An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cancers (Basel)


Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review
Sergiusz Łukasiewicz.
1 Department of Surgical Oncology, Center of Oncology of the Lublin Region St. Jana z Dukli, 20-091 Lublin, Poland; lp.lzoc@zciweisakulS (S.Ł.); [email protected] (A.S.)
Marcin Czeczelewski
2 Department of Forensic Medicine, Medical University of Lublin, 20-090 Lublin, Poland; [email protected] (M.C.); lp.teno@amrofa (A.F.)
Alicja Forma
3 Department of Human Anatomy, Medical University of Lublin, 20-090 Lublin, Poland; [email protected]
Robert Sitarz
Andrzej stanisławek.
4 Department of Oncology, Chair of Oncology and Environmental Health, Medical University of Lublin, 20-081 Lublin, Poland
Simple Summary
Breast cancer is the most common cancer among women. It is estimated that 2.3 million new cases of BC are diagnosed globally each year. Based on mRNA gene expression levels, BC can be divided into molecular subtypes that provide insights into new treatment strategies and patient stratifications that impact the management of BC patients. This review addresses the overview on the BC epidemiology, risk factors, classification with an emphasis on molecular types, prognostic biomarkers, as well as possible treatment modalities.
Breast cancer (BC) is the most frequently diagnosed cancer in women worldwide with more than 2 million new cases in 2020. Its incidence and death rates have increased over the last three decades due to the change in risk factor profiles, better cancer registration, and cancer detection. The number of risk factors of BC is significant and includes both the modifiable factors and non-modifiable factors. Currently, about 80% of patients with BC are individuals aged >50. Survival depends on both stage and molecular subtype. Invasive BCs comprise wide spectrum tumors that show a variation concerning their clinical presentation, behavior, and morphology. Based on mRNA gene expression levels, BC can be divided into molecular subtypes (Luminal A, Luminal B, HER2-enriched, and basal-like). The molecular subtypes provide insights into new treatment strategies and patient stratifications that impact the management of BC patients. The eighth edition of TNM classification outlines a new staging system for BC that, in addition to anatomical features, acknowledges biological factors. Treatment of breast cancer is complex and involves a combination of different modalities including surgery, radiotherapy, chemotherapy, hormonal therapy, or biological therapies delivered in diverse sequences.
1. Introduction
Being characterized by six major hallmarks, carcinogenesis might occur in every cell, tissue, and organ, leading to the pathological alternations that result in a vast number of cancers. The major mechanisms that enable its progression include evasion of apoptosis, limitless capacity to divide, enhanced angiogenesis, resistance to anti-growth signals and induction of own growth signals, as well as the capacity to metastasize [ 1 ]. Carcinogenesis is a multifactorial process that is primarily stimulated by both—genetic predispositions and environmental causes. The number of cancer-related deaths is disturbingly increasing every year ranking them as one of the major causes of death worldwide. Even though a significant number of cancers do not always need to result in death, they significantly lower the quality of life and require larger costs in general.
Breast cancer is currently one of the most prevalently diagnosed cancers and the 5th cause of cancer-related deaths with an estimated number of 2.3 million new cases worldwide according to the GLOBOCAN 2020 data [ 2 ]. Deaths due to breast cancer are more prevalently reported (an incidence rate approximately 88% higher) in transitioning countries (Melanesia, Western Africa, Micronesia/Polynesia, and the Caribbean) compared to the transitioned ones (Australia/New Zealand, Western Europe, Northern America, and Northern Europe). Several procedures such as preventive behaviors in general as well as screening programs are crucial regarding a possible minimization of breast cancer incidence rate and the implementation of early treatment. Currently, it is the Breast Health Global Initiative (BHGI) that is responsible for the preparation of proper guidelines and the approaches to provide the most sufficient breast cancer control worldwide [ 3 ]. In this review article, we have focused on the female breast cancer specifically since as abovementioned, it currently constitutes the most prevalent cancer amongst females.
2. Breast Cancer Epidemiology
According to the WHO, malignant neoplasms are the greatest worldwide burden for women, estimated at 107.8 million Disability-Adjusted Life Years (DALYs), of which 19.6 million DALYs are due to breast cancer. [ 4 ]. Breast cancer is the most frequently diagnosed cancer in women worldwide with 2.26 million [95% UI, 2.24–2.79 million] new cases in 2020 [ 5 ]. In the United States, breast cancer alone is expected to account for 29% of all new cancers in women [ 6 ]. The 2018 GLOBOCAN data shows that age-standardized incidence rates (ASIR) of breast cancer are strongly and positively associated with the Human Development Index (HDI) [ 7 ]. According to 2020 data, the ASIR was the highest in very high HDI countries (75.6 per 100,000) while it was more than 200% lower in medium and low HDI countries (27.8 per 100,000 and 36.1 per 100,000 respectively) [ 5 ].
Besides being the most common, breast cancer is also the leading cause of cancer death in women worldwide. Globally, breast cancer was responsible for 684,996 deaths [95% UI, 675,493–694,633] at an age-adjusted rate of 13.6/100,000 [ 5 ]. Although incidence rates were the highest in developed regions, the countries in Asia and Africa shared 63% of total deaths in 2020 [ 5 ]. Most women who develop breast cancer in a high-income country will survive; the opposite is true for women in most low-income and many middle-income countries [ 8 ].
In 2020 breast cancer mortality-to-incidence ratio (MIR) as a representative indicator of 5-year survival rates [ 9 ] was 0.30 globally [ 5 ]. Taking into consideration the clinical extent of breast cancer, in locations with developed health care (Hong-Kong, Singapore, Turkey) the 5-year survival was 89.6% for localized and 75.4% for regional cancer. In less developed countries (Costa Rica, India, Philippines, Saudi Arabia, Thailand) the survival rates were 76.3% and 47.4% for localized and regional breast cancer respectively [ 10 ].
Breast cancer incidence and death rates have increased over the last three decades. Between 1990 and 2016 breast cancer incidence has more than doubled in 60/102 countries (e.g., Afghanistan, Philippines, Brazil, Argentina), whereas deaths have doubled in 43/102 countries (e.g., Yemen, Paraguay, Libya, Saudi Arabia) [ 11 ]. Current projections indicate that by 2030 the worldwide number of new cases diagnosed reach 2.7 million annually, while the number of deaths 0.87 million [ 12 ]. In low- and medium-income countries, the breast cancer incidence is expected to increase further due to the westernization of lifestyles (e.g., delayed pregnancies, reduced breastfeeding, low age at menarche, lack of physical activity, and poor diet), better cancer registration, and cancer detection [ 13 ].
3. Risk Factors of Breast Cancer
The number of risk factors of breast cancer is significant and includes both modifiable factors and non-modifiable factors ( Table 1 ).
Modifiable and non-modifiable risk factors of breast cancer.
| Non-Modifiable Factors | Modifiable Factors |
|---|---|
| Female sex | Hormonal replacement therapy |
| Older age | Diethylstilbestrol |
| Family history (of breast or ovarian cancer) | Physical activity |
| Genetic mutations | Overweight/obesity |
| Race/ethnicity | Alcohol intake |
| Pregnancy and breastfeeding | Smoking |
| Menstrual period and menopause | Insufficient vitamin supplementation |
| Density of breast tissue | Excessive exposure to artificial light |
| Previous history of breast cancer | Intake of processed food |
| Non-cancerous breast diseases | Exposure to chemicals |
| Previous radiation therapy | Other drugs |
3.1. Non-Modifiable Factors
3.1.1. female sex.
Female sex constitutes one of the major factors associated with an increased risk of breast cancer primarily because of the enhanced hormonal stimulation. Unlike men who present insignificant estrogen levels, women have breast cells which are very vulnerable to hormones (estrogen and progesterone in particular) as well as any disruptions in their balance. Circulating estrogens and androgens are positively associated with an increased risk of breast cancer [ 14 ]. The alternations within the physiological levels of the endogenous levels of sex hormones result in a higher risk of breast cancer in the case of premenopausal and postmenopausal women; these observations were also supported by the Endogenous Hormones and Breast Cancer Collaborative Group [ 15 , 16 , 17 ].
Less than 1% of all breast cancers occur in men. However, breast cancer in men is a rare disease that’s at the time of diagnosis tends to be more advanced than in women. The average age of men at the diagnosis is about 67. The important factors increase a man’s risk of breast cancer are: older age, BRCA2/BRCA1 mutations, increased estrogen levels, Klinefelter syndrome, family history of breast cancer, and radiation exposure [ 18 ].
3.1.2. Older Age
Currently, about 80% of patients with breast cancer are individuals aged >50 while at the same time more than 40% are those more than 65 years old [ 19 , 20 , 21 ]. The risk of developing breast cancer increases as follows—the 1.5% risk at age 40, 3% at age 50, and more than 4% at age 70 [ 22 ]. Interestingly, a relationship between a particular molecular subtype of cancer and a patient’s age was observed –aggressive resistant triple-negative breast cancer subtype is most commonly diagnosed in groups under 40 age, while in patients >70, it is luminal A subtype [ 21 ]. Generally, the occurrence of cancer in older age is not only limited to breast cancer; the accumulation of a vast number of cellular alternations and exposition to potential carcinogens results in an increase of carcinogenesis with time.
3.1.3. Family History
A family history of breast cancer constitutes a major factor significantly associated with an increased risk of breast cancer. Approximately 13–19% of patients diagnosed with breast cancer report a first-degree relative affected by the same condition [ 23 ]. Besides, the risk of breast cancer significantly increases with an increasing number of first-degree relatives affected; the risk might be even higher when the affected relatives are under 50 years old [ 24 , 25 , 26 ]. The incidence rate of breast cancer is significantly higher in all of the patients with a family history despite the age. This association is driven by epigenetic changes as well as environmental factors acting as potential triggers [ 27 ]. A family history of ovarian cancer—especially those characterized by BRCA1 and BRCA2 mutations—might also induce a greater risk of breast cancer [ 28 ].
3.1.4. Genetic Mutations
Several genetic mutations were reported to be highly associated with an increased risk of breast cancer. Two major genes characterized by a high penetrance are BRCA1 (located on chromosome 17) and BRCA2 (located on chromosome 13). They are primarily linked to the increased risk of breast carcinogenesis [ 29 ]. The mutations within the above-mentioned genes are mainly inherited in an autosomal dominant manner, however, sporadic mutations are also commonly reported. Other highly penetrant breast cancer genes include TP53 , CDH1 , PTEN , and STK11 [ 30 , 31 , 32 , 33 , 34 ]. Except for the increased risk of breast cancer, carriers of such mutations are more susceptible to ovarian cancer as well. A significant number of DNA repair genes that can interact with BRCA genes including ATM , PALB2 , BRIP1 , or CHEK2 , were reported to be involved in the induction of breast carcinogenesis; those are however characterized by a lower penetrance (moderate degree) compared to BRCA1 or BRCA2 ( Table 2 ) [ 29 , 35 , 36 , 37 , 38 ]. According to quite recent Polish research, mutations within the XRCC2 gene could also be potentially associated with an increased risk of breast cancer [ 39 ].
Major genes associated with an increased risk of breast cancer occurrence.
| Penetration | Gene | Chromosome Location | Associated Syndromes/Disorders | Major Functions | Breast Cancer Risk | Ref. |
|---|---|---|---|---|---|---|
| 17q21.31 | Breast cancer Ovarian cancer Pancreatic cancer Fanconi anemia | DNA repair Cell cycle control | 45–87% | [ ] | ||
| 13q13.1 | Breast cancer Ovarian cancer Pancreatic cancer Prostate cancer Fallopian tube cancer Biliary cancer Melanoma Fanconi anemia Glioblastoma Medulloblastoma Wilms tumor | DNA repair Cell cycle control | 50–85% | [ ] | ||
| 17p13.1 | Breast cancer Colorectal cancer Hepatocellular carcinoma Pancreatic cancer Nasopharyngeal carcinoma Li-Fraumeni syndrome Osteosarcoma Adrenocortical carcinoma | DNA repair Cell cycle control Induction of apoptosis Induction of senescence Maintenance of cellular metabolism | 20–40% (even up to 85%) | [ ] | ||
| 16q22.1 | Breast cancer Ovarian cancer Endometrial carcinoma Gastric cancer Prostate cancer | Regulation of cellular adhesions Control of the epithelial cells (proliferation and motility) | 63–83% | [ ] | ||
| 10q23.31 | Breast cancer Prostate cancer Autism syndrome Cowden syndrome 1 Lhermitte-Duclos syndrome | Cell cycle control | 50–85% | [ ] | ||
| 19p13.3 | Breast cancer Pancreatic cancer Testicular tumor Melanoma Peutz-Jeghers syndrome | Cell cycle control Maintenance of energy homeostasis | 32–54% | [ ] | ||
| 11q22.3 | Breast cancer Lymphoma T-cell prolymphocytic leukemia Ataxia-teleangiectasia | DNA repair Cell cycle control | 20–60% | [ ] | ||
| 16p12.2 | Breast cancer Pancreatic cancer Fanconi anemia | DNA repair | 33–58% | [ ] | ||
| 17q23.2 | Breast cancer Fanconi anemia | Involvement in the activity | ND | [ ] | ||
| 22q12.1 | Breast cancer Li-Fraumeni syndrome Prostate cancer Osteosarcoma | Cell cycle control | 20–25% | [ ] | ||
| 7q36.1 | Fanconi anemia Premature ovarian failure Spermatogenic failure | DNA repair | ND | [ ] |
3.1.5. Race/Ethnicity
Disparities regarding race and ethnicity remain widely observed among individuals affected by breast cancer; the mechanisms associated with this phenomenon are not yet understood. Generally, the breast cancer incidence rate remains the highest among white non-Hispanic women [ 51 , 52 ]. Contrarily, the mortality rate due to this malignancy is significantly higher among black women; this group is also characterized by the lowest survival rates [ 53 ].
3.1.6. Reproductive History
Numerous studies confirmed a strict relationship between exposure to endogenous hormones—estrogen and progesterone in particular—and excessive risk of breast cancer in females. Therefore, the occurrence of specific events such as pregnancy, breastfeeding, first menstruation, and menopause along with their duration and the concomitant hormonal imbalance, are crucial in terms of a potential induction of the carcinogenic events in the breast microenvironment. The first full-term pregnancy at an early age (especially in the early twenties) along with a subsequently increasing number of births are associated with a reduced risk of breast cancer [ 54 , 55 ]. Besides, the pregnancy itself provides protective effects against potential cancer. However, protection was observed at approximately the 34th pregnancy week and was not confirmed for the pregnancies lasting for 33 weeks or less [ 56 ]. Women with a history of preeclampsia during pregnancy or children born to a preeclamptic pregnancy are at lower risk of developing breast cancer [ 57 ]. No association between the increased breast cancer risk and abortion was stated so far [ 58 ].
The dysregulated hormone levels during preeclampsia including increased progesterone and reduced estrogen levels along with insulin, cortisol, insulin-like growth factor-1, androgens, human chorionic gonadotropin, corticotropin-releasing factor, and IGF-1 binding protein deviating from the physiological ranges, show a protective effect preventing from breast carcinogenesis. The longer duration of the breastfeeding period also reduces the risk of both the ER/PR-positive and -negative cancers [ 59 ]. Early age at menarche is another risk factor of breast cancer; it is possibly also associated with a tumor grade and lymph node involvement [ 60 ]. Besides, the earlier age of the first menstruation could result in an overall poorer prognosis. Contrarily, early menopause despite whether natural or surgical, lowers the breast cancer risk [ 61 ].
3.1.7. Density of Breast Tissue
The density of breast tissue remains inconsistent throughout the lifetime; however, several categories including low-density, high-density, and fatty breasts have been established in clinical practice. Greater density of breasts is observed in females of younger age and lower BMI, who are pregnant or during the breastfeeding period, as well as during the intake of hormonal replacement therapy [ 62 ]. Generally, the greater breast tissue density correlates with the greater breast cancer risk; this trend is observed both in premenopausal and postmenopausal females [ 63 ]. It was proposed that screening of breast tissue density could be a promising, non-invasive, and quick method enabling rational surveillance of females at increased risk of cancer [ 64 ].
3.1.8. History of Breast Cancer and Benign Breast Diseases
Personal history of breast cancer is associated with a greater risk of a renewed cancerous lesions within the breasts [ 65 ]. Besides, a history of any other non-cancerous alternations in breasts such as atypical hyperplasia, carcinoma in situ, or many other proliferative or non-proliferative lesions, also increases the risk significantly [ 66 , 67 , 68 ]. The histologic classification of benign lesions and a family history of breast cancer are two factors that are strongly associated with breast cancer risk [ 66 ].
3.1.9. Previous Radiation Therapy
The risk of secondary malignancies after radiotherapy treatment remains an individual matter that depends on the patient’s characteristics, even though it is a quite frequent phenomenon that arises much clinical concern. Cancer induced by radiation therapy is strictly associated with an individual’s age; patients who receive radiation therapy before the age of 30, are at a greater risk of breast cancer [ 69 ]. The selection of proper radiotherapy technique is crucial in terms of secondary cancer risk—for instance, tangential field IMRT (2F-IMRT) is associated with a significantly lower risk compared to multiple-field IMRT (6F-IMRT) or double partial arcs (VMAT) [ 70 ]. Besides, the family history of breast cancer in patients who receive radiotherapy additionally enhances the risk of cancer occurrence [ 71 ]. However, Bartelink et al. showed that additional radiation (16 Gy) to the tumor bed combined with standard radiotherapy might decrease the risk of local recurrence [ 72 ].
3.2. Modifiable Factors
3.2.1. chosen drugs.
Data from some research indicates that the intake of diethylstilbestrol during pregnancy might be associated with a greater risk of breast cancer in children; this, however, remains inconsistent between studies and requires further evaluation [ 73 , 74 ]. The intake of diethylstilbestrol during pregnancy is associated with an increased risk of breast cancer not only in mothers but also in the offspring [ 75 ]. This relationship is observed despite the expression of neither estrogen nor progesterone receptors and might be associated with every breast cancer histological type. The risk increases with age; women at age of ≥40 years are nearly 1.9 times more susceptible compared to women under 40. Moreover, breast cancer risk increases with greater diethylstilbestrol doses [ 76 ]. Numerous researches indicate that females who use hormonal replacement therapy (HRT) especially longer than 5 or 7 years are also at increased risk of breast cancer [ 77 , 78 ]. Several studies indicated that the intake of chosen antidepressants, mainly paroxetine, tricyclic antidepressants, and selective serotonin reuptake inhibitors might be associated with a greater risk of breast cancer [ 79 , 80 ]. Lawlor et al. showed that similar risk might be achieved due to the prolonged intake of antibiotics; Friedman et al. observed that breast risk is mostly elevated while using tetracyclines [ 81 , 82 ]. Attempts were made to investigate a potential relationship between hypertensive medications, non-steroidal anti-inflammatory drugs, as well as statins, and an elevated risk of breast cancer, however, this data remains highly inconsistent [ 83 , 84 , 85 ].
3.2.2. Physical Activity
Even though the mechanism remains yet undeciphered, regular physical activity is considered to be a protective factor of breast cancer incidence [ 86 , 87 ]. Chen et al. observed that amongst females with a family history of breast cancer, physical activity was associated with a reduced risk of cancer but limited only to the postmenopausal period [ 88 ]. However, physical activity is beneficial not only in females with a family history of breast cancer but also in those without such a history. Contrarily to the above-mentioned study, Thune et al. pointed out more pronounced effects in premenopausal females [ 89 ]. There are several hypotheses aiming to explain the protective role of physical activity in terms of breast cancer incidence; physical activity might prevent cancer by reducing the exposure to the endogenous sex hormones, altering immune system responses or insulin-like growth factor-1 levels [ 88 , 90 , 91 ].
3.2.3. Body Mass Index
According to epidemiological evidence, obesity is associated with a greater probability of breast cancer. This association is mostly intensified in obese post-menopausal females who tend to develop estrogen-receptor-positive breast cancer. Yet, independently to menopausal status, obese women achieve poorer clinical outcomes [ 92 ]. Wang et al. showed that females above 50 years old with greater Body Mass Index (BMI) are at a greater risk of cancer compared to those with low BMI [ 93 ]. Besides, the researchers observed that greater BMI is associated with more aggressive biological features of tumor including a higher percentage of lymph node metastasis and greater size. Obesity might be a reason for greater mortality rates and a higher probability of cancer relapse, especially in premenopausal women [ 94 ]. Increased body fat might enhance the inflammatory state and affects the levels of circulating hormones facilitating pro-carcinogenic events [ 95 ]. Thus, poorer clinical outcomes are primarily observed in females with BMI ≥ 25 kg/m 2 [ 96 ]. Interestingly, postmenopausal women tend to present poorer clinical outcomes despite proper BMI values but namely due to excessive fat volume [ 97 ]. Greater breast cancer risk with regards to BMI also correlates with the concomitant family history of breast cancer [ 98 ].
3.2.4. Alcohol Intake
Numerous evidences confirm that excessive alcohol consumption is a factor that might enhance the risk of malignancies within the gastrointestinal tract; however, it was proved that it is also linked to the risk of breast cancer. Namely, it is not alcohol type but rather the content of alcoholic beverages that mostly affect the risk of cancer. The explanation for this association is the increased levels of estrogens induced by the alcohol intake and thus hormonal imbalance affecting the risk of carcinogenesis within the female organs [ 99 , 100 ]. Besides, alcohol intake often results in excessive fat gain with higher BMI levels, which additionally increases the risk. Other hypotheses include direct and indirect carcinogenic effects of alcohol metabolites and alcohol-related impaired nutrient intake [ 101 ]. Alcohol consumption was observed to increase the risk of estrogen-positive breast cancers in particular [ 102 ]. Consumed before the first pregnancy, it significantly contributes to the induction of morphological alterations of breast tissue, predisposing it to further carcinogenic events [ 103 ].
3.2.5. Smoking
Carcinogens found in tobacco are transported to the breast tissue increasing the plausibility of mutations within oncogenes and suppressor genes ( p53 in particular). Thus, not only active but also passive smoking significantly contributes to the induction of pro-carcinogenic events [ 104 ]. Besides, longer smoking history, as well as smoking before the first full-term pregnancy, are additional risk factors that are additionally pronounced in females with a family history of breast cancer [ 105 , 106 , 107 , 108 ].
3.2.6. Insufficient Vitamin Supplementation
Vitamins exert anticancer properties, which might potentially benefit in the prevention of several malignancies including breast cancer, however, the mechanism is not yet fully understood. Attempts are continually made to analyze the effects of vitamin intake (vitamin C, vitamin E, B-group vitamins, folic acid, multivitamin) on the risk of breast cancer, nevertheless, the data remains inconsistent and not sufficient to compare the results and draw credible data [ 108 ]. In terms of breast cancer, most studies are currently focused on vitamin D supplementation confirming its potentially protective effects [ 109 , 110 , 111 ]. High serum 25-hydroxyvitamin D levels are associated with a lower incidence rate of breast cancer in premenopausal and postmenopausal women [ 110 , 112 ]. Intensified expression of vitamin D receptors was shown to be associated with lower mortality rates due to breast cancer [ 113 ]. Even so, further evaluation is required since data remains inconsistent in this matter [ 108 , 114 ].
3.2.7. Exposure to Artificial Light
Artificial light at night (ALAN) has been recently linked to increased breast cancer risk. The probable causation might be a disrupted melatonin rhythm and subsequent epigenetic alterations [ 115 ]. According to the studies conducted so far, increased exposure to ALAN is associated with a significantly greater risk of breast cancer compared to individuals with lowered ALAN exposure [ 116 ]. Nonetheless, data regarding the excessive usage of LED electronic devices and increased risk of breast cancer is insufficient and requires further evaluation as some results are contradictory [ 116 ].
3.2.8. Intake of Processed Food/Diet
According to the World Health Organization (WHO), highly processed meat was classified as a Group 1 carcinogen that might increase the risk of not only gastrointestinal malignancies but also breast cancer. Similar observations were made in terms of an excessive intake of saturated fats [ 117 ]. Ultra-processed food is rich in sodium, fat, and sugar which subsequently predisposes to obesity recognized as another factor of breast cancer risk [ 118 ]. It was observed that a 10% increase of ultra-processed food in the diet is associated with an 11% greater risk of breast cancer [ 118 ]. Contrarily, a diet high in vegetables, fruits, legumes, whole grains, and lean protein is associated with a lowered risk of breast cancer [ 119 ]. Generally, a diet that includes food containing high amounts of n-3 PUFA, vitamin D, fiber, folate, and phytoestrogen might be beneficial as a prevention of breast cancer [ 120 ]. Besides, lower intake of n-6 PUFA and saturated fat is recommended. Several in vitro and in vivo studies also suggest that specific compounds found in green tea might present anti-cancer effects which has also been studied regarding breast cancer [ 121 ]. Similar properties were observed in case of turmeric-derived curcuminoids as well as sulforaphane (SFN) [ 122 , 123 ].
3.2.9. Exposure to Chemical
Chronic exposure to chemicals can promote breast carcinogenesis by affecting the tumor microenvironment subsequently inducing epigenetic alterations along with the induction of pro-carcinogenic events [ 124 ]. Females chronically exposed to chemicals present significantly greater plausibility of breast cancer which is further positively associated with the duration of the exposure [ 125 ]. The number of chemicals proposed to induce breast carcinogenesis is significant; so far, dichlorodiphenyltrichloroethane (DDT) and polychlorinated biphenyl (PCB) are mostly investigated in terms of breast cancer since early exposure to those chemicals disrupts the development of mammary glands [ 126 , 127 ]. A potential relationship was also observed in the case of increased exposure to polycyclic aromatic hydrocarbons (PAH), synthetic fibers, organic solvents, oil mist, and insecticides [ 128 ].
3.2.10. Other Drugs
Other drugs that might constitute potential risk factors for breast cancer include antibiotics, antidepressants, statins, antihypertensive medications (e.g., calcium channel blockers, angiotensin II-converting enzyme inhibitors), as well as NSAIDs (including aspirin, ibuprofen) [ 129 , 130 , 131 , 132 , 133 ].
4. Breast Cancer Classification
4.1. histological classification.
Invasive breast cancers (IBC) comprise wide spectrum tumors that show a variation concerning their clinical presentation, behavior, and morphology. The World Health Organization (WHO) distinguish at least 18 different histological breast cancer types [ 134 ].
Invasive breast cancer of no special type (NST), formerly known as invasive ductal carcinoma is the most frequent subgroup (40–80%) [ 135 ]. This type is diagnosed by default as a tumor that fails to be classified into one of the histological special types [ 134 ]. About 25% of invasive breast cancers present distinctive growth patterns and cytological features, hence, they are recognized as specific subtypes (e.g., invasive lobular carcinoma, tubular, mucinous A, mucinous B, neuroendocrine) [ 136 ].
Molecular classification independently from histological subtypes, invasive breast cancer can be divided into molecular subtypes based on mRNA gene expression levels. In 2000, Perou et al. on a sample of 38 breast cancers identified 4 molecular subtypes from microarray gene expression data: Luminal, HER2-enriched, Basal-like, and Normal Breast-like [ 137 ]. Further studies allowed to divide the Luminal group into two subgroups (Luminal A and B) [ 138 , 139 ]. The normal breast-like subtype has subsequently been omitted, as it is thought to represent sample contamination by normal mammary glands. In the Cancer Genome Atlas Project (TCGA) over 300 primary tumors were thoroughly profiled (at DNA, RNA, and protein levels) and combined in biological homogenous groups of tumors. The consensus clustering confirmed the distinction of four main breast cancer intrinsic subtypes based on mRNA gene expression levels only (Luminal A, Luminal B, HER2-enriched, and basal-like) [ 140 ]. Additionally, the 5th intrinsic subtype—claudin-low breast cancer was discovered in 2007 in an integrated analysis of human and murine mammary tumors [ 141 ].
In 2009, Parker et al. developed a 50-gene signature for subtype assignment, known as PAM50, that could reliably classify particular breast cancer into the main intrinsic subtypes with 93% accuracy [ 142 ]. PAM50 is now clinically implemented worldwide using the NanoString nCounter ® , which is the basis for the Prosigna ® test. The Prosigna ® combines the PAM50 assay as well as clinical information to assess the risk of distant relapse estimation in postmenopausal women with hormone receptor-positive, node-negative, or node-positive early-stage breast cancer patients, and is a daily-used tool assessing the indication of adjuvant chemotherapy [ 143 , 144 , 145 ].
4.2. Luminal Breast Cancer
Luminal breast cancers are ER-positive tumors that comprise almost 70% of all cases of breast cancers in Western populations [ 146 ]. Most commonly Luminal-like cancers present as IBC of no special subtype, but they may infrequently differentiate into invasive lobular, tubular, invasive cribriform, mucinous, and invasive micropapillary carcinomas [ 147 , 148 ]. Two main biological processes: proliferation-related pathways and luminal-regulated pathways distinguish Luminal-like tumors into Luminal A and B subtypes with different clinical outcomes.
Luminal A tumors are characterized by presence of estrogen-receptor (ER) and/or progesterone-receptor (PR) and absence of HER2. In this subtype the ER transcription factors activate genes, the expression of which is characteristic for luminal epithelium lining the mammary ducts [ 149 , 150 ]. It also presents a low expression of genes related to cell proliferation [ 151 ]. Clinically they are low-grade, slow-growing, and tend to have the best prognosis.
In contrast to subtype A, Luminal B tumors are higher grade and has worse prognosis. They are ER positive and may be PR negative and/or HER2 positive. Additionally, it has high expression of proliferation-related genes (e.g., MKI67 and AURKA) [ 152 , 153 , 154 ]. This subtype has lower expression of genes or proteins typical for luminal epithelium such as the PR [ 150 , 155 ] and FOXA1 [ 146 , 156 ], but not the ER [ 157 ]. ER is similarly expressed in both A and B subtypes and is used to distinguish luminal from non-luminal disease.
4.3. HER2-Enriched Breast Cancer
The HER2-enriched group makes up 10–15% of breast cancers. It is characterized by the high expression of the HER2 with the absence of ER and PR. This subtype mainly expresses proliferation—related genes and proteins (e.g., ERBB2/HER2 and GRB7), rather than luminal and basal gene and protein clusters [ 154 , 156 , 157 ]. Additionally, in the HER2-enriched subtype there is evidence of mutagenesis mediated by APOBEC3B. APOBEC3B is a subclass of APOBEC cytidine deaminases, which induce cytosine mutation biases and is a source of mutation clusters [ 158 , 159 , 160 ].
HER2-enriched cancers grow faster than luminal cancers and used to have the worst prognosis of subtypes before the introduction of HER2-targeted therapies. Importantly, the HER2-enriched subtype is not synonymous with clinically HER2-positive breast cancer because many ER-positive/HER2-positive tumors qualify for the luminal B group. Moreover, about 30% of HER2-enriched tumors are classified as clinically HER2-negative based on immunohistochemistry (IHC) and/or fluorescence in situ hybridization (FISH) methods [ 161 ].
4.4. Basal-Like/Triple-Negative Breast Cancer
The Triple-Negative Breast Cancer (TNBC) is a heterogeneous collection of breast cancers characterized as ER-negative, PR-negative, and HER2-negative. They constitute about 20% of all breast cancers. TNBC is more common among women younger than 40 years of age and African-American women [ 161 ]. The majority (approximately 80%) of breast cancers arising in BRCA1 germline mutation are TNBC, while 11–16% of all TNBC harbor BRCA1 or BRCA2 germline mutations. TNBC tends to be biologically aggressive and is often associated with a worse prognosis [ 162 ]. The most common histology seen in TNBC is infiltrating ductal carcinoma, but it may also present as medullary-like cancers with a prominent lymphocytic infiltrate; metaplastic cancers, which may show squamous or spindle cell differentiation; and rare special type cancers like adenoid cystic carcinoma (AdCC) [ 163 , 164 , 165 ].
The terms basal-like and TNBC have been used interchangeably; however, not all TNBC are of the basal type. On gene expression profiling, TNBCs can be subdivided into six subtypes: basal-like (BL1 and BL2), mesenchymal (M), mesenchymal stem-like (MSL), immunomodulatory (IM), and luminal androgen receptor (LAR), as well as an unspecified group (UNS) [ 166 , 167 ]. However, the clinical relevance of the subtyping still unclear, and more research is needed to clarify its impact on TNBC treatment decisions [ 168 ].
4.5. Claudin-Low Breast Cancer
Claudin-low (CL) breast cancers are poor prognosis tumors being mostly ER-negative, PR-negative, and HER2-negative. CL tumors account for 7–14% of all invasive breast cancers [ 147 ]. No differences in survival rates were observed between claudin-low tumors and other poor-prognosis subtypes (Luminal B, HER2-enriched, and Basal-like). CL subtype is characterized by the low expression of genes involved in cell-cell adhesion, including claudins 3, 4, and 7, occludin, and E-cadherin. Besides, these tumors show high expression of epithelial-mesenchymal transition (EMT) genes and stem cell-like gene expression patterns [ 169 , 170 ]. Moreover, CL tumors have marked immune and stromal cell infiltration [ 171 ]. Due to their less differentiated state and a preventive effect of the EMT-related transcription factor, ZEB1 CL tumors are often genomically stable [ 172 , 173 ].
4.6. Surrogate Markers Classification
In clinical practice, the key question is the discrimination between patients who will or will not benefit from particular therapies. By using molecular assays, more patients can be spared adjuvant chemotherapy, but these tests are associated with significant costs. Therefore, surrogate subgroups based on pathological morphology and widely available immunohistochemical (IHC) markers are used as a tool for risk stratification and guidance of adjuvant therapy [ 174 ]. A combination of the routine pathological markers ER, PR, and HER2 is used to classify tumors into intrinsic subtypes [ 175 ]. Semiquantitative evaluation of Ki-67 and PR is helpful for further typing of the Luminal subtype [ 176 , 177 ]. Moreover, evaluation of cytokeratin 5/6 and epidermal growth factor receptor is utilized to identify the Basal-like breast cancer among the TNBC [ 178 ].
In St. Gallen’s 2013 guidelines the IHC-based surrogate subtype classification was recommended for clinical decision making [ 179 ]. However, these IHC-based markers are only a surrogate and cannot establish the intrinsic subtype of any given cancer, with discordance rates between IHC-based markers and gene-based assays as high as 30% [ 180 ].
4.7. American Joint Committee on Cancer Classification
The baseline tool to estimate the likely prognosis of patients with breast cancer is the AJCC staging system that includes grading, immunohistochemistry biomarkers, and anatomical advancement of the disease. Since its inception in 1977, the American Joint Committee on Cancer (AJCC) has published an internationally accepted staging system based on anatomic findings: tumor size (T), nodal status (N), and metastases (M). However, gene expression profiling has identified several molecular subtypes of breast cancer [ 181 ]. The eighth edition of the AJCC staging manual (2018), outlines a new prognostic staging system for breast cancer that, in addition to anatomical features, acknowledges biological factors [ 182 ]. These factors—ER, PR, HER2, grade, and multigene assays—are recommended in practice to define prognosis [ 183 , 184 ].
The most widely used histologic grading system of breast cancer is the Elston-Ellis modification [ 185 ] of Scarff-Bloom-Richardson grading system [ 186 ], also known as the Nottingham grading system. The grade of a tumor is determined by assessing morphologic features: (a) formation of tubules, (b) mitotic count, (c) variability, and the size and shape of cellular nuclei. A score between 1 (most favorable) and 3 (least favorable) is assigned for each feature. Grade 1 corresponds to combined scores between 3 and 5, grade 2 corresponds to a combined score of 6 or 7, and grade 3 corresponds to a combined score of 8 or 9.
In addition to grading and biomarkers, the commercially available multigene assays provide additional prognostic information suitable for incorporation in the AJCC 8th edition. The 21-gene assay Oncotype DX ® assessed by reverse transcription-polymerase chain reaction (RT-PCR) was the only assay sufficiently evaluated and included in the staging system. This assay is valuable in the staging of patients with hormone receptor-positive, HER2-negative, node-negative tumors that are <5 cm. Patients with results of the assay (Recurrence Score) less than 11 had excellent disease-free survival at 6.9 years of 98.6% with endocrine therapy alone [ 187 ]. Hence, adjuvant systemic chemotherapy can be safely omitted in patients with a low-risk multigene assay [ 188 ].
The AJCC staging manual includes a pathological and a clinical-stage group. The clinical prognostic stage group should be utilized in all patients on initial evaluation before any systemic therapy. Clinical staging uses the TNM anatomical information, grading, and expression of these three biomarkers. When patients undergo surgical resection of their primary tumor, the post-resection anatomic information coupled with the pretreatment biomarker findings results in the final Pathologic Prognostic Stage Group.
The recent update of breast cancer staging by the biologic markers improved the outcome prediction in comparison to prior staging based only on anatomical features of the disease. The validation studies involving the reassessment of the Surveillance, Epidemiology, and End Results (SEER) database ( n = 209,304, 2010–2014) and the University of Texas MD Anderson Cancer Center database ( n = 3327, years of treatment 2007–2013) according to 8th edition AJCC manual proved the more accurate prognostic information [ 189 , 190 ].
5. Prognostic Biomarkers
5.1. estrogen receptor.
Estrogen receptor (ER) is an important diagnostic determinant since approximately 70–75% of invasive breast carcinomas are characterized by significantly enhanced ER expression [ 191 , 192 ]. Current practice requires the measurement of ER expression on both—primary invasive tumors and recurrent lesions. This procedure is mandatory to provide the selection of those patients who will most benefit from the implementation of the endocrine therapy mainly selective estrogen receptor modulators, pure estrogen receptor downregulators, or third-generation aromatase inhibitors [ 193 ]. Even though the diagnosis of altered expression of ER is particularly relevant in terms of the proper therapy selection, ER expression might also constitute a predictive factor—patients with high ER expression usually present significantly better clinical outcomes [ 194 ]. A relationship was observed between ER expression and the family history of breast cancer which further facilitates the utility of ER expression as a diagnostic biomarker of breast cancer especially in cases of familial risk [ 195 ]. Besides, Konan et al. reported that ERα-36 expression could constitute one of the potential targets of PR-positive cancers and a prognostic marker at the same time [ 196 ].
5.2. Progesterone Receptor
PR is highly expressed (>50%) in patients with ER-positive while quite rarely in those with ER-negative breast cancer [ 197 ]. PR expression is regulated by ER therefore, physiological values of PR inform about the functional ER pathway [ 197 ]. However, both ER and PR are abundantly expressed in breast cancer cells and both are considered as diagnostic and prognostic biomarkers of breast cancer (especially ER-positive ones) [ 198 ]. Greater PR expression is positively associated with the overall survival, time to recurrence, and time to either treatment failure or progression while lowered PR levels are usually related to a more aggressive course of the disease as well as poorer recurrence and prognosis [ 199 ]. Thus, favorable management of breast cancer patients highly depends on the assessment of PR expression. Nevertheless, the predictive value of PR expression still remains controversial [ 200 ].
5.3. Human Epidermal Growth Factor Receptor 2
The expression of human epidermal growth factor receptor 2 (HER2) accounts for approximately 15–25% of breast cancers and its status is primarily relevant in the choice of proper management with breast cancer patients; HER2 overexpression is one of the earliest events during breast carcinogenesis [ 201 ]. Besides, HER2 increases the detection rate of metastatic or recurrent breast cancers from 50% to even more than 80% [ 202 ]. Serum HER2 levels are considered to be a promising real-time marker of tumor presence or recurrence [ 203 ]. HER2 amplification leads to further overactivation of the pro-oncogenic signaling pathways leading to uncontrolled growth of cancer cells which corresponds with poorer clinical outcomes in the case of HER2-positive cancers [ 204 ]. Overexpression of HER2 also correlates with a significantly shorter disease-free period [ 205 ] as well as histologic type, pathologic state of cancer, and a number of axillary nodes with metastatic cancerous cells [ 205 ].
5.4. Antigen Ki-67
The Ki-67 protein is a cellular marker of proliferation and the Ki-67 proliferation index is an excellent marker to provide information about the proliferation of cancerous cells particularly in the case of breast cancer. The proliferative activities determined by Ki-67 reflect the aggressiveness of cancer along with the response to treatment and recurrence time [ 206 ]. Thus, Ki-67 is crucial in terms of the choice of the proper treatment therapy and the potential follow-ups due to recurrence. Though, due to several limitations of the analytical validity of Ki-67 immunohistochemistry, Ki-67 expression levels should be considered benevolently in terms of definite treatment decisions. Ki-67 might be considered as a potential prognostic factor as well; according to a meta-analysis of 68 studies involving 12,155 patients, the overexpression of Ki-67 is associated with poorer clinical outcomes of patients [ 207 ]. High expression of Ki-67 also reflects poorer survival rates of breast cancer patients [ 208 ]. There are speculations whether Ki-67 could be considered as a potential predictive marker, however, such data is still limited and contradictory.
Mib1 (antibody against Ki-67) proliferation index remains a reliable diagnostic biomarker of breast cancer, similarly to Ki-67. A decrease in both Mib1 and Ki-67 expression levels is associated with a good response of breast cancer patients to preoperative treatment [ 209 ]. Mib1 levels are significantly greater in patients with concomitant p53 mutations [ 210 ]. Mib1 assessment might be especially useful in cases of biopsy specimens small in size, inappropriate for neither mitotic index nor S-phase fraction evaluation [ 211 ].
5.6. E-Cadherin
E-cadherin is a critical protein in the epithelial-mesenchymal transition (EMT); loss of its expression leads to the gradual transformation into mesenchymal phenotype which is further associated with increased risk of metastasis. The utility of E-cadherin as a breast biomarker is yet questionable, however, some research indicated that its expression is potentially associated with several breast cancer characteristics such as tumor size, TNM stage, or lymph node status [ 212 ]. Low or even total loss of E-cadherin expression might be potentially useful in the determination of histologic subtype of breast cancer [ 213 , 214 ]. E-cadherin levels do not seem to be promising in terms of patients’ survival rates assessment, however, there are some reports indicating that higher levels of E-cadherin were associated with shorter survival rates in patients with invasive breast carcinoma [ 213 , 215 ]. Lowered E-cadherin expression is positively associated with lymph node metastasis [ 216 ].
5.7. Circulating Circular RNA
Circulating circular RNAs (circRNAs) belong to the group of non-coding RNA and were quite recently shown to be crucial in terms of several hallmarks of breast carcinogenesis including apoptosis, enhanced proliferation, or increased metastatic potential [ 217 ]. One of the most comprehensively described circRNAs, mostly specific to breast cancer include circFBXW7—which was proposed as a potential diagnostic biomarker as well as therapeutic tool for patients with triple-negative breast cancer (TNBC), as well as hsa_circ_0072309 which is abundantly expressed in breast cancer patients and usually associated with poorer survival rates [ 218 ]. Has_circ_0001785 is considered to be promising as a diagnostic biomarker of breast cancer [ 219 ]. The number of circRNAs dysregulated during breast carcinogenesis is significant; their expression might be either upregulated (e.g., has_circ_103110, circDENND4C) or downregulated (e.g., has_circ_006054, circ-Foxo3) [ 220 ]. Besides, specific circRNAs have been reported in different types of breast cancer such as TNBC, HER2-positive, and ER-positive [ 221 ]. Recently it was showed that an interaction between circRNAs and micro-RNA—namely in the form of Cx43/has_circ_0077755/miR-182 post-transcriptional axis, might predict breast cancer initiation as well as further prognosis. Cx43 is transmembrane protein responsible for epithelial homeostasis that mediates junction intercellular communication and its loss dysregulates post-transcriptional axes in breast cancer initiation [ 222 ].
Loss-of-function mutations in the TP53 (P53) gene have been found in numerous cancer types including osteosarcomas, leukemia, brain tumors, adrenocortical carcinomas, and breast cancers [ 223 , 224 ]. P53 protein is essential for normal cellular homeostasis and genome maintenance by mediating cellular stress responses including cell cycle arrest, apoptosis, DNA repair, and cellular senescence [ 225 ]. The silencing mutation of the P53 gene is evident at an early stage of cancer progression. In breast cancer, the prevalence of TP53 mutations is present in approximately 80% of patients with the TNBC and 10% of patients with Luminal A disease [ 226 ].
There have been many studies showing the prognostic role of p53 loss-of-function mutation in breast cancer [ 227 , 228 ]. However, the missense mutations may alters p53 properties causing not only a loss of wild-type function, but also acquisition novel activities-gain of function [ 229 ]. The IHC status of p53 has been proposed as a specific prognostic factor in TNBC, and a feature that divides TNBC into 2 distinct subgroups: a p53-negative normal breast-like TN subgroup, and a p53-positive basal-like subgroup with worse overall survival [ 230 , 231 , 232 ]. However, there is not enough evidence to utilize p53 gene mutational status or immunohistochemically measured protein for determining standardized prognosis in patients with breast cancer [ 233 ].
5.9. MicroRNA
MicroRNAs (miRNA) are a major class of endogenous non-coding RNA molecules (19–25 nucleotides) that have regulatory roles in multiple pathways [ 234 ]. Some miRNAs are related to the development, progression, and response of the tumor to therapy [ 235 ]. Several studies have investigated abnormally expressed miRNAs as biomarkers in breast cancer tissue samples. According to meta-analysis by Adhami et al. two miRNAs (miRNA-21 and miRNA-210) were upregulated consistently and six miRNAs (miRNA-145, miRNA-139-5p, miRNA-195, miRNA-99a, miRNA-497, and miRNA-205) were downregulated consistently in at least three studies [ 236 ].
The miRNA-21 overexpression was observed in TNBC tissues and was associated with enhanced invasion and proliferation of TNBC cells as well as downregulation of the PTEN expression [ 237 ]. Similarly, the high expression of miRNA-210 is related to tumor proliferation, invasion, and poor survival rates in breast cancer patients [ 238 , 239 ].
The miRNA-145 is an anti-cancer agent having the property of inhibiting migration and proliferation of breast cancer cells via regulating the TGF-β1 expression [ 240 ]. However, the miRNA-145 is downregulated in both plasma and tumors of breast cancer patients [ 241 ]. Similarly, miRNA-139-5p and miRNA-195 have tumor suppressor activity in various cancers [ 242 , 243 ].
Nevertheless, further clinical researches focusing on these miRNAs are needed to utilize them as reproducible, disease-specific markers that have a high level of specificity and sensitivity.
5.10. Tumor-Associated Macrophages
Macrophages are known for their immunomodulatory effects and they can be divided according to their phenotypes into M1- or M2-like states [ 244 , 245 ]. M1 macrophages secrete IL-12 and tumor necrosis factor with antimicrobial and antitumor effects. M2 macrophages produce cytokines, including IL-10, IL-1 receptor antagonist type II, and IL-1 decoy receptor. Therefore, macrophages with M1-like phenotype have been linked to good disease course while M2-like phenotype has been associated with adverse outcome, potentially through immunosuppression and the promotion of angiogenesis and tumor cell proliferation and invasion [ 246 , 247 ]. In literature, tumor-associated macrophages (TAMs) are associated with M2 macrophages which promote tumor growth and metastasis.
For breast cancer, studies have shown that the density of TAMs is related to hormone receptor status, stage, histologic grade, lymph node metastasis, and vascular invasion [ 248 , 249 , 250 , 251 ]. According to meta-analysis conducted by Zhao et al. high density of TAMs was related to overall survival disease-free survival [ 252 ].
Conversely, M1 polarized macrophages are linked to favorable prognoses in various cancers [ 253 , 254 , 255 ]. In breast cancer, the high density of M1-like macrophages predicted improved survival in patients with HER2+ phenotype and may be a potential prognostic marker [ 256 ].
However, further studies are needed to clarify the influence of macrophages on breast cancer biology as well as investigate the role of their intratumoral distribution and surface marker selection.
5.11. Inflammation-Based Models
The host inflammatory and immune responses in the tumor and its microenvironment are critical components in cancer development and progression [ 257 ]. The tumor-induced systemic inflammatory response leads to alterations of peripheral blood white blood cells [ 258 ]. Therefore, the relationship between peripheral blood inflammatory cells may serve as an accessible and early method of predicting patient prognosis. Recent studies have reported the predictive role of the inflammatory cell ratios: neutrophil-to-lymphocyte ratio, the lymphocyte-to-monocyte ratio, and the platelet-to-lymphocyte ratio for prognosis in different cancers [ 258 , 259 , 260 , 261 ].
5.11.1. The Neutrophil-to-Lymphocyte Ratio (NLR)
In an extensive study on 27,031 cancer patients, Proctor et al. analyzed the prognostic value of NLR and found a significant relationship between NLR and survival in various cancers including breast cancer [ 262 ]. There are pieces of evidence of the role of lymphocytes in breast cancer immunosurveillance [ 263 , 264 ]. Opposingly neutrophils suppress the cytolytic activity of lymphocytes, leading to enhanced angiogenesis and tumor growth and progression [ 265 ].
Azab et al. first reported that NLR before chemotherapy was an independent factor for long-term mortality and related it to age and tumor size in breast cancer [ 266 ]. In a recent meta-analysis by Guo et al., performed on 17,079 individuals, the high NLR level was associated with both poor overall survival as well as disease-free survival for breast cancer patients. Moreover, it was reported that association between NLR and overall survival was stronger in TNBC patients than in HER2-positive ones [ 267 ].
5.11.2. Lymphocyte-to-Monocyte Ratio
The association of the lymphocyte-to-monocyte ratio (LMR) with patients’ prognosis has been reported for several cancers [ 268 , 269 ]. As lymphocytes have an antitumor activity by inducing cytotoxic cell death and inhibiting tumor proliferation [ 270 ], the monocytes are involved in tumorigenesis, including differentiation into TAMs [ 246 , 247 , 271 ]. In the tumor microenvironment, cytokines, and free radicals that are secreted by monocytes and macrophages are associated with angiogenesis, tumor cell invasion, and metastasis [ 271 ].
A meta-analysis investigating the prognostic effect of LMR showed that low LMR levels are associated with shorter overall survival outcomes in Asian populations, TNBC patients, and patients with non-metastatic and mixed stages [ 272 ]. Moreover, high LMR levels are associated with favorable disease-free survival of breast cancer patients under neoadjuvant chemotherapy [ 273 ].
5.11.3. Platelet-to-Lymphocyte Ratio (PLR)
A high platelet count has been associated with poor prognosis in several types of cancers [ 274 , 275 , 276 ]. Platelets contain both pro-inflammatory molecules and cytokines (P-selectin, CD40L, and interleukin (IL)-1, IL-3, and IL-6) and many anti-inflammatory cytokines. Tumor angiogenesis and growth may be stimulated by the secretion of platelet-derived growth factor, vascular endothelial growth factor, transforming growth factor-beta, and platelet factor 4 [ 277 , 278 , 279 ].
A meta-analysis study investigated the prognostic importance of PLR by analyzing 5542 breast cancer patients. High PLR level was associated with poor prognosis (overall survival and disease-free survival), yet, its prognostic value was not determined for molecular subtypes of breast cancer. Nevertheless, an association was found between PLR and clinicopathological features of the tumor, including stage, lymph node metastasis, and distant metastasis [ 280 ]. In the aforementioned meta-analysis, there was a difference in the incidence of high levels of PLR between HER2 statuses [ 280 ], while other studies found a difference between hormone ER or PR statuses [ 281 , 282 ].
6. Treatment Strategies
6.1. surgery.
There are two major types of surgical procedures enabling the removal of breast cancerous tissues and those include (1) breast-conserving surgery (BCS) and (2) mastectomy. BCS—also called partial/segmental mastectomy, lumpectomy, wide local excision, or quadrantectomy—enables the removal of the cancerous tissue with simultaneous preservation of intact breast tissue often combined with plastic surgery technics called oncoplasty. Mastectomy is a complete removal of the breast and is often associated with immediately breast reconstruction. The removal of affected lymph nodes involves sentinel lymph node biopsy (SLNB) and axillary lymph node dissection (ALND). Even though BCS seems to be highly more beneficial for patients, those who were treated with this technique often show a tendency for a further need for a complete mastectomy [ 283 ]. However, usage of BCS is mostly related to significantly better cosmetic outcomes, lowered psychological burden of a patient, as well as reduced number of postoperative complications [ 284 ]. Guidelines of the European Society for Medical Oncology (ESMO) for patients with early breast cancer make the choice of therapy dependent to tumor size, feasibility of surgery, clinical phenotype, and patient’s willingness to preserve the breast [ 285 ].
6.2. Chemotherapy
Chemotherapy is a systemic treatment of BC and might be either neoadjuvant or adjuvant. Choosing the most appropriate one is individualized according to the characteristics of the breast tumor; chemotherapy might also be used in the secondary breast cancer. Neoadjuvant chemotherapy is used for locally advanced BC, inflammatory breast cancers, for downstaging large tumors to allow BCS or in small tumors with worse prognostics molecular subtypes (HER2 or TNBC) which can help to identify prognostics and predictive factors of response and can be provided intravenously or orally. Currently, treatment includes a simultaneous application of schemes 2–3 of the following drugs—carboplatin, cyclophosphamide, 5-fluorouracil/capecitabine, taxanes (paclitaxel, docetaxel), and anthracyclines (doxorubicin, epirubicin). The choice of the proper drug is of major importance since different molecular breast cancer subtypes respond differently to preoperative chemotherapy [ 286 ]. Preoperative chemotherapy is comparably effective to postoperative chemotherapy [ 287 ].
Even though chemotherapy is considered to be effective, its usage very often leads to several side effects including hair loss, nausea/vomiting, diarrhea, mouth sores, fatigue, increased susceptibility to infections, bone marrow supression, combined with leucopenia, anaemia, easier bruising or bleeding; other less frequent side effects include cardiomyopathy, neuropathy, hand-foot syndrome, impaired mental functions. In younger women, disruptions of the menstrual cycle and fertility issues might also appear. Special form of chemotherapy is electrochemotherapy which can be used in patients with breast cancer that has spread to the skin, however, it is still quite uncommon and not available in most clinics.
6.3. Radiation Therapy
Radiotherapy is local treatment of BC, typically provided after surgery and/or chemotherapy. It is performed to ensure that all of the cancerous cells remain destroyed, minimizing the possibility of breast cancer recurrence. Further, radiation therapy is favorable in the case of metastatic or unresectable breast cancer [ 288 ]. Choice of the type of radiation therapy depends on previous type of surgery or specific clinical situation; most common techniques include breast radiotherapy (always applied after BC), chest-wall radiotherapy (usually after mastectomy), and ‘breast boost’ (a boost of high-dose radiotherapy to the place of tumor bed as a complement of breast radiotherapy after BCS). Regarding breast radiotherapy specifically, several types are distinguished including
- (1) intraoperative radiation therapy (IORT)
- (2) 3D-conformal radiotherapy (3D-CRT)
- (3) intensity-modulated radiotherapy (IMRT)
- (4) brachytherapy—which refers to internal radiation in contrast to other above-mentioned techniques.
Irritation and darkening of the skin exposed to radiation, fatigue, and lymphoedema are one of the most common side effects of radiation therapy applied in breast cancer patients. Nonetheless, radiation therapy is significantly associated with the improvement of the overall survival rates of patients and lowered risk of recurrence [ 289 ].
6.4. Endocrinal (Hormonal) Therapy
Endocrinal therapy might be used either as a neoadjuvant or adjuvant therapy in patients with Luminal–molecular subtype of BC; it is effective in cases of breast cancer recurrence or metastasis. Since the expression of ERs, a very frequent phenomenon in breast cancer patients, its blockage via hormonal therapy is commonly used as one of the potential treatment modalities. Endocrinal therapy aims to lower the estrogen levels or prevents breast cancer cells to be stimulated by estrogen. Drugs that block ERs include selective estrogen receptor modulators (SERMs) (tamoxifen, toremifene) and selective estrogen receptor degraders (SERDs) (fulvestrant) while treatments that aim to lower the estrogen levels include aromatase inhibitors (AIs) (letrozole, anastrazole, exemestane) [ 290 , 291 ]. In the case of pre-menopausal women, ovarian suppression induced by oophorectomy, luteinizing hormone-releasing hormone analogs, or several chemotherapy drugs, are also effective in lowering estrogen levels [ 292 ]. However, approximately 50% of hormonoreceptor-positive breast cancer become progressively resistant to hormonal therapy during such treatment [ 293 ]. Endocrinal therapy combined with chemotherapy is associated with the reduction of mortality rates amongst breast cancer patients [ 294 ].
6.5. Biological Therapy
Biological therapy (targeted therapy) can be provided at every stage of breast therapy– before surgery as neoadjuvant therapy or after surgery as adjuvant therapy. Biological therapy is quite common in HER2-positive breast cancer patients; major drugs include trastuzumab, pertuzumab, trastuzumab deruxtecan, lapatinib, and neratinib [ 295 , 296 , 297 , 298 , 299 ]. Further, the efficacy of angiogenesis inhibitors such as a recombinant humanized monoclonal anti-VEGF antibody (rhuMAb VEGF) or bevacizumab are continuously investigated [ 300 ].
In the case of Luminal, HER2-negative breast cancer, pre-menopausal women more often receive everolimus -TOR inhibitor with exemestane while postmenopausal women often receive CDK 4–6 inhibitor palbociclib or ribociclib simultaneously, combined with hormonal therapy [ 301 , 302 , 303 ]. Two penultimate drugs along with abemaciclib and everolimus can also be used in HER2-negative and estrogen-positive breast cancer [ 304 , 305 ]. Atezolizumab is approved in triple-negative breast cancer, while denosumab is approved in case of metastasis to the bones [ 306 , 307 , 308 ].
7. Conclusions
In this review, we aimed to summarize and update the current knowledge about breast cancer with an emphasis on its current epidemiology, risk factors, classification, prognostic biomarkers, and available treatment strategies. Since both the morbidity and mortality rates of breast cancer have significantly increased over the past decades, it is an urgent need to provide the most effective prevention taking into account that modifiable risk factors might be crucial in providing the reduction of breast cancer incidents. So far, mammography and sonography is the most common screening test enabling quite an early detection of breast cancer. The continuous search for prognostic biomarkers and targets for the potential biological therapies has significantly contributed to the improvement of management and clinical outcomes of breast cancer patients.
Author Contributions
Conceptualization, A.F., R.S. and A.S.; critical review of literature, S.Ł., M.C., A.F., J.B., R.S., A.S.; writing—original draft preparation, M.C., A.F.; writing—review and editing, S.Ł., M.C., A.F., J.B., R.S., A.S.; supervision, R.S. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Breast Cancer - Free Essay Examples And Topic Ideas
Breast cancer is a type of cancer that develops from breast tissue. Essays on this topic could explore the causes, diagnosis, treatment, and prevention of breast cancer. Additionally, discussions might delve into the psychological and social impact of breast cancer on patients and their families, the ongoing research towards finding a cure, and the broader societal awareness and support systems available for those affected. We have collected a large number of free essay examples about Breast Cancer you can find at Papersowl. You can use our samples for inspiration to write your own essay, research paper, or just to explore a new topic for yourself.

Micro Needle Thermocouple for Detection of Breast Cancer
Hundreds and thousands of people are affected by cancer each year; it is one of the most fatal diseases and a leading cause of death and disability for humans (Iranifam 2014). There are several types of cancer than can affect different areas of the body, some being less life-threatening than others. A vast amount of patients suffer from late diagnosis or recurrence of their disease in spite of all the advances in diagnosis and treatment of breast cancer. Modern cancer […]
The Role of Histology in the Breast Cancer
Breast cancer is an uncontrolled growth of breast cell that can be benign, not dangerous, but it can also metastasize and invade different and distant tissues in our body. Breast Cancer is the most common cancer in female of any age and although the risk increases, as you get older, many different factors affect the chance of a woman to get breast cancer. I chose this specific topic because breast cancer is something that I’ve dealt with in my personal […]
Corporate Social Responsibility against Cancer
Abstract As an assistant manager at Kenta Law Firm, based in Monroe, I intend to collaborate with the Susan B. Komen Foundation a non-organization corporation that is interested in reducing issues of breast cancer among women. Kenta law firm has noted that a significant populace of Monroe’s youth especially women and young children specifically those who are homeless are suffering from breast cancer. In this CSR partnership, our law firm will collaborate with the Susan B. Komen Foundation in addressing […]
We will write an essay sample crafted to your needs.
Why is Screening for Breast Cancer Important
The impact this disease has, on not only the individual but the people around them, is powerful. Even though the tests show cancer, I am thankful that I had the annual test. It is true that stress, anxiety, and money can be saved by waiting until the age of 50 years old because of misinterpretation and overdiagnosis. However, early detection is the key to success in the battle against breast cancer. There are many different options for detection scans that […]
Breast Cancer: Casuses and Treatment
Cancer is defined as “when the body’s cells begin to divide without stopping and spread into surrounding tissues.” (“What is cancer?”, 2017), caused by mutations that lead to the cell cycle to proceed, regardless if the cell is qualified to. The mutations block the use of the G1, G2, and M checkpoints in the cell cycle. These checkpoints are important in “sensing defects that occur during essential processes, and induce a cell cycle arrest in response until the defects are […]
Breast Reconstruction after Mastectomy
Breast cancer is always personal. As a physician who counsels women at different steps during the healing process, I am acutely aware of this undeniable fact. Every decision she makes from the point at which she is diagnosed with breast cancer will require her focused engagement and a physician who is central to understanding her need for clarity of options. It is an intimate relationship where trust is a requirement and every woman faced with the many unknowns ahead will […]
Breast Cancer History Research Paper
Breast cancer is a disease in which most commonly occurs in all women no matter their size, shape, race, or ethnicity. About one in eight women will be diagnosed with breast cancer every year, a fatal disease if not discovered early. Early detection of breast cancer is key so that cancerous cells found in the breast do not spread through other parts of the body. With an increasing prevalence in breast cancer today, the evolution of technology has been improved […]
New Healthcare Inventions on Breast Cancer
Abstract Background: The Ki67 labeling index (LI) for breast carcinoma is essential for therapy. It is determined by visual assessment under a microscope which is subjective, thus has limitations due to inter-observer variability. A standardized method for evaluating Ki67 LI is necessary to reduce subjectivity and improve precision. Therefore, automated Digital Image Analysis (DIA) has been attempted as a potential method for evaluating the Ki67 index. Materials and Method: We included 48 cases of invasive breast carcinoma in this study. […]
Understanding Breast Cancer
This paper will clarify what Breast Cancer is. It will explain the symptoms, treatment options, and other useful information regarding this disease. The first thing to know about Breast Cancer is understanding what it is. According to the Cancer.org website, breast cancer begins when cells in the bosom begin to spread out of control. The tumor that is formed from these cells may be detected on an x-ray or can be felt as a lump. Malignancy can advance into neighboring […]
Breast Cancer in African American Women
Summary Despite the fact that Caucasian women in the United States have a higher incidence rate of breast cancer than any other racial group, African-Americans succumb notably worse to the disease and record the highest mortality rate. To comprehend the barriers and challenges that predispose African-American women to these disparities, this research was conducted to get a better understanding from the perspective of oncologists. With diverse ethnicity and gender representation, the participation of seven medical, surgical and radiation oncologists that […]
Essential Breast Cancer Screening Techniques and their Complements
It is with great distress that each year a large number of females suffer and die from breast cancer. Medicine practitioners and researchers have been striving to save lives from breast cancer, and how they manage to do this includes two major parts—diagnosis and treatment. What comes first on the stage of diagnosis is the detection of tumor. Thus, the development of breast imaging techniques is at the highest priority for diagnosing breast cancer, and individuals’ focus is on earlier […]
Breast Cancer Prevention and Treatment
The human body is made up of cells. When a cell dies the body automatically replaces it with a new healthy cell, but sometimes the cell is not healthy and grows out of control. These cells group together and form a lump that can be seen on an x-ray. Breast cancer is a tumor in the cells of person’s breast. It can spread throughout the breast to the person’s lymph nodes and other parts of the body. Sometimes it occurs […]
Breast Cancer Diagnosis
I. Executive Summary Breast cancer is concerning a large number of female individuals worldwide. This disease comes from abnormally developed breast tissue, which usually begins in either lobules or ducts of the breast. Generally speaking, breast cancer is divided into two types—non-invasive and invasive. The core criteria to distinguish in between these two types of breast cancers is the location of cancer cells. Cancer cells remain on their initial positions for a non-invasive breast cancer, whereas they grow, or “invade”, […]
Understanding a Breast Cancer Diagnosis
Breast cancer is often known as an aggressive cancer. It forms when cells grow uncontrollably in the tissues of the breast, leading to a tumor. Over 190,000 individuals are diagnosed yearly (Cancer Center). Breast cancer is the second leading cause of death, and the rate increases every year in women, and occasionally in men. Over 12 percent of women in the United States of America will face breast cancer in their lifetime. It is the most common cause of death […]
Breast Cancer in the Era of Precision Medicine
Introduction: Precision medicine is concerned with the diagnosis of patients according to their biological, genetic, and molecular status. As cancer is a genetic disease, its treatment comes among the first medical disciplines as an application of precision medicine. Breast cancer is a highly complex, heterogeneous, and multifactorial disease; it is also one of the most common diseases among women in the world. Usually, there are no clear symptoms, so regular screening is important for early detection. Scientists recently started using […]
Exome Sequencing to Identify Rare Mutations Associated with Breast Cancer Susceptibility
Abstract Background - Breast cancer predisposition has been known to be caused by hereditary factors. New techniques particularly exome sequencing have allowed/ helped us to identify new and novel variants that exhibit a phenotype. Method - In this review we discuss the advantages of exome sequencing and how it could help in understanding the familial breast cancer. In particular, we will discuss about the studies by Noh et al.(1), Thompson et al.(2), and Kiiski et al.(3), on how they have […]
A Novel Therapeutic Strategy for HER2 Breast Cancer by Nanoparticles Combined with Macrophages
Abstract:In recent years, the cell membrane bionic nanoparticles as a new drug delivery system is widely used in small molecule drugs, vaccines and targeted delivery of macromolecular drugs, because of its inherited the specific receptors on the cell membrane and membrane proteins can be used to implement specific targeted delivery, and the tumor showed a good treatment effect on the disease such as model, this topic with a huge bite cell membrane of the role of tumor capture, chemical modification, […]
Essays About Breast Cancer Breast Cancer is one of the most common cancers in women and is a disease by which the cells in the breast area grow out of control. Breast cancer tends to begin in the ducts or lobules of a breast and there are different types of cancer. In the US alone 1 in 8 women will develop breast cancer at some stage in their lives. In many academic fields; from science to medicine the study of breast cancer and essays about breast cancer are required as part of the curriculum. An essay on breast cancer can seem daunting due to the amount of research and several varying scientific approaches used to talk about the topic. We offer essay examples, or research paper guidance and free essay samples. These can be used to gauge how to approach the topic and are an informative look at all factors that contribute to breast cancer and prevention. We also factor breast cancer awareness into our essay samples and ensure essays for both university and college build a strong foundation to understanding the disease, but also draw criticism when necessary and a strong conclusion on whatever element of breast cancer the focus of the essay is on.
1. Tell Us Your Requirements
2. Pick your perfect writer
3. Get Your Paper and Pay
Hi! I'm Amy, your personal assistant!
Don't know where to start? Give me your paper requirements and I connect you to an academic expert.
short deadlines
100% Plagiarism-Free
Certified writers
Fact sheets
- Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- HIV and AIDS
- Hypertension
- Mental disorders
- Top 10 causes of death
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- Data collection tools
- Global Health Observatory
- Insights and visualizations
- COVID excess deaths
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment case
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Fact sheets /
Breast cancer
- Breast cancer caused 670 000 deaths globally in 2022.
- Roughly half of all breast cancers occur in women with no specific risk factors other than sex and age.
- Breast cancer was the most common cancer in women in 157 countries out of 185 in 2022.
- Breast cancer occurs in every country in the world.
- Approximately 0.5–1% of breast cancers occur in men.
Breast cancer is a disease in which abnormal breast cells grow out of control and form tumours. If left unchecked, the tumours can spread throughout the body and become fatal.
Breast cancer cells begin inside the milk ducts and/or the milk-producing lobules of the breast. The earliest form (in situ) is not life-threatening and can be detected in early stages. Cancer cells can spread into nearby breast tissue (invasion). This creates tumours that cause lumps or thickening.
Invasive cancers can spread to nearby lymph nodes or other organs (metastasize). Metastasis can be life-threatening and fatal.
Treatment is based on the person, the type of cancer and its spread. Treatment combines surgery, radiation therapy and medications.
Scope of the problem
In 2022, there were 2.3 million women diagnosed with breast cancer and 670 000 deaths globally. Breast cancer occurs in every country of the world in women at any age after puberty but with increasing rates in later life. Global estimates reveal striking inequities in the breast cancer burden according to human development. For instance, in countries with a very high Human Development Index (HDI), 1 in 12 women will be diagnosed with breast cancer in their lifetime and 1 in 71 women die of it.
In contrast, in countries with a low HDI; while only 1 in 27 women is diagnosed with breast cancer in their lifetime, 1 in 48 women will die from it.
Who is at risk?
Female gender is the strongest breast cancer risk factor. Approximately 99% of breast cancers occur in women and 0.5–1% of breast cancers occur in men. The treatment of breast cancer in men follows the same principles of management as for women.
Certain factors increase the risk of breast cancer including increasing age, obesity, harmful use of alcohol, family history of breast cancer, history of radiation exposure, reproductive history (such as age that menstrual periods began and age at first pregnancy), tobacco use and postmenopausal hormone therapy. Approximately half of breast cancers develop in women who have no identifiable breast cancer risk factor other than gender (female) and age (over 40 years).
Family history of breast cancer increases the risk of breast cancer, but most women diagnosed with breast cancer do not have a known family history of the disease. Lack of a known family history does not necessarily mean that a woman is at reduced risk.
Certain inherited high penetrance gene mutations greatly increase breast cancer risk, the most dominant being mutations in the genes BRCA1, BRCA2 and PALB-2. Women found to have mutations in these major genes may consider risk reduction strategies such as surgical removal of both breasts or chemoprevention strategies.
Signs and symptoms
Most people will not experience any symptoms when the cancer is still early hence the importance of early detection.
Breast cancer can have combinations of symptoms, especially when it is more advanced. Symptoms of breast cancer can include:
- a breast lump or thickening, often without pain
- change in size, shape or appearance of the breast
- dimpling, redness, pitting or other changes in the skin
- change in nipple appearance or the skin surrounding the nipple (areola)
- abnormal or bloody fluid from the nipple.
People with an abnormal breast lump should seek medical care, even if the lump does not hurt.
Most breast lumps are not cancer. Breast lumps that are cancerous are more likely to be successfully treated when they are small and have not spread to nearby lymph nodes.
Breast cancers may spread to other areas of the body and trigger other symptoms. Often, the most common first detectable site of spread is to the lymph nodes under the arm although it is possible to have cancer-bearing lymph nodes that cannot be felt.
Over time, cancerous cells may spread to other organs including the lungs, liver, brain and bones. Once they reach these sites, new cancer-related symptoms such as bone pain or headaches may appear.
Treatment for breast cancer depends on the subtype of cancer and how much it has spread outside of the breast to lymph nodes (stages II or III) or to other parts of the body (stage IV).
Doctors combine treatments to minimize the chances of the cancer coming back (recurrence). These include:
- surgery to remove the breast tumour
- radiation therapy to reduce recurrence risk in the breast and surrounding tissues
- medications to kill cancer cells and prevent spread, including hormonal therapies, chemotherapy or targeted biological therapies.
Treatments for breast cancer are more effective and are better tolerated when started early and taken to completion.
Surgery may remove just the cancerous tissue (called a lumpectomy) or the whole breast (mastectomy). Surgery may also remove lymph nodes to assess the cancer’s ability to spread.
Radiation therapy treats residual microscopic cancers left behind in the breast tissue and/or lymph nodes and minimizes the chances of cancer recurring on the chest wall.
Advanced cancers can erode through the skin to cause open sores (ulceration) but are not necessarily painful. Women with breast wounds that do not heal should seek medical care to have a biopsy performed.
Medicines to treat breast cancers are selected based on the biological properties of the cancer as determined by special tests (tumour marker determination). The great majority of drugs used for breast cancer are already on the WHO Essential Medicines List (EML).
Lymph nodes are removed at the time of cancer surgery for invasive cancers. Complete removal of the lymph node bed under the arm (complete axillary dissection) in the past was thought to be necessary to prevent the spread of cancer. A smaller lymph node procedure called “sentinel node biopsy” is now preferred as it has fewer complications.
Medical treatments for breast cancers, which may be given before (“neoadjuvant”) or after (“adjuvant”) surgery, is based on the biological subtyping of the cancers. Certain subtypes of breast cancer are more aggressive than others such as triple negative (those that do not express estrogen receptor (ER), progesterone receptor (PR) or HER-2 receptor). Cancer that express the estrogen receptor (ER) and/or progesterone receptor (PR) are likely to respond to endocrine (hormone) therapies such as tamoxifen or aromatase inhibitors. These medicines are taken orally for 5–10 years and reduce the chance of recurrence of these “hormone-positive” cancers by nearly half. Endocrine therapies can cause symptoms of menopause but are generally well tolerated.
Cancers that do not express ER or PR are “hormone receptor negative” and need to be treated with chemotherapy unless the cancer is very small. The chemotherapy regimens available today are very effective in reducing the chances of cancer spread or recurrence and are generally given as outpatient therapy. Chemotherapy for breast cancer generally does not require hospital admission in the absence of complications.
Breast cancers that independently overexpress a molecule called the HER-2/neu oncogene (HER-2 positive) are amenable to treatment with targeted biological agents such as trastuzumab. When targeted biological therapies are given, they are combined with chemotherapy to make them effective at killing cancer cells.
Radiotherapy plays a very important role in treating breast cancer. With early-stage breast cancers, radiation can prevent a woman having to undergo a mastectomy. With later stage cancers, radiotherapy can reduce cancer recurrence risk even when a mastectomy has been performed. For advanced stages of breast cancer, in some circumstances, radiation therapy may reduce the likelihood of dying of the disease.
The effectiveness of breast cancer therapies depends on the full course of treatment. Partial treatment is less likely to lead to a positive outcome.
Global impact
Age-standardized breast cancer mortality in high-income countries dropped by 40% between the 1980s and 2020 (1) . Countries that have succeeded in reducing breast cancer mortality have been able to achieve an annual breast cancer mortality reduction of 2–4% per year.
The strategies for improving breast cancer outcomes depend on fundamental health system strengthening to deliver the treatments that are already known to work. These are also important for the management of other cancers and other non-malignant noncommunicable diseases (NCDs). For example, having reliable referral pathways from primary care facilities to district hospitals to dedicated cancer centres.
The establishment of reliable referral pathways from primary care facilities to secondary hospitals to dedicated cancer centres is the same approach as is required for the management of cervical cancer, lung cancer, colorectal cancer and prostate cancer. To that end, breast cancer is a so-called index disease whereby pathways are created that can be followed for the management of other cancers.
WHO response
The objective of the WHO Global Breast Cancer Initiative (GBCI) is to reduce global breast cancer mortality by 2.5% per year, thereby averting 2.5 million breast cancer deaths globally between 2020 and 2040. Reducing global breast cancer mortality by 2.5% per year would avert 25% of breast cancer deaths by 2030 and 40% by 2040 among women under 70 years of age. The three pillars toward achieving these objectives are: health promotion for early detection; timely diagnosis; and comprehensive breast cancer management.
By providing public health education to improve awareness among women of the signs and symptoms of breast cancer and, together with their families, understand the importance of early detection and treatment, more women would consult medical practitioners when breast cancer is first suspected, and before any cancer present is advanced. This is possible even in the absence of mammographic screening that is impractical in many countries at the present time.
- Age-standardization is a technique used to allow populations to be compared when the age profiles of the populations are quite different.
Global Breast Cancer Initiative
More on cancer
Coronavirus (COVID-19): Latest Updates | Visitation Policies Visitation Policies Visitation Policies Visitation Policies Visitation Policies | COVID-19 Testing | Vaccine Information Vaccine Information Vaccine Information
Health Encyclopedia
Breast cancer: introduction, what is cancer.
Cancer starts when cells in the body change (mutate) and grow out of control. Your body is made up of tiny building blocks called cells. Normal cells grow when your body needs them, and die when your body doesn't need them any longer. Cancer is made up of abnormal cells that grow even though your body doesn’t need them. In most types of cancer, the abnormal cells grow to form a lump or mass called a tumor.
Understanding the breast
The breast is made up of lobules and ducts. The lobules are the glands that can make milk. The ducts are thin tubes that carry the milk from the lobules to the nipple. The breast is also made of fat, connective tissue, lymph nodes, and blood vessels.
What is breast cancer?
Breast cancer is cancer that starts in cells in the breast. The ducts and the lobules are the two parts of the breast where cancer is most likely to start.
Breast cancer is one of the most common types of cancer in the U.S. Healthcare providers don't yet know exactly what causes it. Once breast cancer forms, cancer cells can spread to other parts of the body (metastasize), making it life-threatening. The good news is that breast cancer is often found early, when it's small and before it has spread.
There are many types of breast cancer. These are the most common types:
Ductal carcinoma. This is the most common type. It starts in the lining of the milk ducts. When breast cancer has not spread outside of the ducts, it's called ductal carcinoma in situ or intraductal carcinoma. This is the most common type of noninvasive breast cancer. Invasive ductal carcinoma is breast cancer that has spread beyond the walls of the breast ducts. It's the most common type of invasive breast cancer.
Invasive lobular carcinoma. This type starts in the milk-producing glands (lobules) and spreads outside the lobules.
Names of specific breast cancer types refer to whether they have spread or not:
Noninvasive (in situ) cancer is only in the ducts. It hasn’t spread to nearby areas. If not treated, it can grow over time into a more serious, invasive type of cancer. If you are diagnosed with noninvasive ductal carcinoma, your chances of surviving are very high if you don’t wait to treat it.
Invasive (infiltrating) cancer has the potential to spread to nearby areas. This type is much more serious than noninvasive cancer. When it starts to spread, it often invades nearby lymph nodes first. It can then spread to other parts of your body through your bloodstream and lymphatic system. Treatment for invasive cancer is often a more difficult, long-term process.
These are a few types of invasive breast cancers that you may hear about:
Inflammatory breast cancer. This is a rare form of invasive breast cancer. Often there is no lump or tumor. Instead, this cancer makes the skin of the breast look red and feel warm. The breast skin also looks thick and pitted, like an orange peel. It tends to be found in younger people and grows and spreads quickly.
Triple negative breast cancer. This is a type of breast cancer that doesn’t have estrogen receptors and progesterone receptors. It also doesn’t have an excess of the HER2 protein on the cancer cell surfaces. This type of breast cancer is most often found in younger people and in African-American people. It tends to grow and spread faster than most other types of breast cancer. Because these cancer cells don't have hormone receptors or excess HER2, medicines that target these changes don't work. The most common kind is triple-negative invasive ductal carcinoma.
Less common types of breast cancer include:
Paget disease. This is a very rare form of breast cancer that starts in the glands in the skin of the nipple. It grows slowly and occurs in only one nipple. Most people with Paget disease also have tumors in the same breast. This type causes symptoms that are like a skin infection. They include inflammation, redness, oozing, crusting, itching, and burning.
Angiosarcoma. This starts in the cells that line the blood vessels or lymph vessels. It may involve the breast tissue or the breast skin.
How breast cancer spreads
Breast cancer can spread by growing into nearby tissues in the breast. It can also spread when the cancer cells get into and travel through the blood or lymph systems. When this happens, cancer cells may be found in nearby lymph nodes, such as in the armpit. These lymph nodes are called axillary lymph nodes. They are often checked for cancer as part of the diagnosis process. If the cancer reaches these nodes, it may have spread to other parts of the body.
Breast cancer that has spread from the breast to other organs of the body is called metastatic breast cancer. When breast cancer spreads, it most often goes to the brain, bones, liver, or lungs.
A key factor in making a breast cancer diagnosis is finding out if it has spread.
Talking with your healthcare provider
If you have questions about breast cancer, talk with your healthcare provider. Your healthcare provider can help you understand more about this cancer.
Medical Reviewers:
- Jessica Gotwals RN BSN MPH
- Sabrina Felson MD
- Todd Gersten MD
- Ask a Medical Librarian Make an Appointment Physicians & Services Physicians who treat Breast Cancer
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 23 September 2019
- Breast cancer
- Nadia Harbeck 1 ,
- Frédérique Penault-Llorca 2 ,
- Javier Cortes 3 , 4 ,
- Michael Gnant 5 ,
- Nehmat Houssami 6 ,
- Philip Poortmans 7 , 8 ,
- Kathryn Ruddy 9 ,
- Janice Tsang 10 &
- Fatima Cardoso 11
Nature Reviews Disease Primers volume 5 , Article number: 66 ( 2019 ) Cite this article
90k Accesses
1560 Citations
448 Altmetric
Metrics details
- Cancer therapy
- Genetic predisposition to disease
- Radiotherapy
- Tumour biomarkers
Breast cancer is the most frequent malignancy in women worldwide and is curable in ~70–80% of patients with early-stage, non-metastatic disease. Advanced breast cancer with distant organ metastases is considered incurable with currently available therapies. On the molecular level, breast cancer is a heterogeneous disease; molecular features include activation of human epidermal growth factor receptor 2 (HER2, encoded by ERBB2 ), activation of hormone receptors (oestrogen receptor and progesterone receptor) and/or BRCA mutations. Treatment strategies differ according to molecular subtype. Management of breast cancer is multidisciplinary; it includes locoregional (surgery and radiation therapy) and systemic therapy approaches. Systemic therapies include endocrine therapy for hormone receptor-positive disease, chemotherapy, anti-HER2 therapy for HER2-positive disease, bone stabilizing agents, poly(ADP-ribose) polymerase inhibitors for BRCA mutation carriers and, quite recently, immunotherapy. Future therapeutic concepts in breast cancer aim at individualization of therapy as well as at treatment de-escalation and escalation based on tumour biology and early therapy response. Next to further treatment innovations, equal worldwide access to therapeutic advances remains the global challenge in breast cancer care for the future.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
92,52 € per year
only 92,52 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
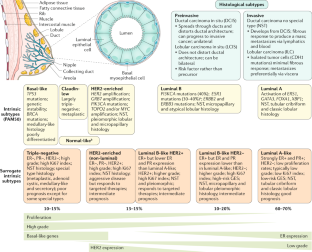
Similar content being viewed by others
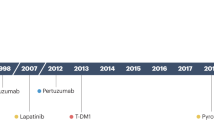
Management of patients with advanced-stage HER2-positive breast cancer: current evidence and future perspectives

A careful reassessment of anthracycline use in curable breast cancer
Emerging systemic therapy options beyond CDK4/6 inhibitors for hormone receptor-positive HER2-negative advanced breast cancer
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406 , 747–752 (2000).
Article CAS PubMed Google Scholar
Cardoso, F. et al. European Breast Cancer Conference manifesto on breast centres/units. Eur. J. Cancer 72 , 244–250 (2017).
Article PubMed Google Scholar
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68 , 394–424 (2018).
Bray, F. et al. Cancer Incidence in Five Continents: inclusion criteria, highlights from Volume X and the global status of cancer registration. Int. J. Cancer 137 , 2060–2071 (2015).
Mariotto, A. B., Etzioni, R., Hurlbert, M., Penberthy, L. & Mayer, M. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol. Biomark. Prev. 26 , 809–815 (2017).
Article Google Scholar
Ren, J.-X., Gong, Y., Ling, H., Hu, X. & Shao, Z.-M. Racial/ethnic differences in the outcomes of patients with metastatic breast cancer: contributions of demographic, socioeconomic, tumor and metastatic characteristics. Breast Cancer Res. Treat. 173 , 225–237 (2019).
Torre, L. A., Siegel, R. L., Ward, E. M. & Jemal, A. Global cancer incidence and mortality rates and trends — an update. Cancer Epidemiol. Biomark. Prev. 25 , 16–27 (2016).
Ginsburg, O. et al. The global burden of women’s cancers: a grand challenge in global health. Lancet 389 , 847–860 (2017).
Allemani, C. et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 385 , 977–1010 (2015).
Winters, S., Martin, C., Murphy, D. & Shokar, N. K. Breast cancer epidemiology, prevention, and screening. Prog. Mol. Biol. Transl Sci. 151 , 1–32 (2017).
Hossain, M. S., Ferdous, S. & Karim-Kos, H. E. Breast cancer in South. Asia: a Bangladeshi perspective. Cancer Epidemiol. 38 , 465–470 (2014).
PubMed Google Scholar
Leong, S. P. L. et al. Is breast cancer the same disease in Asian and western countries? World J. Surg. 34 , 2308–2324 (2010).
Article PubMed PubMed Central Google Scholar
Bhoo Pathy, N. et al. Breast cancer in a multi-ethnic Asian setting: results from the Singapore–Malaysia hospital-based breast cancer registry. Breast 20 , S75–S80 (2011).
Raina, V. et al. Clinical features and prognostic factors of early breast cancer at a major cancer center in North India. Indian J. Cancer 42 , 40 (2005).
Agarwal, G., Pradeep, P. V., Aggarwal, V., Yip, C.-H. & Cheung, P. S. Y. Spectrum of breast cancer in Asian women. World J. Surg. 31 , 1031–1040 (2007).
Li, C. I., Malone, K. E. & Daling, J. R. Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer Epidemiol. Biomark. Prev. 11 , 601–607 (2002).
Google Scholar
Wong, F. Y., Tham, W. Y., Nei, W. L., Lim, C. & Miao, H. Age exerts a continuous effect in the outcomes of Asian breast cancer patients treated with breast-conserving therapy. Cancer Commun. 38 , 39 (2018).
Kohler, B. A. et al. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J. Natl Cancer Inst . 107 , https://doi.org/10.1093/jnci/djv048 (2015).
DeSantis, C. E. et al. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women: Breast Cancer Statistics, 2015. CA Cancer J. Clin. 66 , 31–42 (2016).
DeSantis, C. E., Ma, J., Goding Sauer, A., Newman, L. A. & Jemal, A. Breast cancer statistics, 2017, racial disparity in mortality by state: Breast Cancer Statistics, 2017. CA Cancer J. Clin. 67 , 439–448 (2017).
Shiovitz, S. & Korde, L. A. Genetics of breast cancer: a topic in evolution. Ann. Oncol. 26 , 1291–1299 (2015).
CAS PubMed PubMed Central Google Scholar
Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58 209 women with breast cancer and 101 986 women without the disease. Lancet 358 , 1389–1399 (2001).
Brewer, H. R., Jones, M. E., Schoemaker, M. J., Ashworth, A. & Swerdlow, A. J. Family history and risk of breast cancer: an analysis accounting for family structure. Breast Cancer Res. Treat. 165 , 193–200 (2017).
Huen, M. S. Y., Sy, S. M. H. & Chen, J. BRCA1 and its toolbox for the maintenance of genome integrity. Nat. Rev. Mol. Cell Biol. 11 , 138–148 (2010).
Kuchenbaecker, K. B. et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 317 , 2402 (2017).
Balmana, J., Diez, O., Rubio, I. T. & Cardoso, F., On behalf of the ESMO Guidelines Working Group. BRCA in breast cancer: ESMO clinical practice guidelines. Ann. Oncol. 22 , vi31–vi34 (2011).
Paluch-Shimon, S. et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann. Oncol. 27 , v103–v110 (2016).
Daly, M. B. et al. Genetic/familial high-risk assessment: breast and ovarian, version 2.2015. J. Natl Compr. Cancer Netw. 14 , 153–162 (2016).
Forbes, C., Fayter, D., de Kock, S. & Quek, R. G. W. A systematic review of international guidelines and recommendations for the genetic screening, diagnosis, GENETIC COUNSELING and treatment of BRCA -mutated breast cancer. Cancer Manag. Res. 2019 , 2321–2337 (2019).
Robson, M. et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl. J. Med. 377 , 523–533 (2017).
Litton, J. K. et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N. Engl. J. Med. 379 , 753–763 (2018).
FDA. FDA approves olaparib germline BRCA-mutated metastatic breast cancer. Fda.gov https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-germline-brca-mutated-metastatic-breast-cancer (2018).
FDA. FDA approves talazoparib for gBRCAm HER2-negative locally advanced or metastatic breast cancer. Fda.gov https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-gbrcam-her2-negative-locally-advanced-or-metastatic-breast-cancer (2018).
Pasche, B. Recent advances in breast cancer genetics. Cancer Treat. Res. 141 , 1–10 (2008).
Cobain, E. F., Milliron, K. J. & Merajver, S. D. Updates on breast cancer genetics: clinical implications of detecting syndromes of inherited increased susceptibility to breast cancer. Semin. Oncol. 43 , 528–535 (2016).
Crawford, B. et al. Multi-gene panel testing for hereditary cancer predisposition in unsolved high-risk breast and ovarian cancer patients. Breast Cancer Res. Treat. 163 , 383–390 (2017).
Article CAS PubMed PubMed Central Google Scholar
Taylor, A. et al. Consensus for genes to be included on cancer panel tests offered by UK genetics services: guidelines of the UK Cancer Genetics Group. J. Med. Genet. 55 , 372–377 (2018).
Althuis, M. D., Dozier, J. M., Anderson, W. F., Devesa, S. S. & Brinton, L. A. Global trends in breast cancer incidence and mortality 1973–1997. Int. J. Epidemiol. 34 , 405–412 (2005).
Colditz, G. A., Sellers, T. A. & Trapido, E. Epidemiology — identifying the causes and preventability of cancer? Nat. Rev. Cancer 6 , 75–83 (2006).
Britt, K., Ashworth, A. & Smalley, M. Pregnancy and the risk of breast cancer. Endocr. Relat. Cancer 14 , 907–933 (2007).
Siwko, S. K. et al. Evidence that an early pregnancy causes a persistent decrease in the number of functional mammary epithelial stem cells — implications for pregnancy-induced protection against breast cancer. Stem Cells 26 , 3205–3209 (2008).
Hilakivi-Clarke, L., de Assis, S. & Warri, A. Exposures to synthetic estrogens at different times during the life, and their effect on breast cancer risk. J. Mammary Gland. Biol. Neoplasia 18 , 25–42 (2013).
Danaei, G., Vander Hoorn, S., Lopez, A. D., Murray, C. J. & Ezzati, M. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet 366 , 1784–1793 (2005).
Chen, W. Y., Rosner, B., Hankinson, S. E., Colditz, G. A. & Willett, W. C. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA 306 , 1884 (2011).
Singletary, K. W. & Gapstur, S. M. Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. JAMA 286 , 2143 (2001).
Smith-Warner, S. A. et al. Alcohol and breast cancer in women: a pooled analysis of cohort studies. JAMA 279 , 535 (1998).
Bandera, E. V., Maskarinec, G., Romieu, I. & John, E. M. Racial and ethnic disparities in the impact of obesity on breast cancer risk and survival: a global perspective. Adv. Nutr. 6 , 803–819 (2015).
Picon-Ruiz, M., Morata-Tarifa, C., Valle-Goffin, J. J., Friedman, E. R. & Slingerland, J. M. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention: breast cancer, inflammation, and obesity. CA Cancer J. Clin. 67 , 378–397 (2017).
Shieh, Y. et al. Body mass index, mammographic density, and breast cancer risk by estrogen receptor subtype. Breast Cancer Res. 21 , 48 (2019).
Suzuki, Y., Tsunoda, H., Kimura, T. & Yamauchi, H. BMI change and abdominal circumference are risk factors for breast cancer, even in Asian women. Breast Cancer Res. Treat. 166 , 919–925 (2017).
Del Pup, L., Codacci-Pisanelli, G. & Peccatori, F. Breast cancer risk of hormonal contraception: counselling considering new evidence. Crit. Rev. Oncol. Hematol. 137 , 123–130 (2019).
Busund, M. et al. Progestin-only and combined oral contraceptives and receptor-defined premenopausal breast cancer risk: the Norwegian Women and Cancer Study. Int. J. Cancer 142 , 2293–2302 (2018).
Mørch, L. S. et al. Contemporary hormonal contraception and the risk of breast cancer. N. Engl. J. Med. 377 , 2228–2239 (2017).
Ganz, P. A. et al. Supportive care after curative treatment for breast cancer (survivorship care): resource allocations in low- and middle-income countries. A Breast Health Global Initiative 2013 consensus statement. Breast 22 , 606–615 (2013).
Burris, J. L., Armeson, K. & Sterba, K. R. A closer look at unmet needs at the end of primary treatment for breast cancer: a longitudinal pilot study. Behav. Med. 41 , 69–76 (2015).
Coughlin, S. S., Yoo, W., Whitehead, M. S. & Smith, S. A. Advancing breast cancer survivorship among African-American women. Breast Cancer Res. Treat. 153 , 253–261 (2015).
Bodai, B. Breast cancer survivorship: a comprehensive review of long-term medical issues and lifestyle recommendations. Perm. J. 19 , 48–79 (2015).
Ho, P. J., Gernaat, S. A. M., Hartman, M. & Verkooijen, H. M. Health-related quality of life in Asian patients with breast cancer: a systematic review. BMJ Open 8 , e020512 (2018).
Miyashita, M. et al. Unmet information needs and quality of life in young breast cancer survivors in japan. Cancer Nurs. 38 , E1–E11 (2015).
Bombonati, A. & Sgroi, D. C. The molecular pathology of breast cancer progression. J. Pathol. 223 , 307–317 (2011).
Ellis, M. J. et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature 486 , 353–360 (2012).
Lopez-Garcia, M. A., Geyer, F. C., Lacroix-Triki, M., Marchió, C. & Reis-Filho, J. S. Breast cancer precursors revisited: molecular features and progression pathways: molecular evolution of breast cancer. Histopathology 57 , 171–192 (2010).
Nik-Zainal, S. et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature 534 , 47–54 (2016).
Yates, L. R. & Desmedt, C. Translational genomics: practical applications of the genomic revolution in breast cancer. Clin. Cancer Res. 23 , 2630–2639 (2017).
Heitzer, E., Haque, I. S., Roberts, C. E. S. & Speicher, M. R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 20 , 71–88 (2019).
Ediriweera, M. K., Tennekoon, K. H. & Samarakoon, S. R. Emerging role of histone deacetylase inhibitors as anti-breast-cancer agents. Drug Discov. Today 24 , 685–702 (2019).
Munster, P. N. et al. A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. Br. J. Cancer 104 , 1828–1835 (2011).
Zhou, Y., Wang, Y., Zhang, K., Zhu, J. & Ning, Z. Reverse effect of chidamide on endocrine resistance in estrogen receptor-positive breast cancer. J. Shenzhen Univ. Sci. Eng. 35 , 339 (2018).
Jiang, Z. et al. Phase III trial of chidamide, a subtype-selective histone deacetylase (HDAC) inhibitor, in combination with exemestane in patients with hormone receptor-positive advanced breast cancer [abstract]. Ann. Oncol. 29 , 283O_PR (2018).
Williams, C. & Lin, C.-Y. Oestrogen receptors in breast cancer: basic mechanisms and clinical implications. Ecancermedicalscience 7 , 370 (2013).
PubMed PubMed Central Google Scholar
Levin, E. R. & Pietras, R. J. Estrogen receptors outside the nucleus in breast cancer. Breast Cancer Res. Treat. 108 , 351–361 (2008).
Santen, R. J. Clinical review: effect of endocrine therapies on bone in breast cancer patients. J. Clin. Endocrinol. Metab. 96 , 308–319 (2011).
Ruffell, B. et al. Leukocyte composition of human breast cancer. Proc. Natl Acad. Sci. USA 109 , 2796–2801 (2012).
Solinas, C., Carbognin, L., De Silva, P., Criscitiello, C. & Lambertini, M. Tumor-infiltrating lymphocytes in breast cancer according to tumor subtype: current state of the art. Breast 35 , 142–150 (2017).
Nagarajan, D. & McArdle, S. Immune landscape of breast cancers. Biomedicines 6 , 20 (2018).
Article PubMed Central CAS Google Scholar
Savas, P. et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat. Rev. Clin. Oncol. 13 , 228–241 (2016).
Dieci, M. V. et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin. Cancer Biol. 52 , 16–25 (2018).
Boudreau, A., van’t Veer, L. J. & Bissell, M. J. An ‘elite hacker’: breast tumors exploit the normal microenvironment program to instruct their progression and biological diversity. Cell Adhes. Migr. 6 , 236–248 (2012).
Smyth, M. J., Dunn, G. P. & Schreiber, R. D. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv. Immunol. 90 , 1–50 (2006).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331 , 1565–1570 (2011).
Buonomo, O. C. et al. New insights into the metastatic behavior after breast cancer surgery, according to well-established clinicopathological variables and molecular subtypes. PLOS ONE 12 , e0184680 (2017).
Article PubMed PubMed Central CAS Google Scholar
Gobbini, E. et al. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur. J. Cancer 96 , 17–24 (2018).
Santé Publique France. Breast cancer [French]. Santepubliquefrance.fr https://www.santepubliquefrance.fr/maladies-et-traumatismes/cancers/cancer-du-sein (2019).
Zhang, K. et al. Clinical value of circulating ESR1 mutations for patients with metastatic breast cancer: a meta-analysis. Cancer Manag. Res. 10 , 2573–2580 (2018).
Yates, L. R. et al. Genomic evolution of breast cancer metastasis and relapse. Cancer Cell 32 , 169–184.e7 (2017).
Gingras, I., Salgado, R. & Ignatiadis, M. Liquid biopsy: will it be the ‘magic tool’ for monitoring response of solid tumors to anticancer therapies? Curr. Opin. Oncol. 27 , 560–567 (2015).
Aurilio, G. et al. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur. J. Cancer 50 , 277–289 (2014).
Independent, U. K. Panel on breast cancer screening. the benefits and harms of breast cancer screening: an independent review. Lancet 380 , 1778–1786 (2012).
Nelson, H. D. et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann. Intern. Med. 164 , 244–255 (2016).
Lauby-Secretan, B. et al. Breast-cancer screening — viewpoint of the IARC Working Group. N. Engl. J. Med. 372 , 2353–2358 (2015).
Houssami, N. Overdiagnosis of breast cancer in population screening: does it make breast screening worthless? Cancer Biol. Med. 14 , 1–8 (2017).
Suhrke, P. et al. Effect of mammography screening on surgical treatment for breast cancer in Norway: comparative analysis of cancer registry data. BMJ 343 , d4692–d4692 (2011).
Stang, A., Kääb-Sanyal, V., Hense, H.-W., Becker, N. & Kuss, O. Effect of mammography screening on surgical treatment for breast cancer: a nationwide analysis of hospitalization rates in Germany 2005–2009. Eur. J. Epidemiol. 28 , 689–696 (2013).
IARC Handbooks of Cancer Prevention. Breast Cancer Screening (Volume 15). Iarc.fr http://publications.iarc.fr/Book-And-Report-Series/Iarc-Handbooks-Of-Cancer-Prevention/Breast-Cancer-Screening-2016 (2016).
Nelson, H. D. et al. Harms of breast cancer screening: systematic review to update the 2009 U.S. Preventive Services Task Force recommendation. Ann. Intern. Med. 164 , 256–267 (2016).
Carter, J. L., Coletti, R. J. & Harris, R. P. Quantifying and monitoring overdiagnosis in cancer screening: a systematic review of methods. BMJ 350 , g7773 (2015).
Saslow, D. et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J. Clin. 57 , 75–89 (2007).
Phi, X.-A. et al. Magnetic resonance imaging improves breast screening sensitivity in BRCA mutation carriers age ≥ 50 years: evidence from an individual patient data meta-analysis. J. Clin. Oncol. 33 , 349–356 (2015).
Sardanelli, F. et al. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur. J. Cancer 46 , 1296–1316 (2010).
Melnikow, J. et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the U.S. preventive services task force. Ann. Intern. Med. 164 , 268–278 (2016).
Houssami, N. & Lee, C. I. The impact of legislation mandating breast density notification — review of the evidence. Breast 42 , 102–112 (2018).
Marinovich, M. L., Hunter, K. E., Macaskill, P. & Houssami, N. Breast cancer screening using tomosynthesis or mammography: a meta-analysis of cancer detection and recall. J. Natl Cancer Inst. 110 , 942–949 (2018).
Irwig, L., Macaskill, P. & Houssami, N. Evidence relevant to the investigation of breast symptoms: the triple test. Breast 11 , 215–220 (2002).
Houssami, N., Ciatto, S., Turner, R. M., Cody, H. S. & Macaskill, P. Preoperative ultrasound-guided needle biopsy of axillary nodes in invasive breast cancer: meta-analysis of its accuracy and utility in staging the axilla. Ann. Surg. 254 , 243–251 (2011).
Morrow, M., Waters, J. & Morris, E. MRI for breast cancer screening, diagnosis, and treatment. Lancet 378 , 1804–1811 (2011).
Srigley, J. R. et al. Standardized synoptic cancer pathology reporting: a population-based approach. J. Surg. Oncol. 99 , 517–524 (2009).
World Heath Organisation. WHO Classification of Tumours of the Breast, Fourth Edition. (World Health Organization, 2012).
Elston, C. W. & Ellis, I. O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19 , 403–410 (1991).
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Nccn.org https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (2018).
Curigliano, G. et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann. Oncol. 28 , 1700–1712 (2017).
Senkus, E. et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 24 (Suppl. 6), vi7-vi23 (2013).
Hammond, M. E. H. et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 28 , 2784–2795 (2010).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J. Clin. Oncol. 36 , 2105–2122 (2018).
Dowsett, M. et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J. Natl Cancer Inst. 103 , 1656–1664 (2011).
Rakha, E. A. et al. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer 118 , 3670–3680 (2012).
Barrio, A. V. & Morrow, M. Appropriate margin for lumpectomy excision of invasive breast cancer. Chin. Clin. Oncol. 5 , 35–35 (2016).
Chung, A. et al. Impact of consensus guidelines by the Society of Surgical Oncology and the American Society for Radiation Oncology on margins for breast-conserving surgery in stages 1 and 2 invasive breast cancer. Ann. Surg. Oncol. 22 , 422–427 (2015).
Schulman, A. M. et al. Reexcision surgery for breast cancer: an analysis of the American Society of Breast Surgeons (ASBrS) Mastery SM database following the SSO-ASTRO “no ink on tumor” guidelines. Ann. Surg. Oncol. 24 , 52–58 (2017).
Morrow, M. et al. Society of Surgical Oncology–American Society for Radiation Oncology–American Society of Clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. Pract. Radiat. Oncol. 6 , 287–295 (2016).
Morrow, M. et al. Society of Surgical Oncology–American Society for Radiation Oncology–American Society of Clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. J. Clin. Oncol. 34 , 4040–4046 (2016).
Moran, M. S. et al. Society of Surgical Oncology–American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 88 , 553–564 (2014).
Amin, M. B. et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more ‘personalized’ approach to cancer staging. CA Cancer J. Clin. 67 , 93–99 (2017).
Tao, L. et al. Breast cancer mortality in older and younger breast cancer patients in California. Cancer Epidemiol. Biomark. Prev. 28 , 303–310 (2018).
Salgado, R. et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann. Oncol. 26 , 259–271 (2015).
Green, A. R. et al. Nottingham Prognostic Index Plus: validation of a clinical decision making tool in breast cancer in an independent series. J. Pathol. Clin. Res. 2 , 32–40 (2016).
Candido dos Reis, F. J. et al. An updated PREDICT breast cancer prognostication and treatment benefit prediction model with independent validation. Breast Cancer Res. 19 , 58 (2017).
Phung, M. T., Tin Tin, S. & Elwood, J. M. Prognostic models for breast cancer: a systematic review. BMC Cancer 19 , 230 (2019).
Senkus, E. et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 26 (Suppl. 5), v8-v30 (2015).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384 , 164–172 (2014).
Cardoso, F. et al. 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N. Engl. J. Med. 375 , 717–729 (2016).
Sparano, J. A. et al. Prospective validation of a 21-gene expression assay in breast cancer. N. Engl. J. Med. 373 , 2005–2014 (2015).
Sparano, J. A. et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N. Engl. J. Med. 379 , 111–121 (2018).
Harris, L. N. et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 34 , 1134–1150 (2016).
Krop, I. et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J. Clin. Oncol. 35 , 2838–2847 (2017).
Nitz, U. et al. West German Study PlanB trial: adjuvant four cycles of epirubicin and cyclophosphamide plus docetaxel versus six cycles of docetaxel and cyclophosphamide in HER2-negative early breast cancer. J. Clin. Oncol. 37 , 799–808 (2019).
Sestak, I. Risk stratification in early breast cancer in premenopausal and postmenopausal women: integrating genomic assays with clinicopathological features. Curr. Opin. Oncol. 1 , 29–34 (2018).
McLaughlin, S. A. Surgical management of the breast: breast conservation therapy and mastectomy. Surg. Clin. North Am. 93 , 411–428 (2013).
Margenthaler, J. A. & Ollila, D. W. Breast conservation therapy versus mastectomy: shared decision-making strategies and overcoming decisional conflicts in your patients. Ann. Surg. Oncol. 23 , 3133–3137 (2016).
Buchholz, T. A., Mittendorf, E. A. & Hunt, K. K. Surgical considerations after neoadjuvant chemotherapy: breast conservation therapy. J. Natl Cancer Inst. Monogr. 2015 , 11–14 (2015).
Houssami, N., Macaskill, P., Luke Marinovich, M. & Morrow, M. The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: a meta-analysis. Ann. Surg. Oncol. 21 , 717–730 (2014).
Morrow, M., Harris, J. R. & Schnitt, S. J. Surgical margins in lumpectomy for breast cancer — bigger is not better. N. Engl. J. Med. 367 , 79–82 (2012). This commentary and the meta-analysis by Houssami et al. (2014) settled the decade-long discussions about surgical resection margins and are, therefore, landmark contributions.
Tan, M. P., Sitoh, N. Y. & Sim, A. S. The value of intraoperative frozen section analysis for margin status in breast conservation surgery in a nontertiary institution. Int. J. Breast Cancer https://doi.org/10.1155/2014/715404 (2014).
Boughey, J. C. et al. Impact of analysis of frozen-section margin on reoperation rates in women undergoing lumpectomy for breast cancer: evaluation of the National Surgical Quality Improvement Program data. Surgery 156 , 190–197 (2014).
Haloua, M. H. et al. A systematic review of oncoplastic breast-conserving surgery: current weaknesses and future prospects. Ann. Surg. 257 , 609–620 (2013).
Benelli, L. A new periareolar mammaplasty: the ‘round block’ technique. Aesthetic Plast. Surg. 14 , 93–100 (1990).
Clough, K. B., Kaufman, G. J., Nos, C., Buccimazza, I. & Sarfati, I. M. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann. Surg. Oncol. 17 , 1375–1391 (2010).
Yao, K., Winchester, D. J., Czechura, T. & Huo, D. Contralateral prophylactic mastectomy and survival: report from the national cancer data base, 1998–2002. Breast Cancer Res. Treat. 142 , 465–476 (2013).
Vila, J., Gandini, S. & Gentilini, O. Overall survival according to type of surgery in young (≤40 years) early breast cancer patients: a systematic meta-analysis comparing breast-conserving surgery versus mastectomy. Breast 24 , 175–181 (2015).
Lucci, A. et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group trial Z0011. J. Clin. Oncol. 25 , 3657–3663 (2007).
Krag, D. N. et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 11 , 927–933 (2010). This large clinical trial confirms that there is no overall survival difference between sentinel lymph node biopsy and axillary lymph node dissection.
Veronesi, U. et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N. Engl. J. Med. 349 , 546–553 (2003).
Giuliano, A. E. et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 randomized trial. Ann. Surg. 264 , 413–420 (2016).
Balic, M., Thomssen, C., Würstlein, R., Gnant, M. & Harbeck, N. St. Gallen/Vienna 2019: a brief summary of the consensus discussion on the optimal primary breast cancer treatment. Breast Care 14 , 1–8 (2019).
Kaidar-Person, O., Meattini, I. & Poortmans, P. M. P. Between uncertainties and overtreatment. Int. J. Radiat. Oncol. 104 , 15–16 (2019).
Kuehn, T. et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 14 , 609–618 (2013).
King, T. A. & Morrow, M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat. Rev. Clin. Oncol. 12 , 335–343 (2015).
Giuliano, A. E. et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305 , 569–575 (2011).
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378 , 1707–1716 (2011). This meta-analysis underlines that the contribution of radiation therapy should always be the standard approach for breast-conserving therapy .
Article CAS Google Scholar
EBCTCG (Early Breast Cancer Trialists’ Collaborative Group). Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 383 , 2127–2135 (2014). This meta-analysis helps us to better identify those patients who would benefit most from radiation therapy after mastectomy .
Jatoi, I., Benson, J. R. & Kunkler, I. Hypothesis: can the abscopal effect explain the impact of adjuvant radiotherapy on breast cancer mortality? NPJ Breast Cancer 4 , 8 (2018).
Bartelink, H. et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 16 , 47–56 (2015).
Poortmans, P. Postmastectomy radiation in breast cancer with one to three involved lymph nodes: ending the debate. Lancet 383 , 2104–2106 (2014).
Poortmans, P. M. et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N. Engl. J. Med. 373 , 317–327 (2015).
Whelan, T. J. et al. Regional nodal irradiation in early-stage breast cancer. N. Engl. J. Med. 373 , 307–316 (2015).
Thorsen, L. B. J. et al. DBCG-IMN: a population-based cohort study on the effect of internal mammary node irradiation in early node-positive breast cancer. J. Clin. Oncol. 34 , 314–320 (2016).
Curigliano, G. et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann. Oncol. 29 , 2153–2153 (2018).
Oliai, C. & Hurvitz, S. A. The debate over post-mastectomy radiotherapy should continue: breast cancer. Nat. Rev. Clin. Oncol. 12 , 567–568 (2015).
Recht, A. et al. Postmastectomy radiotherapy: an American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology focused guideline update. Ann. Surg. Oncol. 24 , 38–51 (2017).
Dodwell, D. et al. Abstract GS4-02: regional lymph node irradiation in early stage breast cancer: an EBCTCG meta-analysis of 13,000 women in 14 trials. in General Session Abstracts GS4-02-GS4-02 https://doi.org/10.1158/1538-7445.SABCS18-GS4-02 (American Association for Cancer Research, 2019).
Kunkler, I. H., Canney, P., van Tienhoven, G. & Russell, N. S. MRC/EORTC (BIG 2-04) SUPREMO Trial Management Group. Elucidating the role of chest wall irradiation in ‘intermediate-risk’. breast cancer: The MRC/EORTC SUPREMO trial. Clin. Oncol. R. Coll. Radiol. 20 , 31–34 (2008).
CAS PubMed Google Scholar
Poortmans, P., Aznar, M. & Bartelink, H. Quality indicators for breast cancer: revisiting historical evidence in the context of technology changes. Semin. Radiat. Oncol. 22 , 29–39 (2012).
Osman, S. O. S., Hol, S., Poortmans, P. M. & Essers, M. Volumetric modulated arc therapy and breath-hold in image-guided locoregional left-sided breast irradiation. Radiother. Oncol. 112 , 17–22 (2014).
Essers, M., Poortmans, P. M., Verschueren, K., Hol, S. & Cobben, D. C. P. Should breathing adapted radiotherapy also be applied for right-sided breast irradiation? Acta Oncol. 55 , 460–465 (2016).
Poortmans, P. M. P., Arenas, M. & Livi, L. Over-irradiation. Breast 31 , 295–302 (2017).
Blamey, R. W. et al. Radiotherapy or tamoxifen after conserving surgery for breast cancers of excellent prognosis: British Association of Surgical Oncology (BASO) II trial. Eur. J. Cancer 49 , 2294–2302 (2013).
McGuire, S. E. et al. Postmastectomy radiation improves the outcome of patients with locally advanced breast cancer who achieve a pathologic complete response to neoadjuvant chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 68 , 1004–1009 (2007).
Mamounas, E. P. et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of national surgical adjuvant breast and bowel project B-18 and B-27. J. Clin. Oncol. 30 , 3960–3966 (2012).
Krug, D. et al. Individualization of post-mastectomy radiotherapy and regional nodal irradiation based on treatment response after neoadjuvant chemotherapy for breast cancer: a systematic review. Strahlenther. Onkol. 194 , 607–618 (2018).
Amoroso, V. et al. International Expert Consensus on Primary Systemic Therapy in the Management of Early Breast Cancer: Highlights of the Fifth Symposium on Primary Systemic Therapy in the Management of Operable Breast Cancer, Cremona, Italy (2013). J. Natl Cancer Inst. Monogr. 2015 , 90–96 (2015).
Offersen, B. V. et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer, version 1.1. Radiother. Oncol. 118 , 205–208 (2016).
Haviland, J. S. et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 14 , 1086–1094 (2013).
Whelan, T. J. et al. Long-term results of hypofractionated radiation therapy for breast cancer. N. Engl. J. Med. 362 , 513–520 (2010).
Wang, S.-L. et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 20 , 352–360 (2019).
Brouwers, P. J. A. M. et al. Predictors for poor cosmetic outcome in patients with early stage breast cancer treated with breast conserving therapy: results of the Young Boost trial. Radiother. Oncol. 128 , 434–441 (2018).
Polgár, C. et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the groupe européen de curiethérapie-european society for therapeutic radiology and oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother. Oncol. 94 , 264–273 (2010).
Correa, C. et al. Accelerated partial breast irradiation: executive summary for the update of an ASTRO Evidence-Based. Consensus Statement. Pract. Radiat. Oncol. 7 , 73–79 (2017).
Miranda, F. A. et al. Accelerated partial breast irradiation: current status with a focus on clinical practice. Breast J. https://doi.org/10.1111/tbj.13164 (2018).
Marta, G. N. et al. Effectiveness of different accelerated partial breast irradiation techniques for the treatment of breast cancer patients: systematic review using indirect comparisons of randomized clinical trials. Rep. Pract. Oncol. Radiother. 24 , 165–174 (2019).
Veronesi, U. et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol. 14 , 1269–1277 (2013).
Vaidya, J. S. et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 383 , 603–613 (2014).
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378 , 771–784 (2011).
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomized trials. Lancet 379 , 432–444 (2012). This meta-analysis demonstrates the benefits of adjuvant chemotherapy in early breast cancer .
Rastogi, P. et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J. Clin. Oncol. 26 , 778–785 (2008).
Francis, P. A. et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N. Engl. J. Med. 379 , 122–137 (2018).
Gnant, M. et al. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann. Oncol. 26 , 313–320 (2015).
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386 , 1341–1352 (2015). This meta-analysis demonstrates the benefit of the two individual options for adjuvant endocrine therapy in postmenopausal patients with early breast cancer .
Pan, H. et al. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med. 377 , 1836–1846 (2017).
Gray, R. et al. Increasing the dose density of adjuvant chemotherapy by shortening intervals between courses or by sequential drug administration significantly reduces both disease recurrence and breast cancer mortality: an EBCTCG meta-analysis of 21,000 women in 16 randomised trials [abstract]. SABCS GS1-GS01 (2018).
Finn, R. S. et al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 375 , 1925–1936 (2016).
Hortobagyi, G. N. et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N. Engl. J. Med. 375 , 1738–1748 (2016).
Goetz, M. P. et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J. Clin. Oncol. 35 , 3638–3646 (2017).
Mackey, J. R. et al. Long-term outcomes after adjuvant treatment of sequential versus combination docetaxel with doxorubicin and cyclophosphamide in node-positive breast cancer: BCIRG-005 randomized trial. Ann. Oncol. 27 , 1041–1047 (2016).
Del Mastro, L. et al. Fluorouracil and dose-dense chemotherapy in adjuvant treatment of patients with early-stage breast cancer: an open-label, 2×2 factorial, randomised phase 3 trial. Lancet 385 , 1863–1872 (2015).
Article PubMed CAS Google Scholar
Blum, J. L. et al. Anthracyclines in early breast cancer: the ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J. Clin. Oncol. 35 , 2647–2655 (2017).
Gray, R. et al. Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet 393 , 1440–1452 (2019).
Gianni, L. et al. 5-Year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 17 , 791–800 (2016).
von Minckwitz, G. et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 380 , 617–628 (2018).
von Minckwitz, G. et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N. Engl. J. Med. 377 , 122–131 (2017).
Martin, M. et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 18 , 1688–1700 (2017).
Tolaney, S. M. et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N. Engl. J. Med. 372 , 134–141 (2015).
Tolaney, S. M. et al. Seven-year (yr) follow-up of adjuvant paclitaxel (T) and trastuzumab (H) (APT trial) for node-negative, HER2-positive breast cancer (BC). J. Clin. Oncol. 35 , 511–511 (2017).
Earl, H. M. et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet 393 , 2599–2612 (2019).
Pivot, X. et al. Either 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 14 , 741–748 (2013).
Joensuu, H. et al. Effect of adjuvant trastuzumab for a duration of 9 weeks vs 1 year with concomitant chemotherapy for early human epidermal growth factor receptor 2–positive breast cancer: the SOLD randomized clinical trial. JAMA Oncol. 4 , 1199 (2018).
Piccart-Gebhart, M. J. et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 353 , 1659–1672 (2005).
Goldhirsch, A. et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet 382 , 1021–1028 (2013).
Hahnen, E. et al. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: secondary analysis of the GeparSixto randomized clinical trial. JAMA Oncol. 3 , 1378–1385 (2017).
Sikov, W. M. et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J. Clin. Oncol. 33 , 13–21 (2015).
Masuda, N. et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N. Engl. J. Med. 376 , 2147–2159 (2017).
Gnant, M. et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 386 , 433–443 (2015).
Gnant, M. et al. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 20 , 339–351 (2019).
Coleman, R. E. et al. Adjuvant denosumab in early breast cancer: first results from the international multicenter randomized phase III placebo controlled D-CARE study [abstract]. J. Clin. Oncol. 36 (Suppl.), a501 (2018).
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 386 , 1353–1361 (2015).
Coleman, R. E. et al. Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer. 10 years follow-up of the AZURE randomized clinical trial (BIG 01/04). J. Bone Oncol. 13 , 123–135 (2018).
Cardoso, F. et al. 4th ESO–ESMO international consensus guidelines for advanced breast cancer (ABC 4)†. Ann. Oncol. 29 , 1634–1657 (2018).
Golse, N. & Adam, R. Liver metastases from breast cancer: what role for surgery? Indications and results. Clin. Breast Cancer 17 , 256–265 (2017).
Xie, Y. et al. Surgery of the primary tumor improves survival in women with stage IV breast cancer in southwest China: a retrospective analysis. Medicine 96 , e7048 (2017).
Shien, T. & Doihara, H. Resection of the primary tumor in stage IV breast cancer. World J. Clin. Oncol. 5 , 82–85 (2014).
Badwe, R. et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol. 16 , 1380–1388 (2015).
Soran, A., Ozbas, S., Kelsey, S. F. & Gulluoglu, B. M. Randomized trial comparing locoregional resection of primary tumor with no surgery in stage IV breast cancer at the presentation (Protocol MF07-01): a study of Turkish Federation of the National Societies for Breast Diseases. Breast J. 15 , 399–403 (2009).
Fitzal, F. et al. Impact of breast surgery in primary metastasized breast cancer: outcomes of the prospective randomized phase III ABCSG-28 POSYTIVE Trial. Ann. Surg . https://doi.org/10.1097/SLA.0000000000002771 (2018).
Barinoff, J. et al. Primary metastatic breast cancer in the era of targeted therapy — prognostic impact and the role of breast tumour surgery. Eur. J. Cancer 83 , 116–124 (2017).
Shien, T. et al. A randomized controlled trial comparing primary tumor resection plus systemic therapy with systemic therapy alone in metastatic breast cancer (JCOG1017 PRIM-BC). J. Clin. Oncol. 35 , TPS588–TPS588 (2017).
Cameron, D. Removing the primary tumour in metastatic breast cancer. Lancet Oncol. 16 , 1284–1285 (2015).
Dare, A. J. et al. Surgical Services for Cancer Care. in Cancer: Disease Control Priorities , Third Edition (Volume 3) (eds. Gelband, H., Jha, P., Sankaranarayanan, R. & Horton, S.) (The International Bank for Reconstruction and Development/The World Bank, 2015).
Phillips, C., Jeffree, R. & Khasraw, M. Management of breast cancer brain metastases: a practical review. Breast 31 , 90–98 (2017).
Thavarajah, N. et al. Continued success in providing timely palliative radiation therapy at the rapid response radiotherapy program: a review of 2008–2012. Curr. Oncol. 20 , e206–e211 (2013).
Chow, E. et al. Single versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol. 15 , 164–171 (2014).
Sologuren, I., Rodríguez-Gallego, C. & Lara, P. C. Immune effects of high dose radiation treatment: implications of ionizing radiation on the development of bystander and abscopal effects. Transl Cancer Res. 3 , 18-31–31 (2014).
Morgan, S. C. & Parker, C. C. Local treatment of metastatic cancer — killing the seed or disturbing the soil? Nat. Rev. Clin. Oncol. 8 , 504–506 (2011).
Morgan, S., Caudrelier, J.-M. & Clemons, M. Radiotherapy to the primary tumor is associated with improved survival in stage IV breast cancer [abstract]. SABCS P4 , 16–06 (2012).
Bernier, J. Immuno-oncology: allying forces of radio- and immuno-therapy to enhance cancer cell killing. Crit. Rev. Oncol. Hematol. 108 , 97–108 (2016).
Fietz, T. et al. Palliative systemic therapy and overall survival of 1,395 patients with advanced breast cancer — rResults from the prospective German TMK cohort study. Breast. 34 , 122–130 (2017).
Rugo, H. S. et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology guideline. J. Clin. Oncol. 34 , 3069–3103 (2016).
Turner, N. C. et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N. Engl. J. Med. 379 , 1926–1936 (2018).
Miles, D. W. et al. First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: pooled and subgroup analyses of data from 2447 patients. Ann. Oncol. 24 , 2773–2780 (2013).
Giordano, S. H. et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 32 , 2078–2099 (2014).
Partridge, A. H. et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 32 , 3307–3329 (2014).
Schmid, P. et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 379 , 2108–2121 (2018).
Marinovich, M. L. et al. Early prediction of pathologic response to neoadjuvant therapy in breast cancer: systematic review of the accuracy of MRI. Breast 21 , 669–677 (2012).
Avril, S. et al. 18 F-FDG PET/CT for monitoring of treatment response in breast cancer. J. Nucl. Med. 57 , 34S–39SS (2016).
Marinovich, M. L. et al. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J. Natl Cancer Inst. 105 , 321–333 (2013).
Marinovich, M. L. et al. Agreement between MRI and pathologic breast tumor size after neoadjuvant chemotherapy, and comparison with alternative tests: individual patient data meta-analysis. BMC Cancer 15 , 662 (2015).
Humbert, O. et al. Role of positron emission tomography for the monitoring of response to therapy in breast cancer. Oncologist 20 , 94–104 (2015).
Pennant, M. et al. A systematic review of positron emission tomography (PET) and positron emission tomography/computed tomography (PET/CT) for the diagnosis of breast cancer recurrence. Health Technol. Assess. 14 , 1–103 (2010).
Shachar, S. S. Assessing treatment response in metastatic breast cancer. Am. J. Hematol. Oncol . 12 , (2016).
Lee, C. I. et al. Comparative effectiveness of imaging modalities to determine metastatic breast cancer treatment response. Breast 24 , 3–11 (2015).
Pagani, O. et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N. Engl. J. Med. 371 , 107–118 (2014).
Francis, P., Regan, M. & Fleming, G. Adjuvant ovarian suppression in premenopausal breast cancer. N. Engl. J. Med. 372 , 1672–1673 (2015).
Mao, J. J. et al. Electroacupuncture versus gabapentin for hot flashes among breast cancer survivors: a randomized placebo-controlled trial. J. Clin. Oncol. 33 , 3615–3620 (2015).
Elkins, G. et al. Randomized trial of a hypnosis intervention for treatment of hot flashes among breast cancer survivors. J. Clin. Oncol. 26 , 5022–5026 (2008).
Loprinzi, C. L. et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 356 , 2059–2063 (2000).
Niravath, P. Aromatase inhibitor-induced arthralgia: a review. Ann. Oncol. 24 , 1443–1449 (2013).
Barton, D. L. et al. Impact of vaginal dehydroepiandosterone (DHEA) on vaginal symptoms in female cancer survivors: Trial N10C1 (Alliance). J. Clin. Oncol. 32 , 9507–9507 (2014).
Razvi, Y. et al. ASCO, NCCN, MASCC/ESMO: a comparison of antiemetic guidelines for the treatment of chemotherapy-induced nausea and vomiting in adult patients. Support. Care Cancer 27 , 87–95 (2019).
Gulati, G. et al. Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy (PRADA): a 2×2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur. Heart J. 37 , 1671–1680 (2016).
Smith, E. M. L. et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA 309 , 1359–1367 (2013).
Hershman, D. L. et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 32 , 1941–1967 (2014).
Hanai, A. et al. Effects of cryotherapy on objective and subjective symptoms of paclitaxel-induced neuropathy: prospective self-controlled trial. J. Natl Cancer Inst. 110 , 141–148 (2018).
Kadakia, K. C., Rozell, S. A., Butala, A. A. & Loprinzi, C. L. Supportive cryotherapy: a review from head to toe. J. Pain Symptom Manage. 47 , 1100–1115 (2014).
Hou, S., Huh, B., Kim, H. K., Kim, K.-H. & Abdi, S. Treatment of chemotherapy-induced peripheral neuropathy: systematic review and recommendations. Pain Physician 21 , 571–592 (2018).
Ahmed, R. L., Schmitz, K. H., Prizment, A. E. & Folsom, A. R. Risk factors for lymphedema in breast cancer survivors, the Iowa Women’s Health Study. Breast Cancer Res. Treat. 130 , 981–991 (2011).
Gillespie, T. C., Sayegh, H. E., Brunelle, C. L., Daniell, K. M. & Taghian, A. G. Breast cancer-related lymphedema: risk factors, precautionary measures, and treatments. Gland. Surg. 7 , 379–403 (2018).
Runowicz, C. D. et al. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. J. Clin. Oncol. 34 , 611–635 (2016).
Velikova, G. et al. Quality of life after postmastectomy radiotherapy in patients with intermediate-risk breast cancer (SUPREMO): 2-year follow-up results of a randomised controlled trial. Lancet Oncol. 19 , 1516–1529 (2018).
Hofmann, D. et al. WSG ADAPT — adjuvant dynamic marker-adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early breast cancer: study protocol for a prospective, multi-center, controlled, non-blinded, randomized, investigator initiated phase II/III trial. Trials 14 , 261 (2013).
Robertson, J. F. R., Dowsett, M. & Bliss, J. M. Peri-operative aromatase inhibitor treatment in determining or predicting long-term outcome in early breast cancer — the POETIC Trial (CRUK/07/015) [abstract]. SABCS GS1-03 (2017).
Ellis, M. J. et al. Ki67 Proliferation index as a tool for chemotherapy decisions during and after neoadjuvant aromatase inhibitor treatment of breast cancer: results from the American College of Surgeons Oncology Group Z1031 trial (Alliance). J. Clin. Oncol. 35 , 1061–1069 (2017).
Hölzel, D. et al. Improved systemic treatment for early breast cancer improves cure rates, modifies metastatic pattern and shortens post-metastatic survival: 35-year results from the munich cancer registry. J. Cancer Res. Clin. Oncol. 143 , 1701–1712 (2017).
Hölzel, D. et al. Survival of de novo stage IV breast cancer patients over three decades. J. Cancer Res. Clin. Oncol. 143 , 509–519 (2017).
Angus, L. et al. The genomic landscape of 501 metastatic breast cancer patients [abstract]. SABCS GS1-07 (2018).
Desmedt, C. et al. Unraveling lobular breast cancer progression and endocrine resistance mechanisms through genomic and immune characterization of matched primary and metastatic samples [abstract]. SABCS GS1–06 (2018).
Baselga, J. et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 18 , 904–916 (2017).
André, F. et al. Alpelisib for PIK3CA -mutated, hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 380 , 1929–1940 (2019).
Baselga, J. et al. Phase III study of taselisib (GDC-0032) + fulvestrant (FULV) v FULV in patients (pts) with estrogen receptor (ER)-positive, PIK3CA-mutant (MUT), locally advanced or metastatic breast cancer (MBC): primary analysis from SANDPIPER. J. Clin. Oncol. 36 , LBA1006–LBA1006 (2018).
Kim, S.-B. et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 18 , 1360–1372 (2017).
Schmid, P. et al. AZD5363 plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (PAKT): a randomised, double-blind, placebo-controlled, phase II trial. J. Clin. Oncol. 36 (15 Suppl.), 1007 (2018).
Jones, R. H. et al. Capivasertib (AZD5363) plus fulvestrant versus placebo plus fulvestrant after relapse or progression on an aromatase inhibitor in metastatic ER-positive breast cancer (FAKTION): a randomized, double-blind, placebo-controlled, phase II trial [abstract]. J. Clin. Oncol. 37 (no. 15_suppl), 1005–1005 (2019).
Yardley, D. A. et al. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J. Clin. Oncol. 31 , 2128–2135 (2013).
Ogitani, Y. et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA Topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. 22 , 5097–5108 (2016).
Tamura, K. et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 20 , 816–826 (2019).
Burris III, H. A., Giaccone, G. & Im, S. A. Updated findings of a first-in-human phase 1 study of margetuximab, an Fc-optimized chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors [abstract]. Am. Soc. Clin. Oncol. Meet. 33 (no. 15_suppl), A523 (2015).
Rugo, H. S. et al. SOPHIA primary analysis: a phase 3 (P3) study of margetuximab (M) + chemotherapy (C) versus trastuzumab (T) + C in patients (pts) with HER2+ metastatic (met) breast cancer (MBC) after prior anti-HER2 therapies (Tx) [abstract]. J. Clin. Oncol. 37 (Suppl.), Abstr 1000 (2019).
Hyman, D. M., Piha-Paul, S. & Rodon, J. Neratinib in HER2- or HER3-mutant solid tumors: SUMMIT, a global, multi-histology, open-label, phase 2 ‘basket’ study [abstract]. Am. Assoc. Cancer Res. Meet . CT001 (2017).
Saura, C. et al. Neratinib + capecitabine versus lapatinib + capecitabine in patients with HER2+ metastatic breast cancer previously treated with ≥2 HER2-directed regimens: findings from the multinational, randomized, phase III NALA trial [abstract]. J. Clin. Oncol. 37 (Suppl.), Abstract 1002 (2019).
Gucalp, A. et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin. Cancer Res. 19 , 5505–5512 (2013).
Cortes, J., Crown, J. & Awada, A. Overall survival (OS) from the phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) signaling inhibitor, in AR+ advanced triple-negative breast cancer (aTNBC) [abstract]. Eur. Cancer Congr. 51 (Suppl. 3), 1802 (2015).
Gelmon, K. A. et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 12 , 852–861 (2011).
Nanda, R. et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 Study. J. Clin. Oncol. 34 , 2460–2467 (2016).
Schmid, P., Cruz, C. & Braiteh, F. S. Atezolizumab in metastatic triple-negative breast cancer: long-term clinical outcomes and biomarker analyses [abstract]. Am. Assoc. Cancer Res. 77 , A2986 (2017).
André, F. et al. Alpelisib (ALP) + fulvestrant (FUL) for advanced breast cancer (ABC): results of the phase 3 SOLAR-1 trial [abstract]. ESMO LBA3 PR (2018).
Hyman, D. M. et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554 , 189–194 (2018).
Hartley, R. L., Stone, J. P. & Temple-Oberle, C. Breast cancer in transgender patients: a systematic review. Part 1: male to female. Eur. J. Surg. Oncol. 44 , 1455–1462 (2018).
Cardoso, F. et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann. Oncol. 29 , 405–417 (2017).
PubMed Central Google Scholar
Di Oto, E. et al. X chromosome gain is related to increased androgen receptor expression in male breast cancer. Virchows Arch. 473 , 155–163 (2018).
Severson, T. M. & Zwart, W. A review of estrogen receptor/androgen receptor genomics in male breast cancer. Endocr. Relat. Cancer 24 , R27–R34 (2017).
Deb, S. et al. PIK3CA mutations are frequently observed in BRCAX but not BRCA2-associated male breast cancer. Breast Cancer Res. 15 , R69 (2013).
Gucalp, A. et al. Male breast cancer: a disease distinct from female breast cancer. Breast Cancer Res. Treat. 173 , 37–48 (2019).
Korde, L. A. et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J. Clin. Oncol. 28 , 2114–2122 (2010).
Cardoso, F. et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 30 , 1194–1220 (2019).
Bareche, Y. et al. Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann. Oncol. 29 , 895–902 (2018).
Lehmann, B. D. & Pietenpol, J. A. Clinical implications of molecular heterogeneity in triple negative breast cancer. Breast 24 , S36–S40 (2015).
Lehmann, B. D. et al. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLOS ONE 11 , e0157368 (2016).
Burstein, M. D. et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin. Cancer Res. 21 , 1688–1698 (2015).
Siu, A. L. & on behalf of the U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 164 , 279 (2016).
Klarenbach, S. et al. Recommendations on screening for breast cancer in women aged 40–74 years who are not at increased risk for breast cancer. Can. Med. Assoc. J. 190 , E1441–E1451 (2018).
Oeffinger, K. C. et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 314 , 1599 (2015).
European Commission Initiative on Breast Cancer. Recommendations from European Breast Guidelines Europa.eu https://ecibc.jrc.ec.europa.eu/recommendations/list/Professional (2019).
Dawood, S. et al. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann. Oncol. 22 , 515–523 (2011).
Cserni, G., Charafe-Jauffret, E. & van Diest, P. J. Inflammatory breast cancer: the pathologists’ perspective. Eur. J. Surg. Oncol. 44 , 1128–1134 (2018).
Cheang, M. C. U. et al. Defining breast cancer intrinsic subtypes by quantitative receptor expression. Oncologist 20 , 474–482 (2015).
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490 , 61–70 (2012). This research establishes the contemporary method of classifying breast cancer into clinically relevant molecular subtypes.
Hoadley, K. A., Andre, F., Ellis, M. J. & Perou, C. M. Breast cancer intrinsic subtypes (Poster). Nat. Rev. Clin. Oncol . https://www.nature.com/documents/nrclinonc_posters_breastcancer.pdf (2014).
Desmedt, C. et al. Genomic characterization of primary invasive lobular breast cancer. J. Clin. Oncol. 34 , 1872–1881 (2016).
Ciriello, G. et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell 163 , 506–519 (2015).
Vasudev, P. & Onuma, K. Secretory breast carcinoma: unique, triple-negative carcinoma with a favorable prognosis and characteristic molecular expression. Arch. Pathol. Lab. Med. 135 , 1606–1610 (2011).
Martelotto, L. G. et al. Genomic landscape of adenoid cystic carcinoma of the breast. J. Pathol. 237 , 179–189 (2015).
Goss, P. E. et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N. Engl. J. Med. 375 , 209–219 (2016).
Liang, M. et al. Association between CHEK2*1100delC and breast cancer: a systematic review and meta-analysis. Mol. Diagn. Ther. 22 , 397–407 (2018).
Wang, X. et al. Breast cancer risk and germline genomic profiling of women with neurofibromatosis type 1 who developed breast cancer. Genes. Chromosomes Cancer 57 , 19–27 (2018).
McCart Reed, A. E. et al. Phenotypic and molecular dissection of metaplastic breast cancer and the prognostic implications: prognostic features of metaplastic breast cancer. J. Pathol. 247 , 214–227 (2019).
Wendt, C. & Margolin, S. Identifying breast cancer susceptibility genes — a review of the genetic background in familial breast cancer. Acta Oncol. 58 , 135–146 (2019).
Couch, F. J. et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 3 , 1190 (2017).
Nguyen, J. et al. EORTC QLQ-BR23 and FACT-B for the assessment of quality of life in patients with breast cancer: a literature review. J. Comp. Eff. Res. 4 , 157–166 (2015).
McLachlan, S. A., Devins, G. M. & Goodwin, P. J. Factor analysis of the psychosocial items of the EORTC QLQ-C30 in metastatic breast cancer patients participating in a psychosocial intervention study. Qual. Life Res. 8 , 311–317 (1999).
Bjelic-Radisic, V. et al. An international update of the EORTC questionnaire for assessing quality of life in breast cancer patients (EORTC QLQ-BC23) — EORTC QLQ-BR45. Ann. Oncol. 29 , viii58–viii86 (2018).
Ganz, P. A., Kwan, L., Stanton, A. L., Bower, J. E. & Belin, T. R. Physical and psychosocial recovery in the year after primary treatment of breast cancer. J. Clin. Oncol. 29 , 1101–1109 (2011).
Revicki, D. A. et al. Predicting EuroQol (EQ-5D) scores from the patient-reported outcomes measurement information system (PROMIS) global items and domain item banks in a United States sample. Qual. Life Res. 18 , 783–791 (2009).
Hays, R. D., Bjorner, J. B., Revicki, D. A., Spritzer, K. L. & Cella, D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual. Life Res. 18 , 873–880 (2009).
Bevans, M., Ross, A. & Cella, D. Patient-reported outcomes measurement information system (PROMIS): efficient, standardized tools to measure self-reported health and quality of life. Nurs. Outlook 62 , 339–345 (2014).
Download references
Acknowledgements
The authors thank N. Radosevic-Robin (Jean Perrin Comprehensive Cancer Centre, France) for her assistance in preparing Fig. 1. N. Houssami receives research support through a National Breast Cancer Foundation (NBCF, Australia) Breast Cancer Research Leadership Fellowship. K.R. acknowledges research funding from the Clinical and Translational Sciences Award (CTSA) grant number KL2 TR002379 from the National Centre for Advancing Translational Sciences, a component of the US National Institutes of Health.
Author information
Authors and affiliations.
LMU Munich, University Hospital, Department of Obstetrics and Gynecology, Breast Center and Comprehensive Cancer Center (CCLMU), Munich, Germany
Nadia Harbeck
Department of Pathology and Biopathology, Jean Perrin Comprehensive Cancer Centre, UMR INSERM 1240, University Clermont Auvergne, Clermont-Ferrand, France
Frédérique Penault-Llorca
IOB Institute of Oncology, Quironsalud Group, Madrid and Barcelona, Spain
Javier Cortes
Vall d´Hebron Institute of Oncology, Barcelona, Spain
Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria
Michael Gnant
Sydney School of Public Health, Faculty of Medicine and Health, University of Sydney, Sydney, Australia
Nehmat Houssami
Department of Radiation Oncology, Institut Curie, Paris, France
Philip Poortmans
Université PSL, Paris, France
Department of Oncology, Mayo Clinic, Rochester, MN, USA
Kathryn Ruddy
Hong Kong Breast Oncology Group, The University of Hong Kong, Hong Kong, China
Janice Tsang
Breast Unit, Champalimaud Clinical Center/Champalimaud Foundation, Lisbon, Portugal
Fatima Cardoso
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (all authors); Epidemiology (J.T.); Mechanisms/pathophysiology (F.P.-L.); Diagnosis, screening and prevention (N. Houssami); Management (N. Harbeck, F.C., M.G., P.P., J.C. and N. Houssami); Quality of life (K.R.); Outlook (all authors); Overview of the Primer (N. Harbeck and F.C.).
Corresponding author
Correspondence to Nadia Harbeck .
Ethics declarations
Competing interests.
N. Harbeck reports honoraria for lectures and/or consulting from Agendia, Amgen, Astra Zeneca, Celgene, Daiichi-Sankyo, Genomic Health, Lilly, MSD, Novartis, Odonate, Pfizer, Roche, Sandoz/Hexal and Seattle Genetics. F.P.-L. declares personal financial interests in Abbvie, Agendia, Astrazeneca, BMS, Genomic Health, Janssen, Lilly, Merck Lifa, MSD, Myriad, Nanostring, Novartis, Pfizer and Roche; institutional financial interests in Astrazeneca, BMS, Genomic Health, MSD, Myriad, Nanostring and Roche; and congress invitations from Abbvie, Astrazeneca, BMS, MSD and Roche. J.C. has received honoraria from Celgene, Chugai, Eisai, Novartis, Pfizer, Roche and Samsung; has served as a consultant for Astrazeneca, Biothera, Celgene, Daichii Sankyo, Erytech Pharma, Merus, Polyphor, Roche and Seattle Genetics; has received research funding from Ariad, Astrazeneca, Baxalta GMBH, Bayer, Eisai, Guardant Health, Merch Sharp & Dohme, Pfizer, Puma and Roche; and has stocks in MedSIR. M.G. reports honoraria from Amgen, AstraZeneca, Celgene, Eli Lilly, Medison, Nanostring Technologies, Novartis and Roche; advisory fees from Accelsoir; research funding from AstraZeneca, Novartis, Pfizer and Roche; and travel expenses from Amgen, AstraZeneca, Celgene, Eli Lilly, Ipsen, Medison, Novartis and Pfizer. K.R. declares previous ownership of Merck and Pfizer stock (October 2016–February 2018). J.T. reports honoraria and consultancy or advisory roles for AstraZeneca, Astellas, De Novo, Eisai, Foundation Medicine, Nanostring, Novartis, Pfizer and Roche. F.C. declares consultancy roles for Amgen, Astellas/Medivation, AstraZeneca, Celgene, Daiichi-Sankyo, Eisai, Genentech, GE Oncology, GlaxoSmithKline, Macrogenics, Medscape, Merck-Sharp, Merus BV, Mylan, Mundipharma, Novartis, Pfizer, Pierre-Fabre, prIME Oncology, Roche, Sanofi, Seattle Genetics and Teva. The remaining authors declare no competing interests.
Additional information
Peer review information.
Nature Reviews Disease Primers thanks T. Howell, P. Neven, M. Toi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ABC Global Alliance: https://www.abcglobalalliance.org
Adjuvant! Online: www.adjuvantonline.com
European Organization for Research and Treatment of Cancer: https://qol.eortc.org/modules/
EuroQol 5-Dimensions: https://euroqol.org/
Functional Assessment of Cancer Therapy: http://www.facit.org/FACITOrg
Patient-Reported Outcomes Measurement Information System: http://www.healthmeasures.net/explore-measurement-systems/promis
Short Form Health Survey-36: http://www.rand.org/health/surveys_tools/mos/36-item-short-form.html
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Harbeck, N., Penault-Llorca, F., Cortes, J. et al. Breast cancer. Nat Rev Dis Primers 5 , 66 (2019). https://doi.org/10.1038/s41572-019-0111-2
Download citation
Accepted : 22 July 2019
Published : 23 September 2019
DOI : https://doi.org/10.1038/s41572-019-0111-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Usp22 supports the aggressive behavior of basal-like breast cancer by stimulating cellular respiration.
- Evangelos Prokakis
- Husam Bamahmoud
- Florian Wegwitz
Cell Communication and Signaling (2024)
Targeting cholesterol impairs cell invasion of all breast cancer types
- Mauriane Maja
- Marie Verfaillie
- Donatienne Tyteca
Cancer Cell International (2024)
Identification of a Notch transcriptomic signature for breast cancer
- Eike-Benjamin Braune
- Felix Geist
- Urban Lendahl
Breast Cancer Research (2024)
Synergistic anticancer effect of Pistacia lentiscus essential oils and 5-Fluorouracil co-loaded onto biodegradable nanofibers against melanoma and breast cancer
- Obaydah Abd Alkader Alabrahim
- Hassan Mohamed El-Said Azzazy
Discover Nano (2024)
Exploring the dynamic interplay between exosomes and the immune tumor microenvironment: implications for breast cancer progression and therapeutic strategies
- Sahar Safaei
- Manouchehr Fadaee
- Tohid Kazemi
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
Home — Essay Samples — Nursing & Health — Oncology — Breast Cancer
Essays About Breast Cancer
Brief description of breast cancer.
Breast cancer is a type of cancer that forms in the cells of the breast. It is the second most common cancer in women and can also affect men. Breast cancer can be invasive or non-invasive and is often detected through screening and self-examination. Early detection and treatment are crucial for improving outcomes and survival rates.
Importance of Writing Essays on This Topic
Essays on breast cancer are significant for academic and personal exploration as they provide an opportunity to raise awareness about the disease, its risk factors, prevention, and treatment options. Writing about breast cancer also allows individuals to share personal experiences, advocate for research and support, and contribute to the ongoing dialogue surrounding this prevalent health issue.
Tips on Choosing a Good Topic
- Consider exploring the latest research and advancements in breast cancer treatment and prevention.
- Reflect on personal experiences or those of loved ones affected by breast cancer for a more personal and impactful essay.
- Investigate the societal and cultural impact of breast cancer, including awareness campaigns, advocacy, and support networks.
Essay Topics
- The Role of Genetic Testing in Breast Cancer Prevention
- The Impact of Lifestyle Choices on Breast Cancer Risk
- The Emotional and Psychological Effects of Breast Cancer Diagnosis and Treatment
- The Importance of Early Detection and Screening for Breast Cancer
- The Societal Stigma and Misconceptions Surrounding Breast Cancer
- Exploring Alternative and Complementary Therapies for Breast Cancer Patients
- The Influence of Support Networks and Advocacy Groups in Breast Cancer Awareness
- Analyzing the Economic and Social Burden of Breast Cancer on Patients and Families
- Debunking Common Myths and Misinformation about Breast Cancer
- The Role of Hormone Therapy in Breast Cancer Treatment
Concluding Thought
By writing essays on breast cancer, individuals can contribute to a better understanding of the disease, its impact, and the importance of ongoing research and support. Engaging with this topic through writing can help raise awareness, provide support, and inspire positive change within the community.
The Stages of Breast Cancer
Treatment and diagnosis of breast cancer, made-to-order essay as fast as you need it.
Each essay is customized to cater to your unique preferences
+ experts online
Breast Cancer: The Physical and Mental Effects
The ways of raising awareness about breast cancer, breast cancer: an unending battle that brought us together, the treatment of breast cancer, let us write you an essay from scratch.
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
The Benefits and Harmful Effects of Chemotherapy as a Treatment to Breast Cancer
Research on correlation of notch signaling pathway in the prognosis of breast cancer, hereditary breast and ovarian cancer, ultrasonography for the diagnosis of cancer, get a personalized essay in under 3 hours.
Expert-written essays crafted with your exact needs in mind
Mbmt Pilot Study: How This Affects The Breast Cancer Patient's Attention
Miracle in my life: my mother's battle with breast cancer, relevant topics.
- Mental Health
- Healthy Food
- Yellow Fever
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
Breast Cancer Risk Factors: Genetic and Nutritional Influences Essay
- To find inspiration for your paper and overcome writer’s block
- As a source of information (ensure proper referencing)
- As a template for you assignment
Introduction
Genetics and the policy issues, nutritional influences for the cause of breast cancer, nutritional assessment and counseling, prevalence rates.
Breast cancer is one of the most common cancers among women of all ages. That is why it attracts the close attention of doctors considering the problem of the occurrence of this disease. For example, much attention is paid to a woman’s genetic predisposition to cancer. However, if nothing can be done with this factor, then there is always the opportunity to change a lifestyle. The purpose of this paper is to study the relationship between various factors, primarily nutrition, with the prognosis, occurrence, and treatment of breast cancer.
Before choosing the appropriate treatment for any disease, including cancer, a doctor needs to identify it. Many factors trigger the development of cancer, and one of them is genetics. This part cannot be taken into account in any other way than by conducting special tests. These analyses used in clinical practice can identify patients who are at increased risk for breast cancer due to a concentration of certain hormones (Smith & Farrah, 2019). However, the problems of genetics contribute to the identification of this disease, since the essence of the problem requires constant monitoring of the state of the mammary glands to detect cancer at an early stage.
Following this, particular policies are introduced in medical institutions related to screenings and regular examinations of women. Such tests are carried out at specified intervals, allowing to identify the disease as early as possible. At the same time, older women are advised to undergo such tests with an increased frequency, as the risk of breast cancer rises with age (Forman, 2020). Besides, there are special tests directly related to genetics, such as gene expression profiling, which identifies the risk of recurrence after surgery (Smith & Farrah, 2019). Thus, due to a possible genetic predisposition, it is necessary to introduce special measures aimed at continually monitoring the condition of the breasts of women. It is also important to develop tests that study their genetics to detect and prevent the development of cancer in time.
In addition to genetics, doctors consider many other factors that can contribute to the development of breast cancer. Various hypotheses and studies show that this disease can be caused by a combination of physical and biochemical factors associated with a patient’s lifestyle (Hanselmann & Welter, 2016). Although, at first glance, the relationship between breast cancer and nutrition is not so obvious, studies show that obese people are much more prone to this disease (Forman, 2020). Women over 50 are in particular danger since, for them, the risk of cancer increases significantly.
However, the need to track weight does not arise in late adulthood, but practically from infancy. According to Forman (2020), the rate and the place of weight gain in infancy naturally affect breast development in the future, and at the same time, the possibility of developing benign tumors. Eating fresh vegetables and fruits, i.e., having a varied and balanced diet, slightly reduces the risk of breast cancer (Bakker et al., 2016). Thus, a direct relationship can be noted between human nutrition and the risk of cancer, which manifests itself in a decrease in danger while maintaining a healthy lifestyle and a balanced diet.
To determine how healthy and balanced a person’s nutrition is, it is necessary to evaluate it. This assessment can be carried out from many points of view, such as the relationship with health, prevention, screening, and diagnosis of cancer, as well as the choice of treatment and monitoring its effectiveness. It is worth noting that the presence and amount of carotenoids and vitamin C in the blood can conclusively indicate the number of vegetables and fruits consumed (Bakker et al., 2016). This allows doctors to conduct a kind of screening and diagnostics, since the presence of certain types of carotenes in the blood, namely alpha and beta carotenes, reduces the risk of breast cancer. Thus, after the assessment, it is possible to adjust the human diet, thus preventing the appearance of the disease. It is also possible to change the nutrition as a measure of additional treatment, since eliminating obesity can help avoid a cancer recession, as well as prevent some other diseases. However, unfortunately, it is impossible to get rid of breast cancer by only applying a diet, as this is just an additional measure.
Various methods are used to predict cancer, including those related to human nutrition. One of these methods, namely the prognostic nutritional index, has previously been used only to identify the long-term effects of non-breast related tumors. However, recent studies show that nutritional assessment using this index also works in cases of breast cancer and is a simple but effective marker for predicting possible consequences (Mohri et al., 2016). Moreover, this evaluation does not depend on the stage of the disease and can be used with an equal degree of effectiveness in the presence of a wide variety of tumors.
The prevalence of breast cancer concerning nutrition, for the most part, comes down to comparing the number of obese people with the number of people with cancer. Although obesity is undoubtedly associated with this disease, for a more accurate analysis of the prevalence of cancer and the number of people with a predisposition to it, several other factors must also be considered. Some of them are also related to genetics, but this leaning can also be caused by the nutritional problems of their parents. For example, issues with an overweight mother can negatively affect a baby and serve as a risk factor for developing breast cancer (Forman, 2020). However, the women’s diet at the stage of breast development directly affects the risk of the disease in the future. Therefore, when assessing the prevalence of this disease, it is also necessary to take into account the number of people who already have a risk of developing cancer due to various factors. The same considerations must be taken into account when making predictions regarding the spread of diseases, conducting tests in women, and choosing treatment, as described above in the previous paragraphs.
Thus, a person’s nutrition is directly related to his health, so the better and more varied a person eats, the healthier he or she will be. This factor can be used as an auxiliary during treatment and also be taken as a general recommendation for a healthy lifestyle. However, it must be remembered that one healthy diet is not a guarantee that a woman cannot get breast cancer. Therefore, it is necessary to regularly pass appropriate tests that affect not only nutrition but also, for example, the genetics of women.
Bakker, M., Peeters, P., Klaasen, V., Bueno-de-Mesquita, H., Jansen, E., Ros, M., Travier, N., Olsen, A., Tjønneland, A., Overvad, K., Rinaldi, S., Romieu, I., Brennan, P., Boutron-Ruault, M.-C., Perquier, F., Cadeau, C., Boeing, H., Aleksandrova, K., Kaaks, R.,…van Gils, C. (2016). Plasma carotenoids, vitamin C, tocopherols, and retinol and the risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition cohort , 2. The American journal of clinical nutrition , 103 (2), 454-464. Web.
Forman, M. R. (2020). Breast cancer and nutrition: A paradigm for prevention in 3D across the life course. Frontiers in Oncology , 10 , 129.
Hanselmann R. G., & Welter, C. (2016). Origin of cancer: An information, energy, and matter disease, Frontiers in Cell and Development Biology, 4 (121). Web.
Mohri, T., Mohri, Y., Shigemori, T., Takeuchi, K., Itoh, Y., & Kato, T. (2016). Impact of prognostic nutritional index on long-term outcomes in patients with breast cancer. World journal of surgical oncology , 14 (1), 170. Web.
Smith, A., & Farrah, K. (2019). Gene expression profiling tests for breast cancer: A rapid qualitative review. Canadian Agency for Drugs and Technologies in Health. Web.
- Treatment Options for Breast Cancer
- Breast Cancer: Moral and Medical Aspects
- Fibrocystic Breast Condition or Breast Cancer?
- The Role Genetics Information Plays in Treating Cancer
- Breast Cancer: The Case of Anne H.
- Leukemia Types: Characteristics, Genetics, and Symptoms
- CRISPR and Cas-9 Technology as the Solution to Cancer
- Colon Cancer: Risk Factors
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2022, February 15). Breast Cancer Risk Factors: Genetic and Nutritional Influences. https://ivypanda.com/essays/breast-cancer-risk-factors-genetic-and-nutritional-influences/
"Breast Cancer Risk Factors: Genetic and Nutritional Influences." IvyPanda , 15 Feb. 2022, ivypanda.com/essays/breast-cancer-risk-factors-genetic-and-nutritional-influences/.
IvyPanda . (2022) 'Breast Cancer Risk Factors: Genetic and Nutritional Influences'. 15 February.
IvyPanda . 2022. "Breast Cancer Risk Factors: Genetic and Nutritional Influences." February 15, 2022. https://ivypanda.com/essays/breast-cancer-risk-factors-genetic-and-nutritional-influences/.
1. IvyPanda . "Breast Cancer Risk Factors: Genetic and Nutritional Influences." February 15, 2022. https://ivypanda.com/essays/breast-cancer-risk-factors-genetic-and-nutritional-influences/.
Bibliography
IvyPanda . "Breast Cancer Risk Factors: Genetic and Nutritional Influences." February 15, 2022. https://ivypanda.com/essays/breast-cancer-risk-factors-genetic-and-nutritional-influences/.

Breast Cancer and the Environment: A Life Course Approach (2012)
Chapter: 1 introduction.
1 Introduction
T he prospect of developing breast cancer is a source of anxiety for many women. Breast cancer remains the most common invasive cancer among women (aside from nonmelanoma skin cancers), accounting in 2011 for an estimated 230,480 new cases among women in the United States and another 2,140 new cases among men (ACS, 2011). After lung cancer, it is the second most common cause of mortality from cancer for women, with about 39,520 deaths expected in the United States in 2011. Another 450 breast cancer deaths are expected among men in 2011 (ACS, 2011). Since the mid-1970s, when the National Cancer Institute (NCI) began compiling continuous cancer statistics, the annual incidence of invasive breast cancer rose from 105 cases per 100,000 women to 142 per 100,000 women in 1999 (NCI, 2011). Since then, however, the incidence has declined. In 2008, the incidence of breast cancer was 129 cases per 100,000 women.
Further reduction of the incidence of breast cancer is a high priority, but finding ways to achieve this is a challenge. As in most types of adult cancer, breast cancer is thought to develop as a result of accumulated damage induced by both internal and external triggers resulting in initial carcinogenic events. The affected cells and tissues then progress through multiple stages, with accompanying alterations in the surrounding tissue likely playing a role in whether the damage leads to a cancer. These events contributing to subsequent cancers may occur spontaneously as a by-product of errors in normal processes, such as DNA replication, or potentially through effects of environmental exposures. The early procarcinogenic events from endogenous and exogenous processes may be sustained and
furthered by physiologic conditions such as obesity. It is likely that many such procarcinogenic events may never be entirely preventable because, although potentially modifiable, they are consequences of basic biologic processes, such as oxidative damage to DNA from endogenous metabolism, or stimulation of cell growth through normal hormonal processes. 1 Although such biological “background” mutagenesis is unavoidable, highly efficient protective pathways, such as DNA repair and immune surveillance, are effective at reducing the impacts of procarcinongenic events (Loeb and Nishimura, 2010; Bissell and Hines, 2011).
Although more needs to be learned about both the mechanisms by which breast cancers arise and the array of factors that influence risk for them, much has been established. Among the factors generally accepted as increasing women’s risk are older age, having a first child at an older age or never having a child, exposure to ionizing radiation, and use of certain forms of postmenopausal hormone therapy (HT). Inherited mutations in the BRCA1 and BRCA2 genes also markedly increase risk for breast cancer (and other cancers as well), but these mutations are rare in the general population and account for only 5 to 10 percent of cases (ACS, 2011).
Even though aging, genetics, and patterns of childbearing account for some of the risk for breast cancer, they are not promising targets for preventive measures. More helpful would be identifying modifiable risk factors. For example, the publication of findings from the Women’s Health Initiative (Writing Group for the Women’s Health Initiative Investigators, 2002) confirming earlier indications that estrogen–progestin HT was contributing to an increase in the risk of postmenopausal breast cancer was followed by a rapid reduction in use of HT and in the incidence of invasive breast cancer. As reflected in NCI data, the incidence in 2002 was 136 cases per 100,000 women, compared with 127 in 2003 (NCI, 2011). A portion of the decline in breast cancer incidence since 1999 is attributed to this reduced use of HT (e.g., Ravdin et al., 2007; Farhat et al., 2010). But there are long-standing and still unresolved concerns that aspects of diet, ambient chemicals, or other potentially modifiable environmental exposures may be contributing to high rates of breast cancer.
At present, a large but incomplete body of evidence is available on the relationship between breast cancer and the wide variety of external factors that can be said to comprise the environment. Information on interactions between genetic susceptibility and environmental factors is particularly sparse. In contrast, knowledge of the complexity of breast cancer is growing, with the characterization of multiple tumor subtypes; the possibility
_________________
1 Loeb and Nishimura (2010, p. 4270) note that each normal cell in a person’s body may be exposed to as many as 50,000 DNA-damaging events each day, and that oxygen free radicals are a major source of DNA damage.
that critical events in the origins of breast cancer can occur very early in life; the variety of pathways through which breast cancer risks may be shaped; and the potential significance of both the timing of exposures and the way combinations of factors determine the effect on risks for different types of breast cancer. This growing knowledge has stimulated a transition in breast cancer research. The new perspectives on breast cancer highlight the limitations of the current understanding of the disease, and innovative ideas are beginning to influence the design and analysis of epidemiologic studies, experimental studies in animals, and mechanistic studies of breast cancer biology, all directed toward elucidating how external factors may influence the etiology of breast cancer.
This report presents the results of a study commissioned to review the current evidence on environmental risk factors for breast cancer, consider gene–environment interactions in breast cancer, explore evidence-based actions that might reduce the risk of breast cancer, and recommend research in these areas.
STUDY CHARGE AND COMMITTEE ACTIVITIES
This study resulted from a request to the Institute of Medicine (IOM) by Susan G. Komen for the Cure and its Scientific Advisory Board. Komen for the Cure funds research on prevention, diagnosis, and treatment of breast cancer, and also provides educational information and support services for the public and health care providers. The Statement of Task for the IOM study appears in Box 1-1 .
The members of the study committee were selected to contribute expertise in epidemiology, toxicology, risk assessment, biostatistics, molecular carcinogenesis, gene–environment interactions, communication of health messages, environmental health science, exposure assessment, and health care. The committee includes a member from the patient advocacy community.
The committee met in person five times from April 2010 through February 2011 and conducted additional deliberations by conference call. During these meetings and calls, the committee reviewed and discussed the existing research literature on the topics central to its charge and developed and revised this report. At three of its meetings, the committee held public sessions during which it heard presentations by researchers, representatives of advocacy organizations, and members of the public.
The committee also commissioned work on two topics. One project was a review of data available to assess temporal changes in the potential for exposure to a selected set of chemicals and other environmental agents. The agents included in this paper have been discussed in the research literature and the popular press as possible contributors to increased risk for
BOX 1-1 Study Charge
In response to a request from Susan G. Komen for the Cure ® , the Institute of Medicine will assemble a committee to:
1. Review the evidentiary standards for identifying and measuring cancer risk factors;
2. Review and assess the strength of the science base regarding the relationship between breast cancer and the environment;
3. Consider the potential interaction between genetic and environmental risk factors;
4. Consider potential evidence-based actions that women could take to reduce their risk of breast cancer;
5. Review the methodological challenges involved in conducting research on breast cancer and the environment; and
6. Develop recommendations for future research in this area.
In addition to reviewing the published literature, the committee will seek input from stakeholders, in part by organizing and conducting a public workshop to examine issues related to the current status of evidentiary standards and the science base, research methods, and promising areas of research. The workshop will focus on the challenges involved in the design, conduct, and interpretation of research on breast cancer and the environment. The committee will generate a technical report with conclusions and recommendations, as well as a summary report for the lay public.
breast cancer. This work served as an information resource for the committee and helped to identify some data presented in Chapter 4 . The other project resulted in a paper examining temporal changes in the United States in exposure to ionizing radiation, with a particular focus on exposure from medical imaging (see Appendix F , available electronically at http://www.nap.edu/catalog.php?record_id=13263 ).
APPROACH TO THE STUDY
The committee began its work with recognition of the potentially vast scope of the study task and the need to develop a perspective and approach that could lead to a useful and timely report. The committee sought to focus its attention in areas that it considered to be the most significant and the most pertinent to the charge placed before it.
For purposes of this report, the committee interpreted “environment” broadly, to encompass all factors that are not directly inherited through
DNA. As a result, this definition includes elements that range from the cellular to the societal: the physiologic and developmental course of an individual, diet and other ingested substances, physical activity, microbial agents, physical and chemical agents encountered at home or at work, medical treatments and interventions, social factors, and cultural practices. This perspective was a foundation for the committee’s work; application of it in its broadest sense is something that the committee hopes will expand the scope of future research. For some readers, this interpretation will differ from their association of the phrase “environmental risk factors” primarily with pollutants and other products of industrial processes (Baralt and McCormick, 2010). Furthermore, throughout the report the term “breast cancer” is used to refer to disease in humans and “mammary cancer” or “mammary tumor” to refer to disease in animals.
The committee explored the available evidence concerning breast cancer risks associated with a varied but limited collection of specific substances and factors ( Chapter 3 ), and it also reviewed the many challenges that researchers have had to contend with in studying breast cancer, including those pertaining to gene–environment interactions ( Chapter 4 ). But in its examination of the relation between breast cancer and the environment, the committee chose to highlight an approach that emphasizes the biologic mechanisms through which environmental factors may be operating and the importance of the changing picture over the life course ( Chapter 5 ). This perspective played a major role in shaping the committee’s conclusions and recommendations.
A Life Course Perspective
Breast cancer is primarily (but far from exclusively) a disease of adult women who are approaching or have reached menopause. In 2009, approximately 90 percent of new cases in U.S. women were diagnosed at age 45 or older (ACS, 2009). But the breast undergoes substantial changes from the time it begins developing in the fetus through old age, especially in response to hormonal changes during puberty, pregnancy, lactation, and menopause. With the timing of these developmental events related to risk for some types of breast cancer, there has been growing interest in exploring whether the timing of a variety of environmental exposures also is important in understanding what influences breast cancer risks. In Chapter 5 , the committee has sought to link its examination of the mechanisms of carcinogenesis with a life course perspective on when and how those pathologic pathways may be particularly relevant in relation to when and how environmental exposures occur. Attention was paid to growing evidence for critical windows of susceptibility (e.g., periods with rapid cell proliferation or maturation)
when specific mechanisms that increase the likelihood of a breast cancer developing may be more likely to be activated.
Identifying Environmental Risks for Breast Cancer
Trying to determine which environmental exposures may be influencing rates of breast cancer poses substantial challenges, many of which are discussed in Chapter 4 . Cancer is a complex disease, and its “causes” are generally harder to trace than the bacteria and viruses that cause infectious diseases. People who are never exposed to the measles virus will never get measles. But the impact of removing a particular environmental exposure associated with breast cancer is less clear because many other factors can still contribute to the development of breast cancer. The role of underlying susceptibility from inherited genes appears to involve both rare variants and common ones, but it is still not well characterized. Moreover, people are exposed to a complex and changing mix of environmental agents over the course of a lifetime, so discerning the effects of an individual agent, or knowing which components of the mixture may influence the development of disease or how the mixture’s components may interact with each other or with genes, is not straightforward.
Observational epidemiologic studies are a critical tool for learning about elevated risks, but they can be difficult to do well. They typically are the basis for demonstrating correlations between risk factors and outcomes, but establishing a causal inference is much more difficult. The challenges in establishing causality in such studies include difficulties with exposure measurement and accounting for undetected or poorly measured differences that may exist between the groups designated as exposed and unexposed. Furthermore, the timing and duration of observational studies may affect whether sufficient time has elapsed to detect differences in the incidence of a cancer that may not appear until many years after an exposure. Randomized controlled trials, which assign participants to a specific exposure or a comparison condition, are easier to interpret. However, for ethical and methodological reasons, such studies are rarely possible, especially when the goal is to determine whether the exposure is associated with an adverse event.
Experimental studies in animal models and in vitro systems offer an important opportunity to study the effects of well-defined exposures and to explore mechanisms of carcinogenicity in ways that are not possible in epidemiologic studies. They can signal potential hazards to human health that cannot be identified in other ways, but their results have to be interpreted with an understanding of differences across species and the comparability of an experimental exposure to the conditions encountered in the human population.
Reviewing Evidence on Specific Risk Factors
The literature on risk factors for cancer in general and breast cancer in particular is large and varied. In the United States, the Environmental Protection Agency (EPA) and the National Toxicology Program (NTP) in the National Institute of Environmental Health Sciences have programs to review the evidence on the carcinogenicity of various substances. 2 The International Agency for Research on Cancer (IARC), which is part of the World Health Organization, is a focal point for major international collaboration in such reviews. 3 In addition, a collaborative project between the World Cancer Research Fund International and the American Institute for Cancer Research has an ongoing program to review evidence on diet, physical activity, and cancer (WCRF/AICR, 2007). 4 All of these review programs consider evidence concerning breast cancer (or mammary cancers in animal studies) when it is available, but it is not their focus. Reviews specifically concerning breast cancer have also been conducted. These reviews include one conducted by the California Breast Cancer Research Program (2007) and a review sponsored by Komen for the Cure and conducted by the Silent Spring Institute (e.g., Brody et al., 2007; Rudel et al., 2007).
Assembling a comprehensive review of evidence on the relation between a complete set of environmental factors and breast cancer was not feasible for this study. Instead, the committee chose to focus on a limited selection of various types of environmental factors and potential routes of exposure. These factors are discussed in Chapter 3 . The committee’s aim was to characterize the available evidence and identify where substantial areas of uncertainty exist.
Observations About Risk
One component of the committee’s task was to comment on actions that can be taken to reduce the risk of breast cancer. Opportunities for action are discussed in Chapter 6 , but it is important to emphasize from the outset the challenge of interpreting evidence regarding risk and risk reduction. The widely quoted estimate that women in the United States have a 1-in-8 chance of being diagnosed with breast cancer during their lifetimes
2 Information on the EPA and NTP review programs is available at http://www.epa.gov/ebtpages/pollcarcinogens.html and http://ntp.niehs.nih.gov/?objectid=72016262-BDB7-CEBA-FA60E922B18C2540 .
3 Information on IARC reviews is available at http://www.iarc.fr/ and http://monographs.iarc.fr/index.php .
4 Information on the review by the World Cancer Research Fund International and the American Institute for Cancer Research is available at http://www.wcrf.org/cancer_research/expert_report/index.php .
can be restated as approximately a 12 percent lifetime risk of developing invasive breast cancer (NCI, 2010). The risk can also be presented for shorter, more comprehensible intervals. For example, among white women who are 50 years old, 2.4 percent are likely to be diagnosed with invasive breast cancer over the next 10 years (NCI, 2010). This 10-year risk is 2.2 percent for 50-year-old black women, 2.0 percent for Asian women, and 1.7 percent for Hispanic women. For 70-year-olds, the 10-year risks are 3.9 percent for white women, 3.2 percent for black women, and 2.4 percent for both Asian and Hispanic women. Estimates for longer follow-up periods (e.g., 20 or 30 years) will only increase those risks. Within average values such as these, there are always groups of women whose particular characteristics give them a higher or lower 10-year risk.
These estimates of risk are a critical reference point for understanding the implications of findings from epidemiologic studies on factors associated with increased or decreased risk of breast cancer. These findings are typically reported in terms of relative risk, which reflects a comparison between the risk in a population exposed to a particular factor and that in a similar population that is not exposed. Thus, a relative risk of 2.0 (a doubling of risk) might mean that for women with that risk factor, the 10-year risk of breast cancer is 5 percent rather than 2.5 percent. Similarly, a relative risk of 0.5 for a protective factor means that women with that characteristic may have a 10-year risk of 1.3 percent rather than 2.5 percent. These examples are offered to illustrate the scale of the change in risk implied by typical epidemiologic findings; they are not a formal analysis.
From a public health perspective, another important piece of information is the prevalence of the risk factor in the population. Finding that an environmental factor is associated with a large relative risk may still mean that it accounts for few cases of disease if the disease or the exposure is rare in that population. Alternatively, an environmental exposure that is associated with only a small increase in risk may be contributing to a large number of cases if the exposure is very common in the population. However, if the exposure is so common that there is little variability across the population (virtually everyone is exposed), it can be extremely difficult to identify the contribution from that exposure.
Virtually all of the epidemiologic evidence regarding breast cancer risk is drawn from population-level analyses. As a result, the conclusions reached on the basis of that evidence apply to an exposed population . With current knowledge, it is not possible to apply those conclusions to predict which individuals within that population are most likely to develop breast cancer. Nevertheless, an understanding of population-based estimates of risk can help people make personal choices that may lead to better health outcomes.
TOPICS BEYOND THE SCOPE OF THE STUDY
Several topics were defined as falling beyond the scope of the study. With the focus on environmental risk factors for breast cancer, the committee chose to devote little attention to the established associations between increased risk for breast cancer and reproductive events such as younger age at menarche, older age at first birth, lack of lactation, and older age at menopause. The committee also chose not to evaluate the established associations between breast cancer risk and higher birth weight and attained stature. Although some of them might fall under the committee’s very broad definition of environmental factors, they were not the focus of its review. Background is provided on many of these other factors in Chapter 2 , and the possibility that some environmental exposures may have an indirect influence on risk for breast cancer because they may affect the timing of these reproductive events is discussed in Chapter 5 .
The committee also agreed that the nature and effectiveness of breast cancer screening, diagnosis, and treatment were generally beyond the scope of the study. It noted but did not analyze the impact of increased mammography and changes in screening practices since the 1970s on the observed incidence of breast cancer. The paper commissioned by the committee on medical sources of exposure to ionizing radiation took into account the contribution of mammography. The committee did not examine the appropriateness of screening recommendations or practices.
The committee decided as well that its charge called for a focus on risk for the initial occurrence of breast cancer and not on recurrence or factors that might be associated with the risk of recurrence. Although environmental exposures may well influence the risk of recurrence, that risk is also influenced by characteristics of tumors at the time of diagnosis and subsequent treatment and follow-up practices. Consideration of clinical practice in the treatment of women (and men) with diagnosed breast cancers is substantially different from the study’s primary focus on prevention of breast cancer through improved understanding of and response to environmental risks. Similarly, the committee concluded that its charge called for a focus on the incidence of breast cancer and not mortality. Influences on breast cancer mortality patterns include factors that affect diagnosis and treatment that are separate from the effects of environmental exposures on the incidence of the disease.
The committee did not explicitly assess environmental risk factors for male breast cancer, beyond the general assumption that some of the risk factors identified through studies in women may also be relevant to the development of breast cancer in men.
THE COMMITTEE’S REPORT
This report reviews the current evidence on the biology of breast cancer, examines the challenges of studying environmental risk factors, and presents the committee’s findings and research recommendations from its review of evidence on environmental risk factors. Specifically, Chapter 2 provides important background for evaluating factors influencing breast cancer risk with a brief review of the biology of breast cancer and trends in incidence in the United States, along with discussion of the kinds of studies used to investigate breast cancer and environmental exposures. Chapter 3 presents the committee’s review of evidence on selected environmental risk factors. Chapter 4 discusses the variety of challenges that complicate the study of environmental risk factors for breast cancer, as well as gene–environment interactions. Chapter 5 examines mechanisms of carcinogenesis and links them to a life course perspective on breast development and the potential for environmental factors to influence risk for breast cancer. In Chapter 6 , the committee examines opportunities for evidence-based action to reduce risks for breast cancer and also considers the challenges of avoiding the unintentional introduction of new risks. Chapter 7 concludes the report with the committee’s recommendations for future research efforts. Included as appendixes are agendas for the committee’s public sessions ( Appendix A ), biographical sketches of committee members ( Appendix B ), a summary of weight-of-evidence categories used by major organizations that evaluate cancer risks ( Appendix C ), a table summarizing reports of population attributable risks for breast cancer ( Appendix D ), a glossary ( Appendix E ), and the paper commissioned on exposure to ionizing radiation ( Appendix F ).
ACS (American Cancer Society). 2009. Breast cancer facts and figures 2009–2010 . Atlanta, GA: ACS. http://www.cancer.org/Research/CancerFactsFigures/BreastCancerFactsFigures/index (accessed November 17, 2010).
ACS. 2011. Breast Cancer facts and figures 2011–2012 . Atlanta, GA: ACS. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-030975.pdf (accessed November 15, 2011).
Baralt, L. B., and S. McCormick. 2010. A review of advocate–scientist collaboration in federally funded environmental breast cancer research centers. Environ Health Perspect 118(12):1668–1675.
Bissell, M. J., and W. C. Hines. 2011. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 17(3):320–329.
Brody, J. G., K. B. Moysich, O. Humblet, K. R. Attfield, G. P. Beehler, and R. A. Rudel. 2007. Environmental pollutants and breast cancer: Epidemiologic studies. Cancer 109(12 Suppl):2667–2711.
California Breast Cancer Research Program. 2007. Identifying gaps in breast cancer research: Addressing disparities and the roles of the physical and social environment . http://cbcrp.org/sri/reports/identifyingGaps/index.php (accessed October 25, 2011).
Farhat, G. N., R. Walker, D. S. Buist, T. Onega, and K. Kerlikowske. 2010. Changes in invasive breast cancer and ductal carcinoma in situ rates in relation to the decline in hormone therapy use. J Clin Oncol 28(35):5140–5146.
Loeb, L. A., and S. Nishimura. 2010. Princess Takamatsu Symposium on DNA repair and human cancers. Cancer Res 70(11):4269–4273.
NCI (National Cancer Institute). 2010. SEER cancer statistics review, 1975–2007 . Edited by S. F. Altekruse, C. L. Kosary, M. Krapcho, N. Neyman, R. Aminou, W. Waldron, J. Ruhl, N. Howlader, Z. Tatalovich, H. Cho, A. Mariotto, M. P. Eisner, D. R. Lewis, K. Cronin, H. S. Chen, E. J. Feuer, D. G. Stinchcomb, and B. K. Edwards. Bethesda, MD:
NCI. http://seer.cancer.gov/csr/1975_2007/ (accessed January 6, 2011).
NCI. 2011. SEER cancer statistics review, 1975–2008. Edited by N. Howlader, A. M. Noone, M. Krapcho, N. Neyman, R. Aminou, W. Waldron, S. F. Altekruse, C. L. Kosary, J. Ruhl, Z. Tatalovich, H. Cho, A. Mariotto, M. P. Eisner, D. R. Lewis, H. S. Chen, E. J. Feuer, K. A. Cronin, and B. K. Edwards. Bethesda, MD: NCI. (Based on November 2010 SEER data submission, posted to the SEER website, 2011.) http://seer.cancer.gov/csr/1975_2008/ (accessed June 1, 2011).
Ravdin, P. M., K. A. Cronin, N. Howlader, C. D. Berg, R. T. Chlebowski, E. J. Feuer, B. K. Edwards, and D. A. Berry. 2007. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med 356(16):1670–1674.
Rudel, R. A., K. R. Attfield, J. N. Schifano, and J. G. Brody. 2007. Chemicals causing mammary gland tumors in animals signal new directions for epidemiology, chemicals testing, and risk assessment for breast cancer prevention. Cancer 109(12 Suppl):2635–2666.
WCRF/AICR (World Cancer Research Fund/American Institute for Cancer Research). 2007. Food, nutrition, physical activity, and the prevention of cancer: A global perspective. Washington, DC: AICR.
Writing Group for the Women’s Health Initiative Investigators. 2002. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288(3):321–333.
This page intentionally left blank.
Breast cancer remains the most common invasive cancer among women. The primary patients of breast cancer are adult women who are approaching or have reached menopause; 90 percent of new cases in U.S. women in 2009 were diagnosed at age 45 or older. Growing knowledge of the complexity of breast cancer stimulated a transition in breast cancer research toward elucidating how external factors may influence the etiology of breast cancer.
Breast Cancer and the Environment reviews the current evidence on a selection of environmental risk factors for breast cancer, considers gene-environment interactions in breast cancer, and explores evidence-based actions that might reduce the risk of breast cancer. The book also recommends further integrative research into the elements of the biology of breast development and carcinogenesis, including the influence of exposure to a variety of environmental factors during potential windows of susceptibility during the full life course, potential interventions to reduce risk, and better tools for assessing the carcinogenicity of environmental factors. For a limited set of risk factors, evidence suggests that action can be taken in ways that may reduce risk for breast cancer for many women: avoiding unnecessary medical radiation throughout life, avoiding the use of some forms of postmenopausal hormone therapy, avoiding smoking, limiting alcohol consumption, increasing physical activity, and minimizing weight gain.
Breast Cancer and the Environment sets a direction and a focus for future research efforts. The book will be of special interest to medical researchers, patient advocacy groups, and public health professionals.
READ FREE ONLINE
Welcome to OpenBook!
You're looking at OpenBook, NAP.edu's online reading room since 1999. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website.
Do you want to take a quick tour of the OpenBook's features?
Show this book's table of contents , where you can jump to any chapter by name.
...or use these buttons to go back to the previous chapter or skip to the next one.
Jump up to the previous page or down to the next one. Also, you can type in a page number and press Enter to go directly to that page in the book.
Switch between the Original Pages , where you can read the report as it appeared in print, and Text Pages for the web version, where you can highlight and search the text.
To search the entire text of this book, type in your search term here and press Enter .
Share a link to this book page on your preferred social network or via email.
View our suggested citation for this chapter.
Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available.
Get Email Updates
Do you enjoy reading reports from the Academies online for free ? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.
SEER Training Modules
of the page. Use an empty to account for some browsers not moving keyboard focus after jump. * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * --> Introduction to Breast Cancer
Breast cancer is a malignant cell growth in the breast . If left untreated, the cancer spreads to other areas of the body. Excluding skin cancer , breast cancer is the most common type of cancer in women in the United States, accounting for one of every three cancer diagnoses.
An estimated 211,240 new invasive cases of breast cancer were expected to occur among women in the United States during 2005. About 1,690 new male cases of breast cancer were expected in 2005.
The incidence of breast cancer rises after age 40. The highest incidence (approximately 80% of invasive cases) occurs in women over age 50.
In addition to invasive breast cancer, 58,590 new cases of in situ breast cancer are expected to occur among women during 2005. Of these, approximately 88% will be classified as ductal carcinoma in situ ( DCIS ). The detection of DCIS cases is a direct result of the increased use of mammography screening . This screening method is also responsible for detection of invasive cancers at a less advanced stage than might have occurred otherwise.
An estimated 40,870 deaths (40,410 women, 460 men) were anticipated from breast cancer in 2005. Breast cancer ranks second among cancer deaths in women. According to the most recent data , mortality rates declined significantly during 1992-1998, with the largest decreases in younger women, both white and black.
Introduction to Breast Cancer
- First Online: 04 March 2023
Cite this chapter

- Manzoor Ahmad Mir ORCID: orcid.org/0000-0003-3297-1402 2 &
- Hina Qayoom 2
471 Accesses
1 Citations
Breast cancer is the leading cause of mortality among women worldwide. Being a heterogenous disease it comprises many different subtypes that differ in their pathological features and clinical importance. These distinct subtypes with different histopathological and biological characteristics are responsible for varied responses to treatments and hence require different therapeutic strategies. Therefore, for therapeutic decision-making and personalized treatment it is imperative to classify the breast cancer into its specific subtypes. There are five intrinsic subgroups of breast cancer such as Luminal A, Luminal B, HER2 overexpression, basal, and normal-like tumors. The Luminal A and Luminal B subtypes are largely distinguished from other subtypes in that they are known to express two main biological processes: proliferation/cell cycle and luminal/hormone-regulated pathways. The HER2 enriched subtype is characterized by the overexpression of HER2-related and proliferation-related genes and protein same as the basal-like subtype. Another, important subtype of breast cancer, triple-negative breast cancer (TNBC) is an aggressive most lethal one defined by the lack of three hormonal receptors (ER, PR, and HER2). TNBC is highly invasive coherent with the reduced survival rates and increased mortality in affected population. It has further been classified into six distinct subtypes: basal-like 1 (BL-1), basal-like 2 (BL-2), mesenchymal (M), mesenchymal stem-like (MSL), immunomodulatory (IM), and luminal androgen receptor (LAR).
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Akram M, Siddiqui SA (2012) Breast cancer management: past, present and evolving. Indian J Cancer 49(3):277
Article CAS PubMed Google Scholar
Anestis A et al (2020) Androgen receptor in breast cancer—clinical and preclinical research insights. Molecules 25(2):358
Article CAS PubMed PubMed Central Google Scholar
Atkins H et al (1972) Treatment of early breast cancer: a report after ten years of a clinical trial. Br Med J 2(5811):423–429
Baclesse F (1984) Roentgen therapy as the sole method of treatment of cancer of the breast. In: Modern radiation oncology, vol 2. Harper and Row Publishers, Inc, New York, pp 115–129
Google Scholar
Bareche Y et al (2018) Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann Oncol 29(4):895–902
Beatson GT (1989) On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment, with illustrative cases. Cancer J (Villejuif) 2(10):347–350
Berardi R et al (2013) Role of maspin in cancer. Clin Transl Med 2(1):1–19
Article Google Scholar
Blows FM et al (2010) Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med 7(5):e1000279
Article PubMed PubMed Central Google Scholar
Bonadonna G et al (1976) Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med 294(8):405–410
Brambilla C et al (1976) Response and survival in advanced breast cancer after two non-cross-resistant combinations. Br Med J 1(6013):801–804
Cancer Genome Atlas Network et al (2012) Comprehensive molecular portraits of human breast tumors. Nature 490(7418):61–70
Burris Iii HA (2004) Dual kinase inhibition in the treatment of breast cancer: initial experience with the EGFR/ErbB-2 inhibitor lapatinib. Oncologist 9(S3):10–15
Burstein MD et al (2015) Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer identification of four unique subtypes of TNBCs. Clin Cancer Res 21(7):1688–1698
Cetin B et al (2014) Lapatinib plus capecitabine for HER2-positive advanced breast cancer: a multicentre study of Anatolian Society of Medical Oncology (ASMO). J Chemother 26(5):300–305
Chaudhary LN et al (2018) Triple-negative breast cancer: who should receive neoadjuvant chemotherapy? Surg Oncol Clin 27(1):141–153
Cheang MCU et al (2009) Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 101(10):736–750
Cheng L et al (2010) Rb inactivation accelerates neoplastic growth and substitutes for recurrent amplification of cIAP1, cIAP2 and Yap1 in sporadic mammary carcinoma associated with p53 deficiency. Oncogene 29(42):5700–5711
Choi M et al (2016) ATM mutations in cancer: therapeutic implications. Mol Cancer Ther 15(8):1781–1791
Chumsri S et al (2011) Aromatase, aromatase inhibitors, and breast cancer. J Steroid Biochem Mol Biol 125(1–2):13–22
Clarke BL, Khosla S (2009) New selective estrogen and androgen receptor modulators. Curr Opin Rheumatol 21(4):374
Dawood SS et al (2008) Prognosis of women with stage IV breast cancer by HER2 status and trastuzumab treatment: an institutional based review. J Clin Oncol 26(15_suppl):1018–1018
De Lena M et al (1975) Adriamycin plus vincristine compared to and combined with cyclophosphamide, methotrexate, and 5-fluorouracil for advanced breast cancer. Cancer 35(4):1108–1115
Article PubMed Google Scholar
Dent R et al (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13(15):4429–4434
Desmedt C et al (2009) Development and validation of gene expression profile signatures in early-stage breast cancer. Cancer Investig 27(1):1–10
Desmedt C et al (2016) Genomic characterization of primary invasive lobular breast cancer. J Clin Oncol 34(16):1872–1881
Dias K et al (2017) Claudin-low breast cancer; clinical & pathological characteristics. PLoS One 12(1):e0168669
Dieci MV et al (2014) Rare breast cancer subtypes: histological, molecular, and clinical peculiarities. Oncologist 19(8):805–813
Drew Y, Calvert H (2008) The potential of PARP inhibitors in genetic breast and ovarian cancers. Ann N Y Acad Sci 1138(1):136–145
Farmer P et al (2005) Identification of molecular apocrine breast tumors by microarray analysis. Breast Cancer Res 7(2):1–1
Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1(1):27–30
Foulkes WD et al (2003) Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst 95(19):1482–1485
Gautam M, Malhotra XZ (2010) Histological, molecular and functional subtypes of breast cancers. Cancer Biol Ther 10:955–960
Gucalp A et al (2013) Phase II trial of bicalutamide in patients with androgen receptor–positive, estrogen receptor–negative metastatic breast cancer. Clin Cancer Res 19(19):5505–5512
Halsted WS (1907) I. The results of radical operations for the cure of carcinoma of the breast. Ann Surg 46(1):1
Hellman S (1993) Dogma and inquisition in medicine. Breast cancer as a case study. Cancer 71(7):2430–2433
Hennessy BT et al (2009) Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res 69(10):4116–4124
Herschkowitz JI et al (2007) Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol 8(5):1–17
Hientz K et al (2017) The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget 8(5):8921
Huggins C, Bergenstal DM (1952) Inhibition of human mammary and prostatic cancers by adrenalectomy. Cancer Res 12(2):134–141
CAS PubMed Google Scholar
Iwamoto T, Pusztai L (2010) Predicting prognosis of breast cancer with gene signatures: are we lost in a sea of data? Genome Med 2(11):1–4
Jézéquel P et al (2015) Gene-expression molecular subtyping of triple-negative breast cancer tumors: importance of immune response. Breast Cancer Res 17(1):1–16
Konecny GE et al (2006) Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res 66(3):1630–1639
Kuong KJ, Loeb LA (2013) APOBEC3B mutagenesis in cancer. Nat Genet 45(9):964–965
Lee Y-M et al (2020) Molecular subtypes of triple-negative breast cancer: understanding of subtype categories and clinical implication. Genes Genom 42(12):1381–1387
Lefebvre C et al (2016) Mutational profile of metastatic breast cancers: a retrospective analysis. PLoS Med 13(12):e1002201
Lehmann BD et al (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121(7):2750–2767
Liu Y-R et al (2016) Comprehensive transcriptome analysis identifies novel molecular subtypes and subtype-specific RNAs of triple-negative breast cancer. Breast Cancer Res 18(1):1–10
Loibl S et al (2016) Integrated analysis of PTEN and p4EBP1 protein expression as predictors for pCR in HER2-positive breast cancer PTEN and p4EBP for pCR prediction. Clin Cancer Res 22(11):2675–2683
Luft R, Olivecrona H (1955) Hypophysectomy in man. Experiences in metastatic cancer of the breast. Cancer 8(2):261–270
Lukong KE (2017) Understanding breast cancer–the long and winding road. BBA Clin 7:64–77
McWhirter R (1948) V. The value of simple mastectomy and radiotherapy in the treatment of cancer of the breast. Br J Radiol 21:583
Mehraj U et al (2021a) Tumor microenvironment promotes breast cancer chemoresistance. Cancer Chemother Pharmacol 87(2):147–158
Mehraj U et al (2021b) The tumor microenvironment as driver of stemness and therapeutic resistance in breast cancer: new challenges and therapeutic opportunities. Cell Oncol 44:1209–1229
Mehraj U et al (2021c) Prognostic significance and targeting tumor-associated macrophages in cancer: new insights and future perspectives. Breast Cancer 28(3):539–555
Mehraj U et al (2022a) Expression pattern and prognostic significance of baculoviral inhibitor of apoptosis repeat-containing 5 (BIRC5) in breast cancer: a comprehensive analysis. Adv Cancer Biol-Metastasis 4:100037
Article CAS Google Scholar
Mehraj U et al (2022b) Cryptolepine targets TOP2A and inhibits tumor cell proliferation in breast cancer cells-an in vitro and in silico study. Anticancer Agents Med Chem 22:3025
Mehraj U et al (2022c) Adapalene inhibits the growth of triple-negative breast cancer cells by S-phase arrest and potentiates the antitumor efficacy of GDC-0941. Front Pharmacol 13:958443
Mehraj U et al (2022d) Chemokines in triple-negative breast cancer heterogeneity: new challenges for clinical implications. Semin Cancer Biol 86(Pt 2):769
Miller K et al (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357(26):2666–2676
Mir MA (2015) Developing costimulatory molecules for immunotherapy of diseases. Academic Press, London. https://doi.org/10.1016/C2014-0-02898-5 . ISBN: 9780128025857
Book Google Scholar
Mir MA, Agrewala JN (2007) Influence of CD80 and CD86 co-stimulation in the modulation of the activation of antigen presenting cells. Curr Immunol Rev 3(3):160–169
Mir MA, Agrewala JN (2008) Signaling through CD80: an approach for treating lymphomas. Expert Opin Ther Targets 12(8):969–979
Mir MA et al (2022) Immuno-onco-metabolism and therapeutic resistance. In: Immuno-oncology crosstalk and metabolism. Springer, Singapore, pp 45–89. https://link.springer.com/chapter/10.1007/978-981-16-6226-3_3
Chapter Google Scholar
Mir MA, Mehraj U (2019) Double-crosser of the immune system: macrophages in tumor progression and metastasis. Curr Immunol Rev 15(2):172–184
Mir MA et al (2020) Targeting different pathways using novel combination therapy in triple negative breast cancer. Curr Cancer Drug Targets 20(8):586–602
Montalto FI, De Amicis F (2020) Cyclin D1 in cancer: a molecular connection for cell cycle control, adhesion and invasion in tumor and stroma. Cells 9(12):2648
Muñoz-Gámez JA et al (2005) PARP inhibition sensitizes p53-deficient breast cancer cells to doxorubicin-induced apoptosis. Biochem J 386(1):119–125
Murphy JB (1912) Surgical clinics of John B. Murphy. University of Chicago, Chicago. Cholelithiasis 1 : 417
Overgaard M (1999) Overview of randomized trials in high risk breast cancer patients treated with adjuvant systemic therapy with or without postmastectomy irradiation. Semin Radiat Oncol 9(3):292
Overgaard M et al (1997) Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med 337(14):949–955
Perou CM et al (2000) Molecular portraits of human breast tumors. Nature 406(6797):747–752
Pfahler GE (1932) Results of radiation therapy in 1022 private cases of carcinoma of the breast from 1902 to 1928. Am J Roentgenol Rad Ther 27:497–408
Prat A et al (2010) Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res 12(5):1–18
Prat A et al (2013) Prognostic significance of progesterone receptor–positive tumor cells within immunohistochemically defined luminal a breast cancer. J Clin Oncol 31(2):203
Prat A et al (2014) Molecular features of the basal-like breast cancer subtype based on BRCA1 mutation status. Breast Cancer Res Treat 147(1):185–191
Prat A et al (2015) Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 24:S26–S35
Pusztai L et al (2008) Effect of molecular disease subsets on disease-free survival in randomized adjuvant chemotherapy trials for estrogen receptor–positive breast cancer. J Clin Oncol 26(28):4679–4683
Qayoom H, Bhat BA (2020) U Mehraj U, Mir MA (2020) Rising trends of cancers in Kashmir valley: distribution pattern, incidence and causes. J Oncol Res Treat 5(150):2
Qayoom H et al (2021a) Integrating immunotherapy with chemotherapy: a new approach to drug repurposing. In: Drug repurposing-molecular aspects and therapeutic applications. IntechOpen, London. https://doi.org/10.5772/intechopen.100183
Qayoom H et al (2021b) An insight into the cancer stem cell survival pathways involved in chemoresistance in triple-negative breast cancer. Future Oncol 17(31):4185–4206
Qayoom H et al (2022) Expression patterns and therapeutic implications of CDK4 across multiple carcinomas: a molecular docking and MD simulation study. Med Oncol 39(10):1–13
Qu S et al (2008) Genetic polymorphisms of metastasis suppressor gene NME1 and breast cancer survival. Clin Cancer Res 14(15):4787–4793
Ragaz J et al (1997) Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med 337(14):956–962
Ray T et al (2021) Therapeutically targeting cancers that overexpress FOXC1: a transcriptional driver of cell plasticity, partial EMT, and cancer metastasis. Front Oncol 11:721959
Reis-Filho JS et al (2010) Molecular profiling: moving away from tumor philately. Sci Transl Med 2(47):47ps43
Rimawi MF et al (2015) Targeting HER2 for the treatment of breast cancer. Annu Rev Med 66:111
Roberts SA et al (2013) An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nat Genet 45(9):970–976
Roberts MR et al (2017) Single nucleotide variants in metastasis-related genes are associated with breast cancer risk, by lymph node involvement and estrogen receptor status, in women with European and African ancestry. Mol Carcinog 56(3):1000–1009
Sabatier R et al (2014) Claudin-low breast cancers: clinical, pathological, molecular and prognostic characterization. Mol Cancer 13(1):1–14
Saha P, Nanda R (2016) Concepts and targets in triple-negative breast cancer: recent results and clinical implications. Ther Adv Med Oncol 8(5):351–359
Samavat H, Kurzer MS (2015) Estrogen metabolism and breast cancer. Cancer Lett 356(2):231–243
Sanga S et al (2009) Gene expression meta-analysis supports existence of molecular apocrine breast cancer with a role for androgen receptor and implies interactions with ErbB family. BMC Med Genet 2(1):1–16
Slamon DJ et al (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785):177–182
Smid M et al (2008) Subtypes of breast cancer show preferential site of relapse. Cancer Res 68(9):3108–3114
Sofi S et al (2022a) Targeting cyclin-dependent kinase 1 (CDK1) in cancer: molecular docking and dynamic simulations of potential CDK1 inhibitors. Med Oncol 39(9):1–15
Sofi S et al (2022b) Cyclin-dependent kinases in breast cancer: expression pattern and therapeutic implications. Med Oncol 39(6):1–16
Sørlie T et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci 98(19):10869–10874
Sørlie T et al (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci 100(14):8418–8423
Sotiriou C, Pusztai L (2009) Gene-expression signatures in breast cancer. N Engl J Med 360(8):790–800
Spitale A et al (2009) Breast cancer classification according to immunohistochemical markers: clinicopathologic features and short-term survival analysis in a population-based study from the south of Switzerland. Ann Oncol 20(4):628–635
Su Y et al (2015) The clinicopathological significance and drug target potential of FHIT in breast cancer, a meta-analysis and literature review. Drug Des Devel Ther 9:5439
PubMed PubMed Central Google Scholar
Tang P et al (2008) Molecular classifications of breast carcinoma with similar terminology and different definitions: are they the same? Hum Pathol 39(4):506–513
Thomas R et al (2021) Immune checkpoint inhibitors in triple negative breast cancer treatment: promising future prospects. Front Oncol 10:600573
Traina TA et al (2018) Enzalutamide for the treatment of androgen receptor–expressing triple-negative breast cancer. J Clin Oncol 36(9):884
Varna M et al (2011) TP53 status and response to treatment in breast cancers. J Biomed Biotechnol 2011:284584
Wakeling AE (2000) Similarities and distinctions in the mode of action of different classes of antioestrogens. Endocr Relat Cancer 7(1):17–28
Weigelt B et al (2010) The contribution of gene expression profiling to breast cancer classification, prognostication and prediction: a retrospective of the last decade. J Pathol 220(2):263–280
Wolff AC et al (2014) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med 138(2):241–256
Yersal O, Barutca S (2014) Biological subtypes of breast cancer: prognostic and therapeutic implications. World J Clin Oncol 5(3):412
Yin L et al (2020) Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res 22(1):1–13
Zhao S et al (2020) Molecular subtypes and precision treatment of triple-negative breast cancer. Ann Transl Med 8(7):499
Download references
Author information
Authors and affiliations.
Department of Bioresources, School of Biological Sciences, University of Kashmir, Srinagar, J&K, India
Manzoor Ahmad Mir & Hina Qayoom
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Manzoor Ahmad Mir .
Editor information
Editors and affiliations.
Department of Bioresources, University of Kashmir, Srinagar, Jammu and Kashmir, India
Manzoor Mir
Rights and permissions
Reprints and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Mir, M.A., Qayoom, H. (2023). Introduction to Breast Cancer. In: Mir, M. (eds) Therapeutic potential of Cell Cycle Kinases in Breast Cancer. Springer, Singapore. https://doi.org/10.1007/978-981-19-8911-7_1
Download citation
DOI : https://doi.org/10.1007/978-981-19-8911-7_1
Published : 04 March 2023
Publisher Name : Springer, Singapore
Print ISBN : 978-981-19-8910-0
Online ISBN : 978-981-19-8911-7
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Search by keyword
- Search by citation
Page 1 of 108
Pre-treatment peripheral blood immunophenotyping and response to neoadjuvant chemotherapy in operable breast cancer
Tumor immune infiltration and peripheral blood immune signatures have prognostic and predictive value in breast cancer. Whether distinct peripheral blood immune phenotypes are associated with response to neoad...
- View Full Text

Hypoxia-mediated repression of pyruvate carboxylase drives immunosuppression
Metabolic plasticity mediates breast cancer survival, growth, and immune evasion during metastasis. However, how tumor cell metabolism is influenced by and feeds back to regulate breast cancer progression are ...

Lasofoxifene as a potential treatment for aromatase inhibitor-resistant ER-positive breast cancer
Breast cancers treated with aromatase inhibitors (AIs) can develop AI resistance, which is often driven by estrogen receptor-alpha (ERα/ ESR1 ) activating mutations, as well as by ER-independent signaling pathways....
NSUN2/YBX1 promotes the progression of breast cancer by enhancing HGH1 mRNA stability through m 5 C methylation
RNA m 5 C methylation has been extensively implicated in the occurrence and development of tumors. As the main methyltransferase, NSUN2 plays a crucial regulatory role across diverse tumor types. However, the preci...
Inflammation at diagnosis and cognitive impairment two years later in breast cancer patients from the Canto-Cog study
Inflammation could be related to cancer-related cognitive impairment (CRCI) and might be used as a predictive marker of long-term CRCI. We evaluated associations between inflammatory markers assessed at diagno...
Increased expression of REG3A promotes tumorigenic behavior in triple negative breast cancer cells
Identifying new targets in triple negative breast cancer (TNBC) remains critical. REG3A (regenerating islet-derived protein 3 A), a calcium-dependent lectin protein, was thoroughly investigated for its express...
Alpha-6 integrin deletion delays the formation of Brca1/p53-deficient basal-like breast tumors by restricting luminal progenitor cell expansion
The aberrant amplification of mammary luminal progenitors is at the origin of basal-like breast cancers associated with BRCA1 mutations. Integrins mediate cell–matrix adhesion and transmit mechanical and chemi...
Deep learning-based risk stratification of preoperative breast biopsies using digital whole slide images
Nottingham histological grade (NHG) is a well established prognostic factor in breast cancer histopathology but has a high inter-assessor variability with many tumours being classified as intermediate grade, N...
Unraveling malignant phenotype of peritumoral tissue: transcriptomic insights into early-stage breast cancer
Early-stage invasive ductal carcinoma displays high survival rates due to early detection and treatments. However, there is still a chance of relapse of 3–15% after treatment. The aim of this study was to unco...
Reproductive characteristics, menopausal status, race and ethnicity, and risk of breast cancer subtypes defined by ER, PR and HER2 status: the Breast Cancer Etiology in Minorities study
Associations between reproductive factors and risk of breast cancer differ by subtype defined by joint estrogen receptor (ER), progesterone receptor (PR), and HER2 expression status. Racial and ethnic differen...
EDI3 knockdown in ER-HER2+ breast cancer cells reduces tumor burden and improves survival in two mouse models of experimental metastasis
Despite progress understanding the mechanisms underlying tumor spread, metastasis remains a clinical challenge. We identified the choline-producing glycerophosphodiesterase, EDI3 and reported its association w...
Elevated expression of wildtype RhoC promotes ErbB2- and Pik3ca- induced mammary tumor formation
Copy number gains in genes coding for Rho activating exchange factors as well as losses affecting genes coding for RhoGAP proteins are common in breast cancer (BC), suggesting that elevated Rho signaling may p...
Optimising the diagnostic accuracy of First post-contrAst SubtracTed breast MRI (FAST MRI) through interpretation-training: a multicentre e-learning study, mapping the learning curve of NHS Breast Screening Programme (NHSBSP) mammogram readers using an enriched dataset
Abbreviated breast MRI (FAST MRI) is being introduced into clinical practice to screen women with mammographically dense breasts or with a personal history of breast cancer. This study aimed to optimise diagno...
Breast cancer patients enrolled in the Swiss mammography screening program “donna” demonstrate prolonged survival
We compared the survival rates of women with breast cancer (BC) detected within versus outside the mammography screening program (MSP) “donna”.
Correction: NSABP FB-10: a phase Ib/II trial evaluating ado-trastuzumab emtansine (T-DM1) with neratinib in women with metastatic HER2-positive breast cancer
The original article was published in Breast Cancer Research 2024 26 :69
Deep learning of mammogram images to reduce unnecessary breast biopsies: a preliminary study
Patients with a Breast Imaging Reporting and Data System (BI-RADS) 4 mammogram are currently recommended for biopsy. However, 70–80% of the biopsies are negative/benign. In this study, we developed a deep lear...
Reporting on patient’s body mass index (BMI) in recent clinical trials for patients with breast cancer: a systematic review
The proportion of patients with breast cancer and obesity is increasing. While the therapeutic landscape of breast cancer has been expanding, we lack knowledge about the potential differential efficacy of most...
Infrared laser moxibustion for cancer-related fatigue in breast cancer survivors: a randomized controlled trial
Cancer-related fatigue (CRF) is a pervasive, persistent, and distressing symptom experienced by cancer patients, for which few treatments are available. We investigated the efficacy and safety of infrared lase...
Association of area- and volumetric-mammographic density and breast cancer risk in women of Asian descent: a case control study
Mammographic density (MD) has been shown to be a strong and independent risk factor for breast cancer in women of European and Asian descent. However, the majority of Asian studies to date have used BI-RADS a...
Fusogenic vesicular stomatitis virus combined with natural killer T cell immunotherapy controls metastatic breast cancer
Metastatic breast cancer is a leading cause of cancer death in woman. Current treatment options are often associated with adverse side effects and poor outcomes, demonstrating the need for effective new treatm...
Enhancing pathological complete response prediction in breast cancer: the role of dynamic characterization of DCE-MRI and its association with tumor heterogeneity
Early prediction of pathological complete response (pCR) is important for deciding appropriate treatment strategies for patients. In this study, we aimed to quantify the dynamic characteristics of dynamic cont...
Proteogenomic characterization of difficult-to-treat breast cancer with tumor cells enriched through laser microdissection
Breast cancer (BC) is the most commonly diagnosed cancer and the leading cause of cancer death among women globally. Despite advances, there is considerable variation in clinical outcomes for patients with non...
CD163 + macrophages in the triple-negative breast tumor microenvironment are associated with improved survival in the Women’s Circle of Health Study and the Women’s Circle of Health Follow-Up Study
Tumor-associated macrophages (TAMs) are a prominent immune subpopulation in the tumor microenvironment that could potentially serve as therapeutic targets for breast cancer. Thus, it is important to characteri...
TRPS1 maintains luminal progenitors in the mammary gland by repressing SRF/MRTF activity
The transcription factor TRPS1 is a context-dependent oncogene in breast cancer. In the mammary gland, TRPS1 activity is restricted to the luminal population and is critical during puberty and pregnancy. Its f...
Breast density knowledge and willingness to delay treatment for pre-operative breast cancer imaging among women with a personal history of breast cancer
Following a breast cancer diagnosis, it is uncertain whether women’s breast density knowledge influences their willingness to undergo pre-operative imaging to detect additional cancer in their breasts. We eval...
siRNA treatment targeting integrin α11 overexpressed via EZH2-driven axis inhibits drug-resistant breast cancer progression
Breast cancer, the most prevalent cancer in women worldwide, faces treatment challenges due to drug resistance, posing a serious threat to patient survival. The present study aimed to identify the key molecule...
Quantitative characterization of breast lesions and normal fibroglandular tissue using compartmentalized diffusion-weighted model: comparison of intravoxel incoherent motion and restriction spectrum imaging
To compare the compartmentalized diffusion-weighted models, intravoxel incoherent motion (IVIM) and restriction spectrum imaging (RSI), in characterizing breast lesions and normal fibroglandular tissue.
AMD1 promotes breast cancer aggressiveness via a spermidine-eIF5A hypusination-TCF4 axis
Basal-like breast cancer (BLBC) is the most aggressive subtype of breast cancer due to its aggressive characteristics and lack of effective therapeutics. However, the mechanism underlying its aggressiveness re...
NSABP FB-10: a phase Ib/II trial evaluating ado-trastuzumab emtansine (T-DM1) with neratinib in women with metastatic HER2-positive breast cancer
We previously reported our phase Ib trial, testing the safety, tolerability, and efficacy of T-DM1 + neratinib in HER2-positive metastatic breast cancer patients. Patients with ERBB2 amplification in ctDNA had...
The Correction to this article has been published in Breast Cancer Research 2024 26 :83
Screening mammography performance according to breast density: a comparison between radiologists versus standalone intelligence detection
Artificial intelligence (AI) algorithms for the independent assessment of screening mammograms have not been well established in a large screening cohort of Asian women. We compared the performance of screenin...
Clustering of HR + /HER2− breast cancer in an Asian cohort is driven by immune phenotypes
Breast cancer exhibits significant heterogeneity, manifesting in various subtypes that are critical in guiding treatment decisions. This study aimed to investigate the existence of distinct subtypes of breast ...
Outcomes of sentinel node biopsy according to MRI response in an association with the subtypes in cN1–3 breast cancer after neoadjuvant systemic therapy, multicenter cohort study
This study investigated the feasibility of sentinel lymph node biopsy (SLNB) after neoadjuvant systemic therapy (NAST) in patients with initially high nodal burden.
Meeting Abstracts from the British Society of Breast Radiology annual scientific meeting 2023
This article is part of a Supplement: Volume 26 Supplement 1
Selective omission of sentinel lymph node biopsy in mastectomy for ductal carcinoma in situ: identifying eligible candidates
Sentinel lymph node biopsy (SLNB) is recommended for patients with ductal carcinoma in situ (DCIS) undergoing mastectomy, given the concerns regarding upstaging and technical difficulties of post-mastectomy SL...
Metabolomics assisted by transcriptomics analysis to reveal metabolic characteristics and potential biomarkers associated with treatment response of neoadjuvant therapy with TCbHP regimen in HER2 + breast cancer
This study aimed to explore potential indicators associated with the neoadjuvant efficacy of TCbHP regimen (taxane, carboplatin, trastuzumab, and pertuzumab) in HER2 + breast cancer (BrCa) patients.
Chitin-mediated blockade of chitinase-like proteins reduces tumor immunosuppression, inhibits lymphatic metastasis and enhances anti-PD-1 efficacy in complementary TNBC models
Chitinase-like proteins (CLPs) play a key role in immunosuppression under inflammatory conditions such as cancer. CLPs are enzymatically inactive and become neutralized upon binding of their natural ligand chi...
Serum protein profiling reveals an inflammation signature as a predictor of early breast cancer survival
Breast cancers exhibit considerable heterogeneity in their biology, immunology, and prognosis. Currently, no validated, serum protein-based tools are available to evaluate the prognosis of patients with early ...
U2AF2-SNORA68 promotes triple-negative breast cancer stemness through the translocation of RPL23 from nucleoplasm to nucleolus and c-Myc expression
Small nucleolar RNAs (snoRNAs) play key roles in ribosome biosynthesis. However, the mechanism by which snoRNAs regulate cancer stemness remains to be fully elucidated.
Clinical factors associated with patterns of endocrine therapy adherence in premenopausal breast cancer patients
Patients with hormone receptor positive breast cancer are recommended at least five years of adjuvant endocrine therapy, but adherence to this treatment is often suboptimal. We investigated longitudinal trends...
Correction: Mcl-1 confers protection of Her2-positive breast cancer cells to hypoxia: therapeutic implications
The original article was published in Breast Cancer Research 2016 18 :26
Exploring the dynamic interplay between exosomes and the immune tumor microenvironment: implications for breast cancer progression and therapeutic strategies
Breast cancer continues to pose a substantial worldwide health concern, demanding a thorough comprehension of the complex interaction between cancerous cells and the immune system. Recent studies have shown th...
Establishing conditions for the generation and maintenance of estrogen receptor-positive organoid models of breast cancer
Patient-derived organoid models of estrogen receptor-positive (ER+) breast cancer would provide a much-needed tool to understand drug resistance and disease progression better. However, the establishment and l...
Factors associated with overall survival in breast cancer patients with leptomeningeal disease (LMD): a single institutional retrospective review
Breast cancer-related leptomeningeal disease (BC-LMD) is a dire diagnosis for 5–8% of patients with breast cancer (BC). We conducted a retrospective review of BC-LMD patients diagnosed at Moffitt Cancer Center...
Paradoxical cancer cell proliferation after FGFR inhibition through decreased p21 signaling in FGFR1-amplified breast cancer cells
Fibroblast growth factors (FGFs) control various cellular functions through fibroblast growth factor receptor (FGFR) activation, including proliferation, differentiation, migration, and survival. FGFR amplific...
Correction: The novel phosphatase NUDT5 is a critical regulator of triple-negative breast cancer growth
The original article was published in Breast Cancer Research 2024 26 :23
Temporal changes in mammographic breast density and breast cancer risk among women with benign breast disease
Benign breast disease (BBD) and high mammographic breast density (MBD) are prevalent and independent risk factors for invasive breast cancer. It has been suggested that temporal changes in MBD may impact futur...
Expression- and splicing-based multi-tissue transcriptome-wide association studies identified multiple genes for breast cancer by estrogen-receptor status
Although several transcriptome-wide association studies (TWASs) have been performed to identify genes associated with overall breast cancer (BC) risk, only a few TWAS have explored the differences in estrogen ...
BIRC5 expression by race, age and clinical factors in breast cancer patients
Survivin/BIRC5 is a proliferation marker that is associated with poor prognosis in breast cancer and an attractive therapeutic target. However, BIRC5 has not been well studied among racially diverse population...
Factors associated with engraftment success of patient-derived xenografts of breast cancer
Patient-derived xenograft (PDX) models serve as a valuable tool for the preclinical evaluation of novel therapies. They closely replicate the genetic, phenotypic, and histopathological characteristics of prima...
TMEM120B strengthens breast cancer cell stemness and accelerates chemotherapy resistance via β1-integrin/FAK-TAZ-mTOR signaling axis by binding to MYH9
Breast cancer stem cell (CSC) expansion results in tumor progression and chemoresistance; however, the modulation of CSC pluripotency remains unexplored. Transmembrane protein 120B (TMEM120B) is a newly discov...
- Editorial Board
- Manuscript editing services
- Instructions for Editors
- Sign up for article alerts and news from this journal
- Collections
- Follow us on Twitter
Annual Journal Metrics
2022 Citation Impact 7.4 - 2-year Impact Factor 7.4 - 5-year Impact Factor 1.764 - SNIP (Source Normalized Impact per Paper) 2.408 - SJR (SCImago Journal Rank)
2023 Speed 20 days submission to first editorial decision for all manuscripts (Median) 129 days submission to accept (Median)
2023 Usage 2,432,781 downloads 1,561 Altmetric mentions
- More about our metrics
Breast Cancer Research
ISSN: 1465-542X
- Submission enquiries: [email protected]
- Open access
- Published: 03 June 2024
Genome-wide in vivo CRISPR screen identifies TGFβ3 as actionable biomarker of palbociclib resistance in triple negative breast cancer
- Sophie Poulet 1 ,
- Meiou Dai 1 ,
- Ni Wang 1 ,
- Gang Yan 1 ,
- Julien Boudreault 1 ,
- Girija Daliah 1 ,
- Alan Guillevin 1 ,
- Huong Nguyen 1 ,
- Soaad Galal 1 ,
- Suhad Ali 1 &
- Jean-Jacques Lebrun 1
Molecular Cancer volume 23 , Article number: 118 ( 2024 ) Cite this article
567 Accesses
Metrics details
Triple negative breast cancer (TNBC) remains exceptionally challenging to treat. While CDK4/6 inhibitors have revolutionized HR + breast cancer therapy, there is limited understanding of their efficacy in TNBC and meaningful predictors of response and resistance to these drugs remain scarce. We conducted an in vivo genome-wide CRISPR screen using palbociclib as a selection pressure in TNBC. Hits were prioritized using microarray data from a large panel of breast cancer cell lines to identify top palbociclib sensitizers. Our study defines TGFβ3 as an actionable determinant of palbociclib sensitivity that potentiates its anti-tumor effects. Mechanistically, we show that chronic palbociclib exposure depletes p21 levels, contributing to acquired resistance, and that TGFβ3 treatment can overcome this. This study defines TGFβ3 as an actionable biomarker that can be used to improve patient stratification for palbociclib treatment and exploits the synergistic interaction between CDK4/6 and TGFβ3 to propose a new combinatorial treatment for TNBC.
Introduction
In normal tissue, cellular proliferation, cellular growth, stress management and survival are carefully controlled by stringent cell cycle checkpoints and robust DNA repair mechanisms. The complex transformation of a cell from normal to oncogenic is driven by its acquired abilities to sustain proliferation and to circumvent signalling aiming to stop proliferation, causing a deregulation of its cell cycle [ 1 ].
Cyclin-dependent kinases (CDKs) and their associated cyclins are evolutionarily conserved, central regulators of the cell cycle. Their activity is initiated by mitogenic signals and is tightly regulated by cyclin-dependent kinase inhibitors and activated cell cycle checkpoints. CDK4 and CDK6 (hereafter referred to as CDK4/6) have been shown to be essential in mediating breast tumor formation [ 2 , 3 ]. Cyclin D canonically associates with and activates CDK4/6, which mediates the transition from the G1-phase to the S-phase by phosphorylating and inactivating the retinoblastoma protein (Rb). This releases the E2F transcription factor and drives the transcription of genes responsible for the S-phase transition, including cyclin E [ 4 ]. Cyclin E, by binding to CDK2, increases its activity and results in Rb hyperphosphorylation, ultimately driving the cell into S-phase and DNA replication. This process is maintained by endogenous CDK inhibitory proteins of either the INK4 or Cip/Kip family. In breast cancer patients, amplification of the CCND1 gene may occur in up to 15% of patients, and overexpression of cyclin D1 protein is even more common, occurring in 50% of tumors [ 5 ]. For this reason, CDK4/6 has been explored as a potential therapeutic target for breast cancer.
Breast cancer is classified into three major clinical subtypes depending on the expression of the hormone receptors (HR) – estrogen receptor (ER) and progesterone receptor (PR) – and the human epidermal growth factor receptor 2 (HER2). The recent FDA approval of three CDK4/6 inhibitors (CDK4/6is), palbociclib, ribociclib, and abemaciclib, has led to the rapid adoption of targeted treatment of CDK4/6 as first-line or second-line therapy in advanced ER + /HER2- breast cancer. The indication of these inhibitors for ER + /HER2- breast cancer can be attributed to the specific dependency of these tumors on cyclin D1 and CDK4/6 [ 6 ]. As is the challenge with many anti-cancer drugs, resistance to CDK4/6 targeted therapies limits their use, ultimately leading to disease spread or relapse. Many studies have been conducted to allow for better clinical decision-making, ranging from identifying the causes of intrinsic resistance, to seeking mechanisms responsible for acquired resistance, and to searching for biomarkers of CDK4/6i efficacy. Patients with triple negative breast cancer (TNBC) have long been ineligible for CDK4/6i therapy because of the absence of ER expression and frequent Rb deletions in TNBC [ 7 ]. A phase II clinical trial by DeMichele et al. evaluating palbociclib monotherapy in Rb + metastatic breast cancer found that all four TNBC patients included were refractory to treatment by the study endpoint [ 8 ]. Although sample size constraints of the study prevented significant conclusions from being drawn from the TNBC patients tested, the trial results highlight that much remains to be understood about the interplay between TNBC tumor biology and the cell cycle. While independence from CDK4/6 signalling due to Rb deficiency is often linked to TNBCs’ resistance to CDK4/6is, only approximately 35% of TNBCs are Rb-deficient. This means that a great majority of these tumors are Rb-proficient and are thus potential candidates for CDK4/6i therapy [ 9 ]. Concordantly, we and others have shown that CDK4/6 inhibition by palbociclib reduces tumor growth in vivo in multiple Rb + TNBC models [ 10 , 11 , 12 ]. These findings indicate that there is an avenue worth exploring for CDK4/6i therapy in TNBC; however, there is an unmet need for better biomarkers of response to CDK4/6is. Such predictive markers of drug effectiveness would allow for the identification of a new subset of patients with TNBC who would likely benefit from treatment with CDK4/6is.
This study sought to identify and characterize predictive markers of sensitivity and resistance to palbociclib in TNBC, and to select actionable targets for improving palbociclib efficacy in both TNBC and the general context of breast cancer, through a combinatorial approach. Here, we conducted an in vivo genome-wide CRISPR loss-of-function screen in TNBC to identify genes that could sensitize cells to palbociclib treatment. The enriched gene set (205 genes) was then cross-referenced with microarray data from 38 breast cancer cell lines ranked based on their sensitivity/resistance levels to palbociclib and allowed us to ensure that the gene set is relevant to the broader context of breast cancer, and not limited only to the TNBC subtype. This is important considering the actual clinical context in which the drug is administered.
We aimed to validate the top candidates in vivo using preclinical xenograft models of Rb + TNBC, to confirm the corresponding genes as potential palbociclib sensitizers. We then showed that our top-ranking candidate gene, TGFB3 , could synergize with palbociclib to generate strong anti-tumor effects both in vitro and in vivo. This synergy is largely achieved through a p21-dependent mechanism, whereby the addition of TGFβ3 induces p21 expression, which further contributes to inhibiting still-active CDK4/6/cyclin D1 and CDK2/cyclin E1 complexes. To further translate our findings to the clinic, we also showed that recombinant human TGFβ3, comparable to avotermin, which has been used in several phase I and II clinical trials for the prophylactic treatment of tissue scarring of the skin, efficiently increased breast tumor response to palbociclib treatments in preclinical models of TNBC.
This study underscores the ability of TGFβ3 levels to predict sensitivity to palbociclib and highlights TGFβ3 as an actionable biomarker capable of improving palbociclib efficacy when administered in combination with palbociclib in TNBC. Our findings also highlight the robustness of the in vivo CRISPR screening and prioritization methods used to identify the effectors of palbociclib sensitivity and pave the way for further investigation into combination treatment approaches.
We aimed to identify clinically relevant genes that mediate palbociclib sensitivity by using an in vivo genome-scale CRISPR/Cas9 loss-of-function screen in a preclinical model of TNBC. We used an Rb-proficient human SUM159PT TNBC cell line [ 13 ]. We selected SUM159PT because it is (i) a well-established tumorigenic and metastatic model in vivo (ii) Rb + [ 14 ] and thus intrinsically sensitive to CDK4/6 inhibitor treatment, and (iii) representative of TNBC as it harbors PIK3CA and TP53 mutations, two of the most frequently observed mutations in TNBC [ 15 , 16 ]. As illustrated in Fig. 1 a, SUM159PT cancer cells were transduced with the lentiviral pooled genome-scale CRISPR/Cas9 knockout (KO) GeCKOv2 library. GeCKOv2 covers the whole genome with three single guide RNAs (sgRNAs) for each of the 19,050 target genes and 1000 non-targeting control sgRNAs [ 17 , 18 ]. A low multiplicity of infection (MOI ~ 0.3) was chosen to ensure the integration of only one sgRNA per cell. Due to the sheer number of cells to be transduced, and the complexity of delivering perturbation reagents directly in the host organs of a large number of mice that would have been required to perform a direct in vivo screen, an indirect screen was chosen. Stable knockout cells were thus injected subcutaneously (s.c.) into severely immunodeficient NOD scid gamma (NSG) mice at approximately 400-fold library coverage for each animal in each of the three independent experiments. Tumors were allowed to grow for seven days, until palpable. Mice were then randomized and subjected to intraperitoneal injections of either vehicle or 30 mg/kg palbociclib once daily for five days/week for 23 days. Tumor volume was monitored over the entire 30-day duration of the experiment. Exposure of GeCKO-derived tumors to palbociclib effectively reduced tumor size, illustrating the potency of palbociclib when administered in the in vivo TNBC setting (Fig. 1 b). The cell representation samples were sequenced on the day during which the cells were transplanted subcutaneously in mice, to examine the evenness of the library representation. The cell population at day 0 harbored a 99% library representation, indicative of an excellent library coverage (data not shown). Sequencing of tumors revealed a high degree of reproducibility, as demonstrated by the close grouping of principal component analysis (PCA) (Fig. 1 c) in six same-condition in vivo biological replicates. PCA again highlighted the relative separation of sgRNA distribution between the untreated and palbociclib-treated samples (Fig. 1 c). sgRNAs that were enriched or depleted after in vivo screening under palbociclib selection pressure were then identified. Enriched sgRNAs in palbociclib-treated tumors define genes conferring sensitivity to palbociclib, where loss-of-function mutations in these genes increase overall cell resistance to drug treatment and would thus present novel markers predictive of the palbociclib response. While we did not obtain any significantly depleted sgRNAs, a total of 205 candidate sgRNAs were positively enriched in the palbociclib-treated tumors (Fig. 1 d). The sgRNA enrichment profile was generated by filtering sgRNAs with false discovery rate (FDR) < 0.05. Any sgRNAs with fewer than 10 control reads were dropped from the analysis to ensure screen quality and reduce the potential for false positive hits. Gene ontology pathway enrichment analysis performed on the 205 gene list revealed no significantly enriched gene sets or pathways.

In vivo genome-wide CRISPR knockout screen in TNBC. a Schematic representation of the approach used for gene discovery and validation. b Average tumor volume in NSG mice measured over 30 days. Intraperitoneal (i.p.) injections of either vehicle or palbociclib started on day 7 post-cell implantation, and lasted 23 days. Mean of three independent infection replicate experiments ( n = 6, 2 mice per biological replicate). Data are represented as mean ± standard deviation (SD). Significance was calculated using two-sided, unpaired t-test, p -value * < 0.05, ** < 0.01, *** < 0.001. c Principal component analysis (PCA) of the sgRNAs from the library sequenced in vehicle-treated tumors ( n = 6), and palbociclib-treated tumor samples ( n = 6) at day 30 after normalization. d 205 sgRNAs were enriched with log2-fold change (LFC) > 0 at false discovery rate (FDR) < 0.05 in palbociclib-treated tumors during the screen. Genes representing significant hits are highlighted in red. e Palbociclib sensitivity data was used to rank 38 breast cancer cell lines of varying subtypes, generating two profiles of cell lines, ‘sensitive’ and ‘resistant’. GSEA was used to determine whether 205 sgRNA gene set was significantly enriched in either group of cell lines. Enrichment plot provides the distribution of the enrichment score (green line) of the 205-gene set in the ranked cell lines (sensitive to resistant, left to right). The final, positive normalized enrichment score (NES) at 1.288 indicates significant enrichment of the 205-gene set at FDR < 0.25 in palbociclib ‘sensitive’ cell lines (FDR = 0.0568, p -value = 0.0568). f Using GSEA, expression levels of the 47 genes (core enrichment subset) are presented here. Cell lines are annotated with clinical information
To shortlist candidate genes that could best predict palbociclib sensitivity in TNBC, we next cross-referenced our CRISPR screen gene dataset with microarray data from a panel of 38 breast cancer cell lines with varying sensitivities to palbociclib [ 19 ]. Cell lines were ranked from most to least sensitive based on palbociclib IC50 values determined in Finn and colleagues' study [ 7 ] and correspondingly divided into two groups: ‘more sensitive’ and ‘less sensitive’ to palbociclib. Using Gene Set Enrichment Analysis (GSEA), we sought to determine if the gene set obtained by our screen was enriched in the ‘more sensitive’ cell lines sorted by sensitivity to palbociclib (IC50) [ 20 ]. As expected, our 205-gene set was significantly upregulated at FDR < 0.25 in cell lines which are sensitive to palbociclib (FDR = 0.0568) (Fig. 1 e). The ‘more sensitive’ cell lines expressed higher levels of genes in our gene set, underscoring the power of our screen to identify genes predictive of palbociclib efficacy across a broad landscape of breast cancer subtypes (Suppl. Figure 1a). Of this gene set, 47 genes formed the ‘core enrichment subset’ as defined by GSEA; genes which contributed most to the positive normalized enrichment score (NES) generated for the entire gene set [ 20 , 21 ].
We hypothesized that this subset would therefore have the strongest association with palbociclib effectiveness and could serve as a predictive gene signature for palbociclib sensitivity and overall clinical outcomes in patients. We associated the 38 cell lines used in the GSEA with corresponding clinical information. As expected, this cell line ranking coincided with clustering of cell lines based on Rb proficiency, hormone receptor (HR)/HER2 status, and molecular subtype classification, such that known CDK4/6 sensitivity phenotype criteria were fulfilled (Fig. 1 f) [ 22 , 23 , 24 ]. Indeed, Rb-deficient cell lines clustered together in the ‘less sensitive’ subgroup, as did most cell lines representing the basal subtype of breast cancer. Conversely, HR + and HER2 + cell lines, and cell lines of luminal or HER2 molecular subtype, largely clustered in the ‘more sensitive’ subgroup (Fig. 1 f). These findings contributed to our confidence in the screening and the prioritization methods used as they allowed us to situate our results in the context of what is already known. Nonetheless, these results also help strengthen our rationale for the study, showing that palbociclib sensitivity is not simply dictated by ER status or Rb mutation status during patient stratification. We next sought to evaluate whether the 47-gene core enrichment subset could serve as a predictive gene signature for palbociclib sensitivity and overall clinical outcomes in publicly available data sets. We evaluated these genes’ expression patterns in a cohort of patients with breast invasive carcinoma (METABRIC) using cBioPortal [ 25 , 26 , 27 ]. We observed a trend towards a decrease in gene expression in the HR-/HER2- (TNBC) subgroup, as compared to the other groups classified by their expression of HR and HER2, although this was not significant (Suppl. Figure 1b). A seemingly lower expression of the 47-gene signature was also observed in the more aggressive basal and claudin-low groups of patient samples, and tended to correlate with higher tumor grade, although this was not significant (Suppl. Figure 1c, d). Taken together, the significant upregulation of the 205-gene set obtained from our in vivo CRISPR/Cas9 screening in the 20 ‘more sensitive’ cell lines underscores the power of the screen to reliably and robustly identify markers of drug effectiveness. These findings strengthen the predictive power of the gene signature defined using our prioritization method, showing that overall lower expression of genes here correlates with poorer clinical outcomes in general, while also promoting palbociclib resistance.
Having evaluated the clinical relevance of the 47-gene signature using patient data, we next assessed these genes' ability to modulate the palbociclib response in vivo, using TNBC xenograft models. For this, the eight top-ranking genes of the 47-gene core enrichment subset ( SLC40A1, TGFB3, SNRPN, ITGB6, BAMBI, TMEM176A, PDGFB and TMEM150A ) were selected for validation. Briefly, each gene was individually knocked-out in SUM159PT using CRISPR/Cas9 before being orthotopically transplanted in the mammary fat pad of NSG mice, as previously described [ 10 , 17 ]. Gene modification efficiency was assessed using a SURVEYOR assay from a bulk population of cells, confirming the indel mutations for each KO (Fig. 2 a). Once tumors became palpable, daily intraperitoneal injections of the vehicle or 30 mg/kg palbociclib were each administered to five mice within each group, where each group consisted of 10–12 mice per gene knockout. As expected, tumor growth in non-targeting (NT) control mice groups was significantly inhibited by palbociclib by study endpoint (Fig. 2 b, c). We found that individual knockout of our target genes effectively made cells more resistant to palbociclib over time (Fig. 2 b). By study endpoint, all eight of the eight individual KOs (SLC40A1g1, TGFB3g1, ITGB6g3, BAMBIg2, TMEM176Ag3, PDGFBg1 and TMEM150Ag2) significantly inhibited the palbociclib anti-tumor effect in vivo, defining these genes as key regulators of TNBC response to palbociclib (Fig. 2 c).

In vivo validation of top candidate genes. a Gene modification detection of individual CRISPR-mediated knockouts of top candidate genes. b Cells transduced with non-targeting (NT) control or top candidate gene ( SLC40A1, TGFB3, SNRPN, ITGB6, BAMBI, TMEM176A or PDGFB, TMEM150A ) KO constructs were transplanted orthotopically into the mammary fat pads of NSG mice. Tumors were palpable before mice from each NT ( n = 10–22) or targeting group ( n = 10–12) were randomized into treatment groups (vehicle, n = 5–11; palbociclib (30 mg/kg), n = 5–11). Mean ± SD tumor volume is shown. Significance was calculated using two-sided, unpaired t-test, p -value ns. = nonsignificant, * < 0.05. c Tumor volumes of individual mice in each group, NT or targeting a candidate gene, either treated with vehicle or palbociclib at experiment endpoint ( n = 5). Midlines indicate median tumor volume. Significance was calculated using two-sided, unpaired t-test, p -value * < 0.05
Having found that the depletion of our top targets generated resistance to palbociclib, we further explored the clinical translatability of our genes to predict the sensitivity of mammary tumors to CDK4/6 inhibitors. Accordingly, we used patient data from the NeoPalAna clinical trial, a single-arm phase II clinical trial evaluating the neoadjuvant use of palbociclib, with an anastrozole backbone, in clinical stage 2 or 3 ER + primary breast cancer [ 28 ]. Upon starting the trial, eligible patients received the aromatase inhibitor anastrozole (1 mg daily) for 28 days (Cycle 0). Palbociclib (125 mg daily on days 1–21, Cycle 1) was then added to the treatment regimen on day 1 of cycle 1 (C1D1). Tumor biopsies were collected on C1D1 and 14 days after the start of palbociclib treatment (C1D15). Although all patients were ER + , the only clinical subtype of breast cancer assumed to be responsive to palbociclib, the response to treatment varied in these patients. This illustrates the inadequacy of relying solely on the predictive power of ER positivity. We therefore posited that varying the expression levels of other genes, such as genes from our shortlist, might better predict these varying responses to palbociclib. Gene expression data from total RNA were generated using an Agilent microarray platform during the trial. Here, we compared data from palbociclib-sensitive patients with data from patients deemed palbociclib-resistant at C1D15 because of an inability to achieve complete cell cycle arrest (Ki67 > 2.7%). At C1D1, analysis of gene expression levels revealed lower levels of SLC40A1 and TGFB3 in resistant versus sensitive patients (Suppl Fig. 2a). This trend of lower SLC40A1 and TGFB3 expression in resistant versus sensitive patients was also observed at C1D15. Some of the remaining genes showed similar trends at both time points, but the overall statistical analysis was difficult to perform given that there were too few patients for whom we had gene expression data in the ‘palbociclib-resistant’ group. These data should therefore be interpreted with caution. Nonetheless, we propose that the trends observed in the expression of the top two genes, SLC40A1 and TGFB3 , hint at the potential clinical relevance of our CRISPR screening results in Rb-proficient TNBC in patients with varying Rb statuses in ER + patients.
Analysis of publicly available clinical data on KM Plotter revealed that many of these genes were also correlated with relapse-free survival (RFS) across all breast cancer subtypes [ 29 ]. Lower gene expression of SLC40A1, TGFB3, SNRPN, TMEM176A and TMEM150A was significantly correlated ( p < 0.05) with lower RFS (Suppl. Figure 2b). This may suggest that lower expression of these genes not only affects the response to palbociclib treatment but is also indicative of a worse overall prognosis for breast cancer patients.
Altogether, these results highlight the robustness of both the prioritization and the screening design used in our study. Furthermore, our in vivo findings may attest to the translatability of these results towards clinical applications, as we found that patients who were resistant to palbociclib did have lower median expression of SLC40A1 and TGFB3 in the NeoPalAna trial.
The high ranking obtained by TGFB3 in the prioritization scheme, the strong negation of the palbociclib effect by TGFB3 knockout in vivo, along with the inverse relationship observed between TGFB3 expression and palbociclib resistance in patients led us to further explore the potential value of TGFβ3 as a sensitizer to the palbociclib response. We hypothesized that the effect of palbociclib would be potentiated in TGFB3 -overexpressing tumors, resulting in a greater growth reduction than in control tumors. Therefore, we applied a gain-of-function approach through activation of the TGFB3 endogenous gene promoter using the CRISPR/dCas9 Synergistic Activation Mediator (SAM) system, as previously described [ 10 , 30 ]. As shown in Fig. 3 a, we strongly induced TGFB3 gene expression in SUM159PT cells using three different sgRNAs targeting the TGFB3 gene promoter, without affecting TGFB1 or TGFB2 expression. TGFB3g2 SAM-infected SUM159PT cells were transplanted into the mammary fat pads of NSG mice.
TGFβ3 potentiates palbociclib anti-tumor effect in vivo. a mRNA expression levels of TGFB1, TGFB2 and TGFB3 in SUM159PT following TGFB3 -specific overexpression using CRISPR activation (CRISPR/dCas9 SAM) ( n = 3). Data are represented as mean ± standard deviation (SD). Significance was calculated using two-sided, unpaired t-test, p -value * < 0.05. b Mice from control (lentiSAMv2) or TGFB3 -overexpressing (TGFB3g2 SAM) groups ( n = 13) were each randomized into treatment groups (vehicle, n = 6; palbociclib, n = 7). I.p. injections of the vehicle treatment or a low dose of palbociclib (10 mg/kg) were administered until study endpoint. Data are represented as mean ± SD. c Reduction in tumor growth presented for each group treated with palbociclib, lentiSAMv2 or TGFB3g2 SAM, as compared to the same groups treated with the vehicle. Data are represented as mean, at each timepoint. d left Tumor volumes of individual mice in each group at study endpoint. right Tumor weights of individual mice in each group at study endpoint. Midlines at median. Significance was calculated using ordinary, one-way ANOVA with Tukey’s multiple comparisons test, p -value * < 0.05, ** < 0.01, *** < 0.001. e Average mRNA expression levels of TGFB3 in tumors derived from the vehicle-treated control mice ( n = 6) and the TGFB3 -overexpressing mice ( n = 6). Data are represented as mean ± SD. Significance was calculated using two-sided, unpaired t-test, p -value * < 0.05, ** < 0.01, *** < 0.001. f Protein levels of TGFB3 (60 kDa) in tumors derived from the vehicle-treated control mice ( n = 6) and the TGFB3 -overexpressing mice ( n = 6). g Spontaneous metastasis to the lungs was assessed. Lung nodules were counted and compared in lungs derived from the vehicle-treated control mice ( n = 7) and the TGFB3 -overexpressing mice ( n = 6). Data represent metastatic nodule count per pair of lungs per mouse. Midlines at median. Significance was calculated using nonparametric Mann–Whitney U-test, p -value * < 0.05, ** < 0.01, *** < 0.001. h The effect of TGFB3 CRISPR-mediated knockout on lung colonization was assessed. Data represent metastatic nodule count per pair of lungs per mouse. Midlines at median. i Schematic representation of the use of recTGFβ3 in combination with palbociclib. MDA-MB-231 TNBC cells were transplanted into the mammary fat pads of NSG mice. Tumors were palpable before mice were randomized into treatment groups: vehicle, n = 9; recTGFβ3, n = 8; palbociclib, n = 8, combo (recTGFβ3 + palbociclib), n = 9. j Average tumor volume was measured over time. Data are represented as mean ± SD. k Tumor volumes of individual mice in each group at study endpoint. Midlines at median. Significance was calculated using ordinary, one-way ANOVA with Tukey’s multiple comparisons test, p -value * < 0.05. l Quantification of Ki67-positive cells stained by immunohistochemistry in tumor tissues from all four groups. Data are represented as mean ± SD ( n = 3–4). Significance was calculated using two-sided, unpaired t-test, p -value * < 0.05. m Representative images of Ki67 staining in two tumors per group
Tumors were grown until palpable and treated daily with a relatively low dose of palbociclib (10 mg/kg, i.p.) or vehicle up to 33 days post-implantation. Here, low-dose palbociclib was used to allow for the observation of a potential synergy between treatment and high TGFβ3 levels. As shown in Fig. 3 b, low-dose (10 mg/kg) palbociclib treatment significantly reduced tumor growth in the lentiSAMv2 control tumors. A similar level of effect was observed when TGFB3 expression was induced in untreated cells (TGFB3g2 SAM vehicle). However, of the mice treated with palbociclib, those with TGFB3 -overexpressing tumors had significantly lower average tumor growth rates than the control mice (Fig. 3 b). Statistical significance of the difference in tumor volume was measured at all timepoints and is provided in Suppl. Figure 3a. This is reflected in the mean palbociclib-mediated tumor growth inhibition in each group of mice at every timepoint investigated, where the palbociclib effect on tumor growth inhibition is significantly greater in TGFB3 -overexpressing tumors as compared to control mice during the entire experiment (Fig. 3 c). This is indicative of a potentiation of the palbociclib effect by TGFβ3. At the study endpoint, palbociclib treatment combined with increased TGFB3 expression greatly reduced tumor volume compared to that in control mice (lentiSAMv2) treated with palbociclib (Fig. 3 d, left panel). Tumors were weighed upon resection, and the results shown in Fig. 3 d (right panel) indicate that the anti-tumor effects of palbociclib were also greatly enhanced when TGFB3 was overexpressed. To verify that the enhanced anti-tumor effect observed in the TGFB3 SAM tumors was attributable to a sustained increase in TGFB3 levels, TGFB3 levels were assessed in excised tumors. TGFB3 SAM tumors exhibited higher levels of TGFB3 at both the mRNA level and the protein level than the control tumors (Fig. 3 e, f). Taken together, these results suggest that an increase in TGFB3 expression activates a synthetic lethal interaction upon CDK4/6 inhibition, allowing for greater growth inhibition.
Having thus far only evaluated TGFβ3’s contribution to tumor suppression, we wanted to address the other, pro-metastatic arm of the TGFβ family’s dual role in cancer – a concern due to frequent extrapolation of data relating to TGFβ1’s role in promoting breast cancer to TGFβ3 [ 31 ]. The role of TGFβ in providing breast cancer cells with metastatic capabilities – such as inducing epithelial-to-mesenchymal transition and priming cells for extravasation, has been well established for TGFβ1 [ 32 , 33 ]. However, the TGFβ3 ligand specifically has not been well studied. Thus, we evaluated the effect of TGFB3 overexpression on the spontaneous metastasis of orthotopically transplanted breast cancer cells to the lungs using the CRISPR/dCas9 SAM system described above. Lung nodules were counted after euthanizing the transplanted mice. Mice overexpressing TGFB3 showed significantly fewer nodules on average than non-targeting control mice (Fig. 3 g). In a follow-up experiment, we assessed the effect of TGFB3 gene silencing on lung colonization. TGFB3 KO SUM159PT cells were injected into the tail veins of NSG mice, and lung nodules were counted 38 days after cell injection. We observed a trend towards an increased number of nodules in TGFB3 KO mice compared to non-targeting control mice (Fig. 3 h). Taken together, these data suggest that inducing TGFB3 gene expression does not adversely affect lung metastasis in vivo, while leading to an increased sensitivity of tumors to palbociclib treatment in vivo . This highlights a possible therapeutic avenue for the administration of exogenous TGFβ3 .
Therefore, we exploited the inherent ease of use of TGFβ3 as a potential treatment, being a naturally occurring ligand. Human recombinant TGFβ3 (recTGFβ3) has previously been developed into an intradermal injectable (avotermin) and has been safely used in phase II and III clinical trials for the prevention of scarring [ 34 ]. To validate our findings in another TNBC model and thereby broaden the scope of the implications of our findings, we assessed recTGFβ3/palbociclib anti-tumorigenic effects when administered alone or in combination in preformed MDA-MB-231-derived mammary tumors. MDA-MB-231 is a poorly differentiated, aggressive TNBC cell line derived from the pleural effusion of a 51-year-old Caucasian female [ 35 ]. These cells were transplanted into the mammary fat pads of NSG mice, which were then randomized into four groups. Either the vehicle, human recTGFβ3 alone (2 µg/kg), palbociclib alone (10 mg/kg), or a combination of recTGFβ3 (2 µg/kg) and palbociclib (10 mg/kg) was administered intraperitoneally to mice in each group (Fig. 3 i). Treatment was initiated 33 days after transplantation, once the tumors were palpable and administered daily. The smallest average tumor volume was observed in the combination group (Fig. 3 j). By the endpoint, mice from the groups treated with suboptimal doses of either recTGFβ3 alone or palbociclib alone showed comparable tumor volumes to mice in the control group, whereas the recTGFβ3 + palbociclib combination group had significantly smaller tumors than the control group (Fig. 3 k, Suppl. Figure 3b). Moreover, analysis of the proliferation index (Ki67) by immunohistochemistry in these tumors revealed that the combination treatment significantly reduced the proportion of proliferating cells as compared to the vehicle (Fig. 3 l, m). This is reflective of tumor volume at endpoint, as neither palbociclib alone nor recTGFβ3 alone significantly reduced cell proliferation in vivo, indicating a potential synergy between the two treatments when administered together. These findings highlight the clinical relevance of TGFβ3 as a synthetic lethal target in our screen for its role in potentiating the anti-tumor effects of palbociclib when administered as a recombinant protein. They indicate the ease with which TGFβ3 could be administered in the clinic in combination with palbociclib to achieve significant tumor growth inhibition using low doses of either treatment. This could potentially help avoid unwanted adverse effects of using high individual doses while allowing for on-target inhibition of tumor growth unachievable at low doses of palbociclib.
Having shown that both TGFB3 overexpression and the use of recTGFβ3 significantly promoted the palbociclib response in reducing tumor growth (Fig. 3 ), we sought to gain insight into the molecular mechanism by which these two drugs work together. To better understand the nature of the relationship between palbociclib and recTGFβ3, we assessed combinatorial synergy using drug matrix assays in multiple Rb + TNBC cell lines: SUM159PT, SUM229PE, and MDA-MB-231. To start to address this, dose–response analyses with TGFβ3 or palbociclib alone were performed in these TNBC cell lines. As shown in Suppl. Figure 4a, TGFβ3 stimulation of the cells only produced a modest effect that plateaued at approximately 20% growth inhibition. Palbociclib efficiently reduced cell viability within a given concentration range (Suppl. Figure 4b). Ultimately, dose ranges of palbociclib (12.5 nM to 400 nM) and recTGFβ3 (3.13 pM to 100 pM) were used alone or in combination and cell proliferation was assessed by crystal violet staining.
We used four reference synergy models to assess combinatorial effects in our study: Bliss, Highest Single Agent (HSA), Loewe, and Zero Interaction Potency (ZIP). Each of these models uses different formulas and assumptions to calculate drug combination synergy [ 36 ]. Interestingly, we found that for all cell lines tested, overall synergy was observed across the dose combinations tested, with scores greater than 10 indicating a strong likelihood of a synergistic relationship [ 36 ] (Fig. 4 a). Notably, cotreatment attained a level of synergy that could be reproducibly obtained using all four models tested. The highest degrees of synergism tended to occur at the lower concentrations used for palbociclib, as denoted by the grey rectangles in each graph and the ‘Most synergistic area score’ (Fig. 4 a, b). The percentages of treatment-induced proliferation inhibition for each pairwise comparison in the drug matrices presented help underscore the impact of the combination treatment in each cell line (Suppl. Figure 4d). This further highlights the clinical relevance of our findings, where submaximal doses of palbociclib could be administered, limiting the associated side effects and reducing the need for treatment cycle delays, along with TGFβ3, to achieve an even greater anti-proliferative effect than palbociclib alone. This is especially relevant in a context where cancer patients are subjected to many treatment-associated toxicities, both with palbociclib and radiotherapy or chemotherapy treatments [ 37 ].

Combination of recombinant TGFβ3 and palbociclib synergistically inhibits TNBC cell proliferation in vitro. a Synergy between palbociclib and recTGFβ3 dose combinations was calculated based on four reference models (Bliss, HSA, Loewe, ZIP) using SynergyFinder in four TNBC cell lines (MDA-MB-231, SUM159PT, SUM229PE, 159-R). Synergy maps highlight areas of synergistic (red) or antagonistic (green) interactions between given concentrations of either agent. Grey boxes indicate the area of maximum synergy observed. Mean of a minimum of three independent replicate experiments for each cell line ( n ≥ 3). b ‘Overall synergy scores’ and ‘Most synergistic area scores’ presented for each drug matrix shown in a . Data are represented as score ± 95% confidence interval. c Dot plots show overall synergy scores (black) or most synergistic area scores (pink) for each cell line, with each dot representing the score obtained using the indicated reference model. Midlines represent median scores. Outer vertical lines correspond to minimum and maximum scores obtained. A zero ‘0’ score indicates no interaction between the two agents
We then investigated whether recTGFβ3 could be used to resensitize cells to palbociclib in a model where cells had become resistant to palbociclib due to chronic exposure to the drug. To this end, we first generated a palbociclib-resistant SUM159PT cell line (159-R) by treating SUM159PT with gradually increasing concentrations of palbociclib over four months. A dose–response curve evaluating palbociclib response in 159-R was used to confirm palbociclib resistance (Suppl. Figure 4c). We performed drug matrix assays using palbociclib concentrations ranging from 78 nM to 2.5 µM, while TGFβ3 concentrations ranged from 3.13 pM to 100 pM. Although higher concentrations of palbociclib were necessary in 159-R to generate a similar level of response to the low doses of palbociclib used in parental SUM159PT, we chose to keep the same range of recTGFβ3 concentrations to determine whether resistant cells could be resensitized to palbociclib at the same low concentrations. We found that not only could resistant cells be resensitized to palbociclib by cotreatment with recTGFβ3, but that TGFβ3 could synergize with the effects of palbociclib. Indeed, in 159-R, overall synergy was achieved for the drug concentration ranges tested using all four algorithms (Fig. 4 a, b, Suppl. Figure 4d). As demonstrated in Fig. 4 c, the robustness of this interaction is made evident by the high synergy scores obtained in all cell lines, regardless of previous exposure to palbociclib, and across all algorithms for the ‘Overall synergy scores’ (black) as well as ‘Most synergistic area scores’ (pink). The potential noninteractive zone (dotted line) was excluded from the range of scores obtained for every synergy score analysis (Fig. 4 c). The synergy demonstrated in the treatment-naïve context helps to characterize the interplay observed in the in vivo study, demonstrating that the combination of recTGFβ3 + palbociclib treatment leads to the greatest tumor growth inhibition. Most importantly, this synergy is still achieved when cells are desensitized to palbociclib through chronic exposure to the drug.
To understand the molecular mechanisms underlying the synergism between palbociclib and TGFβ3 growth inhibitory effects in TNBC, we examined the effects of palbociclib on the expression levels of cell cycle regulators. Palbociclib treatment of SUM159PT cells over 24 h led to significant time-dependent increases in established resistance markers, such as CDK4, cyclin D1 and cyclin E1, along with concomitant decreases in Rb and phospho-Rb (Ser780) (Fig. 5 a). The various times at which these changes in protein levels occurred may reflect the indirect nature of these changes in protein levels. Of note, observable and significant changes in phosphorylation of Rb occurred earlier in the time course, whereas a significant decrease in Rb levels was observed after 24 h only (Fig. 5 a). We observed no consistent changes in CDK6 nor the CDK inhibitor CDKN1B (p27) over 24 h. For CDKN2A (p16) and CDKN1C (p57), we found there was no detectable signal. However, there were changes in protein levels of the other phases of the cell cycle, especially later in the time course (Suppl. Figure 5a). Accordingly, these decreases in CDK1, cyclin A1, cyclin B1, and PLK1 were in line with the decrease in proportion of cells which proceeded to S-phase and continued cycling through the cell cycle after addition of palbociclib (Suppl. Figure 5b). Indeed, following cell cycle analysis by flow cytometry, it is clear that treatment with palbociclib arrests cells in G1, but that the induction of G1 arrest is strongest and significant upon the addition of recTGFβ3, which also entails a significant decrease in the proportion of cells in S-phase (Suppl. Figure 5b).

TGFβ3 synergizes with palbociclib in a p21-dependent way . a SUM159PT cells were treated with palbociclib (100 nM) for 2 h, 8 h, 16 h and 24 h and protein lysates were assessed for known CDK4/6i resistance markers (CDK4, cyclin D1, cyclin E1, Rb, phospho-Rb (S780)) by immunoblotting. Relative fold changes in protein levels, compared to untreated cells at each timepoint, were calculated ( n = 3). Data are represented as mean ± SD. Significance was calculated using two-sided, unpaired t-test, p -value * < 0.05, ** < 0.01, *** < 0.001. b SUM159PT (159) and 159-R cells were assessed for known CDK4/6i resistance markers, as well as p21, by immunoblotting. c top SUM159PT and 159-R cells were treated with recTGFβ3 (100 pM) for 24 h and resulting changes in known CDK4/6i resistance markers and p21 were measured by immunoblotting. bottom MDA-MB-231 (231) and SUM229PE (229) cells were treated with recTGFβ3 (200 pM) for 24-48 h and resulting changes in p21 were measured by immunoblotting. d SUM159PT cells were transduced with plasmids encoding control (scramble, scr), Smad2-specific, or Smad3-specific short hairpin RNAs (shRNA). Protein levels of p21 and total Smad2/3 were measured by immunoblotting. e SUM159PT cells were transduced with plasmids encoding control (scr) and p21-specific shRNA. Protein levels of p21 were measured by immunoblotting. f SUM159PT scr shRNA-infected or p21 shRNA-infected cells were treated with varying combinations of palbociclib and recTGFβ3 concentrations. Synergy between dose combinations was calculated using SynergyFinder. upper Synergy maps highlight areas of synergistic (red) or antagonistic (green) interactions between given concentrations of either agent. Grey boxes indicate the area of maximum synergy observed between given recTGFβ3 and palbociclib dose combinations. lower ‘Overall synergy scores’ and ‘Most synergistic area scores’ presented for each drug matrix shown above. Data are represented as score ± 95% confidence interval ( n = 3). Percentage variation in synergy score (score obtained in p21 shRNA cells/score obtained in scr shRNA cells) is also shown (red)
To next determine whether these changes in cell cycle marker expression would be transposed in the long-term palbociclib acquired resistance context, we compared their levels in naïve and resistant cells that had undergone chronic exposure to the drug, in SUM159PT and 159-R, respectively. As shown in Fig. 5 b, strong increases in CDK4, cyclin D1, and cyclin E1, along with a stark decrease in Rb and p-Rb expression, were observed in the resistant cells, indicating that the effects of chronic palbociclib exposure mimicked the changes in marker levels observed in the short-term acquired context. We also found that palbociclib decreased the expression of the cell cycle inhibitor CDKN1A (p21). This defines p21 as a palbociclib target and is consistent with decreased palbociclib efficacy and short-term acquired resistance.
The TGFβ family of ligands acts as potent tumor suppressors notably by inducing CDK inhibitors (CDKIs) [ 38 ]. Thus, we examined whether TGFβ3 could modulate the expression of the CDK inhibitor p21 in both parental and palbociclib-resistant SUM159 cells. As shown in Fig. 5 c, TGFβ3 strongly induced p21 expression in multiple TNBC cell lines, as demonstrated in SUM159PT, MDA-MB-231 and SUM229PE. Furthermore, it restored p21 levels in palbociclib-resistant cells, suggesting that TGFβ3-mediated p21 expression induction contributes to the synergism observed between palbociclib and recTGFβ3. This is also exhibited at the mRNA level, where treatment with recTGFβ3 significantly induces p21 levels in SUM159PT and, to an even greater extent, in 159-R (Suppl. Figure 5c). At the basal level, without recTGFβ3 treatment, there is a significant decrease in p21 in cells chronically exposed to palbociclib, 159-R, at the mRNA level (Suppl. Figure 5c). This is reflected at the protein level as well (Fig. 5 c). Therefore, we further addressed the specific role and contribution of p21 in mediating these effects. First, we determined that the effect of p21 upregulation by TGFβ3 was Smad2/3-dependent. When Smad2 and Smad3 were knocked down individually in SUM159 cells, the TGFβ3-mediated increase in p21 level was diminished (Fig. 5 d). Given that Smad2/3 induction of p21 occurs through the well-established canonical Smad signaling pathway shared by all TGFβ isoforms, we asked whether the synergy observed between TGFβ3 and palbociclib could also be observed between TGFβ1 and TGFβ2 with palbociclib. We tested whether these isoforms could confer similar synergistic effects on palbociclib efficacy in MDA-MB-231 and SUM159PT cell lines and found that all three TGFβ isoforms demonstrate a similar effect on palbociclib efficacy (Suppl. Figure 5d). This is in line with the proposed mechanism of action underlying the synergy between TGFβ3 and palbociclib, which occurs through a mechanism common to all three isoforms.
In defining this relationship between Smad2/3 signaling and p21 expression, we examined whether the decrease in p21 observed in palbociclib-treated cells was also mediated through canonical TGFβ Smad signaling. We observed no added contribution to phosphorylation of Smad2/3 or change in total Smad2/3 following palbociclib treatment alone or in combination with recTGFβ3 (Suppl. Figure 5e). Next, we sought to determine whether p21 was at least partially responsible for the synergy observed between palbociclib and TGFβ3 by knocking down p21 in 159-R cells using a p21-specific shRNA (Fig. 5 e). Using a drug matrix to characterize the drug-response relationship between a range of pairs of recTGFβ3-palbociclib doses, we found that the synergy scores for the entire matrix tested (‘Overall synergy scores’) strongly decreased with all algorithms – by as much as 34.3% (Bliss) – in the absence of p21 (Fig. 5 f). Similarly, all ‘Most synergistic area scores’ in p21 knockdown cells decreased by as much as 39.2% (Bliss) for a given algorithm (Fig. 5 f), highlighting the dependence, albeit partial, of TGFβ3-palbociclib synergy on p21.
Altogether, we showed that known cell cycle markers, such as CDK4, cyclin D1 and cyclin E1, are upregulated as early as 2 h following palbociclib treatment, leading to an overall increase in the components necessary for active cyclin/CDK complexes. We also observed a striking decrease in the level of p21 upon chronic exposure to palbociclib, highlighting an additional route by which cells may become desensitized to palbociclib treatment over time. Stimulation of these chronically exposed cells (159-R) with TGFβ3 increased p21 levels and overcame the downregulation of p21 induced by chronic exposure to palbociclib. Finally, we showed that the TGFβ3-mediated increase in p21 is Smad2/3-dependent and plays an important role in the synergism observed between palbociclib and TGFβ3 in TNBC.
Based on these findings and previous literature, we propose a mechanistic model for the synergism between TGFβ3 and palbociclib. First, in the basal context, cells maintain a balance between active (green) and p21-bound inactive (red) CDK/cyclin complexes. In the presence of palbociclib, CDK4/6 kinase activity is blocked by the inhibitor, while p21 bound to CDK4 is released and displaced to CDK2, inactivating CDK2/cyclin E complexes, and leading to cell cycle arrest [ 39 ] (Fig. 6 a). However, upon prolonged exposure to palbociclib, the expression of key cell cycle regulators (CDK4, cyclins D and E) is induced while p21 expression is strongly inhibited, as demonstrated in Fig. 5 b. Considering that the increase in the individual expression of key regulators known to bind together, we propose that this implies an increase in the number of complexes formed, and notably, an imbalance in active CDK4/cyclin D1 and CDK2/cyclin E1 complexes (Fig. 6 b, upper panel). This progressively leads to acquired palbociclib resistance and reduced drug efficacy. In the presence of both palbociclib and TGFβ3, synergy occurs, where p21 expression levels are restored through TGFβ3, allowing for inactivation of all remaining active CDK/cyclin complexes and thus an increase in p21-bound – thus inactivated – complexes (Fig. 6 b, lower panel). This leads to an improved palbociclib response and cell cycle arrest in vitro, ultimately leading to the greater inhibitory effect of the combination treatment observed in vivo .
Schematic diagram depicting TGFβ3-palbociclib synergy. a In the basal context, cells maintain a balance between active (green) and p21-bound, inactive (red) CDK/cyclin complexes. In the presence of palbociclib (orange capsule), CDK4/6 kinase activity is inactivated, and p21 (pink box) bound to CDK4 is released and preferentially displaced to CDK2. This inactivates CDK2/cyclin E complexes and leads to overall cell cycle arrest. b upper When cells undergo prolonged exposure to palbociclib, key cell cycle regulators (CDK4, cyclins D and E) are upregulated, while p21 expression is strongly inhibited. Some CDK/cyclin complexes are inactivated (red), but the overall imbalance in active CDK4/cyclinD1 and CDK2/cyclinE1 complexes (green) leads to decreased responsiveness of cells to palbociclib, acquired resistance to the drug, and continued cell cycling. lower When TGFβ3 is added in the presence of palbociclib, p21 expression levels are restored through TGFβ3 signalling. The increase in p21 by TGFβ3 synergizes with palbociclib’s mechanism of action, allowing for the inactivation of all remaining active CDK/cyclin complexes (red), and ultimately leading to cell cycle arrest
Over the last decade, an increasing amount of evidence supporting a clear clinical benefit of CDK4/6is has led to a rising rate of prescription of these drugs for ER + /HER2- breast cancer. However, there is limited understanding of their efficacy in triple negative breast cancer (TNBC). Therefore, there is an urgent need for proper patient stratification as well as relevant markers of sensitivity and resistance to CDK4/6 inhibitors. To address this, we performed a genome-wide loss-of-function CRISPR screen using palbociclib as a selection pressure to identify markers of sensitivity for CDK4/6is. The advent of CRISPR technology use in eukaryotic cells has revolutionized the way forward genetic screens are performed to answer biological questions, and large-scale in vitro CRISPR screens have been instrumental in identifying common essential genes [ 40 , 41 , 42 ] and new markers of drug sensitivity or resistance in vitro [ 43 , 44 , 45 ] . In vivo CRISPR screens are considered superior models, as they better recapitulate and more closely resemble the patient 3D tumor microenvironment [ 46 , 47 ]. Our screen was performed in vivo to increase the translatability and clinical relevance of the results by better modeling the tumorigenic process.
Using GSEA, we cross-referenced our screening results with existing palbociclib sensitivity data from a panel of 38 breast cancer cell lines. This allowed us to validate that our screening results in TNBC were indeed viable in the larger context of other subtypes of breast cancer, including the well-established HR + /HER2- subtype. Our prioritization strategy notably attributed certain cell lines typifying the classically ‘CDK4/6 inhibitor-resistant’ phenotype to the ‘palbociclib more sensitive’ subgroup, paving the way for further studies to re-evaluate the criteria for choosing potential recipients of palbociclib treatment. Of note, past studies have often excluded TNBC on the basis of HR negativity, but, as witnessed here, other markers used together or alone could better predict the response to CDK4/6i treatment. Our screen identified several hundred candidate genes associated with sensitivity to palbociclib. Eight of the eight top candidate genes identified in our screen were found to mediate the loss of sensitivity to palbociclib, highlighting the robustness of our screening and hit prioritization approaches. Interestingly, 4/8 of our top targets ( TGFB3, ITGB6, BAMBI, PDGFB ) belong to the TGFβ signalling pathway, highlighting this pathway as an important regulator of the palbociclib response in TNBC.
Using available clinical trial data for ER + /HER2- BC patients with known clinical outcomes following palbociclib treatment (NeoPalAna) [ 28 ], we found that low expression of the top two validated genes, SLC40A1 and TGFB3 , correlated with resistance to palbociclib. This correlation validates the applicability of our results generated in a TNBC model, albeit Rb + , to other subtypes of breast cancer, namely ER + /HER2- breast cancer. This is also supported by the GSEA results. Ultimately, this reflects the usefulness of such screens in identifying clinically predictive molecular markers of responses to therapy in the future. These findings are especially relevant, given that the current predictive markers of response to CDK4/6 inhibitors are not foolproof. Markers, such as the presence of ER, are used as inclusion criteria in clinical trials for breast cancer and fail to reliably translate into meaningful clinical outcomes for many patients. Indeed, 20% of ER + patients enrolled in the phase III PALOMA-3 trial evaluating palbociclib efficacy were initially refractory to treatment (PFS < 6 months). An additional 50% of patients developed resistance to palbociclib during the first 24 months of treatment [ 48 ].
We retained TGFB3 because of its remarkable effect in mediating sensitivity to palbociclib in vivo and its clinical relevance in predicting palbociclib resistance in the trial dataset. Despite the scarcity of information regarding the role of TGFβ3 in tumorigenesis [ 31 ], its function in normal tissues is relatively well defined. TGFβ3 plays an important role in embryogenesis, wound healing, scarless injury repair, and tissue homeostasis. This, in fact, led to the enrolment of recombinant human TGFβ3 (avotermin) in several phase I and II clinical trials for the prophylactic treatment of tissue scarring of the skin [ 31 , 34 ]. Notably, TGFβ3 distinguishes its anti-scarring role from TGFβ1 and TGFβ2’s pro-scarring effects [ 34 ]. No safety concerns were raised before the termination of trials due to failure to show efficacy in phase III trials (possibly due to a change in the standard used to assay avotermin dosage, which ultimately led to much lower doses being used in phase III trials) [ 49 ]. In normal mammary tissue, it has been shown that TGFβ3 expression is increased during pregnancy, falling during lactation and peaking after weaning, during mammary gland involution. The massive induction of TGFβ3 after lactation, during mammary gland involution, contributes to the striking difference seen in expression levels as compared to TGFβ1 and TGFβ2 at this time [ 50 , 51 , 52 ]. TGFβ3’s distinct role in wound healing may explain how TGFβ3 relates to the tumorigenic process after mammary gland involution. Indeed, a parallel between mammary gland involution and tissue remodeling can be proposed; where TGFβ3, as opposed to TGFβ1 and TGFβ2, limits stromal activation associated with tissue scarring and pro-tumorigenic properties in this context [ 53 ]. In fact, in general breast cancer datasets, TGFβ3 seems to be protective against breast cancer [ 53 ]. Consistent with this, our results clearly highlight recTGFβ3 as a potential new combination treatment for patients with breast cancer receiving palbociclib.
To explore the predictive biomarker potential and clinical relevance of TGFβ3, we used the CRISPR activation system to overexpress endogenous TGFβ3 in TNBC tumors. We found that the anti-tumor effects of palbociclib were potentiated in TGFB3 -overexpressing tumors, highlighting the value of TGFB3 in predicting palbociclib response in TNBC. Collectively, these results help demonstrate that better patient stratification, for example through the inclusion of patients with higher TGFB3 levels, during clinical trial enrolment may allow for patients with classically ‘unresponsive’ tumors, such as TNBC, to benefit from CDK4/6is. Future studies are required to determine whether measurement of TGFβ3 in liquid biopsies, for example, is feasible. The identification of biomarkers could have wider implications and be especially useful, given the current efforts being made to test the efficacy of CDK4/6is in other types of cancers.
We found that recTGFβ3 significantly potentiated the palbociclib-mediated inhibitory effects on cell proliferation and tumor growth, highlighting the clinical potential of recTGFβ3/palbociclib combination therapy for TNBC. TGFβ signalling is known to affect treatment sensitivity in breast cancer [ 54 , 55 , 56 , 57 ]. Of note, suppression of the TGFβ signalling pathway has previously been associated with resistance to CDK4/6 inhibitors through an extracellular miRNA-mediated mechanism in ER + breast cancer [ 58 ]. It would be interesting to further investigate whether the synergy observed between TGFβ3 and palbociclib is observable in other cancer types in which palbociclib treatment is being studied.
TGFβ induces the expression of the INK4 family of CDK inhibitors, including p21 CIP1 (p21) [ 38 , 59 ]. It has been shown that CDK4/6 inhibitors, including palbociclib, selectively redistribute p21 from CDK4/cyclin D1 complexes to inhibit CDK2 activity [ 39 ]. The role of p21 in the CDK4/6 inhibitor mechanism of action is not yet well established, but numerous reports indicate that low levels of p21 do seem to contribute to resistance to CDK4/6 inhibitors [ 60 , 61 , 62 , 63 ]. A study by Dean and colleagues demonstrated that prolonged exposure of cells to CDK4/6 inhibition leads to loss of the CDKIs p21 and p27 at the protein level only – not at the transcript level – implying that posttranscriptional mechanisms were responsible for this loss [ 62 ]. This decrease in p21 protein level may be likened to the loss in Rb protein, but not mRNA, following CDK4/6 exposure. While Rb degradation appears in many studies to be proteasome-dependent, it is unclear whether this process is dependent on ubiquitination [ 64 , 65 , 66 , 67 , 68 ]. Thus, it cannot be excluded that Rb is degraded by multiple mechanisms. We demonstrate that basal p21 levels are significantly lower in palbociclib resistant cells at the mRNA level, and that treatment with TGFβ3 leads to a significant increase in both p21 mRNA and protein levels in this context. Further studies elucidating how p21 levels are decreased by CDK4/6 inhibition, and indeed how this may compare to decreased Rb levels would be valuable. We demonstrated that the synergy observed between TGFβ3 and palbociclib was largely achieved through a p21-dependent mechanism, whereby the addition of recTGFβ3 induces p21 expression, which we posit helps inhibit still-active CDK4/6/cyclin D1 and CDK2/cyclin E1 complexes (Fig. 6 ). This dependence on p21 to achieve synergy between recTGFβ3 treatment and palbociclib treatment is further illustrated by the fact that administration of TGFβ1 and TGFβ2, which are also known to induce p21 in a Smad-dependent manner, equally potentiated the palbociclib effect in vitro. It is possible that the knockout efficiency of the other two TGFβ isoforms was not sufficient to produce a functional reduction of palbociclib sensitivity in the CRISPR/Cas9 screen used to identify TGFβ3, explaining why these other two isoforms did not appear enriched in the screen. The demonstration that stronger anti-tumorigenic effects could be achieved upon treatment with both palbociclib and recTGFβ3 simultaneously in multiple TNBC cell lines is of clinical relevance, especially considering the low concentrations of palbociclib at which this was achieved. Using lower concentrations of palbociclib, while still achieving comparable or even stronger anti-tumor responses while TGFβ3 levels are elevated, could help prevent some of the associated on-target toxicity in patient [ 37 ].
Patients often begin CDK4/6i treatment and become resistant to therapy over time. To address whether TGFβ3 could resensitize cells that had become insensitive to palbociclib treatment over time, we generated a palbociclib-resistant cell line over four months, and then treated the cells with recTGFβ3. We found that not only could TGFβ3 resensitize cells to palbociclib, but the combined effect of both TGFβ3 and palbociclib was significantly greater than the effect of either agent alone. Combination treatment with TGFβ3 and palbociclib achieved a synergistic anti-proliferative effect, indicating that administration of recTGFβ3 could be a relevant therapeutic strategy in the context of acquired resistance to palbociclib over time.
Altogether, this study exploited the synthetic lethal interaction between CDK4/6 and TGFβ3 and defined a new combinatorial treatment for TNBC using CDK4/6i and recombinant human TGFβ3. In addition, our study highlights TGFβ3 as a predictive marker to inform patient stratification for palbociclib treatment in breast cancer, underscoring the robustness of in vivo genome-wide CRISPR screening approaches to identify actionable biomarkers of drug response.
Materials and methods
Experimental design.
This study used a genome-wide CRISPR/Cas9 loss-of-function screen to reveal markers of sensitivity and resistance to palbociclib in a CDK4/6 inhibitor-sensitive TNBC model. SUM159PT TNBC cells were infected with a genome-wide CRISPR library and transplanted into NSG mice. Palbociclib was administered to mice as tumors grew, and tumors were extracted and sequenced. Biological and technical replicates were measured. The aim was to identify candidate genes which could predict sensitivity or resistance to palbociclib across all molecular types of breast cancer. Therefore, candidates identified by sequencing were cross-referenced with their respective expression levels in publicly available microarray data from 38 breast cancer cell lines which were categorized based on known sensitivity to palbociclib. Using GSEA, top candidate genes were determined, and validation was performed orthotopically in vivo in NSG mice with daily injections of palbociclib . Loss of TGFβ3 using an individual CRISPR knockout in SUM159 was shown to generate resistance to palbociclib. TGFβ3 was further explored for its role in mediating palbociclib resistance, and it was demonstrated that treating cells with recombinant human TGFβ3 synergized with palbociclib in vivo in another model of TNBC, using preformed orthotopic mammary tumors derived from MDA-MB-231. This was also shown in the context of multiple palbociclib-naïve and palbociclib-resistant TNBC cell lines, and found to be p21-dependent. All experiments were performed with a minimum of three biological replicates. Tumor volumes were measured blindly with a digital caliper. Tumors were always randomized into vehicle and treatment groups, before treatment began.
Cell lines and cell culture
SUM159PT and SUM229PE were cultured in Ham’s F-12, 1X (WISENT INC.) containing 5% fetal bovine serum (FBS, Gibco), 5 µg/mL insulin and 1 µg/mL hydrocortisone. More information about these cell lines is available at Breast Cancer Cell Line Knowledge Base ( www.sumlineknowledgebase.com ). MDA-MB-231 and HEK293T were cultured in Dulbecco’s Modified Eagle Medium (DMEM, WISENT INC.) supplemented with 10% FBS (Gibco). Cell lines were routinely tested by the Diagnostic Laboratory of the Comparative Medicine and Animal Resources Centre (McGill University) and are mycoplasma negative.
Generation of 159-R cell line
SUM159PT cells were initiated to palbociclib isethionate (MedChemExpress, HY-A0065) exposure at a low concentration (100 nM) of the drug. Cells were passaged before reaching confluence and treated with incrementally higher concentrations of palbociclib (+ 100 nM every week for 12 weeks). After Week 12, the concentration was increased to 2 µM and was increased by 1 µM each week until 5 µM was reached.
Genome-wide library (GeCKOv2) infection and in vivo transplantation
Human genome-scale CRISPR knockout pooled library (GeCKOv2, Addgene plasmid #1,000,000,048) was amplified according to manufacturer’s instructions and as shown previously [ 17 ]. 3 × 10 6 SUM159PT cells were seeded per well in 12-well plates and polybrene (8 μg/mL) (EMD Millipore Corp. #TR-1003-G) was added to complete medium. Cells were spin-infected with previously titered lentivirus (MOI 0.3–0.5) at 800 × g for 2 h at 32 °C. Cells were then incubated overnight and subsequently detached, pooled and seeded into T225 flasks. 24 h following infection, puromycin (2 µg/mL) (InvivoGen) was added to medium and cells underwent selection over 9 days. 3 × 10 7 cells were then collected and frozen at -80 °C for subsequent genomic DNA extraction. For each replicate of the screen, 3 × 10 7 cells were transplanted subcutaneously in 4 nod-scid gamma (NSG) mice. Seven days later, once tumors were palpable, 2 mice were assigned to each treatment group. The vehicle (75% saline + 25% Tween-80) or palbociclib isethionate (MedChemExpress, HY-A0065) (30 mg/kg) dissolved in the vehicle was administered intraperitoneally 5 days/week for 23 days. Mice were sacrificed once it was no longer ethical to continue the experiment, when vehicle tumors became too large (experiment endpoint) and tumors were then collected and frozen at -80 °C for subsequent genomic DNA extraction.
Genomic DNA extraction
For each sample, 3 × 10 7 cells (cell representation sample) or 200 mg mechanically grinded tumor tissue (tumor sample) was lysed in 6 mL of NK Lysis Buffer (50 mM Tris, 50 mM EDTA, 1% SDS, pH 8) and 30 μL of 20 mg/mL Proteinase K (Qiagen). Cell lysates were incubated at 55 °C for 1 h (cell pellet) and tumor tissue was incubated overnight. RNAse A (QIAGEN) was added (0.05 mg/mL) and samples were incubated at 37 °C for 30 min, and then on ice for 10 min. 2 mL of ice-cold 7.5 M ammonium acetate (Sigma) was added to each sample before samples were briefly vortexed and centrifuged (4000 × g for 10 min). Supernatants were collected and isopropanol was added for DNA precipitation. Samples were centrifuged and remaining pellets were washed in 70% cold ethanol and resuspended in 1 × TE Buffer.
Library preparation and deep sequencing
Next generation sequencing library was generated by two-step PCR. All PCR reactions were performed using Herculase II Fusion DNA Polymerase (Agilent). PCR1 reactions were prepared by mixing 20 μL Herculase 5 × Buffer, 1 μL of 100 mM dNTP, 2.5 μL of Adapter Primer F, 2.5 μL of Adapter Primer R, 1 μL Herculase II Fusion Enzyme, 10 μg of gDNA and completing to 100 μL with PCR-grade water. After individual validation, PCR1 reactions were pooled and stored at − 20 °C. PCR2 reactions were prepared by mixing 20 μL Herculase 5 × Buffer, 1 μL of 100 mM dNTP, 2.5 μL of Adapter Primer F, 2.5 μL of Adapter Primer R, 1 μL Herculase II Fusion Enzyme, 5 μL of PCR1 amplicon and completing to 100 μL with PCR-grade water. Final PCR products were migrated on a 2% agarose gel, extracted and purified using the QIAquick PCR & Gel Cleanup Kit (QIAGEN). Samples were sequenced (20 million reads) at Génome Québec ( https://www.genomequebec.com/ ).
Data processing and bioinformatics
MAGeCK and MAGeCK-VISPR were used to perform read count mapping, normalization, quality control and to identify sgRNA/gene hits [ 69 ]. sgRNA enrichment profile was generated by filtering for sgRNAs with false discovery rate (FDR) < 0.05. sgRNAs with mean control reads < 10 were removed, to reduce the potential for false positive hits included in the profile. Non-targeting and miRNA-targeting sgRNAs were further excluded from the profile. Significant hits were selected on the basis of having one or more specific gRNA out of the 3 sgRNAs/target present in the library, using a false discovery rate cutoff of < 0.05. It was also ensured that for each significantly enriched sgRNA targeting a given gene, no other gRNA targeting this gene was found to be depleted.
Gene set enrichment analysis
Palbociclib sensitivity data from Finn et al. was used to rank 38 breast cancer cell lines, generating two profiles of cell lines, ‘sensitive’ (palbociclib IC50 < median) and ‘resistant’ (palbociclib IC50 > median) [ 7 ]. Gene expression data from the 38 cell lines was obtained from Kao et al [ 19 ]. The gene set used for gene set enrichment analysis was composed of the genes encoded by the 205 sgRNAs enriched (FDR < 0.05) in the in vivo CRISPR screen.
CRISPR individual knockout and CRISPR activation plasmid cloning
For generation of knockout constructs, lentiCRISPRv2 backbone vector was obtained as a gift from Feng Zhang (Addgene plasmid # 52,961). For generation of activation constructs, lentiSAMv2 (Addgene plasmid # 75,112) and lentiMPHv2 (Addgene plasmid # 89,308) were used. Oligonucleotide sequences for KO and SAM sgRNAs are listed in Supplementary Table 1.
Genomic DNA cleavage assay
Genomic DNA cleavage detection assays were performed for each individual gene knockout using the GeneArt Genomic Cleavage Detection Kit (Invitrogen, cat. no. A24372) according to the manufacturer’s protocol. Briefly, 5 × 10 5 knockout cells were harvested and lysed. Genomic DNA was extracted and the specific Cas9/sgRNA genetically modified region was PCR-amplified using primers listed in Supplementary Table 1. Insertions or deletions (indels) to the region of interest were then detected.
In vivo orthotopic xenograft studies
For individual gene knockout or activation validation, transduced SUM159PT knockout or activation cells (1 × 10 6 /mouse) were diluted 1:1 in Matrigel (BD Bioscience) and then transplanted in the mammary fat pads of 8-week-old, female NSG mice. Tumors were measured with an electronic caliper three times per week and allowed to reach a maximum volume of approximately 1000 mm 3 prior to euthanasia. Tumor volumes were calculated according to the following formula: [4/3 × π × (length/2) × (width/2) 2 ]. For treatments with palbociclib and/or recombinant human TGFβ3 ligand, SUM159PT- or MDA-MB-231- derived tumors were allowed to grow for 3–4 weeks until palpable. Palbociclib isethionate was dissolved in 75% saline and 25% Tween 80 (Sigma-Aldrich, P1754) solution. Palbociclib was administered in 10 mg/kg or 30 mg/kg doses. Recombinant human TGFβ3 ligand (PeproTech, Inc, cat. no. 100-36E) was dissolved in 10 mM citric acid buffer with 0.1% BSA. TGFβ3 was administered in 2 µg/kg doses. Volumes of all solutions injected were adjusted based on individual weight of each mouse. All injections were intraperitoneal. In the case where mice received combination treatment, a 4 h delay between palbociclib and TGFβ3 injections was respected to reduce the potential for formulation interactions between the two treatments. All mice were housed and handled in accordance with the approved guidelines of the Canadian Council on Animal Care (CCAC) “Guide to the Care and Use of Experimental Animals”.
In vivo lung colonization studies
Individual CRISPR-mediated knockouts were generated in SUM159PT cells, and 1 × 10 6 cells were injected into the tail vein of NSG mice to allow for lung colonization. Mice were euthanized and lung tissue was collected. Lungs were fixed and stained in Bouin’s solution and metastatic lesions were manually counted.
NeoPalAna clinical trial
The NeoPalAna phase II clinical trial evaluated the efficacy of neoadjuvant palbociclib + anastrazole treatment in stage II-III ER + primary breast cancer [ 28 ]. The trial enrolled 50 patients. Patients received anastrozole (1 mg, daily) alone for the first 28 days (cycle 0), after which palbociclib (125 mg, daily) was added to the treatment regimen, on day 1 of cycle 1 of treatment (C1D1). Tumor biopsies were collected at C1D1, and 14 days following the start of palbociclib treatment (C1D15). If complete cell cycle arrest (Ki67 > 2.7%) was not achieved by C1D15, patients were deemed ‘resistant’ to treatment.
Quantitative PCR
Frozen tumor tissues (50 mg) were homogenized in 1 mL TriZOL Reagent, and extraction proceeded according to the manufacturer’s protocol. RNA was reverse-transcribed using M-MLV Reverse Transcriptase (Invitrogen). Real-time PCR was performed using SsoFast EvaGreen Supermix (Bio-Rad) on a Rotor-Gene 6000 PCR analyzer (Corbett).
Immunohistochemistry and scoring
Tumors were fixed in 10% formalin for minimum of 24 h. Tissues were paraffin embedded before they were mounted on slides. Following deparaffinization and rehydration, slides were immersed in retrieval solution (sodium citrate 10 mM, pH 6.0 buffer). The slides were incubated in hydrogen peroxide blocks, followed by Ultra V Block. Slides were incubated with Ki67 antibody. Ultra-Vision LP Detection System HRP Polymer & DAB Plus Chromogen (ThermoFisher Scientific) was used for detection. The slides were scanned using Aperio ScanScope XT slide (Leica Biosystems). Quantification of Ki67-positive tumor cells was performed using the Aperio Positive Pixel Count algorithm.
Cell proliferation assay
Cells were seeded on 96-well plates and treated with palbociclib isethionate and/or recTGFβ3 at the indicated concentrations in complete medium for 5–7 days. Cells were then washed with PBS and stained and fixed with a 0.5% crystal violet solution in 25% methanol for 20 min at room temperature. Cell proliferation was assessed by absorbance at 570 nm. The percentage growth inhibition was used to calculate synergy scores using SynergyFinder https://synergyfinder.fimm.fi/ .
shRNA knockdown
Scramble, p21-specific, Smad2-specific and Smad3-specific shRNA plasmids were purchased from Sigma. Transfer vectors were transfected into HEK293T cells along with packaging plasmids p.MD2G and psPAX2. Virus was collected and used to infect 4.5 × 10 5 SUM159 or SUM159 palbociclib-resistant (159-R) cells previously seeded in 6-cm plates and left to attach overnight. Cells were puromycin-selected (2 µg/mL) for 48 h and seeded for downstream analysis.
Immunoblotting
Total protein were extracted in ice-cold lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 100 mM Na3VO4, 1 × protease inhibitor cocktail and 1 × PhosStop Phosphatase Inhibitor Cocktail (Roche), diluted in 5 × loading buffer and boiled at 95 °C for 5 min. Samples were separated by SDS-PAGE, transferred onto nitrocellulose before being assessed by immunoblotting with the indicated antibodies.
Flow cytometry
For cell synchronization, cells were serum starved for 24 h. Cells were released from arrest by addition of complete medium including 5% FBS for 24 h. Cells were treated with indicated agent palbociclib alone (100 nM), recTGFb3 alone (100 pM) or a combination of both (100 nM palbociclib + 100 pM recTGFb3). For propidium iodide (PI) staining, cells were detached, centrifuged at low speed and then counted. Following fixation with 70% ethanol, cells were washed twice with 1 × PBS. 100 µg/mL RNAase A and 50 µg/mL PI in 1 × PBS was added to 1 × 10 6 cells for 30 min at 37°C, and cells were analyzed using the BD FACSCanto ™ II flow cytometer (BD Biosciences).
Statistical analyses
Multiple groups were compared using regular, one-way ANOVA with Tukey’s multiple comparisons tests. Difference between two group means was analyzed using unpaired, two-sided t-tests, with Holm-Šídák correction for multiple comparisons when applicable. Kaplan–Meier survival was analyzed using the log-rank test and presented as hazard ratios with 95% confidence intervals. P-values were considered significant when p < 0.05.
Availability of data and materials
The data generated in this study are available within the article and its supplementary data files.
Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell. 2000;100:57–70.
Article CAS PubMed Google Scholar
Malumbres M, et al. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell. 2004;118:493–504.
Landis MW, Pawlyk BS, Li T, Sicinski P, Hinds PW. Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell. 2006;9:13–22.
Massagué J. G1 cell-cycle control and cancer. Nature. 2004;432:298.
Article PubMed Google Scholar
Gillett C, et al. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Can Res. 1994;54:1812–7.
CAS Google Scholar
Foster JS, Henley DC, Bukovsky A, Seth P, Wimalasena J. Multifaceted regulation of cell cycle progression by estrogen: regulation of Cdk inhibitors and Cdc25A independent of cyclin D1-Cdk4 function. Mol Cell Biol. 2001;21:794–810.
Article CAS PubMed PubMed Central Google Scholar
Finn RS, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast cancer research : BCR. 2009;11:R77.
Article PubMed PubMed Central Google Scholar
DeMichele A, et al. CDK4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:995–1001.
Treré D, et al. High prevalence of retinoblastoma protein loss in triple-negative breast cancers and its association with a good prognosis in patients treated with adjuvant chemotherapy. Ann Oncol. 2009;20:1818–23.
Dai M, et al. Differential Regulation of Cancer Progression by CDK4/6 Plays a Central Role in DNA Replication and Repair Pathways. Can Res. 2021;81:1332–46.
Article CAS Google Scholar
Shu S, et al. Synthetic Lethal and Resistance Interactions with BET Bromodomain Inhibitors in Triple-Negative Breast Cancer. Mol Cell. 2020;78:1096-1113.e1098.
Ge JY, et al. Acquired resistance to combined BET and CDK4/6 inhibition in triple-negative breast cancer. Nat Commun. 2020;11:2350.
Flanagan L, Weelden KV, Ammerman C, Ethier SP, Welsh J. SUM-159PT cells: a novel estrogen independent human breast cancer model system. Breast Cancer Res Treat. 1999;58:193–204.
Hollestelle A, et al. Distinct gene mutation profiles among luminal-type and basal-type breast cancer cell lines. Breast Cancer Res Treat. 2010;121:53–64.
Lehmann BD, Pietenpol JA. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J Pathol. 2014;232:142–50.
Shah SP, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486:395–9.
Dai M, et al. In vivo genome-wide CRISPR screen reveals breast cancer vulnerabilities and synergistic mTOR/Hippo targeted combination therapy. Nat Commun. 2021;12:3055.
Shalem O, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–7.
Kao J, et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS ONE. 2009;4: e6146.
Subramanian A, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci. 2005;102:15545–50.
Mootha VK, et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–73.
Dai X, Cheng H, Bai Z, Li J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J Cancer. 2017;8:3131–41.
Jiang G, et al. Comprehensive comparison of molecular portraits between cell lines and tumors in breast cancer. BMC Genomics. 2016;17:525.
Neve RM, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–27.
Curtis C, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–52.
Cerami E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4.
Gao J, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:1.
Article Google Scholar
Ma CX, et al. NeoPalAna: Neoadjuvant Palbociclib, a Cyclin-Dependent Kinase 4/6 Inhibitor, and Anastrozole for Clinical Stage 2 or 3 Estrogen Receptor-Positive Breast Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23:4055–65.
Lánczky A, Győrffy B. Web-based survival analysis tool tailored for medical research (KMplot): development and implementation. J Med Internet Res. 2021;23: e27633.
Konermann S, et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517:583–8.
Laverty HG, Wakefield LM, Occleston NL, O’Kane S, Ferguson MWJ. TGF-β3 and cancer: A review. Cytokine Growth Factor Rev. 2009;20:305–17.
Kang Y, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–49.
Lebrun, J.J. The Dual Role of TGF in Human Cancer: From Tumor Suppression to Cancer Metastasis. ISRN Molecular Biology 2012;28.
Durani P, Occleston N, O’Kane S, Ferguson MW. Avotermin: a novel antiscarring agent. Int J Low Extrem Wounds. 2008;7:160–8.
Cailleau R, Young R, Olivé M, Reeves WJ Jr. Breast tumor cell lines from pleural effusions. J Natl Cancer Inst. 1974;53:661–74.
visual analytics of multi-drug combination synergies. Ianevski, A., Giri, A.K. & Aittokallio, T. SynergyFinder 2.0. Nucleic Acids Res. 2020;48:W488–93.
Google Scholar
Finn RS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375:1925–36.
Reynisdottir I, Polyak K, Iavarone A, Massague J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 1995;9:1831–45.
Pack LR, Daigh LH, Chung M, Meyer T. Clinical CDK4/6 inhibitors induce selective and immediate dissociation of p21 from cyclin D-CDK4 to inhibit CDK2. Nat Commun. 2021;12:3356.
Marcotte R, et al. Functional Genomic Landscape of Human Breast Cancer Drivers, Vulnerabilities, and Resistance. Cell. 2016;164:293–309.
Tsherniak A, et al. Defining a Cancer Dependency Map. Cell. 2017;170:564–76 e516.
Meyers RM, et al. Computational correction of copy number effect improves specificity of CRISPR-Cas9 essentiality screens in cancer cells. Nat Genet. 2017;49:1779–84.
Pettitt SJ, et al. Genome-wide and high-density CRISPR-Cas9 screens identify point mutations in PARP1 causing PARP inhibitor resistance. Nat Commun. 2018;9:1849.
Makhov P, et al. CRISPR/Cas9 genome-wide loss-of-function screening identifies druggable cellular factors involved in sunitinib resistance in renal cell carcinoma. Br J Cancer. 2020;123:1749–56.
Barghout SH, et al. A genome-wide CRISPR/Cas9 screen in acute myeloid leukemia cells identifies regulators of TAK-243 sensitivity. JCI Insight. 2021;6:e141518.
Kuhn M, Santinha AJ, Platt RJ. Moving from in vitro to in vivo CRISPR screens. Gene and Genome Editing. 2021;2: 100008.
Shalem O, Sanjana NE, Zhang F. High-throughput functional genomics using CRISPR–Cas9. Nat Rev Genet. 2015;16:299.
Cristofanilli M, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–39.
Little JA, et al. TGF β 3 immunoassay standardization: comparison of NIBSC reference preparation code 98/608 with avotermin lot 205–0505-005. J Immunoassay Immunochem. 2012;33:66–81.
Faure E, Heisterkamp N, Groffen J, Kaartinen V. Differential expression of TGF-β isoforms during postlactational mammary gland involution. Cell Tissue Res. 2000;300:89–95.
Atwood CS, Ikeda M, Vonderhaar BK. Involution of Mouse Mammary Glands in Whole Organ Culture: A Model for Studying Programmed Cell Death. Biochem Biophys Res Commun. 1995;207:860–7.
Nguyen AV, Pollard JW. Transforming growth factor beta3 induces cell death during the first stage of mammary gland involution. Development. 2000;127:3107–18.
Flanders KC, Wakefield LM. Transforming growth factor-(beta)s and mammary gland involution; functional roles and implications for cancer progression. J Mammary Gland Biol Neoplasia. 2009;14:131–44.
Bhola NE, et al. TGF-β inhibition enhances chemotherapy action against triple-negative breast cancer. J Clin Investig. 2013;123:1348–58.
Stüber T, et al. Inhibition of TGF-β-receptor signaling augments the antitumor function of ROR1-specific CAR T-cells against triple-negative breast cancer. J Immunother Cancer. 2020;8: e000676.
Liu L, et al. TGFβ induces “BRCAness” and sensitivity to PARP inhibition in breast cancer by regulating DNA-repair genes. Mol Cancer Res. 2014;12:1597–609.
Xu X, et al. TGF-β plays a vital role in triple-negative breast cancer (TNBC) drug-resistance through regulating stemness, EMT and apoptosis. Biochem Biophys Res Commun. 2018;502:160–5.
Cornell L, Wander SA, Visal T, Wagle N, Shapiro GI. MicroRNA-Mediated Suppression of the TGF-β Pathway Confers Transmissible and Reversible CDK4/6 Inhibitor Resistance. Cell Rep. 2019;26:2667-2680.e2667.
Datto MB, et al. Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci USA. 1995;92:5545–9.
Pennycook BR, Barr AR. Palbociclib-mediated cell cycle arrest can occur in the absence of the CDK inhibitors p21 and p27. Open Biol. 2021;11: 210125.
Vilgelm AE, et al. MDM2 antagonists overcome intrinsic resistance to CDK4/6 inhibition by inducing p21. Sci Transl Med. 2019;11(505):eaav7171.
Dean JL, Thangavel C, McClendon AK, Reed CA, Knudsen ES. Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene. 2010;29:4018–32.
AbuHammad S, et al. Regulation of PRMT5-MDM4 axis is critical in the response to CDK4/6 inhibitors in melanoma. Proc Natl Acad Sci USA. 2019;116:17990–8000.
Sdek P, et al. MDM2 promotes proteasome-dependent ubiquitin-independent degradation of retinoblastoma protein. Mol Cell. 2005;20:699–708.
Kim S, et al. Sequential activation of E2F via Rb degradation and c-Myc drives resistance to CDK4/6 inhibitors in breast cancer. Cell Rep. 2023;42: 113198.
Dang F, et al. Inhibition of CK1ε potentiates the therapeutic efficacy of CDK4/6 inhibitor in breast cancer. Nat Commun. 2021;12:5386.
Liu H, et al. Human U3 protein14a is a novel type ubiquitin ligase that binds RB and promotes RB degradation depending on a leucine-rich region. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2018;1865:1611–20.
Wang Y, et al. A Novel Retinoblastoma Protein (RB) E3 Ubiquitin Ligase (NRBE3) Promotes RB Degradation and Is Transcriptionally Regulated by E2F1 Transcription Factor. J Biol Chem. 2015;290:28200–13.
Li W, et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 2014;15:554.
Download references
Acknowledgements
The authors thank Dr. Stephen Ethier for help with providing SUM159PT and SUM229PE cell line. JB received a scholarship from the Fonds de Recherche du Québec Santé (FRQS) and SG received a scholarship from the Egyptian Ministry of Higher Education, Mission Sector.
The Canadian Institutes for Health Research (CIHR) provided the funding for this study (CIHR operating grant to JJL).
Author information
Authors and affiliations.
Department of Medicine, Cancer Research Program, McGill University Health Centre, Montreal, QC, Canada
Sophie Poulet, Meiou Dai, Ni Wang, Gang Yan, Julien Boudreault, Girija Daliah, Alan Guillevin, Huong Nguyen, Soaad Galal, Suhad Ali & Jean-Jacques Lebrun
You can also search for this author in PubMed Google Scholar
Contributions
SP, MD, and JJL were involved in designing all experiments and analyzing and interpreting the data. SP performed experiments and prepared the manuscript. MD, NW, GY, JB, GD, AG, HN and SG assisted in the experiments. SA and JJL assisted with data analysis and manuscript preparation. All authors have read and approved the final manuscript.
Corresponding author
Correspondence to Jean-Jacques Lebrun .
Ethics declarations
Ethics approval and consent to participate.
All animals were housed and handled in accordance with the approved guidelines of the Canadian Council on Animal Care (CCAC) “Guide to the Care and Use of Experimental Animals”. All experiments were performed in accordance with the approved McGill University Animal Care protocol (AUP # 7497 to JJL).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary file 1., supplementary file 2., supplementary file 3., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Poulet, S., Dai, M., Wang, N. et al. Genome-wide in vivo CRISPR screen identifies TGFβ3 as actionable biomarker of palbociclib resistance in triple negative breast cancer. Mol Cancer 23 , 118 (2024). https://doi.org/10.1186/s12943-024-02029-4
Download citation
Received : 08 September 2023
Accepted : 22 May 2024
Published : 03 June 2024
DOI : https://doi.org/10.1186/s12943-024-02029-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Molecular Cancer
ISSN: 1476-4598
- General enquiries: [email protected]
- Open access
- Published: 19 March 2024
Pan-cancer analysis of NUP155 and validation of its role in breast cancer cell proliferation, migration, and apoptosis
- Zi-qiong Wang 1 , 2 , 3 na1 ,
- Zhi-xuan Wu 2 na1 ,
- Zong-pan Wang 1 ,
- Jing-xia Bao 2 ,
- Hao-dong Wu 2 ,
- Di-yan Xu 2 ,
- Hong-feng Li 2 ,
- Yi-Yin Xu 1 ,
- Rong-xing Wu 1 &
- Xuan-xuan Dai 1 , 2 , 3
BMC Cancer volume 24 , Article number: 353 ( 2024 ) Cite this article
915 Accesses
Metrics details
NUP155 is reported to be correlated with tumor development. However, the role of NUP155 in tumor physiology and the tumor immune microenvironment (TIME) has not been previously examined. This study comprehensively investigated the expression, immunological function, and prognostic significance of NUP155 in different cancer types. Bioinformatics analysis revealed that NUP155 was upregulated in 26 types of cancer. Additionally, NUP155 upregulation was strongly correlated with advanced pathological or clinical stages and poor prognosis in several cancers. Furthermore, NUP155 was significantly and positively correlated with DNA methylation, tumor mutational burden, microsatellite instability, and stemness score in most cancers. Additionally, NUP155 was also found to be involved in TIME and closely associated with tumor infiltrating immune cells and immunoregulation-related genes. Functional enrichment analysis revealed a strong correlation between NUP155 and immunomodulatory pathways, especially antigen processing and presentation. The role of NUP155 in breast cancer has not been examined. This study, for the first time, demonstrated that NUP155 was upregulated in breast invasive carcinoma (BRCA) cells and revealed its oncogenic role in BRCA using molecular biology experiments. Thus, our study highlights the potential value of NUP155 as a biomarker in the assessment of prognostic prediction, tumor microenvironment and immunotherapeutic response in pan-cancer.
Peer Review reports
Introduction
Cancer adversely affects human health and quality of life worldwide. In addition to the number of newly diagnosed cancer cases, the burden of cancer is increasing due to rapid population aging [ 1 , 2 ]. The breakthrough in immune checkpoint inhibitor (ICI) therapy has enabled the development of immunotherapy, which is a novel therapeutic approach that improves the clinical outcomes of patients with cancer [ 3 , 4 ]. Therefore, there is a need to explore novel immunotherapeutic targets and their roles in tumor physiology and tumor immune microenvironment (TIME).
The nuclear pore complex (NPC), a specific protein complex for transmembrane transport, functions as a channel for importing and exporting nuclear molecules [ 5 , 6 , 7 ]. Dysfunctional NPC can lead to various diseases, including cancer [ 6 , 8 ]. Nucleoproteins, which are the structural components of the NPC, regulate the progression of cancer through the following three main mechanisms: modulation of protein expression levels, induction of chromosomal translocations that result in the generation of fusion proteins, and induction of single point mutations [ 9 , 10 ]. Various cancer cells, especially multidrug-resistant and aggressive tumor cells, exhibit upregulated levels of nuclear proteins, high rates of nucleoplasmic translocation, and dependency on the nuclear translocation system. This indicates that the nuclear translocation machinery can be a potential therapeutic target for cancer [ 11 ]. Additionally, nucleoplasmic transport inhibitors have been subjected to partial clinical trials as they are reported to effectively induce cancer cell death [ 12 , 13 ]. NUP155 is actively involved in nuclear pore formation, as well as in selective gene regulation in pathological conditions [ 14 , 15 , 16 ]. Besides, a previously published study demonstrated that NUP155 mutations can result in specific phenotypes associated with atrial fibrillation in mice and humans [ 17 ]. Recent studies have reported that NUP155 expression is correlated with the prognosis of various cancers [ 18 , 19 ]. Additionally, NUP155 activates the cell cycle protein-dependent kinase inhibitor p21 in the p53 (tumor suppressor) pathway and has a key role in the transcriptional response to DNA damage [ 20 , 21 ]. Basit et al. demonstrated that the cGAS-STING-TBK1-IRF3 signaling-mediated regulation of p21 in the innate immune response affected chromosomal stability [ 22 ]. Thus, there is growing evidence linking NUP155 to tumor development. However, previous studies have not examined the role of NUP155 in tumor physiology and TIME in pan-cancer datasets.
This study aimed to comprehensively analyze the expression pattern, prognostic value, and immunological functions of NUP155 across 33 types of cancer. The correlation of NUP155 expression with DNA promoter methylation, somatic mutations, tumor mutational burden (TMB), microsatellite instability (MSI), tumor stemness, mismatch repair (MMR), TIME, infiltrating immune cell profile, and immune-related biomarkers was further investigated. Additionally, single-cell RNA sequencing dataset and immunotherapy cohort data analyses indicated that NUP155 is a potential biomarker for predicting the efficacy of immunotherapy. Furthermore, the oncogenic role of NUP155 in breast invasive carcinoma (BRCA) was validated using molecular biology experiments.
Data collection
The RNA sequencing and clinical data were downloaded from TCGA and GTEx databases with the UCSC Xena browser [ 23 ]. The expression data of tumor cell lines and tissues downloaded from the CCLE database were analyzed according to tissue origin. The UALCAN database [ 24 ] was used to examine the DNA methylation and protein levels of NUP155 between cancer and corresponding normal tissues. Tumor Immunology Single Cell Center (TISCH) [ 25 ], a single-cell RNA (scRNA) sequencing database of gene expression levels in the TIME, was used for characterizing NUP155 expression profiles in the microenvironment at the single-cell level. The response to immunotherapy was examined using two immunotherapy cohorts (GSE78220 cohort: patients with melanoma; Imvigor210 cohort: patients with metastatic uroepithelial carcinoma).
Pathological or clinical stage and prognosis
NUP155 expression in TCGA dataset was investigated at different pathological or clinical stages of pan-cancer using statistical methods, including Kruskal-Wallis Test and Dunn’s test [ 26 , 27 , 28 ]. When the data comprised < 3 samples or the standard deviation of the data was 0, stages I and II were combined for early-stage tumors or stages III and IV were combined for late-stage tumors before performing statistical analysis. The prognostic significance of NUP155 was examined using the univariate Cox proportional hazard model and Kaplan-Meier (KM) survival analysis with “survminer” R package. The best cut-off scores were used to determine the overall survival (OS), disease-specific survival (DSS), and progression-free survival (PFS) in the high-expression and low-expression cohorts.
TMB, MSI, and MMR analyses
The Simple Nucleotide Variation dataset of all TCGA samples processed using MuTect2 software was downloaded from Genomics Data Commons (GDC) [ 29 ]. The TMB for each tumor was determined using the “maftools” R package. Additionally, the MSI score was obtained from a previous study [ 30 ]. The expression level of MMR genes was assessed based on the expression profile data from TCGA [ 31 , 32 ].
Somatic mutation and stemness score analyses
The cBioPortal website [ 33 , 34 ] was used to analyze the correlation between NUP155 expression and somatic mutations among pan-cancer. To investigate the correlation between NUP155 expression and tumor stemness score, the gene expression data obtained from previous studies were integrated with the stemness score of each tumor, and the methylation feature was calculated.
Immune cell infiltration and immune modulator gene analyses
The immune and stromal fraction scores for various tumor samples were determined using the ESTIMATE algorithm. The correlation between NUP155 expression and the immune and stromal fraction scores was determined using the ‘estimate’ and ‘limma’ R packages. For reliable immune score assessment, xCell and CIBERSORT analyses were performed using the ‘IOBR’ R package. Next, co-expression analysis of NUP155 and immunoregulation-related genes was performed.
Drug sensitivity analysis
The correlation between NUP155 expression and drug sensitivity was analyzed using the Genomics of Drug Sensitivity in Cancer (GDSC) and Cancer Therapeutics Response Portal (CTRP) databases with the Gene Set Cancer Analysis (GSCA) platform [ 35 ]. Additionally, the correlation between NUP155 expression and sensitivity to 263 drugs approved by the Food and Drug Administration or undergoing clinical trials was examined using the CellMiner (NCI-60) database.
Construction of protein-protein interaction network (PPI) and functional annotation
GeneMANIA [ 36 ], which is a website designed to build PPI networks, provides gene function prediction hypotheses and identifies comparable genes. In this study, the PPI network for NUP155 was constructed using GeneMANIA to explore the interactions between NUP155 and NUP155-related genes.
The biological function of NUP155 in pan-cancer was examined using gene set enrichment analysis (GSEA). The gene sets of Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), and REACTOME were downloaded from the GSEA website. The top 100 co-expressed genes were mapped using the R package ‘clusterProfiler’ for enrichment analysis.
Cell culture and quantitative real-time polymerase chain reaction (qRT-PCR) and western blotting analyses
Normal human breast cells (MCF-10 A cells) and breast cancer cell lines (BT-549, MDA-MB-231, and T-47D cells) were purchased from the National Collection of Authenticated Cell Cultures. The cells were cultured in a humidified atmosphere containing 5% CO 2 . The culture medium was regularly replaced until the cells achieved 80–90% confluency. The primer sequences for the human target gene NUP155 that were purchased from Biosepur were as follows: 5′-CTTAGTGTCTACCTGGCTGCTTGG-3′ (forward primer); 5′-TGATGCTGATGCTGATGCTTCTGG-3′ (reverse primer). Total RNA was extracted from the four cell lines using an RNA extraction kit (Takara). The extracted RNA was then reverse-transcribed to complementary DNA using the reverse transcription kit (Beyotime). qRT-PCR analysis was performed using an Exicycler 96 instrument (BIONEER). The expression levels of NUP155 were normalized to those of GAPDH . The relative expression levels of the target gene were calculated using the ΔΔCq method [ 37 ].
The small interfering RNA (siRNA) oligonucleotides targeting NUP155 (si-NUP155) and scrambled siRNA were designed and synthesized by General Biol Corporation (Anhui, China). The cells were transfected with si-NUP155, washed thrice with phosphate-buffered saline (PBS), and harvested using centrifugation. Total proteins were extracted using radioimmunoprecipitation assay buffer supplemented with a protease inhibitor cocktail (R0010, Solarbio). Western blotting analysis was performed using anti-NUP155 (66359-1-Ig, Proteintech), anti-ACTB (66009-1-Ig, Proteintech), anti-BAX (50599-2-Ig, Proteintech), and anti-BCL2 (68103-1-Ig, Proteintech) antibodies, following the manufacturer’s instructions. The blots were cut prior to hybridisation with antibodies during blotting. The secondary antibodies used in this study were horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (SA00001-1, Proteintech) or HRP-conjugated goat anti-rabbit IgG (SA00001-2, Proteintech). ACTB was used as a loading control. Immunoreactive signals were developed using an enhanced chemiluminescence reagent (4 A Biotech, China).
Cell viability assay
Cells were seeded at a density of 2000 cells/well in 96-well plates and cultured for 0, 24, 48, and 72 h. Next, the cells were incubated with 10 µL of cell counting kit-8 (CCK-8) solution for 120 min. The absorbance of the sample at 450 nm was measured.
Transwell assay
Cells were seeded in the upper chamber containing serum-free medium at a density of 2 × 10 6 cells/well. In the lower chamber, 500 µL of medium containing 20% fetal bovine serum was added. After incubation at room temperature and 5% (v/v) CO 2 for 24 h, non-invasive cells in the upper chamber were removed. Meanwhile, the cells on the bottom surface were fixed using a 10% neutral buffered formalin solution and stained with 0.1% crystal violet. The invasive cells were counted in five randomly selected microscopic fields.
Wound healing assay
After treating logarithmic growth phase cells from the third to the fifth passage, the cells were seeded in six-well plates at a density of 1 × 10 6 cells/mL and cultured for 24 h in a CO 2 incubator until they reached approximately 70% confluency. A sterile pipette tip was used to gently generate a horizontal scratch in the monolayer. The cells were gently washed thrice with PBS to remove detached cells. Next, the cells were cultured in a serum-free medium for 24 h in a CO 2 incubator and fixed using a methanol solution. The closure of the cell scratch was monitored using an inverted microscope after crystal violet staining.
Statistical analyses
All statistical analyses were performed using R software (version 4.0.2) and GraphPad Prism 7. As the gene expression levels exhibited highly right-skewed distribution in TCGA dataset, the gene expression data were normalized using log-2 transformation (X to Log2(X + 1)). Survival was analyzed using Cox regression analysis, the KM method, and log-rank tests. The correlation between two variables was analyzed using Spearman or Pearson tests. To analyze the molecular biology experiment data, means between two groups were compared using the two-tailed Student’s t-test. Data are expressed as mean ± standard error of the mean. Differences were considered significant at P < 0.05. The R-scripts and online tools used in this study are shown in Supplementary Table S1 .
Differential expression ofNUP155 between normal and cancer tissues
Analysis of GTEx datasets revealed that the mRNA expression levels of NUP155 were comparable in all organs, except bone marrow and testis ( Fig. 1 A ) . The NUP155 expression levels were downregulated in most healthy tissues. Figure 1 B shows the relative expression levels of NUP155 in different cell lines in the CCLE dataset. The NUP155 expression levels varied in different cancer cell lines with the small cell lung cancer cell line exhibiting upregulated expression levels. Analysis of NUP155 protein expression using the UALCAN database revealed that the NUP155 expression levels in head and neck squamous cell carcinoma (HNSC), glioblastoma multiforme (GBM), colon cancer, lung adenocarcinoma (LUAD), hepatocellular carcinoma (HCC), and clear cell renal cell carcinoma (RCC) were significantly upregulated when compared with those in the corresponding non-cancerous tissues ( Fig. 2 A ) . The NUP155 mRNA expression levels varied between tumor and non-cancerous tissue in 29 cancers (samples for which non-cancerous tissue data were not available were excluded) ( Fig. 2 B ) . Compared with those in non-cancerous tissues, the NUP155 expression levels were upregulated in adrenocortical carcinoma (ACC), bladder urothelial carcinoma (BLCA), BRCA, cervical squamous cell carcinoma and endocervical adeno carcinoma (CESC), cholangiocarcinoma (CHOL), colon adenocarcinoma (COAD), lymphoid neoplasm diffuse large B-cell lymphoma (DLBC), esophageal carcinoma (ESCA), GBM, HNSC, kidney chromophobe (KICH), kidney renal clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP), brain lower grade glioma (LGG), liver hepatocellular carcinoma (LIHC), LUAD, lung squamous cell carcinoma (LUSC), ovarian serous cystadenocarcinoma (OV), pancreatic adenocarcinoma (PAAD), prostate adenocarcinoma (PRAD), rectum adenocarcinoma (READ), skin cutaneous melanoma (SKCM), stomach adenocarcinoma (STAD), thymoma (THYM), uterine corpus endometrial carcinoma (UCEC), and uterine carcinosarcoma (UCS) tissues. In contrast, the NUP155 expression levels in acute myeloid leukemia (LAML), testicular germ cell tumor (TGCT), and thyroid carcinoma (THCA) tissues were downregulated when compared with those in non-cancerous tissues. The differential expression of NUP155 between cancer and non-cancerous tissues was the most pronounced in DLBC and THYM. However, NUP155 expression was not significantly different between cancer and non-cancerous tissues in mesothelioma (MESO), pheochromocytoma and paraganglioma (PCPG), and sarcoma (SARC).
Differential expression of NUP155 in pan-cancer. A NUP155 expression in normal tissues. B NUP155 expression in tumor cell lines
Differential expression of NUP155 in pan-cancer. A NUP155 protein expression level in normal tissues and primary tissues of HNSC, GBM, COAD, LUAD, HCC and RCC. B Comparison of NUP155 expression between tumor and normal tissues. * p < 0.05; ** p < 0.01; *** p < 0.001
Differential expression of NUP155 between normal and cancer tissues
NUP155 expression was significantly correlated with pathological or clinical stage in ACC, KICH, KIRC, KIRP, LIHC, OV, SKCM, and UCS (Supplementary Fig. 1 ). In particular, NUP155 expression was positively correlated with advanced tumor stage in ACC, KICH, KIRP, and LIHC.
Methylation profile and genetic alterations of NUP155
DNA methylation alterations in cancer are powerful diagnostic and prognostic targets. Analysis of the UALCAN dataset revealed that compared with those in non-cancerous tissues, the methylation levels of NUP155 were upregulated in BRCA, CESC, ESCA, HNSC, KIRC, LIHC, LUAD, LUSC, PAAD, SARC, and UCEC tissues and downregulated in COAD, PRAD, READ, and TGCT tissues ( Fig. 3 A and Supplementary Table S2 ) . The cBioPortal database was used to investigate the NUP155 alterations in pan-cancer. The frequency of NUP155 alterations was the highest in non-small cell lung cancer (approximately 10%) ( Fig. 3 B ) . Amplifications and mutations were the most frequent genetic alterations.
DNA methylation and mutation features of NUP155 in pan-cancer. A Promoter methylation level of NUP155 . B The alteration frequency and different mutation types of NUP155 . * p < 0.05; ** p < 0.01; *** p < 0.001
Furthermore, the landscape of NUP155 copy number variation (CNV) in pan-cancer was examined. This study analyzed the correlation between NUP155 CNV and NUP155 mRNA levels using the GSCA online website. The NUP155 methylation levels were closely associated with NUP155 mRNA expression levels in various cancer types, including LUSC, LUAD, HNSC, SARC, BLCA, OV, BRCA, ESCA, CESC, STAD, SKCM, UCS, KIRC, KICH, COAD, LIHC, UCEC, KIRP, PCPG, READ, and LGG (Supplementary Fig. 2 and Supplementary Table S3 ).
Prognostic value of NUP155 expression in different cancer types
Next, this study examined the prognostic value of NUP155 in different cancer types using survival analyses. The three endpoints of this study were OS, DSS, and PFS. NUP155 expression was significantly correlated with OS in the following 19 types of cancer: ACC, BLCA, BRCA, COAD, ESCA, GBM, HNSC, KICH, KIRC, KIRP, LGG, LIHC, MESO, OV, PAAD, READ, SARC, THYM, and UCEC ( Fig. 4 A ) . The KM survival curves revealed that NUP155 upregulation was significantly correlated with poor OS in ACC, BRCA, KICH, KIRP, LGG, LIHC, MESO, and UCEC and favorable OS in KIRC, READ, and THYM ( Fig. 4 B–L ) . NUP155 expression was correlated with DSS in the following nine types of cancer: ACC, KICH, KIRC, KIRP, LGG, LIHC, MESO, THYM, and UCEC (Supplementary Fig. 3 A). The KM survival curves indicated that NUP155 upregulation was associated with poor DSS in ACC, KICH, KIRP, LGG, LIHC, MESO, and UCEC and favorable DSS in KIRC, THYM, and UCEC (Supplementary Fig. 3 B–J). Additionally, the effect of NUP155 dysregulation on PFS was investigated (Supplementary Fig. 4 A). Univariate Cox regression analysis revealed that NUP155 expression is a risk factor for PFS in ACC, BRCA, KICH, KIRP, LGG, LIHC, MESO, UCEC, and uveal melanoma (UVM) and an active factor for PFS in KIRC (Supplementary Fig. 4 B–K). KM analysis suggested that NUP155 upregulation was associated with unfavorable PFS in patients with ACC, BLCA, KICH, KIRP, LIHC, LGG, MESO, UCEC, and UVM and favorable PFS in patients with KIRC. Thus, NUP155 upregulation is associated with poor prognosis in most cancers.
Association between NUP155 expression levels and OS in TCGA pan-cancer. A Forest plot of association of NUP155 expression and OS. B – L Kaplan-Meier analysis of the association between NUP155 expression and OS.
Correlation of NUP155 expression with DNA methylation-based stem score (DNAss) and RNA methylation-based stem score (RNAss)
The upregulation of stem cell marker expression in tumor cells is strongly correlated with tumor recurrence, metastasis, and drug resistance. The expression of NUP155 exhibited varying degrees of correlation with DNAss ( Fig. 5 A ) and RNAss ( Fig. 5 B ) in different cancer types. NUP155 expression was associated with DNAss in 19 tumors. In particular, NUP155 expression was positively correlated with DNAss in BRCA, CESC, CHOL, glioma (GBMLGG), HNSC, KIRC, KIRP, pan-kidney cohort (KICH + KIRC + KIRP) (KIPAN), LGG, LUAD, LUSC, MESO, PAAD, stomach and esophageal carcinoma (STES), SARC, SKCM, STAD, and TGCT and negatively correlated with DNAss in BLCA. Additionally, NUP155 expression was positively correlated with RNAss in 30 tumors. Thus, NUP155 expression was correlated with DNAss and RNAss in several tumors and may potentially promote the activation of tumor stem cells and facilitate tumor recurrence and proliferation.
Associations between NUP155 expression and stemness score, MSI, TMB, and MMR in pan-cancer. A - B Bar charts illustrating the relationship between NUP155 expression and DNAss and RNAss. C - D Radar plots illustrating the relationship between NUP155 expression and TMB as well as MSI. E The heat map illustrating the relationship between the expression of NUP155 and MMR genes
Correlation of NUP155 expression with TMB, MSI, and MMR genes
Immunotherapy markers are useful for screening patients who may benefit from the treatment as some patients do not respond to immunotherapy and experience severe immune-related side effects. Several clinical studies have demonstrated the promising predictive value of TMB. Tumor cells with a high TMB are easily recognized by the immune system. Consequently, immunotherapy increases the response rates and the survival rates in patients with a high TMB [ 38 ]. NUP155 expression was positively correlated with TMB in ACC, BLCA, BRCA, KICH, LAML, LGG, LUAD, LUSC, MESO, PAAD, SARC, SKCM, STAD, and UCEC and negatively correlated with TMB in THCA ( Fig. 5 D ) . MSI, which is characterized by deficiencies in the MMR proteins, is a well-recognized biomarker for ICI response. NUP155 expression was positively correlated with MSI in ACC, CESC, KIRC, LIHC, LUSC, MESO, OV, SARC, STAD, and UCEC and negatively correlated with MSI in DLBC, PRAD, SKCM, and THCA ( Fig. 5 C ) . We further explored the relationship between NUP155 expression and MMR genes (namely MLH1, MSH2, MSH6, PMS2, EPCAM). As shown in Fig. 5 E, NUP155 expression was correlated with MMR genes in almost all cancers. These results indicate that NUP155 expression may determine the outcomes of ICI therapy in patients with cancer by influencing TMB, MSI, and MMR.
Correlation between NUP155 and TIME
Previous studies have demonstrated that the complexity and diversity of TIME regulate tumorigenesis and tumor progression. Thus, this study examined the correlation between NUP155 expression and TIME in pan-cancer. The eight tumors with the highest correlation coefficients are shown in Supplementary Fig. 5 . Among these eight cancers, NUP155 expression was negatively correlated with both stromal and immune scores in GBM, STES, STAD, and SKCM. Meanwhile, NUP155 expression was negatively correlated with immune scores in TGCT, SARC, and KIPAN. These findings suggested a close correlation between NUP155 expression and the tumor microenvironment in different types of cancer.
Correlation of NUP155 expression with tumor-infiltrating immune cells (TIICs) and immune modulator genes
Comprehensive analysis of the correlation between NUP155 expression and the degree of immune cell infiltration in various cancer types was performed using the xCell database. NUP155 expression was negatively correlated with the levels of infiltrating immune cells, except CD4 + memory T cells, CD4 + T cells, common lymphoid precursors, granulocyte/macrophage precursors, myocytes, and Th2 cells ( Fig. 6 B ) . Moreover, the levels of 26 immune cell types were examined using the “CIBERSORT” algorithm. Correlation analysis revealed that NUP155 expression was positively correlated with the levels of infiltrating naïve B cells, CD4 + memory resting T cells, CD4 + memory activated T cells, dendritic cells, mast cells, macrophages, NK cells (resting), and neutrophils. In contrast, the levels of memory B cells, CD4 + naïve T cells, CD8 + T cells, follicular helper T cells, plasma cells, Treg cells, and activated NK cells were negatively correlated with NUP155 expression ( Fig. 6 A ) . Additionally, analysis at the single-cell level revealed the expression of NUP155 in various immune cells, including CD4 + T cells, CD8 + T cells, B cells, natural killer (NK) cells, monocytes, dendritic cells, and T regulatory (Treg) cells. In particular, NUP155 expression was upregulated in immune cells, especially in proliferative T cells (T prolif cells), Treg cells, and CD8 + exhausted T (Tex) cells, of patients with CRC, LIHC, SKCM, and NSCLC (Supplementary Fig. 6 ).
Correlation of NUP155 expression with immune infiltration. A The heat map showing that NUP155 expression correlates significantly with tumor infiltration of different immune cells from the CIBERSORT database. B The heat map showing that NUP155 expression correlates significantly with tumor infiltration of different immune cells based on the xCell database
Tumor-induced immunosuppression is the primary mechanism through which cancers evade immune surveillance and attack. Tumors manipulate the immune response by modulating the immune checkpoint (ICP) pathway. In this study, gene co-expression analysis was performed to investigate the correlation between NUP155 expression and immune-related genes in various cancers. The heatmaps of the analyzed genes, including those encoding major histocompatibility complex (MHC) ( Fig. 7 A ) , immunosuppressive factors ( Fig. 7 B ) , chemokine receptors ( Fig. 7 C ) , immune activation factors ( Fig. 7 D ) , and chemokines ( Fig. 7 E ) , revealed a strong co-expression pattern between NUP155 and immune-related genes. NUP155 expression was positively correlated with the expression of immune-related genes in ACC, BLCA, HNSC, KICH, KIRC, KIRP, LIHC, PAAD, PCPG, PRAD, and UVM. However, a limited number of immune-related genes exhibited co-expression with NUP155 in CHOL.

Co-expression of NUP155 and immune-related genes in pan-cancer. A - E The heatmap represents the correlation between NUP155 expression and MHC genes, immunosuppressive genes, chemokine receptors, immune activation genes and chemokines
PPI network of NUP155 and effect of NUP155 on drug response
A PPI network of NUP155 was constructed using the GeneMANIA online program to investigate the potential role of NUP155 in carcinogenesis. As shown in Fig. 8 A and Supplementary Table S7 , NUP155 physically interacted with NUP133 , GLE1 , REG1B , SNX5 , and TACC2 . Next, the correlation between NUP155 expression levels and drug sensitivity was analyzed using the CTRP and GDSC databases. In the CTRP dataset, NUP155 expression was negatively correlated with the sensitivity to drugs, such as trametinib, tivantinib, dinaciclib, and docetaxel ( Fig. 8 D and Supplementary Table S4 ) . Meanwhile, in the GDSC dataset, NUP155 expression was positively correlated with the sensitivity to drugs, such as nutlin-3a (-) and 5-Fu ( Fig. 8 E and Supplementary Table S5 ). To further investigate the correlation between NUP155 expression and drug sensitivity in various cancer cell lines, the Cell Miner database was used. As shown in Fig. 8 F, NUP155 expression was positively correlated with sensitivity to AT-13,387, allopurinol, and bosutinib and negatively correlated with sensitivity to isotretinoin.
A a PPI network for NUP155 . B - C Kaplan-Meier analysis of the association between NUP155 expression and OS in the GSE78220 and Imvigor210 immunotherapy cohorts. D - F Correlation of NUP155 expression with drug sensitivity in CTRP, GDSC and Cell Miner databases
Additionally, the correlation between NUP155 expression and patient prognosis after PD-1/PD-L1 immunotherapy was examined by analyzing two immunotherapy cohort datasets (GSE78220 and Imvigor210). GSE78220 comprises the data of patients with malignant melanoma who received anti-PD-1 immunotherapy, while Imvigor210 comprises the data of patients with urothelial carcinoma who received anti-PD-L1 therapy. The KM survival curve of the GSE78220 cohort revealed that NUP155 upregulation was associated with poor OS in patients with malignant melanoma ( Fig. 8 B ) . Meanwhile, the KM survival curve of the Imvigor210 cohort revealed that NUP155 upregulation was associated with beneficial OS in patients with urothelial carcinoma ( Fig. 8 C ) .
GSEA revealed that NUP155 was enriched in multiple GO terms, including the negative regulation of NIK/NF-κB signaling, intermediate filaments, and RNA-mediated gene silencing. (Fig. 9 A-E) KEGG analysis indicated that NUP155 was enriched in immune-related pathways, such as antigen processing and presentation, toll-like receptor signaling pathway, RIG-I-Like receptor signaling pathway, and allograft rejection. (Fig. 10 A-E) GSEA of the REACTOME gene set collection suggested the enrichment of several immune and inflammatory functional pathways, including the class I MHC-mediated antigen processing and presentation pathway, adaptive immune system pathway, interleukin-1 signaling pathway, antigen processing via ubiquitination and proteasome degradation pathway, and MHC Class II antigen presentation pathway, in various cancers. NUP155 was enriched in cell cycle, mitotic spindle checkpoint, regulation of TP53 activity, DNA repair, and other pathways (Supplementary Fig. 7 and Supplementary Table S6 ). These findings suggest that NUP155 has a crucial role in the inflammatory response and TIME.
Results of GSEA. GO functional annotation of NUP155 in various cancers, including ( A ) KICH, ( B ) LUAD, ( C ) OV, ( D ) TGCT, ( E ) UCS
Results of GSEA. KEGG pathway analysis of NUP155 in various cancers, including ( A ) PAAD, ( B ) READ, ( C ) SARC, ( D ) SKCM, ( E ) STAD
Differential expression of NUP155 in breast cancer cells and normal breast cells
According to Cancer Statistics 2022, breast, lung, and colorectal cancers account for 51% of all newly diagnosed cases in women. In particular, breast cancer accounts for approximately one-third of cases. Therefore, the differential expression of NUP155 between healthy breast cells (MCF-10 A cells) and three breast cancer cell lines (BT-549, MDA-MB-231, and T-47D cells) was examined using qRT-PCR analysis (Supplementary Fig. 8 ). The results of qRT-PCR analysis were consistent with those of bioinformatics analysis. The expression of NUP155 mRNA in breast cancer cell lines was significantly higher than that in healthy breast cells. Triple-negative breast cancer (TNBC) has the worst prognosis and poses significant treatment challenges among breast cancer subtypes, with a 5-year survival rate of only 11% in advanced stages [ 39 ]. Two TNBC cell lines (MDA-MB-231 and BT-549 cells) were used in subsequent in vitro experiments.
Effect of NUP155 on the proliferation, migration, and apoptosis of TNBC cells
To investigate the effect of NUP155 on TNBC, si- NUP155 was transfected into MDA-MB-231 and BT-549 cells. Transfection with si- NUP155 downregulated the mRNA and protein expression levels of NUP155 ( Fig. 11 A–F ) . Western blotting analysis revealed that the BCL2/BAX expression ratio was significantly downregulated in si-NUP155-transfected TNBC cells ( Fig. 11 J–M ) . The CCK-8 assay results revealed that transfection with si- NUP155 significantly decreased tumor cell proliferation ( Fig. 11 H–I ) . Furthermore, the wound healing and transwell assay results revealed that NUP155 knockdown significantly impaired the wound healing ( Fig. 11 G ) and migratory ( Fig. 11 N ) abilities of TNBC cells.
Effect of NUP155 silencing on TNBC cell lines MDA-MB-231 and BT-549. A - B RT-PCR validation of NUP155 silencing efficiency. Western blot analysis to verify NUP155 silencing efficiency in MDA-MB-231 cells ( C - D ) and BT-549 cells ( E - F ). G Wound healing assay to analyze the impact of NUP155 silencing on TNBC cell healing ability. H - I CCK8 assay to analyze the effects of NUP155 silencing on the proliferation of MDA-MB-231 and BT-549 cells. Western blot analysis of the decrease in the BCL2/BAX expression ratio in MDA-MB-231 cells ( J - K ) and BT-549 cells ( L - M ) due to NUP155 siRNA. N Transwell assay to analyze the impact of NUP155 silencing on cell migration. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001. The blots were cut prior to hybridisation with antibodies during blotting, and the three replicates of original blots of Fig. 11 C, E and J, and Fig. 11 L are presented in the Supplementary material
The NPC, a giant protein complex embedded in the nuclear envelope, mediates selective nucleocytoplasmic transport [ 40 ]. Deficiency in NPC, which has a crucial role in gene expression and growth and development, is associated with the pathogenesis of various pathological conditions, such as viral infections, cancer, and neurodegenerative diseases. Thus, the nuclear transport machinery is a therapeutic target for several diseases [ 41 ]. Previous studies have reported that the NPC promotes tumorigenesis in hematological cancers and non-hematological malignancies, such as skin, lung, pancreatic, prostate, and colon cancers [ 42 ]. Among nuclear pore proteins, NUP155 is critical for assembling the structure of the NPC [ 43 ]. NUP155 is involved in mitotic arrest mediated by the novel anti-tumor drug NP-10 [ 44 ] and regulates mRNA translation for the cell cycle protein-dependent kinase inhibitor p21 [ 20 ]. Therapeutic approaches for cancer mainly target the proliferation of cancer cells, impairing the assembly of the mitotic spindle to arrest cancer cell division and death. This approach is considered to be the most effective therapeutic strategy. This is the reason why we focus on the gene NUP155 . The role of NUP155 in different cancer types has not been systematically examined using bioinformatic approaches. This study aimed to comprehensively analyze the differential expression, prognostic value, and biological function of NUP155 in different cancer types. The correlation of NUP155 with TIME, TIICs, and immune-related genes was also investigated.
This study demonstrated that NUP155 was under-expressed in normal human tissues, except for bone marrow and testis. We hypothesized that NUP155 upregulation is related to enhanced cell proliferation and turnover in the bone marrow and testis. NUP155 is upregulated in most cancer types but is downregulated in LAML and TGCT. Bone marrow contains hematopoietic stem cells, while testis contains spermatogonial stem cells. Several studies have reported that NPC is important for maintaining stem cell homeostasis [ 45 ]. For example, the inhibition of NUP153 can lead to the derepression of developmental genes and the induction of early differentiation in stem cells [ 46 , 47 ]. Therefore, we hypothesize that NUP155 upregulation in healthy bone marrow and testis is necessary to maintain stem cell homeostasis and that the suppression of NUP155 expression in LAML and TGCT leads to aberrant proliferation and differentiation of stem cells. The NPC plays a major role in cell fate determination. NUP98 mutations contributing to leukemia development have been extensively studied. Mutations in multiple nucleoporin-encoding genes can cause tissue-specific defects or lethality in animals [ 48 , 49 , 50 ]. Based on the data shown in Fig. 3 B, we speculate that NUP155 may also influence leukemia through gene mutations. Although the expression level of NUP155 is downregulated in TGCT, the data in Fig. 3 A revealed that the NUP155 promoter methylation level is downregulated in TGCT, indicating gene instability.
Cancer cells are characterized by an overall loss of methylation modifications and aberrant methylation sites within the enhancer and promoter regions [ 51 , 52 ]. The NUP155 promoter methylation level is downregulated in COAD, PRAD, READ, and TGCT, which is consistent with the classical model [ 53 ]. However, the NUP155 promoter hypermethylation upregulates NUP155 expression in BRCA, CESC, ESCA, HNSC, KIRC, LIHC, LUAD, LUSC, PAAD, SARC, and UCEC tissues. A review by Jim Smith et al. in ‘Trends in Cancer’ suggested that promoter DNA hypermethylation promotes aberrant gene activation. The authors further discussed the potential molecular mechanisms underlying this aberrant regulation [ 54 ]. Therefore, the correlation between NUP155 expression and DNA methylation identified in this study warrants further investigation.
Somatic mutations that accumulate in normal tissues are associated with aging and disease. Additionally, somatic mutations enable the development of novel therapeutic approaches for cancer [ 55 ]. Similarly, tumor-specific antigens derived from somatic mutations have provided new approaches for developing cancer therapy [ 56 ]. Designing vaccines based on patient-specific mutations is a potential strategy for developing personalized tumor therapy [ 57 ]. In this study, NUP155 was frequently mutated in various tumors, especially melanoma, endometrial carcinoma, cervical adenocarcinoma, BLCA, and cervical squamous cell carcinoma. These findings demonstrated that NUP155 is a potential target for cancer vaccines, especially for melanoma, which was the most frequent tumor type. Cox regression analysis of TCGA dataset revealed that NUP155 upregulation is a risk factor for OS in 13 types of cancer. Additionally, NUP155 upregulation was a risk factor for DSS and PFS in nine types of tumors and a favorable factor for DSS and PFS in KIRC. These findings suggest that NUP155 can be used to stratify patients with cancer.
TMB is a valuable predictive biomarker for immunotherapy response in various cancer types [ 58 ]. Meanwhile, MSI is an important biomarker for ICI response [ 59 ]. The upregulation of MSI or TMB can lead to the generation of potent neoantigens, which elicit enhanced immune responses and contribute to an enhanced immunotherapeutic response [ 59 , 60 ]. The findings of this study indicate a strong correlation between NUP155 expression and the levels of TMB and MSI in various cancer types. Hence, NUP155 expression can aid in predicting patient response to ICI therapy.
The results of this study suggest that NUP155 plays a crucial role in cancer immunity. The ESTIMATE score revealed a negative correlation between NUP155 expression and the levels of stromal and immune cells in the tumor microenvironment of 15 different cancer types. TIICs regulate tumorigenesis and tumor progression [ 61 ]. Under physiological conditions, the immune system can recognize and destroy tumor cells in the TIME. However, tumor cells can evade the immune system through various mechanisms that promote their survival and growth. Cytotoxic T cells expressing CD8 receptors on their surface play a pivotal role in the response to cancer immunotherapies. CD8 receptors are the most potent effectors in the anti-cancer immune response [ 62 ]. Treg cells contribute to resistance against ICI therapies, promoting cancer progression [ 63 ]. Th1 cytokines stimulate immune cells to eliminate tumor cells, while Th2 cytokines inhibit tumor immune responses [ 64 , 65 ]. Analysis of immune cell infiltration using the xCell database revealed that NUP155 expression was negatively correlated with the infiltration levels of CD8 + cells and Th1 cells and positively correlated with the infiltration levels of Treg cells and Th2 cells. T cell exhaustion refers to the impaired state of CD8 + T cells, which can identify and eliminate tumor cells, leading to a diminished response against tumor cells [ 66 , 67 ] Dysfunctional CD8 + Tex cells in the tumor microenvironment exhibit the expression of immune co-inhibitory receptors, including LAG3, CD160, CTLA4, and TIGIT [ 68 , 69 ]. CD8 + Tex cells with enhanced expression of ICP receptors exhibit an exhausted phenotype [ 70 ]. As shown in Fig. 7 B, NUP155 was positively correlated and co-expressed with these ICP receptors in most tumors. Hence, we hypothesized that NUP155 may upregulate ICP receptors, regulating the levels of CD8 + Tex cells and consequently modulating the TIME. Additionally, analysis of the TISCH dataset revealed that NUP155 was upregulated in T prolif cells and Treg cells. Analysis of the xCell dataset and the TISCH dataset revealed that NUP155 upregulation may modulate the tumor microenvironment status by upregulating the levels of Tregs and regulating the balance of Th1 and Th2 cells. GSEA revealed that NUP155 was significantly enriched in immune-related pathways, especially those involved in antigen processing and expression. Tumor cells can evade immune recognition by disrupting antigen processing and expression through the suppression of dendritic cell function and the downregulation of HLA-1 [ 71 ]. Immune cell infiltration analysis revealed that NUP155 expression was negatively correlated with the infiltration levels of dendritic cells. These findings suggested that NUP155 expression is a prognostic risk factor in most tumor types. ICIs exert potent growth-inhibitory effects against various cancers, improving the clinical outcomes of patients with cancer [ 72 ]. In this study, NUP155 expression was correlated with genes encoding MHC, immune suppressors, immune activators, chemokines, and chemokine receptors. In particular, NUP155 was negatively correlated with genes encoding ICPs. Thus, NUP155 may mediate the effects of immunotherapy in patients with cancer by regulating TIICs and ICPs.
In the PPI network of NUP155 , the top five genes that were most strongly correlated with NUP155 were NUP133 , GLE1 , REG1B , SNX5 , and TACC2 . The structure of NUP133, a nucleoporin, is similar to that of NUP155 [ 73 , 74 ]. NUP133 functions as a gene regulator and promotes the expression of the oncogene MYC [ 75 ]. The amino (N)-terminal region of GLE1 interacts with NUP155 [ 76 ]. GLE1, an RNA export protein, is crucial for multiple steps in gene expression, from mRNA export to translation [ 77 ]. Mutations in GLE1 can lead to developmental and neurodegenerative disorders and some cancers [ 78 , 79 , 80 ]. REG1B , SNX5 , and TACC2 are reported to be oncogenes [ 81 , 82 , 83 , 84 , 85 ]. Additionally, the PPI network revealed that NUP155 was mainly related to functions, such as nuclear transport, nucleocytoplasmic transport, regulation of ATP metabolic process, and RNA transport as shown in Supplementary Table S8 . Therefore, aberrant NUP155 expression may interfere with these functions and activate oncogenes, such as REG1B , SNX5 , and TACC2 to exert carcinogenic effects.
NUP155 can also serve as a predictive biomarker of immunotherapeutic response in some cancers. ICI therapy is associated with survival benefits in patients with upregulated ICP expression. PD-1(PDCD1) and PD-L1(CD274) are the most widely recognized prognostic predictors of immunotherapy [ 86 , 87 ]. KM survival analysis of the immunotherapy cohort revealed that the prognosis of patients with SKCM exhibiting NUP155 upregulation was poor, which may be related to the correlation between NUP155 , ICP-encoding genes, and the degree of immune cell infiltration. In SKCM, PD-1 expression and dendritic cell levels were negatively correlated with NUP155 expression. Thus, the group exhibiting NUP155 upregulation may not benefit from PD-1 inhibitor therapy. In BLCA, the survival benefit of immunotherapy was significant in the group with NUP155 upregulation. PD-1/PD-L1 and dendritic cells were positively correlated with NUP155 expression in BLCA. Hence, we hypothesized that the correlation between NUP155 expression, ICP-encoding gene expression, and the degree of immune cell infiltration affects the response of patients with cancer to immunotherapy.
The establishment of the sensitivity of tumors with differential expression levels of NUP155 to anti-tumor drugs may guide tumor treatment. For example, trametinib, a representative MEK inhibitor, is used as a monotherapy for unresectable or metastatic melanoma with BRAF-V600E or V600K mutations [ 88 , 89 ]. The sensitivity to trametinib is significantly and positively correlated with the expression of NUP155. Therefore, patients with drug-resistant melanoma exhibiting NUP155 upregulation may be suitable for treatment with trametinib. Paclitaxel and 5-fluorouracil (5-FU) are also common chemotherapy drugs [ 90 , 91 ]. NUP155 is negatively correlated with the sensitivity to paclitaxel and positively correlated with the sensitivity to 5-FU. Therefore, tumors with NUP155 upregulation may be resistant to paclitaxel but not to 5-FU. Analysis of NUP155 expression can aid in selecting anti-tumor drugs in clinical practice, especially for drug-resistant tumors.
The role of NUP155 in BRCA was validated using molecular biology methods. qRT-PCR analysis revealed that the NUP155 mRNA level was upregulated in BRCA cells. TNBC, which accounts for 10–20% of all diagnosed breast cancers [ 92 ], is characterized by the absence of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 [ 93 , 94 ]. Additionally, TNBC exhibits high recurrence, metastasis, and resistance to conventional treatments. Thus, the treatment of TNBC is challenging when compared with that of other types of breast cancer [ 95 ]. Clinically, TNBC is often classified as “difficult-to-treat breast cancer” and is a research hotspot in the field of breast cancer research [ 96 , 97 ]. Therefore, this study selected TNBC cells for subsequent in vitro experiments to validate the findings of bioinformatics analysis. Cellular experiments revealed that NUP155 knockdown significantly inhibited the proliferation and migration and promoted apoptosis in TNBC cells. These findings confirm the accuracy and reliability of the pan-cancer bioinformatics analysis in BRCA. The specific pathogenic mechanism of NUP155 in breast cancer will be validated in the future.
This study has some limitations. Although NUP155 expression was demonstrated to be associated with the immune microenvironment and prognosis of human malignancies, the regulatory effect of NUP155 on the clinical survival rates mediated through the immune-related pathway is unclear. Additionally, this study performed preliminary experiments on BRCA but did not examine the molecular mechanisms of NUP155 in BRCA. This systematic pan-cancer analysis suggested that NUP155 was differentially expressed between non-cancerous and cancer tissues and that NUP155 dysregulation is associated with tumor staging and can be used to predict the prognosis. Additionally, DNA methylation, TMB, MSI, cancer stemness, TIME, and immune cell infiltration may be correlated with NUP155 dysregulation in cancer. These findings can aid in determining the role of NUP155 in tumor development and progression and facilitate the application of precise and personalized immunotherapies.
Availability of data and materials
Publicly available database analyzed in this study can be found in the TCGA ( https://tcgadata.nci.nih.gov/tcga/ ) UCSC ( https://xenabrowser.net/datapages/ ), CellMiner ( https://discover.nci.nih.gov/cellminer/home.do ) and GSEA ( https://www.gsea-msigdb.org/gsea/downloads.jsp ).
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Article PubMed Google Scholar
Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135(5):584–90.
Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39.
Article CAS PubMed Google Scholar
Wieder T, Eigentler T, Brenner E, Röcken M. Immune checkpoint blockade therapy. J Allergy Clin Immunol. 2018;142(5):1403–14.
Davis L. The nuclear pore complex. Annu Rev Biochem. 1995;64:865–96.
Sakuma S, D’Angelo MA. The roles of the nuclear pore complex in cellular dysfunction, aging and disease. Semin Cell Dev Biol. 2017;68:72–84.
Article CAS PubMed PubMed Central Google Scholar
Hurt E, Beck M. Towards understanding nuclear pore complex architecture and dynamics in the age of integrative structural analysis. Curr Opin Cell Biol. 2015;34:31–8.
Simon DN, Rout MP. Cancer and the nuclear pore complex. Adv Exp Med Biol. 2014;773:285–307.
Jamali T, Jamali Y, Mehrbod M, Mofrad M. Nuclear pore complex: biochemistry and biophysics of nucleocytoplasmic transport in health and disease. Int Rev cell Mol Biology. 2011;287:233–86.
Article CAS Google Scholar
Sun J, Shi Y, Yildirim E. The nuclear pore complex in cell type-specific chromatin structure and gene regulation. Trends Genet. 2019;35(8):579–88.
Sakuma S, Raices M, Borlido J, Guglielmi V, Zhu E, D’Angelo M. Inhibition of nuclear pore complex formation selectively induces cancer cell death. Cancer Discov. 2021;11(1):176–93.
Jans D, Martin A, Wagstaff K. Inhibitors of nuclear transport. Curr Opin Cell Biol. 2019;58:50–60.
Taylor J, Sendino M, Gorelick A, Pastore A, Chang M, Penson A, Gavrila E, Stewart C, Melnik E, Herrejon Chavez F, et al. Altered nuclear export signal recognition as a driver of oncogenesis. Cancer Discov. 2019;9(10):1452–67.
Kosinski J, Mosalaganti S, von Appen A, Teimer R, DiGuilio A, Wan W, Bui K, Hagen W, Briggs J, Glavy J, et al. Molecular architecture of the inner ring scaffold of the human nuclear pore complex. Sci (New York NY). 2016;352(6283):363–5.
Article ADS CAS Google Scholar
von Appen A, Kosinski J, Sparks L, Amanda DG, Vollmer B. In situ structural analysis of the human nuclear pore complex. Nature. 2015;526:140.
Article ADS Google Scholar
De Magistris P, Tatarek-Nossol M, Dewor M, Antonin W. A self-inhibitory interaction within Nup155 and membrane binding are required for nuclear pore complex formation. J Cell Sci. 2018;131(1):jcs208538.
PubMed Google Scholar
Zhang X, Chen S, Yoo S, Chakrabarti S, Zhang T, Ke T, Oberti C, Yong S, Fang F, Li L, et al. Mutation in nuclear pore component NUP155 leads to atrial fibrillation and early sudden cardiac death. Cell. 2008;135(6):1017–27.
Savci-Heijink C, Halfwerk H, Koster J, van de Vijver M. A novel gene expression signature for bone metastasis in breast carcinomas. Breast Cancer Res Treat. 2016;156(2):249–59.
Engqvist H, Parris T, Kovács A, Rönnerman E, Sundfeldt K, Karlsson P, Helou K. Validation of Novel Prognostic biomarkers for early-stage Clear-Cell, endometrioid and mucinous ovarian carcinomas using immunohistochemistry. Front Oncol. 2020;10:162.
Article PubMed PubMed Central Google Scholar
Holzer K, Ori A, Cooke A, Dauch D, Drucker E, Riemenschneider P, Andres-Pons A, DiGuilio A, Mackmull M, Baßler J, et al. Nucleoporin Nup155 is part of the p53 network in liver cancer. Nat Commun. 2019;10(1):2147.
Article ADS PubMed PubMed Central Google Scholar
Boyault S, Rickman D, de Reyniès A, Balabaud C, Rebouissou S, Jeannot E, Hérault A, Saric J, Belghiti J, Franco D, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology (Baltimore, MD). 2007;45(1):42–52.
Basit A, Cho M, Kim E, Kwon D, Kang S, Lee J. The cGAS/STING/TBK1/IRF3 innate immunity pathway maintains chromosomal stability through regulation of p21 levels. Exp Mol Med. 2020;52(4):643–57.
Goldman MJ, Craft B, Hastie M, Repečka K, McDade F, Kamath A, Banerjee A, Luo Y, Rogers D, Brooks AN, et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol. 2020;38(6):675–8.
Chandrashekar DS, Karthikeyan SK, Korla PK, Patel H, Shovon AR, Athar M, Netto GJ, Qin ZS, Kumar S, Manne U, et al. UALCAN: an update to the integrated cancer data analysis platform. Neoplasia. 2022;25:18–27.
Sun D, Wang J, Han Y, Dong X, Ge J, Zheng R, Shi X, Wang B, Li Z, Ren P, et al. TISCH: a comprehensive web resource enabling interactive single-cell transcriptome visualization of tumor microenvironment. Nucleic Acids Res. 2021;49(D1):D1420-1430.
Laue C, Papazova E, Pannenbeckers A, Schrezenmeir J. Effect of a probiotic and a Synbiotic on Body Fat Mass, Body Weight and traits of metabolic syndrome in individuals with abdominal overweight: a human, Double-Blind, randomised, controlled clinical study. Nutrients. 2023;15(13):3039.
Wenthe J, Eriksson E, Hellström AC, Moreno R, Ullenhag G, Alemany R, Lövgren T, Loskog A. Immunostimulatory gene therapy targeting CD40, 4-1BB and IL-2R activates DCs and stimulates antigen-specific T-cell and NK-cell responses in melanoma models. J Transl Med. 2023;21(1):506.
El-Adili F, Lui JK, Najem M, Farina G, Trojanowska M, Sam F, Bujor AM. Periostin overexpression in scleroderma cardiac tissue and its utility as a marker for disease complications. Arthritis Res Ther. 2022;24(1):251.
Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463(7283):899–905.
Article ADS CAS PubMed PubMed Central Google Scholar
Bonneville R, Krook MA, Kautto EA, Miya J, Wing MR, Chen HZ, Reeser JW, Yu L, Roychowdhury S. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;2017:PO.17.00073.
Sacks D, Baxter B, Campbell BCV, Carpenter JS, Cognard C, Dippel D, Eesa M, Fischer U, Hausegger K, Hirsch JA, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13(6):612–32.
D’Andrea AD. DNA repair pathways and human cancer. In: The Molecular Basis of Cancer: Fourth Edition. Elsevier; 2015. p. 47-66, e42. https://doi.org/10.1016/B978-1-4557-4066-6.00004-4 .
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–4.
Liu C-J, Hu F-F, Xia M-X, Han L, Zhang Q, Guo A-Y. GSCALite: a web server for gene set cancer analysis. Bioinformatics. 2018;34(21):3771–2.
Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT, et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38(suppl2):W214-220.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–8.
Steuer C, Ramalingam S. Tumor mutation burden: leading immunotherapy to the era of precision medicine? J Clin Oncol. 2018;36(7):631–2.
Ji P, Gong Y, Jin ML, Wu HL, Guo LW, Pei YC, Chai WJ, Jiang YZ, Liu Y, Ma XY, et al. In vivo multidimensional CRISPR screens identify Lgals2 as an immunotherapy target in triple-negative breast cancer. Sci Adv. 2022;8(26):eabl8247.
Pascual-Garcia P, Capelson M. The nuclear pore complex and the genome: organizing and regulatory principles. Curr Opin Genet Dev. 2021;67:142–50.
Kim S, Fernandez-Martinez J, Nudelman I, Shi Y, Zhang W, Raveh B, Herricks T, Slaughter B, Hogan J, Upla P, et al. Integrative structure and functional anatomy of a nuclear pore complex. Nature. 2018;555(7697):475–82.
Azmi AS, Mohammad RM. Targeting cancer at the nuclear pore. J Clin Oncol. 2016;34(34):4180–2.
Franz C, Askjaer P, Antonin W, Iglesias CL, Haselmann U, Schelder M, de Marco A, Wilm M, Antony C, Mattaj IW. Nup155 regulates nuclear envelope and nuclear pore complex formation in nematodes and vertebrates. Embo j. 2005;24(20):3519–31.
Yokoyama T, Yukuhiro M, Iwasaki Y, Tanaka C, Sankoda K, Fujiwara R, Shibuta A, Higashi T, Motoyama K, Arima H, et al. Identification of candidate molecular targets of the novel antineoplastic antimitotic NP-10. Sci Rep. 2019;9(1):16825.
Colussi C, Grassi C. Epigenetic regulation of neural stem cells: the emerging role of nucleoporins. Stem Cells. 2021;39(12):1601–14.
Jacinto FV, Benner C, Hetzer MW. The nucleoporin Nup153 regulates embryonic stem cell pluripotency through gene silencing. Genes Dev. 2015;29(12):1224–38.
Toda T, Hsu JY, Linker SB, Hu L, Schafer ST, Mertens J, Jacinto FV, Hetzer MW, Gage FH. Nup153 interacts with Sox2 to enable bimodal gene regulation and maintenance of neural progenitor cells. Cell Stem Cell. 2017;21(5):618-634e617.
Nakamura T. NUP98 fusion in human leukemia: dysregulation of the nuclear pore and homeodomain proteins. Int J Hematol. 2005;82(1):21–7.
Article MathSciNet CAS PubMed Google Scholar
Domingo-Reinés J, Montes R, Garcia-Moreno A, Gallardo A, Sanchez-Manas JM, Ellson I, Lamolda M, Calabro C, López-Escamez JA, Catalina P, et al. The pediatric leukemia oncoprotein NUP98-KDM5A induces genomic instability that may facilitate malignant transformation. Cell Death Dis. 2023;14(6):357.
Bertrums EJM, Smith JL, Harmon L, Ries RE, Wang YJ, Alonzo TA, Menssen AJ, Chisholm KM, Leonti AR, Tarlock K, et al. Comprehensive molecular and clinical characterization of NUP98 fusions in pediatric acute myeloid leukemia. Haematologica. 2023;108(8):2044–58.
Nishiyama A, Nakanishi M. Navigating the DNA methylation landscape of cancer. Trends Genet. 2021;37(11):1012–27.
Papanicolau-Sengos A, Aldape K. DNA methylation profiling: an emerging paradigm for Cancer diagnosis. Annu Rev Pathol. 2022;17:295–321.
Heichman KA, Warren JD. DNA methylation biomarkers and their utility for solid cancer diagnostics. Clin Chem Lab Med. 2012;50(10):1707–21.
Smith J, Sen S, Weeks RJ, Eccles MR, Chatterjee A. Promoter DNA hypermethylation and paradoxical gene activation. Trends Cancer. 2020;6(5):392–406.
Gold B. Somatic mutations in cancer: stochastic versus predictable. Mutat Res Genet Toxicol Environ Mutagen. 2017;814:37–46.
Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359(6382):1355–60.
Article ADS CAS PubMed Google Scholar
Lang F, Schrörs B, Löwer M, Türeci Ö, Sahin U. Identification of neoantigens for individualized therapeutic cancer vaccines. Nat Rev Drug Discov. 2022;21(4):261–82.
Sha D, Jin Z, Budczies J, Kluck K, Stenzinger A, Sinicrope F. Tumor Mutational Burden as a predictive biomarker in solid tumors. Cancer Discov. 2020;10(12):1808–25.
Chang L, Chang M, Chang HM, Chang F. Microsatellite instability: a predictive biomarker for cancer immunotherapy. Appl Immunohistochem Mol Morphol. 2018;26(2):e15–21.
Goodman AM, Sokol ES, Frampton GM, Lippman SM, Kurzrock R. Microsatellite-stable tumors with high mutational burden benefit from immunotherapy. Cancer Immunol Res. 2019;7(10):1570–3.
Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–70.
Raskov H, Orhan A, Christensen JP, Gögenur I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br J Cancer. 2021;124(2):359–67.
Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: can Treg cells be a new therapeutic target? Cancer Sci. 2019;110(7):2080–9.
Nakayama T, Hirahara K, Onodera A, Endo Y, Hosokawa H, Shinoda K, Tumes DJ, Okamoto Y. Th2 cells in health and disease. Annu Rev Immunol. 2017;35:53–84.
Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8(3):223–46.
McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and Cancer. Annu Rev Immunol. 2019;37:457–95.
Huang Y, Jia A, Wang Y, Liu G. CD8(+) T cell exhaustion in anti-tumour immunity: the new insights for cancer immunotherapy. Immunology. 2023;168(1):30–48.
Dolina JS, Van Braeckel-Budimir N, Thomas GD, Salek-Ardakani S. CD8(+) T cell exhaustion in Cancer. Front Immunol. 2021;12:715234.
Tietscher S, Wagner J, Anzeneder T, Langwieder C, Rees M, Sobottka B, de Souza N, Bodenmiller B. A comprehensive single-cell map of T cell exhaustion-associated immune environments in human breast cancer. Nat Commun. 2023;14(1):98.
Belk JA, Daniel B, Satpathy AT. Epigenetic regulation of T cell exhaustion. Nat Immunol. 2022;23(6):848–60.
Wang Y, Xiang Y, Xin VW, Wang XW, Peng XC, Liu XQ, Wang D, Li N, Cheng JT, Lyv YN, et al. Dendritic cell biology and its role in tumor immunotherapy. J Hematol Oncol. 2020;13(1):107.
Bagchi S, Yuan R, Engleman EG. Immune Checkpoint inhibitors for the treatment of Cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49.
Shen W, Gong B, Xing C, Zhang L, Sun J, Chen Y, Yang C, Yan L, Chen L, Yao L, et al. Comprehensive maturity of nuclear pore complexes regulates zygotic genome activation. Cell. 2022;185(26):4954-4970e4920.
Schwartz M, Travesa A, Martell SW, Forbes DJ. Analysis of the initiation of nuclear pore assembly by ectopically targeting nucleoporins to chromatin. Nucleus. 2015;6(1):40–54.
Scholz BA, Sumida N, de Lima CDM, Chachoua I, Martino M, Tzelepis I, Nikoshkov A, Zhao H, Mehmood R, Sifakis EG, et al. WNT signaling and AHCTF1 promote oncogenic MYC expression through super-enhancer-mediated gene gating. Nat Genet. 2019;51(12):1723–31.
Rayala HJ, Kendirgi F, Barry DM, Majerus PW, Wente SR. The mRNA export factor human Gle1 interacts with the nuclear pore complex protein Nup155. Mol Cell Proteom. 2004;3(2):145–55.
Lin DH, Correia AR, Cai SW, Huber FM, Jette CA, Hoelz A. Structural and functional analysis of mRNA export regulation by the nuclear pore complex. Nat Commun. 2018;9(1):2319.
Culjkovic-Kraljacic B, Baguet A, Volpon L, Amri A, Borden KL. The oncogene eIF4E reprograms the nuclear pore complex to promote mRNA export and oncogenic transformation. Cell Rep. 2012;2(2):207–15.
Fellenberg J, Sähr H, Kunz P, Zhao Z, Liu L, Tichy D, Herr I. Restoration of mir-127-3p and miR-376a-3p counteracts the neoplastic phenotype of giant cell tumor of bone derived stromal cells by targeting COA1, GLE1 and PDIA6. Cancer Lett. 2016;371(1):134–41.
Tzschach A, Grasshoff U, Schäferhoff K, Bonin M, Dufke A, Wolff M, Haas-Lude K, Bevot A, Riess O. Interstitial 9q34.11-q34.13 deletion in a patient with severe intellectual disability, hydrocephalus, and cleft lip/palate. Am J Med Genet A. 2012;158a(7):1709–12.
Liu Z, Zhang Y, Xie J, Li C, Wang X, Shen J, Zhang Y, Wang S, Cheng N. Regenerating gene 1B silencing inhibits colon cancer cell HCT116 proliferation and invasion. Int J Biol Markers. 2015;30(2):e217-225.
Zhou Q, Li J, Ge C, Chen J, Tian W, Tian H. SNX5 suppresses clear cell renal cell carcinoma progression by inducing CD44 internalization and epithelial-to-mesenchymal transition. Mol Ther Oncolytics. 2022;24:87–100.
Zhou Q, Huang T, Jiang Z, Ge C, Chen X, Zhang L, Zhao F, Zhu M, Chen T, Cui Y, et al. Upregulation of SNX5 predicts poor prognosis and promotes hepatocellular carcinoma progression by modulating the EGFR-ERK1/2 signaling pathway. Oncogene. 2020;39(10):2140–55.
Cheng S, Douglas-Jones A, Yang X, Mansel RE, Jiang WG. Transforming acidic coiled-coil-containing protein 2 (TACC2) in human breast cancer, expression pattern and clinical/prognostic relevance. Cancer Genomics Proteom. 2010;7(2):67–73.
CAS Google Scholar
Shakya M, Zhou A, Dai D, Zhong Q, Zhou Z, Zhang Y, Li X, Bholee AK, Chen M. High expression of TACC2 in hepatocellular carcinoma is associated with poor prognosis. Cancer Biomark. 2018;22(4):611–9.
Majidpoor J, Mortezaee K. The efficacy of PD-1/PD-L1 blockade in cold cancers and future perspectives. Clin Immunol. 2021;226: 108707.
Wang X, Wang F, Zhong M, Yarden Y, Fu L. The biomarkers of hyperprogressive disease in PD-1/PD-L1 blockage therapy. Mol Cancer. 2020;19(1):81.
Atkins MB, Lee SJ, Chmielowski B, Tarhini AA, Cohen GI, Truong TG, Moon HH, Davar D, O’Rourke M, Stephenson JJ, et al. Combination Dabrafenib and Trametinib versus combination Nivolumab and Ipilimumab for patients with advanced BRAF-mutant melanoma: the DREAMseq Trial-ECOG-ACRIN EA6134. J Clin Oncol. 2023;41(2):186–97.
Long GV, Hauschild A, Santinami M, Atkinson V, Mandalà M, Chiarion-Sileni V, Larkin J, Nyakas M, Dutriaux C, Haydon A, et al. Adjuvant dabrafenib plus Trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377(19):1813–23.
Vodenkova S, Buchler T, Cervena K, Veskrnova V, Vodicka P, Vymetalkova V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: past, present and future. Pharmacol Ther. 2020;206: 107447.
Zhao S, Tang Y, Wang R, Najafi M. Mechanisms of cancer cell death induction by paclitaxel: an updated review. Apoptosis. 2022;27(9–10):647–67.
Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N. Triple-negative breast cancer–current status and future directions. Ann Oncol. 2009;20(12):1913–27.
Vagia E, Mahalingam D, Cristofanilli M. The Landscape of targeted therapies in TNBC. Cancers (Basel). 2020;12(4):916.
Yin L, Duan JJ, Bian XW, Yu SC. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020;22(1):61.
Bai X, Ni J, Beretov J, Graham P, Li Y. Triple-negative breast cancer therapeutic resistance: where is the Achilles’ heel? Cancer Lett. 2021;497:100–11.
Tray N, Taff J, Adams S. Therapeutic landscape of metaplastic breast cancer. Cancer Treat Rev. 2019;79:101888.
So JY, Ohm J, Lipkowitz S, Yang L. Triple negative breast cancer (TNBC): non-genetic tumor heterogeneity and immune microenvironment: emerging treatment options. Pharmacol Ther. 2022;237: 108253.
Download references
Acknowledgements
Not applicable.
This study was supported by Key Laboratory of Clinical Laboratory Diagnostics (Ministry of Education) [Grant number: 2022E10022], Young Talent Program [grant number: qnyc108] and Zhejiang Medical Health Science and technology Program [Grant number: 2019RC204].
Author information
Zi-qiong Wang and Zhi-xuan Wu contributed equally to this work.
Authors and Affiliations
Quzhou People’s Hospital, The Quzhou Affiliated Hospital of Wenzhou Medical University, 100 Minjiang Avenue, Quzhou, Zhejiang, 324000, Zhejiang, China
Zi-qiong Wang, Zong-pan Wang, Yi-Yin Xu, Rong-xing Wu & Xuan-xuan Dai
Department of Breast Surgery, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325035, Zhejiang, China
Zi-qiong Wang, Zhi-xuan Wu, Jing-xia Bao, Hao-dong Wu, Di-yan Xu, Hong-feng Li & Xuan-xuan Dai
Key Laboratory of Clinical Laboratory Diagnostics (Ministry of Education), The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325035, China
Zi-qiong Wang & Xuan-xuan Dai
You can also search for this author in PubMed Google Scholar
Contributions
ZQ W, ZP W, XX D and RX W designed the study. ZX W and DY X performed the data analysis. ZQ W and JX B wrote the manuscript and helped with the validation. HD W, HF L and YY X helped the revision. All authors contributed to the article and approved the submitted version.
Corresponding authors
Correspondence to Rong-xing Wu or Xuan-xuan Dai .
Ethics declarations
Ethics approval and consent to participate.
The research did not involve animal experiments or human specimens or any ethics-related issues. The study methodologies conformed to the standards set by the Declaration of Helsinki.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:.
The original blots of western blotting.
Additional file 2:
Supplementary figure 1. The correlation between NUP155 expression and the pathological or clinical stages of cancers, including (A) ACC, (B) KICH, (C) KIRC, (D) KIRP, (E) LIHC, (F) OV, (G) SKCM, (H) UCS.
Additional file 3:
Supplementary figure 2. (A) Association between NUP155 CNV and mRNA in pan-cancer. (B) The top six with the highest correlation scores between NUP155 CNV and mRNA.
Additional file 4:
Supplementary figure 3. Association between NUP155 expression levels and Disease-free survival (DSS) in TCGA pan-cancer. (A) Forest plot of association of NUP155 expression and DSS. (B–J) Kaplan-Meier analysis of the association between NUP155 expression and DSS.
Additional file 5:
Supplementary figure 4. Association between NUP155 expression levels and Progression-free survival (PFS) in TCGA pan-cancer. (A) Forest plot of association of NUP155 expression and PFS. (B–K) Kaplan-Meier analysis of the association between NUP155 expression and PFS.
Additional file 6:
Supplementary figure 5. Eight tumors with the highest correlation coefficients between NUP155 expression and the tumor microenvironment. (A) Correlation between NUP155 and stromal scores in GBM, STES, TGCT, SKCM, SARC, KIPAN, STAD, KIRC. (B) Correlation between NUP155 and immune scores in GBM, STES, UCEC, THYM, ESCA, STAD, LUSC, SKCM.
Additional file 7:
Supplementary figure 6. Association between NUP155 gene and the TIME in pan-cancer tissues, using the TISCH database.
Additional file 8:
Supplementary figure 7. Reactome functional annotation of NUP155 in various cancers, including (A) CHOL, (B) PCPG, (C) KICH, (D) TGCT, (E) KIRC, (F) THCA, (G) PAAD, (H) THYM.
Additional file 9:
Supplementary figure 8. Relative mRNA expression of NUP155 in normal breast cell and breast cancer cell lines. * p < 0.05 and ** p < 0.01.
Additional file 10:
Supplementary table S1. The R-Scripts and online tools in this study. Supplementary table S2. The detail results of the methylation levels of NUP155 in pan-cancer. Supplementary table S3. The landscape of NUP155 copy number variation (CNV) in different cancer types. Supplementary table S4. The correlation between NUP155 expression levels and drug sensitivity in the CTRP database. Supplementary table S5. The correlation between NUP155 expression levels and drug sensitivity in the GDSC database. Supplementary table S6. The enrichment analysis of NUP155 in the REACTOME pathway. Supplementary table S7. Genes interacting with NUP155 in the PPI network. Supplementary table S8. Functional enrichment of genes interacting with NUP155 in the PPI network.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wang, Zq., Wu, Zx., Wang, Zp. et al. Pan-cancer analysis of NUP155 and validation of its role in breast cancer cell proliferation, migration, and apoptosis. BMC Cancer 24 , 353 (2024). https://doi.org/10.1186/s12885-024-12039-6
Download citation
Received : 19 October 2023
Accepted : 21 February 2024
Published : 19 March 2024
DOI : https://doi.org/10.1186/s12885-024-12039-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pan-cancer analysis
- Immune infiltration
- Immune checkpoints
- Molecular biology experiments
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Introduction. Being characterized by six major hallmarks, carcinogenesis might occur in every cell, tissue, and organ, leading to the pathological alternations that result in a vast number of cancers. ... Breast cancer is currently one of the most prevalently diagnosed cancers and the 5th cause of cancer-related deaths with an estimated number ...
17 essay samples found. Breast cancer is a type of cancer that develops from breast tissue. Essays on this topic could explore the causes, diagnosis, treatment, and prevention of breast cancer. Additionally, discussions might delve into the psychological and social impact of breast cancer on patients and their families, the ongoing research ...
The mammogram is the first indication of breast cancer, even though other indications such as the presence of the lymph nodes in the armpits are also the early indications of breast cancer. We will write. a custom essay specifically for you by our professional experts. 809 writers online. Learn More.
INTRODUCTION BREAST cancer is currently the most common cancer affecting women worldwide [1]. In European women, it is the leading cause of cancer death, causing one in six of all deaths from cancers [2].In the U.S., a woman has a 12.15% (about one in eight) risk of developing breast cancer during her lifetime [3].
The breast is an organ that sits on top of the upper ribs and chest muscles. There is a left and right breast and each one has mainly glands, ducts, and fatty tissue. In women, the breast makes and delivers milk to feed newborns and infants. The amount of fatty tissue in the breast determines the size of each breast.
Breast cancer is a disease in which abnormal breast cells grow out of control and form tumours. If left unchecked, the tumours can spread throughout the body and become fatal. Breast cancer cells begin inside the milk ducts and/or the milk-producing lobules of the breast. The earliest form (in situ) is not life-threatening and can be detected ...
Surgery for breast cancer. Most women with breast cancer have some type of surgery. Common types of breast surgery are lumpectomy, mastectomy, and taking out lymph nodes from the underarm. Women who have breast surgery may also decide to have the breast shape rebuilt, either at the same time or later on.
Breast cancer is cancer that starts in cells in the breast. The ducts and the lobules are the two parts of the breast where cancer is most likely to start. Breast cancer is one of the most common types of cancer in the U.S. Healthcare providers don't yet know exactly what causes it. Once breast cancer forms, cancer cells can spread to other ...
The lifetime risk for breast cancer in men is 1 in 833 compared with 1 in 10 for a woman. Of affected men, 20% have a first-degree family history of cancer; 4-14% of cases in males are ...
Essays on breast cancer are significant for academic and personal exploration as they provide an opportunity to raise awareness about the disease, its risk factors, prevention, and treatment options. Writing about breast cancer also allows individuals to share personal experiences, advocate for research and support, and contribute to the ...
Facts and Figures. Breast cancer (BC) is regarded as the most common type of cancer globally. According to Mascara and Constantinou (2021), "about 2.3 million people are diagnosed with the disease each year" (p. 9). In the U.S., approximately 264000 and 2400 cancer cases are diagnosed each year among women and men, respectively (Mascara and ...
For example, issues with an overweight mother can negatively affect a baby and serve as a risk factor for developing breast cancer (Forman, 2020). However, the women's diet at the stage of breast development directly affects the risk of the disease in the future.
1 Introduction. T he prospect of developing breast cancer is a source of anxiety for many women. Breast cancer remains the most common invasive cancer among women (aside from nonmelanoma skin cancers), accounting in 2011 for an estimated 230,480 new cases among women in the United States and another 2,140 new cases among men (ACS, 2011).
Introduction to Breast Cancer. Breast cancer is a malignant cell growth in the breast.If left untreated, the cancer spreads to other areas of the body. Excluding skin cancer, breast cancer is the most common type of cancer in women in the United States, accounting for one of every three cancer diagnoses.. An estimated 211,240 new invasive cases of breast cancer were expected to occur among ...
Chapter 1: An Introduction to Breast Cancer. 1.1 Introduction related to Cancer and its Treatment. Methods. There are many ter minologies regard ing cancer available in t he lite-. rature [1 ...
An Introduction to Breast Cancer • 11 minutes • Preview module. Genetics • 20 minutes. Prevention • 16 minutes. Interview with Erin Hofstatter, Co-Director of High Risk Program at Yale Cancer Center • 9 minutes. 1 quiz • Total 30 minutes. Module #1: Risks and Prevention • 30 minutes.
Breast cancer is a life-threatening cancer and a leading cause of death among women. Breast cancer cases are increasing constantly due to the risk factors including age, menopause, obesity, use of hormone replacement therapy, family history, along with the environment and lifestyle factors. The increased awareness and newer diagnosis techniques ...
1.1 Introduction. Breast cancer is the leading cause of death among women worldwide (Fig. 1.1) (Spitale et al. 2009 ). It is a heterogenous disease that comprises many different subgroups that differ in their distinct pathological features and clinical significance (Tang et al. 2008; Desmedt et al. 2009; Sotiriou and Pusztai 2009; Spitale et al ...
Identifying new targets in triple negative breast cancer (TNBC) remains critical. REG3A (regenerating islet-derived protein 3 A), a calcium-dependent lectin protein, was thoroughly investigated for its express... Xiaoxia Jin, Shuyun Yang, Xiaoyun Lu, Xudong Chen and Wencheng Dai. Breast Cancer Research 2024 26 :92.
1 INTRODUCTION. Metaplastic breast cancer is a unique and aggressive subtype of breast cancer which comprises up to 1% of all breast cancer cases. 1 It represents a heterogenous group of tumors containing at least one cell type which has undergone transformation to a nonglandular morphology and usually consists of epithelial and mesenchymal components. 2 The World Health Organization ...
Abstract. Introduction: Synthetically lethal mutations offer unique molecular targets for oncologic therapy. At scale, synthetic lethality (SL) is observed when alterations to one gene alone do not correspond with worse overall survival (OS), but simultaneous expression with another gene does correspond with worse OS. Mutually exclusive gene expression is not a requirement for SL. Existing ...
Breast cancer is a complex disease exhibiting a great degree of heterogeneity due to different molecular subtypes. Notch signaling regulates the differentiation of breast epithelial cells during normal development and plays a crucial role in breast cancer progression through the abnormal expression of the Notch up-and down-stream effectors. To date, there are only a few patient-centered ...
Background Associations between reproductive factors and breast cancer (BC) risk vary by molecular subtype (i.e., luminal A, luminal B, HER2, and triple negative/basal-like [TNBC]). In this systematic review and meta-analysis, we summarized the associations between reproductive factors and BC subtypes. Methods Studies from 2000 to 2021 were included if BC subtype was examined in relation to ...
We leveraged paired tumor and normal sequencing data from 4918 primary and 611 metastatic breast cancer patients, as well as somatic genomic profiles from 341 patients with ductal carcinoma in situ (DCIS), and evaluated the relationship between germline-derived epitope burden (GEB) and subtype commitment, defined by the acquisition of focal oncogenic amplifications.
Introduction: Breast cancer (BC), the predominant non-melanoma cancer among women, is projected to escalate to more than 3 million new cases and ~1 million fatalities globally by 2040. Chronic and fluctuating low oxygen (O2) conditions within tumors favor malignant growth, decreased responsiveness to therapeutic interventions, and reduced patient survivability. Treatment strategies to combat ...
Triple negative breast cancer (TNBC) remains exceptionally challenging to treat. While CDK4/6 inhibitors have revolutionized HR + breast cancer therapy, there is limited understanding of their efficacy in TNBC and meaningful predictors of response and resistance to these drugs remain scarce. We conducted an in vivo genome-wide CRISPR screen using palbociclib as a selection pressure in TNBC.
NUP155 is reported to be correlated with tumor development. However, the role of NUP155 in tumor physiology and the tumor immune microenvironment (TIME) has not been previously examined. This study comprehensively investigated the expression, immunological function, and prognostic significance of NUP155 in different cancer types. Bioinformatics analysis revealed that NUP155 was upregulated in ...