

A systematic literature review on the humanistic burden of cytomegalovirus
Cytomegalovirus (CMV) infection is typically asymptomatic in healthy individuals; however, certain populations are vulnerable to infection and may develop serious sequelae. CMV infection may also have a broad impact on humanistic outcomes, including patient health status and quality of life (QoL). We conducted a systematic literature review (SLR) to describe the global humanistic burden of CMV and congenital CMV (cCMV) infections across all age groups.
Medline, Embase, and LILACS were searched to identify studies on humanistic outcomes following CMV infection, including health status/QoL and any outcomes in domains such as auditory performance, cognitive ability, developmental status, intelligence, language, memory, mental health, motor performance, social communication, speech, and vocabulary. The SLR included articles published from 2000 to 2020 and focused geographically on Australia, Europe, Israel, Japan, Latin America, and North America.
Sixty-three studies met the inclusion criteria. In general, individuals with symptomatic cCMV infection experience a greater burden of disease and more substantial impact on QoL versus those with asymptomatic cCMV infection. Children with hearing loss due to cCMV infection, both symptomatic and asymptomatic, showed improved auditory outcomes following cochlear implantation. Newborns, infants, and children with cCMV infections had worse cognitive outcomes in psychological development, sequential and simultaneous processing, phonological working memory, and attention control versus age-matched controls without cCMV infection. CMV infection was also associated with cognitive decline in elderly populations.
CMV infection can have substantial, lifelong, heterogenous impacts on humanistic outcomes, including health status and QoL, which should be considered when developing and implementing treatment and prevention strategies.
A plain language summary of this manuscript can be found here .
Usage metrics

- Biotechnology
- Biological Sciences not elsewhere classified
- Science Policy
- Mental Health
- Infectious Diseases
- Computational Biology

Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.
Search life-sciences literature (43,815,450 articles, preprints and more)
- Available from publisher site using DOI. A subscription may be required. Full text
- Citations & impact
- Similar Articles
A systematic literature review on the humanistic burden of cytomegalovirus.
Author information, affiliations.
- Diaz-Decaro J 1
- Natenshon A 1
- Talarico C 1
- Lewandowski W 3
- Kaczanowska M 3
- Neumann M 4
- Schmidt E 4
ORCIDs linked to this article
- Schmidt E | 0000-0001-6159-4787
- Diaz-Decaro J | 0000-0001-6487-2113
- Neumann M | 0000-0001-8052-5429
- Talarico C | 0000-0001-9107-5873
- Buck PO | 0000-0002-3898-3669
Current Medical Research and Opinion , 23 Apr 2023 , 39(5): 739-750 https://doi.org/10.1080/03007995.2023.2191477 PMID: 36938652
Abstract
Conclusions, full text links .
Read article at publisher's site: https://doi.org/10.1080/03007995.2023.2191477
Citations & impact
Impact metrics, alternative metrics.

Article citations
Cytomegalovirus and pregnancy: a narrative review..
Pontes KFM , Nardozza LMM , Peixoto AB , Werner H , Tonni G , Granese R , Araujo Júnior E
J Clin Med , 13(2):640, 22 Jan 2024
Cited by: 0 articles | PMID: 38276146 | PMCID: PMC10816506
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A systematic literature review of the economic and healthcare resource burden of cytomegalovirus.
Diaz-Decaro J , Myers E , Mucha J , Neumann M , Lewandowski W , Kaczanowska M , Schmidt E , Natenshon A , Talarico C , Buck PO
Curr Med Res Opin , 39(7):973-986, 03 Jul 2023
Cited by: 0 articles | PMID: 37395088
A systematic literature review of the global seroprevalence of cytomegalovirus: possible implications for treatment, screening, and vaccine development.
Fowler K , Mucha J , Neumann M , Lewandowski W , Kaczanowska M , Grys M , Schmidt E , Natenshon A , Talarico C , Buck PO , Diaz-Decaro J
BMC Public Health , 22(1):1659, 01 Sep 2022
Cited by: 24 articles | PMID: 36050659 | PMCID: PMC9435408
The Burden of Congenital Cytomegalovirus Infection: A Prospective Cohort Study of 20 000 Infants in Finland.
Puhakka L , Lappalainen M , Lönnqvist T , Niemensivu R , Lindahl P , Nieminen T , Seuri R , Nupponen I , Pati S , Boppana S , Saxen H
J Pediatric Infect Dis Soc , 8(3):205-212, 01 Jul 2019
Cited by: 18 articles | PMID: 29554325
Screening for seemingly healthy newborns with congenital cytomegalovirus infection by quantitative real-time polymerase chain reaction using newborn urine: an observational study.
Yamaguchi A , Oh-Ishi T , Arai T , Sakata H , Adachi N , Asanuma S , Oguma E , Kimoto H , Matsumoto J , Fujita H , Uesato T , Fujita J , Shirato K , Ohno H , Kizaki T
BMJ Open , 7(1):e013810, 20 Jan 2017
Cited by: 21 articles | PMID: 28110288 | PMCID: PMC5253530
The Current Challenges in Developing Biological and Clinical Predictors of Congenital Cytomegalovirus Infection.
Tanimura K , Uchida A , Imafuku H , Tairaku S , Fujioka K , Morioka I , Yamada H
Int J Mol Sci , 22(24):13487, 15 Dec 2021
Cited by: 2 articles | PMID: 34948284 | PMCID: PMC8704566
Europe PMC is part of the ELIXIR infrastructure
Research Library
A systematic literature review on the humanistic burden of cytomegalovirus
- Open access
- Published: 01 September 2022
A systematic literature review of the global seroprevalence of cytomegalovirus: possible implications for treatment, screening, and vaccine development
- Karen Fowler 1 ,
- Jacek Mucha 2 ,
- Monika Neumann 3 ,
- Witold Lewandowski 2 ,
- Magdalena Kaczanowska 2 ,
- Maciej Grys 2 ,
- Elvira Schmidt 3 ,
- Andrew Natenshon 4 ,
- Carla Talarico 4 ,
- Philip O. Buck 4 &
- John Diaz-Decaro 4
BMC Public Health volume 22 , Article number: 1659 ( 2022 ) Cite this article
7718 Accesses
51 Citations
143 Altmetric
Metrics details
Cytomegalovirus (CMV) is a common pathogen that affects individuals of all ages and establishes lifelong latency. Although CMV is typically asymptomatic in healthy individuals, infection during pregnancy or in immunocompromised individuals can cause severe disease. Currently, treatments are limited, with no prophylactic vaccine available. Knowledge of the current epidemiologic burden of CMV is necessary to understand the need for treatment and prevention. A systematic literature review (SLR) was conducted to describe the most recent epidemiologic burden of CMV globally.
Medline, Embase, and LILACS were searched to identify data on CMV prevalence, seroprevalence, shedding, and transmission rates. The SLR covered the time period of 2010–2020 and focused geographically on Australia, Europe, Israel, Japan, Latin America (LATAM), and North America. Studies were excluded if they were systematic or narrative reviews, abstracts, case series, letters, or correspondence. Studies with sample sizes < 100 were excluded to focus on studies with higher quality of data.
Twenty-nine studies were included. Among adult men, CMV immunoglobulin G (IgG) seroprevalence ranged from 39.3% (France) to 48.0% (United States). Among women of reproductive age in Europe, Japan, LATAM, and North America, CMV IgG seroprevalence was 45.6-95.7%, 60.2%, 58.3-94.5%, and 24.6-81.0%, respectively. Seroprevalence increased with age and was lower in developed than developing countries, but data were limited. No studies of CMV immunoglobulin M (IgM) seroprevalence among men were identified. Among women of reproductive age, CMV IgM seroprevalence was heterogenous across Europe (1.0-4.6%), North America (2.3-4.5%), Japan (0.8%), and LATAM (0-0.7%). CMV seroprevalence correlated with race, ethnicity, socioeconomic status, and education level. CMV shedding ranged between 0% and 70.2% depending on age group. No findings on CMV transmission rates were identified.
Conclusions
Certain populations and regions are at a substantially higher risk of CMV infection. The extensive epidemiologic burden of CMV calls for increased efforts in the research and development of vaccines and treatments.
Trial registration
Peer Review reports
Cytomegalovirus (CMV), a member of the herpesvirus family ( Herpesviridae ), is a pathogen common worldwide that infects a substantial number of individuals at some point in their lives [ 1 ]. In the United States, it is estimated that the virus will infect approximately 30% of children by 5 years of age and more than 50% of adults by 40 years of age [ 2 ]. Generally, CMV seroprevalence is higher among women, those in older age groups, persons of lower socioeconomic status, and in developing countries [ 3 ]. Among women of reproductive age in particular, global CMV seroprevalence ranges from 45 to 100% [ 3 ].
CMV can be transmitted through contact with infectious bodily fluids such as blood, saliva, urine, tears, seminal fluid, cervical secretions, and breast milk. In addition, infection is possible following solid organ and stem cell transplantation [ 2 ], with CMV representing the most common opportunistic infection among solid organ transplant recipients [ 4 ]. After initial CMV infection in a previously seronegative individual (primary infection), reactivation of persistent latent virus or infection with a different CMV strain (nonprimary infection) can occur.
In healthy individuals, CMV infection is typically asymptomatic or causes mild illness [ 5 ]; however, CMV transmission from a pregnant woman to her fetus in utero may cause congenital CMV (cCMV), which can result in serious long-term sequelae or death [ 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 ]. Acquisition of primary CMV infection during pregnancy poses the greatest risk to infants; approximately a third of infants born to mothers with primary CMV infection during pregnancy have cCMV infection [ 14 , 15 ]. Serious CMV-related sequelae can also occur in those with compromised immune systems, including solid organ or stem cell transplant recipients, individuals on immunosuppressive therapy, or those infected with human immunodeficiency virus (HIV) [ 16 ]. CMV is the most common cause of vision loss in individuals with HIV, even while on highly active antiretroviral therapy [ 17 ]. Currently, treatments for CMV are limited and no vaccine is available [ 18 ]. Thus, development of a CMV vaccine to prevent infection remains a high public health priority.
Given that CMV infection is common globally yet has a variable clinical course and a potential for long-term sequalae, a greater understanding of CMV epidemiologic data worldwide is needed, which can support the development of CMV vaccines and justify vaccine introduction into immunization schedules. Previously conducted systematic literature reviews (SLRs) on CMV prevalence/seroprevalence [ 1 , 19 , 20 , 21 , 22 ], transmission rate [ 23 , 24 ], or long-term sequelae [ 7 , 25 , 26 ] have been published; however, these SLRs included historical data, and thus, more current estimates of CMV burden are warranted. A thorough understanding of the epidemiologic impact of CMV is also hampered by the variation of burden between countries, within countries, and within subpopulations [ 1 , 3 , 24 ]. Therefore, there is a need to highlight seroprevalence, shedding, and transmission in specific populations affected by CMV. Here, we performed a SLR to describe the most recent (2010–2020) epidemiologic data on CMV seroprevalence, shedding, and transmission across several countries/regions according to population characteristics such as sex, age, at-risk status, socioeconomic status, educational level, and race/ethnicity.
A systematic review of the epidemiologic burden of CMV was conducted based on peer-reviewed articles published in the Medline, Embase, and Latin American and Caribbean Health Sciences Literature (LILACS) databases from the year 2000 through December 14, 2020 (an initial search was performed on October 27, 2020, and a widened search with supplemental search terms and additional outcomes of interest was performed on December 14, 2020; Fig. 1 ; Supplemental Tables 1–6 in Additional File 1 ). Our search strategy consisted of subject headings (ie, medical subject header [MeSH] and Emtree), keywords, free text terms, and their synonyms and was adapted to the requirements of each queried database (detailed in Supplemental Tables 1–6 in Additional File 1 ). Medline and Embase were searched for the following themes: ((CMV / cytomegalovirus infections) AND epidemiology AND (epidemiologic studies AND Countries) OR (systematic reviews / meta-analysis)); in LILACS, the search was restricted to CMV AND epidemiology. Each record was assessed for relevance against predefined eligibility criteria (Supplemental Tables 7–8 in Additional File 1 ). Double independent record selection was performed during title/abstract and full text screening. Discrepancies concerning inclusion or exclusion were resolved after discussion between reviewers or through reconciliation by a third reviewer.
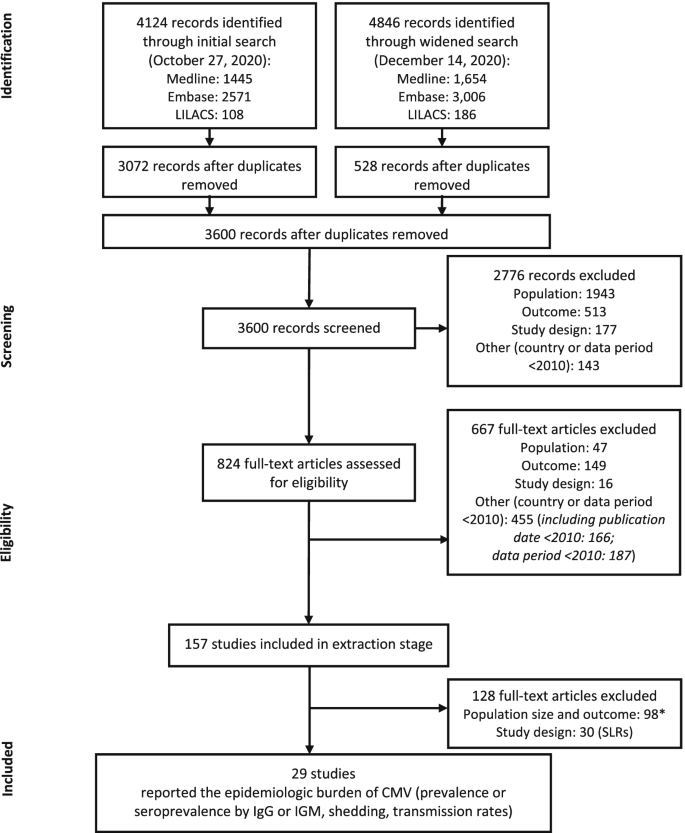
Flow diagram of screening process. *Reasons for exclusion: population size < 100; prevalence or seroprevalence based on a non-IgM or -IgG diagnostic method (eg, reverse transcriptase polymerase chain reaction); or data out of scope of review (incidence, infection rate, mortality, or long-term sequelae). CMV, cytomegalovirus; IgG, immunoglobulin G; IgM, immunoglobulin M; SLR, systematic literature review
The extensive search of bibliographic databases covered the time period of 2000–2020 (2017–2020 for conference abstracts) and was restricted to English language and the following countries and regions: Australia, Latin America (LATAM), Canada, Europe, Israel, Japan, United States, and global (international, worldwide). We included all age groups, mothers and infants with HIV, and specific subpopulations or immunocompromised groups. From this extensive search, we then focused on the most recent data and only extracted data from publications with study data between 2010–2020. If a particular article did not provide information on the study period, the year of publication was considered. For the purpose of this report, SLRs, narrative reviews, abstracts, case series, letters, and correspondence were excluded. Studies with sample sizes < 100 were also excluded in order to focus on studies with higher data quality and an adequate sample size for estimating prevalence.
Initial outcomes of interest were CMV infection rate, force of infection (the rate at which susceptible individuals in a population acquire an infectious disease in that population, per unit time [ 30 ]), reactivation, prevalence/seroprevalence, incidence, vertical and horizontal transmission, mortality, pregnancy loss, prevalence of shedding, morbidity, and long-term sequelae/effects. From this initially broad set of outcomes, we focused on CMV prevalence, seroprevalence, shedding, and transmission rate. Outcomes were divided into categories based on the elements of the research methodology or subpopulation; Table 1 presents the adopted data categorizations and definitions for age, at-risk population, sex, social status, education level, race/ethnicity, and developed or developing country. Seroprevalence outcomes were evaluated as region-specific seroprevalence of CMV (immunoglobulin G [IgG] or immunoglobulin M [IgM]) according to sex, age, at-risk population, socioeconomic status, education level, and race/ethnicity. At-risk populations were defined as individuals with primary immunodeficiencies, individuals with secondary immunodeficiencies caused by diseases of the immune system, critically sick intensive care unit patients, and recipients of drugs suppressing the immune system (Table 1 ). Additionally, CMV seroprevalences were assessed within 10-year age increments for men, women of reproductive age, and adults. CMV shedding and transmission outcomes by age categories were also evaluated.
Outcomes were presented as ranges (minimum–maximum). For single measure estimates wherein it was not possible to determine the interval, the confidence interval was provided (if available within the source reference).
A total of 4124 records were retrieved through the initial search and 4846 records were retrieved through the widened search of the bibliographic databases (Fig. 1 ). After removal of duplicates, 3600 records remained for screening. Of these, 2766 irrelevant records were excluded, with a total of 824 full-text articles assessed for eligibility. A total of 157 references were included in the data extraction stage; 128 references were subsequently excluded either because they had a population size of < 100, had outcomes not relevant for the purpose of this report, or were SLRs. In total, 29 studies were included in this epidemiology review (Fig. 1 ; Supplemental Table 9 in Additional File 1 ).
The included studies covered data from a total of 14 countries: 2 countries from North America (Canada and the United States), 9 countries from Europe (Bosnia and Herzegovina, Croatia, France, Italy, Norway, Poland, Romania, Spain, and the United Kingdom), 2 countries from LATAM (Brazil and Mexico), and 1 country from other regions (Japan). Most studies were from Mexico ( n = 4), the United States ( n = 4), Japan ( n = 3), Poland ( n = 3), and the United Kingdom ( n = 3). Recent epidemiologic data (2015 onwards) were reported in 6 studies; 17 studies presented data before 2015, 5 studies had a data period within 2010–2020, and 1 study did not indicate a data period. Further details of the included studies are shown in Supplemental Table 9 in Additional File 1 .
CMV seroprevalence by sex and age group
Men and women of reproductive age, igg antibodies.
The presence of CMV IgG antibodies in the absence of IgM antibodies indicates previous, but not acute, infection [ 31 ]. Two studies reported CMV IgG seroprevalence specifically for male populations (Table 2 ; Fig. 2 ): 39.3% (95% CI: 34.9-43.8%) in a cross-sectional survey from a nationally representative population-based sample from France (Europe) and 48.0% in a US-based study that utilized a cross-sectional serosurvey among adult residents in North Carolina [ 32 , 33 ].
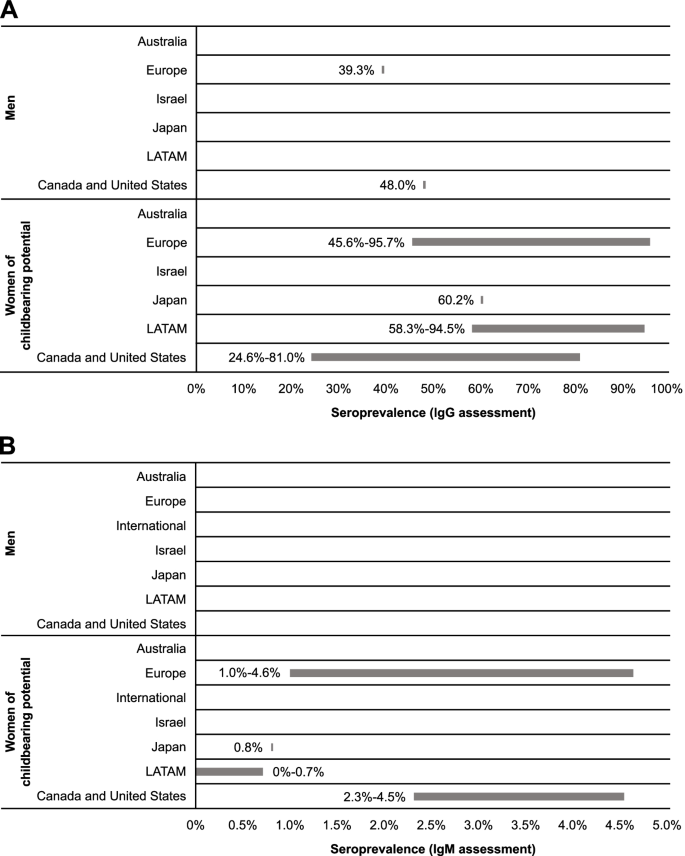
Region-specific cytomegalovirus IgG and IgM seroprevalence among men and women of childbearing potential. IgG, immunoglobulin G; IgM, immunoglobulin M; LATAM, Latin America
Comparatively, 15 studies from Japan, Europe, LATAM, Canada, and the United States reported CMV IgG seroprevalence estimates for women of reproductive age (Table 2 ; Fig. 2 ). In Japan, seroprevalence was estimated as 60.2% [ 34 ]. In Europe and LATAM, seroprevalence was similar across studies, ranging from 45.6 to 95.7% in Europe [ 32 , 35 , 36 , 37 , 38 , 39 , 40 , 41 ] and 58.3 to 94.5% in LATAM [ 42 , 43 , 44 ]. In North America, seroprevalence ranged from 24.6 to 81.0% [ 45 , 46 , 47 ].
No data were found for CMV IgG seroprevalence across age categories in men. Seroprevalence among women of reproductive age suggests a potential increase with age; however, these findings are limited by the small dataset. Seroprevalence of CMV infection in pregnant women in Mexico was higher in those aged 20 to 30 years than those aged ≤ 20 years (91.3 vs 86.5%, respectively) [ 44 ]. Studies from Canada and the United States indicate that seroprevalence was higher among women aged > 40 years compared with women aged ≤ 40 years [ 45 , 46 , 47 ]. No noticeable age-related trends were identified among European studies [ 36 , 37 , 40 , 41 ].
In developing countries of the European and LATAM regions included in this report, reported CMV IgG seroprevalences among women of reproductive age were similar. Studies conducted in Mexico reported CMV IgG seroprevalences of 58.3 to 94.5% for women of reproductive age [ 37 , 42 , 43 , 44 ]. In Europe, CMV IgG seroprevalence ranged from 57.3% among women of reproductive age in Poland [ 40 ] to 95.7% in Romania [ 37 ]. In comparison, in developed countries of Europe, CMV IgG seroprevalence among women of reproductive age ranged between 45.6 and 65.9% [ 32 , 36 , 39 ].
IgM antibodies
The presence of CMV IgM antibodies may be indicative of recent infection (ie, primary, reactivation, or reinfection) [ 31 ]. No studies with data on CMV IgM seroprevalence among men were identified. Among women of reproductive age, estimates suggest burden of primary and secondary CMV infection was similar in Europe (1.0-4.6%) [ 36 , 41 ] and North America (2.3-4.5%) [ 45 ]; these seroprevalences were higher than those observed in Japan (0.8%) [ 34 ] and LATAM (0-0.7%) [ 42 , 43 , 44 ] (Table 2 ; Fig. 2 ). While regional differences in CMV seroprevalence have historically been documented, the small number of studies in this SLR showed seroprevalence to be heterogenous with regional patterns difficult to discern.
Adults and the elderly
Twenty-two references were included for assessing seroprevalence across age categories based on detection of CMV IgG antibodies (Table 2 ). These articles reported data for general populations, healthy or immunocompetent populations (ie, without specific diseases and otherwise healthy), and adults. Among adults, seroprevalence ranged most broadly in European countries (44.4-95.7%) [ 32 , 35 , 36 , 37 , 40 , 41 , 48 , 49 , 50 , 51 ], with the narrowest range observed for Japanese studies (67.2-70.9%; Fig. 3 ) [ 52 , 53 ]. LATAM and North America had notable differences in seroprevalence, with ranges of 59.1 to 91.3% [ 42 , 44 , 54 , 55 ] and 33.0 to 81.0% [ 33 , 45 , 46 , 47 ], respectively. Comparing the range maximums, Europe appeared to have the highest seroprevalence of CMV among adults. Similarly, among the elderly in Europe, multiple articles indicated approximately 2% of the population is seronegative for CMV IgG, suggesting a high seroprevalence of CMV among the elderly in this region [ 35 , 51 , 56 , 57 ].
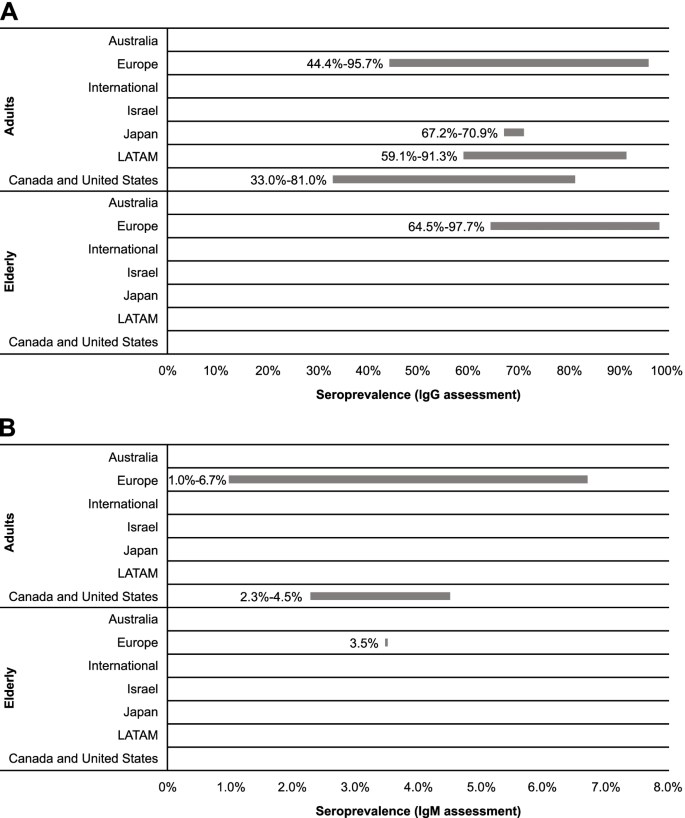
Region-specific cytomegalovirus IgG and IgM seroprevalence among adults and the elderly. IgG, immunoglobulin G; IgM, immunoglobulin M; LATAM, Latin America
Recent data reported within the identified studies suggested differences in seroprevalence ranges across age categories (Table 2 ). Across European studies, the maximum values of the ranges did not substantially vary, but the minimum values increased with age intervals. Data from LATAM indicated that seroprevalence was higher in the 20- to 30-year age group in comparison to the 12- to 20-year age group (91.3 vs 86.5%) [ 44 ]. Age-related increases in seroprevalence were also noticeable in North American studies [ 33 , 45 , 46 ].
CMV IgG seroprevalence was higher among developing than developed countries included in this report. Among adults, CMV IgG seroprevalence ranged from 33.0 to 81.0% for developed countries [ 32 , 33 , 36 , 45 , 46 , 47 , 48 , 49 , 50 , 52 , 53 ] and 59.1 to 95.7% for developing countries [ 35 , 37 , 40 , 42 , 44 , 51 , 54 , 55 ]. For the elderly, seroprevalence was 64.5 to 96.2% for developed countries [ 56 , 57 ] and 93.8 to 97.7% for developing countries [ 35 , 51 ].
We identified 3 studies published since 2010 that provided data on CMV IgM seroprevalence in various adult age categories in general populations; within these studies, no clear regional trend was observed (Table 2 ; Fig. 3 ). Across European studies, CMV IgM seroprevalence was reported as 1.0 to 6.7% for adults [ 41 , 51 ] and 3.5% (95% CI, 1.7-6.3%) for elderly populations [ 51 ]. Within the US study identified, CMV IgM seroprevalence among adults was reported as 2.3 to 4.5% [ 45 ].
Overall, CMV IgM seroprevalence was similar for developing and developed countries. Seroprevalence of CMV IgM was estimated as 2.3 to 4.5% among adults in developed countries [ 45 ] and 1.0 to 6.7% in developing countries [ 41 , 51 ]. Among the elderly, only data for developing countries were available, with a seroprevalence of 3.5% (95% CI, 1.7-6.3%) reported for the elderly population in Croatia [ 51 ].
A total of 3 publications provided data on both IgM and IgG seroprevalences. In a Polish population of pregnant women aged 16 to 45 years, IgM seroprevalence was 2.2% and IgG seroprevalence was 62.4% [ 41 ]. In US women 12 to 49 years of age, the seroprevalence of CMV IgM and IgG was 3.0% and 57.9%, respectively [ 45 ]. CMV IgG seroprevalence generally increased with age, whereas IgM seroprevalence did not show a clear age-related trend in these populations; however, the correlations between age and IgM or IgG seroprevalence were not statistically analyzed [ 45 ]. The study from Croatia reported that among the general population (aged 1 month to 82 years), the seroprevalence of IgM and IgG was 4.3 and 74.4%, respectively; among the elderly, seroprevalence was 3.5 and 93.8%, respectively [ 51 ]. Neither the Croatian nor US studies assessed the statistical correlations between IgM antibody titers and IgG antibody titers [ 45 , 51 ].
As CMV IgM is not a precise indicator of primary versus nonprimary CMV infection, the presence of low CMV IgG avidity can be a useful serologic indicator of primary CMV infection. One study from the United States provided data on the low CMV IgG avidity in the context of CMV IgM prevalence, with the authors stating that primary CMV infection was estimated in 14 to 18% of CMV IgM-positive women, as they had low IgG avidity [ 45 ]. While IgM was not correlated with age, the prevalence of low CMV IgG avidity decreased with age [ 45 ].
CMV seroprevalence by risk factors
At-risk populations.
We identified only 1 article published within the last decade (2010–2020) among at-risk populations [ 51 ] (Table 3 ), which was defined as critically sick intensive care unit patients, those with primary immunodeficiencies, those with secondary immunodeficiencies caused by disease of the immune system, and recipients of immunosuppressing drugs. During a 3-year period (2013–2015), serum samples were collected from Croatian (Europe) residents (of any age) and screened for CMV IgM and IgG antibodies. Among hemodialysis patients, hemodialysis was the main predictor for CMV IgG seropositivity, with CMV seroprevalences reported at 91.4% (95% Cl, 87.7-94.2%) [ 51 ]. Interestingly, CMV reactivation/reinfection was most common in this population (92.3%). Overall, these reported seroprevalences among hemodialysis patients appeared higher than those estimates across European adult populations (44.4-95.7%; Table 2 ). CMV IgM seropositivity seroprevalences were reported as 8.6% (95% CI, 5.7-12.3%) [ 51 ].
Socioeconomic status
Recent studies from North America have evaluated the relationship between CMV seroprevalence and household income and poverty level (Table 3 ). A study from Canada indicated that CMV IgG seroprevalence among a cohort of pregnant women was 58.5%, 34.5%, and 27.1% for household incomes of $0 to $59,999, $60,000 to $99,999, and ≥ $100,000 (Canadian dollars), respectively [ 46 ]. In a separate single-center study in Canada of women from low-, middle-, and high-income families, CMV IgG seroprevalence was 81.0%, 54.0%, and 35.0%, respectively [ 47 ]. In a study among US children aged 1 to 5 years during 2011–2012, CMV IgG seroprevalence was 2-times higher among children from households with a family income to poverty ratio below the poverty level (< 1.0) than those from households above the poverty level (≥ 1.0; 31.1 vs 14.9%, respectively) [ 61 ]; however, this trend was not observed in 2017–2018 (26.4 vs 27.6%, respectively) [ 61 ]. This lack of observable difference likely reflects an increase in CMV seroprevalence among children at or above the poverty level from 2011–2012 to 2017–2018. In Poland, CMV IgG and IgM seroprevalences did not differ significantly by financial status [ 41 ].
Education level
In a Canadian study of pregnant women, CMV IgG seroprevalence was 60.0% among adults with a non-university education level and 51.0% among adults with a university education level [ 47 ] (Table 3 ). When evaluating associations between education level groups, non-university educated women were more likely to be CMV IgG seropositive than university educated women (OR, 2.43; 95% CI, 1.37–4.32) [ 47 ]. Similar results were reported for a cohort of pregnant Polish (European) adult women, where CMV IgG prevalence was evaluated according to education level; seroprevalence was 58.0% among women with higher education, 64.5% among women with secondary education, and 72.9% among women with primary/vocational education [ 41 ]. Interestingly, there was no descriptive or inferential trend observed for evaluations using CMV IgM positive serologic data (2.1% among adults with higher education, 1.9% among adults with secondary education, 2.1% among adults with primary/vocational education) [ 41 ]. These results were likely due to the small sample size of CMV IgM seropositive women ( n = 25). Among US children aged 1 to 5 years, the range of CMV IgG seroprevalence was reported as higher for households with survey participants whose education level was less than a high school diploma (31.3%) versus those households with participants with a high school diploma and some college education (16.7%) or with a college degree or more (17.8%) from 2011–2012 [ 61 ]. Among households with lower education levels, CMV IgG seroprevalence did not significantly increase from 2011–2012 to 2017–2018 (prevalence difference of 5.9 points); however, a substantial increase in seroprevalence during this time frame was observed for households with individuals with a college degree or more (prevalence difference of 16.8) [ 61 ].
Race and ethnicity
Our review identified recent articles from Spain (Europe), Mexico (LATAM), and the United States that explored the relationship between CMV IgG seroprevalence and race/ethnicity (Table 3 ). In a single-center case control study in Spain among a predominantly White study population, CMV IgG seroprevalence among elderly patients was reported as 96.2% [ 57 ]. In a cross-sectional study of pregnant women in Mexico, CMV IgG seroprevalence was 89.6% in a population predominantly of Mestizo ethnic descent [ 44 ]. From these studies, it is difficult to confirm the role of ethnicity as a risk factor for CMV infection. Utilizing the National Health and Nutrition Examination Survey data collected in the United States during 2011–2012, CMV IgG seroprevalence by race/ethnicity of children 1 to 5 years of age were reported as 37.0% among non-Hispanic other/multiracial, 31.0% among Hispanic, 15.9% among Non-Hispanic Black, and 10.6% among non-Hispanic White ethnicities [ 61 ]. When comparing to CMV IgG seroprevalence estimates from 2017–2018, an increase was observed across each race/ethnicity category, with a notable difference among non-Hispanic White children (10.6-24.2%) [ 61 ]. The only exception was a decrease in seroprevalence observed in Non-Hispanic Black children (24.6-15.9%).
CMV shedding and transmission
No studies reporting data for CMV transmission rate were identified. Between 2010 and 2020, only 4 studies provided data on the prevalence of CMV shedding, which were from developed countries (England, France, Spain, and the United States; Table 2 ). Studying urine samples in the British population, shedding was reported as 0% among newborns and infants aged < 2 weeks, 11.0% among those aged 2 weeks to 5 years, 5.2% among those aged 6 to 10 years, and 0% among adolescents (aged 11–15 years) [ 48 ]. In the United States, shedding was reported as 17.0% among children aged 0 to 47 months [ 58 ]. In a feasibility study conducted in French daycare centers among children aged 3 months to 6 years, saliva specimens confirmed CMV shedding in 51.9% of sites [ 59 ]. In a study conducted in the United States, which utilized saliva as well as urine specimens from children aged 0 to 47 months, half of seropositive children were shedding CMV in at least 1 fluid [ 58 ]. Breast milk was screened for CMV DNA in a prospective Spanish study, which found 70.2% of specimens positive for CMV [ 60 ].
This SLR aimed to provide an updated understanding of the current epidemiology of CMV, including prevalence/seroprevalence, shedding, and transmission, across regions and subpopulations. Compiling and assessing these datasets highlights the current knowledge gaps and may aid in guiding policy decisions within the healthcare sector, including those related to CMV clinical guidelines, screening, and potential future treatment and prevention options.
A total of 29 studies were included in our review, with the majority reported from Europe and North America (Canada and the United States). Among women of reproductive age, CMV IgG seroprevalence ranged from 24.6 to 95.7%, which was generally in line with estimates from a recent meta-regression analysis that estimated the global CMV seroprevalence among women of reproductive age as 86%, with the highest seroprevalence observed in the World Health Organization (WHO) Eastern Mediterranean region (92%; 95% uncertainty interval [UI], 88-95%) and the lowest seroprevalence observed in the WHO European region (70%; 95% UI, 63-76%) [ 1 ]. However, our observed ranges were wider than the uncertainty range in this prior report. Among men, we identified only 2 studies reporting CMV IgG seroprevalence and no studies reporting CMV IgM seroprevalence. Thus, similar to reports before 2010, seroprevalence studies continue to primarily focus on women [ 1 , 3 , 62 ]. Overall, our available data indicated that CMV seroprevalence was higher among women of reproductive age than men, in agreement with a prior systematic review from 2010 [ 3 ]. Childcare is generally believed to contribute to higher seroprevalence among women [ 41 , 63 , 64 , 65 ].
Our review also evaluated seroprevalence using IgG and IgM diagnostic methods. CMV IgM antibodies can be used as a marker for primary CMV infection and reactivation/reoccurrence or reinfection (nonprimary infection) and as a potential marker for prevalence of transmission at the time of testing [ 66 ]. In studies utilizing IgM as a diagnostic method, ranges for prevalence were narrower and lower compared with those utilizing IgG methods. This result is expected, as IgM production occurs first after CMV infection, while IgG levels begin to rise several weeks after infection and remain in the blood throughout a person’s lifetime. Therefore, outcomes based on IgM may be more representative of new and active infections, whereas IgG would indicate the overall number of infected patients. However, CMV IgM antibodies can also be associated with both primary and nonprimary CMV infection; thus, distinguishing primary CMV infection requires the detection of low CMV IgG avidity. One study from the United States reported low CMV IgG avidity estimates in 14% to 18% of CMV IgM-positive women, suggesting primary CMV infection [ 45 ]. Limitations of IgM assays should be considered when interpreting IgM data reported throughout this SLR; variation exists between IgM diagnostic assays, indicating assays are less standardized and therefore potentially less reliable than assays for anti-CMV IgG [ 67 ]. In addition, there is a risk that CMV IgM assays may be confounded by antibody cross-reactivity, for example, to Epstein Barr virus [ 68 ].
Previous research has implicated socioeconomic disparities, race, and ethnicity as risk factors of CMV infection and disease [ 69 , 70 , 71 , 72 ]. Our review also indicates an association between CMV seroprevalence and education level, social status, household income, and race and ethnicity [ 3 , 32 , 38 , 41 , 42 , 44 , 46 , 47 , 55 , 58 , 61 ]. This potential association may be based on lifestyle, population density, sexual activity, number of children per family, and child-rearing practices that may be rooted in culture or economics (ie, frequency and duration of breastfeeding, childcare arrangements, and customs that increase saliva sharing with young children) [ 3 , 73 ]. For example, it has been estimated that CMV occurs in 32% of children attending daycare centers worldwide, with a 2.7-times higher chance of CMV positivity among children attending daycare centers [ 22 ]. An analytical model also indicated that hygiene education was greatly effective in prevention of poor outcomes related to CMV infection, estimating that hygiene promotion was associated with a 50% risk reduction for fetal infections in CMV-seronegative populations [ 74 ]. Overall, additional insight into the epidemiologic burden of CMV across different risk factors is needed, which can help guide targeted strategies for those populations at greatest risk for infection and disease.
Only 4 studies evaluating CMV shedding were identified in our review, which indicated shedding prevalence ranged from 11.0 to 51.9% in newborns to children aged 10 years. Due to the low number of included studies, no definite conclusions about the prevalence of CMV shedding across age groups could be drawn. However, prior findings have indicated that shedding of CMV is more prevalent among younger age groups, particularly those < 2 years of age [ 75 ]. Further, no studies on CMV transmission rates were identified in our review. Taken together, our findings underscore the current need for more recent assessments of CMV shedding and transmission.
Our systematic review was strengthened by focusing on the most recent data on CMV epidemiology (2010–2020) and only including studies with sample sizes > 100 to collate data from studies that provide high-quality data and avoid selection bias. However, we did not synthesize data (eg, by meta-analysis) as provided by prior publications [ 1 , 3 , 19 , 20 , 22 , 62 ]. Additionally, statistical comparisons were not included in this report, as any analysis between countries or across time is outside the scope of our systematic review and would require additional analyses to account for the heterogeneity of studies and changes in technology and methodology. Notably, a minimal number of studies from the Asia–Pacific region were identified in our review, which was expected due to our limited review time frame to capture the most current evidence and geographic search restrictions. Additional work is needed to review the CMV seroprevalence literature from China and India, as well as Africa. Further, although permitted within the geographic scope, no studies were identified from Australia or Israel and future research on CMV seroprevalence is warranted in these countries. In the case of the Israel, initial studies identified in our broad search reported prevalence but not CMV IgM or IgG seroprevalence or were congress abstracts and, thus, did not meet the pre-defined inclusion criteria. In addition, although our review suggests CMV seropositivity increased with age and was lower for developed than developing countries (in alignment with previous data [ 3 ]), only limited information was available for these comparisons, thereby restricting an in-depth analysis and inferences. Additional studies that evaluate the age-related seroprevalence of CMV are essential, while studies that evaluate CMV epidemiology in the context of developing regions would aid in deciphering this burden and could guide local clinical recommendations and policy-making decisions towards future interventions such as vaccines.
While our systematic review aimed to elucidate the current epidemiologic burden of CMV across global regions and subpopulations, our study also highlights the lack of recent studies investigating the seroprevalence of CMV among key demographics and countries. Overall, the lack of surveillance and existing evidence in the general population limits our understanding of the causal pathways between CMV infection, disease, and clinically diagnosed outcomes that are critical for the healthcare and policy sectors. Although CMV infection is mild in severity or asymptomatic for the majority of the population, individuals who are immunocompromised (including those undergoing transplant surgery) and neonates have a unique risk of severe disease [ 5 ] and would benefit from improved options for managing the risk of CMV infection. Currently, treatments for CMV are limited and no vaccines are available to prevent against CMV infection and disease, although multiple candidate vaccines are currently in clinical development [ 76 ]. Routine screening of pregnant women for CMV infection is also not recommended or is subject to debate [ 77 ], and interventions to reduce the risk of maternal CMV infection are limited to behavioral practices (ie, hand washing, avoiding contact with a young child’s saliva/urine, etc.)[ 78 , 79 ]. Targeted newborn screening for CMV has been implemented in some states within the United States, as well as some integrated health systems; however, universal routine newborn screening for CMV is not performed globally [ 80 ]. Thus, comprehensive CMV epidemiologic studies are imperative toward furthering our understanding of CMV and associated disease, which in turn can guide public health strategies to reduce disease burden in vulnerable populations through screening, treatment, and vaccine development.
Availability of data and materials
The data summarized in this review are from published articles and are publicly available.
Abbreviations
- Congenital cytomegalovirus
Confidence interval
- Cytomegalovirus
General educational development
Human immunodeficiency virus
Immunoglobulin G
Immunoglobulin M
Latin America
Medical subject header
Not reported
Reverse transcriptase polymerase chain reaction
Systematic literature review
Zuhair M, Smit GSA, Wallis G, Jabbar F, Smith C, Devleesschauwer B, Griffiths P. Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Rev Med Virol. 2019;29(3):e2034.
Article PubMed Google Scholar
Centers for Disease Control and Prevention. Cytomegalovirus (CMV) and Congenital CMV Infection - Clinical Overview. 2020.
Google Scholar
Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20(4):202–13.
Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients-Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13512.
Taylor GH. Cytomegalovirus. Am Fam Physician. 2003;67(3):519–24.
PubMed Google Scholar
Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis. 2013;57(suppl 4):S178–181.
Buca D, Di Mascio D, Rizzo G, Giancotti A, D’Amico A, Leombroni M, Makatsarya A, Familiari A, Liberati M, Nappi L, et al. Outcome of fetuses with congenital cytomegalovirus infection and normal ultrasound at diagnosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2021;57(4):551–9.
Article CAS PubMed Google Scholar
Korndewal MJ, Oudesluys-Murphy AM, Kroes ACM, van der Sande MAB, de Melker HE, Vossen A. Long-term impairment attributable to congenital cytomegalovirus infection: a retrospective cohort study. Dev Med Child Neurol. 2017;59(12):1261–8.
Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17(5):355–63.
Goderis J, De Leenheer E, Smets K, Van Hoecke H, Keymeulen A, Dhooge I. Hearing loss and congenital CMV infection: a systematic review. Pediatrics. 2014;134(5):972–82.
Lanzieri TM, Leung J, Caviness AC, Chung W, Flores M, Blum P, Bialek SR, Miller JA, Vinson SS, Turcich MR, et al. Long-term outcomes of children with symptomatic congenital cytomegalovirus disease. J Perinatol. 2017;37(7):875–80.
Article CAS PubMed PubMed Central Google Scholar
Townsend CL, Forsgren M, Ahlfors K, Ivarsson SA, Tookey PA, Peckham CS. Long-term outcomes of congenital cytomegalovirus infection in Sweden and the United Kingdom. Clin Infect Dis. 2013;56(9):1232–9.
Article PubMed PubMed Central Google Scholar
Colonna AT, Buonsenso D, Pata D, Salerno G, Chieffo DPR, Romeo DM, Faccia V, Conti G, Molle F, Baldascino A, et al. Long-term clinical, audiological, visual, neurocognitive and behavioral outcome in children with symptomatic and asymptomatic congenital cytomegalovirus infection treated with valganciclovir. Front Med (Lausanne). 2020;7:268.
Article Google Scholar
Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17(4):253–76.
Fowler KB, Stagno S, Pass RF, Britt WJ, Boll TJ, Alford CA. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326(10):663–7.
Griffiths P, Baraniak I, Reeves M. The pathogenesis of human cytomegalovirus. J Pathol. 2015;235(2):288–97.
Sugar EA, Jabs DA, Ahuja A, Thorne JE, Danis RP, Meinert CL. Incidence of cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol. 2012;153(6):1016-1024.e1015.
Krishna BA, Wills MR, Sinclair JH. Advances in the treatment of cytomegalovirus. Br Med Bull. 2019;131(1):5–17.
Coppola T, Mangold JF, Cantrell S, Permar SR. Impact of maternal immunity on congenital cytomegalovirus birth prevalence and infant outcomes: a systematic review. Vaccines (Basel). 2019;7(4):129.
Dadashi M, Hajikhani B, Ghazi M, Yazdani S, Goudarzi M, Nasiri MJ, Shokouhi S, Owlia P, Yaslianifard S. The global prevalence of Chlamydia pneumoniae, Helicobacter pylori, Cytomegalovirus and Herpes simplex virus in patients with coronary artery disease: a systematic review and meta-analysis. Microb Pathog. 2021;152:104572.
Romero Starke K, Kofahl M, Freiberg A, Schubert M, Groß ML, Schmauder S, Hegewald J, Kämpf D, Stranzinger J, Nienhaus A, et al. The risk of cytomegalovirus infection in daycare workers: a systematic review and meta-analysis. Int Arch Occup Environ Health. 2020;93(1):11–28.
Zheng QY, Huynh KT, van Zuylen WJ, Craig ME, Rawlinson WD. Cytomegalovirus infection in day care centres: A systematic review and meta-analysis of prevalence of infection in children. Rev Med Virol. 2019;29(1):e2011.
Lanzieri TM, Dollard SC, Josephson CD, Schmid DS, Bialek SR. Breast milk-acquired cytomegalovirus infection and disease in VLBW and premature infants. Pediatrics. 2013;131(6):e1937–1945.
Hamilton ST, van Zuylen W, Shand A, Scott GM, Naing Z, Hall B, Craig ME, Rawlinson WD. Prevention of congenital cytomegalovirus complications by maternal and neonatal treatments: a systematic review. Rev Med Virol. 2014;24(6):420–33.
Fletcher KT, Horrell EMW, Ayugi J, Irungu C, Muthoka M, Creel LM, Lester C, Bush ML. The natural history and rehabilitative outcomes of hearing loss in congenital cytomegalovirus: a systematic review. Otol Neurotol. 2018;39(7):854–64.
Riga M, Korres G, Chouridis P, Naxakis S, Danielides V. Congenital cytomegalovirus infection inducing non-congenital sensorineural hearing loss during childhood; a systematic review. Int J Pediatr Otorhinolaryngol. 2018;115:156–64.
Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7:1–16.
Women of reproductive age (15-49 years) population [ https://www.who.int/data/maternal-newborn-child-adolescent-ageing/indicator-explorer-new/mca/women-of-reproductive-age-(15-49-years)-population-(thousands )]
World Economic Outlook. April 2020. [ https://www.imf.org/external/pubs/ft/weo/2020/01/weodata/groups.htm#lac ]
Kaslow DC. Force of infection: a determinant of vaccine efficacy? NPJ Vaccines. 2021;6(1):51.
Laboratory Testing. https://www.cdc.gov/cmv/clinical/lab-tests.html
Antona D, Lepoutre A, Fonteneau L, Baudon C, Halftermeyer-Zhou F. Y LES, Lévy-Bruhl D: Seroprevalence of cytomegalovirus infection in France in 2010. Epidemiol Infect. 2017;145(7):1471–8.
Styles JN, Converse RR, Griffin SM, Wade TJ, Klein E, Nylander-French LA, Stewart JR, Sams E, Hudgens E, Egorov AI. Human cytomegalovirus infections are associated with elevated biomarkers of vascular injury. Front Cell Infect Microbiol. 2020;10:334.
Toriyabe K, Morikawa F, Minematsu T, Ikejiri M, Suga S, Ikeda T. Anti-cytomegalovirus immunoglobulin M titer for congenital infection in first-trimester pregnancy with primary infection: a multicenter prospective cohort study. J Perinatol. 2017;37(12):1272–7.
Arapović J, Rajič B, Pati S, Brizić I, Azinović I, Šušak B, Ostojić M, Tutiš B, Raguž AB, Tomić V, et al. Cytomegalovirus seroprevalence and birth prevalence of congenital CMV infection in Bosnia and Herzegovina: a single-center experience. Pediatr Infect Dis J. 2020;39(2):140–4.
Barlinn R, Vainio K, Samdal HH, Nordbø SA, Nøkleby H, Dudman SG. Susceptibility to cytomegalovirus, parvovirus B19 and age-dependent differences in levels of rubella antibodies among pregnant women. J Med Virol. 2014;86(5):820–6.
Gorun F, Motoi S, Malita D, Navolan DB, Nemescu D, Olariu TR, Craina M, Vilibic-Cavlek T, Ciohat I, Boda D, et al. Cytomegalovirus seroprevalence in pregnant women in the western region of Romania: a large-scale study. Exp Ther Med. 2020;20(3):2439–43.
CAS PubMed PubMed Central Google Scholar
Plewik D, Tokarska-Rodak M, Paszkiewicz J, Szepeluk A. Seroprevalence of rubella and cytomegalia in young women from Biała Podlaska District. Pol J Microbiol. 2017;66(4):543–5.
Puccio G, Cajozzo C, Canduscio LA, Cino L, Romano A, Schimmenti MG, Giuffrè M, Corsello G. Epidemiology of Toxoplasma and CMV serology and of GBS colonization in pregnancy and neonatal outcome in a Sicilian population. Ital J Pediatr. 2014;40:23.
Siennicka J, Dunal-Szcepaniak M, Trzcińska A, Godzik P, Rosińska M. High seroprevalence of CMV among women of childbearing age implicates high burden of congenital cytomegalovirus infection in Poland. Pol J Microbiol. 2017;65(4):425–32.
Wujcicka W, Gaj Z, Wilczyński J, Sobala W, Spiewak E, Nowakowska D. Impact of socioeconomic risk factors on the seroprevalence of cytomegalovirus infections in a cohort of pregnant Polish women between 2010 and 2011. Eur J Clin Microbiol Infect Dis. 2014;33(11):1951–8.
Alvarado-Esquivel C, Hernández-Tinoco J, Sánchez-Anguiano LF, Ramos-Nevárez A, Cerrillo-Soto SM, Estrada-Martínez S, Martínez-Ramírez L, Pérez-Álamos AR, Guido-Arreola CA. Seroepidemiology of cytomegalovirus infection in pregnant women in Durango City. Mexico BMC Infect Dis. 2014;14:484.
Alvarado-Esquivel C, Sandoval-Carrillo AA, Vazquez-Alaniz F, Salas-Pacheco JM, Hernández-Tinoco J, Sánchez-Anguiano LF, Antuna-Salcido EI. Lack of association between cytomegalovirus infection and hypertensive disorders in pregnancy: a case-control study in Durango, Mexico. Eur J Microbiol Immunol (Bp). 2017;7(3):229–33.
Article CAS Google Scholar
Alvarado-Esquivel C, Terrones-Saldivar MDC, Hernandez-Tinoco J, Munoz-Terrones MDE, Gallegos-Gonzalez RO, Sanchez-Anguiano LF, Reyes-Robles ME, Antuna-Salcido EI. Seroepidemiology of cytomegalovirus infection in pregnant women in the Central Mexican City of Aguascalientes. J Clin Med Res. 2018;10(4):337–44.
Dollard SC, Staras SA, Amin MM, Schmid DS, Cannon MJ. National prevalence estimates for cytomegalovirus IgM and IgG avidity and association between high IgM antibody titer and low IgG avidity. Clin Vaccine Immunol. 2011;18(11):1895–9.
Lamarre V, Gilbert NL, Rousseau C, Gyorkos TW, Fraser WD. Seroconversion for cytomegalovirus infection in a cohort of pregnant women in Québec, 2010–2013. Epidemiol Infect. 2016;144(8):1701–9.
Wizman S, Lamarre V, Coic L, Kakkar F, Le Meur J-B, Rousseau C, Boucher M, Tapiero B. Awareness of cytomegalovirus and risk factors for susceptibility among pregnant women, in Montreal, Canada. BMC Pregnancy Childbirth. 2016;16(1):54.
Article PubMed PubMed Central CAS Google Scholar
Abdel Hamid SES, Abdel-Wahab KSE, Saleh LH, Davis GE, Hastie I, Booth JC. Comparative epidemiology of infection with human cytomegalovirus in Cairo and South London. Int J Virol. 2011;7(3):116–22.
Alari-Pahissa E, Moreira A, Zabalza A, Alvarez-Lafuente R, Munteis E, Vera A, Arroyo R, Alvarez-Cermeno JC, Villar LM, Lopez-Botet M, et al. Low cytomegalovirus seroprevalence in early multiple sclerosis: a case for the “hygiene hypothesis”? Eur J Neurol. 2018;25(7):925–33.
Maple PAC, Tanasescu R, Gran B, Constantinescu CS. A different response to cytomegalovirus (CMV) and Epstein-Barr virus (EBV) infection in UK people with multiple sclerosis (PwMS) compared to controls. J Infect. 2020;80(3):320–5.
Vilibic-Cavlek T, Kolaric B, Beader N, Vrtar I, Tabain I, Mlinaric-Galinovic G. Seroepidemiology of cytomegalovirus infections in Croatia. Wien Klin Wochenschr. 2017;129(3–4):129–35.
Takao M, Yoshioka N, Hagiya H, Deguchi M, Kagita M, Tsukamoto H, Hidaka Y, Tomono K, Tobe T. Risk for the occupational infection by cytomegalovirus among health-care workers. J Infect Chemother. 2020;26(7):681–4.
Takemoto K, Nishimura N, Kozawa K, Hibino H, Kawaguchi M, Takeuchi S, Fujishiro N, Arai S, Gotoh K, Hosono H, et al. Time-series analysis comparing the prevalence of antibodies against nine viral species found in umbilical cord blood in Japan. Jpn J Infect Dis. 2016;69(4):314–8.
De la Tejera-Hernández C, Noyola D, Sánchez-Vargas L, Nava-Zárate N, De La Cruz-Mendoza E, Gómez-Hernández A, Aranda-Romo S. Analysis of risk factors associated to cytomegalovirus infection in dentistry students. J Oral Res (Impresa). 2015;4(3):197–204.
Tiguman GMB, Poll LB, de Castro Alves CE, Pontes GS, Silva MT, Galvao TF. Seroprevalence of cytomegalovirus and its coinfection with Epstein-Barr virus in adult residents from Manaus: a population-based study. Revista da Sociedade Brasileira de Medicina Tropical. 2020;53:e20190363.
Firth C, Harrison R, Ritchie S, Wardlaw J, Ferro CJ, Starr JM, Deary IJ, Moss P. Cytomegalovirus infection is associated with an increase in systolic blood pressure in older individuals. QJM. 2016;109(9):595–600.
González-Quijada S, Mora-Simón MJ, Martin-Ezquerro A. Association between serological evidence of past Coxiella burnetii infection and atherosclerotic cardiovascular disease in elderly patients. Clin Microbiol Infect. 2014;20(9):873–8.
Stowell JD, Mask K, Amin M, Clark R, Levis D, Hendley W, Lanzieri TM, Dollard SC, Cannon MJ. Cross-sectional study of cytomegalovirus shedding and immunological markers among seropositive children and their mothers. BMC Infect Dis. 2014;14:568.
Grosjean J, Trapes L, Hantz S, Mengelle C, Virey B, Undreiner F, Messager V, Denis F, Marin B, Alain S. Human cytomegalovirus quantification in toddlers saliva from day care centers and emergency unit: a feasibility study. J Clin Virol. 2014;61(3):371–7.
Romero-Gómez MP, Cabrera M, Montes-Bueno MT, Cendejas-Bueno E, Segovia C, Pastrana N, Mingorance J, Omeñaca F. Evaluation of cytomegalovirus infection in low-birth weight children by breast milk using a real-time polymerase chain reaction assay. J Med Virol. 2015;87(5):845–50.
Article PubMed CAS Google Scholar
Petersen MR, Patel EU, Abraham AG, Quinn TC, Tobian AAR. Changes in cytomegalovirus seroprevalence among U.S. children aged 1–5 years: the national health and nutrition examination surveys. Clin Infect Dis. 2020;72(9):e408–11.
Article PubMed Central Google Scholar
Ssentongo P, Hehnly C, Birungi P, Roach MA, Spady J, Fronterre C, Wang M, Murray-Kolb LE, Al-Shaar L, Chinchilli VM, et al. Congenital cytomegalovirus infection burden and epidemiologic risk factors in countries with universal screening: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(8):e2120736.
Adler SP. Cytomegalovirus and child day care: risk factors for maternal infection. Pediatr Infect Dis J. 1991;10(8):590–4.
van Zuylen WJ, Zheng QY, Hamilton ST, Egilmezer EE, Craig ME, Gralton J, Rawlinson WD. Prevalence of cytomegalovirus carriage among childcare staff. J Paediatr Child Health. 2017;53(7):724.
van Rijckevorsel GG, Bovee LP, Damen M, Sonder GJ. Schim van der Loeff MF, van den Hoek A: Increased seroprevalence of IgG-class antibodies against cytomegalovirus, parvovirus B19, and varicella-zoster virus in women working in child day care. BMC Public Health. 2012;12:475.
Wang C, Dollard SC, Amin MM, Bialek SR. Cytomegalovirus IgM seroprevalence among women of reproductive age in the United States. PLoS One. 2016;11(3):e0151996.
Prince HE, Lape-Nixon M, Brenner A, Pitstick N, Couturier MR. Potential impact of different cytomegalovirus (CMV) IgM assays on an algorithm requiring IgM reactivity as a criterion for measuring CMV IgG avidity. Clin Vaccine Immunol. 2014;21(6):813–6.
Miendje Deyi Y, Goubau P, Bodeus M. False-positive IgM antibody tests for cytomegalovirus in patients with acute Epstein-Barr virus infection. Eur J Clin Microbiol Infect Dis. 2000;19(7):557–60.
Dowd JB, Aiello AE, Alley DE. Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiol Infect. 2009;137(1):58–65.
Lawrence GM, Friedlander Y, Calderon-Margalit R, Enquobahrie DA, Huang JY, Tracy RP, Manor O, Siscovick DS, Hochner H. Associations of social environment, socioeconomic position and social mobility with immune response in young adults: the Jerusalem Perinatal Family Follow-Up Study. BMJ Open. 2017;7(12):e016949.
Messinger CJ, Lipsitch M, Bateman BT, He M, Huybrechts KF, MacDonald S, Mogun H, Mott K, Hernandez-Diaz S. Association between congenital cytomegalovirus and the prevalence at birth of microcephaly in the United States. JAMA Pediatr. 2020;174(12):1159–67.
Fowler KB, Ross SA, Shimamura M, Ahmed A, Palmer AL, Michaels MG, Bernstein DI, Sanchez PJ, Feja KN, Stewart A, et al. Racial and ethnic differences in the prevalence of congenital cytomegalovirus infection. J Pediatr. 2018;200(196–201):e191.
Pribakovic JA, Katanic N, Radevic T, Tasic MS, Kostic M, Stolic B, Radulovic A, Minic V, Bojovic K, Katanic R. Serological status of childbearing-aged women for Toxoplasma gondii and cytomegalovirus in Northern Kosovo and Metohija. Rev Soc Bras Med Trop. 2019;52:e-20170313.
De BilletteVillemeur A, Tattevin P, Salmi LR. Hygiene promotion might be better than serological screening to deal with cytomegalovirus infection during pregnancy: a methodological appraisal and decision analysis. BMC Infect Dis. 2020;20(1):418.
Cannon MJ, Hyde TB, Schmid DS. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol. 2011;21(4):240–55.
Scarpini S, Morigi F, Betti L, Dondi A, Biagi C, Lanari M. Development of a vaccine against human cytomegalovirus: advances, barriers, and implications for the clinical practice. Vaccines (Basel). 2021;9(6):551.
Lazzarotto T, Blázquez-Gamero D, Delforge ML, Foulon I, Luck S, Modrow S, Leruez-Ville M. Congenital cytomegalovirus infection: a narrative review of the issues in screening and management from a panel of European experts. Front Pediatr. 2020;8:13.
Stowell JD, Forlin-Passoni D, Radford K, Bate SL, Dollard SC, Bialek SR, Cannon MJ, Schmid DS. Cytomegalovirus survival and transferability and the effectiveness of common hand-washing agents against cytomegalovirus on live human hands. Appl Environ Microbiol. 2014;80(2):455–61.
Cannon MJ, Davis KF. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health. 2005;5:70.
Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev. 2013;26(1):86–102.
Download references
Acknowledgements
Medical writing and editorial assistance were provided by Emily Stackpole, PhD, and Kate Russin, PhD, of MEDiSTRAVA in accordance with Good Publication Practice (GPP3) guidelines, funded by Moderna, Inc., and under the direction of the authors.
This study was funded by Moderna, Inc.
Author information
Authors and affiliations.
University of Alabama at Birmingham, Birmingham, AL, USA
Karen Fowler
Certara, Inc., Krakow, Poland
Jacek Mucha, Witold Lewandowski, Magdalena Kaczanowska & Maciej Grys
Certara, Inc., Lörrach, Germany
Monika Neumann & Elvira Schmidt
Moderna, Inc., 200 Technology Square, Cambridge, MA, 02139, USA
Andrew Natenshon, Carla Talarico, Philip O. Buck & John Diaz-Decaro
You can also search for this author in PubMed Google Scholar
Contributions
JM, MN, ES, AN, CT, and PB contributed to the study concept and design. Data collection was performed by JM, MN, WL, MK, ES, MG, CT, PB, and JD-D; data analysis and interpretation was performed by KF, JM, WL, MK, ES, MG, CT, PB, and JD-D. All authors contributed to the preparation of the manuscript and approved the final draft.
Corresponding author
Correspondence to John Diaz-Decaro .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
AN, CT, JD-D, and PB are employees of Moderna, Inc. and hold stock/stock options in the company. ES, JM, MG, MK, MN, and WL are employees of Certara and were paid consultants for Moderna, Inc. for conduct of this research and development of the manuscript.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Fowler, K., Mucha, J., Neumann, M. et al. A systematic literature review of the global seroprevalence of cytomegalovirus: possible implications for treatment, screening, and vaccine development. BMC Public Health 22 , 1659 (2022). https://doi.org/10.1186/s12889-022-13971-7
Download citation
Received : 28 January 2022
Accepted : 08 August 2022
Published : 01 September 2022
DOI : https://doi.org/10.1186/s12889-022-13971-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Epidemiology
- Seroprevalence
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 27 June 2022
Prevalence, incidence, and risk factors associated with cytomegalovirus infection in healthcare and childcare worker: a systematic review and meta-analysis
- Safari Joseph Balegamire ORCID: orcid.org/0000-0001-9657-3791 1 , 2 ,
- Elisabeth McClymont 3 ,
- Agathe Croteau 4 ,
- Philippe Dodin 2 ,
- Soren Gantt 2 , 5 ,
- Amir Abbas Besharati 2 ,
- Christian Renaud 2 , 5 ,
- Benoît Mâsse 1 , 6 &
- Isabelle Boucoiran 1 , 2 , 7
Systematic Reviews volume 11 , Article number: 131 ( 2022 ) Cite this article
4534 Accesses
2 Citations
4 Altmetric
Metrics details
Cytomegalovirus (CMV) is transmitted by direct contact with body fluids from infected individuals. Transmission of CMV in households, particularly those with young children, contributes significantly to CMV infection in the general population. However, little is known about the contribution of occupational healthcare or childcare exposure to risk of CMV infection.
To determine CMV seroprevalence, incidence of primary infection, and associated risk factors in healthcare and childcare workers.
Six electronic databases were searched systematically for publications on CMV infection in healthcare and childcare workers until March 7, 2022. Two authors independently evaluated the literature for quality and inclusion in our analyses. The pooled results for seroprevalence, incidence, and relative risk (RR) were determined using a random effects model. Heterogeneity among studies was quantified and further investigated in subgroup analysis and meta-regression. Publication bias was assessed using funnel plot. Statistical analyses were preformed using R version 4.05.
Forty-eight articles were included in this meta-analysis (quality assessment: 18 good, 14 fair, and 16 poor). Pooled CMV seroprevalence was 59.3% (95% CI : 49.8–68.6) among childcare workers and 49.5% (95% CI : 40.3–58.7) among healthcare workers, and pooled incidences of primary CMV infection per 100 person-years were respectively 7.4 (95% CI : 3.9–11.8) and 3.1 (95% CI : 1.3–5.6). RR for primary infection compared to controls were 3.4 (95% CI : 1.3–8.8) and 1.3 (95% CI : 0.6–2.7) for healthcare and childcare workers, respectively. The odds of CMV seropositivity were 1.6 (95% CI : 1.2–2.3) times higher for childcare workers compared to controls, but not significantly different between healthcare workers and controls (0.9; 95% CI : 0.6–1.2). CMV seropositivity in both groups was significantly associated with having one or more children residing at home, marital status, ethnicity, and age.
Conclusions
Childcare workers, but not healthcare workers, have an increased risk of prevalent and incident CMV infection, a risk that is further increased with the presence of at least one child living at home. These findings suggest that enforcing simple, conventional hygienic measures in childcare settings could help reduce transmission of CMV, and that special precautionary measures for preventing CMV infection may not be required for pregnant healthcare workers.
Systematic review registration
PROSPERO CRD42020139756
Peer Review reports
Introduction
Cytomegalovirus (CMV) is a common infection with a seroprevalence ranging from 45 to 100% [ 1 ], depending on the population and existing risk factors [ 2 , 3 , 4 ].
CMV is a member of the herpesvirus family that characteristically produce latent and persistent infections in human hosts [ 5 , 6 ]. CMV is transmitted horizontally through contact with biological fluids (saliva, urine, tears, semen, vaginal secretions, blood, and breast milk) from an infected individual or vertically (mother to fetus) by placental transfer [ 7 ]. CMV infection can be diagnosed directly with polymerase chain reaction (PCR), virus culture tests, and pp65 viral antigen detection or indirectly with serology and with IgG and IgM avidity tests [ 8 ].
CMV is the most common vertically transmitted infection, and congenital CMV infection is a major health concern as the leading cause of nongenetic hearing loss in children and its association with high rates of severe abnormal neurodevelopment [ 9 , 10 , 11 ].
Young children congenitally or postnatally infected with CMV, especially aged from 1 to 3 years, shed large amounts of virus in biological fluids over prolonged periods and represent important vectors of CMV transmission and infection [ 12 , 13 , 14 ]. Thus, exposure to young children in the workplace may predispose to the risk for CMV infection [ 15 ].
Due to the considerable global health burden of congenital CMV infection, exposure of women of childbearing age to CMV is of great interest to policy makers. To reduce the risk of CMV transmission/acquisition, certain countries, including Germany and parts of Canada, require that women exposed to young children in the workplace, namely healthcare and childcare workers, be reassigned or given leave of absence during pregnancy [ 16 , 17 , 18 ]. A meta-analysis studying CMV prevalence and risk of seropositivity and occupational exposure to children has recently been published that focused on childcare workers only and restricted the selection of studies to certain countries published beginning since the year 2000 [ 19 ]. The present study provides estimates of the prevalence and incidence of CMV, based on relevant studies from any country, whenever they were published, and identifies risk factors for seropositivity and primary infection in healthcare and childcare workers compared to respective control groups. Our findings reveal important differences between study groups and suggest ways to reduce the incidence of CMV infection.
Protocol design and registration
This is a systematic review and meta-analysis of prevalence, incidence, and risk factors associated with CMV infection in healthcare and childcare workers. PRISMA 2020 and MOOSE protocols were used as references for the search strategy and for reporting results (Additional file, Table S 1 ) [ 20 , 21 ]. The study protocol was registered under the International Prospective Register of Systematic Reviews—Prospero, number CRD42020139756.
Study eligibility and selection
Two examiners (SJB, EM) blinded to one another independently evaluated all articles, and those pertaining to prevalence, to incidence, and to risk factors associated with CMV seropositivity were selected for inclusion (Table 1 ). Only studies in human published in French or English were considered. Non-original papers, case reports, and redundant articles were excluded. Any disagreements between article inclusion or exclusion were resolved by a third independent examiner (IB).
Study outcomes
The main outcomes of interest were to estimate the prevalence, incidence, relative risks, and risk factors associated with CMV infection in healthcare and childcare workers.
Search strategy
Systematic searches of the databases PubMed (NLM), Ovid MEDLINE, Ovid All EBM Reviews, Ovid Embase, ISI Web of Science, and EBSCO CINAHL Complete were performed by a trained librarian (PD) who retrieved all publications related to occupational exposure to CMV up until the cutoff date of March 7, 2022. The MeSH terms related to “cytomegalovirus” and “occupational exposure” were defined and combined for the search (Additional file, Table S 2 ).
We also manually searched bibliographies from prior systematic reviews and meta-analyses, thoroughly reviewed articles cited in scientific reports, presentations of the experts from the “Institut National de Santé Publique de Québec,” and searched for articles in Google Scholar. Neither approach identified additional publications.
Quality appraisal of included studies
The quality of included studies was evaluated using the NIH Study Quality Assessment Tools [ 22 ]. These tools were designed to help reviewers focus on concepts that are essential for critically assessing the internal validity of a study, but not to provide a list of factors that includes a numerical score; the guide to using this practical quality assessment tool is explained elsewhere [ 22 ]. A “good” quality study has the least risk of bias, and the results are considered valid. A study of “fair” quality is likely to have some risk of bias, but not enough to invalidate results. A study of “poor” quality indicates a high probability of risk of bias.
The quality of the studies included in the present review was assessed in a blinded manner by two independent examiners (SJB, EM). If opinions differed, an additional assessment was performed by a third examiner (IB), and a consensus was reached.
Data extraction
Data were extracted independently by two examiners (SJB and EM) and compared to ensure accuracy. Data consisted of author, year of data collection, year of publication, article title, geographic location, risk factors for CMV, type of occupation, study setting, number of study sites, number of participants, and diagnostic method used for CMV. The populations of interest were healthcare and childcare workers, and the control groups represented participants with other jobs reported in the studies. The number of CMV seropositive cases and the number of cases that seroconverted were recorded, including, whenever possible, for the control populations. When relevant information was unavailable in the articles themselves, the authors were directly contacted.
Statistical analysis
For each study included in the analyses, the prevalence of CMV was estimated in each group by dividing the number of seropositive cases by the total number of individuals tested. Similarly, the incidence of primary CMV infection expressed in person-years was calculated by dividing the number of individuals that seroconverted during a specified time frame by the total number of individuals tested during that time. The odds ratio (OR) for CMV seropositivity among healthcare and childcare workers was estimated by comparing their odds with those of the control groups. RR for primary CMV infection were calculated by dividing incidences in healthcare and childcare workers by the incidence in the respective control populations. Risk factors for CMV seropositivity were determined based on the odds of CMV seropositivity in each study group compared to these controls.
Statistical analyses were preformed using R version 4.05. A p- value less than 0.05 was considered statistically significant. Pooled prevalence and incidence estimates were obtained using the R metapro and metarate statistics packages [ 23 ] using a random effects model with a restricted maximum likelihood and Freeman-Tukey double-arcsine transformation to stabilize variances [ 24 , 25 ].
Pooled OR and RR measures of CMV seropositivity and primary infection among healthcare and childcare workers versus controls were estimated using meta-analyses of binary outcome data (R metabin ) [ 23 , 26 ]. In the random effects model, variance between studies was estimated using the Paule and Mandel method [ 26 ]. To estimate the percentage of the total variation attributed to study heterogeneity, and not chance, we used the I 2 statistic [ 27 ]; when I 2 was greater than 40%, we studied the heterogeneity in subgroup and post-stratification analyses with the variables, occupational group (childcare workers versus healthcare staff), diagnostic method for CMV, study design, and study quality. Sensitivity analyses based on the study quality and sample size were performed. Meta-regression was employed to further explore study heterogeneity [ 28 ]. Funnel plot was used to assess publication bias, and Begg’s test rank correlation [ 29 ] or Egger’s linear regression method [ 30 ] was used to evaluate its asymmetry.
Study characteristics
A PRISMA chart depicting the number of records identified, included, and excluded is provided in Fig. 1 . Forty-eight articles (18 good quality, 14 fair quality, and 16 poor quality) were included in our analyses (Additional file, Table S 3 ). From the 48 studies included in our analyses, 27 reported prevalence, 20 reported incidence and prevalence, and 1 reported on incidence only. Forty-seven studies were used to estimate pooled CMV seroprevalence, and 21 served to estimate the incidence of primary CMV infection (Additional file, Table S 4 for a description of each article). A total of 29,486 healthcare and childcare workers from 16 countries (median: 183, range: 4–17,130 participants per study) were included in the analysis. Studies were primarily from the USA (44%), Canada (10%), France (8%), and Germany (6%); 35% were cohort studies, and 65% were cross-sectional studies. No experimental or clinical studies were identified that met our inclusion criteria. Forty-five percent (22/48) of the studies involved childcare workers, 50% (24/48) concerned healthcare workers, and two studies included both [ 31 ]. Methodologies used to assess CMV serostatus varied across studies and included ELIS (19/48), latex agglutination (6/48), complement fixation (7/48), anticomplement immunofluorescence (4/48), restriction endonuclease (2/48), unspecified (3/48), and methods defined as “other” (7/48).
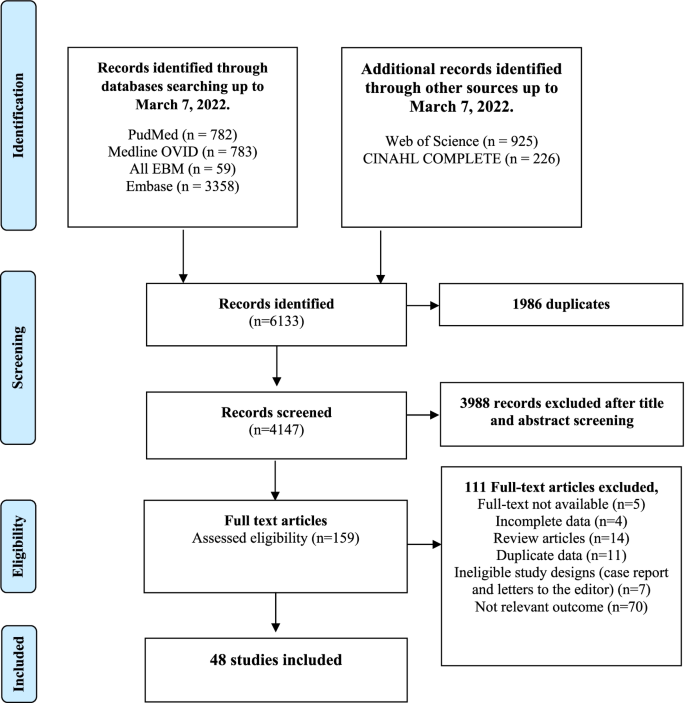
Flowchart describing the selection of studies on prevalence, incidence, and risk factors associated with CMV in childcare and healthcare workers
CMV seroprevalence and incidence of primary CMV infection among healthcare and childcare workers
As shown in Table 2 , the overall pooled seroprevalence among healthcare and childcare workers was 53.3% (95% CI : 46.5–60.0) and differed significantly ( p < 0.0001) between continent (61% Europe, 78% Asia, 47% North America, 52% Oceania, and 48% Africa) (Fig. 2 ). When analyzed by subgroup, the seroprevalences among healthcare and childcare workers were 59.3% and 49.5%, respectively (Fig. 3 ), and these rates did not change significantly (59.2% and 54.6%) in the sensitivity analysis that excluded poor quality studies.
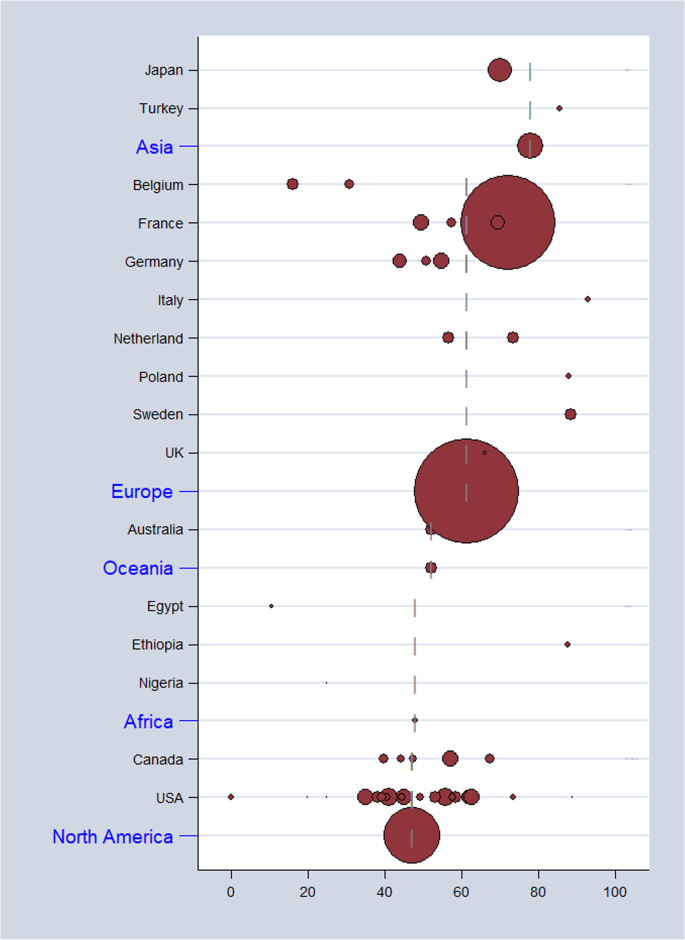
Estimates of seroprevalence of CMV infection by geographical location. Overall CMV seroprevalence 53.3% (95% CI : 46.5–60.0). Bubble plots of CMV pooled seroprevalence in populations exposed to children in the workplace. Individual study data are shown at the country level and pooled by continent. The size of bubble is proportional to the number of participants studied
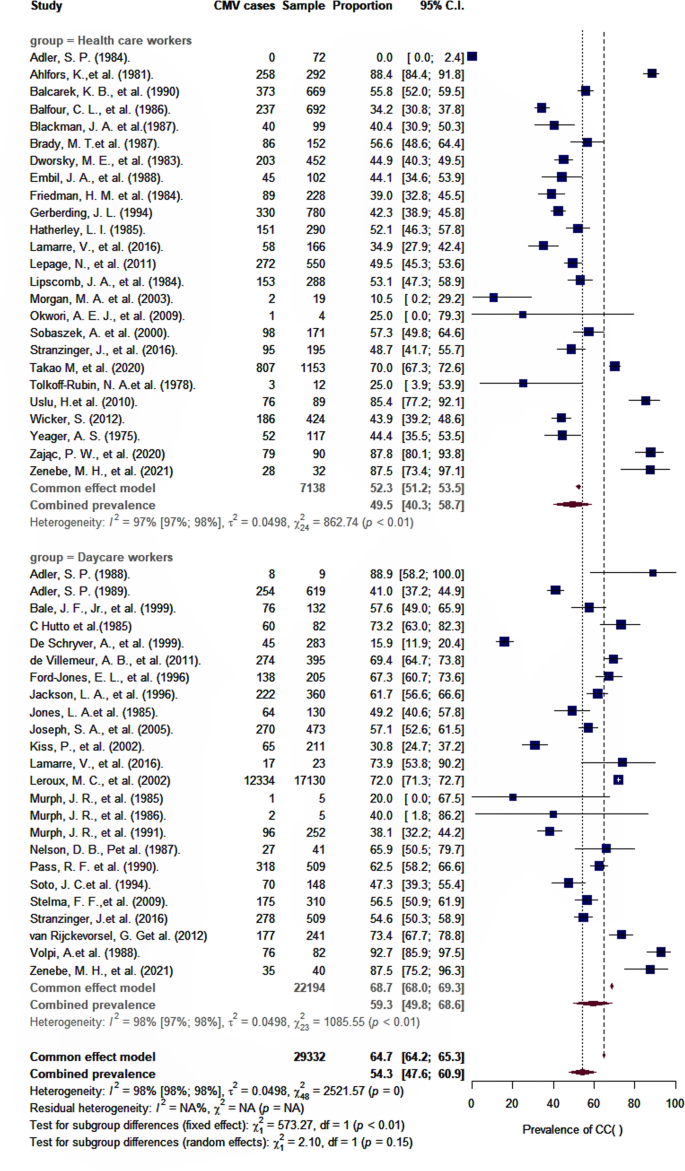
Pooled seroprevalence of CMV infection among childcare and healthcare workers
The overall pooled incidence of primary CMV infection among healthcare and childcare workers was 4.6 per 100 person-years (95% CI : 2.6–7.1). Results of subgroup analysis are presented in Table 2 . Consistent with seroprevalence rates, the pooled annual incidence of primary CMV infection was statistically significantly higher among childcare workers than among healthcare workers (7.4 per 100 person-years versus 3.1 per 100 person-years; p < 0.0001; Fig. 4 ). A sensitivity analysis that omitted studies of poor quality did not significantly change these estimates (7.5 per 100 person-years versus 3.3 per 100 person-years).
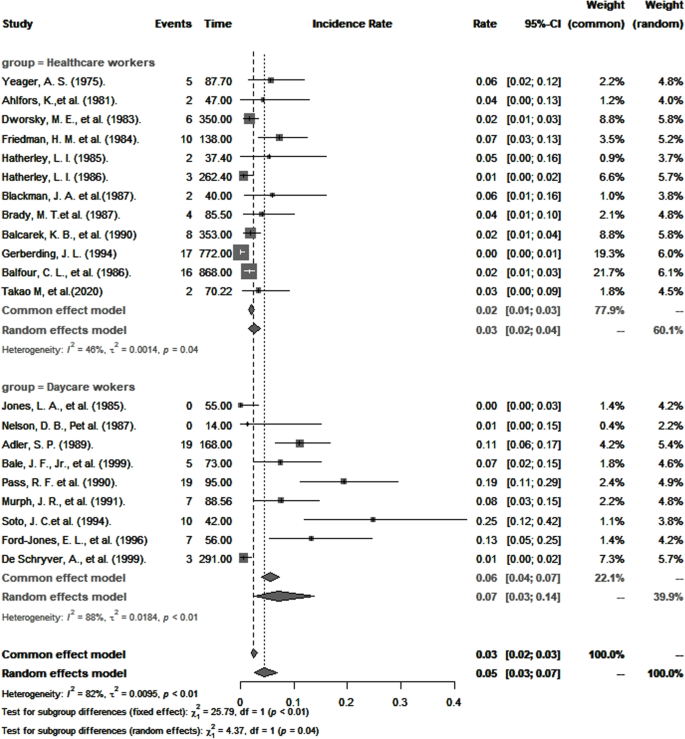
Pooled annual incidence of primary CMV infection
CMV seropositivity and primary infection among healthcare and childcare workers compared to control groups
CMV seropositivity and primary infection rates in healthcare and childcare workers were compared with those of controls (Table 3 ). The odds of CMV seropositivity were significantly higher among childcare workers ( OR 1.6, 95% CI : 1.2–2.3), but not healthcare workers ( OR 0.9, 95% CI : 0.6–1.2) (Fig. 5 ) when compared to their respective controls, and as shown in Fig. 6 , so were the RR for CMV primary infection (3.4, 95% CI : 1.3–8.8 versus 1.3, 95% CI : 0.6–2.7) (Fig. 6 ). Sensitivity analysis that excluded poor quality studies had no significant impact on either the OR estimates for CMV seropositivity ( OR 1.5; 95% CI : 1.1–2.2) versus ( OR 0.9; 95% CI : 0.7–1.2, [ p = 0.8530]) or RR estimates for primary infection ( RR 3.4; 95% CI : 1.3–8.8) and ( RR 1.3; 95% CI : 0.6–2.7, [ p = 0.7425]) compared to respective controls.
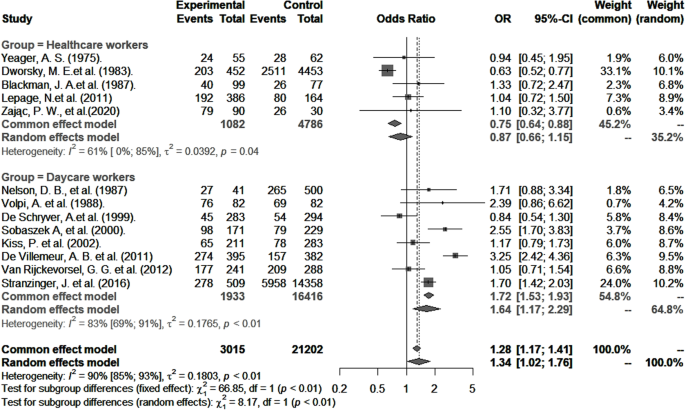
Risk of CMV seropositivity among healthcare and childcare workers compared to controls
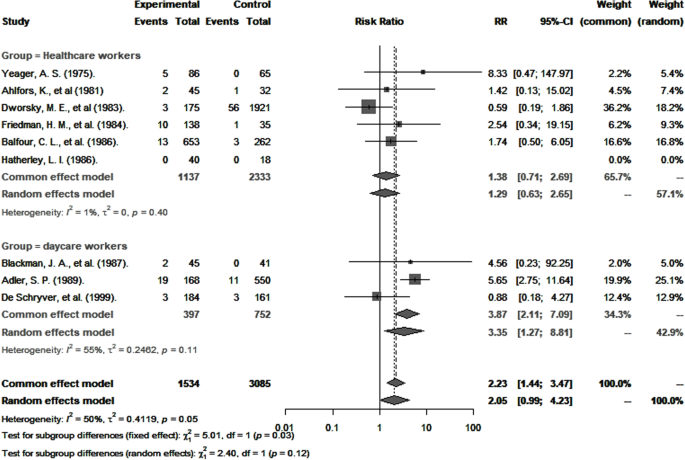
Risk ratio of primary CMV infection in healthcare and childcare workers compared to controls
Risk factors for CMV seropositivity among healthcare and childcare workers
Studies that categorized risk factors similarly were included in the pooled analyses of OR for CMV seropositivity (Table 4 ). In both groups, this OR was significantly higher in households with one or more children (childcare workers: OR 1.9; 95% CI : 1.3–2.7; healthcare workers: OR 2.2; 95% CI : 1.6–3.8). Other factors significantly associated with higher OR for CMV seropositivity were ethnicity other than Caucasian ( OR 2.3; 95% CI : 1.7–3.1), marriage and common-law partnership ( OR 1.7; 95% CI : 1.4–2.1), and, unique to healthcare workers, age ≥ 30 years ( OR 2.6; 95% CI : 1.8–3.8).

Publication bias and meta-regression
Funnel plot asymmetry analysis using the Begg’s and Egger’s tests did not reveal evidence of publication bias (Additional file, Table S 5 ).
Multivariate meta-regression models were used to explore heterogeneity across studies (Additional file, Table S 6 ). More specifically, they were employed to analyze heterogeneity in pooled analyses of CMV prevalence, incidence, risk of seropositivity, and primary CMV infection from non-stratified data. Multivariable meta-regression analysis was not possible to assess the source(s) of heterogeneity in risk factors due to a paucity of studies. We found that heterogeneity for CMV prevalence was largely attributed to studies that had not specified the diagnostic method for CMV, whereas heterogeneity in OR for CMV seropositivity could be explained by diagnostic method, study design, and study quality. Regarding incidence and RR for CMV primary infection, none of the variables tested were associated with heterogeneity.
This meta-analysis of 48 studies from 16 countries provides updated estimates for CMV seroprevalence, OR for seropositivity, and RR of primary infection in healthcare and childcare workers compared to their respective controls and extends our knowledge of the risk factors associated with CMV seropositivity in populations exposed to children in the workplace.
The results show that about half of all healthcare and childcare workers (53%) were seropositive for CMV. This estimate is considerably lower than worldwide (83%), European (70%), southeast Asian (89%), and African (89%) estimates [ 5 ] but comparable to rates estimated for the general adult population in the USA (50%) [ 32 ].
CMV transmission from infected children occurs primarily through direct inoculation of virus shed from body fluids, particularly saliva and urine, in host mucous membranes, and increased transmission appears to be associated with poor hygienic practices [ 33 ]. Compared to controls, our findings show a statistically significant increased odds for CMV seropositivity among childcare, but not healthcare workers, and are consistent with Starke et al. [ 34 ] who reported an increased risk of CMV infection in childcare workers compared to the general population and with Bale et al. [ 15 ] who showed that in addition to childcare workers, this risk was significantly greater among parents with children in childcare, compared with healthcare workers. This might be explained by better adherence to universal infection prevention protocols in healthcare versus childcare workplace settings [ 35 ]. It is worth noting that because healthcare workers are not uniformly exposed to the same risk of exposure to CMV, it is likely that their overall level of exposure was less than for childcare workers. Indeed, studies have shown that the prevalence and incidence rates of CMV infection are different in healthcare staff working in different hospital departments [ 36 , 37 , 38 , 39 ] depending on the nature of the patients they are exposed to.
The overall incidence of primary CMV infection from pooled studies of healthcare and childcare workers was 4.7 per 100 person-years and is within the range of estimates for pregnant women in the general population [ 40 , 41 , 42 ]. Among healthcare workers only, this incidence was 3.1 per 100 person-years and is comparable to that reported for nurses working in pediatric wards [ 43 ].
The significance of the higher risk of CMV primary infection observed in childcare workers compared to controls should be regarded with some caution because for controls, the risk was derived from the unadjusted analysis of only three studies, and major confounders, particularly age, socioeconomic status, and number of children living at home, were not considered. Nevertheless, that identical strains of CMV are observed in children attending daycare and in childcare workers [ 40 , 44 , 45 , 46 ] suggest that the latter acquire it from the former. While the actual risk of congenital CMV infection in pregnant childcare workers has not been established, preventive measures and screening strategies should be implemented, especially for pregnancy [ 47 , 48 ], until effective vaccines become available [ 49 , 50 ].
In both healthcare and childcare workers, having one or more children residing at home doubled the risk of CMV seropositivity (Table 4 ). Therefore, in addition to exposure to children in the workplace, exposure to children at home may significantly contribute to overall risk. Certainly, CMV transmission occurs between occupationally exposed workers, their children, and children in childcare, and that, in any direction. A vicious cycle of viral transmission [ 51 ] can easily be envisioned; approximately, 50% of CMV-positive women transmit the virus through breast milk to their children [ 44 , 52 , 53 ] who may then reinfect the mother, a phenomenon described as “ping-pong” transmission [ 51 ]. The practice of proper infection prevention control measures, such as frequent hand washing, wearing protective gloves, avoiding kissing children on the mouth/cheeks, and not sharing utensils, foods, drinks, and washcloths, decreases the likelihood of CMV infection [ 50 , 54 , 55 , 56 , 57 , 58 ]. Educating childcare workers to adhere to these simple preventive strategies at home and in the workplace should help reduce the transmission of CMV, the likelihood of primary and non-primary infection, and ultimately the risk of congenital CMV infection, as has been described for pregnant women and parents [ 54 , 59 , 60 ]. Consistent with earlier studies [ 1 , 32 , 61 ], we observed greater odds for CMV seropositivity among non-Caucasian ethnicities. Additionally, childcare workers who are married or in a common-law civil union had a significantly greater risk of CMV infection compared with people who are single, probably because couples are more likely to have children residing at home and attending daycare. Among healthcare workers, age greater than 30 years was associated with CMV seropositivity, agreeing with previous reports [ 1 , 62 ].
The main strength of this meta-analysis is that it included studies in healthcare and childcare workers from all countries, without an inferior cutoff for year of publication. This contrasts with the work of Stark et al. [ 34 ] who reported on childcare workers only and included articles published since the year 2000. As shown in Fig. 1 (also supplemental Table S 4 ), 70% of the studies (Fig. 1 & Additional file Table S 4 ) included in this study were published prior to that year, making the present study more complete [ 19 ]. To our knowledge, this is the first comprehensive study to provide pooled estimates of CMV prevalence and incidence of primary infection and to compare the risk of CMV seropositivity and primary infection in healthcare workers versus controls.
The results presented here should be considered in the context of certain limitations. First, most of the studies included did not stratify data according to age, gender, or age of children in daycare, and we could therefore not specifically study women of reproductive age working at childcare and exposed to children younger than 3 years old (when risk of transmission is highest), nor provide data on the incidence of congenital infection. Second, we included articles in French and English only, and studies were predominantly North American and European, thus imposing some limit on generalizability. Third, subgroup analyses of pooled studies stratified by continent, diagnostic method, study design, and quality did not eliminate heterogeneity in our study outcomes. Heterogeneity observed across continents could be explained by local daycare policies that may differ regarding the number of children assigned per daycare worker and the amount of time spent with the children. Different study designs with differing methods of data collection, sampling, statistical analyses, and parameters assessed to determine the quality of the studies could also contribute to heterogeneity across studies. Other factors included the inability to fully distinguish the level of exposure to young children from exposure to other people at risk of CMV infection and the fact that no clear distinctions could be made between large childcare centers and home-based childcare centers. A major objective of meta-analysis was to identify and compare trends among studies rather than to synthesize data from studies to obtain a single conclusive estimate [ 63 , 64 , 65 , 66 ], and this has been achieved in our analyses. Lastly, although the methods used to diagnose CMV were reliable, each has its own limitations [ 67 ] such as how active vs. latent CMV infection is interpreted [ 67 ].
This meta-analysis provides updated estimates of indicators of CMV infection in healthcare and childcare and workers. Prevalence and incidence of CMV infection was more common in childcare than in healthcare workers, which we believe is due to better adherence to infection prevention measures in the healthcare environment. Healthcare and childcare workers having one or more children living at home, being non-Caucasian, and being married or in a common-law relationship were positively associated CMV seropositivity. The relative contribution of these risk factors to the overall risk of CMV primary infection and congenital CMV infection among healthcare and childcare workers remains to be established. Our results suggest that attention to good hygienic measures can reduce the risk for CMV transmission in childcare.
Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20(4):202–13.
Article PubMed Google Scholar
Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17(4):253–76.
Ibrahim S, Siddiqui AA, Siddiqui AR, Ahmed W, Moss PA, Lalani E-NM. Sociodemographic factors associated with IgG and IgM seroprevalence for human cytomegalovirus infection in adult populations of Pakistan: a seroprevalence survey. BMC Public Health. 2016;16(1):1112.
Article PubMed PubMed Central CAS Google Scholar
Lanzieri TM, Dollard SC, Bialek SR, Grosse SD. Systematic review of the birth prevalence of congenital cytomegalovirus infection in developing countries. Int J Infect Dis. 2014;22:44–8.
Article PubMed PubMed Central Google Scholar
Zuhair M, Smit GSA, Wallis G, Jabbar F, Smith C, Devleesschauwer B, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Rev Med Virol. 2019;29(3):e2034.
Griffiths P, Reeves M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat Rev Microbiol. 2021;19(12):759–73.
Article CAS PubMed PubMed Central Google Scholar
Centers—Alabama D-CJP. Prevalence of cytomegalovirus excretion from children in five day-care centers—Alabama. MMWR Morb Mortal Wkly Rep. 1985;34(4):49–51.
Google Scholar
Ross SA, Boppana SB. Congenital cytomegalovirus infection: outcome and diagnosis. Seminars in pediatric infectious diseases. Elsevier; 2005. p. 44–9.
Fowler KB, Boppana SB. Congenital cytomegalovirus infection. Seminars in perinatology. Elsevier; 2018. p. 149–54.
Boppana SB, Pass RF, Britt WJ, Stagno S, Alford CA. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992;11(2):93–8.
Article CAS PubMed Google Scholar
Fowler KB, Stagno S, Pass RF, Britt WJ, Boll TJ, Alford CA. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326(10):663–7.
Murph JR, Bale JF. The natural history of acquired cytomegalovirus infection among children in group day care. Am J Dis Child. 1988;142(8):843–6.
CAS PubMed Google Scholar
Adler SP. Hospital transmission of cytomegalovirus. Infect Agents Dis. 1992;1(1):43–9.
Pass RF, Little EA, Stagno S, Britt WJ, Alford CA. Young children as a probable source of maternal and congenital cytomegalovirus infection. N Engl J Med. 1987;316(22):1366–70.
Bale J, Murph J. The risk of cytomegalovirus infection in hospital personnel and child care providers: a review. Pediatr Rev Commun. 1991;4:233–46.
Stranzinger J, Kindel J, Henning M, Wendeler D, Nienhaus A. Prevalence of CMV infection among staff in a metropolitan children’s hospital–occupational health screening findings. GMS Hyg Infect Control. 2016;11:Doc20.
PubMed PubMed Central Google Scholar
Stranzinger J, Kozak A, Schilgen B, Paris D, Niessen T, Schmidt L, et al. Are female daycare workers at greater risk of cytomegalovirus infection? A secondary data analysis of CMV seroprevalence between 2010 and 2013 in Hamburg, Germany. GMS Hyg Infect Control. 2016;11:Doc09.
Bouchard P, Turcotte G. La maternité en milieu de travail ou pourquoi les Québécoises sont-elles si nombreuses à demander un retrait préventif? Sociologie et Sociétés. 1986;18(2):113–28.
Article Google Scholar
Romero Starke K, Kofahl M, Freiberg A, Schubert M, Gross ML, Schmauder S, et al. The risk of cytomegalovirus infection in daycare workers: a systematic review and meta-analysis. Int Arch Occup Environ Health. 2019;93(1):11–28.
Article PubMed CAS Google Scholar
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Jama. 2000;283(15):2008–12.
Health NIo. Quality assessment tool for observational cohort and cross-sectional studies. 2014;https://wwwnhlbinihgov/health-topics/study-quality-assessment-tools . Accessed 31 Dec 2020.
Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7(3):40–5.
Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2(2):121–45.
Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974–8.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–60.
Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions: Wiley; 2019. p. 257–64
Baker DE. New drugs approved by the FDA; New dosage forms and indications approved by the FDA; agents pending FDA approval; new drug/biologics license applications filed by manufacturer; significant labeling changes or “dear health care professional” letters related to safety. Hosp Pharm. 2009;44(11):1010–8.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Lamarre V, Gilbert N, Rousseau C, Gyorkos TW, Fraser W. Seroconversion for cytomegalovirus infection in a cohort of pregnant women in Québec, 2010–2013. Epidemiol Infect. 2016;144(8):1701–9.
Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988–2004. Clin Infect Dis. 2010;50(11):1439–47.
CMAJ. Cytomegalovirus infection in day-care centres: risks to pregnant women. Infectious Diseases and Immunization Committee, Canadian Paediatric Society. 1990;142:547–9.
Starke KR, Kofahl M, Freiberg A, Schubert M, Groß ML, Schmauder S, et al. The risk of cytomegalovirus infection in daycare workers: a systematic review and meta-analysis. Int Arch Occup Environ Health. 2020;93(1):11–8.
Article CAS Google Scholar
Pittet D, Allegranzi B, Sax H, Dharan S, Pessoa-Silva CL, Donaldson L, et al. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis. 2006;6(10):641–52.
Yeager AS. Longitudinal, serological study of cytomegalovirus infections in nurses and in personnel without patient contact. J Clin Microbiol. 1975;2(5):448–52.
Tolkoff-Rubin NE, Rubin RH, Keller EE, Baker GP, Stewart JA, Hirsch MS. Cytomegalovirus infection in dialysis patients and personnel. Annals of internal medicine. 1978;89(5_Part_1):625–8.
Brady MT, Demmler GJ, Anderson DC. Brief report: cytomegalovirus infection in pediatric house officers: susceptibility to and rate of primary infection. Infect Control Hosp Epidemiol. 1987;8(8):329–32.
Hatherley LI. Prevalence of cytomegalovirus antibodies in obstetric nurses. Med J Aust. 1985;142(3):186–9.
Adler SP. Cytomegalovirus and child day care. N Engl J Med. 1989;321(19):1290–6.
Stagno S, Whitley RJ. Herpesvirus infections of pregnancy: cytomegalovirus and Epstein–Barr virus infections. N Engl J Med. 1985;313(20):1270–4.
Hyde TB, Schmid DS, Cannon MJ. Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMV. Rev Med Virol. 2010;20(5):311–26.
Flowers R, Torner JC, Farr BM. Primary cytomegalovirus infection in pediatric nurses: a meta-analysis. Infect Control Hosp Epidemiol. 1988;9(11):491–6.
Adler S. Cytomegalovirus transmission and child day care. Adv Pediatr Infect Dis. 1992;7:109.
Isaacs D. Cytomegalovirus infection and hospital staff. J Paediatr Child Health. 1991;27(6):317–8.
Adler SP. Molecular epidemiology of cytomegalovirus: viral transmission among children attending a day care center, their parents, and caretakers. J Pediatr. 1988;112(3):366–72.
Zając P, Czarkowska-Pączek B. Risk of CMV infection in nurses in Poland: is it worth performing screening tests to estimate the prevalence of cytomegalovirus antibodies in Polish nurses? Pielęgniarstwo i Zdrowie Publiczne Nurs Public Health. 2020;10(1):43–7.
Leruez-Ville M, Ville Y. Is it time for routine prenatal serological screening for congenital cytomegalovirus? Prenat Diagn. 2020;40(13):1671–80.
Boussemart T, Baudon J, Garbarg-Chenon A, Costil J. Cytomégalovirus, grossesse et risque professionnel. Archives de pédiatrie. 1998;5(1):24–6.
Revello MG, Tibaldi C, Masuelli G, Frisina V, Sacchi A, Furione M, et al. Prevention of primary cytomegalovirus infection in pregnancy. EBioMedicine. 2015;2(9):1205–10.
Boucoiran I, Mayer BT, Krantz EM, Marchant A, Pati S, Boppana S, et al. Non-primary maternal CMV infection following viral shedding in infants. Pediatr Infect Dis J. 2018;37(7):627.
Kumar ML, Nankervis GA, Cooper AR, Gold E. Postnatally acquired cytomegalovirus infections in infants of CMV-excreting mothers. J Pediatr. 1984;104(5):669–73.
Dworsky M, Yow M, Stagno S, Pass RF, Alford C. Cytomegalovirus infection of breast milk and transmission in infancy. Pediatrics. 1983;72(3):295–9.
Adler SP, Finney JW, Manganello AM, Best AM. Prevention of child-to-mother transmission of cytomegalovirus by changing behaviors: a randomized controlled trial. Pediatr Infect Dis J. 1996;15(3):240–6.
Adler SP, Finney JW, Manganello AM, Best AM. Prevention of child-to-mother transmission of cytomegalovirus among pregnant women. J Pediatr. 2004;145(4):485–91.
Law B. Risk of acquiring cytomegalovirus infection while working in out-of-home child care centres. Can J Infect Dis. 1992;3(4):159.
CAS PubMed PubMed Central Google Scholar
Harvey J, Dennis CL. Hygiene interventions for prevention of cytomegalovirus infection among childbearing women: systematic review. J Adv Nurs. 2008;63(5):440–50.
Vauloup-Fellous C, Picone O, Cordier A-G, Parent-du-Châtelet I, Senat M-V, Frydman R, et al. Does hygiene counseling have an impact on the rate of CMV primary infection during pregnancy?: Results of a 3-year prospective study in a French hospital. J Clin Virol. 2009;46:S49–53.
Finney JW, Miller KM, Adler SP. Changing protective and risky behaviors to prevent child-to-parent transmission of cytomegalovirus. J Appl Behav Anal. 1993;26(4):471–2.
Price SM, Bonilla E, Zador P, Levis DM, Kilgo CL, Cannon MJ. Educating women about congenital cytomegalovirus: assessment of health education materials through a web-based survey. BMC Womens Health. 2014;14(1):1–10.
Colugnati FAB, Staras SAS, Dollard SC, Cannon MJ. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infecti Dis. 2007;7:10.
Lachmann R, Loenenbach A, Waterboer T, Brenner N, Pawlita M, Michel A, et al. Cytomegalovirus (CMV) seroprevalence in the adult population of Germany. PloS one. 2018;13(7):e0200267.
Greenland S. Can meta-analysis be salvaged? Am J Epidemiol. 1994;140(9):783–7.
Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9(1):1–30.
Light RJ, Pillemer DB. Summing up; the science of reviewing research; 1984.
Book Google Scholar
Rubin DB. Meta-analysis: literature synthesis or effect-size surface estimation? J Educ Stat. 1992;17(4):363–74.
Drew WL. Diagnosis of cytomegalovirus infection. Rev Infect Dis. 1988;10(Supplement_3):S468–S76.
Download references
Acknowledgements
The authors thank Dr. Christian Band for his critical appraisal and editing of the manuscript.
This work is funded by the Canadian Institutes for Health Research. Dr. Boucoiran is a recipient of a salary award (chercheur-boursier) from FRQ-S (Quebec’s Health Research fund). Dr. Balegamire is a recipient of a scholarship from Altona and FRQ-S. Dr. McClymont is a recipient of the CANFAR/CTN Postdoctoral Fellowship Award and MSFHR Research Trainee Award.
Author information
Authors and affiliations.
Department of Social and Preventive Medicine, École de Santé Publique de Université de Montréal, Montreal, QC, Canada
Safari Joseph Balegamire, Benoît Mâsse & Isabelle Boucoiran
Women and Children’s Infectious Diseases Center, CHU Sainte-Justine Research Center, Montreal, QC, Canada
Safari Joseph Balegamire, Philippe Dodin, Soren Gantt, Amir Abbas Besharati, Christian Renaud & Isabelle Boucoiran
Department of Pediatrics, University of British Columbia, Vancouver, BC, Canada
Elisabeth McClymont
National Institute of Public Health of Québec, Québec City, QC, Canada
Agathe Croteau
Department of Microbiology, CHU Sainte-Justine, Université de Montréal, Montréal, QC, Canada
Soren Gantt & Christian Renaud
Applied Clinical Research Unit, CHU Sainte Justine Research Center, Montreal, QC, Canada
Benoît Mâsse
Division of Maternofetal Medicine, Department of Obstetrics and Gynecology, Université de Montréal, Montreal, QC, Canada
Isabelle Boucoiran
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: IB, AC, SJB, and BM. Data curation: SJB, EM, AAB, PD, and IB. Methodology and formal analysis: SJB and BM. Supervision: IB, BM, and SG. Writing — original draft preparation: SJB, EM, and IB. Writing — review and editing: SJB, EM, AC, PD, SG, CR, and BM. The authors read and approved the final manuscript.
Corresponding author
Correspondence to Safari Joseph Balegamire .
Ethics declarations
Competing interests.
Dr. Gantt reports receiving research funding and consulting fees from Merck, Moderna, VBI vaccines, and Meridian Bioscience related to CMV. The other authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: supplementary table 1..
PRISMA 2020 check lists. Supplementary Table 2. Search strategy. Supplementary Table 3. Results of quality assessment of the observational included studies. Supplementary Table 4. Characteristics of the selected articles. Supplementary Table 5. Funnel plot asymmetry test for publication bias. Supplementary Table 6. Meta-regression
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Balegamire, S.J., McClymont, E., Croteau, A. et al. Prevalence, incidence, and risk factors associated with cytomegalovirus infection in healthcare and childcare worker: a systematic review and meta-analysis. Syst Rev 11 , 131 (2022). https://doi.org/10.1186/s13643-022-02004-4
Download citation
Received : 25 June 2021
Accepted : 11 June 2022
Published : 27 June 2022
DOI : https://doi.org/10.1186/s13643-022-02004-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cytomegalovirus
- Healthcare workers
- Young children
- Primary infection
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
A systematic literature review on the humanistic burden of cytomegalovirus
1,351 citations
751 citations
497 citations
368 citations
333 citations
Related Papers (5)
Ask Copilot
Related papers
Related topics
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Int Med Res
- v.49(8); 2021 Aug
Cytomegalovirus seroprevalence among blood donors: a systematic review and meta-analysis
Screening for cytomegalovirus (CMV)-specific antibodies is not routine in some settings. Thus, transfusion of blood products poses risks for susceptible individuals.
To investigate the global pooled CMV seroprevalence among volunteer blood donors.
This systematic review and meta-analysis was performed according to PRISMA guidelines. The databases searched included Embase, Google Scholar, Medline, PubMed, Web of Science, and Cochrane Library. Data were extracted independently and analyzed using STATA version 11.
The global seroprevalence of CMV IgG, CMV IgM, and both CMV IgM and IgG was 83.16% (95% confidence interval [CI]: 78.55–87.77%, I 2 = 99.5%), 13.77% (95% CI: 11.59–15.95%, I 2 = 98.8%), and 23.78% (95% CI: 10.50–37.06%, I 2 = 98.7), respectively.
The global seroprevalence of CMV was high among blood donors. Therefore, regular CMV screening should be conducted to identify CMV-seronegative blood donors.
Blood transfusion is a lifesaving component of many therapeutic interventions. 1 However, transmission of infectious diseases is a major challenge in transfusion services worldwide. 2 Cytomegalovirus (CMV), also known as human herpesvirus 5, is a large virus that infects humans. 3 CMV is a highly cell-associated virus and normally causes asymptomatic infections in immunocompetent individuals. Transmission of the virus can occur vertically or horizontally through contact with virus-containing body fluids including blood. 4 An important route of infection for high-risk groups is transfusion of blood products from latently infected donors (transfusion-transmitted [TT]-CMV). 5 Transfusion of contaminated blood products can result in primary infection in CMV-seronegative recipients or reinfection by a new CMV strain in CMV-seropositive recipients. 6 TT-CMV was first described by Kääriäinen and co-workers in 1966. 7 TT-CMV infections have traditionally been explained by transfer of latently infected white blood cells (WBCs). 8 The incidence of TT-CMV infection was reported to be as high as 13% to 37% in immunocompromised patients. Thus, the prevention of TT-CMV has become an important priority, especially in high-risk groups. 9
CMV is a complex pathogen with distinct pathobiology. 3 CMV is one of the most common opportunistic pathogens in immunocompromised patients. These patients have high risks of complications following primary CMV infection, reinfection, and reactivation of latent virus. The presence of anti-CMV immunoglobulin G (IgG) indicates a previous infection by CMV, while presence of anti-CMV IgM reflects new infection, acute infection, or re‐activation of CMV. 10 Donor IgM positivity is associated with higher risk of TT-CMV because of higher CMV DNA loads in both whole blood and plasma samples. 11
CMV infection causes significant morbidity and mortality in immunocompromised patients who receive contaminated blood products. 3 , 12 Because CMV can cause severe illness and death in these patients, spread of the virus through blood products should be actively prevented. 13 Studies have demonstrated a high prevalence of CMV infection among various groups, including blood donors. 14 The risk of CMV transmission through blood products can be limited by improved selection of donors. However, the high prevalence of CMV seropositivity in the donor populations of many countries represents a significant problem: increasing demand for CMV-free blood products may be difficult to meet if CMV-seropositive donors are excluded. 13 In addition, use of CMV-seronegative blood cannot completely eliminate the risk of TT-CMV because of the possibility of window period donations. 15
Leukoreduction (LR) of blood products is a common method used to decrease the risk of TT-CMV. Because latent CMV infection is restricted to small numbers of WBCs, removal of these cells significantly decreases the risk of TT-CMV. 16 , 17 Although LR is very effective in removing leukocyte-associated CMV, it cannot remove free CMV in plasma. As a result, newly infected blood donors could transmit CMV despite effective LR. 18 Persistence of CMV DNA following WBC removal explains rare TT-CMV in recipients of LR blood components. 19 In the era of universal LR of blood products, screening for CMV-negative blood products is thought to be unnecessary for hematopoietic stem cell transplantation because no cases of TT-CMV have been detected in some studies. 20 – 22 LR blood products from donors with active CMV infection have very low infectivity. 23
CMV-seronegative products can result in TT-CMV during the window period between infection and positive results of antibody screening tests 6 to 8 weeks later. LR blood products can result in TT-CMV because of incomplete removal of WBCs in a small proportion of units. Therefore, both LR and CMV-seronegative units have low residual risks of TT-CMV. Interestingly, the few centers without dual inventories have a relatively high prevalence of CMV seropositive blood donors within their regional populations. Some countries use both CMV-seronegative and LR products for neonatal, intrauterine, and pregnancy-associated transfusion. Other countries use CMV-seronegative and LR products for all high-risk groups, while others use LR products alone. 5 , 24 , 25
CMV seroprevalence varies significantly (from 40–100%) in different parts of the world. 26 The aim of this systematic review and meta-analysis was to estimate the pooled prevalence of CMV among blood donors worldwide.
Study setting and design
This systematic review and meta-analysis was conducted in a global setting. The study was designed according to the PRISMA-P 2015 Guidelines. 27
Search strategy
We searched Embase, PubMed, Google Scholar, Medline, Web of Science, and Cochrane Library for articles published before 18 January 2021. The search terms were “Prevalence” OR “seroprevalence” OR “frequency” AND “CMV” OR “cytomegalovirus” OR “anti-cytomegalovirus antibody” AND “blood donors” OR “volunteer blood donors”.
Study selection and eligibility criteria
Studies were eligible if they met the following criteria: (1) peer-reviewed original articles in English; (2) cross-sectional and cohort studies reporting prevalence of CMV among blood donors; (3) publication between 1 January 2000 and 18 January 2021. Case reports, case-control studies, and editorial articles were excluded. Published articles reporting CMV seroconversion and incidence rates among blood donors were also excluded.
Data extraction
Two authors (TA and SG) screened references and retrieved articles according to the eligibility criteria. The selected papers were scrutinized and discrepancies between reviewers were resolved by discussion and consensus. Additionally, the reference lists of original and review articles were checked in detail to identify additional relevant studies that were not obtained via database searching. For all included studies, the following information was extracted: name of the first author, year of publication, country, study year, sample size, diagnostic methods used, mean age, and type of blood donor.
Study quality assessment
The Newcastle–Ottawa Scale (modified for prevalence studies) was used for methodological quality assessment. 28
Meta-analysis
For every included study, point prevalence and 95% CI were calculated. A random-effects model was applied to assess the effects of heterogeneity among selected studies. I 2 values of 25%, 50%, and 75% were considered to reflect low, moderate, and high heterogeneity, respectively. 29 Forest plots were used to summarize the effect sizes and 95% CIs for all studies. A subgroup analysis was conducted to identify potential sources of heterogeneity among included studies. Funnel plots and Egger’s test were used to investigate potential publication bias. 30 , 31 All statistical analyses were performed using STATA version 11.0 (StataCorp, College Station, TX, USA).
A total of 1420 articles were retrieved by literature searching. Among these articles, 310 were excluded after duplicate removal, 1036 were irrelevant to the aim of this study, and 18 did not meet the eligibility criteria. Forty-three studies were included in the meta-analysis ( Figure 1 ).

Flow chart of study selection for the systematic review and meta-analysis of the prevalence of anti-CMV antibodies among blood donors.
Study characteristics
Twenty studies were conducted in Africa, 21 in Asia, and two in South America. The countries with the largest number of studies were Nigeria (10 studies) and Iraq (5 studies). Thirty-seven studies used enzyme linked immunosorbent assay (ELISA) to assess anti-CMV antibody titers (IgM and IgG), two studies used enzyme immunoassay, one study used a microparticle enzyme immunoassay, one study used latex particle agglutination, one study used chemiluminescence, and the one used a chromatographic immunoassay. The number of blood donors ranged from 75 in Sudan 32 to 2400 in Japan. 18 The mean age of donors ranged from 19 years to 45 years. Thirty-three studies examined volunteer blood donors, four studies examined healthy male donors, two studies examined blood bags, one study examined family replacement donors, one study examined volunteer blood donors and family replacement donors, one study examined medical staff and volunteer donors, and one study examined regular donors ( Table 1 ).
Characteristics of included studies.
NR, not reported; ELISA, enzyme-linked immunosorbent assay; EIA, enzyme immunoassay; MEIA, microparticle enzyme immunoassay; LPA, latex particle agglutination.
CMV seroprevalence among blood donors
Thirty-eight articles estimated the prevalence of anti-CMV IgG among blood donors. Among these studies, the highest prevalence of anti-CMV IgG antibodies was 99.2% among 1008 blood donors from Iran in 2009. 63 The lowest prevalence of anti-CMV IgG antibodies was 4.82% among 290 blood donors in Nigeria. 47 The estimated global pooled prevalence of anti-CMV IgG among blood donors was 83.16% (95% CI: 78.55%–87.77%, I 2 = 99.5%) ( Figure 2 ).

Forest plot of the prevalence of anti-CMV IgG among blood donors.
Twenty-eight articles estimated the prevalence of anti-CMV IgM among blood donors. The global pooled prevalence of anti-CMV IgM among blood donors using a random effects model was 13.77% (95% CI: 11.59%–15.95%, I 2 = 98.8%). The highest prevalence of anti-CMV IgM was 85% among healthy blood donors in Iran ( Figure 3 ) ( Figure 4 ). 66

Forest plot of the prevalence of anti-CMV IgM among blood donors.

Estimated global CMV seroprevalence among blood donors.
Four studies estimated the prevalence of both anti-CMV IgG and IgM among blood donors. The global pooled prevalence of both CMV IgM and IgG among blood donors using a random effects model was 23.78% (95% CI: 10.50%–37.06%, I 2 = 98.7%) ( Figure 5 ).

Forest plot of the prevalence of anti-CMV IgM and IgG among blood donors.
Subgroup analysis by region and method of detection
The pooled prevalence of anti-CMV IgG in Africa, Asia, and South America was 82.64% (95% CI 67.47%–97.81%), 82.75% (95% CI 78.20%–87.30%), and 99.23% (95% CI 83.90%–100.56%), respectively. The pooled prevalence of anti-CMV IgM in Africa, Asia, and South America was 22.52% (95% CI 15.89%–29.16%), 8.06% (95% CI 5.70%–10.43%), and 59.00% (95% CI 52.54%–65.48%), respectively. The pooled prevalence of anti-CMV IgG and IgM CMV measured by ELISA was higher compared with other methods of detection ( Table 2 ).
Prevalence of anti-CMV antibodies among blood donors.
CMV, cytomegalovirus; CI, confidence interval; ELISA, enzyme-linked immunosorbent assay.
Publication bias
Potential publication bias among the included studies were assessed statistically and graphically using Egger’s test and funnel plots, respectively. Funnel plots of the prevalence of both anti-CMV IgG ( Figure 6 ) and IgM ( Figure 7 ) were non-symmetrical, suggesting the presence of publication bias. Egger’s test also indicated publication bias in both anti-CMV IgG (P < 0.001) and IgM (P < 0.001).

Funnel plot of the prevalence of anti-CMV IgG among blood donors in the included studies.

Funnel plot of the prevalence of anti-CMV IgM among blood donors in the included studies.
The presence of anti-CMV antibodies (IgM and IgG) among blood donors is a sign of potentially infectious virus in transfused blood products. 49 According to this systematic review and meta-analysis, the global prevalence of anti-CMV IgG and IgM among blood donors was 83.16% (95% CI: 78.55%–87.77%, I 2 = 99.5%) and 13.77% (11.59%–15.95%, I 2 = 98.8%), respectively. The global prevalence of both anti-CMV IgM and IgG among blood donors was 23.78% (95% CI: 10.50%–37.06%, I 2 = 98.7%). The high prevalence of anti-CMV IgG identified in this meta-analysis reflects the fact that CMV infection is endemic in different parts of the world. 51 However, the pooled prevalence estimated in the current study was lower than another worldwide estimate of among blood and organ donors (86% seroprevalence). 69 The prevalence of anti-CMV IgG among blood donors varies according to local infection rates in the general population as well as the socioeconomic characteristics of the blood donors. 70 The high seroprevalence of IgG indicates frequent past exposure to CMV. Low socioeconomic status is associated with increased exposure to CMV because of factors such as large household size, crowding, child care practices, and sexual practices. 51 We found that 14.8% of blood donors were positive for anti-CMV IgM, indicating the presence of recent acute CMV infection. 71 This type of infection could be either primary or recurrent. 52 Because of the sensitivity of detection assays, IgM may be detectable both prior to the appearance of IgG and shortly after IgG seroconversion, and remains positive for several months. 72 , 73
In this study, the prevalence of anti-CMV IgG in Africa, Asia, and South America was 82.64% (95% CI: 67.47%–97.81%), 82.75% (95% CI: 78.20%–87.30%), and 99.23% (95% CI: 83.90%–100.56%), respectively. The prevalence of anti-CMV IgM was 22.52% (95% CI: 15.89%–29.16%), 8.06% (95% CI: 5.70%–10.43%), and 59.00% (95% CI: 52.54%–65.48%) in Africa, Asia, and South America, respectively. CMV seroprevalence varies geographically across the world. 49 A systematic review and meta-analysis conducted in Iran by Shaiegan et al. 10 showed that the prevalence of anti-CMV IgG and IgM was 92% (95% CI: 90%–94%) and 2.6% (95% CI: 1.7%–3.6%), respectively. Another single center study conducted in Nigeria by Gwarzo et al. 38 showed that the prevalence of anti-CMV IgG was 100% among blood donors.
The prevalence of anti-CMV IgG among blood donors observed using ELISA and rapid kits was 85.34% (95% CI: 82.44%–88.24%) and 75.51% (95% CI: 47.87%–103.15%), respectively. The prevalence of anti-CMV IgM among blood donors observed using ELISA and rapid kits was 13.41% (95% CI: 10.97%–15.85%) and 1.87% (95% CI: −1.55% to 5.30%), respectively. We found that the prevalence of anti-CMV IgG and IgM among blood donors was higher using ELISA compared with rapid kits. This might be because rapid screening kits are associated with more false negative results compared with ELISA. 74 Moreover, a study conducted by Chameera et al. 75 showed that rapid kits had lower sensitivity and negative predictive values compared with ELISA.
LR of cellular blood products and/or selection of CMV-seronegative donors are measures used to reduce the risk of TT-CMV. The risk of TT-CMV is closely associated with transfer of leukocytes from infected donors to the recipient. 76 However, because of the window period between CMV infection and seroconversion, apparently seronegative donors with transient viremia may be able to transfer CMV. 77 CMV-seropositive blood donors are CMV carriers and latently infected cells may be present in their blood that can be reactivated after transfusion and thus may be infectious. 76 Blood donations from newly CMV IgG-positive donors should have the highest risks of TT-CMV because they contain the highest levels of CMV DNA and early anti-CMV antibodies cannot neutralize the virus. 70 However, because of the high rate of CMV seropositivity in different parts of the world and the need for screening of large numbers of blood donations, use of exclusively CMV-seronegative blood is not practical. 78 Use of pathogen-inactivated blood products is another strategy to reduce the risk of TT-CMV and many other infections. 5
The findings of this systematic review and meta-analysis should be considered in the context of some important limitations. Heterogeneity was observed in all analyses, including subgroup analyses. High heterogeneity may have arisen from inclusion of studies only in the English language. We also did not explore potential risk factors associated with presence of anti-CMV IgG and IgM among blood donors because this information was not available in most of the included studies.
Conclusion and recommendations
This study revealed that CMV seroprevalence was high among blood donors globally. CMV seropositivity among blood donors is a challenge for safe blood transfusion and can lead to high mortality and morbidity in high-risk transfusion recipients. Therefore, routine CMV screening should be performed to identify CMV-seronegative blood donors.
Acknowledgement
We would like to acknowledge the authors of the studies included in this systematic review and meta-analysis.
Availability of data and materials: The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contributions: TA and SG were involved in the literature search, statistical analysis, study quality assessment, and manuscript drafting, review, and final approval. Both authors critically revised the paper and agree to be accountable for all aspects of the work.
A systematic literature review of the economic and healthcare resource burden of cytomegalovirus
Affiliations.
- 1 Moderna, Inc., Cambridge, MA, USA.
- 2 Department of Obstetrics and Gynecology, Duke University Medical Center, Durham, NC, USA.
- 3 Certara, Inc., Krakow, Poland.
- 4 Certara, Inc., Lörrach, Germany.
- PMID: 37395088
- DOI: 10.1080/03007995.2023.2222583
Objective: Cytomegalovirus (CMV) can infect individuals at any age, including infants, who may contract it from infected mothers (congenital CMV [cCMV]). Whereas CMV infection is typically asymptomatic or causes mild illness in healthy individuals, infection can result in severe outcomes in immunocompromised individuals and in infants with cCMV. This systematic review aims to characterize the economic impact of CMV and cCMV infections.
Methods: Medline, Embase, and LILACS databases were searched for publications reporting the economic impact of cCMV and CMV infections across all age groups. Manuscripts published between 2010 and 2020 from Australia, Latin America, Canada, Europe, Israel, Japan, the United States, and global (international, worldwide) studies were included; congress materials were excluded. Outcomes of interest included cCMV- and CMV-attributable direct costs/charges, resource utilization, and indirect/societal costs.
Results: Of 751 records identified, 518 were excluded based on duplication, population, outcome, study design, or country. Overall, 55 articles were eligible for full-text review; 25 were further excluded due to population, outcome, study design, or congress abstract. Two publications were additionally identified, resulting in economic impact data compiled from 32 publications. Of these, 24 publications reported cost studies of cCMV or CMV, including evaluation of direct costs/charges, healthcare resource utilization, and indirect/societal costs, and 7 publications reported economic evaluations of interventions. The populations, methods and outcomes used across these studies varied widely.
Conclusions: CMV and cCMV infections impose a considerable economic impact on different countries, populations, and outcomes. There are substantial evidence gaps where further research is warranted.
Keywords: CMV; Cytomegalovirus; cCMV; congenital cytomegalovirus; economic burden; healthcare resource utilization.
Publication types
- Systematic Review
- Research Support, Non-U.S. Gov't
- Costs and Cost Analysis
- Cytomegalovirus Infections* / epidemiology
- Cytomegalovirus Infections* / therapy
- Cytomegalovirus*
- Patient Acceptance of Health Care
- Open access
- Published: 29 February 2024
Hygiene-based measures for the prevention of cytomegalovirus infection in pregnant women: a systematic review
- María F. Rodríguez-Muñoz 1 ,
- Clara Martín-Martín 2 ,
- Katina Kovacheva 1 ,
- Maria Eugenia Olivares 3 ,
- Nuria Izquierdo 3 ,
- Pilar Pérez-Romero 4 &
- Estéfani García-Ríos 2 , 5
BMC Pregnancy and Childbirth volume 24 , Article number: 172 ( 2024 ) Cite this article
616 Accesses
4 Altmetric
Metrics details
Human Cytomegalovirus (HCMV) is the most frequent congenital infection worldwide causing important sequelae. However, no vaccine or antiviral treatments are currently available, thus interventions are restricted to behavioral measures. The aim of this systematic review was to assess evidence from available intervention studies using hygiene-based measures to prevent HCMV infection during pregnancy.
Studies published from 1972 to 2023 were searched in Medline, PsycInfo, and Clinical Trials (PROSPERO, CRD42022344840) according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Methodological quality was assessed by two authors, using ROBE-2 and MINORS.
After reviewing 6 selected articles, the outcome analysis suggested that implementation of hygiene-based interventions during pregnancy prevent, to some extent, the acquisition of congenital HCMV.
Conclusions
However, these conclusions are based on limited and low-quality evidence available from few studies using this type of intervention in clinical practice. Thus, it would be necessary to perform effective and homogeneous intervention studies using hygiene-based measures, evaluated in high-quality randomized controlled trials (RCTs).
Peer Review reports
Human Cytomegalovirus (HCMV) belongs to the Herpesviridae family (HHV-5), and it is the most frequent congenital infection worldwide (0.5 to 5%), causing important sequelae such as sensorineural hearing loss (SNHL) and neurodevelopmental disabilities in newborns [ 1 , 2 , 3 ]. In reproductive age women, HCMV seroprevalence varies based on socioeconomic factors [ 4 ]. While in developed countries in Europe and North America, seroprevalence ranges from 40 to 83% [ 5 , 6 ], in developing countries from Asia, Africa, and Latin America, seroprevalence can reach 100% [ 7 ]. The prevalence of congenital infection (cHCMV) is therefore associated with seroprevalence among pregnant women, with a higher seroprevalence correlating with ahigher prevalence of cHCMV infection [ 8 ]. In developed countries where up to 50% of childbearing age women are seronegative, cHCMV infection usually occurs due to frequent contact with small children (< 3 years of age) [ 9 , 10 ].
During primary infection, viral shedding can occur through saliva, urine, breast milk, semen, and blood [ 11 ]. A primary infection takes place when an individual with no previous HCMV infection (HCMV seronegative individuals) are infected through contact with a HCMV infected individual, triggering a broad immune response and establishing lifelong latency [ 12 ]. Seropositive individuals can develop new HCMV infection episodes from viral reactivation of latent infection or through re-infection with different viral strains [ 12 , 13 , 14 ]. During pregnancy, HCMV transmission to the fetus can occur both from mothers with primary infection (seronegative women) and from mothers with reactivated latent infection where hormonal changes associated with pregnancy and lactation may stimulate the reactivation [ 15 , 16 ].
The greatest risk of vertical transmission is associated with primary infection ranging from 30 to 35%, compared to 1.1 to 1.7% for non-primary infections [ 17 ]. However, it is crucial to consider the likelihood of acquiring an infection. The risk appears to be relatively low for seronegative women and relatively high for seropositive women [ 18 ]. This observation is supported by earlier studies that modelled the force of infection, estimating a higher incidence in highly seropositive populations [ 19 , 20 ], likely due to environmental and behavioural differences. Some studies indicate that the risk of re-infection among seropositive women surpasses the combined risks of both acquisition and maternal-to-fetal transmission among seronegative women [ 21 ]. Recent serological studies examining strain-specific HCMV antibody responses have revealed that maternal re-infection with a new strain is a significant factor in congenital infection among seropositive women, with re-infections occurring in 8% of seroimmune pregnancies [ 22 ].
Other authors have reported a much greater proportion of symptomatic cHCMV linked to reinfection during pregnancy [ 23 , 24 ]. Up to 10% of neonates with cHCMV infection are symptomatic and develop different sequelae. cHCMV is the leading cause of sensorineural hearing loss (SNHL) and neurodevelopmental delay, with a large number of symptomatic children presenting some degree of psychomotor and cognitive disabilities, and with visual impairment in up to 50% of infants [ 25 , 26 , 27 , 28 ]. Likewise, several studies have demonstrated that the risk of long-term neurodevelopmental sequelae, specifically hearing loss, is comparable in infants born to women with primary HCMV and those with non-primary HCMV infections during pregnancy [ 29 , 30 , 31 ]. The burden of disease related to cHCMV infection is notable, and as a consequence, infants often require special care related to therapeutic and educational needs [ 3 , 32 ].
Currently, there is no licensed vaccine to prevent HCMV infection and no approved treatments to prevent viral transmission from mother to fetus [ 19 , 33 , 34 , 35 , 36 , 37 , 38 ]. Neonates with symptomatic infection can be treated with ganciclovir and/or valganciclovir for 6 months [ 28 ]. Although this therapy has been shown to modestly reduce the incidence of hearing loss [ 39 ], follow-up duration is limited [ 40 ] and further research may be needed. Furthermore, paediatric administration of ganciclovir or valganciclovir is associated with neutropenia, and monitorization of neutrophil counts is recommended in treated children [ 39 , 41 ]. Thus, to reduce transmission from the mother to the fetus and consequently reduce the global burden of HCMV disease in this population, current interventions are restricted to behavioural changes to promote prevention measures. The literature has highlighted three types of prevention measures: universal (targeting the general population, not directed to a specific risk group), selective (targeting individuals at higher-than-average risk for HCMV infection) and indicated (individuals who are identified as having an increased vulnerability for HCMV infection). In our case, the universal group would be pregnant women; the selective group would be seropositive pregnant women for HCMV infection, and the indicated group those pregnant seronegative to HCMV and, thus they are at higher risk of transmission. Selective and indicated prevention strategies might involve more intensive interventions. To identify effective intervention studies, the studies should have described which types of hygiene prevention measures are adequate, determined the preventive effect of the interventions to avoid infection, and generated high level evidence.
To the best of our knowledge, only two review manuscripts describing HCMV preventive interventions have been conducted to date in the general population [ 42 , 43 ]. However, neither of the systematic reviews established which type of intervention is most appropriate for preventing HCMV infection. The first review focused exclusively on trials published before 2004 [ 42 ] while the second focused on trials published before 2019 [ 43 ]. Although both studies included content on behavior modifications, none of them used the Psyinfo database specialized in this field.
Thus, the existing evidence on preventive interventions for HCMV infection in the perinatal period remain inconclusive. For this reason, the aim of this review is to collate evidence relating hygienic measurements acquisition and counselling during pregnancy in order to reduce cHCMV infection.
Search procedures and eligibly criteria
This systematic review of published studies was conducted following the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) statement [ 20 , 44 ]. A systematic review between 1972 and 2023, written in English (due to resource limits), assessing preventive intervention for HCMV infection was carried out. Database search was conducted in September 2023 by two authors (MFR and EGR) independently.
A protocol was elaborated to implement different steps underlying this systematic review and was registered on PROSPERO, the International Prospective Register of Systematic Reviews (ID CRD42022344840). No deviations from the protocol have occurred.
A total of three electronic databases were searched: MEDLINE, PsycINFO. In addition, CLINICAL TRIALS.gov was used to identify unpublished studies or studies still ongoing. The following search terms were combined: (“cytomegalovirus” OR “CMV” OR “CCMV” OR “HCMV” OR “Human betaherpesvirus 5” OR “cytomegalovirus infection*” OR “CMV infection*” OR “cCMV infection*” OR “HCMV infection*” OR “Human betaherpesvirus 5” infection*” OR “congenital cytomegalovirus infection*” OR “congenital infection*” OR “congenital CMV” OR “congenital HCMV” ) AND (“prenatal*” OR “pre-natal*” OR “pre natal*” OR “antenatal* OR “ante-natal*” OR “antepartum” OR “ante-partum” OR “pregnancy” OR “pregnant*” OR “mother*” OR “childbearing” OR “woman” OR “women”) AND (“prenatal education*” OR “antenatal education*” OR “birth preparation*” OR “prenatal class*” OR “antenatal class*” OR “health education*” OR “health promotion*” OR “counselling*” OR “hygiene*” OR “hand wash*” OR “wash hand*” OR “program” OR prevention” OR “control” OR “hygiene-based” OR “control” OR “hygiene education” OR “behavioral intervention” OR “Vertical prevention” ).
Inclusion and exclusion criteria
Analysis of the articles was performed based on previously established inclusion and exclusion criteria and the availability of the full text in English. Randomized controlled trial (RCT) and non-RCT about the effectiveness of HCMV acquisition were eligible for inclusion in the systematic review. The review included articles studying adult pregnant women or attempting pregnancy to whom preventive intervention based on hygiene education and control groups receiving standard treatment or information. The primary outcome of the review was the measurement of seroconversion rates and the secondary was the adherence of pregnant women to the intervention.
Search results were exported to an Excel file and duplicate manuscripts were removed. Two authors (MFR and EGR) independently screened titles and abstracts for eligibility and the full text of the potentially eligible articles were screened. Studies were excluded if they did not evaluate the effectiveness of preventive intervention for HCMV, or did not include psychological or biological outcomes. Any disagreement was resolved by discussion.
Data extraction
Two reviewers (MFR and EGR) independently extracted data from each included study and checked for accuracy using a data extraction excel spreadsheet. The following data were extracted: aim of study, author, year of publication, country of study, time of study, study design, inclusion/exclusion criteria, characteristics of cohort, description of interventions’ characteristics (data were collected as narrative results) – type (information or counselling); delivery format (face-to-face, written, video, individual, group, online); time of intervention (pregnancy or attempting pregnancy); duration of intervention (range); number of sessions (range); follow-up duration; providers; outcome measures and main findings. Summary tables were made to create the extracted information in an organized presentation. Excluded studies and reason for the exclusion has been included in Supplementary material (Table S 1 ).
Risk of bias assessment
Methodological quality of the included studies was independently assessed by two reviewers (MFR and EGR) using ROB-2 [ 45 ], a tool developed for assessing the quality of randomized health care interventions and MINOR [ 46 ] for non/randomized intervention [ 47 ]. ROB-2 evaluates five domains of research validity and bias: randomization process, deviations from intended interventions, missing outcome data, outcome measurement and selection of the reported results. Studies were evaluated as either low, some concerns or high risk of bias for each domain. MINORS contained 12 items, the first eight being specifically for non-comparative studies. The items are scored 0 (not reported), 1 (reported but inadequate) or 2 (reported and adequate). The global ideal score being 16 for non-comparative studies and 24 for comparative studies.
Risk of bias was categorized as low or high. Disagreements on quality rating were discussed and a consensus was reached.
Identification of studies
Search results are summarized in the PRISMA flowchart (Fig. 1 ). The initial search identified a total of 150 references and 3 additional records were collected based on experts in the field. After removing duplicate references, the title and abstract, a total of 145 references were screened (first screening) and a total 135 studies were excluded. A full-text review was performed for the remaining 16 references (second screening) and based on eligibility assessment 10 records were excluded (exclusion reasons are presented in Table S 1 ) and six articles were included in the systematic review. The methodological quality and bias risk of the included studies are shown in Table 1 . All four non-RCT papers [ 48 , 49 , 50 , 51 ] were classified as critically low-quality using MINORS, mainly due to unbiased assessment of the study, for lacking a follow-up period or a prospective calculation of the sample size. In contrast, the two RCT papers [ 52 , 53 ] were evaluated with a high quality based on ROBE-2 criteria.
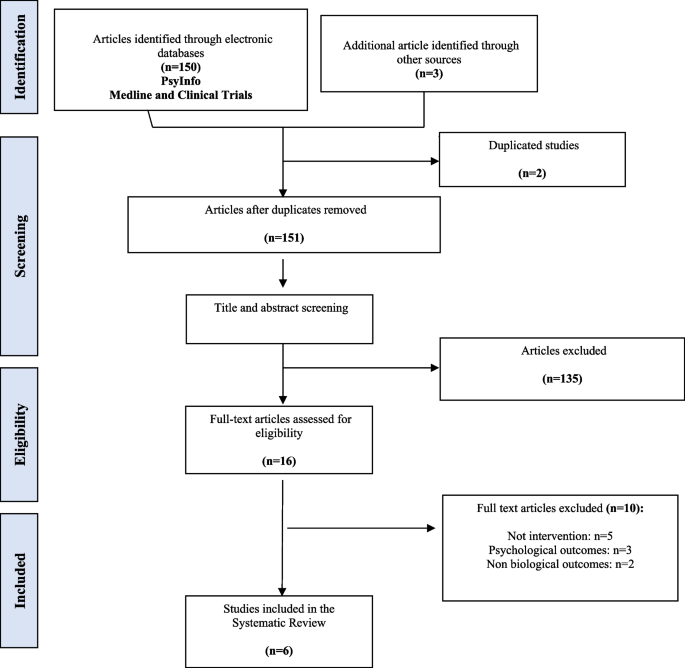
PRISMA flow-chart
Study characteristics
Characteristics of the six included papers are shown in Table 2 . All the studies were published between 2004 and 2021 reporting the findings from a total number of 10,197 participants (i.e., pregnant women or women who attempt to be pregnant). The studies were carried out in United States [ 52 ]; Italy [ 49 ]; Israel [ 48 ]; United Kingdom [ 53 ] and France [ 50 , 51 ]. Sample size ranged from 103 to 5173 women with a median of 545 and a mean of 1699.5.
As previously stated, two of the studies were RCTs [ 52 , 53 ] while the remaining four paper were a retrospective cohort study [ 48 ]; an observational controlled study [ 49 ] and two prospective cohort studies [ 50 , 51 ]. Four studies focused exclusively on pregnant women [ 49 , 50 , 51 , 53 ]. One study on women who were planning to be pregnant [ 48 ] and the last one included both, pregnant women and women who were planning to be pregnant [ 52 ].
Regarding the type of prevention approach used, five studies included selective prevention in seronegative women [ 49 , 50 , 51 , 52 , 53 ] and finally one paper focused on universal prevention [ 48 ].
Regarding the type of personnel providing the interventions, in four of the studies the interventions were mainly provided by health professionals (nurses, midwives, gynecologists) while in two of the studies this information was not reported [ 49 , 53 ].
Characteristics of the interventions
All six studies evaluated the effectiveness of the preventive intervention on the reduction of HCMV acquisition (seroconversion). Most of the papers focused on informing patients of preventive measures [ 49 , 50 , 51 , 52 , 53 ]. However, some of the studies in addition to informing patients of preventive measures, included other parameters such as adherence to follow up visits [ 52 ], patient follow up by telephone calls [ 51 ] and patient reinforcement [ 49 ]. Only one of the selected studies included psychological support through counseling [ 48 ].
Table 3 shows a summary of the main results. In more detail, Adler et al., (2004) analyzed 166 seronegative women with a child below 36 months of age. In this work, participants were randomly assigned to either the control group (intervention) or the intervention group (full intervention). In the control group, women received basic information about HCMV infection but they were not aware of their serological status or whether the child was shedding HCMV or not. On the contrary, women assigned to the intervention group received the same information and indications as in the control group but additionally they were aware of their serological status and whether their child was shedding HCMV or not, and their implications. In addition, home visits were carried out every 3 months in order to assess adherence to the measures of both groups.
Calvert et al., 2021 enrolled 103 pregnant women living with children less than four years old that were randomly divided in the control and the intervention groups. The control group received information through a series of slides about influenza vaccination during pregnancy while intervention group watched a digital educational film with detailed information about HCMV infection and its prevention.
Picone et al., (2009) recruited 3665 seronegative pregnant women during the first trimester visit to the obstetrician. Detailed oral and written information about HCMV infection and its prevention were given to both parents and at around 36 weeks of gestation, a second HCMV serologic test was performed. Following the same procedure for the intervention, Vauloup-Fellous et al., (2009) enrolled 5173 seronegative pregnant women during their first trimester visit to the obstetrician.
Reichman et al., (2014) carried out a retrospective cohort study of 444 women who were attempting pregnancy and were referred to a fertility clinic. Seventy-two seronegative women received detailed preconception counselling about HCMV infection and its preventive measures and every 3–4 months they had a follow-up HCMV serology test.
Finally, Revello et al., (2015) included 646 pregnant women with a control group integrated by women enrolled at delivery who were not informed about HCMV infection, while the intervention group received information about hygiene measures and were prospectively tested for HCMV infection until delivery. Furthermore, in this study authors carried out a reinforcement strategy through sessions during follow-up visits at 18 weeks of gestation and questionnaires every 6 weeks.
Effectiveness of the interventions on HCMV acquisition
The six selected studies reported the HCMV-specific seroconversion rates as a function of the intervention. Of the three studies with a reported control group [ 49 , 52 , 53 ], only one indicated significantly lower HCMV infection after the intervention (4/331, 1.2%) compared with the control group (24/315, 7.6%) [ 49 ]. While no significant reduction of seroconversion was found in Adler et al., (2004) and Calvert et al., (2021) when comparing the control and intervention groups. Regarding the study performed by Adler et al., (2004), a significant reduction in HCMV infection was reported in pregnant women with children younger than 36 months of age who were shedding HCMV (1/17, 5.9%) compared to women with children younger than 36 months of age shedding HCMV attempting pregnancy (10/24, 41.6%). On the other hand, Calvert et al., (2021) reported no significant differences in the seroconversion rate between the end of the first trimester and 34 gestational weeks was 4.55% (2/44) in the intervention group and 4.65% (2/43) in the control group.
The study performed by Picone et al., (2009) reported a reduction in seroconversion after the intervention (5/1951, 0.26%) between 12 and 36 weeks of gestation compared with the first trimester of pregnancy (9/1960, 0.46%). Assuming the nine patients with primary infections had negative serology at 0 weeks of gestation (WG), the count of women without prior HCMV exposure at the start of pregnancy would be the sum of seronegative women at 12 WG (1951) plus nine, totaling 1960.
Similar results were obtained in the study reported by Vauloup-Fellous et al., (2009) in which a significant reduction was also observed after intervention at 12 weeks of gestation (5/2583, 0.19%) when compared with the period before the intervention (11/2594, 0.42%). If we consider that the 11 patients with primary infection (indicated by a low HCMV-G avidity index) had negative serology at 0 weeks of gestation, the number of women without prior HCMV exposure at the beginning of pregnancy would be the total of seronegative women at 12 weeks of gestation (2583) plus 11, amounting to 2594.
Finally, although no comparison was made in the study performed by Reichman et al., (2014), none of the 69 seronegative women who were followed-up until the end of the study seroconverted after receiving counselling at the preconception visits.
Adherence, changes in behavior and HCMV perception
Most of the studies did not report information related to behavioral changes of perception of HCMV [ 48 , 50 , 51 , 53 ] as secondary outcomes. Regarding adherence, it is important to evaluate how well pregnant women follow recommended preventive measures as advised by healthcare providers. Two studies provided information regarding adherence to treatment [ 49 , 52 ]. In Adler et al., (2004), authors reported no significant differences in adherence to the intervention between the groups of participants (infected and uninfected women and pregnant and attempting pregnancy women). On the other study, 745/932 (80%) of respondents women described following the recommendations often 492/745 (66%) or always 253/745 (14%) during pregnancy being the lack of time the major cause to reduce adherence to the prevention measures [ 49 ].
Despite the great Public Health impact caused by HCMV congenital infections as a leading cause of stillbirth, neurodevelopmental problems and hearing loss worldwide, there are no vaccines or therapies commercially available to prevent the infection [ 19 , 33 , 34 , 35 ]. With this regard, the implementation of hygienic measures in the population at risk stands as the cornerstone to prevent HCMV transmission from the mother to the fetus during pregnancy.
In summary, the findings from this systematic review indicate that incorporating hygiene-focused interventions during pregnancy can to some degree reduce the likelihood of acquiring congenital HCMV infection. Nevertheless, the review highlights a scarcity of studies on preventive measures, and the existing ones vary significantly in terms of target populations, assessed outcomes, and the nature and conditions of implemented interventions. This heterogeneity poses challenges in drawing conclusive insights from the available evidence.
The prevalence of cHCMV infection varies from 0.2 to 2% (average 0.65%) depending on maternal seroprevalence [ 54 ]. However, this data come mainly from studies performed in developed regions such as Europe, the USA, and Japan. In low income countries the cHCMV prevalence is higher varying from 6 to 14% [ 55 , 56 , 57 , 58 , 59 ]. The 6 studies included in this systematic review were carried out in five developed countries, and results may therefore not be applicable to other countries with higher prevalence rates. Our results highlight the urgent need to conduct new studies implementing preventive measures in the population at higher risk of infection and transmission. Furthermore, in developing countries in addition to the higher prevalence rates, promoting and implementing hygiene-based measures may be more difficult based on lower socio-economic conditions. In fact, it has been reported that higher educational and social levels are associated with improved patient possibilities to change health behaviours [ 60 , 61 ].
Regarding the HCMV transmission, in three out of the six studies, seroconversion rates were significantly lower either in the intervention group [ 49 ] or after the implementation of hygiene-based measures [ 50 , 51 ]. It is important to mention that, similarly to previously reported results, the three studies with a significant reduction in HCMV transmission rate were conducted exclusively in pregnant women, [ 43 ]. Pregnancy has been commonly defined as a ‘teachable moment” since women are more motivated to improve both the lifestyle and healthy habits compared with non-pregnant women [ 60 , 62 , 63 ]. The response based on emotions during early pregnancy leading to concerns about the fetus health together with the new social role of becoming a mother can motivate pregnant women to modify their lifestyle habits [ 64 ]. Based on pregnant women’s interest in maintaining healthy behaviours in this period, implementing protocols to improve the knowledge regarding the HCMV transmission will be ideal and will also increase available evidence of the effectiveness of the preventive intervention. More information is required regarding the following aspects: moderators and mediators of the prevention treatment response, contents, format and adherence to the preventive measures.
In addition to the significant reduction in the seroconversion rate observed after our analysis, the extrapolation of results may not be possible due to the limited number of RCTs, the small sample size and the heterogeneity of the sample. Thus, as previously stated, our results highlight the urgent need to carry out new RCTs involving pregnant women from different socio-economic backgrounds. In addition, except for one study [ 48 ], the interventions were based on informing patients of preventive measures instead of counselling and active behavior-changing interventions which have already proven to be effective promoting heathy habits during pregnancy for other pathologies [ 65 , 66 , 67 ]. Additionally, counselling is a complex process and the effectiveness of the intervention may be dependent on the specific training and experience of the provider. In the six selected studies, interventions were primarily administered by healthcare professionals such as nurses, midwives, and gynaecologists, potentially lacking specialized training for HCMV infection. Furthermore, these studies lacked clear delineation of the specific interventions, often providing imprecise descriptions, thereby complicating the drawing of definitive conclusions. Consequently, the involvement of professionals specialized in behavior modification could prove instrumental in crafting effective health prevention strategies, proposing and implementing tailored counselling plans during pregnancy.
Limitations
Some limitations must be considered to interpret our results correctly. (i) Our results may be biased since studies were carried out in high-income regions which make difficult the extrapolation of the results to developing countries. (ii) The number of available studies was small and in some studies the sample size was also reduced, leading to limited representativeness [ 52 , 53 ]. (iii) It was not possible to conduct a meta-analysis due to the clinical and methodological heterogeneity of the included studies.
The findings presented in this review highlight the limited and low-quality published evidence currently available, limiting the possibility to make recommendations for clinical intervention. There are only six studies that met the criteria, mainly non-RCT, to study interventions aiming to prevent HCMV infection during pregnancy. It is urgent to develop effective and homogeneous interventions, evaluated in high-quality RCTs. The high number of pregnant women developing complications associated with cHCMV infection worldwide and their clinical burden highlights the need that policymakers should seek to promote research efforts in this area, for example, supporting specialized funding calls. This is particularly important due to the healthcare-related costs associated to this infection. In this sense, further efforts should be done to inform and raise awareness in society about HCMV infection during pregnancy, regardless the serostatus of women, because the associated risk cannot be minimized if they are unknown for the population at risk. Furthermore, interventions need to be replicable, based on theory and evidence, and the study of their effectiveness should be assessed in terms of time, contents and format of the intervention.
Implementation of hygienic measures in pregnant women has potential as the cornerstone to prevent HCMV transmission from the mother to the fetus during pregnancy. Nonetheless, due to the lack of evidence related to the small number and low-quality studies carried out to date, it is not possible to indicate its clinical use, and further studies are proposed with the purpose of clarifying the possible benefits.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
Human cytomegalovirus
Randomized controlled trials
Sensorineural hearing loss
Congenital infection
Preferred Reporting Items for Systematic reviews and Meta-Analysis
Dreher AM, Arora N, Fowler KB, Novak Z, Britt WJ, Boppana SB, et al. Spectrum of disease and outcome in children with symptomatic congenital cytomegalovirus infection. J Pediatr. 2014;164:855–9.
Article PubMed PubMed Central Google Scholar
Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The Silent global burden of congenital cytomegalovirus. Clin Microbiol Rev. 2013;26:86–102.
Article CAS PubMed PubMed Central Google Scholar
Britt WJ. Congenital human cytomegalovirus infection and the enigma of maternal immunity. J Virol. 2017;91:e02392-02316.
Zuhair M, Smit GSA, Wallis G, Jabbar F, Smith C, Devleesschauwer B, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Rev Med Virol. 2019;29:e2034.
Article PubMed Google Scholar
Yinon Y, Farine D, Yudin MH. Screening, diagnosis, and management of cytomegalovirus infection in pregnancy. Obstet Gynecol Surv. 2010;65:736–43.
Puhakka L, Lappalainen M, Lönnqvist T, Niemensivu R, Lindahl P, Nieminen T, et al. The burden of congenital cytomegalovirus infection: a prospective cohort study of 20 000 infants in Finland. J Pediatr Infect Dis Soc. 2019;8:205–12.
Article Google Scholar
Marin LJ, Santos De Carvalho Cardoso E, Bispo Sousa SM, Debortoli De Carvalho L, Marques Filho MF, Raiol MR, et al. Prevalence and clinical aspects of CMV congenital infection in a low-income population. Virol J. 2016;13:1–5.
de Vries JJC, van Zwet EW, Dekker FW, Kroes ACM, Verkerk PH, Vossen ACTM. The apparent paradox of maternal seropositivity as a risk factor for congenital cytomegalovirus infection: a population-based prediction model. Rev Med Virol. 2013;23:241–9.
Pinninti SG, Ross SA, Shimamura M, Novak Z, Palmer AL, Ahmed A, et al. Comparison of saliva PCR assay versus rapid culture for detection of congenital cytomegalovirus infection for the national institute on deafness and other communication disorders CMV and hearing multicenter screening (CHIMES) Study. Pediatr Infect Dis J. 2015;34:536–7.
Boppana SB, Ross SA, Shimamura M, Palmer AL, Ahmed A, Michaels MG, et al. Saliva polymerase-chain-reaction assay for Cytomegalovirus Screening in newborns. N Engl J Med. 2011;364:2111–8.
Mocarski ES, Shenk TE, Pass RF. Cytomegaloviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. 5th ed. Philadelphia: Wilkins, Lippincott Williams; 2007. p. 2702–72.
Google Scholar
Forte E, Zhang Z, Thorp EB, Hummel M. Cytomegalovirus latency and reactivation: an intricate interplay with the host Immune Response. Front Cell Infect Microbiol. 2020;10: 130.
Arora N, Novak Z, Fowler KB, Boppana SB, Ross SA. Cytomegalovirus viruria and DNAemia in healthy seropositive women. J Infect Dis. 2010;202:1800–3.
Britt WJ. Maternal immunity and the natural history of congenital human cytomegalovirus infection. Viruses. 2018;10:405.
Marsico C, Kimberlin DW. Congenital cytomegalovirus infection: advances and challenges in diagnosis, prevention and treatment. Ital J Pediatr. 2017;43:1–8.
Dorfman JR, Balla SR, Pathirana J, Groome MJ, Madhi SA, Moore PL. In Utero Human Cytomegalovirus infection is Associated with increased levels of putatively protective maternal antibodies in Nonprimary infection: evidence for boosting but not Protection. Clin Infect Dis. 2021;73:E981-987.
Article CAS PubMed Google Scholar
Pereira L, Maidji E, McDonagh S, Tabata T. Insights into viral transmission at the uterine-placental interface. Trends Microbiol. 2005;13:164–74.
Britt W. Controversies in the natural history of congenital human cytomegalovirus infection: the paradox of infection and disease in offspring of women with immunity prior to pregnancy. Med Microbiol Immunol. 2015;204:263–71.
Nigro G, Adler SP, La Torre R. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350–62.
Cajal B, Jiménez R, Gervilla E, Montaño JJ. Doing a systematic review in health sciences. Clin Y Salud. 2020;31:77–83.
Shaamash AH, Mohamed IS, Hasan MA, Ibrahim MA. Preconceptional immunity to cytomegalovirus and the risk of symptomatic congenital infection. Int J Gynecol Obstet. 2003;83:199–201.
Article CAS Google Scholar
Yamamoto AY, Mussi-Pinhata MM, Boppana SB, Novak Z, Wagatsuma VM, de Oliveira P, et al. F, Human cytomegalovirus reinfection is associated with intrauterine transmission in a highly cytomegalovirus-immune maternal population. Am J Obstet Gynecol. 2010;202:297.e1-297.e8.
Mack I, Burckhardt M-A, Heininger U, Prüfer F, Schulzke S, Wellmann S. Symptomatic congenital cytomegalovirus infection in children of Seropositive Women. Front Pediatr. 2017;5: 134.
Puhakka L, Renko M, Helminen M, Peltola V, Heiskanen-Kosma T, Lappalainen M, et al. Primary versus non-primary maternal cytomegalovirus infection as a cause of symptomatic congenital infection–register-based study from Finland. Infect Dis (Auckl). 2017;49:445–53.
Foulon I, Naessens A, Foulon W, Casteels A, Gordts F. A 10-Year prospective study of Sensorineural hearing loss in children with congenital cytomegalovirus infection. J Pediatr. 2008;153:84–8.
Xia W, Yan H, Zhang Y, Wang C, Gao W, Lv C, et al. Congenital human cytomegalovirus infection inducing Sensorineural hearing loss. Front Microbiol. 2021;12: 649690.
Pesch MH, Saunders NA, Abdelnabi S. Cytomegalovirus infection in pregnancy: Prevention, Presentation, Management and neonatal outcomes. J Midwifery Women’s Heal. 2021;66:397–402.
Rawlinson WD, Boppana SB, Fowler KB, Kimberlin DW, Lazzarotto T, Alain S, et al. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis. 2017;17:e177-188.
Townsend CL, Forsgren M, Ahlfors K, Ivarsson SA, Tookey PA, Peckham CS. Long-term outcomes of congenital cytomegalovirus infection in Sweden and the United Kingdom. Clin Infect Dis. 2013;56:1232–9.
Ross SA, Fowler KB, Ashrith G, Stagno S, Britt WJ, Pass RF, et al. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J Pediatr. 2006;148:332–6.
Demmler-Harrison GJ, Miller JA. Maternal cytomegalovirus immune status and hearing loss outcomes in congenital cytomegalovirus-infected offspring. PLoS ONE. 2020;15: e0240172.
Chiopris G, Veronese P, Cusenza F, Procaccianti M, Perrone S, Daccò V, et al. Congenital cytomegalovirus infection: update on diagnosis and treatment. Microorganisms. 2020;8: 1516.
Griffiths P. New vaccines and antiviral drugs for cytomegalovirus. J Clin Virol. 2019;116:58–61.
Plotkin SA, Boppana SB. Vaccination against the human cytomegalovirus. Vaccine. 2018. https://doi.org/10.1016/j.vaccine.2018.02.089 .
Revello MG, Lazzarotto T, Guerra B, Spinillo A, Ferrazzi E, Kustermann A, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med. 2014;370:1316–26.
De Santis M, Apicella M, De Luca C, D’Oria L, Valentini P, Sanguinetti M, et al. Valacyclovir in primary maternal CMV infection for prevention of vertical transmission: a case-series. J Clin Virol. 2020;127(March):104351.
Shahar-Nissan K, Pardo J, Peled O, Krause I, Bilavsky E, Wiznitzer A, et al. Valaciclovir to prevent vertical transmission of cytomegalovirus after maternal primary infection during pregnancy: a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:779–85.
Egloff C, Sibiude J, Vauloup-Fellous C, Benachi A, Bouthry E, Biquard F, et al. New data on efficacy of valacyclovir in secondary prevention of maternal–fetal transmission of cytomegalovirus. Ultrasound Obstet Gynecol. 2023;61:59–66.
Kimberlin DW, Jester PM, Sánchez PJ, Ahmed A, Arav-Boger R, Michaels MG, et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med. 2015;372:933–43.
Leung J, Grosse SD, Yockey B, Lanzieri TM. Ganciclovir and Valganciclovir Use among infants with congenital cytomegalovirus: data from a Multicenter Electronic Health Record Dataset in the United States. J Pediatr Infect Dis Soc. 2022;11:379–82.
Nigro G, Scholz H, Bartmann U. Ganciclovir therapy for symptomatic congenital cytomegalovirus infection in infants: a two-regimen experience. J Pediatr. 1994;124:318–22.
Harvey J, Dennis CL. Hygiene interventions for prevention of cytomegalovirus infection among childbearing women: systematic review. J Adv Nurs. 2008;63:440–50.
Barber V, Calvert A, Vandrevala T, Star C, Khalil A, Griffiths P, et al. Prevention of Acquisition of Cytomegalovirus infection in pregnancy through Hygiene-based behavioral interventions: a systematic review and Gap Analysis. Pediatr Infect Dis J. 2020;39:949–54.
Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–6.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Reichman O, Miskin I, Sharoni L, Eldar-Geva T, Goldberg D, Tsafrir A, et al. Preconception screening for cytomegalovirus: an effective preventive approach. Biomed Res Int. 2014;2014:14–6.
Revello MG, Tibaldi C, Masuelli G, Frisina V, Sacchi A, Furione M, et al. Prevention of primary cytomegalovirus infection in pregnancy. EBioMedicine. 2015;2:1205–10.
Picone O, Vauloup-Fellous C, Cordier A, Parent Du Châtelet I, Senat M, Frydman R, et al. A 2‐year study on cytomegalovirus infection during pregnancy in a French hospital. BJOG Int J Obstet Gynaecol. 2009;116:818–23.
Vauloup-Fellous C, Picone O, Cordier AG, Parent-du-Châtelet I, Senat MV, Frydman R, et al. Does hygiene counseling have an impact on the rate of CMV primary infection during pregnancy? J Clin Virol. 2009;46(SUPPL. 4):S49-53.
Adler SP, Finney JW, Manganello AM, Best AM. Prevention of child-to-mother transmission of cytomegalovirus among pregnant women. J Pediatr. 2004;145:485–91.
Calvert A, Vandrevala T, Parsons R, Barber V, Book A, Book G, et al. Changing knowledge, attitudes and behaviours towards cytomegalovirus in pregnancy through film-based antenatal education: a feasibility randomised controlled trial of a digital educational intervention. BMC Pregnancy Childbirth. 2021;21:565.
Lanzieri TM, Dollard SC, Bialek SR, Grosse SD. Systematic review of the birth prevalence of congenital cytomegalovirus infection in developing countries. Int J Infect Dis. 2014;22:44–8.
Zhang XW, Li F, Yu XW, Shi XW, Shi J, Zhang JP. Physical and intellectual development in children with asymptomatic congenital cytomegalovirus infection: a longitudinal cohort study in Qinba mountain area, China. J Clin Virol. 2007;40:180–5.
Yang TH, Huang HM, Hsu WC, Tsao PN, Liu TC, Hsu CJ, et al. The prevalence and demographic features of congenital cytomegalovirus infection in an urban area of East Asia: a population-based study. PLoS ONE. 2021;16: e0248801.
Rico A, Dollard SC, Valencia D, Corchuelo S, Tong VT, Laiton-Donato K, et al. Epidemiology of cytomegalovirus infection among mothers and infants in Colombia. J Med Virol. 2021;93:6393–7.
Pathirana J, Groome M, Dorfman J, Kwatra G, Boppana S, Cutland C, et al. Prevalence of congenital cytomegalovirus infection and Associated Risk of in Utero Human Immunodeficiency Virus (HIV) Acquisition in a High-HIV prevalence setting, South Africa. Clin Infect Dis. 2019;69:1789–96.
Mwaanza N, Chilukutu L, Tembo J, Kabwe M, Musonda K, Kapasa M, et al. High rates of congenital cytomegalovirus infection linked with maternal HIV infection among neonatal admissions at a large referral center in sub-saharan Africa. Clin Infect Dis. 2014;58:728–35.
Lindqvist M, Lindkvist M, Eurenius E, Persson M, Mogren I. Change of lifestyle habits – motivation and ability reported by pregnant women in northern Sweden. Sex Reprod Healthc. 2017;13:83–90.
Bookari K, Yeatman H, Williamson M. Falling short of dietary guidelines – what do Australian pregnant women really know? A cross sectional study. Women Birth. 2017;30:9–17.
Rockliffe L, Peters S, Heazell AEP, Smith DM. Understanding pregnancy as a teachable moment for behaviour change: a comparison of the COM-B and teachable moments models. Heal Psychol Behav Med. 2022;10:41–59.
O’Brien OA, Lindsay KL, McCarthy M, McGloin AF, Kennelly M, Scully HA, et al. Influences on the food choices and physical activity behaviours of overweight and obese pregnant women: a qualitative study. Midwifery. 2017;47:28–35.
Jackson RA, Stotland NE, Caughey AB, Gerbert B. Improving diet and exercise in pregnancy with Video Doctor counseling: a randomized trial. Patient Educ Couns. 2011;83:203–9.
Cantor AG, Jungbauer RM, McDonagh M, Blazina I, Marshall NE, Weeks C, et al. Counseling and behavioral interventions for healthy weight and weight gain in pregnancy: evidence report and systematic review for the US Preventive Services Task Force. JAMA - J Am Med Assoc. 2021;325:2094–109.
Murphy M, McHugh S, O’Keeffe LM, Greene RA, Corcoran P, Kearney PM. Preventive health counselling during antenatal care using the pregnancy risk assessment monitoring system (PRAMS) in Ireland. BMC Pregnancy Childbirth. 2020;20:98.
Esfandiari M, Faramarzi M, Nasiri-Amiri F, Parsian H, Chehrazi M, Pasha H, et al. Effect of supportive counseling on pregnancy-specific stress, general stress, and prenatal health behaviors: a multicenter randomized controlled trial. Patient Educ Couns. 2020;103:2297–304.
Download references
Acknowledgements
Authors thank to Michael J. McConnell for his valuable support.
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was supported by “Convocatoria IMIENS de ayudas para la realización de Proyectos de Iniciación a la Investigación 2022” awarded to E.G-R and M.R-M. E.G-R. is supported by the Sara Borrell Program (CD18CIII/00007), Instituto de Salud Carlos III, Ministerio de Ciencia, Innovación y Universidades and the Generalitat Valenciana plan GenT grant No. CIDEIG/2022/028. C.M-M. is supported by the PFIS Program (FI22CIII/0029), Instituto de Salud Carlos III, Ministerio de Ciencia, Innovación y Universidades. The accreditation to the IATA-CSIC as center of Excellence Severo Ochoa CEX2021-001189-S funded by MCIU/ https://doi.org/10.13039/501100011033 is also fully acknowledged.
Author information
Authors and affiliations.
Faculty of Psychology, Universidad Nacional de Educación a Distancia, (UNED), Madrid, Spain
María F. Rodríguez-Muñoz & Katina Kovacheva
National Centre for Microbiology, Instituto de Salud Carlos III (ISCIII), Carretera Majadahonda - Pozuelo km. 2, Majadahonda, Madrid, 28220, Spain
Clara Martín-Martín & Estéfani García-Ríos
Department of Gynecology and Obstetrics, Hospital Clínico San Carlos, Madrid, Spain
Maria Eugenia Olivares & Nuria Izquierdo
Department of Biological Sciences, University of Notre Dame, Notre Dame, IN, 46556, USA
Pilar Pérez-Romero
Department of Food Biotechnology, Instituto de Agroquimica y Tecnologia de los Alimentos (IATA), CSIC, Agustín Escardino 7, Paterna, Valencia, 46980, Spain
Estéfani García-Ríos
You can also search for this author in PubMed Google Scholar
Contributions
MRF: Conceptualization, Methodology, Formal analysis, Investigation, Writing - Original Draft, Writing - Review & Editing, Supervision, Funding acquisition. CMM: Investigation, Original Draft, Writing. KK: Investigation, Original Draft, Writing. MEO: Investigation, Original Draft, Writing. NI: Investigation, Original Draft, Writing. PPR: Investigation, Original Draft, Writing. EGR: Conceptualization, Methodology, Formal analysis, Investigation, Writing - Original Draft, Writing - Review & Editing, Supervision, Funding acquisition.
Corresponding author
Correspondence to Estéfani García-Ríos .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Rodríguez-Muñoz, M.F., Martín-Martín, C., Kovacheva, K. et al. Hygiene-based measures for the prevention of cytomegalovirus infection in pregnant women: a systematic review. BMC Pregnancy Childbirth 24 , 172 (2024). https://doi.org/10.1186/s12884-024-06367-5
Download citation
Received : 20 December 2023
Accepted : 23 February 2024
Published : 29 February 2024
DOI : https://doi.org/10.1186/s12884-024-06367-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Systematic review
- Cytomegalovirus
- Counselling
- Seroconversion
BMC Pregnancy and Childbirth
ISSN: 1471-2393
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
CMV infection may also have a broad impact on humanistic outcomes, including patient health status and quality of life (QoL). We conducted a systematic literature review (SLR) to describe the global humanistic burden of CMV and congenital CMV (cCMV) infections across all age groups. Methods: Medline, Embase, and LILACS were searched to identify ...
We conducted a systematic literature review (SLR) to describe the global humanistic burden of CMV and congenital CMV (cCMV) infections across all age groups. Methods: Medline, Embase, and LILACS ...
DOI: 10.1080/03007995.2023.2191477 Corpus ID: 257630702; A systematic literature review on the humanistic burden of cytomegalovirus @article{DiazDecaro2023ASL, title={A systematic literature review on the humanistic burden of cytomegalovirus}, author={John D Diaz-Decaro and Evan Myers and Jacek Mucha and Monika Neumann and Witold Lewandowski and Magdalena Kaczanowska and Elvira Schmidt and ...
Cytomegalovirus (CMV) infection is typically asymptomatic in healthy individuals; however, certain populations are vulnerable to infection and may develop serious sequelae. CMV infection may also have a broad impact on humanistic outcomes, including patient health status and quality of life (QoL). We conducted a systematic literature review (SLR) to describe the global humanistic burden of CMV ...
REVIEW ARTICLE A systematic literature review on the humanistic burden of cytomegalovirus John Diaz-Decaroa, Evan Myersb, Jacek Muchac, Monika Neumannd, Witold Lewandowskic, Magdalena Kaczanowskac, Elvira Schmidtd, Andrew Natenshona, Carla Talaricoa† and Philip O. Bucka aModerna, Inc., Cambridge, MA, USA; bDepartment of Obstetrics and Gynecology, Duke University Medical Center, Durham, NC, USA;
A systematic literature review on the humanistic burden of cytomegalovirus. Sign in | Create an account. https://orcid.org. Europe PMC. Menu ... Search life-sciences literature (42,504,035 articles, preprints and more) Search. Advanced search. Feedback Complete Survey Survey.
A systematic literature review on the humanistic burden of cytomegalovirus Introduction Human cytomegalovirus (CMV), a member of the Herpesviridae family of viruses, is a common viral pathogen that can infect individuals of all ages Citation 1 , Citation 2 .
Cytomegalovirus (CMV) infection is typically asymptomatic in healthy individuals; however, certain populations are vulnerable to infection and may develop serious sequelae. CMV infection may also have a broad impact on humanistic outcomes, including patient health status and quality of life (QoL). We conducted a systematic literature review (SLR) to describe the global humanistic burden of CMV ...
CMV infection may also have a broad impact on humanistic outcomes, including patient health status and quality of life (QoL). We conducted a systematic literature review (SLR) to describe the global humanistic burden of CMV and congenital CMV (cCMV) infections across all age groups.
Objective: This review assessed the patient-reported humanistic burden associated with moderate to very severe COPD, specifically the impact on health-related quality of life (HRQoL), symptoms, limitations in daily life, and emotional implications, through the use of HRQoL instruments. Methods: A systematic review was conducted to retrieve ...
A systematic review of the epidemiologic burden of CMV was conducted based on peer-reviewed articles published in the Medline, Embase, and Latin American and Caribbean Health Sciences Literature (LILACS) databases from the year 2000 through December 14, 2020 (an initial search was performed on October 27, 2020, and a widened search with supplemental search terms and additional outcomes of ...
Cytomegalovirus (CMV) is a common infection with a seroprevalence ranging from 45 to 100% [], depending on the population and existing risk factors [2,3,4].CMV is a member of the herpesvirus family that characteristically produce latent and persistent infections in human hosts [5, 6].CMV is transmitted horizontally through contact with biological fluids (saliva, urine, tears, semen, vaginal ...
In this systematic review and meta-analysis including 77 studies from 36 countries comprising 515 646 infants younger than 3 weeks, the pooled overall prevalence of congenital cytomegalovirus was 0.67%. The infection burden was 3-fold greater in low- and middle-income countries than in high-income countries.
A systematic literature review of the global seroprevalence of cytomegalovirus: possible implications for treatment, screening, and vaccine development ... Knowledge of the current epidemiologic burden of CMV is necessary to understand the need for treatment and prevention. A systematic literature review (SLR) was conducted to describe the most ...
Abstract Objective Cytomegalovirus (CMV) infection is typically asymptomatic in healthy individuals; however, certain populations are vulnerable to infection and may develop serious sequelae. CMV infection may also have a broad impact on humanistic outcomes, including patient health status and quality of life (QoL). We conducted a systematic literature review (SLR) to describe the global ...
Background. Blood transfusion is a lifesaving component of many therapeutic interventions. 1 However, transmission of infectious diseases is a major challenge in transfusion services worldwide. 2 Cytomegalovirus (CMV), also known as human herpesvirus 5, is a large virus that infects humans. 3 CMV is a highly cell-associated virus and normally causes asymptomatic infections in immunocompetent ...
Kidney transplant recipients (KTRs) have increased risk for cytomegalovirus (CMV) infection/disease given the necessity of drug-induced immunosuppression. A comprehensive review of published literature reporting real-world data on prevention strategies utilized and associated CMV burden outcomes is limited.
Diaz-Decaro J, Meyers E, Mucha J, et al. A Systematic Literature Review of the Economic and Healthcare Resource Burden of Cytomegalovirus. In preparation. Milewska-Bobula B, Med Wieku Rozwoj, № 14, с. 370 National CMV Foundation. Newborn Screening. 2022 [cited 2023 Jan 5].
This systematic review aims to characterize the economic impact of CMV and cCMV infections. Methods: Medline, Embase, and LILACS databases were searched for publications reporting the economic impact of cCMV and CMV infections across all age groups. Manuscripts published between 2010 and 2020 from Australia, Latin America, Canada, Europe ...
1 INTRODUCTION. Following primary infection, human cytomegalovirus (HCMV) can establish life-long latency in an infected individual and may reactivate, known as non-primary HCMV infection. 1 Non-primary HCMV infection can also occur from reinfection with a different HCMV strain. 1 The current standard for diagnosing primary HCMV infection is the detection of HCMV immunoglobulin G (IgG) with or ...
A systematic review was therefore conducted to provide a qualitative synthesis of existing knowledge on the epidemiology, management, and burden of CMV infection/disease in SOT recipients, accounting for differences in disease definitions. The review covered 15 countries across the regions of Asia, Pacific, and Latin America.
DOI: 10.1080/03007995.2023.2222583 Corpus ID: 259316051; A systematic literature review of the economic and healthcare resource burden of cytomegalovirus @article{DiazDecaro2023ASL, title={A systematic literature review of the economic and healthcare resource burden of cytomegalovirus}, author={John D Diaz-Decaro and Evan Myers and Jacek Mucha and Monika Neumann and Witold Lewandowski and ...
Background Human Cytomegalovirus (HCMV) is the most frequent congenital infection worldwide causing important sequelae. However, no vaccine or antiviral treatments are currently available, thus interventions are restricted to behavioral measures. The aim of this systematic review was to assess evidence from available intervention studies using hygiene-based measures to prevent HCMV infection ...