

Understanding Nursing Research
- Primary Research
- Qualitative vs. Quantitative Research
- Experimental Design
- Is it a Nursing journal?
- Is it Written by a Nurse?
Secondary Research and Systematic Reviews
Comparative table.
- Quality Improvement Plans
Secondary Research is when researchers collect lots of research that has already been published on a certain subject. They conduct searches in databases, go through lots of primary research articles, and analyze the findings in those pieces of primary research. The goal of secondary research is to pull together lots of diverse primary research (like studies and trials), with the end goal of making a generalized statement. Primary research can only make statements about the specific context in which their research was conducted (for example, this specific intervention worked in this hospital with these participants), but secondary research can make broader statements because it compiled lots of primary research together. So rather than saying, "this specific intervention worked at this specific hospital with these specific participants, a piece of secondary research can say, "This intervention works at hospitals that serve this population."
Systematic Reviews are a kind of secondary research. The creators of systematic reviews are very intentional about their inclusion/exclusion criteria, or which articles they'll include in their review and the goal is to make a generalized statement so other researchers can build upon the practices or interventions they recommend. Use the chart below to understand the differences between a systematic review and a literature review.
Check out the video below to watch the Nursing and Health Sciences librarian describe the differences between primary and secondary research.
| Literature Review | Systematic Review | Meta-Analysis |
|---|---|---|
|
|
- "Literature Reviews and Systematic Reviews: What Is the Difference?" This article explains in depth the differences between Literature Reviews and Systematic Reviews. It is from the journal RADIOLOGIC TECHNOLOGY, Nov/Dec 2013, v. 85, #2. It is one to which Bell Library subscribes and meets copyright clearance requirements through our subscription to CCC.
- << Previous: Is it Written by a Nurse?
- Next: Permalinks >>
- Last Updated: May 23, 2024 4:16 PM
- URL: https://guides.library.tamucc.edu/nursingresearch
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Yearb Med Inform
- v.26(1); 2017 Aug

Secondary Use of Patient Data: Review of the Literature Published in 2016
D. r. schlegel.
1 Department of Computer Science, SUNY Oswego, Oswego NY, USA
2 Department of Medical Informatics, EA 2694, Lille University Hospital, France
Objectives: To summarize recent research and emerging trends in the area of secondary use of healthcare data, and to present the best papers published in this field, selected to appear in the 2017 edition of the IMIA Yearbook.
Methods: A literature review of articles published in 2016 and related to secondary use of healthcare data was performed using two bibliographic databases. From this search, 941 papers were identified. The section editors independently reviewed the papers for relevancy and impact, resulting in a consensus list of 14 candidate best papers. External reviewers examined each of the candidate best papers and the final selection was made by the editorial board of the Yearbook.
Results: From the 941 retrieved papers, the selection process resulted in four best papers. These papers discuss data quality concerns, issues in preserving privacy of patients in shared datasets, and methods of decision support when consuming large amounts of raw electronic health record (EHR) data.
Conclusion: In 2016, a significant effort was put into the development of new systems which aim to avoid significant human understanding and pre-processing of healthcare data, though this is still only an emerging area of research. The value of temporal relationships between data received significant study, as did effective information sharing while preserving patient privacy.
Introduction
Reuse, or secondary use, of data concerns the use of clinical data for a different purpose than the one for which it was originally collected. The data being reused are usually those owned by hospitals and health systems - large databases containing administrative, claims, and patient health data. Oftentimes this data is reused for research and applications in quality of care and patient safety. Techniques relying on the reuse of data are in opposition to conventional clinical research using data collected prospectively using pre-defined cohorts.
The literature surrounding the reuse of data is large and continues to grow - the problem is difficult and remains interesting even after some success has been obtained. The difficulties faced include the need to refactor and manage the data (sometimes across sites using different data formats which must interoperate), large numbers of variables and categories to be aggregated, issues with data quality (e.g. missing data), and maintaining security and privacy. These difficulties continue to be the subject of research, and developing solutions is becoming increasingly interdisciplinary - recently we have seen the use of deep neural networks [ 1 ] from the field of artificial intelligence employed in solving data reuse classification problems in medicine.
The reuse of medico-administrative data has also, for several years, been of interest in epidemiology and in particular in pharmacoepidemiology. Projects such as the Observational Medical Outcomes Partnership (OMOP) have empirically demonstrated the value of this data compared to the more traditional pharmacovigilance databases [ 2 ]. These same projects also confirmed, for the control of confounding factors in this observational context, the quality of cohort designs with the use of high-dimensional propensity scores and the particular interest of cross-over designs. Moreover, randomized control trials (RCTs) and routinely collected data have recently been considered together through “registry-based RCTs” [ 3 ], which is an active area of research.
Achieving reliable data reuse is a challenge worthy of our time and research, allowing for the identification of patients of interest for retrospective research (electronic phenotyping [ 4 ]). Electronic Health Record (EHR) data is huge (and therefore has a high statistical power), can be used without interfering with patient care, and is real data that can play a central role within a learning healthcare system.
The papers selected as best papers involve the development of systems which aim to predict patient outcomes, explore the value of temporal relationships in data, and discuss effective information sharing while preserving patient privacy. The following sections discuss the best papers selection method and emphasize notable characteristics of the best papers in the context of the wider literature.
Paper Selection Method
The databases PubMed/Medline and Web of Science® were searched for peer-reviewed papers published in the English language and which pertain to data reuse. The following Boolean expression was used: “secondary use” OR “data reuse” OR (“big data” AND (“health” OR “medicine” OR “medical”)). In addition, a complementary condition was used on Pubmed/Medline regarding the date of publication in 2016 (“2016/01/01”[Date - Publication]: “2016/12/31”[Date - Publication]). Three filters were added in the case of Web of Science® about the date of publication: “2016”, the field of interest: “Medical Informatics”, and the type of paper: “Article OR Proceedings”. Data reuse is an extremely large topic area and these query terms have the potential of excluding some papers, for example in epidemiology, in which data reuse is performed, but not discussed explicitly. Still, a total of 941 papers were retrieved. Papers were independently analyzed by the section editors on the basis of titles and abstracts. The documents were classified into two categories: “accept” or “reject” based on relevance and perceived impact. Each article labeled “accept” was examined in detail to finally reach a consensus list of 14 candidate best papers. In accordance with the IMIA Yearbook selection process [ 5 ], the candidate best papers were assessed by the two section editors and by two additional reviewers. Four papers were selected as the best papers ( Table 1 ). A summary for each of them is given as an appendix.
Best paper selection of articles for the IMIA Yearbook of Medical Informatics 2017 in the section ‘Learning from Experience: Secondary Use of Patient Data’. The articles are listed in alphabetical order of the first author’s surname.
Conclusions and Outlook
In 2016, noteworthy papers discussing secondary use of patient data focused on studying and improving the quality of clinical data, issues in sharing data, and predicting health outcomes using clinical data.
Secondary use of clinical data relies on the data being consistent across time and across departments and/or sites under study. A frequently used approach which continues to be applied to new domains is the use of standard ontologies and terminologies to apply a common semantic model to healthcare data. Sahoo et al ., implemented an informatics platform for epilepsy by surveying existing outcome data, identifying common data elements, and developing an epilepsy domain ontology to resolve issues of data heterogeneity [ 6 ]. Quality of clinical data goes hand-in-hand with the quality of the semantic resources the data is mapped with, which also has seen continued work this year (e.g., [ 7 ]).
Sauer et al . [ 8 ], found that the United States Veterans Administration (VA) stores the results of pulmonary function tests (PFT) in structured, semi-structured, and unstructured forms. They used a natural language processing system to extract PFT results with a high degree of accuracy (F >.98). In this case, despite the different representations, they were able to avoid being formal about their semantic representations because of the narrow focus.
While the VA uses common data formats across sites, many who wish to share data between sites do not. Saez et al . [ 9 ], applied novel methods based on information theory and geometry to assess variability among multiple data sources and changes over time. In particular, they empirically studied data quality issues stemming from variation in probability distributions (due to population differences, biased practices, etc.) and time, concluding that “even if semantic and integration aspects are addressed in data sharing infrastructures, probabilistic variability may still be present.”
Work on identifying issues in sharing data between sites was another trend this year. Saez et al . [ 9 ], discussed the issue of data quality, but other issues addressed include the difficulty in understanding what’s in a large data collection, and issues in maintaining patient privacy when datasets are shared publically. Demner-Fushman’s paper on preparing radiology examination documents for distribution, including de-identification and indexing, noted that “an important step in facilitating secondary use of clinical document collections is easy access to descriptions and samples that represent the content of the collections” [ 10 ]. This is a part of the larger academic discussion of how researchers should handle scientific data in general [ 11 ], as is being studied by the CEDAR group at Stanford.
De-identification of data for sharing has become a significant concern as more clinical datasets are provided on the web for research. Prasser et al ., empirically examined the issue from the point of view of minimizing risk of re-identification, balancing increases in privacy with data quality, all while considering the data sharing context and the aims of the potential attackers [ 12 ]. Their use of risk models decreased information lost in de-identification by 10–24% depending on the strength of adversary they were protecting against.
This year reuse of clinical data trended on predicting outcomes. The contrast of two opposing approaches becomes clear when examining the literature as a whole: (i) identification by experts of the required data elements to build predictors (electronic phenotyping) for outcome prediction, and (ii) the “firehose” approach without pre-processing in which many more variables than may be needed are used. As with Saez et al ., above, Goldstein, et al . [ 13 ], continued a theme of focusing on temporal issues, identifying different data elements most predictive of mortality in patients receiving hemodialysis over different time horizons: vital signs in the near term, demographics and comorbidities in the long-term. In contrast, the OrderRex system [ 14 ] takes the “firehose” approach -automatically ingesting around 1,500 of the most common data elements from inpatient notes and performing association statistics in order to predict next order recommendations and outcomes. Importantly, they found that using temporal relationships between orders in their database improves results, from a precision at 10 recommendations of 33% to 38%.
A team at Mount Sinai has also developed an unsupervised method for learning directly from EHR data, this time using state-of-the-art artificial intelligence (AI) techniques such as feature learning and deep neural networks, called Deep Patient [ 15 ]. This system was used to predict whether patients would develop various diseases using random forest classifiers after using a deep neural network for feature extraction. The system was found to outperform other unsupervised learning mechanisms such as Principal Component Analysis (PCA) and Gaussian mixture models. Accuracy of the system was found to be quite high (.929) but the F-Score was still rather low (.181), even though it was better than all comparison systems. These “firehose”-based approaches are sure to continue gaining popularity as more structured and free text EHR data is annotated with standardized semantic resources for input into such systems. This strategy is related to those used in many papers reviewed by the section editors based on the extraction of large number of quantitative features in medical images (i.e. radiomics), and on the use of raw EHR data to build predictors, as in the work of Singh, et al . [ 16 ], identifying novel predictors of kidney failure from concepts extracted directly from clinical notes.
Summary of the Best Papers Selected for the 2017 Edition of the IMIA Yearbook, Special Section “Learning from Experience: Secondary Use of Patient Data“
Chen J, Podchiyska T, Altman R OrderRex: clinical order decision support and outcome predictions by data-mining electronic medical records J Am Med Inform Assoc 2016;23:339-48
Compliance with evidence-based guidelines is low and a majority of clinical decisions are not supported by randomized control trials. Thus, a large part of medical practice is thus driven by individual expert opinion. The authors present a clinical order recom-mender system which operates on a database which has been mined from existing patient data. The input to the data mining system is around 1,500 common electronic medical record (EMR) data elements (out of 5.4 million structured data elements) from labs results, orders, and diagnosis codes, including temporal separation in the form of patient timelines. This data was extracted for 18 thousand patients and stored in an association matrix. Queries to the database come in the form of clinical terms for the captured data elements for a patient. A ranking of suggested orders based on the input data and the association matrix is output to the user. By mixing outcomes such as death and hospital readmission in with the order results, the system also acts as a predictor of outcomes. The authors observe that including the temporal data increased precision from 33 to 38%, but also note that continued work is required to differentiate simply common behaviors on certain data from the correct ones.
Miotto R, Li L, Kidd BA, Dudley JT Deep Patient: An Unsupervised Representation to Predict the Future of Patients from the Electronic Health Records Sci Rep 2016;6:26094
Proposed in this paper is a novel unsupervised deep feature learning method to derive a patient representation from EHR data that facilitates the prediction of clinical outcomes. Deep learning techniques, using neural networks with more than one hidden layer, have not previously been broadly used with EHR data. The authors used aggregated medical records from the Mount Sinai data warehouse with a stack of denoising autoencoders to capture stable structures and regular patterns from pre-processed EHR data. Then, they implemented random forest classifiers (one-vs.-all learning) to predict the probability that patients might develop a certain disease. On 76,214 test patients comprising 78 diseases from diverse clinical domains and temporal windows, the results significantly outperformed those achieved using representations based on raw EHR data and alternative feature learning strategies such as principal component analysis and Gaussian mixture models.
Saez C, Zurriaga O, Perez-Panades J, Melchor I, Robles M, Garcia-Gomez JM Applying probabilistic temporal and multisite data quality control methods to a public health mortality registry in Spain: a systematic approach to quality control of repositories J Am Med Inform Assoc 2016;23:1085-95
The authors propose the evaluation of variability in data distributions as a criterion which could be used systematically in assessing data quality. This variability is assessed first on different sources of data (i.e., from different sites), and second, over time. The authors proposed a novel statistics-based assessment method providing data quality metrics and exploratory visualizations. The method is empirically driven on a public health mortality registry of the region of Valencia, Spain, with >500,000 entries from 2000 to 2012, separated into 24 health departments. The repository was partitioned into two temporal subgroups following a change in the Spanish National Date certificate in 2009. Several types of data quality issues were identified including punctual temporal anomalies, and outlying or clustered health departments. The authors note that these issues can occur because of biases in practice, different populations, and changes in protocols or guidelines over time - none of which are solved through usual techniques of mapping to standard semantics.
Prasser F, Kohlmayer F, Kuhn KA The Importance of Context: Risk-based De-identification of Biomedical Data Methods Inf Med 2016;55:347-55
As data sharing becomes more common, concerns about maintaining the privacy of patients in such data sets is growing as well. International laws, such as HIPAA, and European Directive on Data Protection emphasize the importance of context when implementing measures for data protection. With methods of de-identification such as k-anonymity (dataset is transformed in such a way that each record is not different from k-1 other records), the degree of protection is high, but it is associated with a loss of information content. Indeed, a major challenge of data sharing is the adequate balance between data quality and privacy. The authors propose a generic de-identification method based on risk models, which assesses the risk of re-identification. An experimental evaluation was performed to assess the impact of different risk models and assumptions about the background knowledge/context of an attacker. Compared with reference methods, the loss of information was between 10% and 24% less, depending on the strength of the adversary being protected against.
Acknowledgements
We would like to acknowledge the support of Brigitte Séroussi and John H. Holmes, along with the reviewers who assisted with the selection process.
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
- Working with sources
- Primary vs. Secondary Sources | Difference & Examples
Primary vs. Secondary Sources | Difference & Examples
Published on June 20, 2018 by Raimo Streefkerk . Revised on May 31, 2023.
When you do research, you have to gather information and evidence from a variety of sources.
Primary sources provide raw information and first-hand evidence. Examples include interview transcripts, statistical data, and works of art. Primary research gives you direct access to the subject of your research.
Secondary sources provide second-hand information and commentary from other researchers. Examples include journal articles, reviews, and academic books . Thus, secondary research describes, interprets, or synthesizes primary sources.
Primary sources are more credible as evidence, but good research uses both primary and secondary sources.
Table of contents
What is a primary source, what is a secondary source, primary and secondary source examples, how to tell if a source is primary or secondary, primary vs secondary sources: which is better, other interesting articles, frequently asked questions about primary and secondary sources.
A primary source is anything that gives you direct evidence about the people, events, or phenomena that you are researching. Primary sources will usually be the main objects of your analysis.
If you are researching the past, you cannot directly access it yourself, so you need primary sources that were produced at the time by participants or witnesses (e.g. letters, photographs, newspapers ).
If you are researching something current, your primary sources can either be qualitative or quantitative data that you collect yourself (e.g. through interviews , surveys , experiments ) or sources produced by people directly involved in the topic (e.g. official documents or media texts).
| Research field | Primary source |
|---|---|
| History | |
| Art and literature | |
| Communication and social studies | |
| Law and politics | |
| Sciences |
Prevent plagiarism. Run a free check.
A secondary source is anything that describes, interprets, evaluates, or analyzes information from primary sources. Common examples include:
- Books , articles and documentaries that synthesize information on a topic
- Synopses and descriptions of artistic works
- Encyclopedias and textbooks that summarize information and ideas
- Reviews and essays that evaluate or interpret something
When you cite a secondary source, it’s usually not to analyze it directly. Instead, you’ll probably test its arguments against new evidence or use its ideas to help formulate your own.
| Primary source | Secondary source |
|---|---|
| Novel | Article analyzing the novel |
| Painting | Exhibition catalog explaining the painting |
| Letters and diaries written by a historical figure | Biography of the historical figure |
| by a philosopher | Textbook summarizing the philosopher’s ideas |
| Photographs of a historical event | Documentary about the historical event |
| Government documents about a new policy | Newspaper article about the new policy |
| Music recordings | Academic book about the musical style |
| Results of an opinion poll | Blog post interpreting the results of the poll |
| Empirical study | that cites the study |
Examples of sources that can be primary or secondary
A secondary source can become a primary source depending on your research question . If the person, context, or technique that produced the source is the main focus of your research, it becomes a primary source.
Documentaries
If you are researching the causes of World War II, a recent documentary about the war is a secondary source . But if you are researching the filmmaking techniques used in historical documentaries, the documentary is a primary source .
Reviews and essays
If your paper is about the novels of Toni Morrison, a magazine review of one of her novels is a secondary source . But if your paper is about the critical reception of Toni Morrison’s work, the review is a primary source .
Newspaper articles
If your aim is to analyze the government’s economic policy, a newspaper article about a new policy is a secondary source . But if your aim is to analyze media coverage of economic issues, the newspaper article is a primary source .
To determine if something can be used as a primary or secondary source in your research, there are some simple questions you can ask yourself:
- Does this source come from someone directly involved in the events I’m studying (primary) or from another researcher (secondary)?
- Am I interested in evaluating the source itself (primary) or only using it for background information (secondary)?
- Does the source provide original information (primary) or does it comment upon information from other sources (secondary)?
Scribbr Citation Checker New
The AI-powered Citation Checker helps you avoid common mistakes such as:
- Missing commas and periods
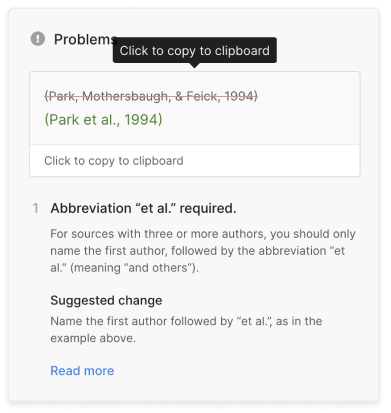
- Incorrect usage of “et al.”
- Ampersands (&) in narrative citations
- Missing reference entries
Most research uses both primary and secondary sources. They complement each other to help you build a convincing argument. Primary sources are more credible as evidence, but secondary sources show how your work relates to existing research. Tertiary sources are often used in the first, exploratory stage of research.
What do you use primary sources for?
Primary sources are the foundation of original research. They allow you to:
- Make new discoveries
- Provide credible evidence for your arguments
- Give authoritative information about your topic
If you don’t use any primary sources, your research may be considered unoriginal or unreliable.
What do you use secondary sources for?
Secondary sources are good for gaining a full overview of your topic and understanding how other researchers have approached it. They often synthesize a large number of primary sources that would be difficult and time-consuming to gather by yourself. They allow you to:
- Gain background information on the topic
- Support or contrast your arguments with other researchers’ ideas
- Gather information from primary sources that you can’t access directly (e.g. private letters or physical documents located elsewhere)
When you conduct a literature review or meta analysis, you can consult secondary sources to gain a thorough overview of your topic. If you want to mention a paper or study that you find cited in a secondary source, seek out the original source and cite it directly.
Remember that all primary and secondary sources must be cited to avoid plagiarism . You can use Scribbr’s free citation generator to do so!
If you want to know more about ChatGPT, AI tools , citation , and plagiarism , make sure to check out some of our other articles with explanations and examples.
- ChatGPT vs human editor
- ChatGPT citations
- Is ChatGPT trustworthy?
- Using ChatGPT for your studies
- What is ChatGPT?
- Chicago style
- Paraphrasing
Plagiarism
- Types of plagiarism
- Self-plagiarism
- Avoiding plagiarism
- Academic integrity
- Consequences of plagiarism
- Common knowledge
Common examples of primary sources include interview transcripts , photographs, novels, paintings, films, historical documents, and official statistics.
Anything you directly analyze or use as first-hand evidence can be a primary source, including qualitative or quantitative data that you collected yourself.
Common examples of secondary sources include academic books, journal articles , reviews, essays , and textbooks.
Anything that summarizes, evaluates or interprets primary sources can be a secondary source. If a source gives you an overview of background information or presents another researcher’s ideas on your topic, it is probably a secondary source.
To determine if a source is primary or secondary, ask yourself:
- Was the source created by someone directly involved in the events you’re studying (primary), or by another researcher (secondary)?
- Does the source provide original information (primary), or does it summarize information from other sources (secondary)?
- Are you directly analyzing the source itself (primary), or only using it for background information (secondary)?
Some types of source are nearly always primary: works of art and literature, raw statistical data, official documents and records, and personal communications (e.g. letters, interviews ). If you use one of these in your research, it is probably a primary source.
Primary sources are often considered the most credible in terms of providing evidence for your argument, as they give you direct evidence of what you are researching. However, it’s up to you to ensure the information they provide is reliable and accurate.
Always make sure to properly cite your sources to avoid plagiarism .
A fictional movie is usually a primary source. A documentary can be either primary or secondary depending on the context.
If you are directly analyzing some aspect of the movie itself – for example, the cinematography, narrative techniques, or social context – the movie is a primary source.
If you use the movie for background information or analysis about your topic – for example, to learn about a historical event or a scientific discovery – the movie is a secondary source.
Whether it’s primary or secondary, always properly cite the movie in the citation style you are using. Learn how to create an MLA movie citation or an APA movie citation .
Articles in newspapers and magazines can be primary or secondary depending on the focus of your research.
In historical studies, old articles are used as primary sources that give direct evidence about the time period. In social and communication studies, articles are used as primary sources to analyze language and social relations (for example, by conducting content analysis or discourse analysis ).
If you are not analyzing the article itself, but only using it for background information or facts about your topic, then the article is a secondary source.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
Streefkerk, R. (2023, May 31). Primary vs. Secondary Sources | Difference & Examples. Scribbr. Retrieved June 18, 2024, from https://www.scribbr.com/working-with-sources/primary-and-secondary-sources/

Is this article helpful?
Raimo Streefkerk
Other students also liked, how to avoid plagiarism | tips on citing sources, the basics of in-text citation | apa & mla examples, how to quote | citing quotes in apa, mla & chicago, get unlimited documents corrected.
✔ Free APA citation check included ✔ Unlimited document corrections ✔ Specialized in correcting academic texts
- Library Guides

The Literature Review
Primary and secondary sources, the literature review: primary and secondary sources.

- Searching the literature
- Grey literature
- Organising and analysing
- Systematic Reviews
- The Literature Review Toolbox
On this page
- Primary vs secondary sources: The differences explained
Can something be both a primary and secondary source?
Research for your literature review can be categorised as either primary or secondary in nature. The simplest definition of primary sources is either original information (such as survey data) or a first person account of an event (such as an interview transcript). Whereas secondary sources are any publshed or unpublished works that describe, summarise, analyse, evaluate, interpret or review primary source materials. Secondary sources can incorporate primary sources to support their arguments.
Ideally, good research should use a combination of both primary and secondary sources. For example, if a researcher were to investigate the introduction of a law and the impacts it had on a community, he/she might look at the transcripts of the parliamentary debates as well as the parliamentary commentary and news reporting surrounding the laws at the time.
Examples of primary and secondary sources
| Diaries | Journal articles |
| Audio recordings | Textbooks |
| Transcripts | Dictionaries and encyclopaedias |
| Original manuscripts | Biographies |
| Government documents | Political commentary |
| Court records | Blog posts |
| Speeches | Newspaper articles |
| Empirical studies | Theses |
| Statistical data | Documentaries |
| Artworks | Critical analyses |
| Film footage | |
| Photographs |
Primary vs secondary sources: The differences explained
Finding primary sources
- VU Special Collections - The Special Collections at Victoria University Library are a valuable research resource. The Collections have strong threads of radical literature, particularly Australian Communist literature, much of which is rare or unique. Women and urban planning also feature across the Collections. There are collections that give you a picture of the people who donated them like Ray Verrills, John McLaren, Sir Zelman Cowen, and Ruth & Maurie Crow. Other collections focus on Australia's neighbours – PNG and Timor-Leste.
- POLICY - Sharing the latest in policy knowledge and evidence, this database supports enhanced learning, collaboration and contribution.
- Indigenous Australia - The Indigenous Australia database represents the collections of the Aboriginal and Torres Strait Islander Commission Library.
- Australian Heritage Bibliography - Aboriginal and Torres Strait Islander Subset (AHB-ATSIS) - AHB is a bibliographic database that indexes and abstracts articles from published and unpublished material on Australia's natural and cultural environment. The AHB-ATSIS subset contains records that specifically relate to the Aboriginal and Torres Strait Islander peoples.include journal articles, unpublished reports, books, videos and conference proceedings from many different sources around Australia. Emphasis is placed on reports written or commissioned by government and non-government heritage agencies throughout the country.
- ATSIhealth - The Aboriginal and Torres Strait Islander Health Bibliography (ATSIhealth), compiled by Neil Thomson and Natalie Weissofner at the School of Indigenous Australian Studies, Kurongkurl Katitjin, Edith Cowan University, is a bibliographic database that indexes published and unpublished material on Australian Indigenous health. Source documents include theses, unpublished articles, government reports, conference papers, abstracts, book chapters, books, discussion and working papers, and statistical documents.
- National Archive of Australia - The National Archives of Australia holds the memory of our nation and keeps vital Australian Government records safe.
- National Library of Australia: Manuscripts - Manuscripts collection that is wide ranging and provides rich evidence of the lives and activities of Australians who have shaped our society.
- National Library of Australia: Printed ephemera - The National Library has been selectively collecting Australian printed ephemera since the early 1960s as a record of Australian life and social customs, popular culture, national events, and issues of national concern.
- National Library of Australia: Oral history and folklore - The Library’s Oral History and Folklore Collection dates back to the 1950’s and includes a rich and diverse collection of interviews and recordings with Australians from all walks of life.
- Historic Hansard - Commonwealth of Australia parliamentary debates presented in an easy-to-read format for historians and other lovers of political speech.
- The Old Bailey Online - A fully searchable edition of the largest body of texts detailing the lives of non-elite people ever published, containing 197,745 criminal trials held at London's central criminal court.
Whether or not a source can be considered both primary and secondary, depends on the context. In some instances, material may act as a secondary source for one research area, and as a primary source for another. For example, Niccolò Machiavelli’s The Prince , published in 1513, is an important secondary source for any study of the various Renaissance princes in the Medici family; but the same book is also a primary source for the political thought that was characteristic of the sixteenth century because it reflects the attitudes of a person living in the 1500s.
Source: Craver, 1999, as cited in University of South Australia Library. (2021, Oct 6). Can something be a primary and secondary source?. University of South Australia Library. https://guides.library.unisa.edu.au/historycultural/sourcetypes
- << Previous: Overview
- Next: Searching the literature >>
- Last Updated: Mar 27, 2024 2:06 PM
- URL: https://libraryguides.vu.edu.au/the-literature-review
Selecting and Evaluating Secondary Data: The Role of Systematic Reviews and Meta-analysis
- First Online: 01 January 2012
Cite this chapter

- Lorenzo Paladino MD 3 &
- Richard H. Sinert DO 3
5734 Accesses
For clinicians to make informed decisions for patient management and research, they must analyze multiple studies for quality and relevance to the population of interest. Secondary sources of information (especially systematic reviews and meta-analyses) help to summarize and reconcile conflicting studies in the literature. By explicitly stating how evidence was found, selected, and evaluated, systematic reviews eliminate many of the biases inherent in narrative reviews. Meta-analysis uses statistical methodology to combine results of several related studies, which affords greater statistical power versus that of individual studies. Though retrievable via traditional online literature search engines, a variety of databases are available that specialize in systematic reviews and meta-analyses. To construct a quality systematic review, one should formulate a clear question, define a comprehensive yet efficient literature searching strategy, include all appropriate studies, summarize results, assess heterogeneity, and consider appropriateness of pooling results if individual studies for meta-analysis. Caution must be exercised when conducting/interpreting a systematic review or meta-analysis to ensure inclusiveness of literature searching, optimization of statistical rigor, minimization of bias, and avoidance of inclusion of multiple publications of the same dataset. The results and conclusions of a systematic review or meta-analysis are only as reliable as the methods used in each of the primary studies; their synthesis does not compensate for errors of methodology in the individual primary studies. Meta-analyses, constructed as they are of multiple nonidentical studies, must be viewed as a hypothesis-generating rather than a hypothesis testing tool especially if major methodological differences or heterogeneity among their components is detected.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others
Conducting and interpreting high-quality systematic reviews and meta-analyses.

The Methodology of Meta-Analyses and Its Potential Contribution to Patient Care

Meta-analysis, Evidence-Based Medicine, and Clinical Guidelines
Lau J, Antman EM, Jimenez-Silva J, Kupelnick B, Mosteller F, Chalmers TC. Cumulative meta-analysis of therapeutic trials for myocardial infarction. N Engl J Med. 1992;327:248–54.
Article PubMed CAS Google Scholar
Collins JA, Fauser BCJM. Balancing the strengths of systematic and narrative reviews. Hum Reprod Update. 2005;11:103–4.
Article PubMed Google Scholar
Pearson K. Report on certain enteric fever inoculation statistics. Br Med J. 1904;3:1243–6.
Google Scholar
Glass GV. Primary, secondary, and meta-analysis of research. Educ Res. 1976;5:3–8.
Sacks HS, Berrier J, Reitman D, Ancona-Berk VA, Chalmers TC. Meta-analyses of randomized controlled trials. N Engl J Med. 1987;316:450–5.
Tuckman BW. Conducting educational research. 3rd ed. New York: Harcourt Brace Jovanovich; 1972.
Wilczynski NL, Haynes RB, Lavis JN, Ramkissoonsingh R, Arnold-Oatley A, HSR Hedges Team. Optimal search strategies for detecting health services research studies in MEDLINE. CMAJ. 2004;171:1179–85.
Oxman AD, Sackett DL, Guyatt GH. Users’ guides to the medical literature. I. How to get started. The Evidence-Based Medicine Working Group. JAMA. 1993;270:2093–5.
Fact Sheet. Medical Subject Headings (Mesh®). U.S. National Library of Medicine. http://www.nlm.nih.gov/pubs/factsheets/mesh.html . Accessed 16 Aug 2011.
Yu CH, Beattie WS. The effects of volatile anesthetics on cardiac ischemic complications and mortality in CABG: a meta-analysis. Can J Anaesth. 2006;53: 906–18.
Centre for Reviews and Dissemination. Systematic reviews: CRD’s guidance for undertaking reviews in healthcare. York: University of York NHS Centre for Reviews & Dissemination; 2009.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12.
ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet. 1988;2:349–60.
Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 10 Nov 2003;3:25. http://www.biomedcentral.com/1471-2288/3/25 . Accessed 16 Sep 2011.
Deeks JJ, Bossuyt PM, Gatsonis C, editors. Cochrane handbook for systematic reviews of diagnostic test accuracy version 1.0. The cochrane collaboration, 2010. http://srdta.cochrane.org/ . Accessed 16 Sep 2011.
Fagan TJ. Letter: nomogram for Bayes theorem. N Engl J Med. 1975;293:257.
PubMed CAS Google Scholar
Stewart LA, Tierney JF. To IPD or not to IPD? Advantages and disadvantages of systematic reviews using individual patient data. Eval Health Prof. 2002;25:76–97.
Matt GE, Cook TD. Threats to the validity of research synthesis. In: Cooper H, Hedges LV, editors. The handbook of research synthesis. NewYork: Russell Sage; 1994.
Lewis S, Clarke C. Forest plots: trying to see the wood and the trees. BMJ. 2001;322:1479–80.
Brandler E, Paladino L, Sinert R. Does the early administration of beta-blockers improve the in-hospital mortality rate of patients admitted with acute coronary syndrome? Acad Emerg Med. 2010;17: 1–10.
L’Abbe KL, Detsky AS, O’Rourke K. Meta-analysis in clinical research. Ann Intern Med. 1987;107:224–33.
PubMed Google Scholar
Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–72.
Egger M, Smith DG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Rosenthal R. The “file drawer problem” and tolerance for null results. Psychol Bull. 1979;86:638–41.
Article Google Scholar
Cooper HM. Statistically combining independent studies: a meta-analysis of sex differences in conformity research. J Pers Soc Psychol. 1979;37:131–46.
Orwin RG. A fail-safe N for effect size in meta-analysis. J Educ Stat. 1983;8:157–9.
Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic; 1969.
Monami M, Bigiarini M, Rotella CM, Mannucci E. Inaccuracy in meta-analysis on rosiglitazone and myocardial infarction. Nutr Metab Cardiovasc Dis. 2011;21:e7–8. Epub 2010 Dec 25.
Claggett B, Wei JL. Analytical issues regarding rosiglitazone meta-analysis. Arch Intern Med. 2011;171:179–80. author reply 180.
Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomized controlled trials: the QUOROM statement. Lancet. 1999;354: 1896–900.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. 2009. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. http://www.plosmedicine.org/article/info%3Adoi%2F10.1371%2Fjournal.pmed.1000097 . Accessed 14 Sep 2011.
Moher D, Tetzlaff J, Tricco AC, Sampson M, Altman DG. Epidemiology and reporting characteristics of systematic reviews. PLoS Med. 2007;4:e78. doi: 10.1371/journal.pmed.0040078 .
Download references
Author information
Authors and affiliations.
Department of Emergency Medicine, SUNY Downstate Medical Center, 450 Clarkson Avenue, 1228, Brooklyn, NY, 11203, USA
Lorenzo Paladino MD & Richard H. Sinert DO
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Richard H. Sinert DO .
Editor information
Editors and affiliations.
, Cardiovascular Medicine, SUNY Downstate Medical Center, Clarkson Avenue, box 1199 450, Brooklyn, 11203, USA
Phyllis G. Supino
, Cardiovascualr Medicine, SUNY Downstate Medical Center, Clarkson Avenue 450, Brooklyn, 11203, USA
Jeffrey S. Borer
Rights and permissions
Reprints and permissions
Copyright information
© 2012 Springer Science+Business Media, LLC
About this chapter
Paladino, L., Sinert, R.H. (2012). Selecting and Evaluating Secondary Data: The Role of Systematic Reviews and Meta-analysis. In: Supino, P., Borer, J. (eds) Principles of Research Methodology. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-3360-6_9
Download citation
DOI : https://doi.org/10.1007/978-1-4614-3360-6_9
Published : 18 April 2012
Publisher Name : Springer, New York, NY
Print ISBN : 978-1-4614-3359-0
Online ISBN : 978-1-4614-3360-6
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

IMAGES
VIDEO
COMMENTS
The general literature review is a synthesis and analysis of published research on a relevant clinical issue, and is a common format for academic theses at the bachelor’s and master’s levels in nursing, physiotherapy, occupational therapy, public health and other related fields.
Use the chart below to understand the differences between a systematic review and a literature review. Check out the video below to watch the Nursing and Health Sciences librarian describe the differences between primary and secondary research.
A systematic review collects secondary data, and is a synthesis of all available, relevant evidence which brings together all existing primary studies for review ( Cochrane 2016 ). A systematic review differs from other types of literature review in several major ways.
If you want to analyze a large amount of readily-available data, use secondary data. If you want data specific to your purposes with control over how it is generated, collect primary data. If you want to establish cause-and-effect relationships between variables , use experimental methods.
In 2016, noteworthy papers discussing secondary use of patient data focused on studying and improving the quality of clinical data, issues in sharing data, and predicting health outcomes using clinical data.
Examples include journal articles, reviews, and academic books. Thus, secondary research describes, interprets, or synthesizes primary sources. Primary sources are more credible as evidence, but good research uses both primary and secondary sources.
This article is organized as follows: The next section presents the methodology adopted by this research, followed by a section that discusses the typology of literature reviews and provides empirical examples; the subsequent section summarizes the process of literature review; and the last section concludes the paper with suggestions on how to ...
Research for your literature review can be categorised as either primary or secondary in nature. The simplest definition of primary sources is either original information (such as survey data) or a first person account of an event (such as an interview transcript).
INTRODUCTION. Different methods for gathering information regarding specific variables of the study aiming to employ them in the data analysis phase to achieve the results of the study, gain the answer of the research .
To construct a quality systematic review, one should formulate a clear question, define a comprehensive yet efficient literature searching strategy, include all appropriate studies, summarize results, assess heterogeneity, and consider appropriateness of pooling results if individual studies for meta-analysis.