- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution


Search form
- Advanced search
- Search responses
- Search blogs
- Advances in the...
Advances in the management of idiopathic pulmonary fibrosis and progressive pulmonary fibrosis
- Related content
- Peer review
- Gabrielle Y Liu , pulmonary and critical care fellow ,
- G R Scott Budinger , professor of medicine , chief of pulmonary and critical care in the Department of Medicine ,
- Jane E Dematte , professor of medicine
- Division of Pulmonary and Critical Care Medicine, Department of Medicine, Northwestern University, Chicago, IL, USA
- Correspondence to: J E Dematte j-dematte{at}northwestern.edu
Similarly to idiopathic pulmonary fibrosis (IPF), other interstitial lung diseases can develop progressive pulmonary fibrosis (PPF) characterized by declining lung function, a poor response to immunomodulatory therapies, and early mortality. The pathophysiology of disordered lung repair involves common downstream pathways that lead to pulmonary fibrosis in both IPF and PPF. The antifibrotic drugs, such as nintedanib, are indicated for the treatment of IPF and PPF, and new therapies are being evaluated in clinical trials. Clinical, radiographic, and molecular biomarkers are needed to identify patients with PPF and subgroups of patients likely to respond to specific therapies. This article reviews the evidence supporting the use of specific therapies in patients with IPF and PPF, discusses agents being considered in clinical trials, and considers potential biomarkers based on disease pathogenesis that might be used to provide a personalized approach to care.
Introduction
The term interstitial lung disease (ILD) encompasses a group of diffuse parenchymal lung diseases with varied clinical, radiographic, and pathologic manifestations reflecting their diverse underlying pathobiology. A subset of ILDs have a progressive fibrosing phenotype. Idiopathic pulmonary fibrosis (IPF) almost invariably has this phenotype. However, other ILDs may also develop this and are thereby termed progressive pulmonary fibrosis (PPF), previously known as progressive fibrosing interstitial lung disease (PF-ILD). 1 2 3 4 In this review, we will use PPF to refer specifically to non-IPF ILDs that have a progressive fibrosing phenotype. IPF and PPF share common downstream mechanistic pathways resulting in self-sustaining fibrosis that may be independent of the initial injury or trigger. However, PPF often begins with an inflammatory phase triggered by either an endogenous autoantigen or an exogenous antigen, such as an environmental trigger. 5 6 7 Therefore, making a distinction between the two is important, particularly when designing clinical trials and research studies for PPF. Connective tissue disease associated ILD (CTD-ILD), including rheumatoid arthritis associated ILD (RA-ILD), systemic sclerosis associated ILD (SSc-ILD), and myositis associated ILD, as well as chronic hypersensitivity pneumonitis (cHP), sarcoidosis, idiopathic nonspecific interstitial pneumonia (iNSIP), and unclassifiable ILD, are the ILDs most likely to develop a progressive fibrosing phenotype. However, the proportion of patients with these ILDs who develop this phenotype can vary significantly—from an estimated 13% of patients with fibrotic iNSIP to an estimated 87% of patients with cHP. 8 9
Beyond prognostication, identifying patients with PPF is clinically important because evidence from randomized placebo controlled clinical trials shows that nintedanib can slow decline in lung function in both patients with IPF and those with PPF. 10 11 12 This review summarizes the epidemiology and pathophysiology of IPF and PPF, their currently approved treatments, and promising therapies in the pipeline. It highlights the need for therapeutic trials based on specific biomarkers to develop a more personalized approach to therapy for patients with IPF and PPF in the future.
Sources and selection criteria
We searched PubMed and Ovid MEDLINE databases from 2000 to April 2021 using the following search terms: progressive fibrosing interstitial lung disease, idiopathic pulmonary fibrosis, pulmonary fibrosis, connective tissue disease associated interstitial lung disease, rheumatoid arthritis associated interstitial lung disease, scleroderma interstitial lung disease, systemic sclerosis interstitial lung disease, sarcoidosis, myositis interstitial lung disease, hypersensitivity pneumonitis, nonspecific interstitial pneumonia, unclassifiable interstitial lung disease, biomarkers interstitial lung disease, and biomarkers idiopathic pulmonary fibrosis. We reviewed published management guidelines from websites of professional societies and governmental bodies, including the American Thoracic Society (ATS), European Respiratory Society (ERS), Japanese Respiratory Society (JRS), Latin American Thoracic Association (ALAT), UK National Institute for Health and Care Excellence (NICE), Thoracic Society of Australia and New Zealand (TSANZ), and Lung Foundation of Australia (LFA). We also searched clinicaltrials.gov for all active phase 3 clinical trials for the treatment of idiopathic pulmonary fibrosis, as well as all active and completed phase 2 and 3 clinical trials of nintedanib and pirfenidone for the treatment of PF-ILDs/PPF. We included only full length, peer reviewed studies published in English. We prioritized phase 3 randomized controlled trials (RCTs), phase 2 RCTs, systematic reviews with meta-analyses, and observational cohort studies, in that order. Case reports were excluded. We also focused on high quality basic science manuscripts that contribute to the understanding of the pathobiology of pulmonary fibrosis and lung injury repair and the key mechanisms of action underlying the therapies reviewed. We reviewed basic science manuscripts with preclinical studies in mouse models of pulmonary fibrosis that provide insights into the pathobiology of lung fibrosis. We determined the quality of basic science papers by their selection for publication in high impact journals, their reproducibility across laboratories, their citations by other investigators, and qualitative assessment by the authors. The abstracts of more than 250 papers were reviewed by at least one of the authors, and more than 169 papers were reviewed in detail.
After the original search date in April 2021, the ATS/ERS/JRS/ALAT clinical practice guideline on IPF (an update) and PPF in adults was published in May 2022. 4 Therefore, this review was updated to use the term “PPF” rather than “PF-ILD,” as determined by this guideline. We updated the algorithm ( fig 1 ) to include the conditional recommendation that transbronchial lung cryobiopsy may be used as an alternative to surgical lung biopsy for making a histopathologic diagnosis in patients with ILD of undertermined type. We also updated the sections on “Conceptualizing and defining PPF,” “Currently approved therapy for IPF: Antacid therapy,” and “Guidelines” to reflect the updated clinical practice guideline.

Suggested algorithm for the evaluation and management of suspect fibrosing interstitial lung disease (ILD). *†American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association guidelines suggest bronchoalveolar lavage (BAL) cellular analysis and surgical lung biopsy or transbronchial lung cryobiopsy in the evaluation of patients in whom IPF is clinically suspected or who have an ILD of uncertain etiology and have a high resolution computed tomography (HRCT) pattern of probable usual interstitial pneumonia (UIP), indeterminate for UIP, or an alternative diagnosis. 13 BAL cellular fluid analysis, surgical lung biopsy, and transbronchial lung biopsy are not recommended in patients in whom idiopathic pulmonary fibrosis (IPF) is clinically suspected and who have an HRCT pattern of UIP. CTD-ILD=connective tissue disease associated interstitial lung disease; DLCO=diffusing capacity of the lung for carbon monoxide; FVC=forced vital capacity; GERD=gastresophageal reflux; iNSIP=idiopathic nonspecific interstitial pneumonia; IPAF=interstitial pneumonia with autoimmune features; LTOT=long term oxygen therapy; PFT=pulmonary function test; PPF=progressive pulmonary fibrosis
- Download figure
- Open in new tab
- Download powerpoint
Conceptualizing and defining PPF
The concept of grouping several non-IPF fibrosing ILDs together grew in part out of the recognition that an unmet need existed for treatment options for these lung diseases. Apart from SSc-ILD, robust RCT data to support the use of immunosuppression in fibrosing ILDs have been lacking. Additionally, many patients with fibrosing ILDs progressed despite conventional treatment. However, a challenge to the design of a robust randomized clinical trial to evaluate new therapies was the fact that the prevalence of each individual fibrosing ILD is relatively low. Thus, the term PF-ILD first came into use in 2017 with the design and development of the INBUILD trial (clinicaltrials.org; NCT02999178 ). INBUILD was a randomized, double blind, placebo controlled trial to study the efficacy and safety of nintedanib in patients with ILD diagnoses that were noted to behave similarly to IPF in that they were characterized by progressive pulmonary fibrosis, declining lung function, resistance to immunomodulatory therapies, and early mortality. 1
Before the publication of the ATS/ERS/JRS/ALAT clinical practice guideline on PPF in 2022, PF-ILD had been largely defined by selection criteria for clinical trials. Three randomized clinical trials—INBUILD, 12 RELIEF (German Clinical Trials Register; DRKS00009822), 14 and a phase 2 clinical trial evaluating the use of pirfenidone in patients with progressive fibrosing unclassifiable ILD ( NCT03099187 ) 15 —have proposed criteria for progressive fibrosis. The Erice ILD Working Group also proposed criteria for defining PPF. 16 These criteria shared several common elements. Firstly, the diagnosis must be an ILD other than IPF. This distinction is particularly important when considering the use of these criteria for the purpose of selecting populations for clinical trials. The ATS/ERS/JRS/ALAT clinical practice guideline underscores that PPF is not a diagnosis, but rather a manifestation of certain ILDs, and is agnostic to the underlying condition. 4 Secondly, evidence of fibrotic changes on high resolution computed tomography (HRCT) imaging must be present. These fibrotic features include coarse reticulation with traction bronchiectasis and honeycombing. INBUILD and the study of pirfenidone in unclassifiable ILD both required that participants have fibrotic changes on HRCT affecting at least 10% of lung volume at the time of enrollment. 12 15 Thirdly, evidence of progression of lung disease despite conventional treatment must exist. Each of these groups and trials had defined progression differently; however, with the 2022 ATS/ERS/JRS/ALAT clinical practice guideline, a consensus definition of PPF was determined and is shown in box 1 .
Identifying progressive pulmonary fibrosis 4
Interstitial lung disease diagnosis other than idiopathic pulmonary fibrosis
Radiologic evidence of pulmonary fibrosis
Evidence of progression, defined as meeting at least two of three criteria within the previous year with no alternative explanation:
Worsening respiratory symptoms
Absolute decline in FVC >5% predicted or absolute decline in DLCOc ≥10% predicted within one year of follow-up
Radiologic evidence of progression, including:
Increased extent or severity of traction bronchiectasis or bronchiolectasis
New ground glass opacity with traction bronchiectasis
New fine reticulation
Increased extent or coarseness of reticulations
New or increased honeycombing
Increased lobar volume loss
DLCOc=diffusing capacity of the lung for carbon monoxide corrected for hemoglobin; FVC=forced vital capacity
Progression of fibrosis may be more relevant than just its presence. A study that followed patients from the Scleroderma Lung Studies I and II for a median of eight years found that decline in forced vital capacity (FVC) and diffusing capacity for carbon monoxide (DLCO) over two years was a better predictor of mortality than baseline FVC and DLCO. 17 However, progression can be determined only by serial testing, which may delay lung preserving therapy. Figure 1 shows a suggested algorithm for the evaluation and management of patients with suspected fibrosing ILD.
Epidemiology
Idiopathic pulmonary fibrosis.
IPF is the first or second most commonly encountered ILD in pulmonary practice and is estimated to account for 17-37% of all ILD diagnoses. 18 19 The incidence of IPF in the US and Europe is estimated to be 3-17 per 100 000 person years. 18 19 20 The lowest incidence rate of IPF globally is in Asia, with rates ranging from 1.2 to 4.6 per 100 000 per year. 21 In a study using Medicare data limited to people in the US aged 65 years and older, the incidence of IPF was as high as 93.7 per 100 000 person years and the prevalence was 494.5 cases per 100 000, reflecting age as the major risk factor for IPF. 22
Most common ILDs manifesting PPF
Rheumatoid arthritis is the most common autoimmune disease worldwide and is estimated to have a prevalence of 400-1000 cases per 100 000. 21 Clinically significant ILD occurs in 8-20% of patients with rheumatoid arthritis and is more common in men and those with greater overall disease severity. 21 23 The proportion of patients with RA-ILD who have progressive decline in lung function is estimated at 40%, on the basis of a study that found that 40% of patients with RA-ILD had a DLCO <40% predicted by five years after diagnosis of ILD. 24 The detection of a usual interstitial pneumonia (UIP) pattern on HRCT scan is associated with increased risk of both progressive lung disease and death, compared with a nonspecific interstitial pneumonia (NSIP) or organizing pneumonia pattern. 24 25
The prevalence of systemic sclerosis is estimated to be 7.2-33.9 cases per 100 000 in Europe and 13.5-44.3 cases per 100 000 in North America. 26 The proportion of people with systemic sclerosis who have SSc-ILD is as high as 90% on the basis of HRCT scanning. 27 Of patients with SSc-ILD, 18-25% have progressive worsening of lung function or HRCT findings. 10 28 29 30 Clinical features that predict progressive ILD include Black/African-American race, older age at disease onset, diffuse cutaneous skin disease, detection of antitopoisomerase antibodies, and lower baseline FVC and DLCO. 31 32 Histologic patterns of NSIP or UIP are not significantly associated with overall mortality in SSc-ILD. 33
Myositis related ILD
Idiopathic inflammatory myopathies are a group of rare systemic autoimmune disorders characterized by inflammation of skeletal muscle and sometimes skin, with a reported incidence of 0.2-0.9 cases per 100 000 person years. 34 The subtypes most commonly associated with ILD are dermatomyositis, polymyositis, and antisynthetase syndrome. 35 The reported prevalence of ILD in myositis ranges widely from 19.9% to 86%. 35 In a single center retrospective study, 31% of patients diagnosed as having myositis had ILD. 36 Of the patients with ILD, 33% had complete resolution of their lung disease with treatment and 16% had deterioration of their ILD after a median 34 months of follow-up. 36 An organizing pneumonia pattern on HRCT often responds to immunosuppressive therapy leading to clinical resolution of disease, whereas a UIP pattern is associated more often with progressive disease and clinical deterioration. 36 37 38 39
Chronic hypersensitivity pneumonitis
In a study using US administrative claims based data, the prevalence of hypersensitivity pneumonitis was estimated to be only 1.67-2.71 cases per 100 000, of which approximately 25% met criteria for fibrotic or chronic hypersensitivity pneumonitis. 40 However, in studies from cohorts of patients with ILD of new onset, a clinical diagnosis of hypersensitivity pneumonitis is made in 18-47% of patients. 41 42 43 Most patients with hypersensitivity pneumonitis who have fibrotic disease at baseline will have progressive disease. 8 44 45 Salisbury and colleagues found that compared with patients with IPF, those with cHP and honeycombing on HRCT had a greater decline in FVC and similar median survival. 8
Idiopathic NSIP
The estimated prevalence of iNSIP is 1-9 cases per 100 000. 46 In a retrospective cohort study of patients with fibrotic iNSIP, 13% had progression of radiologic findings on HRCT, 36% had radiologic improvement, and 23% had stable findings. 9 The prognosis of fibrotic iNSIP is generally better than that of IPF, with a five year survival rate ranging from 45% to 90%. 47 48 49
Sarcoidosis
In the US, the prevalence of sarcoidosis is 141.4 per 100 000 in people identifying as Black or African-American, 49.8 in those identifying as white, 21.7 in those identifying as Hispanic, and 18.9 in those identifying as Asian. 50 Fibrotic (stage IV) lung disease is estimated to occur in less than 20% of people with pulmonary sarcoidosis. 51 52 In a retrospective cohort study of patients with stage IV sarcoid, 24.8% had worse lung function after a mean 6.2 years of follow-up, whereas lung function was improved in 39.3% and stable in 35.9%. 53
Unclassifiable ILD
The proportion of patients with new onset ILD who are deemed to have unclassifiable ILD after multidisciplinary discussion was 10% in one single center retrospective study. 54 In this study, 52% of patients had significant progressive decline in lung function or death. Additionally, patients with unclassifiable ILD had longer survival rates compared with IPF and similar survival compared with other ILDs with progressive fibrosis. 54
Pathophysiology
Pulmonary fibrosis is increasingly recognized to begin with damage to the epithelium, possibly induced by environmental insults including cigarette smoke, viruses, environmental dusts (for example, silica or asbestos), or, perhaps, autoimmune injury ( fig 2 ). 55 56 In support of this hypothesis, some genetic mutations associated with pulmonary fibrosis involve genes that are exclusively expressed in the lung epithelium. These include a mutation in the promoter region of MUC5B that enhances its expression and mutations in SFTPC that lead to production of a misfolded protein. 57 58 59 Furthermore, genetic studies in mice localize the fibrotic effects of mutations in genes associated with pulmonary fibrosis that are expressed in all cells to the lung epithelium. Important examples include deficiency in genes that maintain telomere length and genes associated with the Hermansky-Pudlak syndrome. 60 61 62

Mechanisms and signals involved in the development of pulmonary fibrosis and therapeutic targets. During normal repair after lung injury, tissue resident alveolar macrophages interact with other cells in the alveolar epithelium to clear apoptotic cells, particulates, and pathogens without disrupting the normal gas exchanging functions of the alveolus. Alveolar type 2 (AT2) cells differentiate into alveolar type 1 (AT1) cells, passing through a transitional state characterized by expression of keratin-17, thereby restoring the normal alveolar epithelium. During disordered repair, recurring injuries to alveolar epithelium, by either environmental insults or antigen stimulation, cause AT1 cell death as well as aberrant activation of AT2 cells. The process of AT2 cells differentiating into AT1 cells is impaired in regions of lung fibrosis. Partially differentiated keratin-17 positive (KRT17+) epithelial cells accumulate, where they are associated with fibrosis. These KRT17+ cells produce large amounts of connective tissue growth factor (CTGF) and express αvβ6 integrin, which has been shown to activate latent transforming growth factor β (TGF-β), both of which promote differentiation of fibroblasts into myofibroblasts. The abnormally activated alveolar epithelial cells also contribute to fibroblast and myofibroblast proliferation through the production of platelet derived growth factor (PDGF), TGF-β, and CTGF. In response to this failed attempt at epithelial repair, circulating monocytes are recruited into the alveolar space and differentiate into profibrotic alveolar macrophages. These monocyte derived alveolar macrophages (Mo-AM) secrete PDGF and other growth factors that promote the activation and proliferation of fibroblasts as well as their differentiation into myofibroblasts. In a reciprocal positive feed-forward loop, fibroblasts secrete macrophage colony stimulating factor (M-CSF), which maintains alveolar macrophages at the site of injury. Myofibroblasts secrete excessive extracellular matrix (ECM) proteins, leading to stiffening of lung tissue. Myofibroblasts over time produce TGF-β in an autocrine manner and lose their need for macrophages in order to proliferate. The stiff matrix inhibits fibroblast apoptosis in another positive feed-forward loop that contributes to self-sustaining fibrosis. Recombinant human pentraxin (rhPTX)-2 has been proposed to inhibit the recruitment of alveolar macrophages to areas of fibrosis, which in turn inhibits myofibroblast activation. Nintedanib (NTB) likely inhibits fibroblasts by blocking PDGF signaling, among other profibrotic signaling pathways. The exact mechanisms by which pirfenidone (PFD) slows the progression of interstitial pulmonary fibrosis remain incompletely understood. Pamrevlumab (Pmab) is an anti-CTGF antibody that also likely inhibits fibroblasts
The advent of single cell RNA sequencing and its application to animal models of lung fibrosis and clinical samples from patients with pulmonary fibrosis have brought the multicellular nature of pulmonary fibrosis into focus. 63 64 65 66 67 68 Repair of the injured alveolar epithelium requires the asymmetric division followed by differentiation of alveolar type 2 cells into alveolar type 1 cells. 69 70 During the process of alveolar type 2 to type 1 cell differentiation, a transitional cell population characterized by expression of keratin-8 in mice and keratin-17 in humans forms. 68 71 72 73 These keratin-8 or keratin-17 positive epithelial cells are found at low concentrations in the normal mouse or human lung, but they increase during pulmonary fibrosis and are specifically localized to fibrotic lung regions in mice and humans. 64 65 68 71 72 74 These results suggest that normal epithelial repair is disrupted in regions of lung fibrosis. In response to this failed repair, circulating monocytes are recruited to the alveolar space where they rapidly differentiate into profibrotic monocyte derived alveolar macrophages. 62 75 76 77 These alveolar macrophages form reciprocal circuits with matrix fibroblasts in which fibroblasts secrete macrophage colony stimulating factor (M-CSF) to maintain alveolar macrophages at the site of injury and alveolar macrophages secrete platelet derived growth factor (PDGF) and other growth factors that drive the differentiation of fibroblasts into myofibroblasts, which excrete excessive matrix proteins. 66 78 In addition, alveolar epithelial injury induces the activation of latent transforming growth factor β (TGF-β) in the matrix. 79 TGF-β is a cytokine that modulates cellular differentiation, proliferation, and apoptosis, as well as extracellular matrix production. 80 It also maintains alveolar macrophages and activates myofibroblasts. 81 82 Over time, myofibroblasts lose their requirement for alveolar macrophages for proliferation and matrix secretion, in part through autocrine production and activation of TGF-β, 83 84 resulting in spatially restricted regions of progressive fibrosis. 78 This model of pulmonary fibrosis suggests a multimodal strategy for treatment. Such a strategy might include therapies to accelerate the differentiation of alveolar type 2 into alveolar type 1 cells through inhibition of the integrated stress response, 68 85 therapies that reduce the recruitment or prevent the maintenance of profibrotic monocyte derived alveolar macrophages in the alveolar space, 86 and therapies that target signaling through TGF-β, PDGF, and other growth factors in myofibroblasts (for example, nintedanib). 11
Management of IPF
Currently approved treatment.
The most recent ATS/ERS/JRS/ALAT clinical practice guideline on the treatment of IPF recommends only two drugs for the treatment of IPF—pirfenidone and nintedanib. 4 The 2015 ATS/ERS/JRS/ALAT guideline also included a conditional recommendation for antacid therapy, and therefore its evidence is also discussed here. 87 Table 1 lists the major clinical trials that examined the use of pirfenidone and nintedanib in the treatment of IPF and PPF.
Major randomized clinical trials evaluating the use of antifibrotic medications in the treatment of IPF and progressive pulmonary fibrosis (PPF)
- View inline
Pirfenidone
The US Food and Drug Administration (FDA) approved pirfenidone for the treatment of IPF in October 2014. The approval was based on data from three phase 3 clinical trials—CAPACITY I, CAPACITY II, and ASCEND. With pooled data from the CAPACITY I and II trials, primary endpoint analysis found that pirfenidone reduced the mean decline in FVC per cent predicted over 72 weeks compared with placebo (−8.5% v −11.0%; P=0.005). 88 The ASCEND trial found that pirfenidone led to a 47.9% reduction in the proportion of particpants who had an absolute decline of 10% or more in the FVC per cent predicted or who died after 52 weeks (16.5% v 31.8%; P<0.001). 89 Prespecified secondary analyses that pooled data with the two CAPACITY trials found that treatment with pirfenidone was associated with decreased all cause mortality (3.5% v 6.7%; P=0.01) and IPF specific mortality (1.1% v 3.5%; P=0.006), compared with placebo. 89 Separate post hoc analysis of pooled data from the CAPACITY and ASCEND trials also found that participants receiving pirfenidone had a lower risk of respiratory related hospital admissions (7% v 12%; P=0.001). 91 The exact mechanisms by which pirfenidone slows the progression of IPF are not known, although several have been proposed. 92
The US FDA also approved nintedanib for the treatment of IPF in October 2014. This was based on two INPULSIS phase 2 clinical trials, which both found that nintedanib reduced the annual rate of decline in FVC at week 52 compared with placebo. 11 In INPULSIS 1, the difference in annual rate of decline in FVC was 125.3 (95% confidence interval 77.7 to 172.8) mL/year (P<0.001); in INPULSIS 2, the difference was 93.7 (44.8 to 142.7) mL/year (P<0.001). In prespecified pooled analyses, no significant difference was seen between nintedanib and placebo groups in the time to first investigator reported acute exacerbation, death from any cause, or death from a respiratory cause. Nintedanib is a tyrosine kinase inhibitor that was originally developed as an anti-angiogenic cancer drug designed to bind and block platelet derived growth factor receptor (PDGFR), fibroblast growth factor receptor 1 (FGFR-1), and vascular endothelial growth factor receptor 2. 93 94 PDGF is made by alveolar macrophages in response to injury and inflammation and contributes to the proliferation, survival, and migration of myofibroblasts, which deposit extracellular matrix proteins in the interstitial space. 94 95 FGF/FGFR signaling also contributes to lung fibrosis, specifically through FGF-2 which induces fibroblast proliferation and collagen synthesis in lung fibroblasts and myofibroblasts. 96 97 Through its inhibition of growth factor signaling, nintedanib is thought to reduce the proliferation and migration of lung fibroblasts, the transdifferentiation of fibroblasts to myofibroblasts, and the deposition of extracellular matrix. 94
Antacid therapy
Abnormal gastroesophageal reflux is common in patients with IPF and is a known risk factor for aspiration and microaspiration. 98 Regular use of antiacid therapy, either with proton pump inhibitors or histamine-2 blockers, is believed to decrease the lung injury induced by microaspiration of acidic gastric juices. 99 Although the 2015 ATS/ERS/JRS/ALAT IPF treatment guidelines give a conditional recommendation for the use of antacid therapy, even in patients without symptoms of gastroesophageal reflux, the 2022 updated guideline makes a conditional recommendation against its use for the purpose of improving respiratory outcomes. 4 The TSANZ/LFA guidelines state that antacid therapy has unclear benefit and do not make a recommendation for or against its use. 101 When examining IPF patients in the placebo arms of RCTs, one study that used the IPFnet trials found that antacid use at baseline was associated with reduced decline in FVC 102 ; however, a more recent study using the CAPACITY and ASCEND trials found that antacid therapy did not improve outcomes and was associated with an increased risk of infection in patients with advanced lung disease. 103 Similarly, the WRAP-IPF trial, a phase II randomized, unblinded, controlled trial, found that laparoscopic antireflux surgery in patients with IPF and abnormal gastroesophageal reflux did not significantly reduce the decline in FVC over 48 weeks. 104 However, in a pilot randomized, placebo controlled trial of participants with IPF and a history of cough, omeprazole use was associated with a reduction in cough frequency of 39.1% (−66.0% to 9.3%), although it was not statistically significant owing to small sample size. 105
Non-drug management
The most recent guidelines from leading international societies of pulmonary medicine recommend long term oxygen therapy for IPF patients with resting hypoxemia, as well as referrals for pulmonary rehabilitation and lung transplant evaluation in appropriate patients. 101 106 107 The current recommendation for supplemental oxygen therapy in IPF is largely based on indirect evidence from two landmark RCTs in obstructive lung disease that showed a survival benefit with long term oxygen therapy in patients with resting hypoxemia (PaO 2 55-65 mm Hg). 108 109 Evidence to directly support the use of supplemental oxygen in people with IPF and resting or exertional hypoxemia is limited. A 2016 Cochrane review that included three RCTs found no evidence to support or refute the use of ambulatory or short burst oxygen in patients with ILD and exertional hypoxemia owing to the limited data 110 ; however, a subsequent systematic review that included studies examining the use of oxygen during exercise or exercise training found that ambulatory oxygen was associated with a consistent increase in exercise capacity. 111
Pulmonary rehabilitation is a comprehensive intervention that includes exercise training, education, and behavior change. 112 A Cochrane review that included five randomized or quasi-randomized controlled trials found that among people with ILD, and IPF specifically, significant improvements in exercise capacity, dyspnea, and quality of life were seen immediately after pulmonary rehabilitation, with the quality of evidence rated as low to moderate. 113 A subsequent meta-analysis of four RCTs found that, in patients with IPF, pulmonary rehabilitation had no detectable benefit at long term follow-up. 114 The current ATS/ERS/JRS/ALAT guidelines recommend that most patients with IPF be treated with pulmonary rehabilitation (weak recommendation, low quality of evidence). 106
Given the progressive natural history of IPF, with a median survival time of 3.8 years after diagnosis, 22 guidelines recommend that appropriate patients undergo lung transplantation and that discussion of transplantation should occur at the time of diagnosis or soon after. 101 106 107 In North America, the percentage of lung transplants performed in patients with IPF has been increasing over the past three decades, and from 2010 to 2018 IPF was the most common indication for lung transplantation. 115 Although post-transplant survival is worse for patients with IPF than for those with COPD and other matched non-IPF patients, 115 116 lung transplantation is associated with a 75% reduction in risk of death. 117 From 1992 to 2017 median survival time for patients with IPF was 5.2 years post-transplant, which increased to 7.3 years among those who survived at least one year post-transplant. 118
Therapies in the pipeline for treatment of IPF
Several drugs for the treatment of IPF—recombinant human pentraxin 2, pamrevlumab, treprostinil, and N-acetylcysteine—have recent phase 3 clinical trials. Table 2 lists these trials along with the data from the phase 2 and 3 trials that support the potential role of these drugs as treatment for IPF.
Recent active phase 3 clinical trials of treatments for idiopathic pulmonary fibrosis and phase 2/3 trials supporting their study
Recombinant human pentraxin 2 (rhPTX-2; PRM-151)
PRM-151 is a recombinant human pentraxin 2 protein (rhPTX-2). Pentraxin 2, also known as serum amyloid P, inhibits the recruitment of profibrotic monocyte derived alveolar macrophages to areas of fibrosis. 123 This is predicted to limit signaling by macrophages that drive matrix remodeling and myofibroblast activation. 63 75
The effect of rhPTX-2 was studied in a phase 2 double blind, randomized, placebo controlled trial of patients with mild to moderate IPF. 86 Concurrent therapy with pirfenidone or nintedanib was permitted. For the primary efficacy endpoint, the least squares mean change in FVC per cent predicted at week 28 in participants treated with rhPTX-2 was −2.5, compared with −4.8 in those given placebo (difference of 2.3, 90% confidence interval 1.1 to 3.5; P=0.001). An open label extension study found a persistent treatment effect in participants who continued taking rhPTX-2, with a decline in the FVC per cent predicted of −3.6% per year. 119 In participants who started taking rhPTX-2, FVC decline improved from −8.7 per cent predicted per year in weeks 0-28 (while taking placebo) to −0.9 per cent predicted per year in weeks 28-52. Thirteen (12%) of 111 participants had adverse events that led to discontinuation of rhPTX-2. Four participants had events that were considered by investigators to be related to rhPTX-2, including IPF exacerbation, tendinitis, dysgeusia, and cardiomyopathy. A phase 3 randomized, double blind, placebo controlled trial to study the efficacy and safety of rhPTX-2 began recruitment in March 2021, with an estimated study completion date in March 2023 ( NCT04552899 ).
Pamrevlumab
Pamrevlumab is an anti-connective tissue growth factor (CTGF) antibody under investigation for the treatment of IPF. CTGF is a mediator of tissue remodeling, acting downstream of TGF-β on connective tissue cells and functioning to stimulate fibroblast proliferation and the production of extracellular matrix. 124 125 CTGF is produced at high concentrations by airway and epithelial cells, as well as by activated fibroblasts in the lung tissue of patients with IPF. 64
The effect of pamrevlumab in patients with IPF was investigated in the phase 2 randomized, double blind, placebo controlled PRAISE trial. 120 Patients included had mild to moderate IPF and were not permitted to be on treatment with pirfenidone or nintedanib. Patients treated with pamrevlumab had a decline in FVC of 2.9 per cent predicted per year compared with 7.2 per cent predicted per year with placebo (difference of 4.3 (0.4 to 8.3) per cent predicted per year; P=0.033). The proportion of patients with disease progression, as defined by decline from baseline FVC per cent predicted ≥10% or death at week 48, was also reduced in the pamrevlumab group compared with the placebo group (10.0% v 31.4%; P=0.013). The frequency of adverse events was similar in the pamrevlumab and placebo groups, and the events were generally mild or moderate in severity and typical of participants’ underlying medical conditions. ZEPHYRUS 1 and 2 are ongoing phase 3 randomized, placebo controlled trials to further evaluate the use of pamrevlumab in patients with IPF and are estimated to complete in 2023 ( NCT03955146; NCT04419558 ).
Inhaled treprostinil
Treprostinil is a prostacyclin analog that is approved by the US FDA as an inhaled solution (Tyvaso) for treatment of pulmonary arterial hypertension and pulmonary hypertension associated with ILD. Inhaled treprostinil causes vasodilation of pulmonary and systemic arterial vascular beds and inhibits platelet aggregation. 126 It has also been shown to reduce collagen deposition in a bleomycin induced mouse model of pulmonary fibrosis, in part by inhibiting TGF-β1 induced expression of collagen mRNA and protein. 127
INCREASE was a randomized, double blind trial that examined the use of inhaled treprostinil in the treatment of pulmonary hypertension in people with ILD. 121 The trial met its primary efficacy endpoint in finding that the least squares mean difference between the inhaled treprostinil group and placebo group in the change from baseline six minute walk distance was 31.12 (16.85 to 45.39) m; P<0.001). Serious adverse events were similar in the inhaled treprostinil and placebo groups. Post hoc analysis found a difference in change in FVC per cent predicted of 1.8% (0.2% to 3.4%; P=0.028), favoring inhaled treprostinil over placebo, by week 16. 128 Notably, this study also found that the largest treatment effect occurred in patients with IPF. Based on these data, a phase 3 randomized, double blind, placebo controlled study began in April 2021 to evaluate the safety and efficacy of inhaled treprostinil in people with IPF, with change in FVC as the primary outcome measure ( NCT04708782).
N-acetylcysteine
N-acetylcysteine is a tripeptide precursor of glutathione that has antioxidant effects in the lung. 129 130 Three randomized, placebo controlled trials have examined the use of N-acetylcysteine monotherapy in the treatment of IPF. 131 132 133 The primary outcome in each of these studies was change in FVC, and none found a significant difference between N-acetylcysteine and placebo groups. Similarly, after the results of these three RCTs were pooled, no significant benefit on mortality, change in FVC, quality of life, or adverse outcomes was seen. 87 Two randomized, placebo controlled studies, including the PANORAMA study, then examined N-acetylcysteine in combination with pirfenidone in patients with IPF. 134 135 Although neither found a significant difference in the incidence of adverse events, both studies found a greater decline in FVC in patients receiving N-acetylcysteine; however, both were limited by small sample size.
However, a post hoc analysis of the PANTHER-IPF trial, which randomized participants with IPF to receive N-acetylcysteine monotherapy, combined prednisone, azathioprine, and N-acetylcysteine, or placebo, identified a subgroup of patients with the TOLLIP TT genotype in which N-acetylcysteine monotherapy was associated with a significant decrease in the composite endpoint of lung disease progression, hospital admission, transplantation, or death (hazard ratio 0.14, 95% confidence interval 0.02 to 0.83; P=0.03). 136 The TOLLIP CC genotype was associated with a non-significant increase in risk of the composite endpoint (hazard ratio 3.23, 0.79 to 13.16; P=0.10), which was significant in replication cohorts. Based on these data, the PRECISIONS trial is a phase 3 clinical trial comparing the effect of N-acetylcysteine plus standard care in patients with IPF who have the TOLLIP TT genotype ( NCT04300920 ).
Treatment of inflammatory ILDs
The currently accepted treatment for inflammatory ILDs, including CTD-ILD, cHP, iNSIP, and unclassifiable ILD, is immunosuppression. However, the only RCT data supporting this approach come from studies in patients with SSc-ILD. 137 138 139 Additionally, the only immunosuppressive drug that is approved by the FDA for the treatment of SSc-ILD is tocilizumab. FaSScinate, a phase 2/3 RCT, and focuSSced, a phase 3 RCT, were the basis for the FDA approval of tocilizumab for the treatment of SSc-ILD. 139 140 The focuSSced trial randomly assigned 210 people with diffuse cutaneous systemic sclerosis to receive tocilizumab or placebo. 139 People with severe ILD were excluded, and the cohort had a mean baseline FVC per cent predicted of 82% and evidence of SSc-ILD on HRCT in 65% of cases. Although the primary endpoint of change in the modified Rodman skin score was not met, on analysis of secondary outcomes participants who received tocilizumab had less decline in FVC per cent predicted than did those who received placebo (absolute difference in least square mean of 4.2%, 2.0% to 6.4%; P=0.0002).
Although cyclophosphamide and mycophenolate mofetil are not approved by the FDA for the treatment of SSc-ILD, their use is supported by the Scleroderma Lung Studies I and II. In the Scleroderma Lung Study I, which randomized 158 patients with SSc-ILD to receive cyclophosphamide or placebo, the mean absolute difference in adjusted FVC per cent predicted at 12 months was 2.53% (0.28% to 4.79%; P<0.03), favoring cyclophosphamide. 138 The Scleroderma Lung Study II subsequently randomized 126 patients with SSc-ILD to receive either cyclophosphamide or mycophenolate mofetil. 137 No significant difference was seen in the primary outcome of FVC per cent predicted at 24 months, but mycophenolate mofetil was associated with fewer toxicities and was better tolerated.
The evidence to support the use of immunotherapies such as steroids, mycophenolate mofetil, azathioprine, cyclophosphamide, tacrolimus, and rituximab for the treatment of other inflammatory ILDs is limited to observational studies and case series. Despite this, immunosuppression remains the standard of care for CTD-ILD and cHP and should be considered as first line therapy. The RECITAL trial is ongoing and has randomized patients with severe and/or progressive CTD-ILD to receive either cyclophosphamide (as standard of care) or rituximab as first line therapy and may further clarify the role of rituximab in CTD-ILD. 141
Despite the use of immunosuppressive treatment, high morbidity and mortality associated with these ILDs remain. Thus, a clear mandate exists for better treatment strategies that may be informed by understanding the progressive fibrosing phenotype and the role of antifibrotics in its treatment.
Treatment of PPF
Although the non-uniformity of the interstitial lung diseases that manifest PPF poses a challenge to designing and conducting clinical trials, several studies have established a role for antifibrotic therapy in PPF ( table 1 ). 10 12 15 90
Strong evidence supports the use of nintedanib for PPF. The SENSCIS trial was a phase 3 RCT that investigated the efficacy of nintedanib versus placebo in 576 people with SSc-ILD. 10 Enrollment did not require evidence of disease progression but included only people who had fibrosis affecting at least 10% of the lungs on baseline HRCT. The primary endpoint, annual rate of decline in FVC over 52 weeks, was lower in the nintedanib arm (difference 41.0 (2.9 to 69.0) mL/year). INBUILD, another phase 3 RCT of nintedanib versus placebo, expanded inclusion criteria to any non-IPF progressive fibrosing ILD. 12 Enrollment required meeting the study criteria for progressive fibrosis, based on FVC decline, or a combination of worsening FVC, symptoms, or imaging findings. The primary endpoint of annual rate of decline in FVC over 52 weeks was again lower in the nintedanib arm (difference 107 (65.4 to 148.5) mL/year). The difference was greater for the nearly two thirds of participants with a radiographic pattern of UIP (difference 128.2 (70.8 to 185.6) mL); however, a definitive treatment effect could not be inferred for other radiographic patterns of fibrosis.
Nearly half of the participants in the SENSCIS trial (48.5%) were concurrently taking mycophenolate mofetil, and subgroup analysis found no heterogeneity in nintedanib’s treatment effect according to baseline mycophenolate mofetil use. 10 142 Although the absolute reduction in FVC decline associated with nintedanib use was less in participants taking mycophenolate mofetil, the relative reduction in FVC decline was similar in those taking and those not taking mycophenolate mofetil (40% v 46%). Notably, participants receiving mycophenolate mofetil and placebo had a similar adjusted mean annual rate of FVC decline to those receiving nintedanib alone (−66.5 v −63.9 mL/year); however, the authors note that this comparison was out of the scope of the trial. The INBUILD trial excluded people who were receiving concomitant immunosuppression for ILD.
The data supporting pirfenidone in PPF are less robust. Pirfenidone was studied in two completed phase 2/2b RCTs. The first enrolled 253 people with unclassifiable ILD, including those with interstitial pneumonia with autoimmune features, and evidence of progressive loss of lung function. 15 The primary endpoint used home spirometry and provided unreliable results that could not be analyzed. The secondary outcome, using on-site spirometry, compared the mean decline in FVC over 24 weeks and showed a treatment difference favoring pirfenidone over placebo (difference 95.3 (35.9 to 154) mL; P=0.002). The RELIEF study enrolled only 127 of the planned 374 people with PPF, including those with CTD-ILD, cHP, iNSIP, and asbestos induced lung fibrosis. 90 The trial was terminated early owing to slow enrollment and for futility. The result was that 47% of participants, in both arms, had imputed data. Despite being underpowered by early termination, when imputed data were included, the primary endpoint of absolute change in FVC per cent predicted from baseline to 48 weeks was lower in participants taking pirfenidone (P=0.049). The median difference in change in FVC per cent predicted per year ranged from 1.69% to 3.53%, depending on the test used. The finding remained significant on multiple sensitivity analyses. Although the analysis of the primary outcome performed without imputation was not statistically significant, these findings may be clinically relevant. Clinical trials of both pirfenidone and nintedanib that are ongoing in a variety of PPF subsets are noted in table 3 .
Ongoing randomized clinical trials of antifibrotic drugs for treatment of idiopathic pulmonary fibrosis and progressive fibrosing interstitial lung disease
Gaps in knowledge in management of PPF
Identifying and treating ppf.
Recognition of a progressive fibrosing phenotype of ILD is important to both treatment strategies and prognosis. However, before May 2022, the diagnosis of PPF had been hampered by the lack of established clinical criteria and biomarkers. Additionally, the proposed criteria do not account for time from disease onset and may identify early inflammatory disease without a progressive fibrosing phenotype. Early decline in FVC in inflammatory ILDs may be remediated with immunosuppressive treatment, and a progressive fibrosing phenotype may never occur despite the proposed criteria being met early in the course of disease. Nevertheless, this needs to be balanced with the consideration that earlier treatment directed toward fibrosis may help to preserve lung function in patients who ultimately develop a progressive phenotype.
When immunosuppressive treatment is efficacious in inflammatory ILDs, it is continued. When an inflammatory ILD has progressive fibrosis despite immunosuppression, the question is whether to escalate immunosuppressive therapy or to start treatment with an antifibrotic drug such as nintedanib. Treatment decisions should consider the time from disease onset, as immunosuppressive therapies may be more likely to be effective early in the disease course. The prospective trials of immunosuppressive treatments for SSc-ILD recruited people early in the disease course and showed stabilization of lung function with cyclophosphamide, mycophenolate, or tociluzimab. 137 138 139 Acute and subacute cases of hypersensitivity pneumonitis may resolve with antigen avoidance with or without a short course of corticosteroids. However, once cHP develops and fibrotic features are present on imaging, five year mortality is similar to that of IPF at 50%. 8 In this setting, immunosuppressive therapy is unlikely to be beneficial and treatment with antifibrotics should be offered. Similarly, in CTD-ILD, antifibrotics should be strongly considered once progressive fibrosis has been established. Whether immunosuppression should continue when antifibrotic therapy is introduced also remains unclear. Although it is associated with worse outcomes in IPF, data in SSc-ILD from the SENSCIS trial suggest that treatment with combined immunosuppression and antifibrotic therapy may be advantageous. 10
Given the complex and multicellular pathobiology of pulmonary fibrosis, defining disease endotypes that can be identified by patterns of clinical characteristics, radiologic features, and biomarkers is important. These endotypes can then be used to guide initial therapy and to modify treatment over time. The recognition of PPF creates a further need to develop biomarkers of progressive disease. A comprehensive review of diagnostic and prognostic biomarkers was recently published. 143 Of the many studies examining biomarkers, most are observational and retrospective in design and few have been validated in separate prospective cohorts. For these reasons, biomarkers are infrequently used in clinical practice. 143 Single cell RNA sequencing and spatial transcriptomic studies conducted on explanted lungs obtained at the time of transplant when fibrosis is well established suggest relatively little heterogeneity between pulmonary fibrosis with differing initiating factors. 63 64 144 These findings suggest the need to obtain samples from patients with early disease to guide the selection of initial therapy and monitor the response to therapy over time.
The first large prospective study to evaluate biomarkers in IPF examined serum specimens from the PROFILE cohort, a longitudinal cohort of treatment-naive patients with IPF. 145 After measuring 123 serum proteins, the investigators focused on surfactant protein D (SFTPD), matrix metalloproteinase-7 (MMP7), CA19-9 (ST6GALNAC6), and CA-125 (MUC16). Including the discovery and validation phases of the trial, the study included 312 participants with IPF (145 with stable disease and 155 with progressive disease at follow-up) and 50 healthy controls. Although MMP7 was higher in patients with IPF compared with controls, it did not predict disease progression or mortality. SFTPD had higher discriminatory power for distinguishing IPF from healthy controls and identifying patients at high risk of progression. Although neither CA19-9 nor CA-125 could distinguish disease from controls, CA19-9 was most highly predictive of progressive fibrosis, and increasing concentrations of CA-125 predicted both disease progression and overall survival. As CA19-9 and CA-125 are relatively new markers in IPF, immunohistochemical localization of these markers was done in control and fibrotic lung tissue to ensure relevance to lung disease. CA19-9 and CA-125 were present in the apical bronchial epithelium in normal lungs, whereas in the fibrotic lung these markers were seen throughout the metaplastic epithelium in fibrotic lesions.
The largest study to examine biomarkers in non-IPF ILD is a retrospective study in 148 people with CTD-ILD, 98 with cHP, and 159 with unclassifiable ILD. 146 Six biomarkers of interest were evaluated with the primary endpoint of progression-free survival defined as survival without lung transplant or ≥10% decline in FVC over two years. The investigators found that increased serum concentrations of CXCL13 were associated with decreased survival in all three disease subgroups, but the optimal threshold concentration varied substantially between subgroups. CXCL13 is a chemokine that is chemotactic for B lymphocyte migration, and increased concentrations have been associated with ectopic germinal centers in autoimmune disease. 147 The authors speculate that the CXCL13 threshold variability may reflect different underlying biology, with inflammatory phenotypes of ILD having a higher baseline concentration overall, and therefore may indicate that CXCL13 could be useful in identifying a population of patients responsive to immunosuppression.
Genetic biomarkers may identify patients at increased risk for pulmonary fibrosis and predict disease progression. Patients with heterozygous mutations of either the TERT gene or the TERC gene, which are part of the telomerase complex genes, are at increased risk of IPF, as are those with shortened telomeres. 148 Although the use of telomere length testing in patients with suspected familial forms of idiopathic ILD varies in clinical practice, no formal recommendations on its use exist. A single nucleotide polymorphism (SNP) in the promotor region of the MUC5B gene (rs35705950) that increases the expression of the gene is associated with the development of IPF but has unclear effects on disease severity and survival. 149 150 Three SNPs in the TOLLIP gene have also been associated with IPF. 151 TOLLIP encodes toll interacting proteins that are linked to the lung’s immune responses, including modulation of TGF-β signaling. 152 Post hoc genotyping of TOLLIP and MUC5B was performed on previously collected samples from people enrolled in the PANTHER trial, 132 and identified polymorphisms within these genes were suggested to modify the effect of treatment with N-acetylcysteine or immunosuppression. 136 The results of this analysis were used to support further investigation of N-acetylcysteine in IPF patients with the TOLLIP rs3750920 TT genotype through the PRECISION trial ( NCT04300920 ).
Emerging therapies and diagnostics
Advanced diagnostics.
Newer methods that exploit advances in transcriptomics and proteomics may not only advance our understanding of the pathobiology of fibrosing lung diseases but may also serve to improve the utility of biomarkers. They offer a personalized approach to the management of PPF by eliciting the specific biologic pathways that are active at a given point in time and thereby might facilitate targeted therapy. Machine learning tools offer promise to iteratively improve the predictive power of these information-rich multi-omics data by incorporating detailed clinical and imaging metadata, including the response to therapy.
Currently available for clinical use, the Envisia Genomic Classifier (EGC) was developed using machine learning methods applied to exome enriched RNA sequencing data from whole lung biopsies (bulk RNA) in combination with histologically confirmed diagnoses. The product of this is an algorithm that differentiates UIP from non-UIP histologic patterns by recognizing the transcriptomic signature of UIP. This classifier was validated using an independent dataset in the BRAVE studies. 153 In these studies, samples were obtained from 84 people with suspected ILD undergoing planned, clinically indicated lung biopsy procedures. The transcriptome analysis showed that biopsy samples histologically classified as UIP were enriched for gene expression pathways associated with cellular metabolism, adhesion, and developmental processes. However, samples histologically classified as non-UIP showed gene expression pathways associated with immune activities, lipid metabolism, stress response, and cell death. Using the developed algorithm and a single transbronchial lung biopsy sample to distinguish UIP from non-UIP histologic patterns, the EGC had a sensitivity of 63% (95% confidence interval 51% to 74%) and a specificity of 86% (71% to 95%). If three to five samples were used, the sensitivity improved to 74% (51% to 90%) and specificity improved to 93% (68% to 100%). The EGC has now been validated in an additional study using the BRAVE cohort, which found that it had a negative predictive value of 60.3% (46.6% to 73.0%) and a positive predictive value of 92.1% (78.6% to 98.3%) for histology proven UIP. 154
The EGC identifies a transcriptomic pattern associated with histologic UIP in patients with indeterminant radiographic patterns. This does not equate to a diagnosis of IPF. Rather, the results from EGC are an additional piece of data that can be incorporated into a multidisciplinary discussion to achieve a consensus diagnosis. The ECG has also not yet been studied in PPF. However, future studies to evaluate the use of transcriptomic tools to identify or predict progressive fibrosis and predict response to antifibrotics in this patient population may be instrumental in developing precise therapeutic targets.
Bulk RNA sequencing like that used in the EGC provides an average measure of gene expression across the heterogenous cell populations that make up the lung. This creates a problem of averaging in which a change in cellular composition (for example, an increased number of inflammatory cells) can drive changes in average gene expression and biologically important signals in cell populations or subpopulations can be missed. Single cell RNA sequencing avoids these problems by measuring gene expression within each individual cell, allowing one to compare cell populations—for example, alveolar type 2 cells—in health and disease. In addition to identifying biomarkers, single cell RNA sequencing allows one to generate hypotheses about which cellular interactions drive fibrosis and can be targeted pharmacologically. Although still too costly and time consuming for clinical practice, single cell RNA sequencing has become an invaluable discovery tool, particularly when applied to small samples from patients with early disease, including those obtained by bronchoscopic lavage or biopsy.
Along with improved tools for exploring the pathobiology of IPF and PPF, several national and international ILD registries are enrolling people. Registries differ from clinical trials in that they are large, they allow for prolonged follow-up time, and enrollment is inclusive and thus more reflective of the general population of patients with a given disease. Participants should be well characterized as to important clinical features of their disease. Insights derived from registries complement clinical trials and may answer questions about the long term effectiveness of treatments. Current registries will need to be expanded to accommodate digitized images and genomic data that will facilitate the training of multimodal machine learning classifiers to predict disease endotypes and responsiveness to therapy.
Resolution of fibrosis
IPF and PPF are characterized by self-sustaining fibrosis and progressive decline in lung function. The therapies approved and undergoing phase 3 clinical trials for the treatment of IPF and PPF have been shown only to slow decline in lung function, and none has shown resolution of fibrosis. However, growing evidence suggests that fibrosis may be reversible, particularly with removal of the underlying cause of injury. 155 A recent review covered the biology of self-sustaining fibrosis and emphasized three processes necessary for resolution of fibrosis—elimination of matrix producing cells, clearance of excess matrix, and regeneration of normal tissue constituents. 5
Metformin has been found to ameliorate pulmonary fibrosis in bleomycin induced mouse models of lung fibrosis. 156 157 Metformin inhibits mitochondrial complex I to activate adenosine monophosphate activated protein kinase (AMPK), which subsequently inhibits TGF-β. 157 158 159 160 Metformin is able to normalize myofibroblast sensitivity to apoptosis and stimulate turnover of collagen via AMPK dependent activation of autophagy. 156 By eliminating matrix producing myofibroblasts and promoting the clearance of excess matrix, metformin, or other AMPK activators, may be able to reverse established fibrosis. Notably, however, when patients who were randomized to placebo in the CAPACITY and ASCEND trials of pirfenidone were stratified by baseline metformin use, no significant difference in disease progression associated with metformin use was seen. 161 One potential reason for the discrepancy between these findings and experimental studies may be the high doses (65-300 mg/kg) of metformin and intraperitoneal route used in the mouse models. 156 157
The resolution of fibrosis requires not only breaking the positive feed-forward loops that sustain and amplify fibrosis but also regenerating normal tissue to occupy the area of former fibrosis. Alveolar type 2 cells are a partially committed stem cell population in the adult lung that undergo asymmetric division and differentiation to replace damaged alveolar type 1 cells 69 74 162 ; however, when alveolar type 2 cells are isolated from IPF lung tissue they have impaired regenerative ability compared with healthy tissue. 163 In single cell RNA sequencing data from lung explants from patients with pulmonary fibrosis, investigators have noted the emergence of a population of epithelial cells characterized by expression of low concentrations of keratin-5 and increased levels of keratin-17. 64 65 These cells also express high levels of genes associated with senescence, including p16 ( CDKN2A ), p21 ( CDKN1A ), and plasminogen activator inhibitor 1 ( SERPINE1 ), among others. A transcriptionally similar population of cells has been observed in murine models of pulmonary fibrosis and in a murine model of alveolar regeneration after pneumonectomy. 68 71 72 73 In all of these studies, these cells are characterized by increased expression of keratin-8, along with similar senescence associated genes. All three initial reports of these cells showed them to be a transitional cell population that forms during the differentiation of alveolar type 2 to type 1 cells. 68 71 72 Strunz and colleagues showed that, during bleomycin induced fibrosis, these cells develop a transcriptomic signature suggestive of activation of the integrated stress response during their differentiation. 68 This is of interest because inhibitors of the integrated stress response have been shown to reduce fibrosis in animal models. 164 Watanabe and colleagues followed up on these results, showing that a small molecule inhibitor of the integrated stress response, ISRIB, accelerated the differentiation of alveolar type 2 cells into alveolar type 1 cells during fibrosis, reducing the number of keratin-8 positive cells. 85 This suggests that a decline in the function of the proteostasis network, as occurs during aging in model organisms, might impair the differentiation of alveolar type 2 cells, predisposing to the development of fibrosis. 165 Future studies are needed to determine whether the emergence of keratin-17 cells explains some of the increase in senescence markers observed in lung fibrosis. 166
Table 4 summarizes the most recent guidelines from the leading international societies on the management of idiopathic pulmonary fibrosis and highlights some of the key commonalities and differences between the recommendations. The ATS/ERS/JRS/ALAT clinical practice guidelines published in 2011 were updated in 2015 and 2022. 4 87 106 The JRS published a separate clinical practice guideline in 2018, which provided additional recommendations not previously included in the 2015 joint guidelines. 167 Specifically, for patients experiencing an acute exacerbation of IPF, they recommend against the use of polymyxin B (weak recommendation, low quality of evidence), neutrophil elastase inhibitors (weak recommendation, very low quality of evidence), and recombinant thrombomodulin (weak recommendation, low quality of evidence) and recommend the use of immunosuppressant drug therapy (weak recommendation, low quality of evidence).
Comparison of guideline recommendations from ATS/ERS/JRS/ALAT, JRS, NICE, and TSANZ/LFA for treatment of idiopathic pulmonary fibrosis
NICE guidelines on the diagnosis and management of IPF were published in 2013 and last updated in 2017. 107 168 169 As seen in table 4 , NICE guidelines have minor differences from the ATS/ERS/JRS/ALAT guidelines, which may reflect the fact the NICE Guideline Development Group is required to make decisions based on the best available evidence of both clinical effectiveness and cost effectiveness. 170 The TSANZ and the LFA published a position statement on the treatment of IPF in 2017, which differs from the ATS/ERS/JRS/ALAT guidelines in its recommendation to use disease severity to guide decisions on antifibrotic therapy and its neutral stance on antacid therapy. 101
The first international gudelines on the treatment of PPF came in May 2022 with the ATS/ERS/JRS/ALAT clinical practice guideline. 4 This guideline suggested nintedanib for the treatment of PPF in patients who have not responded to standard management for non-IPF fibrotic ILD (conditional recommendation, low quality evidence). The committee made no recommendation for or against the use of pirfenidone for the treatment of PPF and recommended further research into the use of pirfenidone in non-IPF ILDs.
Tremendous advances have been made in elucidating the biologic processes that promote and sustain pulmonary fibrosis. The recognition that ILDs other than IPF may also have a progressive fibrosing phenotype has also been instrumental in moving forward the treatment options for patients with PPF and conceptualizing how to best manage these patients in the future. Importantly, nintedanib has been shown to slow progression of disease in patients with PPF, and several ongoing clinical trials are examining whether pirfenidone may also be beneficial. Several promising therapies are in the pipeline that may offer novel ways of treating IPF that could potentially be used instead of or in addition to the currently available antifibrotics. However, significant gaps in knowledge surrounding the treatment of IPF and PPF remain. Notably, we lack biomarkers and other diagnostic tests that can be used early in the disease course (before functional decline is present) to determine when patients with PPF may benefit from antifibrotics. Additionally, more studies are necessary to examine whether antifibrotics should be used in lieu of or in addition to immunosuppression when no extrapulmonary indications for immunosuppressive therapy are present. The essential question of whether and how established fibrotic disease can actually be reversed and normal lung tissue and function restored also remains. Future research must consider these questions to continue advancing the care for patients with these devasting diseases.
Glossary of abbreviations
ALAT—Latin American Thoracic Association
AMPK—adenosine monophosphate activated protein kinase
ATS—American Thoracic Society
cHP—chronic hypersensitivity pneumonitis
CTD-ILD—connective tissue disease associated ILD
CTGF—connective tissue growth factor
DLCO—diffusing capacity for carbon monoxide
EGC—Envisia Genomic Classifier
ERS—European Respiratory Society
FDA—Food and Drug Administration
FGFR-1—fibroblast growth factor receptor 1
FVC—forced vital capacity
HRCT—high resolution computed tomography
ILD—interstitial lung disease
iNSIP—idiopathic nonspecific interstitial pneumonia
IPF—idiopathic pulmonary fibrosis
JRS—Japanese Respiratory Society
LFA—Lung Foundation of Australia
M-CSF—macrophage colony stimulating factor
MMP7—matrix metalloproteinase-7
NICE—National Institute for Health and Care Excellence
NSIP—nonspecific interstitial pneumonia
PDGF—platelet derived growth factor
PDGFR—platelet derived growth factor receptor
PF-ILD—progressive fibrosing interstitial lung disease
PPF—progressive pulmonary fibrosis
RA-ILD—rheumatoid arthritis associated ILD
RCTs—randomized controlled trials
rhPTX-2—recombinant human pentraxin 2
SFTPD—surfactant protein D
SNP—single nucleotide polymorphism
SSc-ILD—systemic sclerosis associated ILD
TGF-β—transforming growth factor β
TSANZ—Thoracic Society of Australia and New Zealand
UIP—usual interstitial pneumonia
Research questions
What drives progressive pulmonary fibrosis in patients with interstitial lung disease (ILD)?
Do biomarkers exist that can predict which patients with ILD will develop progressive pulmonary fibrosis before they have lung function decline?
What is the optimal timing for starting antifibrotics in patients with non-idiopathic pulmonary fibrosis fibrotic? Should antifibrotics be started only after patients have shown progression on immunosuppression?
Should immunosuppression be continued in patients with progressive pulmonary fibrosis who start treatment with antifibrotics?
Do therapies exist that can reverse or resolve pulmonary fibrosis?
Series explanation: State of the Art Reviews are commissioned on the basis of their relevance to academics and specialists in the US and internationally. For this reason they are written predominantly by US authors
Contributors: All authors contributed to the intellectual content, did the literature search, and participated in the preparation, editing, and critical review of the manuscript.
Funding: GYL is supported by NIH grant F32-HL162318 and North Western University’s Lung Sciences Training Program 5T32HL076139-17. GRSB is supported by supported by NIH grants ES013995, HL071643, and AG049665 and the Veterans Administration grant BX000201.
Competing interests: We have read and understood the BMJ policy on declaration of interests and declare: none.
Patient involvement: No patients or members of the public were involved in the design, conduct, reporting, or dissemination plans of this manuscript.
Provenance and peer review: Commissioned; externally peer reviewed.
- Flaherty KR ,
- Hirani NA ,
- Hotchkin DL ,
- Fischer A ,
- Quaresma M ,
- Stowasser S ,
- Remy-Jardin M ,
- Richeldi L ,
- Podolsky MJ
- Spagnolo P ,
- Distler O ,
- Ryerson CJ ,
- King TE Jr .
- Salisbury ML ,
- Highland KB ,
- Gahlemann M ,
- SENSCIS Trial Investigators
- du Bois RM ,
- INPULSIS Trial Investigators
- INBUILD Trial Investigators
- American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society
- George PM ,
- Kreuter M ,
- Erice ILD working group
- Volkmann ER ,
- Tashkin DP ,
- SLS I and SLS II study groups
- Nalysnyk L ,
- Cid-Ruzafa J ,
- Rotella P ,
- Hutchinson J ,
- Fogarty A ,
- Hubbard R ,
- Gifford AH ,
- Fernández Pérez ER ,
- Bongartz T ,
- Nannini C ,
- Medina-Velasquez YF ,
- Zamora-Legoff JA ,
- Krause ML ,
- Crowson CS ,
- Matteson EL
- Elicker BM ,
- Maldonado F ,
- Bergamasco A ,
- Hartmann N ,
- Wallace L ,
- Verpillat P
- Solomon JJ ,
- Davidyock T ,
- Ferguson LT ,
- Hoyles RK ,
- Denton CP ,
- Assassi S ,
- Volkmann ER
- Nicholson AG ,
- Blumbergs P ,
- Roberts-Thomson P
- Morisset J ,
- Johnson C ,
- Collard HR ,
- Hatron PY ,
- Dominique S ,
- Mouthon L ,
- Reynaud Q ,
- Tazelaar HD ,
- Viggiano RW ,
- Pickersgill J ,
- Raimundo K ,
- Koelsch TL ,
- Kulkarni R ,
- Collins BF ,
- Sharma BB ,
- Doménech G ,
- De Gracia J ,
- Lacasse Y ,
- Costabel U ,
- HP Study Group
- Vasakova M ,
- Belloli EA ,
- Beckford R ,
- Flaherty KR
- Travis WD ,
- Hunninghake G ,
- King TE Jr . ,
- Hansell DM ,
- Baughman RP ,
- Culver DA ,
- Patterson KC ,
- Clinical Features and Outcomes
- Brillet PY ,
- Letoumelin P ,
- Urbania TH ,
- Kropski JA ,
- Lawson WE ,
- Blackwell TS
- Seibold MA ,
- Degryse AL ,
- Nureki SI ,
- Naikawadi RP ,
- Disayabutr S ,
- Mallavia B ,
- Barkauskas CE ,
- Limjunyawong N ,
- Gulleman PM ,
- Bridges JP ,
- Reyfman PA ,
- Walter JM ,
- Habermann AC ,
- Gutierrez AJ ,
- Schupp JC ,
- Watanabe S ,
- Nabhan AN ,
- Brownfield DG ,
- Harbury PB ,
- Krasnow MA ,
- Zacharias WJ ,
- Kobayashi Y ,
- Konkimalla A ,
- Gil de Rubio R ,
- Hrycaj SM ,
- Querrey M ,
- Markov NS ,
- Misharin AV ,
- Morales-Nebreda L ,
- McCubbrey AL ,
- Barthel L ,
- Mohning MP ,
- Scott MKD ,
- Munger JS ,
- Kawakatsu H ,
- Fernandez IE ,
- Eickelberg O
- Sheppard D ,
- Buttgereit A ,
- Monclus EA ,
- van den Blink B ,
- Hamblin MJ ,
- Rochwerg B ,
- American Thoracic Society ,
- European Respiratory society ,
- Japanese Respiratory Society ,
- Latin American Thoracic Association
- Bradford WZ ,
- CAPACITY Study Group
- Castro-Bernardini S ,
- ASCEND Study Group
- RELIEF investigators
- Swigris J ,
- Ruwanpura SM ,
- Thomas BJ ,
- Hilberg F ,
- Pautsch A ,
- Tinkle SS ,
- Dockstader K ,
- Freudenberger TD ,
- Günther A ,
- Bonella F ,
- Anstrom KJ ,
- IPFnet Investigators
- Renzoni E ,
- Pellegrini CA ,
- Funston W ,
- ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis
- ↵ National Institute for Health and Care Excellence. Idiopathic pulmonary fibrosis in adults: diagnosis and management. 2017. https://www.nice.org.uk/guidance/cg163/chapter/1-Recommendations#management .
- Nocturnal Oxygen Therapy Trial Group
- ↵ Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party . Lancet 1981 ; 1 : 681 - 6 . pmid: 6110912 OpenUrl CrossRef PubMed Web of Science
- Adamali H ,
- Rochester CL ,
- Vogiatzis I ,
- Holland AE ,
- ATS/ERS Task Force on Policy in Pulmonary Rehabilitation
- Chambers DC ,
- Zuckermann A ,
- International Society for Heart and Lung Transplantation
- Brizzio ME ,
- Alster JM ,
- Castier Y ,
- ↵ International Society for Heart & Lung Transplantation. Registry Data Slides. 2021. https://ishlt.org/research-data/registries/ttx-registry/ttx-registry-slides .
- Restrepo-Jaramillo R ,
- Thenappan T ,
- Restrepo R ,
- Castaño AP ,
- Igarashi A ,
- Bradham DM ,
- Grotendorst GR
- Yamauchi K ,
- Whittle BJ ,
- Silverstein AM ,
- Mottola DM ,
- Corboz MR ,
- Nathan SD ,
- Rajagopal S ,
- Gillissen A ,
- Degenkolb B ,
- Krombach F ,
- Vogelmeier C
- Taniguchi H ,
- Japan NAC Clinical Study Group
- Martinez FJ ,
- Idiopathic Pulmonary Fibrosis Clinical Research Network
- Tomioka H ,
- Imanaka K ,
- Bendstrup E ,
- Crestani B ,
- Sakamoto S ,
- Kataoka K ,
- Diffuse Lung Diseases Research Group of the Ministry of Health, Labour and Welfare, Japan
- Oldham JM ,
- Clements PJ ,
- Elashoff R ,
- Scleroderma Lung Study Research Group
- focuSSced investigators
- Saunders P ,
- Tsipouri V ,
- SENSCIS trial investigators
- Kaminski N ,
- Allard JD ,
- Pittet JF ,
- Simpson JK ,
- Alqalyoobi S ,
- Adegunsoye A ,
- Linderholm A ,
- Fingerlin TE ,
- Dudbridge F ,
- Sheehan NA ,
- Pankratz DG ,
- Scholand MB ,
- Rangarajan S ,
- Zmijewska AA ,
- Takasaka N ,
- Yoshida M ,
- Viswanadhapalli S ,
- Kheirollahi V ,
- Wasnick RM ,
- Wheaton WW ,
- Weinberg SE ,
- Hamanaka RB ,
- Tanjore H ,
- Sznajder JI ,
- Budinger S ,
- Schafer MJ ,
- Ministry of Health, Labour and Welfare, the Study Group on Diffuse Pulmonary Disorders, Scientific Research/Research on Intractable Diseases, and Japanese Respiratory Society
- ↵ National Institute for Health and Care Excellence. Pirfenidone for treating idiopathic pulmonary fibrosis. 2018. https://www.nice.org.uk/guidance/ta504 .
- ↵ National Institute for Health and Care Excellence. Nintedanib for treating idiopathic pulmonary fibrosis. 2016. https://www.nice.org.uk/guidance/TA379/chapter/1-Recommendations .
- ↵ National Institute for Health and Care Excellence. The guidelines manual. 2012. https://www.nice.org.uk/process/pmg6/chapter/assessing-cost-effectiveness .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 02 March 2021
Occupational and environmental risk factors of idiopathic pulmonary fibrosis: a systematic review and meta-analyses
- Yeonkyung Park 1 na1 ,
- Chiwon Ahn 2 na1 &
- Tae-Hyung Kim 3
Scientific Reports volume 11 , Article number: 4318 ( 2021 ) Cite this article
6501 Accesses
51 Citations
12 Altmetric
Metrics details
- Environmental sciences
- Environmental social sciences
- Health occupations
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, fibrosing interstitial lung disease of unknown cause. It has a high risk of rapid progression and mortality. We conducted a systematic review and meta-analysis to evaluate the risk factor of IPF. We searched Medline, Embase, and the Cochrane library from the earliest record to March, 2020. Case–control studies on occupational and environmental risk factors or on jobs with a risk of IPF were searched for. From 2490 relevant records, 12 studies were included. Any occupational or environmental exposure to metal dust (OR 1.83, 95% CI 1.15–2.91, I 2 = 54%), wood dust (OR 1.62 5% CI 1.04–2.53, I 2 = 5%) and pesticide (OR 2.07, 95% CI 1.24–3.45, I 2 = 0%) were associated with an increased risk of IPF. Farming or agricultural work (OR 1.88, 95% CI 1.17–3.04, I 2 = 67%) was also associated with an increased risk of IPF. Moreover, smoking increased IPF risk with an odds ratio of 1.39 (95% CI 1.01–1.91, I 2 = 29%). In conclusion, metal dust, wood dust, pesticide, occupational history of farming or agriculture and ever smoking increased the risk of IPF.
Similar content being viewed by others
Lung cancer mortality attributable to residential radon: a systematic scoping review
Wood dust exposure and small cell lung cancer: a systematic review and meta-analysis
A systematic review and meta-analysis on the association between ambient air pollution and pulmonary tuberculosis
Introduction.
Interstitial lung disease (ILD) causes abnormal collagen deposition by proliferation of interstitial compartments and infiltration of various inflammatory cells, and fibrotic changes. Idiopathic pulmonary fibrosis (IPF) is a special form of chronic ILD of unknown cause that occurs mainly in the lungs with increasing age and is associated with histopathological or radiological form of usual interstitial pneumonia (UIP). To diagnose IPF, other types of ILD should be ruled out, including drug-induced ILD, ILD through environmental exposure, or systemic disease-related ILD 1 . It has been reported that increasing age, genetic predisposing factors, smoking, or continuous exposure to various environmental and occupational factors can cause physical irritation and damage to the lungs 2 . IPF prevalence is higher in men and increases with age. According to a national survey in Korea, 72.4% of IPF patients are men and the average age at diagnosis is 69 years 3 .
Several studies conducted in various countries have investigated the association between occupational and environmental exposure factors, and IPF over the past decades. According to the 2015 Korean National Health and Nutrition Survey, those exposed to occupational and environmental dust were diagnosed with IPF at a younger age and had a longer period of symptomatic symptoms at the time of diagnosis. Moreover, it has been reported to be related to increase the mortality rate of IPF patients in exposed group 4 .
Case–control studies have investigated the association of IPF incidence with each of the exposure factors that can cause IPF in various countries like the UK 5 , 6 , 7 , 8 , the USA 9 , 10 , Sweden 11 , Mexico 12 , Egypt 13 , South Korea 14 , 15 , and Southern Europe 16 . Exposure to metal dust 5 , 6 , 7 , 9 , 15 , 16 , 17 , wood dust 6 , 7 , 11 , 13 , stone or sand dust 9 , 14 , and raising of birds or livestock and working in agriculture 6 , 9 , 13 , 16 are associated with IPF incidence. Although smoking has not been established as a causative agent, it has been shown to increase the risk of IPF 18 . In 2006, Taskar and Coultas et al. reported a significant increase in risk of IPF on stone/sand/soil exposure in a meta-analysis of four papers 18 . Additionally, a survey of occupational burdens in benign respiratory diseases, jointly conducted by the American Respiratory Society and the European Respiratory Society in 2019, revealed that exposure to silica, wood dust, metal dust, agricultural dust, and vapors, gas, dust, or fumes increased the risk of IPF 19 .
Workers in agriculture 9 , 13 , 16 , livestock industry 9 , chemical, petrochemical industry 13 , woodworking industry 13 , and steel industry 16 had higher risk of IPF. On the other hand, a study on the occupational burden in benign respiratory diseases conducted by the American Respiratory Society and the European Respiratory Society showed no significant association between IPF incidence with these specific occupational groups 19 .
IPF is ILD due to unknown causes. Case–control studies have been conducted on the relationship between various occupational and environmental risk factors and IPF. However, the results of such studies are inconsistent. Therefore, in this study, through systematic literature review, the effects of occupational and environmental exposure factors on IPF incidence and the influence of the individual’s past or present occupation and IPF incidence were investigated. The relationship between smoking history and the incidence of IPF was also investigated.
Search result
In total, 2852 studies were included: Medline (n = 1413); Embase (n = 1423); the Cochrane Library (n = 15); additionally identified in the literature review process (n = 1). By reviewing the title and the abstract, a total of 73 papers were analyzed, excluding documents that did not meet the purpose of this study. In total, 8 case–control studies were included. Fifty-nine articles with unclear data or not with a case–control study design were excluded. Two abstracts, one of which was later published as an article, were excluded; another abstract sharing the same case–control cohort was excluded. Three case–control studies diagnosed IPF based on chest X-ray and physical exam were excluded. In total, 8 case–control studies were included (Fig. 1 ).
PRISMA (preferred reporting items for systematic reviews and meta-analyses) flow diagram.
Characteristics of studies and participants included
Table 1 summarizes the characteristics of the eight studies and participants included. The studies were conducted in various countries, such as the United States, Japan, Sweden, Southern Europe, Mexico, Egypt, and South Korea. In four of the eight studies, the non-IPF control group included healthy adults from the community or hospital patients without lung disease including IPF. However, in their non-IPF control group Miyake et al. included patients who visited the hospital with acute bacterial pneumonia or cold; Garcia-Sancho et al., with asthma, chronic obstructive pulmonary disease (COPD), lung cancer, and otorhinolaryngology problems; Awadalla et al., with chest infection, bronchial asthma, COPD, bronchiectasis, pulmonary embolism, and bronchogenic carcinoma; Koo et al., with pulmonary tuberculosis and community acquired pneumonia. In two studies, the survey was conducted using organized questions through a self-reporting questionnaire or a phone call or mail. All studies analyzed occupational and environmental exposure risk factors and five studies analyzed occupation types. In the included studies, the mean age of the subjects ranged between 50 and 75 years, and in four of the eight studies, the age-sex distribution between the IPF patient group and the non-IPF control group was matched without statistically significant differences. Four studies did not provide data or did not match the age and sex proportions between the two groups. High rates of smoking were observed in the IPF patient group, except for the study by Awadalla et al. which matched smoking history in advance.
Quality assessment of studies
Among the eight studies included, five studies score less than 1 with high quality, one study in moderate quality, and two studies in low quality. In the measurement of intervention category, two studies were evaluated as “high” because the questionnaire was self-reported by postal questionnaire or telephone interview. In the confounding variables category, two studies showing differences in age or sex composition between the IPF and non-IPF groups were evaluated as “high”. In the selective outcome-reporting category, one study was evaluated as “high” because only statistically significant exposure risk factor results were mentioned (Supplementary Fig. S1 ).
Occupational and environmental exposure factors and risk of IPF
Seven studies (2845 subjects) investigated metal dust exposure. Three papers 9 , 15 , 16 had increased the risk of IPF and four studies had no relationship 11 , 13 , 14 , 17 . Awadalla et al., Gustafon et al., Kim et al. and Paolocci et al. investigated the metal dust and metal fumes as one category. Baumgartner et al. with metal dust excluding aluminum, beryllium, and cobalt and Koo et al. investigated metal dust and fumes separately. With seven studies on analysis, metal dust increased the risk of IPF with an odds ratio of 1.83 (95% CI 1.15–2.91, p = 0.01, I 2 = 54%) (Fig. 2 A, Supplementary Table S2 ).
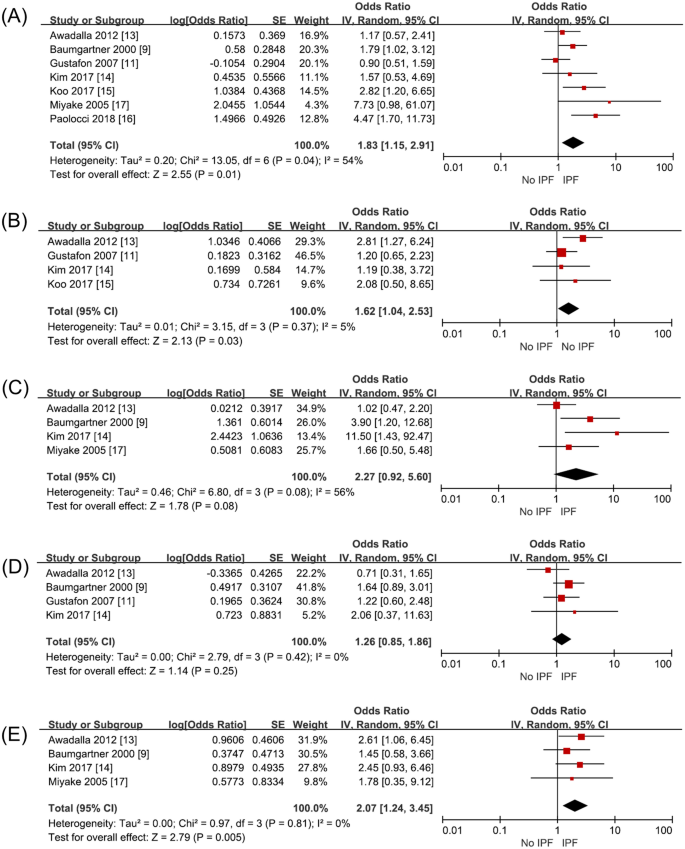
Risk of IPF in exposure to occupational and environmental risk factors compared with non-IPF subjects. ( A ) metal dust, ( B ) wood dust, ( C ) stone and sand dust, ( D ) textile dust, and ( E ) pesticide.
Four studies (1599 subjects) investigated wood dust exposure. Among them, Awadalla et al. investigated exposure to wood dust and to wood preservatives as one exposure factor and Gustafon et al. investigated exposure to wood dust, hardwood dust and birch into different risk factor. Exposure to wood dust statistically significantly increased the risk of IPF with an odds ratio of 1.62 (95% CI 1.04–2.53, p = 0.03, I 2 = 5%) (Fig. 2 B, Supplementary Table S2 ).
Four studies (1446 subjects) investigated stone/sand dust exposure. Miyake et al. and Baumgather et al. investigated stone and sand dust exposure; Awadalla et al., stone, glass, and concrete dust exposure. Kim et al. surveyed exposure to stone and sand dust containing silica. On combining all their results, the risk of IPF was not increased with an odds ratio of 2.27 (95% CI 0.92–5.60, p = 0.06, I 2 = 56%) when exposed to stone/sand dust (Fig. 2 C, Supplementary Table S2 ).
Four studies (2182 subjects) investigated textile dust exposure. The risk of IPF did not increase on exposure to textile dust with an odds ratio of 1.26 (95% CI 0.85–1.86, p = 0.25, I 2 = 0%) (Fig. 2 D, Supplementary Table S2 ).
Four studies (1446 subjects) investigated pesticide exposure, which on meta-analysis was found to increase IPF risk with an odds ratio of 2.07 (95% CI 1.24–3.45, p = 0.005, I 2 = 0%) (Fig. 2 E, Supplementary Table S2 ).
Job and risk of IPF
Five studies (1792 subjects) investigated exposure through working in the construction industry, including working at building construction and sites. IPF risk on such exposure increased with an odds ratio of 1.39 (95% CI 0.89–2.18, p = 0.15, I 2 = 20%), but it was not statistically significant (Fig. 3 A, Supplementary Table S3 ).
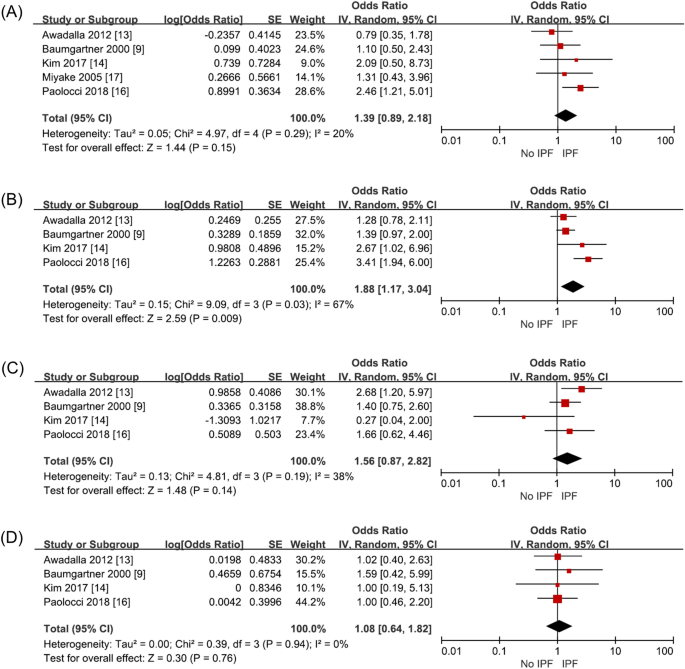
Risk of IPF in occupation compared to non-IPF controls. ( A ) building construction and demolition workers, ( B ) farming or agriculture workers, ( C ) carpentry and wood workers, and ( D ) textile making workers.
Four studies (1631 subjects) investigated exposure through working in the agriculture sector. Paolocci et al. classified agriculture, veterinarians, and gardeners into one occupation group. Miyake et al. unified agriculture and fisheries into one occupational category. While, Awadalla et al., separated agriculture and fisheries into different occupational categories. Therefore, the study by Miyake et al. was excluded from the analysis. On meta-analysis, exposure as agricultural workers increased IPF risk statistically significantly (OR 1.88, 95% CI 1.17–3.04, p = 0.009, I 2 = 67%) (Fig. 3 B, Supplementary Table S3 ). Heterogeneity was high in this analysis. When the sensitivity analysis was performed excluding Paolocci et al., the heterogeneity decreased to I 2 = 0 (OR 1.43, 95% CI 1.08–1.90, p = 0.01) and it was as statistically significant as the previous results. This was confirmed because Paolocci et al. included veterinarians and gardeners into the same occupation group as agricultural workers, unlike the other 3 studies.
Four studies (1631 subjects) analyzed exposure through working in the wood working industry. This factor tends to increase IPF risk with an odds ratio of 1.56 (95% CI 0.87–2.82, p = 0.14, I 2 = 38%), which was below statistically significant level (Fig. 3 C, Supplementary Table S3 ).
Four studies (1631 subjects) investigated exposure through working in the textile industry. This included work involving manufacturing or repairing of textiles. This factor did not increase IPF risk significantly with an odds ratio of 1.08 (95% CI 0.64–1.82, p = 0.76, I 2 = 0%) (Fig. 3 D, Supplementary Table S3 ).
Smoking and the risk of IPF
Among the studies, Awadalla et al. was excluded because it was a smoking-matched case–control study. Meta-analysis showed that smoking increased IPF risk with an odds ratio of 1.38 (95% CI 1.09–1.74, p = 0.008, I 2 = 10%), which was statistically significant (Fig. 4 , Supplementary Table S4 ).
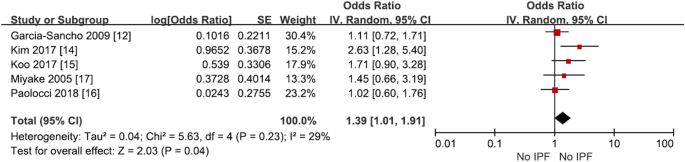
Risk of IPF in ever smoker compared with never smoker.
From previous case-controls studies, we found that metal dust 6 , 7 , 9 , 15 , 16 , 17 increased the risk of IPF and wood dust 6 , 7 , 11 , 13 increased IPF risk with statistical significance. Additionally, the exposure to livestock like cattle and birds, livestock feed, pesticides, mold, soil dust, stone dust, stone polishes, and smoke increases IPF prevalence.
On our analysis metal dust, wood dust and pesticide increased the risk of IPF. From the previous case control studies with metal dust and IPF some studies had significant relationship with disease but some other studies did not 11 , 12 , 14 . A cohort study of United Kingdom engineering company, increased proportional mortality increased in relation to the duration of metal-working 5 . Metal dust and metal related fumes can deposit in the lung by inhalation or ingestion of the particles and interfere with the pulmonary immune system but specific pathogenesis is not known 20 .
Although a recent, informal meta-analysis, conducted by the international pulmonology conference, meaningful results were found about IPF risk on exposure to the wood dust, metal dust, silica dust, agriculture dust and vapors, gas, dust or fumes 19 . In our study, agricultural dust was not included because only occupational and environmental exposure factors that were included in more than four studies were considered, and silica was excluded because silica was already widely known with silica related lung disease.
Unlike previous studies, our meta-analysis showed statistically significant increase in IPF risk on pesticide exposure (Fig. 2 E). Earlier, a case control study about pesticides from Egypt had shown increased risk of IPF 13 . Pesticide exposure can directly and indirectly increase the risk of COPD and asthma 21 . The chemicals can persist in soil for decades. The specific pathogenesis related to ILD is unknown.
A longer occupational exposure period is known to increase IPF risk 16 . Exposure through working in agriculture, livestock, beauty, chemical/petrochemical, woodworking, and steel industries was reported to increase IPF risk previously 9 , 13 , 14 , 16 , 17 . On meta-analysis, we found statistically significantly increased IPF risk in only agricultural workers. Additionally, the risk of IPF increased through working in building, woodworking, and textile industries, but did not reach statistically significant level.
Only two of the seven included studies showed that individuals’ smoking history statistically significantly increased the risk of developing IPF, but when meta-analysis was conducted, we found that smoking increased IPF risk with an odds ratio of 1.38 (Fig. 4 , Supplementary Table S4 ).
Smoking has been found to increase the risk of IPF in several studies 9 , 14 , 16 , 22 . Studies to date suggest that the increased oxidative stress caused by smoking affects IPF progression in former and current smokers compared to non-smokers 23 . In a study conducted using a population-based registry in Sweden, the risk of IPF increased with an odds ratio of 2.10 (95% CI 1.20–3.68) when subjects smoked for 10–19 pack years and with an odds ratio of 2.25 (95% CI 1.26–4.02) when subjects smoked more for than 20 pack years. Dose-dependent increase was reported for smoking as a risk factor for IPF 23 . Our study also confirmed that the risk of IPF increased in smoker compared to never smoker from meta-analysis on case–control studies.
There were several limitations in this study. First, recall bias may be a limitation of this study. Because the subject’s occupational and environmental risk factors were collected retrospectively, the quality of information may deteriorate because they rely on recall. In four studies, the questionnaire was minimized by direct questionnaire by specialized researchers like occupational environment experts. But remain studies were conducted by the patient himself or herself. Second, a quantitative analysis considering the degree and frequency of exposure would be more informative when investigating risk factors, rather than a simple exposure analysis. However, such an analysis could not be conducted in this study. In terms of occupation type exposure, the actual amount, duration, and frequency of risk factor exposure during the period of exposure in a particular job type was neither conducted nor comparatively analyzed. Third, although studies from various countries are included, they did not have national representation because each study covers a specific region of the country. Also, mainly in the studies in the United States, some European countries, and Asia (where only Japan and Korea are included), racial differences may not be reflected. Fourth, as the diagnostic definition of IPF has been changing for decades. Heterogeneity among the included cases may exist due to the development of imaging technology which may have affected the diagnosis. The first international guidelines 24 , based on expert opinions on the diagnosis and treatment of IPF, were published in 2000 and evidence-based revised new treatment guidelines have been published in 2011, integrating the patient’s clinical symptoms, pathogenesis, and natural course 25 . Later, as new drugs for IPF treatment were developed focusing on early treatment and diagnosis, the new IPF diagnostic criteria, complemented with high-resolution computed tomography imaging and related pathological findings, were presented in 2018 26 , 27 . This study includes about 30 years of research from 1990 to recent studies. To minimize of misdiagnosis and overdiagnosis of IPF, case control studies that had diagnosed IPF mainly based on chest X-ray were removed 6 , 7 , 10 . Finally, in some studies, the control group was not a healthy adult control group. Inclusion of patients with respiratory diseases, such as acute bacterial pneumonia, chronic obstructive pulmonary disease, lung cancer, and pulmonary tuberculosis other than IPF may have affected the interpretation of the results. The effect of smoking as a multiplicative risk for the development of IPF cannot be omitted.
In conclusion, meta-analysis of patient-control studies revealed that exposure to pesticides, metal dust, and wood dust increases the risk of IPF. Additionally, the risk of IPF was more in agricultural workers. Lastly, smoking also increased the risk of IPF.
Searching strategy
The Patient populations, Intervention, Comparison, Outcomes (PICO) of this study are as follows:
Adult, IPF cases.
Environmental and occupational exposure, occupation.
Non-IPF controls.
Risk of IPF depending on exposure to each factor.
This study was conducted according to the systematic literature review reporting guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 28 . Medline, Embase, and the Cochrane Library were searched for papers published by March 2020 using the Ovid interface. The search terms were “Idiopathic Pulmonary Fibrosis” [ALL] OR “Idiopathic Pulmonary Fibroses” [ALL] OR “Cryptogenic Fibrosing Alveolitis” [ALL] OR “Cryptogenic Alveolitides” [ALL] OR “Idiopathic Fibrosing Alveolitis” [ALL] OR “Idiopathic Fibrosing Alveolitides” [ALL] OR “Usual Interstitial Pneumonitis” [ALL] OR “Usual Interstitial Pneumonitides” [ALL] OR “Usual Interstitial Pneumonia” [ALL] OR “Usual Interstitial Pneumonias” [ALL]. The studies not recorded in the databases but existing in previous meta-analysis studies were directly searched and added. Additional details are listed in Supplementary Table 1 .
Inclusion and exclusion criteria
Two investigators independently selected the studies after confirming the title and abstract according to the inclusion and exclusion criteria of this study. Duplicate papers were excluded.
The inclusion criteria were: (1) study on adult population over 18 years of age; (2) IPF diagnosis criteria based on the clinician’s judgment of the patient’s symptoms, clinical findings, and imaging findings (histological diagnosis may not have been necessarily included in the diagnosis); (3) categorization of the surveyed jobs or occupational and environmental exposure factors that could lead to risk of IPF. The survey methods for occupational and environmental exposure factors included any method that is systematic and planned, ranging from self-reporting by mail or telephone to face-to-face surveys with experts. Additionally, the effect of cigarettes was analyzed by studying individuals who had smoked in the past or who are currently smoking.
Reviews, letters, editorials, case reports, studies on animals or children, theoretical studies on the medical system itself, revisions after the medical system were introduced, papers or papers not related to the research purpose, papers written in languages other than English were excluded. Additionally, studies that only focused on known risk factors, such as asbestosis, coal worker’s pneumoconiosis, and silicosis, were excluded.
Evaluation of paper quality
Quality evaluation was conducted for papers that met the inclusion criteria, which was quantitatively evaluated using Risk of Bias Assessment Tool for Nonrandomized Studies (RoBANS) 29 . This tool includes 6 items, including selection of participants, confounding variables, exposure measurement, outcome assessment blinding, incomplete outcome data, and selective reporting. Each item was rated as “high”, “low”, and “uncertain”; 0 for “low”, + 1 for “uncertain”, and + 2 for “high”. On summation, a score of 1 or less meant the paper was of high quality; 2–3, of moderate quality; 4 or more, low in quality.
Extraction of data
Authors, publication year, location of study, multi-center study, research method, number of experimental and control groups, age, sex, and smoking status were extracted and summarized for the finally included papers.
The number of exposure factors examined in each study was varied. Among them, if more than four studies investigated a common exposure factor, that exposure factor was analyzed. Finally, our analysis was conducted on five exposure factors.
We analyzed four occupations types which were included in four or more studies. Five of the 12 studies included occupational classification in the case–control group 9 , 13 , 14 , 16 , 17 . Among them, researchers such as Kim and Miyake conducted research according to the Korean Standard Classification of Occupations and Japanese Standard Occupational Classification standards, respectively. The analyzed occupations were classified in each study according to the classification criteria set by the researchers.
The individual's smoking history was classified into two groups, the smoking group including both past and present smoking, and the non-smoking group who had never smoked.
Statistical analysis
We used Review Manager Version 5.3 (The Cochrane library, Oxford, UK) was used. To minimize the influence of other variables as much as possible, the unadjusted odds ratio value was used. When raw data were presented in the paper, the unadjusted odds ratio was calculated using the presented values. If multiple adjusted odds ratios were presented, the odds ratio values corresponding to the same criteria were used after consultation between authors. Statistical meta-analysis was then performed using the extracted ratio values.
In the main analysis, the occupational and environmental exposure factors included were five types of metal dust, wood dust, stone/sand dust, textile dust, and pesticides. The occupation types included were construction, agriculture, woodworking, and textile. Additionally, the relationship between the individual's smoking history and disease was investigated.
To evaluate statistical heterogeneity in each study, I 2 test of Higgins was calculated with a 95% confidence interval (CI). Statistical heterogeneity was considered low if I 2 value was less than 25%, moderate if it was between 25 and 50%, high if it was between 50 and 75%, and very high if it was more than 75%.
After obtaining the odds ratios of each factor, the pooled effect size was estimated using the inverse variance weighted method 30 . The 95% confidence interval and weight are presented as a forest plot.
Sensitivity analysis was conducted to exclude studies of low quality or which included specific conditions or characteristics. If more than 10 studies included in the analysis, a statistical analysis of the asymmetry was performed using Egger’s test to confirm the publication error, and a visual analysis of the asymmetry was performed using a funnel plot.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Travis, W. D. et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 188 , 733–748. https://doi.org/10.1164/rccm.201308-1483ST (2013).
Article PubMed PubMed Central Google Scholar
Wolters, P. J., Collard, H. R. & Jones, K. D. Pathogenesis of idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 179 , 157–179. https://doi.org/10.1146/annurev-pathol-012513-104706 (2014).
Article CAS Google Scholar
Cho, S. J. & Stout-Delgado, H. W. Aging and lung disease. Annu. Rev. Physiol. 82 , 433–459. https://doi.org/10.1146/annurev-physiol-021119-034610 (2020).
Article CAS PubMed Google Scholar
Lee, S. H. et al. Association between occupational dust exposure and prognosis of idiopathic pulmonary fibrosis: A Korean national survey. Chest 147 , 465–474. https://doi.org/10.1378/chest.14-0994 (2015).
Article PubMed Google Scholar
Hubbard, R. et al. Risk of cryptogenic fibrosing alveolitis in metal workers. Lancet 355 , 466–467. https://doi.org/10.1016/S0140-6736(00)82017-6 (2000).
Scott, J., Johnston, I. & Britton, J. What causes cryptogenic fibrosing alveolitis? A case-control study of environmental exposure to dust. BMJ 301 , 1015–1017. https://doi.org/10.1136/bmj.301.6759.1015 (1990).
Article CAS PubMed PubMed Central Google Scholar
Hubbard, R., Johnston, I., Coultas, D. B. & Britton, J. Mortality rates from cryptogenic fibrosing alveolitis in seven countries. Thorax 51 , 711–716. https://doi.org/10.1136/thx.51.7.711 (1996).
Reynolds, C., Sisodia, R., Barber, C. & Cullinan, P. S123 occupational exposures to wood, metal, and stone in IPF; findings from the idiopathic pulmonary fibrosis job exposures study (IPFJES). Thorax 74 , A78 (2019).
Google Scholar
Baumgartner, K. B. et al. Occupational and environmental risk factors for idiopathic pulmonary fibrosis: A multicenter case-control study. Collaborating Centers. Am. J. Epidemiol. 152 , 307–315. https://doi.org/10.1093/aje/152.4.307 (2000).
Mullen, J., Hodgson, M. J., DeGraff, C. A. & Godar, J. Case-control study of idiopathic pulmonary fibrosis and environmental exposures. J. Occup. Environ. Med. 40 , 363–367. https://doi.org/10.1097/00043764-199804000-00011 (1998).
Gustafson, T. et al. Occupational exposure and severe pulmonary fibrosis. Respir. Med. 101 , 2207–2212. https://doi.org/10.1016/j.rmed.2007.02.027 (2007).
Garcia-Sancho, F. M. C. et al. Risk factors for idiopathic pulmonary fibrosis in a Mexican population. A case-control study. Respir. Med. 104 , 305–309. https://doi.org/10.1016/j.rmed.2009.08.013 (2010).
Article Google Scholar
Awadalla, N. J., Hegazy, A., Elmetwally, R. A. & Wahby, I. Occupational and environmental risk factors for idiopathic pulmonary fibrosis in Egypt: A multicenter case-control study. Int. J. Occup. Environ. Med. 3 , 107–116 (2012).
CAS PubMed Google Scholar
Kim, S. Y. et al. Occupational and environmental risk factors for chronic fibrosing idiopathic interstitial pneumonia in South Korea. J. Occup. Environ. Med. 59 , e221–e226. https://doi.org/10.1097/JOM.0000000000001153 (2017).
Koo, J.-W. et al. Occupational exposure and idiopathic pulmonary fibrosis: A multicentre case-control study in Korea. Int. J. Tuberc. Lung Dis. 21 , 107–112. https://doi.org/10.5588/ijtld.16.0167 (2017).
Paolocci, G. et al. Occupational risk factors for idiopathic pulmonary fibrosis in Southern Europe: A case-control study. BMC Pulm. Med. 18 , 75. https://doi.org/10.1186/s12890-018-0644-2 (2018).
Miyake, Y. et al. Occupational and environmental factors and idiopathic pulmonary fibrosis in Japan. Ann. Occup. Hyg. 49 , 259–265. https://doi.org/10.1093/annhyg/meh090 (2005).
Taskar, V. S. & Coultas, D. B. Is idiopathic pulmonary fibrosis an environmental disease?. Proc. Am. Thorac. Soc. 3 , 293–298. https://doi.org/10.1513/pats.200512-131TK (2006).
Blanc, P. D. et al. The occupational burden of nonmalignant respiratory diseases. An Official American Thoracic Society and European Respiratory Society Statement. Am. J. Respir. Crit. Care Med. 199 , 1312–1334. https://doi.org/10.1164/rccm.201904-0717ST (2019).
Assad, N. et al. Metal-induced pulmonary fibrosis. Curr. Environ. Health Rep. 5 , 486–498. https://doi.org/10.1007/s40572-018-0219-7 (2018).
Nordgren, T. M. & Bailey, K. L. Pulmonary health effects of agriculture. Curr. Opin. Pulm. Med. 22 , 144–149. https://doi.org/10.1097/MCP.0000000000000247 (2016).
Ekstrom, M. et al. Effects of smoking, gender and occupational exposure on the risk of severe pulmonary fibrosis: A population-based case-control study. BMJ Open 4 , e004018. https://doi.org/10.1136/bmjopen-2013-004018 (2014).
Oh, C. K., Murray, L. A. & Molfino, N. A. Smoking and idiopathic pulmonary fibrosis. Pulm. Med. 2012 , 808260. https://doi.org/10.1155/2012/808260 (2012).
American Thoracic Society. Idiopathic pulmonary fibrosis: Diagnosis and treatment International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am. J. Respir. Crit. Care Med. 161 , 646–664. https://doi.org/10.1164/ajrccm.161.2.ats3-00 (2000).
Raghu, G. et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 183 , 788–824. https://doi.org/10.1164/rccm.2009-040GL (2011).
Lynch, D. A. et al. Diagnostic criteria for idiopathic pulmonary fibrosis: A Fleischner Society white paper. Lancet Respir. Med. 6 , 138–153. https://doi.org/10.1016/S2213-2600(17)30433-2 (2018).
Raghu, G. et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 198 , e44–e68. https://doi.org/10.1164/rccm.201807-1255ST (2018).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 62 , e1–e34. https://doi.org/10.1016/j.jclinepi.2009.06.006 (2009).
Kim, S. Y. et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J. Clin. Epidemiol. 66 , 408–414. https://doi.org/10.1016/j.jclinepi.2012.09.016 (2013).
Borenstein, M., Hedges, L. V., Higgins, J. P. & Rothstein, H. R. Introduction to Meta-analysis (Wiley, New York, 2011).
MATH Google Scholar
Download references
Author information
These authors contributed equally: Yeonkyung Park and Chiwon Ahn.
Authors and Affiliations
Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Veterans Health Service Medical Center, Seoul, South Korea
Yeonkyung Park
Department of Emergency Medicine, College of Medicine, Chung-Ang University, Seoul, South Korea
Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, College of Medicine, Hanyang University Guri Hospital, 153, Gyeongchun-ro, Guri-si, Gyeonggi-do, 11923, South Korea
Tae-Hyung Kim
You can also search for this author in PubMed Google Scholar
Contributions
All authors conceived the study and designed the review. Y.P. and C.A. performed the searches and screened studies for eligibility. All authors assessed the quality of the papers and Y.P. and C.A. performed the statistical analysis. Y.P. and C.A. drafted the manuscript, and all authors contributed substantially to its revision. T.-H.K. takes responsibility for the paper as a whole.
Corresponding author
Correspondence to Tae-Hyung Kim .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author (s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Park, Y., Ahn, C. & Kim, TH. Occupational and environmental risk factors of idiopathic pulmonary fibrosis: a systematic review and meta-analyses. Sci Rep 11 , 4318 (2021). https://doi.org/10.1038/s41598-021-81591-z
Download citation
Received : 09 September 2020
Accepted : 05 January 2021
Published : 02 March 2021
DOI : https://doi.org/10.1038/s41598-021-81591-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Targeting transitioning lung monocytes/macrophages as treatment strategies in lung disease related to environmental exposures.
- Aaron D. Schwab
- Todd A. Wyatt
- Jill A. Poole
Respiratory Research (2024)
Apolipoprotein E controls Dectin-1-dependent development of monocyte-derived alveolar macrophages upon pulmonary β-glucan-induced inflammatory adaptation
- H. Theobald
- D. A. Bejarano
- A. Schlitzer
Nature Immunology (2024)
NHLRC2 expression is increased in idiopathic pulmonary fibrosis
- Mervi Kreus
- Siri Lehtonen
- Riitta Kaarteenaho
Respiratory Research (2022)
Importance of physician history taking in complementing patient-reported interstitial lung disease questionnaire
- Tal Moshe Perluk
- Inbal Friedman Regev
- Avraham Unterman
BMC Pulmonary Medicine (2022)
Prevalence of idiopathic pulmonary fibrosis in Japan based on a claims database analysis
- Yasuhiro Kondoh
- Takafumi Suda
- Sakae Homma
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Anthropocene newsletter — what matters in anthropocene research, free to your inbox weekly.
- Patient Care & Health Information
- Diseases & Conditions
- Pulmonary fibrosis
Pulmonary fibrosis is scarred and thickened tissue around and between the air sacs called alveoli in the lungs, as shown on the right. A healthy lung with healthy alveoli is shown on the left.
Pulmonary fibrosis is a lung disease that occurs when lung tissue becomes damaged and scarred. This thickened, stiff tissue makes it harder for the lungs to work properly. Pulmonary fibrosis worsens over time. Some people can stay stable for a long time, but the condition gets worse faster in others. As it gets worse, people become more and more short of breath.
The scarring that happens in pulmonary fibrosis can be caused by many things. Often, doctors and other healthcare professionals cannot pinpoint what's causing the problem. When a cause cannot be found, the condition is called idiopathic pulmonary fibrosis.
Idiopathic pulmonary fibrosis usually occurs in middle-aged and older adults. Sometimes pulmonary fibrosis is diagnosed in children and infants, but this is not common.
The lung damage caused by pulmonary fibrosis cannot be repaired. Medicines and therapies can sometimes help slow down the rate of fibrosis, ease symptoms and improve quality of life. For some people, a lung transplant might be an option.
Pulmonary fibrosis care at Mayo Clinic
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Symptoms of pulmonary fibrosis may include:
- Shortness of breath.
- Extreme tiredness.
- Weight loss that's not intended.
- Aching muscles and joints.
- Widening and rounding of the tips of the fingers or toes, called clubbing.
How fast pulmonary fibrosis worsens over time and how severe the symptoms are can vary greatly from person to person. Some people become ill very quickly with severe disease. Others have moderate symptoms that worsen more slowly, over months or years.
When symptoms suddenly get worse
In people with pulmonary fibrosis, especially idiopathic pulmonary fibrosis, shortness of breath can suddenly get worse over a few weeks or days. This is called an acute exacerbation. It can be life-threatening. The cause of an acute exacerbation may be another condition or an illness, such as a lung infection. But usually the cause is not known.
When to see a doctor
If you have symptoms of pulmonary fibrosis, contact your doctor or other healthcare professional as soon as possible. If your symptoms get worse, especially if they get worse fast, contact your healthcare team right away.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
Get the latest lung transplant-related health information from Mayo Clinic
Sign up for free, and receive lung transplant and pulmonary fibrosis content, plus expertise on lung health.
Error Select a location
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing
You will receive the first lung transplant and pulmonary fibrosis email in your inbox shortly. When seeking answers, people often look to experts for clear and accurate information. By subscribing to pulmonary fibrosis and lung transplant content from Mayo Clinic, you have taken an important first step in gaining knowledge and using it for your overall health and well-being.
If you don't receive our email within 5 minutes, check your SPAM folder, then contact us at [email protected] .
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Pulmonary fibrosis is scarring and thickening of the tissue around and between the air sacs called alveoli in the lungs. These changes make it harder for oxygen to pass into the bloodstream.
Damage to the lungs that results in pulmonary fibrosis may be caused by many different things. Examples include long-term exposure to certain toxins, radiation therapy, some medicines and certain medical conditions. In some cases, the cause of pulmonary fibrosis is not known.
Your work and surroundings
The type of work you do and where you work or live could be the cause or part of the cause for pulmonary fibrosis. Having continuous or repeated contact with toxins or pollutants — substances that harm the quality of water, air or land — can damage your lungs, especially if you do not wear protective gear. Examples include:
- Silica dust.
- Asbestos fibers.
- Hard metal dusts.
- Wood, coal and grain dusts.
- Bird and animal droppings.
Radiation treatments
Some people who receive radiation therapy to the chest, such as for lung or breast cancer, show signs of lung damage months or sometimes years after treatment. How severe the damage is may depend on:
- How much of the lung was exposed to radiation.
- The total amount of radiation given.
- Whether chemotherapy also was used.
- Whether there is underlying lung disease.
Many medicines can damage the lungs. Some examples include:
- Chemotherapy. Medicines designed to kill cancer cells, such as methotrexate (Trexall, Otrexup, others), bleomycin and cyclophosphamide (Cytoxan), can damage lung tissue.
- Heart medicines. Some medicines used to treat irregular heartbeats, such as amiodarone (Nexterone, Pacerone), may harm lung tissue.
- Some antibiotics. Antibiotics such as nitrofurantoin (Macrobid, Macrodantin) or ethambutol (Myambutol) can cause lung damage.
- Anti-inflammatory medicines. Certain anti-inflammatory medicines such as rituximab (Rituxan) or sulfasalazine (Azulfidine) can cause lung damage.
Medical conditions
Lung damage can also result from a number of conditions, including:
- Dermatomyositis, an inflammatory disease marked by muscle weakness and a skin rash.
- Lupus, a disease that occurs when the body's immune system attacks its own tissues and organs.
- Mixed connective tissue disease, which has a mix of symptoms of different disorders, such as lupus, scleroderma and polymyositis.
- Pneumonia, an infection that inflames the air sacs in one or both lungs.
- Polymyositis, an inflammatory disease that causes muscle weakness on both sides of the body.
- Rheumatoid arthritis, an inflammatory disease that affects joints and other body systems.
- Sarcoidosis, an inflammatory disease that most often affects the lungs and lymph nodes.
- Scleroderma, a group of rare diseases that involve hardening and tightening of the skin as well as problems inside the body.
Idiopathic pulmonary fibrosis
Many substances and conditions can lead to pulmonary fibrosis. Even so, in many people, the cause is never found. But risk factors such as smoking or exposure to air pollution could be related to the condition, even if the cause cannot be confirmed. Pulmonary fibrosis with no known cause is called idiopathic pulmonary fibrosis.
Many people with idiopathic pulmonary fibrosis also may have gastroesophageal reflux disease, also called GERD. This condition occurs when acid from the stomach flows back into the esophagus. GERD may be a risk factor for idiopathic pulmonary fibrosis or cause the condition to worsen faster. But more studies are needed.
Risk factors
Pulmonary fibrosis has been found in children and infants, but this is not common. Idiopathic pulmonary fibrosis is much more likely to affect middle-aged and older adults. Other types of pulmonary fibrosis, such as that caused by connective tissue disease, can occur in younger people.
Factors that can raise your risk of pulmonary fibrosis include:
- Smoking. If you smoke now or used to smoke, you're at a higher risk of pulmonary fibrosis than people who never smoked. People with emphysema are at higher risk, too.
- Certain types of work. You have a higher risk of developing pulmonary fibrosis if you work in mining, farming or construction. The risk also is higher if you have continuous or repeated contact with pollutants known to damage the lungs.
- Cancer treatments. Having radiation treatments to your chest or using certain chemotherapy medicines can raise your risk of pulmonary fibrosis.
- Genetics. Some types of pulmonary fibrosis run in families, so genes may play a role.
Complications
Complications of pulmonary fibrosis may include:
- High blood pressure in the lungs. Called pulmonary hypertension, this type of high blood pressure affects the arteries in the lungs. These are the pulmonary arteries. Stiff and thick arteries may slow down or block blood flow through the lungs. This raises the pressure inside the pulmonary arteries and the lower right heart chamber, called the right ventricle.
- Right-sided heart failure. This serious condition occurs when your heart's right chamber has to pump harder than usual to move blood through partly blocked pulmonary arteries.
- Respiratory failure. This is often the last stage of long-term lung disease. It occurs when blood oxygen levels fall dangerously low.
- Lung cancer. Long-standing pulmonary fibrosis increases your risk of developing lung cancer.
- Other lung problems. As pulmonary fibrosis gets worse over time, it may lead to serious problems such as blood clots in the lungs, a collapsed lung or lung infections.
Living with pulmonary fibrosis?
Connect with others like you for support and answers to your questions in the Transplants support group on Mayo Clinic Connect, a patient community.
Transplants Discussions

387 Replies Sat, Jun 01, 2024

42 Replies Thu, May 23, 2024

29 Replies Sat, May 18, 2024
- Raghu G, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: An official ATS/ERS/JRS/ALAT clinical practice guideline. American Journal of Respiratory and Critical Care Medicine. 2022; doi:10.1164/rccm.202202-0399stt.
- Broaddus VC, et al., eds. Idiopathic pulmonary fibrosis. In: Murray and Nadel's Textbook of Respiratory Medicine. 7th ed. Elsevier; 2022. https://www.clinicalkey.com. Accessed April 5, 2023.
- Broaddus VC, et al., eds. Pleural fibrosis and unexpandable lung. In: Murray and Nadel's Textbook of Respiratory Medicine. 7th ed. Elsevier; 2022. https://www.clinicalkey.com. Accessed April 5, 2023.
- Idiopathic pulmonary fibrosis. National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health/idiopathic-pulmonary-fibrosis. Accessed April 5, 2023.
- Baqir M, et al. Idiopathic pulmonary fibrosis and gastroesophageal reflux disease: A population-based, case-control study. Respiratory Medicine. 2021; doi:10.1016/j.rmed.2021.106309.
- Strykowski R, et al. Idiopathic pulmonary fibrosis and progressive pulmonary fibrosis. Immunology and Allergy Clinics of North America. 2023; doi:10.1016/j.iac.2023.01.010.
- Ahmad K, et al. Lung disease-related pulmonary hypertension. Cardiology Clinics. 2022; doi:10.1016/j.ccl.2021.08.005.
- Collins BF, et al. Diagnosis and management of fibrotic interstitial lung diseases. Clinics in Chest Medicine. 2021; doi:10.1016/j.ccm.2021.03.008.
- Lee JYT, et al. Self-management for pulmonary fibrosis: Insights from people living with the disease and healthcare professionals. Patient Education and Counseling. 2022; doi:10.1016/j.pec.2021.07.005.
- Wuyts WA, et al. Idiopathic pulmonary fibrosis: Best practice in monitoring and managing a relentless fibrotic disease. Respiration. 2020; doi:10.1159/000504763.
- Glass DS, et al. Idiopathic pulmonary fibrosis: Current and future treatment. Clinical Respiratory Journal. 2022; doi:10.1111/crj.13466.
- Dempsey TM, et al. Pulmonary function tests for the generalist: A brief review. Mayo Clinic Proceedings. 2018; doi:10.1016/j.mayocp.2018.04.009.
- Pulmonary rehabilitation. National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health/pulmonary-rehabilitation. Accessed April 5, 2023.
- Park Y, et al. Occupational and environmental risk factors of idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Scientific Reports. 2021; doi:10.1038/s41598-021-81591-z.
- Jarzebska N, et al. Scarred lung. An update on radiation-induced pulmonary fibrosis. Frontiers in Medicine. 2021; doi:10.3389/frmed.2020.585756.
- Broaddus VC, et al., eds. Drug-induced pulmonary disease. In: Murray and Nadel's Textbook of Respiratory Medicine. 7th ed. Elsevier; 2022. https://www.clinicalkey.com. Accessed April 11, 2023.
- Table: Substances with toxic pulmonary effects. Merck Manual Professional Version. https://www.merckmanuals.com/professional/pulmonary-disorders/interstitial-lung-diseases/drug-induced-pulmonary-disease. Accessed April 11, 2023.
- Baqir M (expert opinion). Mayo Clinic. June 16, 2023.
- Allscripts EPSi. Mayo Clinic.
Associated Procedures
- Bronchoscopy
- Chest X-rays
- Echocardiogram
- Lung transplant
- Stress test
News from Mayo Clinic
- Shortened telomeres heighten risk of serious lung disease Dec. 16, 2023, 12:00 p.m. CDT
- Science Saturday: AI enables early identification, intervention in debilitating lung disease Oct. 29, 2022, 11:00 a.m. CDT
Mayo Clinic in Rochester, Minnesota, Mayo Clinic in Phoenix/Scottsdale, Arizona, and Mayo Clinic in Jacksonville, Florida, have been recognized among the top Pulmonology hospitals in the nation for 2023-2024 by U.S. News & World Report.
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.

- Departments
- Anesthesiology
- Biomedical Sciences
- Cardiac Surgery
- Computational Biomedicine
- Neurosurgery
- Obstetrics & Gynecology
- Orthopaedics
- Pathology & Laboratory Medicine
- Physical Medicine & Rehabilitation
- Psychiatry & Behavioral Neurosciences
- Radiation Oncology
- Board of Governors Regenerative Medicine Institute
- F. Widjaja Inflammatory Bowel Disease Institute
- Samuel Oschin Comprehensive Cancer Institute
- Smidt Heart Institute
- Advanced Clinical Biosystems Research Institute
- Biomedical Imaging Research Institute
- Diabetes & Obesity Research Institute
- Geri & Richard Brawerman Nursing Institute
- Human Microbiome Research Institute
- Kao Autoimmunity Institute
- Maxine Dunitz Neurosurgical Institute
- Women's Guild Lung Institute
- Research Topics
- Laboratories
- Research Cores
- Clinical Trials
- Office of Research Administration
- Technology & Innovations
- Clinical & Translational Research Center
- News & Breakthroughs
- Graduate Medical Education
- Graduate School of Biomedical Sciences
- Continuing Medical Education
- Professional Training Programs
- Women's Guild Simulation Center
- Center for the Arts and Humanities in Medicine
- Medical Library
- Campus Life
- Office of the Dean
- Academic Calendar
- Back to Research
- Industry Relations & Conflicts of Interest Policy
- Meet the Team
- Grant & Contract Services
- Clinical Trials Administration Office
- Post Award Reporting & Compliance
- Areas of Research
- Publications
- Conferences & Meetings
- Research Grants & Competitions
- RECOOP Grant Awards
- Institutional Review Board/IRB Operations
- Research Compliance & Post-Approval Monitoring
- Quality Improvement & Education
- Research Areas, Centers & Programs
- Instrumentation
- Bioinformatics
- Request Form
- Core Services
- New User Information
- Services and Prices
- Ordering Supplies
- Our Policies
- User Information
- Software and Data Tools
- Facility Access
- Funding Opportunities
- Frequently Asked Questions
- Technologies/Resources
- Vendor List
- A Letter From the Director
- Administration
- Advisory Boards
- Training & Education
- About Clinical Trials
- OnCore & Clinical Trial Informatics
- Our Catchment Area
- Disease Research Groups
- Cancer Research Center for Health Equity
- Molecular Twin
- Cancer Biology Program
- Cancer Prevention and Control Program
- Cancer Therapeutics Program
- Determinants of Liver Metastasis Program
- Shared Resources
- Clinical Care
- Obstetrics and Gynecology
- Department of Computational Biomedicine
- Urology Department
- Lab Members
- Research Areas
- Personal Statement
- Reagents and Resources
- Achievements
- Turkson Lab
New Findings on Idiopathic Pulmonary Fibrosis
Cedars-Sinai investigators have made significant progress in identifying the mechanisms behind idiopathic pulmonary fibrosis, a deadly disease that scars the lungs and impairs breathing. The findings appeared in a study published this summer in the journal Science Advances .
Idiopathic pulmonary fibrosis affects more than 100,000 people in the U.S. The condition results in a buildup of fibrosis–fibrous scar tissue–that blocks the lungs from performing their primary function of transporting oxygen to the bloodstream. Although the disease progresses at variable rates, most patients die within five years after being diagnosed unless they are a candidate for and receive a lung transplant, according to the National Institutes of Health.

Paul Noble, MD
"This research is an important step forward in really understanding what causes disease progression in idiopathic pulmonary fibrosis and how we can better treat it," said Paul Noble, MD , professor of Medicine and chair of the Department of Medicine , director of the Women's Guild Lung Institute and the Vera and Paul Guerin Family Distinguished Chair in Pulmonary Medicine at Cedars-Sinai . Noble is the corresponding author of the study.
In a normal lung, a specialized cell known as a type 2 alveolar epithelial cells (ATII) function as progenitor cells, or stem cells, meaning that they regenerate to support the renewal and overall health of the lung. Other cells, known as mesenchymal cells, support the progenitor function of the ATII cells, secreting growth factors that promote the ATII renewal.
The interactions between the mesenchymal cells and ATII cells can either deter or promote cell renewal in the lung. When functioning properly, the two cell types work together to maintain healthy alveoli, air sacs where gas exchange occurs. For reasons not fully understood, that protective and regenerative mechanism is lost in idiopathic pulmonary fibrosis. Instead, the mesenchymal cells become invasive fibroblasts and destroy the progenitor cell niche, resulting in fibrous scars. In this capacity, they behave similarly to cancer cells that invade tissues.
This process inhibits the function of the lungs, as ATII cells are needed to provide the environment where oxygen can be transferred to the bloodstream. The fibrous scar that forms supplants them and can't perform this function.
"This communication is essential for normal repair after injury, and if it’s lost, your lungs will progressively fibrose," Noble said.
Investigators in the study created a mouse model of pulmonary fibrosis in which they eliminated the growth hormone receptor in the mesenchymal cells. They found that the mice without the receptors were more prone to developing fibrosis. The results suggest that the growth hormone receptors have a key role to play in maintaining healthy lung tissue. Interestingly, the prevalence of IPF dramatically increases with age–most patients are over 60 at the time of diagnosis–and it is well known that growth hormone levels decrease with age.
"So, communication matters, and when it's lost, disarray happens," Noble said. "We think that's one of the mechanisms for idiopathic pulmonary fibrosis progression, but it is not the only one."
Further, investigators found that in mice that received injections of growth hormone receptor-enriched extracellular vesicles, the hormone receptor-enriched vesicles were readily taken up by the ATII cells and used effectively within the lung.
"These findings provide evidence that mesenchymal growth hormone receptor deficiency contributes to pulmonary fibrosis. They also demonstrate that a vesicle mechanism of communication between mesenchymal cells and ATII cells can benefit ATII renewal and work against pulmonary fibrosis in mice," said Ting Xie, PhD , lead author of the study.
Their data suggest that vesicles, nano-sized carriers that transport molecular substances within or between cells, can carry functional growth hormone receptor that originated outside of the ATII cells, and that treatment with growth hormone receptor may improve lung function.
Noble said that while treatment with growth hormone receptor using a vesicle delivery mechanism may eventually prove to be an option for patients with pulmonary fibrosis, more study is needed.
"Our next step will be to better understand how this communication works and to explore whether restoring the growth hormone receptor would be a possible treatment option," Noble said.
Volume 6 Supplement 1
IPF in 2011 - Key updates on guidelines and therapeutics. Case studies
- Case Report
- Open access
- Published: 16 April 2013
Clinical case: Differential diagnosis of idiopathic pulmonary fibrosis
- Carlos Robalo Cordeiro 1 , 2 ,
- Tiago M Alfaro 1 , 2 &
- Sara Freitas 1 , 2
BMC Research Notes volume 6 , Article number: S1 ( 2013 ) Cite this article
7180 Accesses
1 Citations
Metrics details
The diagnosis of idiopathic pulmonary fibrosis can be quite challenging, even after careful clinical evaluation, imaging and pathological tests. This case report intends to demonstrate and discuss these difficulties, especially those concerning the differential diagnosis with chronic hypersensitivity pneumonitis.
Case presentation
A 58-year-old white male presented with shortness of breath, dry cough, fatigue and weight loss for two months. He was a former smoker and had regular exposure to a parakeet and poultry. Physical examination revealed bilateral basal crackles and chest imaging showed subpleural cystic lesions and traction bronchiectasis with a right side and upper level predominance. Auto-antibodies and IgG immunoglobulins to parakeet and fungal proteins were negative. Lung function tests displayed moderate restriction, low diffusion capacity and resting hypoxaemia. Bronchoalveolar lavage showed increased lymphocytes (28%) and neutrophils (12%) and surgical lung biopsy was compatible with a pattern of usual interstitial pneumonia. According to the possibility of either idiopathic pulmonary fibrosis or chronic hypersensitivity pneumonitis, treatment included prednisolone, azathioprine, acetylcysteine and avoidance of contact with the parakeet, but there was an unfavorable response and the patient was subsequently referred for lung transplant.
Chronic hypersensitivity pneumonitis and idiopathic pulmonary fibrosis can present with the same clinical and radiological manifestations In this case, despite careful evaluation, no definite diagnosis could be achieved.
Brief introduction
This case demonstrates the difficulties that can occur during the diagnosis of patients with Idiopathic Pulmonary Fibrosis (IPF), and the importance of careful clinical evaluation followed by the appropriate tests.
Patient history
A 58-year old male was referred to our outpatient consultation centre with complaints of shortness of breath, dry cough and fatigue over the previous two months. He also reported anorexia and involuntary weight loss for the same period of time. His primary care physician had treated him with antibiotics, but no response or improvement in symptoms were noted. The patient’s past medical history included an episode of pesticide poisoning 35 years ago for which no information was available and occasional gout that responded to anti-inflammatory medication. The patient was an ex-smoker of 80-pack years and a moderate drinker. No known allergies were reported. His occupational history included working as a stacker in a warehouse for 20 years, with moderate dust exposure, and following this, as an administrative worker for 20 years. He was regularly exposed to a parakeet (Melopsittacus undulatus), chickens, and cats. The patient was unaware of any exposure to tuberculosis patients, recent trips abroad or family history of respiratory disease.
Physical examination
On physical examination, he was in good general condition, but crackles were heard in both lung bases. No other changes were noted.
Diagnostic tests
The patient’s chest X-ray showed bilateral diffuse interstitial infiltrates with a predominant reticular pattern and no spared areas (Figure 1 ). This was followed by a high resolution computed tomography (HRCT) scan of the chest that showed several areas of subpleural cystic lesions and traction bronchiectasis affecting all lobes, but having an upper and middle level predominance and being much more extensive in the right lung. There were also multiple mediastinal enlarged lymph nodes and an enlargement of the pulmonary artery (3.2 cm diameter) and right cardiac cavities (Figure 2 ). Cardiac tests were performed, including an electrocardiogram and echocardiogram, and no other signs of cardiac disease were found. Blood tests, including those for auto-antibodies and IgG immunoglobulins (to parakeet and fungal proteins) were negative. Lung function tests suggested moderate restriction (percentage predicted forced vital capacity [FVC], 57.5%), low diffusion capacity ([DLco] 36% of the predicted value) and resting hypoxaemia (PaO2, 69.7 mmHg). The decision was made to perform bronchoscopy with bronchoalveolar lavage and transbronchial biopsy. Upon examination, the bronchial mucosa showed moderate signs of inflammation, but no other morphological changes. Bronchoalveolar lavage showed an increase in the total cell count (300 cells/µL), and increased percentage of lymphocytes (28%) and neutrophils (12%). The CD4/CD8 ratio was 0.2. Transbronchial biopsy showed no specific findings. A transthoracic biopsy was then performed, but the results were also inconclusive. The patient was referred for surgical lung biopsy. The pathology of the surgical specimen was compatible with a pattern of usual interstitial pneumonia (Figure 3 ).
Chest X-ray showing bilateral diffuse interstitial infiltrates with a predominantly reticular pattern and no spared areas.
HRCT scans showing honeycombing and traction bronchiectasis affecting all lobes of the lungs, enlarged mediastinal lymph nodes and enlargement of the pulmonary artery (3.2 cm in diameter) and the right cardiac cavities.
Surgical lung biopsy showing aspects compatible with a pattern of usual interstitial pneumonia.
Treatment and patient management
At this time no definite diagnosis could be made, as the clinical, radiological and pathological findings were compatible with both chronic hypersensitivity pneumonitis and IPF. Nevertheless, treatment with prednisolone, azathioprine and acetylcysteine commenced. The patient was also instructed to avoid any contact with the parakeet. Despite the treatment, the patient got progressively worse, and has been referred for lung transplantation.
Chronic hypersensitivity pneumonitis and IPF can present with the same clinical and radiological manifestations [ 1 ]. A careful clinical evaluation is therefore fundamental, and the surgical pulmonary biopsy is usually helpful in performing the differential diagnosis [ 2 ], but not in this case. A UIP pattern can be seen on biopsy (and/or CT) in both IPF and chronic HP. The addition of BAL can give a decisive contribution to the diagnostic procedures [ 3 ]. A cut-off level of 30% for lymphocytes in BAL demonstrated a favorable discriminative power for the diagnosis of IPF [ 4 ]. In this case, despite careful evaluation, no definite diagnosis could be achieved.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al: An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011, 183 (6): 788-824. 10.1164/rccm.2009-040GL.
Article PubMed Google Scholar
Costabel U, Bonella F, Guzman J: Chronic hypersensitivity pneumonitis. Clin Chest Med. 2012, 33 (1): 151-163. 10.1016/j.ccm.2011.12.004.
Cordeiro CR, Jones JC, Alfaro T, Ferreira AJ: Bronchoalveolar lavage in occupational lung diseases. Semin Respir Crit Care Med. 2007, 28 (5): 504-513. 10.1055/s-2007-991523.
Ohshimo S, Bonella F, Cui A, Beume M, Kohno N, Guzman J, Costabel U: Significance of bronchoalveolar lavage for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009, 179 (11): 1043-1047. 10.1164/rccm.200808-1313OC.
Download references
Acknowledgements
The author thanks C. Trenam, I. Mandic and M. Smith of IntraMed Communications for editorial assistance in the preparation of the manuscript. Development of this article was supported by InterMune AG.
Declarations
This article has been published as part of BMC Research Notes Volume 6 Supplement 1, 2013:IPF in 2011 – Key updates on guidelines and therapeutics. The full contents of the supplement are available online at http://www.biomedcentral.com/bmcresnotes/supplements/6/S1 . This supplement originates from presentations given at the symposium “AIR: Advancing IPF Research. Working together to translate IPF research into practice” held in Berlin in November 2011. The publication was supported by InterMed Communications with funding from InterMune, AG. The content was proposed by InterMed Communications and developed with the journal. All articles in the supplement have undergone the journal’s standard peer review process.
Author information
Authors and affiliations.
Centre of Pulmonology of the University of Coimbra, Portugal
Carlos Robalo Cordeiro, Tiago M Alfaro & Sara Freitas
Department of Pulmonology, University Hospital of Coimbra, Portugal
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Carlos Robalo Cordeiro .
Additional information
Competing interests.
CRC was a speaker at the AIR meeting, receiving fees. SF and TMA reported no competing interests.
Authors’ contributions
TMA and SF performed the data collection and drafted the manuscript. CRC conceived and supervised the whole study and made the final revision to the manuscript. All authors read and approved the final manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Cordeiro, C.R., Alfaro, T.M. & Freitas, S. Clinical case: Differential diagnosis of idiopathic pulmonary fibrosis. BMC Res Notes 6 (Suppl 1), S1 (2013). https://doi.org/10.1186/1756-0500-6-S1-S1
Download citation
Published : 16 April 2013
DOI : https://doi.org/10.1186/1756-0500-6-S1-S1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Idiopathic Pulmonary Fibrosis
- High Resolution Compute Tomography
- Acetylcysteine
- Lung Function Test
- Usual Interstitial Pneumonia

BMC Research Notes
ISSN: 1756-0500
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Clinical Trials
Pulmonary fibrosis.
Displaying 42 studies
The primary purpose of this study is to see how GLPG1690 works together with current standard treatment on lung function and IPF disease in general. The study will also investigate how well GLPG1690 is tolerated (side effects).
The purpose of this study is to assess the feasibility of Folate scan in patients with Idiopathic Pulmonary Fibrosis (IPF).
The purpose of this study is to evaluate the safety profile and tolerability of ORIN1001 alone and/or in combination with the local standard of care (SOC) in adult subjects with idiopathic pulmonary fibrosis (IPF).
The primary objective of this study is to evaluate the safety and effectiveness of inhaled treprostinil in subjects with Idiopathic Pulmonary Fibrosis (IPF).
The purpose of this study is to compare the effect of n-acetylcysteine (NAC) plus standard care with matched placebo plus standard of care in patients diagnosed with idiopathic pulmonary fibrosis (IPF) who have the TOLLIP rs3750920 TT genotype. The study will compare the time to a composite endpoint of relative decline in lung function [10% relative decline in forced vital capacity (FVC), first respiratory hospitalization, lung transplantation, or all-cause mortality] The secondary objectives will be to examine the effect of NAC on the components of the primary composite endpoint, the rates of clinical events, change in physiology, change in ...
The purpose of this study is to evaluate the effectiveness and safety of 30 mg/kg intravenous (IV) infusions of pamrevlumab administered every 3 weeks as compared to placebo in subjects with Idiopathic Pulmonary Fibrosis.
The purpose of this study is to confirm the long-term safety, effectiveness, and pharmacokinetics of PRM-151 in the treatment of eligible patients with IPF who have taken part in Study PRM-151-202 and received the open-label study drug or completed the Phase III Study WA42293 with PRM-151. Additionally, patients who have discontinued treatment from or have completed Study WA42293 and do not want to receive open-label PRM-151 in this study, will be invited to enroll in survival follow-up. Patients in Cohort C will not receive any treatment and will not undergo any safety or efficacy assessments during the study.
The purpose of this study is to determine if Simtuzumab (GS-6624) is safe and effective in treating Idiopathic Pulmonary Fibrosis.
This phase 2 clinical study will be a randomized, double-blind, placebo-controlled, multicenter study to evaluate the safety, tolerability, biological activity, and pharmacokinetics (PK) of ND-L02-s0201 for Injection in subjects with idiopathic pulmonary fibrosis (IPF).
The purpose of this study is to learn more about pulmonary fibrosis and how it develops to determine if the disease can be detected early, before the lung is permanently scarred. This study will enroll participants who are not currently diagnosed with pulmonary fibrosis, but who have family members with pulmonary fibrosis. Because there is an increased risk within affected families, this cohort will allow us to learn how pulmonary fibrosis develops, and how the lungs change over time.
The main objectives of this study are to determine the difference in change from baseline in Six Minute Walk Distance (6MWD) when pulmonary rehabilitation (PR) is added to stable underlying nintedanib therapy in patients with idiopathic pulmonary fibrosis (IPF), to determine the difference in change in Quality of Life (QoL) when pulmonary rehabilitation (PR) is added to stable underlying nintedanib therapy in patients with idiopathic pulmonary fibrosis (IPF), and to determine if there is an enduring effect in 6MWD, QoL and lung function from pulmonary rehabilitation (PR) when pulmonary rehabilitation (PR) is added to stable underlying nintedanib therapy in patients with idiopathic pulmonary ...
To study the safety and effectiveness of multiple-doses of tralokinumab on pulmonary function in adults with mild to moderate idiopathic pulmonary fibrosis (IPF). IPF is a chronic, progressive, irreversible, and usually fatal lung disease of unknown cause.
The purpose of this study is to evaluate the effectiveness, safety, and tolerability of BI 1015550 compared to placebo in patients with Idiopathic Pulmonary Fibrosis (IPF) in addition to patient’s standard of care over the course of at least 52 weeks.
The primary purpose of this study is to evaluate the effectiveness of BG00011 compared with placebo in participants with Idiopathic Pulmonary Fibrosis (IPF).
The secondary objectives of this study are: to evaluate the efficacy of BG00011 compared with placebo in participants with IPF as determined by change in percent predicted forced (expiratory) vital capacity (FVC); to assess progression-free survival in participants who receive BG00011 compared with placebo; to assess the occurrence of IPF exacerbation in participants who receive BG00011 compared with placebo; to assess the incidence of absolute decline in FVC ≥10% in participants who receive BG00011 compared with placebo; ...
The purpose of this study is to determine if study drug (BMS-986020) dose of 600 mg once daily or 600 mg twice daily for 26 weeks compared with placebo will reduce the decline in forced vital capacity (FVC) and will be well tolerated in subjects with idiopathic pulmonary fibrosis (IPF).
This randomized, multicenter, double-blind, placebo-controlled, parallel-group study will evaluate the efficacy and safety of lebrikizumab as monotherapy in the absence of background idiopathic pulmonary fibrosis (IPF) therapy or as combination therapy with pirfenidone background therapy in participants with idiopathic pulmonary fibrosis. Participants will be randomized to receive either lebrikizumab or placebo subcutaneously (SC) every 4 weeks.
The purpose of this study is to assess the safety and tolerability of single- and multiple-inhaled doses of TRK-250 in subjects with idiopathic pulmonary fibrosis (IPF).
TRK-250 is a nucleic acid medicine that inhibits the progression of pulmonary fibrosis by selectively suppressing the expression of transforming growth factor-beta 1 (TGF-β1) protein, at the gene expression level.
Primary Objective: To evaluate, in comparison with placebo, the efficacy of 2 dose levels of SAR156597 administered subcutaneously during 52 weeks on lung function of patients with Idiopathic Pulmonary Fibrosis (IPF). Secondary Objectives: To evaluate the efficacy of 2 dose levels of SAR156597 compared to placebo on IPF disease progression. To evaluate the safety of 2 dose levels of SAR156597 compared to placebo in patients with IPF.
The purpose of this study is to compare the effect of standard care, versus standard of care plus antimicrobial therapy (co-trimoxazole or doxycycline), on clinical outcomes in patients diagnosed with idiopathic pulmonary fibrosis (IPF).
The purpose of this trial is to demonstrate proof of concept of clinical activity of BI 1015550 on the change of Forced Vital Capacity (FVC) between baseline and 12 weeks. New treatments are needed that further reduce the decline in FVC, positively affect symptoms and improve quality of life in patients with Idiopathic Pulmonary Fibrosis. This trial will investigate BI 1015550 to be used in this patient population either as stand-alone treatment or in addition to local standard of care (SoC), which may or may not include currently approved antifibrotic treatments (nintedanib or pirfenidone).
Mayo Clinic will not be participating in ...
This is an open-label, multi-center, extension study for patients with IPF who complete a qualifying InterMune clinical trial of pirfenidone. The purpose of this study is to obtain additional safety data for pirfenidone 2403 mg/day in patients with IPF who complete a qualifying InterMune clinical trial of pirfenidone.
The purpose of this study is to evaluate the long-term safety and tolerability of inhaled treprostinil in subjects with fibrotic interstitial lung disease
The purpose of the Pulmonary Fibrosis Foundation Patient Registry is to collect data on well-characterized patients with interstitial lung disease, especially idiopathic pulmonary fibrosis, for participation in retrospective and prospective research.
This is an open label multi-center program to allow patients in the US with IPF access to treatment with pirfenidone.
The purpose of this study is to evaluate the effectiveness, safety, and pharmacokinetics of PRM-151 compared with placebo in patients with idiopathic pulmonary fibrosis (IPF). Specific objectives and corresponding endpoints for the study are outlined below.
The purpose of this trial is to evaluate the effectiveness and safety of pamrevlumab in subjects with idiopathic pulmonary fibrosis (IPF). Subjects who were previously treated with approved IPF therapies (i.e., nintedanib or pirfenidone; unless neither treatment is available in the host country) may be eligible for screening, provided that the subject is not currently receiving treatment with an approved IPF therapy.
The overall objective of this trial is to evaluate the effectiveness and safety of pamrevlumab as compared to placebo in subjects with Idiopathic Pulmonary Fibrosis.
The purpose of this study is to evaluate Inhaled RVT-1601 (formerly, PA101B), a new inhalation formulation of cromolyn sodium delivered via the eFlow® Closed System (CS) nebulizer, for the treatment of persistent cough in patients with idiopathic pulmonary fibrosis (IPF).
ORV-PF-01 is a two way, placebo controlled, cross-over study, to evaluate the effect of two doses of orvepitant on cough in patients with Idiopathic Pulmonary Fibrosis (IPF).
The purpose of this study is to assess the safety and effectiveness of AF-219 in patients with idiopathic pulmonary fibrosis with persistent cough.
The purpose of this study is to assess the safety and efffectiveness of pulsed inhaled nitric oxide (iNO) in subjects at risk for pulmonary hypertension associated with pulmonary fibrosis on long term oxygen therapy.
The main purpose of this study is to see how GLPG1690 works together with current standard treatment on lung function and IPF disease in general. The study will also investigate how well GLPG1690 is tolerated; i.e., side effects while on study drug).
This is a multicenter, randomized (1:1 inhaled treprostinil: placebo), double-blinded, placebo-controlled trial to evaluate the safety and efficacy of inhaled treprostinil in subjects with pre-capillary pulmonary hypertension (PH) associated with interstitial lung disease (ILD) including combined pulmonary fibrosis and emphysema (CPFE). The study will include about 314 patients at approximately 120 clinical trial centers. The treatment phase of the study will last approximately 16 weeks. Patients who complete all required assessments will also be eligible to enter an open-label, extension study (RIN-PH-202).
To develop a repository of blood, urine and tissue samples from patients with ILD to support future studies into the development of such biomarkers.
The purpose of this study is to investigate the possibility that B lymphocytes (a kind of white blood cell ) may be contributing to the development of fibrosis in the lungs. This study will examine if B lymphocytes, isolated from blood, can induce the stimulation of fibroblasts. Fibroblasts are cells that are responsible for the formation of scarring in the lungs. Specific markers found in the surface of B lymphocytes will also be investigated to see if it can be identified why these cells may induce the development of fibrosis.
The purpose of this study is to assess the long-term tolerability and safety of oral nintedanib treatment in patients with Progressive Fibrosing Interstitial Lung Disease who have completed (and did not prematurely discontinue trial medication in) the phase III parent trial, 1199.247 (INBUILD®).
The purpose of this research study is to establish a pool of healthy donors who will regularly participate in our research studies of human immunity. The studies in our laboratory investigate the role of human blood cells in immunity to fungal disease and lung fibrosis. Most of our studies require blood that is freshly drawn. Thus, we propose to draw blood on an as needed, ongoing basis.
The purpose of this study is to determine the effectiveness of a new computer-aided analysis tool in identifying treatment response in idiopathic pulmonary fibrosis. Computer-Aided Lung Informatics for Pathology Evaluation and Ratings (CALIPER) is an image analysis tool for characterizing and measuring mixed parenchymal diseases such as emphysema and interstitial lung diseases and has been shown to correlate with idiopathic pulmonary fibrosis mortality.
The main objective is to assess long term safety of treatment with oral nintedanib in patients with Systemic Sclerosis associated Interstitial Lung Disease (SSc-ILD).
This is a multicenter, open-label trial to evaluate the safety and efficacy of inhaled treprostinil in subjects with pre-capillary pulmonary hypertension (PH) associated with interstitial lung disease (ILD) including combined pulmonary fibrosis and emphysema (CPFE). The study will include about 266 patients who completed all required assessments in the RIN-PH-201 study at approximately 100 clinical trial centers. The study will continue Your participation in this study is voluntary and will last until you discontinue from the study or the study ends. The study will continue until each subject reaches the Week 108 visit or until inhaled treprostinil become commercially available ...
The purpose of this study is to evaluate the effectiveness of inhaled molgramostim, administered open-label, to adult cystic fibrosis subjects with chronic pulmonary nontuberculous mycobacterial (NTM) infection, with or without ongoing antimycobacterial guideline based combination therapy.
The purpose of this study is to determine the usefulness of palliative care consultations by a specialty physician and a pulmonologist for patients who have advanced lung disease.
The purpose of this study is to evaluate the effietiveness of remdesivir (RDV) in reducing the rate of of all-cause medically attended visits (MAVs; medical visits attended in person by the participant and a health care professional) or death in non-hospitalized participants with early stage coronavirus disease 2019 (COVID-19) and to evaluate the safety of RDV administered in an outpatient setting.
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
- Advanced Search
- Teaching Cases
- Submit a Case
A case of idiopathic pulmonary fibrosis
Chest imaging
Clinical Cases
A. Strutynskaya 1 , M. Karnaushkina 2
1 Federal state autonomous institution “National Medical Research Center for Children's Health” of the Russian Federation Ministry of Health. Lomonosov Avenue, 2, building 1. Moscow, Russia 2 I.M. Sechenov First Moscow State Medical University. Bolschaya Pirogovskaya street, 2 building 4. Moscow, Russia Corresponding author – A. Strutynskaya. Email: [email protected]
64 years, female
A 64-year-old female patient complaining of cough with scarce yellowish sputum, severe exertional dyspnoea and weakness in the last 3 weeks and treating with aminopenicillins and ipratropium bromide+fenoterol for 7 days with minimal effect. Her lung function had progressively deteriorated during last 2 years. She had stopped smoking 6 years ago. Clinical examination revealed dyspnoea and tachypnoea with multiple predominantly bibasal crackles, O2 saturation was 85%, restrictive changes were identified at spirometry.
Inspiratory chest CT showed inhomogeneous decrease of pneumatisation, multiple foci of irregularly spaced reticulation, honeycombing pattern (clustered cystic air spaces of variable diameters, occasionally up to 15 mm, with thick, dense walls) and traction bronchiectasis. The latter is defined as irregular bronchial dilatation surrounding retractile pulmonary fibrosis. It is important that all the reticular abnormalities are predominantly in subpleural zones and there is craniocaudal gradient of the lesions, seen on coronal images. Also sliding hiatal hernia (no contrast enema was given) is observed. Described features are consistent with usual interstitial pneumonia (UIP) pattern. Considering the appropriate anamnesis and clinical findings, idiopathic pulmonary fibrosis (IPF) was diagnosed.
Background IPF is a chronic, progressive, fibrotic interstitial lung disease of unknown cause [1]. Repeated alveolar micro-injury superimposed on pro-fibrotic epigenetic reprogramming, impaired mechanisms of alveolar epithelium repair and dysfunction of surfactant leads to development of fibrosis [3-5]. IPF requires differentiation with alternative causes of pulmonary fibrosis, preferentially with connective tissue disorders (e.g. rheumathoid arthritis, antisynthetase and Sjogren's syndromes), chronic hypersensitivity pneumonitis, occupational lung diseases and drug toxicity [1,2].
Clinical Perspective A diagnosis of IPF requires multidisciplinary discussion among clinician, radiologist, pathologist and other specialists if it’s needed (e.g. in cases of connective tissue disorders suspected). Especially when clinical history or radiological patterns are not definite. A diagnostic search begins with clinician’s work, who has to establish a probability of interstitial lung diseases (ILD) presence and exclude their known causes like occupational exposure, connective tissue disorders, drug addiction. The probability of the diagnosis is increased in male patients, smokers, over 60 y/o with a family history of ILD and/or comorbid lung pathology [1,2,6]. Physical examination can reveal unexplained exertional dyspnoea, progressing with time, chronic dry cough, fine high-pitched bibasilar inspir¬atory crackles (so called velcro-like sounds). Spirometry typically detects restrictive changes and plethysmography - a reduction in diffusing capacity of the lung for carbon monoxide [1,2,5]. In our case, a diagnosis of IPF was proposed at the stage of clinical examination due to the anamnesis and clinical findings. Although in a differential list, there was also pneumonia, chronic bronchitis and COPD. After revising CT results according to ATS/ERS guidelines, serological testing was provided, and connective tissue diseases were excluded.
Imaging perspective
High-resolution CT protocols are required with the thinnest collimation and should include both inspiratory and expiratory images. All consequences of pathophysiologic processes can be clearly seen on chest CT. Fibrotic changes implicate interstitium inside the secondary pulmonary lobule, which appears on CT as intralobular reticular pattern with irregular thickening of the interstitium. Because of aberrant alveolar repair and continuous micro-injuries, acinar structure is completely destroyed and alveoli become deformed. They evolve into cysts of different sizes and shapes, surrounded by walls of variable thickness. In total all these changes are named as honeycombing pattern. Patchy, basal subpleural predominant distribution of honeycombing, fit by presence of reticular abnormalities, traction bronchiectasis or bronchioloectasis represents UIP pattern. In some cases, these lesions may be associated with ground glass opacity (GGO). If all features of UIP except for honeycombing are presented, such pulmonary lesion is regarded as probable UIP pattern. In both cases definite diagnosis of IPF can be made, considering appropriate anamnesis (patients over 60 years, smokers, with progressive deterioration of lung function, absence of other potential causes of ILD) and clinical data (worsening of dyspnoea, cough with sputum, restrictive changes at spirometry). When there is no strong evidence of UIP pattern – only subtle reticulation with basal subpleural predominant distribution and probable mild GGO is presented, intermediate for UIP pattern should be assigned. In such cases, and when an alternative diagnosis is suggested, a biopsy is required [1,2,4,5]. Although according to ATS/ERS guidelines even in cases of probable UIP pattern with inappropriate anamnesis and clinical data biopsy is recommended [6]. In the described case there are quite extensive areas of GGO, which match up with honeycombing distribution and aren’t as pronounced as reticulation abnormalities. Such changes may be regarded as demonstration of fibrotic changes and as sign of infection. The letter is more probable in our case in the background of clinical examination.
Treatment As first-line therapy, Nintedanib e.g. pirfenidone, having a number of anti-inflammatory and antifibrotic effects, are recommended [1 3-5]. Several options for non-pharmacologic treatment are available: smoking cessation, supplemental oxygen therapy, administration, special complex of pulmonary rehabilitation exercises, and age-appropriate vaccines [1,4,5].
Take home massages: 1. At least brief knowledge of IPF pathogenesis is crucial for understanding the nature of pathologic findings from the CT image. 2. Since IPF has no single pathognomonic feature, it should be diagnosed based on complex assessment of anamnesis, symptoms, chest CT data and pathologic findings. 3. In cases of typical or probable UIP patterns, considering exact IPF anamnesis and clinics, no biopsy is required for diagnosis.
Written informed patient consent for publication has been obtained.
[1] DA Lynch, N Sverzellati, WD Travis, KK Brown, TV Colby, JR Galvin, JG Goldin, DM Hansell, Y Inoue, T Johkoh, AG Nicholson, SL Knight, S Raoof, L Richeldi, CJ Ryerson, JH Ryu, AU Wells (2018) Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med 6:138–53. (PMID: 29154106 )
[2] DJ Lederer, FJ Martinez (2018) Idiopathic Pulmonary Fibrosis. N Engl J Med;378:1811-23. (PMID: 29742380 )
[3] SL Barratt, A Creamer, C Hayton, N Chaudhuri (2018) Idiopathic Pulmonary Fibrosis (IPF): An Overview. J. Clin. Med 201(7): e21 (PMID: 30082599 )
[4] G Sgalla, B Iovene, M Calvello, M Ori, F Varone, L Richeldi. (2018). Idiopathic pulmonary fibrosis: pathogenesis and management. Respiratory Research 19(1):32 (PMID: 29471816 )
[5] FJ Martinez, HR Collard, A Pardo, G Raghu, L Richeldi, M Selman, JJ Swigris, H Taniguchi, AU. Wells (2017). Idiopathic pulmonary fibrosis. Nat Rev Dis Primers 3: e19 (PMID: 29052582 )
[6] G Raghu, M Remy-Jardin, JL Myers, L Richeldi, CJ Ryerson, DJ Lederer, J Behr, V Cottin SK Danoff, F Morell, KR Flaherty, A Wells, FJ Martinez, A Azuma , TJ Bice, D Bouros, KK Brown, HR Collard, A Duggal, L Galvin, Y Inoue, RG Jenkins, T Johkoh, EA Kazerooni, M Kitaichi, SL Knight, G Mansour, AG Nicholson, SNJ Pipavath, I Buendía-Roldán, M Selman, WD Travis, S Walsh, KC Wilson. (2018). Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 198(5):e44-e68. (PMID: 30168753 )
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License .

Axial CT image

Coronal CT image

Sagittal CT image

Incidence and Progression of Fibrotic Lung Disease in an At-Risk Cohort
Affiliations.
- 1 Department of Medicine.
- 2 Center for Advanced Heart and Lung Disease, Baylor University Medical Center at Dallas, Dallas, Texas.
- 3 Department of Radiology.
- 4 Department of Radiology, and.
- 5 Department of Diagnostic Imaging, Oncological Radiotherapy, and Hematology, Fondazione Policlinico University Gemelli, Rome, Italy.
- 6 Department of Regional Radiology, Cleveland Clinic Imaging Institute, Cleveland, Ohio; and.
- 7 Department of Medicine, Vanderbilt University, Nashville, Tennessee.
- 8 Department of Medicine, and.
- 9 Center for Genes, Environment, and Health, National Jewish Health, Denver, Colorado.
- 10 Department of Microbiology and Immunology, University of Colorado School of Medicine, Aurora, Colorado.
- PMID: 36094461
- PMCID: PMC10870916
- DOI: 10.1164/rccm.202206-1075OC
Rationale: Relatives of patients with familial interstitial pneumonia (FIP) are at increased risk for pulmonary fibrosis and develop preclinical pulmonary fibrosis (PrePF). Objectives: We defined the incidence and progression of new-onset PrePF and its relationship to survival among first-degree relatives of families with FIP. Methods: This is a cohort study of family members with FIP who were initially screened with a health questionnaire and chest high-resolution computed tomography (HRCT) scan, and approximately 4 years later, the evaluation was repeated. A total of 493 asymptomatic first-degree relatives of patients with FIP were evaluated at baseline, and 296 (60%) of the original subjects participated in the subsequent evaluation. Measurements and Main Results: The median interval between HRCTs was 3.9 years (interquartile range, 3.5-4.4 yr). A total of 252 subjects who agreed to repeat evaluation were originally determined not to have PrePF at baseline; 16 developed PrePF. A conservative estimate of the annual incidence of PrePF is 1,023 per 100,000 person-years (95% confidence interval, 511-1,831 per 100,000 person-years). Of 44 subjects with PrePF at baseline, 38.4% subjects had worsening dyspnea compared with 15.4% of those without PrePF ( P = 0.002). Usual interstitial pneumonia by HRCT ( P < 0.0002) and baseline quantitative fibrosis score ( P < 0.001) are also associated with worsening dyspnea. PrePF at the initial screen is associated with decreased survival ( P < 0.001). Conclusions: The incidence of PrePF in this at-risk population is at least 100-fold higher than that reported for sporadic idiopathic pulmonary fibrosis (IPF). Although PrePF and IPF represent distinct entities, our study demonstrates that PrePF, like IPF, is progressive and associated with decreased survival.
Keywords: familial idiopathic pulmonary fibrosis; idiopathic pulmonary fibrosis; pulmonary fibrosis.
Publication types
- Research Support, N.I.H., Extramural
- Cohort Studies
- Idiopathic Pulmonary Fibrosis*
- Lung Diseases, Interstitial*
- Retrospective Studies
Grants and funding
- P01 HL092870/HL/NHLBI NIH HHS/United States
- R01 HL158668/HL/NHLBI NIH HHS/United States
- UH2/3-HL151865/HL/NHLBI NIH HHS/United States
- I01 BX005295/BX/BLRD VA/United States
- UG3-HL151865/HL/NHLBI NIH HHS/United States
- P01 HL162607/HL/NHLBI NIH HHS/United States
- PO1-HL0928701/HL/NHLBI NIH HHS/United States
- R01 HL149836/HL/NHLBI NIH HHS/United States
- UH2 HL123442/HL/NHLBI NIH HHS/United States
- UH3 HL151865/HL/NHLBI NIH HHS/United States
- RO1-HL149836/HL/NHLBI NIH HHS/United States
- UH3 HL123442/HL/NHLBI NIH HHS/United States
- UG3 HL151865/HL/NHLBI NIH HHS/United States
- Virtual cOaching in making Informed Choices on Elder Mistreatment Self-Disclosure
- Integrated Model for Personal Assistant Research and Training
- Community Complex Case Response Team
- Health Information Technology to Prevent Abuse, Neglect, and Exploitation
- Virtual Multimedia Interactive Informed Consent
- Radiotherapy For Older Women
- Lab Members
- Publications
INFORMATION FOR
- Residents & Fellows
- Researchers
Single-Cell Profiling Reveals Insights About Immunity in Idiopathic Pulmonary Fibrosis
Listen to "single-cell profiling reveals insights about immunity in idiopathic pulmonary fibrosis".
Possible immune cell responses in idiopathic pulmonary fibrosis (IPF), a chronic and deadly lung disease characterized by lung scarring, have been suggested for years. Yet, until now, detailed profiles of these cells have been lacking.
In a new study , published in the American Journal of Respiratory and Critical Care Medicine , researchers used single-cell RNA sequencing to measure the gene expressions of individual peripheral blood mononuclear cells in patients with progressive IPF, patients with stable IPF, and lung-disease-free patients. Previously, the researchers had studied bulk samples of these cells but not examined them individually and in detail.
This new data should encourage the research community to better understand immune cells with the aim of developing new precision therapies. Naftali Kaminski, MD
"Using this state-of-the-art single-cell profiling technology on around 10 to 20,000 cells per patient, we applied a very advanced method to get the profile of every cell,” said Naftali Kaminski, MD , Boehringer Ingelheim Pharmaceuticals, Inc. Professor of Medicine (Pulmonary). “We applied cutting-edge computer science and deep learning methods in this computationally intensive process.”
The multidisciplinary team was able to obtain detailed profiles of monocytic cells, building on prior studies from the Kaminski Lab showing that this type of immune cell is higher in patients with IPF, and even higher in individuals with progressive IPF, Kaminski said.
One unexpected finding was an elevated level of regulatory T-cells (Tregs), a type of white blood cell that controls inflammation, in those with progressive IPF.
“Unlike most types of lymphocytes that decrease in progressive compared to stable IPF, Tregs are actually increased in progressive disease, both in the blood and the lungs,” said first author Avraham Unterman, MD, MBA, formerly of the Kaminski Lab, who currently leads the Pulmonary Fibrosis Center and Genomic Research Laboratory for Lung Fibrosis at Tel Aviv Medical Center. “They interact with most immune cells in the IPF lung and are likely involved in disease pathogenesis.”
The researchers used data from the study to create a model of the interplay between cells of the peripheral immune system and the lung. To accelerate advancements, before publishing their results the team made their findings publicly available through the ILD Immune Cell Atlas, a multi-institutional collaboration that seeks to facilitate the exploration of single-cell RNA sequencing datasets related to IPF through an accessible web tool.
“Through these efforts, we provided the first comprehensive single-cell peripheral blood atlas for idiopathic pulmonary fibrosis, revealing key differences in cell populations associated with disease progression and proposing mechanisms for how these immune cells contribute to lung fibrosis,” said Amy Zhao , an MD/PhD student in the Kaminski Lab. “Seeing how these findings relate to the disease mechanisms and severity prediction in idiopathic pulmonary fibrosis was particularly enlightening."
“In addition to the IPF cell atlas we have previously published , we now have comprehensive, detailed maps of what’s happening in the immune system available to investigators all over the world,” Kaminski added.
Kaminski emphasizes that this type of translational research could not have been done without the collaboration among clinicians, immunologists, molecular biologists, and genomics experts from many institutions.
“Through our joint efforts, we are providing evidence that our findings are worth analyzing further to gain insights about IPF,” he said. “This new data should encourage the research community to better understand immune cells with the aim of developing new precision therapies.”
The Section of Pulmonary, Critical Care and Sleep Medicine is one of the eleven sections within Yale School of Medicine’s Department of Internal Medicine. To learn more about Yale-PCCSM, visit PCCSM's website , or follow them on Facebook and Twitter .
- Basic Science Research
- Internal Medicine
- Pulmonary, Critical Care and Sleep Medicine
Featured in this article
- Naftali Kaminski, MD Boehringer Ingelheim Pharmaceuticals, Inc. Professor of Medicine (Pulmonary); Section Chief, Pulmonary, Critical Care & Sleep Medicine
Related Links
- ILD Immune Cell Atlas
- Open access
- Published: 23 May 2024
Identifying potential drug targets for idiopathic pulmonary fibrosis: a mendelian randomization study based on the druggable genes
- Zetao Liu 1 , 2 ,
- Zhiyu Peng 1 , 2 ,
- Huahang Lin 1 , 2 ,
- Ke Zhou 1 , 2 ,
- Linchuan Liang 1 , 2 ,
- Jie Cao 1 , 2 ,
- Zhaokang Huang 1 , 2 &
- Jiandong Mei 1 , 2
Respiratory Research volume 25 , Article number: 217 ( 2024 ) Cite this article
387 Accesses
Metrics details
Idiopathic pulmonary fibrosis (IPF) is a chronic fibrotic interstitial lung disease characterized by progressive dyspnea and decreased lung function, yet its exact etiology remains unclear. It is of great significance to discover new drug targets for IPF.
We obtained the cis-expression quantitative trait locus (cis-eQTL) of druggable genes from eQTLGen Consortium as exposure and the genome wide association study (GWAS) of IPF from the International IPF Genetics Consortium as outcomes to simulate the effects of drugs on IPF by employing mendelian randomization analysis. Then colocalization analysis was performed to calculate the probability of both cis-eQTL of druggable genes and IPF sharing a causal variant. For further validation, we conducted protein quantitative trait locus (pQTL) analysis to reaffirm our findings.
The expression of 45 druggable genes was significantly associated with IPF susceptibility at FDR < 0.05. The expression of 23 and 15 druggable genes was significantly associated with decreased forced vital capacity (FVC) and diffusing capacity of the lungs for carbon monoxide (DLco) in IPF patients, respectively. IPF susceptibility and two significant genes ( IL-7 and ABCB2 ) were likely to share a causal variant. The results of the pQTL analysis demonstrated that high levels of IL-7 in plasma are associated with a reduced risk of IPF (OR = 0.67, 95%CI: 0.47–0.97).
IL-7 stands out as the most promising potential drug target to mitigate the risk of IPF. Our study not only sheds light on potential drug targets but also provides a direction for future drug development in IPF.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive fibrotic interstitial lung disease with poor prognosis characterized by progressive dyspnea and decline in lung function [ 1 ]. In the past two decades, the incidence of IPF has increased, especially among the elderly [ 2 ]. Unfortunately, the exact etiology and pathogenesis of IPF remain elusive, with potential risk factors including genetic variations, long-term exposure to air pollution, smoking, certain viral infections, and gastroesophageal reflux disease [ 3 ]. Although anti-fibrotic drugs like pirfenidone and nintedanib, recommended by current guidelines, have displayed modest ability in slowing disease progression, halting or reversing the process of IPF remains a challenge [ 4 ]. Thus, the identification of novel drug targets capable of preventing IPF or delaying its progression assumes paramount significance.
Mendelian randomization (MR) is an approach that employs genetic variants associated with specific exposures as instrumental variables to estimate causal relationships between the exposure of interest and the desired outcome (Fig. 1 ) [ 4 ]. Guided by the laws of gene segregation and independent assortment, alleles segregate and genes on non-homologous chromosomes recombine freely during gamete formation. Subsequently, the combination of parental gametes determines the presence or absence of certain genes, facilitating the random distribution of lifetime-long exposures. MR analysis has similar power to randomized controlled trial (RCT) with less bias and no reverse causality [ 5 ].
Overview of MR analysis. Choose cis-eQTL of druggable genes as instrumental variables (IVs) to investigate the causal relationship between the expression of druggable genes and IPF. The “X” between IVs and confounders indicates that the IVs are independent of any confounding factors
The “X” between IVs and outcome indicates that the IVs only affect the outcome through the exposure rather than other potential pathways. IV, instrumental variable; SNP, single-nucleotide polymorphisms; IPF, Idiopathic pulmonary fibrosis
In drug target MR analysis, single nucleotide polymorphisms (SNPs) associated with gene expression levels, known as expression quantitative trait loci (eQTL), are employed as instrumental variables to examine the effects of druggable genes. Specifically, cis-eQTLs in the genomic regions proximal to the target gene are often selected due to their close relationship with gene expression. This methodology has garnered widespread application across various diseases, including Parkinson’s disease, aortic aneurysms, and even the COVID-19 [ 6 , 7 , 8 ].
Building upon this foundation, the present study aims to leverage the power of MR analysis to unearth potential drug targets for IPF from a pool of 4,479 druggable genes encoding drug targets or proteins related to drug targets through MR method, whether to prevent disease or delay the progression.
Study design
The flowchart visually describing the overall of the study is shown in Fig. 2 . In short, we performed a two-sample MR analysis utilizing cis-eQTL of druggable genes in the blood as exposure and the genome wide association study (GWAS) of IPF as outcomes to investigate the causal relationship between the expression of druggable genes and susceptibility and progression of IPF. According to strict inclusion and exclusion criteria, appropriate SNPs were selected as instrumental variables (IVs). A series of sensitivity analyses was conducted to control the quality of MR analysis. For the druggable genes that exhibited significant MR results, we performed colocalization analysis to assess whether the same causal variant was shared by both the cis-eQTL and IPF. Additionally, we conducted protein quantitative trait locus (pQTL) analysis, which provided further validation of these druggable genes by examining the effect of the protein levels on the outcome.

The flowchart of our study design. MR, mendelian randomization; eQTL, expression quantitative trait locus; pQTL, protein quantitative trait locus; FVC, forced vital capacity; DLco, diffusing capacity of the lungs for carbon monoxide
Exposure data
The druggable genes are defined as a set of genes encoding proteins with potential to be modulated by a drug-like small molecule based on sequence and structural similarity to the targets of existing drugs [ 9 ]. A total of 4,479 druggable genes were identified by Finan et al. including 1,427 genes encoding approved or clinical-phase drug targets, 682 genes encoding proteins that bind to known drug molecules or are similar to approved drug targets and 2,370 genes that were members of key druggable gene families or encoding proteins with distant similarity to approved drug targets [ 9 ]. This diverse collection of druggable genes offered a wide range of potential targets for investigation (Supplementary material: Table S2 ).
The cis-eQTL data in the blood for only 2,525 genes out of 4,479 druggable genes was obtained by searching in eQTLGen Consortium [ 10 ]. This consortium incorporates 37 datasets with a total of 31,684 individuals, predominantly of European ancestry. The eQTL data facilitates the identification of genetic variants associated with gene expression levels in blood samples, situated within a 1 Mb distance from the central location of each gene. The minor allele frequency (MAF) of every variant is greater than 0.01.
The pQTL data was available from the INTERVAL study encompassing 3,301 healthy participants of European descent [ 11 ]. In this study, a total of 1,927 pQTLs about 1,478 plasma proteins were identified. We selected the pQTL for druggable genes significantly colocalized with IPF outcomes to further investigate the relationship between levels of protein encoded by druggable genes and outcomes.
Instrumental variables (IVs) selection
To ensure the reliability and accuracy of our results, it is crucial to satisfy three important assumptions in MR analysis: (1) The IVs are strongly associated with exposure; (2) The IVs are independent of any confounding factors; (3) There is no presence of horizontal pleiotropy, meaning that the IVs only affect the outcome through the exposure and not through any other potential pathways.
In line with these assumptions, a rigorous selection process was implemented for each druggable gene in our study. Firstly, we employed a stringent threshold and selected SNPs from the cis-eQTL data, ensuring that only those with P -values lower than the genome-wide significance threshold (5.0 × 10 − 8 ) were considered. Next, in order to achieve a set of mutually independent SNPs, the SNPs for every druggable genes were clumped based on the 1,000 Genomes Project European population and the linkage disequilibrium (LD) threshold was set to r 2 < 0.1 with a clumping window of 10,000kb [ 12 ]. Thirdly, incompatible SNPs between the exposures and outcomes (e.g., A/G vs. A/C) were excluded and positive strand alleles were inferred using allele frequencies for palindromes or the palindromic SNPs were excluded directly if there were no allele frequencies. Finally, the following formula was used to calculate the F -statistic [ 13 ].
The F -statistic serve as an essential metric in MR analysis, determining the strength of the IVs’ association with the exposure variable and aiding in the assessment of possible bias or weak instrument issues. In this formula, R 2 is the proportion of variance explained by the IVs, N is the sample size, and k is the number of IVs. The SNPs with F -statistic less than 20 were excluded to avoid weak instrument bias [ 13 ].
Outcome data
The GWAS statistics for IPF susceptibility and progression were obtained from the International IPF Genetics Consortium. For the GWAS of IPF susceptibility, a meta-analysis was conducted across five studies, comprising a total of 4,125 cases and 20,464 controls [ 14 ]. For the GWAS of IPF progression, two key measurements, namely forced vital capacity (FVC) and diffusing capacity of the lungs for carbon monoxide (DLco), were employed to identify variants that may contribute to a more rapid decline in lung capacity or gas transfer among IPF patients. There were 1,048 cases a total of 4,560 FVC measures and 729 cases with a total of 2,795 DLco measures [ 15 ].
Mendelian randomization and colocalization
MR analysis was conducted using the R package “TwoSampleMR” (version 0.5.6). For the MR analysis, Wald ratio method was used when there was only one SNP as the IV. And inverse variance weighted (IVW), MR-Egger, weighted median, simple mode and weighted mode five methods were utilized if the IV contained two or more SNPs. Previous research has indicated that the IVW method is more conservative but robust compared to the other four methods [ 16 ]. Therefore, whether or not there is heterogeneity, the results were mainly based on the IVW method, supplemented by the others. To account for multiple testing, FDR (false discovery rate) corrections were applied to identify significant MR results.
Then the sensitivity analysis was performed by several methods. The potential heterogeneity of IVs was examined by Cochrane’s Q test [ 17 ]. If the P -value of Cochrane’s Q test was less than 0.05, it was indicative of heterogeneity. And MR-Egger regression was used to detect potential pleiotropy in the association between the exposures and outcomes [ 17 ]. If the P -value of MR-Egger regression intercept was less than 0.05, it suggested the presence of pleiotropy and rendered the MR analysis results unreliable.
For the druggable genes exhibiting significant MR results, colocalization analysis was conducted using R package “coloc” (version 5.1.0.1) [ 18 ]. The default prior probability was P 1 = 1.0 × 10 − 4 , P 2 = 1.0 × 10 − 4 , P 12 = 1.0 × 10 − 5 , representing respectively a SNP is associated with the expression of the druggable genes, the outcome, or both. The posterior probabilities for the following 5 hypotheses were generated from colocation analysis: PPH0, no association with either expression of the druggable genes or outcome; PPH1, association with expression of the druggable genes, but not outcome; PPH2, association with outcome, but not expression of the druggable genes; PPH3, association with expression of the druggable genes and outcome, with different causal variants; PPH4, association with expression of the druggable genes and outcome, with a shared causal variant. PPH4 > 0.80 was considered strong evidence for colocalization and the genes colocalized with IPF were regarded as potential targets. The variant most closely associated with exposure (with the lowest P -value) was selected as the reference variant and variants ± 500 kb of the reference variant were included in colocalization analysis.
According to the selection criteria of IVs, a total of 4,0356 SNPs were used as IVs for 2,525 druggable genes. The F -statistic of IVs all exceeded 20, indicating no evidence of weak instrument bias. Details about the IVs are shown in Supplementary material: Table S3 .
- Mendelian randomization
Based on the IVW method, we found the expression of 45 druggable genes was significantly associated with IPF susceptibility at FDR < 0.05. The expression of 23 and 15 druggable genes was significantly associated with decreased FVC and DLco levels in IPF patients, respectively (Figs. 3 and 4 ).

Significant MR results between the expression of druggable genes and IPF susceptibility after FDR correction

Significant MR results between the expression of druggable genes and DLco decline after FDR correction
The results of Cochran’s Q test showed no heterogeneity in IVs for significant genes (Supplementary material: Table S4-6 ). Furthermore, for some significant genes, pleiotropy was detected by MR-Egger regression methods and the corresponding results for these genes were considered unreliable (Supplementary material: Table S4-6 ).
Colocalization
For the druggable genes with significant MR results, we conducted colocalization analysis to calculate probability of cis-eQTL and IPF outcomes sharing a causal variant. The results of colocalization analysis indicated IPF susceptibility and two significant genes ( IL-7 and ABCA2 ) were likely to share a causal variant, with a posterior probability of PP.H4 > 0.80% ( IL-7 : 84.00%, ABCA2 : 81.50%). But there was no evidence of colocalization between IPF progression and the significant genes (Supplementary material: Table S7 ). Therefore, IL-7 and ABCA2 were identified as potential drug targets for reducing IPF risk based on MR and colocalization analyses.
pQTL analysis for IL-7
To verify the effect of druggable gene expression on IPF susceptibility, we further investigated plasma protein levels using pQTL data. The pQTL data for IL-7 was obtained from the INTERVAL study. Unfortunately, we could not find any pQTL data for ABCA2 .
We filtered out the SNPs with P -values less than the genome-wide significance threshold and clumped with r 2 < 0.001 and clumping window of 10,000 kb. Only one SNP (rs72673751) was screened as IV representing IL-7 protein level for pQTL analysis (Supplementary material: Table S8 ). To ensure the validity of result, we searched on PhenoScanner website to exclude the existence of pleiotropy which could affect outcome through potentially other pathways.
The results of the pQTL analysis demonstrated that high levels of IL-7 in plasma are associated with a reduced risk of IPF (OR = 0.67, 95%CI: 0.47–0.97, P = 0.035), which is consistent with the findings of eQTL analysis (Supplementary material: Table S9 ).
In order to identify potential drug targets for IPF, we conducted a large-scale MR analysis to evaluate the role of 2,429 druggable gene expression in IPF susceptibility and progression. After a series of sensitivity analyzes and further analyses, including Cochrane’s Q test, MR-Egger regression, colocalization analysis, pQTL analysis, we have discovered that IL-7 holds the most promising potential as a therapeutic target for IPF susceptibility. However, it is important to note that the therapeutic effect of IL-7 was not replicated in the IPF progression cohort.
Although the pathogenesis of IPF has not been fully elucidated, there is sufficient evidence that transforming growth factor–β (TGF-β) plays a key role. Overexpressed TGF-β induces epithelial-mesenchymal transition (EMT) and promotes abnormal deposition of extracellular matrix (ECM), leading to pulmonary fibrosis [ 19 ]. There have been some studies exploring how IL-7 affects TGF-β to reduce the risk of IPF. Huang et al. [ 20 ] demonstrated that IL-7 can not only down-regulate the synthesis of TGF-β in lung fibroblasts but also block TGF-β signaling through the intact JAK1/STAT1 pathway to reduce collagen synthesis. In addition, further studies found that IL-7 also inhibited PKC-δ activity to reduce TGF-β-induced expression of collagen genes COL1A1 and COL3A1 [ 21 ]. They also found that IL-7 was able to alleviate bleomycin-induced pulmonary fibrosis in vivo [ 20 ]. In an observational study using direct hemoperfusion with a polymyxin B-immobilized fiber column (PMX-DHP) for acute exacerbations of IPF, plasma IL-7 level was significantly higher in survivors compared with non-survivors on day 30 after treatment, which may indicate IL-7 has potential anti-fibrotic effects [ 22 ]. These previous studies suggest that IL-7 has therapeutic potential for IPF. Different from the perspective of the above studies, our study proved this genetically through MR analysis.
Some MR analyzes about IPF have been published, including lung cancer, gastroesophageal reflux disease, allergic rhinitis, but our study is the first to apply drug target MR analysis using eQTL to IPF. One of the strengths of our study lies in the size and diversity of the GWAS data used. To the best of our knowledge, these GWAS data are currently the largest available for IPF research. Furthermore, we ensured that there was no overlap between the population samples used in different GWAS, which adds to the reliability and validity of our findings. We implemented strict screening criteria during the IVs selection process. By following these stringent procedures and ensuring the fulfillment of key assumptions, we aimed to minimize the risk of bias and obtain reliable results in our MR analysis. These rigorous steps were essential in upholding the validity and integrity of our findings, thereby bolstering the overall robustness of our study. Colocalization analysis showed that IL-7 and IPF are likely to share the same causal variant, which strengthens the causal relationship. Of course, this result may be caused by pleiotropy [ 23 , 24 ]. But our study using cis-eQTL variants is supported by a clear and unidirectional biological principle (the central dogma) with less likelihood of other pathways, reducing potential horizontal pleiotropy [ 15 ]. In addition to IL-7, our study also identified other targets. Although they were not supported by colocalization analysis, their potential value cannot be completely denied, still providing broad possibilities for the development of IPF drugs.
There are several limitations in our study. Drug target MR only simulates the lifetime low-dose exposure of drugs under ideal conditions, and the actual situation will be more complicated due to the interference of other factors, so it cannot completely replace clinical trials and the actual efficacy of drugs is uncertain. Therefore, clinical trials remain necessary, and our study provides valuable insight and direction for the development of new drugs for IPF. Secondly, MR can only evaluate the impact of single druggable gene expression on outcome separately. However, many drugs exert their effects through the superposition of multiple targets. Thirdly, this study only included eQTL in blood, because we did not obtain appropriate eQTL data in the lung tissue. In case of unavailability of eQTL data in the lung tissue, biomarkers from the lungs will be released into the blood in the context of disease and blood serves as a valuable proxy tissue that offers a systemic perspective on disease processes. Blood carries molecular signals and cellular components from various organs and tissues, to a certain extent reflecting the dynamic interplay of systemic processes. The choice of blood has its limitations, including the dilution effect of systemic circulation and the potential masking of tissue-specific signals. Some molecular signals of the disease may not be fully revealed in blood eQTLs. Fortunately, some experiments [ 20 , 21 ] have demonstrated the anti-fibrotic effect of IL-7 in lung tissue, which makes up for this deficiency in our study. Furthermore, it is important to note that some studies have pointed out that the inhibition of TGF-β will show a variety of side effects, due to its wide range of effects [ 19 ]. And high levels of IL-7 are associated with autoimmune diseases such as rheumatoid arthritis [ 25 ], whether boosting IL-7 would have similar side effects as inhibiting TGF-β or more is not known, which may limit the application of IL-7 boosting strategy to IPF patients. Finally, the participants in the GWAS used were almost exclusively of European ancestry. This restriction may limit the generalizability of our results to other populations.
Conclusions
Drug target MR opens a new avenue for identifying potential drug targets utilizing druggable genetic data and disease GWAS data. In conclusion, through the drug target MR analysis based on the druggable genes, we have found that IL-7 holds promise as a potential target to reduce the risk of IPF in high-risk population. However, it is imperative to conduct further research to validate the effect of IL-7 in preventing IPF.
Data availability
All data used in this study are publicly available and listed in Table S1 . The cis-eQTL data were obtained from the eQTLGen Consortium ( https://www.eqtlgen.org/cis-eqtls.html ). The pQTL data was available from the INTERVAL study ( https://gwas.mrcieu.ac.uk/datasets/prot-a-1543/ ). The GWAS statistics for IPF susceptibility and progression were obtained from the International IPF Genetics Consortium ( https://github.com/genomicsITER/PFgenetics ).
Abbreviations
- Idiopathic pulmonary fibrosis
Expression quantitative trait locus
Protein quantitative trait locus
Genome wide association study
Forced vital capacity
Diffusing capacity of the lungs for carbon monoxide
Single nucleotide polymorphism
Instrumental variable
Inverse variance weighted
False discovery rate
Transforming growth factor–β
Raghu G, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2022;205(9):e18–47.
Article PubMed PubMed Central Google Scholar
Podolanczuk AJ et al. Idiopathic pulmonary fibrosis: state of the art for 2023. Eur Respir J, 2023. 61(4).
Cui F et al. Air pollutants, genetic susceptibility and risk of incident idiopathic pulmonary fibrosis. Eur Respir J, 2023. 61(2).
Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol. 2000;29(4):722–9.
Article CAS PubMed Google Scholar
Emdin CA, Khera AV, Kathiresan S. Mendelian Randomization Jama. 2017;318(19):1925–6.
Article PubMed Google Scholar
Storm CS, et al. Finding genetically-supported drug targets for Parkinson’s disease using mendelian randomization of the druggable genome. Nat Commun. 2021;12(1):7342.
Article CAS PubMed PubMed Central Google Scholar
Chen Y, et al. Genetic insights into therapeutic targets for aortic aneurysms: a mendelian randomization study. EBioMedicine. 2022;83:104199.
Gaziano L, et al. Actionable druggable genome-wide mendelian randomization identifies repurposing opportunities for COVID-19. Nat Med. 2021;27(4):668–76.
Finan C et al. The druggable genome and support for target identification and validation in drug development. Sci Transl Med, 2017. 9(383).
Võsa U, et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat Genet. 2021;53(9):1300–10.
Sun BB, et al. Genomic atlas of the human plasma proteome. Nature. 2018;558(7708):73–9.
Gkatzionis A, Burgess S, Newcombe PJ. Statistical methods for cis-mendelian randomization with two-sample summary-level data. Genet Epidemiol. 2023;47(1):3–25.
Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for mendelian randomization studies using multiple genetic variants. Int J Epidemiol. 2011;40(3):740–52.
Allen RJ, et al. Genome-wide association study across five cohorts identifies five novel loci associated with idiopathic pulmonary fibrosis. Thorax. 2022;77(8):829–33.
Allen RJ, et al. Longitudinal lung function and gas transfer in individuals with idiopathic pulmonary fibrosis: a genome-wide association study. Lancet Respir Med. 2023;11(1):65–73.
Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 2016;35(11):1880–906.
Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in mendelian randomization studies. Hum Mol Genet. 2018;27(R2):R195–208.
Giambartolomei C, et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014;10(5):e1004383.
Peng D, et al. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol Cancer. 2022;21(1):104.
Huang M, et al. IL-7 inhibits fibroblast TGF-beta production and signaling in pulmonary fibrosis. J Clin Invest. 2002;109(7):931–7.
Zhang L, et al. Interleukin-7 and transforming growth factor-beta play counter-regulatory roles in protein kinase C-delta-dependent control of fibroblast collagen synthesis in pulmonary fibrosis. J Biol Chem. 2004;279(27):28315–9.
Tachibana K, et al. Polymyxin-B hemoperfusion for acute exacerbation of idiopathic pulmonary fibrosis: serum IL-7 as a prognostic marker. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28(2):113–22.
CAS PubMed Google Scholar
Cano-Gamez E, Trynka G. From GWAS to function: using Functional Genomics to identify the mechanisms underlying Complex diseases. Front Genet. 2020;11:424.
Zuber V, et al. Combining evidence from mendelian randomization and colocalization: review and comparison of approaches. Am J Hum Genet. 2022;109(5):767–82.
Meyer A, Parmar PJ, Shahrara S. Significance of IL-7 and IL-7R in RA and autoimmunity. Autoimmun Rev. 2022;21(7):103120.
Download references
Acknowledgements
We would like to thank all members of the eQTLGen Consortium, the International IPF Genetics Consortium and the author of INTERVAL study for making the data publicly available.
No funding.
Author information
Authors and affiliations.
Department of Thoracic Surgery, West China Hospital, Sichuan University, Chengdu, 610041, China
Zetao Liu, Zhiyu Peng, Huahang Lin, Ke Zhou, Linchuan Liang, Jie Cao, Zhaokang Huang & Jiandong Mei
Western China Collaborative Innovation Center for Early Diagnosis and Multidisciplinary Therapy of Lung Cancer, Sichuan University, Chengdu, China
You can also search for this author in PubMed Google Scholar
Contributions
Z.L. and J.M. contributed to the design of this study. Z.P., H.L. and K.Z. contributed to data acquisition. L.L., J.C. and Z.H. contributed to analyze of data and draft the manuscript. All authors participated in revisions and reviewed the manuscript before submission.
Corresponding author
Correspondence to Jiandong Mei .
Ethics declarations
Ethics statements.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Liu, Z., Peng, Z., Lin, H. et al. Identifying potential drug targets for idiopathic pulmonary fibrosis: a mendelian randomization study based on the druggable genes. Respir Res 25 , 217 (2024). https://doi.org/10.1186/s12931-024-02848-5
Download citation
Received : 26 September 2023
Accepted : 13 May 2024
Published : 23 May 2024
DOI : https://doi.org/10.1186/s12931-024-02848-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Druggable genes
Respiratory Research
ISSN: 1465-993X
- General enquiries: [email protected]
- Open access
- Published: 28 May 2024
The long-term effect of elexacaftor/tezacaftor/ivacaftor on cardiorespiratory fitness in adolescent patients with cystic fibrosis: a pilot observational study
- Nela Stastna 1 , 2 ,
- Lenka Hrabovska 3 ,
- Pavel Homolka 3 ,
- Lukas Homola 4 ,
- Michal Svoboda 5 ,
- Kristian Brat 6 &
- Libor Fila 1
BMC Pulmonary Medicine volume 24 , Article number: 260 ( 2024 ) Cite this article
129 Accesses
1 Altmetric
Metrics details
Physical activity is a crucial demand on cystic fibrosis treatment management. The highest value of oxygen uptake (VO 2peak ) is an appropriate tool to evaluate the physical activity in these patients. However, there are several other valuable CPET parameters describing exercise tolerance (W peak , VO 2VT1 , VO 2VT2, VO 2 /HR peak , etc.), and helping to better understand the effect of specific treatment (V E , V T , V D /V T etc.). Limited data showed ambiguous results of this improvement after CFTR modulator treatment. Elexacaftor/tezacaftor/ivacaftor medication improves pulmonary function and quality of life, whereas its effect on CPET has yet to be sufficiently demonstrated.
We performed a single group prospective observational study of 10 adolescent patients with cystic fibrosis who completed two CPET measurements between January 2019 and February 2023. During this period, elexacaftor/tezacaftor/ivacaftor treatment was initiated in all of them. The first CPET at the baseline was followed by controlled CPET at least one year after medication commencement. We focused on interpreting the data on their influence by the novel therapy. We hypothesized improvements in cardiorespiratory fitness following treatment. We applied the Wilcoxon signed-rank test. The data were adjusted for age at the time of CPET to eliminate bias of aging in adolescent patients.
We observed significant improvement in peak workload, VO 2 peak , VO 2VT1 , VO 2VT2 , V E /VCO 2 slope, V E , V T , RQ, VO 2 /HR peak and RR peak. The mean change in VO 2 peak was 5.7 mL/kg/min, or 15.9% of the reference value (SD ± 16.6; p = 0.014). VO 2VT1 improved by 15% of the reference value (SD ± 0.1; p = 0.014), VO 2VT2 improved by 0.5 (SD ± 0.4; p = 0.01). There were no differences in other parameters.
Exercise tolerance improved after elexacaftor/tezacaftor/ivacaftor treatment initiation. We suggest that the CFTR modulator alone is not enough for recovering physical decondition, but should be supplemented with physical activity and respiratory physiotherapy. Further studies are needed to examine the effect of CFTR modulators and physical therapy on cardiopulmonary exercise tolerance.
Peer Review reports
Cystic fibrosis (CF) is the genetic disorder affecting the lungs worldwide. There is substantial heterogeneity of clinical manifestation in patients with CF. Cystic fibrosis transmembrane conductance regulator (CFTR) mutation results in the development of bronchiectasis, recurrent infectious exacerbations and lung function decline. Thus, patients moreover have increased dead space (V D ), which is why the alveolar ventilation is lower compared to healthy subjects. During exercise in CF patients, tidal volume (V T ) increases inadequately, the respiratory rate (RR) then increases to heighten minute ventilation (V E ) and reach an adequate oxygen uptake (VO 2 ). Response to physical activity is inefficient [ 1 , 2 ]. Muscle function and muscle mass, the same as cardiac abnormalities, are mostly limitations in mild or moderate CF, patients with severe lung disease, oxygen delivery and non-physiological respiratory mechanics limit exercise capacity [ 1 , 3 , 4 , 5 , 6 , 7 ].
Cardiopulmonary exercise testing (CPET) measures aerobic exercise capacity and provides a comprehensive assessment of respiratory, cardiac and musculoskeletal function during exercise and recovery and may be used for prognosis and risk assessment [ 8 ]. Not just VO 2 peak is a predictor of survival, but also peak workload (W peak), V E /VO 2 peak (ventilatory equivalent for oxygen, EQO 2 ) and V E /VCO 2 peak (ventilatory equivalent for carbon dioxide, EQCO 2 ) may be significant [ 8 ].
Ivacaftor and lumacaftor/ivacaftor combination had no effect on VO 2 peak, only sets of case reports in tezacaftor/ivacaftor demonstrated minor improvements [ 9 , 10 , 11 , 12 , 13 ]. The novel triple combination of the modulators of CFTR channel, elexacaftor/tezacaftor/ivacaftor (ETI) provides substantial clinical improvement and prolonged median predicted survival, improves lung functions (ppFEV 1 increases by 14.3%); also promotes over-nutrition and overweight, which might affect the physical activity attitude otherwise [ 14 , 15 ]. The exact quantitative evaluation is still not well known because there is not enough documented evidence of CPET-derived measures of triple combination. The data published so far are not sufficiently convincing. ETI seem to improve VO 2 peak, but still lack more detailed data about the physical tolerability mechanism [ 16 ]. By performing this trial, we try to fill the gap in the knowledge of additional CPET parameters, than just VO 2 peak. These are important tools to evaluate the effect of the new treatment on prognosis and survival.
We hypothesize that patients with CF will improve their physical activity by several adaption mechanisms, e.g. higher myocardial contractility and cardiac output (HR, higher AT which correlate with decreasing values of RQ and V E /VO 2 ), more effective gas exchange (increased VO 2 peak and VCO 2 ), lower work of breathing (improved V E due to increased V T , RR and decreased V D /V T ), and lower hyperventilation following elexacaftor/tezacaftor/ivacaftor use. Also, the aim of this study is to define other CPET parameters suitable for evaluating the cardiorespiratory demands in cystic fibrosis.
This is a prospective observational, non-randomised study. Inclusion criteria comprised a diagnosis of CF based on current guidelines, EMA-approved genotype for ETI indication and informed consent provided by the patient or their legal representative. All the patients were ETI treatment-naïve. For this study, we analysed data of patients with CF aged 8–19 years at the time of the first testing, who had a full CPET meeting between January 23, 2019, and February 9, 2023. The follow-up measurement was performed at least 12 months after ETI commencement (Kaftrio ®, elexacaftor 100 mg, tezacaftor 50 mg, ivacaftor 75 mg; always used in combination with Kalydeko ®, ivacaftor 150 mg). This was the only intervention made; patients haven’t engaged in standardized exercise training.
All the testing was performed during a clinically stable period. We used an exhaustive ramp incremental (10-25W/min) cycling CPET (Ergoline, Ergoselect, Bitz, Germany) protocol. We selected the ramp protocol based on the patient’s physical activity level, body weight and sex. After a 3-min warm-up (10-40W), all the participants completed a test to the point of exhaustion. The protocol was tailored to the individual to yield a fatigue-limited exercise duration of 8–12 min. A five-minute active cool down period followed CPET. Breath-by-breath analysis was provided, and the O 2 and CO 2 concentrations of exhaled air with ventilatory volume was measured via face mask with connected gas and flow spirometer sensors. The stress test was performed on the ergometer ERGOLINE, and the exhaled gases was analysed by POWER CUBE – Schiller (Switzerland). A ramp protocol was used while VO 2 , VO 2VT1 , VO 2VT2 , V E /VCO 2 , V E /VO 2 , V E /VCO 2 slope, V E , V T , RQ, VO 2 /HR, RR parameters were measured every 10 s, and peak values taken as the highest 15 s achieved during the test. Blood pressure and Sp0 2 were measured during CPET monitoring.
We evaluated anthropometric parameters: height (cm), weight (kg). Our outcome was to evaluate respiratory CPET-derived parameters: maximal workload (W, W/kg, % ref.), RQ max., VO 2 peak (L, mL/kg/min, % ref.,), VO 2VT1 (L/min, % ref.), VO 2VT2 , (L/min), V E /VCO 2 slope, V E (L/min, % ref.), V T (L, % ref.), V D /V T in rest and in maximal effort, RR (min −1 ), VO 2 /HR (ml/beats per minute).
Statistical methods
Numerical parameters are described by mean (standard deviation = SD). Change during two times is tested by paired Wilcoxon signed-rank test. All tests are two-sided on level of significance 5%. Analysis was prepared in the software R (v4.2) (Bell Laboratories, Inc., Windsor, WI, US).
Thirteen patients were eligible, but only 10 patients with CF met the criteria for ETI therapy and were included in the final analysis. The mean age was 14 years, mean ppFEV 1 89.4%, 70% of the patients with CF were F508del homozygotes. Baseline patients’ characteristics are presented in Table 1 .
We observed significant improvements in VO 2 peak , the mean change was 0.8 L (SD ± 0.6; p = 0.002), 15.9% of the reference value (SD ± 16.6; p = 0.014). As well as VO 2VT1 improved by 15% of the reference value (SD ± 0.1; p = 0.014) and VO 2VT2 improved by 0.5 (SD ± 0.4; p = 0.01). Patients achieved also better VO 2VT2 , VE/VCO 2 slope, V E , V T , RQ, VO 2 /HR peak , and RR peak values. Even V D /V T marker improved. Only RQ remained unchanged.
Complete data are presented in Table 2 . The parameters were recalculated based on age and current weight (% ref.), so the impact of aging was eliminated.
During controlled CPET, all the patients indicated the fatigue of lower extremities as their reason for stopping. We did not observe any exercise-induced arrythmia.
In this study, we report improvement in most of the parameters, which are valuable predictors of death or lung transplant in CF (VO 2 peak, max. effort, peak work rate, V E /VCO 2 slope) and parameters valuable to understand the ventilatory efficiency (VO 2VT1 , VO 2VT2 , V E , V T , V D /V T VO 2 /HR peak and RR peak) [ 8 ]. An abnormally low exercise capacity and deconditioning in CF results in VO 2 peak < 82% predicted and/or peak workload < 93% predicted, VO 2VT1 occurring < 50% predicted VO 2 peak [ 17 ]. Patients in this cohort achieved improvement in two of these parameters, beyond deconditioning. This might suggest improved prognosis in patients with CF treated with ETI for at least one year.
These data provided on triple combination of CFTR modulators therapy are higher than those achieved on double combination (lumacaftor/ivacaftor or tezacaftor/ivacaftor) in Danish patients with CF followed for the same period of use (VO 2 peak 1.07 mL/min/kg, maximal workload change 14.2W) [ 18 ]. Therefore, ETI might be more effective in improving CPET-derived parameters, but improvement is likely multifactorial, and further investigation in a larger patient cohort is necessary.
Older patients with CF aged > 40 years deal with specific comorbidities, while younger patients with CF are healthier than ever due to the variant treatment strategies. Still, physical fitness in CF takes an indisputable position to effect quality of life and prognosis. Medication which reduces the amount of mucus in the respiratory tract and improve pulmonary function could not be the only reason for improved exercise tolerance. Patients eligible into our study were mostly teenagers; the maximal VO 2 peak increased significantly in absolute but also in relative values, leaving minimal doubts about the role of ageing bias over the study period [ 17 ]. Other CPET variables, VO 2VT1 and VO 2VT2 , demonstrate improved aerobic capacity, physical fitness, and more effective training to the maximal effort. Improvement of V D /V T suggests more effective ventilation and decreased V/Q mismatch, as well as improved lung function in general. This would also hint at statistically significant improvement in VO 2 /HR.
In this cohort, the mean ppFEV 1 is 89.4%; research suggests that in mild lung disease, the respiratory limitation of exercise capacity is rather low [ 13 ]. Pulmonary function improvement alone in CFTR modulator users would then be unlikely to change the exercise tolerance. The impact of ETI on the exercise tolerance improvement is hypothesized by several mechanisms [ 19 ]. CFTR protein is expressed in myocardial cells, vascular smooth muscle cells, and sarcolemma and sarcoplasm of skeletal muscle cells [ 20 , 21 , 22 ]. Impaired CFTR function results in local vasoconstriction and affects nitric oxide production [ 23 ].Therefore, the CFTR modulators might improve reduced peripheral O 2 extraction during exercise and utilisation of O 2 not only by skeletal muscle, but it is also suggested, by reduced V E / VO 2 peak [ 16 ]. CFTR protein is hypothesized to be involved in regulating mitochondrial oxidative stress and mitochondrial function in adenosine triphosphate production [ 24 , 25 ].
Decreased systemic inflammation by reducing several interleukins and pro-inflammatory mediators after CFTR modulator use affects cardiorespiratory fitness. Inter alia, chronic inflammation (especially Pseudomonas aeruginosa airway infection) relates to impaired aerobic capacity [ 26 , 27 ]. Systemic inflammation is demonstrated to lead to muscle atrophy and impaired contractility [ 28 ].
To examine body composition change, it is necessary to distinguish the mechanism of VO 2 peak change. The pattern of weight gain (whether muscle or fat) due to triple therapy must be studied. This is because increased adiposity has been suggested to contribute to the decreased VO 2 outcome [ 19 ]. Even in this cohort, the patient who gained the most weight (+ 20.4 kg), where BMI increased from 21.86 kg/m 2 to 28.38 kg/m 2 , reported the highest VO 2 peak decrease (41.9 mL/kg/min to 35.2 mL/kg/min). Last but not least, it is necessary to consider the change in the mental state of patients on triple therapy and the awareness of new life horizons and possibilities, along with the awareness of the need for more intensive care for overall fitness.
There are several limitations of this study. First, the baseline testing was performed during the COVID-19 pandemic era, so we were not able to recruit more patients in this trial. Even a change in the patient’s physical activity manners during the pandemic era changed to a more sedentary style, which is difficult to quantify. Second, we did not perform a capillary blood gas analysis, and we were therefore not able to assess the V/Q mismatch. Third, the increase in absolute values of certain parameters (e.g. VO 2 peak) might partly be attributed to the given adolescent’s growth. However, there were significant improvements also in relative values (% of predicted), therefore aging bias (if any) appears to be limited. Comparative trials with a cohort of same-aged CF patients ineligible for ETI would reveal other perspectives, and the risk of a worsened overall status due to severe CFTR pathogenic variants could bias the results.
Conclusions
We demonstrated improvements in cardiorespiratory fitness in adolescent patients with cystic fibrosis following at least one year of ETI therapy by performing controlled CPET testing. CFTR modulator treatment alone might not be effective in transforming all the mechanisms of exercise intolerance. Understanding the impact of new therapeutical strategies in cystic fibrosis is important for better therapeutical evaluation and survival assessment. Further comparative trials with a larger cohort need to be performed to streamline our results.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
Anaerobic threshold
Body mass index
Cystic fibrosis transmembrane conductance regulator
- Cystic fibrosis
Carbon dioxide
Coronavirus disease of 2019
- Cardiopulmonary exercise testing
Ventilatory equivalent for oxygen
Ventilatory equivalent for carbon dioxide
- Elexacaftor/tezacaftor/ivacaftor
Probability value
Percent predicted forced expiratory volume in one second
Revolution per minute
Respiratory quotient
Respiratory rate
Standard deviation
Ventilation/perfusion
Carbon dioxide elimination
Minute ventilation
Tidal volume
Oxygen uptake
Oxygen uptake on the first ventilatory threshold
Oxygen uptake on the second ventilatory threshold
Cerny FJ, Pullano TP, Cropp GJ. Cardiorespiratory adaptations to exercise in cystic fibrosis. Am Rev Respir Dis. 1982;126(2):217–20.
CAS PubMed Google Scholar
Thin AG, Dodd JD, Gallagher CG, Fitzgerald MX, Mcloughlin P. Effect of respiratory rate on airway deadspace ventilation during exercise in cystic fibrosis. Respir Med. 2004;98(11):1063–70.
Article CAS PubMed Google Scholar
Regnis JA, Donnelly PM, Robinson M, Alison JA, Bye PT. Ventilatory mechanics at rest and during exercise in patients with cystic fibrosis. Am J Respir Crit Care Med. 1996;154(5):1418–25.
Nixon PA, Joswiak ML, Fricker FJ. A six-minute walk test for assessing exercise tolerance in severely ill children. J Pediatr. 1996;129(3):362–6.
Pastré J, Prévotat A, Tardif C, Langlois C, Duhamel A, Wallaert B. Determinants of exercise capacity in cystic fibrosis patients with mild-to-moderate lung disease. BMC Pulm Med. 2014;14:74.
Article PubMed PubMed Central Google Scholar
Pianosi P, Pelech A. Stroke volume during exercise in cystic fibrosis. Am J Respir Crit Care Med. 1996;153(3):1105–9.
Szollosi I, King SJ, Wilson JW, Naughton MT. Tachycardia in adults with cystic fibrosis is associated with normal autonomic function. Intern Med J. 2011;41(6):455–61.
Hebestreit H, Hulzebos EHJ, Schneiderman JE, Karila C, Boas SR, Kriemler S, et al. Cardiopulmonary exercise testing provides additional prognostic information in cystic fibrosis. Am J Respir Crit Care Med. 2019;199(8):987–95.
Article PubMed Google Scholar
Edgeworth D, Keating D, Ellis M, Button B, Williams E, Clark D, et al. Improvement in exercise duration, lung function and well-being in G551D-cystic fibrosis patients: a double-blind, placebo-controlled, randomized, cross-over study with ivacaftor treatment. Clin Sci (Lond). 2017;131(15):2037–45.
Wilson J, You X, Ellis M, Urquhart DS, Jha L, Duncan M, et al. VO2max as an exercise tolerance endpoint in people with cystic fibrosis: lessons from a lumacaftor/ivacaftor trial. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2021;20(3):499–505.
Article CAS Google Scholar
Saynor ZL, Barker AR, Oades PJ, Williams CA. The effect of ivacaftor in adolescents with cystic fibrosis (G551D mutation): an exercise physiology perspective. Pediatr Phys Ther Off Publ Sect Pediatr Am Phys Ther Assoc. 2014;26(4):454–61.
Google Scholar
Savi D, Schiavetto S, Simmonds NJ, Righelli D, Palange P. Effects of Lumacaftor/Ivacaftor on physical activity and exercise tolerance in three adults with cystic fibrosis. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2019;18(3):420–4.
Ahmed MI, Dayman N, Madge J, Gaillard E. P91 Cardiopulmonary exercise testing in CF adolescents after starting Tezacaftor/Ivacaftor. Thorax. 2021;76(Suppl 1):A136–A136.
Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381(19):1809–19.
Article CAS PubMed PubMed Central Google Scholar
Snowball JE, Flight WG, Heath L, Koutoukidis DA. A paradigm shift in cystic fibrosis nutritional care: clinicians’ views on the management of patients with overweight and obesity. J Cyst Fibros. 2023;0(0). https://doi.org/10.1016/j.jcf.2023.03.011 .
Causer AJ, Shute JK, Cummings MH, Shepherd AI, Wallbanks SR, Pulsford RM, et al. Elexacaftor-Tezacaftor-Ivacaftor improves exercise capacity in adolescents with cystic fibrosis. Pediatr Pulmonol. 2022;57(11):2652–8.
Hebestreit H, Arets HGM, Aurora P, Boas S, Cerny F, Hulzebos EHJ, et al. Statement on exercise testing in cystic fibrosis. Respir Int Rev Thorac Dis. 2015;90(4):332–51.
Rysgaard UK, Pedersen CL, Jensen JH, Sørensen L, Philipsen LKD, Leo-Hansen C, et al. Change in exercise capacity measured by Cardio-pulmonary Exercise Testing (CPET) in Danish people with cystic fibrosis after initiation of treatment with Lumacaftor/Ivacaftor and Tezacaftor/Ivacaftor. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2022;21(5):844–9.
Caterini JE, Ratjen F, Barker AR, Williams CA, Rendall K, Schneiderman JE, et al. Exercise intolerance in cystic fibrosis-the role of CFTR modulator therapies. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2022;21(2):282–92.
Warth JD, Collier ML, Hart P, Geary Y, Gelband CH, Chapman T, et al. CFTR chloride channels in human and simian heart. Cardiovasc Res. 1996;31(4):615–24.
Robert R, Norez C, Becq F. Disruption of CFTR chloride channel alters mechanical properties and cAMP-dependent Cl- transport of mouse aortic smooth muscle cells. J Physiol. 2005;568(Pt 2):483–95.
Lamhonwah AM, Bear CE, Huan LJ, Kim Chiaw P, Ackerley CA, Tein I. Cystic fibrosis transmembrane conductance regulator in human muscle: Dysfunction causes abnormal metabolic recovery in exercise. Ann Neurol. 2010;67(6):802–8.
Malik FA, Meissner A, Semenkov I, Molinski S, Pasyk S, Ahmadi S, et al. Sphingosine-1-phosphate is a novel regulator of cystic fibrosis transmembrane conductance regulator (CFTR) activity. PLoS One . 2015;10(6):e0130313.
Madácsy T, Pallagi P, Maleth J. Cystic fibrosis of the pancreas: the role of CFTR channel in the regulation of intracellular Ca2+ signaling and mitochondrial function in the exocrine pancreas. Front Physiol. 2018;9:1585.
Velsor LW, Kariya C, Kachadourian R, Day BJ. Mitochondrial oxidative stress in the lungs of cystic fibrosis transmembrane conductance regulator protein mutant mice. Am J Respir Cell Mol Biol. 2006;35(5):579–86.
van de Weert-van Leeuwen PB, Slieker MG, Hulzebos HJ, Kruitwagen CLJJ, van der Ent CK, Arets HGM. Chronic infection and inflammation affect exercise capacity in cystic fibrosis. Eur Respir J. 2012;39(4):893–8.
Casey M, Gabillard-Lefort C, McElvaney OF, McElvaney OJ, Carroll T, Heeney RC, et al. Effect of elexacaftor/tezacaftor/ivacaftor on airway and systemic inflammation in cystic fibrosis. Thorax. 2023;78(8):835–9.
Divangahi M, Balghi H, Danialou G, Comtois AS, Demoule A, Ernest S, et al. Lack of CFTR in skeletal muscle predisposes to muscle wasting and diaphragm muscle pump failure in cystic fibrosis mice. PLoS Genet. 2009;5(7):e1000586.
Download references
Acknowledgements
We appreciate the willingness of all patients and medical staff to participate in clinical trials and their processing.
Publication will be supported by the Czech Pulmonological and Phthisiological Society (open access publication free grant).
Author information
Authors and affiliations.
Department of Pulmonology, Second Faculty of Medicine, Charles University and Motol University Hospital, Prague, Czech Republic
Nela Stastna & Libor Fila
Faculty of Medicine, Masaryk University, Brno, Czech Republic
Nela Stastna
Department of Sports Medicine and Rehabilitation, St. Anne’s University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic
Lenka Hrabovska & Pavel Homolka
Department of Paediatric Infectious Diseases, University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic
Lukas Homola
Institute of Biostatistics and Analyses Ltd. and Faculty of Medicine, Masaryk University, Brno, Czech Republic
Michal Svoboda
Department of Pulmonology, University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic
Kristian Brat
You can also search for this author in PubMed Google Scholar
Contributions
N.S.: writing – original draft, methodology, visualization, formal analysis, data curation, L.H.: supervision, methodology, P.H.: writing – original draft, methodology, formal analysis, data curation, L.H.: methodology data curation, M.S.: data curation, formal analysis, K.B.: supervision, writing – review, editing, L.F.: supervision, writing – review, editing.
Corresponding author
Correspondence to Nela Stastna .
Ethics declarations
Ethics approval and consent to participate.
Approved by University Hospital Brno committee.
Informed consent was obtained from all subject or their legal guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Stastna, N., Hrabovska, L., Homolka, P. et al. The long-term effect of elexacaftor/tezacaftor/ivacaftor on cardiorespiratory fitness in adolescent patients with cystic fibrosis: a pilot observational study. BMC Pulm Med 24 , 260 (2024). https://doi.org/10.1186/s12890-024-03069-8
Download citation
Received : 19 March 2024
Accepted : 21 May 2024
Published : 28 May 2024
DOI : https://doi.org/10.1186/s12890-024-03069-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
BMC Pulmonary Medicine
ISSN: 1471-2466
- Submission enquiries: [email protected]
- General enquiries: [email protected]

- News Releases
From fibrosis and cancer to obesity, Alzheimer’s and aging: New paper reveals broad potential of TNIK as a therapeutic target
InSilico Medicine
Research reveals that TNIK has broad potential as a therapeutic target for some of the most pervasive aging-related diseases, including fibrosis, cancer, obesity, and Alzheimer’s. The findings could guide the development of new therapeutics. The lead drug in Insilico’s pipeline, INS018_055, is an AI-designed TNIK inhibitor being advanced as a treatment for the deadly lung disease idiopathic pulmonary fibrosis (IPF) and is currently in Phase II trials with patients.
Credit: Insilico Medicine
A new paper in Trends in Pharmacological Sciences from researchers at generative artificial intelligence (AI)- and robotics-powered clinical stage drug discovery company Insilico Medicine (“Insilico”) and ETH Zurich reveals the broad potential of TNIK as a therapeutic target for some of the most pervasive aging-related diseases, including fibrosis, cancer, obesity, and Alzheimer’s. The findings could guide the development of new therapeutics. The lead drug in Insilico’s pipeline, INS018_055, is an AI-designed TNIK inhibitor being advanced as a treatment for the deadly lung disease idiopathic pulmonary fibrosis (IPF) and is currently in Phase II trials with patients.
TNIK, a member of the germinal center kinase (GCK) family, has been found to play a significant role in biological processes relevant to disease states, including cell migration, cytoskeletal organization, and cell proliferation in malignant and healthy cells.
TNIK is a reasonably novel but trending therapeutic target with around 150 papers referencing it on PubMed – most of them published since 2020. Few papers refer to it as a therapeutic target.
In cancer, TNIK has been found to drive cancer cell proliferation, treatment resistance, and cell migration across multiple cancer types – including colorectal, ovarian, thyroid, osteosarcoma, and lung squamous cell carcinoma (LSCC) – due to its activation of the Wnt signaling pathway. Aberrant Wnt signaling enhances T-cell factor 4 (TCF4) transcription factor activity and transcription of TCF4 target genes, promoting cancer cell growth and resistance to standard-of-care treatments. Targeting TNIK via an inhibitor has been shown to block these treatment-resistant properties.
“TNIK amplification is observed in a number of human cancers and has been shown to affect solid tumor progression,” says paper author Frank Pun, PhD, Head of Insilico Hong Kong. “We see great opportunity for exploring TNIK inhibition – particularly in conjunction with other cancer agents – to improve therapeutic efficacy.”
Researchers also looked at TNIK’s role as a metabolic controller – controlling the change of dietary sugars into lipids – which could lead to TNIK inhibitors designed to combat obesity and Type 2 diabetes. The researchers speculate that TNIK could be the previously unknown regulator of DHAP-mTORC1 signaling. The protein complex mTORC1, or mammalian target of rapamycin complex 1, is the “canonical nutrient and energy sensor” the authors write, and its activation by DHAP suggests that TNIK may indirectly regulate its ability to sense glucose and coordinate de novo lipogenesis, or the metabolic formation of fat, making it a promising target for obesity. They note that when TNIK knockout (KO) mice were placed on a high-fat, high-sugar diet, the animals were resistant to diet-induced weight gain. TNIK inhibition may even contribute to increased physical activity, as the TNIK KO mice also exhibited increased activity.
The paper also considered TNIK’s emerging role in neurodegeneration. While research in this space is still in the early stages, there is a known association between neurotransmission and metabolic homeostasis, and several studies have shown TNIK’s role in regulating neuronal function, including axon guidance and cell migration. There’s also evidence that TNIK interacts with Tau protein, a pathogenic protein that accumulates in the brain of Alzheimer’s patients, which damages neuronal signaling and neuronal repair.
The TNIK-Aging Association
To understand why TNIK plays a critical role in so many diseases, the researchers point to its association with several major hallmarks of aging – in particular, chronic inflammation, deregulated nutrient sensing, cellular senescence, and altered intercellular communication.
“There is a need for high-quality targeted drugs that may address a very broad spectrum of diseases. And initiatives like the Inflation Reduction Act (IRA) provide additional incentives to go after more novel therapeutics working in multiple biological processes that can be tested in and purposed toward a broad range of diseases and have substantial combination potential,” says Alex Zhavoronkov, PhD, founder and CEO of Insilico Medicine. “There are numerous examples of such therapeutics in history including aspirin and rapamycin that are very inexpensive and the new wave of comparatively safe and commercially-viable drugs targeting GLP-1, a high value therapeutic target discovered almost 40 years ago, where the breadth of therapeutic potential is still being explored. We wanted to find similar-class targets and drugs with broad therapeutic potential by looking at multiple conserved biological processes at the same time and prioritizing the data types that change significantly during aging and are implicated in age-related diseases."
Insilico researchers first identified TNIK as a therapeutic target utilizing a combination of multiple computational target discovery approaches while studying aging and fibrosis. In 2022, the Company published the hallmarks of aging assessment of multiple therapeutic targets ranked by novelty, confidence and druggability, showing that TNIK is implicated in multiple biological processes associated with aging-related diseases.
“In 2024, we published a very important experimental paper showing several years of preclinical and clinical work on this target. In this latest paper, we are able to draw connections between aging, TNIK, and a number of diseases, including cancer, obesity and Alzheimer’s,” Zhavoronkov says. “This adds to our already extensive understanding of the kinase’s role in fibrosis and opens the possibility for many more treatments focused on this target.”
Fibrosis, a common aging-related condition, is known to induce cellular senescence, in which cells stop dividing over time. In the case of IPF, an often fatal disease involving progressive scarring of the lungs, TNIK has been identified as a crucial profibrotic and proinflammatory agonist. Insilico’s AI-designed novel inhibitor for IPF, INS018_055, meanwhile, has been shown to dramatically improve pulmonary function in rat and mice models. The drug is now in Phase II clinical trials with patients. As this AI drug progresses, it holds promise not only for IPF patients who have limited treatment options, but also potential in other aging-related disease indications.
Insilico Medicine is a pioneer in using generative AI for drug discovery and development. The Company first described the concept of using generative AI for the design of novel molecules in a peer-reviewed journal in 2016. Insilico then developed and validated multiple approaches and features for its generative adversarial network (GAN)-based AI platform and integrated those algorithms into the commercially available Pharma.AI platform, which includes generative biology, chemistry, and medicine and has been used to produce a robust pipeline of promising therapeutic assets in multiple disease areas, including fibrosis, cancer, immunology and aging-related disease, a number of which have been licensed. Since 2021, Insilico has nominated 18 preclinical candidates in its comprehensive portfolio of over 30 assets and has advanced seven pipelines to the clinical stage.
About Insilico Medicine Insilico Medicine, a global clinical-stage biotechnology company powered by generative AI, connects biology, chemistry, and clinical trial analysis using next-generation AI systems. The company has developed AI platforms that utilize deep generative models, reinforcement learning, transformers, and other modern machine learning techniques for novel target discovery and generating novel molecular structures with desired properties. Insilico Medicine is developing breakthrough solutions to discover and develop innovative drugs for cancer, fibrosis, immunity, central nervous system diseases, infectious diseases, autoimmune diseases, and aging-related diseases. www.insilico.com
Trends in Pharmacological Sciences
10.1016/j.tips.2024.04.010
Article Title
TNIK’s emerging role in cancer, metabolism, and age-related diseases
Article Publication Date
21-May-2024
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system.
Translations
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Springer Nature - PMC COVID-19 Collection

Pulmonary Fibrosis
David a. zisman.
4 Division of Pulmonary and Critical Care Medicine, David Geffen School of Medicine at University of California, Los Angleles, CA
Michael P. Keane
John a. belperio, robert m. strieter.
5 Division of Pulmonary and Critical Care Medicine, Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of Callifornia, Los Angeles, CA
Joseph P. Lynch, III
Idiopathic pulmonary fibrosis (IPF) is a chronic fibrosing lung disease limited to the lungs and associated with the histologic appearance of usual interstitial pneumonia (UIP) on surgical lung biopsy. The estimated prevalence in the United States is between 35,000 and 55,000 cases, and evidence suggests that the prevalence is increasing for IPF. Risk factors associated with pulmonary fibrosis include smoking, environmental exposures, gastroesophageal reflux disease, commonly prescribed drugs, diabetes mellitus, infectious agents, and genetic factors. The diagnosis requires a careful history and physical examination, characteristic physiological and radiological studies, and, in some cases, a surgical lung biopsy. The natural history of IPF is not known, but evidence supports the concept of a continuum of idiopathic interstitial pneumonias that may overlap in time. Most patients with IPF succumb to respiratory failure, cardiovascular disease, lung cancer, pulmonary embolism, infection, and other health problems. The median survival time for patients with IPF is less than 3 yr. Factors that predict poor outcome include older age, male gender, severe dyspnea, history of cigarette smoking, severe loss of lung function, appearance and severity of fibrosis on radiological studies, lack of response to therapy, and prominent fibroblastic foci on histopathologic evaluation. Conventional therapy (corticosteroids, azathioprine, cyclophosphamide) provides only marginal benefit. Lung transplantation should be considered for patients with IPF refractory to medical therapy. In light of the poor prognosis and lack of response to available anti-inflammatory therapy, alternative approaches to therapy are being pursued. Emerging strategies to treat patients with IPF include agents that inhibit epithelial injury or enhance repair, anticytokine approaches, agents that inhibit fibroblast proliferation or induce fibroblast apoptosis, and other novel approaches.
Introduction
The diagnosis and management of idiopathic interstitial pneumonias (IIPs) remains a challenge to the clinician. Recently, there have been substantial changes in our understanding and approach to theses diseases. With greater comprehension of the clinical relevance of the different histopathological subgroups that make up the idiopathic interstitial pneumonias, the term idiopathic pulmonary fibrosis (IPF) is now reserved to patients with idiopathic usual interstitial pneumonia (UIP) on surgical lung biopsy. The following review will provide an updated discussion of the epidemiology, risk factors, diagnosis, natural history, morbidity and mortality, prognosis, and conventional therapy of IPF, as well as emerging strategies for the treatment of patients with this disease.
Epidemiology
The true prevalence of IPF, also known as cryptogenic fibrosing alveolitis (CFA), is unknown. Despite the poor quality of the data and the changes in diagnostic criteria and classification, there is evidence suggesting that IPF is increasing ( 1 ). There are approx 3 to 20.2 cases of IPF per 100,000 in the general population ( 1 – 5 ). The prevalence increases with older age, history of smoking, and male gender ( 3 , 4 , 6 ). In a population-based study in Bernalillo County, New Mexico, the prevalence for adults from age 35 to 44 yr was 2.7 per 100,000, but surpassed 175 per 100,000 for individuals older than 75 yr ( 3 ). The prevalence of IPF is higher in men (20.2 cases per 100,000) than in women (13.2 cases per 100,000) ( 3 ). The estimated prevalence in the United States is between 35,000 and 55,000 cases ( 3 ).
The incidence of IPF is estimated at 10.7 cases per 100,000 per year for males and 7.4 cases per 100,000 per year for females ( 3 ).
Vital statistics figures are limited and incomplete. In 1988, there were 30,000 hospitalizations and 4851 deaths in the United States owing to pulmonary fibrosis (compared with 665,000 hospitalizations owing to chronic obstructive pulmonary disease and asthma). In Japan, the mortality rate for IPF per 100,000 population was estimated to be 3.3 in men and 2.5 in women, with an overall rate of 3.0 in both sexes ( 4 ). In the United Kingdom, the annual number of deaths from IPF increased twofold between 1979 and 1988. The age-adjusted rate of pulmonary fibrosis among deceased in the United States increased from 48.6 per 100,000 in 1979 to 50.9 per 100,000 in 1991 in males, and from 21.4 per 100,000 in 1979 to 27.2 per 100,000 in 1991 in females ( 7 ). Pulmonary fibrosis listed as a cause of death increased from 40% in 1979 to 56% in 1991. In the United States, the age-adjusted mortality rates are highest in the western and southwestern states and lowest in the midwest and northeast. In the United Kingdom, highest mortality rates are found in industrialized areas of England and Wales ( 7 , 8 ).
Risk Factors
In case-control studies, smoking has been identified as a possible risk factor for IPF with an odds ratio (OR) of 1.6 (95% confidence interval [CI] 1.1–2.4) for ever-smoking, and 1.9 (95% CI 1.3–2.9) for former-smokers ( 4 , 9 , 10 ). Smokers of 21 to 40 pack-years have an OR of 2.3 (95% CI 1.3–3.8) for pulmonary fibrosis ( 9 ). In a recent study in Japan, the adjusted odds ratio for cigarette smoking was estimated at 5.40 (95% CI 2.30–12.66) ( 11 ). Interestingly, three studies reported improved survival among current or former smokers with UIP compared to never-smokers ( 12 – 14 ). However, others found no such effect ( 15 – 17 ). It is possible that the apparent protective effect of cigarette smoking may relate to the following: lead time bias; alterations in the balance of proteinases and antiproteinases that would influence net deposition of extracellular matrix in the lung; or inhibitory effects of cigarette smoke on lung fibroblast proliferation and chemotaxis ( 12 , 18 ).
Gastroesophageal Reflux
Instillation of acid in several animal models results in aspiration-induced lung injury and pulmonary fibrosis ( 19 ). Clinical data suggest that a high percentage of patients with IPF have clinically silent gastroesophageal reflux disease (GERD) ( 19 , 20 ). Small tracheobronchial aspirations of gastric acid may play a role in the pathogenesis of IPF; however, a causal relationship has not been established ( 19 , 20 ). Investigators at the University of Washington Medical Center are conducting an ongoing prospective study of 65 patients with IPF. Interim results indicate that the prevalence of IPF patients with GERD is 95%, with only 40% of patients reporting symptoms ( 19 ). However, the perpherial pattern of UIP in the lung would be unusual if GERD was truly the cause, as compared with an association.
Commonly Prescribed Drugs
In one case-control study, IPF was associated with exposure to antidepressants with an OR of 1.79 (95% CI 1.09–2.95), and specifically to imipramine (OR of 4.79 [95% CI 1.50–15.3]), dothiepin OR of 2.37 [95% CI 0.99–5.69]), and mianserin OR of 3.27 [95% CI 1.11–9.61]). These associations were independent of smoking and occupational dust exposure. The authors concluded that exposure to antidepressants may be responsible for approx 10% of cases of IPF seen in their population. No significant association was noted between IPF and the other drug groups tested (anticonvulsants, β-blockers, antibiotics, and nonsteroidal anti-inflammatory drugs [NSAIDs]) ( 21 ).
Diabetes Mellitus
In a recent study, clinical and demographic data were extracted from medical records of 65 consecutive patients with IPF admitted to a Japanese hospital. IPF was associated with diabetes mellitus (DM) with and OR of 4.06 (95% CI 1.80–9.15). The authors concluded that DM might be a risk factor for IPF ( 11 ).
Environmental Exposures
The etiology of IPF is unknown, but environmental factors may play a causative role. Metal and wood dust environments may be important risk factors for pulmonary fibrosis. In one study, metal dust exposure was identified as a risk factor with an odds ratio (OR) of 1.11 (95% CI 1.06–1.16), and wood dust exposure with an OR of 1.12 (95% CI 1.02–1.24). In that study, metal and wood dust exposure may have caused up to 13% and 10% of pulmonary fibrosis cases, respectively. Dust containing brass, lead, cobalt, aluminum, zinc, cadmium, mercury, and pine dusts were associated with pulmonary fibrosis ( 10 ).
Certain occupations may predispose to pulmonary fibrosis. In one study, farming was identified as a potential risk factor with and OR of 1.6 (95% CI 1.0–2.5) and exposure to livestock was associated with an OR of 2.7 (95% CI 1.3–5.5). Hairdressing was linked to pulmonary fibrosis with an OR of 4.4 (95% CI 1.2–16.3), metal dust exposure with an OR of 2.0 (95% CI 1.0–4.0), raising birds with an OR of 4.5 (95% CI 1.6–14.1), stone cutting/polishing with an OR of 3.9 (95% CI 1.2–12.7), and vegetable/animal dust exposure with an OR of 4.7 (95% CI 2.1–10.4) ( 22 ).
Infectious Agents
A number of viruses have been associated with pulmonary fibrosis, but true cause-effect relationships remain unproven. A serological survey found an association between active Epstein-Barr virus (EBV) infection and IPF ( 23 ). Egan and colleagues reported immunohistochemical evidence of EBV-productive cycle antigens in type II alveolar epithelial cells in IPF ( 24 ). Subsequently, these investigators detected EBV DNA by polymerase chain reaction (PCR) in the lung tissue of patients with IPF ( 25 ). Further study demonstrated that productive EBV replication is common in IPF and it is not associated with immunosuppressive therapy ( 26 ). Tang and co-workers detected one or more of four herpesviruses (cytomegalovirus [CMV], EBV, human herpesvirus 7 [HH-7], and human herpesvirus 8 [HHV-8]) in 97% of patients with IPF and 36% of controls, suggesting that a herpesvirus could be a source of chronic antigenic stimulation in IPF ( 27 ). Relatively high prevalence of serum antibodies to hepatitis C was demonstrated in patients with pulmonary fibrosis, but this finding was not confirmed in other studies ( 28 – 30 ). Adenovirus DNA was found in transbronchial lung biopsies (TBBx) from patients with IPF, and it was more prevalent in IPF patients treated with corticosteroids (67%) than in those who were not (10%), suggesting that this virus may newly infect or reactivate following corticosteroid therapy ( 31 ). A higher incidence of influenza ( 32 – 34 ), parainfluenza ( 35 , 36 ), CMV ( 37 ), human immunodeficiency virus (HIV)-1 ( 38 ), measles ( 39 ), herpes simplex virus-6, Mycoplasma, and Legionella has been reported in IPF ( 23 , 24 , 30 , 40 ).
In a recent study, human T-lymphtropic virus type I (HTVL-I) positive IPF patients had more affected lung parenchyma, demonstrated traction bronchiectasis with honeycomb change, and exhibited increased levels of specific cytokines that correlated with activated T-cells in the bronchoalveolar lavage fluid (BALF). These findings suggested that HTLV-I infection might contribute to the development of IPF via activation of T-cells ( 41 ). The latent nature together with episodic reactivation of many of these viruses may provide a scenario for the concept of “multiple hits” host defense followed by repair that these patients may experience during the course of their disease.
Genetic Factors
The genetics of familial IPF have not been elucidated. An autosomal dominant trait with variable penetrance may account for approx 70% of cases; there is no clear mode of transmission in the remaining 30% ( 2 , 42 ). Investigators have linked IPF to an increase in MZ phenotype for α1-antitrypsin inhibition on chromosome 14 ( 43 – 45 ). Using a candidate gene approach, researchers identified surfactant protein C gene mutations in large familial pulmonary fibrosis kindred, including adults with UIP and children with nonspecific interstitial pneumonia (NSIP) ( 46 ). In a separate study, Selman and co-workers demonstrated that Surfactant protein A and B genetic variants predispose to IPF ( 47 ). Genetic polymorphisms for interleukin-1 receptor antagonist (IL-1ra) and tumor necrosis factor (TFN)-α appear to be important in determining risk ( 48 ). In contrast, transforming growth factor (TGF)-β polymorphisms do not predispose to IPF, but these polymorphisms may affect the course of the disease ( 49 ).
It is unknown what proportion of IPF is familial, but it is estimated that 0.5 to 2.2% of cases have a genetic basis ( 42 ). Thirty-eight families affected by pulmonary fibrosis have been identified and are currently under active investigation. The familial aggregation in those families is consistent with a genetic basis in at least a subset of patients with IPF ( 50 ).
IPF is a specific form of chronic fibrosing interstitial pneumonia limited to the lungs and associated with the histological pattern of UIP on surgical lung biopsy ( 2 ). Many earlier studies included various other idiopathic interstitial pneumonias under the term idiopathic pulmonary fibrosis, but the clinical term IPF is now reserved to patients with idiopathic UIP.
History and Physical Examination
The diagnostic approach to any patient with diffuse lung disease must include a thorough history and physical examination with attention to symptoms or signs suggestive of a connective tissue disease, occupational or environmental exposures, use of fibrogenic drugs, and family history of pulmonary fibrosis. Patient age at disease onset is generally between 50 and 70 yr of age and IPF is more common in males than females. IPF typically presents insidiously, with gradual onset of a nonproductive cough and dyspnea ( 2 ). Patients are often treated for other conditions such as congestive heart failure, “walking pneumonia,” bronchitis, or asthma before the diagnosis is made. The physical examination in most patients (>80%) reveals fine bibasilar inspiratory crackles (“Velcro rales”), and clubbing is noted in up to 50% of patients ( 51 , 52 ). Signs of right heart failure are evident in advanced cases ( 2 ).
Laboratory and Serological Tests
Laboratory abnormalities are mild and nonspecific. One early study described “autoimmune factors” in blood of 8 patients (4 of whom had autoimmune-associated diseases) among 17 patients with diffuse fibrosing alveolitis ( 53 ). A positive rheumatoid factor occurred in 3 of 20 patients with unexplained pulmonary fibrosis and positive antinuclear factor in the serum (ANA) ( 54 ). A later study examined serum specimens from 122 patients with IPF and compared them with specimens from age- and sex-matched controls; ANAs were present in 21% of patients with IPF and in 6% of the control subjects ( 55 ). Positive circulating ANAs or rheumatoid factor occur in 10% to 20% of patients with IPF, but titers are rarely high ( 2 , 53 , 54 , 56 ). An elevated erythrocyte sedimentation rate, lactate dehydrogenase, or hypergammaglobulinemia may be found in patients with IPF, but are nondiagnostic ( 2 ). Serological findings do not correlate with extent or severity of disease, and have no prognostic value ( 2 , 57 ). In the absence of symptoms of connective tissue disease, the presence of autoantibodies does not imply an underlying systemic disorder. Recently, investigators reported the occurrence of low fasting triglyceride and high free fatty acid levels in patients with pulmonary fibrosis. Because insulin-like growth factor (IGF)-I is known to lower triglycerides and increase free fatty acids, the authors hypothesized that the reported increased production of IGF-I in patients with IPF may explain such findings ( 58 ).
Radiological Studies
Chest radiograph.
Classic chest radiographic findings in IPF include a basal predominant reticular, or reticulonodular, pattern associated with decreased lung volumes, and in later stages, cystic areas representing honeycomb (HC) lung ( 2 , 59 – 61 ). When a “confident” diagnosis of IPF is made on the basis of the chest radiograph, it is correct in 48 to 87% of cases ( 60 , 62 , 63 ). Most patients with IPF will have an abnormal chest radiograph but, rarely, patients may present with a normal plain film ( 2 ). It is important to review all previous chest films to assess the rate of change in disease activity. In addition, radiographs are indicated if clinical deterioration occurs in order to identify superimposed infection or malignancy ( 2 , 64 ). Pleural effusions, upper lobe predominant disease, airbronchograms, or prominent lymphadenopathy should suggest an alternative diagnosis.
High-Resolution Chest Computed Tomography
Typical high-resolution chest computed tomography (HRCT) features of IPF/UIP include patchy, predominantly peripheral, subpleural, and symmetrical bibasilar honeycombing, reticular abnormalities, and limited “groundglass” opacities (GGO) ( 65 – 68 ). Several studies have shown that experienced radiologists can make a “confident” diagnosis of UIP with specificity greater than 95%, provided CT features are typical ( 65 , 69 – 72 ). Although a characteristic HRCT is highly specific for IPF/UIP, “typical” HRCT identifies only 37 to 67% of patients with histological UIP; therefore, a surgical lung biopsy (SLB) is recommended when clinical and radiological information result in an uncertain diagnosis ( 14 , 65 , 70 , 73 ). One study evaluated the proficiency of physicians with expertise in interstitial lung diseases to identify accurately the HRCT scans from patients with biopsy-proven IPF/UIP. When these investigators made a “confident” diagnosis of IPF based on HRCT scan and clinical data, they were right in more than 80% of the cases. However, more than half of the patients with IPF had an uncertain diagnosis on the basis of HRCT and clinical assessment ( 70 ). In a subsequent analysis of these data, investigators identified HRCT features associated with a pathological diagnosis of UIP. On multivariate analysis, lower lobe honeycombing (OR, 5.36), and upper-lung irregular lines (OR, 6.28) were the only independent predictors of UIP. When they combined those two factors, a diagnosis of UIP was established with a sensitivity of 74%, a specificity of 81%, and a positive predictive value of 85% ( 65 ). Interestingly, in that study, adenopathy was observed in 55% and 21% of patients with UIP and without UIP, respectively. This finding suggests that patients with IPF generate a marked lymphoproliferative response to an unknown antigen or antigens; we believe that this observation requires further investigation as it may provide important clues to further our understanding of the pathogenesis the disease. In a separate study, two radiologists independently assessed CT scans from a cohort of patients with either UIP. CT features were “typical” for UIP in only 37% patients, and all of them had histological UIP on SLB. Typical CT features of UIP are associated with advanced, latestage disease. Among patients with earlier phases of UIP, CT features may be atypical ( 74 ) or indeterminate ( 69 ).
Extensive GGO is not a major feature of UIP, and suggests an alternative diagnosis such as desquamative interstitial pneumonia (DIP), NSIP, lymphocytic interstitial pneumonia (LIP), cryptogenic organizing pneumonia (COP), hypersensitivity pneumonia (HP), or pulmonary alveolar proteinosis (PAP) ( 75 ). In contrast, honeycomb change is a cardinal feature of UIP, and is rare in other IIPs ( 71 , 76 ).
Physiological Studies
Pulmonary function testing.
Pulmonary function tests (PFTs) characteristically reveal a restrictive ventilatory defect with impaired gas exchange; however, smokers may have preserved lung volumes or airflow obstruction in the initial stages of the disease ( 2 , 77 , 78 ). Impairments in gas exchange (i.e., carbon monoxide diffusing capasity [DL CO ]) and oxygenation may be evident early in the course of the disease, even when spirometry and lung volumes are normal ( 79 ). The most appropriate and simple tests are vital capacity and DL CO ; these are most useful for assessing the extent and monitoring the progression of the disease ( 80 ).
Exercise Testing
Cardiopulmonary exercise testing (CPET) demonstrates hypoxemia, widened A-a O 2 gradient, submaximal exercise endurance, reduced oxygen consumption (VO 2 ), high respiratory frequency, low tidal volume (V T ) breathing pattern, increased dead space (V D /V T ), increased minute ventilation for the level of VO 2 , and a low O 2 pulse ( 80 – 82 ). Arterial desaturation and abnormal widening of A-a O 2 gradient with exercise may be elicited with relatively simple tests, such as the 6-min walk test ( 83 , 84 ).
Bronchoalveolar Lavage Fluid
BALF may play a role in the diagnosis of inorganic dust diseases, suspected malignancy, infections, some hematological disorders, drug-induced diseases, pulmonary alveolar proteinosis, Langerhans’ cell histiocytosis, and alveolar hemorrhage ( 85 ). BALF is useful in research studies but of limited clinical application when evaluating IIPs ( 85 ). Increases in polymorphonuclear leukocytes, eosinophils, mast cells, alveolar macrophages, and countless cytokines are noted in BALF from patients with IPF/UIP; lymphocyte numbers are usually normal ( 2 ). BALF neutrophilia is present in 67 to 90% of patients with IPF/CFA ( 86 , 87 ), but does not predict prognosis or therapeutic responsiveness. Elevations in BALF eosinophils were associated with more severe clinical impairment ( 86 , 87 ), but BALF eosinophil counts do not correlate consistently with prognosis ( 86 , 87 ). By contrast, the presence of BALF lymphocytosis, found in fewer than 15% of cases, was associated with a greater responsiveness to corticosteroid therapy, a more cellular biopsy, and less honeycombing ( 86 , 87 ). Data compiled from two studies documented favorable responses to corticosteroids in 12 of 13 patients exhibiting BALF lymphocytosis, but in only 4 of 37 without lymphocytosis ( 86 , 87 ). Because the studies citing BALF lymphocytosis in steroid-responsive patients with IPF ( 87 ) or CFA ( 86 ) antedated the description of NSIP, it is possible that BALF lymphocytosis reflects disorders distinct from UIP (such as cellular NSIP). Nevertheless, in a recent study, researchers hypothesized that BALF findings may distinguish between UIP and NSIP; BALF total and differential cell counts were not different between the two groups, and in neither group, were BALF findings predictive of survival or changes in lung function ( 88 ).
The American Thoracic Society (ATS) and the European Respiratory Society (ERS) in collaboration with American College of Chest Physicians (ACCP) published an international consensus statement on the diagnosis and treatment of IPF. This statement stated that the definite diagnosis of IPF requires an SLB showing the UIP pattern. However, an SLB is not recommended in patients with suspected IPF in whom the clinical or radiographic information are stereotypical of IPF/UIP. It has been suggested that, in the absence of an SLB, the presence of all four major diagnostic criteria and at least three minor criteria increases the likelihood of an accurate diagnosis of IPF/UIP ( 2 ). However, these criteria have not been prospectively validated.
An SLB, preferably by video-assisted thoracoscopy (VATS), is recommended in patients with suspected IPF in whom the clinical or radiographic information are not typical of IPF/UIP ( 2 ). Given the patchy and heterogeneous nature of the UIP lesion, a large piece of lung tissue is required and TBBx are used mainly to rule out other disorders that mimic IPF. However, emerging data suggest that TBBx findings may be more useful in diagnosing UIP than previously recognized; characteristic histological features of UIP (interstitial fibrosis with fibroblastic foci [FF] and/or HC change) can be appreciated even in a small sample obtained by TBBx; these findings in a patient with characteristic clinical and radiographic features may indicate a diagnosis of UIP. However, these findings require prospective validation ( 89 ).
Significant advances have been made in our understanding of the idiopathic interstitial pneumonias (IIPs). The most important advancement has been the greater appreciation of the clinical relevance of the different histopathological subgroups that make up the IIPs. Until recently, inflammatory or fibrotic lung disorders of unknown etiology were “lumped” under the term IPF ( 2 , 75 , 90 ). However, with the advent of VATS lung biopsies and the greater availability and quality of surgical specimens, pathologists have recognized the heterogeneous nature of these disorders and described specific histopathological patterns that predict response to therapy and survival ( 74 , 91 , 92 ). IPF/UIP is the most common of the IIPs, comprised of 47 to 71% of the cases ( 76 , 91 , 93 , 94 ). The UIP lesion is characterized by temporal and geographic heterogeneity, with areas of old scar and HC change, admixed with granulation tissue and normal lung; the lesion has predilection for the subpleural and basilar regions of the lung, there is scant inflammation, and prominent aggregates of fibroblast and myofibroblasts, so-called “fibroblastic foci,” which actively secreting extracellular matrix ( 75 , 92 , 95 ). Additional features include smooth muscle hypertrophy, metaplasia and hyperplasia of type II pneumocytes, destroyed and disrupted alveolar architecture, traction bronchiectasis and bronchioloectasis, and secondary pulmonary hypertension changes ( 75 , 92 , 95 ).
Other categories of IIP that must be distinguished from IPF/UIP include NSIP, DIP, respiratory bronchiolitis-associated interstitial lung disease (RBILD), acute interstitial pneumonia (AIP), COP, and LIP ( 75 ). NSIP is observed in approx 25% of patients with IIP ( 75 ). This provisional category is used to describe a temporally homogeneous lesion with varying degrees of inflammation and fibrosis with favorable response to therapy and prognosis. NSIP can be subdivided into NSIP-cellular and NSIP-fibrotic varieties depending on the degree of inflammation and fibrosis present in the surgical specimen ( 93 ). This subclassification provides important prognostic information; patients with idiopathic NSIP, cellular pattern have a better 5- and 10-yr survival than those with idiopathic NSIP, fibrosing pattern (100% vs 90% and 100% vs 35%, respectively) ( 93 ).
In many patients, however, histological overlap between UIP and NSIP is evident. Flaherty and co-workers reviewed SLBs from 109 patients with IIP who had multiple lobes biopsied and reported histopathological variability between lobes in 26% of patients. Importantly, in that study, UIP in at least one lobe defined prognosis ( 94 ). In a later study, the pathological findings in biopsy and subsequent explant specimens from 20 patients with UIP were reviewed to refine histological criteria and to assess the relationship between UIP and NSIP. The important new finding was that NSIP-like areas were present in the majority of UIP patients (80%) in both biopsy and explants specimens, and in some, these areas were extensive, making accurate diagnosis of UIP difficult (cases were misdiagnosed as NSIP). The most useful feature for diagnosing UIP in difficult cases is the presence of a distinct “patchwork” or variegated pattern of parenchymal involvement ( 95 ).
RBILD and DIP comprise approx 15% of IIPs ( 75 ). These entities are thought to be smoking-related diseases and, like NSIP, tend to be responsive to anti-inflammatory therapy. This is not surprising, as pathologically, these lesions are characterized by varying degrees of intra-alveolar and/or peribronchial pigmented macrophage infiltration with scant or no fibrosis. AIP is characterized by active fibrosis consisting of proliferating fibroblasts and myofibroblasts with minimal collagen deposition resembling the organizing stage of diffuse alveolar damage (DAD) ( 92 ). Finally within the IIPs, some include COP (previously termed bronchiolitis obliterans organizing pneumonia, or BOOP), and LIP. Both are relatively steroid-responsive lesions; pathologically, COP is characterized by the presence of intra-alveolar plugs of granulation tissue, and LIP by lymphocytic infiltration of the alveolar walls. It should be noted, however, that many experts argue that LIP should not be included within the IIPs because it is considered as lymphoproliferative disorder which, in turn, is rarely idiopathic and is mostly observed in association with infections (e.g., HIV) or collagen vascular diseases (CVDs) ( 75 ).
Natural History
The natural history of the pathogenesis of IPF is not known. The description of temporal heterogeneity for the histopathological entity of UIP would suggest that this process occurs over a significant period of time within the same low-power microscopic field of the lung. Moreover, the description supports the notion that the lesions are not homogeneous for both the timing of the orginal injury and the subsequent response to the injury and repair. In addition, the histopathological entity of UIP is not unique to IPF, and can be found in patients with connective tissue diseases, end-stage asbestosis, and end-stage hypersensitivity pneumonia. Therefore, UIP may represent an end-stage of a “process,” not the beginning, intermediate, and end-stage of a disease. The only insight into the natural history of this process comes from two recent studies that suggest the potential concept of a continuum of IIPs that may overlap in time.
In a study of 109 patients whom had multiple lobes biopsied, histological variability was evident in 26% of the patients. Patients concordant for UIP were older (63 ± 9 yr) than those discordant for UIP (57 ±12 yr) or with fibrotic NSIP (56 ±11 yr) or cellular NSIP (50 ±9 yr), suggesting that NSIP may be an early lesion that progresses with time to UIP ( 94 ). In a separate study, investigators found discordant histological diagnoses between lobes in 20% of the patients. NSIP-like reactions were evident in 80% of patients with UIP, suggesting that NSIP may evolve into UIP ( 95 ). In a genetic study, two different histopathological patterns of interstitial pneumonia were found to exist in members of a family who shared protein C gene mutations: adults with UIP and children with NSIP; this supports the notion that NSIP may be a precursor lesion to UIP ( 46 ).
It has been suggested that most patients with IPF progress in a relentless and insidious manner with a median survival of less than 3 yr ( 91 ). This concept was challenged in a recent study of subcutaneous interferon (IFN)-γ1b (200 µg thrice weekly) in 330 patients with mild to moderate IPF (forced vital capacity [FVC] > 50% and DL CO > 30%), where a trend toward lower mortality was seen in IFN-γ1b-treated patients compared with placebo-treated patients ( 96 ). Interestingly, there were no significant differences in lung function or gas exchange between IFN-γ1b-treated and placebo-treated patients at 48 wk of follow-up. In both study groups, 70% of patients remained stable, 25% deteriorated, and the rest improved, suggesting that most patients with mild to moderate IPF remain stable for at least 1 yr on no specific therapy. The so-called “IPF exacerbations” may explain the trend toward lower mortality seen in this study, but this requires further study. IPF exacerbations may be defined as an accelerated deterioration of IPF in the absence of apparent infectious agents and heart failure ( 97 , 98 ). It is often a terminal event, with features of DAD or organizing pneumonia on lung biopsy or autopsy ( 99 , 100 ). This syndrome is indistinguishable from idiopathic AIP ( 101 ), and is similar to acute respiratory distress syndrome (ARDS). The factors responsible for this accelerated phase of IPF are unknown, but viral infections, high concentrations of oxygen, or drug reactions are plausible etiological factors ( 101 ).
Morbidity and Mortality
Only one study has addressed mechanism of mortality of patients with IPF. Panos and colleagues ( 102 ) found that most patients with IPF succumb to respiratory failure (39%), cardiovascular disease (27%), lung cancer (6–13%), pulmonary embolism (3%), infection (3%), or other health problems (18%) ( 102 ). Although infection would appear unlikely as a cause of mortality in IPF patients, clinically we can only determine the micro-organism cause of community acquired pneumonia in 30% of all patients. Therefore, we do not know the true incidence and prevalence of infection as a cause of mortality in patients with IPF, and perhaps a significant portion of the respiratory failure mortalities had infectious etiologies. With regard to cardiovascular disease, congestive heart failure and coronary artery disease (CAD) account for 30% of deaths ( 102 ). Patients with IPF appear to be at increased risk of developing CAD. In a cross-sectional study of 630 patients referred for lung transplantation, fibrotic lung diseases were associated with an increased prevalence of CAD compared with nonfibrotic diseases after adjustment for traditional risk factors (OR 2.18; 95% CI, 1.17–4.06); the authors theorized that the fibroproliferative process may influence cells beyond the pulmonary compartment, and that mediator molecules produced in these disorders might promote atherogenesis ( 67 ).
Pulmonary arterial hypertension occurs in 70% of patients with advanced IPF and its presence correlates with a vital capacity (VC) below 50% of predicted or a DL CO under 45% of predicted ( 102 ). Left ventricular (LV) dysfunction occurs in less than 10% of patients and it is mostly (66%) a result of coexisting right heart failure. Other causes of LV dysfunction include ischemic and hypertensive heart disease ( 5 , 102 ). Six to 13% of patients with IPF develop bronchogenic carcinoma. Lung cancer in patients with IPF typically presents as a peripheral squamous cell carcinoma in older male smokers ( 103 ). Predisposing factors include squamous metaplasia, atypical epithelial cells, or occupational exposures ( 13 , 57 , 102 ). Hubbard and colleagues, in a population-based cohort in the United Kingdom, studied 890 patients with IPF and 5884 controls. The risk ratio for IPF and lung cancer was 7.31 (95% CI 4.5–11.9). Importantly, adjusting for previous smoking had little effect on this ratio, suggesting that IPF is an independent risk factor for lung cancer ( 104 ).
Pulmonary embolism occurs in approx 3 to 7% of patients; inactivity, heart failure, bronchogenic carcinoma, and possibly corticosteroid therapy predispose patients to thrombosis ( 102 ). Pulmonary infection causes 2 to 4% of deaths in patients with IPF; immunosuppressive therapy, traction bronchiectasis, and possibly GERD are predisposing factors ( 102 ). Pneumothorax occurs in up to 10% of patients with IPF and tends to be less responsive to tube thoracostomy, often necessitating surgical intervention ( 102 ).
Complications of Therapy
Corticosteroids (CS) can cause a myriad of side effects including myopathy, peptic ulcer disease, cataracts, osteoporosis, compression fractures, fluid and electrolyte abnormalities, adrenal insufficiency, and infection ( 105 , 106 ). In a cross-sectional study in patients with asthma, chronic obstructive pulmonary disease, or “alveolitis” taking oral corticosteroids ( n = 367) vs controls ( n = 734), the OR for bone fractures was 1.8 (95% CI 1.3–2.6 [vertebral fracture OR 10, hip fracture OR 6, and ribs or sternum fracture OR 3.2]). Patients taking corticosteroids experienced more cataracts, used more antacids, had more muscle weakness, back pain, bruising, and oral candidiasis, and had fewer teeth compared with controls ( 106 ). One prospective study included 41 patients with IPF; 24 received 100 mg/d of prednisone for 3 mo and 17 received 60 mg/d for 1 mo followed by 40 mg/d for 2 mo. Patients were monitored monthly for steroid-related side effects. All patients experienced at least one side effect. Common side effects included insomnia (76%), cushingoid change (73%), weight gain (71%), irritability (61%), infection (49%), blurred vision (41%), abdominal bloating (34%), glucose intolerance (24%), and fractures or avascular necrosis (10%) ( 105 ).
Cytotoxic agents (cyclophosphamide [CP], azathioprine [AZA]) can cause infections, bone marrow suppression, hepatitis, hemorrhagic cystitis (CP) and/or malignancies ( 107 , 108 ). In a prospective uncontrolled study, 19 patients with biopsy-proven UIP who were unresponsive or intolerant to CS therapy were treated with oral CP (1–2 mg/kg/d) for 6 mo. Nearly two-thirds of patients reported adverse effects, and 50% of patients discontinued therapy because of intolerable side effects. Common side effects related to CP include nausea/vomiting (26%), anorexia (26%), cytopenias (21%), weight loss (21%), alopecia (10%), infection (Herpes zoster) (10%), and ovarian failure (5%) ( 108 ).
Several factors have been shown to predict poor outcome in IPF. These include older age at presentation, male gender, severe dyspnea at presentation, history of cigarette smoking, severe loss of lung function, severity of reticular opacities or honeycomb change on HRCT, characteristic HRCT appearance, lack of response to conventional therapy, and histopathological findings showing prominent FF ( 2 , 17 , 69 , 74 , 109 ). Investigators from the University of Michigan evaluated the impact of histological diagnosis, baseline clinical, physiological, and radiographical factors on survival in 168 patients with suspected IIP. The presence of histological UIP was the most important risk factor for mortality (risk ratio [RR] of 28.46 [95% CI 5.5–148]), followed by the presence of honeycombing on HRCT, a radiographic feature that was shown to be a good surrogate for histological UIP (sensitivity of 90%, and a specificity of 86%) ( 14 ). The same group evaluated the impact of HRCT appearance on survival in patients with IIP. Patients with histological UIP and stereotypical HRCT appearance of UIP had a shorter survival (median survival 2.08 yr) when compared with patients with histological UIP and indeterminate HRCT scans (median survival 5.76 yr) ( 69 ).
Three studies have shown that a higher HRCT-fibrosis score identify patients with worse prognosis. Gay and colleagues did not find any measure of pulmonary function to be predictive of survival, but did find both the HRCT-fibrosis score and the pathological fibrosis score to be useful in predicting survival ( 109 ). Similarly, investigators from the United Kingdom found that baseline percent-predicted DL CO and HRCT-fibrosis score were independent predictors of mortality ( 110 ). Japanese investigators demonstrated that the baseline HC score and the rate of HC progression were both predictive of worse survival in patients with IPF ( 111 ).
Histopathological findings showing prominent FF identify patients with poor outcome. Nicholson and associates retrospectively studied ( 53 ) patients with IPF/UIP and analyzed the prognostic significance of four specific microscopic features of UIP. Multivariate analysis revealed that increasing FF and mononuclear cell infiltrate scores were associated with worsening lung function. Higher profusion of FF and a lower DL CO were independent predictors of mortality ( 112 ). These results supported the findings by King and co-workers, who also demonstrated that an increase in the number of FF correlated highly with mortality in patients with IPF/UIP ( 13 ). In a separate study, expert pathologists reviewed SLB from 108 patients with idiopathic or CVD-associated UIP and assigned a score for FF. Patients with idiopathic UIP had more FF and worse survival compared with patients with CVD-associated UIP ( 113 ).
A number of composite scoring systems have been developed with which to predict survival in IPF. King and co-workers studied 238 patients with IPF/UIP and derived a clinical-radiological-physiological (CRP) scoring system using clinical (age, smoking status, clubbing), radiographical (extent of interstitial opacities, presence of pulmonary hypertension on chest radiographs), and physiological parameters (reduced lung volume, abnormal gas exchange during maximal exercise). These investigators demonstrated that the CRP score correlated with important histopathological findings and was helpful in predicting survival in patients with IPF. A second abbreviated CRP scoring system that excluded Pa O2 during maximal exercise was inferior in predicting survival ( 12 , 114 ). Similarly, investigators from the Brompton Hospital devised a composite physiological index (CPI) using radiographical and physiological information that predicted mortality more accurately than individual PFT in patients with IPF ( 115 ). Even though these composite scoring systems are accurate in their predictive ability, they are expensive and cumbersome to generate in clinical practice. With this in mind, the prognostic value of oxygen desaturation during a 6-min walking test was evaluated in patients with IPF. Desaturation defined as a fall in oxygen saturation to 88% or less during the 6-min walk test identified patients with higher mortality compared with patients who did not desaturate. The 4-yr survival rate of IPF patients who desaturated to that level was 34.5% compared with 69.1% in patients who did not desaturate ( 84 ).
Predicting survival in IPF has been centered on baseline radiographical, pathological, and/or physiological testing. Recently, researchers have focused on the association of serial changes in pulmonary function or radiographical features and prognosis. One study determined that a decrease in FVC (>10% from baseline) during the initial 6 mo of follow-up was associated with increased mortality (hazard ratio 2.06; CI 1.09–3.89) ( 116 ). In a separate study, investigators concluded that at 6 and 12 mo of follow-up, serial pulmonary function trends (change in DL CO , FVC, forced expiratory volume in 1 s [FEV 1 ], and the CPI) provided important prognostic information in IPF ( 117 ). A third study showed that assessment of changes in clinical and physiological variables (dyspnea score, total lung capacity, thoracic gas volume, FVC, FEV 1 , DL CO , p O2 , oxygen saturation, and alveolar-arterial oxygen gradient) at 6 and 12 mo provide clinicians with more accurate prognostic information than baseline values alone ( 118 ).
Serum markers and nuclear medicine testing may have a predictive role in IPF. Greene and colleagues found that serum levels of surfactant protein-A (SP-A) were predictive of survival in patients with IPF ( 119 ).
In a prospective study, investigators analyzed the usefulness of inhaled 99m-labeled diethylenetriamine penta-acetic acid ([99m] Tc-DTPA) aerosol clearance and survival in a cohort of 106 patients with UIP. Multiple stepwise Cox regression analysis identified fast clearance as an independent predictor of mortality ( 120 ).
Conventional Therapy
Conventional therapy (CS, AZA, or CP) for IPF provides only marginal benefit. Unfortunately, in many studies, diagnoses were not based on the findings of lung biopsies or were not classified by current pathological criteria; thus, there is uncertainty as to the nature of the disease being treated. Two recent meta-analyses searched two large databases for randomized controlled trials (RCT) and controlled clinical trials (CCT) using CS or non-CS agents in patients with histological UIP or who fulfilled all ATS criteria for IPF; the authors could not find RCTs or CCTs evaluating CS alone in IPF and concluded that there are scant good-quality data regarding the efficacy of non-CS agents in IPF ( 121 , 122 ). The following is a brief discussion of anti-inflammatory (conventional) therapy in the treatment of IPF.
Corticosteroids
CS were the mainstay of therapy for more than four decades, but are of unproven efficacy, and are associated with significant toxicities ( 105 , 123 , 124 ). Early studies of patients with IPF/CFA cited response rates of 10 to 30% with CS (alone or combined with immunosuppressive agents), but complete or sustained remissions were rare ( 105 , 125 – 127 ). More importantly, many responders likely had IIPs other than UIP (e.g., NSIP or RBILD/DIP).
In recent studies, response rates to CS among patients with histological evidence for UIP are low (0–17%) ( 16 , 57 , 74 , 76 , 123 ). Large retrospective studies of patients with IPF showed no survival benefit with CS ( 12 ; 15 , 123 , 128 ). In one retrospective study from England, survival was worse among IPF patients treated with CS or CP, although this likely reflects a selection bias ( 15 ). Given the potential severe toxicities associated with CS ( 105 , 124 ), recent international consensus statements argue that high-dose CS should not be used to treat IPF ( 2 , 61 ). However, because anecdotal responses to CS are occasionally noted in patients with IPF/UIP ( 14 ), these statements acknowledge that selected patients with clinical or physiological impairment or worsening PFTs should be treated ( 2 , 61 ). Both statements ( 2 , 61 ) advocate an individualized approach to treating IPF/UIP. Among patients requiring treatment, both statements recommend combining therapy with either oral AZA or CP plus low-dose prednisone or prednisolone (0.5 mg/kg [lean body weight per d] for 4 wk, then 0.25 mg/kg for 8 wk, then 0.125 mg/kg). This represents a substantial departure from earlier regimens advocating high-dose prednisone (e.g., ≥1 mg/kg/d for ≥6–12 wk) ( 114 , 125 , 126 ). Combined therapy should be continued for 6 mo in the absence of adverse effects. Treatment should be continued beyond 6 or 12 mo or later time points only if patients improve or remain stable. It should be emphasized that these recommendations ( 2 , 61 ) reflect expert opinion, but have not been validated in clinical trials. We believe CS should not be given to patients at high risk for adverse effects (e.g., age > 70 yr, osteoporosis, DM, extreme obesity, and so on).
Azathioprine
Two prospective studies evaluated AZA for IPF ( 125 , 126 ). In both studies, AZA was combined with prednisone. In the first study, 20 patients with progressive IPF were initially treated with prednisone alone for 3 mo ( 125 ). At that point, AZA (3 mg/kg/d) was added and both agents were continued for an additional 9 mo or longer. Twelve patients (60%) responded. The independent effect of AZA was difficult to assess, because all patients received prednisone concomitantly. In a second, double-blind trial, Raghu and associates compared the effect of AZA plus prednisone on lung function with that of prednisone alone in previously untreated patients with IPF (the study population may have included patients with IIP other than UIP). Forty-three percent of patients randomly assigned to AZA plus prednisone died during the 9-yr follow-up period, compared with 77% of patients randomly assigned to prednisone alone. The difference became statistically significant only after adjustment for age ( p = 0.02) ( 126 ).
Cyclophosphamide
Two randomized trials evaluated CP for IPF ( 127 , 129 ). In one 6-mo trial, 28 patients with “mid-course” IPF were randomized to prednisone alone ( n = 16); prednisone plus oral CP (1.5 mg/kg/d) ( n = 9); or CP alone ( n = 5) (129). Mean BALF neutrophil counts declined in the cohort receiving CP, but PFTs did not change in any group. Johnson and colleagues compared the effect of prednisolone alone with that of prednisolone plus CP on breathlessness, radiographic appearance, and lung function in patients with IPF (the study population included patients with CVD and with IIP other than UIP). Initial improvement occurred in 7 of the 22 patients in the prednisolone-only group and in 5 of the 21 patients in the CP plus prednisolone group. However, at 36 mo, only 2 of the 22 patients in the prednisolone-only group remained improved, and only 1 of the 21 patients in the CP-prednisolone group remained improved. Life-table analysis suggested better survival in patients in the CP-prednisolone group, but this was not statistically significant ( 127 ). In a prospective uncontrolled study, Zisman and associates studied the efficacy of CP in 19 patients with biopsyproven UIP who were unresponsive or intolerant to CS therapy. Only 1 patient improved; 7 remained stable, and 11 deteriorated. Nearly two-thirds of the patients developed drug-related side effects and one half of the patients discontinued therapy due to intolerable side effects ( 108 ). Intermittent, intravenous “pulse” CP, administered every 2 to 4 wk, has been tried for IPF refractory to CS in nonrandomized studies, but benefit was not convincing ( 130 – 132 ).
Lung Transplantation
Lung transplantation should be considered for patients with IPF refractory to medical therapy ( 133 , 134 ). Two-year survival following single lung transplant (SLT) ranges from 60 to 80%; 5-yr survival is 40 to 60% ( 134 – 136 ). In one study, lung transplantation reduced the risk of death by 75% ( 135 ). In addition, patients surviving lung transplantation appear to achieve considerable improvement in most dimensions of health-related quality of life ( 137 – 139 ). Unfortunately, owing to a shortage of donor organs, waiting time may be prolonged (up to 2–3 yr) and many patients with IPF die while awaiting transplantation ( 134 , 135 ). One study evaluated baseline PFT and HRCT fibrosis scores and the relationship to 2-yr survival in patients with IPF younger than 65 yr of age; the optimal points on the receiving operator characteristics (ROC) curves for discriminating between survivors and nonsurvivors corresponded to a combination of DL CO of 39% predicted with HRCT-fibrosis score of 2.25 ( 110 ). In a separate study, investigators reviewed all transplant referrals for IIP that were listed for lung transplantation at their center. The aim of the study was to determine a parameter that would discriminate between patients who survived and patients who died awaiting transplantation. The severity of hypoxemia at rest was the only significant difference between both groups ( 140 ). Unless contraindications exist, patients with severe functional impairment (e.g., FVC <60% predicted, DL CO <40% predicted), oxygen dependency, and a deteriorating course refractory to medical therapy should be listed promptly for transplantation ( 133 , 134 ).
Antifibrotic Therapy
Historically, the fibrotic process in IPF has been thought to be preceded by a chronic inflammatory process that injures the lung and modulates fibrogenesis ( 141 ). Conventional management of IPF has been primarily based on the notion that suppressing inflammation may prevent progression to fibrosis. Evidence against the notion that inflammation plays an important role in the pathogenesis of IPF comes from the lack of correlation of most markers of inflammation with disease stage or outcome, and the recognition that inflammation is not a prominent histopathological finding in UIP ( 92 , 141 ). Additionally, emerging evidence suggests that inflammation is not required for the development of a fibrotic response ( 141 ). In light of the poor prognosis and lack of response to available anti-inflammatory therapy, alternative approaches to the treatment of IPF are being pursued. The following is a brief discussion of antifibrotic therapy and other promising agents in the treatment of IPF.
Colchicine is an alkaloid derivative of the plant Colchicum autumnale , which has been used in acute attacks of gout. It is known to bind microtubular proteins necessary for intracellular trafficking and cellular mitosis, thus adversely affecting secretion of proteins from cells and cellular proliferation ( 142 , 143 ). Its antifibrotic activity was described following the discovery that colchicine inhibits secretion of collagen and other important growth factors necessary for fibroblast proliferation ( 144 ). However, further studies ( 145 ) with or without additional therapeutic agents, such as steroids, failed to document efficacy of colchicine in the treatment of human pulmonary fibrosis. It is thus not currently recommended for use in therapy of IPF.
Penicillamine
The D -isomer of penicillamine has been extensively studied in animal models of fibrosis, in which it has been shown to prevent accumulation of collagen in the lung by interrupting cross-linking of collagen molecules ( 146 ). This observation has led to its use in treating fibrotic lung disease associated with systemic sclerosis with good results ( 147 ). However, its efficacy in the treatment of IPF has been disappointing ( 148 ), and it is known to have toxic and significant adverse effects ( 2 ). Thus it is currently not recommended as therapy for IPF.
Pirfenidone
Pirfenidone is a novel agent with broad-spectrum antifibrotic activity. Numerous in vitro and animal model studies have demonstrated its effectiveness as an antifibrotic agent. In vitro studies have shown that pirfenidone significantly reduced mRNA levels of type I and type III collagen, and may act at the transcriptional or translational level of collagen synthesis ( 149 ). In vivo, it has been shown to inhibit TGF-β1-induced collagen synthesis, decrease extracellular matrix deposition, and suppress the overexpression of TGF-βin the bleomycin model of pulmonary fibrosis ( 150 , 151 ). Because IPF is becoming increasingly recognized as primarily a fibrotic process, the potential role of pirfenidone as a therapeutic agent is being explored.
In a prospective open-label study, Raghu and colleagues ( 152 ) treated 54 (42 biopsy-proven) consecutive patients with pirfenidone who were either unwilling to receive or unresponsive to conventional therapy. Survival rates of 78% at 1 yr and 63% at 2 yr compared favorably with historical controls. In addition, 83% of patients discontinued prednisone therapy and the remaining 17% were able to reduce their daily dose. All patients treated with immunosuppressive therapy tolerated discontinuation of the drug. Interestingly, patients whose lung function had deteriorated before enrollment appeared to stabilize after beginning pirfenidone. Side effects were relatively common, with patients reporting nausea (44%), fatigue (44%), and photosensitivity (24%). Despite these encouraging observations, the results of this study are difficult to interpret owing to the lack of appropriate controls, incomplete pre-entry and follow-up pulmonary function test data, a small study population, and a bias associated with survivorship effect (pulmonary function data of patients who died were not included in the analysis, and this could have biased the results). Furthermore, the observed steroid- and immunosuppressive-sparing effects of pirfenidone may have simply reflected lack of efficacy of conventional therapy rather than a true effect of pirfenidone. In a second open-label trial, Japanese investigators evaluated oral pirfenidone in eight patients with IPF and two with diffuse lung disease associated with systemic sclerosis; after 1 yr of therapy, there was no change in chest radiographic scores and arterial oxygen tension; the drug was well tolerated ( 153 ). Early treatment with pirfenidone appears to slow the progression of pulmonary fibrosis in patients with Hermansky-Pudlak syndrome ( 154 ). A randomized-controlled trial focusing on early treatment is warranted to test the efficacy and safety of this agent in IPF.
Interferon-γ1b
IFNs play an integral role in the regulation of fibroblast proliferation and collagen synthesis, but the mechanism by which they exert their effect is not clearly understood. Recent observations have shown that IFN-γ has antiproliferative, immunomodulatory, and antifibrotic effects ( 155 ), and thus may play a crucial role in the pathogenesis of IPF. IFN-γ decreases collagen content in the bleomycin model of lung fibrosis by inhibiting TGF-β transcription and subsequent procollagen mRNA production ( 156 ). In addition, IFN-γ inhibits fibroblast proliferation in cultures derived from normal and fibrotic human lung, making an argument that IFN-γ may have therapeutic applications ( 157 ). Lower levels of IFN-γ have been found in patients with IPF compared with patients with less fibrotic diseases such as pulmonary sarcoidosis ( 158 ). Kuroki and associates ( 159 ) measured levels of type III collagen in patients with progressive pulmonary fibrosis and found an inverse correlation with IFN-γ levels, particularly in patients with IPF. These studies suggest that patients with IPF may have a defect in IFN-γ production or function, which predisposes them to develop fibrosis following injury. However, the potential for developing fibrosis is not likely to be dependent on one factor; rather it is likely the result of a complex interplay of fibrotic mediators, differential gene expression, and feedback mechanisms. A study by Shaw and colleagues ( 160 ) showed that alveolar macrophages from patients with interstitial lung disease had increased production of platelet-derived growth factor, which is a potent mitogen for fibroblasts. This increase in platelet-derived growth factor was upregulated following treatment with IFN-γ, suggesting that IFN-γ may act to potentiate fibrosis in certain cellular environments.
Clearly, there is a complex regulatory mechanism in place with regard to whether fibrosis occurs or not, and conflicting in vitro studies must be interpreted with caution. A randomized, prospectively controlled trial was conducted in 18 patients with IPF comparing IFN-γ1b and low-dose prednisolone with prednisolone alone for 12 mo ( 161 ). The results were remarkable in that patients with progressive pulmonary fibrosis treated with IFN-γ1b plus lowdose prednisolone demonstrated improvement in pulmonary function, whereas those who received prednisolone alone experienced further decline in pulmonary function. The authors showed that all patients treated had almost undetectable levels of IFN-γ mRNA, and increased levels of both TGF-β and connective tissue growth factor mRNA in lung tissue. Furthermore, after treatment with IFN-γ1b, transcription of TGF-β and connective tissue growth factor were both significantly decreased. Several concerns have been raised regarding the findings reported by Ziesche and co-workers ( 162 ), particularly the unexpectedly good results with IFN-γ1b. To address some of the issues raised, an outside panel of experts reanalyzed the study data by reviewing each patient’s lung function studies, CT scans, and SLBs to assess the clinical course and diagnosis of IPF according to the International Consensus Statement ( 2 , 163 ). Fifteen of 18 patients had either definite ( n = 9) or probable ( n = 6) IPF. The panel reanalyzed treatment response using published criteria and eliminated the patients who definitely did not have IPF. Patients treated with IFN-γ1b plus low-dose prednisolone demonstrated either stability or improvement in pulmonary function and gas exchange after 1 yr of treatment, whereas treatment with prednisolone alone was associated with no improvement in all patients ( 163 ). The observed benefit of IFN-γ1b on lung function has not been reproduced in subsequent studies. In one retrospective uncontrolled observation of 21 patients with IPF treated with IFN-γ1b, only one patient experienced objective improvement, 7 discontinued therapy (owing to lack of perceived benefit), and 11 died after 6 mo of therapy ( 164 ). In a separate study of five patients with IPF treated with IFN-γ1b, only one patient improved, two discontinued treatment owing to adverse effects and decline in lung function, and one died after 3 mo of therapy ( 165 ).
In a recent prospective, randomized, placebo-controlled, double-blind, multicenter phase III clinical trial of subcutaneous IFN-γ1b (200 µg thrice weekly) in 330 patients with mild to moderate idiopathic pulmonary fibrosis (FVC >50% and DL CO >30%), a trend toward lower mortality was seen in IFN-γ1b-treated patients compared with placebo-treated patients. However, there were no significant differences in lung function or gas exchange between IFN-γ1-treated and placebo-treated patients after 48 wk of therapy ( 96 ). A prospective controlled multinational trial is planned to verify the possible survival benefit observed with IFN-γ1b therapy in IPF.
Interferon β -1a
IFN-β is used for the treatment of chronic hepatitis C and multiple sclerosis. In vitro IFN-β1a has been shown to reduce fibroblast proliferation ( 166 ), inhibit collagen production by fibroblasts ( 167 ), increase collagenase mRNA ( 168 ), decrease pro-collagen mRNA ( 169 ), and increase collagenase activity ( 167 ). Further, IFN-β inhibits irradiation-induced pulmonary fibrosis in mice ( 170 ). A multicenter randomized, double-blind clinical trial examining the efficacy of IFNβ-1a was recently completed. Patients were randomized into four groups: placebo or IFNβ-1a at 15, 30, or 60 µg intramuscularly twice per week for a minimum of 12 mo and up to 2.5 yr. Preliminary results suggest that IFNβ-1a lacks significant efficacy ( 171 ).
Emerging Strategies
With a dismal response to existing therapy and its accompanied toxicity, the search for additional therapies has intensified in the past decade. The following is an overview of other therapeutic approaches, most of which are undergoing investigation and have not been adequately studied in humans.
Agents That Inhibit Epithelial Injury or Enhance Repair
The prior discussion has centered on fibroblast proliferation and collagen deposition as two areas of therapeutic intervention. It is becoming more apparent that the fibrotic process has multiple pathogenetic mechanisms. One of the fundamental hypotheses of pulmonary fibrosis involves an imbalanced response to injury, in which the capacity of the alveolar epithelium to repair itself is compromised, ultimately leading to fibrosis. Some investigators propose that the alveolar epithelium itself has antifibrotic properties, and that chronic loss of alveolar epithelium leads to an environment conducive to the development of fibrosis ( 172 ). Support for this notion exists in the fact that induction of apoptosis of alveolar epithelium has been shown to occur following administration of bleomycin ( 173 ). Therapies that either inhibit epithelial injury or enhance repair may limit the fibrotic response. In this regard, captopril, an angiotensin-converting enzyme inhibitor widely used in clinical practice, may have a role in the treatment of IPF. In vitro, captopril inhibits fibroblast proliferation, and in models of bleomycin-induced pulmonary fibrosis, it has been shown to reduce alveolar epithelial cell apoptosis and fibroproliferation. In addition, captopril abrogates Fas-induced apoptosis in human alveolar epithelial cells ( 141 , 174 ). There is currently an ongoing clinical trial at the National Institute of Respiratory Diseases in Mexico testing the efficacy of captopril in patients with IPF ( 141 ).
Another agent that may protect the alveolar epithelium is keratinocyte growth factor (KGF). This class of growth factor stimulates type II cell proliferation with no direct effects on fibroblasts ( 175 ). Keratinocyte growth factor increases surfactant protein gene expression and sodium/potassium adenosine triphosphatase, factors that may protect the alveolar epithelium ( 175 ). In vivo, KGF has been shown to protect animals from injury and subsequent development of fibrosis caused by a variety of insults ( 175 ).
There is evidence that an exaggerated oxidant stress may play a role in the pathogenesis of pulmonary fibrosis by injuring the alveolar epithelial cells. This oxidant burden is thought to be a consequence of both increased levels of reactive oxygen species and a defective antioxidant response. A major protector of oxidant-induced injury of the alveolar epithelium is glutathione, which has been shown to be deficient in the BALF of patients with IPF ( 176 ). Moreover, in vitro studies have shown that N -acetylcysteine (NAC), a precursor for glutathione synthesis, may augment the antioxidant defense system and protect the alveolar epithelium from free radical-induced injury ( 177 ). In vivo, Hagiwara and associates ( 178 ) reported a significant inhibition of bleomycininduced lung fibrosis in mice following aerosolized NAC during the early inflammatory phase of injury. Whether this effect was secondary to NAC inhibition of cellular inflammation, or its role as a scavenger of reactive oxygen species, is not clear. German investigators evaluated oral NAC as a strategy to augment lung glutathione levels in 17 patients with biopsy-proven IPF. Following therapy with NAC, glutathione levels in BALF were significantly increased compared with pretreatment levels ( 177 ). In a separate study, Behr and colleagues ( 179 ) prospectively studied 18 patients with IPF and assessed the redox balance of the lung and changes in lung function following high-dose NAC therapy for 12 wk. They reported an increase in the total and reduced form of glutathione concentration in the BALF, and significant improvement in pulmonary function. The authors suggest that NAC may be considered as an adjunct in the treatment of IPF. Currently, there is a clinical trial in Europe to evaluate the potential benefits of NAC in IPF.
Anticytokine Approaches
As mechanisms of fibrosis at the cellular and molecular level become elucidated, their application to the development of novel therapeutic strategies appears promising. Given the temporal heterogeneity of the UIP lesion, early histopathological abnormalities may be present even in patients with advanced IPF. If early cytokine release is relevant to the initiation of this pathogenic response, then the targeting of early cytokines such as TNF-α should be considered. TNF-α appears to be upregulated soon after bleomycin-induced injury and has been implicated in a variety of inflammatory processes ( 180 ). Sime and colleagues ( 181 ) showed that transient overexpression of TNF-α in rat lung led to fibrosis associated with concomitant TGF-β expression and proliferation of myofibroblasts ( 181 ). Furthermore, upregulation of TNF-α expression has been shown to occur in inbred murine strains that are sensitive to bleomycin-induced lung fibrosis, with similar expression being absent in resistant strains ( 180 ). In addition, Ortiz and colleagues ( 182 ) showed that TNF receptor-deficient mice did not develop pulmonary fibrosis following exposure to bleomycin despite increased TNF expression. Studies of human lung biopsy specimens of patients with IPF have shown an upregulation of TNF-α mRNA and protein ( 183 ). These observations along with other studies in animal models demonstrating abrogation of pulmonary fibrosis following treatment with soluble TNF receptors suggest that TNF-α may play an important role in the pathogenesis of pulmonary fibrosis ( 184 ). Several agents that can block TNF-α are now available for human use ( 185 ). There is currently an ongoing clinical trial evaluating the safety and efficacy of etanercept, a TNF-α receptor antagonist, in patients with IPF.
The expression of TNF-α is inhibited by certain cytokines such as IL-10, which is produced by a variety of cells including T-helper (Th) cells, monocytes, and alveolar macrophages ( 186 , 187 ). It is conceivable that IL-10 may be useful in blunting the action of increased TNF-α observed following bleomycin-induced lung injury, therefore possibly inhibiting progression to fibrosis. Arai and colleagues ( 188 ) investigated the possible inhibitory effects of IL-10 by introducing the IL-10 gene into mice exposed to bleomycin and found that bleomycin-induced pulmonary fibrosis was suppressed. These results suggest that treatment with IL-10 during the early inflammatory phases of lung injury may be promising and requires further investigation. Concerns regarding the role of this agent in treating pulmonary fibrosis exist in that IL-10 is a type-2 Th (Th2) cytokine that could suppress IFN-γ expression and promote fibrogenesis ( 189 ). A clinical trial to study the effect of this cytokine is now underway in the United States.
The realization that Th cell subsets could be categorized on the basis of cytokine profiles has helped clarify our understanding of chronic cell-mediated immune responses. The type-1 (Th1) cytokines include IFN-γ, IL-2, IL-12, IL-18, and Th2 cytokines include IL-4, IL-5, IL-10, and IL-13. Analysis of subset populations of Th cells within the interstitium of patients with IPF reveal a predominantly Th2-type pattern of cytokine production, suggesting that alterations in T-cell subpopulations of Th1 and Th2 cells and their associated pattern of cytokine production may contribute to progression of IPF ( 190 ). Supporting evidence comes from studies demonstrating that IFN-γ (a Th1 cytokine) has profound antifibrotic effects in IPF possibly because it shifts the balance away from a Th2-dependent profibrotic environment. Thus, it seems reasonable to target therapy to correct the Th imbalance by either favoring a Th1 phenotype or abrogating the predominant Th2 response (e.g., administration of IL-12 to promote IFN-γ expression, or inhibition of IL-4, IL-13, and so on).
TGF-β is a critical cytokine for the promotion of fibrosis. In bleomycininduced pulmonary fibrosis, passive immunization with neutralizing antibodies against TGF-β reduces collagen deposition ( 191 ). In addition, the overexpression of TGF-β results in a fibrogenic response resembling UIP (i.e., abundant FF) ( 192 ). In patients with IPF, increased expression of TGF-β is localized to bronchiolar epithelial cells, epithelial cells of HC cysts, and hyperplastic type II pneumocytes ( 193 ). It is possible that therapy with neutralizing antibodies against TGF-β1, or utilization of a TGF-β1 inhibitor such as decorin, may become useful in the treatment of IPF ( 194 ).
TGF-β signals through a receptor that activates transcription factors Smad2 and Smad3 promoting TGF-β gene transcription. Interestingly, IFN-γ inhibits the activation of Smad3 and induces the expression of Smad7, an antagonistic molecule that inhibits TGF-β expression. Smad7 can be produced in the laboratory and may become a useful molecule in the treatment of IPF ( 195 ).
Monocyte chemoattractant protein (MCP)-1 is a member of the C-C subfamily of chemokines involved in monocyte/macrophage mediated inflammation ( 196 , 197 ). In addition, MCP-1 has been shown to stimulate pulmonary fibroblasts, TGF-β synthesis, and collagen production ( 198 ). Analysis of serum, BALF, and lung biopsy specimens from patients with IPF reveal increased levels of this chemokine ( 196 , 197 , 199 ). Furthermore, serum MCP-1 levels correlate with clinical course in patients with interstitial lung disease ( 199 ). Further investigation into the clinical significance of MCP-1 and its contribution into the pathogenesis of IPF are necessary, and may provide the groundwork for novel therapies.
Another potential mediator of fibrosis produced in a variety of cells in the lung is the vasoactive peptide endothelin (ET)-1 ( 200 , 201 ). First thought to be primarily a vasoactive agent, ET-1 has been shown to stimulate fibroblast proliferation, activate monocytes, induce collagen production, and regulate cytokine production ( 202 ). Mutsaers and colleagues ( 203 ) revealed that ET-1 levels are augmented following administration of bleomycin with increased localization of the agent in areas of fibrosis. With increases in ET-1 synthesis following TNF-α and TGF-β stimulation, one may speculate that ET-1 may play an important role in the cascade of events leading to pulmonary fibrosis ( 204 , 205 ). Additional support for its role in pulmonary fibrosis comes from studies in animals in which fibrosis was attenuated following treatment with bosentan, an ET receptor antagonist ( 206 ). ET-1 has also been associated with pulmonary fibrosis in humans. In a study examining the expression of ET-1 in patients with interstitial lung fibrosis, Giaid and associates ( 200 ) found a striking expression of ET-1 in lung tissue that correlated with parameters of disease activity in patients with IPF. There is currently an ongoing clinical trial evaluating the safety and efficacy of bosentan in patients with IPF.
Other Agents That Inhibit Fibroblast Proliferation or Induce Fibroblast Apoptosis
Some have hypothesized that inducing fibroblast apoptosis may curb progression of fibrosis. Lovastatin is a pharmacological agent widely used in the treatment of hypercholesterolemia that inhibits 3-hydroxy-3-methylglutarylcoenzyme A, therefore affecting many cellular functions essential for normal cell homeostasis including proliferation and cell survival. Tan and associates showed that clinically achievable concentrations of lovastatin induced apoptosis of human lung fibroblasts in vitro, and in vivo reduced granulation tissue formation and induced fibroblast apoptosis in a guinea pig wound model of fibroproliferation ( 207 ). With its known safety profile and potential antifibrotic effect, lovastatin is an attractive candidate in the treatment of IPF.
Suramin is a sulfonated napthylurea that has been used to treat onchocerciasis, acquired immunodeficiency virus, and prostate cancer. In vitro, suramin antagonizes the effects of a number of growth factors that promote fibrogenesis such as TGF-β, insulin-like growth factor-1, platelet-derived growth factor, epidermal-like growth factor, and fibroblast growth factor. In vivo, suramin has been shown to delay wound healing ( 175 ).
Relaxin is a protein secreted by the gravid uterus responsible for remodeling of the interpubic ligament and cervix during the later phases of pregnancy. Relaxin inhibits the TGF-β-mediated overexpression of extracellular matrix, stimulates the expression of collagenases by lung fibroblasts in vitro, and has been shown to block bleomycin-induced fibrosis in mice ( 141 ).
The eicosanoids are potential candidates for therapeutic intervention. The prostaglandin PGE 2 is a potent inhibitor of fibroblast proliferation and extracellular matrix deposition and may ameliorate the fibrotic process in IPF ( 175 ). Indomethacin, an inhibitor of cyclo-oxygenase, has been shown to decrease bleomycin-induced pulmonary fibrosis in an animal model but to our knowledge, it has not been evaluated in human IPF ( 175 ). The profibrotic leukotriene B4 has been shown to be increased in BALF and lung tissue of patients with IPF ( 175 ). Inhibition of leukotriene production may be an effective adjuvant therapy, and drugs are now available to block leukotriene synthesis.
Gene-specific antisense therapy against proteins known to be important in human lung fibroblast proliferation may become an effective approach in treating IPF patients. In vitro, Chen and associates showed that gene-specific oligonucleotides (oligos) against c-Ki-RAS substantially inhibited the proliferation of human fibroblasts ( 141 ).
Beractan is a natural bovine lung extract containing phospholipids, neutral lipids, fatty acids, and surfactant-associated proteins. In vitro, beractan provoked fibroblast apoptosis, induced collagenase-1 expression, and decreased type I collagen ( 141 ).
Other Novel Strategies
Pulmonary fibrosis can be complicated by pulmonary hypertension limiting exercise tolerance and survival. German investigators performed a randomized-controlled, open-label trial in 16 individuals with pulmonary hypertension secondary to pulmonary fibrosis. They compared oral sildenafil with inhaled nitric oxide and infused epoprostenol. A single dose of sildenafil reduced pulmonary vascular resistance by nearly one-third and increased the mean arterial blood oxygen tension by 14 mmHg. The drug was well tolerated with no adverse effects on ventilation-perfusion matching ( 208 ). A clinical trial evaluating sildenafil in patients with IPF and pulmonary hypertension will be conducted soon.
Conclusions
In recent years, significant advances have been made in our understanding and management of IPF. However, in order to further our knowledge and make significant progress in the care of these patients, it is critical that we improve our understanding of the natural history and pathogenesis of IPF. In addition, we need to pursue novel imaging and diagnostic technologies to improve earlier diagnosis and we must also educate primary care physicians and pulmonologists to refer patients early to an interstitial lung disease specialist or lung transplant center. Therapeutic strategies must target specific aberrant pathways during the natural history of the pathogenesis of pulmonary fibrosis. Only when these issues are in place will we be able to improve the prognosis of disorders associated with progressive pulmonary fibrosis.

IMAGES
VIDEO
COMMENTS
INTRODUCTION: Idiopathic pulmonary fibrosis (IPF) is an interstitial lung disease characterized by chronic, progressive scarring of the lungs and the pathological hallmark of usual interstitial pneumonia. For patients with interstitial lung disease (ILD) with apparently unknown cause and have a chest high resolution computered tomography (HRCT) pattern of probable UIP, indeterminate for UIP ...
Background. The diagnosis of idiopathic pulmonary fibrosis can be quite challenging, even after careful clinical evaluation, imaging and pathological tests. This case report intends to demonstrate and discuss these difficulties, especially those concerning the differential diagnosis with chronic hypersensitivity pneumonitis.
Walsh SLF, Maher TM, Kolb M, et al. Diagnostic accuracy of a clinical diagnosis of idiopathic pulmonary fibrosis: an international case-cohort study. Eur Respir J 2017;50(2):1700936-1700936. Crossref
The different types of pulmonary fibrosis are a subgroup of the interstitial lung diseases (ILDs). They are associated with a chronic and often progressive course. ... Montero MÁ, et al. Chronic hypersensitivity pneumonitis in patients diagnosed with idiopathic pulmonary fibrosis: a prospective case-cohort study. Lancet Respir Med. 2013; 1:685 ...
Honeycomb fibrosis that is detected on imaging or on gross examination is a marker of end-stage fibrosing lung disease, including idiopathic pulmonary fibrosis. 2,33-36 In this case, ground ...
1. Introduction. Idiopathic pulmonary fibrosis (IPF) is a progressive disease with many suggested etiologies and with an uncertain incidence [ 1, 2 ]. The diagnosis of IPF is mostly made by radiology if a pattern of usual interstitial pneumomia (UIP) is seen. It has been suggested that IPF incidence is higher in Europe and North America, while ...
Introduction. Idiopathic pulmonary fibrosis (IPF) is a chronic lung disease in which progressive fibrosis and scarring of the lung parenchyma leads to symptoms such as dyspnoea on exertion, dry cough and, ultimately, respiratory failure [ 1 ]. IPF is a complex disease with several aspects that are not fully understood, including its ...
Idiopathic Pulmonary Fibrosis: Prospective, Case-Controlled Study of Natural History and Circulating Biomarkers Chest. 2018 Dec;154(6):1359-1370. doi: 10.1016/j.chest.2018.08.1083. ... Idiopathic pulmonary fibrosis (IPF) is a fatal lung disease with 3 to 5 years' survival. Although FVC is used to assess disease progression and treatment ...
Occupational and environmental risk factors for idiopathic pulmonary fibrosis in Australia: case-control study. Thorax. ... in telomerase genes and their association with MUC5B common variant status in patients with idiopathic pulmonary fibrosis: a candidate gene sequencing study. Lancet Respir Med 6: 603-614, 2018. doi: ...
Similarly to idiopathic pulmonary fibrosis (IPF), other interstitial lung diseases can develop progressive pulmonary fibrosis (PPF) characterized by declining lung function, a poor response to immunomodulatory therapies, and early mortality. The pathophysiology of disordered lung repair involves common downstream pathways that lead to pulmonary fibrosis in both IPF and PPF. The antifibrotic ...
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, fibrosing interstitial lung disease of unknown cause. It has a high risk of rapid progression and mortality. We conducted a ...
Abstract. A supervised combined training program was applied to a sedentary 56-year-old man with idiopathic pulmonary fibrosis (IPF) along three years, until lung transplantation. It included: (a) aerobic continuous (CT) and interval training (IT), (b) high load resistance training (RT) and (c) inspiratory muscle training (IMT).
Pulmonary fibrosis is scarring and thickening of the tissue around and between the air sacs called alveoli in the lungs. These changes make it harder for oxygen to pass into the bloodstream. ... Baqir M, et al. Idiopathic pulmonary fibrosis and gastroesophageal reflux disease: A population-based, case-control study. Respiratory Medicine. 2021 ...
Cedars-Sinai investigators have made significant progress in identifying the mechanisms behind idiopathic pulmonary fibrosis, a deadly disease that scars the lungs and impairs breathing. The findings appeared in a study published this summer in the journal Science Advances. Idiopathic pulmonary fibrosis affects more than 100,000 people in the U.S.
Case #1: IPF. We will start off by discussing an IPF case. As we know, the I stands for idiopathic, which means that all other possible causes of lung disease have to be excluded, rather than making the diagnosis by including a specific set of criteria. Review this case study describing a patient diagnosed with idiopathic pulmonary fibrosis ...
The diagnosis of idiopathic pulmonary fibrosis can be quite challenging, even after careful clinical evaluation, imaging and pathological tests. This case report intends to demonstrate and discuss these difficulties, especially those concerning the differential diagnosis with chronic hypersensitivity pneumonitis. A 58-year-old white male presented with shortness of breath, dry cough, fatigue ...
Idiopathic pulmonary fibrosis (IPF) is a progressive lung disorder characterized by scarring of the lungs from an unknown cause. The condition has a poor long-term prognosis. ... Sverzellati N. Deep learning for classifying fibrotic lung disease on high-resolution computed tomography: a case-cohort study. Lancet Respir Med. 2018 Nov; 6 (11):837 ...
Idiopathic pulmonary fibrosis (IPF) is associated with increased risk of respiratory-related hospitalizations. Studies suggest mechanical ventilation (MV) use in IPF does not improve outcomes and guidelines recommend against its general use. ... As is the case for all diagnoses in this study, these conditions were not confirmed clinically. MV ...
A Study to Evaluate PRM-151 in Patients with Idiopathic Pulmonary Fibrosis Rochester, MN. The purpose of this study is to evaluate the effectiveness, safety, and pharmacokinetics of PRM-151 compared with placebo in patients with idiopathic pulmonary fibrosis (IPF). Specific objectives and corresponding endpoints for the study are outlined below.
A case of idiopathic pulmonary fibrosis. 1 Federal state autonomous institution "National Medical Research Center for Children's Health" of the Russian Federation Ministry of Health. Lomonosov Avenue, 2, building 1. Moscow, Russia. 2 I.M. Sechenov First Moscow State Medical University. Bolschaya Pirogovskaya street, 2 building 4. Moscow ...
Rationale: Relatives of patients with familial interstitial pneumonia (FIP) are at increased risk for pulmonary fibrosis and develop preclinical pulmonary fibrosis (PrePF).Objectives: We defined the incidence and progression of new-onset PrePF and its relationship to survival among first-degree relatives of families with FIP.Methods: This is a cohort study of family members with FIP who were ...
Community Complex Case Response Team. Health Information Technology to Prevent Abuse, Neglect, and Exploitation ... Possible immune cell responses in idiopathic pulmonary fibrosis (IPF), a chronic and deadly lung disease characterized by lung scarring, have been suggested for years. ... The researchers used data from the study to create a model ...
The development of pulmonary fibrosis is a rare complication of the novel coronavirus disease 2019 (COVID-19). Limited information is available in the literature about that, and the present study aimed to address this gap. This case-control study included 64 patients with post-COVID-19 pulmonary fibrosis who were hospitalized for COVID-19.
Idiopathic pulmonary fibrosis (IPF) is a chronic fibrotic interstitial lung disease characterized by progressive dyspnea and decreased lung function, yet its exact etiology remains unclear. It is of great significance to discover new drug targets for IPF. We obtained the cis-expression quantitative trait locus (cis-eQTL) of druggable genes from eQTLGen Consortium as exposure and the genome ...
In this study, we report improvement in most of the parameters, which are valuable predictors of death or lung transplant in CF (VO 2 peak, max. effort, peak work rate, V E /VCO 2 slope) and parameters valuable to understand the ventilatory efficiency (VO 2VT1, VO 2VT2, V E, V T, V D /V T VO 2 /HR peak and RR peak) [].An abnormally low exercise capacity and deconditioning in CF results in VO 2 ...
The lead drug in Insilico's pipeline, INS018_055, is an AI-designed TNIK inhibitor being advanced as a treatment for the deadly lung disease idiopathic pulmonary fibrosis (IPF) and is currently ...
The incidence of COVID-induced pulmonary fibrosis caused by COVID can be estimated based on a 15-year observational study of lung pathology after SARS. Most SARS patients with fibrotic lung damage recovered within the first year and then remained healthy; however, in 20% of the cases, significant fibrosis progression was found in 5-10 years.
Idiopathic pulmonary fibrosis is a fatal interstitial lung disease for which effective drug therapies are lacking. Senegenin, an effective active compound from the traditional Chinese herb Polygala tenuifolia Willd, has been shown to have a wide range of pharmacological effects. In this study, we investigated the therapeutic effects of senegenin on pulmonary fibrosis and their associated ...
In case-control studies, smoking has been identified as a possible risk factor for IPF with an odds ratio (OR) of 1.6 (95% confidence interval [CI] ... Additional support for its role in pulmonary fibrosis comes from studies in animals in which fibrosis was attenuated following treatment with bosentan, an ET receptor antagonist . ET-1 has also ...