- Open access
- Published: 20 October 2020

A qualitative analysis of vaccine decision makers’ conceptualization and fostering of ‘community engagement’ in India
- Tapati Dutta ORCID: orcid.org/0000-0002-3272-1115 1 ,
- Beth E. Meyerson 2 ,
- Jon Agley 3 ,
- Priscilla A. Barnes 4 ,
- Catherine Sherwood-Laughlin 5 &
- Jill Nicholson-Crotty 6
International Journal for Equity in Health volume 19 , Article number: 185 ( 2020 ) Cite this article
4566 Accesses
14 Citations
28 Altmetric
Metrics details
Globally, and in India, research has highlighted the importance of community engagement in achieving national vaccination goals and in promoting health equity. However, community engagement is not well-defined and remains an underutilized approach. There is also paucity of literature on community engagement’s effectiveness in achieving vaccination outcomes. To address that gap, this study interviewed Indian vaccination decision makers to derive a shared understanding of the evolving conceptualization of community engagement, and how it has been fostered during India’s Decade of Vaccines (2010-2020).
Semi-structured interviews were conducted with 25 purposefully sampled national-level vaccine decision makers in India, including policymakers, immunization program heads, and vaccine technical committee leads. Participants were identified by their ‘elite’ status among decisionmakers in the Indian vaccination space. Schutz’ Social Phenomenological Theory guided development of an a priori framework derived from the Social Ecological Model. The framework helped organize participants’ conceptualizations of communities, community engagement, and related themes. Inter-rater reliability was computed for a subsample of coded interviews, and findings were validated in a one-day member check-in meeting with study participants and teams.
The interviews successfully elucidated participants’ understanding of key terminology (“community”) and approaches to community engagement propagated by the vaccine decision makers. Participants conceptualized ‘communities’ as vaccine-eligible children, their parents, frontline healthcare workers, and vaccination influencers. Engagement with those communities was understood to mean vaccine outreach, capacity-building of healthcare workers, and information dissemination. However, participants indicated that there were neither explicit policy guidelines defining community engagement nor pertinent evaluation metrics, despite awareness that community engagement is complex and under-researched. Examples of different approaches to community engagement ranged from vaccine imposition to empowered community vaccination decision-making. Finally, participants proposed an operational definition of community engagement and discussed concerns related to implementing it.
Conclusions
Although decision makers had different perceptions about what constitutes a community, and how community engagement should optimally function, the combined group articulated its importance to ensure vaccination equity and reiterated the need for concerted political will to build trust with communities. At the same time, work remains to be done both in terms of research on community engagement as well as development of appropriate implementation and outcome metrics.
The Global Vaccine Action Plan 2011–2020 lists equity as one of its six guiding principles [ 1 ]. Resonating this ethos, various national vaccination policies and programs have acknowledged vaccines’ contribution to preventing high-cost treatments and averting medical impoverishment, while striving to extend the benefits of immunization to all [ 2 , 3 ]. Correspondingly, community engagement (CE) for vaccinations has increasingly been recognized by decision makers [ 4 ] as a core component of working toward health equity, with a focus on community-based participatory research [ 5 , 6 , 7 ]. CE has been lauded for its facilitation of research translation [ 8 ] and for fostering positive perceptions of vaccines and immunization-related interventions [ 9 ], while decreasing the likelihood of therapeutic misconception [ 10 ]. CE also has been recognized for its assertion that research and interventions with people but without their input is unethical [ 11 ]. Further, recurring incidents of vaccine backlash by communities, as demonstrated by skepticism, resistance, and lack of vaccine support, are often attributed to ‘inappropriate CE’ [ 12 ]. Despite this salutogenic understanding of CE, which has been hypothesized to be a pathway through which population health goals related to public health equity can be met [ 13 ], several studies have suggested that CE has not been clearly defined or explicated in the context of vaccination programs [ 14 , 15 ]. It is important to understand how CE may be utilized to ensure that vaccines are translated into affordable and globally accessible public health solutions, which are acceptable by all communities [ 16 , 17 , 18 ].
To do so, this study examined CE in the context of India’s Universal Immunization Program. India has made tremendous progress during the “Decade of Vaccines” (2010-2020) by introducing multiple new vaccines along with striving to increase access to new and underused vaccines in the country [ 19 ]. The National Vaccine Policy of India mentions ethical use and equitable access as its basic mantra (Ch 5, p 28). However, vaccine decisionmakers are increasingly concerned with the 62% vaccination uptake prevalence among vaccine-eligible children (12-23 months), compared to the 90% target set under government’s Universal Immunization Program, to be achieved by the end of 2020 [ 20 ].
Vaccine studies also indicate the need to embed CE within India’s immunization programs [ 19 , 21 , 22 ]. This growing sensitization about CE among Indian vaccine decision makers has been bolstered by the Supreme Court advisory which recommends meaningful dialogue with communities to accelerate vaccination uptake [ 21 ]. CE is also perceived to be an important step in addressing communities’ vaccine resistance, which leads to delays that inhibit timely vaccination. For example, the cervical cancer-preventing human papilloma virus vaccine was suspended by the Supreme Court of India in 2010. Later, the country's right-wing groups wrote to the Prime Minister expressing concerns about pharmaco-governance and asserting that foreign companies were pushing the vaccine onto an unsuspecting public. Through July 2020, despite advice by the National Technical Advisory Group on Immunization, the Federation of Obstetric & Gynecological Societies of India, and the Indian Academy of Pediatrics for its inclusion in the Universal Immunization Program, substantial community resistance remains. As a result, the vaccine has been sporadically rolled out in three (Sikkim, Punjab and Delhi) out of 36 states and Union Territories [ 23 , 24 ].
Community skepticism about vaccines has a long history in India, evidenced by covert and overt vaccine resistance. As early as the mid 1800’s, some Hindus resisted the smallpox vaccine on religious grounds, because the material used for vaccines was drawn from the lymph of a cow, which is considered a sacred animal by the community [ 25 ]. During the National Polio Surveillance Program, community resistance ranged from people closing their doors and windows when they heard vaccinators approaching their houses, to vaccine backlash such as physical conflict between vaccinators and communities [ 26 ]. Recently, in 2017, there was decreased uptake of measles-rubella vaccination in certain Indian states amidst community uproar following social media rumors of political conspiracy and safety concerns about the vaccine [ 27 , 28 ]. Thus, work to articulate a shared conceptualization of CE is critical at this juncture to establish concerted and strategic CE that can facilitate transparent vaccine communication between communities and decisionmakers and build on existing technology, interventions, and healthcare systems to address inequities in vaccination coverage, especially among under-reached and underserved populations. This may be especially useful in overcoming communities’ myths and fears about new vaccines, which are often considerably more expensive than existing ones, and target relatively ‘hidden’ diseases [ 21 , 23 ]. However, current CE evidence is limited to a few systematic examinations focused on community counselling and vaccination campaigns, often in pockets of high vaccine resistance and low vaccination coverage [ 29 , 30 ]. These reviews have also focused on public opposition rather than involvement, and no data have been collected to indicate ‘if’ and ‘how’ communities are engaged beyond individuals’ decisions to vaccinate themselves and their children [ 31 , 32 , 33 ]. The wider body of academic literature attributes this dearth of CE related studies to the variously premised and sometimes conflicting definitions of and rationales for CE [ 34 ] and the absence of CE metrics [ 35 ]. Other studies mention that evaluating CE is challenging, as such activities often occur in the context of ongoing work and throughout the process of adopting more collaborative engagement approaches [ 36 , 37 ].
It is our perception that typifying an understanding of CE may lead to contextual and ethical application of CE within a complex system of relationships among researchers, policymakers, implementation scientists, and vaccine users. It may also prevent erroneous assumptions about its value and utility, or lack thereof, and inform research and data needs related to CE. This may, in turn, trigger a policy dialogue focused on robust measures to assess what works, how it works, and, over time, if CE efforts have improved vaccination rates and thereby bolstered national efforts to reach out to every vaccine eligible child and adult. Therefore, this study aimed to examine elite Indian vaccine decision makers’ individual perspectives and collective understanding about CE, the circumstances in which CE has been implemented, and how they have fostered CE for effective vaccination.
Schutz’s Social Phenomenology Theory was used as an underlying approach because it is consistent with the belief that ‘conceptualizations’ are socially constructed and appropriated to explore participatory action [ 38 ]. This theory also helped direct attention toward considering the dynamic contexts in which CE was conceived and operationalized [ 39 ]. Social Phenomenology further helped to treat CE conceptualization and its fostering as intersubjective, integral to institutions and systems, all embedded in history, time, and space [ 40 ]. The lead author’s (TD) professional role was that of a translational researcher, supporting evidence-based programs and policy through examination of ecological frameworks using a community-based participatory approach. Her a priori assumption was that community engagement can foster social, relational, and ethical progress toward health equity [ 41 ]. However, few assumptions were made about how decisionmakers would conceptualize CE and community, as these issues infrequently are described in formal, written documents and must instead be intuited from distally related activities.
In preparing for this study, the lead author (TD) purposefully identified 30 individuals who had authoritative roles related to vaccine discovery, development and delivery, such as national-level vaccine decision makers who were policymakers, program heads and/or associates in the government, private sector, non-governmental organizations, and country-offices of international donor and UN agencies. Thus, these individuals, by virtue of their knowledge and positions, were the ‘elites’ [ 42 ] and were able to provide a unique ‘big-picture perspective’ [ 43 ] about CE strategizing and implementation during India’s Decade of Vaccines. Interviewees were approached with this status differential in mind [ 44 ]. In keeping with the assumptions and beliefs of social phenomenology, a two-step participatory approach for data collection was used: (1) semi-structured elite interviews followed by (2) a member check-in meeting [ 42 ]. All interactions used a community-engaged approach, including emphasis on mutual respect and recognition of the knowledge and expertise of study participants. This included adhering to participants’ preferred meeting dates on December 25 and January 1, even though these were national holidays. Further, the member check-in meeting was democratically conducted rather than using the researcher as a moderator. In addition, the researcher was sensitive that issues related to vaccine resistance were occurring in real time, wherein trust building with the study participants was necessary to obtain ‘good data’ and completion of the project.
Access to participants (distinct from identification of the sample) was obtained using a snowball methodology, beginning from the professional network of the principal investigator (TD). Recruitment emails were sent by TD in December 2017 to each of the 30 potential participants, followed up with phone calls to identify interest and availability for an in-person interview. Interviews were conducted by TD with 25 individuals who agreed to participate in the study from December 2017 to February 2018. Each interview lasted for 50 to 90 minutes, was carried out in English, and conducted in the country-offices of the respective agencies, institutions, and organizations located in or around New Delhi, the capital city of India. Interviews were audio recorded and transcribed verbatim. All personalized information was anonymized. Data also included field-notes written within 24 hours of each interview. The interview topics drew from earlier studies focusing on CE as a strategic tool for vaccine research and rollout [ 45 , 46 ]. Accordingly, the inquiries explored participants’: (i) conceptualization of community and CE, (ii) evolution of CE, (iii) fostering support for CE, (iv) resources available for CE, (v) partnerships for CE, (vi) enablers to CE, and (vii) barriers to actualize CE. The interview guide used for this study, including questions and probes, is available as a digital supplement ( 1 ) to this article.
Once a preliminary analysis of the interview data was completed, TD presented the findings in a one-day member check-in meeting among the study participants and their teams (who held second-line leadership positions) in January 2018. Study participants and their team members who participated in the member check-in meeting were knowledgeable about the issue and were comfortable validating and candidly critiquing the primary findings. All study participants and their teams were nationally known; thus, in order to maintain confidentiality, identities, names, and organizational affiliations were not used in reporting the findings. Therefore, although participants in the follow-up meeting knew each other, no specific responses were linked to any individual or organization. This meeting ensured that the overall summation and meaning making of the findings prepared by TD and the research team conformed with what the study participants had mentioned in their interviews and made sense to both the vaccine decision makers and their teams in India (e.g., validity). This was a participatory way to verify both data saturation and completeness of the findings, as well as archival document review (part of the overall project, but not of this study). The study was approved by Indiana University’s Institutional Review Board.
Data analysis
First, all data were transcribed verbatim and entered in NVivo12 (QSR International, Melbourne, Australia) for qualitative data management.
An a priori coding structure was used to categorize individual participants’ conceptualization of CE, how their interests in CE for vaccination evolved by overcoming barriers and optimizing facilitators, while integrating 'policy push for vaccine uptake' and 'generating vaccination demand pull' approaches for different vaccines under the UIP. Based on the interpretive analysis used in social phenomenology, first-level broad construction of CE was done, followed by second-level typical constructs, deliberated through critical events or performance of CE ‘duties’ and ‘responsibilities’ throughout the tenure of the decision makers [ 47 ]. Categories conceptually corresponded with the Social Ecological Model, which has been used to study vaccination uptake and health disparities [ 41 ]. Given this loose pre-existing framework, a general inductive approach was used [ 46 ]. To reach intercoder-reliability (>90%), two coders joined TD, iteratively reviewed, and re-reviewed data for existing and emerging themes and/or patterns, and ultimately crystallized a holistic interpretation through multiple coding conferences. Thereafter the three coders independently coded five interviews to test, reject, accept, or refine the codes [ 43 ]. The final coding structure contained 7 multi-dimensional CE themes with 42 nodes. Exemplar interview excerpts illustrate the findings, although the analysis drew from the entire dataset. The coding structure is available in full as a digital supplemental ( 2 ) file.
All study participants held national and multi-regional leadership roles in vaccine policymaking, financing, and/or program planning and management across vaccine research, development, and roll-out stages for at least ten years in India. In addition to their roles in India, five participants reported managing programs in multiple countries in Asia, Africa, and Latin America. Table 1 describes the study participants.
This section sequentially shares results organized by the following categories and subcategories. Because the results are extensive, we list many of the key themes in brief here as well.
conceptualization of community, and how stakeholders define community;
community was typically understood to be one or more of the following: vaccine-eligible children and their parents and vaccine-eligible adults, frontline healthcare providers, local-level stakeholders, vaccine gatekeepers, and local-level implementing organizations.
conceptualization of CE, with particular attention to analyzing extant efforts, which generally fell into three categories:
capacity building of frontline stakeholders as CE;
capacity building most often was expressed as training, training-of-trainers, and course offerings;
vaccine-related information dissemination as CE;
participants described a wide variety of different communication methods, as well as perceived benefits and disadvantages to each;
targeted community interventions as CE;
participants provided examples of ways in which community
interventions had been carried out;
different tangible ways in which CE might be fostered;
fostering CE was viewed on a broad spectrum that ranged from highly participatory approaches to direct imposition of vaccination services;
evolution and transformation of CE;
all participants acknowledged the need for a better understanding of CE and, in the member check-in meeting, came to a consensus on a definition of CE.
Conceptualization of community
Most participants defined communities as ‘beneficiaries of the UIP,’ with a notion of transactional exchange of vaccine related information between the providers and the communities, always with the aim of vaccination uptake. Communities consisted of the following categories of people: (1) vaccine-eligible children, vaccine-eligible young adults, and their parents and guardians who make vaccination-decisions for the former; (2) healthcare providers who deliver vaccines and sensitize vaccine-eligible populations and their guardians for improved vaccination rates and herd immunity; (3) local-level stakeholders who disseminate information to encourage vaccination uptake; (4) gatekeepers who resist a particular vaccine or vaccination per se , and; (5) local-level implementing organizations of community health workers, groups that includes what are known in India as the 3As. These are Auxiliary Nurse Midwifes who are based at a sub-center and are multipurpose workers responsible for administering vaccines among communities of < 5000 people; Accredited Social Health Activists, who are local women trained to act as health educators in their communities, catering to 700 people in tribal areas and 1000 in rural villages; and Anganwadi Workers, resident workers in the village rural child care centers in India who are responsible for promoting maternal and child health, including interpersonal communication for full immunization coverage, among communities of <1000 people. A few participants took a broader perspective: “ It is the whole communities in which those individuals were living.”
Most of the participants acknowledged their distance from the community, mentioning “if I went to the community nobody will accept me,” while comparing the sense of community with local organizations because they “help raise community demand for routine immunization. ” These organizations included grassroots non-profit organizations (NPOs), community-based organizations (CBOs) like women’s self-help groups, local-level representatives of occupational groups like brick-kiln workers and barbers, and the local-chapters of technical and youth organizations such as the Indian Association of Pediatricians and Nehru Yuva Kendras Sangathan (autonomous organization for youth development under the Government of India, Ministry of Youth Affairs and Sports). Several NGO heads identified themselves as ‘communities’ for their people-centric approach, though, in most of these expressions, fractious relationships and issues of incompatibility between decision makers [mostly government or donors] and NPOs were evident.
“ … .they [Government or donors] want to clip our wings. This is very sad because we [NPOs] bring up issues [local issues of the communities] , which you [Government or donors because of being at the national-level] might never know.”
Some participants identified vaccine-gatekeepers, people who were suspicious that vaccination is a political agenda against minority groups, as communities. Interventions targeting their positive vaccination decisions came across as an area of CE.
“ … in Mallapuram the mother generally said ‘no’ to vaccination because their husband lived in the Middle East [who was proxy decision-makers for their child’s vaccination] .”
Finally, it was unclear whether the media was part of the community, or a driver of communities’ vaccination decisions. Most participants indicated that the media spread misinformation and promulgated negative sentiments among vaccine priority populations about vaccines, and thus expressed the need “to stop negative media so that they [media] do not “blindly publish” , or “ over-sensationalize when it is not an Adverse Event Following Immunization.”
Conceptualization of CE
The participants perceived CE both as a strategy and tool in implementation terms, and variously defined CE as segments of processes comprising of: (1) vaccine policy and program formulation; (2) capacity-building of frontline stakeholders; (3) vaccine information dissemination among communities to promote vaccination uptake, and; (4) targeted community-level interventions to curtail the recurring incidents of vaccine-related community backlash. There was evidence of relational goals of CE, like “longer-term trust building” [between the vaccine decision makers and the communities], and “ … .understand what is going on in people’s minds [regarding vaccinations] ” .
Intuitively, all the participants proposed ongoing and early instantiation of CE for better vaccination outcomes:
“We always go to the communities earlier and have media campaigns, and interpersonal communications to sensitize people on what [vaccine] we would give to their children.”
However, several participants critiqued that CE interventions came in waves, mostly during vaccine introductions, before and during vaccine trials, and in response to a disease outbreak. They also noted that there were no tools or metrics to measure its impact. They speculated that these deficits may be because:
“The Immunization Technical Unit was not built with a CE model [CE frame] for immunization. Like, you [Government] compensate Accredited Social Health Activists for fully immunizing children and trainings attended, but not for doing CE.”
Participants described a top-down and decentralized vaccine governance structure where vaccine policy formulation and vaccine introductions were conducted at the Ministry, considering disease burden, vaccine cost, cold-chain, and supply chain issues. These efforts were completely funded by the Ministry of Health and Family Welfare (MoHFW) and international donors.
“ … . [CE is like] a chandelier, the [MoHFW] is the hook. The different lights are the different partners, they are held at right distances in the right manner. In immunization, the roles and partnerships [of national level decisionmakers] are clearly defined .”
The development of vaccine policies and operational guidelines in English and Hindi (one of the 22 scheduled languages of the Republic of India, and also one of the official languages of India which is understood, spoken, and read by more people than English) by the technical bodies of MoHFW, such as the Immunization Technical Support Unit, and the Mission Steering Group, was conceptualized as CE too. Participants mentioned that the “ state translated and modified [these documents] if they think that something is to be added or deleted,” though no such example of any such revisions incorporated based on communities’ recommendations was cited.
Except the Vaccine Policy (2011), which recommended enhancing communities’ vaccination acceptance and confidence, and vaccine-specific Operational Guidelines, which recommended community-facing strategies, participants did not identify any sub-population-based CE-specific policy. Almost half of the participants cited the Communication Strategy for Polio Eradication , published by the UNICEF and USAID CORE Group, detailing intensive outreach for polio vaccination, as nearest to any CE guideline. Three participants, considering India’s diversity where “every mile the language changes, the culture changes” suggested having a “village-level communication strategy.” Participants noted strategic programs like Mission Indradhanush and Intensified Mission Indradhanush to achieve 90% immunization “to the last child” as CE.
The heads of organizations and technical bodies often criticized chasms in this one-way, top-down approach as “ working in silos” and “not real CE,” and feared that it would ultimately “hinder an integrated approach.” A few participants identified CE as activities occurring in spaces like Village Nutrition and Sanitation Days, which are organized monthly at rural childcare centers. There, communities can ask questions about vaccines and vaccination strategies. However, these participants were doubtful that communities possessed any emancipated voice beyond seeking or resisting vaccines.
Capacity building of frontline stakeholders
Some participants mentioned ‘cascade training of trainers’ for the 3As and local Master Trainers as CE, since the goal is to motivate communities for full immunization. Notably, the CE roles of the 3As and other local stakeholders were different. The Auxiliary Nurse Midwives and Anganwadi Workers are salaried staff for vaccine administration among communities and the Accredited Social Health Activists receive honoraria for counselling and escorting the communities to vaccinations. However, the local NPOs and CBOs appeared to be instrumental in carrying out community-based activities to motivate each community’s vaccination decisions, and, in the case of vaccine trial conducting organizations, act as conduits between researchers and vaccine clinical trial participants.
Participants conceptualized the 18 months training for ANMs, and 3–4 weeks trainings for AWWs and ASHA workers respectively, with additional trainings such as the 3-day Boosting Routine Immunization Demand Generation course for the 3As, and vaccination sensitization trainings for the local-level vaccine-champions (community advisory boards, local religious leaders, barbers, and CBO members), as CE. In these instances, it appeared that some interpersonal tactics were imparted to frontline stakeholders, and tasks were later delegated to them. However, a few participants questioned the ‘quality of CE outcomes’ from these trainings:
“So, you [Government] piggy back everything on the Community Healthcare Worker, who talks to communities about everything: immunization, family planning, maternal health, school health, adolescent health, non-communicable diseases, and cancer … [but] you are not actually engaging or doing CE.”
Vaccine-related information dissemination
Most respondents mentioned “ bilateral information transfer [interpersonal and behavior change communication] sent down to communities” as CE. In the same vein, most participants denoted the Communications Officer as the CE human resource. In fact, one participant said, “The role of communication, I mean CE, sorry using the wrong word again.”
Some participants highlighted the need to be creative and explore web-based media, considering its ease of use, cost-effectiveness, and penetration to interior locations:
“Nobody is interested to read your mobile texts. So, use GIF messaging.”
There were a few examples where bottom-up information, going from the community to the government which facilitated realizing the vaccine program goals, was acknowledged:
“In a construction site we [participant’s organization] did the mapping. But when we reached the community after a fortnight, they [community] have already migrated. The local person would tell us the whereabouts of the mobile community and we could then reach them through the Accredited Social Health Activist network.”
Some participants highlighted campaign-related booklets like the area-based ‘ Underserved Strategy,’ developed after a polio outbreak in Uttar Pradesh in 2002 among the Muslim populations, the ‘ Social Mobilization Network’ formed in 2001 to sensitize families to polio immunization, ‘ My Village my Home’ , a pictographic vaccination tracking method in the shape of a hut, where each column of the hut contains vaccination details of each new-born in the village, and media trainings of “State Immunization Officers on how to handle the media and stop negative media,” as CE.
Vaccine champion engagement and celebrity engagement to motivate communities’ vaccination decisions came across as another form of CE, though there were mixed reactions regarding this strategy.
“Our communication campaigns are pathetic. What is the point in having [a film star in his 70s ] there? We have no way of measuring CE. Does he convey safety of the product? To sell a toothpaste or a phone we spend hundreds of millions of dollars. How much is going into selling something far more important as vaccines?”
Targeted community interventions
Some participants perceived CE as a [right of the communities], “communities want the leadership to come to them. … just sit with them [communities], work with them and that is CE. The leader needs to go to the community at least once or twice. It really increases the communities’ motivation and trust.”
Others suggested a more emancipatory understanding of CE:
“[Vaccine] demand generation is another thing [than CE] . It means that you [government/vaccine providers] are giving and we [vaccine-eligible community] are accepting. Policy influencing is that where the [empowered] community thinks that certain things needs to be changed [and advocates for that] .”
Intervention programs reflected a range, between vaccine imposition and respectful engagement with community stakeholders, where participants’ responses reflected balanced trade-offs between CE’s time and resource investments and feasibility, emphasizing that it is a “ marathon, and not a sprint,” “an expensive process” and “took 20 years to learn about community and how to do CE.”
“In XXXX district community was very resistant and started beating the vaccination team. Then we had to contact a local muscleman, briefed him that this [carrying on with the vaccination drive] is important, and then told him to make an announcement that vaccination is not a bad thing.”
“We engaged with the staff of Aligarh Muslim University, Jamia Milia Islamia and Jamia Hamdard [institutions of higher education that were created to manifest indigenous ethos and spirit of diversity in India] , who went to the field. That helped to address the issue of vaccine hesitancy among religious leaders [especially the Muslim religious leaders] .”
Later, in the member check-in meeting, participants reiterated that effective CE conceptualization and conduct will require developing CE performance and outcome indicators and advocating for their incorporation in immunization surveillance instruments in India. Herein, all the participants emphasized the need to document CE effectiveness and its relational gains:
“ … as a country, I will be ashamed … ., very poor in documentation. You will hardly see any papers from the learnings of polio eradication. This is so because the people who are doing CE do not have the time to document.”
Range of approaches to fostering CE
Though a strict categorization of responses by organizations would not be accurate, participants endorsed a wide variety of types of approaches to fostering CE. These methods generally fell on a spectrum ranging from empowered (‘1’) to disempowered (‘7’). Table 2 provides exemplar quotes illustrating efforts or actions that might be categorized into these different levels.
All participants acknowledged “ decision makers’ good intention for CE but they were not matched with recipes of successful CE models.” Most of the CE interventions reported occurred during the National Polio Surveillance Program (a campaign of the World Health Organization and MoHFW initiated in 1995 to ensure polio eradication through house-to-house poliovirus vaccine delivery), with minimal evidence of institutionalization, replication, or scale-up of these during introduction of other vaccines.
Evolution and transformation of CE
All participants indicated that CE was still a “very poorly understood space,” “complex,” and there were “several gaps to understand this puzzle.” Three participants from NPOs critiqued that it is “ offhand,” “ad-hoc practices to douse the fire,” “firefight,” or “control big chaos and help put things back to normal” and recommended “real community engagement” and a “scientific approach to CE.” Recollecting CE’s evolution, participants noted that the earlier paternalistic prevention impositions has built a negative community memory, and jeopardized communities’ trust on vaccine authorities:
“..the vaccine fear was connected to the family planning program wherein women were forcibly sterilized.”
There was some evidence of pragmatic pressures by global provider/donor organizations (e.g., “GAVI funding went partly for community mobilization” ) that reinforced renewed systems-thinking and inclusive bottom-up- models, like:
“We were not really very serious and formed a small community group. [Initially, the community group ] came, had some snacks and went off. CE really didn’t go beyond that. But by then the NIH and USAID wanted Community Advisory Boards or CABs … and then we learnt how necessary it was.”
Consequently, several participants described recent and direct interactions between vaccine decisionmakers and communities while referring to “ The Prime Minister’s Office invites suggestion from the public” and “ Health Minister issues letters to each Accredited Social Health Activist and Auxiliary Nurse Midwife encouraging them to vaccinate every child.”
In the day-long member check-in meeting, the summary of analysis from the interviews was presented. Study participants and their teams agreed with the findings, and jointly came up with a robust definition of CE, which can be summarized as:
“CE is an upstream policy imperative rather than downstream interventions to build trustworthy relationships between vaccine decisionmakers and communities. It involves demystifying vaccine science and transparent communication for empowered community agency. This would enable communities to critically analyze vaccine related myths and misinformation and enable knowledge co-production in building community sensitive vaccine policies and programs. [CE] is incumbent to sustained political-will and resources to ensure evidence-informed, tailored, vaccine policies and programs, providing equitable, quality, and tangible vaccination and capacity building benefits to community members.”
Meeting participants recognized the need to carry out interventions in ways such that trustworthy relationships between communities and decision makers are established. There were comments reflecting realizations like “If we [decisionmakers] close the doors once again to the community, we might lose their trust, and not get the communities back, ever again.” They also recommended creating more opportunities for relationship-building and group discussions between community healthcare workers and vaccine decision makers. Meeting participants were especially interested in addressing inequities in vaccination coverage by building on the existing range of interventions while innovating newer mechanisms such as community mobilization for vaccination, strategic interventions with vaccine gatekeepers, providing immunization information using traditional, digital, and social media, and dispelling vaccine misinformation and disinformation while formulating rumor management strategies.
This study was able to identify elite decision makers’ core conceptualizations of community, CE, and both extant and aspirational approaches to CE related to vaccination programs in India. In reviewing these findings with study participants, a core definition of CE emerged, focused on upstream relationships (bidirectional), fostering trust, transparent communication, capacity building, and political will to ensure such approaches. Participants indicated that much of the extant work being conceptualized as CE is primarily downstream delivery and even imposition of services for vaccination uptake. While such things can be beneficial (e.g., vaccination), it likely matters to whom they done, in what way, and with what level of community voice (e.g., changing “to whom” to “with whom”). Given that direct imposition has resulted in community backlash against vaccination campaigns both in India and other parts of the world, including violence and hiding children from vaccinators, achieving national policy goals and fostering equitable distribution of public health outcomes may be difficult without a revised approach to CE. Concomitant to this must be an increased focus on CE metrics to promote greater understanding of processes and goals. Importantly, each of the different approaches to CE, including direct imposition, appeared to have been done with the primary goal of increasing equitable access to vaccinations (e.g., supporting community immunization). Thus, the underlying question discussed in this study did not focus on whether individuals should have equitable access to vaccinations, but rather on how such an outcome might best be achieved – that is, the degree to which a revised understanding of CE can support bilateral improvements in both vaccination dissemination by the government and vaccine confidence among communities.
Notably, being an Indian but performing the research at an American university, mitigated reflexivity issues and gave TD the identity of an 'informed outsider,' which allowed her to gain increased access to elites [ 43 ]. Being an implementation researcher allowed TD to deeply engage in analyzing the data while utilizing NVivo predominately as a data management tool. The member check-in meeting facilitated a participatory approach to the interviews, providing considerable interpretive latitude, and probing opportunities. It also allowed participants to critically review CE in UIP with a diversity-equity-inclusion focus. This was particularly important because studies on ‘elite interviewing’ mention that such access can be rare, because such people are hard to reach, surrounded by gatekeepers, and have power and ability to protect themselves from intrusion and criticism [ 44 , 48 ].
This study also benefited from the fact that none of the CE strategies/interventions were ranked as ‘best practice’ over another by institutional mandate or leadership, unlike the traditional ranking of engagement models in Holland Matrix (1997) [ 49 ], or Arnestein’s Ladder [ 50 ]. This helped reduce social desirability issues among the participants, who would not be perceived as ignoring a best-practice approach when answering honestly about CE.
In most cases, decision makers did not identify themselves or their families as ‘community’, and in some cases only a section of the public was perceived as ‘community’. Ensuring full immunization to communities under UIP was considered the most important CE goal and a step toward equitable health outcomes. However, as noted in other literature [ 35 , 51 ], a non-immersive and reductionist approach to conceptualizing communities may inhibit formation of trusted collaborations with the communities, ultimately compromising the creation of communities’ agency [ 52 ]. Some authors have described this as ‘conservative corporatism’ which, contrary to the ‘whole community approach’ [ 53 ], can lead to fragmented health governance, introduce barriers to building comprehensive people-centered vaccine policy reform [ 36 ], and risk defining communities as internally homogenous entities, which is unlikely to be the case given the diversities prevalent in India [ 54 ]. This may also undermine tailored CE strategies for particular sub-populations, leading to reductions in their trust of vaccinators and empowered vaccination decision making, especially among those for whom vaccine hesitancies are high, and/or vaccination uptake is low [ 55 , 56 ].
While findings supported current iterations of CE in making substantive contributions to vaccine demand generation and disease eradication, communities were often seen as offering ‘passive demand.’ Ideally, communities would actively seek vaccines and there would be community demand reflecting social support for vaccination as a norm [ 11 ]. Head’s research goes so far as to suggest that utilitarian CE may foster health inequities [ 57 ]. Gopichandran’s work looks at the relational gains (more intrinsic in nature, rather than transactional relationship building) from CE and posits development of trust between vaccine decision makers and communities as a result of shared CE goals integrated into vaccination targets [ 56 ]. Accordingly, doing empowered CE may require a paradigm-shift to perceive communities as integral parts of the policy and delivery systems, incorporate CE metrics into vaccine surveillance, and create new roles with a focused responsibility to coordinate CE.
It appears that many facets of the national-level CE response were an equilibrating reaction to appease community outrage rather than an integral approach set in place a priori . Adhikari et. al. has defined such CE as 'short-hand' [ 31 ], often resulting in wasted resources, with the potential to create mistrust rather than enhance benefits, create legitimacy, or share responsibility [ 31 , 56 ]. Other authors have envisaged that such CE can eventually give rise to communities as agents of the government, and CE becoming an ‘involvement industry’ ‘procured from external organizations’ [ 57 , 58 ]. To alleviate this, Folayan et. al. (2019) have recommended memoranda signed between the government and local partner organizations at the study design stage [ 54 ]. That noted, Webber seems to doubt whether national government-based public health initiatives might ever be able to stray too far from a top-down approach, postulated as the ‘two-community thesis’ [ 58 ]. Other authors suggest that deviation from this paradigm will require transformative leadership which is difficult to achieve in the public service sector with the prevailing traditional organizational thinking, policies, and management techniques [ 59 ].
While frontline local stakeholders played a role in Indian vaccination efforts as two-way conduits between decision makers and the community, more studies are recommended to examine complex issues derived thereof, such as internal chasms and accountability mechanisms between the 3As, and motivational erosion when CE work is not compensated financially (adequately). Prior research would not suggest, though, that social media could replace this in-person work. Ramsbottom’s et al.’s study found that, although social-media messaging is a cost-effective mechanism for vaccine information dissemination, it might not be the best approach for India, and could leave out social media illiterate populations, those with erratic and sporadic internet connectivity, and areas where vaccine communication needs to be translated to local dialects [ 53 ].
Limitations
Ensuring open discussion with vaccine decision makers and their team members on a potentially controversial topic like CE for vaccination was not always easy; it took time to convince the potential participants to participate. Some of the elites were difficult to access because of the ongoing community uproars around Measles-Rubella and HPV vaccines which were playing out in real time in the country during the study’s time period [ 60 , 61 ]. Despite these structural impediments, theoretical saturation was ensured by virtue of interviewing nearly the entire group of elite vaccine decision makers in the country. This was achieved by utilizing TD’s professional networking and familiarity with some of the study participants, use of a sensitive mix of knowledge and intercultural humility, flexibility to re-schedule appointments after office hours or on national holidays, and use appropriately persuasive multiple communication channels like Facebook Messenger, or WhatsApp [ 43 ], in addition to emails and phone calls. Nonetheless, the study findings were limited by the inherent limitations of a qualitative study design. However, generalizability within India might be more strongly inferred than would be typical given the high percentage of decision makers who provided data. In addition, all the study participants were interviewed in or around New Delhi. While the individuals who were interviewed each had a national or international scope to their decision making, this centrality may have influenced the findings in some way. Finally, some of the findings related to intended actions in the future rather than things that had already been completed; this hampered the ability to ascribe definite actions in some cases. However, existing literature demonstrates that intentions are moderately good predictors of future behavior [ 62 , 63 ].
The results from this study can be used both to understand past CE challenges and successes and to prospectively plan community-led, tailored CE initiatives for better vaccination outcomes. Of note, there appears to be conceptual tension between multiple vaccination-related goals, such that each can be perceived as CE for health equity; namely, top-down vaccination programs may be successful in achieving some short-term immunization, but there may be backlash, and longer-term increases in immunization rates may suffer as a result. At this stage, it will be critical to devise CE process and outcome indicators for vaccination programs in India, and to advocate for their incorporation in vaccination surveillance datasets. As of now, the suggestions herein are theoretical – and evaluation metrics would allow for demonstrations of how CE impacts a variety of important outcomes, and, ultimately, foster replicability of successful efforts within India and internationally.
Availability of data and materials
All twenty-five qualitative interviews (audio recording and transcripts) are available from the lead author (TD) and can be shared on request.
Plan WG. Plan 2011–2020. Geneva: World Health Organization. 2013. http://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011_2020/en/ .
Boyce T, Gudorf A, de Kat C, Muscat M, Butler R, Habersaat KB. Towards equity in immunisation. Eurosurveillance. 2019;24(2):1800204.
Article PubMed Central Google Scholar
Chang AY, Riumallo-Herl C, Perales NA, Clark S, Clark A, Constenla D, Garske T, Jackson ML, Jean K, Jit M, Jones EO. The equity impact vaccines may have on averting deaths and medical impoverishment in developing countries. Health Affairs. 2018;37(2):316–24.
Article PubMed Google Scholar
Boxelaar, L, Paine, M, Beilin, R. Community Engagement: for Whom? Proceedings of International Conference on Engaging Communities. Brisbane: Queensland Department of Main Roads, 2005.
Boston PQ, Mitchell MM, Collum K, Gravlee CC. Community engagement and health equity. Practicing Anthropol. 2015;37(4):28–32.
Article Google Scholar
Wallerstein N, Duran B. Community-based participatory research contributions to intervention research: the intersection of science and practice to improve health equity. Am J Public Health. 2010;100(S1):S40–6.
Article PubMed PubMed Central Google Scholar
Wallerstein NB, Yen IH, Syme SL. Integration of social epidemiology and community-engaged interventions to improve health equity. Am J Public Health. 2011;101(5):822–30.
Sariola S, Reynolds L. The Ethics and Politics of Community Engagement in Global Health Research. Crit Public Health. 2018;28(3):257–68.
Pramanik S, Ghosh A, Nanda RB, De Rouw M, Forth P, Albert S. Impact evaluation of a community engagement intervention in improving childhood immunization coverage: a cluster randomized controlled trial in Assam, India. BMC Public Health. 2018;18(1):1–3.
Fregonese F. Community involvement in biomedical research conducted in the global health context; what can be done to make it really matter? BMC Medical Ethics. 2018;19(1):44.
Gibson A, Britten N, Lynch J. Theoretical directions for an emancipatory concept of patient and public involvement. Health. 2012;16(5):531–47.
Larson HJ, De Figueiredo A, Xiahong Z, Schulz WS, Verger P, Johnston IG, Cook AR, Jones NS. The state of vaccine confidence 2016: global insights through a 67-country survey. EBioMedicine. 2016;12:295–301.
Fawcett S, Schultz J, Watson-Thompson J, Fox M, Bremby R. Peer reviewed: Building multisectoral partnerships for population health and healthequity. Prev Chronic Dis. 2010;7(6):1–7.
Kagee A, De Wet A, Kafaar Z, Lesch A, Swartz L, Newman PA. Caveats and pitfalls associated with researching community engagement in the context of HIV vaccine trials. J Health Psychol. 2020;25(1):82–91.
Tindana P, de Vries J, Campbell M, Littler K, Seeley J, Marshall P, Troyer J, Ogundipe M, Alibu VP, Yakubu A, Parker M. Community engagement strategies for genomic studies in Africa: a review of the literature. BMC Medical Ethics. 2015;16(1):24.
Pratt B, de Vries J. Community engagement in global health research that advances health equity. Bioethics. 2018;32(7):454–63.
Goldstein S, MacDonald NE, Guirguis S. SAGE Working Group on Vaccine Hesitancy. Health communication and vaccine hesitancy. Vaccine. 2015;33(34):4212–4.
Obregón R, Chitnis K, Morry C, Feek W, Bates J, Galway M, Ogden E. Achieving polio eradication: a review of health communication evidence and lessons learned in India and Pakistan. Bull World Health Organization. 2009;87:624–30.
Paul S, Sahoo J. Four new vaccines for routine immunization in India: What about hemophilus influenza B and pneumococcal vaccine? J Family Med Primary Care. 2015;4(1):9.
IIPS I. National Family Health Survey (NFHS-4), 2015–16. International Institute for Population Sciences (IIPS), Mumbai, India. 2017.
Gurnani V, Haldar P, Aggarwal MK, Das MK, Chauhan A, Murray J, Arora NK, Jhalani M, Sudan P. Improving vaccination coverage in India: lessons from Intensified Mission Indradhanush, a cross-sectoral systems strengthening strategy. BMJ. 2018;363.
Laxminarayan R, Ganguly NK. India’s vaccine deficit: why more than half of Indian children are not fully immunized, and what can—and should—be done. Health Affairs. 2011;30(6):1096–103.
Zimet GD, Meyerson BE, Dutta T, Forster A, Corcoran B, Hanley S. Political and Public Responses to Human Papillomavirus Vaccination. InHuman Papillomavirus 2020 Jan 1 (pp. 363-377). Academic Press.
Sankaranarayanan R, Basu P, Kaur P, Bhaskar R, Singh GB, Denzongpa P, Grover RK, Sebastian P, Saikia T, Oswal K, Kanodia R. Current status of human papillomavirus vaccination in India's cervical cancer prevention efforts. Lancet Oncol. 2019;20(11):e637–44.
Lahariya C. A brief history of vaccines & vaccination in India. Indian J Med Res. 2014;139(4):491.
PubMed PubMed Central Google Scholar
Solomon R. Involvement of civil society in India’s polio eradication program: lessons learned. Am J Tropical Med Hygiene. 2019;101(4_Suppl):15–20.
Das MK, Singh D. Vaccine news in India: trend and content analysis of online mass media. Int J Community Med Public Health. 2018;5(9):3951.
Rao M & Govindarajan V, Scroll in, Feb 24, 2017 WhatsApp rumours about vaccinations hamper India's drive to halt measles and rubella. Accessed 1 Mar 2017. https://scroll.in/pulse/830129/rumours-about-measles-rubella-vaccine-hit-coverage . Accessed on July 25, 2020.
Dimala CA, Kika BT, Kadia BM, Blencowe H. Current challenges and proposed solutions to the effective implementation of the RTS, S/AS01 Malaria Vaccine Program in sub-Saharan Africa: A systematic review. PloS one. 2018;13(12):e0209744.
Article CAS PubMed PubMed Central Google Scholar
Sarrami-Foroushani P, Travaglia J, Debono D, Braithwaite J. Implementing strategies in consumer and community engagement in health care: results of a large-scale, scoping meta-review. BMC Health Services Research. 2014;14(1):402.
Adhikari B, James N, Newby G, Von Seidlein L, White NJ, Day NP, Dondorp AM, Pell C, Cheah PY. Community engagement and population coverage in mass anti-malarial administrations: a systematic literature review. Malaria J. 2016;15(1):523.
Farmer J, Taylor J, Stewart E, Kenny A. Citizen participation in health services co-production: a roadmap for navigating participation types and outcomes. Australian Journal of Primary Health. 2018;23(6):509–15.
Ministry of Health and Family Welfare. Government of India Drugs and Cosmetics (First Amendment) Rules. New Delhi: Government of India Press; 2013.
Google Scholar
Enria L, Lees S, Smout E, Mooney T, Tengbeh AF, Leigh B, Greenwood B, Watson-Jones D, Larson H. Power, fairness and trust: understanding and engaging with vaccine trial participants and communities in the setting up the EBOVAC-Salone vaccine trial in Sierra Leone. BMC Public Health. 2016;16(1):1140.
MacQueen KM, Bhan A, Frohlich J, Holzer J, Sugarman J, Ethics Working Group of the HIV Prevention Trials Network. Evaluating community engagement in global health research: the need for metrics. BMC Medical Ethics. 2015;16(1):44.
Martin GP, Carter P, Dent M. Major health service transformation and the public voice: conflict, challenge or complicity? J Health Services Res Policy. 2018;23(1):28–35.
Forsythe LP, Carman KL, Szydlowski V, Fayish L, Davidson L, Hickam DH, Hall C, Bhat G, Neu D, Stewart L, Jalowsky M. Patient engagement in research: early findings from the Patient-Centered Outcomes Research Institute. Health Affairs. 2019;38(3):359–67.
Schutz A. The problem of personality in the social world. InCollected papers VI. Literary reality and relationships 2013 (pp. 199-240). Springer, Dordrecht.
Uddin J, Sarma H, Bari TI, Koehlmoos TP. Introduction of new vaccines: decision-making process in Bangladesh. J Health Population Nutrition. 2013;31(2):211.
Weston C, Gandell T, Beauchamp J, McAlpine L, Wiseman C, Beauchamp C. Analyzing interview data: The development and evolution of a coding system. Qualitative Sociol. 2001;24(3):381–400.
McElfish PA, Post J, Rowland B. A social ecological and community-engaged perspective for addressing health disparities among Marshallese in Arkansas. International Journal of Nursing & Clinical Practices. 2016;30:2016.
Aberbach JD, Rockman BA. Conducting and coding elite interviews. Political Science and Politics. 2002;35(4):673–6.
Harvey WS. Strategies for conducting elite interviews. Qualitative Res. 2011;11(4):431–41.
Lancaster K. Confidentiality, anonymity and power relations in elite interviewing: conducting qualitative policy research in a politicised domain. Int J Soc Res Methodol. 2017;20(1):93–103.
Kumar S, Quinn SC, Kim KH, Musa D, Hilyard KM, Freimuth VS. The social ecological model as a framework for determinants of 2009 H1N1 influenza vaccine uptake in the United States. Health Education Behavior. 2012;39(2):229–43.
Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qualitative Health Res. 2005;15(9):1277–88.
Elo S, Kyngäs H. The qualitative content analysis process. J Advanced Nurs. 2008;62(1):107–15.
Hochschild JL. Conducting intensive interviews and elite interviews. InWorkshop on interdisciplinary standards for systematic qualitative research 2009. National Science Foundation. .
Holland B. Factors and strategies that influence faculty involvement in public service. Building the Field of Higher Education Engagement: Foundational Ideas and Future Directions. 2019.
Arnstein SR. A ladder of citizen participation. City Reader. 2015;279.
O'Mara-Eves A, Brunton G, McDaid G, Oliver S, Kavanagh J, Jamal F, Matosevic T, Harden A, Thomas J. Community engagement to reduce inequalities in health: a systematic review, meta-analysis and economic analysis. Pub Health Res. 2013;1(4). https://doi.org/10.3310/phr01040 .
Kuhlmann E, Burau V. Strengthening stakeholder involvement in health workforce governance: why we need to talk about power. J Health Services Res Policy. 2018;23(1):66–8.
Ramsbottom A, O’Brien E, Ciotti L, Takacs J. Enablers and barriers to community engagement in public health emergency preparedness: a literature review. J Community Health. 2018;43(2):412–20.
Folayan MO, Oyedeji KS, Fatusi OA. Community members' engagement with and involvement in genomic research: Lessons to learn from the field. Developing World Bioethics. 2015;15(1):1–7.
Howard-Grabman L, Miltenburg AS, Marston C, Portela A. Factors affecting effective community participation in maternal and newborn health programme planning, implementation and quality of care interventions. BMC Pregnancy Childbirth. 2017;17(1):268.
Gopichandran V. Public trust in vaccination: an analytical framework. Indian J Med Ethics. 2017;2(2):98–104.
PubMed Google Scholar
Head BW. Community engagement: participation on whose terms? Australian J Political Sci. 2007;42(3):441–54.
Webber DJ. Explaining policymakers' use of policy information: The relative importance of the two-community theory versus decision-maker orientation. Knowledge. 1986;7(3):249–90.
Newman PA, Rubincam C. Advancing community stakeholder engagement in biomedical HIV prevention trials: principles, practices and evidence. Expert Review Vaccines. 2014;13(12):1553–62.
Article CAS Google Scholar
Firstpost, Nair N, 2017 Won't take Modi-RSS vaccine’: Myths, quacks derail Malappuram vaccination drive putting lakhs of children at risk. Accessed 20 Mar 2018. https://www.firstpost.com/india/wont-take-modi-rss-vaccine-myths-quacks-derail-malappuram-vaccination-drive-putting-lakhs-of-children-at-risk-4236543.html .
Cheatham A, February 26, 2018, Duke Global Reproductive Health, Despite Government Policy, Cervical Cancer Progress Stalls in India. Accessed 20 Mar 2018. http://dukecenterforglobalreproductivehealth.org/2018/02/26/despite-government-policy-cervical-cancer-progress-stalls-in-india/ .
Frew PM, Archibald M, Martinez N, del Rio C, Mulligan MJ. Promoting HIV vaccine research in African American communities: does the theory of reasoned action explain potential outcomes of involvement? Challenge (Atlanta, Ga.). 2007;13(2):61.
Albarracin D, Wyer RS Jr. The cognitive impact of past behavior: influences on beliefs, attitudes, and future behavioral decisions. J Personality Social Psychol. 2000;79(1):5.
Download references
Acknowledgements
We thank all the study participants and their teams for providing the information and data used in this study and for hosting the member check-in in meeting in New Delhi, India.
The lead author (TD) received a scholarship from Dhar India Studies Program, Indiana University, to undertake travel to India for the member check-in meeting.
Author information
Authors and affiliations.
Room No. 469, Berndt Hall, Public Health Department, Health Sciences Division, Fort Lewis College, Durango, CO, 81301, USA
Tapati Dutta
Southwest Institute for Research on Women, College of Social & Behavioral Sciences, Family & Community Medicine, College of Medicine, University of Arizona, Tucson, USA
Beth E. Meyerson
Department of Applied Health Science, Prevention Insights, Indiana University School of Public Health-Bloomington, 809 E 9th Street, Room 101, Bloomington, IN, 47405, USA
Department of Applied Health Science, Indiana University School of Public Health-Bloomington, 809 E 9th Street, Room 203, Bloomington, IN, 47405, USA
Priscilla A. Barnes
Department of Applied Health Science, Indiana University School of Public Health-Bloomington, 801 E 7th Street, Room 102, Bloomington, IN, 47405, USA
Catherine Sherwood-Laughlin
School of Public and Environmental Affairs, Indiana University, Room 351, 1315 E 10th Street, Bloomington, IN, 47405, USA
Jill Nicholson-Crotty
You can also search for this author in PubMed Google Scholar
Contributions
As the first and corresponding author, TD was primarily responsible for conceptualizing the study, conducting the data collection and analysis, coordinating and facilitating the check-in validation meetings, writing the manuscript, and adding the revisions addressing the reviewer comments in this version of the manuscript. Other authors (dissertation committee members) offered extensive input at the proposal and study design stages and throughout the dissertation (BEM, JA, PB, CSL, JNC). JA additionally contributed intensively to revisions and preparation of this manuscript for publication, and revisions addressing reviewer comments. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Tapati Dutta .
Ethics declarations
Ethics approval and consent to participate.
This manuscript is one of the papers from the lead author’s (TD) doctoral dissertation. That study, and all related manuscripts, were reviewed and approved under the 'Exempt' category by the Indiana University's Institutional Review Board (protocol 1710654732). Consent to participate was obtained from all study participants via email.
Consent for publication
The co-authors here are the former Dissertation Committee members (BEM, JA, PB, CSL, JNC) of the lead author (TD), and have consented to be co-authors for this manuscript.
Competing interests
The study does not have any conflict of interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1., additional file 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Dutta, T., Meyerson, B.E., Agley, J. et al. A qualitative analysis of vaccine decision makers’ conceptualization and fostering of ‘community engagement’ in India. Int J Equity Health 19 , 185 (2020). https://doi.org/10.1186/s12939-020-01290-5
Download citation
Received : 28 May 2020
Accepted : 24 September 2020
Published : 20 October 2020
DOI : https://doi.org/10.1186/s12939-020-01290-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
International Journal for Equity in Health
ISSN: 1475-9276
- General enquiries: [email protected]
- Frontiers in Immunology
- Vaccines and Molecular Therapeutics
- Research Topics
A Global Perspective on Vaccines: Priorities, Challenges and Online Information
Total Downloads
Total Views and Downloads
About this Research Topic
Vaccines have been one of the most important medical discoveries of the last three centuries. Thanks to their development, millions of deaths have been avoided. Together with improved hygiene and antibiotics, vaccines have significantly contributed to the prolongation of life expectancy in high-income ...
Keywords : Vaccine, Vaccination
Important Note : All contributions to this Research Topic must be within the scope of the section and journal to which they are submitted, as defined in their mission statements. Frontiers reserves the right to guide an out-of-scope manuscript to a more suitable section or journal at any stage of peer review.
Topic Editors
Topic coordinators, recent articles, submission deadlines.
Submission closed.
Participating Journals
Total views.
- Demographics
No records found
total views article views downloads topic views
Top countries
Top referring sites, about frontiers research topics.
With their unique mixes of varied contributions from Original Research to Review Articles, Research Topics unify the most influential researchers, the latest key findings and historical advances in a hot research area! Find out more on how to host your own Frontiers Research Topic or contribute to one as an author.
Effectiveness of COVID-19 vaccines and their challenges (Review)
Affiliations.
- 1 Department of Environmental, Biological and Pharmaceutical Sciences and Technologies, University of Campania 'Luigi Vanvitelli', 81100 Caserta, Italy.
- 2 School of Biomedical Convergence Engineering, Pusan National University, Yangsan, Gyeongsangnam-do 50612, Republic of Korea.
- 3 Inventra Medclin Biomedical Healthcare and Research Center, Katemanivli, Kalyan, Thane, Maharashtra 421306, India.
- PMID: 34676000
- PMCID: PMC8524740
- DOI: 10.3892/etm.2021.10843
At the end of 2019, a new disease recognized such as severe acute respiratory syndrome (SARS), was reported in Wuhan, China. This disease was caused by an unknown SARS coronavirus 2 (SARS-CoV-2); a virus is characterized by high infectivity among humans. In some cases, this disease can be asymptomatic, while in other cases can induce flu-like symptoms or acute respiratory distress syndrome, pneumonia and death. For this reason, the World Health Organization and Public Health Emergency of International Concern declared a pandemic status in January 2020. Currently, numerous countries have been involved in the development of effective vaccines to protect humans against SARS-CoV-2 infection. The present review will discuss the four vaccines, AZD1222 (AstraZeneca or Vaxzevria), Janssen (Ad26.COV2.S), Moderna/mRNA-1273 and BioNTech/Fosun/Pfizer BNT162b1, that are currently in use worldwide to understand their efficacy, but also evaluate the difficulties and challenges of vaccine development. Although several questions should be addressed regarding these vaccines, the current review will examine the viral elements used in the coronavirus-19 vaccine that can play a crucial role in inducing a strong immune response, as well as the different adverse effects that they can cause to individuals.
Keywords: adverse effects; coronavirus-19; immunity; mRNA; pandemic; vaccines.
Copyright: © Marfe et al.
Publication types
- Open access
- Published: 05 January 2022
Hesitancy in COVID-19 vaccine uptake and its associated factors among the general adult population: a cross-sectional study in six Southeast Asian countries
- Roy Rillera Marzo 1 , 2 , 3 ,
- Waqas Sami 4 , 5 ,
- Md. Zakiul Alam 6 ,
- Swosti Acharya 7 ,
- Kittisak Jermsittiparsert 8 ,
- Karnjana Songwathana 9 ,
- Nhat Tan Pham 10 , 11 ,
- Titik Respati 12 ,
- Erwin Martinez Faller 13 ,
- Aries Moralidad Baldonado 14 ,
- Yadanar Aung 15 , 16 ,
- Sharmila Mukund Borkar 17 ,
- Mohammad Yasir Essar 18 ,
- Sunil Shrestha 19 &
- Siyan Yi 20 , 21 , 22
Tropical Medicine and Health volume 50 , Article number: 4 ( 2022 ) Cite this article
64k Accesses
67 Citations
23 Altmetric
Metrics details
Vaccines are effective and reliable public health interventions against viral outbreaks and pandemics. However, hesitancy regarding the Coronavirus disease (COVID-19) vaccine is evident worldwide. Therefore, understanding vaccination-related behavior is critical in expanding the vaccine coverage to flatten the infection curve. This study explores the public perception regarding COVID-19 vaccination and identifies factors associated with vaccine hesitancy among the general adult populations in six Southeast Asian countries.
Using a snowball sampling approach, we conducted a descriptive cross-sectional study among 5260 participants in Indonesia, Malaysia, Myanmar, Philippines, Thailand, and Vietnam between February and May 2021. Binary logistic regression analysis with a backward conditional approach was applied to identify factors associated with COVID-19 vaccine hesitancy.
Of the total, 50.6% were female, and the median age was 30 years (range: 15–83 years). The majority of the participants believed that vaccination effectively prevents and controls COVID-19 (81.2%), and 84.0% would accept COVID-19 vaccines when they become available. They agreed that health providers’ advice (83.0%), vaccination convenience (75.6%), and vaccine costs (62.8%) are essential for people to decide whether to accept COVID-19 vaccines. About half (49.3%) expressed their hesitancy to receive the COVID-19 vaccines. After adjustment for other covariates, COVID-19 vaccine hesitancy was significantly associated with age, residential area, education levels, employment status, and family economic status. Participants from Indonesia, Myanmar, Thailand, and Vietnam were significantly more likely to express hesitancy in receiving COVID-19 vaccines than those from Philippines.
Conclusions
In general, participants in this multi-country study showed their optimistic perception of COVID-19 vaccines’ effectiveness and willingness to receive them. However, about half of them still expressed their hesitancy in getting vaccinated. The hesitation was associated with several socioeconomic factors and varied by country. Therefore, COVID-19 vaccination programs should consider these factors essential for increasing vaccine uptake in the populations.
Introduction
The ongoing global pandemic of coronavirus disease 2019 (COVID-19) has already infected 257 million population and of them, 5.1 million already died . Both therapeutic and non-therapeutic measures were taken to flatten the numbers of COVID-19 confirmed cases and reduce the deaths. However, the non-pharmaceutical interventions taken worldwide to tackle the pandemic have become tranquil with time [ 1 , 2 ]. Therefore, it becomes essential to achieve herd immunity or implement effective vaccination. Achieving herd immunity for COVID-19 by natural means or allowing a large number of people to become infected will cause an unprecedented strain on healthcare resources and will also result in up to 30 million deaths worldwide [ 3 ]. Thus, mass vaccination has become the only way to manage COVID-19 transmission.
Vaccines other than COVID-19 are one of the most effective and reliable public health interventions ever implemented that prevent millions of deaths from viral infections every year [ 4 , 5 , 6 ]. Although anti-vaccination attitudes and associated misconceptions are prevalent worldwide [ 7 , 8 ], vaccination programs have been developed and progressed significantly in the global health era. Currently, the vaccine from the Pfizer/BioNTech, the SII/Covishield and AstraZeneca/AZD1222 developed by AstraZeneca/Oxford, the Janssen/Ad26.COV 2.S developed by Johnson & Johnson, the Moderna COVID-19 vaccine (mRNA 1273), the Sinopharm COVID-19 vaccine from China National Biotec Group, and the Sinovac-CoronaVac are listed for WHO Emergency Use Listing (EUL). All the vaccines have some sort of mild to moderate side effects, but all of them are safe and effective (60–95%). COVID-19 vaccines save from not only infection but also severe illness and death. Though mass vaccination programs have already been started globally, the effectiveness of vaccination programs has been affected by a hesitancy to receive the vaccines in populations [ 9 , 10 , 11 ], where vaccine hesitancy is defined as the delay in acceptance or refusal of available vaccines [ 12 ].
The hesitancy regarding COVID-19 vaccines is prominently evident worldwide [ 13 , 14 , 15 ]. Studies have identified several factors associated with the COVID-19 vaccine hesitancy in different domains. The identified factors included various socioeconomic and demographic characteristics (e.g., age, sex, residence, income, occupation, and marital status) [ 14 , 16 , 17 , 18 , 19 ] constructs of the health belief model [ 20 , 21 ], constructs of theory of planned behavior and the 5c psychological antecedents [ 20 , 22 ], vaccines-related knowledge [ 14 , 23 , 24 ], attitude towards COVID-19 vaccination [ 14 , 18 , 24 ], conspiracy beliefs [ 14 , 25 , 26 , 27 ], trust and confidence [ 9 ], COVID-19 preventive behavioral practices [ 28 , 29 , 30 ], and the perceived safety and side effects of the vaccines [ 31 , 32 , 33 , 34 ]. Despite vaccine hesitancy, the demand for vaccines increases over time, and disparities in vaccine access within and across the countries are remarkable [ 35 ]. Even though the primary drivers of vaccine hesitancy are often context-specific, there are some agreements that confidence and trust in the COVID-19 vaccine play a critical role in increasing vaccine acceptance [ 9 , 36 ].
COVID-19 cases have been increasing in Southeast Asian countries [ 37 ], and the COVID-19 pandemic impacted the lives of everyone, including health care workers, in many ways, including mental health [ 8 , 38 , 39 , 40 , 41 ]. As of November 21, 2021, around 4.25, 2.82, 2.58, 2.06, 1.09, and 0.52 million confirmed cases have already been in Indonesia, Philippine, Malaysia, Thailand, Vietnam, Myanmar, respectively. The government of all the countries has been trying to mitigate the infection with several measures, including mass vaccination. Understanding vaccination-related behavior is critical in expanding the vaccine coverage to flatten the infection curve. Unfortunately, studies related to the COVID-19 vaccine hesitancy are limited in the context of these nations. As of November 21, 2021, the proportion of the general population fully vaccinated was 32.2% in Indonesia, 79.9% in Malaysia, 17.9% in Myanmar, 38.3% in Philippines, 54.73% in Thailand, and 39.6% in Vietnam [ 37 ]. Though started with AstraZeneca in the first phase, Pfizer, Sinovac, and Covovax vaccines are available in east Asian countries. The hesitancy to receive the COVID-19 vaccine may pose critical challenges in the fight against the pandemic and the global shortage of vaccines. To address this gap, we conducted a multi-country study to assess the perception of the COVID-19 vaccine effectiveness, acceptance, and hesitancy in the context of Southeast Asian countries. We also explored factors associated with the hesitation in the vaccine uptake.
Study design and sites
This descriptive cross-sectional study was conducted in six Southeast Asian countries i.e., Indonesia, Malaysia, Myanmar, Philippines, Thailand, and Vietnam for 4 months from February to May 2021.
Participants and sampling
The target participants were adult citizens from the participating countries aged 18 years and above, who could read and understand local languages or English. Due to the limitations in employing face-to-face methods during the outbreak, the survey was prepared in a Google form and disseminated to the participants using a snowball sampling method. First, we recruited 50 primary participants and asked them to share the questionnaire link to individuals in their social networks who met the inclusion criteria. We chose these social media platforms, because they are widely used across socio-demographic characteristics. The response rate ranged from 30–45%.
Data collection procedures
We distributed the questionnaire using personal contacts using word of mouth or emails and through web-based applications and social media, such as Facebook, Instagram, LinkedIn, Telegram, Twitter, and WhatsApp. Participants were reminded to respond only once. We employed unique identifiers for use only in a single account by settings that allow only one response per user. In addition, the Google form will not allow another entry from the same Google Account. Participants were ensured the confidentiality and privacy of their responses to reduce potential bias introduced by self-reported data.
Tool development and measures
We developed the questionnaire through participatory discussion with the research team of participating countries. Through Zoom meetings, the principal author discussed research objectives and methodology with all country representatives. The questionnaire was initially developed in English and translated into local languages. Then, the questionnaire was back-translated, pre-tested, and revised by the research team in the individual country. A group of expert panels in the respective countries which included psychiatrists, clinical psychologists, physicians, clinicians and public health experts translated and culturally validated into their national. Pilot testing comprised of 15 participants in each country to test face validity and 50 participants in each country to test the internal consistency. The Cronbach's alpha value ranging from 0.824 to 0.925 indicated that the questionnaire has a good to excellent internal consistency across all countries. It took approximately 10 mins to complete the survey.
The questionnaire had 15 items divided into two sections, namely, Section A had nine items and Section B had six questions regarding factors influencing the acceptability of COVID-19 vaccination. The socio-demographic characteristics of the participants were age (continuous), sex (male, female), place of residence (rural, urban), the education level (illiterate, secondary, post-secondary education, tertiary education), employment status (employed, student, unemployed), marital status (never married, married, widowed/divorced/separated), and family economic status (low, medium, high). The economic status was classified according to income classification from Department of Statistics for each country.
We used yes/no questions to assess the participants’ perceived COVID-19 vaccine effectiveness, acceptance, and factors believed to be essential for deciding whether to accept COVID-19 vaccines. Participants were asked whether they think COVID-19 vaccination can effectively prevent and control COVID-19. They were also asked whether they would accept COVID-19 vaccines when they become available. We asked whether the participant agreed that vaccination convenience (methods, frequency, distance to vaccination sites), health providers’ advice, and costs of vaccines are essential for deciding whether to accept COVID-19 vaccines. Hesitancy in COVID-19 vaccine uptake was measured by asking whether the participant would take COVID-19 vaccines as soon as they become available in the country.
Statistical analyses
We used Statistical Product and Service Solutions (SPSS) 26.0 (IBM Corp., Armonk, N.Y., USA) for data analyses. One-sample Kolmogorov–Smirnov test was used to assess the normality of age distribution, and it was non-normally distributed. Therefore, median and range were used as a measure of central tendency. Categorical variables are presented as frequencies and percentages. Pearson’s Chi-square test was used to observe the association between socio-demographics and the COVID-19 vaccine-related variables and between-country differences. The Bonferroni-adjusted post hoc test was applied for significant results in the Pearson’s Chi-square tests. After fulfilling the assumptions (relationship between variables and absence of multicollinearity), we conducted binary logistic regression analyses with a backward conditional approach to explore the relationship between vaccine hesitancy (yes, no) and socio-demographic characteristics controlling for the covariates included in the models. All variables associated with vaccine hesitancy in bivariate analyses at a level of p value < 0.05 were included in the multivariable regression analyses.
Each study country representative obtained ethical clearance. The format of informed consent forms for all selected studies adhered to the guidelines recommended by the SOMREC which, at the minimum, stipulate inclusion of sections on purpose of the research, study procedures, discomforts and risks, potential benefits, privacy and confidentiality, compensation for participation, voluntary participation, investigators’ contact information for questions about study, and ethics committee contact for questions about rights and welfare of participants.
Table 1 shows the socio-demographic characteristics of the participants. A total of 5260 participants completed the questionnaire—339 from Indonesia, 1273 from Malaysia, 300 from Myanmar, 311 from Philippines, 2367 from Thailand, and 670 from Vietnam. The median age of the participants was 30 years (range: 15–83 years). Slightly more than half of the participants were female (50.6%) and never married (55.4%). About two-thirds of the participants were employed (61.7%), and 69.6% resided in urban areas. More than half of the participants (55.6%) had tertiary education. Almost half of the participants (46.6%) reported medium family economic status, and 45% were from Thailand.
As shown in Table 2 , 81.2% of the participants agreed that vaccination could effectively prevent and control COVID-19, and 84.0% would accept the vaccines when they become available. The majority believed that vaccination convenience (75.6%), health providers’ advice (83.0%), and costs of vaccines (62.8%) are essential for deciding whether to accept COVID-19 vaccines. However, about half (50.7%) still expressed their hesitation to take the COVID-19 vaccines.
Table 3 shows that males were significantly more likely to agree that vaccines could effectively prevent and control COVID-19, responded that they would accept the vaccines when they become available, and believed that health providers’ advice and costs of vaccines are important for deciding whether to accept COVID-19 vaccines than females. Participants living in urban areas were significantly more likely to agree that vaccines can effectively prevent and control COVID-19, responded that they would accept the vaccines when they become available, and believed that vaccination convenience, health providers’ advice, and costs of vaccines are important for deciding whether to accept COVID-19 vaccines than those living in rural areas. Participants living in rural areas were significantly more likely to express hesitancy in receiving COVID-19 vaccines than those living in urban areas.
Participants with tertiary education were significantly more likely to agree that vaccines can effectively prevent and control COVID-19, responded that they would accept the vaccines when they become available, and believed that vaccination convenience, health providers’ advice, and costs of vaccines are important for deciding whether to accept COVID-19 vaccines than participants with lower education. Participants with lower education were significantly more likely to express hesitancy in receiving COVID-19 vaccines than participants with tertiary education. Compared to unemployed and employed participants, students were significantly more likely to agree that vaccines can effectively prevent and control COVID-19, responded that they would accept the vaccines when they become available, and believed that vaccination convenience, health providers’ advice, and costs of vaccines are important for deciding whether to accept COVID-19 vaccines. Unemployed participants were significantly more likely to express hesitancy in receiving COVID-19 vaccines than students and employed participants.
Never-married participants were significantly more likely to agree that vaccines can effectively prevent and control COVID-19, responded that they would accept the vaccines when they become available, and believed that vaccination convenience, health providers’ advice, and costs of vaccines are important for deciding whether to accept COVID-19 vaccines than married and widowed, divorced or separated participants. Participants with a high family economic status were significantly more likely to agree that vaccines can effectively prevent and control COVID-19, responded that they would accept the vaccines when they become available, and believed that health providers’ advice is important for deciding whether to accept COVID-19 vaccines than participants with a low and medium family economic status. Participants with a medium family economic status were significantly more likely to believe that vaccination convenience is important for deciding whether to accept COVID-19 vaccines than participants with a low and high family economic status. Participants with a low family economic status were significantly more likely to believe that vaccine costs are important for deciding whether to accept COVID-19 vaccines than participants with a medium and high family economic status. Participants with a low and high family economic status were significantly more likely to express hesitancy in receiving COVID-19 vaccines than participants with a medium family economic status. The differences between countries were all statistically significant.
Table 4 presents the association between countries and COVID-19 vaccine effectiveness, acceptance, convenience, recommendation, price, and hesitancy. Results showed a significant association between all vaccine factors and countries ( p < 0.001), respectively.
Table 5 shows factors associated with hesitancy in COVID-19 vaccine uptake in the logistic regression model. After adjustment, having no hesitation was significantly associated with living in rural areas (AOR: 1.40, 95% CI: 1.24–1.59), lower education (AOR: 7.74, 95% CI: 2.72–22.05 for illiterate, AOR: 1.19, 95% CI: 1.01–1.41 for secondary education, and AOR: 1.29, 95% CI: 1.13–1.47 for post-secondary relative to tertiary education), family economic status (AOR: 1.23, 95% CI 1.09–1.39 for lower and AOR: 1.39, 95% CI: 1.19–1.63 for higher relative to medium-income), and employment status (AOR: 1.21, 95% CI 1.03–1.42 for being employed and AOR: 1.85, 95% CI: 1.14–2.60 for being unemployed relative to being students). Compared to those from Philippines, participants from Indonesia (AOR: 6.81, 95% CI: 4.81 9.64), Myanmar (AOR 3.20, 95% CI: 2.30–4.46), Thailand (AOR: 1.06, 95% CI: 1.06–1.74), and Vietnam (AOR: 11.28, 95% CI: 8.22–1.50) were significantly more likely to express no hesitancy in receiving COVID-19 vaccines.
Our multi-country study of six countries of the Southeast Asian region provides essential insight into the perception of COVID-19 vaccines, acceptability, hesitancy, and factors associated with hesitation in the vaccine uptake. Most participants believed that vaccination effectively prevents and controls COVID-19 and would accept COVID-19 vaccines when they become available. They agreed that health providers’ advice, vaccination convenience, and vaccine costs are essential for deciding whether to accept COVID-19 vaccines. However, about half expressed their hesitancy to receive the COVID-19 vaccines. The highest rate of vaccine hesitancy has been observed in Russia (72%), whereas the lowest in Vietnam (27%) [ 9 ].
We have identified several socio-demographic factors associated with hesitancy in COVID-19 vaccine uptake, including age, residential area, education level, family economic status, employment status, and country of residence. The existing studies from Southeast Asian countries [ 19 , 42 ] also show that the older populations are more likely to express their hesitation in receiving the vaccines than the younger populations. In addition, participants from low and high family economic backgrounds were more likely to show uncertainty in receiving COVID-19 vaccines than those with medium family financial status. Previous studies have reported several factors that may explain the populations’ hesitancy in COVID-19 vaccine acceptance. The factors include lower economic level [ 43 ], concerns about the possibly damaging outcome of the COVID-19 vaccines to developing babies in the womb [ 44 ], conspiracy beliefs regarding the COVID-19 vaccine might cause infertility and miscarriages [ 45 ], and less perceived susceptibility [ 46 ].
Place of residence was one of the significant factors that may determine COVID-19 acceptance and uptake. In this study, urban residents were more likely to support COVID-19 vaccines’ effectiveness and uptake. They were more likely to believe that vaccination convenience, advice from health providers, and vaccine costs are important for people deciding whether to receive the vaccines than rural residents. Similarly, rural residents had a higher level of hesitation in the COVID-19 vaccine than urban residents. These findings are similar to other studies conducted in Bangladesh and Philippines [ 14 ]. Higher levels of accessibility, affordability, education, and standard of living are related to vaccine acceptability among people living in urban areas. Having more exposure to the different sources of information, urban residents can create more comprehensive access to more accurate information through media and other reliable sources regarding vaccines. Exposure to negative information about the vaccines was associated with a high level of vaccine hesitancy in Philippines [ 47 ]. There is the need for accurate information on the COVID-19 vaccine, which is very important for its proper management [ 48 ].
Education level was also associated with hesitancy in COVID-19 uptake in this study. People with tertiary education were more likely to support COVID-19 vaccines’ effectiveness and uptake than those with lower education. They were also more likely to believe that vaccination convenience, health providers’ advice, and costs of vaccines are important for people to decide whether to receive COVID-19 vaccines. Similarly, people with lower education more hesitated when asked whether they would accept COVID-19 vaccines than people with tertiary education. Higher educated populations generally possess better knowledge about the vaccines and vaccination process [ 49 ], which creates more heightened awareness regarding the risks and benefits of the vaccination. The level of hesitancy decreases when the level of knowledge about the COVID-19 vaccine and its associated processes increases [ 14 ]. Better knowledge of the vaccination process was a significant factor associated with vaccine hesitancy in previous studies in Bangladesh, Malaysia, India, Kenya, Myanmar, and Thailand [ 14 , 16 , 50 ].
Strengths and limitations
To our knowledge, this is the first multi-country study examining the factors associated with COVID-19 vaccine hesitancy in Southeast Asia. We collected data from a large sample in six countries assessing vaccine effectiveness, acceptance, and hesitation in various populations from different contexts, cultures, and backgrounds. Despite these strengths, this study has several limitations. Response biases could be one of the critical limitations of the study. In addition, data were collected using the snowball technique, which could hamper the heterogeneity in the sample. Another significant limitation is the representativeness of the sample population. A higher proportion of the sampled population were highly educated and residing in urban areas. Since, hesitancy was slightly lower among educated and urban residents, overrepresentation of these groups could lead to underestimation of vaccine hesitancy.
This study provides a crucial understanding of the populations’ perception required to design effective COVID-19 vaccine programs in Southeast Asia. Participants in this multi-country study generally showed their optimistic perception of COVID-19 vaccines’ effectiveness and willingness to receive them. However, about half of them still expressed their hesitancy in getting vaccinated. The hesitation was associated with several socioeconomic factors and varied by country. COVID-19 vaccination promotion campaign should consider these factors as essential elements for increasing vaccine uptake in the populations in the region. Further studies on COVID-19 vaccine acceptance and hesitancy should be a priority. We can use the studies' findings to inform contextualized vaccination programs and information-sharing, ultimately resulting in increased confidence in and uptake of the available vaccines.
Availability of data and materials
The data relating to this manuscript are available upon request.
Omer SB, Yildirim I, Forman HP. Herd immunity and implications for SARS-CoV-2 control. JAMA. 2020;324(20):2095–6.
CAS PubMed Google Scholar
Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, Agha M, Agha R. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg. 2020;78:185–93.
PubMed PubMed Central Google Scholar
Randolph HE, Barreiro LB. Herd immunity: understanding COVID-19. Immunity. 2020;52(5):737–41.
CAS PubMed PubMed Central Google Scholar
Ehreth J. The value of vaccination: a global perspective. Vaccine. 2003;21(27–30):4105–17.
PubMed Google Scholar
Hajj Hussein I, Chams N, Chams S, El Sayegh S, Badran R, Raad M, Gerges-Geagea A, Leone A, Jurjus A. Vaccines through centuries: major cornerstones of global health. Front Public Health. 2015;3:269.
Rodrigues CMC, Plotkin SA. Impact of vaccines; health, economic and social perspectives. Front Microbiol. 2020;11:1526.
Larson HJ, Jarrett C, Eckersberger E, Smith DM, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012. Vaccine. 2014;32(19):2150–9.
Khatiwada AP, Shrestha N, Shrestha S. Will COVID-19 lead to a resurgence of vaccine-preventable diseases? Infect Drug Resist. 2021;14:119–24.
Rozek L, Jones P, Menon A, Hicken A, Apsley S, King E. Understanding vaccine hesitancy in the context of COVID-19: the role of trust and confidence in a seventeen-country survey. Int J Public Health. 2021;66:48.
Google Scholar
Wiysonge CS, Ndwandwe D, Ryan J, Jaca A, Batouré O, Anya B-PM, Cooper S. Vaccine hesitancy in the era of COVID-19: could lessons from the past help in divining the future? Hum Vaccines Immunother. 2021; 1–3.
Marzo RR, Ahmad A, Abid K, Khatiwada AP, Ahmed A, Kyaw TM, Abidin IBZ, Srithar M, Sinnathamby S, Sarvasundram AP, et al. Factors influencing the acceptability of COVID-19 vaccination: a cross-sectional study from Malaysia. Vacunas. 2021. https://doi.org/10.1016/j.vacun.2021.07.007 .
Article PubMed PubMed Central Google Scholar
MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161–4.
Lin C, Tu P, Beitsch LM. Confidence and receptivity for COVID-19 vaccines: a rapid systematic review. Vaccines (Basel). 2020;9(1):16.
Hossain MB, Alam MZ, Islam MS, Sultan S, Faysal MM, Rima S, Hossain MA, Mamun AA. COVID-19 vaccine hesitancy among the adult population in Bangladesh: a nationally representative cross-sectional survey. medRxiv. 2021:2021.2004.2023.21255844.
Sallam M. COVID-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines. 2021;9(2):160.
Bono SA, de Villela FME, Siau CS, Chen WS, Pengpid S, Hasan MT, Sessou P, Ditekemena JD, Amodan BO, Hosseinipour MC, et al. Factors affecting COVID-19 vaccine acceptance: an international survey among low- and middle-income countries. Vaccines. 2021;9(5):515.
Vicente NE, Cordero DA. In the service of the Filipino: the role of Catholic higher education institutions in promoting COVID-19 vaccines in the Philippines. J Public Health (Oxf). 2021;43(2):e377–8.
Soares P, Rocha JV, Moniz M, Gama A, Laires PA, Pedro AR, Dias S, Leite A, Nunes C. Factors associated with COVID-19 vaccine hesitancy. Vaccines. 2021;9(3):300.
Huynh G, Tran T, Nguyen H, Pham L. COVID-19 vaccination intention among healthcare workers in Vietnam. Asian Pac J Trop Med. 2021;14(4):159–64.
CAS Google Scholar
Lin Y, Hu Z, Zhao Q, Alias H, Danaee M, Wong LP. Understanding COVID-19 vaccine demand and hesitancy: a nationwide online survey in China. PLoS Negl Trop Dis. 2020;14(12):e0008961.
Mercadante AR, Law AV. Will they, or won’t they? Examining patients’ vaccine intention for flu and COVID-19 using the Health Belief Model. Res Social Adm Pharm. 2021;17(9):1596–605.
Hossain MB, Alam MZ, Islam MS, Sultan S, Faysal MM, Rima S, Hossain MA, Mamun AA. Health belief model, theory of planned behavior, or psychological antecedents: what predicts COVID-19 vaccine hesitancy better among the Bangladeshi adults? Front Public Health. 2021;9:1172.
Ruiz JB, Bell RA. Predictors of intention to vaccinate against COVID-19: results of a nationwide survey. Vaccine. 2021;39(7):1080–6.
Paul E, Steptoe A, Fancourt D: Anti-vaccine attitudes and risk factors for not agreeing to vaccination against COVID-19 amongst 32,361 UK adults: implications for public health communications. medRxiv. 2020:2020.2010.2021.20216218.
Khan YH, Mallhi TH, Alotaibi NH, Alzarea AI, Alanazi AS, Tanveer N, Hashmi FK. Threat of COVID-19 vaccine hesitancy in Pakistan: the need for measures to neutralize misleading narratives. Am J Trop Med Hyg. 2020;103(2):603–4.
Dubé E, Vivion M, MacDonald NE. Vaccine hesitancy, vaccine refusal and the anti-vaccine movement: influence, impact and implications. Expert Rev Vaccines. 2015;14(1):99–117.
Sallam M, Dababseh D, Eid H, Al-Mahzoum K, Al-Haidar A, Taim D, Yaseen A, Ababneh NA, Bakri FG, Mahafzah A. High rates of COVID-19 vaccine hesitancy and its association with conspiracy beliefs: a study in Jordan and Kuwait among other Arab countries. Vaccines (Basel). 2021;9(1):42.
Latkin CA, Dayton L, Yi G, Colon B, Kong X. Mask usage, social distancing, racial, and gender correlates of COVID-19 vaccine intentions among adults in the US. PLoS ONE. 2021;16(2):e0246970.
Momplaisir F, Haynes N, Nkwihoreze H, Nelson M, Werner RM, Jemmott J. Understanding drivers of COVID-19 vaccine hesitancy among blacks. Clin Infect Dis. 2021;73:1784.
Bhuvan KC, Shrestha R, Leggat PA, Ravi Shankar P, Shrestha S. Safety of air travel during the ongoing COVID-19 pandemic. Travel Med Infect Dis. 2021;43:102103–102103.
Neumann-Böhme S, Varghese NE, Sabat I, Barros PP, Brouwer W, van Exel J, Schreyögg J, Stargardt T. Once we have it, will we use it? A European survey on willingness to be vaccinated against COVID-19. Eur J Health Econ. 2020;21(7):977–82.
Rhodes A, Hoq M, Measey M-A, Danchin M. Intention to vaccinate against COVID-19 in Australia. Lancet Infect Dis. 2021;21(5):e110–e110.
Sah R, Shrestha S, Mehta R, Sah SK, Rabaan AA, Dhama K, Rodriguez-Morales AJ. AZD1222 (Covishield) vaccination for COVID-19: experiences, challenges, and solutions in Nepal. Travel Med Infect Dis. 2021;40:101989–101989.
Shrestha S, Khatri J, Shakya S, Danekhu K, Khatiwada AP, Sah R, Kc B, Paudyal V, Khanal S, Rodriguez-Morales AJ. Adverse events related to COVID-19 vaccines: the need to strengthen pharmacovigilance monitoring systems. Drugs Therapy Perspect. 2021;37(8):376–82.
Kothari A, Pfuhl G, Schieferdecker D, Harris CT, Tidwell C, Fitzpatrick KM, Godleski S, Sanjay S. The barrier to vaccination is not vaccine hesitancy: patterns of COVID-19 vaccine acceptance over the course of the pandemic in 23 countries. medRxiv. 2021:2021.2004.2023.21253857.
King I, Heidler P, Marzo RR. The long and winding road: uptake, acceptability, and potential influencing factors of COVID-19 vaccination in Austria. Vaccines. 2021;9(7):790.
Center for Strategic and International Studies (CSIS). Southeast Asia Covid-19 Tracker. 2021. https://www.csis.org/programs/southeast-asia-program/projects/southeast-asia-covid-19-tracker .
Kamberi F, Sinaj E, Jaho J, Subashi B, Sinanaj G, Jaupaj K, Stramarko Y, Arapi P, Dine L, Gurguri A, et al. Impact of COVID-19 pandemic on mental health, risk perception and coping strategies among health care workers in Albania—evidence that needs attention. Clin Epidemiol Global Health. 2021;12:100824.
Htay MNN, Marzo RR, Bahari R, AlRifai A, Kamberi F, El-Abasiri RA, Nyamache JM, Hlaing HA, Hassanein M, Moe S, et al. How healthcare workers are coping with mental health challenges during COVID-19 pandemic?—a cross-sectional multi-countries study. Clin Epidemiol Global Health. 2021;11:100759.
Marzo RR, Ismail Z, Nu Htay MN, Bahari R, Ismail R, Villanueva EQ, Singh A, Lotfizadeh M, Respati T, Irasanti SN, et al. Psychological distress during pandemic Covid-19 among adult general population: result across 13 countries. Clin Epidemiol Global Health. 2021;10:100708.
Ghatak N, Marzo RR, Saleem SM, Sharma N, Singh A, Bhattacharya S. Impact on routine immunization services during the lockdown period in india: implications and future recommendations. Eur J Mol Clin Med. 2020;7(5):35–40.
Syed Alwi SAR, Rafidah E, Zurraini A, Juslina O, Brohi IB, Lukas S. A survey on COVID-19 vaccine acceptance and concern among Malaysians. BMC Public Health. 2021;21(1):1129.
Chang W-H. A review of vaccine effects on women in light of the COVID-19 pandemic. Taiwanese J Obstet Gynecol. 2020;59:812.
Skjefte M, Ngirbabul M, Akeju O, Escudero D, Hernandez-Diaz S, Wyszynski DF, Wu JW. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. 2021;36(2):197–211.
Murewanhema G. Vaccination hesitancy among women of reproductive age in resource-challenged settings: a cause for public health concern. Pan Afri Med J. 2021;38:336.
Jahangiry L, Bakhtari F, Sohrabi Z, Reihani P, Samei S, Ponnet K, Montazeri A. Risk perception related to COVID-19 among the Iranian general population: an application of the extended parallel process model. BMC Public Health. 2020;20(1):1571.
Migriño J Jr, Gayados B, Birol KRJ, De Jesus L, Lopez CW, Mercado WC, Tolosa J-MC, Torreda J, Tulagan G. Factors affecting vaccine hesitancy among families with children 2 years old and younger in two urban communities in Manila, Philippines. Western Pac Surveill Response J. 2020;11(2):20–6.
Khatiwada AP, Shakya S, Shrestha S. Paradigm shift of drug information centers during the COVID-19 pandemic. Drugs Therapy Perspect. 2020;36(9):389–95.
Eitze S, Heinemeier D, Schmid-Küpke NK, Betsch C. Decreasing vaccine hesitancy with extended health knowledge: evidence from a longitudinal randomized controlled trial. Health Psychol. 2021;40(2):77–88.
Adugna K, Robert K, Asrat Dibaba T, Md Abul K, Thomas D, Heidi L. Determinants of COVID-19 Vaccine Acceptance in Six Lower-and Middle-Income Countries. Research square; Preprint. 2021.
Download references
Acknowledgements
The authors would like to thank the participants of this study.
The authors received no funding for this work.
Author information
Authors and affiliations.
Department of Community Medicine, International Medical School, Management and Science University, Shah Alam, Selangor, Malaysia
Roy Rillera Marzo
Department of Community Medicine, Faculty of Medicine, Asia Metropolitan University, Masai, Johor, Malaysia
Global Public Health, Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia, Jalan Lagoon Selatan, 47500, Subang Jaya, Selangor, Malaysia
Department of Community Medicine and Public Health, College of Medicine, Majmaah University, Almajmaah, 11952, Saudi Arabia
Azra Naheed Medical College, Superior University, Lahore, 54000, Pakistan
Department of Population Sciences, University of Dhaka, Dhaka, Bangladesh
Md. Zakiul Alam
Department of Nursing, Nepal Health Research and Innovation Foundation, Lalitpur, Province Bagmati, Nepal
Swosti Acharya
College of Innovative Business and Accountancy, Dhurakij Pundit University, Bangkok, Thailand
Kittisak Jermsittiparsert
Faculty of Economics and Investment, Bangkok University, Bangkok, Thailand
Karnjana Songwathana
International University, Ho Chi Minh City, Vietnam
Nhat Tan Pham
Vietnam National University Ho Chi Minh City, Ho Chi Minh City, Vietnam
Department of Public Health, Faculty of Medicine and Faculty of Graduate Studies, Universitas Islam Bandung, Bandung, Indonesia
Titik Respati
Department of Pharmacy, Faculty of Pharmacy, San Pedro College, Davao City, Philippines
Erwin Martinez Faller
College of Nursing, Saint Alexius College, Koronadal, South Cotabato, Philippines
Aries Moralidad Baldonado
Medical Statistics Division, Department of Medical Research, Pyin Oo Lwin, Myanmar
Yadanar Aung
Institute for Population and Social Research, Mahidol University, Bangkok, Thailand
Department of Economics, Sridora Caculo College, Mapusa, Goa, India
Sharmila Mukund Borkar
Kabul University of Medical Sciences, Kabul, Afghanistan
Mohammad Yasir Essar
Department of Pharmaceutical and Health Service Research, Nepal Health Research and Innovation Foundation, Lalitpur, Province Bagmati, Nepal
Sunil Shrestha
Saw Swee Hock School of Public Health, National University of Singapore and National University Health System, Singapore, Singapore
KHANA Center for Population Health Research, Phnom Penh, Cambodia
Center for Global Health Research, Touro University California, Vallejo, California, USA
You can also search for this author in PubMed Google Scholar
Contributions
RRM conceived and designed the study. RRM and MZA performed validation and reliability of the questionnaire. RRM, KJ, KS, NTP, TR, EMF, AMB, YA, SMB collected the data. WS and RRM conducted the statistical analysis and interpretation of the findings. RRM, SY, MZA, WS, MYE, and SA wrote the initial draft. RRM, WS, MZA, SS and SY critically reviewed and finalized the manuscript. All authors read and approved the final version of this manuscript.
Corresponding author
Correspondence to Mohammad Yasir Essar .
Ethics declarations
Ethic approval and consent to participate, consent for publication.
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Marzo, R.R., Sami, W., Alam, M.Z. et al. Hesitancy in COVID-19 vaccine uptake and its associated factors among the general adult population: a cross-sectional study in six Southeast Asian countries. Trop Med Health 50 , 4 (2022). https://doi.org/10.1186/s41182-021-00393-1
Download citation
Received : 14 September 2021
Accepted : 20 December 2021
Published : 05 January 2022
DOI : https://doi.org/10.1186/s41182-021-00393-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Immunization
- Vaccine hesitancy
- Multi-country study
- Southeast Asia
Tropical Medicine and Health
ISSN: 1349-4147
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Loading metrics
Open Access
Peer-reviewed
Research Article
COVID-19 vaccine uptake and associated factors among adolescents and youths: Findings and implications for future vaccination programmes
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Department of Pharmacy, School of Health Sciences, University of Zambia, Lusaka, Zambia
Roles Project administration, Writing – original draft, Writing – review & editing
Affiliations Department of Public Health Pharmacy and Management, School of Pharmacy, Sefako Makgatho Health Sciences University, Pretoria, South Africa, South African Vaccination and Immunisation Centre, Sefako Makgatho Health Sciences University, Pretoria, South Africa
Affiliations Department of Pharmacology and Therapeutics, Ekiti State University, Ado Ekiti, Nigeria, Department of Medicine, Ekiti State University Teaching Hospital, Ado Ekiti, Nigeria
Roles Project administration, Validation, Writing – original draft, Writing – review & editing
Affiliations Department of Pharmacology, Therapeutics and Toxicology, Lagos State University College of Medicine, Ikeja, Lagos, Nigeria, Department of Medicine, Lagos State University Teaching Hospital, Ikeja, Lagos, Nigeria
Affiliation Department of Pharmacy Practice, Faculty of Pharmacy, Bahauddin Zakariya University, Multan, Pakistan
Roles Resources, Validation, Writing – original draft, Writing – review & editing
Affiliation Clinical Research Department, Faculty of Infectious and Tropical Diseases, London School of Hygiene &Tropical Medicine, London, United Kingdom
Roles Formal analysis, Investigation, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing
Affiliation Department of Public Health, Michael Chilufya Sata School of Medicine, Copperbelt University, Ndola, Zambia
Roles Supervision, Validation, Writing – original draft, Writing – review & editing
Affiliation Department of Medicines Control, Zambia Medicines Regulatory Authority, Lusaka, Zambia
Roles Conceptualization, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing
Roles Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing
Affiliations Department of Pharmacy, School of Health Sciences, University of Zambia, Lusaka, Zambia, HIV and Women’s Health Research Group, University Teaching Hospital, Lusaka, Zambia
Affiliation Department of Clinical Pharmacy, School of Pharmacy, Sefako Makgatho Health Sciences University, Pretoria, South Africa
Roles Project administration, Software, Validation, Writing – original draft, Writing – review & editing
Affiliation Department of Pharmaceutical Sciences, School of Pharmacy, Sefako Makgatho Health Sciences University, Pretoria, South Africa
Roles Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing
Roles Investigation, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing
- [ ... ],
Roles Formal analysis, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing
Affiliations Department of Public Health Pharmacy and Management, School of Pharmacy, Sefako Makgatho Health Sciences University, Pretoria, South Africa, Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow, United Kingdom
- [ view all ]
- [ view less ]
- Steward Mudenda,
- Johanna C. Meyer,
- Joseph O. Fadare,
- Olayinka O. Ogunleye,
- Zikria Saleem,
- Scott K. Matafwali,
- Victor Daka,
- Billy Chabalenge,
- Jacob Chama,

- Published: September 20, 2023
- https://doi.org/10.1371/journal.pgph.0002385
- Reader Comments
Adolescents and youths are a key part of the population that needs to be protected against the coronavirus disease 2019 (COVID-19). This is because they are more likely to spread the virus to vulnerable individuals. In view of these concerns, this study investigated the uptake of COVID-19 vaccines and associated factors among adolescents and youths attending secondary schools in Zambia. This cross-sectional study was conducted among 1500 school-going adolescents in Lusaka from September 2022 to November 2022. Overall, 1409 participants took part giving a response rate of 94%. Only 29.2% (n = 411) of the participants were vaccinated against COVID-19 at the time of the study. Compared to their unvaccinated counterparts, vaccinated adolescents and youths scored higher for knowledge (66.2% vs 57.8%) and attitudes (76.7% vs 39.4%) regarding COVID-19 vaccines. Healthcare workers, family/friends and social media were key sources of information regarding the vaccine. Factors associated with increased vaccine uptake were positive attitudes (AOR = 33.62, 95% CI: 19.92–56.73), indicating it was stressful to follow COVID-19 preventive measures (AOR = 1.47, 95% CI: 1.09–1.99), participants in Grade 12 (AOR = 3.39, 95% CI: 1.94–5.91), Grade 11 (AOR = 2.59, 95% CI: 1.94–5.91), Grade 10 (AOR = 3.48, 95% CI: 1.98–6.11) and Grade 9 (AOR = 3.04, 95% CI: 1.74–5.32) compared to Grade 8. This study found a relatively low uptake of COVID-19 vaccines among adolescents and youths in Zambia. There is a need to provide adequate strategies to address knowledge and attitude gaps regarding COVID-19 vaccines to improve uptake and reduce future morbidity and mortality.
Citation: Mudenda S, Meyer JC, Fadare JO, Ogunleye OO, Saleem Z, Matafwali SK, et al. (2023) COVID-19 vaccine uptake and associated factors among adolescents and youths: Findings and implications for future vaccination programmes. PLOS Glob Public Health 3(9): e0002385. https://doi.org/10.1371/journal.pgph.0002385
Editor: Collins Otieno Asweto, University of Embu, KENYA
Received: July 5, 2023; Accepted: August 22, 2023; Published: September 20, 2023
Copyright: © 2023 Mudenda et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All data is freely available for access and is part of the supplementary materials in this manuscript.
Funding: The authors received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic remains a serious burden and priority globally increasing morbidity, mortality and costs including economic costs from lockdwn measures especially in low- and middle-income countries (LMICs) [ 1 – 3 ]. In addition, placing an unparalleled burden on the education sector across countries, especially during the earlier stages of the pandemic [ 4 – 8 ]. Before the development of effective vaccines and treatments, countries typically introduced a variety of public health preventative measures [ 9 – 12 ]. These included restrictions on gatherings, social distancing and lockdown measures incorporating the closure of borders, transport restrictions, closure of clinics as well as the mandatory wearing of face masks in public places [ 9 – 11 , 13 – 16 ]. In many countries, lockdown measures also included the closure of classes with face-to-face learning in schools, colleges and universities [ 4 – 6 , 17 ]. These measures were instigated in order to minimise the spread of infection among pupils, students, their teachers, and family members [ 17 – 20 ]. Despite these measures, COVID-19 continued to spread across countries, exacerbated not only by the variable implementation of public health measures but also concerns regarding their effectiveness [ 9 , 12 , 21 , 22 ]. Consequently, there was an appreciable impetus globally to develop effective vaccines against COVID-19. One important impact in the case of children would be to restore routine immunisation programmes, which had been severely affected by the lockdown measures with considerable impact on future morbidity and mortality [ 23 – 25 ]. The primary goal of vaccination is to prevent pathogen-driven infections [ 26 – 29 ]. This maintains homeostasis by preventing infection-induced undesirable outcomes and training the immune system to re-program innate immune system cells to respond more effectively to future threats of the same antigenic type [ 30 ]. Their use to promote immunity and reduce the impact of infectious diseases has been reported in many areas and studies [ 30 – 35 ]. Following risk-benefit considerations, regulatory and medical decision-makers across countries sought to accelerate the development of COVID-19 vaccines to reduce the impact of the virus, which included the unintended consequences of lockdown and other measures [ 23 , 36 – 38 ]. This feat was unprecedented considering vaccine development traditionally takes decades [ 39 ]. The rapid development of COVID-19 vaccines was made possible through previous research experience and tools supported by new manufacturing platforms, structure-based antigen design, computational biology, protein engineering and gene synthesis [ 39 , 40 ].
The uptake of effective COVID-19 vaccines has varied across different populations and countries [ 41 – 47 ]. In general, COVID-19 vaccines have been well-received, with many people eager to be vaccinated in order to protect themselves and their communities from the virus [ 43 , 48 – 51 ]. However, some groups across countries have been hesitant due to a variety of reasons, which have been fuelled by misinformation and/ or distrust of the vaccines [ 52 – 57 ]. The reasons for vaccine hesitancy are complex and vary across groups, time and regions [ 43 , 46 , 47 , 58 ]. For example, surveys conducted among healthcare workers (HCWs) and students in various African countries found persistent vaccine hesitancy, which was principally due to concerns regarding their effectiveness and safety [ 47 , 59 – 63 ]. However, higher acceptance rates have been reported among some European countries compared to the United States (US) and some African countries [ 43 , 47 , 51 , 58 , 64 , 65 ].
Demographic and socio-economic characteristics also appear to influence the uptake of COVID-19 vaccines. Certain racial and ethnic groups, alongside lower-educated and lower-income people, are typically less likely to receive vaccines. This is potentially due to a lack of access as well as longstanding mistrust of the medical system [ 43 , 66 , 67 ]. Public health officials need to address these disparities, alongside ongoing misinformation, to ensure vaccines are readily available, accessible, and administered to all members of society to maximise the benefit of vaccines [ 47 ].
Children, adolescents, and youths attending school are key among the vulnerable populations at risk of contracting COVID-19 and subsequently spreading the virus, especially as vaccination in populations aged 12 to 17 years has only recently been approved, with hospitalisation rates increasing in countries pre-vaccination [ 68 , 69 ]. To date over 17,000 children worldwide have died from COVID-19, and in the USA, COVID-19 was the seventh leading cause of death among children aged 5–11 years, and even higher for children aged 12–18 years leading to calls to vaccinate all children above the age of 5 [ 70 , 71 ]. They also remain a key group for herd immunity, and encouraging vaccination in this population will help protect children and the community in the future [ 72 ]. There are also growing concerns about possibly increased mortality in this group in LMICs, enhanced by new variants [ 73 , 74 ]. In addition, there is currently overuse of antibiotics when children with actual or suspected COVID-19 are admitted to the hospital, increasing antimicrobial resistance (AMR) [ 75 – 78 ]. AMR is a key concern in sub-Saharan Africa with high and growing rates, further increasing morbidity, mortality and costs [ 79 – 81 ].
Consequently, vaccination against COVID-19 is of increasing importance in this population to protect them from severe disease, including subsequent hospital admission, if contracted [ 68 , 72 , 82 , 83 ]. However, vaccine hesitancy has been reported among adolescents in sub-Saharan Africa [ 84 ]. This is a concern as low acceptance of the COVID-19 vaccines may eventually lead to low uptake as seen with other vaccines [ 85 , 86 ]. In addition, low uptake of this vaccine may reduce the uptake of other vaccines against infectious diseases in Africa, with vaccination rates already severely affected by lockdown and other measures in the early stages of the pandemic [ 23 , 24 ].
A low acceptance rate of COVID-19 vaccines was reported among pupils attending secondary schools in Zambia, despite most of them having good knowledge and attitudes towards the vaccine [ 87 ]. However, there is currently insufficient information regarding the actual uptake of COVID-19 vaccines among adolescents and youths attending secondary schools in Zambia to further refine policies to improve future vaccination rates in this critical group. We also believe it is critical to compare and contrast attitudes towards the vaccine among both vaccinated and unvaccinated populations to provide future guidance to key stakeholders in Zambia if concerns persist. Alongside this, the Zambian government recently (January 2022) commenced COVID-19 vaccination for all children from the age of 12 to 17 years [ 47 ], with 222,300 doses Pfizer-BioNTech vaccine being the first to be received for this role [ 88 ]. Consequently, this study investigated the uptake of COVID-19 vaccines and associated factors among adolescents and youths attending secondary schools in Lusaka, Zambia. The findings from this comprehensive approach can be used to develop more robust age-appropriate interventions to promote confidence in the vaccines in this important sub-population in Zambia and beyond given the ongoing public health concerns.
Materials and methods
Study design, setting and population.
This cross-sectional study involved both vaccinated and unvaccinated pupils aged 12 years and older attending secondary schools in Lusaka. Lusaka was chosen for this study because it is Zambia’s capital city and the country’s first COVID-19 epicentre [ 89 , 90 ]. The findings will help determine the COVID-19 vaccine uptake among the secondary school population in Lusaka city, and not just intentions, and to more comprehensively explore potential associations between vaccination uptake and participants’ knowledge and attitudes. Secondary schools in Zambia provide education (Levels: Grade 8 to Grade 12) to adolescents (defined as persons aged 10 to 19 years) and youths (defined as persons aged 15 to 35 years) [ 91 ]. We appreciate that up to 35 years is typically seen as old for youths; however, this is the current situation in Zambia.
Sampling method and sample size calculation
Multi-stage sampling was used to select 32 out of 111 secondary schools in Lusaka city, representative of the four sub-districts, with two to four classes selected, randomly and proportional to the school size [ 87 ]. Participants from the selected classes were recruited using simple random sampling (computer-generated random numbers without replacement). The effective sample size for a single population was estimated at 543, using the Raosoft software http://www.raosoft.com/samplesize.html (accessed on 22 July 2022). A 10% provision was made for possible losses, incomplete, or non-responses and a design effect of 1.5 was used to account for the loss in precision due to clustering.
Data collection process and tool
A pre-validated, self-administered questionnaire was used to collect data [ 87 ]. The questionnaire consisted of five parts, collecting data on the following components: i) Sociodemographic characteristics of the students; ii) their knowledge of COVID-19 vaccines; iii) Attitudes towards COVID-19; iv) Acceptance and uptake of COVID-19 vaccines; v) and key factors that could influence the acceptance and uptake of COVID-19 vaccines ( S1 Text ).
A pilot study was undertaken before the full study to check for the feasibility and simplicity of the questionnaire to enhance its robustness. No changes were subsequently made to the questionnaire. The final questionnaire had acceptable reliability (Cronbach’s alpha of 0.842 for attitude and 0.762 for knowledge) and required 20 to 30 minutes to complete. The findings from the pilot study were not included in the main study findings and statistical analysis. Data regarding the vaccinated and unvaccinated participants were collected between September 2022 and November 2022 by two trained data collectors. Participants were first informed about the study. Upon providing written assent from the youths or obtaining written consent from the parents and/or guardians of adolescents, the questionnaire was subsequently distributed to the adolescents and youths attending the selected secondary schools. The findings were subsequently compared to the previous study on intentions to vaccinate against COVID-19 to help formulate pertinent strategies to improve vaccination rates in this population where concerns were identified [ 87 ].
Data management and analysis
Data were entered and cleaned for consistency and range checks in Microsoft Excel® (Redmond, WA) ( S1 Data ). The data were subsequently exported to Stata® version 17/BE (Stata Corp., College Station, Texas, USA) for statistical analysis. Knowledge and attitude questions were assigned a score of one for “Yes” responses and a zero for “No or don’t know” responses. The item scores were summed to create a total score for knowledge and attitude. Descriptive analyses were subsequently performed to assess knowledge and attitude scores for vaccinated participants.
Robust estimations of standard errors were used to account for the clustering of participants within schools in all analyses. Clopper-Pearson’s exact method was subsequently used to calculate the 95% binomial confidence interval (CI) of the overall proportion of vaccinated pupils. Following this, we fitted univariable logistic regression models with robust estimation of standard errors with ‘COVID-19 vaccine uptake’ as the outcome variable and one of the predictor variables at a time, to assess evidence of an association with COVID-19 vaccine uptake by assessing crude odds ratios (CORs). Subsequently, a multiple logistic regression model was fitted with variables that were significant in the univariable model at p<0.2 using investigator-led stepwise regression, with only knowledge and attitudes set as priori variables.
Interactions between significant predictors in the final model were subsequently considered; however, none reached statistical significance considered as p≤0.05. Finally, adjusted odds ratios (AORs) at 95% CI were used to identify statistically significant predictors of COVID-19 vaccine uptake. The goodness of fit of the model was assessed using the Hosmer-Lemeshow test.

Ethical approval
Ethical approval was granted by the University of Zambia Health Sciences Research Ethics Committee (UNZAHSREC). The study was approved under the protocol ID: 202211231183, IORG no: 0009227, IRB no: 00011000. Clearance to collect data was also obtained from the Lusaka District Education Board (DEB) and the management of the selected schools. We informed the participants of the purpose of this study and they assented to be part of the study. Additionally, we obtained consent from the participant’s parents or guardians for the adolescents to participate in the study. The youths provided consent on their own to be part of the study.
Inclusivity in global research
Additional information regarding the ethical, cultural, and scientific considerations specific to inclusivity in global research is included in the S2 Text .
Response rate
In total, 1500 questionnaires were distributed to the combined study population, of which 1409 completed questionnaires were returned (94% response rate). The final sample size provided more than 80% power to detect the minimum difference in the rates of COVID-19 vaccine uptake among secondary school-attending adolescents and youths with precision ±5% and 95% CIs.
COVID-19 vaccine uptake, sociodemographic characteristics, knowledge and attitudes
Overall, 29.2% (95% CI: 26.8%-31.6%) of participants had received at least one dose of the COVID-19 vaccine. The mean age of the participants was 15.8 years [Standard Deviation (SD) = 1.9], ranging from 12 to 26 years. The largest proportion of participants (n = 929, 65.9%) were females, 1336 (94.8%) were living at home with their parents and 363(25.8%) were in Grade 11 ( Table 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pgph.0002385.t001
Approximately three in five participants reported HCWs, family/friends and social media as the principal sources of information regarding the COVID-19 pandemic. Overall, participants reported a mean COVID-19 vaccine knowledge and attitude percentage score of 60.3% (SD = 28.4) and 50.3% (SD = 37.2), respectively. Compared to participants who were not vaccinated, the vaccinated participants had greater knowledge (66.2% vs 57.8%) and attitude (76.7% vs 39.4%) scores regarding the COVID-19 vaccine ( Table 1 ).
COVID-19 experience
The majority (n = 1187, 84.2%) of the participants had not suffered from COVID-19, did not know a relative/friend who died from COVID-19 (n = 1009, 71.6%) and had never been quarantined due to COVID-19 (n = 991, 70.3%).
Approximately half (n = 680, 48.3%) of the participants knew a relative/friend who suffered from COVID-19 disease, and just over half (n = 765, 54.3%) reported that preventive measures were not stressful to follow. A large proportion of participants (n = 1087, 77.2%) were able to practice social distancing, and an appreciable number (n = 1244, 88.3%) had never suffered from a chronic condition ( Table 2 ).
https://doi.org/10.1371/journal.pgph.0002385.t002
Predictors of COVID-19 vaccine uptake
The multivariable logistic regression model showed that independent factors associated with COVID-19 vaccine uptake were attitude, school grade and COVID-19 preventive measures being stressful to follow ( Table 3 ).
https://doi.org/10.1371/journal.pgph.0002385.t003
Participants with higher attitude scores (AOR = 33.62, 95% CI: 19.92–56.73) and those who reported that COVID-19 preventive measures were stressful to follow (AOR = 1.47, 95% CI: 1.09–1.99) were more likely to be vaccinated against COVID 19. In addition, participants who were in Grade 12 (AOR = 3.39, 95% CI: 1.94–5.91), Grade 11 (AOR = 2.59, 95% CI: 1.94–5.91), Grade 10 (AOR = 3.48, 95% CI: 1.98–6.11) or Grade 9 (AOR = 3.04, 95% CI: 1.74–5.32) were more likely to be vaccinated against COVID 19 versus those in Grade 8 ( Table 3 ).
We believe this is the first study in Zambia to investigate the current uptake of COVID-19 vaccines among adolescents and youths attending secondary schools, where the national mass COVID-19 vaccine administration program is taking place in the country. Overall, the vaccine uptake among this population at the time of the study was 29.2% (n = 411). The vaccine uptake was higher among participants with better COVID-19 knowledge and attitude scores as well as higher school grades. Encouragingly, the actual uptake of the COVID-19 vaccine was higher than the findings of an earlier study among unvaccinated school children and adolescents in Zambia where only 12.7% of participants indicated that they would accept to be vaccinated with COVID-19 vaccines [ 87 ]. This shows the benefit of conducting studies not only about intentions but also about subsequent actions to guide future strategies.
However, the low uptake of COVID-19 vaccines among school-going adolescents and youths in our study is a public health concern. Having said this, the uptake rate was similar to the findings from a multi-country study conducted in the Eastern Mediterranean region at 32% [ 92 ], and in the US where only 25.6% of the adolescents in the study were vaccinated [ 93 ]. This though has increased in the US in recent months [ 69 , 94 ]. We are also aware in the study of Wang et al. (2022) that only 12% to 14% of adolescents in Tanzania were willing to be vaccinated, and only 35% of adolescents in Nigeria [ 84 ]. These combined findings could be due to limited knowledge and negative attitudes among school adolescents and youths regarding COVID-19 vaccines. Alongside this, the overall attitude of adult residents in their communities regarding COVID-19 vaccination may have some influence on our study population. This is a concern as low uptake of COVID-19 vaccines can affect national goals of protecting the majority of school-going children, adolescents and youths against the coronavirus and its effects.
Encouragingly, a recent study in Italy reported a vaccination rate of 87.3% among school-going adolescents [ 95 ]. Additionally, in Turkey, the vaccination rate among youths was 80% [ 96 ]. Alongside this in Kersa, Ethiopia, 86% of adolescents stated they would seek vaccination once available [ 84 ]. In Turkey, the youths wanted to be vaccinated to protect their health as well as the health of their families and relatives [ 96 ]. In Brazil, a recent study reported that 88.7% of caregivers wanted their children to be vaccinated while 77.6% of parents in China wanted their children to be vaccinated against COVID-19 [ 97 , 98 ].
In the US, the authors found that parental confidence in the COVID-19 vaccines was critical to increasing the number of children and adolescents vaccinated [ 69 ]. This is similar to other countries and other vaccines for children, including HPV vaccines [ 84 , 92 , 99 – 101 ]. These findings indicate a key role of parents and caregivers in improving the acceptance and uptake of COVID-19 vaccines among children and adolescents.
The main sources of information on COVID-19 among the study participants were mass media, HCWs, and social media. Others have also reported that these sources of information are influential in spreading correct information and misinformation about COVID-19 vaccines [ 56 , 102 – 107 ]. Similarly in Taiwan, among university students in Jordan, and among adolescents across Africa, the internet and social media were the most common sources of information regarding COVID-19 and its vaccines [ 84 , 108 , 109 ]. This shows that mass media should increasingly be used as a platform for sharing credible information about COVID-19 vaccines and their benefits as well as addressing misinformation to reduce the morbidity, mortality and costs associated with COVID-19 [ 110 , 111 ].
Concerns regarding the effectiveness and safety of COVID-19 vaccines among HCWs need also to be addressed going forward as HCWs play a critical role in sharing information about COVID-19 vaccines [ 47 , 112 – 115 ]. Traditional sources of information such as television and radio have also been used as key sources of information on COVID-19 and its vaccines in many settings [ 116 – 118 ]; however, their role is changing in favour of social media and other platforms. This needs to be taken into consideration when health authorities and Governments are developing and refining their strategies to address vaccine hesitancy generally.
We found that most of the vaccinated adolescents and youths in our study had relatives or friends that had suffered or died from COVID-19. This suggests that individuals who have been directly impacted by the virus due to sickness or the death of loved ones may be more inclined to seek vaccination as a means of protecting themselves and family members, which is similar to other studies [ 92 , 115 , 119 – 121 ]. Public health campaigns need to recognise and address this motivation in order to effectively promote vaccination and mitigate the spread of COVID-19 amongst this key group.
Additionally, we found that some of the vaccinated participants had previously been quarantined due to COVID-19 exposure or had a pre-existing chronic condition. This suggests that individuals who are at higher risk for severe illness from COVID-19, especially those with co-morbidities, may be more likely to prioritise vaccination to protect their health, again similar to other studies [ 120 , 122 – 125 ]. Consequently, future public health campaigns need to consider these additional risk factors as well and target outreach and education efforts towards individuals who may be more vulnerable to severe COVID-19 illness. Together with this, parents alongside key HCWs are important targets for vaccinating children, adolescents, and youths.
We also found that participants who had higher odds of being vaccinated were in higher grades (Grade 9, -10, -11, and -12 compared to Grade 8). In addition, those that found prevention measures stressful to follow had higher odds of being vaccinated, with a likely reduction in preventative measures as more of the population become vaccinated. These findings imply that promoting vaccine uptake can be achieved by targeting appropriate socio-demographic populations and those with negative attitudes and poor knowledge concerning COVID-19 vaccines [ 126 – 128 ]. This is also evidenced by previous studies that have shown that parents’ and caregivers’ positive attitudes can promote vaccine acceptance and uptake[ 121 , 129 – 132 ]. This further emphasises the role of governments, HCWs, teachers and other stakeholders in promoting positive messages regarding the positive effects of COVID-19 vaccines among school children, adolescents and youths as well as adults to counteract ongoing misinformation.
We are aware of a number of limitations of this study. Firstly, this study was only conducted in a population of adolescents and youths attending secondary schools in Lusaka, hence, generalisation of the findings should be done with caution. Consequently, future studies should investigate the entire population of schooling and non-schooling adolescents and youths in Zambia. Secondly, this was a survey as opposed to an in-depth qualitative discussion with participants. Thirdly, being a cross-sectional study in nature, our findings may be affected by recall bias. Finally, we did not ask for the name of the COVID-19 vaccine that each vaccinated participant had received. However, despite this, we consider our findings robust given the choice of the setting and the extensive methodology involved. Future studies can be conducted to investigate further factors that contribute to vaccine hesitancy among schooling and non-schooling adolescents and youths in Zambia as the roll-out of the vaccine continues in this younger age group. The findings can subsequently be used to develop future strategies, including social media strategies, to enhance vaccine acceptance and uptake in this critical population, with implications for other infectious diseases.
We found a relatively low uptake of the COVID-19 vaccines among adolescents and youths attending secondary schools in Zambia. The low uptake of the COVID-19 vaccine generally among adolescents and youth is a public health concern as this may hinder the goals of vaccination programmes against COVID-19 as well as generally among this population. Consequently, there is a need to develop mainstream youth-friendly educational interventions and associated communication programs to enhance the acceptance and uptake of COVID-19 vaccines in adolescents, and youths. Further studies should be undertaken to monitor this.
Supporting information
S1 text. study questionnaire..
https://doi.org/10.1371/journal.pgph.0002385.s001
S1 Checklist. STROBE statement—checklist of items that should be included in reports of observational studies.
https://doi.org/10.1371/journal.pgph.0002385.s002
S1 Data. Study dataset.
https://doi.org/10.1371/journal.pgph.0002385.s003
S2 Text. Plos GPH questionnaire.
https://doi.org/10.1371/journal.pgph.0002385.s004
Acknowledgments
We are grateful to the adolescents and youths who participated in this overall study. We would also like to acknowledge the Lusaka District Education Board (DEBs) for accepting our request to collect data from the schools in Lusaka.
- 1. WHO. WHO Coronavirus (COVID-19) Dashboard. 2022. Available at URL: https://covid19.who.int/ .
- View Article
- PubMed/NCBI
- Google Scholar
- 70. UNICEF. Child mortality and COVID-19. March 2023. Available at URL: https://data.unicef.org/topic/child-survival/covid-19/ .
- 71. Johns Hopkins Bloomberg School of Public Health. 10 Reasons Your Child Should Get Vaccinated for COVID-19 as Soon as Possible. Available online: https://publichealth.jhu.edu/2021/10-reasons-your-child-should-get-vaccinated-for-covid-19-as-soon-as-possible .
- 88. Kunda, J. Zambia Receives over 222,000 More COVID Jabs to Vaccinate Children Available online: https://www.aa.com.tr/en/africa/zambia-receives-over-222-000-more-covid-jabs-to-vaccinate-children/2473218 .
- 91. Department of Public Health, Ministry of Health, GOVERNMENT OF THE REPUBLIC OF ZAMBIA. Adolescent Health Strategy 2017 to 2021. 2017 Available at URL: https://aaazambia.org/download/adolescent-health-strategy-2017-21/ .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 31 August 2023
People’s perceptions on COVID-19 vaccination: an analysis of twitter discourse from four countries
- Manah Verma 1 na1 ,
- Nikhil Moudgil 1 na1 ,
- Gaurav Goel 2 ,
- Peehu Pardeshi 3 , 4 ,
- Jacquleen Joseph 3 ,
- Neeraj Kumar 1 , 5 , 6 , 7 , 8 ,
- Kulbir Singh 9 ,
- Hari Singh 10 &
- Prakash Babu Kodali 11
Scientific Reports volume 13 , Article number: 14281 ( 2023 ) Cite this article
2768 Accesses
1 Citations
5 Altmetric
Metrics details
- Health care
- Health policy
- Health services
- Public health
More than six and half million people have died as a result of the COVID-19 pandemic till Dec 2022. Vaccination is the most effective means to prevent mortality and infection attributed to COVID-19. Identifying public attitudes and perceptions on COVID-19 vaccination is essential to strengthening the vaccination programmes. This study aims to identify attitudes and perceptions of twitter users towards COVID-19 vaccinations in four different countries. A sentiment analysis of 663,377 tweets from October 2020 to September 2022 from four different countries (i.e., India, South Africa, UK, and Australia) was conducted. Text mining using roBERTA (Robustly Optimized Bert Pretraining approach) python library was used to identify the polarity of people’s attitude as "negative", "positive" or "neutral" based on tweets. A sample of 2000 tweets (500 from each country) were thematically analysed to explore the people’s perception concerning COVID-19 vaccines across the countries. The attitudes towards COVID-19 vaccines varied by countries. Negative attitudes were observed to be highest in India (58.48%), followed by United Kingdom (33.22%), Australia (31.42%) and South Africa (28.88%). Positive attitudes towards vaccines were highest in the United Kingdom (21.09%). The qualitative analysis yielded eight themes namely (i) vaccine shortages, (ii) vaccine side-effects, (iii) distrust on COVID-19 vaccines, (iv) voices for vaccine equity, (v) awareness about vaccines, (vi) myth busters, (vii) vaccines work and (viii) vaccines are safe. The twitter discourse reflected the evolving situation of COVID-19 pandemic and vaccination strategies, lacunae and positives in the respective countries studied.
Similar content being viewed by others
Harnessing Twitter data to survey public attention and attitudes towards COVID-19 vaccines in the UK
Evaluation of Twitter data for an emerging crisis: an application to the first wave of COVID-19 in the UK

Political context of the European vaccine debate on Twitter
Introduction.
Vaccination plays an important role in prevention of mortality and morbidity associated with infectious diseases. The ongoing Corona Virus Disease (COVID-19) had an unprecedented impact on global health systems and economies 1 . Achieving significant coverage of vaccination is the only long-term solution for the COVID-19 crisis. Internationally, the countries are focusing towards increasing the coverage of COVID-19 vaccination with booster dose being administered. COVID vaccines are now available for administration to individuals aged 5 years and above providing a significant protection from infection 2 , 3 . Studies indicate that Vaccines are effective in preventing COVID-19. However, despite vaccines being available in several countries, evidence of hesitancy towards COVID-19 vaccines emerge indicating it as a major public health challenge 4 .
Vaccine hesitancy is characterized as a delay in accepting or refusing vaccines despite the availability of vaccine services. It is impacted by factors such as complacency, indifference, and fear 5 . The World Health Organization (WHO) named “vaccine hesitancy” as one of the top global health threats 6 . Vaccine hesitancy and vaccine refusal is known to be associated with the outbreaks and reemergence of vaccine preventable diseases 4 . Intentional under vaccination was documented to be a reason for measles outbreaks even in highly vaccinated population 7 . Modelling studies reported that vaccine refusal can potentially lead to resurgence of infectious diseases such as measles, chickenpox and rubella with greater or severe illness 4 . Vaccine hesitancy is a key challenge in tackling the ongoing COVID-19 pandemic. Low vaccine coverage and vaccine hesitancy prolongs high incidence of COVID-19 cases, result in pocketed outbreaks and poses the risk of emergence of new strains of the infectious virus (e.g. BF.7). Evidence from European region reported the presence of mistrust and negative attitudes towards COVID-19 vaccines, even before the vaccines were publicly available 8 . Before the first vaccine was released, the percentage of individuals hesitant to take COVID-19 vaccines in Europe ranged between 13.9% and 43.7% 9 , 10 , 11 , 12 . In India, vaccine hesitancy rates among medical students were around 10.6% 13 . A 2020 global vaccine survey of 19 major economies reported that India and South Africa had vaccine acceptance rates of 74.53% and 79.79%, whereas UK had the acceptance rate of 71.48% 12 . In this regard, studying people’s attitudes towards vaccination is essential to inform public health decision making.
Social media is a major tool in new age public discourse including on key matters of public health importance such as vaccination. Studies have observed that the surge in public discourse on social media is linked to recent outbreaks of Ebola and Zika 14 , 15 . A recent systematic review concluded that individual networks within social media allow misinformation to flourish in the like-minded circles often resulting in pseudoscientific practices, conspiracy theories and hesitancy towards interventions such as vaccination 16 . Social media such as Twitter and Facebook served as key platforms for public discussion on the matters concerning COVID-19. A study analyzing over 45 million tweets during January 2020 to January 2021 reported a greater amount of twitter discourse on vaccine hesitancy and rejection compared to vaccine acceptance 17 . A recent study investigating 1.49 million unique tweets from 583,499 users from 11th March 2020 to 31st January 2021 reported that trust was the predominant emotion, followed by anticipation, fear and sadness 18 .
While the studies analyzing twitter discourse reported the varied underlying emotions, belief systems and attitudes towards COVID-19 vaccines, majority of the studies focused on twitter discourse up to January 2021. However, the accessibility and availability of COVID-19 vaccination globally improved only after March 2021. Moreover, several countries (including India & UK) witnessed multiple waves and variants of COVID-19 (Fig. 1 ). Understanding social media discourse in corroboration with developments in field of COVID-19 vaccination is helpful making conclusive interpretations of people’s perceptions towards COVID-19 vaccination programmes. Moreover, the observations can also facilitate improving the essential vaccination campaigns and predict the direction of public discourse in future outbreaks. The social media posts, specifically the twitter tweets are an excellent source of data to understand behavioral attitudes of a larger population concerning a specific idea.
Figure outlining the timeline of events concerning COVID-19 in studied countries.
Machine learning tools such as sentiment analysis are popularly used to analyse social media post and analyse people’s perception towards shared experiences such as vaccination. It is a systematic procedure of extracting, preparing and analyzing large volume of text data sharing similar characteristics (such as keywords and hashtags#). The countries India, South Africa, Australia, and United Kingdom are among the largest uses of twitter platform. The countries also account for among the highest doses of COVID-19 vaccines administered in their respective regions. Employing the tool of sentiment analysis, to analyse the tweets on COVID-19 vaccination in these countries the current study aims at addressing the following objectives.
To assess the attitudes towards COVID-19 vaccination in selected developed and developing countries.
To explore public perceptions on COVID-19 vaccination in the selected countries.
Materials and methods
This research paper uses twitter data (i.e., tweets) to study the attitudes of people towards vaccination in different countries. Twitter is one of the most popular microblogging platforms, with a user base of 360 million active users in 2022. Countries were chosen so that analysis can be performed to study public perception towards COVID-19 vaccines in developed and developing country settings. India and South Africa were countries chosen to represent the developing countries whereas Australia and United Kingdom represented developed countries.
The data collected included the tweets extracted from the date of release of the first vaccine in the respective country till 30 September 2022. The dataset included all the tweets written in the English language related to the COVID-19 vaccine. The English language was preferred since the model used for sentimental analysis works only on tweets written in English. The tweets were analysed numerically and qualitatively.
Numerical analysis
The numerical analysis of tweets was conducted to assess the attitudes towards COVID-19 vaccines across the countries. The numerical analysis followed the stages of (i) extraction, (ii) pre-processing and (iii) processing.
(i) Extraction
For extracting tweets, an automated web scraping tool known as “snscrape” [ https://github.com/JustAnotherArchivist/snscrape ] was used. Due to high pliancy, snscrape was the most appropriate and advanced scraping tool which directly searches the web for analyzing tweets of a certain region for a certain period. Snscrape has no limit on the number of tweets that can be extracted which makes it better than twitter’s own application programming interface.
Snscrape was configured according to the geographical location of the country, the date when the first vaccine was released there, and the language preferences. The raw data consisted of the union of all tweets, extracted using the query ‘COVID-19 Vaccine’ for each country.
(ii) Pre-processing
A tweet is a post on Twitter that can be up to 140 characters long, including spaces, emoji or Emoticon, hashtags, URLs, "@" mentions (Twitter handles), etc. Using REGEX (Regular expressions), these strings were removed from the tweets to make the data less chaotic before giving it as an input to machine learning algorithms. Regular expressions, also called REGEX, are used to search, extract, manipulate, and validate specific string patterns 19 . The tweets (which were not refined by snscrape) containing words from any other languages other than English were filtered out. After complete processing, a final number of 663,377 tweets were extracted for analysis. Country-wise distribution of the extracted tweets is shown in Table 1 .
(iii) Processing
We conducted sentiment analysis to identify attitudes of people regarding vaccination in developed and developing countries 20 . Based on the sentiments identified the attitudes of the twitter users based on the tweet was categorized into one of the three categories, i.e., positive, negative, or neutral. The RoBERTa (Robustly Optimized Bert Pre-training Approach) pre-trained model was used in this study. The pre-trained version of RoBERTa was trained on approximately 58 Million tweets 21 . Previously RoBERTa was found to have an 89% accuracy and 87% precision in undertaking sentiment analysis of COVID-19 tweets 22 .
The pre-processed tweets are passed to the tokenizer, which was a part of the pre-trained model. The encoded input is later passed into the RoBERTa model (pre-trained on tweets). The output was weights for the sentence to be negative, neutral, or positive, present at the[0][0] index of the output. The weights were extracted using detach function and converted into a numerical python array. Later softmax transformation was used to generate probabilistic overview for the country specific tweets being negative, neutral and positive. To ensure consistency and accuracy of the RoBERTa model in our study settings, we analysed a random sample of 400 tweets (100 per country) both manually and using RoBERTa and compared the findings. We computed Krippendorff’s alpha (α) to compare the agreement between manual estimation and RoBERTa. The value of α for positive tweets was 0.73, negative tweets was 0.74, and neutral tweets was 0.67 indicating a substantial agreement 23 .
Word cloud was generated from the tweets processed from each country to provide a visual representation of most frequent terms represented in tweets concerning COVID-19 vaccines.
Qualitative analysis
The qualitative analysis was conducted to explore the views of public towards COVID-19 vaccination. The tweets were analysed manually to generate themes reflecting the public perception towards COVID-19 vaccines 24 .
A random sample of 500 tweets from each country (i.e., total of 2000 tweets) were extracted manually and analysed. Tweets which were in English, and having more than five words were included. Tweets which exclusively used non-alphabetical characters, religious/political campaigns and those with fewer words (less than five) were excluded. The extracted textual data in the form of tweets was inductively coded to develop initial codes. The codes were then processed to yield subthemes. The subthemes were amalgamated to form themes.
Ethics declarations
Ethical approval and informed consent.
This study is a secondary analysis of a large dataset of over 0.66 million tweets between December 2020 and September 2022. Due to the vast volume of tweets and secondary nature of the data, obtaining informed consent from individual users was not practically feasible. As the data were publicly available tweets, there was no direct data collection from human participants. This eliminated the need for ethical clearance, typically required when collecting data directly from individuals. However, we ensured that all relevant ethical guidelines were adhered to while handling, analysing, and presenting the data.
First, all data extraction and curation processes were designed to exclude any personally identifiable information (PII) from the dataset. Specifically, the USER ID was removed from the dataset and replaced with a specific alphanumeric ID based on county and the serial number of extracted tweet. This was crucial to maintaining user anonymity and confidentiality. Moreover, during the analysis and presentation of the findings, great care was taken to present the results at a country level or in an aggregated form. This approach minimized the possibility of identifying any single user, further safeguarding the privacy of individuals contributing to the tweets in the dataset. Furthermore, the extracted data files were archived and stored securely after removing personal identifiers to maintain data security and confidentiality. Only the research team members had access to the data, and no information was shared with any third party outside of the research team. This strict control over data access and sharing contributed to the protection of user privacy. We ensured data minimization, extracting only the data directly relevant to the research questions, reducing the potential impact on user privacy. We respected user settings and adhered to the terms of service and privacy settings provided by the social media platform.
Numerical findings
A total of 663,377 tweets were extracted and analysed. Figure 2 depicts the results of sentiment analysis of tweets from each country in terms of positive, negative as well as neutral tweets. While the maximum number of neutral tweets (have very little subjectivity linked to them) are from South Africa (63.88%), the minimum number of positive tweets (7.40%), as well as negative tweets (28.88%), were also extracted from South Africa only. India had highest percentage of negative tweets (58.48%) as compared to South Africa (28.88%). United Kingdom reported highest percentage of positive tweets (21.09%) as opposed to South Africa (7.40%).
Graphical representation of percentage of negative, positive and neutral attitudes.
The word cloud reflects the most frequently reported terms. In India, the words “government”, “Patients”, “Stay Safe”, “Second Wave”, “Hospital”, “Situation”, and “Help” were most frequently observed. In contrary, in Australia “Fully vaccinated”, “get vaccinated”, “variants”, “public health”, “support” etc. were most frequent words. Figure 3 outlines the word clouds from the four countries studied.
Word cloud generated by analysing the tweets. Footnotes: (1) India; (2) South Africa; (3) United Kingdom; (4) Australia.
Qualitative findings
Several key themes emerged from the analysis of tweets sampled (Fig. 4 ). It was observed that the twitter served as a platform for advocacy, community engagement, information sharing, myth busting, awareness building, and reporting COVID-19 vaccination shortcomings. The themes reflected on the positive/negative attitudes of individuals towards COVID-19 Vaccines. Figure 4 outlines the themes emerged from the analysis of the tweets.
Figure outlining the themes identified across the polarity.
Theme 1: awareness about vaccines
One of the commonly observed positive sentiment towards vaccination was spreading awareness about the COVID-19 vaccines, their availability and vaccination drives. Specifically, the twitter handles of national and local government machineries, civil society bodies and celebrities focused on sharing the information on vaccination, which were actively retweeted by other individuals. Such awareness about COVID-19 vaccines was observed alike for initial and booster doses of vaccines. One of the tweets from UK a country with a high vaccination rate said the following:
“Time to take up your #COVID19 booster jabs or your first or second vaccinations if you have not had them. If you have just become eligible for you #boosterjab wait to be contacted to invite you for vaccination. See below for current walk in vaccination sites. https://t.co/lLIkNlhlfx ” (UK 05).
While countries like India faced vaccine shortages initially, the vaccination programmes amped as time progressed. Technology such as CoWIN platform was leveraged to aid the vaccination drive and develop awareness about vaccination.
“Book your vaccination on the given link, https://t.co/VkLfexCFiO , Current availability slot #Mumbai”(India 79583)
The information shared using the twitter handles served in better transmission & awareness about COVID-19 vaccination, positively contributing to vaccine coverage.
Theme 2: vaccines work
A commonly observed positive tweets among twitter users is concerning the effectiveness of vaccines. The idiom “Vaccines Work” was a common catchphrase used by the national and regional governments, politicians, bureaucrats, health workers and celebrities across the countries. The users shared posters, web links, videos, statistics and research articles reporting efficacy and effectiveness of vaccines contributing to spreading a positive word about COVID-19 vaccines. Individuals even shared their real-life experiences touting that being unvaccinated increases the risk of COVID-19.
“I was shocked at the proportion of unvaccinated pregnant women now in ICU..….. Remember the vaccines work and are safer than encountering COVID19 first” (UK 7142). “Half a million lives saved because of the COVID-19 vaccine. Vaccines work!” (Australia 108693).
As the COVID-19 pandemic progressed and new variants emerged the tweets were also observed to be reporting the effectiveness of vaccines on the multiple variants of COVID. Some of the tweets reporting effectiveness of vaccines towards new COVID-19 variants said the following.
“Good news! “Two doses of Pfizer jab are 90% effective against Covid-19 hospital admission for all variants for at least six months, according to a study”.”(UK 4670) “Before anyone jumps to conclusion that @Pfizer-BioNTech #vaccine doesn't work against #COVID19 #DeltaVariant 3rd wave in #SouthAfrica. Data from #Israel show that it does. Spread the word. https://t.co/NbJ4XvJwNK ” (UK 11971).
These messages enabled a positive discourse on the effectiveness of the COVID-19 vaccines, despite of the fears of new variants.
Theme 3: vaccines are safe
The safety of the vaccines was another important domain found to be repetitive across the countries studied. Given the apprehensions surrounding the safety of COVID-19 vaccines, the twitter users largely advocated that the vaccines are safe to use. As the science of COVID-19 vaccines evolved the specific information concerning the safety of vaccines (among younger age groups, pregnant women etc.) was also reported. Twitter handles from India and South Africa tweeted the following.
“in South Africa, 71 k people have died of Covid-19, 7 million are Vaccinated and are fine a few with some side effects. It’s not rocket science, vaccinate.”(South Africa 42666). “The fourth and the biggest phase of #COVID19 vaccination in India has commenced & according to #WHO, vaccines are safe to be administered to women on their periods. Don't delay your vaccinations. Don't fall for myths.#VaccineForAll #VaccinationForAll https://t.co/r05KsgLpRg ” (India 81029).
In India specifically, while initial concerns on vaccines such as “Covi-shield” were observed, the discourse gradually shifted to safety and efficacy of the vaccines. Twitter handles of popular leaders also facilitated in developing a positive discourse towards safety and efficacy of vaccines.
“There have been rumors about #vaccines made in India being unreliable. I would like to request all of you to stay aware and know that our vaccines are safe and effective: #PMModi”(India 71370).
Twitter served as a platform of advocacy for vaccine safety. Particularly the twitter handles of popular leaders and cultural icons vouching for vaccine safety improved trust and acceptance of vaccines in the community.
Theme 4: voices for vaccine equity
The twitter users also advocated for vaccine sharing and vaccine equity to better control COVID-19 at a global scale. Specifically, the tweets were found to be advocating for vaccine equity by tagging the policy makers to share their stockpiles of vaccines with low-income countries through COVAX initiatives. One of the several tweets concerning vaccine equity tagging world leaders reads as follows.
“@BorisJohnson@JustinTrudeau@POTUS@EUCouncil@RegSprecher @ScottMorrisonMP the world needs #COVID19 vaccine access now. The #G7 must step up to #EndThePandemic for all by sharing 1 billion doses through #COVAX by September. https://t.co/ha2zi5IJEH ” (UK 14594).
After emergence of new variants such as Omicron, the dialogue for vaccine equity was more strongly observed in the twitter discourse. The users voiced for vaccine sharing and vaccine equity for the developing countries to prevent emergence of newer variants of the virus.
One of the key lessons from the emergence of the new B.1.1.159 variant in Southern Africa is that if we don’t address the enormous global inequity in access to Covid-19 vaccines, other variants may emerge that are highly infectious or against which vaccines work less well. (Australia 89403).
Theme 5: myth busters
A large proportion of positive tweets on vaccines were concerned with busting popular myths on COVID-19 vaccines. Specifically, in both developed and developing country settings, popular vaccine myths on conspiracy around COVID-19 vaccines, viability of COVID-19 vaccines for specific population groups, timing of vaccines and disease status etc. were debunked. Some of the popular tweets debunking COVID-19 vaccine myths are as follows.
“Covid-19 vaccines do not contain microchips https://t.co/kyWSAeqzDf via @harakahdailyHD” (India 980). “MYTH: The vaccine alters your DNA. FACT: Vaccines do not change a person’s DNA. Vaccines work by stimulating the body the same way the virus would if someone was infected.”(Australia 98087).
Debunking COVID-19 vaccine myths was also actively done by the Government agencies across the countries. For example, the Government of India as part of its COVID-19 prevention campaign, launched “Jan Andolan” to bust myths and spread awareness about COVID-19 vaccines.
“Mythbusters on #COVID19Vaccines. Myth: Vaccine not needed for #COVID19 recovered persons. Fact: Advisable to receive a complete schedule of vaccine despite recovery from COVID-19, to enhance the immune response. #JanAndolan @SpokespersonMoD @diprjk @PIB_India @rajnathsingh https://t.co/hkWDXoJOFZ ” (India 120860).
The active participation of individuals, government and civil society bodies, prevented fear & panic and contributed to the positive discourse on COVID-19 vaccines.
Theme 6: vaccine shortages
Experiences of shortage of vaccines were among the common negative discourse observed in developing countries. In Indian context, the tweets on vaccine shortages raised exponentially during the April-June 2021 quarter. One tweet concerning suspension of vaccination drive read as follows.
“COVID-19 vaccination drive in Mumbai suspended for two days due to vaccine shortage https://t.co/K6eSNlssJx ” (India 52738).
In developed countries like UK while the vaccine shortages are substantially less visible, the tweets reflected the user’s advocacy to tackle vaccine shortages in developing countries, specifically in Africa.
“Everyone vaccinated on an remote island in Scotland that had no #COVID19 cases… instead of giving the vaccine to individuals in #COVID19 UK hotspots or sharing vaccine with other countries? There is a global vaccine shortage! This is #vaccinenationalism at its finest! https://t.co/HxZyhvOf09 ” (UK 19740).
The tweets concerning vaccine shortages also reflected on the supply chain & health system issues within the countries and larger vaccine inequity between the developed and developing countries. One twitter handle from South Africa said.
“WHO warns lack of COVID-19 vaccine supply in Africa could make it breeding ground for new variants and send the whole world back to square one” (South Africa 29430).
Theme 7: distrust on vaccines
The negative discourse on COVID-19 vaccines also reflected the general distrust of COVID-19 vaccines in the communities. The distrust of vaccines is largely reported in the developed country settings, quoting concerns around expedited process of vaccine development, capitalism and big pharma lobbying. The twitter discourse reflected the user’s skepticism on COVID-19 vaccines and their development process. Some tweets from Australia and the UK read as follows.
“So it seems people are finally realizing the covid 19 vaccines aren’t vaccines. They just call these shots vaccine so people who get serious side effects can’t sue the gov. Covid19 is just a shot people.#shots#vaccines#thanksgivingconvos https://t.co/lRhqrOW9gu ” (Australia 46606). “People are not linking a drug and vaccine. They're linking what can happen if pharmaceutical companies don't trial and test a product adequately. AIDS has been around for 50 years but no vaccine whereas COVID-19 about a year. Public have a right to be concerned” (UK 86542). “There are no vaccines available for most of NTDs, Malaria, HIV and others, but there are many vaccines developed and manufactured in less than one year time against #COVID19. #RuleOf3Ps Fact speaks itself” https://t.co/01C0kFaQju ” (UK 20113).
In developing countries, the vaccine distrust was largely observed in villages and areas with limited awareness. In rural India, distrust was mainly surrounded by the myths that COVID-19 vaccines will cause infertility and impotency.
“We are hearing the rural population is scared of the vaccine—they think it will make women infertile and men impotent. Children are being sent away when there is a vaccine campaign in villages.#COVID19 #VaccineHesitancy #mythbusting” (India 79298).
Theme 8: vaccine side-effects
The distrust on vaccines was seen to be largely linked to the fear of vaccine side-effects. In this regard it was observed that twitter also served as platform to share reliable information and updates on rare events such as vaccine side-effects. The platform served to communicate any recommendation, report, news concerning vaccine side-effects such as allergic reactions, bell’s palsy, and even death in the countries studied. Some of the tweets studied are as follows.
“2 people have experienced allergic reactions and 4 people have developed Bells' palsy to Pfizer's COVID19 Vaccine so far”. (UK 70882). “Covid Has A 99% Survival Rate. Now I personally know of at least two otherwise healthy people who died of blood clots after getting the vaccine & three others who developed Bells Palsy. https://t.co/40z6yv39n3 ″ (Australia 116251). “#COVID19 | Government’s review of side effects confirms 1 death after vaccine dose” (India 68330).
The study analysed the twitter discourse & people’s sentiments on COVID-19 vaccines in four selected countries. Across the counties there was a general negative sentiment on COVID-19 vaccines ranging between 28.88 and 58.48%. The highest percentage of tweets with negative sentiments were identified from India, whereas the highest percentage of positive tweets were identified in UK i.e., 21.09%.
The tweets reflected the general reality of vaccination status and the overall discourse around the vaccination in specific country during the time period. For example, the tweets in India reflected the words “Second wave”, “Delhi”, and “Stay safe” a reminiscence of the deadly second wave the country witnessed in mid-2021 25 . In UK the prominent words “Get”, “Work” reflects the government’s advocacy that “vaccines work” and “get vaccinated” the prominent messages communicated by the UK government during that time. In South Africa, the prominent words were “detected south”, “identified south”, “million doses” and “Johnson & Johnson” conveying the messages concerning the challenges in vaccine roll out in the country 26 .
From the overall discourse we observed that the major negative themes emerged included (i) vaccine shortages, (ii) distrust on vaccines and (iii) side effects. In developing countries, the discourse was largely concerning the supply side factors of vaccines i.e., shortage of vaccines. The attitudes observed in twitter discourse were confirmatory with the COVID-19 status and vaccine rollout in respective countries. The data on weekly confirmed COVID-19 mortality indicates that India witnessed the highest number of deaths during the study timeline (supplementary Fig. 1A), explaining the high percentage of negative tweets. The COVID-19 vaccine doses remained administered per 100 people in South Africa remained less than 65/100 during the study period (supplementary Fig. 1B), indicating substantial challenges in vaccine delivery. Our findings suggest that high number of COVID-19 deaths, coupled with systematic inefficiencies resulted in negative attitudes. This is further supported by our supplementary analysis reflecting the tweets across COVID-19 timeline in India (Fig. 5 ). We found that the negative tweets peaked during the months of April to June 2021, during which India witnessed a massive spike in deaths due to COVID-19. The content of these tweets was “medicine stock outs”, “vaccine shortages”, “requests for help”, “shortage of hospital beds”, and “systematic failure”. The word cloud for India (Fig. 2 (1)) supports the same.
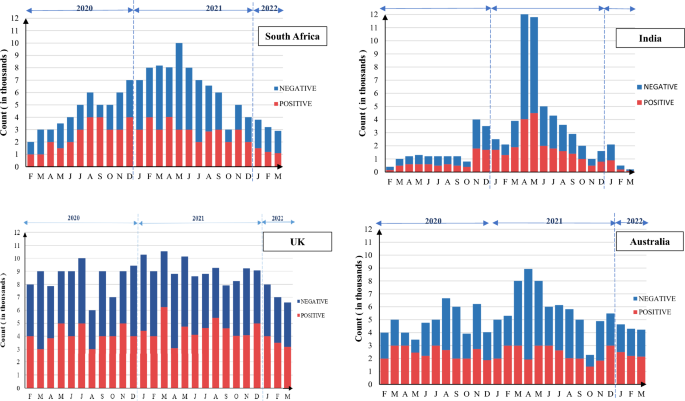
Positive and negative tweets across COVID-19 timeline in selected countries.
Our findings reflect that twitter was also used as a platform to voice for vaccine equity. Users from developed countries were observed to call out the leaders to share their surplus vaccines with developing world through COVAX initiative. Studies argue that health system inadequacies, coupled with limited vaccine supplies, high percentage of susceptible population, and increase in virulent variants of virus can result in potential outbreaks of COVID-19 in the developing countries 27 . While Vaccination, is the most effective strategy to prevent infection, the unavailability of COVID-19 vaccines still remains a major problem three years into the pandemic. While 68.7% of the world population received at least one dose of COVID-19 vaccination, less than 26% of those in low-income countries received it 28 . Vaccination shortages and logistic inefficiencies are reported as reasons for inadequate vaccination rates in low- and middle-income countries of Asia and Africa 29 , 30 . Our findings reflect that from a developing country perspective, availability and accessibility of vaccines should be of prime focus.
In Australia and the United Kingdome which had a high vaccination rates (supplementary Fig. 1B), the discourse was largely concerning the trust-worthiness and side effects of vaccines. Specifically, the swiftness at which the COVID-19 vaccines were developed, coupled with the myths surrounding them created a sense of distrust. Existing research from UK and elsewhere corroborate with our study findings. A study conducted in France reported that 28.8% of working age adults out rightly refused the COVID-19 vaccination 31 . Another study reported that 40.9% of the US population had a low level of trust on COVID-19 vaccine 32 . In India among the population groups such as medical community who were priority recipients of COVID-19 vaccines during India’s initial COVID-19 vaccination drive, vaccine hesitancy was reported to be around 10.6% 13 . A recent study on vaccine hesitancy among UK adults reported that existing misinformation, concerns about vaccine safety and personal beliefs impacted vaccination decision making 33 .
To improve uptake of COVID-19 vaccines, countries have adopted multiple strategies to spread awareness about safety of vaccines, and busting the myths around vaccination. Individual and governmental effects were observed towards communicating a positive word on COVID-19 vaccines through platforms like twitter. In developed and developing countries alike, there were several myths such as “vaccines having microchips”, “those with prior COVID-19 infection need not take vaccine”, “COVID-19 vaccine causes infertility” etc., which hindered vaccine acceptance at regional and national levels 33 , 34 . Twitter served as one of the platforms to bust the myths. Specifically, the official handles of government agencies were found to bust specific myths surrounding COVID-19 vaccines across the countries. Earlier studies also report the use of social media to counter misinformation and disseminate accurate information 35 , 36 , 37 .
Similarly, twitter served as a platform to disseminate information on vaccine safety by popular leaders and celebrities. Popular leaders such as the Indian and UK prime ministers posted on twitter taking their first doses of COVID-19 vaccines creating a positive image for vaccines. Earlier studies reported that politicians and celebrities personally taking the vaccine and urging the fellow countrymen to take the vaccine created a positive image and confidence on COVID-19 vaccines 38 , 39 . Additionally, it can be argued that twitter served as a means to undertake persuasive messaging, by spreading the positive messages such as “vaccines work”, “get the jab done”, “vaccines are safe” etc. Studies report that persuasive messaging is effective in increasing the vaccination uptake and improve awareness 40 .
Limitations
This study focused solely on four countries and may not provide a generalizable view to global discourse on COVID-19 vaccines. Twitter was the primary data source for the study. Non-inclusion of other social media platforms, restrictions on length of tweets, and confirming to tweets in English language are some of the limitations with the data. The study could not establish causal associations given its use of a single data source and descriptive focus of objectives and analysis. Moreover, social media data such as twitter possess the risk of sampling bias, language and cultural biases, geographical bias, and echo chamber effect which might limit representativeness and generalisability. We attempted to overcome these limitations by analysing a large sample more than 100,000 tweets per country, purposively identifying the major economies from Europe, Oceania, South Asia, and Sub-Saharan Africa, and stratifying the results of our sentiment analysis by geography. Albeit a smaller sample, by thematically analysing 500 randomly selected tweets from each country, and contextualising our analysis with the real word COVID-19 data we complemented our numerical analysis and minimized the potential bias of echo chamber effect. Future studies triangulating the sentiment analysis with multi-site qualitative interviews or thematic analysis of a larger sample of tweets could provide further insights. Studies employing longitudinal approaches are needed to establish temporality and causation. In spite of limitations, this study provides a crucial insight to public opinion on COVID-19 vaccines in the countries studied.
Our findings suggest that twitter discourse reflected the general trend in progression of COVID-19 epidemic and vaccinations in the studied countries. While, there exist a substantial negative perception among the population, the negative sentiments were usually backed by vaccine shortages and supply chain inefficiencies in developing countries and safety concerns and myths surrounding vaccines in developed countries. These findings reflect the core issues which are faced in the specific countries studied. In developed countries with stronger healthcare systems and better availability of vaccines, the focus should be to improve public perception on vaccination. In contrary, more efforts are needed to strengthen the vaccine availability, delivery systems, and improving public perception in developing countries. The findings also suggest the role of social media as platforms to share a positive word about vaccination and suggest a future potential role of social media in tackling misinformation. Given that the threat of COVID-19 is still looming and inadequate vaccination is a persistent challenge, social media should be continued to be used by concerned authorities to report positive messages on COVID-19. Also, the findings point towards the prominence of analysing the discourse in social media to inform public health strategies towards disease prevention and management.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Goel, S. et al. Resilient and agile engineering solutions to address societal challenges such as coronavirus pandemic. Mater. Today Chem. 17 , 100300 (2020).
Article CAS PubMed PubMed Central Google Scholar
Chenchula, S. et al. Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: A systematic review. J. Med. Virol. 94 (7), 2969–2976 (2022).
Singh, A. et al. The safety profile of COVID-19 vaccinations in the United States. Am. J. Infect. Control 50 (1), 15–19 (2022).
Article MathSciNet CAS PubMed Google Scholar
Fefferman, N. H. & Naumova, E. N. Dangers of vaccine refusal near the herd immunity threshold: A modelling study. Lancet. Infect. Dis 15 (8), 922–926 (2015).
Article PubMed Google Scholar
Bassey, O. & Rotimi, O. Vaccine hesitancy. Br. Dent. J. 230 (5), 271–272 (2021).
Harrison, E. A. & Wu, J. W. Vaccine confidence in the time of COVID-19. Eur. J. Epidemiol. 35 (4), 325–330 (2020).
Sugerman, D. E. et al. Measles outbreak in a highly vaccinated population, San Diego, 2008: Role of the intentionally undervaccinated. Pediatrics 125 (4), 747–755 (2010).
Wawrzuta, D. et al. What arguments against COVID-19 vaccines run on facebook in Poland: Content analysis of comments. Vaccines (Basel) 9 (5), 1 (2021).
Google Scholar
Barello, S. et al. “Vaccine hesitancy” among university students in Italy during the COVID-19 pandemic. Eur. J. Epidemiol. 35 (8), 781–783 (2020).
Mercadante, A. R. & Law, A. V. Will they, or Won’t they? Examining patients’ vaccine intention for flu and COVID-19 using the Health Belief Model. Res. Social Adm. Pharm. 17 (9), 1596–1605 (2021).
Freeman, D. et al. COVID-19 vaccine hesitancy in the UK: The Oxford coronavirus explanations, attitudes, and narratives survey (Oceans) II. Psychol. Med. 52 (14), 3127–3141 (2022).
Lazarus, J. V. et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 27 (2), 225–228 (2021).
Article CAS PubMed Google Scholar
Jain, J. et al. COVID-19 vaccine hesitancy among medical students in India. Epidemiol. Infect. 149 , e132 (2021).
Abouzahra, M. & Tan, J. Twitter vs. Zika—The role of social media in epidemic outbreaks surveillance. Health Policy Technol. 10 (1), 174–181 (2021).
Article Google Scholar
Hossain, L. et al. Social media in Ebola outbreak. Epidemiol. Infect. 144 (10), 2136–2143 (2016).
Wang, Y. et al. Systematic literature review on the spread of health-related misinformation on social media. Soc. Sci. Med. 240 , 112552 (2019).
Article PubMed PubMed Central Google Scholar
Yousefinaghani, S. et al. An analysis of COVID-19 vaccine sentiments and opinions on Twitter. Int. J. Infect. Dis. 108 , 256–262 (2021).
Lyu, J. C., Le Han, E. & Luli, G. K. COVID-19 vaccine–related discussion on Twitter: topic modeling and sentiment analysis. J. Med. Internet Res. 23 (6), e24435 (2021).
Larson, E. Automatic checking of regular expressions . in 2018 IEEE 18th International Working Conference on Source Code Analysis and Manipulation (SCAM) . 2018. IEEE.
Fang, X. & Zhan, J. Sentiment analysis using product review data. J. Big Data 2 (5), 1 (2015).
CAS Google Scholar
Barbieri, F., et al. Unified Benchmark and Comparative Evaluation for Tweet Classification (Findings of the Association for Computational Linguistics, 2020).
Ayon, S. S., et al. Sentiment Analysis on COVID-19 Tweets. in 2022 25th International Conference on Computer and Information Technology (ICCIT) (IEEE, 2022).
Saura, J. R., Herráez, B. R. & Reyes-Menendez, A. Comparing a traditional approach for financial brand communication analysis with a big data analytics technique. IEEE Access 7 , 37100–37108 (2019).
Miller, W. R. et al. Nursing in the spotlight: Talk about nurses and the nursing profession on Twitter during the early COVID-19 pandemic. Nurs. Outlook 70 (4), 580–589 (2022).
Chakraborty, C. et al. The current second wave and COVID-19 vaccination status in India. Brain Behav. Immun. 96 , 1–4 (2021).
Dzinamarira, T. et al. COVID-19 vaccine roll-out in South Africa and Zimbabwe: Urgent need to address community preparedness, fears and hesitancy. Vaccines (Basel) 9 (3), 1 (2021).
Ciotti, M. et al. The COVID-19 pandemic: Viral variants and vaccine efficacy. Crit. Rev. Clin. Lab. Sci. 59 (1), 66–75 (2022).
Edouard Mathieu, et al. Coronavirus Pandemic (COVID-19). 2020: OurWorldInData.org.
Chu, D.-T. et al. COVID-19 in Southeast Asia: Current status and perspectives. Bioengineered 13 (2), 3797–3809 (2022).
Masresha, B. et al. The first year of COVID-19 vaccine roll-out in Africa: Challenges and lessons learned. Pan Afr Med J 41 (Suppl 2), 2 (2022).
PubMed PubMed Central Google Scholar
Schwarzinger, M. et al. COVID-19 vaccine hesitancy in a representative working-age population in France: A survey experiment based on vaccine characteristics. Lancet Public Health 6 (4), e210–e221 (2021).
Latkin, C. A. et al. Trust in a COVID-19 vaccine in the U.S.: A social-ecological perspective. Soc. Sci. Med. 270 , 1184 (2021).
Lockyer, B. et al. Understanding COVID-19 misinformation and vaccine hesitancy in context: Findings from a qualitative study involving citizens in Bradford, UK. Health Expect 24 (4), 1158–1167 (2021).
Ullah, I. et al. Myths and conspiracy theories on vaccines and COVID-19: Potential effect on global vaccine refusals. Vacunas 22 (2), 93–97 (2021).
Rashid, A. A., Mohamad, I. & Mohd Haris, A. F. The role of social media in making an impact to health knowledge and behaviour on COVID-19 in Malaysia. Malays. J. Med. Sci. 28 (3), 155–157 (2021).
Vraga, E. K. & Bode, L. Addressing COVID-19 misinformation on social media preemptively and responsively. Emerg. Infect. Dis. 27 (2), 396–403 (2021).
Olapeju, B. et al. Addressing COVID-19 rumors and behaviors using theory in Guyana: A program case study. Glob. Health Sci. Pract. 10 (4), 1 (2022).
Tolia, V. et al. Understanding factors to COVID-19 vaccine adoption in Gujarat, India. Int J Environ Res Public Health 19 (5), 1 (2022).
Sanghavi, N. & Neiterman, E. COVID-19 vaccine hesitancy in middle-aged and older adults in India: A mixed-methods study. Cureus 14 (10), e30362 (2022).
James, E. K. et al. Persuasive messaging to increase COVID-19 vaccine uptake intentions. Vaccine 39 (49), 7158–7165 (2021).
Download references
Acknowledgements
The authors would like to thank their respective institutes for providing the necessary facilities.
Author information
These authors contributed equally: Manah Verma and Nikhil Moudgil.
Authors and Affiliations
Department of Computer Science and Engineering, Thapar Institute of Engineering and Technology, Patiala, Punjab, 147004, India
Manah Verma, Nikhil Moudgil & Neeraj Kumar
School of Energy and Environment, Thapar Institute of Engineering and Technology, Patiala, Punjab, 147004, India
Gaurav Goel
Jamsetji Tata School of Disaster Studies, Tata Institute of Social Sciences, Deonar, Mumbai, 400088, India
Peehu Pardeshi & Jacquleen Joseph
Tata Center for Technology and Design, Indian Institute of Technology Bombay, Mumbai, India
Peehu Pardeshi
School of Computer Science, University of Petroleum and Energy Studies, Dehradun, India
Neeraj Kumar
Faculty of computing and IT, King Abdulaziz University, Jeddah, Saudi Arabia
Department of Computer Science and Engineering, Graphics Era University, Dehradun, India
Department of Electrical and Computer Engineering, Lebanese American University, Beirut, Lebanon
Department of Civil Engineering, MM Engineering College, Maharishi Markandeshwar (Deemed to Be University), Mullana-Ambala, 133207, Haryana, India
Kulbir Singh
Chemistry Department, RIMT UNIVERSITY, Mandi Gobindgarh, Punjab, 147301, India
Department of Public Health and Community Medicine, Central University of Kerala, Kasaragod, Kerala, 671320, India
Prakash Babu Kodali
You can also search for this author in PubMed Google Scholar
Contributions
The authors M.V., N.M., G.G. and P.B.K. conceptualized the study. M.V. & N.M. extracted the data. M.V., N.M. & G.G. under the supervision of N.K. processed the data for sentiment analysis. M.V. & N.M. prepared the Figs. 1 , 2 , 3 and 5 . P.B.K. undertook qualitative analysis. P.P. & J.J. provided necessary intellectual input from the conceptualization stage to drafting of the manuscript. G.G., N.K., K.S. & H.S. developed the initial draft of the manuscript. PBK revised the manuscript and developed the submission draft. All authors reviewed the manuscript.
Corresponding author
Correspondence to Prakash Babu Kodali .
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Verma, M., Moudgil, N., Goel, G. et al. People’s perceptions on COVID-19 vaccination: an analysis of twitter discourse from four countries. Sci Rep 13 , 14281 (2023). https://doi.org/10.1038/s41598-023-41478-7
Download citation
Received : 10 February 2023
Accepted : 27 August 2023
Published : 31 August 2023
DOI : https://doi.org/10.1038/s41598-023-41478-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Perspect Clin Res
- v.4(1); Jan-Mar 2013
Current topics in research ethics in vaccine studies
Prasad s. kulkarni.
Serum Institute of India Ltd, Pune, India
About 7.6 million children under the age of five die every year, according to 2010 figures,[ 1 ] out of these 2.4 million children die from vaccine preventable diseases.[ 2 ] The problem is compounded by the absence of effective therapies for many infectious diseases. Obviously, new, more cost-effective and improved vaccines are needed today and in the future.
Vaccines have some distinct features than drugs. Unlike therapeutic molecules, vaccines have preventive role against specific infectious diseases. The target population is healthy people, mostly children and infants; as a result, tolerability of adverse events is less. Additionally, vaccines are highly complex substances derived from living microorganisms and their quality and safety needs to be demonstrated on a lot-to-lot basis. Naturally, these factors have some bearing on the clinical trials of vaccines. Here we discuss some of the current ethical issues in vaccine clinical trials.
Pediatric trials
Most of the vaccine studies are conducted in children, some of them in infants and even in newborns because that is where you want to catch them for prevention of an infection. However, children by themselves are unable to consent, and the vaccinator has to accept a legal guardian's agreement. Also, one would expect children to experience more adverse reactions than adults. For these and many other reasons, it is generally agreed that vaccine studies are, at least primarily, unethical in children if the relevant investigation can be done among adults. The main problem here is, however, that many infections are characteristically only pediatric diseases, or at least, those infections are specially harmful to the youngest.
One therefore needs to seek for a difficult balance between the true and ostensible need of a vaccine in the pediatric population. The CIOMS rightly states that “Before undertaking research involving children, the investigator must ensure that-the research might not be equally well be carried out in adults; and the purpose of the research is to obtain knowledge relevant to the health needs of children.”[ 3 ]
Parental consent
More in developing countries than elsewhere, parents or guardians of children may have little or no understanding of research trials. They may be unfamiliar with concepts such as “informed consent” and “confidentiality” and may not understand the scientific terms and processes involved in trials, including the use of randomization and placebos. Yet these parents will be called upon to give consent on behalf of their small children, or to explain to their older heirs (children) what is happening in the trial.
Another concern is consent of an appropriate legal representative in the absence of parental consent. Recently a demonstration project on a vaccine was conducted in India. An investigation was prompted after press reports of some deaths. Though the deaths were not found caused by the vaccine, consent obtained from hostel wardens in some subjects living in hostels was questioned.[ 4 ]
Need for the trial
Before launching a trial in children one must show that there is compelling need to use children to establish safety, immunogenicity, effectiveness or efficacy of the vaccine. Such a trial would not be justified if the child comes from population in which that particular disease is not a problem. Malaria vaccine cannot be tested soon in Europe or North America.
An absolute care must be taken to ensure that socioeconomic inequalities between industrialized and developing countries are not exploited i.e., that children in a poor country are not asked to undertake risks to produce a vaccine that, for economic or other reasons, would primarily benefit their counterparts in industrialized countries. At the same time, research should not be impeded that aims to reduce the inequality of health care and to benefit pediatric populations in need in developing countries.
Selection of control
If a good vaccine is already in use in some other country or community which is more or less comparable to site where the trial is planned, that vaccine should be used as the comparator. If such a vaccine does not exist, a placebo “vaccine” may be used, provided the set-up is thoroughly explained to the participants, their families and the community. Placebo controls are ethically acceptable when there is no proven vaccine for the indication for which the candidate vaccine is to be tested.[ 5 , 6 ]
A modification of this setting is that the placebo recipients receive the true vaccine later–but all this has to be explained in understandable words to the participants.
An alternative to the use of placebo is to give another vaccine that provides comparable benefit against another disease, or more willingly, against similar disease caused by different agents. This was the approach in Finland in the 1970s, when the first vaccines against bacterial meningitis (due to Neisseria meningitidis and H. influenza) were tested in children.[ 7 ] Here it was important these two types of meningitis were equally common in that community. For some vaccines, the choice is not difficult since there are no effective interventions so far, e.g., malaria or HIV vaccines.
In Indonesia, an exceptional approach was taken on 1998-2002.[ 8 ] Half of children received traditional DTP (diphtheria-tetanus-pertussis) vaccine, whereas the other half of children got DTP with H. influenza type B (Hib) component. Thus, all children were not in an equivalent position, but the setup was considered justified because in the absence of disease burden data and vaccine efficacy data in the region, the trial was deemed helpful for the decision whether or not to introduce Hib vaccination in Indonesia and the whole region.
When, instead of clinical efficacy, “only” immunogenicity (antibody production) is measured, the rules of equipoise are looser. The comparator vaccine may function more as “compensation” to the child in the trial's control arm. For instance, meningococcal C conjugate vaccine in a pneumococcal vaccine trial, or rabies vaccine in a Japanese encephalitis vaccine trial does not restore equipoise but benefit the child who would not otherwise receive that vaccine.
Age de-escalation
Age de-escalation means that phase I and II trials are conducted first in adults, then in older children, and finally, if relevant, in small children. Epidemiology of the disease, the risks/benefits of the vaccine for each age group, and the safety profile are all factors to be taken into account in de-escalation.
However, if a new vaccine is only for infants, trials in older children may expose them to unnecessary risks without giving any benefit to these too “old” vaccinees. Rotavirus vaccines are good examples in this category. Sometimes adult participants can be used in the first trials, although they are of no help in the efficacy trials.
Sometimes there are grounds to use child participants already in phase I trials. This is the case if the new vaccine would likely cause problems in adults (but not in children) because of prior immunity in adults e.g., DTP vaccine.
Participation of adolescents
Only a few vaccines as targeted just for adolescents: examples are human papillomavirus (HPV) and herpes virus (HSV) vaccines. However, adolescents may be used in the de-escalation studies before progressing to small children. The participation of adolescents often involves complex legal, ethical issues and operational issues.
Informed consent is problematic, because adolescents often have the intellectual and emotional capacity to provide consent, but do not have the legal right to consent. Also their views may not be the same as their parents’ views, and appropriate confidentiality can be difficult to maintain. An extreme would be a situation in which the youth disagrees but the parents agree the trial, or in which a willing adolescent would be included in the absence of parental consent.
The participation of adolescent girls is further complicated by the potential or soon materializing pregnancy. Not only would it perhaps risk the young mother and the fetus, but also raise complex issues regarding the consent, confidentiality and legal liability. Routine pregnancy testing of adolescent girls prior to the inclusion in a trial would also have its cultural problems.
Limitations of informed consent
Obtaining informed consent in a developing country has its own problems and should be seen as a process which begins from the voluntary decision to participate in the study. The decision should be based on sufficient information prior to the trial entry. The informed consent form should be simple enough to be understood by the often not-too-educated individual, or in case of a child, by parents or legal guardian, but still comprehensive to explain the concepts, potential risks and benefits, implications of the use of a placebo or other comparator, care that will be provided, and the indemnity for injury or death arising from the trial. Importantly, it must be stated clearly that a withdrawal from study is allowed at any time without giving an explanation for the decision. If the circumstances of the trial change significantly, the consent form is to be changed accordingly, and the whole study warrant discussion with the already enrolled participants. Another consent is then to be obtained.
The problems in getting valid consent are heightened in developing countries where people may be unfamiliar with scientific research, concepts and vocabulary. Thus, the expectations may be unrealistic. Also the individual's full autonomy might become endangered because of the society's cultural and/or gender norms, or the family or spousal pressure. All of these challenges are further complicated when the trial deals with children.
Child's assent
In the case of a child, every effort should be made to explain to him/her also, in language that is understandable to the child, what the participation means, as regards to potential risks (discomfort, time spent, etc.) and benefits, The investigators should document the child's assent.
Community consent
Since an informed consent may be culturally sensitive, family or community discussions are sometimes necessary, albeit the community consent should not be considered as a substitute for the individual consent. There may also be tension between the ethical responsibility to maintain individual confidentiality, and cultural norms that press for “shared confidentiality”. Within appropriate boundaries of confidentiality, it may be useful to have an impartial witness/observer present during an oral consent particularly if verbal rather than signed consent is sought. Such witnessed consent must be recorded in the trial files.
Potential for inducement
The improved medical care provided during the trial may constitute an inducement and may impact on the willingness to participate. Indeed, trial participants often accept the trial in the belief that they will receive improved treatment. It is important to explain that participation will not necessarily ensure protection against disease. In case of a study using placebo, the entire set-up and the meaning of randomization should be explained, including the fact that the participant might fall in the placebo group. Any care or other benefits that perhaps are offered should be described.
Another concern is if the parents see an opportunity for economic benefits, they may encourage enrolling their and perhaps other children in trials in which those should not necessarily be included. All efforts should be made to avoid any exploitation, and to minimize all mental, emotional and physical harm.
Standard of care
In case of vaccine trials in developing countries, the situation is tricky because of a high burden of disease and low standards of health care in that community. With the contribution of local authorities, a standard of care should be offered. This means an improvement in the health conditions of participants, and that it is sustainable. These efforts need an approval from the local ethics committees.
Duration of follow-up
An active follow-up should extend at least to the end of the trial. In case of an adverse effect, the, follow-up should be continued for an additional six months. In high mortality populations, it may be desirable to analyse long-term mortality changes and to follow-up participants for a number of years. Passive follow-up is advisable even longer, and if existing mechanisms can be used for this purpose.
Long-term follow-up may complicate a trial substantially and greatly increase the costs. Therefore, gathering only passive data may suffice. Creative follow-up should be contemplated, both for safety and long-term protection. The high titer measles vaccine was studied in some African countries, however on a long term follow up, it was discovered that female mortality was higher following the vaccine,[ 9 ] which resulted in abandoning the use of the vaccine. This important finding was detected only because of long-term follow-up.
Screening of subjects
Vaccine trials need to be conducted in healthy people and hence, the screening for inclusion/exclusion criteria is very critical. Enrolment of children with underlying medical conditions can complicate the safety outcomes. A recent vaccine trial in India brought forth this issue. A death was reported in the study after an infant had received a licensed vaccine used as a control. The investigation revealed that the infant who died had a pre-existing medical condition.[ 10 ] It is recognized that physical screening of young infants has limitations; however, every effort should be made to ascertain the health status. In case of suspicious cases, it is better to err on the safer side.
CONCLUSIONS
Vaccine clinical research needs to deal with certain ethical issues because of the inherent nature of these trials. The issues are more complicated since the research mostly happens in pediatric populations in developing countries. Keeping in mind these issues while designing research on vaccines is critical.
Source of Support: Nil
Conflict of Interest: None declared

COMMENTS
Vaccine Confidence, Coverage, and Hesitancy Worldwide Hammond 2 many of the unvaccinated are a result of rising vaccine hesitancy of parents in conjunction with the anti-vaccination movement. Vaccine hesitancy is defined as "a delay in acceptance or refusal of vaccines despite availability" (Macdonald, 2015, p. 34).
Two doses of the vaccine or placebo were given 21 days apart to the respective groups. 21 The mean age of the participants was 45.3 years, and the majority of participants were Caucasian (98.5%). 21 From 21 days after the first dose of the vaccine, efficacy against symptomatic COVID-19 illness was 91.6% (95% CI, 85.6-95.2%) with 16 confirmed ...
uncertainty around the virus and consequently the vaccine. This thesis aims to explore nurses' decision-making around COVID-19 vaccination, with a specific focus on the role of uncertainty both about the pandemic and available vaccines. In what follows, this study first reviews the state of the COVID-19 pandemic, and specifically the
Vaccines work by exposing a subject to part of or a whole pathogen, thus activating the immune system described above. They play a critical role in global health by preventing infection and transmission of multiple diseases worldwide [4]. The World Health Organization estimates that vaccines prevent the death of 2-3 million people every year [5].
Brief History of Vaccine Development. Human use of preparations to prevent specific infections have been described since 1500 AD, beginning in China (Needham, 2000) where smallpox was prevented by variolation, which is the introduction of material from scabs into the skin.In 1796 in the United Kingdom, Edward Jenner observed the immunity to smallpox of milkmaids having previously had natural ...
The Global Vaccine Action Plan 2011-2020 lists equity as one of its six guiding principles [].Resonating this ethos, various national vaccination policies and programs have acknowledged vaccines' contribution to preventing high-cost treatments and averting medical impoverishment, while striving to extend the benefits of immunization to all [2, 3].
Higher degrees of vaccine efficacy significantly increased individuals' willingness to receive a COVID-19 vaccine, while a high incidence of minor side effects, a co-pay, and Emergency Use ...
Metrics. Vaccines have revolutionized modern medicine by preventing infectious diseases and safeguarding public health. This Collection showcases cutting-edge research on advancements in vaccine ...
Introduction. Vaccinations are among the most important public health tools for reducing the spread and harm caused by dangerous diseases [].The World Health Organization estimates that vaccines prevented at least 10 million deaths between 2010-2015 worldwide [].Despite considerable evidence showing vaccines are safe [3, 4], there is increasing skepticism toward vaccination [5, 6].
Vaccines have been one of the most important medical discoveries of the last three centuries. Thanks to their development, millions of deaths have been avoided. Together with improved hygiene and antibiotics, vaccines have significantly contributed to the prolongation of life expectancy in high-income countries to a current average of 85 years, compared to 47 years in 1900.
The race for COVID‐19 vaccine invention and development against the spread and catastrophic effects of the disease is real. WHO released a draft list of COVID‐19 candidate vaccines on 3 September 2020. At least 34 vaccine candidates are in clinical evaluation to date . Several new technologies are used as COVID‐19 vaccine development ...
In wealthier countries, the ethical issues that surround vaccination tend to focus on the rights of individuals versus government or society. In poorer countries, the fundamental issue is the lack ...
The present review will discuss the four vaccines, AZD1222 (AstraZeneca or Vaxzevria), Janssen (Ad26.COV2.S), Moderna/mRNA-1273 and BioNTech/Fosun/Pfizer BNT162b1, that are currently in use worldwide to understand their efficacy, but also evaluate the difficulties and challenges of vaccine development. Although several questions should be ...
Vaccines are effective and reliable public health interventions against viral outbreaks and pandemics. However, hesitancy regarding the Coronavirus disease (COVID-19) vaccine is evident worldwide. Therefore, understanding vaccination-related behavior is critical in expanding the vaccine coverage to flatten the infection curve. This study explores the public perception regarding COVID-19 ...
A deep and heartfelt thank you to my phenomenal thesis advisor - Dr.Tan. I am incredibly grateful to have you as a teacher, advisor, and mentor. ... Vaccine hesitancy is important to examine to understand disparities in vaccine uptake and ... people worldwide and has earned the title of the deadliest pandemic in United States history (CDC, 2020 ...
Abstract. The coronavirus disease 2019 (COVID-19) pandemic is a global crisis, with devastating health, business and social impacts. Vaccination is a safe, simple, and effective way of protecting a person against COVID-19. By the end of August 2021, only 24.6% of the world population has received two doses of a COVID-19 vaccine.
Abstract. Vaccine hesitancy (VH) is considered a top-10 global health threat. The concept of VH has been described and applied inconsistently. This systematic review aims to clarify VH by ...
Adolescents and youths are a key part of the population that needs to be protected against the coronavirus disease 2019 (COVID-19). This is because they are more likely to spread the virus to vulnerable individuals. In view of these concerns, this study investigated the uptake of COVID-19 vaccines and associated factors among adolescents and youths attending secondary schools in Zambia. This ...
The availability of a safe and effective vaccine for COVID-19 is well-recognized as an additional tool to contribute to the control of the pandemic. At the same time, the challenges and efforts needed to rapidly develop, evaluate and produce this at scale are enormous. It is vital that we evaluate as many vaccines as possible as we cannot ...
It was found that during the first pandemic outbreak period (23.1.2020-08.04.2020), the public information needs for the COVID-19 vaccine were high, mainly focusing on the vaccine safety, vaccination necessity, adverse effects of vaccines, and the vaccine potency of coping with the variation of novel coronavirus.
More than six and half million people have died as a result of the COVID-19 pandemic till Dec 2022. Vaccination is the most effective means to prevent mortality and infection attributed to COVID-19.
In a human challenge vaccine study, healthy volunteers are given an experimental vaccine, and then deliberately exposed to the organism causing the disease to see if the vaccine works. Some scientists believe that this approach could accelerate COVID-19 vaccine development, in part because it would require far fewer volunteers than a typical study.
About 7.6 million children under the age of five die every year, according to 2010 figures, [ 1] out of these 2.4 million children die from vaccine preventable diseases. [ 2] The problem is compounded by the absence of effective therapies for many infectious diseases. Obviously, new, more cost-effective and improved vaccines are needed today ...