CHEM101: General Chemistry I
Course introduction.
- Time: 36 hours
- College Credit Recommended ($25 Proctor Fee) -->
- Free Certificate

Course Syllabus
First, read the course syllabus. Then, enroll in the course by clicking "Enroll me". Click Unit 1 to read its introduction and learning outcomes. You will then see the learning materials and instructions on how to use them.
Unit 1: Matter and Measurements
Chemistry is the study of matter and how we can change matter chemically and physically. What is matter? Matter is everything around us that has mass and volume. Matter can be any phase - solid, liquid, or gas. In this unit, we explore the properties, phases, and how we measure matter. We review the standard units of measurement and how to report our measurements using significant figures.
Completing this unit should take you approximately 3 hours.
Unit 2: The Atom
The atom is the basic unit of matter and serves as our starting point for the study of chemistry. The atom is composed of the subatomic particles protons, neutrons, and electrons. Scientists have studied atoms for hundreds of years and have developed a number of different models to describe them, as experimental technology has improved and new discoveries have been made. Chemists currently use the quantum mechanical model of the atom. In this unit, we explore the structure and properties of atoms. We also study some of the basic tenets of quantum mechanics, and how quantum mechanics describes atomic structure. Finally, we learn about the structure and organization of the periodic table of the elements.
Completing this unit should take you approximately 5 hours.
Unit 3: Bonding
Bonds are connections between atoms. A solid grasp of valence shell electron pair repulsion (VSEPR) theory will help you understand how elements that differ by one or two atomic numbers behave. According to VSEPR theory, the number of electrons an element has corresponds with its chemical properties. For example, sodium differs from neon and potassium by one atomic number, but it resembles potassium, not neon. Sodium and potassium both have one valence electron, which explains their similar properties, while neon is a stable element with eight valence electrons. We use VSEPR to predict the three-dimensional structure, or geometry, of molecules.
Unit 4: Chemical Formulas and Equations
Chemists need to write out formulas and equations to solve chemistry problems. It is important that chemists have a common set of rules for writing formulas and equations so they can communicate with other scientists. In this unit, we begin to name and write formulas for compounds, and learn how to write and balance chemical equations. Equations enable us to describe chemistry topics in mathematical terms and predict the outcomes of reactions. For example, what volume of steam is created if we turn one kilogram of ice into pure steam, at 200 degrees Celsius and sea-level air pressure? We can calculate the precise answer when we write the reaction out in the form of an equation!
Completing this unit should take you approximately 4 hours.
Unit 5: States of Matter
In this unit, we explore how matter behaves in terms of the three main phases of matter: solids, liquids, and gases. We investigate gases first because their properties are described by well-defined equations. Next, we study phase changes, which we describe in terms of a graph known as a phase diagram. We finish this unit with an exploration of the properties of solids.
Completing this unit should take you approximately 7 hours.
Unit 6: Thermochemistry and Thermodynamics
In this unit, we study thermodynamics and thermochemistry. Thermodynamics is the study of heat transfer. Thermochemistry is specifically the study of heat transfer in chemical reactions. We were introduced to thermodynamics in Unit 5 when we learned about the energy associated with phase changes. Thermodynamics and thermochemistry allow us to predict whether a reaction will produce heat, such as the burning of a candlewick, or if a reaction will require heat to proceed, such as the reaction that occurs inside a disposable cold pack. In this unit we also learn about Gibbs Free Energy, which tells us whether a reaction is spontaneous, meaning the reaction will occur without external "help".
Completing this unit should take you approximately 6 hours.
Unit 7: Acid-Base and Oxidation-Reduction Reactions
In this unit, we study two important types of chemical reactions: acid-base and oxidation-reduction. We will discuss how these types of reactions occur in all aspects of science and in everyday life. We will also review the properties of acids and bases and introduce two acid-base definitions: Arrhenius and Brønsted-Lowry. We will perform pH calculations and learn how to use the pH scale to identify acidic and alkaline solutions. Then, we will discuss oxidation and reduction, also known as electron transfer reactions. We will also learn how to write and balance equations for oxidation-reduction reactions and introduce some common oxidizing and reducing agents.
Unit 8: Nuclear Chemistry
Finally, we'll examine the processes of nuclear decay, nuclear fusion, and nuclear fission. Unlike all other types of chemical reactions, which involve electrons, nuclear reactions involve the nucleus of the atom. In this unit we discuss different types of nuclear decay, learn how to write equations that describe nuclear reactions, review the concept of half-life in the context of radioactive decay, and learn how we use nuclear fission to generate electric energy.
Completing this unit should take you approximately 2 hours.
Study Guide
This study guide will help you get ready for the final exam. It discusses the key topics in each unit, walks through the learning outcomes, and lists important vocabulary. It is not meant to replace the course materials!
Course Feedback Survey
Please take a few minutes to give us feedback about this course. We appreciate your feedback, whether you completed the whole course or even just a few resources. Your feedback will help us make our courses better, and we use your feedback each time we make updates to our courses. If you come across any urgent problems, email [email protected].
Certificate Final Exam
Take this exam if you want to earn a free Course Completion Certificate.
To receive a free Course Completion Certificate, you will need to earn a grade of 70% or higher on this final exam. Your grade for the exam will be calculated as soon as you complete it. If you do not pass the exam on your first try, you can take it again as many times as you want, with a 7-day waiting period between each attempt.
Once you pass this final exam, you will be awarded a free Course Completion Certificate .
Saylor Direct Credit
Take this exam if you want to earn college credit for this course . This course is eligible for college credit through Saylor Academy's Saylor Direct Credit Program .
The Saylor Direct Credit Final Exam requires a proctoring fee of $5 . To pass this course and earn a Credly Badge and official transcript , you will need to earn a grade of 70% or higher on the Saylor Direct Credit Final Exam. Your grade for this exam will be calculated as soon as you complete it. If you do not pass the exam on your first try, you can take it again a maximum of 3 times , with a 14-day waiting period between each attempt.
We are partnering with SmarterProctoring to help make the proctoring fee more affordable. We will be recording you, your screen, and the audio in your room during the exam. This is an automated proctoring service, but no decisions are automated; recordings are only viewed by our staff with the purpose of making sure it is you taking the exam and verifying any questions about exam integrity. We understand that there are challenges with learning at home - we won't invalidate your exam just because your child ran into the room!
Requirements:
- Desktop Computer
- Chrome (v74+)
- Webcam + Microphone
- 1mbps+ Internet Connection
Once you pass this final exam, you will be awarded a Credly Badge and can request an official transcript .
Saylor Direct Credit Exam
This exam is part of the Saylor Direct College Credit program. Before attempting this exam, review the Saylor Direct Credit page for complete requirements.
Essential exam information:
- You must take this exam with our automated proctor. If you cannot, please contact us to request an override.
- The automated proctoring session will cost $5 .
- This is a closed-book, closed-notes exam (see allowed resources below).
- You will have two (2) hours to complete this exam.
- You have up to 3 attempts, but you must wait 14 days between consecutive attempts of this exam.
- The passing grade is 70% or higher.
- This exam consists of 50 multiple-choice questions.
Some details about taking your exam:
- Exam questions are distributed across multiple pages.
- Exam questions will have several plausible options; be sure to pick the answer that best satisfies each part of the question.
- Your answers are saved each time you move to another page within the exam.
- You can answer the questions in any order.
- You can go directly to any question by clicking its number in the navigation panel.
- You can flag a question to remind yourself to return to it later.
- You will receive your grade as soon as you submit your answers.
Allowed resources:
Gather these resources before you start your exam.
- Blank paper
- basic: https://www.desmos.com/fourfunction 18
- graphing: https://www.desmos.com/calculator 6
- scientific: https://www.desmos.com/scientific 35
- This formulas sheet: https://learn.saylor.org/mod/page/view.php?id=18848
What should I do before my exam?
- Gather these before you start your exam:
- A photo I.D. to show before your exam.
- A credit card to pay the automated proctoring fee.
- (optional) Blank paper and pencil.
- (optional) A glass of water.
- Make sure your work area is well-lit and your face is visible.
- We will be recording your screen, so close any extra tabs!
- Disconnect any extra monitors attached to your computer.
- You will have up to two (2) hours to complete your exam. Try to make sure you won't be interrupted during that time!
- You will require at least 1mbps of internet bandwidth. Ask others sharing your connection not to stream during your exam.
- Take a deep breath; you got this!
Browse Course Material
Course info.
- Prof. Catherine Drennan
Departments
As taught in.
- Inorganic Chemistry
- Organic Chemistry
- Physical Chemistry
Learning Resource Types
Principles of chemical science, course description.
This course provides an introduction to the chemistry of biological, inorganic, and organic molecules. The emphasis is on basic principles of atomic and molecular electronic structure, thermodynamics, acid-base and redox equilibria, chemical kinetics, and catalysis. One year of high school chemistry is the expected …
This course provides an introduction to the chemistry of biological, inorganic, and organic molecules. The emphasis is on basic principles of atomic and molecular electronic structure, thermodynamics, acid-base and redox equilibria, chemical kinetics, and catalysis. One year of high school chemistry is the expected background for this freshman-level course.
The aims include developing a unified and intuitive view of how electronic structure controls the three-dimensional shape of molecules, the physical and chemical properties of molecules in gases, liquids and solids, and ultimately the assembly of macromolecules as in polymers and DNA. Relationships between chemistry and other fundamental sciences such as biology and physics are emphasized, as are the relationships between the science of chemistry to its applications in environmental science, atmospheric chemistry and electronic devices.

Acknowledgements
Professor Drennan would like to acknowledge the contributions of MIT Lecturer Dr. Elizabeth Vogel Taylor, Professor Sylvia Ceyer, and Professor Robert Silbey to the development of this course and its materials.

You are leaving MIT OpenCourseWare
Forgot password? New user? Sign up
Existing user? Log in
- Number Theory
- Probability
- Everyday Math
- Classical Mechanics
- Electricity and Magnetism
- Computer Science
- Quantitative Finance
Take a guided, problem-solving based approach to learning Chemistry. These compilations provide unique perspectives and applications you won't find anywhere else.
The Chemical Reaction
What's inside.
- Introduction
- Chemical Kinetics
- Reaction Principles
- Elements of Reactivity
Science Essentials
- Introduction to Science
- The Scientific Process
- Measurement
Physics of the Everyday
- In the House
- On the Field
- Fuel the World
- Out in Nature
- Infrastructure

Community Wiki
Browse through thousands of Chemistry wikis written by our community of experts.
Fundamentals
- Chemistry in action
- Atoms, Molecules, Elements, Compounds
- Dalton's Atomic Model
- Bohr's Model
- Periodic Table of the Elements
- Atomic and Molecular Weights
- Mole Concept
- Concentration Terminology
- Nuclear Decay
- Balancing Chemical Reactions
- Limiting Reagents
- Laws of Chemical Combination
- Try It At Home Chemistry Experiments
- Chemistry Of Drugs
Chemical Bonding
- Ionic Compounds
- Lewis Structure
- Hybridisation
- Valence Shell Electron Pair Repulsion (VSPER) Theory
- Sigma and Pi Bonds
- Molecular Orbital Theory
- Coordinate Bonds
- Polarity of a Molecule
- Van der Waals Force
- Dipole Interactions
- Hydrogen Bonds
- Surface Tension
- Strength of Intermolecular Forces
- Chemistry of Diamonds
Reaction Mechanics
- Chemical Equilibrium
- Basics of Statistical Mechanics
- Le Chatelier's Principle
- Acids and Bases
- Acid/Base Equilibrium
- Thermal Expansion
- Rate of Chemical Reactions
- Order of Chemical Reactions
- Activation Energy
- Chemistry of Fireworks
- Thermometry
- Electrolytic Cells and Electrolysis
- Galvanic Cells
Organic Chemistry
- Structural Representations of Organic Compounds
- IUPAC Nomenclature - Branched Chain Alkanes, Cyclic Compounds Unsaturated Compounds
- Structural Isomerism
- Geometric Isomerism
- Optical Isomerism
- Electrophiles and Nucleophiles
- Inductive Effect, Electromeric Effect, Resonance Effects, and Hyperconjugation
- Reactive Intermediates
- Common Types of Organic Reactions
- Grignard Reagent
- Alkanes, Alkenes, Alkynes
- Do objects gain protons to become positive?
- Chemistry Of Poisons
- Chemistry Of Nutrition
- Reverse Transcription
- Mutation and DNA repair
- Viral Replication
Problem Loading...
Note Loading...
Set Loading...
Nominate your advisor for Advisor of the Year 2024
Five advisors will each receive $500, and five Sophia learners will each get a free month of Sophia.
- Introduction to Chemistry
- All courses
- Science courses

Introduction to Chemistry reviews
Gain competencies in the fundamental principles, theories, and concepts of chemistry in this course designed for the non-science major. Take this self-paced, online course anytime, anywhere, and most courses can be accessed from any device.
7218 students successfully completed
48 partners accept credit transfer.* See Partners
3.0 semester credits
Recommended for credit to the ACE® and DEAC college and university networks.

Download Syllabus
Fill in the form to recieve a syllabus for your course..
By providing your information, you consent to receive occasional special promotional offers and education opportunities by email from Sophia Learning or one of its affiliates.
Learning outcomes
Partners who accept this course.
This course transfers into a degree program at these featured institutions
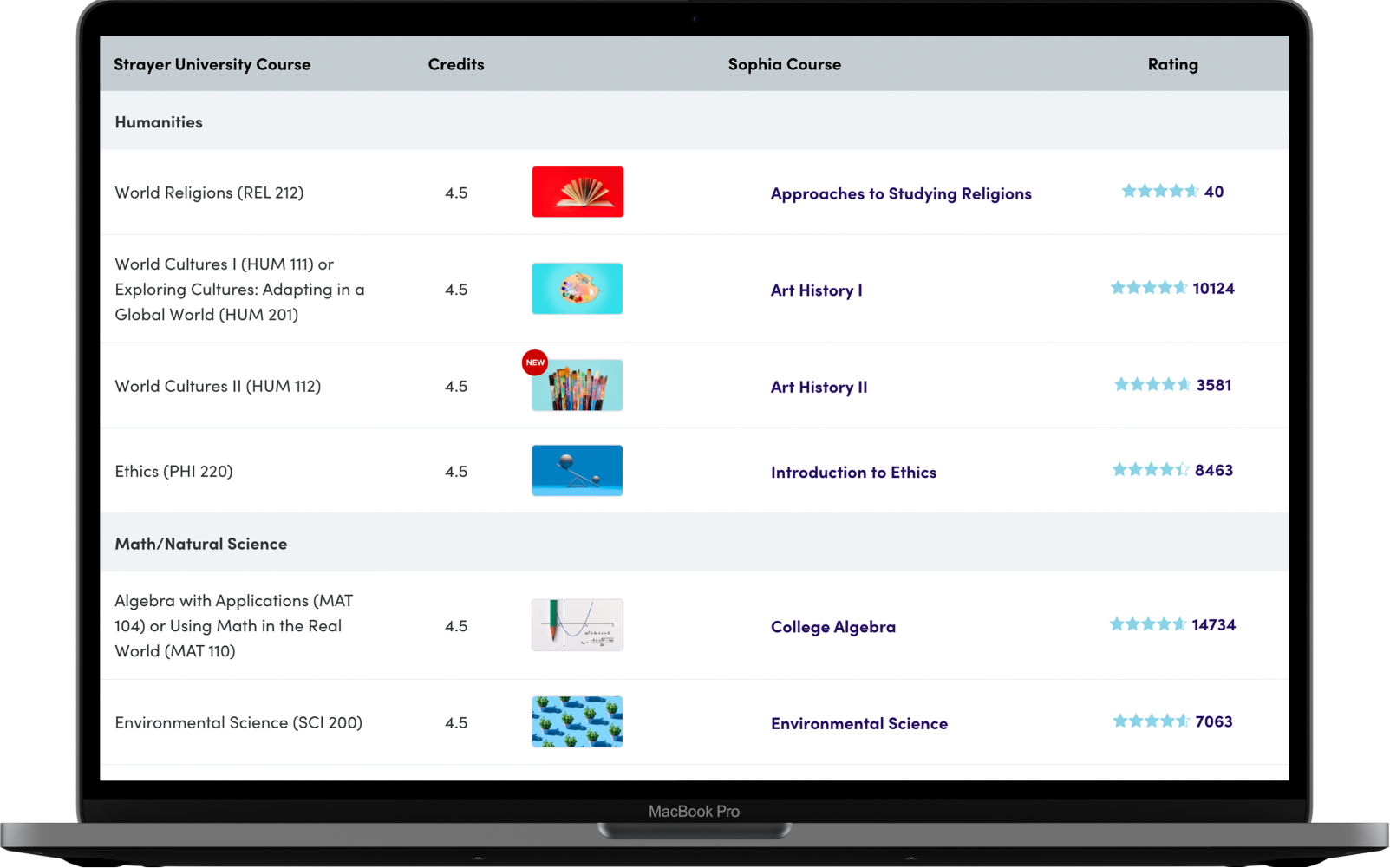
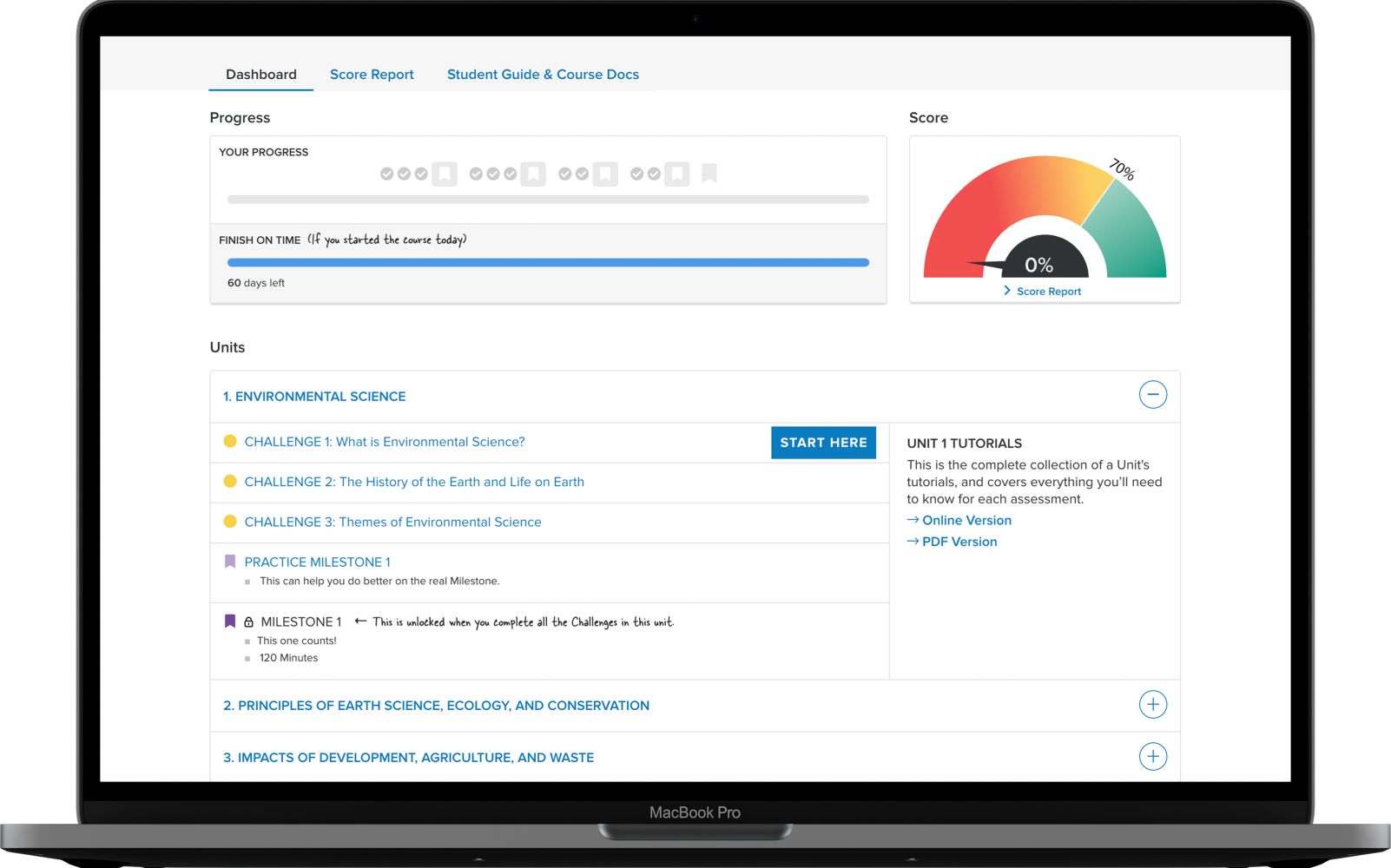
Assignments & grading
This is a pass/fail course. You must complete 12 Challenge assessments (these are like quizzes), and 4 Milestone assessments (these are like tests), with an overall score of 70% or better. The assessments for this course may require access to the periodic table of elements, a calculator, and other equations and charts provided within the tutorial associated with those questions. An accessible version of the periodic table provided by WebElements and can be accessed at https://webelements.com/. Desmos free online scientific calculator can be found at https://www.desmos.com/scienti...

- No credit card required!
- Sophia membership starts with a risk-free trial
* All fields are required.
Inside the Sophia courseroom
Knock out your general education requirements on your terms. Sophia courses are available anytime, anywhere, and most can be accessed from any device.
A revolutionary way to satisfy general education requirements for your degree. On demand. ACE ®-recommended. Low cost.
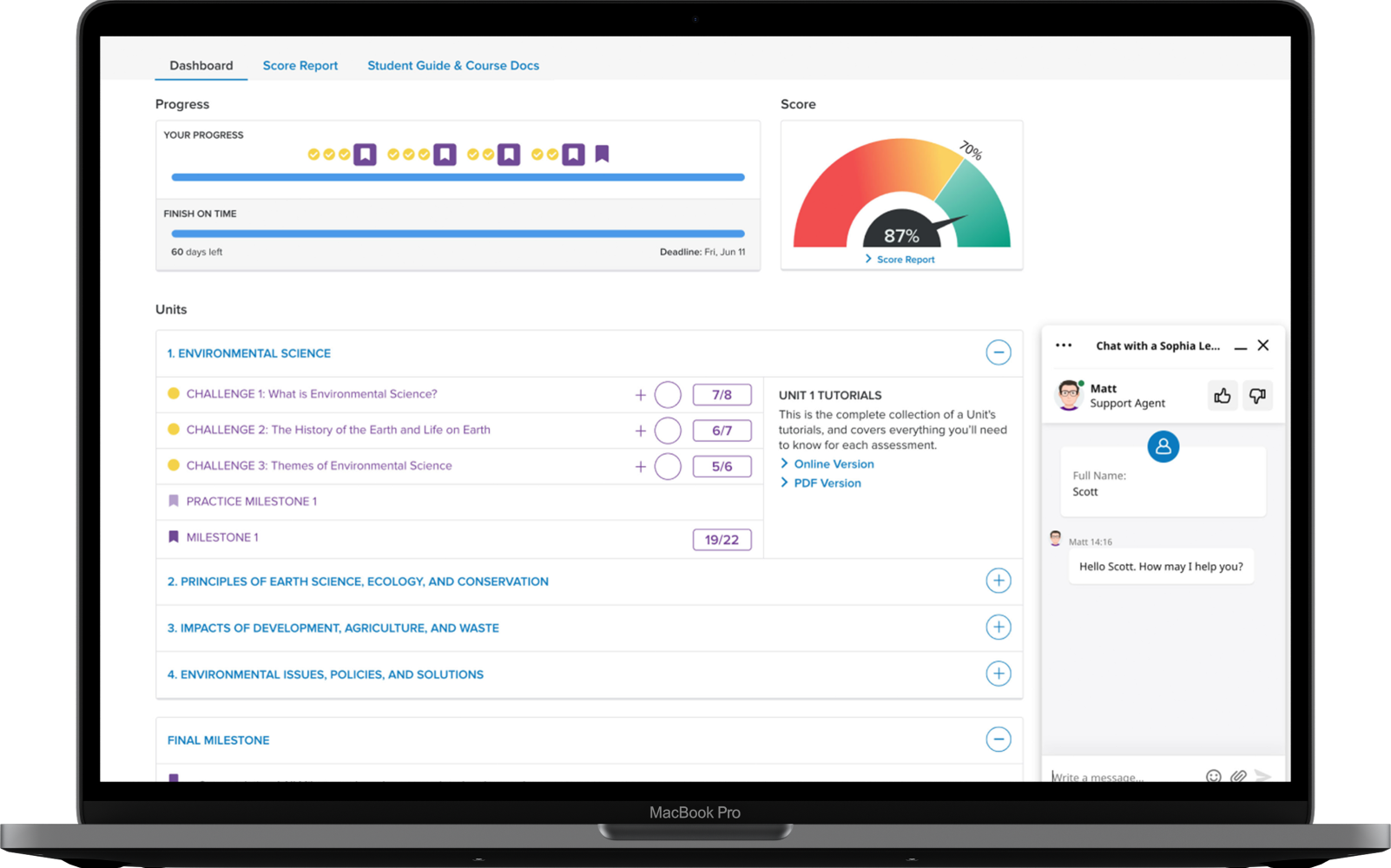
Sophia's Learning Coaches are here for you by phone, email or online chat from the start of your course to ordering your final transcript.
How many courses can I take with the free trial?
Access the course content through the first Challenge of any of Sophia’s 50+ courses.
How do I confirm transfer credit with my school?
Check Sophia’s list of partner schools to see if your school is on the list. If not, contact your registrar to learn about your school’s transfer credit policy and if Sophia coursework can be submitted for transfer.
Can I take courses for credit if I’m not enrolled at a school?
Sophia course completions do not expire and will be available to submit for transfer when you’re ready. At that time, check with your school’s registrar for their credit transfer policies.
What happens to the coursework I’ve already completed once I become a Sophia member?
If you’ve completed the first Challenge during your free trial, you can pick up right where you left off after you become a Sophia member.
What happens to my coursework if I cancel my Sophia membership?
Don’t worry. Your completed courses won’t disappear if you cancel your membership. Those courses will be there for you when you’re ready to submit for transfer.
Will my HR benefits cover the cost of Sophia?
If you have an education benefit through your employer, it may cover your subscription to Sophia. Check with your benefits administrator to find out if you qualify.
- Higher education partnerships
- Corporate partnerships
- Social impact partnerships
- Privacy policy
- Cookie policy
- Terms of use
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
AP®︎/College Chemistry
Welcome to ap®︎/college chemistry, unit 1: atomic structure and properties, unit 2: molecular and ionic compound structure and properties, unit 3: intermolecular forces and properties, unit 4: chemical reactions, unit 5: kinetics, unit 6: thermodynamics, unit 7: equilibrium, unit 8: acids and bases, unit 9: applications of thermodynamics.
You must enable JavaScript in order to use this site.
Chemistry Center
The UI Department of Chemistry has established the Chemistry Center to serve all students taking chemistry courses and provide support for the course instructors and teaching assistants. The Chemistry Center is a good place to start if you have any questions about undergraduate chemistry courses, want TA or instructor information, or need help with a specific chemistry course.
Student services include:
- Signing add/drop slips for the instructor
- Returning exams, labs, and homework
- Make-up exams
- Student disability exams
- Make-up labs: Please check your ICON course site for information on making up labs
- Scheduling and maintaining faculty and teaching assistant office hours
- Scheduling conference rooms for office hours, examinations, group tutoring, and One Button Studio
- Arranging accommodations for those students with disabilities or other academic accommodations
- Managing the department’s digital signage and social media sites
- Lost and found
- Reserving rooms for chemistry student organizations
Chemistry Center hours
Our hours are as follows, but we may not be in the office during this time. If we aren't in the office, you can reach us via email at [email protected] or phone at 319-335-1341 . If you need any assistance, please reach out to us; we know these are stressful times and are here to help in any way we can.
Monday through Thursday: 8 a.m. to noon and 1 to 5 p.m. Fridays: 8 a.m. to noon and 12:30 to 4:30 p.m.
Chemistry Center facilities
The Chemistry Building (CB) has the following resources for students:
- Computer labs–W238 and W241
- Resource Room (TA office hours)−E208, W215
- Study Commons−W207
- Majors Study Lounge and One Button Studio−W234
- Desks, booths, study corrals and communal tables located around the East, North, and West corridors of the second floor
- Instructional labs−W328, E324, E340, E364, W368, W428, E424, E440, E464, E468, E414
Learn more about the Chemistry Building
E225 Chemistry Building (CB) Department of Chemistry University of Iowa Iowa City, IA 52242
319-335-1341 [email protected]
Chemistry Center resources
Course waiting list sign-up.
Use the Department of Chemistry waitlist application to add yourself to a waiting list for a particular course and section. The Chemistry Center has access to the waitlists in real time and will be monitoring the lists as registration progresses.
Be sure your information is accurate with the Registrar (including a current email-address) as it will facilitate your movement to the top of the list and help get you into the course you want.
When a space becomes available for the time(s) you selected, you will receive an e-mail. These may not all be sent until the "eight day rule" takes effect.
Join a waitlist
Regression list
Regression occurs when a student takes a lower-level or prerequisite course after having satisfactorily completed a more advanced course in the same or related subject. Hours earned by regression do not count toward the total number of hours required for graduation. Regression is identified only at the time of final graduation analysis.
Regression occurs when a course course in the left column is taken after any of the courses in the right column of the following table. These are the only cases considered to be regression.
Office hours
Graduate teaching assistants hold office hours for their courses in the Chemistry Resource Center. These instructors are available to assist students in their chemistry courses. Students utilize this room to prepare for exams, request help on homework, work on laboratory reports and general study for chemistry. Graduate teaching assistants give priority to the students in their course but will also assist with other chemistry courses when time is available. Other resources available in the room are student computers, alternate text books, and modeling kits.
Supplemental Instruction (SI) at the Academic Resource Center
SI is academic support that is focused in active and collaborative learning strategies. SI sessions are facilitated by SI leaders, fellow undergraduate students. SI is offered for several chemistry courses every semester.
Learn more about SI
Tutor Iowa is a resource to find free academic assistance and a listing of available private tutors for your University of Iowa chemistry courses. Fees for private tutors will vary.
Search tutors
TRIO offers personalized tutoring assistance to first-generation students, income-eligible students, and students with disabilities.
Learn more about TRIO
Tutoring for student athletes
Tutoring is available to all student athletes, free of charge. Tutors can help you with course content as well as study strategies.
The UI Department of Chemistry is committed to providing a safe working environment for undergraduate students working in research and teaching laboratories. Safety information is provided by instructors for all lab courses and by research advisors.
We strive to maintain safe facilities by following regulations provided by the Occupational Safety and Health Administration, the University of Iowa Environmental Health and Safety Office , and the Department of Chemistry Safety Committee.
- You are here:
- American Chemical Society
- Students & Educators
- Standards & Guidelines
- ACS Approval Program
- Institutional Environment
- Faculty & Staff
- Infrastructure
Undergraduate Research
- Creating a Safety Culture
- Professional Skills & Competencies
- Diversity, Equity, Inclusion, and Respect
- Appendix 1: Natural Disaster and Emergency Guidelines
- Apply for Approval
- Maintain Approval
- CPARS Training
- Data & Reports
- Teaching Labs in COVID-19
- Supplements
- CPT Member Highlight: Anna, Irwin, and Muchalski
- Chemistry Communities of Practice
- ACS Adds Green Concepts to Approval Guidelines
Coursework Section 5.1
The curriculum of an approved program provides both a broad background in chemical principles and an in-depth study of chemistry or chemistry-related areas that build on this background. Student learning progresses from beginner to expert knowledge and comprises introductory, foundation, and in-depth experiences. Foundation experiences are designed to provide students with an intellectual framework that covers the breadth of modern chemistry. In-depth experiences are designed to provide students with deeper development of critical thinking and problem-solving.
< Previous Section: Infrastructure
Next Section: Laboratory - Curriculum & Skills >
Critical Requirements
Introductory Courses
Prior to beginning foundation-level coursework, students must have an introductory chemistry experience that addresses basic chemical concepts such as stoichiometry, states of matter, atomic structure, molecular structure and bonding, thermodynamics, equilibria, and kinetics.
Foundation Courses
- Definition: Foundation courses require an introductory chemistry prerequisite and use textbooks or other specialized materials that are beyond the introductory chemistry experience. Course content and exams should reflect coverage at a higher level than general chemistry.
- (Semester) 5 one-semester courses of at least 3 credits each.
- (Quarter) 8 one-quarter courses.
- Coverage: The foundation courses must cover all areas of ABIOP , either as stand-alone courses or with content distributed across courses.
In-depth Courses
- Definition: In-depth courses require a foundation or in-depth course prerequisite.
- Course content and exams include coverage at a higher level than foundation courses, with a focus on critical thinking and problem-solving skills.
- (Semester) 4 courses that add to at least 12 credits.
- (Quarter) 6 courses that correspond to at least 18 credits.
- Undergraduate research (on or off campus) can satisfy one in-depth course for students who wish to have a certified degree. ( See Section 6 - Undergraduate Research for more details ).
- Courses in other disciplines with a chemical perspective (atomic/molecular-level perspective, rely on the tools of chemical measurement and analysis, and have a prerequisite of a full year of introductory chemistry) could be considered as an in-depth course.
- Seminar classes cannot count towards foundation or in-depth coursework .
- It must represent an advanced laboratory experience that includes the integration of student skills and builds on the foundation laboratory experiences.
- In these courses, students are typically in the laboratory for at least six hours a week.
- A lab associated with a lecture course, even if it has a separate course number, is not considered a separate in-depth course.
Course Frequency Foundation Courses
- (Semester) 4 foundation courses each academic year covering 4/5 ABIOP.
- (Quarters) 6 foundation courses each academic year covering 4/5 ABIOP.
- If one of the foundation courses is taught by faculty outside of chemistry, then the chemistry faculty must teach the other 4 courses.
In-Depth Courses
- (Semester): Three, 3-credit, in-depth courses per academic year, exclusive of research.
- (Quarters) Five, 3-credit, in-depth courses, exclusive of research.
- Frequency of in-depth courses must allow students to graduate in 4 years.
MSN Requirement
- Coverage of synthetic polymers, biological macromolecules, supramolecular aggregates, meso- or nanoscale materials (MSN) must be part of the curriculum, by using either a course dedicated to MSN content or within a distributed model across more than one course. At least two of the four types of systems must be covered.
- In the distributed model, coverage of MSN should constitute a minimum of 15 hours . Coverage of these systems be reasonably balanced.
- Instruction should encompass the preparation, characterization, and physical properties of the systems.
Green Chemistry & Sustainability
The curriculum must provide students with a working knowledge of the Twelve Principles of Green Chemistry.
- Must complete the equivalent of 2 semesters of math including calculus I and a second math course, such as calculus II, linear algebra, statistics, or data science. The second math course may not be a prerequisite for calculus I.
- Must complete the equivalent of 2 semesters of physics with labs.
Normal Expectations
- The curriculum includes the operation and theory of modern instruments and their use to solve chemical problems.
- Five foundation courses are taught each academic year.
- Undergraduate research opportunities are available within the curriculum.
- The curriculum includes two semesters of calculus-based physics with lab.
- Case studies are used to demonstrate to students the interplay of chemical, environmental health, regulatory, and business considerations that dictate chemical processes and product design.
Markers of Excellence
- Curriculum includes integrative experiences that require students to synthesize the knowledge and skills introduced across the curriculum. These integrative experiences could be provided in an existing upper-level, designated capstone course (e.g., senior seminar) or distributed among several courses taught in the chemistry department.
- Students have opportunities to develop expertise at the interface of chemistry to help them solve problems that span scientific disciplines.
- A variety of in-depth courses are offered. Some examples could include catalysis, environmental chemistry, green/sustainable chemistry, materials science, or toxicology.
- Mentored opportunities exist for undergraduate students to integrate their knowledge and skills through peer instruction.
Cognate Courses
- The curriculum includes cognate courses beyond the critical requirement expectation.
- Students are given the opportunity to assess chemical products and processes and design greener alternatives when appropriate.
- Students understand and can evaluate the environmental, social, and health impacts of a chemical product over the life cycle of the product, from synthesis to disposal.
Accept & Close The ACS takes your privacy seriously as it relates to cookies. We use cookies to remember users, better understand ways to serve them, improve our value proposition, and optimize their experience. Learn more about managing your cookies at Cookies Policy .
1155 Sixteenth Street, NW, Washington, DC 20036, USA | service@acs.org | 1-800-333-9511 (US and Canada) | 614-447-3776 (outside North America)
- Terms of Use
- Accessibility
Copyright © 2024 American Chemical Society
- Skip to Main Content
- Undergraduate
- News & Events
MS Chemistry
- Message from the Department Head
- Biochemistry Concentration
- Student Central
- Financial Support
- FAQs – Admission
- Two Year Track
You will need to complete 30 semester hours with a B average (GPA 3.0) to graduate. If you have an assistantship, you will need to maintain a GPA of 3.0 at all times to keep the assistantship. To complete the program in two years, you will need to take 7-8 credits in each semester excluding Summer. You do not need to register for any courses during the Summer Semester, unless you graduate during Summer. The following courses are required:
Four core courses in the major areas of chemistry are required for the Master of Science degree:
- CHE 553 – Advanced Organic Chemistry I
- CHE 632 – Advanced Analytical Chemistry or Bioanalytical Chemistry
- CHE 641 – Advanced Inorganic Chemistry II
- CHE 661 – Advanced Physical Chemistry I
Note that CHE 633 can often replace CHE 632.
In addition, students must select two electives from the courses in chemistry or biochemistry, which may include organometallic chemistry, medicinal chemistry, forensic chemistry, enzyme mechanisms, spectroscopy, computational chemistry, and others.
Students attend the Department’s weekly seminar series and present two seminars during the course of their Master’s degree studies. One seminar is based on a topic from the recent literature. The first is a presentation of the thesis proposal. The second seminar is a presentation of the thesis research and should generally be given in the semester or summer of graduation. These seminar experiences provide strong preparation for future professional presentations. The seminars are presented in the context of courses:
- CHE 601 – Graduate Seminar I
- CHE 602 – Graduate Seminar II
Research and Thesis
Each graduate student in the M.S. Chemistry program must carry out a research project and write a Master’s thesis based on the research. Course credit for research is accumulated through two courses, which may be taken for up to 6 credits each:
- CHE 680 – Research Problems in Chemistry and Biochemistry
- CHE 691 – Introduction to Graduate Research
- CHE 699 – Thesis
Students choose a research advisor and begin research under the course number CHE 680. Before registering for CHE 699, students must write a research proposal and present it to his or her thesis committee. The research project and thesis are an important part of the M.S. program, accounting for at least a third of the credit hours earned. Actual effort invested in the research project may be much greater, since students must work toward completion of a project with meaningful results. During the summer, students supported by assistantships should devote full-time effort to making major progress on the research project.
Electives and additional research
(10 or more credits) You will need to take 10 semester hours of electives and research. Research Problems (CHE 680), which will get you started on research, can be taken for up to 6 hours. You will need to take 4 or more credits of elective courses.
All graduate students, regardless of whether they are registered for a seminar course, are required to attend all seminars related to CHE 602, as well as additional symposia and guest seminars. This is part of your broad training in chemistry.
Department of Chemistry and Biochemistry The University of North Carolina at Greensboro Patricia A. Sullivan Science Building PO Box 26170 | Greensboro, NC 27402-6170 Phone: 336.334.5714 | Fax: 336.334.5402 Copyright © 2022. The University of North Carolina at Greensboro. All rights reserved

COMMENTS
Learn Chemistry With Our Online Practice Tests, Study Guides & Video Lessons. Learn Chemistry With Our Online Practice Exams, Study Guides & Video Lessons.
Learn Chemistry online at your own pace. Start today and improve your skills. Join millions of learners from around the world already learning on Udemy.
Join for free and get personalized recommendations, updates and offers. Learn Chemistry or improve your skills online today. Choose from a wide range of Chemistry courses offered from top universities and industry leaders. Our Chemistry courses are perfect for individuals or for corporate Chemistry training to upskill your workforce.
Chemistry course curriculum. Get an introduction to chemistry with online courses from universities and institutions worldwide. edX offers both individual courses and advanced programs designed to help you learn about basic chemistry concepts and chemical science fundamentals in an engaging and effective online learning environment. Learners can also earn certificates in chemistry courses.
Chemistry is the study of matter and the changes it undergoes. Here you can browse chemistry videos, articles, and exercises by topic. We keep the library up-to-date, so you may find new or improved material here over time. Unit 1: Atoms, compounds, and ions.
This course provides an introduction to the chemistry of biological, inorganic, and organic molecules. Add to list. MIT OpenCourseWare. 25 hours. On-Demand. Free Online Course. Load the next 15 courses of 733. Best online courses in Chemistry from Harvard, Stanford, MIT, University of Pennsylvania and other top universities around the world.
Chemistry. CHEM101: General Chemistry I. Learn new skills or earn credit towards a degree at your own pace with no deadlines, using free courses from Saylor Academy. Join the 1,839,519 students that started their journey with us. We're committed to removing barriers to education and helping you build essential skills to advance your career goals.
Module 1 • 1 hour to complete. <p>Welcome! Over the eight weeks of the Introduction to Chemistry: Structures and Solutions course, we will begin discussions about the electronic structure of the atom, structures of molecules, phases of matter, and solutions. This week, we included some basic concept videos in Chemistry.
This course provides an introduction to the chemistry of biological, inorganic, and organic molecules. The emphasis is on basic principles of atomic and molecular electronic structure, thermodynamics, acid-base and redox equilibria, chemical kinetics, and catalysis. One year of high school chemistry is the expected background for this freshman-level course. The aims include developing a ...
This course is designed to cover subjects in advanced high school chemistry courses, correlating to the standard topics as established by the American Chemical Society. This course is a precursor to the Advanced Chemistry Coursera course. Areas that are covered include atomic structure, periodic trends, compounds, reactions and stoichiometry ...
Welcome to High school chemistry! In this course, you'll explore the fascinating world of atoms and molecules. Learn about atomic structure, isotopes and ions, the periodic table, chemical bonding, chemical reactions, thermochemistry, solutions, acids and bases, and nuclear chemistry. Give us feedback.
Organic Chemistry. Structural Representations of Organic Compounds. IUPAC Nomenclature - Branched Chain Alkanes, Cyclic Compounds Unsaturated Compounds. Structural Isomerism. Geometric Isomerism. Optical Isomerism. Electrophiles and Nucleophiles. Inductive Effect, Electromeric Effect, Resonance Effects, and Hyperconjugation. Reactive Intermediates.
Take Introduction to Chemistry online at your own pace. Gain competencies in the fundamental principles, theories, and concepts of chemistry in this course designed for the non-science major. Take this self-paced, online course anytime, anywhere, and most courses can be accessed from any device. Start your free trial.
This course is a precursor to the Advanced Chemistry Coursera course. Areas that are covered include atomic structure, periodic trends, compounds, reactions and stoichiometry, bonding, and thermochemistry. ★★★★★ ( 6 ratings) Ideal Gases. University of Colorado Boulder via Coursera.
Learn AP Chemistry using videos, articles, and AP-aligned practice. Review the fundamentals of atomic structure, intermolecular forces and bonding, chemical reactions, kinetics, thermodynamics, and equilibrium. ... Course challenge Test your knowledge of the skills in this course. Start Course challenge. Community questions. AP® is a ...
To help you find the best courses available, we researched and reviewed the 10 best chemistry online courses. edX — Basic Analytical Chemistry by The University of Tokyo — Top Pick. edX — Introduction to Solid State Chemistry by MIT — Best Full-Length University Course. Coursera — Chemistry by The University of Kentucky — Best for ...
Chemistry 2e is designed to meet the scope and sequence requirements of the two-semester general chemistry course. The textbook provides an important opportunity for students to learn the core concepts of chemistry and understand how those concepts apply to their lives and the world around them. The book also includes a number of innovative ...
General Chemistry 1 covers all of the topics typically covered in first semester General Chemistry and includes both formative assessments, with high scaffolding, and end of unit and module quizzes. This course offers highly contextualized, engaging content, designed in a logical flow that transitions smoothly between relatively small amounts of expository text, worked examples, activities,
There are 12 modules in this course. This course will cover the topics of a full year, two semester General Chemistry course. We will use a free on-line textbook, Concept Development Studies in Chemistry, available via Rice's Connexions project. The fundamental concepts in the course will be introduced via the Concept Development Approach ...
The content is based on the International GCSE specification as followed by thousands of students around the world, and will prepare you thoroughly for progression to advanced programmes. In Section 1, The Principles of Chemistry, there are five video lessons, each one lasting about 20 minutes. The first lesson introduces the states of matter ...
Top Chemistry Courses Online - Updated [April 2024] Development. Web Development Data Science Mobile Development Programming Languages Game Development Database Design & Development Software Testing Software Engineering Software Development Tools No-Code Development. Business.
Preq: 300-level chemistry course or high school teaching experience or consent of instructor. CH 4990 Creative Inquiry—Chemistry IV 1-4(1-4,0) In consultation with and under the direction of a faculty member, students pursue scholarly activities individually or in teams. These creative inquiry projects may be interdisciplinary.
The Chemistry Center is a good place to start if you have any questions about undergraduate chemistry courses, want TA or instructor information, or need help with a specific chemistry course. Student services include: Signing add/drop slips for the instructor; Returning exams, labs, and homework; Scheduling alternative exams. Make-up exams
Coursework Section 5.1. The curriculum of an approved program provides both a broad background in chemical principles and an in-depth study of chemistry or chemistry-related areas that build on this background. Student learning progresses from beginner to expert knowledge and comprises introductory, foundation, and in-depth experiences.
Introduction. Module 1 • 3 hours to complete. Over the seven weeks of Introduction to Chemistry: Reactions and Ratios, you will be able to progress from a most basic knowledge of matter and energy to solving interesting real world chemical reaction stoichiometry problems. Each lesson in the course introduces some new concepts that allow you ...
Course credit for research is accumulated through two courses, which may be taken for up to 6 credits each: CHE 680 - Research Problems in Chemistry and Biochemistry. CHE 691 - Introduction to Graduate Research. CHE 699 - Thesis. Students choose a research advisor and begin research under the course number CHE 680.
Class Schedule FALL SESSION 2024 (Aug 26-Dec 19): COURSE SCHEDULE (3/13/2024) FALL EXAM DATES (midterm and final exams) => CLICK HERE WINTER SESSION 2025 (January 2025) CHE 327: Organic Chemistry Laboratory (2 credits, TWTF 8:30am-12:30pm or TWTF 1:00-5:00pm, + asynchronous online lecture; Lab experiments are in-person.
coursework. Inorganic Chemistry Required Y 8 Y Y Y We will accept Pass/Fail and online coursework Organic Chemistry Required Y 8 Y Y Y We will accept both Pass/Fail and online coursework. Physics Required Y 8 Y Y Y This requirement can be met with 8 credit hours in any physical science course that includes lab (physics, geology, ecology, etc ...
In the field of education, ChatGPT has become a topic of debate for its usefulness as a learning tool. This article focuses on non-science majors' (n = 29) perceptions of a ChatGPT enabled final exam, where, prior to the exam, students wrote papers on science and sustainability and, during the final exam, students were asked to compare their paper to one produced on the same topic by ChatGPT ...