- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution


Search form
- Advanced search
- Search responses
- Search blogs
- Performing a...
Performing a literature review
- Related content
- Peer review
- Gulraj S Matharu , academic foundation doctor ,
- Christopher D Buckley , Arthritis Research UK professor of rheumatology
- 1 Institute of Biomedical Research, College of Medical and Dental Sciences, School of Immunity and Infection, University of Birmingham, UK
A necessary skill for any doctor
What causes disease, which drug is best, does this patient need surgery, and what is the prognosis? Although experience helps in answering these questions, ultimately they are best answered by evidence based medicine. But how do you assess the evidence? As a medical student, and throughout your career as a doctor, critical appraisal of published literature is an important skill to develop and refine. At medical school you will repeatedly appraise published literature and write literature reviews. These activities are commonly part of a special study module, research project for an intercalated degree, or another type of essay based assignment.
Formulating a question
Literature reviews are most commonly performed to help answer a particular question. While you are at medical school, there will usually be some choice regarding the area you are going to review.
Once you have identified a subject area for review, the next step is to formulate a specific research question. This is arguably the most important step because a clear question needs to be defined from the outset, which you aim to answer by doing the review. The clearer the question, the more likely it is that the answer will be clear too. It is important to have discussions with your supervisor when formulating a research question as his or her input will be invaluable. The research question must be objective and concise because it is easier to search through the evidence with a clear question. The question also needs to be feasible. What is the point in having a question for which no published evidence exists? Your supervisor’s input will ensure you are not trying to answer an unrealistic question. Finally, is the research question clinically important? There are many research questions that may be answered, but not all of them will be relevant to clinical practice. The research question we will use as an example to work through in this article is, “What is the evidence for using angiotensin converting enzyme (ACE) inhibitors in patients with hypertension?”
Collecting the evidence
After formulating a specific research question for your literature review, the next step is to collect the evidence. Your supervisor will initially point you in the right direction by highlighting some of the more relevant papers published. Before doing the literature search it is important to agree a list of keywords with your supervisor. A source of useful keywords can be obtained by reading Cochrane reviews or other systematic reviews, such as those published in the BMJ . 1 2 A relevant Cochrane review for our research question on ACE inhibitors in hypertension is that by Heran and colleagues. 3 Appropriate keywords to search for the evidence include the words used in your research question (“angiotensin converting enzyme inhibitor,” “hypertension,” “blood pressure”), details of the types of study you are looking for (“randomised controlled trial,” “case control,” “cohort”), and the specific drugs you are interested in (that is, the various ACE inhibitors such as “ramipril,” “perindopril,” and “lisinopril”).
Once keywords have been agreed it is time to search for the evidence using the various electronic medical databases (such as PubMed, Medline, and EMBASE). PubMed is the largest of these databases and contains online information and tutorials on how to do literature searches with worked examples. Searching the databases and obtaining the articles are usually free of charge through the subscription that your university pays. Early consultation with a medical librarian is important as it will help you perform your literature search in an impartial manner, and librarians can train you to do these searches for yourself.
Literature searches can be broad or tailored to be more specific. With our example, a broad search would entail searching all articles that contain the words “blood pressure” or “ACE inhibitor.” This provides a comprehensive list of all the literature, but there are likely to be thousands of articles to review subsequently (fig 1). ⇓ In contrast, various search restrictions can be applied on the electronic databases to filter out papers that may not be relevant to your review. Figure 2 gives an example of a specific search. ⇓ The search terms used in this case were “angiotensin converting enzyme inhibitor” and “hypertension.” The limits applied to this search were all randomised controlled trials carried out in humans, published in the English language over the last 10 years, with the search terms appearing in the title of the study only. Thus the more specific the search strategy, the more manageable the number of articles to review (fig 3), and this will save you time. ⇓ However, this method risks your not identifying all the evidence in the particular field. Striking a balance between a broad and a specific search strategy is therefore important. This will come with experience and consultation with your supervisor. It is important to note that evidence is continually becoming available on these electronic databases and therefore repeating the same search at a later date can provide new evidence relevant to your review.

Fig 1 Results from a broad literature search using the term “angiotensin converting enzyme inhibitor”
- Download figure
- Open in new tab
- Download powerpoint

Fig 2 Example of a specific literature search. The search terms used were “angiotensin converting enzyme inhibitor” and “hypertension.” The limits applied to this search were all randomised controlled trials carried out in humans, published in English over the past 10 years, with the search terms appearing in the title of the study only

Fig 3 Results from a specific literature search (using the search terms and limits from figure 2)
Reading the abstracts (study summary) of the articles identified in your search may help you decide whether the study is applicable for your review—for example, the work may have been carried out using an animal model rather than in humans. After excluding any inappropriate articles, you need to obtain the full articles of studies you have identified. Additional relevant articles that may not have come up in your original search can also be found by searching the reference lists of the articles you have already obtained. Once again, you may find that some articles are still not applicable for your review, and these can also be excluded at this stage. It is important to explain in your final review what criteria you used to exclude articles as well as those criteria used for inclusion.
The National Institute for Health and Clinical Excellence (NICE) publishes evidence based guidelines for the United Kingdom and therefore provides an additional resource for identifying the relevant literature in a particular field. 4 NICE critically appraises the published literature with recommendations for best clinical practice proposed and graded based on the quality of evidence available. Similarly, there are internationally published evidence based guidelines, such as those produced by the European Society of Cardiology and the American College of Chest Physicians, which can be useful when collecting the literature in a particular field. 5 6
Appraising the evidence
Once you have collected the evidence, you need to critically appraise the published material. Box 1 gives definitions of terms you will encounter when reading the literature. A brief guide of how to critically appraise a study is presented; however, it is advisable to consult the references cited for further details.
Box 1: Definitions of common terms in the literature 7
Prospective—collecting data in real time after the study is designed
Retrospective—analysis of data that have already been collected to determine associations between exposure and outcome
Hypothesis—proposed association between exposure and outcome. If presented in the negative it is called the null hypothesis
Variable—a quantity or quality that changes during the study and can be measured
Single blind—subjects are unaware of their treatment, but clinicians are aware
Double blind—both subjects and clinicians are unaware of treatment given
Placebo—a simulated medical intervention, with subjects not receiving the specific intervention or treatment being studied
Outcome measure/endpoint—clinical variable or variables measured in a study subsequently used to make conclusions about the original interventions or treatments administered
Bias—difference between reported results and true results. Many types exist (such as selection, allocation, and reporting biases)
Probability (P) value—number between 0 and 1 providing the likelihood the reported results occurred by chance. A P value of 0.05 means there is a 5% likelihood that the reported result occurred by chance
Confidence intervals—provides a range between two numbers within which one can be certain the results lie. A confidence interval of 95% means one can be 95% certain the actual results lie within the reported range
The study authors should clearly define their research question and ideally the hypothesis to be tested. If the hypothesis is presented in the negative, it is called the null hypothesis. An example of a null hypothesis is smoking does not cause lung cancer. The study is then performed to assess the significance of the exposure (smoking) on outcome (lung cancer).
A major part of the critical appraisal process is to focus on study methodology, with your key task being an assessment of the extent to which a study was susceptible to bias (the discrepancy between the reported results and the true results). It should be clear from the methods what type of study was performed (box 2).
Box 2: Different study types 7
Systematic review/meta-analysis—comprehensive review of published literature using predefined methodology. Meta-analyses combine results from various studies to give numerical data for the overall association between variables
Randomised controlled trial—random allocation of patients to one of two or more groups. Used to test a new drug or procedure
Cohort study—two or more groups followed up over a long period, with one group exposed to a certain agent (drug or environmental agent) and the other not exposed, with various outcomes compared. An example would be following up a group of smokers and a group of non-smokers with the outcome measure being the development of lung cancer
Case-control study—cases (those with a particular outcome) are matched as closely as possible (for age, sex, ethnicity) with controls (those without the particular outcome). Retrospective data analysis is performed to determine any factors associated with developing the particular outcomes
Cross sectional study—looks at a specific group of patients at a single point in time. Effectively a survey. An example is asking a group of people how many of them drink alcohol
Case report—detailed reports concerning single patients. Useful in highlighting adverse drug reactions
There are many different types of bias, which depend on the particular type of study performed, and it is important to look for these biases. Several published checklists are available that provide excellent resources to help you work through the various studies and identify sources of bias. The CONSORT statement (which stands for CONsolidated Standards Of Reporting Trials) provides a minimum set of recommendations for reporting randomised controlled trials and comprises a rigorous 25 item checklist, with variations available for other study types. 8 9 As would be expected, most (17 of 25) of the items focus on questions relating to the methods and results of the randomised trial. The remaining items relate to the title, abstract, introduction, and discussion of the study, in addition to questions on trial registration, protocol, and funding.
Jadad scoring provides a simple and validated system to assess the methodological quality of a randomised clinical trial using three questions. 10 The score ranges from zero to five, with one point given for a “yes” in each of the following questions. (1) Was the study described as randomised? (2) Was the study described as double blind? (3) Were there details of subject withdrawals, exclusions, and dropouts? A further point is given if (1) the method of randomisation was appropriate, and (2) the method of blinding was appropriate.
In addition, the Critical Appraisal Skills Programme provides excellent tools for assessing the evidence in all study types (box 2). 11 The Oxford Centre for Evidence-Based Medicine levels of evidence is yet another useful resource for assessing the methodological quality of all studies. 12
Ensure all patients have been accounted for and any exclusions, for whatever reason, are reported. Knowing the baseline demographic (age, sex, ethnicity) and clinical characteristics of the population is important. Results are usually reported as probability values or confidence intervals (box 1).
This should explain the major study findings, put the results in the context of the published literature, and attempt to account for any variations from previous work. Study limitations and sources of bias should be discussed. Authors’ conclusions should be supported by the study results and not unnecessarily extrapolated. For example, a treatment shown to be effective in animals does not necessarily mean it will work in humans.
The format for writing up the literature review usually consists of an abstract (short structured summary of the review), the introduction or background, methods, results, and discussion with conclusions. There are a number of good examples of how to structure a literature review and these can be used as an outline when writing your review. 13 14
The introduction should identify the specific research question you intend to address and briefly put this into the context of the published literature. As you have now probably realised, the methods used for the review must be clear to the reader and provide the necessary detail for someone to be able to reproduce the search. The search strategy needs to include a list of keywords used, which databases were searched, and the specific search limits or filters applied. Any grading of methodological quality, such as the CONSORT statement or Jadad scoring, must be explained in addition to any study inclusion or exclusion criteria. 6 7 8 The methods also need to include a section on the data collected from each of the studies, the specific outcomes of interest, and any statistical analysis used. The latter point is usually relevant only when performing meta-analyses.
The results section must clearly show the process of filtering down from the articles obtained from the original search to the final studies included in the review—that is, accounting for all excluded studies. A flowchart is usually best to illustrate this. Next should follow a brief description of what was done in the main studies, the number of participants, the relevant results, and any potential sources of bias. It is useful to group similar studies together as it allows comparisons to be made by the reader and saves repetition in your write-up. Boxes and figures should be used appropriately to illustrate important findings from the various studies.
Finally, in the discussion you need to consider the study findings in light of the methodological quality—that is, the extent of potential bias in each study that may have affected the study results. Using the evidence, you need to make conclusions in your review, and highlight any important gaps in the evidence base, which need to be dealt with in future studies. Working through drafts of the literature review with your supervisor will help refine your critical appraisal skills and the ability to present information concisely in a structured review article. Remember, if the work is good it may get published.
Originally published as: Student BMJ 2012;20:e404
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
- ↵ The Cochrane Library. www3.interscience.wiley.com/cgibin/mrwhome/106568753/HOME?CRETRY=1&SRETRY=0 .
- ↵ British Medical Journal . www.bmj.com/ .
- ↵ Heran BS, Wong MMY, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database Syst Rev 2008 ; 4 : CD003823 , doi: 10.1002/14651858.CD003823.pub2. OpenUrl PubMed
- ↵ National Institute for Health and Clinical Excellence. www.nice.org.uk .
- ↵ European Society of Cardiology. www.escardio.org/guidelines .
- ↵ Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th ed). Chest 2008 ; 133 : 381 -453S. OpenUrl CrossRef
- ↵ Wikipedia. http://en.wikipedia.org/wiki .
- ↵ Moher D, Schulz KF, Altman DG, Egger M, Davidoff F, Elbourne D, et al. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001 ; 357 : 1191 -4. OpenUrl CrossRef PubMed Web of Science
- ↵ The CONSORT statement. www.consort-statement.org/ .
- ↵ Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996 ; 17 : 1 -12. OpenUrl CrossRef PubMed Web of Science
- ↵ Critical Appraisal Skills Programme (CASP). www.sph.nhs.uk/what-we-do/public-health-workforce/resources/critical-appraisals-skills-programme .
- ↵ Oxford Centre for Evidence-based Medicine—Levels of Evidence. www.cebm.net .
- ↵ Van den Bruel A, Thompson MJ, Haj-Hassan T, Stevens R, Moll H, Lakhanpaul M, et al . Diagnostic value of laboratory tests in identifying serious infections in febrile children: systematic review. BMJ 2011 ; 342 : d3082 . OpenUrl Abstract / FREE Full Text
- ↵ Awopetu AI, Moxey P, Hinchliffe RJ, Jones KG, Thompson MM, Holt PJ. Systematic review and meta-analysis of the relationship between hospital volume and outcome for lower limb arterial surgery. Br J Surg 2010 ; 97 : 797 -803. OpenUrl CrossRef PubMed
- University of Detroit Mercy
- Health Professions
Health Services Administration
- Writing a Literature Review
- Find Articles (Databases)
- Evidence-based Practice
- eBooks & Articles
- General Writing Support
- Creating & Printing Posters
- Research Project Web Resources
- Statistics: Health / Medical
- Searching Tips
- Streaming Video
- Database & Library Help
- Medical Apps & Mobile Sites
- Faculty Publications
Literature Review Overview
What is a Literature Review? Why Are They Important?
A literature review is important because it presents the "state of the science" or accumulated knowledge on a specific topic. It summarizes, analyzes, and compares the available research, reporting study strengths and weaknesses, results, gaps in the research, conclusions, and authors’ interpretations.
Tips and techniques for conducting a literature review are described more fully in the subsequent boxes:
- Literature review steps
- Strategies for organizing the information for your review
- Literature reviews sections
- In-depth resources to assist in writing a literature review
- Templates to start your review
- Literature review examples
Literature Review Steps

Graphic used with permission: Torres, E. Librarian, Hawai'i Pacific University
1. Choose a topic and define your research question
- Try to choose a topic of interest. You will be working with this subject for several weeks to months.
- Ideas for topics can be found by scanning medical news sources (e.g MedPage Today), journals / magazines, work experiences, interesting patient cases, or family or personal health issues.
- Do a bit of background reading on topic ideas to familiarize yourself with terminology and issues. Note the words and terms that are used.
- Develop a focused research question using PICO(T) or other framework (FINER, SPICE, etc - there are many options) to help guide you.
- Run a few sample database searches to make sure your research question is not too broad or too narrow.
- If possible, discuss your topic with your professor.
2. Determine the scope of your review
The scope of your review will be determined by your professor during your program. Check your assignment requirements for parameters for the Literature Review.
- How many studies will you need to include?
- How many years should it cover? (usually 5-7 depending on the professor)
- For the nurses, are you required to limit to nursing literature?
3. Develop a search plan
- Determine which databases to search. This will depend on your topic. If you are not sure, check your program specific library website (Physician Asst / Nursing / Health Services Admin) for recommendations.
- Create an initial search string using the main concepts from your research (PICO, etc) question. Include synonyms and related words connected by Boolean operators
- Contact your librarian for assistance, if needed.
4. Conduct searches and find relevant literature
- Keep notes as you search - tracking keywords and search strings used in each database in order to avoid wasting time duplicating a search that has already been tried
- Read abstracts and write down new terms to search as you find them
- Check MeSH or other subject headings listed in relevant articles for additional search terms
- Scan author provided keywords if available
- Check the references of relevant articles looking for other useful articles (ancestry searching)
- Check articles that have cited your relevant article for more useful articles (descendancy searching). Both PubMed and CINAHL offer Cited By links
- Revise the search to broaden or narrow your topic focus as you peruse the available literature
- Conducting a literature search is a repetitive process. Searches can be revised and re-run multiple times during the process.
- Track the citations for your relevant articles in a software citation manager such as RefWorks, Zotero, or Mendeley
5. Review the literature
- Read the full articles. Do not rely solely on the abstracts. Authors frequently cannot include all results within the confines of an abstract. Exclude articles that do not address your research question.
- While reading, note research findings relevant to your project and summarize. Are the findings conflicting? There are matrices available than can help with organization. See the Organizing Information box below.
- Critique / evaluate the quality of the articles, and record your findings in your matrix or summary table. Tools are available to prompt you what to look for. (See Resources for Appraising a Research Study box on the HSA, Nursing , and PA guides )
- You may need to revise your search and re-run it based on your findings.
6. Organize and synthesize
- Compile the findings and analysis from each resource into a single narrative.
- Using an outline can be helpful. Start broad, addressing the overall findings and then narrow, discussing each resource and how it relates to your question and to the other resources.
- Cite as you write to keep sources organized.
- Write in structured paragraphs using topic sentences and transition words to draw connections, comparisons, and contrasts.
- Don't present one study after another, but rather relate one study's findings to another. Speak to how the studies are connected and how they relate to your work.
Organizing Information
Options to assist in organizing sources and information :
1. Synthesis Matrix
- helps provide overview of the literature
- information from individual sources is entered into a grid to enable writers to discern patterns and themes
- article summary, analysis, or results
- thoughts, reflections, or issues
- each reference gets its own row
- mind maps, concept maps, flowcharts
- at top of page record PICO or research question
- record major concepts / themes from literature
- list concepts that branch out from major concepts underneath - keep going downward hierarchically, until most specific ideas are recorded
- enclose concepts in circles and connect the concept with lines - add brief explanation as needed
3. Summary Table
- information is recorded in a grid to help with recall and sorting information when writing
- allows comparing and contrasting individual studies easily
- purpose of study
- methodology (study population, data collection tool)
Efron, S. E., & Ravid, R. (2019). Writing the literature review : A practical guide . Guilford Press.
Literature Review Sections
- Lit reviews can be part of a larger paper / research study or they can be the focus of the paper
- Lit reviews focus on research studies to provide evidence
- New topics may not have much that has been published
* The sections included may depend on the purpose of the literature review (standalone paper or section within a research paper)
Standalone Literature Review (aka Narrative Review):
- presents your topic or PICO question
- includes the why of the literature review and your goals for the review.
- provides background for your the topic and previews the key points
- Narrative Reviews: tmay not have an explanation of methods.
- include where the search was conducted (which databases) what subject terms or keywords were used, and any limits or filters that were applied and why - this will help others re-create the search
- describe how studies were analyzed for inclusion or exclusion
- review the purpose and answer the research question
- thematically - using recurring themes in the literature
- chronologically - present the development of the topic over time
- methodological - compare and contrast findings based on various methodologies used to research the topic (e.g. qualitative vs quantitative, etc.)
- theoretical - organized content based on various theories
- provide an overview of the main points of each source then synthesize the findings into a coherent summary of the whole
- present common themes among the studies
- compare and contrast the various study results
- interpret the results and address the implications of the findings
- do the results support the original hypothesis or conflict with it
- provide your own analysis and interpretation (eg. discuss the significance of findings; evaluate the strengths and weaknesses of the studies, noting any problems)
- discuss common and unusual patterns and offer explanations
- stay away from opinions, personal biases and unsupported recommendations
- summarize the key findings and relate them back to your PICO/research question
- note gaps in the research and suggest areas for further research
- this section should not contain "new" information that had not been previously discussed in one of the sections above
- provide a list of all the studies and other sources used in proper APA 7
Literature Review as Part of a Research Study Manuscript:
- Compares the study with other research and includes how a study fills a gap in the research.
- Focus on the body of the review which includes the synthesized Findings and Discussion
Literature Reviews vs Systematic Reviews
Systematic Reviews are NOT the same as a Literature Review:
Literature Reviews:
- Literature reviews may or may not follow strict systematic methods to find, select, and analyze articles, but rather they selectively and broadly review the literature on a topic
- Research included in a Literature Review can be "cherry-picked" and therefore, can be very subjective
Systematic Reviews:
- Systemic reviews are designed to provide a comprehensive summary of the evidence for a focused research question
- rigorous and strictly structured, using standardized reporting guidelines (e.g. PRISMA, see link below)
- uses exhaustive, systematic searches of all relevant databases
- best practice dictates search strategies are peer reviewed
- uses predetermined study inclusion and exclusion criteria in order to minimize bias
- aims to capture and synthesize all literature (including unpublished research - grey literature) that meet the predefined criteria on a focused topic resulting in high quality evidence
Literature Review Examples
- Breastfeeding initiation and support: A literature review of what women value and the impact of early discharge (2017). Women and Birth : Journal of the Australian College of Midwives
- Community-based participatory research to promote healthy diet and nutrition and prevent and control obesity among African-Americans: A literature review (2017). Journal of Racial and Ethnic Health Disparities
- Vitamin D deficiency in individuals with a spinal cord injury: A literature review (2017). Spinal Cord
Resources for Writing a Literature Review
These sources have been used in developing this guide.
Resources Used on This Page
Aveyard, H. (2010). Doing a literature review in health and social care : A practical guide . McGraw-Hill Education.
Purdue Online Writing Lab. (n.d.). Writing a literature review . Purdue University. https://owl.purdue.edu/owl/research_and_citation/conducting_research/writing_a_literature_review.html
Torres, E. (2021, October 21). Nursing - graduate studies research guide: Literature review. Hawai'i Pacific University Libraries. Retrieved January 27, 2022, from https://hpu.libguides.com/c.php?g=543891&p=3727230
- << Previous: General Writing Support
- Next: Creating & Printing Posters >>
- Last Updated: May 21, 2024 11:23 AM
- URL: https://udmercy.libguides.com/hsa
- USC Libraries
- Research Guides
- Health Sciences
Medical Students Scholarly Project Course
- Literature Review
What is a literature review?
Systematic reviews vs literature reviews, literature reviews - articles, writing literature reviews, frequently used journal article databases.
- Conference Posters This link opens in a new window
- Soft Skills
The literature review is the qualitative summary of evidence on a topic using informal or subjective methods to collect and interpret studies.The literature review can inform a particular research project or can result in a review article publication.

- Aaron L. Writing a literature review article. Radiol Technol. 2008 Nov-Dec; 80(12): 185-6.
- Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int. 2011 Nov; 31(11): 1409-17.
- Matharu GS, Buckley CD. Performing a literature review: a necessary skill for any doctor. Student BMJ. 2012; 20:e404. Requires FREE site registration
- Literature Reviews The Writing Center at University of North Carolina at Chapel Hill has created a succinct handout that explains what a literature review is and offer insights into the form and construction of a literature review in the humanities, social sciences, and sciences.
- Review Articles (Health Sciences) Guide Identifies the difference between a systematic review and a literature review. Connects to tools for research, writing, and publishing.
- Systematic Approaches to a Successful Literature Review by Andrew Booth; Diana Papaioannou; Anthea Sutton Call Number: Norris Medical Library, Upper Level, LB 1047.3 B725s 2012
- Documenting your search This resource provides guidance on how to document and save database search strategies.
- Cochrane Database of Systematic Reviews
- Embase This link opens in a new window
- Google Scholar This link opens in a new window
- PsycINFO This link opens in a new window
- Scopus This link opens in a new window
- Web of Science This link opens in a new window
- Next: Data Mgmt >>
- Last Updated: Nov 1, 2023 3:17 PM
- URL: https://libguides.usc.edu/healthsciences/spc
Health (Nursing, Medicine, Allied Health)
- Find Articles/Databases
- Reference Resources
- Evidence Summaries & Clinical Guidelines
- Drug Information
- Health Data & Statistics
- Patient/Consumer Facing Materials
- Images and Streaming Video
- Grey Literature
- Mobile Apps & "Point of Care" Tools
- Tests & Measures This link opens in a new window
- Citing Sources
- Selecting Databases
- Framing Research Questions
- Crafting a Search
- Narrowing / Filtering a Search
- Expanding a Search
- Cited Reference Searching
- Saving Searches
- Term Glossary
- Critical Appraisal Resources
- What are Literature Reviews?
- Conducting & Reporting Systematic Reviews
- Finding Systematic Reviews
- Tutorials & Tools for Literature Reviews
- Finding Full Text
What are Systematic Reviews? (3 minutes, 24 second YouTube Video)
Systematic Literature Reviews: Steps & Resources
These steps for conducting a systematic literature review are listed below .
Also see subpages for more information about:
- The different types of literature reviews, including systematic reviews and other evidence synthesis methods
- Tools & Tutorials
Literature Review & Systematic Review Steps
- Develop a Focused Question
- Scope the Literature (Initial Search)
- Refine & Expand the Search
- Limit the Results
- Download Citations
- Abstract & Analyze
- Create Flow Diagram
- Synthesize & Report Results
1. Develop a Focused Question
Consider the PICO Format: Population/Problem, Intervention, Comparison, Outcome
Focus on defining the Population or Problem and Intervention (don't narrow by Comparison or Outcome just yet!)
"What are the effects of the Pilates method for patients with low back pain?"
Tools & Additional Resources:
- PICO Question Help
- Stillwell, Susan B., DNP, RN, CNE; Fineout-Overholt, Ellen, PhD, RN, FNAP, FAAN; Melnyk, Bernadette Mazurek, PhD, RN, CPNP/PMHNP, FNAP, FAAN; Williamson, Kathleen M., PhD, RN Evidence-Based Practice, Step by Step: Asking the Clinical Question, AJN The American Journal of Nursing : March 2010 - Volume 110 - Issue 3 - p 58-61 doi: 10.1097/01.NAJ.0000368959.11129.79
2. Scope the Literature
A "scoping search" investigates the breadth and/or depth of the initial question or may identify a gap in the literature.
Eligible studies may be located by searching in:
- Background sources (books, point-of-care tools)
- Article databases
- Trial registries
- Grey literature
- Cited references
- Reference lists
When searching, if possible, translate terms to controlled vocabulary of the database. Use text word searching when necessary.
Use Boolean operators to connect search terms:
- Combine separate concepts with AND (resulting in a narrower search)
- Connecting synonyms with OR (resulting in an expanded search)
Search: pilates AND ("low back pain" OR backache )
Video Tutorials - Translating PICO Questions into Search Queries
- Translate Your PICO Into a Search in PubMed (YouTube, Carrie Price, 5:11)
- Translate Your PICO Into a Search in CINAHL (YouTube, Carrie Price, 4:56)
3. Refine & Expand Your Search
Expand your search strategy with synonymous search terms harvested from:
- database thesauri
- reference lists
- relevant studies
Example:
(pilates OR exercise movement techniques) AND ("low back pain" OR backache* OR sciatica OR lumbago OR spondylosis)
As you develop a final, reproducible strategy for each database, save your strategies in a:
- a personal database account (e.g., MyNCBI for PubMed)
- Log in with your NYU credentials
- Open and "Make a Copy" to create your own tracker for your literature search strategies
4. Limit Your Results
Use database filters to limit your results based on your defined inclusion/exclusion criteria. In addition to relying on the databases' categorical filters, you may also need to manually screen results.
- Limit to Article type, e.g.,: "randomized controlled trial" OR multicenter study
- Limit by publication years, age groups, language, etc.
NOTE: Many databases allow you to filter to "Full Text Only". This filter is not recommended . It excludes articles if their full text is not available in that particular database (CINAHL, PubMed, etc), but if the article is relevant, it is important that you are able to read its title and abstract, regardless of 'full text' status. The full text is likely to be accessible through another source (a different database, or Interlibrary Loan).
- Filters in PubMed
- CINAHL Advanced Searching Tutorial
5. Download Citations
Selected citations and/or entire sets of search results can be downloaded from the database into a citation management tool. If you are conducting a systematic review that will require reporting according to PRISMA standards, a citation manager can help you keep track of the number of articles that came from each database, as well as the number of duplicate records.
In Zotero, you can create a Collection for the combined results set, and sub-collections for the results from each database you search. You can then use Zotero's 'Duplicate Items" function to find and merge duplicate records.

- Citation Managers - General Guide
6. Abstract and Analyze
- Migrate citations to data collection/extraction tool
- Screen Title/Abstracts for inclusion/exclusion
- Screen and appraise full text for relevance, methods,
- Resolve disagreements by consensus
Covidence is a web-based tool that enables you to work with a team to screen titles/abstracts and full text for inclusion in your review, as well as extract data from the included studies.

- Covidence Support
- Critical Appraisal Tools
- Data Extraction Tools
7. Create Flow Diagram
The PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) flow diagram is a visual representation of the flow of records through different phases of a systematic review. It depicts the number of records identified, included and excluded. It is best used in conjunction with the PRISMA checklist .

Example from: Stotz, S. A., McNealy, K., Begay, R. L., DeSanto, K., Manson, S. M., & Moore, K. R. (2021). Multi-level diabetes prevention and treatment interventions for Native people in the USA and Canada: A scoping review. Current Diabetes Reports, 2 (11), 46. https://doi.org/10.1007/s11892-021-01414-3
- PRISMA Flow Diagram Generator (ShinyApp.io, Haddaway et al. )
- PRISMA Diagram Templates (Word and PDF)
- Make a copy of the file to fill out the template
- Image can be downloaded as PDF, PNG, JPG, or SVG
- Covidence generates a PRISMA diagram that is automatically updated as records move through the review phases
8. Synthesize & Report Results
There are a number of reporting guideline available to guide the synthesis and reporting of results in systematic literature reviews.
It is common to organize findings in a matrix, also known as a Table of Evidence (ToE).

- Reporting Guidelines for Systematic Reviews
- Download a sample template of a health sciences review matrix (GoogleSheets)
Steps modified from:
Cook, D. A., & West, C. P. (2012). Conducting systematic reviews in medical education: a stepwise approach. Medical Education , 46 (10), 943–952.
- << Previous: Critical Appraisal Resources
- Next: What are Literature Reviews? >>
- Last Updated: May 31, 2024 10:32 AM
- URL: https://guides.nyu.edu/health
- Systematic Review
- Open access
- Published: 23 May 2024
Systematic literature review of real-world evidence for treatments in HR+/HER2- second-line LABC/mBC after first-line treatment with CDK4/6i
- Veronique Lambert ORCID: orcid.org/0000-0002-6984-0038 1 ,
- Sarah Kane ORCID: orcid.org/0009-0006-9341-4836 2 na1 ,
- Belal Howidi ORCID: orcid.org/0000-0002-1166-7631 2 na1 ,
- Bao-Ngoc Nguyen ORCID: orcid.org/0000-0001-6026-2270 2 na1 ,
- David Chandiwana ORCID: orcid.org/0009-0002-3499-2565 3 ,
- Yan Wu ORCID: orcid.org/0009-0008-3348-9232 1 ,
- Michelle Edwards ORCID: orcid.org/0009-0001-4292-3140 3 &
- Imtiaz A. Samjoo ORCID: orcid.org/0000-0003-1415-8055 2 na1
BMC Cancer volume 24 , Article number: 631 ( 2024 ) Cite this article
411 Accesses
1 Altmetric
Metrics details
Cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i) combined with endocrine therapy (ET) are currently recommended by the National Comprehensive Cancer Network (NCCN) guidelines and the European Society for Medical Oncology (ESMO) guidelines as the first-line (1 L) treatment for patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative, locally advanced/metastatic breast cancer (HR+/HER2- LABC/mBC). Although there are many treatment options, there is no clear standard of care for patients following 1 L CDK4/6i. Understanding the real-world effectiveness of subsequent therapies may help to identify an unmet need in this patient population. This systematic literature review qualitatively synthesized effectiveness and safety outcomes for treatments received in the real-world setting after 1 L CDK4/6i therapy in patients with HR+/ HER2- LABC/mBC.
MEDLINE®, Embase, and Cochrane were searched using the Ovid® platform for real-world evidence studies published between 2015 and 2022. Grey literature was searched to identify relevant conference abstracts published from 2019 to 2022. The review was conducted in accordance with PRISMA guidelines (PROSPERO registration: CRD42023383914). Data were qualitatively synthesized and weighted average median real-world progression-free survival (rwPFS) was calculated for NCCN/ESMO-recommended post-1 L CDK4/6i treatment regimens.
Twenty records (9 full-text articles and 11 conference abstracts) encompassing 18 unique studies met the eligibility criteria and reported outcomes for second-line (2 L) treatments after 1 L CDK4/6i; no studies reported disaggregated outcomes in the third-line setting or beyond. Sixteen studies included NCCN/ESMO guideline-recommended treatments with the majority evaluating endocrine-based therapy; five studies on single-agent ET, six studies on mammalian target of rapamycin inhibitors (mTORi) ± ET, and three studies with a mix of ET and/or mTORi. Chemotherapy outcomes were reported in 11 studies. The most assessed outcome was median rwPFS; the weighted average median rwPFS was calculated as 3.9 months (3.3-6.0 months) for single-agent ET, 3.6 months (2.5–4.9 months) for mTORi ± ET, 3.7 months for a mix of ET and/or mTORi (3.0–4.0 months), and 6.1 months (3.7–9.7 months) for chemotherapy. Very few studies reported other effectiveness outcomes and only two studies reported safety outcomes. Most studies had heterogeneity in patient- and disease-related characteristics.
Conclusions
The real-world effectiveness of current 2 L treatments post-1 L CDK4/6i are suboptimal, highlighting an unmet need for this patient population.
Peer Review reports
Introduction
Breast cancer (BC) is the most diagnosed form of cancer in women with an estimated 2.3 million new cases diagnosed worldwide each year [ 1 ]. BC is the second leading cause of cancer death, accounting for 685,000 deaths worldwide per year [ 2 ]. By 2040, the global burden associated with BC is expected to surpass three million new cases and one million deaths annually (due to population growth and aging) [ 3 ]. Numerous factors contribute to global disparities in BC-related mortality rates, including delayed diagnosis, resulting in a high number of BC cases that have progressed to locally advanced BC (LABC) or metastatic BC (mBC) [ 4 , 5 , 6 ]. In the United States (US), the five-year survival rate for patients who progress to mBC is three times lower (31%) than the overall five-year survival rate for all stages (91%) [ 6 , 7 ].
Hormone receptor (HR) positive (i.e., estrogen receptor and/or progesterone receptor positive) coupled with negative human epidermal growth factor 2 (HER2) expression is the most common subtype of BC, accounting for ∼ 60–70% of all BC cases [ 8 , 9 ]. Historically, endocrine therapy (ET) through estrogen receptor modulation and/or estrogen deprivation has been the standard of care for first-line (1 L) treatment of HR-positive/HER2-negative (HR+/HER2-) mBC [ 10 ]. However, with the approval of the cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) palbociclib in combination with the aromatase inhibitor (AI) letrozole in 2015 by the US Food and Drug Administration (FDA), 1 L treatment practice patterns have evolved such that CDK4/6i (either in combination with AIs or with fulvestrant) are currently considered the standard of care [ 11 , 12 , 13 , 14 , 15 , 16 , 17 ]. Other CDK4/6i (ribociclib and abemaciclib) in combination with ET are approved for the treatment of HR+/HER2- LABC/mBC; 1 L use of ribociclib in combination with an AI was granted FDA approval in March 2017 for postmenopausal women (with expanded approval in July 2018 for pre/perimenopausal women and for use in 1 L with fulvestrant for patients with disease progression on ET as well as for postmenopausal women), and abemaciclib in combination with fulvestrant was granted FDA approval in September 2017 for patients with disease progression following ET and as monotherapy in cases where disease progression occurs following ET and prior chemotherapy in mBC (with expanded approval in February 2018 for use in 1 L in combination with an AI for postmenopausal women) [ 18 , 19 , 20 , 21 ].
Clinical trials investigating the addition of CDK4/6i to ET have demonstrated significant improvement in progression-free survival (PFS) and significant (ribociclib) or numerical (palbociclib and abemaciclib) improvement in overall survival (OS) compared to ET alone in patients with HR+/HER2- advanced or mBC, making this combination treatment the recommended option in the 1 L setting [ 22 , 23 , 24 , 25 , 26 , 27 ]. However, disease progression occurs in a significant portion of patients after 1 L CDK4/6i treatment [ 28 ] and the optimal treatment sequence after progression on CDK4/6i remains unclear [ 29 ]. At the time of this review (literature search conducted December 14, 2022), guidelines by the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) recommend various options for the treatment of HR+/HER2- advanced BC in the second-line (2 L) setting, including fulvestrant monotherapy, mammalian target of rapamycin inhibitors (mTORi; e.g., everolimus) ± ET, alpelisib + fulvestrant (if phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha mutation positive [PIK3CA-m+]), poly-ADP ribose polymerase inhibitors (PARPi) including olaparib or talazoparib (if breast cancer gene/partner and localizer of BRCA2 positive [BRCA/PALB2m+]), and chemotherapy (in cases when a visceral crisis is present) [ 15 , 16 ]. CDK4/6i can also be used in 2 L [ 16 , 30 ]; however, limited data are available to support CDK4/6i rechallenge after its use in the 1 L setting [ 15 ]. Depending on treatments used in the 1 L and 2 L settings, treatment in the third-line setting is individualized based on the patient’s response to prior treatments, tumor load, duration of response, and patient preference [ 9 , 15 ]. Understanding subsequent treatments after 1 L CDK4/6i, and their associated effectiveness, is an important focus in BC research.
Treatment options for HR+/HER2- LABC/mBC continue to evolve, with ongoing research in both clinical trials and in the real-world setting. Real-world evidence (RWE) offers important insights into novel therapeutic regimens and the effectiveness of treatments for HR+/HER2- LABC/mBC. The effectiveness of the current treatment options following 1 L CDK4/6i therapy in the real-world setting highlights the unmet need in this patient population and may help to drive further research and drug development. In this study, we conducted a systematic literature review (SLR) to qualitatively summarize the effectiveness and safety of treatment regimens in the real-world setting after 1 L treatment with CDK4/6i in patients with HR+/HER2- LABC/mBC.
Literature search
An SLR was performed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions [ 31 ] and reported in alignment with the Preferred Reporting Items for Systematic Literature Reviews and Meta-Analyses (PRISMA) statement [ 32 ] to identify all RWE studies assessing the effectiveness and safety of treatments used for patients with HR+/HER2- LABC/mBC following 1 L CDK4/6i therapy and received subsequent treatment in 2 L and beyond (2 L+). The Ovid® platform was used to search MEDLINE® (including Epub Ahead of Print and In-Process, In-Data-Review & Other Non-Indexed Citations), Ovid MEDLINE® Daily, Embase, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews by an experienced medical information specialist. The MEDLINE® search strategy was peer-reviewed independently by a senior medical information specialist before execution using the Peer Review of Electronic Search Strategies (PRESS) checklist [ 33 ]. Searches were conducted on December 14, 2022. The review protocol was developed a priori and registered with the International Prospective Register of Systematic Review (PROSPERO; CRD42023383914) which outlined the population, intervention, comparator, outcome, and study design (PICOS) criteria and methodology used to conduct the review (Table 1 ).
Search strategies utilized a combination of controlled vocabulary (e.g., “HER2 Breast Cancer” or “HR Breast Cancer”) and keywords (e.g., “Retrospective studies”). Vocabulary and syntax were adjusted across databases. Published and validated filters were used to select for study design and were supplemented using additional medical subject headings (MeSH) terms and keywords to select for RWE and nonrandomized studies [ 34 ]. No language restrictions were included in the search strategy. Animal-only and opinion pieces were removed from the results. The search was limited to studies published between January 2015 and December 2022 to reflect the time at which FDA approval was granted for the first CDK4/6i agent (palbociclib) in combination with AI for the treatment of LABC/mBC [ 35 ]. Further search details are presented in Supplementary Material 1 .
Grey literature sources were also searched to identify relevant abstracts and posters published from January 2019 to December 2022 for prespecified relevant conferences including ESMO, San Antonio Breast Cancer Symposium (SABCS), American Society of Clinical Oncology (ASCO), the International Society for Pharmacoeconomics and Outcomes Research (ISPOR US), and the American Association for Cancer Research (AACR). A search of ClinicalTrials.gov was conducted to validate the findings from the database and grey literature searches.
Study selection, data extraction & weighted average calculation
Studies were screened for inclusion using DistillerSR Version 2.35 and 2.41 (DistillerSR Inc. 2021, Ottawa, Canada) by two independent reviewers based on the prespecified PICOS criteria (Table 1 ). A third reviewer was consulted to resolve any discrepancies during the screening process. Studies were included if they reported RWE on patients aged ≥ 18 years with HR+/HER2- LABC/mBC who received 1 L CDK4/6i treatment and received subsequent treatment in 2 L+. Studies were excluded if they reported the results of clinical trials (i.e., non-RWE), were published in any language other than English, and/or were published prior to 2015 (or prior to 2019 for conference abstracts and posters). For studies that met the eligibility criteria, data relating to study design and methodology, details of interventions, patient eligibility criteria and baseline characteristics, and outcome measures such as efficacy, safety, tolerability, and patient-reported outcomes (PROs), were extracted (as available) using a Microsoft Excel®-based data extraction form (Microsoft Corporation, WA, USA). Data extraction was performed by a single reviewer and was confirmed by a second reviewer. Multiple publications identified for the same RWE study, patient population, and setting that reported data for the same intervention were linked and extracted as a single publication. Weighted average median real-world progression-free survival (rwPFS) values were calculated by considering the contribution to the median rwPFS of each study proportional to its respective sample size. These weighted values were then used to compute the overall median rwPFS estimate.
Quality assessment
The Newcastle-Ottawa scale (NOS) for nonrandomized (cohort) studies was used to assess the risk of bias for published, full-text studies [ 36 ]. The NOS allocates a maximum of nine points for the least risk of bias across three domains: (1) Formation of study groups (four points), (2) Comparability between study groups (two points), (3) Outcome ascertainment (three points). NOS scores can be categorized in three groups: very high risk of bias (0 to 3 points), high risk of bias (4 to 6), and low risk of bias (7 to 9) [ 37 ]. Risk of bias assessment was performed by one reviewer and validated by a second independent reviewer to verify accuracy. Due to limited methodological data by which to assess study quality, risk of bias assessment was not performed on conference abstracts or posters. An amendment to the PROSPERO record (CRD42023383914) for this study was submitted in relation to the quality assessment method (specifying usage of the NOS).
The database search identified 3,377 records; after removal of duplicates, 2,759 were screened at the title and abstract stage of which 2,553 were excluded. Out of the 206 reports retrieved and assessed for eligibility, an additional 187 records were excluded after full-text review; most of these studies were excluded for having patients with mixed lines of CDK4/6i treatment (i.e., did not receive CDK4/6i exclusively in 1 L) (Fig. 1 and Table S1 ). The grey literature search identified 753 records which were assessed for eligibility; of which 752 were excluded mainly due to the population not meeting the eligibility criteria (Fig. 1 ). In total, the literature searches identified 20 records (9 published full-text articles and 11 conference abstracts/posters) representing 18 unique RWE studies that met the inclusion criteria. The NOS quality scores for the included full-text articles are provided in Table S2 . The scores ranged from four to six points (out of a total score of nine) and the median score was five, indicating that all the studies suffered from a high risk of bias [ 37 ].
Most studies were retrospective analyses of chart reviews or medical registries, and all studies were published between 2017 and 2022 (Table S3 ). Nearly half of the RWE studies (8 out of 18 studies) were conducted in the US [ 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ], while the remaining studies included sites in Canada, China, Germany, Italy, Japan, and the United Kingdom [ 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 ]. Sample sizes ranged from as few as 4 to as many as 839 patients across included studies, with patient age ranging from 26 to 86 years old.
Although treatment characteristics in the 1 L setting were not the focus of the present review, these details are captured in Table S3 . Briefly, several RWE studies reported 1 L CDK4/6i use in combination with ET (8 out of 18 studies) or as monotherapy (2 out of 18 studies) (Table S3 ). Treatments used in combination with 1 L CDK4/6i included letrozole, fulvestrant, exemestane, and anastrozole. Where reported (4 out of 18 studies), palbociclib was the most common 1 L CDK4/6i treatment. Many studies (8 out of 18 studies) did not report which specific CDK4/6i treatment(s) were used in 1 L or if its administration was in combination or monotherapy.
Characteristics of treatments after 1 L CDK4/6i therapy
Across all studies included in this review, effectiveness and safety data were only available for treatments administered in the 2 L setting after 1 L CDK4/6i treatment. No studies were identified that reported outcomes for patients treated in the third-line setting or beyond after 1 L CDK4/6i treatment. All 18 studies reported effectiveness outcomes in 2 L, with only two of these studies also describing 2 L safety outcomes. The distribution of outcomes reported in these studies is provided in Table S4 . Studies varied in their reporting of outcomes for 2 L treatments; some studies reported outcomes for a group of 2 L treatments while others described independent outcomes for specific 2 L treatments (i.e., everolimus, fulvestrant, or chemotherapy agents such as eribulin mesylate) [ 42 , 45 , 50 , 54 , 55 ]. Due to the heterogeneity in treatment classes reported in these studies, this data was categorized (as described below) to align with the guidelines provided by NCCN and ESMO [ 15 , 16 ]. The treatment class categorizations for the purpose of this review are: single-agent ET (patients who exclusively received a single-agent ET after 1 L CDK4/6i treatment), mTORi ± ET (patients who exclusively received an mTORi with or without ET after 1 L CDK4/6i treatment), mix of ET and/or mTORi (patients who may have received only ET, only mTORi, and/or both treatments but the studies in this group lacked sufficient information to categorize these patients in the “single-agent ET” or “mTOR ± ET” categories), and chemotherapy (patients who exclusively received chemotherapy after 1 L CDK4/6i treatment). Despite ESMO and NCCN guidelines indicating that limited evidence exists to support rechallenge with CDK4/6i after 1 L CDK4/6i treatment [ 15 , 16 ], two studies reported outcomes for this treatment approach. Data for such patients were categorized as “ CDK4/6i ± ET ” as it was unclear how many patients receiving CDK4/6i rechallenge received concurrent ET. All other patient groups that lacked sufficient information or did not report outcome/safety data independently (i.e., grouped patients with mixed treatments) to categorize as one of the treatment classes described above were grouped as “ other ”.
The majority of studies reported effectiveness outcomes for endocrine-based therapy after 1 L CDK4/6i treatment; five studies for single-agent ET, six studies for mTORi ± ET, and three studies for a mix of ET and/or mTORi (Fig. 2 ). Eleven studies reported effectiveness outcomes for chemotherapy after 1 L CDK4/6i treatment, and only two studies reported effectiveness outcomes for CDK4/6i rechallenge ± ET. Eight studies that described effectiveness outcomes were grouped into the “other” category. Safety data was only reported in two studies: one study evaluating the chemotherapy agent eribulin mesylate and one evaluating the mTORi everolimus.
Effectiveness outcomes
Real-world progression-free survival
Median rwPFS was described in 13 studies (Tables 2 and Table S5 ). Across the 13 studies, the median rwPFS ranged from 2.5 months [ 49 ] to 17.3 months [ 39 ]. Out of the 13 studies reporting median rwPFS, 10 studies reported median rwPFS for a 2 L treatment recommended by ESMO and NCCN guidelines, which ranged from 2.5 months [ 49 ] to 9.7 months [ 45 ].
Weighted average median rwPFS was calculated for 2 L treatments recommended by both ESMO and NCCN guidelines (Fig. 3 ). The weighted average median rwPFS for single-agent ET was 3.9 months ( n = 92 total patients) and was derived using data from two studies reporting median rwPFS values of 3.3 months ( n = 70) [ 38 ] and 6.0 months ( n = 22) [ 40 ]. For one study ( n = 7) that reported outcomes for single agent ET, median rwPFS was not reached during the follow-up period; as such, this study was excluded from the weighted average median rwPFS calculation [ 49 ].
The weighted average median rwPFS for mTORi ± ET was 3.6 months ( n = 128 total patients) and was derived based on data from 3 studies with median rwPFS ranging from 2.5 months ( n = 4) [ 49 ] to 4.9 months ( n = 25) [ 54 ] (Fig. 3 ). For patients who received a mix of ET and/or mTORi but could not be classified into the single-agent ET or mTORi ± ET treatment classes, the weighted average median rwPFS was calculated to be 3.7 months ( n = 17 total patients). This was calculated based on data from two studies reporting median rwPFS values of 3.0 months ( n = 5) [ 46 ] and 4.0 months ( n = 12) [ 49 ]. Notably, one study of patients receiving ET and/or everolimus reported a median rwPFS duration of 3.0 months; however, this study was excluded from the weighted average median rwPFS calculation for the ET and/or mTORi class as the sample size was not reported [ 53 ].
The weighted average median rwPFS for chemotherapy was 6.1 months ( n = 499 total patients), calculated using data from 7 studies reporting median rwPFS values ranging from 3.7 months ( n = 249) [ 38 ] to 9.7 months ( n = 121) [ 45 ] (Fig. 3 ). One study with a median rwPFS duration of 5.6 months was not included in the weighted average median rwPFS calculation as the study did not report the sample size [ 53 ]. A second study was excluded from the calculation since the reported median rwPFS was not reached during the study period ( n = 7) [ 41 ].
Although 2 L CDK4/6i ± ET rechallenge lacks sufficient information to support recommendation by ESMO and NCCN guidelines, the limited data currently available for this treatment have shown promising results. Briefly, two studies reported median rwPFS for CDK4/6i ± ET with values of 8.3 months ( n = 302) [ 38 ] and 17.3 months ( n = 165) (Table 2 ) [ 39 ]. The remaining median rwPFS studies reported data for patients classified as “Other” (Table S5 ). The “Other” category included median rwPFS outcomes from seven studies, and included a myriad of treatments (e.g., ET, mTOR + ET, chemotherapy, CDK4/6i + ET, alpelisib + fulvestrant, chidamide + ET) for which disaggregated median rwPFS values were not reported.
Overall survival
Median OS for 2 L treatment was reported in only three studies (Table 2 ) [ 38 , 42 , 43 ]. Across the three studies, the 2 L median OS ranged from 5.2 months ( n = 3) [ 43 ] to 35.7 months ( n = 302) [ 38 ]. Due to the lack of OS data in most of the studies, weighted averages could not be calculated. No median OS data was reported for the single-agent ET treatment class whereas two studies reported median OS for the mTORi ± ET treatment class, ranging from 5.2 months ( n = 3) [ 43 ] to 21.8 months ( n = 54) [ 42 ]. One study reported 2 L median OS of 24.8 months for a single patient treated with chemotherapy [ 43 ]. The median OS data in the CDK4/6i ± ET rechallenge group was 35.7 months ( n = 302) [ 38 ].
Patient mortality was reported in three studies [ 43 , 44 , 45 ]. No studies reported mortality for the single-agent ET treatment class and only one study reported this outcome for the mTORi ± ET treatment class, where 100% of patients died ( n = 3) as a result of rapid disease progression [ 43 ]. For the chemotherapy class, one study reported mortality for one patient receiving 2 L capecitabine [ 43 ]. An additional study reported eight deaths (21.7%) following 1 L CDK4/6i treatment; however, this study did not disclose the 2 L treatments administered to these patients [ 44 ].
Other clinical endpoints
The studies included limited information on additional clinical endpoints; two studies reported on time-to-discontinuation (TTD), two reported on duration of response (DOR), and one each on time-to-next-treatment (TTNT), time-to-progression (TTP), objective response rate (ORR), clinical benefit rate (CBR), and stable disease (Tables 2 and Table S5 ).
Safety, tolerability, and patient-reported outcomes
Safety and tolerability data were reported in two studies [ 40 , 45 ]. One study investigating 2 L administration of the chemotherapy agent eribulin mesylate reported 27 patients (22.3%) with neutropenia, 3 patients (2.5%) with febrile neutropenia, 10 patients (8.3%) with peripheral neuropathy, and 14 patients (11.6%) with diarrhea [ 45 ]. Of these, neutropenia of grade 3–4 severity occurred in 9 patients (33.3%) [ 45 ]. A total of 55 patients (45.5%) discontinued eribulin mesylate treatment; 1 patient (0.83%) discontinued treatment due to adverse events [ 45 ]. Another study reported that 5 out of the 22 patients receiving the mTORi everolimus combined with ET in 2 L (22.7%) discontinued treatment due to toxicity [ 40 ]. PROs were not reported in any of the studies included in the SLR.
The objective of this study was to summarize the existing RWE on the effectiveness and safety of therapies for patients with HR+/HER2- LABC/mBC after 1 L CDK4/6i treatment. We identified 18 unique studies reporting specifically on 2 L treatment regimens after 1 L CDK4/6i treatment. The weighted average median rwPFS for NCCN- and ESMO- guideline recommended 2 L treatments ranged from 3.6 to 3.9 months for ET-based treatments and was 6.1 months when including chemotherapy-based regimens. Treatment selection following 1 L CDK4/6i therapy remains challenging primarily due to the suboptimal effectiveness or significant toxicities (e.g., chemotherapy) associated with currently available options [ 56 ]. These results highlight that currently available 2 L treatments for patients with HR+/HER2- LABC/mBC who have received 1 L CDK4/6i are suboptimal, as evidenced by the brief median rwPFS duration associated with ET-based treatments, or notable side effects and toxicity linked to chemotherapy. This conclusion is aligned with a recent review highlighting the limited effectiveness of treatment options for HR+/HER2- LABC/mBC patients post-CDK4/6i treatment [ 56 , 57 ]. Registrational trials which have also shed light on the short median PFS of 2–3 months achieved by ET (i.e., fulvestrant) after 1 L CDK4/6i therapy emphasize the need to develop improved treatment strategies aimed at prolonging the duration of effective ET-based treatment [ 56 ].
The results of this review reveal a paucity of additional real-world effectiveness and safety evidence after 1 L CDK4/6i treatment in HR+/HER2- LABC/mBC. OS and DOR were only reported in two studies while other clinical endpoints (i.e., TTD, TTNT, TTP, ORR, CBR, and stable disease) were only reported in one study each. Similarly, safety and tolerability data were only reported in two studies each, and PROs were not reported in any study. This hindered our ability to provide a comprehensive assessment of real-world treatment effectiveness and safety following 1 L CDK4/6i treatment. The limited evidence may be due to the relatively short period of time that has elapsed since CDK4/6i first received US FDA approval for 1 L treatment of HR+/HER2- LABC/mBC (2015) [ 35 ]. As such, almost half of our evidence was informed by conference abstracts. Similarly, no real-world studies were identified in our review that reported outcomes for treatments in the third- or later-lines of therapy after 1 L CDK4/6i treatment. The lack of data in this patient population highlights a significant gap which limits our understanding of the effectiveness and safety for patients receiving later lines of therapy. As more patients receive CDK4/6i therapy in the 1 L setting, the number of patients requiring subsequent lines of therapy will continue to grow. Addressing this data gap over time will be critical to improve outcomes for patients with HR+/HER2- LABC/mBC following 1 L CDK4/6i therapy.
There are several strengths of this study, including adherence to the guidelines outlined in the Cochrane Handbook to ensure a standardized and reliable approach to the SLR [ 58 ] and reporting of the SLR following PRISMA guidelines to ensure transparency and reproducibility [ 59 ]. Furthermore, the inclusion of only RWE studies allowed us to assess the effectiveness of current standard of care treatments outside of a controlled environment and enabled us to identify an unmet need in this patient population.
This study had some notable limitations, including the lack of safety and additional effectiveness outcomes reported. In addition, the dearth of studies reporting PROs is a limitation, as PROs provide valuable insight into the patient experience and are an important aspect of assessing the impact of 2 L treatments on patients’ quality of life. The studies included in this review also lacked consistent reporting of clinical characteristics (e.g., menopausal status, sites of metastasis, prior surgery) making it challenging to draw comprehensive conclusions or comparisons based on these factors across the studies. Taken together, there exists an important gap in our understanding of the long-term management of patients with HR+/HER2- LABC/mBC. Additionally, the effectiveness results reported in our evidence base were informed by small sample sizes; many of the included studies reported median rwPFS based on less than 30 patients [ 39 , 40 , 41 , 46 , 49 , 51 , 60 ], with two studies not reporting the sample size at all [ 47 , 53 ]. This may impact the generalizability and robustness of the results. Relatedly, the SLR database search was conducted in December 2022; as such, novel agents (e.g., elacestrant and capivasertib + fulvestrant) that have since received FDA approval for the treatment of HR+/HER2- LABC/mBC may impact current 2 L rwPFS outcomes [ 61 , 62 ]. Finally, relative to the number of peer-reviewed full-text articles, this SLR identified eight abstracts and one poster presentation, comprising half (50%) of the included unique studies. As conference abstracts are inherently limited by how much content that can be described due to word limit constraints, this likely had implications on the present synthesis whereby we identified a dearth of real-world effectiveness outcomes in patients with HR+/HER2- LABC/mBC treated with 1 L CDK4/6i therapy.
Future research in this area should aim to address the limitations of the current literature and provide a more comprehensive understanding of optimal sequencing of effective and safe treatment for patients following 1 L CDK4/6i therapy. Specifically, future studies should strive to report robust data related to effectiveness, safety, and PROs for patients receiving 2 L treatment after 1 L CDK4/6i therapy. Future studies should also aim to understand the mechanism underlying CDK4/6i resistance. Addressing these gaps in knowledge may improve the long-term real-world management of patients with HR+/HER2- LABC/mBC. A future update of this synthesis may serve to capture a wider breadth of full-text, peer-reviewed articles to gain a more robust understanding of the safety, effectiveness, and real-world treatment patterns for patients with HR+/HER2- LABC/mBC. This SLR underscores the necessity for ongoing investigation and the development of innovative therapeutic approaches to address these gaps and improve patient outcomes.
This SLR qualitatively summarized the existing real-world effectiveness data for patients with HR+/HER2- LABC/mBC after 1 L CDK4/6i treatment. Results of this study highlight the limited available data and the suboptimal effectiveness of treatments employed in the 2 L setting and underscore the unmet need in this patient population. Additional studies reporting effectiveness and safety outcomes, in addition to PROs, for this patient population are necessary and should be the focus of future research.
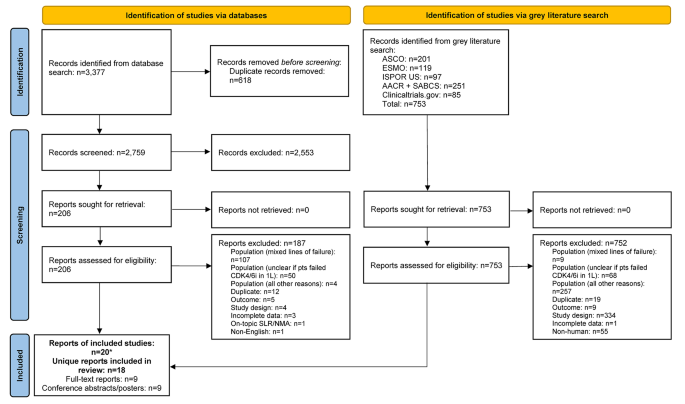
PRISMA flow diagram. *Two included conference abstracts reported the same information as already included full-text reports, hence both conference abstracts were not identified as unique. Abbreviations: 1 L = first-line; AACR = American Association of Cancer Research; ASCO = American Society of Clinical Oncology; CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; ESMO = European Society for Medical Oncology; ISPOR = Professional Society for Health Economics and Outcomes Research; n = number of studies; NMA = network meta-analysis; pts = participants; SABCS = San Antonio Breast Cancer Symposium; SLR = systematic literature review.
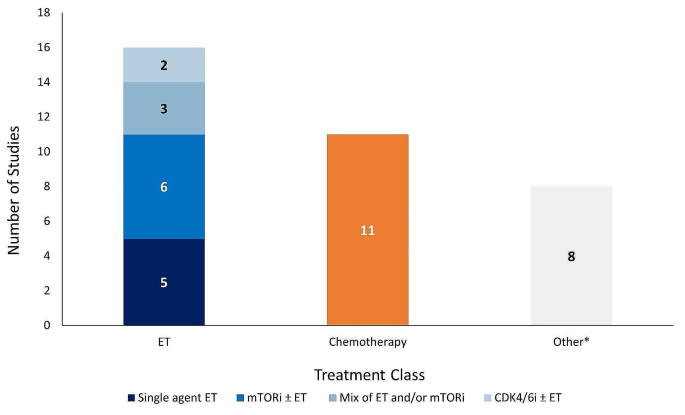
Number of studies reporting effectiveness outcomes exclusively for each treatment class. *Studies that lack sufficient information on effectiveness outcomes to classify based on the treatment classes outlined in the legend above. Abbreviations: CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; ET = endocrine therapy; mTORi = mammalian target of rapamycin inhibitor.
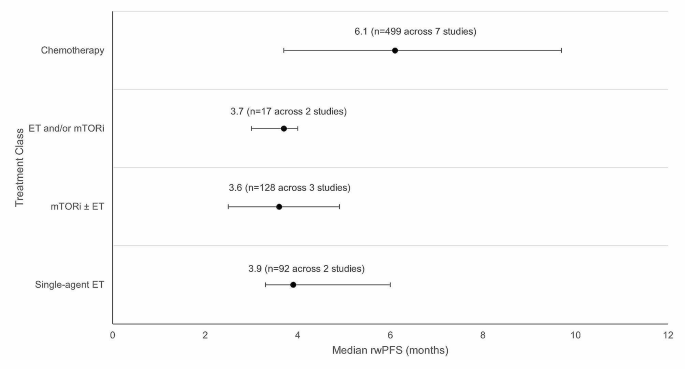
Weighted average median rwPFS for 2 L treatments (recommended in ESMO/NCCN guidelines) after 1 L CDK4/6i treatment. Circular dot represents weighted average median across studies. Horizontal bars represent the range of values reported in these studies. Abbreviations: CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; ESMO = European Society for Medical Oncology; ET = endocrine therapy, mTORi = mammalian target of rapamycin inhibitor; n = number of patients; NCCN = National Comprehensive Cancer Network; rwPFS = real-world progression-free survival.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files]. This study is registered with PROSPERO (CRD42023383914).
Abbreviations
Second-line
Second-line treatment setting and beyond
American Association of Cancer Research
Aromatase inhibitor
American Society of Clinical Oncology
- Breast cancer
breast cancer gene/partner and localizer of BRCA2 positive
Clinical benefit rate
Cyclin-dependent kinase 4/6 inhibitor
Complete response
Duration of response
European Society for Medical Oncology
Food and Drug Administration
Human epidermal growth factor receptor 2
Human epidermal growth factor receptor 2 negative
Hormone receptor
Hormone receptor positive
Professional Society for Health Economics and Outcomes Research
Locally advanced breast cancer
Metastatic breast cancer
Medical Literature Analysis and Retrieval System Online
Medical subject headings
Mammalian target of rapamycin inhibitor
National Comprehensive Cancer Network
Newcastle Ottawa Scale
Objective response rate
Poly-ADP ribose polymerase inhibitor
Progression-free survival
Population, Intervention, Comparator, Outcome, Study Design
Partial response
Preferred Reporting Items for Systematic Literature Reviews and Meta-Analyses
Patient-reported outcomes
- Real-world evidence
San Antonio Breast Cancer Symposium
- Systematic literature review
Time-to-discontinuation
Time-to-next-treatment
Time-to-progression
United States
Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A, Breast, Cancer—Epidemiology. Risk factors, classification, prognostic markers, and current treatment Strategies—An. Updated Rev Cancers. 2021;13(17):4287.
Google Scholar
World Health Organization (WHO). Breast Cancer Facts Sheet [updated July 12 2023. https://www.who.int/news-room/fact-sheets/detail/breast-cancer .
Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. 2022;66:15–23.
Article PubMed PubMed Central Google Scholar
Wilkinson L, Gathani T. Understanding breast cancer as a global health concern. Br J Radiol. 2022;95(1130):20211033.
Article PubMed Google Scholar
Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A et al. Breast Cancer Statistics, 2022. CA: A Cancer Journal for Clinicians. 2022;72(6):524– 41.
National Cancer Institute (NIH). Cancer Stat Facts: Female Breast Cancer [updated 2020. https://seer.cancer.gov/statfacts/html/breast.html .
American Cancer Society. Key Statistics for Breast Cancer [ https://www.cancer.org/cancer/types/breast-cancer/about/how-common-is-breast-cancer.html .
Zagami P, Carey LA. Triple negative breast cancer: pitfalls and progress. npj Breast Cancer. 2022;8(1):95.
Article CAS PubMed PubMed Central Google Scholar
Matutino A, Joy AA, Brezden-Masley C, Chia S, Verma S. Hormone receptor-positive, HER2-negative metastatic breast cancer: redrawing the lines. Curr Oncol. 2018;25(Suppl 1):S131–41.
Lloyd MR, Wander SA, Hamilton E, Razavi P, Bardia A. Next-generation selective estrogen receptor degraders and other novel endocrine therapies for management of metastatic hormone receptor-positive breast cancer: current and emerging role. Ther Adv Med Oncol. 2022;14:17588359221113694.
Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, et al. 4th ESO-ESMO International Consensus guidelines for advanced breast Cancer (ABC 4)†. Ann Oncol. 2018;29(8):1634–57.
Article CAS PubMed Google Scholar
US Food Drug Administration. Palbociclib (Ibrance) 2017 [updated March 31, 2017. https://www.fda.gov/drugs/resources-information-approved-drugs/palbociclib-ibrance .
US Food Drug Administration. FDA expands ribociclib indication in HR-positive, HER2-negative advanced or metastatic breast cancer 2018 [updated July 18. 2018. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-expands-ribociclib-indication-hr-positive-her2-negative-advanced-or-metastatic-breast-cancer .
US Food Drug Administration. FDA approves abemaciclib for HR positive, HER2-negative breast cancer 2017 [updated Sept 28. 2017. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abemaciclib-hr-positive-her2-negative-breast-cancer .
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Breast Cancer 2022 [ https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf .
Gennari A, André F, Barrios CH, Cortés J, de Azambuja E, DeMichele A, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–95.
Beaver JA, Amiri-Kordestani L, Charlab R, Chen W, Palmby T, Tilley A, et al. FDA approval: Palbociclib for the Treatment of Postmenopausal Patients with estrogen Receptor-Positive, HER2-Negative metastatic breast Cancer. Clin Cancer Res. 2015;21(21):4760–6.
US Food Drug Administration. Ribociclib (Kisqali) [ https://www.fda.gov/drugs/resources-information-approved-drugs/ribociclib-kisqali#:~:text=On%20March%2013%2C%202017%2C%20the,hormone%20receptor%20(HR)%2Dpositive%2C .
US Food Drug Administration. FDA approves new treatment for certain advanced or metastatic breast cancers [ https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-certain-advanced-or-metastatic-breast-cancers .
US Food Drug Administration. FDA expands ribociclib indication in HR-positive, HER2-negative advanced or metastatic breast cancer. 2018 [ https://www.fda.gov/drugs/resources-information-approved-drugs/fda-expands-ribociclib-indication-hr-positive-her2-negative-advanced-or-metastatic-breast-cancer .
US Food Drug Administration. FDA approves abemaciclib as initial therapy for HR-positive, HER2-negative metastatic breast cancer [ https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abemaciclib-initial-therapy-hr-positive-her2-negative-metastatic-breast-cancer .
Turner NC, Slamon DJ, Ro J, Bondarenko I, Im S-A, Masuda N, et al. Overall survival with Palbociclib and fulvestrant in advanced breast Cancer. N Engl J Med. 2018;379(20):1926–36.
Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III randomized study of Ribociclib and Fulvestrant in hormone Receptor-Positive, human epidermal growth factor receptor 2-Negative advanced breast Cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465–72.
Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast Cancer. J Clin Oncol. 2017;35(32):3638–46.
Gopalan PK, Villegas AG, Cao C, Pinder-Schenck M, Chiappori A, Hou W, et al. CDK4/6 inhibition stabilizes disease in patients with p16-null non-small cell lung cancer and is synergistic with mTOR inhibition. Oncotarget. 2018;9(100):37352–66.
Watt AC, Goel S. Cellular mechanisms underlying response and resistance to CDK4/6 inhibitors in the treatment of hormone receptor-positive breast cancer. Breast Cancer Res. 2022;24(1):17.
Goetz M. MONARCH 3: final overall survival results of abemaciclib plus a nonsteroidal aromatase inhibitor as first-line therapy for HR+, HER2- advanced breast cancer. SABCS; 2023.
Munzone E, Pagan E, Bagnardi V, Montagna E, Cancello G, Dellapasqua S, et al. Systematic review and meta-analysis of post-progression outcomes in ER+/HER2– metastatic breast cancer after CDK4/6 inhibitors within randomized clinical trials. ESMO Open. 2021;6(6):100332.
Gennari A, André F, Barrios CH, Cortés J, de Azambuja E, DeMichele A, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Annals of Oncology. 2021;32(12):1475-95.
European Society for Medical Oncology (ESMO). ESMO Metastatic Breast Cancer Living Guideline: ER-positive HER2-negative Breast Cancer [updated May 2023. https://www.esmo.org/living-guidelines/esmo-metastatic-breast-cancer-living-guideline/er-positive-her2-negative-breast-cancer .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Welch PM VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). www.training.cochrane.org/handbook : Cochrane; 2021.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583.
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 Guideline Statement. J Clin Epidemiol. 2016;75:40–6.
Fraser C, Murray A, Burr J. Identifying observational studies of surgical interventions in MEDLINE and EMBASE. BMC Med Res Methodol. 2006;6(1):41.
US Food Drug Administration. Palbociclib (Ibrance). Silver Spring, MD: US Food and Drug Administration; 2017.
Book Google Scholar
GA Wells BS, D O’Connell J, Peterson V, Welch M, Losos PT. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [ https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
Lo CK-L, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14(1):45.
Martin JM, Handorf EA, Montero AJ, Goldstein LJ. Systemic therapies following progression on first-line CDK4/6-inhibitor treatment: analysis of real-world data. Oncologist. 2022;27(6):441–6.
Kalinsky KM, Kruse M, Smyth EN, Guimaraes CM, Gautam S, Nisbett AR et al. Abstract P1-18-37: Treatment patterns and outcomes associated with sequential and non-sequential use of CDK4 and 6i for HR+, HER2- MBC in the real world. Cancer Research. 2022;82(4_Supplement):P1-18-37-P1-18-37.
Choong GM, Liddell S, Ferre RAL, O’Sullivan CC, Ruddy KJ, Haddad TC, et al. Clinical management of metastatic hormone receptor-positive, HER2-negative breast cancer (MBC) after CDK 4/6 inhibitors: a retrospective single-institution study. Breast Cancer Res Treat. 2022;196(1):229–37.
Xi J, Oza A, Thomas S, Ademuyiwa F, Weilbaecher K, Suresh R, et al. Retrospective Analysis of Treatment Patterns and effectiveness of Palbociclib and subsequent regimens in metastatic breast Cancer. J Natl Compr Canc Netw. 2019;17(2):141–7.
Rozenblit M, Mun S, Soulos P, Adelson K, Pusztai L, Mougalian S. Patterns of treatment with everolimus exemestane in hormone receptor-positive HER2-negative metastatic breast cancer in the era of targeted therapy. Breast Cancer Res. 2021;23(1):14.
Bashour SI, Doostan I, Keyomarsi K, Valero V, Ueno NT, Brown PH, et al. Rapid breast Cancer Disease Progression following cyclin dependent kinase 4 and 6 inhibitor discontinuation. J Cancer. 2017;8(11):2004–9.
Giridhar KV, Choong GM, Leon-Ferre R, O’Sullivan CC, Ruddy K, Haddad T, et al. Abstract P6-18-09: clinical management of metastatic breast cancer (MBC) after CDK 4/6 inhibitors: a retrospective single-institution study. Cancer Res. 2019;79:P6–18.
Article Google Scholar
Mougalian SS, Feinberg BA, Wang E, Alexis K, Chatterjee D, Knoth RL, et al. Observational study of clinical outcomes of eribulin mesylate in metastatic breast cancer after cyclin-dependent kinase 4/6 inhibitor therapy. Future Oncol. 2019;15(34):3935–44.
Moscetti LML, Riggi L, Sperduti I, Piacentini FOC, Toss A, Barbieri E, Cortesi L, Canino FMA, Zoppoli G, Frassoldati A, Schirone A, Dominici MECF. SEQUENCE OF TREATMENTS AFTER CDK4/6 THERAPY IN ADVANCED BREAST CANCER (ABC), A GOIRC MULTICENTER RETRO/ PROSPECTIVE STUDY. PRELIMINARY RESULTS IN THE RETROSPECTIVE SERIES OF 116 PATIENTS. Tumori. 2022;108(4S):80.
Menichetti AZE, Giorgi CA, Bottosso M, Leporati R, Giarratano T, Barbieri C, Ligorio F, Mioranza E, Miglietta F, Lobefaro R, Faggioni G, Falci C, Vernaci G, Di Liso E, Girardi F, Griguolo G, Vernieri C, Guarneri V, Dieci MV. CDK 4/6 INHIBITORS FOR METASTATIC BREAST CANCER: A MULTICENTER REALWORLD STUDY. Tumori. 2022;108(4S):70.
Marschner NW, Harbeck N, Thill M, Stickeler E, Zaiss M, Nusch A, et al. 232P Second-line therapies of patients with early progression under CDK4/6-inhibitor in first-line– data from the registry platform OPAL. Annals of Oncology. 2022;33:S643-S4
Gousis C, Lowe KMH, Kapiris M. V. Angelis. Beyond First Line CDK4/6 Inhibitors (CDK4/6i) and Aromatase Inhibitors (AI) in Patients with Oestrogen Receptor Positive Metastatic Breast Cancer (ERD MBC): The Guy’s Cancer Centre Experience. Clinical Oncology2022. p. e178.
Endo Y, Yoshimura A, Sawaki M, Hattori M, Kotani H, Kataoka A, et al. Time to chemotherapy for patients with estrogen receptor-positive breast Cancer and cyclin-dependent kinase 4 and 6 inhibitor use. J Breast Cancer. 2022;25(4):296–306.
Li Y, Li W, Gong C, Zheng Y, Ouyang Q, Xie N, et al. A multicenter analysis of treatment patterns and clinical outcomes of subsequent therapies after progression on palbociclib in HR+/HER2- metastatic breast cancer. Ther Adv Med Oncol. 2021;13:17588359211022890.
Amaro CP, Batra A, Lupichuk S. First-line treatment with a cyclin-dependent kinase 4/6 inhibitor plus an aromatase inhibitor for metastatic breast Cancer in Alberta. Curr Oncol. 2021;28(3):2270–80.
Crocetti SPM, Tassone L, Marcantognini G, Bastianelli L, Della Mora A, Merloni F, Cantini L, Scortichini L, Agostinelli V, Ballatore Z, Savini A, Maccaroni E. Berardi R. What is the best therapeutic sequence for ER-Positive/HER2- Negative metastatic breast cancer in the era of CDK4/6 inhibitors? A single center experience. Tumori. 2020;106(2S).
Nichetti F, Marra A, Giorgi CA, Randon G, Scagnoli S, De Angelis C, et al. 337P Efficacy of everolimus plus exemestane in CDK 4/6 inhibitors-pretreated or naïve HR-positive/HER2-negative breast cancer patients: A secondary analysis of the EVERMET study. Annals of Oncology. 2020;31:S382
Luhn P, O’Hear C, Ton T, Sanglier T, Hsieh A, Oliveri D, et al. Abstract P4-13-08: time to treatment discontinuation of second-line fulvestrant monotherapy for HR+/HER2– metastatic breast cancer in the real-world setting. Cancer Res. 2019;79(4Supplement):P4–13.
Mittal A, Molto Valiente C, Tamimi F, Schlam I, Sammons S, Tolaney SM et al. Filling the gap after CDK4/6 inhibitors: Novel Endocrine and Biologic Treatment options for metastatic hormone receptor positive breast Cancer. Cancers (Basel). 2023;15(7).
Ashai N, Swain SM. Post-CDK 4/6 inhibitor therapy: current agents and novel targets. Cancers (Basel). 2023;15(6).
Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). www.training.cochrane.org/handbook : Cochrane; 2022.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Serdar CC, Cihan M, Yücel D, Serdar MA. Sample size, power and effect size revisited: simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem Med (Zagreb). 2021;31(1):010502.
US Food Drug Administration. FDA approves elacestrant for ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer [updated January 27 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-elacestrant-er-positive-her2-negative-esr1-mutated-advanced-or-metastatic-breast-cancer .
US Food Drug Administration. FDA approves capivasertib with fulvestrant for breast cancer [updated November 16 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-capivasertib-fulvestrant-breast-cancer .
Download references
Acknowledgements
The authors would like to acknowledge Joanna Bielecki who developed, conducted, and documented the database searches.
This study was funded by Pfizer Inc. (New York, NY, USA) and Arvinas (New Haven, CT, USA).
Author information
Sarah Kane, Belal Howidi, Bao-Ngoc Nguyen and Imtiaz A. Samjoo contributed equally to this work.
Authors and Affiliations
Pfizer, 10017, New York, NY, USA
Veronique Lambert & Yan Wu
EVERSANA, Burlington, ON, Canada
Sarah Kane, Belal Howidi, Bao-Ngoc Nguyen & Imtiaz A. Samjoo
Arvinas, 06511, New Haven, CT, USA
David Chandiwana & Michelle Edwards
You can also search for this author in PubMed Google Scholar
Contributions
VL, IAS, SK, BH, BN, DC, YW, and ME participated in the conception and design of the study. IAS, SK, BH and BN contributed to the literature review, data collection, analysis, and interpretation of the data. VL, IAS, SK, BH, BN, DC, YW, and ME contributed to the interpretation of the data and critically reviewed for the importance of intellectual content for the work. VL, IAS, SK, BH, BN, DC, YW, and ME were responsible for drafting or reviewing the manuscript and for providing final approval. VL, IAS, SK, BH, BN, DC, YW, and ME meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Corresponding author
Correspondence to Imtiaz A. Samjoo .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors of this manuscript declare that the research presented was funded by Pfizer Inc. and Arvinas. While the support from Pfizer Inc. and Arvinas was instrumental in facilitating this research, the authors affirm that their interpretation of the data and the content of this manuscript were conducted independently and without bias to maintain the transparency and integrity of the research. IAS, SK, BH, and BN are employees of EVERSANA, Canada, which was a paid consultant to Pfizer in connection with the development of this manuscript.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lambert, V., Kane, S., Howidi, B. et al. Systematic literature review of real-world evidence for treatments in HR+/HER2- second-line LABC/mBC after first-line treatment with CDK4/6i. BMC Cancer 24 , 631 (2024). https://doi.org/10.1186/s12885-024-12269-8
Download citation
Received : 26 January 2024
Accepted : 16 April 2024
Published : 23 May 2024
DOI : https://doi.org/10.1186/s12885-024-12269-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- First-line CDK4/6i
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Patient contracts for chronic medical conditions: Scoping review
Affiliations.
- 1 Assistant Professor in the Department of Family Medicine at McMaster University in Hamilton, Ont. [email protected].
- 2 Assistant Professor in the Department of Health Research Methods, Evidence and Impact at McMaster University.
- 3 Master of public health candidate at McMaster University.
- 4 Associate Professor in the Department of Family Medicine and the Department of Health Research Methods, Evidence and Impact at McMaster University.
- 5 Associate Clinical Professor in the Department of Family Medicine at McMaster University.
- 6 Associate Professor in the Department of Medicine at the University of Toronto in Ontario.
- PMID: 35552216
- PMCID: PMC9097748
- DOI: 10.46747/cfp.6805e169
Abstract in English, French
Objective: To describe how and why patient contracts are used for the management of chronic medical conditions.
Data sources: A scoping review was conducted in the following databases: MEDLINE, Embase, AMED, PsycInfo, Cochrane Library, CINAHL, and Nursing & Allied Health. Literature from 1997 to 2017 was included.
Study selection: Articles were included if they were written in English and described the implementation of a patient contract by a health care provider for the management of a chronic condition. Articles had to present an outcome as a result of using the contract or an intervention that included the contract.
Synthesis: Of the 7528 articles found in the original search, 76 met the inclusion criteria for the final review. Multiple study types were included. Extensive variety in contract elements, target populations, clinical settings, and cointerventions was found. Purposes for initiating contracts included behaviour change and skill development, including goal development and problem solving; altering beliefs and knowledge, including motivation and perceived self-efficacy; improving interpersonal relationships and role clarification; improving quality and process of chronic care; and altering objective and subjective health indices. How contracts were developed, implemented, and assessed was inconsistently described.
Conclusion: More research is required to determine whether the use of contracts is accomplishing their intended purposes. Questions remain regarding their rationale, development, and implementation.
Objectif: Décrire comment et pourquoi les contrats avec les patients sont utilisés pour la gestion de problèmes de santé chroniques.
Sources d’information: Une revue exploratoire a été effectuée dans les bases de données suivantes : MEDLINE, Embase, AMED, PsycInfo, Bibliothèque Cochrane, CINAHL et Nursing & Allied Health. Les articles parus de 1997 à 2017 ont été inclus.
Sélection des études: Les articles ont été retenus s’ils étaient rédigés en anglais et s’ils décrivaient la mise en œuvre d’un contrat avec un patient par un professionnel de la santé pour la gestion d’un problème chronique. Les articles devaient présenter une issue comme étant le résultat de l’utilisation du contrat ou d’une intervention qui incluait le contrat.
Synthèse: Des 7528 articles recensés dans la recherche initiale, 76 répondaient aux critères d’inclusion dans la revue finale. De multiples types d’études ont été inclus. Une diversité considérable dans les éléments de contrat, les populations visées, les milieux cliniques et les interventions conjointes a été observée. Les raisons de conclure un contrat incluaient des changements comportementaux et le perfectionnement des compétences, y compris l’établissement d’objectifs et la solution de problèmes; la modification des croyances et des connaissances, notamment la motivation et l’auto-efficacité perçue; l’amélioration des relations interpersonnelles et la clarification des rôles; l’amélioration de la qualité et des processus des soins chroniques; la modification des indicateurs objectifs et subjectifs de la santé. Les descriptions de l’élaboration, de la mise en œuvre et de l’évaluation des contrats manquaient de cohérence.
Conclusion: Il faut plus de recherches pour déterminer si l’utilisation de contrats permet d’atteindre les objectifs prévus. Des questions demeurent sans réponses quant à leur justification, leur élaboration et leur mise en œuvre.
Copyright © 2022 the College of Family Physicians of Canada.
Publication types
- Chronic Disease
- Health Personnel*
- Interpersonal Relations
- Motivation*
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Med Cannabis Cannabinoids
- v.4(1); 2021 Jun

A Mapping Literature Review of Medical Cannabis Clinical Outcomes and Quality of Evidence in Approved Conditions in the USA from 2016 to 2019
Sebastian jugl.
a Pharmaceutical Outcomes and Policy, University of Florida, Gainesville, Florida, USA
b Center for Drug Evaluation and Safety (CoDES), University of Florida, Gainesville, Florida, USA
Aimalohi Okpeku
Brianna costales, earl j. morris, golnoosh alipour-haris, juan m. hincapie-castillo, nichole e. stetten.
c Occupational Therapy, University of Florida, Gainesville, Florida, USA
Ruba Sajdeya
d Epidemiology, University of Florida, Gainesville, Florida, USA
Shailina Keshwani
Verlin joseph, yahan zhang, lauren adkins.
e Health Sciences Center Libraries, University of Florida, Gainesville, Florida, USA
Almut G. Winterstein
Amie goodin, associated data.
In 2017, a National Academies of Sciences, Engineering, and Medicine (NASEM) report comprehensively evaluated the body of evidence regarding cannabis health effects through the year 2016. The objectives of this study are to identify and map the most recently (2016–2019) published literature across approved conditions for medical cannabis and to evaluate the quality of identified recent systematic reviews, published following the NASEM report. Following the literature search from 5 databases and consultation with experts, 11 conditions were identified for evidence compilation and evaluation: amyotrophic lateral sclerosis, autism, cancer, chronic noncancer pain, Crohn's disease, epilepsy, glaucoma, human immunodeficiency virus/AIDS, multiple sclerosis (MS), Parkinson's disease, and posttraumatic stress disorder. A total of 198 studies were included after screening for condition-specific relevance and after imposing the following exclusion criteria: preclinical focus, non-English language, abstracts only, editorials/commentary, case studies/series, and non-U.S. study setting. Data extracted from studies included: study design type, outcome definition, intervention definition, sample size, study setting, and reported effect size. Few completed randomized controlled trials (RCTs) were identified. Studies classified as systematic reviews were graded using the Assessing the Methodological Quality of Systematic Reviews-2 tool to evaluate the quality of evidence. Few high-quality systematic reviews were available for most conditions, with the exceptions of MS (9 of 9 graded moderate/high quality; evidence for 2/9 indicating cannabis improved outcomes; evidence for 7/9 indicating cannabis inconclusive), epilepsy (3 of 4 graded moderate/high quality; 3 indicating cannabis improved outcomes; 1 indicating cannabis inconclusive), and chronic noncancer pain (12 of 13 graded moderate/high quality; evidence for 7/13 indicating cannabis improved outcomes; evidence from 6/7 indicating cannabis inconclusive). Among RCTs, we identified few studies of substantial rigor and quality to contribute to the evidence base. However, there are some conditions for which significant evidence suggests that select dosage forms and routes of administration likely have favorable risk-benefit ratios (i.e., epilepsy and chronic noncancer pain). The body of evidence for medical cannabis requires more rigorous evaluation before consideration as a treatment option for many conditions, and evidence necessary to inform policy and treatment guidelines is currently insufficient for many conditions.
Introduction
Medical cannabis is available to patients by physician order in 33 states and territories in the USA as of 2020. However, at the federal level, cannabis remains classified as a schedule I controlled substance, which limits efficacy and safety investigations [ 1 ]. Collectively, “medical cannabis” encompasses various terms used in reference to medical marijuana, cannabis-derived products from the cannabis plant (including cannabinoids), and synthetic cannabinoids (e.g., synthetic delta-9-tetrahydrocannabinol (THC) or dronabinol). States that permit physician-ordered medical cannabis typically require a diagnosed medical condition that is considered qualifying by respective state law permitting its use as treatment or adjuvant. Currently, over 50 medical conditions have been granted a qualifying medical condition status by individual state laws, though there is significant variation between each state's approved conditions [ 2 ]. The most frequent medical conditions for approved medical cannabis use nationally are chronic noncancer pain, multiple sclerosis (MS) and other motor neuron disorders, epilepsy, cancer and cancer symptoms, mental health disorders (primarily anxiety disorders such as posttraumatic stress disorder [PTSD]), glaucoma, and symptoms related to irritable bowel diseases [ 3 , 4 ].
Approximately 12.9% of Americans report past-year cannabis use, with 90.2% using for nonmedical purposes only, 6.2% for medical purposes only, and 3.6% for both purposes [ 5 ]. The amount of medical-only cannabis users is higher in states that have enacted medical marijuana laws, where around 17% of cannabis users consumed cannabis for medical reasons in those states [ 6 ]. The most common routes of administration of cannabis use in the USA are oral/peroral (e.g., edibles), pulmonary (e.g., smoking, or vaping), and topical [ 7 ].
In 2017, the National Academies of Sciences, Engineering, and Medicine (NASEM) published a comprehensive scientific review on the effects of cannabis and cannabinoids in the treatment of medical conditions frequently cited for medical cannabis use [ 8 ]. The NASEM report included an evidence review of studies evaluating the efficacy and safety of cannabis for selected conditions based on the frequency of use, hypothesized effectiveness, and/or eligibility of the condition for medical cannabis certification across several states. The NASEM report evaluated the body of evidence published in the literature through the year 2016, and the objective of this study is to further expand this work by examining the most recently available evidence. Therefore, the objectives of this review are to (1) identify and map the most recently published clinical and scientific evidence across approved conditions for medical cannabis and (2) evaluate the quality of identified recent systematic reviews.
Topic Selection
Clinical conditions were selected based on inclusion within the NASEM report, relevance to current trends in medical cannabis-eligible diagnoses, and consultation with subject matter experts and relevant stakeholders (e.g., physicians, patients, and community input). Relevant stakeholders perceived needs in research priorities, and evidence gaps as related to clinical outcomes were assessed via preliminary surveys, interviews, and open-ended discussion. Stakeholders recommended including medical conditions approved in the US state jurisdiction of the study team, in addition to emerging trends in use of medical cannabis applications based on discussion with physicians who were certified to order medical cannabis in this locale. Based on this process, the clinical conditions determined for inclusion for this review were amyotrophic lateral sclerosis (ALS), autism, cancer, chronic noncancer pain, Crohn's disease, epilepsy, glaucoma, human immunodeficiency virus (HIV)/AIDS, MS, Parkinson's disease, and PTSD.
Literature Search and Identification
The search strategy was developed in collaboration with the University of Florida Health Sciences Center Library. For this mapping review, we conducted a systematic search using the following databases: PubMed, Embase, Web of Science, the Cochrane Library, and clinicaltrials.gov . We restricted our search to studies that were published after the NASEM report's inclusion period, between May 2016 and October 2019. Search strings from the NASEM report were replicated, and additional keywords and Medical Subject Headings terms were identified in collaboration with subject matter experts and through literature cross-referencing. Since autism was the only included condition that was not evaluated by NASEM, we employed rapid review strategies and adjusted our date restriction inclusion period from the year 2000 to October 2019 for this condition. We limited our search to English language literature only. Complete search strings are available for all conditions in the see online suppl. files. (For all online suppl. material, see www.karger.com/doi/10.1159/000515069 .)
Literature Screening
Screening for eligible studies was conducted in 2 phases. In each phase, publications were either classified as include, exclude, or uncertain. In the first phase, for each clinical indication, one reviewer screened the identified abstracts for eligibility. Abstracts that were classified as “uncertain” were then screened by a second reviewer. If the second abstract reviewer also classified the abstract as uncertain, the publication was advanced for full-text screening. In the second phase, full-text publications were screened for eligibility for each clinical indication. Publications classified as “uncertain” during full-text screening were then screened by a second reviewer. If the publication was still classified as “uncertain” following a second full-text screening, group review and discussion were required until consensus regarding eligibility was achieved. Other discrepancies between reviewers were resolved via discussion and by a third reviewer, when necessary. Publications were included in qualitative synthesis if they were published between 27 May 2016 and 22 September 2019 and investigated the therapeutic effect, a patient or provider perspective, or utilization of medical cannabis in any form in one of the identified 11 indications or conditions. Additionally, the study had to be conducted in humans. Publications were excluded if they included only preclinical data, if the primary research was conducted exclusively outside the USA, clinical case studies, abstracts-only, letters to the editors, opinion pieces, or editorials.
Data Extraction
The study team created a standardized data extraction tool in Microsoft Excel to capture elements from all included studies. An initial pilot run with the underlying data extraction table was performed in a group setting for training purposes and to ensure consistency. Afterward, for each condition, one reviewer extracted the following data from the eligible studies into the tool: study design, study setting, cannabis intervention type, study period, inclusion and exclusion criteria, indicators for whether special populations were included (e.g., pediatrics and geriatrics), outcomes assessed, outcome definition, change in outcome, and summary of findings. Reviewers presented uncertainties in data extraction in a group discussion meeting for resolution. In instances where a single study was identified as eligible for data extraction for multiple conditions, data were independently extracted as relevant for each condition covered within the study; however, these studies were not counted more than once in overall counts of assessed studies.
Quality of Evidence Assessment
Studies that were classified as systematic reviews with or without meta-analysis were evaluated using the Assessing the Methodological Quality of Systematic Reviews-2 (AMSTAR-2) instrument. The Assessing the Methodological Quality of Systematic Reviews-2 tool was developed to grade the quality of evidence reviewed, organized, and presented within systematic reviews [ 9 ]. It consists of 16 items that evaluate the methodological quality of systematic reviews and the risk of bias via a checklist, and each item can be answered with “yes,” “partial yes,” “no,” or “no meta-analysis conducted.” Based on weaknesses in critical domains, systematic reviews are then rated as a high-, moderate-, low-, or critically low-quality review. Two reviewers for each condition conducted the evidence grading independently. Disagreements were resolved by a third reviewer, and when necessary, classifications of study design were re-evaluated. Additional reviewers examined studies when needed until the majority consensus on both study design classification and quality of evidence rating was achieved.
Evidence Synthesis
Findings from identified studies were reported in accordance with PRISMA guidelines. Search, screening, and evaluation were conducted in accordance with systematic literature review best practices; however, the structure of this review is more appropriately classified as a mapping review to allow for its broad scope [ 10 ].
Studies in each condition were classified according to whether they assessed efficacy and/or safety outcomes. (See online suppl. Tables for outcome definitions.) Studies assessing relevant efficacy outcomes were classified into 1 of 5 categories based on the following classification scheme. Studies were classified as “outcome improved” when the condition improved following medical cannabis treatment; as “outcome worsened” when the condition worsened; as “none” when there was no significant observable change; as “inconclusive” if they specifically indicated that results were inconclusive in their results and discussion section and/or there were multiple outcomes assessed but not all reported in findings; or as “mixed” in cases where multiple outcomes were assessed, but some indicated improvement and others indicated no change or worsening. Study outcome definitions for efficacy by condition were summarized (online suppl. Table 1 ).
Studies reporting safety outcomes were classified into 4 different categories. Studies were classified as “worsening” when an increase in adverse events as compared to placebo, active comparator, or both groups were reported, or single-arm studies reported side effects or adverse events that might be associated with exposure; as “mixed” when different safety outcomes were assessed, but some indicated no change, while others indicated worsening; as “no change” when no significant changes in safety outcomes when measured against the comparator group were reported, or in the case of single-arm studies, studies not reporting any side effects that might be associated with exposure; or as “inconclusive” when studies specifically described results as inconclusive in the results and discussion section and/or if there were multiple outcomes assessed, but not all reported in findings were classified analogous to the efficacy outcome.
Studies that did not fit into the presented classification scheme assessed outcomes unrelated to efficacy and safety, employed a cross-sectional design, or were utilization studies, all of which were summarized separately. Cross-sectional studies were not included in the classification scheme due to their lack of longitudinal assessment, thus limiting the interpretability of findings for quantifying the evidence base in regard to efficacy and safety. Studies that were classified as “other nonsystematic reviews” (e.g., clinical, narrative, scoping, or undefined) were captured in our search strategy but were not evaluated using the classification schemes described herein.
For visualization purposes, all systematic reviews assessing safety or efficacy outcomes were compiled into an evidence map figure consisting of 5 different dimensions (Fig. (Fig.1). 1 ). The bubble size is proportional to the number of included studies within each condition topic area. The bubble color represents the underlying medical condition. The x -axis describes the effect of cannabis in each condition. The y -axis represents the quality of evidence assessment score, and notations within the bubbles indicate whether the systematic reviews included meta-analysis. For a more comprehensive insight into the efficacy and safety-related findings of eligible studies, studies were finally organized by the condition-specific outcome, study design type, and directions of findings.

Quality of evidence among systematic reviews assessing medical cannabis efficacy, effectiveness, and safety outcomes in selected conditions. MS, multiple sclerosis; ALS, amyotrophic lateral sclerosis; PTSD, posttraumatic stress disorder; HIV, human immunodeficiency virus.
A total of 15,917 studies were identified across all searched databases during the study period, where searches were conducted for each of the included clinical conditions. Following stratification by clinical condition relevance and screening for eligibility, 438 studies remained (see online suppl. materials for PRISMA flow diagrams for individual clinical conditions). We then further restricted qualitative synthesis to studies that reported primary results or systematically reviewed prior work ( n = 198), meaning that 240 studies were narrative reviews or other types of nonsystematic reviews. Table Table1 1 summarizes efficacy findings as stratified by study design type and condition, and Table Table2 2 summarizes the same for safety findings. Table Table3 3 summarizes cannabis agents administered or observed in randomized controlled trials (RCTs) and observational studies by agent and route of administration for each condition. Below, we summarize condition-specific findings.
Medical cannabis study efficacy outcome findings, 1 by condition and study design type
ALS, amyotrophic lateral sclerosis; HIV, human immunodeficiency virus; PTSD, posttraumatic stress disorder; RCT, randomized controlled trial; MS, multiple sclerosis. 1 Findings for efficacy outcomes were classified for cannabis/cannabinoid treatment relative to placebo or active comparator according to the following: “improvement” if outcome improved, “worsening” if outcome worsened, “mixed” if multiple efficacy outcomes were assessed with divergent findings for each, “no change” if no change observed, and “inconclusive” if outcomes were unable to be assessed.
Medical cannabis safety outcome findings, 1 by condition and study design type
ALS, amyotrophic lateral sclerosis; HIV, human immunodeficiency virus; PTSD, posttraumatic stress disorder; RCT, randomized controlled trial; MS, multiple sclerosis. 1 Safety outcomes were defined in all studies as proportion of adverse events relative to placebo/active comparator, frequency of adverse events, or severity of adverse events relative to placebo/active comparator. Findings for safety outcomes were classified for cannabis/cannabinoid treatment according to the following: “worsening” if outcome worsened, “mixed” if multiple safety outcomes were assessed with divergent findings for each, “no change” if no change observed, and “inconclusive” if outcomes were unable to be assessed. 2 A secondary endpoint from one RCT, was deemed appropriate for inclusion in safety outcomes [ 136 ].
Counts of agents in reviewed studies by routes of administration and condition
No studies were eligible in the area of ALS, glaucoma, and MS. THCA, tetrahydrocannabinolic acid; ALS, amyotrophic lateral sclerosis; HIV, human immunodeficiency virus; PTSD, posttraumatic stress disorder; THC, tetrahydrocannabinol. 1 Buccal, tincture, oromucosal, rectal, and other not specified. 2 When more than one agent was investigated, but the route of administration was not distinguished between the agents. 3 THCA oil.
Amyotrophic Lateral Sclerosis
As depicted in the flow diagrams (online suppl. files), the use of medical cannabis in patients with ALS was investigated in 9 eligible publications. Among those were 2 systematic reviews without meta-analysis, 2 observational/quasi-experimental studies, and 5 other types of reviews. Of all studies investigating medical cannabis and ALS, 2 studies used cramp intensity/frequency as the primary outcome [ 11 , 12 ] and 2 investigated other outcomes or used a cross-sectional design [ 13 , 14 ]. Among those studies that investigated cramp intensity/frequency, one indicated no change [ 11 ] and one study indicated inconclusive findings [ 11 , 12 ]. (More detailed information about each study type and summary of findings can be found in Tables Tables1 1 and and2 2 and in the online suppl. files.) Other outcomes assessed in this condition included an examination of trajectories of ALS cases [ 13 ], and one cross-sectional study assessed patient characteristics in a dispensary and dispensary staff recommendations [ 14 ].
Medical cannabis in patients with autism was investigated in 17 eligible publications. Among those were one systematic review with meta-analysis, 8 observational/quasi-experimental studies, and 8 other types of reviews. Of all studies investigating medical cannabis and autism, 3 studies used symptom mitigation (see online suppl. Table 1 for outcome definitions) as the primary outcome [ 15 , 16 , 17 ] and 6 investigated other outcomes or used a cross-sectional design [ 18 , 19 , 20 , 21 , 22 , 23 ]. The latter studies and other types of reviews are summarized in the online suppl. files. Among those studies that investigated symptom mitigation, 2 indicated an improvement [ 16 , 17 ] and one study indicated no change in symptoms [ 15 ]. Other outcomes assessed in this condition were assessed in 6 studies, of which one used a cross-sectional study design. Among those outcomes that were assessed by more than 1 study, 2 studies assessed the brain activity in response to CBD with functional magnetic resonance imaging and magnetic resonance spectroscopy [ 22 , 23 ].
Medical cannabis in patients with cancer was investigated in 138 eligible publications. Among those were 6 systematic reviews with meta-analysis, 10 systematic reviews without meta-analysis, 4 RCTs, 31 observational/quasi-experimental studies, and 86 other types of reviews. Of all studies investigating medical cannabis and cancer, 13 studies investigated cancer-related pain reduction as the primary outcome [ 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 ]; 2 studies investigated cancer-related nausea and vomiting [ 27 , 36 ]; 3 studies investigated weight change, appetite increase, or caloric intake [ 27 , 37 ]; 17 studies investigated safety outcomes [ 24 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 34 , 35 , 36 , 38 , 39 , 40 , 41 , 42 , 43 ]; and 31 studies investigated other outcomes or used a cross-sectional design [ 3 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 ]. Among studies that investigated cancer-related pain, 5 indicated an improvement [ 24 , 25 , 28 , 29 , 36 ], 2 studies indicated no change [ 34 , 35 ], and 6 were inconclusive [ 26 , 27 , 30 , 32 , 74 , 75 ]. Among studies that investigated cancer-related nausea and vomiting, one indicated an improvement [ 36 ] and one was inconclusive [ 27 ]. In studies that investigated weight change, appetite increase, or caloric intake, one indicated an improvement [ 36 ] and 2 were inconclusive [ 27 , 37 ]. Of the 17 studies assessing safety outcomes of medical cannabis in cancer patients, 11 studies indicated worsening [ 24 , 26 , 28 , 29 , 34 , 35 , 36 , 38 , 39 , 40 , 43 ], one indicated mixed findings [ 41 ], and 5 studies were inconclusive [ 27 , 30 , 32 , 42 , 74 ]. For 2 RCTs, results are still pending at this time [ 76 , 77 ]. Other outcomes assessed in this condition were assessed in 31 studies, of which 24 used a cross-sectional study design. Among those outcomes that were assessed by more than one study, 10 studies investigated patients or provider perceptions of cannabis benefits and side effects [ 47 , 52 , 53 , 56 , 60 , 62 , 64 , 67 , 68 , 69 ] and 7 investigated patterns of cannabis consumption [ 48 , 49 , 55 , 57 , 63 , 71 , 72 ].
Chronic Noncancer Pain
Medical cannabis in patients with chronic noncancer pain was investigated in 120 publications. Among those were 8 systematic reviews with meta-analysis, 8 systematic reviews without meta-analysis, 3 RCTs, 36 observational/quasi-experimental studies, and 63 other types of reviews. Of all studies investigating medical cannabis and chronic noncancer pain, 17 studies investigated pain reduction or quality of life as the primary outcome, 9 studies investigated safety outcomes, and 35 investigated other outcomes or used a cross-sectional design [ 14 , 68 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 ]. Among those studies that investigated pain reduction or quality of life, 10 indicated an improvement [ 25 , 111 , 112 , 113 , 114 , 115 ], one study indicated mixed findings [ 42 ], 3 studies indicated no change [ 116 , 117 , 118 ], and 3 were inconclusive [ 30 , 119 , 120 ]. Of the 9 studies investigating safety outcomes of medical cannabis in patients with chronic noncancer pain, 6 studies indicated a worsening [ 111 , 112 , 117 , 119 , 121 , 122 ], 1 indicated mixed findings [ 25 ], and 2 were inconclusive [ 30 , 114 ]. For 3 RCTs, results are still pending (see online suppl. Table 2 ) [ 123 , 124 , 125 ]. Thirty-five eligible studies, including 27 cross-sectional studies, investigated other outcomes. Among those outcomes that were assessed by more than 1 study, 9 studies investigated patients or provider perceptions of cannabis benefits and side effects [ 84 , 85 , 88 , 92 , 100 , 102 , 103 , 106 , 107 ], 8 studies investigated different relationships between cannabis use and opioid use [ 79 , 81 , 87 , 96 , 101 , 105 , 108 , 109 ], 6 studies investigated cannabis use patterns [ 78 , 82 , 83 , 90 , 97 , 110 ], 2 examined consumer characteristics [ 89 , 93 ], and 2 explored reasons for medical cannabis use [ 68 , 99 ].
Crohn's Disease
Twenty-five publications investigated medical cannabis in patients with Crohn's disease. Among those were 2 systematic reviews without meta-analysis, 1 RCT, and 8 observational/quasi-experimental studies. Of all studies investigating medical cannabis in patients with Crohn's disease, 3 studies investigated symptom mitigation as the primary outcome, 1 study investigated safety outcomes, and 6 investigated other outcomes or used a cross-sectional design [ 14 , 116 , 126 , 127 , 128 , 129 ]. In studies that investigated symptom mitigation, 1 study indicated an improvement [ 130 ], 1 study indicated mixed findings [ 61 ], and one was inconclusive [ 131 ]. Safety outcomes were reported by one study, which indicated worsening safety outcomes [ 132 ]. The RCT has recently been withdrawn due to inadequate funding [ 133 ]. Six eligible studies, including 3 cross-sectional studies, investigated other outcomes. Outcomes that were assessed by more than one study included patient perceptions of cannabis benefits and side effects, which was assessed by 2 studies [ 127 , 128 ], and cannabis use patterns, which was investigated by 2 studies [ 126 , 129 ].
Medical cannabis in patients with epilepsy was investigated in 72 eligible publications. Among those were 3 systematic reviews with meta-analysis, 2 systematic reviews without meta-analysis, 3 RCTs, 17 observational/quasi-experimental studies, and 47 other types of reviews. Of all studies investigating medical cannabis and epilepsy, 19 studies investigated the effect on seizures (i.e., reductions in number of seizures and seizure frequency) as the primary outcome, 2 studies assessed health-related quality of life, 18 studies investigated safety outcomes, and 3 studies investigated other outcomes or used a cross-sectional design. Among those studies that investigated the effect on seizures as outcomes, 13 studies indicated an improvement [ 116 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 ], 4 studies indicated no change [ 144 , 146 , 147 , 148 ], and 2 studies were inconclusive [ 149 , 150 ]. In those studies that investigated health-related quality of life or quality of life as the primary outcome, both studies indicated an improvement [ 144 , 146 ] and one study indicated no change [ 146 ]. Among those 18 studies that investigated safety outcomes, 10 studies indicated worsening [ 134 , 136 , 138 , 140 , 142 , 145 , 151 , 152 , 153 , 154 ], 1 indicated mixed findings [ 155 ], 5 indicated no change [ 135 , 141 , 144 , 156 ], and 2 were inconclusive [ 149 , 150 ]. Three eligible studies, including 1 cross-sectional study, investigated other outcomes. One study assessed potential pharmacokinetic interactions [ 157 ], one investigated perception about cannabis use and benefits [ 158 ], and the third assessed doses of cannabidiol [ 116 ].
Medical cannabis in patients with glaucoma was investigated in 14 eligible publications, including one systematic review without meta-analysis and one book section. (Detailed information about the latter and the 12 other types of reviews can be found in the online suppl. files.) Of all studies, one investigated the effect of medical cannabis on intraocular pressure, and this study indicated no change in the outcome [ 116 ].
Human Immunodeficiency Virus/AIDS
Medical cannabis in patients with HIV/AIDS was investigated in 25 eligible publications, among those were 3 systematic reviews with meta-analysis, 19 observational/quasi-experimental studies, and 3 other types of reviews. Of all studies within this section, 2 studies investigated symptom mitigation (see online suppl. material) as the primary outcome, 4 studies investigated the effect on adherence to antiretroviral therapy, 2 studies investigated the effect on viral suppression, 5 studies investigated safety outcomes, and 12 studies investigated other outcomes or used a cross-sectional design [ 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 ]. Among the 2 studies that investigated symptom mitigation, one indicated an improvement [ 120 ] and one was inconclusive [ 115 ]. Among the 5 studies examining the effect of cannabis use on adherence to antiretroviral therapy, 2 indicated worsening [ 171 , 172 ], 2 reported no change [ 160 , 173 ], and 1 reported inconclusive findings [ 174 ]. One study examining the effect on viral suppression indicated no change [ 173 ], and 1 study indicated inconclusive findings [ 174 ]. Of the 5 studies investigating safety outcomes, 3 studies indicated worsening [ 120 , 175 , 176 ] and 2 studies indicated no change [ 176 , 177 ]. Twelve eligible studies, including 6 cross-sectional studies, investigated other outcomes. Among those outcomes that were assessed by more than one study, 5 studies assessed aspects of HIV care continuum measures [ 160 , 161 , 162 , 163 , 168 ] and 2 studies assessed the prevalence and correlates of substance use [ 165 , 178 ].
Multiple Sclerosis
Medical cannabis in patients with MS or related motor neuron disorders was investigated in 25 eligible publications. Among those were 5 systematic reviews with meta-analysis, 4 systematic reviews without meta-analysis, and 16 other types of reviews. Of all studies within this section, 6 studies investigated spasticity and spasm as the primary outcome, 4 studies investigated efficacy on MS-related pain, 3 studies investigated bladder function, 1 study examined the effect on gait function, and 6 studies investigated safety outcomes. (More information about the 17 other types of reviews can be found in the online suppl. files.) Among the 6 studies investigating spasticity and spasm, 3 indicated an improvement [ 179 , 180 , 181 ], one indicated mixed findings [ 182 ], one study reported no change [ 183 ], and one was inconclusive [ 30 ]. Among the 4 studies examining MS-related pain, one indicated improvement [ 180 ], one reported no change [ 183 ], and 2 reported inconclusive findings [ 30 , 115 ]. Of studies examining the effect on bladder function, 2 indicated improvement [ 180 , 184 ] and one reported no change [ 183 ]. One study investigating gait function reported inconclusive findings [ 185 ]. In studies investigating safety outcomes, 3 studies indicated worsening [ 180 , 183 , 184 ], 2 studies indicated no change [ 181 , 182 ], and 1 study reported inconclusive findings [ 30 ].
Parkinson's Disease
Medical cannabis in patients with Parkinson's disease was investigated in 17 eligible publications. Among those were one RCT and 4 observational/quasi-experimental studies. Of all studies, one study investigated the effect of medical cannabis on safety outcomes and indicated no change in the outcome [ 186 ]. For one RCT, results are still forthcoming [ 187 ]. Other outcomes were investigated by 3 cross-sectional studies. All of these studies investigated physicians or patient expectations or perceived benefits of cannabis on Parkinson's disease-related symptoms [ 83 , 188 , 189 ]. (More information about the studies that assessed other outcomes and the 12 other types of reviews can be found in the online suppl. files.)
Posttraumatic Stress Disorder
Medical cannabis in patients with PTSD was investigated in 50 eligible publications. Among those were 10 systematic reviews without meta-analysis, 5 RCTs, 3 observational/quasi-experimental studies, and 31 other types of reviews. Of all studies investigating medical cannabis in patients with PTSD, 8 studies investigated symptom mitigation (e.g., sleep disturbances, dissociative reactions or flashbacks, and hyperarousal) as the primary outcome, 3 studies investigated safety outcomes, and 3 assessed other outcomes or used a cross-sectional design [ 14 , 190 , 191 ]. Among those studies that investigated symptom mitigation, 2 indicated mixed findings [ 192 , 193 ] and 6 were inconclusive [ 12 , 122 , 194 , 195 , 196 , 197 ]. One study investigating safety outcomes indicated mixed findings [ 198 ], and 2 studies reported inconclusive findings [ 195 , 199 ]. Among those 5 RCTs, 1 study has been terminated, 2 were completed, but publications were not available at the time of literature search, and for 2, results are still pending. For 5 RCTs, results are still pending [ 200 , 201 , 202 , 203 , 204 ]. Three eligible studies, including 2 cross-sectional studies, investigated other outcomes. Two of 3 investigated cannabis dispensary staff or healthcare provider practices [ 14 , 191 ] and 1 study investigated cannabis use patterns and associated problems [ 190 ].
Cannabis Agents
The vast majority of RCTs and observational studies (including cross-sectional studies) that investigated the safety or efficacy of cannabis did not further specify the type of cannabis product that was investigated. A specific route of administration was also often not reported. Among those publications that specified the cannabis product, CBD was the most frequent investigated agent and mostly for investigations related to epilepsy or other seizure disorders. Whole plant cannabis was the least investigated drug. With respect to route of administration, studies investigating THC, CBD, or THC and CBD combinations typically employed oral/peroral, buccal, or sublingual administration. This is in contrast to those studies assessing unspecified agents, in which pulmonary and oral/peroral administrations were most common. We encountered only one study that assessed minor cannabinoids, namely, tetrahydrocannabinolic acid.
Evidence Map
The majority of identified systematic reviews were conducted on the topic areas of chronic noncancer pain, cancer, MS, epilepsy, and PTSD. The evidence map includes indications for conditions that were determined to have scarce recent evidence available. The quality of evidence varied widely among all eligible systematic reviews and differed between each condition. Reviews graded as either critically low or low quality, indicating serious risks of biases and/or methodological limitations, were mainly conducted in the areas of cancer, PTSD, and HIV/AIDS. Moderate-quality systematic reviews were represented in all conditions. Only the areas of chronic noncancer pain, epilepsy, and MS included systematic reviews graded as high quality. In terms of safety and efficacy outcomes, only a few systematic reviews in the area of ALS, cancer, chronic noncancer pain, Crohn's disease, glaucoma, and MS indicated worsening or no difference. The majority of included reviews reported inconclusive or mixed results, and only publications in the area of chronic noncancer pain, cancer, epilepsy, and MS reported improved outcomes. Furthermore, among high-quality reviews, only chronic noncancer pain and epilepsy reported improved outcomes (see Fig. Fig.1 1 ).
Referring to the 11 investigated conditions, the NASEM report in 2017 concluded that there is conclusive or substantial evidence for cannabis in treating chronic noncancer pain, chemotherapy-induced nausea and vomiting (oral cannabinoids), and MS spasticity symptoms (via oral cannabinoids). In addition, limited evidence was reported for the efficacy of cannabis and cannabinoids for the purposes of increasing appetite and decreasing weight loss in patients with HIV/AIDS, improving clinician measures of MS spasticity symptoms (specifically, via oral cannabinoids), and improving symptoms of PTSD (specifically, with nabilone). NASEM also concluded that limited evidence was available that cannabis and cannabinoids were ineffective in improving intraocular pressure associated with glaucoma (specifically via cannabinoids). Furthermore, insufficient or no evidence existed to support or refute the effectiveness of cannabis or cannabinoids for a majority of examined indications. Those indications included cancer (cannabinoids), cancer-associated anorexia-cachexia syndrome and anorexia nervosa (cannabinoids), symptoms of irritable bowel syndrome (dronabinol), epilepsy (cannabinoids), symptoms associated with ALS, or Parkinson's disease-related symptoms or levodopa-induced dyskinesia (cannabinoids).
In the 4 years since the NASEM report, much has been published in the clinical and scientific literature regarding the safety and efficacy of cannabis and cannabinoids, but we identified few recent studies conducted within US populations and were of substantial rigor and quality to move the evidence base forward for many clinical conditions. In fact, across all condition topic areas, the most frequently identified study design was clinical/narrative review with a nonsystematic approach, and these reviews only recounted and compiled previous RCT and observational study findings. Many other identified studies, particularly observational studies, also had significant limitations when assessing the safety and efficacy of cannabis that potentially affected validity. Detailed information about the history of cannabis use, other substance use, concomitant medications, comorbidities, types of cannabis product (THC, CBD, THC/CBD, and whole plant), route of administration, and dosage was not captured in the majority of observational studies due to unavailable data or limited subject knowledge. Thus, confounding was a recurring threat to validity in many identified studies. Several observational studies, for example, suggest that cancer patients using medical cannabis tend to have more severe symptoms than those who did not consume medical or recreational cannabis [ 57 , 60 , 71 ]. However, it is unclear if cannabis is contributing to more severe symptoms or if the presence of severe symptoms prompted increases in cannabis utilization. In addition, patient-reported outcomes and behaviors may be more susceptible to recall bias and/or inaccurate reporting of dosage, duration, and frequency of use [ 205 , 206 ]. Patients also might not report nonmedical cannabis use due to perceived social norms.
Quality of Evidence
Our assessment of the quality of systematic reviews determined that high-quality systematic reviews were conducted only among the conditions of chronic noncancer pain, epilepsy, and MS. In the area of chronic noncancer pain, the most recent systematic reviews are in alignment with findings of the NASEM report, which reported substantial evidence for the use of cannabis as a treatment for chronic pain in adults.
In the area of epilepsy, one recently published high-quality systematic review included several newly published RCTs focusing on pediatrics and found significantly reduced seizure frequency with adjunctive CBD use in pediatric drug-resistant Dravet and Lennox-Gastaut syndromes, aligning with the FDA approval of Epidiolex. High-quality systematic reviews in the field of MS did not include any RCT results following the publication of the NASEM report and are, therefore, not expanding the evidence base.
Only 7 systematic reviews were graded as high quality, whereas almost one-third were graded as low- or critically low-quality systematic reviews. Common reasons for being rated as a moderate- or low-quality review were due to the absence of a prior established protocol, lack of a comprehensive literature search strategy, failing to report the source of funding of included studies, missing an adequate detailed description of excluded studies, inadequate accounting for the risk of bias assessment within result interpretation and discussion, absence of adequate discussion of heterogeneity, and absence of a quantitative synthesis or meta-analysis. In addition to these limitations, many identified systematic reviews also consisted of few RCTs.
Despite the limited evidence available from recent high-quality systematic reviews, it is promising that we identified 12 RCTs with registered protocols and trial registrations. The studies are covering the field of Crohn's disease, chronic noncancer pain, cancer, Parkinson's disease, and PTSD [ 76 , 77 , 123 , 124 , 125 , 133 , 187 , 200 , 201 , 202 , 203 , 204 ], and 2 of them have recently been withdrawn or terminated [ 133 , 202 ]. However, the remaining 10 RCTs have the potential to expand the evidence base. In addition, our review identified many studies that reported an increase in adverse events relative to placebo or an active comparator, which was consistent across most of the assessed medical conditions. Nevertheless, the vast majority of the reviewed studies reported that adverse event severity ranged from mild to moderate, and most adverse events were reversible with dose reduction or discontinuation. Medical cannabis was often referred to as “generally well tolerated.” However, information about long-term safety outcomes was scarce.
Gaps in Literature
We identified several persistent gaps in the literature during this review. Recent observational studies often lacked specific information about the route of administration, dosage, frequency, and cannabis product used. Clinical trials were mainly limited to peroral, oral, or sublingual administration and represented few formulations of available cannabis products. Studies investigating whole-plant cannabis products are needed to better understand the risks and benefits of cannabis in real-world settings as patients receiving medical cannabis in practice are typically receiving whole-plant products. In order to provide valuable information about the effectiveness and safety of medical cannabis, real-world studies must define cannabis products, the route of administration, and dosage precisely. In addition, it is unclear whether or not standardized products provided in RCTs are comparable to those products offered by dispensaries, where consistency in product dosing, concentrations, and even routes of administration offered are not necessarily guaranteed and are subject to variations in state regulations [ 207 ]. Furthermore, there remain other questions about the generalizability of existing evidence raised. For example, patients with substance use disorder histories were often excluded from randomized studies across several conditions, even though use by patients with these or similar underlying conditions is common (e.g., PTSD and chronic noncancer pain) [ 208 ].
Implications for Research, Clinicians, and Policy
The prevalence of medical cannabis and cannabis use for nonmedical reasons is increasing [ 209 ], while perceived risks associated with cannabis use are decreasing, particularly among younger persons [ 210 ]. Therefore, it is important to evaluate and disseminate the evidence widely to both clinicians and patients. Interestingly, there is also some evidence suggesting that the legalization of cannabis might not necessarily affect the compliance rate of primary therapies in patients with chronic noncancer pain under opioid therapy [ 211 ], so it is unclear whether the changing availability of licit nonmedical cannabis will impact clinical outcomes in patients receiving medical cannabis.
There remains a need for well-designed and conducted RCTs for most of the assessed medical conditions. However, there are several methodological and practical challenges in conducting RCTs specific to investigating efficacy and safety of cannabis and cannabinoids, including placebo effects, practical limitations in conducting blinding for cannabis products, and regulatory barriers. Expense and complicated implementation, meanwhile, render it difficult to design and perform high-quality RCTs even in the absence of cannabis-related regulatory barriers [ 212 , 213 ]. Studies assessing cannabis efficacy and safety for these conditions, or any condition, must consider the effect that different routes of administration can have on systemic exposure and ultimately on study outcomes. Studies must also clearly and precisely quantify active metabolites and ratio of metabolites (i.e., THC:CBD) with the same rigor as applied to other medication studies.
Questions also remain about medical cannabis safety, especially in terms of rare adverse drug events, long-term effects, the effects on patients with comorbidities (e.g., people with history of substance abuse), and the potential for interactions with prescription medications and other substances, particularly among patients most susceptible to adverse events from drug-drug interactions (e.g., geriatric populations). Future research will require the utilization of a combination of approaches and techniques to overcome the barriers associated with capturing these rare or long-term outcomes, including the use of real-world data and sophisticated pharmacoepidemiologic methods to overcome current limitations in reported studies for ascertaining exposures and outcomes.
The evolving and challenging legal status of cannabis remains a significant obstacle to the expansion of cannabis research in the USA. The schedule I controlled substance designation of whole-plant cannabis restricts research in this area due to regulatory barriers and limited feasibility, along with scarce federal research funding allocated to the investigation of constituent compounds [ 214 ]. Furthermore, only a minority of the National Institute of Health's budget is earmarked for therapeutic cannabis research, while more is available for investigations of problematic uses and/or abuse potential, making it challenging to get US funding for investigation of therapeutic potential [ 215 , 216 ]. The complicated legal status of cannabis in the USA restricts cultivation and production to a single federally permitted institution; thus, a narrow amount of cannabis products can be tested, and these may not mirror constituents and concentrations of products available to consumers on the market [ 217 , 218 ]. Thus, policies would need revision to permit handling or production of dispensary-available cannabis products for research purposes and expand funding mechanism to support urgently needed research on clinical outcomes of medical cannabis.
Limitations and Strengths
Our review has several limitations that should be considered in the interpretation of the findings. First, we restricted our search strategy to studies published between July 2016 and October 2019 and for our rapid review to studies published between 2000 and October 2019. Therefore, we assessed only a narrow period of the most recently available literature. Second, we excluded articles reporting primary research conducted exclusively outside the USA, in order to account for differences in cannabis product availability internationally as well as differences in regulatory barriers and access. We, therefore, have excluded potentially relevant recent literature conducted in countries with robust scientific and clinical research programs evaluating cannabis efficacy and safety. However, studies originating from the USA accounted for almost 2/3 of all publications between 2000 and 2017 [ 219 ]. Third, even though we conducted pilot runs and training with reviewers on the use of the data extraction tool, the data extraction step was only conducted by one reviewer with review by a second reviewer in cases of uncertainty. In addition, the screening process for each topic area was only conducted by a second reviewer for those articles categorized as “uncertain”; thus, selection bias might have been introduced during both stages. However, weekly meetings throughout the review process were used to clarify any questions and uncertainties throughout the screening and extraction process. Fourth, systematic reviews and meta-analyses were not excluded if they partially included studies that were not matching our criteria (e.g., a systematic review consisting of studies that were conducted between 2016 and 2019 but also prior to 2016 was still considered as eligible, since it was not feasible to disentangle the evidence synthesis without examining the underlying primary study). Therefore, our findings based on systematic reviews and meta-analyses might not be restricted to our country and time criteria. In order to account for this limitation, we stratified our findings by study design and also restricted our summary of cannabis agents to RCTs and observational studies. Fifth, we did not assess whether medical cannabis was used as adjuvant treatment or primary therapy. Subsequently, different directions of findings might be based on variation in co-medications. However, the regulatory environment in the US mainly restricts the use of medical cannabis products to adjuvants, and the objective of this study was not to assess safety and efficacy of medical cannabis. Last, although a standardized classification scheme was applied to categorize the outcomes, inter-rater variability might have introduced misclassification of the outcomes.
There are also several strengths of this review to consider, including the broad scope of assessed medical conditions, comprehensive search strategy that extended beyond RCTs, and adherence to the PRISMA statement for gathering and reporting findings. Furthermore, this review highlights recent research efforts by medical condition, and directions of findings, thus creating a comprehensive picture of the scientific landscape of clinical studies about cannabis. Moreover, we also identified several literature gaps that could be addressed in future research, and we assessed the quality of evidence available, which is essential information for policymaking. Additionally, input from an external expert panel ensured a wide range in scope of the literature covered, and this review gives an up-to-date overview about the current state of evidence quality in a readily interpretable map.
The large body of the literature recently published regarding medical cannabis masks a paucity of evidence related to efficacy and safety as treatment options for several conditions for which it is commonly prescribed. Across 11 conditions, we identified few studies of substantial rigor and quality to contribute to the evidence base. However, there are some conditions for which significant evidence suggests that certain dosage forms and routes of medical cannabis products likely have favorable risk-benefit ratios (i.e., epilepsy and chronic noncancer pain). Gaps in the evidence remain significant for most examined conditions, but the identification of several registered forthcoming RCTs suggests that improved evidence will be available in the coming years.
Conflict of Interest Statement
The Consortium for Medical Marijuana Clinical Outcomes Research provided funding support for 4 contributors to this study, where S.J. and B.C. received graduate student stipend support in 2019–2020, and A.G., J.H.C., and A.W. received salary offset for serving as University of Florida faculty leads in 2019–2020. No other authors have conflicts of interest to declare.
Funding Sources
The consortium (described above) provided material support for 4 authors during the period of study completion (2019–2020). The funder did not have a role in decisions related to the preparation of data or the contents of this manuscript.
Author Contributions
S.J. prepared protocols for literature search, screening, and data extraction and drafted the manuscript. A.G. designed the study, supervised contributors, and critically revised the manuscript. L.A. performed literature searches and curated the reference library. The following contributors were topic lead reviewers for the following conditions: A.G. in amyotrophic lateral sclerosis, A.O. in human immunodeficiency virus (HIV)/AIDS, B.C. in Crohn's disease and posttraumatic stress disorder, E.J.M. in Parkinson's disease, G.A.H. in epilepsy, S.K. and S.J. in cancer, S.J. in chronic noncancer pain, Y.S. in autism and multiple sclerosis, and Y.Z. in glaucoma. R.S. developed figures. All other contributors were positioned in various roles as screeners, in data extraction, in reviewer resolutions, and in qualitative synthesis. All contributors critically revised and approved the manuscript.
Supplementary Material
Supplementary data
Acknowledgement
We thank and acknowledge the panel of scientific and clinical subject matter experts who provided comments on the protocols for topic selection, literature identification search strategies, literature screening, and data extraction procedures. Expert panelists were recruited as part of the Consortium for Medical Marijuana Clinical Outcomes Research activities, but the panelists have not contributed to the interpretation of the review findings.
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals
What you need to know.
- An updated list of high-risk underlying conditions, along with their associated evidence, is provided below. The conditions are grouped by the level of evidence, with the highest level shown in the top section.
- The list of underlying medical conditions is not exhaustive and will be updated as the science evolves.
- This list should not be used to exclude people with underlying conditions from recommended measures for prevention or treatment of COVID-19.
Summary of Conditions with Evidence
Actions healthcare professionals can take, key findings from one large cross-sectional study.
This webpage provides an evidence-based resource for healthcare professionals caring for patients with underlying medical conditions who are at higher risk of experiencing severe outcomes of COVID-19. Severe outcomes of COVID-19 are defined as hospitalization, admission to the intensive care unit (ICU), intubation or mechanical ventilation, or death.
This page summarizes data from published reports, scientific articles in press, unreviewed pre-prints, and internal data that were included in literature reviews conducted by subject matter experts. Evidence used to inform the list of underlying conditions was determined by CDC reviewers based on available literature about COVID-19 at time of review. The information reflects evidence regarding underlying medical conditions and is intended to help healthcare professionals make informed decisions about patient care and to increase the awareness of risk among their patients.
The methods used to assess the conditions have changed during the pandemic as the amount of literature and types of studies increased. For instance, preliminary versions of this list focused on providing the latest information based on descriptive data. As the literature grew, CDC investigators categorized the literature by study design.
Since May 2021, the process has been updated to include a CDC-led review process that uses rigorous systematic review methods. To learn more about the process of CDC’s systematic reviews, see CDC systematic review process .
Age is the strongest risk factor for severe COVID-19 outcomes. Patients with one or multiple of certain underlying medical conditions are also at higher risk. ( 1 – 3 )
Additionally, being unvaccinated or not being up to date on COVID-19 vaccinations also increases the risk of severe COVID-19 outcomes.
Providers should consider the patient’s age, presence of underlying medical conditions and other risk factors, and vaccination status in determining the risk of severe COVID-19-associated outcomes for any patient.
Demographic Factors
Studies have shown that COVID-19 does not affect all population groups equally. Three important factors are age, race, and ethnicity.
Age remains the strongest risk factor for severe COVID-19 outcomes, with risk of severe outcomes increasing markedly with increasing age. Based on data from the National Vital Statistics System (NVSS) at NCHS ( Risk for COVID-19 Infection, Hospitalization, and Death By Age Group ), compared with ages 18–29 years, the risk of death is 25 times higher in those ages 50–64 years, 60 times higher in those ages 65–74 years, 140 times higher in those ages 75–84 years, and 340 times higher in those ages 85+ years. Notably, these data include all deaths in the United States that occurred throughout the pandemic, from February 2020 to July 1, 2022, including deaths among unvaccinated individuals.
Risk of severe outcomes is increased in people of all ages with certain underlying medical conditions and in people who are 50 years and older, with risk increasing substantially at ages >65 years. 4,5 Residents of long-term care facilities are also at increased risk, making up less than 1% of the U.S. population but accounting for more than 35% of all COVID-19 deaths. 6-10
Race and Ethnicity
The COVID-19 pandemic has highlighted racial, ethnic, and socioeconomic disparities in COVID-19 illnesses, hospitalizations, and deaths. 11-13 Some racial and ethnic minority groups are also more likely to face multiple barriers to accessing health care including lack of insurance, transportation, child care, or ability to take time off from work.
Studies have identified racial and ethnic differences in at-home COVID-19 test use, vaccination coverage, and access to outpatient therapeutics.14-16 Data has shown that compared to non-Hispanic White people, people from racial and ethnic minority groups are more likely to be infected with SARS-CoV-2 (the virus that causes COVID-19). Once infected, people from racial and ethnic minority groups are more likely to be hospitalized, be admitted to the ICU, and die from COVID-19 at younger ages. 17
We are still learning about how the environments where people live, learn, and work can influence the risk for infection and severe COVID-19 outcomes.
Evidence used to inform the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19 is presented in alphabetical order by study design section. Conditions are categorized as higher risk, suggestive higher risk, and mixed evidence.
Higher Risk (conclusive)
Higher risk is defined as an underlying medical condition or risk factor that has a published meta-analysis or systematic review or underwent the CDC systematic review process . The meta-analysis or systematic review demonstrates a conclusive increase in risk for at least one severe COVID-19 outcome.
Evidence of Impact on COVID-19 Severity [Reference number]
CDC Systematic Review [K]
- Hematologic Malignancies
CDC Systematic Review [O] Meta-Analysis/ Systematic Review 18-22 Cohort Study 23-25 Case Series 26-28 Case Control Study 29
Cerebrovascular disease
Meta-Analysis 30-33 Synthesis of Evidence 34 Cohort Study 35-37
Chronic kidney disease*
- People receiving dialysis 38,39 ^
Meta-Analysis 33,40 Cohort Studies 36,41-62, 63 * Case Series 64-66
Chronic lung diseases limited to:
- Bronchiectasis
- COPD (Chronic obstructive pulmonary disease)
- Interstitial lung disease
- Pulmonary embolism
- Pulmonary hypertension
- CDC Systematic Review [A]
- CDC Systematic Review [L]
- CDC Systematic Review [D]
- CDC Systematic Review [G]
Chronic liver diseases limited to:
- Non-alcoholic fatty liver disease
- Alcoholic liver disease
- Autoimmune hepatitis
CDC Systematic Review [B]
Cystic fibrosis
CDC Systematic Review [M]
Diabetes mellitus, type 1
Meta-Analysis 67 Case Series 65 Cohort Study 35,68-73
Diabetes mellitus, type 2*
Meta-Analysis 74 Systematic Review 75 * Gestational Diabetes Systematic Review 76 * Case Series 65 Longitudinal Study 77 Cohort Study 67,71,77-82
Disabilities‡, including Down syndrome
For the list of all conditions that were part of the review, see the module below
CDC Systematic Review [C]
Heart conditions (such as heart failure, coronary artery disease, or cardiomyopathies)
Meta-Analysis 83-85 Cohort Study 35,36
HIV (Human immunodeficiency virus)
Meta-Analysis/ Systematic Review 86 Cohort Study 54 , 87-89 Case Series 90-92
Mental health conditions limited to:
- Mood disorders, including depression
- Schizophrenia spectrum disorders
Meta-Analysis/ Systematic Review 93 , 94
Neurologic conditions limited to dementia‡
Meta-Analysis/ Systematic Review 95-98 Cross-Sectional Study 99 Cohort Study 36,100
Obesity (BMI > 30 kg/m 2 or > 95 th percentile in children)
Meta-Analysis 101-103 Systematic Review 75 * Cohort 46 , 104-112 ; 63,113-116 *
Physical inactivity
CDC Systematic Review [E]
Pregnancy and recent pregnancy
Meta-Analysis/ Systematic Review 75,117 Case Control 118 , 119 Case Series 120-122 Cohort Study 123-126
Primary immunodeficiencies
CDC Systematic Review [F]
Smoking, current and former
Meta-Analysis 83,127 , 128-135
Solid organ or blood stem cell transplantation
Meta-Analysis 108 Case Series 136-147 Cohort 148-151
Tuberculosis
CDC Systematic Review [H]
Use of corticosteroids or other immunosuppressive medications
Meta-Analysis/ Systematic Review 152 Cohort Study 153 Cross-Sectional 154 Case Series 155-157
- Attention-deficit/hyperactivity disorder (ADHD)
- Cerebral palsy
- Charcot foot
- Chromosomal disorders
- Chromosome 17 and 19 deletion
- Chromosome 18q deletion
- Cognitive impairment
- Congenital hydrocephalus
- Congenital malformations
- Deafness/hearing loss
- Disability indicated by Barthel Index
- Down syndrome
- Fahr’s syndrome
- Fragile X syndrome
- Gaucher disease
- Hand and foot disorders
- Learning disabilities
- Leber's hereditary optic neuropathy (LHON) or Autosomal dominant optic atrophy (ADOA)
- Leigh syndrome
- Limitations with self-care or activities of daily living
- Maternal inherited diabetes and deafness (MIDD)
- Mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) and risk markers
- Mobility disability
- Movement disorders
- Multiple disability (referred to in research papers as “bedridden disability”)
- Multisystem disease
- Myoclonic epilepsy with ragged red fibers (MERRF)
- Myotonic dystrophy
- Neurodevelopmental disorders
- Neuromuscular disorders
- Neuromyelitis optica spectrum disorder (NMOSD)
- Neuropathy, ataxia, and retinitis pigmentosa (NARP)
- Perinatal spastic hemiparesis
- Primary mitochondrial myopathy (PMM)
- Progressive supranuclear palsy
- Senior-Loken syndrome
- Severe and complex disability (referred to in research papers as “polyhandicap disability”)
- Spina bifida and other nervous system anomalies
- Spinal cord injury
- Tourette syndrome
- Traumatic brain injury
- Visual impairment/blindness
- Wheelchair use
Suggestive Higher Risk
Suggestive higher risk is defined as an underlying medical condition or risk factor that did not have a published meta-analysis or systematic review or did not undergo the CDC systematic review process . The evidence is supported by mostly cohort, case-control, or cross-sectional studies. (Systematic reviews are available for some conditions for children with underlying conditions.)
Children with certain underlying conditions
Read More: Information for Pediatric Healthcare Providers
Systematic Review 158,159 Cross-Sectional Study 99 , 160,161 Cohort Study 100 , 162-169 Case Series 170,171
Overweight (BMI > 25 kg/m 2 but <30 kg/m 2 )
Cohort Study 111 Case Series 110
Sickle cell disease
Cohort 170-173 Case Series 170,173-188
Substance use disorders
Case-Control Study 189-191 Cohort Study 192,193
Mixed Evidence (inconclusive: no conclusions can be drawn from the evidence)
Mixed evidence is defined as an underlying medical condition or risk factor that has a published meta-analysis or systematic review or underwent the CDC systematic review process . The meta-analysis or systematic review is inconclusive, either because the aggregated data on the association between an underlying condition and severe COVID-19 outcomes are inconsistent in direction or there are insufficient (or limited) data on the association between an underlying condition and severe COVID-19 outcomes.
- Limited: The evidence consists of one study, or several small studies with no comparison group, limiting the conclusions that can be drawn.
- Inconsistent: The evidence suggests no clear direction of association, meaning no firm conclusions can be drawn.
Alpha 1 antitrypsin deficiency
Limited: CDC Systematic Review [I]
Bronchopulmonary dysplasia
Limited: CDC Systematic Review [J]
Hepatitis B
Inconsistent: CDC Systematic Review [B]
Hepatitis C
Limited: CDC Systematic Review [B]
Hypertension*
Inconsistent Meta-Analysis 83,194-197 Systematic Review 198 , 75 * Cohort Study 35,36,41,199-205 Case Series 206
Thalassemia
Limited: CDC Systematic Review [N]
Footnotes: * Indicates presence of evidence for pregnant and non-pregnant people
‡ Underlying conditions for which there is evidence in pediatric patients
^ Risk may be further increased for people receiving dialysis
- Recommend vaccination with approved and authorized COVID-19 vaccines (updated 2023-2024 COVID-19 vaccine), which are safe and effective. Check out the Interim Clinical Considerations for Use of COVID-19 Vaccines as well as Stay Up to Date with Your Vaccines and locations for COVID-19 vaccination for patients for more information.
- Prescribe antivirals , which have been shown to significantly decrease the risk of hospitalization and death when treating patients with mild to moderate illness and risk factors for severe illness. Outcomes are improved if therapeutics are started within the first 5-7 days of symptom onset.
- Consider pemivibart (Pemgarda™), a monoclonal antibody for COVID-19 pre-exposure prophylaxis in people who are moderately or severely immunocompromised and unlikely to mount an adequate immune response to COVID-19 vaccination and who meet the FDA-authorized conditions for use . Pemivibart may provide another layer of protection against COVID-19 in addition to vaccination and can be given at least 2 weeks after receiving a COVID-19 vaccine. For more information, please see the FDA Fact Sheet for Providers .
- Remind older patients and those with underlying medical conditions that wearing a mask is an additional prevention strategy they can choose to further protect themselves.
- Encourage patients to keep appointments for routine care and adhere to treatment regimens for their medical conditions.
- Consider use of telehealth when appropriate.
- Check out additional information for your patients .
Considerations for Patients Within Racial and Ethnic Minority Groups
- Ask patients about their concerns about vaccines and therapy. Consider using an evidence-based and culturally sensitive approach, such as motivational interviewing . Try to provide trusted sources of information and other resources.
- Encourage testing, as well as early treatment , for patients who are eligible.
- Facilitate access to culturally and linguistically appropriate resources.
- Reduce barriers to accessing current outpatient treatments.
CDC strongly encourages healthcare professionals, patients and their advocates, and health system administrators to regularly consult the Infectious Diseases Society of America (IDSA) COVID-19 Treatment Guidelines .
Underlying Medical Conditions and Severe Illness Among 540,667 Adults Hospitalized With COVID-19, March 2020–March 2021
This study used data from the Premier Healthcare Database, which represents approximately 20% of all inpatient admissions in the United States since 2000. This cross-sectional study of 540,667 adults hospitalized with COVID-19 included both inpatients and hospital-based outpatients with laboratory-diagnosed COVID-19 from March 1, 2020, through March 31, 2021. The database included reports from 592 acute care hospitals in the United States. The study was designed to examine risk factors associated with severe outcomes of COVID-19 including admission to an ICU or stepdown unit, invasive mechanical ventilation, and death.
Main Findings:
- Certain underlying medical conditions were associated with an increased risk for severe COVID-19 illness in adults.
- Having multiple conditions was also associated with severe COVID-19 illness.
- Obesity, diabetes with complications, and anxiety and fear-related disorders had the strongest association with death.
- The number of frequent underlying medical conditions (present in ≥10.0% of patients) increased with age. 207
Adapted from Sources:
- Kompaniyets L, Pennington AF, Goodman AB, Rosenblum HG, Belay B, Ko JY, et al. Underlying Medical Conditions and Severe Illness Among 540,667 Adults Hospitalized With COVID-19, March 2020–March 2021. To learn more, visit the Preventing Chronic Disease article: https://www.cdc.gov/pcd/issues/2021/21_0123.htm
- Pennington AF, Kompaniyets L, Summers AD, Danielson ML, Goodman AB, Chevinsky JR, Preston LE, Schieber LZ, Namulanda G, Courtney J, Strosnider HM, Boehmer TB, Mac Kenzie WR, Baggs J, Gundlapalli AV, Risk of Clinical Severity by Age and Race/Ethnicity Among Adults Hospitalized for COVID-19—United States, March–September 2020, Open Forum Infectious Diseases , Volume 8, Issue 2, February 2021. To learn more, visit: https://doi.org/10.1093/ofid/ofaa638
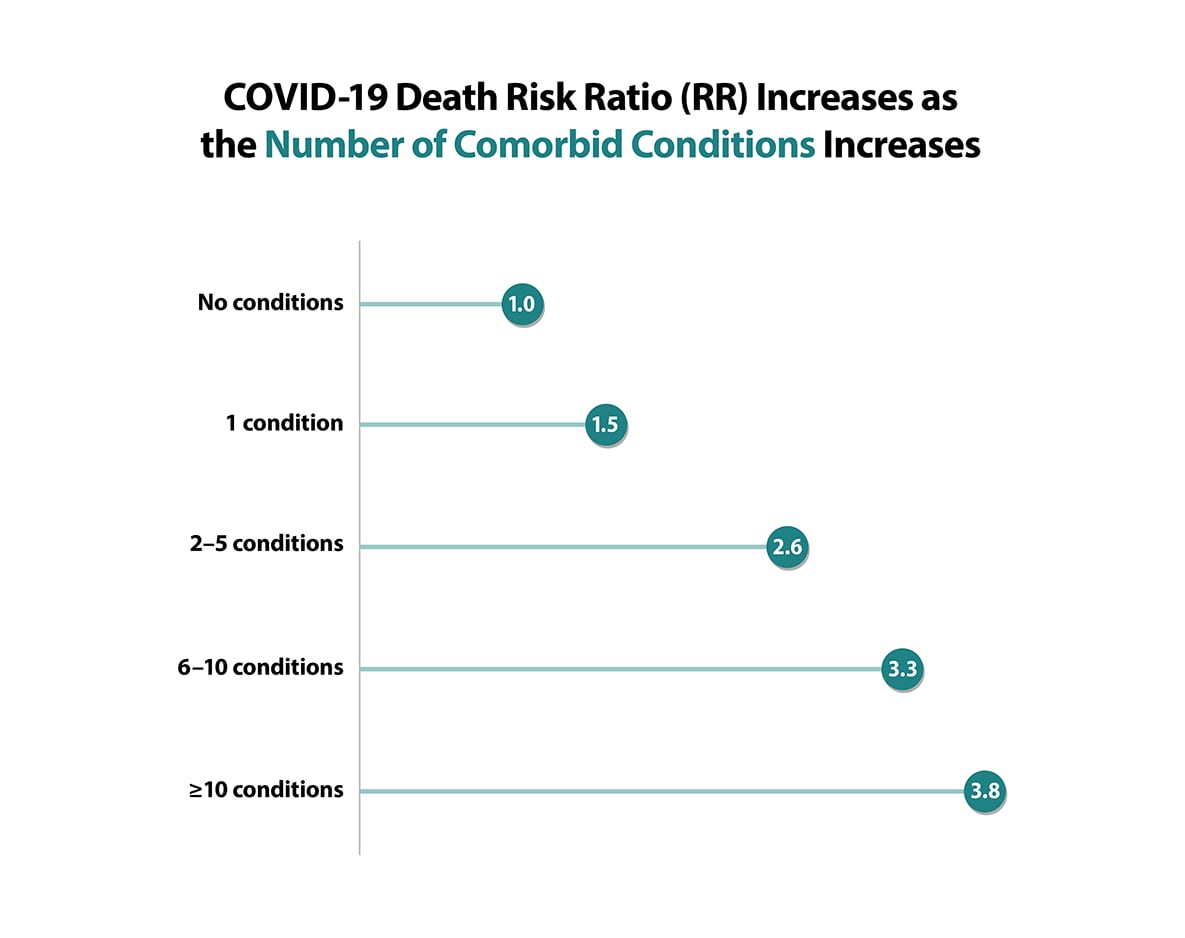
Source: Kompaniyets L, Pennington AF, Goodman AB, Rosenblum HG, Belay B, Ko JY, et al. Underlying Medical Conditions and Severe Illness Among 540,667 Adults Hospitalized With COVID-19, March 2020–March 2021. To learn more, visit the Preventing Chronic Disease article: https://www.cdc.gov/pcd/issues/2021/21_0123.htm
- Methods for the Underlying Conditions ICD-10 List [PDF, 2 pages, 112K]
- COVID-19 Treatment Guidelines: What’s New
- COVID-19 Therapeutics
- Clinical Care Considerations
- COVID-19 Treatment in Outpatients
- COVID-19 Cases, Deaths, and Laboratory Testing (NAATs) by State, Territory, and Jurisdiction
- Demographic Trends of COVID-19 Cases and Deaths in the U.S. by Race and Ethnicity
- Health Equity: Promoting Fair Access to Health
- How Do I Find a COVID-19 Vaccine
- COVID-19 Vaccination Clinical & Professional Resources
- Stone EC, Weissman D, Mazurek J, et al. Brief Summary of Findings on the Association Between Underlying Bronchiectasis and Severe COVID-19 Outcomes. [print only, 476K, 18 pages] CDC COVID-19 Scientific Brief. October 2021.
- Stone EC, Hofmeister M, Okasako-Schmucker DL, et al. Brief Summary of Findings on the Association Between Underlying Liver Diseases and Severe COVID-19 Outcomes. [print only, 1462K, 111 pages] CDC COVID-19 Scientific Brief. October 2021.
- So CN, Ryerson AB, Yeargin-Allsopp M, Kristie EN et al. Brief Summary of Findings on the Association Between Disabilities and Severe COVID-19 Outcomes. [1984K, 165 pages] CDC COVID-19 Scientific Brief.
- Okasako-Schmucker DL, Weissman D, Mazurek J et al. Brief Summary of Findings on the Association Between Interstitial Lung Diseases and Severe COVID-19 Outcomes. [print only, 837K, 67 pages] CDC COVID-19 Scientific Brief. October 2021.
- Hill AL, Whitfield G, Morford M et al. Brief Summary of Findings on the Association Between Physical Inactivity and Severe COVID-19 Outcomes. [931 KB, 63 pages] CDC COVID-19 Scientific Brief.
- Morford M, Green RF, Drzymalla E et al. Brief Summary of Findings on the Association Between Underlying Primary Immunodeficiency and Severe COVID-19 Outcomes. [print only, 705K, 41 pages] CDC COVID-19 Scientific Brief.
- Wassef M, Weissman D, Mazurek J et al. Brief Summary of Findings on the Association Between a History of Pulmonary Embolism or Pulmonary Hypertension and Severe COVID-19 Outcomes. [print only, 506K, 16 pages] CDC COVID-19 Scientific Brief. October 2021.
- Kumasaka JK, Jereb JA, Stone E et al. Brief Summary of Findings on the Association Between Tuberculosis and Severe COVID-19 Outcomes. [print only, 443K, 17 pages] CDC COVID-19 Scientific Brief. October 2021.
- Morford M, Weissman, D, Mazurek J, et al. Brief Summary of Findings on the Association Between Alpha-1 Antitrypsin Deficiency and Severe COVID-19 Outcomes. [print only, 533K, 27 pages] CDC COVID-19 Scientific Brief. October 2021.
- Henry MC, Weissman D, Mazurek J, et al. Brief Summary of Findings on the Association Between Underlying Bronchopulmonary Dysplasia (BPD) and Severe COVID-19 Outcomes. [print only, 375K, 12 pages] CDC COVID-19 Scientific Brief. October 2021.
- Okasako-Schmucker DL, Cornwell C, Mirabelli M, et al. Brief Summary of Findings on the Association Between Asthma and Severe COVID-19 Outcomes. [print only, 2,000KB, 142 pages] CDC COVID-19 Scientific Brief. June 2022.
- Kumasaka JK, Weissman D, Mazurek J, et al . Brief Summary of Findings on the Association Between COPD and Severe COVID-19 Outcomes. [print only, 2,000KB, 176 pages] CDC COVID-19 Scientific Brief. June 2022.
- So CN, Green RF, Drzymalia E, et al. Brief Summary of Findings on the Association Between Cystic Fibrosis and Severe COVID-19 Outcomes. [print only, 788K, 54 pages] CDC COVID-19 Scientific Brief. June 2022.
- Hill AL, Payne AB, Schieve LA, et al. Brief Summary of Findings on the Association Between Thalassemia and Severe COVID-19 Outcomes. [print only, 619 KB, 26 pages] CDC COVID-19 Scientific Brief. June 2022.
- Supplement: Morford M, Koumans E, Giovanni J, et al. Brief Summary of Findings on the Association Between Secondary Immunosuppression from B-Cell-Depleting Therapy and Severe COVID-19 Outcomes [482 KB – 28 pages] CDC COVID-19 Clinical Care. January 2023.
- Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk Factors Associated With In-Hospital Mortality in a US National Sample of Patients With COVID-19. JAMA Network Open . 2020;3(12):e2029058-e2029058. doi:10.1001/jamanetworkopen.2020.29058
- De Giorgi A, Fabbian F, Greco S, et al. Prediction of in-hospital mortality of patients with SARS-CoV-2 infection by comorbidity indexes: an Italian internal medicine single center study. Eur Rev Med Pharmacol Sci . Oct 2020;24(19):10258-10266. doi: https://dx.doi.org/10.26355/eurrev_202010_23250
- Dominguez-Ramirez L, Rodriguez-Perez F, Sosa-Jurado F, Santos-Lopez G, Cortes-Hernandez P. The role of metabolic comorbidity in COVID-19 mortality of middle-aged adults. The case of Mexico. 2020:2020.12.15.20244160. doi:10.1101/2020.12.15.20244160 %J medRxiv
- Ahmad FB, Cisewski JA, Minino A, Anderson RN. Provisional Mortality Data – United States, 2020. MMWR Morb Mortal Wkly Rep . Apr 9 2021;70(14):519-522. doi:10.15585/mmwr.mm7014e1
- Prevention CDC. CDC COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/#demographics
- Abrams HR, Loomer L, Gandhi A, Grabowski DC. Characteristics of US Nursing Homes with COVID‐19 Cases. Journal of the American Geriatrics Society . 2020;
- Grabowski DC, Mor V. Nursing Home Care in Crisis in the Wake of COVID-19. Journal of the American Medical Association . 2020;
- Brown KA, Jones A, Daneman N, et al. Association between nursing home crowding and COVID-19 infection and mortality in Ontario, Canada. Journal of the American Medical Association, Internal Medicine . 2020;
- Sarah HY, See I, Kent AG, et al. Characterization of COVID-19 in assisted living facilities—39 states, October 2020. Morbidity and Mortality Weekly Report . 2020;69(46):1730.
- Fisman DN, Bogoch I, Lapointe-Shaw L, McCready J, Tuite AR. Risk factors associated with mortality among residents with coronavirus disease 2019 (COVID-19) in long-term care facilities in Ontario, Canada. Journal of the American Medical Association . 2020;3(7):e2015957-e2015957.
- Ko JY, Danielson ML, Town M, et al. Risk Factors for Coronavirus Disease 2019 (COVID-19)–Associated Hospitalization: COVID-19–Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clinical Infectious Diseases . 2021;72(11):e695-e703. doi:10.1093/cid/ciaa1419
- Wortham JM, Lee JT, Althomsons S, et al. Characteristics of Persons Who Died with COVID-19 – United States, February 12-May 18, 2020. MMWR Morb Mortal Wkly Rep . Jul 17 2020;69(28):923-929. doi:10.15585/mmwr.mm6928e1
- Yang X, Zhang J, Chen S, et al. Demographic Disparities in Clinical Outcomes of COVID-19: Data From a Statewide Cohort in South Carolina. Open Forum Infect Dis . Sep 2021;8(9):ofab428. doi:10.1093/ofid/ofab428
- Rader B.; Gertz AL, D.; Gilmer, M.; Wronski, L.; Astley, C.; Sewalk, K.; Varrelman, T.; Cohen, J.; Parikh, R.; Reese, H.; Reed, C.; Brownstein J. Use of At-Home COVID-19 Tests — United States, August 23, 2021–March 12, 2022. MMWR Morb Mortal Wkly Rep . April 1, 2022;71(13):489–494. doi: http://dx.doi.org/10.15585/mmwr.mm7113e1
- Pingali C, Meghani M, Razzaghi H, et al. COVID-19 Vaccination Coverage Among Insured Persons Aged >/=16 Years, by Race/Ethnicity and Other Selected Characteristics – Eight Integrated Health Care Organizations, United States, December 14, 2020-May 15, 2021. MMWR Morb Mortal Wkly Rep . Jul 16 2021;70(28):985-990. doi:10.15585/mmwr.mm7028a1
- Wiltz JL, Feehan AK, Molinari NM, et al. Racial and Ethnic Disparities in Receipt of Medications for Treatment of COVID-19 – United States, March 2020-August 2021. MMWR Morb Mortal Wkly Rep . Jan 21 2022;71(3):96-102. doi:10.15585/mmwr.mm7103e1
- Prevention CDC. Health Disparities: Race and Hispanic Origin. Provisional Death Counts for Coronavirus Disease 2019 . February 9, 2022. https://www.cdc.gov/nchs/nvss/vsrr/covid19/health_disparities.htm
- Saini KS, Tagliamento M, Lambertini M, et al. Mortality in patients with cancer and coronavirus disease 2019: A systematic review and pooled analysis of 52 studies. Eur J Cancer . Nov 2020;139:43-50. doi:10.1016/j.ejca.2020.08.011
- Zhou Y, Yang Q, Chi J, et al. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta-analysis. Int J Infect Dis . Oct 2020;99:47-56. doi:10.1016/j.ijid.2020.07.029
- Venkatesulu BP, Chandrasekar VT, Girdhar P, et al. A Systematic Review and Meta-Analysis of Cancer Patients Affected by a Novel Coronavirus. JNCI Cancer Spectrum . 2021;5(2)doi:10.1093/jncics/pkaa102
- Salunke AA, Nandy K, Pathak SK, et al. Impact of COVID -19 in cancer patients on severity of disease and fatal outcomes: A systematic review and meta-analysis. Diabetes & Metabolic Syndrome: Clinical Research & Reviews . 2020/09/01/ 2020;14(5):1431-1437. doi: https://doi.org/10.1016/j.dsx.2020.07.037
- Gao Y, Liu M, Shi S, et al. Cancer is associated with the severity and mortality of patients with COVID-19: a systematic review and meta-analysis. medRxiv . 2020:2020.05.01.20087031. doi:10.1101/2020.05.01.20087031
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. The Lancet Oncology . Mar 2020;21(3):335-337. doi:10.1016/s1470-2045(20)30096-6
- Nepogodiev D, Bhangu A, Glasbey JC, et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. The Lancet . 2020;396(10243):27-38. doi:10.1016/S0140-6736(20)31182-X
- Lee LY, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet . Jun 20 2020;395(10241):1919-1926. doi:10.1016/S0140-6736(20)31173-9
- Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nature medicine . Aug 2020;26(8):1218-1223. doi:10.1038/s41591-020-0979-0
- Zhang H, Wang L, Chen Y, et al. Outcomes of novel coronavirus disease 2019 (COVID-19) infection in 107 patients with cancer from Wuhan, China. Cancer . Sep 1 2020;126(17):4023-4031. doi:10.1002/cncr.33042
- Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet . Jun 20 2020;395(10241):1907-1918. doi:10.1016/S0140-6736(20)31187-9
- Wang Q, Berger NA, Xu R. Analyses of Risk, Racial Disparity, and Outcomes Among US Patients With Cancer and COVID-19 Infection. JAMA oncology . Dec 10 2020;doi:10.1001/jamaoncol.2020.6178
- Pranata R, Huang I, Lim MA, Wahjoepramono EJ, July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19-systematic review, meta-analysis, and meta-regression. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association . Aug 2020;29(8):104949. doi:10.1016/j.jstrokecerebrovasdis.2020.104949
- Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging . Apr 8 2020;12(7):6049-6057. doi:10.18632/aging.103000
- Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS One . 2020;15(8):e0238215. doi:10.1371/journal.pone.0238215
- Khan MMA, Khan MN, Mustagir MG, Rana J, Islam MS, Kabir MI. Effects of underlying morbidities on the occurrence of deaths in COVID-19 patients: A systematic review and meta-analysis. Journal of global health . Dec 2020;10(2):020503. doi:10.7189/jogh.10.020503
- Martins-Filho PR, Tavares CSS, Santos VS. Factors associated with mortality in patients with COVID-19. A quantitative evidence synthesis of clinical and laboratory data. European journal of internal medicine . Jun 2020;76:97-99. doi:10.1016/j.ejim.2020.04.043
- Chen R, Liang W, Jiang M, et al. Risk Factors of Fatal Outcome in Hospitalized Subjects With Coronavirus Disease 2019 From a Nationwide Analysis in China. Chest . Jul 2020;158(1):97-105. doi:10.1016/j.chest.2020.04.010
- Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature . Aug 2020;584(7821):430-436. doi:10.1038/s41586-020-2521-4
- Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. The Journal of infection . Jun 2020;80(6):639-645. doi:10.1016/j.jinf.2020.03.019
- Pilgram L, Eberwein L, Wille K, et al. Clinical course and predictive risk factors for fatal outcome of SARS-CoV-2 infection in patients with chronic kidney disease. Infection . Aug 2021;49(4):725-737. doi:10.1007/s15010-021-01597-7
- Kang SH, Kim SW, Kim AY, Cho KH, Park JW, Do JY. Association between Chronic Kidney Disease or Acute Kidney Injury and Clinical Outcomes in COVID-19 Patients. J Korean Med Sci . Dec 28 2020;35(50):e434. doi:10.3346/jkms.2020.35.e434
- Fajgenbaum DC, Khor JS, Gorzewski A, et al. Treatments Administered to the First 9152 Reported Cases of COVID-19: A Systematic Review. Infect Dis Ther . Sep 2020;9(3):435-449. doi:10.1007/s40121-020-00303-8
- Gottlieb M, Sansom S, Frankenberger C, Ward E, Hota B. Clinical Course and Factors Associated With Hospitalization and Critical Illness Among COVID-19 Patients in Chicago, Illinois. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine . Oct 2020;27(10):963-973. doi:10.1111/acem.14104
- Fernandes DM, Oliveira CR, Guerguis S, et al. Severe Acute Respiratory Syndrome Coronavirus 2 Clinical Syndromes and Predictors of Disease Severity in Hospitalized Children and Youth. The Journal of pediatrics . Nov 14 2020;doi:10.1016/j.jpeds.2020.11.016
- Hernandez-Galdamez DR, Gonzalez-Block MA, Romo-Duenas DK, et al. Increased Risk of Hospitalization and Death in Patients with COVID-19 and Pre-existing Noncommunicable Diseases and Modifiable Risk Factors in Mexico. Archives of Medical Research . 2020;doi: http://dx.doi.org/10.1016/j.arcmed.2020.07.003
- Menezes Soares RDC, Mattos LR, Raposo LM. Risk Factors for Hospitalization and Mortality due to COVID-19 in Espirito Santo State, Brazil. American Journal of Tropical Medicine and Hygiene . 2020;103(3):1184-1190. doi: http://dx.doi.org/10.4269/ajtmh.20-0483
- Oetjens MT, Luo JZ, Chang A, et al. Electronic health record analysis identifies kidney disease as the leading risk factor for hospitalization in confirmed COVID-19 patients. PloS one . 2020;15(11):e0242182. doi: https://dx.doi.org/10.1371/journal.pone.0242182
- Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ . May 22 2020;369:m1966. doi:10.1136/bmj.m1966
- Reilev M, Kristensen KB, Pottegard A, et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. International journal of epidemiology . 2020;doi: http://dx.doi.org/10.1093/ije/dyaa140
- Suleyman G, Fadel RA, Malette KM, et al. Clinical Characteristics and Morbidity Associated With Coronavirus Disease 2019 in a Series of Patients in Metropolitan Detroit. JAMA Netw Open . Jun 1 2020;3(6):e2012270. doi:10.1001/jamanetworkopen.2020.12270
- Rastad H, Ejtahed HS, Mahdavi-Ghorabi A, et al. Factors associated with the poor outcomes in diabetic patients with COVID-19. Journal of Diabetes and Metabolic Disorders . 2020;doi: http://dx.doi.org/10.1007/s40200-020-00646-6
- Fried MW, Crawford JM, Mospan AR, et al. Patient Characteristics and Outcomes of 11,721 Patients with COVID19 Hospitalized Across the United States. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America . 2020;doi: http://dx.doi.org/10.1093/cid/ciaa1268
- Kolhe NV, Fluck RJ, Selby NM, Taal MW. Acute kidney injury associated with COVID-19: A retrospective cohort study. PLoS Med . Oct 2020;17(10):e1003406. doi:10.1371/journal.pmed.1003406
- Bowe B, Cai M, Xie Y, Gibson AK, Maddukuri G, Al-Aly Z. Acute Kidney Injury in a National Cohort of Hospitalized US Veterans with COVID-19. Clin J Am Soc Nephrol . Nov 16 2020;doi:10.2215/CJN.09610620
- McKeigue PM, Weir A, Bishop J, et al. Rapid Epidemiological Analysis of Comorbidities and Treatments as risk factors for COVID-19 in Scotland (REACT-SCOT): A population-based case-control study. PLoS medicine . 2020;17(10):e1003374. doi: https://dx.doi.org/10.1371/journal.pmed.1003374
- Boulle A, Davies MA, Hussey H, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis . Aug 29 2020;doi:10.1093/cid/ciaa1198
- Parra-Bracamonte GM, Lopez-Villalobos N, Parra-Bracamonte FE. Clinical characteristics and risk factors for mortality of patients with COVID-19 in a large data set from Mexico. Annals of Epidemiology . 2020;doi: http://dx.doi.org/10.1016/j.annepidem.2020.08.005
- Ng JH, Hirsch JS, Wanchoo R, et al. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney international . 2020;doi: http://dx.doi.org/10.1016/j.kint.2020.07.030
- Omrani AS, Almaslamani MA, Daghfal J, et al. The first consecutive 5000 patients with Coronavirus Disease 2019 from Qatar; a nation-wide cohort study. BMC Infectious Diseases . 2020;20(1):777. doi: http://dx.doi.org/10.1186/s12879-020-05511-8
- Iaccarino G, Borghi C, Carugo S, et al. Gender differences in predictors of intensive care units admission among COVID-19 patients: The results of the SARS-RAS study of the italian society of hypertension. PLoS ONE . 2020;15(10 October):e0237297. doi: http://dx.doi.org/10.1371/journal.pone.0237297
- Gu T, Chu Q, Yu Z, et al. History of coronary heart disease increased the mortality rate of patients with COVID-19: a nested case-control study. BMJ open . Sep 17 2020;10(9):e038976. doi:10.1136/bmjopen-2020-038976
- Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of Hospitalized Adults With COVID-19 in an Integrated Health Care System in California. Jama . Jun 2 2020;323(21):2195-2198. doi:10.1001/jama.2020.7202
- Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int . Jul 2020;98(1):209-218. doi:10.1016/j.kint.2020.05.006
- Gold JAW, Wong KK, Szablewski CM, et al. Characteristics and Clinical Outcomes of Adult Patients Hospitalized with COVID-19 – Georgia, March 2020. MMWR Morb Mortal Wkly Rep . May 8 2020;69(18):545-550. doi:10.15585/mmwr.mm6918e1
- Jering KS, Claggett BL, Cunningham JW, et al. Clinical Characteristics and Outcomes of Hospitalized Women Giving Birth With and Without COVID-19. JAMA Intern Med . Jan 15 2021;doi:10.1001/jamainternmed.2020.9241
- Garg S, Kim L, Whitaker M, et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 – COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep . Apr 17 2020;69(15):458-464. doi:10.15585/mmwr.mm6915e3
- Richardson S, Hirsch JS, Narasimhan M, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA . 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775 %J JAMA
- Lee JY, Hong SW, Hyun M, et al. Epidemiological and clinical characteristics of coronavirus disease 2019 in Daegu, South Korea. Int J Infect Dis . Sep 2020;98:462-466. doi:10.1016/j.ijid.2020.07.017
- Fadini GP, Morieri ML, Boscari F, et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res Clin Pract . Oct 2020;168:108374. doi:10.1016/j.diabres.2020.108374
- Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol . Oct 2020;8(10):813-822. doi:10.1016/s2213-8587(20)30272-2
- Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 Severity Is Tripled in the Diabetes Community: A Prospective Analysis of the Pandemic’s Impact in Type 1 and Type 2 Diabetes. Diabetes Care . Dec 2 2020;doi:10.2337/dc20-2260
- Duarte-Salles T, Vizcaya D, Pistillo A, et al. Baseline characteristics, management, and outcomes of 55,270 children and adolescents diagnosed with COVID-19 and 1,952,693 with influenza in France, Germany, Spain, South Korea and the United States: an international network cohort study. medRxiv . Oct 30 2020;doi:10.1101/2020.10.29.20222083
- Bode B, Garrett V, Messler J, et al. Glycemic Characteristics and Clinical Outcomes of COVID-19 Patients Hospitalized in the United States. Journal of diabetes science and technology . Jul 2020;14(4):813-821. doi:10.1177/1932296820924469
- Vangoitsenhoven R, Martens P-J, van Nes F, et al. No Evidence of Increased Hospitalization Rate for COVID-19 in Community-Dwelling Patients With Type 1 Diabetes. 2020;43(10):e118-e119. doi:10.2337/dc20-1246 %J Diabetes Care
- Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes . Nov 17 2020;doi:10.1111/pedi.13158
- Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. Journal of endocrinological investigation . Jun 2020;43(6):867-869. doi:10.1007/s40618-020-01236-2
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ . Sep 1 2020;370:m3320. doi:10.1136/bmj.m3320
- Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. Cmaj . Apr 19 2021;193(16):E540-e548. doi:10.1503/cmaj.202604
- Zhu L, She ZG, Cheng X, et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab . Jun 2 2020;31(6):1068-1077.e3. doi:10.1016/j.cmet.2020.04.021
- Chen Y, Yang D, Cheng B, et al. Clinical Characteristics and Outcomes of Patients With Diabetes and COVID-19 in Association With Glucose-Lowering Medication. Diabetes Care . Jul 2020;43(7):1399-1407. doi:10.2337/dc20-0660
- Sathish T, Kapoor N, Cao Y, Tapp RJ, Zimmet P. Proportion of newly diagnosed diabetes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Obes Metab . Nov 27 2020;doi:10.1111/dom.14269
- de Almeida-Pititto B, Dualib PM, Zajdenverg L, et al. Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: a meta-analysis. Diabetol Metab Syndr . 2020;12:75. doi:10.1186/s13098-020-00586-4
- Kow CS, Hasan SS. Mortality risk with preadmission metformin use in patients with COVID-19 and diabetes: A meta-analysis. J Med Virol . Sep 9 2020;doi:10.1002/jmv.26498
- Perez-Belmonte LM, Torres-Pena JD, Lopez-Carmona MD, et al. Mortality and other adverse outcomes in patients with type 2 diabetes mellitus admitted for COVID-19 in association with glucose-lowering drugs: a nationwide cohort study. BMC Med . Nov 16 2020;18(1):359. doi:10.1186/s12916-020-01832-2
- Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. The Journal of infection . Aug 2020;81(2):e16-e25. doi:10.1016/j.jinf.2020.04.021
- Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis . May 2020;94:91-95. doi:10.1016/j.ijid.2020.03.017
- Del Sole F, Farcomeni A, Loffredo L, et al. Features of severe COVID-19: A systematic review and meta-analysis. European journal of clinical investigation . 2020;50(10):e13378. doi: https://doi.org/10.1111/eci.13378
- Ssentongo P, Heilbrunn ES, Ssentongo AE, et al. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: a systematic review and meta-analysis. Scientific Reports . 2021/03/18 2021;11(1):6283. doi:10.1038/s41598-021-85359-3
- Bhaskaran K, Rentsch CT, MacKenna B, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV . Dec 11 2020;doi:10.1016/s2352-3018(20)30305-2
- Hadi YB, Naqvi SFZ, Kupec JT, Sarwari AR. Characteristics and outcomes of COVID-19 in patients with HIV: a multicentre research network study. AIDS (London, England) . 2020;34(13):F3-F8. doi:10.1097/qad.0000000000002666
- Miyashita H, Kuno T. Prognosis of coronavirus disease 2019 (COVID-19) in patients with HIV infection in New York City. HIV medicine . 2021;22(1):e1-e2. doi: https://doi.org/10.1111/hiv.12920
- Härter G, Spinner CD, Roider J, et al. COVID-19 in people living with human immunodeficiency virus: a case series of 33 patients. Infection . Oct 2020;48(5):681-686. doi:10.1007/s15010-020-01438-z
- Altuntas Aydin O, Kumbasar Karaosmanoglu H, Kart Yasar K. HIV/SARS-CoV-2 coinfected patients in Istanbul, Turkey. J Med Virol . Nov 2020;92(11):2288-2290. doi:10.1002/jmv.25955
- Ho H-e, Peluso MJ, Margus C, et al. Clinical Outcomes and Immunologic Characteristics of Coronavirus Disease 2019 in People With Human Immunodeficiency Virus. The Journal of Infectious Diseases . 2020;223(3):403-408. doi:10.1093/infdis/jiaa380
- Fond G, Nemani K, Etchecopar-Etchart D, et al. Association Between Mental Health Disorders and Mortality Among Patients With COVID-19 in 7 Countries: A Systematic Review and Meta-analysis. JAMA Psychiatry . 2021;doi:10.1001/jamapsychiatry.2021.2274
- Ceban F, Nogo D, Carvalho IP, et al. Association Between Mood Disorders and Risk of COVID-19 Infection, Hospitalization, and Death: A Systematic Review and Meta-analysis. JAMA Psychiatry . Oct 1 2021;78(10):1079-1091. doi:10.1001/jamapsychiatry.2021.1818
- Herman C, Mayer K, Sarwal A. Scoping review of prevalence of neurologic comorbidities in patients hospitalized for COVID-19. Neurology . Jul 14 2020;95(2):77-84. doi:10.1212/wnl.0000000000009673
- Zuin M, Guasti P, Roncon L, Cervellati C, Zuliani G. Dementia and the risk of death in elderly patients with COVID-19 infection: Systematic review and meta-analysis. International Journal of Geriatric Psychiatry . 2021;36(5):697-703. doi: https://doi.org/10.1002/gps.5468
- Liu N, Sun J, Wang X, Zhao M, Huang Q, Li H. The Impact of Dementia on the Clinical Outcome of COVID-19: A Systematic Review and Meta-Analysis. Journal of Alzheimer’s Disease . 2020;78:1775-1782. doi:10.3233/JAD-201016
- Saragih ID, Saragih IS, Batubara SO, Lin C-J. Dementia as a mortality predictor among older adults with COVID-19: A systematic review and meta-analysis of observational study. Geriatric Nursing . 2021/09/01/ 2021;42(5):1230-1239. doi: https://doi.org/10.1016/j.gerinurse.2021.03.007
- Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and Outcomes of Children With Coronavirus Disease 2019 (COVID-19) Infection Admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr . Sep 1 2020;174(9):868-873. doi:10.1001/jamapediatrics.2020.1948
- Parri N, Lenge M, Buonsenso D. Children with Covid-19 in Pediatric Emergency Departments in Italy. New England Journal of Medicine . 2020;383(2):187-190. doi:10.1056/NEJMc2007617
- Yang J, Hu J, Zhu C. Obesity aggravates COVID-19: A systematic review and meta-analysis. J Med Virol . Jun 30 2020;doi:10.1002/jmv.26237
- Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 Infection and Pediatric Comorbidities: A Systematic Review and Meta-Analysis. Int J Infect Dis . Nov 20 2020;103:246-256. doi:10.1016/j.ijid.2020.11.163
- Földi M, Farkas N, Kiss S, et al. Obesity is a risk factor for developing critical condition in COVID-19 patients: A systematic review and meta-analysis. Obesity Reviews . 2020;21(10):e13095. doi: https://doi.org/10.1111/obr.13095
- Lighter J, Phillips M, Hochman S, et al. Obesity in Patients Younger Than 60 Years Is a Risk Factor for COVID-19 Hospital Admission. Clin Infect Dis . Jul 28 2020;71(15):896-897. doi:10.1093/cid/ciaa415
- Tartof SY, Qian L, Hong V, et al. Obesity and Mortality Among Patients Diagnosed With COVID-19: Results From an Integrated Health Care Organization. Ann Intern Med . Nov 17 2020;173(10):773-781. doi:10.7326/m20-3742
- Hur K, Price CPE, Gray EL, et al. Factors Associated With Intubation and Prolonged Intubation in Hospitalized Patients With COVID-19. Otolaryngology–head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery . Jul 2020;163(1):170-178. doi:10.1177/0194599820929640
- Simonnet A, Chetboun M, Poissy J, et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity (Silver Spring) . Jul 2020;28(7):1195-1199. doi:10.1002/oby.22831
- Aziz F, Mandelbrot D, Singh T, et al. Early Report on Published Outcomes in Kidney Transplant Recipients Compared to Nontransplant Patients Infected With Coronavirus Disease 2019. Transplantation proceedings . Nov 2020;52(9):2659-2662. doi:10.1016/j.transproceed.2020.07.002
- Ko JY, Danielson ML, Town M, et al. Risk Factors for COVID-19-associated hospitalization: COVID-19-Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clin Infect Dis . Sep 18 2020;doi:10.1093/cid/ciaa1419
- Nakeshbandi M, Maini R, Daniel P, et al. The impact of obesity on COVID-19 complications: a retrospective cohort study. International journal of obesity (2005) . Sep 2020;44(9):1832-1837. doi:10.1038/s41366-020-0648-x
- Hamer M, Gale CR, Kivimaki M, Batty GD. Overweight, obesity, and risk of hospitalization for COVID-19: A community-based cohort study of adults in the United Kingdom. Proc Natl Acad Sci U S A . Sep 1 2020;117(35):21011-21013. doi:10.1073/pnas.2011086117
- Palaiodimos L, Kokkinidis DG, Li W, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism: clinical and experimental . Jul 2020;108:154262. doi:10.1016/j.metabol.2020.154262
- Di Martino D, Chiaffarino F, Patanè L, et al. Assessing risk factors for severe forms of COVID-19 in a pregnant population: A clinical series from Lombardy, Italy. International Journal of Gynecology & Obstetrics . 2021;152(2):275-277. doi: https://doi.org/10.1002/ijgo.13435
- Khoury R, Bernstein PS, Debolt C, et al. Characteristics and Outcomes of 241 Births to Women With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection at Five New York City Medical Centers. Obstet Gynecol . Aug 2020;136(2):273-282. doi:10.1097/AOG.0000000000004025
- Metz TD, Clifton RG, Hughes BL, et al. Disease Severity and Perinatal Outcomes of Pregnant Patients With Coronavirus Disease 2019 (COVID-19). Obstet Gynecol . Apr 1 2021;137(4):571-580. doi:10.1097/AOG.0000000000004339
- Galang RR, Newton SM, Woodworth KR, et al. Risk factors for illness severity among pregnant women with confirmed SARS-CoV-2 infection – Surveillance for Emerging Threats to Mothers and Babies Network, 20 state, local, and territorial health departments, March 29, 2020 -January 8, 2021. medRxiv . 2021:2021.02.27.21252169. doi:10.1101/2021.02.27.21252169
- Yang Z, Wang M, Zhu Z, Liu Y. Coronavirus disease 2019 (COVID-19) and pregnancy: a systematic review. The Journal of Maternal-Fetal & Neonatal Medicine . 2022/04/18 2022;35(8):1619-1622. doi:10.1080/14767058.2020.1759541
- Collin J, Bystrom E, Carnahan A, Ahrne M. Public Health Agency of Sweden’s Brief Report: Pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand . Jul 2020;99(7):819-822. doi:10.1111/aogs.13901
- Li N, Han L, Peng M, et al. Maternal and Neonatal Outcomes of Pregnant Women With Coronavirus Disease 2019 (COVID-19) Pneumonia: A Case-Control Study. Clin Infect Dis . Nov 19 2020;71(16):2035-2041. doi:10.1093/cid/ciaa352
- Chen L, Li Q, Zheng D, et al. Clinical Characteristics of Pregnant Women with Covid-19 in Wuhan, China. New England Journal of Medicine . 2020;382(25):e100. doi:10.1056/NEJMc2009226
- Breslin N, Baptiste C, Gyamfi-Bannerman C, et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. American Journal of Obstetrics & Gynecology MFM . 2020/05/01/ 2020;2(2, Supplement):100118. doi: https://doi.org/10.1016/j.ajogmf.2020.100118
- Lokken EM, Walker CL, Delaney S, et al. Clinical characteristics of 46 pregnant women with a severe acute respiratory syndrome coronavirus 2 infection in Washington State. American Journal of Obstetrics and Gynecology . 2020/12/01/ 2020;223(6):911.e1-911.e14. doi: https://doi.org/10.1016/j.ajog.2020.05.031
- Pierce-Williams RAM, Burd J, Felder L, et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. Am J Obstet Gynecol MFM . Aug 2020;2(3):100134. doi:10.1016/j.ajogmf.2020.100134
- Savasi VM, Parisi F, Patane L, et al. Clinical Findings and Disease Severity in Hospitalized Pregnant Women With Coronavirus Disease 2019 (COVID-19). Obstet Gynecol . Aug 2020;136(2):252-258. doi:10.1097/AOG.0000000000003979
- Ellington S, Strid P, Tong VT, et al. Characteristics of Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status – United States, January 22-June 7, 2020. MMWR Morb Mortal Wkly Rep . Jun 26 2020;69(25):769-775. doi:10.15585/mmwr.mm6925a1
- Zambrano LD, Ellington S, Strid P, et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status – United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep . Nov 6 2020;69(44):1641-1647. doi:10.15585/mmwr.mm6944e3
- Lippi G, Henry BM. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respir Med . Jun 2020;167:105941. doi:10.1016/j.rmed.2020.105941
- Patanavanich R, Glantz SA. Smoking Is Associated With COVID-19 Progression: A Meta-analysis. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco . Aug 24 2020;22(9):1653-1656. doi:10.1093/ntr/ntaa082
- Guo FR. Active smoking is associated with severity of coronavirus disease 2019 (COVID-19): An update of a meta-analysis. Tobacco induced diseases . 2020;18:37. doi:10.18332/tid/121915
- Zhao Q, Meng M, Kumar R, et al. The impact of COPD and smoking history on the severity of COVID-19: A systemic review and meta-analysis. J Med Virol . Oct 2020;92(10):1915-1921. doi:10.1002/jmv.25889
- Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). European journal of internal medicine . May 2020;75:107-108. doi:10.1016/j.ejim.2020.03.014
- Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, Severity and Mortality associated with COPD and Smoking in patients with COVID-19: A Rapid Systematic Review and Meta-Analysis. PloS one . 2020;15(5):e0233147. doi:10.1371/journal.pone.0233147
- Li J, He X, Yuan Y, et al. Meta-analysis investigating the relationship between clinical features, outcomes, and severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia. American journal of infection control . Jan 2021;49(1):82-89. doi:10.1016/j.ajic.2020.06.008
- Farsalinos K, Barbouni A, Poulas K, Polosa R, Caponnetto P, Niaura R. Current smoking, former smoking, and adverse outcome among hospitalized COVID-19 patients: a systematic review and meta-analysis. Therapeutic advances in chronic disease . 2020;11:2040622320935765. doi:10.1177/2040622320935765
- Sanchez-Ramirez DC, Mackey D. Underlying respiratory diseases, specifically COPD, and smoking are associated with severe COVID-19 outcomes: A systematic review and meta-analysis. Respir Med . Sep 2020;171:106096. doi:10.1016/j.rmed.2020.106096
- Akalin E, Azzi Y, Bartash R, et al. Covid-19 and Kidney Transplantation. N Engl J Med . Jun 18 2020;382(25):2475-2477. doi:10.1056/NEJMc2011117
- Ketcham SW, Adie SK, Malliett A, et al. Coronavirus Disease-2019 in Heart Transplant Recipients in Southeastern Michigan: A Case Series. J Card Fail . Jun 2020;26(6):457-461. doi:10.1016/j.cardfail.2020.05.008
- Latif F, Farr MA, Clerkin KJ, et al. Characteristics and Outcomes of Recipients of Heart Transplant With Coronavirus Disease 2019. JAMA cardiology . Oct 1 2020;5(10):1165-1169. doi:10.1001/jamacardio.2020.2159
- Zhu L, Xu X, Ma K, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant . Jul 2020;20(7):1859-1863. doi:10.1111/ajt.15869
- Fernández-Ruiz M, Andrés A, Loinaz C, et al. COVID-19 in solid organ transplant recipients: A single-center case series from Spain. American Journal of Transplantation . 2020;20(7):1849-1858. doi: https://doi.org/10.1111/ajt.15929
- Travi G, Rossotti R, Merli M, et al. Clinical outcome in solid organ transplant recipients with COVID-19: A single-center experience. Am J Transplant . Sep 2020;20(9):2628-2629. doi:10.1111/ajt.16069
- Tschopp J, L’Huillier AG, Mombelli M, et al. First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study. Am J Transplant . Oct 2020;20(10):2876-2882. doi:10.1111/ajt.16062
- Yi SG, Rogers AW, Saharia A, et al. Early Experience With COVID-19 and Solid Organ Transplantation at a US High-volume Transplant Center. Transplantation . 2020;104(11):2208-2214.
- Fung M, Chiu CY, DeVoe C, et al. Clinical outcomes and serologic response in solid organ transplant recipients with COVID-19: A case series from the United States. American Journal of Transplantation . 2020;20(11):3225-3233. doi: https://doi.org/10.1111/ajt.16079
- Hoek RAS, Manintveld OC, Betjes MGH, et al. COVID-19 in solid organ transplant recipients: a single-center experience. Transpl Int . 2020;33(9):1099-1105.
- Iacovoni A, Boffini M, Pidello S, et al. A case series of novel coronavirus infection in heart transplantation from 2 centers in the pandemic area in the North of Italy. J Heart Lung Transplant . 2020;39(10):1081-1088.
- Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant . 2020;20(7):1800-1808.
- Kates OS, Haydel BM, Florman SS, et al. Coronavirus Disease 2019 in Solid Organ Transplant: A Multicenter Cohort Study. Clin Infect Dis . Dec 6 2021;73(11):e4090-e4099. doi:10.1093/cid/ciaa1097
- Sharma A, Bhatt NS, St Martin A, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. The Lancet Haematology . Mar 2021;8(3):e185-e193. doi:10.1016/s2352-3026(20)30429-4
- Ljungman P, de la Camara R, Mikulska M, et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia . 2021/10/01 2021;35(10):2885-2894. doi:10.1038/s41375-021-01302-5
- Jering KS, McGrath MM, Mc Causland FR, Claggett B, Cunningham JW, Solomon SD. Excess mortality in solid organ transplant recipients hospitalized with COVID-19: A large-scale comparison of SOT recipients hospitalized with or without COVID-19. Clin Transplant . Jan 2022;36(1):e14492. doi:10.1111/ctr.14492
- Yekedüz E, Utkan G, Ürün Y. A systematic review and meta-analysis: the effect of active cancer treatment on severity of COVID-19. Eur J Cancer . Dec 2020;141:92-104. doi:10.1016/j.ejca.2020.09.028
- Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, But Not TNF Antagonists, Are Associated With Adverse COVID-19 Outcomes in Patients With Inflammatory Bowel Diseases: Results From an International Registry. Gastroenterology . Aug 2020;159(2):481-491.e3. doi:10.1053/j.gastro.2020.05.032
- Michelena X, Borrell H, López-Corbeto M, et al. Incidence of COVID-19 in a cohort of adult and paediatric patients with rheumatic diseases treated with targeted biologic and synthetic disease-modifying anti-rheumatic drugs. Seminars in arthritis and rheumatism . Aug 2020;50(4):564-570. doi:10.1016/j.semarthrit.2020.05.001
- Di Giorgio A, Nicastro E, Speziani C, et al. Health status of patients with autoimmune liver disease during SARS-CoV-2 outbreak in northern Italy. Journal of hepatology . Sep 2020;73(3):702-705. doi:10.1016/j.jhep.2020.05.008
- Marlais M, Wlodkowski T, Vivarelli M, et al. The severity of COVID-19 in children on immunosuppressive medication. The Lancet Child & adolescent health . Jul 2020;4(7):e17-e18. doi:10.1016/s2352-4642(20)30145-0
- Montero-Escribano P, Matías-Guiu J, Gómez-Iglesias P, Porta-Etessam J, Pytel V, Matias-Guiu JA. Anti-CD20 and COVID-19 in multiple sclerosis and related disorders: A case series of 60 patients from Madrid, Spain. Multiple sclerosis and related disorders . Jul 2020;42:102185. doi:10.1016/j.msard.2020.102185
- Alsaied T, Aboulhosn JA, Cotts TB, et al. Coronavirus Disease 2019 (COVID-19) Pandemic Implications in Pediatric and Adult Congenital Heart Disease. J Am Heart Assoc . Jun 16 2020;9(12):e017224. doi:10.1161/jaha.120.017224
- Sanna G, Serrau G, Bassareo PP, Neroni P, Fanos V, Marcialis MA. Children’s heart and COVID-19: Up-to-date evidence in the form of a systematic review. Eur J Pediatr . Jul 2020;179(7):1079-1087. doi:10.1007/s00431-020-03699-0
- Sabatino J, Ferrero P, Chessa M, et al. COVID-19 and Congenital Heart Disease: Results from a Nationwide Survey. J Clin Med . Jun 8 2020;9(6)doi:10.3390/jcm9061774
- Bellino S, Punzo O, Rota MC, et al. COVID-19 Disease Severity Risk Factors for Pediatric Patients in Italy. Pediatrics . Oct 2020;146(4)doi:10.1542/peds.2020-009399
- DeBiasi RL, Song X, Delaney M, et al. Severe Coronavirus Disease-2019 in Children and Young Adults in the Washington, DC, Metropolitan Region. The Journal of pediatrics . Aug 2020;223:199-203.e1. doi:10.1016/j.jpeds.2020.05.007
- Chao JY, Derespina KR, Herold BC, et al. Clinical Characteristics and Outcomes of Hospitalized and Critically Ill Children and Adolescents with Coronavirus Disease 2019 at a Tertiary Care Medical Center in New York City. The Journal of pediatrics . Aug 2020;223:14-19.e2. doi:10.1016/j.jpeds.2020.05.006
- Kim DW, Byeon KH, Kim J, Cho KD, Lee N. The Correlation of Comorbidities on the Mortality in Patients with COVID-19: an Observational Study Based on the Korean National Health Insurance Big Data. J Korean Med Sci . Jul 6 2020;35(26):e243. doi:10.3346/jkms.2020.35.e243
- González-Dambrauskas S, Vásquez-Hoyos P, Camporesi A, et al. Pediatric Critical Care and COVID-19. Pediatrics . Sep 2020;146(3)doi:10.1542/peds.2020-1766
- Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health . Sep 2020;4(9):653-661. doi:10.1016/s2352-4642(20)30177-2
- Zachariah P, Johnson CL, Halabi KC, et al. Epidemiology, Clinical Features, and Disease Severity in Patients With Coronavirus Disease 2019 (COVID-19) in a Children’s Hospital in New York City, New York. JAMA Pediatr . Oct 1 2020;174(10):e202430. doi:10.1001/jamapediatrics.2020.2430
- Verma S, Lumba R, Dapul HM, et al. Characteristics of Hospitalized Children With SARS-CoV-2 in the New York City Metropolitan Area. Hosp Pediatr . Jan 2021;11(1):71-78. doi:10.1542/hpeds.2020-001917
- Leon-Abarca JA. Obesity and immunodeficiencies are the main pre-existing conditions associated with mild to moderate COVID-19 in children. Pediatr Obes . Dec 2020;15(12):e12713. doi:10.1111/ijpo.12713
- Oualha M, Bendavid M, Berteloot L, et al. Severe and fatal forms of COVID-19 in children. Archives de pediatrie : organe officiel de la Societe francaise de pediatrie . Jul 2020;27(5):235-238. doi:10.1016/j.arcped.2020.05.010
- Heilbronner C, Berteloot L, Tremolieres P, et al. Patients with sickle cell disease and suspected COVID-19 in a paediatric intensive care unit. British journal of haematology . 2020;190(1):e21-e24. doi: https://doi.org/10.1111/bjh.16802
- Arlet J-B, de Luna G, Khimoud D, et al. Prognosis of patients with sickle cell disease and COVID-19: a French experience. The Lancet Haematology . 2020/09/01/ 2020;7(9):e632-e634. doi: https://doi.org/10.1016/S2352-3026(20)30204-0
- Odièvre MH, de Marcellus C, Ducou Le Pointe H, et al. Dramatic improvement after tocilizumab of severe COVID-19 in a child with sickle cell disease and acute chest syndrome. American journal of hematology . Aug 2020;95(8):E192-e194. doi:10.1002/ajh.25855
- McCloskey KA, Meenan J, Hall R, Tsitsikas DA. COVID-19 infection and sickle cell disease: a UK centre experience. British journal of haematology . Jul 2020;190(2):e57-e58. doi:10.1111/bjh.16779
- Nur E, Gaartman AE, van Tuijn CFJ, Tang MW, Biemond BJ. Vaso-occlusive crisis and acute chest syndrome in sickle cell disease due to 2019 novel coronavirus disease (COVID-19). American journal of hematology . Jun 2020;95(6):725-726. doi:10.1002/ajh.25821
- Hussain FA, Njoku FU, Saraf SL, Molokie RE, Gordeuk VR, Han J. COVID-19 infection in patients with sickle cell disease. British journal of haematology . Jun 2020;189(5):851-852. doi:10.1111/bjh.16734
- Panepinto JA, Brandow A, Mucalo L, et al. Coronavirus Disease among Persons with Sickle Cell Disease, United States, March 20-May 21, 2020. Emerg Infect Dis . Oct 2020;26(10):2473-2476. doi:10.3201/eid2610.202792
- Al-Hebshi A, Zolaly M, Alshengeti A, et al. A Saudi family with sickle cell disease presented with acute crises and COVID-19 infection. Pediatric blood & cancer . Sep 2020;67(9):e28547. doi:10.1002/pbc.28547
- Allison D, Campbell-Lee S, Crane J, et al. Red blood cell exchange to avoid intubating a COVID-19 positive patient with sickle cell disease? Journal of clinical apheresis . Aug 2020;35(4):378-381. doi:10.1002/jca.21809
- Appiah-Kubi A, Acharya S, Fein Levy C, et al. Varying presentations and favourable outcomes of COVID-19 infection in children and young adults with sickle cell disease: an additional case series with comparisons to published cases. British journal of haematology . Aug 2020;190(4):e221-e224. doi:10.1111/bjh.17013
- Azerad MA, Bayoudh F, Weber T, et al. Sickle cell disease and COVID-19: Atypical presentations and favorable outcomes. EJHaem . Aug 4 2020;doi:10.1002/jha2.74
- Chakravorty S, Padmore-Payne G, Ike F, et al. COVID-19 in patients with sickle cell disease – a case series from a UK Tertiary Hospital. Haematologica . Jun 11 2020;105(11)doi:10.3324/haematol.2020.254250
- De Luna G, Habibi A, Deux JF, et al. Rapid and severe Covid-19 pneumonia with severe acute chest syndrome in a sickle cell patient successfully treated with tocilizumab. American journal of hematology . Jul 2020;95(7):876-878. doi:10.1002/ajh.25833
- Ershler WB, Holbrook ME. Sickle cell anemia and COVID-19: Use of voxelotor to avoid transfusion. Transfusion . Dec 2020;60(12):3066-3067. doi:10.1111/trf.16068
- Jacob S, Dworkin A, Romanos-Sirakis E. A pediatric patient with sickle cell disease presenting with severe anemia and splenic sequestration in the setting of COVID-19. Pediatric blood & cancer . Dec 2020;67(12):e28511. doi:10.1002/pbc.28511
- Justino CC, Campanharo FF, Augusto MN, Morais SC, Figueiredo MS. COVID-19 as a trigger of acute chest syndrome in a pregnant woman with sickle cell anemia. Hematology, transfusion and cell therapy . Jul-Sep 2020;42(3):212-214. doi:10.1016/j.htct.2020.06.003
- Morrone KA, Strumph K, Liszewski MJ, et al. Acute chest syndrome in the setting of SARS-COV-2 infections-A case series at an urban medical center in the Bronx. Pediatric blood & cancer . Nov 2020;67(11):e28579. doi:10.1002/pbc.28579
- Balanchivadze N, Kudirka AA, Askar S, et al. Impact of COVID-19 Infection on 24 Patients with Sickle Cell Disease. One Center Urban Experience, Detroit, MI, USA. Hemoglobin . Jul 2020;44(4):284-289. doi:10.1080/03630269.2020.1797775
- Allen B, El Shahawy O, Rogers ES, Hochman S, Khan MR, Krawczyk N. Association of substance use disorders and drug overdose with adverse COVID-19 outcomes in New York City: January-October 2020. Journal of public health (Oxford, England) . Dec 26 2020;doi:10.1093/pubmed/fdaa241
- Ji W, Huh K, Kang M, et al. Effect of Underlying Comorbidities on the Infection and Severity of COVID-19 in Korea: a Nationwide Case-Control Study. J Korean Med Sci . Jun 29 2020;35(25):e237. doi:10.3346/jkms.2020.35.e237
- Wang QQ, Kaelber DC, Xu R, Volkow ND. COVID-19 risk and outcomes in patients with substance use disorders: analyses from electronic health records in the United States. Molecular psychiatry . Sep 14 2020:1-10. doi:10.1038/s41380-020-00880-7
- Lee SW, Yang JM, Moon SY, et al. Association between mental illness and COVID-19 susceptibility and clinical outcomes in South Korea: a nationwide cohort study. The lancet Psychiatry . Dec 2020;7(12):1025-1031. doi:10.1016/s2215-0366(20)30421-1
- Baillargeon J, Polychronopoulou E, Kuo YF, Raji MA. The Impact of Substance Use Disorder on COVID-19 Outcomes. Psychiatr Serv . Nov 3 2020:appips202000534. doi:10.1176/appi.ps.202000534
- Matsushita K, Ding N, Kou M, et al. The Relationship of COVID-19 Severity with Cardiovascular Disease and Its Traditional Risk Factors: A Systematic Review and Meta-Analysis. Global heart . Sep 22 2020;15(1):64. doi:10.5334/gh.814
- Wu T, Zuo Z, Kang S, et al. Multi-organ Dysfunction in Patients with COVID-19: A Systematic Review and Meta-analysis. Aging and disease . Jul 2020;11(4):874-894. doi:10.14336/ad.2020.0520
- Guo X, Zhu Y, Hong Y. Decreased Mortality of COVID-19 With Renin-Angiotensin-Aldosterone System Inhibitors Therapy in Patients With Hypertension: A Meta-Analysis. Hypertension . Aug 2020;76(2):e13-e14. doi:10.1161/HYPERTENSIONAHA.120.15572
- Zhang J, Wu J, Sun X, et al. Association of hypertension with the severity and fatality of SARS-CoV-2 infection: A meta-analysis. Epidemiol Infect . May 28 2020;148:e106. doi:10.1017/S095026882000117X
- Pranata R, Lim MA, Huang I, Raharjo SB, Lukito AA. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: A systematic review, meta-analysis and meta-regression. Journal of the renin-angiotensin-aldosterone system : JRAAS . Apr-Jun 2020;21(2):1470320320926899. doi:10.1177/1470320320926899
- Javanmardi F, Keshavarzi A, Akbari A, Emami A, Pirbonyeh N. Prevalence of underlying diseases in died cases of COVID-19: A systematic review and meta-analysis. PLoS One . 2020;15(10):e0241265. doi:10.1371/journal.pone.0241265
- Iaccarino G, Grassi G, Borghi C, et al. Age and Multimorbidity Predict Death Among COVID-19 Patients: Results of the SARS-RAS Study of the Italian Society of Hypertension. Hypertension . 08 2020;76(2):366-372. doi: https://dx.doi.org/10.1161/HYPERTENSIONAHA.120.15324
- Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. The European respiratory journal . May 2020;55(5)doi:10.1183/13993003.00547-2020
- Kim L, Garg S, O’Halloran A, et al. Risk Factors for Intensive Care Unit Admission and In-hospital Mortality among Hospitalized Adults Identified through the U.S. Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin Infect Dis . Jul 16 2020;doi:10.1093/cid/ciaa1012
- Ran J, Song Y, Zhuang Z, et al. Blood pressure control and adverse outcomes of COVID-19 infection in patients with concomitant hypertension in Wuhan, China. Hypertension research : official journal of the Japanese Society of Hypertension . Nov 2020;43(11):1267-1276. doi:10.1038/s41440-020-00541-w
- Yanover C, Mizrahi B, Kalkstein N, et al. What Factors Increase the Risk of Complications in SARS-CoV-2-Infected Patients? A Cohort Study in a Nationwide Israeli Health Organization. JMIR public health and surveillance . Aug 25 2020;6(3):e20872. doi:10.2196/20872
- Killerby ME, Link-Gelles R, Haight SC, et al. Characteristics Associated with Hospitalization Among Patients with COVID-19 – Metropolitan Atlanta, Georgia, March-April 2020. MMWR Morb Mortal Wkly Rep . Jun 26 2020;69(25):790-794. doi:10.15585/mmwr.mm6925e1
- Chen R, Yang J, Gao X, et al. Influence of blood pressure control and application of renin-angiotensin-aldosterone system inhibitors on the outcomes in COVID-19 patients with hypertension. J Clin Hypertens (Greenwich) . Nov 2020;22(11):1974-1983. doi:10.1111/jch.14038
- Kompaniyets L, Pennington AF, Goodman AB, et al. Underlying Medical Conditions and Severe Illness Among 540,667 Adults Hospitalized With COVID-19, March 2020-March 2021. Preventing chronic disease . Jul 1 2021;18:E66. doi:10.5888/pcd18.210123
To receive email updates about COVID-19, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
A systematic review into the assessment of medical apps: motivations, challenges, recommendations and methodological aspect
- Review Paper
- Published: 23 June 2020
- Volume 10 , pages 1045–1061, ( 2020 )
Cite this article

- A. H. Alamoodi ORCID: orcid.org/0000-0003-4393-5570 1 ,
- Salem Garfan 1 ,
- B. B. Zaidan 1 ,
- A. A. Zaidan 1 ,
- Moceheb Lazam Shuwandy 2 ,
- Mussab Alaa 1 ,
- M. A. Alsalem 3 ,
- Ali Mohammed 4 ,
- A. M. Aleesa 5 ,
- O. S. Albahri 1 ,
- Ward Ahmed Al-Hussein 6 &
- O. R. Alobaidi 4
1288 Accesses
28 Citations
5 Altmetric
Explore all metrics
Recent years have shown significantly pervasive interest in mobile applications (hereinafter “apps”). The number and popularity of these apps are dramatically increasing. Even though mobile apps are diverse, countless ones are available through many platforms. Some of these apps are not useful nor do they possess rich content, which benefits end users as expected, especially in medical-related cases. This research aims to review and analyze articles associated with medical app assessment across different platforms. This research also aimed to provide the best practices and identify the academic challenges, motivations and recommendations related with quality assessments. In addition, a methodological approach followed in previous research in this domain was also discussed to give some insights for future comers with what to expect. We systematically searched articles on topics related to medical app assessment. The search was conducted on five major databases, namely, Science Direct, Springer, Web of Science, IEEE Xplore and PubMed from 2009 to September 2019. These indices were considered sufficiently extensive and reliable to cover our scope of the literature. Articles were selected on the basis of our inclusion and exclusion criteria ( n = 72 ). Medical app assessment is considered a major topic which warrants attention. This study emphasizes the current standpoint and opportunities for research in this area and boosts additional efforts towards the understanding of this research field.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others
Qualitative evaluation of mobile cancer apps with particular attention to the target group, content, and advertising.

Evaluating and selecting mobile health apps: strategies for healthcare providers and healthcare organizations
Mobile applications in the Philippines during the COVID-19 pandemic: systematic search, use case mapping, and quality assessment using the Mobile App Rating Scale (MARS)
Holl K, Elberzhager F. Mobile application quality assurance. Adv Comput. 2019;112:1–77 Elsevier.
Google Scholar
Bahadori S, Wainwright TW, Ahmed OH. Smartphone apps for total hip replacement and total knee replacement surgery patients: a systematic review. Disabil Rehabil. 2018:1–6.
Orsini G, Bade D, Lamersdorf W. Context-aware computation offloading for mobile cloud computing: requirements analysis, survey and design guideline. Procedia Comput Sci. 2015;56:10–7.
Beam L, Burrows B, Dobey Z, Poser R, Sopko M. RecycMe: the Ohio State University recycling phone application. 2013.
Ouhbi S, Fernández-Alemán JL, Pozo JR, El Bajta M, Toval A, Idri A. Compliance of blood donation apps with mobile OS usability guidelines. J Med Syst. 2015;39(6):63.
Kim MS, Park JH, Park K-Y. Development and effectiveness of a drug dosage calculation training program using cognitive loading theory based on smartphone application. J Korean Acad Nurs. 2012;42(5):689–98.
Nayebi F, Desharnais J-M, Abran A. The state of the art of mobile application usability evaluation. In 2012 25th IEEE Canadian Conference on Electrical and Computer Engineering (CCECE). IEEE; 2012, p. 1–4.
Chen Z-S, Li R, Chen X, Xu H. A survey study on consumer perception of mobile-commerce applications. Procedia Environ Sci. 2011;11:118–24.
Tang AK. A systematic literature review and analysis on mobile apps in m-commerce: implications for future research. Electron Commer Res Appl. 2019;37:100885.
Darras KE, van Merriënboer JJG, Toom M, Roberson ND, de Bruin ABH, Nicolaou S, et al. Developing the evidence base for M-learning in undergraduate radiology education: identifying learner preferences for Mobile apps. Can Assoc Radiol J. 2019;70(3):320–6.
Albrecht U-V, Hillebrand U, von Jan U. Relevance of trust marks and CE labels in German-language store descriptions of health apps: analysis. JMIR mHealth and uHealth. 2018;6(4):e10394.
Anderson N, Steele J, O'Neill L-A, Harden LA. Pokémon Go: mobile app user guides. Br J Sports Med . 2016;2016:096762.
Ahtinen A, Isomursu M, Huhtala Y, Kaasinen J, Salminen J, Häkkilä J. Tracking outdoor sports–user experience perspective. In: Aarts E, Crowley JL, Ruyter B, Gerhäuser H, Pflaum A, Schmidt J, Wichert R, editors. European Conference on Ambient Intelligence. Berlin: Springer; 2008. p. 192–209.
Lior LN. Writing for interaction: crafting the information experience for web and software apps. Newnes. 2013, Writing Text for Interaction.
Cortimiglia MN, Ghezzi A, Renga F. Mobile applications and their delivery platforms. IT Prof. 2011;13(5):51–6.
Beimborn D, Palitza M. Enterprise app stores for mobile applications-development of a benefits framework. 2013.
Harman M, Jia Y, Zhang Y. App store mining and analysis: MSR for app stores. In Proceedings of the 9th IEEE Working Conference on Mining Software Repositories. IEEE Press; 2012, p. 108–111.
Freier A. App revenue reaches $92.1 billion in 2018 driven by mobile gaming apps. Business Apps. 2018;13(09).
Cheney S, Thompson E. The 2017–2022 app economy forecast: 6 billion devices, $157 billion in spend & more. App Annie. 2018.
Chen J, Cade JE, Allman-Farinelli M. The most popular smartphone apps for weight loss: a quality assessment. JMIR mHealth uHealth. 2015;3(4):e104.
Kim BY, Sharafoddini A, Tran N, Wen EY, Lee J. Consumer mobile apps for potential drug-drug interaction check: systematic review and content analysis using the mobile app rating scale (MARS). JMIR mHealth uHealth. 2018;6(3):e74.
Haskins BL, Lesperance D, Gibbons P, Boudreaux ED. A systematic review of smartphone applications for smoking cessation. Transl Behav Med. 2017;7(2):292–9.
Ali EE, Teo AKS, Goh SXL, Chew L, Yap KY-L. MedAd-AppQ: a quality assessment tool for medication adherence apps on iOS and android platforms. Res Soc Adm Pharm. 2018;14(12):1125–33.
Muntaner-Mas A, Martinez-Nicolas A, Lavie CJ, Blair SN, Ross R, Arena R, et al. A systematic review of fitness apps and their potential clinical and sports utility for objective and remote assessment of cardiorespiratory fitness. Sports Med. 2019;49(4):587–600.
Zhao J, Freeman B, Li M. How do infant feeding apps in China measure up? A Content Quality Assessment. JMIR mHealth uHealth. 2017;5(12):e186.
Weekly T, Walker N, Beck J, Akers S, Weaver M. A review of apps for calming, relaxation, and mindfulness interventions for pediatric palliative care patients. Children . 2018;5(2):16.
Nicholas J, Larsen ME, Proudfoot J, Christensen H. Mobile apps for bipolar disorder: a systematic review of features and content quality. J Med Internet Res. 2015;17(8):e198.
Huckvale K, Car M, Morrison C, Car J. Apps for asthma self-management: a systematic assessment of content and tools. BMC Med. 2012;10(1):144.
Zhang MW, Ho RC, Hawa R, Sockalingam S. Analysis of the information quality of bariatric surgery smartphone applications using the silberg scale. Obes Surg. 2016;26(1):163–8.
Bergeron D, et al. Mobile applications in neurosurgery: a systematic review, quality audit, and survey of Canadian neurosurgery residents. World neurosurg. 2019.
van Galen L, Xu X, Koh M, Thng S, Car J. Eczema apps conformance with clinical guidelines: a systematic assessment of functions, tools and content. Br J Dermatol. 2019.
Larco A, Enríquez F, Luján-Mora S. Review and evaluation of special education iOS Apps using MARS. In 2018 IEEE World Engineering Education Conference (EDUNINE). IEEE; 2018, p. 1–6.
Larco A, Montenegro C, Luján-Mora S, Quality improvement criteria of apps in Spanish for people with disabilities. In 2018 4th International Conference on Information Management (ICIM). IEEE; 2018, p. 260–264.
Alamoodi A, et al. A review of data analysis for early-childhood period: taxonomy, motivations, challenges, recommendation, and methodological aspects. IEEE Access. 2019;7:51069–103.
Burgers C, Brugman BC, Boeynaems A. Systematic literature reviews: four applications for interdisciplinary research. J Pragmat. 2019;145:102–9.
Loureiro SMC, Romero J, Bilro RG. Stakeholder engagement in co-creation processes for innovation: a systematic literature review and case stud. J Bus Res. 2019.
Kushwah S, Dhir A, Sagar M, Gupta B. Determinants of organic food consumption. A systematic literature review on motives and barriers. Appetite. 2019;143:104402.
Daigneault P-M, Jacob S, Ouimet M. Using systematic review methods within a Ph. D. Dissertation in political science: challenges and lessons learned from practice. Int J Soc Res Methodol. 2014;17(3):267–83.
Ain N, Vaia G, DeLone WH, Waheed M. Two decades of research on business intelligence system adoption, utilization and success – a systematic literature review. Decis Support Syst. 2019;125:113113.
Dani VS, Freitas CMDS, Thom LH. Ten years of visualization of business process models: a systematic literature review. Comput Stand Inter. 2019.
Owens OL, Beer JM, Reyes LI, Thomas TL. Systematic review of commercially available mobile phone applications for prostate cancer education. Am J Men’s Health. 2019;13(1):1557988318816912.
Huckvale K, Morrison C, Ouyang J, Ghaghda A, Car J. The evolution of mobile apps for asthma: an updated systematic assessment of content and tools. BMC Med. 2015;13(1):58.
El-Gayar O, Timsina P, Nawar N, Eid W. Mobile applications for diabetes self-management: status and potential. J Diabetes Sci Technol. 2013;7(1):247–62.
Huang Z, Lum E, Jimenez G, Semwal M, Sloot P, Car J. Medication management support in diabetes: a systematic assessment of diabetes self-management apps. BMC Med. 2019;17(1):127.
Dayer L, Heldenbrand S, Anderson P, Gubbins PO, Martin BC. Smartphone medication adherence apps: potential benefits to patients and providers. J Am Pharm Assoc. 2013;53(2):172–81.
Nguyen AD, Baysari MT, Kannangara DRW, Tariq A, Lau AYS, Westbrook JI, et al. Mobile applications to enhance self-management of gout. Int J Med Inform. 2016;94:67–74.
Payo RM, Harris J, Armes J. Prescribing fitness apps for people with cancer: a preliminary assessment of content and quality of commercially available apps. J Cancer Surviv. 2019;13:1–9.
Rusin M, Årsand E, Hartvigsen G. Functionalities and input methods for recording food intake: a systematic review. Int J Med Inform. 2013;82(8):653–64.
Bhattarai P, Newton-John T, Phillips JL. Quality and usability of arthritic pain self-management apps for older adults: a systematic review. Pain Med. 2017;19(3):471–84.
Devan H, Farmery D, Peebles L, Grainger R. Evaluation of self-management support functions in apps for people with persistent pain: systematic review. JMIR mHealth uHealth. 2019;7(2):e13080.
Metelmann B, Metelmann C, Schuffert L, Hahnenkamp K, Brinkrolf P. Medical correctness and user friendliness of available apps for cardiopulmonary resuscitation: systematic search combined with guideline adherence and usability evaluation. JMIR mHealth uHealth. 2018;6(11):e190.
Taki S, Campbell KJ, Russell CG, Elliott R, Laws R, Denney-Wilson E. Infant feeding websites and apps: a systematic assessment of quality and content. Interact J Med Res. 2015;4(3):e18.
Lee H, Sullivan SJ, Schneiders AG, Ahmed OH, Balasundaram AP, Williams D, et al. Smartphone and tablet apps for concussion road warriors (team clinicians): a systematic review for practical users. Br J Sports Med. 2015;49(8):499–505.
Moglia ML, Nguyen HV, Chyjek K, Chen KT, Castaño PM. Evaluation of smartphone menstrual cycle tracking applications using an adapted APPLICATIONS scoring system. Obstet Gynecol. 2016;127(6):1153–60.
Brouard B, Bardo P, Bonnet C, Mounier N, Vignot M, Vignot S. Mobile applications in oncology: is it possible for patients and healthcare professionals to easily identify relevant tools? Ann Med. 2016;48(7):509–15.
Latorre GF, de Fraga R, Seleme MR, Mueller CV, Berghmans B. An ideal e-health system for pelvic floor muscle training adherence: systematic review. Neurourol Urodyn. 2019;38(1):63–80.
Luo D, Wang P, Lu F, Elias J, Sparks JA, Lee YC. Mobile apps for individuals with rheumatoid arthritis: a systematic review. JCR: J Clin Rheumatol. 2019;25(3):133–41.
Sun C, Malcolm JC, Wong B, Shorr R, Doyle M-A. Improving glycemic control in adults and children with type 1 diabetes with the use of smartphone-based mobile applications: a systematic review. Can J Diabetes. 2019;43(1):51–8. e3.
Bry LJ, Chou T, Miguel E, Comer JS. Consumer smartphone apps marketed for child and adolescent anxiety: a systematic review and content analysis. Behav Ther. 2018;49(2):249–61.
Milani P, Coccetta CA, Rabini A, Sciarra T, Massazza G, Ferriero GJP. Mobile smartphone applications for body position measurement in rehabilitation: a review of goniometric tools. PM&R. 2014;6(11):1038–43.
Kalz M, et al. Smartphone apps for cardiopulmonary resuscitation training and real incident support: a mixed-methods evaluation study. J Med Internet Res. 2014;16(3):e89.
Piran P, et al. Medical mobile applications for stroke survivors and caregivers. J Stroke Cerebrovasc Dis. 2019;28(11):104318.
Adam A, Hellig JC, Perera M, Bolton D, Lawrentschuk N. ‘Prostate Cancer risk Calculator’ mobile applications (apps): a systematic review and scoring using the validated user version of the Mobile application rating scale (uMARS). World J Urol. 2018;36(4):565–73.
Schumer H, Amadi C, Joshi A. Evaluating the dietary and nutritional apps in the Google play store. Healthcare Inform Res. 2018;24(1):38–45.
Torous J, Levin ME, Ahern DK, Oser ML. Cognitive behavioral mobile applications: clinical studies, marketplace overview, and research agenda. Cogn Behav Pract. 2017;24(2):215–25.
Anthony Berauk VL, Murugiah MK, Soh YC, Chuan Sheng Y, Wong TW, Ming LC. Mobile health applications for caring of older people: review and comparison. Ther Innov Regul Sci. 2018;52(3):374–82.
Meghani SH, MacKenzie MA, Morgan B, Kang Y, Wasim A, Sayani S. Clinician-targeted mobile apps in palliative care: a systematic review. J Palliat Med. 2017;20(10):1139–47.
Rincon E, Monteiro-Guerra F, Rivera-Romero O, Dorronzoro-Zubiete E, Sanchez-Bocanegra CL, Gabarron E. Mobile phone apps for quality of life and well-being assessment in breast and prostate cancer patients: systematic review. JMIR mHealth uHealth. 2017;5(12):e187.
Chen E, Mangone ER. A systematic review of apps using mobile criteria for adolescent pregnancy prevention (mCAPP). JMIR mHealth uHealth. 2016;4(4):e122.
Alyami M, Giri B, Alyami H, Sundram F. Social anxiety apps: a systematic review and assessment of app descriptors across mobile store platforms. Evid-Based Ment Health. 2017;20(3):65–70.
Park JYE, Li J, Howren A, Tsao NW, De Vera M. Mobile phone apps targeting medication adherence: quality assessment and content analysis of user reviews. JMIR mHealth uHealth. 2019;7(1):e11919.
Byambasuren O, Sanders S, Beller E, Glasziou P. Prescribable mHealth apps identified from an overview of systematic reviews. Digit Med. 2018;1(1):12.
Short CE, Finlay A, Sanders I, Maher C. Development and pilot evaluation of a clinic-based mHealth app referral service to support adult cancer survivors increase their participation in physical activity using publicly available mobile apps. BMC Health Serv Res. 2018;18(1):27.
Bender JL, Yue RYK, To MJ, Deacken L, Jadad AR. A lot of action, but not in the right direction: systematic review and content analysis of smartphone applications for the prevention, detection, and management of cancer. J Med Internet Res. 2013;15(12):e287.
Bychkov D, Young SD. Facing up to nomophobia: a systematic review of mobile phone apps that reduce Smartphone usage. In Big data in engineering applications. Springer; 2018, p. 161–171.
Olivero E, Bert F, Thomas R, Scarmozzino A, Raciti IM, Gualano MR, et al. E-tools for hospital management: an overview of smartphone applications for health professionals. Int J Med Inform. 2019;124:58–67.
Rodríguez AQ, Wägner AM. Mobile phone applications for diabetes management: a systematic review. Endocrinol Diab Nutr. 2019;66(5):330–7.
Reynoldson C, Stones C, Allsop M, Gardner P, Bennett MI, Closs SJ, et al. Assessing the quality and usability of smartphone apps for pain self-management. Pain Med. 2014;15(6):898–909.
Rajani NB, Weth D, Mastellos N, Filippidis FT. Adherence of popular smoking cessation mobile applications to evidence-based guidelines. BMC Public Health. 2019;19(1):743.
Xiao Q, Wang Y, Sun L, Lu S, Wu Y. Current status and quality assessment of cardiovascular diseases related smartphone apps in China. Nurs Inform. 2016;225:1030–1.
Sedrati H, Nejjari C, Chaqsare S, Ghazal H. Mental and physical mobile health apps. Procedia Comput Sci. 2016;100:900–6.
Huckvale K, Prieto JT, Tilney M, Benghozi P-J, Car J. Unaddressed privacy risks in accredited health and wellness apps: a cross-sectional systematic assessment. BMC Med. 2015;13(1):214.
Larco A, Yanez C, Almendáriz V, Luján-Mora S. Thinking about inclusion: Assessment of multiplatform apps for people with disability. In 2018 IEEE Global Engineering Education Conference (EDUCON). IEEE; 2018, pp. 350–354.
Seabrook HJ, Stromer JN, Shevkenek C, Bharwani A, de Grood J, Ghali WA. Medical applications: a database and characterization of apps in Apple iOS and Android platforms. BMC Res Notes. 2014;7(1):573.
Buechi R, et al. Evidence assessing the diagnostic performance of medical smartphone apps: a systematic review and exploratory meta-analysis. BMJ Open. 2017;7(12):e018280.
Mangone ER, Lebrun V, Muessig KE. Mobile phone apps for the prevention of unintended pregnancy: a systematic review and content analysis. JMIR mHealth uHealth. 2016;4(1):e6.
Williams JP, Schroeder D. Popular glucose tracking apps and use of mHealth by Latinos with diabetes. JMIR mHealth uHealth. 2015;3(3):e84.
Con D, De Cruz P. Mobile phone apps for inflammatory bowel disease self-management: a systematic assessment of content and tools. JMIR mHealth uHealth. 2016;4(1):e13.
Linares-Del Rey M, Vela-Desojo L, Cano-de la Cuerda R. Mobile phone applications in Parkinson’s disease: a systematic review. Neurología (English Edition). 2018.
Larsen ME, Nicholas J, Christensen H. A systematic assessment of smartphone tools for suicide prevention. PloS One. 2016;11(4):e0152285.
Huckvale K, Adomaviciute S, Prieto JT, Leow MK-S, Car J. Smartphone apps for calculating insulin dose: a systematic assessment. BMC Med. 2015;13(1):106.
Bachmann DJ, Jamison NK, Martin A, Delgado J, Kman NE. Emergency preparedness and disaster response: there’s an app for that. Prehosp Dis Med. 2015;30(5):486–90.
Bakker D, Kazantzis N, Rickwood D, Rickard N. Mental health smartphone apps: review and evidence-based recommendations for future developments. JMIR Ment Health. 2016;3(1):e7.
Wurzer P, Parvizi D, Lumenta DB, Giretzlehner M, Branski LK, Finnerty CC, et al. Smartphone applications in burns. Burns. 2015;41(5):977–89.
Hayes W, Naziri Q, De Tolla JE, Akamnonu CP, Merola AA, Paulino C. A systematic review of all smart phone applications specifically aimed for use as a scoliosis screening tool. Spine J. 2013;13(9):S38.
Download references
this research is supported by Universiti Pendidikan Sultan Idris under University Research Grant (2017–0310–107-01).
Author information
Authors and affiliations.
Department of Computing, Sultan Idris University of Education (UPSI), Tanjong Malim, Malaysia
A. H. Alamoodi, Salem Garfan, B. B. Zaidan, A. A. Zaidan, Mussab Alaa & O. S. Albahri
Computer Science Department, College of Computer Science and Mathematics, Tikrit University (TU), Tikrit, Iraq
Moceheb Lazam Shuwandy
Department of Management Information System, College of Administration and Economics, University of Mosul, Mosul, Iraq
M. A. Alsalem
Faculty of Engineering and Built Environment, Universiti Kebangaan Malaysia (UKM), Bangi, Selangor, Malaysia
Ali Mohammed & O. R. Alobaidi
Department of Engineering Technology, Universiti Tun Hussein Onn (UTHM), Batu Pahat, Malaysia
A. M. Aleesa
Faculty of Computer Science and Information Technology, University of Malaya (UM), Kuala Lumpur, Malaysia
Ward Ahmed Al-Hussein
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to A. H. Alamoodi .
Ethics declarations
Disclosure of potential conflicts of interest.
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no competing of interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
(DOCX 14 kb)
Rights and permissions
Reprints and permissions
About this article
Alamoodi, A.H., Garfan, S., Zaidan, B.B. et al. A systematic review into the assessment of medical apps: motivations, challenges, recommendations and methodological aspect. Health Technol. 10 , 1045–1061 (2020). https://doi.org/10.1007/s12553-020-00451-4
Download citation
Received : 27 April 2020
Accepted : 11 June 2020
Published : 23 June 2020
Issue Date : September 2020
DOI : https://doi.org/10.1007/s12553-020-00451-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Apps assessment
- Mobile apps
- Medical apps
- Find a journal
- Publish with us
- Track your research
This paper is in the following e-collection/theme issue:
Published on 31.5.2024 in Vol 26 (2024)
Use of Patient-Generated Health Data From Consumer-Grade Devices by Health Care Professionals in the Clinic: Systematic Review
Authors of this article:

- Sharon Guardado 1 , MSc ;
- Maria Karampela 1 , PhD ;
- Minna Isomursu 1 , PhD ;
- Casandra Grundstrom 2 , PhD
1 Faculty of Information Technology and Electrical Engineering, University of Oulu, Oulu, Finland
2 Department of Computer Science, Norwegian University of Science and Technology, Trondheim, Norway
Corresponding Author:
Sharon Guardado, MSc
Faculty of Information Technology and Electrical Engineering
University of Oulu
Pentti Kaiteran katu 1
Oulu, 90570
Phone: 358 504388396
Email: [email protected]
Background: Mobile health (mHealth) uses mobile technologies to promote wellness and help disease management. Although mHealth solutions used in the clinical setting have typically been medical-grade devices, passive and active sensing capabilities of consumer-grade devices like smartphones and activity trackers have the potential to bridge information gaps regarding patients’ behaviors, environment, lifestyle, and other ubiquitous data. Individuals are increasingly adopting mHealth solutions, which facilitate the collection of patient-generated health data (PGHD). Health care professionals (HCPs) could potentially use these data to support care of chronic conditions. However, there is limited research on real-life experiences of HPCs using PGHD from consumer-grade mHealth solutions in the clinical context.
Objective: This systematic review aims to analyze existing literature to identify how HCPs have used PGHD from consumer-grade mobile devices in the clinical setting. The objectives are to determine the types of PGHD used by HCPs, in which health conditions they use them, and to understand the motivations behind their willingness to use them.
Methods: A systematic literature review was the main research method to synthesize prior research. Eligible studies were identified through comprehensive searches in health, biomedicine, and computer science databases, and a complementary hand search was performed. The search strategy was constructed iteratively based on key topics related to PGHD, HCPs, and mobile technologies. The screening process involved 2 stages. Data extraction was performed using a predefined form. The extracted data were summarized using a combination of descriptive and narrative syntheses.
Results: The review included 16 studies. The studies spanned from 2015 to 2021, with a majority published in 2019 or later. Studies showed that HCPs have been reviewing PGHD through various channels, including solutions portals and patients’ devices. PGHD about patients’ behavior seem particularly useful for HCPs. Our findings suggest that PGHD are more commonly used by HCPs to treat conditions related to lifestyle, such as diabetes and obesity. Physicians were the most frequently reported users of PGHD, participating in more than 80% of the studies.
Conclusions: PGHD collection through mHealth solutions has proven beneficial for patients and can also support HCPs. PGHD have been particularly useful to treat conditions related to lifestyle, such as diabetes, cardiovascular diseases, and obesity, or in domains with high levels of uncertainty, such as infertility. Integrating PGHD into clinical care poses challenges related to privacy and accessibility. Some HCPs have identified that though PGHD from consumer devices might not be perfect or completely accurate, their perceived clinical value outweighs the alternative of having no data. Despite their perceived value, our findings reveal their use in clinical practice is still scarce.
International Registered Report Identifier (IRRID): RR2-10.2196/39389
Introduction
The term “mobile health” (mHealth) has been in use for nearly 2 decades to refer to the application of mobile technologies in delivering health services and collecting data pertinent to disease diagnosis, prevention, and management [ 1 , 2 ]. In the last decade, the scope of mHealth has expanded to include consumer-grade devices, such as smartphones, wearable, sensors, and other quasi-medical devices, while it increasingly targets specific health conditions, in addition to wellness [ 2 , 3 ]. Whereas medical-grade mobile devices require clinical evidence for certification, often requiring years to bring a device to the market [ 4 ], consumer-grade mobile devices evolving at a rapid pace, and open numerous possibilities through their capacity for ubiquitous data collection [ 5 ]. mHealth solutions have become integral to many people’s lives, serving as tools for tracking health and well-being. Research has found that mHealth solutions can benefit individuals in general by fostering moderate increases in physical activity [ 6 ] or by being a convenient tool for self-management of health issues [ 7 ]. For individuals with chronic diseases, mHealth solutions have been particularly effective in offering support for condition management, goal setting, and enhancing overall satisfaction [ 7 , 8 ]. In addition to supporting people’s efforts to manage their health, mHealth solutions also enable the collection of electronic patient-generated health data (PGHD), which can be used in the clinical context. PGHD refer to health-related data created, recorded, and gathered by and from patients outside of the clinical settings [ 9 , 10 ]. PGHD encompasses a broad range of data types from both passive and active sensing [ 1 , 11 ]. Passive data collection usually involves sensors that are connected to a mobile device that may be worn or embedded, limiting the patient’s participation to wearing, carrying, or activating the device [ 12 ]. Active data collection requires patients to manually enter information or interact with an external device such as a peak flowmeter, glucometer, or thermometer to generate information. These data are “patient-generated” since the patient has actively participated in collecting and recording [ 12 ]. It has been hypothesized that through both passively and actively collected PGHD, health care professionals (HCPs) could gain insights into patients’ activities, lifestyle, and physical condition to inform care decisions and personalize care approaches [ 13 ].
In countries with medium or high levels of digitalization, more than 56% of people appear willing to share their personal health data, even if the purpose of sharing them is not directly related to the improvement of their health [ 14 ]. Similarly, 46.3% of individuals who owned a wearable medical device indicated having shared data with a health provider in 2019 [ 15 ]. With mHealth solutions becoming increasingly accessible, it can be expected that more people may be interested in sharing their health data with HCPs if they believe that it could help them improve health care. However, a recent study found that although providers of mHealth solutions for chronic condition self-management encourage data sharing with HCPs, few solutions are designed to facilitate HCPs’ review of these data [ 4 ]. This issue, in combination with already known challenges such as interoperability, data privacy issues, data validity, and the added burden of reviewing [ 9 , 16 ], makes the use of PGHD in the clinic an unrealistic possibility for many HCPs.
HCPs might have different approaches and goals when deciding to ponder PGHD collected through nonmedical mobile devices. According to Nittas et al [ 17 ], when integrating PGHD into the care process, HCPs can take the supporter or the reviewer role. In the supporter role, they limit themselves to motivating patients to use mHealth, whereas in the reviewer role, HCPs assess PGHD to complement medical data. Taking the reviewer role implies an active stance, and though some might value PGHD’s contribution to care, this type of data may still be a new and unfamiliar source of information for some HCPs [ 18 ]. For PGHD for mobile devices to be feasible as a complementary tool in the clinical setting, their use should benefit both patients and HCPs. Though the adoption of mHealth solutions by patients supports their well-being and enables the availability of PGHD, such availability does not automatically equate to usefulness for HCPs. Despite the acceptance and adoption of mHealth solutions by HCPs being one of the most influential factors regarding the success of those solutions [ 19 , 20 ], there has not been significant research on the role HCPs are expected to take in the use of mHealth solutions [ 4 , 17 ] or on the concrete experiences and motivators of those willing to review PGHD.
The main objective of our review is to systematically analyze existing scientific literature to identify what types of PGHD and in what health conditions HCPs have been using PGHD from consumer-grade mobile devices, as well as further context information for their motivations to use these types of data as a complementary tool in the clinic.
To attain these objectives the proposed research questions for our review are as follows: (1) In what health conditions have PGHD from consumer-grade mobile been a suitable tool for HCP? (2) What types of PGHD have HCPs found useful in the care of chronic conditions? (3) What are the main motivations behind HCPs’ decision to review PGHD from consumer-grade devices?
Study Design
A systematic literature review (SLR) was selected as our main research method to comprehensively synthesize evidence and prior research on HCPs’ experiences reviewing PGHD from consumer-grade mobile devices to address our research questions. We wanted to follow a transparent and systematic method to inform further research on this topic. We adopted methodologies from the Guidelines for Performing Systematic Literature Reviews in Software Engineering [ 21 ] and the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement) [ 22 , 23 ], both of which provide reliable methodologies to perform SLRs in the fields of computer science and medicine, respectively ( Multimedia Appendix 1 [ 24 ]). We deemed it pertinent to combine methodological traditions from both computer science and medicine, as our research topic combines technical and care viewpoints and is interdisciplinary by nature [ 24 ].
To perform this SLR, we adhered to a systematic review protocol that was prepared before starting the searches and screening process. The protocol has been published elsewhere [ 25 ] and provides an ample description of the methods used in the search strategy and the inclusion and exclusion criteria used. The review adhered closely to the original protocol, with no significant deviations.
Search Strategy
Eligible studies were identified through comprehensive literature searches we conducted in bibliographical databases on health and biomedicine and information technology domains. The searched databases included PubMed, ACM Digital Library (including the ACM Guide to Computing Literature), IEEE Xplore, and Scopus. The searches were carried out in May 2022.
To ensure the identification of relevant papers, we constructed the search strategy in an iterative way [ 25 ]. The search string used for each database is available in Multimedia Appendix 2 . Based on the specific objectives of our review, and after conducting a pilot search in PubMed, we determined that the literature search should be constructed around 3 specific key topics: “patient-generated health data,” “health personnel,” and “mobile technologies.” We used the corresponding Medical Subject Headings (MeSH) and their possible variants to construct the final search query. Once the query had been tested, it was validated by a research librarian from the University of Oulu. After completing the electronic searches, we performed a supplementary hand search of the citations found within other SLRs and scoping reviews that were retrieved during the literature searches.
Eligibility Criteria
The defined eligibility criteria ( Table 1 ) aimed to include original papers that reported on the use of PGHD created via consumer-grade mHealth solutions by HCPs. PGHD reported in the studies should have been collected outside of the clinical setting, through either the patients’ use of mobile health apps or the wearable devices such as smartwatches, smart rings, fitness trackers, and similar wearable trackers; studies reporting on PGHD collected by HCPs during appointments or inside the clinical settings were excluded. Studies were limited to those involving consumer-grade devices to focus on, excluding those solely focusing on PGHD from medical-grade devices. The included papers report on the experiences of HCPs who have experience using PGHD in their clinical practice, as part of a stand-alone mHealth solution, by personal initiative, or for any other reasons. We excluded papers that focus solely on the perceptions or perspectives of HCPs as potential users of PGHD. Eligible publications were restricted to those accepted in peer-reviewed journals and conference proceedings written in the English language.
a PGHD: patient-generated health data.
b HCP: health care professional.
Though current consumer-grade mobile and wearable technologies started to become more accessible in the first half of the last decade, their impact on the health care scene started to become evident only years later. In 2013, it was acknowledged that only a few studies had assessed the impact of mobile apps in the health context, and all those studies referred to apps that had been created only for research purposes and were not available to the public at that time [ 26 ]. Therefore, we limited our search to papers with publication dates starting in 2013. Our search criteria did not delimit aspects such as the medical profile or specialties of the HCPs participating in the studies, or the health conditions treated, as we aimed to ascertain whether PGHD usage by HCPs would be more prevalent in the treatment of certain medical conditions or certain medical fields.
Selection Process
After the electronic search, the resulting papers were imported into Covidence (Veritas Health Innovation) for screening. The screening process was divided into 2 stages carried out independently by 2 researchers (SG and MK) with computer science backgrounds and previous research experience with mHealth and PGHD. Initially, the screening was limited to titles and abstracts. Before starting this stage, the reviewers completed a joint exercise to validate the review methodology and ensure that the inclusion and exclusion criteria were correctly understood. The disagreements that arose during the initial stage were all discussed and resolved between the 2 reviewers before starting the second screening stage. The second screening round included the review of the full text of all the preliminarily included papers.
Data Extraction
The relevant information of the included papers was collected using a structured data extraction form constructed in Covidence ( Multimedia Appendix 3 ). The most relevant data extracted for each paper included the professions of the participants; health conditions treated; mobile technologies used; the type of PGHD collected; and the channels used for visualization. In addition, to understand what motivated HCPs to review PGHD quotes related to their motivations and conclusions related to the topic were extracted from each study.
The data extraction task was completed by the 2 original reviewers (SG and MK) and 2 additional reviewers (CG and MI), all of whom have previous research experience with the topic of this review. Each paper was randomly assigned to be examined by 2 of the reviewers. Each reviewer performed the data extraction independently. Upon completion, the data extracted by both reviewers were compared. Discrepancies were resolved through discussion between the reviewers and a final consensus was reached in all cases.
Data Analysis and Synthesis
Quantitative and qualitative studies were included in this review. Due to the significant heterogeneity observed in the studies’ design, types of health conditions, types of PGHD, and types of mHealth solutions, methods such as meta-analysis or meta-synthesis were not deemed the most appropriate approach for the data synthesis. The extracted data were summarized using a combination of descriptive and narrative syntheses [ 27 ]. The descriptive analysis was conducted to summarize data from the different studies. This involved classifying the studies based on the type of mobile technologies used, health conditions treated, and the types of PGHD reviewed. This approach arranged the studies into more homogenous subgroups, which aided in synthesizing different types of data. The data related to the motivations of HCPs were examined using a thematic analysis, from which different categories were derived. For the narrative synthesis, similarities, and differences between the findings of different studies were identified. The analysis and synthesis comprised three major steps: (1) organization of the included studies, (2) descriptive analysis of the findings within studies, and (3) a narrative synthesis aiming at exploring interconnections between the studies.
Quality Assessment
The quality of the included studies was assessed in parallel to the data extraction process. From the checklists, the quality of studies proposed by Kitchenham and Charters [ 21 ] were assessed. As the included studies were both qualitative and quantitative, we selected the questions that were most appropriate for our specific research questions that were present in both the qualitative and quantitative checklists.
Upon assessment, all reviewers agreed that the included studies had credible findings; proper data collection methods; clear and coherent reporting; and clear links between data, interpretation, and conclusions ( Figure 1 ).

The single topic that produced some uncertainty during the quality assessment was the lack of clarity on whether some of the selected studies had explored enough diversity of perspective and context. This can likely be attributed to the fact that almost all included studies were performed in developed countries, predominantly in the United States or Europe, which is a typical setting for digital health studies. Hence, the findings of these investigations will provide the most accurate depiction of the state of health care systems in developed countries.
Ethical Considerations
The Ethics Committee of Human Sciences of the University of Oulu guidelines state that as no human or animal subjects were involved in the study, no separate ethics statement is required. However, the general ethical guidelines from the Finnish National Board on Research Integrity [ 28 ] guided the ethics of the study.
Our search across electronic databases and supplementary hand searches identified 1696 papers. Covidence automatically removed 374 duplicates. We screened 1322 titles and abstracts, resulting in 86 papers for full-text screening. Following the completion of this second screening stage, 18 papers met all the inclusion criteria. However, upon closer examination, it was observed that 2 pairs of papers ([ 29 , 30 ] and [ 31 , 32 ]) had similar authors and identical samples and methodologies. Each pair was merged into a single study for analysis, resulting in the final inclusion of 16 studies for our SLR ( Figure 2 ).
During full-text screening, papers were primarily excluded for focusing exclusively on PGHD from medical-grade devices (31/68, 46%); evaluating the usability of specific mHealth solutions, rather than PGHD use (27/68, 39%); lacking data collection from HCPs (9/68, 13%); and discussing potential rather than actual use of PGHD (1/68, 1%).

Characteristics of the Included Studies
We included studies spanning 2015-2021. Notably, more than two-thirds of the papers (11/16, 69%) were published in 2019 or later, indicating a growing interest in the topic both before and during the COVID-19 pandemic. The predominant location was North America (11/16, 69%), specifically the United States and Canada; within Europe (3/16, 19%), Sweden and the United Kingdom were the primary locations; and 1 study was conducted in Asia and 1 in a multicountry setting. The authors used diverse methodologies for data collection, with interviews (8/16, 50%) and mixed methods (4/16, 25%) being the most common. More comprehensive insights into the specific study designs and data collection methods are available in Table 2 . A complete summary of the included studies can be found in Multimedia Appendix 4 [ 29 , 30 , 32 - 46 ].
a HCP: health care professional.
b PGHD: patient-generated health data.
Medical Profiles and Specialties
Although some of the studies examined data collected from various stakeholders such as patients, researchers, hospital managers, or solution providers, our focus centered on data collected from HCPs. Collectively, the studies in our review had 355 HCPs as participants. Among the represented professions, physicians accounted for the largest number of participants, present in 81% (13/16) of the studies. While approximately half of those studies referred to physicians using a general term, the other half provided clear information about the medical specialties of the physicians. Nurses were the second most represented profession, participating in 62% (10/16) of the studies. Physiotherapists were the third most represented, participating in 38% (6/16) of the studies. Other health professions present were psychologists (3/16, 19%) and surgeons, dietitians, health coaches, and assistant practitioners, each mentioned in 12% (2/16) of the studies ( Table 2 ).
All the studies reported the medical specialties where PGHD was being used. Those specialties included geriatrics, anesthesiology, orthopedic surgery, gastroenterology, dietetics and nutrition, behavioral and clinical psychology, psychiatry, obstetrics and gynecology, infertility, endocrinology, internal medicine, family medicine, rehabilitation, pediatric nephrology, otorhinolaryngology, and audiology.
Health Conditions Treated
The studies examined a wide range of health conditions, classified according to the WHO International Classification of Diseases , Eleventh Revision ( ICD-11 ), into categories such as endocrine, nutritional, or metabolic diseases; mental, behavioral, or neurodevelopmental disorders; diseases of the nervous, circulatory respiratory, and digestive systems, and diseases of the musculoskeletal system or connective tissue. In addition, some studies reported the use of PGHD for other types of medical tasks including perioperative care and care of older adults.
The most cited health conditions for which PGHD from mobile devices were reviewed by HCPs were diabetes and obesity, each mentioned in at least 3 studies. A quarter of the studies did not address a specific health condition. In those cases, the contextual information provided was limited to medical specialties or professions ( Table 2 ).
Types of mHealth Solutions
Among the 16 included studies, 5 mentioned specific mHealth solutions patients had been using to self-manage their health condition. The remaining studies mentioned commercial mHealth solutions in general. In half of the studies, HCPs reported using PGHD derived from a combination of diverse mHealth solutions, which included 1 or multiple mobile health apps and wearable devices. The remaining half of the studies addressed the experience of HCPs using PGHD exclusively generated through mobile health apps installed in patients’ smartphones (4/16, 25%) or captured from wearable devices (4/16, 25%).
Types of PGHD
Various classifications of PGHD have been proposed in terms of purpose (self-use, behavior change, clinical use, and research), management of a condition (eg, diabetes, hypertension), data type (physiological, behavioral, or environmental), mode of data capture (using sensors, external devices, implanted devices, patient portals, web-based surveys, and manual entry), and whether the process is active, passive, or mixed [ 12 ]. In this study, we focused on classifying PGHD based on data types.
Physiological data were reviewed in all studies. In 7 of 16 studies, at least 3 different types of physiological data were collected. Weight was the most frequently mentioned physiological data, reported in 44% (7/16) of the studies, followed by mood (6/16, 38%) and vital signs (5/16, 31%). Other less commonly reviewed types of data were pain, blood glucose level, and other symptoms ( Table 3 ).
Behavioral data constituted the most used category of PGHD. More than 80% (13/16) of the studies indicated that HCPs had reviewed some form of behavioral data, although always in combination with physiological data. Physical activity seems to be the most reviewed type of PGHD produced by consumer-grade devices, with 75% of the studies reporting its use, followed by food intake (9/16, 56%), sleep quality or quantity (8/16, 50%), and medication adherence (6/16, 38%).
Only 12% (2/16) of the studies reported the use of environmental data, which were primarily collected through passive sensing, using wearables, whereas physiological and behavioral types of data were reported to be collected through either passive or active sensing or by a combination of both. For instance, certain types of PGHD, such as sleep, physical activity, or sedentariness, were collected through active sensing in some studies and through passive sensing in others.
Access to PGHD
Diverse channels for PGHD access were presented. Notably, 19% (3/16) of the papers did not describe the precise channels HCPs used to access PGHD. Dashboards or solution portals were used in 56% (9/16) of the studies. The second most common channel was the patient’s mobile device (5/16, 31%). In a few studies, HCPs accessed PGHD through integration with the electronic health record (EHR; 2/16, 12%), by email (2/16, 12%), or from patients’ verbal summaries of data from their mobile devices (1/16, 6%).
Motivation for Reviewing PGHD
Although not all studies cited the reasons behind HCPs’ willingness to review PGHD from consumer-grade devices, motivation for reviewing them centered into 3 main categories: benefits for the patient, supporting their clinical roles, and strengthening the patient-HCP relationship ( Figure 3 ). Key motivations that showed how PGHD supported HCPs included topics such as accessing additional data types, identifying health patterns, and reducing data collection workload.

Principal Findings
Our review underlines a growing interest in understanding the experiences of HCPs who are using PGHD in the clinic. We aimed to identify how PGHD from consumer-grade mobile devices have been used to assist them in clinical practice. HCPs, who were primarily physicians and nurses, shared their experience on the topic. The health conditions for which HCPs most resorted to PGHD were diabetes and obesity. We found that physiological data, such as weight, mood, and vital signs, and behavioral data, such as physical activity, food intake, and sleep quality, have been frequently used. HCPs had access to PGHD through different channels, such as web portals provided by the mHealth solutions or through integration with the EHR.
Previous reviews have explored the role of PGHD in facilitating prevention and health promotion [ 17 ], their use in clinical practice [ 18 ], and their effect on patient-clinician relationships [ 47 ]. However, those studies have concentrated on PGHD from medical-grade devices, which tend to be more accurate and more accepted in the medical community. PGHD created through consumer-grade mHealth solutions, although praised for their potential to transform health care, have typically not been deemed reliable or accurate enough for the clinical context [ 3 , 48 , 49 ]. Despite concerns over PGHD accuracy and reliability, HCPs recognized that their clinical value outweighs the absence of data [ 40 ]. This value comes with a caveat, as recent studies indicate that PGHD must be curated by HCPs to ascribe actionable clinical value, but even then, they can be treated as supplementary to data collected through clinically recognized standards such as through laboratory tests [ 50 , 51 ]. PGHD from consumer-grade solutions have been used by HCPs in the treatment of a wide variety of health conditions, although it seems common only in the care of diabetes, cardiovascular diseases, and obesity ( Table 2 ).
The most frequently used types of data (physical activity, food intake, sleep quantity, and weight) are highly associated with lifestyle health risks, implying that access to lifestyle-related data can provide valuable insights into the control of lifestyle-related diseases. Furthermore, patients having these conditions are more willing to share PGHD, therefore, fostering HCPs’ familiarity with those types of data [ 15 ]. Our findings reveal that PGHD’s use in clinical practice remains relatively scarce [ 29 , 36 , 42 ], pointing out a gap between their potential and their current use. This finding is in line with a recent study suggesting that in comparison with the expectations of policies related to the European Health Data Space, the prompting and reviewing of PGHD from consumer-grade devices seem still relatively rare [ 50 ]. It is plausible that these types of PGHD have been used by HCPs in practice, but research on the practicalities of this phenomenon has only increased in the last 5 years.
HCPs indicated that PGHD are useful in the identification of patterns, to support certain diagnoses, and for certain types of monitoring. For example, lifestyle diseases [ 36 ], irritable bowel syndrome [ 42 ], or infertility [ 37 ] requires long-term management or presents a high level of uncertainty. In these cases, PGHD can provide longitudinal insights into patients’ health between clinic visits or even before they start treatment, saving time in identifying patterns. It is worth noting that, although HCPs in those studies acknowledged the value of PGHD, they also indicated engaging with PGHD infrequently and only with a few specific data types, in comparison with the substantial amount of data some patients want to share. For patients with chronic diseases, knowing that HCPs are reviewing their PGHD can be a comfortable way to know that they are being monitored and can provide data at the right time to facilitate decision-making and early intervention [ 41 , 52 ].
Multiple types of data were collected in all the studies, which signifies that as more data are collected, the need for analytical strategies that can support HCPs in reviewing and analyzing the potential relationships between different categories of data will be higher. Most existing mHealth solutions for self-monitoring lack standardized formats and mechanisms for patients to control and share PGHD [ 40 , 45 ]. Support for HCPs’ data access and use requires standardization and, in some cases, EHR integration [ 44 , 45 ].
Limitations
We limited our inclusion to papers written in English. However, this approach may have excluded relevant papers from developing regions where English is not the primary language for scientific dissemination but where the interest and potential for mHealth solutions and PGHD are growing. Similarly, a gap in the current body of research regarding these topics in developing regions is highlighted, since all studies came from countries with high economic and digitalization levels.
PGHD is a relatively recent definition, and some relevant papers published prior to its official designation as a MeSH term may have employed alternative terminology to describe the same concept of PGHD used in our study.
The shift toward digital health solutions the COVID-19 pandemic potentiated may have modified HCPs’ perceptions of PGHD use. However, no studies explicitly examining this relationship were identified in our prior searches or a later search. Therefore, future research could explore whether the shift toward digital health has catalyzed the adoption of consumer-grade technologies and PGHD in clinical settings.
Conclusions
Despite skepticism regarding the reliability and accuracy of PGHD and the multiple challenges that they convey, our study highlights a noticeable shift toward recognizing their practical value in health care, particularly in managing chronic conditions such as diabetes, obesity, and cardiovascular diseases. Yet, their impact in supporting the clinical practice is not clear from the literature. Many HCPs in the study, predominantly physicians and nurses, showed interest in using PGHD in the clinical workflows, albeit with a cautious approach that considers them as supplementary to traditional clinical data only. While they acknowledged the benefit of reviewing PGHD for the patient-HCP relationship, it was also noted that only certain types of PGHD are truly deemed useful and even then, they are not regularly used by HCPs. The findings call for continued research and innovation in mHealth, with a focus on enhancing the reliability, usability, and clinical relevance of PGHD, which in return can foster a culture of trust and collaboration between patients and HCPs.
Acknowledgments
We would like to acknowledge the More Stamina Project research group for supporting the development of this work. We acknowledge the use of ChatGPT version 4 and Grammarly to identify improvements in the organization of our text and to improve the writing style in the Introduction and Discussion sections.
Data Availability
This literature review synthesizes findings from peer-reviewed journal papers and conference papers. Given the nature of this review, it does not generate new primary data; instead, it compiled and analyzed existing publications on the use of PGHD from mobile technologies by HCPs. The reviewed papers are all available in public scientific databases. The data extraction form and the extracted data are available in the Multimedia Appendix section. These resources aim to ensure the reproducibility of our methods and facilitate future research in this area.
Conflicts of Interest
None declared.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist.
Search strategies for all searched databases.
Data extraction form.
Summary of the included studies.
- Sim I. Mobile devices and health. N Engl J Med. 2019;381(10):956-968. [ CrossRef ] [ Medline ]
- Istepanian RSH. Mobile health (m-Health) in retrospect: the known unknowns. Int J Environ Res Public Health. 2022;19(7):3747. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Omoloja A, Vundavalli S. Patient generated health data: benefits and challenges. Curr Probl Pediatr Adolesc Health Care. 2021;51(11):101103. [ CrossRef ] [ Medline ]
- Bradway M, Gabarron E, Johansen M, Zanaboni P, Jardim P, Joakimsen R, et al. Methods and measures used to evaluate patient-operated mobile health interventions: scoping literature review. JMIR Mhealth Uhealth. 2020;8(4):e16814. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Dunn J, Runge R, Snyder M. Wearables and the medical revolution. Per Med. 2018;15(5):429-448. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Mönninghoff A, Kramer JN, Hess AJ, Ismailova K, Teepe GW, Tudor Car L, et al. Long-term effectiveness of mHealth physical activity interventions: systematic review and meta-analysis of randomized controlled trials. J Med Internet Res. 2021;23(4):e26699. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Vo V, Auroy L, Sarradon-Eck A. Patients' perceptions of mHealth apps: meta-ethnographic review of qualitative studies. JMIR Mhealth Uhealth. 2019;7(7):e13817. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Feng S, Mäntymäki M, Dhir A, Salmela H. How self-tracking and the quantified self promote health and well-being: systematic review. J Med Internet Res. 2021;23(9):e25171. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lavallee DC, Lee JR, Austin E, Bloch R, Lawrence SO, McCall D, et al. mHealth and patient generated health data: stakeholder perspectives on opportunities and barriers for transforming healthcare. Mhealth. 2020;6:8. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Figueiredo MC, Chen Y. Patient-generated health data: dimensions, challenges, and open questions. Found Trends Hum–Comput Interact. 2020;13(3):165-297. [ CrossRef ]
- Jim HSL, Hoogland AI, Brownstein NC, Barata A, Dicker AP, Knoop H, et al. Innovations in research and clinical care using patient-generated health data. CA Cancer J Clin. 2020;70(3):182-199. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Mars M, Scott RE. Electronic patient-generated health data for healthcare. In: Linwood SL, editor. Digital Health. Brisbane, Australia. Exon Publications; 2022:1-16.
- Li X, Dunn J, Salins D, Zhou G, Zhou W, Schüssler-Fiorenza Rose SM, et al. Digital health: tracking physiomes and activity using wearable biosensors reveals useful health-related information. PLoS Biol. 2017;15(1):e2001402. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Karampela M, Ouhbi S, Isomursu M. Connected health user willingness to share personal health data: questionnaire study. J Med Internet Res. 2019;21(11):e14537. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Turner K, Jo A, Wei G, Tabriz AA, Clary A, Jim HSL. Sharing patient-generated data with healthcare providers: findings from a 2019 national survey. J Am Med Inform Assoc. 2021;28(2):371-376. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Gurupur VP, Wan TTH. Challenges in implementing mHealth interventions: a technical perspective. Mhealth. 2017;3:32. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Nittas V, Lun P, Ehrler F, Puhan MA, Mütsch M. Electronic patient-generated health data to facilitate disease prevention and health promotion: scoping review. J Med Internet Res. 2019;21(10):e13320. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Demiris G, Iribarren SJ, Sward K, Lee S, Yang R. Patient generated health data use in clinical practice: a systematic review. Nurs Outlook. 2019;67(4):311-330. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Jacob C, Sanchez-Vazquez A, Ivory C. Social, organizational, and technological factors impacting clinicians' adoption of mobile health tools: systematic literature review. JMIR Mhealth Uhealth. 2020;8(2):e15935. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Gagnon MP, Ngangue P, Payne-Gagnon J, Desmartis M. m-Health adoption by healthcare professionals: a systematic review. J Am Med Inform Assoc. 2016;23(1):212-220. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kitchenham B, Charters S. Guidelines for performing systematic literature reviews in software engineering. Researchgate. 2007. URL: https://www.researchgate.net/profile/Barbara-Kitchenham/publication/302924724_Guidelines_for_performing_Systematic_Literature_Reviews_in_Software_Engineering/links/61712932766c4a211c03a6f7/Guidelines-for-performing-Systematic-Literature-Reviews-in-Software-Engineering.pdf [accessed 2024-05-07]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. BMJ. 2009;339:b2535-b2535. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. Mar 29, 2021;372:n71. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Karampela M, Ouhbi S, Isomursu M. Personal health data: a systematic mapping study. Int J Med Inform. 2018;118:86-98. [ CrossRef ] [ Medline ]
- Medina SG, Isomursu M. The use of patient-generated health data from consumer-grade mobile devices in clinical workflows: protocol for a systematic review. JMIR Res Protoc. 2023;12:e39389. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Fiordelli M, Diviani N, Schulz PJ. Mapping mHealth research: a decade of evolution. J Med Internet Res. 2013;15(5):e95. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Evans D. Systematic reviews of interpretive research: interpretive data synthesis of processed data. Aust J Adv Nurs. 2002;20(2):22-26. [ FREE Full text ]
- Guidelines for ethical review in human sciences. Publications of the Finnish National Board on Research Integrity. 2019. URL: https://tenk.fi/sites/default/files/2023-11/RI_Guidelines_2023.pdf
- Alpert JM, Manini T, Roberts M, Kota NSP, Mendoza TV, Solberg LM, et al. Secondary care provider attitudes towards patient generated health data from smartwatches. NPJ Digit Med. 2020;3(1):27. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Alpert JM, Kota NSP, Ranka S, Mendoza TV, Solberg LM, Rashidi P, et al. A simulated graphical interface for integrating patient-generated health data from smartwatches with electronic health records: usability study. JMIR Hum Factors. 2020;7(4):e19769. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lindroth T, Islind AS, Steineck G, Lundin J. From narratives to numbers: data work and patient-generated health data in consultations. Stud Health Technol Inform. 2018;247:491-495. [ Medline ]
- Cerna K, Grisot M, Islind AS, Lindroth T, Lundin J, Steineck G. Changing categorical work in healthcare: the use of patient-generated health data in cancer rehabilitation. Comput Supported Coop Work. 2020;29(5):563-586. [ FREE Full text ] [ CrossRef ]
- Abdolkhani R, Gray K, Borda A, DeSouza R. Patient-generated health data management and quality challenges in remote patient monitoring. JAMIA Open. 2019;2(4):471-478. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Austin E, Lee JR, Amtmann D, Bloch R, Lawrence SO, McCall D, et al. Use of patient-generated health data across healthcare settings: implications for health systems. JAMIA Open. 2020;3(1):70-76. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Chung CF, Cook J, Bales E, Zia J, Munson SA. More than telemonitoring: health provider use and nonuse of life-log data in irritable bowel syndrome and weight management. J Med Internet Res. 2015;17(8):e203. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Cohen DJ, Keller SR, Hayes GR, Dorr DA, Ash JS, Sittig DF. Integrating patient-generated health data into clinical care settings or clinical decision-making: lessons learned from project HealthDesign. JMIR Hum Factors. 2016;3(2):e26. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Costa Figueiredo M, Su HI, Chen Y. Using data to approach the unknown. Proc ACM Hum-Comput Interact. 2021;4(CSCW3):1-35. [ FREE Full text ] [ CrossRef ]
- Gupta A, Heng T, Shaw C, Gromala D, Leese J, Li L. Oh, I didn't do a good job: how objective data affects physiotherapist-patient conversations for arthritis patients. In: Proceedings of the 14th EAI International Conference on Pervasive Computing Technologies for Healthcare. 2020. Presented at: Proceedings of the 14th EAI International Conference on Pervasive Computing Technologies for Healthcare; May 18-20, 2020:156-165; Atlanta, GA, USA. [ CrossRef ]
- Holtz B, Vasold K, Cotten S, Mackert M, Zhang M. Health care provider perceptions of consumer-grade devices and apps for tracking health: a pilot study. JMIR Mhealth Uhealth. 2019;7(1):e9929. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kim B, Ghasemi P, Stolee P, Lee J. Clinicians and older adults' perceptions of the utility of patient-generated health data in caring for older adults: exploratory mixed methods study. JMIR Aging. 2021;4(4):e29788. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kim Y, Ji S, Lee H, Kim JW, Yoo S, Lee J. "My doctor is keeping an eye on me!": exploring the clinical applicability of a mobile food logger. 2016. Presented at: Proceedings of the 2016 CHI Conference on Human Factors in Computing Systems; May 7-12, 2016:5620-5631; San Jose, CA. [ CrossRef ]
- Ng A, Kornfield R, Schueller SM, Zalta AK, Brennan M, Reddy M. Provider perspectives on integrating sensor-captured patient-generated data in mental health care. Proc ACM Hum Comput Interact. 2019;3(CSCW):1-25. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Tendedez H, Ferrario MA, McNaney R. 'The issue with that sort of data?': clinicians' accountability concerns around COPD self-monitoring tools. 2019. Presented at: Companion Publication of the 2019 Conference on Computer Supported Cooperative Work and Social Computing; November 9-13, 2019:382-386; Austin, TX. [ CrossRef ]
- West P, Van Kleek M, Giordano R, Weal MJ, Shadbolt N. Common barriers to the use of patient-generated data across clinical settings. 2018. Presented at: Proceedings of the 2018 CHI Conference on Human Factors in Computing System; April 21-26, 2018:1-13; Montreal, QC. [ CrossRef ]
- Wu DTY, Xin C, Bindhu S, Xu C, Sachdeva J, Brown JL, et al. Clinician perspectives and design implications in using patient-generated health data to improve mental health practices: mixed methods study. JMIR Form Res. 2020;4(8):e18123. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zhu H, Colgan J, Reddy M, Choe EK. Sharing patient-generated data in clinical practices: an interview study. AMIA Annu Symp Proc. 2016;2016:1303-1312. [ FREE Full text ] [ Medline ]
- Lordon RJ, Mikles SP, Kneale L, Evans HL, Munson SA, Backonja U, et al. How patient-generated health data and patient-reported outcomes affect patient-clinician relationships: a systematic review. Health Informatics J. 2020;26(4):2689-2706. [ FREE Full text ] [ CrossRef ] [ Medline ]
- West P, Van Kleek M, Giordano R, Weal M, Shadbolt N. Information quality challenges of patient-generated data in clinical practice. Front Public Health. 2017;5:284. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Park JI, Lee HY, Kim H, Lee J, Shinn J, Kim HS. Lack of acceptance of digital healthcare in the medical market: addressing old problems raised by various clinical professionals and developing possible solutions. J Korean Med Sci. 2021;36(37):e253. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Haase CB, Ajjawi R, Bearman M, Brodersen JB, Risor T, Hoeyer K. Data as symptom: doctors' responses to patient-provided data in general practice. Soc Stud Sci. 2023;53(4):522-544. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Reading MJ, Merrill JA. Converging and diverging needs between patients and providers who are collecting and using patient-generated health data: an integrative review. J Am Med Inform Assoc. 2018;25(6):759-771. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Gaveikaite V, Grundstrom C, Winter S, Schonenberg H, Isomursu M, Chouvarda I, et al. Challenges and opportunities for telehealth in the management of chronic obstructive pulmonary disease: a qualitative case study in Greece. BMC Med Inform Decis Mak. 2020;20(1):216. [ FREE Full text ] [ CrossRef ] [ Medline ]
Abbreviations
Edited by A Mavragani; submitted 26.05.23; peer-reviewed by CM Chu, P Dunn, A Brigden, C Baxter; comments to author 08.02.24; revised version received 05.04.24; accepted 11.04.24; published 31.05.24.
©Sharon Guardado, Maria Karampela, Minna Isomursu, Casandra Grundstrom. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 31.05.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research, is properly cited. The complete bibliographic information, a link to the original publication on https://www.jmir.org/, as well as this copyright and license information must be included.

- Collections
- Recent Additions
- Public Access
- Submit Content
- home Rosa P
Medical Conditions and Driving: A Review of the Literature (1960-2000)
Search our Collections & Repository
- Advanced Search
- Custom Query
All these words:
For very narrow results
This exact word or phrase:
When looking for a specific result
Any of these words:
Best used for discovery & interchangable words
None of these words:
Recommended to be used in conjunction with other fields
Publication Date Range:
Document Type:
Collection:
Query Builder
For additional assistance using the Custom Query please check out our Help Page
- By Dobbs, Bonnie M. ; Wodzin, Elaine (Petrucelli) ; Vegega, Maria
- Alternative Title: Medical Conditions and Driving: a Review of the Literature (1960-2000)
- Creators: Dobbs, Bonnie M. ; Wodzin, Elaine (Petrucelli) ; Vegega, Maria Dobbs, Bonnie M. ; Wodzin, Elaine (Petrucelli) ; Vegega, Maria Less -
- Corporate Creators: Association for the Advancement of Automotive Medicine
- Corporate Contributors: United States. Department of Transportation. National Highway Traffic Safety Administration
- Subject/TRT Terms: [+] Aged Aged Drivers Automotive Medicine Diseases And Medical Conditions Older Automobile Drivers--United States Traffic Safety Traffic Safety--United States
- Publication/ Report Number: DOT HS 809 690
- DOI: https://doi.org/10.21949/1525693
- Resource Type: Tech Report
- Geographical Coverage: United States
- Contracting Officer: John Eberhard
- Corporate Publisher: United States. National Highway Traffic Safety Administration
- Abstract: This report reviews the contribution of medical conditions and functional limitations (e.g., sensory, motor, or cognitive functioning) to motor vehicle crashes. It provides a comprehensive and up-to-date review of the international research literature on the effects of medical and functional conditions on driving performance. The report is divided into 15 sections (Introduction, Vision, Hearing, Cardiovascular Diseases, Cerebrovascular Diseases, Peripheral Vascular Diseases, Diseases of the Nervous System, Respiratory Diseases, Metabolic Diseases, Renal Diseases, Muscuoloskeletal Disabilities, Psychiatric Diseases, Drugs, The Aging Driver, and the Effects of Anesthesia and Surgery). Each section contains a brief overview of the condition/illness; prevalence information; a review of the medical, gerontological, and epidemiological literature relevant to the condition/illness, followed by current fitness to drive guidelines for the condition/illness from Canada and Australia. The Appendix presents preliminary guidelines for physicians to assess medical fitness-to-drive. The report is a scholarly but practical compendium that can serve as a valuable resource for physicians, rehabilitation practitioners, other allied health care professionals and educators, Department of Motor Vehicle personnel, road and traffic safety personnel, transportation planners, highway safety researchers, and public policymakers. Its value is particularly relevant as the driving population increases in size and age. More ▼ -->
- Format: PDF
- Funding: DTNH22-94-G-05297
- Collection(s): NHTSA - Behavioral Safety Research
- Main Document Checksum: [+] urn:sha256:119b8dce2f1cae08afb42b41a771b3f3e6b796d93f119cec5b679212ecf7ec60
- Download URL: https://rosap.ntl.bts.gov/view/dot/1902/dot_1902_DS1.pdf
Supporting Files
You may also like.
Checkout today's featured content at rosap.ntl.bts.gov
Exit Notification/Disclaimer Policy
Thank you for visiting.
You are about to access a non-government link outside of the U.S. Department of Transportation's National Transportation Library.
Please note: While links to Web sites outside of DOT are offered for your convenience, when you exit DOT Web sites, Federal privacy policy and Section 508 of the Rehabilitation Act (accessibility requirements) no longer apply. In addition, DOT does not attest to the accuracy, relevance, timeliness or completeness of information provided by linked sites. Linking to a Web site does not constitute an endorsement by DOT of the sponsors of the site or the products presented on the site. For more information, please view DOT's Web site linking policy.
To get back to the page you were previously viewing, click your Cancel button.
U.S. DEPARTMENT OF TRANSPORTATION
Bureau of Transportation Statistics
1200 NEW JERSEY AVENUE, SE
WASHINGTON, DC 20590
800-853-1351
Subscribe to email updates
- Case Report
- Open access
- Published: 28 May 2024
Fistula of the mitral-aortic intervalvular fibrosa in a patient with bacterial endocarditis: a case report and systematic literature review
- Rafael Figueroa-Casanova ORCID: orcid.org/0000-0002-5511-2806 1 ,
- Juan D. Saavedra-Henao ORCID: orcid.org/0000-0001-7997-8113 2 ,
- Juan S. Figueroa-Laverde ORCID: orcid.org/0000-0003-4857-0320 3 ,
- Diego A. Beltrán-Gonzales ORCID: orcid.org/0000-0002-1504-8264 2 ,
- José G. Labrador-Rosales 2 ,
- Sara Eslait-Olaciregui ORCID: orcid.org/0000-0001-6537-6648 4 &
- Carlos J. Pérez Rivera ORCID: orcid.org/0000-0001-7937-7676 5
Journal of Cardiothoracic Surgery volume 19 , Article number: 300 ( 2024 ) Cite this article
86 Accesses
Metrics details
A fistulous tract in the mitro-aortic intervalvular fibrosa (MAIVF) is a rare entity, which presents as a complication of endocarditis or surgical trauma. Generally, it is associated to a pseudoaneurysm of the MAIVF (p-MAIVF) or aortic abscesses. MAIVF fistulas could potentially lead to devastating complications and a high mortality rate. This condition is managed surgically, either by a percutaneous closure or an open surgical approach. Herein we report the complex case of a patient with a MAIVF fistula secondary to bacterial endocarditis. Further clinical deterioration was caused by severe aortic valve insufficiency and hemodynamic compromise, requiring surgical intervention.
Case presentation
A 74-year-old male patient was admitted to a primary care center with complaints of malaise, asthenia, adynamia, hyporexia, and lower limb edema over the past eight days. His past medical history is positive for arterial hypertension and being monorenal. A transesophageal echocardiogram (TEE) was performed, exhibiting a 56% left ventricle ejection fraction (LVEF) and complicated aortic valve endocarditis. Surgical management through an open approach included vegetation resection, valve replacement, and closure of the MAIVF fistula. After completing antibiotic therapy, the patient was discharged without complications. During postoperative follow-up, the patient remained asymptomatic, and the control echocardiogram showed no signs of MAIVF fistula.4.
Conclusions
The clinical case of a patient with a MAIVF fistula secondary to endocarditis by Streptococcus Anginous was presented. The fistulous tract was not associated to p-MAIVF or aortic abscess, findings which further deteriorate the patient’s condition and increase the likelihood of fatality. This case reinforces the importance of a prompt diagnosis through cardiac imaging and timely surgical closure of the defect.
Peer Review reports
A fistulous tract in the mitro-aortic intervalvular fibrosa (MAIVF) is a rare entity, which presents as a complication of endocarditis or surgical trauma. Generally, it is associated to a pseudoaneurysm of the MAIVF (p-MAIVF) or aortic abscesses [ 1 ]. MAIVF fistulas could potentially lead to devastating complications and a high mortality rate; additionality, the defect is related to severe regurgitation due to aortic valve dehiscence [ 2 ]. Clinical features may vary depending on the underlying cause, the spectrum ranges from asymptomatic patients to cases presenting fever spikes and symptoms of congestive heart failure along with pulmonary edema [ 2 , 3 ]. Most commonly, cases are identified incidentally during an echocardiographic evaluation, in which case a transesophageal echocardiogram (TEE) would optimize characterization of the condition. The selection of the approach for the surgical management, either percutaneous or open closure, depends on the clinical presentation, structural valve damage and/or presence of vegetations [ 1 ]. Herein we describe the complex case of a patient with a MAIVF fistula secondary to endocarditis by Streptococcus Anginous which provoked severe aortic valve insufficiency. The condition was not associated to previously observed p-MAIVF or aortic abscess. Surgical repair and closure were indicated.
A 74-year-old male patient was admitted to a primary care center with complaints of malaise, asthenia, adynamia, hyporexia and lower limb edema over the past eight days. His past medical history is positive for arterial hypertension and being monorenal. Decompensated heart failure was suspected; thus, the patient was transferred to our high complexity medical center where he would be assessed by the internal medicine department. Upon arrival severe lower limb edema was observed; therefore, blood samples for laboratory tests were withdrawn, revealing an elevated NT-proBNP (7620 pg/ml), abnormal nitrogen levels (Creatinine 1.46 mg/dL and urea nitrogen 28.3 mg/dL). Additionally, a chest X-ray showed evidence of cardiomegaly and pulmonary edema. The case was assessed by the cardiology team, who concluded that the clinical presentation was compatible with congestive heart failure, characterized as class IV according to the New York Heart Association (NYHA) and as Stevenson C for the hemodynamic profile. Pharmacological management consisting of furosemide, spironolactone, dapagliflozin and sacubitril/valsartan, along with the conduction of a TEE (Fig. 1 ) were deemed necessary. The TEE exhibited 56% left ventricle ejection fraction (LVEF) and a complicated aortic valve endocarditis: Severe insufficiency due to the rupture of the left coronary leaflet, along with the presence of a mitro-aortic fistula with flow from the outflow tract to the left atrium), associated with bi-atrial dilation and moderate tricuspid insufficiency caused by a probable rupture of the anterior valve, which is why surgical management through cardiovascular surgery was recommended (Fig. 1 ). Coronary angiography was conducted to rule out secondary coronary artery disease, and no significant lesions were found in the epicardial coronary arteries.

Preoperative transesophageal echocardiogram (A) Two-chamber apical view. Evidence of fistula in the mitro-aortic fibrous portion (yellow circle) with competitive flow. (B) Longitudinal or long-axis view. Evidence of fistula in the mitro-aortic fibrous portion (yellow circle) with competitive flow.
Parallelly, blood cultures confirmed the presence of Streptococcus Anginous leading to the infectious disease team determination to suspend broad-spectrum empirical antibiotherapy and establish a new regime with ceftriaxone for 28 days. Considering the structural compromise observed in the TEE and etiologic confirmation of bacteriemia, a definite diagnosis of infective endocarditis was appropriate. Furthermore, contemplating the extent of the structural defect and hemodynamic consequences, surgical management including vegetation resection, valve replacement and closure of MAIVF fistula was indicated. Additionally, the structure of the tricuspid valve was assessed, as it could be managed by repairing its leaflet through tricuspid valve plasty or, alternatively, tricuspid valve replacement.
Surgical intervention was conducted with extracorporeal circulation support, featuring pump and clamp times of 120 min and 89 min, respectively. Initially, the aortic valve was excised and extracted (Fig. 2 ), followed by the closure of the mitro-aortic fistula using an autologous pericardial patch (Fig. 3 ). Subsequently, an aortic valve replacement was performed using a 25 mm bioprosthesis, The anatomical structure of the tricuspid valve was assessed, revealing no involvement of the chordae tendineae of the anterior leaflet. An in vivo mechanical test was also conducted, which did not show retrograde flow in the tricuspid valve. Therefore, neither valve repair nor replacement was deemed necessary. As a result, the patient was decannulated, and layered closure was performed. The patient was transferred to intensive care unit (ICU) under orotracheal intubation and vasoactive support was provided with low doses of noradrenaline and vasopressin. The infectious disease team recommended a 28-day continuation of antibiotherapy with Ceftriaxone.

Intraoperative view of the MAIVF fistula
(A) Fistula located in the mitro-aortic fibrous portion (Black circle) resected through a left ventricular approach. (B) Mitro-aortic fistula sealed with an autologous pericardial patch (Black circle).
Surgical specimen
Resected aortic valve with a vegetation secondary to bacterial endocarditis.
Two days postoperatively, a follow-up TTE was performed, revealing an ejection fraction of 26%, a normally functioning #25 aortic bioprosthesis, mild secondary mitral insufficiency, and mild tricuspid valve regurgitation. Extubation proceeded without complications, and vasopressor support was discontinued. On the seventh postoperative day, the patient was transferred to the hospital, presenting hypotensive blood pressure readings. Consequently, an urgent TEE was recommended, confirming a LVEF of 26%, functional #25 aortic bioprosthesis with no evidence of mitro-aortic fistula (Fig. 4 ). Additionally, a severe circumferential pericardial effusion of approximately 520 ml with signs of cardiac tamponade was noted. Urgent pericardiocentesis was performed, draining 150 cc of hematic fluid, and a catheter was left in place for continued drainage.
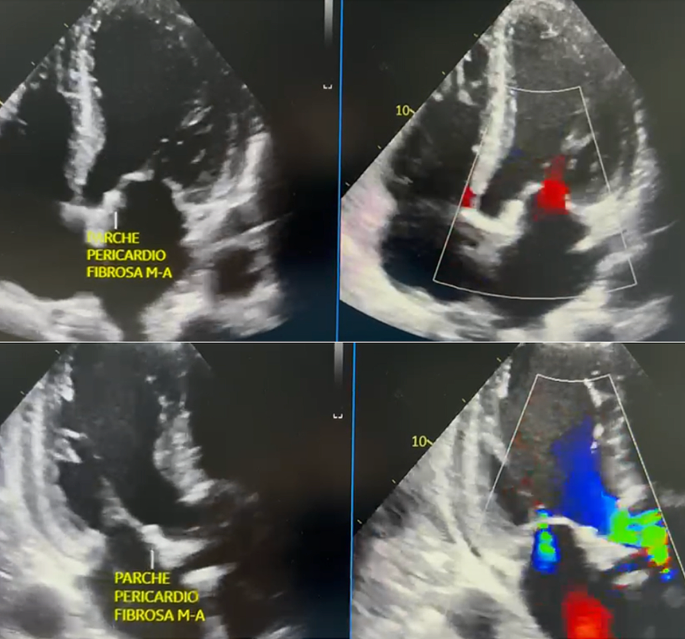
Postoperative transesophageal echocardiogram (A) Four-chamber apical view. Evidence of pericardial patch in the mitro-aortic fibrous portion occluding the fistulous tract, with no flow. (B) Two-chamber apical view. Evidence of pericardial patch in the mitro-aortic fibrous portion occluding the fistulous tract, with no flow.
Consequently, the patient was readmitted to the intensive care unit, and a gradual adjustment of hemodynamic support was initiated, leading to a progressive improvement. Ultimately, after 30 days postoperatively and upon completing the aforementioned antibiotic regimen, the decision was made to discharge the patient with medical orders. At the 30-day follow-up appointment with our specialty, a review of the cardiac Holter report revealed no evidence of arrhythmias. The echocardiogram showed the absence of a mitro-aortic fistula, a normally functioning aortic bioprosthesis with an ejection fraction of 35%, cardiovascular asymptomatic status, and good tolerance to physical activity. Thus, the patient was discharged from cardiovascular surgery care. However, continued follow-up under cardiology supervision persisted until 8 months post-procedure, during which a transthoracic echocardiogram reported an ejection fraction of 50%, a normally functioning biological prosthesis in the aortic valve position. During the clinical evaluation the patient indicated NYHA class I-II symptoms.
Systematic literature review
A systematic literature review was carried out in order to identify the available resources pertaining to the clinical case. 1 were used by applying a search strategy consisting of keywords and pre-established criteria of inclusion. All authors agreed on the application of a multi-string search strategy with the terms: “(mitral-aortic fibrosa) AND (fistula)”, “(intervalvular fibrosa) AND (fistula)”, “(mitral-aortic junction) AND (fistula), ((aortomitral) OR (aorto-Mitral) or (Mitro-Aortic) AND fistula)). The search was restricted to articles written in English and Spanish, without additional filters for the publication date. Articles herby included were published before January 16th, 2023. Methodological quality of the obtained publications was assessed by the Grading of Recommendations, Assessment, Development and Evaluation (GRADE). Figure 5 exhibits a flowchart of the study selection process.
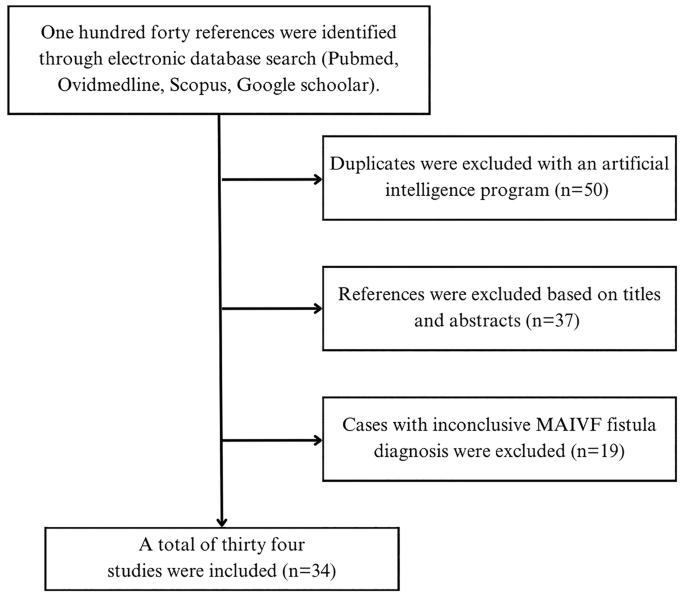
Flowchart illustrating study selection process
Even though transversal studies were identified; information was insufficient for the analysis of MAIVF fistula specific presentation and outcomes. Additionally, no controlled randomized trials or controlled cohort studies were retrieved. Thus, thirty-four case reports about MAIVF fistulas were scrutinized and the pertinent demographical and clinical features were summarized on Table 1 (available in the document “Additional file 1 ”).
Case-reports on the matter were retrieved from a diverse spectrum of countries. Notably, reports from Latin American countries were not identified, which would imply that the clinical case hereby presented will be the first report of its kind in our region. The mean age at the time of diagnosis was 50.06 years old, with a range between 20 and 81 years old. The ratio male to female was 29:5. Importantly, eighteen patients had a past medical history of cardiovascular surgery and previous infective endocarditis caused by microorganisms such as Corynebacterium, Group C beta hemolytic Streptococcus and Neisseria gonorrhea [ 4 , 5 , 6 ].
MAIVF fistula has been described as a rare and critical complication of infective endocarditis (IE); however, our research points out that such a structural defect could also be attributed to a surgical complication. 67.6% ( n = 23) of the cases were confirmed to be associated to IE. The microbiological agents were distributed as follows: Staphylococcus aureus was detected in eight cases (47.1%) [ 1 , 7 , 8 , 9 , 10 , 11 ]. 29.4%( n = 5) of the cases were caused by microorganisms from the Streptococcus genus [ 12 , 13 , 14 , 15 , 16 ]. One exceptional case was caused by enterococcus [ 17 ]. Interestingly, three cases were taken as the complication of an IE that occurred months prior [ 18 , 19 , 20 ]. MAIVF fistulas associated to prior cardiac interventions were confirmed in five clinical cases; time interval from the surgical procedures to the new event were significantly different between cases.
Concerning the therapeutic approach exhibited in the review, 90% of authors considered surgical intervention as the most appropriate management for each patient. 42.9% of the interventions involved patch repair; including bovine, porcine and unspecified origin patches were used [ 4 , 5 , 7 , 8 , 11 , 21 , 22 ]. Repair with an autologous patch was described in only one reference [ 5 ]. More than 57% of surgical cases required mitral and/or aortic valve replacement.
Most commonly, fistulous tracts in the MAIVF are observed in association to other morphological and functional changes. As exhibited by this case, fistulous tracts may be present in the absence of other defects. Interestingly, MAIVF fistulas and pseudoaneurysms may lead to compression of structures. For instance, 3D TTE and angiography revealed a fistulous communication in the MAIVF, a pseudoaneurysm in the same region associated to systolic compression and narrowing of the left main trunk [ 18 ].
Postoperative (POP) outcomes are favorable in most cases, 73.9% of the surgical interventions were successful throughout follow-up. Negative results included dehiscence of the MAIVF patch and the subsequent recurrence of the fistula in one case, and early mortality (first 24 h after the procedure) due to multiorgan failure, malignant ventricular arrythmia. Notably, the clinical case in which antibiotherapy without surgical intervention was preferred resulted in mortality [ 23 ].
The MAIVF is located between the anterior leaflet of the mitral valve and the non-coronary and left coronary cusps. A mitral aortic fistula is described as the presence of an anomalous pathway within this region. Most frequently, the occurrence of this structural defect is associated to a preexisting or concomitant pseudoaneurysm, which is referred as p-MAIVF. Up to 20% of the patients with p-MAIVF also develop fistulas at this level [ 24 ], phenomenon which was further reinforced by the case reports we reviewed [ 1 , 7 ]. However, in the case we described the TEE nor direct intraoperative observation led to the identification of a pseudoaneurysm in this region. Meaning that this report represents a unique clinical presentation of a rare condition, an isolated MAIVF fistula [ 1 , 3 , 7 ]. What is more, structural complications secondary to the presence of the p-MAIVF have been described in the literature; they include coronary artery occlusion, cardiac tamponade, and intra-aneurysmal thrombosis with the potential to cause systemic embolism and stroke. None of these were present in our clinical case, further reinforcing its peculiarity.
Having established that most of MAIVF fistulas are the result of a ruptured pseudoaneurysm; it is worth detailing that IE and previous aortic valve surgery are the most common etiologies of pseudoaneurysms in the fibrosa. The underlying mechanism for this condition articulates two concepts: 1). The MAIVF is an avascular tissue, which conditions higher propensity to infections. 2). The cardiac cycle generates shear forces that provoke remodeling of the fibrosa. Both events take place when there is an aortic valve infection or by the surgical replacement of the valve. However, congenital defects of the MAIVF and blunt thoracic trauma are less frequent conditions that might lead to the formation of p-MAIVF [ 21 , 25 ]. Particularly, in the clinical case herein presented the patient was diagnosed with bacterial endocarditis due to Streptococcus Anginous , which was presumed as the direct cause of the fistula even though there was no previous history of valve surgery. The latter differentiates this case from other case reports; where patients presented infective endocarditis by Staphylococcus Aureus summed to a past medical history of valvular surgery [ 1 , 7 ].
An accurate diagnosis of life-threatening structural defects of the heart will likely increase the success of a surgical intervention and patient survival. Therefore, cardiac diagnostic imaging used on patients with suspected valvular anomalies should yield the highest sensitivity possible. TEE has shown of 90%, which corresponds to a higher diagnostic power compared to transthoracic echocardiography (TTE). In our review, the diagnostic tool most frequently used was the TEE. It should be noticed that recent technological advances developed new strategies such as color flow doppler and 3D rendition to maximize the sensitivity of this imaging modality. Three of the clinical cases were diagnosed by TEE with color flow doppler [ 5 , 6 , 12 ]. Despite being less frequently observed on the reports, TTE and TTE enhanced with 3D technology and color doppler was also reported on the literature. In specific scenarios ventriculography and aortography were utilized to make a preoperative diagnosis, yet, in one particular case an intraoperative view was necessary to confirm the presence of a MAIVF fistula. In addition to the TEE, diagnostic imaging techniques such as computed tomography (CT) and cardiac magnetic resonance (CMR) could be used to characterize the lesion, identify potential complications, and direct the therapeutic approach [ 26 , 27 ].
The definite treatment of a fistulous tract in the MAIVF involves the prompt closure of the defect, which can be achieved through either a percutaneous or an open surgical approach. Failure to correct this structural abnormality in a timely manner can lead to a range of adverse clinical outcomes. These may include hemodynamic instability, septic shock, cardiac tamponade and, in severe cases, may even result in a fatal outcome. Such is the case of the patient reported by Fazlinezah et al., the aortography confirmed the presence of a large fistula between the aortic root and left ventricular outflow tract, and severe aortic regurgitation [ 28 ]. Unfortunately, the patient died due to rapid decompensation and insufficient time for OR transport. Additionally, to the selection of the surgical approach, the team must decide which type of patch will be used for closure. In our case, similarly to Spampinato 2012, an open surgical repair was achieved by an autologous pericardial patch. Kahraman 2023 et al. were also inclined for an open surgical approach, but in contrast to our case, a porcine pericardial patch was used. Another occlusive method involves an Amplatzer Vascular Plug II, its use is described in a patient with history of two cardiac interventions without associated valvopathy [ 1 , 3 , 7 ].
In general terms, postoperative follow-up is focused on the evaluation of cardiac symptoms and tolerance to physical activity, interpretation of a Holter report in order to exclude arrythmias and the observation of structural and hemodynamic parameters on the echocardiogram. The case reports we reviewed confirmed at follow-up improvement or resolution of symptoms, as well as the absence of residual flow tracts and valve dysfunction [ 3 , 7 ]. Similarly, our clinical case had a favorable clinical evolution. The patient was asymptomatic at the time, the Holter did not exhibit significant arrythmias and the TEE reported 35% LVEF without MAIVF fistula. It is worth mentioning that not all outcomes are as fortunate; during the postoperative monitoring of a percutaneous closure, the TEE only showed a mild decrease of pseudoaneurysm flow [ 1 ].
This systematic review provides valuable information and insights into the demographics and clinical features of MAIVF fistulas. Additionally, this manuscript reinforces the significance of accurate diagnostic imaging modalities and their impact in the timely medical attention. From a surgical standpoint, this review highlights the technical complexity embedded in the reconstruction of cardiac tissue, especially, when there are multiple fibrous structures involved. Despite the identification of a substantial number of case-reports, further research should include enhanced epidemiological methodologies and larger populations. This will allow a more comprehensive understanding of MAIVF fistula and its surgical implications.
This case is unique because the fistula occurred in the absence of a p-MAIVF and an aortic abscess; furthermore, the patient was endangered by the subsequent severe aortic valve insufficiency. A prompt closure was attained by the placement of an autologous pericardial patch during an open surgical approach. Further epidemiological and clinical studies with robust methodologies are required to fully elucidate this entity. Nevertheless, this clinical case contributes with the existing body of literature and reinforces the importance of timely diagnosis and appropriate treatment selection in complex and potentially mortal conditions such as this one.
Data availability
Availability of the data used and analyzed during the writing of the case report is under the responsibility of the corresponding author, and its distribution is authorized upon reasonable request.
Abbreviations
Coronary Bypass Time
Cardiac Magnetic Resonance
Computerized Tomography
Intensive Care Unit
Infective Endocarditis
Left Ventricular Ejection Fraction
Mitro-Aortic Intervalvular Fibrosa
New York Heart Association
Operating Room
Pseudo Aneurysm of the Mitro-Aortic Intervalvular Fibrosa
Postoperative
Pro–B-Type Natriuretic Peptide
Transesophageal Echocardiogram
Transthoracic Echocardiogram
Cakal S, Cakal B, Yildirim A. Closure of a paravalvular communication originating from the pseudoaneurysm of the mitral-aortic intervalvular fibrosa following prosthetic valve infective endocarditis. Anatol J Cardiol. 2021;25(10):741–2.
Article PubMed PubMed Central Google Scholar
Bozbuga N, Erentug V, Erdogan HB, Kirali K, Ardal H, Tas S, et al. Surgical treatment of aortic abscess and fistula. Tex Heart Inst J. 2004;31(4):382–6.
PubMed PubMed Central Google Scholar
Spampinato RA, Borger MA, Strotdrees E, Mohr FW. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa as a complication after minimally invasive mitral valve repair. Interact Cardiovasc Thorac Surg. 2013;16(3):396–8.
Article PubMed Google Scholar
Yong MS, Saxena P, Killu AM, Coffey S, Burkhart HM, Wan SH, et al. The preoperative evaluation of infective endocarditis via 3-Dimensional Transesophageal Echocardiography. Tex Heart Inst J. 2015;42(4):372–6.
Silbiger JJ, Krasner A, Chikwe J, Marino T, Mathewkutty S, Marcali M, et al. Pseudoaneurysm Formation Infective Endocarditis Echocardiography. 2013;30(10):E319–21.
PubMed Google Scholar
Sheppard RC, Chandrasekaran K, Ross J, Mintz GS. An Acquired Interatrial Fistula secondary to para-aortic abscess documented by Transesophageal Echocardiography. J Am Soc Echocardiogr. 1991;4(3):271–6.
Article CAS PubMed Google Scholar
Kahraman N, Topal D, Altunal AM, Tiryakioğlu SK, Taner T, Demir D, et al. A rare complication of double prosthetic valve endocarditis; reconstructive surgical treatment of mitral-aortic intervalvular fibrosa pseudoaneurysm and left atrial fistula. Echocardiography. 2023;40(1):51–6.
Goodwin EM, Sinner GJ, Goodwin RP, Kotter J. An Open and Shut Case: a lesson in infective endocarditis complications with a focus on the mitral-aortic intervalvular Fibrosa. CASE. 2019;3(3):115–9.
Agrawal Y, Konda M, Kalavakunta JK. Aorto-left atrial fistula: rare cause of acute cardiac failure in a previously healthy individual. J Saudi Heart Assoc. 2016;28(4):270–3.
Chandra S, Ameta D, Kharwar RB, Goyal M, Kumar D, Dwivedi SK, et al. Three-dimensional echocardiographic delineation of an Acquired Aorto-Left Atrial Fistula complicating native aortic valve endocarditis - advantage of three dimensions. Echocardiography. 2013;30(10):E326–30.
Kunavarapu C, Olkovsky Y, Lafferty JC, Homayuni AR, Mohan SS, McGinn J. Unusual Cardiac Complications of Staphylococcus aureus Endocarditis. Journal of the American Society of Echocardiography. 2008;21(2):187.e3-187.e5.
Bagh I, Marino TM, Silbiger JJ. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa with fistulous communication to the left atrium causing congestive heart failure. Clin Case Rep. 2021;9(6).
Pereira VH, Português J, Calvo L, Machado I, Azevedo O, Lourenço A. Aortic root to left atrium fistula: a rare complication of infective endocarditis in a native aortic valve. Int J Cardiovasc Imaging. 2014;30(6):1203–4.
Hasebe H, Takanohashi A, Shirota K, Nakamura H. Infective endocarditis with intermittent atrioventricular block and pseudoaneurysm of the mitral-aortic intervalvular fibrosa in a patient with severe aortic stenosis. Intern Med. 2016;55(19):2825–9.
Çimen T, Doğan M, Kızıltepe U, Akyel A, Sunman H, Yeter E. Mitral-aortic intervalvular fibrosa pseudoaneurysm with rupture into the left atrium: a three-dimensional trans-esophageal echocardiographic approach. Anatol J Cardiol. 2015;15(12):1030–1.
Tak T. Pseudoaneurysm of Mitral-Aortic Intervalvular Fibrosa [Internet]. Vol. 1, Clinical Medicine & Research. 2001. www.mfldclin.edu/clinmedres .
Velibey Y, Güngör B, Bolca O, Eren M. Mitral-aortik intervalvüler fibroza psödoanevrizmasi{dotless} ve 3b transezofageal ekokardiografik görüntülemenin tamamlayi{dotless}ci{dotless} rolü. 13, Anadolu Kardiyoloji Dergisi. 2013.
Grande AM, Amoroso F, Crimi G, Ferrario M, Mazzola A. Mitral-aortic intervalvular Fibrosa Pseudoaneurysm Causing Systolic Compression of Left Main trunk. Ann Thorac Surg. 2017;103(5):e461.
Agirbasli M, Fadel BM. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa: a long-term complication of infective endocarditis. Echocardiography. 1999;16(3):253–7.
Apostolidou E, Beale C, Poppas A, Stockwell P. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa: a Case Series with Literature Review. CASE. 2017;1(6):221–6.
Abdallah H, Michetti J, Demers P. Fistula formation following repair of a pseudoaneurysm of the mitral-aortic intervalvular fibrosa — a rare complication. J Cardiol Cases. 2017;15(5):170–2.
Zencirkıran Ağuş H. Infective endocarditis of bicuspit aortic valve complicated by septal aneurysm and mitral-aortic intervalvular fibrosa pseudoaneurysm. Turk Kardiyoloji Dernegi Arsivi-Archives of the Turkish Society of Cardiology; 2017.
Giannoccaro P, Ascah KJ, Sochowski RA, Chan KL, Ruddy TD. Spontaneous drainage of Paravalvular Abscess diagnosed by Transesophageal Echocardiography. J Am Soc Echocardiogr. 1991;4(4):397–400.
Lin A, Poppas A, Mansoor A, Fernandez AB. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa. J Cardiovasc Med. 2015;16:S33–4.
Article Google Scholar
Salerno D, Donati G, Forconi S, Gori T. Giant pseudoaneurysm of the mitro-aortic intervalvular fibrosa: incidental diagnosis. Intern Emerg Med. 2008;3(3):279–82.
Koch R, Kapoor A, Spencer KT. Stroke in patient with an intervalvular fibrosa pseudoaneurysm and aortic pseudoaneurysm. J Am Soc Echocardiogr. 2003;16(8):894–6.
Xie M, Li Y, Cheng TO, Wang X, Lu Q, He L, et al. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa. Int J Cardiol. 2013;166(1):2–7.
Fazlinezhad A, Fatehi H, Tabaee S, Alavi M, Hoseini L, Yousefzadeh H. Pseudoaneurysm of mitro-aortic intervalvular fibrosa during the course of mitral valve endocarditis with aorto-left ventricle outflow tract fistula. J Saudi Heart Assoc. 2012;24(3):201–4.
Article CAS PubMed PubMed Central Google Scholar
Download references
Acknowledgements
Not applicable.
No funding was received for the production of this work.
Author information
Authors and affiliations.
Cardiovascular Surgery Department, Clínica Avidanti, Tolima, Ibagué, Colombia
Rafael Figueroa-Casanova
Clínica Avidanti, Tolima, Ibagué, Colombia
Juan D. Saavedra-Henao, Diego A. Beltrán-Gonzales & José G. Labrador-Rosales
Universidad del Bosque, Bogotá, DC, Colombia
Juan S. Figueroa-Laverde
Universidad del Rosario, Bogotá, Colombia
Sara Eslait-Olaciregui
Surgery, Universidad El Bosque, Cundinamarca, Bogotá, Colombia
Carlos J. Pérez Rivera
You can also search for this author in PubMed Google Scholar
Contributions
RF analyzed the patient data and revised the work. JDS collected clinical information and was a contributor in writing the manuscripts. CJP analyzed and reviewed the manuscript. DAB retrieved patient information and contributed to the writing process. SE contributed to the design and drafting of the work. JGL reviewed and corrected the paper. JSF acquired clinical information and contributed with the drafting. All authors read and approved the final manuscript and agreed to be personally accountable for the authors own contribution.
Corresponding author
Correspondence to Carlos J. Pérez Rivera .
Ethics declarations
Ethical approval and consent.
The approval was granted by the bioethics committee of “Clínica Avidanti” in the city of Ibagué, Colombia with the reference number.
Consent for publication
Informed consent was signed and given by the patient’s parent.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Figueroa-Casanova, R., Saavedra-Henao, J.D., Figueroa-Laverde, J.S. et al. Fistula of the mitral-aortic intervalvular fibrosa in a patient with bacterial endocarditis: a case report and systematic literature review. J Cardiothorac Surg 19 , 300 (2024). https://doi.org/10.1186/s13019-024-02736-5
Download citation
Received : 06 October 2023
Accepted : 29 March 2024
Published : 28 May 2024
DOI : https://doi.org/10.1186/s13019-024-02736-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Endocarditis
- Mitral-aortic intervalvular fibrosa
- Transesophageal echocardiography
Journal of Cardiothoracic Surgery
ISSN: 1749-8090
- General enquiries: [email protected]
- Case report
- Open access
- Published: 31 May 2024
Surgical intervention of Lemierre’s syndrome: a case report and review of the literature
- Yiqi Pan 1 na1 ,
- Zhihong Shi 1 na1 ,
- Qian Da 2 ,
- Chaofu Wang 2 ,
- Yilin Shen 1 &
- Mingliang Xiang ORCID: orcid.org/0000-0001-9253-9852 1
Journal of Medical Case Reports volume 18 , Article number: 265 ( 2024 ) Cite this article
Metrics details
Lemierre’s syndrome is a fatal and rare disease that is typically characterized by oropharyngeal infection and internal jugular vein thrombosis. Timely institution of appropriate antibiotics is the standard treatment.
Case presentation
The authors report a case of Lemierre’s syndrome. A 67-year-old male patient of Han ethnicity in China suffered from a large inflammatory neck mass involving left internal jugular vein thrombosis diagnosed as Lemierre’s syndrome and finally cured by surgical treatment. In addition, a literature review was carried out through PubMed using the terms “Lemierre’s syndrome/disease and review, meta-analysis or retrospective study” and “Lemierre’s syndrome/disease and internal jugular vein”. This search yielded six articles that recorded surgical methods such as drainage, craniotomy, tooth extraction, and ligation of the occluded vein to give clinicians more ideas about the treatment of the Lemierre’s syndrome.
This is the first review to summarize the conditions under which surgical treatment are conducted. Additionally, this is the first report of such a large inflammatory neck mass that was completely cured by surgical resection and internal jugular vein ligation. The authors also offer several conclusions regarding surgical intervention in Lemierre’s syndrome for the first time.
Peer Review reports
Internal jugular vein (IJV) thrombosis is a relatively rare and urgent disease. In a retrospective study, the number of such cases occurring from 2001 to 2008 was 2.5 times that occurring from 1991 to 2000 and 20 times that occurring from 1980 to 1990 [ 1 ]. The reasons behind the increasing incidence of IJV thrombosis include the increase in antibiotic resistance, widespread use of hemodialysis, general application of central venous catheters, expansion of assisted reproductive technology, and increasing incidence of cancer [ 2 ]. In a 9-year retrospective study of 1948 patients with deep vein thrombosis, only 29 patients developed IJV thrombosis, of whom 23 had IJV thrombosis secondary to another condition, such as a malignancy (for example, Trousseau syndrome), central venous catheter implantation, or ovarian hyperstimulation syndrome (OHSS) [ 3 ]. In addition, bilateral internal jugular vein thrombosis is an important indicator of malignant tumors. In a 5-year retrospective study of 41 patients with IJV thrombosis in Germany, paraneoplastic thrombosis accounted for 54% of cases; of these cases, otolaryngology head and neck diseases accounted for 68%. The other patients mostly had inflammatory diseases [ 4 ].
In the ear, nose, and throat (ENT) field, IJV thrombosis is commonly associated with Lemierre’s syndrome (LS), which is a complication of infectious diseases, such as otitis media and oropharyngeal abscess or infection. LS is commonly defined by the following diagnostic criteria: (1) oropharyngeal infection; (2) internal jugular vein thrombophlebitis or thrombosis; (3) septic emboli at a remote site, more frequently the lungs; and (4) isolation of Fusobacterium nucleatum on blood culture [ 5 ]. LS is usually accompanied by septic emboli in the lungs or other organs [ 6 ]. Under some rare conditions, LS can also be triggered by tooth extraction [ 7 ]. Pulmonary embolism, with an incidence of approximately 10%, and postthrombotic syndromes, such as limb pain, heaviness, venous dilatation, edema, pigmentation, nutritional skin changes, and venous ulcers, are complications of IJV thrombosis [ 4 ]. Therefore, ENT doctors should give enough attention to patients with IJV thrombosis to avoid the disastrous results caused by pulmonary or cerebral thrombosis.
To the best of our knowledge, this is the first case of such a large infectious neck mass with internal jugular vein thrombosis that was completely cured by surgical intervention.
A 67-year-old Chinese male of Han ethnicity developed pain in the left neck 14 days prior after eating mud fish. He was healthy and denied a history of infectious diseases, chronic diseases, thrombotic disease, surgical trauma, blood transfusion, allergies, or contact with poisonous substances. Physical examination on admission revealed the following: fever, chills, fatigue, mild dysuria, diffusive swelling pain of the neck on the left side, and high skin temperature. His left neck was tender and edematous with cellulitis. The mass was scleroid with a liquefied center. Other parameters were as follows: white blood cell (WBC) count, 12.2 × 10 9 /L; neutrophil count, 11.76 × 10 9 /L; platelet (PLT) count, 51 × 10 9 /L; C-reactive protein (CRP) level, 146 mg/L; procalcitonin (PCT), 156.99 ng/mL; temperature, 39.5 °C; respiratory rate, 24 breaths per minute; pulse, 118 beats per minute; and blood pressure (BP), 109/61 mmHg. The patient stated that he had been to many hospitals in the last 2 weeks and that the use of antibiotics such as ceftriaxone and metronidazole slightly alleviated his neck pain at first. However, the effect was temporary and no longer present after he transferred to Shanghai, and 3 days before he presented to our hospital, he noticed extreme swelling and felt increased pain in the area of his neck mass (Fig. 1 ). A series of further examinations were performed, with the following results: glucose, 22.13 mmol/L; activated partial thromboplastin time (APTT), 43.1 seconds; prothrombin time (PT), 17.3 seconds; fibrinogen (Fg), 5.0 g/L; fibrin/fibrinogen degradation products (FDP), 7.3 mg/L; and D-dimer (D-D), 2.1 mg/L. Urine analysis was positive for glucose, blood, protein, and white blood cells. Infectious diseases and acute nephrology were considered. The culture of blood and fluid obtained from the mass was negative for any bacteria including anaerobic bacteria, mycoplasma, or fungus. Ultrasound revealed mixed echogenicity in the left neck mass that was irregular in shape. The mass was approximately 74 × 37 mm in size. Color Doppler revealed generalized thrombosis of the internal jugular vein. CT of the chest and neck was conducted and suggested that the cervical abscess extended to the thorax and superior mediastinum, without a signal from the left internal jugular vein. Multiple enlarged cervical lymph nodes were observed. The trachea and left thyroid were also compressed (Fig. 2 ). Video laryngoscopy excluded the possibility of pyriform sinus fistula or any foreign body. Pus obtained from the mass showed many neutrophils and large amounts of necrotic tissue, and 14 days of combination antibiotic treatment (imipenem and teicoplanin) and regular insulin therapy in our hospital returned the patient’s temperature, routine blood markers, CRP and PCT levels, and coagulation function to normal. However, the neck mass remained. Therefore, the surgery department was consulted.
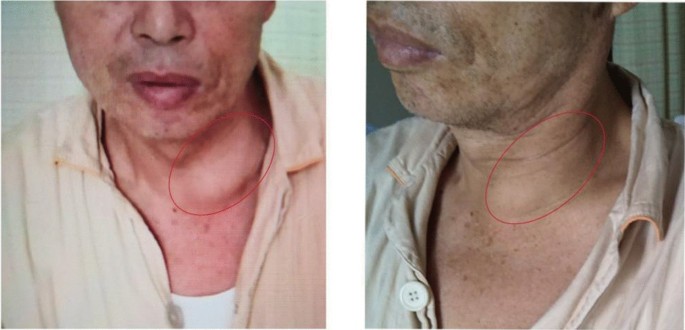
Picture of the patient’s neck showed a huge mass with tenderness (red circles)
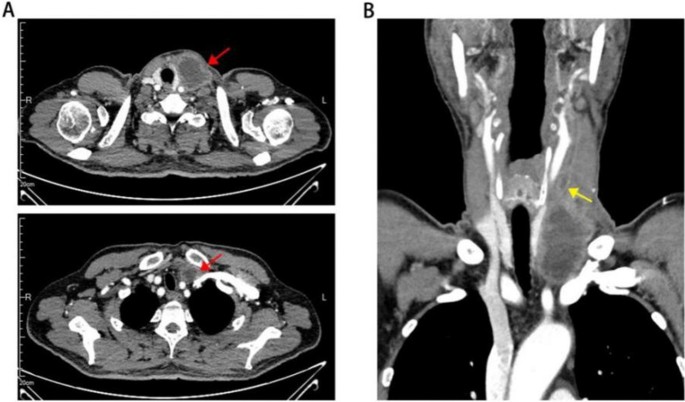
A Coronal plane of computed tomography showed neck mass spread down to superior mediastinum with iquefactive necrosis in the center (red arrows). Trachea was oppressed to the right side. B Significant intraluminal filling defect and thrombosis was found in internal jugular vein (yellow arrow)
The patient was taken to the operating room. The neck mass adhered tightly to the surrounding tissue, and the involved segment of the left internal jugular vein was exposed by sharp dissection (Fig. 3 A). The proximal part of the mass needed to be ligated first to avoid small thrombus detachment. There was a large amount of inflammation and fibrosis present in the involved area. The involved segment of the internal jugular vein and the whole neck mass were completely resected, and the distal part of the internal jugular vein was fully ligated (Fig. 3 B). The pathological examination showed hemorrhagic necrosis with the proliferation of fibrous and granulation tissue and the accumulation of foam-like cells and multinuclear giant cells (Fig. 4 ). The patient recovered and was discharged a week after surgery. No other adverse events happened.
Intraoperative view showed ligation of internal jugular vein and separation of the mass ( A ). The resected neck mass as well as left internal jugular vein was shown. Intraluminal thrombosis could be seen clearly when opening the internal jugular vein ( B )
Representative pathological photomicrograph demonstrated thrombus ( A ) and abscess formation ( B )
Literature review
It is rare that patients with Lemierre’s syndrome require surgical intervention when antibiotics and anticoagulant therapies fail. This is the first study to summarize cases of LS requiring surgical treatment (not including abscess drainage). First, we decided to collect previous reviews and meta-analyses to obtain good knowledge of the rate of surgery in LS. Search strategy and selection criteria were as follows. A search of the literature in MEDLINE was performed through PubMed to identify relevant English language articles from 1980 to 2022. The following search terms were used: “Lemierre’s syndrome/disease and review, meta-analysis or retrospective study” and “Lemierre’s syndrome/disease and internal jugular vein”. The references of the retrieved articles were also reviewed to identify additional sources. Through reading the abstract and the full text, we found a total of six reviews and meta-analyses (Table 1 ) that included detailed descriptions of patients who underwent surgery (not including abscess drainage). In a retrospective review from 1998 to 2010 at a local tertiary referral hospital, 17 of the 23 patients underwent surgical treatment of the primary infection site [ 8 ]. In a 5-year systematic review, surgical procedures, such as tooth extraction, craniotomy, and ligation of the occluded vein, were performed in five patients to prevent further septic emboli [ 9 ]. A retrospective study from June 2000 to May 2016 showed that IJV ligation was performed in only one of five LS cases at the Children’s Hospital of Alabama [ 10 ]. In an 8-year Swedish nationwide retrospective study, three patients with peritonsillitis were surgically treated by tonsillectomy [ 11 ]. In the latest meta-analysis of 394 patients in 2020, only 10 patients underwent IJV ligation/excision, only 1 patient underwent ligation/excision of the thrombosed external carotid artery, 3 underwent endoscopic sinus surgery, and 11 underwent mastoidectomy [ 12 ]. In the latest systematic review, which included the most LS cases reported, surgical procedures were performed in 101 patients, and 31 patients underwent IJV ligation/embolectomy [ 13 ].
In this study, we report a case of Lemierre’s syndrome in an elderly male caused by an acute infectious neck mass. Timely comprehensive medical and surgical treatments were given to avoid serious complications.
Internal jugular vein thrombosis is a rare and serious emergent disease that needs to be identified early in the course, as it can lead to catastrophic consequences, such as stroke or pulmonary embolism. The main pathological basis of internal jugular vein thrombosis is as follows: (1) injury of venous intima; (2) slowing down of blood flow; and (3) hypercoagulability. The common causes are as follows [ 14 , 15 , 16 ]: (1) facial infection, such as furuncle and carbuncle, sinusitis, otitis media, and suppurative tonsillitis; bacteria can spread through the damaged mucosa and parapharyngeal space or invade the jugular vein through the lymphatic and venous systems, leading to infectious phlebitis and bacterial embolism information; (2) long duration of internal jugular vein catheterization; (3) head and neck surgery; (4) head and neck tumor; (5) pulmonary embolism; and (6) other systemic diseases, such as polycythemia. Doctors need to take care of patients immediately when encountering such cases.
Lemierre’s syndrome can show the typical symptoms and signs of progressive infection, including sore throat, fever, or neck pain. A systematic review of Lemierre’s syndrome by Peter et al . found that in 84 patients, the most common first clinical presentation was a sore throat (33%), followed by a neck mass (23%) and neck pain (20%) [ 1 ]. In the current case, the patient presented with fever and neck pain at first, followed by a neck mass. The use of antibiotics before he was transferred to our hospital was ineffective. The white blood cell count, PCT level, and erythrocyte sedimentation rate (ESR) were elevated, and blood appeared in his urine. We adjusted the treatment to the combined application of broad-spectrum antibiotics, including imipenem and teicoplanin, for another 2 weeks. The patient’s body temperature returned to normal and laboratory testing showed that the patient’s infectious condition had been controlled, but the neck mass and internal jugular vein thrombosis persisted and required surgical treatment.
Fusobacterium necrophorum is the main pathogen of Lemierre’s syndrome [ 1 ]. However, in this case, the culture of both blood and fluid obtained from the mass was negative for bacteria, which might be because the patient had been treated with antibiotics (mainly including ceftriaxone and metronidazole) for nearly 2 weeks before coming to our hospital. This also suggests that it is particularly important for doctors to culture blood or fluid from the mass before any use of antibiotics in these patients. In many reviews, a large proportion of the cases also did not report any microbiological agent [ 9 , 12 , 13 ] and thus far a clinical diagnosis of LS is still valid if the bacteria go undetected. As for the reason about how this patient got such infectious disease, considering that he had eaten mud fish before the onset of disease and CT showed that mucous of left pyriform sinus was edematous and enhanced (Fig. 5 ), we considered that neck infection might be secondary to the infection of mucous scratch in left pyriform sinus, and uncontrolled hyperglycemia was an important factor to cause serious infection.
Coronal plane of computed tomography showed disappearance of left pyriform sinus surrounding by abnormal enhancement (yellow arrow)
The diagnosis of internal jugular vein thrombosis in Lemierre’s syndrome relies on imaging examination. Ultrasound is the first choice for the diagnosis of LS, and CT and magnetic resonance imaging (MRI) are currently implemented in general practice when necessary. Albertyn et al . first summarized the classic imaging features of internal jugular vein thrombosis. Ultrasonography shows the vein to be distended and nonpulsatile, with internal echoes. CT shows swelling of the adjacent soft tissues, distension of the vein with wall enhancement, and low-attenuation intraluminal filling defects. However, ultrasound has limitations and cannot display the anatomy behind the clavicle or mandible [ 16 ]. In this case, we found that although US can clearly show internal jugular vein thrombosis, knowing its boundaries and connection with the tumor still depends on CT or MRI, especially when there is an urgent need for surgery. A full assessment by preoperative imaging is of great importance. This is also consistent with the views of Charles et al . [ 17 ].
Priority treatment for LS includes antibiotic therapy and drainage of the infected site. Here in our hospital, considering that cephalosporins such as ceftriaxone did not control the fever and pain in this patient, imipenem and teicoplanin were used according to experience to cover wide varieties of Gram-negative and Gram-positive bacteria, including both anaerobic and aerobic bacteria. In addition, teicoplanin has lower side effects than vancomycin [ 18 ]. Rarely, other surgical procedures, such as ligation of the occluded vein, craniotomy, and tooth extraction, are performed. Antithrombotic therapy, including novel oral anticoagulants (DOACs), is also recommended depending on the individual’s condition. However, it remains controversial whether anticoagulation or antithrombotics are effective in Lemierre’s syndrome. Some scholars think that thrombosis is due to the infection process and can be resolved when the infection has been controlled [ 19 ]. In this case, we did not immediately apply anticoagulant or thrombolytic therapies considering that the APTT of this patient was significantly prolonged at the time he came to our hospital and the consumption of platelets was relatively high; emergency anticoagulant therapy may have increased his risk of bleeding. To date, there have been no sufficient clinical studies and no sufficient evidence suggesting the necessity for anticoagulant therapy in Lemierre’s syndrome [ 20 ]. Previous studies have also reported the occurrence of extensive suppurative thrombophlebitis of the bilateral IJV and superior vena cava in patients with Lemierre’s syndrome despite the use of antibiotics and anticoagulant therapy; adjunctive catheter-directed thrombolysis and superior vena cava stenting were performed to help these patients completely recover [ 21 ]. Anticoagulation therapy has not been shown to reduce the complications of Lemierre’s syndrome, such as sepsis [ 17 ]. Meanwhile, Johannesen et al . did not find that anticoagulation therapy decreased the mortality rate or course of the disease or reduced the duration of antibiotic use [ 9 ]. However, anticoagulation therapy is recommended in patients with a poor clinical response despite antibiotic therapy and with a high risk of intracranial thrombosis or recurrent thrombophlebitis [ 22 , 23 , 24 ]. In this case, the cause of internal jugular vein thrombosis was largely infection, so surgical treatment was the best choice when antibiotics could not completely cure the infection and thrombosis. Through previous retrospective studies, systematic reviews and meta-analyses obtained by database searches, we summarized the following points regarding surgical intervention in Lemierre’s syndrome:
When patients do not respond to conservative medical therapy and continue to show extensive septic thrombosis or uncontrolled severe sepsis, surgical treatments need to be considered.
Abscess drainage is the most common and convenient surgical treatment for abscesses upon formation.
Surgical treatment of the primary infection site is effective for controlling the spread of infection and sepsis.
IJV ligation or excision is suitable for patients with persistent septic embolization after treatment with antibiotics and anticoagulants.
IJV ligation or excision is also appropriate to avoid thrombus detachment when anticoagulation therapy or catheter-directed thrombolysis is ineffective.
Lemierre’s syndrome is an extremely rare disease, but the fatality rate can reach 15%, even with escalating antibiotic therapy [ 21 ] . Therefore, early diagnosis is particularly important, and the timely institution of appropriate antibiotics is the standard treatment. Surgical intervention may be the only effective option for controlling the source of infection or when conservative medical treatment fails.
Availability of data and materials
Available from corresponding author on reasonable request.
Abbreviations
Internal jugular vein
Ear, nose, and throat
- Lemierre’s syndrome
White blood cell
C-reactive protein
Procalcitonin
Blood pressure
Computed tomography
Magnetic resonance imaging
Karkos PD, Asrani S, Karkos CD, et al . Lemierre’s syndrome: a systematic review. Laryngoscope. 2009;119(8):1552–9.
Article PubMed Google Scholar
Leibman Y, Ayalon M, Steiner IP. Internal jugular venous thrombosis after in vitro fertilization. J Emerg Med. 2009;37(1):29–31.
Gbaguidi X, Janvresse A, Benichou J, Cailleux N, Levesque H, Marie I. Internal jugular vein thrombosis: outcome and risk factors. QJM. 2011;104(3):209–19.
Article CAS PubMed Google Scholar
Hahn J, Nordmann-Kleiner M, Hoffmann TK, Greve J. Thrombosis of the internal jugular vein in the ENT-department—Prevalence, causes and therapy: a retrospective analysis. Auris Nasus Larynx. 2019;46(4):624–9.
Kuppalli K, Livorsi D, Talati NJ, Osborn M. Lemierre’s syndrome due to Fusobacterium necrophorum. Lancet Infect Dis. 2012;12(10):808–15.
Yombi JC, Bogaert T, Tribak K, Danse E. Lemierre syndrome of the femoral vein, related to Fusobacterium necrophorum abscess of vastus lateralis. J Emerg Med. 2016;50(4):e191–3.
Miyamoto S, Toi T, Kotani R, et al . Lemierre syndrome associated with ipsilateral recurrent laryngeal nerve palsy: a case report and review. NMC Case Rep J. 2016;3(3):53–7.
Article PubMed PubMed Central Google Scholar
Schubert AD, Hotz MA, Caversaccio MD, Arnold A. Septic thrombosis of the internal jugular vein: Lemierre’s syndrome revisited. Laryngoscope. 2015;125(4):863–8.
Johannesen KM, Bodtger U. Lemierre’s syndrome: current perspectives on diagnosis and management. Infect Drug Resist. 2016;9:221–7.
Jariwala RH, Srialluri S, Huang MZ, Boppana SB. Methicillin-resistant Staphylococcus aureus as a cause of Lemierre’s syndrome. Pediatr Infect Dis J. 2017;36(4):429–31.
Nygren D, Holm K. Invasive infections with Fusobacterium necrophorum including Lemierre’s syndrome: an 8-year Swedish nationwide retrospective study. Clin Microbiol Infect. 2020;26(8):1089.e7-1089.e12.
Gore MR. Lemierre syndrome: a meta-analysis. Int Arch Otorhinolaryngol. 2020;24(3):e379–85.
Valerio L, Zane F, Sacco C, et al . Patients with Lemierre syndrome have a high risk of new thromboembolic complications, clinical sequelae and death: an analysis of 712 cases. J Intern Med. 2021;289(3):325–39.
Riordan T. Human infection with Fusobacterium necrophorum (Necrobacillosis), with a focus on Lemierre’s syndrome. Clin Microbiol Rev. 2007;20(4):622–59.
Article CAS PubMed PubMed Central Google Scholar
Chirinos JA, Lichtstein DM, Garcia J, Tamariz LJ. The evolution of Lemierre syndrome: report of 2 cases and review of the literature. Medicine (Baltimore). 2002;81(6):458–65.
Albertyn LE, Alcock MK. Diagnosis of internal jugular vein thrombosis. Radiology. 1987;162(2):505–8.
Charles K, Flinn WR, Neschis DG. Lemierre’s syndrome: a potentially fatal complication that may require vascular surgical intervention. J Vasc Surg. 2005;42(5):1023–5.
Liu CY, Lee WS, Fung CP, et al . Comparative study of teicoplanin vs vancomycin for the treatment of methicillin-resistant Staphylococcus aureus bacteraemia. Clin Drug Investig. 1996;12(2):80–7.
Campo F, Fusconi M, Ciotti M, et al . Antibiotic and anticoagulation therapy in Lemierre’s syndrome: case report and review. J Chemother. 2019;31(1):42–8.
Chapman R, Tully A. A life-threatening sore throat. Lancet. 2004;364(9428):112.
Choi BM, Son SW, Park CK, Lee SH, Yoon HK. Extensive bilateral Lemierre syndrome due to methicillin-resistant staphylococcus epidermidis in a patient with lung adenocarcinoma. Tuberc Respir Dis (Seoul). 2015;78(3):289–92.
Phan T, So TY. Use of anticoagulation therapy for jugular vein thrombus in pediatric patients with Lemierre’s syndrome. Int J Clin Pharm. 2012;34(6):818–21.
Lakshminarayana PH, Woodske ME. A unique case of lemierre syndrome associated with thrombophilia in an adult and the role of anticoagulation. Case Rep Med. 2010;2010:1.
Article Google Scholar
Schmid T, Miskin H, Schlesinger Y, Argaman Z, Kleid D. Respiratory failure and hypercoagulability in a toddler with Lemierre’s syndrome. Pediatrics. 2005;115(5):e620–2.
Download references
Acknowledgements
Not applicable.
This study was supported by the National Natural Science Foundation of China (grant nos. 82101212, 82101209, 82301296, 82301297), Science and Technology Commission of Shanghai Municipality (grant nos. 23ZR1440200, 21ZR1440200, SHDC2020CR1044B-003), Shanghai “Rising Stars of Medical Talents” Youth Development Program, and Shanghai Municipal Hospital ENT Specialist Alliance.
Author information
Yiqi Pan and Zhihong Shi contributed equally to this work.
Authors and Affiliations
Department of Otolaryngology and Head and Neck Surgery, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Yiqi Pan, Zhihong Shi, Bin Ye, Yilin Shen & Mingliang Xiang
Department of Pathology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Qian Da & Chaofu Wang
You can also search for this author in PubMed Google Scholar
Contributions
Mingliang Xiang performed the whole operation and checked the final review. Yilin Shen revised and reviewed the original manuscript. Yiqi Pan wrote the whole manuscript. Zhihong Shi took part in the whole operation and carried out the literature review. Bin Ye reviewed the manuscript. Chaofu Wang and Qian Da provided the pathological images. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Yilin Shen or Mingliang Xiang .
Ethics declarations
Ethics approval and consent to participate.
The studies involving human participants were reviewed and approved by Human Ethics Committee, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. The patient provided his written informed consent for the use of his images and other clinical information in this study. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
There are no conflicts of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Pan, Y., Shi, Z., Ye, B. et al. Surgical intervention of Lemierre’s syndrome: a case report and review of the literature. J Med Case Reports 18 , 265 (2024). https://doi.org/10.1186/s13256-024-04584-2
Download citation
Received : 15 March 2024
Accepted : 22 April 2024
Published : 31 May 2024
DOI : https://doi.org/10.1186/s13256-024-04584-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Head and neck infection
- Internal jugular vein thrombosis
- Surgical treatment
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Search Menu
- Sign in through your institution
- Volume 2024, Issue 5, May 2024 (In Progress)
- Volume 2024, Issue 4, April 2024
- Case of the Year
- MSF Case Reports
- Audiovestibular medicine
- Cardiology and cardiovascular systems
- Critical care medicine
- Dermatology
- Emergency medicine
- Endocrinology and metabolism
- Gastroenterology and hepatology
- Geriatrics and gerontology
- Haematology
- Infectious diseases and tropical medicine
- Medical ophthalmology
- Medical disorders in pregnancy
- Paediatrics
- Palliative medicine
- Pharmacology and pharmacy
- Radiology, nuclear medicine, and medical imaging
- Respiratory disorders
- Rheumatology
- Sexual and reproductive health
- Sports medicine
- Substance abuse
- Author Guidelines
- Submission Site
- Open Access
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, case presentation, acknowledgements, conflict of interest statement.
- < Previous
A peculiar foreign body ingestion in 2-year-old girl complicated by esophageal perforation: case report and review of the literature
- Article contents
- Figures & tables
- Supplementary Data
Danah Albarrak, Suliman Alrajhi, Mohammed Naeem, A peculiar foreign body ingestion in 2-year-old girl complicated by esophageal perforation: case report and review of the literature, Oxford Medical Case Reports , Volume 2024, Issue 5, May 2024, omae040, https://doi.org/10.1093/omcr/omae040
- Permissions Icon Permissions
Foreign body ingestion is a common pediatric gastrointestinal emergency, which should be suspected in all patients who present with signs of airway obstruction or upper GI bleeding, especially if it developed after the child was left unwitnessed for a while. The most common foreign bodies identified in the literature are button batteries or coins. Early identification and management of suspected foreign body ingestion is crucial as it can lead to devastating complications including bleeding, fistula formation, perforation, mediastinitis, or abscess. Here we report a case of a peculiar foreign body ingestion resulting in esophageal perforation in a 2-year-old girl.
Foreign body (FB) ingestion is considered a common gastrointestinal (GI) emergency in the pediatric population with the majority of cases occurring in children aged 6 months to 5 years [ 1 , 2 ]. The most common FB identified are coins, however, button batteries, magnets, toys, jewelry, and less commonly sharp objects have been reported in the literature [ 1 , 2 ]. Based on the 2021 Annual American Association of Poison Control Center report, FB ingestion in children less than 5 years accounted for approximately 55 000 cases with higher rates observed among boys [ 3 ]. Clinical presentation varies depending on the type and location of the FB ingested however common symptoms may include drooling, vomiting, dysphagia, throat, or chest pain [ 4 ]. Fortunately, the mortality rate is low and the majority of FBs ingested are spontaneously passed [ 1 ]. However, based on current guidelines endoscopic retrieval is indicated if the FB is impacted in the esophagus within 2hrs in case of battery ingestion with or without symptoms. A coin, magnet, or sharp foreign bodies impacted in the esophagus in an asymptomatic patient can be removed within 24hrs [ 5 ]. Any long foreign (more than 6-cm) in the esophagus should be removed within 24hrs even if the patient is asymptomatic. Only < 1% of patients may require further surgical intervention [ 1 ]. Possible complications of FB ingestion include the development of ulceration, bleeding, fistula formation, perforation, mediastinitis, or abscess [ 1 ]. Here we report a case of esophageal perforation caused by FB ingestion in a 2-year-old girl.
A 2-year-old developmentally normal girl with no significant past medical or surgical history presented to the emergency department (ED) with a three-day history of vomiting large amounts of fresh blood with clots and a single episode of dark stool. The family denied any history of fever, abdominal pain, upper respiratory tract symptoms, bleeding from other sites, easy bruising, or trauma. Two days prior to this presentation the family sought medical attention in another hospital, the patient was found to have a low hemoglobin (Hb) level of 5 g/dl. She was suspected to have foreign body ingestion and was recommended to proceed with an endoscopy. However, the family refused and the patient was discharged against medical advice.
Upon physical examination, vital signs were as follows HR 127 beats/minute, BP 84/39 mmHg, RR 26 breaths/minute, afebrile, and oxygen saturation 98% on room air. The patient was alert and active, with intact pulses, and warm extremities with no bleeding, bruises, or skin changes; the rest of the physical examination was unremarkable. The repeated Hb level was 4 g/dl, thus the patient received 10 ml/kg (-O) packed red blood cells (PRBC) transfusion. Initial chest x-ray (CXR) revealed a round radiolucent object seen lateral to the trachea on the left side with mild widening of the mediastinum, and lung fields were clear ( Fig. 1 ). The patient was anticipated to have a difficult airway and therefore was intubated to proceed with imaging safely, CXR was repeated revealing an opacity in the right upper lung likely due to aspiration ( Fig. 1 ). Computed tomography (CT) showed a hyperdense object measuring 18.1 mm, seen in the posterior mediastinum, consolidation in the right upper lobe due to aspiration and mediastinal hematoma ( Fig. 2 ).

( A ) initial CXR in the ED: reveals a round radiolucent object seen lateral to the trachea on the left side with mild widening of the mediastinum and clear lung fields. ( B ) Repeated CXR 4hrs after, an endotracheal tube is observed and there is an opacity in the right upper lung likely due to aspiration.

Findings of chest CT on the day of patient presentation (red arrows). ( A ) axial view: shows hyperdense object seen in the posterior mediastinum likely within the esophagus. ( B ) Axial and lung window view: hyperdense subject seen in the posterior mediastinum and there is consolidation in the right upper lobe (aspiration). ( C ) Coronal view: hyperdense object (FB) seen in the posterior mediastinum. ( D ) Sagittal view: hyperdense FB seen in the posterior mediastinum. Maximum intensity projection (MIP) imaging revealed a round hyperdense FB measure it 18.1 mm seen in the posterior mediastinum in multiple views, ( E ) Coronal view, ( F ) sagittal view.
The patient was rushed for exploratory thoracotomy and FB retrieval with concurrent esophagogastroduodenoscopy (EGD). Initially, rigid endoscopy was performed and revealed pooling of blood inside the esophageal lumen with bulging of the mucosa, there was no active bleeding. Left thoracotomy revealed multiple perforated feeding vessels to the pleura, which were ligated using a clip. There was no evidence of fistula, active bleeding, or inflammatory process in that area. The esophagus was skeletonized and showed no pus collection, hematoma, or cyst. However, it couldn’t be skeletonized more proximally as the arch of the aorta was intervening. The area was reexamined using rigid endoscopy and there was no evidence of perforation or active bleeding: therefore; proceeded with a fluoroscopic esophagogram which showed an esophageal pouch at the left second rib, suspicious of esophageal duplication cyst. The gastrology department was consulted to perform flexible endoscopy, which revealed a cystic-like lesion in the esophagus at almost 15 cm from the mouth with no evidence of perforation, guidewire was inserted in the cyst for the surgeon to continue.
Left thoracotomy was closed and converted to right thoracotomy which revealed a cystic-like structure, esophageal pouch, which was flimsy and macerated. An enterotomy was performed in the anterior esophageal wall almost 3 cm in length. The esophageal lumen was examined from the inside, and old digested blood coming from the esophageal lumen was noted: however; there was no active bleeding. Additionally, the cyst was followed to its origin and proximal to the enterotomy made, upon closer inspection and dissection the FB was found eroding the esophagus coming from the esophageal mucosa which was easily retrieved ( Fig. 3 ). The operation was concluded by a primary repair of the esophageal side perforation and repair of the esophageal enterotomy.

Picture of the foreign body after retrieval.
Following the surgery, the patient was admitted to PICU for close monitoring. Initially, the patient was doing well: however; on the third day she suddenly developed emesis and epistaxis and had systolic hypotension, tachycardia, and tachypnea. She improved after being managed with 20 ml/kg stat bolus, PRPCs, and platelet transfusion. Additionally, she developed left followed by right-sided pneumothorax which improved the next day. Moreover, the patient’s blood culture came back positive for gram cocci in chains (anginosus group), infectious disease department was consulted and recommended treatment with tazocin. Fluoroscopy was performed seven days postop revealing a narrowing of the upper esophagus with passing contrast to the lower esophagus and stomach ( Fig. 4 ). On day 12, the patient improved clinically and was discharged.

Fluoroscopy on day 7 post-op: revealed a narrowing of the upper esophagus (white arrows) with passing contrast to the lower esophagus and stomach.
FB ingestion is frequently encountered in pediatric emergencies, patients might present with a wide array of symptoms including drooling, dysphagia, vomiting, or emesis [ 4 ]. Timely identification of FB and management is essential to avoid possible complications which can include but are not limited to esophageal ulceration, bleeding, fistula formation, and perforation [ 1 ].
Based on the review of the literature 12 cases reported esophageal perforation secondary to FB ingestion in children aged 24 months or less ( Table 1 ). The most common FBs were button batteries and metal objects, none of the FBs identified was similar to the one retrieved from our patient. Delayed presentation and prolonged duration of impaction, especially > 1 week resulted in the development of multiple complications. Most patients were alive and well on follow-up with no complications, 2 lost follow-up, and 2 developed complications including vocal cord paralysis and intermittent croup [ 6–11 ].
Literature review of esophageal perforation secondary to FB ingestion in children aged 24 months or less
In our case, the patient had delayed presentation of FB ingestion as she started to develop signs of Upper GI bleeding 3 days prior. CT confirmed the presence of a round hyperdense FB in the posterior mediastinum. Flexible endoscopy was only able to identify a spot in the esophagus that looked cystic-like lesion. An emergency thoracotomy retrieved the FB that had eroded the esophagus. This foreign body was found to be a cork that is widely used in the caps of disposable bottles. Postoperatively the patient was admitted to the PICU for close monitoring and later discharged once she was clinically stable.
FB ingestion should be suspected in all pediatric patients who present with signs of airway obstruction or upper GI bleeding, especially if it developed after the child was left unwitnessed for a while. Parental education regarding prevention, signs and symptoms of FB ingestion is imminent in order to prevent delayed presentation and the development of devastating complications.
Primary care and emergency healthcare workers should consider FB ingestion as a differential diagnosis in any pediatric patient presenting with symptoms of airway compromise or GI bleeding, especially if there is a history of leaving the child unchaperoned. Additionally, community education on the topic is recommended to allow early recognition, and treatment and to avoid the development of any complications.
Not applicable.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The author(s) received no financial support for the research, authorship, and/or publication of this article.
The patient provided informed consent for publication of the case report.
Eisen GM , Baron TH , Dominitz JA , Faigel DO , Goldstein JL , Johanson JF . et al. Guideline for the management of ingested foreign bodies . Gastrointest Endosc 2002 ; 55 : 802 – 6 .
Google Scholar
Dorterler ME , Günendi T . Foreign body and caustic substance ingestion in childhood . Open Access Emerg Med 2020 ; 12 : 341 – 52 .
Gummin DD , Mowry JB , Beuhler MC , Spyker DA , Rivers LJ , Feldman R . et al. 2021 annual report of the national poison data system© (npds) from america’s poison centers: 39th annual report . Clin Toxicol (Phila) 2022 ; 60 : 1381 – 643 .
Rybojad B , Niedzielska G , Niedzielski A , Drozak ER , Rybojad P . Esophageal foreign bodies in pediatric patients: a thirteen-year retrospective study . ScientificWorldJournal 2012 ; 2012 : 102642 .
Kramer RE , Lerner DG , Lin T , Manfredi M , Shah M , Stephen TC . et al. Management of ingested foreign bodies in children: a clinical report of the naspghan endoscopy committee . J Pediatr Gastroenterol Nutr 2015 ; 60 : 562 – 74 .
Leinwand K , Brumbaugh DE , Kramer RE . Button battery ingestion in children: a paradigm for management of severe pediatric foreign body ingestions . Gastrointest Endosc Clin N Am 2016 ; 26 : 99 – 118 .
Shah PV , Wathen J , Keyes J , Osborne C , Messacar K , Stence N . et al. Foreign body esophageal perforation leading to multifocal brain abscesses: a case report . J Emerg Med 2020 ; 59 : 131 – 5 .
Chang MY , Chang ML , Wu CT . Esophageal perforation caused by fish vertebra ingestion in a seven-month-old infant demanded surgical intervention: a case report . World J Gastroenterol 2006 ; 12 : 7213 – 5 .
Kimball SJ , Park AH , Rollins MD , Grimmer JF , Muntz H . A review of esophageal disc battery ingestions and a protocol for management . Arch Otolaryngol Head Neck Surg 2010 ; 136 : 866 – 71 .
Panella NJ , Kirse DJ , Pranikoff T , Evans AK . Disk battery ingestion: case series with assessment of clinical and financial impact of a preventable disease . Pediatr Emerg Care 2013 ; 29 : 165 – 9 .
Oliveros L , McIntosh C , Wilsey A , Karjoo S , Wilsey M . "Jingle all the way!": sharp foreign bodies embedded within the esophageal mucosa during the holiday season . Cureus 2022 ; 26 : e24493 .
Email alerts
Citing articles via, affiliations.
- Online ISSN 2053-8855
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Case Report
- Open access
- Published: 29 May 2024
Repeated acute coronary syndrome caused by a mind-bending mural thrombus in ascending aorta: a case report and review of the literature
- Hanxuan Liu 1 na1 ,
- Zhangjie Yu 2 na1 ,
- Ying Xu 1 ,
- Yan Zhou 2 ,
- Juntao Yang 1 ,
- Yinyin Qiu 1 ,
- Yangbo Xing 2 ,
- Fang Peng 2 &
- Weiliang Tang 2
BMC Cardiovascular Disorders volume 24 , Article number: 281 ( 2024 ) Cite this article
49 Accesses
Metrics details
Acute coronary syndrome due to coronary artery embolism in the setting of ascending aortic thrombus is an uncommon condition, even rarer when there is no aortic pathology such as aneurysm, severe atherosclerosis, aortic dissection, or thrombophilia (whether inherited or acquired).
Case presentation
We report a case of a 58-year-old male presented with acute chest pain, electrocardiogram showing non-ST-elevation acute coronary syndrome. The computed tomography angiography of coronary artery revealed a mural thrombus in the proximal part of ascending aorta, located above the left coronary artery ostium, without any aortic pathologies. With the exception of hypertension and cigarette smoking, no other risk factors were identified in this patient that may increase the risk of thrombosis. Given the life-threatening risk of interventional therapy and surgery, the patient determinedly opted for anticoagulant and dual antiplatelet therapy. Then he experienced the reoccurrence of chest pain after 6-day treatment, progressed to anterior and inferior ST-segment elevation myocardial infarction. Coronary artery embolism originating from the ascending aortic thrombus was suspected. Considering the hemodynamic instability of the patient, the medical treatment was continued and bridged to warfarin and aspirin after discharge. Follow-up computed tomography angiography at 6 months showed no obstruction in coronary artery and complete resolution of the thrombus. No thromboembolic events occurred henceforward.
Conclusions
Acute coronary syndrome could be a manifestation of secondary coronary embolism due to ascending aortic thrombus. Currently, there is no standardized guideline for the treatment of aortic mural thrombus, individualized treatment is recommended. When surgical therapy is not applicable for the patient, anticoagulation and dual antiplatelet treatment are alternative treatments that may successfully lead to the resolution of the aortic thrombus.
Peer Review reports
Acute coronary syndrome (ACS) is commonly attributable to coronary atherosclerotic plaque rupture, resulting in thrombosis and obstruction in coronary artery. Nevertheless, coronary artery embolism (CAE), as an infrequent nonatherosclerotic cause of ACS, should also be taken into account. It was reported that the incidence of acute myocardial infarction caused by CAE is 2.9% [ 1 ].One systemic review demonstrated that the most commonly documented etiologies of CAE included infective endocarditis (22.4%), atrial fibrillation (17.0%), and prosthetic heart valve thrombosis (16.3%), only one CAE case was caused by an ascending aortic thrombus (0.6%) [ 2 ]. Ascending aortic thrombus is usually associated with aortic pathologies, including aortic aneurysm, severe aortic atherosclerosis, and aortic dissection [ 3 ]. Ascending aortic thrombus in the absence of the lesions above is very uncommon. This report presents a rare case of the ACS secondary to a mural thrombus sited in a normal ascending aorta, ultimately well-treated with anticoagulation and antiplatelet agents.
A 58-year-old male presented to our emergency department with excruciating chest pain lasting for over 30 min. He had a smoking addiction (over 300 packs of cigarettes per year for more than 20 year) and a history of hypertension that was well-controlled with antihypertensive drugs (including nifedipine, telmisartan and hydrochlorothiazide), with no previous history of atrial fibrillation, valvular diseases or thromboembolic events. On admission, his blood pressure was too low to detect, and the oxygen saturation level in atmospheric air dropped to 90.1%. The initial electrocardiogram (ECG) showed persistent ST-segment depression in leads II, III, AVF and V1-V6, indicating ongoing massive myocardial ischemia and acute non-ST elevation myocardial infarction (non-STEMI) (Fig. 1 ). The serum troponin I (TnI) level increased to 2.46 ng/mL. Echocardiography showed left ventricular dyskinesia with an enlarged left atrium and an ejection fraction of 27%, negative for endocarditis, valve abnormalities, or any thrombus in the cardiac chambers (Supplementary File 1 and File 2 ). Based on the latest guideline for the management of ACS [ 4 ], the patient was advised for invasive coronary angiography and emergency percutaneous coronary intervention, which was rejected by the patient’s dependents for the high risk of intraoperative adverse events. Emergency treatments were immediately performed including a loading dose of aspirin and clopidogrel, dopamine boost, norepinephrine and low molecular weight heparin (LMWH).

Emergency room 12-Lead Electrocardiogram presenting ST-segment depression in leads II, III, AVF and V1-V6, indicating acute non-STEMI
The patient was transferred to the department of cardiology after the symptoms were palliated. Laboratory data showed a high D-dimer level (2.25 µg/mL). Blood cell count, tumor markers antinuclear antibody and blood lipid profile were normal. The computed tomography angiography (CTA) of coronary artery demonstrated a completely normal coronary artery, with no obstructions or stenosis (Fig. 3 ). Unexpectedly, the CTA identified a lobulated mass in the proximal part of ascending aorta, located above the left coronary ostium, measuring 1.5 × 1.1 × 0.9 cm, suggestive for a mural thrombus in the ascending aorta (Fig. 4 ). The pursued CTA of aorta obtained the consistent result that the coronary artery showed no obvious abnormality, and that no signs of atherosclerotic deposits, aneurysms or aortic dissection were found in the ascending aorta (Fig. 5 ). The patient had received short-term anticoagulant and antiplatelet therapy before receiving the aortic CTA, which showed that the original thrombus decreased in size and became indistinct at the edges. His Society of Thoracic Surgery score and European System for Cardiac Operative Risk Evaluation (EuroSCORE) II were 59.8% and 20.81% respectively, for isolated coronary artery bypass grafting (CABG). Considering the life-threatening risk of interventional therapy and surgery, the patient was determined on conservative treatment. Therefore, dual antiplatelet and anticoagulant therapy (aspirin 100 mg, clopidogrel 75 mg and LMWH) were still administered, which subsequently relieved his chest pain. Echocardiography revealed LVEF of 45%, and left atrial diameter (LAD) 32 mm, left ventricular internal dimension at end-diastole (LVIDd) 50 mm (shown in Supplementary Fig. 1A and Fig. 1 B in the revised manuscript). However, after the 6 days of conservative treatment, the patient was attacked by a recurrence of chest pain and became hemodynamically unstable. Bedside ECG showed ST segment elevation in precordial leads V1 to V5 and limb leads II, III, AVF, indicating extensive anterior and inferior myocardial infarction (Fig. 2 ). Serum troponin I level increased significantly to 36.17ng/mL. CAE originating from the ascending aortic thrombus accounting for the ACS was suspected. In such condition, coronary intervention carries a hazard of catheter-induced thrombus dislodgement resulting in life-threatening thromboembolic events, including but not limited to acute coronary occlusion and cerebral embolism. As the thrombus had been present for several days (over 24 h), it already passed the time window for intravenous thrombolysis. Therefore, intravenous thrombolytic therapy not only has little effect on resolving such a subacute thrombus but also contributes to bleeding tendencies. Even worse, the subsequent dissolution and dislodgement of the thrombus might pose a risk of secondary arterial embolism. In order to properly get rid of the thrombus, the patient was referred to the department of cardiovascular surgery for surgical thrombectomy. However, considering the huge traumatic stress that the surgery brings, as well as the possible risk of the thrombus dislodgement during operation, the patient rejected the surgical thrombectomy in the end. He continued the conservative treatment consisting of Vasodilators, dual antiplatelet and anticoagulant agents for the remaining days of the hospital stay, which fortunately alleviated the chest pain. Afterwards, the serum troponin I turned to normal level and repeat coronary CTA showed complete resolution of the lobulated thrombus without any abnormality in the coronary artery (Fig. 6 ). The patient was discharged with warfarin (2.5 mg) and aspirin(100 mg), followed up biweekly to ensure the international normalized ratio (INR) ranged between 2.0 and 3.0. Repeat echocardiography at 1month indicated his cardiac function was basically recovered (shown in Supplementary Fig. 2 A and Fig. 2 B). Follow-up CTA at 6 months revealed no thrombus in the aortic root or ascending aorta (Fig. 7 ). No thromboembolic events occurred henceforward.

Bedside Electrocardiogram after the reoccurrence of chest pain shows ST-segment elevation in leads II, III, AVF and V1-V5, with Q waves in leads I II, III, AVF, indicating acute ST-segment elevation myocardial infarction in extensive anterior and inferior walls

Coronary CTA images in ( A ) axial and ( B ) sagittal planes showing a lobulated filling defect suggestive of a thrombus, located in the proximal portion of ascending aorta. The thrombus occurs just above the left coronary ostium, closed to the sinotubular junction and the sinus of Valsalva. No abnormality was found in the coronary artery

3D reconstruction based on coronary CTA revealing the presence of a large thrombus in the proximal portion of the ascending aorta, measuring 1.5 × 1.1 × 0.9 cm. Red structure indicates the thrombus

( A ) and ( B ): The pursued CTA of aorta showed patency of coronary artery. The original thrombus decreased in size and became indistinct at the edges after the short-term anticoagulant and dual antiplatelet therapy. ( C ) Three-dimensional reconstruction of the aorta. Yellow arrow indicates the dissolving thrombus

( A ) and ( B ): Repeated coronary CTA before discharge showed no obvious abnormalities

( A ) and ( B ): Follow-up CTA at 6 months demonstrating complete resolution of the previous thrombus
ACS due to an aortic mural thrombus (AMT) sited in the ascending aorta is a distinctive and uncommon cause of acute myocardial infarction. In many cases, AMT is usually associated with aortic pathologies, including severe atherosclerosis, vasculitis, aneurysm and dissection of the aorta. Primary aortic mural thrombosis (PAMT), defined as a thrombus attached to the aortic wall in the absence of any atherosclerotic or aneurysm disease in the aorta, is a relatively rare entity. Machleder et al. reported an incidence rate of 0.45% for non-aneurysmal AMT based on a consecutive series of 10,671 autopsies, none of which involves ascending aorta [ 5 ]. In addition, the proximal portion of the ascending aorta is an uncommon site for PAMT. Existing literature acknowledged that the descending thoracic aorta was the most common location of the AMT (38%), while the ascending aorta were the least common location (12%) [ 6 ]. Verma et al. documented 19 patients of PAMT manifested as peripheral embolic events, of whom only 2 patients had a thrombus located in the ascending aorta [ 7 ].
The presented case has attracted much interest because of the infrequent manifestation of ACS caused by an ascending aortic thrombus. Most cases of ascending AMT are clinically silent, usually diagnosed after an embolic event or occasionally identified through echocardiography and CTA [ 8 ]. The typical embolic events caused by AMT include ischemic stroke, visceral and distal embolization, while the coronary events are relatively rare [ 6 ]. What’s unexpected, our patient underwent repeated ACS in his hospital stay. As reported in previous cases, both thrombi in the ascending aorta originating from the ostium of coronary artery and an aortic root thrombus extending into the coronary sinus of Valsalva can account for ACS [ 9 , 10 ]. The occurrence of ACS could arise from the distal CAE caused by the detachment of the aortic thrombus. Ascending aortic thrombus occluding the coronary ostium is also a possible condition. The differential diagnosis relying on effective investigation seems rather critical. Though transesophageal echocardiography (TEE) can precisely localize aortic thrombi and atherosclerosis, TEE still has limitations in presenting the ascending aorta and the proximal aortic arch due to the interference of the trachea, making CTA a superior examination to detect ascending aortic thrombus [ 8 ]. Coronary CTA possess high diagnostic value in excluding significant coronary artery disease (CAD) [ 11 ]. As the first-line anatomic test, Coronary CTA could reduce the total costs of diagnosis in stable CAD patients by avoiding invasive coronary angiography and hospitalization [ 12 ]. We ultimately ruled out the cardiogenic thrombus resulting in ACS, for no evidence of atrial fibrillation and left ventricular thrombus was revealed in the CTA. Then thorough investigations about the etiologies of ascending aortic thrombosis were planned on this patient.
Although the pathophysiological mechanism of an ascending aortic mural thrombosis has not been fully explored, the principle of Virchow’s triad for thrombogenesis remains closely related. Virchow’s triad describes the three factors related to thrombosis: hemodynamics (fluid stasis), endothelial injury (associated with foreign materials), and hypercoagulability (abnormal blood chemistry) [ 13 ]. The high-flow environment of the ascending aorta, characterized by the large blood flow, the high blood pressure and high shear stress, theoretically serves as a protective mechanism against stasis and thrombosis, and contributes to the scarcity of mural thrombi at the proximal ascending aorta. Also, the helical blood flow in the ascending aorta protects the aortic wall from atherosclerosis, thrombus formation, and intimal proliferation [ 14 ]. On the other hand, the Valsalva sinuses in the aortic root complex has been reported to alleviate the high sheer stress of the aortic root. The Valsalva sinuses create a space between the aortic valve leaflets and the aortic wall. As the valve leaflets move towards the aortic wall in the early systole, the space make it possible for blood to flow along the sinotubular junction and enters the Valsalva sinuses, facilitating the eddy current development and promoting thrombosis in the ascending aorta [ 15 ]. In our case, the thrombus was located just around the sinotubular junction and the sinus of Valsalva, somehow making sense of the ascending aortic thrombosis in the patient. In addition to hemodynamics, endothelial injury and hypercoagulable states are closely associated with aortic thrombosis. Prior studies indicated that aortic endothelial injury could arise from prosthetic aortic valve implantation, aortic stent implantation, open aortic surgery, aortitis and infective endocarditis, potentially leading to thrombus-related CAE [ 16 , 17 , 18 , 19 , 20 ]. Therefore, actively searching for evidence of hypercoagulative conditions is our clinical strategy to determine the probable causes of ascending aortic thrombosis in a normal aorta. The hypercoagulable states that have been reported to be associated with the pathogenesis of ascending aortic thrombi include active cancer, inherited thrombophilia, thrombocythemia, polycythemia, antiphospholipid syndrome, systemic infectious diseases [ 5 , 21 , 22 , 23 , 24 , 25 , 26 ]. However, in our case, neither associations with aortic endothelial injury and aortic pathologies such as aortic atherosclerosis, aneurysm, or dissection were found through CTA, nor were any signs indicating risk factors for the aforementioned hypercoagulable states observed during post-admission examinations. Predisposing factors for aortic thrombosis include old age, diabetes, hypertension, hyperlipidemia, smoking addiction, trauma, oral contraceptive use, hormone replacement therapy, and use of exogenous steroids [ 7 ]. Our patient unexpectedly has no other marked predisposing factors but only history of hypertension and smoking. Since hypertension was well controlled, the possible explanation is a transient hypercoagulable state induced by smoking. Also, nicotine in tobacco can cause to vascular endothelial injury, increasing the risk of aortic thrombosis. But the exact cause of thrombosis in the ascending aorta remains mysterious. The interaction of Virchow’s triad potentially led to the ascending aortic thrombosis.
A systematic review indicates that ACS due to coronary embolism has a worse prognosis than atherosclerotic ACS [ 2 ], for the former type has larger myocardial infarct areas. Early differential diagnosis and coronary revascularization are crucial. One must rule out a thrombus within an aneurysm and aortic atherosclerotic thrombus, for the different therapeutic avenues. Aortic mural thrombi associated with aneurysmal formation are most sited in the abdominal aorta, known as intraluminal thrombus (ILT). ILT adheres firmly to the aortic wall, so it’s rare the ever-growing ILT leads to distal occlusion. Anticoagulation is notoriously difficult to reverse in the event of hemorrhage. Currently, neither antiplatelet nor anticoagulant therapy is clinically used except aspirin recommended as secondary prevention for abdominal aortic aneurysm-induced cardiovascular diseases [ 27 ]. Aortic atherosclerotic thrombosis involves thrombus building up on complex or ulcerated aortic plaques. The present evidence supports that dual antiplatelet therapy or moderate intensity anticoagulation with warfarin as secondary prevention of aortic atheroma could decrease the recurrence of thromboembolic stroke [ 28 ]. There is no consensus or properly evolved guidelines about the management of ascending aortic thrombus because of its scarcity, so the management of AMT rely much on clinical experience. Treatment options reported so far include anticoagulation, antiplatelet therapy, the combination of anticoagulation and antiplatelet agents, thrombolysis, endovascular stent graft placement, and surgical therapy. Individualized treatment is recommended, taking into consideration factors including the size, location, mobility and implantation base of the thrombus, hemodynamic stability of the patient, and the medical standard of the hospital. Surgery is the recommended treatment for AMT in a normal or mildly atherosclerotic aorta and thrombus located in the ascending aorta, with lower incidence of recurrent thrombus, thromboembolic complication, and limb loss [ 6 ]. As for thrombus located in the aortic arch, descending aorta, and abdominal aorta, endovascular treatment or medical treatment are the preferred option [ 29 ]. Aortic mural thrombi are classified as sessile and pedunculated based on the implantation base. For large and pedunculated thrombi bear a higher risk of secondary embolism, surgical treatment may obtain better clinical results [ 30 ]. In contrast, small and sessile thrombi are appropriate for anticoagulation [ 31 ]. Thrombi over 1 cm in diameter were reported with higher risks of embolic events [ 32 ]. Previous studies postulated that anticoagulation may lysing the thin attachment site of the pedunculated thrombus before lysing the thrombus itself, thus triggering the subsequent embolic events [ 31 ]. Laperche et al. reported 23 patients with isolated ascending aortic arch thrombus, among whom 17 patients were treated with anticoagulants, and the aortic arch thrombi in 11 patients (92%) disappeared from 3 days to 6 months after the initial embolic event, indicating the therapeutic potential of anticoagulants in the management of ascending aortic thrombus. Choukron et al. recommended anticoagulation as initial therapy and resorting to surgical thrombectomy only when anticoagulation proves ineffective [ 33 ]. Some cases also reported successful resolution of aortic thrombi under the combination of anticoagulation and antiplatelet therapy, but it brings an increased tendency of bleeding [ 34 ]. Currently, the optimal drug, dose, and duration of anticoagulation therapy for ascending aortic thrombus have not been standardized, and no study has assessed direct oral anticoagulants in aortic thrombus so far [ 28 ]. We referred to the management for left ventricular thrombus and prescribed the patient warfarin with stable INR (within the range of 2.0–3.0) [ 35 ]. Endovascular stent graft is less invasive than surgery, also possibly decreasing the size of residual aortic thrombus with a reduced risk of recurrent embolization in comparison to anticoagulation. But when dealing with ascending aortic thrombus, endovascular treatment may pose the risk of dislodging the thrombus during the stent placement, giving rise to fatal embolism [ 7 ]. Thrombolysis, though infrequently performed in managing aortic thrombus, has also provided successful treatments in some cases [ 36 ]. Conventional surgery can remove the aortic thrombus once for all and avoid recurrent embolism, but as a more invasive choice, it involves sternotomy procedure, cardiopulmonary bypass, deep hypothermia and a subsequent series of post-operative complications, including arrhythmias and even post-thrombectomy mortality [ 37 ]. Pre-operative hemodynamic instability is usually associated with the increased risks of surgical mortality. Considering our patient’s hemodynamic instability and his strong preference for conservative treatment, we determined on a safer management, which consisted of anticoagulant and dual antiplatelet agents. Fortunately, the patient’s symptoms and quality of life were all improved at the six-month follow-up.
ACS due to coronary artery embolism in the setting of a mural thrombus sited in a normal ascending aorta is rather rare. This case report has attempted to elaborate the potential mechanisms of ascending aortic thrombosis from the perspective of the well-known Virchow’s triad. Active hypercoagulable investigations on the patients are essential. Our patient was successfully treated with antiplatelet and anticoagulant therapy, it should be noted that AMT sited in the ascending aorta is a hazardous condition. When surgery therapy was unavailable or inapplicable, antiplatelet and anticoagulant therapy could bring a chance of resolving ascending mural thrombi. However, it also comes with possibilities of secondary embolic events, including CAE as one of the worst conditions.
Data availability
All data generated or analysed during this study are included in this article.
Abbreviations
Acute Coronary Syndrome
Coronary Artery Embolism
Electrocardiogram
non-ST elevation myocardial infarction
Low Molecular Weight Heparin
Computed Tomography Angiography
Coronary Artery Bypass Grafting
European System for Cardiac Operative Risk Evaluation
International Normalized Ratio
Aortic Mural Thrombus
Primary Aortic Mural Thrombosis
Transesophageal Echocardiography
Coronary Artery Disease
Intraluminal Thrombus
Shibata T, Kawakami S, Noguchi T, Tanaka T, Asaumi Y, Kanaya T, et al. Prevalence, clinical features, and prognosis of Acute myocardial infarction attributable to coronary artery embolism. Circulation. 2015;132(4):241–50.
Article CAS PubMed Google Scholar
Lacey MJ, Raza S, Rehman H, Puri R, Bhatt DL, Kalra A. Coron Embolism: Syst Rev Cardiovasc Revasc Med. 2020;21(3):367–74.
Google Scholar
Pang PYK, Nathan VB. Successful thrombectomy for an idiopathic floating ascending aortic Thrombus. Ann Thorac Surg. 2016;102(3):e245–7.
Article PubMed Google Scholar
Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–367.
Machleder HI, Takiff H, Lois JF, Holburt E. Aortic mural thrombus: an occult source of arterial thromboembolism. J Vasc Surg. 1986;4(5):473–8.
Fayad ZY, Semaan E, Fahoum B, Briggs M, Tortolani A. M D’Ayala. Aortic mural thrombus in the normal or minimally atherosclerotic aorta. Ann Vasc Surg. 2013;27(3):282–90.
Verma H, Meda N, Vora S, George RK, Tripathi RK. Contemporary management of symptomatic primary aortic mural thrombus. J Vasc Surg. 2014;60(6):1524–34.
Yang S, Yu J, Zeng W, Yang L, Teng L, Cui Y, et al. Aortic floating thrombus detected by computed tomography angiography incidentally: five cases and a literature review. J Thorac Cardiovasc Surg. 2017;153(4):791–803.
Shahin GM, Bosker HA, Knaepen PJ, Morshuis WJ, Lindeboom JE. Organized thrombus in the ascending aorta originating from the ostium of the right coronary artery. Cardiovasc Surg. 2002;10(1):62–4.
Abubakar H, Ahmed AS, Subahi A, Yassin AS. Thrombus in the right coronary sinus of Valsalva originating from the Left Atrial Appendage Causing Embolic Inferior Wall Myocardial Infarction. J Investig Med High Impact Case Rep. 2018;6:2324709618792023.
PubMed PubMed Central Google Scholar
Kruk M, Rudziński P, Demkow M, Kępka C. Is the Majority Benefitting at the costs of the minority among patients undergoing CTA as the First-Line Diagnostic in highly suspected coronary artery disease? JACC Cardiovasc Imaging. 2019;12(5):944.
Rudziński PN, Kruk M, Kępka C, Schoepf UJ, Otani K, Leonard TJ, et al. Assessing the value of coronary artery computed tomography as the first-line anatomical test for stable patients with indications for invasive angiography due to suspected coronary artery disease. Initial cost analysis in the CAT-CAD randomized trial. J Cardiovasc Comput Tomogr. 2020;14(1):75–9.
Raghav V, Midha P, Sharma R, Babaliaros V, Yoganathan A. Transcatheter aortic valve thrombosis: a review of potential mechanisms. J R Soc Interface. 2021;18(184):20210599.
Article PubMed PubMed Central Google Scholar
Liu X, Sun A, Fan Y, Deng X. Physiological significance of helical flow in the arterial system and its potential clinical applications. Ann Biomed Eng. 2015;43(1):3–15.
Ozaki N, Yuji D, Sato M, Inoue K, Wakita N. A floating thrombus in the ascending aorta complicated by acute myocardial infarction. Gen Thorac Cardiovasc Surg. 2017;65(4):213–5.
Bolton A, Hajj G, Payvandi L, Komanapalli C. ST segment elevation caused by ostial right coronary artery obstruction in infective endocarditis: a case report. BMC Cardiovasc Disord. 2020;20(1):412.
De Roeck F, Abdulmajid L, Haine S. Prosthetic aortic valve thrombosis complicated by Left Main Coronary Artery Bifurcation Embolism: Case Report and Review of Literature. Cardiovasc Revasc Med. 2021;28s:72–4.
Hebbo E, Khoury AE, Iskandarani D, Sawaya F. Case report: acute myocardial infarction in the setting of acute transcatheter aortic valve thrombus. Front Cardiovasc Med. 2023;10:1164668.
Tzanninis I, Elserwey A, Mason A, Rawlins J. A rare case report of granulomatosis with polyangiitis presenting with thrombus of the ascending aorta. Clin Med (Lond). 2022;22(Suppl 4):42–3.
Virk HU, Inayat F, Farooq S, Ghani AR, Mirrani GA, Athar MW. Prosthetic aortic valve endocarditis with left main coronary artery embolism: a Case Report and Review of the literature. N Am J Med Sci. 2016;8(6):259–62.
Ye S, Xia WJ, Chen P. Case Report: a rare case of Acute Anterior myocardial infarction simultaneously Associated with aortic mural thrombosis due to essential thrombocytosis. Front Cardiovasc Med. 2022;9:840906.
Takemoto K, Atagi K. Aortic artery thrombosis Associated with inherited Thrombophilia. Intern Med. 2023;62(10):1565–6.
Shah NC, Munir SM, Alp NJ. Spontaneous aortic thrombosis causing left main coronary occlusion in a man with secondary polycythemia. JACC Cardiovasc Interv. 2011;4(8):934–5.
Otsuka R, Saito S, Yamamoto T, Ohno T, Koyama A, Morimae H, et al. Recurrent mural thrombosis of the Ascending Aorta in a patient with antiphospholipid syndrome. Ann Vasc Dis. 2022;15(1):77–80.
Marin-Acevedo JA, Koop AH, Diaz-Gomez JL, Guru PK. Non-atherosclerotic aortic mural thrombus: a rare source of embolism. BMJ Case Rep. 2017; 2017.
Geana RC, Nayyerani R, Dragulescu RP, Marinica I, Bacalbasa N, Balescu I, et al. COVID-19-related floating Thrombus in the Ascending Aorta - A Case Report. Vivo. 2023;37(5):2381–6.
Article Google Scholar
Cameron SJ, Russell HM, Owens AP 3. Antithrombotic therapy in abdominal aortic aneurysm: beneficial or detrimental? Blood. 2018;132(25):2619–28.
Article CAS PubMed PubMed Central Google Scholar
Caron F, Anand SS. Antithrombotic therapy in aortic diseases: a narrative review. Vasc Med. 2017;22(1):57–65.
Moldovan H, Bulescu C, Sibișan AM, Țigănașu R, Cacoveanu C, Nica C et al. A large ascending aorta Thrombus in a patient with Acute myocardial infarction-case report. Med (Kaunas). 2021; 57(11).
Geha AS, El-Zein C, Massad MG, Bagai J, Khoury F, Evans A, et al. Surgery for aortic arch thrombosis. Thorac Cardiovasc Surg. 2004;52(3):187–90.
Weiss S, Bühlmann R, von Allmen RS, Makaloski V, Carrel TP, Schmidli J, et al. Management of floating thrombus in the aortic arch. J Thorac Cardiovasc Surg. 2016;152(3):810–7.
Pagni S, Trivedi J, Ganzel BL, Williams M, Kapoor N, Ross C, et al. Thoracic aortic mobile thrombus: is there a role for early surgical intervention? Ann Thorac Surg. 2011;91(6):1875–81.
Choukroun EM, Labrousse LM, Madonna FP, Deville C. Mobile thrombus of the thoracic aorta: diagnosis and treatment in 9 cases. Ann Vasc Surg. 2002;16(6):714–22.
Abissegue YG, Lyazidi Y, Chtata H, Bakkali T, Taberkant M. Acute systemic embolism due to an idiopathic floating thrombus of the thoracic aorta: success of medical management: a case report. BMC Res Notes. 2015;8:181.
Levine GN, McEvoy JW, Fang JC, Ibeh C, McCarthy CP, Misra A, et al. Management of patients at risk for and with left ventricular Thrombus: a Scientific Statement from the American Heart Association. Circulation. 2022;146(15):e205–23.
Hausmann D, Gulba D, Bargheer K, Niedermeyer J, Comess KA, Daniel WG. Successful thrombolysis of an aortic-arch thrombus in a patient after mesenteric embolism. N Engl J Med. 1992;327(7):500–1.
Abdallah H. Unexpected outcome of a floating Thrombus in the Ascending Aorta. Jurnalul De Chirurgie. 2015; 11Legend.
Download references
Acknowledgements
The authors would like to thank the patient for his participation in this study.
This research was funded by Zhejiang Traditional Chinese Medicine Science and Technology Program (2023ZL184).
Author information
Hanxuan Liu and Zhangjie Yu shared first authorship.
Authors and Affiliations
School of Medicine, Shaoxing University, Shaoxing City 312000, Zhejiang Province, China
Hanxuan Liu, Ying Xu, Juntao Yang & Yinyin Qiu
Department of Cardiology, Shaoxing People’s Hospital, NO. 568 North Zhongxing Road, Yuecheng district, Shaoxing City 312000, Zhejiang Province, China
Zhangjie Yu, Yan Zhou, Yangbo Xing, Fang Peng & Weiliang Tang
You can also search for this author in PubMed Google Scholar
Contributions
Hanxuan Liu and Zhangjie Yu equally contributed and authored the manuscript. Concept – Hanxuan Liu, Zhangjie Yu, Weiliang Tang; Design – Hanxuan Liu, Zhangjie Yu, Ying Xu; Supervision – Zhangjie Yu, Weiliang Tang; Funding – Zhejiang Traditional Chinese Medicine Science and Technology Program (2023ZL184); Materials –Zhangjie Yu, Juntao Yang; Data collection and/or processing – Zhangjie Yu, Juntao Yang, Yinyin Qiu; Analysis and/or interpretation – Hanxuan Liu, Ying Xu, Weiliang Tang; Literature review – Hanxuan Liu, Fang Peng, Weiliang Tang; Writer – Hanxuan Liu, Zhangjie Yu; Critical review – Fang Peng, Yan Zhou; Other – None.
Corresponding author
Correspondence to Weiliang Tang .
Ethics declarations
Ethics approval and consent to participate.
The case report was approved by the Ethics Committee of Shaoxing People’s Hospital (Ethical Determination for Case Report No: 003, Date: 27 − 12 -2023). The study was conducted in accordance with the local legislation and institutional requirements.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any potentially identifiable images or data.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, supplementary material 3, supplementary material 4, supplementary material 5, supplementary material 6, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Liu, H., Yu, Z., Xu, Y. et al. Repeated acute coronary syndrome caused by a mind-bending mural thrombus in ascending aorta: a case report and review of the literature. BMC Cardiovasc Disord 24 , 281 (2024). https://doi.org/10.1186/s12872-024-03956-2
Download citation
Received : 30 January 2024
Accepted : 22 May 2024
Published : 29 May 2024
DOI : https://doi.org/10.1186/s12872-024-03956-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Ascending aortic thrombus
- Primary aortic mural thrombus
- Acute coronary syndrome
- Coronary artery embolism
BMC Cardiovascular Disorders
ISSN: 1471-2261
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Introduction. Literature review is an essential feature of academic research. Fundamentally, knowledge advancement must be built on prior existing work. To push the knowledge frontier, we must know where the frontier is. By reviewing relevant literature, we understand the breadth and depth of the existing body of work and identify gaps to explore.
An earlier NHTSA review of the effects of medical conditions on driving considered expert and stakeholder opinion when selecting medical conditions for review (Lococoet al., 2018). This earlier work culminated in a systematic review of literature published between 2000-2011 and
Literature reviews are most commonly performed to help answer a particular question. While you are at medical school, there will usually be some choice regarding the area you are going to review. Once you have identified a subject area for review, the next step is to formulate a specific research question. This is arguably the most important ...
In the literature review for each medical condition are sections that describe its prevalence in the U.S. population, effects on the functional abilities needed for safe driving, effects on driving performance, and relationships with motor vehicle crash and violation risk. A
Doing a Literature Review in Health33 This chapter describes how to undertake a rigorous and thorough review of the literature and is divided into three sections. The first section examines the two main types of review: the narrative and the systematic review. The second section describes some techniques for undertaking a comprehensive search,
Purpose and Importance of the Literature Review. An understanding of the current literature is critical for all phases of a research study. Lingard 9 recently invoked the "journal-as-conversation" metaphor as a way of understanding how one's research fits into the larger medical education conversation. As she described it: "Imagine yourself joining a conversation at a social event.
Run a few sample database searches to make sure your research question is not too broad or too narrow. If possible, discuss your topic with your professor. 2. Determine the scope of your review. The scope of your review will be determined by your professor during your program. Check your assignment requirements for parameters for the Literature ...
This effort presents updated research findings describing the effect of medical conditions on driving performance and safety. Researchers conducted a preliminary literature search and discussion with driving safety professionals providing information that was used to select the medical conditions for review: attention deficit hyperactive disorder (ADHD); autism spectrum disorder (ASD ...
The literature review is the qualitative summary of evidence on a topic using informal or subjective methods to collect and interpret studies.The literature review can inform a particular research project or can result in a review article publication. ... Norris Medical Library, Upper Level, LB 1047.3 B725s 2012. Documenting your search.
Background: Various patient demographic and clinical characteristics have been associated with poor outcomes for individuals with coronavirus disease 2019 (COVID-19). To describe the importance of age and chronic conditions in predicting COVID-19-related outcomes. Methods: Search strategies were conducted in PubMed/MEDLINE. Daily alerts were created.
Kavakiotis et al. conducted a systematic review regarding the machine learning applications, data mining techniques, and tools used in the diabetes field to showcase the prediction and diagnosis of diabetes, its complications, and genetic conditions and situation, including the physical condition care management. After the in-depth search, it ...
Additionally, the assessment and determination of the clinical meaning of fatigue and its multidimensionality may vary by medical condition. Methods: A scoping literature review was conducted to investigate how fatigue is defined and measured, including its dimensions, in non-oncologic medical conditions. The PubMed database was searched using ...
These steps for conducting a systematic literature review are listed below. Also see subpages for more information about: What are Literature Reviews? ... Cook, D. A., & West, C. P. (2012). Conducting systematic reviews in medical education: a stepwise approach. Medical Education, 46(10), 943-952. << Previous: Critical Appraisal Resources ...
This literature review relates changes in performance or safety outcome measures for older drivers to their medical conditions or medication use, and associated functional impairments. It was carried out as an initial task in the project, The Effects of Medical Conditions on Driving Performance. Researchers conducted a search of peer-reviewed ...
We have directed this review according to the preferred reporting items for systematic reviews and Meta-Analysis guidelines. The survey offers the readers wide-ranging knowledge of the literature on AI (decision tree, which breaks down the dataset into smaller subsets and to build it, two types of entropy using frequencies are calculated in which X, S is a discrete random variable which occurs ...
conditions as medical conditions that have occurred for at least three months and involve activities of daily living deficits or increased medical needs that are exacerbated for . ... systematic literature review, each article was screened by the three categories established exclusively for this research. The three categories are listed as ...
Background Cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i) combined with endocrine therapy (ET) are currently recommended by the National Comprehensive Cancer Network (NCCN) guidelines and the European Society for Medical Oncology (ESMO) guidelines as the first-line (1 L) treatment for patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative, locally ...
A systematic review provides a comprehensive review of Good syndrome, a rare condition characterized by the association of thymoma and immunodeficiency. Despite being documented over 50 years ago, GS remains a clinical enigma. ... Notably, this study marks a significant milestone in medical literature as the first reported case of Long COVID-19 ...
Objective: To describe how and why patient contracts are used for the management of chronic medical conditions. Data sources: A scoping review was conducted in the following databases: MEDLINE, Embase, AMED, PsycInfo, Cochrane Library, CINAHL, and Nursing & Allied Health. Literature from 1997 to 2017 was included. Study selection: Articles were included if they were written in English and ...
The most frequent medical conditions for approved medical cannabis use nationally are chronic noncancer pain, multiple sclerosis (MS) and other motor neuron ... We identified several persistent gaps in the literature during this review. Recent observational studies often lacked specific information about the route of administration, dosage ...
Evidence used to inform the list of underlying conditions was determined by CDC reviewers based on available literature about COVID-19 at time of review. The information reflects evidence regarding underlying medical conditions and is intended to help healthcare professionals make informed decisions about patient care and to increase the ...
This study is based on systematic review approach or systematic literature review (SLR).This type of review enables a comprehensive understanding of a certain topic of interest or phenomenon, which provides details and important insights for policies and future studies [].SLR is also a structured approach to research synthesis, following a number of pre-determined steps to evaluate the state ...
Objective: This systematic review aims to analyze existing literature to identify how HCPs have used PGHD from consumer-grade mobile devices in the clinical setting. The objectives are to determine the types of PGHD used by HCPs, in which health conditions they use them, and to understand the motivations behind their willingness to use them.
All-trans retinoic acid (ATRA) is an indispensable part of the treatment of acute promyelocytic leukemia (APL). Although, mild cutaneous toxicities like mucocutaneous xerosis, rash, and pruritus are well reported, ATRA associated severe dermatological toxicities are extremely rare. ATRA is primary metabolized by cytochrome P450 (CYP450) enzyme system, and triazole antifungals are notorious for ...
Wandering spleen is a rare clinical entity in which the spleen is hypermobile and migrate from its normal left hypochondriac position to any other abdominal or pelvic position as a result of absent or abnormal laxity of the suspensory ligaments (Puranik in Gastroenterol Rep 5:241, 2015, Evangelos in Am J Case Rep. 21, 2020) which in turn is due to either congenital laxity or precipitated by ...
This report reviews the contribution of medical conditions and functional limitations (e.g., sensory, motor, or cognitive functioning) to motor vehicle crashes. It provides a comprehensive and up-to-date review of the international research literature on the effects of medical and functional conditions on driving performance.
A fistulous tract in the mitro-aortic intervalvular fibrosa (MAIVF) is a rare entity, which presents as a complication of endocarditis or surgical trauma. Generally, it is associated to a pseudoaneurysm of the MAIVF (p-MAIVF) or aortic abscesses. MAIVF fistulas could potentially lead to devastating complications and a high mortality rate. This condition is managed surgically, either by a ...
Lemierre's syndrome is a fatal and rare disease that is typically characterized by oropharyngeal infection and internal jugular vein thrombosis. Timely institution of appropriate antibiotics is the standard treatment. The authors report a case of Lemierre's syndrome. A 67-year-old male patient of Han ethnicity in China suffered from a large inflammatory neck mass involving left ...
A peculiar foreign body ingestion in 2-year-old girl complicated by esophageal perforation: case report and review of the literature Danah Albarrak, Danah Albarrak College of Medicine, King Saud bin Abdulaziz University for Health Sciences ... case report and review of the literature, Oxford Medical Case Reports, Volume 2024, Issue 5, May 2024, ...
Background Acute coronary syndrome due to coronary artery embolism in the setting of ascending aortic thrombus is an uncommon condition, even rarer when there is no aortic pathology such as aneurysm, severe atherosclerosis, aortic dissection, or thrombophilia (whether inherited or acquired). Case presentation We report a case of a 58-year-old male presented with acute chest pain ...