
- News/Events
- Arts and Sciences
- Design and the Arts
- Engineering
- Global Futures
- Health Solutions
- Nursing and Health Innovation
- Public Service and Community Solutions
- University College
- Thunderbird School of Global Management
- Polytechnic
- Downtown Phoenix
- Online and Extended
- Lake Havasu
- Research Park
- Washington D.C.
- Biology Bits
- Bird Finder
- Coloring Pages
- Experiments and Activities
- Games and Simulations
- Quizzes in Other Languages
- Virtual Reality (VR)
- World of Biology
- Meet Our Biologists
- Listen and Watch
- PLOSable Biology
- All About Autism
- Xs and Ys: How Our Sex Is Decided
- When Blood Types Shouldn’t Mix: Rh and Pregnancy
- What Is the Menstrual Cycle?
- Understanding Intersex
- The Mysterious Case of the Missing Periods
- Summarizing Sex Traits
- Shedding Light on Endometriosis
- Periods: What Should You Expect?
- Menstruation Matters
- Investigating In Vitro Fertilization
- Introducing the IUD
- How Fast Do Embryos Grow?
- Helpful Sex Hormones
- Getting to Know the Germ Layers
- Gender versus Biological Sex: What’s the Difference?
- Gender Identities and Expression
- Focusing on Female Infertility
- Fetal Alcohol Syndrome and Pregnancy
- Ectopic Pregnancy: An Unexpected Path
- Creating Chimeras
- Confronting Human Chimerism
- Cells, Frozen in Time
- EvMed Edits
- Stories in Other Languages
- Virtual Reality
- Zoom Gallery
- Ugly Bug Galleries
- Ask a Question
- Top Questions
- Question Guidelines
- Permissions
- Information Collected
- Author and Artist Notes
- Share Ask A Biologist
- Articles & News
- Our Volunteers
- Teacher Toolbox


show/hide words to know
ATP: adenosine triphosphate. ATP is the energy-carrying molecule of all cells...... more
Cellulose: the structural material found in the cell wall in most plants. Cellulose is used to make many products, including paper and cloth... more
Electron transport chain: cell process that uses electrons to generate chemical energy... more
Ion: an atom or molecule that does not have the same number of electrons as it has protons. This gives the atom or molecule a negative or positive charge... more
Light-dependent reaction: the first part of photosynthesis where (sun)light energy is captured and stored by a plant... more
Molecule: a chemical structure that has two or more atoms held together by a chemical bond. Water is a molecule of two hydrogen atoms and one oxygen atom (H2O)... more
Protein: a type of molecule found in the cells of living things, made up of special building blocks called amino acids.
Starch: made by all green plants and used to store energy for later use... more
Thylakoid: the disk-shaped parts of a plant cell where light-dependent reactions occur... more
In with One Energy and out with Another
The light-dependent reactions take place in the thylakoid membrane, inside chloroplasts. Since they are light 'dependent' reactions, you can guess that these reactions need light to work. Remember that the purpose of this first part of photosynthesis is to convert sunlight energy into other forms of energy?

The light-dependent reactions of photosynthesis require sunlight. Image by Mell27.
Plants cannot use light energy directly to make sugars. Instead, the plant changes the light energy into a form it can use: chemical energy. Chemical energy is all around us. For example, cars need the chemical energy from gasoline to run. The chemical energy that plants use are stored in ATP and NADPH. ATP and NADPH are two kinds of energy-carrying molecules. These two molecules are not only in plants, as animals use them as well.
A Recipe for Energy
Plants need water to make NADPH. This water is broken apart to release electrons (negatively charged subatomic particles). When water is broken it also creates oxygen, a gas that we all breathe.
The electrons must travel through special proteins stuck in the thylakoid membrane. They go through the first special protein (the photosystem II protein) and down the electron transport chain. Then they pass through a second special protein (photosystem I protein).
Photosystem I and Photosystem II
Wait a second... first electrons go through the second photosystem and second they go through the first? That seems really confusing. Why would they name the photosystems that way?

Water molecules are broken down to release electrons. These electrons then move down a gradient, storing energy in ATP in the process. Image by Jina Lee.
Photosystem I and II don't align with the route electrons take through the transport chain because they weren't discovered in that order. Photosystem I was discovered first. Later, photosystem II was discovered and found to be earlier in the electron transport chain. But it was too late, the name stuck. Electrons first travel through photosystem II and then photosystem I.
The Electron Transport Chain
While at photosystem II and I, the electrons gather energy from sunlight. How do they do that? Chlorophyll, which is present in the photosystems, soaks up light energy. The energized electrons are then used to make NADPH. The electron transport chain is a series of molecules that accept or donate electrons easily. By moving step-by-step through these, electrons are moved in a specific direction across a membrane. The movement of hydrogen ions are coupled with this. This means that when electrons are moved, hydrogen ions move too. ATP is created when hydrogen ions are pumped into the inner space (lumen) of the thylakoid. Hydrogen ions have a positive charge. Like in magnets, the same charges repel, so the hydrogen ions want to get away from each other. They escape the thylakoid through a membrane protein called ATP synthase. By moving through the protein they give it power, like water moving through a dam. When hydrogen ions move through the protein and down the electron transport chain, ATP is created. This is how plants turn to sunlight into chemical energy that they can use.
The Calvin Cycle: Building Life from Thin Air
How does something like air become the wood of a tree? The answer lies in what makes up the air.

How can the air surrounding a tree be turned into tree material? Through a complex set of reactions that use the carbon from the air to make other materials. Image by André Karwath.
The air holds different elements like oxygen, carbon, and nitrogen. These elements make up molecules like carbon dioxide (CO2). Carbon dioxide is made out of one carbon atom and two oxygen atoms. Plants take the carbon atom from carbon dioxide and use it to build sugars. This is done using the Calvin cycle. The Calvin cycle occurs inside chloroplasts, but outside the thylakoids (where ATP was created). The ATP and NADPH from the light-dependent reactions are used in the Calvin cycle. Parts of the Calvin cycle are sometimes called light-independent reactions. But don't let the name fool you... those reactions do require sunlight to work. The protein RuBisCO also helps in the process to change carbon from the air into sugars. RuBisCO works slowly, so plants need a lot of it. In fact, RuBisCO is the most abundant protein in the world! The products of the Calvin cycle are used to make the simple sugar glucose. Glucose is used to build more complex sugars like starch and cellulose. Starch stores energy for the plant and cellulose is the stuff of which plants are made.
Images via Wikimedia Commons. Seedling image by Bff.
Read more about: Snacking on Sunlight
View citation, bibliographic details:.
- Article: Photosynthesis
- Author(s): Heather Kropp, Angela Halasey
- Publisher: Arizona State University School of Life Sciences Ask A Biologist
- Site name: ASU - Ask A Biologist
- Date published: May 25, 2017
- Date accessed: May 28, 2024
- Link: https://askabiologist.asu.edu/photosynthesis
Heather Kropp, Angela Halasey. (2017, May 25). Photosynthesis. ASU - Ask A Biologist. Retrieved May 28, 2024 from https://askabiologist.asu.edu/photosynthesis
Chicago Manual of Style
Heather Kropp, Angela Halasey. "Photosynthesis". ASU - Ask A Biologist. 25 May, 2017. https://askabiologist.asu.edu/photosynthesis
MLA 2017 Style
Heather Kropp, Angela Halasey. "Photosynthesis". ASU - Ask A Biologist. 25 May 2017. ASU - Ask A Biologist, Web. 28 May 2024. https://askabiologist.asu.edu/photosynthesis

Plants need chemical energy to grow and survive. But how do they convert energy in sunlight into chemical energy?
Snacking on Sunlight
Be Part of Ask A Biologist
By volunteering, or simply sending us feedback on the site. Scientists, teachers, writers, illustrators, and translators are all important to the program. If you are interested in helping with the website we have a Volunteers page to get the process started.
Share to Google Classroom
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Biochemistry, electron transport chain.
Maria Ahmad ; Adam Wolberg ; Chadi I. Kahwaji .
Affiliations
Last Update: September 4, 2023 .
- Introduction
The electron transport chain is a series of four protein complexes that couple redox reactions, creating an electrochemical gradient that leads to the creation of ATP in a complete system named oxidative phosphorylation. It occurs in mitochondria in both cellular respiration and photosynthesis. In the former, the electrons come from breaking down organic molecules, and energy is released. In the latter, the electrons enter the chain after being excited by light, and the energy released is used to build carbohydrates.
- Fundamentals
Aerobic cellular respiration is made up of three parts: glycolysis, the citric acid (Krebs) cycle, and oxidative phosphorylation. In glycolysis, glucose metabolizes into two molecules of pyruvate, with an output of ATP and nicotinamide adenine dinucleotide (NADH). Each pyruvate oxidizes into acetyl CoA and an additional molecule of NADH and carbon dioxide (CO2). The acetyl CoA is then used in the citric acid cycle, which is a chain of chemical reactions that produce CO2, NADH, flavin adenine dinucleotide (FADH2), and ATP. In the final step, the three NADH and one FADH2 amassed from the previous steps are used in oxidative phosphorylation, to make water and ATP.
Oxidative phosphorylation has two parts: the electron transport chain (ETC) and chemiosmosis. The ETC is a collection of proteins bound to the inner mitochondrial membrane and organic molecules, which electrons pass through in a series of redox reactions, and release energy. The energy released forms a proton gradient, which is used in chemiosmosis to make a large amount of ATP by the protein ATP-synthase.
Photosynthesis is a metabolic process that converts light energy into chemical energy to build sugars. In the light-dependent reactions, light energy and water are used to make ATP, NADPH, and oxygen (O2). The proton gradient used to make the ATP forms via an electron transport chain. In the light-independent reactions, sugar is made from the ATP and NADPH from the previous reactions.
- Cellular Level
In the electron transport chain (ETC), the electrons go through a chain of proteins that increases its reduction potential and causes a release in energy. Most of this energy is dissipated as heat or utilized to pump hydrogen ions (H+) from the mitochondrial matrix to the intermembrane space and create a proton gradient. This gradient increases the acidity in the intermembrane space and creates an electrical difference with a positive charge outside and a negative charge inside. The ETC proteins in a general order are complex I, complex II, coenzyme Q, complex III, cytochrome C, and complex IV.
- (NADH + H+) + CoQ + 4 H+(matrix) -> NAD+ + CoQH2 + 4 H+(intermembrane)
- Succinate + FAD -> Fumarate + 2 H+(matrix) + FADH2
- Glycerol-3-Phosphate dehydrogenase and Acyl-CoA dehydrogenase also accept electrons from glycerol-3-P and fatty acyl-CoA, respectively. Inclusion of these protein complexes allows for the donation to the ETC by cytosolic NADH (glycerol-3-P acts as a shuttle to regenerate cytosolic NAD from NADH) and fatty acids undergoing beta-oxidation within the mitochondria (acyl-CoA is oxidized to enoyl-CoA in the first step, producing FADH2). [7] [8]
- Coenzyme Q, also known as ubiquinone (CoQ), is made up of quinone and a hydrophobic tail. Its purpose is to function as an electron carrier and transfer electrons to complex III. Coenzyme Q undergoes reduction to semiquinone (partially reduced, radical form CoQH-) and ubiquinol (fully reduced CoQH2) through the Q cycle. This process receives further elaboration under Complex III.
- Step 1 in the Q cycle involves ubiquinol (CoQH2) and ubiquinone (CoQ) binding to two separate sites on complex III. CoQH2 transfers each electron to a different path. One electron goes to Fe-S and then cytochrome c, while the second electron is transferred to cytochrome b and then to CoQ bound at the other site. While this occurs, 2 H+ ions are released into the intermembrane space, contributing to the proton gradient. CoQH2 is now oxidized to ubiquinone and dissociates from the complex. The CoQ bound at the second site enters a transitional CoQH- radical state from accepting one of the electrons.
- The second step of the cycle involves a repeat of the first: a new CoQH2 binds to the first site and transfers two electrons like before (and 2 more H+ ions released). Again, one electron passes to cytochrome c and one to cytochrome b, which this time works to reduce CoQH- to CoQH2 before it dissociates from complex III and can be recycled. In this way, one full cycle appears as follows: [12]
- 2 CoQH2(site 1) + CoQ(site 2) + 2 Cyt c(ox) + 2 H+(matrix) -> 2 CoQ(site 1) + CoQH2(site 2) + 2 Cyt c(red) + 4 H+(intermembrane)
- 2 cytochrome c(red) + ½O2 + 4 H+(matrix) -> 2 cytochrome c(ox) + 1 H2O + 2 H+(intermembrane)
ATP synthase, also called complex V, uses the ETC generated proton gradient across the inner mitochondrial membrane to form ATP. ATP-synthase contains up of F0 and F1 subunits, which act as a rotational motor system. F0 is hydrophobic and embedded in the inner mitochondrial membrane. It contains a proton corridor that is protonated and deprotonated repeatedly as H+ ions flow down the gradient from intermembrane space to matrix. The alternating ionization of F0 causes rotation, which alters the orientation of the F1 subunits. F1 is hydrophilic and faces the mitochondrial matrix. Conformational changes in F1 subunits catalyze the formation of ATP from ADP and Pi. For every 4 H+ ions, 1 ATP is produced. ATP-synthase can also be forced to run in reverse, consuming ATP to produce a hydrogen gradient, as is seen in some bacteria. [15] [16] [17]
- Molecular Level
Nicotinamide adenine dinucleotide has two forms: NAD+ (oxidized) and NADH (reduced). It is a dinucleotide connected by phosphate groups. One nucleoside has an adenine base and the other nicotinamide. When involved in metabolic redox reactions, the mechanism is as shown in Reaction 1.
- Reaction 1: RH2 + NAD+ -> R + H+ + NADH
R is the reactant, for example, sugar.
NADH enters the ETC at complex I and produces a total of 10 H+ ions through the ETC (4 from complex I, 4 from complex III, and 2 from complex IV). ATP-synthase synthesizes 1 ATP for 4 H+ ions. Therefore, 1 NADH = 10 H+, and 10/4 H+ per ATP = 2.5 ATP per NADH (**some sources round up**). When NADH is oxidized, it breaks into NAD+, H+, and 2 e- as shown in Reaction 2.
- Reaction 2: NADH -> H+ + NAD+ + 2 e-
Flavin adenine dinucleotide has 4 redox states, 3 of them being FAD (quinone, fully oxidized form), FADH- (semiquinone, partially oxidized), and FADH2 (hydroquinone, fully reduced). FAD is made up of an adenine nucleotide and a flavin mononucleotide (FMN), connected by phosphate groups. FMN is synthesized in part from vitamin B2 (riboflavin). FAD contains a highly stable aromatic ring, and FADH2 does not. When FADH2 oxidizes, it becomes aromatic and releases energy, as seen in Reaction 3. This state makes FAD a potent oxidizing agent, with an even more positive reduction potential than NAD. FADH2 enters the ETC at complex II and creates a total of 1.5 ATP (4 H+ from complex III, and 2 H+ from complex IV; 6/4 H+ per ATP = 1.5 ATP per FADH2 **some sources round up**). [18]
- Reaction 3: FADH2 -> FAD + 2 H+ + 2 e-
FAD also functions in several metabolic pathways outside of the ETC, including DNA repair (MTHF repair of UV damage), fatty acid beta-oxidation (acyl-CoA dehydrogenase), and synthesis of coenzymes (CoA, CoQ, heme).
- Clinical Significance
Uncoupling Agents
An uncoupling agent dissociates the electron transport chain from phosphorylation by ATP-synthase, preventing the formation of ATP. Disruption of the phospholipid bilayer of membranes causes a fluid-like and disorganized state, which allows protons to flow through more freely. This proton leak weakens the electrochemical gradient, while also transferring protons without the use of ATP-synthase such that no ATP is produced.
While the cell becomes starved of ATP, the ETC will overwork in an attempt to shuttle more and more electrons to ATP-synthase without success. The ETC regularly produces heat as the electrons transfer from one carrier to the next, and this overactivity will raise the body temperature as a result. Additionally, cells will adapt to utilizing fermentation as if in anaerobic conditions; this may cause a type B lactic acidosis in affected patients. [19]
Aspirin (Salicylic Acid)
- Salicylic acid is an uncoupler. Unique to salicylate poisoning, however, are signs of tinnitus and early respiratory alkalosis, which transitions to a mixed metabolic acidosis and respiratory alkalosis as the process progresses. Early treatment involves activated charcoal if presenting within 1 hour of ingestion, or sodium bicarbonate otherwise. [20]
Thermogenin
- Thermogenin, also known as uncoupling protein 1 (UCP1), is found in brown adipose tissue. Brown adipose tissue has many small lipid droplets and a high concentration of mitochondria (which provide the "brown" color), in contrast to white adipose tissue, which has a single droplet. This difference supports that brown fat is classically abundantly present in hibernating animals or newborns, who have delayed neurologic thermoregulation (ex. shivering) and are therefore at risk for hypothermia. These brown fat mitochondria contain more thermogenin than other cells, allowing for increased inner mitochondrial membrane disruption and proton leakage. [21] [22]
Oxidative Phosphorylation Inhibitors
Certain poisons can inhibit cellular oxidative phosphorylation such as rotenone, carboxin, antimycin A, cyanide, carbon monoxide (CO), sodium azide, and oligomycin. Rotenone inhibits complex I, carboxin inhibits complex II, antimycin A inhibits complex III, and cyanide and CO inhibit complex IV. Oligomycin inhibits ATP synthase. [23] [24]
Rotenone (and some barbiturates) – inhibits complex I (coenzyme Q binding site)
- Rotenone is a broadly used pesticide, but more often in the US as a piscicide (fish). Rotenone blocks complex I from passing electrons from the Fe-S clusters to ubiquinone. It is poorly absorbed through the skin, but rarely deadly as poisoning can cause vomiting and removal of the substance. However, purposeful ingestion can be fatal. [25] [26]
Carboxin – inhibits complex II (coenzyme Q binding site)
- Carboxin is a fungicide that is no longer in use because of newer, more broad-spectrum agents. Similar to rotenone, carboxin interferes with ubiquinone at the binding site.
Doxorubicin – coenzyme Q (theoretical)
- Doxorubicin is used in cancer chemotherapy, typically breast and bladder carcinomas, and lymphoma. A well-known side effect of doxorubicin is dilated cardiomyopathy. One proposed mechanism of causation is the generation of reactive oxygen species within myocardial tissue as the drug interferes with electron transfer by coenzyme Q. [27]
Antimycin A – inhibits complex III (cytochrome c reductase)
- Antimycin A is a piscicide that binds to cytochrome c reductase at the Qi binding site. This activity prevents ubiquinone from binding and accepting an electron, thereby blocking the recycling of ubiquinol (CoQH2) by the Q cycle.
Carbon Monoxide (CO) – inhibits complex IV (cytochrome c oxidase)
- Carbon monoxide binds to and inhibits cytochrome c oxidase (complex IV). In addition to the disruption of the ETC, carbon monoxide also binds to hemoglobin at an oxygen-binding site converting it to carboxyhemoglobin. In this state, oxygen is displaced from hemoglobin, effectively blocking delivery to body tissues. The cardiac and central nervous systems, both organ systems which are highly dependent on oxygen consumption, manifest the common signs of CO poisoning. Symptoms such as tachycardia, hypotension, or arrhythmias may couple with fatigue, headache, nausea, vomiting, and changes in vision. More serious cases may display seizure, coma, retinal hemorrhages, or a characteristic cherry-red blood hue of the skin, though more often useful on autopsy (caution is critical: some patients may appear "normal" rather than pale/dusky because of inadequate tissue oxygenation). [28]
- Sources of CO are paint strippers, house fires, wood-burning stoves, automobile exhaust, and other gasoline- or propane-fueled equipment. A CO saturation monitor can detect CO levels. Ratios of carboxyhemoglobin to hemoglobin greater than 10% are likely to show as symptomatic. Regular pulse oximetry devices read the percent of bound hemoglobin, irrespective of what is bound. Therefore, when CO is bound rather than O2, a patient's pulse Ox may still appear normal and cannot be used reliably. Instead, a co-oximeter should be used. Treatment for CO poisoning is to dissociate the bound CO with O2. Providing 100% supplemental oxygen via non-rebreather or administering hyperbaric oxygen are options. [29] [30] [31]
Cyanide (CN) – inhibits complex IV (cytochrome c oxidase)
- Cyanide also binds to and inhibits cytochrome c oxidase (complex IV). Similar symptoms as a result of tissue hypoxia can present in affected patients. In contrast, these patients tend to have hypoxia that is not responsive to supplemental O2 and an almond breath odor. Typical sources of cyanide include house fires (furniture or rugs), jewelry cleaning solutions, plastic or rubber manufacturing, iatrogenic from prescribed nitroprusside, or even some fruit seeds (apricots, peaches, apples).
- Treatment can include nitrites to oxidize hemoglobin iron from Fe2+ to Fe3+, also known as methemoglobin, a conformation that binds cyanide, preventing it from contacting the ETC. However, this prevents blood cells from transporting oxygen, therefore requiring further treatment with methylene blue to reduce Fe3+ back to Fe2+. Another option is administering hydroxocobalamin, a form of vitamin B12, or thiosulfate, although thiosulfate is not time efficient and typically requires combination therapy with nitrites. [32]
Oligomycin – inhibits ATP-synthase (complex V)
- Oligomycin is a macrolide antibiotic synthesized by Streptomyces species that inhibits the F0 subunit of ATP-synthase, preventing ATP production. Its predominant use is for research purposes. [33]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Electron Transport Chain graphic. Shows Inter-membrane space, inner membrane and matrix areas Illustration by Emma Gregory
Disclosure: Maria Ahmad declares no relevant financial relationships with ineligible companies.
Disclosure: Adam Wolberg declares no relevant financial relationships with ineligible companies.
Disclosure: Chadi Kahwaji declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Ahmad M, Wolberg A, Kahwaji CI. Biochemistry, Electron Transport Chain. [Updated 2023 Sep 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Biochemistry, Oxidative Phosphorylation. [StatPearls. 2024] Biochemistry, Oxidative Phosphorylation. Deshpande OA, Mohiuddin SS. StatPearls. 2024 Jan
- Electron transport and photophosphorylation by Photosystem I in vivo in plants and cyanobacteria. [Photosynth Res. 1993] Electron transport and photophosphorylation by Photosystem I in vivo in plants and cyanobacteria. Fork DC, Herbert SK. Photosynth Res. 1993 Jun; 36(3):149-68.
- Light-driven production of ATP catalysed by F0F1-ATP synthase in an artificial photosynthetic membrane. [Nature. 1998] Light-driven production of ATP catalysed by F0F1-ATP synthase in an artificial photosynthetic membrane. Steinberg-Yfrach G, Rigaud JL, Durantini EN, Moore AL, Gust D, Moore TA. Nature. 1998 Apr 2; 392(6675):479-82.
- Review Biogenesis of the bc(1) Complex of the Mitochondrial Respiratory Chain. [J Mol Biol. 2018] Review Biogenesis of the bc(1) Complex of the Mitochondrial Respiratory Chain. Ndi M, Marin-Buera L, Salvatori R, Singh AP, Ott M. J Mol Biol. 2018 Oct 19; 430(21):3892-3905. Epub 2018 May 4.
- Review Mitochondrial Respiratory Chain Complexes. [Subcell Biochem. 2018] Review Mitochondrial Respiratory Chain Complexes. Sousa JS, D'Imprima E, Vonck J. Subcell Biochem. 2018; 87:167-227.
Recent Activity
- Biochemistry, Electron Transport Chain - StatPearls Biochemistry, Electron Transport Chain - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

8.15: Electron Transport Chain
- Last updated
- Save as PDF
- Page ID 43600
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
You have just read about two pathways in cellular respiration—glycolysis and the citric acid cycle—that generate ATP. However, most of the ATP generated during the aerobic catabolism of glucose is not generated directly from these pathways. Rather, it is derived from a process that begins with moving electrons through a series of electron transporters that undergo redox reactions: the electron transport chain . This causes hydrogen ions to accumulate within the matrix space. Therefore, a concentration gradient forms in which hydrogen ions diffuse out of the matrix space by passing through ATP synthase. The current of hydrogen ions powers the catalytic action of ATP synthase, which phosphorylates ADP, producing ATP.
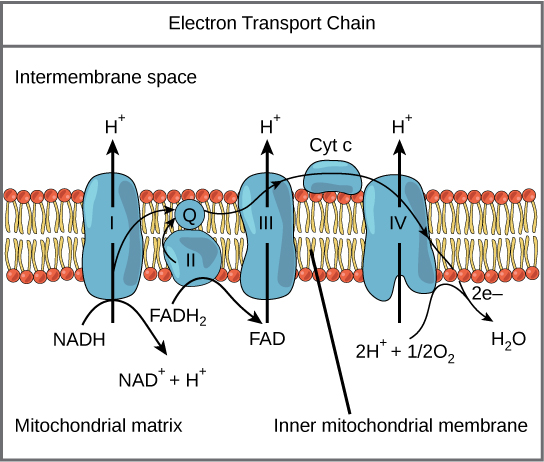
The electron transport chain (Figure 1) is the last component of aerobic respiration and is the only part of glucose metabolism that uses atmospheric oxygen. Oxygen continuously diffuses into plants; in animals, it enters the body through the respiratory system. Electron transport is a series of redox reactions that resemble a relay race or bucket brigade in that electrons are passed rapidly from one component to the next, to the endpoint of the chain where the electrons reduce molecular oxygen, producing water. There are four complexes composed of proteins, labeled I through IV in Figure 1, and the aggregation of these four complexes, together with associated mobile, accessory electron carriers, is called the electron transport chain. The electron transport chain is present in multiple copies in the inner mitochondrial membrane of eukaryotes and the plasma membrane of prokaryotes. Note, however, that the electron transport chain of prokaryotes may not require oxygen as some live in anaerobic conditions. The common feature of all electron transport chains is the presence of a proton pump to create a proton gradient across a membrane.
To start, two electrons are carried to the first complex aboard NADH. This complex, labeled I, is composed of flavin mononucleotide (FMN) and an iron-sulfur (Fe-S)-containing protein. FMN, which is derived from vitamin B 2 , also called riboflavin, is one of several prosthetic groups or co-factors in the electron transport chain. A prosthetic group is a non-protein molecule required for the activity of a protein. Prosthetic groups are organic or inorganic, non-peptide molecules bound to a protein that facilitate its function; prosthetic groups include co-enzymes, which are the prosthetic groups of enzymes. The enzyme in complex I is NADH dehydrogenase and is a very large protein, containing 45 amino acid chains. Complex I can pump four hydrogen ions across the membrane from the matrix into the intermembrane space, and it is in this way that the hydrogen ion gradient is established and maintained between the two compartments separated by the inner mitochondrial membrane.
Q and Complex II
Complex II directly receives FADH 2 , which does not pass through complex I. The compound connecting the first and second complexes to the third is ubiquinone (Q). The Q molecule is lipid soluble and freely moves through the hydrophobic core of the membrane. Once it is reduced, (QH 2 ), ubiquinone delivers its electrons to the next complex in the electron transport chain. Q receives the electrons derived from NADH from complex I and the electrons derived from FADH 2 from complex II, including succinate dehydrogenase. This enzyme and FADH 2 form a small complex that delivers electrons directly to the electron transport chain, bypassing the first complex. Since these electrons bypass and thus do not energize the proton pump in the first complex, fewer ATP molecules are made from the FADH 2 electrons. The number of ATP molecules ultimately obtained is directly proportional to the number of protons pumped across the inner mitochondrial membrane.
Complex III
The third complex is composed of cytochrome b, another Fe-S protein, Rieske center (2Fe-2S center), and cytochrome c proteins; this complex is also called cytochrome oxidoreductase. Cytochrome proteins have a prosthetic group of heme. The heme molecule is similar to the heme in hemoglobin, but it carries electrons, not oxygen. As a result, the iron ion at its core is reduced and oxidized as it passes the electrons, fluctuating between different oxidation states: Fe + + (reduced) and Fe + + + (oxidized). The heme molecules in the cytochromes have slightly different characteristics due to the effects of the different proteins binding them, giving slightly different characteristics to each complex. Complex III pumps protons through the membrane and passes its electrons to cytochrome c for transport to the fourth complex of proteins and enzymes (cytochrome c is the acceptor of electrons from Q; however, whereas Q carries pairs of electrons, cytochrome c can accept only one at a time).
The fourth complex is composed of cytochrome proteins c, a, and a 3 . This complex contains two heme groups (one in each of the two cytochromes, a, and a 3 ) and three copper ions (a pair of Cu A and one Cu B in cytochrome a 3 ). The cytochromes hold an oxygen molecule very tightly between the iron and copper ions until the oxygen is completely reduced. The reduced oxygen then picks up two hydrogen ions from the surrounding medium to make water (H 2 O). The removal of the hydrogen ions from the system contributes to the ion gradient used in the process of chemiosmosis.
Chemiosmosis
In chemiosmosis, the free energy from the series of redox reactions just described is used to pump hydrogen ions (protons) across the membrane. The uneven distribution of H + ions across the membrane establishes both concentration and electrical gradients (thus, an electrochemical gradient), owing to the hydrogen ions’ positive charge and their aggregation on one side of the membrane.
If the membrane were open to diffusion by the hydrogen ions, the ions would tend to diffuse back across into the matrix, driven by their electrochemical gradient. Recall that many ions cannot diffuse through the nonpolar regions of phospholipid membranes without the aid of ion channels. Similarly, hydrogen ions in the matrix space can only pass through the inner mitochondrial membrane through an integral membrane protein called ATP synthase (Figure 2). This complex protein acts as a tiny generator, turned by the force of the hydrogen ions diffusing through it, down their electrochemical gradient. The turning of parts of this molecular machine facilitates the addition of a phosphate to ADP, forming ATP, using the potential energy of the hydrogen ion gradient.
Practice Question
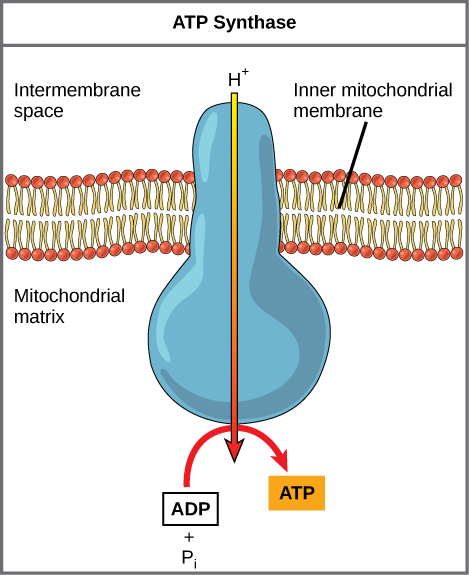
Dinitrophenol (DNP) is an uncoupler that makes the inner mitochondrial membrane leaky to protons. It was used until 1938 as a weight-loss drug. What effect would you expect DNP to have on the change in pH across the inner mitochondrial membrane? Why do you think this might be an effective weight-loss drug?
[practice-area rows=”2″][/practice-area] [reveal-answer q=”346232″] Show Answer [/reveal-answer] [hidden-answer a=”346232″]After DNP poisoning, the electron transport chain can no longer form a proton gradient, and ATP synthase can no longer make ATP. DNP is an effective diet drug because it uncouples ATP synthesis; in other words, after taking it, a person obtains less energy out of the food he or she eats. Interestingly, one of the worst side effects of this drug is hyperthermia, or overheating of the body. Since ATP cannot be formed, the energy from electron transport is lost as heat.[/hidden-answer]
Chemiosmosis (Figure 3) is used to generate 90 percent of the ATP made during aerobic glucose catabolism; it is also the method used in the light reactions of photosynthesis to harness the energy of sunlight in the process of photophosphorylation. Recall that the production of ATP using the process of chemiosmosis in mitochondria is called oxidative phosphorylation. The overall result of these reactions is the production of ATP from the energy of the electrons removed from hydrogen atoms. These atoms were originally part of a glucose molecule. At the end of the pathway, the electrons are used to reduce an oxygen molecule to oxygen ions. The extra electrons on the oxygen attract hydrogen ions (protons) from the surrounding medium, and water is formed.
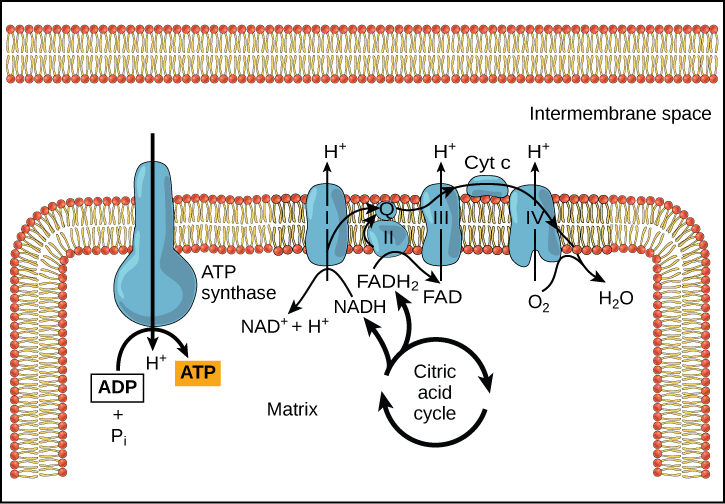
Cyanide inhibits cytochrome c oxidase, a component of the electron transport chain. If cyanide poisoning occurs, would you expect the pH of the intermembrane space to increase or decrease? What effect would cyanide have on ATP synthesis?
[practice-area rows=”2″][/practice-area] [reveal-answer q=”26135″] Show Answer [/reveal-answer] [hidden-answer a=”26135″]After cyanide poisoning, the electron transport chain can no longer pump electrons into the intermembrane space. The pH of the intermembrane space would increase, the pH gradient would decrease, and ATP synthesis would stop.[/hidden-answer]
The number of ATP molecules generated from the catabolism of glucose varies. For example, the number of hydrogen ions that the electron transport chain complexes can pump through the membrane varies between species. Another source of variance stems from the shuttle of electrons across the membranes of the mitochondria. (The NADH generated from glycolysis cannot easily enter mitochondria.) Thus, electrons are picked up on the inside of mitochondria by either NAD + or FAD + . As you have learned earlier, these FAD + molecules can transport fewer ions; consequently, fewer ATP molecules are generated when FAD + acts as a carrier. NAD + is used as the electron transporter in the liver and FAD + acts in the brain.
Another factor that affects the yield of ATP molecules generated from glucose is the fact that intermediate compounds in these pathways are used for other purposes. Glucose catabolism connects with the pathways that build or break down all other biochemical compounds in cells, and the result is somewhat messier than the ideal situations described thus far. For example, sugars other than glucose are fed into the glycolytic pathway for energy extraction. Moreover, the five-carbon sugars that form nucleic acids are made from intermediates in glycolysis. Certain nonessential amino acids can be made from intermediates of both glycolysis and the citric acid cycle. Lipids, such as cholesterol and triglycerides, are also made from intermediates in these pathways, and both amino acids and triglycerides are broken down for energy through these pathways. Overall, in living systems, these pathways of glucose catabolism extract about 34 percent of the energy contained in glucose.
Learning Objectives
The electron transport chain is the portion of aerobic respiration that uses free oxygen as the final electron acceptor of the electrons removed from the intermediate compounds in glucose catabolism. The electron transport chain is composed of four large, multiprotein complexes embedded in the inner mitochondrial membrane and two small diffusible electron carriers shuttling electrons between them. The electrons are passed through a series of redox reactions, with a small amount of free energy used at three points to transport hydrogen ions across a membrane. This process contributes to the gradient used in chemiosmosis. The electrons passing through the electron transport chain gradually lose energy, High-energy electrons donated to the chain by either NADH or FADH 2 complete the chain, as low-energy electrons reduce oxygen molecules and form water. The level of free energy of the electrons drops from about 60 kcal/mol in NADH or 45 kcal/mol in FADH 2 to about 0 kcal/mol in water. The end products of the electron transport chain are water and ATP. A number of intermediate compounds of the citric acid cycle can be diverted into the anabolism of other biochemical molecules, such as nonessential amino acids, sugars, and lipids. These same molecules can serve as energy sources for the glucose pathways.
Contributors and Attributions
- Biology. Provided by : OpenStax CNX. Located at : http://cnx.org/contents/[email protected] . License : CC BY: Attribution . License Terms : Download for free at http://cnx.org/contents/[email protected]
8.2 The Light-Dependent Reaction of Photosynthesis
Learning objectives.
In this section, you will explore the following questions:
- How do plants absorb energy from sunlight?
- What are the differences between short and long wavelengths of light? What wavelengths are used in photosynthesis?
- How and where does photosynthesis occur within a plant?
Connection for AP ® Courses
Photosynthesis consists of two stages: the light-dependent reactions and the light-independent reactions or Calvin cycle. The light-dependent reactions occur when light is available. The overall equation for photosynthesis shows that is it a redox reaction; carbon dioxide is reduced and water is oxidized to produce oxygen:
The light-dependent reactions occur in the thylakoid membranes of chloroplasts, whereas the Calvin cycle occurs in the stroma of chloroplasts. Embedded in the thylakoid membranes are two photosystems (PS I and PS II), which are complexes of pigments that capture solar energy. Chlorophylls a and b absorb violet, blue, and red wavelengths from the visible light spectrum and reflect green. The carotenoid pigments absorb violet-blue-green light and reflect yellow-to-orange light. Environmental factors such as day length and temperature influence which pigments predominant at certain times of the year. Although the two photosystems run simultaneously, it is easier to explore them separately. Let’s begin with photosystem II.
A photon of light strikes the antenna pigments of PS II to initiate photosynthesis. In the noncyclic pathway, PS II captures photons at a slightly higher energy level than PS I. (Remember that shorter wavelengths of light carry more energy.) The absorbed energy travels to the reaction center of the antenna pigment that contains chlorophyll a and boosts chlorophyll a electrons to a higher energy level. The electrons are accepted by a primary electron acceptor protein and then pass to the electron transport chain also embedded in the thylakoid membrane. The energy absorbed in PS II is enough to oxidize (split) water, releasing oxygen into the atmosphere; the electrons released from the oxidation of water replace the electrons that were boosted from the reaction center chlorophyll. As the electrons from the reaction center chlorophyll pass through the series of electron carrier proteins, hydrogen ions (H + ) are pumped across the membrane via chemiosmosis into the interior of the thylakoid. (If this sounds familiar, it should. We studied chemiosmosis in our exploration of cellular respiration in Cellular Respiration.) This action builds up a high concentration of H+ ions, and as they flow through ATP synthase, molecules of ATP are formed. These molecules of ATP will be used to provide free energy for the synthesis of carbohydrate in the Calvin cycle, the second stage of photosynthesis. The electron transport chain connects PS II and PS I. Similar to the events occurring in PS II, this second photosystem absorbs a second photon of light, resulting in the formation of a molecule of NADPH from NADP + . The energy carried in NADPH also is used to power the chemical reactions of the Calvin cycle.
Information presented and the examples highlighted in the section support concepts and learning objectives outlined in Big Idea 2 of the AP ® Biology Curriculum Framework, as shown in the table. The learning objectives listed in the Curriculum Framework provide a transparent foundation for the AP ® Biology course, an inquiry-based laboratory experience, instructional activities, and AP ® exam questions. A learning objective merges required content with one or more of the seven science practices.
Teacher Support
This section deals with the first half of photosynthesis. These reactions capture light energy and store it in chemicals for short periods of time to fuel the second half of photosynthesis. This is also where free oxygen can be released, but carbon dioxide is not captured or fixed.
The Science Practice Challenge Questions contain additional test questions for this section that will help you prepare for the AP exam. These questions address the following standards: [APLO 2.5][APLO 2.16][APLO 2.18][APLO 1.9][APLO 1.32][APLO 4.14][APLO 2.2][APLO 2.3][APLO 2.23][APLO 1.15][APLO 1.29]
How can light be used to make food? When a person turns on a lamp, electrical energy becomes light energy. Like all other forms of kinetic energy, light can travel, change form, and be harnessed to do work. In the case of photosynthesis, light energy is converted into chemical energy, which photoautotrophs use to build carbohydrate molecules ( Figure 8.9 ). However, autotrophs only use a few specific components of sunlight.
What Is Light Energy?
Everybody knows what a rainbow is, but some students may not be able to connect it to actual light sources. Obtain some way of refracting light, such as a prism, and use it to separate the components of several light sources, such as an older, incandescent light bulb, a new fluorescent type of light bulb and actual sunlight.
When discussing the electromagnetic spectrum, include the fact that when someone sets a radio station to its number, such as 92.1 or 1450 on the dial, they are really setting the radio to the specific wavelength of spectrum used by the station.
The sun emits an enormous amount of electromagnetic radiation (solar energy). Humans can see only a fraction of this energy, which portion is therefore referred to as “visible light.” The manner in which solar energy travels is described as waves. Scientists can determine the amount of energy of a wave by measuring its wavelength , the distance between consecutive points of a wave. A single wave is measured from two consecutive points, such as from crest to crest or from trough to trough ( Figure 8.10 ).
Visible light constitutes only one of many types of electromagnetic radiation emitted from the sun and other stars. Scientists differentiate the various types of radiant energy from the sun within the electromagnetic spectrum. The electromagnetic spectrum is the range of all possible frequencies of radiation ( Figure 8.11 ). The difference between wavelengths relates to the amount of energy carried by them.
Each type of electromagnetic radiation travels at a particular wavelength. The longer the wavelength (or the more stretched out it appears in the diagram), the less energy is carried. Short, tight waves carry the most energy. This may seem illogical, but think of it in terms of a piece of moving a heavy rope. It takes little effort by a person to move a rope in long, wide waves. To make a rope move in short, tight waves, a person would need to apply significantly more energy.
The electromagnetic spectrum ( Figure 8.11 ) shows several types of electromagnetic radiation originating from the sun, including X-rays and ultraviolet (UV) rays. The higher-energy waves can penetrate tissues and damage cells and DNA, explaining why both X-rays and UV rays can be harmful to living organisms.
Absorption of Light
Stress the differences in the amount of energy at each wavelength, and the usefulness of the wavelengths for energy capture. Discuss what is in a “grow light” (artificial light source for plants grown indoors).
Light energy initiates the process of photosynthesis when pigments absorb the light. Organic pigments, whether in the human retina or the chloroplast thylakoid, have a narrow range of energy levels that they can absorb. Energy levels lower than those represented by red light are insufficient to raise an orbital electron to a populatable, excited (quantum) state. Energy levels higher than those in blue light will physically tear the molecules apart, called bleaching. So retinal pigments can only “see” (absorb) 700 nm to 400 nm light, which is therefore called visible light. For the same reasons, plants pigment molecules absorb only light in the wavelength range of 700 nm to 400 nm; plant physiologists refer to this range for plants as photosynthetically active radiation.
The visible light seen by humans as white light actually exists in a rainbow of colors. Certain objects, such as a prism or a drop of water, disperse white light to reveal the colors to the human eye. The visible light portion of the electromagnetic spectrum shows the rainbow of colors, with violet and blue having shorter wavelengths, and therefore higher energy. At the other end of the spectrum toward red, the wavelengths are longer and have lower energy ( Figure 8.12 ).
Understanding Pigments
Concentrate on the types and functions of chlorophylls and carotenoids that are found in leaves. Discuss how all of them are always there even though they are not visible in the summer. They are visible in the fall.
Ask the class what color coats people tend to wear in the summer and in the winter. Discuss why they do this.
Different kinds of pigments exist, and each absorbs only certain wavelengths (colors) of visible light. Pigments reflect or transmit the wavelengths they cannot absorb, making them appear in the corresponding color.
Chlorophylls and carotenoids are the two major classes of photosynthetic pigments found in plants and algae; each class has multiple types of pigment molecules. There are five major chlorophylls: a , b , c and d and a related molecule found in prokaryotes called bacteriochlorophyll. Chlorophyll a and chlorophyll b are found in higher plant chloroplasts and will be the focus of the following discussion.
With dozens of different forms, carotenoids are a much larger group of pigments. The carotenoids found in fruit—such as the red of tomato (lycopene), the yellow of corn seeds (zeaxanthin), or the orange of an orange peel (β-carotene)—are used as advertisements to attract seed dispersers. In photosynthesis, carotenoids function as photosynthetic pigments that are very efficient molecules for the disposal of excess energy. When a leaf is exposed to full sun, the light-dependent reactions are required to process an enormous amount of energy; if that energy is not handled properly, it can do significant damage. Therefore, many carotenoids reside in the thylakoid membrane, absorb excess energy, and safely dissipate that energy as heat.
Each type of pigment can be identified by the specific pattern of wavelengths it absorbs from visible light, which is the absorption spectrum . The graph in Figure 8.13 shows the absorption spectra for chlorophyll a , chlorophyll b , and a type of carotenoid pigment called β-carotene (which absorbs blue and green light). Notice how each pigment has a distinct set of peaks and troughs, revealing a highly specific pattern of absorption. Chlorophyll a absorbs wavelengths from either end of the visible spectrum (blue and red), but not green. Because green is reflected or transmitted, chlorophyll appears green. Carotenoids absorb in the short-wavelength blue region, and reflect the longer yellow, red, and orange wavelengths.
Many photosynthetic organisms have a mixture of pigments; using them, the organism can absorb energy from a wider range of wavelengths. Not all photosynthetic organisms have full access to sunlight. Some organisms grow underwater where light intensity and quality decrease and change with depth. Other organisms grow in competition for light. Plants on the rainforest floor must be able to absorb any bit of light that comes through, because the taller trees absorb most of the sunlight and scatter the remaining solar radiation ( Figure 8.14 ).
When studying a photosynthetic organism, scientists can determine the types of pigments present by generating absorption spectra. An instrument called a spectrophotometer can differentiate which wavelengths of light a substance can absorb. Spectrophotometers measure transmitted light and compute from it the absorption. By extracting pigments from leaves and placing these samples into a spectrophotometer, scientists can identify which wavelengths of light an organism can absorb. Additional methods for the identification of plant pigments include various types of chromatography that separate the pigments by their relative affinities to solid and mobile phases.
How Light-Dependent Reactions Work
Photosystems I and II can be confusing. Obtain diagrams of both systems and use them to go through the steps of the pathways. Discuss why some plants use the cyclic form of the systems and some the linear form. Discuss why oxygen is released during one pathway, but not the other.
The overall function of light-dependent reactions is to convert solar energy into chemical energy in the form of NADPH and ATP. This chemical energy supports the light-independent reactions and fuels the assembly of sugar molecules. The light-dependent reactions are depicted in Figure 8.15 . Protein complexes and pigment molecules work together to produce NADPH and ATP.
The actual step that converts light energy into chemical energy takes place in a multiprotein complex called a photosystem , two types of which are found embedded in the thylakoid membrane, photosystem II (PSII) and photosystem I (PSI) ( Figure 8.16 ). The two complexes differ on the basis of what they oxidize (that is, the source of the low-energy electron supply) and what they reduce (the place to which they deliver their energized electrons).
Both photosystems have the same basic structure; a number of antenna pigments to which the chlorophyll molecules are bound surround the reaction center where the photochemistry takes place. Each photosystem is serviced by the light-harvesting complex , which passes energy from sunlight to the reaction center; it consists of multiple antenna pigments that contain a mixture of 300–400 chlorophyll a and b molecules as well as other pigments like carotenoids. The absorption of a single photon or distinct quantity or “packet” of light by any of the chlorophylls pushes that molecule into an excited state. In short, the light energy has now been captured by biological molecules but is not stored in any useful form yet. The energy is transferred from chlorophyll to chlorophyll until eventually (after about a millionth of a second), it is delivered to the reaction center. Up to this point, only energy has been transferred between molecules, not electrons.

Visual Connection
- carbon dioxide
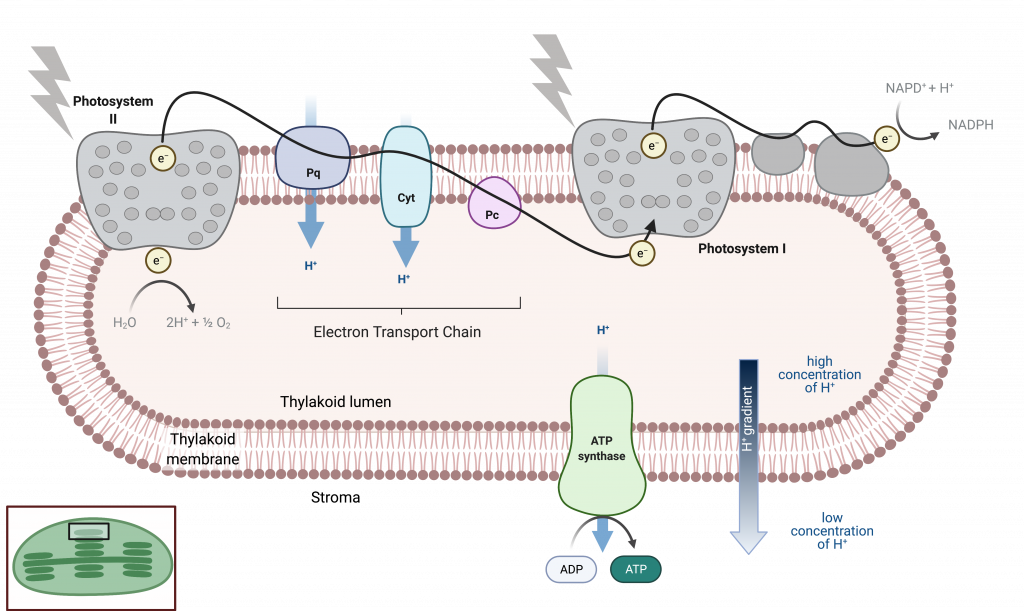
The reaction center contains a pair of chlorophyll a molecules with a special property. Those two chlorophylls can undergo oxidation upon excitation; they can actually give up an electron in a process called a photoact . It is at this step in the reaction center, this step in photosynthesis, that light energy is converted into an excited electron. All of the subsequent steps involve getting that electron onto the energy carrier NADPH for delivery to the Calvin cycle where the electron is deposited onto carbon for long-term storage in the form of a carbohydrate.PSII and PSI are two major components of the photosynthetic electron transport chain , which also includes the cytochrome complex . The cytochrome complex, an enzyme composed of two protein complexes, transfers the electrons from the carrier molecule plastoquinone (Pq) to the protein plastocyanin (Pc), thus enabling both the transfer of protons across the thylakoid membrane and the transfer of electrons from PSII to PSI.
The reaction center of PSII (called P680 ) delivers its high-energy electrons, one at the time, to the primary electron acceptor , and through the electron transport chain (Pq to cytochrome complex to plastocyanine) to PSI. P680’s missing electron is replaced by extracting a low-energy electron from water; thus, water is split and PSII is re-reduced after every photoact. Splitting one H 2 O molecule releases two electrons, two hydrogen atoms, and one atom of oxygen. Splitting two molecules is required to form one molecule of diatomic O 2 gas. About 10 percent of the oxygen is used by mitochondria in the leaf to support oxidative phosphorylation. The remainder escapes to the atmosphere where it is used by aerobic organisms to support respiration.
As electrons move through the proteins that reside between PSII and PSI, they lose energy. That energy is used to move hydrogen atoms from the stromal side of the membrane to the thylakoid lumen. Those hydrogen atoms, plus the ones produced by splitting water, accumulate in the thylakoid lumen and will be used to synthesize ATP in a later step. Because the electrons have lost energy prior to their arrival at PSI, they must be re-energized by PSI, hence, another photon is absorbed by the PSI antenna. That energy is relayed to the PSI reaction center (called P700 ). P700 is oxidized and sends a high-energy electron to NADP + to form NADPH. Thus, PSII captures the energy to create proton gradients to make ATP, and PSI captures the energy to reduce NADP + into NADPH. The two photosystems work in concert, in part, to guarantee that the production of NADPH will roughly equal the production of ATP. Other mechanisms exist to fine tune that ratio to exactly match the chloroplast’s constantly changing energy needs.
Generating an Energy Carrier: ATP
Discuss the similarities between ATP production in the light dependent reactions and in cellular respiration.
As in the intermembrane space of the mitochondria during cellular respiration, the buildup of hydrogen ions inside the thylakoid lumen creates a concentration gradient. The passive diffusion of hydrogen ions from high concentration (in the thylakoid lumen) to low concentration (in the stroma) is harnessed to create ATP, just as in the electron transport chain of cellular respiration. The ions build up energy because of diffusion and because they all have the same electrical charge, repelling each other.
To release this energy, hydrogen ions will rush through any opening, similar to water jetting through a hole in a dam. In the thylakoid, that opening is a passage through a specialized protein channel called the ATP synthase. The energy released by the hydrogen ion stream allows ATP synthase to attach a third phosphate group to ADP, which forms a molecule of ATP ( Figure 8.16 ). The flow of hydrogen ions through ATP synthase is called chemiosmosis because the ions move from an area of high to an area of low concentration through a semi-permeable structure.
Link to Learning
Visit this site and click through the animation to view the process of photosynthesis within a leaf.
- Electrons from PS I cause the reduction of NADPH to NADP + .
- Electrons from PSII cause the reduction of NADP + to NADPH.
- Electrons from PS I cause the reduction of NADP + to NADPH.
- Electrons are gained which causes the oxidation of NADP + .
Everyday Connection for AP® Courses
If the stomata were sealed, what would happen to oxygen ( O 2 ) and carbon dioxide ( CO 2 ) levels in a photosynthesizing leaf?
- O 2 levels would increase and CO 2 levels would decrease.
- CO 2 levels would increase and O 2 levels would decrease.
- O 2 and CO 2 levels would both decrease.
- O 2 and CO 2 levels would both increase.
Science Practice Connection for AP® Courses
Think about it.
On a hot, dry day, plants close their stomata to conserve water. Predict the impact of this on photosynthesis and justify your prediction.
The Think About It question is an application of Learning Objective 4.4 and Science Practice 6.4 because students are making a prediction about how interactions of cellular organelles and structures affect the rate of photosynthesis.
Possible answer:
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/biology-ap-courses/pages/1-introduction
- Authors: Julianne Zedalis, John Eggebrecht
- Publisher/website: OpenStax
- Book title: Biology for AP® Courses
- Publication date: Mar 8, 2018
- Location: Houston, Texas
- Book URL: https://openstax.org/books/biology-ap-courses/pages/1-introduction
- Section URL: https://openstax.org/books/biology-ap-courses/pages/8-2-the-light-dependent-reaction-of-photosynthesis
© Apr 26, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
22.4 Electron Transport Chains in Respiration and Photosynthesis
Chemiosmosis produces ATP in both chloroplasts and mitochondria using the same mechanism. In both cases, an electron transport chain maintains the hydrogen ion gradient that powers chemiosmosis. There are differences between the electron transport chains in the mitochondrion and the chloroplast, however. The original source of the electrons, the specific components of the electron transport chain, and the final electron acceptor all differ.
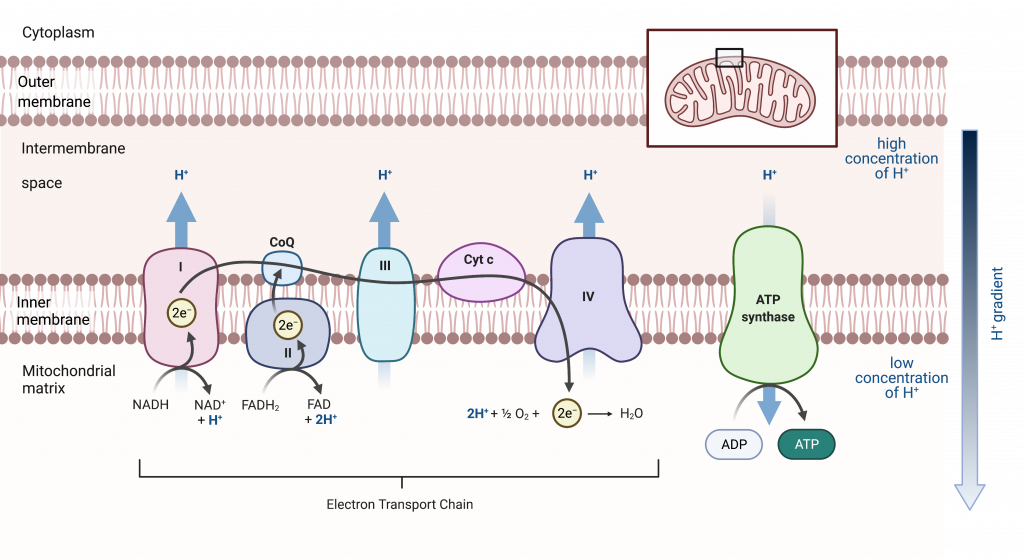
The Electron Transport Chain in the Mitochondrion
The electron transport chain in mitochondria is the last component of aerobic respiration and is the only part of glucose metabolism that directly uses atmospheric oxygen. In each mitochondrion of a eukaryotic cell, the electron transport chain is present in many copies within the inner mitochondrial membrane. Electrons are shuttled to the first complex of the electron transport chain by NADH and FADH 2 , which are the reduced forms of the two electron carriers used in respiration.
The electrons are then passed from protein complex to protein complex. Each complex has a greater electronegativity than the one before it such that energy is release with each transfer. Finally, electrons are passed to oxygen, which is more electronegative than any of the protein complexes. Two electrons combine with an oxygen atom and two hydrogen ions to form water, which is a byproduct of aerobic respiration.
This is the reason that organisms like us require oxygen to live. Without oxygen, the electron transport chain would not function, the hydrogen ion gradient between the intermembrane space and the matrix would equalize, and most ATP production would cease. Cessation of ATP production is incompatible with life.
The Electron Transport Chain in the Chloroplast
Photosynthesis functions as a series of two major steps, the light-dependent reactions and the Calvin cycle. The light-dependent reactions transform light energy into the chemical energy of ATP and NADPH. The Calvin cycle then uses these molecules to build a carbohydrate. ATP is produced via chemiosmosis, the same mechanism as in cellular respiration; thus the light-dependent reactions include an electron transport chain.
As in the intermembrane space of the mitochondria during cellular respiration, the buildup of hydrogen ions inside the thylakoid lumen creates a concentration gradient. The passive diffusion of hydrogen ions from high concentration (in the thylakoid lumen) to low concentration (in the stroma) is harnessed to create ATP.
Comparing the Electron Transport Chain in the Mitochondrion and Chloroplast
The electron transport chains in the mitochondrion and chloroplast have the same function — to maintain a gradient of hydrogen ions that can be used to power the endergonic process of producing ATP by chemiosmosis. There are differences between the two processes, which are summarized below.
process in which organisms convert energy in the presence of oxygen
ability of an atom to attract electrons
College Biology I Copyright © by Melissa Hardy is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
8. Photosynthesis
The light-dependent reactions of photosynthesis, learning objectives.
By the end of this section, you will be able to do the following:
- Explain how plants absorb energy from sunlight
- Describe short and long wavelengths of light
- Describe how and where photosynthesis takes place within a plant
How can light energy be used to make food? When a person turns on a lamp, electrical energy becomes light energy. Like all other forms of kinetic energy, light can travel, change form, and be harnessed to do work. In the case of photosynthesis, light energy is converted into chemical energy, which photoautotrophs use to build basic carbohydrate molecules ( (Figure) ). However, autotrophs only use a few specific wavelengths of sunlight.

Figure 1. Photoautotrophs can capture visible light energy in specific wavelengths from the sun, converting it into the chemical energy used to build food molecules. (credit: Gerry Atwell)
What Is Light Energy?
The sun emits an enormous amount of electromagnetic radiation (solar energy in a spectrum from very short gamma rays to very long radio waves). Humans can see only a tiny fraction of this energy, which we refer to as “visible light.” The manner in which solar energy travels is described as waves. Scientists can determine the amount of energy of a wave by measuring its wavelength (shorter wavelengths are more powerful than longer wavelengths)—the distance between consecutive crest points of a wave. Therefore, a single wave is measured from two consecutive points, such as from crest to crest or from trough to trough ( (Figure) ).

Figure 2. The wavelength of a single wave is the distance between two consecutive points of similar position (two crests or two troughs) along the wave.
Visible light constitutes only one of many types of electromagnetic radiation emitted from the sun and other stars. Scientists differentiate the various types of radiant energy from the sun within the electromagnetic spectrum. The electromagnetic spectrum is the range of all possible frequencies of radiation ( (Figure) ). The difference between wavelengths relates to the amount of energy carried by them.

Figure 3. The sun emits energy in the form of electromagnetic radiation. This radiation exists at different wavelengths, each of which has its own characteristic energy. All electromagnetic radiation, including visible light, is characterized by its wavelength.
Each type of electromagnetic radiation travels at a particular wavelength. The longer the wavelength, the less energy it carries. Short, tight waves carry the most energy. This may seem illogical, but think of it in terms of a piece of moving heavy rope. It takes little effort by a person to move a rope in long, wide waves. To make a rope move in short, tight waves, a person would need to apply significantly more energy.
The electromagnetic spectrum ( (Figure) ) shows several types of electromagnetic radiation originating from the sun, including X-rays and ultraviolet (UV) rays. The higher-energy waves can penetrate tissues and damage cells and DNA, which explains why both X-rays and UV rays can be harmful to living organisms.
Absorption of Light
Light energy initiates the process of photosynthesis when pigments absorb specific wavelengths of visible light. Organic pigments, whether in the human retina or the chloroplast thylakoid, have a narrow range of energy levels that they can absorb. Energy levels lower than those represented by red light are insufficient to raise an orbital electron to a excited (quantum) state. Energy levels higher than those in blue light will physically tear the molecules apart, in a process called bleaching. Our retinal pigments can only “see” (absorb) wavelengths between 700 nm and 400 nm of light, a spectrum that is therefore called visible light. For the same reasons, plants, pigment molecules absorb only light in the wavelength range of 700 nm to 400 nm; plant physiologists refer to this range for plants as photosynthetically active radiation.
The visible light seen by humans as white light actually exists in a rainbow of colors. Certain objects, such as a prism or a drop of water, disperse white light to reveal the colors to the human eye. The visible light portion of the electromagnetic spectrum shows the rainbow of colors, with violet and blue having shorter wavelengths, and therefore higher energy. At the other end of the spectrum toward red, the wavelengths are longer and have lower energy ( (Figure) ).

Figure 4. The colors of visible light do not carry the same amount of energy. Violet has the shortest wavelength and therefore carries the most energy, whereas red has the longest wavelength and carries the least amount of energy. (credit: modification of work by NASA)
Understanding Pigments
Different kinds of pigments exist, and each absorbs only specific wavelengths (colors) of visible light. Pigments reflect or transmit the wavelengths they cannot absorb, making them appear a mixture of the reflected or transmitted light colors.
Chlorophylls and carotenoids are the two major classes of photosynthetic pigments found in plants and algae; each class has multiple types of pigment molecules. There are five major chlorophylls: a , b , c and d and a related molecule found in prokaryotes called bacteriochlorophyll . Chlorophyll a and chlorophyll b are found in higher plant chloroplasts and will be the focus of the following discussion.
With dozens of different forms, carotenoids are a much larger group of pigments. The carotenoids found in fruit—such as the red of tomato (lycopene), the yellow of corn seeds (zeaxanthin), or the orange of an orange peel (β-carotene)—are used as advertisements to attract seed dispersers. In photosynthesis, carotenoids function as photosynthetic pigments that are very efficient molecules for the disposal of excess energy. When a leaf is exposed to full sun, the light-dependent reactions are required to process an enormous amount of energy; if that energy is not handled properly, it can do significant damage. Therefore, many carotenoids reside in the thylakoid membrane, absorb excess energy, and safely dissipate that energy as heat.
Each type of pigment can be identified by the specific pattern of wavelengths it absorbs from visible light: This is termed the absorption spectrum. The graph in (Figure) shows the absorption spectra for chlorophyll a , chlorophyll b , and a type of carotenoid pigment called β-carotene (which absorbs blue and green light). Notice how each pigment has a distinct set of peaks and troughs, revealing a highly specific pattern of absorption. Chlorophyll a absorbs wavelengths from either end of the visible spectrum (blue and red), but not green. Because green is reflected or transmitted, chlorophyll appears green. Carotenoids absorb in the short-wavelength blue region, and reflect the longer yellow, red, and orange wavelengths.

Figure 5. (a) Chlorophyll a, (b) chlorophyll b, and (c) β-carotene are hydrophobic organic pigments found in the thylakoid membrane. Chlorophyll a and b, which are identical except for the part indicated in the red box, are responsible for the green color of leaves. β-carotene is responsible for the orange color in carrots. Each pigment has (d) a unique absorbance spectrum.
Many photosynthetic organisms have a mixture of pigments, and by using these pigments, the organism can absorb energy from a wider range of wavelengths. Not all photosynthetic organisms have full access to sunlight. Some organisms grow underwater where light intensity and quality decrease and change with depth. Other organisms grow in competition for light. Plants on the rainforest floor must be able to absorb any bit of light that comes through, because the taller trees absorb most of the sunlight and scatter the remaining solar radiation ( (Figure) ).

Figure 6. Plants that commonly grow in the shade have adapted to low levels of light by changing the relative concentrations of their chlorophyll pigments. (credit: Jason Hollinger)
When studying a photosynthetic organism, scientists can determine the types of pigments present by generating absorption spectra. An instrument called a spectrophotometer can differentiate which wavelengths of light a substance can absorb. Spectrophotometers measure transmitted light and compute from it the absorption. By extracting pigments from leaves and placing these samples into a spectrophotometer, scientists can identify which wavelengths of light an organism can absorb. Additional methods for the identification of plant pigments include various types of chromatography that separate the pigments by their relative affinities to solid and mobile phases.
How Light-Dependent Reactions Work
The overall function of light-dependent reactions is to convert solar energy into chemical energy in the form of NADPH and ATP. This chemical energy supports the light-independent reactions and fuels the assembly of sugar molecules. The light-dependent reactions are depicted in (Figure) . Protein complexes and pigment molecules work together to produce NADPH and ATP. The numbering of the photosystems is derived from the order in which they were discovered, not in the order of the transfer of electrons.

Figure 7. A photosystem consists of 1) a light-harvesting complex and 2) a reaction center. Pigments in the light-harvesting complex pass light energy to two special chlorophyll a molecules in the reaction center. The light excites an electron from the chlorophyll a pair, which passes to the primary electron acceptor. The excited electron must then be replaced. In (a) photosystem II, the electron comes from the splitting of water, which releases oxygen as a waste product. In (b) photosystem I, the electron comes from the chloroplast electron transport chain discussed below.
The actual step that converts light energy into chemical energy takes place in a multiprotein complex called a photosystem, two types of which are found embedded in the thylakoid membrane: photosystem II (PSII) and photosystem I (PSI) ( (Figure) ). The two complexes differ on the basis of what they oxidize (that is, the source of the low-energy electron supply) and what they reduce (the place to which they deliver their energized electrons).
Both photosystems have the same basic structure; a number of antenna proteins to which the chlorophyll molecules are bound surround the reaction center where the photochemistry takes place. Each photosystem is serviced by the light-harvesting complex, which passes energy from sunlight to the reaction center; it consists of multiple antenna proteins that contain a mixture of 300 to 400 chlorophyll a and b molecules as well as other pigments like carotenoids. The absorption of a single photon or distinct quantity or “packet” of light by any of the chlorophylls pushes that molecule into an excited state. In short, the light energy has now been captured by biological molecules but is not stored in any useful form yet. The energy is transferred from chlorophyll to chlorophyll until eventually (after about a millionth of a second), it is delivered to the reaction center. Up to this point, only energy has been transferred between molecules, not electrons.
Art Connection

Figure 8. In the photosystem II (PSII) reaction center, energy from sunlight is used to extract electrons from water. The electrons travel through the chloroplast electron transport chain to photosystem I (PSI), which reduces NADP+ to NADPH. The electron transport chain moves protons across the thylakoid membrane into the lumen. At the same time, splitting of water adds protons to the lumen, and reduction of NADPH removes protons from the stroma. The net result is a low pH in the thylakoid lumen, and a high pH in the stroma. ATP synthase uses this electrochemical gradient to make ATP.
What is the initial source of electrons for the chloroplast electron transport chain?
- carbon dioxide
The reaction center contains a pair of chlorophyll a molecules with a special property. Those two chlorophylls can undergo oxidation upon excitation; they can actually give up an electron in a process called a photoact. It is at this step in the reaction center during photosynthesis that light energy is converted into an excited electron. All of the subsequent steps involve getting that electron onto the energy carrier NADPH for delivery to the Calvin cycle where the electron is deposited onto carbon for long-term storage in the form of a carbohydrate. PSII and PSI are two major components of the photosynthetic electron transport chain, which also includes the cytochrome complex . The cytochrome complex, an enzyme composed of two protein complexes, transfers the electrons from the carrier molecule plastoquinone (Pq) to the protein plastocyanin (Pc), thus enabling both the transfer of protons across the thylakoid membrane and the transfer of electrons from PSII to PSI.
The reaction center of PSII (called P680) delivers its high-energy electrons, one at the time, to the primary electron acceptor, and through the electron transport chain (Pq to cytochrome complex to plastocyanine) to PSI. P680’s missing electron is replaced by extracting a low-energy electron from water; thus, water is “split” during this stage of photosynthesis, and PSII is re-reduced after every photoact. Splitting one H 2 O molecule releases two electrons, two hydrogen atoms, and one atom of oxygen. However, splitting two molecules is required to form one molecule of diatomic O 2 gas. About 10 percent of the oxygen is used by mitochondria in the leaf to support oxidative phosphorylation. The remainder escapes to the atmosphere where it is used by aerobic organisms to support respiration.
As electrons move through the proteins that reside between PSII and PSI, they lose energy. This energy is used to move hydrogen atoms from the stromal side of the membrane to the thylakoid lumen. Those hydrogen atoms, plus the ones produced by splitting water, accumulate in the thylakoid lumen and will be used synthesize ATP in a later step. Because the electrons have lost energy prior to their arrival at PSI, they must be re-energized by PSI, hence, another photon is absorbed by the PSI antenna. That energy is relayed to the PSI reaction center (called P700). P700 is oxidized and sends a high-energy electron to NADP + to form NADPH. Thus, PSII captures the energy to create proton gradients to make ATP, and PSI captures the energy to reduce NADP + into NADPH. The two photosystems work in concert, in part, to guarantee that the production of NADPH will roughly equal the production of ATP. Other mechanisms exist to fine-tune that ratio to exactly match the chloroplast’s constantly changing energy needs.
Generating an Energy Carrier: ATP
As in the intermembrane space of the mitochondria during cellular respiration, the buildup of hydrogen ions inside the thylakoid lumen creates a concentration gradient . The passive diffusion of hydrogen ions from high concentration (in the thylakoid lumen) to low concentration (in the stroma) is harnessed to create ATP, just as in the electron transport chain of cellular respiration. The ions build up energy because of diffusion and because they all have the same electrical charge, repelling each other.
To release this energy, hydrogen ions will rush through any opening, similar to water jetting through a hole in a dam. In the thylakoid, that opening is a passage through a specialized protein channel called the ATP synthase. The energy released by the hydrogen ion stream allows ATP synthase to attach a third phosphate group to ADP, which forms a molecule of ATP ( (Figure) ). The flow of hydrogen ions through ATP synthase is called chemiosmosis because the ions move from an area of high to an area of low concentration through a semi-permeable structure of the thylakoid.
Link to Learning
Visit this site and click through the animation to view the process of photosynthesis within a leaf.
Section Summary
The pigments of the first part of photosynthesis, the light-dependent reactions, absorb energy from sunlight. A photon strikes the antenna pigments of photosystem II to initiate photosynthesis. The energy travels to the reaction center that contains chlorophyll a and then to the electron transport chain, which pumps hydrogen ions into the thylakoid interior. This action builds up a high concentration of hydrogen ions. The hydrogen ions flow through ATP synthase during chemiosmosis to form molecules of ATP, which are used for the formation of sugar molecules in the second stage of photosynthesis. Photosystem I absorbs a second photon, which results in the formation of an NADPH molecule, another energy and reducing carrier for the light-independent reactions.
Art Connections
(Figure) What is the source of electrons for the chloroplast electron transport chain?
- Carbon dioxide
(Figure) A.
Review Questions
Which of the following structures is not a component of a photosystem?
- ATP synthase
- antenna molecule
- reaction center
- primary electron acceptor
How many photons does it take to fully reduce one molecule of NADP + to NADPH?
Which complex is not involved in the establishment of conditions for ATP synthesis?
- photosystem I
- photosystem II
- cytochrome complex
From which component of the light-dependent reactions does NADPH form most directly?
Three of the same species of plant are each grown under a different colored light for the same amount of time. Plant A is grown under blue light, Plant B is grown under green light, and Plant C is grown under orange light. Assuming the plants use only chlorophyll a and chlorophyll b for photosynthesis, what would be the predicted order of the plants from most growth to least growth?
Show Solution
Plants containing only chlorophyll b are exposed to radiation with the following wavelengths: 10nm (x-rays), 450nm (blue light), 670nm (red light), and 800nm (infrared light). Which plants harness the most energy for photosynthesis?
- X-ray irradiated plants
- Blue light irradiated plants
- Red light irradiated plants
- Infrared irradiated plants
Free Response
Describe the pathway of electron transfer from photosystem II to photosystem I in light-dependent reactions.
A photon of light hits an antenna molecule in photosystem II, and the energy released by it travels through other antenna molecules to the reaction center. The energy causes an electron to leave a molecule of chlorophyll a to a primary electron acceptor protein. The electron travels through the electron transport chain and is accepted by a pigment molecule in photosystem I.
What are the roles of ATP and NADPH in photosynthesis?
Both of these molecules carry energy; in the case of NADPH, it has reducing power that is used to fuel the process of making carbohydrate molecules in light-independent reactions.
How and why would the end products of photosynthesis be changed if a plant had a mutation that eliminated its photosystem II complex?
Knocking out photosystem II would eliminate the production of oxygen and ATP during photosynthesis. Photosystem II splits water into oxygen atoms, hydrogen protons that remain in the thylakoid lumen, and hydrogen-derived electrons that move from the reaction center into the electron transport chain. The transfer of an electron through the electron transport chain provides the energy to pump more protons into the thylakoid lumen to maintain a higher concentration of protons there. Moving protons across the thylakoid membrane back to the stroma provides the energy for ATP synthase to produce ATP. Without this proton gradient, ATP will not be synthesized.
- Biology 2e. Provided by : OpenStax. Located at : https://openstax.org/details/books/biology-2e . License : CC BY: Attribution . License Terms : Download for free at http://cnx.org/contents/[email protected]

Privacy Policy

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

20.9: The Electron-Transport Chain and ATP Production
- Last updated
- Save as PDF
- Page ID 293831
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Outcomes
- Summarize the electron transport chain.
- Recognize that electron transport chain is the third and final stage of aerobic cellular respiration.
- Identify the products of the citric acid cycle.
What do trains, trucks, boats, and planes all have in common? They are ways to transport. And they all use a lot of energy. To make ATP, energy must be "transported" - first from glucose to NADH, and then somehow passed to ATP. How is this done? With an electron transport chain, the third stage of aerobic respiration. This third stage uses energy to make energy.
The Electron Transport Chain: ATP for Life in the Fast Lane
At the end of the Krebs Cycle, energy from the chemical bonds of glucose is stored in diverse energy carrier molecules: four ATPs, but also two FADH\(_2\) and ten NADH molecules. The primary task of the last stage of cellular respiration, the electron transport chain , is to transfer energy from the electron carriers to even more ATP molecules, the "batteries" which power work within the cell.
Pathways for making ATP in stage 3 of aerobic respiration closely resemble the electron transport chains used in photosynthesis. In both electron transport chains, energy carrier molecules are arranged in sequence within a membrane so that energy-carrying electrons cascade from one to another, losing a little energy in each step. In both photosynthesis and aerobic respiration, the energy lost is harnessed to pump hydrogen ions into a compartment, creating an electrochemical gradient or chemiosmotic gradient across the enclosing membrane. And in both processes, the energy stored in the chemiosmotic gradient is used with ATP synthase to build ATP.
For aerobic respiration, the electron transport chain or "respiratory chain" is embedded in the inner membrane of the mitochondria (see figure below). The FADH\(_2\) and NADH molecules produced in glycolysis and the Krebs Cycle, donate high-energy electrons to energy carrier molecules within the membrane. As they pass from one carrier to another, the energy they lose is used to pump hydrogen ions into the mitochondrial intermembrane space, creating an electrochemical gradient. Hydrogen ions flow "down" the gradient - from outer to inner compartment - through the ion channel/enzyme ATP synthase, which transfers their energy to ATP. Note the paradox that it requires energy to create and maintain a concentration gradient of hydrogen ions that are then used by ATP synthase to create stored energy (ATP). In broad terms, it takes energy to make energy. Coupling the electron transport chain to ATP synthesis with a hydrogen ion gradient is chemiosmosis , first described by Nobel laureate Peter D. Mitchell. This process, the use of energy to phosphorylate ADP and produce ATP is also known as oxidative phosphorylation .

After passing through the electron transport chain, low-energy electrons and low-energy hydrogen ions combine with oxygen to form water. Thus, oxygen's role is to drive the entire set of ATP-producing reactions within the mitochondrion by accepting "spent" hydrogens. Oxygen is the final electron acceptor, no part of the process - from the Krebs Cycle through the electron transport chain- can happen without oxygen.
The electron transport chain can convert the energy from one glucose molecule's worth of \(FADH_2\) and \(NADH\) + \(\ce{H^+}\) into as many as 34 ATP. When the four ATP produced in glycolysis and the Krebs Cycle are added, the total of 38 ATP fits the overall equation for aerobic cellular respiration:
\[ \ce{6O2} + \underbrace{\ce{C6H12O6}}_{\text{stored chemical energy}} + \ce{38 ADP} + \text{39 P}_\text{i} \rightarrow \underbrace{\ce{38 ATP}}_{\text{stored chemical energy}} + \ce{6CO2} + \ce{6 H2O}\]
Aerobic respiration is complete. If oxygen is available, cellular respiration transfers the energy from one molecule of glucose to 38 molecules of ATP, releasing carbon dioxide and water as waste. "Deliverable" food energy has become energy which can be used for work within the cell - transport within the cell, pumping ions and molecules across membranes, and building large organic molecules. Can you see how this could lead to "life in the fast lane" compared to anaerobic respiration (glycolysis alone)?
Contributors and Attributions
Allison Soult , Ph.D. (Department of Chemistry, University of Kentucky)
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
AP®︎/College Biology
Course: ap®︎/college biology > unit 3.
- Cellular respiration introduction
- Introduction to cellular respiration and redox
- Steps of cellular respiration
- Overview of cellular respiration
Oxidative phosphorylation and the electron transport chain
- Oxidative phosphorylation
- Fermentation and anaerobic respiration
- ATP synthase
- Cellular respiration
Want to join the conversation?
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

Video transcript

IMAGES
VIDEO
COMMENTS
The electron transport chain is a series of molecules that accept or donate electrons easily. By moving step-by-step through these, electrons are moved in a specific direction across a membrane. The movement of hydrogen ions are coupled with this. This means that when electrons are moved, hydrogen ions move too.
All cells use an electron transport chain (ETC) to oxidize substrates in exergonic reactions. The electron flow from reduced substrates through an ETC is like the movement of electrons between the poles of a battery. In the case of the battery, the electron flow releases free energy to power a motor, light, cell phone, etc.
In this article, we'll explore the light-dependent reactions as they take place during photosynthesis in plants. We'll trace how light energy is absorbed by pigment molecules, how reaction center pigments pass excited electrons to an electron transport chain, and how the energetically "downhill" flow of electrons leads to synthesis of ATP and NADPH.
Figure \(\PageIndex{8}\): In the photosystem II (PSII) reaction center, energy from sunlight is used to extract electrons from water. The electrons travel through the chloroplast electron transport chain to photosystem I (PSI), which reduces NADP + to NADPH. The electron transport chain moves protons across the thylakoid membrane into the lumen.
The electron transport chain is a series of four protein complexes that couple redox reactions, creating an electrochemical gradient that leads to the creation of ATP in a complete system named oxidative phosphorylation. It occurs in mitochondria in both cellular respiration and photosynthesis. In the former, the electrons come from breaking down organic molecules, and energy is released.
The electron transport chain is a series of electron transporters embedded in the inner mitochondrial membrane that shuttles electrons from NADH and FADH 2 to molecular oxygen. In the process, protons are pumped from the mitochondrial matrix to the intermembrane space, and oxygen is reduced to form water. The electron transport chain (Figure 1 ...
The electron transport chain is a series of electron transporters embedded in the inner mitochondrial membrane that shuttles electrons from NADH and FADH 2 to molecular oxygen. In the process, protons are pumped from the mitochondrial matrix to the intermembrane space, and oxygen is reduced to form water. The electron transport chain (Figure 1 ...
Photosynthesis consists of two stages: the light-dependent reactions and the light-independent reactions or Calvin cycle. The light-dependent reactions occur when light is available. The overall equation for photosynthesis shows that is it a redox reaction; carbon dioxide is reduced and water is oxidized to produce oxygen:
The electron transport chain is a series of electron transporters embedded in the inner mitochondrial membrane that shuttles electrons from NADH and FADH2 to molecular oxygen. In the process, protons are pumped from the mitochondrial matrix to the intermembrane space, and oxygen is reduced to form water. (Electron Transport Chain by Melissa ...
A photon strikes the antenna pigments of photosystem II to initiate photosynthesis. The energy travels to the reaction center that contains chlorophyll a and then to the electron transport chain, which pumps hydrogen ions into the thylakoid interior. This action builds up a high concentration of hydrogen ions.
In the Calvin cycle, carbon atoms from CO 2 are fixed (incorporated into organic molecules) and used to build three-carbon sugars. This process is fueled by, and dependent on, ATP and NADPH from the light reactions. Unlike the light reactions, which take place in the thylakoid membrane, the reactions of the Calvin cycle take place in the stroma ...
The electron transport chain is symbolized by the red staircase, representing the successive release of energy from the electrons. ... photosynthesis uses an electron transport chain of its own to carry energy from sunlight into the bonds of sugar molecules. The plant can then use these molecules to feed other cells within its body. Just as an ...
The Electron Transport System also called the Electron Transport Chain, is a chain of reactions that converts redox energy available from oxidation of NADH and FADH 2, into proton-motive force which is used to synthesize ATP through conformational changes in the ATP synthase complex through a process called oxidative phosphorylation.. Oxidative phosphorylation is the last step of cellular ...
The electron transport chain is a collection of membrane-embedded proteins and organic molecules, most of them organized into four large complexes labeled I to IV. In eukaryotes, many copies of these molecules are found in the inner mitochondrial membrane. In prokaryotes, the electron transport chain components are found in the plasma membrane.
The electron transport chain is a cluster of proteins that transfer electrons through a membrane within mitochondria to form a gradient of protons that drives the creation of adenosine triphosphate (ATP). ... By-products from other cycles and processes, like the citric acid cycle, amino acid oxidation, and fatty acid oxidation, are used in the ...
Coupling the electron transport chain to ATP synthesis with a hydrogen ion gradient is chemiosmosis, first described by Nobel laureate Peter D. Mitchell. This process, the use of energy to phosphorylate ADP and produce ATP is also known as oxidative phosphorylation. Figure 15.4.1: The third stage of cellular respiration uses the energy stored ...
Coupling the electron transport chain to ATP synthesis with a hydrogen ion gradient is chemiosmosis, first described by Nobel laureate Peter D. Mitchell. This process, the use of energy to phosphorylate ADP and produce ATP is also known as oxidative phosphorylation. Figure 20.9.1 20.9. 1: The third stage of cellular respiration uses the energy ...
At the end of the electron transport chain, oxygen accepts electrons and takes up protons to form water. Glycolysis can take place without oxygen in a process called fermentation. The other three stages of cellular respiration—pyruvate oxidation, the citric acid cycle, and oxidative phosphorylation—require oxygen in order to occur. Only ...
Mitochondrial electron transport chains The electron transport chain in the mitochondrion is the site of oxidative phosphorylation in eukaryotes.It mediates the reaction between NADH or succinate generated in the citric acid cycle and oxygen to power ATP synthase.. Most eukaryotic cells have mitochondria, which produce ATP from reactions of oxygen with products of the citric acid cycle, fatty ...
Introduction. Photosynthesis is one of the most important chemical reactions for sustaining life on earth. Principally, photosynthesis can be divided into two steps, namely the light reactions and the Calvin cycle (also known as the dark reactions) (Fischer et al., 2016; Johnson, 2016).The light reactions constitute a light-driven electron transport process, which is generally classified into ...
The electron transport pathways in the thylakoids of cyanobacteria (A) and microalgae (B) branches into auxiliary electron transport routes, to distribute excess electrons when the sink capacity of the CBB cycle is insufficient. The major electron distribution hub is Fd, and the electron transport route toward the CBB-cycle converges there with ...
The electron carriers take the electrons to a group of proteins in the inner membrane of the mitochondrion, called the electron transport chain. As electrons move through the electron transport chain, they go from a higher to a lower energy level and are ultimately passed to oxygen (forming water).
Stage 3: Electron Transport Chain Occurs in the: The electron transport chain (ETC) occurs in the inner mitochondrial membrane. More specifically, it takes place within the folds of the inner mitochondrial membrane known as the cristae. The cristae provide a large surface area for the proteins and complexes involved in the electron transport chain.
Transcript. Oxidative phosphorylation produces ATP (energy) in our cells. NADH, a molecule produced during cellular respiration, gets oxidized and releases electrons. These electrons pass through a series of acceptors in the electron transport chain, releasing energy. This energy pumps hydrogen protons across a membrane, creating a gradient.