AssignmentsBag.com

Assignments For Class 11 Chemistry
Solve CBSE Class 11 Chemistry Assignment pdf Free with answers. These Class 11 Chemistry assignment pdf is prepared by our professional teachers focusing on the latest syllabus of the CBSE Board Exam. We have provided you CBSE Class 11 Chemistry Assignments PDF with answers to help students to make their preparation better.
Assignments for Class 11 Chemistry have been developed for Standard 11 students based on the latest syllabus and textbooks applicable in CBSE, NCERT and KVS schools. Parents and students can download the full collection of class assignments for class 11 Chemistry from our website as we have provided all topic wise assignments free in PDF format which can be downloaded easily. Students are recommended to do these assignments daily by taking printouts and going through the questions and answers for Grade 11 Chemistry. You should try to do these test assignments on a daily basis so that you are able to understand the concepts and details of each chapter in your Chemistry book and get good marks in class 11 exams.
Assignments for Class 11 Chemistry as per CBSE NCERT pattern
All students studying in Grade 11 Chemistry should download the assignments provided here and use them for their daily routine practice. This will help them to get better grades in Chemistry exam for standard 11. We have made sure that all topics given in your textbook for Chemistry which is suggested in Class 11 have been covered ad we have made assignments and test papers for all topics which your teacher has been teaching in your class. All chapter wise assignments have been made by our teachers after full research of each important topic in the textbooks so that you have enough questions and their solutions to help them practice so that they are able to get full practice and understanding of all important topics. Our teachers at https://www.assignmentsbag.com have made sure that all test papers have been designed as per CBSE, NCERT and KVS syllabus and examination pattern. These question banks have been recommended in various schools and have supported many students to practice and further enhance their scores in school and have also assisted them to appear in other school level tests and examinations. Its easy to take print of thee assignments as all are available in PDF format.
Some advantages of Free Assignments for Class 11 Chemistry
- Solving Assignments for Chemistry Class 11 helps to further enhance understanding of the topics given in your text book which will help you to get better marks
- By solving one assignments given in your class by Chemistry teacher for class 11 will help you to keep in touch with the topic thus reducing dependence on last minute studies
- You will be able to understand the type of questions which are expected in your Chemistry class test
- You will be able to revise all topics given in the ebook for Class 11 Chemistry as all questions have been provided in the question banks
- NCERT Class 11 Chemistry Workbooks will surely help you to make your concepts stronger and better than anyone else in your class.
- Parents will be able to take print out of the assignments and give to their child easily.
All free Printable practice assignments are in PDF single lick download format and have been prepared by Class 11 Chemistry teachers after full study of all topics which have been given in each chapter so that the students are able to take complete benefit from the worksheets. The Chapter wise question bank and revision assignments can be accessed free and anywhere. Go ahead and click on the links above to download free CBSE Class 11 Chemistry Assignments PDF.
Free PDF download of 11th Chemistry Assignment PDF with answers created by master educators from the latest syllabus of CBSE Boards. By practicing these Class 11 Chemistry Assignment Pdf will help you to score more marks in your CBSE Board Examinations. We also give free NCERT Solutions and other study materials for students to make their preparation better.
Students who are searching for better solutions can download the 11th Chemistry Assignment PDF with answers to assist you with revising the whole syllabus and score higher marks in your exam.
This 11th Chemistry Assignment PDF shows up with an answer key with step-by-step answers for students to comprehend the problem at each level and not retain it.
We hope this CBSE Chemistry Assignment Topics for Class 11 with Answers shared with you will help you to score good marks in your exam. We have also other study material like NCERT Solutions, NCERT Book, Exam Question, and simpler paper of Class 11 Chemistry. If You want to score higher in your exam, So practice all this study material and if you have any problem in regard to Chemistry Assignment for Class 11, write it in the comment box and we will guide you as much as possible.

You can download free assignments for class 11 Chemistry from https://www.assignmentsbag.com
You can get free PDF downloadable assignments for Grade 11 Chemistry from our website which has been developed by teachers after doing extensive research in each topic.
On our website we have provided assignments for all subjects in Grade 11, all topic wise test sheets have been provided in a logical manner so that you can scroll through the topics and download the worksheet that you want.
You can easily get question banks, topic wise notes and questions and other useful study material from https://www.assignmentsbag.com without any charge
Yes all test papers for Chemistry Class 11 are available for free, no charge has been put so that the students can benefit from it. And offcourse all is available for download in PDF format and with a single click you can download all assignments.
https://www.assignmentsbag.com is the best portal to download all assignments for all classes without any charges.
Related Posts

Assignments For Class 9 Mathematics Polynomials

Assignments For Class 6 Tamil
Assignments class 11 biology digestion and absorption.
CBSE NCERT Solutions
NCERT and CBSE Solutions for free
Class 11 Chemistry Assignments
We have provided below free printable Class 11 Chemistry Assignments for Download in PDF. The Assignments have been designed based on the latest NCERT Book for Class 11 Chemistry . These Assignments for Grade 11 Chemistry cover all important topics which can come in your standard 11 tests and examinations. Free printable Assignments for CBSE Class 11 Chemistry , school and class assignments, and practice test papers have been designed by our highly experienced class 11 faculty. You can free download CBSE NCERT printable Assignments for Chemistry Class 11 with solutions and answers. All Assignments and test sheets have been prepared by expert teachers as per the latest Syllabus in Chemistry Class 11. Students can click on the links below and download all Pdf Assignments for Chemistry class 11 for free. All latest Kendriya Vidyalaya Class 11 Chemistry Assignments with Answers and test papers are given below.
Chemistry Class 11 Assignments Pdf Download
We have provided below the biggest collection of free CBSE NCERT KVS Assignments for Class 11 Chemistry . Students and teachers can download and save all free Chemistry assignments in Pdf for grade 11th. Our expert faculty have covered Class 11 important questions and answers for Chemistry as per the latest syllabus for the current academic year. All test papers and question banks for Class 11 Chemistry and CBSE Assignments for Chemistry Class 11 will be really helpful for standard 11th students to prepare for the class tests and school examinations. Class 11th students can easily free download in Pdf all printable practice worksheets given below.
Topicwise Assignments for Class 11 Chemistry Download in Pdf
Advantages of Class 11 Chemistry Assignments
- As we have the best and largest collection of Chemistry assignments for Grade 11, you will be able to easily get full list of solved important questions which can come in your examinations.
- Students will be able to go through all important and critical topics given in your CBSE Chemistry textbooks for Class 11 .
- All Chemistry assignments for Class 11 have been designed with answers. Students should solve them yourself and then compare with the solutions provided by us.
- Class 11 Students studying in per CBSE, NCERT and KVS schools will be able to free download all Chemistry chapter wise worksheets and assignments for free in Pdf
- Class 11 Chemistry question bank will help to improve subject understanding which will help to get better rank in exams
Frequently Asked Questions by Class 11 Chemistry students
At https://www.cbsencertsolutions.com, we have provided the biggest database of free assignments for Chemistry Class 11 which you can download in Pdf
We provide here Standard 11 Chemistry chapter-wise assignments which can be easily downloaded in Pdf format for free.
You can click on the links above and get assignments for Chemistry in Grade 11, all topic-wise question banks with solutions have been provided here. You can click on the links to download in Pdf.
We have provided here topic-wise Chemistry Grade 11 question banks, revision notes and questions for all difficult topics, and other study material.
We have provided the best collection of question bank and practice tests for Class 11 for all subjects. You can download them all and use them offline without the internet.
Related Posts

Class 11 Mathematics Set Theory Assignments

Class 11 Entrepreneurship Assignments

Class 11 Mathematics Probability Assignments
NCERT Books and Solutions for all classes
Assignments Class 11 Chemistry Pdf Download
Students can refer to Assignments for Class 11 Chemistry available for download in Pdf. We have given below links to subject-wise free printable Assignments for Chemistry Class 11 which you can download easily. All assignments have a collection of questions and answers designed for all topics given in your latest NCERT Books for Class 11 Chemistry for the current academic session. All Assignments for Chemistry Grade 11 have been designed by expert faculty members and have been designed based on the type of questions asked in standard 11 class tests and exams. All Free printable Assignments for NCERT CBSE Class 11, practice worksheets, and question banks have been designed to help you understand all concepts properly. Practicing questions given in CBSE NCERT printable assignments for Class 11 with solutions and answers will help you to further improve your understanding. Our faculty have used the latest syllabus for Class 11. You can click on the links below to download all Pdf assignments for class 11 for free. You can get the best collection of Kendriya Vidyalaya Class 11 Chemistry assignments and questions workbooks below.
Class 11 Chemistry Assignments Pdf Download
CBSE NCERT KVS Assignments for Chemistry Class 11 have been provided below covering all chapters given in your CBSE NCERT books. We have provided below a good collection of assignments in Pdf for Chemistry standard 11th covering Class 11 questions and answers for Chemistry. These practice test papers and workbooks with question banks for Class 11 Chemistry Pdf Download and free CBSE Assignments for Class 11 are really beneficial for you and will support in preparing for class tests and exams. Standard 11th students can download in Pdf by clicking on the links below.
Subjectwise Assignments for Class 11 Chemistry

Benefits of Solving Class 11 Chemistry Assignments
- The best collection of Grade 11 assignments for Chemistry have been provided below which will help you in getting better marks in class tests and exams.
- The solved question for Class 11 Chemistry will help you to gain more confidence to attempt all types of problems in exams
- Latest NCERT Books for Class 11 Chemistry have been referred to for designing these assignments
- We have provided step by step solutions for all questions in the Class 11 assignments so that you can understand the solutions in detail.
- We have provided single click download links to all chapterwise worksheets and assignments in Pdf.
- Class 11 practice question banks will support to enhance subject knowledge and therefore help to get better marks in exam
FAQs by Chemistry Students in Class 11
At https://www.ncertbooksolutions.com is the best website that has the biggest collection of free printable assignments for Class 11 Chemistry.
We provide here Standard 11 subject-wise assignments which can be easily downloaded in Pdf format for free. Our teachers have provided these Grade 11 Chemistry test sheets for Chemistry given in your books.
You can click on the links above and get assignments for Chemistry in Grade 11, all chapters and topic-wise question banks with solutions have been provided here. You can click on the links to download in Pdf.
We have provided here subject-wise Grade 11 Chemistry question banks, revision notes and questions for all difficult topics, and other study material. You can download it all without any charge by clicking on the links provided above.
We have provided the best quality question bank for Class 11 for Chemistry available for Pdf Download. You can download them all and use them offline without the internet.
Related Posts:

Related Posts

Assignments Class 11 Home Science Pdf Download

Assignments Class 11 Engineering Graphics Pdf Download

Assignments Class 11 Sociology Pdf Download
Talk to our experts
1800-120-456-456
NCERT Solutions for Class 11 Chemistry Chapter 2 - Structure Of Atom
- NCERT Solutions
- Chapter 2 Structure Of Atom

NCERT Solutions for Class 11 Chemistry Chapter 2: A Valuable Resource for In-depth Understanding
NCERT Solutions for Class 11 Chemistry Chapter 2 are available for you to download online in PDF format. The Class 11 Chemistry NCERT Solutions Chapter 2 are prepared by the experts of Vedantu who are working in this field for decades now. Our subject matter experts will get the right answers for all the questions that are asked in the examinations relating to Class 11 Chemistry Chapter 2. You can also download the Structure of Atom Class 11 questions and answers PDF, and go through them.
Note: ➤ Calculate your potential NEET rank based on marks with our NEET Rank Predictor by Marks !
Structure of Atom Chapter at a Glance - Class 11 NCERT Solutions
Constituents of atom: Atom is no longer considered as indivisible. It is made up of electrons, protons and neutrons called fundamental particles.
Electron: A fundamental particle which carries one unit negative charge and has a mass nearly equal to 1/1837th of that of hydrogen atom.
Proton: A fundamental particle which carries one unit positive charge and has a mass nearly equal to that of hydrogen atom.
Neutron: A fundamental particle which carries no charge but has a mass nearly equal to that of hydrogen atom.
Thomson’s model of atom: An atom is a sphere of positive electricity in which sufficient number of electrons were embedded to neutralize the positive charge just as seeds in a melon or raisins in pudding. It could not explain results of
Rutherford’s -rays scattering experiments.
Rutherford’s model of atom: A thin foil of gold was bombarded with -particles. Most of the particles passed through the foil undeflected, a few were deflected through small angle while very few were deflected back. It was therefore, concluded that there was sufficient empty space within the atom and small heavy positively charged body at the center called nucleus. Thus, atom consists of a heavy positively charged nucleus in the centre containing all protons and the electrons were revolving around the nucleus so that the centrifugal force balances the force of attraction.
Atomic number and mass number: The general notation that is used to represent the mass number and atomic number of a given atoms is $_{A}^{Z}\textrm{X}$
Where, X – symbol of element
A – Mass number
Z – atomic number
Isotopes: Isotopes are the atoms of the same element having identical atomic number but different mass number. The difference is due to the difference in number of neutrons.
Isobars: Atoms of different elements having different atomic numbers but same mass numbers are called isobars.
Isotones: Atoms of different elements which contain the same number of neutrons are called isotones.
Isoelectronic species: The species (atoms or ions) containing the same number of electrons are called isoelectronic.
Electromagnetic radiation: Energy is emitted continuously from any source in the form of radiations travelling in the form of waves and associated with electric and magnetic fields, oscillating perpendicular to each other and to the Direction of propagation of waves. All of them travel with the velocity of light.
Relationship between velocity, frequency & wavelength:
c =$\nu\lambda$
where c : speed of light i.e. 3 × 10 8 m/s in vacuum
$\nu$: frequency; $\lambda$ : wavelength
Electromagnetic spectrum: When all the electromagnetic radiations are arranged in increasing order of wavelength or decreasing frequency the band of radiations obtained is termed as electromagnetic spectrum.
Black body radiation: If the substance being heated is a black body (which is a perfect absorber and perfect radiator of energy) the radiation emitted is called black body radiation.
Photoelectric effect: When radiation of certain minimum frequency ($\nu_0$) strike the surface of a metal, electrons are ejected. This minimum energy ($h\nu_0$) is called wave function (W 0 ).
$h\nu =h\nu_0+(\frac{1}{2})m_e\nu^{2}$
Planck’s quantum theory: This theory was put forward to explain the limitations of electromagnetic wave theory. It suggests that radiant energy is emitted or absorbed discontinuously in the form of small packets of energy called quanta (called photons in case of light). Energy of each quantum (E) = hv where ‘h’ is Planck’s constant (= 6.626 × 10 -34 Js). Total energy emitted or absorbed = nhv where n is an interger.
Emission and Absorption Spectra: When light emitted from any source is directly passed on to prism and resolved, the spectrum obtained is called emission spectrum. In case of white light, e.g., from sun, it is resolved into seven colours (VIBGYOR). The spectrum obtained is called contiuous spectrum. If light emitted from a discharge tube is resolved, some coloured lines are obtained. The spectrum obtained is called line spectrum. It white light is first passed through the solution of a compound or vapour of a substance and then resolved, the spectrum obtained is called absorption spectrum. It has dark lines in the continuous spectrum.
Absorption spectrum of hydrogen: When H 2 gas is taken in the discharge tube, series of lines obtained and the regions in which they lie are as under:
$\text{Series:} \underbrace{Lyman}\;\underbrace{ Balmer}\;\underbrace{\text{Paschen Brackett Pfund}}$
Region:$\text{UV Visible Infrared}$
Rydberg formula: This formula is used to calculate wave number of different series of lines of the spectrum of hydrogen or hydrogen like particles as :
$\bar{\nu }=R\left ( \frac{1}{n^{2}_{i}} -\frac{1}{n^{2}_{f}}\right )\;Z^{2}\(\text{Z = 1 for hydrogen})$
where R = Rydberg constant = 109677 cm -1 or 1.097×10 7 m -1
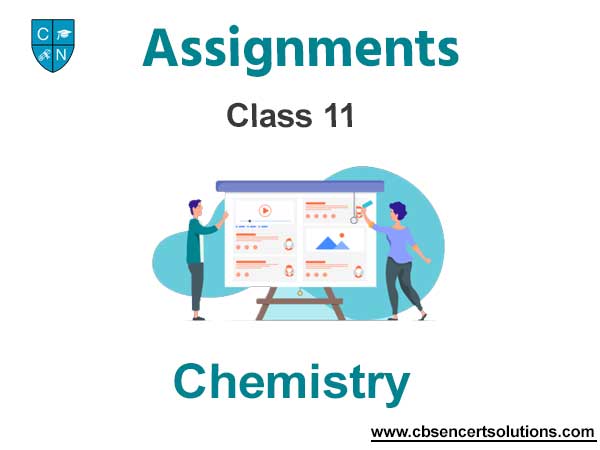
Bohr’s Model:
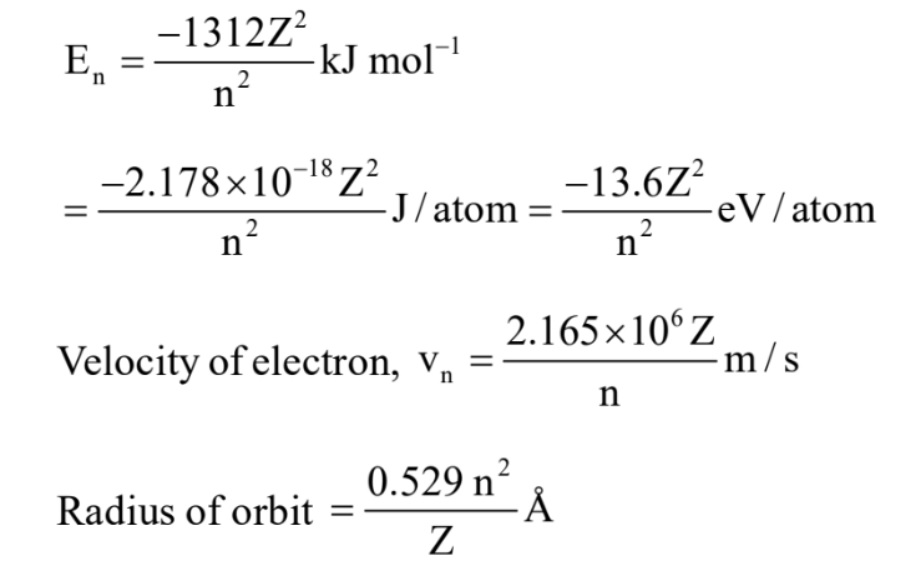
Dual Behaviour of Particle: According to de Broglie, every object in motion has a wave character. The wavelengths associated with ordinary objects are so short (because of their large masses) that their wave properties cannot be detected. The wavelengths associated with electrons and other subatomic particles (with very small mass) can however be detected experimentally.
$\lambda =\frac{h}{mv}=\frac{h}{p}=\frac{h}{\sqrt{2m(KE)}}$
Heisenberg’s Uncertainty Principle: It is impossible to measure simultaneously the position and momentum of a small particle with absolute accuracy. If an attempt is made to measure any of these two quantities with higher accuracy, the other becomes less accurate. The product of the uncertainty in the position (\Delta x) and the uncertainty in momentum (\Delta p) is always a constant and is equal to or greater than h/4\pi.
$(\Delta x).(\Delta p)\geq h/4\pim$
Quantum mechanical Model of atom: Quantum mechanics is a theoretical science that deals with the study of the motion of microscopic objects which have both particle like and wave like properties. The fundamental equation of quantum mechanics was developed by Schrodinger.
Quantum number: It is a set of four numbers which give complete information about any electron in an atom. These are:
Principal quantum number (n): It determines the size of the orbital. Its values are 1, 2, 3, etc. or K, L, M, etc. It also determines the energy of the main shell in which the elecron is present and maximum number of electrons present in the n th shell (= 2 n 2 ).
Azimuthal quantum number (l): It determines the number of subshells present in any main shell (n) and the shape of the subshell. For a given value of n, l = 0 to n - 1. Thus, for n = 1, l = 0 (one subshell), for n = 2 , l = 0, 1, (2 subshell), for n = 3, l = 0, 1, 2 (3 subshells), for n = 4, l = 0, 1, 2, 3 (4 subshells). For l = 0, 1, 2 and 3. designation are s, p, d and f respectively. Thus, subshells present are : n = 1 (1s), n = 2 (2s, 2p), n = 3 (3s, 3p, 3d), n = 4 (4s, 4p, 4d, 4f).
Magnetic quantum number (m): It determines the number of orbitals present in any subshell and the orientation of each orbital. For a given value of l, m = - l to + l including ‘0’.
Spin quantum number (s): It tells about the spinning motion of the electron, i.e., clockwise or anti-clockwise. For a given value of m,s=+\frac{1}{2} and -\frac{1}{2}. It helps to explain magnetic properties of the substances.
Shapes of atomic orbitals: The shape of an orbital is found by finding the probability (\psi^{2}) of the electron in that orbital at different points around the nucleus and representing by the density of points. The shape of the electron cloud thus obtained gives the shape of the orbital. Some orbitals are found to have a region of space within it where probability is zero. This is called a node. It may be spherical/radial or planar/angular.
Rules for filling of electrons in orbitals:
Aufbau principle: Orbitals are filled in order of their increasing energy. The order of energy and hence that of filling orbitals is found by (n + l) rule. It states “lower the (n + l) value, lower is the energy. If two orbitals have same (n + l) value, orbital with lower value of n has lower energy.” Thus, the order is:
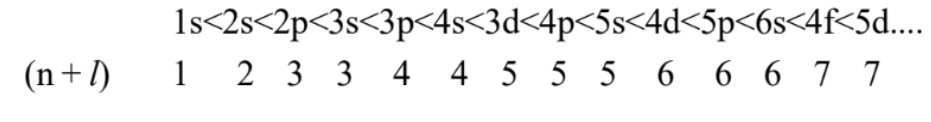
Hund’s rule of maximum multiplicity: Pairing of electrons does not occur in orbitals of the same subshell (degenerate orbitals) until each of them is first singly occupied. Pauli exclusion principle: No two electrons in an atom can have the same set of four quantum numbers or an orbital can have maximum two electrons and these must have opposite spin.
Electronic configuration of elements: Distribution of electrons of an atom into different shells, subshells and orbitals is called its electronic configuration. Complete electronic configuration is obtained by following the above rules, e.g.,
17 Cl = 1s 2 2s 2 2p 6 3s 2 3p 2 x 3p 2 y 3p 1 z

Access NCERT solutions for Class 11 Chemistry Chapter – 2 Structure of Atom
(i). Calculate the Number of Electrons Which Will Together Weigh One Gram.
Ans: Mass of one electron = \[9.10939\times {{10}^{-31}}kg\]
Number of electrons that weigh \[9.10939\times {{10}^{-31}}kg\]= 1
Number of electrons that will weigh 1 g = \[1\times {{10}^{-3}}kg\]
\[\frac{1}{9.10939\times {{10}^{-31}}kg}(1\times {{10}^{-3}}kg)=0.1098\times {{10}^{-3+31}}\]
\[=1.098\times {{10}^{27}}\]
(ii).Calculate the Mass and Charge of One Mole of Electrons.
Mass of one mole of electron = \[(6.022\times {{10}^{23}})\times (9.10939\times {{10}^{-31}}kg)\] = \[5.48\times {{10}^{-7}}kg\]
Charge on one electron = \[1.6022\times {{10}^{-19}}coulomb\]
Charge on one mole of electron = \[(1.6022\times {{10}^{-19}})(6.022\times {{10}^{23}})\] = \[9.65\times {{10}^{4}}C\]
(i).Calculate the Total Number of Electrons Present in One Mole of Methane.
Ans: Number of electrons present in 1 molecule of methane (\[C{{H}_{4}}\])= \[\{1(6)+4(1)\}=10\]
Number of electrons present in 1 mole i.e., \[6.023\times {{10}^{23}}\] molecules of methane = \[6.023\times {{10}^{23}}\times 10=6.023\times {{10}^{24}}\]
(ii). a) Find the Total Number of Neutrons in 7mg of ${}^{14}C$ . (Assume the Mass of a Neutron = $1.675\times {{10}^{-27}}kg$ )
Ans: Number of atoms of \[{}^{14}C\]in 1 mole = \[6.023\times {{10}^{23}}\]
Since 1 atom of \[{}^{14}C\] contains (14-6) i.e., 8 neutrons, the number of neutrons in 14g of \[{}^{14}C\] is \[(6.023\times {{10}^{23}})\times 8\]
Number of neutrons in 7 mg
\[=\frac{6.022\times {{10}^{23}}\times 8\times 7}{1400mg}\]
\[=2.4092\times {{10}^{21}}\]
(b) Find the Total Mass of Neutrons in 7mg of ${}^{14}C$ . (Assume the Mass of a Neutron = $1.675\times {{10}^{-27}}kg$ )
Ans: Mass of one neutron = \[1.67493\times {{10}^{-27}}kg\] Mass of total neutrons in 7g of \[{}^{14}C\]
\[=(2.4092\times {{10}^{21}})(1.67493\times {{10}^{-27}}kg)\]
\[=4.0352\times {{10}^{-6}}kg\]
(iii). (a) Find the Total Number of Protons in 34mg of $N{{H}_{3}}$ at STP.
Ans: 1 mole of \[N{{H}_{3}}\] = {1(14) +3(1)} g of \[N{{H}_{3}}\] = 17g of \[N{{H}_{3}}\]
\[=6.023\times {{10}^{23}}\] Molecules of \[N{{H}_{3}}\]
Total number of protons present in 1 molecule of \[N{{H}_{3}}\]
= [1(7) +1(3)] =10
Number of protons in \[6.023\times {{10}^{23}}\] molecules of \[N{{H}_{3}}\]
\[=(6.023\times {{10}^{23}})(10)=6.023\times {{10}^{24}}\]
17g of \[N{{H}_{3}}\] contains \[(6.023\times {{10}^{24}})\] protons.
Number of protons in 34mg of \[N{{H}_{3}}\]
\[=\frac{6.022\times {{10}^{24}}\times 34mg}{1700mg}\]
\[=1.2046\times {{10}^{22}}\]
(b) Find the total mass of protons in 34mg of $N{{H}_{3}}$ at STP. Will the answer change if the temperature and pressure changed?
Ans: Mass of one proton = \[1.67493\times {{10}^{-27}}kg\]
Total mass of protons in 34mg of \[N{{H}_{3}}\]
\[=(1.67493\times {{10}^{-27}}kg)(1.2046\times {{10}^{22}})\]
\[=2.0176\times {{10}^{-5}}kg\]
The number of protons, electrons, and neutrons in an atom is independent of temperature and pressure conditions. Hence, the obtained values will remain unchanged if the temperature and pressure is changed.
3. How Many Neutrons and Protons are There in the Following Nuclei? \[_{6}^{13}C,_{8}^{16}O,_{12}^{24}Mg,_{26}^{56}Fe,_{38}^{88}Sr\]
Ans: $_{6}^{13}C$ :
Atomic mass =13
Atomic number = Number of Protons = 6
Number of neutrons = (Atomic Mass)-(Atomic Number)=13-6=7
$_{8}^{16}O$ :
Atomic mass =16
Atomic number = Number of Protons = 8
Number of neutrons = (Atomic Mass)-(Atomic Number) =16-8=8
$_{12}^{24}Mg$ :
Atomic mass =24
Atomic number = Number of Protons = 12
Number of neutrons = (Atomic Mass)-(Atomic Number)=24-12 =12
$_{26}^{56}Fe$ :
Atomic mass = 56
Atomic number = Number of Protons = 26
Number of neutrons = (Atomic Mass)-(Atomic Number) = 56-26=30
$_{38}^{88}Sr$ :
Atomic mass = 88
Atomic number = Number of Protons = 38
Number of neutrons = (Atomic Mass)-(Atomic Number)= 88-38=50
4. Write the Complete Symbol for the Atom with the Given Atomic Number (Z) and Atomic mass (A)
a. Z = 17, A = 35
Ans: $_{17}^{35}Cl$
b. Z = 92, A = 233
Ans: $_{92}^{233}U$
c. Z = 4, A = 9
Ans: $_{4}^{9}Be$
5. Yellow light emitted from a sodium lamp has a wavelength ($\lambda $ ) of 580 nm. Calculate the frequency ($\upsilon $) and wave number ( $\overset{-}{\mathop{\upsilon }}\,$ ) of the yellow light.
From the expression,
\[\lambda =\frac{c}{\upsilon }\]
$\upsilon =\frac{c}{\lambda }$ ----- (i)
Where,
$\upsilon $ = frequency of yellow light
c = velocity of light in vacuum = $3\times {{10}^{8}}m/s$
$\lambda $ = wavelength of yellow light = 580nm = $580\times {{10}^{-9}}m$
Substituting the values in the expression (i)
\[\upsilon =\frac{3\times {{10}^{8}}}{580\times {{10}^{-9}}}=5.17\times {{10}^{14}}{{S}^{-1}}\]
Thus, frequency of yellow light emitted from sodium lamp
= $5.17\times {{10}^{14}}{{S}^{-1}}$
Wave number of yellow light, \[\overset{-}{\mathop{\upsilon }}\,=\frac{1}{\lambda }=\frac{1}{580\times {{10}^{-9}}m}=1.72\times {{10}^{6}}{{m}^{-1}}\]
(i). Find energy of each of the photons which correspond to light of frequency $3\times {{10}^{15}}Hz$
Ans: Energy (E) of a photon is given by the expression,
\[E=h\upsilon \]
Where, h = Planck’s constant = $6.626\times {{10}^{-34}}Js$
$\upsilon $ = frequency of light = $3\times {{10}^{15}}Hz$
Substituting the values in the given expression of Energy, $E=(6.626\times {{10}^{-34}})(3\times {{10}^{15}})=1.988\times {{10}^{-18}}J$
(ii). Find energy of each of the photons which have wavelength of $0.50{{A}^{o}}$
Ans: Energy (E) of a photon having wavelength ($\lambda $) is given by the expression,
\[E=\frac{hc}{\lambda }\]
Where, h = Planck’s constant = $6.626\times {{10}^{-34}}Js$
c = velocity of light in vacuum = $3\times {{10}^{8}}m/s$
$\lambda $ = wavelength of yellow light = $0.50{{A}^{o}}$ = $0.5\times {{10}^{-10}}m$
\[E=\frac{(6.626\times {{10}^{-34}})(3\times {{10}^{8}})}{0.5\times {{10}^{-10}}}J\]
\[E=3.98\times {{10}^{-15}}J\]
7. Calculate the wavelength, frequency and wave number of a light wave whose period is $2.0\times {{10}^{-10}}s$
Ans: Frequency ($\upsilon $) of light = $\frac{1}{period}=\frac{1}{2\times {{10}^{-10}}s}=5\times {{10}^{9}}{{s}^{-1}}$
Wavelength of light, \[\lambda =\frac{c}{\upsilon }\]
Substitution the value in the given expression $\lambda $ ,
\[\lambda =\frac{3\times {{10}^{8}}}{5\times {{10}^{9}}}=6.0\times {{10}^{-2}}m\]
Wave number of light, $\overset{-}{\mathop{\upsilon }}\,=\frac{1}{\lambda }=\frac{1}{6.0\times {{10}^{-2}}}=16.66m$
8. What is the number of photons of light with a wavelength of 4000 pm that provide 1 J of energy?
Energy of ‘n’ photons,
\[{{E}_{n}}=nh\upsilon \]
\[\Rightarrow n=\frac{{{E}_{n}}\lambda }{hc}\]
Where, $\lambda $ = wavelength of yellow light = $4000pm=4000\times {{10}^{-12}}m$
h = Planck’s constant = $6.626\times {{10}^{-34}}Js$
Substituting the values in the given expression ‘n’,
\[n=\frac{(1)\times (4000\times {{10}^{-12}})}{(6.626\times {{10}^{-34}})(3\times {{10}^{8}})}=2.012\times {{10}^{16}}\]
Hence, the number of photons with a wavelength of 4000 pm and energy of 1 J are $2.012\times {{10}^{16}}$
9. A photon of wavelength $4\times {{10}^{7}}m$ strikes on metal surface, the work function of the metal being 2.13eV.
(i). Calculate the energy of photon (eV)
Ans: Energy of photon, $E=h\upsilon =\frac{hc}{\lambda }$
Where, c = velocity of light in vacuum = $3\times {{10}^{8}}m/s$
$\lambda $ = wavelength of yellow light = $4\times {{10}^{-7}}m$
Substituting the values in the given expression of E,
\[E=\frac{(6.626\times {{10}^{-34}})(3\times {{10}^{8}})}{4\times {{10}^{-7}}}=4.9695\times {{10}^{-19}}J\]
Hence, the energy of photon is $4.9695\times {{10}^{-19}}J$
(ii). Calculate the kinetic energy of the emission.
Ans: the kinetic energy of emission is given by,
\[{{E}_{k}}=h\upsilon -h{{\upsilon }_{0}}=(E-W)eV\]
\[=[\frac{4.9696\times {{10}^{-19}}}{1.6020\times {{10}^{-19}}}]-2.13eV=0.9720eV\]
Hence, the kinetic energy of emission is 0.97eV
(iv). Calculate the velocity of the photoelectron ($1eV=1.6020\times {{10}^{-19}}J$ )
Ans: The velocity of a photoelectron (ν) can be calculated by the expression,
\[\frac{1}{2}m{{v}^{2}}=h\upsilon -h{{\upsilon }_{0}}\]
\[\Rightarrow v=\sqrt{\frac{2(h\upsilon -h{{\upsilon }_{0}})}{m}}\]
Where, $h\upsilon -h{{\upsilon }_{0}}$ is the kinetic energy of emission in Joules and ‘m’ is the mass of the photoelectron. Substituting the values in the given expression of v:
\[v=\sqrt{\frac{2(0.9720\times 1.6020\times {{10}^{-19}})}{9.10939\times {{10}^{-31}}kg}J}\]
\[\Rightarrow v=\sqrt{0.3418\times {{10}^{12}}{{m}^{2}}{{s}^{-2}}}=5.84\times {{10}^{5}}m{{s}^{-1}}\]
Hence, the velocity of the photoelectron is $5.84\times {{10}^{5}}m{{s}^{-1}}$
10. Electromagnetic radiation of wavelength 242 nm is just sufficient to ionize the sodium atom. Calculate the ionization energy of sodium in $kJmo{{l}^{-1}}$
Ans: Energy of sodium, $E=\frac{{{N}_{A}}hc}{\lambda }$
\[E=\frac{(6.023\times {{10}^{23}}mo{{l}^{-1}})(6.626\times {{10}^{-34}}Js)(3\times {{10}^{8}}m{{s}^{-1}})}{242\times {{10}^{-9}}m}=4.947\times {{10}^{5}}Jmo{{l}^{-1}}\]
Hence, the ionization energy of sodium is $494kJmo{{l}^{-1}}$
11. A 25 watt bulb emits monochromatic yellow light of wavelength of $0.57\mu m$ . Calculate the rate of emission of quanta per second.
Ans: power of Bulb, P = 25 Watt = $25J{{s}^{-1}}$
Energy of one photon, $E=h\upsilon =\frac{hc}{\lambda }$
\[E=\frac{(6.626\times {{10}^{-34}})(3\times {{10}^{8}})}{0.57\times {{10}^{-6}}}=34.87\times {{10}^{-20}}J\]
Rate of emission of quanta per second,
\[=\frac{25}{34.87\times {{10}^{-20}}}=7.169\times {{10}^{19}}{{s}^{-1}}\]
12. Electrons are emitted with zero velocity from a metal surface when it is exposed to radiation of wavelength$6800{{A}^{0}}$. Calculate threshold frequency (${{\upsilon }_{0}}$) and the work function (${{w}_{0}}$) of the metal.
Ans: Threshold wavelength of radian (${{\lambda }_{0}}$) = $6800{{A}^{0}}=6800\times {{10}^{10}}m$
Threshold frequency of metal (${{\upsilon }_{0}}$) = $\frac{c}{{{\lambda }_{0}}}=\frac{3\times {{10}^{8}}m{{s}^{-1}}}{6.8\times {{10}^{-7}}m}=4.41\times {{10}^{14}}{{s}^{-1}}$
Thus, threshold frequency of the metal is $4.41\times {{10}^{14}}{{s}^{-1}}$
Hence, the work function of the metal is,
\[{{w}_{0}}=h{{v}_{0}}=(6.626\times {{10}^{-34}}Js)(4.41\times {{10}^{14}}{{s}^{-1}})=2.922\times {{10}^{-19}}J\]
13. What is the wavelength of light emitted when the electron in a hydrogen atom undergoes transition from an energy level with n = 4 to an energy level with n =2?
Ans: The ${{n}_{i}}=4$ to ${{n}_{f}}=2$ transition will rise to a spectral line of the Balmer series. The energy involved in the transition is given by the relation,
\[E=2.18\times {{10}^{-18}}\left[ \frac{1}{{{n}_{i}}}-\frac{1}{{{n}_{f}}} \right]\]
\[E=2.18\times {{10}^{-18}}\left[ \frac{1}{{{n}_{i}}^{2}}-\frac{1}{{{n}^{2}}_{f}} \right]=2.18\times {{10}^{-18}}\left[ \frac{1}{{{4}^{2}}}-\frac{1}{{{2}^{2}}} \right]=-4.0875\times {{10}^{-19}}J\]
The negative sign indicates the energy of emission.
Wavelength of light emitted, \[\lambda =\frac{hc}{E}\]
Substituting the values in the given expression of \[\lambda \] :
\[\lambda =\frac{(6.626\times {{10}^{-34}})(3\times {{10}^{8}})}{4.0875\times {{10}^{-19}}}\]
\[\lambda =4.8631\times {{10}^{-7}}m\]
\[\lambda =486nm\]
14. How much energy is required to ionize a H atom if the electron occupies n = 5 orbit? Compare your answer with the ionization enthalpy of the H atom (energy required to remove the electron from n =1 orbit).
Ans: The expression of energy is given by,
\[{{E}_{n}}=\frac{-(2.18\times {{10}^{-18}}){{Z}^{2}}}{{{n}^{2}}}\]
Where, Z = atomic number of atom
n = principal quantum number
For ionization from ${{n}_{1}}=5$ to ${{n}_{2}}=\infty $
\[\Delta E={{E}_{\infty }}-{{E}_{5}}\]
\[\Rightarrow \Delta E=\left[ \left( \frac{(-2.18\times {{10}^{-18}}J)({{1}^{2}})}{{{\infty }^{2}}} \right)-\left( \frac{(-2.18\times {{10}^{-18}}J)({{1}^{2}})}{{{5}^{2}}} \right) \right]=2.18\times {{10}^{-18}}J\]
Hence, the energy required for ionization from \[{{n}_{1}}=5\] to \[{{n}_{2}}=\infty \] is \[8.72\times {{10}^{20}}J\]
The energy required for ionization from \[{{n}_{1}}=1\] to \[{{n}_{2}}=\infty\]
\[\Delta E={{E}_{\infty }}-{{E}_{1}}\]
\[\Rightarrow \Delta E=\left[ \left( \frac{(-2.18\times {{10}^{-18}}J)({{1}^{2}})}{{{\infty }^{2}}} \right)-\left( \frac{(-2.18\times {{10}^{-18}}J)({{1}^{2}})}{{{1}^{2}}} \right) \right]=2.18\times {{10}^{-18}}J\]
Hence, less energy is required to ionize an electron in the ${{5}^{th}}$ orbital of a hydrogen atom as compared to that in the ground state.
15. What is the maximum number of emission lines when the excited electron of an H atom in n = 6 drops to the ground state?
Ans: When the excited electron of an H atom in n = 6 drops to the ground state, the following transitions are possible:
(Image will be uploaded soon)
Hence, a total number of (5 + 4 + 3 + 2 + 1) 15 lines will be obtained in the emission spectrum. The number of spectral lines produced when an electron in the ${{n}^{th}}$ level drops down to the ground state is given by. $\frac{n(n-1)}{2}$
Number of spectral lines for n=6, $\frac{6(6-1)}{2}=15$
(i). The energy associated with the first orbit in the hydrogen atom is $-2.18\times {{10}^{18}}J $&$ ato{{m}^{-1}}$. What is the energy associated with the fifth orbit?
Ans: Energy associated with the fifth orbit of hydrogen atom is calculated as:
\[{{E}_{5}}=\frac{-(2.18\times {{10}^{-18}})}{{{(5)}^{2}}}=\frac{-2.18\times {{10}^{-18}}}{25}\]
\[{{E}_{5}}=-8.72\times {{10}^{-20}}J\]
(ii). Calculate the radius of Bohr’s fifth orbit for hydrogen atom.
Ans: Radius of Bohr’s ${{n}^{th}}$ orbit for hydrogen atom is given by,
\[{{r}_{n}}=(0.0529nm){{n}^{2}}\]
\[{{r}_{5}}=(0.0529nm)\times {{5}^{2}}\]
\[{{r}_{5}}=1.3225nm\]
17. Calculate the wave number for the longest wavelength transition in the Balmer series of atomic hydrogen.
Ans: For the Balmer series, ${{n}_{i}}=2$ . Thus, the expression of wave number ($\overset{-}{\mathop{\upsilon }}\,$) is given by
\[\overset{-}{\mathop{\upsilon }}\,=\left( \frac{1}{{{(2)}^{2}}}-\frac{1}{{{n}^{2}}_{f}} \right)(1.097\times {{10}^{7}}{{m}^{-1}})\]
Wave number ($\overset{-}{\mathop{\upsilon }}\,$) is inversely proportional to wavelength of transition. Hence, for the longest wavelength transition, ($\overset{-}{\mathop{\upsilon }}\,$) has to be the smallest.
For ($\overset{-}{\mathop{\upsilon }}\,$) to be minimum, ${{n}_{f}}$ should be minimum. For the Balmer series, a transition from ${{n}_{i}}$ = 2 to ${{n}_{f}}$= 3 is allowed. Hence, taking ${{n}_{f}}$ = 3, we get:
\[\overset{-}{\mathop{\upsilon }}\,=\left( \frac{1}{{{(2)}^{2}}}-\frac{1}{{{(3)}^{2}}} \right)(1.097\times {{10}^{7}}{{m}^{-1}})\]
\[\overset{-}{\mathop{\upsilon }}\,=\left( \frac{1}{4}-\frac{1}{9} \right)(1.097\times {{10}^{7}}{{m}^{-1}})\]
\[\overset{-}{\mathop{\upsilon }}\,=1.5236\times {{10}^{6}}{{m}^{-1}}\]
18. What is the energy in joules required to shift the electron of the hydrogen atom from the first Bohr orbit to the fifth Bohr orbit and what is the wavelength of the light emitted when the electron returns to the ground state? The ground state electron energy is $-2.18\times {{10}^{-11}}ergs$
Ans: Energy (E) of the ${{n}^{th}}$ Bohr orbit of an atom is given by,
\[{{E}_{n}}=\frac{-(2.18\times {{10}^{-18}}){{Z}^{2}}}{{{n}^{2}}}\]
Where, Z = atomic number of the atom
Ground state energy= $-2.18\times {{10}^{-11}}ergs=-2.18\times {{10}^{-11}}\times {{10}^{-7}}J=-2.18\times {{10}^{-18}}J$
Energy required to shift the electron from n = 1 to n = 5 is given as:
\[\Delta E={{E}_{5}}-{{E}_{1}}\]
\[=\frac{-(2.18\times {{10}^{-18}}){{(1)}^{2}}}{{{(5)}^{2}}}-(-2.18\times {{10}^{-18}})\]
\[=(2.18\times {{10}^{-18}})\left( \frac{24}{25} \right)=2.0928\times {{10}^{-18}}\]
Weight of emitted light = $\frac{hc}{E}$
\[=\frac{(6.626\times {{10}^{-34}})(3\times {{10}^{8}})}{(2.0928\times {{10}^{-18}})}=9.498\times {{10}^{-8}}m\]
19. The electron energy in hydrogen atom is given by ${{E}_{n}}=\frac{(-2.18\times {{10}^{-18}})}{{{n}^{2}}}J$ Calculate the energy required to remove an electron completely from the n = 2 orbit. What is the longest wavelength of light in cm that can be used to cause this transition?
Ans: ${{E}_{n}}=\frac{(-2.18\times {{10}^{-18}})}{{{n}^{2}}}J$
Given,
\[\Delta E={{E}_{\infty }}-{{E}_{2}}\]
\[\Delta E=\left( \frac{(-2.18\times {{10}^{-18}})}{{{\infty }^{2}}} \right)-\left( \frac{(-2.18\times {{10}^{-18}})}{{{2}^{2}}} \right)=0.545\times {{10}^{-18}}J\]
Energy required for ionization from n = 2 is given by, \[0.545\times {{10}^{-18}}J\]
\[\Delta E=5.45\times {{10}^{-19}}J\]
\[\lambda =\frac{hc}{\Delta E}\] , here $\lambda $ is the longest wavelength causing the transition.
\[\lambda =\frac{(6.626\times {{10}^{-34}})(3\times {{10}^{8}})}{5.45\times {{10}^{-19}}}=3.647\times {{10}^{-7}}m\]
\[\lambda =3647{{A}^{0}}\]
20. Calculate the wavelength of an electron moving with a velocity of $2.05\times {{10}^{7}}m{{s}^{-1}}$
Ans: According to de Broglie’s equation, $\lambda =\frac{h}{mv}$
Where,$\lambda $ = wavelength of moving particle
m = mass of particle, v = velocity of particle, h = Planck’s constant
Substituting the values in the expression of $\lambda $:
\[\lambda =\frac{6.626\times {{10}^{-34}}Js}{(9.10939\times {{10}^{-31}}kg)(2.05\times {{10}^{7}}m{{s}^{-1}})}=3.548\times {{10}^{-11}}m\]
Hence, the wavelength of an electron moving with a velocity of $2.05\times {{10}^{7}}m{{s}^{-1}}$ is $3.548\times {{10}^{-11}}m$
21. The mass of an electron is $9.1\times {{10}^{-31}}kg$ . If it is K.E. is $3.0\times {{10}^{-25}}J$ . Calculate its wavelength.
Ans : According to de Broglie’s equation, $\lambda =\frac{h}{mv}$
Given, the K.E. of electrons is$3.0\times {{10}^{-25}}J$.
Since, $K.E=\frac{1}{2}m{{v}^{2}}$
Velocity (v) = $\sqrt{\frac{2K.E}{m}}$
\[\Rightarrow v=\sqrt{\frac{2(3.0\times {{10}^{-25}}J)}{9.10939\times {{10}^{-31}}kg}=}\sqrt{6.5866\times {{10}^{4}}}\]
V= $811.579m{{s}^{-1}}$
Substituting the value in the expression of $\lambda $ :
\[\lambda =\frac{6.626\times {{10}^{-34}}Js}{(9.10939\times {{10}^{-31}}kg)(811.579m{{s}^{-1}})}\]
\[\lambda =8.9625\times {{10}^{-7}}m\]
22. Which of the following are isoelectronic species i.e., those having the same number of electrons? $N{{a}^{+}},{{K}^{+}},M{{g}^{+2}},C{{a}^{+2}},{{S}^{-2}},Ar$
Ans: Isoelectronic species have the same number of electrons.
Number of electrons in sodium (Na) = 11
Number of electrons in ($N{{a}^{+}}$) = 10
A positive charge denotes the loss of an electron.
Similarly,
Number of electrons in ${{K}^{+}}$ = 18
Number of electrons in $M{{g}^{+2}}$ = 10
Number of electrons in $C{{a}^{+2}}$ = 18
A negative charge denotes the gain of an electron by a species.
Number of electrons in sulphur (S) = 16
$\therefore $ Number of electrons in ${{S}^{-2}}$ = 18
Number of electrons in argon (Ar) = 18
Hence, the following are isoelectronic species:
(1) $N{{a}^{+}}$and $M{{g}^{+2}}$ (10 electrons each)
(2) ${{K}^{+}}$,$C{{a}^{+2}}$, ${{S}^{-2}}$and Ar (18 electrons each).
(i). Write the Electronic Configurations of the Following ions:
(a) ${{H}^{-}}$ ion
Ans: The electronic configuration of H atom is $1{{s}^{1}}$
A negative charge on the species indicates the gain of an electron by it.
$\therefore $ Electronic configuration of ${{H}^{-}}$ is $1{{s}^{2}}$
(b) $N{{a}^{+}}$ion
Ans: The electronic configuration of Na atom is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{1}}$
A positive charge on the species indicates the loss of an electron by it.
$\therefore $Electronic configuration of $N{{a}^{+}}$is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{0}}or1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}$
(c) ${{O}^{-2}}$ ion
Ans: The electronic configuration of O atom is $1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}$
A negative charge on the species indicates the gain of two electrons by it.
$\therefore $Electronic configuration of ${{O}^{-2}}$is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}$
(d) ${{F}^{-}}$ ion
Ans: The electronic configuration of F atom is $1{{s}^{2}}2{{s}^{2}}2{{p}^{5}}$
$\therefore $Electronic configuration of ${{F}^{-}}$is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}$
(ii). What are the Atomic Numbers of Elements Whose Outermost Electrons are Represented by
(a) $3{{s}^{1}}$
Ans: Completing the electron configuration of the element as $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{1}}$
$\therefore $ Number of electrons present in the atom of the element
= 2 + 2 + 6 + 1 = 11
$\therefore $Atomic number of the element = 11
(b) $2{{p}^{3}}$
Ans: Completing the electron configuration of the element as $1{{s}^{2}}2{{s}^{2}}2{{p}^{3}}$
$\therefore $Atomic number of the element = 7
(c) $3{{p}^{5}}$
Ans: Completing the electron configuration of the element as $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{5}}$
= 2+2+6+2+5=17
$\therefore $Atomic number of the element = 17
(iii).Which Atoms are Indicated by the Following Configurations?
(a) $[He]2{{s}^{1}}$
Ans: The electronic configuration of the element is $[He]2{{s}^{1}}$= $1{{s}^{2}}2{{s}^{1}}$
$\therefore $ Atomic number of the element = 3
Hence, the element with the electronic configuration $[He]2{{s}^{1}}$is lithium (Li).
(b) $[Ne]3{{s}^{2}}3{{p}^{3}}$
Ans: The electronic configuration of the element is $[Ne]3{{s}^{2}}3{{p}^{3}}$= $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{3}}$ .
$\therefore $ Atomic number of the element = 15
Hence, the element with the electronic configuration $[Ne]3{{s}^{2}}3{{p}^{3}}$is phosphorus (P).
(c) $[Ar]4{{s}^{2}}3{{d}^{1}}$
Ans: The electronic configuration of the element is $[Ar]4{{s}^{2}}3{{d}^{1}}$=$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{1}}$ .
$\therefore $Atomic number of the element = 21
Hence, the element with the electronic configuration $[Ar]4{{s}^{2}}3{{d}^{1}}$is scandium (Sc).
24. What is the Lowest Value of n That Allows G Orbitals To Exist?
Ans: For g-orbitals, l = 4.
As for any value ‘n’ of principal quantum number, the Azimuthal quantum number (l) can have a value from zero to (n – 1).
$\therefore $ For l = 4, minimum value of n = 5.
25. An Electron Is in One of the 3D Orbitals. Give the Possible Values of n, l and m, for This Electron.
Ans: For the 3d orbital:
Principal quantum number (n) = 3
Azimuthal quantum number (l) = 2
Magnetic quantum number (${{m}_{l}}$) = –2, –1, 0, 1, 2
(i). An Atom of an Element Contains 29 Electrons and 35 Neutrons. Deduce the Number of Protons.
Ans: For an atom to be neutral, the number of protons is equal to the number of electrons.
$\therefore $ Number of protons in the atom of the given element = 29
(II). An Atom of an Element Contains 29 Electrons and 35 Neutrons. Deduce the Electronic Configuration of the Given Element.
Ans: The electronic configuration of the atom is $1{{s}^{2}}2{{s}^{2}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{10}}$
The name of the element is Copper $_{29}^{35}Cu$
27. Give the Number of Electrons in the Species ${{H}_{2}}^{+}$ , ${{H}_{2}}$ and ${{O}_{2}}^{+}$
Ans: ${{H}_{2}}^{+}$:
Number of electrons present in hydrogen molecule (${{H}_{2}}$) = 1 + 1 = 2
$\therefore$Number of electrons in ${{H}_{2}}^{+}$= 2 – 1 = 1
${{H}_{2}}$:
Number of electrons in${{H}_{2}}$ = 1 + 1 = 2
${{O}_{2}}^{+}$:
Number of electrons present in oxygen molecule (${{O}_{2}}$) = 8 + 8 = 16
$\therefore$ Number of electrons in ${{O}_{2}}^{+}$= 16-1 = 15
(i). An atomic orbital has n = 3. What are the possible values of l and ${{m}_{l}}$ ?
Ans: n = 3 (Given)
For a given value of n, l can have values from 0 to (n – 1).
$\therefore$For n = 3
l = 0, 1, 2
For a given value of l, ml can have (2l + 1) values.
$\therefore$For l = 0, m = 0
l = 1, m = –1, 0, 1
l = 2, m = –2, –1, 0, 1, 2
${{m}_{0}}$ = 0
${{m}_{1}}$ = –1, 0, 1
${{m}_{2}}$ = –2, –1, 0, 1, 2
(ii). List the quantum numbers (${{m}_{l}}$ and l) of electrons for 3d orbital.
Ans: For 3d orbital, l = 2.
For a given value of l, ${{m}_{l}}$ can have (2l + 1) values i.e., 5 values.
$\therefore$For l = 2
${{m}_{2}}$ = –2, –1, 0, 1, 2
(iii). Which of the following orbitals are possible?
1p, 2s, 2p and 3f
Ans: Among the given orbitals only 2s and 2p are possible. 1p and 3f cannot exist. For p-orbital, l = 1.
For a given value of n, l can have values from zero to (n – 1).
Therefore for l is equal to 1, the minimum value of n is 2.
For f-orbital, l = 4.
For l = 4, the minimum value of n is 5. Hence, 1p and 3f do not exist.
29. Using s, p, d notations, describes the orbital with the following quantum numbers.
a) n = 1, l = 0;
Ans: n = 1, l = 0 (Given) the orbital is 1s.
b) n = 3; l =1
Ans: For n = 3 and l = 1 the orbital is 3p.
c) n = 4; l = 2;
Ans: For n = 4 and l = 2 the orbital is 4d.
d) n = 4; l =3.
Ans: For n = 4 and l = 3 the orbital is 4f.
30. Explain, Giving Reasons, Which of the Following Sets of Quantum Numbers are Not
a) The given set of quantum numbers is not possible because the value of the principal quantum number (n) cannot be zero.
b) The given set of quantum numbers is possible.
c) The given set of quantum numbers is not possible. For a given value of n, ‘l’ can have values from zero to (n – 1). For n = 1, l = 0 and not 1.
d) The given set of quantum numbers is possible.
e) The given set of quantum numbers is not possible. For n = 3,
l = 0 to (3 – 1)
l = 0 to 2 i.e., 0, 1, 2
f) The given set of quantum numbers is possible.
31. How Many Electrons in an Atom May Have the Following Quantum Numbers?
a) n = 4, and ${{m}_{s}}=-\frac{1}{2}$
Total number of electrons in an atom for a value of n = $2{{n}^{2}}$
$\therefore $ For n = 4,
Total number of electrons = $2{{(4)}^{2}}=32$
The given element has a fully filled orbital as $1{{s}^{2}}2{{s}^{2}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{10}}$
Hence, all the electrons are paired.
$\therefore $ Number of electrons (having n = 4 and ${{m}_{s}}=-\frac{1}{2}$ ) = 16
b) n = 3, l = 0
Ans: n = 3, l = 0 indicates that the electrons are present in the 3s orbital. Therefore, the number of electrons having n = 3 and l = 0 is 2.
32. Show That the Circumference of the Bohr Orbit for the Hydrogen Atom is an Integral Multiple of the De Broglie Wavelength Associated With the Electron Revolving Around the Orbit.
Ans: Since a hydrogen atom has only one electron, according to Bohr’s postulate, the angular momentum of that electron is given by:
$mvr=n\frac{h}{2\pi }$ ----------- (i)
Where, n = 1, 2, 3 …
According to de Broglie’s equation:
$\lambda =\frac{h}{mv}$ Or $mv=\frac{h}{\lambda }$ ------- (ii)
Substituting the value of ‘mv’ from expression (ii) in expression (i):
$\frac{hr}{\lambda }=n\frac{h}{2\pi }$ ----------------- (iii)
Since $2\pi r$ represents the circumference of the Bohr orbit (r), it is proved by equation (iii) that the circumference of the Bohr orbit of the hydrogen atom is an integral multiple of de Broglie’s wavelength associated with the electron revolving around the orbit.
33. What Transition in the Hydrogen Spectrum Would have the Same Wavelength as the Balmer Transition n = 4 to n = 2 of $H{{e}^{+}}$ spectrum?
Ans: for $H{{e}^{+}}$ion, the wave number $\overset{-}{\mathop{v}}\,$ associated with the Balmer transition, n=4 to n =2 given by:
\[\overset{-}{\mathop{v}}\,=\frac{1}{\lambda }=R{{Z}^{2}}\left( \frac{1}{{{n}_{1}}^{2}}-\frac{1}{{{n}^{2}}_{2}} \right)\]
Where, ${{n}_{1}}=2and{{n}_{2}}=4$ , Z = atomic number of Helium
\[\overset{-}{\mathop{v}}\,=\frac{1}{\lambda }=R{{2}^{2}}\left( \frac{1}{{{2}^{2}}}-\frac{1}{{{4}^{2}}} \right)=\frac{3R}{4}\]
\[\Rightarrow \lambda =\frac{4}{3R}\]
According to the question, the desired transition for hydrogen will have the same wavelength as that of $H{{e}^{+}}$.
\[R{{(1)}^{2}}\left( \frac{1}{{{n}_{1}}^{2}}-\frac{1}{{{n}_{2}}^{2}} \right)=\frac{3R}{4}\]
\[\left( \frac{1}{{{n}_{1}}^{2}}-\frac{1}{{{n}_{2}}^{2}} \right)=\frac{3}{4}\] -------- (1)
By hit and trial method, the equality given by equation (1) is true only when ${{n}_{1}}=1$ and ${{n}_{2}}=2$
The transition for ${{n}_{2}}=2$to ${{n}_{1}}=1$ in hydrogen spectrum would have the same wavelength as Balmer transition n = 4 to n = 2 of $H{{e}^{+}}$spectrum.
34. Calculate the energy required for the process $H{{e}^{+}}_{(g)}\to H{{e}_{(g)}}^{2+}+{{e}^{-}}$
The ionization energy for the H atom in the ground state is $2.18\times {{10}^{-18}}Jato{{m}^{-1}}$ .
Ans: Energy associated with hydrogen-like species is given by,
\[{{E}_{n}}=\frac{-(2.18\times {{10}^{-18}}){{Z}^{2}}}{{{n}^{2}}}\]
For ground state of hydrogen atom,
\[\Delta E={{E}_{\infty }}-{{E}_{1}}\]
\[\Rightarrow \Delta E=0-\left[ -2.18\times {{10}^{-18}}\left( \frac{{{(1)}^{2}}}{{{(1)}^{2}}} \right) \right]J\]
\[\therefore \Delta E=2.18\times {{10}^{-28}}J\]
For the given process, $H{{e}^{+}}_{(g)}\to H{{e}_{(g)}}^{2+}+{{e}^{-}}$
An electron is removed from n = 1 to n = ∞.
\[\Delta E={{E}_{\infty }}-{{E}_{1}}\]
\[\Rightarrow \Delta E=0-\left[ -2.18\times {{10}^{-18}}\left( \frac{{{(2)}^{2}}}{{{(1)}^{2}}} \right) \right]J\]
\[\therefore \Delta E=8.72\times {{10}^{-18}}J\]
The energy required for the process $8.72\times {{10}^{-18}}J$
35. If the Diameter of a Carbon Atom Is 0.15 nm, Calculate the Number of Carbon Atoms Which Can Be Placed Side by Side in a Straight Line Across a Length of Scale of Length 20 cm Long.
Ans: 1 m = 100 cm
1cm = ${{10}^{-2}}m$
Length of the scale = 20 cm = $20\times {{10}^{-2}}m$
Diameter of a carbon atom = 0.15 nm = $0.15\times {{10}^{-9}}m$
One carbon atom occupies $0.15\times {{10}^{-9}}m$
Number of carbon atoms that can be placed in a straight line = \[\frac{20\times {{10}^{-2}}m}{0.15\times {{10}^{-9}}m}=1.33\times {{10}^{9}}\]
36. $2\times {{10}^{8}}$ atoms of carbon are arranged side by side. Calculate the radius of the carbon atom if the length of this arrangement is 2.4 cm.
Ans: Length of the given arrangement = 2.4 cm
Number of carbon atoms present = $2\times {{10}^{8}}$
$\therefore $ Diameter of carbon atom = $=\frac{2.4\times {{10}^{-2}}}{2\times {{10}^{8}}}=1.2\times {{10}^{-16}}m$
Radius of carbon atom = $\frac{diameter}{2}=\frac{1.2\times {{10}^{-10}}m}{2}=6\times {{10}^{-11}}m$
37. The diameter of the zinc atom is $2.6{{A}^{0}}$ .
a) Calculate radius of zinc atom in pm
Ans: Radius of zinc atom = $\frac{diameter}{2}=\frac{2.6{{A}^{0}}}{2}=1.3\times {{10}^{-11}}m=13\times {{10}^{-12}}m=13pm$
b) Calculate the number of atoms present in a length of 1.6 cm if the zinc atoms are arranged side by side lengthwise.
Ans: Length of the arrangement = 1.6 cm = $1.6\times {{10}^{-2}}m$
Diameter of zinc atom = $2.6{{A}^{0}}$= $2.6\times {{10}^{-10}}m$
Number of zinc atoms present in the arrangement =
\[\frac{1.6\times {{10}^{-2}}m}{2.6\times {{10}^{-10}}m}=0.6153\times {{10}^{8}}m\]
38. A certain particle carries $2.5\times {{10}^{-16}}C$ of static electric charge. Calculate the number of electrons present in it.
Ans: Charge on one electron = $1.6022\times {{10}^{-19}}C$
\[\Rightarrow 1.6022\times {{10}^{-19}}C\] charge is carried by 1 electron.
Number of electrons carrying a charge of $2.5\times {{10}^{-16}}C$
\[=1\left( \frac{2.5\times {{10}^{-16}}C}{1.6022\times {{10}^{-19}}} \right)=1.560\times {{10}^{3}}C=1560C\]
39. In Milikan’s experiment, static electric charge on the oil drops has been obtained by shining X-rays. If the static electric charge on the oil drop is $-1.282\times {{10}^{-18}}C$ , calculate the number of electrons present in it.
Ans: Charge on the oil drop = $-1.282\times {{10}^{-18}}C$
Charge on one electron = $1.6022\times {{10}^{-19}}C$
$\therefore $ Number of electrons present on the oil drop = $=\frac{1.282\times {{10}^{-18}}C}{1.6022\times {{10}^{-19}}C}=0.8001\times {{10}^{1}}=8.0$
40. In Rutherford’s experiment, generally the thin foil of heavy atoms, like gold, platinum etc. have been used to be bombarded by the $\alpha $-particles. If the thin foil of light atoms like aluminium etc. is used, what difference would be observed from the above results?
Ans: A thin foil of lighter atoms will not give the same results as given with the foil of heavier atoms.
Lighter atoms would be able to carry very little positive charge. Hence, they will not cause enough deflection of $\alpha $-particles (positively charged).
41. Symbols $_{35}^{79}Br$ and ${}^{79}Br$ can be written. Whereas symbols $_{79}^{35}Br$ and ${}^{35}Br$are not acceptable. Answer briefly.
Ans: The general convention of representing an element along with its atomic mass (A) and atomic number (Z) is $_{Z}^{A}X$
Hence, $_{35}^{79}Br$is acceptable, but $_{79}^{35}Br$is not acceptable.
${}^{79}Br$ can be written but ${}^{35}Br$ cannot be written because the atomic number of an element is constant, but the atomic mass of an element depends upon the relative abundance of its isotopes. Hence, it is necessary to mention the atomic mass of an element.
42. An element with mass number 81 contains 31.7% more neutrons as compared to protons. Assign the atomic symbol.
Ans: Let the number of protons in the element be x.
$\therefore $Number of neutrons in the element = $x+0.317x=1.317x$
According to the question,
Mass number of the element = 81
(Number of protons + number of neutrons) = 81
\[\Rightarrow x+1.317x=81\]
\[2.317x=81\]
\[x=\frac{81}{2.317}=35\]
Hence, the number of protons in the element i.e., x is 35.
Since the atomic number of an atom is defined as the number of protons present in its nucleus, the atomic number of the given element is 35.
The atomic symbol of the element is $_{35}^{81}Br$
43. An ion with mass number 37 possesses one unit of negative charge. If the ion contains 11.1% more neutrons than the electrons, find the symbol of the ion.
Ans: Let the number of electrons in the ion carrying a negative charge be x.
Then,
Number of neutrons present = $=x+0.111x=1.111x$
Number of electrons in the neutral atom = (x – 1)
(When an ion carries a negative charge, it carries an extra electron)
$\therefore $ Number of protons in the neutral atom = x – 1
Mass number of the ion = 37
\[\therefore (x-1)+1.111x=37\]
\[2.111x=38\]
Number of electrons = 18; Number of protons = 18 – 1 = 17
Atomic number of the ion = 17; Atom correspondence to ion = $Cl$
$\therefore $The symbol of the ion is ${17}^{37}C{{l}^{-1}}$
44. An ion with mass number 56 contains 3 units of positive charge and 30.4% more neutrons than electrons. Assign the symbol to this ion.
Ans: Let the number of electrons present in ion ${{A}^{+3}}$ be ‘x’
Number of neutrons in it = $x+0.304x=1.304x$
Since the ion is tripositive,
Number of electrons in neutral atom = x + 3
$\therefore $Number of protons in neutral atom = x + 3
Mass number of the ion = 56
(x + 3) + (1.304x) = 56
2.304x = 53
Number of protons = x + 3 = 23 + 3 = 26
The symbol of the ion ${26}^{56}F{{e}^{3+}}$
45. Arrange the Following Type of Radiations in Increasing Order of Frequency:
radiation from microwave oven
amber light from traffic signal
radiation from FM radio
cosmic rays from outer space and
Ans: The increasing order of frequency is as follows:
Radiation from FM radio < amber light < radiation from microwave oven < X- rays < cosmic rays
The increasing order of wavelength is as follows:
Cosmic rays < X-rays < radiation from microwave ovens < amber light < radiation of FM radio.
46. Nitrogen laser produces radiation at a wavelength of 337.1 nm. If the number of photons emitted is $5.6\times {{10}^{24}}$ , calculate the power of the laser.
Ans: Power of laser = Energy with which it emits photons
Power = $E=\frac{Nhc}{\lambda }$
Where, N = number of photons emitted
h = Planck’s constant
c = velocity of radiation
$\lambda $= wavelength of radiation
\[E=\frac{(5.6\times {{10}^{24}})(6.626\times {{10}^{-34}}Js)(3\times {{10}^{8}}m{{s}^{-1}})}{(337.1\times {{10}^{-9}}m)}=3.33\times {{10}^{6}}J\]
Hence, the power of laser is $3.33\times {{10}^{6}}J$
47. Neon Gas is Generally Used in Sign Boards. If it Emits Strongly at 616 nm,
a) Calculate the frequency of emission.
Ans: Wavelength of radiation emitted = 616 nm = $616\times {{10}^{-9}}m$ (Given)
Frequency of emission, $v=\frac{c}{\lambda }$
Where, c = velocity of radiation
$\lambda =$ Wavelength of radiation
Substituting the values in the given expression of frequency of emission,
\[v=\frac{3.0\times {{10}^{8}}m/s}{616\times {{10}^{-9}}m}=4.87\times {{10}^{14}}{{s}^{-1}}\]
Hence, Frequency of emission, $v=4.87\times {{10}^{14}}{{s}^{-1}}$
b) Calculate Distance Traveled by this Radiation in 30 s
Ans: velocity of radiation, c = $3\times {{10}^{8}}m/s$
Distance travelled by this radiation in 30 s = $(3\times {{10}^{8}}m{{s}^{-1}})(90s)=9\times {{10}^{9}}m$
c) Calculate energy of quantum…
Ans: energy of Quantum, $E=hv=(6.626\times {{10}^{-34}}Js)(4.87\times {{10}^{14}}{{s}^{-1}})=32.27\times {{10}^{-20}}J$
Energy of Quantum (E) = $32.27\times {{10}^{-20}}J$
d) Calculate number of quanta present if it produces 2 J of energy.
Ans: Energy of one photon (E) = $32.27\times {{10}^{-20}}J$
Therefore, $32.27\times {{10}^{-20}}J$ of energy is present in 1 quantum number of quanta in 2J of energy = $\frac{2J}{32.27\times {{10}^{-20}}J}=6.19\times {{10}^{18}}$
48. In astronomical observations, signals observed from distant stars are generally weak. If the photon detector receives a total of $3.15\times {{10}^{-18}}$J from the radiations of 600 nm, calculate the number of photons received by the detector.
Ans: From the expression of energy of one photon, $E=\frac{hc}{\lambda }$
Where, $\lambda $ = wavelength of radiation
c= velocity of radiation
Substituting the values in the given expression of E:
\[E=\frac{(6.626\times {{10}^{-34}}Js)(3\times {{10}^{8}}m{{s}^{-1}})}{(600\times {{10}^{-9}}m)}\]
Energy of one photon, E = $3.313\times {{10}^{-19}}J$
Number of photons with received with $3.15\times {{10}^{-18}}$J energy,
\[=\frac{3.15\times {{10}^{-18}}J}{3.313\times {{10}^{-19}}J}=9.5\approx 10\] .
Hence, the number of photons received by the detector = 10
49. Lifetimes of the molecules in the excited states are often measured by using pulsed radiation source of duration nearly in the nanosecond range. If the radiation source has a duration of 2 ns and the number of photons emitted during the pulse source is $2.5\times {{10}^{15}}$ , calculate the energy of the source.
Ans: frequency of radiation, $v=\frac{1}{2.0\times {{10}^{-9}}s}=5\times {{10}^{8}}{{s}^{-1}}$
Energy of source, $E=Nhv$
v = frequency of radiation
\[E=(2.5\times {{10}^{15}})(6.626\times {{10}^{-34}}Js)(5.0\times
{{10}^{8}}{{s}^{-1}})=8.282\times {{10}^{-10}}J\]
Hence, the energy of source, $E=8.282\times {{10}^{-10}}J$
50. The longest wavelength doublet absorption transition is observed at 589 and 589.6 nm. Calculate the frequency of each transition and energy difference between two excited states.
Ans: Given,
Wavelength associated with first transition, ${{\lambda }_{1}}=589nm=589\times {{10}^{-9}}m$
Wavelength associated with second transition, ${{\lambda }_{2}}=589.6nm=589.6\times {{10}^{-9}}m$
Frequency of first wavelength is, ${{v}_{1}}=\frac{c}{{{\lambda }_{1}}}=\frac{3\times {{10}^{8}}m{{s}^{-1}}}{589\times {{10}^{-9}}m}=5.093\times {{10}^{14}}{{s}^{-1}}$
And, frequency of second wavelength is, ${{v}_{2}}=\frac{c}{{{\lambda }_{2}}}=\frac{3\times {{10}^{8}}m{{s}^{-1}}}{589.6\times {{10}^{-9}}m}=5.088\times {{10}^{14}}{{s}^{-1}}$
Energy difference between two excited states is given as,
\[\Delta E=h{{v}_{1}}-h{{v}_{2}}=h({{v}_{1}}-{{v}_{2}})\]
\[\Rightarrow \Delta E=(6.626\times {{10}^{-34}}Js)(5.093\times {{10}^{14}}{{s}^{-1}}-5.088\times {{10}^{14}}{{s}^{-1}})=3.31\times {{10}^{-22}}J\]
51. The Work Function for Cesium Atoms is 1.9 eV.
a) Calculate the threshold wavelength
Ans: It is given that the work function (${{W}_{0}}$) for caesium atom is 1.9 eV.
From the expression, ${{W}_{o}}=\frac{hc}{{{\lambda }_{0}}}$
We get, ${{\lambda }_{o}}=\frac{hc}{{{W}_{0}}}$
Where, ${{\lambda }_{0}}$ = threshold wavelength
h = Planck’s constant
Substituting the values in the given expression of${{\lambda }_{0}}$:
${{\lambda }_{0}}=\frac{(6.626\times {{10}^{-1}}Js)(3.0\times {{10}^{8}}m{{s}^{-1}})}{1.9\times 1.602\times {{10}^{-19}}J}=6.53\times {{10}^{-7}}m$
Hence, threshold wavelength ${{\lambda }_{0}}$is 653 nm
b) Calculate the threshold frequency of the radiation.
Ans: From the expression, ${{W}_{0}}=h{{v}_{0}}$
we get, ${{v}_{0}}=\frac{h}{{{W}_{0}}}$
Where, ${{v}_{0}}$ = threshold frequency
Substituting the values in the given expression of${{v}_{0}}$,
${{v}_{0}}=\frac{1.9\times 1.602\times {{10}^{-19}}J}{6.626\times {{10}^{-34}}Js}=4.593\times {{10}^{14}}{{s}^{-1}}$
Hence, the threshold frequency of the radiation, ${{v}_{0}}=4.593\times {{10}^{14}}{{s}^{-1}}$
c) If the cesium element is irradiated with a wavelength 500 nm, calculate the kinetic energy and the velocity of the ejected photoelectron.
Ans: According to the question:
Wavelength used in irradiation, $\lambda $ = 500nm
Kinetic energy = $h(v-{{v}_{0}})=hc\left( \frac{1}{\lambda }-\frac{1}{{{\lambda }_{0}}} \right)$
\[\Rightarrow K.E=(6.626\times {{10}^{-34}}Js)(3.0\times {{10}^{8}}m{{s}^{-1}})\left( \frac{1}{500\times {{10}^{-9}}m}-\frac{1}{653\times {{10}^{-9}}m} \right)\]
\[=\frac{(1.9878\times {{10}^{-26}})(153\times {{10}^{9}})}{653\times 500}=9.3149\times {{10}^{-20}}J\]
Kinetic energy of the ejected photoelectron = $9.3149\times {{10}^{-20}}J$
Since, kinetic energy = $\frac{1}{2}m{{v}^{2}}=9.3149\times {{10}^{-20}}J$
\[v=\sqrt{\frac{2(9.3149\times {{10}^{-20}}J)}{9.10939\times {{10}^{11}}{{m}^{2}}{{s}^{-2}}}}=4.52\times {{10}^{5}}m{{s}^{-1}}\]
Hence, the velocity of the ejected photoelectron (v) is $4.52\times {{10}^{5}}m{{s}^{-1}}$
52. Following Results are Observed When Sodium Metal is Irradiated With Different Wavelengths.
a) Calculate threshold wavelength
Ans: Assuming the threshold wavelength to be ${{\lambda }_{0}}nm$ , the kinetic energy of the radiation is given as:
\[h(v-{{v}_{0}})=\frac{1}{2}m{{v}^{2}}\]
Three different equalities can be formed by the given value as:
\[hc\left( \frac{1}{\lambda }-\frac{1}{{{\lambda }_{0}}} \right)=\frac{1}{2}m{{v}^{2}}\]
\[hc\left( \frac{1}{500\times {{10}^{9}}}-\frac{1}{{{\lambda }_{0}}\times {{10}^{-9}}m} \right)=\frac{1}{2}m{{(2.55\times {{10}^{5}}\times {{10}^{-2}}m{{s}^{-1}})}^{2}}\]
$\frac{hc}{{{10}^{-9}}m}\left( \frac{1}{500}-\frac{1}{{{\lambda }_{0}}} \right)=\frac{1}{2}m{{(2.55\times {{10}^{3}}m{{s}^{-1}})}^{2}}$ ---------- (1)
$\frac{hc}{{{10}^{-9}}m}\left( \frac{1}{450}-\frac{1}{{{\lambda }_{0}}} \right)=\frac{1}{2}m{{(3.45\times {{10}^{3}}m{{s}^{-1}})}^{2}}$ -------------- (2)
$\frac{hc}{{{10}^{-9}}m}\left( \frac{1}{400}-\frac{1}{{{\lambda }_{0}}} \right)=\frac{1}{2}m{{(5.35\times {{10}^{3}}m{{s}^{-1}})}^{2}}$ --------------- (3)
Dividing equation (3) by equation (1):
\[\frac{\left[ \frac{{{\lambda }_{0}}-400}{400{{\lambda }_{0}}} \right]}{\left[ \frac{{{\lambda }_{0}}-500}{500{{\lambda }_{0}}} \right]}=\frac{{{(5.35\times {{10}^{3}}m{{s}^{-1}})}^{2}}}{{{(2.55\times {{10}^{3}}m{{s}^{-1}})}^{2}}}\]
\[\frac{5{{\lambda }_{0}}-2000}{4{{\lambda }_{0}}-2000}={{\left( \frac{5.35}{2.55} \right)}^{2}}=\frac{28.6225}{6.5025}\]
\[\frac{5{{\lambda }_{0}}-2000}{4{{\lambda }_{0}}-2000}=4.40177\]
\[17.6070{{\lambda }_{0}}-5{{\lambda }_{0}}=8803.537-2000\]
\[{{\lambda }_{0}}=\frac{6805.537}{12.607}=539.8nm=540nm\]
Threshold wavelength = 540 nm
b) Calculate Planck’s constant
Ans: the question is not done due to the incorrect values of velocity given in the question.
53. The Ejection of the Photoelectron From the Silver Metal in the Photoelectric Effect Experiment Can Be Stopped by Applying the Voltage of 0.35 v When the Radiation 256.7 nm Is Used. Calculate the Work Function for Silver Metal.
Ans: From the principle of conservation of energy, the energy of an incident photon (E) is equal to the sum of the work function (${{W}_{0}}$) of radiation and its kinetic energy (K.E.) i.e., $E={{W}_{0}}+K.E$
we get, ${{W}_{0}}=E-K.E$
Energy of incident photon, $E=\frac{hc}{\lambda }$
C = velocity of radiation
\[E=\frac{(6.626\times {{10}^{-34}}Js)(3.0\times {{10}^{8}}m{{s}^{-1}})}{256.7\times {{10}^{-9}}m}=7.744\times {{10}^{-19}}J\]
\[E=\frac{7.744\times {{10}^{-19}}}{1.602\times {{10}^{-19}}C}=4.83eV\]
The potential applied to silver metal changes to kinetic energy (K.E) of the photoelectron. Hence, K.E = 0.35 eV
Work function, ${{W}_{0}}=E-K.E$
\[{{W}_{0}}=4.83eV-0.35eV=4.48eV\]
54. If the Photon of the Wavelength 150 PM Strikes an Atom and One of Its Inner Bound Electrons Is Ejected Out With a Velocity of $1.5\times {{10}^{7}}m{{s}^{-1}}$ , Calculate the energy with which it is bound to the nucleus
Ans: Energy of incident photon (E) is given by, $E=\frac{hc}{\lambda }$
\[E=\frac{(6.626\times {{10}^{-34}}Js)(3.0\times {{10}^{8}}m{{s}^{-1}})}{(150\times {{10}^{-12}}m)}=13.252\times {{10}^{-16}}J\]
Energy of the electron ejected (K.E) = $\frac{1}{2}{{m}_{e}}{{v}^{2}}$
\[=\frac{1}{2}(9.10939\times {{10}^{-31}}kg)(1.5\times {{10}^{7}}m{{s}^{-1}})=1.025\times {{10}^{-16}}J\]
Hence, the energy with which the electron is bound to the nucleus can be obtained as: = E – K.E
=$13.252\times {{10}^{-16}}J-1.02\times {{10}^{-16}}J=12.227\times {{10}^{-16}}J$
\[=\frac{12.227\times {{10}^{-16}}}{1.602\times {{10}^{-19}}C}=7.6\times {{10}^{3}}eV\]
The energy with which it is bound to the nucleus is $7.6\times {{10}^{3}}eV$
55. Emission Transitions in the Paschen Series end at orbit n = 3 and start from orbit n and can be represented as $v=3.29\times {{10}^{5}}(Hz)\left[ \frac{1}{{{3}^{2}}}-\frac{1}{{{n}^{2}}} \right]$ Calculate the value of n if the transition is observed at 1285 nm. Find the region of the spectrum.
Ans: Wavelength of transition = 1285 nm = $1285\times {{10}^{-9}}m$$v=3.29\times {{10}^{5}}(Hz)\left[ \frac{1}{{{3}^{2}}}-\frac{1}{{{n}^{2}}} \right]$
Since, $v=\frac{c}{\lambda }=\frac{3.0\times {{10}^{8}}m{{s}^{-1}}}{1285\times {{10}^{-9}}m}=2.33\times {{10}^{14}}{{s}^{-1}}$
Substituting the value of v in the given expression,
$2.33\times {{10}^{14}}=3.29\times {{10}^{5}}(Hz)\left[ \frac{1}{{{3}^{2}}}-\frac{1}{{{n}^{2}}} \right]$
\[\Rightarrow \left( \frac{1}{9}-\frac{1}{{{n}^{2}}} \right)=\frac{2.33\times {{10}^{14}}}{3.29\times {{10}^{15}}}\]
\[\Rightarrow \frac{1}{{{n}^{2}}}=1.1\times {{10}^{-1}}-0.7082\times {{10}^{-1}}\]
\[\Rightarrow \frac{1}{{{n}^{2}}}=4.029\times {{10}^{-2}}\]
\[n=\sqrt{\frac{1}{4.029\times {{10}^{-2}}}}=4.98\approx 5\]
Hence, for the transition to be observed at 1285 nm, n = 5. The spectrum lies in the infra-red region.
56. Calculate the Wavelength for the emission transition if it starts from the orbit having radius 1.3225 nm and ends at 211.6 pm. Name the series to which this transition belongs and the region of the spectrum.
Ans: The radius of the ${{n}^{th}}$ orbit of hydrogen-like particles is given by,
\[r=\frac{0.529{{n}^{2}}}{Z}{{A}^{0}}\]
\[r=\frac{52.9{{n}^{2}}}{Z}pm\]
For radius, ${{r}_{1}}=1.3225nm=1.3225\times {{10}^{-9}}m=1322.5pm$
\[{{n}_{1}}^{2}=\frac{{{r}_{1}}Z}{52.9}=\frac{1322.5Z}{52.9}\] Similarly,
\[{{n}_{2}}^{2}=\frac{211.6Z}{52.9}\]
\[\frac{{{n}_{1}}^{2}}{{{n}_{2}}^{2}}=\frac{1322.5}{211.6}=6.25\] \[\frac{{{n}_{1}}}{{{n}_{2}}}=2.5=\frac{25}{10}=\frac{5}{2}\]
\[\Rightarrow {{n}_{1}}=5\And {{n}_{2}}=2\]
Thus, the transition is from the ${{5}^{th}}$ orbit to the ${{2}^{nd}}$ orbit. It belongs to the Balmer series. Wave number ($\overset{-}{\mathop{v}}\,$) for the transition is given by,
\[=1.097\times {{10}^{7}}\left( \frac{1}{{{2}^{2}}}-\frac{1}{{{5}^{2}}} \right)\]
\[=1.097\times {{10}^{7}}\left( \frac{21}{100} \right)=2.303\times {{10}^{6}}{{m}^{-1}}\]
Wavelength associated with the emission transition is given by,
\[\lambda =\frac{1}{\overset{-}{\mathop{v}}\,}=\frac{1}{2.303\times {{10}^{6}}{{m}^{-1}}}=0.434\times {{10}^{6}}m=434nm\]
57. Dual Behavior of Matter Proposed by De Broglie Led to the Discovery of Electron Microscopes Often Used for the Highly Magnified Images of Biological Molecules and Other Types of Material. If the velocity of the electron in this microscope is $1.6\times {{10}^{6}}m{{s}^{-1}}$ , calculate the de Broglie wavelength associated with this electron.
Ans: From de Broglie’s equation,
\[\lambda =\frac{h}{mv}=\frac{6.626\times {{10}^{-34}}Js}{(9.10939\times {{10}^{-31}}kg)(1.6\times {{10}^{6}}m{{s}^{-1}})}=4.55\times {{10}^{-10}}m=455pm\]
de Broglie’s wavelength associated with the electron is 455 pm.
58. Similar to Electron Diffraction, Neutron Diffraction Microscope is Also Used for the Determination of the Structure of Molecules. If the Wavelength Used Here is 800 PM, Calculate the Characteristic Velocity Associated With the Neutron
Ans: From de Broglie’s equation,
\[\lambda =\frac{h}{mv}\]
Then, $v=\frac{h}{m\lambda }$
v = velocity of particle (neutron)
m = mass of particle (neutron)
$\lambda $ = wavelength
Substituting the values in the expression of velocity (v),
\[v=\frac{(6.626\times {{10}^{-34}})Kg{{m}^{2}}{{s}^{-1}}}{(1.675\times {{10}^{-27}})(8\times {{10}^{-10}}m)}=\frac{6.626\times {{10}^{3}}}{1.675\times 8}=4.94\times {{10}^{2}}m{{s}^{-1}}=494m{{s}^{-1}}\]
Velocity associated with the neutron = $494m{{s}^{-1}}$
59. If the velocity of the electron in Bohr’s first orbit is $2.19\times {{10}^{6}}m{{s}^{-1}}$ calculate the de Broglie wavelength associated with it.
\[\lambda =\frac{h}{mv}\]
Substituting the values in the expression of λ:
\[\lambda =\frac{(6.626\times {{10}^{-34}}Js)}{(9.10939\times {{10}^{-31}}kg)(2.19\times {{10}^{6}}m{{s}^{-1}})}=3.32\times {{10}^{-10}}m=332\times {{10}^{-12}}m=332pm\]
Wavelength associated with the electron = 332 pm
60. The velocity associated with a proton moving in a potential difference of 1000 V is $4.37\times {{10}^{5}}m{{s}^{-1}}$ . If the hockey ball of mass 0.1 kg is moving with this velocity, calculate the wavelength associated with this velocity.
Substituting the values in the expression,
\[\lambda =\frac{(6.626\times {{10}^{-34}}Js)}{(0.1kg)(4.37\times {{10}^{5}}m{{s}^{-1}})}=1.516\times {{10}^{-38}}m\]
61. If the position of the electron is measured within an accuracy of +0.002 nm, calculate the uncertainty in the momentum of the electron. Suppose the momentum of the electron is $\frac{h}{4\pi m\times 0.05nm}$ . Is there any problem in defining this value?
Ans: From Heisenberg’s uncertainty principle,
\[\Delta x\times \Delta p=\frac{h}{4\pi }\Rightarrow \Delta p=\frac{1}{\Delta x}\frac{h}{4\pi }\]
Where, $\Delta x$ = uncertainty in position of the electron
$\Delta p$ = uncertainty in momentum of the electron
Substituting the values in the expression of$\Delta p$,
$\Delta p=\frac{1}{0.002nm}\frac{6.626\times {{10}^{-34}}Js}{4\times 3.14}$
$\Rightarrow \Delta p=2.637\times {{10}^{-23}}kgm{{s}^{-1}}$
Actual momentum = $\frac{h}{4\pi m\times 0.05nm}$= $\frac{6.626\times {{10}^{-34}}Js}{4\times 3.14\times 5.0\times {{10}^{-11}}m}=1.055\times {{10}^{-24}}kgm{{s}^{-1}}$
Since the magnitude of the actual momentum is smaller than the uncertainty, the value cannot be defined.
62. The quantum numbers of six electrons are given below. Arrange them in order of increasing energies. If any of these combination(s) has/have the same energy lists:
n = 4, l = 2, ${{m}_{l}}=-2,{{m}_{s}}=-\frac{1}{2}$
n = 3, l = 2, ${{m}_{l}}=1,{{m}_{s}}=+\frac{1}{2}$
n = 4, l = 1, ${{m}_{l}}=0,{{m}_{s}}=+\frac{1}{2}$
n = 3, l = 2, ${{m}_{l}}=-2,{{m}_{s}}=-\frac{1}{2}$
n = 3, l = 1, ${{m}_{l}}=-1,{{m}_{s}}=+\frac{1}{2}$
Ans: For n = 4 and l = 2, the orbital occupied is 4d.
For n = 3 and l = 2, the orbital occupied is 3d.
For n = 4 and l = 1, the orbital occupied is 4p.
Hence, the six electrons i.e., 1, 2, 3, 4, 5, and 6 are present in the 4d, 3d, 4p, 3d, 3p, and 4p orbitals respectively.
Therefore, the increasing order of energies is 5(3p) < 2(3d) = 4(3d) < 3(4p) = 6(4p) < 1 (4d).
63. The bromine atom possesses 35 electrons. It contains 6 electrons in 2p orbital, 6 electrons in 3p orbital and 5 electrons in 4p orbital. Which of these electrons experiences the lowest effective nuclear charge?
Ans: Nuclear charge experienced by an electron (present in a multi-electron atom) is dependent upon the distance between the nucleus and the orbital, in which the electron is present. As the distance increases, the effective nuclear charge also decreases.
Among p-orbitals, 4p orbitals are farthest from the nucleus of bromine atoms with (+35) charge. Hence, the electrons in the 4p orbital will experience the lowest effective nuclear charge. These electrons are shielded by electrons present in the 2p and 3p orbitals along with the s-orbitals. Therefore, they will experience the lowest nuclear charge.
64. Among the following pairs of orbitals which orbital will experience the larger effective nuclear charge?
(i) 2s and 3s
Ans: Nuclear charge is defined as the net positive charge experienced by an electron in the orbital of a multi-electron atom. The closer the orbital, the greater is the nuclear charge experienced by the electron(s) in it.
The electron(s) present in the 2s orbital will experience greater nuclear charge (being closer to the nucleus) than the electron(s) in the 3s orbital.
(ii) 4d and 4f
Ans: 4d will experience greater nuclear charge than 4f since 4d is closer to the nucleus.
(iii) 3d and 3p
Ans: 3p will experience greater nuclear charge since it is closer to the nucleus than 3f.
65. The unpaired electrons in Al and Si are present in 3p orbital. Which electrons will experience more effective nuclear charge from the nucleus?
Ans: Nuclear charge is defined as the net positive charge experienced by an electron in a multi-electron atom.
The higher the atomic number, the higher is the nuclear charge.
Silicon has 14 protons while aluminium has 13 protons.
Hence, silicon has a larger nuclear charge of (+14) than aluminium, which has a nuclear charge of (+13).
Thus, the electrons in the 3p orbital of silicon will experience a more effective nuclear charge than aluminium.
66. Indicate the number of unpaired electrons
a) Phosphorous (P)
Ans: Atomic number = 15
The electronic configuration of P is: $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{3}}$
The orbital picture of P can be represented as:
From the orbital picture, phosphorus has three unpaired electrons.
b) Silicon (Si)
Ans: Atomic number = 14
The electronic configuration of Si is: $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{2}}$
The orbital picture of Si can be represented as:
From the orbital picture, phosphorus has two unpaired electrons.
c) Chromium (Cr)
Ans: Atomic number = 24
The electronic configuration of Cr is: $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{1}}3{{d}^{5}}$
The orbital picture of chromium is:
From the orbital picture, chromium has six unpaired electrons.
d) Iron (Fe)
Ans: Atomic number = 26
The electronic configuration is: $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{6}}$
The orbital picture of iron is:
From the orbital picture, iron has four unpaired electrons.
e) Krypton (Kr)
Ans: Atomic number = 36
The electronic configuration is:$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{10}}4{{p}^{6}}$
The orbital picture of krypton is:
Since all orbitals are fully occupied, there are no unpaired electrons in krypton.
67. Answer the following.
a) How many subshells are associated with n = 4?
Ans: n = 4 (Given)
For a given value of ‘n’, ‘l’ can have values from zero to (n – 1).
Hence, l = 0, 1, 2, 3
Thus, four subshells are associated with n = 4, which are s, p, d and f.
b) How many electrons will be present in the sub-shells having ${{m}_{s}}$ value of $-\frac{1}{2}$ for n = 4?
Ans: Number of orbitals in the ${{n}^{th}}$ shell =${{n}^{2}}$
Number of orbitals = 16
If each orbital is taken fully, then it will have 1 electron with ${{m}_{s}}$ value of $-\frac{1}{2}$
$\therefore $ Number of electrons with ${{m}_{s}}$ value of $-\frac{1}{2}$ = 16
Chapter 2 – Structure of Atom
Chapter 2 chemistry class 11 covers the below topics:.
Discovery of Subatomic Particles
Discovery of Electron
Charge to mass ratio of electron, charge on the electron, discovery of protons and neutrons, atomic models.
Thomson Model of Atom
Rutherford’s Nuclear Model of Atom
Atomic number and mass number, isobars and isotopes, drawbacks of rutherford model, developments leading to the bohr’s model of atom, wave nature of electromagnetic radiation, particle nature of electromagnetic radiation: planck’s quantum theory, photoelectric effect, dual behaviour of electromagnetic radiation.
Evidence for the quantized* Electronic Energy Levels: Atomic spectra
Emission and Absorption Spectra
Line spectrum of hydrogen, the spectral lines for atomic hydrogen.
Bohr’s Model for Hydrogen Atom
Explanation of Line Spectrum of Hydrogen
Limitations of bohr’s model.
Towards Quantum Mechanical Model Of the Atom
Dual Behaviour of Matter
Heisenberg’s Uncertainty Principle
Significance of the Uncertainty Principle
Reasons for the Failure of the Bohr Model
Quantum Mechanical Model Of Atom
Hydrogen Atom and the Schrödinger Equation
Orbitals and quantum numbers, shapes of atomic orbitals, energies of orbital, filling of orbitals in atom, aufbau principle, pauli exclusion principle, hund’s rule of maximum multiplicity, electronic configuration of atoms, stability of completely filled and half-filled subshells, introduction.
In Ch 2 Chemistry Class 11, it will focus on explaining everything about the structure of Atom to the students. For example, the chapter will focus on making them learn about the existence of atoms, the fundamentals, theorems related to atoms, characteristics, understanding the features of quantum mechanical model of atoms, the nature of electromagnetic radiation, Planck’s quantum theory, describing the photoelectric effect and features of atomic spectra, stating the De Broglie relation and Heisenberg uncertainty principle, defining the atomic orbital quantum number, stating the principle of Aufbau, Pauli exclusion principle, Hund’s rule of maximum multiplicity, and writing the electronic configurations of atoms.
Discovery of Sub-Atomic Particles
In Class 11 Chemistry Chapter 2 Structure of Atom, the first segment, that is, Discovery of Sub-Atomic Particles will give the students more insight on the structure of atoms that were obtained using the experiments done on electrical discharge through gases.
You can also refer to Vedantu; NCERT Solutions for Class 11 Chemistry Chapter 2 to find out the answers related to the discovery of atoms.
In Chemistry Class 11 Chapter 2, you will get to know in detail how electrons were discovered. you will be asked questions in the examinations regarding the discovery of electrons for which you can refer to Vedantu to get the right answers.
In this chapter, you will get the information about the ratio of electrical charge to the mass of the electron. Experts from Vedantu will help you understand the experiment and process through which this value was derived with great clarity.
This part is all about interpreting the value of the charge on the electron. To understand how this value was derived Vedantu is very helpful and can be used for better insight for its elaborated explanations of the experiments.
This section informs you about the process which led to the discovery of Protons and Neutrons. To grip the essence of this discovery becomes easy if you are guided by Vedantu.
In Class 11 Chemistry Chapter 2, the student gets an idea regarding the structure of two different atomic models which has been proposed for explaining the distribution of the charged particles inside an atom. you should refer to the experts of Vedantu for getting a clear idea of the structure of atom Class 11 NCERT Solutions.
Thompson Model of Atom
This segment explains the structure of the model of an atom proposed as the plum pudding model by scientist JJ Thomson. With Vedantu you can get a deeper understanding of this model to understand the complexity of the subject.
This segment in Class 11 Chemistry Chapter 2 Structure of the Atom, explores another proposal for the structure of the atom as suggested by scientists Rutherford through experiments he conducted with his students. Only Vendantu’s NCERT Class 11 Chemistry Chapter 2 exercise Solutions can help you understand such complicated experiments in simplicity.
This chapter explains the concept of Atomic Number and Mass Number of every element and how to calculate them. If you want to calculate the atomic number and mass number perfectly in chemistry Class 11 chapter 2, then using Vedantu is very helpful.
This chapter explains the primary difference between Isobars and Isotopes. Very often there are chances of confusing the two each other. It is wise to get Vedantu’s NCERT solutions for Class 11 chemistry chapter 2 pdf download to avoid technical confusion which strongly affects the understanding of the subject.
This section imparts students with the drawbacks associated with the structure of the model of an atom suggested by Rutherford and his students. With the help of Vedantu, you can understand the flaws of the model in greater detail.
This chapter informs the students how Rutherford’s model of the structure of the atom contributed to the development of BOHR’s Model of Atom. Vedantu’s Class 11 Chemistry Chapter 2 solutions are the best way to understand this.
This part talks about the discovery of the wave nature of electromagnetic radiation. Vedantu’s Class 11 Chemistry Chapter 2 NCERT solutions explain this in a lot more detail.
This segment explains the Quantum Theory for which scientist Plank was awarded the Nobel Prize. The intricate details can be confusing for a young student. In such situations, Vedantu’s solutions can be of a lot of help.
This section explains the definitions of the photoelectric effect and the experiments through which it was discovered. Experts from Vedantu help you get the clearest picture regarding its concepts.
This chapter raises the problem of electromagnetic Radiation having dual nature and makes you understand its concepts. Chemistry Class 11 Chapter 2 NCERT solutions by Vedantu are a great help during such times of difficulty.
Evidence for the Quantized* Electronic Energy Levels: Atomic Spectra
The concept of Atomic spectra is made clear to the students in this part of the course. If the student faces any trouble to understand this phenomenon, we suggest them to take help of Vedantu’s study materials.
This part explains the radiation effect technically known as emission spectra. Often having help from experts such as Vedantu help in getting a better grip of the subject which may seem complex to students.
This segment talks about the reaction of when an electric discharge is passed through hydrogen. Vedantu’s solutions are a great guide for a good understanding of various types of reactions.
This part informs the students of the different spectral lines which have been named after their respective scientist who discovered. NCERT Solutions for Class 11 Chemistry Chapter 2 offered by Vedantu removes all possible confusion that can arise in a student.
Bohr’s Model For Hydrogen Atom
This section talks about the first explanation of the general feature of an atom of hydrogen. Vedantu understands that this is very crucial and provides perfect materials for students to understand it.
This chapter uses the Bohr Model to explain the different lines of the spectrum of hydrogen. Vedantu’s chemistry Chapter 2 Class 11 NCERT solutions are a great help for a strong understanding of this concept.
This portion tells about the limitations faced by the Bohr’s Model, this enables us with a strong understanding of this crucial subject. If you still have doubts, then referring to Vedantu’s team of experts is a very simple solution.
Towards Quantum Mechanical Model of The Atom
This segment talks about theories which tried to solve the problem faced by Bohr’s Model. This is an important area in the syllabus and referring to Vedantu is very much recommended to every student.
This part explains how dual behaviour of the matter was discovered which was a big leap in the field of academics. With Vedantu such important matters can be understood simply.
This section explores the theory put forward by Heinsberg who said that it was not possible to determine exactly the position or the speed of an electron in an atom. Vedantu’s experts explore this theory to give you strong clarity on the matter.
The many reasons why Bohr Model failed are explained in this chapter. But for more details, it is helpful if the students refer Vedantu.
Quantum Mechanical Model of Atom
This portion takes on a new structure of atom referred to as the Quantum Mechanical Model. NCERT solution Class 11 chemistry chapter 2 by Vedantu makes this complex subject easy.
This segment gives a good explanation of how hydrogen and the Schrodinger equation work. We encourage you to consult Vedanta if there remains any confusion.
It is quantum numbers that help to distinguish atomic orbitals, as the students will read in this part. Vedantu provides the best study material for tough topics.
This chapter explains the factors on which the shape of atomic orbitals depends in a very interesting way. if any information is left out, it is covered in the Vedanta study guide.
This part informs students how the energy of an electron in a hydrogen atom can be measured and what are the factors it depends upon. The student should further consult Vedantu experts if there occurs a confusion.
This section dwells on how electrons fill the atomic orbitals which are made more interesting by Vedantu in their study guides.
It is a theory about how electrons fill orbitals in order of increasing energy. This theory is explained in perfectly by the team of Vedantu, you need another source of reference.
The Exclusion principle influences how electrons fill the orbital space which is talked about in this part. Vedantu gives good information on such important topics.
This segment explains how the electrons fill in the same subshell orbits. this reading is made more interesting by Vedantu’s study materials.
The process of how the electron is distributed in the orbitals is called the electronic configuration and that is the main topic of this chapter. In case you need more study material for Chemistry Class 11 NCERT solutions Chapter 2, the Vedantu can be of great help.
This part explains the process of stability of electrons which have filled and half-filled subshells and the causes of it. This can be and tricked but Vedantu’s experts can make you understand it in very simple ways.

Chemistry NCERT Solutions for Class 11 - Chapter wise PDFs
Chapter 1 - Some Basic Concepts of Chemistry
Chapter 2 - Structure of Atom
Chapter 3 - Classification of Elements and Periodicity in Properties
Chapter 4 - Chemical Bonding and Molecular Structure
Chapter 5 - States of Matter
Chapter 6 - Thermodynamics
Chapter 7 - Equilibrium
Chapter 8 - Redox Reactions
Chapter 9 - Hydrogen
Chapter 10 - The s-Block Elements
Chapter 11 - The p-Block Elements
Chapter 12 - Organic Chemistry - Some Basic Principles and Techniques
Chapter 13 - Hydrocarbons
Chapter 14 - Environmental Chemistry
You may receive aid comprehending all major ideas from Vedantu specialists, and you can quickly obtain its free PDF online guide on all topics to achieve amazing outcomes in exams. Getting a second view on many issues is really beneficial since sometimes a theory appears simple but is extremely tough to understand. Trusting Vedantu's NCERT answers for Class 11 Chemistry Chapter 2 as a reference is the best option in such instances.
Students may benefit from the availability of free PDF downloads of NCERT Answers for Class 11 Chemistry Chapter 2. These answers include extensive explanations, step-by-step solutions, and key insights into the chapter's themes. Students may improve their comprehension, boost their problem-solving abilities, and thrive in their chemistry studies by using these answers. The ease of use of free PDF downloads allows students to have access to and use these resources for excellent test preparation and general academic achievement.
Types of Questions Asked in NCERT Class 11 Chemistry Chapter 2 Structure of Atom
The following are examples of the types of questions asked in the NCERT exercise section for this chapter:
Fundamental calculations for subatomic particles such as protons, electrons, and neutrons
Numericals based on the calculating the energy associated with electromagnetic radiation
Numericals based on the relations between frequency and wavelength
Problems related to the transition of electrons to different shells
Questions related to writing the electron configurations
Problems related to quantum numbers and their combinations for electrons

FAQs on NCERT Solutions for Class 11 Chemistry Chapter 2 - Structure Of Atom
Q1: What are the important topics of Structure of Atoms?
Ans: The topics covered are
1. Subatomic Particles
Discovery Of Electron
Charge To Mass Ratio Of Electron
Charge On The Electron
Discovery Of Protons And Neutrons
2. Atomic Models
Thomson Model Of Atom
Rutherford’s Nuclear Model Of Atom
Atomic Number And Mass Number
Isobars And Isotopes
Drawbacks Of Rutherford Model
3. Developments Leading To The Bohr’s Model Of Atom
Wave Nature Of Electromagnetic Radiation
Particle Nature Of Electromagnetic Radiation: Planck’s Quantum Theory
The Quantized* Electronic Energy Levels: Atomic Spectra
4. Bohr’s Model For Hydrogen Atom
Explanation Of Line Spectrum Of Hydrogen
Limitations Of Bohr’s Model
5. Towards Quantum Mechanical Model Of The Atom
Dual Behaviour Of Matter
6. Quantum Mechanical Model Of Atom
Orbitals And Quantum Numbers
Shapes Of Atomic Orbitals
Energies Of Orbitals
Filling Of Orbitals In Atom
Electronic Configuration Of Atoms
Stability Of Completely Filled And Half Filled Subshells.
Q2: What is discussed in the chapter?
Ans: This chapter introduces the concept of atoms, electrons, protons, and neutrons. Also, the students will understand the concepts of isotopes, atomic number, and isobars. These concepts are important for them to understand, as these will build a strong foundation of Chemistry. This chapter will also introduce the students to advanced theories like Rutherford's model, Thomson's model and Bohr's model, their uses and their limitations.
Q3: What is the photoelectric effect in simple words?
Ans: The photoelectric effect is a physical phenomena. The outcome will be based on the notion that electromagnetic radiation is made up of photons, a type of subatomic particle. When an electron collides with a photon on a metal surface, electron emission can be detected. The electrons that are emitted are known as photoelectrons.
The "photoelectric effect" describes how light causes electron emission or ejection from a surface, most commonly a metal. Understanding the photoelectric effect may help one understand the quantum nature of electrons and light, as well as how the concept of wave-particle duality is generated.
Q4: How to prepare for Chemistry with Vedantu?
Ans: Preparing in a step by step and structured manner will help in understanding the concepts in Chemistry easier. The most scoring and difficult concept in this subject is the Structure of the Atom . The weightage of this topic is also high. You may think of Qualitatives analysis towards the end, as it needs very less time.
Our NCERT Solutions gives you an in-depth knowledge of the conceptual topics. NCERT Solutions have been drafted as per the latest CBSE Class 11 Science Syllabus. Students will feel that the solutions are in a simple language and can understand the difficult topics easily.
Q5. What type of questions is present in NCERT Solutions for Class 11 Chemistry Chapter 2 Structure of Atom?
Ans: In Class 11 Chemistry Chapter 2: Structure of Atoms, you will learn about the existence of atoms, fundamental theories about atoms, their characteristics, features of the quantum mechanical model of atoms, the nature of electromagnetic radiations, Planck's quantum theory, describing the photoelectric effect, features of atomic spectra, and much more. You will be asked questions on the topics covered in the chapter.
Q6. Why should I opt for NCERT Solutions for Class 11 Chemistry Chapter 2?
Ans: Chemistry is a scoring subject where if you understand the concept and the type of questions you can come across during the exam, you can easily answer. Having deep knowledge of the subject is very important. It is equally important to format your answers to score good marks. NCERT Solutions for Class 11 Chemistry Chapter 2 will give you well-structured solutions for each question. You will get a proper idea of how to prepare your responses for better marks. The solutions are available on the Vedantu Mobile app hence accessible anywhere and anytime.
Q7. What topics are covered in NCERT Solutions for Class 11 Chemistry Chapter 2?
Ans: The topics covered in NCERT Solutions for Class 11 Chemistry Chapter 2 Structure of Atoms are Discovery of Subatomic Particles, Electron, Protons and Neutrons, Charge to Mass Ratio on Electrons, Atomic Models, Atomic Number and Mass Number, Wave Nature of Electromagnetic Radiation, Photoelectric Effect, Emission and Absorption Spectra and much more.
Q8. What is the importance of understanding NCERT Class 11 Chemistry Chapter 2?
Ans: NCERT Class 11 Chemistry is the most scoring subject if understood properly. Chapter 2, Structure of Atoms is the base of Chemistry, where you will learn and understand about the smallest unit of any substance. Hence, it is very important to understand the chapter and practice thoroughly. You can practice various types of questions to understand every tangent of the chapter.
Q9. Where can I find accurate solutions for NCERT Class 11 Chemistry Chapter 2?
Ans: You can find the most accurate and reliable solutions for NCERT Class 11 Chemistry Chapter 2 by following these steps:
Click on the link NCERT Solutions for Class 11 Chemistry Chapter 2 Structure of Atom The Solutions page of Vedantu for Class 11 Chemistry Chapter 2 Structure of Atoms will open.
At the top of the page, you will find the link to download the solutions for Chapter 2 Class 11 NCERT. The solutions are free of cost.
You can also find important questions to practice several types of questions to deeply understand the chapter.
NCERT Solutions for Class 11 Chemistry
- NCERT Solutions
- NCERT Class 11
- NCERT 11 Chemistry
NCERT Solutions for Class 11 Chemistry
Ncert solutions for class 11 chemistry download chapter-wise pdf for 2023-24.
NCERT Solutions for Class 11 Chemistry is a study material which is developed by the faculty at BYJU’S by keeping in mind the grasping power of Class 11 students. NCERT Solutions for Class 11 are drafted in a simple and understandable manner to help students ace the exam without fear. Chemistry is a subject which contains a lot of chemical reactions and symbols. It might be difficult for the students to remember all these concepts effectively if not revised on a regular basis. So, making use of proper study material is very important.
At BYJU’S, students can access chapter-wise NCERT Solutions for Class 11 to get their doubts clarified instantly. The faculty has provided both online and offline modes of solutions which can be used free of cost. The chapter-wise links of NCERT Solutions for Class 11 Chemistry are as follows:
Chapter-Wise NCERT Solutions for Class 11 Chemistry
Chapter 1: Some Basic Concepts of Chemistry
Chapter 2: structure of atom, chapter 3: classification of elements and periodicity in properties, chapter 4: chemical bonding and molecular structure.
- Chapter 5: Thermodynamics
- Chapter 6: Equilibrium
- Chapter 7: Redox Reactions
- Chapter 8: Organic Chemistry – Some Basic Principles & Techniques
- Chapter 9: Hydrocarbons
The following chapters have been removed from the NCERT Class 11 Chemistry textbook 2023-24.
- States of Matter
- The s-Block Elements
- The p-Block Elements
- Environmental Chemistry
From the NCERT Solutions at BYJU’S, students will clearly learn about all the chemical reactions which occur in our day-to-day activities. The solutions explain each and every minute concept in the best way possible so that students do not face any problems in the exam. It not only boosts their exam preparation, but also provides a strong foundation of fundamental concepts, which frequently appear in various competitive exams. Using these solutions, students will understand how to approach complex questions that would appear in the exam and answer them with confidence.
Download NCERT Solutions for Class 11 Chemistry PDF to revise all the concepts.
NCERT Solutions Class 11 Chemistry
NCERT Solutions for Class 11 Chemistry at BYJU’S is designed in such a way that students will be able to grasp all the concepts instantly. Each and every chemical reaction is explained with suitable examples and explanations in order to provide a quality learning experience for the students. For a student of Class 11, learning all the concepts from the NCERT Textbook would be a difficult task if they do not have a proper understanding of the concepts. The main objective of creating these solutions is to help students face the annual exam without fear. It not only enables logical and analytical thinking approach among students, but also helps them to attain good grades.
NCERT Solutions Class 11 Chemistry Chapter Details and Exercises
This chapter explains the importance of chemistry, and concepts like molecular mass and atomic mass. A few basic theories and laws, like Avogadro’s law , Dalton’s atomic theory and the law of conservation of mass, are explained in brief in this chapter. You will also solve problems based on determining the molecular weight of compounds, mass percent and concentration. You will get a clear idea about the empirical and molecular formulae, molarity, molality and mole concept.
Topics Covered in Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry :
General Introduction: Importance and scope of chemistry. Historical approach to particulate nature of matter, laws of chemical combination, Dalton’s atomic theory: the concept of elements, atoms and molecules. Atomic and molecular masses. Mole concept and molar mass; percentage composition and empirical and molecular formula; chemical reactions, stoichiometry and calculations based on stoichiometry.
Also, access the following resources for Class 11 Chapter 1 Some Basic Concepts of Chemistry at BYJU’S:
- CBSE Class 11 Notes Chapter 1 – Some Basic Concepts of Chemistry
- Chemistry Revision Notes for Class 11 Chapter 1 Some Basic Concepts of Chemistry
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 1 – Some Basic Concepts Of Chemistry Solutions
- Important Questions for Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry
Students will learn about Thomson’s atomic model, subatomic particles, Bohr’s model , Rutherford’s atomic model and quantum mechanical model of atom. Problems on relationship between frequency and wavelength, energy associated with electromagnetic radiation and subatomic particles are present in this chapter. You will get to know how to write the electron configurations and the electron transition in different shells which is important for the exam.
Topics Covered in Class 11 Chemistry Chapter 2 Structure of Atom :
Discovery of electron, proton and neutron; atomic number, isotopes and isobars. Thomson’s model and its limitations, Rutherford’s model and its limitations, Bohr’s model and its limitations, concept of shells and subshells, dual nature of matter and light, de Broglie’s relationship, Heisenberg uncertainty principle, concept of orbitals, quantum numbers, shapes of s, p, and d orbitals, rules for filling electrons in orbitals – Aufbau principle, Pauli exclusion principle and Hund’s rule, electronic configuration of atoms, stability of half filled and completely filled orbitals.
Also, access the following resources for Class 11 Chapter 2 Structure of Atom at BYJU’S:
- Structure of Atom Class 11 Notes
- Chemistry Revision Notes for Class 11 Chapter 2 Structure of Atom
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 2 – Structure of Atom
From the exam point of view, classification of elements is very important. Most of the questions in the final exam and various competitive exams appear from this chapter. So learning all the concepts from this chapter is important. From this chapter, students will also learn about the s-block, p-block, d-block and f-block elements from the periodic table , trends in physical and chemical properties and chemical reactivity. By learning this chapter thoroughly, students will obtain good score in the exam.
Topics Covered in Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties :
Significance of classification, brief history of the development of periodic table, modern periodic law and the present form of periodic table, periodic trends in properties of elements – atomic radii, ionic radii, inert gas radii, ionization enthalpy, electron gain enthalpy, electronegativity, valence.
Also, access the following resources for Class 11 Chapter 3 Classification of Elements and Periodicity in Properties at BYJU’S:
- Classification of Elements and Periodicity in Properties Class 11 Notes – Chapter 3
- Chemistry Revision Notes for Class 11 Chapter 3 Classification of Elements and Periodicity in Properties
- Important Questions Chemistry Class 11 Chapter 3 Classification of Elements and Periodicity in Properties
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 3 – Classification of Elements and Periodicity in Properties
Chemistry is based on the things which we observe in our surroundings and is called the central science. The topics discussed in this chapter are – VSEPR Theory, Lewis structures, Valence Bond Theory , polar character of covalent bonds and hydrogen bonding. Students will learn how to draw Lewis dot symbols for molecules, atoms and polyatomic ions. The solutions at BYJU’S contain diagrams for each concept to provide visual learning experience for the students.
Topics Covered in Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure :
Valence electrons, ionic bond, covalent bond, bond parameters, Lewis structure, polar character of covalent bond, covalent character of ionic bond, valence bond theory, resonance, geometry of covalent molecules, VSEPR theory, concept of hybridization involving s, p and d orbitals and shapes of some simple molecules, molecular orbital theory of homonuclear diatomic molecules (qualitative idea only), hydrogen bond.
Also, access the following resources for Class 11 Chapter 4 Chemical Bonding and Molecular Structure at BYJU’S:
- Chemical Bonding Class 11 Notes
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 4 – Chemical Bonding and Molecular Structure
Chapter 5: States of Matter
This chapter explains the intermolecular forces and how they affect the physical state of a substance. It deals with other concepts associated with gaseous and liquid states of matter. This is the main reason why this chapter is important for the exam. Students will be able to understand the Boyle’s Law , Charle’s Law, Gay Lussac’s Law and Avogadro Law which carry more marks as per the exam pattern. Problems on finding partial pressure, critical temperature and pressure, Van der Waals force and other intermolecular forces are present in this chapter.
Topics Covered in Class 11 Chemistry Chapter 5 States of Matter :
Three states of matter, intermolecular interactions, type of bonding, melting and boiling points, role of gas laws in elucidating the concept of the molecule, Boyle’s law, Charles’ law, Gay Lussac’s law, Avogadro’s law, ideal behaviour, empirical derivation of gas equation, Avogadro’s number, ideal gas equation, deviation from ideal behaviour, liquefaction of gases, critical temperature. Liquid State – Vapour pressure, viscosity and surface tension (qualitative idea only, no mathematical derivations).
Also, access the following resources for Class 11 Chapter 5 States of Matter at BYJU’S:
- States Of Matter Class 11 Notes – Chapter 5
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 5 – States of Matter
Chapter 6: Thermodynamics
It is a branch of science which explains the relationship between heat and the other energy forms. On the basis of exchange of matter and energy, thermodynamic systems are divided into three types – open, closed and isolated systems. Students will learn more about the concepts like terms in thermodynamics, applications, calorimetry, enthalpies, spontaneity and Gibbs energy change and equilibrium. The problems in the NCERT Solutions are answered in a systematic manner completely based on the latest syllabus of the CBSE board.
Topics Covered in Class 11 Chemistry Chapter 6 Thermodynamics :
Concepts of system, types of systems, surroundings, work, heat, energy, extensive and intensive properties, state functions. First law of thermodynamics – internal energy and enthalpy, heat capacity and specific heat, measurement of ΔU and ΔH, Hess’s law of constant heat summation, enthalpy of bond dissociation, combustion, formation, atomization, sublimation, phase transition, ionization, and dilution. Introduction of entropy as a state function, free energy change for spontaneous and nonspontaneous process, equilibrium.
Also, access the following resources for Class 11 Chapter 6 Thermodynamics at BYJU’S:
- Thermodynamics Class 11 Notes – Chapter 6
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 6 – Thermodynamics
Chapter 7: Equilibrium
From this chapter, students will learn buffer solutions, equilibrium constant and the common ion effect . As this chapter contains a lot of concepts, a thorough understanding of each of them is important to perform well in the annual exam. The solutions contain clear cut explanations for each and every concept so that all the queries of the students are clarified. The concepts discussed in this chapter are – solid liquid equilibrium, applications of equilibrium constant, factors affecting equilibria, acids, bases and salts, buffer solutions etc.
Topics Covered in Class 11 Chemistry Chapter 7 Equilibrium:
Equilibrium in physical and chemical processes, dynamic nature of equilibrium, law of mass action, equilibrium constant, factors affecting equilibrium – Le Chatelier’s principle; ionic equilibrium – ionization of acids and bases, strong and weak electrolytes, degree of ionization, concept of pH. Hydrolysis of salts (elementary idea), buffer solutions, solubility product, common ion effect (with illustrative examples).
Also, access the following resources for Class 11 Chapter 7 Equilibrium at BYJU’S:
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 7 – Equilibrium
- Equilibrium Class 11 Notes – Chapter 7
Chapter 8: Redox Reactions
The fact here is that the field of electrochemistry deals with the redox reactions . It is important for the students to learn this chapter in order to score good marks in the final exam. Students will get questions based on the classical idea of redox reaction, redox reactions in terms of electron transfer reactions, oxidation number and electrode processes. Students are recommended to first complete the entire chapter using the NCERT textbook prescribed by the CBSE board.
Topics Covered in Class 11 Chemistry Chapter 8 Redox Reaction :
Concept of oxidation and reduction, redox reactions, oxidation number, balancing redox reactions, applications of redox reactions.
Also, access the following resources for Class 11 Chapter 8 Redox Reactions at BYJU’S:
- Redox Reactions Class 11 Notes – Chapter 8
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 8 – Redox Reactions
Chapter 9: Hydrogen
Hydrogen is the lightest element with one electron and proton. The most abundant element in the entire universe is dihydrogen . Hydrogen is an important element which makes up 80% of the entire mass of the universe. It is very important for the students to know about this element in order to secure good marks in the final exam. Students will also learn about the preparation and properties of dihydrogen and its applications in our daily life. It might be difficult for the students to grasp all these concepts at a stretch, so making use of a perfect study material will help you through it.
Topics Covered in Class 11 Chemistry Chapter 9 Hydrogen :
Position of hydrogen in periodic table, occurrence, isotopes, preparation, properties and uses of hydrogen; hydrides – ionic, covalent and interstitial; physical and chemical properties of water, heavy water; hydrogen peroxide – preparation, reactions and structure; hydrogen as a fuel.
Also, access the following resources for Class 11 Chapter 9 Hydrogen at BYJU’S:
- Hydrogen Class 11 Notes – Chapter 9
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 9 – Hydrogen
- Chemistry Revision Notes for Class 11 Chapter 9 Hydrogen
Chapter 10: The s-Block Elements
Students will learn about the Alkali metals and Alkaline earth metals which are the group 1 and group 2 in the periodic table. Some of the concepts like physical and chemical properties of s-block elements, general characteristics of compounds and about important compounds like calcium. The biological importance of calcium and magnesium is also explained clearly in this chapter. Periodic table and the elements present in them is a hot topic in the exam perspective. For this reason, students are highly recommended to learn all these concepts thoroughly to score well.
Topics Covered in Class 11 Chemistry Chapter 10 The s-Block Elements :
Group 1 and Group 2 elements: General introduction, electronic configuration, occurrence, anomalous properties of the first element of each group, diagonal relationship, trends in the variation of properties (such as ionization enthalpy, atomic and ionic radii), trends in chemical reactivity with oxygen, water, hydrogen and halogens; uses.
Preparation and properties of some important compounds: Sodium carbonate, sodium chloride, sodium hydroxide and sodium hydrogen carbonate, biological importance of sodium and potassium. CaO, CaCO 3 , and industrial use of lime and limestone, biological importance of Mg and Ca.
Also, access the following resources for Class 11 Chapter 10 The s-Block Elements at BYJU’S:
- The s-Block Elements Class 11 Notes – Chapter 10
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 10 – The S Block Elements
Chapter 11: The p-Block Elements
The elements present between the 13th and 18th group are called the p-block elements. As this chapter has a lots of concepts, it is very important for the students to revise it regularly. Students will get a gist of the p-block elements, where the last electron enters or is found on the p-subshell. Some of the concepts which you will learn in this chapter are – boron family , important compounds of boron, uses of aluminium and boron, carbon family, allotropes of carbon and important compounds of silicon and carbon.
Topics Covered in Class 11 Chemistry Chapter 11 The p-Block Elements :
General Introduction to p-Block Elements Group 13 elements: General introduction, electronic configuration, occurrence, variation of properties, oxidation states, trends in chemical reactivity, anomalous properties of first element of the group; Boron – physical and chemical properties, some important compounds: borax, boric acids, boron hydrides. Aluminium: uses, reactions with acids and alkalies. Group 14 elements: General introduction, electronic configuration, occurrence, variation of properties, oxidation states, trends in chemical reactivity, anomalous behaviour of first element. Carbon – catenation, allotropic forms, physical and chemical properties; uses of some important compounds: oxides. Important compounds of silicon and a few uses: silicon tetrachloride , silicones, silicates and zeolites.
Also, access the following resources for Class 11 Chapter 11 The p-Block Elements at BYJU’S:
- What Are P-Block Elements?
- The p-Block Elements Class 11 Notes – Chapter 11
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 11 – The P Block Elements
Chapter 12: Organic Chemistry – Some Basic Principles and Techniques
When it comes to exam preparation, both organic and inorganic chemistry plays an important role in attaining good marks. This chapter will introduce you to concepts such as organic compounds, structural representation, classification, nomenclature, isomerism , purification of organic compounds and quantitative analysis. If students are not able to learn these concepts using the NCERT textbook they can download the PDF of solutions which are available online for absolutely free of cost.
Topics Covered in Class 11 Chemistry Chapter 12 Organic Chemistry – Some Basic Principles :
General introduction, methods of purification, qualitative and quantitative analysis, classification and IUPAC nomenclature of organic compounds. Electronic displacements in a covalent bond: inductive effect, electromeric effect, resonance and hyper conjugation. Homolytic and heterolytic fission of a covalent bond: free radicals, carbocations, carbanions; electrophiles and nucleophiles, types of organic reactions
Also access the following resources for Class 11 Chapter 12 Organic Chemistry – Some Basic Principles and Techniques at BYJU’S:
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 12 – Organic Chemistry Solutions Some Basic Principles and Techniques
- Chemistry Revision Notes for Class 11 Chapter 12 Organic Chemistry – Some Basic Principles and Techniques
Chapter 13: Hydrocarbons
The scientific study of properties, structure, reactions, composition and synthesis of organic compounds is called organic chemistry. It teaches the students about concepts like alkanes and its preparation from alkyl halides, unsaturated hydrocarbons and carboxylic acids. Students will also learn about the physical and chemical properties of alkanes as per the prescribed CBSE syllabus. As this chapter is important from the exam point of view, students must highly concentrate on all the concepts and memorise it thoroughly to perform well in the exam.
Topics Covered in Class 11 Chemistry Chapter 13 Hydrocarbons :
Classification of hydrocarbons Alkanes: Nomenclature, isomerism, conformations (ethane only), physical properties, chemical reactions including free radical mechanism of halogenation, combustion and pyrolysis. Alkenes: Nomenclature, structure of double bond (ethene), geometrical isomerism, physical properties, methods of preparation; chemical reactions: addition of hydrogen, halogen, water, hydrogen halides (Markovnikov’s addition and peroxide effect), ozonolysis, oxidation, mechanism of electrophilic addition. Alkynes: Nomenclature, structure of triple bond (ethyne), physical properties, methods of preparation, chemical reactions: acidic character of alkynes, addition reaction of – hydrogen, halogens, hydrogen halides and water. Aromatic hydrocarbons: Introduction, IUPAC nomenclature; Benzene: resonance, aromaticity; chemical properties: mechanism of electrophilic substitution – nitration sulphonation, halogenation, Friedel Craft’s alkylation and acylation; directive influence of functional group in mono-substituted benzene; carcinogenicity and toxicity
Also access the following resources for Class 11 Chapter 13 Hydrocarbons at BYJU’S:
- Hydrocarbons Class 11 Notes – Chapter 13
- Chapter 13 – Hydrocarbons
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 13 – Hydrocarbons
Chapter 14: Environmental Chemistry
Various concepts which are related to environment like water pollution , atmospheric pollution, soil pollution and its reasons are explained briefly in this chapter. Along with this students will also learn about the use of pesticides to reduce soil pollution and strategies to control pollution like waste disposal. By learning this chapter thoroughly, students will get to know about the pollution occurring in nature by our activities.
Topics Covered in Class 11 Chemistry Chapter 14 Environmental Chemistry :
Environmental pollution : Air, water and soil pollution, chemical reactions in atmosphere, smogs, major atmospheric pollutants; acid rain, ozone and its reactions, effects of depletion of ozone layer, greenhouse effect and global warming – pollution due to industrial wastes; green chemistry as an alternative tool for reducing pollution, strategy for control of environmental pollution.
Also access the following resources for Class 11 Chapter 14 Environmental Chemistry at BYJU’S:
- Environmental Chemistry Class 11 Notes – Chapter 14
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 14 – Environmental Chemistry Solutions
In the NCERT Textbook of Chemistry, there are about 14 chapters which contains various concepts which are crucial for the exam. It might be difficult for the students to remember all the chemical formulas and reactions during the final exam so regular practise is necessary. To understand the syllabus students can make use of the NCERT Class 11 Books from BYJU’S.
Features of NCERT Solutions for Class 11 Chemistry
The NCERT Solutions for Class 11 Chemistry provided by BYJU’S features:
- Brief explanations are provided for each question.
- The numerical equations are solved in a step wise manner.
- The answers are clear cut and precise completely based on the CBSE syllabus.
- Subject matter experts design the solutions after conducting a vast research.
- PDFs are available with a free download option.
Note: CBSE Class 11 Chemistry students can bookmark this page in their browser to easily access NCERT Solutions for Class 11 Chemistry in the future.
CBSE Marking Scheme 2023-24
The entire syllabus as per CBSE board is divided portions containing all the concepts divided equally. By doing this, the faculty explain the relationship between the various topics which are of higher importance. At the end of each academic year, the CBSE conducts exams in accordance to the syllabus allotted for each year. This mainly aims to provide students the ability to learn the various concepts that are crucial from the exam perspective.
Toppers Prefer BYJU’S NCERT Solutions. Here’s Why…
The highly experienced subject experts at BYJU’S design the solutions with the aim of helping students ace the exams. The solutions contain explanations in simple language so that students can grasp the concepts effectively. Students can rely on these concepts as they strictly follow the NCERT Class 11 Chemistry Syllabus . The solutions offered by BYJU’S give students a competitive edge by meeting the following criteria:
1. The solutions are created by the best faculty in the respective field
The highly qualified academic tutors design the solutions with the main objective of providing the best educational content to the students across the country. The solutions are very elaborate so that students get all their doubts cleared instantly.
2. Helps students answer complex questions
The explanatory solutions provided by the experts at BYJU’S are student friendly. The complex questions and numericals are solved in a step wise manner in order to make students grasp the method of solving them. The solutions are concept focused, which in turn, provides a strong foundation of fundamental concepts which would help students in their future studies.
3. Best study material for revision purpose
Among the various study materials available online, students can refer to the NCERT Solutions at BYJU’S to speed up their exam preparation. All the concepts are covered in the solutions so that students do not miss any concept which is important for the exam. From theory questions to numerical problems, NCERT Solutions provides the best knowledge to the students.
After going through our NCERT Solutions for Class 11 Chemistry, Also Check Out:
- NCERT Exemplar Problems for Class 11 Chemistry
- CBSE Revision Notes for Class 11 Chemistry
Have any questions/issues regarding our NCERT Solutions for Class 11 Chemistry? Our support team is always available to resolve your queries. Register with BYJU’S and get in touch with them NOW!
Frequently Asked Questions on NCERT Solutions for Class 11 Chemistry
Is the ncert solutions for class 11 chemistry free of cost, can i get my doubts clarified using the ncert solutions for class 11 chemistry at byju’s, how many chapters are present in the ncert solutions for class 11 chemistry , is the ncert solutions for class 11 chemistry the best study material for chapter revision, leave a comment cancel reply.
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
NCERT Solutions for Class 11 Chemistry
NCERT Solutions for Class 11 Chemistry in PDF format English Medium MCQ, Extra Questions for CBSE and State Board. As per the new textbook published for academic year 2024-25, there are only 9 chapters in class 11 chemistry Syllabus. Chapter wise NCERT Solutions for Class 11 Chemistry Chapter 1. Some Basic Concepts of Chemistry Chapter 2. Structure of Atom Chapter 3. Classification of Elements and Periodicity in Properties Chapter 4. Chemical Bonding and Molecular Structure Chapter 5. Thermodynamics Chapter 6. Equilibrium Chapter 7. Redox Reactions Chapter 8. Organic Chemistry – Some Basic Principles and Techniques Chapter 9. Hydrocarbons
NCERT books Solutions, notes and assignments according to current CBSE syllabus, are also available to download along with the answers given at the end of the book. If you are having any suggestion for the improvement, your are welcome. The improvement of the website and its contents are based on your suggestion and feedback.
The branch of science under which the structure of matter, its properties, uses and interaction with other types of a matter and various forms of energy are studied is called chemistry.
1. Since times immortal. The man come on earth his curious nature urge him. Due to this he made new discoveries. In Indus Valley cultures making of metals techniques were found. They were make copper and bronze articles. In Egyptian culture the methods to preserve dead bodies as ‘mummies. Chemicals used for it remain mystery. Even today we do not know about the chemicals. Egyptians had the knowledge of making soap, dyes, glass, etc. Pyramids of Egypt are the examples of their progress. Chemist’ of Egypt discovered touch stone, universal solvent. They did not succeed in their attempts. They learnt about process like distillation and sublimation. These are help us even today.
2. For treatment, different systems of medicine were found. Allopathy in Europe, Unani in Greece and Chinese in China. In India, many expert in Ayurveda contributed notably. Sage Kanad termed the smallest part of as particle matter/elements. On this basis, John Dalton gave his Atomic Theory in 1808 AD. For example:. This body made from Earth, Water, Air, Fire and sky. The concept of five elements is the Research of Indian scientist only.
3. Ancient Indians had the knowledge of metallurgy, fermentation. They can prepare bases, extracts and distillates. The polish done on the ‘Iron Pillar’ of Mehrauli in Delhi. This is a subject of research. There is no rusting has occurred on it till date.
Provide us feedback to improve the quality of the content. Class 11 Chemistry NCERT Solutions are in the form of PDF and Videos. Download NCERT Books and Offline Apps 2024-25 based on new CBSE Syllabus. Ask your doubts related to NIOS or UP Board, MP Board or other Boards and share your knowledge with your friends and other users through Discussion Forum.
Copyright 2024 by Tiwari Academy | A step towards Free Education


NCERT Solutions for Class 11 Chemistry in PDF

NCERT Solutions for Class 11 Chemistry
- NCERT Exercises Solutions Chapter 1
- NCERT Books for Class 11 in PDF file
- NCERT Books for Class 11 in ZIP file
- Assignment chapter 1 – Solved
- Notes of chapter 1
- Study Material chapter 1
- Book for Revision Chapter 1
- Revision Notes for 2021 – 2022 (From Chapter 1)
- NCERT Exercises Solutions of Chapter 2
- Assignment for Chapter 2 (Solved)
- Notes of chapter 2
- Study Material chapter 2
- Book for Revision Chapter 2
- Revision Notes for 2021 – 2022 (From Chapter 2)
- NCERT Exercises Solutions of Chapter 3
- Assignment for Chapter 3 (Solved)
- Notes of chapter 3
- Study Material chapter 3
- Book for Revision Chapter 3
- Revision Notes for 2021 – 2022 (From Chapter 3)
- NCERT Exercises Solutions Chapter 4
- Notes based on chapter 4
- Study Material chapter 4
- Book for Revision of Chapter 4
- Revision Notes for 2021 – 2022 (From Chapter 4)
- NCERT Exercises Solutions of Chapter 5
- Assignment based on chapter 5 (Solved)
- Notes on chapter 5 (Revision)
- Study Material chapter 5
- Book for better Revision on Chapter 5
- Revision Notes for 2021 – 2022 Exams (From Chapter 5)
- NCERT Exercises Solutions of Chapter 6
- Notes for Revision of chapter 6
- Study Material on chapter 6
- Revision Book Chapter 6
- Revision Notes for 2021 – 2022 (From Chapter 6)
- NCERT Exercises Q1 – Q20
- NCERT Exercises Q21 – Q40
- NCERT Exercises Q41 – Q60
- NCERT Exercises Q61 – Onward
- Notes for Revision of Equilibrium
- Study Material base on Equilibrium
- Revision Book Chapter 7 Equilibrium
- Revision Notes for 2021 – 2022
- NCERT Exercises Solutions Chapter 8
- Solved Assignment chapter 8
- Study Material chapter 8
- Revision Book Chapter 8
- Revision Notes for 2021 – 2022 (Chapter 8)
- NCERT Exercises Solutions Chapter 9
- Study Material chapter 9
- Revision Book Chapter 9
- Revision Notes for 2021 – 2022 (From Chapter 9)
- NCERT Exercises Solutions Chapter 10
- Study Material chapter 10
- Revision Book Chapter 10
- Revision Notes for 2021 – 2022 (Based on Chapter 10)
- NCERT Exercises chapter 11
- Study Material chapter 11
- Revision Book Chapter 11
- Revision Notes for CBSE Exams 2021 – 2022
- NCERT Exercises Q1 – Q18
- NCERT Exercises Q19 – Onward
- Solved Assignment Chapter 12
- Study Material chapter 12
- Revision Book Chapter 12
- Revision Notes for 2021 – 2022 CBSE Exams
- NCERT Exercises Solutions Chapter 13
- Study Material chapter 13
- Revision Book Chapter 13
- Revision Notes for 2021 – 2022 base on Chapter 13
- NCERT Exercises Solutions Chapter 14
- Study Material chapter 14
- Revision Book Chapter 14
- Revision Notes for 2021 – 2022 Chapter 14
Law of Chemical Combination
Law of Conservation of Mass: “Matter can neither be created nor destroyed.” This law was put forth by Antoine Lavoisier in 1789. This law formed the basis for several later developments in chemistry. In fact this was the result of planned experiments performed by Lavoisier.
Dalton’s Atomic Theory : We know that Dalton’s atomic theory can explain the laws of chemical combination. According to John Dalton (1776 – 1884), “Matter is made up of small indivisible particles.” These smallest indivisible particles are called atoms. Dalton’s atomic theory is helpful to know chemical reaction but during his time the fundamental particles of atom like proton, electron and neutron were not discovered.
Law of Combining Weights : According to this law, masses of two elements which separately react chemically with identical masses of a third element are also the masses which react with each other and are in simple multiples of combining weight of an element which is either equal to its atomic weight or simple multiple of it.
NCERT Solutions for Class 11 (All Subjects)
- NCERT Solutions for Class 6
- NCERT Solutions for Class 7
- NCERT Solutions For Class 8
- NCERT Solutions for Class 9
- NCERT Solution for Class 10
- NCERT Solutions for Class 11
- NCERT Solutions for Class 12
Class 10 Maths Solutions Chapter 1 Class 10 Maths Solutions Chapter 2 Class 10 Maths Solutions Chapter 3 Class 10 Maths Solutions Chapter 4 Class 10 Maths Solutions Chapter 5
Class 10 Maths Solutions Chapter 6 Class 10 Maths Solutions Chapter 7 Class 10 Maths Solutions Chapter 8 Class 10 Maths Solutions Chapter 9 Class 10 Maths Solutions Chapter 10
Class 10 Maths Solutions Chapter 11 Class 10 Maths Solutions Chapter 12 Class 10 Maths Solutions Chapter 13 Class 10 Maths Solutions Chapter 14 Class 10 Maths Solutions Chapter 15
Class 9 Maths Solutions Chapter 1 Class 9 Maths Solutions Chapter 2 Class 9 Maths Solutions Chapter 3 Class 9 Maths Solutions Chapter 4 Class 9 Maths Solutions Chapter 5
Class 9 Maths Solutions Chapter 6 Class 9 Maths Solutions Chapter 7 Class 9 Maths Solutions Chapter 8 Class 9 Maths Solutions Chapter 9 Class 9 Maths Solutions Chapter 10
Class 9 Maths Solutions Chapter 11 Class 9 Maths Solutions Chapter 12 Class 9 Maths Solutions Chapter 13 Class 9 Maths Solutions Chapter 14 Class 9 Maths Solutions Chapter 15
- 10th Quarterly Exam Question 2024
- 11th 2nd Mid Term Question
- 9th 2nd Mid term Question
- 8th All subjects - 2nd Mid-Term Question 2023
- _Error Page
- Privacy policy
.jpeg)
- TNPSC GROUP 4 Government Study Material 2024 PDF Download
- 11th Supplementary Exam Question paper 2024 & Important questions
- 12th Supplementary Exam Question paper 2024 & Important questions
- 10th First Mid-term Question paper 2024 & Important questions
TN 11th std Assignment PDF , Question,Answer Key 2021 worksheet
Tn 11th std assignment pdf , question,answer key 2021.
Tamilnadu state board samacheer kalvi 11th std all subjects assignment writing work sheet and answer guide PDF Download
TNSCERT 11th Assignment PDF Download with answer Tamil medium , English medium
11th assignment tamil medium question paper ,answer ke.
- Tamil – – Unit -1 -Tamil Medium Assignment
- Accountancy – Unit -1 -Tamil Medium Assignment
- Advance Tamil – Unit -1 -Tamil Medium Assignment
- Agricultural Science – Unit -1 -Tamil Medium Assignment
- Basic Automobile Engg– Unit -1 -Tamil Medium Assignment
- Basic Civil Engg- – Unit -1 -Tamil Medium Assignment
- Basic Electrical Engg – Unit -1 -Tamil Medium Assignment
- Basic Electronics Engineering – Unit -1 -Tamil Medium Assignment
- Basic Mechanical Engg – Unit -1 -Tamil Medium Assignment
- Bio botany– Unit -1 -Tamil Medium Assignment
- Bio Chemistry – Unit -1 -Tamil Medium Assignment
- Bio- zoology – Unit -1 -Tamil Medium Assignment
- Botany– Unit -1 -Tamil Medium Assignment
- Business Mathematics– Unit -1 -Tamil Medium Assignment
- Chemistry – Unit -1 -Tamil Medium Assignment
- Commerce – Unit -1 -Tamil Medium Assignment
- Computer Applicatioin – Unit -1 -Tamil Medium Assignment
- Computer Science– Unit -1 -Tamil Medium Assignment
- Computer Technology – Unit -1 -Tamil Medium Assignment
- Economics– Unit -1 -Tamil Medium Assignment
- Ethics and Indian Culture– Unit -1 -Tamil Medium Assignment
- Geography – Unit -1 -Tamil Medium Assignment
- History – Unit -1 -Tamil Medium Assignment
- Home Science – Unit -1 -Tamil Medium Assignment
- Mathematics – Unit -1 -Tamil Medium Assignment
- Microbiology– Unit -1 -Tamil Medium Assignment
- Nursing General – Unit -1 -Tamil Medium Assignment
- Nursing Vocationa – Unit -1 -Tamil Medium Assignment
- Nutrition and Dietetics – Unit -1 -Tamil Medium Assignment
- Office Management – Unit -1 -Tamil Medium Assignment
- Physics– Unit -1 -Tamil Medium Assignment
- Political Science – Unit -1 -Tamil Medium Assignment
- Statistics– Unit -1 -Tamil Medium Assignment
- Textile and dress designing – Unit -1 -Tamil Medium Assignment
- Zoology– Unit -1 -Tamil Medium Assignment
- உணவக மேலாண்மை – Unit -1 -Tamil Medium Assignment
- நெசவியல் தொழிற்நுட்பம்– Unit -1 -Tamil Medium Assignment
11th Assignment English Medium PDF Download, Question paper ,answer key
- Tamil – Unit 1 Assignment
- Accountancy – Unit 1 Assignment English Medium
- Basic Automobile Engg – Unit 1 Assignment English Medium
- Basic Civil Engg- – Unit 1 Assignment English Medium
- Basic Electrical Engineering – Unit 1 Assignment English Medium
- Basic Electronics Engg – – Unit 1 Assignment English Medium
- Basic Mechanical Engg – Unit 1 Assignment English Medium
- Bio Botany – – Unit 1 Assignment English Medium
- Bio -Chemistry – Unit 1 Assignment English Medium
- Bio Zoology- EM- – Unit 1 Assignment English Medium
- Botany- EM- – Unit 1 Assignment English Medium
- Business Mathematics – Unit 1 Assignment English Medium
- Chemistry – Unit 1 Assignment English Medium
- Commerce – – Unit 1 Assignment English Medium
- Communcative English – Unit 1 Assignment English Medium
- Computer Application– Unit 1 Assignment English Medium
- Computer Science – Unit 1 Assignment English Medium
- Computer Technology– Unit 1 Assignment English Medium
- Economics – Unit 1 Assignment English Medium
- English – Unit 1 Assignment English Medium
- Food Service Management – Unit 1 Assignment English Medium
- Geography – Unit 1 Assignment English Medium
- History– Unit 1 Assignment English Medium
- Home Science – Unit 1 Assignment English Medium
- Maths Assign – Unit 1 Assignment English Medium
- Microbiology– Unit 1 Assignment English Medium
- Nursing General – Unit 1 Assignment English Medium
- Nursing Vocational – Unit 1 Assignment English Medium
- NUTRITION – Unit 1 Assignment English Medium
- Office Management-– Unit 1 Assignment English Medium
- physics – Unit 1 Assignment English Medium
- Political Science – Unit 1 Assignment English Medium
- Zoology – – Unit 1 Assignment English Medium
இந்த பயனுள்ள பக்கத்தை உங்கள் நண்பர்களுக்கும் share செய்யுங்கள்
Also Read: Related Post
12th All Subjects Assignment Question and Answer - Download Link
11th All Subjects Assignment Question and Answer - Download Link
10th All Subjects Assignment Question and Answer - Download Link
9th All Subjects Assignment Question and Answer - Download Link
8th All Subjects Assignment Question and Answer - Download Link
7th All Subjects Assignment Question and Answer - Download Link
6th All Subjects Assignment Question and Answer - Download Link
5th All Subjects Assignment Question and Answer - Download Link
4th All Subjects Assignment Question and Answer - Download Link
3rd All Subjects Assignment Question and Answer - Download Link
2nd std All Subjects Assignment Question and Answer - Download Link
1st std All Subjects Assignment Question and Answer - Download Link
- Copy Link Copied
கல்விகவி வலைப்பூ நண்பர்களே.. வாசகர்களின் கருத்து சுதந்திரத்தை வரவேற்கும் இந்தப்பகுதியை ஆரோக்கியமாக பயன்படுத்திக் கொள்ள அன்புடன் வேண்டுகிறோம். உங்கள் கருத்தையும் ,study Mateials தேவையை சுதந்திரமாக பகிரலாம். நண்பர்களுக்கு Share செய்யலாம். குறிப்பு: 1. இங்கு பதிவாகும் கருத்துக்கள் வாசகர்களின் சொந்த கருத்துக்களே. kalvikavi வலைப்பக்கங்கள்பூ இதற்கு எவ்வகையிலும் பொறுப்பல்ல. 2. கருத்தை நிராகரிக்கவோ, குறைக்கவோ, தணிக்கை செய்யவோ கல்விகவி குழுவுக்கு முழு உரிமை உண்டு. 3. தனிமனித தாக்குதல்கள், நாகரிகமற்ற வார்த்தைகள், படைப்புக்கு பொருத்தமில்லாத கருத்துகள் நீக்கப்படும். 4. தங்களின் பெயர் மற்றும் சரியான மின்னஞ்சல் முகவரியை பயன்படுத்தி கருத்தை பதிவிட அன்புடன் வேண்டுகிறோம். -அன்புடன் KALVIAVI வலைப்பூ ( Join our telegram & WhatsApp Get instant Study Materials & கல்விச்செய்திகள் )
English medium answers are not supported
P Vijaya Murugan

Kalvi Arts Youtube & Kalvi kavi.com
மாணவர்களுக்கு தேவையான Study Materials, Important Questions,Model Question Papers ,தினந்தோறும் கல்விச்செய்திகள் இங்கே கிடைக்கும்

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Chemistry Worksheets and Handouts (PDF for Printing)

This is a collection of free chemistry worksheets and handouts to print. Most of the printables are PDF files, although some are available as JPG or PNG files. All of these worksheets print cleanly on normal printer paper, plus you can resize them to fit your needs.
Here is a list of worksheets. This site also has articles explaining these topics in detail.
- Label Parts of the Atom [ Google Apps worksheet ][ worksheet PDF ][ worksheet PNG ][ answers PNG ]
- Acid formulas [ PDF ][ Answers ]
- Balancing equations Worksheet #1 [ PDF ][ Answers ] Worksheet #2 [ PDF ][ Answers ] Worksheet #3 [ PDF ][ Answers ] Worksheet #4 [ PDF ][ Answers ]
- Chemical and Physical Changes [ PDF ][ Answers ]
- Chemistry scavenger hunt [ PDF clues ][ Answers ]
- Element names crossword [ PDF ][ Answers ]
- Element symbols – Symbols that make words [ PDF worksheet ][ Answers ]
- Element symbols – Countries of the world [ PDF ][ Answers ]
- More element symbol worksheets
- Homogeneous or Heterogeneous Mixtures [ PDF ][ Answers ]
- Intensive and Extensive Properties [ Worksheet ][ Answer Key ]
- Intrinsic and Extrinsic Properties [ PDF ][ Answers ]
- Ionic and Covalent Compounds (Names and Identification) [ PDF Worksheet ][ Answer Key ]
- Ionic Compound Names and Formulas [ PDF Worksheet ][ Answer Key ]
- Metric to English Unit Conversions [ PDF Worksheet ][ Answer Key ]
- Mixtures [ PDF ][ Answers ]
- Periodic table scavenger hunt [ PDF clues ][ Answers ]
- Reading a meniscus [ PDF ][ Answers ]
- Reading periodic table element information Worksheet #1 [ PDF ][ Answers ] Worksheet #2 [ PDF ][ Answers ]
- Scientific Notation [ PDF ][ Answers ]
- Significant digits Rules [ PDF ][ Answers ] Addition and subtraction [ PDF ][ Answers ] Multiplication and division [ PDF ][ Answers ]
- Types of Chemical Reactions [ Worksheet ][ Answers ]
In addition to these chemistry worksheets, there is a collection of word search puzzles .
Chemistry Handouts
These chemistry handouts illustrate chemistry concepts and offer examples.
- Amino acid side chains [ PDF ]
- Antimatter examples [ PNG ]
- Atom facts [ PNG ]
- Chemical properties [ JPG ]
- Colligative properties [ JPG ]
- Electron configurations [ PDF ]
- Element electronegativities [ PDF ]
- 118 Element Flash Cards [ PDF ]
- Element list [ PDF ]
- Endothermic reactions [ PNG ]
- Error calculations [ JPG ]
- Exothermic reactions [ JPG ]
- Heterogeneous mixtures [ JPG ]
- Hydrocarbon prefixes [ JPG ]
- Ionic compound properties [ PNG ]
- Genetic codons [ PDF ]
- Lewis structures [ JPG ]
- Litmus test [ PNG ]
- Magnetic vs non-magnetic metals [ JPG ]
- Mole ratio [ JPG ]
- Organic vs inorganic [ JPG ]
- Oxidation numbers [ JPG ]
- Periodic table Bingo game [ PDF ]
- pH indicators [ PNG ]
- Physical change [ JPG ]
- Physical properties [JPG ]
- Noble metals [ JPG ]
- Reactants and products [ JPG ]
- RNA vs DNA [ JPG ]
- States of matter [ JPG ]
- Visible spectrum [ JPG ]
Periodic Tables
There’s a printable periodic table for just about any purpose, but some of the most popular are listed here.

- 118 element vibrant periodic table [ PNG ]
- Actinides [ JPG ]
- Blank periodic table [ PDF ]
- Element charges [ JPG ]
- Element density [ PDF ]
- Element electrical conductivity [ PDF ]
- Element state of matter [ PDF ]
- Muted color 118 element periodic table [ PDF ]
- Native elements [ JPG ]
- Valence [ JPG ]

Biology Worksheets and Handouts
Is biology more your thing? We’ve got similar resources for the life sciences, including biology, biochemistry, cell biology, and anatomy.
Chemistry Worksheets Terms of Use
You are welcome to print these resources for personal or classroom use. They may be used as handouts or posters. They may not be posted elsewhere online, sold, or used on products for sale.
This page doesn’t include all of the assets on the Science Notes site. If there’s a table or worksheet you need but don’t see, just let us know!
Related Posts

IMAGES
VIDEO
COMMENTS
021022_11TH Chemistry_Assignment 01_States of Matter_Questions & answers_Origional (1) - Free download as PDF File (.pdf) or read online for free.
TNSCERT 11th Chemistry Assignment PDF Download with answer TN 11th Chemistry Assignment Question paper ,answer key Chemistry - Unit -1 -Tamil Medium Assignment
Free PDF download of 11th Chemistry Assignment PDF with answers created by master educators from the latest syllabus of CBSE Boards. By practicing these Class 11 Chemistry Assignment Pdf will help you to score more marks in your CBSE Board Examinations. ... May 2, 2021 August 17, 2022 admin. Assignments Class 11 Mathematics Permutations and ...
July 28, 2021 November 2, 2022 admin. ... All latest Kendriya Vidyalaya Class 11 Chemistry Assignments with Answers and test papers are given below. Chemistry Class 11 Assignments Pdf Download. We have provided below the biggest collection of free CBSE NCERT KVS Assignments for Class 11 Chemistry. Students and teachers can download and save all ...
11th CHEMISTRY Assignment-1 1 2021-2022 | 👇pdf link 👇https://drive.google.com/file/d/1DMUhkMN8nzNg-70tA7usspQGT4MlK8QV/view?usp=drivesdk
NCERT Solutions for Class 11 Chemistry Free PDF Download (Chapter-wise) Chapter 1 Some Basic Concepts of Chemistry. Chapter 2 Structure of Atom. Chapter 3 Classification of Elements and Periodicity in Properties. Chapter 4 Chemical Bonding and Molecular Structure. Chapter 5 States of Matter. Chapter 6 Thermodynamics.
Tamilnadu State Board Samacheer Kalvi 11th Chemistry Book Volume 1 Solutions. Chapter 1 Basic Concepts of Chemistry and Chemical Calculations. Chapter 2 Quantum Mechanical Model of Atom. Chapter 3 Periodic Classification of Elements. Chapter 4 Hydrogen. Chapter 5 Alkali and Alkaline Earth Metals. Chapter 6 Gaseous State. Chapter 7 Thermodynamics.
Students can refer to Assignments for Class 11 Chemistry available for download in Pdf. We have given below links to subject-wise free printable Assignments for Chemistry Class 11 which you can download easily. All assignments have a collection of questions and answers designed for all topics given in your latest NCERT Books for Class 11 Chemistry for the current academic session.
NCERT Solutions for Class 11 Chemistry Chapter 2 are available for you to download online in PDF format. The Class 11 Chemistry NCERT Solutions Chapter 2 are prepared by the experts of Vedantu who are working in this field for decades now. Our subject matter experts will get the right answers for all the questions that are asked in the ...
NCERT Solutions for Class 11 Chemistry Download Chapter-wise PDF for 2023-24. NCERT Solutions for Class 11 Chemistry is a study material which is developed by the faculty at BYJU'S by keeping in mind the grasping power of Class 11 students.NCERT Solutions for Class 11 are drafted in a simple and understandable manner to help students ace the exam without fear.
Chapter 9: Hydrogen. Chapter 10: The s - Block Elements. Chapter 11: The p - Block Elements. Chapter 12: Organic Chemistry - Some Basic Principles and Techniques. Chapter 13: Hydrocarbons. Chapter 14: Environmental Chemistry. NCERT Answers Part 1. NCERT Answers Part 2. Class 11 Chemistry NCERT Book PDF Download Part I.
on August 19, 2023, 11:26 AM. NCERT Solutions for Class 11 Chemistry in PDF format English Medium MCQ, Extra Questions for CBSE and State Board. As per the new textbook published for academic year 2024-25, there are only 9 chapters in class 11 chemistry Syllabus. Chapter wise NCERT Solutions for Class 11 Chemistry.
NCERT Solutions for Class 11 Chemistry in PDF. NCERT Solutions for Class 11 Chemistry in PDF form to free download. NCERT Solutions of other subjects based on latest CBSE Curriculum for 2021 - 2022 is also available to free download. Along with NCERT sols, books for revision, assignments - solved and unsolved, notes and special notes for ...
Dear teachers and Students you can download the (HSC) +1 Public Exam model Question Paper PDF .your Mobile and PC. These HSC first YEAR 11TH Class Public Exam 2020-2021 Model question paper help for your Public Examination. You can get good marks in 2020-2021 11th std. public Examination.
Chemistry Answer Key Unit 1-4. Assignments None. 1. Lolsomething chemical-equations-and-reactions. ... Unit 3 assignment; Chemistry Unit 1 Electronegativity and Polarity; Show 8 more documents Show all 219 documents ... Grade 11 Chemistry (3U) EXAM Review. 23 pages 2018/2019 89% (44) 2018/2019 89% (44) Save. ...
It is correct to say "the molarity is one molar" since: MOLARITY is a QUANTITY and MOLAR is the UNIT of Molarity. CHEMISTRY 11. Unit 5: Solubility and Solutions. Many chemical compounds are stored, measured, and used in reactions as solutions. Recall the definition of a solution: Types of solutions: Solid solute Liquid solute Gas solute.
11th Assignment English Medium PDF Download, Question paper ,answer key. Tamil - Unit 1 Assignment. Accountancy - Unit 1 Assignment English Medium. Basic Automobile Engg - Unit 1 Assignment English Medium.
Print free chemistry worksheets and handouts to enhance student learning. This is a collection of free chemistry worksheets and handouts to print. Most of the printables are PDF files, although some are available as JPG or PNG files. All of these worksheets print cleanly on normal printer paper, plus you can resize them to fit your needs.
2020-2021 Welcome to AP Chemistry! I am happy that you have chosen to take this class. ... the assignment and answer. If you do not have access to a printer, you can write your answers on loose-leaf. 1. Summer Assignment Review Checklist: Read through and review these topics 1. Periodic Table Use and Understanding: ... 5/27/2020 11:48:47 AM ...
Tamilnadu State Board Samacheer Kalvi 11th Chemistry Book Volume 1 Solutions. Chapter 1 Basic Concepts of Chemistry and Chemical Calculations. Chapter 2 Quantum Mechanical Model of Atom. Chapter 3 Periodic Classification of Elements. Chapter 4 Hydrogen. Chapter 5 Alkali and Alkaline Earth Metals. Chapter 6 Gaseous State.
Our resource for Mcgraw-Hill Ryerson Chemistry 11 includes answers to chapter exercises, as well as detailed information to walk you through the process step by step. With Expert Solutions for thousands of practice problems, you can take the guesswork out of studying and move forward with confidence. Find step-by-step solutions and answers to ...
To address these questions, we developed and assessed several assignments across the chemistry curriculum. In General Chemistry, students critiqued laboratory reports generated by ChatGPT to learn about report structure and generative AI's capabilities. In upper-division lab courses, students used ChatGPT for writing assistance with lab ...