A multi-site case study of community-clinical linkages for promoting HPV vaccination
Affiliations.
- 1 a Department of Health Promotion, Education, and Behavior, University of South Carolina Arnold School of Public Health , Columbia , SC , USA.
- 2 b Department of Health, Behavior and Society, University of Kentucky College of Public Health , Lexington , KY , USA.
- 3 c Department of Health Management and Policy, University of Iowa , Iowa City , USA.
- 4 d Oregon Health and Science University School of Public Health , Portland , QR , USA.
- 5 e Department of Community & Behavioral Health, University of Iowa College of Public Health , Iowa City , IA , USA.
- 6 f Department of Health Services, University of Washington School of Public Health , Seattle , WA , USA.
- PMID: 31158042
- PMCID: PMC6746520
- DOI: 10.1080/21645515.2019.1616501
Human papillomavirus (HPV) vaccination rates in the U.S. are suboptimal, requiring innovative partnerships between community and clinical entities to remedy this issue. A rigorous evaluation of HPV-related community-clinical linkages (CCLs) was conducted to understand their components, processes, and outcomes to increase HPV vaccination. Cancer Prevention and Control Research Network (CPCRN) investigators explored CCLs in their communities employing an iterative, case study approach. Information describing nine CCLs on HPV vaccination was collected from representatives from the community organization and clinical setting. Thematic content analysis was used to analyze and interpret data. Five CCLs included a federally qualified health center as the clinical partner, and five included a non-profit organization as the community partner. Five reflected clinically focused integration wherein engagement occurs in the community but vaccine delivery and follow-up occur in the clinical setting. The main impetus was the need to improve HPV vaccination and a community's strong interest in preventing cancer. Noted critical components were a designated person to support the CCL and funding. Results will guide HPV vaccination promotion, education, and intervention efforts. CCLs provide an opportunity to study the adaption, integration, and enhancement of evidence-based approaches to increase HPV vaccination.
Keywords: Community health services; cervical cancer; community networks; health services; prevention; vaccination.

Publication types
- Multicenter Study
- Research Support, N.I.H., Extramural
- Research Support, Non-U.S. Gov't
- Community Health Services*
- Delivery of Health Care / methods*
- Health Knowledge, Attitudes, Practice
- Papillomavirus Infections / prevention & control*
- Papillomavirus Vaccines / administration & dosage*
- Patient Acceptance of Health Care
- Uterine Cervical Neoplasms / prevention & control
- Vaccination / methods*
- Vaccination / psychology
- Papillomavirus Vaccines
Grants and funding
- U48 DP005006/DP/NCCDPHP CDC HHS/United States
- U48 DP005021/DP/NCCDPHP CDC HHS/United States
- P30 CA086862/CA/NCI NIH HHS/United States
- U48 DP005013/DP/NCCDPHP CDC HHS/United States
- U48 DP005000/DP/NCCDPHP CDC HHS/United States
- U48DP005014/ACL/ACL HHS/United States
Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.
Search life-sciences literature (44,143,407 articles, preprints and more)
- Free full text
- Citations & impact
- Similar Articles
A multi-site case study of community-clinical linkages for promoting HPV vaccination.
Author information, affiliations.
- Brandt HM 1
- Zubizarreta M 1
- Vanderpool RC 2
- Stradtman LR 2
- Daniel-Ulloa J 5
- Seegmiller L 5
ORCIDs linked to this article
- McClam M | 0000-0002-0953-8945
Human Vaccines & Immunotherapeutics , 03 Jun 2019 , 15(7-8): 1599-1606 https://doi.org/10.1080/21645515.2019.1616501 PMID: 31158042 PMCID: PMC6746520
Free full text in Europe PMC
Abstract
Free full text , a multi-site case study of community-clinical linkages for promoting hpv vaccination, heather m. brandt.
a Department of Health Promotion, Education, and Behavior, University of South Carolina Arnold School of Public Health, Columbia, SC, USA
Robin C. Vanderpool
b Department of Health, Behavior and Society, University of Kentucky College of Public Health, Lexington, KY, USA
Susan J. Curry
c Department of Health Management and Policy, University of Iowa, Iowa City, USA
Paige Farris
d Oregon Health and Science University School of Public Health, Portland, QR, USA
Jason Daniel-Ulloa
e Department of Community & Behavioral Health, University of Iowa College of Public Health, Iowa City, IA, USA
Laura Seegmiller
Lindsay r. stradtman.
f Department of Health Services, University of Washington School of Public Health, Seattle, WA, USA
Victoria Taylor
Maria zubizarreta.
Human papillomavirus (HPV) vaccination rates in the U.S. are suboptimal, requiring innovative partnerships between community and clinical entities to remedy this issue. A rigorous evaluation of HPV-related community-clinical linkages (CCLs) was conducted to understand their components, processes, and outcomes to increase HPV vaccination. Cancer Prevention and Control Research Network (CPCRN) investigators explored CCLs in their communities employing an iterative, case study approach. Information describing nine CCLs on HPV vaccination was collected from representatives from the community organization and clinical setting. Thematic content analysis was used to analyze and interpret data. Five CCLs included a federally qualified health center as the clinical partner, and five included a non-profit organization as the community partner. Five reflected clinically focused integration wherein engagement occurs in the community but vaccine delivery and follow-up occur in the clinical setting. The main impetus was the need to improve HPV vaccination and a community’s strong interest in preventing cancer. Noted critical components were a designated person to support the CCL and funding. Results will guide HPV vaccination promotion, education, and intervention efforts. CCLs provide an opportunity to study the adaption, integration, and enhancement of evidence-based approaches to increase HPV vaccination.
- 1. Introduction
Human papillomavirus (HPV) accounts for a large proportion of cervical, anal, penile, vaginal, vulvar, and oropharyngeal cancers in the United States (U.S.). 1 HPV vaccination is recommended for females and males aged 9–26; however, the primary target for HPV vaccination is children aged 11–12 as part of the guideline-recommended adolescent immunization platform covered by public and private insurers. 2 – 6 Despite these recommendations and financial coverage, uptake remains well below the Healthy People 2020 national goal of 80% for adolescents aged 13–17 with only 65% of adolescent girls and 56% of boys having received 1 dose and only 50% of girls and 38% of boys having completed the three-dose series (up-to-date) in 2016. 7 , 8
To date, the majority of HPV vaccination interventions have largely focused on clinical setting implementation, increasing community demand, or improving access to vaccination. 9 , 10 In particular, interventions have targeted provider recommendation through clinical setting changes, such as provider reminders, standing orders, or provider assessment and feedback, because of the noted influence on parental/adolescent immunization behaviors, including initiation and uptake. 10 Most community-based HPV vaccination interventions have occurred in school settings or have been linked in some way to schools. 9 In addition, interventions aimed at expanding access to and community awareness of the HPV vaccine have occurred in pharmacy, mobile clinic, and dental settings, and through the use of strategic health communication campaigns. 11 , 12 Despite previous HPV vaccination interventions, uptake, and completion of the HPV vaccine series remains a public health challenge. 13 – 15 Innovative and coordinated partnerships between vested stakeholders and implementation of evidence-based prevention strategies in clinical and community settings offers a way to connect these settings. 16 Community-clinical linkages (CCLs), which are “…collaborations between health care practitioners in clinical settings and programs in the community – both working to improve the health of people and the communities in which they live”, 17 build upon this concept by linking community programs and clinical practice settings together, thereby capitalizing on the synergy of these two settings and their collective resources. 18 – 21
CCLs have been used to enhance referral services and train providers on methods to improve clinical practices, 22 including heart disease among women, cancer prevention and control, obesity prevention, promotion of physical activity, tobacco cessation, and influenza immunizations. 17 , 22 – 26 The integration of CCLs can vary, with either a mutual focus, or one that more heavily favors the clinical or community setting. 27 Building on the success of CCLs in other health domains and in connecting providers and community organizations, one of the overarching goals of this study was to provide a formal assessment of CCLs focused specifically on HPV vaccination. 17 , 22 , 28 Effective CCLs may improve HPV vaccine promotion and programmatic sustainability, HPV awareness and knowledge among linkage constituents, and patient/parent demand resulting in increased HPV vaccination rates. Therefore, the purpose of this study was to assess the mechanics of CCLs dedicated to HPV vaccination, including types of clinical and community partners, CCL impetus, available HPV vaccination services, outcomes evaluation, linkage sustainability, and partnership dynamics 18 , 22 , 29 in five diverse geographic sites.
Table 2 presents a summary of descriptive results of the nine HPV vaccination-focused CCLs examined for this analysis. Following is a more detailed description of the results by construct.
Construct table and interview guide questions for community and clinical linkage leaders.
Descriptive characteristics of HPV vaccination community-clinical linkages.
2.1. Descriptive information
Community sites were defined broadly and diversely, and reflected where people live, work, learn, play, and pray. Clinical sites were defined as having the ability to administer clinical services (i.e. HPV vaccination). Five of the nine CCLs included a FQHC as the clinical site. The missions of the community and clinical sites varied; however, the mission of all clinical sites included a focus on delivering health care to patients/clients. The mission of all community sites reflected health and human services interests. The missions of both community and clinical sites permitted a focus on HPV vaccination, and in some cases, the mission mandated a focus on vaccination (e.g., immunization coalition). Staffing reflected the nature of the site. For example, clinical settings were staffed with health-care providers. The populations served by the CCLs varied. Two of the nine CCLs focused on a targeted population (e.g., Hispanic/Latino). A focus on uninsured or underserved populations was less clear. There was some mention of this in the transcripts, but it was not clearly communicated whether vaccination of these populations was a priority among the interviewed CCL representatives. Geographical characteristics of locations served were mixed, as three of nine CCLs were rural-located.
2.2. Type of integration
When examining engagement (i.e., engaging individuals in need of a service), delivery (i.e., administering the service), and follow up as key indicators of the type of CCL integration, six CCLs exhibited clinically focused integration and three exhibited equally shared integration. In clinically focused integration, engagement occurs in the community setting but delivery and follow-up occur in the clinical setting. For example, a community site may work to increase HPV vaccination awareness and provide referrals to a clinical site. The clinical site delivers HPV vaccination and follows up with patient care. In equally shared integration, engagement, delivery, and follow-up occur in both the community and clinical spaces. For example, a pharmacy (community) and primary care clinic (clinical) both identify individuals in need of vaccination (i.e., engagement), provide education and administer HPV vaccines (i.e., delivery), and follow-up occurs by ensuring accurate documentation of vaccination and plan for subsequent doses.
2.3. Impetus
Impetus examines the influences behind the CCL (e.g., in response to leadership or policies at the national, state, or local level), and whether a focus on HPV vaccination is supported (i.e., both encouraged and resourced). Across all CCLs, the need to address HPV vaccination was noted as a priority with varying degrees of emphasis. In at least one example, a needs assessment was conducted revealing the necessity to focus on HPV vaccination. Other examples influencing the level of priority included the influence of an elected official (governor), state policy, and the efforts of state public health departments. At the local level, CCLs conducted work on a local level but due to a national initiative (e.g., national non-profit organization with a HPV vaccination initiative conducted in partnership with a local FQHC). CCLs reported wide-ranging differences in financial and personnel resources available. Resources contributed to the level of priority a community and/or clinical partner was able to devote to partnerships and increasing HPV vaccination.
2.4. Types of services offered
All clinical sites offered HPV9 as well as all ACIP-recommended vaccines. In some cases, CCLs had existing relationships on which a HPV vaccination focus was built. Community and clinical sites had previously partnered to educate individuals around various health issues, including HPV vaccination, through media and promotional campaigns. Responses about planned or future efforts demonstrated an interest in continuing existing efforts to increase HPV vaccination coverage as well as extend/expand efforts, but resources were identified as an important element influencing the feasibility and sustainability of these efforts. CCLs reported that limited training was offered, especially on HPV vaccination linkages, but there was some mention of national conferences, webinars, or community town hall meetings as training activities. Clinical settings referenced more training opportunities, especially for clinical staff to meet continuing education requirements.
2.5. Spanning support
Spanning support refers to the arrangements, processes, tools, resources, information systems, and surveillance data required to facilitate collaboration between the community and clinical entities. In order for community and clinical sites to work well together and achieve mutual goals, overcoming institutional boundaries is necessary. The main type of spanning support reported by CCLs was staff-related. A designated and dedicated person to oversee the CCL and ensure good communication and collaboration was essential. No specific information on the qualifications of the designated and dedicated person was provided by respondents; however, there was great emphasis on the need for a person to provide spanning support. Desired types of support focused on processes, resources, policies, and data required for the HPV vaccination CCLs. Examples of desired spanning support included: school mandates for vaccination, increased awareness and knowledge of HPV and need for vaccination among the general public, increased awareness about low HPV vaccination coverage among providers, effective routine communication between partners, organizational communication, stable or increased funding, improved administrative support, and enhanced data connectivity for the CCLs’ efforts to increase HPV vaccination.
2.6. Facilitators
Facilitating factors ranged from historical to substantive. The mutual interest and desire to increase HPV vaccination was a common theme across CCLs. Additional facilitating factors included a history of collaboration between entities, personal relationships between key staff members in both community and clinical sites, administrative support, and routine communication.
2.7. Barriers
CCL representatives from both community and clinical respondents identified several barriers, which reflected practical challenges associated with working across organizational boundaries on common issues and HPV vaccination. Barriers identified included the public’s lack of knowledge about HPV and need for vaccination, challenges talking about HPV due to its sexually transmitted nature, poor technology linkage between partners impeded expeditious communication, competing staff priorities, limited planning time to focus on the CCL and HPV vaccination, staff turnover, absence of protocols to guide work, and evaluation challenges due to data infrastructure issues or other factors influencing available data and usability. For three of these barriers, respondents provided expanded descriptions. Examples of lack of knowledge about HPV include a general sense of misunderstanding about the HPV vaccine, lack of familiarity with the needs for HPV vaccine, and unclear about recommendations for HPV vaccination (e.g., such as recommended for boys as well as girls). Competing priorities among staff included directives to staff to focus on other issues, and HPV vaccination was not always viewed as a priority. For example, in FQHC settings, HPV vaccination is not a current quality measure. FQHC staff perceived a need to focus on current quality measures. In terms of poor technology linkages between partners, the main focus was on challenges posed by where and who documents vaccination. For example, immunization registries, pharmacy records, and/or medical records are not always concordant or updated.
2.8. Evaluation
Limited information on evaluation plans and processes was provided by the collective interviewees. Eight of nine CCLs conducted some evaluation activities related to their collaborative efforts. The most common activities included the collection of clinical data on HPV vaccination, including patient-level data, dose completion data, and dose ordering data. Additional evaluation activities included surveys examining the public’s knowledge and beliefs about HPV and HPV vaccination, assessing the feasibility of pharmacy–clinic relationship for increasing HPV vaccinations, and estimated the reach of advertisements to promote HPV vaccination.
- 3. Discussion
This preliminary, qualitative case study provides insights from nine community-clinical collaborations across five geographic areas focused on improving HPV vaccination rates. We used a landscape assessment to explore potential CCLs in place, at various stages of collaboration, to further explore the CCL in terms of evidence indicating there was some work related to HPV vaccination being conducted. Community sites spanned non-profit organizations, faith-based organizations, and pharmacies, aligning with other HPV vaccination initiatives found in the literature. 30 – 33 Five of the nine clinical sites were FQHCs, an indicator of the commitment among clinical and community sites to providing preventive care to economically disadvantaged populations and a common site for research and quality improvement projects focused on HPV vaccination across the country. 34 , 35 The profiled linkages included urban and rural settings; inclusion of rural communities is particularly important given the disparities in HPV-related cancers in rural areas of the U.S. 11 Each CCL had unique characteristics, but several common themes emerged from these profiles.
First, the collaborations build on a strong national commitment to cancer prevention through effective vaccination. Seven CCLs indicated the importance of a national initiative as the impetus for the collaboration, either directly or through the local level. National HPV vaccination initiatives organized by the National Cancer Institute, Centers for Disease Control and Prevention, and American Cancer Society were particularly salient among these CCLs. Second, most CCLs relied on funding, with a majority being funded by both the clinical and community site. Third, there is a strong commitment to CCL evaluation efforts, reflected in eight of the nine collaborations indicating that they have conducted evaluations to determine input, process, and outcome indicators. 36 Finally, CCLs focused on HPV vaccination are strengthened when there are other areas of previous and/or current collaboration between organizations, suggesting the importance of sustained relationships and communication across partner sites.
Most CCLs were classified as clinically focused, meaning that the clinical site provides all of the vaccination services and follow-up, with community sites engaged primarily in outreach to target populations. Even with clinically focused CCLs, the most common barriers and needs described focused on creating demand and enhancing the public’s awareness and knowledge about HPV, which according to national surveillance data is still suboptimal, and particularly limited among minority and medically underserved populations. 37 – 39 This may reflect the need for communication tools and messages specifically targeting populations served in both the community and clinical settings, such as culturally appropriate and literacy-appropriate tools. It could also suggest the need for fewer barriers between outreach and behavior (i.e., more CCLs with equally shared integration that make vaccination available at the time of education and outreach).
Interviews with CCL leaders revealed a robust commitment to collaborative activities. All of the interviewees emphasized the importance of accountable staff, such as “HPV vaccination champions,” at each site that are recognized across organizations for their role in enhancing vaccination efforts. Champions – recognized as an implementation strategy – are commonly used to support, market, and drive changes across an organization and have been identified as an important player in creating a climate supportive of HPV vaccination. 40 – 42 This ‘spanning support’ needs to be strategically developed and encouraged by organizational leadership in both settings. Because CCLs fit well with the overarching concept of population health, establishing and maintaining CCLs can be encouraged as an effective approach to bring prevention and health care beyond the clinical setting and into the community.
This study has limitations to be noted. This assessment of the mechanics of CCLs was selective and should not be construed as representative of the total range of clinical-community collaborations focused on HPV vaccination. In addition, the qualitative approach to data collection was limited in terms of gathering specific details such as the composition of each community, the patient population of each clinical entity, the disciplinary and sectoral cultures of the community and clinic staff, and the clinics’ HPV vaccination rates. CPCRN site leaders knew the CCLs profiled and some of them involved university collaboration. Nonetheless, the assessment indicates that CCLs for HPV vaccination can be robust, sustained, and can provide generalizable best practices for broader dissemination.
Results will inform future HPV vaccination promotion, education, and interventional efforts across CPCRN sites and contribute to dissemination and implementation science focused on CCLs to improve public health, including the identification of opportunities to disseminate clear descriptions of effective CCLs as implementation strategies aimed at increasing HPV vaccination. 42 Specifically, the results may guide the development of strategies for community and clinical partners to collaborate in order to increase HPV vaccination rates. Indeed, CCLs are ripe for further study using an implementation science lens, including theoretical considerations, study designs, and implementation outcomes. 43 The focus on CCLs is novel given most HPV vaccination intervention-related research has occurred in one, but not both, clinical and community settings. 9 , 10 CCLs offer an opportunity to facilitate connections between previously developed clinical interventions and community-based interventions to, as one example, increase provider recommendation while also increasing community demand for vaccination 9 , 10 in a coordinated manner to maximize outcomes. It is important to note in this study we were unable to determine differentiation between CCLs for initiation and/or completion of the HPV vaccination series. As a result, examining the role of CCLs specifically in initiation and completion is an important area of exploration. CCLs provide an opportunity to study the adaption, integration, and enhancement of evidence-based approaches to increase HPV vaccination.
4.1. Setting
The Cancer Prevention and Control Research Network (CPCRN) is a network of academic, public health, and community partners, with a mission of accelerating adoption of evidence-based cancer prevention and control strategies, to ultimately reduce cancer burden. 44 CPCRN is a thematic research network of the Prevention Research Centers (PRC), a program funded by CDC and the National Cancer Institute. The network is comprised of eight institutions, and investigators from participating sites collaborate on cancer-related signature projects. The current study is the result of the CPCRN HPV Vaccination Signature Project workgroup, represented by the Oregon Health and Science University, University of Iowa, University of Kentucky, University of South Carolina, and University of Washington. The aim of the workgroup is to contribute to the science and evidence-base supporting innovative CCLs to increase HPV vaccination rates. To achieve this aim, the workgroup chose to focus on understanding the current status of HPV vaccination-focused CCLs across participating CPCRN sites to obtain in-depth information about existing CCLs in five sites, Iowa, Kentucky, Oregon, South Carolina, and Washington.
4.2. Selection of CCLs
Prior to CCL selections, the five CPCRN sites participated in a brief landscape assessment to identify HPV vaccination-related CCLs in which they are participating, of which they were aware, and/or interested in learning more about. Using an online survey, one respondent from each site responded to a cross-tabulated list of possible clinical and community dyads dedicated to HPV vaccination. Possible clinical sites included: primary care, federally qualified health center (FQHC), pediatric, obstetrics/gynecology, internal medicine, immunization clinic, sexually transmitted disease clinic, community hospital, school health center, and other. Community sites included faith-based entities, schools, pharmacies, community centers, community-based organizations, state/local health departments, and others. For each possible CCL, respondents indicated whether the CPCRN site was actively involved in the linkage, aware of but not actively involved in the linkage, not aware but interested in the linkage, or not interested in the CCL. Of the original 10 categories, three clinical sites – primary care, FQHCs, and internal medicine – were frequently endorsed for existing collaborations or aware of but not actively involved in collaborations. Faith-based organizations, state and local health departments, and pharmacies were frequently endorsed as community linkage sites. Based on landscape assessment results, the five CPCRN site respondents were then asked to identify two CCLs to be contacted for further insight into their HPV vaccination activities. To be selected, the CPCRN site confirmed that each of the two chosen CCLs were engaged in activities that promote, educate about, and/or deliver HPV vaccination services among their target populations and were actively working in this space (or recently completed work). For all sites, only two CCLs met these criteria. The CPCRN site respondent was either directly involved in the CCL or was familiar with/connected to the community and/or clinical partner through other cancer control collaborations. One site was unable to profile one of their selected CCLs due to confidentiality concerns (i.e., vulnerable population), therefore results from nine CCLs are included. The study protocol was approved by the Institutional Review Board at each of the five CPCRN sites.
4.3. Case examples
A conceptually driven case study approach was used to collect information describing the chosen CCLs from each of the five CPCRN sites. Krist and colleagues’ 27 integrative CCL framework guided the development of a standard interview protocol. This framework highlights three core components for effective collaboration in service delivery: engaging individuals in need of service (i.e. HPV vaccination), administering the service, and follow-up. Further, the model outlines critical stakeholders for achieving integration that spans each of these effective collaboration components: clinicians; community members and organizations; spanning personnel and infrastructure; national and/or state leadership; local leadership; and funders and purchasers. 27 As summarized in Table 1 , the semi-structured interview guide assessed descriptive information about each of the community and clinical sites, type of CCL integration (based on the Krist et al. 27 categories of mutual, community-focused, and clinically focused), impetus for the CCL, types of HPV vaccination services offered, spanning support, partnership facilitators and barriers, and evaluation activities.
Interviews were conducted with a knowledgeable leader at the clinical and community organizations, respectively, that were involved in each CCL. The respondents were most often directly involved with the CCL and could speak to site characteristics and specifics of the collaboration. To lessen participant burden and increase participation, interviews could be completed via email, telephone, in person, or a combination of these modes. Following the completion of the qualitative interviews, each CPCRN site independently completed an abstract form designed to facilitate retrieval of emergent information on the primary constructs identified in Table 1 for each interview and uploaded the original transcript and abstract form to a password-protected shared workspace. Abstract forms were independently reviewed by two investigators to confirm accuracy of the information extracted from the transcript. If discrepancies were noted, individual sites were asked to submit revised abstract forms and these were reviewed again by the investigators. The final abstract forms were used to guide thematic content analysis conducted by two investigators with a third investigator serving as an independent source to resolve conflicting interpretations. The first step in thematic content analysis was to use the Krist et al. framework to organize responses based on the conceptual model. 27 The interview guide was highly structured and allowed for organizing the data by type of CCL integration, impetus for the CCL, types of HPV vaccination services offered, spanning support, partnership facilitators and barriers, and evaluation activities. From there, two investigators carefully reviewed the data to glean emergent similarities and differences across the CCLs. This process was iterative and involved the two investigators conferring throughout the process to ensure a similar approach. However, a third investigator was instrumental in providing insight into interpretations when there were differences in the interpretation of the two investigators who conducted the analysis. This process continued until results were compiled and then presented to representatives from all five CPCRN sites for further discussion and confirmation.
- Funding Statement
This research is the result of work conducted by five of the Cancer Prevention and Control Research Network sites funded by the Centers for Disease Control and Prevention (CDC) and the National Cancer Institute (NCI). The research was supported by the following cooperative agreements from the CDC’s Prevention Research Centers (PRC) Program and the NCI. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the CDC and NCI. • Oregon Health & Science University: Dissemination, Implementation and Evaluation of Native STAND in American Indian Communities [U48 DP005006; PI: Becker] • University of Iowa: Health Promotion and Disease Prevention Research Centers [U48 DP005021; PI: Curry] • University of Kentucky: Appalachian Center for Cancer Education, Screening and Support [U48DP005014-01S2; PI: Vanderpool] • University of South Carolina: Multi-Level, Community-Clinical Cancer Prevention and Control Interventions [U48 DP005000-01S2; PI: Friedman] • University of Washington: Alliance for Reducing Cancer, Northwest [U48 DP005013; PI: Hannon] .
- Acknowledgments
Madisen Cotter, BUILD EXITO student at Portland State University in Oregon, actively participated in and contributed to the analyses of CPCRN CCL interviews for this manuscript. Kerri Lopez (Tolowa), Director of the Northwest Tribal Cancer Control Project at NPAIHB provided valuable insight into tribal considerations throughout the collaboration between authors and workgroup members. Jessica Seel, MPH, project coordinator at the University of South Carolina contributed to data collection. We would also like to thank Alexander Krist, MD, MPH at the Virginia Commonwealth University for his guidance on community-clinical linkage research and his review of this manuscript. This manuscript is dedicated to Frances Lee-Lin, PhD, RN, OCN, CNS of Oregon Health & Science University. Her contributions to our research were instrumental in the development of this manuscript.
- Disclosure of potential conflicts of interest
Dr. Heather Brandt has served as a member of the Merck US HPV Advisory Board. There are no other conflicts of interest to report.
Full text links
Read article at publisher's site: https://doi.org/10.1080/21645515.2019.1616501
Citations & impact
Impact metrics, citations of article over time, smart citations by scite.ai smart citations by scite.ai include citation statements extracted from the full text of the citing article. the number of the statements may be higher than the number of citations provided by europepmc if one paper cites another multiple times or lower if scite has not yet processed some of the citing articles. explore citation contexts and check if this article has been supported or disputed. https://scite.ai/reports/10.1080/21645515.2019.1616501, article citations, twenty years of collaborative research to enhance community practice for cancer prevention and control..
White A , Sabatino SA , White MC , Vinson C , Chambers DA , Richardson LC
Cancer Causes Control , 34(suppl 1):1-5, 16 May 2023
Cited by: 0 articles | PMID: 37191768 | PMCID: PMC10185931
Development and Feasibility Testing of the Clinical-Community Linkage Self-Assessment Survey for Community Organizations.
Fishleder S , Harris JR , Petrescu-Prahova M , Kohn M , Helfrich CD
Front Public Health , 10:797468, 20 May 2022
Cited by: 1 article | PMID: 35669755 | PMCID: PMC9163549
The Impact of a Comic Book Intervention on East African-American Adolescents' HPV Vaccine-Related Knowledge, Beliefs and Intentions.
Shin MB , Ko LK , Ibrahim A , Mohamed FB , Lin J , Celentano I , Shankar M , Amsalu F , Ali AA , Richardson BA , Taylor VM , Winer RL
J Immigr Minor Health , 24(6):1489-1500, 31 Mar 2022
Cited by: 0 articles | PMID: 35357620 | PMCID: PMC10129048
Disentangling the Role of Religiosity in Human Papillomavirus Vaccination Amidst COVID-19 Pandemic.
Olagoke AA , Floyd B , Caskey R , Hebert-Beirne J , Boyd AD , Molina Y
J Relig Health , 61(2):1734-1749, 03 Feb 2022
Cited by: 3 articles | PMID: 35112233 | PMCID: PMC8810213
Disparities in healthcare access and utilization and human papillomavirus (HPV) vaccine initiation in the United States.
Goel K , Vasudevan L
Hum Vaccin Immunother , 17(12):5390-5396, 04 Nov 2021
Cited by: 2 articles | PMID: 34736353 | PMCID: PMC8903982
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
- http://www.ebi.ac.uk/biostudies/studies/S-EPMC6746520?xr=true
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A Qualitative Study of Parental Knowledge and Perceptions of Human Papillomavirus and Cervical Cancer Prevention in Rural Central Java, Indonesia: Understanding Community Readiness for Prevention Interventions.
Spagnoletti BRM , Bennett LR , Wahdi AE , Wilopo SA , Keenan CA
Asian Pac J Cancer Prev , 20(8):2429-2434, 01 Aug 2019
Cited by: 12 articles | PMID: 31450917 | PMCID: PMC6852813
Evaluation of fotonovela to increase human papillomavirus vaccine knowledge, attitudes, and intentions in a low-income Hispanic community.
Chan A , Brown B , Sepulveda E , Teran-Clayton L
BMC Res Notes , 8:615, 29 Oct 2015
Cited by: 27 articles | PMID: 26514184 | PMCID: PMC4625467
Provider perceptions of barriers and facilitators of HPV vaccination in a high-risk community.
Javanbakht M , Stahlman S , Walker S , Gottlieb S , Markowitz L , Liddon N , Plant A , Guerry S
Vaccine , 30(30):4511-4516, 03 May 2012
Cited by: 38 articles | PMID: 22561142
Overcoming the barriers to HPV vaccination in high-risk populations in the US.
Downs LS , Scarinci I , Einstein MH , Collins Y , Flowers L
Gynecol Oncol , 117(3):486-490, 29 Mar 2010
Cited by: 55 articles | PMID: 20303156
"HPV? Never heard of it!": a systematic review of girls' and parents' information needs, views and preferences about human papillomavirus vaccination.
Hendry M , Lewis R , Clements A , Damery S , Wilkinson C
Vaccine , 31(45):5152-5167, 09 Sep 2013
Cited by: 86 articles | PMID: 24029117
Funding
Funders who supported this work.
ACL HHS (1)
Grant ID: U48DP005014
11 publication s
Centers for Disease Control and Prevention (5)
Grant ID: U48 DP005013
2 publication s
Grant ID: U48 DP005000-01S2
3 publication s
Grant ID: U48 DP005006; PI
1 publication
Grant ID: U48DP005014-01S2
Grant ID: U48 DP005021
NCCDPHP CDC HHS (4)
30 publication s
53 publication s
Grant ID: U48 DP005006
24 publication s
Grant ID: U48 DP005000
50 publication s
NCI NIH HHS (1)
Grant ID: P30 CA086862
2079 publication s
Europe PMC is part of the ELIXIR infrastructure
- Study protocol
- Open access
- Published: 07 September 2022
An overview of implementing an evidence based program to increase HPV vaccination in HIV community clinics
- Jessica Wells ORCID: orcid.org/0000-0003-1995-0383 1 ,
- James L. Klosky 2 , 3 ,
- Yuan Liu 4 , 5 &
- Theresa Wicklin Gillespie 5 , 6
BMC Public Health volume 22 , Article number: 1696 ( 2022 ) Cite this article
1419 Accesses
1 Altmetric
Metrics details
HPV-related anal cancer occurs in excess rates among people living with HIV (PLWH) and has been increasing in incidence. The HPV vaccine is an effective and safe approach to prevent and reduce the risk of HPV-related disease. Yet, HPV vaccine programs tailored and implemented in the HIV population are lagging for this high-risk group.
A pre-post intervention study design will be used to tailor, refine, and implement the 4 Pillars™ Practice Transformation Program to increase HPV vaccination among PLWH. Guided by the RE-AIM framework, the CHAMPS study will provide training and motivation to HIV providers and clinic staff to recommend and administer the HPV vaccination within three HIV clinics in Georgia. We plan to enroll 365 HIV participants to receive HPV education, resources, and reminders for HPV vaccination. Sociodemographic, HPV knowledge, and vaccine hesitancy will be assessed as mediators and moderators for HPV vaccination. The primary outcome will be measured as an increase in uptake rate in initiation of the HPV vaccine and vaccine completion (secondary outcome) compared to historical baseline vaccination rate (control).
The proposed study is a novel approach to address a serious and preventable public health problem by using an efficacious, evidence-based intervention on a new target population. The findings are anticipated to have a significant impact in the field of improving cancer outcomes in a high-risk and aging HIV population.
Trial registration
NCT05065840; October 4, 2021.
HPV-related anal cancer occurs in excess rates among people living with HIV (PLWH) [ 1 ], and has been increasing in incidence [ 1 ]. Notably, the incidence of anal cancer among men who have sex with men (MSM) is 20- to 40- fold greater relative to non-MSMs [ 2 ]. The Human Papillomavirus (HPV) is responsible for 90% of anal cancers where oncogenic HPV type 16 is responsible for 90% of anal cancers [ 3 ]. It is presumed the increased risk for anal cancer among PLWH is due to an impaired ability to clear HPV infections and increased reactivation of latent HPV infection. Of note, highly active antiretroviral therapy (HAART) has modest to no effect on HPV clearance or persistence; thus, other mechanisms may be involved that result in cellular immune dysfunction [ 4 ].
The safety and efficacy of the HPV vaccine has been evaluated in PLWH and is shown to be safe and highly immunogenic against oncogenic HPV types 16 and 18 [ 5 , 6 , 7 , 8 ]. The HPV vaccine also has been shown to decrease the risk of HPV-related anal intraepithelial neoplasia in a sample of MSMs [ 9 ]. Thus, anal cancer can be potentially a preventable disease through the use of the HPV vaccine [ 3 ]. However, very limited research has been conducted on the uptake of HPV vaccination among PLWH. One study found among a sample of young MSM’s who self-reported as HIV-positive, HPV vaccine initiation was 13.4% [ 10 ]. Although uptake is low, studies of the acceptability of the HPV vaccine has been found to be high among high risk groups like MSMs [ 11 , 12 , 13 ].
The United States’ Advisory Committee on Immunization Practices (ACIP) recommends vaccination up to age 26 years and recently FDA (Food and Drug Association) approved up to age 45 years for women and men [ 14 ]. ACIP also advises individuals who are immunocompromised to receive the 3-dose series of the HPV vaccine up to age 26 years of age and with shared clinical decision making for those 26 years and older. The Center for Disease Control and Prevention (CDC) urges catchup vaccination for adults who have not been previously vaccinated and remain vulnerable to develop preventable HPV-related cancers [ 15 ]. Yet, there is a dearth of studies that have tailored and implemented evidence-based approaches to promote HPV vaccination among PLWH and eligible for catchup vaccination. Since intervention development is costly, complex, and time consuming, we seek to refine and tailor an existing, evidence-based intervention and integrate in a new population and new setting. The CDC’s 4 Pillars™ Practice Transformation Program (4 Pillars™ Program) is a robust and empirically supported strategic approach that promotes the uptake of adult vaccinations and addresses facilitators and barriers at the patient, provider, and clinic level [ 16 ]. The 4 Pillars™ Program incorporates these recommendations via “a menu” of strategies to promote the establishment and maintenance of vaccination into routine practice (Table 1 ).
The 4 Pillars™ Program has shown to improve vaccination rates among high risk adults in primary care practices that successfully implemented strategies across the program [ 17 , 18 ]. A randomized controlled cluster trial (RCCT) found the 4 Pillars Program significantly increased HPV vaccination among a cohort of 10,861 adolescent patients in primary care practices [ 19 ]. The intervention sites increased baseline HPV vaccination by 10.2 percentage points (PP) versus 7.3 PP in the control sites ( p < .001) [ 19 ]. Furthermore, another large RCCT of adolescents found the 4 Pillars™ Program significantly increased baseline initiation of HPV vaccination by 17.1 PP ( p < .001) and increased HPV completion by 14.8 PP ( p < .001) [ 20 ]. These findings highlight the effectiveness of the 4 Pillars™ Program to increase HPV vaccination in the general population.
The Advancing HPV vaccination for HIV Positive Adults (CHAMPS) study seeks to expand the success of the 4 Pillars™ Program and tailor, refine, and implement in the HIV positive population, who are at high risk for HPV-related cancers and can obtain the most benefit from the vaccine. The strategies selected from the 4 Pillars™ Program are based on an extensive review of the HIV and related literature (Table 2 ) [ 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 ]. The intervention will be implemented in three HIV community clinics in Georgia, USA and enroll n = 365 PLWH, age 18–45 years, from those clinics. Guided by the RE-AIM framework, the proposed specific aims are:
Tailor and refine the 4 Pillars™ program for implementation in three HIV community clinics in Georgia.
Test the effectiveness of the 4 Pillars™ program as measured by an increase in uptake rate in initiation of the HPV vaccine (primary outcome) and vaccine completion (secondary outcome) compared to historical baseline vaccination rate (control) among PLWH. It is hypothesized after implementation of the 4 Pillars™ program, we estimate an uptake rate of > = 13.5% in initiation of HPV vaccination.
Identify mediators and potential moderators (HPV knowledge and awareness and vaccine hesitancy) of the intervention effects on HPV vaccination.
Assess the sustainability of the intervention in vaccine uptake post-intervention and assess scalability of the program for wider implementation via a future national randomized control trial.
Methods/design
A pre-post intervention study design is used where HPV vaccination initiation and completion rates are measured before and after the intervention across the same clinics and enrolled participants (Fig. 1 ). HPV vaccination uptake 18 months before intervention will be queried from electronic medical records (EMR) and Georgia Registry of Immunization Transactions and Services (GRITS), which will serve as the historical control. GRITS is a population-based web application containing consolidated demographic and immunization history information. The use of a concurrent control is to reassess the background HPV uptake rate among PLWH during an adjacent time-period to post-intervention and similar population resources. The comparison of HPV vaccination rates pre- and post-intervention in the three selected representative HIV clinics in Georgia will be an exploratory goal of the trial due to the retrospective approach in the control phase and the prospective approach in the post intervention phase. A “within” analysis will be conducted to compare sites both pre- and post-intervention.
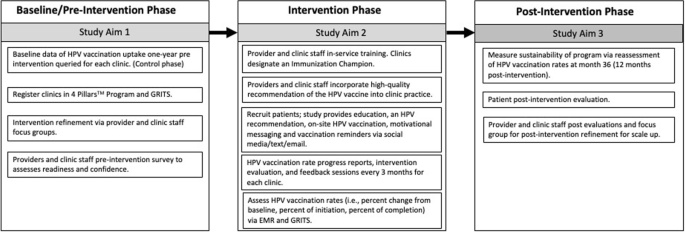
Schematic overview of the CHAMPS study
Clinic selection and patient eligibility
Three HIV community clinics for this study were selected due to agreement to participate in the study, granted study access to electronic medical records, and willingness to make office changes to increase vaccination rates. Patients will be recruited from these three clinics and enrolled in the study based on the following eligibility criteria: 1) HIV positive; 2) 18–45 years of age; 3) understand English; 4) capable of informed consent; 5) have not received or completed the three dose HPV vaccine; 6) no contraindications to receiving the HPV vaccine (i.e., history of an anaphylactic allergy to latex, an immediate hypersensitivity to yeast, current moderate or severe acute illness, and/or are currently pregnant).
Pre-implementation approach
During the pre-intervention phase of the trial, each clinic site enrolls patients who receive immunizations using the GRITS system. The GRITS system offers a variety of functions for health care providers including recording immunizations, validating immunization history, providing immunization recommendations, producing recall and reminder notices, generating vaccine usage and client reports, and performing data extraction. Clinics will register with the 4 Pillars™ Program and clinic staff will complete a pre-intervention survey that assesses readiness and confidence in increasing HPV vaccination and current vaccination practices. Providers and clinic staff will be asked to participate in a focus group for feedback on tailoring the intervention for their clinic population prior to program implementation.
Clinic-level intervention approach
The 4 Pillars™ Program offers providers and clinic staff evidence-based strategies to increase HPV vaccination uptake via training and educational resources. This program will be refined to provide tailored training and motivation to HIV providers and clinic staff to recommend and administer the HPV vaccine to HIV patients at the infectious disease clinic. Providers and clinic staff who are interested in participating will “enroll” online and complete an electronic informed consent before participating in the focus groups and completing the evaluation surveys. Providers and clinic staff are offered an opportunity to attend an in-service training that will provide education, training, and resources to help increase HPV vaccination at their clinic. Participation in the in-service will be offered to the entire clinic with opportunity for continuing education (CE) units to be earned. The in-service component is delivered under the purpose of quality improvement and does not require informed consent to attend.
Components of the in-service training consist of education and resources related to the 4 Pillars™ program, epidemiology of HPV-related cancers among HIV positive individuals, ACIP’s guidelines for HPV vaccination for immunosuppressed patients, safety profile of the vaccine, and the importance and effectiveness of delivering evidence-based recommendations for HPV vaccination. Providers and clinic staff who are within scope of practice to administer the HPV vaccine are asked to recommend and administer the HPV vaccine to eligible patients during each routine clinic visit. Consenting providers and clinic staff are asked to complete pre-intervention evaluations, an intervention evaluation every 3 months, and post-intervention evaluations via a secured link to complete online. Alternatively, paper copies will be provided to the providers and clinic staff and administered by an Immunization Champion to those who choose not to access the evaluations by email.
Each clinic site identifies an Immunization Champion (a medical assistant, nurse, or clinic manager) who will work and motivate the clinic staff and participate in biweekly updates of progress with the research coordinator. The Immunization Champion (IC) will encourage, remind, and ensure timely documentation of the HPV vaccination within the clinic’s electronic medical records and within GRITS. The IC helps maintain stock and storage of the vaccine and identify and address any issues with the vaccine inventory. The IC assists patients to complete Merck’s Patient Assistance Program to cover vaccination for those who are uninsured and qualify for the program. Lastly, the IC will contact patients on the monthly call list to schedule appointments or assist in reminders to patients to schedule the next visit for the follow up HPV vaccine.
Clinics receive a progress report that documents the clinic’s vaccination progress every three months. The research coordinator schedules group feedback sessions via in-person or webinar with the IC every three months to discuss: 1) the intervention evaluations completed by the providers and staff; 2) to learn of any barriers to HPV vaccination at the clinic; 3) brainstorm strategies to overcome the barriers; and 4) quality assurance of intervention fidelity.
Patient-level intervention approach
Eligible and consenting participants ( n = 365) will be part of the intervention group and will receive recommendation for the HPV vaccine from providers and clinic staff. Enrolled participants will also complete a self-administered questionnaire at enrollment on a HIPPA compliant online data management database on a tablet device. The survey questions will include sociodemographic characteristics, knowledge of and attitudes towards HPV, HPV vaccination, and anal cancer, and vaccine hesitancy. Participants will be requested to provide consent for their HPV vaccination history to be verified with electronic EMR and GRITS. Participants will then watch a short video on HPV and HPV vaccination that can be viewed on their phones (or the study’s tablet device) while waiting to be seen. Potential participants will be asked to follow the study’s private Facebook page which offers additional educational information tailored towards individuals with HIV on HPV-related disease and general health promotion, and risk reduction tips. The Facebook page will also utilize Facebook Messenger (commonly known as Messenger). The proposed study will utilize Messenger to send reminders for follow up appointments for the next shot in the series and motivational messaging to encourage and promote receipt of the HPV vaccine. Participants will be contacted to complete a post-evaluation survey administered via online, telephone, or a mailed paper copy at 6–9 months after baseline. Participants will receive $25 incentive for completion of baseline and follow up study activities.
Data analysis plan
Study outcomes.
Initiation of the HPV vaccine is the primary outcome endpoint (Fig. 2 ). Initiation of the HPV vaccine is defined as receiving the first or second immunization from the series. This variable will be measured by electronic medical records and GRITS at baseline (historical control) and 24 months post baseline. We hypothesize the initiation rate will be higher than the historical control rate. Completion of the HPV vaccine is the secondary outcome variable. Completion is defined as receiving all three immunizations from the series, regardless of time. This variable will be measured by electronic medical records and GRITS baseline (control) and 24 months post baseline.
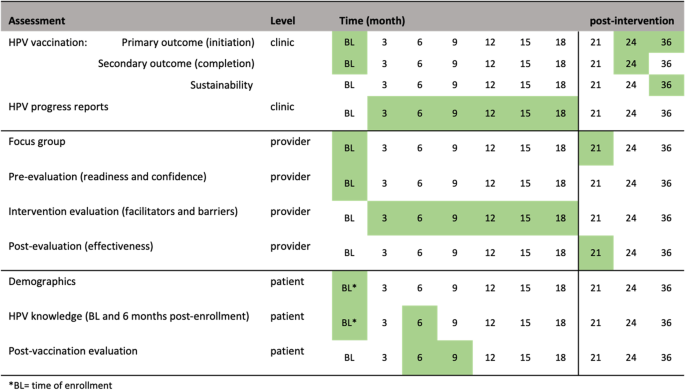
Summary of study assessments and time of collection
Process evaluation
The RE-AIM (Reach, Effectiveness/Efficacy, Adoption, Implementation, Maintenance) framework will guide planning, implementation, and evaluation of the 4 Pillars™ program. The study’s reach will be estimated from a quantitative perspective by estimating the target population that was exposed to the intervention. The clinic’s patient census data during the period of the implementation phase will be used to estimate the likely reach of the program across sites. Intervention effectiveness and efficacy will be measured by the change in uptake rate of vaccination (i.e., intervention effectiveness). We will calculate the percent change in initiation of the vaccine and percent change in completion of the vaccine from the control phase and 24 months post intervention, after adjusting for demographics differences (age, gender, race, healthcare insurance) in population. Providers and clinic staff will be asked to complete an evaluation of HPV vaccination progress every 3-months. We will also collect qualitative data from feedback sessions with the immunization champions to assess opportunities for and barriers to adoption. The post-evaluation interviews with providers and staff who implemented the program will assess extent of involvement, acceptance of the intervention, implementation fidelity, and extent of organizational spread of the intervention. We will assess the frequency, duration, and the extent to which the intervention was implemented as planned, participation attendance, and costs of implementation, as measured via the intervention and post-intervention evaluations. Intervention sustainability will be measured as the gains or maintenance of HPV vaccination rates post-delivery of the intervention. HPV vaccination rates will be calculated via EMR and GRITS at month 36 (12 months post intervention) and compare to HPV vaccination rates at month 24. Follow up assessments will measure penetration or the extent to which recommendation and administration of the HPV vaccination is integrated within the clinic.
Data analyses of primary outcomes
For analysis of the primary (HPV vaccination initiation) and secondary endpoints (HPV vaccination completion), the uptake rate of HPV vaccination pre- and post-intervention will be estimated separately with a 95% exact confidence interval using all eligible cases from both phases. The one-sample binomial exact test will be used to test whether the rate after the intervention is higher than the baseline rate (P0 = 13.5%). We will also perform the Chi-square test to compare the rate change between control and interventional phases, which will be exploratory. Logistic regression will be used to further adjust background difference in study population in pre- and post- intervention phases. For the longitudinal data collected from the intervention phase, the data structure holds multi-level information from patients, providers, and clinic levels. The goal of the analyses is to identify the factors that might impact HPV uptake from each level of information, which might lead to the future improvement of implementation strategy. Data will be described using summary statistics (e.g., quartiles, median, mean, standard deviation) for continuous variables and marginal distribution (frequency and percentage) for categorical variables. The univariate association with the HPV vaccination (yes vs. no) will be tested in logistic regression for each variable separately. The change of survey response between baseline and a follow-up time point will be tested by paired tests (e.g., paired t-test, McNemar test). Along with data visualization, all above mentioned descriptive and univariate association analyses will be repeated within each of the three clinics. Data will be pooled to build the multilevel analysis models, we mainly consider using the mixed-effect model and/or Bayesian multilevel modeling, in which the random effect will be considered at provider and clinical levels. We will follow the key modeling considerations listed by J.J. Hox [ 29 ] to identify the significant mediators and moderators at different levels that impact the uptake of HPV vaccination.
Secondary aims
To assess sustainability, HPV vaccination rates will be calculated at month 36 (12 months post-intervention). The change in HPV vaccination rates between month 24 and month 36 (12 months post-intervention) will be tested by McNemar test. The intervention will be deemed as sustaining its effect if rates of HPV vaccine initiation rates at month 36 remains or increases from month 24’s vaccination rates. To inform future scalability of the program, data from the evaluation and post-evaluation surveys will be described using summary statistics (e.g., quartiles, median, mean, standard deviation) for continuous variables and marginal distribution (frequency and percentage) for categorical variables. The similar analyses will be repeated within each of the three clinics. Additionally, we will conduct a post-intervention focus group consisting of the providers and clinic staff that participated in the program. The qualitative evaluation explore how implementation took place; the barriers to and facilitators of implementation success; ways to address any problems that may have occurred; and recommendations to refine the intervention for scale-up. The focus session will be recorded and transcribed where themes will be extracted and used for adaptation, scale-up considerations, and future research directions.
Statistical power
Based on our preliminary data, we found that HPV vaccination rate is around 13.5% (P0) in general, and another larger study in the literature found a very similar rate of 13.6% [ 10 ]. We powered the study to detect an uptake rate > 13.5% after the intervention. Thus, a sample size of 317 achieves 80% power to detect a superiority difference of 5% (PB-P0) using an exact one-sided test with a significance level (alpha) of 0.05. We anticipate a 5% superiority difference is reasonable to achieve with an uptake rate of 18.5% (PB) after the intervention. We will be able to reject the Null hypothesis and claim the uptake rate of > 13.5% after 54 patients have initiated the HPV test. After taking about 15% of the drop-off rate into account, we plan to include 365 participants among 3 clinics for the intervention phase. The calculation was by PASS 2020 for testing superiority of one proportion using the Exact test. The Null hypothesis is P < = 13.5% and the alternative hypothesis is P > 13.5%. For the control phase, we will query all eligible subjects from EMR database among the 3 clinics at 18 months pre-intervention, which could be approximately 2300 subjects [ 30 ]. Assuming we end up with the same number of subjects in both control ( N = 317) and intervention phase ( N = 317), we will have 81% statistical power to detect an HPV uptake rate difference of 9% (22.5% vs. 13.5%) by two-sided Fishers’ Exact Test and under significance level of 0.05. We anticipate such a difference would be feasible based on our best knowledge and the literature. Regarding the patient- level component of the intervention, all incoming eligible patients will be influenced, and hence 365 participants are the minimum number to capture the follow-up information, the actual number of sample size used for the calculation of HPV vaccination rate will be larger as the vaccination status will be captured automatically in EMR without a consent.
The underutilization of HPV vaccination is a national problem that has been identified by the President’s Cancer Panel as a serious but correctable threat to the progress against cancer [ 31 ]. However, few studies have focused on the high-risk HIV population—an aging population that is increasingly managing other co-morbidities with their HIV diagnoses, including cancer. HPV vaccination is a form of primary cancer prevention that is imperative for a successful cancer control plan that may reduce the untimely death and clinical burden of HIV patients from several potentially vaccine-preventable HPV-related cancers including anal, cervical, vulvar, vaginal, penile, and oropharyngeal cancers. With an aging HIV population, it is an essential public health goal to provide the necessary resources and cancer prevention strategies for PLWH to achieve a normal life expectancy and quality of life. The CHAMPS study is the next step to achieving this goal for high-risk HIV-positive populations.
NCT05065840; Registered on October 4, 2021.
Availability of data and materials
The datasets used and/or analyzed during the current study will be available from the corresponding author on reasonable request.
Abbreviations
Human Papillomavirus
Human Immunodeficiency virus/ Acquired immunodeficiency syndrome
People living with HIV
Men who have sex with men
Highly active antiretroviral therapy
Food and Drug Administration
Centers for disease control and prevention
Percentage points
Advancing HPV vaccination in HIV positive adults
Reach, Effectiveness/Efficacy, Adoption, Implementation, Maintenance
Electronic medical records
Georgia Registry of Immunization Transactions and Services
Continuing education
Immunization champion
Power analysis & Sample size
Khandwala P, Singhal S, Desai D, Parsi M, Potdar R. HIV-Associated Anal Cancer Cureus. 2021;13(5):e14834.
PubMed Google Scholar
Silverberg MJ, Lau B, Justice AC, Engels E, Gill MJ, Goedert JJ, et al. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis. 2012;54(7):1026–34.
Article PubMed PubMed Central Google Scholar
De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta analysis. Int J Cancer. 2009;124(7):1626–36.
Article PubMed Google Scholar
Shrestha S, Sudenga SL, Smith JS, Bachmann LH, Wilson CM, Kempf MC. The impact of highly active antiretroviral therapy on prevalence and incidence of cervical human papillomavirus infections in HIV-positive adolescents. BMC Infect Dis. 2010;10(1):295.
Giuliano AR, Palefsky JM, Goldstone S, Moreira ED Jr, Penny ME, Aranda C, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364(5):401–11.
Article CAS PubMed PubMed Central Google Scholar
Palefsky JM, Gillison ML, Strickler HD. HPV vaccines in immunocompromised women and men. Vaccine. 2006;24:S140–S6.
Article Google Scholar
Kahn JA, Xu J, Kapogiannis BG, Rudy B, Gonin R, Liu N, et al. Immunogenicity and safety of the human papillomavirus 6, 11, 16, 18 vaccine in HIV-infected young women. Clin Infect Dis. 2013;57(5):735–44.
Wilkin T, Lee JY, Lensing SY, Stier EA, Goldstone SE, Berry JM, et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis. 2010;202(8):1246–53.
Palefsky JM, Giuliano AR, Goldstone S, Moreira ED Jr, Aranda C, Jessen H, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365(17):1576–85.
Article CAS PubMed Google Scholar
Meites E, Markowitz LE, Paz-Bailey G, Oster AM, Group NS. HPV vaccine coverage among men who have sex with men–national HIV behavioral surveillance system, United States, 2011. Vaccine. 2014;32(48):6356–9.
Cummings T, Kasting ML, Rosenberger JG, Rosenthal SL, Zimet GD, Stupiansky NW. Catching up or missing out? Human papillomavirus vaccine acceptability among 18- to 26-year-old men who have sex with men in a US National Sample. Sex Transm Dis. 2015;42(11):601–6.
Nadarzynski T, Smith H, Richardson D, Pollard A, Llewellyn C. Perceptions of HPV and attitudes towards HPV vaccination amongst men who have sex with men: a qualitative analysis. Br J Health Psychol. 2017;22(2):345–61.
Reiter PL, Brewer NT, McRee A-L, Gilbert P, Smith JS. Acceptability of HPV vaccine among a national sample of gay and bisexual men. Sex Transm Dis. 2010;37(3):197.
FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old [press release]. 2018.
Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, et al. Human papillomavirus vaccination: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2014;63(5):1–30.
Abdool Karim SS, Passmore JS, Baxter C. The microbiome and HIV prevention strategies in women. Curr Opin HIV AIDS. 2018;13(1):81–7.
Nowalk MP, Moehling KK, Zhang S, Raviotta JM, Zimmerman RK, Lin CJ. Using the 4 pillars™ to increase vaccination among high-risk adults: who benefits? Am J Manag Care. 2017;23(11):651.
PubMed PubMed Central Google Scholar
Zimmerman RK, Brown AE, Pavlik VN, Moehling KK, Raviotta JM, Lin CJ, et al. Using the 4 pillars practice transformation program to increase pneumococcal immunizations for older adults: a cluster-randomized trial. J Am Geriatr Soc. 2017;65(1):114–22.
Zimmerman RK, Moehling KK, Lin CJ, Zhang S, Raviotta JM, Reis EC, et al. Improving adolescent HPV vaccination in a randomized controlled cluster trial using the 4 pillars™ practice transformation program. Vaccine. 2017;35(1):109–17.
Zimmerman RK, Raviotta JM, Nowalk MP, Moehling KK, Reis EC, Humiston SG, et al. Using the 4 pillars™ practice transformation program to increase adolescent human papillomavirus, meningococcal, tetanus-diphtheria-pertussis and influenza vaccination. Vaccine. 2017;35(45):6180–6.
Apaydin KZ, Fontenot HB, Shtasel D, Dale SK, Borba CP, Lathan CS, et al. Facilitators of and barriers to HPV vaccination among sexual and gender minority patients at a Boston community health center. Vaccine. 2018;36(26):3868–75.
Gorbach PM, Cook R, Gratzer B, Collins T, Parrish A, Moore J, et al. Human papillomavirus vaccination among young men who have sex with men and transgender women in 2 US cities, 2012–2014. Sex Transm Dis. 2017;44(7):436.
Klosky JL, Hudson MM, Chen Y, Connelly JA, Wasilewski-Masker K, Sun C-L, et al. Human papillomavirus vaccination rates in young cancer survivors. J Clin Oncol. 2017;35(31):3582.
Reiter PL, Katz ML, Bauermeister JA, Shoben AB, Paskett ED, McRee A-L. Recruiting young gay and bisexual men for a human papillomavirus vaccination intervention through social media: the effects of advertisement content. JMIR Public Health Surveill. 2017;3(2):e33.
Subasinghe AK, Nguyen M, Wark JD, Tabrizi SN, Garland SM. Targeted Facebook advertising is a novel and effective method of recruiting participants into a human papillomavirus vaccine effectiveness study. JMIR Res Protocols. 2016;5(3):e154.
Wells JS, Flowers L, Paul S, Nguyen ML, Sharma A, Holstad M. Knowledge of anal cancer, anal cancer screening, and hpv in hiv-positive and high-risk hiv-negative women. J Cancer Educ. 2020;35(3):606–15.
York JM, Klosky JL, Chen Y, Connelly JA, Wasilewski-Masker K, Giuliano AR, et al. Patient-level factors associated with lack of health care provider recommendation for the human papillomavirus vaccine among young Cancer survivors. J Clin Oncol. 2020;38(25):2892–901.
Rand CM, Vincelli P, Goldstein NP, Blumkin A, Szilagyi PG. Effects of phone and text message reminders on completion of the human papillomavirus vaccine series. J Adolesc Health. 2017;60(1):113–9.
Hox JJ, Moerbeek M. Van de Schoot R. multilevel analysis: techniques and applications: Routledge; 2017.
Book Google Scholar
Georgia Department of Health. HIV surveillance summary, Georgia 2018. In: Section HAE, editor. 2020.
Panel PsC. HPV vaccination for cancer prevention: progress, opportunities, and a renewed call to action. A report to the president of the United States from the chair of the President’s Cancer Panel 2018.
Download references
Acknowledgments
The 4 Pillars™ Immunization Toolkit Materials are an evidence-based educational and support program developed by the University of Pittsburgh and funded by the Centers for Disease Control to increase immunizations.
This study was supported by National Institutes of Health, National Institute of Nursing Research, R01NR020154. The funders had no role in the study design, data collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and affiliations.
Nell Hodgson Woodruff School of Nursing, Emory University, 1520 Clifton Road, NE, RM. 230, Atlanta, GA, 30324, USA
Jessica Wells
Department of Pediatrics, School of Medicine, Emory University, Atlanta, GA, USA
James L. Klosky
Aflac Cancer and Blood Disorders Center, Children’s Healthcare of Atlanta, Atlanta, GA, USA
Departments of Biostatistics and Bioinformatics, Emory University, Atlanta, GA, USA
Winship Cancer Institute, Emory University, Atlanta, GA, USA
Yuan Liu & Theresa Wicklin Gillespie
Department of Surgery, Division of Surgical Oncology, School of Medicine, Emory University, Atlanta, GA, USA
Theresa Wicklin Gillespie
You can also search for this author in PubMed Google Scholar
Contributions
JW is responsible for the study design, oversight of the study and draft of the manuscript. JW, JK, YL, TG contributed to the writing and critical review of the manuscript. YL contributed the statistical analysis plan of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Jessica Wells .

Ethics declarations
Ethics approval and consent to participate.
Approval from Emory University’s Institutional Review Board and ethical approval from the participating clinic sites was obtained before data collection (IRB00002469). All eligible participants will provide written informed consent before study enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wells, J., Klosky, J.L., Liu, Y. et al. An overview of implementing an evidence based program to increase HPV vaccination in HIV community clinics. BMC Public Health 22 , 1696 (2022). https://doi.org/10.1186/s12889-022-14100-0
Download citation
Received : 18 August 2022
Accepted : 31 August 2022
Published : 07 September 2022
DOI : https://doi.org/10.1186/s12889-022-14100-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- HPV vaccination
- Implementation
BMC Public Health
ISSN: 1471-2458
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 17 May 2024
Epidemiology of HPV-associated cancers past, present and future: towards prevention and elimination
- Talía Malagón ORCID: orcid.org/0000-0002-9505-3564 1 , 2 , 3 ,
- Eduardo L. Franco ORCID: orcid.org/0000-0002-4409-8084 1 , 3 ,
- Romina Tejada ORCID: orcid.org/0000-0003-4420-5289 1 , 3 &
- Salvatore Vaccarella 4
Nature Reviews Clinical Oncology ( 2024 ) Cite this article
686 Accesses
22 Altmetric
Metrics details
- Cancer epidemiology
- Cervical cancer
- Head and neck cancer
- Risk factors
Cervical cancer is the first cancer deemed amenable to elimination through prevention, and thus lessons from the epidemiology and prevention of this cancer type can provide information on strategies to manage other cancers. Infection with the human papillomavirus (HPV) causes virtually all cervical cancers, and an important proportion of oropharyngeal, anal and genital cancers. Whereas 20th century prevention efforts were dominated by cytology-based screening, the present and future of HPV-associated cancer prevention relies mostly on HPV vaccination and molecular screening tests. In this Review, we provide an overview of the epidemiology of HPV-associated cancers, their disease burden, how past and contemporary preventive interventions have shaped their incidence and mortality, and the potential for elimination. We particularly focus on the cofactors that could have the greatest effect on prevention efforts, such as parity and human immunodeficiency virus infection, as well as on social determinants of health. Given that the incidence of and mortality from HPV-associated cancers remain strongly associated with the socioeconomic status of individuals and the human development index of countries, elimination efforts are unlikely to succeed unless prevention efforts focus on health equity, with a commitment to both primary and secondary prevention.
Human papillomavirus (HPV) infection is a necessary cause for virtually all cervical cancers and an attributable cause for variable proportions of anal, oropharyngeal, vaginal, vulvar and penile cancers worldwide.
Cervical cancer screening led to substantial declines in cervical cancer incidence and mortality in many countries during the 20th century.
The advent of HPV vaccines and screening approaches has created the opportunity to eliminate cervical cancer, a recognized public health problem, by the end of the 21st century.
HPV vaccination programmes will probably prevent HPV-associated cancers other than cervical cancer, although research into the optimal screening approaches for these cancers is still ongoing.
Parity, tobacco use and human immunodeficiency virus infections are major cofactors that influence the epidemiology of HPV-associated cancers.
Cervical cancer elimination will require combined primary and secondary prevention approaches as well as a focus on reducing health inequities within and between countries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Concomitant human papillomavirus (HPV) vaccination and screening for elimination of HPV and cervical cancer
Durability of single-dose HPV vaccination in young Kenyan women: randomized controlled trial 3-year results
Vaccination and screening strategies to accelerate cervical cancer elimination in Norway: a model-based analysis
Sporn, M. B. The war on cancer. Lancet 347 , 1377–1381 (1996).
Article CAS PubMed Google Scholar
Bailar, J. C. & Smith, E. M. Progress against cancer? N. Engl. J. Med. 314 , 1226–1232 (1986).
Article PubMed Google Scholar
Vineis, P. & Wild, C. P. Global cancer patterns: causes and prevention. Lancet 383 , 549–557 (2014).
World Health Organization. Global strategy to accelerate the elimination of cervical cancer as a public health problem. World Health Organization www.who.int/publications/i/item/9789240014107 (2020).
Walboomers, J. M. et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189 , 12–19 (1999).
Muñoz, N. Human papillomavirus and cancer: the epidemiological evidence. J. Clin. Virol. 19 , 1–5 (2000).
Bosch, F. X., Lorincz, A., Munoz, N., Meijer, C. J. & Shah, K. V. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 55 , 244–265 (2002).
Article CAS PubMed PubMed Central Google Scholar
Inglis, S., Shaw, A. & Koenig, S. Chapter 11: HPV vaccines: commercial research & development. Vaccine 24 , S99–S105 (2006).
Article Google Scholar
Petry, K. U., Liebrich, C., Luyten, A., Zander, M. & Iftner, T. Surgical staging identified false HPV-negative cases in a large series of invasive cervical cancers. Papillomavirus Res. 4 , 85–89 (2017).
Article PubMed PubMed Central Google Scholar
Kaliff, M. et al. HPV-negative tumors in a Swedish cohort of cervical cancer. Int. J. Gynecol. Pathol. 39 , 279–288 (2020).
Brisson, M. et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 395 , 575–590 (2020).
de Martel, C., Plummer, M., Vignat, J. & Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 141 , 664–670 (2017).
Doll, R., Muir, C. & Waterhouse, J. Cancer Incidence in Five Continents: Volume II – 1970 Vol. 2 (Springer, 2012).
Vaccarella, S. et al. 50 years of screening in the Nordic countries: quantifying the effects on cervical cancer incidence. Br. J. Cancer 111 , 965–969 (2014).
Sigurdsson, K. The Icelandic and Nordic cervical screening programs: trends in incidence and mortality rates through 1995. Acta Obstet. Gynecol. Scand. 78 , 478–485 (1999).
Peto, J., Gilham, C., Fletcher, O. & Matthews, F. E. The cervical cancer epidemic that screening has prevented in the UK. Lancet 364 , 249–256 (2004).
Kyndi, M., Frederiksen, K. & Krüger Kjær, S. Cervical cancer incidence in Denmark over six decades (1943–2002). Acta Obstet. Gynecol. Scand. 85 , 106–111 (2006).
Dickinson, J. A. et al. Reduced cervical cancer incidence and mortality in Canada: national data from 1932 to 2006. BMC Public. Health 12 , 992 (2012).
Gatta, G. et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur. J. Cancer 47 , 2493–2511 (2011).
Ervik, M., Lam, F., Laversanne, M., Ferlay, J. & Bray, F. Global Cancer Observatory: Cancer Over Time gco.iarc.fr/overtime (2021).
Vizcaino, A. P. et al. International trends in incidence of cervical cancer: II. Squamous-cell carcinoma. Int. J. Cancer 86 , 429–435 (2000).
Vizcaino, A. P. et al. International trends in the incidence of cervical cancer: I. Adenocarcinoma and adenosquamous cell carcinomas. Int. J. Cancer 75 , 536–545 (1998).
Gustafsson, L., Ponten, J., Zack, M. & Adami, H. O. International incidence rates of invasive cervical cancer after introduction of cytological screening. Cancer Causes Control. 8 , 755–763 (1997).
Smith, M. & Canfell, K. Impact of the Australian national cervical screening program in women of different ages. Med. J. Aust. 205 , 359–364 (2016).
Wingo, P. A. et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer 97 , 3133–3275 (2003).
Miller, A. B., Lindsay, J. & Hill, G. B. Mortality from cancer of the uterus in Canada and its relationship to screening for cancer of the cervix. Int. J. Cancer 17 , 602–612 (1976).
Cramer, D. W. The role of cervical cytology in the declining morbidity and mortality of cervical cancer. Cancer 34 , 2018–2027 (1974).
Lyon, J. L. & Gardner, J. W. The rising frequency of hysterectomy: its effect on uterine cancer rates. Am. J. Epidemiol. 105 , 439–443 (1977).
Laukkanen, P. et al. Time trends in incidence and prevalence of human papillomavirus type 6, 11 and 16 infections in Finland. J. Gen. Virol. 84 , 2105–2109 (2003).
Ryser, M. D., Rositch, A. & Gravitt, P. E. Modeling of US human papillomavirus (HPV) seroprevalence by age and sexual behavior indicates an increasing trend of HPV infection following the sexual revolution. J. Infect. Dis. 216 , 604–611 (2017).
Desai, S. et al. Prevalence of human papillomavirus antibodies in males and females in England. Sex. Transm. Dis. 38 , 622–629 (2011).
Francesca, P. & Peter, S. Impact of screening on cervical cancer incidence in England: a time trend analysis. BMJ Open. 9 , e026292 (2019).
Shen, X., Cheng, Y., Ren, F. & Shi, Z. The burden of cervical cancer in China. Front. Oncol. 12 , 979809 (2022).
Baldur-Felskov, B. et al. Trends in the incidence of cervical cancer and severe precancerous lesions in Denmark, 1997–2012. Cancer Causes Control. 26 , 1105–1116 (2015).
Adegoke, O., Kulasingam, S. & Virnig, B. Cervical cancer trends in the United States: a 35-year population-based analysis. J. Womens Health 21 , 1031–1037 (2012).
Lönnberg, S. et al. Cervical cancer prevented by screening: long-term incidence trends by morphology in Norway. Int. J. Cancer 137 , 1758–1764 (2015).
Islami, F., Fedewa, S. A. & Jemal, A. Trends in cervical cancer incidence rates by age, race/ethnicity, histological subtype, and stage at diagnosis in the United States. Prev. Med. 123 , 316–323 (2019).
Sasieni, P., Castanon, A. & Cuzick, J. Screening and adenocarcinoma of the cervix. Int. J. Cancer 125 , 525–529 (2009).
Sherman, M. E., Wang, S. S., Carreon, J. & Devesa, S. S. Mortality trends for cervical squamous and adenocarcinoma in the United States. Cancer 103 , 1258–1264 (2005).
Sundqvist, A., Moberg, L., Dickman, P. W., Högberg, T. & Borgfeldt, C. Time trends for incidence and net survival of cervical cancer in Sweden 1960–2014 – a nationwide population-based study. Cancer Epidemiol. Biomark. Prev. 31 , 1572–1581 (2022).
Wright, J. D. et al. Population-level trends in relative survival for cervical cancer. Am. J. Obstet. Gynecol. 213 , 670.e1–670.e7 (2015).
Muñoz, N. et al. Role of parity and human papillomavirus in cervical cancer: the IARC multicentric case-control study. Lancet 359 , 1093–1101 (2002).
Dhillon, P. K., Yeole, B. B., Dikshit, R., Kurkure, A. P. & Bray, F. Trends in breast, ovarian and cervical cancer incidence in Mumbai, India over a 30-year period, 1976–2005: an age–period–cohort analysis. Br. J. Cancer 105 , 723–730 (2011).
Xiao, Z., Mehrotra, P. & Zimmerman, R. Sexual revolution in China: implications for Chinese women and society. AIDS Care 23 , 105–112 (2011).
Jedy-Agba, E. et al. Trends in cervical cancer incidence in sub-Saharan Africa. Br. J. Cancer 123 , 148–154 (2020).
Stelzle, D. et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob. Health 9 , e161–e169 (2021).
de Sanjose, S. et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect. Dis. 7 , 453–459 (2007).
Bruni, L. et al. Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: a review and synthetic analysis. Lancet Glob. Health 10 , e1115–e1127 (2022).
Zumsteg, Z. S. et al. Global epidemiologic patterns of oropharyngeal cancer incidence trends. J. Natl Cancer Inst. 115 , 1544–1554 (2023).
Chaturvedi, A. K. et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J. Clin. Oncol. 31 , 4550–4559 (2013).
Forte, T., Niu, J., Lockwood, G. A. & Bryant, H. E. Incidence trends in head and neck cancers and human papillomavirus (HPV)-associated oropharyngeal cancer in Canada, 1992–2009. Cancer Causes Control. 23 , 1343–1348 (2012).
Larønningen S. et al. NORDCAN: cancer incidence, mortality, prevalence and survival in the Nordic countries, version 9.3 (02.10.2023). nordcan.iarc.fr/ (accessed 5 December 2023).
Klussmann, J. P. et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am. J. Pathol. 162 , 747–753 (2003).
Lundberg, M., Leivo, I., Saarilahti, K., Mäkitie, A. A. & Mattila, P. S. Increased incidence of oropharyngeal cancer and p16 expression. Acta Otolaryngol. 131 , 1008–1011 (2011).
Habbous, S. et al. The changing incidence of human papillomavirus-associated oropharyngeal cancer using multiple imputation from 2000 to 2010 at a Comprehensive Cancer Centre. Cancer Epidemiol. 37 , 820–829 (2013).
Zamani, M. et al. The current epidemic of HPV-associated oropharyngeal cancer: an 18-year Danish population-based study with 2,169 patients. Eur. J. Cancer 134 , 52–59 (2020).
Chaturvedi, A. K. et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 29 , 4294–4301 (2011).
Rettig, E. M. et al. Oropharyngeal cancer is no longer a disease of younger patients and the prognostic advantage of human papillomavirus is attenuated among older patients: analysis of the National Cancer Database. Oral. Oncol. 83 , 147–153 (2018).
Tota, J. E. et al. Evolution of the oropharynx cancer epidemic in the United States: moderation of increasing incidence in younger individuals and shift in the burden to older individuals. J. Clin. Oncol. 37 , 1538–1546 (2019).
Lu, Y. et al. Global burden of oropharyngeal cancer attributable to human papillomavirus by anatomical subsite and geographic region. Cancer Epidemiol. 78 , 102140 (2022).
Shield, K. D. et al. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J. Clin. 67 , 51–64 (2017).
Tam, S. et al. The epidemiology of oral human papillomavirus infection in healthy populations: a systematic review and meta-analysis. Oral. Oncol. 82 , 91–99 (2018).
Islami, F., Ferlay, J., Lortet-Tieulent, J., Bray, F. & Jemal, A. International trends in anal cancer incidence rates. Int. J. Epidemiol. 46 , 924–938 (2016).
Google Scholar
Bray, F., Laversanne, M., Weiderpass, E. & Arbyn, M. Geographic and temporal variations in the incidence of vulvar and vaginal cancers. Int. J. Cancer 147 , 2764–2771 (2020).
Robinson, D., Coupland, V. & Møller, H. An analysis of temporal and generational trends in the incidence of anal and other HPV-related cancers in Southeast England. Br. J. Cancer 100 , 527–531 (2009).
Huang, J. et al. Incidence, risk factors, and temporal trends of penile cancer: a global population-based study. BJU Int. 133 , 314–323 (2023).
Hansen, B. T., Orumaa, M., Lie, A. K., Brennhovd, B. & Nygård, M. Trends in incidence, mortality and survival of penile squamous cell carcinoma in Norway 1956–2015. Int. J. Cancer 142 , 1586–1593 (2018).
Arya, M. et al. Long-term trends in incidence, survival and mortality of primary penile cancer in England. Cancer Causes Control. 24 , 2169–2176 (2013).
Mignozzi, S. et al. Global trends in anal cancer incidence and mortality. Eur. J. Cancer Prev. 33 , 77–86 (2023).
Shiels, M. S., Kreimer, A. R., Coghill, A. E., Darragh, T. M. & Devesa, S. S. Anal cancer incidence in the United States, 1977–2011: distinct patterns by histology and behavior. Cancer Epidemiol. Biomark. Prev. 24 , 1548–1556 (2015).
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73 , 17–48 (2023).
Statistics Canada. Cancer incidence and mortality trends, 1984 to 2020. Statistics Canada www150.statcan.gc.ca/n1/daily-quotidien/220204/dq220204b-eng.htm (2022).
Clifford, G. M. et al. A meta-analysis of anal cancer incidence by risk group: toward a unified anal cancer risk scale. Int. J. Cancer 148 , 38–47 (2021).
Shiels, M. S., Pfeiffer, R. M., Chaturvedi, A. K., Kreimer, A. R. & Engels, E. A. Impact of the HIV epidemic on the incidence rates of anal cancer in the United States. J. Natl Cancer Inst. 104 , 1591–1598 (2012).
Goodman, M. T. et al. Acquisition of anal human papillomavirus (HPV) infection in women: the Hawaii HPV Cohort study. J. Infect. Dis. 197 , 957–966 (2008).
World Health Organization. Human papillomavirus vaccines: WHO position paper, no. 50 (2022 update). Wkly. Epidemiol. Rec. 97 , 645–672 (2022).
Schiller, J. T. & Kreimer, A. R. An HPV vaccine from India: broadening possibilities for cervical cancer control. Lancet Oncol. 24 , 1288–1289 (2023).
Zhao, X.-L. et al. Tackling barriers to scale up human papillomavirus vaccination in China: progress and the way forward. Infect. Dis. Poverty 12 , 81–86 (2023).
Li, N., Franceschi, S., Howell-Jones, R., Snijders, P. J. & Clifford, G. M. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int. J. Cancer 128 , 927–935 (2011).
de Sanjose, S. et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 11 , 1048–1056 (2010).
Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. Engl. J. Med. 356 , 1915–1927 (2007).
Harper, D. M. et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 364 , 1757–1765 (2004).
Paavonen, J. et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 374 , 301–314 (2009).
The World Bank. Prevalence of current tobacco Use (% of adults). The World Bank https://genderdata.worldbank.org/indicators/sh-prv-smok/?gender=female&geos=EAS_LCN_NAC_SSF_EMU&view=trend (2024).
Ho, L. et al. The genetic drift of human papillomavirus type 16 is a means of reconstructing prehistoric viral spread and the movement of ancient human populations. J. Virol. 67 , 6413–6423 (1993).
Wheeler, C. M. et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 13 , 100–110 (2012).
Wheeler, C. M. et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16-26 years. J. Infect. Dis. 199 , 936–944 (2009).
World Health Organization. Human Papillomavirus (HPV) Vaccination Coverage. World Health Organization https://immunizationdata.who.int/global/wiise-detail-page/human-papillomavirus-(hpv)-vaccination-coverage (2023).
Khieu, M. & Butler, S. L. High-grade Squamous Intraepithelial Lesion of the Cervix (StatPearls, 2024).
Patel, C. et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonavalent vaccine prevent? Eur. Surveill. 23 , 1700737 (2018).
Rosenblum, H. G. et al. Declines in prevalence of human papillomavirus vaccine-type infection among females after introduction of vaccine – United States, 2003–2018. MMWR Morb. Mortal. Wkly. Rep. 70 , 415–420 (2021).
Wang, W. et al. Real-world impact and effectiveness of the quadrivalent HPV vaccine: an updated systematic literature review. Expert. Rev. Vaccines 21 , 1799–1817 (2022).
Mesher, D. et al. The impact of the national HPV vaccination program in England using the bivalent HPV vaccine: surveillance of type-specific HPV in young females, 2010–2016. J. Infect. Dis. 218 , 911–921 (2018).
Herweijer, E. et al. Quadrivalent HPV vaccine effectiveness against high-grade cervical lesions by age at vaccination: a population-based study. Int. J. Cancer 138 , 2867–2874 (2016).
Baldur-Felskov, B., Dehlendorff, C., Munk, C. & Kjaer, S. K. Early impact of human papillomavirus vaccination on cervical neoplasia – nationwide follow-up of young Danish women. J. Natl Cancer Inst. 106 , djt460 (2014).
Pollock, K. G. et al. Reduction of low- and high-grade cervical abnormalities associated with high uptake of the HPV bivalent vaccine in Scotland. Br. J. Cancer 111 , 1824–1830 (2014).
Dong, L., Nygård, M., Støer, N. C., Klungsøyr, O. & Hansen, B. T. Real-world effectiveness of HPV vaccination against cervical neoplasia among birth cohorts ineligible for routine vaccination. Int. J. Cancer 153 , 399–406 (2023).
Falcaro, M. et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet 398 , 2084–2092 (2021).
Palmer, T. J. et al. Invasive cervical cancer incidence following bivalent human papillomavirus vaccination: a population-based observational study of age at immunization, dose, and deprivation. J. Natl Cancer Inst . djad263, https://doi.org/10.1093/jnci/djad263 (2024).
Lei, J. et al. HPV vaccination and the risk of invasive cervical cancer. N. Engl. J. Med. 383 , 1340–1348 (2020).
Kjaer, S. K., Dehlendorff, C., Belmonte, F. & Baandrup, L. Real-world effectiveness of human papillomavirus vaccination against cervical cancer. J. Natl Cancer Inst. 113 , 1329–1335 (2021).
Burger, E. A., Kim, J. J., Sy, S. & Castle, P. E. Age of acquiring causal human papillomavirus (HPV) infections: leveraging simulation models to explore the natural history of HPV-induced cervical cancer. Clin. Infect. Dis. 65 , 893–899 (2017).
Gheit, T. et al. Impact of HPV vaccination on HPV-related oral infections. Oral. Oncol. 136 , 106244 (2023).
Palefsky, J. M. et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N. Engl. J. Med. 365 , 1576–1585 (2011).
Kreimer, A. R. et al. Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica Vaccine Trial. Lancet Oncol. 12 , 862–870 (2011).
Xu, L. et al. Prophylactic vaccination against human papillomaviruses to prevent vulval and vaginal cancer and their precursors. Expert. Rev. Vaccines 18 , 1157–1166 (2019).
Baandrup, L., Maltesen, T., Dehlendorff, C. & Kjaer, S. K. Human papillomavirus vaccination and anal high-grade precancerous lesions and cancer – a real-world effectiveness study. J. Natl Cancer Inst. 116 , 283–287 (2023).
Chaturvedi, A. K. et al. Prevalence of oral HPV infection in unvaccinated men and women in the United States, 2009-2016. JAMA 322 , 977–979 (2019).
Matti, L. et al. Human papillomavirus vaccine efficacy against invasive, HPV-positive cancers: population-based follow-up of a cluster-randomised trial. BMJ Open. 11 , e050669 (2021).
Nanda, K. et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann. Intern. Med. 132 , 810–819 (2000).
Nessa, A., Anwar, B. R. & Begum, S. A. in Preventive Oncology for the Gynecologist (eds Sumita, M. & Anshuja, S.) 167–185 (Springer, 2019).
Catarino, R., Petignat, P., Dongui, G. & Vassilakos, P. Cervical cancer screening in developing countries at a crossroad: emerging technologies and policy choices. World J. Clin. Oncol. 6 , 281–290 (2015).
Cuzick, J. et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int. J. Cancer 119 , 1095–1101 (2006).
Salazar, K. L., Duhon, D. J., Olsen, R. & Thrall, M. A review of the FDA-approved molecular testing platforms for human papillomavirus. J. Am. Soc. Cytopathol. 8 , 284–292 (2019).
Poljak, M., Oštrbenk Valenčak, A., Gimpelj Domjanič, G., Xu, L. & Arbyn, M. Commercially available molecular tests for human papillomaviruses: a global overview. Clin. Microbiol. Infect. 26 , 1144–1150 (2020).
Arbyn, M. et al. 2020 list of human papillomavirus assays suitable for primary cervical cancer screening. Clin. Microbiol. Infect. 27 , 1083–1095 (2021).
World Health Organization. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. World Health Organization www.who.int/publications/i/item/9789240030824 (2021).
Polman, N. J., Snijders, P. J. F., Kenter, G. G., Berkhof, J. & Meijer, C. HPV-based cervical screening: rationale, expectations and future perspectives of the new Dutch screening programme. Prev. Med. 119 , 108–117 (2019).
Smith, M. A. et al. National experience in the first two years of primary human papillomavirus (HPV) cervical screening in an HPV vaccinated population in Australia: observational study. BMJ 376 , e068582 (2022).
Cuzick, J. et al. Impact of HPV testing in opportunistic cervical screening: support for primary HPV screening in the United States. Int. J. Cancer 153 , 83–93 (2023).
Aitken, C. A. et al. Introduction of primary screening using high-risk HPV DNA detection in the Dutch cervical cancer screening programme: a population-based cohort study. BMC Med. 17 , 228 (2019).
Zhao, Y. et al. Real-world effectiveness of primary screening with high-risk human papillomavirus testing in the cervical cancer screening programme in China: a nationwide, population-based study. BMC Med. 19 , 164 (2021).
Kaljouw, S. et al. Reducing unnecessary referrals for colposcopy in hrHPV-positive women within the Dutch cervical cancer screening programme: a modelling study. Gynecol. Oncol. 160 , 713–720 (2021).
Rijkaart, D. C. et al. Evaluation of 14 triage strategies for HPV DNA-positive women in population-based cervical screening. Int. J. Cancer 130 , 602–610 (2012).
Isidean, S. D. et al. Comparison of triage strategies for HPV-positive women: Canadian Cervical Cancer Screening Trial results. Cancer Epidemiol. Biomark. Prev. 26 , 923–929 (2017).
Article CAS Google Scholar
Wentzensen, N., Schiffman, M., Palmer, T. & Arbyn, M. Triage of HPV positive women in cervical cancer screening. J. Clin. Virol. 76 , S49–S55 (2016).
Taghavi, K., Zhao, F., Downham, L., Baena, A. & Basu, P. Molecular triaging options for women testing HPV positive with self-collected samples. Front. Oncol. 13 , 1243888 (2023).
Tota, J. E. et al. Approaches for triaging women who test positive for human papillomavirus in cervical cancer screening. Prev. Med. 98 , 15–20 (2017).
Lei, J. et al. Impact of HPV vaccination on cervical screening performance: a population-based cohort study. Br. J. Cancer 123 , 155–160 (2020).
Rebolj, M. et al. The impact of catch-up bivalent human papillomavirus vaccination on cervical screening outcomes: an observational study from the English HPV primary screening pilot. Br. J. Cancer 127 , 278–287 (2022).
Palmer, T. J. et al. HPV immunisation and cervical screening – confirmation of changed performance of cytology as a screening test in immunised women: a retrospective population-based cohort study. Br. J. Cancer 114 , 582–589 (2016).
Franco, E. L. & Cuzick, J. Cervical cancer screening following prophylactic human papillomavirus vaccination. Vaccine 26 , A16–A23 (2008).
Franco, E. L., Mahmud, S. M., Tota, J., Ferenczy, A. & Coutlee, F. The expected impact of HPV vaccination on the accuracy of cervical cancer screening: the need for a paradigm change. Arch. Med. Res. 40 , 478–485 (2009).
Arbyn, M. & Castle, P. E. Offering self-sampling kits for HPV testing to reach women who do not attend in the regular cervical cancer screening program. Cancer Epidemiol. Biomark. Prev. 24 , 769–772 (2015).
Schmeink, C. E., Bekkers, R. L. M., Massuger, L. F. A. G. & Melchers, W. J. G. The potential role of self-sampling for high-risk human papillomavirus detection in cervical cancer screening. Rev. Med. Virol. 21 , 139–153 (2011).
Elfström, M., Gray, P. G. & Dillner, J. Cervical cancer screening improvements with self-sampling during the COVID-19 pandemic. eLife 12 , e80905 (2023).
Serrano, B. et al. Worldwide use of HPV self-sampling for cervical cancer screening. Prev. Med. 154 , 106900 (2022).
Canfell, K. et al. Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 395 , 591–603 (2020).
Burmeister, C. A. et al. Cervical cancer therapies: current challenges and future perspectives. Tumour Virus Res. 13 , 200238 (2022).
Pang, S. S., Murphy, M. & Markham, M. J. Current management of locally advanced and metastatic cervical cancer in the United States. JCO Oncol. Prac. 18 , 417–422 (2022).
Douglas, E., Wardle, J., Massat, N. J. & Waller, J. Colposcopy attendance and deprivation: a retrospective analysis of 27 193 women in the NHS cervical screening programme. Br. J. Cancer 113 , 119–122 (2015).
Ezechi, O. C. et al. Predictors of default from follow-up care in a cervical cancer screening program using direct visual inspection in south-western Nigeria. BMC Health Serv. Res. 14 , 143 (2014).
Desai, K. T. et al. The development of “automated visual evaluation” for cervical cancer screening: the promise and challenges in adapting deep-learning for clinical testing. Int. J. Cancer 150 , 741–752 (2022).
Watson, A. J. M., Smith, B. B., Whitehead, M. R., Sykes, P. H. & Frizelle, F. A. Malignant progression of anal intra-epithelial neoplasia. Anz. J. Surg. 76 , 715–717 (2006).
Scholefield, J. H., Castle, M. T. & Watson, N. F. Malignant transformation of high-grade anal intraepithelial neoplasia. Br. J. Surg. 92 , 1133–1136 (2005).
Palefsky, J. M. et al. Treatment of anal high-grade squamous intraepithelial lesions to prevent anal cancer. N. Engl. J. Med. 386 , 2273–2282 (2022).
Clarke, M. A. et al. A systematic review and meta-analysis of cytology and HPV-related biomarkers for anal cancer screening among different risk groups. Int. J. Cancer 151 , 1889–1901 (2022).
Jin, F. et al. The performance of anal cytology as a screening test for anal HSILs in homosexual men. Cancer Cytopathol. 124 , 415–424 (2016).
Cohen, C. M. & Clarke, M. A. Anal cancer and anal cancer screening. Clin. Obstet. Gynecol. 66 , 516–533 (2023).
Palefsky, J. M. & Rubin, M. The epidemiology of anal human papillomavirus and related neoplasia. Obstet. Gynecol. Clin. North. Am. 36 , 187–200 (2009).
Hillman, R. J. et al. 2016 IANS international guidelines for practice standards in the detection of anal cancer precursors. J. Low. Genit. Tract. Dis. 20 , 283–291 (2016).
Clarke, M. A. & Wentzensen, N. Strategies for screening and early detection of anal cancers: a narrative and systematic review and meta-analysis of cytology, HPV testing, and other biomarkers. Cancer Cytopathol. 126 , 447–460 (2018).
Nyitray, A. G., D’Souza, G., Stier, E. A., Clifford, G. & Chiao, E. Y. The utility of digital anal rectal examinations in a public health screening program for anal cancer. J. Low. Genit. Tract. Dis. 24 , 192–196 (2020).
Stier, E. A. et al. International Anal Neoplasia Society’s consensus guidelines for anal cancer screening. Int. J. Cancer 154 , 1694–1702 (2024).
Kreimer, A. R. et al. Screening for human papillomavirus-driven oropharyngeal cancer: considerations for feasibility and strategies for research. Cancer 124 , 1859–1866 (2018).
Day, A. T., Fakhry, C., Tiro, J. A., Dahlstrom, K. R. & Sturgis, E. M. Considerations in human papillomavirus-associated oropharyngeal cancer screening: a review. JAMA Otolaryngol. Head. Neck Surg. 146 , 656–664 (2020).
Holzinger, D. et al. Sensitivity and specificity of antibodies against HPV16 E6 and other early proteins for the detection of HPV16-driven oropharyngeal squamous cell carcinoma. Int. J. Cancer 140 , 2748–2757 (2017).
Kreimer, A. R. et al. Timing of HPV16-E6 antibody seroconversion before OPSCC: findings from the HPVC3 Consortium. Ann. Oncol. 30 , 1335–1343 (2019).
Rosenthal, M. et al. Detection of HPV related oropharyngeal cancer in oral rinse specimens. Oncotarget 8 , 109393–109401 (2017).
Gipson, B. J., Robbins, H. A., Fakhry, C. & D’Souza, G. Sensitivity and specificity of oral HPV detection for HPV-positive head and neck cancer. Oral. Oncol. 77 , 52–56 (2018).
Koch, W. M. Clinical features of HPV-related head and neck squamous cell carcinoma: presentation and work-up. Otolaryngol. Clin. North. Am. 45 , 779–793 (2012).
Tota, J. E., Isidean, S. D. & Franco, E. L. Defining benchmarks for tolerable risk thresholds in cancer screening: impact of HPV vaccination on the future of cervical cancer screening. Int. J. Cancer 147 , 3305–3312 (2020).
Qaseem, A., Humphrey, L. L., Harris, R., Starkey, M. & Denberg, T. D. Screening pelvic examination in adult women: a clinical practice guideline from the American College of Physicians. Ann. Intern. Med. 161 , 67–72 (2014).
Marcello, T., Sarah Connor, G., Ainsley, M. & Brett, D. T. Recommendations on routine screening pelvic examination. Can. Fam. Physician 62 , 211 (2016).
Olawaiye, A. B., Cuello, M. A. & Rogers, L. J. Cancer of the vulva: 2021 update. Int. J. Gynecol. Obstet. 155 , 7–18 (2021).
Maclean, A. B. Vulval cancer: prevention and screening. Best. Pract. Res. Clin. Obstet. Gynaecol. 20 , 379–395 (2006).
Chesson, H. W., Dunne, E. F., Hariri, S. & Markowitz, L. E. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex. Transm. Dis. 41 , 660–664 (2014).
Zheng, R. et al. Global, regional, and national lifetime probabilities of developing cancer in 2020. Sci. Bull. 68 , 2620–2628 (2023).
Castellsagué, X. & Muñoz, N. Chapter 3: Cofactors in human papillomavirus carcinogenesis – role of parity, oral contraceptives, and tobacco smoking. J. Natl Cancer Inst. Monogr . (31), 20–28 (2003).
Aguayo, F. et al. High-risk human papillomavirus and tobacco smoke interactions in epithelial carcinogenesis. Cancers 12 , 2201 (2020).
Almonte, M. et al. Risk factors for human papillomavirus exposure and co-factors for cervical cancer in Latin America and the Caribbean. Vaccine 26 , L16–L36 (2008).
Kelly, H. et al. Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: a systematic review and meta-analysis. Lancet HIV 5 , e45–e58 (2018).
Ojha, P. S., Maste, M. M., Tubachi, S. & Patil, V. S. Human papillomavirus and cervical cancer: an insight highlighting pathogenesis and targeting strategies. Virusdisease 33 , 132–154 (2022).
Cohen, P. A., Jhingran, A., Oaknin, A. & Denny, L. Cervical cancer. Lancet 393 , 169–182 (2019).
Hildesheim, A. et al. HPV co-factors related to the development of cervical cancer: results from a population-based study in Costa Rica. Br. J. Cancer 84 , 1219–1226 (2001).
de Araujo Souza, P. S., Sichero, L. & Maciag, P. C. HPV variants and HLA polymorphisms: the role of variability on the risk of cervical cancer. Future Oncol. 5 , 359–370 (2009).
Choi, S., Ismail, A., Pappas-Gogos, G. & Boussios, S. HPV and cervical cancer: a review of epidemiology and screening uptake in the UK. Pathogens 12 , 298 (2023).
He, W.-Q. & Li, C. Recent global burden of cervical cancer incidence and mortality, predictors, and temporal trends. Gynecol. Oncol. 163 , 583–592 (2021).
Senapati, R., Senapati, N. N. & Dwibedi, B. Molecular mechanisms of HPV mediated neoplastic progression. Infect. Agent. Cancer 11 , 59 (2016).
Muwonge, R. et al. Socio-demographic and reproductive determinants of cervical neoplasia in seven sub-Sahara African countries. Cancer Causes Control. 27 , 1437–1446 (2016).
Husain, R. S. & Ramakrishnan, V. Global variation of human papillomavirus genotypes and selected genes involved in cervical malignancies. Ann. Glob. Health 81 , 675–683 (2015).
Vaccarella, S. et al. Reproductive factors, oral contraceptive use, and human papillomavirus infection: pooled analysis of the IARC HPV prevalence surveys. Cancer Epidemiol. Biomark. Prev. 15 , 2148–2153 (2006).
Louie, K. S. et al. Early age at first sexual intercourse and early pregnancy are risk factors for cervical cancer in developing countries. Br. J. Cancer 100 , 1191–1197 (2009).
Bosch, F. X., Qiao, Y. L. & Castellsague, X. CHAPTER 2 The epidemiology of human papillomavirus infection and its association with cervical cancer. Int. J. Gynaecol. Obstet. 94 , S8–S21 (2006).
Dugué, P. A., Rebolj, M., Garred, P. & Lynge, E. Immunosuppression and risk of cervical cancer. Expert. Rev. Anticancer. Ther. 13 , 29–42 (2013).
International Collaboration of Epidemiological Studies of Cervical Cancer Cervical carcinoma and reproductive factors: collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33,542 women without cervical carcinoma from 25 epidemiological studies. Int. J. Cancer 119 , 1108–1124 (2006).
The World Bank. Fertility rate, total (births per woman). The World Bank https://genderdata.worldbank.org/indicators/sp-dyn-tfrt-in/?view=trend (2024).
Singh, D. et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 11 , e197–e206 (2023).
Pérez-González, A., Cachay, E., Ocampo, A. & Poveda, E. Update on the epidemiological features and clinical implications of human papillomavirus infection (HPV) and human immunodeficiency virus (HIV) coinfection. Microorganisms 10 , 1047 (2022).
The World Bank. World Development Indicators. The World Bank databank.worldbank.org/source/world-development-indicators (2024).
Ferlay, J., Ervik, M., Lam, F., Colombert, M., Mery, L., Piñeros, M., Znaor, A., Soerjomataram, I. & Bray, F. Global Cancer Observatory: Cancer Today . gco.iarc.fr/today (2020).
The Global Health Observatory. HIV – Prevalence of HIV among adults aged 15 to 49 (%). World Health Organization https://www.who.int/data/gho/data/indicators/indicator-details/GHO/prevalence-of-hiv-among-adults-aged-15-to-49-(-) (2023).
The Global Health Observatory. HIV – New HIV infections (per 1000 uninfected population). World Health Organization https://www.who.int/data/gho/data/indicators/indicator-details/GHO/new-hiv-infections-(per-1000-uninfected-population) (2023).
Castle, P. E., Einstein, M. H. & Sahasrabuddhe, V. V. Cervical cancer prevention and control in women living with human immunodeficiency virus. CA Cancer J. Clin. 71 , 505–526 (2021).
Clifford, G. M. et al. Immunodeficiency and the risk of cervical intraepithelial neoplasia 2/3 and cervical cancer: a nested case-control study in the Swiss HIV Cohort Study. Int. J. Cancer 138 , 1732–1740 (2016).
Szarewski, A. et al. Effect of smoking cessation on cervical lesion size. Lancet 347 , 941–943 (1996).
Castle, P. E. How does tobacco smoke contribute to cervical carcinogenesis? J. Virol. 82 , 6084–6085 (2008); author’s reply 82 , 6085–6086 (2008).
Guan, P. et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int. J. Cancer 131 , 2349–2359 (2012).
Clifford, G. M., Smith, J. S., Aguado, T. & Franceschi, S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br. J. Cancer 89 , 101–105 (2003).
Smith, J. S. et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int. J. Cancer 121 , 621–632 (2007).
Mirabello, L. et al. The intersection of HPV epidemiology, genomics and mechanistic studies of HPV-mediated carcinogenesis. Viruses 10 , 80 (2018).
Burk, R. D., Harari, A. & Chen, Z. Human papillomavirus genome variants. Virology 445 , 232–243 (2013).
Cornet, I. et al. HPV16 genetic variation and the development of cervical cancer worldwide. Br. J. Cancer 108 , 240–244 (2013).
Villa, L. L. et al. Molecular variants of human papillomavirus types 16 and 18 preferentially associated with cervical neoplasia. J. Gen. Virol. 81 , 2959–2968 (2000).
Sichero, L. et al. High grade cervical lesions are caused preferentially by non-European variants of HPVs 16 and 18. Int. J. Cancer 120 , 1763–1768 (2007).
Zehbe, I. et al. Human papillomavirus 16 E6 polymorphisms in cervical lesions from different European populations and their correlation with human leukocyte antigen class II haplotypes. Int. J. Cancer 94 , 711–716 (2001).
Castellsagué, X. et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J. Natl Cancer Inst. 108 , djv403 (2016).
Serrano, B. et al. Human papillomavirus genotype attribution for HPVs 6, 11, 16, 18, 31, 33, 45, 52 and 58 in female anogenital lesions. Eur. J. Cancer 51 , 1732–1741 (2015).
Bloss, J. D. et al. Clinical and histologic features of vulvar carcinomas analyzed for human papillomavirus status: evidence that squamous cell carcinoma of the vulva has more than one etiology. Hum. Pathol. 22 , 711–718 (1991).
Gillison, M. L. et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J. Natl Cancer Inst. 100 , 407–420 (2008).
Lekoane, K. M. B., Kuupiel, D., Mashamba-Thompson, T. P. & Ginindza, T. G. The interplay of HIV and human papillomavirus-related cancers in sub-Saharan Africa: scoping review. Syst. Rev. 9 , 88 (2020).
Chaturvedi, A. K., Madeleine, M. M., Biggar, R. J. & Engels, E. A. Risk of human papillomavirus-associated cancers among persons with AIDS. J. Natl Cancer Inst. 101 , 1120–1130 (2009).
Lechner, M., Liu, J., Masterson, L. & Fenton, T. R. HPV-associated oropharyngeal cancer: epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 19 , 306–327 (2022).
Kesic, V. et al. The European Society of Gynaecological Oncology (ESGO), the International Society for the Study of Vulvovaginal Disease (ISSVD), the European College for the Study of Vulval Disease (ECSVD), and the European Federation for Colposcopy (EFC) consensus statement on the management of vaginal intraepithelial neoplasia. Int. J. Gynecol. Cancer 33 , 446–461 (2023).
Frisch, M., Biggar, R. J. & Goedert, J. J. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J. Natl Cancer Inst. 92 , 1500–1510 (2000).
Wei, F. et al. Epidemiology of anal human papillomavirus infection and high-grade squamous intraepithelial lesions in 29 900 men according to HIV status, sexuality, and age: a collaborative pooled analysis of 64 studies. Lancet HIV 8 , e531–e543 (2021).
Lin, C., Franceschi, S. & Clifford, G. M. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infect. Dis. 18 , 198–206 (2018).
Piketty, C. et al. Incidence of HIV-related anal cancer remains increased despite long-term combined antiretroviral treatment: results from the French Hospital Database on HIV. J. Clin. Oncol. 30 , 4360–4366 (2012).
Hernandez-Ramirez, R. U., Shiels, M. S., Dubrow, R. & Engels, E. A. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV 4 , e495–e504 (2017).
Kelly, H. et al. Association of antiretroviral therapy with anal high-risk human papillomavirus, anal intraepithelial neoplasia, and anal cancer in people living with HIV: a systematic review and meta-analysis. Lancet HIV 7 , e262–e278 (2020).
Wang, C. J. & Palefsky, J. M. HPV-associated anal cancer in the HIV/AIDS patient. Cancer Treat. Res. 177 , 183–209 (2019).
Sunesen, K. G., Nørgaard, M., Thorlacius-Ussing, O. & Laurberg, S. Immunosuppressive disorders and risk of anal squamous cell carcinoma: a nationwide cohort study in Denmark, 1978–2005. Int. J. Cancer 127 , 675–684 (2010).
Fehr, M. K. et al. Disease progression and recurrence in women treated for vulvovaginal intraepithelial neoplasia. J. Gynecol. Oncol. 24 , 236–241 (2013).
Anantharaman, D. et al. Combined effects of smoking and HPV16 in oropharyngeal cancer. Int. J. Epidemiol. 45 , 752–761 (2016).
Applebaum, K. M. et al. Lack of association of alcohol and tobacco with HPV16-associated head and neck cancer. J. Natl Cancer Inst. 99 , 1801–1810 (2007).
Farsi, N. J. et al. Aetiological heterogeneity of head and neck squamous cell carcinomas: the role of human papillomavirus infections, smoking and alcohol. Carcinogenesis 38 , 1188–1195 (2017).
Auguste, A. et al. Joint effect of tobacco, alcohol, and oral HPV infection on head and neck cancer risk in the French West Indies. Cancer Med. 9 , 6854–6863 (2020).
United Nations Development Programme. Human development report 2021-22: Uncertain times, unsettled lives: shaping our future in a transforming world. UNDP https://hdr.undp.org/content/human-development-report-2021-22 (United Nations Development Programme, 2022).
Braaten, T., Weiderpass, E., Kumle, M. & Lund, E. Explaining the socioeconomic variation in cancer risk in the Norwegian Women and Cancer Study. Cancer Epidemiol. Biomark. Prev. 14 , 2591–2597 (2005).
Jensen, K. E. et al. Social inequality and incidence of and survival from cancer of the female genital organs in a population-based study in Denmark, 1994-2003. Eur. J. Cancer 44 , 2003–2017 (2008).
de Vries, E., Arroyave, I. & Pardo, C. Re-emergence of educational inequalities in cervical cancer mortality, Colombia 1998–2015. J. Cancer Policy 15 , 37–44 (2018).
Vaccarella, S. et al. Socioeconomic inequalities in cancer mortality between and within countries in Europe: a population-based study. Lancet Reg. Health Eur. 25 , 100551 (2023).
Drolet, M. et al. Sociodemographic inequalities in sexual activity and cervical cancer screening: implications for the success of human papillomavirus vaccination. Cancer Epidemiol. Biomark. Prev. 22 , 641–652 (2013).
Damiani, G. et al. Socioeconomic disparities in the uptake of breast and cervical cancer screening in Italy: a cross sectional study. BMC Public. Health 12 , 99 (2012).
Walsh, B. & O’Neill, C. Socioeconomic disparities across ethnicities: an application to cervical cancer screening. Am. J. Manag. Care 21 , e527–e536 (2015).
PubMed Google Scholar
Coughlin, S. S., King, J., Richards, T. B. & Ekwueme, D. U. Cervical cancer screening among women in metropolitan areas of the United States by individual-level and area-based measures of socioeconomic status, 2000 to 2002. Cancer Epidemiol. Biomark. Prev. 15 , 2154–2159 (2006).
Lee, M. et al. Socioeconomic disparity in cervical cancer screening among Korean women: 1998–2010. BMC Public. Health 13 , 553 (2013).
Tomi, A., Kemi, O., Swati, S., Valentine, O. & Dejana, B. Life-course socioeconomic status and breast and cervical cancer screening: analysis of the WHO’s Study on Global Ageing and Adult Health (SAGE). BMJ Open 6 , e012753 (2016).
Nuche-Berenguer, B. & Sakellariou, D. Socioeconomic determinants of cancer screening utilisation in Latin America: a systematic review. PLoS ONE 14 , e0225667 (2019).
Palencia, L. et al. Socio-economic inequalities in breast and cervical cancer screening practices in Europe: influence of the type of screening program. Int. J. Epidemiol. 39 , 757–765 (2010).
Cotton, S. C. et al. Lifestyle and socio-demographic factors associated with high-risk HPV infection in UK women. Br. J. Cancer 97 , 133–139 (2007).
Shi, R., Devarakonda, S., Liu, L., Taylor, H. & Mills, G. Factors associated with genital human papillomavirus infection among adult females in the United States, NHANES 2007–2010. BMC Res. Notes 7 , 544 (2014).
Franceschi, S. et al. Differences in the risk of cervical cancer and human papillomavirus infection by education level. Br. J. Cancer 101 , 865–870 (2009).
Bosch, F. X. & de Sanjose, S. The epidemiology of human papillomavirus infection and cervical cancer. Dis. Markers 23 , 213–227 (2007).
Fisher, H., Trotter, C. L., Audrey, S., MacDonald-Wallis, K. & Hickman, M. Inequalities in the uptake of human papillomavirus vaccination: a systematic review and meta-analysis. Int. J. Epidemiol. 42 , 896–908 (2013).
Fisher, H., Audrey, S., Mytton, J. A., Hickman, M. & Trotter, C. Examining inequalities in the uptake of the school-based HPV vaccination programme in England: a retrospective cohort study. J. Public. Health 36 , 36–45 (2014).
Barbaro, B. & Brotherton, J. M. L. Assessing HPV vaccine coverage in Australia by geography and socioeconomic status: are we protecting those most at risk? Aust. N. Zealand J. Public. Health 38 , 419–423 (2014).
de Munter, A. C. et al. Determinants of HPV-vaccination uptake and subgroups with a lower uptake in the Netherlands. BMC Public. Health 21 , 1848 (2021).
Malagon, T. et al. The impact of differential uptake of HPV vaccine by sexual risks on health inequalities: a model-based analysis. Vaccine 31 , 1740–1747 (2013).
Wang, J. et al. Mode of HPV vaccination delivery and equity in vaccine uptake: a nationwide cohort study. Prev. Med. 120 , 26–33 (2019).
Devotta, K., Vahabi, M., Prakash, V. & Lofters, A. Reach and effectiveness of an HPV self-sampling intervention for cervical screening amongst under- or never-screened women in Toronto, Ontario Canada. BMC Women’s Health 23 , 36 (2023).
Pretsch, P. K. et al. Effect of HPV self-collection kits on cervical cancer screening uptake among under-screened women from low-income US backgrounds (MBMT-3): a phase 3, open-label, randomised controlled trial. Lancet Public. Health 8 , e411–e421 (2023).
Conway, D. I. et al. Estimating and explaining the effect of education and income on head and neck cancer risk: INHANCE Consortium pooled analysis of 31 case-control studies from 27 countries. Int. J. Cancer 136 , 1125–1139 (2015).
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 , 209–249 (2021).
Bilimoria, K. Y. et al. Outcomes and prognostic factors for squamous-cell carcinoma of the anal canal: analysis of patients from the National Cancer Data Base. Dis. Colon. Rectum 52 , 624–631 (2009).
Lin, D. et al. Impact of socioeconomic status on survival for patients with anal cancer. Cancer 124 , 1791–1797 (2018).
Cruz, A. et al. Racial and gender disparities in the incidence of anal cancer: analysis of the Nationwide Inpatient Sample (NIS). J. Gastrointest. Oncol. 10 , 37–41 (2019).
Damgacioglu, H. et al. State variation in squamous cell carcinoma of the anus incidence and mortality, and association with HIV/AIDS and smoking in the United States. J. Clin. Oncol. 41 , 1228–1238 (2023).
National Cancer Intelligence Network. Cancer by Deprivation in England, Incidence, 1996-2010, Mortality, 1997-2011 (NCIN, 2014).
Svahn, M. F., Munk, C., von Buchwald, C., Frederiksen, K. & Kjaer, S. K. Burden and incidence of human papillomavirus-associated cancers and precancerous lesions in Denmark. Scand. J. Public. Health 44 , 551–559 (2016).
Benard, V. B. et al. Examining the association between socioeconomic status and potential human papillomavirus-associated cancers. Cancer 113 , 2910–2918 (2008).
Baekhøj Kortsen, D., Predbjørn Krarup, K. & Jakobsen, J. K. DaPeCa-9 – cohabitation and socio-economic conditions predict penile cancer-specific survival in a national clinical study from Denmark. Scand. J. Urol. 55 , 486–490 (2021).
Broutet, N. et al. Implementation research to accelerate scale-up of national screen and treat strategies towards the elimination of cervical cancer. Prev. Med. 155 , 106906 (2022).
Gravitt, P. E. et al. Achieving equity in cervical cancer screening in low- and middle-income countries (LMICs): strengthening health systems using a systems thinking approach. Prev. Med. 144 , 106322 (2021).
Boily, M.-C. et al. Estimating the effect of HIV on cervical cancer elimination in South Africa: comparative modelling of the impact of vaccination and screening. eClinicalMedicine 54 , 101754 (2022).
Simms, K. T. et al. Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020–99: a modelling study. Lancet Oncol. 20 , 394–407 (2019).
Bruni, L. et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev. Med. 144 , 106399 (2021).
Morales-Campos, D. Y., Zimet, G. D. & Kahn, J. A. Human papillomavirus vaccine hesitancy in the United States. Pediatr. Clin. North. Am. 70 , 211–226 (2023).
Milondzo, T., Meyer, J. C., Dochez, C. & Burnett, R. J. Human papillomavirus vaccine hesitancy highly evident among caregivers of girls attending South African private schools. Vaccines 10 , 506 (2022).
Nogueira-Rodrigues, A. et al. HPV vaccination in Latin America: coverage status, implementation challenges and strategies to overcome it. Front. Oncol. 12 , 984449 (2022).
Hanley, S. J., Yoshioka, E., Ito, Y. & Kishi, R. HPV vaccination crisis in Japan. Lancet 385 , 2571 (2015).
Hansen, P. R., Schmidtblaicher, M. & Brewer, N. T. Resilience of HPV vaccine uptake in Denmark: decline and recovery. Vaccine 38 , 1842–1848 (2020).
Simas, C., Munoz, N., Arregoces, L. & Larson, H. J. HPV vaccine confidence and cases of mass psychogenic illness following immunization in Carmen de Bolivar, Colombia. Hum. Vaccin. Immunother. 15 , 163–166 (2019).
Muhoza, P. et al. Routine vaccination coverage – worldwide, 2020. MMWR Morb. Mortal. Wkly. Rep. 70 , 1495–1500 (2021).
Bhutta, Z. A. Conflict and polio: winning the polio wars. JAMA 310 , 905–906 (2013).
Paniz-Mondolfi, A. E. et al. Resurgence of vaccine-preventable diseases in venezuela as a regional public health threat in the Americas. Emerg. Infect. Dis. 25 , 625–632 (2019).
Landy, R., Windridge, P., Gillman, M. S. & Sasieni, P. D. What cervical screening is appropriate for women who have been vaccinated against high risk HPV? A simulation study. Int. J. Cancer 142 , 709–718 (2018).
Simms, K. T. et al. Will cervical screening remain cost-effective in women offered the next generation nonavalent HPV vaccine? Results for four developed countries. Int. J. Cancer 139 , 2771–2780 (2016).
Kim, J. J., Burger, E. A., Sy, S. & Campos, N. G. Optimal cervical cancer screening in women vaccinated against human papillomavirus. J. Natl Cancer Inst. 109 , djw216 (2017).
Moscicki, A.-B. et al. Screening for anal cancer in women. J. Low. Genit. Tract. Dis. 19 , S27–S42 (2015).
Barroso, L. F., Stier, E. A., Hillman, R. & Palefsky, J. Anal cancer screening and prevention: summary of evidence reviewed for the 2021 Centers for Disease Control and Prevention sexually transmitted infection guidelines. Clin. Infect. Dis. 74 , S179–S192 (2022).
Timbang, M. R. et al. HPV-related oropharyngeal cancer: a review on burden of the disease and opportunities for prevention and early detection. Hum. Vaccin. Immunother. 15 , 1920–1928 (2019).
Hall, M. T. et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public. Health 4 , e19–e27 (2019).
Moore, S. P. et al. Cancer incidence in indigenous people in Australia, New Zealand, Canada, and the USA: a comparative population-based study. Lancet Oncol. 16 , 1483–1492 (2015).
Spencer, J. C. et al. Reducing poverty-related disparities in cervical cancer: the role of HPV vaccination. Cancer Epidemiol. Biomark. Prev. 30 , 1895–1903 (2021).
Whop, L. J., Cunningham, J., Garvey, G. & Condon, J. R. Towards global elimination of cervical cancer in all groups of women. Lancet Oncol. 20 , e238 (2019).
Whop, L. J. et al. Achieving cervical cancer elimination among Indigenous women. Prev. Med. 144 , 106314 (2021).
Canadian Partnership Against Cancer. Action plan for the elimination of cervical cancer in Canada, 2020–2030. Canadian Partnership Against Cancer https://www.partnershipagainstcancer.ca/topics/elimination-cervical-cancer-action-plan/ (2020).
Tranberg, M. et al. HPV self-sampling in cervical cancer screening: the effect of different invitation strategies in various socioeconomic groups – a randomized controlled trial. Clin. Epidemiol. 10 , 1027–1036 (2018).
Tope, P., Morais, S., El-Zein, M., Franco, E. L. & Malagón, T. Differences in site-specific cancer incidence by individual- and area-level income in Canada from 2006 to 2015. Int. J. Cancer 153 , 1766–1783 (2023).
Clegg, L. X. et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 20 , 417–435 (2009).
Hodge, J. M., Patel, A. V., Islami, F., Jemal, A. & Hiatt, R. A. Educational attainment and cancer incidence in a large nationwide prospective cohort. Cancer Epidemiol. Biomark. Prev. 32 , 1747–1755 (2023).
National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General (Centers for Disease Control and Prevention, 2014).
Doll R., Payne P. & Waterhouse J. Cancer Incidence in Five Continents: a Technical Report (Springer, 1966).
Eurostat. Revision of the European Standard Population: report of Eurostat’s task force. 2013 edition. European Commission ec.europa.eu/eurostat/web/products-manuals-and-guidelines/-/ks-ra-13-028 (2013).
UNESCO Institute for Statistics. International Standard Classification of Education: ISCED 2011 (UNESCO Institute for Statistics, 2011).
Download references
Author information
Authors and affiliations.
Department of Oncology, McGill University, Montréal, Quebec, Canada
Talía Malagón, Eduardo L. Franco & Romina Tejada
St Mary’s Research Centre, Montréal West Island CIUSSS, Montréal, Quebec, Canada
Talía Malagón
Department of Epidemiology Biostatistics, and Occupational Health, McGill University, Montréal, Quebec, Canada
International Agency for Research on Cancer, Lyon, France
Salvatore Vaccarella
You can also search for this author in PubMed Google Scholar
Contributions
R.T. and S.V. researched data for the article. T.M. and E.L.F. contributed substantially to discussion of the content. All authors wrote, reviewed and/or edited the manuscript before submission.
Corresponding author
Correspondence to Talía Malagón .
Ethics declarations
Competing interests.
T.M. is a board member of the International Papillomavirus Society. E.L.F. has received personal fees from Merck, and holds a patent related to the discovery of DNA methylation markers for the early detection of cervical cancer, which is registered at the Office of Innovation and Partnerships, McGill University, Montreal, Quebec, Canada. R.T. and S.V. declare no competing interests.
Peer review
Peer review information.
Nature Reviews Clinical Oncology thanks K. Canfell, who co-reviewed with S. Yuill; T. Fenton; and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Disclaimer Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Malagón, T., Franco, E.L., Tejada, R. et al. Epidemiology of HPV-associated cancers past, present and future: towards prevention and elimination. Nat Rev Clin Oncol (2024). https://doi.org/10.1038/s41571-024-00904-z
Download citation
Accepted : 30 April 2024
Published : 17 May 2024
DOI : https://doi.org/10.1038/s41571-024-00904-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Integrating Clinical, Community, and Policy Perspectives on HPV Vaccination
María e. fernández.
1 University of Texas Health Science Center at Houston, School of Public Health, Houston, TX
Jennifer D. Allen
2 Dana-Farber Cancer Institute and Harvard School of Public Health, Boston, MA
3 William Connell School of Nursing, Boston College, Chestnut Hill, MA
Ritesh Mistry
4 University of California at Los Angeles School of Public Health, Los Angeles, CA
Jessica A. Kahn
5 Cincinnati Children's Hospital Medical Center and the University of Cincinnati College of Medicine, Cincinnati, OH
Infection with genital human papillomavirus (HPV) may cause anogenital cancers, oropharyngeal cancers, anogenital warts, and respiratory papillomas. Two prophylactic vaccines (a bivalent and a quadrivalent vaccine) are now licensed and currently in use in a number of countries. Both vaccines prevent infection with HPV-16 and HPV-18, which together cause approximately 70% of cervical cancers, and clinical trials have demonstrated 90%-100% efficacy in preventing precancerous cervical lesions attributable to HPV-16 and HPV-18. One vaccine also prevents HPV-6 and HPV-11, which cause 90% of genital warts. A growing literature describes associations between psychosocial, interpersonal, organizational, and societal factors that influence HPV vaccination acceptability. This paper summarizes the current literature and presents an integrated perspective, taking into account these diverse influences. The resulting integrated model can be used as a heuristic tool for organizing factors at multiple levels to guide intervention development and future research.
Introduction
Human papillomaviruses (HPVs) are DNA viruses that infect the skin or mucosa. Genital HPVs are highly contagious and usually acquired through sexual contact, both penetrative and non-penetrative. Infection often occurs within the first few months after sexual initiation ( 112 ), and peak incidence occurs in 15- to 24-year-old men and women ( 36 , 110 ). Infection with high-risk or cancer-associated HPV types (e.g. HPV-16, HPV-18) may cause anogenital cancers such as cervical cancer as well as oropharyngeal cancers. Infection with low-risk types (e.g. HPV-6, HPV-11) may cause anogenital warts and respiratory papillomas ( 15 ).
Two prophylactic vaccines, a bivalent (HPV-16, -18) vaccine and a quadrivalent (HPV-6, -11, -16, -18) vaccine, are now licensed and currently in use in a number of countries. Both vaccines prevent infection with HPV-16 and HPV-18, which together cause approximately 70% of cervical cancers. The quadrivalent HPV vaccine also prevents infection with HPV-6 and HPV-11, which cause approximately 90% of anogenital warts.
While both HPV vaccines are expected to decrease morbidity and mortality related to HPV-associated diseases, the public health impact will depend upon vaccine uptake in the general population and in particular among groups disproportionately affected by these conditions. Recent estimates indicate current HPV vaccination rates are low among girls in the target age range (approximately 25%) and even lower among those at highest risk ( 37 ). HPV vaccine acceptability among clinicians, parents, and adolescents is likely to have a substantial impact on vaccine uptake. To date, the vast majority of studies have examined individual characteristics associated with vaccine acceptability ( 5 , 9 , 10 , 16 , 19 , 20 , 26 , 28 , 31 , 32 , 33 , 34 , 35 , 38 , 50 , 52 , 61 , 72 , 81 , 83 , 85 , 87 , 89 , 96 , 98 , 103 , 104 , 105 , 113 , 114 , 116 ). Other studies have identified external influences on vaccine uptake including provider recommendation and vaccine availability ( 30 , 66 , 73 , 77 , 78 , 88 , 93 , 95 ). Successful vaccination programs require an understanding of individual factors influencing vaccination decisions; broader issues related to access, processes of care delivery, societal vaccine policies; and the relationships among these various factors. Therefore, a comprehensive framework to understand HPV vaccine utilization and guide future research and vaccine promotion activities is needed ( 76 ).
The purpose of this paper is to 1) present a review of clinical data related to HPV vaccination; 2) summarize personal, interpersonal, provider, organizational, and policy factors influencing HPV vaccination uptake; and 3) propose a conceptual model for integrating the factors that affect access to and uptake of the vaccine. In this paper we organize existing findings related to determinants of HPV vaccination, discuss implications of available evidence for the development of comprehensive programs that address mediators of vaccine uptake across multiple levels of influence, and discuss remaining challenges and areas of needed research.
HPV Vaccines
Globally, cervical cancer is the second most common cancer among women and is thought to be the largest single cause of years of life lost to cancer in low-income countries ( 3 , 24 ). Approximately 490,000 women are diagnosed and 270,000 die from cervical cancer each year ( 24 ). In the U.S., despite organized cervical cancer prevention programs involving regular Pap screening in reproductive-age women, approximately 11,000 women are still diagnosed with cervical cancer and 4,000 women die from the disease annually ( http://seer.cancer.gov/cgi-bin/csr/1975_2005/search.pl#results ). Substantial racial, ethnic, and socioeconomic disparities exist for HPV infection, cervical cancer incidence, and cervical cancer mortality ( 45 , 74 ). Direct costs associated with the prevention and treatment of HPV-related disease are at least 4 billion U.S. dollars (USD) per year, and indirect costs such as productivity losses add 1.3 USD per year ( 63 , 64 ).
Clinical trials of the bivalent vaccine have been conducted in women 10-55 and men 10-18 years of age; trials of the quadrivalent vaccine have been conducted in women 9-45 and men 10-26 years of age ( 12 , 18 , 47 , 48 , 57 , 58 , 71 , 92 , 94 ). The clinical endpoints in efficacy studies of both vaccines include cervical intraepithelial neoplasia (CIN) 2/3 and adenocarcinoma in-situ (AIS), generally considered to be cervical cancer precursors, as well as other anogenital cancer precursors (e.g. anal intraepithelial neoplasia). Anogenital warts are also an endpoint for clinical trials of the quadrivalent vaccine.
Information about the immunogenicity and efficacy of the bivalent and quadrivalent HPV vaccines is available from several international, randomized clinical trials. Seroconversion rates are ≥ 97.5% for both vaccines ( 18 , 48 , 92 , 94 ). Studies suggest that although immune responses tend to be durable for at least five years after vaccination, antibody levels do eventually decline, especially for HPV-18 ( 44 , 57 , 58 , 108 ). However, lower antibody levels do not appear to lead to breakthrough disease, possibly due to immune memory ( 70 ). Data from ongoing clinical trials will be needed to confirm the duration of immunity and to determine whether there will be a need for a booster dose. Both HPV vaccines appear to generate cross-neutralizing antibodies against related HPV types not targeted by the vaccines, and therefore may provide protection against disease caused by those types ( 7 , 23 , 69 ).
Clinical trials of both the bivalent and quadrivalent vaccines have demonstrated 90%-100% efficacy in preventing precancerous cervical lesions attributable to HPV-16 and -18, among women who were uninfected with those HPV types before vaccination and who received all three vaccine doses ( 6 , 47 , 48 , 57 , 71 , 108 ). The quadrivalent vaccine is also highly effective in preventing anogenital disease caused by HPV-6 and -11 ( 48 ). Efficacy is substantially lower among women who may be HPV-infected at the time of vaccination ( 47 ), because vaccination does not protect against HPV-16 or -18 infection or disease in women infected with those HPV types at the time of vaccination ( 47 , 48 , 92 ). In addition, vaccination does not facilitate clearance of HPV-16 and -18 in infected women ( 59 ). Thus, HPV vaccines will have the most significant impact on individual and population health if vaccination is targeted to early adolescents, prior to sexual initiation and thus before HPV acquisition.
Extensive clinical trials data and post-marketing safety monitoring data have demonstrated that both HPV vaccines are generally safe and well-tolerated. The Centers for Disease Control and Prevention (CDC) in the U.S. ( http://www.cdc.gov/vaccinesafety/vaers/gardasil.htm ) and the World Health Organization (WHO) Global Advisory Committee on Vaccine Safety have concluded that data support the safety of both HPV vaccines ( http://www.who.int/vaccine_safety/reports/June_2007/en/index.html ). Mild injection-site adverse events (e.g. pain, redness, itching, and swelling) are fairly common, while mild systemic adverse events such as low-grade fevers are uncommon. Serious adverse events were rare in the clinical trials, and did not occur more frequently in vaccine than placebo participants ( 47 , 92 ). Post-licensing studies to evaluate quadrivalent vaccine safety, and especially to detect rare adverse events, have been conducted by the CDC, other public health organizations globally, and the vaccine manufacturers since 2006. Rare post-marketing reports of serious illness or death occurring at some point after receipt of the quadrivalent vaccine have been reported, but none of these events appear to be caused by the vaccine ( http://www.fda.gov/cber/safety/gardasil071408.htm ).
Both the quadrivalent and bivalent vaccines have been licensed in many countries, and in those countries with national immunization programs that have established recommendations for HPV vaccination, there is general consensus that young adolescent girls should be targeted for vaccination ( 80 ). In the U.S., it is recommended that HPV vaccination be targeted to 11- to 12-year-old girls, and catch-up immunization is also recommended for 13- to 26-year-old women ( 84 ).
In summary, HPV vaccines are available and recommended for use in many countries. The data thus far suggest that they are safe, and are highly effective when administered to young women who are uninfected with HPV types included in the vaccine. However, the public health impact of vaccination can only be realized if vaccine uptake is high in young women who have not yet initiated sexual intercourse, and are therefore unlikely to be infected with HPV. Thus, integrating community and policy perspectives related to HPV vaccine access and uptake is critical to maximizing the potential health impact of these vaccines.
Factors Influencing HPV Vaccination
The existing literature on HPV vaccination includes many studies that describe associations between various individual and healthcare factors and HPV vaccination. However, no logic model or conceptual framework has been described that presents an integrated perspective of HPV vaccination and that can be used to guide intervention development and future research. The majority of studies that describe psychosocial factors associated with vaccine acceptability or intent measure constructs from theories including the Health Belief Model ( 17 ), Theory of Reasoned Action ( 4 ), and Social Cognitive Theory ( 11 ), yet often there is no mention of the theoretical model that guided selection of hypothesized determinants (JD Allen, GD Coronado, RS Williams, B Glenn, C Escoffery, M Fernandez, RA Tuff, KM Wilson, PD Mullen. A systematic review of measures used in studies to assess acceptability of the human papillomavirus (HPV) vaccine. Manuscript under review.) Studies of personal, organizational, or societal factors that are associated with vaccine acceptability, which will be summarized next, each provide only a partial perspective on the multitude of factors that may influence vaccine uptake. The evidence-based conceptual model that will be described following the literature review facilitates consideration of multiple determinants of vaccination across a number of theoretical models of health behavior. As such, it provides a useful framework for examining the impact of personal, interpersonal; organizational; and broader community and societal factors on vaccination ( 11 , 13 , 56 , 86 ). The model also provides a framework for understanding the complex interrelationships between these multiple levels of influence, which often have a profound impact on individual decision-making and behavior.
Personal Factors Influencing HPV Vaccination
Parental acceptability for hpv vaccination.
Because the HPV vaccine is recommended for young adolescent girls, parents will have authority for making most decisions about vaccination. Therefore, the success of programs designed to maximize vaccine uptake will largely depend upon parental decision-making. A number of quantitative studies have examined parental willingness to vaccinate their daughters. Most were conducted prior to licensure of the vaccine ( 9 , 20 , 26 , 31 , 32 , 33 , 50 , 81 , 98 , 103 , 114 , 116 ). Approximately half of these were conducted in the U.S. ( 9 , 28 , 31 , 33 , 38 , 50 , 72 , 96 , 103 , 104 , 116 ). Most have been conducted in clinical settings ( 9 , 26 , 31 , 33 , 34 , 38 , 50 , 96 , 98 , 105 , 116 ), and many have been conducted in schools ( 20 , 32 , 83 , 85 , 105 , 114 ). Only a few have been population-based ( 20 , 28 , 72 , 89 ).
Overall, knowledge about HPV and its relationship to cervical cancer among parents is low. In quantitative studies conducted post-licensure and that assessed knowledge, 60%-89% of parents reported that they were not aware of HPV until the time of the survey ( 20 , 34 , 38 , 83 , 89 ). In some cross-sectional studies, an association between HPV knowledge and vaccine acceptability has been observed ( 38 , 81 , 114 ). In addition, increased willingness to vaccinate following provision of factual information about HPV has been observed in some ( 26 , 31 , 32 ), but not all, studies ( 33 ).
Despite low levels of knowledge demonstrated across these studies ( 79 ), the majority of parents (70% to 85%) expressed a willingness to vaccinate their daughter(s) ( 9 , 20 , 28 , 89 ). Two recent studies have reported lower levels of willingness to vaccinate (48% and 52%, respectively)( 26 , 72 ). Some ( 33 , 72 , 85 ) but not all ( 96 , 116 ) studies have shown parental acceptability of vaccination may vary with the child's age, with parents being more willing to vaccinate daughters who are older than the age recommended by the Advisory Committee on Immunization Practices (ACIP) ( 11 - 12 ).
Parental vaccine acceptability has also been associated with a number of beliefs and attitudes suggested by theories of health behavior, the Health Belief Model ( 17 ), being the most common. Parents who feel their child is susceptible to HPV or to cervical cancer ( 33 , 38 , 50 ), worry about HPV or sexually transmitted infections ( 20 , 26 ), believe the vaccine is efficacious and will afford benefits ( 9 , 20 , 31 , 32 , 33 , 38 , 50 , 72 , 81 , 116 ), and perceive few barriers to accessing the vaccine ( 38 , 98 ) are more likely to report willingness to vaccinate. Perceived severity of HPV and associated health consequences was related to parental attitudes toward vaccination in some ( 9 , 38 , 72 , 85 , 116 ), but not all ( 33 ) studies. Positive attitudes toward vaccines in general have also been associated with parental intention to obtain the HPV vaccine for one's daughter ( 72 ). Social influences, such as the approval of significant others or the perception that vaccination is normative, have been associated with vaccine acceptability ( 33 , 34 , 72 , 85 , 89 ). Across studies, the importance of provider endorsement of the vaccine is clear ( 33 , 34 , 50 , 98 ).
By far, the major concerns expressed by parents are related to vaccine safety and potential adverse behavioral consequences. Those who believe the vaccine to be safe ( 20 , 31 , 32 , 50 ), or have more positive attitudes toward vaccines ( 28 , 72 , 89 , 114 ) are substantially more likely to report that they would vaccinate their children. In a number of studies, parents expressed concern that vaccination would send a message to their children condoning sex or could encourage earlier age of sexual initiation, and this was associated with lower rates of vaccine intentions ( 26 , 28 , 31 , 114 ). The proportion of parents reporting concerns about sexual disinhibition varies considerably across studies, with some studies reporting extremely low prevalence of this concern ( 34 , 83 , 89 ) and others reporting this to be an issue for as much as a quarter to a third of study subjects ( 98 , 105 ). In a study of Texas physicians, at least half noted that parents in their practice had refused the HPV vaccine because of concerns about vaccine safety, lack of parental education, negative media reports about the vaccine, and parental concern that their consent would imply that they condone premarital sexual intercourse ( 73 ).
Few studies have systematically examined the association between socio-demographic characteristics and acceptability of vaccination. Those examining these relationships have yielded mixed results, with some finding no impact of parental age, educational level or marital status ( 83 ), and others finding differences across race/ethnicity ( 28 , 85 ), education and income levels ( 31 ).
Acceptability of receiving the vaccine for oneself
Although personal intentions to receive the HPV vaccine and vaccine acceptability among adults, young adults or adolescents vary substantially, from 48% ( 72 ) to 96% ( 16 ), generally acceptability is high, in the range of 66% to 86% ( 19 , 35 , 38 , 50 , 52 , 61 , 76 , 87 , 98 , 104 , 114 ).
Some studies have reported factors associated with vaccine acceptability or intent for oneself to receive the vaccine. Constructs most often assessed have included knowledge, perceived susceptibility, perceived severity, social influence, perceived effectiveness, barriers to vaccination, and perceived benefits. ( 5 , 19 , 22 , 33 , 38 , 39 , 40 , 46 , 49 , 50 , 51 , 52 , 53 , 60 , 75 , 76 , 87 , 113 , 114 ).
Social influences, such as the perception that one's parents approve of vaccination, predicted vaccine acceptability among adolescents and young adults ( 19 , 27 ). Other studies found perceived provider endorsement of the vaccine was associated with increased vaccine acceptability ( 27 , 50 , 117 ). Perceived social norms (the belief that others like them will get vaccinated) also has been shown to predict vaccine acceptability ( 5 , 49 ). In a recent study, subjective norms (belief one's parents, partners, and/or clinicians supported vaccination) influenced receipt of at least one HPV vaccine dose ( 27 ).
Some studies have demonstrated a relationship between perceived severity of HPV or cervical cancer and higher vaccine acceptability ( 40 , 76 ). Other studies, however, have not shown this relationship ( 19 , 33 , 75 ).
Beliefs about the safety of the HPV vaccine were positively associated with vaccine acceptability in some studies ( 19 , 50 ), and concerns about potential side effects were negatively associated with acceptability in others ( 31 , 87 , 103 , 113 ). Perceived effectiveness of the vaccine ( 19 , 38 , 39 , 50 , 118 ) and low cost ( 19 , 39 , 49 , 98 , 113 , 117 ) were also associated with acceptability.
Environmental Factors Influencing HPV Vaccination
Interpersonal.
It is well-established that clinician recommendation is one of the most important predictors of a parent's decision to accept vaccination for his or her child ( 29 , 55 , 97 ). Therefore, understanding clinicians' intention to recommend HPV vaccines and actual HPV vaccine recommendations is crucial, as identification of those factors predicting intentions and recommendations may help to guide the design of interventions. Studies conducted prior to vaccine licensure demonstrated that physicians reported high intention to recommend HPV vaccines, although some studies suggested that intentions varied; clinicians reported higher intention to recommend a vaccine to girls compared to boys, and to older compared to younger children ( 30 , 66 , 77 , 78 , 95 ). In a qualitative study of pediatricians, preferences for vaccinating girls were based primarily on the belief that girls would derive a greater health benefit from vaccination than boys, and the concern that it would be difficult to convince boys and their parents that boys should be vaccinated ( 77 ). Those who intended to vaccinate pre-adolescents cited the importance of vaccinating prior to sexual initiation, while those who preferred to vaccinate older adolescents perceived their patients to be at low risk for HPV or were concerned about the need to discuss sexuality when recommending the HPV vaccine.
Studies conducted after HPV vaccine licensing generally confirm the findings of earlier studies. In a study of Texas primary care physicians conducted two years after the HPV vaccine was licensed in the U.S., fewer than half reported that they always recommended the HPV vaccine to 11- to 12-year-old girls ( 73 ). Two-thirds reported always vaccinating 13- to 17-year-old girls, suggesting that parents or physicians may be delaying vaccination until girls are older than 12 years of age. The finding that providers are reluctant to vaccinate young adolescents is problematic because by ninth grade, approximately one-third of U.S. adolescents have initiated sexual intercourse ( 25 ). Prophylactic HPV vaccines will not prevent HPV-related disease caused by a vaccine-type HPV if a young woman is infected with that HPV type at the time of vaccination. Thus, the public health impact of vaccination will not be maximized if vaccination is delayed in young women until after sexual initiation, as HPV is often acquired soon after sexual initiation ( 112 ).
Research has also shown that clinicians' personal characteristics, practice characteristics, knowledge about HPV, and attitudes are associated with intention to recommend and actual recommendation of HPV vaccines. Key attitudes include: belief that influential organizations endorse vaccination, perceived susceptibility of one's patients to HPV-related disease, perceived severity of HPV-related disease (particularly cervical cancer), and benefits and barriers to vaccination ( 77 , 78 , 88 , 93 , 95 ). In one study, factors that independently predicted a clinician's recommendation to vaccinate an 11-12 year-old girl included: percentage of patients with Medicaid, academic vs. non-academic practice, office procedures to maximize vaccination, HPV knowledge, valuing HPV vaccine information from professional organizations and professional conferences, belief in mandated HPV vaccination, and barriers to vaccination ( 73 ).
Organizational
In most countries, the successful introduction of a new vaccine depends upon the following steps: the vaccine is licensed by a regulatory agency, a national immunization program establishes vaccination recommendations, professional organizations provide guidance for vaccination, new infrastructure is developed or existing infrastructure utilized to ensure vaccine delivery, the cost of vaccination is financed by public or private mechanisms, and individuals at risk for disease then access the vaccine ( 102 ).
Both the quadrivalent and bivalent vaccines have been licensed in many countries and some national immunization programs have established recommendations for vaccination( 80 ). However, there are two major organizational barriers to HPV vaccine implementation. First, the infrastructure for implementation of an adolescent vaccination program is poorly developed in many countries, particularly low- and middle-income countries. School-based programs may be an efficient mechanism for adolescent vaccination ( 21 ); however, vaccine coverage will be limited in countries where young adolescent girls are not likely to be in school. Public health campaigns, such as the Expanded Program on Immunization (EPI), generally are designed to target infants or young children, and may not effectively reach adolescents. Thus, each country must develop a service delivery strategy for HPV vaccines, based upon what is feasible and culturally acceptable ( 115 ).
Second, even in countries where adolescent vaccination programs are well-established, evidence-based systems to maximize vaccine recommendations and uptake are not in place in many offices, schools, public health clinics, and other settings where vaccination takes place. These include recall and reminder systems (e.g. calls or letters to patients or families to remind them to make or keep vaccine appointments); clinician reminders for vaccination; auditing and feedback to clinicians; standing orders for vaccination; and regional immunization registries ( 107 ). Such systems appear to be effective in increasing vaccination rates in both children and adults ( 68 , 106 ). The establishment of a country-specific service delivery strategies as well as the implementation of specific systems to ensure high rates of vaccination will be critical for maximizing HPV vaccine uptake ( 100 ).
Community and societal
The currently available quadrivalent HPV vaccine is one of the most costly of all available pediatric and adult vaccines. According to the CDC, the cost of the vaccine for the private sector is estimated at about 130 USD per dose (390 USD for three doses). The CDC negotiated price is currently 100 USD per dose ( http://www.cdc.gov/vaccines/programs/vfc/cdc-vac-price-list.htm ). High cost is an important barrier to obtaining HPV vaccination ( 22 ), as has been reported by studies in a variety of populations ( 19 , 46 , 76 , 117 ). Insurance coverage can help mitigate the financial costs of vaccination; lack of coverage is a frequently cited barrier to delivery of adolescent immunization ( 27 , 91 , 99 ). In the U.S., the cost of vaccination is covered for many girls with private insurance, and is also covered for many low-income girls by public financing (Medicaid and the Vaccines for Children Program or VFC). However, not all women in the recommended age group for vaccination can afford vaccination; not all commercial insurance plans cover all or the full cost of vaccination; and adolescents from low-income families – especially those older than 18 years of age – may not qualify for vaccination under Medicaid or VFC ( 1 , 2 ). Private insurance coverage and coverage through federal and state programs may partially address cost as a barrier to vaccination; however, there are costs associated with clinic visits and the time spent on obtaining the HPV vaccine ( 14 ). One study suggests that to reach optimum vaccine coverage levels, subsidies would need to be offered to cover these additional costs; alternatively, the cost of the quadrivalent vaccine would have to decrease by $55 per dose ( 14 ).
Since the ACIP recommendations for HPV vaccination were published ( 84 ), there has been a flurry of policy and regulatory activities that could potentially impact uptake of the vaccine. The activities focus on financing HPV vaccination particularly through the Vaccine for Children program, Medicaid and private insurance; legislation regarding mandatory HPV vaccination; and education about HPV vaccination.
In the U.S., HPV vaccine financing requirements have in large part been borne by private health insurance companies, that tend to cover the majority of costs associated with obtaining recommended vaccines for their members. However, not all insurance companies cover all recommended vaccines. To increase coverage, many states have introduced legislation to require private insurance companies to cover the HPV vaccine, but only a handful such as Iowa, Colorado, Illinois, Nevada, New Mexico, Rhode Island, and Texas have enacted such laws. For children 18 years or younger who are eligible for Medicaid and do not have other health insurance, the federally funded VFC program covers the cost of immunizations. Unfortunately, significant numbers of children are under-insured and not eligible for the VFC program. For them, Section 317 of the Public Health Service Act provides funds to states to cover vaccination costs, but funding from this source has not kept pace with the rising cost of vaccines making it difficult for states for cover cost of HPV vaccination for these children and for other uninsured and under-uninsured groups ( 65 ). In addition, there are no widespread federal programs to assist with the costs of HPV vaccination for low-income or uninsured women between the ages of 19-26 years who cannot afford to pay for the vaccine. In response, some states such as New Hampshire, South Dakota and Washington, are supplementing federal funds to increase HPV vaccine uptake by providing vaccines at low or no cost.
A policy strategy to increase uptake of vaccines has been to use vaccination mandates, particularly for school entry ( 67 , 111 ) and immigration. Bills requiring HPV vaccination for school entry have been introduced with much controversy in 28 states and the District of Columbia. In February, 2007, Texas became the first state to enact a school entry mandate by executive order from the governor. The Texas legislature, however, overrode the governor's order and in fact later passed a law to prohibit mandates for HPV vaccination. Also in 2007, Virginia passed a school vaccine requirement but later removed the requirement. The District of Columbia remains the only place in the United States that has enacted and kept a school entry mandate for the HPV vaccine (with an opt-out option).
Some states have taken the approach of using policies to increase educational efforts regarding the HPV vaccine. For example, Iowa, Colorado and North Carolina enacted legislation that requires HPV vaccine education in schools through sex education classes or by providing educational materials to parents. Other states, such as Texas and North Dakota have enacted legislation to require their state department of health to educate the public about the HPV vaccine, so that parents are fully informed about vaccine decisions.
Conceptual Framework/Logic Model for Integrating Clinical, Community and Policy Interventions for Promoting HPV Vaccine Uptake
Figure 1 presents an evidence-based logic model reflecting the existing literature on determinants of HPV vaccination and represents relationships between various factors. This type of conceptual model, described by Bartholomew, et al., ( 13 ) and derived from the PRECEDE model ( 56 ), provides a framework for organizing the causal factors associated with the health behavior of interest. Additionally, this model allows the depiction of multiple interacting levels of the environment, which often have a profound impact on individual decision-making and behavior.

Logic Model of Factors Influencing HPV Vaccination
The model proposes that willingness or intention to vaccinate one's daughter or oneself (“behavioral factors”) influence HPV vaccination, which in turn leads to a reduction in HPV-related disease. “Environmental factors” (interpersonal, organizational, and community/societal) also influence willingness or intention to vaccinate one's daughter or oneself. Finally, factors influencing behavioral factors and environmental factors are shown in the boxes to the left. The relationships represented in Figure 1 are proposed pathways that are supported by the evidence reviewed above. However, since many of the findings are based on cross-sectional studies and measures of vaccine acceptability rather than actual uptake, it is important to view this model as a proposed framework that may be further supported or modified based on future studies.
Recommendations for Interventions and Future Research
Implications for interventions.
A goal of this paper has been to integrate findings from studies that have examined personal, interpersonal, organizational and societal factors that influence HPV uptake to inform a conceptual model ( Figure 1 ) for intervention planning and evaluation. Multi-component intervention approaches that include methods and strategies to effect change at multiple levels are likely to have the greatest impact on HPV vaccination behavior.
Educational interventions and messages for both parents and young women should stress the low rate of serious adverse events associated with vaccination, and emphasize evidence of durable efficacy. Promoting awareness of the high prevalence of HPV infection and the fact that young women are vulnerable to HPV-associated illnesses, including cervical cancer, will also be needed given perceptions of low susceptibility among both women and parents. Given the importance of social and subjective norms in studies both of these groups, it may be useful to develop peer-led interventions (for young women) or educational campaigns that depict vaccination as normative. Specific educational interventions for parents must also stress that vaccination is most effective if administered prior to sexual initiation and should also address concerns about potential adverse effects on adolescent sexual behaviors. Interventions that promote effective communication between parents and their children about sexual health issues may lessen these concerns. Intervention approaches and messages that are effective for low literacy audiences are critical to avoid disparities in vaccine uptake ( 109 ). Finally, removing or reducing cost barriers, by providing information about federal vaccine programs that offer free vaccine for families who qualify, and alerting individuals to the potential for insurance coverage by many carriers, would also be an important aspect of interventions.
Providers clearly play an important role in the vaccine decisions of parents and other individuals, and are an important resource in efforts to improve accuracy of risk perceptions, promote and endorse vaccination prior to sexual initiation, and educate parents about issues related to the sexual health of their children. Organizational and provider-level interventions should address factors such as vaccine policies and procedures; knowledge and awareness of HPV vaccines/vaccine guidelines; communication about vaccines; and potential barriers such as vaccine cost ( 77 ).
Office- or clinic-based vaccination, school-based vaccination programs, and public health campaigns are some of the methods by which adolescent vaccines may be successfully delivered. Clinic procedures to improve vaccination rates, such as recall and reminder systems, are effective, particularly if these systems are automated. In addition, clinician reminders about vaccination, as well as chart-auditing with feedback to clinicians, can be an effective strategy for changing provider practices ( 68 , 106 , 107 ). School-based programs have been shown to be feasible and acceptable in high-income countries ( 21 ). Schools can also provide education about vaccines for students and distribute information to parents.
Ensuring that HPV vaccination is affordable and accessible will be critical to ensure high uptake rates and avoid inequities in vaccination. Reducing costs for the individual via insurance coverage and through state and federal programs will be crucial. More importantly, federal efforts will be needed to negotiate cost reductions for the public health system. Both will likely be complicated, given broader economic pressures. Federal programs such as VFC and funds from Section 317 of the Public Health Service Act will likely need to be augmented by state funds to ensure greater access and equitable delivery of the vaccine.
Policy strategies will also play a critical role in HPV vaccine uptake. While the role of mandates remains controversial, school-entry requirements have been extremely effective in attaining high rates of vaccination for other diseases ( 8 , 90 ). Mandates may not be politically feasible in the U.S. now, but state legislation that requires private and public insurance coverage for the vaccine, as well as requirements for public education about the vaccine may be useful in addressing some of the personal and organizational barriers noted above.
Future research
The logic model in Figure 1 depicts a broad perspective for considering a number of factors influencing HPV vaccination and helps identify gaps in the evidence. Based on this review, it is evident that additional research is needed to: 1) document HPV vaccination behavior and monitor potential variability across population subgroups; 2) provide stronger evidence of the association between behavioral and/or environmental factors and vaccination behavior, and examine the interrelationships between factors that influence vaccination at various levels; 3) examine the impact of vaccination on sexual behavior and cervical cancer screening; and 4) evaluate the effectiveness of multilevel interventions.
Since the majority of studies on both parental and individual attitudes and beliefs about the HPV vaccine were conducted prior to licensure, studies examining factors related to actual vaccine receipt are needed. Theoretical and empirical evidence suggest a strong link between intention and behavior ( 41 , 42 , 43 ); however, some studies have failed to show this relationship ( 27 ). Prospective studies that identify predictors of actual vaccine uptake (that is, receipt of at least one vaccine dose) and completion of the series will provide stronger evidence of the causal relationships between personal and external factors and vaccination. Monitoring to ensure equitable distribution and uptake of the vaccine is also critical.
A better understanding of factors that influence organizational and community/societal predictors of HPV vaccination is needed. Studies that address questions such as why organizational and policy changes take place, who is responsible, and what factors would influence decision-makers to make these changes are all needed to develop interventions that can impact this level of the environment. Moreover, studies that simultaneously examine personal and environmental factors and their interaction are needed to better understand their reciprocal relationships on vaccination.
Research on the impact of vaccination on perceived risk and sexual behaviors is another area of needed study ( 14 ). While studies of parental beliefs indicate that some parents are concerned that vaccination may lead to riskier sexual behaviors, studies of other interventions that protect against potential negative consequences of sexual activity do not support this notion ( 54 , 101 ). Studies that examine the impact of vaccination on sexual behavior are needed to construct evidence-based messages that can address the concern parents express and to design interventions to minimize adverse behavioral responses to vaccination. In addition, since continued Pap test screening is needed for individuals who have been vaccinated studies should examine how vaccination impacts screening behaviors and interventions should emphasize the importance of continued routine screening after HPV vaccination.
Perhaps one of the most significant gaps in the literature exists is in the development and evaluation of interventions designed to increase HPV vaccination. Health communication studies that test various characteristics of vaccine promotion messages including message framing, source, delivery channel, and content are needed ( 52 , 53 , 62 , 82 ). Information as to the most effective way to present the vaccine (e.g., as an STI vaccine vs. a cervical cancer vaccine) ( 62 ) in order to increase uptake, and whether preferences for information differ between parents and young women, can inform intervention approaches. Additionally, studies that explore the relative efficacy of interventions that utilize an “informed decision-making” approach that aims to inform individuals of the risks, benefits and limitations of vaccination, versus a “persuasion approach” that aims to maximize vaccine uptake without decision support are also needed.
Additional studies are needed to understand the variety, scope and impact of legislative activities at the state and federal level in the United States, and of the driving forces behind policy formation and enactment. Research is also recommended to determine the optimal cost for the vaccine, so that it is affordable enough to ensure that vaccination rates are high enough to achieve herd immunity as well as to reduce the potential inequities in vaccine uptake and cervical cancer. Finally, research in developing countries and among groups at highest risk for HPV-associated disease is needed. Women who are not typically screened for cervical cancer are also likely not to receiving the vaccine for themselves and their daughters. Specific research and intervention approaches are needed to encourage vaccination in these groups so as not to exacerbate existing disparities in HPV-associated diseases.
Summary Points
- HPV vaccines are safe and effective; maximizing the public health benefits of HPV vaccines will depend on uptake.
- Both personal and external factors influence vaccine acceptability and intent.
- Using a multilevel socio-ecological model to organize factors influencing HPV vaccination can help in the design of interventions and guide future research.
- Interventions that affect change in parental and personal knowledge and attitudes about the HPV vaccine are needed to increase uptake.
- Multilevel interventions impacting personal, interpersonal, organizational and societal change are likely to be the most effective in increasing HPV vaccination uptake.
- Future research should focus on evidence of the association between behavioral and/or environmental factors and actual vaccination behavior, examine the interrelationships between factors that influence vaccination at various levels, and evaluate the effectiveness of multilevel interventions.
Acknowledgements
We thank Karyn Popham, Dolores Proubasta, Jon Kerner, Matthew Kreuter
Research for this publication was supported by the Centers for Disease Control and Prevention (CDC) and the National Cancer Institute (NCI) cooperative agreements for the Cancer Prevention and Control Research Networks (CPCRN) at University of Texas School of Public Health ((1-U48-DP-000057); Harvard School of Public Health/Boston School of Public Health (1-U48-DP000064); University of California at Los Angeles School of Public Health (1U48DP000056: SIP 16-04); and by R01 AI073713 and the Charlotte R. Schmidlapp Award, Fifth-Third Bank, Cincinnati, OH.
Key Terms and Definitions
Literature cited.
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Role of human...
Role of human papillomavirus (HPV) vaccination on HPV infection and recurrence of HPV related disease after local surgical treatment: systematic review and meta-analysis
- Related content
- Peer review
- Konstantinos S Kechagias , doctoral student 1 ,
- Ilkka Kalliala , senior lecturer 1 2 ,
- Sarah J Bowden , postdoctoral research fellow 1 ,
- Antonios Athanasiou , doctoral student 1 ,
- Maria Paraskevaidi , postdoctoral research fellow 1 ,
- Evangelos Paraskevaidis , professor 3 ,
- Joakim Dillner , professor 4 ,
- Pekka Nieminen , associate professor 2 ,
- Bjorn Strander , associate professor 5 ,
- Peter Sasieni , professor 6 ,
- Areti Angeliki Veroniki , assistant professor 1 7 ,
- Maria Kyrgiou , professor 1 8
- 1 Department of Metabolism, Digestion, and Reproduction and Department of Surgery and Cancer, Faculty of Medicine, Imperial College London, London, UK
- 2 Department of Obstetrics and Gynaecology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland
- 3 Department of Obstetrics and Gynaecology, University of Ioannina, Ioannina, Greece
- 4 Centre for Cervical Cancer Prevention, Medical Diagnostics Karolinska, Karolinska University Hospital and Karolinska Institutet, Stockholm, Sweden
- 5 Department of Obstetrics and Gynaecology, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
- 6 King’s Clinical Trials Unit, King's College London, London, UK
- 7 Li Ka Shing Knowledge Institute, St Michael’s Hospital, Unity Health Toronto, Toronto, ON, Canada
- 8 Imperial College Healthcare NHS Trust, London, UK
- Correspondence to: M Kyrgiou m.kyrgiou{at}imperial.ac.uk
- Accepted 14 June 2022
Objective To explore the efficacy of human papillomavirus (HPV) vaccination on the risk of HPV infection and recurrent diseases related to HPV infection in individuals undergoing local surgical treatment.
Design Systematic review and meta-analysis
Data sources PubMed (Medline), Scopus, Cochrane, Web of Science, and ClinicalTrials.gov were screened from inception to 31 March 2021.
Review methods Studies reporting on the risk of HPV infection and recurrence of disease related to HPV infection after local surgical treatment of preinvasive genital disease in individuals who were vaccinated were included. The primary outcome measure was risk of recurrence of cervical intraepithelial neoplasia grade 2 or higher (CIN2+) after local surgical treatment, with follow-up as reported by individual studies. Secondary outcome measures were risk of HPV infection or other lesions related to HPV infection. Independent and in duplicate data extraction and quality assessment were performed with ROBINS-I and RoB-2 tools for observational studies and randomised controlled trials, respectively. Grading of Recommendations Assessment, Development, and Evaluation (GRADE) was implemented for the primary outcome. Observational studies and randomised controlled trials were analysed separately from post hoc analyses of randomised controlled trials. Pooled risk ratios and 95% confidence intervals were calculated with a random effects meta-analysis model. The restricted maximum likelihood was used as an estimator for heterogeneity, and the Hartung-Knapp-Sidik-Jonkman method was used to derive confidence intervals.
Results 22 articles met the inclusion criteria of the review; 18 of these studies also reported data from a non-vaccinated group and were included in the meta-analyses (12 observational studies, two randomised controlled trials, and four post hoc analyses of randomised controlled trials). The risk of recurrence of CIN2+ was reduced in individuals who were vaccinated compared with those who were not vaccinated (11 studies, 19 909 participants; risk ratio 0.43, 95% confidence interval 0.30 to 0.60; I 2 =58%, τ 2 =0.14, median follow-up 36 months, interquartile range 24-43.5). The effect estimate was even stronger when the risk of recurrence of CIN2+ was assessed for disease related to HPV subtypes HPV16 or HPV18 (six studies, 1879 participants; risk ratio 0.26, 95% confidence interval 0.16 to 0.43; I 2 =0%, τ 2 =0). Confidence in the meta-analysis for CIN2+ overall and CIN2+ related to HPV16 or HPV18, assessed by GRADE, ranged from very low to moderate, probably because of publication bias and inconsistency in the studies included in the meta-analysis. The risk of recurrence of CIN3 was also reduced in patients who were vaccinated but uncertainty was large (three studies, 17 757 participants; 0.28, 0.01 to 6.37; I 2 =71%, τ 2 =1.23). Evidence of benefit was lacking for recurrence of vulvar, vaginal, and anal intraepithelial neoplasia, genital warts, and persistent and incident HPV infections, although the number of studies and participants in each outcome was low.
Conclusion HPV vaccination might reduce the risk of recurrence of CIN, in particular when related to HPV16 or HPV18, in women treated with local excision. GRADE assessment for the quality of evidence indicated that the data were inconclusive. Large scale, high quality randomised controlled trials are required to establish the level of effectiveness and cost of HPV vaccination in women undergoing treatment for diseases related to HPV infection.
Systematic review registration PROSPERO CRD42021237350.
Introduction
The introduction of human papillomavirus (HPV) vaccines has revolutionised the prevention of cervical cancer. 1 HPV vaccines are highly effective in preventing HPV infection and diseases related to HPV infection when given to prepubertal girls and boys, but do not clear or reduce persistence of the virus in women with ongoing infections. 2
The cost effectiveness of HPV vaccination declines substantially after the age of 26. 3 Women undergoing local surgical treatment for cervical intraepithelial neoplasia (CIN), however, have been identified as a target high risk population that would benefit from adjuvant vaccination to reduce the risk of cervical cancer. Women who develop high grade CIN are particularly sensitive to infection with HPV and can rapidly re-acquire infections after local surgical treatment. 4 These women have an increased risk of recurrent CIN and other malignancies related to HPV infection, 5 6 and repeat conisations have been associated with adverse reproductive outcomes. 7 8 9 10 11 12
A previous study has shown that HPV vaccines are much more immunogenic than the infection. 13 Natural immunity from induced antibodies seems to wane over time, and vaccines have been reported to provide protection from reinfection or reactivation in individuals who are seropositive with a previous cleared infection. 14 15 The vaccine might have beneficial effects against new infections and reinfections from the same HPV subtype soon after treatment, although it is less likely that this effect promotes clearance of an existing infection in isolation. Whether the vaccine can boost the effect of local surgical treatment and promote viral clearance is unclear.
Evidence of the efficacy of HPV vaccination during conisation procedures is contradictory. Post hoc analyses of randomised controlled trials provided indirect evidence of a reduction in the recurrence of high grade CIN for individuals who had been vaccinated and subsequently required conisation compared with those who had been vaccinated with a placebo. 16 17 Although some observational studies found a reduction in the recurrence of high grade CIN of up to 80% in women who were vaccinated at the time of treatment, 18 other studies refuted these findings and suggested no benefit. 19 20 Also, two randomised controlled trials suggested a reduction in the recurrence of CIN but both studies were grossly underpowered with less than 250 patients recruited in each study. 21 22 In this systematic review and meta-analysis, our aim was to review the existing literature and examine the effect of HPV vaccination on the risk of HPV infection and recurrence of preinvasive disease related to HPV infection after local surgical treatment for cervical disease or other diseases related to HPV infection.
Our systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 23
Literature search
We searched PubMed (Medline), Scopus, Cochrane, Web of Science, and ClinicalTrials.gov from inception to 31 March 2021. Two of the authors (KSK and AA) developed the search strategy and screened the articles independently (supplementary material).
Eligibility criteria
The systematic review included all studies reporting on HPV infection rates and recurrence of diseases related to HPV infection after local surgical treatment for genital diseases related to HPV in individuals who were vaccinated. In the meta-analysis, we only included studies that also reported results from a cohort who were not vaccinated. Studies were included irrespective of study design, type of vaccine, timing of vaccination, and surgical technique (supplementary material).
Data extraction and risk of bias
Data were independently extracted by KSK and AA in prespecified forms. Discrepancies were discussed and resolved by other authors (IK and MK). Risk of bias was assessed by two authors independently (KSK and SJB) with the ROBINS-I tool (Risk Of Bias In Non-randomised Studies–of Interventions) for observational studies and the RoB-2 tool (Risk of Bias for randomised trials) for randomised controlled trials 24 25 (supplementary material).
The GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) tool was used to assess the quality of evidence for the primary outcome. The assessment was based on five parameters: risk of bias, inconsistency (or heterogeneity) between studies, indirectness, imprecision (risk of random errors), and publication bias. The evidence for each item was rated as high, moderate, low, or very low (supplementary material). 26
Definitions of outcome
Our primary outcome was recurrence of cervical intraepithelial neoplasia grade 2 or higher (CIN2+) after local surgical treatment related to all HPV types. Secondary outcomes included recurrence of CIN grade 1 or higher (CIN1+), CIN grade 3 (CIN3), CIN2, CIN1, CIN2+ related to HPV16 or HPV18 subtypes, and CIN1+ related to HPV16 or HPV18. We also examined the risk of recurrence of vulvar, vaginal, and anal intraepithelial neoplasia, genital warts, the risk of abnormal cytology (defined as atypical squamous cells of undetermined significance or worse, based on the Bethesda System, or borderline changes or worse, based on the British Association of Cytopathology), and HPV infections (persistent or incident) after treatment.
Statistical analysis
We conducted two separate meta-analyses based on the design of the studies. For our main analysis, we pooled data from observational studies and randomised controlled trials reporting on the efficacy of vaccination when given shortly before, at the time of, or up to 12 months after the local surgical treatment. Our secondary analysis included post hoc analyses of randomised controlled trials reporting on the risk of recurrence in individuals who had undergone local surgical treatment and who were vaccinated at study entry and randomisation, and then developed preinvasive disease. We also performed an analysis that included all studies, irrespective of study design (supplementary material). We used adjusted data in preference to unadjusted data, when available, for the primary outcome (risk of recurrence of CIN2+ after local surgical treatment), and sensitivity and subgroup analyses. The median of the median length of follow-up across studies, along with its interquartile range, were calculated for the primary outcome.
We combined study effect sizes using risk ratios and corresponding 95% confidence intervals with the random effects model (inverse variance method). 27 In the random effects model, we estimated each summary effect size and 95% confidence interval with the Hartung-Knapp-Sidik-Jonkman method 28 29 30 to deal with meta-analyses that included a small number of studies. We also calculated prediction intervals as a way of expressing heterogeneity compared with confidence intervals.
Visual inspection of the contour enhanced funnel plot and Egger’s test were used to assess the effects of small studies and publication bias when more than 10 studies were available for each outcome. 31 When funnel plot asymmetry was detected, we performed the Copas selection model to look for publication bias. 32 Statistical heterogeneity was assessed with the χ 2 test (P<0.10 indicating significant heterogeneity), and I 2 and τ 2 (to quantify the degree of heterogeneity); the restricted maximum likelihood estimator was used to estimate variance (τ 2 ) between studies. 33 34 35 36 37
To determine possible sources of heterogeneity, we performed predefined sensitivity analyses for the primary outcome (risk of recurrence of CIN2+ after local surgical treatment) restricted to: peer reviewed articles, studies with a moderate or low risk of bias, studies with low attrition bias (<10% loss during follow-up), studies with histopathological confirmation of the outcome, and studies with unadjusted data. Because our meta-analysis examined rare events and publication bias was possible, we performed a fixed effects meta-analysis with the Mantel-Haenszel method; this method provides more robust estimates of the summary effect but ignores heterogeneity. 38
We also conducted a series of subgroup analyses to determine sources of heterogeneity and differences in summary estimates for the primary outcome (risk of recurrence of CIN2+ after local surgical treatment) according to: continent, timing of vaccination (up to three months before v at the time of or up to 12 months after treatment), type of vaccine (Gardasil v Gardasil or Cervarix v unknown type), age (mean age >35 v mean age ≤35), median follow-up (<24 months v ≥24 months), and study design (randomised controlled trials excluding post hoc analyses of randomised controlled trials v observational studies). Analyses were performed with the R software (meta package), 39 and the selection model to assess publication bias was performed in R with the rjags package. 32 40
Patient and public involvement
Patients and the wider public were involved from the outset through informal interviews in the clinic and through patient advocate representative bodies. We formed a group of patient representatives that met regularly every six months and will support dissemination of the results. The research questions and outcomes were developed based on the patients’ concerns and priorities. Patients were not involved in the interpretation of results or writing of the article.
We identified 10 662 articles; 22 were eligible for the systematic review ( fig 1 and table S1). 16 17 18 19 20 21 22 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 Eighteen studies reported on a non-vaccinated group (no vaccine or placebo) and were included in the meta-analyses. We retrieved two randomised controlled trials, 12 observational studies (six retrospective and six prospective), and four post hoc analyses of randomised controlled trials (supplementary material). The median of the median length of follow-up for the primary outcome (risk of recurrence of CIN2+ after local surgical treatment) was 36 months (interquartile range 24-43.5) for observational studies and randomised controlled trials, and 27 months (21-39) for post hoc analyses of randomised controlled trials (table S2).

Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart
- Download figure
- Open in new tab
- Download powerpoint
Risk of bias
The two randomised controlled trials were classified as low risk of bias with adequate design (RoB-2 tool). In the observational studies and post hoc analyses, risk of bias was moderate for seven studies, serious for seven, and critical for two, based on the ROBINS-I tool (table S3). Asymmetry was not present in the funnel plot when adjusted data were used (fig S1a), but asymmetry was found with unadjusted data, indicating the possibility of publication bias (fig S1b).
CIN recurrence and risk of HPV infection after local surgical treatment for CIN
Our main analysis of observational studies and randomised controlled trials showed that women who were vaccinated at the time of treatment had a reduction in the risk of recurrence of CIN2+ compared with those who were not vaccinated (11 studies, 19 909 participants; risk ratio 0.43, 95% confidence interval 0.30 to 0.60; I 2 =58%, τ 2 =0.14; median follow-up 36 months, interquartile range 24-43.5) ( fig 2 and table 1 ). Confidence in this meta-analysis ranged from very low to moderate according to GRADE because of the presence of small study effects indicating potential publication bias (table S4). The risk of recurrence of CIN2+ related to the HPV subtypes HPV16 or HPV18 was also reduced with vaccination compared with no vaccination (six studies, 1879 participants; 0.26, 0.16 to 0.43; I 2 =0%, τ 2 =0); a similar result was found for the risk of recurrence of CIN1+ related to HPV16 or HPV18 (one study, 178 participants; 0.25, 0.05 to 1.14) ( fig 2 and table 1 ). Confidence in the meta-analysis of CIN2+ related to the HPV subtypes HPV16 or HPV18 ranged from very low to moderate according to GRADE because of the high risk of bias in the included studies and inconsistency (only one randomised controlled trial provided data) (table S4). The risk of recurrence of CIN3 was reduced among patients who were vaccinated but uncertainty was large (three studies, 17 757 participants; 0.28, 0.01 to 6.37; I 2 =71%, τ 2 =1.23) ( table 1 ).

Forest plots assessing risk of recurrence of cervical intraepithelial neoplasia grade 2 or higher (CIN2+) between human papillomavirus (HPV) vaccinated (V) and non-vaccinated control (C) groups after local conservative treatment for cervical intraepithelial neoplasia, irrespective of HPV type (top) and related to HPV subtypes HPV16 and HPV18 (bottom) (randomised controlled trials and observational studies)
Effect of human papillomavirus (HPV) vaccination on risk of recurrence of lesions related to HPV infection and HPV clearance after local surgical treatment for genital disease (randomised controlled trials and observational studies)
- View inline
The effect estimates for the primary outcome (risk of recurrence of CIN2+ after local surgical treatment) were similar in all sensitivity analyses (table S5). Heterogeneity was reduced compared with the main analysis after excluding studies with a high and serious overall risk of bias (fig S3a). In contrast, heterogeneity remained similar to the main analysis after the exclusion of grey literature, of studies with high attrition bias, and of studies that did not use histopathological confirmation of diagnosis. A sensitivity analysis with only unadjusted data showed a similar effect estimate as the main analysis with slightly reduced heterogeneity (11 studies, 19 909 participants; risk ratio 0.41, 95% confidence interval 0.30 to 0.56; I 2 =51%, τ 2 =0.12) (fig S3g).
The results for the primary outcome (risk of recurrence of CIN2+ after local surgical treatment) were largely consistent with a series of subgroup analyses and were not substantially affected by continent, study design, type of vaccine, or timing of vaccination (table S6). The risk of recurrence of CIN2+ was similar in studies that used the vaccine Gardasil (four studies, 1501 participants; risk ratio 0.38, 95% confidence interval 0.23 to 0.60; I 2 =0%, τ 2 =0) and those that used the Gardasil or Cervarix vaccine (five studies, 1205 participants; 0.36, 0.23 to 0.57; I 2 =0%, τ 2 =0) (fig S4a). Cervarix alone was not reported in any study, and two studies did not state the type of vaccine (unknown). The point estimates were similar when the vaccine was given at the time of or after treatment (10 studies, 15 546 participants; 0.41, 0.28 to 0.60; I 2 =62%, τ 2 =0.17), and when the vaccine was given before treatment (two studies, 4183 participants; 0.37, 0.02 to 6.43; I 2 =0%, τ 2 =0) (figure S4b). The effect estimate was comparable in studies with a median follow-up of >24 months (seven studies, 19 214 participants; 0.44, 0.26 to 0.73; I 2 =61%, τ 2 =0.19) versus ≤24 months (two studies, 507 participants; 0.33, 0 to 28.56; I 2 =47%, τ 2 =0.13) (fig S4f).
The second meta-analysis of post hoc analyses of randomised controlled trials showed similar point estimates ( table 2 ). Although HPV vaccination reduced the risk of recurrence of CIN2+ compared with no vaccination, uncertainty was large (four studies, 2268 participants; risk ratio 0.45, 95% confidence interval 0.13 to 1.57; I 2 =14%, τ 2 =0.05; median follow-up 27 months, interquartile range 21-39) (fig S5a). HPV vaccination was also associated with a reduced risk of recurrence of CIN1+ (three studies, 1958 participants; 0.64, 0.45 to 0.90; I 2 =0%, τ 2 =0) (fig S5b).
Effect of human papillomavirus (HPV) vaccination on risk of recurrence risk of lesions related to HPV infection and HPV clearance after local surgical treatment for genital disease (post hoc analyses of randomised controlled trials)
In the analysis of all studies irrespective of design (observational studies, randomised controlled trials, and post hoc analyses of randomised controlled trials), we found that the effect estimates and heterogeneity were similar to the main analysis for recurrence of CIN2+ (15 studies, 22 177 participants; risk ratio 0.43, 95% confidence interval 0.32 to 0.59; I 2 =51%, τ 2 =0.12; median follow-up 36 months, interquartile range 24-45) (fig S7a). To investigate the effect of timing of vaccination, we performed a subgroup analysis irrespective of study design that showed similar point estimates when the vaccine was given before (six studies, 6451 participants; 0.39, 0.24 to 0.64; I 2 =0%, τ 2 =0) versus at the time of or after treatment (10 studies, 15 726 participants; 0.41, 0.28 to 0.60; I 2 =62%, τ 2 =0.17) (fig S7b).
Recurrence of non-cervical diseases related to HPV infection after local surgical treatment of non-cervical disease
The risk of recurrence of vulvar or vaginal intraepithelial neoplasia grade 2 or higher was reduced in women who were vaccinated and treated locally for cervical, vulvar or vaginal intraepithelial neoplasia grade 2 or higher compared with women not vaccinated, but uncertainty was large (two studies, 296 participants; risk ratio 0.56, 95% confidence interval 0.01 to 35.16; I 2 =0%, τ 2 =0) ( table 1 and fig S2e). Only one study assessed risk of vulvar intraepithelial neoplasia or vaginal intraepithelial neoplasia grade 1 or higher in women who were vaccinated and treated locally for CIN2+, and no differences were found. Also, one study assessed the risk of recurrence of high grade anal intraepithelial neoplasia in men treated locally for anal intraepithelial neoplasia and found no difference ( table 1 and supplementary material).
The second meta-analysis of post-hoc analyses of randomised controlled trials showed no benefit of HPV vaccination on recurrence of lesions of vulvar or vaginal intraepithelial neoplasia grade 1 or higher, or vulvar or vaginal intraepithelial neoplasia grade 2 or higher, in women treated for genital HPV related disease ( table 2 , figs S5i and S5j, and supplementary material).
The findings of our meta-analysis suggest that adjuvant HPV vaccination at the time of local excision for CIN might lead to a reduction in the risk of recurrence of high grade preinvasive disease (CIN2+). The effect estimate was even more pronounced for CIN2+ related to HPV16 or HPV18. Publication bias might be present, however, affecting the overall quality of evidence, indicating that the evidence is inconclusive. Evidence was lacking on the benefit of HPV vaccination for recurrence of vulvar, vaginal, or anal intraepithelial lesions, genital warts, or for incident or persistent HPV infections, although the data were scarce with small numbers of studies and participants. Analysis of the post hoc studies from randomised controlled trial data with historic vaccination at randomisation before the development of the disease reported inconsistent results. The effect size on secondary outcomes in the main analysis of our study showed possible beneficial effects, although the evidence was inconclusive because the number of included studies was small.
Four meta-analyses have attempted to combine the evidence so far. 56 57 58 59 Although our findings on the summary estimate were in agreement with recent meta-analyses examining similar questions, 56 57 58 59 our analysis raised concerns about the quality of the data and we concluded that the evidence is inconclusive. Some of the previous meta-analyses published in 2020 and 2021 only included a fraction of the published literature, failed to include grey literature and unpublished data, had exclusion criteria that affected the final number of studies with potentially relevant data, and the presence of publication bias was not assessed and was not associated with the overall quality of the data. These studies did not include extensive sensitivity and subgroup analyses to determine the effect of risk of bias, type of vaccine, heterogeneity between studies, and attrition rate on the primary outcome, and the tools used to assess the quality of studies and risk of bias were not optimal.
Lichter et al 56 included six studies in their systematic review and meta-analysis (three post hoc analyses of randomised controlled trials, two observational studies, and one randomised controlled trial) and reported on a series of CIN and non-cervical outcomes with consistent estimates. However, the post hoc analyses of randomised controlled trials were misreported as randomised controlled trials in the analysis, ignoring the fact that randomisation in these studies was not made for treatment as intervention. The risk of bias and timing of the vaccine were also not examined, and most of the data were extracted from one to three studies, with very low to moderate quality of evidence based on GRADE.
Another systematic review and meta-analysis 57 published in 2020 included 10 studies and reported similar estimates to our study for the risk of recurrence of CIN2+. A subgroup analysis based on study design was not included, however, and risk of bias for observational studies was assessed with the RoB-2 tool, commonly used for randomised controlled trials. Di Donato et al’s meta-analysis 58 included 11 studies and subgroup analysis was performed based only on study design. Risk of bias was assessed with the ROBINS-I tool for all studies, although RoB-2 is more suitable for randomised controlled trials; GRADE assessment was not carried out. Bartels et al 59 retrieved data from only five studies for their meta-analysis on the risk of recurrence of CIN2+ and did not include sensitivity or subgroup analyses. GRADE assessment was not performed, and the RoB tools used (MINORS and JADAD) are not recommended by the Cochrane Library.
Strengths and limitations
Our systematic review and meta-analysis reported on the role of HPV vaccination at the time of local surgical treatment for cervical and other non-cervical diseases related to HPV infection, with rigorous methodological assessment of risk of bias, heterogeneity, and appropriate data pooling from different study designs. Post hoc analyses of randomised controlled trials with historic vaccination were analysed separately from observational or randomised studies that administered the vaccine at or around the time of local treatment, removing potential inaccurate assessment of effect estimates and reducing heterogeneity. We used established risk of bias tools for observational studies and randomised controlled trials (ROBINS-I and RoB-2) to examine the quality of the studies included in the meta-analysis. We also investigated the possibility of publication bias in our meta-analysis with a selection model and performed analyses irrespective of HPV type and subgroup analyses for HPV16 and HPV18 disease. We performed a thorough assessment of the grey literature and we also assessed our findings with fixed and random effects meta-analyses, as well as with a series of subgroup and sensitivity analyses that controlled for risk of bias, timing of vaccination, type of vaccine, and attrition bias.
Our findings should be interpreted with caution, however. Most of the published literature were observational studies that are at risk of bias, and the two randomised controlled trials included only 178 21 and 242 patients, 22 respectively. The observational studies were of low to moderate quality based on the ROBINS-I bias assessment tool, and only five studies provided adjusted data. The low to moderate quality could be attributed, at least partially, to the presence of confounders, differences in baseline characteristics between intervention and control groups, and suboptimal selection of participants in the included studies. Specifically, the mean age of participants was not provided in most studies and age differences between women who were vaccinated and those who were not vaccinated might affect the risk of recurrence of disease. Women who are vaccinated might be younger than those who are not vaccinated, and increased recurrence of disease could partly be a result of older age. Also, confounders such as smoking, associated with a higher risk of recurrence, were not controlled for in many studies.
Variability in diagnostic methods, length of follow-up, and HPV vaccine types and timing among the studies could also influence the accuracy of the effect estimate. The median of median length of follow-up was 36 months and therefore we could not assess whether the effect estimate would be sustained in the long term. Although subgroup analyses according to median length of follow-up (<24 v ≥24 months) showed similar effect estimates, pooling risk ratios at different time points was not possible. The short follow-up is an intrinsic limitation of our meta-analysis and similar meta-analyses, and this factor needs to be looked at in randomised studies with a long follow-up interval. Most recurrences present in the first 24 months, 60 however, and no clear biological reason exists to explain why a longer length of follow-up would reverse this trend.
Heterogeneity in several meta-analyses was high, as indicated by the τ 2 estimates and the wide prediction intervals, highlighting the uncertainty around the observed effect estimates. Also, the difference in effect estimates between the random and fixed effect models indicates the presence of small study effects. The use of adjusted study specific effects, where available, might have also contributed to this heterogeneity. Assessment of publication bias was restricted by the limited number of studies for some comparisons, further highlighting the need for a randomised trial. Although the subgroup and sensitivity analyses according to type of vaccine, timing of vaccination, attrition bias, and overall risk of bias showed consistent results and reduced this heterogeneity to some extent, the number of included studies in these subgroups was low. Furthermore, distinguishing between incident and persistent infections was not possible because persistence was assessed in only one study. Studies that used the nonavalent vaccine Gardasil 9 that might offer wider protection were not available. An analysis with data from an intention to treat analysis of randomised controlled trials was also not possible because of lack of data. For many of the secondary outcomes, the number of studies and participants was also low, precluding clear conclusions to be drawn on the effectiveness of the vaccine. The quality of evidence ranged from very low to moderate, as assessed by GRADE, owing to the possible presence of publication bias, high risk of bias of the included studies, and inconsistency.
Conclusions
Women who have been treated for high grade CIN have a lifelong residual high risk of cervical cancer and other malignancies related to HPV infection. New strategies to reduce the risk in these women might shorten and simplify screening programmes and eliminate cervical cancer more rapidly. 5 6 Large, appropriately powered randomised controlled trials are required to establish the effectiveness of adjuvant HPV vaccination at the time of local surgical treatment of CIN. Given that the incidence of recurrence of high grade disease is low in quality assured national screening programmes, such as in the UK, absolute risks and a cost effectiveness analysis would be important in determining the implementation strategy of HPV vaccination after treatment. The NOVEL (Nonavalent Prophylactic HPV Vaccine (Gardasil 9) After Local Conservative, NCT03979014 ) trial, an ongoing, large, appropriately powered randomised controlled trial, is expected to report results and give further insight on the effect of Gardasil 9 on persistent incident, recurrent, and prevalent HPV infections and cost effectiveness. 61 If the study reports benefit, the results could be applicable to women with multifocal disease, other diseases related to HPV infection, and individuals with a diagnosis of malignancies related to HPV infection.
What is already known on this topic
Women with cervical intraepithelial neoplasia (CIN) undergoing local surgical treatment have a high risk of recurrent preinvasive and invasive cervical disease and other diseases related to human papillomavirus (HPV) infection
Women who develop high grade CIN might be particularly sensitive to HPV infection and rapidly re-acquire infections
The use of prophylactic HPV vaccination at the time of local surgical treatment for CIN might reduce the risk of future recurrence
What this study adds
Prophylactic HPV vaccination at the time of local treatment for CIN might reduce the risk of recurrence of high grade preinvasive cervical, but the evidence is inconclusive and the GRADE assessment for the quality of evidence ranged from very low to moderate
The effect of HPV vaccination on the risk of HPV infection and other non-cervical diseases related to HPV infection treated surgically is unclear because of the scarcity of data and the moderate to high overall risk of bias of the available studies
Large, appropriately powered randomised controlled trials are required to establish the effectiveness of HPV vaccination at the time of surgical treatment of cervical preinvasive disease based on failure rates and costs in different settings
Ethics statements
Ethical approval.
Not required.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Contributors: The study was conceived by MK and designed by MK, KSK, AAV, and IK. The data were acquired and collated by KSK, AA, SJB, MP, and MK, and analysed by KSK, IK, AA, SJB, MP, MK, and AAV. The manuscript was drafted and revised critically for important intellectual content by all authors (KSK, IK, SJB, AA, MP, EP, JD, PN, BS, PS, AAV, and MK). All authors gave final approval of the version to be published. The corresponding author (MK) attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. The guarantor (MK) accepts full responsibility for the work, had access to the data, and controlled the decision to publish.
Funding: This work was supported by the National Institute for Health and Care Research (NIHR) EME (17/11/45). The researchers were also supported by Imperial Healthcare NHS Trust NIHR Biomedical Research Centre (P83204) (MK and KSK); Imperial Charity Fellowship (RFP02122_8) (MP); and CRUK Early Detection (EDDPJT-May21/100001) (MK and MP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the NIHR EME for the submitted work; the authors declare no conflict of interest with regards to the presented work; a number of authors are investigators of the NIHR EME funded NOVEL trial (MK, KSK, PS, JD, BS, PN, and IK); this trial is also supported by MSD who supplied the vaccines for the trial.
The lead authors (KSK and MK) affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Dissemination to participants and related patient and public communities: The results will be disseminated to the lay audience through a press release, social media, through the Imperial College website, and through the authors' involvement with charities, public presentations, and interviews.
Provenance and peer review: Not commissioned; externally peer reviewed.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
- Schiller JT ,
- Paavonen J ,
- Salmerón J ,
- HPV PATRICIA Study Group
- Szilagyi PG ,
- Chesson HW ,
- Soutter WP ,
- Sasieni P ,
- Panoskaltsis T
- Kalliala I ,
- Athanasiou A ,
- Veroniki AA ,
- Strander B ,
- Hällgren J ,
- Kyrgiou M ,
- Koliopoulos G ,
- Martin-Hirsch P ,
- Prendiville W ,
- Paraskevaidis E
- Paraskevaidi M ,
- Kalliala IEJ ,
- Veroniki A ,
- Efthimiou O ,
- Pang Y-YS ,
- Olsson S-E ,
- Sigurdsson K ,
- Gonzalez M ,
- Hildesheim A ,
- Gonzalez P ,
- Kreimer AR ,
- Costa Rica HPV Vaccine Trial (CVT) Group
- Garland SM ,
- FUTURE I and II Study Group
- Ghelardi A ,
- Parazzini F ,
- Martella F ,
- Frederiksen K ,
- Dehlendorff C
- Pieralli A ,
- Bianchi C ,
- Karimi-Zarchi M ,
- Allahqoli L ,
- Nehmati A ,
- Taghipour-Zahir S ,
- McKenzie J ,
- Bossuyt P ,
- Sterne JA ,
- Hernán MA ,
- Reeves BC ,
- Sterne JAC ,
- Savović J ,
- Higgins JP ,
- Chandler J ,
- Jackson D ,
- Viechtbauer W ,
- Hartung J ,
- Sutton AJ ,
- Ioannidis JP ,
- Mavridis D ,
- Cipriani A ,
- Thompson SG ,
- Huedo-Medina TB ,
- Sánchez-Meca J ,
- Marín-Martínez F ,
- Boutron I ,
- DerSimonian R ,
- Viechtbauer W
- Schwarzer G
- Spiegelhalter D ,
- Coskuner ER ,
- Karakose A ,
- Dillioglugil O ,
- Swedish KA ,
- Factor SH ,
- Goldstone SE
- Grzes BHJ ,
- Ortega-Quiñonero P ,
- Remezal-Solano M ,
- Carazo-Díaz M ,
- Vinnytska A
- Petrillo M ,
- Dessole M ,
- Tinacci E ,
- Del Pino M ,
- Raspagliesi F ,
- Sopracordevole F ,
- Jaisamrarn U ,
- Giannella L ,
- Moscato GM ,
- Di Matteo G ,
- Di Bonito P ,
- Andreoni M ,
- ↵ Şenol T, Yerçok Y, Özkaya E, et al. Efficacy of quadrivalent HPV vaccine for vulvar condyloma recurrence. 2016. https://acikerisim.medipol.edu.tr/xmlui/handle/20.500.12511/1038
- Kreuter A ,
- Lichter K ,
- Jentschke M ,
- Kampers J ,
- Sibbertsen P ,
- Hillemanns P
- Di Donato V ,
- Bartels HC ,
- Rogers AC ,
- Redman CWE ,
- Verdoodt F ,
- ↵ Kyrgiou M. Nonavalent prophylactic HPV vaccine (GARDASIL9) after local conservative The NOVEL Trial (NOVEL). 2019. https://clinicaltrials.gov/ct2/show/NCT03979014?term=NOVEL&draw=2&rank=1 .

IMAGES
VIDEO
COMMENTS
Abstract. Human papillomavirus (HPV) vaccination rates in the U.S. are suboptimal, requiring innovative partnerships between community and clinical entities to remedy this issue. A rigorous evaluation of HPV-related community-clinical linkages (CCLs) was conducted to understand their components, processes, and outcomes to increase HPV vaccination.
A rigorous evaluation of HPV-related community-clinical linkages (CCLs) was conducted to understand their components, processes, and outcomes to increase HPV vaccination. Cancer Prevention and Control Research Network (CPCRN) investigators explored CCLs in their communities employing an iterative, case study approach.
Request PDF | A multi-site case study of community-clinical linkages for promoting HPV vaccination | Human papillomavirus (HPV) vaccination rates in the U.S. are suboptimal, requiring innovative ...
A rigorous evaluation of HPV-related community-clinical linkages was conducted to understand their components, processes, and outcomes to increase HPV vaccination, and found CCLs provide an opportunity to study the adaption, integration, and enhancement of evidence-based approaches. ABSTRACT Human papillomavirus (HPV) vaccination rates in the U.S. are suboptimal, requiring innovative ...
A multi-site case study of community-clinical linkages for promoting HPV vaccination Heather M. Brandt, Robin C. Vanderpool, Susan J. Curry, Paige Farris, Jason Daniel-Ulloa, Laura Seegmiller, Lindsay R. Stradtman, Thuy Vu, Victoria Taylor, Maria Zubizarreta ...
Table 2. Descriptive Characteristics of HPV Vaccination Community‐Clinical Linkages • HPV vaccination rates remain well below national Healthy People 2020 goals of 80% coverage for adolescent females and ma les aged 13-17. Nationally, in 2015, 63% of adoles cent girls had received
A multi-site case study of community-clinical linkages for promoting HPV vaccination. Brandt H; Vanderpool R; Curry S; et al. See more; Human Vaccines and Immunotherapeutics (2019) 15(7-8) 1599-1606. DOI: 10.1080/21645515.2019.1616501. 11 Citations. Citations of this article.
A multi-site case study of community-clinical linkages for promoting HPV vaccination. Hum Vaccin Immunother. Hum Vaccin Immunother. 2019 ;15(7-8): 1599 - 1606 . doi: 10.1080/21645515.2019.1616501 .
Community-Clinical Linkages Community-clinical linkages (CCLs)are defined by the CDC as "…collaborations between health care practitioners in the clinical settings and programs in the community -both working to improve the health of the people and the communities in which they live." Sources: CDC Developing Community-Clinical Linkages ...
The study found that the intervention had no effect on the uptake of HPV vaccine (RR 1.03, 95% CI 1.01 to 1.05; 5912 participants). We judged the certainty of the evidence as moderate because of a high risk of bias in the included study. Three studies measured the costs of the intervention [ 25, 45, 47 ].
School-based vaccination programs 12,13, education about HPV and the vaccine at the outpatient clinic 14,15, reminder letters to students and parents about upcoming vaccination appointments 16,17 ...
A rigorous evaluation of HPV-related community-clinical linkages (CCLs) was conducted to understand their components, processes, and outcomes to increase HPV vaccination. Cancer Prevention and Control Research Network (CPCRN) investigators explored CCLs in their communities employing an iterative, case study approach.
A multi-state case study of community-clinical linkages for promoting HPV vaccination. Hum Vaccin Immunother Jun 3:1-8, 2019. doi: 10.1080/21645515.2019.1616501. Farr DE, Brandt HM, Friedman DB, Adams SA, Armstead CA, Fulton JK, Bull DM. False positive mammography and mammography screening intentions among Black women: the influence of emotions ...
A multi- site case study of community-clinical lin kages for promoting HPV vaccination. Hum Vaccin Immunother. 2019;15(7-8):1599-1606. doi: 10.1080/21645515.2019.1616501. Epub 2019 Jun 3. PMID: 31158042; PMCID: PMC6746520 . Limited focus on community-clinical linkages to address supply and demand challenges.
Europe PMC is an archive of life sciences journal literature. https://orcid.org
Background Despite the human papillomavirus (HPV) vaccine being a safe, effective cancer prevention method, its uptake is suboptimal in the United States (U.S.). Previous research has found a variety of intervention strategies (environmental and behavioral) to increase its uptake. The purpose of the study is to systematically review the literature on interventions that promote HPV vaccination ...
Background HPV-related anal cancer occurs in excess rates among people living with HIV (PLWH) and has been increasing in incidence. The HPV vaccine is an effective and safe approach to prevent and reduce the risk of HPV-related disease. Yet, HPV vaccine programs tailored and implemented in the HIV population are lagging for this high-risk group. Methods A pre-post intervention study design ...
A multi-site case study of community-clinical linkages for promoting HPV vaccination. Hum Vaccin Immunother. 2019; 15 (7-8):1599-606. doi: 10.1080/21645515.2019.1616501. [PMC free article] [Google Scholar] 41. Kaul S, Do TQN, Hsu E, Schmeler KM, Montealegre JR, Rodriguez AM. School-based human ...
Our findings suggested that countries with a relatively higher cervical cancer burden and thereby a relatively higher need for HPV vaccination had relatively lower coverage during 2010-22. Further, there were significant inequities in HPV vaccination coverage within the Americas, Europe, and Western Pacific regions, and in high- and low-income countries with a pro-advantaged and regressive ...
Cervical cancer is the first cancer deemed amenable to elimination through prevention, and thus lessons from the epidemiology and prevention of this cancer type can provide information on ...
Both vaccines prevent infection with HPV-16 and HPV-18, which together cause approximately 70% of cervical cancers, and clinical trials have demonstrated 90%-100% efficacy in preventing precancerous cervical lesions attributable to HPV-16 and HPV-18. One vaccine also prevents HPV-6 and HPV-11, which cause 90% of genital warts.
Eleven articles describe mixed delivery locations Citation 10-28, Citation 29-43 - Citation 61-75 - Citation 80 where in the HPV vaccination is offered in more than one location, commonly with schools as the primary vaccination site. Health facilities or special community sites serve as secondary locations for students who missed a ...
Objective To explore the efficacy of human papillomavirus (HPV) vaccination on the risk of HPV infection and recurrent diseases related to HPV infection in individuals undergoing local surgical treatment. Design Systematic review and meta-analysis Data sources PubMed (Medline), Scopus, Cochrane, Web of Science, and ClinicalTrials.gov were screened from inception to 31 March 2021. Review ...