Your Article Library
Cell: essay on cells in human body.
ADVERTISEMENTS:
Read this Essay on Cells in Human Body !
The body of any living organism is made up of cells. Cells are very minute in size and extremely complicated in structure. Human is no exception. Each cell is basically a unit of protoplasm, which is said to be the “material basis of life”. The protoplasm is the combination of cytoplasm and nucleus.
The cytoplasm is semi-fluid, jelly-like, hyaline substance; its outer surface is much more thick to form the boundary of the cell. This specialized boundary of the cell is known as cell membrane or plasma membrane. The cell membrane controls the in-and-out of various chemical substances. Various living and non-living bodies are found in the cytoplasm.
Of the living bodies the mitochondria and the Golgi body are very important. The mitochondria are the minute, semi-solid body enclosed in a membrane with a complex internal structure. Although the main constituents of mitochondria are protein and fat, it also contains several enzymes, notably oxidative enzyme systems. The Golgi body is the local clump of material present in the cytoplasm.
Its functions are not clearly known, but seem to be associated with the formation of secretions. The vacuole is important as the fluid-filled space within the cytoplasm of a cell, which controls the cell sap isotonic with cytoplasm by expelling or entering the water from the environment, following the process of osmosis. The centrosome is the region of differentiated cytoplasm containing centriole. The centriole is the minute granule present in many resting cells, just outside the nuclear membrane.

The nucleus is denser than the cytoplasm and it is regarded as the dynamic centre of life. Its shape is absolutely spherical. A membrane, known as nuclear membrane surrounds it to keep the core protected. The nuclear cavity remains filled up with a dense jelly-like substance, known as nucleoplasm. Some delicate thread-like structures that suspend in the nucleoplasm are called nuclear reticulum or chromatin network.
The threads are easily stainable by basic dyes; they are made up of a substance, called chromatin. Apart from these, one or two small spherical bodies are found in the nucleoplasm, known as nucleolus. The nucleus exerts a direct influence on different activities of the cell and plays a great role in transmitting hereditary characters from the parents to offspring.
The cell can be divided into two types – the somatic cell or body cell and the germ cell or reproductive cell. The somatic cell helps in construction and maintenance of different bodily structures. It can also be sub-divided into nerve cells, muscle cells, etc. in accordance with their nature and function.
The germ cell is useful in reproducing new species. It can be sub-divided into sperms or male sex cells and ova or female sex cells. The zygote is also a cell, which is formed by the fusion of an egg or ovum, and a sperm. The human ovum is spherical in shape, and about 1/7th of a millimeter or about 1/175th of an inch in diameter.
Though it is the largest of all cells in human body, still not visible in naked eye. In premature stage, a sperm cell is similar to a somatic cell consisting of a nucleus and a mass of cytoplasm. But a mature sperm cell is long and thread-like with an enlarged head formed by the nucleus. It also possesses a long slender tail and a conical middle piece. The tail is used in movement.
A new life starts when a sperm fertilizes an ovum. After the union of the sperm and the ovum, the fertilization takes place when the head and the middle piece of the sperm sink into the egg. The tail of the sperm is left outside. However, the head of the sperm starts functioning as a normal nucleus by absorbing fluid from the cytoplasm of the ovum.
Fertilization is thus a process of the nuclear fusion. The sperm loses its identity and the fertilized ovum or egg is called a zygote, which begins to divide into two, four, eight and so on, by the process of cell division. From the early stage, some of these cells are set apart to form the germ cells while others go to form various body parts, known as somatic cells.
The cell division is not a simple process; complicated changes have been noted in the substances of the nucleus. In this regard it is necessary to know about the chromosomes. The chromosomes are the slender, rope-like bodies that usually occur in pairs and found in the nucleus. The number of chromosomes remains constant in each species, which generally ranges between 2 to 200.
The size and form of chromosomes also vary from species to species. In fact, differences of chromosome constitution mark off one species from another.In case of man, the usual number of chromosomes is 46. There are altogether 23 pairs of chromosome; 22 pairs act as autosomes and the other pair is known as sex-determining chromosome or sex chromosome.
Chromosomes are composed of two kinds of nucleic acids and two main types of proteins. Chromatin is the most important nucleo-protein in chromosomes. The main nucleic acid of the chromosome is Deoxyribonucleic acid or DNA. In 1951, a renowned biologist James Watson with his chemist friend Francis Crick proposed the structure of DNA.
The other kind of nucleic acid in the chromosomes is the Ribonucleic acid or RNA, which exists in cytoplasm. DNA samples from different tissues of the same species are all identical in composition. RNA not only differs from DNA; the amount of ribonucleic acid in the chromosomes varies from tissue to tissue.
It is found in large quantities in the cytoplasmic particles of the cell. In some cases, the nucleolus also contains large quantities of RNA. It is believed by the scholars that RNA plays an essential role in protein synthesis and carries instruction to the cytoplasm of a cell. In general, the DNA content of an egg is greater than that of a sperm. A variety of suggestions have been made regarding the relationship between the DNA and protein components of the chromosomes but no steady conclusion has yet been achieved.
As a matter of fact the nucleus and the cytoplasm exist in a state of symbiosis. The nucleus depends on the cytoplasm for its energy supply and also for the supply of materials out of which new nucleic acid molecules and chromosomal proteins can be synthesized. On the other hand, the cytoplasm is fully dependent on the nucleus for the maintenance of its essential biosynthetic mechanisms.

Related Articles:
- Fixed Cells: Useful notes on the Structure of Fixed Cells
- Prokaryotic Cells: 7 Most Important Characteristics of Prokaryotic Cells
No comments yet.
Leave a reply click here to cancel reply..
You must be logged in to post a comment.
Introduction to the Human Body
The human body is a complex, highly organized structure made up of unique cells that work together to accomplish the specific functions necessary for sustaining life.
The biology of the human body includes
Physiology (how the body functions)
Anatomy (how the body is structured)
Anatomy is organized by levels, from the smallest components of cells to tissues and organs and to organ systems .
Gross anatomy is the study of the body's organs as seen with the naked eye during visual inspection and when the body is cut open for examination (dissection).
Cellular anatomy is the study of cells and their components, which can be observed only with the use of special techniques and special instruments such as microscopes.
Molecular anatomy (often called molecular biology) is the study of the smallest components of cells at the biochemical level.
Anatomy and physiology change remarkably between fertilization and birth. After birth, the rate of anatomic and physiologic changes slows, but childhood is still a time of remarkable growth and development ( see Physical Growth of Infants and Children ). Some anatomic changes occur past adulthood, but the physiologic changes in the body's cells and organs are what contribute most to what we experience as aging ( see Changes in the Body With Aging ).

- Cookie Preferences

Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

1.3: Structural Organization of the Human Body
- Last updated
- Save as PDF
- Page ID 22247

- Whitney Menefee, Julie Jenks, Chiara Mazzasette, & Kim-Leiloni Nguyen
- Reedley College, Butte College, Pasadena City College, & Mt. San Antonio College via ASCCC Open Educational Resources Initiative
By the end of the section, you will be able to:
- Describe the structure of the human body in terms of six levels of organization
- List the eleven organ systems of the human body and identify at least one organ and one major function of each
Before you begin to study the different structures and functions of the human body, it is helpful to consider its basic architecture; that is, how its smallest parts are assembled into larger structures. It is convenient to consider the structures of the body in terms of fundamental levels of organization that increase in complexity: atoms, molecules, organelles, cells, tissues, organs, organ systems, and organisms (Figure \(\PageIndex{1}\)).

The Levels of Organization
To study the smallest level of organization, scientists consider the simplest building blocks of matter: atoms and molecules. The chemical level of organization considers these two building block as atoms bond to form molecules with three dimensional structures. All matter in the universe is composed of one or more unique pure substances called elements, familiar examples of which are hydrogen, oxygen, carbon, nitrogen, calcium, and iron. The smallest unit of any of these pure substances (elements) is an atom. Atoms are made up of subatomic particles such as the proton, electron and neutron. Two or more atoms combine to form a molecule, such as the water molecules, proteins, and sugars found in living things. Molecules are the chemical building blocks of all body structures.
The cellular level is considered when a variety of molecules combine to form the fluid and organelles of a body cell. A cell is the smallest independently functioning unit of a living organism. Even bacteria, which are extremely small, independently-living organisms, have a cellular structure. Each bacterium is a single cell. All living structures of human anatomy contain cells, and almost all functions of human physiology are performed in cells or are initiated by cells. A human cell, such as a smooth muscle cell, typically consists of flexible membranes that enclose cytoplasm , a water-based cellular fluid together with a variety of tiny functioning units called organelles .
The tissue level can be studied when a community of similar cells form a body tissue. A tissue is a group of many similar cells (though sometimes composed of a few related types) that work together to perform a specific function. For example, when many smooth muscle cells come together both structurally and functionally, these cell collectively form a layer of smooth muscle tissue.
An organ is an anatomically distinct structure of the body composed of two or more tissue types, which forms the organ level of organization. Each organ performs one or more specific physiological functions. The human bladder, which is composed of smooth muscle tissue, transitional epithelial tissue, and several types of connective tissue serves the function of storing urine produced by the kidneys.
An organ system level is a group of organs that work together to perform major functions or meet physiological needs of the body. In the organ example above, both the kidneys and the bladder are organs of the urinary system. The kidneys produce urine, which is moved to the bladder by the ureters. Urine can then leave the bladder, and the body, through the urethra. These four organs work together to rid the body of liquid waste.
This book covers eleven distinct organ systems in the human body (Figure \(\PageIndex{2}\) and Figure \(\PageIndex{3}\)). Assigning organs to organ systems can be imprecise since organs that “belong” to one system can also have functions integral to another system. In fact, most organs contribute to more than one system.
.jpg?revision=1&size=bestfit&width=470&height=777)
The eleven distinct organ systems in the human body covered in this book seen in Figure \(\PageIndex{2}\) and Figure \(\PageIndex{3}\) include:
- The integumentary system functions to enclose internal body structures and is the site of many sensory receptors. Some organs of this system include skin, hair, and nails.
- The skeletal system supports the body and enables movement (with the help of the muscular system). Bones are the major organs of this system.
- The muscular system enables movement (with the help of the skeletal system) and also helps to maintain body temperature. Skeletal muscles are the major organs of this system, which are connected to bones by tendons.
- The nervous system detects and processes sensory information and activates bodily responses. The main organs that perform these functions are the brain, spinal cord, and peripheral nerves.
- The endocrine system is responsible for secreting hormones to regulate bodily processes. Some major organs of the endocrine system include the pituitary gland, thyroid gland, pancreas, adrenal glands, testes, and ovaries.
- The cardiovascular system delivers oxygen and nutrients to tissues while also removing wastes, and helps to equalize temperature in the body. This is accomplished by the heart and blood vessels.
- The lymphatic system functions to return fluids back to the blood and defends the body against pathogens. Major structures and organs of this system include the thymus, lymph nodes, the spleen, and lymphatic vessels.
- The respiratory system delivers oxygen to blood and removes carbon dioxide from the body. The nasal cavity, trachea, and lungs all work together to carry out these functions.
- The digestive system processes food for use by the body and removes wastes from undigested foods. Some organs of the digestive system include the stomach, liver, gallbladder, large intestine, and small intestine.
- The urinary system controls water balance in the body and removes wastes from the blood and excretes them. The kidneys and bladder are major organs of this system.
- The reproductive system produces sex hormones and gametes. The male reproductive system also functions to deliver gametes to the female, while the female reproductive system supports an embryo/fetus until birth and produces milk for an infant. Organs of the male reproductive system include the epididymis and testes. Organs of the female reproductive system include the mammary glands, ovaries, and the uterus.
While only some functions and major organs of each system have been listed above, each of these organ systems will be covered in much greater detail in the following chapters of this book.
The organism level , when many organ systems work harmoniously together to perform the functions of an independent organism, is the highest level of organization in the study of human anatomy. An organism is a living being that has a cellular structure and that can independently perform all physiologic functions necessary for life. In multicellular organisms, including humans, all cells, tissues, organs, and organ systems of the body work together to maintain the life and health of the organism.
Concept Review
Life processes of the human body are maintained at several levels of structural organization. These include the chemical, cellular, tissue, organ, organ system, and the organism level. Higher levels of organization are built from lower levels. Therefore, molecules combine to form cells, cells combine to form tissues, tissues combine to form organs, organs combine to form organ systems, and organ systems combine to form organisms.
Review Questions
Q. The smallest independently functioning unit of an organism is a(n) ________.
B. molecule
Q. A collection of tissues that performs a specific function is an ________.
B. organelle
C. organism
D. organ system
Q. The body system responsible for structural support and movement is the ________.
A. cardiovascular system
B. endocrine system
C. muscular system
D. skeletal system
Critical Thinking Questions
Q. Name the six levels of organization of the human body.
A. Chemical, cellular, tissue, organ, organ system, organism.
Q. The female ovaries and the male testes are a part of which body system? Can these organs be members of more than one organ system? Why or why not?
A. The female ovaries and the male testes are parts of the reproductive system. But they also secrete hormones, as does the endocrine system, therefore ovaries and testes function within both the endocrine and reproductive systems.
Contributors and Attributions
OpenStax Anatomy & Physiology (CC BY 4.0). Access for free at https://openstax.org/books/anatomy-and-physiology

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

10.8: Case Study Conclusion: Pressure and Chapter Summary
- Last updated
- Save as PDF
- Page ID 17076

- Suzanne Wakim & Mandeep Grewal
- Butte College
Case Study Conclusion: Under Pressure
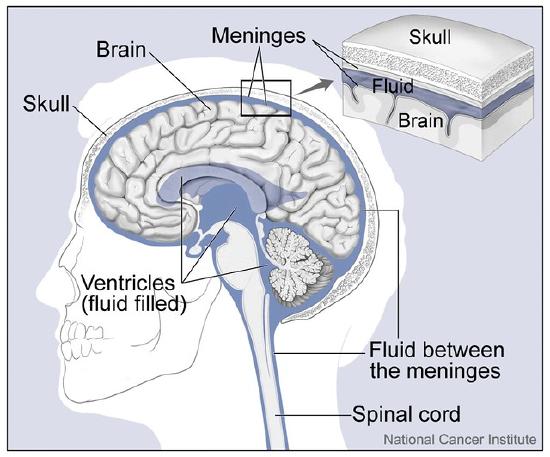
As you learned in this chapter, the human body consists of many complex systems that normally work together efficiently like a well-oiled machine to carry out life’s functions. For example, Figure \(\PageIndex{1}\) illustrates how the brain and spinal cord are protected by layers of membrane called meninges and fluid that flows between the meninges and in spaces called ventricles inside the brain. This fluid is called cerebrospinal fluid (CSF) and as you have learned, one of its important functions is to cushion and protect the brain and spinal cord, which make up most of the central nervous system (CNS). CSF additionally circulates nutrients and removes waste products from the CNS. CSF is produced continually in the ventricles, circulates throughout the CNS, and then is reabsorbed by the bloodstream. If too much CSF is produced, its flow blocked, or if not enough is reabsorbed, the system becomes out of balance, and CSF can build up in the ventricles. This causes an enlargement of the ventricles called hydrocephalus that can put pressure on the brain, resulting in the types of neurological problems that former professional football player, Dayo, described at the beginning of this chapter, is suffering from.
Recall that Dayo’s symptoms included loss of bladder control, memory loss, and difficulty walking. The cause of their symptoms was not immediately clear, although their doctors suspected that it related to the nervous system since the nervous system acts as the control center of the body, controlling and regulating many other organ systems. Dayo’s memory loss directly implicated the involvement of the brain, since that is the site of thoughts and memory. The urinary system is also controlled in part by the nervous system, and the inability to hold urine appropriately can be a sign of a neurological issue. Dayo’s trouble walking involved the muscular system, which works alongside the skeletal system to enable movement of the limbs. In turn, the contraction of muscles is regulated by the nervous system. You can see why a problem in the nervous system can cause a variety of different symptoms by affecting multiple organ systems in the human body.
To try to find the exact cause of Dayo’s symptoms, their doctors performed a lumbar puncture, or spinal tap, which is the removal of some CSF through a needle inserted into the lower part of the spinal canal. Doctors then analyzed Dayo’s CSF for the presence of pathogens such as bacteria to determine whether an infection was the cause of their neurological symptoms. When no evidence of infection was found, Doctors used an MRI to observe the structures of Dayo's brain. This is when Doctors discovered Dayo's enlarged ventricles, which are a hallmark of hydrocephalus.
To treat Dayo’s hydrocephalus, a surgeon implanted a device called a shunt in Dayo's brain to remove the excess fluid (Figure \(\PageIndex{2}\)). One side of the shunt consists of a small tube, called a catheter , which was inserted into Dayo’s ventricles. Excess CSF is then drained through a one-way valve to the other end of the shunt, which was threaded under their skin to their abdominal cavity, where the CSF is released and can be reabsorbed by the bloodstream.
Implantation of a shunt is the most common way to treat hydrocephalus, and for some people, it can allow them to recover almost completely. However, there can be complications associated with a brain shunt. The shunt can have mechanical problems or cause an infection. Also, the rate of draining must be carefully monitored and adjusted to balance the rate of removal of CSF with the rate of its production. If it is drained too fast, it is called overdraining, and if it is drained too slowly, it is called underdraining . In the case of underdraining, the pressure on the brain and associated neurological symptoms will persist. In the case of overdraining, the ventricles can collapse, which can cause serious problems such as the tearing of blood vessels and hemorrhaging. To avoid these problems, some shunts have an adjustable pressure valve where the rate of draining can be adjusted by placing a special magnet over the scalp. You can see how the proper balance between CSF production and removal is so critical – both in the causes of hydrocephalus and in its treatment.
In what other ways does your body regulate balance, or maintain a state of homeostasis? In this chapter, you learned about the feedback loops that keep body temperature and blood glucose within normal ranges. Other important examples of homeostasis in the human body are the regulation of the pH in the blood and the balance of water in the body. You will learn more about homeostasis in different body systems in the coming chapters.
Thanks to Dayo’s shunt, their symptoms are starting to improve, but they have not fully recovered. Time may tell whether the removal of the excess CSF from their ventricles will eventually allow them to recover normal functioning or whether permanent damage to their nervous system has already been done. The flow of CSF might seem simple but when it gets out of balance, it can easily wreak havoc on multiple organ systems because of the intricate interconnectedness of the systems within the human “machine."
Chapter Summary
This chapter provided an overview of the organization and functioning of the human body. You learned that:
- The human body consists of multiple parts that function together to maintain life. The biology of the human body incorporates the body’s structure, or anatomy, and the body’s functioning, or physiology.
- The organization of the human body is a hierarchy of increasing size and complexity, starting at the level of atoms and molecules and ending at the level of the entire organism.
- Variations in cell function are generally reflected in variations in cell structure.
- Some cells are unattached to other cells and can move freely; others are attached to each other and cannot move freely. Some cells can divide readily and form new cells; others can divide only under exceptional circumstances. Many cells are specialized to produce and secrete particular substances.
- All the different cell types within an individual have the same genes. Cells can vary because different genes are expressed depending on the cell type.
- Many common types of human cells consist of several subtypes of cells, each of which has a special structure and function. For example, subtypes of bone cells include osteocytes, osteoblasts, osteogenic cells, and osteoclasts.
- Connective tissues, such as bone and blood, are made up of cells that are separated by non-living material, called the extracellular matrix.
- Epithelial tissues, such as skin and mucous membranes, protect the body and its internal organs and secrete or absorb substances.
- Muscle tissues are made up of cells that have the unique ability to contract. They include skeletal, smooth, and cardiac muscle tissues.
- Nervous tissues are made up of neurons, which transmit electrical messages, and glial cells of various types, which play supporting roles. Types of nervous tissues include gray matter, white matter, nerves, and ganglia.
- Many organs are composed of a major tissue that performs the organ’s main function, as well as other tissues that play supporting roles.
- The human body contains five organs that are considered vital for survival. They are the heart, brain, kidneys, liver, and lungs. If any of these five organs stops functioning, the death of the organism is imminent without medical intervention.
- There are 11 major organ systems in the human organism. They are the integumentary, skeletal, muscular, nervous, endocrine, cardiovascular, lymphatic, respiratory, digestive, urinary, and reproductive systems. Only the reproductive system varies significantly between males and females.
- The ventral cavity is at the anterior, or front, of the trunk. It is subdivided into the thoracic cavity and abdominopelvic cavity.
- The dorsal cavity is at the posterior, or back, of the body, and includes the head and the back of the trunk. It is subdivided into the cranial cavity and spinal cavity.
- Cellular respiration is a good example of organ system interactions because it is a basic life process that occurs in all living cells. It is the intracellular process that breaks down glucose with oxygen to produce carbon dioxide and energy. Cellular respiration requires the interaction of the digestive, cardiovascular, and respiratory systems.
- The fight-or-flight response is a good example of how the nervous and endocrine systems control other organ system responses. It is triggered by a message from the brain to the endocrine system and prepares the body for flight or a fight. Many organ systems are stimulated to respond, including the cardiovascular, respiratory, and digestive systems.
- Digesting food requires teamwork between the digestive system and several other organ systems, including the nervous, cardiovascular, and muscular systems.
- Playing softball or doing other voluntary physical activities may involve the interaction of nervous, muscular, skeletal, respiratory, and cardiovascular systems.
- For any given variable, such as body temperature, there is a particular set point that is the physiological optimum value. The spread of values around the setpoint that is considered insignificant is called the normal range.
- Homeostasis is generally maintained by a negative feedback loop that includes a stimulus, sensor, control center, and effector. Negative feedback serves to reduce an excessive response and to keep a variable within the normal range. Negative feedback loops control body temperature and blood glucose level.
- Sometimes homeostatic mechanisms fail, resulting in homeostatic imbalance. Diabetes is an example of a disease caused by homeostatic imbalance. Aging can bring about a reduction in the efficiency of the body’s control system, making the elderly more susceptible to disease.
- Positive feedback loops are not common in biological systems. Positive feedback serves to intensify a response until an endpoint is reached. Positive feedback loops control blood clotting and childbirth.
The severe and broad impact of hydrocephalus on the body’s systems highlights the importance of the nervous system and its role as the master control system of the body. In the next chapter, you will learn much more about the structures and functioning of this fascinating and important system.
Chapter Summary Review
- Compare and contrast tissues and organs.
- Osteocyte cells are part of which type of tissue and organ system?
- mucous membranes
- gray matter
- Which type of tissue lines the inner and outer surfaces of the body?
- True or False. The extracellular matrix that surrounds cells is always solid.
- True or False. Skin is an organ.
- What is a vital organ? What happens if a vital organ stops working?
- Name three organ systems that transport or remove wastes from the body.
- Name two types of tissue in the digestive system.
- Processes sensory information
- Secretes hormones
- Releases carbon dioxide from the body to the outside world
- Produces gametes
- Controls water balance in the body
- Integumentary
- Describe one way in which the integumentary and cardiovascular systems work together to regulate homeostasis in the human body.
- Name the two largest body cavities in humans and describe their general locations.
- What are the names given to the three body cavity divisions where the reproductive organs are located?
- True or False. There are two pleural cavities.
- True or False. Body cavities are filled with air.
- The pituitary gland is in which organ system? Describe how the pituitary gland increases metabolism.
- When the level of thyroid hormone in the body gets too high, it acts on other cells to reduce the production of more thyroid hormone. What type of feedback loop does this represent?
- What is the stimulus in this feedback loop?
- If the level of B1 falls significantly below the setpoint, what do you think happens to the production of A1? Why?
- What is the effector in this feedback loop?
- If organs A and B are part of the endocrine system, what type of molecules do you think A1 and B1 are likely to be?
- What are the two main systems that allow various organ systems to communicate with each other?
- spinal cord
- thoracic cavity
- What are two functions of the hypothalamus that you learned about in this chapter?
Attributions
- Brain and Nearby Structures by NIH Image Gallery , public domain via Flickr
- Diagram showing a brain shunt by Cancer Research UK , CC BY 4.0 via Wikimedia Commons
- Text adapted from Human Biology by CK-12 licensed CC BY-NC 3.0
Human Body Essay
Introduction.
It is surprising to see how a human body functions with maximum capability. Whether we are talking, walking or seeing, there are distinct parts in our body that are destined to perform a particular function. The importance of each part is discussed in this human body essay. When we feel tired, we often take a rest and lie down for a moment. But our body continues to work, even when we take a break. Even if you are tired, your heart will not stop beating. It pumps blood and transports nutrients to your body.
The human body is made up of many parts and organs that work together to sustain life in our body. No organ or body part is more important than the other, and if you ignore one of them, then the whole body will be in pain. So, let us teach the significance of different parts of the body to our children through this essay on human body parts in English. To explore other exciting content for kids learning , head to our website.

Different Systems in the Human Body
The human body looks very simple from the outside with hands, legs, face, eyes, ears and so on. But, there is a more complex and significant structure inside the body that helps us to live. The human body is made up of many small structures like cells, tissues, organs and systems. It is covered by the skin, beneath which you could find muscles, veins, and blood. This structure is formed on the base of a skeleton, which consists of many bones. All these are arranged in a specific way to help the body function effectively. In this human body essay, we will see the different systems in the human body and their functions.
The circulatory system, respiratory system, digestive system and nervous system are the main systems of the human body. Each system has different organs, and they function together to accomplish several tasks. The circulatory system consists of organs like the heart, blood and blood vessels, and its main function is to pump blood from the heart to the lungs and carry oxygen to different parts of the body.
Next, we will understand the importance of the respiratory system through this human body essay in English. The respiratory system enables us to breathe easily, and it includes organs like the lungs, airways, windpipe, nose and mouth. While the digestive system helps in breaking down the food we eat and gives the energy to work with the help of organs like the mouth, food pipe, stomach, intestines, pancreas, liver, and anus, the nervous system controls our actions, thoughts and movements. It mainly consists of organs like the brain, spinal cord and nerves.
All these systems are necessary for the proper functioning of the human body, which is discussed in this essay on human body parts in English. By inculcating good eating habits, maintaining proper hygiene and doing regular exercises, we can look after our bodies. You can refer to more essays for kids on our website.
Frequently Asked Questions on Human Body Essay
Why should we take care of our bodies.
Most of the tasks we do like walking, running, eating etc., are only possible if we have a healthy body. To ensure we have a healthy body, all the systems must function properly, which is determined by our lifestyle and eating habits. Only a healthy body will have a healthy mind, and hence, we must take good care of our bodies.
What are some of the body parts and their functions?
We see with our eyes, listen with our ears, walk with our legs, touch with our hands, breathe through our nose and taste with our tongue.
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

Essay on Human Body
Students are often asked to write an essay on Human Body in their schools and colleges. And if you’re also looking for the same, we have created 100-word, 250-word, and 500-word essays on the topic.
Let’s take a look…
100 Words Essay on Human Body
The marvel of the human body.
The human body is a complex, wonderful machine. It consists of many parts, all working together.
Body Systems
There are many systems in our body, like the skeletal system that provides structure, and the muscular system that allows movement.
Organs and Cells
Organs like the heart and lungs perform vital functions. Our body is also made up of trillions of tiny cells.
Body’s Defenses
The immune system protects us from disease, while the skin serves as a barrier against the outside world.
In conclusion, the human body is a fascinating subject, full of wonder and complexity.
250 Words Essay on Human Body
Introduction.
The human body, a complex biological system, is a marvel of evolution. It comprises numerous organs, tissues, and cells, all working in harmony to maintain life. This essay delves into the intricacies of the human body, highlighting its major components and their functions.
Structural Organization
At the most basic level, cells form the building blocks of the human body. These cells group together to form tissues, which further combine to create organs. Each organ has a specific function, contributing to the body’s overall health and survival.

Systems of the Human Body
The body is divided into several systems, including the nervous, circulatory, respiratory, digestive, endocrine, and musculoskeletal systems. Each of these systems plays a crucial role. For instance, the nervous system, which includes the brain and spinal cord, controls body functions and enables cognition and consciousness.
Maintenance and Regulation
The human body’s homeostasis is fundamental to its functioning. This involves maintaining a constant internal environment, such as body temperature and pH balance. The endocrine system, with its hormones, and the nervous system play significant roles in this regulation.
In conclusion, the human body is an intricate and efficient system, a testament to the wonders of nature and evolution. Its complexity and functionality are a constant subject of study, offering endless possibilities for research and advancement in the field of medicine and biology.
500 Words Essay on Human Body
The human body is a complex and fascinating entity that is the epitome of biological engineering. It is a marvel of evolution, honed over millions of years to become a highly efficient machine capable of extraordinary feats. This essay delves into the intricacies of the human body, exploring its structure, function, and the symbiotic relationship between its various systems.
Structural Complexity
The human body is composed of several levels of structural organization. At the most basic level, we find cells – the building blocks of life. These cells group together to form tissues, which in turn combine to form organs. The organs then work together in organ systems to perform specific functions. The human body comprises eleven organ systems, each with its own unique role, yet they all work in unison to maintain homeostasis.
Functional Dynamics
The functionality of the human body is a testament to the intricate design and coordination of its systems. The circulatory system, for instance, is responsible for the transportation of nutrients, oxygen, and waste products around the body. The nervous system, on the other hand, acts as the body’s control center, sending and receiving signals to and from different parts of the body. The respiratory system facilitates the exchange of gases, while the digestive system breaks down food into nutrients that the body can use. These systems, among others, work in a coordinated fashion to ensure the smooth functioning of the body.
The Symbiotic Relationship
The relationship between the various systems of the human body is symbiotic in nature. Each system relies on the others to function optimally. For instance, the respiratory and circulatory systems work together to deliver oxygen to cells and remove carbon dioxide. The nervous system controls the rate of breathing and heart rate based on the body’s needs. The endocrine system, with its hormones, influences almost every other system, affecting growth, metabolism, mood, and more. This interdependence underscores the complexity and efficiency of the human body.
Adaptability and Resilience
One of the most remarkable characteristics of the human body is its adaptability and resilience. It can adapt to various environmental conditions, from the freezing temperatures of the Arctic to the scorching heat of the desert. The immune system, a complex network of cells, tissues, and organs, defends the body against harmful microorganisms, demonstrating the body’s resilience. Furthermore, the body has remarkable healing capabilities, with systems in place to repair damage and restore function.
In conclusion, the human body is an intricately designed system that showcases the marvels of evolution. Its structural complexity, functional dynamics, symbiotic relationship between systems, and adaptability and resilience are awe-inspiring. Understanding the human body not only allows us to appreciate the marvel that it is but also equips us with the knowledge to take better care of it. Indeed, the human body is a testament to the sophistication and beauty of biological engineering.
That’s it! I hope the essay helped you.
If you’re looking for more, here are essays on other interesting topics:
- Essay on How to Make Sandwitch
- Essay on How to Prepare Tea
- Essay on How to Make a Cup of Tea
Apart from these, you can look at all the essays by clicking here .
Happy studying!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.23(9); 2020 Sep 25
The Whole Body as the System in Systems Immunology
Maya m.l. poon.
1 Columbia Center for Translational Immunology, Columbia University Medical Center, New York, NY 10032, USA
2 Department of Microbiology and Immunology, Columbia University Medical Center, New York, NY 10032, USA
Donna L. Farber
3 Department of Surgery, Columbia University Medical Center, New York, NY 10032, USA
The human immune system is comprised of a diverse and interactive network of specialized cells localized in diverse tissues throughout the body, where they mediate protection against pathogens and environmental insults while maintaining tissue homeostasis. Although much of our understanding of human immunology has derived from studies of peripheral blood, recent work utilizing human tissue resources and innovative computational methods have employed a whole-body, systems-based approach, revealing tremendous complexity and heterogeneity of the immune system within individuals and across the population. In this review, we discuss how tissue localization, developmental and age-associated changes, and conditions of health and disease shape the immune response, as well as how improved understanding of interindividual and tissue-specific immunity can be leveraged for developing targeted therapeutics.
Graphical Abstract

Immunology; Complex System Biology
Introduction
The human immune system protects the body from infection by pathogens, exposure to environmental toxins and allergens, and cellular damage that may lead to cancer. The immune system also plays a critical role in maintaining homeostasis by mediating tissue repair and controlling inflammation. Unlike other organ systems that are contained within a specific tissue or structure (for example, the heart and blood vessels in the cardiovascular system), the immune system is comprised of a diverse population of specialized immune cells that provide surveillance to all tissues, forming a complex network across the human body. The two arms of the immune response comprise innate immunity, which provides a crucial immediate response triggered by signals from pathogens or dying cells, and adaptive immunity, which generates memory responses to specific antigens for long-term protective immunity. Cells of the innate immune system include macrophages and dendritic cells that largely reside in tissues, as well as neutrophils and other granulocytes that predominate in circulation. T and B lymphocytes form the cellular components of adaptive immunity and populate blood, lymphoid tissues, and non-lymphoid sites as circulating and tissue-resident subsets. Thus, understanding the complexity of the immune response requires examination of multiple cell subsets within diverse compartments across the body.
This vast and intricate system of cell-cell interactions across multiple anatomic sites has proven particularly challenging to study in humans. Although most of our understanding of human immunology is based on studies of peripheral blood, such analyses provide a limited view as the majority of innate and adaptive immune cells localize in tissues. Identifying differences in immune cells between blood and individual tissue sites has been accomplished with samples from biopsies and surgical resections, with the caveat that tissues are often associated with disease states ( Alcántara-Hernández et al., 2017 ; Giesecke et al., 2014 ; McGovern et al., 2014 ; Medina et al., 2002 ; See et al., 2017 ; Sen et al., 2014 ; Simoni et al., 2017 ). By contrast, obtaining samples from deceased organ donors enables acquisition of multiple nondiseased tissue sites from an individual, such that tissue- and subset-specific influences on immune cells can be assessed ( Boor et al., 2019 ; James et al., 2020 ; Schoettler et al., 2019 ; Senda et al., 2019 ; Snyder et al., 2019 ; Stewart et al., 2019 ). Analyzing multiple sites within an individual and among individuals can also provide insight into how the immune system functions as a network across diverse organ tissues within the human body across decades of life ( Carpenter et al., 2018 ; Dogra et al., 2020 ; Granot et al., 2017 ; Meng et al., 2017 ; Sathaliyawala et al., 2013 ; Szabo et al., 2019a ; Thome et al., 2014 , Thome et al., 2016a ; Yudanin et al., 2019 ). Advancements in cell profiling technology and computational techniques applied to these samples have proven indispensable for building a whole-body, system-based perspective of the human immune system, providing a new baseline from which to understand the immune response in disease. This review will examine recent progress in the field of human immunology and systems biology that have revealed new insights into the multiple levels of cellular and tissue interactions that underlie immune protection, regulation, and homeostasis and how they are altered due to age and disease.
Levels of Heterogeneity in the Human Immune System
The immune system controls how we respond to pathogens, environmental antigens, cancer, and other insults; therefore, understanding the pathways, mechanism, cells, and molecules is central to human health. There is much to learn about the factors that impact the immune response, including variations among individuals over the broad range of ages of human life and as a feature of health and disease ( Figure 1 ). Because the immune response is localized in multiple sites across the body and also in the blood, it is essential to sample key tissues that contain diverse immune cell populations including primary and secondary lymphoid organs (bone marrow, lymph nodes, spleen, tonsil), mucosal and barrier sites (skin, intestines, lungs), and metabolic organs, such as the liver and pancreas ( Figure 1 ). Within each of these tissues are multiple innate and adaptive immune cell types and subsets; notably, similar immune cell lineages exhibit distinct features depending on whether they circulate in blood or localize in tissues. Adding to the complexity are tissue-specific adaptations that immune cells adopt within certain sites. Described below and in Figure 1 and Table 1 are the key cellular components of innate and adaptive immunity, their segregation in blood and different tissue sites, and their roles in the immune response.

Immune System Heterogeneity of the Human Body
The human immune system is characterized by multilevel heterogeneity. Within an individual, immune cells are localized in multiple sites, including blood, primary and secondary lymphoid organs, organs of the respiratory and GI tract (lungs, intestines, pancreas liver), and barrier sites (skin, mucosal surfaces). Within tissues are diverse types of innate and adaptive immune cells—some that are present in circulation and migrate through blood and different tissues and others that establish and maintain residence within specific tissue sites. The composition, phenotype, function, and tissue-specific adaptations of diverse populations of immune cells differ between sites. Between individuals, immune responses vary depending on factors, such as age and conditions of health or disease. Understanding the whole body as a system requires investigation of the human immune system at all levels and integration of data from single cells to tissues to individuals.
Circulating and Tissue-Localized Immune Cell Types and Their Role in Immunity
In peripheral blood, innate immune cells comprise the majority of the immune cell compartment, with neutrophils being particularly abundant and T and B lymphocytes found in smaller proportions ( Figure 1 ). Within organs and barrier sites, the innate and adaptive immune cells coordinate responses to provide tissue-specific immunity ( Figure 1 ). The key tissue innate cells are macrophages, professional phagocytes that ingest cellular debris, pathogens, and other foreign substances. Long-lived tissue macrophages are established prenatally from embryonic precursors, self-renew in situ , and are highly specialized for their tissue of residence ( Ginhoux and Guilliams, 2016 ; Gomez Perdiguero et al., 2015 ; Hashimoto et al., 2013 ; Yona et al., 2013 ). These include brain microglia, lung alveolar macrophages, spleen red pulp macrophages, and liver Kupffer cells ( Ginhoux et al., 2010 ; Ginhoux and Jung, 2014 ; Hoeffel et al., 2012 ; Lavin et al., 2014 ; Merad et al., 2002 ; Nagelkerke et al., 2018 ) ( Table 1 ).
Dendritic cells (DCs) are the innate immune cells that link innate and adaptive immunity through their role as antigen-presenting cells (APCs) crucial for T cell activation. Because of their unique ability to carry antigens from mucosal sites to T cell zones of tissue-draining lymph nodes, conventional DCs (cDCs), including cDC1 and cDC2 subsets perform the crucial task of presenting antigens to induce T cell responses ( Table 1 ) ( Audiger et al., 2017 ; Austyn, 2016 ; Bigley et al., 2015 ; Granot et al., 2017 ; Kashem et al., 2017 ; Klechevsky et al., 2008 ; Randolph et al., 2008 ). Circulating plasmacytoid DCs (pDCs) represent a small subset of DCs that infiltrate tissues to secrete large amounts of type I and type III interferons (IFNs) in response to viral infection and are particularly abundant in human tonsil at steady state ( Bigley et al., 2015 ; Merad et al., 2013 ; Reizis et al., 2011b , Reizis et al., 2011a ; Segura et al., 2013 ). Other innate cell types are innate lymphoid cells, which include natural killer (NK) cells abundant in human blood and tissues, as well as helper-type innate lymphoid cells (ILCs) ILC1, ILC2, and ILC3 that are mostly tissue-resident in different sites ( Figure 1 ) ( Brüggen et al., 2016 ; Dogra et al., 2020 ; Fuchs et al., 2013 ; Peng and Tian, 2017 ; Simoni et al., 2017 ; Yudanin et al., 2019 ). NK cells are important for anti-tumor and anti-viral immunity, whereas ILCs may act to promote tissue repair ( Ishizuka et al., 2016 ; Sun and Lanier, 2011 ). Although innate immune cells have classically been described as having a rapid, short-lived response, studies in the past decade have shown that the function of myeloid and innate lymphoid cells can be shaped and enhanced by prior antigenic exposure through epigenetic and metabolic changes or modulation of surface receptor expression ( Netea et al., 2020 ; O'Sullivan et al., 2015 ; Rodriguez et al., 2019 ; Sun et al., 2009 ; Wang et al., 2020 ).
The adaptive immune system is characterized by its diverse repertoire of antigen-specific receptors expressed by T and B lymphocytes and ability to retain immunological memory over decades. V(D)J recombination (recombination of receptor gene segments) and somatic hypermutation (mutation affecting the variable regions of immunoglobulin genes) allow a limited number of genes to generate a diverse repertoire of antigen receptors. T lymphocytes recognize specific antigens through interactions between their unique surface T cell receptor (TCR) and peptide-bound MHC molecules on APCs ( Rossjohn et al., 2015 ). Conventional T cells are categorized into CD4 + helper and CD8 + cytotoxic T cells. Upon activation, naive CD4 + T cells differentiate into T helper cell lineages defined by their function and cytokine production, including Th1, Th2, Th9, Th17, Th22, T follicular helper cells (Tfh), and regulatory T cells (Treg) (for a review, see ( Geginat et al., 2013 )) ( Table 1 ). CD8 + cytotoxic T cells comprise a less diverse group and, when activated, can secrete proinflammatory cytokines and cytotoxic mediators for killing of infected cells and tumor cells ( Zhang and Bevan, 2011 ). Following the effector phase, during which expanded clones of antigen-specific T cell subsets coordinate antigen clearance, most T cells undergo apoptosis. However, a small number of T cells survive and differentiate into heterogeneous subsets of memory T cells, which provide long-term immunosurveillance and enhanced recall responses.
Diverse memory T cell subsets populate blood and virtually every tissue site; circulating subsets include central memory T cells (T CM ) expressing the lymph node-homing receptor CCR7, effector memory T cells (T EM ) that migrate through diverse tissues, and terminally differentiated effector T cells (T EMRA ) ( Sallusto et al., 2004 ; Thome et al., 2014 ), whereas tissue-resident memory T cells (T RM ), characterized by their expression of CD69 (and CD103 in barrier sites), are noncirculating, localize within peripheral tissues, and provide optimal protective immunity to pathogens in situ ( Clark et al., 2006 ; Heath and Carbone, 2013 ; Kumar et al., 2017 ; Masopust and Soerens, 2019 ; Mueller and Mackay, 2016 ; Sathaliyawala et al., 2013 ; Szabo et al., 2019b ; Watanabe et al., 2015 ; Zhu et al., 2013 ). Other types of T cells with more restricted recognition properties and invariant TCR, such as γδ and mucosal-associated invariant T cells (MAIT) cells, also exhibit tissue residence ( Table 1 ), and their role and interaction with conventional αβ T cells in mediating tissue immune responses is an active area of study ( Bendelac et al., 2007 ; Chien et al., 2014 ; Xiao and Cai, 2017 ).
B cells can also be found in circulation and in tissues, but they are largely confined to lymphoid sites (lymph nodes, spleen, bone marrow) and comprise a relatively rare immune population in human nonlymphoid organs, often far outnumbered by T cells ( Carpenter et al., 2018 ; Egbuniwe et al., 2015 ; Nihal et al., 2000 ; Sathaliyawala et al., 2013 ). B cells express a unique membrane-bound B cell antigen receptor (BCR) immunoglobulin that, when activated, is secreted as antibodies in plasma that can directly bind antigens. In secondary lymphoid organs (SLOs) and in isolated lymphoid follicles (ILFs) found along the length of the gastrointestinal (GI) tract and in Peyer patches (PPs), follicular B cells form clusters surrounded by a T cell zone, whereas marginal zone B cells populate the interface between nonlymphoid red pulp and lymphoid white pulp in the spleen and in other lymphoid tissues ( Pillai and Cariappa, 2009 ). During an active immune response, B cell follicles form germinal centers that facilitate T-B cell interactions, immunoglobulin class switching, and B cell differentiation to antibody secreting plasma cells and memory B cells. Humoral immunity is maintained through maintenance of memory B cells in lymphoid sites, plasmablasts in circulation, and long-lived plasma cells in bone marrow ( Nutt et al., 2015 ; Slifka et al., 1998 ).
Methods and Computational Approaches in Systems Immunology
Defining the heterogeneity of different immune cell lineages and their variability between and within sites, over age, and in disease requires high-dimensional experimental and computational methods. Assays of increasing resolution have led to new insights into immune cell populations on the protein, transcriptomic, and genomic level, including single-cell technologies that newly define heterogeneity of immune cells across tissues, among individuals, and over development ( Figure 2 ). Cytometry is one of the most tried and true methods for analyzing immune populations at the population and single-cell level ( Figure 2 ). Flow cytometry utilizes fluorophore-conjugated antibodies that bind to surface and intracellular proteins expressed by immune cells, which can then be analyzed based on the emission spectrum upon illumination with specific wavelengths. Previously, the number of parameters was limited by the spectral overlap of fluorescent markers, but with advances in instrumentation and fluorochromes, current technologies have the capability of analyzing up to 50 different parameters at high resolution ( Dogra et al., 2020 ; Mair and Prlic, 2018 ; Saeys et al., 2016 ). Most recently, spectral flow cytometry, which captures the entire spectrum of fluorescence, has enabled the use of fluorochromes with closely overlapping emission spectra and allows for an ever increasing number of parameters ( Nolan and Condello, 2013 ). Flow cytometry also enables isolation of populations defined based on these multiple parameters using different types of fluorescent-activated cells sorters.

Single-Cell Methods for Systems Immunology
Diagram shows the different single-cell technologies, such as cytometry and genomic sequencing techniques, and computational approaches that have been instrumental to systems-wide study of the human immune system. Flow cytometry and mass cytometry enable characterization of immune cell phenotype and function through quantification of cell surface proteins, intracellular proteins, and cytokine production. Single-cell RNA sequencing measures differentially expressed genes among single cells to elucidate heterogeneity within an immune population and identify distinct functional modules and regulatory networks. Antigen receptor sequencing characterizes lymphocyte repertoires, which inform the connectivity of adaptive immunity across diverse tissues within an individual through assessment of clonal overlap between sites, gene segment usage, and clonal abundance. Computational approaches for data visualization and analysis (bottom row) include methods for dimensionality reduction and unsupervised clustering (e.g. t-SNE, UMAP), trajectory inference analysis, and data projection onto existing datasets to directly compare immune parameters between tissues, individuals, under different conditions, and in health and disease.
An alternative to flow cytometry is mass cytometry, or cytometry by time-of-fight (CyTOF), which replaces fluorophore labels with heavy metal tags and analyzes cells using a time-of-flight mass spectrometer ( Figure 2 ). Eliminating the issue of spectral overlap encountered in flow cytometry, CyTOF allows for analysis of up to 100 parameters although is often limited to 40–50 parameters ( Amir et al., 2013 ; Miron et al., 2018 ; Nyman et al., 2017 ; Stewart et al., 2020 ). Studies using CyTOF technology have provided new insights in human CD8 + T cell activation, monocyte heterogeneity, DC development and interindividual variation, memory T cell maintenance in lymph nodes, remodeling of T cell populations following viral infection, and compartmentalization of immune cells in fetal tissues ( Alcántara-Hernández et al., 2017 ; Hamers et al., 2019 ; Li et al., 2019 ; Miron et al., 2018 ; Newell et al., 2012 ; See et al., 2017 ; Sen et al., 2014 ). Limitations of CyTOF are that a smaller, less dynamic range in expression can be discerned compared with flow cytometry, and the cells, once analyzed, cannot be sorted for isolation.
Advances in detection of gene expression on the population and single-cell level has increased the depth and breadth at which we can analyze the human immune system. Whole transcriptome profiling by RNA sequencing (RNA-seq) generates read counts of all the transcripts within a population and differential gene expression analysis can reveal signatures that define specific subsets ( Stark et al., 2019 ). RNA-seq has been used to define T RM as a distinct subset in mice and humans ( Kumar et al., 2017 ; Mackay and Kallies, 2017 ; Mackay et al., 2016 ) and distinct features of human B cell subsets in blood and tissues ( Weisel et al., 2020 ). Applying whole transcriptome profiling to single cells (scRNA-seq) enables identification and stratification of cell subsets based on differential gene expression and can lead to de novo discovery of new cell types and cell states, as well as tissue-specific adaptations of cell types within and across tissues ( Figure 2 ). Since the introduction of scRNA-seq in 2009 ( Tang et al., 2009 ), new technological advances in scRNA-seq methods including plate-based approaches and the 10X Genomics approach employing microdroplet-based systems enable rapid and efficient capture of high transcript numbers per cell ( Bush et al., 2017 ; Hwang et al., 2018 ; Jaitin et al., 2014 ). Recent studies using scRNA-seq approaches have led to identification of cell types and progenitors, developmental processes, activation trajectories, and functional signatures for diverse immune cell lineages ( Papalexi and Satija, 2018 ; Paul et al., 2015 ; Popescu et al., 2019 ; Schultze and Aschenbrenner, 2019 ; See et al., 2017 ; Stewart et al., 2019 , Stewart et al., 2020 ; Szabo et al., 2019a ; Villani et al., 2017 ; Yu et al., 2016 ).
T and B cells exhibit an additional level of genomic complexity in their expression of uniquely rearranged antigen receptor genes. Each T and B cell that develops expresses a distinct TCR or BCR that can be identified by high-throughput sequencing of the variable portion (containing the complementarity region 3 (CDR3) of the TCRβ chain for T cells and the IGH gene for B cells) ( Bradley and Thomas, 2019 ; Chaudhary and Wesemann, 2018 ). Sequencing methods capturing beyond the CDR3 region also exist ( Rosati et al., 2017 ). Common methods for high-throughput antigen receptor sequencing include PCR amplification of genomic DNA and sequencing or reverse transcription of mRNA transcripts to cDNA ( Heather et al., 2018 ; Nielsen and Boyd, 2018 ). Other approaches include multiomic single-cell technologies, such as 10X Genomics single-cell immune profiling with V(D)J sequencing ( Park et al., 2020 ), and innovative analysis methods that allow researchers to extract receptor sequences from RNA sequencing data ( Bolotin et al., 2015 , 2017 ). These approaches have enabled parallel analysis of TCR/BCR repertoire and transcription profile. Although the 10X Genomics approach involves a primer-based amplification of the antigen receptor locus, extraction of receptor sequences from RNA sequencing data ensures that there is no primer bias ( Mose et al., 2016 ). However, in both methods, the data likely represent only a fraction of the total clonal diversity due to limited sampling. Sequencing TCR and BCR can identify how unique B and T cell clones of the adaptive immune response are expanded and maintained across the human body in health, as well as their role in inflammatory and autoimmune immune diseases and in cancer ( Figure 2 ) ( de Jong et al., 2018 ; James et al., 2020 ; Meng et al., 2017 ; Schoettler et al., 2019 ; Thome et al., 2014 ).
Along with the innovations in instrumentation for single-cell profiling, advancements in computational techniques have been crucial to analyze rapidly expanding datasets and extracting novel insights. Dimensionality reduction techniques have allowed for unbiased and more intuitive visualization of high-dimensional data. Common dimensionality reduction techniques include principle component analysis (PCA), t-distributed stochastic neighbor embedding (t-SNE), and Uniform Approximation and Projection (UMAP) method ( Figure 2 ) ( Becht et al., 2018 ; Luecken and Theis, 2019 ; van der Maaten and Hinton, 2008 ). Cluster analysis methods group cells based on similarity of gene expression profiles, providing structure to heterogeneous immune populations. Unsupervised clustering methods are particularly useful, because they provide an unbiased, data-driven approach to organizing unlabeled data that has led to discoveries of distinct immune subsets ( Li et al., 2019 ; See et al., 2017 ; Sweatt et al., 2019 ). Longitudinal analyses of human immune responses are less readily accomplished compared with animal models; however, trajectory inference analysis, such as “pseudotime” or “pseudospace,” of single-cell data is a technique that orders individual cells along a trajectory based on expression profiles to infer a continuum of cell states from a static time point ( Chen et al., 2018 ; Kunz et al., 2018 ; Saelens et al., 2019 ). This technique has been used to understand dynamic immune responses, capture transition states, define gene regulatory networks, and follow immune cell development and differentiation ( Figure 2 ) ( Bendall et al., 2014 ; Lonnberg et al., 2017 ; Paul et al., 2015 ; Setty et al., 2019 ; Stubbington et al., 2017 ; Velten et al., 2017 ). Finally, emerging computational strategies allow projection of data onto existing datasets, enabling direct comparison of immune responses under different conditions and in health and disease ( Figure 2 ) ( Szabo et al., 2019a ).
Emerging single-cell technologies now enable simultaneous analysis of multiple data sources, including genomic DNA and mRNA transcription ( Dey et al., 2015 ), gene and protein expression using DNA-labeled antibodies as in cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq) ( Peterson et al., 2017 ; Stoeckius et al., 2017 ), gene expression and TCR/BCR repertoire ( Ferguson and Chen, 2020 ; James et al., 2020 ), and gene expression along with DNA methylation ( Cheow et al., 2016 ; Macaulay et al., 2017 ). In addition, new technologies pairing single-cell transcriptomics and spatial profiling—spatial transcriptomics—enable deeper understanding of the role of tissue topography and environment in the function and organization of the human immune system ( Eng et al., 2019 ; Moncada et al., 2020 ; Rodriques et al., 2019 ). As single-cell profiling technologies become more advanced and datasets become larger and more complex, the integration of systems biology is essential for identifying key features of the human immune system in health and disease.
Immunity in Space and Time
At the whole-body level, the integration of the multiple approaches described earlier has revealed new insights into the development, function, and maintenance of the immune response. We and others have used in-depth profiling that integrates flow or mass cytometry-based approaches with transcriptomics as applied to multiple sites obtained from individuals to assess how innate and adaptive immune cells are distributed and maintained in blood and tissue sites over decades of life ( Carpenter et al., 2018 ; Dogra et al., 2020 ; Granot et al., 2017 ; Kumar et al., 2018 , Kumar et al., 2017 ; Miron et al., 2018 ; Sathaliyawala et al., 2013 ; Senda et al., 2019 ; Thome et al., 2014 , Thome et al., 2016a ; Weisberg et al., 2019 ; Yudanin et al., 2019 ). These studies have revealed that the immune cell composition is largely a feature of the tissue site and that the distribution of subsets for each lineage type is also influenced by the developmental origin. Single-cell technologies have elucidated developmental and functional heterogeneity within lineages and how immune cells in blood relate to those in tissue sites. Finally, high-level analyses integrating multiple readouts are showing which aspects of the immune response change with age and the key drivers of individual difference between immune responses.
Innate Immune Cell Distribution, Maintenance, and Development
For innate cells, studies combining high-dimensional and basic flow cytometry methods, whole transcriptome profiling, and other approaches have shown that monocytes, granulocytes, DCs, and innate lymphoid cells, including NK cells and ILCs, exhibit tissue-intrinsic distribution. Monocytes and granulocytes are largely confined to blood and blood-rich tissues sites, and their distribution and frequencies are features of the specific site and do not show significant age-associated or interindividual variations ( Carpenter et al., 2018 ; Granot et al., 2017 ). For DCs, studies in multiple tissues, including skin, lung, and intestine, and in SLOs, such as spleen, lymph nodes, and tonsil, have shown that the phenotype, function, localization, and composition of DC subsets are specialized to the tissue site ( Bigley et al., 2015 ; Chu et al., 2012 ; Granot et al., 2017 ; Haniffa et al., 2012 ; Kashem et al., 2017 ; Klechevsky et al., 2008 ; Mittag et al., 2011 ; Sada-Ovalle et al., 2012 ; Segura et al., 2013 ; Steiniger, 2015 ). These tissue-intrinsic distribution patterns that are largely independent of age also apply to cDC subsets (in all sites and at highest frequencies in SLOs) and pDCs, which are rare subsets present in blood and lymphoid sites ( Carpenter et al., 2018 ; Granot et al., 2017 ).
Studies of site-specific features of DCs have provided insights into their development and function. Mature cDC subsets, which upregulate MHC class II for antigen presentation and exhibit markers of tissue migration, are predominantly cDC2 and enriched in lymph nodes draining mucosal tissue sites, such as the lung and intestines ( Granot et al., 2017 ), indicating active sites of DC-mediated immune surveillance during homeostasis. They identified bone-marrow-derived circulating cDC precursors present in both cord blood and adult peripheral blood and found that cDC1 and cDC2 populations are derived from distinct lineage-primed circulating precursors, which can be distinguished based on CD172a ( Breton et al., 2016 ). Tissue-specific features of DCs have been identified, including Langerhans cells (LCs) in the skin, characterized by their expression of Langerin (CD207), and follicular DCs in B cell follicles of SLOs, essential for proper germinal center formation and memory B cell development ( Heesters et al., 2014 ; Merad et al., 2008 ). Although these features of cDC tissue distribution are largely conserved over age and between individuals, interindividual heterogeneity among blood and skin cDC2s have also been found ( Alcántara-Hernández et al., 2017 ), suggesting differences in DC-mediated surveillance between individuals. The developmental origin of DCs has been analyzed by scRNA-seq of blood DCs demonstrating a differentiation trajectory of human DCs, as well as precursors for the DC lineage and specific cDC subsets ( Breton et al., 2016 ; See et al., 2017 ). Whether certain of these newly defined DC precursors are seeded or maintained in tissues remains to be established.
Innate lymphoid cells include NK cells that are abundant in many sites and subsets of helper-type ILC1, ILC2, and ILC3 that are rarer and mostly tissue-resident. A recent comprehensive analysis of NK cell subsets of different maturation and functional states demonstrated that characteristics of NK cell subsets are primarily driven by tissue site; mature, cytolytic, and terminally differentiated NK cells are enriched in circulation and in blood-rich tissues, such as the lung, bone marrow, and spleen, whereas less functionally mature NK cells are present in lymphoid sites and intestines and exhibit transcriptional and phenotypic features of tissue residency ( Dogra et al., 2020 ). Analysis of multiple markers of NK cell development revealed that NK cells in lymph nodes and intestines are immature and also contain NK-like precursors with novel phenotypes ( Dogra et al., 2020 ), suggesting that lymph nodes and intestines may be reservoirs for maintenance of immature NK cells for seeding and replenishing the mature NK cell pool. Complementary studies examining maintenance of NK cells and helper-type ILCs in diverse tissue sites demonstrated that innate lymphoid cell subsets display considerable phenotypic and transcriptional heterogeneity driven by tissue site and conserved across donors and age ( Simoni et al., 2017 ; Yudanin et al., 2019 ). Together, these studies on cells of the innate immune system reveal tissue distribution based on site and developmental stage that is largely maintained over the lifetime of an individual.
Adaptive Immunity: T Cell Development, Differentiation, and Memory Maintenance
For adaptive immune cells, the majority of studies have focused on T cells, dissecting how the different subsets are distributed in tissue sites and the impact of tissue site and age in their functional regulation. T cells are unique among immune cells in that they have a specialized organ for their development and output—the thymus—which is highly active at birth, declines during childhood, and is nascently active in adulthood with evidence pointing to thymic shutdown in middle age (40–50 years) ( Haynes et al., 2000 ; Thapa and Farber, 2019 ; Thome et al., 2016b ). In the thymus, bone-marrow-derived T cell precursors undergo further development to mature T cells, involving rearrangement of T cell antigen receptor genes and selection for T cells that lack overt self-reactivity for export to the periphery. Applying scRNA-seq and systems analysis to T cell development in the human thymus has recently been reported across fetal development, childhood, and adult life ( Park et al., 2020 ). Paired analysis of scRNA-seq data with TCR data revealed a developmental trajectory for human T cells starting from CD4 − CD8 − double-negative (DN) cells to mature CD4 + and CD8 + single positive (SP) cells and elucidated the process of TCRβ selection, providing evidence for progressive recombination of the TCRα allele starting with proximal V-J pairs followed by distal pairs ( Park et al., 2020 ). In addition, Park, et al. described a diverging lineage of γδ T cells between the DN and DP stages and Tregs branching from αβ T cells ( Park et al., 2020 ). This in-depth analysis revealed a developmental trajectory for human T cells that uniquely occurs within thymic tissue and prior to export of mature naive CD4 + and CD8 + T cells to peripheral sites.
Upon export from the thymus, mature T cells populate blood and virtually every tissue site in the body. Human T cell subsets also follow a tissue-dependent pattern of distribution and maintenance, but unlike innate cells, tissue intrinsic subset composition exhibits age-associated changes, as reviewed elsewhere ( Kumar et al., 2018 ). Naive T cells are the predominant population in lymphoid tissue sites in early life and childhood, whereas memory T cells populate mucosal, exocrine, and barrier sites with the accumulation of antigen experience during childhood ( Kumar et al., 2018 ; Thome et al., 2014 , Thome et al., 2016a ). As a result, for most of adult life, there is a stable segregation of naive T cells being only found in blood and lymphoid tissue (lymph node, spleen), whereas memory T cells predominate in virtually every nonlymphoid tissue examined, including mucosal sites (lungs, intestines), skin, exocrine tissues, liver, and brain ( Kumar et al., 2017 ; Pallett et al., 2017 ; Smolders et al., 2018 ; Watanabe et al., 2015 ; Weisberg et al., 2019 ). The majority of these tissue memory cells comprise noncirculating T RM cells that exhibit distinct phenotypic and transcriptional profiles that enable their retention in tissue sites ( Hombrink et al., 2016 ; Kumar et al., 2017 ); tissue-specific proportions of T RM in each site are stably maintained with age ( Senda et al., 2019 ; Thome et al., 2014 ). Given that T RM are formed early in life and can be detected in mucosal sites in infants and children and during an active infection ( Connors et al., 2018 ; Thome et al., 2016a ), it is possible that T RM maintain long-term immunity for maintenance of tissue homeostasis.
T RM exhibit heterogeneity and tissue-specific properties that are starting to be defined using transcriptomics and single-cell approaches. In lymphoid sites, such as lymph nodes, tissue memory T cells exhibit increased expression of transcription factors and functional markers associated with stemness and denoting maintenance in a more quiescent state compared with memory T cells in spleen and bone marrow, which exhibit a more differentiated state with increased turnover ( Miron et al., 2018 ). Conversely, T RM localized across the GI tract exhibit distinct phenotypes and metabolic signatures whether they localize to the small intestine, associated lymphoid tissue, or the pancreas ( Weisberg et al., 2019 ), and unique localization of T RM in liver sinusoids requires specific adaptations ( Holz et al., 2018 ; McNamara et al., 2017 ). Further elucidation of tissue memory T cell heterogeneity and tissue adaptations will be facilitated by applying scRNA-seq technologies.
Functional Regulation
Single-cell technologies are providing new insights into the diverse functions and subtypes of T cells and how these vary as a function of tissue site. A recent study using scRNA-seq profiling of resting and activated T cells isolated from blood, bone marrow, lung, and lung lymph node (LLN) from human donors identified functional modules conserved across all sites and specific signatures that distinguish blood and tissue T cells ( Szabo et al., 2019a ). Unsupervised clustering of scRNA-seq data from bone marrow, lung, and LLN from two organ tissue donors revealed three unique CD4 + T cell activation states distinguished by differential expression of IL2, TNF, and IL4R and two major functional states for CD8 + T cells characterized by differential expression of genes associated with pro-inflammatory cytokines and chemokines and cytotoxic mediators ( Szabo et al., 2019a ). Applying single-cell Hierarchical Poisson Factorization (scHPF) ( Levitin et al., 2019 ) revealed activation and functional “modules” that were highly conserved across tissues and donors, including a proliferation module, an IFN response module, and two unique cytotoxicity modules. Moreover, projection of blood T cells profiles onto UMAP embeddings of T cells from tissue donors revealed that tissue T cells upregulated genes associated with cell structure, extracellular matrix, adhesion, and tissue residency, suggesting that T cells adopt structural changes that facilitate interactions with tissue matrix ( Szabo et al., 2019a ). Together, these analyses demonstrate that T cells adopt a spectrum of functional states when activated and are able to adapt when localized in peripheral tissues.
Functional heterogeneity of T cell subsets, such as Tregs and antigen-specific T cells, is also being elucidated by scRNA-seq profiling. A recent study of scRNA-seq profiling identified the activation trajectory of Tregs between draining lymph nodes and colon ( James et al., 2020 ). They showed that there is a continuous range of functional states from resting Tregs in mesenteric lymph nodes to colonic Tregs, which express high levels of functional regulatory molecules ( James et al., 2020 ). Variations in T cell functional states are hallmarks of diverse diseases, and scRNA-seq analysis can uncover new insights important for understanding disease pathogenesis and new therapeutic targets. A recent study identified specific functional states for Tregs and allergen-specific CD4 + T cells isolated from the blood of individuals with atopy and asthma, revealing type 2 cytokines, along with an interferon (IFN) response state ( Seumois et al., 2020 ), similar to the IFN signature identified by scRNA-seq analysis of activated CD4 + T cells from blood and tissues ( Szabo et al., 2019a ). These findings highlight how scRNA-seq can reveal new cell states and identify whether they are associated with disease or are part of the healthy spectrum of functional states.
Clinical Applications
Advancements in sequencing technologies and computational techniques not only provide essential tools in understanding the human immune system in health, but also are crucial for the development of improved therapeutic strategies for human disease. In particular, vaccinology and cancer immunotherapies are two areas that have greatly benefited from our increased knowledge of human immunity as a system that spans the entire body.
Systems Vaccinology
A systems-biology-based approach to human immunology offers unique insights into vaccine development. In the clinical setting, neutralizing antibody titers measured in peripheral blood serve as the primary correlate of protection. However, immune responses within peripheral blood are not indicative of what is occurring or maintained at tissue sites. In the context of pathogen-specific responses, human-influenza-specific lung T RM have been shown to be abundant, polyfunctional, and diverse in their receptor sequences ( Pizzolla et al., 2018 ; Purwar et al., 2011 ), whereas mouse studies of acute and persistent viral infections have revealed that a large portion of T cells reside in tissue as T RM and provide optimal protection long after viral clearance (see ( Masopust and Soerens, 2019 ; Szabo et al., 2019b ) for reviews). Thus, it is crucial to design strategies that will ensure the development and maintenance of immunity within the appropriate tissue sites.
There are two major challenges for generating vaccines that target tissue responses. First, optimal strategies for targeting durable and long-lasting tissue-resident responses need to be defined; and second, methods are needed for monitoring the efficacy of tissue-directed vaccines in peripheral blood. Although the former challenge requires more studies using animal models, progress in high-resolution analysis of blood has revealed that T cells with tissue signatures are detectable in circulation. For example, circulating forms of Tfh cells (resident in lymphoid follicles) are present in increased frequencies after vaccinations and can be used for assessing vaccine efficacy ( Huber et al., 2020 ; Koutsakos et al., 2018 ). During steady state, T cells with a skin-homing phenotype have been detected in peripheral blood ( Klicznik et al., 2019 ), and a small fraction of circulating T cells exhibit a transcriptional signature for tissue T cells ( Szabo et al., 2019a ). These findings suggest that perturbations in tissue responses may be possible to monitor in blood using sensitive single-cell technologies.
Innovative systems-based computational approaches to vaccinology have also enabled a more comprehensive understanding of the mechanisms that drive protective immunity. For instance, systems vaccinology approaches enable high-level analysis of innate and adaptive immune responses to vaccination ( Hagan et al., 2015 ; Zak et al., 2014 ). Vaccine responses and efficacy can be further evaluated at the population level, addressing the challenge of designing vaccines that are effective among a diverse population ( Koff et al., 2013 ; Li et al., 2017 ; Tsang et al., 2014 ). Combined data from serum analyte profiling, multiparameter cytometry, multiomics technologies, and adaptive immune receptor repertoire profiling identify shared molecular signatures and biomarkers to predict vaccine responsiveness and immunogenicity ( Kazmin et al., 2017 ; Nakaya et al., 2015 ; Natrajan et al., 2019 ; Ramos, 2020 ; Sullivan et al., 2019 ; Team and Consortium, 2017 ). By incorporating molecular signatures of tissue responses, systems vaccinology approaches can inform development and monitoring of vaccines that ensure effective establishment of tissue immunity.
Cancer and Personalized Therapeutics
Understanding the human immune system beyond peripheral blood is essential for developing effective cancer immunotherapies, particularly therapeutic strategies that successfully target solid tumors. Progress has been made in defining the tumor-associated T cell populations in terms of their tissue residence and correlation to prognosis and responses to therapy. Recent studies of tumor infiltrating lymphocytes (TILs) have suggested that tissue localization is crucial for effective tumor killing. In fact, studies have shown that the expression of certain chemokines are positively correlated with the abundance of TILs and with postoperative survival in melanoma and colorectal cancer ( Kistner et al., 2017 ; Martinet et al., 2012 ). In addition, TILs expressing T RM marker CD103 have been shown to be enriched in tumor tissue, particularly in cancers of epithelial origin, and often correlate with improved patient survival ( Boddupalli et al., 2016 ; Djenidi et al., 2015 ; Edwards et al., 2018 ; Komdeur et al., 2017 ; Smazynski and Webb, 2018 ; Wang et al., 2016 ; Webb et al., 2015 ).
In recent years, adoptive T cell therapy has emerged as a promising option for treating malignancies. Among the various forms of adoptive T cell therapy, TCR-engineered T (TCR-T) cell therapy and chimeric antigen receptor T (CAR-T) cell therapy are two precision medicine strategies that use genetically modified T cells to treat cancers based on tumor antigen specificity ( June, 2007 ; Kershaw et al., 2013 ). CAR-T cells, genetically engineered T cells that express an antigen-recognition domain and costimulatory signaling molecules, have proven remarkably successful in the treatment of hematological malignancies, especially when paired with checkpoint blockade ( June and Sadelain, 2018 ; Kalos et al., 2011 ; Porter et al., 2011 ). However, their success in targeting solid tumors has been limited ( Martinez and Moon, 2019 ; Newick et al., 2017 ). One major barrier to treatment efficacy is understanding how to target CAR-T cells to the affected tissue and maintain them there for elimination of tumor cells. This process requires identification of the homing receptors and residency markers that control tissue-specific trafficking and retention. Thus, understanding the role of unique tissue environments in determining human immune response is essential for designing cancer immunotherapies that can effectively reach target tissues and treat solid tumors.
An alternative to CAR-T cell therapy is TCR-T cell therapy, which has been shown to display greater sensitivity than CAR-T cell therapy, enabling more rapid destruction of tumor cells, as well as improved solid tumor penetration ( Garber, 2018 ). TCR sequencing techniques have been crucial for understanding the immune landscape of tumors, as well as identifying TCR candidates for personalized precision immunotherapy. In particular, high-throughput paired sequencing of bulk DNA (pairSEQ) has enabled more rapid identification of TCR α and β chain sequences of T cells that recognize antigens unique to an individual tumor, which can then be used to engineer TCR-T cells ( Howie et al., 2015 ; Pasetto et al., 2016 ; Ping et al., 2018 ).
Unfortunately, for both CAR-T cell and TCR-T cell therapy, a devastating consequence of unsuccessful clinical trials has been off-target effects in triggering inflammation at remote tissue sites such as the skin, gut, and pancreas ( Kunert et al., 2013 ; Lamers et al., 2006 ; Linette et al., 2013 ; Morgan et al., 2013 ; Morgan et al., 2010 ; Parkhurst et al., 2011 ; van den Berg et al., 2015 ). Such inflammation may in part be due to the combination of adoptive T cell therapy and immune checkpoint inhibitor therapy. Because T RM express PD-1 during homeostasis and maintenance in tissues, anti-PD-1 therapy can potentially disrupt endogenous tissue T cell homeostasis ( Weisberg et al., 2019 ). Given the crucial requirements for ensuring engineered T cells traffic not only to tissues but also to specific tumor tissue, approaching human immunology from a whole-body, system-based perspective is essential to facilitating more targeted strategies to enhance the efficacy and tissue specificity of engineered T cells for optimal anti-tumor responses.
Conclusions and Future Directions
The human immune system is a unique cellular network that spans the entire human body. Diverse and dynamic populations of specialized immune cells migrate within and across tissues to provide immune protection against infection and malignancy while ensuring tissue homeostasis. Studies of human tissues discussed in this review have highlighted the importance of tissue localization in determining the characteristics of immune cell interactions and functions. Integrating of systems biology approaches has shed light on the vast complexities and intricacies of the human immune system, providing new insights on immune heterogeneity, development, and function previously unexplored. As the field of systems immunology grows and progresses, one of the major challenges is consistency: with new technologies and computational methods emerging daily, it is crucial to develop principles of best practice when it comes to (1) how to analyze datasets such that results are reproducible when using different analysis pipelines, (2) how to define immune cell types and subtypes, and (3) how to integrate new insights into a growing framework of human immunology. In addition, as we amass large datasets, such information can be used to determine how age, sex, and race/ethnicity may influence immune responses, which is important for the development of personalized therapeutics and vaccine strategies. Although tremendous efforts have already been made in building a comprehensive systems-based perspective of the human immune system, advancements in single-cell profiling technologies, adaptive immune cell repertoire analysis, and computational strategies continue to pave new paths of discovery in the field of human immunology.
Acknowledgments
This work was supported by NIH grants AI AI106697 and AI128949 awarded to D.L.F. M.M.L.P. was supported by NIH T32GM007367.
Author Contributions
Conceptualization, Writing—Original Draft, Review and Editing, and Visualization were performed by M.M.L.P. and D.L.F.
- Alcántara-Hernández M., Leylek R., Wagar L.E., Engleman E.G., Keler T., Marinkovich M.P., Davis M.M., Nolan G.P., Idoyaga J. High-dimensional phenotypic mapping of human dendritic cells reveals interindividual variation and tissue specialization. Immunity. 2017; 47 :1037–1050.e6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Amir E.-A., Davis K.L., Tadmor M.D., Simonds E.F., Levine J.H., Bendall S.C., Shenfeld D.K., Krishnaswamy S., Nolan G.P., Pe'er D. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat. Biotechnol. 2013; 31 :545–552. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Audiger C., Rahman M.J., Yun T.J., Tarbell K.V., Lesage S. The importance of dendritic cells in maintaining immune tolerance. J. Immunol. 2017; 198 :2223–2231. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Austyn J.M. Dendritic cells in the immune system-history, lineages, tissues, tolerance, and immunity. Microbiol. Spectr. 2016; 4 :1–50. [ PubMed ] [ Google Scholar ]
- Becht E., McInnes L., Healy J., Dutertre C.A., Kwok I.W.H., Ng L.G., Ginhoux F., Newell E.W. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 2018; 37 :38–44. [ PubMed ] [ Google Scholar ]
- Bendall S.C., Davis K.L., Amir e.-A., Tadmor M.D., Simonds E.F., Chen T.J., Shenfeld D.K., Nolan G.P., Pe'er D. Single-cell trajectory detection uncovers progression and regulatory coordination in human B cell development. Cell. 2014; 157 :714–725. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bendelac A., Savage P.B., Teyton L. The biology of NKT cells. Annu. Rev. Immunol. 2007; 25 :297–336. [ PubMed ] [ Google Scholar ]
- Bigley V., McGovern N., Milne P., Dickinson R., Pagan S., Cookson S., Haniffa M., Collin M. Langerin-expressing dendritic cells in human tissues are related to CD1c+ dendritic cells and distinct from Langerhans cells and CD141high XCR1+ dendritic cells. J. Leukoc. Biol. 2015; 97 :627–634. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Boddupalli C.S., Bar N., Kadaveru K., Krauthammer M., Pornputtapong N., Mai Z., Ariyan S., Narayan D., Kluger H., Deng Y. Interlesional diversity of T cell receptors in melanoma with immune checkpoints enriched in tissue-resident memory T cells. JCI Insight. 2016; 1 :e88955. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bolotin D.A., Poslavsky S., Davydov A.N., Frenkel F.E., Fanchi L., Zolotareva O.I., Hemmers S., Putintseva E.V., Obraztsova A.S., Shugay M. Antigen receptor repertoire profiling from RNA-seq data. Nat. Biotechnol. 2017; 35 :908–911. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bolotin D.A., Poslavsky S., Mitrophanov I., Shugay M., Mamedov I.Z., Putintseva E.V., Chudakov D.M. MiXCR: software for comprehensive adaptive immunity profiling. Nat. Methods. 2015; 12 :380–381. [ PubMed ] [ Google Scholar ]
- Boor P.P.C., Bosma B.M., Tran K.T.C., van der Laan L.J.W., Hagenaars H., IJzermans J.N.M., Metselaar H.J., Kwekkeboom J. Characterization of antigen-presenting cell subsets in human liver-draining lymph nodes. Front. Immunol. 2019; 10 :441. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bradley P., Thomas P.G. Using T cell receptor repertoires to understand the principles of adaptive immune recognition. Annu. Rev. Immunol. 2019; 37 :547–570. [ PubMed ] [ Google Scholar ]
- Breton G., Zheng S., Valieris R., Tojal da Silva I., Satija R., Nussenzweig M.C. Human dendritic cells (DCs) are derived from distinct circulating precursors that are precommitted to become CD1c+ or CD141+ DCs. J. Exp. Med. 2016; 213 :2861–2870. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brüggen M.C., Bauer W.M., Reininger B., Clim E., Captarencu C., Steiner G.E., Brunner P.M., Meier B., French L.E., Stingl G. In situ mapping of innate lymphoid cells in human skin: evidence for remarkable differences between normal and inflamed skin. J. Invest. Dermatol. 2016; 136 :2396–2405. [ PubMed ] [ Google Scholar ]
- Bush E.C., Ray F., Alvarez M.J., Realubit R., Li H., Karan C., Califano A., Sims P.A. PLATE-Seq for genome-wide regulatory network analysis of high-throughput screens. Nat. Commun. 2017; 8 :105. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Carpenter D.J., Granot T., Matsuoka N., Senda T., Kumar B.V., Thome J.J.C., Gordon C.L., Miron M., Weiner J., Connors T. Human immunology studies using organ donors: impact of clinical variations on immune parameters in tissues and circulation. Am. J. Transplant. 2018; 18 :74–88. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chaudhary N., Wesemann D.R. Analyzing immunoglobulin repertoires. Front. Immunol. 2018; 9 :462. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen J., Rénia L., Ginhoux F. Constructing cell lineages from single-cell transcriptomes. Mol. Aspects Med. 2018; 59 :95–113. [ PubMed ] [ Google Scholar ]
- Cheow L.F., Courtois E.T., Tan Y., Viswanathan R., Xing Q., Tan R.Z., Tan D.S., Robson P., Loh Y.H., Quake S.R. Single-cell multimodal profiling reveals cellular epigenetic heterogeneity. Nat. Methods. 2016; 13 :833–836. [ PubMed ] [ Google Scholar ]
- Chien Y.H., Meyer C., Bonneville M. γδ T cells: first line of defense and beyond. Annu. Rev. Immunol. 2014; 32 :121–155. [ PubMed ] [ Google Scholar ]
- Chu C.C., Ali N., Karagiannis P., Di Meglio P., Skowera A., Napolitano L., Barinaga G., Grys K., Sharif-Paghaleh E., Karagiannis S.N. Resident CD141 (BDCA3)+ dendritic cells in human skin produce IL-10 and induce regulatory T cells that suppress skin inflammation. J. Exp. Med. 2012; 209 :935–945. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Clark R.A., Chong B., Mirchandani N., Brinster N.K., Yamanaka K., Dowgiert R.K., Kupper T.S. The vast majority of CLA+ T cells are resident in normal skin. J. Immunol. 2006; 176 :4431–4439. [ PubMed ] [ Google Scholar ]
- Connors T.J., Baird J.S., Yopes M.C., Zens K.D., Pethe K., Ravindranath T.M., Ho S.H., Farber D.L. Developmental regulation of effector and resident memory T cell generation during pediatric viral respiratory tract infection. J. Immunol. 2018; 201 :432–439. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- de Jong A., Jabbari A., Dai Z., Xing L., Lee D., Li M.M., Duvic M., Hordinsky M., Norris D.A., Price V. High-throughput T cell receptor sequencing identifies clonally expanded CD8+ T cell populations in alopecia areata. JCI Insight. 2018; 3 :e121949. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dey S.S., Kester L., Spanjaard B., Bienko M., van Oudenaarden A. Integrated genome and transcriptome sequencing of the same cell. Nat. Biotechnol. 2015; 33 :285–289. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Djenidi F., Adam J., Goubar A., Durgeau A., Meurice G., de Montpréville V., Validire P., Besse B., Mami-Chouaib F. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J. Immunol. 2015; 194 :3475–3486. [ PubMed ] [ Google Scholar ]
- Dogra P., Rancan C., Ma W., Toth M., Senda T., Carpenter D.J., Kubota M., Matsumoto R., Thapa P., Szabo P.A. Tissue determinants of human NK cell development, function, and residence. Cell. 2020; 180 :749–763.e13. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Edwards J., Wilmott J.S., Madore J., Gide T.N., Quek C., Tasker A., Ferguson A., Chen J., Hewavisenti R., Hersey P. CD103(+) tumor-resident CD8(+) T cells are associated with improved survival in immunotherapy-naive melanoma patients and expand significantly during anti-PD-1 treatment. Clin. Cancer Res. 2018; 24 :3036–3045. [ PubMed ] [ Google Scholar ]
- Egbuniwe I.U., Karagiannis S.N., Nestle F.O., Lacy K.E. Revisiting the role of B cells in skin immune surveillance. Trends Immunol. 2015; 36 :102–111. [ PubMed ] [ Google Scholar ]
- Eng C.L., Lawson M., Zhu Q., Dries R., Koulena N., Takei Y., Yun J., Cronin C., Karp C., Yuan G.C. Transcriptome-scale super-resolved imaging in tissues by RNA seqFISH. Nature. 2019; 568 :235–239. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ferguson A., Chen K. Analysis of transcriptional profiling of immune cells at the single-cell level. Methods Mol. Biol. 2020; 2111 :47–57. [ PubMed ] [ Google Scholar ]
- Fuchs A., Vermi W., Lee J.S., Lonardi S., Gilfillan S., Newberry R.D., Cella M., Colonna M. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity. 2013; 38 :769–781. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Garber K. Driving T-cell immunotherapy to solid tumors. Nat. Biotechnol. 2018; 36 :215–219. [ PubMed ] [ Google Scholar ]
- Geginat J., Paroni M., Facciotti F., Gruarin P., Kastirr I., Caprioli F., Pagani M., Abrignani S. The CD4-centered universe of human T cell subsets. Semin. Immunol. 2013; 25 :252–262. [ PubMed ] [ Google Scholar ]
- Giesecke C., Frölich D., Reiter K., Mei H.E., Wirries I., Kuhly R., Killig M., Glatzer T., Stölzel K., Perka C. Tissue distribution and dependence of responsiveness of human antigen-specific memory B cells. J. Immunol. 2014; 192 :3091–3100. [ PubMed ] [ Google Scholar ]
- Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M.F., Conway S.J., Ng L.G., Stanley E.R. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010; 330 :841–845. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ginhoux F., Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016; 44 :439–449. [ PubMed ] [ Google Scholar ]
- Ginhoux F., Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014; 14 :392–404. [ PubMed ] [ Google Scholar ]
- Gomez Perdiguero E., Klapproth K., Schulz C., Busch K., Azzoni E., Crozet L., Garner H., Trouillet C., de Bruijn M.F., Geissmann F. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015; 518 :547–551. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Granot T., Senda T., Carpenter D.J., Matsuoka N., Weiner J., Gordon C.L., Miron M., Kumar B.V., Griesemer A., Ho S.H. Dendritic cells display subset and tissue-specific maturation dynamics over human life. Immunity. 2017; 46 :504–515. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hagan T., Nakaya H.I., Subramaniam S., Pulendran B. Systems vaccinology: enabling rational vaccine design with systems biological approaches. Vaccine. 2015; 33 :5294–5301. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hamers A.A.J., Dinh H.Q., Thomas G.D., Marcovecchio P., Blatchley A., Nakao C.S., Kim C., McSkimming C., Taylor A.M., Nguyen A.T. Human monocyte heterogeneity as revealed by high-dimensional mass cytometry. Arterioscler. Thromb. Vasc. Biol. 2019; 39 :25–36. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Haniffa M., Shin A., Bigley V., McGovern N., Teo P., See P., Wasan P.S., Wang X.N., Malinarich F., Malleret B. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity. 2012; 37 :60–73. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hashimoto D., Chow A., Noizat C., Teo P., Beasley M.B., Leboeuf M., Becker C.D., See P., Price J., Lucas D. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013; 38 :792–804. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Haynes B.F., Markert M.L., Sempowski G.D., Patel D.D., Hale L.P. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu. Rev. Immunol. 2000; 18 :529–560. [ PubMed ] [ Google Scholar ]
- Heath W.R., Carbone F.R. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat. Immunol. 2013; 14 :978–985. [ PubMed ] [ Google Scholar ]
- Heather J.M., Ismail M., Oakes T., Chain B. High-throughput sequencing of the T-cell receptor repertoire: pitfalls and opportunities. Brief. Bioinform. 2018; 19 :554–565. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Heesters B.A., Myers R.C., Carroll M.C. Follicular dendritic cells: dynamic antigen libraries. Nat. Rev. Immunol. 2014; 14 :495–504. [ PubMed ] [ Google Scholar ]
- Hoeffel G., Wang Y., Greter M., See P., Teo P., Malleret B., Leboeuf M., Low D., Oller G., Almeida F. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 2012; 209 :1167–1181. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Holz L.E., Prier J.E., Freestone D., Steiner T.M., English K., Johnson D.N., Mollard V., Cozijnsen A., Davey G.M., Godfrey D.I. CD8(+) T cell activation leads to constitutive formation of liver tissue-resident memory T cells that seed a large and flexible niche in the liver. Cell Rep. 2018; 25 :68–79.e4. [ PubMed ] [ Google Scholar ]
- Hombrink P., Helbig C., Backer R.A., Piet B., Oja A.E., Stark R., Brasser G., Jongejan A., Jonkers R.E., Nota B. Programs for the persistence, vigilance and control of human CD8+ lung-resident memory T cells. Nat. Immunol. 2016; 17 :1467–1478. [ PubMed ] [ Google Scholar ]
- Howie B., Sherwood A.M., Berkebile A.D., Berka J., Emerson R.O., Williamson D.W., Kirsch I., Vignali M., Rieder M.J., Carlson C.S. High-throughput pairing of T cell receptor α and β sequences. Sci. Transl. Med. 2015; 7 :301ra131. [ PubMed ] [ Google Scholar ]
- Huber J.E., Ahlfeld J., Scheck M.K., Zaucha M., Witter K., Lehmann L., Karimzadeh H., Pritsch M., Hoelscher M., von Sonnenburg F. Dynamic changes in circulating T follicular helper cell composition predict neutralising antibody responses after yellow fever vaccination. Clin. Transl. Immunol. 2020; 9 :e1129. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hwang B., Lee J.H., Bang D. Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp. Mol. Med. 2018; 50 :96. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ishizuka I.E., Constantinides M.G., Gudjonson H., Bendelac A. The innate lymphoid cell precursor. Annu. Rev. Immunol. 2016; 34 :299–316. [ PubMed ] [ Google Scholar ]
- Jaitin D.A., Kenigsberg E., Keren-Shaul H., Elefant N., Paul F., Zaretsky I., Mildner A., Cohen N., Jung S., Tanay A. Massively parallel single-cell RNA-seq for marker-free decomposition of tissues into cell types. Science. 2014; 343 :776–779. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- James K.R., Gomes T., Elmentaite R., Kumar N., Gulliver E.L., King H.W., Stares M.D., Bareham B.R., Ferdinand J.R., Petrova V.N. Distinct microbial and immune niches of the human colon. Nat. Immunol. 2020; 21 :343–353. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- June C.H. Adoptive T cell therapy for cancer in the clinic. J. Clin. Invest. 2007; 117 :1466–1476. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- June C.H., Sadelain M. Chimeric antigen receptor therapy. N. Engl. J. Med. 2018; 379 :64–73. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kalos M., Levine B.L., Porter D.L., Katz S., Grupp S.A., Bagg A., June C.H. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011; 3 :95ra73. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kashem S.W., Haniffa M., Kaplan D.H. Antigen-presenting cells in the skin. Annu. Rev. Immunol. 2017; 35 :469–499. [ PubMed ] [ Google Scholar ]
- Kazmin D., Nakaya H.I., Lee E.K., Johnson M.J., van der Most R., van den Berg R.A., Ballou W.R., Jongert E., Wille-Reece U., Ockenhouse C. Systems analysis of protective immune responses to RTS, S malaria vaccination in humans. Proc. Natl. Acad. Sci. U S A. 2017; 114 :2425–2430. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kershaw M.H., Westwood J.A., Darcy P.K. Gene-engineered T cells for cancer therapy. Nat. Rev. Cancer. 2013; 13 :525–541. [ PubMed ] [ Google Scholar ]
- Kistner L., Doll D., Holtorf A., Nitsche U., Janssen K.P. Interferon-inducible CXC-chemokines are crucial immune modulators and survival predictors in colorectal cancer. Oncotarget. 2017; 8 :89998–90012. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Klechevsky E., Morita R., Liu M., Cao Y., Coquery S., Thompson-Snipes L., Briere F., Chaussabel D., Zurawski G., Palucka A.K. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008; 29 :497–510. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Klicznik M.M., Morawski P.A., Höllbacher B., Varkhande S.R., Motley S.J., Kuri-Cervantes L., Goodwin E., Rosenblum M.D., Long S.A., Brachtl G. Human CD4+ CD103+ cutaneous resident memory T cells are found in the circulation of healthy individuals. Sci. Immunol. 2019; 4 :eaav8995. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Koff W.C., Burton D.R., Johnson P.R., Walker B.D., King C.R., Nabel G.J., Ahmed R., Bhan M.K., Plotkin S.A. Accelerating next-generation vaccine development for global disease prevention. Science. 2013; 340 :1232910. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Komdeur F.L., Prins T.M., van de Wall S., Plat A., Wisman G.B.A., Hollema H., Daemen T., Church D.N., de Bruyn M., Nijman H.W. CD103+ tumor-infiltrating lymphocytes are tumor-reactive intraepithelial CD8+ T cells associated with prognostic benefit and therapy response in cervical cancer. Oncoimmunology. 2017; 6 :e1338230. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Koutsakos M., Wheatley A.K., Loh L., Clemens E.B., Sant S., Nüssing S., Fox A., Chung A.W., Laurie K.L., Hurt A.C. Circulating TFH cells, serological memory, and tissue compartmentalization shape human influenza-specific B cell immunity. Sci. Transl. Med. 2018; 10 :eaan8405. [ PubMed ] [ Google Scholar ]
- Kumar B.V., Connors T.J., Farber D.L. Human T cell development, localization, and function throughout life. Immunity. 2018; 48 :202–213. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kumar B.V., Ma W., Miron M., Granot T., Guyer R.S., Carpenter D.J., Senda T., Sun X., Ho S.H., Lerner H. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep. 2017; 20 :2921–2934. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kunert A., Straetemans T., Govers C., Lamers C., Mathijssen R., Sleijfer S., Debets R. TCR-engineered T cells meet new challenges to treat solid tumors: choice of antigen, T cell fitness, and sensitization of tumor milieu. Front. Immunol. 2013; 4 :363. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kunz D.J., Gomes T., James K.R. Immune cell dynamics unfolded by single-cell technologies. Front. Immunol. 2018; 9 :1435. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lamers C.H., Sleijfer S., Vulto A.G., Kruit W.H., Kliffen M., Debets R., Gratama J.W., Stoter G., Oosterwijk E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J. Clin. Oncol. 2006; 24 e20–22. [ PubMed ] [ Google Scholar ]
- Lavin Y., Winter D., Blecher-Gonen R., David E., Keren-Shaul H., Merad M., Jung S., Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014; 159 :1312–1326. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Levitin H.M., Yuan J., Cheng Y.L., Ruiz F.J., Bush E.C., Bruce J.N., Canoll P., Iavarone A., Lasorella A., Blei D.M. Gene signature identification from single-cell RNA-seq with hierarchical Poisson factorization. Mol. Syst. Biol. 2019; 15 :e8557. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Li N., van Unen V., Guo N., Abdelaal T., Somarakis A., Eggermont J., Mahfouz A., Chuva de Sousa Lopes S.M., Lelieveldt B.P.F., Koning F. Early-life compartmentalization of immune cells in human fetal tissues revealed by high-dimensional mass cytometry. Front. Immunol. 2019; 10 :1932. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Li S., Sullivan N.L., Rouphael N., Yu T., Banton S., Maddur M.S., McCausland M., Chiu C., Canniff J., Dubey S. Metabolic phenotypes of response to vaccination in humans. Cell. 2017; 169 :862–877.e17. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Linette G.P., Stadtmauer E.A., Maus M.V., Rapoport A.P., Levine B.L., Emery L., Litzky L., Bagg A., Carreno B.M., Cimino P.J. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013; 122 :863–871. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lonnberg T., Svensson V., James K.R., Fernandez-Ruiz D., Sebina I., Montandon R., Soon M.S., Fogg L.G., Nair A.S., Liligeto U. Single-cell RNA-seq and computational analysis using temporal mixture modelling resolves Th1/Tfh fate bifurcation in malaria. Sci. Immunol. 2017; 2 :eaal2192. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Luecken M.D., Theis F.J. Current best practices in single-cell RNA-seq analysis: a tutorial. Mol. Syst. Biol. 2019; 15 :e8746. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Macaulay I.C., Ponting C.P., Voet T. Single-cell multiomics: multiple measurements from single cells. Trends Genet. 2017; 33 :155–168. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mackay L.K., Kallies A. Transcriptional regulation of tissue-resident lymphocytes. Trends Immunol. 2017; 38 :94–103. [ PubMed ] [ Google Scholar ]
- Mackay L.K., Minnich M., Kragten N.A., Liao Y., Nota B., Seillet C., Zaid A., Man K., Preston S., Freestone D. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. 2016; 352 :459–463. [ PubMed ] [ Google Scholar ]
- Mair F., Prlic M. OMIP-044: 28-color immunophenotyping of the human dendritic cell compartment. Cytometry A. 2018; 93 :402–405. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Martinet L., Le Guellec S., Filleron T., Lamant L., Meyer N., Rochaix P., Garrido I., Girard J.P. High endothelial venules (HEVs) in human melanoma lesions: major gateways for tumor-infiltrating lymphocytes. Oncoimmunology. 2012; 1 :829–839. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Martinez M., Moon E.K. CAR T cells for solid tumors: new strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front. Immunol. 2019; 10 :128. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Masopust D., Soerens A.G. Tissue-resident T cells and other resident leukocytes. Annu. Rev. Immunol. 2019; 37 :521–546. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- McGovern N., Schlitzer A., Gunawan M., Jardine L., Shin A., Poyner E., Green K., Dickinson R., Wang X.N., Low D. Human dermal CD14⁺ cells are a transient population of monocyte-derived macrophages. Immunity. 2014; 41 :465–477. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- McNamara H.A., Cai Y., Wagle M.V., Sontani Y., Roots C.M., Miosge L.A., O'Connor J.H., Sutton H.J., Ganusov V.V., Heath W.R. Up-regulation of LFA-1 allows liver-resident memory T cells to patrol and remain in the hepatic sinusoids. Sci. Immunol. 2017; 2 :eaaj1996. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Medina F., Segundo C., Campos-Caro A., González-García I., Brieva J.A. The heterogeneity shown by human plasma cells from tonsil, blood, and bone marrow reveals graded stages of increasing maturity, but local profiles of adhesion molecule expression. Blood. 2002; 99 :2154–2161. [ PubMed ] [ Google Scholar ]
- Meng W., Zhang B., Schwartz G.W., Rosenfeld A.M., Ren D., Thome J.J.C., Carpenter D.J., Matsuoka N., Lerner H., Friedman A.L. An atlas of B-cell clonal distribution in the human body. Nat. Biotechnol. 2017; 35 :879–884. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Merad M., Ginhoux F., Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat. Rev. Immunol. 2008; 8 :935–947. [ PubMed ] [ Google Scholar ]
- Merad M., Manz M.G., Karsunky H., Wagers A., Peters W., Charo I., Weissman I.L., Cyster J.G., Engleman E.G. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 2002; 3 :1135–1141. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Merad M., Sathe P., Helft J., Miller J., Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013; 31 :563–604. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Miron M., Kumar B.V., Meng W., Granot T., Carpenter D.J., Senda T., Chen D., Rosenfeld A.M., Zhang B., Lerner H. Human lymph nodes maintain TCF-1hi memory T cells with high functional potential and clonal diversity throughout life. J. Immunol. 2018; 201 :2132–2140. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mittag D., Proietto A.I., Loudovaris T., Mannering S.I., Vremec D., Shortman K., Wu L., Harrison L.C. Human dendritic cell subsets from spleen and blood are similar in phenotype and function but modified by donor health status. J. Immunol. 2011; 186 :6207–6217. [ PubMed ] [ Google Scholar ]
- Moncada R., Barkley D., Wagner F., Chiodin M., Devlin J.C., Baron M., Hajdu C.H., Simeone D.M., Yanai I. Integrating microarray-based spatial transcriptomics and single-cell RNA-seq reveals tissue architecture in pancreatic ductal adenocarcinomas. Nat. Biotechnol. 2020; 38 :333–342. [ PubMed ] [ Google Scholar ]
- Morgan R.A., Chinnasamy N., Abate-Daga D., Gros A., Robbins P.F., Zheng Z., Dudley M.E., Feldman S.A., Yang J.C., Sherry R.M. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 2013; 36 :133–151. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Morgan R.A., Yang J.C., Kitano M., Dudley M.E., Laurencot C.M., Rosenberg S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010; 18 :843–851. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mose L.E., Selitsky S.R., Bixby L.M., Marron D.L., Iglesia M.D., Serody J.S., Perou C.M., Vincent B.G., Parker J.S. Assembly-based inference of B-cell receptor repertoires from short read RNA sequencing data with V'DJer. Bioinformatics. 2016; 32 :3729–3734. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mueller S.N., Mackay L.K. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 2016; 16 :79–89. [ PubMed ] [ Google Scholar ]
- Nagelkerke S.Q., Bruggeman C.W., den Haan J.M.M., Mul E.P.J., van den Berg T.K., van Bruggen R., Kuijpers T.W. Red pulp macrophages in the human spleen are a distinct cell population with a unique expression of Fc-γ receptors. Blood Adv. 2018; 2 :941–953. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nakaya H.I., Hagan T., Duraisingham S.S., Lee E.K., Kwissa M., Rouphael N., Frasca D., Gersten M., Mehta A.K., Gaujoux R. Systems analysis of immunity to influenza vaccination across multiple years and in diverse populations reveals shared molecular signatures. Immunity. 2015; 43 :1186–1198. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Natrajan M.S., Rouphael N., Lai L., Kazmin D., Jensen T.L., Weiss D.S., Ibegbu C., Sztein M.B., Hooper W.F., Hill H. Systems vaccinology for a live attenuated tularemia vaccine reveals unique transcriptional signatures that predict humoral and cellular immune responses. Vaccines (Basel) 2019; 8 :4. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Netea M.G., Domínguez-Andrés J., Barreiro L.B., Chavakis T., Divangahi M., Fuchs E., Joosten L.A.B., van der Meer J.W.M., Mhlanga M.M., Mulder W.J.M. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020; 20 :375–388. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Newell E.W., Sigal N., Bendall S.C., Nolan G.P., Davis M.M. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity. 2012; 36 :142–152. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Newick K., O'Brien S., Moon E., Albelda S.M. CAR T cell therapy for solid tumors. Annu. Rev. Med. 2017; 68 :139–152. [ PubMed ] [ Google Scholar ]
- Nielsen S.C.A., Boyd S.D. Human adaptive immune receptor repertoire analysis-Past, present, and future. Immunol. Rev. 2018; 284 :9–23. [ PubMed ] [ Google Scholar ]
- Nihal M., Mikkola D., Wood G.S. Detection of clonally restricted immunoglobulin heavy chain gene rearrangements in normal and lesional skin: analysis of the B cell component of the skin-associated lymphoid tissue and implications for the molecular diagnosis of cutaneous B cell lymphomas. J. Mol. Diagn. 2000; 2 :5–10. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nolan J.P., Condello D. Spectral flow cytometry. Curr. Protoc. Cytom. 2013 Chapter 1, Unit1.27. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nutt S.L., Hodgkin P.D., Tarlinton D.M., Corcoran L.M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 2015; 15 :160–171. [ PubMed ] [ Google Scholar ]
- Nyman T.A., Lorey M.B., Cypryk W., Matikainen S. Mass spectrometry-based proteomic exploration of the human immune system: focus on the inflammasome, global protein secretion, and T cells. Expert Rev. Proteomics. 2017; 14 :395–407. [ PubMed ] [ Google Scholar ]
- O'Sullivan T.E., Sun J.C., Lanier L.L. Natural killer cell memory. Immunity. 2015; 43 :634–645. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pallett L.J., Davies J., Colbeck E.J., Robertson F., Hansi N., Easom N.J.W., Burton A.R., Stegmann K.A., Schurich A., Swadling L. IL-2high tissue-resident T cells in the human liver: sentinels for hepatotropic infection. J. Exp. Med. 2017; 214 :1567–1580. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Papalexi E., Satija R. Single-cell RNA sequencing to explore immune cell heterogeneity. Nat. Rev. Immunol. 2018; 18 :35–45. [ PubMed ] [ Google Scholar ]
- Park J.E., Botting R.A., Domínguez Conde C., Popescu D.M., Lavaert M., Kunz D.J., Goh I., Stephenson E., Ragazzini R., Tuck E. A cell atlas of human thymic development defines T cell repertoire formation. Science. 2020; 367 :eaay3224. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Parkhurst M.R., Yang J.C., Langan R.C., Dudley M.E., Nathan D.A., Feldman S.A., Davis J.L., Morgan R.A., Merino M.J., Sherry R.M. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther. 2011; 19 :620–626. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pasetto A., Gros A., Robbins P.F., Deniger D.C., Prickett T.D., Matus-Nicodemos R., Douek D.C., Howie B., Robins H., Parkhurst M.R. Tumor- and neoantigen-reactive T-cell receptors can Be identified based on their frequency in fresh tumor. Cancer Immunol. Res. 2016; 4 :734–743. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Paul F., Arkin Y., Giladi A., Jaitin D.A., Kenigsberg E., Keren-Shaul H., Winter D., Lara-Astiaso D., Gury M., Weiner A. Transcriptional heterogeneity and lineage commitment in myeloid progenitors. Cell. 2015; 163 :1663–1677. [ PubMed ] [ Google Scholar ]
- Peng H., Tian Z. Diversity of tissue-resident NK cells. Semin. Immunol. 2017; 31 :3–10. [ PubMed ] [ Google Scholar ]
- Peterson V.M., Zhang K.X., Kumar N., Wong J., Li L., Wilson D.C., Moore R., McClanahan T.K., Sadekova S., Klappenbach J.A. Multiplexed quantification of proteins and transcripts in single cells. Nat. Biotechnol. 2017; 35 :936–939. [ PubMed ] [ Google Scholar ]
- Pillai S., Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat. Rev. Immunol. 2009; 9 :767–777. [ PubMed ] [ Google Scholar ]
- Ping Y., Liu C., Zhang Y. T-cell receptor-engineered T cells for cancer treatment: current status and future directions. Protein Cell. 2018; 9 :254–266. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pizzolla A., Nguyen T.H., Sant S., Jaffar J., Loudovaris T., Mannering S.I., Thomas P.G., Westall G.P., Kedzierska K., Wakim L.M. Influenza-specific lung-resident memory T cells are proliferative and polyfunctional and maintain diverse TCR profiles. J. Clin. Invest. 2018; 128 :721–733. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Popescu D.M., Botting R.A., Stephenson E., Green K., Webb S., Jardine L., Calderbank E.F., Polanski K., Goh I., Efremova M. Decoding human fetal liver haematopoiesis. Nature. 2019; 574 :365–371. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Porter D.L., Levine B.L., Kalos M., Bagg A., June C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011; 365 :725–733. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Purwar R., Campbell J., Murphy G., Richards W.G., Clark R.A., Kupper T.S. Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS One. 2011; 6 :e16245. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ramos I. mSphere of influence: predicting immune responses and susceptibility to influenza virus-may the data Be with you. mSphere. 2020; 5 :e00085-20. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Randolph G.J., Ochando J., Partida-Sánchez S. Migration of dendritic cell subsets and their precursors. Annu. Rev. Immunol. 2008; 26 :293–316. [ PubMed ] [ Google Scholar ]
- Reizis B., Bunin A., Ghosh H.S., Lewis K.L., Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu. Rev. Immunol. 2011; 29 :163–183. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Reizis B., Colonna M., Trinchieri G., Barrat F., Gilliet M. Plasmacytoid dendritic cells: one-trick ponies or workhorses of the immune system? Nat. Rev. Immunol. 2011; 11 :558–565. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rodriguez R.M., Suarez-Alvarez B., Lopez-Larrea C. Therapeutic epigenetic reprogramming of trained immunity in myeloid cells. Trends Immunol. 2019; 40 :66–80. [ PubMed ] [ Google Scholar ]
- Rodriques S.G., Stickels R.R., Goeva A., Martin C.A., Murray E., Vanderburg C.R., Welch J., Chen L.M., Chen F., Macosko E.Z. Slide-seq: a scalable technology for measuring genome-wide expression at high spatial resolution. Science. 2019; 363 :1463–1467. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rosati E., Dowds C.M., Liaskou E., Henriksen E.K.K., Karlsen T.H., Franke A. Overview of methodologies for T-cell receptor repertoire analysis. BMC Biotechnol. 2017; 17 :61. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rossjohn J., Gras S., Miles J.J., Turner S.J., Godfrey D.I., McCluskey J. T cell antigen receptor recognition of antigen-presenting molecules. Annu. Rev. Immunol. 2015; 33 :169–200. [ PubMed ] [ Google Scholar ]
- Sada-Ovalle I., Talayero A., Chavéz-Galán L., Barrera L., Castorena-Maldonado A., Soda-Merhy A., Torre-Bouscoulet L. Functionality of CD4+ and CD8+ T cells from tonsillar tissue. Clin. Exp. Immunol. 2012; 168 :200–206. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Saelens W., Cannoodt R., Todorov H., Saeys Y. A comparison of single-cell trajectory inference methods. Nat. Biotechnol. 2019; 37 :547–554. [ PubMed ] [ Google Scholar ]
- Saeys Y., Van Gassen S., Lambrecht B.N. Computational flow cytometry: helping to make sense of high-dimensional immunology data. Nat. Rev. Immunol. 2016; 16 :449–462. [ PubMed ] [ Google Scholar ]
- Sallusto F., Geginat J., Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 2004; 22 :745–763. [ PubMed ] [ Google Scholar ]
- Sathaliyawala T., Kubota M., Yudanin N., Turner D., Camp P., Thome J.J., Bickham K.L., Lerner H., Goldstein M., Sykes M. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013; 38 :187–197. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Schoettler N., Hrusch C.L., Blaine K.M., Sperling A.I., Ober C. Transcriptional programming and T cell receptor repertoires distinguish human lung and lymph node memory T cells. Commun. Biol. 2019; 2 :411. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Schultze J.L., Aschenbrenner A.C. Systems immunology allows a new view on human dendritic cells. Semin. Cell Dev. Biol. 2019; 86 :15–23. [ PubMed ] [ Google Scholar ]
- See P., Dutertre C.A., Chen J., Günther P., McGovern N., Irac S.E., Gunawan M., Beyer M., Händler K., Duan K. Mapping the human DC lineage through the integration of high-dimensional techniques. Science. 2017; 356 :eaag3009. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Segura E., Durand M., Amigorena S. Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells. J. Exp. Med. 2013; 210 :1035–1047. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sen N., Mukherjee G., Sen A., Bendall S.C., Sung P., Nolan G.P., Arvin A.M. Single-cell mass cytometry analysis of human tonsil T cell remodeling by varicella zoster virus. Cell Rep. 2014; 8 :633–645. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Senda T., Dogra P., Granot T., Furuhashi K., Snyder M.E., Carpenter D.J., Szabo P.A., Thapa P., Miron M., Farber D.L. Microanatomical dissection of human intestinal T-cell immunity reveals site-specific changes in gut-associated lymphoid tissues over life. Mucosal Immunol. 2019; 12 :378–389. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Setty M., Kiseliovas V., Levine J., Gayoso A., Mazutis L., Pe'er D. Characterization of cell fate probabilities in single-cell data with Palantir. Nat. Biotechnol. 2019; 37 :451–460. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Seumois G., Ramirez-Suastegui C., Schmiedel B.J., Liang S., Peters B., Sette A., Vijayanand P. Single-cell transcriptomic analysis of allergen-specific T cells in allergy and asthma. Sci. Immunol. 2020; 5 :eaba6087. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Simoni Y., Fehlings M., Kløverpris H.N., McGovern N., Koo S.L., Loh C.Y., Lim S., Kurioka A., Fergusson J.R., Tang C.L. Human innate lymphoid cell subsets possess tissue-type based heterogeneity in phenotype and frequency. Immunity. 2017; 46 :148–161. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Slifka M.K., Antia R., Whitmire J.K., Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998; 8 :363–372. [ PubMed ] [ Google Scholar ]
- Smazynski J., Webb J.R. Resident memory-like tumor-infiltrating lymphocytes (TILRM): latest players in the immuno-oncology repertoire. Front. Immunol. 2018; 9 :1741. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Smolders J., Heutinck K.M., Fransen N.L., Remmerswaal E.B.M., Hombrink P., Ten Berge I.J.M., van Lier R.A.W., Huitinga I., Hamann J. Tissue-resident memory T cells populate the human brain. Nat. Commun. 2018; 9 :4593. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Snyder M.E., Finlayson M.O., Connors T.J., Dogra P., Senda T., Bush E., Carpenter D., Marboe C., Benvenuto L., Shah L. Generation and persistence of human tissue-resident memory T cells in lung transplantation. Sci. Immunol. 2019; 4 :eaav5581. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stark R., Grzelak M., Hadfield J. RNA sequencing: the teenage years. Nat. Rev. Genet. 2019; 20 :631–656. [ PubMed ] [ Google Scholar ]
- Steiniger B.S. Human spleen microanatomy: why mice do not suffice. Immunology. 2015; 145 :334–346. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stewart B.J., Ferdinand J.R., Clatworthy M.R. Using single-cell technologies to map the human immune system - implications for nephrology. Nat. Rev. Nephrol. 2020; 16 :112–128. [ PubMed ] [ Google Scholar ]
- Stewart B.J., Ferdinand J.R., Young M.D., Mitchell T.J., Loudon K.W., Riding A.M., Richoz N., Frazer G.L., Staniforth J.U.L., Vieira Braga F.A. Spatiotemporal immune zonation of the human kidney. Science. 2019; 365 :1461–1466. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stoeckius M., Hafemeister C., Stephenson W., Houck-Loomis B., Chattopadhyay P.K., Swerdlow H., Satija R., Smibert P. Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods. 2017; 14 :865–868. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stubbington M.J.T., Rozenblatt-Rosen O., Regev A., Teichmann S.A. Single-cell transcriptomics to explore the immune system in health and disease. Science. 2017; 358 :58–63. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sullivan N.L., Eberhardt C.S., Wieland A., Vora K.A., Pulendran B., Ahmed R. Understanding the immunology of the Zostavax shingles vaccine. Curr. Opin. Immunol. 2019; 59 :25–30. [ PubMed ] [ Google Scholar ]
- Sun J.C., Beilke J.N., Lanier L.L. Adaptive immune features of natural killer cells. Nature. 2009; 457 :557–561. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sun J.C., Lanier L.L. NK cell development, homeostasis and function: parallels with CD8⁺ T cells. Nat. Rev. Immunol. 2011; 11 :645–657. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sweatt A.J., Hedlin H.K., Balasubramanian V., Hsi A., Blum L.K., Robinson W.H., Haddad F., Hickey P.M., Condliffe R., Lawrie A. Discovery of distinct immune phenotypes using machine learning in pulmonary arterial hypertension. Circ. Res. 2019; 124 :904–919. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Szabo P.A., Levitin H.M., Miron M., Snyder M.E., Senda T., Yuan J., Cheng Y.L., Bush E.C., Dogra P., Thapa P. Single-cell transcriptomics of human T cells reveals tissue and activation signatures in health and disease. Nat. Commun. 2019; 10 :4706. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Szabo P.A., Miron M., Farber D.L. Location, location, location: tissue resident memory T cells in mice and humans. Sci. Immunol. 2019; 4 :eaas9673. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tang F., Barbacioru C., Wang Y., Nordman E., Lee C., Xu N., Wang X., Bodeau J., Tuch B.B., Siddiqui A. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods. 2009; 6 :377–382. [ PubMed ] [ Google Scholar ]
- Team H.-C.S.P., Consortium H.-I. Multicohort analysis reveals baseline transcriptional predictors of influenza vaccination responses. Sci. Immunol. 2017; 2 :eaal4656. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Thapa P., Farber D.L. The role of the thymus in the immune response. Thorac. Surg. Clin. 2019; 29 :123–131. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Thome J.J., Bickham K.L., Ohmura Y., Kubota M., Matsuoka N., Gordon C., Granot T., Griesemer A., Lerner H., Kato T. Early-life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nat. Med. 2016; 22 :72–77. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Thome J.J., Grinshpun B., Kumar B.V., Kubota M., Ohmura Y., Lerner H., Sempowski G.D., Shen Y., Farber D.L. Longterm maintenance of human naive T cells through in situ homeostasis in lymphoid tissue sites. Sci. Immunol. 2016; 1 :aah6506. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Thome J.J., Yudanin N., Ohmura Y., Kubota M., Grinshpun B., Sathaliyawala T., Kato T., Lerner H., Shen Y., Farber D.L. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 2014; 159 :814–828. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tsang J.S., Schwartzberg P.L., Kotliarov Y., Biancotto A., Xie Z., Germain R.N., Wang E., Olnes M.J., Narayanan M., Golding H. Global analyses of human immune variation reveal baseline predictors of postvaccination responses. Cell. 2014; 157 :499–513. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- van den Berg J.H., Gomez-Eerland R., van de Wiel B., Hulshoff L., van den Broek D., Bins A., Tan H.L., Harper J.V., Hassan N.J., Jakobsen B.K. Case report of a fatal serious adverse event upon administration of T cells transduced with a MART-1-specific T-cell receptor. Mol. Ther. 2015; 23 :1541–1550. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- van der Maaten L., Hinton G. Visualizing Data using t-SNE. J. Mach. Learn. Res. 2008; 9 :2579–2605. [ Google Scholar ]
- Velten L., Haas S.F., Raffel S., Blaszkiewicz S., Islam S., Hennig B.P., Hirche C., Lutz C., Buss E.C., Nowak D. Human haematopoietic stem cell lineage commitment is a continuous process. Nat. Cell Biol. 2017; 19 :271–281. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Villani A.C., Satija R., Reynolds G., Sarkizova S., Shekhar K., Fletcher J., Griesbeck M., Butler A., Zheng S., Lazo S. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017; 356 :eaah4573. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang X., Tian Z., Peng H. Tissue-resident memory-like ILCs: innate counterparts of TRM cells. Protein Cell. 2020; 11 :85–96. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang Z.Q., Milne K., Derocher H., Webb J.R., Nelson B.H., Watson P.H. CD103 and intratumoral immune response in breast cancer. Clin. Cancer Res. 2016; 22 :6290–6297. [ PubMed ] [ Google Scholar ]
- Watanabe R., Gehad A., Yang C., Scott L.L., Teague J.E., Schlapbach C., Elco C.P., Huang V., Matos T.R., Kupper T.S. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci. Transl. Med. 2015; 7 :279ra239. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Webb J.R., Milne K., Nelson B.H. PD-1 and CD103 are widely coexpressed on prognostically favorable intraepithelial CD8 T cells in human ovarian cancer. Cancer Immunol. Res. 2015; 3 :926–935. [ PubMed ] [ Google Scholar ]
- Weisberg S.P., Carpenter D.J., Chait M., Dogra P., Gartrell-Corrado R.D., Chen A.X., Campbell S., Liu W., Saraf P., Snyder M.E. Tissue-resident memory T cells mediate immune homeostasis in the human pancreas through the PD-1/PD-L1 pathway. Cell Rep. 2019; 29 :3916–3932.e5. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Weisel N.M., Weisel F.J., Farber D.L., Borghesi L.A., Shen Y., Ma W., Luning Prak N.T., Shlomchik M.J. Comprehensive analysis of B cell compartments across the human body reveals novel subsets and a gut resident memory phenotype. Blood. 2020 doi: 10.1182/blood.2019002782. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Xiao X., Cai J. Mucosal-associated invariant T cells: new insights into antigen recognition and activation. Front. Immunol. 2017; 8 :1540. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yona S., Kim K.W., Wolf Y., Mildner A., Varol D., Breker M., Strauss-Ayali D., Viukov S., Guilliams M., Misharin A. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013; 38 :79–91. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yu Y., Tsang J.C., Wang C., Clare S., Wang J., Chen X., Brandt C., Kane L., Campos L.S., Lu L. Single-cell RNA-seq identifies a PD-1. Nature. 2016; 539 :102–106. [ PubMed ] [ Google Scholar ]
- Yudanin N.A., Schmitz F., Flamar A.L., Thome J.J.C., Tait Wojno E., Moeller J.B., Schirmer M., Latorre I.J., Xavier R.J., Farber D.L. Spatial and temporal mapping of human innate lymphoid cells reveals elements of tissue specificity. Immunity. 2019; 50 :505–519.e4. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zak D.E., Tam V.C., Aderem A. Systems-level analysis of innate immunity. Annu. Rev. Immunol. 2014; 32 :547–577. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhang N., Bevan M.J. CD8(+) T cells: foot soldiers of the immune system. Immunity. 2011; 35 :161–168. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhu J., Peng T., Johnston C., Phasouk K., Kask A.S., Klock A., Jin L., Diem K., Koelle D.M., Wald A. Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature. 2013; 497 :494–497. [ PMC free article ] [ PubMed ] [ Google Scholar ]

Snapsolve any problem by taking a picture. Try it in the Numerade app?
Unilever’s Short Cycle Detergent Is Big Test for CEO’s Shakeup
By Dasha Afanasieva

A liter of sweat, a gram of salt, a billion dead skin cells, 15 grams of body oil, plus extra cellular DNA. That’s what the human body sheds every day, according to Keith Rutherford, innovation boss for the homecare unit of Unilever Plc .
It’s a cocktail of emissions that’s mostly invisible, meaning that even when clothes aren’t stained they may not be clean, he said, speaking at a lab in Northern England.

Consumers are increasingly keen to tackle such soiling, Rutherford added, using daily, quick 15- to 30-minute washing machine cycles. And in a bid to cash in ...
Learn more about Bloomberg Law or Log In to keep reading:
Learn about bloomberg law.
AI-powered legal analytics, workflow tools and premium legal & business news.
Already a subscriber?
Log in to keep reading or access research tools.

COMMENTS
Read this Essay on Cells in Human Body ! The body of any living organism is made up of cells. Cells are very minute in size and extremely complicated in structure. Human is no exception. Each cell is basically a unit of protoplasm, which is said to be the "material basis of life". The protoplasm is the combination of cytoplasm and nucleus.
A cell is the smallest living organism and the basic unit of life on earth. Together, trillions of cells make up the human body. Cells have three parts: the membrane, the nucleus, and the cytoplasm.
essay human anatomy the human body, the physical substance of the human organism, is composed of living cells and extracellular materials and organized into ... The human body consists of trillions of cells, each capable of growth, metabolism, response to stimuli, and, with some exceptions, reproduction. Although there are some 200 different ...
After you eat a meal, your blood glucose levels rise, triggering the secretion of insulin from β cells in the pancreas. Insulin acts as a signal that triggers cells of the body, such as fat and muscle cells, to take up glucose for use as fuel. Insulin also causes glucose to be converted into glycogen—a storage molecule—in the liver.
For example, epithelial cells protect the outside surface of the body as part of the skin and cover the organs and body cavities within. Bone cells build up bones to provide support for the body. Cells of the immune system fight invading bacteria. Blood and blood cells carry nutrients and oxygen throughout the body while removing carbon dioxide.
The cell is the basic living unit of the human body—indeed, of all organisms. The human body consists of trillions of cells, each capable of growth, metabolism, response to stimuli, and, with some exceptions, reproduction. Although there are some 200 different types of cells in the body, these can be grouped into four basic classes.
The body has levels of organization that build on each other. Cells make up tissues, tissues make up organs, and organs make up organ systems. The function of an organ system depends on the integrated activity of its organs. For instance, digestive system organs cooperate to process food. The survival of the organism depends on the integrated ...
The adult human body is composed of ∼37 trillion cells of human origin and at least as many again as part of the human microbiota ( Bianconi et al., 2013; Sender et al., 2016 ). Historically, cells have been defined based on their origin and morphology and more recently based on cell surface proteins, resulting in 200-500 defined major cell ...
The human body is a complex, highly organized structure made up of unique cells that work together to accomplish the specific functions necessary for sustaining life. The biology of the human body includes. Anatomy is organized by levels, from the smallest components of cells to tissues and organs and to organ systems .
A multicellular organism is made of many cells. A higher animal or plant contains billions of cells. For example, a newly born human infant has 2 x 1012 cells. The number increases to 100 trillion (100 x 1012 or 1014) cells in the body of 60 kg human being. About 25% (25 x 1012) of them are found in the blood.
The eleven distinct organ systems in the human body covered in this book seen in Figure 1.3.2 1.3. 2 and Figure 1.3.3 1.3. 3 include: The integumentary system functions to enclose internal body structures and is the site of many sensory receptors. Some organs of this system include skin, hair, and nails.
Muscular system The muscular system consists of all the body muscles. There are three muscle types; smooth, cardiac and skeletal muscles. Smooth muscle is found within walls of blood vessels and hollow organs such as the stomach or intestines.Cardiac muscle cells form the heart muscle, also called the false. Skeletal muscles attach to the bones of the body.Among these three, only skeletal ...
Cells duplicate themselves and results in two daughter cells that have the exact same genetic material than the original cells. Lastly, all cells need energy to function. Energy typically takes the form of adenosine troposphere or ATP. In most cells, glucose reacts with oxygen to product ATP.
Case Study Conclusion: Under Pressure. As you learned in this chapter, the human body consists of many complex systems that normally work together efficiently like a well-oiled machine to carry out life's functions. For example, Figure 10.8.1 10.8. 1 illustrates how the brain and spinal cord are protected by layers of membrane called meninges ...
The human body is made up of many small structures like cells, tissues, organs and systems. It is covered by the skin, beneath which you could find muscles, veins, and blood. This structure is formed on the base of a skeleton, which consists of many bones. All these are arranged in a specific way to help the body function effectively.
250 Words Essay on Human Body Introduction. The human body, a complex biological system, is a marvel of evolution. It comprises numerous organs, tissues, and cells, all working in harmony to maintain life. This essay delves into the intricacies of the human body, highlighting its major components and their functions. Structural Organization
Antibodies alone are often not enough to protect the body against pathogens. In these instances, the immune system uses cell-mediated immunity to destroy infected body cells. T cells are responsible for cell-mediated immunity. Killer T cells (cytotoxic T cells) assist with the elimination of infected body cells by releasing toxins into them and ...
The immune system is the body's tool for preventing or limiting infection. Its complex network of cells, organs, proteins, and tissues enable the immune system to defend the body from pathogens ...
The human immune system is comprised of a diverse and interactive network of specialized cells localized in diverse tissues throughout the body, where they mediate protection against pathogens and environmental insults while maintaining tissue homeostasis. Although much of our understanding of human immunology has derived from studies of ...
The human body is a complex system of organ systems and cells that work together to maintain life and support the body's various functions. Understanding human anatomy and physiology is essential for understanding how the body works and how to maintain good health. One of the main topics covered in a chapter on human anatomy and physiology is ...
Human Body Essay: Human body is truly a marvel. It is perhaps the most evolved living thing. It is, in fact, like a highly sophisticated machine. You can read more Essay Writing about articles, events, people, sports, technology many more. Short Essay on Human Body 200 Words for Kids and Students in English Below we have given a […]
S a need to have content. Okay, so if you want to draft an essay about cells and the importance in living organisms. You need to first draft content. Or will your essay contain So we need to have an introduction of sales. You need to have a proper introduction and introduction. You look at your defined cells. Okay, defined cells.
As a result, the mature erythrocyte is among the smallest cells of the body. In humans, the average red blood cell has a volume of only about 100 µm 3. The natural shape of the cell is that of a biconcave disk (Fig. 24-9), although the cell is flexible and its shape is easily distorted when the cell passes through narrow capillaries.
CEO Hein Schumacher launched turnaround plan in October. A liter of sweat, a gram of salt, a billion dead skin cells, 15 grams of body oil, plus extra cellular DNA. That's what the human body sheds every day, according to Keith Rutherford, innovation boss for the homecare unit of Unilever Plc. It's a cocktail of emissions that's mostly ...