Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms

- Advanced Search
- See Studies by Topic
- See Studies on Map
- How to Search
- How to Use Search Results
- How to Find Results of Studies
- How to Read a Study Record

- Learn About Studies
- Other Sites About Studies
- Glossary of Common Site Terms

- Submit Studies to ClinicalTrials.gov PRS
- Why Should I Register and Submit Results?
- FDAAA 801 and the Final Rule
- How to Apply for a PRS Account
- How to Register Your Study
- How to Edit Your Study Record
- How to Submit Your Results
- Frequently Asked Questions
- Support Materials
- Training Materials

- Selected Publications
- Clinical Alerts and Advisories
- Trends, Charts, and Maps
- Downloading Content for Analysis

- ClinicalTrials.gov Background
- About the Results Database
- History, Policies, and Laws
- ClinicalTrials.gov Modernization
- Media/Press Resources
- Linking to This Site
- Terms and Conditions

ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted around the world.
Explore 496,616 research studies in all 50 states and in 222 countries..
ClinicalTrials.gov is a resource provided by the U.S. National Library of Medicine.
IMPORTANT : Listing a study does not mean it has been evaluated by the U.S. Federal Government. Read our disclaimer for details.
Before participating in a study, talk to your health care provider and learn about the risks and potential benefits .
- Studies by Topic
- Studies on Map
Patients and Families
Researchers, study record managers.
- Customer Support
- Accessibility
- Viewers and Players
- Freedom of Information Act
- HHS Vulnerability Disclosure
- U.S. National Library of Medicine
- U.S. National Institutes of Health
- U.S. Department of Health and Human Services
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.15(2); 2023 Feb
- PMC10023071

Clinical Trials and Clinical Research: A Comprehensive Review
Venkataramana kandi.
1 Clinical Microbiology, Prathima Institute of Medical Sciences, Karimnagar, IND
Sabitha Vadakedath
2 Biochemistry, Prathima Institute of Medical Sciences, Karimnagar, IND
Clinical research is an alternative terminology used to describe medical research. Clinical research involves people, and it is generally carried out to evaluate the efficacy of a therapeutic drug, a medical/surgical procedure, or a device as a part of treatment and patient management. Moreover, any research that evaluates the aspects of a disease like the symptoms, risk factors, and pathophysiology, among others may be termed clinical research. However, clinical trials are those studies that assess the potential of a therapeutic drug/device in the management, control, and prevention of disease. In view of the increasing incidences of both communicable and non-communicable diseases, and especially after the effects that Coronavirus Disease-19 (COVID-19) had on public health worldwide, the emphasis on clinical research assumes extremely essential. The knowledge of clinical research will facilitate the discovery of drugs, devices, and vaccines, thereby improving preparedness during public health emergencies. Therefore, in this review, we comprehensively describe the critical elements of clinical research that include clinical trial phases, types, and designs of clinical trials, operations of trial, audit, and management, and ethical concerns.
Introduction and background
A clinical trial is a systematic process that is intended to find out the safety and efficacy of a drug/device in treating/preventing/diagnosing a disease or a medical condition [ 1 , 2 ]. Clinical trial includes various phases that include phase 0 (micro-dosing studies), phase 1, phase 2, phase 3, and phase 4 [ 3 ]. Phase 0 and phase 2 are called exploratory trial phases, phase 1 is termed the non-therapeutic phase, phase 3 is known as the therapeutic confirmatory phase, and phase 4 is called the post-approval or the post-marketing surveillance phase. Phase 0, also called the micro-dosing phase, was previously done in animals but now it is carried out in human volunteers to understand the dose tolerability (pharmacokinetics) before being administered as a part of the phase 1 trial among healthy individuals. The details of the clinical trial phases are shown in Table Table1 1 .
This table has been created by the authors.
MTD: maximum tolerated dose; SAD: single ascending dose; MAD: multiple ascending doses; NDA: new drug application; FDA: food and drug administration
Clinical research design has two major types that include non-interventional/observational and interventional/experimental studies. The non-interventional studies may have a comparator group (analytical studies like case-control and cohort studies), or without it (descriptive study). The experimental studies may be either randomized or non-randomized. Clinical trial designs are of several types that include parallel design, crossover design, factorial design, randomized withdrawal approach, adaptive design, superiority design, and non-inferiority design. The advantages and disadvantages of clinical trial designs are depicted in Table Table2 2 .
There are different types of clinical trials that include those which are conducted for treatment, prevention, early detection/screening, and diagnosis. These studies address the activities of an investigational drug on a disease and its outcomes [ 4 ]. They assess whether the drug is able to prevent the disease/condition, the ability of a device to detect/screen the disease, and the efficacy of a medical test to diagnose the disease/condition. The pictorial representation of a disease diagnosis, treatment, and prevention is depicted in Figure Figure1 1 .

This figure has been created by the authors.
The clinical trial designs could be improvised to make sure that the study's validity is maintained/retained. The adaptive designs facilitate researchers to improvise during the clinical trial without interfering with the integrity and validity of the results. Moreover, it allows flexibility during the conduction of trials and the collection of data. Despite these advantages, adaptive designs have not been universally accepted among clinical researchers. This could be attributed to the low familiarity of such designs in the research community. The adaptive designs have been applied during various phases of clinical trials and for different clinical conditions [ 5 , 6 ]. The adaptive designs applied during different phases are depicted in Figure Figure2 2 .

The Bayesian adaptive trial design has gained popularity, especially during the Coronavirus Disease-19 (COVID-19) pandemic. Such designs could operate under a single master protocol. It operates as a platform trial wherein multiple treatments can be tested on different patient groups suffering from disease [ 7 ].
In this review, we comprehensively discuss the essential elements of clinical research that include the principles of clinical research, planning clinical trials, practical aspects of clinical trial operations, essentials of clinical trial applications, monitoring, and audit, clinical trial data analysis, regulatory audits, and project management, clinical trial operations at the investigation site, the essentials of clinical trial experiments involving epidemiological, and genetic studies, and ethical considerations in clinical research/trials.
A clinical trial involves the study of the effect of an investigational drug/any other intervention in a defined population/participant. The clinical research includes a treatment group and a placebo wherein each group is evaluated for the efficacy of the intervention (improved/not improved) [ 8 ].
Clinical trials are broadly classified into controlled and uncontrolled trials. The uncontrolled trials are potentially biased, and the results of such research are not considered as equally as the controlled studies. Randomized controlled trials (RCTs) are considered the most effective clinical trials wherein the bias is minimized, and the results are considered reliable. There are different types of randomizations and each one has clearly defined functions as elaborated in Table Table3 3 .
Principles of clinical trial/research
Clinical trials or clinical research are conducted to improve the understanding of the unknown, test a hypothesis, and perform public health-related research [ 2 , 3 ]. This is majorly carried out by collecting the data and analyzing it to derive conclusions. There are various types of clinical trials that are majorly grouped as analytical, observational, and experimental research. Clinical research can also be classified into non-directed data capture, directed data capture, and drug trials. Clinical research could be prospective or retrospective. It may also be a case-control study or a cohort study. Clinical trials may be initiated to find treatment, prevent, observe, and diagnose a disease or a medical condition.
Among the various types of clinical research, observational research using a cross-sectional study design is the most frequently performed clinical research. This type of research is undertaken to analyze the presence or absence of a disease/condition, potential risk factors, and prevalence and incidence rates in a defined population. Clinical trials may be therapeutic or non-therapeutic type depending on the type of intervention. The therapeutic type of clinical trial uses a drug that may be beneficial to the patient. Whereas in a non-therapeutic clinical trial, the participant does not benefit from the drug. The non-therapeutic trials provide additional knowledge of the drug for future improvements. Different terminologies of clinical trials are delineated in Table Table4 4 .
In view of the increased cost of the drug discovery process, developing, and low-income countries depend on the production of generic drugs. The generic drugs are similar in composition to the patented/branded drug. Once the patent period is expired generic drugs can be manufactured which have a similar quality, strength, and safety as the patented drug [ 9 ]. The regulatory requirements and the drug production process are almost the same for the branded and the generic drug according to the Food and Drug Administration (FDA), United States of America (USA).
The bioequivalence (BE) studies review the absorption, distribution, metabolism, and excretion (ADME) of the generic drug. These studies compare the concentration of the drug at the desired location in the human body, called the peak concentration of the drug (Cmax). The extent of absorption of the drug is measured using the area under the receiver operating characteristic curve (AUC), wherein the generic drug is supposed to demonstrate similar ADME activities as the branded drug. The BE studies may be undertaken in vitro (fasting, non-fasting, sprinkled fasting) or in vivo studies (clinical, bioanalytical, and statistical) [ 9 ].
Planning clinical trial/research
The clinical trial process involves protocol development, designing a case record/report form (CRF), and functioning of institutional review boards (IRBs). It also includes data management and the monitoring of clinical trial site activities. The CRF is the most significant document in a clinical study. It contains the information collected by the investigator about each subject participating in a clinical study/trial. According to the International Council for Harmonisation (ICH), the CRF can be printed, optical, or an electronic document that is used to record the safety and efficacy of the pharmaceutical drug/product in the test subjects. This information is intended for the sponsor who initiates the clinical study [ 10 ].
The CRF is designed as per the protocol and later it is thoroughly reviewed for its correctness (appropriate and structured questions) and finalized. The CRF then proceeds toward the print taking the language of the participating subjects into consideration. Once the CRF is printed, it is distributed to the investigation sites where it is filled with the details of the participating subjects by the investigator/nurse/subject/guardian of the subject/technician/consultant/monitors/pharmacist/pharmacokinetics/contract house staff. The filled CRFs are checked for their completeness and transported to the sponsor [ 11 ].
Effective planning and implementation of a clinical study/trial will influence its success. The clinical study majorly includes the collection and distribution of the trial data, which is done by the clinical data management section. The project manager is crucial to effectively plan, organize, and use the best processes to control and monitor the clinical study [ 10 , 11 ].
The clinical study is conducted by a sponsor or a clinical research organization (CRO). A perfect protocol, time limits, and regulatory requirements assume significance while planning a clinical trial. What, when, how, and who are clearly planned before the initiation of a study trial. Regular review of the project using the bar and Gantt charts, and maintaining the timelines assume increased significance for success with the product (study report, statistical report, database) [ 10 , 11 ].
The steps critical to planning a clinical trial include the idea, review of the available literature, identifying a problem, formulating the hypothesis, writing a synopsis, identifying the investigators, writing a protocol, finding a source of funding, designing a patient consent form, forming ethics boards, identifying an organization, preparing manuals for procedures, quality assurance, investigator training and initiation of the trial by recruiting the participants [ 10 ].
The two most important points to consider before the initiation of the clinical trial include whether there is a need for a clinical trial, if there is a need, then one must make sure that the study design and methodology are strong for the results to be reliable to the people [ 11 ].
For clinical research to envisage high-quality results, the study design, implementation of the study, quality assurance in data collection, and alleviation of bias and confounding factors must be robust [ 12 ]. Another important aspect of conducting a clinical trial is improved management of various elements of clinical research that include human and financial resources. The role of a trial manager to make a successful clinical trial was previously reported. The trial manager could play a key role in planning, coordinating, and successfully executing the trial. Some qualities of a trial manager include better communication and motivation, leadership, and strategic, tactical, and operational skills [ 13 ].
Practical aspects of a clinical trial operations
There are different types of clinical research. Research in the development of a novel drug could be initiated by nationally funded research, industry-sponsored research, and clinical research initiated by individuals/investigators. According to the documents 21 code of federal regulations (CFR) 312.3 and ICH E-6 Good Clinical Practice (GCP) 1.54, an investigator is an individual who initiates and conducts clinical research [ 14 ]. The investigator plan, design, conduct, monitor, manage data, compile reports, and supervise research-related regulatory and ethical issues. To manage a successful clinical trial project, it is essential for an investigator to give the letter of intent, write a proposal, set a timeline, develop a protocol and related documents like the case record forms, define the budget, and identify the funding sources.
Other major steps of clinical research include the approval of IRBs, conduction and supervision of the research, data review, and analysis. Successful clinical research includes various essential elements like a letter of intent which is the evidence that supports the interest of the researcher to conduct drug research, timeline, funding source, supplier, and participant characters.
Quality assurance, according to the ICH and GCP guidelines, is necessary to be implemented during clinical research to generate quality and accurate data. Each element of the clinical research must have been carried out according to the standard operating procedure (SOP), which is written/determined before the initiation of the study and during the preparation of the protocol [ 15 ].
The audit team (quality assurance group) is instrumental in determining the authenticity of the clinical research. The audit, according to the ICH and GCP, is an independent and external team that examines the process (recording the CRF, analysis of data, and interpretation of data) of clinical research. The quality assurance personnel are adequately trained, become trainers if needed, should be good communicators, and must handle any kind of situation. The audits can be at the investigator sites evaluating the CRF data, the protocol, and the personnel involved in clinical research (source data verification, monitors) [ 16 ].
Clinical trial operations are governed by legal and regulatory requirements, based on GCPs, and the application of science, technology, and interpersonal skills [ 17 ]. Clinical trial operations are complex, time and resource-specific that requires extensive planning and coordination, especially for the research which is conducted at multiple trial centers [ 18 ].
Recruiting the clinical trial participants/subjects is the most significant aspect of clinical trial operations. Previous research had noted that most clinical trials do not meet the participant numbers as decided in the protocol. Therefore, it is important to identify the potential barriers to patient recruitment [ 19 ].
Most clinical trials demand huge costs, increased timelines, and resources. Randomized clinical trial studies from Switzerland were analyzed for their costs which revealed approximately 72000 USD for a clinical trial to be completed. This study emphasized the need for increased transparency with respect to the costs associated with the clinical trial and improved collaboration between collaborators and stakeholders [ 20 ].
Clinical trial applications, monitoring, and audit
Among the most significant aspects of a clinical trial is the audit. An audit is a systematic process of evaluating the clinical trial operations at the site. The audit ensures that the clinical trial process is conducted according to the protocol, and predefined quality system procedures, following GCP guidelines, and according to the requirements of regulatory authorities [ 21 ].
The auditors are supposed to be independent and work without the involvement of the sponsors, CROs, or personnel at the trial site. The auditors ensure that the trial is conducted by designated professionally qualified, adequately trained personnel, with predefined responsibilities. The auditors also ensure the validity of the investigational drug, and the composition, and functioning of institutional review/ethics committees. The availability and correctness of the documents like the investigational broacher, informed consent forms, CRFs, approval letters of the regulatory authorities, and accreditation of the trial labs/sites [ 21 ].
The data management systems, the data collection software, data backup, recovery, and contingency plans, alternative data recording methods, security of the data, personnel training in data entry, and the statistical methods used to analyze the results of the trial are other important responsibilities of the auditor [ 21 , 22 ].
According to the ICH-GCP Sec 1.29 guidelines the inspection may be described as an act by the regulatory authorities to conduct an official review of the clinical trial-related documents, personnel (sponsor, investigator), and the trial site [ 21 , 22 ]. The summary report of the observations of the inspectors is performed using various forms as listed in Table Table5 5 .
FDA: Food and Drug Administration; IND: investigational new drug; NDA: new drug application; IRB: institutional review board; CFR: code of federal regulations
Because protecting data integrity, the rights, safety, and well-being of the study participants are more significant while conducting a clinical trial, regular monitoring and audit of the process appear crucial. Also, the quality of the clinical trial greatly depends on the approach of the trial personnel which includes the sponsors and investigators [ 21 ].
The responsibility of monitoring lies in different hands, and it depends on the clinical trial site. When the trial is initiated by a pharmaceutical industry, the responsibility of trial monitoring depends on the company or the sponsor, and when the trial is conducted by an academic organization, the responsibility lies with the principal investigator [ 21 ].
An audit is a process conducted by an independent body to ensure the quality of the study. Basically, an audit is a quality assurance process that determines if a study is carried out by following the SPOs, in compliance with the GCPs recommended by regulatory bodies like the ICH, FDA, and other local bodies [ 21 ].
An audit is performed to review all the available documents related to the IRB approval, investigational drug, and the documents related to the patient care/case record forms. Other documents that are audited include the protocol (date, sign, treatment, compliance), informed consent form, treatment response/outcome, toxic response/adverse event recording, and the accuracy of data entry [ 22 ].
Clinical trial data analysis, regulatory audits, and project management
The essential elements of clinical trial management systems (CDMS) include the management of the study, the site, staff, subject, contracts, data, and document management, patient diary integration, medical coding, monitoring, adverse event reporting, supplier management, lab data, external interfaces, and randomization. The CDMS involves setting a defined start and finishing time, defining study objectives, setting enrolment and termination criteria, commenting, and managing the study design [ 23 ].
Among the various key application areas of clinical trial systems, the data analysis assumes increased significance. The clinical trial data collected at the site in the form of case record form is stored in the CDMS ensuring the errors with respect to the double data entry are minimized.
Clinical trial data management uses medical coding, which uses terminologies with respect to the medications and adverse events/serious adverse events that need to be entered into the CDMS. The project undertaken to conduct the clinical trial must be predetermined with timelines and milestones. Timelines are usually set for the preparation of protocol, designing the CRF, planning the project, identifying the first subject, and timelines for recording the patient’s data for the first visit.
The timelines also are set for the last subject to be recruited in the study, the CRF of the last subject, and the locked period after the last subject entry. The planning of the project also includes the modes of collection of the data, the methods of the transport of the CRFs, patient diaries, and records of severe adverse events, to the central data management sites (fax, scan, courier, etc.) [ 24 ].
The preparation of SOPs and the type and timing of the quality control (QC) procedures are also included in the project planning before the start of a clinical study. Review (budget, resources, quality of process, assessment), measure (turnaround times, training issues), and control (CRF collection and delivery, incentives, revising the process) are the three important aspects of the implementation of a clinical research project.
In view of the increasing complexity related to the conduct of clinical trials, it is important to perform a clinical quality assurance (CQA) audit. The CQA audit process consists of a detailed plan for conducting audits, points of improvement, generating meaningful audit results, verifying SOP, and regulatory compliance, and promoting improvement in clinical trial research [ 25 ]. All the components of a CQA audit are delineated in Table Table6 6 .
CRF: case report form; CSR: clinical study report; IC: informed consent; PV: pharmacovigilance; SAE: serious adverse event
Clinical trial operations at the investigator's site
The selection of an investigation site is important before starting a clinical trial. It is essential that the individuals recruited for the study meet the inclusion criteria of the trial, and the investigator's and patient's willingness to accept the protocol design and the timelines set by the regulatory authorities including the IRBs.
Before conducting clinical research, it is important for an investigator to agree to the terms and conditions of the agreement and maintain the confidentiality of the protocol. Evaluation of the protocol for the feasibility of its practices with respect to the resources, infrastructure, qualified and trained personnel available, availability of the study subjects, and benefit to the institution and the investigator is done by the sponsor during the site selection visit.
The standards of a clinical research trial are ensured by the Council for International Organizations of Medical Sciences (CIOMS), National Bioethics Advisory Commission (NBAC), United Nations Programme on Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome (HIV/AIDS) (UNAIDS), and World Medical Association (WMA) [ 26 ].
Recommendations for conducting clinical research based on the WMA support the slogan that says, “The health of my patient will be my first consideration.” According to the International Code of Medical Ethics (ICME), no human should be physically or mentally harmed during the clinical trial, and the study should be conducted in the best interest of the person [ 26 ].
Basic principles recommended by the Helsinki declaration include the conduction of clinical research only after the prior proof of the safety of the drug in animal and lab experiments. The clinical trials must be performed by scientifically, and medically qualified and well-trained personnel. Also, it is important to analyze the benefit of research over harm to the participants before initiating the drug trials.
The doctors may prescribe a drug to alleviate the suffering of the patient, save the patient from death, and gain additional knowledge of the drug only after obtaining informed consent. Under the equipoise principle, the investigators must be able to justify the treatment provided as a part of the clinical trial, wherein the patient in the placebo arm may be harmed due to the unavailability of the therapeutic/trial drug.
Clinical trial operations greatly depend on the environmental conditions and geographical attributes of the trial site. It may influence the costs and targets defined by the project before the initiation. It was noted that one-fourth of the clinical trial project proposals/applications submit critical data on the investigational drug from outside the country. Also, it was noted that almost 35% of delays in clinical trials owing to patient recruitment with one-third of studies enrolling only 5% of the participants [ 27 ].
It was suggested that clinical trial feasibility assessment in a defined geographical region may be undertaken for improved chances of success. Points to be considered under the feasibility assessment program include if the disease under the study is related to the population of the geographical region, appropriateness of the study design, patient, and comparator group, visit intervals, potential regulatory and ethical challenges, and commitments of the study partners, CROs in respective countries (multi-centric studies) [ 27 ].
Feasibility assessments may be undertaken at the program level (ethics, regulatory, and medical preparedness), study level (clinical, regulatory, technical, and operational aspects), and at the investigation site (investigational drug, competency of personnel, participant recruitment, and retention, quality systems, and infrastructural aspects) [ 27 ].
Clinical trials: true experiments
In accordance with the revised schedule "Y" of the Drugs and Cosmetics Act (DCA) (2005), a drug trial may be defined as a systematic study of a novel drug component. The clinical trials aim to evaluate the pharmacodynamic, and pharmacokinetic properties including ADME, efficacy, and safety of new drugs.
According to the drug and cosmetic rules (DCR), 1945, a new chemical entity (NCE) may be defined as a novel drug approved for a disease/condition, in a specified route, and at a particular dosage. It also may be a new drug combination, of previously approved drugs.
A clinical trial may be performed in three types; one that is done to find the efficacy of an NCE, a comparison study of two drugs against a medical condition, and the clinical research of approved drugs on a disease/condition. Also, studies of the bioavailability and BE studies of the generic drugs, and the drugs already approved in other countries are done to establish the efficacy of new drugs [ 28 ].
Apart from the discovery of a novel drug, clinical trials are also conducted to approve novel medical devices for public use. A medical device is defined as any instrument, apparatus, appliance, software, and any other material used for diagnostic/therapeutic purposes. The medical devices may be divided into three classes wherein class I uses general controls; class II uses general and special controls, and class III uses general, special controls, and premarket approvals [ 28 ].
The premarket approval applications ensure the safety and effectiveness, and confirmation of the activities from bench to animal to human clinical studies. The FDA approval for investigational device exemption (IDE) for a device not approved for a new indication/disease/condition. There are two types of IDE studies that include the feasibility study (basic safety and potential effectiveness) and the pivotal study (trial endpoints, randomization, monitoring, and statistical analysis plan) [ 28 ].
As evidenced by the available literature, there are two types of research that include observational and experimental research. Experimental research is alternatively known as the true type of research wherein the research is conducted by the intervention of a new drug/device/method (educational research). Most true experiments use randomized control trials that remove bias and neutralize the confounding variables that may interfere with the results of research [ 28 ].
The variables that may interfere with the study results are independent variables also called prediction variables (the intervention), dependent variables (the outcome), and extraneous variables (other confounding factors that could influence the outside). True experiments have three basic elements that include manipulation (that influence independent variables), control (over extraneous influencers), and randomization (unbiased grouping) [ 29 ].
Experiments can also be grouped as true, quasi-experimental, and non-experimental studies depending on the presence of specific characteristic features. True experiments have all three elements of study design (manipulation, control, randomization), and prospective, and have great scientific validity. Quasi-experiments generally have two elements of design (manipulation and control), are prospective, and have moderate scientific validity. The non-experimental studies lack manipulation, control, and randomization, are generally retrospective, and have low scientific validity [ 29 ].
Clinical trials: epidemiological and human genetics study
Epidemiological studies are intended to control health issues by understanding the distribution, determinants, incidence, prevalence, and impact on health among a defined population. Such studies are attempted to perceive the status of infectious diseases as well as non-communicable diseases [ 30 ].
Experimental studies are of two types that include observational (cross-sectional studies (surveys), case-control studies, and cohort studies) and experimental studies (randomized control studies) [ 3 , 31 ]. Such research may pose challenges related to ethics in relation to the social and cultural milieu.
Biomedical research related to human genetics and transplantation research poses an increased threat to ethical concerns, especially after the success of the human genome project (HGP) in the year 2000. The benefits of human genetic studies are innumerable that include the identification of genetic diseases, in vitro fertilization, and regeneration therapy. Research related to human genetics poses ethical, legal, and social issues (ELSI) that need to be appropriately addressed. Most importantly, these genetic research studies use advanced technologies which should be equally available to both economically well-placed and financially deprived people [ 32 ].
Gene therapy and genetic manipulations may potentially precipitate conflict of interest among the family members. The research on genetics may be of various types that include pedigree studies (identifying abnormal gene carriers), genetic screening (for diseases that may be heritable by the children), gene therapeutics (gene replacement therapy, gene construct administration), HGP (sequencing the whole human genome/deoxyribonucleic acid (DNA) fingerprinting), and DNA, cell-line banking/repository [ 33 ]. The biobanks are established to collect and store human tissue samples like umbilical tissue, cord blood, and others [ 34 ].
Epidemiological studies on genetics are attempts to understand the prevalence of diseases that may be transmitted among families. The classical epidemiological studies may include single case observations (one individual), case series (< 10 individuals), ecological studies (population/large group of people), cross-sectional studies (defined number of individuals), case-control studies (defined number of individuals), cohort (defined number of individuals), and interventional studies (defined number of individuals) [ 35 ].
Genetic studies are of different types that include familial aggregation (case-parent, case-parent-grandparent), heritability (study of twins), segregation (pedigree study), linkage study (case-control), association, linkage, disequilibrium, cohort case-only studies (related case-control, unrelated case-control, exposure, non-exposure group, case group), cross-sectional studies, association cohort (related case-control, familial cohort), and experimental retrospective cohort (clinical trial, exposure, and non-exposure group) [ 35 ].
Ethics and concerns in clinical trial/research
Because clinical research involves animals and human participants, adhering to ethics and ethical practices assumes increased significance [ 36 ]. In view of the unethical research conducted on war soldiers after the Second World War, the Nuremberg code was introduced in 1947, which promulgated rules for permissible medical experiments on humans. The Nuremberg code suggests that informed consent is mandatory for all the participants in a clinical trial, and the study subjects must be made aware of the nature, duration, and purpose of the study, and potential health hazards (foreseen and unforeseen). The study subjects should have the liberty to withdraw at any time during the trial and to choose a physician upon medical emergency. The other essential principles of clinical research involving human subjects as suggested by the Nuremberg code included benefit to the society, justification of study as noted by the results of the drug experiments on animals, avoiding even minimal suffering to the study participants, and making sure that the participants don’t have life risk, humanity first, improved medical facilities for participants, and suitably qualified investigators [ 37 ].
During the 18th world medical assembly meeting in the year 1964, in Helsinki, Finland, ethical principles for doctors practicing research were proposed. Declaration of Helsinki, as it is known made sure that the interests and concerns of the human participants will always prevail over the interests of the society. Later in 1974, the National Research Act was proposed which made sure that the research proposals are thoroughly screened by the Institutional ethics/Review Board. In 1979, the April 18th Belmont report was proposed by the national commission for the protection of human rights during biomedical and behavioral research. The Belmont report proposed three core principles during research involving human participants that include respect for persons, beneficence, and justice. The ICH laid down GCP guidelines [ 38 ]. These guidelines are universally followed throughout the world during the conduction of clinical research involving human participants.
ICH was first founded in 1991, in Brussels, under the umbrella of the USA, Japan, and European countries. The ICH conference is conducted once every two years with the participation from the member countries, observers from the regulatory agencies, like the World Health Organization (WHO), European Free Trade Association (EFTA), and the Canadian Health Protection Branch, and other interested stakeholders from the academia and the industry. The expert working groups of the ICH ensure the quality, efficacy, and safety of the medicinal product (drug/device). Despite the availability of the Nuremberg code, the Belmont Report, and the ICH-GCP guidelines, in the year 1982, International Ethical Guidelines for Biomedical Research Involving Human Subjects was proposed by the CIOMS in association with WHO [ 39 ]. The CIOMS protects the rights of the vulnerable population, and ensures ethical practices during clinical research, especially in underdeveloped countries [ 40 ]. In India, the ethical principles for biomedical research involving human subjects were introduced by the Indian Council of Medical Research (ICMR) in the year 2000 and were later amended in the year 2006 [ 41 ]. Clinical trial approvals can only be done by the IRB approved by the Drug Controller General of India (DGCI) as proposed in the year 2013 [ 42 ].
Current perspectives and future implications
A recent study attempted to evaluate the efficacy of adaptive clinical trials in predicting the success of a clinical trial drug that entered phase 3 and minimizing the time and cost of drug development. This study highlighted the drawbacks of such clinical trial designs that include the possibility of type 1 (false positive) and type 2 (false negative) errors [ 43 ].
The usefulness of animal studies during the preclinical phases of a clinical trial was evaluated in a previous study which concluded that animal studies may not completely guarantee the safety of the investigational drug. This is noted by the fact that many drugs which passed toxicity tests in animals produced adverse reactions in humans [ 44 ].
The significance of BE studies to compare branded and generic drugs was reported previously. The pharmacokinetic BE studies of Amoxycillin comparing branded and generic drugs were carried out among a group of healthy participants. The study results have demonstrated that the generic drug had lower Cmax as compared to the branded drug [ 45 ].
To establish the BE of the generic drugs, randomized crossover trials are carried out to assess the Cmax and the AUC. The ratio of each pharmacokinetic characteristic must match the ratio of AUC and/or Cmax, 1:1=1 for a generic drug to be considered as a bioequivalent to a branded drug [ 46 ].
Although the generic drug development is comparatively more beneficial than the branded drugs, synthesis of extended-release formulations of the generic drug appears to be complex. Since the extended-release formulations remain for longer periods in the stomach, they may be influenced by gastric acidity and interact with the food. A recent study suggested the use of bio-relevant dissolution tests to increase the successful production of generic extended-release drug formulations [ 47 ].
Although RCTs are considered the best designs, which rule out bias and the data/results obtained from such clinical research are the most reliable, RCTs may be plagued by miscalculation of the treatment outcomes/bias, problems of cointerventions, and contaminations [ 48 ].
The perception of healthcare providers regarding branded drugs and their view about the generic equivalents was recently analyzed and reported. It was noted that such a perception may be attributed to the flexible regulatory requirements for the approval of a generic drug as compared to a branded drug. Also, could be because a switch from a branded drug to a generic drug in patients may precipitate adverse events as evidenced by previous reports [ 49 ].
Because the vulnerable population like drug/alcohol addicts, mentally challenged people, children, geriatric age people, military persons, ethnic minorities, people suffering from incurable diseases, students, employees, and pregnant women cannot make decisions with respect to participating in a clinical trial, ethical concerns, and legal issues may prop up, that may be appropriately addressed before drug trials which include such groups [ 50 ].
Conclusions
Clinical research and clinical trials are important from the public health perspective. Clinical research facilitates scientists, public health administrations, and people to increase their understanding and improve preparedness with reference to the diseases prevalent in different geographical regions of the world. Moreover, clinical research helps in mitigating health-related problems as evidenced by the current Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) pandemic and other emerging and re-emerging microbial infections. Clinical trials are crucial to the development of drugs, devices, and vaccines. Therefore, scientists are required to be up to date with the process and procedures of clinical research and trials as discussed comprehensively in this review.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
- Clinical Trials
About Clinical Studies
Research: it's all about patients.
Mayo's mission is about the patient, the patient comes first. So the mission and research here, is to advance how we can best help the patient, how to make sure the patient comes first in care. So in many ways, it's a cycle. It can start with as simple as an idea, worked on in a laboratory, brought to the patient bedside, and if everything goes right, and let's say it's helpful or beneficial, then brought on as a standard approach. And I think that is one of the unique characteristics of Mayo's approach to research, that patient-centeredness. That really helps to put it in its own spotlight.
At Mayo Clinic, the needs of the patient come first. Part of this commitment involves conducting medical research with the goal of helping patients live longer, healthier lives.
Through clinical studies, which involve people who volunteer to participate in them, researchers can better understand how to diagnose, treat and prevent diseases or conditions.
Types of clinical studies
- Observational study. A type of study in which people are observed or certain outcomes are measured. No attempt is made by the researcher to affect the outcome — for example, no treatment is given by the researcher.
- Clinical trial (interventional study). During clinical trials, researchers learn if a new test or treatment works and is safe. Treatments studied in clinical trials might be new drugs or new combinations of drugs, new surgical procedures or devices, or new ways to use existing treatments. Find out more about the five phases of non-cancer clinical trials on ClinicalTrials.gov or the National Cancer Institute phases of cancer trials .
- Medical records research. Medical records research involves the use of information collected from medical records. By studying the medical records of large groups of people over long periods of time, researchers can see how diseases progress and which treatments and surgeries work best. Find out more about Minnesota research authorization .
Clinical studies may differ from standard medical care
A health care provider diagnoses and treats existing illnesses or conditions based on current clinical practice guidelines and available, approved treatments.
But researchers are constantly looking for new and better ways to prevent and treat disease. In their laboratories, they explore ideas and test hypotheses through discovery science. Some of these ideas move into formal clinical trials.
During clinical studies, researchers formally and scientifically gather new knowledge and possibly translate these findings into improved patient care.
Before clinical trials begin
This video demonstrates how discovery science works, what happens in the research lab before clinical studies begin, and how a discovery is transformed into a potential therapy ready to be tested in trials with human participants:
How clinical trials work
Trace the clinical trial journey from a discovery research idea to a viable translatable treatment for patients:
See a glossary of terms related to clinical studies, clinical trials and medical research on ClinicalTrials.gov.
Watch a video about clinical studies to help you prepare to participate.
Let's Talk About Clinical Research
Narrator: This presentation is a brief introduction to the terms, purposes, benefits and risks of clinical research.
If you have questions about the content of this program, talk with your health care provider.
What is clinical research?
Clinical research is a process to find new and better ways to understand, detect, control and treat health conditions. The scientific method is used to find answers to difficult health-related questions.
Ways to participate
There are many ways to participate in clinical research at Mayo Clinic. Three common ways are by volunteering to be in a study, by giving permission to have your medical record reviewed for research purposes, and by allowing your blood or tissue samples to be studied.
Types of clinical research
There are many types of clinical research:
- Prevention studies look at ways to stop diseases from occurring or from recurring after successful treatment.
- Screening studies compare detection methods for common conditions.
- Diagnostic studies test methods for early identification of disease in those with symptoms.
- Treatment studies test new combinations of drugs and new approaches to surgery, radiation therapy and complementary medicine.
- The role of inheritance or genetic studies may be independent or part of other research.
- Quality of life studies explore ways to manage symptoms of chronic illness or side effects of treatment.
- Medical records studies review information from large groups of people.
Clinical research volunteers
Participants in clinical research volunteer to take part. Participants may be healthy, at high risk for developing a disease, or already diagnosed with a disease or illness. When a study is offered, individuals may choose whether or not to participate. If they choose to participate, they may leave the study at any time.
Research terms
You will hear many terms describing clinical research. These include research study, experiment, medical research and clinical trial.
Clinical trial
A clinical trial is research to answer specific questions about new therapies or new ways of using known treatments. Clinical trials take place in phases. For a treatment to become standard, it usually goes through two or three clinical trial phases. The early phases look at treatment safety. Later phases continue to look at safety and also determine the effectiveness of the treatment.
Phase I clinical trial
A small number of people participate in a phase I clinical trial. The goals are to determine safe dosages and methods of treatment delivery. This may be the first time the drug or intervention is used with people.
Phase II clinical trial
Phase II clinical trials have more participants. The goals are to evaluate the effectiveness of the treatment and to monitor side effects. Side effects are monitored in all the phases, but this is a special focus of phase II.
Phase III clinical trial
Phase III clinical trials have the largest number of participants and may take place in multiple health care centers. The goal of a phase III clinical trial is to compare the new treatment to the standard treatment. Sometimes the standard treatment is no treatment.
Phase IV clinical trial
A phase IV clinical trial may be conducted after U.S. Food and Drug Administration approval. The goal is to further assess the long-term safety and effectiveness of a therapy. Smaller numbers of participants may be enrolled if the disease is rare. Larger numbers will be enrolled for common diseases, such as diabetes or heart disease.
Clinical research sponsors
Mayo Clinic funds clinical research at facilities in Rochester, Minnesota; Jacksonville, Florida; and Arizona, and in the Mayo Clinic Health System. Clinical research is conducted in partnership with other medical centers throughout the world. Other sponsors of research at Mayo Clinic include the National Institutes of Health, device or pharmaceutical companies, foundations and organizations.
Clinical research at Mayo Clinic
Dr. Hugh Smith, former chair of Mayo Clinic Board of Governors, stated, "Our commitment to research is based on our knowledge that medicine must be constantly moving forward, that we need to continue our efforts to better understand disease and bring the latest medical knowledge to our practice and to our patients."
This fits with the term "translational research," meaning what is learned in the laboratory goes quickly to the patient's bedside and what is learned at the bedside is taken back to the laboratory.
Ethics and safety of clinical research
All clinical research conducted at Mayo Clinic is reviewed and approved by Mayo's Institutional Review Board. Multiple specialized committees and colleagues may also provide review of the research. Federal rules help ensure that clinical research is conducted in a safe and ethical manner.
Institutional review board
An institutional review board (IRB) reviews all clinical research proposals. The goal is to protect the welfare and safety of human subjects. The IRB continues its review as research is conducted.
Consent process
Participants sign a consent form to ensure that they understand key facts about a study. Such facts include that participation is voluntary and they may withdraw at any time. The consent form is an informational document, not a contract.
Study activities
Staff from the study team describe the research activities during the consent process. The research may include X-rays, blood tests, counseling or medications.
Study design
During the consent process, you may hear different phrases related to study design. Randomized means you will be assigned to a group by chance, much like a flip of a coin. In a single-blinded study, participants do not know which treatment they are receiving. In a double-blinded study, neither the participant nor the research team knows which treatment is being administered.
Some studies use an inactive substance called a placebo.
Multisite studies allow individuals from many different locations or health care centers to participate.
Remuneration
If the consent form states remuneration is provided, you will be paid for your time and participation in the study.
Some studies may involve additional cost. To address costs in a study, carefully review the consent form and discuss questions with the research team and your insurance company. Medicare may cover routine care costs that are part of clinical trials. Medicaid programs in some states may also provide routine care cost coverage, as well.
When considering participation in a research study, carefully look at the benefits and risks. Benefits may include earlier access to new clinical approaches and regular attention from a research team. Research participation often helps others in the future.
Risks/inconveniences
Risks may include side effects. The research treatment may be no better than the standard treatment. More visits, if required in the study, may be inconvenient.
Weigh your risks and benefits
Consider your situation as you weigh the risks and benefits of participation prior to enrolling and during the study. You may stop participation in the study at any time.
Ask questions
Stay informed while participating in research:
- Write down questions you want answered.
- If you do not understand, say so.
- If you have concerns, speak up.
Website resources are available. The first website lists clinical research at Mayo Clinic. The second website, provided by the National Institutes of Health, lists studies occurring in the United States and throughout the world.
Additional information about clinical research may be found at the Mayo Clinic Barbara Woodward Lips Patient Education Center and the Stephen and Barbara Slaggie Family Cancer Education Center.
Clinical studies questions
- Phone: 800-664-4542 (toll-free)
- Contact form
Cancer-related clinical studies questions
- Phone: 855-776-0015 (toll-free)
International patient clinical studies questions
- Phone: 507-284-8884
- Email: [email protected]
Clinical Studies in Depth
Learning all you can about clinical studies helps you prepare to participate.
- Institutional Review Board
The Institutional Review Board protects the rights, privacy, and welfare of participants in research programs conducted by Mayo Clinic and its associated faculty, professional staff, and students.
More about research at Mayo Clinic
- Research Faculty
- Laboratories
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Postdoctoral Fellowships
- Training Grant Programs
- Publications
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih clinical research trials and you, finding a clinical trial, around the nation and worldwide.

NIH conducts clinical research trials for many diseases and conditions, including cancer , Alzheimer’s disease , allergy and infectious diseases , and neurological disorders . To search for other diseases and conditions, you can visit ClinicalTrials.gov.
ClinicalTrials.gov [ How to Use Search ] This is a searchable registry and results database of federally and privately supported clinical trials conducted in the United States and around the world. ClinicalTrials.gov gives you information about a trial's purpose, who may participate, locations, and phone numbers for more details. This information should be used in conjunction with advice from health care professionals.
Listing a study does not mean it has been evaluated by the U.S. Federal Government. Read the disclaimer on ClinicalTrials.gov for details.
Before participating in a study, talk to your health care provider and learn about the risks and potential benefits.
At the NIH Clinical Center in Bethesda, Maryland

Search NIH Clinical Research Studies The NIH maintains an online database of clinical research studies taking place at its Clinical Center, which is located on the NIH campus in Bethesda, Maryland. Studies are conducted by most of the institutes and centers across the NIH. The Clinical Center hosts a wide range of studies from rare diseases to chronic health conditions, as well as studies for healthy volunteers. Visitors can search by diagnosis, sign, symptom or other key words.
Join a National Registry of Research Volunteers

ResearchMatch This is an NIH-funded initiative to connect 1) people who are trying to find research studies, and 2) researchers seeking people to participate in their studies. It is a free, secure registry to make it easier for the public to volunteer and to become involved in clinical research studies that contribute to improved health in the future.
This page last reviewed on November 6, 2018
Connect with Us
- More Social Media from NIH
Clinical Trial Templates to Start Your Clinical Research
By Kate Eby | May 13, 2019
- Share on Facebook
- Share on LinkedIn
Link copied
In this article, you will find everything you need to start your clinical research trials, with easy-to-understand guidance and terminology, 26 adaptable templates, and project plans in Microsoft Word, Excel, Project, and SharePoint formats.
Included on this page, you'll find details on what a research protocol is, project management for clinical trials , research compliance templates , and post-clinical study research documentation and templates
What Is the Research Protocol?
All clinical research starts with the research protocol , a document that details all aspects of the trial: its background, rationale, objectives, design, methodology, statistical analysis plan, and organization. With the protocol, you can make sure you protect the participants and collect the data. Using protocol templates, you can start thinking through what you need to meet compliance standards with the Food and Drug Administration (FDA) and clinical study best practices.

Download Research Protocol Template - Word
The full research protocol includes the following sections and topics:
- Title Pages: These pages provide general information about the protocol, including name, number, version number and date, trial phase, investigational product name, investigational new drug (IND) number, sponsor (or principal investigator in academia), funding organization, medical monitor, and coordinating center. The pages include the principal investigator’s signature (or sponsor), as well as site-specific information, such as the agreement, and protocol details. They also detail the study team and site, particularly in the case of multiple teams and sites.
- Objectives: List the study’s primary and secondary objectives.
- Background Information: Describe the problem under study and priority. Include the medical and scientific rationale that justifies researching the problem. Include data from other studies relevant to this proposed research. Include the name and description of the proposed intervention, including the dosage, route of administration, period, and frequency of intervention.
- Study Design: Describe the methodology and how it will answer the study question. This should include the type of study, primary and secondary outcome(s), population, sample size, study location, period of enrollment and follow-up, intervention and route of administration, randomization (as necessary), and any other relevant protocol information.
- Selection and Exclusion of Subjects: Provide statements describing how the participants must meet all the inclusion and exclusion criteria, and list the criteria. Clearly define the study population. For example, list the demographic criteria, required laboratory data, any prior therapies allowed or disallowed, ability to understand and meet all study requirements, if contraception is necessary, exclusion criteria such as specific health status, use of excluded drugs, cancer status, and chemical dependency status.
- Study Enrollment Procedures: Describe the methods and procedures for identifying and enrolling subjects, how they are documented, how consent is obtained, and any randomization procedures.
- Study Intervention, Duration, and Route of Administration: This section should describe each intervention and duration, as well as how each is administered. List expected adverse effects and dose escalation, if applicable. Discuss how the intervention is acquired, stored, and disposed of, as well as documentation for intervention accountability. In addition, note the medications restricted, allowed, and required, along with the extent to which these medications are tracked and documented.
- Study Procedures: This section includes a study evaluation schedule (presented as a chart) and explanations of the required assessments, what each period is, and any special considerations or instructions necessary. These should match what is available in the column headers of the chart above, and they should include information on the screening or baseline assessments, randomization, blinding, follow-up visits, and final assessments.
- Safety Assessment: List any expected adverse events, and how these could be managed. Mention any toxicities seen in earlier IND studies here. Also, include safety measures as identified in laboratory findings, methods and timing for safety parameters based on the risk profile, definitions for adverse events (AE) and serious adverse events (SAE) and laboratory values used to identify their possibility, timeframes for reporting and collecting information on AEs and SAEs, the reporting system, how you will follow up on AEs, and the specific guidelines for independent monitoring.
- Intervention Discontinuation: List criteria for intervention discontinuation and how you could meet them. Also list possible reasons for discontinuation, any modifications to the schedule should it be discontinued, duration of follow-up, any temporary discontinuation criteria, or any evaluations should participants be temporarily or permanently discontinued from the study.
- Statistical and Analytical Considerations: Include primary and secondary statistical hypotheses, why you chose the study design, the primary and secondary outcome measures, and the validity and reliability of these measures. Also discuss sample size and randomization, treatment assignment procedures, how you define the population, any interim analyses, primary and secondary outcome analyses, the statistical methods you use to consider any necessary intervention effect between groups, and if necessary, the expected positive within group correlations among different study arms.
- Data Collection: Detail how you will gather the data, the required forms, how to keep these forms confidential, and what source data to expect. Note site responsibility for data collection and management, and (if necessary) the responsibilities of the coordinating center.
- Quality Assurance: Describe training for study staff, whether there is a control committee and their required practices, any quality control metrics, how you will identify and document protocol deviations, how you will assure protocol compliance, and the schedule for reviews. If you have a manual of procedures (MOP), reference it here.
- Participants Rights: Include references to the Institutional Review Board (IRB) requirements, informed consent documents, procedures for participant confidentiality, and study discontinuation requirements.
- Committees: List any committees associated with the study, along with their roles.
- Publication: Outline the requirements and procedures for publication.
- References: List any citations referenced in this protocol.
- Supplements/Appendices: Include any additional documentation.
To track every aspect of the proposed research for each participant, create a case report form (CRF) that you can use in both paper and electronic formats. With CRFs, you can collect and analyze data for analysis, and then generate a conclusion for your study. For more information on the distinct phases of clinical trials, see “ Understanding the Phases of Clinical Trials .”
Concept Protocol Template
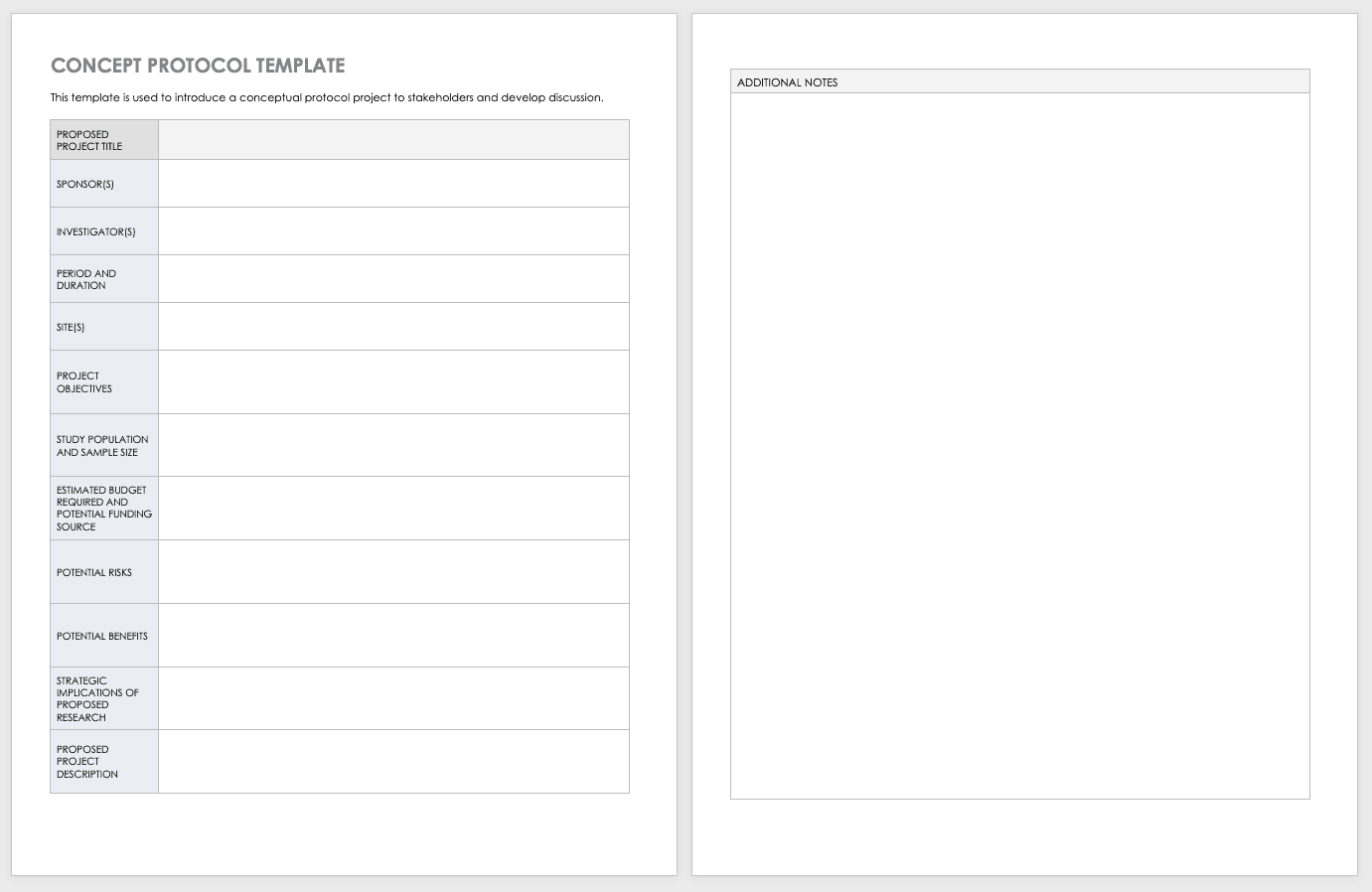
Before you start your full protocol, consider putting together a concept protocol. A concept protocol helps you introduce an abstract project to stakeholders and encourage discussion around the proposed project.
Download Concept Protocol Template for Clinical Research
Phase 1 Clinical Trial Protocol Template

For nonclinical research or clinical trials that are Phase 0 or Phase 1, use this free template. Phase 1 or nonclinical trials do not require the same amount of detail as a full study protocol.
Download Phase 1 Clinical Trial Protocol Template - Word
Research Compliance Templates
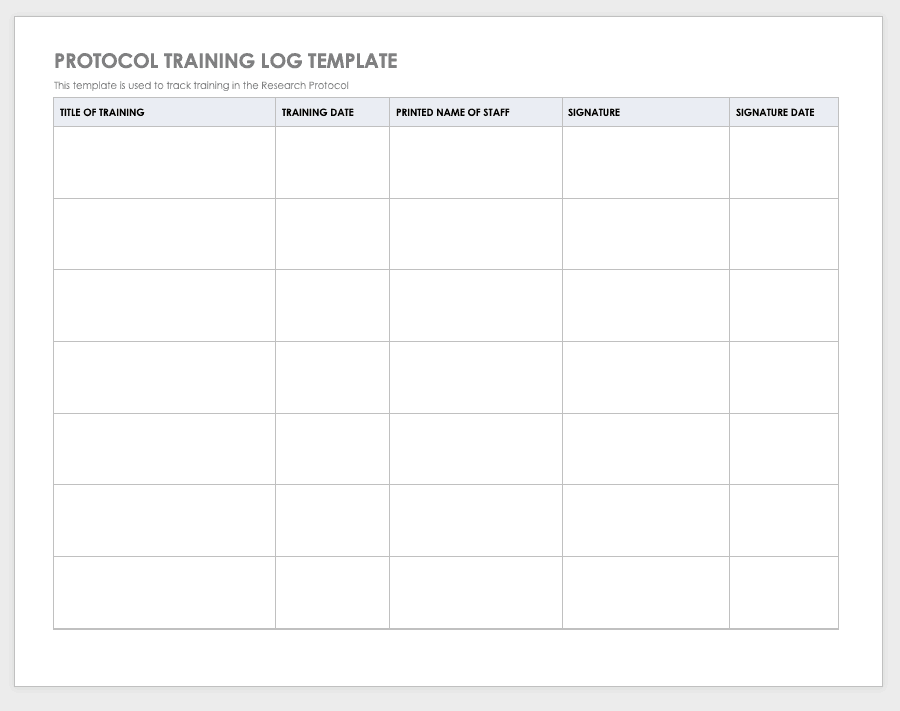
By training staff members on the research protocol, you’ll help them meet compliance standards and understand the purpose and details of the study. Use a training log to record all training that the site study staff completes, signing the log entry for verification.
Download Protocol Training Log Template
Excel | Word | PDF | Smartsheet
Protocol Deviation Template
Protocol deviations are inadvertent or unplanned changes or noncompliance with the research protocol. These events do not increase risk or decrease benefit, nor do they impinge on participants’ safety or rights. They do not compromise study data, but you should capture the deviation for reference.
Download Protocol Deviation Log Template
Excel | Word | PDF
Delegation of Authority Log Template
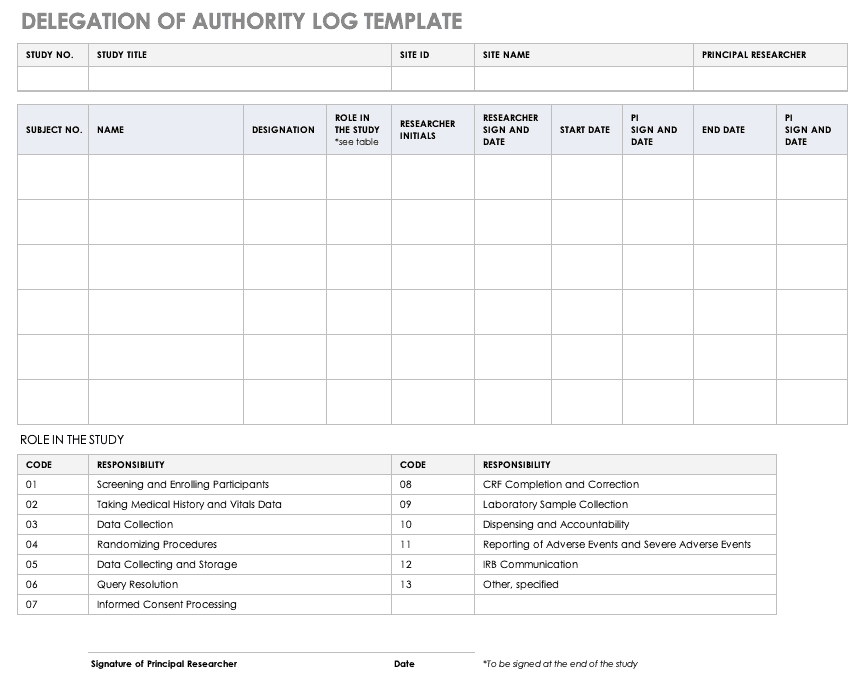
Once you’ve trained your staff and figured out their roles and responsibilities, the principal investigator must delegate authority. The delegation of authority log should be filled out and signed prior to the study’s start.
Download Delegation of Authority Log Template
Site Selection Visit Form Template
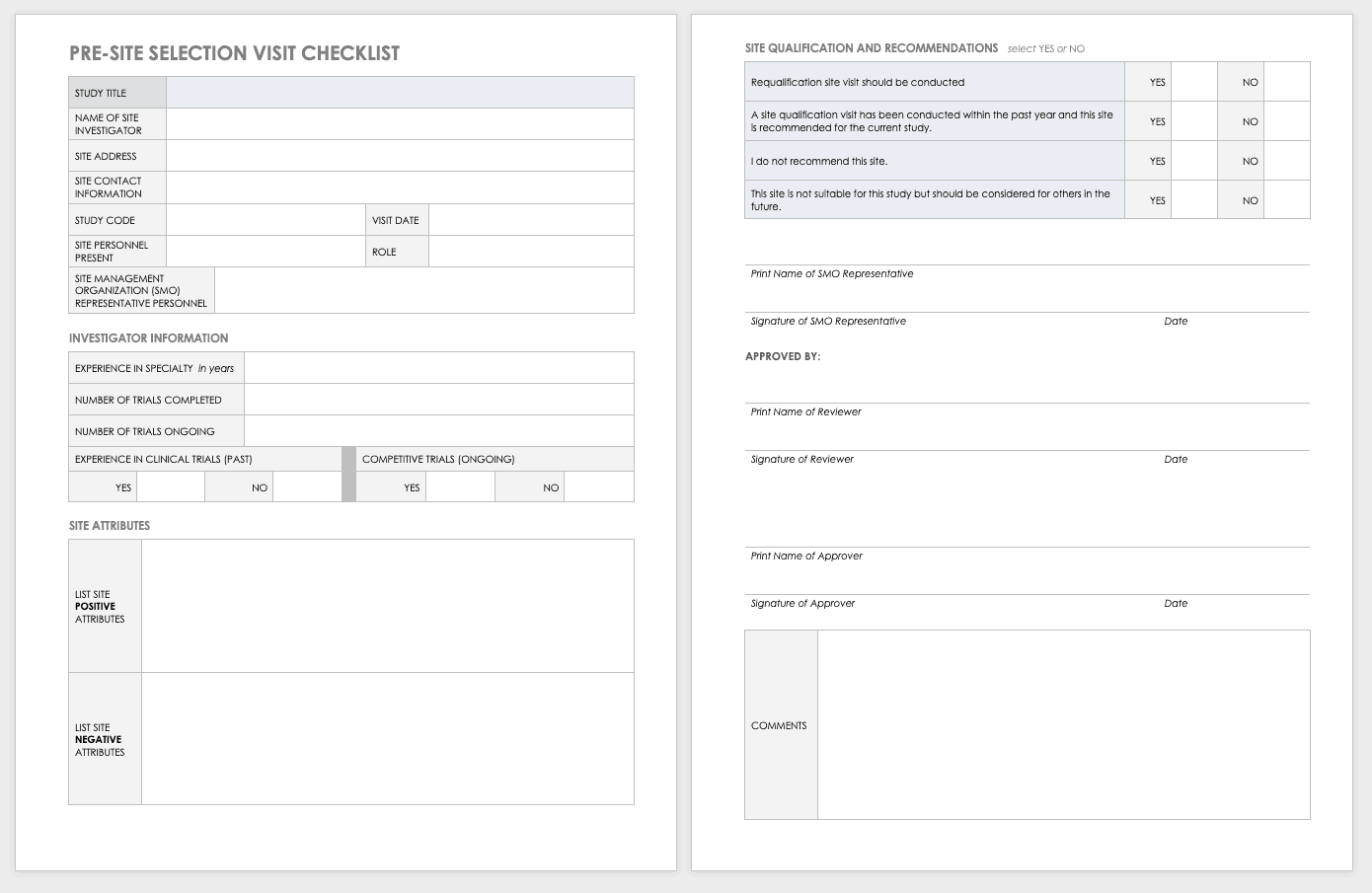
The sponsor must perform a site visit to determine its suitability as part of a multisite study. This means taking a tour to determine whether the site has the capabilities to meet the sponsor’s goals.
Download Site Selection Visit Form Template
Word | PDF | Smartsheet
Study Site Initiation Checklist
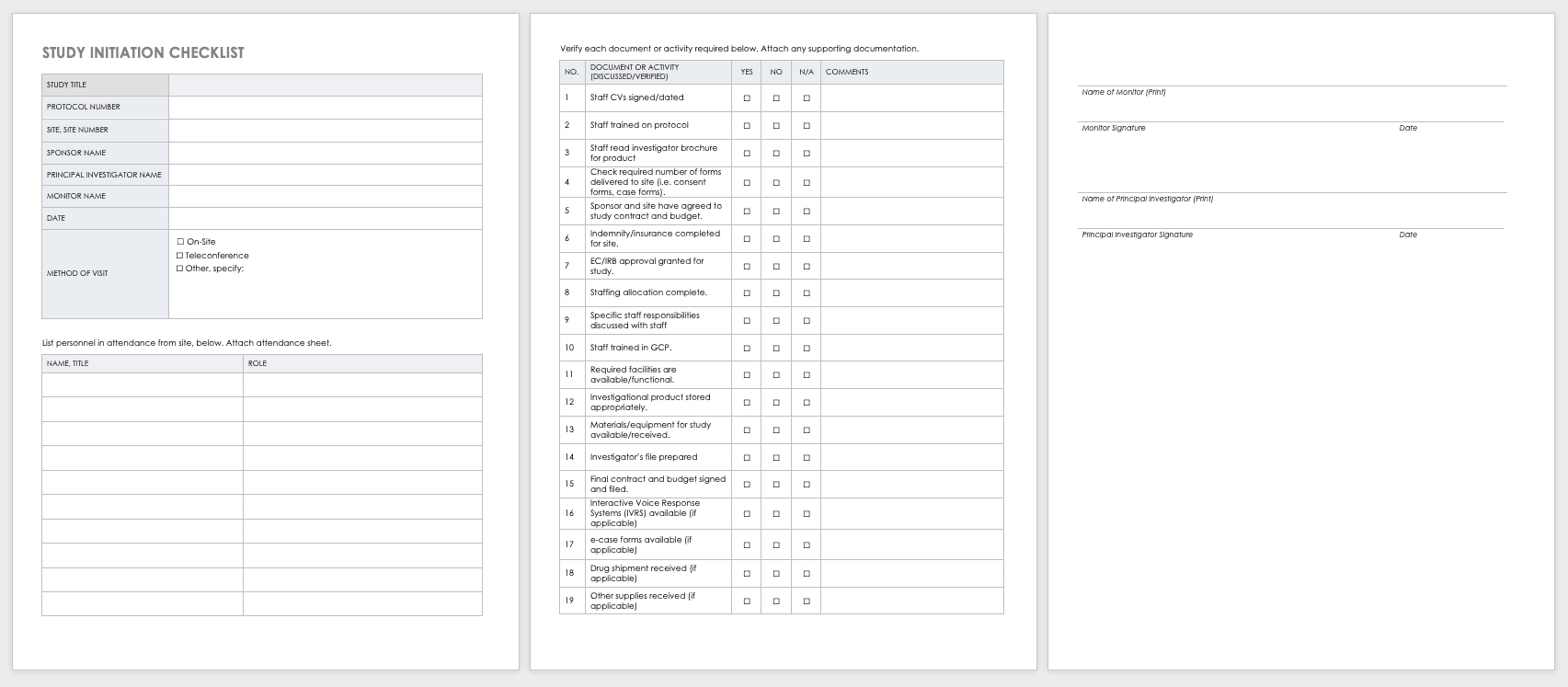
Teams must also perform an inspection to determine if a site has the appropriate staff, training, equipment, and supplies to be part of a multisite trial.
Download Study Site Initiation Checklist
Project Management for Clinical Trials, Practices, Templates, and Documents
Clinical trials are big projects. If the organization is not used to planning and wants to conduct clinical research, it must hire a project manager and work with senior leadership to introduce planning into the organization.
Together, they should develop the main goals and define their limits and the terms of success. They should set out a strategy for which tasks and sets of tasks to perform and in what manner. Test any planning tools or software before the trials start. When possible, use templates to ensure consistency and best practices.
Once the trial starts, evaluate your systems with standardized metrics. The project manager can track study deviations and apply corrective actions. Use the lessons learned from past and current projects to help guide future projects. Employing consistent tools gives you the opportunity to draw from a reservoir of data.
Clinical research can cost billions of dollars and years of time, resources, and effort. As
such, project management best practices and methodologies are critical to the success of a clinical trial, according to experts .
Many software systems are available to manage clinical trials. When very specialized, these are referred to as clinical trial management systems (CTMSs). However, other platforms can also manage clinical trials and may already be embedded with your information technology. Regardless of the platform you use, you should have full project management functionality, such as planning and reporting modules, as well as the ability to track participant contact information, deadlines, and milestones.
You may want to consider the following project management documents for your clinical research.
Project Management Plan (PMP) for Clinical Trials
A PMP delineates and acts as an agreed-upon document of scope, responsibilities, and guidance. You can use it throughout the project to help stay on track. Every clinical trial has difficult milestones, but a good project management plan can help you sidestep some of the regular issues.
You have many PMP software platforms to choose from, but regardless of your ultimate decision, your PMP must focus on protocol adherence, subject care, and service quality, along with how to achieve each standard. Here are the sections you should include in your PMP for a clinical trial:
- Project Objectives: This is an outline of the research objectives for the study, your quantifying standards, and your goals.
- Background and Strategic Context: By documenting background and context, you establish a foundation for decisions and discussion to follow.
- Study Governance: The governance covers the roles and responsibilities in the project, encouraging open communication, sharing, and accountability.
- Stakeholder Management Plan: This plan details how the staff and investigators will collaborate and effectively communication with stakeholders. This could include (as per the roles and responsibilities) regular emails, newsletters, consultation, oversight, training, and documentation.
- Scope: This document delineates assumptions, constraints, and deliverables (and their expected dates).
- Project Risk Assessment: This document helps you prepare for risks and decide on the risk profile.
Clinical Research Project Activity List
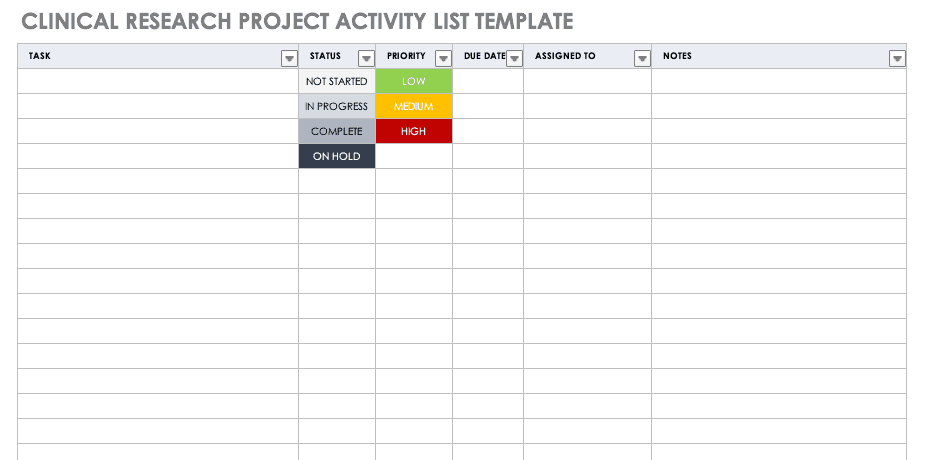
A project activity list is an itemized documentation of all the activities scheduled as part of the project. This list should be very detailed, including the status and priority of the task, when it is due, and to whom it is assigned.
Download Clinical Research Project Activity List Template
Excel | Smartsheet
Clinical Trial Timeline Template
A timeline enables you and your staff to track each major portion or milestone of your clinical trial. Your timeline should include these steps:
- Choose Research Questions and Study Design: Research always begins with questions. Your research question will determine how you design your study.
- Choose Outcomes: The outcomes for any trial are dependent on many factors, including scope, health conditions under study, target population, type of intervention. One resource to help develop outcomes is Core Outcome Measures in Effectiveness Trials (COMET) . This database details core outcome sets for comparison in clinical trials.
- Prospectively Register the Trial: Whether you are working through the FDA, World Health Organization (WHO), or another national agency, study transparency is critical. Prospective registration of trials is recommended. One resource for registration is the ISRCTN registry .
- Obtain Ethics Approval: Any trial involving human participants must go through an ethics review to safeguard the subjects’ rights, safety, well-being, and dignity. There are many options for institutional review, including through a university or a private or governmental organization. Without this step, research cannot commence.
- Prospectively Publish Protocol and Analysis Plan: Before a clinical trial, you must complete some pilot research. When you publish the research leading up to a clinical trial, along with the protocol and analysis for the trial itself, you increase transparency and accountability of the research.
- Planning for the Trial and Data Management: Many clinical research professionals recommend including patients in the planning phase of clinical trials, at least as stakeholders to review the plan. By completing the plan early and allowing potential participants to review it, you help improve recruitment and retention during the trial.
- Recruitment and Retention: Recruitment is getting the right people to take part in your trial, and retention is about keeping their interest and trust. A source of unending frustration for researchers, recruitment and retention can make or break a trial.
- Identify and Manage Trial Sites and Staff: This process is not as straightforward as it is often thought to be. Study coordinators must use feasibility checklists to choose sites and figure out how to get bring on staff who have the bandwidth to recruit for the study.
- Data Collection: The methods for collecting data are critical to any study. Advance planning and structure help you stay organized, comprehensive, and transparent so that your study can have a seamless analysis and solid conclusions.
- Data analysis: Flaws in analysis can generate poor, biased, or erroneous outcomes. In advance, researchers should consider patient blinding, randomization procedures, and sequence generation.
- Findings dissemination: Some researchers recommend threading all research on a trial topic. One resource for this is CrossRef , a database that links similar research. Regardless, the point of research is to capitalize on scientific progress and move it along. By having a plan to disseminate your results, you ensure that others capitalize on your research and move the knowledge forward.
Use this free template to develop your own clinical trial timeline. Add your own steps, milestones, and dates for a comprehensive, expansive view.

Download Clinical Trial Timeline Template
For a different perspective, add your project details to this free template so you can view your timeline visually.
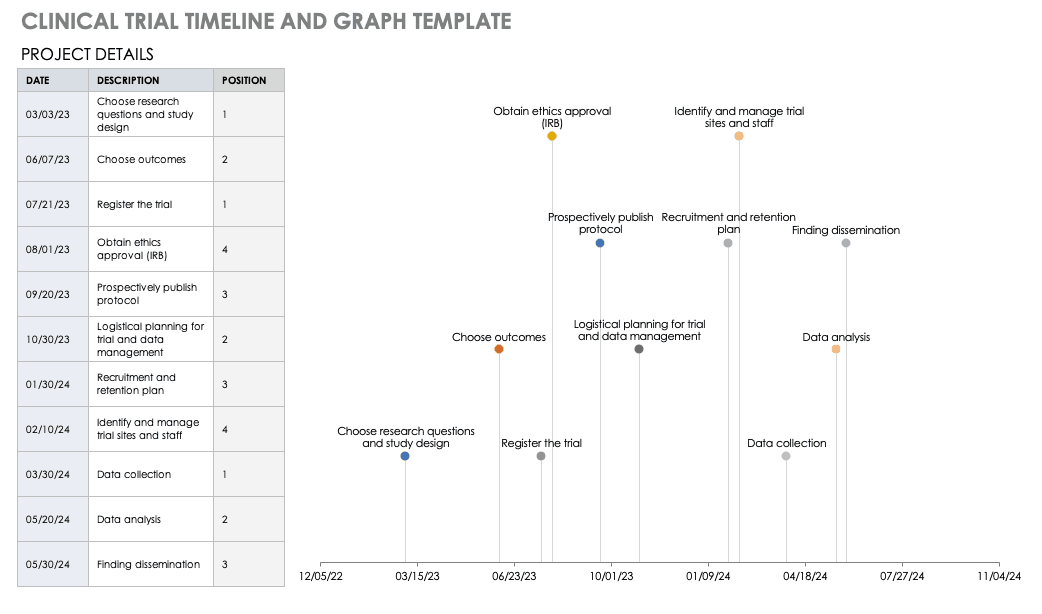
Download Trial Timeline and Graph Template

Microsoft Project Management for Clinical Trials
First released in 1985, Project is a well-respected Microsoft product for project management. Microsoft Project was not traditionally available as a part of Office Suites, a package of programs for professionals and professional organizations. However, Microsoft recently included it as a part of the Windows 2016 suite.
Microsoft Project Management has the following features:
- Built-in templates
- Project portfolio management
- IT management
- Presentations
- Out-of-the-box reports
- Multiple timelines
- Real-time reporting
- Dependency management
- Priority assignment
- Lean management
- Gantt charts/project mapping
- Calendar views
- Setting baselines/KPIs
- Project budgeting
- Issue tracking
- Task creation
- Resource management
- Cloud access
Microsoft Project has built-in templates that you can apply to clinical trial management.
Microsoft SharePoint for Clinical Trials
SharePoint is a collaboration platform that is integrated with Microsoft Office. SharePoint manages and stores documents , and it enables multiple users to access the documents via their own site or a standardized Microsoft site. A subscription to Microsoft Office 365’s SharePoint does not require a server, but customization options are limited; the flexible authentication and authorization systems are built in.
SharePoint Server, available in Standard or Enterprise versions, can be developed as either
virtual or hosted services in a business’s IT department. SharePoint Server enables the organization to control the SharePoint features available to staff, and you can scale it to meet different numbers of users.
Windows SharePoint Services 3.0 is a Microsoft-hosted version that comes with Microsoft Office. Microsoft provides a template in SharePoint for Clinical Trials: Clinical Trial Initiation and Management application template for Windows SharePoint Services 3.0 . You can download and add this template to your SharePoint Services, which enables you to create the following:
- Clinical Trial Protocols: This includes the objectives, study design, project plan, subject selection, and budget.
- Protocol Documents: This includes additional documents relative to your study.
- Calendar: Track milestones in the project.
- Threaded Document Discussions: Team members can start and track discussions within documents.
- Task Creation and Assignment: You can create and assign tasks to users, who receive email notifications.
- Archiving: You can move documents or groups of documents to archive status, keeping them but not making them visible.
The clinical trial template has site lists of libraries for clinical trial protocols, protocol documents, announcements, calendars, issues, tasks, and document discussions. These can be further customized with different versions of SharePoint. To download this template, you will need access to SharePoint Server 3.0.
Clinical Research Budget Plan Template
In many instances, you set the clinical trial budget after much negotiation with a sponsor. Other times, you need to build a budget before the sponsor is even on board, as a way to convince them of the project’s feasibility. The key cost drivers for any clinical research project are the following:
- Patient Grants: These include the costs for screening failures, baseline patient measurements, and procedural costs.
- Site Costs: This covers any expenses associated with the site, such as start-up fees, IRB fees, storage fees, and site management costs.
- Non-Patient Costs: This includes consultation fees, monitoring board fees, and any medical device costs.
- Labor Costs: You must account for all the staff required for the project and their full-time equivalency (FTE).
- Site Management: These costs include pre-study visits, initiation fees, monitoring, and close-out fees.
- Miscellaneous: These include investigator meetings, any technology needs, and ad hoc travel.
- Unexpected Costs: These are costs resulting from protocol amendments, value added tax (VAT), delays, and inflation.
Before you start putting together your research budget, you must gather the following:
- Schedule of assessments from the protocol
- Standard institutional fees from your institution, if applicable
- Evaluation and procedural costs
- Staff allocation and their hourly rates
- Indirect cost rate
- Subject compensation costs
- Data storage fee estimate
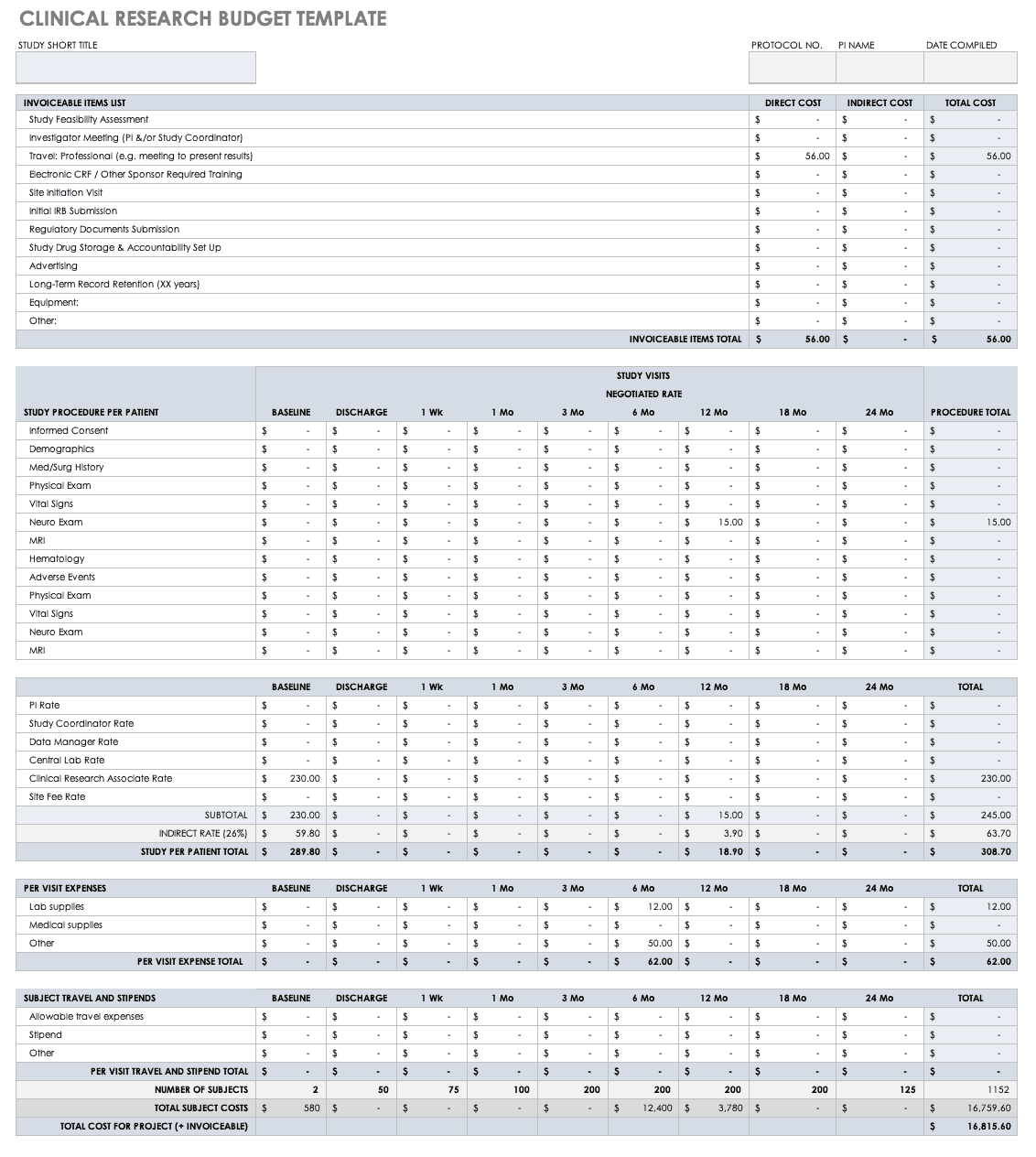
Put together your own clinical trial budget with this free clinical research budget template.
Download Clinical Research Budget Template - Excel
Clinical Research Tracking Log Templates
Clinical research requires scrupulous planning, a well-developed team, regulatory adherence, and above all, excellent documentation. It is therefore critical for clinical trial project managers to have a completed scope of work and to develop all the forms and templates before the trial begins. Some of these documents are for planning, and some, like those included below, are for operational purposes.
Regulatory Binder Checklist

Strong clinical practice thrives with a regulatory binder checklist. This checklist keeps track of all paper versions of essential regulatory study documents. Each document should also include any electronic locations. This document should be regularly updated, customized for unique studies, and stored in reverse chronological order.
Download Regulatory Binder Checklist
Clinical Study Document Tracking Log
It is important to not only track all paperwork related to a clinical trial, but also be able to locate it easily between various staff and sites. A clinical trial document tracking log can help you keep a written trail of the documents and when they were submitted and approved. You should also keep copies of the documents with the log. Use this free template to develop your own clinical study document tracking log. You can also adapt the log for specific correspondence, such as documents relating to FDA or IRB submissions, but it should not be mixed with regulatory documentation.
Download Clinical Study Document Tracking Log
Data and Safety Monitoring Plan (DSMP) Template
Before you can undertake a study, you must develop a DSMP for how to keep participants safe and how to secure data and ensure accuracy. The DSMP has several sections:
- The study purpose
- An adherence statement
- Any protocol amendments
- Multisite agreements
- A plan for subject privacy
- Confidentiality during adverse event reporting
- Expected risks
- Adverse events, unanticipated problems, and serious adverse events: how they are defined, their relation to the study, expectations, severity grading, and reporting procedures in single-site and multisite trials, and whether they are IND or non-IND studies
- Events of special interest
- Pregnancy reporting
- Rules to halt the study for participants
- Quality control and quality assurance
- Subject accrual and compliance
- Sample size justification
- Stoppage rules
- Monitoring committee designation
- Safety review plan
- Study report plan for independent monitors
- Plan to submit reports from onsite monitoring and audits
- Data handling and record keeping
- Informed consent
- Reporting changes in study status

Create your own data and safety monitoring plan using this free template. It lays out each section so you can specify them for your research. The principal investigator should sign and date this document once it is complete so that it may be filed.
Download Data and Safety Monitoring Plan Template - Word
Research Communication Plan Template
A communication plan should describe how you will converse with internal and external stakeholders during your project. Your communication plan should include a brief overview of your project and a breakdown of the messages you need to get out. You should adapt the messages for different audiences and define who will deliver these messages. The messages should include the following:
- The purpose and benefits of the research
- The known effectiveness of the intervention, or (if the intervention is under study) the disclosure that the effectiveness is unknown
- How participants will be protected
- The risks and benefits of participating

Develop your own communication plan using this free clinical trial communication plan template. This template also includes a section for situation analysis and risk analysis that asks for inputs on strengths, weaknesses, opportunities, and threats.
Download Clinical Trial Communication Plan Template - Word
Participant Management in Clinical Trials Using Templates
A few main documents help ensure that your participants are tracked and well-cared for before and during your research study.
Enrollment Log for Clinical Trials Template
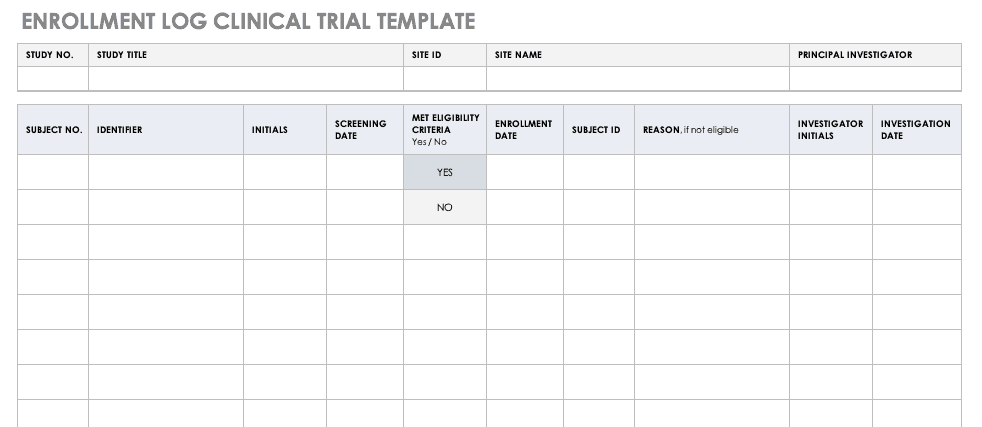
This log keeps track of everyone that has been enrolled for participation in your study. This does not mean that they have met the eligibility requirements or have been otherwise screened, but it is a record that they have signed up to be admitted.
Download Enrollment Log for Clinical Trials Template
Informed Consent Form Templates
Informed consent is the central tenet of ethical research with human subjects. The consent process typically involves a researcher delineating what is involved in the study, its risks and benefits, what a participant’s duties entail, and answering any questions they have. Before you perform any research, make sure the informed consent document is signed and the participant receives a copy, unless the informed consent document has been waived by an institutional review board (IRB). Federal regulations 45 CFR 46.116 govern what you must provide in the informed consent process in the United States.
To prepare informed consent documentation, researchers must do the following:
- Use plain, easily understandable language no higher than an 8th-grade reading level.
- Tailor documents to the potential population.
- Avoid technical jargon.
- Use the second or third person (you/he/she) to present study details.
- Include a statement of agreement.
- Ensure that the consent document is consistent with information in the IRB application.
These templates assist the principal investigator in the design of their informed consent forms (ICFs). You can adapt them to accommodate the details of any study and include both the information sheet and the consent form. Modify each section with the appropriate description described in italics. Use the general template for any type of research.
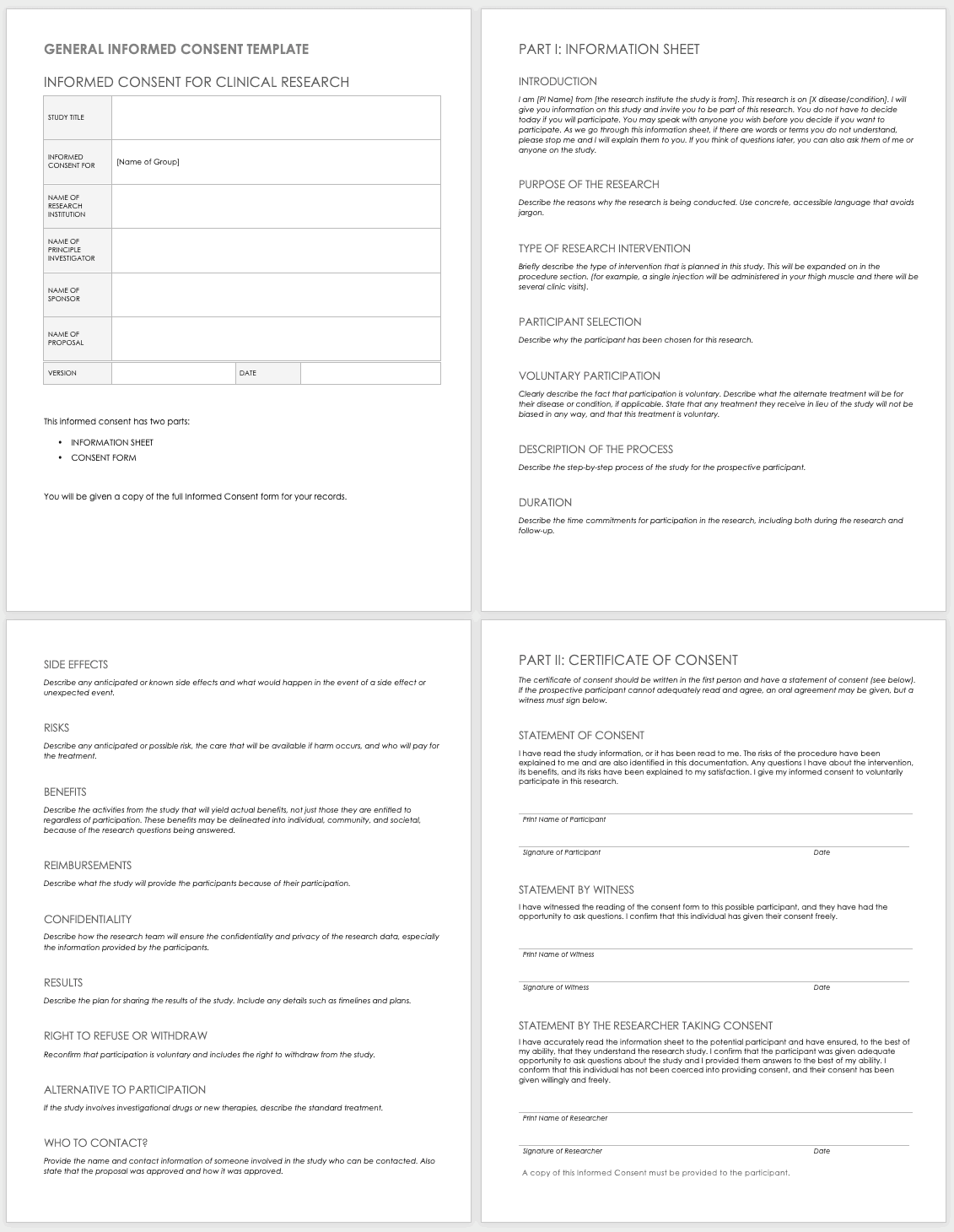
Download General Informed Consent Template - Word
Use the clinical trial template for medical research.

Download Informed Consent for Clinical Trials Template - Word
Eligibility Criteria (Inclusion/Exclusion) Checklist
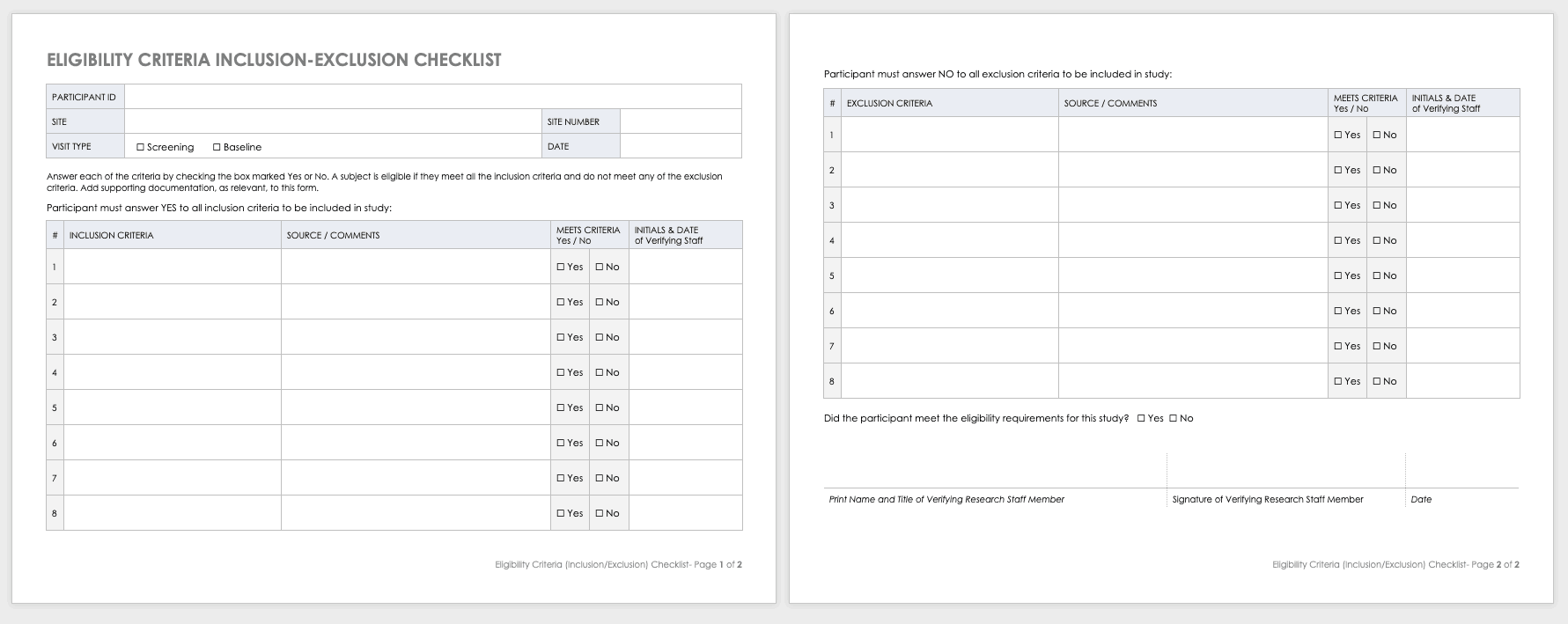
Eligibility criteria are an essential part of clinical trials. They define the population under investigation.
Inclusion criteria are the standards that participants must meet to enroll in the study. For example, in a study on a new diabetes medication, you would likely want participants who have already been diagnosed with diabetes.
Exclusion criteria specify the characteristics that disqualify participants from taking part in the research. For example, in the diabetes study above, the proposed diabetes drug may target a specific age demographic. One exclusion criterion could be a participant whose age falls outside of the range.
Download Eligibility Checklist Inclusion-Exclusion Template
Concomitant Medication Log Template
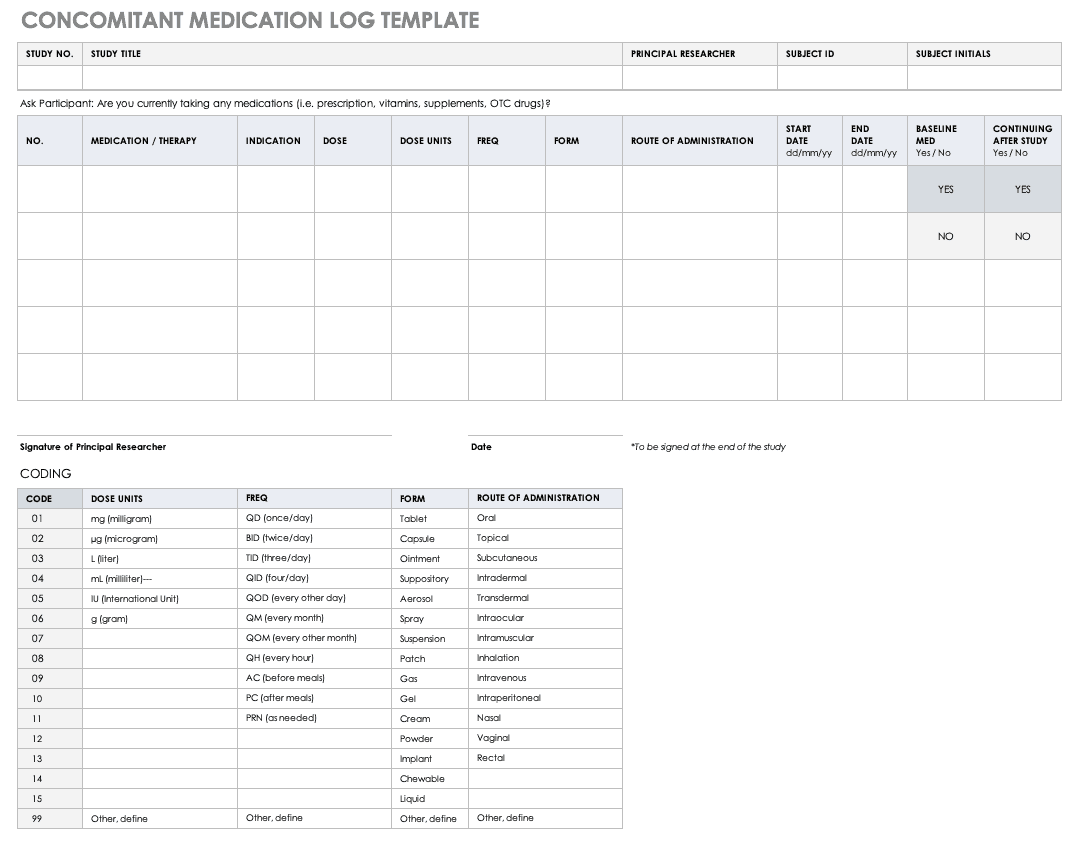
Properly documenting any medications that participants are taking is imperative to understanding the reactions occurring in their bodies, as well as what could spur adverse and severe adverse events during the study. Fill out a concomitant medication log for every participant and account for everything participants take, even seemingly innocuous items like multivitamins.
Download Concomitant Medication Log Template
Excel | Word | PDF
Adverse Event Form
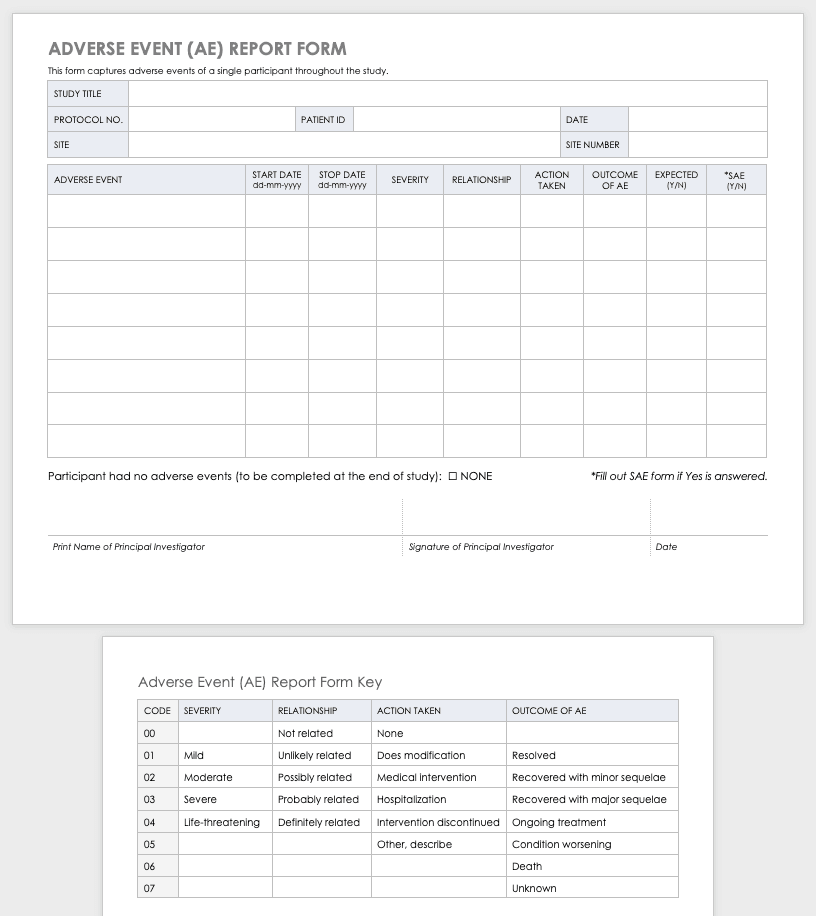
Clinical research can result in complications for the participants and trigger an adverse or severe adverse event. An adverse or severe adverse event is when participants in a clinical trial have negative medical symptoms that can be shown in laboratory or physical testing. Each participant in a clinical trial should have an adverse event log that tracks any adverse events through the duration of the study.
Download Adverse Event Form Template
Severe Adverse Event Form

A severe adverse event (SAE) is a special case of an adverse event in which the outcomes are acute. Examples of SAEs include death, life-threatening complications, or anything leading to immediate hospitalization, physical disability, or congenital abnormalities. Log SAEs in the AE form, but fill out an additional SAE form.
Download Severe Adverse Event Form Template
Word | PDF | Smartsheet
Post-Clinical Study Research Documentation and Templates
After you complete or terminate a clinical trial, you should prepare several additional documents. Here are some examples of this documentation:
- Investigational Product Accountability Log: You generally provide an accountability log to the authorities that tracks drug products to show product disposition and accountability per participant. It also helps you track the drug product stock and any imbalance at the end of the study.
- Investigational Product Destruction: Due to regulations governing the proper disposition of investigational products in clinical research, you must properly dispose of products left at the end of a study (as evidenced by the product accountability log). This form describes and ensures that you have properly handled any leftover products.
- Close-out Checklist/Report: A study close-out checklist and report helps ensure that you complete all closing procedures, archive the paperwork, and resolve electronic data.
Clinical Study Summary Report Template

Assemble the summary report at the end of a study to get results into the sponsor’s or public’s hands while you complete the full report. A summary report is typically about 2-3 page-long document that encompasses the highlights from the trial.
Download Study Summary Report Template - Word
Clinical Study Report (Full) Template

The full clinical study report (CSR) encompasses all aspects and details of the research you’ve conducted. It is not a sales or marketing tool; instead, it is a scientific report details the methodology and shows scientific rigor.
Download Clinical Study Report Template - Word
Public Links and Resources for Clinical Trials
The following are publicly available resources, tools, and links for clinical trial practitioners and principal investigators:
- PROMIS : Patient-Reported Outcomes Measurement Information System (PROMIS) software gives clinicians health status patient measures that are physical, mental, and social patient-reported metrics. Funded by the National Institutes of Health (NIH), PROMIS can be used in clinical trials as measures of conditions and disease and as a comparison to the general population. The measures in PROMIS are free to administer on paper, by computer (computer adaptive tests), or with an app. The computer adaptive tests may be conducted on REDCap , Assessment Center , or Epic .
- REDCap: REDCap (Research Electronic Data Capture) is an electronic data capture system that works on browsers to develop research databases. It was developed at Vanderbilt University to support clinical research data collection and is a free resource to nonprofit organizations. It is limited to organizations joining the REDCap consortium and is not open-source or available for commercial use.
- Good Clinical Practice (GCP) Training: GCP is an international quality standard designed for use by staff involved in clinical trials. The guidelines for this are from the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). These regulate the ethical guidelines, documentation, record keeping, training, facilities, technology, and inspections. The purpose of these guidelines is to keep clinical trials scientifically rigorous and to delineate the roles and responsibilities of research staff. The National Institutes of Health administers training for GCP.
- Quality Management Study-wide Review Tool: Developed by the NIH, this review tool is for PIs and study teams to manage their quality reviews, and may be customized for unique studies.
- Quality Management Subject Review Tool: Also developed by the NIH, this review tool provides study teams the structure for review of participant data, and may be customized for the unique study. This should be developed in concert with the DSMP.
- AccrualNet: AccrualNet is sponsored by the National Cancer Institute (NCI), and offers advice and training to staff on how to recruit study participants.
- Regulatory Education for Industry (REdI): The FDA offers a Clinical Investigator Training Course for researchers conducting investigational new drug (IND) or device exemption (IDE) studies.
- ResearchMatch: Available to volunteers and researchers affiliated with the NIH Clinical and Translational Science Award (CTSA) program, this site helps match prospective participants with specific studies.
- Grant Policies and Guidance: The NIH and National Center for Complementary and Integrative Health (NCCIH) offer links to many resources that are policy- and grant-specific to the NIH and NCCIH, updated regularly.
- Protocol Amendments: The NIH and NCCIH offer regularly updated guidance for NIH policy and protocol changes.
- Clinical Terms of Award for Human Subjects Research: The NIH and NCCIH offer guidance for clinical trial grant awardees for compliance.
- NIH Single IRB (sIRB) Policy for Multisite Research: The NIH offers a FAQ page for multisite research that includes policy, contract and application information, responsibilities, exceptions, and costs.
- Dictionary of Cancer Terms: The National Cancer Institute (NCI) offers a dictionary of cancer terms for researchers and laypersons. You can add this dictionary to your website as a widget.
- Informed Consent FAQs: The U.S. Department of Health and Human Services (HHS) and the Office for Human Research Protections (OHRP) offer a FAQ page about informed consent for researchers and lay persons.\Informed Consent Language (ICL) Database: The National Comprehensive Cancer Network (NCCN) offers a database to help write informed consents. This database is specific to medical conditions and different risk language.
Improve Clinical Trial Research with Smartsheet for Healthcare
Empower your people to go above and beyond with a flexible platform designed to match the needs of your team — and adapt as those needs change.
The Smartsheet platform makes it easy to plan, capture, manage, and report on work from anywhere, helping your team be more effective and get more done. Report on key metrics and get real-time visibility into work as it happens with roll-up reports, dashboards, and automated workflows built to keep your team connected and informed.
When teams have clarity into the work getting done, there’s no telling how much more they can accomplish in the same amount of time. Try Smartsheet for free, today.
Discover why over 90% of Fortune 100 companies trust Smartsheet to get work done.

- ▮ Home
- Latest Headlines
- Top Stories
- Breaking News
- Stock Alerts
- Key Wallstreet Events
- Stock Splits
- Conference Calls
- Earnings Calendar
- Pos Pre-announcements
- Profit Warnings
- Positive Surprise
- Negative Surprise
- Latest Earnings
- Drug Approvals
Clinical Trial Calendar
- Cov Initiations
- Cov. Reiterated
- Economic Calendar
- Economic Scorecard
- Fed Members
- Cryptocurrency
- Morning Mkt Analysis
- US Commentary
- European Commentary
- Asian Commentary
- Canadian Commentary
- Indian Commentary
- Commodities
- White House
- General News
- FX Top Stories
- Currency Analysis
- Currency Alerts
- Coronavirus
- COVID-19 Calendar
- Diet & Fitness
- Kids Health
- Men's Health
- Women's Health
- Cancer News
- Drug Development
- Mental Health
- ▮ Slide Shows
- Game of Thrones
- Classic Rock
- Rap/Hip-Hop
- Alternative
- Newswires & Feeds
- Content Syndication
- Digital Signage Services
- Radio News Services
- Intelligent Investor
- Biotech Investor NEW
- Latest News Videos
- Free Content
- Press Releases
- Slide Shows

- UB Directory
- Clinical and Translational Science Institute >
- Clinical Research Facilitation >
- Educational Modules >
Introduction to Central Study Registration

The Central Study Registration (CSR) system was designed to alleviate some of the frustrations investigators experience as they conduct their research. This UB CTSI Educational Modules video provides:
- An overview and origins of the CSR system
- Goals of study registration
- A breakdown of when your study should be registered
- Advantages of using the system
Developed by Kimberly Brunton, RN, MSN, Associate Director of Operations, UB Clinical Research Office
Presented by Alexis O’Brien, UB CTSI Clinical Research Facilitator
Running Time: 9 minutes
Questions? Contact Kimberly Brunton RN, MSN, Associate Director of Operations, UB Clinical Research Office, at [email protected] , or Alexis O’Brien, UB CTSI Clinical Research Facilitator, at [email protected] .
Additional resources: Central Study Registration login: https://www.research.buffalo.edu/studyregistration/main/login Open Research Office session presentation by Brunton: https://www.youtube.com/watch?v=FMsgwIRwwxU
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- For Patients
- Learn About Drug and Device Approvals
- The Drug Development Process
Step 3: Clinical Research
While preclinical research answers basic questions about a drug’s safety, it is not a substitute for studies of ways the drug will interact with the human body. “Clinical research” refers to studies, or trials, that are done in people. As the developers design the clinical study, they will consider what they want to accomplish for each of the different Clinical Research Phases and begin the Investigational New Drug Process (IND), a process they must go through before clinical research begins.
On this page you will find information on:
Designing Clinical Trials
Clinical Research Phase Studies
The Investigational New Drug Process
Asking for FDA Assistance
FDA IND Review Team
Researchers design clinical trials to answer specific research questions related to a medical product. These trials follow a specific study plan, called a protocol , that is developed by the researcher or manufacturer. Before a clinical trial begins, researchers review prior information about the drug to develop research questions and objectives. Then, they decide:
Who qualifies to participate (selection criteria)
How many people will be part of the study
How long the study will last
Whether there will be a control group and other ways to limit research bias
How the drug will be given to patients and at what dosage
What assessments will be conducted, when, and what data will be collected
How the data will be reviewed and analyzed
Clinical trials follow a typical series from early, small-scale, Phase 1 studies to late-stage, large scale, Phase 3 studies.
What are the Clinical Trial Phases?
Watch this video to learn about the three phases of clinical trials.
Study Participants: 20 to 100 healthy volunteers or people with the disease/condition.
Length of Study: Several months
Purpose: Safety and dosage
During Phase 1 studies, researchers test a new drug in normal volunteers (healthy people). In most cases, 20 to 80 healthy volunteers or people with the disease/condition participate in Phase 1. However, if a new drug is intended for use in cancer patients, researchers conduct Phase 1 studies in patients with that type of cancer.
Phase 1 studies are closely monitored and gather information about how a drug interacts with the human body. Researchers adjust dosing schemes based on animal data to find out how much of a drug the body can tolerate and what its acute side effects are.
As a Phase 1 trial continues, researchers answer research questions related to how it works in the body, the side effects associated with increased dosage, and early information about how effective it is to determine how best to administer the drug to limit risks and maximize possible benefits. This is important to the design of Phase 2 studies.
Approximately 70% of drugs move to the next phase
Study Participants: Up to several hundred people with the disease/condition.
Length of Study: Several months to 2 years
Purpose: Efficacy and side effects
In Phase 2 studies, researchers administer the drug to a group of patients with the disease or condition for which the drug is being developed. Typically involving a few hundred patients, these studies aren't large enough to show whether the drug will be beneficial.
Instead, Phase 2 studies provide researchers with additional safety data. Researchers use these data to refine research questions, develop research methods, and design new Phase 3 research protocols.
Approximately 33% of drugs move to the next phase
Study Participants: 300 to 3,000 volunteers who have the disease or condition
Length of Study: 1 to 4 years
Purpose: Efficacy and monitoring of adverse reactions
Researchers design Phase 3 studies to demonstrate whether or not a product offers a treatment benefit to a specific population. Sometimes known as pivotal studies, these studies involve 300 to 3,000 participants.
Phase 3 studies provide most of the safety data. In previous studies, it is possible that less common side effects might have gone undetected. Because these studies are larger and longer in duration, the results are more likely to show long-term or rare side effects
Approximately 25-30% of drugs move to the next phase
Study Participants: Several thousand volunteers who have the disease/condition
Purpose: Safety and efficacy
Phase 4 trials are carried out once the drug or device has been approved by FDA during the Post-Market Safety Monitoring
Learn more about Clinical Trials .
Drug developers, or sponsors , must submit an Investigational New Drug (IND) application to FDA before beginning clinical research.
In the IND application, developers must include:
Animal study data and toxicity (side effects that cause great harm) data
Manufacturing information
Clinical protocols (study plans) for studies to be conducted
Data from any prior human research
Information about the investigator
Drug developers are free to ask for help from FDA at any point in the drug development process, including:
Pre-IND application, to review FDA guidance documents and get answers to questions that may help enhance their research
After Phase 2, to obtain guidance on the design of large Phase 3 studies
Any time during the process, to obtain an assessment of the IND application
Even though FDA offers extensive technical assistance, drug developers are not required to take FDA’s suggestions. As long as clinical trials are thoughtfully designed, reflect what developers know about a product, safeguard participants, and otherwise meet Federal standards, FDA allows wide latitude in clinical trial design.
The review team consists of a group of specialists in different scientific fields. Each member has different responsibilities.
Project Manager: Coordinates the team’s activities throughout the review process, and is the primary contact for the sponsor.
Medical Officer: Reviews all clinical study information and data before, during, and after the trial is complete.
Statistician: Interprets clinical trial designs and data, and works closely with the medical officer to evaluate protocols and safety and efficacy data.
Pharmacologist: Reviews preclinical studies.
Pharmakineticist: Focuses on the drug’s absorption, distribution, metabolism, and excretion processes.Interprets blood-level data at different time intervals from clinical trials, as a way to assess drug dosages and administration schedules.
Chemist: Evaluates a drug’s chemical compounds. Analyzes how a drug was made and its stability, quality control, continuity, the presence of impurities, etc.
Microbiologist: Reviews the data submitted, if the product is an antimicrobial product, to assess response across different classes of microbes.
The FDA review team has 30 days to review the original IND submission. The process protects volunteers who participate in clinical trials from unreasonable and significant risk in clinical trials. FDA responds to IND applications in one of two ways:
Approval to begin clinical trials.
Clinical hold to delay or stop the investigation. FDA can place a clinical hold for specific reasons, including:
Participants are exposed to unreasonable or significant risk.
Investigators are not qualified.
Materials for the volunteer participants are misleading.
The IND application does not include enough information about the trial’s risks.
A clinical hold is rare; instead, FDA often provides comments intended to improve the quality of a clinical trial. In most cases, if FDA is satisfied that the trial meets Federal standards, the applicant is allowed to proceed with the proposed study.
The developer is responsible for informing the review team about new protocols, as well as serious side effects seen during the trial. This information ensures that the team can monitor the trials carefully for signs of any problems. After the trial ends, researchers must submit study reports.
This process continues until the developer decides to end clinical trials or files a marketing application. Before filing a marketing application, a developer must have adequate data from two large, controlled clinical trials.
Services & Specialties Clinical Trials / Research Studies
Improving care through clinical studies.
Cincinnati Children’s involvement in clinical trials / research studies is crucial to understanding diseases and developing ways to prevent or treat health problems in children and adults throughout the world. Research at Cincinnati Children’s has led to medical developments such as the Sabin oral polio vaccine and the first heart-lung machine.
In this section we provide an offering of information in research studies for children and adults, including studies for healthy children and adults . We also provide test site information for sponsors and contract research organizations (CROs), and additional resources for study and research participants.
If you are looking for research studies for your child:
- Visit our online study database
- Email us at [email protected]
- Call us at 513-636-0098
Industry partners may call us at 513-636-3232 or email [email protected] .
Current Research Studies
> connect to current research studies at cincinnati children's, information for participants.
Cincinnati Children’s is involved in research because it is very important to understanding diseases and developing ways to prevent and treat health problems in children, as well as adults. Learn more about how you can help.
> INFORMATION FOR PARENTS OF PARTICIPANTS > INFORMATION FOR ADULT PARTICIPANTS
Information for Sponsors / CROs / Professionals
The Office for Clinical and Translational Research (OCTR) at Cincinnati Children’s provides sponsors and investigators with comprehensive support services, research tools, personnel and facilities to conduct or facilitate pediatric and adult clinical research studies.
> INFORMATION FOR SPONSORS / CROs / PROFESSIONALS
Watch: Research Study Helps Family of Patient with Tuberous Sclerosis Give Back
Join Us on the Web
Connect on instagram.

Connect With Us
3333 Burnet Avenue, Cincinnati, Ohio 45229-3026
© 1999-2024 Cincinnati Children's Hospital Medical Center. All rights reserved.

For UCI Researchers interested in learning more about CCR resources and support, please contact us.
Investigators, starting a trial.
Contact our Business Development Specialist
At the UCI Center for Clinical Research (CCR), we recognize the challenges that investigators can face when navigating the intricacies of the clinical research system. That's why we're here to help. Our highly specialized Start-Up , Clinical Operations, and Regulatory Team s are fully dedicated to supporting our esteemed investigators throughout the entire process, from initial communication to site initiation and every crucial step in between. With their unwavering focus, our team members are well-versed in overcoming the complexities of the system to ensure that our investigators receive the comprehensive support they require to achieve success . Below outlines the processes that occur when starting a clinical research project at UCI , with the activation timeline goal of 120 days (starting from when the study is reviewed by our Internal Feasibility Committee) .
Initial Communication
Confidential disclosure agreement (cda).
- Sponsored Projects Administration (SPA) for clinical trials
- Applied Innovation (AI) for clinical research
- Once the CDA is executed, the sponsor will send a draft study protocol for CCR review
Protocol Acceptance
Site qualification visit (sqv), ccr internal feasibility committee (ifc) review, parallel start-up submissions.
The respective IRB Submission, Coverage Analysis/Budget Negotiation, and Contract Negotiation processes all run concurrently for optimal efficiency in study activation. These processes culminate in Document Alignment to ensure the accuracy of details across all documents before final signoffs are conducted.
The Contract Track includes the KR Proposal Process as well as a convenient DocuSign workflow to expedite contract execution:

Document Alignment
- Document alignment is an important and institutional requirement for PIs to ensure that all documents related to a clinical trial are accurate, complete, and consistent with one another to ensure financial risk is mitigated. Document alignment involves reviewing and comparing all study-related documents to identify discrepancies, inconsistencies, or errors that could impact the study.
- Once alignment is complete, the CCR Start-Up Team submits KR signoff in order to execute the contract and allow the study to open.
News & Events
- In the News
Screening and Preparing for a Study Visit
There are several steps that must be taken prior to conducing a study visit..
Many human subject protocols require participants to be scheduled for specific research visits. The number and length of time required for a research appointment to occur is highly dependent on the specifics of the study design. The specifics of the appointment are highly dependent on the location of the appointment. Appointments can occur in inpatient units, outpatient clinics, lab draw locations, diagnostic testing locations, research labs, Clinical Research Center (CRC), at the participant’s home or a neutral site convenient for the participant. The visits where the study will occur must be listed with the IRB.
It is important to set the expectations of each visit for the participant. For example, where the visit will take place, how long the visit will take, tasks to be completed at the visit, what tasks are clinical vs. what is research related, special instructions the participant must follow prior to the visit, directions and parking information, whether or not compensation for time or parking will take place and who will be involved in the visit. This should all be included in a confirmation letter.
Access Screening Letter
Access Confirmation Letter
Access Confirmation Letter 2
Once the visit is scheduled, follow-up contact within a week prior to the visit by a phone call to the participant. It is essential to reinforce the requirements of the study and ensure there has not been any changes since the last time you spoke (broke a leg, new infection, changed medication). Some of these changes might make the patient ineligible and would be better to catch ahead of time.
It is important to be understanding of the participant’s personal schedule and to help identify research visit times that are convenient for the individual to commit to without sacrificing protocol compliance. If the study lasts a while and you know the study visit windows, it is good to provide it to the subject to help them plan ahead of when you might want them to come back for visits.
Access Schedule Template
Access Subject Schedule by Enrollment
Prior to each study visit, the research team must be prepared for all known and unknown tasks that may need to be completed per protocol. If applicable, physician orders need to be completed and authorized for lab draws, study medication and additional testing; research lab kits should be prepared and available to the appropriate clinical team drawing the samples; participant questionnaires should be prepared; flowsheets required for research documentation should be made available to the appropriate team members; and any end of study visit items should be readily available if the participant decides to withdraw from the study or is removed from the study due to adverse events or investigator discretion, etc.
Access Checklist Screening
Access Checklist Follow-up
Obtaining a Medical Record Number
A medical record number (MRN) needs to be assigned to a research participant if they will be admitted to the hospital in the outpatient or inpatient setting or if they will undergo any medical tests that need to be processed by a hospital lab. When first scheduling the participant, research staff can check whether a medical record number already exists for the individual by checking IHIS. If they have a medical record number, that number will be used to identify them for any hospital-related admissions or tests. If they do not have a medical record number you are able to create a new patient in IHIS. Talk with your clinical research manager about how your department wants the new MRN to be created.
Clinic Visits
Many studies at Ohio State recruit research participants from patients that are already scheduled for healthcare visits in the medical center or cancer center. If the study design is such that these visits can serve the dual purpose of study visit and doctor visits, then scheduling is relatively straightforward and involves coordinating with the clinical treatment team. Clinic and diagnostic testing appointments are scheduled in IHIS, the provider scheduling database. The research team can review the electronic medical record, for specific information on the visits scheduled. It is important to review the participant visits often, as they could be altered or canceled by someone else which may result in protocol compliance concerns or impact the anticipated activities for that day.
For research appointments that need to be scheduled in the Ohio State hospital or clinics, independent of the patient’s medical appointments, most of the scheduling is done by the hospital schedulers or you might have a point person to ensure you have the correct staffing needed for the research visit. However, it is still the responsibility of the research staff to communicate the specifics of the protocol visit, including the timing of the visits scheduled, how long visits are expected to take, complete any paperwork necessary, obtain physician order for lab or special testing that needs to be performed at a given visit.
Research Appointments
Many studies require that research visits take place in a location that is specifically equipped to carry out the research protocol. Space may be designed to administer specific computer questionnaires, to conduct interviews in a private setting, to be proximal to labs for obtaining and processing blood or other biological specimen or to medical equipment like research CT scans or MRI equipment that cannot be moved. Sometimes certain controlled environment or conditional experiences are part of the research visit. These visits will be arranged with the key research staff involved in the research visit and may not be formally scheduled in the medical center scheduling system. This can cause patients to get reminder calls about a research procedure that happens later in the day and it is separate from the time/location you had discussed for the consent procedure visit to take place. It is essential to send a research confirmation letter that lists the procedures to help clarify to the who, what when and where for the entire study visit.
Scheduling Off-Site Research Visits
There are research studies that allow the visits to take place in the participant’s home or another neutral location, more convenient for the individual. Off-site study visits pose some additional challenges to the research staff in the form of feasibility and safety.
Before scheduling a home visit, the research staff must assess if the visit tasks can be accomplished in the specific location. For example, is there a workspace sufficient to carry out lab draws and physical examinations, are there electrical outlets for medical equipment or laptop computers, etc.
The distance away from the study center needs to be taken into consideration as many studies have a travel limit for outreach research staff. Distance can also cause some feasibility issues such as timeframe that blood specimens must be processed to maintain the integrity of the sample. Other things to consider include how the data will be transported to and from the home or off-site study visit. If laptops are used to transport data, they need to be encrypted to ensure HIPAA compliance.
Safety is also a necessary consideration when conducting home visits. When a member of the research team goes to an unfamiliar area to conduct a study visit, at least one other coworker should know that the appointment is occurring. The researcher should communicate with the research team immediately before and after the study visit occurs. The safety of the research staff should always be the primary consideration over the completion of a study visit outside the research study center and appropriate judgment should be utilized.
Lab Result Reviews
When you receive lab reports your investigator will need to document that they have been reviewed. On any abnormal lab value the investigator must document if it is clinically significant. If it is documented as clinically significant then you will need to create an Adverse Event form. Sometimes the lab is related to other areas of the patient’s medical history so if it is abnormality, it can be documented as such. Great investigator write on the first batch of labs what the abnormal lab value correlates with in subject’s medical history (ie elevated glucose=subject is diabetic) so that reviewers can see that they reviewed all abnormalities seriously.
Below is a link to a sticker you can attach to printed reports that quickly documents that the investigator has reviewed the labs and then have your investigator sign off on it.
Abnormal Lab Result Sticker
If you have a disability and experience difficulty accessing this content, please submit an email to [email protected] for assistance.

InterSECT Job Simulations
Interactive Simulation Exercises for Career Transitions
Clinical Trials: Coordinating Schedules
Coordinate the schedule of clinical trial events for medical staff and study enrollees, the exercise.
Create a schedule for the clinical trial for the clinical staff and the enrollees. As a CRC, you organize and schedule all the interactions between patients, nurses and doctors to fulfill the requirements of the study including analysis and drug administration.
For this exercise, you are part of the team responsible for the initiation of a Phase III randomized study that will compare the efficacy of the combination of two immunotherapy drugs to single therapy in the context of untreated unresectable or metastatic melanoma.
Scientific Background
Nivolumab and Ipilimumab are novel biological drugs called checkpoint inhibitor antibodies. Nivolumab is a human anti-PD-1 antibody, while Ipilimumab specifically recognizes human CTLA-4. Both PD-1 and CTLA-4 are immune-inhibitory receptors that dampen and downregulate the functions of the immune system, particularly of T cells. In the context of cancer, PD-1 and CTLA-4 prevent the proper activation of T cells, hindering T cells from attacking and clearing cancer cells. Nivolumab and Ipilimumab block the interaction of the receptors PD-1 and CTLA-4 with their targets, thus enabling proper T cell activation. Blockade of PD-1 and CTLA-4 allows T cells to fulfill their cytotoxic function against cancer cells.
Task 1: Coordinate calendar for study staff
Set a schedule or calendar addressed to the nurses that includes the pre-screening assessment, the drug administration and the subsequent follow-up analyses. This must include the list of analyses that the clinic and nurses need to perform. We suggest using an Excel file for this task – see this example . For this exercise, use the abbreviated study protocol provided .
Summary of the abbreviated study protocol:
- Synopsis (p6-10)
- Drug administration information (Tables 4.3-1 and 4.3-2)
- Medical examinations before, during, and after drug administration (section 5.1, p46)
IMPORTANT: This is a double-blind study: make sure the details of the drug administration, like the type of drug, are not included.
Task 2: Coordinate calendar for a patient
Craft a calendar for a new patient, Jane Micro. Prepare a prospect of the timeline of all the examinations and appointments she will need to attend to before, during and after the completion of the drug administration. Divide the appointments according to type: pre-screening, drug-infusion, follow-ups. For this exercise, we suggest using Excel to help you create the first draft, in a similar way to Task 1.
For this task, provide information about the procedure to help the patient prepare for the examination. Examples could include whether there will be a blood draw and the volume or whether there will be a scan for that visit. For this task, you will not need to include information about the length of the visit. The level of detailed information may depend on the organization and the team for which you are working.
The Deliverable
Instructions.
A standard deliverable is an excel file or electronic calendar. If you’re interested in viewing an example, please refer to this example of a patient’s calendar. Feel free to organize the information in a way that makes sense to you. There is no correct way for this task. Note that the format for calendars and timelines will depend on the site and its procedures.
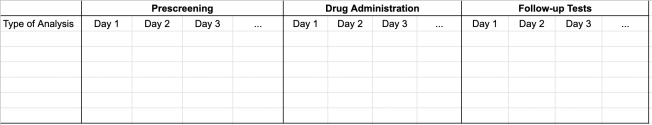
The timeline in Task 1 will give the CRC a complete picture of the scheduling s/he needs to organise. The timeline in Task 2 will serve as a reminder to the patient of all his/her appointments. A more experienced CRC might not need to draft a table (Task 1) or a timeline (Task 2); they may instead gather the information they need directly from the study protocol.
A new CRC starting out may receive guidance from a colleague or the Clinical Research Manager (CRM) or the PI of the study who is responsible for oversight of the trial and for patient safety. After this introductory period, an experienced CRC will mostly work independently and run the study.
General resources to help you get started:
- Before starting the simulation, read the general research principles – Point 1 on the Drug Development Process and Point 3 on Investigational New Drug (The Ohio State)
- Information on the role of the research coordinator and overview of good clinical practice and ethical principles (UCSF)
- List of abbreviations
- NIH’s Office of Clinical Research: free, self-paced online clinical research course
Additional information on the study at hand:
- This is the full study protocol. As you can see, a protocol can be very long and additional modification can be added during the course of the study PDF of the complete protocol .
- This is the resulting scientific publication of the clinical study the simulation refers to. Interesting read if you want more information on the results of the trial and on the science behind the trial PDF of the publication of the study.
Skills used to perform this task:
- Organizational skills
- Ability to communicate effectively with clinical staff and patients
- Simplifying complex information for a lay audience
Skills needed in clinical research careers:
- Excellent organizational skills
- Multitasking
- Quick problem solving
Learn about competencies for CRCs from The Association of Clinical Research Professionals
To view detailed lists of skills in job descriptions for policy careers, please see workforce data generated by Boston University’s BEST program.
Additional responsibilities of clinical research professionals:
- Consent participants for a clinical trial (see simulation for Responding to Patient’s Concerns)
- Collect samples from the patient and deliver them to the research laboratory for analysis
- Keep track of the status of all the patients and updates colleagues and the clinicians involved in the study on the status of the patients
You are viewing a job simulation. To get started, set up SMART Goals to perform this simulation in a reasonable timeline. If you have completed the task, fill out the Self-Reflection Sheet .
Simulation author: Chiara Rancan, PhD
Simulation vetted by professionals from Genentech and UCSF

- Already have a WordPress.com account? Log in now.
- Subscribe Subscribed
- Copy shortlink
- Report this content
- View post in Reader
- Manage subscriptions
- Collapse this bar
- Introduction
- Conclusions
- Article Information
PNE indicates pain neuroscience education.
The KOOS 4 primary outcome includes the subscales pain, symptoms, function of daily living, and knee-related quality of life; scores range from 0 to 100, with higher scores indicating better outcomes. Data points are means; error bars represent 95% CI. PNE indicates pain neuroscience education.
The KOOS 4 primary outcome includes the subscales pain, symptoms, function of daily living, and knee-related quality of life; scores range from 0 to 100, with higher scores indicating better outcomes. Positive scores indicate improvements in KOOS 4 , and negative scores indicate a decline in KOOS 4 . PNE indicates pain neuroscience education.
Trial Protocol and Statistical Analysis Plan
eAppendix 1. CONSORT Checklist for Randomized Trials
eAppendix 2. TIDieR Checklist for Information to Include When Describing an Intervention
eAppendix 3. CERT Checklist for What to Include When Reporting Exercise Programs
eMethods 1. Pain Neuroscience Education Session 1
eMethods 2. Pain Neuroscience Education Session 2
eTable 1. Patient Baseline Characteristics for Those Attending the 12-Month Follow-up Assessment and Those who Did Not Attend
eTable 2. Intention-to-Treat Analysis for Risk Ratios for Usage of Pain Medication From Baseline to 12 Months
eTable 3. Per-Protocol Analysis for the Primary and Secondary Outcomes for Change From Baseline to 12 Months
eTable 4. Per-Protocol Analysis for the Changes in Usage of Pain Medication From Baseline to 12 Months
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Larsen JB , Skou ST , Laursen M , Bruun NH , Arendt-Nielsen L , Madeleine P. Exercise and Pain Neuroscience Education for Patients With Chronic Pain After Total Knee Arthroplasty : A Randomized Clinical Trial . JAMA Netw Open. 2024;7(5):e2412179. doi:10.1001/jamanetworkopen.2024.12179
Manage citations:
© 2024
- Permissions
Exercise and Pain Neuroscience Education for Patients With Chronic Pain After Total Knee Arthroplasty : A Randomized Clinical Trial
- 1 Musculoskeletal Health and Implementation, Department of Health Science and Technology, Faculty of Medicine, Aalborg University, Aalborg, Denmark
- 2 Research Unit for Musculoskeletal Function and Physiotherapy, Department of Sports Science and Clinical Biomechanics, University of Southern Denmark, Odense, Denmark
- 3 The Research and Implementation Unit PROgrez, Department of Physiotherapy and Occupational Therapy, Næstved-Slagelse-Ringsted Hospitals, Region Zealand, Denmark
- 4 Orthopedic Surgery Research Unit, Aalborg University Hospital, Aalborg, Denmark
- 5 Research Data and Biostatistics, Aalborg University Hospital, Aalborg, Denmark
- 6 Translational Pain Biomarkers, Department of Health Science and Technology, Faculty of Medicine, Aalborg University, Aalborg, Denmark
- 7 ExerciseTech, Department of Health Science and Technology, Faculty of Medicine, Aalborg University, Aalborg, Denmark
Question What is the effect of neuromuscular exercise and pain neuroscience education compared with pain neuroscience education alone on pain and function in patients with chronic pain for more than 1 year after total knee arthroplasty?
Findings In this randomized clinical trial of 69 patients, neuromuscular exercise and pain neuroscience education did not provide superior pain and function outcomes compared with pain neuroscience education alone, although approximately one-third of all patients experienced clinically important improvements.
Meaning Findings from this study suggest that neuromuscular exercise and pain neuroscience education do not provide superior pain and function outcomes compared with pain neuroscience education alone, but clinically important improvements in pain and function can be elicited in patients with chronic pain after total knee arthroplasty.
Importance Up to 20% of patients develop chronic pain after total knee arthroplasty (TKA), yet there is a scarcity of effective interventions for this population.
Objective To evaluate whether neuromuscular exercise and pain neuroscience education were superior to pain neuroscience education alone for patients with chronic pain after TKA.
Design, Setting, and Participants A superiority randomized clinical trial was conducted at 3 outpatient clinics at Aalborg University Hospital in Denmark. Participants with moderate-to-severe average daily pain intensity and no signs of prosthesis failure at least 1 year after primary TKA were included. Participant recruitment was initiated on April 12, 2019, and completed on October 31, 2022. The 12-month follow-up was completed on March 21, 2023.
Interventions The study included 24 sessions of supervised neuromuscular exercise (2 sessions per week for 12 weeks) and 2 total sessions of pain neuroscience education (6 weeks between each session) or the same pain neuroscience education sessions alone. The interventions were delivered in groups of 2 to 4 participants.
Main Outcomes and Measures The primary outcome was change from baseline to 12 months using the mean score of the Knee Injury and Osteoarthritis Outcome Score, covering the 4 subscales pain, symptoms, activity of daily living, and knee-related quality of life (KOOS 4 ; scores range from 0 to 100, with higher scores indicating better outcomes). The outcome assessors and statistician were blinded. All randomized participants were included in the intention-to-treat analysis.
Results Among the 69 participants (median age, 67.2 years [IQR, 61.2-71.9 years]; 40 female [58%]) included in the study, 36 were randomly assigned to the neuromuscular exercise and pain neuroscience education group, and 33 to the pain neuroscience education–alone group. The intention-to-treat analysis showed no between-group difference in change from baseline to 12 months for the KOOS 4 (7.46 [95% CI, 3.04-11.89] vs 8.65 [95% CI, 4.67-12.63] points; mean difference, −1.33 [95% CI, −7.59 to 4.92]; P = .68). Among the 46 participants who participated in the 12-month assessment in the 2 groups, 16 (34.8%) experienced a clinically important improvement (a difference of ≥10 points on the KOOS 4 ) with no between-group difference. No serious adverse events were observed.
Conclusions and Relevance In this randomized clinical trial, the results demonstrated that neuromuscular exercises and pain neuroscience education were not superior to pain neuroscience education alone in participants with chronic pain after TKA. Approximately one-third of the participants, regardless of intervention, experienced clinically important improvements. Future studies should investigate which patient characteristics indicate a favorable response to exercises and/or pain neuroscience education.
Trial Registration ClinicalTrials.gov Identifier: NCT03886259
End-stage knee osteoarthritis is commonly treated with total knee arthroplasty (TKA). 1 In 2018, more than 715 000 TKAs were performed in the US, 2 and the number is expected to rise to 1.9 million annually by 2030. 3 Most patients undergoing TKA surgery will experience a positive outcome in terms of pain relief and improved functional performance, but 15% to 20% of patients will develop chronic pain after TKA. 4 , 5 Chronic pain after TKA is defined as pain present for at least 3 to 6 months following surgery. 6
Patients have described the chronic pain after TKA as extreme, constant, and requiring maximal effort to endure. 7 Furthermore, activities of daily living (eg, walking and stair climbing) are impaired in patients with chronic pain after TKA when compared with patients with knee osteoarthritis prior to surgery. 8
Chronic pain after TKA is considered multifactorial and can be influenced by physiological factors, such as central pain mechanisms, and psychosocial factors. 6 , 8 There is a scarcity of high-quality evidence and guidelines on effective treatments of chronic pain after TKA. 6 , 9 The lack of evidence-based treatment guidelines leads to inadequate access to optimal treatment and the risk of patients feeling abandoned by the health care system. 10
Studies have evaluated the inclusion of early postoperative exercises to avoid patients developing chronic pain after TKA but have not found this approach effective. 11 , 12 However, a combination of exercise and education treatment modalities could induce beneficial treatment effects in patients with chronic pain after TKA, 13 but to our knowledge, this has never been investigated.
Therefore, we conducted a superiority randomized clinical trial with the purpose of investigating whether a 12-week treatment consisting of neuromuscular exercise and pain neuroscience education (PNE) would prove superior in terms of improving pain and function compared with receiving PNE alone. It was hypothesized that the participants randomized to neuromuscular exercise and PNE would improve significantly more from baseline to 12 months compared with participants randomized to PNE alone.
The study was designed as a parallel-group superiority randomized clinical trial, entitled the NEPNEP (Neuromuscular Exercises and Pain Neuroscience Education for Chronic Pain) trial. An open access study protocol was published to ensure research quality and transparency. 14 The trial followed the Consolidated Standards of Reporting Trials ( CONSORT ) reporting guideline for randomized clinical trials. 15 The patient flow diagram is provided as Figure 1 , and the trial protocol is provided in Supplement 1 . The CONSORT, Template for Intervention Description and Replication (TIDieR), and Consensus on Exercise Reporting Template (CERT) checklists are provided in eAppendices 1-3, respectively, in Supplement 2 . The trial was approved by the North Denmark Region Committee on Health Research Ethics. All participants signed informed consent before inclusion in the trial.
Participants were recruited from Aalborg University Hospital (Aalborg, Denmark), which included 3 hospital sites in Farsoe, Thisted, and Aalborg. The hospital research database was used to identify participants who underwent TKA at least 1 year before recruitment. Eligible participants were contacted by mail and telephone and invited to participate in the study. Participants willing to enroll and meeting the eligibility criteria of primary TKA due to knee osteoarthritis 12 months or longer after their surgery and, in the index knee, chronic pain for longer than 6 months and an average daily pain score of 4 or more (moderate to severe pain) on a numeric rating scale (ranging from 0 to 10, in which 0 is no pain, and 10 is maximum pain) over the last week were included. The major exclusion criteria were chronic pain due to loosening of an implant or a prosthesis failure requiring revision surgery or primary pain area other than the index knee (eg, low back pain or upper extremity pain). A full list of eligibility criteria can be found in the study protocol ( Supplement 1 ). 14 Participants received the interventions at 1 of the 3 outpatient clinics at Aalborg University Hospital (Farsoe, Thisted, and Aalborg) dependent on their geographical preferences and on which day and time for exercise and PNE suited them best. Recruitment was initiated on April 12, 2019, and completed on October 31, 2022. The 12-month follow-up was completed on March 21, 2023.
Two patients with chronic pain after TKA assisted in designing the trial from a patient perspective. The patients gave feedback concerning study procedures, interventions, and outcome measures and how to describe and explain the study in layperson’s terms to possible participants.
The participants were randomized in a 1:1 ratio and allocated to 1 of 2 intervention arms, neuromuscular exercises and PNE or PNE alone. Randomization with treatment group concealment was done by the project manager (J.B.L.) by using computer-generated random numbers in permuted blocks of 4 to 8 participants. Outcome assessment was performed by trained outcome assessors (not involved in the study), who were masked toward treatment allocation. The statistician (N.H.B.) conducting the analysis was masked toward group allocation.
The neuromuscular exercises and PNE group received a 12-week neuromuscular exercise program 16 and PNE. The neuromuscular exercise program has previously been found feasible for patients following TKA surgery. 17 One-hour group-based sessions consisting of 2 to 4 participants were held twice a week (24 sessions in total). Sessions were supervised by trained physiotherapists and included individualization of the exercise difficulty considering each participant’s physical ability and pain intensity. Full details of the neuromuscular exercise program can be found in the study protocol ( Supplement 1 ). 14
The PNE consisted of two 1-hour group-based educational sessions. The first session was held before the first exercise session for the neuromuscular exercise and PNE group, and the second session took place 6 weeks later. A physiotherapist trained in PNE (J.B.L.) delivered the sessions to both groups. Both intervention groups received the same content in the PNE sessions. The overall aim of PNE was to change maladaptive pain cognitions, enabling the participants to reconceptualize their pain 18 and thereby engage in self-management of their symptoms. Following both PNE sessions, a short information leaflet, summarizing the PNE topics, was given to the participants. Content for the PNE sessions can be found in eMethods 1 and 2 in Supplement 2 . Assessments of outcomes were conducted at baseline and at 3, 6, and 12 months.
The primary outcome was prespecified and reported in the study protocol 14 and the statistical analysis plan. 19 The primary outcome was the between-group change from baseline to 12 months for the Knee Injury and Osteoarthritis Outcome Score (KOOS), using the mean score of the 4 subscales: pain, symptoms, activities of daily living, and knee-related quality of life (KOOS 4 ). The subscales, which include a fifth dimension—sport and recreation—are scored on a 5-point Likert scale; the total is converted into a range of 0 (worst) to 100 (best). 20 , 21 A prespecified minimum clinically important difference of 10 points was used to indicate whether a clinically relevant between-group improvement from baseline to the 12-month follow-up had occurred. 22 The KOOS questionnaire has shown validity, reliability, and responsiveness as a patient-reported outcome measure following TKA. 23
Six prespecified secondary outcomes were evaluated as between-group changes using the mean difference from baseline to a 12-month follow-up. 19 All 5 KOOS subscales, including the sport and recreation subscale, were reported individually to support the clinical interpretation of the primary outcome. 22 The overall change in a participant’s knee condition was measured using the global perceived effect scale by their answer to the question: “How are your knee problems now compared with before you entered this study?” The global perceived effect scale was administered on a 7-point Likert scale ranging from 1 (improved, an important improvement) to 7 (worse, an important worsening). The global perceived effect scale has shown excellent reliability. 24 Three physical performance tests were included. 25 The time to complete the 40-m fast-paced walk test and the stair-climb test, a test of ascending and descending 9 steps on a staircase, was recorded. For the 30-second chair-stand test, the maximum number of chair-rise repetitions within 30 seconds was registered. 25 The physical performance tests have been found reliable. 26 , 27 Use of pain medication was evaluated by asking participants whether they had used pain medication over last week (yes or no). Adverse events occurring during the trial period were registered as either serious or nonserious events by participant self-report and/or by the physiotherapists supervising the neuromuscular exercises. Serious adverse events were defined according to the definitions from the US Food and Drug Administration, and nonserious adverse events comprised all other events. 28 Other treatments initiated because of the index knee received during the trial period were registered by self-reporting from the participants.
A statistical analysis plan was published and available before the 12-month follow-up, and any analyses were initiated. 19 The analyses were conducted as predefined in the statistical analysis plan. To avoid the risk of misleading interpretation, the results from the intention-to-treat analysis were presented to the author group in a blinded version (coded as group A and group B). In writing, the authors agreed on 2 separate interpretations of the results, 29 and documentation for the interpretations was registered online. 30 After finalizing the interpretations, the randomization code was broken, and the appropriate interpretation was chosen.
For this superiority randomized clinical trial, a sample-size calculation was conducted to estimate the sample size required to detect a between-group minimum clinically important difference in change of 10 points from baseline to the 12-month follow-up for the KOOS 4 (with an SD of 15). 17 , 22 The calculation revealed that 49 participants were required in both groups to achieve a study power of 90% from baseline to the 12-month follow-up for the between-group comparison, using a 2-sided significance level of .05. To account for a possible loss to follow-up of 20%, a total of 60 participants in each group were planned to be enrolled. However, the trial was impacted by the COVID-19 pandemic, making recruitment particularly difficult and causing a higher dropout rate than anticipated. Therefore, we were not able to recruit the preplanned number of participants and decided to stop recruitment after recruiting for 42 months.
The main analysis consisted of the between-group differences in mean change from baseline to the 12-month follow-up. Analysis of all outcomes was performed according to the intention-to-treat principle. Furthermore, a prespecified per-protocol analysis was conducted, including participants who participated in at least 18 of 24 (75%) neuromuscular exercise sessions and participated in both PNE sessions (valid for both groups).
Data were checked for normal distribution by reviewing data frequency in histograms and tests for normality (Shapiro-Wilk). Based on the observations, median and IQR were recorded. For the primary and secondary outcomes (except use of pain medication), repeated measures mixed-effects models were applied, with participants as the random effect and time for visit (baseline and 3, 6, and 12 months) and treatment arm (neuromuscular exercises and PNE or PNE alone) as fixed effects, with adjustment for baseline imbalance. Interaction between follow-up and treatment arm was also included in the models. Two models are reported: model 1, adjusted for participant, follow-up, treatment arm, and interaction between follow-up and treatment arm; model 2 further included adjustment for age, sex, and body mass index. The between-group comparison for use of pain medication within the last week was dichotomized as yes or no, and relative risks were analyzed using a Poisson regression model with robust error variance. No analysis for difference in adverse events was required because no adverse events were registered in the PNE-alone group.
A prespecified responder analysis was conducted to illustrate the proportion of participants in the 2 intervention groups who experienced a minimum clinically important difference of at least 10 points in KOOS 4 . The proportions were compared using a χ 2 test.
For all outcomes, 95% CIs are presented. A 95% CI, including 10 points or more for the primary outcome, KOOS 4 , was interpreted as a clinically meaningful difference. 22 A 2-sided P < .05 was considered significant. All analyses were performed in Stata, version 18 (StataCorp LLC).
A total of 69 patients (median age, 67.2 years [IQR, 61.2-71.9 years]; 40 female [58%]) and 29 male [42%]) were recruited. Overall, 435 patients were assessed for eligibility ( Figure 1 ). Of these, 364 were excluded, leaving 71 eligible for inclusion; 2 patients withdrew before randomization. Thirty-six participants were randomized to receive neuromuscular exercises and PNE and 33 participants to receive PNE alone. The participants’ baseline characteristics were comparable ( Table 1 ). 31 The mean body mass index in our population was greater than 33 (calculated as weight in kilograms divided by height in meters squared), most participants had at least 1 comorbidity, and there was a group-average score in the Hospital Anxiety and Depression Scale 31 that indicated clinical depression.
All participants were included in the intention-to-treat analysis. Twenty-three participants (64%) in the neuromuscular exercises and PNE group and 26 (79%) in the PNE-alone group adhered to the intervention and were included in the per-protocol analysis. The completion rates for the 12-month follow-up assessment were 24 of 36 participants (67%) for the neuromuscular exercises and PNE group and 22 of 33 (67%) for the PNE-alone group. Dropout reasons are reported in Figure 1 . The baseline characteristics for the participants adhering to the 12-month assessment and the participants lost to follow-up were comparable (eTable 1 in Supplement 2 ).
The intention-to-treat analysis showed no between-group difference in improvement from baseline to the 12-month follow-up for the primary outcome KOOS 4 , illustrated by an adjusted mean difference of −1.33 (95% CI, −7.59 to 4.92; P = .68) ( Figure 2 ). Both groups experienced significant improvements in KOOS 4 from baseline to the 12-month follow-up, with the neuromuscular exercise and PNE group improving 7.46 points (95% CI, 3.04-11.89; P = .001) and the PNE-alone group improving 8.65 points (95% CI, 4.67-12.63; P < .001) ( Table 2 ).
The responder analysis showed that 8 of 24 participants (33.3%) in the neuromuscular exercise and PNE group and 8 of 22 participants (36.4%) in the PNE-alone group (16 of 46 total participants [34.8%]) experienced clinically important improvements (10 points) from baseline to the 12-month follow-up for the primary outcome KOOS 4 . Individual changes in KOOS 4 from baseline to 12 months are shown in Figure 3 . There was no difference in the proportion of responders between the groups (relative risk, 1.09; 95% CI, 0.49-2.41; P = .83).
There were no significant between-group differences in change in the 5 KOOS subscales of pain, symptoms, activity of daily living, sport and recreation, and knee-related quality of life; the global perceived effect; time to complete the 40-m fast-paced walk test and the stair-climb test; or numbers of repetitions in the 30-second chair-stand test ( Table 2 ). Nor was there a significant between-group difference for use of pain medication (relative risk, 1.02; 95% CI, 0.73-1.43; P = .92) (eTable 2 in Supplement 2 ). Both groups experienced significant within-group improvements in all outcomes except use of pain medication, in which neither group showed an improvement; the KOOS subscale sport and recreation, in which the neuromuscular exercise and PNE group showed no improvement; and the 40-m fast-paced walk test, in which the PNE-alone group showed no improvement.
No serious adverse events were registered in either of the intervention groups during the trial. For the neuromuscular exercise and PNE group, 5 nonserious adverse events were registered during the trial: 4 participants experienced increased pain intensity, and 1 participant experienced swelling in the index knee following a neuromuscular exercise session, which subsided after a few days and did influence the next neuromuscular exercise session. No nonserious adverse events were registered in the PNE-alone group. No participants in either group reported that they had received other treatments during the trial period. The per-protocol analysis revealed no differences in changes from baseline to 12 months for neither the primary nor the secondary outcomes (eTables 3 and 4 in Supplement 2 ).
To our knowledge, the NEPNEP trial is the first randomized clinical trial evaluating exercise and education for patients with chronic pain after TKA. Our results revealed that neuromuscular exercise and PNE were not superior to PNE alone for the primary outcome KOOS 4 in patients with chronic pain after TKA or for any of the secondary outcomes. Consequently, the results did not support the hypothesis that neuromuscular exercises and PNE would lead to greater improvements in pain and function than would PNE alone. We observed clinically important improvements in approximately one-third (34.7%) of the participants with chronic pain after TKA, regardless of treatment allocation.
Studies evaluating the effect of treatments introduced in the early postoperative period 32 - 37 have not considered that patients who undergo TKA often experience spontaneous improvements in pain between 3 and 9 months after surgery. 38 Hence, the observed treatment effects could have been influenced by the natural course of improvement after TKA and are therefore not generalizable to patients with chronic pain more than 1 year after TKA. Our findings contribute insight into the treatment of the patients who do not experience spontaneous improvements postoperatively and still experience chronic pain for at least 1 year after their TKA surgery.
Qualitative research has shown that patients with chronic pain after TKA feel abandoned by the health care system and the lack of treatment options. Therefore, patients experience their pain as something they are stuck with and that nothing more can be done. 10 Our results challenge that perception. Given that both intervention groups experienced similar outcomes, the introduction of PNE as treatment could be of particular importance. By providing PNE, patients might realize the factors they can influence themselves, which could lead to improved self-management.
As illustrated in Figure 2 , the neuromuscular exercise and PNE group exhibited an improvement in KOOS 4 immediately after the 3-month supervised exercise therapy program. While the neuromuscular exercise and PNE-alone group largely maintained their improvements until the 12-month follow-up, the PNE group gradually improved from baseline to 12 months. This could indicate that exercising is effective when performed with effects diminishing over time, similarly to findings within hip and knee osteoarthritis. 39 , 40 Therefore, it would be valuable to investigate whether a longer period of exercise therapy or booster sessions could provide sustained improvements.
The KOOS was chosen as the primary outcome, as it is imperative to consider the patient perspective when evaluating treatment effect. 41 , 42 The psychometric properties of KOOS have been scrutinized, with some findings indicating the need for further validation 42 and other findings consolidating its validity and reliability. 23 , 41 However, the KOOS remains a frequently used patient-reported outcome measure for patients undergoing TKA. 17 , 43 , 44
As illustrated in Figure 3 , participants from both groups experienced large improvements in KOOS 4 , highlighting that some participants benefited substantially from neuromuscular exercise and PNE or PNE alone. On the contrary, other participants in both groups experienced little improvement or even a worsening in KOOS 4 . This supports the need for individualized approaches when seeking the best possible treatment. Future research should investigate which patient characteristics indicate a favorable response to exercises and PNE and who might not benefit from either. 45
The mean body mass index in our population was greater than 33, most participants had at least 1 comorbidity, and there was a group-average score in the Hospital Anxiety and Depression Scale 31 that indicated clinical depression. These factors have previously been associated with chronic pain after TKA 45 and emphasize the complexity of the studied population. Given the multiple factors influencing chronic pain and the characteristics of the population, a biopsychosocial and multimodal treatment approach should be considered for patients with chronic pain after TKA. 6 , 10
This trial has some limitations. The study was affected by the COVID-19 pandemic and failed in recruiting the target sample size. However, when taking the small between-group differences into consideration, it seems unlikely that a fully powered study would change the conclusion of no between-group differences. Moreover, considering that the study did not include a no-treatment control group, the true effects of neuromuscular exercises and/or PNE could not be determined. Therefore, the findings could represent fluctuations in pain intensity over time. Long-term follow-up studies have observed that some patients experience pain fluctuations after TKA, whereas other patients’ chronic pain remains stable over time. 38
The results of this randomized clinical trial suggest that neuromuscular exercises and PNE were not superior to PNE alone for the primary outcome on pain, symptoms, function, and knee-related quality of life or any of the secondary outcomes in participants with chronic pain after TKA. The study demonstrated clinically relevant improvements in approximately one-third of the participants, regardless of intervention group. This finding challenges the perception that nothing can be done to relieve pain in patients with chronic pain after TKA. Therefore, the results could have important implications for the future management of patients with chronic pain after TKA. Despite the contributions of this study, an evidence gap for the treatment and management of patients with chronic pain after TKA remains and should be further addressed in future research.
Accepted for Publication: March 15, 2024.
Published: May 24, 2024. doi:10.1001/jamanetworkopen.2024.12179
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Larsen JB et al. JAMA Network Open .
Corresponding Author: Jesper B. Larsen, PhD, Musculoskeletal Health and Implementation, Department of Health Science and Technology, Aalborg University, Selma Lagerløfs Vej 249, 9260 Gistrup, Denmark ( [email protected] ).
Author Contributions: Dr Larsen and Mr Bruun had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Larsen, Skou, Laursen, Arendt-Nielsen, Madeleine.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Larsen, Skou, Arendt-Nielsen, Madeleine.
Critical review of the manuscript for important intellectual content: All authors.
Statistical analysis: Larsen, Bruun.
Obtained funding: Larsen, Skou, Arendt-Nielsen, Madeleine.
Administrative, technical, or material support: Larsen, Laursen, Madeleine.
Supervision: Skou, Laursen, Arendt-Nielsen, Madeleine.
Conflict of Interest Disclosures: Prof Skou reported receiving grants from the European Research Council as payment to the University of Southern Denmark and from Region Zealand (Exercise First) for payment to the Næstved-Slagelse-Ringsted Hospital, receiving personal fees from Munksgaard as royalties for book chapters and from TrustMe-Ed as royalties for online lectures, and receiving honoraria from Nestlé Health Science for 1 presentation at a webinar on osteoarthritis outside the submitted work and reported being cofounder of GLA:D, a not-for-profit initiative hosted at the University of Southern Denmark aimed at implementing clinical guidelines for osteoarthritis in clinical practice. No other disclosures were reported.
Funding/Support: This work was supported by grant R168-A5619 from the Danish Rheumatism Association and by the Svend Andersen Foundation and the Lions Club Danmark (Dr Larsen) and by grants 801790 for payment to the University of Southern Denmark and 945377 for payment to the Næstved-Slagelse-Ringsted Hospital from the European Union’s Horizon 2020 research and innovati on program (Prof Skou).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 3 .
Additional Contributions: We thank the patients for their participation in the trial. We acknowledge the Department of Occupational Therapy and Physiotherapy, Aalborg University Hospital, Denmark, for administrative and logistic support and the Department of Orthopedic Surgery, Aalborg University Hospital, Denmark, for its involvement in recruiting patients.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK

Clinical Research Coordinator I
- Obstetrics and Gynecology
- Columbia University Medical Center
- Opening on: May 30 2024
- Job Type: Officer of Administration
- Bargaining Unit:
- Regular/Temporary: Regular
- End Date if Temporary:
- Hours Per Week: 35
- Standard Work Schedule:
- Salary Range: $62,400.00 - $67,428.00
Position Summary
This candidate will join the Family Planning Research team to coordinate several ongoing research studies and assist in the supervision of additional staff and management of the research office. The primary role of this position is to support clinical research involving Family Planning Research. The employee will interact with women, their partners or families and clinical staff as it relates to clinical research protocols and clinical trials, industry funded and grant funded, being implemented at PH 16.
Responsibilities
- Completion of GCP, HIPAA and applicable regulatory training.
- Complete certification requirements for assigned protocols.
- Screen designated schedules or patient lists for eligible subjects.
- Approach and verify eligibility subjects.
- Enroll and consent eligible subjects.
- Complete research study visits as delineated in assigned protocol and manual of operations set forth by sponsor and supervisor.
- Complete Telephone follow-up and telephone reminder calls for study participants, during these phone calls the person will need to administer study questionnaire as assigned.
- Coordinate the collection of all research data points as assigned, whether through research visits, chart abstraction or telephone.
- Scheduling of research visits.
- Collection through venipuncture, processing, transporting and shipping of biological specimens as assigned and by steps delineated in the protocol or manual of operations.
- Completion study documents and files some examples might include case report forms, worksheets and medical record notes.
- Maintain confidentiality of documents and files such as HIPAA.
- Informing relevant clinical staff regarding subject protocol participation.
- Assist in other research related activities and projects as needed.
- Regular collaboration with the PI and other research staff.
- Lead CRC of project or work area.
- Train, supervise, and serve as contact person for summer youth and student casual employees.
- Serve as liaison with Biomed and Central Sterilization to ensure compliance for study-related equipment and instruments.
- Serve as back-up ARC custodian for processing participant compensation, ordering supplies, and reconciling procurement card expenses.
- Independently manage and maintain regulatory records for research studies under their purview including trial master files and IRB submissions.
- Ensure research exam space is compliant by monitoring dates for equipment calibration, checking for expired supplies, and collecting up-to-date documents for staff master binders, training binders, and temperature log binders.
- Assist in preparation for site visits including collection of documents and booking of conference rooms.
- Perform other related duties and responsibilities as assigned/requested.
Minimum Qualifications
- Bachelor's degree or equivalent in education and experience, plus (3) three years of related experience.
- Experience in a patient care setting.
- Excellent interpersonal, written/oral communication, and organizational skills.
- Proficiency in Microsoft Office.
Preferred Qualifications
- Phlebotomy Certified (or other forms of certification in lieu of phlebotomy such as certified medical assistant, nursing degree, medical degree) - current or obtained within 3 months.
- Incumbent must be self-directed and able to make independent decision within the parameters of all federal, state, institutional and departmental guidelines.
- Experience in a patient care setting and clinical research experience.
- Complete proficiency in written and spoken English and Spanish.
- Ability to work night shift 11pm-7am after training period.
- Master’s or another advanced degree may substitute in part for experience.
- Excellent interpersonal, written/oral communication, and organizational skills bullet.
Equal Opportunity Employer / Disability / Veteran
Columbia University is committed to the hiring of qualified local residents.
Commitment to Diversity
Columbia university is dedicated to increasing diversity in its workforce, its student body, and its educational programs. achieving continued academic excellence and creating a vibrant university community require nothing less. in fulfilling its mission to advance diversity at the university, columbia seeks to hire, retain, and promote exceptionally talented individuals from diverse backgrounds. , share this job.
Thank you - we'll send an email shortly.
Other Recently Posted Jobs
Administrative Assistant
Admissions officer, research assistant.
Refer someone to this job

- ©2022 Columbia University
- Accessibility
- Administrator Log in
Wait! Before you go, are you interested in a career at Columbia University? Sign up here!
Thank you, for sharing your information. A member of our team will reach out to you soon!

This website uses cookies as well as similar tools and technologies to understand visitors' experiences. By continuing to use this website, you consent to Columbia University's usage of cookies and similar technologies, in accordance with the Columbia University Website Cookie Notice .
- Fortrea Holdings-stock
- News for Fortrea Holdings
Fortrea Introduces Comprehensive Solution to Improve Diversity and Inclusion in Clinical Research
DURHAM, N.C., May 30, 2024 (GLOBE NEWSWIRE) -- Fortrea (Nasdaq: FTRE) (the “Company”), a leading global contract research organization (CRO), today announced its comprehensive and integrated solution to improve the diversity and inclusion (D&I) of participants in clinical trials. Fortrea’s D&I solution is designed to expand patient access to participate in clinical trials and address the U.S. Food and Drug Administration (FDA) requirements, under The Food and Drug Omnibus Reform Act, to increase enrollment of underrepresented populations in clinical trials.
Fortrea’s comprehensive process integrates five components of diversity action planning and execution:
- Real-world evidence advisors research relevant real-world data sets to inform diversity planning.
- Regulatory, development and clinical operational experts design the Diversity Action Plan, validate with patient groups and negotiate with regulators.
- Operational teams access multiple data platforms, Fortrea’s Site Advisory Board and technology-enabled solutions to implement the Diversity Action Plan as an integral part of Fortrea’s clinical trial execution.
- Monitoring and reporting are enabled by Fortrea’s exclusive Diversity and Inclusion Study Insights Dashboard, providing actionable data and visualizations for ongoing study management.
- Experienced report technical writers compile data and prepare reports for regulatory submission, with ongoing regulatory support provided as part of the D&I solution.
"Clinical research that reflects a representative population provides better insight into how a potential treatment will work in a real-world setting,” said John Doyle, DrPH, president Fortrea Consulting. "Recent regulatory requirements codify progress over the last few years in biopharma’s approach to improving the inclusion of diverse populations in their development programs. Fortrea’s solution brings deep, real-world data expertise to design D&I plans that are effective and realistic, along with more than 30 years of experience across more than 20 therapeutic areas in trial execution. We also bring a steadfast commitment to D&I, not just in clinical trials but across our entire company as we pursue our purpose of bringing life-changing treatments to patients faster."
Fortrea’s D&I solution incorporates a series of proprietary tools, including epidemiological and feasibility assessments that leverage an exclusive combination of large data sets. The solution also integrates inputs from patient groups to create insights into protocol tolerance and study conduct support requirements in different patient populations across multiple therapy areas and geographies. These insights inform global and local patient recruitment and retention plans to reach under-represented patient populations and address barriers to participation in clinical trials.
“Ensuring the inclusion of diverse patient populations in clinical trials must go beyond a plan, it takes insight and action,” said Mark Morais, chief operating officer, Fortrea. “Because of our comprehensive Voice of Patient program and our collaboration with diverse investigator sites and site networks, we have a deep understanding of what it takes to be successful in reaching populations that have traditionally been under-represented in clinical trials. At Fortrea, we are informed by real-world data, enabled by innovative technologies, and driven by our passion to deliver new therapies for all patients.”
Please visit Diversity and Inclusion in Clinical Trials on Fortrea.com for more information.
About Fortrea
Fortrea (Nasdaq: FTRE) is a leading global provider of clinical development and patient access solutions to the life sciences industry. We partner with emerging and large biopharmaceutical, biotechnology, medical device and diagnostic companies to drive healthcare innovation that accelerates life-changing therapies to patients. Fortrea provides phase I-IV clinical trial management, clinical pharmacology, consulting services, differentiated technology enabled trial solutions and post-approval services.
Fortrea’s solutions leverage three decades of experience spanning more than 20 therapeutic areas, a passion for scientific rigor, exceptional insights and a strong investigator site network. Our talented and diverse team working in more than 90 countries is scaled to deliver focused and agile solutions to customers globally.
Learn more about how Fortrea is becoming a transformative force from pipeline to patient at Fortrea.com and follow us on LinkedIn and X (formerly Twitter) @Fortrea.
Fortrea Contacts: Fortrea Media: Galen Wilson – 703-298-0802, [email protected] Fortrea Media: Kate Dillon – 646-818-9115, [email protected]

Fortrea Holdings News MORE
Related stocks.

IMAGES
VIDEO
COMMENTS
Schedule of Events Template Always download and save the latest version of any form or resource from this website before compiling study documents.. Schedule of Events Template - Version 09/15/2023; Purpose. All protocols that require an OnCore calendar need to have a Schedule of Events (SOE) that is in the form of a table listing the study visits as a timeline, and the procedures and events ...
Michigan Institute for Clinical & Health Research (MICHR)1600 Huron Parkway, Building 400 Ann Arbor, MI 48109(734) [email protected]. Cite ItPlease help us continue our support for clinical and translational research by citing our grant number in relevant publications: UL1TR002240.
The purpose of the NIA Clinical Research Toolbox is to provide a Web-based informational repository for investigators and staff involved in clinical research. The Toolbox contains templates, sample forms, guidelines, regulations and informational materials to assist investigators in the development and conduct of high quality clinical research ...
ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. Explore 496,616 research studies in all 50 states and in 222 countries. See listed clinical studies related to the coronavirus disease (COVID-19)
UCLA conducts research for a wide range of medical disorders, and offers patients the opportunity to participate in clinical trials and research. Connect with us to see if you're a good fit for a clinical trial, and gain access to the latest treatments while contributing to a healthier society. Call 855-731-6040. Match Now.
8600 Rockville Pike, Bethesda, MD 20894. ClinicalTrials.gov. An official website of the U.S. Department of Health and Human Services, National Institutes of Health, National Library of Medicine, and National Center for Biotechnology Information.
A service of the National Institutes of Health. The Website provides patients, family members, and members of the public easy and free access to information on clinical studies for a wide range of ...
Hence, this requires a well‐designed clinical research study that rests on a strong foundation of a detailed methodology and is governed by ethical principles. 2. From an epidemiological standpoint, ... Hence, the case and control are matched on calendar time and length of follow‐up. When this study design is implemented, it is possible for ...
The clinical study is conducted by a sponsor or a clinical research organization (CRO). A perfect protocol, time limits, and regulatory requirements assume significance while planning a clinical trial. ... In accordance with the revised schedule "Y" of the Drugs and Cosmetics Act (DCA) (2005), a drug trial may be defined as a systematic study ...
Observational study. A type of study in which people are observed or certain outcomes are measured. No attempt is made by the researcher to affect the outcome — for example, no treatment is given by the researcher. Clinical trial (interventional study). During clinical trials, researchers learn if a new test or treatment works and is safe.
This is an NIH-funded initiative to connect 1) people who are trying to find research studies, and 2) researchers seeking people to participate in their studies. It is a free, secure registry to make it easier for the public to volunteer and to become involved in clinical research studies that contribute to improved health in the future.
The Clinical Trial Protocol incorporates all the aspects of what is needed to define how the study is to be conducted and reviewed; for the purposes of this first iteration of the Implementation Guide we are constraining the scope to focus just on the elements incorporated in the Schedule of Activities. PSS Link: PSS-2003 - Getting issue details...
Clinical trials are conducted for many reasons: to determine whether a new drug or device is safe and effective for people to use. to study different ways to use standard treatments or current ...
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn ...
All clinical research starts with the research protocol, a document that details all aspects of the trial: its background, rationale, objectives, design, methodology, statistical analysis plan, and organization.With the protocol, you can make sure you protect the participants and collect the data. Using protocol templates, you can start thinking through what you need to meet compliance ...
Get the latest information on Clinical Trials, FDA Drug Approvals, FDA Calendar, FDA Events and more on RTTNews ... Clinical Trial Calendar. Tweet. Results Date. Company Name. Ticker. Event. Indication. Outcome. Details. Aug 2024. AN2 Therapeutics, Inc. ANTX. Topline Phase 2 results of EBO-301.
This 10-session course introduces the basics of clinical research design, including: biostatistics, design of diagnostic and predictive test studies, required/desired elements of clinical trial protocols, the regulatory aspects of clinical research conduct and oversight, Good Clinical Practice (GCP), and ethical dimensions of clinical research. Open to faculty, trainees, students and staff. A ...
The Central Study Registration (CSR) system was designed to alleviate some of the frustrations investigators experience as they conduct their research. This UB CTSI Educational Modules video provides: An overview and origins of the CSR system; Goals of study registration; A breakdown of when your study should be registered; Advantages of using ...
Watch this video to learn about the three phases of clinical trials. Clinical Research Phase Studies. Phase 1. Study Participants: 20 to 100 healthy volunteers or people with the disease/condition ...
We also provide test site information for sponsors and contract research organizations (CROs), and additional resources for study and research participants. If you are looking for research studies for your child: Visit our online study database. Email us at [email protected]. Call us at 513-636-0098.
The CCR Start-Up Team manages the process of building the protocol calendar in OnCore, completing the Coverage Analysis, and oversees Budget and Budget Negotiations. The CCR Start-Up Team submits the study documents (i.e., protocol, draft budget and contract) to Sponsored Projects Administration (SPA) or Applied Innovation (AI) in Kuali ...
Whether it's treating rare tumor types, leveraging precision medicine or finding new ways to improve existing oncology treatments, we're searching for ways to make even greater differences in the lives of people living with cancer. Learn more about Lilly's Cancer Research or view a complete list of our cancer trials.
Access Subject Schedule by Enrollment. Prior to each study visit, the research team must be prepared for all known and unknown tasks that may need to be completed per protocol. If applicable, physician orders need to be completed and authorized for lab draws, study medication and additional testing; research lab kits should be prepared and ...
Coordinate the schedule of clinical trial events for medical staff and study enrollees Background The Clinical Research Coordinator (CRC) is a specialized research professional whose goal is to ensure the smooth and accurate progress of clinical studies. A CRC is involved in all stages of a clinical study, from the planning and approval stages through…
Life Cycle of a Clinical Trial. Services and rates. Study team resources. Phase 1 trials. Support to stem cell research. CTRC Project Launch shows you how to implement and perform your clinical trials with the UCLA CTRC. STEP 1: Project Pre-Award. STEP 2: Project Application. STEP 3: Project Planning.
11066 Background: There is a large variation in activation times for cancer clinical trials, delaying the opportunity for patients to benefit from cutting edge treatments. Academic medical centers typically take longer to activate a clinical trial as compared to community settings. Some academic medical centers have adopted quality improvement processes to reduce clinical trial start-up time ...
An open access study protocol was published to ensure research quality and transparency. 14 The trial followed the Consolidated Standards of Reporting Trials reporting guideline for randomized clinical trials. 15 The patient flow diagram is provided as Figure 1, and the trial protocol is provided in Supplement 1.
While the biologic benefits of biospecimen research have been well established, the benefit of translational research on the Veteran care experience has yet to be defined. In this study, we evaluated: 1. Whether Veteran enrollment in a biospecimen-based prostate cancer research study improved their VA patient care experience and satisfaction and 2.
Job Type: Officer of Administration Bargaining Unit: Regular/Temporary: Regular End Date if Temporary: Hours Per Week: 35 Standard Work Schedule: Building: Salary Range: $62,400.00 - $67,428.00 The salary of the finalist selected for this role will be set based on a variety of factors, including but not limited to departmental budgets, qualifications, experience, education, licenses, specialty ...
Fortrea provides phase I-IV clinical trial management, clinical pharmacology, consulting services, differentiated technology enabled trial solutions and post-approval services.