| We are talking here about a mixture of a weak base and one of its salts - for example, a solution containing ammonia and ammonium chloride. The modern, and easy, way of doing these calculations is to re-think them from the point of view of the ammonium ion rather than of the ammonia solution. Once you have taken this slightly different view-point, everything becomes much the same as before. So how would you find the pH of a solution containing 0.100 mol dm of ammonia and 0.0500 mol dm of ammonium chloride? The mixture will contain lots of unreacted ammonia molecules and lots of ammonium ions as the essential ingredients. The ammonium ions are weakly acidic, and this equilibrium is set up whenever they are in solution in water: You can write a K expression for the ammonium ion, and make the same sort of assumptions as we did in the previous case: The presence of the ammonia in the mixture forces the equilibrium far to the left. That means that you can assume that the ammonium ion concentration is what you started off with in the ammonium chloride, and that the ammonia concentration is all due to the added ammonia solution. The value for K for the ammonium ion is 5.62 x 10 mol dm . Remember that we want to calculate the pH of a buffer solution containing 0.100 mol dm of ammonia and 0.0500 mol dm of ammonium chloride. Just put all these numbers in the K expression, and do the sum: | If this is the first set of questions you have done, please read the before you start. You will need to use the BACK BUTTON on your browser to come back here afterwards. | Where would you like to go now? To the acid-base equilibria menu . . . To the Physical Chemistry menu . . . To Main Menu . . . © Jim Clark 2002 (last modified January 2016) Module 14: Acid-Based EquilibriaLearning outcomes. - Describe the composition and function of acid–base buffers
- Calculate the pH of a buffer before and after the addition of added acid or base
A solution containing appreciable amounts of a weak conjugate acid-base pair is called a buffer solution, or a buffer . Buffer solutions resist a change in pH when small amounts of a strong acid or a strong base are added (Figure 1). A solution of acetic acid and sodium acetate (CH 3 COOH + CH 3 COONa) is an example of a buffer that consists of a weak acid and its salt. An example of a buffer that consists of a weak base and its salt is a solution of ammonia and ammonium chloride (NH 3 ( aq ) + NH 4 Cl( aq )).  Figure 1. (a) The unbuffered solution on the left and the buffered solution on the right have the same pH (pH 8); they are basic, showing the yellow color of the indicator methyl orange at this pH. (b) After the addition of 1 mL of a 0.01-M HCl solution, the buffered solution has not detectably changed its pH but the unbuffered solution has become acidic, as indicated by the change in color of the methyl orange, which turns red at a pH of about 4. (credit: modification of work by Mark Ott) How Buffers WorkTo illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium acetate. The presence of a weak conjugate acid-base pair in the solution imparts the ability to neutralize modest amounts of added strong acid or base. For example, adding strong base to this solution will neutralize hydronium ion and shift the acetic acid ionization equilibrium to the right, partially restoring the decreased H 3 O + concentration: [latex]{\text{CH}}_{3}{\text{CO}}_{2}\text{H}\left(aq\right)+{\text{H}}_{2}\text{O}\left(l\right)\leftrightharpoons {\text{H}}_{3}{\text{O}}^{\text{+}}\left(aq\right)+{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\left(aq\right)[/latex] Likewise, adding strong acid to this buffer solution will neutralize acetate ion, shifting the above ionization equilibrium right and returning [H 3 O + ] to near its original value. Figure 2 provides a graphical illustration of the changes in conjugate-partner concentration that occur in this buffer solution when strong acid and base are added. The buffering action of the solution is essentially a result of the added strong acid and base being converted to the weak acid and base that make up the buffer’s conjugate pair. The weaker acid and base undergo only slight ionization, as compared with the complete ionization of the strong acid and base, and the solution pH, therefore, changes much less drastically than it would in an unbuffered solution. 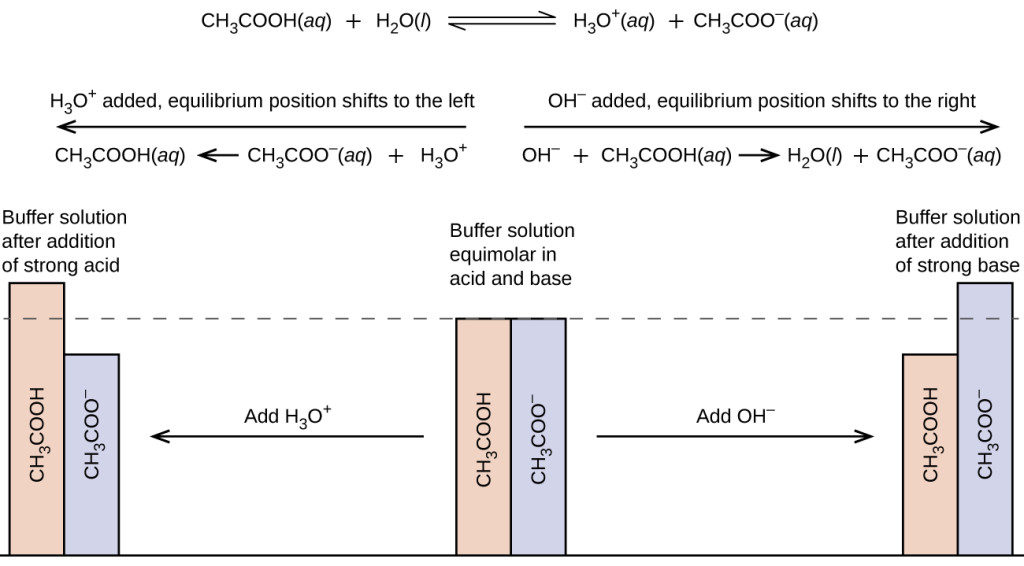 Figure 2. Buffering action in a mixture of acetic acid and acetate salt. Example 1: pH Changes in Buffered and Unbuffered SolutionsAcetate buffers are used in biochemical studies of enzymes and other chemical components of cells to prevent pH changes that might affect the biochemical activity of these compounds. (a) Calculate the pH of an acetate buffer that is a mixture with 0.10 M acetic acid and 0.10 M sodium acetate. (b) Calculate the pH after 1.0 mL of 0.10 NaOH is added to 100 mL of this buffer. (c) For comparison, calculate the pH after 1.0 mL of 0.10 M NaOH is added to 100 mL of a solution of an unbuffered solution with a pH of 4.74. To determine the pH of the buffer solution we use a typical equilibrium calculation (as illustrated in earlier Examples):  Step 1. Determine the direction of change. [latex]{\text{CH}}_{3}{\text{CO}}_{2}\text{H}\left(aq\right)+{\text{H}}_{2}\text{O}\left(l\right)\rightleftharpoons {\text{H}}_{3}{\text{O}}^{\text{+}}\left(aq\right)+{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\left(aq\right)[/latex] We look it up in Ionization Constants of Weak Acids : K a = 1.8 [latex]\times [/latex] 10 −5 . With [CH 3 CO 2 H] = [latex]\left[{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\right][/latex] = 0.10 M and [latex]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right][/latex] = ~0 M , the reaction shifts to the right to form [latex]{\text{H}}_{3}{\text{O}}^{\text{+}}[/latex]. ![assignment of buffer solution This table has two main columns and four rows. The first row for the first column does not have a heading and then has the following in the first column: Initial concentration ( M ), Change ( M ), Equilibrium concentration ( M ). The second column has the header of “[ C H subscript 3 C O subscript 2 H ] [ H subscript 2 O ] equilibrium arrow H subscript 3 O superscript plus sign [ C H subscript 3 C O subscript 2 superscript negative sign ].” Under the second column is a subgroup of four columns and three rows. The first column has the following: 0.10, negative x, 0.10 minus sign x. The second column is blank. The third column has the following: approximately 0, positive x, x. The fourth column has the following: 0.10, positive x, 0.10 plus sign x.](https://openstax.org/resources/c6cd395413486614bd489c119fd3913a299faa7e) Step 3. Solve for x and the equilibrium concentrations. [latex]x=1.8\times {10}^{-5}M[/latex] [latex]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]=0+x=1.8\times {10}^{-5}M[/latex] [latex]\text{pH}=-\text{log}\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]=-\text{log}\left(1.8\times {10}^{-5}\right)[/latex] [latex]=4.74[/latex] Step 4. Check the work . If we calculate all calculated equilibrium concentrations, we find that the equilibrium value of the reaction coefficient, Q = K a . ![assignment of buffer solution Eight tan rectangles are shown in four columns of two rectangles each that are connected with right pointing arrows. The first rectangle in the upper left is labeled “Volume of N a O H solution.” An arrow points right to a second rectangle labeled “Moles of N a O H added.” A second arrow points right to a third rectangle labeled “Additional moles of N a C H subscript 3 C O subscript 2.” Just beneath the first rectangle in the upper left is a rectangle labeled “Volume of buffer solution.” An arrow points right to another rectangle labeled “Initial moles of C H subscript 3 C O subscript 2 H.” This rectangle points to the same third rectangle, which is labeled “ Additional moles of N a C H subscript 3 C O subscript 2.” An arrow points right to a rectangle labeled “ Unreacted moles of C H subscript 3 C O subscript 2 H.” An arrow points from this rectangle to a rectangle below labeled “[ C H subscript 3 C O subscript 2 H ].” An arrow extends below the “Additional moles of N a C H subscript 3 C O subscript 2” rectangle to a rectangle labeled “[ C H subscript 3 C O subscript 2 ].” This rectangle points right to the rectangle labeled “[ C H subscript 3 C O subscript 2 H ].”](https://s3-us-west-2.amazonaws.com/courses-images/wp-content/uploads/sites/219/2016/08/09043740/CNX_Chem_14_06_steps2_img.jpg) Step 1. Determine the moles of NaOH. One milliliter (0.0010 L) of 0.10 M NaOH contains [latex]0.0010\cancel{\text{L}}\times \left(\dfrac{0.10\text{mol NaOH}}{1\cancel{\text{L}}}\right)=1.0\times {10}^{-4}\text{mol NaOH}[/latex] Step 2. Determine the moles of CH 2 CO 2 H. Before reaction, 0.100 L of the buffer solution contains [latex]0.100\cancel{\text{L}}\times \left(\dfrac{0.100\text{mol}{\text{ CH}}_{3}{\text{CO}}_{2}\text{H}}{1\cancel{\text{L}}}\right)=1.00\times {10}^{-2}\text{mol}{\text{CH}}_{3}{\text{CO}}_{2}\text{H}[/latex] Step 3. Solve for the amount of NaCH 3 CO 2 produced. The 1.0 [latex]\times [/latex] 10 −4 mol of NaOH neutralizes 1.0 [latex]\times [/latex] 10 −4 mol of CH 3 CO 2 H, leaving [latex]\left(1.0\times {10}^{-2}\right)-\left(0.01\times {10}^{-2}\right)=0.99\times {10}^{-2}\text{mol}{\text{CH}}_{3}{\text{CO}}_{2}\text{H}[/latex] and producing 1.0 [latex]\times [/latex] 10 −4 mol of NaCH 3 CO 2 . This makes a total of [latex]\left(1.0\times {10}^{-2}\right)+\left(0.01\times {10}^{-2}\right)=1.01\times {10}^{-2}\text{mol}{\text{NaCH}}_{3}{\text{CO}}_{2}[/latex] Step 4. Find the molarity of the products. After reaction, CH 3 CO 2 H and NaCH 3 CO 2 are contained in 101 mL of the intermediate solution, so: [latex]\left[{\text{CH}}_{3}{\text{CO}}_{2}\text{H}\right]=\dfrac{9.9\times {10}^{-3}\text{mol}}{0.101\text{L}}=0.098M[/latex] [latex]\left[{\text{NaCH}}_{3}{\text{CO}}_{2}\right]=\dfrac{1.01\times {10}^{-2}\text{mol}}{0.101\text{L}}=0.100M[/latex]  This 1.8 [latex]\times [/latex] 10 −5 – M solution of HCl has the same hydronium ion concentration as the 0.10- M solution of acetic acid-sodium acetate buffer described in part (a) of this example. The solution contains: [latex]0.100\text{L}\times \left(\dfrac{1.8\times {10}^{-5}\text{mol HCl}}{1\text{L}}\right)=1.8\times {10}^{-6}\text{mol HCl}[/latex] As shown in part (b), 1 mL of 0.10 M NaOH contains 1.0 [latex]\times [/latex] 10 −4 mol of NaOH. When the NaOH and HCl solutions are mixed, the HCl is the limiting reagent in the reaction. All of the HCl reacts, and the amount of NaOH that remains is: [latex]\left(1.0\times {10}^{-4}\right)-\left(1.8\times {10}^{-6}\right)=9.8\times {10}^{-5}M[/latex] - The concentration of NaOH is: [latex]\dfrac{9.8\times {10}^{-5}M\text{NaOH}}{0.101\text{L}}=9.7\times {10}^{-4}M[/latex]
- The pOH of this solution is: [latex]\text{pOH}=-\text{log}\left[{\text{OH}}^{-}\right]=-\text{log}\left(9.7\times {10}^{-4}\right)=3.01[/latex]
- The pH is: [latex]\text{pH}=14.00-\text{pOH}=10.99[/latex]
The pH changes from 4.74 to 10.99 in this unbuffered solution. This compares to the change of 4.74 to 4.75 that occurred when the same amount of NaOH was added to the buffered solution described in part (b). Check Your LearningShow that adding 1.0 mL of 0.10 M HCl changes the pH of 100 mL of a 1.8 [latex]\times [/latex] 10 −5 M HCl solution from 4.74 to 3.00. Initial pH of 1.8 [latex]\times [/latex] 10 −5 M HCl; pH = −log [latex]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right][/latex] = −log[1.8 [latex]\times [/latex] 10 −5 ] = 4.74 Moles of [latex]{\text{H}}_{3}{\text{O}}^{\text{+}}[/latex] in 100 mL 1.8 [latex]\times [/latex] 10 −5 M HCl; 1.8 [latex]\times [/latex] 10 −5 moles/L [latex]\times [/latex] 0.100 L = 1.8 [latex]\times [/latex] 10 −6 Moles of [latex]{\text{H}}_{3}{\text{O}}^{\text{+}}[/latex] added by addition of 1.0 mL of 0.10 M HCl: 0.10 moles/L [latex]\times [/latex] 0.0010 L = 1.0 [latex]\times [/latex] 10 −4 moles; final pH after addition of 1.0 mL of 0.10 M HCl: [latex]\text{pH}=-\text{log}\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]=-\text{log}\left(\dfrac{\text{total moles}{\text{ H}}_{3}{\text{O}}^{\text{+}}}{\text{total volume}}\right)=-\text{log}\left(\dfrac{1.0\times {10}^{-4}\text{mol}+1.8\times {10}^{-6}\text{mol}}{101\text{mL}\left(\frac{1\text{L}}{1000\text{mL}}\right)}\right)=3.00[/latex] If we add an acid or a base to a buffer that is a mixture of a weak base and its salt, the calculations of the changes in pH are analogous to those for a buffer mixture of a weak acid and its salt. Buffer CapacityBuffer solutions do not have an unlimited capacity to keep the pH relatively constant (Figure 3). If we add so much base to a buffer that the weak acid is exhausted, no more buffering action toward the base is possible. On the other hand, if we add an excess of acid, the weak base would be exhausted, and no more buffering action toward any additional acid would be possible. In fact, we do not even need to exhaust all of the acid or base in a buffer to overwhelm it; its buffering action will diminish rapidly as a given component nears depletion.  Figure 3. The indicator color (methyl orange) shows that a small amount of acid added to a buffered solution of pH 8 (beaker on the left) has little affect on the buffered system (middle beaker). However, a large amount of acid exhausts the buffering capacity of the solution and the pH changes dramatically (beaker on the right). (credit: modification of work by Mark Ott) The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the pH changes significantly, usually by one unit. Buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer mixture. For example, 1 L of a solution that is 1.0 M in acetic acid and 1.0 M in sodium acetate has a greater buffer capacity than 1 L of a solution that is 0.10 M in acetic acid and 0.10 M in sodium acetate even though both solutions have the same pH. The first solution has more buffer capacity because it contains more acetic acid and acetate ion. Selection of Suitable Buffer MixturesThere are two useful rules of thumb for selecting buffer mixtures: ![assignment of buffer solution A graph is shown with a horizontal axis labeled “Added m L of 0.10 M N a O H” which has markings and vertical gridlines every 10 units from 0 to 110. The vertical axis is labeled “p H” and is marked every 1 unit beginning at 0 extending to 11. A break is shown in the vertical axis between 0 and 4. A red curve is drawn on the graph which increases gradually from the point (0, 4.8) up to about (100, 7) after which the graph has a vertical section up to about (100, 11). The curve is labeled [ C H subscript 3 C O subscript 2 H ] is 11 percent of [ C H subscript 3 CO subscript 2 superscript negative].](https://s3-us-west-2.amazonaws.com/courses-images/wp-content/uploads/sites/219/2016/08/09044040/CNX_Chem_14_06_buffer.jpg) Figure 4. The graph, an illustration of buffering action, shows change of pH as an increasing amount of a 0.10- M NaOH solution is added to 100 mL of a buffer solution in which, initially, [CH 3 CO 2 H] = 0.10 M and [CH 3 CO 2 − ]=0.10M. Note the greatly diminished buffering action occurring after the buffer capacity has been reached, resulting in drastic rises in pH on adding more strong base. - Weak acids and their salts are better as buffers for pHs less than 7; weak bases and their salts are better as buffers for pHs greater than 7.
Blood is an important example of a buffered solution, with the principal acid and ion responsible for the buffering action being carbonic acid, H 2 CO 3 , and the bicarbonate ion, [latex]{\text{HCO}}_{3}{}^{-}[/latex]. When an excess of hydrogen ion enters the blood stream, it is removed primarily by the reaction: [latex]{\text{H}}_{3}{\text{O}}^{\text{+}}\left(aq\right)+{\text{HCO}}_{3}{}^{-}\left(aq\right)\longrightarrow {\text{H}}_{2}{\text{CO}}_{3}\left(aq\right)+{\text{H}}_{2}\text{O}\left(l\right)[/latex] When an excess of the hydroxide ion is present, it is removed by the reaction: [latex]{\text{OH}}^{-}\left(aq\right)+{\text{H}}_{2}{\text{CO}}_{3}\left(aq\right)\longrightarrow {\text{HCO}}_{3}{}^{-}\left(aq\right)+{\text{H}}_{2}\text{O}\left(l\right)[/latex] The added strong acid or base is thus effectively converted to the much weaker acid or base of the buffer pair (H 3 O + is converted to H 2 CO 3 and OH – is converted to HCO 3 – ). The pH of human blood thus remains very near the value determined by the buffer pairs pKa, in this case, 7.35. Normal variations in blood pH are usually less than 0.1, and pH changes of 0.4 or greater are likely to be fatal. The Henderson-Hasselbalch EquationThe ionization-constant expression for a solution of a weak acid can be written as: [latex]{K}_{\text{a}}=\dfrac{\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]\left[{\text{A}}^{-}\right]}{\text{[HA]}}[/latex] Rearranging to solve for [latex]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right][/latex], we get: [latex]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]={K}_{\text{a}}\times \dfrac{\text{[HA]}}{\left[{\text{A}}^{-}\right]}[/latex] Taking the negative logarithm of both sides of this equation, we arrive at: [latex]-\text{log}\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]=-\text{log}{K}_{\text{a}}\text{- log}\dfrac{\left[\text{HA}\right]}{\left[{\text{A}}^{-}\right]}[/latex], which can be written as - [latex]\text{pH}=\text{p}{K}_{\text{a}}+\text{log}\dfrac{\left[{\text{A}}^{-}\right]}{\left[\text{HA}\right]}[/latex]
where p K a is the negative of the common logarithm of the ionization constant of the weak acid (p K a = −log K a ). This equation relates the pH, the ionization constant of a weak acid, and the concentrations of the weak acid and its salt in a buffered solution. Scientists often use this expression, called the Henderson-Hasselbalch equation , to calculate the pH of buffer solutions. It is important to note that the “ x is small” assumption must be valid to use this equation. Lawrence Joseph Henderson and Karl Albert HasselbalchLawrence Joseph Henderson (1878–1942) was an American physician, biochemist and physiologist, to name only a few of his many pursuits. He obtained a medical degree from Harvard and then spent 2 years studying in Strasbourg, then a part of Germany, before returning to take a lecturer position at Harvard. He eventually became a professor at Harvard and worked there his entire life. He discovered that the acid-base balance in human blood is regulated by a buffer system formed by the dissolved carbon dioxide in blood. He wrote an equation in 1908 to describe the carbonic acid-carbonate buffer system in blood. Henderson was broadly knowledgeable; in addition to his important research on the physiology of blood, he also wrote on the adaptations of organisms and their fit with their environments, on sociology and on university education. He also founded the Fatigue Laboratory, at the Harvard Business School, which examined human physiology with specific focus on work in industry, exercise, and nutrition. In 1916, Karl Albert Hasselbalch (1874–1962), a Danish physician and chemist, shared authorship in a paper with Christian Bohr in 1904 that described the Bohr effect, which showed that the ability of hemoglobin in the blood to bind with oxygen was inversely related to the acidity of the blood and the concentration of carbon dioxide. The pH scale was introduced in 1909 by another Dane, Sørensen, and in 1912, Hasselbalch published measurements of the pH of blood. In 1916, Hasselbalch expressed Henderson’s equation in logarithmic terms, consistent with the logarithmic scale of pH, and thus the Henderson-Hasselbalch equation was born. Medicine: The Buffer System in BloodThe normal pH of human blood is about 7.4. The carbonate buffer system in the blood uses the following equilibrium reaction: [latex]{\text{CO}}_{2}\left(g\right)+2{\text{H}}_{2}\text{O}\left(l\right)\rightleftharpoons {\text{H}}_{2}{\text{CO}}_{3}\left(aq\right)\rightleftharpoons {\text{HCO}}_{3}{}^{-}\left(aq\right)+{\text{H}}_{3}{\text{O}}^{\text{+}}\left(aq\right)[/latex] The concentration of carbonic acid, H 2 CO 3 is approximately 0.0012 M , and the concentration of the hydrogen carbonate ion, [latex]{\text{HCO}}_{3}{}^{-}[/latex], is around 0.024 M . Using the Henderson-Hasselbalch equation and the p K a of carbonic acid at body temperature, we can calculate the pH of blood: [latex]\text{pH}=\text{p}{K}_{\text{a}}+\text{log}\dfrac{\left[\text{base}\right]}{\left[\text{acid}\right]}=6.1+\text{log}\dfrac{0.024}{0.0012}=7.4[/latex] The fact that the H 2 CO 3 concentration is significantly lower than that of the [latex]{\text{HCO}}_{3}{}^{-}[/latex] ion may seem unusual, but this imbalance is due to the fact that most of the by-products of our metabolism that enter our bloodstream are acidic. Therefore, there must be a larger proportion of base than acid, so that the capacity of the buffer will not be exceeded. Lactic acid is produced in our muscles when we exercise. As the lactic acid enters the bloodstream, it is neutralized by the [latex]{\text{HCO}}_{3}{}^{-}[/latex] ion, producing H 2 CO 3 . An enzyme then accelerates the breakdown of the excess carbonic acid to carbon dioxide and water, which can be eliminated by breathing. In fact, in addition to the regulating effects of the carbonate buffering system on the pH of blood, the body uses breathing to regulate blood pH. If the pH of the blood decreases too far, an increase in breathing removes CO 2 from the blood through the lungs driving the equilibrium reaction such that [latex]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right][/latex] is lowered. If the blood is too alkaline, a lower breath rate increases CO 2 concentration in the blood, driving the equilibrium reaction the other way, increasing [H + ] and restoring an appropriate pH. Key Concepts and SummarySolutions that contain appreciable amounts of a weak conjugate acid-base pair are called buffers. A buffered solution will experience only slight changes in pH when small amounts of acid or base are added. Addition of large amounts of acid or base can exceed the buffer capacity, consuming most of one conjugate partner and preventing further buffering action. Key Equations- [latex]\text{p}K_{\text{a}}=−\text{log}K_{\text{a}}[/latex]
- [latex]\text{p}K_{\text{b}}=−\text{log}K_{\text{b}}[/latex]
- Explain why a buffer can be prepared from a mixture of NH 4 Cl and NaOH but not from NH 3 and NaOH.
- Explain why the pH does not change significantly when a small amount of an acid or a base is added to a solution that contains equal amounts of the acid H 3 PO 4 and a salt of its conjugate base NaH 2 PO 4 .
- Explain why the pH does not change significantly when a small amount of an acid or a base is added to a solution that contains equal amounts of the base NH 3 and a salt of its conjugate acid NH 4 Cl.
- What is [latex]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right][/latex] in a solution of 0.25 M CH 3 CO 2 H and 0.030 M NaCH 3 CO 2 ? [latex]{\text{CH}}_{3}{\text{CO}}_{2}\text{H}\left(aq\right)+{\text{H}}_{2}\text{O}\left(l\right)\rightleftharpoons {\text{H}}_{3}{\text{O}}^{\text{+}}\left(aq\right)+{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\left(aq\right){K}_{\text{a}}=1.8\times {10}^{-5}[/latex]
- What is [latex]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right][/latex] in a solution of 0.075 M HNO 2 and 0.030 M NaNO 2 ? [latex]{\text{HNO}}_{2}\left(aq\right)+{\text{H}}_{2}\text{O}\left(l\right)\rightleftharpoons {\text{H}}_{3}{\text{O}}^{\text{+}}\left(aq\right)+{\text{NO}}_{2}{}^{-}\left(aq\right){K}_{\text{a}}=4.5\times {10}^{-5}[/latex]
- What is [OH − ] in a solution of 0.125 M CH 3 NH 2 and 0.130 M CH 3 NH 3 Cl? [latex]{\text{CH}}_{3}{\text{NH}}_{2}\left(aq\right)+{\text{H}}_{2}\text{O}\left(l\right)\rightleftharpoons {\text{CH}}_{3}{\text{NH}}_{3}{}^{\text{+}}\left(aq\right)+{\text{OH}}^{-}\left(aq\right){K}_{\text{b}}=4.4\times {10}^{-4}[/latex]
- What is [OH − ] in a solution of 1.25 M NH 3 and 0.78 M NH 4 NO 3 ? [latex]{\text{NH}}_{3}\left(aq\right)+{\text{H}}_{2}\text{O}\left(l\right)\rightleftharpoons {\text{NH}}_{4}{}^{\text{+}}\left(aq\right)+{\text{OH}}^{-}\left(aq\right){K}_{\text{b}}=1.8\times {10}^{-5}[/latex]
- What concentration of NH 4 NO 3 is required to make [OH − ] = 1.0 [latex]\times [/latex] 10 −5 in a 0.200- M solution of NH 3 ?
- What concentration of NaF is required to make [latex]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right][/latex] = 2.3 [latex]\times [/latex] 10 −4 in a 0.300- M solution of HF?
- CH 3 CO 2 H
- What will be the pH of a buffer solution prepared from 0.20 mol NH 3 , 0.40 mol NH 4 NO 3 , and just enough water to give 1.00 L of solution?
- Calculate the pH of a buffer solution prepared from 0.155 mol of phosphoric acid, 0.250 mole of KH 2 PO 4 , and enough water to make 0.500 L of solution.
- How much solid NaCH 3 CO 2 •3H 2 O must be added to 0.300 L of a 0.50- M acetic acid solution to give a buffer with a pH of 5.00? (Hint: Assume a negligible change in volume as the solid is added.)
- What mass of NH 4 Cl must be added to 0.750 L of a 0.100- M solution of NH 3 to give a buffer solution with a pH of 9.26? (Hint: Assume a negligible change in volume as the solid is added.)
2. Excess [latex]{\text{H}}_{3}{\text{O}}^{\text{+}}[/latex] is removed primarily by the reaction [latex]{\text{H}}_{3}{\text{O}}^{\text{+}}\left(aq\right)+{\text{H}}_{2}{\text{PO}}_{4}{}^{-}\left(aq\right)\longrightarrow {\text{H}}_{3}{\text{PO}}_{4}\left(aq\right)+{\text{H}}_{2}\text{O}\left(l\right)[/latex] Excess base is removed by the reaction [latex]{\text{OH}}^{-}\left(aq\right)+{\text{H}}_{3}{\text{PO}}_{4}\left(aq\right)\longrightarrow {\text{H}}_{2}{\text{PO}}_{4}{}^{-}\left(aq\right)+{\text{H}}_{2}\text{O}\left(l\right)[/latex] 4. The equilibrium expression is [latex]{K}_{\text{a}}=\frac{\left[{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\right]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]}{\left[{\text{CH}}_{3}{\text{CO}}_{2}\text{H}\right]}=1.8\times {10}^{-5}[/latex] The initial and equilibrium concentrations for this system can be written as follows: | [CH CO H] | [H O ] | [CH CO ] | | Initial concentration ( ) | 0.25 | 0 | 0.030 | | Change ( ) | − | | | | Equilibrium ( ) | 0.25 − | | 0.030 + | Substituting the equilibrium concentrations into the equilibrium expression, and making the assumptions that (0.25 − x ) ≈ 0.25 and (0.030 − x ) ≈ 0.030, gives: [latex]\frac{\left[{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\right]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]}{\left[{\text{CH}}_{3}{\text{CO}}_{2}\text{H}\right]}=\frac{\left(x\right)\left(0.030-x\right)}{\left(0.25-x\right)}\approx \frac{\left(x\right)\left(0.030\right)}{0.25}=1.8\times {10}^{-5}[/latex] Solving for x gives 1.50 [latex]\times [/latex] 10 −4 M . Because this value is less than 5% of both 0.25 and 0.030, our assumptions are correct. Therefore, [latex]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right][/latex] = 1.5 [latex]\times [/latex] 10 −4 M . This problem can also be solved using the Henderson-Hasselbalch equation: [latex]\text{pH}=\text{p}{K}_{\text{a}}+\text{log}\frac{\left[{\text{A}}^{-}\right]}{\left[\text{HA}\right]}[/latex]; p K a = −log( K a ) = −log(1.8 [latex]\times [/latex] 10 −5 ) = 4.74; [HA] ≈ [HA] 0 = [CH 3 CO 2 H] 0 = 0.25 M ; [A − ] ≈ [NaCH 3 CO 2 ] = 0.030 M . Using these data: [latex]\text{pH}=4.74-\text{log}\left(\frac{0.030M}{0.25M}\right)=3.82[/latex]; [latex]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right][/latex] = 10 −pH M = 10 −3.82 M = 1.5 [latex]\times [/latex] 10 −4 M 6. The equilibrium expression is: [latex]{K}_{\text{b}}=\frac{\left[{\text{CH}}_{3}{\text{NH}}_{3}{}^{\text{+}}\right]\left[{\text{OH}}^{-}\right]}{\left[{\text{CH}}_{3}{\text{NH}}_{2}\right]}=4.4\times {10}^{-4}[/latex] | [CH NH ] | [CH NH ] | [OH ] | | Initial concentration ( ) | 0.125 | 0.130 | 0 | | Change ( ) | − | | | | Equilibrium ( ) | 0.125 − | 0.130 + | | Substituting the equilibrium concentrations into the equilibrium expression, and making the assumptions that (0.125 − x ) ≈ 0.125 and (0.130 − x ) ≈ 0.130, gives: [latex]\frac{\left[{\text{CH}}_{3}{\text{NH}}_{3}{}^{\text{+}}\right]\left[{\text{OH}}^{-}\right]}{\left[{\text{CH}}_{3}{\text{NH}}_{2}\right]}=\frac{\left(0.130-x\right)\left(x\right)}{\left(0.125-x\right)}\approx \frac{\left(0.130\right)\left(x\right)}{0.125}=4.4\times {10}^{-4}[/latex] Solving for x gives 4.23 [latex]\times [/latex] 10 −4 M . Because this value is less than 5% of both 0.125 and 0.130, our assumptions are correct. Therefore, [OH − ] = 4.2 [latex]\times [/latex] 10 −4 M . 8. The reaction and equilibrium constant are [latex]{\text{NH}}_{3}\left(aq\right)+{\text{H}}_{2}\text{O}\left(l\right)\rightleftharpoons {\text{NH}}_{4}{}^{\text{+}}\left(aq\right)+{\text{OH}}^{-}\left(aq\right)\,\,\,\,\,\,\,\,\,\,\,{K}_{\text{b}}=1.8\times {10}^{-4}[/latex] The equilibrium expression is [latex]{K}_{\text{b}}=\frac{\left[{\text{NH}}_{4}{}^{\text{+}}\right]\left[{\text{OH}}^{-}\right]}{\left[{\text{NH}}_{3}\right]}=1.8\times {10}^{-5}[/latex] Let x = the concentration of NH 4 NO 3 required. The initial and equilibrium concentrations for this system can be written as follows: | [NH ] | [NH ] | [OH ] | | Initial concentration ( ) | 0.200 | 0.78 | 0 | | Change ( ) | − | | | | Equilibrium ( ) | 0.200 − | | = 1.0 × 10 | Substituting the equilibrium concentrations into the equilibrium expression, and making the assumption that ( x + x ) ≈ x , gives: [latex]\frac{\left[{\text{NH}}_{4}{}^{\text{+}}\right]\left[{\text{OH}}^{-}\right]}{\left[{\text{NH}}_{3}\right]}=\frac{\left(x-x\right)\left(1.0\times {10}^{-5}\right)}{\left(0.200 - 1.0\times {10}^{-5}\right)}\approx \frac{\left(x\right)\left(1.0\times {10}^{-5}\right)}{0.200}=1.8\times {10}^{-5}[/latex] Solving for x gives 0.360 M . Because x is less than 5% of this value, our assumption is correct. Therefore, [latex]\left[{\text{NH}}_{4}{}^{\text{+}}\right][/latex] = [NH 4 NO 3 ] = 0.36 M . 10. The reaction and equilibrium constant are [latex]{\text{CH}}_{3}{\text{CO}}_{2}\text{H}\left(aq\right)+{\text{H}}_{2}\text{O}\left(l\right)\rightleftharpoons {\text{H}}_{3}{\text{O}}^{\text{+}}\left(aq\right)+{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\left(aq\right)\,\,\,\,\,\,\,\,\,\,\,{K}_{\text{a}}=1.8\times {10}^{-5}[/latex] - (a) The added HCl will increase the concentration of [latex]{\text{H}}_{3}{\text{O}}^{\text{+}}[/latex] slightly, which will react with [latex]{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}[/latex] and produce CH 3 CO 2 H in the process. Thus, [latex]\left[{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\right][/latex] decreases and [CH 3 CO 2 H] increases.
- (b) The added KCH 3 CO 2 will increase the concentration of [latex]\left[{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\right][/latex] which will react with [latex]{\text{H}}_{3}{\text{O}}^{\text{+}}[/latex] and produce CH 3 CO 2 H in the process. Thus, [latex]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right][/latex] decreases slightly and [CH 3 CO 2 H] increases.
- (c) The added NaCl will have no effect on the concentration of the ions.
- (d) The added KOH will produce OH − ions, which will react with the [latex]{\text{H}}_{3}{\text{O}}^{\text{+}}[/latex], thus reducing [latex]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right][/latex]. Some additional CH 3 CO 2 H will dissociate, producing [latex]\left[{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\right][/latex] ions in the process. Thus, [CH 3 CO 2 H] decreases slightly and [latex]\left[{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\right][/latex] increases.
- (e) The added CH 3 CO 2 H will increase its concentration, causing more of it to dissociate and producing more [latex]\left[{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\right][/latex] and [latex]{\text{H}}_{3}{\text{O}}^{\text{+}}[/latex] in the process. Thus, [latex]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right][/latex] increases slightly and [latex]\left[{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\right][/latex] increases.
12. The reaction and equilibrium constant are: [latex]{\text{NH}}_{3}\left(aq\right)+{\text{H}}_{2}\text{O}\left(l\right)\rightleftharpoons {\text{NH}}_{4}{}^{\text{+}}\left(aq\right)+{\text{OH}}^{-}\left(aq\right)\,\,\,\,\,\,\,\,\,\,\,{K}_{\text{b}}=1.8\times {10}^{-5}[/latex] The equilibrium expression is: [latex]{K}_{\text{b}}=\frac{\left[{\text{NH}}_{4}{}^{\text{+}}\right]\left[{\text{OH}}^{-}\right]}{\left[{\text{NH}}_{3}\right]}=1.8\times {10}^{-5}[/latex] The initial concentrations of NH 3 and [latex]{\text{NH}}_{4}{}^{\text{+}}[/latex] are 0.20 M and 0.40 M , respectively. The equilibrium concentrations for this system can be written as follows: | [NH ] | NH ] | [OH ] | | Initial concentration ( ) | 0.20 | 0.40 | 0 | | Change ( ) | | | | | Equilibrium ( ) | 0.20 − | 0.40 + | | Substituting the equilibrium concentrations into the equilibrium expression, and making the assumptions that (0.20 − x ) ≈ 0.20 and (0.40 + x ) ≈ 0.40, gives: [latex]\frac{\left[{\text{NH}}_{4}{}^{\text{+}}\right]\left[{\text{OH}}^{-}\right]}{\left[{\text{NH}}_{3}\right]}=\frac{\left(0.40+x\right)\left(x\right)}{\left(0.20-x\right)}\approx \frac{\left(0.40\right)\left(x\right)}{0.20}=1.8\times {10}^{-5}[/latex] Solving for x gives 9.00 [latex]\times [/latex] 10 −6 M . Because this value is less than 5% of both 0.20 and 0.40, our assumptions are correct. Therefore, [OH − ] = 9.00 [latex]\times [/latex] 10 −6 M . Thus: - pOH = −log(9.00 [latex]\times [/latex] 10 −6 ) = 5.046
- pH = 14.000 − pOH = 14.000 − 5.046 = 8.954 = 8.95
14. The reaction and equilibrium constant are: [latex]{\text{CH}}_{3}{\text{CO}}_{2}\text{H}\left(aq\right)+{\text{H}}_{2}\text{O}\left(l\right)\rightleftharpoons {\text{H}}_{3}{\text{O}}^{\text{+}}\left(aq\right)+{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\left(aq\right)\,\,\,\,\,\,\,\,\,\,\,{K}_{\text{a}}=1.8\times {10}^{-5}[/latex] The equilibrium expression is [latex]{K}_{\text{a}}=\frac{\left[{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\right]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]}{\left[{\text{CH}}_{3}{\text{CO}}_{2}\text{H}\right]}=1.8\times {10}^{-5}[/latex] Let x be the concentration of [latex]{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}[/latex]. The hydronium ion concentration at equilibrium is [latex]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right][/latex] = 10 −pH = 10 −5.00 = 1.00 [latex]\times [/latex] 10 −5 M | [CH CO H] | [H O ] | [CH CO ] | | Initial concentration ( ) | 0.50 | 0 | | | Change ( ) | − | | | | Equilibrium ( ) | 0.50 − | = 10 × 10 | | [latex]\frac{\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]\left[{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\right]}{\left[{\text{CH}}_{3}{\text{CO}}_{2}\text{H}\right]}=\frac{\left(1.0\times {10}^{-5}\right)\left(x+x\right)}{\left(0.50 - 1.0\times {10}^{-5}\right)}\approx \frac{\left(1.0\times {10}^{-5}\right)\left(x\right)}{0.50}=1.8\times {10}^{-5}[/latex] Solving for x gives 0.900 M . Because x is less than 5% of this value, our assumption is correct. Therefore, [latex]\left[{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\right][/latex] = 0.900 M . Using the molar mass of NaC 2 H 3 O 2 •3H 2 O (136.080 /mol) and the volume gives the mass required: [latex]\frac{0.900\text{mol}}{1\text{L}}\times 0.300\text{L}\times \frac{136.080\text{g}}{1\text{mol}}=36.7=37\text{g}\left(0.27\text{mol}\right)[/latex] - What is the pH of the solution?
- Is the solution acidic or basic?
- What is the pH of a solution that results when 3.00 mL of 0.034 M HCl is added to 0.200 L of the original buffer?
- What is the pH of this buffer solution?
- What is the pH of a solution that results when 3.00 mL of 0.034 M HCl is added to the solution?
The reaction and equilibrium constant are [latex]{\text{CH}}_{3}{\text{CO}}_{2}\text{H}\left(aq\right)+{\text{H}}_{2}\text{O}\left(l\right)\rightleftharpoons {\text{H}}_{3}{\text{O}}^{\text{+}}\left(aq\right)+{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\left(aq\right)\,\,\,\,\,\,\,\,\,\,\,{K}_{\text{a}}=1.8\times {10}^{-5}[/latex] The molar mass of NH 4 Cl is 53.4912 g/mol. The moles of NH 4 Cl are: [latex]\frac{5.36\text{g}}{53.4912\text{g}{\text{mol}}^{-1}}=0.1002\text{mol}[/latex] Assume 0.500 L of each solution is present The total volume is thus 1.000 L. The initial concentrations of the ions is obtained using M 1 V 1 = M 2 V 2 , or: [latex]\begin{array}{l} \left[{\text{CH}}_{3}{\text{CO}}_{2}\text{H}\right]={M}_{1}\times \frac{{V}_{1}}{{V}_{2}}=\left(0.200\right)\times \frac{0.500\text{L}}{1.000\text{L}}=0.100M\\ \left[{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\right]={M}_{1}\times \frac{{V}_{1}}{{V}_{2}}=\left(0.600\right)\times \frac{0.500\text{L}}{1.000\text{L}}=0.300M\end{array}[/latex] The initial and equilibrium concentrations of this system can be written as follows: | [CH CO H] | [H O ] | [CH CO ] | | Initial concentration ( ) | 0.100 | 0 | 0.300 | | Change ( ) | − | | | | Equilibrium ( ) | 0.100 − | | 0.300 + | Substituting the equilibrium concentrations into the equilibrium expression, and making the assumptions that (0.100 − x ) ≈ 0.100 and (0.300 − x ) ≈ 0.300, gives: [latex]\frac{\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]\left[{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\right]}{\left[{\text{CH}}_{3}{\text{CO}}_{2}\text{H}\right]}=\frac{\left(x\right)\left(0.300+x\right)}{\left(0.100-x\right)}\approx \frac{\left(x\right)\left(0.300\right)}{0.100}=1.80\times {10}^{-5}[/latex] Solving for x gives 6.000 [latex]\times [/latex] 10 −6 M . Because this value is less than 5% of both 0.100 and 0.300, our assumptions are correct. Therefore [latex]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right][/latex] = 6.000 [latex]\times [/latex] 10 −6 M : pH = −log(6.000 [latex]\times [/latex] 10 −6 ) = 5.2218 = 5.222; The solution is acidic. Assume that the added H + reacts completely with an equal amount of [latex]{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}[/latex], forming an equal amount of CH 3 CO 2 H in the process. The moles of H + added equal 0.034 M [latex]\times [/latex] 0.00300 L = 1.02 [latex]\times [/latex] 10 −4 mol. For the acetic acid, the initial moles present equal 0.2000 M [latex]\times [/latex] 0.500 L = 0.1000 mol, and for acetate ion, 0.600 M [latex]\times [/latex] 0.500 L = 0.3000 mol. Thus: mol CH 3 CO 2 H = 0.1000 + 1.02 [latex]\times [/latex] 10 −4 = 0.1001 mol [latex]\text{mol}{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}=0.3000 - 1.02\times {10}^{-4}=0.2999\text{mol}[/latex] Final volume = 1.000 L + 3.00 [latex]\times [/latex] 10 −3 L = 1.0030 L The initial concentrations are therefore: - [latex]\left[{\text{CH}}_{3}{\text{CO}}_{2}\text{H}\right]=\frac{0.1001\text{mol}}{1.0030\text{L}}=0.09980M[/latex]
- [latex]\left[{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\right]=\frac{0.2999\text{mol}}{1.0030\text{L}}=0.2990M[/latex]
| [CH CO H] | [H O ] | [CH CO ] | | Initial concentration ( ) | 0.09980 | 0 | 0.2990 | | Change ( ) | − | | | | Equilibrium ( ) | 0.09980 − | | 0.2990 + | Substituting the equilibrium concentrations into the equilibrium expression, and making the assumptions that (0.09980 − x ) ≈ 0.09980 and (0.2990 − x ) ≈ 0.2990, gives: [latex]\frac{\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]\left[{\text{CH}}_{3}{\text{CO}}_{2}{}^{-}\right]}{\left[{\text{CH}}_{3}{\text{CO}}_{2}\text{H}\right]}=\frac{\left(x\right)\left(0.2990+x\right)}{\left(0.09980-x\right)}\approx \frac{\left(x\right)\left(0.2990\right)}{0.09980}=1.80\times {10}^{-5}[/latex] Solving for x gives 6.008 [latex]\times [/latex] 10 −6 M . Because this value is less than 5% of both 0.09980 and 0.2990, our assumptions are correct. Therefore, [latex]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right][/latex] = 6.008 [latex]\times [/latex] 10 −6 M . pH = −log(6.008 [latex]\times [/latex] 10 −6 ) = 5.2213 = 5.221 - Which acid in Table 1 of Relative Strengths of Acids and Bases is most appropriate for preparation of a buffer solution with a pH of 3.1? Explain your choice.
- Which acid in Table 1 of Relative Strengths of Acids and Bases is most appropriate for preparation of a buffer solution with a pH of 3.7? Explain your choice.
- Which base in Table 2 of Relative Strengths of Acids and Bases is most appropriate for preparation of a buffer solution with a pH of 10.65? Explain your choice.
- Which base in Table 2 of Relative Strengths of Acids and Bases is most appropriate for preparation of a buffer solution with a pH of 9.20? Explain your choice.
- Saccharin, C 7 H 4 NSO 3 H, is a weak acid ( K a = 2.1 [latex]\times [/latex] 10 −2 ). If 0.250 L of diet cola with a buffered pH of 5.48 was prepared from 2.00 [latex]\times [/latex] 10 −3 g of sodium saccharide, Na(C 7 H 4 NSO 3 ), what are the final concentrations of saccharine and sodium saccharide in the solution?
- What is the pH of 1.000 L of a solution of 100.0 g of glutamic acid (C 5 H 9 NO 4 , a diprotic acid; K 1 = 8.5 [latex]\times [/latex] 10 −5 , K 2 = 3.39 [latex]\times [/latex] 10 −10 ) to which has been added 20.0 g of NaOH during the preparation of monosodium glutamate, the flavoring agent? What is the pH when exactly 1 mol of NaOH per mole of acid has been added?
1. To prepare the best buffer for a weak acid HA and its salt, the ratio [latex]\frac{\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]}{{K}_{\text{a}}}[/latex] should be as close to 1 as possible for effective buffer action. The [latex]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right][/latex] concentration in a buffer of pH 3.1 is [latex]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right][/latex] = 10 −3.1 = 7.94 [latex]\times [/latex] 10 −4 M We can now solve for K a of the best acid as follows: [latex]\begin{array}{l}{ }\frac{\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]}{{K}_{\text{a}}}=1\\ {K}_{\text{a}}=\frac{\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]}{1}=7.94\times {10}^{-4}\end{array}[/latex] In Table 1 of Relative Strengths of Acids and Bases , the acid with the closest K a to 7.94 [latex]\times [/latex] 10 −4 is HF, with a K a of 7.2 [latex]\times [/latex] 10 −4 . 3. For buffers with pHs > 7, you should use a weak base and its salt. The most effective buffer will have a ratio [latex]\frac{\left[{\text{OH}}^{-}\right]}{{K}_{\text{b}}}[/latex] that is as close to 1 as possible. The pOH of the buffer is 14.00 − 10.65 = 3.35. Therefore, [OH − ] is [OH − ] = 10 −pOH = 10 −3.35 = 4.467 [latex]\times [/latex] 10 −4 M . We can now solve for K b of the best base as follows: [latex]\frac{\left[{\text{OH}}^{-}\right]}{{K}_{\text{b}}}=1[/latex] K b = [OH − ] = 4.47 [latex]\times [/latex] 10 −4 In Table 2 of Relative Strengths of Acids and Bases , the base with the closest K b to 4.47 [latex]\times [/latex] 10 −4 is CH 3 NH 2 , with a K b = 4.4 [latex]\times [/latex] 10 −4 . 5. The molar mass of sodium saccharide is 205.169 g/mol. Using the abbreviations HA for saccharin and NaA for sodium saccharide the number of moles of NaA in the solution is: [latex]2.00\times {10}^{-3}\text{g}\times \frac{1\text{mol}}{205.169\text{g}}=9.75\times {10}^{-6}\text{mol}[/latex] This ionizes initially to form saccharin ions, A − , with: [latex]\left[{\text{A}}^{-}\right]=\frac{9.75\times {10}^{-6}\text{mol}}{0.250\text{L}}=3.9\times {10}^{-5}M[/latex] but A − reacts with water: [latex]\begin{array}{l}{\text{A}}^{-}\left(aq\right)+{\text{H}}_{2}\text{O}\left(l\right)\rightleftharpoons \text{HA}\left(aq\right)+{\text{OH}}^{-}\left(aq\right)\\ {K}_{\text{b}}=\frac{{K}_{\text{w}}}{{K}_{\text{a}}}=\frac{1.0\times {10}^{-14}}{2.1\times {10}^{-12}}=4.8\times {10}^{-3}\\ =4.8\times {10}^{-3}=\frac{\left[\text{HA}\right]\left[{\text{OH}}^{-}\right]}{\left[{\text{A}}^{-}\right]}\end{array}[/latex] The pH of the solution is 5.48, so pOH = 14.00 − 5.48 = 8.52, and [OH − ] = 10 −8.52 = 3.02 [latex]\times [/latex] 10 −9 M Because of the small size of K b , almost all the A − will be in the form of HA. Therefore, [latex]4.8\times {10}^{-3}=\frac{x\left(3.02\times {10}^{-9}\right)}{3.9\times {10}^{-5}-x}[/latex], where x ≈ 3.9 [latex]\times [/latex] 10 −5 M = [HA] = [C 7 H 4 NSO 3 H] Consequently, [A − ] is extremely small. Therefore, solve for [A − ] from the equilibrium expression: [latex]\left[{\text{A}}^{-}\right]=\frac{\left[\text{HA}\right]\left[{\text{OH}}^{-}\right]}{{K}_{\text{b}}}=\frac{\left(3.9\times {10}^{-5}\right)\left(3.02\times {10}^{-9}\right)}{4.8\times {10}^{-3}}=2.5\times {10}^{-11}M=\left[\text{Na}\left({\text{C}}_{7}{\text{H}}_{4}{\text{NSO}}_{3}\right)\right][/latex] buffer capacity: amount of an acid or base that can be added to a volume of a buffer solution before its pH changes significantly (usually by one pH unit) buffer: mixture of a weak acid or a weak base and the salt of its conjugate; the pH of a buffer resists change when small amounts of acid or base are added Henderson-Hasselbalch equation: equation used to calculate the pH of buffer solutions - Chemistry 2e. Provided by : OpenStax. Located at : https://openstax.org/ . License : CC BY: Attribution . License Terms : Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
- IIT JEE Study Material
- Buffer Solutions
Buffer SolutionA buffer solution is a water solvent-based solution which consists of a mixture containing a weak acid and the conjugate base of the weak acid or a weak base and the conjugate acid of the weak base. They resist a change in pH upon dilution or upon the addition of small amounts of acid/alkali to them. Download Complete Chapter Notes of Equilibrium Download Now The pH of buffer solutions shows minimal change upon the addition of a very small quantity of strong acid or strong base. They are therefore used to keep the pH at a constant value. Table of Contents Handerson-Hasselbalch EquationpH MaintenanceWhat Is a Buffer Solution?The buffer solution is a solution able to maintain its hydrogen ion concentration (pH) with only minor changes in the dilution or addition of a small amount of either acid or base. Buffer solutions are used in fermentation , food preservatives, drug delivery, electroplating, printing and the activity of enzymes, and the blood oxygen-carrying capacity needs specific hydrogen ion concentration (pH). Solutions of a weak acid and its conjugate base or weak base and its conjugate acid are able to maintain pH and are buffer solutions. Types of Buffer SolutionsThe two primary types into which buffer solutions are broadly classified are acidic and alkaline buffers. Acidic BuffersAs the name suggests, these solutions are used to maintain acidic environments. Acid buffer has acidic pH and is prepared by mixing a weak acid and its salt with a strong base. An aqueous solution of an equal concentration of acetic acid and sodium acetate has a pH of 4.74. - The pH of these solutions is below seven.
- These solutions consist of a weak acid and a salt of a weak acid.
- An example of an acidic buffer solution is a mixture of sodium acetate and acetic acid (pH = 4.75).
Alkaline BuffersThese buffer solutions are used to maintain basic conditions. A basic buffer has a basic pH and is prepared by mixing a weak base and its salt with strong acid. The aqueous solution of an equal concentration of ammonium hydroxide and ammonium chloride has a pH of 9.25. - The pH of these solutions is above seven.
- They contain a weak base and a salt of the weak base.
- An example of an alkaline buffer solution is a mixture of ammonium hydroxide and ammonium chloride (pH = 9.25).
- Acid and Base
- pH Scale and Acidity
- pH and Solutions
 Mechanism of a Buffering ActionIn solution, the salt is completely ionised, and the weak acid is partly ionised. - CH 3 COONa ⇌ Na + + CH 3 COO –
- CH 3 COOH ⇌ H + + CH 3 COO –
On Addition of Acid and Base1. On addition of acid, the released protons of acid will be removed by the acetate ions to form an acetic acid molecule. H + + CH 3 COO – (from added acid) ⇌ CH 3 COOH (from buffer solution) 2. On addition of the base, the hydroxide released by the base will be removed by the hydrogen ions to form water. HO – + H + (from added base) ⇌ H 2 O (from buffer solution) Preparation of a Buffer SolutionIf the dissociation constant of the acid (pK a ) and of the base (pK b ) is known, a buffer solution can be prepared by controlling the salt-acid or the salt-base ratio. As discussed earlier, these solutions are prepared by mixing the weak bases with their corresponding conjugate acids or by mixing weak acids with their corresponding conjugate bases. An example of this method of preparing buffer solutions can be given by the preparation of a phosphate buffer by mixing HPO 4 2- and H 2 PO 4- . The pH maintained by this solution is 7.4. Preparation of Acid BufferConsider an acid buffer solution containing a weak acid (HA) and its salt (KA) with a strong base (KOH). Weak acid HA ionises, and the equilibrium can be written as HA + H 2 O ⇋ H + + A − Acid dissociation constant = Ka = [H + ] [A – ]/HA Taking the negative log of RHS and LHS,  pH of acid buffer = pKa + ([salt]/[acid]) The equation is the Henderson-Hasselbalch equation , popularly known as the Henderson equation. Preparation of a Base BufferConsider a base buffer solution containing a weak base (B) and its salt (BA) with strong acid. pOH, can be derived as above. - pOH of a basic buffer = pKb + log ([salt]/[acid])
- pH of a basic buffer = pKa – log ([salt]/[acid])
Significance of the Handerson EquationHanderson equation can be used to - Calculate the pH of the buffer prepared from a mixture of salt and weak acid/base.
- Calculate the pKa value.
- Prepare buffer solution of needed pH.
Limitations of Henderson-Hasselbalch EquationThe Henderson-Hasselbalch equation cannot be used for strong acids and strong bases. Buffering CapacityThe number of millimoles of acid or base to be added to a litre of buffer solution to change the pH by one unit is the buffer capacity of the buffer. Β = millimoles /(ΔpH) Problems on Buffer SolutionProblem 1: What is the ratio of base to acid when pH = p K a in buffer solution? How about when pH = P K a + 1? pH = p K a when the ratio of base to acid is 1 because log 1 = 0 When log (base/acid) = 1, then the ratio of base to acid is 10:1 Problem 2: What is the pH of a buffered solution of 0.5 M ammonia and 0.5 M ammonium chloride when enough hydrochloric acid corresponds to make 0.15 M HCl? The p K b of ammonia is 4.75. p K a = 14 – p K b . = 9.25 0.15 M H + reacts with 0.15 M ammonia to form 0.15 M more ammonium. So, the ammonium ion is 0.65 M and 0.35 M remaining ammonia (base). Using the Henderson-Hasselbalch equation, pKa – log ([salt]/[acid]) = 9.25 – log (.65/.35) = 9.25 – .269 = 8.98 Problem 3: How many moles of sodium acetate and acetic acid must you use to prepare 1.00 L of a 0.100 mol/L buffer with pH 5.00? pH = pKa + log([A−][HA]) 5.00 = 4.74 + log([A−][HA]) log([A−][HA]) = 0.26 Also, [A⁻] + [HA] = 0.100 mol/L 1.82[HA] + [HA] = 0.100 mol/L 2.82[HA] = 0.100 mol/L 0.0355 mol of acetic acid and 0.0645 mol of sodium acetate is required to prepare 1 L of the buffer solution. In order to understand how buffer solutions maintain a constant pH, let us consider the example of a buffer solution containing sodium acetate and acetic acid. In this example, it can be noted that the sodium acetate almost completely undergoes ionisation, whereas the acetic acid is only weakly ionised. These equilibrium reactions can be written as - CH 3 COOH ⇌ H + + CH 3 COO –
- CH 3 COONa ⇌ Na + + CH 3 COO –
When strong acids are added, the H + ions combine with the CH 3 COO – ions to give a weakly ionised acetic acid, resulting in a negligible change in the pH of the environment. When strongly alkaline substances are introduced to this buffer solution, the hydroxide ions react with the acids which are free in the solution to yield water molecules, as shown in the reaction given below. CH 3 COOH + OH – ⇌ CH 3 COO – + H 2 O Therefore, the hydroxide ions react with the acid to form water, and the pH remains the same. Uses of Buffer Solutions- There exist a few alternate names that are used to refer to buffer solutions, such as pH buffers or hydrogen ion buffers.
- An example of the use of buffers in pH regulation is the use of bicarbonate and carbonic acid buffer system in order to regulate the pH of animal blood.
- Buffer solutions are also used to maintain an optimum pH for enzyme activity in many organisms.
- The absence of these buffers may lead to the slowing of the enzyme action, loss in enzyme properties , or even denaturing of the enzymes. This denaturation process can even permanently deactivate the catalytic action of the enzymes.
 Frequently Asked Questions – FAQsWhat is a buffer solution, what is the ph range of an acidic solution, what is the ph range of a basic solution, what ph value is considered neutral, what is the drawback of the henderson-hasselbach equation.  Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin! Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz Visit BYJU’S for all JEE related queries and study materials Your result is as below Request OTP on Voice Call Leave a Comment Cancel replyYour Mobile number and Email id will not be published. Required fields are marked * Post My Comment  helpful information l now understand better than before  Register with Aakash BYJU'S & Download Free PDFsRegister with byju's & watch live videos. Talk to our experts 1800-120-456-456  Introduction: What is a Buffer Solution?A buffer is an aqueous solution that consists of a mixture of a weak acid and its salt (acid buffer) or a weak base with its salt (basic buffer). Its pH changes very little when a small amount of strong acid or base is added to it and is thus used to prevent a solution's pH change. Buffer solutions are used for a wide range of chemical applications. Blood is one example of a buffer solution found in nature. Human blood has a natural pH of 7.4. Many people experience severe anxiety and suffer from alkalosis. Alkalosis is a disease in which blood pH is excessively high. The reverse condition is called acidosis-a blood, pH greater than 7.4 Some chemical reactions only occur at a certain pH. Other households and consumer items need to monitor their pH values, such as shampoo to combat the soap's alkalinity to avoid inflammation, baby lotion to retain a pH of around 6 to discourage multiplication of bacteria, washing powder, eye drops, fizzy lemonade etc. Buffer Solution DefinitionSolutions with the stable concentration of hydrogen ions and thus typically with no change in pH which is almost independent of dilution and which change very little with small additions of a strong acid or alkali are called buffers. It can also be described in simple terms as a solution that prevents any pH change when a small amount of a strong acid or a strong base is applied to it, which is called a buffer solution or simply as a buffer. Both buffers have acidity and alkalinity balance. Any compounds, such as ammonium acetate, tend to resist any change in their concentration of hydronium ions or pH, whenever a small amount of a strong acid or a strong base is applied to it. Buffer solutions usually consist of a mixture of a weak acid and salt with a strong base like CH 3 COOH and CH 3 COONa, or a weak base with a strong acid like NH 4 OH and NH 4 Cl and salt. Mechanism of Buffering ActionConsider the example of a buffer solution made by dissolving sodium acetate into acetic acid, to consider how a buffer functions. As you can see from the name, acetate acid is an acid: CH 3 COOH, while sodium acetate dissociates in solution to yield the conjugate base, CH 3 COO-acetate ions. The reaction equation is: CH 3 COOH (aq) + OH-(aq) 🡪CH 3 COO-(aq) + H 2 O (aq) If this solution is combined with a strong acid, the acetate ion can neutralise. CH 3 COO-(aq) + H+(aq) 🡪CH 3 COOH (aq) It changes the original buffer reaction equilibrium, thereby holding the pH steady. Preparation of Buffer SolutionThere are a few methods to prepare a buffer solution with a different pH. Prepare a solution with acid and its conjugate base in the first approach by dissolving the acid component of the buffer in around 60 per cent of the amount of water used to produce the final volume of solution. Instead, use a pH detector to test the pH of the solution. Using a strong base like NaOH the pH can be changed to the desired value. If a base and its conjugate acid are used to make the buffer, the pH can be modified using a strong acid, like HCl. Dilute the solution to the final desired volume, once the pH is right. Additionally, you should prepare solutions for both the solution's acid type and base form. Both solutions must have the same quantity of buffer as in the final solution. Add one solution to the other while tracking the pH to get the final buffer. In a third method, using the Henderson-Hasselbach equation, you can determine the exact amount of acid and conjugate base required to make a buffer of a certain pH: pH = pKa + log|A−||HA| Types of Buffer SolutionThere are two buffer forms, acid buffer, and base buffer. Acid Buffer A buffer solution that contains large quantities of a weak acid, and its salt with a strong base, is called an acid buffer. On the acidic side, such buffer solutions have pH, i.e.pH is below 7 at 298 K. The equation gives the pH of an acid buffer. CH 3 COOH, with CH 3 COONa. pH = pKa + ln(Salt)Acid Where Ka -----acid dissociation constant of the weak acid Basic Buffer A buffer solution that contains relatively large quantities of a weak base and its salt with a strong acid is called a simple buffer. On the alkaline side, these buffers have pH, i.e., pH is higher than 7 at 298 K. For example, NH 4 OH and NH 4 Cl. The pH of an appropriate buffer is determined by the equation pOH = pKb + ln(Salt)Acid Where, Kb ------base dissociation constant. These equations are called Henderson Hasselbalch equations Buffer Solution ExamplesBlood - contains a bicarbonate buffer system Tris buffer Phosphate buffer As mentioned, buffers are beneficial over specific pH ranges. For example, here is the pH range of common buffering agents: While making a buffer solution, the pH of the solution is changed to get it within the right effective range. A strong acid, such as hydrochloric acid (HCl), is usually added to reduce the pH of acidic buffers. A strong base such as sodium hydroxide (NaOH) solution is added to increase the pH of the alkaline buffers. Importance of BuffersThe acidity of the solution in which they occur affects a lot of chemical reactions. The pH of the reaction medium must be controlled for a given reaction to occur or to occur at a suitable rate. This control is provided by buffer solutions, which are solutions that preserve a certain pH. Biochemical reactions are particularly sensitive to pH. Most biological molecules contain groups of atoms that can be charged or neutral based on pH, and whether these groups are charged or neutral has a significant effect on the molecule's biological activity. The fluid within the cell and the fluids around the cells have a characteristic and almost constant pH in all multicellular organisms. This pH is preserved in several ways, and one of the most important is through buffer systems.  FAQs on Buffer Solution1. What are Buffer Solutions Examples? Acid buffers are liquids with a pH of below 7, containing a weak acid and one of its salts. A combination of acetic acid and sodium acetate for example serves as a buffer solution with a pH of about 4.75. 2. What are Buffer Solutions Used for? Buffer solutions are used in a wide variety of chemical applications as a means of keeping pH to an almost constant value. There are many systems in nature which use buffering for regulating pH. The bicarbonate buffering system for example is used to regulate the pH of the blood. 3. How Do You Make a Buffer Solution? Add water to 1 litre. (Alternatively, dilute 10 times 100 mM of phosphoric acid (sodium) buffer solution (pH=6.8). Add water to 1 litre. Add water to 1 litre. 4. Why are Buffer Solutions Important? A buffer is a solution that can tolerate pH change when an acidic or basic component is applied. It can neutralise small amounts of added acid or base and thus retain a fairly steady pH of the solution. This is important for processes and/or reactions where unique and stable pH ranges are needed. (Image will be Updated soon) 5. What is a buffer solution? A buffer solution is an aqueous mixture of a weak acid and its conjugate base. When a normal quantity of strong acid or base is introduced to it, the pH hardly changes. Buffer solutions are widely used in chemical applications to keep pH at a constant value. Many natural systems rely on buffering to maintain pH balance. The bicarbonate buffering mechanism, for example, is used to maintain blood pH, and bicarbonate also serves as a buffer in the ocean. Visit Vedantu website to learn more. 6. What are the applications of buffers? Regardless of what else is in the solution, the pH of a solution containing a buffering agent can only vary within a narrow range. This is a requirement for enzymes to function properly in biological systems. The plasma component of human blood, for example, contains a mixture of carbonic acid and bicarbonate, which is the fundamental mechanism for keeping blood pH between 7.35 and 7.45. Acidosis and alkalosis metabolic states occur quickly outside of this restricted range (7.40 0.05 pH unit), eventually leading to death if the proper buffering capacity is not quickly restored. The efficacy of an enzyme declines when the pH of a solution rises or falls too much, a process known as denaturation, which is usually irreversible. The bulk of biological samples used in research is stored in a buffer solution, which is usually phosphate-buffered saline (PBS) with a pH of 7.4. Buffering agents are used in the industry in fermentation processes and to set the proper conditions for dyes used in fabric colouring. They are also employed in chemical analysis and pH metre calibration. The pH of buffers in acidic environments can be changed to a desirable value by adding a strong acid to the buffering agent, such as hydrochloric acid. A strong base, such as sodium hydroxide, can be used to make alkaline buffers. A buffer combination can also be created by combining an acid and its conjugate base. An acetate buffer, for example, can be prepared from acetic acid and sodium acetate. A mixture of the base and its conjugate acid can also be used to make an alkaline buffer. 7. What are monoprotic acids? Make a note of the equilibrium expression first. This demonstrates that when the acid dissociates, it produces an equal quantity of hydrogen ions and anion. An ICE table can be used to compute the equilibrium concentrations of these three components (ICE stands for "initial, change, equilibrium"). The initial circumstances are listed in the first row, labelled I: the acid concentration is C0, originally undissociated, so A and H+ concentrations are zero; y is the initial concentration of added strong acid, such as hydrochloric acid. When a strong alkali, such as sodium hydroxide, is introduced, y becomes negative because the alkali eliminates hydrogen ions from the solution. The changes that occur when the acid dissociates are specified in the second row, which is labelled C for "change." The acid concentration falls by x units, but the concentrations of A and H+ both rise by x units. The Vedantu app and website offers free study materials. What is Buffer Solution?Buffer solution. A buffer solution refers to an aqueous solution . Furthermore, it consists of a mixture of a weak acid and its conjugate base or vice-versa. This solution is quite important in the field of chemistry. You can explore more about buffer solutions here. Definition of Buffer SolutionA buffer solution certainly consists of an acid and a base . This solution comes into existence by taking weak acid and then adding to its conjugate base. Another way to form it is by taking combining a weak base with its conjugate acid. The use of conjugate solutions is significantly important in buffer solutions. We used buffer solutions in order to keep pH at a somewhat constant value. This has a wide variety of chemistry applications. The use of conjugates gives buffer solutions their resistance to pH changes. Furthermore, it creates an equilibrium between the acid and the base. Creating equilibrium is something that is difficult for other acids and bases to overcome. Even when strong acids or bases are used, the equilibrium between the weak acid/base and its conjugate reduces the effect of addition on overall solution pH. Application of Buffer SolutionBuffer solutions certainly have a massive range of applications. The applications of buffer solutions are for both the real world and the lab. A buffered pH is a necessity of most enzymes to function efficiently and correctly. Furthermore, buffering is important for ensuring proper colour concentration when using dyes. A buffer solution is required for calibrating equipment. It is especially required for pH meters that may be in the miscalibrated in the absence of a buffer. Buffer solutions whose preparation takes place from acetic acid, citric acid, ammonia , can have pH values as high as 10 or as low as 2. This allows buffer solutions to be worked with very strong bases or acids. Properties of Buffer SolutionBuffer solutions are certainly resistant to changes in pH. However, the pH of a buffer solution can change if there is an addition of sufficient strong acid or strong base. Buffer capacity refers to the amount of strong acid or base a buffer solution can take before significant pH changes take place. It is a measure of the resistance of a buffer solution to pH change on the addition of hydroxide ions. Buffer capacity differs in accordance with the core components of the buffer solution and the amount of strong acid or base. If adding a strong acid to buffer solutions, the capacity is equal to the base’s amount. If adding a strong base, the capacity is equal to the acid’s amount. Solved Question For YouQ1 Which of the following statements is not true when it comes to buffer solutions? A. Buffer solutions are organic in nature B. Buffer solutions are aqueous solutions C. These solutions consist of a mixture of a weak acid and its conjugate base D. The use of conjugates gives buffer solutions their resistance to pH changes A1 The correct answer is option A. which is “buffer solutions are organic in nature”. This is not true because buffer solutions consist of a mixture of a weak acid and its conjugate base or vice-versa. Customize your course in 30 secondsWhich class are you in.  Acids, Bases and Salts- Acid Strength
- Tartaric Acid
- Sulfuric Acid
- Inorganic Chemistry
- Formic Acid
- Benzoic Acid
- Hydrochloric Acid
- Phosphoric Acid
- Omega 3 Fatty Acids
Leave a Reply Cancel replyYour email address will not be published. Required fields are marked * Download the App  You are here: - Acid-Base Chemistry / Virtual Labs >
- Creating a Buffer Solution
Virtual Lab: Creating a Buffer Solution- International languages available on our previous website
- Español
- Català
- Português (Br)
- Français
We are pleased to announce a new HTML5 based version of the virtual lab. Please use FireFox or Chrome web browser to access this page, errors have been reported when using Internet Explorer. Introductory Video and Support Information The ChemCollective site and its contents are licensed under a Creative Commons Attribution 3.0 NonCommercial-NoDerivs License. The Evolution of Assessment Methods: From Paper-Based to Digital SolutionsThe evolution of assessment methods in education has been marked by a transformative journey from traditional paper-based practices to the integration of digital solutions. While there has been a significant shift towards digital assessment methods, many educators still see the benefits of handwritten assessments. This raises the question: How do we effectively balance the advantages of both approaches to meet diverse educational needs? How have assessment methods shifted from paper to digital? For many years, paper-based assessment methods have been the cornerstone of education, offering educators a familiar and tactile way to evaluate student understanding. Handwritten assignments and exams can sometimes provide a physical connection to the material being studied, allowing students to engage more deeply with the content . The transition from paper-based to digital assessment methods marks a significant shift in education, driven by advancements in technology over the past few decades. As the internet became more accessible and technology advanced, digital assessment solutions evolved to encompass a wide range of functionalities. Online quizzes and tests became commonplace, offering educators a way to assess student understanding in real-time and provide immediate feedback.  Automated grading systems reduce the burden of manual grading, saving educators valuable time and allowing for more frequent assessments. They also have the means to provide data-driven insights into student performance allowing educators to identify trends, strengths, and areas needing improvement. Is there a continued demand for paper-based assessment methods? Despite the widespread adoption of digital solutions, traditional paper-based assessment methods continue to exist in education. Paper-based assessment methods are held in high regard by students and educators. Many educators still use printed materials for assessments due to familiarity, accessibility, and perceived reliability. They feel that paper grading allows for flexibility in grading and the ability to offer personalized feedback tailored to individual student needs. For students, handwritten assignments and exams can also offer a physical representation of their work, fostering a deeper engagement with the content. However, grading handwritten assignments can be time-consuming for educators, especially when they have large classes to oversee. This may delay the feedback process. Paper-based assignments are also known to offer limited insights into student performance beyond the final grade, making it challenging for educators to identify patterns or trends in student understanding without the aid of digital analytics. Despite these limitations, the rapid emergence of generative AI has educators around the world reconsidering the power of paper and how it can be used in tandem with digital solutions to ensure learning comprehension and inspire student success with more impactful feedback. Can traditional paper-based and digital assessment methods coexist? Some countries have struggled to fully embrace the revolution of digital solutions in education. Factors such as limited access to technology, inadequate infrastructure, and lack of digital literacy among educators and students have hindered progress in certain regions. Software solutions that digitize paper assessments are now becoming more prevalent, allowing educators to overcome barriers associated with the paper and digital divide, allowing the two to coexist in education. These tools enable educators to scan and digitize paper-based assignments, providing an equitable way for students to be assessed. Students can continue to use paper and educators can utilize digital solutions to grade, ensuring efficient and effective assessment practices. The coexistence of traditional and digital assessment methods highlights the diverse needs and preferences of educators and students. While digital platforms offer efficiency and scalability, paper-based assessments provide a tangible format that some learners find beneficial. Overview: The evolution of paper to digital assessment methods The coexistence and balance of paper and digital assessment methods leverages the strengths of both traditional and technological tools. While digital solutions have transformed assessment practices by introducing efficiency, real-time feedback, and detailed analytics , paper-based assessments continue to offer unique benefits such as tactile engagement and accessibility. Innovative technologies, such as OCR software, facilitate the seamless integration of paper-based work into digital platforms. By blending the familiarity and reliability of paper with the dynamic capabilities of digital tools, educators can provide more comprehensive and personalized feedback, enhance student engagement, and make data-driven decisions to support learning outcomes. This holistic approach to assessment ensures that educational practices not only stay relevant and effective but also embrace the best of both worlds to foster student success. Bridge the gap between paper assignments and digital efficiency. Sponsored Content Disclaimer:Sponsored Content in Partnership With NASSP NASSP allows select groups to share information and thought leadership with our program audiences.  About the AuthorLaura Young is a content marketing specialist at Turnitin. A version of this post originally appeared on the Turnitin Blog . Leave a Reply Cancel replyYour email address will not be published. Required fields are marked * Save my name, email, and website in this browser for the next time I comment.   | 








![assignment of buffer solution Eight tan rectangles are shown in four columns of two rectangles each that are connected with right pointing arrows. The first rectangle in the upper left is labeled “Volume of N a O H solution.” An arrow points right to a second rectangle labeled “Moles of N a O H added.” A second arrow points right to a third rectangle labeled “Additional moles of N a C H subscript 3 C O subscript 2.” Just beneath the first rectangle in the upper left is a rectangle labeled “Volume of buffer solution.” An arrow points right to another rectangle labeled “Initial moles of C H subscript 3 C O subscript 2 H.” This rectangle points to the same third rectangle, which is labeled “ Additional moles of N a C H subscript 3 C O subscript 2.” An arrow points right to a rectangle labeled “ Unreacted moles of C H subscript 3 C O subscript 2 H.” An arrow points from this rectangle to a rectangle below labeled “[ C H subscript 3 C O subscript 2 H ].” An arrow extends below the “Additional moles of N a C H subscript 3 C O subscript 2” rectangle to a rectangle labeled “[ C H subscript 3 C O subscript 2 ].” This rectangle points right to the rectangle labeled “[ C H subscript 3 C O subscript 2 H ].”](https://s3-us-west-2.amazonaws.com/courses-images/wp-content/uploads/sites/219/2016/08/09043740/CNX_Chem_14_06_steps2_img.jpg)

![assignment of buffer solution A graph is shown with a horizontal axis labeled “Added m L of 0.10 M N a O H” which has markings and vertical gridlines every 10 units from 0 to 110. The vertical axis is labeled “p H” and is marked every 1 unit beginning at 0 extending to 11. A break is shown in the vertical axis between 0 and 4. A red curve is drawn on the graph which increases gradually from the point (0, 4.8) up to about (100, 7) after which the graph has a vertical section up to about (100, 11). The curve is labeled [ C H subscript 3 C O subscript 2 H ] is 11 percent of [ C H subscript 3 CO subscript 2 superscript negative].](https://s3-us-west-2.amazonaws.com/courses-images/wp-content/uploads/sites/219/2016/08/09044040/CNX_Chem_14_06_buffer.jpg)










IMAGES
VIDEO
COMMENTS
A buffer is a solution that resists sudden changes in pH. If a strong acid—a source of H + ions—is added to the buffer solution, the H + ions will react with the anion from the salt. Because HC 2 H 3 O 2 is a weak acid, it is not ionized much. This means that if lots of hydrogen ions and acetate ions (from sodium acetate) are present in the same solution, they will come together to make ...
Introduction to Buffers. Page ID. A buffer is a solution that can resist pH change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus maintaining the pH of the solution relatively stable. This is important for processes and/or reactions which require specific and stable pH ranges.
An aqueous solution that resists any change in pH by adding a small amount of acid or base is called a buffer solution. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer solution can resist pH change because of an equilibrium between the acid (HA) and its conjugate base (A - ).
A solution containing appreciable amounts of a weak conjugate acid-base pair is called a buffer solution, or a buffer.Buffer solutions resist a change in pH when small amounts of a strong acid or a strong base are added (Figure 14.14).A solution of acetic acid and sodium acetate (CH 3 COOH + CH 3 COONa) is an example of a buffer that consists of a weak acid and its salt.
Transcript. In this video, we'll explore two common methods for preparing buffer solutions. In the first approach, a certain amount of a weak acid (or weak base) is neutralized with a strong base (or strong acid), forming a conjugate acid-base pair in solution. In the second approach, a weak acid (or weak base) is combined with a salt ...
A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. Buffer solutions resist a change in pH when small amounts of a strong acid or a strong base are added (Figure 11.8.1 11.8. 1 ). A solution of acetic acid ( CH3COOH CH 3 COOH and sodium acetate CH3COONa ...
Buffer - Chemistry - The solution which opposes the change in their pH value on addition of small amount of strong acid or strong base is known as buffer solution. These are mainly acidic buffer and basic buffer. To learn more about the Buffer Actions, Hendersion's Equation with Videos and FAQs of buffer, Visit BYJU'S
A buffer solution is a solution that only changes slightly when an acid or a base is added to it. For an acid-buffer solution, it consists of a week acid and its conjugate base. For a basic-buffer solution, it consists of a week base and its conjugate acid. The main purpose of a buffer solution is just to resist the change in pH so that the pH ...
• Explain why a solution of a strong acid and a strong base will not resist changes in pH . • Write the equilibrium expression for a weak acid . • Evaluate the ability of a solution of a weak acid and strong base to create an effective buffer . • Brainstorm factors that should be considered when designing a buffer solution .
Definition. A buffer solution is one which resists changes in pH when small quantities of an acid or an alkali are added to it. Acidic buffer solutions. An acidic buffer solution is simply one which has a pH less than 7. Acidic buffer solutions are commonly made from a weak acid and one of its salts - often a sodium salt.
However, with a buffer present, since there is a weak acid, HA, present to neutralize the added hydroxide anions, the buffer solution resists a change to the pH. So let's summarize how buffer solutions work. If we add a small amount of an acid, H+, to a buffer solution, the conjugate base that's present, A-, neutralizes the added acid.
A solution containing appreciable amounts of a weak conjugate acid-base pair is called a buffer solution, or a buffer . Buffer solutions resist a change in pH when small amounts of a strong acid or a strong base are added (Figure 1). A solution of acetic acid and sodium acetate (CH 3 COOH + CH 3 COONa) is an example of a buffer that consists of ...
A buffer solution resists a change in pH from the addition of a small amount of acid or base. A buffer is a solution that maintains the stability of a system's pH level when adding small quantities of acids or bases. This characteristic makes buffers important in biological and chemical applications where pH stability is crucial.
Calculate the volume of each stock solution you need to prepare 30 - 50 mL buffer solution of your target pH. (Hint, choose either acid or base to begin with 20 mL, and determine the volume you will need of the other solution to have the correct base/acid ratio for your target pH). Record your calculations.
An aqueous solution of an equal concentration of acetic acid and sodium acetate has a pH of 4.74. The pH of these solutions is below seven. These solutions consist of a weak acid and a salt of a weak acid. An example of an acidic buffer solution is a mixture of sodium acetate and acetic acid (pH = 4.75).
BUFFER SOLUTIONS. Objectives. At the end of this unit , the student is expected to be able to : 1- Understand the concept of the buffer , its importance in chemistry and in real life and its types . 2- Realize the way by which the buffer stabilizes the pH . 3- Calculate the pH of all sorts of buffer solutions . 4- Know how to prepare all types ...
The buffer "works" because the HCl reacts with the base component of the buffer (A−) according to the equation below. HCl + A− → HA + Cl−. Thus the buffer solution eliminates all of the HCl (or the H+ or H3O+) and sacrifices some of the weak base (A−) making more weak acid, HA.
A buffer solution that contains large quantities of a weak acid, and its salt with a strong base, is called an acid buffer. On the acidic side, such buffer solutions have pH, i.e.pH is below 7 at 298 K. The equation gives the pH of an acid buffer. CH3COOH, with CH3COONa. pH = pKa + ln (Salt)Acid.
Phosphoric Acid. Omega 3 Fatty Acids. Boric Acid. A buffer solution refers to an aqueous solution. Furthermore, it consists of a mixture of a weak acid and its conjugate base or vice-versa. It consists of an acid and a base. This solution comes into existence by taking weak acid and then adding to its conjugate base.
LAB lab 11: examination of buffer solutions indicators purpose buffer solutions contain weak acid and its conjugate base, or weak base and its conjugate acid, Skip to document. University; High School. ... Homework Assignment - OCC - Prof. Helen Maughan. General Chemistry B 100% (4) 3. Written HW 13 - Homework Assignment - OCC - Prof. Helen ...
Workbench 1. Tip 1/2. Welcome to the new HTML5 version of the Virtual Lab! In this version, the Solution Information Panel is now located on the left and appears when a solution is selected. Virtual Lab.
Buffer Calculations: Formula and Equations. Molar solution equation: desired molarity × formula weight × solution final volume (L) = grams needed Percentage by weight (w/v): (% buffer desired / 100) × final buffer volume (mL) = g of starting material needed Henderson-Hasselbach equation: pH = pKa + log [A-]/[HA] The Henderson-Hasselbalch equation enables determination of a buffer solution's ...
Title: investigating buffer solutions Aim: To investigate the effect of buffer solutions in maintaining the PH and also to prepare buffer solutions with a set PH. Hypothesis: A buffer solution maintains the PH when exposed to an acid or a base. Background: A buffer solution is a solution that is formed from the mixture of a weak acid and its conjugate base or vice versa.
Assignment buffer solutions and reagents often you see protocols where are needed instead of pure water. this is because using pure water in experiments, Skip to document. Ask AI. ... There are multiple variations of buffer solutions, depending on the experimental circumstances. They are mainly used to control the pH and the osmolarity of the ...
Software solutions that digitize paper assessments are now becoming more prevalent, allowing educators to overcome barriers associated with the paper and digital divide, allowing the two to coexist in education. These tools enable educators to scan and digitize paper-based assignments, providing an equitable way for students to be assessed.