- Open access
- Published: 14 February 2024

Genome sequencing as a generic diagnostic strategy for rare disease
- Gaby Schobers ORCID: orcid.org/0000-0003-1380-4254 1 , 2 ,
- Ronny Derks 1 ,
- Amber den Ouden 1 ,
- Hilde Swinkels 1 ,
- Jeroen van Reeuwijk 1 , 2 ,
- Ermanno Bosgoed 1 ,
- Dorien Lugtenberg 1 ,
- Su Ming Sun 3 ,
- Jordi Corominas Galbany 1 , 2 ,
- Marjan Weiss 1 ,
- Marinus J. Blok 3 ,
- Richelle A. C. M. Olde Keizer 1 , 2 ,
- Tom Hofste 1 ,
- Debby Hellebrekers 3 ,
- Nicole de Leeuw 1 ,
- Alexander Stegmann 3 ,
- Erik-Jan Kamsteeg 1 ,
- Aimee D. C. Paulussen 3 ,
- Marjolijn J. L. Ligtenberg 1 , 2 ,
- Xiangqun Zheng Bradley 4 ,
- John Peden 4 ,
- Alejandra Gutierrez 4 ,
- Adam Pullen 4 ,
- Tom Payne 4 ,
- Christian Gilissen 1 , 2 ,
- Arthur van den Wijngaard 3 ,
- Han G. Brunner 1 , 2 , 3 ,
- Marcel Nelen 1 na1 ,
- Helger G. Yntema 1 , 2 na1 &
- Lisenka E. L. M. Vissers 1 , 2 na1
Genome Medicine volume 16 , Article number: 32 ( 2024 ) Cite this article
2925 Accesses
8 Altmetric
Metrics details
To diagnose the full spectrum of hereditary and congenital diseases, genetic laboratories use many different workflows, ranging from karyotyping to exome sequencing. A single generic high-throughput workflow would greatly increase efficiency. We assessed whether genome sequencing (GS) can replace these existing workflows aimed at germline genetic diagnosis for rare disease.
We performed short-read GS (NovaSeq™6000; 150 bp paired-end reads, 37 × mean coverage) on 1000 cases with 1271 known clinically relevant variants, identified across different workflows, representative of our tertiary diagnostic centers. Variants were categorized into small variants (single nucleotide variants and indels < 50 bp), large variants (copy number variants and short tandem repeats) and other variants (structural variants and aneuploidies). Variant calling format files were queried per variant, from which workflow-specific true positive rates (TPRs) for detection were determined. A TPR of ≥ 98% was considered the threshold for transition to GS. A GS-first scenario was generated for our laboratory, using diagnostic efficacy and predicted false negative as primary outcome measures. As input, we modeled the diagnostic path for all 24,570 individuals referred in 2022, combining the clinical referral, the transition of the underlying workflow(s) to GS, and the variant type(s) to be detected.
Overall, 95% (1206/1271) of variants were detected. Detection rates differed per variant category: small variants in 96% (826/860), large variants in 93% (341/366), and other variants in 87% (39/45). TPRs varied between workflows (79–100%), with 7/10 being replaceable by GS. Models for our laboratory indicate that a GS-first strategy would be feasible for 84.9% of clinical referrals (750/883), translating to 71% of all individuals (17,444/24,570) receiving GS as their primary test. An estimated false negative rate of 0.3% could be expected.
Conclusions
GS can capture clinically relevant germline variants in a ‘GS-first strategy’ for the majority of clinical indications in a genetics diagnostic lab.
Although human genetic diseases are rare, they account for an important public health burden [ 1 , 2 ]. Diagnostic approaches to detect the underlying genetic causes of these diseases require a broad spectrum of technologies, ranging from traditional approaches such as karyotyping, genomic microarrays, FISH, MLPA, and Sanger sequencing, to more advanced technologies, such as exome sequencing and transcriptomics. Each of these technologies is dedicated to detecting one or multiple variant types [ 3 , 4 , 5 , 6 ]. In clinical genomics, (de novo) single nucleotide and copy number variants (SNV/CNV) are the most commonly found aberrations [ 7 , 8 , 9 ], but to a lesser extent aneuploidy, expansions of short tandem repeats (STR), and (copy-neutral) structural variants (SV) also contribute to disease. To molecularly diagnose a rare disease, multiple workflows are often used, as a single disease can often be caused by multiple variant types [ 10 , 11 , 12 , 13 , 14 ]. Importantly, for diagnostic purposes, every technology needs to prove clinical, as well as analytical, validity [ 3 , 15 ].
Genome sequencing (GS) promises comprehensive variant calling of all variant types from a single experiment, allowing for all types of molecular diagnoses [ 16 , 17 ]. This (potentially) not only leads to an increased diagnostic yield but also provides a higher efficiency for genetic diagnostic laboratories that would no longer need to maintain multiple workflows to capture the various variant types. So far, however, widespread implementation of GS is lagging as the increase in diagnostic yield has been limited while incurring higher costs compared to routine workflows [ 18 , 19 , 20 ].
Several studies have performed direct comparisons between GS and one or a few techniques to explore concordance and utility [ 18 , 19 , 20 , 21 ], and GS has meanwhile been implemented for diagnosis and discovery in a few countries [ 22 , 23 ]. A less explored scenario for effective implementation of GS as a routine diagnostic test is the impact of GS replacing all currently used diagnostic workflows. For instance, in our tertiary referral centers for genetic diagnostic testing at the Radboud University Medical Center (Radboudumc) and Maastricht University Medical Center + (MUMC +), approximately 25,000 individuals with a rare disease are tested annually, requiring > 10 molecular and cytogenetic workflows to capture all genetic variant types. Replacing these workflows with a single GS-based workflow would increase efficiency. To determine the feasibility of transitioning to a generic GS diagnostic workflow, we performed a benchmarking study using GS on 1000 individuals previously molecularly diagnosed with a rare genetic disease, representative of the myriad of genetic variant types identified across 10 different workflows and modeled the impact of a GS-first diagnostic strategy for rare disease in our centers.
Cohort selection
We retrospectively selected archival residual DNA material from a cohort ( n = 1000) with known clinically relevant variants ( n = 1271) from genome diagnostic laboratories of the Radboudumc in Nijmegen and the MUMC + in Maastricht. The cohort was selected from all positive reports in 2018, taking into account the distribution of molecular and cytogenetic workflows used in these departments for the primary diagnosis of germline variants underlying hereditary and congenital diseases using blood-derived DNA (based on the total number of requests and diagnostic yield), as well as to include a myriad of different genes, chromosomes and variant types ( n = 979 cases, 1249 variants; Additional file 1 : Table S1 and S2). The cohort was complemented with a few interesting cases for which DNA was extracted from another source than EDTA blood ( n = 21, 22 variants; Additional file 1 : Table S1 and S2). Of note, the cohort included 62 cases with diagnostic referrals that are under suspicion of harboring variants that are at risk to fail detection in a 30 × short-read genome. These cases had variants ( n = 119 in genes or regions with a high level of sequence homology ( n = 63), or possible mosaic variants ( n = 56, range 2.4–54%), where the primary diagnostic referral was not always aimed at germline testing, but EDTA blood samples were available (Additional file 1 : Table S2). Based on the selection criteria, the cohort is considered representative for our diagnostic centers.
- Genome sequencing
GS, using 150 bp paired-end short-reads, was performed as defined by the manufacturer (Illumina, San Diego, CA, USA). In brief, 1000 ng DNA was used for library preparation using the Illumina DNA PCR-free protocol and DNA was tagmented to an average insert size of 450 bp using bead-linked transposomes [ 24 ]. To allow equimolar pooling of samples, barcoded dual indexing was used after which the Illumina index correction strategy was applied (Additional file 1 : Table S1). Sequencing was performed on an Illumina NovaSeq6000™ Instrument (24 samples on a S4 flowcell) to an anticipated genome-wide coverage of 30-fold minimal.
Data analysis
Raw output was stored in Illumina’s BaseSpace Sequence Hub and data was analyzed using the Germline Pipeline of Illumina’s DRAGEN™ (Dynamic Read Analysis for GENomics) Bio-IT platform v3.7.5 [ 25 , 26 ]. In short, after data is demultiplexed, mapped, and aligned (GRCh37), the DRAGEN Germline Pipeline provides a comprehensive analysis, including small variant (SNV and indels < 50 bp), ROH, CNV, and SV calling, as well as repeat expansion detection and genotyping through Illumina Expansion Hunter [ 27 ]. In addition, we used newly developed DRAGEN SMA [ 28 ] and CYP21A2 (DRAGEN v3.9) callers for those specific cases in which the genetic variants located in SMN1/2 or CYP21A2 ( n = 19 cases, 34 variants).
Variant detection strategy
Variant detection was divided into two phases. First, variant call format files (VCF) generated by the DRAGEN Germline Pipeline were assessed by automated (including clinical filters) or manual targeted queries using Illumina’s TruSight Software Suite v2.5 (TSS) to identify the variants of interest, resulting in a positive “ + ” (detected) or negative “ − ” (not detected) result. Variant detection was based on matching of chromosomal coordinates of small variants, or reciprocal overlap of genomic event intervals for large variants (CNV, ROH, STR). Other variants (structural variants and aneuploidies) were only investigated manually using VCFs and the Integrative Genomics Viewer (IGV) genome browser [ 29 ] in TSS, as TSS did not support automated features (clinical filters) at the time of analysis. Second, variants that failed detection were further assessed to determine why they were absent from the VCFs.
Sensitivity analysis
Sensitivity analysis was performed in two ways. First, we assessed the overall sensitivity of GS by calculating the true positive rate (TPR) for each workflow, defined as the number of true positive variants (TP) divided by the total number of variants ( n = 1271 in 1000 cases) including the false negatives (FN; TPR = TP/(TP + FN). Second, we repeated the analysis after exclusion of the cases ( n = 62) with variants ( n = 123) which were a priori known to fail detection in a 30 × short-read genome to better approximate the TPR.
Impact analysis
We modeled a scenario of the overall impact of GS implementation as a generic workflow. Hereto we performed three in silico analyses.
First, we determined the sequence depth at genomic positions that are known to harbor (likely) pathogenic variation. Sequence depth was calculated from 35 randomly selected genomes. The median coverages were subsequently intersected with genomic positions (coordinates) of all known pathogenic variants reported in the repository of the Dutch Association of Clinical Laboratory Geneticists [ 30 , 31 ] and ClinVar [ 32 ]. In addition, we determined the median coverages for all coding positions of genes with well-established rare disease associations [ 33 ]. Under the assumption that sequence coverage is one of the main determinants for being able to reliably call a variant, we next calculated the fraction of variants with sufficient coverage. Minimal threshold for presumed detection of a variant was set at tenfold coverage at the respective genomic coordinate. Assuming a binomial distribution with probability 0.5 of sequencing the variant allele at a heterozygous position, at least 10 reads are required to obtain a 99% probability that at least two reads contain the variant allele [ 34 ].
Secondly, we extrapolated and modeled the obtained workflow-based TPRs and GS variant detection limitations from our experimental data to a real-life scenario of our genetic diagnostic laboratories. In line with guidelines for assuring the quality of diagnostic next-generation sequencing [ 35 , 36 ], we used a TPR of ≥ 98% as threshold for replacing workflows by GS. As input for our model, we used anonymized data of all 41,691 individuals tested in our genetic diagnostic laboratories in 2022 (Additional file 2 : Fig. S1). For each diagnostic referral ( n = 54,680), we evaluated the reason for referral and eligibility for inclusion in our model. A total of 24,166 referrals were excluded, as these either represented cascade screening ( n = 7854) or were not within the current scope of replacing by GS ( n = 16,312), such as for instance non-DNA based and/or biochemical assays (Additional file 2 : Fig. S1). For the remaining 30,514 referrals for testing, performed in 24,570 individuals, we determined the experiments and workflows used to address the diagnostic referral as input for the model. Combining the workflow and variant type detected per clinical indication, we modeled the impact of substituting eligible experiments (clinical indications) for GS in the diagnostic trajectory of these individuals. Of note, for individuals with multiple referrals that could be replaced by GS, a maximum of one GS was considered, with subsequent diagnostic referrals involving reanalysis of existing data.
Finally, to determine the impact of the GS-first strategy on overall diagnostic yield, the outcome per individual was projected under the following assumptions:
Negative diagnostic results remained negative, regardless of the underlying workflow, thus also not considering a possible added diagnostic value of GS.
For individuals whose diagnostic track would not include GS, or where GS was supplemented with an additional non-GS transferable clinical referral, the original diagnostic outcome was maintained.
For individuals with a conclusive ((highly) likely pathogenic variant), or possible (variant of unknown significance) diagnosis, the GS diagnostic outcome was offset with the TPRs per workflow. Of note, for individuals with multiple diagnostic referrals, it was first determined which experimental workflow led to the initial possible/conclusive diagnosis.
We subsequently determined the number of individuals negatively impacted by the GS-first strategy as proxy for false negatives [FN]. The false negative rate (FNR) was determined by FNR = [FN]/[FN] + [TP], in which [TP] was defined as the original diagnostic yield in the cohort of 24,570 individuals minus the [FN].
Genome diagnostics and cohort demographics
This local 1000 genome project included archival DNA samples of 505 males and 495 females who were genetically tested in our laboratories using 10 different workflows (Additional file 1 : Table S1; Additional file 2 : Fig. S2). For 378 individuals, this included analysis of specific variants, a single gene, or a few genes, whereas in 617 individuals, extensive gene panels or other genome-wide analyses were used. For the remaining five individuals, a combination of both approaches was employed (Additional file 2 : Fig. S2). A total of 1271 diagnostically relevant variants were reported (Additional file 1 : Table S2; Additional file 2 : Fig. S2). All variants were called complying to specifications of DRAGEN variant calling, grouping them in three categories: a category for small variants ( n = 860), including SNVs and indels up to 50 bp in size, a second one for large variants ( n = 366), i.e., CNVs and STRs, leaving a third category for all other variants ( n = 45), involving SVs and chromosome anomalies (CA) (Additional file 1 : Table S2; Additional file 2 : Fig. S2). For our 1000 genomes, we reached an average sequencing depth of 37 × (Additional file 2 : Fig. S3).
GS technical validation and feasibility assessment of replacing workflows by GS
In total, 94.9% (1206/1271) of all variants were detected with GS (Fig. 1 ; Additional file 1 : Table S2). Small variants were detected in 96.1% (826/860), large variants (123 bp–72.8 Mb) in 93.2% (341/366), and other variants in 86.7% (39/45) (Additional file 2 : Fig. S4 and S5). Subdividing the cohort by the variants we expected to readily identify ( n = 1152) and those that we would not ( n = 119), indeed confirmed the prior knowledge of the technical challenges in detecting mosaic variants and variants located in homologous regions or genes with short-read 30 × GS: 1138 of 1152 variants (98.8%) were detected as expected, whereas only 68/119 (57.1%) of challenging variants were identified (Fisher’s exact test p < 0.001; Additional file 1 : Table S2). The variants that remained undetected after manual curation ( n = 65), could be categorized into four categories: mosaic variants ( n = 27, including somatic and mitochondrial variants), homologous regions ( n = 25, e.g., variants in STRC or the Opsin gene family), short tandem repeats/repetitive sequence ( n = 10, such as FMR1 and Robertsonian translocations), and other variants ( n = 3). Of note, the detection limit of small mosaic variants was 13%.
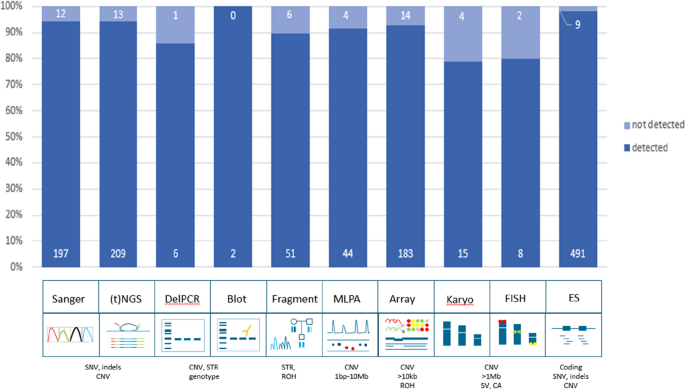
Technical validation of 1271 variants. Schematic representation of detection rates of previously identified pathogenic variants across multiple different workflows. In total, 94.9% (1206/1271) of all variants were detected in GS data. The distribution of variants across the ten workflows shows a detection rate ranging between 79 and 100%. Abbreviations: targeted next-generation sequencing ((t)NGS), deletion polymerase chain reaction (DelPCR), multiplex ligation-dependant probe amplification (MLPA), fluorescence in situ hybridization (FISH), exome sequencing (ES), single nucleotide variants (SNV), copy number variants (CNV), short tandem repeat expansions (STRs), region of homozygosity (ROH), structural variants (SV), chromosome anomalies (CA)
We next reconstituted the 1271 variants to their original workflows to determine the overall performance of detection of different variant types per workflow, which ranged from 79% for karyotyping to 100% for Southern blots (Fig. 1 ). Subsequent analysis of the TPR per workflow revealed that all workflows, except repeat length analysis, karyotyping, and FISH, were determined to have a TPR > 98% (Additional file 2 : Table S3).
In silico extrapolation of detection rates to 58,393 variants and 4266 disease genes
Assessing the available coverage data of 794 detected SNVs in our cohort showed that 99.1% had a ≥ 10 × coverage (Additional file 1 : Table S2; Additional file 2 : Fig. S6). We next leveraged the observations onto a larger in silico data set of variants. Hereto, we obtained 58,393 genomic coordinates from variants known in the VKGL and/or ClinVar databases to cause autosomal dominant/recessive disease (Additional file 1 : Table S4) and determined the sequence coverage for those positions across 35 genomes. For 99.5% of variants, the minimal coverage across 35 genomes was ≥ 10 × (Additional file 2 : Fig. S6). Generation of similar coverage statistics for all coding bases of 4,266 disease-associated genes showed that the average coverage was 45 × (Additional file 1 : Table S5; Additional file 2 : Fig. S5), with 88.1% of genes (3759/4266) having a coverage of ≥ 10 × for all protein-coding bases (Additional file 2 : Fig. S6).
Modeling the impact of GS implementation in clinical practice
We next set out to model the impact of GS implementation on everyday practice in our clinical centers, from both the clinical point of view, as well as from the laboratory point of view. In addition, we determined the impact on overall diagnostic yield obtained from a GS-first perspective.
In 2022, our tertiary genetic diagnostic laboratory received 30,514 diagnostic referrals to identify the primary germline DNA defect in 24,570 individuals with rare disease (Fig. 2 ; Additional file 2 : Fig. S7). In total, 883 different reasons for referral were observed, with the top 10 ranking clinical indications being responsible for 21% of all referrals. On average, per individual 1.24 referrals were noted, and 82% of individuals were referred only once (Additional file 2 : Fig. S7). Of note, for 966 individuals, the diagnostic referral ( n = 2072) consisted of reanalysis of existing exome data and did not require the generation of novel experimental data. For the other 28,442 referrals, 36,633 wet lab experiments were performed using 11 different workflows (Fig. 2 ).
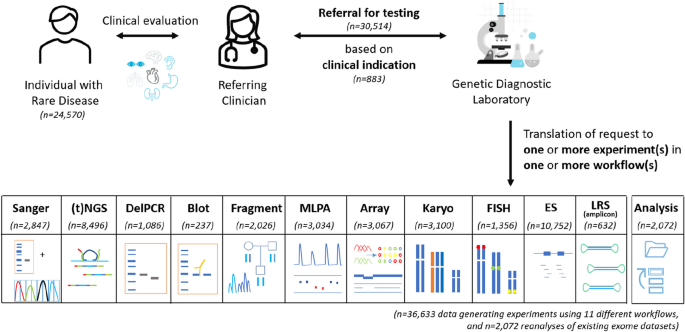
Diagnostic referrals for genetic testing in 2022. In total, 24,570 individuals were referred, together requiring 36,633 data-generating experiments (in 23,604 individuals) in 11 different workflows, and 2072 reanalyses of existing (exome) datasets (in 966 individuals). Abbreviations: targeted next-generation sequencing ((t)NGS), deletion polymerase chain reaction (DelPCR), multiplex ligation-dependant probe amplification (MLPA), fluorescence in situ hybridization (FISH), exome sequencing (ES), long-read sequencing (LRS)
From a clinical point of view, 750 of 883 (85%) clinical reasons for referral could be addressed via GS (Fig. 3 ). The remaining 133 could not be performed via GS for various reasons, of which somatic variant detection (53%) and detection of variants in homologous regions (13%) are the most prominent (Fig. 3 ). From a laboratory point of view, this GS-first strategy would not only fully replace the exome workflow and all Southern blots but would also considerably reduce the use of other workflows, such as Sanger sequencing (by 89%), MLPA (by 80%) and targeted NGS approaches (by 70%; Fig. 3 ). Importantly, applying these observations to the diagnostic trajectory of all individuals shows that GS can be used as first-tier test for 16,777 (68%; Fig. 3 ) of individuals.
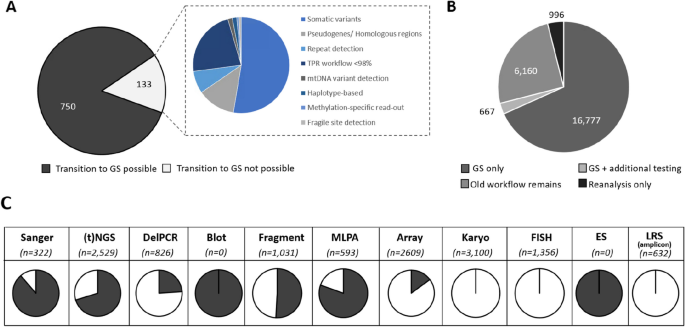
Assessing the impact of a GS-first transition. A From 833 different clinical reasons for referral in 2022, 750 can be transitioned to GS. B This transition would result in 16,777 individuals receiving GS as the only workflow. For 667 (3%), the GS should be supplemented by an additional test, whereas for the remaining 7126 (29%) GS would not be suited, either because for them the clinical indications included experiments not transferable to GS ( n = 6160; 25%), or because the referral did not require data generation ( n = 966; 4%). C The use of GS as a primary test has a significant impact on reducing the experimental workload in the original workflows. Proportions of the transferable number of tests per workflow are indicated in black. Abbreviations: targeted next-generation sequencing ((t)NGS), deletion polymerase chain reaction (DelPCR), multiplex ligation-dependant probe amplification (MLPA), fluorescence in situ hybridization (FISH), exome sequencing (ES), long-read sequencing (LRS)
Finally, we modeled the impact on the overall diagnostic yield. In 2022, a conclusive molecular diagnosis was obtained in 2652 of 24,570 individuals (10.79%), and for another 3597 (14.64%) a possible diagnosis was identified. Extrapolation of TPRs for individuals whose diagnostic trajectory would include GS, resulted in an anticipated conclusive diagnosis in 2643 individuals (10.76%) and a possible diagnosis in 3589 (14.61%; Additional file 2 : Fig. S8). Collectively, a generic GS-first strategy would thus possibly negatively impact the diagnostic outcome for 17 (0.07%) individuals (FN = 17), translating to a possible false negative diagnostic rate of 0.3%.
Over the last decade, the use of GS as a routine diagnostic test has been debated in the context of a higher potential diagnostic yield by interpreting non-coding DNA variants, as well as the potential to diagnose individuals with rare disease more efficiently, as GS allows the identification of virtually all genetic variants in a single experiment. Widespread diagnostic implementation has however been hampered by the costs involved with GS, given that the anticipated higher diagnostic yield has so far not materialized. An increased diagnostic yield is however still expected for unexplained rare genetic disease, especially when looking beyond SNV and CNV detection in the exome only. To ultimately benefit from the advantages of GS, costs need to be reduced for a generic genetic diagnostic laboratory. In this study, we focused on the potential for GS as a generic diagnostic rare disease test, replacing the full spectrum of workflows available in a genetic diagnostic laboratory. With our cohort of 1000 genomes, representative of 10 different workflows and a multitude of genetic variant types, we found that GS detected > 95% of all pathogenic variants, albeit with variable efficacy across variant types and workflows. We also modeled the impact of a transition to a generic GS workflow for our diagnostic laboratories and concluded that for 68% of individuals diagnostically referred to our departments a generic GS workflow would be possible.
In our series of 1000 samples, we noted differences in the detection of different variant types; 96.1% of small variants (< 50 bp) were detected, whereas only 93.3% of large variants, and 86.7% of other variants were recovered from GS. Interestingly, one of the arguments generally used as benefit from GS is its ability to better detect structural variation compared to ES [ 37 ]. Conceptually, this is true from having a more uniform coverage across the genome [ 38 ]. In addition, we, and others, have previously shown that additional diagnoses obtained via GS compared to routine care, not only are often SVs, but also that the resolution of SV complexity identified, often (far) exceeds that of other technologies [ 20 , 39 ]. However, our data now show that the capture of SNVs/indels from GS is more complete than of SVs (Fisher’s exact, p = 0.006). Irrespectively, it must be noted that the overall number of SVs evaluated was limited due to our inclusion criteria which required DNA from EDTA blood. Further retrospective analysis of pathogenic SVs might provide additional insights. Also, given the assumption and prior work showing that GS excels in SV detection, one might also speculate on GS now uncovering variants where the initial gold standard technology might have been wrong.
Any technology comes with technical limitations. Here, we did not identify any unexpected limitations in variant calling for the 5% (65/1271) of undetected variants, besides the already known limitations of variant calling in short-read GS data. For instance, it is known that mapping short reads in homologous regions is difficult, and also, short-read GS at ~ 30 × will pose difficulties in detecting mosaic variants. Solutions to recover these variants from short read data are, however, possible: for mosaic variants, increasing GS sequence depth may be able to recover all clinically relevant variation, while bioinformatic callers might help in the successful retrieval of (likely) pathogenic variants in complex homologous genomic regions. Currently, such dedicated callers already exist, e.g., we successfully used callers for the SMA [ 28 ] and CYP21A2 loci in our analyses, and also, other tools calling variation in paralogous regions have been developed [ 40 ]. On a positive note, we already recovered 68 of 119 variants that we a priori expected to be beyond the technical limitations of 30 × GS, without further optimization. These included variants located in highly homologous regions ( STRC and OTOA ), as well as variants present in mosaic state (> 14%), for which in the SOC trajectory dedicated tests are deployed that are designed to detect specific variants of interest.
Diagnostic efficacy can be enhanced by reducing the complexity of sample handling and the number of workflows. In our laboratory set-up, one clinical referral is often translated into experiments in multiple workflows; for example, to molecularly diagnose CHARGE syndrome, caused by CHD7 haploinsufficiency, both Sanger sequencing and MLPA analysis are needed to allow the detection of SNV/indels as well as of (partial) gene deletions. The introduction of a generic GS workflow would allow for calling both SNV/indels, CNVs, and other SVs affecting CHD7 from a single experiment. For other disorders, for instance, those caused by the expansion of short tandem repeats, it might be more challenging, as short-read sequencing technologies may be unable to capture the full length of the extension. However, our data shows that although for some repeats the exact length cannot be obtained, a generic GS workflow is able to identify those individuals with repeat lengths outside of the normal range. This result can be followed with dedicated tests to determine the size of the repeat. From an efficacy point of view, one may argue that a second workflow is still required. While this is a valid point, in a generic GS workflow, the subsequent use of a second workflow is much more efficient, as it will only be used for those individuals with a high a priori chance of a positive outcome (given their abnormal GS results).
Whether or not it is efficient for laboratories to make a transition towards a generic GS workflow may depend on lab-specific factors, including size of the lab, number of workflows in use, and type of diagnostic referrals received. From our series of 1000 genomes tested, we showed that ES can technically be replaced by GS (TPR > 98%), in line with previous reports on comparing diagnostic outcomes of ES and GS [ 18 , 19 , 20 ]. Hence, diagnostic laboratories, whose expertise is to only perform ES, could easily move towards GS with the benefit of a faster workflow as enrichment is no longer needed [ 24 ]. Yet, for laboratories specialized in the use of karyotyping (TPR < 98%) for the detection of somatic copy number changes, routine 30 × GS might not be sufficient. Additionally, we showed that there are some genes with lower coverage (for part) of all protein-coding bases, which warrants caution when these genes are of specific interest related to a specific clinical differential diagnosis. The results of our study should therefore be carefully examined and extrapolated to local infrastructure and clinical expertise. Of note, a site-specific (early) health economic impact analysis is also recommended prior to large-scale implementation, in which evaluation of cost-effectiveness is key. These studies are mostly performed in the context of proving that an early diagnosis also has a beneficial impact on overall health care cost expenditure [ 21 , 41 , 42 , 43 ]. In light of implementing a generic GS workflow, a complementary micro-costing study could be of relevance [ 44 , 45 ]. Such studies would allow to weigh possible cost-reductions from phasing out workflows and changes in workforce against potential increase of per-sample sequencing costs, as well as the costs associated with (ease of) clinical data interpretation.
Here, we report on our laboratories, which together maintain > 10 workflows, representative for most core technologies used in genetic testing [ 16 ], and enabling detection of all variant types. The scenario models for our centers showed that 750/883 (85%) diagnostic referrals can be completed using GS, which would result in 68% of all individuals referred to our diagnostic laboratory making use of a single workflow and a single experiment, and 3% needing additional testing, suggesting that for 71% of individuals ( n = 17,444) a GS-first strategy would be beneficial. Whereas our analysis did not include a full micro-costing study, a generic GS-first workflow for such volume of samples might become within reach, especially with prices announced for germline GS in the range of 100 to 200 dollars per genome [ 46 , 47 , 48 ]. For the 15% of clinical indications not transferable to GS (responsible for 29% of individuals referred), we noted trends, such that most of these required somatic structural variant detection, currently assayed via karyotyping, FISH and/or arrays, or variants were located in complex regions of the genome, currently assessed by amplicon-based long-read sequencing strategies [ 49 ]. These technical challenges can — in part — be overcome by sequencing at higher depth (e.g., ~ 100 × or even ~ 350 ×) to allow better somatic SNV/indel detection [ 50 ]. Yet, it could also be considered to maintain workflows for dedicated diagnostic referrals. Alternatively, technological innovations specifically targeting these more challenging variant types and regions would constitute a worthwhile investment. For somatic variant detection via karyotyping, FISH and/or arrays, optical genome mapping [ 51 , 52 ] could replace these workflows as a second major generic assay, available in parallel to GS, but used for mutually exclusive clinical referrals. Similarly, a more generic use of long read genomes [ 53 , 54 ] may provide a costs-effective strategy for diagnostic referrals involving variants in complex regions in the genome, or where variant size exceeds those detectable from short reads (such as for repeat expansions). For either technical solution, a careful evaluation of the required coverage, as well as the impact on the false negative rate when compared to the old technique [ 38 , 55 , 56 , 57 , 58 ].
The implementation of a novel technology requires careful balancing of the pros and cons. For GS, our study has highlighted advantages related to laboratory efficiency, but also showed that not all previously detected (likely) pathogenic germline variants were also identifiable from GS. Hence, if a generic GS workflow were to be used, it is to be expected that some individuals who would receive a conclusive diagnosis with the old diagnostic test strategy, would no longer do so with the implementation of a generic GS. In our objective quantification of the false negative rate from GS, using all diagnoses obtained by the current diagnostic strategy as the gold standard, we modeled that the transition to a generic GS in our laboratory might result in an additional diagnostic false negative rate of 0.3%. Whereas this is undesirable for the individual patient, previous experience has shown that there may be trade-offs. For instance, with the introduction of genomic microarrays at the expense of karyotyping, no longer detecting apparently balanced chromosomal rearrangements had to be accepted. Further, with the introduction of ES as replacement for Sanger sequencing for genetically and clinically heterogeneous disorders, one lost sensitivity at base pair level while gaining in mutation target size. Both innovations changed diagnostic testing, because despite losing out on a few positive diagnoses, they still improved the overall diagnostic yield [ 59 , 60 ]. So far, the overall diagnostic advantage of GS is still limited. Disease-specific evaluations of diagnostic yield of GS have, however, reported on an increase in diagnostic yield, ranging from 1.3% for neurodevelopmental disorders [ 20 ] to 17% for congenital limb malformations [ 17 ]. Additionally, it has been reported that cytogenetically found apparently balanced chromosomal rearrangements appear to be genomic imbalances in ~ 1/3 of patients with de novo translocations and inversions [ 61 , 62 ], and that ~ 2/3 of balanced chromosomal abnormalities are involved in pathogenic mechanisms [ 63 ]. With growing experience in detecting and interpreting structural variants in GS data, we also expect to identify more inversions, translocations, and other structural variants as underlying causes of human genetic disease. The use of GS over current workflows would provide an added value for which individuals with rare disease would immediately benefit, thus potentially compensating for the 0.3% diagnostic loss from introducing a generic GS workflow.
Finally, our study is designed as technical benchmarking, which did not include an evaluation of variant prioritization. We and others have, however, recently shown in prospective parallel and randomized GS studies that similar variant types and diagnostic yield are obtained when comparing GS to current (non-GS) standard-of-care diagnostic workflows [ 18 , 20 ]. In light of this, it is also worthwhile to underscore that even though analytically a full genome sequence is provided, a targeted interpretation of variants, in line with the clinical request, could still be pursued. For instance, initially, variants in single genes can be prioritized using in silico enrichment strategies when the GS is performed instead of a Sanger test, or, alternatively, only CNVs can be visualized when otherwise an array would have been analyzed. If negative, a more agnostic approach for the interpretation of genetic variation can performed, where the existing and available GS data provide a valuable resource for efficient reanalysis and reinterpretation strategies. We noted that 6.8% of our referrals ( n = 2072) already involved reanalysis of existing exome data. With increasing knowledge on the role of (rare) non-coding variants in relation to disease and improvement in the bioinformatic detection of variants in complex regions of the genome from short reads, it can be expected that the availability of GS provides more flexibility in adapting reanalysis strategies towards these loci and variant types in the near future.
In summary, our benchmarking study provides detailed insights into the technical possibilities and limitations of GS and its use as a generic diagnostic workflow. We show that > 95% of known pathogenic variants, selected across the full spectrum of genetic variation, are readily detectable from GS. Modeling the impact of the transition to a generic GS strategy for our laboratory resulted in a more efficient workflow for 71% of individuals by reducing overall test complexity. A possible false negative rate of 0.3% was observed. It is possible that this potential diagnostic loss will be offset by an increase in diagnostic yield expected from GS over standard care, enabled by an evolving GS workflow, guided by better bioinformatic tools to further improve the detection of a wide variety of genomic variants and a greater understanding of non-coding and structural variant interpretation. GS thus appears a suitable generic first tier test to diagnose individuals with rare diseases.
Availability of data and materials
The consent of the samples from which data was generated does not allow for broad sharing of raw FASTQ Files, and re-use of data is limited. Nonetheless, all data obtained of relevance to support the conclusions are presented in the supplementary datafiles, for which more details are available upon reasonable request from the authors.
Abbreviations
Chromosome anomaly
Copy number variants
Deoxyribonucleic acid
Exome sequencing
Fluorescence in situ hybridization
False negative
False negative rate
Multiplex ligation-dependent probe amplification
Next-generation sequencing
Region of homozygosity
Southern blot
Spinal muscular atrophy
Single nucleotide variant
Short tandem repeat
Structural variant
True positive
True positive rate
Variant calling format
Blencowe H, Moorthie S, Petrou M, Hamamy H, Povey S, Bittles A, et al. Rare single gene disorders: estimating baseline prevalence and outcomes worldwide. J Community Genet. 2018;9(4):397–406.
Article PubMed PubMed Central Google Scholar
Ontario H. Genome-wide sequencing for unexplained developmental disabilities or multiple congenital anomalies: a health technology assessment. Ont Health Technol Assess Ser. 2020;20(11):1–178.
Google Scholar
Katsanis SH, Katsanis N. Molecular genetic testing and the future of clinical genomics. Nat Rev Genet. 2013;14(6):415–26.
Article CAS PubMed PubMed Central Google Scholar
Hantash FM, Goos DG, Tsao D, Quan F, Buller-Burckle A, Peng M, et al. Qualitative assessment of FMR1 (CGG)n triplet repeat status in normal, intermediate, premutation, full mutation, and mosaic carriers in both sexes: implications for fragile X syndrome carrier and newborn screening. Genet Med. 2010;12(3):162–73.
Article CAS PubMed Google Scholar
Shi X, Tang H, Lu J, Yang X, Ding H, Wu J. Prenatal genetic diagnosis of omphalocele by karyotyping, chromosomal microarray analysis and exome sequencing. Ann Med. 2021;53(1):1285–91.
Article PubMed Google Scholar
Slater H, Bruno D, Ren H, La P, Burgess T, Hills L, et al. Improved testing for CMT1A and HNPP using multiplex ligation-dependent probe amplification (MLPA) with rapid DNA preparations: comparison with the interphase FISH method. Hum Mutat. 2004;24(2):164–71.
Lupski JR. Clan genomics: From OMIM phenotypic traits to genes and biology. Am J Med Genet A. 2021;185(11):3294–313.
Brunet T, Jech R, Brugger M, Kovacs R, Alhaddad B, Leszinski G, et al. De novo variants in neurodevelopmental disorders—experiences from a tertiary care center. Clin Genet. 2021;100(1):14–28.
Kaplanis J, Samocha KE, Wiel L, Zhang Z, Arvai KJ, Eberhardt RY, et al. Evidence for 28 genetic disorders discovered by combining healthcare and research data. Nature. 2020;586(7831):757–62.
Article ADS CAS PubMed PubMed Central Google Scholar
Jansen S, Vissers L, de Vries BBA. The genetics of intellectual disability. Brain Sci. 2023;13(2):231.
Strom SP, Hossain WA, Grigorian M, Li M, Fierro J, Scaringe W, et al. A streamlined approach to prader-willi and angelman syndrome molecular diagnostics. Front Genet. 2021;12:608889.
Pignatelli D, Carvalho BL, Palmeiro A, Barros A, Guerreiro SG, Macut D. The complexities in genotyping of congenital adrenal hyperplasia: 21-hydroxylase deficiency. Front Endocrinol. 2019;10:432.
Article Google Scholar
Lindstrand A, Davis EE, Carvalho CM, Pehlivan D, Willer JR, Tsai IC, et al. Recurrent CNVs and SNVs at the NPHP1 locus contribute pathogenic alleles to Bardet-Biedl syndrome. Am J Hum Genet. 2014;94(5):745–54.
Spedicati B, Santin A, Nardone GG, Rubinato E, Lenarduzzi S, Graziano C, et al. The enigmatic genetic landscape of hereditary hearing loss: a multistep diagnostic strategy in the italian population. Biomedicines. 2023;11(3):703.
Pum J. A practical guide to validation and verification of analytical methods in the clinical laboratory. Adv Clin Chem. 2019;90:215–81.
Lalonde E, Rentas S, Lin F, Dulik MC, Skraban CM, Spinner NB. Genomic diagnosis for pediatric disorders: revolution and evolution. Front Pediatr. 2020;8:373.
Elsner J, Mensah MA, Holtgrewe M, Hertzberg J, Bigoni S, Busche A, et al. Genome sequencing in families with congenital limb malformations. Hum Genet. 2021;140(8):1229–39.
Brockman DG, Austin-Tse CA, Pelletier RC, Harley C, Patterson C, Head H, et al. Randomized prospective evaluation of genome sequencing versus standard-of-care as a first molecular diagnostic test. Genet Med. 2021;23(9):1689–96.
Clark MM, Stark Z, Farnaes L, Tan TY, White SM, Dimmock D, et al. Meta-analysis of the diagnostic and clinical utility of genome and exome sequencing and chromosomal microarray in children with suspected genetic diseases. NPJ Genom Med. 2018;3:16.
van der Sanden B, Schobers G, Corominas Galbany J, Koolen DA, Sinnema M, van Reeuwijk J, et al. The performance of genome sequencing as a first-tier test for neurodevelopmental disorders. Eur J Hum Genet. 2023;31(1):81–8.
Runheim H, Pettersson M, Hammarsjö A, Nordgren A, Henriksson M, Lindstrand A, et al. The cost-effectiveness of whole genome sequencing in neurodevelopmental disorders. Sci Rep. 2023;13(1):6904.
Turro E, Astle WJ, Megy K, Gräf S, Greene D, Shamardina O, et al. Whole-genome sequencing of patients with rare diseases in a national health system. Nature. 2020;583(7814):96–102.
Stranneheim H, Lagerstedt-Robinson K, Magnusson M, Kvarnung M, Nilsson D, Lesko N, et al. Integration of whole genome sequencing into a healthcare setting: high diagnostic rates across multiple clinical entities in 3219 rare disease patients. Genome Med. 2021;13(1):40.
Bruinsma S, Burgess J, Schlingman D, Czyz A, Morrell N, Ballenger C, et al. Bead-linked transposomes enable a normalization-free workflow for NGS library preparation. BMC Genomics. 2018;19(1):722.
Zhao S, Agafonov O, Azab A, Stokowy T, Hovig E. Accuracy and efficiency of germline variant calling pipelines for human genome data. Sci Rep. 2020;10(1):20222.
Miller NA, Farrow EG, Gibson M, Willig LK, Twist G, Yoo B, et al. A 26-hour system of highly sensitive whole genome sequencing for emergency management of genetic diseases. Genome Medicine. 2015;7(1):100.
Dolzhenko E, Deshpande V, Schlesinger F, Krusche P, Petrovski R, Chen S, et al. ExpansionHunter: a sequence-graph-based tool to analyze variation in short tandem repeat regions. Bioinformatics. 2019;35(22):4754–6.
Chen X, Sanchis-Juan A, French CE, Connell AJ, Delon I, Kingsbury Z, et al. Spinal muscular atrophy diagnosis and carrier screening from genome sequencing data. Genet Med. 2020;22(5):945–53.
Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29(1):24–6.
Fokkema I, van der Velde KJ, Slofstra MK, Ruivenkamp CAL, Vogel MJ, Pfundt R, et al. Dutch genome diagnostic laboratories accelerated and improved variant interpretation and increased accuracy by sharing data. Hum Mutat. 2019;40(12):2230–8.
MOLGENIS. Available from: https://vkgl.molgeniscloud.org/ . Accessed Jan 2023.
ClinVar. Available from: https://www.ncbi.nlm.nih.gov/clinvar/ . Accessed 19 Mar 2023.
Gene panels DG3.60. Available from: https://www.radboudumc.nl/patientenzorg/onderzoeken/erfelijkheidsonderzoek-exoomsequencing-wes/informatie-voor-verwijzers/exoom-panels .
de Ligt J, Willemsen MH, van Bon BWM, Kleefstra T, Yntema HG, Kroes T, et al. Diagnostic exome sequencing in persons with severe intellectual disability. N Engl J Med. 2012;367(20):1921–9.
Matthijs G, Souche E, Alders M, Corveleyn A, Eck S, Feenstra I, et al. Guidelines for diagnostic next-generation sequencing. Eur J Hum Genet. 2016;24(1):2–5.
Gargis AS, Kalman L, Berry MW, Bick DP, Dimmock DP, Hambuch T, et al. Assuring the quality of next-generation sequencing in clinical laboratory practice. Nat Biotechnol. 2012;30(11):1033–6.
Hehir-Kwa JY, Pfundt R, Veltman JA. Exome sequencing and whole genome sequencing for the detection of copy number variation. Expert Rev Mol Diagn. 2015;15(8):1023–32.
Lelieveld SH, Spielmann M, Mundlos S, Veltman JA, Gilissen C. Comparison of exome and genome sequencing technologies for the complete capture of protein-coding regions. Hum Mutat. 2015;36(8):815–22.
Smedley D, Smith KR, Martin A, Thomas EA, McDonagh EM, Cipriani V, et al. 100,000 Genomes Pilot on Rare-Disease Diagnosis in Health Care - Preliminary Report. N Engl J Med. 2021;385(20):1868–80.
Steyaert W, Haer-Wigman L, Pfundt R, Hellebrekers D, Steehouwer M, Hampstead J, et al. Systematic analysis of paralogous regions in 41,755 exomes uncovers clinically relevant variation. Nat Commun. 2023;14(1):6845.
Lunke S, Eggers S, Wilson M, Patel C, Barnett CP, Pinner J, et al. Feasibility of ultra-rapid exome sequencing in critically ill infants and children with suspected monogenic conditions in the Australian Public Health Care System. JAMA. 2020;323(24):2503–11.
Stark Z, Schofield D, Alam K, Wilson W, Mupfeki N, Macciocca I, et al. Prospective comparison of the cost-effectiveness of clinical whole-exome sequencing with that of usual care overwhelmingly supports early use and reimbursement. Genet Med. 2017;19(8):867–74.
Vissers L, van Nimwegen KJM, Schieving JH, Kamsteeg EJ, Kleefstra T, Yntema HG, et al. A clinical utility study of exome sequencing versus conventional genetic testing in pediatric neurology. Genet Med. 2017;19(9):1055–63.
Santos Gonzalez F, Mordaunt D, Stark Z, Dalziel K, Christodoulou J, Goranitis I. Microcosting diagnostic genomic sequencing: a systematic review. Genet Med. 2023;25(6):100829.
Soilly AL, Robert-Viard C, Besse C, Bruel AL, Gerard B, Boland A, et al. Cost of exome analysis in patients with intellectual disability: a micro-costing study in a French setting. BMC Health Serv Res. 2023;23(1):386.
Illumina's revolutionary NovaSeq X exceeds 200th order milestone in first quarter 2023 [press release]. 2023.
A $100 genome? New DNA sequencers could be a ‘game changer’ for biology, medicine [press release]. 2022.
China’s BGI says it can sequence a genome for just $100 [press release]. 2020.
Haer-Wigman L, den Ouden A, van Genderen MM, Kroes HY, Verheij J, Smailhodzic D, et al. Diagnostic analysis of the highly complex OPN1LW/OPN1MW gene cluster using long-read sequencing and MLPA. NPG Genom Med. 2022;7(1):65.
Article CAS Google Scholar
Truty R, Rojahn S, Ouyang K, Kautzer C, Kennemer M, Pineda-Alvarez D, et al. Patterns of mosaicism for sequence and copy-number variants discovered through clinical deep sequencing of disease-related genes in one million individuals. Am J Hum Genet. 2023;110(4):551–64.
Mantere T, Neveling K, Pebrel-Richard C, Benoist M, van der Zande G, Kater-Baats E, et al. Optical genome mapping enables constitutional chromosomal aberration detection. Am J Hum Genet. 2021;108(8):1409–22.
Neveling K, Mantere T, Vermeulen S, Oorsprong M, van Beek R, Kater-Baats E, et al. Next-generation cytogenetics: comprehensive assessment of 52 hematological malignancy genomes by optical genome mapping. Am J Hum Genet. 2021;108(8):1423–35.
Logsdon GA, Vollger MR, Eichler EE. Long-read human genome sequencing and its applications. Nat Rev Genet. 2020;21(10):597–614.
Pauper M, Kucuk E, Wenger AM, Chakraborty S, Baybayan P, Kwint M, et al. Long-read trio sequencing of individuals with unsolved intellectual disability. Eur J Hum Genet. 2021;29(4):637–48.
Yaldiz B, Kucuk E, Hampstead J, Hofste T, Pfundt R, Corominas Galbany J, et al. Twist exome capture allows for lower average sequence coverage in clinical exome sequencing. Hum Genomics. 2023;17(1):39.
Corominas J, Smeekens SP, Nelen MR, Yntema HG, Kamsteeg EJ, Pfundt R, et al. Clinical exome sequencing-Mistakes and caveats. Hum Mutat. 2022;43(8):1041–55.
Barbitoff YA, Polev DE, Glotov AS, Serebryakova EA, Shcherbakova IV, Kiselev AM, et al. Systematic dissection of biases in whole-exome and whole-genome sequencing reveals major determinants of coding sequence coverage. Sci Rep. 2020;10(1):2057.
Meienberg J, Zerjavic K, Keller I, Okoniewski M, Patrignani A, Ludin K, et al. New insights into the performance of human whole-exome capture platforms. Nucleic Acids Res. 2015;43(11):e76.
Miller DT, Adam MP, Aradhya S, Biesecker LG, Brothman AR, Carter NP, et al. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet. 2010;86(5):749–64.
Srivastava S, Love-Nichols JA, Dies KA, Ledbetter DH, Martin CL, Chung WK, et al. Meta-analysis and multidisciplinary consensus statement: exome sequencing is a first-tier clinical diagnostic test for individuals with neurodevelopmental disorders. Genet Med. 2019;21(11):2413–21.
De Gregori M, Ciccone R, Magini P, Pramparo T, Gimelli S, Messa J, et al. Cryptic deletions are a common finding in “balanced” reciprocal and complex chromosome rearrangements: a study of 59 patients. J Med Genet. 2007;44(12):750–62.
Tabet A-C, Verloes A, Pilorge M, Delaby E, Delorme R, Nygren G, et al. Complex nature of apparently balanced chromosomal rearrangements in patients with autism spectrum disorder. Molecular Autism. 2015;6(1):19.
Redin C, Brand H, Collins RL, Kammin T, Mitchell E, Hodge JC, et al. The genomic landscape of balanced cytogenetic abnormalities associated with human congenital anomalies. Nat Genet. 2017;49(1):36–45.
Download references
Acknowledgements
Part of the reagents and software pipelines required for the study were kindly provided to us by Illumina. We are most grateful to our Radboudumc Genome Technology Center for sequencing the genomes and our bioinformatic team for data processing and storage.
The study was in part funded through grants from the Dutch Organisation for Health Research and Development (015.014.066 to LELMV). In addition, the aims of this study contribute to the Solve-RD project (to HGB and LELMV), which has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 779257.
Author information
Marcel Nelen, Helger G. Yntema, and Lisenka E. L. M. Vissers contributed equally to this work.
Authors and Affiliations
Department of Human Genetics, Radboudumc, Nijmegen, Netherlands
Gaby Schobers, Ronny Derks, Amber den Ouden, Hilde Swinkels, Jeroen van Reeuwijk, Ermanno Bosgoed, Dorien Lugtenberg, Jordi Corominas Galbany, Marjan Weiss, Richelle A. C. M. Olde Keizer, Tom Hofste, Nicole de Leeuw, Erik-Jan Kamsteeg, Marjolijn J. L. Ligtenberg, Christian Gilissen, Han G. Brunner, Marcel Nelen, Helger G. Yntema & Lisenka E. L. M. Vissers
Research Institute for Medical Innovation, Radboudumc, Nijmegen, Netherlands
Gaby Schobers, Jeroen van Reeuwijk, Jordi Corominas Galbany, Richelle A. C. M. Olde Keizer, Marjolijn J. L. Ligtenberg, Christian Gilissen, Han G. Brunner, Helger G. Yntema & Lisenka E. L. M. Vissers
Department of Clinical Genetics, Maastricht University Medical Center, Maastricht, Netherlands
Su Ming Sun, Marinus J. Blok, Debby Hellebrekers, Alexander Stegmann, Aimee D. C. Paulussen, Arthur van den Wijngaard & Han G. Brunner
Illumina Inc., Cambridge, UK
Xiangqun Zheng Bradley, John Peden, Alejandra Gutierrez, Adam Pullen & Tom Payne
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: HB, HY, LV; data curation: JCG, JP, AG, APu, TP; formal analysis: GS, LV; investigation: GS, RD, AO, HS, JR, ROK, AG, APu, TP; resources: MN, EB, DL, SS, MW, MB, TH, DH, NL, AS, EK, APa, ML, XZB, CG, AW; software: JR, JCG, XZB, JP, AG, APu; supervision: HB, HY, LV; visualization: GS, LV; writing — original draft: GS, LV; writing — review and editing: CG, HB, HY, LV, GS. All authors have read and approved the final version of the manuscript.
Corresponding author
Correspondence to Lisenka E. L. M. Vissers .
Ethics declarations
Ethics approval and consent to participate.
The study confirmed to the principles of the Helsinki declaration. In addition, the study was performed as part of a local validation study for the implementation of GS under ISO15189 accreditation and assessed as a diagnostic innovation by the Medical Ethics Review Committee Arnhem-Nijmegen under dossier number 2020–7142.
Consent for publication
Not applicable.
Competing interests
Illumina co-authors (X.B., A.P., T.P.) are employees and shareholders of Illumina. All other authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: table s1..
Online excel file providing overview of 1,000 individuals and workflows used. Table S2. Online excel file providing 1,271 genetic variants in 1,000 individuals. Table S4. Online excel file providing coverage statistics for 58,393 variants for which previously (likely) pathogenic variants were described. Table S5. Online excel file providing coverage statistics for 4,266 disease-associated genes.
Additional file 2: Figure S1.
Scenario model to determine individuals eligible for GS-first strategy. Figure S2. A cohort of 1000 cases with clinically relevant variants spanning the broad range of genome diagnostics. Figure S3. The average output of 1000 genomes. Figure S4. GS Technical validation by variant type and assessment of why variants were not identified. Figure S5. Examples of comprehensive GS. Figure S6. In silico coverage statistics at variant level and disease genes. Figure S7. Schematic representation of referrals to Radboudumc and MUMC+ in 2022. Figure S8. Schematic overview of assumptions made to evaluate the impact on diagnostic yield from transition to a generic GS approach. Table S3. GS sensitivity: Overview of TPRs per workflow.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Schobers, G., Derks, R., den Ouden, A. et al. Genome sequencing as a generic diagnostic strategy for rare disease. Genome Med 16 , 32 (2024). https://doi.org/10.1186/s13073-024-01301-y
Download citation
Received : 21 September 2023
Accepted : 02 February 2024
Published : 14 February 2024
DOI : https://doi.org/10.1186/s13073-024-01301-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Rare disease
- Impact modeling
- Reducing workflow complexity
- Genetic diagnostic laboratories
- Germline variant detection
Genome Medicine
ISSN: 1756-994X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
REVIEW article
Review: understanding rare genetic diseases in low resource regions like jammu and kashmir – india.

- 1 Human Genetics Research Group, School of Biotechnology, Shri Mata Vaishno Devi University, Katra, India
- 2 Bioinformatics Infrastructure Facility, School of Biotechnology, Shri Mata Vaishno Devi University, Katra, India
- 3 Shri Mata Vaishno Devi Narayana Superspeciality Hospital, Katra, India
- 4 Institute of Human Genetics, University of Jammu, Jammu, India
- 5 Independent Researcher, Health Clinic, Jammu, India
Rare diseases (RDs) are the clinical conditions affecting a few percentage of individuals in a general population compared to other diseases. Limited clinical information and a lack of reliable epidemiological data make their timely diagnosis and therapeutic management difficult. Emerging Next-Generation DNA Sequencing technologies have enhanced our horizons on patho-physiological understanding of many of the RDs and ushered us into an era of diagnostic and therapeutic research related to this ignored health challenge. Unfortunately, relevant research is meager in developing countries which lack a reliable estimate of the exact burden of most of the RDs. India is to be considered as the “Pandora’s Box of genetic disorders.” Owing to its huge population heterogeneity and high inbreeding or endogamy rates, a higher burden of rare recessive genetic diseases is expected and supported by the literature findings that endogamy is highly detrimental to health as it enhances the degree of homozygosity of recessive alleles in the general population. The population of a low resource region Jammu and Kashmir (J&K) – India, is highly inbred. Some of its population groups variably practice consanguinity. In context with the region’s typical geographical topography, highly inbred population structure and unique but heterogeneous gene pool, a huge burden of known and uncharacterized genetic disorders is expected. Unfortunately, many suspected cases of genetic disorders remain undiagnosed or misdiagnosed due to lack of appropriate clinical as well as diagnostic resources in the region, causing patients to face a huge psycho-socio-economic crisis and many a time suffer life-long with their ailment. In this review, the major challenges associated with RDs are highlighted in general and an account on the methods that can be adopted for conducting fruitful molecular genetic studies in genetically vulnerable and low resource regions is also provided, with an example of a region like J&K – India.
Introduction
Rare diseases (RDs) are progressive, chronically debilitating and/or life-threatening heterogeneous clinical conditions that affect a limited fraction of individuals from the general population in comparison to other prevalent diseases ( Schieppati et al., 2008 ; Richter et al., 2015 ; Danese and Lippi, 2018 ). However, these have been recently recognized as one of the major public health concerns prevalent across the globe. It has been estimated that RDs are altogether afflicting a significantly larger proportion of the global population (translating to billion individuals) ( Schieppati et al., 2008 ). There are several challenges that make diagnosis and therapeutic management of RDs cumbersome and impede RDs-related research. Despite several global initiatives to address the RDs-associated challenges, a lot of work needs to be carried out in order to deal with this ignored health sector. In a developing country like India, the RD-related research has been hampered by limited advanced clinical resources and far-to-approach, sporadically localized genetic services centers ( Pradhan et al., 2011 ; Aggarwal and Phadke, 2015 ; Kasthuri, 2018 ).
India’s primitive migration history and highly diverse population architecture which is epitomized by socio-cultural, geographical, linguistic, and religious isolation have been suggested as the main contributor to the country’s genetic diversity ( Basu et al., 2016 ). However, biological isolation of several endogamous population groups might have resulted in a relatively higher prevalence of genetic disorders in India ( Pradhan et al., 2011 ). One of India’s highly diverse and conglomeration of various inbred population groups, the population of Jammu and Kashmir (J&K) region, is expected to be an unexplored reservoir of genetic disorders. The J&K population is characterized by several endogamous groups with specific marital affinities, including the practice of consanguinity. In addition, the geographical isolation of various groups due to complex terrain may provide J&K a unique genetic architecture and disease profile. Unfortunately, the region has largely remained unexplored in context of genetic-based research. This has potentially contributed to the ignorance of a large number of hereditary diseases which are likely restricted to specific extended families or communities on a whole. Advanced high-throughput genomics-based approaches have, however, outpaced genetic research in J&K and precise diagnosis of some monogenic disorders, which are otherwise rare in prevalence across the globe but are likely to have attained higher incidence in the region due to higher inbreeding rates ( Rai et al., 2016 ; Kuchay et al., 2019 ). We propose that the population of J&K, thus, offers a special niche to understand yet-to-be-explored underpinning molecular etiology of genetic diseases. With respect to the population of J&K, genetic research holds a huge potential in designing diagnostic protocols for prevailing genetic diseases and development of therapeutics.
This review provides a brief account on RDs and their prevalence, followed by a discussion on the major RDs-associated challenges in general, an account on the methods that can be adopted for conducting fruitful molecular genetic studies of monogenic diseases, and the experiences of genetic research in Indian context with a special reference to a genetically vulnerable and low resource region like J&K.
Rare Diseases: Some Facts
Rare diseases are caused by function-altering variation(s) in a single gene, and hence are referred to as “single gene disorders” or “monogenic disorders” ( Boycott et al., 2017 ). There is no single universal definition for prevalence of known RDs. The base prevalence rate of RDs set by the World Health Organization (WHO) is approximately 1 in 2,000 people ( Lopes et al., 2018 ). However, different nations have their own definitions for the prevalence of RDs which is mostly based on the prevalence of a disease in their own population, status of health care system and availability of resources. A genetic disorder prevalent in the European Union (EU) is considered rare only if it affects 5 or less per 10,000 cases, whereas the incidence rate for RDs in the United States is 7 or less per 10,000 individuals ( Lang and Wood, 1999 ; Hughes et al., 2005 ). These numbers translate to nearly 30 million Europeans and 25 million North Americans (approximately 1 in every 10) affected by any of the known RDs ( Haffner et al., 2002 ; Wastfelt et al., 2006 ). The incidence rate is estimated to be ≤2.5 cases in 10,000 -and 1 in 10,000 individuals for Japan and Australia, respectively ( Lang and Wood, 1999 ; Hughes et al., 2005 ; Zurynski et al., 2008 ).
Nearly 7,000 distinct RDs have been delineated and new ones are being consistently reported in the literature ( Ng et al., 2010b ). It is believed that a majority of RDs (80% or more) are genetic in origin, whereas distinct underpinning causes for the remaining disorders are not well understood ( Mckusick, 2007 ; Song et al., 2012a ). The remaining RDs may be caused by environmental (for instance, Jamaican vomiting sickness, mesothelioma), infectious (for instance, maternofetal measles) or immunological (for instance, juvenile chronic arthritis) factors ( Guillem et al., 2008 ; Lopes et al., 2018 ). There is a wide variability in severity and expression of distinct RDs. Many of these are congenital on onset and continue to exist with poor prognosis (lifelong-disability and/or early death) over the lifetime of the afflicted individual, while in some individuals the symptoms of the disease (whether same or other) may appear later in life, thus, presenting difficulties in their diagnosis. It has been estimated that nearly 50% of reported RDs occur in children, 30% of RDs patients die during infancy (before the age of 5), and 12% of them die between 5 and 15 years of age ( Song et al., 2012b ).
Although RDs are distinctly defined as rare on the basis of their low prevalence, yet their cumulative burden on the public health is huge. The known RDs altogether affect a substantial number of estimated 350 million individuals across the globe which translates to approximately 10% of the global population ( Song et al., 2012b ; Boycott et al., 2013 ). About 80% of them are cumulatively affected by merely lesser than 100 known RDs ( Luzzatto et al., 2015 ). Nonetheless, the available figures for their global prevalence are alarming indicating that these altogether affect more individuals than those suffering with common diseases; for instance diabetes has an estimated incidence of 20.8 million and 1.4 million among Americans and Australians, respectively ( Dunstan et al., 2002 ; Zurynski et al., 2008 ).
Challenges Associated With RDs and Related Progress
Different types of RDs altogether constitute a major exigent global public-health issue and present several formidable challenges which are largely related to diagnosis, access to health care services and disease-specific interventions, lack of specialized clinical personnel and specific infrastructure, challenges faced by the RD patients and their families, and lack of ample resources for RD-associated research and development (R&D). Further these challenges inevitably result in unreliability of the available patient-registries and, therefore, vague epidemiological data. To address these challenging issues, a drive toward universal health coverage is required so that the needs of RD patients get fulfilled and public and government (national or international) agencies invest their funds into fundamental biomedical research for understanding the etiology of diseases and discovering their novel therapeutic targets and strategies. However, a significant scientific and technological progress has been witnessed over the last few decades that have notably filled our knowledge gaps on the understanding of several RDs and made their diagnosis and management a bit simple task. A brief account on major RDs-associated challenges and their related progress has been provided in this section.
Clinical Challenges, Lack of Reliable Patient-Registries and Inaccurate Epidemiological Data
Clinical challenges include limited or no availability of appropriate clinical resources, lack of specific literature and evidence-based knowledge or difficulty in assessing knowledge sources, and has rendered RDs diagnosis and management troublesome ( Schieppati et al., 2008 ). Inadequate clinical resources include lack of clinicians/experts having a sound knowledge and experience in Clinical Genetics, lack of standardized clinical guidelines and specific clinical infrastructure which, thereby, usually require the needy patients to undergo unavoidable clinical investigations that end up getting them multiple indefinite diagnoses ( Knight and Senior, 2006 ; Zurynski et al., 2008 ). It is obvious that a clinician’s disease management expertise is proportional to the frequency of clinician-patient encounters. Since RDs have a relatively less frequency, such clinical encounters are assumed to be negligible which subsequently contributes to a huge clinical knowledge gap. This knowledge gap is also contributed by insufficient availability of sources of clinical information on the underpinning cause, patho-physiology and natural course of most of the RDs, and limited availability and endorsement of relevant guidelines of the allied clinical societies. Clinical heterogeneity of distinct RDs results in many subtypes leading to different clinical manifestations and course which along with lack of relevant clinical information usually make the diagnosis of several RDs troublesome. No diagnosis or misdiagnosis to the patients ultimately result in no or lack of reliable patient-registries which subsequently ends up in the lack of an accurate epidemiological data on RDs ( Zurynski et al., 2008 ). Unfortunately, of the estimated ≥7,000 disorders defined as “single gene disorders,” a detailed phenotypic information on about 5,551 of these have been currently reported in the Online Mendelian Inheritance in Man ® database (OMIM ® ), 1 while phenotypic information on the remaining disorders is scanty till now. According to the data available from the Orphanet database, 2 epidemiology of only 29% of ≥7,000 RDs has been reported.
Problems Faced by the Patients and Their Families
RDs patients and their families face huge psycho-socio-economic burden due to social isolation, difficulty in accessing appropriate health care services, delay in diagnosis, and uncertainty about their future and financial hardships. Most of the RDs are often severely disabling, impair the overall abilities of the patients, and substantially reduce the quality of their life and life expectancy. About half of the RDs appear in early childhood which makes it hard or impossible for the young patients to attain their education in the schools or colleges ( Zurynski et al., 2008 ). The patients and their families also have to experience social stigma in the form of social isolation and overall discrimination. Due to fear of social stigma compounded by lack of awareness on their health condition (whether being an inherited or genetic disorder), many a times patients intentionally do not get a clinical consultation. This, in turn, directly impacts the reliability of the patient-registries since these patients do not get registered in the hospitals. Patients generally struggle to find specialized clinicians having sound knowledge and experience in Clinical Genetics. Clinicians having deep knowledge and expertise in management of RDs are usually concentrated in geographically dispersed specialized centers which may remain beyond the patient’s access or require most of the patients to travel long distances or to shift their residence to a new place for getting a diagnosis ( Zurynski et al., 2008 ). These factors altogether result in diagnostic delays, misdiagnosis or no diagnosis and ultimately no effective treatments to the patients. All this complicates the medical condition of the patients as they are only left with an option of suffering with the primary or secondary consequences of their disease and its late sequelae ( Yang et al., 2013 ). Besides, a long-term search for an accurate diagnosis of RDs, referred to as the “diagnostic odyssey,” usually incur a huge medical expenditure with unsuccessful attempts and consumption of limited resources which has its own financial implications on the patient’s family as raising a disabled child is relatively expensive than for a normal child ( Zurynski et al., 2008 ; Yang et al., 2013 ).
Diagnostic Challenges of Rare Diseases
Establishing the precise diagnoses for RDs is usually difficult. Their diagnosis is highly dependent on the access to diagnostic testing and requires determination of the underpinning genetic cause ( Boycott and Ardigo, 2018 ). Factors including clinical heterogeneity, co-morbidity and varying disease course among different RDs patients highly demand a differential diagnosis of the disease with which they suffer life-long ( Romdhane et al., 2016 ; Benjamin et al., 2017 ). However, establishing differential diagnosis is a meticulous and time-consuming task incurring a diagnostic odyssey that usually relies on the skills of concerned clinician and the diagnostic tests that a patient has to undergo, as discussed earlier.
Since 2010, Next-Generation Sequencing (NGS) has accelerated the rate of RDs diagnosis. Although NGS has significantly accelerated the rate of precision diagnoses in RDs patients, but with a diagnostic yield of only 25–50% ( Li et al., 2018 ). For the remaining significant fraction of patients comprising of the ones presenting complex phenotypes, it fails to yield any confirmed diagnosis due to several technical limitations ( Wenger et al., 2017 ). However, many approaches pertaining to genetic diagnosis have recently emerged. An amalgamation of comparative reanalysis of clinical as well as non-clinical NGS data using various newly emerged data analysis pipelines and software in consideration with updated scientific literature can be employed for improving the diagnostic yield through NGS. For instance, a recent study on reanalysis of 40 unsolved exome reports later led to a precise diagnosis in about 10% of cases ( Wenger et al., 2017 ).
Challenges Faced in RD-Related R&D and Therapeutics
The RDs-related R&D is highly complicated and impeded by several challenging issues including a huge knowledge gap about the underpinning causes of distinct RDs, lack of an international standard code for their classification, assembling cohorts of patients for conducting a research study owing to their distinct rare prevalence, and insufficient funding opportunities on RDs-research. These challenges, further, compound the determination of suitable therapeutic interventions and development of particular drug molecules for targeting a specific clinical condition. No single institution and/or country have a sufficient figure on the number of affected individuals for carrying out a generalized clinical and translational research. This could be mainly attributed to the International Classification of Diseases (ICD) system used in many countries for disease classification. The ICD is not suitable for most of the RDs which further hampers inclusion of national and international patients’ registries into reliable epidemiological databases and lead to non-reliable assessment of their economic and social burden ( Schieppati et al., 2008 ). The other major reason is that some RDs occur so infrequently (<1 in 1,000,000 population) that only by conducting international population-based study can sufficient numbers of geographically dispersed patients be accrued for a clinical investigation so that a higher power study could be yielded. Recruitment of such a number of patients into a research study is further impeded by the lack of reliable patient registries which subsequently lead to non-reliable assessment of disease burden, imprecise cost estimations of resource consumption involved in the whole process of research, drug development and clinical trials for developing a suitable disease management or therapeutic strategy, and missing out a potential funding opportunity ( Schieppati et al., 2008 ). Funding and policy-making has also been a major obstacle in establishing infrastructure for maintaining registries of the patients ( Forrest et al., 2011 ). Although for some of the disorders, national and/or international patient registries have been regularly maintained by different associations, yet there is no recognition for most of these at the Government level due to lack of or limited documentation of RDs patients in the local hospitals ( Schieppati et al., 2008 ).
Taking into consideration of a dire need for the formulation of a universal RDs classification system that would provide comprehensive information on known RDs, the European Rare Disease Task Force of the Health and Consumers Protection Directorate General of the European Commission in collaboration with WHO has set up the ICD-10. Besides, several other classification systems like the Orphanet, the OMIM ® , the Systemized Nomenclature of Medicine – Clinical Terms (SNOMED-CT) are available for coding of these diseases, with each of these have their own advantages and disadvantages ( Ayme et al., 1998 ; Mckusick, 2007 ; De Silva et al., 2011 ). Another international group called the “Rare Disease Terminology and Definitions Used in Outcomes Research Working Group” under aegis of the “International Society for Pharmacogenomics and Outcomes Research (ISPOR) Rare Disease Special Interest Group” has been established for the development of a universal RDs definition ( Richter et al., 2015 ). With regular up-gradation of clinical infrastructure and updating of clinical databases via collaborative efforts, knowledge of these diseases is also improving. Such an increase in focus over RDs has been mainly facilitated by the relentless work of a significant number of legislations, NGO committees and patients’ organizations which have highlighted the plight of RDs patients and stressed over their timely therapeutic management. This has further paved the impetus to RDs research by incentivizing pharmaceutical and biotechnology companies, and in turn, has proven instrumental in figuring out diagnostic, effective therapeutics and preventative modalities for a variety of RDs ( Richter et al., 2015 ). Unless before the US Orphan Drug Act (1983) and the European Union (EU) Regulation 142/2000 (2000) on medicinal products came into effect, the pharmaceutical industry was ignorant to the development of “orphan drugs” or drugs for the treatment of distinct RDs ( Haffner et al., 2002 ; Richter et al., 2015 ; Auvin et al., 2018 ). With their enactment, incentives were provided to pharmaceutical companies for the development of RDs diagnostics and therapeutic strategies in the United States and the EU, and since then, their success could be defined by the United States Food and Drug Administration (FDA) and the European Medicine Agency (EMA) approval for marketing of several hundreds of therapeutic drugs and biological products for the treatment of merely 5% RDs mainly including rare forms of cancers ( Haffner et al., 2002 ; Haffner, 2006 ; Braun et al., 2010 ; Richter et al., 2015 ; Auvin et al., 2018 ). Although these approved drugs have significantly transformed the treatment of only 5% of these diseases for which fewer or no treatment options were available earlier, yet there is a lack of availability of treatment options for a significantly higher percentage (95%) of RDs. Since, the available drugs are extremely expensive, they pose a huge financial stress on the affected families, health-care systems and donor agencies.
However, for creating an advanced integrated research pipeline for the development of novel therapeutics for RDs, several governmental and non-governmental organizations and their programs including the United States National Institutes of Health’s (NIH) “Therapeutics for Rare and Neglected Diseases (TRND)” program, the “Genetic and Rare Diseases (GARD) Information Center” (a collaborative effort of two NIH centers namely the National Human Genome Research Institute (NHGRI) and the National Center for Advancing Translational Sciences (NCATS)), the NIH’s “Office of Rare Diseases Research” (ORDR), the “National Organization for Rare Diseases” (NARD), the “European Organization for Rare Diseases” (EURORDIS), the “International Rare Diseases Research Consortium” (IRDiRC), the “Genetic Alliance,” the “Vereniging Spierziekten Nederland” (VSN), etc., have been initiated during the past few years for advocating RDs patients’ need for national/international RDs policies. These initiatives are providing a platform for patients’ advocacy, research funding and development of new advanced amenities for addressing the challenges pertaining to ignored RDs with a common aim of dissemination of the related data and information to the scientific community and demonstration of their overall usefulness in RDs diagnostics and therapeutics. Despite, it is assumed that the current rate of R&D would not be able to generate therapeutics for most of the RDs for the next several years. This universal RD-challenge would only be addressed by an unprecedented, large scale international cooperation between different geographically scattered government and non-government agencies and R&D units of different pharmaceutical companies.
Understanding Rare Genetic Disorders: Methodologies for Studying Molecular Etiology
In general, the first-line diagnostics includes detection of pathognomonic phenotypic changes, disease-phenotype correlation and biochemical analysis of the known disease biomarkers through newborn disease screening methods, hematological evaluation, metabolic testing, and radiographic examinations. Once a preliminary diagnosis is established, its authenticity primarily relies on the determination of molecular etiology of query disease through genetic screening of the patients. Different genetic screening methods include traditional as well as advanced cytogenetic techniques, single-gene sequencing, and sequencing of a panel of genes associated with specific disease types. Though each of these have their own limitations, yet are widely practiced for the diagnosis of genetic diseases in many countries and are helping risked families in early detection of a possible disease, its early intervention and preventative/palliative care.
For many previous years, underpinning genetic reasons for several human diseases have been primarily deciphered through linkage and association-based genetic epidemiological studies. However, with further advancements in several biological techniques, genetic epidemiological studies have seen a dramatic shift from conventional study approaches (such as twin studies, family-based linkage studies) to population-based genome-wide association studies (GWAS) to the studies based on the most-advanced Next-Generation DNA Sequencing (NGS) technologies. On one hand where population-based study designs like GWAS and Twin-based Epidemiological Studies remain uninformative in understanding RDs, specific methodologies to facilitate determination of their distinct molecular etiology also exist but are cumbersome. With advent of new, high-throughput technologies, these efforts have improved significantly. Brief accounts of these study designs are provided below:
Genetic Linkage Studies
Genetic linkage studies are generally used for mapping/determining the most probable chromosomal/genomic loci co-segregating with a disease phenotype through studies either based on the information of disease’s mode of inheritance (dominant or recessive) in the family pedigrees (parametric or model-based linkage analysis) or on some genome-wide polymorphic genetic markers such as microsatellites in the selected families (non-parametric or model-free linkage analysis) ( Dawn Teare and Barrett, 2005 ; Teare and Santibanez Koref, 2014 ). The main principle of these family-based studies is that physically close genetic loci on a chromosome remain highly linked during meiosis (that is, the probability of recombination between them is <50%) and are inherited independently from parents to offspring ( Pulst, 1999 ; Dawn Teare and Barrett, 2005 ). If a set of variations are linked together in a same haplotype in a particular population, they are considered to be in “linkage disequilibrium” (LD) and the genetic loci in LD are considered to be linked ( Dawn Teare and Barrett, 2005 ). Studies based on linkage mapping in affected and unaffected siblings or affected individuals and their parents (child-parent trio) in multi-case extended families (loaded families) or pedigrees provide information on the co-segregation of genetic variations with the disease phenotype, and minimize genotyping error and maximize power for assertion ( Whittemore and Nelson, 1999 ; Bailey-Wilson and Wilson, 2011 ; Barrett, 2014 ). In the first course of a genetic characterization of a clinical phenotype or trait for which exact causal genetic component is unknown, linkage studies are extremely useful in the identification of a genetic locus that might be harboring a disease-causing gene being shared between affected individuals and needs just a very few markers to carry out the same. However, this does not mean that linkage studies could map the causative gene alone. For this purpose, these mostly rely on “positional cloning” workflows ( Teare and Santibanez Koref, 2014 ). The other limitation of linkage studies is that the power of a linkage study may get highly reduced when both incomplete penetrance and locus heterogeneity exists in the study subjects ( Teare and Santibanez Koref, 2014 ).
Linkage studies are the most powerful tool in the identification of highly penetrant, rare variations underpinning rare monogenic Mendelian disorders and birth defects ( Bailey-Wilson and Wilson, 2011 ). Previously, several linkage workflows have helped in delineating/mapping the underpinning genes for a number of RDs in large multi-case families ( Barrett, 2014 ). It is worth mentioning that molecular etiology of nearly 3,500 known RDs have been delineated primarily through conventional positional cloning methods based on linkage analysis and “homozygosity mapping” in which inheritance pattern of specific DNA markers such as single nucleotide variations (SNVs) and microsatellite repeats was used for ascertaining recombination events in extended multi-case pedigrees. Nevertheless, the remaining disorders have been refractory to these classical genetic screening methods for several reasons: locus heterogeneity, phenotypic heterogeneity, reduced penetrance, availability of only a small number of patients or families which may not be sufficient enough to attain a high-power study, and substantially reduced reproductive fitness in the patients due to early disease onset and severe effect ( Lander and Botstein, 1987 ; Vink and Boomsma, 2002 ; Botstein and Risch, 2003 ; Ng et al., 2010c ; Boycott et al., 2013 ). These classical approaches remain uninformative in case of spontaneous and non-inherited disorders and are expensive, labor intensive and time consuming.
Next-Generation DNA Sequencing
Several recently emerged state-of-the-art molecular biological techniques such as chip-based DNA arrays and several “massively parallel” or high throughput NGS technologies has helped overcome the shortcomings of previously described studies. The initial information on the human genome came into limelight with completion of the Human Genome Project that mostly relied on the “hierarchical shotgun strategy” carried out through the classic Sanger biochemistry ( Lander et al., 2001 ; Shendure et al., 2017 ). After 2–3 decades of gradual improvements in sequencing biochemistry and technology, NGS technologies have vastly outpaced our ability in a way that presently several samples can be sequenced simultaneously on a single platform (multiplexing) with a higher accuracy and lesser cost ( Shendure et al., 2017 ). This has subsequently lead to an era of unprecedented productivity – that is, progressive accumulation and availability of genomic data of individuals (personal genomes or exomes) of genetically varied populations that has facilitated comprehensive understanding of several human diseases and their susceptibility among different population groups ( Wang et al., 2013 ; Middha et al., 2015 ). Over the past decade, NGS has emerged as a leading player in the odyssey of finding the underpinning causes of several RDs. It has created a paradigm shift in clinical genetics through a relatively easier discovery of the underpinning genetic causes of a number of RDs at a much lesser cost, thereby, obtaining a precise delineation for many uncharacterized RDs cases ( Sawyer et al., 2016 ; Nambot et al., 2018 ). Discovery of new disease-associated genes and novel genetic variations through different NGS platforms has efficiently reinvigorated our understanding on the etiology of several genetic disorders, especially those of rare Mendelian disorders, which has led to the development of disease-specific diagnostic procedures and therapeutics ( Shendure et al., 2017 ).
Since the previous decade, NGS helped overcome challenges associated with traditional gene discovery approaches, made it easier to identify the underpinning genetic etiology of several RDs and provided insights into their distinct underpinning biological mechanisms ( Shendure et al., 2017 ). Due to the wider span, intrinsic complexity and greater cost of whole genome sequencing (WGS), whole exome sequencing (WES) has relatively gained more popularity in the identification of RDs genetics since 2010. It is based on the sequence analysis of exome (the total protein-coding content of the genome) which represents about 1% of the human genome and is known to harbor nearly 85% of the genetic variations that have large effects on the human physiology and cause disease-related phenotype ( Choi et al., 2009 ). With continuous refinements in the sequencing technology and having aided in discovery of more than 550 distinct novel disease-associated genes, WES has emerged as an amazing technological advancement that has enhanced our understanding of the structure and function of the human genome and the molecular pathways underpinning human development and disease biology ( Boycott et al., 2013 ).
The initial proof-of concept for the potential role of WES in RDs diagnostics and research came into limelight with the identification of genes responsible for Kabuki Syndrome (OMIM: 147920) and Miller syndrome (OMIM: 263750). Using WES and targeted sequencing, the researchers at University of Washington had identified and reported distinct MLL2 gene (currently known as KMT2D gene) and DHODH gene variants responsible for Kabuki Syndrome and Miller syndrome, respectively, in majority of the afflicted individuals ( Ng et al., 2010a , b ). Later, protein truncating PRRT2 variations were identified in a familial case of Paroxysmal Kinsesigenic Dyskinesia through WES ( Chen et al., 2011 ). Variations in SMARCB1 , SMARCA4 , SMARCA2 , SMARCE1 , ARID1A , and ARID1B genes encoding proteins belonging to SWI/SWF chromatin-remodeling complex were identified using WES and implicated in a rare congenital anomaly called Coffin-Siris syndrome (OMIM: 135900) ( Tsurusaki et al., 2012 ). A case of ectrodactyly and lethal pulmonary acinar dysplasia associated with FGFR2 variations in a 5 day old baby born to normal consanguineous was identified using WES ( Barnett et al., 2016 ). Evidently, there has been a proportional flurry of reports at an accelerating rate on discovery of RDs-associated genes and novel variations which, in turn, has accelerated precise diagnosis of a number of suspected characterized as well as uncharacterized RDs since the advent of WES in 2010 ( Yang et al., 2014 ; Shendure et al., 2017 ). WES has been successful in leading to precise diagnoses in an estimated 30–50% of RDs cases in clinical settings ( Fresard and Montgomery, 2018 ). Despite, the molecular etiology of nearly one-third of RDs is still unknown and remains to be discovered ( Boycott and Ardigo, 2018 ). WES is still not the gold-standard diagnostic approach for clinically uncharacterized diseases owing to its technical limitations that restrict the determination of variations in non-coding and/or regulatory genomic regions, structural variations and complex genetic mechanisms such as somatic mosaicism and gene imprinting underlying a substantial fraction of RDs ( Fresard and Montgomery, 2018 ). Here, coming to rescue from the limitations of WES is another NGS technology, that is, the WGS. Besides the identification of SNVs, WGS is also capable of determining structural as well as epigenetic changes in the genome ( Wang et al., 2015 ).
Success of NGS mostly relies on the accuracy of data mining tools used for the analysis of sequencing data. There is ample availability of raw NGS data processing and variant calling tools. Initially, processing of raw NGS data includes several steps such as quality check, adapter trimming, post-trimming quality check, PCR duplicate removal, alignment of sequencing reads to the reference genome and variant calling. FastQC is a tool used for checking quality reports of pre- and post-trimming sequencing data ( Andrews, 2010 ). Trimmomatic is used for the initial quality trimming of the reads ( Bolger et al., 2014 ). MarkDuplicates utility of Picard tool is used for PCR duplicates removal ( Wysoker et al., 2013 ). Bowtie ( Langmead et al., 2009 ) and Burrows-Wheeler Alignment (BWA) tool ( Li and Durbin, 2009 ) are used for alignment of the reads to the reference genomes. Tools that are used for variant calling from NGS data include DISCOVAR ( Weisenfeld et al., 2014 ), genome analysis toolkit (GATK) ( Mckenna et al., 2010 ) for SNVs, DELLY ( Rausch et al., 2012 ), GASV ( Sindi et al., 2009 ), LUMPY ( Layer et al., 2014 ) for Structural Variations, CoNIFER ( Krumm et al., 2013 ), CONTRA ( Li et al., 2012 ), XHMM ( Fromer et al., 2012 ) for Copy Number Variations, Platypus ( Rimmer et al., 2014 ) for SNVs, indels, repeat elements, de novo variations, and SAMtools ( Li et al., 2009 ) for SNVs and short indels. These tools are capable of mining different types of variations from NGS data with a higher accuracy.
Burden of Genetic Disorders in India
Population stratification in India has added to the country’s population diversity and gene pool. A high inbreeding rate in some specific Indian population clusters hint toward a relatively higher burden of specific genetic diseases and founder variations. India is, thus, considered as a unique hotspot of inherited genetic disorders and variations. With India’s recent accelerating clinical demographic switch to non-communicable diseases, congenital malformations/birth defects and genetic disorders have emerged as the major causes of mortality in the perinatal period ( Verma and Bijarnia, 2002 ). A higher burden of inherited genetic disorders and variations highlights the importance of dissecting the genetic etiology and pathogenesis of several recessive disorders and complex diseases in India.
Brief accounts on the population stratification, inbreeding, genetic disorders, and genetic services in India are as follow:
Population Architecture of India
India, the world’s second most populous country, holds the distinction of being the sixth largest home to more than one-sixth of the global human population ( Aggarwal and Phadke, 2015 ). During prehistoric and historic times, the country has served as a major corridor for different migratory waves of anatomically modern humans ( Majumder and Basu, 2014 ). These migratory events have significantly contributed to high heterogenic population stratification in the inhabiting Indian population groups in terms of their religious, socio-cultural, linguistic and racial backgrounds. In an evolutionary context, the population diversity in India has been considered as a result of admixture of multiple migratory populations and invaders belonging to the northwestern and eastern corners of the globe that had entered into the country by following land and coastal routes ( Basu et al., 2016 ). Genetic studies have indicated that the modern Indian population is as an admixture of five large ancestral, genetically divergent, heterogeneous population groups. The ancestral groups comprising the Indian mainlanders include the “Ancestral North Indian (ANI),” “Ancestral South Indian (ASI),” “Ancestral Austro-Asiatic (AAA)” and “Ancestral Tibeto-Burman (ATB),” and a separate ancestral group named the “Ancient Ancestral South Indian (AASI) – related” for the people of the Andaman archipelago ( Basu et al., 2016 ; Narasimhan et al., 2018 ). However, the current Indian population can also be categorized into four ethno-racial groups namely the “Australoids,” “Caucasoids,” “Mongoloids,” and “Negritos,” stratified into more than 4,000 anthropologically distinct population groups having their individual linguistic profiles ( Aggarwal and Phadke, 2015 ). Based on religious-socio-cultural backgrounds, the Indian population is further sub-classified into different religious groups, castes and tribes. A vast majority (∼80%) of the Indian population comprises of the Hindu population groups which is further sub-divided into castes and sub-castes, about 8% is represented by the tribal populations while the rest of the population is comprised of other religious groups such as Muslims, Christians, Buddhists, Jews, Sikhs, and others ( Indian Genome Variation Consortium, 2005 ).
Inbreeding in India
The contemporary Indian population groups is an agglomeration of several thousands of separate endogamous groups (>50,000) residing in topographically alienated pockets, many of which have been in existence for at least 100 generations ( Mcelreavey et al., 2005 ). These groups represent distinct conservative breeding pools in which marriages are usually restricted within same religion, caste and biraderi according to the customs dating back to some 3,000 years ( Bittles et al., 2002 ). In India, the establishment of marital relationships among individual population groups is guided by different regulations which are usually based on their distinct religious-socio-cultural norms. For instance, the Hindu religious group is structured into several hierarchical socio-cultural groups called varnas (Brahmins, Kshatriyas, Vaishyas, Shudras) which are sub-divided into castes (or jatis) ( Indian Genome Variation Consortium, 2005 ). The population groups based on varnas and castes are usually endogamous. Each caste group is sub-divided into patrilineal groups or sub-castes known as gotras, each representing an exogamous group. The tribal sections or the ancestor-worshippers are mainly endogamous ( Indian Genome Variation Consortium, 2005 ).
Nevertheless, consanguinity is also practiced as a custom in some specific Indian population groups, with rate of consanguinity ranging between 20 and 30% ( Bittles, 2002a ). Among the Indian Hindus, non-uniform views pertaining to consanguinity subsist with more complex marriage regulations ( Bittles and Black, 2010a ). According to a general prohibition dating back to 200 BC, the majority Hindu population in the northern, eastern, and north-eastern states rigorously forbid consanguinity by avoiding same “gotra” marriage including those between kins and between a man and his father’s sister’s or mother’s sister’s or mother’s brother’s daughter; though a long tradition of first-cousin marital union, uncle-niece marriage and marriage between a man and his maternal uncle’s daughter is prevalent among Dravidian Hindus belonging to southern India and in most Christian denominations, mostly reported from rural communities and among the underprivileged (including the poorest, illiterate, and least educated) groups ( Hussain and Bittles, 2000 ; Bittles, 2002b ). First-cousin marriage, particularly between a man and his maternal uncle’s daughter, is generally preferred in Andhra Pradesh, Karnataka, and Tamil Nadu and in Kerala, Goa, and southern Maharashtra to a lesser extent ( Bittles, 2002a ). The Muslim religious group practice consanguinity at a higher rate with no comparable north-south distinction in consanguinity, as indicated in Table 1 ( Bittles, 2002b ). Although consanguineous marriages are forbidden in the Sikh religion, some minority Sikh groups in India appear to exercise flexibility in the observance of this proscription by allowing first- or second-cousin marriages ( Table 1 ) ( Bittles, 2002b ).
Table 1. Rate of consanguineous marriages among various religious groups in India during 1992–1993 ( Bittles, 2002b ).
India as a Trove of Genetic Disorders
Of the 350 million global estimate of RDs patients, India alone is a home to approximately 70 million (equating to 1 in 20) patients with any of the known progressive, life-threatening and chronically debilitating rare health anomalies which encompass a wide range of systemic disorders including immunodeficiency syndromes, blood disorders, skeletal disorders, neurological disorders, and many more ( Kumar H. et al., 2017 ). This indicates that the cumulative burden of RDs is quite significant in India in comparison to the world average owing to its highly inbred population structure ( Kumar H. et al., 2017 ). Unfortunately, there is no standard definition to describe the prevalence of a RD in India; though Organization for Rare Diseases India (ORDI) has suggested a threshold for defining a disease as rare if it afflicts 1 in 5,000 individuals in India ( Rajasimha et al., 2014 ).
Religious restrictions compounded by the geographical isolation of some Indian habitats due to the country’s diverse topography have contributed significantly to a relatively higher rate of inbreeding (population-inbreeding coefficient of India = 0.00–0.20) and, thus, served as barriers to random mating and free gene flow leading to the distinct gene pool of the Indian sub-populations ( Bittles et al., 2002 ). In parallel, there is also a relatively higher burden of specific diseases usually restricted and/or unique to specific Indian ethnic groups, sub-castes, tribes or clans under the influence of founder events that have occurred 50–100 generations back ( Bittles, 2002a ; Mcelreavey et al., 2005 ; Pradhan et al., 2011 ; Dixit et al., 2015 ). A list of some of the disease-associated founder variations determined from the Indian population has been indicated in Table 2 . One of the biggest examples supporting the association of endogamy and community-specific disease burden in India is a highly endogamous Agarwal community. Genetic diseases such as Megalencephalic Leukodystrophy with sub-cortical cysts (OMIM#604004), Panthothenate Kinase-Associated Neurodegeneration with Brain Iron Accumulation (PKAN; OMIM#234200) and Spinocerebellar ataxia type 12 (OMIM#604326) associated with a number of founder variations in CAPN3 (OMIM#114240), MLC1 (OMIM#605908), PANK2 (OMIM#606157), PPP2R2B (OMIM# 604325) genes, respectively, are frequently observed in this Indian community ( Table 3 ). Genetic disorders reported from India are enlisted in Table 4 . Besides a higher rate of inbreeding and founder effects in some Indian sub-population groups, other factors including lack of diagnostic, disease management and rehabilitation infrastructure in the country, its large population size and high birth rate have contributed significantly to a relatively higher incidence of recessive disorders in India ( Verma, 2000 ; Verma and Bijarnia, 2002 ).
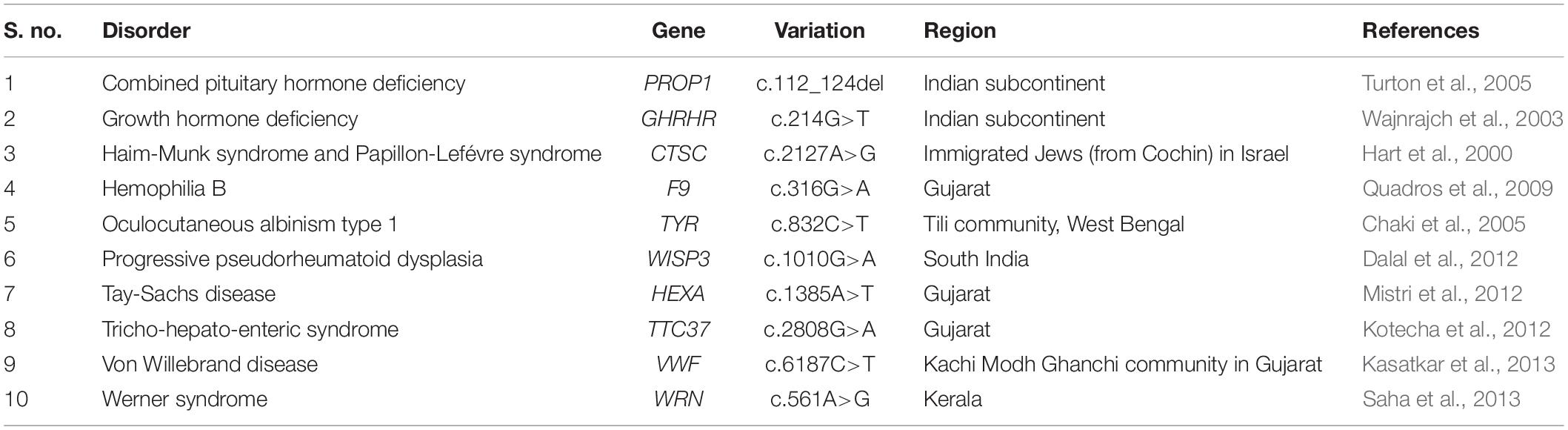
Table 2. A list of some of the disease-associated founder variations prevalent in India.
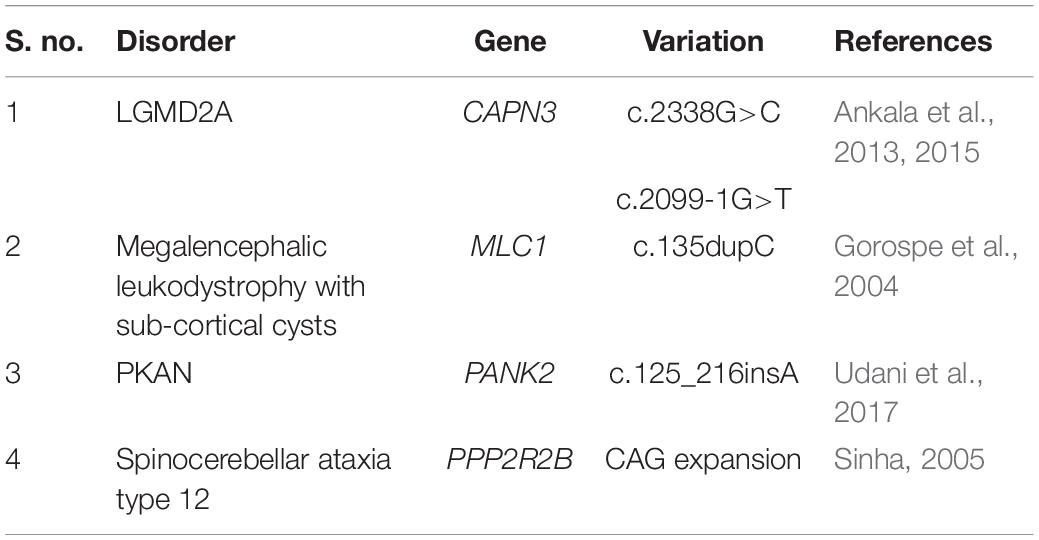
Table 3. A table representing some of the founder variations prevalent in a highly endogamous Indian Agrawal community.

Table 4. The estimated rate of incidence of common genetic disorders in India ( Ankala et al., 2015 ).
Status of RDs-Genetic Services in India
Like other developing countries, India too faces a number of challenges in dealing with RDs as a major public health issue. There is a lack of true, reliable quantitative data on individual as well as cumulative prevalence of RDs at the national and regional level and the epidemiology of associated morbidity and mortality. These factors have further impeded the RDs-related reliable cost estimations and the implementation of relevant research and development programs for RDs therapeutic management in the country. Other challenges include lack of availability of specialized medical personnel, molecular diagnostic infrastructure, and standard therapeutic drugs and protocols committed to understanding and management of RDs. The genetic background of the Indian population is also not well understood due to its under-representation in the major global genomic studies.
Several individual research groups, pharmaceutical companies and patient assistance organizations and their charitable programs are consistently working on advocacy of the importance of RDs diagnosis, research and drug development and framing of a national RDs policy in India. These include ORDI, Indian Organization for Rare Diseases (I-ORD), Foundation for Research on Rare Diseases and Disorders- Rare Diseases India (FRRDD-RDI), Open Platform for Rare Diseases (OPFORD), Genomics for Understanding Rare Diseases India Alliance Network (GUaRDIAN) and many more ( Rajasimha et al., 2014 ). Several case reports for distinct RDs from India have been published during the recent years. These include a series of reports on accurate diagnosis of RDs cases including Acid Sphingomyelinase (ASM)-Deficient Niemann-Pick Disease, Allgrove or Triple A (AAA) syndrome, Ethylmalonic Encephalopathy, Fanconi-Bickel Syndrome, Fructose-1,6-biphophatase Deficiency, Homozygous familial hypercholesterolemia, Mucopolysaccharidoses type I and type II, Rhizomelic chondroplasia punctata type 1, progressive pseudorheumatoid dysplasia and many more using Sanger Sequencing of disease-associated genes ( Phadke et al., 2010 ; Dalal et al., 2012 ; Kehar et al., 2014 ; Bijarnia-Mahay et al., 2016 ; Ranganath et al., 2016 ; Setia et al., 2016 ; Uttarilli et al., 2016 ; Angural et al., 2018 ; Bhai et al., 2018 ). Besides, there are several case reports indicating the major application of NGS technology in RDs diagnostics including that of ichthyosis, rare syndromes of mineralocorticoid excess, dystrophic epidermolysis bullosa, cone dystrophy, sporadic acrokeratosis verruciformis, Dowling-Degos disease, Spastic Paraplegia 79 and many more, ( Gupta et al., 2015 , 2016 , 2017 ; Karuthedath Vellarikkal et al., 2016 ; Narayanan et al., 2016 ; Das Bhowmik et al., 2018 ; Virmani et al., 2018 ). Many of these diagnosed RDs are so rare that they have been reported either distinctly or as spectrum disorders for the first time from India. In this context, there is a dire need of conducting genetic screening of the uncharacterized RDs patients and their families and population genetic studies for elucidating the load of pathogenic variations harbored by the Indian population and establishing its own population-based genetic variation database.
Jammu and Kashmir: a Model Population for Studying Rare Genetic Disorders
Despite continuous global efforts, a very little attention has been paid to the most challenging health issue of RDs in India with special reference to region like J&K. J&K is located in the northern part of the Indian sub-continent in the vicinity of the Karakoram and westernmost Himalayan mountain ranges (outer hills, middle Himalayas, and inner Himalayas) ( Bhasin and Nag, 2002b ). The region is being bordered by countries such as Pakistan in the west, Afghanistan in the north-west and China in the north-east ( Bhasin and Nag, 2002a ). Topographically, J&K is divided into three main isolated divisions, namely Jammu, Kashmir, and Ladakh ( Sharma et al., 2018 ). The region is characterized by tough mountainous terrains that have rendered geographical isolation of its heterogeneous population. J&K has been consistently under geo-political turbulences and terrorism and, in general, has low resources including basic facilities of education, feeding, healthcare, electricity, sanitation, and transportation. Owing to geographical isolation and religious socio-cultural norms, majority of the inhabiting population is highly endogamous and consanguineous.
A huge burden of RDs has been suspected in J&K. According to the information available from Rare Diseases India Organization, more than 0.7 million individuals from J&K are likely suffering from RDs. Nevertheless, this information appears to be vague due to lack of a centralized patient-registry which has resulted in an unfortunate lack of accurate epidemiological data on distinct RDs prevalent in the region. Adding further to this is the lack of appropriate clinical information on these diseases, lack of awareness among the general population and basic facilities such as tertiary care hospitals, medical personnel, diagnostic facilities, and scanty R&D centers. Although there is a magnanimous quantitative burden of RDs in J&K, but these have mostly remained clinically ignored owing to aforementioned issues. Unfortunately, the population groups of J&K have also largely remained under-represented in the previous Indian genetic studies until recently, resulting in a lack of information on the genetic make-up of the J&K population strata and their disease heritage. In context with the region’s geographical topography and higher inbreeding rate, the unexplored cases of genetic disorders are likely to be associated with founder events unique to different population groups or families which may also hold clues for their evolutionary perspectives. The genetic assessment of the affected individuals from the region is, thus, a need-of-the-hour and essential for the development of appropriate therapeutic interventions. This would aid in genetic counseling and management of the reported genetic diseases.
In this section of the review, brief accounts on the population architecture of J&K, burden of genetic disorders and genetic studies conducted in the region have been provided.
Population Architecture of J&K
The population of J&K is typically heterogeneous with many anthropologically well-defined distinct ethnic and religious groups. The ethnic groups residing in J&K mainly include Arghuns, Bakerwals, Baltis, Bedas, Bodhs, Brokpas, Changpas, Dogras, Garras, Gujjars, Harijans, Kashmiris (Pandits and Muslims), Khatris, Kishtwaris, Ladakhis, Mahajans, Mons, Paharis and Purigpas ( Bhasin and Nag, 2002a ). The major religious groups of J&K include the Buddhists, Christians, Hindus, Jains, Muslims, Sikhs and others. According to the Population Census of India – 2011, Muslims (68.31%) constitute the major inhabiting population strata in J&K, followed by Hindus (28.44%), Sikhs (1.87%), Christians (0.28%), Jains (0.02%) and others (0.17%). The contemporary population of J&K speaks languages belonging to three different linguistic families – the Indo-European, Tibeto-Burman (in Ladakh) and various dialect of Dardic (in Kashmir) from Indo-Aryan language group ( Sharma et al., 2018 ). These population groups usually reside in small, geographically and socially isolated pockets since many centuries and are variably reinforced by their societal or religious customs to practice endogamy or consanguinity. Endogamy is preferred by almost all the population groups of J&K, whereas the consanguinity is mostly preferred by the Muslim population groups ( Bhasin and Nag, 2002b ). A study from the Rajouri and Poonch areas of J&K has indicated that the rate of consanguinity is quite high among the native Muslim populations which account nearly 35–50% with nearly 70–80% of the inbreeding occurring among first cousins ( Fareed and Afzal, 2014a , 2017 ). However, the reported figures were limited to the Muslim population of only two areas in J&K and might vary between different regions and communities.
Burden of Genetic Diseases in J&K
The population stratification of J&K holds a high significance in the historical, religious, socio-cultural and linguistic diversification of the Indian population. It has been suggested that various pre-historic and historic events of migrations and immigrations toward the Indian sub-continent have occurred through J&K along the north-eastern and north-western routes ( Bhasin and Nag, 2002a ; Pandith et al., 2015 ; Sharma et al., 2018 ). Intense endogamy within the population groups of J&K has resulted in restricted gene flow and genetic isolation for several centuries, thus, making them unique in terms of their gene pool and disease heritage. It is known that genetically isolated and highly endogamous population groups have higher levels of genetic homozygosity and, thus, are relatively more prone to have a higher burden of genetic disorders, especially recessive RDs ( Woods et al., 2006 ; Bittles and Black, 2010 ). In context to J&K, this fact could be supported by a study conducted on 995 individuals belonging to six Muslim population groups (including Gujjars and Bakerwals, Khans, Maliks, Mirs, Mughals, and Syed) from Rajouri and Pooch areas in J&K which has indicated a relatively higher level of homozygosity for Rhesus factor alleles ( Fareed et al., 2014 ).
Since past few years, a number of suspected cases of genetic disorders (including new/known monogenic diseases and other known genetic diseases with atypical clinical features) have been reported from J&K region. Cases of chromosomal genetic disorders (such as Down Syndrome, Turner Syndrome, Klinefelter Syndrome, Patau Syndrome; Table 5 ), anemias, blood disorders (including Thalassemia), congenital anomalies, disorders of sex development, metabolic disorders like G6PD deficiency, neurological disorders ( Table 6 ) and others are frequently reported in J&K ( Razdan et al., 1994 ; Kumar et al., 2010 ; Upma et al., 2010 ; Vasudev and Sawhney, 2014 ; Ara et al., 2018 ; Dar et al., 2018 ; Hockham et al., 2018 ). The detrimental effects of consanguinity and inbreeding depression on child health and mortality, cognitive behavior and fertility and an increased risk of cardiovascular diseases in small population groups from J&K has also been reported ( Bhasin and Nag, 2002b ; Fareed and Afzal, 2014a , b , 2016 ; Fareed et al., 2017 ). However, the J&K population has been largely under-represented in the surveys/screening studies conducted in context with disease incidence. These studies were usually restricted to small regional pockets in different areas of J&K and, therefore, have created a huge gap in the literature on incidence of the prevalent diseases.
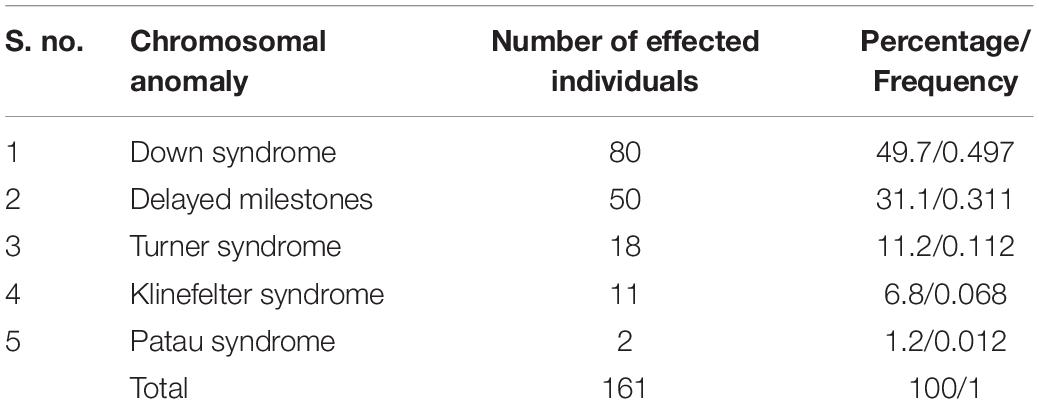
Table 5. Figures on the reported cases of chromosomal anomalies in J&K ( Kumar et al., 2010 ).
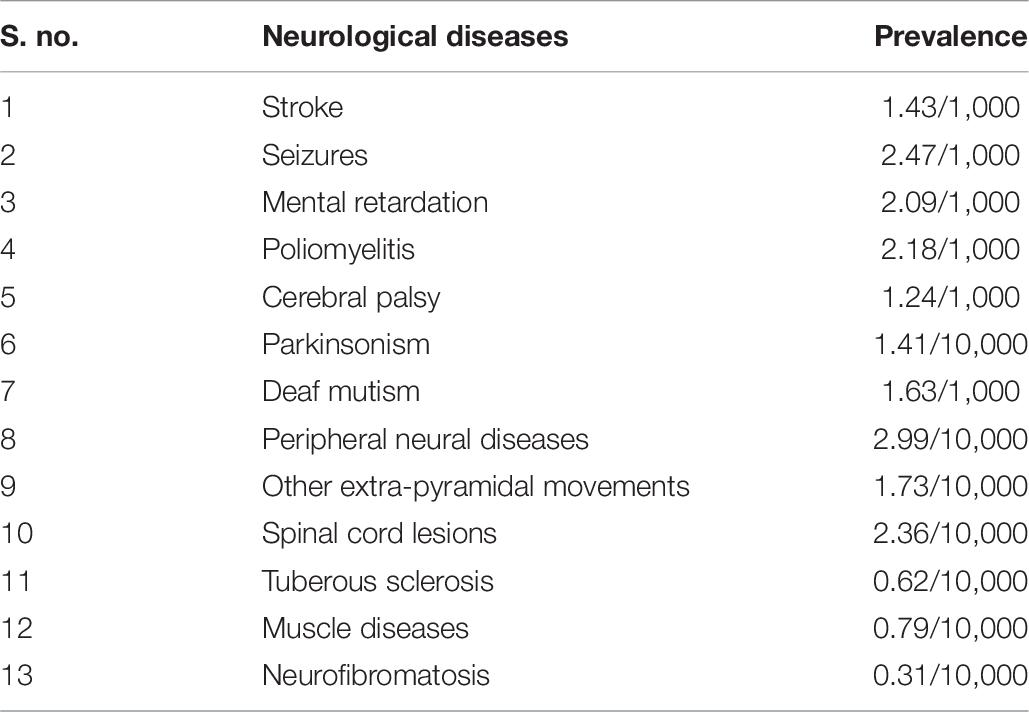
Table 6. Prevalence of various neurological diseases, as reported in rural Kashmir during 1986 ( Razdan et al., 1994 ).
There are some curious cases of certain clinical conditions that are highly prevalent in different hotspots or villages located in isolated remote areas of J&K. These reports indicate a higher burden of some diseases in the region restricted to individual villages or families. Information on these cases has been mostly portrayed over narrow approaches in the form of generalized or journalistic information. Summarized reports on some of these have been provided as follows:
Report 1 – The Village of Silence, J&K: Dadhkai village of Bhalessa, often known as the “Village of Silence,” in Doda area has been reported to have a high incidence of hearing loss. As per the reports (both journalistic as well as literary), this hamlet is inhabited by more than 2,500 individuals with over 95 members being deaf and mute. At least there is one member in each family who can neither hear or is mute. However, it has been reported that there is an increase in number of affected individuals in the region during the past decades. The village is located in a remote isolated area characterized by a very tough terrain in the Himalayan Mountains. It lacks basic facilities of nutrition, healthcare, immunization and rehabilitation, education, electricity, roads, and transportation. It is connected to the mainland through a foot-bridge. Some of the individuals have been found to be iodine- and salt-deficient by the clinicians. The residents of the village are of the opinion that the community might have been under some curse. The majority of the population in Dadhkai village belongs to a scheduled tribe “Gujjar” community of Muslim religion. However, given impetus to prolonged endogamous practices among this community, the clinical condition must have genetic origin and, thus, attained a relatively high incidence in the village. Pedigrees of four families from Dadhkai are already available in the literature ( Raina et al., 2017 ).
Report 2 – Arai village in Poonch, J&K: There are reports on a mysterious skeletal disease highly prevalent in Arai, a cluster of three villages, in Mandi area of Poonch – J&K. The symptoms of the disease usually appear between 4 and 8 years of age and progress in severity with advancing age. The affected individuals develop enlarged joints that look knobby in appearance, pain in joints, gait disturbance, abnormal posture, and short stature. More than 100 individuals over two extended families in the village have been rendered crippled for their life with this disease. Arai is located in the remote mountainous terrains of the Himalayas and has remained isolated due to lack of transport facilities until the roads were laid recently in the village area. The region also lacks basic facilities of education, electricity, healthcare, and sanitation. The residents of Arai are economically poor. Owing to their incurable ailment and lack of medical awareness, local belief of a curse resulting in the skeletal disorder in families has remained for decades and, thus, families have given up on medical consultations. Recent extensive efforts and a study reported from Arai village characterized the disorder as progressive pseudorheumatoid dysplasia (PPD), an autosomal recessive genetic disease with variants in gene WISP3 as the cause ( Rai et al., 2016 ).
Report 3 – Village of deaf and mute in Paralkot, J&K: In a remote village named Paralkot, about 80% of the population has been reported to be deaf and mute. The village falls in Sawjian area of Mandi sub-division, Poonch, J&K near the Line of Control (LoC), and lacks basic facilities of education, health-care and transportation. Majority of the inhabiting population is poor and are laborers. The villagers have different telltales regarding their clinical condition. Some believe that the person who sees the fairies residing in a nearby mountain become deaf and mute, while others believe there are evil spirits in the area or some curse shadowed over their families. It has been reported that owing to their clinical condition, about 30 families from this village had migrated to Pakistan occupied Kashmir (PoK) during 1990–1991. However, the villagers marry their close relatives within the same village. Owing to their endogamous background, there could be a likely genetic cause for their hearing disability as indicated by our preliminary (unpublished) findings.
There are further series of case reports on RDs including Ellis-van Creveld Syndrome, Epidermolysis Bullosa, Fabry’s disease, Fahr’s disease, Hereditary Stomatocytosis, Holt-Oram Syndrome, Ollier’s disease, different types of Porphyria, Rogers Syndrome, Wolfram syndrome, congenital anomalies, and many more from different regions of J&K ( Bhat J. I. et al., 2010 ; Bhat Y. J. et al., 2010 ; Bhat et al., 2015 ; Qayoom et al., 2010 ; Ganie et al., 2011 , 2012 ; Majid et al., 2012 ; Shoib et al., 2012 ; Hassan and Keen, 2013 ; Rasool et al., 2015 ; Wani et al., 2016 ; Kumar S. et al., 2017 ; Nazir and Chalkoo, 2017 ; Ilyas et al., 2019 ). Most of these are merely presented as case reports and the underpinning molecular etiology of many of the reported as well as unreported RDs cases from J&K have been due for years. Further there is a likelihood of more under-represented or yet to be identified RDs cases from J&K.
RDs-Associated Genetic Studies Conducted in the Region so Far
With advances in the genome research technologies, researchers have been recently successful in delineating some cases of RDs prevalent in J&K. The findings of these studies have immense contribution in expanding the genotype-phenotype and geographical spectrum of the reported RDs. Interestingly, these reports have been variably reported from different regions across the globe in high numbers from consanguineous/endogamous population groups in association with specific founder events. So far, these reported RDs cases have been reported for the first time from J&K in association with variations restricted to individual families or population groups of which a few indicates the presence of likely founder events.
Two studies based on CFTR (OMIM# 602421) variation analysis by Sher-e-Kashmir Institute of Medical Sciences, Kashmir in the Kashmiri population suspected with Cystic Fibrosis (OMIM# 219700) has revealed disease’s association with highly frequent CFTR . ΔF508 (c.1521_1523delCTT; p.Phe508del) and CFTR . 3,849+10 kb C > T variations ( Kawoosa et al., 2014 ; Pandith et al., 2015 ). The major limitation of these studies was that these were based on screening of only two CFTR variations in limited sample size. Considering the heterogeneity of the J&K population, it would have been highly informative if these studies could have screened the whole CFTR gene. Nevertheless, it is pertinent to mention that until these molecular studies had been conducted, Cystic Fibrosis was earlier considered to be uncommon in J&K. A team of researchers from Jammu had conducted a pilot study on screening of coding region exon 2 of GJB2 gene (OMIM# 121011) in randomly selected 17 affected individuals from the previously mentioned Dadhkai village (Village of Silence) in Doda – J&K ( Razdan et al., 2012 ). Through this study, GJB2 variations in only 4 out 17 subjects were detected which included p.G12V, p.L6L, p.R165W, p.L214P, and Del T at nt 636 variations. The study could have benefitted and informative if screening of other genes associated with hearing loss been performed. A further interesting study on eight families (one large and seven small families) representing about 50% of the affected individuals from the same village was conducted jointly by the Department of Biotechnology in University of Kashmir (Kashmir), Molecular Biology and Genetics Unit in Jawaharlal Nehru Centre for Advanced Scientific Research (Bengaluru) and Department of Audiology in Ali Yavar Jung National Institute for the Hearing Handicapped (Mumbai) ( Pandey et al., 2017 ). The families were screened using methods like Genome-wide scan and linkage analysis, mutation analysis of OTOF (OMIM# 603681), Cx26 or GJB2 , TMIE (OMIM# 607237), CLDN14 (OMIM# 605608), SLC26A4 (OMIM# 605646), TMPRSS3 (OMIM# 605511), TMC1 (OMIM# 606706), and USH1C (OMIM# 605242) genes. The findings of this study suggested genetic heterogeneity underpinning hearing loss among inhabitants of the highly endogamous Dadhkai village. Genome-wide scan and linkage analysis of the large family extending upto six generations mapped deafness to a chromosome loci 2p24-p22. The findings of the mutation analysis indicated a novel, founder variation NM_194248.2:c.2122C>T (NP_919224.1:p.R708 ∗ ) in exon 18 of OTOF gene, NM_144492.2:c.254T>A (NP_652763.1:p.V85D) in exon 7 of CLDN14 gene (a founder variation with origin in Pakistan) and a novel NM_00441.1:c.1668T>A (NP_000432.1:p.Y556 ∗ ) in exon 16 of SLC26A4 gene causing hearing loss in 94% of the cases genetically screened through this study. Furthermore, an unidentified underpinning genetic cause in one of the families was suspected to be the fourth cause.
A case of Autosomal Recessive Spastic Ataxia of Charlevoix-Saguenay (ARSACS; OMIM# 270550) in individual born to consanguineous parents of tribal origin from Rajouri area of J&K has been recently delineated through WES and reported in a reputed peer-reviewed journal ( Kuchay et al., 2019 ). The study identified a novel, frame-shift variation NM_014363: c.8605delT (p.Cys2869ValfsTer15) in exon 10 of SACS gene (OMIM# 604490), a well known causative gene of ARSACS. The proband was found to be homozygous for the reported variation, whereas his consanguineous parents were carriers.
Our research group (Human Genetics Research Group) at Shri Mata Vaishno Devi University, Katra, J&K – India is actively engaged in elucidating the underlying genetic causes for various complex disorders including type 2 Diabetes, Scoliosis as well as RDs and understanding the evolutionary perspectives of the population of J&K. In context with RDs, we have collected clinical information and samples from 60 distinct extended families suspected with uncharacterized genetic disorders from different regions of J&K. Of these recruited families, the exact genetic etiology of three distinct RDs have been determined and reported recently. The Arai village with a high incidence of a mysterious skeletal disorder (as mentioned earlier) was identified as a rare skeletal disorder “Progressive Pseudorheumatoid Dysplasia” (PPD; OMIM# 208230) using WES by our research group ( Rai et al., 2016 ). Both affected as well as unaffected members of two highly extended consanguineous families in Arai village were recruited for their genetic screening. Through WES in three members (affected siblings and their distant uncle) belonging to one of the families revealed two co-segregating, highly autosomal recessive variations NM_003880.3:c.156C>A (NP_003871.1:p.Cys52 ∗ ; rs121908901) and NM_003880.3:c.248G>A (NP_003871.1:p.Gly83Glu; rs147337485) in exon 3 of WISP3 gene (OMIM# 603400). These variations are already known to co-segregate with the disease phenotype in some families belonging to different parts of the world and might be an outcome in a region as a founder event, most likely of a middle-eastern origin ( Delague et al., 2005 ). Both of these variations have also been reported in some South Indian families ( Dalal et al., 2012 ). However, these variations were not found in the other recruited family for which sequencing of whole WISP3 gene was carried out and it was found that the second family is harboring a novel, autosomal recessive, splice-site variation NM_003880.3:c.643+1G>A (rs879255273) at the WISP3 exon 4 – intron 4 junction. Interestingly, PPD is a rare disorder having a prevalence of 1 in 1 million individuals in the United Kingdom, but have attained a higher prevalence in Arai village (upto 1000 times) due to the residing community’s consanguineous marital practices ( Wynne-Davies et al., 1982 ). This was the first ever study from the region that exploited a NGS technique (WES) for the identification and characterization of an unknown disease.
In an independent study using a PCR-based Direct Sanger Sequencing Strategy, our research group has identified an autosomal recessive variant NM_153638.3:c.1069C>T (NP_705902.2:p.Arg357Trp; rs753376100) located in exon 3 of PANK2 gene in a clinically suspected familial case of neurodegenerative disorder “Pantothenate Kinase-Associated Neurodegeneration” (PKAN; OMIM# 234000) ( Angural et al., 2017 ). PKAN is a progressive neurodegenerative disorder characterized by an abnormal accumulation of iron in the basal ganglia in brain and extra-pyramidal manifestation ( Hayflick et al., 2003 ). Thus, through this study the suspected PKAN diagnosis among two affected siblings, belonging to a remote village in Doda – J&K, was confirmed nearly within a decade of their preliminary diagnosis. The variation identified is located at a highly conserved region in codon 357 of hPank2 protein and predicted to be pathogenic through in silico pathogenicity prediction tools. Through a comparative molecular dynamics study of the wild-type and variant hPank2 protein models, it was observed that the reported variation has rendered rigidity to the other highly dynamic protein structure which might have caused a functional compromise in hPank2 molecules ( Angural et al., 2017 ).
Recently, our group has also successfully identified an uncharacterized neurological case in a 9 years old boy as an atypical form of Leigh Syndrome (LS; OMIM# 256000) through Whole Mitochondrial Genome Sequencing, and was found to be associated with a novel heteroplasmic MTP6 gene (OMIM# 516060) variant m.8936T>A ( Angural et al., 2019 ). LS is a progressive neurodegenerative disorder of infancy or early childhood with a clinical and genetically heterogeneous background ( Sofou et al., 2014 ). The proband in this study depicted an atypical feature of calcification in basal ganglia which was reported for the first time through this study.
Bottom-Up Approach
For carrying out genetic screening of cases of suspected genetic disorders, our research group has adopted the “Bottom-up Approach.” This approach is highly efficient in delineating the genetic etiology of various genetic disorders, especially RDs, prevalent in different global population groups. An outline of the designed Bottom-up Approach has been provided in Figure 1 . The strategy begins with the collection of clinical information of the patient and his/her family’s clinical history in the form of a pedigree. This is followed by a strategic collection of samples (blood, saliva, and tissue) from the patients and some of their unaffected family members, so that a comparative genetic screening is conducted. Based on the clinical suspicion and prior knowledge on the molecular etiology of the suspected disorders, two distinct approaches are considered for the genetic screening. In case of clinically suspected but genetically characterized RDs, targeted screening of known disease-associated candidate genes through a PCR-based Direct Sanger Sequencing approach (candidate gene screening) is suggested. On the other hand, WES approach is adopted in case of clinically suspected but genetically uncharacterized RDs, the results of which are further validated through a targeted PCR-based Direct Sanger Sequencing approach to screen the identified variations in the recruited subjects. After rigorous analyses and interpretation of the raw sequencing data through various tools, the identified variations should be further analyzed for their plausible pathogenicity through various online in silico prediction tools, the brief details of which have been provided in Table 1 . The plausible pathogenic variations are then marked for establishing their correlation with the clinical phenotype of the patients followed by analyses of their co-segregation with the suspected disorder in the recruited family. However, the final proof for variant-disease phenotype correlation is obtained through molecular functional studies based on DNA-RNA, DNA-Protein, RNA–RNA, and protein–protein interactions and expression studies dealing with the elucidation of query variation in a gene in model organisms. Once a variation is identified as pathogenic, a “Bottom-up Approach” is then followed which includes collection of more clinical information of the patient and his/her affected family members, and a comprehensive clinical evaluation in order to establish a differential diagnosis of the disease, followed by recruitment and genetic screening of other family individuals (including both affected as well as unaffected) for assessing the carrier frequency of the identified genetic variation.
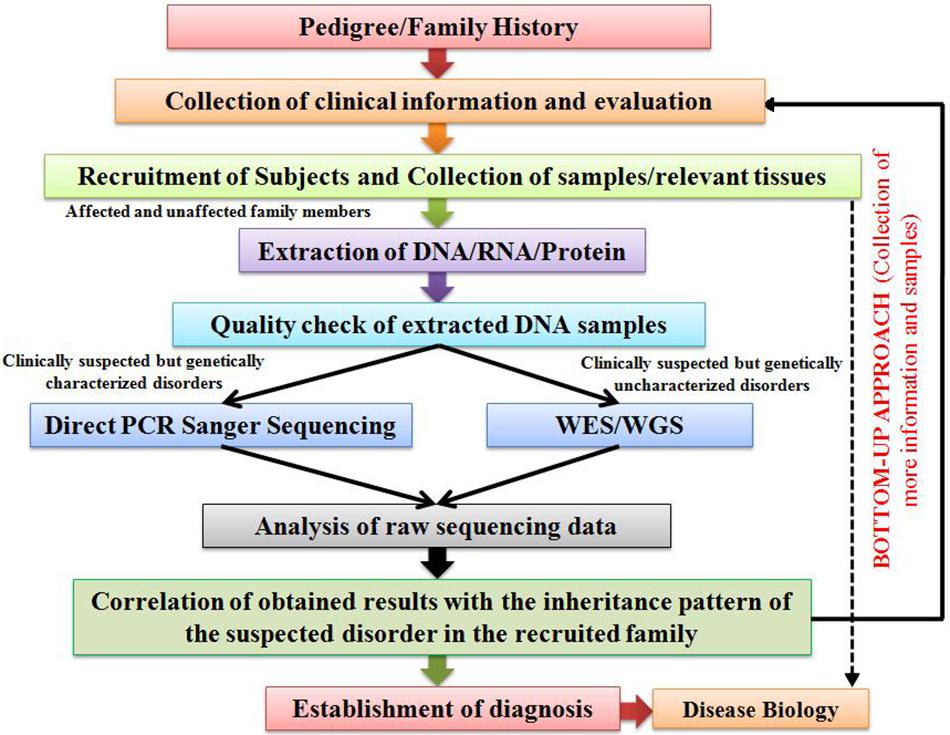
Figure 1. An illustration outlining the proposed “Bottom-up Approach” for the characterization of genetic disorders.
RDs are an important public health issue which needs to be overcome. To address RDs-associated challenges, a drive toward universal health coverage to fulfill RDs patients’ needs is required along with investment of public and government (national or international) funding in fundamental biomedical research for understanding the disease etiology, discovery of novel diagnostic biomarkers and therapeutic targets, and development of personalized intervention strategies for individual RDs patients. In promoting RDs-related R&D, significant progress has been made across the globe in recent years and many opportunities have been developed to build on the successful programs, projects and collaborations. For several RDs, remarkable fundamental research into the disease process has increased our understanding of RDs patho-physiology and led to development of suitable orphan drugs, healthcare innovation, and therapeutic interventions. Despite, there are still several hurdles in RDs research and healthcare and more emphasis is required to support appropriate RDs-related R&D and policy programs within individual countries so that all global patients would have equal access to therapeutic interventions. It is an irony that unlike other developing countries, India is lagging behind in context to regulation of RDs-based R&D due to several key issues that needs to be urgently addressed. The precise delineation of distinct RDs is possible by a meticulous clinical evaluation of the patients and their genetic screening. Recognition of carriers harboring clinically pathogenic genetic variations is important as to provide proper genetic counseling to the suspected individuals/families and an appropriate management of the disorder in an affected individual in a timely manner. For this purpose, we propose a highly potential workflow named “Bottom-up Approach” which could possibly aid in addressing this challenge not only for the Indian population, but for several other endogamous/consanguineous population groups existing across the globe. We further propose that the heterogeneous population of J&K could inspire future genetic studies and could serve as an interesting population-model for the same purpose. Besides, strong legislative policies and initiatives are also required from government and other institutions for carrying out RDs-related research.
Future Perspectives
Although the main source of information on RDs support and research groups for the patients and their families remains the internet, yet national RDs support websites are still needed in many countries ( Cullen, 2002 ; Lasker et al., 2005 ; Zurynski et al., 2008 ). To address the clinical challenges associated with RDs, it becomes imperative that different sources of clinical information and the clinical infrastructure should be updated regularly. A separate course on Clinical Genetics must be included in the academic curriculum of medical students in order to provide them knowledge on the basic concepts of Genetics and its applications in human health.
In context to the limitations of WES, it becomes imperative to use WGS and other “omics” platform as an alternative for determining the underpinning complex molecular etiology of RDs. This should also be accompanied with the development of innovative approaches that could possibly maintain and accelerate the current pace of clinical as well as genetic discoveries and inform future therapeutic developments.
We also emphasize that genetic screening of suspected population groups in J&K through the “Bottom-up Approach” based on the state-of-the-art biological techniques in amalgamation with clinical expertise should be carried out. In order to ascertain the genetic profile of the J&K population and the burden of carriers harboring pathogenic variations, a baseline database targeting each and every endogamous group from the region needs to be created, a daunting task undertaken by our Human Genetics Research Group at SMVDU but coming out with promising outcomes.
Author Contributions
AA and ASp primarily wrote the manuscript and prepared the figure and tables. SS and ER critically edited the manuscript, planned various studies, and developed procedures and execution pipelines. AA, ASp, AM, VV, ASh, PK, MD, KP, ER, and SS were involved in carrying out various studies. MD facilitated Sanger Sequencing at DNA Sequencing Facility, University of Jammu.
Conflict of Interest
SS has recently founded as a Chief Scientific Advisor a start-up “Biodroid Innovations Pvt. Ltd.,” involved in developing genetic and Med tech solutions.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank patients and their families who have been actively participating in the genetic studies being carried out at Human Genetics Research Group, School of Biotechnology, SMVDU. AA, ASp, and SS thank SMVDU for providing doctoral fellowship to AA and ASp. AM and SS thank DST-SERB for NPDF to AM.
- ^ https://www.omim.org/
- ^ https://www.orpha.net/consor/cgi-bin/index.php
Aggarwal, S., and Phadke, S. R. (2015). Medical genetics and genomic medicine in India: current status and opportunities ahead. Mol. Genet. Genomic Med. 3, 160–171. doi: 10.1002/mgg3.150
PubMed Abstract | CrossRef Full Text | Google Scholar
Andrews, S. (2010). FastQC: A Quality Control Tool for High Throughput Sequence Data. Available at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed October 6, 2011).
Google Scholar
Angural, A., Sharma, I., Pandoh, P., Sharma, V., Spolia, A., Rai, E., et al. (2018). A case report on a novel MT-ATP6 gene variation in atypical mitochondrial Leigh syndrome associated with bilateral basal ganglia calcifications. Mitochondrion 46, 209–213. doi: 10.1016/j.mito.2018.06.005
Angural, A., Sharma, I., Pandoh, P., Sharma, V., Spolia, A., Rai, E., et al. (2019). A case report on a novel MT-ATP6 gene variation in atypical mitochondrial Leigh syndrome associated with bilateral basal ganglia calcifications. Mitochondrion 46, 209–213. doi: 10.1016/j.mito.2018.06.005
Angural, A., Singh, I., Mahajan, A., Pandoh, P., Dhar, M. K., Kaul, S., et al. (2017). A variation in PANK2 gene is causing Pantothenate kinase-associated Neurodegeneration in a family from Jammu and Kashmir - India. Sci. Rep. 7:4834. doi: 10.1038/s41598-017-05388-9
Ankala, A., Kohn, J. N., Dastur, R., Gaitonde, P., Khadilkar, S. V., and Hegde, M. R. (2013). Ancestral founder mutations in calpain-3 in the Indian Agarwal community: historical, clinical, and molecular perspective. Muscle Nerve 47, 931–937. doi: 10.1002/mus.23763
Ankala, A., Tamhankar, P. M., Valencia, C. A., Rayam, K. K., Kumar, M. M., and Hegde, M. R. (2015). Clinical applications and implications of common and founder mutations in Indian subpopulations. Hum. Mutat. 36, 1–10. doi: 10.1002/humu.22704
Ara, A., Kumar, D., Dewan, D., and Digra, N. (2018). Incidence of congenital anomalies in a rural population of Jammu - A prospective study. Indian J. Public Health 62, 188–192. doi: 10.4103/ijph.IJPH_77_17
Auvin, S., Irwin, J., Abi-Aad, P., and Battersby, A. (2018). The problem of rarity: estimation of prevalence in rare disease. Value Health 21, 501–507. doi: 10.1016/j.jval.2018.03.002
Ayme, S., Urbero, B., Oziel, D., Lecouturier, E., and Biscarat, A. C. (1998). Information on rare diseases: the Orphanet project. Rev. Med. Interne 19(Suppl. 3), 376S–377S.
Bailey-Wilson, J. E., and Wilson, A. F. (2011). Linkage analysis in the next-generation sequencing era. Hum. Hered. 72, 228–236. doi: 10.1159/000334381
Barnett, C. P., Nataren, N. J., Klingler-Hoffmann, M., Schwarz, Q., Chong, C. E., Lee, Y. K., et al. (2016). Ectrodactyly and lethal pulmonary acinar dysplasia associated with homozygous FGFR2 mutations identified by exome sequencing. Hum. Mutat. 37, 955–963. doi: 10.1002/humu.23032
Barrett, J. H. (2014). Rare variants and disease. Brief. Funct. Genomics 13, 351–352.
Basu, A., Sarkar-Roy, N., and Majumder, P. P. (2016). Genomic reconstruction of the history of extant populations of India reveals five distinct ancestral components and a complex structure. Proc. Natl. Acad. Sci. U.S.A. 113, 1594–1599. doi: 10.1073/pnas.1513197113
Benjamin, K., Vernon, M. K., Patrick, D. L., Perfetto, E., Nestler-Parr, S., and Burke, L. (2017). Patient-reported outcome and observer-reported outcome assessment in rare disease clinical trials: an ISPOR COA emerging good practices task force report. Value Health 20, 838–855. doi: 10.1016/j.jval.2017.05.015
Bhai, P., Bijarnia-Mahay, S., Puri, R. D., Saxena, R., Gupta, D., Kotecha, U., et al. (2018). Clinical and molecular characterization of Indian patients with fructose-1, 6-bisphosphatase deficiency: identification of a frequent variant (E281K). Ann. Hum. Genet. doi: 10.1111/ahg.12256 [Epub ahead of print].
CrossRef Full Text | PubMed Abstract | Google Scholar
Bhasin, M., and Nag, S. (2002a). A demographic profile of the people of Jammu and Kashmir 1. Population Structure. J. Hum. Ecol. 13, 1–55. doi: 10.1080/09709274.2002.11905524
CrossRef Full Text | Google Scholar
Bhasin, M. K., and Nag, S. (2002b). Consanguinity and its effects on fertility and child survival among Muslims of Ladakh in Jammu and Kashmir. Anthropologist 4, 131–140. doi: 10.1080/09720073.2002.11890739
Bhat, J. I., Qureeshi, U. A., and Bhat, M. A. (2010). Acute intermittent porphyria with transient cortical blindness. Indian Pediatr. 47, 977–978. doi: 10.1007/s13312-010-0152-9
Bhat, Y., Hassan, I., Sajad, P., and Wani, R. (2015). Pretibial epidermolysis bullosa in a Kashmiri girl. Indian J. Paediat. Dermatol. 16, 203–206.
Bhat, Y. J., Baba, A. N., Manzoor, S., Qayoom, S., Javed, S., and Ajaz, H. (2010). Ellis-van Creveld syndrome with facial hemiatrophy. Indian J. Dermatol. Venereol. Leprol. 76, 266–269. doi: 10.4103/0378-6323.62968
Bijarnia-Mahay, S., Gupta, D., Shigematsu, Y., Yamaguchi, S., Saxena, R., and Verma, I. C. (2016). Ethylmalonic Encephalopathy in an Indian Boy. Indian Pediatr. 53, 914–916. doi: 10.1007/s13312-016-0959-0
Bittles, A. H. (2002a). Endogamy, consanguinity and community genetics. J. Genet. 81, 91–98. doi: 10.1007/bf02715905
Bittles, A. H. (2002b). The impact of consanguinity on the Indian population. Indian J. Hum. Genet. 8, 45–51.
Bittles, A. H., and Black, M. L. (2010). The impact of consanguinity on neonatal and infant health. Early Hum. Dev. 86, 737–741. doi: 10.1016/j.earlhumdev.2010.08.003
Bittles, A. H., and Black, M. L. (2010a). Consanguinity, human evolution, and complex diseases. Proc. Natl. Acad. Sci. U.S.A. 107, 1779–1786. doi: 10.1073/pnas.0906079106
Bittles, A. H., Savithri, H. S., and Appaji Rao, N. (2002). Community genetics in developing countries. Community Genet. 5, 151–152. doi: 10.1159/000066327
Bolger, A. M., Lohse, M., and Usadel, B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. doi: 10.1093/bioinformatics/btu170
Botstein, D., and Risch, N. (2003). Discovering genotypes underlying human phenotypes: past successes for mendelian disease, future approaches for complex disease. Nat. Genet. 33(Suppl.), 228–237. doi: 10.1038/ng1090
Boycott, K. M., and Ardigo, D. (2018). Addressing challenges in the diagnosis and treatment of rare genetic diseases. Nat. Rev. Drug Discov. 17, 151–152. doi: 10.1038/nrd.2017.246
Boycott, K. M., Rath, A., Chong, J. X., Hartley, T., Alkuraya, F. S., Baynam, G., et al. (2017). International cooperation to enable the diagnosis of all rare genetic diseases. Am. J. Hum. Genet. 100, 695–705. doi: 10.1016/j.ajhg.2017.04.003
Boycott, K. M., Vanstone, M. R., Bulman, D. E., and Mackenzie, A. E. (2013). Rare-disease genetics in the era of next-generation sequencing: discovery to translation. Nat. Rev. Genet. 14, 681–691. doi: 10.1038/nrg3555
Braun, M. M., Farag-El-Massah, S., Xu, K., and Cote, T. R. (2010). Emergence of orphan drugs in the United States: a quantitative assessment of the first 25 years. Nat. Rev. Drug Discov. 9, 519–522. doi: 10.1038/nrd3160
Chaki, M., Mukhopadhyay, A., Chatterjee, S., Das, M., Samanta, S., and Ray, K. (2005). Higher prevalence of OCA1 in an ethnic group of eastern India is due to a founder mutation in the tyrosinase gene. Mol. Vis. 11, 531–534.
PubMed Abstract | Google Scholar
Chen, W. J., Lin, Y., Xiong, Z. Q., Wei, W., Ni, W., Tan, G. H., et al. (2011). Exome sequencing identifies truncating mutations in PRRT2 that cause paroxysmal kinesigenic dyskinesia. Nat. Genet. 43, 1252–1255. doi: 10.1038/ng.1008
Choi, M., Scholl, U. I., Ji, W., Liu, T., Tikhonova, I. R., Zumbo, P., et al. (2009). Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc. Natl. Acad. Sci. U.S.A. 106, 19096–19101. doi: 10.1073/pnas.0910672106
Cullen, R. J. (2002). In search of evidence: family practitioners’ use of the Internet for clinical information. J. Med. Libr. Assoc. 90, 370–379.
Dalal, A., Bhavani, G. S., Togarrati, P. P., Bierhals, T., Nandineni, M. R., Danda, S., et al. (2012). Analysis of the WISP3 gene in Indian families with progressive pseudorheumatoid dysplasia. Am. J. Med. Genet. A 158A, 2820–2828. doi: 10.1002/ajmg.a.35620
Danese, E., and Lippi, G. (2018). Rare diseases: the paradox of an emerging challenge. Ann. Transl. Med. 6:329. doi: 10.21037/atm.2018.09.04
Dar, S., Nazir, M., Lone, R., Sameen, D., Ahmad, I., Wani, W., et al. (2018). Clinical spectrum of disorders of sex development: a cross-sectional observational study. Indian J. Endocr. Metab. 22, 774–779. doi: 10.4103/ijem.IJEM_159_18
Das Bhowmik, A., Patil, S. J., Deshpande, D. V., Bhat, V., and Dalal, A. (2018). Novel splice-site variant of UCHL1 in an Indian family with autosomal recessive spastic paraplegia-79. J. Hum. Genet. 63, 927–933. doi: 10.1038/s10038-018-0463-6
Dawn Teare, M., and Barrett, J. H. (2005). Genetic linkage studies. Lancet 366, 1036–1044.
De Silva, T. S., Macdonald, D., Paterson, G., Sikdar, K. C., and Cochrane, B. (2011). Systematized nomenclature of medicine clinical terms (SNOMED CT) to represent computed tomography procedures. Comput. Methods Programs Biomed. 101, 324–329. doi: 10.1016/j.cmpb.2011.01.002
Delague, V., Chouery, E., Corbani, S., Ghanem, I., Aamar, S., Fischer, J., et al. (2005). Molecular study of WISP3 in nine families originating from the Middle-East and presenting with progressive pseudorheumatoid dysplasia: identification of two novel mutations, and description of a founder effect. Am. J. Med. Genet. A 138A, 118–126. doi: 10.1002/ajmg.a.30906
Dixit, S., Sahu, P., Kar, S. K., and Negi, S. (2015). Identification of the hot-spot areas for sickle cell disease using cord blood screening at a district hospital: an Indian perspective. J. Community Genet. 6, 383–387. doi: 10.1007/s12687-015-0223-7
Dunstan, D. W., Zimmet, P. Z., Welborn, T. A., De Courten, M. P., Cameron, A. J., Sicree, R. A., et al. (2002). The rising prevalence of diabetes and impaired glucose tolerance: the Australian Diabetes. Obesity and Lifestyle Study. Diabetes Care 25, 829–834. doi: 10.2337/diacare.25.5.829
Fareed, M., and Afzal, M. (2014a). Estimating the inbreeding depression on cognitive behavior: a population based study of child cohort. PLoS One 9:e109585. doi: 10.1371/journal.pone.0109585
Fareed, M., and Afzal, M. (2014b). Evidence of inbreeding depression on height, weight, and body mass index: a population-based child cohort study. Am. J. Hum. Biol. 26, 784–795. doi: 10.1002/ajhb.22599
Fareed, M., and Afzal, M. (2016). Increased cardiovascular risks associated with familial inbreeding: a population-based study of adolescent cohort. Ann. Epidemiol. 26, 283–292. doi: 10.1016/j.annepidem.2016.03.001
Fareed, M., and Afzal, M. (2017). Genetics of consanguinity and inbreeding in health and disease. Ann. Hum. Biol. 44, 99–107. doi: 10.1080/03014460.2016.1265148
Fareed, M., Hussain, R., Shah, A., and Afzal, M. (2014). A 1 A 2 BO and Rh gene frequencies among six populations of Jammu and Kashmir, India. Transfus. Apher. Sci. 50, 247–252. doi: 10.1016/j.transci.2014.01.014
Fareed, M., Kaisar Ahmad, M., Azeem Anwar, M., and Afzal, M. (2017). Impact of consanguineous marriages and degrees of inbreeding on fertility, child mortality, secondary sex ratio, selection intensity, and genetic load: a cross-sectional study from Northern India. Pediatr. Res. 81, 18–26. doi: 10.1038/pr.2016.177
Forrest, C. B., Bartek, R. J., Rubinstein, Y., and Groft, S. C. (2011). The case for a global rare-diseases registry. Lancet 377, 1057–1059. doi: 10.1016/s0140-6736(10)60680-0
Fresard, L., and Montgomery, S. B. (2018). Diagnosing rare diseases after the exome. Cold Spring Harb. Mol. Case Stud. 4:a003392. doi: 10.1101/mcs.a003392
Fromer, M., Moran, J. L., Chambert, K., Banks, E., Bergen, S. E., Ruderfer, D. M., et al. (2012). Discovery and statistical genotyping of copy-number variation from whole-exome sequencing depth. Am. J. Hum. Genet. 91, 597–607. doi: 10.1016/j.ajhg.2012.08.005
Ganie, M. A., Ali, I., Ahangar, A. G., Wani, M. M., Ahmed, S., Bhat, M. A., et al. (2012). Thiamine responsive megaloblastic anemia syndrome associated with patent ductus arteriosus: first case report from Kashmir Valley of the Indian subcontinent. Indian J. Endocrinol. Metab. 16, 646–650. doi: 10.4103/2230-8210.98033
Ganie, M. A., Laway, B. A., Nisar, S., Wani, M. M., Khurana, M. L., Ahmad, F., et al. (2011). Presentation and clinical course of Wolfram (DIDMOAD) syndrome from North India. Diabet. Med. 28, 1337–1342. doi: 10.1111/j.1464-5491.2011.03377.x
Gorospe, J. R., Singhal, B. S., Kainu, T., Wu, F., Stephan, D., Trent, J., et al. (2004). Indian Agarwal megalencephalic leukodystrophy with cysts is caused by a common MLC1 mutation. Neurology 62, 878–882. doi: 10.1212/01.wnl.0000115106.88813.5b
Guillem, P., Cans, C., Robert-Gnansia, E., Ayme, S., and Jouk, P. S. (2008). Rare diseases in disabled children: an epidemiological survey. Arch. Dis. Child 93, 115–118. doi: 10.1136/adc.2006.104455
Gupta, A., Sharma, Y., Deo, K., Vellarikkal, S., Jayarajan, R., Dixit, V., et al. (2015). Case Report: Whole exome sequencing helps in accurate molecular diagnosis in siblings with a rare co-occurrence of paternally inherited 22q12 duplication and autosomal recessive non-syndromic ichthyosis. F1000Res. 4:446. doi: 10.12688/f1000research.6779.1
Gupta, A., Sharma, Y. K., Vellarikkal, S. K., Jayarajan, R., Dixit, V., Verma, A., et al. (2016). Whole-exome sequencing solves diagnostic dilemma in a rare case of sporadic acrokeratosis verruciformis. J. Eur. Acad. Dermatol. Venereol. 30, 695–697. doi: 10.1111/jdv.12983
Gupta, S., Chaurasia, A., Pathak, E., Mishra, R., Chaudhry, V. N., Chaudhry, P., et al. (2017). Whole exome sequencing unveils a frameshift mutation in CNGB3 for cone dystrophy: a case report of an Indian family. Medicine 96:e7490. doi: 10.1097/MD.0000000000007490
Haffner, M. E. (2006). Adopting orphan drugs–two dozen years of treating rare diseases. N. Engl. J. Med. 354, 445–447. doi: 10.1056/nejmp058317
Haffner, M. E., Whitley, J., and Moses, M. (2002). Two decades of orphan product development. Nat. Rev. Drug Discov. 1, 821–825. doi: 10.1038/nrd919
Hart, T. C., Hart, P. S., Michalec, M. D., Zhang, Y., Firatli, E., Van Dyke, T. E., et al. (2000). Haim-Munk syndrome and Papillon-Lefevre syndrome are allelic mutations in cathepsin C. J. Med. Genet. 37, 88–94. doi: 10.1136/jmg.37.2.88
Hassan, I., and Keen, M. A. (2013). Severe generalized recessive dystrophic epidermolysis bullosa (Hallopeau-Siemen’s): a case report. J. Pediatr. Sci. 5:e190.
Hayflick, S. J., Westaway, S. K., Levinson, B., Zhou, B., Johnson, M. A., Ching, K. H. L., et al. (2003). Genetic, clinical, and radiographic delineation of Hallervorden–Spatz syndrome. N. Engl. J. Med. 348, 33–40. doi: 10.1056/nejmoa020817
Hockham, C., Bhatt, S., Colah, R., Mukherjee, M. B., Penman, B. S., Gupta, S., et al. (2018). The spatial epidemiology of sickle-cell anaemia in India. Sci. Rep. 8, 17685. doi: 10.1038/s41598-018-36077-w
Hughes, D. A., Tunnage, B., and Yeo, S. T. (2005). Drugs for exceptionally rare diseases: do they deserve special status for funding? QJM 98, 829–836. doi: 10.1093/qjmed/hci128
Hussain, R., and Bittles, A. H. (2000). Sociodemographic correlates of consanguineous marriage in the Muslim population of India. J. Biosoc. Sci. 32, 433–442. doi: 10.1017/s0021932000004338
Ilyas, M., Wani, A., Ahmad, Z., Ahmad Kazimi, M., and Choh, N. (2019). Holt-oram syndrome - case series of two reports. CHRISMED J. Health Res. 6, 119–122. doi: 10.1002/ajmg.a.36783
Indian Genome Variation Consortium (2005). The Indian Genome Variation database (IGVdb): a project overview. Hum. Genet. 118, 1–11. doi: 10.1007/s00439-005-0009-9
Lander, E. S., Linton, L. M., Birren, B., Nusbaum, C., Zody, M. C., Baldwin, J., et al. (2001). Initial sequencing and analysis of the human genome. Nature 409, 860–921. doi: 10.1038/35057062
Karuthedath Vellarikkal, S., Jayarajan, R., Verma, A., Nair, S., Ravi, R., Senthivel, V., et al. (2016). Case Report: whole exome sequencing reveals a novel frameshift deletion mutation p.G2254fs in COL7A1 associated with autosomal recessive dystrophic epidermolysis bullosa. F1000Res. 5:900. doi: 10.12688/f1000research.8380.2
Kasatkar, P., Ghosh, K., and Shetty, S. (2013). A common founder mutation p.P2063S in exon 36 of VWF in 11 unrelated Indian von Willebrand disease (VWD) families. Ann. Hematol. 92, 1147–1148. doi: 10.1007/s00277-013-1680-x
Kasthuri, A. (2018). Challenges to Healthcare in India - The Five A’s. Indian J. Community Med. 43, 141–143.
Kawoosa, M. S., Bhat, M. A., Ali, S. W., Hafeez, I., and Shastri, S. (2014). Clinical and mutation profile of children with cystic fibrosis in Jammu and Kashmir. Indian Pediatr. 51, 185–189. doi: 10.1007/s13312-014-0381-4
Kehar, M., Bijarnia, S., Ellard, S., Houghton, J., Saxena, R., Verma, I. C., et al. (2014). Fanconi-Bickel syndrome - mutation in SLC2A2 gene. Indian J. Pediatr. 81, 1237–1239. doi: 10.1007/s12098-014-1487-3
Knight, A. W., and Senior, T. P. (2006). The common problem of rare disease in general practice. Med. J. Aust. 185, 82–83. doi: 10.5694/j.1326-5377.2006.tb00477.x
Kotecha, U. H., Movva, S., Puri, R. D., and Verma, I. C. (2012). Trichohepatoenteric syndrome: founder mutation in asian indians. Mol. Syndromol. 3, 89–93. doi: 10.1159/000339896
Krumm, N., O’roak, B. J., Karakoc, E., Mohajeri, K., Nelson, B., Vives, L., et al. (2013). Transmission disequilibrium of small CNVs in simplex autism. Am. J. Hum. Genet. 93, 595–606. doi: 10.1016/j.ajhg.2013.07.024
Kuchay, R. A. H., Mir, Y. R., Zeng, X., Hassan, A., Musarrat, J., Parwez, I., et al. (2019). ARSACS as a worldwide disease: novel SACS mutations identified in a consanguineous family from the remote tribal Jammu and Kashmir Region in India. Cerebellum 18, 807–812. doi: 10.1007/s12311-019-01028-2
Kumar, H., Sarma, P., and Medhi, B. (2017). Orphan drugs: Indian perspective. Indian J. Pharmacol. 49, 267–269.
Kumar, P., Gupta, A., Fotra, R., Upma, Raina, S., Sethi, S., et al. (2010). Chromosomal anomalies in referred cases with suspected genetic disorders: first report from Jammu and Kashmir. Int. J. Hum. Genet. 10, 41–47. doi: 10.1080/09723757.2010.11886083
Kumar, S., Bhalla, A., Sharma, N., Dhibar, D. P., Kumari, S., and Varma, S. (2017). Clinical, biochemical characteristics and hospital outcome of acute intermittent porphyria patients: a descriptive study from North India. Ann. Indian Acad. Neurol. 20, 263–269.
Lander, E. S., and Botstein, D. (1987). Homozygosity mapping: a way to map human recessive traits with the DNA of inbred children. Science 236, 1567–1570. doi: 10.1126/science.2884728
Lang, J., and Wood, S. C. (1999). Development of orphan vaccines: an industry perspective. Emerg. Infect. Dis. 5, 749–756. doi: 10.3201/eid0506.990602
Langmead, B., Trapnell, C., Pop, M., and Salzberg, S. L. (2009). Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10:R25. doi: 10.1186/gb-2009-10-3-r25
Lasker, J. N., Sogolow, E. D., and Sharim, R. R. (2005). The role of an online community for people with a rare disease: content analysis of messages posted on a primary biliary cirrhosis mailinglist. J. Med. Internet Res. 7:e10. doi: 10.2196/jmir.7.1.e10
Layer, R. M., Chiang, C., Quinlan, A. R., and Hall, I. M. (2014). LUMPY: a probabilistic framework for structural variant discovery. Genome Biol. 15:R84. doi: 10.1186/gb-2014-15-6-r84
Li, D., Tian, L., and Hakonarson, H. (2018). Increasing diagnostic yield by RNA-Sequencing in rare disease-bypass hurdles of interpreting intronic or splice-altering variants. Ann. Transl. Med. 6:126. doi: 10.21037/atm.2018.01.14
Li, H., and Durbin, R. (2009). Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760. doi: 10.1093/bioinformatics/btp324
Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., et al. (2009). The sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079. doi: 10.1093/bioinformatics/btp352
Li, J., Lupat, R., Amarasinghe, K. C., Thompson, E. R., Doyle, M. A., Ryland, G. L., et al. (2012). CONTRA: copy number analysis for targeted resequencing. Bioinformatics 28, 1307–1313. doi: 10.1093/bioinformatics/bts146
Lopes, M. T., Koch, V. H., Sarrubbi-Junior, V., Gallo, P. R., and Carneiro-Sampaio, M. (2018). Difficulties in the diagnosis and treatment of rare diseases according to the perceptions of patients, relatives and health care professionals. Clinics 73:e68. doi: 10.6061/clinics/2018/e68
Luzzatto, L., Hollak, C. E., Cox, T. M., Schieppati, A., Licht, C., Kaariainen, H., et al. (2015). Rare diseases and effective treatments: are we delivering? Lancet 385, 750–752. doi: 10.1016/s0140-6736(15)60297-5
Majid, S., Ahmad, Q. M., and Shah, I. H. (2012). Porphyrias amongst patients with cutaneous photosensitivity in Kashmir: a seven year experience. Indian J. Dermatol. Venereol. Leprol. 78:520. doi: 10.4103/0378-6323.98097
Majumder, P. P., and Basu, A. (2014). A genomic view of the peopling and population structure of India. Cold Spring Harb. Perspect. Biol. 7:a008540. doi: 10.1101/cshperspect.a008540
Mcelreavey, K., Wang, W., and Bittles, A. H. (2005). First Asian workshop on genomics and community genetics. Community Genet. 8, 130–132. doi: 10.1159/000084784
Mckenna, A., Hanna, M., Banks, E., Sivachenko, A., Cibulskis, K., Kernytsky, A., et al. (2010). The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303. doi: 10.1101/gr.107524.110
Mckusick, V. A. (2007). Mendelian Inheritance in Man and its online version, OMIM. Am. J. Hum. Genet. 80, 588–604. doi: 10.1086/514346
Middha, S., Lindor, N. M., Mcdonnell, S. K., Olson, J. E., Johnson, K. J., Wieben, E. D., et al. (2015). How well do whole exome sequencing results correlate with medical findings? A study of 89 Mayo Clinic Biobank samples. Front. Genet. 6:244. doi: 10.3389/fgene.2015.00244
Mistri, M., Tamhankar, P. M., Sheth, F., Sanghavi, D., Kondurkar, P., Patil, S., et al. (2012). Identification of novel mutations in HEXA gene in children affected with Tay Sachs disease from India. PLoS One 7:e39122. doi: 10.1371/journal.pone.0039122
Nambot, S., Thevenon, J., Kuentz, P., Duffourd, Y., Tisserant, E., Bruel, A. L., et al. (2018). Clinical whole-exome sequencing for the diagnosis of rare disorders with congenital anomalies and/or intellectual disability: substantial interest of prospective annual reanalysis. Genet. Med. 20, 645–654. doi: 10.1038/gim.2017.162
Narasimhan, V. M., Patterson, N. J., Moorjani, P., Lazaridis, I., Mark, L., Mallick, S., et al. (2018). The genomic formation of South and Central Asia. bioRxiv [Preprint]. doi: 10.1101/292581
Narayanan, R., Karuthedath Vellarikkal, S., Jayarajan, R., Verma, A., Dixit, V., Scaria, V., et al. (2016). Case Report: Application of whole exome sequencing for accurate diagnosis of rare syndromes of mineralocorticoid excess. F1000Res. 5:1592. doi: 10.12688/f1000research.8779.2
Nazir, N., and Chalkoo, A. H. (2017). Oral manifestations of a patient with Epidermolysis Bullosa. Biomed. J. Sci. Tech. Res. 1, 1562–1565.
Ng, S. B., Bigham, A. W., Buckingham, K. J., Hannibal, M. C., Mcmillin, M. J., Gildersleeve, H. I., et al. (2010a). Exome sequencing identifies MLL2 mutations as a cause of Kabuki syndrome. Nat. Genet. 42, 790–793. doi: 10.1038/ng.646
Ng, S. B., Buckingham, K. J., Lee, C., Bigham, A. W., Tabor, H. K., Dent, K. M., et al. (2010b). Exome sequencing identifies the cause of a mendelian disorder. Nat. Genet. 42, 30–35. doi: 10.1038/ng.499
Ng, S. B., Nickerson, D. A., Bamshad, M. J., and Shendure, J. (2010c). Massively parallel sequencing and rare disease. Hum. Mol. Genet. 19, R119–R124. doi: 10.1093/hmg/ddq390
Orphan Drug Act (1983). Pub L. No. 97-414, 96 Stat. 2049.
Pandey, N., Rashid, T., Jalvi, R., Sharma, M., Rangasayee, R., Andrabi, K. I., et al. (2017). Mutations in OTOF, CLDN14 & SLC26A4 genes as major causes of hearing impairment in Dhadkai village, Jammu & Kashmir, India. Indian J. Med. Res. 146, 489–497.
Pandith, A. A., Sheikh, S. A., Faheem, S., Zargar, M. H., Malla, T. M., Shah, Z. A., et al. (2015). Identification of unique pattern of CFTR gene mutations in cystic fibrosis in an ethnic Kashmiri Population (North India). J. Genet. Syndr. Gene Ther. 6, 2157–7412.
Phadke, S. R., Gupta, N., Girisha, K. M., Kabra, M., Maeda, M., Vidal, E., et al. (2010). Rhizomelic chondrodysplasia punctata type 1: report of mutations in 3 children from India. J. Appl. Genet. 51, 107–110. doi: 10.1007/BF03195717
Pradhan, S., Sengupta, M., Dutta, A., Bhattacharyya, K., Bag, S. K., Dutta, C., et al. (2011). Indian genetic disease database. Nucleic Acids Res. 39, D933–D938. doi: 10.1093/nar/gkq1025
Pulst, S. M. (1999). Genetic linkage analysis. Arch. Neurol. 56, 667–672.
Qayoom, S., Masood, Q., Sultan, J., Hassan, I., Jehangir, M., Bhat, Y. J., et al. (2010). Epidermolysis bullosa: a series of 12 patients in kashmir valley. Indian J. Dermatol. 55, 229–232. doi: 10.4103/0019-5154.70668
Quadros, L., Ghosh, K., and Shetty, S. (2009). Novel mutations in factor IX gene from western India with reference to their phenotypic and haplotypic attributes. J. Pediatr. Hematol. Oncol. 31, 157–160. doi: 10.1097/MPH.0b013e31818b3759
Rai, E., Mahajan, A., Kumar, P., Angural, A., Dhar, M. K., Razdan, S., et al. (2016). Whole exome screening identifies novel and recurrent WISP3 mutations causing progressive Pseudorheumatoid Dysplasia in Jammu and Kashmir-India. Sci. Rep. 6:27684. doi: 10.1038/srep27684
Raina, S., Saroch, M., Yadav, G., and Bhardwaj, A. (2017). Establishing ancestry through pedigree of a village with high prevalence of hearing-impaired. J. Indian Speech Lang. Hear. Assoc. 31, 1–4.
Rajasimha, H. K., Shirol, P. B., Ramamoorthy, P., Hegde, M., Barde, S., Chandru, V., et al. (2014). Organization for rare diseases India (ORDI) - addressing the challenges and opportunities for the Indian rare diseases’ community. Genet. Res. 96:e009. doi: 10.1017/S0016672314000111
Ranganath, P., Matta, D., Bhavani, G. S., Wangnekar, S., Jain, J. M., Verma, I. C., et al. (2016). Spectrum of SMPD1 mutations in Asian-Indian patients with acid sphingomyelinase (ASM)-deficient Niemann-Pick disease. Am. J. Med. Genet. A 170, 2719–2730. doi: 10.1002/ajmg.a.37817
Rasool, J., Manzoor, F., Bhat, S., Bashir, N., and Geelani, S. (2015). Hereditary stomatocytosis: First case report from Valley of Kashmir. Med. J. Dr. DY Patil. Univ. 8, 347–349.
Rausch, T., Zichner, T., Schlattl, A., Stutz, A. M., Benes, V., and Korbel, J. O. (2012). DELLY: structural variant discovery by integrated paired-end and split-read analysis. Bioinformatics 28, i333–i339. doi: 10.1093/bioinformatics/bts378
Razdan, S., Kaul, R. L., Motta, A., Kaul, S., and Bhatt, R. K. (1994). Prevalence and pattern of major neurological disorders in rural Kashmir (India) in 1986. Neuroepidemiology 13, 113–119. doi: 10.1159/000110368
Razdan, S., Raina, S. K., Pandita, K. K., Razdan, S., Nanda, R., Kaul, R., et al. (2012). Inbreeding as a cause for deafness: Dadhkai study. Indian J. Hum. Genet. 18, 71–74. doi: 10.4103/0971-6866.96655
Richter, T., Nestler-Parr, S., Babela, R., Khan, Z. M., Tesoro, T., Molsen, E., et al. (2015). Rare disease terminology and definitions-a systematic global review: report of the ISPOR rare disease special interest group. Value Health 18, 906–914. doi: 10.1016/j.jval.2015.05.008
Rimmer, A., Phan, H., Mathieson, I., Iqbal, Z., Twigg, S. R. F., Consortium, W. G. S., et al. (2014). Integrating mapping-, assembly- and haplotype-based approaches for calling variants in clinical sequencing applications. Nat. Genet. 46, 912–918. doi: 10.1038/ng.3036
Romdhane, L., Messaoud, O., Bouyacoub, Y., Kerkeni, E., Naouali, C., Cherif Ben Abdallah, L., et al. (2016). Comorbidity in the Tunisian population. Clin. Genet. 89, 312–319. doi: 10.1111/cge.12616
Saha, B., Lessel, D., Nampoothiri, S., Rao, A. S., Hisama, F. M., Peter, D., et al. (2013). Ethnic-specific WRN mutations in south Asian Werner syndrome patients: potential founder effect in patients with Indian or Pakistani ancestry. Mol. Genet. Genomic Med. 1, 7–14. doi: 10.1002/mgg3.1
Sawyer, S. L., Hartley, T., Dyment, D. A., Beaulieu, C. L., Schwartzentruber, J., Smith, A., et al. (2016). Utility of whole-exome sequencing for those near the end of the diagnostic odyssey: time to address gaps in care. Clin. Genet. 89, 275–284. doi: 10.1111/cge.12654
Schieppati, A., Henter, J. I., Daina, E., and Aperia, A. (2008). Why rare diseases are an important medical and social issue. Lancet 371, 2039–2041. doi: 10.1016/s0140-6736(08)60872-7
Setia, N., Saxena, R., Arora, A., and Verma, I. C. (2016). Spectrum of mutations in homozygous familial hypercholesterolemia in India, with four novel mutations. Atherosclerosis 255, 31–36. doi: 10.1016/j.atherosclerosis.2016.10.028
Sharma, I., Sharma, V., Khan, A., Kumar, P., Rai, E., Bamezai, R. N. K., et al. (2018). Ancient human migrations to and through Jammu Kashmir- India were not of males exclusively. Sci. Rep. 8:851. doi: 10.1038/s41598-017-18893-8
Shendure, J., Balasubramanian, S., Church, G. M., Gilbert, W., Rogers, J., Schloss, J. A., et al. (2017). DNA sequencing at 40: past, present and future. Nature 550, 345–353. doi: 10.1038/nature24286
Shoib, S., Maqbool Dar, M., Arif, T., Bashir, H., and Ahmed, J. (2012). Fahr’s disease presenting as mania: a case report. Iran. J. Psychiatry Behav. Sci. 6, 102–104.
Sindi, S., Helman, E., Bashir, A., and Raphael, B. J. (2009). A geometric approach for classification and comparison of structural variants. Bioinformatics 25, i222–i230. doi: 10.1093/bioinformatics/btp208
Sinha, K. (2005). Spinocerebellar Ataxia 12 (SCA12). A Tremor Dominant Disease, Typically Seen in India. Mumbai: Association of Physicians of India, 600–602.
Sofou, K., De Coo, I. F., Isohanni, P., Ostergaard, E., Naess, K., De Meirleir, L., et al. (2014). A multicenter study on Leigh syndrome: disease course and predictors of survival. Orphanet. J. Rare Dis. 9:52. doi: 10.1186/1750-1172-9-52
Song, P., Gao, J., Inagaki, Y., Kokudo, N., and Tang, W. (2012a). Intractable and rare diseases research in Asia. Biosci Trends 6, 48–51.
Song, P., Gao, J., Inagaki, Y., Kokudo, N., and Tang, W. (2012b). Rare diseases, orphan drugs, and their regulation in Asia: current status and future perspectives. Intractable Rare Dis. Res. 1, 3–9.
Teare, M. D., and Santibanez Koref, M. F. (2014). Linkage analysis and the study of Mendelian disease in the era of whole exome and genome sequencing. Brief. Funct. Genomics 13, 378–383. doi: 10.1093/bfgp/elu024
Tsurusaki, Y., Okamoto, N., Ohashi, H., Kosho, T., Imai, Y., Hibi-Ko, Y., et al. (2012). Mutations affecting components of the SWI/SNF complex cause Coffin-Siris syndrome. Nat. Genet. 44, 376–378. doi: 10.1038/ng.2219
Turton, J. P., Mehta, A., Raza, J., Woods, K. S., Tiulpakov, A., Cassar, J., et al. (2005). Mutations within the transcription factor PROP1 are rare in a cohort of patients with sporadic combined pituitary hormone deficiency (CPHD). Clin. Endocrinol. 63, 10–18. doi: 10.1111/j.1365-2265.2005.02291.x
Udani, V., Das, S., and Chhabria, R. (2017). Panthotenate Kinase-associated neurodegeneration has a founder mutation (c.215_216insa) in Indian Agrawal patients. Mov. Disord. Clin. Pract. 4, 96–99. doi: 10.1002/mdc3.12341
Upma, Kumar, P., Raina, T. R., Sharma, K., and Gupta, S. (2010). Clinical and cytogenetic analysis of human anemias from Jammu region of Jammu and Kashmir state. Asian J. Transfus. Sci. 4, 99–101. doi: 10.4103/0973-6247.67031
Uttarilli, A., Ranganath, P., Matta, D., Md Nurul Jain, J., Prasad, K., Babu, A. S., et al. (2016). Identification and characterization of 20 novel pathogenic variants in 60 unrelated Indian patients with mucopolysaccharidoses type I and type II. Clin. Genet. 90, 496–508. doi: 10.1111/cge.12795
Vasudev, R., and Sawhney, V. (2014). Thalassemia major and Intermedia in Jammu and Kashmir, India: a regional transfusion centre experience. Indian J. Hematol. Blood Transfus. 30, 297–300. doi: 10.1007/s12288-013-0301-0
Verma, I. (2000). Burden of genetic disorders in India. Indian J. Pediatr. 67, 893–898. doi: 10.1007/bf02723953
Verma, I., and Bijarnia, S. (2002). The burden of genetic disorders in India and a framework for community control. Public Health Genomics 5, 192–196. doi: 10.1159/000066335
Vink, J. M., and Boomsma, D. I. (2002). Gene finding strategies. Biol. Psychol. 61, 53–71. doi: 10.1016/s0301-0511(02)00052-2
Virmani, N., Vellarikkal, S. K., Verma, A., Jayarajan, R., Sakhiya, J., Desai, C., et al. (2018). Whole exome sequencing in a multi-generation family from India reveals a genetic variation c.10C > T (p.Gln4Ter) in keratin 5 gene associated with Dowling-Degos disease. Indian J. Dermatol. Venereol. Leprol. 84, 344–346.
Wajnrajch, M. P., Gertner, J. M., Sokoloff, A. S., Ten, I., Harbison, M. D., Netchine, I., et al. (2003). Haplotype analysis of the growth hormone releasing hormone receptor locus in three apparently unrelated kindreds from the Indian subcontinent with the identical mutation in the GHRH receptor. Am. J. Med. Genet. A 120A, 77–83. doi: 10.1002/ajmg.a.10209
Wang, Q., Lu, Q., and Zhao, H. (2015). A review of study designs and statistical methods for genomic epidemiology studies using next generation sequencing. Front. Genet. 6:149. doi: 10.3389/fgene.2015.00149
Wang, Z., Liu, X., Yang, B. Z., and Gelernter, J. (2013). The role and challenges of exome sequencing in studies of human diseases. Front. Genet. 4:160. doi: 10.3389/fgene.2013.00160
Wani, M. M., Khan, I., Bhat, R. A., and Ahmad, M. (2016). Fabry’s disease: case series and review of literature. Ann. Med. Health Sci. Res. 6, 193–197. doi: 10.4103/2141-9248.183935
Wastfelt, M., Fadeel, B., and Henter, J. I. (2006). A journey of hope: lessons learned from studies on rare diseases and orphan drugs. J. Intern. Med. 260, 1–10. doi: 10.1111/j.1365-2796.2006.01666.x
Weisenfeld, N. I., Yin, S., Sharpe, T., Lau, B., Hegarty, R., Holmes, L., et al. (2014). Comprehensive variation discovery in single human genomes. Nat. Genet. 46, 1350–1355. doi: 10.1038/ng.3121
Wenger, A. M., Guturu, H., Bernstein, J. A., and Bejerano, G. (2017). Systematic reanalysis of clinical exome data yields additional diagnoses: implications for providers. Genet. Med. 19, 209–214. doi: 10.1038/gim.2016.88
Whittemore, A. S., and Nelson, L. M. (1999). Study design in genetic epidemiology: theoretical and practical considerations. J. Natl. Cancer Inst. Monogr. 61–69. doi: 10.1093/oxfordjournals.jncimonographs.a024228
Woods, C. G., Cox, J., Springell, K., Hampshire, D. J., Mohamed, M. D., Mckibbin, M., et al. (2006). Quantification of homozygosity in consanguineous individuals with autosomal recessive disease. Am. J. Hum. Genet. 78, 889–896. doi: 10.1086/503875
Wynne-Davies, R., Hall, C., and Ansell, B. M. (1982). Spondylo-epiphysial dysplasia tarda with progressive arthropathy. A “new” disorder of autosomal recessive inheritance. J. Bone Joint Surg. Br. 64, 442–445. doi: 10.1302/0301-620x.64b4.6807993
Wysoker, A., Tibbetts, K., and Fennell, T. (2013). Picard Tools version 1.90. Available online at: http://picard.sourceforge.net (accessed March 10, 2020).
Yang, Y., Muzny, D. M., Reid, J. G., Bainbridge, M. N., Willis, A., Ward, P. A., et al. (2013). Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N. Engl. J. Med. 369, 1502–1511. doi: 10.1056/NEJMoa1306555
Yang, Y., Muzny, D. M., Xia, F., Niu, Z., Person, R., Ding, Y., et al. (2014). Molecular findings among patients referred for clinical whole-exome sequencing. JAMA 312, 1870–1879. doi: 10.1001/jama.2014.14601
Zurynski, Y., Frith, K., Leonard, H., and Elliott, E. (2008). Rare childhood diseases: how should we respond? Arch. Dis. Child 93, 1071–1074. doi: 10.1136/adc.2007.134940
Keywords : rare diseases, Next-Generation DNA Sequencing, endogamy, consanguinity, India, Jammu and Kashmir, Bottom-up Approach
Citation: Angural A, Spolia A, Mahajan A, Verma V, Sharma A, Kumar P, Dhar MK, Pandita KK, Rai E and Sharma S (2020) Review: Understanding Rare Genetic Diseases in Low Resource Regions Like Jammu and Kashmir – India. Front. Genet. 11:415. doi: 10.3389/fgene.2020.00415
Received: 03 August 2019; Accepted: 01 April 2020; Published: 30 April 2020.
Reviewed by:
Copyright © 2020 Angural, Spolia, Mahajan, Verma, Sharma, Kumar, Dhar, Pandita, Rai and Sharma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ekta Rai, [email protected] ; Swarkar Sharma, [email protected]
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 14 March 2022
Rare diseases, common challenges
Nature Genetics volume 54 , page 215 ( 2022 ) Cite this article
11k Accesses
10 Citations
9 Altmetric
Metrics details
- Clinical genetics
- Medical research
The genetics community has a particularly important part to play in accelerating rare disease research and contributing to improving diagnosis and treatment. Innovations in sequencing technology and machine learning approaches have positively affected diagnostic success, but more coordinated efforts are needed to move towards effective therapies or even cures for these important, and sometimes overlooked, class of diseases.
Rare Disease Day was recently held on 28 February 2022, which aimed to raise awareness and promote advocacy for rare disease research. Globally, there are more than 300 million people living with rare diseases and there are no approved therapies for over 90% of these disorders. Because around 80% of rare diseases have a genetic basis, recent advances in genomic sequencing technologies and molecular gene therapies have enhanced diagnosis and expanded treatments. To ensure that these advances are benefitting as many patients as possible and doing so in an equitable manner, unified efforts that span different stakeholders across rare disease communities should be supported.
In this issue of Nature Genetics , Halley and colleagues present a Comment that calls for an integrated approach for rare disease research in the United States. The authors argue that rare diseases are an important public health issue that should be given commensurate attention for their collective effects on individual patients, disease communities and healthcare systems. As such, the approach to rare disease research needs to broader for maximum benefits to a greater number of patients. The authors call for integrated approaches to research infrastructure that would minimize barriers to making connections, whether biological, therapeutic or societal, within and between rare diseases.
The authors highlight that rare disease research is currently very siloed and often organized around single disorders. Although efforts such as the Rare Disease Clinical Research Network have taken a broader approach, overall, there is limited coordination across rare disease research networks. The single-disorder focus creates challenges for jointly combining efforts, sharing data, assessing outcomes and capturing knowledge that could be relevant across diseases. A more integrated structure with appropriate support for researchers to coordinate across rare diseases would minimize redundant efforts and increase efficiency, potentially accelerating development and the implementation of successful therapies.
Importantly, no recommendations intended to promote rare disease research can ignore equity; indeed, ensuring fair practices in funding and equitable benefits of research outcomes must be a central focus of any research initiatives into rare diseases. It is challenging to achieve greater parity across rare diseases within the current research infrastructure, as analyzing how outcomes vary within or across rare diseases in different populations or socioeconomic groups is not straightforward. A more integrated approach to rare disease research will enable the assessment of how various factors (such as income level, insurance status, or racism in health care) affect participation in rare disease research or access to its benefits.
Altogether, the authors advocate for moving towards a more coordinated approach to rare disease research that would enable analysis of the similarities and differences across diseases in terms of etiology, treatment and outcomes. Although this article is specifically focused on the United States, the authors also recognize existing international efforts, such as the Global Genes and Genetic Alliance and the International Rare Disease Research Consortium that are leading the way in facilitating coordinated research efforts and data sharing.
We are excited by new technical advances in rare disease genetics research that apply the latest technologies to improve diagnosis. As an example, also in this issue of Nature Genetics , Hsieh and colleagues report a tool that uses deep convolutional neural networks to aid in diagnosing ultra-rare disorders based on facial morphology. GestaltMatcher defines a Clinical Face Phenotype Space based on over 17,000 photographs of patients representing more than 1,100 rare disorders. An advantage of using this method is that patients who share the same genetic diagnosis can be matched, even in cases when the disorder is not part of the training set. This helps with the clinical diagnosis of both known and new phenotypes. The concept of matching patients with rare disease is also conveyed on our cover, with actual matches forming the shape of a human face.
Rare disease research encompasses passionate individuals who span different sectors of interest: clinicians, patients, genetic counselors, biologists, technicians, advocates, funders and educators. We hope that the common challenges facing rare disease research can be combatted through enhanced coordination and cooperation across research communities, with the goal of accelerating diagnosis, maximizing therapeutic benefits and reducing inefficiencies.

Rights and permissions
Reprints and permissions
About this article
Cite this article.
Rare diseases, common challenges. Nat Genet 54 , 215 (2022). https://doi.org/10.1038/s41588-022-01037-8
Download citation
Published : 14 March 2022
Issue Date : March 2022
DOI : https://doi.org/10.1038/s41588-022-01037-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Landscape analysis of available european data sources amenable for machine learning and recommendations on usability for rare diseases screening.
- Ralitsa Raycheva
- Kostadin Kostadinov
- Rumen Stefanov
Orphanet Journal of Rare Diseases (2024)
Revealing myopathy spectrum: integrating transcriptional and clinical features of human skeletal muscles with varying health conditions
- Huahua Zhong
- Veronica Sian
Communications Biology (2024)
Highly efficient capture approach for the identification of diverse inherited retinal disorders
- Hsiao-Jung Kao
- Ting-Yi Lin
- Shun-Ping Huang
npj Genomic Medicine (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Rare Disease News
- Resource Library
- Rare Disease Facts and Statistics
- NORD’s Rare Disease Database
- Rare Disease Video Library
- What It Means To Be Undiagnosed
- Find A Rare Disease Organization
- Stories That Inspire
- RareEdu ® – Online Learning Platform
- Rare Disease Day
- State Resource Center – Find Local Resources
- Publications On Rare Disease
- Getting Help & Support
- Managing Your Disease
- How NORD Can Help
- Speak To Someone at NORD
- Rare Disease Center Of Excellence
- Patient Assistance Programs
- Explore Clinical Trials
- Find A Patient Organization
- Caregiver Resources
- State Resource Center – Discover Local Resources
- Rare Diseases Defined
- Financial & Medical Assistance
- Call Center & Information Services
- Bringing Together Your Community
- NORD Member List
- Start a Rare Disease Organization
- Membership Program
- Becoming Research Ready
- Launching Registries & Natural History Studies
- Patient-Focused Drug Development
- Rare Disease Centers of Excellence
- Continuing Medical Education (CME)
- Corporate Council
- National Partnerships
- Global Parnerships
- Diversity, Equity & Inclusion
- List of Rare Diseases
- Gene Therapy for Rare Disease
- Find Clinical Trials & Research Studies
- Request for Proposals
- Research Grant Programs
- Data Standards for Rare Diseases
- Resources for Patients
- Find a Rare Disease Care Center
- IAMRARE ® Program Powered by NORD
- Rare Disease Cures Accelerator (RDCA-DAP)
- Add Your Expertise
- Today’s Policy Issues
- NORD’s Policy Statements
- Rare Disease Advisory Councils
- NORD State Report Card
- Join the Rare Action Network®
- Policy & Advocacy Taskforce
- Contact your Representative
- Take Action on Key Issues
- Learn about our current policy goals
- Do-It-Yourself NORD Fundraiser
- Students for Rare
- Sports & Fitness Fundraisers
- Media Inquiries
- Attend An Upcoming Event
- Find a Rare Disease Patient Organization
- Stay Informed With NORD’s Email Newsletter
- Rare Disease Day®
- Share Your Story
- Careers At NORD
- Intern At NORD
- Jobs At Patient Disease Organizations
- Donate to NORD
- Volunteer with NORD
- Visit the NORD Store
- For Clinicians & Researchers
- For Patient Organizations
Rare Disease Database
Explore more.
- Getting Help and Support
- Rare Disease Information
- Financial Assistance
- Caregiver Aid
- Educational Support
- Patient & Caregiver FAQs
- Sobre los Centros de Excelencia
- Apply (Medical Institutions only)
- Finding a Support Organization
- Speak to Someone at NORD
What Is the NORD Rare Disease Database?
With more than 1,200 rare disorders, you can explore rare disease reports that include information on symptoms, causes, treatments, clinical trials, and sources of help such as patient advocacy organizations. Each report has a list of references, such as textbooks, articles, and government agency reports.
What Is a Rare Disease?
A rare disorder is a disease or condition that affects fewer than 200,000 Americans. Cumulatively, there are more than 10,000 rare diseases affecting more than 30 million Americans. NORD is committed to the identification, treatment, and cure of rare diseases through education, advocacy, research, and service programs.
Need more help?
One of the first places many newly diagnosed rare disease patients and their families turn to is the NORD Rare Disease Database. If you didn’t find what you’re looking for, NORD can help.
47, XXX (Trisomy X)
Also known as: 47, XXX 47, XXX karyotype 47, XXX syndrome triple X syndrome triple X XXX syndrome
47, XXY (Klinefelter Syndrome)
Also known as: KS 47,XXY male
* También disponible en español
48, XXYY Syndrome
Also known as: XXYY syndrome 48, XXYY variant of Klinefelter syndrome 48, XXYY Klinefelter syndrome
5,10 – Methenyltetrahydrofolate Synthetase Deficiency
Also known as: neurodevelopmental disorder with microcephaly, epilepsy and hypomyelination NEDMEHM MTHFS-related developmental delay-microcephaly-short stature-epilepsy syndrome
Aarskog Syndrome
Also known as: Aarskog disease Aarskog-Scott syndrome AAS faciodigitogenital syndrome faciogenital dysplasia FGDY Scott Aarskog syndrome
Abetalipoproteinemia
Also known as: ABL Bassen-Kornzweig syndrome low density lipoprotein deficiency microsomal triglyceride transfer protein deficiency MTP deficiency familial hypobetalipoproteinemia due to secretion defect 1 (FHBL-SD1)
Ablepharon-Macrostomia Syndrome
Also known as: AMS
Acanthocheilonemiasis
Also known as: Acanthocheilonemiasis perstans Dipetalonema perstans Dipetalonemiasis Mansonella perstans
Aceruloplasminemia
Also known as: familial apoceruloplasmin deficiency hereditary ceruloplasmin deficiency
Also known as: cardiospasm dyssynergia esophagus esophageal aperistalsis megaesophagus
Achard Thiers Syndrome
Also known as: Diabetic Bearded Woman Syndrome
Achondrogenesis
Achondroplasia.
Also known as: ACH achondroplastic dwarfism dwarf, achondroplastic
Acid Sphingomyelinase Deficiency
Also known as: ASMD ASM Deficiency Acid Sphingomyelinase-deficient Niemann-Pick Disease ASM-deficient Niemann-Pick Disease
Acoustic Neuroma
Also known as: acoustic neurilemoma acoustic neurinoma fibroblastoma, perineural neurinoma of the acoustic nerve neurofibroma of the acoustic nerve schwannoma of the acoustic nerve vestibular schwannoma
- For Patients & Caregivers
- For Organizations
Every donation matters.
Your support helps to ensure everyone’s free access to NORD’s rare disease reports.
Why the Pandemic Probably Started in a Lab, in 5 Key Points

By Alina Chan
Dr. Chan is a molecular biologist at the Broad Institute of M.I.T. and Harvard, and a co-author of “Viral: The Search for the Origin of Covid-19.”
This article has been updated to reflect news developments.
On Monday, Dr. Anthony Fauci returned to the halls of Congress and testified before the House subcommittee investigating the Covid-19 pandemic. He was questioned about several topics related to the government’s handling of Covid-19, including how the National Institute of Allergy and Infectious Diseases, which he directed until retiring in 2022, supported risky virus work at a Chinese institute whose research may have caused the pandemic.
For more than four years, reflexive partisan politics have derailed the search for the truth about a catastrophe that has touched us all. It has been estimated that at least 25 million people around the world have died because of Covid-19, with over a million of those deaths in the United States.
Although how the pandemic started has been hotly debated, a growing volume of evidence — gleaned from public records released under the Freedom of Information Act, digital sleuthing through online databases, scientific papers analyzing the virus and its spread, and leaks from within the U.S. government — suggests that the pandemic most likely occurred because a virus escaped from a research lab in Wuhan, China. If so, it would be the most costly accident in the history of science.
Here’s what we now know:
1 The SARS-like virus that caused the pandemic emerged in Wuhan, the city where the world’s foremost research lab for SARS-like viruses is located.
- At the Wuhan Institute of Virology, a team of scientists had been hunting for SARS-like viruses for over a decade, led by Shi Zhengli.
- Their research showed that the viruses most similar to SARS‑CoV‑2, the virus that caused the pandemic, circulate in bats that live r oughly 1,000 miles away from Wuhan. Scientists from Dr. Shi’s team traveled repeatedly to Yunnan province to collect these viruses and had expanded their search to Southeast Asia. Bats in other parts of China have not been found to carry viruses that are as closely related to SARS-CoV-2.

The closest known relatives to SARS-CoV-2 were found in southwestern China and in Laos.
Large cities
Mine in Yunnan province
Cave in Laos
South China Sea

The closest known relatives to SARS-CoV-2
were found in southwestern China and in Laos.
philippines

The closest known relatives to SARS-CoV-2 were found
in southwestern China and Laos.
Sources: Sarah Temmam et al., Nature; SimpleMaps
Note: Cities shown have a population of at least 200,000.

There are hundreds of large cities in China and Southeast Asia.

There are hundreds of large cities in China
and Southeast Asia.

The pandemic started roughly 1,000 miles away, in Wuhan, home to the world’s foremost SARS-like virus research lab.

The pandemic started roughly 1,000 miles away,
in Wuhan, home to the world’s foremost SARS-like virus research lab.

The pandemic started roughly 1,000 miles away, in Wuhan,
home to the world’s foremost SARS-like virus research lab.
- Even at hot spots where these viruses exist naturally near the cave bats of southwestern China and Southeast Asia, the scientists argued, as recently as 2019 , that bat coronavirus spillover into humans is rare .
- When the Covid-19 outbreak was detected, Dr. Shi initially wondered if the novel coronavirus had come from her laboratory , saying she had never expected such an outbreak to occur in Wuhan.
- The SARS‑CoV‑2 virus is exceptionally contagious and can jump from species to species like wildfire . Yet it left no known trace of infection at its source or anywhere along what would have been a thousand-mile journey before emerging in Wuhan.
2 The year before the outbreak, the Wuhan institute, working with U.S. partners, had proposed creating viruses with SARS‑CoV‑2’s defining feature.
- Dr. Shi’s group was fascinated by how coronaviruses jump from species to species. To find viruses, they took samples from bats and other animals , as well as from sick people living near animals carrying these viruses or associated with the wildlife trade. Much of this work was conducted in partnership with the EcoHealth Alliance, a U.S.-based scientific organization that, since 2002, has been awarded over $80 million in federal funding to research the risks of emerging infectious diseases.
- The laboratory pursued risky research that resulted in viruses becoming more infectious : Coronaviruses were grown from samples from infected animals and genetically reconstructed and recombined to create new viruses unknown in nature. These new viruses were passed through cells from bats, pigs, primates and humans and were used to infect civets and humanized mice (mice modified with human genes). In essence, this process forced these viruses to adapt to new host species, and the viruses with mutations that allowed them to thrive emerged as victors.
- By 2019, Dr. Shi’s group had published a database describing more than 22,000 collected wildlife samples. But external access was shut off in the fall of 2019, and the database was not shared with American collaborators even after the pandemic started , when such a rich virus collection would have been most useful in tracking the origin of SARS‑CoV‑2. It remains unclear whether the Wuhan institute possessed a precursor of the pandemic virus.
- In 2021, The Intercept published a leaked 2018 grant proposal for a research project named Defuse , which had been written as a collaboration between EcoHealth, the Wuhan institute and Ralph Baric at the University of North Carolina, who had been on the cutting edge of coronavirus research for years. The proposal described plans to create viruses strikingly similar to SARS‑CoV‑2.
- Coronaviruses bear their name because their surface is studded with protein spikes, like a spiky crown, which they use to enter animal cells. T he Defuse project proposed to search for and create SARS-like viruses carrying spikes with a unique feature: a furin cleavage site — the same feature that enhances SARS‑CoV‑2’s infectiousness in humans, making it capable of causing a pandemic. Defuse was never funded by the United States . However, in his testimony on Monday, Dr. Fauci explained that the Wuhan institute would not need to rely on U.S. funding to pursue research independently.

The Wuhan lab ran risky experiments to learn about how SARS-like viruses might infect humans.
1. Collect SARS-like viruses from bats and other wild animals, as well as from people exposed to them.

2. Identify high-risk viruses by screening for spike proteins that facilitate infection of human cells.

2. Identify high-risk viruses by screening for spike proteins that facilitate infection of
human cells.

In Defuse, the scientists proposed to add a furin cleavage site to the spike protein.
3. Create new coronaviruses by inserting spike proteins or other features that could make the viruses more infectious in humans.

4. Infect human cells, civets and humanized mice with the new coronaviruses, to determine how dangerous they might be.

- While it’s possible that the furin cleavage site could have evolved naturally (as seen in some distantly related coronaviruses), out of the hundreds of SARS-like viruses cataloged by scientists, SARS‑CoV‑2 is the only one known to possess a furin cleavage site in its spike. And the genetic data suggest that the virus had only recently gained the furin cleavage site before it started the pandemic.
- Ultimately, a never-before-seen SARS-like virus with a newly introduced furin cleavage site, matching the description in the Wuhan institute’s Defuse proposal, caused an outbreak in Wuhan less than two years after the proposal was drafted.
- When the Wuhan scientists published their seminal paper about Covid-19 as the pandemic roared to life in 2020, they did not mention the virus’s furin cleavage site — a feature they should have been on the lookout for, according to their own grant proposal, and a feature quickly recognized by other scientists.
- Worse still, as the pandemic raged, their American collaborators failed to publicly reveal the existence of the Defuse proposal. The president of EcoHealth, Peter Daszak, recently admitted to Congress that he doesn’t know about virus samples collected by the Wuhan institute after 2015 and never asked the lab’s scientists if they had started the work described in Defuse. In May, citing failures in EcoHealth’s monitoring of risky experiments conducted at the Wuhan lab, the Biden administration suspended all federal funding for the organization and Dr. Daszak, and initiated proceedings to bar them from receiving future grants. In his testimony on Monday, Dr. Fauci said that he supported the decision to suspend and bar EcoHealth.
- Separately, Dr. Baric described the competitive dynamic between his research group and the institute when he told Congress that the Wuhan scientists would probably not have shared their most interesting newly discovered viruses with him . Documents and email correspondence between the institute and Dr. Baric are still being withheld from the public while their release is fiercely contested in litigation.
- In the end, American partners very likely knew of only a fraction of the research done in Wuhan. According to U.S. intelligence sources, some of the institute’s virus research was classified or conducted with or on behalf of the Chinese military . In the congressional hearing on Monday, Dr. Fauci repeatedly acknowledged the lack of visibility into experiments conducted at the Wuhan institute, saying, “None of us can know everything that’s going on in China, or in Wuhan, or what have you. And that’s the reason why — I say today, and I’ve said at the T.I.,” referring to his transcribed interview with the subcommittee, “I keep an open mind as to what the origin is.”
3 The Wuhan lab pursued this type of work under low biosafety conditions that could not have contained an airborne virus as infectious as SARS‑CoV‑2.
- Labs working with live viruses generally operate at one of four biosafety levels (known in ascending order of stringency as BSL-1, 2, 3 and 4) that describe the work practices that are considered sufficiently safe depending on the characteristics of each pathogen. The Wuhan institute’s scientists worked with SARS-like viruses under inappropriately low biosafety conditions .

In the United States, virologists generally use stricter Biosafety Level 3 protocols when working with SARS-like viruses.
Biosafety cabinets prevent
viral particles from escaping.
Viral particles
Personal respirators provide
a second layer of defense against breathing in the virus.
DIRECT CONTACT
Gloves prevent skin contact.
Disposable wraparound
gowns cover much of the rest of the body.

Personal respirators provide a second layer of defense against breathing in the virus.
Disposable wraparound gowns
cover much of the rest of the body.
Note: Biosafety levels are not internationally standardized, and some countries use more permissive protocols than others.

The Wuhan lab had been regularly working with SARS-like viruses under Biosafety Level 2 conditions, which could not prevent a highly infectious virus like SARS-CoV-2 from escaping.
Some work is done in the open air, and masks are not required.
Less protective equipment provides more opportunities
for contamination.

Some work is done in the open air,
and masks are not required.
Less protective equipment provides more opportunities for contamination.
- In one experiment, Dr. Shi’s group genetically engineered an unexpectedly deadly SARS-like virus (not closely related to SARS‑CoV‑2) that exhibited a 10,000-fold increase in the quantity of virus in the lungs and brains of humanized mice . Wuhan institute scientists handled these live viruses at low biosafet y levels , including BSL-2.
- Even the much more stringent containment at BSL-3 cannot fully prevent SARS‑CoV‑2 from escaping . Two years into the pandemic, the virus infected a scientist in a BSL-3 laboratory in Taiwan, which was, at the time, a zero-Covid country. The scientist had been vaccinated and was tested only after losing the sense of smell. By then, more than 100 close contacts had been exposed. Human error is a source of exposure even at the highest biosafety levels , and the risks are much greater for scientists working with infectious pathogens at low biosafety.
- An early draft of the Defuse proposal stated that the Wuhan lab would do their virus work at BSL-2 to make it “highly cost-effective.” Dr. Baric added a note to the draft highlighting the importance of using BSL-3 to contain SARS-like viruses that could infect human cells, writing that “U.S. researchers will likely freak out.” Years later, after SARS‑CoV‑2 had killed millions, Dr. Baric wrote to Dr. Daszak : “I have no doubt that they followed state determined rules and did the work under BSL-2. Yes China has the right to set their own policy. You believe this was appropriate containment if you want but don’t expect me to believe it. Moreover, don’t insult my intelligence by trying to feed me this load of BS.”
- SARS‑CoV‑2 is a stealthy virus that transmits effectively through the air, causes a range of symptoms similar to those of other common respiratory diseases and can be spread by infected people before symptoms even appear. If the virus had escaped from a BSL-2 laboratory in 2019, the leak most likely would have gone undetected until too late.
- One alarming detail — leaked to The Wall Street Journal and confirmed by current and former U.S. government officials — is that scientists on Dr. Shi’s team fell ill with Covid-like symptoms in the fall of 2019 . One of the scientists had been named in the Defuse proposal as the person in charge of virus discovery work. The scientists denied having been sick .
4 The hypothesis that Covid-19 came from an animal at the Huanan Seafood Market in Wuhan is not supported by strong evidence.
- In December 2019, Chinese investigators assumed the outbreak had started at a centrally located market frequented by thousands of visitors daily. This bias in their search for early cases meant that cases unlinked to or located far away from the market would very likely have been missed. To make things worse, the Chinese authorities blocked the reporting of early cases not linked to the market and, claiming biosafety precautions, ordered the destruction of patient samples on January 3, 2020, making it nearly impossible to see the complete picture of the earliest Covid-19 cases. Information about dozens of early cases from November and December 2019 remains inaccessible.
- A pair of papers published in Science in 2022 made the best case for SARS‑CoV‑2 having emerged naturally from human-animal contact at the Wuhan market by focusing on a map of the early cases and asserting that the virus had jumped from animals into humans twice at the market in 2019. More recently, the two papers have been countered by other virologists and scientists who convincingly demonstrate that the available market evidence does not distinguish between a human superspreader event and a natural spillover at the market.
- Furthermore, the existing genetic and early case data show that all known Covid-19 cases probably stem from a single introduction of SARS‑CoV‑2 into people, and the outbreak at the Wuhan market probably happened after the virus had already been circulating in humans.

An analysis of SARS-CoV-2’s evolutionary tree shows how the virus evolved as it started to spread through humans.
SARS-COV-2 Viruses closest
to bat coronaviruses
more mutations

Source: Lv et al., Virus Evolution (2024) , as reproduced by Jesse Bloom

The viruses that infected people linked to the market were most likely not the earliest form of the virus that started the pandemic.

- Not a single infected animal has ever been confirmed at the market or in its supply chain. Without good evidence that the pandemic started at the Huanan Seafood Market, the fact that the virus emerged in Wuhan points squarely at its unique SARS-like virus laboratory.
5 Key evidence that would be expected if the virus had emerged from the wildlife trade is still missing.

In previous outbreaks of coronaviruses, scientists were able to demonstrate natural origin by collecting multiple pieces of evidence linking infected humans to infected animals.
Infected animals
Earliest known
cases exposed to
live animals
Antibody evidence
of animals and
animal traders having
been infected
Ancestral variants
of the virus found in
Documented trade
of host animals
between the area
where bats carry
closely related viruses
and the outbreak site

Infected animals found
Earliest known cases exposed to live animals
Antibody evidence of animals and animal
traders having been infected
Ancestral variants of the virus found in animals
Documented trade of host animals
between the area where bats carry closely
related viruses and the outbreak site

For SARS-CoV-2, these same key pieces of evidence are still missing , more than four years after the virus emerged.

For SARS-CoV-2, these same key pieces of evidence are still missing ,
more than four years after the virus emerged.
- Despite the intense search trained on the animal trade and people linked to the market, investigators have not reported finding any animals infected with SARS‑CoV‑2 that had not been infected by humans. Yet, infected animal sources and other connective pieces of evidence were found for the earlier SARS and MERS outbreaks as quickly as within a few days, despite the less advanced viral forensic technologies of two decades ago.
- Even though Wuhan is the home base of virus hunters with world-leading expertise in tracking novel SARS-like viruses, investigators have either failed to collect or report key evidence that would be expected if Covid-19 emerged from the wildlife trade . For example, investigators have not determined that the earliest known cases had exposure to intermediate host animals before falling ill. No antibody evidence shows that animal traders in Wuhan are regularly exposed to SARS-like viruses, as would be expected in such situations.
- With today’s technology, scientists can detect how respiratory viruses — including SARS, MERS and the flu — circulate in animals while making repeated attempts to jump across species . Thankfully, these variants usually fail to transmit well after crossing over to a new species and tend to die off after a small number of infections. In contrast, virologists and other scientists agree that SARS‑CoV‑2 required little to no adaptation to spread rapidly in humans and other animals . The virus appears to have succeeded in causing a pandemic upon its only detected jump into humans.
The pandemic could have been caused by any of hundreds of virus species, at any of tens of thousands of wildlife markets, in any of thousands of cities, and in any year. But it was a SARS-like coronavirus with a unique furin cleavage site that emerged in Wuhan, less than two years after scientists, sometimes working under inadequate biosafety conditions, proposed collecting and creating viruses of that same design.
While several natural spillover scenarios remain plausible, and we still don’t know enough about the full extent of virus research conducted at the Wuhan institute by Dr. Shi’s team and other researchers, a laboratory accident is the most parsimonious explanation of how the pandemic began.
Given what we now know, investigators should follow their strongest leads and subpoena all exchanges between the Wuhan scientists and their international partners, including unpublished research proposals, manuscripts, data and commercial orders. In particular, exchanges from 2018 and 2019 — the critical two years before the emergence of Covid-19 — are very likely to be illuminating (and require no cooperation from the Chinese government to acquire), yet they remain beyond the public’s view more than four years after the pandemic began.
Whether the pandemic started on a lab bench or in a market stall, it is undeniable that U.S. federal funding helped to build an unprecedented collection of SARS-like viruses at the Wuhan institute, as well as contributing to research that enhanced them . Advocates and funders of the institute’s research, including Dr. Fauci, should cooperate with the investigation to help identify and close the loopholes that allowed such dangerous work to occur. The world must not continue to bear the intolerable risks of research with the potential to cause pandemics .
A successful investigation of the pandemic’s root cause would have the power to break a decades-long scientific impasse on pathogen research safety, determining how governments will spend billions of dollars to prevent future pandemics. A credible investigation would also deter future acts of negligence and deceit by demonstrating that it is indeed possible to be held accountable for causing a viral pandemic. Last but not least, people of all nations need to see their leaders — and especially, their scientists — heading the charge to find out what caused this world-shaking event. Restoring public trust in science and government leadership requires it.
A thorough investigation by the U.S. government could unearth more evidence while spurring whistleblowers to find their courage and seek their moment of opportunity. It would also show the world that U.S. leaders and scientists are not afraid of what the truth behind the pandemic may be.
More on how the pandemic may have started

Where Did the Coronavirus Come From? What We Already Know Is Troubling.
Even if the coronavirus did not emerge from a lab, the groundwork for a potential disaster had been laid for years, and learning its lessons is essential to preventing others.
By Zeynep Tufekci

Why Does Bad Science on Covid’s Origin Get Hyped?
If the raccoon dog was a smoking gun, it fired blanks.
By David Wallace-Wells

A Plea for Making Virus Research Safer
A way forward for lab safety.
By Jesse Bloom
The Times is committed to publishing a diversity of letters to the editor. We’d like to hear what you think about this or any of our articles. Here are some tips . And here’s our email: [email protected] .
Follow the New York Times Opinion section on Facebook , Instagram , TikTok , WhatsApp , X and Threads .
Alina Chan ( @ayjchan ) is a molecular biologist at the Broad Institute of M.I.T. and Harvard, and a co-author of “ Viral : The Search for the Origin of Covid-19.” She was a member of the Pathogens Project , which the Bulletin of the Atomic Scientists organized to generate new thinking on responsible, high-risk pathogen research.
- Share full article
Advertisement
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Can Fam Physician
- v.56(4); 2010 Apr
Language: English | French
Genetic screening
To provide a primer for primary care professionals who are increasingly called upon to discuss the growing number of genetic screening services available and to help patients make informed decisions about whether to participate in genetic screening, how to interpret results, and which interventions are most appropriate.
QUALITY OF EVIDENCE
As part of a larger research program, a wide literature relating to genetic screening was reviewed. PubMed and Internet searches were conducted using broad search terms. Effort was also made to identify the gray literature.
MAIN MESSAGE
Genetic screening is a type of public health program that is systematically offered to a specified population of asymptomatic individuals with the aim of providing those identified as high risk with prevention, early treatment, or reproductive options. Ensuring an added benefit from screening, as compared with standard clinical care, and preventing unintended harms, such as undue anxiety or stigmatization, depends on the design and implementation of screening programs, including the recruitment methods, education and counseling provided, timing of screening, predictive value of tests, interventions available, and presence of oversight mechanisms and safeguards. There is therefore growing apprehension that economic interests might lead to a market-driven approach to introducing and expanding screening before program effectiveness, acceptability, and feasibility have been demonstrated. As with any medical intervention, there is a moral imperative for genetic screening to do more good than harm, not only from the perspective of individuals and families, but also for the target population and society as a whole.
Primary care professionals have an important role to play in helping their patients navigate the rapidly changing terrain of genetic screening services by informing them about the benefits and risks of new genetic and genomic technologies and empowering them to make more informed choices.
Résumé
Fournir un guide original aux professionnels des soins primaires qui sont de plus en plus appelés à discuter avec leurs patients des tests génétiques de plus en plus nombreux désormais disponibles, et de les aider à prendre des décisions éclairées sur l’intérêt de participer à ce genre de dépistage, sur la façon d’interpréter les résultats et sur le choix des interventions les plus appropriées.
QUALITÉ DES PREUVES
Dans le cadre d’un programme de recherche plus large, on a effectué une revue minutieuse de la littérature sur le dépistage génétique. On a consulté PubMed et Internet à l’aide d’un vaste éventail de termes de recherche. On s’est aussi efforcé d’identifier la documentation parallèle.
PRINCIPAL MESSAGE
Le dépistage génétique est un programme de santé publique qui est systématiquement offert à une population spécifique de personnes asymptomatiques, dans le but d’offrir aux personnes à risque élevé des mesures préventives, un traitement précoce ou des choix concernant la reproduction. Pour profiter des avantages supplémentaires du dépistage comparativement aux soins cliniques courants, et prévenir des préjudices involontaires tels que de l’anxiété ou une stigmatisation inutiles, il faut bien concevoir et exécuter les programmes de dépistage, notamment les méthodes de recrutement, les services d’information et de counseling, le moment du dépistage, la valeur prédictive des tests, les interventions disponibles, et la présence de mécanismes d’encadrement et de sauvegardes. On craint donc de plus en plus que des intérêts économiques puissent mener à une démarche axée sur le marché visant à adopter et élargir les programmes de dépistage avant que ne soient démontrés leur efficacité, leur acceptabilité et leur faisabilité. Comme pour toute intervention médicale, il est moralement impératif que le dépistage génétique comporte plus d’avantages que de risques, du point de vue non seulement des individus et des familles, mais aussi de la population ciblée et de la société dans son ensemble.
Les professionnels des soins primaires ont un rôle important à jouer pour aider leurs patients à comprendre le domaine en rapide évolution des services de dépistage génétique, en les informant des avantages et des risques des nouvelles technologies génétiques et génomiques, et en les rendant aptes à faire des choix plus éclairés.
Genetic screening is often touted as an important vehicle for translating genetic and genomic advances into population health gains. 1 , 2 This has contributed to increasing pressures from various sources to introduce or expand population-based genetic screening programs. 3 , 4 However, the availability of new tests for genetic screening is outpacing our ability to adequately integrate these into services, as the epidemiologic data, regulatory frameworks, infrastructure, clinical capacity, and public debate often lag far behind. 5 – 9
Deciding whether or not to introduce or expand population-based screening programs is complex and involves systematic analysis and synthesis of different kinds of evidence to evaluate the risks, benefits, and costs of screening from various viewpoints. 10 Because the introduction of new screening tests involves more than scientific judgment alone, there has been a call for greater public engagement with and debate about the moral issues and societal values at stake. Far-reaching implications have indeed been described, ranging from the psychological effects of living with risk and the potential for discrimination, to being denied insurance or suffering loss of employment. The technological imperative and the increasingly broadening conception of benefit are rapidly increasing the number of screening tests being offered, 11 in spite of the fact that each has its own distinct implications and needs to be considered on a case-by-case basis. This primer was therefore developed to assist primary care professionals in the complex task of discussing the growing number of genetic screening services with their patients and with the communities that they serve, thereby facilitating informed choices. 12 In particular, the primer begins by clarifying the nature of a genetic screening program, then explores the implications of genetic screening for different types of genetic conditions using different screening tests at different phases of the life cycle, and finally highlights key features of the decision-making process.
Quality of evidence
As part of a larger research program, a wide literature relating to multiple aspects of genetic screening policy-making was reviewed. PubMed and Internet searches were conducted using broad search terms such as genetic screening, prenatal screening, newborn screening, and population-based screening . Identified abstracts were scanned for relevance. Reference lists of retrieved documents were used to identify further sources. A special effort was also made to identify the gray literature through attendance at genetics conferences and discussions with key informants in the field.
Genetic diseases and hereditary diseases
Diseases caused by alterations in the genetic makeup of an individual (eg, single-gene mutations, chromosomal aberrations) are considered genetic diseases . 13 For instance, cancer is a genetic disease resulting from an accumulation of genetic mutations over time. However, not all genetic diseases are hereditary. Only diseases passed down from parents to offspring, according to laws first described by Gregor Mendel in the 19th century, are hereditary diseases. 14 Thus, although cancers are genetic diseases, less than 10% or 20% are due to inherited predispositions that can be transmitted from one generation to the next. 15
Traditionally, the discourse on genetic diseases referred to rare single-gene Mendelian conditions, which are both genetic and hereditary, often causing severe disability and death at an early age. 16 However, discussion of genetic diseases is becoming increasingly complex as a growing number of genetic alterations are being discovered for several common late-onset diseases with complex, multifactorial inheritance, such as the nonfamilial forms of cancer, type 2 diabetes, heart disease, and many psychiatric conditions. 17
Single-gene diseases and complex genetic diseases
Most diseases result from a combination of genetic and environmental factors. 18 There is, however, an etiologic spectrum on which diseases at one end are mostly due to genetic factors and diseases at the other end are mostly due to environmental factors.
At the “genetic” end of the spectrum, there are more than a thousand single-gene diseases (eg, Huntington disease, cystic fibrosis, and hemochromatosis), 19 which share certain features: they tend to be rare conditions; they are inherited in a Mendelian fashion; and genetic factors are strong determinants of the disease. 16
At the “environmental” end of the spectrum, multifactorial or complex genetic diseases (eg, heart disease, psychiatric conditions, and cancers) are caused by the interplay of multiple low-penetrance genes with various behavioural and environmental factors. 17 , 20
Although there are high expectations that personalized medicine could be used to screen individuals for multiple low-penetrance disease genes associated with common late-onset conditions, this remains highly controversial. 21 – 23 For now, genetic screening might be most promising for rare diseases, as more than 80% are single-gene diseases, each with a strong genetic basis, inherited in a more predictable fashion.
Rare diseases and orphan diseases
Rare diseases have been defined as having a low prevalence of less than 1 in 2000. 24 There exist between 5000 and 7000 distinct rare diseases that together affect between 6% and 8% of the world’s population, including an estimated 54 million people in Europe and North America combined. Thus, taken together, rare diseases are in fact not so rare. 25
Owing to the low prevalence of each disease in isolation, however, rare diseases have not traditionally been considered a public health concern. Some progress has been made, 26 but it remains difficult to get rare diseases onto the agendas of policy makers and pharmaceutical companies. 27 , 28 Many rare diseases are therefore also orphan diseases, which receive little attention in terms of research focus, market interest, and public health policies. 27 , 29 Special efforts are needed to reduce morbidity and mortality related to orphan diseases. 30
Screening as a strategy to improve health outcomes
Screening forms part of a continuum of approaches for improving population health, ranging from health promotion and disease prevention to treatment and rehabilitation. 31 Screening has been defined as a health service in which members of a specified population, who do not necessarily perceive themselves to be at risk of a disease or its complications, are asked a question or offered a test with the aim of identifying those individuals who are more likely to be helped than harmed by further tests or treatments. 32
Screening is known in public health terms as a secondary prevention strategy , 33 which identifies disease before symptoms develop, as early intervention might lead to improved health outcomes. Such benefits do not always occur, however, and screening can also have disadvantages. 34 – 36 Many factors must be considered, often through the use of established criteria, 37 to determine whether or not to introduce or expand screening programs.
Genetic screening refers to screening for genetic diseases; however, the term is not used in a consistent manner. 38 Depending on how genetic screening programs are organized, the recruitment strategies used, the timing of screening, the predictive value of the screening tests, and the interventions available for those with positive results, there can be very different activities involved with a range of implications.
Genetic screening is broadly defined here as a systematic program offered to a specified population of asymptomatic individuals whereby a variety of test methods can be used to make a risk estimate regarding an inherited predisposition to disease, to detect an inherited disease at an early stage, or to make a risk estimate regarding the possibility of transmitting a disease to offspring, for the purpose of disease prevention, early treatment, or family planning.
Genetic screening programs
Genetic screening programs are a type of public health program. 39 Public health programs are systematically offered to most or all members of a specified population, with the aim of delivering a net benefit to the population, as well as benefits to individuals. 40
Genetic screening involves more than just tests, but rather encompasses a complex and systematic program of services offered to a defined population who are informed of the potential risks and benefits through extensive education and counseling. Genetic screening programs thus require coordination among the testing, the clinical services, and the program management levels to enable the overall objectives of the program to be achieved and to ensure accountability. 41
Genetic screening recruitment strategies
In mass screening ( Figure 1 ), a test is offered to all individuals within a defined target population who are recruited through systematic outreach efforts; in opportunistic screening, individuals are recruited when they consult the health system for unrelated medical services. 42 Genetic screening should not be confused with genetic testing, 43 which is part of a diagnostic workup within a clinical setting for individuals who present with health-related concerns. Cascade screening, however, which involves the systematic identification and testing of asymptomatic relatives of those affected by a genetic disorder or previously identified as a carrier, 13 constitutes a gray zone between population-based genetic screening and genetic testing in a clinical setting.

Genetic screening continuum
Genetic screening tests
Genetic screening tests can involve molecular, 33 biochemical, 38 and other types of analyses, or even the use of family history questionnaires, 44 to predict which individuals are at risk of developing or transmitting (or both) a genetic condition. 45 Some tests are strong predictors of disease occurrence, 46 but many have a high degree of uncertainty. It can be difficult for those who have positive screening results to decide how best to proceed, as the proposed interventions vary greatly depending on the disease in question, they are not always highly effective, and might also involve certain risks. 47
Predictive value of genetic tests
Not all genetic tests have the same predictive value. This largely depends on whether the disease is caused by a single gene or chromosomal abnormality, as opposed to complex gene-gene and gene-environment interactions. Penetrance is a way of quantifying to what extent a given genetic alteration will be expressed as signs and symptoms of disease. 48 The greater the penetrance, the more likely an individual carrying a genetic alteration will develop the disease and become symptomatic. Genetic tests can thus be classified into presymptomatic, predisposition, and susceptibility tests.
Presymptomatic tests. Presymptomatic tests (eg, for Huntington disease) 49 test for rare conditions caused by single genes with autosomal dominant inheritance and very high penetrance (eg, more than 90% of those with the genetic alteration will develop the disease during their lifetimes). Nonetheless, the severity of the disease and the age at onset of symptoms can vary (ie, as a result of genotype-phenotype heterogeneity).
Predisposition tests. Predisposition tests (eg, for hereditary breast or ovarian cancer) 50 test for rare forms of otherwise common conditions that in a small subset of cases (usually less than 5% or 10%) are each caused by a single gene with autosomal dominant inheritance and an intermediate level of penetrance (eg, approximately 20% to 80% of those with the genetic alteration will develop the disease).
Susceptibility tests. Susceptibility tests (eg, for heart disease) 51 test for common conditions caused by complex gene-gene and gene-environment interactions, in which each individual gene has a low penetrance (eg, 5% or 10% of those with the gene will develop the disease). The overall risk profile for a set of markers might have a higher predictive value, although it still remains to be demonstrated whether genetic information provides any added value to more traditional environmental and behavioural risk factors of common chronic diseases (eg, advancing age, sedentary habits, obesity, smoking) and whether it will be useful in promoting preventive behaviours.
Carrier screening
In autosomal recessive conditions, offspring are only at risk of becoming ill if they receive 2 copies of a mutant gene, 1 from each parent. 13 Generally, however, the birth of an affected child comes as a surprise, as parents are often healthy carriers, with 1 normal copy of a gene and 1 mutated copy. 52 If both parents are carriers, there is a 1 in 4 risk that the child will be affected by the disease (depending on the disease penetrance and environmental factors) and a 1 in 2 risk that the child will be a carrier. In carrier screening, a test is used to identify couples who might be at risk of transmitting a genetic condition to their offspring.
Timing of genetic screening
The rationale underlying why certain conditions are screened for at specific times during the life course is generally linked to the optimal time for intervening that maximizes benefit and minimizes harm. Thus, the timing of screening is often used to divide genetic screening programs into 3 main types: preconception screening (ie, before having children), prenatal screening (ie, during pregnancy), and newborn screening (ie, after birth).
Preconception, prenatal, and newborn screening programs have long existed. More recently, screening for adult-onset conditions has been envisioned. However, whether such programs (eg, screening for early-onset Alzheimer disease or hereditary hemochromatosis) should be developed at all is highly controversial, largely owing to uncertainty about the predictive value of tests, the lack of preventive and early treatment options, and the fact that there is as yet no proven added benefit compared with standard care. For now, population-based genetic screening programs for adult-onset conditions are limited to the research context. However, with advances in knowledge and technology, this area might evolve rapidly.
Preconception screening. Preconception screening occurs before having children, and generally involves screening for carriers or identifying couples in which both individuals are asymptomatic carriers of a recessive condition (eg, cystic fibrosis), 53 to better predict whether their future offspring could be affected and to offer reproductive choices. Carrier screening is generally recommended in the preconception period, as it offers the widest range of reproductive options. In practice, however, carrier screening also occurs during pregnancy, when individuals are more conscious of reproductive issues. It would even be possible to determine carrier status in the newborn period; however, there are many ethical issues involved, and the general consensus is that screening newborns should only be carried out if it is directly relevant to their health and well-being during infancy and childhood. 54 , 55 Carrier screening programs are generally limited to specific high-risk groups, such as Tay-Sachs screening in Ashkenazi Jewish 56 and French Canadian populations. However, the primary care team can also identify couples planning to start families who have family histories of hereditary disease (particularly for diseases in which the gene is known) and who would be interested in referral to genetic counseling services for more detailed information and nondirective counseling tailored to their specific situations.
Prenatal screening. Prenatal screening, also known as antenatal screening , is carried out during pregnancy and generally identifies whether an unborn fetus has or is at risk of having a congenital condition (eg, chromosomal anomalies, such as Down syndrome, and structural anomalies, such as neural tube disorders or cardiac malformations). 57 The parents generally do not have identifiable genetic risk factors for these conditions; rather these conditions are associated with certain environmental influences (eg, advanced maternal age for Down syndrome, insufficient maternal intake of folic acid for neural tube disorders). Prenatal screening often involves a number of preliminary screening tests, followed by a confirmatory diagnostic test for those identified as high risk. The primary care team plays a key role in informing pregnant couples of the availability of such screening tests, which are generally time-sensitive. Prenatal screening offered to the general population should not be confused with clinical testing or cascade screening offered during pregnancy to a parent who might be at increased risk on account of having an affected relative with a single-gene disorder, for instance. Although here again, the primary care team can identify candidates who warrant referral to genetic counseling services by eliciting detailed family histories with respect to hereditary disease.
Newborn screening. Newborn screening, also known as neonatal screening, is usually carried out shortly after a baby is born and identifies whether the newborn is at risk of developing a disease in childhood for which prevention or early treatment exists (eg, a low-phenylalanine diet for phenylketonuria or hormone-replacement medication for congenital hypothyroidism). 58 Blood-spot screening has existed in many countries around the world for several decades. The most common form of newborn screening occurs a few days after birth, when a drop of blood from the heel of the baby is placed on a piece of absorbent paper (known as a Guthrie card ) to be analyzed using traditional biochemical techniques or newer tandem mass spectrometry methods. 59 In some countries, newborn screening is mandatory by law, and in other jurisdictions it is universal with implicit consent (with the option to opt out). Originally, diseases being screened for had very severe consequences (ie, profound mental retardation or death), which could be easily prevented if detected early with minimal or no risk to the child. However, over the years, the list of conditions being screened for has expanded from the initial 2 mentioned above to 29 conditions or more in certain jurisdictions, 60 making the estimation of risks and benefits even more complex. 61 This rapid expansion also poses a challenge for primary care teams who will be increasingly called upon to participate in the process of informing pregnant couples of what to expect after the birth and, at the very least, to make them aware of the existence of screening programs. Many new parents are not even aware that their newborns are being screened, as historically the benefits so greatly outweighed the risks that consent was considered to be implicit. As programs and times change, keeping up to date and informing parents will be increasingly important.
Decision making
As with any medical intervention, there is a moral imperative for genetic screening to do more good than harm. Introducing new genetic tests into clinical practice for diagnostic purposes when patients present with clinical indications (eg, symptoms of disease or high risk owing to family history) entails complex consideration of the analytical validity, clinical validity, and clinical utility of the tests. 62 , 63 However, the moral imperative is even more pronounced in the case of screening, which involves offering unsolicited services to asymptomatic individuals at baseline risk of developing the disease.
Population-based genetic screening has both individual and collective implications, thus the balance of risks and benefits has to be considered not only from the perspective of individuals and families, but also from that of the target population and of society as a whole. Even when there is scientific evidence that screening provides an added benefit to individuals and families, implementing a population-based screening program requires evaluation of the potential for the realization of these benefits and the minimization of risks in a given context, as well as consideration of the opportunity cost of funding the screening program.
The benefits of genetic screening programs stem from providing high-risk individuals with prevention, early treatment, or reproductive options. As science advances, making it possible to screen for a growing number of genetic conditions, it is important to consider the added value of genetic screening, as compared, for instance, to addressing the social, behavioural, and environmental determinants of health. 64 , 65
Critics are concerned that the “geneticization” of health and “routinization” of genetic information are being used to justify the introduction of new technologies before their potential effects are fully understood. 66 – 68 There are concerns that this might fail to improve health at a population level, that it could draw attention away from interventions with greater potential for disease prevention, and that it might exacerbate health inequities. 69
There is also growing apprehension that economic interests, with additional pressures from consumer groups, 70 might lead to a market-driven approach to genetic screening policy development 71 before the value of screening has been demonstrated. Governments must therefore balance the many different perspectives and needs of society, while promoting greater equity and supporting vulnerable groups, 72 such as individuals and families bearing the burden of rare and orphan diseases.
Even in genetic screening for rare diseases, there are many complex considerations to take into account. Risk information pertaining to genetic conditions, especially those caused by highly penetrant single genes, can have important implications for family members who might also be at risk. 73 – 75 In some instances, entire communities have been subjected to discrimination or stigmatization, particularly when there was insufficient community involvement or education when developing screening programs. Therefore, to avoid the premature introduction of new technologies and to ensure that concerns about genetic screening are adequately addressed, there needs to be a more “balanced and informed approach to the development of genetic policies and regulations” 76 through greater consultation, transparency, and public participation. 77 Primary care professionals have an important role to play in helping their patients navigate the rapidly changing terrain of genetic screening services, by informing and empowering them on how to maximize the benefits of new genetic and genomic technologies, where appropriate, while minimizing the risks. 78
EDITOR’S KEY POINTS
- The growing number of genetic tests now available are rapidly being incorporated into genetic screening services—both public and private—often before the far-reaching implications of such tests can be fully determined.
- Patients are faced with an increasing number of complex decisions about whether to participate in genetic screening, how to interpret their test results, and what action to take in the event of positive or indeterminate result. Primary care professionals will increasingly be called upon to help their patients assess whether there is an added benefit from screening that outweighs the risks, as well as to better navigate the screening process.
- To promote informed and balanced decision making, this primer explains key terms and concepts related to genetic screening and highlights the often complex implications of the type of condition screened, the timing of screening, the recruitment strategy used, the predictive value of the screening tests, and the effectiveness of interventions offered to those with positive test results.
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
Contributors
Drs Andermann and Blancquaert contributed to the literature search and the preparation of the article.
Competing interests
None declared

IMAGES
VIDEO
COMMENTS
This review explores the genetic basis of human disease, including single gene disorders, chromosomal imbalances, epigenetics, cancer and complex disorders, and considers how our understanding and technological advances can be applied to provision of appropriate diagnosis, management and therapy for patients.
Genetic disorders are of different types i.e. single-gene. disorders, chromosomal disorders, complex disorder s. This paper intends to be as an introductory paper for the project "Human genetic ...
This review explores. the genetic basis of human disease, including single gene disorders, chromosomal imbal-. ances, epigenetics, cancer and complex disorders, and considers how our understanding ...
Diseases stemming from alterations in the human genetic code pose a substantial burden, with recognised genetic diseases affecting over 5% of live births and more than two-thirds of miscarriages [11].
Genomic research has evolved from seeking to understand the fundamentals of the human genetic code to examining the ways in which this code varies among people, and then applying this knowledge to ...
Simultaneous assessment of multiple lipid derangements revealed nuanced differences in coronary artery disease risk and genetic architectures across dyslipoproteinemia subtypes. These findings highlight the importance of looking beyond single lipid traits to better understand combined lipid and lipoprotein phenotypes and implications for ...
With the completion of the Human Genome Project in 2004 [1] human genetics moved into a new era of exploring the whole genome and its relation to the causes of genetic disorders. New approaches ...
This Review charts recent milestones in the history of human disease genetics and provides an opportunity to reflect on lessons learned by the human genetics community.
The genetic tractability of monogenic defects, their penetrance in early life, and the ex- panding collection of human mutations and curated phenotypes make rare diseases ideal targets of study for discovering evolutionarily conserved biological mechanisms that go awry in common human diseases.
Learn about the advances and challenges in identifying the genetic causes of rare diseases, and the opportunities for future research and clinical applications.
A primary goal of human genetics is to identify DNA sequence variants that influence biomedical traits, particularly those related to the onset and progression of human disease. Over the past 25 years, progress in realizing this objective has been transformed by advances in technology, foundational genomic resources and analytical tools, and by ...
Abstract | The majority of rare diseases affect children, most of whom have an underlying genetic cause for their condition. However, making a molecular diagnosis with current technologies and ...
The genetic-dependence discloses the mechanisms underlying pathogenicity of gene/variants and common diseases. One sentence summary Genetic dependent feature differs in genes and determines pathogenicity of genes/variants and the clinical features of genetic diseases.
A plethora of network-based approaches within the Systems Biology universe have been applied, to date, to investigate the underlying molecular mechanisms of various human diseases. In the present study, we perform a bipartite, topological and clustering graph analysis in order to gain a better understanding of the relationships between human genetic diseases and the relationships between the ...
Genomics includes the scientific study of complex diseases such as heart disease, asthma, diabetes, and cancer because these diseases are typically caused more by a combination of genetic and ...
Background To diagnose the full spectrum of hereditary and congenital diseases, genetic laboratories use many different workflows, ranging from karyotyping to exome sequencing. A single generic high-throughput workflow would greatly increase efficiency. We assessed whether genome sequencing (GS) can replace these existing workflows aimed at germline genetic diagnosis for rare disease. Methods ...
Birth defects may have a genetic infection or environmental sources but about 50 % of all congenital disorders still have no exact cause. This review article focuses on the causes of genetic ...
We propose that the population of J&K, thus, offers a special niche to understand yet-to-be-explored underpinning molecular etiology of genetic diseases. With respect to the population of J&K, genetic research holds a huge potential in designing diagnostic protocols for prevailing genetic diseases and development of therapeutics.
Rare genetic diseases are the result of a continuous forward genetic screen that nature is conducting on humans. Here, we present epistemological and systems biology arguments highlighting the importance of studying these rare genetic diseases. We contend that the expanding catalog of mutations in ∼4,000 genes, which cause ∼6,500 diseases ...
Ethical Issues in Genetic Research, Screening, and Testing, with Particular Reference to Developing Countries. . . . . .147 ... nicable and genetic diseases as well as other common killers or causes of chronic ill health including cardiovascular diseases, cancer, diabetes, and
The genetics community has a particularly important part to play in accelerating rare disease research and contributing to improving diagnosis and treatment. Innovations in sequencing technology ...
With more than 1,200 rare disorders, you can explore rare disease reports that include information on symptoms, causes, treatments, clinical trials, and sources of help such as patient advocacy organizations. Each report has a list of references, such as textbooks, articles, and government agency reports.
PDF | Background: As gene therapy is one of the hottest topics of the new century, it carries the excitement of a cure to most of diseases, the... | Find, read and cite all the research you need ...
Patients with type 2 diabetes and chronic kidney disease are at high risk for kidney failure, cardiovascular events, and death. Whether treatment with semaglutide would mitigate these risks is unkn...
The world must not continue to bear the intolerable risks of research with the potential to cause pandemics.
To provide a primer for primary care professionals who are increasingly called upon to discuss the growing number of genetic screening services available and to help patients make informed decisions about whether to participate in genetic screening, how to interpret results, and which interventions are most appropriate.
This test's results make it possible to identify changes or mutations in one or more chromosomes, genes, and proteins to determine the possibility of genetic disease [5].