ENCYCLOPEDIC ENTRY
Photosynthesis.
Photosynthesis is the process by which plants use sunlight, water, and carbon dioxide to create oxygen and energy in the form of sugar.

Loading ...
Learning materials, instructional links.
- Photosynthesis (Google doc)
Most life on Earth depends on photosynthesis .The process is carried out by plants, algae, and some types of bacteria, which capture energy from sunlight to produce oxygen (O 2 ) and chemical energy stored in glucose (a sugar). Herbivores then obtain this energy by eating plants, and carnivores obtain it by eating herbivores.
The process
During photosynthesis, plants take in carbon dioxide (CO 2 ) and water (H 2 O) from the air and soil. Within the plant cell, the water is oxidized, meaning it loses electrons, while the carbon dioxide is reduced, meaning it gains electrons. This transforms the water into oxygen and the carbon dioxide into glucose. The plant then releases the oxygen back into the air, and stores energy within the glucose molecules.
Chlorophyll
Inside the plant cell are small organelles called chloroplasts , which store the energy of sunlight. Within the thylakoid membranes of the chloroplast is a light-absorbing pigment called chlorophyll , which is responsible for giving the plant its green color. During photosynthesis , chlorophyll absorbs energy from blue- and red-light waves, and reflects green-light waves, making the plant appear green.
Light-dependent Reactions vs. Light-independent Reactions
While there are many steps behind the process of photosynthesis, it can be broken down into two major stages: light-dependent reactions and light-independent reactions. The light-dependent reaction takes place within the thylakoid membrane and requires a steady stream of sunlight, hence the name light- dependent reaction. The chlorophyll absorbs energy from the light waves, which is converted into chemical energy in the form of the molecules ATP and NADPH . The light-independent stage, also known as the Calvin cycle , takes place in the stroma , the space between the thylakoid membranes and the chloroplast membranes, and does not require light, hence the name light- independent reaction. During this stage, energy from the ATP and NADPH molecules is used to assemble carbohydrate molecules, like glucose, from carbon dioxide.
C3 and C4 Photosynthesis
Not all forms of photosynthesis are created equal, however. There are different types of photosynthesis, including C3 photosynthesis and C4 photosynthesis. C3 photosynthesis is used by the majority of plants. It involves producing a three-carbon compound called 3-phosphoglyceric acid during the Calvin Cycle, which goes on to become glucose. C4 photosynthesis, on the other hand, produces a four-carbon intermediate compound, which splits into carbon dioxide and a three-carbon compound during the Calvin Cycle. A benefit of C4 photosynthesis is that by producing higher levels of carbon, it allows plants to thrive in environments without much light or water. The National Geographic Society is making this content available under a Creative Commons CC-BY-NC-SA license . The License excludes the National Geographic Logo (meaning the words National Geographic + the Yellow Border Logo) and any images that are included as part of each content piece. For clarity the Logo and images may not be removed, altered, or changed in any way.
Media Credits
The audio, illustrations, photos, and videos are credited beneath the media asset, except for promotional images, which generally link to another page that contains the media credit. The Rights Holder for media is the person or group credited.
Production Managers
Program specialists, last updated.
March 20, 2024
User Permissions
For information on user permissions, please read our Terms of Service. If you have questions about how to cite anything on our website in your project or classroom presentation, please contact your teacher. They will best know the preferred format. When you reach out to them, you will need the page title, URL, and the date you accessed the resource.
If a media asset is downloadable, a download button appears in the corner of the media viewer. If no button appears, you cannot download or save the media.
Text on this page is printable and can be used according to our Terms of Service .
Interactives
Any interactives on this page can only be played while you are visiting our website. You cannot download interactives.
Related Resources
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Recent advances in understanding and improving photosynthesis
Alicia v perera-castro.
1 Department of Biology, Universitat de les Illes Balears, INAGEA, Palma de Mallorca, Spain
Jaume Flexas
Since 1893, when the word “photosynthesis” was first coined by Charles Reid Barnes and Conway MacMillan, our understanding of the elements and regulation of this complex process is far from being entirely understood. We aim to review the most relevant advances in photosynthesis research from the last few years and to provide a perspective on the forthcoming research in this field. Recent discoveries related to light sensing, harvesting, and dissipation; kinetics of CO 2 fixation; components and regulators of CO 2 diffusion through stomata and mesophyll; and genetic engineering for improving photosynthetic and production capacities of crops are addressed.
Introduction
Photosynthesis is the chemical reaction that sustains most life on Earth. Since the description of the Hill reaction and the Calvin-Benson cycle 1 – 3 , knowledge about their components, regulation, and limitations experienced a vertiginous increase. It is widely known that plants have important handicaps related to photosynthesis. First, the photosynthetic apparatus that harvests and transforms light energy into electron transport for the generation of ATP and NADPH 2 must cope with the generation of dangerous reactive oxygen species (ROS) 4 and most of the energy must be “wasted” in dynamic heat dissipation mechanisms 5 . Second, the enzyme that catalyzes CO 2 fixation in the Calvin-Benson cycle—ribulose 1,5-bisphosphate carboxylase oxidase or rubisco—is inefficient owing to several intrinsic characteristics, the most notable being the competitiveness between carboxylation and oxidation processes, since the oxidation of D-ribulose-1,5-bisphosphate results in the energetically expensive but perhaps convenient photorespiratory pathway 6 . And, third, the diffusion of CO 2 from the atmosphere surrounding leaves through stomata and the leaf tissues to the carboxylation sites in the chloroplast stroma, where rubisco is located, is a dynamic pathway that is full of barriers and includes gaseous, lepidic, and aqueous phases, the latter with a small solubility and diffusivity for CO 2 .
In the last few years, researchers have tried to determine the limitations and components of the processes described above. Engineering photosynthesis targeting different aspects of photosynthesis and its regulation has also advanced. The aim of this review is to compile and organize these advances in photosynthesis from the last few years and suggest a next horizon for plant physiologists, ecologists, and geneticists.
Light harvesting and use
Light energy is absorbed and transferred to the photosystem II (PSII) core by the light-harvesting complex II (LHCII). The way this absorption is regulated is relevant, since excessive and/or unbalanced exposure to light can lead to the generation of ROS and, in the long term, to the initiation of senescence processes 7 . Some isoforms of LHCII upregulate its transcription and translation as a response to high irradiance 8 , 9 , and their interaction with PsbS—a protein that plays a special role in photoprotection—has been described in detail 10 . Furthermore, Janil et al . 11 discussed the enhanced dimerization of LHCII under strong light conditions as a photoprotective response partially responsible for the dissipation of excess excitation. In line with this, Albanese et al . 12 recently described how the organization of PSII–LHCII supercomplexes changed with the diversification of land plants, contributing to their adaptability to different light environments. However, photoprotective processes and their ecophysiological implications remain far from fully characterized 5 . At the extreme opposite to excess light, shaded leaves within the canopy exhibit lower photosynthesis rates and slower activation of rubisco, stomata opening, and relaxation of photoprotection states. These delays, especially in rubisco activation, have been estimated to decrease wheat assimilation by 21% in shade to sun transitions 13 . Indeed, the fact that light is often in excess in the most illuminated leaves while limited in the shaded leaves within the canopy has led to the suggestion that lowering chlorophyll content may result not only in negligible effects on leaf-level photosynthesis rates but also in a higher distribution of light harvesting through the canopy, hence potentially enhancing whole plant photosynthesis rates and yield 14 , 15 . On the other hand, alterations of the canopy structure have also been suggested as a mechanism to improve light interception and canopy assimilation (see the recent review by Morales et al . 16 and references therein), mainly through long-term breeding but also through hormonal and/or genetic means 17 .
Besides studies on the photosynthetic management of light amount , the effect of light quality on photosynthesis-related issues has also been addressed. It is widely known that growing under blue light conditions induces lower photosynthetic rates, increases the synthesis of carotenoids and anthocyanins and the photoprotection capacity, and decreases stomata size while increasing their density 18 . Light quality also affects the level of ROS and the expression of antioxidant enzymes 19 . Recently, Górecka et al . 20 demonstrated that PsbS is not only a compulsory protein for enhancing dissipation of the excess of light energy as heat but also relevant for the red/blue light-associated enhancement of tolerance to UV-C and chloroplast signaling for light memory. A recent study has also described a species-specific response of photosynthesis to the quality of light independent of its intensity 21 . These interspecific differences in light response represent an opportunity to deeply understand the elements of light harvesting and their adaptation to different light environments.
Rubisco kinetics and CO 2 -concentrating mechanisms
Interspecific variation of rubisco kinetics has also been a focus over the last several years. In two almost simultaneously published works, Hermida-Carrera et al . 22 and Orr et al . 23 assessed rubisco kinetics, their temperature dependency, and the aminoacidic replacements in the large subunit of rubisco in many crop species. Orr et al . 23 extended their study to include 75 angiosperm species and found that some undomesticated plants presented inherently better rubisco kinetics, being thus a potential source for crop photosynthesis improvement. Iñiguez et al . 24 and Flamholz et al . 25 extended the analysis of differences in rubisco catalysis across the phylogeny and correlated them with the incidence of CO 2 concentration mechanisms (CCMs), showing that organisms that had evolved CCMs tended to have faster rubiscos yet with lower affinity and specificity for CO 2 . Hermida-Carrera et al . 26 found similar results when comparing rubisco catalytic traits of orchids and bromeliads with and without CCMs. These results suggest that equipping C 3 crops with CCMs could be another strategy for fueling their photosynthetic capacity.
C 4 photosynthesis is often envisaged as an efficient CCM and thus converting typical C 3 crops into C 4 has been a long-standing goal, resulting in the development of large-scale projects like the ongoing C4Rice ( https://c4rice.com/ ), yet the goal has not been fully accomplished yet 27 . Furthermore, transitioning from mostly C 3 to mostly C 4 crops may be an efficient way to enhance productivity in a world exhibiting increased global aridity 28 , 29 , as it has been shown that in some cases C 4 plants performed better under drought than did C 3 species 30 . In the same vein, introducing crassulacean acid metabolism (CAM) into C 3 crops has been suggested as a strategy to increase water use efficiency, i.e. to maximize CO 2 fixation with minimum water loss through transpiration 31 , 32 . On the other hand, other CCMs like those found in algae and other aquatic organisms (e.g. pyrenoids and carboxysomes) have been reported to concentrate more CO 2 around rubisco than C 4 photosynthesis. Hence, while the C 4 mechanism allows CO 2 concentrations around rubisco of at least 10-times higher than those of the surrounding atmosphere 33 , eukaryotic algae like Chlamydomonas containing pyrenoids can concentrate CO 2 40-times 34 and prokaryotic cyanobacteria possessing carboxysomes 100-times 35 higher than the surrounding atmosphere. Consequently, the potential expression of cyanobacterial and algal CCMs in crop plants has been proposed as an opportunity to improve their photosynthesis 36 .
Despite the inefficiencies of light harvesting and rubisco, photochemical and/or biochemical limitations to photosynthesis are not larger than the diffusional limitations related to both stomatal and mesophyll resistances to CO 2 in most of the studied species 37 – 45 . Gago et al . 46 recently presented a compilation of photosynthetic limitations across land plants’ phylogenies, in which angiosperms showed a well-balanced distribution among biochemical, stomatal, and mesophyll limitations; photosynthesis in gymnosperms and ferns was co-limited mostly by stomatal and mesophyll limitations; and in bryophytes and lycophytes the mesophyll limitation largely predominated.
Mesophyll conductance components
Mesophyll conductance to CO 2 ( g m ) depends on several leaf structures that comprise the pathway from sub-stomatal cavities to carboxylation sites of rubisco. Intercellular air spaces, cell walls, plasma membranes, cytosol, double chloroplast membranes, and stroma offer resistance to CO 2 diffusion. Values of g m vary strongly among species, and short-term changes in g m have been reported in response to many different environmental variables 46 – 49 , although a part of them could reflect methodological errors or uncertainties 50 – 52 . While interspecific differences are largely explained by anatomical traits 37 – 39 , 53 , 54 , short-term changes cannot be explained either by variable leaf anatomy or by the temperature coefficient reported for CO 2 diffusion 55 – 57 . Consequently, it has been suggested that a biochemically facilitated CO 2 diffusion must contribute to g m instead of solely physical diffusion 56 , 58 – 60 . Short-term chloroplast movement, aquaporins, and carbonic anhydrases have been indicated as candidates 53 , 56 , 61 , although their actual involvement is far away from being conclusive.
For instance, despite the fact that chloroplast surface area facing intercellular airspaces per unit leaf area ( S c / S ) is one of the anatomical parameters more correlated with g m 37 , 53 , 54 , no evidence for an association between short-term changes of g m and chloroplast movement or leaf anatomy has been found 57 , 62 , 63 , with the exception of Arabidopsis mutants with phytochrome-mediated impairment of the chloroplast avoidance response 64 . In a similar way, the contribution of carbonic anhydrases to g m variations remains elusive and is a matter of ongoing debate 65 . The most recent studies showed that latitudinal variation of g m correlates with variations in carbonic anhydrase activity 66 , 67 and that a coupled inhibition of both g m and carbonic anhydrases is obtained with treatment with mercuric chloride 68 . Han et al . 69 also reported a decrease in the expression of carbonic anhydrase ( CA1 ) during drought. On the contrary, Kolbe and Cousins 70 did not find any variation in g m in five lines of maize despite their differences in carbonic anhydrase activity.
The role of aquaporins as enhancers of CO 2 diffusion across membranes has been widely reported 48 , 71 . Changes in g m had been induced by inhibitors of aquaporins 68 , 72 in transgenics 73 – 76 and in mutants 77 – 80 . Direct measurement of the CO 2 permeability of chloroplasts also revealed a 50% reduction in chloroplasts of an Arabidopsis aquaporin mutant as compared to the wild-type 81 . Despite these findings, Kromdijk et al . 82 recently reported null differences in g m among several knockout aquaporin mutants and wild-type, probably due to functional redundancy of aquaporin isoforms.
Additionally, the relative importance of these biochemical processes and anatomical traits in regulating g m remains unknown. Furthermore, recent studies showed uncertainty about estimating some relevant anatomical parameters from microscopic images of 2D cross-sections compared to 3D microscopy, especially the mesophyll surface area exposed to air-filled spaces 83 and chloroplast volume 84 . This could partially explain the differences in the g m calculated from chlorophyll fluorescence and/or gas exchange and g m calculated so far from anatomical models 38 , 39 , 53 , 85 , 86 . Earles et al . 87 have emphasized the need to improve 3D techniques and models to properly characterize leaf-level photosynthesis in its whole complexity.
Within the anatomical components, S c / S and cell wall thickness ( T cw ) have been recognized as especially determinant for g m 46 . Besides the effect of T cw , an effect of cell wall composition and porosity in short- and long-term variations of g m has been suggested 88 , 89 , and recently the first empirical evidence was provided. Thus, a reduction of g m was observed by Ellsworth et al . 90 in mutants with disrupted β-glucosyl polysaccharides of the cell wall. More recent studies have shown that the decrease of g m provoked by drought, salinity, and low temperatures is coupled with variations in the relative levels of cellulose, hemicelluloses, and pectins 91 , 92 . More evidence is needed to understand how cell wall composition affects porosity and CO 2 diffusion.
Stomatal conductance
As mentioned above, an additional important limiting factor of photosynthesis is the stomatal conductance ( g s ). Several internal and environmental factors are widely known to affect g s . Stomatal shape, size, density, and clustering influence g s and therefore photosynthesis 93 . These traits are established during leaf development and regulated by several phytohormones, especially abscisic acid (ABA) 94 . Light, CO 2 , and water supply also affect g s 95 , 96 .
The speed of g s responses to light and CO 2 has been recently compared among phylogenetic plant groups. Although fern and lycophyte stomata are not insensitive to light and CO 2 , their response is lower and slower than that observed in angiosperms 97 – 100 . Furthermore, unlike angiosperms, fern and lycophyte stomata do not respond to endogenous levels of ABA 97 , 98 and their closure is based on a passive response of guard cells to dehydration 101 . The mechanism that explains this different response remains unclear, although it is likely related to differences in the molecular mechanisms operating in the guard cells along the phylogeny. Among other factors affecting g s (kinases, anion channels, etc.), it is known that carbonic anhydrases can be involved in the biochemical mechanism by which guard cells of angiosperms sense CO 2 (see the review by Engineer et al . 95 ), although details of signal transduction and the identity of the second messengers (bicarbonate, protons) are still debated. Furthermore, a higher CO 2 assimilation related to phosphoenolpyruvate carboxylase activity followed by gluconeogenesis and maybe sucrose synthesis has been described for guard cells in comparison to those of mesophyll cells of C 3 plants 102 .
In addition, recent studies suggest that stomata movement is regulated by mesophyll-derived signals. Sucrose has been identified as an important metabolite for the regulation of stomatal opening and closure 100 , 103 , 104 . Wang et al . 105 reported that the maize mutant cst1 —with an impaired membrane glucose transporter CST1 located in the subsidiary cell membrane—presented lower g s , lower photosynthesis, and earlier senescence than the wild-type. In line with this, Fujita et al . 106 demonstrated that stomatal responses are disrupted when a membrane excluding molecules of 100–500 Da is transplanted between mesophyll and guard cells, which would avoid the transport of sucrose, malate, and ABA. In a study of ABA-regulated genes in Arabidopsis , Yoshida et al . 107 found highly expressed genes in guard cells related to the tricarboxylic acid cycle and sucrose and hexose transport and metabolism. These studies support the hypothesis of stomatal regulation driven by carbohydrate/hormone-related mesophyll signals. However, the differences in the mechanism of mesophyll cell signaling and in guard cell metabolism among fern, lycophytes, and angiosperms—both anisohydric and isohydric species—remain unknown.
Even in angiosperms, the predominance of hormonal vs . hydraulic stomatal regulation is currently under debate 108 – 110 . Traditionally, stomatal closure has been understood as a safety valve to prevent cavitation (see Hochberg et al . 111 and references therein). However, a detailed chronological description of the drought response of g s and hydraulic conductance ( K leaf ) in rice revealed that the decline in K leaf preceded and probably triggered the decline of g s and g m 108 . Nadal et al . 112 suggested that both types of drought response are not necessarily incompatible and can be related to the spectrum of the iso-anisohydric response of angiosperms.
Engineering photosynthesis
While there are some opposing views 113 , improving photosynthesis is often envisaged as an important goal for improving crop yields 114 – 117 , including the cultivation of photosynthetic microorganisms, which constitutes a huge and important branch of bioengineering for bioenergy production 118 , 119 . Regarding land plant bioengineering, optimizing production with a minimum investment of resources (water, land, and nutrients) is the aim of ongoing large-scale projects, such as the already mentioned C4rice or the RIPE project ( https://ripe.illinois.edu/ ). Several targets for manipulation—including all those mentioned in the above sections—have been proposed with the aim of improving photosynthesis and crop yield 120 , 121 . Neglecting which are the main limitations for photosynthesis when targeting genes for improving photosynthesis is an example of the mutual disregard that ecophysiologists and biotechnologists have had for each other in the last few decades 122 , i.e. biotechnologists attempting to improve photosynthetic targets that ecophysiologists were showing to be non-limiting for photosynthesis. Using a model approach, Flexas 116 showed that only modest improvements of photosynthesis can be expected from relaxing only one limiting factor, since photosynthetic limitations are generally well-balanced in angiosperms 46 . Nevertheless, even with this relatively modest approach, increases of yield of >40% have been reported in some successful attempts 117 .
Rubisco kinetics have been among the most common targets for improving photosynthesis. All the advances in rubisco engineering have implied important improvements in our understanding of rubisco regulation and assembly but unsuccessfully improved the catalytic performance of rubisco 123 , 124 or photosynthesis 125 . While faster rubisco from cyanobacteria have been successfully engineered in transplastomic tobacco 126 , post-transcriptional assembly of functional rubisco in large enough quantities remains a limiting factor, likely due to the inability of local chaperones to deal with foreign rubisco fragments (see Whitney et al . 127 and their attempt to solve this problem by the use of ancillary chaperone genes). For this reason, this is a very active area of ongoing research 127 , 128 . Rubisco activase is another potential limiting factor, as Fukuyama et al . 129 also showed how increased expression of rubisco activase resulted in a negative correlation with rubisco content.
Besides achieving more efficient rubiscos, an alternative strategy has been to increase CO 2 concentration by either introducing elements of algal CCMs or bypassing photorespiration by different processes. While theoretically CCMs should increase photosynthesis 130 , introducing CCMs into either tobacco 131 or Arabidopsis failed to increase photosynthesis 132 , 133 , probably because of insufficient encapsulation of local rubisco in the foreign carboxysomes, which can be improved by simultaneously replacing the native large subunit of rubisco 134 . Additional elements might also be essential for a proper assemblage of fully functional carboxysome–rubisco CCMs, as recently demonstrated for bestrophin-like proteins 135 .
More successful results have been obtained when the photorespiration pathway has been manipulated in Arabidopsis and tobacco 136 , 137 . While photosynthesis increases 136 , biomass production has been shown to vary from decreasing through unaffected to increasing by 10–50% 117 , 138 . Recently, South et al . 137 obtained a 24% maximum increase of biomass when glycolate byproducts of photorespiration are processed by foreign malate synthase and a green algal glycolate dehydrogenase, substituting the native pathway. Tissue-specific overexpression of one of the subunits forming in the glycine dehydrogenase system also increased biomass yield by 13–38% in tobacco 139 . This is a very promising approach for improving grain crop yields in the near future.
Also, modifications of the Calvin-Benson cycle have resulted in improved photosynthesis and yield. Overexpression or transgenic insertion of several enzymes involved in the cycle (mostly sucrose bis-phosphatase—SBPase—and fructose bis-phosphatase—FBPase—but also FBPaldolase) has also resulted in increased photosynthesis and dry weights, although generally not in improved yield. However, Driever et al . 140 showed an up to 40% increase in grain yield in wheat, and Simkin et al . 141 a 35–53% increase in seed yield in Arabidopsis . Furthermore, overexpression of FBP/SBPase has been recently combined with an improved electron transport by the addition of the algae cytochrome C 6 , which also resulted in up to 53% of increase of biomass 142 . These results open up the possibility of using this approach for improving crop yields in the very near future.
Few attempts have focused on modifying CO 2 diffusive characteristics of leaves. Altered stomatal density in epidermal patterning factor (EPF) mutants of Arabidopsis 143 and wheat 144 resulted in an increased photosynthetic water-use efficiency (WUE) but not increased photosynthesis itself. Similarly, Yang et al . 145 showed that overexpression of the ABA receptors RCAR6/PYL12 increases the sensitivity of the stomata in Arabidopsis lines, reducing g s even in the absence of water stress without affecting photosynthesis, thus also enhancing WUE. As described in previous sections, g s was also enhanced by overexpression of glucose transporters in subsidiary cell membranes 105 .
Generally speaking, increasing stomatal conductance does not result in enhanced photosynthesis because stomatal limitations are generally minor in the absence of stress. However, during leaf development, the presence of well-developed and functional stomata appears to be the main driver of the development of mesophyll porosity, which is an essential anatomical trade favoring g m and hence photosynthesis 146 . This finding is remarkable as it implies that, while it is likely that a mesophyll signal is involved in stomata regulation (see above sections), stomata define the developmental set-up of the mesophyll structure, hence establishing a very intricate co-dependency between g s and g m limitations at different time scales that deserves further study. In line with this, Lehmeier et al . 147 showed that it is possible to genetically modify cell density and the arrangement of the air channels with an overall decreased path tortuosity in the palisade air spaces in a way that facilitates g m without affecting g s . Similarly, alteration of leaf mesophyll anatomy of Eucalyptus has been attempted by the overexpression of the transcription factor EcHB1 , which is involved in multiple genes related to cell wall biosynthesis and cell growth, increasing the number of chloroplasts per unit leaf area and therefore enhancing CO 2 diffusion into chloroplasts and photosynthesis 148 . These results offer new possibilities in improving photosynthesis by reducing CO 2 diffusion limitations. Advances in the understanding of cell wall composition determinants of g m may open complementary doors in the near future.
While significant and important in some cases, the above-described manipulations aimed to improve maximum photosynthesis rates, i.e. light-saturated photosynthesis in the absence of abiotic and biotic stresses. However, photosynthesis in nature occurs in largely variable conditions, e.g. in fluctuating light. For instance, De Souza et al . 43 showed in cassava that, while under steady-state high-light conditions, g m and biochemical limitations accounted for up to 84% of the total photosynthetic limitation and, under non-steady state conditions during shade to sun transition, g s became the most dominant limitation. Thus, in recent years, research has focused on improving photosynthesis and efficiency under non-steady-state conditions by decreasing the excess absorption of light 15 , 149 or increasing the relaxing velocity of photoprotection 150 – 152 . More surprisingly, overexpressing PsbS in transgenic tobacco resulted in enhanced WUE by reducing g s , not increasing photosynthesis, again pointing to potential mesophyll signals in stomata regulation 153 . Recently, Papanatsiou et al . 154 used an optogenetic approach to improve photosynthesis, WUE, and growth in Arabidopsis . They expressed a synthetic light-gated K + channel in stomatal guard cells (BLINK1), which improved the speed of stomata kinetics in response to varying light. Increased velocity of stomata opening from a dark-to-light transition and closing from a light-to-dark transition resulted in increased plant growth and WUE by approximately 30% 154 .
Light sensing, photoprotection, CO 2 diffusion, and its fixation involve numerous and complex processes that are far from fully understood. In the last few years, new insights have been obtained into how interaction and conformation of light-harvesting complexes and photosystems affect photoprotection and heat dissipation. Advances have been made also in the understanding of the variability in rubisco kinetics and photosynthetic limitations at steady state along the plant’s phylogeny, of the genetics and mechanistic aspects of carbon-concentrating mechanisms, and of the major anatomical determinants of g m and the metabolic determinants of stomatal conductance and kinetics. Important links between mesophyll and stomatal cells have been revealed, although the signaling between mesophyll cells and guard cells that regulates g s requires further research, as does understanding the chemical and biochemical determinants of g m .
Nevertheless, owing to the new knowledge acquired, engineering efforts for improving photosynthesis and photosynthetic WUE have been attempted, some of them with significant success, which open up the opportunity for photosynthesis-mediated improvement of crop productivity in the forthcoming years. To achieve this goal, a close collaboration among plant physiologists, molecular biologists, geneticists, and agronomists might be essential for generating multiple new photosynthetic genotypes and evaluating them under realistic conditions, both under steady- and non-steady-state conditions, from a photosynthetic limitations perspective to a yield and WUE perspective 122 . Technical advances in analytical tools, like the recently implemented rapid CO 2 response curves of gas exchange 155 – 159 , would be crucial to allow in-depth phenotyping of photosynthesis in record times.
Funding Statement
Alicia V. Perera-Castro and Jaume Flexas’s research was supported by the project EREMITA (PGC-2018-093824-B-C41) from the Ministerio de Economía y Competitividad (MINECO, Spain) and the ERDF (FEDER). The Ministerio de Educación, Cultura y Deporte (MECD, Spain) supported a pre-doctoral fellowship (FPU-02054) awarded to Alicia V. Perera-Castro. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The peer reviewers who approve this article are:
- Asaph B. Cousins , School of Biological Sciences, Washington State University, WA, USA No competing interests were disclosed.
- Esa Tyystjärvi , Department of Biochemistry/Molecular Plant Biology, University of Turku, Turku, Finland No competing interests were disclosed.
This page has been archived and is no longer updated
Photosynthetic Cells
Cells get nutrients from their environment, but where do those nutrients come from? Virtually all organic material on Earth has been produced by cells that convert energy from the Sun into energy-containing macromolecules. This process, called photosynthesis, is essential to the global carbon cycle and organisms that conduct photosynthesis represent the lowest level in most food chains (Figure 1).
What Is Photosynthesis? Why Is it Important?
Most living things depend on photosynthetic cells to manufacture the complex organic molecules they require as a source of energy. Photosynthetic cells are quite diverse and include cells found in green plants, phytoplankton, and cyanobacteria. During the process of photosynthesis, cells use carbon dioxide and energy from the Sun to make sugar molecules and oxygen. These sugar molecules are the basis for more complex molecules made by the photosynthetic cell, such as glucose. Then, via respiration processes, cells use oxygen and glucose to synthesize energy-rich carrier molecules, such as ATP, and carbon dioxide is produced as a waste product. Therefore, the synthesis of glucose and its breakdown by cells are opposing processes.
However, photosynthesis doesn't just drive the carbon cycle — it also creates the oxygen necessary for respiring organisms. Interestingly, although green plants contribute much of the oxygen in the air we breathe, phytoplankton and cyanobacteria in the world's oceans are thought to produce between one-third and one-half of atmospheric oxygen on Earth.
What Cells and Organelles Are Involved in Photosynthesis?
Chlorophyll A is the major pigment used in photosynthesis, but there are several types of chlorophyll and numerous other pigments that respond to light, including red, brown, and blue pigments. These other pigments may help channel light energy to chlorophyll A or protect the cell from photo-damage. For example, the photosynthetic protists called dinoflagellates, which are responsible for the "red tides" that often prompt warnings against eating shellfish, contain a variety of light-sensitive pigments, including both chlorophyll and the red pigments responsible for their dramatic coloration.
What Are the Steps of Photosynthesis?
Photosynthesis consists of both light-dependent reactions and light-independent reactions . In plants, the so-called "light" reactions occur within the chloroplast thylakoids, where the aforementioned chlorophyll pigments reside. When light energy reaches the pigment molecules, it energizes the electrons within them, and these electrons are shunted to an electron transport chain in the thylakoid membrane. Every step in the electron transport chain then brings each electron to a lower energy state and harnesses its energy by producing ATP and NADPH. Meanwhile, each chlorophyll molecule replaces its lost electron with an electron from water; this process essentially splits water molecules to produce oxygen (Figure 5).
Once the light reactions have occurred, the light-independent or "dark" reactions take place in the chloroplast stroma. During this process, also known as carbon fixation, energy from the ATP and NADPH molecules generated by the light reactions drives a chemical pathway that uses the carbon in carbon dioxide (from the atmosphere) to build a three-carbon sugar called glyceraldehyde-3-phosphate (G3P). Cells then use G3P to build a wide variety of other sugars (such as glucose) and organic molecules. Many of these interconversions occur outside the chloroplast, following the transport of G3P from the stroma. The products of these reactions are then transported to other parts of the cell, including the mitochondria, where they are broken down to make more energy carrier molecules to satisfy the metabolic demands of the cell. In plants, some sugar molecules are stored as sucrose or starch.
This page appears in the following eBook
Topic rooms within Cell Biology

Within this Subject (25)
- Basic (25)
Other Topic Rooms
- Gene Inheritance and Transmission
- Gene Expression and Regulation
- Nucleic Acid Structure and Function
- Chromosomes and Cytogenetics
- Evolutionary Genetics
- Population and Quantitative Genetics
- Genes and Disease
- Genetics and Society
- Cell Origins and Metabolism
- Proteins and Gene Expression
- Subcellular Compartments
- Cell Communication
- Cell Cycle and Cell Division
© 2014 Nature Education
- Press Room |
- Terms of Use |
- Privacy Notice |

Visual Browse
- Biology Article
Photosynthesis
Photosynthesis is a process by which phototrophs convert light energy into chemical energy, which is later used to fuel cellular activities. The chemical energy is stored in the form of sugars, which are created from water and carbon dioxide.

Table of Contents
- What is Photosynthesis?
- Site of photosynthesis
Photosynthesis definition states that the process exclusively takes place in the chloroplasts through photosynthetic pigments such as chlorophyll a, chlorophyll b, carotene and xanthophyll. All green plants and a few other autotrophic organisms utilize photosynthesis to synthesize nutrients by using carbon dioxide, water and sunlight. The by-product of the photosynthesis process is oxygen.Let us have a detailed look at the process, reaction and importance of photosynthesis.
What Is Photosynthesis in Biology?
The word “ photosynthesis ” is derived from the Greek words phōs (pronounced: “fos”) and σύνθεσις (pronounced: “synthesis “) Phōs means “light” and σύνθεσις means, “combining together.” This means “ combining together with the help of light .”
Photosynthesis also applies to other organisms besides green plants. These include several prokaryotes such as cyanobacteria, purple bacteria and green sulfur bacteria. These organisms exhibit photosynthesis just like green plants.The glucose produced during photosynthesis is then used to fuel various cellular activities. The by-product of this physio-chemical process is oxygen.
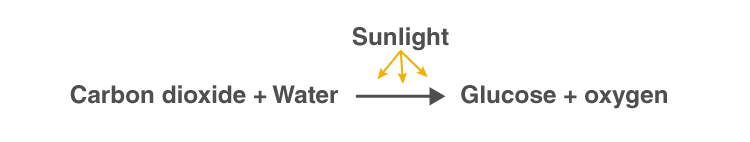
A visual representation of the photosynthesis reaction
- Photosynthesis is also used by algae to convert solar energy into chemical energy. Oxygen is liberated as a by-product and light is considered as a major factor to complete the process of photosynthesis.
- Photosynthesis occurs when plants use light energy to convert carbon dioxide and water into glucose and oxygen. Leaves contain microscopic cellular organelles known as chloroplasts.
- Each chloroplast contains a green-coloured pigment called chlorophyll. Light energy is absorbed by chlorophyll molecules whereas carbon dioxide and oxygen enter through the tiny pores of stomata located in the epidermis of leaves.
- Another by-product of photosynthesis is sugars such as glucose and fructose.
- These sugars are then sent to the roots, stems, leaves, fruits, flowers and seeds. In other words, these sugars are used by the plants as an energy source, which helps them to grow. These sugar molecules then combine with each other to form more complex carbohydrates like cellulose and starch. The cellulose is considered as the structural material that is used in plant cell walls.
Where Does This Process Occur?
Chloroplasts are the sites of photosynthesis in plants and blue-green algae. All green parts of a plant, including the green stems, green leaves, and sepals – floral parts comprise of chloroplasts – green colour plastids. These cell organelles are present only in plant cells and are located within the mesophyll cells of leaves.
Also Read: Photosynthesis Early Experiments
Photosynthesis Equation
Photosynthesis reaction involves two reactants, carbon dioxide and water. These two reactants yield two products, namely, oxygen and glucose. Hence, the photosynthesis reaction is considered to be an endothermic reaction. Following is the photosynthesis formula:
Unlike plants, certain bacteria that perform photosynthesis do not produce oxygen as the by-product of photosynthesis. Such bacteria are called anoxygenic photosynthetic bacteria. The bacteria that do produce oxygen as a by-product of photosynthesis are called oxygenic photosynthetic bacteria.
Structure Of Chlorophyll
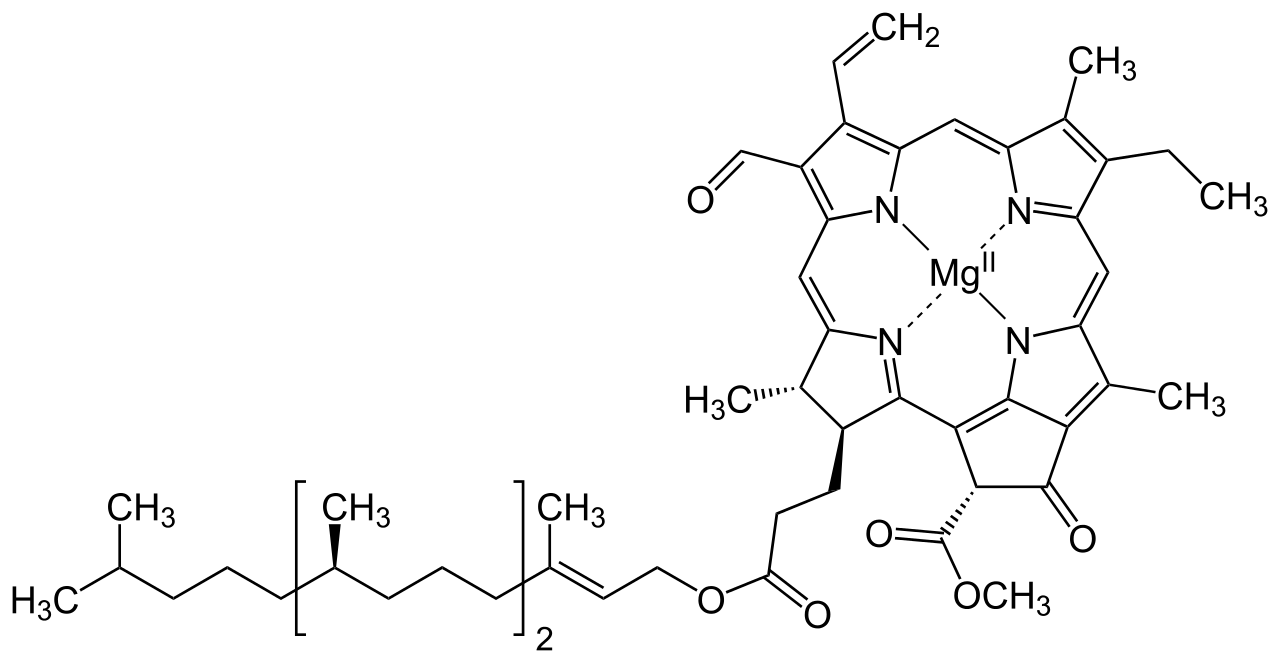
The structure of Chlorophyll consists of 4 nitrogen atoms that surround a magnesium atom. A hydrocarbon tail is also present. Pictured above is chlorophyll- f, which is more effective in near-infrared light than chlorophyll- a
Chlorophyll is a green pigment found in the chloroplasts of the plant cell and in the mesosomes of cyanobacteria. This green colour pigment plays a vital role in the process of photosynthesis by permitting plants to absorb energy from sunlight. Chlorophyll is a mixture of chlorophyll- a and chlorophyll- b .Besides green plants, other organisms that perform photosynthesis contain various other forms of chlorophyll such as chlorophyll- c1 , chlorophyll- c2 , chlorophyll- d and chlorophyll- f .
Also Read: Biological Pigments
Process Of Photosynthesis
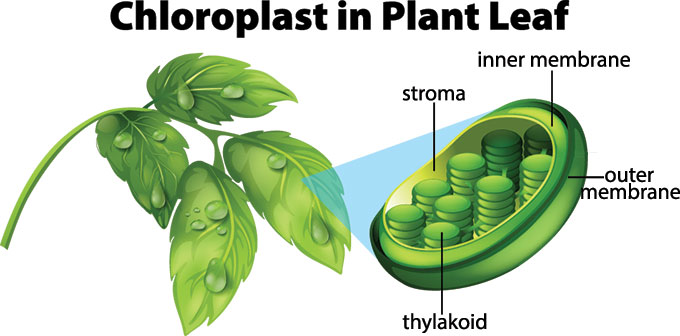
At the cellular level, the photosynthesis process takes place in cell organelles called chloroplasts. These organelles contain a green-coloured pigment called chlorophyll, which is responsible for the characteristic green colouration of the leaves.
As already stated, photosynthesis occurs in the leaves and the specialized cell organelles responsible for this process is called the chloroplast. Structurally, a leaf comprises a petiole, epidermis and a lamina. The lamina is used for absorption of sunlight and carbon dioxide during photosynthesis.
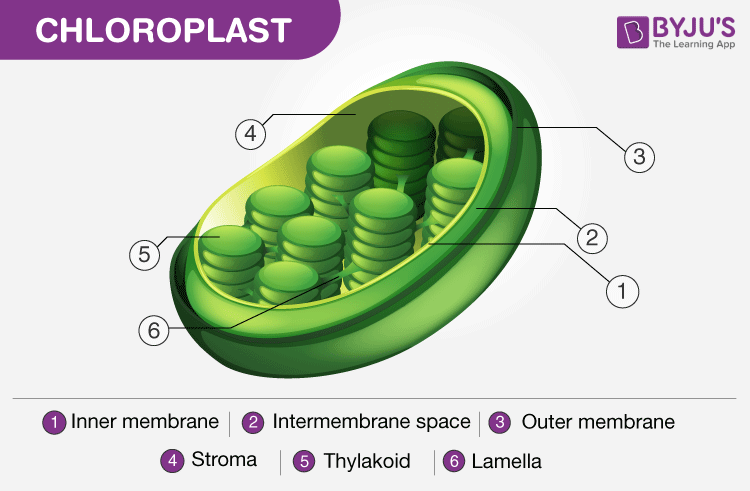
Structure of Chloroplast. Note the presence of the thylakoid
“Photosynthesis Steps:”
- During the process of photosynthesis, carbon dioxide enters through the stomata, water is absorbed by the root hairs from the soil and is carried to the leaves through the xylem vessels. Chlorophyll absorbs the light energy from the sun to split water molecules into hydrogen and oxygen.
- The hydrogen from water molecules and carbon dioxide absorbed from the air are used in the production of glucose. Furthermore, oxygen is liberated out into the atmosphere through the leaves as a waste product.
- Glucose is a source of food for plants that provide energy for growth and development , while the rest is stored in the roots, leaves and fruits, for their later use.
- Pigments are other fundamental cellular components of photosynthesis. They are the molecules that impart colour and they absorb light at some specific wavelength and reflect back the unabsorbed light. All green plants mainly contain chlorophyll a, chlorophyll b and carotenoids which are present in the thylakoids of chloroplasts. It is primarily used to capture light energy. Chlorophyll-a is the main pigment.
The process of photosynthesis occurs in two stages:
- Light-dependent reaction or light reaction
- Light independent reaction or dark reaction
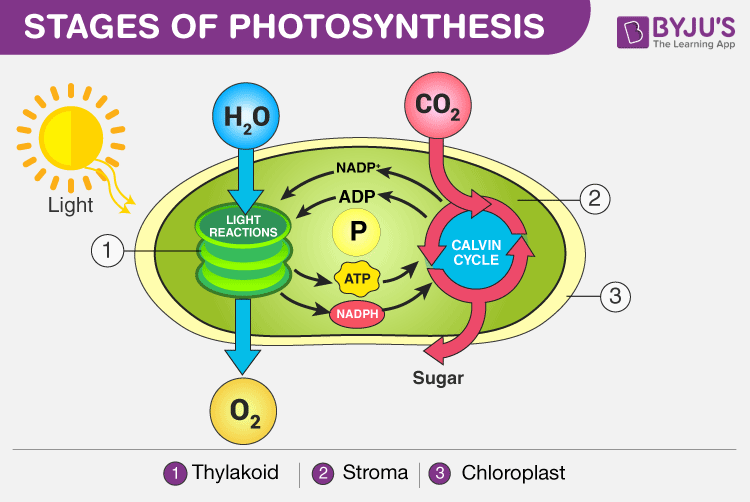
Stages of Photosynthesis in Plants depicting the two phases – Light reaction and Dark reaction
Light Reaction of Photosynthesis (or) Light-dependent Reaction
- Photosynthesis begins with the light reaction which is carried out only during the day in the presence of sunlight. In plants, the light-dependent reaction takes place in the thylakoid membranes of chloroplasts.
- The Grana, membrane-bound sacs like structures present inside the thylakoid functions by gathering light and is called photosystems.
- These photosystems have large complexes of pigment and proteins molecules present within the plant cells, which play the primary role during the process of light reactions of photosynthesis.
- There are two types of photosystems: photosystem I and photosystem II.
- Under the light-dependent reactions, the light energy is converted to ATP and NADPH, which are used in the second phase of photosynthesis.
- During the light reactions, ATP and NADPH are generated by two electron-transport chains, water is used and oxygen is produced.
The chemical equation in the light reaction of photosynthesis can be reduced to:
2H 2 O + 2NADP+ + 3ADP + 3Pi → O 2 + 2NADPH + 3ATP
Dark Reaction of Photosynthesis (or) Light-independent Reaction
- Dark reaction is also called carbon-fixing reaction.
- It is a light-independent process in which sugar molecules are formed from the water and carbon dioxide molecules.
- The dark reaction occurs in the stroma of the chloroplast where they utilize the NADPH and ATP products of the light reaction.
- Plants capture the carbon dioxide from the atmosphere through stomata and proceed to the Calvin photosynthesis cycle.
- In the Calvin cycle , the ATP and NADPH formed during light reaction drive the reaction and convert 6 molecules of carbon dioxide into one sugar molecule or glucose.
The chemical equation for the dark reaction can be reduced to:
3CO 2 + 6 NADPH + 5H 2 O + 9ATP → G3P + 2H+ + 6 NADP+ + 9 ADP + 8 Pi
* G3P – glyceraldehyde-3-phosphate
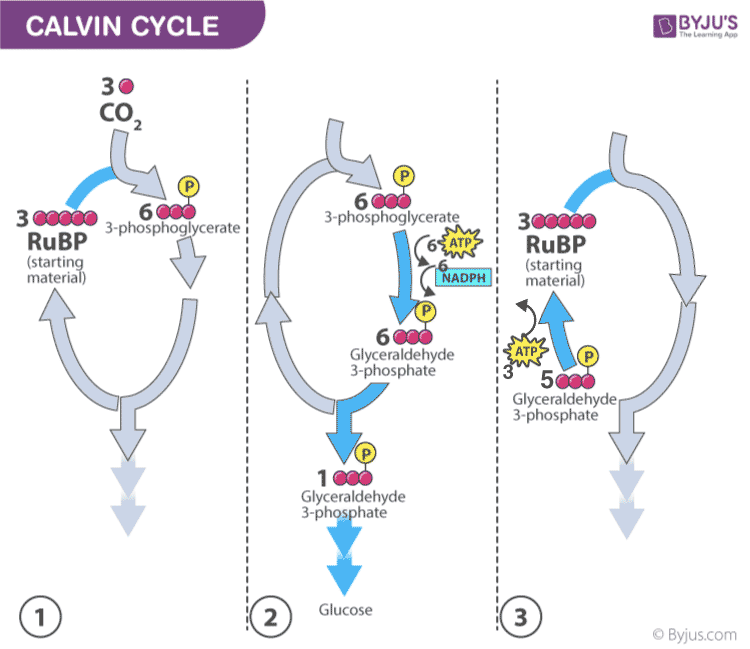
Calvin photosynthesis Cycle (Dark Reaction)
Also Read: Cyclic And Non-Cyclic Photophosphorylation
Importance of Photosynthesis
- Photosynthesis is essential for the existence of all life on earth. It serves a crucial role in the food chain – the plants create their food using this process, thereby, forming the primary producers.
- Photosynthesis is also responsible for the production of oxygen – which is needed by most organisms for their survival.
Frequently Asked Questions
1. what is photosynthesis explain the process of photosynthesis., 2. what is the significance of photosynthesis, 3. list out the factors influencing photosynthesis., 4. what are the different stages of photosynthesis, 5. what is the calvin cycle, 6. write down the photosynthesis equation..

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Biology related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
very useful
It’s very helpful ☺️
Please What Is Meant By 300-400 PPM
PPM stands for Parts-Per-Million. It corresponds to saying that 300 PPM of carbon dioxide indicates that if one million gas molecules are counted, 300 out of them would be carbon dioxide. The remaining nine hundred ninety-nine thousand seven hundred are other gas molecules.
Thank you very much Byju’s! I couldn’t find the answer anywhere. But luckily I hit upon this website. Awesome explanation and illustration.
byjus = Wow!
It helps me a lot thank you
Thanks in a million I love Byjus!
Super Byjus
Thanks helped a lot
Very interesting and helpful site.
Nice it is very uesful
It’s very useful 👍 Thank you Byju’s
Thank you very much Byju’s! I couldn’t find the answer anywhere. But luckily I hit upon this website. Awesome explanation and illustration.
Thank you BYJU’S for helping me in further clarifying my concepts
Excellent material easy to understand
Indeed, it’s precise and understandable. I like it.
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

It’s a wonderful world — and universe — out there.
Come explore with us!
Science News Explores
Explainer: how photosynthesis works.
Plants make sugar and oxygen with the power of water, carbon dioxide and sunlight

Green plants take in light from the sun and turn water and carbon dioxide into the oxygen we breathe and the sugars we eat.
Jeja/E+/Getty Images
Share this:
- Google Classroom
By Bethany Brookshire
October 28, 2020 at 6:30 am
Take a deep breath. Then thank a plant. If you eat fruit, vegetables, grains or potatoes, thank a plant too. Plants and algae provide us with the oxygen we need to survive, as well as the carbohydrates we use for energy. They do it all through photosynthesis.
Photosynthesis is the process of creating sugar and oxygen from carbon dioxide, water and sunlight. It happens through a long series of chemical reactions. But it can be summarized like this: Carbon dioxide, water and light go in. Glucose, water and oxygen come out. (Glucose is a simple sugar.)
Photosynthesis can be split into two processes. The “photo” part refers to reactions triggered by light. “Synthesis” — the making of the sugar — is a separate process called the Calvin cycle.
Both processes happen inside a chloroplast. This is a specialized structure, or organelle, in a plant cell. The structure contains stacks of membranes called thylakoid membranes. That’s where the light reaction begins.
Let the light shine in
When light hits a plant’s leaves, it shines on chloroplasts and into their thylakoid membranes. Those membranes are filled with chlorophyll , a green pigment. This pigment absorbs light energy. Light travels as electromagnetic waves . The wavelength — distance between waves — determines energy level. Some of those wavelengths are visible to us as the colors we see . If a molecule, such as chlorophyll, has the right shape, it can absorb the energy from some wavelengths of light.
Chlorophyll can absorb light we see as blue and red. That’s why we see plants as green. Green is the wavelength plants reflect, not the color they absorb.
While light travels as a wave, it also can be a particle called a photon . Photons have no mass. They do, however, have a small amount of light energy.
When a photon of light from the sun bounces into a leaf, its energy excites a chlorophyll molecule. That photon starts a process that splits a molecule of water. The oxygen atom that splits off from the water instantly bonds with another, creating a molecule of oxygen, or O 2 . The chemical reaction also produces a molecule called ATP and another molecule called NADPH. Both of these allow a cell to store energy. The ATP and NADPH also will take part in the synthesis part of photosynthesis.
Notice that the light reaction makes no sugar. Instead, it supplies energy — stored in the ATP and NADPH — that gets plugged into the Calvin cycle. This is where sugar is made.
But the light reaction does produce something we use: oxygen. All the oxygen we breathe is the result of this step in photosynthesis, carried out by plants and algae (which are not plants ) the world over.
Give me some sugar
The next step takes the energy from the light reaction and applies it to a process called the Calvin cycle. The cycle is named for Melvin Calvin, the man who discovered it.
The Calvin cycle is sometimes also called the dark reaction because none of its steps require light. But it still happens during the day. That’s because it needs the energy produced by the light reaction that comes before it.
While the light reaction takes place in the thylakoid membranes, the ATP and NADPH it produces end up in the stroma. This is the space inside the chloroplast but outside the thylakoid membranes.
The Calvin cycle has four major steps:
- carbon fixation : Here, the plant brings in CO 2 and attaches it to another carbon molecule, using rubisco. This is an enzyme , or chemical that makes reactions move faster. This step is so important that rubisco is the most common protein in a chloroplast — and on Earth. Rubisco attaches the carbon in CO 2 to a five-carbon molecule called ribulose 1,5-bisphosphate (or RuBP). This creates a six-carbon molecule, which immediately splits into two chemicals, each with three carbons.
- reduction : The ATP and NADPH from the light reaction pop in and transform the two three-carbon molecules into two small sugar molecules. The sugar molecules are called G3P. That’s short for glyceraldehyde 3-phosphate (GLIH- sur-AAL-duh-hide 3-FOS-fayt).
- carbohydrate formation : Some of that G3P leaves the cycle to be converted into bigger sugars such as glucose (C 6 H 12 O 6 ).
- regeneration : With more ATP from the continuing light reaction, leftover G3P picks up two more carbons to become RuBP. This RuBP pairs up with rubisco again. They are now ready to start the Calvin cycle again when the next molecule of CO 2 arrives.
At the end of photosynthesis, a plant ends up with glucose (C 6 H 12 O 6 ), oxygen (O 2 ) and water (H 2 O). The glucose molecule goes on to bigger things. It can become part of a long-chain molecule, such as cellulose; that’s the chemical that makes up cell walls. Plants also can store the energy packed in a glucose molecule within larger starch molecules. They can even put the glucose into other sugars — such as fructose — to make a plant’s fruit sweet.
All of these molecules are carbohydrates — chemicals containing carbon, oxygen and hydrogen. (CarbOHydrate makes it easy to remember.) The plant uses the bonds in these chemicals to store energy. But we use the these chemicals too. Carbohydrates are an important part of the foods we eat, particularly grains, potatoes, fruits and vegetables.
More Stories from Science News Explores on Plants

Experiment: Can plants stop soil erosion?

On hot summer days, this thistle stays cool to the touch

Rampaging vines are slowly strangling tropical forests

This urban gardener is mimicking nature to create healthier plants

To spy this palm’s blooms and fruits, start digging underground

Here’s why blueberries aren’t blue — but appear to be

Scientists Say: Marcescence

Pikmin ’s plant-animal mashups don’t exist — but sun-powered animals do
- OC Test Preparation
- Selective School Test Preparation
- Maths Acceleration
- English Advanced
- Maths Standard
- Maths Advanced
- Maths Extension 1
- Maths Standard 2
- Maths Extension 2
- UCAT Preparation
- UCAT Preparation Course Online
Select a year to see available courses
- Level 7 English
- Level 7 Maths
- Level 8 English
- Level 8 Maths
- Level 9 English
- Level 9 Maths
- Level 9 Science
- Level 10 English
- Level 10 Maths
- Level 10 Science
- VCE English Units 1/2
- VCE Biology Units 1/2
- VCE Chemistry Units 1/2
- VCE Physics Units 1/2
- VCE Maths Methods Units 1/2
- VCE English Units 3/4
- VCE Maths Methods Units 3/4
- VCE Biology Unit 3/4
- VCE Chemistry Unit 3/4
- VCE Physics Unit 3/4
- Castle Hill
- Strathfield
- Sydney City
- Inspirational Teachers
- Great Learning Environments
- Proven Results
- Jobs at Matrix
- 1300 008 008
- Book a Free Trial
How to Write a Scientific Report | Step-by-Step Guide
Got to document an experiment but don't know how? In this post, we'll guide you step-by-step through how to write a scientific report and provide you with an example.
Get free study tips and resources delivered to your inbox.
Join 75,893 students who already have a head start.
" * " indicates required fields
You might also like
- What Tense Should I Use?
- 20 Words Your Year 7 Child Must Know | Vocabulary Test
- Jarif’s Hacks: How I Stopped Wasting Time and Began Acing My Subjects
- Year 9 Maths Algebra: Factorisation Techniques [Free Algebra Worksheet]
- How to Write a Topic Sentence for Years 9 and 10 Students
Related courses
Year 9 science, year 10 science.
Is your teacher expecting you to write an experimental report for every class experiment? Are you still unsure about how to write a scientific report properly? Don’t fear! We will guide you through all the parts of a scientific report, step-by-step.
How to write a scientific report:
- What is a scientific report
- General rules to write Scientific reports
- Syllabus dot point
- Introduction/Background information
- Risk assessment

What is a scientific report?
A scientific report documents all aspects of an experimental investigation. This includes:
- The aim of the experiment
- The hypothesis
- An introduction to the relevant background theory
- The methods used
- The results
- A discussion of the results
- The conclusion
Scientific reports allow their readers to understand the experiment without doing it themselves. In addition, scientific reports give others the opportunity to check the methodology of the experiment to ensure the validity of the results.
A scientific report is written in several stages. We write the introduction, aim, and hypothesis before performing the experiment, record the results during the experiment, and complete the discussion and conclusions after the experiment.
But, before we delve deeper into how to write a scientific report, we need to have a science experiment to write about! Read our 7 Simple Experiments You Can Do At Home article and see which one you want to do.

General rules about writing scientific reports
Learning how to write a scientific report is different from writing English essays or speeches!
You have to use:
- Passive voice (which you should avoid when writing for other subjects like English!)
- Past-tense language
- Headings and subheadings
- A pencil to draw scientific diagrams and graphs
- Simple and clear lines for scientific diagrams
- Tables and graphs where necessary
Structure of scientific reports:
Now that you know the general rules on how to write scientific reports, let’s look at the conventions for their structure!
The title should simply introduce what your experiment is about.
The Role of Light in Photosynthesis
2. Introduction/Background information
Write a paragraph that gives your readers background information to understand your experiment.
This includes explaining scientific theories, processes and other related knowledge.
Photosynthesis is a vital process for life. It occurs when plants intake carbon dioxide, water, and light, and results in the production of glucose and water. The light required for photosynthesis is absorbed by chlorophyll, the green pigment of plants, which is contained in the chloroplasts.
The glucose produced through photosynthesis is stored as starch, which is used as an energy source for the plant and its consumers.
The presence of starch in the leaves of a plant indicates that photosynthesis has occurred.

The aim identifies what is going to be tested in the experiment. This should be short, concise and clear.
The aim of the experiment is to test whether light is required for photosynthesis to occur.
4. Hypothesis
The hypothesis is a prediction of the outcome of the experiment. You have to use background information to make an educated prediction.
It is predicted that photosynthesis will occur only in leaves that are exposed to light and not in leaves that are not exposed to light. This will be indicated by the presence or absence of starch in the leaves.
5. Risk assessment
Identify the hazards associated with the experiment and provide a method to prevent or minimise the risks. A hazard is something that can cause harm, and the risk is the likelihood that harm will occur from the hazard.
A table is an excellent way to present your risk assessment.
Remember, you have to specify the type of harm that can occur because of the hazard. It is not enough to simply identify the hazard.
- Do not write: “Scissors are sharp”
- Instead, you have to write: “Scissors are sharp and can cause injury”

The method has 3 parts:
- A list of every material used
- Steps of what you did in the experiment
- A scientific diagram of the experimental apparatus
Let’s break down what you need to do for each section.
6a. Materials
This must list every piece of equipment and material you used in the experiment.
Remember, you need to also specify the amount of each material you used.
- 1 geranium plant
- Aluminium foil
- 2 test tubes
- 1 test tube rack
- 1 pair of scissors
- 1 250 mL beaker
- 1 pair of forceps
- 1 10 mL measuring cylinder
- Iodine solution (5 mL)
- Methylated spirit (50ml)
- Boiling water
- 2 Petri dishes

The rule of thumb is that you should write the method in a clear way so that readers are able to repeat the experiment and get similar results.
Using a numbered list for the steps of your experimental procedure is much clearer than writing a whole paragraph of text. The steps should:
- Be written in a sequential order, based on when they were performed.
- Specify any equipment that was used.
- Specify the quantity of any materials that were used.
You also need to use past tense and passive voice when you are writing your method. Scientific reports are supposed to show the readers what you did in the experiment, not what you will do.
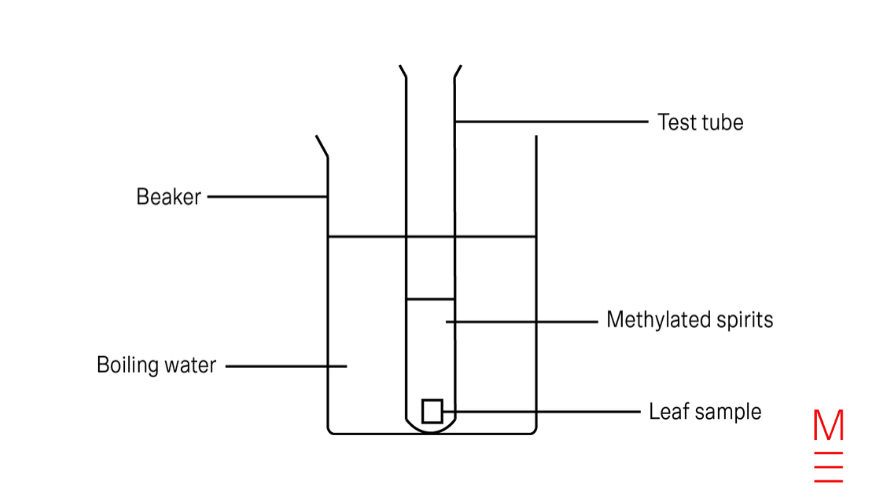
- Aluminium foil was used to fully cover a leaf of the geranium plant. The plant was left in the sun for three days.
- On the third day, the covered leaf and 1 non-covered leaf were collected from the plant. The foil was removed from the covered leaf, and a 1 cm square was cut from each leaf using a pair of scissors.
- 150 mL of water was boiled in a kettle and poured into a 250 mL beaker.
- Using forceps, the 1 cm square of covered leaf was placed into the beaker of boiling water for 2 minutes. It was then placed in a test tube labelled “dark”.
- The water in the beaker was discarded and replaced with 150 mL of freshly boiled water.
- Using forceps, the 1 cm square non-covered leaf was placed into the beaker of boiling water for 2 minutes. It was then placed in a test tube labelled “light”
- 5 mL of methylated spirit was measured with a measuring cylinder and poured into each test tube so that the leaves were fully covered.
- The water in the beaker was replaced with 150 mL of freshly boiled water and both the “light” and “dark” test tubes were immersed in the beaker of boiling water for 5 minutes.
- The leaves were collected from each test tube with forceps, rinsed under cold running water, and placed onto separate labelled Petri dishes.
- 3 drops of iodine solution were added to each leaf.
- Both Petri dishes were placed side by side and observations were recorded.
- The experiment was repeated 5 times, and results were compared between different groups.
6c. Diagram
After you finish your steps, it is time to draw your scientific diagrams! Here are some rules for drawing scientific diagrams:
- Always use a pencil to draw your scientific diagrams.
- Use simple, sharp, 2D lines and shapes to draw your diagram. Don’t draw 3D shapes or use shading.
- Label everything in your diagram.
- Use thin, straight lines to label your diagram. Do not use arrows.
- Ensure that the label lines touch the outline of the equipment you are labelling and not cross over it or stop short of it
- The label lines should never cross over each other.
- Use a ruler for any straight lines in your diagram.
- Draw a sufficiently large diagram so all components can be seen clearly.
This is where you document the results of your experiment. The data that you record for your experiment will generally be qualitative and/or quantitative.
Qualitative data is data that relates to qualities and is based on observations (qualitative – quality). This type of data is descriptive and is recorded in words. For example, the colour changed from green to orange, or the liquid became hot.
Quantitative data refers to numerical data (quantitative – quantity). This type of data is recorded using numbers and is either measured or counted. For example, the plant grew 5.2 cm, or there were 5 frogs.
You also need to record your results in an appropriate way. Most of the time, a table is the best way to do this.
Here are some rules to using tables
- Use a pencil and a ruler to draw your table
- Draw neat and straight lines
- Ensure that the table is closed (connect all your lines)
- Don’t cross your lines (erase any lines that stick out of the table)
- Use appropriate columns and rows
- Properly name each column and row (including the units of measurement in brackets)
- Do not write your units in the body of your table (units belong in the header)
- Always include a title
Note : If your results require calculations, clearly write each step.
Observations of the effects of light on the amount of starch in plant leaves.

If quantitative data was recorded, the data is often also plotted on a graph.
8. Discussion
The discussion is where you analyse and interpret your results, and identify any experimental errors or possible areas of improvements.
You should divide your discussion as follows.
1. Trend in the results
Describe the ‘trend’ in your results. That is, the relationship you observed between your independent and dependent variables.
The independent variable is the variable that you are changing in the experiment. In this experiment, it is the amount of light that the leaves are exposed to.
The dependent variable is the variable that you are measuring in the experiment, In this experiment, it is the presence of starch in the leaves.
Explain how a particular result is achieved by referring to scientific knowledge, theories and any other scientific resources you find. 2. Scientific explanation:
The presence of starch is indicated when the addition of iodine causes the leaf to turn dark purple. The results show that starch was present in the leaves that were exposed to light, while the leaves that were not exposed to light did not contain starch.
2. Scientific explanation:
Provide an explanation of the results using scientific knowledge, theories and any other scientific resources you find.
As starch is produced during photosynthesis, these results show that light plays a key role in photosynthesis.
3. Validity
Validity refers to whether or not your results are valid. This can be done by examining your variables.
VA lidity = VA riables
Identify the independent, dependent, controlled variables and the control experiment (if you have one).
The controlled variables are the variables that you keep the same across all tests e.g. the size of the leaf sample.
The control experiment is where you don’t apply an independent variable. It is untouched for the whole experiment.
Ensure that you never change more than one variable at a time!
The independent variable of the experiment was amount of light that the leaves were exposed to (the covered and uncovered geranium leaf), while the dependent variable was the presence of starch. The controlled variables were the size of the leaf sample, the duration of the experiment, the amount of time the solutions were heated, and the amount of iodine solution used.
4. Reliability
Identify how you ensured the reliability of the results.
RE liability = RE petition
Show that you repeated your experiments, cross-checked your results with other groups or collated your results with the class.
The reliability of the results was ensured by repeating the experiment 5 times and comparing results with other groups. Since other groups obtained comparable results, the results are reliable.
5. Accuracy
Accuracy should be discussed if your results are in the form of quantitative data, and there is an accepted value for the result.
Accuracy would not be discussed for our example photosynthesis experiment as qualitative data was collected, however it would if we were measuring gravity using a pendulum:
The measured value of gravity was 9.8 m/s 2 , which is in agreement with the accepted value of 9.8 m/s 2 .
6. Possible improvements
Identify any errors or risks found in the experiment and provide a method to improve it.
If there are none, then suggest new ways to improve the experimental design, and/or minimise error and risks.

Possible improvements could be made by including control experiments. For example, testing whether the iodine solution turns dark purple when added to water or methylated spirits. This would help to ensure that the purple colour observed in the experiments is due to the presence of starch in the leaves rather than impurities.
9. Conclusion
State whether the aim was achieved, and if your hypothesis was supported.
The aim of the investigation was achieved, and it was found that light is required for photosynthesis to occur. This was evidenced by the presence of starch in leaves that had been exposed to light, and the absence of starch in leaves that had been unexposed. These results support the proposed hypothesis.
Written by Matrix Science Team
© Matrix Education and www.matrix.edu.au, 2023. Unauthorised use and/or duplication of this material without express and written permission from this site’s author and/or owner is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to Matrix Education and www.matrix.edu.au with appropriate and specific direction to the original content.
Year 9 Science tutoring at Matrix is known for helping students build a strong foundation before studying Biology, Chemistry or Physics in senior school.
Learning methods available
Level 9 Science course that covers every aspect of the new Victorian Science Curriculum.
Level 10 Science course that covers every aspect of the new Victorian Science Curriculum.
Year 10 Science tutoring at Matrix is known for helping students build a strong foundation before studying Biology, Chemistry or Physics in Year 11 and 12.
Related articles

Year 7 & 8 (Stage 4) Recommended Reading List
A list of recommended reading for Year 7 and 8 students.

Daniel’s Hacks: How I Got Into James Ruse in Year 11
Find out about Daniel's experience moving to James Ruse Agricultural High School in Year 11 as well as tips for the application process.

Should I Study HSC English Standard or Advanced?
Unsure about which level of English you should take for Stage 6? In this post we give you the information you need to make an informed decision.
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Biology library
Course: biology library > unit 13.
- Conceptual overview of light dependent reactions
- Light dependent reactions actors
- Photosynthesis: Overview of the light-dependent reactions
Light and photosynthetic pigments
- The light-dependent reactions
Introduction
What is light energy, pigments absorb light used in photosynthesis, chlorophylls, carotenoids, what does it mean for a pigment to absorb light, attribution:.
- “ The light-dependent reactions of photosynthesis ,” by OpenStax College ( CC BY 3.0 ). Download the original article for free at http://cnx.org/contents/f829b3bd-472d-4885-a0a4-6fea3252e2b2@11 .
- " Bis2A 06.3 Photophosphorylation: the light reactions of photosynthesis ," by Mitch Singer ( CC BY 4.0 ). Download the original article for free at http://cnx.org/contents/c8fa5bf4-1af7-4591-8d76-711d0c1f05f9@2 .
Works cited:
- Chlorophyll a. (2015, October 11). Retrieved October 22, 2015 from Wikipedia: https://en.wikipedia.org/wiki/Chlorophyll_a .
- Speer, B.R., (1997, July 9) Photosynthetic pigments. In UCMP glossary . Retrieved from http://www.ucmp.berkeley.edu/glossary/gloss3/pigments.html .
- Bullerjahn, G. S. and A. F. Post. (1993). The prochlorophytes: are they more than just chlorophyll a/b-containing cyanobacteria? Crit. Rev. Microbiol. 19(1), 43. http://dx.doi.org/10.3109/10408419309113522 .
- Reece, J. B., Urry, L. A., Cain, M. L., Wasserman, S. A., Minorsky, P. V., and Jackson, R. B. (2011). Photosynthesis. In Campbell biology (10th ed.). San Francisco, CA: Pearson, 193.
Additional references:
Want to join the conversation.
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

Photosynthesis
Affiliation.
- 1 Department of Molecular Biology and Biotechnology, University of Sheffield, Firth Court, Western Bank, Sheffield S10 2TN, U.K. [email protected].
- PMID: 27784776
- PMCID: PMC5264509
- DOI: 10.1042/EBC20160016
Photosynthesis sustains virtually all life on planet Earth providing the oxygen we breathe and the food we eat; it forms the basis of global food chains and meets the majority of humankind's current energy needs through fossilized photosynthetic fuels. The process of photosynthesis in plants is based on two reactions that are carried out by separate parts of the chloroplast. The light reactions occur in the chloroplast thylakoid membrane and involve the splitting of water into oxygen, protons and electrons. The protons and electrons are then transferred through the thylakoid membrane to create the energy storage molecules adenosine triphosphate (ATP) and nicotinomide-adenine dinucleotide phosphate (NADPH). The ATP and NADPH are then utilized by the enzymes of the Calvin-Benson cycle (the dark reactions), which converts CO 2 into carbohydrate in the chloroplast stroma. The basic principles of solar energy capture, energy, electron and proton transfer and the biochemical basis of carbon fixation are explained and their significance is discussed.
Keywords: membrane; photosynthesis; thylakoid.
© 2016 The Author(s).
Publication types
- Electron Transport*
- Photosynthesis*
- Photosynthetic Reaction Center Complex Proteins / chemistry
- Photosynthetic Reaction Center Complex Proteins / genetics
- Photosynthetic Reaction Center Complex Proteins / metabolism*
- Plants / metabolism*
- Photosynthetic Reaction Center Complex Proteins

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

13.3: Lab Report
- Last updated
- Save as PDF
- Page ID 105849
Exercise 1 Data:
Make a sketch of your chromatogram using the template that follows. Using colored pencils, note the color of the bands. Use Table 1 to help you identify each pigment. On your sketch, label each band with the name of the pigment. Mark the distance (in cm) from the initial pigment band to each colored band as well as the total distance from the initial pigment band to the solvent front.
Sketch of Resulting Chromatogram
- Use the data from your sketch to complete the data table below.
- Calculate the value and include that in your data table.
Exercise 1 Review Questions:
- Which plant pigments are most polar?
- Which are least polar?
- How do these differences in polarity affect the movement of the pigments up the chromatography paper? Explain why this is observed.
4. What does the abbreviation Rf stand for? How is it calculated? What does it tell us?
- If Solution A moves 4 cm and Solution B moves 4.5 cm on a piece of chromatography paper, when the solvent moves 10 cm, which is the most polar solution? Explain your answer.
Exercise 2 Data and Review Questions:
*peaks may vary and are pH-dependent.
- How do you think the knowledge obtained from your chromatogram and the spectrograms relate to our understanding of plant pigments?
- Why do leaves change color in the fall?
Exercise 3 Employing Steps in the Scientific Method:
- Record the Question that is being investigated in this experiment. ________________________________________________________________
- Record a Hypothesis for the question stated above. ________________________________________________________________
- Predict the results of the experiment based on your hypothesis (if/then). ________________________________________________________________
Exercise 3 Review Questions:
- Complete the following sentence.
Photosynthesis is a set of ____________________________ in which ____________energy is converted to __________________energy.
- Why do some trees appear green in the summer but change colors in the fall?
3. What optimal wavelengths (or peaks) did you observe for both chlorophyll a and b ?
4. Do your leaf disks float? Use the information in this diagram of a cross-section of a leaf to explain why a leaf disk would float.
- Where does photosynthesis occur in a leaf? State which organelles carry out photosynthesis and which type or types of leaf cells have this organelle.
- Explain why it is useful to the plant to have air spaces around the spongy mesophyll cells in the leaves. (Hint: Recall the chemical equation for photosynthesis.)
- What was the purpose of the sodium bicarbonate in this experiment?
- Leaf disks normally float. What caused the leaf disks to sink?
- To measure the rate of photosynthesis, you replaced the air in the spongy mesophyll in your leaf disks with a liquid. This caused the leaf disks to sink. Then you put these leaf disks in water with dissolved CO2 and measured the amount of time it took for the leaf disks to float. Which product of photosynthesis accumulated in the spongy mesophyll and caused the leaf disks to float?
- Suppose that a leaf disk that has had the air sucked out is placed in a bicarbonate solution under a dim light that results in a low rate of photosynthesis that just equals the rate of cellular respiration. Would you expect this leaf disk to float? Explain why or why not.
Exercise 4: Observing and Quantifying Stomata (Optional)
- Develop a hypothesis about the number of open stomata found on the upper side of a leaf as compared to the lower side of the leaf. ___________________________________________________________
- Develop a hypothesis about the number of open stomata found on the upper side of a leaf as compared to the lower side of the leaf. Write your hypothesis in the space below. ____________________________________________________________
Extension Activity: (Optional)
The results of this experiment can be presented graphically. The presentation of your data in a graph will assist you in interpreting your results. Based on your results, you can complete the final step of scientific investigation, in which you must be able to propose a logical argument that either allows you to support or reject your initial hypothesis.
- Graph your results using the data from Table 3.
- What is the dependent variable? Which axis is used to graph this data? ______________________________________________________________________
- What is your independent variable? Which axis is used to graph this data? _____________________________________________________________________
Exercise 4 Review Questions:
- What are stomata?
2. What is the importance of stomata in photosynthesis?
3. What is the function of the guard cells?
4. During the lab activity, were more stomata observed on the upper surface of the leaf or the lower surface of the leaf? Explain why you think this distribution exists.
ORIGINAL RESEARCH article
Vegetation communities and soil properties along the restoration process of the jinqianghe mine site in the qilian mountains, china.

- 1 Key Laboratory of Ecohydrology of Inland River Basin, Alax Desert Eco-hydrology Experimental Research Station, Qilian Mountains Eco-Environment Research Center in Gansu Province, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, Lanzhou, China
- 2 Technology Innovation Center for Mine Geological Environment Restoration in the Alpine and Arid Regions, Ministry of Natural Resources, Lanzhou, China
The study explores the impact of mine grassland restoration on plant communities and soil properties in alpine grasslands, a subject of significant interest due to the observed relationship between grassland changes, plant communities, and soil properties. While prior research has mainly focused on the consequences of grassland degradation on plant diversity and soil characteristics, the specific effects of varying restoration degrees in alpine mining grasslands at the regional scale remain poorly understood. To address this knowledge gap, we established 15 sampling plots (0.5m×0.5m) across five different restoration degrees within alpine mining grasslands in the Qilian Mountains, China. Our objective was to assess the variations in plant diversity and soil properties along these restoration gradients. We conducted comprehensive analyses, encompassing soil properties [soil water content (SWC), available nitrogen (AN), total phosphorus (TP), nitrate nitrogen (NO 3 -N), ammonium nitrogen (NH 4 -N), total nitrogen (TN), available phosphorus (AP), soil organic carbon (SOC), nitrate nitrogen, soil pH, and electrical conductivity (EC)], plant characteristics (height, density, frequency, coverage, and aboveground biomass), and plant diversity indices (Simpson, Shannon-Wiener, Margalef, Dominance, and Evenness indexes). Our findings included the identification and collection of 18 plant species from 11 families and 16 genera across the five restoration degrees: Very Low Restoration Degree (VLRD), Low Restoration Degree (LRD), Moderate Restoration Degree (MRD), High Restoration Degree (HRD), and Natural Grassland (NGL). Notably, species like Carex duriuscula , Cyperus rotundus , and Polygonum viviparum showed signs of recovery. Principal component analysis and Pearson correlation analysis revealed that soil pH, SWC, SOC, NO 3 -N, and AN were the primary environmental factors influencing plant communities. Specifically, soil pH and EC decreased as restoration levels increased, while SWC, AN, TP, NH 4 -N, TN, AP, SOC, and NO 3 -N exhibited a gradual increase with greater restoration efforts. Furthermore, the HRD plant community demonstrated similarities to the NGL, indicating the most effective natural recovery. In conclusion, our study provides valuable insights into the responses of plant community characteristics, plant diversity, and soil properties across varying restoration degrees to environmental factors. It also elucidates the characteristics of plant communities along recovery gradients in alpine grasslands.
1 Introduction
Alpine grasslands in Northwest China serve as a critical ecological buffer, offering vital ecosystem functions, including livestock grazing, landscape aesthetics, and vegetation production ( Wang et al., 2018 ). However, the alpine grassland ecosystem is notably sensitive to human activities and climate fluctuations, rendering it highly susceptible to issues like grassland degradation, soil contamination, and declining vegetation cover ( Lei et al., 2020 ; Wang and Ali, 2021 ). Once an ecological imbalance occurs, ecosystem recovery becomes a formidable challenge ( Dudley et al., 2020 ). Northwest China boasts abundant mineral resources, yet imprudent or excessive mining practices can trigger a range of ecological problems, including soil quality deterioration due to human interventions ( Wu et al., 2021 ). Consequently, this contributes to the degradation of mining sites. Achieving equilibrium between the alpine grassland ecosystem and mining grassland ecosystems has emerged as a significant scientific endeavor ( Xie et al., 2017 ).
Plant communities and soil properties share a close relationship, with soil properties serving as a determinant of plant composition ( Jochum et al., 2020 ). Human activities, climate conditions, and soil properties collectively influence the development of alpine grassland ecosystems. Mining, as a significant factor, contributes to grassland degradation and alters plant community composition ( Wiegand et al., 2007 ; Chang et al., 2015 ). However, it’s important to note that variations in soil properties primarily define the plant community in mining grasslands ( Zhang et al., 2018 ; Gao et al., 2021 ). Although prior research has acknowledged the impact of excessive mining on alpine grassland ecosystems, there remains a gap in understanding the mechanisms behind the changes in grassland restoration levels ( Guo et al., 2021 ; Huang et al., 2021a ; Castro et al., 2023 ). In particular, studying how the plant community evolves can shed light on grassland restoration mechanisms ( Kang et al., 2020 ). Plant community characteristics can provide insights into both plant diversity, encompassing variety and function, and how they respond to different restoration levels ( Harrison et al., 2020 ). Examining the diversity of plant species and their functions across various restoration levels is a compelling avenue of inquiry. While previous studies have predominantly focused on individual plant communities or soil properties at different restoration levels ( Feyissa et al., 2021 ; Stokes et al., 2021 ), there is a limited body of research on how plant communities and plant diversity change across these levels ( Zhou et al., 2019 ; Schmid et al., 2021 ). Quantifying plant community and soil properties at different restoration levels can enhance our understanding of how plant communities and plant diversity evolve in alpine grasslands.
The Qilian Mountains National Nature Reserve is a crucial national ecological sanctuary in China, meticulously preserved and protected. It plays an indispensable role in shaping and efforts have been made to enhance the ecological environment in the Western region ( Feng et al., 2019 ). However, mining activities in the Qilian Mountains Reserve have led to ecological damage, resulting in the degradation of the original grassland and soil erosion ( Kong et al., 2021 ). Presently, numerous researchers have been actively involved in managing the ecological environment of mining areas and implementing scientific and rational approaches for the restoration of degraded grassland in these regions. The ecological balance of moderate restoration of its productivity and economic benefits is particularly important ( Chen et al., 2020 ). The mining area in the Qilian Mountains in the Tianzhu region of China has expanded rapidly in recent years, and the largest open mine in the Gansu Province is located in this region. The ecological and environmental damage to the nature reserve has aroused considerable attention. However, there have been few studies on how plant community features and soil properties changes in mining areas have affected the mining grasslands ( Chen et al., 2022 ).
While some studies have explored the restoration of mining areas, there is a notable gap in understanding how restored plant community features vary. Additionally, there has been limited research on plant community features and soil properties across different restoration levels in alpine grasslands ( Lorite et al., 2021 ; Siebert et al., 2021 ). To address this knowledge gap, our study investigates plant communities and soil properties at various restoration levels within the primary gold mining area of alpine grasslands in the Qilian Mountains, China. We hypothesize that plant communities, plant diversity, and soil properties strongly influence the outcomes at different restoration levels in alpine grasslands. Specifically, our study seeks to achieve the following objectives: (1) Assess the restoration outcomes of alpine mining grassland ecosystems compared to natural grasslands; (2) Investigate how plant communities, plant diversity, functional diversity, and soil properties change at various levels of restoration within the mining area. The findings from this study aim to provide valuable insights into how different restoration levels respond to environmental factors. Additionally, our research intends to establish a scientific foundation for the rational restoration of alpine grassland resources in mining areas.
2 Materials and methods
2.1 study area description.
The Jinqianghe mining area is located in the part of Daiqian village, Zhuaxixiulong Town, Wuwei City, Gansu Province. The geographical coordinates are 37°25’30”-37°25’63”N, 102°51’48”-102°57’82”E ( Figures 1A, B ), and the altitude is 3267-3323m. This area is located at the junction of Gansu and Qinghai provinces, Zhuanglang River Jinqiang River source of the typical gold mine restoration demonstration area, in Qilian Mountains National Nature Reserve. Soil types in the project area are mainly grassland meadow soils, followed by large black soils and black hemp soils. There is no treatment of the original soil because the sampling places are in the meadow of the Jinqianghe mining area. Jinqianghe upstream alluvial gold mining area is in the headwaters of the Jinqianghe, the source of the Zhuanglang River, the overall topography of the northwest high southeast low, the topography of the undulating changes, elevation generally 3190~4100 m, the relative height difference of the mountains are more than 500 m, the slope of the mountain slope is generally 30~60°, the local section of the slope is greater than 70°. The valley of Jinqianghe is generally 200m~300m wide, and the widest part can reach 1000m, with the first and second terraces developed and third terraces sporadically developed.
Figure 1 Location of the study area on the Chinese Qilian Mountains (A, B) and VLRD, very low recovered degree (C) , and LRD, low recovered degree (D) and MRD, middle recovered degree (E) and HRD, high recovered degree (F) and NGL, CK natural grassland (G) .
The ecological environment was fragile before the mine was restored, the land was barren and the degree of soil erosion was severe, clearly improving the ecological environment after the mine restoration. The region has an alpine semi-arid climate, with seasonal changes of temperature in the region being obvious, with the highest temperature being in July, and the lowest temperature being in January, and a huge diurnal temperature difference. The average annual temperature is 4.8°C. The average annual rainfall and potential evaporation are 650mm and 1400mm, separately, and the rainy season is mainly focused on the June-September. The distinctive feature is clear vertical zoning, low temperatures, high precipitation (more topographic rain) and short frost-free periods. The Northwest winds are prevalent in the area, with the annual average wind speed is 2.1m/s. The main soil type is grassland meadow soil, followed by big black soil, black hemp soil, and other soil types. Before restoration, due to the serious damage to the ecological environment caused by mining activities, a large amount of dumped soil and slag were piled up randomly, the soil was pressed and dug up, and the vegetation cover was extremely low, mainly through the natural restoration method of slag heap leveling, sowing grass seeds and fencing protection. With the expansion of the scale within the area of mining, the waste slag after mining destroyed the vegetation and degraded the vegetation on both sides of the river valley and the ditch, and the vegetation coverage on both sides of the river valley and the ditch before treatment was lower, the mine restoration from 2018 by the same restoration method of natural restoration ( Yang et al., 2023a ). The primary methods for natural restoration include the leveling of slag heaps, sowing of grass seeds, fence protection, and the facilitation of natural vegetation recovery. Removal of residue piles, backfilling of mining pits, restoration of vegetation in pressure-occupied areas and backfilled areas, and restoration of topography and ecological environment to the greatest extent possible. The approach for restoring succession sequences is determined according to the “Soil Erosion Classification and Grading Standard” (SL190-2007) of China. In accordance with the coverage of natural restoration, we used the temporal dynamics of restoration process by replacing the time scale with space, for the investigation, representative grassland restoration in the Qilian Mountains Tianzhu Jinqianghe gold mine was selected. The study encompassed five different levels of grassland recovery based on plant coverage, classified as follows: Very Low Recovered Degree (VLRD), Low Recovered Degree (LRD), Middle Recovered Degree (MRD), High Recovered Degree (HRD), and a reference of Natural Grassland (NGL), with Natural Grassland (NGL) serving as the control (CK), as indicated in Table 1 . The vegetation primarily consists of Cyperus rotundus, Polygonum viviparum, Oxytropis ochrocephala and Elymus nutans , and the animal husbandry industry is the leading industry.
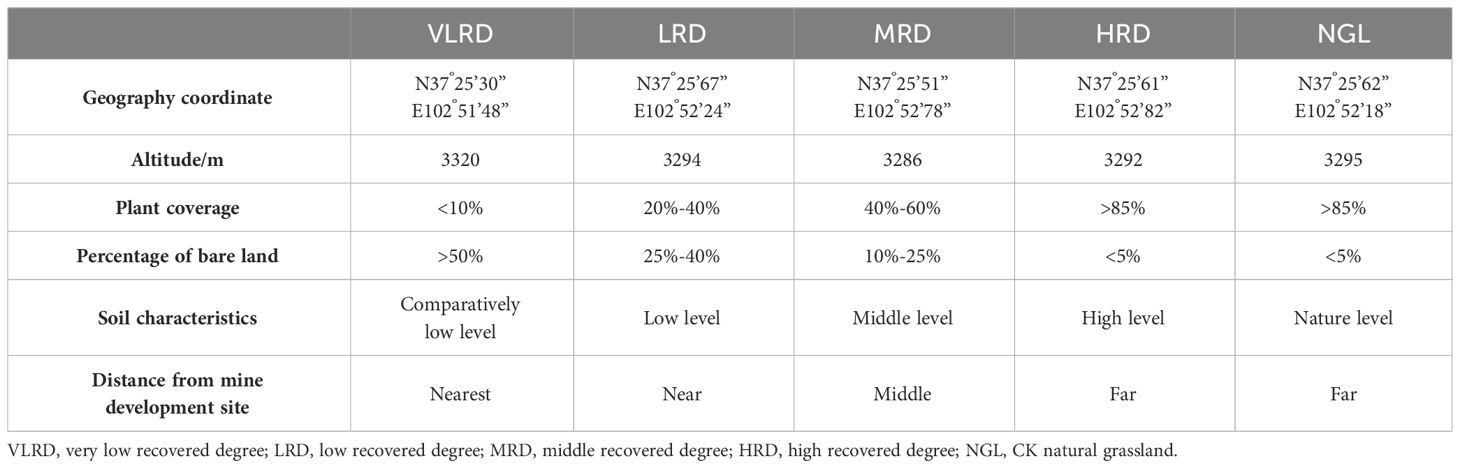
Table 1 Sample plots settings.
2.2 Sample collection and testing
This study primarily focuses on investigating various soil parameters, including pH, electrical conductivity (EC), soil water content (SWC), soil organic carbon (SOC), total nitrogen (TN), nitrate nitrogen (NO 3 -N), ammonium nitrogen (NH 4 -N), available nitrogen (AN), total phosphorus (TP), and available phosphorus (AP). The data collection was conducted in August 2022. Three study sites with similar geographic conditions, vegetation composition, and several years of restoration management were selected. To ensure consistency and representativeness in vegetation and soil sampling, we established three large quadrats within each of the five different restoration grassland areas. Within each large quadrat, five small sampling plots (measuring 50×50 cm) were randomly chosen in the mining area for sample collection, with precise GPS coordinates recorded for accurate positioning ( Figure 1 ). Differences in vegetation restoration were observed, particularly in areas closer to the road, due to varying distances from the mine’s pollution source. Despite employing the same restoration method, disparities in vegetation recovery persisted. We measured plant circumference, plant height using a steel tape measure, and recorded the plant altitude, longitude, and latitude for each location using GPS devices. Plant community characteristics, including plant frequency, plant density, plant coverage, and plant composition, were documented ( Gao et al., 2021 ). For each sampling plot, we collected soil samples (at depths of 0-20 cm and 20-40 cm) from five points, which were subsequently mixed thoroughly to create a composite sample. These soil samples were transported to the laboratory, where they were naturally air-dried, and any rocks, plant roots, or debris were removed. Afterward, the samples were ground, passed through a 120-mesh sieve, properly labeled, and prepared for testing.
The SWC was measured by the drying method. The soil pH, EC, SOC, TN, NO 3 -N, NH 4 -N, AN, TP and AP were determined by the electrode potential ( Pansu and Gautheyrou, 2006 ), conductivity meter, dichromate oxidation, element analyzer, Interval-flow analysis, Flow-Injection, alkaline solution-diffusion, Sommers-Nelson, and molybdenum antimony anti-colorimetric methods ( Pei et al., 2021 ), respectively.
2.3 Data processing methods
The indexes of species diversity and functional diversity within the plant characteristics were examined to explore the changing of plant diversity across restoration gradients. Community Importance Value (IV) serves as a crucial criterion for distinguishing various communities and efficiently determining the major components of each community. The calculation method for the Importance Value (IV) is as follows:
IV = (Relative Height + Relative Cover + Relative Density)/3 ( Zhang, 2004 ).
Species diversity is evaluated through factors like species richness, diversity indices, and evenness. This is expressed using measures such as degree and dominance degree ( Ma, 1994 ). The calculation is as follows:
In the preceding formula, the symbols are defined as follows: H denotes the Shannon-Wiener species diversity index (1) , Ma represents the Margalef richness index (2) , D stands for the Simpson diversity index (3) , Eve corresponds to the Evenness index (4) , Do signifies the Dominance index (5) , Pi denotes the species “i” important value ratio (6) , N is the sum of the important values of plants in the transect, Ni represents the important value of plant “i” in the plot, and S is the total number of species in the plot.
2.4 Statistical analysis
Soil physical and chemical properties for data analysis were obtained from average measurements at depths of 0-20 cm and 20-40 cm per sample. Plant biomass values were determined for the underlying biomass. To assess differences among restored grassland communities, one-way analysis of variance (ANOVA) was applied. Two-tailed relationships between plant community and soil properties were analyzed using Spearman correlation coefficients in SPSS version 25.0 (IBM, Chicago, USA). Principal Component Analysis (PCA) in CANOCO 5.0 ( ter Braak and Smilauer, 2012 ) was employed to explore diversity variations along recovery degrees for enhanced plant distribution pattern assessment. Important value and diversity index for each herb layer plant were computed using Microsoft Excel 2010. Correlation analysis and graph plotting were performed in Origin 9.0 (Origin Lab Corporation, Northampton, MA, USA), while bar charts and additional data analysis were carried out in Sigmaplot 10.0 (Sigmaplot Lab Corporation, Northampton, MA, USA).
3.1 Characteristics of plants at various restoration degrees
The plant characteristics of alpine mining grassland communities exhibited significant variations across different levels of restoration ( Figure 2 ). Vegetation features displayed notable distinctions ( P <0.05) among the various restoration levels, with the ranking being NGL > HRD > MRD > LRD > VLRD. The height of HRD was 1.66, 2.52, and 4.58 times that of MRD, LRD, and VLRD, respectively ( Figure 2A ). Additionally, its density exceeded that of MRD, LRD, and VLRD by 1.06, 4.54, and 9.42 times, as shown in Figure 2B . The frequency of HRD surpassed that of MRD, LRD, and VLRD by 1.17, 2.18, and 3.07 times ( Figure 2C ), while its coverage was 1.48, 3.08, and 4.81 times greater than MRD, LRD, and VLRD ( Figure 2D ), respectively. Moreover, the aboveground biomass of HRD was 1.64, 2.76, and 3.79 times that of MRD, LRD, and VLRD ( Figure 2E ). Notably, natural restoration methods did not achieve the level of NGL (CK).
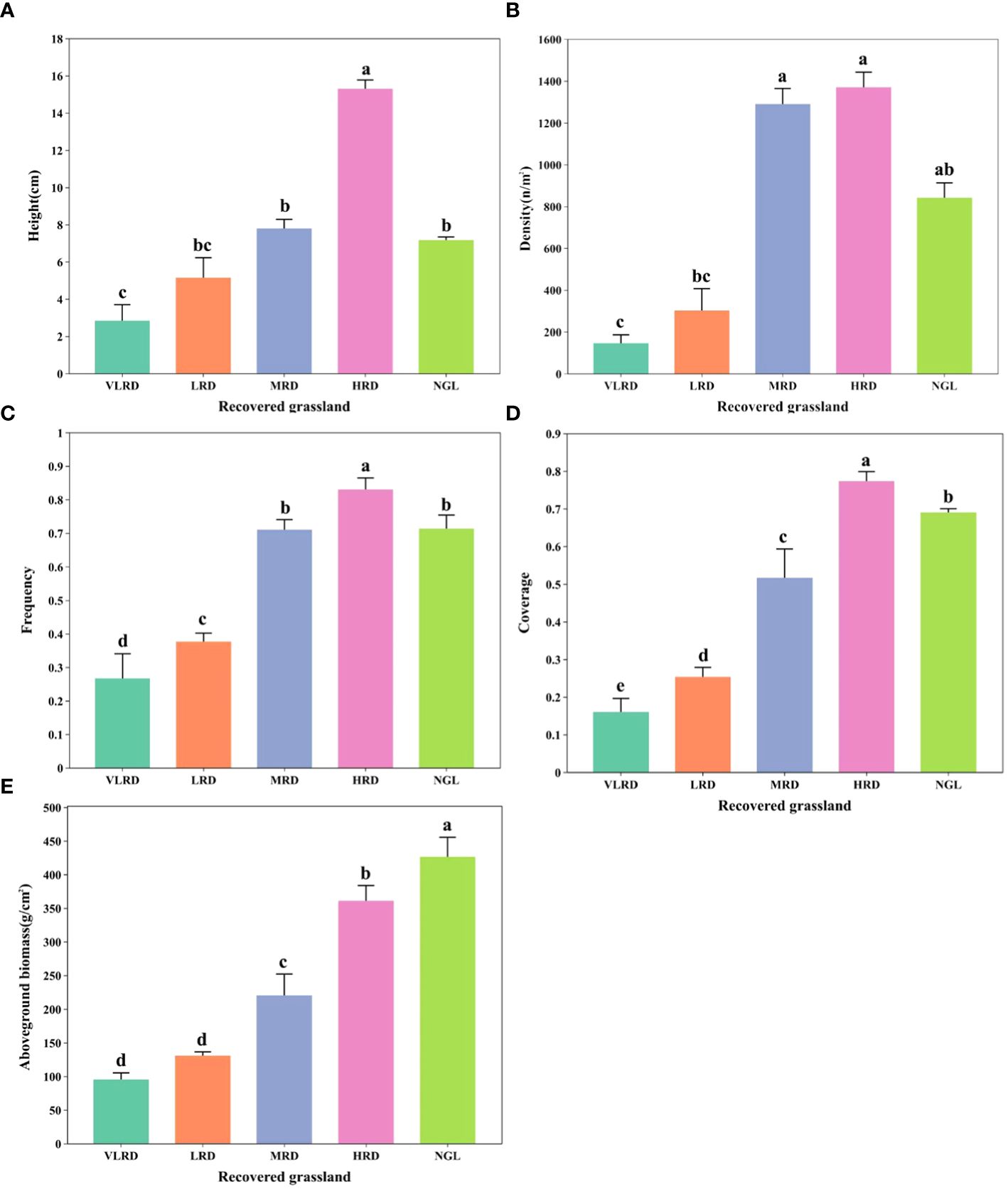
Figure 2 Illustrates the vegetation characteristics of alpine grassland at various stages of restoration, with five key attributes: (A) Height, (B) Density, (C) Frequency, (D) Coverage, and (E) Aboveground biomass. The different restoration levels are represented by the following abbreviations: VLRD (Very Low Recovery Degree), LRD (Low Recovery Degree), MRD (Middle Recovery Degree), HRD (High Recovery Degree), and NGL (CK Natural Grassland). Significant differences at the 0.05 level are denoted by different letters above error bars between the treatments.
3.2 The composition of plant families, genera, and species varies across different restoration degrees
Table 2 presents the taxonomic structure of alpine mining grassland at various stages of restoration. It is evident that the order of species composition varies with different restoration degrees, with HRD > MRD > LRD > VLRD. The total number of individual genera in the various community types aligns with the species composition pattern. According to the statistical data from our sample survey, the study plot contained 18 herbaceous plant species, which were distributed across 11 families and 16 genera. Among these, Asteraceae had the highest species diversity with 6 genera and 6 species. This was followed by Leguminosae and Cyperaceae, each with 2 genera and 2 species, while Rosaceae, Graminaceae, Ranunculus, Polygonum, Chenopodium, Plantago, Apiaceae, and Geraniaceae each had 1 genus and 1 species. This demonstrates the simplicity of the plant genus and species structure, with a relatively dispersed distribution of plant families. Across all samples, three species— Carex duriuscula , Cyperus rotundus , and Polygonum viviparum —were consistently present. Plant species from five different recovery degrees belonged to the Leguminosae, Asteraceae, Compositae, Rosaceae, and Cyperaceae families, and these families exhibited a high degree of representation. Among them, these five major plant families constituted 67% of the total plant species in the HRD community, 55% in the MRD, 86% in the LRD, and 50% in the VLRD. This pattern is largely attributed to the adaptability of Leguminosae and Graminaceae to the challenging environmental conditions characterized by low temperatures and drought in alpine grassland mining areas. Notably, the plant composition in the NGL (CK Natural Grassland) demonstrated the highest degree of diversity in alpine mining grassland areas.
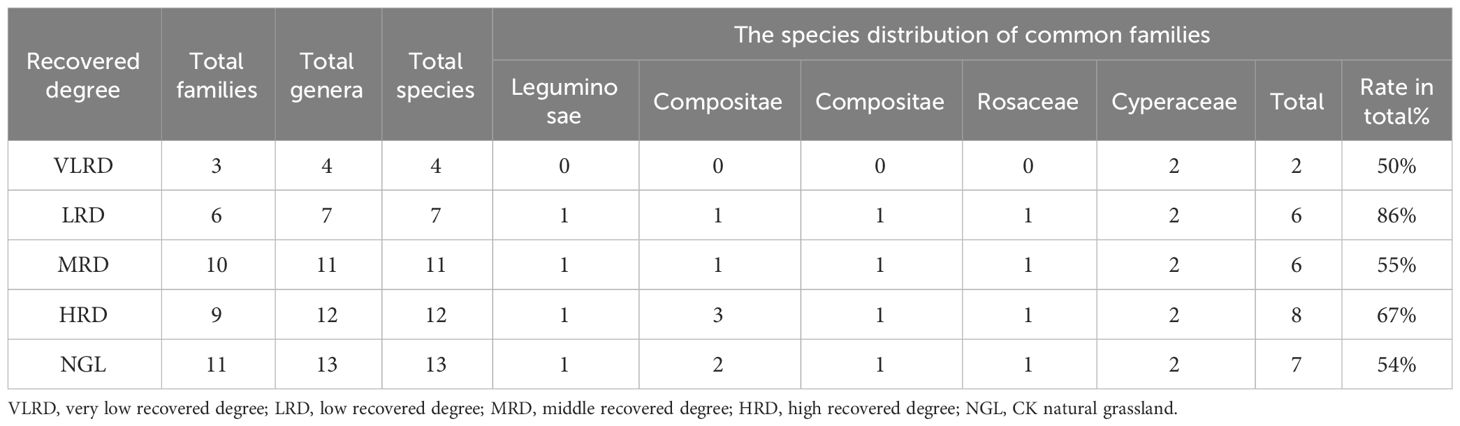
Table 2 Composition of dominant plant families, genera, and species at various levels of recovery.
3.3 Life-form structure characteristics of plant communities
The life-form structure characteristics of plant communities at various restoration levels in alpine mining grassland are shown in Table 3 . The life-form classification follows the Whitaker growth type system, which is based on the degree of stem lignification in the community, as described by Yang et al. (2023a) . This study categorizes the herbaceous plant community into two groups: perennial plants and annual herbaceous plants. Among the life-form structure characteristics, perennial herbaceous species predominate, with 12 species distributed across 9 families and 12 genera, while annual herbaceous species are less dominant, consisting of 4 species from 3 families and 4 genera. Perennial herbaceous species account for 75% of the total species, while annual herbaceous species make up the remaining 25% ( Table 3 ). There is a significant disparity in the abundance of perennial and annual herbaceous species among various restoration levels in alpine mining grassland, with perennial herbaceous species being abundant and annual herbaceous species relatively scarce. Particularly in the NGL, perennial herbaceous species dominate at 97%, and they also constitute a significant portion in HRD, MRD, LRD, and VLRD, with proportions of 88%, 77%, 96%, and 100%, respectively. Conversely, MRD has the largest proportion of annual herbaceous species, amounting to 23%. It’s worth mentioning that the presence of perennial herbaceous species significantly influences the structure, system function, and stability of the grassland community. Although the species structure of NGL grassland is slightly higher than that of HRD, HRD exhibits the most stable species community structure, followed by MRD, LRD, and VLRD grasslands.
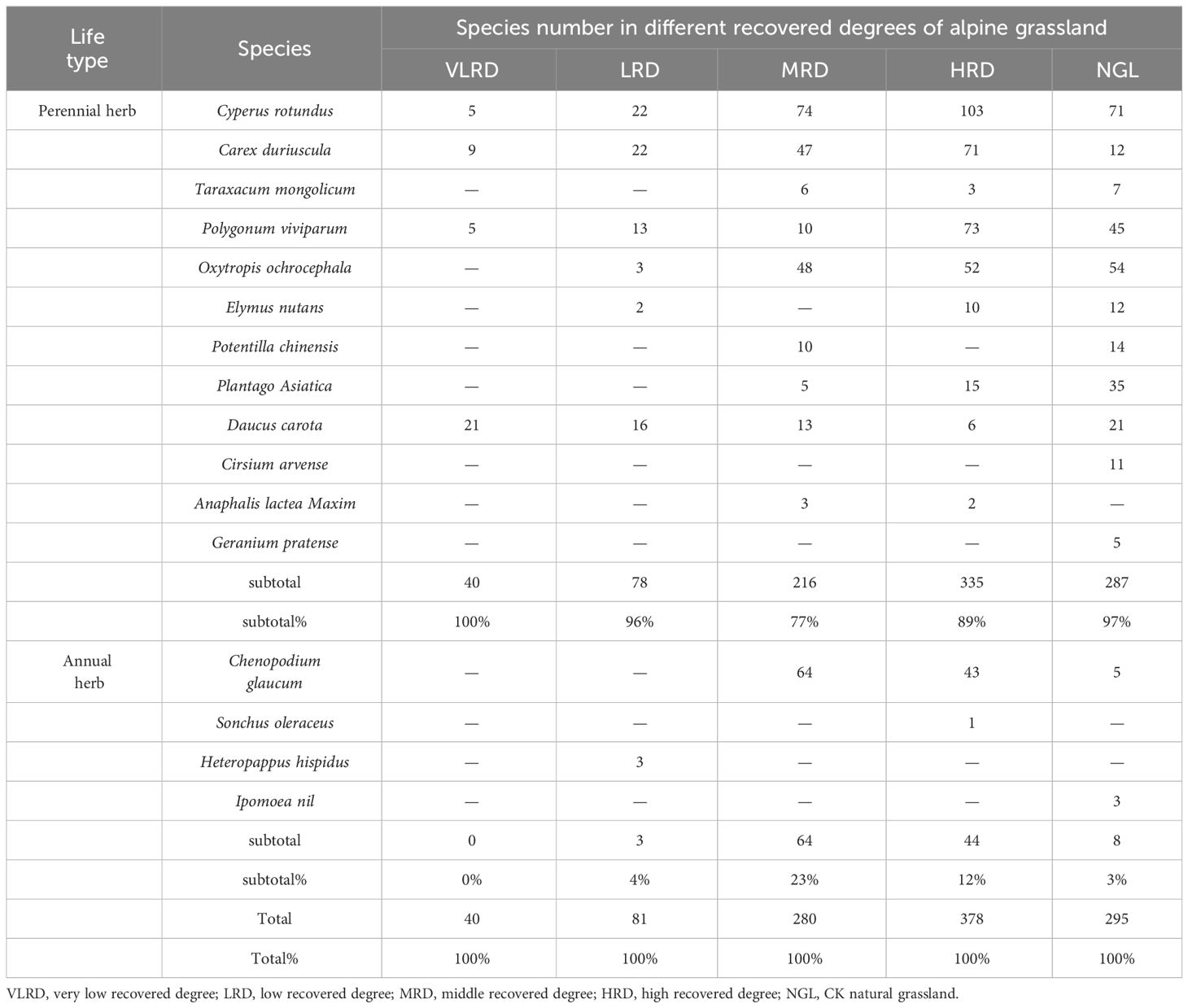
Table 3 Composition and quantitative traits of communities at different recovery stages.
3.4 Plant community value of different restoration degrees
Perennial herbaceous plants occupy a dominant position within various communities, playing a crucial role in shaping community structure, ecosystem function, and overall stability. The importance of plant communities at different restoration levels in alpine grassland is detailed in Table 4 . In HRD, LRD, and VLRD, Carex duriuscula stands out as the dominant species with importance values of 18.53, 27.33, and 39.22, respectively. The sub-dominant species in these communities are Cyperus rotundus and Polygonum viviparum . Notably, the importance values for HRD are 16.70 and 15.64 for Cyperus rotundus and Polygonum viviparum, respectively. In MRD, the dominant species is Carex duriuscula with an importance value of 21.40, while the sub-dominant species is represented by Cyperus rotundus and Polygonum viviparum with respective importance values of 4.53. In HRD, the primary companion species include Chenopodium glaucum and Sonchus oleraceus , boasting importance values of 7.71 and 1.11, respectively. For VLRD, Carex duriuscula and Daucus carota are the dominant species with importance values of 39.22 and 27.11. The importance values of other companion species are relatively similar and remain below 10. When we compare alpine grassland herbaceous communities across four restoration-degree mining areas, it becomes evident that the importance values of perennial herbs in these communities consistently exceed 10. This underscores the significant role played by perennial herbs in the recovery of alpine grassland mining areas, with HRD approaching the level of NGL, suggesting that HRD has a highly favorable recovery status.
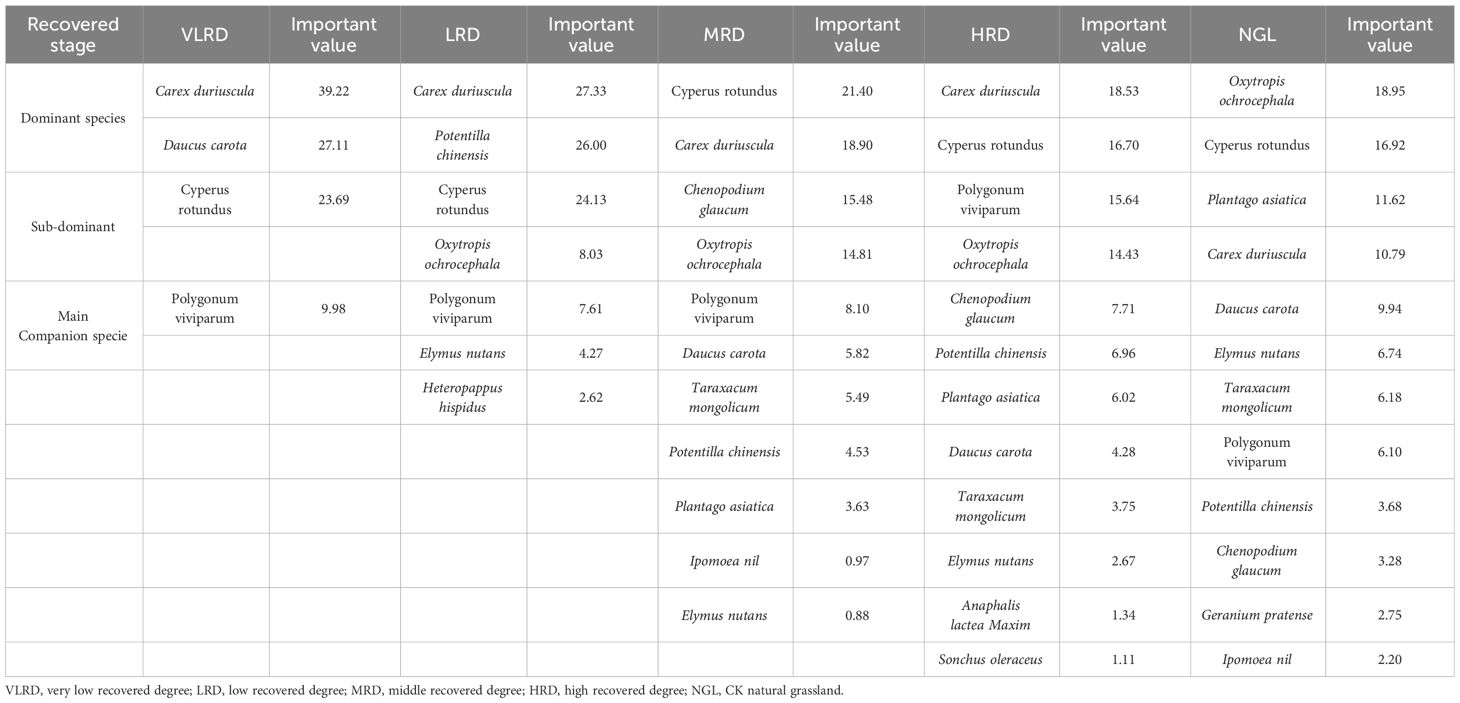
Table 4 Important value of dominant and sub-dominant species under different recovered stages.
3.5 Variations in community life structure characteristics across different restoration levels
In terms of community life structure, the characteristics of different restoration degrees in alpine mining grassland exhibited a consistent trend between the two life forms—perennial herbaceous plants were significantly dominant, collectively representing more than 83.56% of the total importance values, while annual herbaceous plants had a smaller share ( Table 5 ). Across the various restoration levels, perennial herbaceous plants consistently held higher importance values than annual herbaceous plants. Notably, alpine grasslands with NGL, HRD, and LRD had relatively high importance values for perennial herbaceous plants. In addition, alpine grasslands with HRD and MRD displayed relatively high importance values for annual herbaceous plants. Perennial herbaceous species in these communities primarily belonged to the Graminaceae, Asteraceae, Leguminosae, Rosaceae, and Cyperaceae families. On the other hand, annual herbs were primarily represented by the following four species: Sonchus oleraceus , Chenopodium glaucum , Heteropappus hispidus , and Ipomoea nil . When examining alpine grasslands with different restoration levels, HRD featured the highest number of plant species, mainly composed of perennial herbaceous plants, while VLRD lacked annual herbaceous species. Within these restoration grassland communities, perennial herbaceous plants were predominantly Carex duriuscula , Cyperus rotundus , Oxytropis ochrocephala , Potentilla chinensis , and Polygonum viviparum . Annual herbaceous plants primarily included Chenopodium glaucum , Sonchus oleraceus , Heteropappus hispidus , and Ipomoea nil .
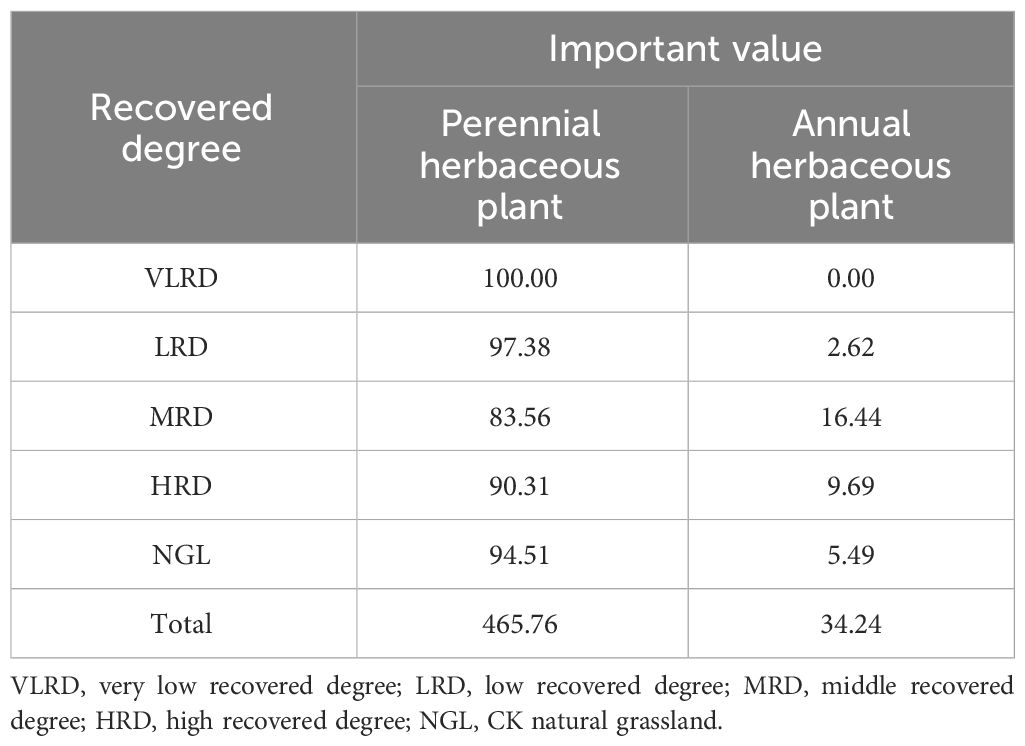
Table 5 Relative importance of life forms in grassland communities at various recovery degrees (%).
3.6 Variations in plant diversity across restoration levels
The variations in plant diversity across different restoration levels in alpine grassland is displayed in Figure 3 . The Simpson index indicates that HRD and MRD have higher diversity indices compared to VLRD, with no statistically significant differences between HRD and MRD, and VLRD ( P >0.05). Their respective values are 0.87, 0.86, and 0.71. Likewise, the Shannon-Wiener index is higher in HRD and MRD compared to VLRD, with no significant differences between HRD and MRD, and LRD ( P >0.05). The values are 2.20, 2.09, and 1.69. The Margalef species richness index in HRD is significantly greater than in LRD and VLRD ( P <0.05). HRD boasts the highest species richness with 12 species, followed by MRD with 11 species, LRD with 7 species, and a minimum of 4 species in VLRD. HRD’s composition closely resembles that of NGL. The Dominance index does not exhibit significant differences among different restoration levels in alpine grassland ( P >0.05). However, the Dominance index in VLRD is higher than in other restoration levels, with a value of 0.29. Regarding the Evenness index, HRD and MRD surpass LRD, but no significant differences exist between HRD and MRD, and LRD ( P >0.05), the values were 0.88, 0.87, and 0.86, respectively.
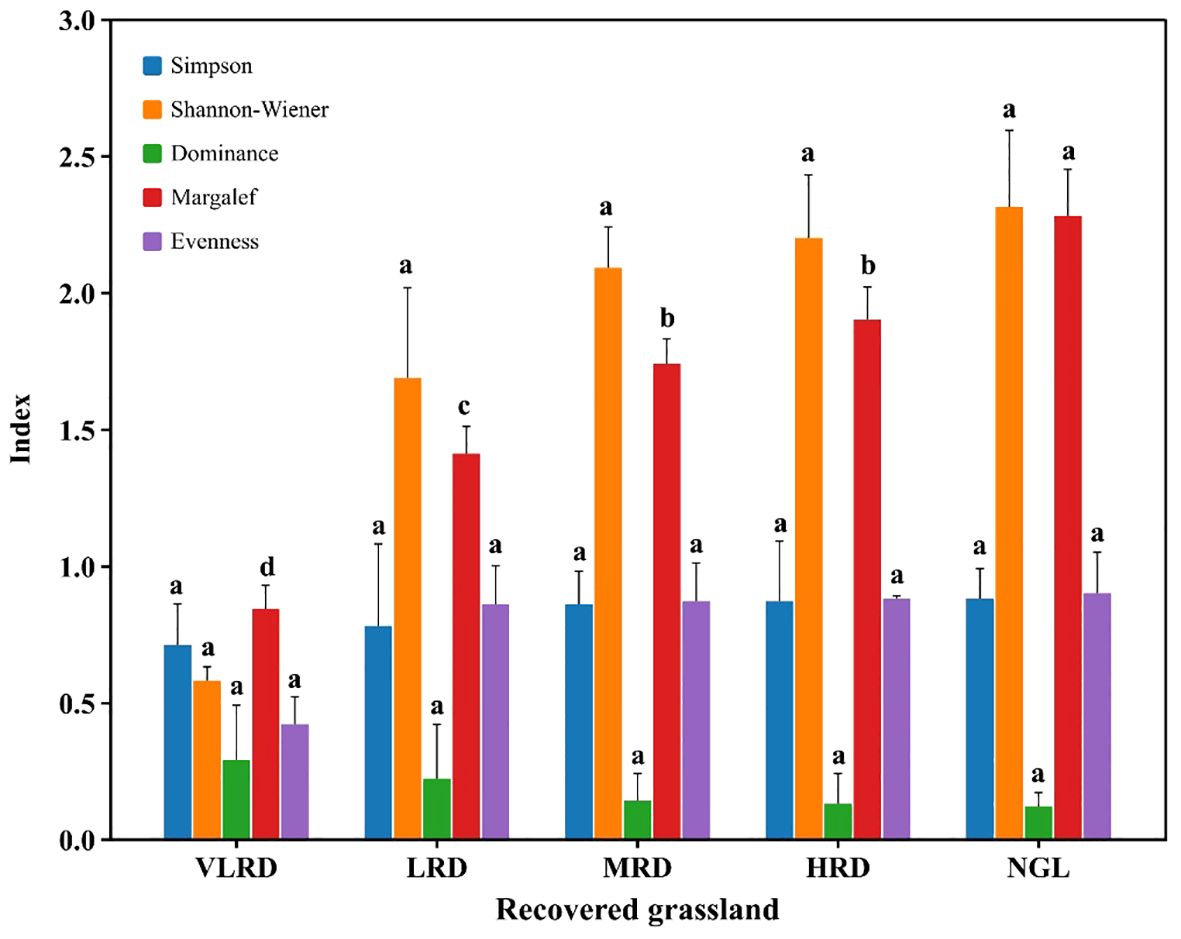
Figure 3 Variation in plant diversity in alpine meadows under different restoration degrees. Different colors represents Simpson and Shannon-Wiener Indices, Dominance, Margalef, and Evenness Indexes. VLRD stands for Very Low Recovered Degree, LRD for Low Recovered Degree, MRD for Middle Recovered Degree, HRD for High Recovered Degree, and NGL for CK Natural Grassland. Different letters above error bars between treatments indicate statistically significant differences at the 0.05 level.
3.7 Statistical overview of soil properties
Soil properties exhibited significant variations across different recovered grasslands ( Figure 4 ). Specifically, soil pH, electrical conductivity (EC), and soil water content (SWC) at the 20-40 cm soil depth were significantly higher than those at the 0-20 cm depth ( P < 0.05). Moreover, soil pH and EC showed a gradual decrease with increasing restoration levels, while soil SWC increased with higher restoration levels. When comparing different restoration levels, the soil pH in VLRD and LRD grasslands at the 0-20 cm soil depth was 44.84% and 33.93% higher, respectively, than that in the NGL. At the 20-40 cm soil depth, the soil pH in VLRD, LRD, MRD, and HRD grasslands was 36.20%, 28.43%, 14.38%, and 7.77% higher, respectively, than in the NGL. Similar trends were observed for soil EC and SWC. Additionally, the HRD exhibited recovery effects that were similar to those of the NGL, indicating the most successful natural recovery. Soil properties, including soil organic carbon (SOC), total nitrogen (TN), nitrate nitrogen (NO 3 -N), ammonium nitrogen (NH 4 -N), available nitrogen (AN), total phosphorus (TP), and available phosphorus (AP), were significantly higher at the 0-20 cm soil depth compared to the 20-40 cm depth ( P < 0.05). In HRD grassland, SOC, TN, NO 3 -N, NH 4 -N, AN, TP, and AP were significantly higher than in VLRD grassland ( P < 0.05). These trends gradually increased with higher restoration levels at 10-20 cm and 20-40 cm soil depths. Once again, the HRD demonstrated recovery effects similar to the NGL, signifying the most successful natural recovery.
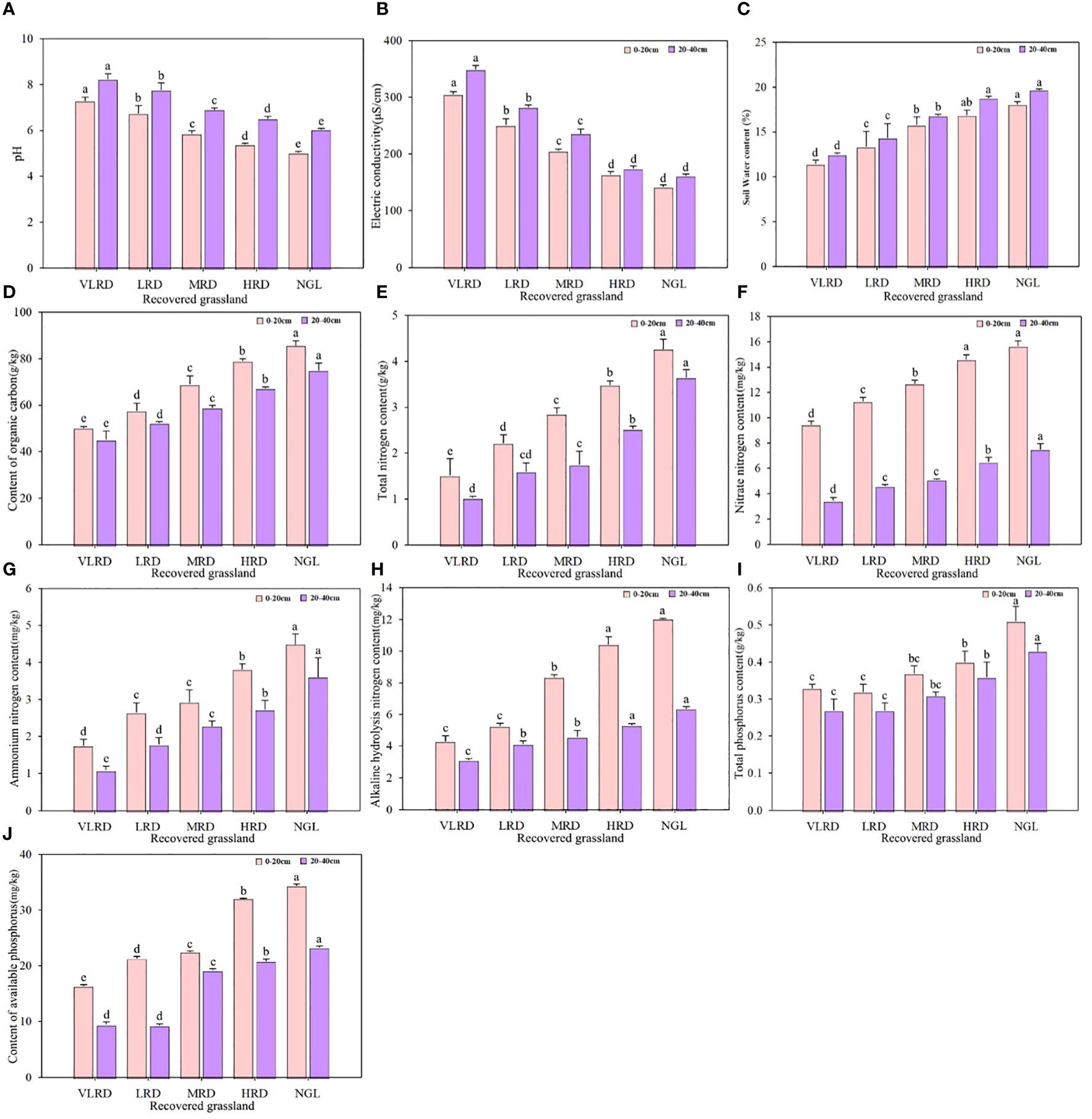
Figure 4 Soil properties of alpine meadows, including pH (A) , EC (B) , SWC (C) , SOC (D) , TN (E) , NO 3 -N (F) , NH 4 -N (G) , AN (H) , TP (I) , and AP (J) , Under Different Restoration Degrees. VLRD, very low recovered degree; LRD, low recovered degree; MRD, middle recovered degree; HRD, high recovered degree; NGL, CK natural grassland. Different letters above error bars between treatments indicate significant differences at the 0.05 level.
3.8 The connection between plant community diversity and soil properties
Pearson correlation analysis was employed to investigate the relationships between plant community diversity and soil properties ( Figure 5 ). The results show that, at the 0-20 cm soil depths, soil properties including soil water content (SWC), available nitrogen (AN), total phosphorus (TP), ammonium nitrogen (NH 4 -N), total nitrogen (TN), available phosphorus (AP), soil organic carbon (SOC), nitrate nitrogen (NO 3 -N), pH, and electrical conductivity (EC) exhibited a significant and positive correlation with plant frequency, coverage, and biomass ( P < 0.01). However, these soil properties were significantly and negatively correlated with plant community diversity indices (Simpson, Shannon, Evenness, and Margalef indices) ( P > 0.05). Furthermore, soil SOC exhibited a significant positive correlation with plant height (R = 0.65, P < 0.01) ( Figure 5A ). At the 20-40 cm soil depths, SWC, AN, TP, NH 4 -N, TN, AP, SOC, NO 3 -N, pH, and EC showed significant and positive correlations with plant frequency, coverage, and biomass ( P< 0.01). However, these soil properties at 20-40 cm depths were significantly and negatively correlated with plant community diversity indices ( P > 0.05). Additionally, soil AP demonstrated a significant positive correlation with plant height (R = 0.66, P < 0.01) ( Figure 5B ).
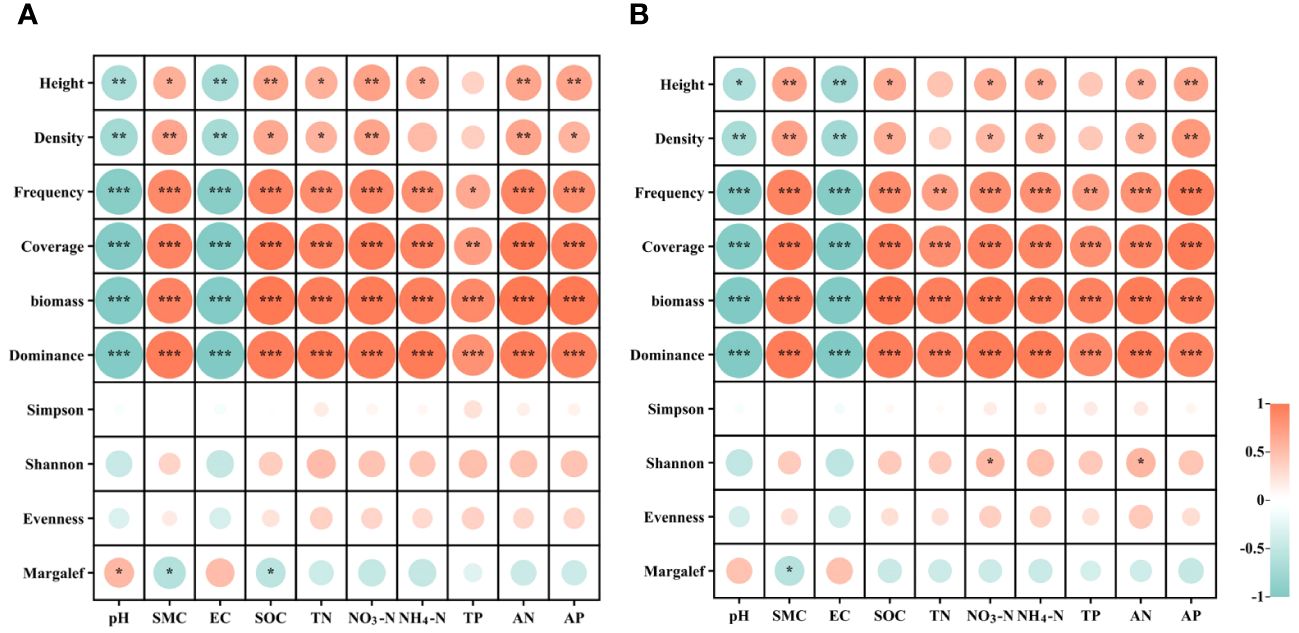
Figure 5 Correlations between plant community diversity and soil physiochemical properties in recovered alpine grassland at soil depths of 0-20 cm (A) and 20-40 cm (B) . pH, soil pH; SWC, soil water content; EC, soil electric conductivity; SOC, soil organic carbon; AP, soil available phosphorus; TP, soil total phosphorus; TN, soil total nitrogen; NO3-N, soil nitrate nitrogen; NH 4 -N, soil ammonium nitrogen; AN, soil alkaline nitrogen. * indicates significant correlation (p < 0.05), ** indicates extremely significant correlation (p < 0.01), *** indicates extremely significant correlation (p < 0.001).
Principal component analysis (PCA) was employed to investigate the associations between soil physicochemical properties and the level of soil recovery. The analysis of soil property factors at a depth of 0-20 cm in the recovered grassland at the Jinqianghe gold mine indicated that the first axis accounted for 93.22% of the variance, while the second axis explained 2.32%, resulting in a cumulative explanation of 95.55% ( Figure 6A ).At 0-20 cm soil depths, soil properties between HRD and NGL displayed positive correlations with soil water content (SWC), electrical conductivity (EC), available nitrogen (AN), total phosphorus (TP), ammonium nitrogen (NH 4 -N), total nitrogen (TN), available phosphorus (AP), soil organic carbon (SOC), and nitrate nitrogen (NO 3 -N). However, HRD exhibited a negative correlation with soil pH at these depths. At 20-40 cm soil depths, the first axis explained 92.05%, with the corresponding second axis explaining 2.94%, resulting in a cumulative explanation of 94.98% ( Figure 6B ). Soil properties at these depths between HRD and NGL exhibited positive correlations with SWC, AN, TP, NH 4 -N, TN, AP, SOC, and NO 3 -N. Conversely, HRD displayed negative correlations with soil pH and EC at these depths.
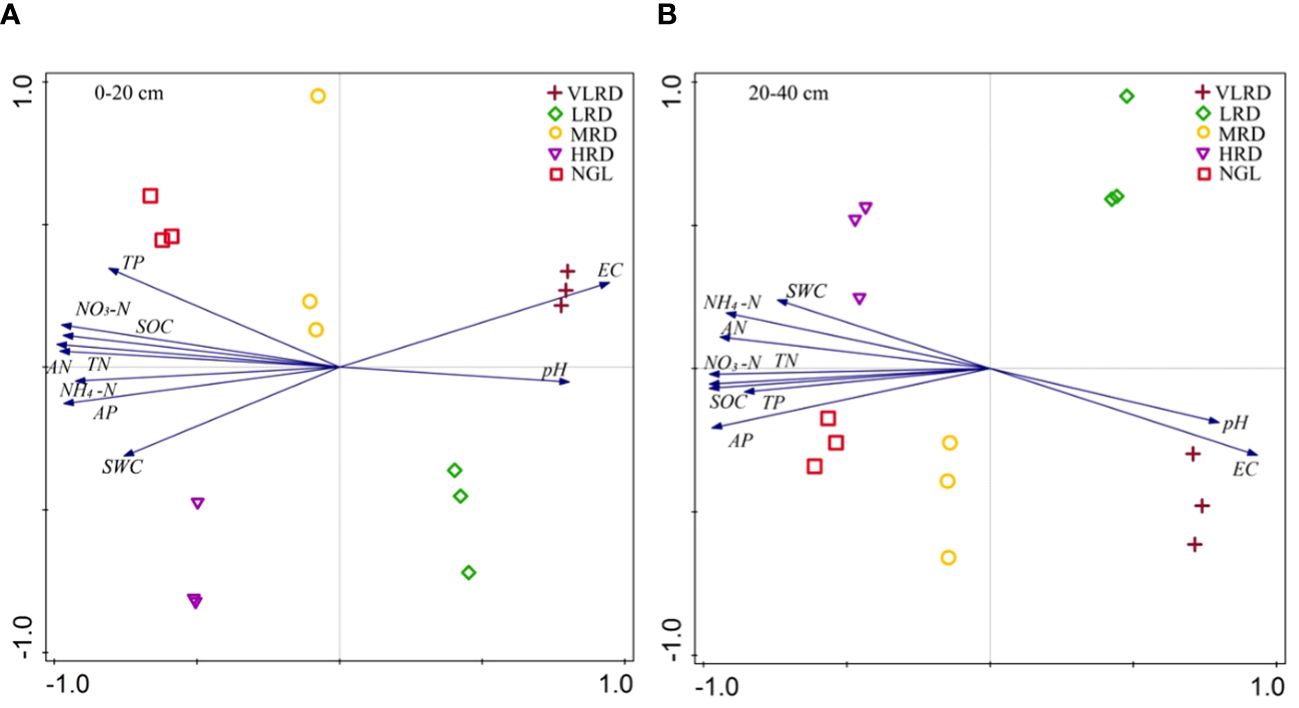
Figure 6 The principal component analysis (PCA) of soil physiochemical properties in alpine recovered grassland at soil depths of 0-20 cm (A) and 20-40 cm (B) was depicted. In the PCA plot, arrows indicate the direction and strength of the associated soil physiochemical indices, with the length of the arrows reflecting the degree of correlation with the variables. Each graph represents the recovered grassland for each sample (three replicates per sample, 1–3). The data are derived from three replicates (1–3) per sample. pH, soil pH; SWC, soil moisture content; EC, soil electric conductivity; SOC, soil organic carbon; AP, soil available phosphorus; TP, soil total phosphorus; TN, soil total nitrogen; NO 3 -N, soil nitrate nitrogen; NH 4 -N, soil ammonium nitrogen; AN, soil alkaline nitrogen.
4 Discussion
4.1 changes in plant community characteristics.
To safeguard the vital aspects of plant diversity, nurture plant growth, and effectively monitor restored grasslands, it is imperative to understand the characteristics of plant communities ( Catorci and Gratti, 2010 ; Tang et al., 2016 ). Many mining areas have undergone restoration using both artificial and natural methods. However, the outcomes have exhibited significant variability, and it has been challenging to distinguish the effects of different mining areas on grassland environments ( Huang et al., 2019 ; Wei et al., 2019 ). Consequently, field studies are essential to assess and enhance the restoration levels in alpine grasslands. The species composition within plant communities can serve as a reflection of the external appearance of the community, which, in turn, represents the comprehensive influence of environmental factors ( Deng et al., 2020 ). In our study, the vegetation types of restored grassland (VLRD, LRD, MRD, HRD, and NGL) in the mine area were Carex duriuscula + Daucus carota , Carex duriuscula + Potentilla chinensis , Cyperus rotundus + Carex duriuscula , Carex duriuscula + Cyperus rotundus ( Table 4 ). With the degree of restored grassland, the vegetation characteristics showed an increasing trend, the aboveground biomass and coverage increased significantly, and productivity increased ( Figure 2 ). Perennial herbaceous plants played the most important role in different restoration degrees of alpine grassland, while annual herbaceous plants were a few plant components ( Table 3 ). The plant species dominated by Leguminosae, Asteraceae, Graminaceae, Rosaceae, and Cyperaceae usually grow in low-temperature, high-altitude and alpine grassland ( Table 2 ). This is consistent with previous studies that show that these environmental factors preferentially support dominant species’ existence and have poor competition among plant species, and the plant community preserves ecological structure with human intervention ( Islam et al., 2022 ).
The impact of major factors influencing plant value perception remains inconclusive due to variations in study subjects across mine restoration research ( Li et al., 2019 ). The relationships between restoration levels and environmental factors are complex, resulting in diverse plant diversity at different restoration stages ( Zhao et al., 2019 ). Plant diversity serves both as a measure of structural characteristics and an indicator of environmental conditions ( Bennett et al., 2020 ). Prior studies have consistently reported that the importance of plants can directly affect plant diversity ( Guo et al., 2020 ; Hailu et al., 2021 ). In our findings, the highest number of species was observed in HRD and NGL, whereas VLRD, LRD, and HRD grasslands had Carex duriuscula as the dominant species, with corresponding values of 39.22, 27.33, and 18.53, respectively ( Table 4 ). The Shannon-Wiener, Simpson, Margalef, and Evenness indexes displayed an upward trend, while the Dominance index exhibited the opposite trend. The HRD Evenness index surpassed that of other restoration levels, possibly influenced by the number of community species and the uniform distribution of individual plants among species. Appropriate replanting and fencing could reduce the likelihood of invasion and enhance other species within the community, consequently promoting community-level plant diversity ( Figure 3 ). Previous research has indicated that climate, degree of recovery, precipitation, and human activities like grazing and mining can impact the phenological periods and diversity of vegetation ( Bao et al., 2014 ). Our results affirm that HRD grasslands exhibit richness in species, stable communities, and high uniformity. These findings suggest that HRD can serve as the primary management model for local restoration-level grasslands, providing a critical theoretical foundation for ecological restoration efforts in mining area grasslands. In the case of VLRD, vegetation cover was limited, mainly concentrated on both sides of roads and susceptible to trampling by cattle and sheep. Although fences were initially erected to enclose the restoration area, the large size of the area led to fence destruction by livestock. This necessitates enhanced management measures, including the installation of taller fences, in the later stages of restoration, especially in key secondary restoration areas.
4.2 Changes in soil characteristics in different soil layers
Differences in soil fertility as well as geographic location determine differences in vegetation, which in turn absorbs nutrients for plant growth from the soil through nutrients absorbed by the roots into the soil, and to a certain extent plant growth promotes soil nutrients, soil properties play a key role in promoting plant growth, and plant growth can also have an impact on the nutrient properties of the soil ( An and Xu, 2013 ). Among the primary environmental factors affecting plant communities, soil stands out ( Yang et al., 2023b ). The physical and chemical attributes of soil significantly shape the growth and development of vegetation. In mining grasslands, both soil properties and vegetation growth transform as restoration levels change. Relationships of considerable significance exist among soil characteristics, restoration levels, plant community structure, and plant diversity ( Zhang et al., 2020 ; Huang et al., 2021b ). Several studies have shown that restored grasslands could reduce soil pH and EC ( Dai et al., 2014 , 2017 ). A similar result was also found in this study, where soil pH and EC were lower from HRD to VLRD due to vegetation growth and sufficient soil nutrients. However, soil SWC, SOC, TN, NO 3 -N, NH 4 -N, AN, TP, and AP were higher from HRD to VLRD ( Figure 4 ). Soil pH, EC, and SWC were significantly higher from 20-40 cm soil depths to 0–20 cm soil depths, while soil SOC, TN, NO 3 -N, NH 4 -N, AN, TP, and AP were significantly lower from 20-40 cm soil depths to 0–20 cm soil depths ( Figure 4 ). Low-quality soil is apt to cause vegetation degradation ( Li et al., 2020 ), the varying degrees of vegetation coverage could play a crucial role, as vegetation affects soil chemical properties through leaf litter, root exudation, and associations with microorganisms, while an increase in grassland diversity and aboveground biomass leads to further soil restoration ( Han et al., 2020 ). Our results indicate that restored plant communities (VLRD and LRD) had lower plant diversity and aboveground biomass, which might not recover well. Therefore, it is essential to explore plant composition characteristics and soil properties variation in different restoration degrees and take timely policy measures to protect restoration grassland vegetation.
4.3 Changes in the correlation between vegetation and soil characteristics
The connection between plant communities and soil properties can vary based on the restoration level, geographic location, and study area ( Springate and Kover, 2014 ; Qin et al., 2019 ). The results of PCA analysis showed that the composition of the HRD plant community significantly differed from that under VLRD and LRD restoration degrees. The dominant controls for the HRD community composition were soil SWC, EC, AN, TP, NH 4 -N, TN, AP, SOC, and NO 3 -N at soil depths of 0-20 cm and 20-40 cm, respectively. The main reason for this was that the HRD was located at the foot of the mountain, away from the roadside, have sufficient rainfall and was free from human and livestock interference ( Figure 5 ). In other studies, soil nutrients have been proven to be the main factor impacting plant growth, distribution and function ( Xu et al., 2016 , 2019 ). Soil nutrients exhibited a strong connection with plant communities in both soil depths ( Yang et al., 2023b ). In the soil properties at 0-20 cm depth, a positive correlation was observed between HRD and NGL with SWC, EC, AN, TP, NH 4 -N, TN, AP, SOC, and NO 3 -N, while HRD exhibited a negative correlation with pH at 0-20 cm soil depths. Similarly, at 20-40 cm depth, the soil properties between HRD and NGL displayed positive correlations with SWC, AN, TP, NH 4 -N, TN, AP, SOC, and NO 3 -N, while HRD showed negative correlations with pH and EC at 20-40 cm soil depths ( Figure 6 ). Furthermore, our results confirmed that the HRD restoration degree was similar to NGL, which could enhance the number of vegetation and soil nutrients, leading to long-term mine grasslands restoration. Knowledge on the impact of mining grassland restoration on plant communities and soil properties can provide a scientific basis for the restoration and sustainable utilization of alpine grasslands.
For future restoration of mining areas, an integrated restoration approach should be applied, based on the findings of this study. Specifically, areas with low vegetation cover, classified as VLRD and LRD, should undergo secondary seeding as part of the restoration efforts. Additionally, fencing should be established to prohibit grazing and trampling, taking into account an integrated evaluation of ecological and socio-economic factors. The use of a diverse mixture of native species is recommended to enhance the resilience and stability of the ecosystem. Soil improvement practices, such as the application of organic materials, should be implemented to enhance soil health and fertility, thereby facilitating quicker vegetation establishment and growth. Monitoring and adaptive management practices should involve the implementation of long-term monitoring plans to track restoration progress, with management strategies adjusted as necessary to ensure the effectiveness of the mining area’s restoration efforts.
5 Conclusions
This study investigates how environmental factors influence plant community characteristics, plant diversity, and soil properties at various restoration levels. It also aims to identify plant community characteristics along the recovery gradient in the Qilian Mountains study sites. In these sites, we observed 18 species belonging to 11 families and 16 genera across five restoration levels. Notably, Carex duriuscula , Cyperus rotundus , and Polygonum viviparum dominated the areas showing significant recovery, as indicated by their high importance values and species count. Our findings revealed that the Shannon and Simpson indexes in HRD were notably higher compared to VLRD, LRD, and MRD. Moreover, the recovery effect in HRD was similar to that of NGL, demonstrating the most effective natural recovery. Among the environmental factors, soil pH, SWC, SOC, NO 3 -N, and AN played a significant role in regulating the plant community. As restoration levels increased, soil pH and EC gradually decreased, while soil SWC, AN, TP, NH 4 -N, TN, AP, SOC, and NO 3 -N progressively increased. The results suggest that the improvement in grassland restoration is closely linked to soil restoration, particularly the increase in soil SOC and AN content. Furthermore, the enhancement of grassland community characteristics and diversity contributes to further soil restoration. This study establishes a theoretical basis for the restoration and preservation of mining grasslands by revealing the connection between plant community attributes and soil properties in alpine grasslands.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
XY: Conceptualization, Data curation, Software, Writing – original draft, Writing – review & editing. QF: Funding acquisition, Project administration, Writing – review & editing. MZ: Data curation, Methodology, Software, Writing – original draft. JZ: Investigation, Supervision, Writing – review & editing. LY: Formal analysis, Methodology, Writing – review & editing. CZ: Conceptualization, Validation, Writing – review & editing. ZW: Supervision, Validation, Writing – review & editing. YF: Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key R&D Program of China (No. 2022YFF1303301, 2022YFF1302603), the National Natural Science Fund of China (No. 52179026, 42001035, 42101115), the Science and Technology Program of Gansu Province (No. 22JR5RA072, 22JR5RA068), the Postdoctoral Funding Program of Gansu Province (No. E339880139), the Natural Science Foundation of Gansu Province (No. E331040901), the Science and Technology Fund of Gansu Province (No. 23JRRA640), the Consulting and Research Project of the Gansu Research Institute of Chinese Engineering Science and Technology Development Strategy (No. GS2022ZDI03), and the Open Fund of Technology Innovation Center for Mine Geological Environment Restoration in the Alpine and Arid Regions (No. HHGCKK2204).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
An, H., Xu, K. (2013). The effect of grazing disturbance on soil properties in desert steppe. Acta Pratacult. Sin. 22 (4), 35–42.
Google Scholar
Bao, G., Qin, Z. H., Bao, Y. H., Zhou, Y., Li, W. J., Sanjjav, A. (2014). NDVI-based long-term vegetation dynamics and its response to climatic change in the Mongolian Plateau. Remote Sens 6, 8337–8358. doi: 10.3390/rs6098337
CrossRef Full Text | Google Scholar
Bennett, A. C., Murugapiran, S. K., Hamilton, T. L. (2020). Temperature impacts community structure and function of phototrophic Chloroflexi and Cyanobacteria in two alkaline hot springs in Yellowstone National Park. Environ. Microbiol. Rep. 12, 503–513. doi: 10.1111/1758-2229.12863
PubMed Abstract | CrossRef Full Text | Google Scholar
Castro, R., Perez, A., Gomez, R. (2023). Evaluating wet muck risk in block caving mines: a new model. Int. J. Rock Mech. Min Sci. 170, 105485. doi: 10.1016/j.ijrmms.2023.105485
Catorci, A., Gratti, R. (2010). Floristic composition and spatial distribution assessment of montane mesophilous grasslands in the central Apennines, Italy:a muti-scale and diachronic approach. Plant Biosyst. 144, 793–804. doi: 10.1080/11263504.2010.513864
Chang, X., Lv, S., Feng, Z., Ye, S. (2015). Impact of topography on the spatial distribution pattern of net primary productivity in a meadow. Acta Ecol. Sin. 35 (10), 3339–3348.
Chen, L., He, Z., Zhao, W., Liu, J., Zhou, H., Li, J., et al. (2020). Soil structure and nutrient supply drive changes in soil microbial communities during conversion of virgin desert soil to irrigated cropland. Eur. J. Soil Sci. 71, 768–781. doi: 10.10.1111/ejss.12901
Chen, Y., Wang, J., Xiong, N., Sun, L., Xu, J. (2022). Impacts of land use changes on net primary productivity in urban agglomerations under multi-scenarios simulation. Remote Sens 14, 1755. doi: 10.3390/rs14071755
Dai, Z., Wang, Y., Muhammad, N., Yu, X., Xiao, K., Meng, J., et al. (2014). The effects and mechanisms of soil acidity changes, following incorporation of biochars in three soils differing in initial pH. Soil Sci. Soc. Am. J. 78, 1606–1614. doi: 10.2136/sssaj2013.08.0340
Dai, Z., Zhang, X., Tang, C., Muhammad, N., Wu, J., Brookes, P. C., et al. (2017). Potential role of biochars in decreasing soil acidification - A critical review. Sci. Total Environ. 581–582, 601–61. doi: 10.1016/j.scitotenv.2016.12.169
Deng, J., Bai, X., Zhou, Y., Zhu, W., Yin, Y. (2020). Variations of soil microbial communities accompanied by different vegetation restoration in an open-cut iron mining area. Sci. Total Environ. 704, 135243. doi: 10.1016/j.scitotenv.2019.135243
Dudley, N., Eufemia, L., Fleckenstein, M., Periago, M. E., Petersen, I., Timmers, J. F. (2020). Grasslands and savannahs in the UN decade on ecosystem restoration. Restor. Ecol. 28, 1313–1317. doi: 10.1111/rec.13272
Feng, Q., Tian, Y. Z., Yu, T. F. (2019). Combating desertification through economic development in northwestern China. Land Degradation Dev. 30, 910–917. doi: 10.1002/ldr.3277
Feyissa, A., Yang, F., Wu, J., Chen, Q., Zhang, D., Cheng, X. (2021). Soil nitrogen dynamics at a regional scale along a precipitation gradient in secondary grassland of China. Sci. Total Environ. 781, 146736–146736. doi: 10.1016/j.scitotenv.2021.146736
Guo, C., Zhang, F., Wang, X., Lu, N. (2020). Effects of meteorology and soil on the herb species diversity in plantations in a reclamation area of coal mine after 6 years. Environ. Sci. pollut. Res. 27, 24231–24241. doi: 10.1007/s11356-020-08402-2
Guo, D., Song, X., Hu, R., Cai, S., Zhu, X., Hao, Y. (2021). Grassland type-dependent spatiotemporal characteristics of productivity in Inner Mongolia and its response to climate factors. Sci. Total Environ. 775, 145644. doi: 10.1016/j.scitotenv.2021.145644
Gao, Y., An, Y., Qi, B., Liu, J., Yu, H., Wang, D. (2021). Grazing exclusion mediates the trade-off between plant diversity and productivity in Leymus chinensis meadows along a chronosequence on the Songnen Plain. China Ecol. Indic. 126, 107655. doi: 10.1016/j.ecolind.2021.107655
Hailu, G. A., Geurts, R., Wolde-Meskel, E., Degefu, T. E., Giller, K., van Heerwaarden, J. (2021). Phylogeographic distribution of rhizobia nodulating common bean ( Phaseolus vulgaris L. ) in Ethiopia. FEMS Microbiol. Ecol. 97, flab046. doi: 10.1093/femsec/fiab046
Han, X., Li, Y., Du, X., Li, Y., Wang, Z., Jiang, S., et al. (2020). Effect of grassland degradation on soil quality and soil biotic community in a semi-arid temperate steppe. Ecol. Process 9, 63. doi: 10.1186/s13717-020-00256-3
Harrison, S., Spasojevic, J. M., Li, D. (2020). Climate and plant community diversity in space and time. Proc. Natl. Acad. Sci. 117, 4464–4470. doi: 10.1073/pnas.1921724117
Huang, Y., Cao, Y., Zhou, W., Kuang, X., Wang, F., Bai, Z. (2021b). Effects of straw biochar on the growth of medicago falcata in the reconstructed soil of grassland mining area. Acta Ecol. Sin. 41, 588–602. doi: 10.5846/stxb202003150552
Huang, W. W., Wang, W., Cao, M., Fu, G., Xia, J. Y., Wang, Z. X., et al. (2021a). Local climate and biodiversity affect the stability of China’s grasslands response to drought. Sci. Total Environ. 768, 145482. doi: 10.1016/j.scitotenv.2021.145482
Huang, X. L., Zhang, T. B., Yi, G. H., He, D., Zhou, X. B., Li, J. J., et al. (2019). Dynamic changes of NDVI in the growing season of the Tibetan Plateau during the past 17 years and its response to climate change. Int. J. Environ. Res. Public Health 16, 3452. doi: 10.3390/ijerph16183452
Islam, M. S., Haque, K. A., Jahan, N., Atikullah, M., Uddin, M. N., Naser, A. M. (2022). Soil salinity mitigation by naturally grown halophytes in seawater affected coastal Bangladesh. Int. J. Environ. Sci. Technol. 19, 11013–11022. doi: 10.1007/s13762-022-03912-7
Jochum, M., Fischer, M., Isbell, F., Roscher, C., van der Plas, F., Boch, S., et al. (2020). The results of biodiversity-ecosystem functioning experiments are realistic. Nat. Ecol. Evol. 4, 1485–1494. doi: 10.1038/s41559-020-1280-9
Kang, S., Niu, J. M., Zhang, Q., Zhang, X. F., Han, G. D., Zhao, M. L. (2020). Niche differentiation is the underlying mechanism maintaining the relationship between community diversity and stability under grazing pressure. Glob. Ecol. Conserv. 24, e01246. doi: 10.1016/j.gecco.2020.e01246
Kong, J. Q., He, Z. B., Chen, L. F., Yang, R., Du, J. (2021). Efficiency of biochar, nitrogen addition, and microbial agent amendments in remediation of soil properties and microbial community in Qilian Mountains mine soils. Ecol. Evol. 11, 9318–9331. doi: 10.1002/ece3.7715
Lei, T., Feng, J., Lv, J., Wang, J., Song, H., Gao, X. (2020). Net primary productivity loss under different drought levels in different grassland ecosystems. J. Environ. Manage. 274, 111144. doi: 10.1016/j.jenvman.2020.111144
Li, Y., Liu, X. J., Xu, W. B., Bongers, F. J., Bao, W. K., Chen, B., et al. (2020). Effects of diversity, climate and litter on soil organic carbon storage in subtropical forests. For Ecol. Manage. 476, 118479. doi: 10.1016/j.foreco.2020.118479
Li, P., Zhang, X., Hao, M., Cui, Y., Zhu, S., Zhang, Y. (2019). Effects of vegetation restoration on soil bacterial communities, enzyme activities, and nutrients of reconstructed soil in a mining area on the Loess Plateau, China. Sustainability 11, 2295. doi: 10.3390/su11082295
Lorite, J., Ballesteros, M., García-Robles, H., Cañadas, E. M. (2021). Economic evaluation of ecological restoration options in gypsum habitats after mining. J. Nat. Conserv. 59, 125935. doi: 10.1016/j.jnc.2020.125935
Ma, K. P. (1994). A measure of biological community diversity A measure of alpha diversity (Top). Biodiversity 2, 162–168.
Pansu, M., Gautheyrou, J. (2006). pH Measurement (Berlin/Heidelberg, Germany: Springer).
Pei, L., Xiao, J., Sun, L. (2021). The effects of reclaimed water irrigation on the soil characteristics and microbial populations of plant rhizosphere. Environ. Sci. pollut. Res. 29, 17570–17579. doi: 10.1007/s11356-021-16983-9
Qin, G. X., Wu, J., Li, C. B., Qin, A. N., Yao, X. Q. (2019). Assland vegetation phenology change and its response to climate changes in North China. Chin. J. Appl. Ecol. 30, 4099–4107. doi: 10.13287/j.1001-9332.201912.015
Schmid, J. S., Huth, A., Taubert, F. (2021). Influences of traits and processes on productivity and functional composition in grasslands: a modeling study. Ecol. Model. 440, 109395. doi: 10.1016/j.ecolmodel.2020.109395
Siebert, F., Van Staden, N., Komape, D. M., Swemmer, A. M., Siebert, S. J. (2021). Effects of land-use change on herbaceous vegetation in a semi-arid Mopaneveld savanna. Bothalia 51, 107–132. doi: 10.38201/btha.abc.v51.i1.8
Springate, D. A., Kover, P. X. (2014). Plant responses to elevated temperatures: a field study on phenological sensitivity and fitness responses to simulated climate warming. Glob. Chang. Biol. 20, 456–465. doi: 10.1111/gcb.12430
Stokes, A., Angeles, G., Anthelme, F., Aranda-Delgado, F., Barois, I., Bounous, M. (2021). Shifts in soil and plant functional diversity along an altitudinal gradient in the French Alps. BMC Res. 14, 54. doi: 10.1186/s13104-021-05468-0
Tang, H. J., Xin, X. P., Li, L. H., Wang, D. L., Yan, Y. C., Zhou, Y. L., et al. (2016). North meadow degraded grassland treatment technology and demonstration. Acta Ecol. Sin. 36 (22), 7034–7039.
ter Braak, C. J., Smilauer, P. (2012). Canoco reference manual and user’s guide: software for ordination, version 5.0. (Ithaca USA: Microcomputer Power). Available at https://research.wur.nl/en/publications/canoco-reference-manual-and-users-guide-software .
Wang, L., Ali, A. (2021). Climate regulates the functional traits aboveground biomass relationships at a community-level in forests: a global meta-analysis. Sci. Total Environ. 761, 143238. doi: 10.1016/j.scitotenv.2020.143238
Wang, D. L., Wang, L., Liu, J. S., Zhu, H., Zhong, Z. W. (2018). Grassland ecology in China: perspectives and challenges. Front. Agric. Sci. Eng. 5, 24–43. doi: 10.15302/J-FASE-2018205
Wei, Z. F., Huang, Q. Y., Zhang, R. (2019). Dynamics of vegetation coverage and response to climate change in China-South Asia-Southeast Asia during 1982-2013. Appl. Ecol. Environ. Res. 17, 2865–2879. doi: 10.15666/aeer/1702-28652879
Wiegand, T., Gunatilleke, S., Gunatilleke, N., Okuda, T. (2007). Analyzing the spatial structure of a Sri Lankan tree species with multiple scales of clustering. Ecology 88, 3088–3102. doi: 10.1890/06-1350.1
Wu, Y., Xu, N., Wang, H., Li, J., Zhong, H., Dong, H., et al. (2021). Variations in the diversity of the soil microbial community and structure under various categories of degraded wetland in Sanjiang Plain, northeastern China. Land Degrad Dev. 32, 2143–2156. doi: 10.1002/ldr.3872
Xie, Y., Crary, D., Bai, Y., Cui, X., Zhang, A. (2017). Modeling grassland ecosystem responses to coupled climate and socioeconomic influences from multi-spatial-and-temporal scales. J. Environ. Inf. 1684–8799. doi: 10.3808/jei.201600337
Xu, X., Du, H., Fan, W., Hu, J., Mao, F., Dong, H. (2019). Long-term trend in vegetation gross primary production, phenology and their relationships inferred from the FLUXNET data. J. Environ. Manage. 246, 605–616. doi: 10.1016/j.jenvman.2019.06.023
Xu, Y., Yang, J., Chen, Y. N. (2016). NDVI-based vegetation responses to climate change in an arid area of China. Theor. Appl. Climatol. 126, 213–222. doi: 10.1007/s00704-015-1572-1
Yang, X. M., Feng, Q., Liu, W., Xia, H. H., Zhang, J. T., Yang, L. S., et al. (2023b). Community structure and plant diversity under different degrees of restored grassland in mining areas of the Qilian Mountains, Northwestern China. Front. Environ. Sci. 11. doi: 10.3389/fenvs.2023.1191599
Yang, X. M., Feng, Q., Zhu, M., Yang, L. S., Zhang, C. Q., Zhang, J. T., et al. (2023a). Changes in nutrient-regulated soil microbial communities in soils concomitant with grassland restoration in the alpine mining region of the Qilian Mountains. Agronomy 13, 3052. doi: 10.3390/agronomy13123052
Zhang, J. T. (2004). Quantitative ecology [M] (Beijing: Science Press).
Zhang, C., Dong, Q., Chu, H., Shi, J., Li, S., Wang, Y., et al. (2018). Grassland community composition response to grazing intensity under different grazing regimes. Rangel. Ecol. Manage. 71, 196–204. doi: 10.1016/j.rama.2017.09.007
Zhang, Q. D., Wei, W., Chen, L. D., Yang, L., Luo, Y. Q., Cai, A. D. (2020). Plant traits in influencing soil moisture in semiarid grasslands of the Loess Plateau, China. Sci. Total Environ. 718, 137355. doi: 10.1016/j.scitotenv.2020.137355
Zhao, S., Liu, J. J., Banerjee, S., White, J. F., Zhou, N., Zhao, Z. Y. (2019). Not by salinity alone: how environmental factors shape fungal communities in saline soils. Soil Sci. Soc. Am. J. 83, 1387–1398. doi: 10.10.2136/sssaj2019.03.0082
Zhou, X., Liu, X., Zhang, P., Guo, Z., Du, G. (2019). Increased community compositional dissimilarity alleviates species loss following nutrient enrichment at large spatial scales. J. Plant Ecol. 12, 376–386. doi: 10.1093/jpe/rty035
Keywords: alpine mining area grassland, grassland ecosystem, restoration, plant diversity, community, Qilian Mountains
Citation: Yang X, Feng Q, Zhu M, Zhang J, Yang L, Zhang C, Wang Z and Feng Y (2024) Vegetation communities and soil properties along the restoration process of the Jinqianghe mine site in the Qilian Mountains, China. Front. Plant Sci. 15:1358309. doi: 10.3389/fpls.2024.1358309
Received: 19 December 2023; Accepted: 28 March 2024; Published: 22 April 2024.
Reviewed by:
Copyright © 2024 Yang, Feng, Zhu, Zhang, Yang, Zhang, Wang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Feng, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

COMMENTS
In chemical terms, photosynthesis is a light-energized oxidation-reduction process. (Oxidation refers to the removal of electrons from a molecule; reduction refers to the gain of electrons by a molecule.) In plant photosynthesis, the energy of light is used to drive the oxidation of water (H 2 O), producing oxygen gas (O 2 ), hydrogen ions (H ...
Note that the above global equation of photosynthesis emphasizes that the oxygen molecules released into the atmosphere originate from water oxidation, not from carbon dioxide, as established using 18 O-labelled water (Ruben et al., 1941).. This process starts in the thylakoid membrane (TM) with two light reactions taking place simultaneously at photosystem (PS) II and PSI reaction centres ...
Most life on Earth depends on photosynthesis.The process is carried out by plants, algae, and some types of bacteria, which capture energy from sunlight to produce oxygen (O 2) and chemical energy stored in glucose (a sugar). Herbivores then obtain this energy by eating plants, and carnivores obtain it by eating herbivores.. The process. During photosynthesis, plants take in carbon dioxide (CO ...
Read the latest Research articles in Photosynthesis from Scientific Reports. ... Photosystem stoichiometry adjustment is a photoreceptor-mediated process in Arabidopsis. Iskander M. Ibrahim, ...
Since 1893, when the word "photosynthesis" was first coined by Charles Reid Barnes and Conway MacMillan, our understanding of the elements and regulation of this complex process is far from being entirely understood. We aim to review the most relevant advances in photosynthesis research from the last few years and to provide a perspective ...
5.1: Overview of Photosynthesis. All living organisms on earth consist of one or more cells. Each cell runs on the chemical energy found mainly in carbohydrate molecules (food), and the majority of these molecules are produced by one process: photosynthesis. Through photosynthesis, certain organisms convert solar energy (sunlight) into chemical ...
The chloroplast is involved in both stages of photosynthesis. The light reactions take place in the thylakoid. There, water (H 2 O) is oxidized, and oxygen (O 2) is released. The electrons that ...
Photosynthesis is the process in which light energy is converted to chemical energy in the form of sugars. In a process driven by light energy, glucose molecules (or other sugars) are constructed from water and carbon dioxide, and oxygen is released as a byproduct. The glucose molecules provide organisms with two crucial resources: energy and ...
Figure 8.1.1 8.1. 1: This world map shows Earth's distribution of photosynthesis as seen via chlorophyll a concentrations. On land, this is evident via terrestrial plants, and in oceanic zones, via phytoplankton. (credit: modification of work by SeaWiFS Project, NASA/Goddard Space Flight Center and ORBIMAGE) The processes in all organisms ...
Photosynthesis is powered by energy from sunlight. This energy is used to rearrange atoms in carbon dioxide and water to make oxygen and sugars. Carbon dioxide and water are inputs of photosynthesis. These inputs come from the environment. Oxygen and sugars are outputs of photosynthesis. The oxygen is released into the environment.
Meaning. Photosynthesis. The process by which plants, algae, and some bacteria convert light energy to chemical energy in the form of sugars. Photoautotroph. An organism that produces its own food using light energy (like plants) ATP. Adenosine triphosphate, the primary energy carrier in living things. Chloroplast.
Photosynthesis ( / ˌfoʊtəˈsɪnθəsɪs / FOH-tə-SINTH-ə-sis) [1] is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their activities.
The word "photosynthesis" is derived from the Greek words phōs (pronounced: "fos") and σύνθεσις (pronounced: "synthesis")Phōs means "light" and σύνθεσις means, "combining together."This means "combining together with the help of light." Photosynthesis also applies to other organisms besides green plants. These include several prokaryotes such as ...
The chemical equation for the entire process can be seen below. Photosynthesis Equation. 6 CO 2 + 6 H 2 O + Light -> C 6 H 12 O 6 + 6 O 2 + 6 H 2 O. Above is the overall reaction for photosynthesis. Using the energy from light and the hydrogens and electrons from water, the plant combines the carbons found in carbon dioxide into more complex ...
photosynthesis: the process by which plants and other photoautotrophs generate carbohydrates and oxygen from carbon dioxide, water, and light energy in chloroplasts. photoautotroph: an organism that can synthesize its own food by using light as a source of energy. chemoautotroph: a simple organism, such as a protozoan, that derives its energy ...
Photosynthesis is the process of creating sugar and oxygen from carbon dioxide, water and sunlight. It happens through a long series of chemical reactions. But it can be summarized like this: Carbon dioxide, water and light go in. Glucose, water and oxygen come out. (Glucose is a simple sugar.) Photosynthesis can be split into two processes.
Now that you know the general rules on how to write scientific reports, let's look at the conventions for their structure! 1. Title. ... Photosynthesis is a vital process for life. It occurs when plants intake carbon dioxide, water, and light, and results in the production of glucose and water. The light required for photosynthesis is ...
Plants, on the other hand, are experts at capturing light energy and using it to make sugars through a process called photosynthesis. This process begins with the absorption of light by specialized organic molecules, called pigments, that are found in the chloroplasts of plant cells.Here, we'll consider light as a form of energy, and we'll also see how pigments - such as the chlorophylls ...
Abstract. Photosynthesis sustains virtually all life on planet Earth providing the oxygen we breathe and the food we eat; it forms the basis of global food chains and meets the majority of humankind's current energy needs through fossilized photosynthetic fuels. The process of photosynthesis in plants is based on two reactions that are carried ...
Make a sketch of your chromatogram using the template that follows. Using colored pencils, note the color of the bands. Use Table 1 to help you identify each pigment. On your sketch, label each band with the name of the pigment. Mark the distance (in cm) from the initial pigment band to each colored band as well as the total distance from the ...
Photosynthesis is a process by which plants prepare their own food in the presence of water, chlorophyll, sunlight, and CO2. This process occurs mainly in the leaves of the plants ("Factors Affecting Photosynthesis", n.). Chlorophyll, light, and carbon dioxide are several factors affecting photosynthesis- its rate and efficiency.
Photosynthesis Lab Report Instructions: In this lab activity, you will investigate the rate of photosynthesis in response to light using the floating leaf disk technique. Then you will create your own investigation to test the effects of a second variable on the rate of photosynthesis. Submit your lab report to your instructor when completed.
1 Key Laboratory of Ecohydrology of Inland River Basin, Alax Desert Eco-hydrology Experimental Research Station, Qilian Mountains Eco-Environment Research Center in Gansu Province, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, Lanzhou, China; 2 Technology Innovation Center for Mine Geological Environment Restoration in the Alpine and Arid Regions, Ministry ...