Res. 2014;2:30.
Google Scholar
Pawson R. The Science of Evaluation: A Realist Manifesto. London: Sage; 2013.
Pollock N, Williams R. Software and organisations. The biography of the enterprise-wide system or how SAP conquered the world. London: Routledge; 2009.
Fitzpatrick G, Ellingsen G. A review of 25 years of CSCW research in healthcare: contributions, challenges and future agendas. Comput Supp Coop Work (CSCW). 2013;22(4-6):609–65.
Vincent, C. and R. Amalberti, Safety strategies in hospitals, in Safer Healthcare. SpringerOpen, 2016, p. 73-91.
Moher D, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Booth A, et al. Towards a methodology for cluster searching to provide conceptual and contextual “richness” for systematic reviews of complex interventions: case study (CLUSTER). BMC Med Res Methodol. 2013;13:118.
Tolson D, et al. Developing a managed clinical network in palliative care: a realistic evaluation. Int J Nurs Stud. 2007;44(2):183–95.
Ball SL, Greenhalgh J, Roland M. Referral management centres as a means of reducing outpatients attendances: how do they work and what influences successful implementation and perceived effectiveness? BMC Fam Pract. 2016;17:37.
Rycroft-Malone J, et al. Improving skills and care standards in the support workforce for older people: a realist review. BMJ Open. 2014;4(5):e005356.
Pawson R. Evidence and policy and naming and shaming. Policy Stud. 2002;23(3):211–30.
Download references
Acknowledgements
This project is funded by the NIHR Health Services and Delivery Research programme, project 16/53/03. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
National Institute for Health Research, Health Services and Delivery Research programme, project 16/53/03.
Author information
Authors and affiliations.
Leeds Institute of Health Sciences, University of Leeds, Worsley Building, Clarendon Way, Leeds, LS2 9NL, England
Justin Keen, Roberta Longo, Jon Fistein, Maysam Abdulwahid, Natalie King & Judy Wright
School of Sociology and Social Policy, University of Leeds, Leeds, England
Joanne Greenhalgh
School of Healthcare, University of Leeds, Leeds, England
Rebecca Randell
School of Psychology, University of Leeds, Leeds, England
Peter Gardner
Health Services Management Centre, University of Birmingham, Birmingham, England
Justin Waring
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to writing the paper. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Justin Keen .
Ethics declarations
Ethics approval and consent to participate.
Ethics letter provided.
Consent for publication
The National Institute for Health Research operates a ‘negative consent’ model, whereby papers are submitted and if—after 30 days—no objections are raised, consent is deemed to have been given. This draft paper has been submitted to NIHR.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1..
Search strategies to identify reports for generating theory.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Keen, J., Greenhalgh, J., Randell, R. et al. Networked information technologies and patient safety: a protocol for a realist synthesis. Syst Rev 8 , 307 (2019). https://doi.org/10.1186/s13643-019-1223-1
Download citation
Received : 13 February 2019
Accepted : 06 November 2019
Published : 05 December 2019
DOI : https://doi.org/10.1186/s13643-019-1223-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Patient safety
- Information technology
- Systems integration
- Health information exchange
- Health services research
- Systematic review
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Help & FAQ
Patient safety risks associated with telecare: A systematic review and narrative synthesis of the literature
Research output : Contribution to journal › Article › Research › peer-review
Background: Patient safety risk in the homecare context and patient safety risk related to telecare are both emerging research areas. Patient safety issues associated with the use of telecare in homecare services are therefore not clearly understood. It is unclear what the patient safety risks are, how patient safety issues have been investigated, and what research is still needed to provide a comprehensive picture of risks, challenges and potential harm to patients due to the implementation and use of telecare services in the home. Furthermore, it is unclear how training for telecare users has addressed patient safety issues. A systematic review of the literature was conducted to identify patient safety risks associated with telecare use in homecare services and to investigate whether and how these patient safety risks have been addressed in telecare training. Methods: Six electronic databases were searched in addition to hand searches of key items, reference tracking and citation tracking. Strict inclusion and exclusion criteria were set. All included items were assessed according to set quality criteria and subjected to a narrative synthesis to organise and synthesize the findings. A human factors systems framework of patient safety was used to frame and analyse the results. Results: 22 items were included in the review. 11 types of patient safety risks associated with telecare use in homecare services emerged. These are in the main related to the nature of homecare tasks and practices, and person-centred characteristics and capabilities, and to a lesser extent, problems with the technology and devices, organisational issues, and environmental factors. Training initiatives related to safe telecare use are not described in the literature. Conclusions: There is a need to better identify and describe patient safety risks related to telecare services to improve understandings of how to avoid and minimize potential harm to patients. This process can be aided by reframing known telecare implementation challenges and user experiences of telecare with the help of a human factors systems approach to patient safety.
- Human factors
- Narrative synthesis
- Patient safety
- Systematic review
This output contributes to the following UN Sustainable Development Goals (SDGs)
Access to Document
- 10.1186/s12913-014-0588-z Licence: CC BY
Other files and links
- Link to publication in Scopus
T1 - Patient safety risks associated with telecare
T2 - A systematic review and narrative synthesis of the literature
AU - Guise, Veslemøy
AU - Anderson, Janet
AU - Wiig, Siri
N1 - Funding Information: We would like to thank Grete Mortensen, special librarian at the University of Stavanger, for valuable assistance during the literature search process. We would also like to acknowledge The Research Council of Norway for funding the Safer@Home research project of which this study is a part (grant number 210799) and thank our partners in the project. Publisher Copyright: © 2014 Guise et al.; licensee BioMed Central Ltd.
PY - 2014/11
Y1 - 2014/11
N2 - Background: Patient safety risk in the homecare context and patient safety risk related to telecare are both emerging research areas. Patient safety issues associated with the use of telecare in homecare services are therefore not clearly understood. It is unclear what the patient safety risks are, how patient safety issues have been investigated, and what research is still needed to provide a comprehensive picture of risks, challenges and potential harm to patients due to the implementation and use of telecare services in the home. Furthermore, it is unclear how training for telecare users has addressed patient safety issues. A systematic review of the literature was conducted to identify patient safety risks associated with telecare use in homecare services and to investigate whether and how these patient safety risks have been addressed in telecare training. Methods: Six electronic databases were searched in addition to hand searches of key items, reference tracking and citation tracking. Strict inclusion and exclusion criteria were set. All included items were assessed according to set quality criteria and subjected to a narrative synthesis to organise and synthesize the findings. A human factors systems framework of patient safety was used to frame and analyse the results. Results: 22 items were included in the review. 11 types of patient safety risks associated with telecare use in homecare services emerged. These are in the main related to the nature of homecare tasks and practices, and person-centred characteristics and capabilities, and to a lesser extent, problems with the technology and devices, organisational issues, and environmental factors. Training initiatives related to safe telecare use are not described in the literature. Conclusions: There is a need to better identify and describe patient safety risks related to telecare services to improve understandings of how to avoid and minimize potential harm to patients. This process can be aided by reframing known telecare implementation challenges and user experiences of telecare with the help of a human factors systems approach to patient safety.
AB - Background: Patient safety risk in the homecare context and patient safety risk related to telecare are both emerging research areas. Patient safety issues associated with the use of telecare in homecare services are therefore not clearly understood. It is unclear what the patient safety risks are, how patient safety issues have been investigated, and what research is still needed to provide a comprehensive picture of risks, challenges and potential harm to patients due to the implementation and use of telecare services in the home. Furthermore, it is unclear how training for telecare users has addressed patient safety issues. A systematic review of the literature was conducted to identify patient safety risks associated with telecare use in homecare services and to investigate whether and how these patient safety risks have been addressed in telecare training. Methods: Six electronic databases were searched in addition to hand searches of key items, reference tracking and citation tracking. Strict inclusion and exclusion criteria were set. All included items were assessed according to set quality criteria and subjected to a narrative synthesis to organise and synthesize the findings. A human factors systems framework of patient safety was used to frame and analyse the results. Results: 22 items were included in the review. 11 types of patient safety risks associated with telecare use in homecare services emerged. These are in the main related to the nature of homecare tasks and practices, and person-centred characteristics and capabilities, and to a lesser extent, problems with the technology and devices, organisational issues, and environmental factors. Training initiatives related to safe telecare use are not described in the literature. Conclusions: There is a need to better identify and describe patient safety risks related to telecare services to improve understandings of how to avoid and minimize potential harm to patients. This process can be aided by reframing known telecare implementation challenges and user experiences of telecare with the help of a human factors systems approach to patient safety.
KW - Homecare
KW - Human factors
KW - Narrative synthesis
KW - Patient safety
KW - Systematic review
KW - Telecare
UR - http://www.scopus.com/inward/record.url?scp=84988602909&partnerID=8YFLogxK
U2 - 10.1186/s12913-014-0588-z
DO - 10.1186/s12913-014-0588-z
M3 - Article
C2 - 25421823
AN - SCOPUS:84988602909
SN - 1472-6963
JO - BMC Health Services Research
JF - BMC Health Services Research
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 12, Issue 2
- Barriers and facilitators to improving patient safety learning systems: a systematic review of qualitative studies and meta-synthesis
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0003-3139-5829 Hassan Assem Mahmoud 1 , 2 ,
- http://orcid.org/0000-0003-4738-8447 Kednapa Thavorn 3 ,
- Sunita Mulpuru 4 ,
- Daniel McIsaac 5 , 6 ,
- Mohamed A Abdelrazek 7 ,
- Amr Assem Mahmoud 8 ,
- http://orcid.org/0000-0003-2942-2891 Alan J Forster 5 , 9
- 1 Epidemiology , University of Ottawa Faculty of Medicine , Ottawa , Ontario , Canada
- 2 Public Health , Canadian Red Cross , Ottawa , Ontario , Canada
- 3 Epidemiology and Preventive Medicine , University of Ottawa , Ottawa , Ontario , Canada
- 4 Respirology , Ottawa Hospital General Campus , Ottawa , Ontario , Canada
- 5 Clinical Epidemiology Program , Ottawa Hospital Research Institute , Ottawa , Ontario , Canada
- 6 Anesthesiology and Pain Medicine , University of Ottawa , Ottawa , Ontario , Canada
- 7 Radiology Department , London Health Sciences Centre , London , Ontario , Canada
- 8 Public Health and Community Medicine , Cairo University Kasr Alainy Faculty of Medicine , Cairo , Egypt
- 9 Department of Medicine , University of Ottawa Faculty of Medicine , Ottawa , Ontario , Canada
- Correspondence to Dr Hassan Assem Mahmoud; dr.hassanassem{at}hotmail.com
Background The implementation and continuous improvement of patient safety learning systems (PSLS) is a principal strategy for mitigating preventable harm to patients. Although substantial efforts have sought to improve these systems, there is a need to more comprehensively understand critical success factors. This study aims to summarise the barriers and facilitators perceived by hospital staff and physicians to influence the reporting, analysis, learning and feedback within PSLS in hospitals.
Methods We performed a systematic review and meta-synthesis by searching MEDLINE (Ovid), EMBASE (Ovid), CINAHL, Scopus and Web of Science. We included English-language manuscripts of qualitative studies evaluating effectiveness of the PSLS and excluded studies evaluating specific individual adverse events, such as systems for tracking only medication side effects, for example. We followed the Joanna Briggs Institute methodology for qualitative systematic reviews.
Results We extracted data from 22 studies, after screening 2475 for inclusion/exclusion criteria. The included studies focused on reporting aspects of the PSLS, however, there were important barriers and facilitators across the analysis, learning and feedback phases. We identified the following barriers for effective use of PSLS: inadequate organisational support with shortage of resources, lack of training, weak safety culture, lack of accountability, defective policies, blame and a punitive environment, complex system, lack of experience and lack of feedback. We identified the following enabling factors: continuous training, a balance between accountability and responsibility, leaders as role models, anonymous reporting, user-friendly systems, well-structured analysis teams, tangible improvement.
Conclusion Multiple barriers and facilitators to uptake of PSLS exist. These factors should be considered by decision makers seeking to enhance the impact of PSLS.
Ethics and dissemination No formal ethical approval or consent were required as no primary data were collected.
- Healthcare quality improvement
- Incident reporting
- Medication safety
- Patient safety
Data availability statement
No data are available.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjoq-2022-002134
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Patient safety learning systems (PSLSs) are viewed as an important tool for improving the quality and outcomes of hospital-based care.
WHAT THIS STUDY ADDS
This review identifies barriers and facilitators for different stages of system implementation.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our findings can inform decision makers in establishing or improving their PSLS. Further research can build on these results.
Patient safety learning systems (PSLSs) support healthcare staff in documenting patient safety events and concerns, facilitate immediate corrective actions including communication, and promote the learning and improvement required to prevent future similar occurrences. 1 Use of PSLS involves four related and sequential efforts 2 : (1) structured reporting, (2) collation of data, (3) analysis and (4) learning from the reported incident. 3 In their 1999 report, the Institute of Medicine strongly recommended the use of incident reporting as a means to improve patient safety.
The success of PSLS in improving patient safety is debatable, 4 as errors and adverse events continue to occur in all healthcare settings. It is also important to note that using percentage of reported incidents as a measure of improvement after implementing PSLS might be unreliable as the true number of incidents (the denominator) is unknown because of under-reporting. 4 Although there are no published reports demonstrating a reduction in adverse events, many studies have evaluated the adoption of PSLS. 5 These studies identified factors associated with improving the use of PSLS, but tended to focus on the reporting of events rather than the factors improving the downstream actions of analysis and learning. As these later steps are the actions that will lead to improved safety, it is important that they are addressed in efforts to improve PSLS. 1 To address this potential gap, we conducted a systematic review to identify the perceived barriers and facilitators for effective PSLS adoption in hospital settings. We assessed factors related to all phases of PSLS, not just the reporting of events. A fulsome description of all aspects of PSLS will help design future studies, support our understanding of ways to improve the effectiveness of PSLS, and enable hospitals and health systems to assess their own efforts. To guide our qualitative evaluation, we meta-characterised factors based on the Patient Safety Toolkit developed by the Canadian Patient Safety Institute (CPSI) ( figure 1 ). 6
- Download figure
- Open in new tab
- Download powerpoint
Shows the different steps in the patient safety management toolkit developed by the CPSI (5). CPSI, Canadian Patient Safety Institute.
To synthesise the large array of qualitative findings in the literature, we first identified an overarching conceptual framework for safety reporting and response; then we mapped the factors related to the barriers and facilitators to this framework. This inductive approach resulted in the development of emerging themes. A protocol was registered a priori (CRD42021220504). 7

Search strategy
An experienced medical librarian (LS) was consulted to develop a search strategy. The strategy was piloted in MEDLINE (Ovid), and then translated to include EMBASE (Ovid), CINAHL, Scopus and Web of Science. We manually searched Google Scholar, Grey Literature Reports, the CPSI and the WHO for unpublished studies. The multifield search query we used in our study is shown in online supplemental appendix 1 .
Supplemental material
We used the PICO approach (suited to qualitative systematic reviews and meta-synthesis) for selecting our primary studies. 8 The participants in these studies were healthcare professionals, quality and safety experts. According to the criteria discussed below, we included qualitative studies that investigated the problem of the continuous occurrence of patient safety incidents and the factors perceived to be responsible for ineffective PSLS.
Inclusion criteria
Studies published in English and discussing the perceived barriers and/or facilitators to the use of PSLS in hospitals were included. No restrictions on the type of incidents, demographic or geographical areas, sampling, or type of participant were applied.
Exclusion criteria
We excluded studies that evaluated the effectiveness of PSLS, those focused on a single incident and those examining the effect of a specific factor on PSLS. Studies investigating the criteria for reporting incidents and the trends in doing so were also excluded.
Study screening and selection
Two reviewers (HAM and MAA) independently screened the titles, abstracts and full text (in that sequence) of citations using Covidence software. 9 Any disagreement between the two reviewers was resolved through discussion with a third reviewer (AAM).
Data extraction
Data were extracted into an Excel spreadsheet for analysis, including the following data points: study characteristics (authors, year of publication, journal); methodology (design, research purpose and/or questions, method of analysis); participant characteristics; country in which the study took place; setting; population descriptors; sample size; barriers, facilitators and conclusions reported. We used the qualitative assessment and review instrument for qualitative data extraction tool from the Joanna Briggs Institute (see online supplemental appendix 2 ). 10 Data were extracted by one reviewer (HAM), and the second (MAA) verified the extracted data. Disagreements were resolved through discussion with a third reviewer (AAM).
Assessment of methodological quality
The purpose of assessing methodological quality for qualitative research is to describe the overall robustness and validity of the findings. It is not to be used to weight results or support the exclusion of studies as it is possible to find important themes from research with relatively weak designs. We; therefore, assessed quality using the Critical Appraisal Skills Programme (CASP) Qualitative Checklist (see online supplemental appendix 3 : CASP Qualitative Checklist) by two independent reviewers (HAM and MAA), and any disagreement was resolved through discussion with a third researcher (AAM). However, this process did not lead to any studies being excluded from the analysis.
Guiding framework
The Patient Safety Toolkit 6 is derived from the best available evidence and expert advice, and can be tailored for any healthcare setting. As such, we thought it might help us identify barriers to the success of PSLS, and used it as a framework for summarising our findings. This framework is comprehensive in that as well as including the four steps of PSLS, it describes other aspects expected to influence the effectiveness of such systems, for example, those ‘before the incident’, which include the organisational culture that encourages reporting and the support from the leadership. The incident management domain within the tool was used to develop our themes and subthemes.
Data synthesis
Two reviewers (HAM and MAA) reviewed all articles to retrieve data describing the participants, study characteristics, and perceived barriers and facilitators. Directed analysis was done, including identification and classification of extracted factors (barriers and/or facilitators), and assigning factors to themes and subthemes according to the CPSI Patient Safety Toolkit. These data were entered into separate spreadsheets. The results from the two reviewers were compared and differences were resolved by consensus with a third reviewer (AAM). We then counted the number of factors identified within each subtheme to inform our narrative synthesis. After removing duplicates, these factors were analysed, merged and new definitions were developed. In the present report, the resulting synthesised factors are narrated and tabulated as a set of main findings. 11
Study inclusion
A total of 3507 studies were identified from searched databases on 1 February 2021, leaving 2475 studies for title and abstract screening after removal of 1032 duplicates ( figure 2 ). Of these, 2352 studies were excluded according to the following criteria: the language was not English, review articles or having different study designs or outcomes. After assessment of the full texts, a further 101 articles were excluded: different study design (70 articles), different outcome (13 articles), full text was not found (7), different intervention (4) or setting (3), specific incident type (2), different indication (1) and conference report (1). After this exclusion process, we included 22 full-text articles.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 flow diagram.
Study characteristics
All studies included were published in 2010–2021, except for three. 12–14 Five were conducted in England, 13 15–18 two in Australia, 12 14 Canada, 19 20 the USA, 21 22 Brazil, 23 24 and Iran, 25 26 and one in the Netherlands, 27 UAE, 28 Sweden, 29 Turkey, 30 Qatar, 31 and South Korea. 32 One included study was multinational. 33
No data were provided regarding the sex, age or work experience of any of the participants. Participants in the 22 included studies were 781 healthcare professionals including 359 nurses from different medical, intensive care and surgical departments, 190 physicians, 49 pharmacists, 42 quality and safety managers, and 5 allied health professionals. The profession of the remaining 136 participants were not mentioned in three of the studies. 17 23 24 Studies were conducted in intensive care units, 12 teaching hospitals, 15 20 23 27 30 university hospitals, 25 tertiary hospitals, 22 31 32 large general public or community hospitals 13 14 17–19 24 29 31 and children’s hospitals 21 ; 1 of the studies involved participants from 111 hospitals. 26
In relation to sampling, eight studies used a purposive sampling technique 14 15 18 21 28–30 33 ; two studies used purposive sampling and snowball sampling 20 31 ; four studies used invitation letters 22 24 25 27 ; the researchers of one study sought out their acquaintances by posting advertisements and using snowball sampling techniques 32 ; one study used posters and nurse leader appeal in selecting their participants 12 ; one study involved all nurses until saturation occurred 23 ; four studies did not clearly describe their sampling methods. 16 17 19 26
All of the included studies clearly described how they were conducted, but eight articles failed to clarify the researchers’ relationships to the participants ( table 1 ). 12 13 19 20 22 23 29 31 In relation to sampling, 11 of the 22 studies carried a risk of selection bias (the researchers selected their participants) and/or a risk of non-representative sampling. 12 16 18 19 21–23 25 26 31 32 One study included only less-experienced residents. 22 Two studies did not report how data saturation was defined or achieved. 16 22 There was no weight to specific questions nor a scoring system for the CASP Qualitative Checklist. As qualitative studies, we included all primary studies regardless of the quality of their methodology, as even methodologically sound studies can be poorly interpreted thereby offering insufficient insight in a particular topic; conversely, studies of lower quality may provide new insights. This heterogeneity contributed to the interpretation of the overall findings. 34
- View inline
A summary of the overall methodological quality of the included studies
Summary of study findings
We extracted 375 reported factors from the 22 studies, all of which were either unequivocal or credible based on the participants’ verbatim transcripts and researcher experience.
Six themes and 16 subthemes were developed based on the Patient Safety Toolkit and are outlined below and in table 2 . Factors under the theme ‘immediate response’ were the most cited as perceived barriers to improving PSLS, cited 170 times out of 375 reported factors (45.3%). Its subthemes ‘reporting the incident’, ‘secure items for confidentiality’ and ‘care for reporter’ were cited 98, 41 and 31 times, respectively. The theme ‘factors before the incident’ was cited 134 times (35.7%) and included subthemes ‘training’ (46 times), ‘organisational and leadership support’ (39 times), ‘cultivate just and safe culture’ (28 times), and ‘availability of resources’ (21 times). Other factors were reported less frequently, however, this does not necessarily reflect lesser importance, as all of the included studies were more focused on reporting procedures of the PSLS and less on the subsequent steps.
Identified themes and subthemes based on the CPSI safety tool kit and their proportions
Theme 1: before the incident (n=134)
Subtheme 1.1: training.
Training was examined by 16 studies, 13–15 19–28 30 32 33 which identified a ‘lack of training’—for both reporters and managers—as a major barrier (46 of 134 responses, 34.3%). Facilitators included planning for and implementation of continuous training, which should be effectively communicated to staff and managers.
Subtheme 1.2: organisational and leadership support
Factors related to organisational and leadership support were mentioned 39 times (29.1%) and included: lack of accountability that would make staff feel more responsible; ineffective reporting systems 19 20 24 25 33 ; lack of definitions, policies or standards 14 18 19 21 26 32 ; poor communication 19 26 ; negative responses to reporting by leaders 13 18 22 26 27 30–32 ; lack of authority 25 and role models. 32 Related facilitators included: incentives and initiatives to report more 31 ; professional accountability and supportive atmospheres 19 20 25 28 29 33 ; writing and dissemination of a guiding manual; increased confidentiality and security of the reporting process 14 ; motivate staff to report through the display of posters 27 ; eliminating blame by seniors or doctors. 29 30
Subtheme 1.3: cultivate a just and safe culture
This subtheme was cited 28 times (20.9%). Barriers included a blaming and punitive culture 14 18 21–23 25 32 ; an attitude of the administration for personalising errors 27 30 ; a lack of awareness about safety culture, which subsequently fostered a lack of responsibility and undervaluing the reporting system 14 30 ; a defective culture unwilling to accept responsibility for errors 25 ; a culture of low expectations, for example, ‘Well they’re in a hospital and things happen’. 22 Facilitators included balancing the need for responsibility and accountability 15 ; building trust in a non-punitive system attained by supporting the reporters, promoting peer reporting, anonymous reporting to an independent body 20 29 31 ; belief that this promotes patient protection 19 ; stimulating role of superiors. 27
Subtheme 1.4: availability of resources
Reported 21 times (15.7%), this subtheme comprised the following barriers: manual reporting systems or defective electronic systems can be time-consuming 18 ; shortage of time and equipment for reporting. 14–16 18 19 21 22 25 26 29 32 Facilitators included carrying a pocket-size plasticised card that outlines the reporting process 27 ; electronic systems together with human and financial resources 18 28 29 ; the option of faster reporting processes (eg, telephone reporting). 14
Theme 2: immediate response to the incident (n=170)
Subtheme 2.1: reporting the incident.
Factors in this subtheme were reported 98 times (57.6%) and were classified further into three categories: ‘user friendly’ (reported 37 times, 37.8%), ‘external and internal reporter influencers’ (32 times, 32.7%) and ‘incident related factors’ (29 times, 29.6%).
Under the ‘user friendly’ category, participants reported the following barriers: complex systems and forms asking for unnecessary data 13 14 16 18 19 22 24 27–30 33 ; poor quality of the incident report 15 ; reporting is time-consuming 12 16 19 25 27 29 32 ; limited access to forms 29 ; the extra work burden on the reporter 19 27 32 ; duplication of work being registered in patient files and in the PSLS or rereporting by other staff. 18 22 24 Facilitators included profession-specific forms 14 18 ; forms that balance the amount of information requested with time. 18 19 27 29
A second category is ‘internal and external influencers to report’, which included the following barriers: juniors are afraid to report seniors’ errors; seniors preventing juniors from reporting; the norm that doctors never report but nurses should 12 18 20 28 ; avoiding reports that might cause internal disputes 12 18 19 22 25 28 32 ; negative attitudes to reporting (eg, it is ‘a waste of time’) 27 29 32 ; reporting yields a feeling of failure 12 ; the perception that reporting brings no personal benefit 12 19 25 ; non-mandatory reporting 19 20 25 ; a lack of perceived ability to report or that staff simply do not think of reporting 27 or consider errors a normal part of daily events. 30 Facilitators included a belief in learning from errors, 20 that this benefits patients 19 25 and showing commitment to adhering to policies. 19
A third category, ‘incident related factors’, includes ‘seriousness of incidents regarding impact, consequences, severity and frequency’, that is, the more serious incidents are more likely to be reported, whereas near misses are perceived to have less chance of being reported 12 18–25 27 29 30 32 33 ; no clear cause for the error. 24 The facilitators included: focusing on reporting serious prioritised incidents, which allows better analysis and management 18 29 ; errors with serious consequences and high frequency of occurrence influence reporting. 25 32
Subtheme 2.2: care for and support for reporter
This subtheme was mentioned 31 times (18.2%) in the primary studies, 25 of which described barriers, including: ‘reporters feel worse, guilt, shame, uncertainty, disloyal to colleagues and emotionally charged’; a lack of a supporting manager 12 13 18 21 22 24 27 30 32 ; the feeling that reporting the error might cause loss of honour, respect, reputation and dignity along with perceived incompetence 19–21 25 ; inappropriate responses by managers. 21 25 Six facilitators included protecting reporters against legal and non-legal actions. 14 19
Subtheme 2.3: confidentiality
This subtheme was reported 41 times (24.1%) and some barriers were directly related to ‘breach of confidentiality that might cause loss of reputation, career, relationships, friendships and respect’ 12 13 15 16 19 20 28 30 ; ‘fear of blame or punishment or legal action’ 12 13 16 19 20 22 25–27 29 30 ; ‘lack of anonymity and lack of trust in system confidentiality’ 18–20 22 25 32 ; ‘fear of economic losses’. 25 Confidentiality and anonymous reporting to independent bodies have been repeatedly reported as positive system influencer. 18 21 27 31
Theme 3: prepare for analysis
Subtheme 3.1: preliminary investigation.
Factors related to preliminary investigations were mentioned five times and were all barriers such as lack of time and resources 15 and poor communication. 18 Furthermore, anonymity was cited as making information about the incident more difficult to obtain. 17 32
Subtheme 3.2: identify team
Factors related to ‘identify team’ were cited six times, and included the following barriers: lack of front-line engagement 15 ; complex organisations, in which it is difficult to assemble the appropriate team 15 17 ; lack of cooperation between units related to the incident. 29 Building fully representative teams was reported as an aspect that might improve PSLS. 31
Subtheme 3.3: plan for interview
In thinking about planning for incident-related interviews, participants only reported fearing loss of reputation as a barrier for participation. 17
Theme 4: analysis process
Subtheme 4.1: investigate what happened.
One barrier and four facilitators were related to investigation: mass reporting might interfere with accurate analysis and capacity for learning 33 ; ‘multidisciplinary meetings ensure equal and fair participation’ 17 ; applying tools and analytical approaches, avoiding analytical myopia, 15 17 and to have open communication. 31
Subtheme 4.2: understand why and how it happened
This subtheme was represented only by one facilitator of PSLS: use of proper analysis tools. 18
Subtheme 4.3: develop and manage recommendations
Factors in this subtheme were reported six times, including poor-quality recommendations such as ‘too much details or too simple’, 15 contradictions with other change agendas and initiatives. 17 ‘Avoid giving undue attention to the report’ 17 was reported as a facilitator.
Theme 5: follow through
Subtheme 5.1: implement.
Reported 11 times, this subtheme includes a lack of effective measures that make tangible changes 22 24 27 29 and also time-consuming recommendations, 29 difficult to implement recommendations, 32 existence of plans that contradict recommendations. 26 Visible change was reported as a facilitator. 18
Subtheme 5.2: follow and assess
Factors in this subtheme were reported five times, four of which were facilitators. Two of these were the use of risk-assessment tools to prioritise actions for follow-up 14 and formal (eg, targets, audits and score cards) and informal (keeping on meeting agenda, management oversight, risk officer spot-checks) performance management for evaluating changes. 15 Only one barrier was reported under this subtheme: data processing does not reflect the real number of reports due to under-reporting. 23
Theme 6: close the loop
This theme was reported 31 times (8.3%) and includes only one subtheme ‘share learning internally and externally’. The barriers are lack or defective feedback resulting in loss of trust in the system. 12–14 16 22 24 26–28 32 Facilitators include: feedback mechanisms that motivate reporting 15 18 19 29 ; improving error-related communication from senior management to healthcare professionals 19 ; to ‘definitely collect less data of better quality of learning and feedback’; to ‘share learning horizontally (with peers), and also for vertical accountability’ 33 ; transparency in sharing data 21 ; the type and timing of feedback depend on the severity of the incident or the degree of risk to the organisation; individual and group feedback about action taken will increase trust in the system. 14
In this systematic review, we identified all barriers and facilitators reported in the literature for PSLS. In those studies, recommendations were reported by participants encountering such barriers. The results also revealed the participants’ perceptions of how these barriers might prevent improvement of the system. By including all the aforementioned factors and recommendations, managers and other decision makers have a wide range of options for taking innovative actions to improve their safety systems.
We included 22 primary studies. We sorted the reported factors (barriers and facilitators) into themes based on a framework derived from the CPSI Patient Safety Toolkit. Facilitators and barriers related to ‘before the incident’ included the preparedness of a hospital to implement PSLS through planning, training, assignment of resources, a well-established safety culture that focuses on systems not people and ensures top-level commitment and support, and well-developed policies and procedures. Related to ‘the immediate response’, some studies recommended that hospitals support reporters emotionally and against legal action. Factors related to ‘analysis process’ and ‘follow through’ included having a well-trained multidisciplinary team to ensure high-quality investigations of safety incidents. Analysis should start with the selection of the most appropriate analytical approaches, followed by investigating the incident and development and implementation of smart recommendations that make tangible system changes. Factors related to ‘closing the loop’ included providing timely feedback to reporters and sharing results of the analysis internally with other departments as well as externally with other hospitals to facilitate learning on a wider scale.
Our results are consistent with other systematic review studies; however, we identified a gap in the research—the predominant focus is the reporting stage, leaving the subsequent stages of the learning system neglected. Vrbnjak et al 35 , stated that ‘a non-blaming, non-punitive and non-fearful learning culture’ is needed to encourage incident reporting. They grouped most of the system barriers into three main areas: the efficiency of the reporting system, management behaviour and staff education. 35 In 2015, Health Quality Ontario identified the barriers and facilitators of PSLS, all of which are consistent with our results, including a non-accusatory environment, improving safety culture, training, enhanced transparency and effective feedback, role models (such as managers), protecting reporters, anonymous reporting, and clear operational policies. Barriers identified for other steps of PSLS have been derived from the WHO guidelines and not primary studies. 36 Similar findings by other studies include a blame-free culture, clear guidelines on how and what to report, user-friendly systems, organisational support for data analysis to generate meaningful learning outcomes and multiple mechanisms to provide feedback through routes to reporters and the wider community. 6 36 37
Gaps in the research
It is important to note that identified barriers to the reporting stage are also pertinent to the three themes not addressed in 19 of our included studies 12–14 16 18–25 27 28 30–33 38 : ‘prepare for analysis’, ‘analysis process’ and ‘follow-up’. These include a lack of time, resources and training for analysis, and a lack of direct communication between responsible departments. Under these circumstances, developing analysis teams is difficult, especially in complex organisations. Also, staff are unwilling to participate in these teams because they are afraid of losing reputation. The corresponding facilitator to these barriers is to bridge the gap in communication through holding regular meetings and structuring well representative teams with front-line engagement and top-level commitment. A newly reported barrier was related to the anonymity of the system, making it difficult to clarify incidents for which data is missing. Modifying an electronic system to not accept incomplete reports was suggested to overcome this barrier. 39 It is also important to mention the problem of under-reporting and its effect on the accuracy of data processing with subsequent failure of follow-up. This can be improved through audits, use of performance scorecards and spot checks.
Participants did not reveal any barriers or facilitators under the ‘select appropriate analysis method’ subtheme. This can be attributed to being outside the scope of the included studies.
Strengths and limitations
Our study is unique as it included a variety of safety incidents including medication-related and device-related incidents. As it is more comprehensive than prior published studies, its results are more applicable to the common setting of a general hospital with a need to track all patient safety events. Our review included 22 primary studies with 781 participants, making it the largest published review on this topic, increasing the likelihood we have not omitted important published information. In addition, the participants represent different departments in hospitals, further increasing the general applicability of our findings. Furthermore, the included studies were done in geographically and economically diverse countries, representing both the developed and the developing world. We included only qualitative studies that gave more chance for participants to express their concerns about the system, which was reflected in the number of perceived system barriers and facilitators. We ensured valid results by including studies of high-quality methodology. Although 9 of the 22 studies involved relationships between the researchers and the participants with a risk of selection bias, no potential bias in their studies was reported. 25
One limitation of our study related to the selection methods of the included studies. More than half of the included studies selected participants non-representative of the healthcare worker population, that is, either only doctors 13 16 27 or only nurses, 12 21 25 or residents. This potentially limits the generality of each study’s conclusions. However, taking the totality of findings across all studies and the consistency of the findings, we are confident our results are valid. Another limitation was an inability to perform subgroup analyses based on healthcare worker characteristics (eg, age, sex, occupation and years of experience of the participants) as studies did not consistently provide that information. Given our findings, we do not feel this limits our conclusions.
Finally, this study is based on what hospital staff perceive as factors affecting PSLS as opposed to actual quantifiable effects. This is a limitation of the existing knowledgebase that needs to be addressed in future studies.
Our review found that most studies on the effectiveness of PSLS relate to aspects ‘before the incident’ and the ‘immediate response’. Themes related to investigations, follow through, and ‘closing the loop’ have received less attention, and it is likely that health systems have spent less time considering them. As it is these stages that lead to improvements in safety, it is not surprising that PSLS have not achieved desired success. We recommend that researchers, safety leaders and health system policy makers focus more of their attention on these aspects of PSLS.
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
All studies were granted ethical approval.
Acknowledgments
We would like to thank everyone who directly or indirectly participated in this research with special thanks to Lindsey Sikora (Health Sciences Research Librarian) for her endless support to develop the Data bases search strategy.
- Ahluwalia J ,
- Shojania KG
- Stavropoulou C ,
- Doherty C ,
- ↵ PROSPERO . 2021 . Available : https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=220504
- Scientific Research
- covidence software
- JBI Qualitative data extraction tool
- Assarroudi A ,
- Heshmati Nabavi F ,
- Armat MR , et al
- Brungs SM ,
- Nagy M , et al
- McArdle D ,
- Kingston MJ ,
- Smith BJ , et al
- Anderson JE ,
- Walters R , et al
- Alkaisy Z ,
- Nicolini D ,
- Williams SD ,
- Phipps DL ,
- Ashcroft DM
- Hartnell N ,
- MacKinnon N ,
- Sketris I , et al
- Szymusiak J ,
- Benson M , et al
- Cunha SGS ,
- Varallo FR ,
- Passos AC ,
- Nadai TR de , et al
- Hashemi F ,
- Nasrabadi AN ,
- Mirbaha F ,
- Shalviri G ,
- Yazdizadeh B , et al
- Martowirono K ,
- Jansma JD ,
- van Luijk SJ , et al
- Alqubaisi M ,
- Strath A , et al
- Carlfjord S ,
- Gunnarsson A
- Soydemir D ,
- Seren Intepeler S ,
- Stewart D ,
- MacLure K ,
- Pallivalapila A , et al
- Lee S-I , et al
- Howell AM ,
- Hull L , et al
- Dixon-Woods M ,
- Agarwal S , et al
- Vrbnjak D ,
- Denieffe S ,
- O’Gorman C , et al
- Pronovost PJ
- Yazdizadeh B ,
- Mirbaha F , et al
- Montobbio G ,
- Pini-Prato A ,
- Guida E , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
Contributors Corresponding author (HAM) was the guarantor responsible for the whole research at its different stages (protocol development, planning, screening of primary studies, extraction and analysis of data, writing, editing and decision of publication. AJF is the research supervisor responsible for planning, supervision and revision of the final manuscript. SM, KT and DM, they all participated in development of the protocol, revision and approval of the analysis, writing the manuscript and revision of the different edits of the manuscript. AAM and MAM they both participated in the screening of primary studies and data extraction and analysis and finally in revisions of the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
- Research article
- Open access
- Published: 12 March 2019
A systematic literature review and narrative synthesis on the risks of medical discharge letters for patients’ safety
- Christine Maria Schwarz 1 ,
- Magdalena Hoffmann ORCID: orcid.org/0000-0003-1668-4294 1 , 2 ,
- Petra Schwarz 3 ,
- Lars-Peter Kamolz 1 ,
- Gernot Brunner 1 &
- Gerald Sendlhofer 1 , 2
BMC Health Services Research volume 19 , Article number: 158 ( 2019 ) Cite this article
27k Accesses
44 Citations
5 Altmetric
Metrics details
The medical discharge letter is an important communication tool between hospitals and other healthcare providers. Despite its high status, it often does not meet the desired requirements in everyday clinical practice. Occurring risks create barriers for patients and doctors. This present review summarizes risks of the medical discharge letter.
The research question was answered with a systematic literature research and results were summarized narratively. A literature search in the databases PubMed and Cochrane Library for Studies between January 2008 and May 2018 was performed. Two authors reviewed the full texts of potentially relevant studies to determine eligibility for inclusion. Literature on possible risks associated with the medical discharge letter was discussed.
In total, 29 studies were included in this review. The major identified risk factors are the delayed sending of the discharge letter to doctors for further treatments, unintelligible (not patient-centered) medical discharge letters, low quality of the discharge letter, and lack of information as well as absence of training in writing medical discharge letters during medical education.
Conclusions
Multiple risks factors are associated with the medical discharge letter. There is a need for further research to improve the quality of the medical discharge letter to minimize risks and increase patients’ safety.
Peer Review reports
The medical discharge letter is an important communication medium between hospitals and general practitioners (GPs) and an important legal document for any queries from insurance carriers, health insurance companies, and lawyers [ 1 ]. Furthermore, the medical discharge letter is an important document for the patient itself.
A timely transmission of the letter, a clear documentation of findings, an adequate assessment of the disease as well as understandable recommendations for follow-up care are essential aspects of the medical discharge letter [ 2 ]. Despite this importance, medical discharge letters are often insufficient in content and form [ 3 ]. It is also remarkable that writing of medical discharge letters is often not a particular subject in the medical education [ 4 ]. Nevertheless, the medical discharge letter is an important medical document as it contains a summary of the patient’s hospital admission, diagnosis and therapy, information on the patient’s medical history, medication, as well as recommendations for continuity of treatment. A rapid transmission of essential findings and recommendations for further treatment is of great interest to the patient (as well as relatives and other persons that are involved in the patients’ caring) and their current and future physicians. In most acute care hospitals, patients receive a preliminary medical discharge letter (short discharge letter) with diagnoses and treatment recommendations on the day of discharge [ 5 ]. Unfortunately, though, the full hospital medical discharge letter, which is often received with great delay, is an area of constant conflict between GPs and hospital doctors [ 1 ]. Thus the medical discharge letter does not only represent a feature of process and outcome quality of a clinic, but also influences confidence building and binding of resident physicians to the hospital [ 6 ].
Beside the transmission of patients’ findings from physician to physician, the delivery of essential information to the patient is an underestimated purpose of the medical discharge letter [ 7 ]. The medical discharge letter is often characterized by a complex medical language that is often not understood by the patients. In recent years, patient-centered/patient-directed medical discharge letters are more in discussion [ 8 ]. Thus, the medical discharge letter points out risks for patients and physicians while simultaneously creating barriers between them.
A systematic review of the literature was undertaken to identify patient safety risks associated with the medical discharge letter.
Search strategy
A systematic literature search was conducted using the electronic databases PubMed and Cochrane Database. Additionally, we scanned the reference lists of selected articles (snowballing). The following search terms were used: “discharge summary AND risks”, “discharge summary AND risks AND patient safety” and “discharge letter AND risks” and “discharge letter AND risks AND patient safety”. We reviewed relevant titles and abstracts on English and German literature published between January 2008 and May 2018 and started the search at the beginning of February 2018 and finished it at the end of May 2018.
Eligibility criteria
In this systematic review, articles were included if the title and/or abstract indicated the report of results of original research studies using quantitative, qualitative, or mixed method approaches. Studies in paediatric settings or studies that do not handle possible risks of the medical discharge letter were excluded, as well as reports, commentaries and letters. Electronic citations, including available abstracts of all articles retrieved from the search, were screened by two authors to select reports for full-text review. Duplicates were removed from the initial search. Nevertheless, during the search of articles the selection, publication as well as language bias must be considered. Thereafter, full-texts of potentially relevant studies were reviewed to determine eligibility for inclusion. In the following Table 1 inclusion and exclusion criteria for the studies are listed. Afterwards, key outcomes and main results were summarized. Differences were resolved by consensus. Finally, a narrative synthesis of studies meeting the inclusion criteria was conducted. Reference management software MENDELEY (Version 1.19.3) was used to organise and store the literature.
Data extraction
The data extraction in form of a table was used to summarize study results. The two authors extracted the data relating to author, country, year, study design, and outcome measure as well as potential risk factors to patient safety directly into a pre-formatted data collection form. After data extraction, the literature was discussed and synthesized into themes. The evaluation of the single studies was done using checklists [STROBE (combined) and the Cochrane Data collection form for intervention reviews (RCTs and non-RCTs)]. Meta-analysis was not considered appropriate for this body of literature because of the wide variability of studies in relation to research design, study population, types of interventions and outcomes.
Then a narrative synthesis was performed to synthesize the findings of the different studies. Because of the range of very different studies that were included in this systematic review, we have decided that a narrative synthesis constitutes the best instrument to synthesise the findings of the studies. First, a preliminary synthesis was undertaken in form of a thematic analysis involving searching of studies, listing and presenting results in tabular form. Then the results were discussed again and structured into themes. Afterwards, summarizing of included studies in a narrative synthesis within a framework was performed by one author.
This framework consisted of the following factors: the individuals and the environment involved in the studies (doctors, hospitals), the tools and technology (such as discharge letter delivery systems), the content of the medical discharge letter (such as missing content, quality of content), the accuracy and timeliness of transfer. These themes were discussed in relation to potential risks for patient’s safety. All articles that were included in this review were published before. The framework of this study was chosen following a previously published systematic review dealing with patient risks associated with telecare [ 9 ].
The initial literature search in the two online databases identified 940 records. From these records, 65 full text articles were screened for eligibility. Then 36 full-text articles were excluded because they pertained to patient transfer within the hospital or to another hospital, or to patient hand-over situations. Finally, 29 studies were included in this review. Included studies are listed in Table 2 . All document types were searched with a focus on primary research studies. The results of the search strategy are shown in Fig. 1 .
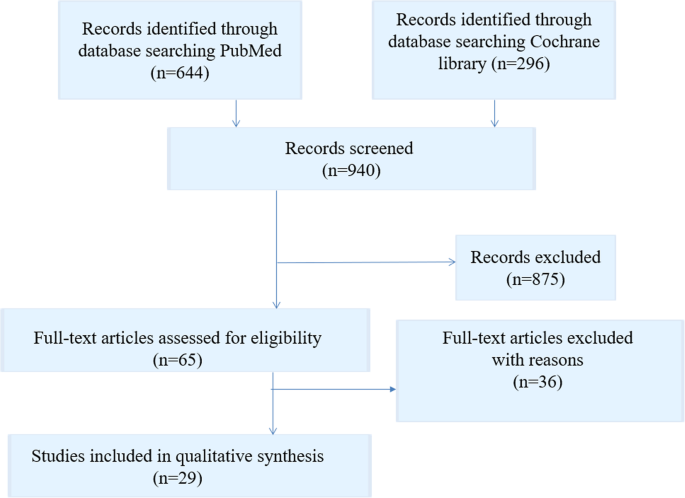
Flow chart literature search strategy
From these 29 studies, 13 studies dealt with the quality analysis of discharge letters, 12 studies with delayed transmission of medical discharge letters and just as many with the lack of information in medical discharge letters. Only few studies dealt with training on writing medical discharge letters and with understanding of patients of their medical discharge letters. The descriptive information of the included articles is presented in Table 2 . Overall quality of the articles was found to be acceptable, with clearly stated research questions and appropriate used methods.
Risk factors
In the following the identified major risk factors concerning the medical discharge letter are presented in a narrative summary.
Delayed delivery
The medical discharge letters should arrive at the GP soon after hospital discharge to ensure the quickest possible further treatment [ 4 ]. If letters are delivered weeks after the hospital stay, a continuous treatment of the patient cannot be ensured. Furthermore, the author of the medical discharge letter will no longer have current data after the discharge of the patient, which may result in a loss of important information [ 10 ]. Interfaces between different treatment areas and organizational units are known to cause a loss of information and a lack of quality in patient handling [ 11 ]. The improvement of information transfer between different healthcare providers during the transition of patients has been recommended to improve patient care [ 12 , 13 ]. Delayed communication of findings may lead to a lack of continuity of care and suboptimal outcomes, as well as decreased satisfaction levels for both patients and GPs [ 14 , 15 , 16 ]. In a review of Kripalani et al., it was shown that 25% of discharge summaries were never received by GPs [ 17 ]. This has several negative consequences for patients. Li et al. [ 18 ] found that a delayed transmission or absence of the medical discharge summary is related to patient readmission, and a study by Gilmore-Bykovskyi [ 19 ] found a strong relationship between patients whose discharge summaries omitted designation of a responsible clinician/clinic for follow-up care and re-hospitalisation and/or death. A Swedish study by Carlsson et al. [ 20 ] points out that a lack of accuracy and continuity in discharge information on eating difficulties may increase risk of undernutrition and related complications. A study of Were et al. [ 18 ] investigated pending lab results in medical discharge summaries and found that only 16% of tests with pending results were mentioned in the discharge summaries, and Walz et al. [ 21 ] found that approximately one third of the sub-acute care patients had pending lab results at discharge, but only 11% of these were documented in the medical discharge summaries.
Quality, lack of information
Medical discharge letters are a key communication tool for patient safety issues [ 17 ]. Incomplete and insufficient medical discharge letters increase the risks of readmission and myriad other complications [ 22 ]. Langelaan et al. (2017) evaluated more than 2000 medical discharge letters and found that in about 60% of the letters essential information was missing, such as a change of the existing medication, laboratory data, and even data on the patients themselves [ 23 ]. Accurate and complete medical discharge summaries are essential for patient safety [ 17 , 24 , 25 ]. Addresses; patient data, including duration of stay; diagnoses; procedures; operations; epicrisis and therapy recommendations; as well as findings in the appendix; are minimum requirements that are supposed to be included in the medical discharge letter [ 4 ]. However, it was found that key components are often lacking in medical discharge letters, including information about follow-up and management plans [ 23 , 26 ], test results [ 27 , 28 , 29 ], and medication adjustments [ 30 , 31 , 32 , 33 , 34 , 35 ]. In a review of Wimsett et al. [ 36 ] key components of a high-quality medical discharge summary were identified in 32 studies. These important components were discharge diagnosis, the received treatment, results of investigations as well as follow-up plans.
Accuracy of patients’ medication information is important to ensure patient safety. Hospital doctors expect GPs to continue with the prescribed (or modified) drug therapy. However, the selection of certain drugs is not always transparent for the GPs. A study by Grimes et al. [ 30 ] found that a discrepancy in medication documentation at discharge occurred in 10.8% of patients. From these patients nearly 65.5% were affected by discrepancies in medication documentation. The most prevalent inconsistency was drug omission (20.9%). Only 2% of patients were contacted, although general patient harm was assessed. A Swedish study of 2009 [ 37 ] investigated the quality improvement of medical discharge summaries. A higher quality of discharge letter led to an average of 45% fewer medication errors per patient.
A recent study by Tong et al. [ 38 ] revealed a reduced rate of medication errors in medical discharge summaries that were completed by a hospital pharmacist. Hospital pharmacists play a key role in preparing the discharge medication information transferred to GPs upon patient discharge and should work closely with hospital doctors to ensure accurate medication information that is quickly communicated to GPs at transitions of care [ 39 ]. Most hospitals have introduced electronic systems to improve the discharge communication, and many studies found a significant overall improvement in electronic transfer systems due to better documentation of information about follow-up care, pending test results, and information provided to patients and relatives [ 40 , 41 , 42 ]. Mehta et al. [ 43 ] found that the changeover to a new electronic system resulted in an increased completeness of discharge summaries from 60.7 to 75.0% and significant improvements in levels of completeness in certain categories.
Writing of medical discharge letter is missing in medical education
Both junior doctors as well as medical students reported that they received inadequate guidance and training on how to write medical discharge summaries [ 44 , 45 ] and recognized that higher priority is often given to pressing clinical tasks [ 46 ]. Research into the causes of prescribing errors by junior doctors at hospitals in the UK has revealed that latent conditions like organizational processes, busy environments, and medical care for complex patients can lead to medication errors in the medical discharge summary [ 47 ].
Fortunately, some study results demonstrate that information and education on writing medical discharge letters would enhance communication to the GPs and prevent errors during the patient discharge process [ 37 ]. Minimal formal teaching about writing medical discharge summaries is common in most medical schools [ 39 , 46 ]; however, a study by Shivji et al. has shown that simple, intensive educational sessions can lead to an improvement in the writing process of medical discharge summaries and communication with primary care [ 48 ].
Since the medical discharge letter should meet specific quality criteria, senior physicians and/or the head physician correct(s) and validate(s) the letter. The medical discharge letter therefore represents an essential learning target [ 8 ]. Training activities and workshops are necessary for junior doctors to improve writing medical discharge letters [ 44 , 49 ]. It might be also useful for young doctors to use checklists or other structured procedures to improve writing [ 4 ]. Maher et al. showed that the use of a checklist enhanced the quality (content, structure, and clarity) of medical discharge letters written by medical students [ 50 ].
In the following Table 3 main risk factors of the medical discharge letter are summarized.
The results of this systematic literature research indicate notable risk factors relating to the medical discharge letter. In a study by Sendlhofer et al., 360 risks were identified in hospital settings [ 51 ]. From these, 176 risks were scored as strategic and clustered into “top risks”. Top risks included medication errors, information errors, and lack of communication, among others. During this review, these potential risk factors were also identified in terms of the medical discharge letter.
Delayed sending and low quality of medical discharge letters to the referring physicians, may adversely affect the further course of treatment. However, a study of Spencer et al. has determined rates of failures in processing actions requested in hospital discharge summaries in general practice. It was found that requested medication changes were not made in 17% and patient harm occurred in 8% in relation to failures [ 52 ].
Despite the existence of reliable standards [ 53 ] many physicians are not adequately trained for writing medical discharge letters during their studies. Regular trainings and workshops and standardized checklists may optimize the quality of the medical discharge letter. Furthermore, electronic discharge letters have the potential to easily and quickly extract important information such as diagnoses, medication, and test results into a structured discharge document, and offer important advantages such as reliability, speed of information transfer, and standardization of content. Comprehensive discharge letters reduce the readmission rate and increase safety and quality by discharging of the patient. A missing structure, as well as a complex language, illegible handwriting, and unknown abbreviations, make reading medical discharge letters more complicated [ 4 ]. At least, poor patient understanding of their diagnosis and treatment plans and incomprehensible recommendations can adversely impact clinical outcome following hospital discharge. Many studies confirm that inadequate communication of findings [ 3 , 39 , 54 ] is an important risk factor in patients’ safety [ 51 ].
Most medical information in the discharge letter is not understood by patients (as well as relatives and other persons that are involved in the patients’ caring) and patients themselves do not receive a comprehensible medical discharge letter. The content of the medical discharge letter is often useless for the patient due to its medical terminology and content that is not matching with the patient’s level of knowledge or health literacy [ 55 , 56 , 57 ]. Poor understanding of diagnoses and related discharge plans are common among patients and family members and often accompanied by unplanned hospital readmissions [ 58 , 59 , 60 , 61 ]. In a study by Lin et al., it was shown that a patient-directed discharge letter enhanced understanding for hospitalization and for recommendations. Furthermore, verbal communication of the letter contents, explanation of every section of the medical discharge letter, and the opportunity for discussion and asking questions improved patient comprehension [ 7 ]. A study by O’Leary et al. showed that roughly 80–95% of patients with breast tumours want to be informed and educated about their illness, treatment, and prognosis [ 62 ].
High quality of care is characterized by a patient-centered communication, where the patient’s personal needs are also in focus [ 63 ]. Translation of medical terms in reports and letters leads to a better understanding of the disease and, interestingly, the avoidance of medical terms did not lead to deterioration in the transmission of information between the treating physicians. Moreover, it was found that the minimisation of medical terminology in medical discharge letters improved understanding and perception of patients’ ability to manage chronic health conditions [ 64 ]. In effect, it is clear that patient-centered communication improves outcome, mental health, patient satisfaction and reduces the use of health services [ 65 ].
Strengths and limitations
We have identified key problems with the medical discharge summaries that negatively impact patients’ safety and wellbeing. However, there is a heterogeneous nature of the included studies in terms of study design, sample size, outcomes, and language. Only two reviewers screened the studies for eligibility and only full-text articles were included in the literature review; furthermore, only the databases Pubmed and Cochrane library were screened for appropriate studies. Due to these constraints, there is a chance that other relevant studies may have been missed.
High-quality medical discharge letters are essential to ensure patient safety. To address this, the current review identified the major risk factors as delayed sending and low quality of medical discharge letters, lack of information and patient understanding, and inadequate training in writing medical discharge letters. In future, research studies should focus on improving the communication of pending test results and findings at discharge, and on evaluating the impact that this improved communication has on patient outcomes. Moreover, a simple patient-centered medical discharge letter may improve the patient’s (as well as family members’ and other caregivers’) understanding of disease, treatment and post-discharge recommendations.
Abbreviations
General practitioner
Randomized Controlled Trial
STrengthening the Reporting of OBservational studies in Epidemiology
United Kingdom
Kreße B, Dinser R. Anforderungen an Arztberichte- ein haftungsrechtlicher Ansatz. Medizinrecht. 2010;28(6):396–400.
Google Scholar
Möller K-H, Makoski K. Der Arztbrief - Rechtliche Rahmen- Bedingungen. 2015;5:186–94.
Van Walraven C, Weinberg AL. Quality assessment of a discharge summary system. CMAJ. 1995;152(9):1437–42.
PubMed Google Scholar
Unnewehr M, Schaaf B, Friederichs H. Die Kommunikation optimieren. Dtsch Arztebl Int. 2013;110(37):831–4.
Roth-Isigkeit A, Harder S. Die Entlassungsmedikation im Arztbrief. Eine explorative Befragung von Hausärzten/−innen. Vol. 100, Medizinische Klinik. 2005. p. 87–93.
Bohnenkamp B. Arbeitsorganisation: Der Arztbrief - Viel mehr als nur lästige Pflicht. Vol. 113, Deutsches Ärzteblatt International. 2016. p. 2–4.
Lin R, Tofler G, Spinaze M, Dennis C, Clifton-Bligh R, Nojoumian H, Gallagher R, et al. Patient-directed discharge letter (PADDLE)-a simple and brief intervention to improve patient knowledge and understanding at time of hospital discharge. Hear Lung Circ. 2012;21:S312.
Hammerer P. Patientenverständliche Arztbriefe und Befunde. Springer Medizin. 2018;33(2):119–23.
Guise V, Anderson J, Wiig S. Patient safety risks associated with telecare: a systematic review and narrative synthesis of the literature. BMC Health Serv Res. 2014;14(1):588.
Raab, A., & Drissner A. Einweiserbeziehungsmanagement: Wie Krankenhäuser erfolgreich Win-Win-Beziehungen zu niedergelassenen Ärzten aufbauen. Kohlhammer Verlag; 2011. 240 p.143.
Hart D. Vertrauen, Kooperation, Organisation. Berlin Heidelberg: Springer Verlag; 2006. p. 845–7.
Cook RI. Gaps in the continuity of care and progress on patient safety. BMJ. 2000;320(7237):791–4.
CAS PubMed Google Scholar
Duggan C, Feldman R, Hough J, Bates I. Reducing adverse prescribing discrepancies following hospital discharge. Int J Pharm Pract. 1998;6(2):77–82.
Polyzotis PA, Suskin N, Unsworth K, Reid RD, Jamnik V, Parsons C, et al. Primary care provider receipt of cardiac rehabilitation discharge summaries are they getting what they want to promote long-term risk reduction. Circ Cardiovasc Qual Outcomes. 2013;6(1):83–9.
Poon EG, Gandhi TK, Sequist TD, Murff HJ, Karson AS, Bates DW. “I wish i had seen this test result earlier!”: Dissatisfaction with test result management systems in primary care. Vol. 164, Archives of Internal Medicine. 2004. p. 2223–8.
Coleman EA, Berenson RA. Lost in transition: Challenges and opportunities for improving the quality of transitional care. Vol. 141, Annals of Internal Medicine. 2004. p. 533–6.
Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–41.
Were MC, Li X, Kesterson J, Cadwallader J, Asirwa C, Khan B, et al. Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow-up providers. J Gen Intern Med. 2009;24(9):1002–6.
Gilmore-Bykovskyi AL, Kennelty KA, Dugoff E, Kind AJH. Hospital discharge documentation of a designated clinician for follow-up care and 30-day outcomes in hip fracture and stroke patients discharged to sub-acute care. BMC Health Serv Res. 2018;18(1).
Carlsson E, Ehnfors M, Eldh AC, Ehrenberg A. Accuracy and continuity in discharge information for patients with eating difficulties after stroke. J Clin Nurs. 2012;21(1–2):21–31.
Walz SE, Smith M, Cox E, Sattin J, Kind AJH. Pending laboratory tests and the hospital discharge summary in patients discharged to sub-acute care. J Gen Intern Med. 2011;26(4):393–8.
Horwitz LI, Jenq GY, Brewster UC, Chen C, Kanade S, Van Ness PH, et al. Comprehensive quality of discharge summaries at an academic medical center. J Hosp Med. 2013;8(8):436–43.
Alderton M, Callen JL. Are general practitioners satisfied with electronic discharge summaries? Vol. 36, Health Information Management Journal. 2007. p. 7–12.
Harel Z, Wald R, Perl J, Schwartz D, Bell CM. Evaluation of deficiencies in current discharge summaries for dialysis patients in Canada. J Multidiscip Healthc. 2012;5:77–84.
Philibert I, Barach P. The European HANDOVER Project: A multi-nation program to improve transitions at the primary care - Inpatient interface. BMJ Quality and Safety. 2012;21(SUPPL. 1).
Greer RC, Liu Y, Crews DC, Jaar BG, Rabb H, Boulware LE. Hospital discharge communications during care transitions for patients with acute kidney injury: a cross-sectional study. BMC Health Serv Res. 2016;16:449.
Belleli E, Naccarella L, Pirotta M. Communication at the interface between hospitals and primary care: a general practice audit of hospital discharge summaries. Aust Fam Physician. 2013;42(12):886–90.
Roy CL, Poon EC, Karson AS, Ladak-Merchant Z, Johnson RE, Maviglia SM, et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med. 2005;143(2):121–8.
Gandara E, Moniz T, Ungar J, Lee J, Chan-Macrae M, O’Malley T, et al. Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals. J Hosp Med. 2009;4(8):E28–33.
Grimes T, Delaney T, Duggan C, Kelly JG, Graham IM. Survey of medication documentation at hospital discharge: implications for patient safety and continuity of care. Ir J Med Sci. 2008;177(2):93–7.
Uitvlugt EB, Siegert CEH, Janssen MJA, Nijpels G, Karapinar-Çarkit F. Completeness of medication-related information in discharge letters and post-discharge general practitioner overviews. Int J Clin Pharm. 2015;37(6):1206–12.
Ooi CE, Rofe O, Vienet M, Elliott RA. Improving communication of medication changes using a pharmacist-prepared discharge medication management summary. Int J Clin Pharm. 2017;39(2):394–402.
Perren A, Previsdomini M, Cerutti B, Soldini D, Donghi D, Marone C. Omitted and unjustified medications in the discharge summary. Qual Saf Heal Care. 2009;18(3):205–8.
CAS Google Scholar
Garcia BH, Djønne BS, Skjold F, Mellingen EM, Aag TI. Quality of medication information in discharge summaries from hospitals: an audit of electronic patient records. Int J Clin Pharm. 2017;39(6):1331–7.
Monfort AS, Curatolo N, Begue T, Rieutord A, Roy S. Medication at discharge in an orthopaedic surgical ward: quality of information transmission and implementation of a medication reconciliation form. Int J Clin Pharm. 2016;38(4):838–47.
Wimsett J, Harper A, Jones P. Review article: Components of a good quality discharge summary: A systematic review. Vol. 26, EMA - Emergency Medicine Australasia. 2014. p. 430–8.
Bergkvist A, Midlöv P, Höglund P, Larsson L, Bondesson Å, Eriksson T. Improved quality in the hospital discharge summary reduces medication errors-LIMM: Landskrona integrated medicines management. Eur J Clin Pharmacol. 2009;65(10):1037–46.
Tong EY, Roman CP, Mitra B, Yip GS, Gibbs H, Newnham HH, et al. Reducing medication errors in hospital discharge summaries: a randomised controlled trial. Med J Aust. 2017;206(1):36–9.
Yemm R, Bhattacharya D, Wright D, Poland F. What constitutes a high quality discharge summary? A comparison between the views of secondary and primary care doctors. Int J Med Educ. 2014;5:125–31.
O’Leary KJ, Liebovitz DM, Feinglass J, Liss DT, Evans DB, Kulkarni N, et al. Creating a better discharge summary: improvement in quality and timeliness using an electronic discharge summary. J Hosp Med. 2009;4(4):219–25.
Chan S. P Maurice a, W pollard C, Ayre SJ, Walters DL, Ward HE. Improving the efficiency of discharge summary completion by linking to preexisiting patient information databases. BMJ Qual Improv Reports. 2014;3(1):1–5.
Lehnbom EC, Raban MZ, Walter SR, Richardson K, Westbrook JI. Do electronic discharge summaries contain more complete medication information? A retrospective analysis of paper versus electronic discharge summaries. Heal Inf Manag J. 2014;43(3):4–12.
Mehta RL, Baxendale B, Roth K, Caswell V, Le Jeune I, Hawkins J, et al. Assessing the impact of the introduction of an electronic hospital discharge system on the completeness and timeliness of discharge communication: A before and after study. BMC Health Serv Res. 2017;17(1).
Heaton A, Webb DJ, Maxwell SRJ. Undergraduate preparation for prescribing: the views of 2413 UK medical students and recent graduates. Br J Clin Pharmacol. 2008;66(1):128–34.
Maxwell S, Walley T. Teaching safe and effective prescribing in UK medical schools: A core curriculum for tomorrow’s doctors. Vol. 55, British Journal of Clinical Pharmacology. 2003. p. 496–503.
Frain JP, Frain AE, Carr PH. Experience of medical senior house officers in preparing discharge summaries. Br Med J. 1996;312(7027):350.
Dornan T, Investigator P, Ashcroft D, Lewis P, Miles J, Taylor D, et al. An in depth investigation into causes of prescribing errors by foundation trainees in relation to their medical education. EQUIP study. Vol. 44, Methods. 2010.
Shivji FS, Ramoutar DN, Bailey C, Hunter JB. Improving communication with primary care to ensure patient safety post-hospital discharge. Br J Hosp Med. 2015;76(1):46–9.
Cresswell A, Hart M, Suchanek O, Young T, Leaver L, Hibbs S. Mind the gap: Improving discharge communication between secondary and primary care. BMJ Qual Improv Reports. 2015;4(1):u207936.w3197.
Maher B, Drachsler H, Kalz M, Hoare C, Sorensen H, Lezcano L, et al. Use of Mobile applications for hospital discharge letters - improving handover at point of practice. Int J Mob Blended Learn. 2013;5(4):29.
Sendlhofer G, Brunner G, Tax C, Falzberger G, Smolle J, Leitgeb K, et al. Systematic implementation of clinical risk management in a large university hospital: the impact of risk managers. Wien Klin Wochenschr [Internet]. 2015; 127:1–11. Available from: https://doi.org/10.1007/s00508-014-0620-7
Spencer RA, Spencer SEF, Rodgers S, Campbell SM, Avery AJ. Processing of discharge summaries in general practice: a retrospective record review. Br J Gen Pract. 2018;68(673):e576–85.
Carpenter I. A Clinician’s guide to record standards - part 2: standards for the structure and content of medical records and communications when patients are admitted to hospital. Acad Med R Coll R Coll Physicians. 2008;10(5):24.
GMC. Good practice in prescribing and managing medicines and devices. Good Medical Practice. 2013. p. 1–11.
Jolly BT, Scott JL, Sanford SM. Simplification of emergency department discharge instructions improves patient comprehension. Ann Emerg Med. 1995;26(4):443–6.
Williams DM, Counselman FL, Caggiano CD. Emergency department discharge instructions and patient literacy: a problem of disparity. Am J Emerg Med. 1996;14(1):19–22.
Jolly BT, Scott JL, Feied CF, Sanford SM. Functional illiteracy among emergency department patients: a preliminary study. Ann Emerg Med. 1993;22(3):573–8.
Soler RS, Juvinyà Canal D, Noguer CB, Poch CG, Brugada Motge N, Garcia D m, Gil M. Continuity of care and monitoring pain after discharge: patient perspective. J Adv Nurs. 2010;66(1):40–8.
Grover G, Berkowitz CD, Lewis RJ. Parental recall after a visit to the emergency department. Clin Pediatr (Phila). 1994;33(4):194–201.
Witherington EMA, Pirzada OM, Avery AJ. Communication gaps and readmissions to hospital for patients aged 75 years and older: observational study. Qual Saf Heal Care. 2008;17(1):71–5.
Marcantonio ER, McKean S, Goldfinger M, Kleefield S, Yurkofsky M, Brennan T. a. Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan. Am J Med. 1999;107(1):13–7.
O’Leary KA, Estabrooks CA, Olson K, Cumming C. Information acquisition for women facing surgical treatment for breast cancer: Influencing factors and selected outcomes. Vol. 69, Patient Education and Counseling. 2007. p. 5–19.
Vogel BA, Helmes AW, Bengel J. Arzt-Patienten-Kommunikation in der Tumorbehandlung: Erwartungen und Erfahrungen aus Patientensicht. Zeitschrift für Medizinische Psychol. 2006;15(4):149–61.
Wernick M, Hale P, Anticich N, Busch S, Merriman L, King B, et al. A randomised crossover trial of minimising medical terminology in secondary care correspondence in patients with chronic health conditions: impact on understanding and patient reported outcomes. Intern Med J. 2016;46(5):596–601.
Woods SS, Schwartz E, Tuepker A, Press NA, Nazi KM, Turvey CL, et al. Patient experiences with full electronic access to health records and clinical notes through the my healthevet personal health record pilot: Qualitative study. J Med Internet Res. 2013;(15, 3).
Weiskopf NG, Rusanov A, Weng C. Sick patients have more data: the non-random completeness of electronic health records. MIA Symp. 2013;2013:1472–7.
Choudhry AJ, Baghdadi YMK, Wagie AE, Habermann EB, Heller SF, Jenkins DH, et al. Readability of discharge summaries: with what level of information are we dismissing our patients? In: Am J Surg. 2016. p. 631–6.
Li JYZ, Yong TY, Hakendorf P, Ben-Tovim D, Thompson CH. Timeliness in discharge summary dissemination is associated with patients’ clinical outcomes. J Eval Clin Pract. 2013;19(1):76–9.
Download references
Acknowledgements
Not applicable.
This research project was part of a project funded by the Gesundheitsfonds Steiermark. The funders had no role in study design, data collection and analyses, decision to publish, or preparation of the manuscript.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Author information
Authors and affiliations.
Research Unit for Safety in Health, c/o Division of Plastic, Aesthetic and Reconstructive Surgery, Department of Surgery, Medical University of Graz, Graz, Austria
Christine Maria Schwarz, Magdalena Hoffmann, Lars-Peter Kamolz, Gernot Brunner & Gerald Sendlhofer
Executive Department for Quality and Risk Management, University Hospital Graz, Auenbruggerplatz 1/3, 8036, Graz, Austria
Magdalena Hoffmann & Gerald Sendlhofer
Carinthia University of Applied Science, Feldkirchen, Austria
Petra Schwarz
You can also search for this author in PubMed Google Scholar
Contributions
CS wrote the manuscript; CS, MH and PS performed the literature search; LK contributed to the conception of this work; GB contributed to the interpretation of data and GS supervised the project. All authors were critically revising the manuscript and all authors have read and approved the final manuscript.
Corresponding author
Correspondence to Magdalena Hoffmann .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Schwarz, C.M., Hoffmann, M., Schwarz, P. et al. A systematic literature review and narrative synthesis on the risks of medical discharge letters for patients’ safety. BMC Health Serv Res 19 , 158 (2019). https://doi.org/10.1186/s12913-019-3989-1
Download citation
Received : 09 August 2018
Accepted : 06 March 2019
Published : 12 March 2019
DOI : https://doi.org/10.1186/s12913-019-3989-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Discharge letter
- Discharge summary
- Patient safety
- Hospital discharge
- Systematic review
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
A systematic literature review and narrative synthesis on the risks of medical discharge letters for patients' safety
Affiliations.
- 1 Research Unit for Safety in Health, c/o Division of Plastic, Aesthetic and Reconstructive Surgery, Department of Surgery, Medical University of Graz, Graz, Austria.
- 2 Research Unit for Safety in Health, c/o Division of Plastic, Aesthetic and Reconstructive Surgery, Department of Surgery, Medical University of Graz, Graz, Austria. [email protected].
- 3 Executive Department for Quality and Risk Management, University Hospital Graz, Auenbruggerplatz 1/3, 8036, Graz, Austria. [email protected].
- 4 Carinthia University of Applied Science, Feldkirchen, Austria.
- 5 Executive Department for Quality and Risk Management, University Hospital Graz, Auenbruggerplatz 1/3, 8036, Graz, Austria.
- PMID: 30866908
- PMCID: PMC6417275
- DOI: 10.1186/s12913-019-3989-1
Background: The medical discharge letter is an important communication tool between hospitals and other healthcare providers. Despite its high status, it often does not meet the desired requirements in everyday clinical practice. Occurring risks create barriers for patients and doctors. This present review summarizes risks of the medical discharge letter.
Methods: The research question was answered with a systematic literature research and results were summarized narratively. A literature search in the databases PubMed and Cochrane Library for Studies between January 2008 and May 2018 was performed. Two authors reviewed the full texts of potentially relevant studies to determine eligibility for inclusion. Literature on possible risks associated with the medical discharge letter was discussed.
Results: In total, 29 studies were included in this review. The major identified risk factors are the delayed sending of the discharge letter to doctors for further treatments, unintelligible (not patient-centered) medical discharge letters, low quality of the discharge letter, and lack of information as well as absence of training in writing medical discharge letters during medical education.
Conclusions: Multiple risks factors are associated with the medical discharge letter. There is a need for further research to improve the quality of the medical discharge letter to minimize risks and increase patients' safety.
Keywords: Discharge letter; Discharge summary; Hospital discharge; Patient safety; Risk; Systematic review.
Publication types
- Systematic Review
- Communication
- Health Personnel
- Medical Records / standards*
- Patient Discharge / standards*
- Patient Safety / standards*
- Professional Practice / standards
Grants and funding
- -/Gesundheitsfonds Steiermark
- Open access
- Published: 18 March 2024
The efficacy and safety of cannabidiol (CBD) in pediatric patients with Dravet Syndrome: a narrative review of clinical trials
- Nicholas Aderinto 1 , 8 ,
- Gbolahan Olatunji 2 ,
- Emmanuel Kokori 2 ,
- Yusuf Ismaila Ajayi 3 ,
- Olumide Akinmoju 4 ,
- Abiola Samuel Ayedun 5 ,
- Oluwapelumi Ikeoluwa Ayoola 6 &
- Noah Oluwaseun Aderinto 7
European Journal of Medical Research volume 29 , Article number: 182 ( 2024 ) Cite this article
201 Accesses
3 Altmetric
Metrics details
Dravet Syndrome (DS) is a rare and severe form of childhood epilepsy that is often refractory to conventional antiepileptic drugs. Emerging evidence suggests that Cannabidiol (CBD) offer therapeutic benefits for DS. This review aims to evaluate the efficacy and safety of CBD in pediatric patients with DS based on data from ten clinical trials.
A review was conducted to identify clinical trials assessing the efficacy and safety of CBD in pediatric patients diagnosed with DS. PubMed, MEDLINE, Scopus, Web of Science, and relevant grey literature were systematically searched for relevant articles up to October 2023, and clinical trials within the last 10 years were included. The search strategy incorporated controlled vocabulary terms and keywords related to "Cannabidiol," "Dravet Syndrome," and "pediatric patients."
The analysis revealed promising efficacy outcomes. Notably, CBD demonstrated substantial reductions in seizure frequency, with some patients achieving seizure freedom. The findings emphasised the consistency of CBD's efficacy across different patient subgroups. The safety profile of CBD was generally acceptable, with adverse events often being manageable.
This review consolidates evidence from multiple clinical trials, affirming the potential of CBD as a promising treatment option for pediatric patients with DS. While further research is needed to address existing knowledge gaps, CBD's efficacy and acceptable safety profile make it a valuable addition to the therapeutic tools for DS.
Introduction
Dravet Syndrome (DS), also known as Severe Myoclonic Epilepsy of Infancy (SMEI), is a rare and debilitating form of epilepsy characterised by recurrent febrile and afebrile seizures, ataxia, cognitive impairment, and developmental delays [ 1 , 2 ]. Onset typically occurs in early infancy and imposes a substantial burden on the quality of life for affected individuals [ 3 ]. It is primarily caused by mutations in the SCN1A gene, leading to neuronal hyperexcitability and intractable seizures [ 4 ]. Additionally, patients with DS are at risk of sudden unexplained death, making early and effective seizure control crucial [ 5 ].
The genetic basis of DS is largely attributed to heterozygous mutations in the NaV1.1 alpha subunit of voltage-gated sodium ion channels encoded by the SCN1A gene [ 6 , 7 ]. These mutations result in the loss of function of NaV1.1 channels, which is critical for normal brain function and leads to seizures and epilepsy [ 8 , 9 ]. In some cases, these pathogenic SCN1A variants can be inherited, while in others, de novo mutations occur [ 10 , 11 ]. Other genes like SCN1B, GABRA1, PCDH19, GABRG2, HCN1, and STXBP1 have also been implicated in DS, although not all cases are genetic, and not all genetic mutations result in DS [ 11 ].
DS typically manifests in the first year of life, often with a normal early childhood development followed by the onset of seizures around 4–12 months of age [ 12 ]. Seizures can be medically refractory, leading to recurrent status epilepticus and various comorbidities, including intellectual disability, ataxia, and an increased risk of early mortality [ 13 ]. Therefore, the impact of DS on affected individuals is profound, encompassing not only seizures but also developmental and cognitive challenges [ 14 , 15 , 16 ]. Despite several decades of research, current treatment options for DS remain limited, often necessitating the use of polypharmacy with antiepileptic drugs [ 17 ]. Medications like sodium valproate, topiramate, and stiripentol are commonly used, but some, like carbamazepine, should be avoided [ 18 ]. Recent studies have demonstrated the efficacy of stiripentol in managing seizures associated with DS [ 19 , 20 ]. Notably, stiripentol has shown potential as an additional therapy, offering a new avenue for improving seizure control in individuals with this challenging condition [ 21 ]. Additionally, dietary therapies, such as the ketogenic diet, and non-pharmacologic strategies, like avoiding seizure triggers, are considered [ 22 ]. In addition, emerging evidence suggests that other antiepileptic drugs with sodium channel-blocking properties, such as oxcarbazepine and lamotrigine, also pose a risk of exacerbating seizures in individuals with DS. While these interventions can reduce seizure frequency and disease severity, they do not address the underlying pathogenesis.
Cannabidiol (CBD), a non-psychoactive compound derived from the cannabis plant, has garnered attention as a potential treatment for DS [ 23 , 24 , 25 ]. Clinical trials, along with the FDA's approval of Epidiolex for DS and Lennox–Gastaut Syndrome, highlight CBD's potential as an alternative therapy [ 26 , 27 ]. Existing studies often focus on specific aspects of the potential treatment, such as seizure reduction or safety profiles, rather than providing a holistic view of CBD's efficacy and safety in managing the multifaceted challenges of DS [ 28 , 29 ].
While the predominant emphasis in the literature lies in investigating the impact of CBD on convulsive seizures in pediatric patients with DS. Studies have begun to shed light on the potential efficacy of CBD in mitigating various seizure manifestations beyond convulsions, such as absence seizures and myoclonic seizures [ 30 , 31 ]. Moreover, CBD has demonstrated a well-documented interaction with clobazam. Studies have consistently reported that co-administration of CBD and clobazam can lead to alterations in the pharmacokinetics of both substances [ 32 , 33 ]. This interaction highlights the necessity for close monitoring and potential dosage adjustments when utilising CBD alongside other anti-epileptics in the treatment of DS. This review aims to examine the existing body of evidence regarding the efficacy and safety of CBD in the management of DS, considering the limitations of current treatment options and the potential benefits of CBD-based therapies.
Methodology
Literature search strategy
In this study, an extensive search was conducted in PubMed, MEDLINE, Scopus, Web of Science, and relevant grey literature. The search incorporated a range of search terms, such as "cannabidiol," "CBD," "Dravet Syndrome," "seizures," and related keywords. Studies published from the inception of each database until the present were included in the search. The search was specifically limited to articles published in the English language. See Fig. 1 .
Inclusion and exclusion criteria
For inclusion, the study must be a clinical trial, published within the last 10 years to provide the latest evidence, published in the English language and focused on the use of CBD in the management of DS in individuals aged 18 or less. This review specifically looked at studies that addressed seizure reduction, safety profiles, and broader impacts on DS. Animal studies, meta-analyses, reviews, and observational studies were excluded.
Data extraction
For each selected study, relevant information, such as study design, sample size, patient demographics, CBD dosing regimen, treatment duration, outcomes measured, and reported results, was extracted. Two reviewers carried out the data extraction process independently, and any discrepancies were resolved through discussion and, if necessary, consultation with a third reviewer.
Data synthesis and analysis
A narrative synthesis approach was employed to summarise and analyse the findings of the selected studies. The effectiveness of CBD in reducing seizures, its impact on cognitive and developmental outcomes, and its safety profile were subject to thorough examination. Heterogeneity in study designs, patient populations, and dosing regimens was carefully considered when concluding, and any inconsistencies or discrepancies in the literature were brought to attention.
This study reviewed data from ten distinct clinical trials. See Table 1 . These interventions varied in terms of the type of CBD administered, which could be a pharmaceutical formulation or plant-derived. The doses of CBD ranged from 2 to 20 mg/kg/d, with varying treatment durations spanning from 4 to 72 weeks. Additionally, the frequency of administration varied, with some studies utilising once-daily dosing and others opting for twice-daily schedules. The duration of follow-up showed significant diversity across these studies, extending from 4 to 72 weeks. In total, these ten studies included 1,724 participants. On average, each study featured approximately 172.4 participants. CBD doses ranged from a minimum of 2.5 mg/kg/day to 30 mg/kg/day, with preparations typically being highly purified CBD in a 100 mg/ml oral solution, and the mean modal dose across the studies was approximately 22 mg/kg/day. The primary efficacy outcomes were evaluated based on the percentage reduction in convulsive and total seizures. Furthermore, clinical improvement was assessed through the Subject/Clinician Global Impression of Change (SCGIC) scale, and quality of life was measured using the Childhood Epilepsy Questionnaire. The reduction in seizure frequency for convulsive seizures ranged from 38 to 74%, and for total seizures, it varied from 40 to 84%. SCGIC scale reported improvements in the range of 81–84%. In addition, 24-h ambulatory EEG was utilised to monitor EEG spikes, aligning these outcome measures with those commonly employed to assess the efficacy of other antiepileptic drugs (AEDs).
Efficacy outcomes
The analysis of the ten clinical trials provides a profound understanding of the efficacy of CBD in pediatric patients with DS. Iannone et al. conducted a randomised open-label extension trial [ 34 ]. Their findings show that CBD at 25 mg/kg per day had a remarkable impact. At the 3-month follow-up, 40.2% of patients substantially reduced seizure frequency, with 1.2% experiencing seizure freedom. A particularly interesting aspect is the observed stability in patient retention across the diagnosis spectrum, suggesting the potential for CBD's consistent efficacy. This study also revealed that CBD's efficacy remained independent of the dosage used, which has implications for treatment optimisation.
Devinsky et al. study demonstrated a 48.7% reduction in convulsive seizure frequency and a 45.7% reduction in total seizure frequency [ 23 ]. Notably, the findings indicated that CBD at 20 mg/kg/ per day did not significantly influence concomitant antiepileptic drug (AED) levels, reinforcing its efficacy as an independent therapeutic agent. The open-label extension trial by Scheffer et al. highlighted the sustainability of CBD's efficacy at 22 mg/kg per day [ 18 ]. Patients in this study experienced sustained, clinically meaningful reductions in seizure frequency. After 12 weeks, add-on CBD treatment led to a 50% reduction in median monthly major motor seizures and a 44% reduction in total seizures. Moreover, the study reported that 83% or more of patients or caregivers noted an improvement in their overall condition. The inclusion of patients taking concomitant valproic acid provided valuable insights into CBD's potential as a long-term treatment option for those with DS.
Miller et al. embarked on a double-anonymized, placebo-controlled, randomised clinical trial involving pediatric patients aged 2 to 18 [ 28 ]. The study underscored the improved safety and tolerability profile of a 10-mg/kg/d CBD dosage, significantly advancing in treating children with treatment-resistant DS. In a study conducted by Halford et al. involving patients with an average age of 9.8 years, significant reductions in convulsive and total seizures were reported with CBD of 100 mg/mL in oral solution [ 35 ]. While over 80% of patients or caregivers noted improvements in their overall condition, it underscores the substantial enhancement in the quality of life for DS patients.
Linda et al. reported substantial reductions in major motor and total seizures with 10 mg/kg per day of CBD [ 24 ]. Devinsky et al. reported statistically significant reductions in convulsive and total seizure frequency with 20 mg/kg per day of CBD [ 36 ]. Additionally, the study noted improvements in Subject/Caregiver Global Impression of Change (S/CGIC) scores, demonstrating CBD's positive impact on seizure control and patients' overall well-being. Similarly, Bláthnaid et al. reported a statistically significant improvement in quality of life, a median motor seizure reduction of 70.6%, and a 50% responder rate of 63%, emphasising the transformative potential of CBD at 2 to 16 mg/kg per day in enhancing the lives of young patients with DS [ 22 ].
Devinsky et al. noted that CBD at 20 mg/kg per day led to a more substantial reduction in convulsive seizure frequency compared to a placebo [ 29 ]. However, it is important to recognise that this increased efficacy was associated with higher rates of adverse events, underscoring the importance of balancing therapeutic benefits with potential risks. In Devinsky et al., the study leading to FDA approval, the findings pointed to a statistically significant reduction in the median frequency of convulsive seizures per month with CBD at 20 mg/kg per day, reaffirming the potential of this treatment in effectively reducing seizure frequency in patients with DS [ 23 ].
In terms of efficacy, a noteworthy observation is the absence of substantial distinctions among different dosages, 5 mg/kg/day, 10 mg/kg/day, and 20 mg/kg/day, compared to the placebo [ 23 , 28 , 34 ]. All treatment groups exhibited considerable enhancements in reducing seizure frequency relative to the placebo; however, discernible variations between these dosage tiers were notably limited.
Safety outcomes
Ensuring the safety of CBD in pediatric patients with DD is paramount. Each of the reviewed clinical trials provides valuable insights into the safety profile of CBD in this patient population. The randomised open-label extension trial conducted by Iannone et al. reported that 31.2% of patients dropped out for various reasons. Common adverse events included somnolence (22.6%), diarrhoea (11.9%), transaminase elevation, and loss of appetite [ 34 ]. Notably, only 1.1% of patients met withdrawal criteria. In the case of Devinsky et al., the study identified common adverse events associated with CBD, including pyrexia, somnolence, decreased appetite, sedation, vomiting, ataxia, and abnormal behaviour [ 23 ]. Intriguingly, six patients taking CBD and valproate experienced elevated transaminases, but none met the criteria for drug-induced liver injury, and all patients eventually recovered. The study indicated that lethargy is particularly common in patients taking CBD alongside clobazam. The study emphasised that exposure to CBD and its metabolites increases proportionally with the dose.
The open-label extension trial by Scheffer et al. reported that adverse events occurred in 97% of patients, with the majority being mild (23%) or moderate (50%) [ 18 ]. Commonly reported adverse events included diarrhoea (43%), pyrexia (39%), decreased appetite (31%), and somnolence (28%). Importantly, 9% of patients experienced liver transaminase elevations greater than three times the upper limit of normal, although none of these cases led to severe liver injury. For Miller et al. specific safety data with percentages were not provided [ 28 ]. However, the study emphasised that long-term add-on CBD treatment for DS was generally well tolerated, with an adverse event profile similar to that observed in controlled trials. Similarly, for Halford et al. specific safety data with percentages were unavailable [ 35 ]. Nevertheless, it was reported that long-term treatment with add-on CBD in patients with DS produced sustained seizure reductions with no new safety concerns.
The data provided by Linda et al. did not specify the percentages of adverse events [ 24 ]. Nonetheless, it was noted that CBD had an acceptable safety profile. Safety data from this study emphasised the overall tolerability of CBD in patients with DS. Miller et al. reported that long-term add-on CBD treatment for DS was generally well tolerated [ 36 ]. McCoy et al. reported that adverse events common during titration included somnolence, anorexia, and diarrhoea [ 22 ]. Abnormalities of liver transaminases and platelets were observed with concomitant valproic acid therapy. Nevertheless, this THC-containing cannabinoid preparation was generally considered safe and well-tolerated.
The review of data from ten distinct clinical trials provides valuable insights into the use of CBD in pediatric patients with DS. These studies varied in terms of CBD type, dosage, treatment duration, and frequency of administration, yet they collectively shed light on the potential of CBD for managing DS.
The variability in CBD interventions across these studies presents challenges and opportunities for future research and clinical practice. While this diversity reflects real-world clinical scenarios, it complicates determining optimal treatment regimens. Whether pharmaceutical or plant-derived, the type of CBD administered could impact efficacy and safety. Moreover, the wide range of CBD doses and treatment durations underscores the need for further investigation into the most effective and sustainable treatment protocols. Additionally, the diverse frequency of administration across studies prompts whether once-daily or twice-daily dosing is more advantageous. Further exploration in this area could provide valuable guidance for treatment optimisation.
The collective findings from the ten clinical trials investigating the efficacy of CBD in pediatric patients with DS provide compelling insights into the potential of CBD as a therapeutic intervention. These trials consistently revealed notable reductions in both convulsive and total seizures, with some achieving remarkable results. For instance, Iannone et al. noted a 40.2% reduction in seizure frequency, with 1.2% of patients experiencing seizure freedom, while Devinsky et al. reported a 48.7% reduction in convulsive seizure frequency and a 45.7% reduction in total seizure frequency [ 23 , 34 ].
These outcomes suggest a promising avenue for treatment. However, there is a pressing need for long-term studies to assess the sustained efficacy and safety of CBD in DS patients. While short-term results are encouraging, understanding the effects of extended CBD treatment is crucial. Additionally, future research must identify optimal dosages, as personalised dosing strategies could enhance treatment outcomes and minimise potential risks. Comparative studies that assess CBD's efficacy in comparison to other treatments or in conjunction with standard antiepileptic drugs (AEDs) can provide additional insights into its role in the treatment landscape. Furthermore, investigating how genetic and clinical factors influence individual responses to CBD treatment is vital. Identifying potential predictors of treatment outcomes can facilitate treatment customisation and improve overall efficacy and safety.
Safety considerations are paramount when assessing the use of CBD in pediatric patients with DS. The trials revealed that while CBD holds promise as a therapeutic intervention, it is not without adverse effects. Common adverse events reported across the trials included somnolence, diarrhoea, pyrexia, decreased appetite, vomiting, ataxia, sedation, and abnormal behaviour. Although relatively common, these adverse events were generally mild to moderate in intensity. Notably, a fraction of patients experienced elevated liver transaminases, albeit without severe liver injury, emphasising the importance of vigilant monitoring for potential liver-related adverse events. Importantly, the dropout rates due to adverse events in these trials were generally low, suggesting that most patients could tolerate CBD treatment. The trials also highlighted that while adverse events were observed, most patients did not meet withdrawal criteria, indicating an overall favourable risk–benefit profile.
These trials' results underscore CBD's promising role in managing DS, providing hope for improved seizure management and quality of life. However, the variability in CBD interventions and the occurrence of adverse events necessitate further investigation. Future research should determine the most effective treatment regimens, considering the type, dose, duration, and frequency of CBD administration. Long-term effects and interactions with other antiepileptic medications also require thorough examination. These findings hold practical significance for clinicians managing pediatric DS patients, emphasising the need for individualised treatment plans and close monitoring for adverse events. CBD-based therapies offer a valuable addition to the existing treatment options for DS, potentially improving patient outcomes and quality of life.
Limitations of review
This review, which analysed data from ten distinct clinical trials involving many pediatric patients with DS, offers valuable insights into the efficacy and safety of CBD treatment. However, it is essential to acknowledge several limitations inherent to this review. The review's restriction to English-language studies poses a notable limitation. By focusing exclusively on English-language research, there is a risk of missing out on valuable non-English literature. This could introduce a language bias, potentially excluding relevant findings from studies conducted in other languages. Also, this review concentrated on clinical trials, thereby excluding observational studies. Despite these limitations, this systematic review offers valuable insights into CBD's potential benefits in managing DS. The synthesis of evidence and clinical implications outlined in the review provides a strong foundation for further research and clinical decision-making.
This review offers a comprehensive and in-depth analysis of the existing evidence on the efficacy and safety of CBD in pediatric patients diagnosed with DS. The findings, compiled from ten distinct clinical trials, consistently point to the potential of CBD as a valuable therapeutic option for managing DS. Notably, CBD remarkably reduces seizure frequency and enhances the overall quality of life for affected patients. One of the most intriguing findings is the consistent efficacy of CBD across various studies, irrespective of the dosage administered. This suggests that CBD holds promise as a treatment that can deliver reliable results for a broad spectrum of DS patients. However, it is crucial to underscore the critical balance between its increased efficacy in some cases and the higher occurrence of adverse events. This balance reinforces the need for a cautious and individualised approach to treatment, ensuring that the therapeutic benefits outweigh potential risks.
The results of this review have significant implications for clinical practice, research endeavours, and healthcare policies. Clinicians managing pediatric patients with DS should consider CBD as a valuable adjunct therapy, particularly for cases refractory to other treatments. However, it is imperative to stay updated with evolving research and best practices to optimise CBD treatment regimens. While this review sheds light on the potential of CBD in transforming the management of DS, it also emphasises the need for further research. Well-designed clinical trials are warranted to refine treatment protocols, explore the optimal CBD dosage, and assess the durability of its therapeutic effects. Addressing long-term safety concerns, especially when CBD is used in conjunction with other antiepileptic drugs, is crucial to ensure the well-being of DS patients. Future research should delve deeper into the underlying mechanisms of CBD's antiseizure effects and its potential interactions with other medications. This will enhance our understanding of CBD's role in DS management and open new avenues for therapeutic innovation.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Abbreviations
- Antiepileptic drugs
Cannabidiol
- Dravet Syndrome
Electroencephalogram
Food and Drug Administration
Sodium Voltage-Gated Channel Alpha Subunit 1
Severe Myoclonic Epilepsy of Infancy
Wirrell EC. Treatment of Dravet syndrome. Can J Neurol Sci. 2016;43(S3):S13–8.
Article PubMed Google Scholar
Lattanzi S, Brigo F, Trinka E, Zaccara G, Striano P, Del Giovane C, Silvestrini M. Adjunctive cannabidiol in patients with Dravet syndrome: a systematic review and meta-analysis of efficacy and safety. CNS Drugs. 2020;34:229–41.
Article CAS PubMed Google Scholar
Wu YW, Sullivan J, McDaniel SS, Meisler MH, Walsh EM, Li SX, Kuzniewicz MW. Incidence of Dravet syndrome in a US population. Pediatrics. 2015;136(5):e1310–5.
Article PubMed PubMed Central Google Scholar
Depienne C, Trouillard O, Saint-Martin C, Gourfinkel-An I, Bouteiller D, Carpentier W, Keren B, Abert B, Gautier A, Baulac S, Arzimanoglou A, Cazeneuve C, Nabbout R, LeGuern E. Spectrum of SCN1A gene mutations associated with Dravet syndrome: analysis of 333 patients. J Med Genet. 2009;46(3):183–91.
Lagae L, Brambilla I, Mingorance A, Gibson E, Battersby A. Quality of life and comorbidities associated with Dravet syndrome severity: a multinational cohort survey. Dev Med Child Neurol. 2018;60(1):63–72.
Jansen FE, Sadleir LG, Harkin LA, Vadlamudi L, McMahon JM, Mulley JC, Scheffer E, Berkovic SF. Severe myoclonic epilepsy of infancy (Dravet syndrome): recognition and diagnosis in adults. Neurology. 2006;67(12):2224–6.
Ziobro J, Eschbach K, Sullivan JE, Knupp KG. Current treatment strategies and future treatment options for Dravet syndrome. Curr Treat Options Neurol. 2018;20:1–15.
Article Google Scholar
White CM. A review of human studies assessing cannabidiol’s (CBD) therapeutic actions and potential. J Clin Pharmacol. 2019;59(7):923–34.
FDA Approves First Drug Comprised of an Active Ingredient Derived from Marijuana to Treat Rare, Severe Forms of Epilepsy (2018) https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-comprised-active-ingredient-derived-marijuana-treat-rare-severe-forms
Janković SM, Janković SV, Vojinović R, Lukić S. Investigational new drugs for the treatment of Dravet syndrome: an update. Expert Opin Investig Drugs. 2023. https://doi.org/10.1080/13543784.2023.2193680 .
Riva A, D’Onofrio G, Amadori E, Tripodi D, Balagura G, Iurilli V, et al. Current and promising therapeutic options for Dravet syndrome. Expert Opin Investig Drugs. 2023;23(15):1727–36. https://doi.org/10.1080/14656566.2022.2127089 .
Article CAS Google Scholar
Cooper MS, Mcintosh A, Crompton DE, McMahon JM, Schneider A, Farrell K, et al. Mortality in Dravet syndrome. Epilepsy Res. 2016;128:43–7. https://doi.org/10.1016/j.eplepsyres.2016.10.006 .
Myers KA. SCN1A as a therapeutic target for Dravet syndrome. Expert Opin Ther Targets. 2023;27(6):459–67.
Strzelczyk A, Lagae L, Wilmshurst J, Brunklaus A, Striano P, Rosenow F, et al. Dravet syndrome: a systematic literature review of the illness burden. Epilepsia Open. 2023;8:1256.
Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55(6):791–802. https://doi.org/10.1111/epi.12631 .
Article CAS PubMed PubMed Central Google Scholar
Morano A, Fanella M, Albini M, Cifelli P, Palma E, Giallonardo AT, et al. Cannabinoids in the treatment of epilepsy: current status and future prospects. Neuropsychiatr Dis Treat. 2020;16:381–96. https://doi.org/10.2147/NDT.S203782 .
Martínez-Aguirre C, Carmona-Cruz F, Velasco AL, Velasco F, Aguado-Carrillo G, Cuéllar-Herrera M, et al. Cannabidiol acts at 5-HT1A receptors in the human brain: relevance for treating temporal lobe epilepsy. Front Behav Neurosci. 2020;14: 611278. https://doi.org/10.3389/fnbeh.2020.611278 .
Scheffer IE, Halford JJ, Miller I, Nabbout R, Sanchez-Carpintero R, Shiloh-Malawsky Y, et al. Add-on cannabidiol in patients with Dravet syndrome: results of a long-term open-label extension trial. Epilepsia. 2021;62(10):2505–17. https://doi.org/10.1111/epi.17036 .
Strzelczyk A, Schubert-Bast S. A practical guide to the treatment of Dravet syndrome with anti-seizure medication. CNS Drugs. 2022;36(3):217–37. https://doi.org/10.1007/s40263-022-00898-1 .
Chiron C. Stiripentol for the treatment of seizures associated with Dravet syndrome. Expert Rev Neurother. 2019;19(4):301–10. https://doi.org/10.1080/14737175.2019.1593142 .
Frampton JE. Stiripentol: a review in Dravet syndrome. Drugs. 2019;79:1785–96. https://doi.org/10.1007/s40265-019-01204-y .
McCoy B, Wang L, Zak M, Al-Mehmadi S, Kabir N, Alhadid K, et al. A prospective open-label trial of a CBD/THC cannabis oil in Dravet syndrome. Ann Clin Transl Neurol. 2018;5(9):1077–88.
Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376(21):2011–20.
Laux LC, Bebin EM, Checketts D, Chez M, Flamini R, Marsh ED, et al. Long-term safety and efficacy of cannabidiol in children and adults with treatment resistant Lennox-Gastaut syndrome or Dravet syndrome: Expanded access program results. Epilepsy Res. 2019;1(154):13–20.
Seiden LG, Connor GS. The importance of drug titration in the management of patients with epilepsy. Epilepsy Behav. 2022;1(128): 108517.
Chalasani N, Bonkovsky HL, Stine JG, Gu J, Barnhart H, Jacobsen E, et al. Clinical characteristics of antiepileptic-induced liver injury in patients from the DILIN prospective study. J Hepatol. 2022;76(4):832–40.
Klotz KA, Schulze-Bonhage A, San Antonio-Arce V, Jacobs J. Cannabidiol for treatment of childhood epilepsy–a cross-sectional survey. Front Neurol. 2018;7(9):731.
Miller I, Scheffer IE, Gunning B, Sanchez-Carpintero R, Gil-Nagel A, Perry MS, et al. Dose-ranging effect of adjunctive oral cannabidiol vs placebo on convulsive seizure frequency in Dravet syndrome: a randomized clinical trial. JAMA Neurol. 2020;77(5):613–21.
Devinsky O, Patel AD, Thiele EA, Wong MH, Appleton R, Harden CL, et al. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90(14):e1204–11.
Miller I, Devinsky O, Nabbout R, Laux L, Zolnowska M, Wright S, et al. Maintenance of long-term safety and efficacy of cannabidiol (CBD) treatment in Dravet Syndrome (DS): results of the open-label extension (OLE) Trial (GWPCARE5) (S19.003). Neurology. 2018. https://doi.org/10.1212/WNL.90.15_supplement.S19.003 .
Bhardwaj AK, Allsop DJ, Copeland J, McGregor IS, Dunlop A, Shanahan M, et al. Randomised Controlled Trial (RCT) of cannabinoid replacement therapy (Nabiximols) for the management of treatment-resistant cannabis dependent patients: a study protocol. BMC Psychiatry. 2018;18(1):140.
Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84(11):2477–82. https://doi.org/10.1111/bcp.13710 .
Anderson LL, Absalom NL, Abelev SV, Low IK, Doohan PT, Martin LJ, Chebib M, McGregor IS, Arnold JC. Coadministered cannabidiol and clobazam: preclinical evidence for both pharmacodynamic and pharmacokinetic interactions. Epilepsia. 2019;60(11):2224–34. https://doi.org/10.1111/epi.16355 .
Iannone LF, Arena G, Battaglia D, Bisulli F, Bonanni P, Boni A, Canevini MP, Cantalupo G, Cesaroni E, Contin M, Coppola A, Cordelli DM, Cricchiuti G, De Giorgis V, De Leva MF, De Rinaldis M, d’Orsi G, Elia M, Galimberti C, Morano A, Granata T, Guerrini R, Lodi MAM, La Neve A, Marchese F, Masnada S, Michelucci R, Nosadini M, Pilolli N, Pruna D, Ragona F, Rosati A, Santucci M, Spalice A, Pietrafusa N, Striano P, Tartara E, Tassi L, Papa A, Zucca C, Russo E, Mecarelli O, CBD LICE Italy Study Group. Results from an Italian expanded access program on cannabidiol treatment in highly refractory Dravet Syndrome and Lennox-Gastaut Syndrome. Front Neurol. 2021;12: 673135. https://doi.org/10.3389/fneur.2021.673135 .
Halford J, Scheffer I, Nabbout R, Sanchez-Carpintero R, Shiloh Malawsky Y, Wong M, Checketts D, Van Landingham K. Long-term safety and efficacy of add-on cannabidiol (CBD) treatment in patients with dravet syndrome in an open-label extension trial (P55–005). Neurology. 2019;92(15 Supplement): P5.5-005.
Devinsky O, Nabbout R, Miller I, Laux L, Zolnowska M, Wright S, Roberts C. Long-term cannabidiol treatment in patients with Dravet syndrome: an open-label extension trial. Epilepsia. 2019;60(2):294–302. https://doi.org/10.1111/epi.14628 .
Download references
Acknowledgements
No funding was received for this study.
Author information
Authors and affiliations.
Department of Medicine and Surgery, Ladoke Akintola University Teaching Hospital, Ogbomoso, Nigeria
Nicholas Aderinto
Department of Medicine and Surgery, University of Ilorin, Ilorin, Nigeria
Gbolahan Olatunji & Emmanuel Kokori
Department of Medicine and Surgery, Obafemi Awolowo University Teaching Hospital, Ife, Nigeria
Yusuf Ismaila Ajayi
Department of Medicine and Surgery, University of Ibadan, Ibadan, Nigeria
Olumide Akinmoju
Department of Medicine, University of Abuja, Abuja, Nigeria
Abiola Samuel Ayedun
Bowen University, Iwo, Nigeria
Oluwapelumi Ikeoluwa Ayoola
Department of Paediatrics and Child Health, UNIOSUN Teaching Hospital, Osogbo, Nigeria
Noah Oluwaseun Aderinto
Department of Medicine and Surgery, Ladoke Akintola University of Technology, PMB 5000, Ogbomosho, Nigeria
You can also search for this author in PubMed Google Scholar
Contributions
NA conceptualised the study; all authors were involved in the literature review; YIA and NA extracted the data from the review studies; all authors wrote the final and first drafts. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Nicholas Aderinto .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Aderinto, N., Olatunji, G., Kokori, E. et al. The efficacy and safety of cannabidiol (CBD) in pediatric patients with Dravet Syndrome: a narrative review of clinical trials. Eur J Med Res 29 , 182 (2024). https://doi.org/10.1186/s40001-024-01788-6
Download citation
Received : 08 November 2023
Accepted : 11 March 2024
Published : 18 March 2024
DOI : https://doi.org/10.1186/s40001-024-01788-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cannabidiol (CBD)
- Pediatric patients
- Seizure frequency
European Journal of Medical Research
ISSN: 2047-783X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Hughes RG, editor. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008 Apr.

Patient Safety and Quality: An Evidence-Based Handbook for Nurses.
Chapter 7 the evidence for evidence-based practice implementation.
Marita G. Titler .
Affiliations
Overview of evidence-based practice.
Evidence-based health care practices are available for a number of conditions such as asthma, heart failure, and diabetes. However, these practices are not always implemented in care delivery, and variation in practices abound. 1–4 Traditionally, patient safety research has focused on data analyses to identify patient safety issues and to demonstrate that a new practice will lead to improved quality and patient safety. 5 Much less research attention has been paid to how to implement practices. Yet, only by putting into practice what is learned from research will care be made safer. 5 Implementing evidence-based safety practices are difficult and need strategies that address the complexity of systems of care, individual practitioners, senior leadership, and—ultimately—changing health care cultures to be evidence-based safety practice environments. 5
Nursing has a rich history of using research in practice, pioneered by Florence Nightingale. 6–9 Although during the early and mid-1900s, few nurses contributed to this foundation initiated by Nightingale, 10 the nursing profession has more recently provided major leadership for improving care through application of research findings in practice. 11
Evidence-based practice (EBP) is the conscientious and judicious use of current best evidence in conjunction with clinical expertise and patient values to guide health care decisions. 12–15 Best evidence includes empirical evidence from randomized controlled trials; evidence from other scientific methods such as descriptive and qualitative research; as well as use of information from case reports, scientific principles, and expert opinion. When enough research evidence is available, the practice should be guided by research evidence in conjunction with clinical expertise and patient values. In some cases, however, a sufficient research base may not be available, and health care decision making is derived principally from nonresearch evidence sources such as expert opinion and scientific principles. 16 As more research is done in a specific area, the research evidence must be incorporated into the EBP. 15
Models of Evidence-Based Practice
Multiple models of EBP are available and have been used in a variety of clinical settings. 16–36 Although review of these models is beyond the scope of this chapter, common elements of these models are selecting a practice topic (e.g., discharge instructions for individuals with heart failure), critique and syntheses of evidence, implementation, evaluation of the impact on patient care and provider performance, and consideration of the context/setting in which the practice is implemented. 15 , 17 The learning that occurs during the process of translating research into practice is valuable information to capture and feed back into the process, so that others can adapt the evidence-based guideline and/or the implementation strategies.
A recent conceptual framework for maximizing and accelerating the transfer of research results from the Agency for Healthcare Research and Quality (AHRQ) patient safety research portfolio to health care delivery was developed by the dissemination subcommittee of the AHRQ Patient Safety Research Coordinating Committee. 37 This model is a synthesis of concepts from scientific information on knowledge transfer, social marketing, social and organizational innovation, and behavior change (see Figure 1 ). 37 Although the framework is portrayed as a series of stages, the authors of this framework do not believe that the knowledge transfer process is linear; rather, activities occur simultaneously or in different sequences, with implementation of EBPs being a multifaceted process with many actors and systems.
AHRQ Model of Knowledge Transfer Adapted from Nieva, V., Murphy, R., Ridley, N., et al. Used with permission. http://www.ahrq.gov/qual/advances/
Steps of Evidence-Based Practice
Steps of promoting adoption of EBPs can be viewed from the perspective of those who conduct research or generate knowledge, 23 , 37 those who use the evidence-based information in practice, 16 , 31 and those who serve as boundary spanners to link knowledge generators with knowledge users. 19
Steps of knowledge transfer in the AHRQ model 37 represent three major stages: (1) knowledge creation and distillation, (2) diffusion and dissemination, and (3) organizational adoption and implementation. These stages of knowledge transfer are viewed through the lens of researchers/creators of new knowledge and begin with determining what findings from the patient safety portfolio or individual research projects ought to be disseminated.
Knowledge creation and distillation is conducting research (with expected variation in readiness for use in health care delivery systems) and then packaging relevant research findings into products that can be put into action—such as specific practice recommendations—thereby increasing the likelihood that research evidence will find its way into practice. 37 It is essential that the knowledge distillation process be informed and guided by end users for research findings to be implemented in care delivery. The criteria used in knowledge distillation should include perspectives of the end users (e.g., transportability to the real-world health care setting, feasibility, volume of evidence needed by health care organizations and clinicians), as well as traditional knowledge generation considerations (e.g., strength of the evidence, generalizability).
Diffusion and dissemination involves partnering with professional opinion leaders and health care organizations to disseminate knowledge that can form the basis of action (e.g., essential elements for discharge teaching for hospitalized patient with heart failure) to potential users. Dissemination partnerships link researchers with intermediaries that can function as knowledge brokers and connectors to the practitioners and health care delivery organizations. Intermediaries can be professional organizations such as the National Patient Safety Foundation or multidisciplinary knowledge transfer teams such as those that are effective in disseminating research-based cancer prevention programs. In this model, dissemination partnerships provide an authoritative seal of approval for new knowledge and help identify influential groups and communities that can create a demand for application of the evidence in practice. Both mass communication and targeted dissemination are used to reach audiences with the anticipation that early users will influence the latter adopters of the new usable, evidence-based research findings. Targeted dissemination efforts must use multifaceted dissemination strategies, with an emphasis on channels and media that are most effective for particular user segments (e.g., nurses, physicians, pharmacists).
End user adoption, implementation, and institutionalization is the final stage of the knowledge transfer process. 37 This stage focuses on getting organizations, teams, and individuals to adopt and consistently use evidence-based research findings and innovations in everyday practice. Implementing and sustaining EBPs in health care settings involves complex interrelationships among the EBP topic (e.g., reduction of medication errors), the organizational social system characteristics (such as operational structures and values, the external health care environment), and the individual clinicians. 35 , 37–39 A variety of strategies for implementation include using a change champion in the organization who can address potential implementation challenges, piloting/trying the change in a particular patient care area of the organization, and using multidisciplinary implementation teams to assist in the practical aspects of embedding innovations into ongoing organizational processes. 35 , 37 Changing practice takes considerable effort at both the individual and organizational level to apply evidence-based information and products in a particular context. 22 When improvements in care are demonstrated in the pilot studies and communicated to other relevant units in the organization, key personnel may then agree to fully adopt and sustain the change in practice. Once the EBP change is incorporated into the structure of the organization, the change is no longer considered an innovation but a standard of care. 22 , 37
In comparison, other models of EBP (e.g., Iowa Model of Evidence-based Practice to Promote Quality of Care 16 ) view the steps of the EBP process from the perspective of clinicians and/or organizational/clinical contexts of care delivery. When viewing steps of the EBP process through the lens of an end user, the process begins with selecting an area for improving care based on evidence (rather than asking what findings ought to be disseminated); determining the priority of the potential topic for the organization; formulating an EBP team composed of key stakeholders; finding, critiquing, and synthesizing the evidence; setting forth EBP recommendations, with the type and strength of evidence used to support each clearly documented; determining if the evidence findings are appropriate for use in practice; writing an EBP standard specific to the organization; piloting the change in practice; implementing changes in practice in other relevant practice areas (depending on the outcome of the pilot); evaluating the EBP changes; and transitioning ongoing quality improvement (QI) monitoring, staff education, and competency review of the EBP topic to appropriate organizational groups as defined by the organizational structure. 15 , 40 The work of EBP implementation from the perspective of the end user is greatly facilitated by efforts of AHRQ, professional nursing organizations (e.g., Oncology Nursing Society), and others that distill and package research findings into useful products and tools for use at the point of care delivery.
When the clinical questions of end users can be addressed through use of existing evidence that is packaged with end users in mind, steps of the EBP process take less time and more effort can be directed toward the implementation, evaluation, and sustainability components of the process. For example, finding, critiquing, and synthesizing the evidence; setting forth EBP recommendations with documentation of the type and strength of evidence for each recommendation; and determining appropriateness of the evidence for use in practice are accelerated when the knowledge-based information is readily available. Some distilled research findings also include quick reference guides that can be used at the point of care and/or integrated into health care information systems, which also helps with implementation. 41 , 42
Translation Science: An Overview
Translation science is the investigation of methods, interventions, and variables that influence adoption by individuals and organizations of EBPs to improve clinical and operational decisionmaking in health care. 35 , 43–46 This includes testing the effect of interventions on promoting and sustaining adoption of EBPs. Examples of translation studies include describing facilitators and barriers to knowledge uptake and use, organizational predictors of adherence to EBP guidelines, attitudes toward EBPs, and defining the structure of the scientific field. 11 , 47–49
Translation science must be guided by a conceptual model that organizes the strategies being tested, elucidates the extraneous variables (e.g., behaviors and facilitators) that may influence adoption of EBPs (e.g., organizational size, characteristics of users), and builds a scientific knowledge base for this field of inquiry. 15 , 50 Conceptual models used in the translating-research-into-practice studies funded by AHRQ were adult learning, health education, social influence, marketing, and organizational and behavior theories. 51 Investigators have used Rogers’s Diffusion of Innovation model, 35 , 39 , 52–55 the Promoting Action on Research Implementation in Health Services (PARIHS) model, 29 the push/pull framework, 23 , 56 , 57 the decisionmaking framework, 58 and the Institute for Healthcare Improvement (IHI) model 59 in translation science.
Study findings regarding evidence-based practices in a diversity of health care settings are building an empirical foundation of translation science. 19 , 43 , 51 , 60–83 These investigations and others 18 , 84–86 provide initial scientific knowledge to guide us in how to best promote use of evidence in practice. To advance knowledge about promoting and sustaining adoption of EBPs in health care, translation science needs more studies that test translating research into practice (TRIP) interventions: studies that investigate what TRIP interventions work, for whom, in what circumstances, in what types of settings; and studies that explain the underlying mechanisms of effective TRIP interventions. 35 , 49 , 79 , 87 Partnership models, which encourage ongoing interaction between researchers and practitioners, may be the way forward to carry out such studies. 56 Challenges, issues, methods, and instruments used in translation research are described elsewhere. 11 , 19 , 49 , 78 , 88–97
- Research Evidence
What Is Known About Implementing Evidence-Based Practices?
Multifaceted implementation strategies are needed to promote use of research evidence in clinical and administrative health care decisionmaking. 15 , 22 , 37 , 45 , 64 , 72 , 77 , 79 , 98 , 99 Although Grimshaw and colleagues 65 suggest that multifaceted interventions are no more effective than single interventions, context (site of care delivery) was not incorporated in the synthesis methodology. As noted by others, the same TRIP intervention may meet with varying degrees of effectiveness when applied in different contexts. 35 , 49 , 79 , 80 , 87 , 100 , 101 Implementation strategies also need to address both the individual practitioner and organizational perspective. 15 , 22 , 37 , 64 , 72 , 77 , 79 , 98 When practitioners decide individually what evidence to use in practice, considerable variability in practice patterns result, 71 potentially resulting in adverse patient outcomes.
For example, an “individual” perspective of EBP would leave the decision about use of evidence-based endotracheal suctioning techniques to each nurse and respiratory therapist. Some individuals may be familiar with the research findings for endotracheal suctioning while others may not. This is likely to result in different and conflicting practices being used as people change shifts every 8 to 12 hours. From an organizational perspective, endotracheal suctioning policies and procedures based on research are written, the evidence-based information is integrated into the clinical information systems, and adoption of these practices by nurses and other practitioners is systematically promoted in the organization. This includes assuring that practitioners have the necessary knowledge, skills, and equipment to carry out the evidence-based endotracheal suctioning practice. The organizational governance supports use of these practices through various councils and committees such as the Practice Committee, Staff Education Committee, and interdisciplinary EBP work groups.
The Translation Research Model, 35 built on Rogers’s seminal work on diffusion of innovations, 39 provides a guiding framework for testing and selecting strategies to promote adoption of EBPs. According to the Translation Research Model, adoption of innovations such as EBPs are influenced by the nature of the innovation (e.g., the type and strength of evidence, the clinical topic) and the manner in which it is communicated (disseminated) to members (nurses) of a social system (organization, nursing profession). 35 Strategies for promoting adoption of EBPs must address these four areas (nature of the EBP topic; users of the evidence; communication; social system) within a context of participative change (see Figure 2 ). This model provided the framework for a multisite study that tested the effectiveness of a multifaceted TRIP intervention designed to promote adoption of evidence-based acute pain management practices for hospitalized older adults. The intervention improved the quality of acute pain management practices and reduced costs. 81 The model is currently being used to test the effectiveness of a multifaceted TRIP intervention to promote evidence-based cancer pain management of older adults in home hospice settings. * This guiding framework is used herein to overview what is known about implementation interventions to promote use of EBPs in health care systems (see Evidence Table ).
*Implementation Model Redrawn from Rogers EM. Diffusion of innovations. 5th ed. New York: The Free Press; 2003; Titler MG, Everett LQ. Translating research into practice: considerations for critical care investigators. Crit Care Nurs Clin North Am 2001a;13(4):587-604. (more...)
Evidence Table
Evidence-Based Practice in Nursing
Nature of the Innovation or Evidence-Based Practice
Characteristics of an innovation or EBP topic that affect adoption include the relative advantage of the EBP (e.g., effectiveness, relevance to the task, social prestige); the compatibility with values, norms, work, and perceived needs of users; and complexity of the EBP topic. 39 For example, EBP topics that are perceived by users as relatively simple (e.g., influenza vaccines for older adults) are more easily adopted in less time than those that are more complex (acute pain management for hospitalized older adults). Strategies to promote adoption of EBPs related to characteristics of the topic include practitioner review and “reinvention” of the EBP guideline to fit the local context, use of quick reference guides and decision aids, and use of clinical reminders. 53 , 59 , 60 , 65 , 74 , 82 , 102–107 An important principle to remember when planning implementation of an EBP is that the attributes of the EBP topic as perceived by users and stakeholders (e.g., ease of use, valued part of practice) are neither stable features nor sure determinants of their adoption. Rather it is the interaction among the characteristics of the EBP topic, the intended users, and a particular context of practice that determines the rate and extent of adoption. 22 , 35 , 39
Studies suggest that clinical systems, computerized decision support, and prompts that support practice (e.g., decisionmaking algorithms, paper reminders) have a positive effect on aligning practices with the evidence base. 15 , 51 , 65 , 74 , 80 , 82 , 102 , 104 , 107–110 Computerized knowledge management has consistently demonstrated significant improvements in provider performance and patient outcomes. 82 Feldman and colleagues, using a just-in-time e-mail reminder in home health care, have demonstrated (1) improvements in evidence-based care and outcomes for patients with heart failure, 64 , 77 and (2) reduced pain intensity for cancer patients. 75 Clinical information systems should deploy the evidence base to the point of care and incorporate computer decision-support software that integrates evidence for use in clinical decisionmaking about individual patients. 40 , 104 , 111–114 There is still much to learn about the “best” manner of deploying evidence-based information through electronic clinical information systems to support evidence-based care. 115
Methods of Communication
Interpersonal communication channels, methods of communication, and influence among social networks of users affect adoption of EBPs. 39 Use of mass media, opinion leaders, change champions, and consultation by experts along with education are among strategies tested to promote use of EBPs. Education is necessary but not sufficient to change practice, and didactic continuing education alone does little to change practice behavior. 61 , 116 There is little evidence that interprofessional education as compared to discipline-specific education improves EBP. 117 Interactive education, used in combination with other practice-reinforcing strategies, has more positive effects on improving EBP than didactic education alone. 66 , 68 , 71 , 74 , 118 , 119 There is evidence that mass media messages (e.g., television, radio, newspapers, leaflets, posters and pamphlets), targeted at the health care consumer population, have some effect on use of health services for the targeted behavior (e.g., colorectal cancer screening). However, little empirical evidence is available to guide framing of messages communicated through planned mass media campaigns to achieve the intended change. 120
Several studies have demonstrated that opinion leaders are effective in changing behaviors of health care practitioners, 22 , 68 , 79 , 100 , 116 , 121–123 especially in combination with educational outreach or performance feedback. Opinion leaders are from the local peer group, viewed as a respected source of influence, considered by associates as technically competent, and trusted to judge the fit between the innovation and the local situation. 39 , 116 , 121 , 124–127 With their wide sphere of influence across several microsystems/units, opinion leaders’ use of the innovation influences peers and alters group norms. 39 , 128 The key characteristic of an opinion leader is that he or she is trusted to evaluate new information in the context of group norms. Opinion leadership is multifaceted and complex, with role functions varying by the circumstances, but few successful projects to implement innovations in organizations have managed without the input of identifiable opinion leaders. 22 , 35 , 39 , 81 , 96 Social interactions such as “hallway chats,” one-on-one discussions, and addressing questions are important, yet often overlooked components of translation. 39 , 59 Thus, having local opinion leaders discuss the EBPs with members of their peer group is necessary to translate research into practice. If the EBP that is being implemented is interdisciplinary in nature, discipline-specific opinion leaders should be used to promote the change in practice. 39
Change champions are also helpful for implementing innovations. 39 , 49 , 81 , 129–131 They are practitioners within the local group setting (e.g., clinic, patient care unit) who are expert clinicians, passionate about the innovation, committed to improving quality of care, and have a positive working relationship with other health care professionals. 39 , 125 , 131 , 132 They circulate information, encourage peers to adopt the innovation, arrange demonstrations, and orient staff to the innovation. 49 , 130 The change champion believes in an idea; will not take “no” for an answer; is undaunted by insults and rebuffs; and, above all, persists. 133 Because nurses prefer interpersonal contact and communication with colleagues rather than Internet or traditional sources of practice knowledge, 134–137 it is imperative that one or two change champions be identified for each patient care unit or clinic where the change is being made for EBPs to be enacted by direct care providers. 81 , 138 Conferencing with opinion leaders and change champions periodically during implementation is helpful to address questions and provide guidance as needed. 35 , 66 , 81 , 106
Because nurses’ preferred information source is through peers and social interactions, 134–137 , 139 , 140 using a core group in conjunction with change champions is also helpful for implementing the practice change. 16 , 110 , 141 A core group is a select group of practitioners with the mutual goal of disseminating information regarding a practice change and facilitating the change by other staff in their unit/microsystem. 142 Core group members represent various shifts and days of the week and become knowledgeable about the scientific basis for the practice; the change champion educates and assists them in using practices that are aligned with the evidence. Each member of the core group, in turn, takes the responsibility for imparting evidence-based information and effecting practice change with two or three of their peers. Members assist the change champion and opinion leader with disseminating the EBP information to other staff, reinforce the practice change on a daily basis, and provide positive feedback to those who align their practice with the evidence base. 15 Using a core-group approach in conjunction with a change champion results in a critical mass of practitioners promoting adoption of the EBP. 39
Educational outreach, also known as academic detailing, promotes positive changes in practice behaviors of nurses and physicians. 22 , 64 , 66 , 71 , 74 , 75 , 77 , 81 , 119 , 143 Academic detailing is done by a topic expert, knowledgeable of the research base (e.g., cancer pain management), who may be external to the practice setting; he or she meets one-on-one with practitioners in their setting to provide information about the EBP topic. These individuals are able to explain the research base for the EBPs to others and are able to respond convincingly to challenges and debates. 22 This strategy may include providing feedback on provider or team performance with respect to selected EBP indicators (e.g., frequency of pain assessment). 66 , 81 , 119
Users of the Innovation or Evidence-Based Practice
Members of a social system (e.g., nurses, physicians, clerical staff) influence how quickly and widely EBPs are adopted. 39 Audit and feedback, performance gap assessment (PGA), and trying the EBP are strategies that have been tested. 15 , 22 , 65 , 66 , 70–72 , 81 , 98 , 124 , 144 PGA and audit and feedback have consistently shown a positive effect on changing practice behavior of providers. 65 , 66 , 70 , 72 , 81 , 98 , 124 , 144 , 145 PGA (baseline practice performance) informs members, at the beginning of change, about a practice performance and opportunities for improvement. Specific practice indicators selected for PGA are related to the practices that are the focus of evidence-based practice change, such as every-4-hour pain assessment for acute pain management. 15 , 66 , 81
Auditing and feedback are ongoing processes of using and assessing performance indicators (e.g., every-4-hour pain assessment), aggregating data into reports, and discussing the findings with practitioners during the practice change. 22 , 49 , 66 , 70 , 72 , 81 , 98 , 145 This strategy helps staff know and see how their efforts to improve care and patient outcomes are progressing throughout the implementation process. Although there is no clear empirical evidence for how to provide audit and feedback, 70 , 146 effects may be larger when clinicians are active participants in implementing change and discuss the data rather than being passive recipients of feedback reports. 67 , 70 Qualitative studies provide some insight into use of audit and feedback. 60 , 67 One study on use of data feedback for improving treatment of acute myocardial infarction found that (1) feedback data must be perceived by physicians as important and valid, (2) the data source and timeliness of data feedback are critical to perceived validity, (3) time is required to establish credibility of data within a hospital, (4) benchmarking improves the validity of the data feedback, and (5) physician leaders can enhance the effectiveness of data feedback. Data feedback that profiles an individual physician’s practices can be effective but may be perceived as punitive; data feedback must persist to sustain improved performance; and effectiveness of data feedback is intertwined with the organizational context, including physician leadership and organizational culture. 60 Hysong and colleagues 67 found that high-performing institutions provided timely, individualized, nonpunitive feedback to providers, whereas low performers were more variable in their timeliness and nonpunitiveness and relied more on standardized, facility-level reports. The concept of useful feedback emerged as the core concept around which timeliness, individualization, nonpunitiveness, and customizability are important.
Users of an innovation usually try it for a period of time before adopting it in their practice. 22 , 39 , 147 When “trying an EBP” (piloting the change) is incorporated as part of the implementation process, users have an opportunity to use it for a period of time, provide feedback to those in charge of implementation, and modify the practice if necessary. 148 Piloting the EBP as part of implementation has a positive influence on the extent of adoption of the new practice. 22 , 39 , 148
Characteristics of users such as educational preparation, practice specialty, and views on innovativeness may influence adoption of an EBP, although findings are equivocal. 27 , 39 , 130 , 149–153 Nurses’ disposition to critical thinking is, however, positively correlated with research use, 154 and those in clinical educator roles are more likely to use research than staff nurses or nurse managers. 155
Social System
Clearly, the social system or context of care delivery matters when implementing EBPs. 2 , 30 , 33 , 39 , 60 , 84 , 85 , 91 , 92 , 101 , 156–163 For example, investigators demonstrated the effectiveness of a prompted voiding intervention for urinary incontinence in nursing homes, but sustaining the intervention in day-to-day practice was limited when the responsibility of carrying out the intervention was shifted to nursing home staff (rather than the investigative team) and required staffing levels in excess of a majority of nursing home settings. 164 This illustrates the importance of embedding interventions into ongoing processes of care.
Several organizational factors affect adoption of EBPs. 22 , 39 , 79 , 134 , 165–167 Vaughn and colleagues 101 demonstrated that organizational resources, physician full-time employees (FTEs) per 1,000 patient visits, organizational size, and whether the facility was located in or near a city affected use of evidence in the health care system of the Department of Veterans Affairs (VA). Large, mature, functionally differentiated organizations (e.g., divided into semiautonomous departments and units) that are specialized, with a focus of professional knowledge, slack resources to channel into new projects, decentralized decisionmaking, and low levels of formalization will more readily adopt innovations such as new practices based on evidence. Larger organizations are generally more innovative because size increases the likelihood that other predictors of innovation adoption—such as slack financial and human resources and differentiation—will be present. However, these organizational determinants account for only about 15 percent of the variation in innovation adoption between comparable organizations. 22 Adler and colleagues 168 hypothesize that while more structurally complex organizations may be more innovative and hence adopt EBPs relatively early, less structurally complex organizations may be able to diffuse EBPs more effectively. Establishing semiautonomous teams is associated with successful implementation of EBPs, and thus should be considered in managing organizational units. 168–170
As part of the work of implementing EBPs, it is important that the social system—unit, service line, or clinic—ensures that policies, procedures, standards, clinical pathways, and documentation systems support the use of the EBPs. 49 , 68 , 72 , 73 , 103 , 140 , 171 Documentation forms or clinical information systems may need revision to support changes in practice; documentation systems that fail to readily support the new practice thwart change. 82
Absorptive capacity for new knowledge is another social system factor that affects adoption of EBPs. Absorptive capacity is the knowledge and skills to enact the EBPs; the strength of evidence alone will not promote adoption. An organization that is able to systematically identify, capture, interpret, share, reframe, and recodify new knowledge, and put it to appropriate use, will be better able to assimilate EBPs. 82 , 103 , 172 , 173 A learning organizational culture and proactive leadership that promotes knowledge sharing are important components of building absorptive capacity for new knowledge. 66 , 139 , 142 , 174 Components of a receptive context for EBP include strong leadership, clear strategic vision, good managerial relations, visionary staff in key positions, a climate conducive to experimentation and risk taking, and effective data capture systems. Leadership is critical in encouraging organizational members to break out of the convergent thinking and routines that are the norm in large, well-established organizations. 4 , 22 , 39 , 122 , 148 , 163 , 175
An organization may be generally amenable to innovations but not ready or willing to assimilate a particular EBP. Elements of system readiness include tension for change, EBP-system fit, assessment of implications, support and advocacy for the EBP, dedicated time and resources, and capacity to evaluate the impact of the EBP during and following implementation. If there is tension around specific work or clinical issues and staff perceive that the situation is intolerable, a potential EBP is likely to be assimilated if it can successfully address the issues, and thereby reduce the tension. 22 , 175
Assessing and structuring workflow to fit with a potential EBP is an important component of fostering adoption. If implications of the EBP are fully assessed, anticipated, and planned for, the practice is more likely to be adopted. 148 , 162 , 176 If supporters for a specific EBP outnumber and are more strategically placed within the organizational power base than opponents, the EBP is more likely to be adopted by the organization. 60 , 175 Organizations that have the capacity to evaluate the impact of the EBP change are more likely to assimilate it. Effective implementation needs both a receptive climate and a good fit between the EBP and intended adopters’ needs and values. 22 , 60 , 140 , 175 , 177
Leadership support is critical for promoting use of EBPs. 33 , 59 , 72 , 85 , 98 , 122 , 178–181 This support, which is expressed verbally, provides necessary resources, materials, and time to fulfill assigned responsibilities. 148 , 171 , 182 , 183 Senior leaders need to create an organizational mission, vision, and strategic plan that incorporate EBP; implement performance expectations for staff that include EBP work; integrate the work of EBP into the governance structure of the health care system; demonstrate the value of EBPs through administrative behaviors; and establish explicit expectations that nurse leaders will create microsystems that value and support clinical inquiry. 122 , 183 , 184
A recent review of organizational interventions to implement EBPs for improving patient care examined five major aspects of patient care. The review suggests that revision of professional roles (changing responsibilities and work of health professionals such as expanding roles of nurses and pharmacists) improved processes of care, but it was less clear about the effect on improvement of patient outcomes. Multidisciplinary teams (collaborative practice teams of physicians, nurses, and allied health professionals) treating mostly patients with prevalent chronic diseases resulted in improved patient outcomes. Integrated care services (e.g., disease management and case management) resulted in improved patient outcomes and cost savings. Interventions aimed at knowledge management (principally via use of technology to support patient care) resulted in improved adherence to EBPs and patient outcomes. The last aspect, quality management, had the fewest reviews available, with the results uncertain. A number of organizational interventions were not included in this review (e.g., leadership, process redesign, organizational learning), and the authors note that the lack of a widely accepted taxonomy of organizational interventions is a problem in examining effectiveness across studies. 82
An organizational intervention that is receiving increasing attention is tailored interventions to overcome barriers to change. 162 , 175 , 185 This type of intervention focuses on first assessing needs in terms of what is causing the gap between current practice and EBP for a specified topic, what behaviors and/or mechanism need to change, what organizational units and persons should be involved, and identification of ways to facilitate the changes. This information is then used in tailoring an intervention for the setting that will promote use of the specified EBP. Based on a recent systematic review, effectiveness of tailored implementation interventions remains uncertain. 185
In summary, making an evidence-based change in practice involves a series of action steps and a complex, nonlinear process. Implementing the change will take several weeks to months, depending on the nature of the practice change. Increasing staff knowledge about a specific EBP and passive dissemination strategies are not likely to work, particularly in complex health care settings. Strategies that seem to have a positive effect on promoting use of EBPs include audit and feedback, use of clinical reminders and practice prompts, opinion leaders, change champions, interactive education, mass media, educational outreach/academic detailing, and characteristics of the context of care delivery (e.g., leadership, learning, questioning). It is important that senior leadership and those leading EBP improvements are aware of change as a process and continue to encourage and teach peers about the change in practice. The new practice must be continually reinforced and sustained or the practice change will be intermittent and soon fade, allowing more traditional methods of care to return. 15
- Practice Implications From Translation Science
Principles of Evidence-Based Practice for Patient Safety
Several translation science principles are informative for implementing patient safety initiatives:
- First, consider the context and engage health care personnel who are at the point of care in selecting and prioritizing patient safety initiatives, clearly communicating the evidence base (strength and type) for the patient safety practice topic(s) and the conditions or setting to which it applies. These communication messages need to be carefully designed and targeted to each stakeholder user group.
- Second, illustrate, through qualitative or quantitative data (e.g., near misses, sentinel events, adverse events, injuries from adverse events), the reason the organization and individuals within the organization should commit to an evidence-based safety practice topic. Clinicians tend to be more engaged in adopting patient safety initiatives when they understand the evidence base of the practice, in contrast to administrators saying, “We must do this because it is an external regulatory requirement.” For example, it is critical to converse with busy clinicians about the evidence-based rationale for doing fall-risk assessment, and to help them understand that fall-risk assessment is an external regulatory agency expectation because the strength of the evidence supports this patient safety practice.
- Third, didactic education alone is never enough to change practice; one-time education on a specific safety initiative is not enough. Simply improving knowledge does not necessarily improve practice. Rather, organizations must invest in the tools and skills needed to create a culture of evidence-based patient safety practices where questions are encouraged and systems are created to make it easy to do the right thing.
- Fourth, the context of EBP improvements in patient safety need to be addressed at each step of the implementation process; piloting the change in practice is essential to determine the fit between the EBP patient safety information/innovation and the setting of care delivery. There is no one way to implement, and what works in one agency may need modification to fit the organizational culture of another context.
- Finally, it is important to evaluate the processes and outcomes of implementation. Users and stakeholders need to know that the efforts to improve patient safety have a positive impact on quality of care. For example, if a new barcoding system is being used to administer blood products, it is imperative to know that the steps in the process are being followed (process indicators) and that the change in practice is resulting in fewer blood product transfusion errors (outcome indicators).
Research Implications
Translation science is young, and although there is a growing body of knowledge in this area, we have, to date, many unanswered questions. These include the type of audit and feedback (e.g., frequency, content, format) strategies that are most effective, the characteristics of opinion leaders that are critical for success, the role of specific context variables, and the combination of strategies that are most effective. We also know very little about use of tailored implementation interventions, or the key context attributes to assess and use in developing and testing tailored interventions. The types of clinical reminders that are most effective for making EBP knowledge available at the point of care require further empirical explanation. We also know very little about the intensity and intervention dose of single and multifaceted strategies that are effective for promoting and sustaining use of EBPs or how the effectiveness differs by type of topic (e.g., simple versus complex). Only recently has the context of care delivery been acknowledged as affecting use of evidence, and further empirical work is needed in this area to understand how complex adaptive systems of practice incorporate knowledge acquisition and use. Lastly, we do not know what strategies or combination of strategies work for whom, in what context, why they work in some settings or cases and not others, and what is the mechanism by which these strategies or combination of strategies work.
This is an exciting area of investigation that has a direct impact on implementing patient safety practices. In planning investigations, researchers must use a conceptual model to guide the research and add to the empirical and theoretical understanding of this field of inquiry. Additionally, funding is needed for implementation studies that focus on evidence-based patient safety practices as the topic of concern. To generalize empirical findings from patient safety implementation studies, we must have a better understanding of what implementation strategies work, with whom, and in what types of settings, and we must investigate the underlying mechanisms of these strategies. This is likely to require mixed methods, a better understanding of complexity science, and greater appreciation for nontraditional methods and realistic inquiry. 87
Although the science of translating research into practice is fairly new, there is some guiding evidence of what implementation interventions to use in promoting patient safety practices. However, there is no magic bullet for translating what is known from research into practice. To move evidence-based interventions into practice, several strategies may be needed. Additionally, what works in one context of care may or may not work in another setting, thereby suggesting that context variables matter in implementation. 80
- Search Strategy
Several electronic databases were searched (MEDLINE ® , CINAHL ® , PubMed ® ) using terms of evidence-based practice research, implementation research, and patient safety. (The terms “quality improvement” or “quality improvement intervention research” were not used.) The Cochrane Collaboration–Cochrane Reviews was also searched to look for systematic reviews of specific implementation strategies, and the Journal of Implementation Science was also reviewed. I also requested the final reports of the TRIP I and TRIP II studies funded by AHRQ. Classic articles known to the author were also included in this chapter (e.g.,Locock et al. 123 ).
*Principal Investigator: Keela Herr (R01 grant no. CA115363-01; National Cancer Institute (NCI))Background
- Cite this Page Titler MG. The Evidence for Evidence-Based Practice Implementation. In: Hughes RG, editor. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008 Apr. Chapter 7.
- PDF version of this page (470K)
In this Page
Other titles in this collection.
- Advances in Patient Safety
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- It Is Not That Simple nor Compelling! Comment on "Translating Evidence Into Healthcare Policy and Practice: Single Versus Multi-faceted Implementation Strategies - Is There a Simple Answer to a Complex Question?". [Int J Health Policy Manag. 2015] It Is Not That Simple nor Compelling! Comment on "Translating Evidence Into Healthcare Policy and Practice: Single Versus Multi-faceted Implementation Strategies - Is There a Simple Answer to a Complex Question?". Bucknall T, Fossum M. Int J Health Policy Manag. 2015 Jul 28; 4(11):787-8. Epub 2015 Jul 28.
- Nursing implementation science: how evidence-based nursing requires evidence-based implementation. [J Nurs Scholarsh. 2008] Nursing implementation science: how evidence-based nursing requires evidence-based implementation. van Achterberg T, Schoonhoven L, Grol R. J Nurs Scholarsh. 2008; 40(4):302-10.
- Review The science of implementation: changing the practice of critical care. [Curr Opin Crit Care. 2008] Review The science of implementation: changing the practice of critical care. Weinert CR, Mann HJ. Curr Opin Crit Care. 2008 Aug; 14(4):460-5.
- Review Integrative review of implementation strategies for translation of research-based evidence by nurses. [Clin Nurse Spec. 2014] Review Integrative review of implementation strategies for translation of research-based evidence by nurses. Wuchner SS. Clin Nurse Spec. 2014 Jul-Aug; 28(4):214-23.
- Review A realist analysis of hospital patient safety in Wales: applied learning for alternative contexts from a multisite case study [ 2015] Review A realist analysis of hospital patient safety in Wales: applied learning for alternative contexts from a multisite case study Herepath A, Kitchener M, Waring J. 2015 Sep
Recent Activity
- The Evidence for Evidence-Based Practice Implementation - Patient Safety and Qua... The Evidence for Evidence-Based Practice Implementation - Patient Safety and Quality
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
VIDEO
COMMENTS
Background: Quality-of-care improvement and prevention of practice errors is dependent on nurses' adherence to the principles of patient safety.Aims: This paper aims to provide a systematic review of the international literature, to synthesise knowledge and explore factors that influence nurses' adherence to patient-safety principles.Methods: Electronic databases in English, Norwegian, and ...
Objectives To produce a narrative synthesis of published academic and grey literature focusing on patient safety outcomes for people with learning disabilities in an acute hospital setting. Design Scoping review with narrative synthesis. Methods The review followed the six stages of the Arksey and O'Malley framework. We searched four research databases from January 2000 to March 2021, in ...
Patient safety is one of the overarching goals of patient care and quality management. Of the many quality management frameworks, Beauchamp and Childress's four principles of biomedical ethics presents aspects of patient centeredness in clinical care. The Institute of Medicine's six aims for improvement encapsulates elements of high-quality patient care. The Institute of Healthcare ...
A patient safety learning system (also called a critical incident reporting system) refers to structured reporting, collation, and analysis of such incidents. 2 Potential benefits of using this system are as follows: learning from adverse events. monitoring of underlying trends and patterns to allow early detection of future adverse events.
Objectives: To produce a narrative synthesis of published academic and grey literature focusing on patient safety outcomes for people with learning disabilities in an acute hospital setting. Design: Scoping review with narrative synthesis. Methods: The review followed the six stages of the Arksey and O'Malley framework. We searched four research databases from January 2000 to March 2021, in ...
Within the field of patient safety research, there is a focus on the incidence of adverse events. ... findings from a meta-synthesis: Patient's experiences bedside shift-to-shift: Abstract review: Tobiano et al. ... The literature presented support the use of a verbal handoff supplemented with written documentation in a structured format or ...
The literature identifies various system, service and clinical factors that support effective patient engagement such as education about their condition, 13, 14 empowerment to engage 15, 16 and the willingness and ability of clinicians and patients to communicate about safety. 17-20 The extent to which an organisation is committed to patient ...
The investigation of patient safety in mental health inpatient settings using secondary data or in non-peer-reviewed formats is an avenue for additional systematic reviews. The last systematic literature search was conducted on 27 June 2019, meaning that literature published since this date will not have been included.
The aim of this review is to establish how, why and in what circumstances interoperable systems affect patient safety. A realist synthesis will be undertaken, to understand how and why inter-organisational systems reduce patients' clinical risks, or fail to do so. ... Literature searches are then designed. It is not usually feasible to ...
Furthermore, it is unclear how training for telecare users has addressed patient safety issues. A systematic review of the literature was conducted to identify patient safety risks associated with telecare use in homecare services and to investigate whether and how these patient safety risks have been addressed in telecare training.
Background The implementation and continuous improvement of patient safety learning systems (PSLS) is a principal strategy for mitigating preventable harm to patients. Although substantial efforts have sought to improve these systems, there is a need to more comprehensively understand critical success factors. This study aims to summarise the barriers and facilitators perceived by hospital ...
This evidence synthesis had 3 objectives: explore the current literature regarding psychological safety, identify methods used in its assessment and investigate for evidence of consequences of a psychologically safe environment. ... Ten studies inferred that low psychological safety negatively impacted patient safety. Nine demonstrated a ...
Aim: To provide an overview of instruments measuring patient safety culture in a hospital setting. Methods: The study has a character of a narrative literature review. The search was performed in ...
Patient safety risk in the homecare context and patient safety risk related to telecare are both emerging research areas. Patient safety issues associated with the use of telecare in homecare services are therefore not clearly understood. ... An Updated Systematic Overview and Synthesis of the Literature. Final Report for the NHS Connecting for ...
A literature search in the databases PubMed and Cochrane Library for Studies between January 2008 and May 2018 was performed. Two authors reviewed the full texts of potentially relevant studies to determine eligibility for inclusion. Literature on possible risks associated with the medical discharge letter was discussed.
A systematic literature review and narrative synthesis on the risks of medical discharge letters for patients' safety ... Literature on possible risks associated with the medical discharge letter was discussed. Results: In total, 29 studies were included in this review. The major identified risk factors are the delayed sending of the discharge ...
FIGURE 1. Procedure for assessing the strength of evidence of nursing‐sensitive patient outcomes (NSPOs) based on results from the included literature reviews. Y = 1 if the association between nurse staffing and an NSPO was coded as "significant" based on evidence from the literature review; otherwise Y = 0. For each literature review, we ...
Strategies for Hospitals to Improve Patient Safety: A Review of the Literature. March 6, 2005. Wong J, Beglaryan H. Toronto, ON: The Change Foundation; February 2004. View more articles from the same authors. A literature review of preventable adverse events in acute-care hospitals. Full analysis and recommendations are provided based on the ...
This systematic review synthesizes prior literature to develop a framework for learning from adverse events and building a safety culture in chiropractic care. A model framework for patient safety training in chiropractic: a literature synthesis. | PSNet
This article summarizes a systematic review of bedside shift reports and relates the support for improving quality of care, patient safety, and patient-centered care. A computer-assisted search using MEDLINE, PubMed, and Ovid databases produced a total of 33 studies that met all inclusion criteria.
We hand-searched 11 key health care journals: The Milbank Quarterly, Social Science and Medicine, Quality and Safety in Health Care, the International Journal of Health Care Quality Assurance, Health, the New England Journal of Medicine, the Journal of the American Medical Association, the British Medical Journal, the Medical Journal of Australia, the Canadian Medical Association Journal, and ...
Patient safety is defined as "the absence of preventable harm to a patient and reduction of risk of unnecessary harm associated with health care to an acceptable minimum." Within the broader health system context, it is "a framework of organized activities that creates cultures, processes, procedures, behaviours, technologies and ...
A review was conducted to identify clinical trials assessing the efficacy and safety of CBD in pediatric patients diagnosed with DS. PubMed, MEDLINE, Scopus, Web of Science, and relevant grey literature were systematically searched for relevant articles up to October 2023, and clinical trials within the last 10 years were included.
Although the science of translating research into practice is fairly new, there is some guiding evidence of what implementation interventions to use in promoting patient safety practices. However, there is no magic bullet for translating what is known from research into practice. To move evidence-based interventions into practice, several strategies may be needed. Additionally, what works in ...