An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

The impact of stress on body function: A review
Habib yaribeygi.
1 Neurosciences Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran
Yunes Panahi
2 Clinical Pharmacy Department, Faculty of Pharmacy, Baqiyatallah University of Medical Sciences, Tehran, Iran
Hedayat Sahraei
Thomas p. johnston.
3 Division of Pharmaceutical Sciences, School of Pharmacy, University of Missouri-Kansas City, Kansas City, Missouri, USA
Amirhossein Sahebkar
4 Biotechnology Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
Any intrinsic or extrinsic stimulus that evokes a biological response is known as stress. The compensatory responses to these stresses are known as stress responses. Based on the type, timing and severity of the applied stimulus, stress can exert various actions on the body ranging from alterations in homeostasis to life-threatening effects and death. In many cases, the pathophysiological complications of disease arise from stress and the subjects exposed to stress, e.g. those that work or live in stressful environments, have a higher likelihood of many disorders. Stress can be either a triggering or aggravating factor for many diseases and pathological conditions. In this study, we have reviewed some of the major effects of stress on the primary physiological systems of humans.
Abbreviations
ACTH: Adrenocorticotropic hormone
CNS: Central nervous system
CRH: Corticotropin releasing hormone
GI: Gastrointestinal
LTP: Long-term potentiation
NMDA : N-methyl-D-aspartate
VTA: Ventral tegmental area
Stress and the Brain Function Complications
For a long time, researchers suggested that hormones have receptors just in the peripheral tissues and do not gain access to the central nervous system (CNS) (Lupien and Lepage, 2001[ 63 ]). However, observations have demonstrated the effect of anti-inflammatory drugs (which are considered synthetic hormones) on behavioral and cognitive disorders and the phenomenon called “Steroid psychosis” (Clark et al., 1952[ 16 ]). In the early sixties, neuropeptides were recognized as compounds devoid of effects on the peripheral endocrine system. However, it was determined that hormones are able to elicit biological effects on different parts of the CNS and play an important role in behavior and cognition (De Kloet, 2000[ 22 ]). In 1968, McEven suggested for the first time that the brain of rodents is capable of responding to glucocorticoid (as one of the operators in the stress cascade). This hypothesis that stress can cause functional changes in the CNS was then accepted (McEwen et al., 1968[ 74 ]). From that time on, two types of corticotropic receptors (glucocorticosteroids and mineralocorticoids) were recognized (de Kloet et al., 1999[ 23 ]). It was determined that the affinity of glucocorticosteroid receptors to cortisol and corticosterone was about one tenth of that of mineralocorticoids (de Kloet et al., 1999[ 23 ]). The hippocampus area has both types of receptors, while other points of the brain have only glucocorticosteroid receptors (de Kloet et al., 1999[ 23 ]).
The effects of stress on the nervous system have been investigated for 50 years (Thierry et al., 1968[ 115 ]). Some studies have shown that stress has many effects on the human nervous system and can cause structural changes in different parts of the brain (Lupien et al., 2009[ 65 ]). Chronic stress can lead to atrophy of the brain mass and decrease its weight (Sarahian et al., 2014[ 100 ]). These structural changes bring about differences in the response to stress, cognition and memory (Lupien et al., 2009[ 65 ]). Of course, the amount and intensity of the changes are different according to the stress level and the duration of stress (Lupien et al., 2009[ 65 ]). However, it is now obvious that stress can cause structural changes in the brain with long-term effects on the nervous system (Reznikov et al., 2007[ 89 ]). Thus, it is highly essential to investigate the effects of stress on different aspects of the nervous system (Table 1 (Tab. 1) ; References in Table 1: Lupien et al., 2001[ 63 ]; Woolley et al., 1990[ 122 ]; Sapolsky et al., 1990[ 99 ]; Gould et al., 1998[ 35 ]; Bremner, 1999[ 10 ]; Seeman et al., 1997[ 108 ]; Luine et al., 1994[ 62 ]; Li et al., 2008[ 60 ]; Scholey et al., 2014[ 101 ]; Borcel et al., 2008[ 9 ]; Lupien et al., 2002[ 66 ]).

Stress and Memory
Memory is one of the important functional aspects of the CNS and it is categorized as sensory, short term, and long-term. Short term memory is dependent on the function of the frontal and parietal lobes, while long-term memory depends on the function of large areas of the brain (Wood et al., 2000[ 121 ]). However, total function of memory and the conversion of short term memory to long-term memory are dependent on the hippocampus; an area of the brain that has the highest density of glucocorticosteroid receptors and also represents the highest level of response to stress (Scoville and Milner, 1957[ 107 ]; Asalgoo et al., 2015[ 1 ]). Therefore, during the past several decades, the relationship between the hippocampus and stress have been hotly debated (Asalgoo et al., 2015[ 1 ]; Lupien and Lepage, 2001[ 63 ]). In 1968, it was proven that there were cortisol receptors in the hippocampus of rats (McEwen et al., 1968[ 74 ]). Later, in 1982, by using specific agonists of glucocorticosteroid and mineralocorticoid receptors, the existence of these two receptors in the brain and hippocampus area of rats was proven (Veldhuis et al., 1982[ 119 ]). It should also be noted that the amygdala is very important to assessing the emotional experiences of memory (Roozendaal et al., 2009[ 91 ]).
The results of past studies have demonstrated the effect of stress on the process of memory (Ghodrat et al., 2014[ 32 ]). Various studies have shown that stress can cause functional and structural changes in the hippocampus section of the brain (McEwen, 1999[ 72 ]). These structural changes include atrophy and neurogenesis disorders (Lupien and Lepage, 2001[ 63 ]). Also, chronic stress and, consequently, an increase in plasma cortisol, leads to a reduction in the number of dendritic branches (Woolley et al., 1990[ 122 ]) and the number of neurons (Sapolsky et al., 1990[ 99 ]), as well as structural changes in synaptic terminals (Sapolsky et al., 1990[ 99 ]) and decreased neurogenesis in the hippocampus tissue (Gould et al., 1998[ 35 ]). Glucocorticosteroids can induce these changes by either effecting the cellular metabolism of neurons (Lawrence and Sapolsky, 1994[ 58 ]), or increasing the sensitivity of hippocampus cells to stimulatory amino acids (Sapolsky and Pulsinelli, 1985[ 98 ]) and/or increasing the level of extracellular glutamate (Sapolsky and Pulsinelli, 1985[ 98 ]).
High concentrations of stress hormones can cause declarative memory disorders (Lupien and Lepage, 2001[ 63 ]). Animal studies have shown that stress can cause a reversible reduction in spatial memory as a result of atrophy of the hippocampus (Luine et al., 1994[ 62 ]). In fact, high plasma concentrations of glucocorticosteroids for extended periods of time can cause atrophy of the hippocampus leading to memory disorders (Issa et al., 1990[ 45 ]). Additionally, people with either Cushing's syndrome (with an increased secretion of glucocorticosteroids), or people who receive high dosages of exogenous synthetic anti-inflammatory drugs, are observed to have atrophy of the hippocampus and associated memory disorders (Ling et al., 1981[ 61 ]). MRI images taken from the brains of people with post-traumatic stress disorder (PTSD) have demonstrated a reduction in the volume of the hippocampus along with neurophysiologic effects such as a weak verbal memory (Bremner, 1999[ 10 ]). Several human studies have suggested that even common therapeutic doses of glucocorticosteroids and dexamethasone can cause problems with explicit memory (Keenan et al., 1995[ 49 ]; Kirschbaum et al., 1996[ 53 ]). Thus, there is an inverse relationship between the level of cortisol and memory (Ling et al., 1981[ 61 ]), such that increasing levels of plasma cortisol following prolonged stress leads to a reduction in memory (Kirschbaum et al., 1996[ 53 ]), which improves when the level of plasma cortisol decreases (Seeman et al., 1997[ 108 ]).
Stress also has negative effects on learning. Results from hippocampus-dependent loading data demonstrate that subjects are not as familiar with a new environment after having been exposed to a new environment (Bremner, 1999[ 10 ]). Moreover, adrenal steroids lead to alteration in long-term potentiation (LTP), which is an important process in memory formation (Bliss and Lømo, 1973[ 7 ]).
Two factors are involved in the memory process during stress. The first is noradrenaline, which creates emotional aspects of memories in the basolateral amygdala area (Joëls et al., 2011[ 47 ]). Secondly, this process is facilitated by corticosteroids. However, if the release of corticosteroids occurs a few hours earlier, it causes inhibition of the amygdala and corresponding behaviors (Joëls et al., 2011[ 47 ]). Thus, there is a mutual balance between these two hormones for creating a response in the memory process (Joëls et al., 2011[ 47 ]).
Stress does not always affect memory. Sometimes, under special conditions, stress can actually improve memory (McEwen and Lupien, 2002[ 71 ]). These conditions include non-familiarity, non-predictability, and life-threatening aspects of imposed stimulation. Under these specific conditions, stress can temporarily improve the function of the brain and, therefore, memory. In fact, it has been suggested that stress can sharpen memory in some situations (Schwabe et al., 2010[ 105 ]). For example, it has been shown that having to take a written examination can improve memory for a short period of time in examination participants. Interestingly, this condition is associated with a decrease in the level of cortisol in the saliva (Vedhara et al., 2000[ 118 ]). Other studies have shown that impending stress before learning occurs can also lead to either an increase in the power of memory (Domes et al., 2002[ 27 ]; Schwabe et al., 2008[ 102 ]), or decrease in the capacity for memory (Diamond et al., 2006[ 26 ]; Kirschbaum et al., 1996[ 53 ]). This paradox results from the type of imposed stress and either the degree of emotional connection to the stressful event (Payne et al., 2007[ 83 ]; Diamond et al., 2007[ 25 ]), or the period of time between the imposing stress and the process of learning (Diamond et al., 2007[ 25 ]).
The process of strengthening memory is usually reinforced after stress (Schwabe et al., 2012[ 103 ]). Various studies on animal and human models have shown that administration of either glucocorticosteroids, or stress shortly after learning has occurred facilitates memory (Schwabe et al., 2012[ 103 ]). Also, it has been shown that glucocorticosteroids (not mineralocorticoids) are necessary to improve learning and memory (Lupien et al., 2002[ 66 ]). However, the retrieval of events in memory after exposure to stress will be decreased (Schwabe et al., 2012[ 103 ]), which may result from the competition of updated data for storage in memory in a stressful state (de Kloet et al., 1999[ 23 ]). Some investigations have shown that either exposure to stress, or injection of glucocorticosteroids before a test to assess retention, decreases the power of memory in humans and rodents (Schwabe and Wolf, 2009[ 104 ]).
In summary, it has been concluded that the effect of stress on memory is highly dependent on the time of exposure to the stressful stimulus and, in terms of the timing of the imposed stress, memory can be either better or worse (Schwabe et al., 2012[ 103 ]). Moreover, recent studies have shown that using a specific-timed schedule of exposure to stress not only affects hippocampus-dependent memory, but also striatum-dependent memory, which highlights the role of timing of the imposed stressful stimulus (Schwabe et al., 2010[ 105 ]).
Stress, Cognition and Learning
Cognition is another important feature of brain function. Cognition means reception and perception of perceived stimuli and its interpretation, which includes learning, decision making, attention, and judgment (Sandi, 2013[ 95 ]). Stress has many effects on cognition that depend on its intensity, duration, origin, and magnitude (Sandi, 2013[ 95 ]). Similar to memory, cognition is mainly formed in the hippocampus, amygdala, and temporal lobe (McEwen and Sapolsky, 1995[ 73 ]). The net effect of stress on cognition is a reduction in cognition and thus, it is said that any behavioral steps undertaken to reduce stress leads to increase in cognition (Scholey et al., 2014[ 101 ]). In fact, stress activates some physiological systems, such as the autonomic nervous system, central neurotransmitter and neuropeptide system, and the hypothalamus-pituitary-adrenal axis, which have direct effects on neural circuits in the brain involved with data processing (Sandi, 2013[ 95 ]). Activation of stress results in the production and release of glucocorticosteroids. Because of the lipophilic properties of glucocorticosteroids, they can diffuse through the blood-brain barrier and exert long-term effects on processing and cognition (Sandi, 2013[ 95 ]).
It appears that being exposed to stress can cause pathophysiologic changes in the brain, and these changes can be manifested as behavioral, cognitive, and mood disorders (Li et al., 2008[ 60 ]). In fact, studies have shown that chronic stress can cause complications such as increased IL-6 and plasma cortisol, but decreased amounts of cAMP responsive element binding protein and brain-derived neurotrophic factor (BDNF), which is very similar to what is observed in people with depression and mood disorders that exhibit a wide range of cognitive problems (Song et al., 2006[ 114 ]). Additionally, the increased concentrations of inflammatory factors, like interleukins and TNF-α (which play an important role in creating cognitive disorders), proves a physiologic relationship between stress and mood-based cognitive disorders (Solerte et al., 2000[ 113 ]; Marsland et al., 2006[ 68 ]; Li et al., 2008[ 60 ]). Studies on animals suggest that cognitive disorders resulting from stress are created due to neuroendocrine and neuroamine factors and neurodegenerative processes (Li et al., 2008[ 60 ]). However, it should be noted that depression may not always be due to the over activation of the physiological-based stress response (Osanloo et al., 2016[ 81 ]).
Cognitive disorders following exposure to stress have been reported in past studies (Lupien and McEwen, 1997[ 64 ]). Stress has effects on cognition both acutely (through catecholamines) and chronically (through glucocorticosteroids) (McEwen and Sapolsky, 1995[ 73 ]). Acute effects are mainly caused by beta-adrenergic effects, while chronic effects are induced in a long-term manner by changes in gene expression mediated by steroids (McEwen and Sapolsky, 1995[ 73 ]). In general, many mechanisms modulate the effects of stress on cognition (McEwen and Sapolsky, 1995[ 73 ]; Mendl, 1999[ 75 ]). For instance, adrenal steroids affect the function of the hippocampus during cognition and memory retrieval in a biphasic manner (McEwen and Sapolsky, 1995[ 73 ]). In chronic stress, these steroids can destroy neurons with other stimulatory neurotransmitters (Sandi, 2013[ 95 ]). Exposure to stress can also cause disorders in hippocampus-related cognition; specifically, spatial memory (Borcel et al., 2008[ 9 ]; Sandi et al., 2003[ 96 ]). Additionally, stress can halt or decrease the genesis of neurons in the dentate gyrus area of the hippocampus (this area is one of the limited brain areas in which neurogenesis occurs in adults) (Gould and Tanapat, 1999[ 34 ]; Köhler et al., 2010[ 54 ]). Although age is a factor known to affect cognition, studies on animals have demonstrated that young rats exposed to high doses of adrenal steroids show the same level of decline in their cognition as older adult animals with normal plasma concentrations of glucocorticoids (Landfield et al., 1978[ 57 ]). Also, a decrease in the secretion of glucocorticosteroids causes preservation of spatial memory in adults and has also been shown to have neuroprotective effects (Montaron et al., 2006[ 78 ]). Other studies have shown that stress (or the injection of adrenal steroids) results in varied effects on cognition. For instance, injection of hydrocortisone at the time of its maximum plasma concentration (in the afternoon) leads to a decrease in reaction time and improves cognition and memory (Lupien et al., 2002[ 66 ]).
In summary, the adverse effects of stress on cognition are diverse and depend on the type, timing, intensity, and duration (Sandi, 2013[ 95 ]). Generally, it is believed that mild stress facilitates an improvement in cognitive function, especially in the case of virtual or verbal memory. However, if the intensity of stress passes beyond a predetermined threshold (which is different in each individual), it causes cognitive disorders, especially in memory and judgment. The disruption to memory and judgment is due to the effects of stress on the hippocampus and prefrontal cortex (Sandi, 2013[ 95 ]). Of course, it must be realized that factors like age and gender may also play a role in some cognitive disorders (Sandi, 2013[ 95 ]). Importantly, it should be emphasized that different people may exhibit varied responses in cognition when exposed to the very same stressful stimulus (Hatef et al., 2015[ 39 ]).
Stress and Immune System Functions
The relationship between stress and the immune system has been considered for decades (Khansari et al., 1990[ 50 ]; Dantzer and Kelley, 1989[ 21 ]). The prevailing attitude between the association of stress and immune system response has been that people under stress are more likely to have an impaired immune system and, as a result, suffer from more frequent illness (Khansari et al., 1990[ 50 ]). Also, old anecdotes describing resistance of some people to severe disease using the power of the mind and their thought processes, has promoted this attitude (Khansari et al., 1990[ 50 ]). In about 200 AC, Aelius Galenus (Galen of Pergamon) declared that melancholic women (who have high levels of stress and, thus, impaired immune function) are more likely to have cancer than women who were more positive and exposed to less stress (Reiche et al., 2004[ 88 ]). This may be the first recorded case about the relationship between the immune system and stress. In an old study in the early 1920's, researchers found that the activity of phagocytes in tuberculosis decreased when emotional stress was induced. In fact, it was also suggested that living with stress increases the risk of tuberculosis by suppressing the immune system (Ishigami, 1919[ 44 ]). Following this study, other researchers suggested that the probability of disease appearance increases following a sudden, major, and extremely stressful life style change (Holmes and Rahe, 1967[ 41 ]; Calabrese et al., 1987[ 12 ]).
Over the past several decades, there have been many studies investigating the role of stress on immune system function (Dantzer and Kelley, 1989[ 21 ]; Segerstrom and Miller, 2004[ 109 ]). These studies have shown that stress mediators can pass through the blood-brain barrier and exert their effects on the immune system (Khansari et al., 1990[ 50 ]). Thus, the effect of stress on the immune system is now an accepted relationship or association.
Stress can affect the function of the immune system by modulating processes in the CNS and neuroendocrine system (Khansari et al., 1990[ 50 ]; Kiecolt-Glaser and Glaser, 1991[ 51 ]). Following stress, some neuroendocrine and neural responses result in the release of corticotropin-releasing hormone (CRH), adrenocorticotropic hormone (ACTH), and other stress mediators (Carrasco and Van de Kar, 2003[ 13 ]). However, evidence suggests that the lymphatic system, which is a part of the immune system, also plays a role in releasing these mediators (Khansari et al., 1990[ 50 ]). For instance, thymus peptides, such as thymopentine, thymopoietin, and thymosin fraction-5, cause an increase in ACTH production (Goya et al., 1993[ 36 ]). Additionally, the existence of CRH in thymus has been proven (Redei, 1992[ 87 ]). It has also been proven that interleukin-1 released from phagocytes has a role in ACTH secretion (Berkenbosch et al., 1987[ 4 ]). On the other hand, natural or synthetic glucocorticosteroids (which are the final stress operators) are known as anti-inflammatory drugs and immune suppressants and their role in the inhibition of lymphocytes and macrophages has been demonstrated as well (Elenkov et al., 1999[ 28 ]; Reiche et al., 2004[ 88 ]). Moreover, their role in inhibiting the production of cytokines and other immune mediators and decreasing their effect on target cells during exposure to stress has also been determined (Reiche et al., 2004[ 88 ]).
In addition to adrenal steroids, other hormones are affected during stress. For example, the secretion of growth hormone will be halted during severe stress. A study showed that long-term administration of CRH into the brain ventricles leads to a cessation in the release of growth hormone (Rivier and Vale, 1985[ 90 ]). Stress also causes the release of opioid peptides to be changed during the time period over which the person is exposed to stress (McCarthy et al., 2001[ 70 ]). In fact, stress modifies the secretion of hormones that play a critical role in the function of the immune system (Khansari et al., 1990[ 50 ]). To date, it has been shown that various receptors for a variety of hormones involved in immune system function are adversely affected by stress. For example, ACTH, vasoactive intestinal peptide (VIP), substance P, growth hormone, prolactin, and steroids all have receptors in various tissues of the immune system and can modulate its function (De la Fuente et al., 1996[ 24 ]; Gala, 1991[ 30 ]; Mantyh, 1991[ 67 ]). In addition, active immune cells are also able to secrete several hormones; thus, some researchers believe that these hormones, as mediators of immune system, play a significant role in balancing its function (Blalock et al., 1985[ 6 ]).
Severe stress can lead to malignancy by suppressing the immune system (Reiche et al., 2004[ 88 ]). In fact, stress can decrease the activity of cytotoxic T lymphocytes and natural killer cells and lead to growth of malignant cells, genetic instability, and tumor expansion (Reiche et al., 2004[ 88 ]). Studies have shown that the plasma concentration of norepinephrine, which increases after the induction stress, has an inverse relationship with the immune function of phagocytes and lymphocytes (Reiche et al., 2004[ 88 ]). Lastly, catecholamines and opioids that are released following stress have immune-suppressing properties (Reiche et al., 2004[ 88 ]).
Stress and the Function of the Cardiovascular System
The existence of a positive association between stress and cardiovascular disease has been verified (Rozanski et al., 1999[ 93 ]). Stress, whether acute or chronic, has a deleterious effect on the function of the cardiovascular system (Rozanski et al., 1999[ 93 ]; Kario et al., 2003[ 48 ]; Herd, 1991[ 40 ]). The effects of stress on the cardiovascular system are not only stimulatory, but also inhibitory in nature (Engler and Engler, 1995[ 29 ]). It can be postulated that stress causes autonomic nervous system activation and indirectly affects the function of the cardiovascular system (Lazarus et al., 1963[ 59 ]; Vrijkotte et al., 2000[ 120 ]). If these effects occur upon activation of the sympathetic nervous system, then it mainly results in an increase in heart rate, strength of contraction, vasodilation in the arteries of skeletal muscles, a narrowing of the veins, contraction of the arteries in the spleen and kidneys, and decreased sodium excretion by the kidneys (Herd, 1991[ 40 ]). Sometimes, stress activates the parasympathetic nervous system (Pagani et al., 1991[ 82 ]). Specifically, if it leads to stimulation of the limbic system, it results in a decrease, or even a total stopping of the heart-beat, decreased contractility, reduction in the guidance of impulses by the heart stimulus-transmission network, peripheral vasodilatation, and a decline in blood pressure (Cohen et al., 2000[ 17 ]). Finally, stress can modulate vascular endothelial cell function and increase the risk of thrombosis and ischemia, as well as increase platelet aggregation (Rozanski et al., 1999[ 93 ]).
The initial effect of stress on heart function is usually on the heart rate (Vrijkotte et al., 2000[ 120 ]). Depending upon the direction of the shift in the sympatho-vagal response, the heart beat will either increase or decrease (Hall et al., 2004[ 38 ]). The next significant effect of stress on cardiovascular function is blood pressure (Laitinen et al., 1999[ 56 ]). Stress can stimulate the autonomic sympathetic nervous system to increase vasoconstriction, which can mediate an increase in blood pressure, an increase in blood lipids, disorders in blood clotting, vascular changes, atherogenesis; all, of which, can cause cardiac arrhythmias and subsequent myocardial infarction (Rozanski et al., 1999[ 93 ]; Vrijkotte et al., 2000[ 120 ]; Sgoifo et al., 1998[ 111 ]). These effects from stress are observed clinically with atherosclerosis and leads to an increase in coronary vasoconstriction (Rozanski et al., 1999[ 93 ]). Of course, there are individual differences in terms of the level of autonomic-based responses due to stress, which depends on the personal characteristics of a given individual (Rozanski et al., 1999[ 93 ]). Thus, training programs for stress management are aimed at reducing the consequences of stress and death resulting from heart disease (Engler and Engler, 1995[ 29 ]). In addition, there are gender-dependent differences in the cardiovascular response to stress and, accordingly, it has been estimated that women begin to exhibit heart disease ten years later that men, which has been attributed to the protective effects of the estrogen hormone (Rozanski et al., 1999[ 93 ]).
Studies have shown that psychological stress can cause alpha-adrenergic stimulation and, consequently, increase heart rate and oxygen demand (Rozanski et al., 1998[ 92 ], 1999[ 93 ]; Jiang et al., 1996[ 46 ]). As a result, coronary vasoconstriction is enhanced, which may increase the risk of myocardial infarction (Yeung et al., 1991[ 124 ]; Boltwood et al., 1993[ 8 ]; Dakak et al., 1995[ 20 ]). Several studies have demonstrated that psychological stress decreases the microcirculation in the coronary arteries by an endothelium-dependent mechanism and increases the risk of myocardial infarction (Dakak et al., 1995[ 20 ]). On the other hand, mental stress indirectly leads to potential engagement in risky behaviors for the heart, such as smoking, and directly leads to stimulation of the neuroendocrine system as part of the autonomic nervous system (Hornstein, 2004[ 43 ]). It has been suggested that severe mental stress can result in sudden death (Pignalberi et al., 2002[ 84 ]). Generally, stress-mediated risky behaviors that impact cardiovascular health can be summarized into five categories: an increase in the stimulation of the sympathetic nervous system, initiation and progression of myocardial ischemia, development of cardiac arrhythmias, stimulation of platelet aggregation, and endothelial dysfunction (Wu, 2001[ 123 ]).
Stress and Gastrointestinal Complications
The effects of stress on nutrition and the gastrointestinal (GI) system can be summarized with two aspects of GI function.
First, stress can affect appetite (Bagheri Nikoo et al., 2014[ 2 ]; Halataei et al., 2011[ 37 ]; Ranjbaran et al., 2013[ 86 ]). This effect is related to involvement of either the ventral tegmental area (VTA), or the amygdala via N-methyl-D-aspartate (NMDA) glutamate receptors (Nasihatkon et al., 2014[ 80 ]; Sadeghi et al., 2015[ 94 ]). However, it should also be noted that nutrition patterns have effects on the response to stress (Ghanbari et al., 2015[ 31 ]), and this suggests a bilateral interaction between nutrition and stress.
Second, stress adversely affects the normal function of GI tract. There are many studies concerning the effect of stress on the function of the GI system (Söderholm and Perdue, 2001[ 112 ]; Collins, 2001[ 18 ]). For instance, studies have shown that stress affects the absorption process, intestinal permeability, mucus and stomach acid secretion, function of ion channels, and GI inflammation (Collins, 2001[ 18 ]; Nabavizadeh et al., 2011[ 79 ]). Stress also increases the response of the GI system to inflammation and may reactivate previous inflammation and accelerate the inflammation process by secretion of mediators such as substance P (Collins, 2001[ 18 ]). As a result, there is an increase in the permeability of cells and recruitment of T lymphocytes. Lymphocyte aggregation leads to the production of inflammatory markers, activates key pathways in the hypothalamus, and results in negative feedback due to CRH secretion, which ultimately results in the appearance of GI inflammatory diseases (Collins, 2001[ 18 ]). This process can reactivate previous silent colitis (Million et al., 1999[ 76 ]; Qiu et al., 1999[ 85 ]). Mast cells play a crucial role in stress-induced effects on the GI system, because they cause neurotransmitters and other chemical factors to be released that affect the function of the GI system (Konturek et al., 2011[ 55 ]).
Stress can also alter the functional physiology of the intestine (Kiliaan et al., 1998[ 52 ]). Many inflammatory diseases, such as Crohn's disease and other ulcerative-based diseases of the GI tract, are associated with stress (Hommes et al., 2002[ 42 ]). It has been suggested that even childhood stress can lead to these diseases in adulthood (Schwartz and Schwartz, 1983[ 106 ]). Irritable bowel syndrome, which is a disease with an inflammatory origin, is highly related to stress (Gonsalkorale et al., 2003[ 33 ]). Studies on various animals suggest the existence of inflammatory GI diseases following induction of severe stress (Qiu et al., 1999[ 85 ]; Collins et al., 1996[ 19 ]). Additionally, pharmacological interventions, in an attempt to decrease the response of CRH to stress, have been shown to result in an increase in GI diseases in rats (Million et al., 1999[ 76 ]).
Altering the permeability of the mucosal membrane by perturbing the functions of mucosal mast cells may be another way that stress causes its effects on the GI system, since this is a normal process by which harmful and toxic substances are removed from the intestinal lumen (Söderholm and Perdue, 2001[ 112 ]). Also, stress can both decrease the removal of water from the lumen, as well as induce sodium and chloride secretion into the lumen. This most likely occurs by increasing the activity of the parasympathetic nervous system (Barclay and Turnberg, 1987[ 3 ]). Moreover, physical stress, such as trauma or surgery, can increase luminal permeability (Söderholm and Perdue, 2001[ 112 ]) (Table 2 (Tab. 2) ; References in Table 2: Halataei et al., 2011[ 37 ]; Ranjbaran et al., 2013[ 86 ]; Mönnikes et al., 2001[ 77 ]; Collins, 2001[ 18 ]; Nabavizadeh et al., 2011[ 79 ]; Barclay and Turnberg, 1987[ 3 ]; Million et al., 1999[ 76 ]; Gonsalkorale et al., 2003[ 33 ]).

Stress also affects movement of the GI tract. In this way, it prevents stomach emptying and accelerates colonic motility (Mönnikes et al., 2001[ 77 ]). In the case of irritable bowel syndrome, stress increases the movement (contractility and motility) of the large intestine (Mönnikes et al., 2001[ 77 ]). Previous studies have revealed that CRH increases movement in the terminal sections of the GI tract and decreases the movements in the proximal sections of the GI tract (Mönnikes et al., 2001[ 77 ]). A delay in stomach emptying is likely accomplished through CRH-2 receptors, while type 1 receptors affect the colon (Mönnikes et al., 2001[ 77 ]). The effects produced by CRH are so prominent that CRH is now considered an ideal candidate for the treatment of irritable bowel syndrome (Martinez and Taché, 2006[ 69 ]). When serotonin is released in response to stress (Chaouloff, 2000[ 14 ]), it leads to an increase in the motility of the colon by stimulating 5HT-3 receptors (Mönnikes et al., 2001[ 77 ]). Moreover, it has also been suggested that stress, especially mental and emotional types of stress, increase visceral sensitivity and activate mucosal mast cells (Mönnikes et al., 2001[ 77 ]). Stimulation of the CNS by stress has a direct effect on GI-specific nervous system ( i.e. , the myenteric system or plexus) and causes the above mentioned changes in the movements of the GI tract (Bhatia and Tandon, 2005[ 5 ]). In fact, stress has a direct effect on the brain-bowel axis (Konturek et al., 2011[ 55 ]). Various clinical studies have suggested a direct effect of stress on irritable bowel syndrome, intestinal inflammation, and peptic ulcers (Konturek et al., 2011[ 55 ]).
In conclusion, the effects of stress on the GI system can be classified into six different actions: GI tract movement disorders, increased visceral irritability, altered rate and extent of various GI secretions, modified permeability of the intestinal barrier, negative effects on blood flow to the GI tract, and increased intestinal bacteria counts (Konturek et al., 2011[ 55 ]).
Stress and the Endocrine System
There is a broad and mutual relationship between stress and the endocrine system. On one hand, stress has many subtle and complex effects on the activity of the endocrine system (Sapolsky, 2002[ 97 ]; Charmandari et al., 2005[ 15 ]), while on the other hand, the endocrine system has many effects on the response to stress (Ulrich-Lai and Herman, 2009[ 117 ]; Selye, 1956[ 110 ]). Stress can either activate, or change the activity of, many endocrine processes associated with the hypothalamus, pituitary and adrenal glands, the adrenergic system, gonads, thyroid, and the pancreas (Tilbrook et al., 2000[ 116 ]; Brown-Grant et al., 1954[ 11 ]; Thierry et al., 1968[ 115 ]; Lupien and McEwen, 1997[ 64 ]). In fact, it has been suggested that it is impossible to separate the response to stress from the functions of the endocrine system. This premise has been advanced due to the fact that even a minimal amount of stress can activate the hypothalamic-pituitary-adrenal axis, which itself is intricately involved with the activation of several different hormone secreting systems (Sapolsky, 2002[ 97 ]). In different locations throughout this article, we have already discussed the effects of stress on hormones and various endocrine factors and, thus, they will not be further addressed.
Altogether, stress may induce both beneficial and harmful effects. The beneficial effects of stress involve preserving homeostasis of cells/species, which leads to continued survival. However, in many cases, the harmful effects of stress may receive more attention or recognition by an individual due to their role in various pathological conditions and diseases. As has been discussed in this review, various factors, for example, hormones, neuroendocrine mediators, peptides, and neurotransmitters are involved in the body's response to stress. Many disorders originate from stress, especially if the stress is severe and prolonged. The medical community needs to have a greater appreciation for the significant role that stress may play in various diseases and then treat the patient accordingly using both pharmacological (medications and/or nutraceuticals) and non-pharmacological (change in lifestyle, daily exercise, healthy nutrition, and stress reduction programs) therapeutic interventions. Important for the physician providing treatment for stress is the fact that all individuals vary in their response to stress, so a particular treatment strategy or intervention appropriate for one patient may not be suitable or optimal for a different patient.
Yunes Panahi and Amirhossein Sahebkar (Department of Medical Biotechnology, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran, P.O. Box: 91779-48564, Iran; Tel: 985118002288, Fax: 985118002287, E-mail: [email protected], [email protected]) contributed equally as corresponding authors.
Conflict of interest
The authors declare that have no conflict of interest in this study.
Acknowledgement
The authors would like to thank the "Neurosciences Research Center of Baqiyatallah University of Medical Sciences" and the “Clinical Research Development Center of Baqiyatallah (a.s.) Hospital” for providing technical supports.
Microbiota-Gut-Brain Axis in Psychiatry: Focus on Depressive Disorders
- Published: 05 June 2024
Cite this article

- I-Ching Wang 1 ,
- Shelly A. Buffington 1 , 2 &
- Ramiro Salas 1 , 3 , 4 , 5
Purpose of Review
Gut microbiota contribute to several physiological processes in the host. The composition of the gut microbiome is associated with different neurological and neurodevelopmental diseases. In psychiatric disease, stress may be a major factor leading to gut microbiota alterations. Depressive disorders are the most prevalent mental health issues worldwide and patients often report gastrointestinal symptoms. Accordingly, evidence of gut microbial alterations in depressive disorders has been growing. Here we review current literature revealing links between the gut microbiome and brain function in the context of depression.
Recent Findings
The gut-brain axis could impact the behavioral manifestation of depression and the underlying neuropathology via multiple routes: the HPA axis, immune function, the enteric nervous system, and the vagus nerve. Furthermore, we explore possible therapeutic interventions including fecal microbiota transplant or probiotic supplementation in alleviating depressive symptoms.
Understanding the mechanisms by which bidirectional communication along the gut-brain axis can be dysregulated in patients with depression could lead to the development of personalized, microbiome-targeted therapies for the treatment of this disorder.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Data Availability
No datasets were generated or analysed during the current study.
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823–36.
Article CAS PubMed Google Scholar
Ganal-Vonarburg SC, Hornef MW, Macpherson AJ. Microbial-host molecular exchange and its functional consequences in early mammalian life. Science (New York, NY). 2020;368(6491):604–7.
Article CAS Google Scholar
Kundu P, Blacher E, Elinav E, Pettersson S. Our gut microbiome: the evolving inner self. Cell. 2017;171(7):1481–93.
Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693(Pt B):128–33.
Article CAS PubMed PubMed Central Google Scholar
Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–84.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63.
Zinocker MK, Lindseth IA. The Western diet-microbiome-host interaction and its role in metabolic disease. Nutrients. 2018;10(3).
Perler BK, Friedman ES, Wu GD. The role of the gut microbiota in the relationship between diet and human health. Annu Rev Physiol. 2023;85:449–68.
Leclercq S, Mian FM, Stanisz AM, Bindels LB, Cambier E, Ben-Amram H, et al. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat Commun. 2017;8:15062.
Desbonnet L, Clarke G, Traplin A, O’Sullivan O, Crispie F, Moloney RD, et al. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav Immun. 2015;48:165–73.
Lopera-Maya EA, Kurilshikov A, van der Graaf A, Hu S, Andreu-Sanchez S, Chen L, et al. Effect of host genetics on the gut microbiome in 7,738 participants of the Dutch Microbiome Project. Nat Genet. 2022;54(2):143–51.
Qin Y, Havulinna AS, Liu Y, Jousilahti P, Ritchie SC, Tokolyi A, et al. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat Genet. 2022;54(2):134–42.
Vuong HE, Yano JM, Fung TC, Hsiao EY. The microbiome and host behavior. Annu Rev Neurosci. 2017;40(1):21–49.
Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013.
Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell. 2016;165(7):1762–75.
Sgritta M, Dooling SW, Buffington SA, Momin EN, Francis MB, Britton RA, et al. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron. 2019;101(2):246-59.e6.
Buffington SA, Dooling SW, Sgritta M, Noecker C, Murillo OD, Felice DF, et al. Dissecting the contribution of host genetics and the microbiome in complex behaviors. Cell. 2021;184(7):1740-56 e16.
Curtis K, Stewart CJ, Robinson M, Molfese DL, Gosnell SN, Kosten TR, et al. Insular resting state functional connectivity is associated with gut microbiota diversity. Eur J Neurosci. 2019;50(3):2446–52.
Article PubMed PubMed Central Google Scholar
Lakosa A, Rahimian A, Tomasi F, Marti F, Reynolds LM, Tochon L, et al. Impact of the gut microbiome on nicotine’s motivational effects and glial cells in the ventral tegmental area in male mice. Neuropsychopharmacology. 2023;48(6):963–74.
Nikolova VL, Smith MRB, Hall LJ, Cleare AJ, Stone JM, Young AH. Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psychiat. 2021;78(12):1343–54.
Article Google Scholar
Sanada K, Nakajima S, Kurokawa S, Barcelo-Soler A, Ikuse D, Hirata A, et al. Gut microbiota and major depressive disorder: a systematic review and meta-analysis. J Affect Disord. 2020;266:1–13.
Moreno-Agostino D, Wu YT, Daskalopoulou C, Hasan MT, Huisman M, Prina M. Global trends in the prevalence and incidence of depression:a systematic review and meta-analysis. J Affect Disord. 2021;281:235–43.
Article PubMed Google Scholar
WHO. Depression and other common mental disorders: global health estimates. 2017. Retrieved from https://policycommons.net/artifacts/546082/depression-and-other-common-mental-disorders/
Santomauro DF, Mantilla Herrera AM, Shadid J, Zheng P, Ashbaugh C, Pigott DM, et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398(10312):1700–12.
Mazza MG, Palladini M, Villa G, Agnoletto E, Harrington Y, Vai B, et al. Prevalence of depression in SARS-CoV-2 infected patients: an umbrella review of meta-analyses. Gen Hosp Psychiatry. 2023;80:17–25.
Blackett JW, Sun Y, Purpura L, Margolis KG, Elkind MSV, O’Byrne S, et al. Decreased gut microbiome tryptophan metabolism and serotonergic signaling in patients with persistent mental health and gastrointestinal symptoms after COVID-19. Clin Transl Gastroenterol. 2022;13(10):e00524.
Ramakrishnan RK, Kashour T, Hamid Q, Halwani R, Tleyjeh IM. Unraveling the mystery surrounding post-acute sequelae of COVID-19. Front Immunol. 2021;12:686029.
Marwaha S, Palmer E, Suppes T, Cons E, Young AH, Upthegrove R. Novel and emerging treatments for major depression. Lancet. 2023;401(10371):141–53.
Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Focus (Am Psychiatr Publ). 2018;16(4):420–9.
PubMed PubMed Central Google Scholar
Aguado A, García Del Álamo M. Gastrointestinal comorbidity and symptoms associated with depression in patients aged over 60 years. Semergen. 2020;46(1):27–32.
Huang J, Cai Y, Su Y, Zhang M, Shi Y, Zhu N, et al. Gastrointestinal symptoms during depressive episodes in 3256 patients with major depressive disorders: findings from the NSSD. J Affect Disord. 2021;286:27–32.
Haj Kheder S, Heller J, Bär JK, Wutzler A, Menge BA, Juckel G. Autonomic dysfunction of gastric motility in major depression. J Affect Disord. 2018;226:196–202.
Zhang B, Wang HE, Bai YM, Tsai SJ, Su TP, Chen TJ, et al. Bidirectional association between inflammatory bowel disease and depression among patients and their unaffected siblings. J Gastroenterol Hepatol. 2022;37(7):1307–15.
Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. 2016;21(6):786–96.
Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–94.
Madan A, Thompson D, Fowler JC, Ajami NJ, Salas R, Frueh BC, et al. The gut microbiota is associated with psychiatric symptom severity and treatment outcome among individuals with serious mental illness. J Affect Disord. 2020;264:98–106.
Radjabzadeh D, Bosch JA, Uitterlinden AG, Zwinderman AH, Ikram MA, van Meurs JBJ, et al. Gut microbiome-wide association study of depressive symptoms. Nat Commun. 2022;13(1):7128.
Kelly JR, Borre Y, O’ Brien C, Patterson E, El Aidy S, Deane J, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;2016(82):109–18.
Jianguo L, Xueyang J, Cui W, Changxin W, Xuemei Q. Altered gut metabolome contributes to depression-like behaviors in rats exposed to chronic unpredictable mild stress. Transl Psychiatry. 2019;9(1):40-.
Bellavance MA, Rivest S. The HPA - immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front Immunol. 2014;5:136.
Malhi GS, Mann JJ. Depression. Lancet. 2018;392(10161):2299–312.
Juruena MF. Early-life stress and HPA axis trigger recurrent adulthood depression. Epilepsy Behav. 2014;38:148–59.
Juruena MF, Bocharova M, Agustini B, Young AH. Atypical depression and non-atypical depression: is HPA axis function a biomarker? A systematic review. J Affect Disord. 2018;233:45–67.
Wu WL, Adame MD, Liou CW, Barlow JT, Lai TT, Sharon G, et al. Microbiota regulate social behaviour via stress response neurons in the brain. Nature. 2021;595(7867):409–14.
Luo Y, Zeng B, Zeng L, Du X, Li B, Huo R, et al. Gut microbiota regulates mouse behaviors through glucocorticoid receptor pathway genes in the hippocampus. Transl Psychiatry. 2018;8(1):187.
O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho A-M, Quigley EMM, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiat. 2009;65(3):263–7.
Moya-Perez A, Perez-Villalba A, Benitez-Paez A, Campillo I, Sanz Y. Bifidobacterium CECT 7765 modulates early stress-induced immune, neuroendocrine and behavioral alterations in mice. Brain Behav Immun. 2017;65:43–56.
Tian P, O’Riordan KJ, Lee YK, Wang G, Zhao J, Zhang H, et al. Towards a psychobiotic therapy for depression: bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol Stress. 2020;12:100216.
Jacobson A, Yang D, Vella M, Chiu IM. The intestinal neuro-immune axis: crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 2021;14(3):555–65.
Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22–34.
Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20(2):145–55.
Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25(3):397–407.
Wei L, Li Y, Tang W, Sun Q, Chen L, Wang X, et al. Chronic unpredictable mild stress in rats induces colonic inflammation. Front Physiol. 2019;10:1228.
Wong ML, Inserra A, Lewis MD, Mastronardi CA, Leong L, Choo J, et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry. 2016;21(6):797–805.
Pearson-Leary J, Zhao C, Bittinger K, Eacret D, Luz S, Vigderman AS, et al. The gut microbiome regulates the increases in depressive-type behaviors and in inflammatory processes in the ventral hippocampus of stress vulnerable rats. Mol Psychiatry. 2019;1068–1079.
Browning KN, Verheijden S, Boeckxstaens GE. The vagus nerve in appetite regulation, mood, and intestinal inflammation. Gastroenterology. 2017;152(4):730–44.
de Lartigue G. Role of the vagus nerve in the development and treatment of diet-induced obesity. J Physiol. 2016;594(20):5791–815.
Bonaz B, Sinniger V, Pellissier S. Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J Physiol. 2016;594(20):5781–90.
Rong P, Liu J, Wang L, Liu R, Fang J, Zhao J, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J Affect Disord. 2016;195:172–9.
Carreno FR, Frazer A. Vagal nerve stimulation for treatment-resistant depression. Neurotherapeutics. 2017;14(3):716–27.
Fang J, Rong P, Hong Y, Fan Y, Liu J, Wang H, et al. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry. 2016;79(4):266–73.
Prechtl JC, Powley TL. The fiber composition of the abdominal vagus of the rat. Anat Embryol. 1990;181(2):101–15.
Paintal AS. Vagal sensory receptors and their reflex effects. Physiol Rev. 1973;53(1):159–227.
Breit S, Kupferberg A, Rogler G, Hasler G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front Psychiatry. 2018;9:44.
Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 2018;12(FEB):1–9.
Google Scholar
Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci. 2011;108(38):16050–5.
Zhang J, Ma L, Chang L, Pu Y, Qu Y, Hashimoto K. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl Psychiatry. 2020;10(1):186.
Pu Y, Tan Y, Qu Y, Chang L, Wang S, Wei Y, et al. A role of the subdiaphragmatic vagus nerve in depression-like phenotypes in mice after fecal microbiota transplantation from Chrna7 knock-out mice with depression-like phenotypes. Brain Behav Immun. 2021;94:318–26.
• Wang S, Ishima T, Qu Y, Shan J, Chang L, Wei Y, et al. Ingestion of Faecalibaculum rodentium causes depression-like phenotypes in resilient Ephx2 knock-out mice: a role of brain-gut-microbiota axis via the subdiaphragmatic vagus nerve. J Affect Disord. 2021;292:565–73. This study demonstrated that probiotics study showing modulation of gut microbiota can affect depessive-like phenotypes.
Wang S, Ishima T, Zhang J, Qu Y, Chang L, Pu Y, et al. Ingestion of Lactobacillus intestinalis and Lactobacillus reuteri causes depression- and anhedonia-like phenotypes in antibiotic-treated mice via the vagus nerve. J Neuroinflammation. 2020;17(1):241.
Shin HC, Jo BG, Lee C-Y, Lee K-W, Namgung U. Hippocampal activation of 5-HT1B receptors and BDNF production by vagus nerve stimulation in rats under chronic restraint stress. Eur J Neurosci. 2019;50(1):1820–30.
Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9(5):286–94.
• Margolis KG, Cryan JF, Mayer EA. The microbiota-gut-brain axis: from motility to mood. Gastroenterology. 2021;160(5):1486–501. This review elaborated on how enteric nervous system modulate the gut microbiota in mood disorders.
McVey Neufeld KA, Bienenstock J, Bharwani A, Champagne-Jorgensen K, Mao Y, West C, et al. Oral selective serotonin reuptake inhibitors activate vagus nerve dependent gut-brain signalling. Sci Rep. 2019;9(1):14290.
Lobo B, Tramullas M, Finger BC, Lomasney KW, Beltran C, Clarke G, et al. The stressed gut: region-specific immune and neuroplasticity changes in response to chronic psychosocial stress. J Neurogastroenterol Motil. 2023;29(1):72–84.
•• Schneider KM, Blank N, Alvarez Y, Thum K, Lundgren P, Litichevskiy L, et al. The enteric nervous system relays psychological stress to intestinal inflammation. Cell. 2023;186(13):2823-38.e20. This study provides evidence of how ENS communicates with CNS via the immune system and glia activation.
Collins J, Borojevic R, Verdu EF, Huizinga JD, Ratcliffe EM. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol Motil. 2014;26(1):98–107.
Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28(2):203–9.
Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–45.
Wu M, Tian T, Mao Q, Zou T, Zhou C-j, Xie J, et al. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl Psychiatry. 2020;10(1):350.
Liu L, Wang H, Chen X, Zhang Y, Zhang H, Xie P. Gut microbiota and its metabolites in depression: from pathogenesis to treatment. EBioMedicine. 2023;90:104527.
Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, et al. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138(5):1772–82.
O’Riordan KJ, Collins MK, Moloney GM, Knox EG, Aburto MR, Fulling C, et al. Short chain fatty acids: microbial metabolites for gut-brain axis signalling. Mol Cell Endocrinol. 2022;546:111572.
van de Wouw M, Boehme M, Lyte JM, Wiley N, Strain C, O’Sullivan O, et al. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J Physiol. 2018;596(20):4923–44.
Del Colle A, Israelyan N, Gross MK. Novel aspects of enteric serotonergic signaling in health and brain-gut disease. Am J Physiol Gastrointest Liver Physiol. 2020;318(1):G130–43.
Israelyan N, Del Colle A, Li Z, Park Y, Xing A, Jacobsen JPR, et al. Effects of serotonin and slow-release 5-hydroxytryptophan on gastrointestinal motility in a mouse model of depression. Gastroenterology. 2019;157(2):507-21.e4.
Tri BD, Shashni B, Matsui H, Nagasaki Y. Designing poly(gamma-aminobutyric acid)-based nanoparticles for the treatment of major depressive disorders. J Control Release. 2023;360:110–21.
Patterson E, Ryan PM, Wiley N, Carafa I, Sherwin E, Moloney G, et al. Gamma-aminobutyric acid-producing lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome. Sci Rep. 2019;9(1):16323.
Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. 2019;102:13–23.
Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170(4):1179–88.
Marin IA, Goertz JE, Ren T, Rich SS, Onengut-Gumuscu S, Farber E, et al. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci Rep. 2017;7:43859.
Schaub AC, Schneider E, Vazquez-Castellanos JF, Schweinfurth N, Kettelhack C, Doll JPK, et al. Clinical, gut microbial and neural effects of a probiotic add-on therapy in depressed patients: a randomized controlled trial. Transl Psychiatry. 2022;12(1):227.
Cai T, Shi X, Yuan LZ, Tang D, Wang F. Fecal microbiota transplantation in an elderly patient with mental depression. Int Psychogeriatr. 2019;31(10):1525–6.
Doll JPK, Vazquez-Castellanos JF, Schaub AC, Schweinfurth N, Kettelhack C, Schneider E, et al. Fecal Microbiota Transplantation (FMT) as an adjunctive therapy for depression-case report. Front Psychiatry. 2022;13:815422.
Sharon G, Cruz NJ, Kang D-W, Gandal MJ, Wang B, Kim Y-M, et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. 2019;177(6):1600-18.e17.
Hu B, Das P, Lv X, Shi M, Aa J, Wang K, et al. Effects of “healthy” fecal microbiota transplantation against the deterioration of depression in fawn-hooded rats. mSystems. 2022;7(3):e0021822.
Rao J, Xie R, Lin L, Jiang J, Du L, Zeng X, et al. Fecal microbiota transplantation ameliorates gut microbiota imbalance and intestinal barrier damage in rats with stress-induced depressive-like behavior. Eur J Neurosci. 2021;53(11):3598–611.
Settanni CR, Ianiro G, Bibbò S, Cammarota G, Gasbarrini A. Gut microbiota alteration and modulation in psychiatric disorders: current evidence on fecal microbiota transplantation. Prog Neuropsychopharmacol Biol Psychiatry. 2021;109:110258.
Martinez-Guryn K, Leone V, Chang EB. Regional diversity of the gastrointestinal microbiome. Cell Host Microbe. 2019;26(3):314–24.
Hollister EB, Gao C, Versalovic J. Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology. 2014;146(6):1449–58.
Gu S, Chen D, Zhang JN, Lv X, Wang K, Duan LP, et al. Bacterial community mapping of the mouse gastrointestinal tract. PLoS ONE. 2013;8(10):e74957.
Rehan M, Al-Bahadly I, Thomas DG, Young W, Cheng LK, Avci E. Smart capsules for sensing and sampling the gut: status, challenges and prospects. Gut. 2023;73(1):186–202.
Dinan K, Dinan T. Antibiotics and mental health: the good, the bad and the ugly. J Intern Med. 2022;292(6):858–69.
Hao W-Z, Li X-J, Zhang P-W, Chen J-X. A review of antibiotics, depression, and the gut microbiome. Psychiatry Res. 2020;284:112691.
Nettis MA, Lombardo G, Hastings C, Zajkowska Z, Mariani N, Nikkheslat N, et al. Augmentation therapy with minocycline in treatment-resistant depression patients with low-grade peripheral inflammation: results from a double-blind randomised clinical trial. Neuropsychopharmacology. 2021;46(5):939–48.
Download references
This study was supported the Veterans Health Administration (grant VHA I01CX001937 to RS) and The Robert & Janice McNair Foundation, NIH NICHD R01 HD109095, NIH NICHD R01 HD109780, and The Brain & Behavior Research Foundation, NARSAD Young Investigator Award 28918 to SAB. This study is in part the result of work supported with resources and the use of facilities at the Michael E. DeBakey Veterans Affairs Medical Center.
Author information
Authors and affiliations.
Department of Neuroscience, Baylor College of Medicine, Houston, TX, USA
I-Ching Wang, Shelly A. Buffington & Ramiro Salas
Center for Precision Environmental Health, Baylor College of Medicine, Houston, TX, USA
Shelly A. Buffington
Psychiatry & Behavioral Sciences, Baylor College of Medicine, Houston, TX, USA
Ramiro Salas
Center for Translational Research On Inflammatory Diseases, Michael E DeBakey VA Medical, Houston, TX, USA
The Menninger Clinic, Houston, TX, USA
You can also search for this author in PubMed Google Scholar
Contributions
I-C W, SAB, RS wrote the review.
Corresponding author
Correspondence to Ramiro Salas .
Ethics declarations
Conflict of interests.
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
The authors do not report new studies/experiments with human or animal subjects.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Genetic Epidemiology
Rights and permissions
Reprints and permissions
About this article
Wang, IC., Buffington, S.A. & Salas, R. Microbiota-Gut-Brain Axis in Psychiatry: Focus on Depressive Disorders. Curr Epidemiol Rep (2024). https://doi.org/10.1007/s40471-024-00349-z
Download citation
Accepted : 21 May 2024
Published : 05 June 2024
DOI : https://doi.org/10.1007/s40471-024-00349-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Gut microbiome
- Immune system
- Vagus nerve
- Fecal microbiota transfer
Advertisement
- Find a journal
- Publish with us
- Track your research
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Study Protocol
The impact of perinatal maternal stress on the maternal and infant gut and human milk microbiomes: A scoping review protocol
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation School of Nursing and Midwifery, University College Cork, Wilton, Cork, Ireland
Roles Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Validation, Visualization, Writing – review & editing
Roles Conceptualization, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing
Affiliation Department of Anatomy and Neuroscience, APC Microbiome Ireland, University College Cork, Ireland
- Niamh Ryan,
- Patricia Leahy-Warren,
- Helen Mulcahy,
- Siobhain O’Mahony,
- Lloyd Philpott

- Published: June 5, 2024
- https://doi.org/10.1371/journal.pone.0304787
- Peer Review
- Reader Comments
The objective of this scoping review is to review the research evidence regarding the impact of perinatal maternal stress on the maternal and infant gut and human milk microbiomes.
Introduction
Perinatal stress which refers to psychological stress experienced by individuals during pregnancy and the postpartum period is emerging as a public health concern. Early exposure of infants to perinatal maternal stress can potentially lead to metabolic, immune, and neurobehavioral disorders that extend into adulthood. The role of the gut and human milk microbiome in the microbiome-gut-brain axis as a mechanism of stress transfer has been previously reported. A transfer of colonised aberrant microbiota from mother to infant is proposed to predispose the infant to a pro- inflammatory microbiome with dysregulated metabolic process thereby initiating early risk of chronic diseases. The interplay of perinatal maternal stress and its relationship to the maternal and infant gut and human milk microbiome requires further systematic examination in the literature.
Inclusion criteria
This scoping review is an exploratory mapping review which will focus on the population of mothers and infants with the exploration of the key concepts of maternal stress and its impact on the maternal and infant gut and human milk microbiome in the context of the perinatal period. It will focus on the pregnancy and the post-natal period up to 6 months with infants who are exclusively breastfed.
This study will be guided by the Joanna Briggs Institute’s (JBI) methodology for scoping reviews along with use of the Prisma Scr reporting guideline. A comprehensive search will be conducted using the following databases, CINAHL Complete; MEDLINE; PsycINFO, Web of Science and Scopus. A search strategy with pre-defined inclusion and exclusion criteria will be used to retrieve peer reviewed data published in English from 2014 to present. Screening will involve a three-step process with screening tool checklists. Results will be presented in tabular and narrative summaries, covering thematic concepts and their relationships. This protocol is registered with Open Science Framework DOI 10.17605/OSF.IO/5SRMV .
Citation: Ryan N, Leahy-Warren P, Mulcahy H, O’Mahony S, Philpott L (2024) The impact of perinatal maternal stress on the maternal and infant gut and human milk microbiomes: A scoping review protocol. PLoS ONE 19(6): e0304787. https://doi.org/10.1371/journal.pone.0304787
Editor: Wafaa Rashed, Faculty of Pharmacy - Ahram Canadian University, EGYPT
Received: April 9, 2024; Accepted: May 17, 2024; Published: June 5, 2024
Copyright: © 2024 Ryan et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All data are in the manuscript and supporting information files.
Funding: The author(s) received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Maternal mental health in the perinatal period refers to the psychological well-being of women during pregnancy and the postnatal period [ 1 ]. It includes conditions such as depression, anxiety, and stress. Perinatal maternal stress in particular is a public health concern as studies worldwide are reporting a rising prevalence of between 5% - 93% [ 2 – 5 ]. Its rise reportedly influenced by societal pressures, social isolation, economic challenges, pregnancy, and birthing complications [ 6 , 7 ].
Maternal mental health not only impacts the health of the mother, but it can also have long lasting implications for infant and child development [ 8 – 12 ]. In the past 30 years, a substantial body of evidence is building on Barker’s theory of the developmental origin of health and disease (DoHD) which demonstrated that adverse maternal conditions, such as stress during the first 1000 days of life can increase the risk of metabolic, immunologic, and neurobehavioral disorders in offspring [ 13 – 22 ]. Given that perinatal maternal stress is a prevalent and modifiable factor that affects maternal, foetal and child health it requires focused attention in research and preventative health care.
There is growing researcher interest in how stress transfers from the mother to the infant and the role that the gut microbiome plays in the process [ 23 , 24 ]. The gut microbiome, composed of trillions of microorganisms in the digestive tract, influences gut, immune and metabolic health [ 25 , 26 ]. The balance of the gut microbiome is crucial for overall well-being, with disruptions linked to various health problems such as cardiometabolic diseases, digestive disorders, neurological disorders [ 25 , 27 – 33 ]. There is now evidence that both the gut and human milk microbiome is altered by many maternal factors such as maternal health, maternal body mass index, mode of delivery, and antibiotic use [ 34 – 38 ]. This alteration or dysbiosis in the microbiome caused by aberrant microbiota is proposed to be transferred to the infant, predisposing them to a pro-inflammatory microbiome with dysregulated metabolic processes leading to adverse health conditions. Focusing on the influence of maternal stress, this review is interested in the brain gut axis (the two-way communication between the central nervous system and the gut microbiome and its impact on altering maternal and infant gut and human milk microbiomes. Limited research has focused on how maternal perinatal stress as a single variable affects the microbiomes, with no comprehensive review of existing evidence. Therefore, conducting a scoping review is timely and warranted to systematically map this research area. Seminal work by Arksey and O’Malley describes a scoping review as beneficial for examining the extent, range, and nature of research, while also identifying gaps in the evidence base [ 39 ]. This review also highlights the importance of perinatal stress for maternal and infant well-being. It emphasizes the necessity for clinician intervention and highlights research gaps. By tackling perinatal stress, immediate risks such as low birth weight and preterm delivery could be reduced, while also fostering stronger mother-child bonding [ 40 – 42 ]. Furthermore, addressing maternal stress may mitigate negative behaviours like smoking and alcohol consumption thus further improving lifestyle [ 43 , 44 ].Given the relative novelty of data in this topic, understanding the depth of evidence through mapping is crucial, as feasibility of a systematic review may be difficult due to a potential lack of research [ 45 ]. It is advised that a protocol is essential prior to a scoping review [ 46 ].
Review question
The review question was formulated using the Participant (P), Concept (C), Context (C) framework following JBI guidance [ 47 ] ( Table 1 ). The review aims to provide an overview of the evidence to address the following question: What is the impact of perinatal maternal stress on the maternal and infant gut and human milk microbiomes? With the objective to; a) Identify the methods and tools used by researchers to measure stress and the microbiome(s), b) Identity if perinatal maternal stress has an impact on bacterial taxa in the microbiome(s), c) Identify if perinatal maternal stress has an impact on bacterial diversity of the microbiome(s).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0304787.t001
The scoping review will be conducted following the guidelines for scoping reviews described by the JBI Manual for Evidence Synthesis and Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for Scoping review (PRISMA-ScR) [ 46 , 47 ].
Study eligibility criteria
The inclusion exclusion criteria for this review will be based on the population-concept-context framework. ( Fig 1 ) ( Table 1 ) recommended by the Johanna Briggs Institute (JBI) [ 47 ].
https://doi.org/10.1371/journal.pone.0304787.g001
Agreement among five researchers regarding inclusion and exclusion criteria at the start of the review process occurred, with each researcher agreeing with the final criteria. The eligibility criteria for this study are outlined in Table 2 where it is matched to population concepts and context.
https://doi.org/10.1371/journal.pone.0304787.t002
Types of sources
This review will include primary peer reviewed papers that explored maternal stress, the human milk microbiome, and maternal and infant gut microbiome. Secondary analyses, such as systematic reviews will be included if they meet the inclusion criteria. Case reports, book chapters, guidelines, commentaries, editorials, letters to editors, and narrative reviews are excluded as these sources do not directly address the review question and pose challenges in data extraction. This review will not include grey literature due to the difficulty in retrieving and extracting the data, but also in evaluating its credibility due to the absence of standardized quality indicators or peer review processes [ 53 ]. The below Table 3 identifies final eligibility criteria for types and sources of evidence to be included in the protocol and full review.
https://doi.org/10.1371/journal.pone.0304787.t003
Search strategy
The review will follow the three-step JBI process: To begin initial searches will be conducted in PubMed and CINAHL to identify relevant papers. Secondly text words, keywords, and index terms will be analysed from identified articles to develop a comprehensive search strategy for all databases (See S1 File ). Finally, reference lists of identified articles will be searched for additional studies. Keywords and search terms will be peer-reviewed using PRESS guidance with a librarian. To ensure comprehensive results, there will be multiple searches, one for each concept (See S1 Table ). All searches will use Boolean operators AND and OR in title (TI) and abstract searches (AB) with CINAHL Headings and Mesh headings used as appropriate. Information will be sourced from the following databases CINAHL Complete, Psych Info, PubMed, Web of Science and Scopus. The review will restrict analysis to English articles, however initial searches will include all languages to determine the number if any in other languages to avoid bias.
Source of evidence selection
Following the search, all included studies will be exported to Covidence, and all duplicates removed. Two reviewers (NR HM LP SOM PLW) will independently screen all papers in 2 stages: title and abstract screening, and full-text screening. Agreement from two reviewers will be required for an article to be excluded at the title-screening stage. The full text of selected papers will be assessed against the inclusion criteria by two reviewers. Reasons for exclusion of sources of evidence at full text that do not meet the inclusion criteria will be recorded and reported in the scoping review. Any conflicts in the screening stage will be resolved through the inclusion of a third reviewer. The results of the search will be presented in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram [ 54 ] ( Fig 2 ).
*Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools. From : Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71 . For more information, visit: http://www.prisma-statement.org/ .
https://doi.org/10.1371/journal.pone.0304787.g002
Data extraction
Data will be extracted from papers included in the scoping review by two reviewers using a data extraction tool developed by the reviewer NR on Covidence ( S2 File ). Data extracted will include author’s name, year, and type of publication. The aims/purpose in relation to the concepts and objectives will be extracted along with methodology and methods, sample (e.g. maternal/ infant or both). Analysis of confounding variables will be noted along with statistical tests and key findings that relate to the scoping review question with limitations/quality issues noted. The extraction table will be piloted on two papers to ensure all relevant results are extracted and to improve transparency and consistency [ 39 , 46 ].
Data analysis and presentation
The results will be presented in a narrative summary, along with tables and charts. Gaps in the evidence will be identified at this stage. Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for Scoping review (PRISMA-ScR) will be completed along with presentation of results to ensure each element is covered within the scoping review ( S3 File ).
Supporting information
S1 file. detailed search strategy..
https://doi.org/10.1371/journal.pone.0304787.s001
S2 File. Data extraction template for covidence.
https://doi.org/10.1371/journal.pone.0304787.s002
S3 File. PRISMA Sr checklist completed.
https://doi.org/10.1371/journal.pone.0304787.s003
S4 File. Completed PRISMA P checklist.
https://doi.org/10.1371/journal.pone.0304787.s004
S1 Table. Keywords in multiple searches.
https://doi.org/10.1371/journal.pone.0304787.s005
Acknowledgments
This scoping review was produced by author Niamh Ryan NR as a contribution to her Doctoral degree.
- 1. World Health Organisation (WHO). 2021. [cited 2024 Mar 12]. Maternal health: fact sheet on Sustainable Development Goals (SDGs): health targets. Available from: https://www.who.int/europe/publications/i/item/WHO-EURO-2017-2363-42118-58039 .
- View Article
- PubMed/NCBI
- Google Scholar
- Open access
- Published: 03 June 2024
Association between oxidative balance score and kidney stones: data from the national health and nutrition examination survey (NHANES)
- Rundong Song 1 ,
- Minghai Ma 1 ,
- Lu Wang 1 ,
- Yunzhong Jiang 1 ,
- Jianpeng Li 1 &
- Jinhai Fan 1
BMC Nephrology volume 25 , Article number: 190 ( 2024 ) Cite this article
Metrics details
Some studies have found that the pathological formation of kidney stones is closely related to injury and inflammatory response. Behaviors such as dietary composition, physical activity, obesity and smoking can all affect the body’s oxidative stress levels. In order to evaluate the effects of various diets and lifestyles on the body’s oxidative and antioxidant systems, an oxidative balance score was developed. To investigate whether the OBS is associated with the development of kidney stones.
Data were taken from the National Health and Nutrition Examination Survey (NHANES) from 2007–2018, followed by retrospective observational studies. The association between kidney stones and OBS was analyzed using survey-weighted logistic regression by adjusting for demographics, laboratory tests, and medical comorbidity covariates. The oxidative balance score is calculated by screening 16 nutrients and 4 lifestyle factors, including 5 prooxidants and 15 antioxidants, based on prior information about the relationship between oxidation levels in the body and nutrients or lifestyle factors.
A total of 26,786 adult participants were included in the study, of which 2,578, or 9.62%, had a history of nephrolithiasis. Weighted logistic regression analysis found an association between OBS and kidney stones. In the fully tuned model, i.e., model 3, the highest quartile array of OBS was associated with the lowest quartile array of OBS (OR = 0.73 (0.57, 0.92)) with the risk of kidney stone ( p = 0.01), and was statistically significant and remained relatively stable in each model. At the same time, the trend test in the model is also statistically significant. With the increase of OBS, the OR value of kidney stones generally tends to decrease.
Conclusions
There is an inverse correlation between OBS and kidney stone disease. At the same time, higher OBS suggests that antioxidant exposure is greater than pro-oxidative exposure in diet and lifestyle, and is associated with a lower risk of kidney stones
Peer Review reports
Introduction
Kidney stones are a common disease in urology, and its incidence has been increasing in recent years [ 1 ]. In the United States, there is a disproportionate increase in the incidence and prevalence of kidney stone disease in women and in black and Hispanic individuals [ 2 ]. The disease has a long course and is prone to recurrence, causing serious damage to kidney function and imposing a huge economic burden on patients and society [ 3 , 4 ]. Previous studies have shown that gender, race, age, lifestyle, and diet are important factors in stone formation [ 5 , 6 ]. The formation of stones is not yet fully understood, and there are various theories, including renal calcified plaque, supersaturated crystals, stone matrix, crystal-inhibiting substances, and heterogeneity-promoting nucleation theory. In most patients, the underlying etiology is thought to be multifactorial, including environmental, dietary, hormonal, and genetic components [ 7 ]. In addition, abnormalities in the body’s metabolism, obstruction of the urinary tract, infections, foreign bodies, and the use of medications are common causes of stone formation. Focusing on and addressing these issues can reduce stone formation and recurrence.
Previous studies have shown that the pathology of kidney stone formation is closely related to injury and inflammatory responses where reactive oxygen species (ROS) -induced oxidative stress is essential [ 8 , 9 ]. ROS originate predominantly in injured mitochondria, and calcium salt crystals significantly damage epithelial cell mitochondria and exacerbate the inflammatory response [ 10 ]. In physiological conditions, there is a balance between oxidants and antioxidant systems. When ROS production exceeds the scavenging capacity of the antioxidant response system, large amounts of proteins are oxidized, and lipid peroxidation occurs. Excessive production of ROS by epithelial cells promotes crystal aggregation, growth, and adhesion, ultimately leading to stone formation [ 11 ].
However, the effect of a single certain factor on the body’s oxidative and antioxidant systems is limited, and various dietary components, physical activity, obesity, and behaviors such as smoking all affect the body’s oxidative stress levels. Therefore, to assess the effects of various diets and lifestyles on the body’s oxidative and antioxidant systems, the Oxidative Balance Score (OBS) was developed to reflect the overall balance of dietary and lifestyle-promoted oxidant and antioxidant exposure [ 12 ]. In general, higher OBS indicates that antioxidants are superior to pro-oxidants. Previous studies have found that OBS is negatively associated with various diseases, including digestive, respiratory, cardiovascular, and type II diabetes. However, no studies have evaluated the relationship between kidney stones and OBS. We hypothesized that OBS might be associated with an increased risk of developing kidney stones, and to answer this question. We examined the relationship between OBS and kidney stones in a nationally representative survey controlling for various known risk factors for kidney stones. This study aimed to assess the association between OBS and kidney stones in U.S. adults using data from the National Health and Nutrition Examination Survey (NHANES).
Material and methods
Study population.
The National Center for Health Statistics (NCHS) annually surveys randomly selected, non-institutionalized U.S. civilians. Certain participant subgroups, such as the Hispanic, black, and elderly populations, are intentionally oversampled to reflect the U.S. population’s demographic composition accurately. The survey assessed the demographics, socioeconomic status, and health status of a nationally representative sample of U.S. residents. We analyzed respondents who completed relevant kidney status questionnaires over 6 NHANES cycles (2007–2018), including questions about kidney stones. Subjects who answered "refused," "missing," or "do not know" to questions assessing kidney stones were excluded. This study is a retrospective observational study.
Definition of kidney stones
Kidney stones were defined as an affirmative response to "Have you ever had kidney stones?" from a single survey question (KIQ026).
Oxidative balance score (OBS)
OBS was calculated based on a priori information on the relationship between O.S. and nutrients or lifestyle factors by screening 16 nutrients and 4 lifestyle factors, including 5 pro-oxidants and 15 antioxidants. Dietary intakes of 16 nutrients, including dietary fiber, carotenoids, riboflavin, niacin, vitamin B6, total folate, vitamin B12, vitamin C, vitamin E, calcium, magnesium, zinc, copper, selenium, total Dietary intakes of 16 nutrients, including total fat and iron, were obtained from the first dietary review interview.4 Lifestyle factors were physical activity, body mass index (BMI), alcohol consumption, and smoking, with the extent of smoking indicated by cotinine. Of these, total fat, iron, BMI, alcohol consumption, and smoking were considered pro-oxidants, and the rest were considered antioxidants. Referring to the method of calculating OBS, alcohol consumption was categorized into 3 groups, heavy drinkers (women ≥ 15 g / d, men ≥ 30 g / d), non-heavy drinkers (women 0 ∼ 15 g / d, men 0 ∼ 30 g / d), and non-drinkers, which were assigned scores of 0, 1, and 2, respectively. After that, the other components were grouped by gender and then divided into 3 groups by tertiles, where antioxidants were assigned a value of 0 ∼ 2, and pro-oxidants were assigned a value of 2 ∼ 0 in groups 1 ∼ 3 [ 12 ]. The higher the OBS scores, the more significant the antioxidant exposure. Subjects with ≥ 16 complete data for each of the 20 OBS components were selected for this study. For OBS with missing components, we assigned a score of 0 corresponding to the missing component, either antioxidant or pro-oxidant.
Assessment of covariates
In our study, covariates were certain factors previously shown or hypothesized to be associated with kidney stones or OBS, including sociodemographic variables, indicators of inflammation, diet quality, and comorbidities. Sociodemographic variables included age, sex (male, female), race (Mexican American, non-Hispanic black, non-Hispanic white, other Hispanic, other race - including multiracial), education (less than high school, high school, greater than high school), marital status (divorced/separated/widowed, married/living with a partner, never married), and the ratio of household income to poverty level (< 1.3, 1.3 ∼ 3.5, > 3.5). Inflammatory indicators reflected their status by the number of leukocytes, neutrophils, lymphocytes, and monocytes. Overall dietary quality was assessed using the 2015 version of the Healthy Eating Index (HEI) and total energy intake [ 13 ]. Comorbidities included hypertension, cardiovascular disease, diabetes mellitus, arthritis, and hyperlipidemia, with hypertension and diabetes mellitus diagnosed by index measurements, medication use, and self-report, and the remaining comorbidities identified by self-report.
Statistical analysis
All statistical analyses were performed using R version 4.3.0 (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org ), and differences were considered statistically significant at a P value < 0.05. Weighting was performed using the NHANES-recommended weight selection and calculation method. Characterization of sociodemographic variables, inflammatory indicators, diet quality, comorbidities, and oxidative balance scores for the prevalence or absence of kidney stones was performed for the weighted data. In the baseline characterization, continuous variables were expressed as weighted means (standard errors), and categorical variables were expressed as sample sizes (weighted percentages). To test for differences in variable characteristics between OBS groups (quartiles), differences in weighted means for continuous variables were analyzed using ANOVA, and differences in weighted percentages for categorical variables were analyzed using the Rao - Scott χ 2 test to characterize the total population. A weighted logistic regression model explored the association between OBS and kidney stones. To validate the correlation between OBS and kidney stones and to explore the possibility of a nonlinear relationship between OBS and kidney stones, the continuous variable OBS was transformed into a categorical variable by quartiles, and a trend P value was calculated. A total of 4 models were used in this study, and the crude model did not adjust for any potential confounders. Model 1 adjusted for age, sex, race/ethnicity, marital status, poverty income ratio, and education. Model 2 further adjusted for white blood cells, neutrophils, lymphocytes, monocyte, Healthy Eating Index, and Total energy intake. Model 3 was additionally adjusted for five comorbidities. The OBS was categorized into dietary OBS and lifestyle OBS, and their associations with kidney stones were discussed separately. Finally, restricted cubic spline (RCS) regression was used to verify the relationship between kidney stones and OBS.
Baseline characteristics
Thirty-four thousand seven hundred seventy survey respondents who responded to the kidney stone questionnaire were first identified from NHANES. Four Thousand Twenty One respondents were removed due to missing data from the questions (Fig. 1 ). When applying survey weights, 2,447 were removed due to missing individual sample weights, and 1,516 were removed due to zero individual sample weights (Fig. 1 ). Baseline demographic information is detailed in Table 1 .9.62% of the survey respondents had a history of kidney stones. The mean age (± S.E.) of the population was 48.17 ± 0.25, and those with kidney stones were somewhat older, with a significant correlation ( p < 0.0001). Of the total population, 47.64% were males and 52.36% were females, and a higher percentage of males in the population with the disease remained significantly correlated ( p < 0.0001).42.82% self-identified as non-Hispanic white, 21.71% self-identified as non-Hispanic black, 14.41% self-identified as Mexican American, 10.17% self-identified as other Hispanic, 10.17% self-identified as other Hispanic, and 10.17% self-identified as other Hispanic and identified as Other Hispanic, and 10.88% self-identified as Other, with a significant correlation ( p < 0.0001) when comparing participants with and without a history of kidney stones. For different marital statuses, there was a significant correlation with having kidney stones ( p < 0.0001). There was no significant correlation between the presence of kidney stones and other sociodemographic variables such as poverty-income ratio and educational attainment. When comparing the presence or absence of kidney stones with our indicators of interest, OBS, diet-related OBS, and lifestyle-related OBS, all were found to be significantly correlated ( p < 0.0001, p < 0.001, p < 0.0001). There was no correlation between the presence of kidney stones and total energy intake, and there was a significant correlation with Healthy Eating Index ( p < 0.0001). In comparing their levels with the inflammation-related indicators, leukocytes, neutrophils, lymphocytes, and monocytes were all correlated. When comparing subjects with and without a history of kidney stones, a significant correlation was found between patients with stones and several comorbidities (Table 1 ), all with p -values less than 0.0001.

Participant selection criteria
Baseline characteristics of individuals grouped by OBS quartile
Compared to the lowest OBS quartile, participants in the highest OBS quartile group had the following characteristics: younger age, non-Hispanic white or other race, higher wealth, married or partnered, higher education level, higher HEI, higher total energy intake, lower white blood cell levels, lower neutrophil levels, lower lymphocyte levels, and lower monocyte levels. The prevalence of kidney stones and several of their comorbidities, including hypertension, diabetes, cardiovascular disease, arthritis, and hyperlipidemia, decreased progressively with increasing OBS. Differences in gender between OBS quartile groups were not statistically significant. (Table 2 )
Association between OBS and kidney stones
Weighted logistic regression analysis revealed an association between OBS and kidney stones, as detailed in Table 3 . A total of 4 models were used, and the crude model did not adjust for any potential confounders. Model 1 adjusted for age, sex, race/ethnicity, marital status, poverty income ratio, and education. Model 2 was further adjusted for white blood cells, neutrophils, lymphocytes, monocyte, Healthy Eating Index, and Total energy intake. Model 3 was additionally adjusted for 5 comorbidities. In Model 3, the highest quartile group of OBS compared with the lowest quartile group of OBS (OR = 0.73 (0.57,0.92)) was correlated with the risk of kidney stone prevalence ( p = 0.01). It was statistically significant, remaining relatively stable across models. Similarly compared to the lowest quartile group of OBS, the second and third quartiles of OBS were correlated with the risk of kidney stone prevalence, and both were statistically significant (Q2: OR = 0.76 (0.61,0.93), p = 0.01; Q3: OR = 0.81 (0.66, 0.98), p < 0.03). Also, the test for trends in the model was statistically significant.
Associations of dietary OBS and lifestyle OBS with kidney stones
Weighted logistic regression analyses revealed the presence or absence of associations between dietary OBS and kidney stones, as detailed in Table 4 . 4 models were still used, and the crude model was not adjusted for any potential confounders. Model 1 adjusted for age, sex, race/ethnicity, marital status, poverty income ratio, and education. Model 2 was further adjusted for white blood cells, neutrophils, lymphocytes, monocyte, Healthy Eating Index, and Total energy intake. Model 3 was additionally adjusted for 5 comorbidities. In Model 3, the highest quartile of OBS compared to the lowest quartile of OBS (OR = 0.80 (0.61,1.05)) may not be correlated with the risk of prevalence of kidney stones ( p = 0.1).
Similarly, compared to the lowest quartile group of OBS, the second and third quartile groups of OBS may not correlate with the risk of kidney stone prevalence. Also, the test for trends in the model was not statistically significant. Weighted logistic regression analysis found an association between lifestyle OBS and kidney stones, as detailed in Table 5 . The model used was the same as before. In model 3, the highest quartile group of OBS compared to the lowest quartile group of OBS (OR = 0.74 (0.62,0.90)) was correlated with the risk of kidney stone prevalence ( p = 0.002). It was statistically significant, remaining relatively stable across models. However, there may be no correlation between the second and third quartiles of OBS and the risk of kidney stone prevalence compared to the lowest quartile of OBS. However, the test for trends in the models was statistically significant.
- Restricted cubic spline regression analysis
In restricted cubic spline regression, adjusting for different covariates, we found a significant nonlinear relationship between OBS and kidney stones (p for nonlinear < 0.0059, Fig. 2 A). Figure 2 A shows an overall trend of decreasing OR for kidney stones with increasing OBS, but between OBS values of 20 and 23, there is again a trend of increasing OR, which is worth pondering and discussing. There was a nonlinear negative correlation between lifestyle OBS and kidney stones (p for nonlinear < 0.0060, Fig. 2 B). Figure 2 B shows an overall trend of decreasing OR for kidney stones with increasing lifestyle OBS. Despite the differences in the results of the nonlinear analysis of the restricted triple spline, the overall trends of the dependent and independent variables were generally consistent across the plots.

Analysis of restricted cubic spline regression
To discuss the relationship between kidney stones and oxidative balance scores, we analyzed data from about 26,786 participants from a nationally representative survey population in the United States. We found a correlation between kidney stones and OBS. Even after adjusting for sociodemographic variables (age, sex, race, education, marital status, and ratio of household income to poverty), indicators of inflammation (number of leukocytes, neutrophils, lymphocytes, and monocytes), the Healthy Eating Index (HEI) and total energy intake to assess overall dietary quality, and covariates for comorbidities including hypertension, cardiovascular disease, diabetes mellitus, arthritis, and hyperlipidemia. This association persisted after the variables.
There are several possible drivers behind our finding of a lower prevalence of kidney stones in the presence of high OBS levels compared to participants with low OBS levels. One possible explanation is the difference in ROS levels in the body of subjects with different oxidative balance scores. Higher OBS levels imply that antioxidants are superior to pro-oxidants in the organism [ 12 ]. Therefore, subjects with high levels of OBS have lower levels of ROS in their bodies, and it has been shown that ROS-induced oxidative stress is essential in the pathogenesis of kidney stones,8,9 so this reason may have contributed to the low prevalence of kidney stones in participants with high levels of OBS. Other researchers have proposed that ROS production and the progression of oxidative stress may be a common pathophysiologic basis for kidney stones and other metabolic diseases [ 14 ]. These findings can provide us with a research idea and direction to study kidney stones and other diseases, which can further investigate the relationship between reactive oxygen species and kidney stones and explore their molecular biological mechanisms.
Because there are no studies to examine the direct relationship between oxidative balance scores and the prevalence of kidney stones, there are studies in previously published articles that have involved examining the relationship between obesity and kidney stones and have found that obesity can independently lead to kidney stones in the absence of metabolic abnormalities and insulin resistance [ 15 ]. In some studies, obesity is also characterized by chronic low-grade inflammation and permanently increased oxidative stress [ 16 ]. In obese animals or humans, adipose tissue is characterized by increased local and systemic production of pro-inflammatory adipocytokines that induce ROS production [ 17 ]. Elevated ROS leads to important changes in adipose tissue, which promote a systemic low-grade inflammatory response with adverse effects throughout the body [ 18 ]. Summarizing the results of these studies, the idea can be put forward that obese people may produce more reactive oxygen species and are, therefore, more likely to suffer from kidney stone disease. The OBS can be used as a good evaluative criterion to determine the oxidative and antioxidant status that the participants are in, which can be used to predict the development of kidney stones through the OBS and also to change the level of the OBS by adjusting the diet and the lifestyle in to prevent the occurrence of kidney stones. Other researchers have found an association between dietary intake of riboflavin and thiamine and kidney stones, with higher riboflavin intake negatively associated with kidney stones [ 19 ]. In our study, riboflavin was considered an antioxidant in the OBS calculations. The higher the intake, the higher the score, so it is consistent with the findings of our study. One study revealed a negative association between the level of dietary selenium intake and the risk of kidney stones in the U.S. population, especially for young adults (< 50 years old), men, and those who are overweight/obese (BMI ≥ 25.0) [ 20 ], which is also consistent with the findings of our study. The next step could be modeling to predict the incidence of kidney stones in the population, and the molecular biology of the relationship between the various factors affecting OBS and kidney stones could be further investigated.
There are also some limitations to this study, as the data were taken from a nationally representative survey population in the United States, so the conclusions drawn may only be appropriate for the U.S. mainland population, and further research on populations in other parts of the world would be needed to obtain a generalizable conclusion. The research used in this study was primarily in the form of a questionnaire, so there is recall bias. Also, there were many participants with varying degrees of missing information in the raw data, and there was non-response bias. With the data in this study, it is difficult to determine the temporal relationship between antecedents and consequences.
In conclusion, our study found a negative association between OBS and the prevalence of kidney stones. Higher OBS, indicating more antioxidant exposure than pro-oxidant exposure in diet and lifestyle, was also associated with a lower risk of kidney stone prevalence. This finding suggests that antioxidant diets and lifestyles are beneficial in reducing the incidence of kidney stones, improving people’s quality of life, and reducing the disease burden.
Data Availability
The data used in this study are publicly available from the National Centers for Health Statistics (NCHS), a branch of the Centers for Disease Control and Prevention (CDC). The website link is: https://www.cdc.gov/nchs/nhanes/index.htm.
Khan SR, Pearle MS, Robertson WG, et al. Kidney stones. Nat Rev Dis Primers. 2016;2:16008. https://doi.org/10.1038/nrdp.2016.8 .
Article PubMed PubMed Central Google Scholar
Crivelli JJ, Maalouf NM, Paiste HJ, et al. Disparities in Kidney Stone Disease: A Scoping Review. J Urol. 2021;206(3):517–25. https://doi.org/10.1097/JU.0000000000001846 .
Morgan MSC, Pearle MS. Medical management of renal stones. BMJ. 2016;352:i52. https://doi.org/10.1136/bmj.i52 .
Article PubMed Google Scholar
Rivera M, Jaeger C, Yelfimov D, Krambeck AE. Risk of Chronic Kidney Disease in Brushite Stone Formers Compared With Idiopathic Calcium Oxalate Stone Formers. Urology. 2017;99:23–6. https://doi.org/10.1016/j.urology.2016.08.041 .
Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010;12(2–3):e86–96.
PubMed PubMed Central Google Scholar
Ziemba JB, Matlaga BR. Epidemiology and economics of nephrolithiasis. Investig Clin Urol. 2017;58(5):299–306. https://doi.org/10.4111/icu.2017.58.5.299 .
Singh P, Harris PC, Sas DJ, Lieske JC. The genetics of kidney stone disease and nephrocalcinosis. Nat Rev Nephrol. 2022;18(4):224–40. https://doi.org/10.1038/s41581-021-00513-4 .
Khan SR. Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl Androl Urol. 2014;3(3):256–76. https://doi.org/10.3978/j.issn.2223-4683.2014.06.04 .
Liu Y, Sun Y, Kang J, et al. Role of ROS-Induced NLRP3 Inflammasome Activation in the Formation of Calcium Oxalate Nephrolithiasis. Front Immunol. 2022;13:818625. https://doi.org/10.3389/fimmu.2022.818625 .
Article CAS PubMed PubMed Central Google Scholar
Veena CK, Josephine A, Preetha SP, Rajesh NG, Varalakshmi P. Mitochondrial dysfunction in an animal model of hyperoxaluria: a prophylactic approach with fucoidan. Eur J Pharmacol. 2008;579(1–3):330–6. https://doi.org/10.1016/j.ejphar.2007.09.044 .
Article CAS PubMed Google Scholar
Khan SR. Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J Urol. 2013;189(3):803–11. https://doi.org/10.1016/j.juro.2012.05.078 .
Zhang W, Peng SF, Chen L, Chen HM, Cheng XE, Tang YH. Association between the Oxidative Balance Score and Telomere Length from the National Health and Nutrition Examination Survey 1999–2002. Oxid Med Cell Longev. 2022;2022:1345071. https://doi.org/10.1155/2022/1345071 .
Krebs-Smith SM, Pannucci TE, Subar AF, et al. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591–602. https://doi.org/10.1016/j.jand.2018.05.021 .
Khan SR. Is oxidative stress, a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome? Urol Res. 2012;40(2):95–112. https://doi.org/10.1007/s00240-011-0448-9 .
Chen W, Man S, Hong Y, et al. Association between metabolically healthy obesity and kidney stones: results from the 2011–2018 National Health and Nutrition Examination Survey. Front Public Health. 2023;11:1103393. https://doi.org/10.3389/fpubh.2023.1103393 .
Marseglia L, Manti S, D’Angelo G, et al. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci. 2014;16(1):378–400. https://doi.org/10.3390/ijms16010378 .
Sengenès C, Miranville A, Lolmède K, Curat CA, Bouloumié A. The role of endothelial cells in inflamed adipose tissue. J Intern Med. 2007;262(4):415–21. https://doi.org/10.1111/j.1365-2796.2007.01853.x .
Rzheshevsky AV. Fatal triad: lipotoxicity, oxidative stress, and phenoptosis. Biochem (Mosc). 2013;78(9):991–1000. https://doi.org/10.1134/S0006297913090046 .
Article CAS Google Scholar
Di XP, Gao XS, Xiang LY, Wei X. The association of dietary intake of riboflavin and thiamine with kidney stone: a cross-sectional survey of NHANES 2007–2018. BMC Public Health. 2023;23(1):964. https://doi.org/10.1186/s12889-023-15817-2 .
Liu M, Cui Z, Chen J, Gao M, Zhu Z, Chen H. Dietary selenium intake and the risk of kidney stones in adults, an analysis of 2007–2018 National Health and Nutrition Examination Survey, a cross-sectional study. Front Nutr. 2022;9:877917. https://doi.org/10.3389/fnut.2022.877917 .
Download references
Acknowledgements
Not applicable.
Key research and development plan in Shaanxi province (No. 2020SF-123), and Medical research program of department of science and technology of Xi’an, Shaanxi Province (No.2019115713YX012SF048(4)).
Author information
Authors and affiliations.
Department of Urology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, 710061, China
Rundong Song, Minghai Ma, Lu Wang, Yunzhong Jiang, Jianpeng Li & Jinhai Fan
The Second Affiliated Hospital of Xi’an Jiaotong University, 710004, Xi’an, Shaanxi, China
You can also search for this author in PubMed Google Scholar
Contributions
R.S wrote the main manuscript text, K.W、M.M、L.W collected information, Y.J、J.L made tables and figures, J.F designed the research. All authors reviewed the manuscript.
Corresponding author
Correspondence to Jinhai Fan .
Ethics declarations
Ethics approval and consent to participate:.
The National Health and Nutrition Examination Survey (NHANES) is a publicly available database and approved by the National Center for Health Statistics institutional review board. All participants provide written informed consent when they did the national survey in the United States. Ethical review and approval were waived for this study since secondary analysis did not require additional institutional review board approval.
Consent for publication:
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Song, R., Wu, K., Ma, M. et al. Association between oxidative balance score and kidney stones: data from the national health and nutrition examination survey (NHANES). BMC Nephrol 25 , 190 (2024). https://doi.org/10.1186/s12882-024-03607-w
Download citation
Received : 02 January 2024
Accepted : 13 May 2024
Published : 03 June 2024
DOI : https://doi.org/10.1186/s12882-024-03607-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Kidney stones
- Oxidative balance score
- Reactive oxygen species
- National health and nutrition examination survey
BMC Nephrology
ISSN: 1471-2369
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 30 June 2021
Targeting oxidative stress in disease: promise and limitations of antioxidant therapy
- Henry Jay Forman ORCID: orcid.org/0000-0001-5838-2791 1 , 2 &
- Hongqiao Zhang 2
Nature Reviews Drug Discovery volume 20 , pages 689–709 ( 2021 ) Cite this article
67k Accesses
1013 Citations
423 Altmetric
Metrics details
- Biochemistry
- Drug discovery
An Author Correction to this article was published on 13 July 2021
This article has been updated
Oxidative stress is a component of many diseases, including atherosclerosis, chronic obstructive pulmonary disease, Alzheimer disease and cancer. Although numerous small molecules evaluated as antioxidants have exhibited therapeutic potential in preclinical studies, clinical trial results have been disappointing. A greater understanding of the mechanisms through which antioxidants act and where and when they are effective may provide a rational approach that leads to greater pharmacological success. Here, we review the relationships between oxidative stress, redox signalling and disease, the mechanisms through which oxidative stress can contribute to pathology, how antioxidant defences work, what limits their effectiveness and how antioxidant defences can be increased through physiological signalling, dietary components and potential pharmaceutical intervention.
Similar content being viewed by others
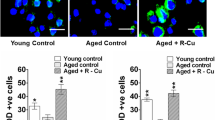
A pro-oxidant combination of resveratrol and copper down-regulates multiple biological hallmarks of ageing and neurodegeneration in mice
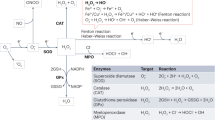

Oxidative stress and the role of redox signalling in chronic kidney disease
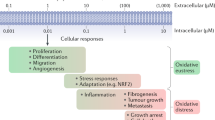
Reactive oxygen species (ROS) as pleiotropic physiological signalling agents
Introduction.
The term ‘ oxidative stress ’ was first coined by Helmut Sies 1 as an imbalance between production of oxidants and antioxidant defences that may result in damage to biological systems. Since then, the field of redox biology has evolved from concepts of oxidative stress in pathology to redox signalling in physiology 2 , 3 , 4 .
Oxidative stress has been shown to participate in a wide range of diseases including atherosclerosis, chronic obstructive pulmonary disease (COPD), Alzheimer disease and cancer, which has revealed the multiple mechanisms by which oxidants contribute to cellular damage 5 . However, the extent to which oxidative stress participates in the pathology of diseases is quite variable, such that the effectiveness of increasing antioxidant defence may be limited in some diseases.
Oxidative stress involves the chemistry of reactions of so-called reactive species derived from oxygen and nitrogen (Box 1 ). Understanding which of these species cause damage to macromolecules helps to provide a rationale for improving therapeutic approaches to antioxidant defence. However, so far, the use of small molecules therapeutically has been disappointing, largely owing to overly optimistic and incorrect assumptions about how antioxidants work 6 . For example, scavenging of hydroxyl radical (•OH) is impractical, but preventing its formation by reducing hydrogen peroxide (H 2 O 2 ) production can provide effective prevention of damage. One of the major misunderstandings in the field of oxidative stress concerns the scavenging of superoxide (O 2 • − ) or H 2 O 2 by small molecules, which are also ineffective inside cells. This is because the antioxidant enzymes react thousands to millions of times more rapidly with those oxidants than small molecules do and provide the predominant antioxidant defence 6 , 7 . However, in extracellular fluids where antioxidant enzymes are absent, scavenging of O 2 • − and H 2 O 2 (but not •OH) is possible with mimics of superoxide dismutase (SOD) and catalase, as discussed below.
It is essential to recognize the limitations that have led to failures in clinical trials and how antioxidant defences can be effective if one is realistic about where, when and to what extent oxidative stress is part of a disease. Indeed, most antioxidant defence within cells is not provided by either exogenous or endogenous small molecules acting as scavengers, but by antioxidant enzymes using their specific substrates to reduce oxidants. Therefore, the major therapeutic opportunities lie in preventing the production of oxidants that cause direct injury to macromolecules, inhibiting downstream signalling by oxidants that results in signalling for inflammation or cell death, and increasing both antioxidant enzymes and their substrates. Currently, there are clinical trials ongoing for ebselen, a glutathione peroxidase (GPX) mimic, for Meniere disease in phase II (NCT02603081); GC4419, a SOD mimic, for squamous cell cancers in phase I (NCT01921426); and sulforaphane, an activator of the NRF2 transcription factor , for COPD in phase II (NCT01335971), among others.
This article reviews the relationships between oxidative stress, redox signalling and disease and presents an overview of the mechanisms through which oxidative stress can contribute to pathology. We focus on current understanding of the mechanisms mediating antioxidant defences and what limits their effectiveness, and highlight emerging approaches to therapeutically modulate them. Through greater understanding of the mechanisms through which oxidants act and the limitations and potential of antioxidant therapies, a rational approach can be developed that will improve therapeutic intervention.
For the purposes of this Review, we refer to oxidative stress as the situation in which oxidants non-enzymatically damage macromolecules, including proteins, nucleic acids and the lipids that compose cell membranes. This Review focuses only on factors that either prevent production of oxidants or allow their efficient removal. The principal targets are O 2 • − , H 2 O 2 and lipid hydroperoxides. By eliminating these targets, production of the more reactive •OH, peroxynitrite (ONOO − ) and the hypohalous acids (HOX) can be prevented. Although ONOO − production can be limited by inhibiting nitric oxide (•NO) production, because •NO is too important in maintaining normal physiology, the better approach is to limit excessive O 2 • − production.
Box 1 Mechanisms of oxidative stress
Both endogenous and exogenous agents cause oxidative stress 276 . The term reactive oxygen species (ROS) encompasses molecules derived from O 2 , including superoxide (O 2 • − ), hydrogen peroxide (H 2 O 2 ), hydroxyl radical (•OH), ozone and singlet oxygen. The use of ROS, as though it were a chemical entity, leads to many imprecise statements because the chemistries of these species are markedly different.
Production of O 2 • − by one-electron reduction of O 2 is primarily through leakage of electrons from the mitochondrial respiratory chain, particularly from ubisemiquinone (QH • − ) 277 (reaction 1):
and the NADPH oxidases that catalyse reaction 2 (refs 232 , 278 ):
The NADPH oxidases (NOX4, DuOX1 and DuOX2) and some other flavoprotein enzymes reduce O 2 to H 2 O 2 , by giving O 2 • − a second electron before it leaves their active sites 279 .
The predominant source of H 2 O 2 is dismutation of O 2 • − , a fast reaction with a rate constant near 10 5 M −1 s −1 that is accelerated to 2 × 10 9 M −1 s −1 by superoxide dismutases (reaction 3):
The rate of H 2 O 2 production largely determines whether redox signalling, oxidative stress or no significant oxidation occurs. H 2 O 2 is reduced enzymatically by 15 enzymes, including catalase (reaction 4):
the five peroxiredoxins that use thioredoxin (a small protein with two crucial cysteines, Trx(SH) 2 ) or the eight glutathione peroxidases and peroxiredoxin 6 that use the tripeptide, glutathione (γ-glutamyl-cysteinyl-glycine, GSH) (reactions 5 and 6):
where TrxS 2 is thioredoxin disulfide and GSSG is glutathione disulfide.
H 2 O 2 does not easily oxidize most molecules but it can react rapidly with transition metals such as iron to produce hydroxyl radical (reaction 7, often referred to as the Fenton reaction) 280 :
The hydroxyl radical is an extraordinarily strong oxidant that will rapidly oxidize whatever molecule it is next to.
One reaction responsible for oxidative stress is the lipid peroxidation chain reaction that can be initiated by •OH (reactions 8–10):
where LH is a lipid with allylic hydrogens, which are present in polyunsaturated fatty acids including arachidonic acid.
Superoxide can cause release of iron from iron–sulfur proteins, which can then catalyse reaction 7. The major way that the relatively weak oxidant O 2 • − contributes to oxidative stress, however, is as a precursor of H 2 O 2 and peroxynitrite (ONOO − ), which is formed in reaction 11:
where •NO is nitric oxide. The danger of producing oxidative stress is not directly from the free radicals, •NO and O 2 • − , but from the protonated form of peroxynitrite, peroxynitrous acid (ONOOH), a non-radical. Peroxynitrous acid is a very strong oxidant that has the reactivity of the intermediates formed in its decomposition (reaction 12):
nitrogen dioxide •NO 2 and •OH. •NO 2 can abstract hydrogen as does •OH or add to some molecules including the tyrosines in proteins producing nitrotyrosine that may alter function. ONOO − can also rapidly cause the release of iron from iron–sulfur proteins 11 , promoting •OH production from H 2 O 2 (reaction 7).
Both •NO 2 and •OH are indiscriminate in what they will oxidize, which creates the havoc called oxidative stress. Again, because of their rapid reactions, the best way this can be addressed is prevention of the formation of •NO 2 and •OH.
The final oxidants we consider are the hypohalous acids (HOX) that are formed from H 2 O 2 in reaction 13, which is catalysed by phagocytic cell myeloperoxidases:
where X − may be Cl − , Br − or even SCN − (ref. 281 ). They play a major role in tissue damage associated with phagocyte-mediated inflammation.
Roles of oxidative stress in disease
There are two major mechanisms through which oxidative stress contributes to disease. The first involves the production of reactive species during oxidative stress — particularly •OH, ONOO − and HOCl — that directly oxidize macromolecules, including membrane lipids, structural proteins, enzymes and nucleic acids, leading to aberrant cell function and death. The second mechanism of oxidative stress is aberrant redox signalling (Box 2 ). Oxidants, particularly H 2 O 2 generated by cells upon physiological stimulation, can act as second messengers 8 . In oxidative stress, non-physiological production of H 2 O 2 can cause redox signalling to go awry 4 . Both types of oxidative stress mechanism can occur in a single disease, such as in diabetes, where both advanced glycation products accumulate and aberrant activation of stress signalling pathways leads to diabetic complications 9 . Also, the increase in H 2 O 2 production and iron release from proteins in oxidative stress by O 2 • − (ref. 10 ) and ONOO − (ref. 11 ) causes a marked elevation in the production of lipid peroxidation products including 4-hydroxy-2-nonenal (HNE), which can also cause aberrant signalling 12 .
Oxidative stress has been associated with a wide range of pathologies. On the basis of the contribution of oxidative stress to the aetiology of these pathologies, they have been grouped into two categories below: first, oxidative stress as the primary cause of pathology (including toxicities caused by radiation and paraquat, and in atherosclerosis); second, oxidative stress as the secondary contributor to disease progression (such as in COPD, hypertension and Alzheimer disease). However, as the role of oxidative stress in many diseases is incompletely understood, this categorization is tentative.
Box 2 Redox signalling, homeostasis and antioxidant defences
Redox signalling is dependent on specific interactions of signalling proteins with hydrogen peroxide (H 2 O 2 ) or other electrophiles that act as second messengers. As with oxidative stress, both endogenous and exogenous sources of H 2 O 2 or other electrophiles may be involved; however, for redox signalling to be physiological rather than pathological, regulation is essential and requires the involvement of specificity that is not part of oxidative stress. An oxidative challenge, as opposed to oxidative stress, involves the stimulation of redox signalling without any damage, a phenomenon that we have called ‘para-hormesis’ 101 . A related term is ‘oxidative eustress’ 3 .
Maintaining redox homeostasis is important for cell function. Despite its name, homeostasis does not imply that nothing is changing. Indeed, a balance between oxidants and reductants, including glutathione, thioredoxin and NADPH, which are the substrates for antioxidant enzymes, is essential for maintaining normal physiology 101 . Thus, diseases that involve oxidative stress can be due to disruption of redox homeostasis, with type 2 diabetes mellitus as one example 9 . Adaptive homeostasis, as defined by Kelvin Davies 282 , involves elevated antioxidant defences brought about by transient alteration of redox homeostasis and redox signalling. However, redox signalling may also occur under pathological conditions, as oxidative stress can stimulate the same pathways as redox signalling under physiological conditions. The difference in this context is that the signalling will be unregulated and accompanied by nonspecific damage.
The effectiveness of this antioxidant system in maintaining the homeostasis relies upon keeping the generation and removal of superoxide (O 2 • − ), H 2 O 2 and nitric oxide (•NO) within a range that does not allow significant production of peroxynitrite (ONOO − ) and hydroxyl radical (•OH) 101 . It is not a perfect system as evidenced by a low rate of oxidized proteins that accumulate with age. Regardless, the ability to induce the enzymes that remove O 2 • − and H 2 O 2 and damaged proteins in what Davies calls ‘adaptive homeostasis’ provides a major means of enhancing antioxidant defences that will be described elsewhere in this Review 282 .
Oxidative stress as the primary cause of pathology
Oxidative stress can be a primary factor in toxicity and disease. However, an important caveat is that once damage begins, antioxidant therapy often fails to inhibit the progression of tissue injury as other factors become dominant in the pathology.
Radiation-induced lung injury
Early pneumonitis followed by fibrosis frequently occur as side effects of radiotherapy for lung and oesophageal cancers 13 . When cells are exposed to radiation, homolytic cleavage of H 2 O directly generates •OH, which then oxidizes macromolecules and triggers an inflammatory response leading to infiltration of inflammatory cells into the lung (pneumonitis) and cell death. Over a longer period, aberrant redox signalling for the continuous production of cytokines causes accumulation of collagen and lung fibrosis 14 . In addition, higher lipid peroxidation and DNA oxidation (8-hydroxy-2′-deoxyguanosine) has been observed in lungs of radiation-induced lung injury in rats, which can persist for months after radiation exposure 15 .
Paraquat poisoning
Oxidative stress is also responsible for the toxicity of the widely used chemical herbicide, paraquat. When ingested, paraquat is actively taken up by alveolar type II cells and leads to pneumonitis and progressive lung fibrosis with poor prognosis. Paraquat also causes injury to other organs including liver and kidney. Long-term exposure to paraquat is associated with Parkinson disease 16 . Paraquat toxicity is initiated by the continuous redox cycling that generates O 2 • − (ref. 17 ).
Atherosclerosis
In atherosclerosis, plaque builds up in the intimal layer of arteries and over time the arteries narrow, leading to infarction and stroke. Substantial evidence indicates that oxidative stress has a crucial role in the pathogenesis of atherosclerosis. Since the first identification of lipid hydroperoxides in human atherosclerotic aorta 18 , many studies have shown an increase in oxidized lipids and other oxidative stress markers in the atherosclerotic lesions. For example, 20% of cholesteryl linoleate (Ch18:2) in freshly isolated human plaque was reported to be oxidized, whereas it was undetectable in normal arteries 19 . In addition, HNE-modified low-density lipoprotein (LDL) was found to be elevated by 50% in plasma of patients with atherosclerosis compared with healthy volunteers 20 . Furthermore, isoprostanes, peroxidation products of arachidonic acid, have been reported to be increased at least fivefold in human atherosclerotic lesions compared with human umbilical veins, and oxidized linoleic acid was detected only in human lesions 21 . Oxidative stress is responsible for the conversion of LDL cholesterol into the atherogenic form of oxidized-LDL (OxLDL), which has a crucial role in initiating and promoting the inflammatory response and recruitment of leukocytes in the lesion site, and contributes to the development of atherosclerosis through activation of smooth muscle cells and reduced •NO bioavailability 22 .
Oxidative stress as a secondary contributor to disease progression
In many diseases, oxidative stress occurs secondary to the initiation of pathology by other factors. Examples of this are the oxidative stress caused by increased production of O 2 • − or H 2 O 2 from NADPH oxidases (NOXs) in the inflammatory response that follows initial tissue injury, and by xanthine oxidase in ischaemia–reperfusion . Oxidative stress can disturb various signalling pathways and affect multiple biological processes through modifying proteins, promoting inflammation, inducing apoptosis, deregulating autophagy , impairing mitochondrial function and many other mechanisms. These effects frequently accelerate pathological progression and exacerbate the symptoms of diseases, as discussed in representative examples below.
Chronic obstructive pulmonary disease
COPD comprises progressive and irreversible chronic bronchitis and/or emphysema. Cigarette smoking, the main cause of COPD, is an abundant source of oxidants. Oxidative stress can lead to oxidation and inhibition of α1-antitrypsin, thus reducing its ability to inhibit neutrophil elastase, a major factor in the pathogenesis of COPD 23 . In addition, chronic exposure to oxidants in cigarette smoke causes and promotes the inflammatory response and other pathological cascades such as cell death and fibrosis in COPD pathogenesis 14 . The sources of oxidants in COPD are both exogenous (for example, cigarette smoking and air pollution) and endogenous (for example, NOX, mitochondria, inducible nitric oxide synthase (iNOS) and myeloperoxidase) 14 . Increased levels of oxidants and lipid peroxidation products, including 8-isoprostane, have been consistently detected in exhaled breath condensate of patients with COPD compared with healthy controls 24 . In addition, HNE (HNE adducts) levels were found to be significantly elevated by at least 50% in airway and alveolar epithelial cells, endothelial cells and neutrophils in patients with COPD compared with healthy controls 25 ; and the urinary level of 8-hydroxydeoxyguanosine (8-OHdG), a marker of DNA oxidation, was significantly elevated in patients with COPD 26 . The level of oxidative stress was inversely correlated with lung function of the patients 25 . Together, these results suggest that oxidative stress occurs both in the lung and systemically in patients with COPD and contributes to disease pathogenesis.
Idiopathic pulmonary fibrosis
The pathology of idiopathic pulmonary fibrosis (IPF) is characterized by diffuse and progressive mesenchymal fibrosis and mild inflammation in the lung with unknown aetiology. Many studies have shown the presence of oxidative stress in IPF. Oxidative stress markers such as H 2 O 2 , 8-isoprostane, 8-isoprostaglandin-F2α (8-iso-PGF2α) and ethane are significantly increased in the exhaled breath condensate of patients with IPF compared with healthy individuals 27 . In addition, 8-isoprostane is elevated fivefold 28 and oxidized proteins twofold 29 in bronchoalveolar lavage fluid (BALF) of patients with IPF. HNE in lung 30 and 8-isoprostane in blood 31 are also significantly elevated in IPF. The glutathione (GSH) level in epithelial lining fluid and sputum of patients with IPF is fourfold lower than in healthy controls 32 , indicating a deficiency of this important component of antioxidant defence in IPF. H 2 O 2 production is apparently mainly from NOX4 (ref. 33 ) and dysfunctional mitochondria 34 , and GSH synthesis is downregulated by TGFβ signalling 35 . Mounting evidence suggests that oxidative stress plays a significant part in IPF, by promoting fibrogenesis through causing apoptosis of alveolar epithelial cells, activating myofibroblasts and inducing an inflammatory response 36 . Besides oxidative stress, IPF pathogenesis involves a number of processes including apoptosis, senescence, epithelial–mesenchymal transition, endothelial–mesenchymal transition, epithelial cell migration, increased production of chemokines, cytokines and growth factors, as well as mitochondrial dysfunction, endoplasmic reticulum stress, hypoxia and inflammation 37 . These mechanisms are interrelated, with oxidative stress representing an important component of the IPF pathogenesis.
Hypertension
Multiple risk factors such as diet, smoking, lifestyle, genetics and comorbidities contribute to hypertension. More than 90% of cases are essential hypertension with unclear cause. At the molecular level, however, oxidative stress is a common feature of this condition. Experimental studies suggest that oxidants are mainly from NOXs in hypertension 38 . Oxidative markers, including H 2 O 2 (ref. 39 ), glutathione disulfide (GSSG) to GSH ratio, malondialdehyde (a lipid peroxidation product) 40 and 8-isoprostanes, are significantly increased in the plasma of patients with hypertension 41 . H 2 O 2 has a role in the development and progression of hypertension, through influencing angiotensin II signalling, NO signalling and other cellular processes 42 . However, a causative role of oxidative stress in hypertension has not yet been established.
Type 2 diabetes mellitus
Patients with type 2 diabetes mellitus display substantial evidence of oxidative stress that results in microvascular and macrovascular complications 43 . Markers of oxidative stress, including OxLDL to LDL ratio 44 , 8-OHdG 45 , 8-iso-PGF2α 46 , protein carbonyls 47 and GSH conjugation to haemoglobin 48 , have been reported to be significantly elevated in the plasma of patients with type 2 diabetes mellitus, as have urine 8-OHdG and 8-iso-PGF2α levels 49 . The increased oxidants in type 2 diabetes mellitus arise from dysfunctional mitochondria 50 and NOX1 (ref. 51 ) activated by the diabetic abnormalities of hyperglycaemia and dyslipidaemia.
Alzheimer disease
Alzheimer disease is characterized by the progressive accumulation of extracellular amyloid-β plaques and neurofibrillary tangles inside neurons. Several risk factors (age, genetics, sex, trauma and air pollution) for Alzheimer disease have been identified, but the exact cause remains unclear. However, accumulating evidence suggests that oxidative stress may have a crucial role through multiple pathways 52 . Many studies have demonstrated increased oxidative stress in the brain of patients with Alzheimer disease, including increased levels of F2-isoprostane-α in cerebrospinal fluid 53 and frontal and temporal poles 54 , acrolein in amygdala and hippocampus/parahippocampal gyrus 55 , and HNE in ventricular fluid 56 , hippocampus and inferior parietal lobule 57 , and cortex 58 . Increased levels of nuclear and mitochondrial DNA oxidation were also found in frontal, parietal and temporal lobes of the brain of patients with Alzheimer disease compared with age-matched control subjects 59 . In addition, protein oxidation in the hippocampus 60 and protein carbonyls in the cerebral cortex 58 were significantly elevated in the brains of patients with Alzheimer disease. Claims have been made that Aβ(1–42) 61 , activated microglia 62 , iron accumulation 63 and dysfunctional mitochondria contribute to increased oxidant production 64 .
Through aberrantly altering signalling transduction pathways that damage DNA and exacerbate inflammation, oxidants are involved in various phases of tumorigenesis, including transformation of normal cells to tumour cells, tumour cell growth, proliferation, invasion, angiogenesis and metastasis 65 . Conversely, oxidative stress can also trigger apoptosis and ferroptosis, and reduce the opportunity for transformation and thereby prevent tumorigenesis 65 . In addition, oxidative stress is the main mechanism of action of radiation (see Radiation-induced lung injury subsection above) and many chemotherapeutic drugs 66 . Therefore, oxidative stress is implicated in almost all phases of cancer. Cancer cells produce more oxidants than normal cells, and therefore cancer cells are exposed to increased oxidative stress in the loci. The increased oxidants in cancer cells are mainly from mitochondria 67 , NOX4 (ref. 68 ) and 5-lipoxygenase 69 . Oxidants in the loci may also come from normal cells in or surrounding the tumour mass, such as endothelial cells and inflammatory immune cells. The increase in oxidative markers has been observed in various types of cancer. For example, patients with non-small-cell lung cancer have been shown to exhale more H 2 O 2 than control individuals 70 . In addition, increased levels of 8-OHdG 71 were detected in breast cancer tissues compared with matched normal tissues, and 8-OHdG was significantly elevated in prostate cancers 72 and lung cancers 73 .
Systemic inflammatory response syndrome
Systemic inflammatory response syndrome (SIRS) is a disorder caused by an exaggerated inflammatory response in the whole body to infectious pathogens or non-infectious insults 74 . SIRS involves the release of oxidants and inflammatory cytokines leading to reversible or irreversible end organ dysfunction and even death. Sepsis is a SIRS caused by infection, which shares common features of inflammation and oxidative stress with SIRS caused by non-infectious insults, and is more frequently studied. Plasma F2-isoprostanes 75 , HNE 76 and 8-OHdG 77 have been reported to be significantly increased in patients with severe sepsis. In patients with acute respiratory distress syndrome from SIRS, the level of 8-iso-PGF2α is increased in exhaled breath condensate 78 as is nitrotyrosine in BALF 79 . Oxidants in sepsis originate from several sources depending on the tissues and/or cells, and include iNOS (also known as NOS2) 80 , NOXs 81 , xanthine oxidase 82 and dysfunctional mitochondria 83 . In addition, the levels of antioxidants such as vitamin C 84 , vitamin E 85 and GSH 86 are decreased in sepsis.
Ischaemia–reperfusion injury
Although timely reperfusion is essential to avoid irreversible injury from ischaemia (interrupted blood flow), extensive damage to both the local and distant organs can occur through initiation of a systemic inflammatory response. Ischaemia–reperfusion injury (IRI) has a major role in the pathophysiological changes of several critical clinical conditions including heart attack, stroke and organ transplantation. The molecular mechanisms underlying IRI are multifactorial and involve the inflammatory response and oxidative stress. In the ischaemic phase, lack of oxygen and nutrients results in accumulation of hypoxanthine, release of calcium, activation of xanthine oxidase and induction of pro-inflammatory cytokines; and in the reperfusion phase, production of NO, ONOO − , O 2 • − and other oxidants is significantly increased from hypoxanthine/xanthine oxidase 87 , mitochondria, iNOS (NOS2) and NOXs 88 in endothelial cells, infiltrated neutrophils and local tissue cells 89 . Markers of oxidative stress including urinary 8-iso-PGF2α are elevated in patients with acute myocardial infarction given thrombolytic therapy, when compared with both age-matched, healthy control subjects and patients with stable coronary heart disease 90 , and in patients with coronary angioplasty following carotid reperfusion 91 . A study involving 66 individuals with stroke and 132 control subjects showed that plasma and urinary F2-isoprostanes were elevated immediately and up to day 7 after onset of ischaemic stroke 92 . Urinary 8-OHdG was also increased after reperfusion in acute myocardial infarction 93 . It should be noted that most oxidative markers measured in IRI studies were systemic and few studies determined the presence of these markers in the lesion site.
Antioxidant defences and therapeutic implications
To defend against oxidative injury, organisms have evolved defences primarily dependent upon antioxidant enzymes, supply of their substrates and repair of injury. In response to oxidants and other electrophiles, these defences increase and thereby boost the capacity to detoxify oxidants and/or electrophiles and repair oxidative damage. Agents that enhance these defences are the principal strategies underlying antioxidant therapy.
Extensive studies on the induction of antioxidant enzymes have focused on the regulatory mechanisms, the implications in diseases and potential inducers with therapeutic purpose. Although several transcription factors are redox sensitive and are involved in the induction of antioxidant genes (for example, the induction of haem oxygenase 1 (HO1, encoded by HMOX1 ) through activator protein 1 (AP-1) 94 and peroxisome proliferator-activated receptor-γ (PPARγ) 95 , and the induction of glutamate–cysteine ligase (GCL) 96 and SOD1 (ref. 97 ) through nuclear factor-κB (NF-κB)), the finding with the broadest effect in this area is the induction of antioxidant genes GCLC , GCLM , HMOX1 , NQO1 , GSTM1 , GPX4 , TXN and PRDX1 through NRF2 (refs 98 , 99 ) (Box 3 ).
Oxidant species that present immediate danger to the structural integrity and function of cells are •OH, ONOO − and HOX. However, these oxidants react too rapidly with membrane lipids, proteins and nucleic acids to be effectively scavenged by exogenous small molecules. Unfortunately, many erroneous claims have been made for •OH scavengers. Although oxidative stress involves the generation of •OH, the proposed scavenging of these radicals in biological systems by exogenous molecules is unsound. All organic compounds react with •OH with similar rate constants approaching diffusion limitation. Thus, no compound has more •OH scavenging activity than the thousands of molecules already present in any biological system. To be 50% effective, any compound would have to be present at equal or greater concentration than all of those endogenous molecules. The only effective strategy preventing damage by •OH is prevention of its formation. Strategies that have the potential to be successful in that endeavour are prevention of the formation of O 2 • − and removal of O 2 • − and H 2 O 2 . The removal of O 2 • − also prevents the formation of ONOO − , and the removal of H 2 O 2 prevents formation of •OH and HOX.
SODs and enzymes that remove H 2 O 2 and lipid hydroperoxides form the front line of defence against oxidative stress. However, there are major differences between the extracellular fluids and within cells, which have therapeutic implications. Extracellular SOD (EC-SOD, SOD3) is generally associated with the outer membrane of cells and is not present in all extracellular fluids. SOD mimics are effective in the extracellular fluids where decreased production of the potentially hazardous ONOO − has the additional advantage of sparing •NO, which participates in vasodilation and other important physiological processes 100 . Although the outer surface of some cells binds to EC-SOD, the additional catalase activity of most SOD mimics also catalyses removal of H 2 O 2 , which EC-SOD cannot achieve. Intracellular defences include cytosolic SOD1 and mitochondrial matrix SOD2, which remove O 2 • − , while catalase in peroxisomes (and cardiac mitochondria), GPXs and peroxiredoxins (PRDXs) remove H 2 O 2 . Some of the GPXs and PRDXs also reduce lipid hydroperoxides, with two of them (PRDX6 and GPX4) being able to reduce phospholipid hydroperoxides. Within cells, scavenging of O 2 • − by small molecules is negligible compared with the rate of removal by endogenous SODs, which have rate constants (~2 × 10 9 M −1 s −1 ) that are millions of times higher than those of most other reactions with O 2 • − . The outer surface of some cells binds to EC-SOD, which also outcompetes any potential O 2 • − scavenger. Nonetheless, SOD mimics are useful in extracellular environments that lack significant EC-SOD. SOD produces H 2 O 2 , which would seem to be not much of a gain in terms of antioxidant defence; however, the removal of O 2 • − prevents formation of the more dangerous ONOO − , while simultaneously sparing physiologically important •NO. Compounds with combined SOD and catalase activities have an advantage over SOD alone.
The second line of antioxidant defence includes the synthesis of thioredoxin (TRX), GCL and glutathione synthetase responsible for the synthesis of GSH, glutathione reductase and thioredoxin reductase, which use NADPH to reduce GSSG and TrxS 2 . It should be noted that both first-line and second-line enzymes also have a role in physiological redox signalling and the maintenance of redox homeostasis, and that total elimination of H 2 O 2 would adversely alter cellular function 101 . Scavenging of H 2 O 2 and other hydroperoxides by small molecules is negligible compared with removal by the 15 enzymes that reduce H 2 O 2 and lipid hydroperoxides and the two enzymes that reduce phospholipid hydroperoxides. Nonetheless, a few mimics of GPX, including ebselen (see below), have rate constants that approach those of the enzymes. In addition, ebselen may also reduce ONOO − . Although GSH is normally in the millimolar range in cells, it can be depleted during oxidative stress. Thus, compounds that increase GSH by either supplying cysteine, which is limiting for GSH synthesis, or are precursors for GSH, increase the effectiveness of endogenous GPXs or GPX mimics. Increasing synthesis of GSH by induction of GCL, the enzyme that kinetically limits GSH synthesis, also offers a therapeutic advantage. Indeed, finding agents that induce GCL through activation of the NRF2 transcription factor has been a major goal for more than two decades.
A third line of antioxidant defence is repair or removal of oxidized macromolecules. This broad area of research is not directly relevant to the present Review; however, the enzymatic systems for removal of oxidized proteins 102 , oxidized fatty acid removal and replacement 103 , and oxidized DNA removal and repair 104 are induced by oxidants.
Box 3 NRF2–EpRE signalling
Nuclear factor E2-related factor (NRF2) is a member of the ‘cap‘n’collar’ family of bZIP transcription factors (CNC-bZIP). It was first identified as a transcription factor regulating the expression of β-globin by Moi et al. in 1994 (ref. 283 ), and soon after was found to be a transcription activator of NQO1 that bound to the antioxidant response element (ARE) in the promoter 284 . Many detailed studies established that NRF2–ARE signalling has a central role in the regulation of antioxidant gene expression 285 . ARE, the cis element of NRF2 binding, is more accurately called the electrophile response element (EpRE) as the ‘antioxidant’ inducers are electrophiles and include hydrogen peroxide (H 2 O 2 ), some components of intermediary metabolism and products derived from dietary polyphenols 6 .
NRF2–EpRE signalling regulates the basal and inducible expression of more than 200 genes that encode proteins involved in antioxidant defence, detoxification, apoptosis, DNA repair, removal of oxidized protein by the proteasome, inflammation and other processes 102 , 286 , 287 . The role of NRF2 in the induction of antioxidant enzymes and defence against oxidative stress has been verified in cell and non-human animal models with NRF2 knockout and/or induction. Mounting evidence suggests that deficiency of NRF2 signalling suppresses the induction of target antioxidant enzymes in response to oxidative stress, increases susceptibility to oxidative damage 288 and accelerates the inflammatory response 289 , whereas enhancing NRF2 activity increases the expression of antioxidant enzymes and the defence against oxidative stress.
The molecular mechanism and regulation of NRF2 activation in response to oxidative stress has been discussed in many recent articles. Most relevant to therapeutics is the recent review by Cuadrado et al. 99 . Thus, we only briefly describe the regulation of NRF2 (Fig. 3 ). Under basal conditions, most NRF2 protein binds to Kelch-like ECH-associated protein 1 (KEAP1) and/or β-transducin repeat-containing protein (βTrCP) and is rapidly degraded by 26S proteasome after ubiquitylation. KEAP1 is an adaptor for Cullin 3-containing ubiquitin ligase E3 complex 290 , and βTrCP is a substrate receptor for Cul1-based ubiquitin ligase 291 . In response to oxidative stimuli, KEAP1 is oxidatively modified and loses the capacity to present NRF2 for degradation. Simultaneously, oxidative inhibition of glycogen synthase kinase 3β (GSK3β)-mediated NRF2 phosphorylation at the Neh6 domain stops the interaction of NRF2 and βTrCP. NRF2 can also be activated through p62-mediated autophagic degradation of KEAP1 (ref. 292 ). With the activation of these pathways, NRF2, both dissociated from KEAP1 and newly synthesized, escapes from degradation and is then translocated into the nucleus where it forms heterodimers with small Maf or Jun family proteins, binds to EpRE in the promoter and increases transcription of target genes. In the nucleus, NRF2 is competitively suppressed by BACH1 (ref. 293 ). ChIP-seq assays identified a considerable overlap of BACH1 (in HEK293 cells) and NRF2 (in mouse MEF cells) target genes 294 . Evidence suggests that the suppressive effect of BACH1 on NRF2 signalling may be gene selective. BACH1 inactivation is required for the induction of HO1 but not for that of thioredoxin reductase 1, even though both genes are regulated by NRF2 (ref. 295 ). In Bach1 -knockout mice, fewer than 10% of the upregulated genes are NRF2 target genes 244 . It should be noted that NRF2 regulation is far more complicated than the simplified pathways, as nuclear factor-κB (NF-κB), PKC, p21, BRCA1, HRD1, CRIF1 and microRNAs are involved in regulating NRF2 signalling by acting on NRF2 expression, protein stability, activation and translocation 99 .
With more regulators and interaction pathways being identified, NRF2 activity is clearly regulated by a network of signalling pathways allowing it to hold important roles in multiple biological processes and response to multiple circumstances. Some puzzles remain for NRF2 regulation, including how NRF2 is transported in and out of the nucleus, and the dysregulation and ceiling effect of NRF2 induction under some pathophysiological conditions.
Antioxidant therapeutic strategies
Multiple antioxidant therapeutic strategies are being explored, some of which are currently undergoing clinical trials. These include removal of O 2 • − before it can react with •NO to form ONOO − (reaction 11) and removal of H 2 O 2 before it can form •OH (reaction 7) or HOX (reaction 13); increasing GSH using precursors; increasing the synthesis of antioxidant enzymes, particularly through NRF2 activation (Box 3 ); inhibition of NOXs (reaction 2); mitochondrial antioxidant defence; supplementing dietary antioxidants; and finally, inhibition of aberrant redox signalling (Box 2 ). See Box 1 for reactions.
SOD and SOD–catalase mimics
Several antioxidant enzyme mimics have been and are currently in clinical trials (Table 1 ). SOD is the only enzyme that can eliminate O 2 • − in mammalian cells and is a key component in defence against oxidative stress and in preserving •NO. The therapeutic potential of SOD has therefore generated interest since its discovery in 1969 (ref. 105 ), and many SOD mimetics have since been developed. These mimetics include the metalloporphyrins, Mn cyclic polyamines, nitroxides, Mn–salen complexes and fullerenes, and their chemical properties have previously been well summarized 106 , 107 .The early studies on SOD mimics primarily focused on metalloporphyrins (that is, MnTM-4-PyP 5+ and FeTM-4-PyP 5+ ) 108 , 109 , 110 , and since the establishment of the structure–activity relationship between metal-site redox ability and SOD activity in the late 1990s 111 , more porphyrins or porphyrin-related mimics with higher SOD activity have been developed. The protective effects of many of these compounds have been demonstrated in non-human animal studies or even clinical trials. Mimics of SOD and catalase have rate constants several orders of magnitude lower than the enzymes. Thus, when they enter cells, their contribution to cytosolic antioxidant defence is relatively minor. However, SOD and catalase mimics appear to be effective in extracellular spaces where the concentrations of antioxidant enzymes and substrates are very low or absent (Fig. 1 ). Some of the mimics may also be effective in the mitochondrial matrix, but they can act as pro-oxidants instead of as protectors of mitochondrial function 112 .
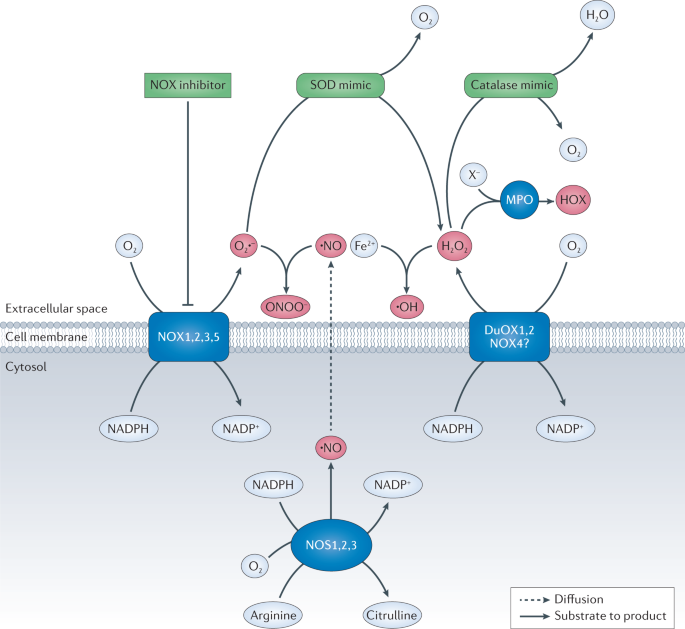
Plasma membrane NADPH oxidase (NOX) production of superoxide (O 2 • − ) outside cells may be prevented by NOX inhibitors. Dismutation of O 2 • − to hydrogen peroxide (H 2 O 2 ) is accelerated by superoxide dismutase (SOD) mimics, preventing the formation of peroxynitrite (ONOO − ), which spares nitric oxide (•NO). Reduction of H 2 O 2 is accelerated by catalase mimics, preventing the formation of hypohalous acids (HOX) by myeloperoxidase (MPO) and hydroxyl radical (•OH) production via the Fenton reaction. Most SOD mimics appear to have catalase activity. Although NOX4, which is primarily in intracellular organelle membranes, has also been found in the plasma membrane, this has only been reported for one cell type 275 and so its extracellular location remains debatable (indicated by the question mark). NOS, nitric oxide synthase.
Although being developed to remove O 2 • − specifically, most SOD mimics are not specific and can also reduce other reactive oxygen or nitrogen species such as ONOO − , peroxyl radical, H 2 O 2 and CO 3 • − (refs 113 , 114 ). In addition, some SOD mimics, such as Mn porphyrins, Mn( ii ) cyclic polyamines and M40403, can act as pro-oxidants and react with thiols 112 , ascorbate 115 and tetrahydrobiopterin 116 , thereby affecting redox-sensitive signalling pathways and cellular transcription 117 , 118 . Therefore, some protective effects of SOD mimics might be attributable to activities other than mimicking SOD.
SOD itself was first developed as a drug called orgotein in the late 1970s, but it has not been approved for human use 119 . However, several clinical trials based on the anti-inflammatory property of orgotein have been conducted. A double-blind, placebo-controlled study has demonstrated that orgotein can be used safely and effectively to ameliorate or prevent the side effects of radiation therapy in patients with bladder cancer, such as the incidence of radio-induced acute cystitis and rectitis 120 , 121 . However, in another clinical trial, orgotein showed no beneficial effect on radiation response or the acute radiation reactions, and caused side effects such as marked subcutaneous infiltration and redness at local injection site in some patients 122 . Currently orgotein is used as an anti-inflammatory agent in non-human animals.
The best-studied class of SOD mimics is probably the Mn porphyrins. Various Mn porphyrin compounds have been synthesized and evaluated for their O 2 • − dismutation activity 114 . Some of them, such as MnTM-2-pYp 5+ and MnTE-2-pYp 5+ , showed very high SOD activity. Although whether the underlying mechanism is via SOD-like activity or another action (for example, pro-oxidant activity) remains elusive in some cases, the protective and therapeutic effects of many Mn porphyrins such as MnTE-2-pYp 5+ and MnTDE-2-ImP 5+ have been demonstrated in non-human animal models of diseases, including stroke 123 , radiation injuries 124 , cancers 125 , 126 , diabetes 127 and cardiovascular system damage 128 . These preclinical results suggest the potential of Mn porphyrins in the clinical therapy of diseases in which oxidative stress plays a significant part. Currently, a phase I clinical trial of MnTDE-2-ImP 5+ in patients with amyotrophic lateral sclerosis showed no toxicity at therapeutic doses 129 .
Another promising SOD mimetic is GC4419, a novel, highly stable Mn( ii )-containing penta-azamacrocyclic. GC4419 selectively removes superoxide anions without reacting with other oxidants 130 . In vitro, GC4419 significantly enhanced the toxicity of AscH − to kill cancer cells 131 . In addition, GC4419 has exhibited therapeutic effects in several non-human animal models of inflammation 132 , joint disease 133 and myocardial IRI 134 . A recent phase I clinical trial in severe oral mucositis of oropharyngeal cancer with radiation and chemotherapy indicates that the safety of GC4419 in patients is acceptable 135 .
Salens, aromatic, substituted ethylenediamine metal complexes, represent an emerging class of SOD mimics. The Mn( iii )-containing salen complexes have both O 2 • − and H 2 O 2 dismutation activity 136 . Salen compounds are not selective and can also react with other peroxides and ONOO − . The typical representative salens are EUK-8, EUK-134 and EUK-189, which have been shown to be protective in many non-human animal models of human diseases, including sepsis 137 , heart ischaemia–reperfusion 138 , cardiomyopathy 139 , haemorrhage 140 and amyotrophic lateral sclerosis 141 (EUK-8); IRI 142 and stroke 143 (EUK-134); radiation lung fibrosis 144 , cognitive impairment 145 , diaphragm muscle weakness in monocrotalin-induced pulmonary hypertension 146 and hyperthermia 147 (EUK-189). However, no human clinical trial for salens has yet been reported.
Glutathione peroxidase mimics
A variety of mimics of GPXs have been developed 148 . Among these mimetics, the selenoorganic compound ebselen (2-phenyl-1,2-benzisoselenazol-3(2H)-one) is best known, with its broad specificity for substrates from H 2 O 2 and smaller organic hydroperoxides to membrane-bound phospholipid and cholesterol hydroperoxides 149 . Ebselen may also induce phase II detoxification enzymes 150 . In non-human animal studies, ebselen has been shown to reduce oxidative damage 150 , prevent the acute loss of outer hair cells and reduce hearing loss 151 , and decrease inflammation 152 . Accordingly, several clinical trials have been conducted in diseases including Meniere disease (phase III, NCT04677972), bipolar disorder 153 , complete occlusion of the middle cerebral artery 154 , delayed neurological deficits after aneurysmal subarachnoid haemorrhage 155 and acute ischaemic stroke 156 . In these studies, oral administration of ebselen was well tolerated, exerted therapeutic effects and displayed favourable bioavailability.
ALT-2074 (BXT-51072) is a newer analogue of ebselen, displaying increased GPX activity and potency. In vitro, ALT-2074 inhibited the inflammatory response in endothelial cells 157 , reduced oxidative damage and prevented neuronal death 158 , and in a mouse model of heart ischaemia–reperfusion it reduced infarct size 159 . A phase II clinical trial of ALT-2074 (NCT00491543) in diabetes and coronary artery disease has been completed but data are not yet available. Another clinical trial on psoriasis (NCT00782613) was terminated but the reasons for this remain unknown.
Chelation of iron
It has long been recognized that when iron and copper are released from proteins, they can participate in •OH production, and that some chelators enhance that activity while others inhibit it 160 . In principle, using the inhibitory chelators would be an excellent strategy to prevent •OH production; however, as iron is essential for many biological activities, chelation therapy is generally restricted to the prevention of iron overload in patients with sickle cell disease and thalassaemia, who require frequent transfusions 161 .
Increasing GSH
Although most cells have a concentration of GSH in the millimolar range, GSH is often significantly decreased by oxidative stress. Thus, approaches to maintaining or replenishing GSH using GSH esters or agents that provide its precursor, cysteine, the limiting amino acid in GSH synthesis, have shown effectiveness in various diseases.
N -acetylcysteine
N -acetylcysteine (NAC) is one of the most studied antioxidant agents for therapeutic treatment (Table 1 ). It is water soluble and quickly absorbed primarily via the anion exchange protein on the cell membrane 162 . NAC in cells is deacetylated to produce cysteine. Evidence indicates that the antioxidant function of NAC is primarily mediated via replenishing GSH 163 . NAC can also reduce cysteine conjugates in plasma 162 . NAC has been used therapeutically for the treatment of many pathologies, including liver paracetamol (also known as acetaminophen) toxicity 164 , cystic fibrosis, where it is delivered through the airways 165 and nephropathy 166 . In non-human animal studies and clinical trials, NAC is being investigated for prevention or treatment of many other diseases and conditions. The results from these studies are conflicting and a consensus has yet to be reached. Failure of NAC to exert a therapeutic effect may be due to oxidative stress being a secondary contributor to the disease being studied.
GSH itself is not effectively transported into most cells, and exogenously administered GSH is rapidly degraded in plasma 167 . Thus, using derivatives of GSH is a strategy for more successful delivery. Ester derivatives of GSH, including monomethyl (GSH-OMe), monoethyl (GSH-MEE), diethyl (GSH-DEE) and isopropyl esters have been synthesized and evaluated for the efficiency of GSH supplementation. In GSH-MEE, the carboxyl group of the glycine residue is esterified (Glu-Cys-Gly-OEt); whereas in GSH-DEE both glutamate and glycine residues are esterified (tEO-Glu-Cys-Gly-OEt). GSH esters are lipophilic, more efficiently transported across the cellular membrane and resistant to degradation by γ-glutamyl transpeptidase in plasma 168 . Once inside cells, GSH esters are rapidly hydrolysed by nonspecific esterases and form GSH. The transport of GSH-DEE into cells seems more efficient than that of the monoester 169 , and human cells can rapidly convert the diethyl ester into the monoester, which is hydrolysed into GSH.
The high efficiency of GSH esters to increase cell and/or tissue GSH has been evidenced in many studies in cell and non-human animal models 170 , 171 , 172 , 173 , 174 , 175 . Subcutaneous or intraperitoneal injection of GSH esters into animals was able to increase GSH levels in various tissues including liver 170 , kidney 170 , spleen, pancreas and heart 176 , but not brain 177 . Brain GSH levels can be increased via intracerebroventricular 174 delivery of GSH-MEE 177 . Although oral administration could also increase tissue GSH levels, this is less effective 176 .
The relative efficacy of various GSH esters to increase tissue GSH remains unclear owing to limited evidence. Some cell culture-based studies suggest that GSH-DEE is more effective than GSH-MEE in increasing GSH levels 169 . GSH-DEE is metabolized differently in the plasma of non-human animals and humans. In mouse and rat, plasma GSH-DEE is rapidly converted into GSH-MEE by plasma α-esterase, whereas human (and many other species including hamster, guinea pig, rabbit and sheep) plasma has no α-esterase activity, meaning that GSH-DEE can be transported into tissues more efficiently than GSH-MEE 169 . However, no direct comparison study has been conducted on the relative efficacy of the different GSH esters in clinical settings. Although the reports above suggest that humans have apparently been treated with GSH without adverse effects, and the efficacy of GSH esters to increase GSH levels and alleviate oxidative damage in cells and non-human animals has been demonstrated, no clinical trials have been reported with any GSH ester. Figure 2 summarizes the strategies for maintaining GSH in cells.
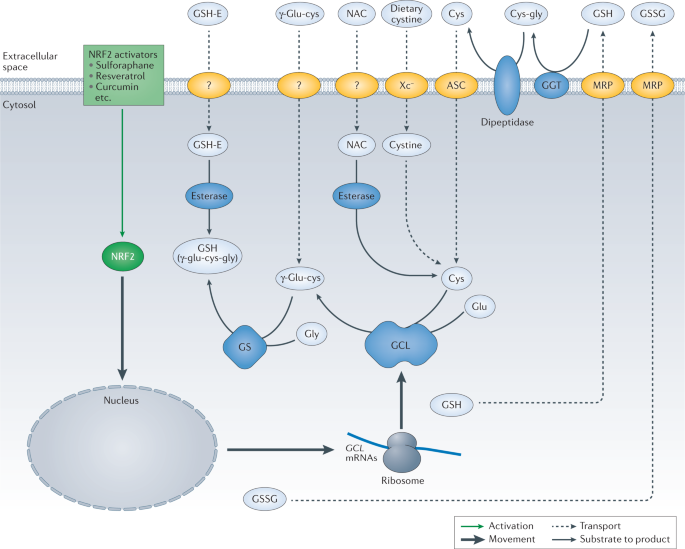
Glutathione (GSH) is synthesized through reactions catalysed by glutamate–cysteine ligase (GCL) and GSH synthetase (GS), with GCL as the rate-limiting enzyme and cysteine as the rate-limiting substrate. Both reduced GSH and glutathione disulfide (GSSG) are exported from cells through multidrug resistance protein (MRP), and extracellular GSH is sequentially metabolized by membrane-bound γ-glutamyl transpeptidase (GGT) into cysteinylglycine and γ-glutamyl products, and dipeptidase hydrolyses cysteinylglycine to cysteine and glycine. The amino acids are transported back into cells and participate in GSH synthesis. N -acetylcysteine (NAC) is deacetylated by esterase action into cysteine, while GSH esters (GSH-E) are directly converted by esterase into GSH. γ-Glutamylcysteine (γ-glu-cys) can bypass GCL, the rate-limiting step for GSH synthesis. Electrophiles cause the activation of NRF2, which regulates the transcription of the two subunits of GCL, and also GS. Some transporters have been identified: ASC, sodium-dependent alanine-serine-cysteine transporter; Xc − , system cystine/glutamate antiporter. Question marks denote the unidentified transporters/channels for GSH-E, γ-glu-cys and NAC.
NRF2 activators
Dysregulation of NRF2 signalling (Box 3 ; Fig. 3 ) is implicated in many oxidative stress-related diseases including cardiovascular diseases 178 , neurodegenerative disorders 179 and pulmonary diseases 180 . Therefore, NRF2 activators are regarded as potential agents to induce antioxidant capacity and alleviate pathology. The induction of antioxidant enzymes, particularly through NRF2, is a major way in which antioxidant therapy is being developed. Indeed, when the small molecules such as polyphenols are effective, they act primarily through antioxidant enzyme induction mediated by NRF2 signalling 6 . NRF2 activators comprise five categories, according to their mechanisms of action (Fig. 3 ): modification of Kelch-like ECH-associated protein 1 (KEAP1; regulates proteasomal degradation of NRF2), which is inactivated when its sensor cysteines form adducts with electrophiles or when they are oxidized to disulfides; disruption of the interaction between β-transducin repeat-containing protein (βTrCP; ubiquitylates NRF2 for degradation) and NRF2, via oxidative inhibition of the axis of glycogen synthase kinase 3β (GSK3β)–NRF2 phosphorylation at the Neh6 domain–βTrCP; KEAP1 sequestration by p62; de novo synthesis of NRF2 that escapes degradation by inactivated KEAP1 (ref. 181 ); and BACH1 inhibitors that reduce NRF2 suppression by BACH1, including agents that inhibit BACH1 translation 182 and promote BACH1 degradation 183 .
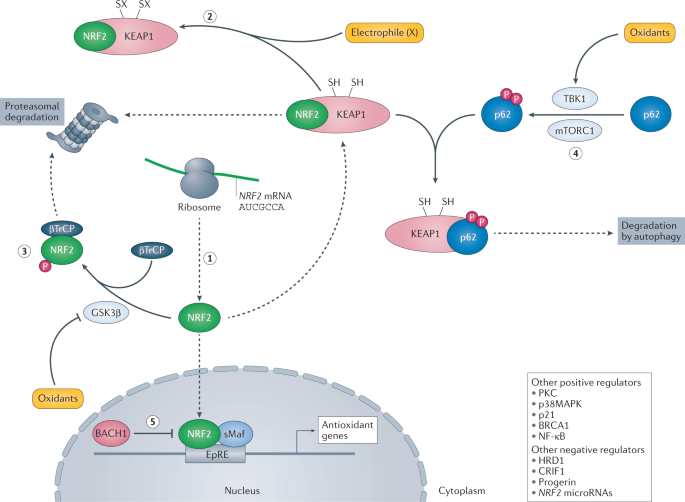
(1) Transcription factor NRF2 is constantly synthesized in cells but its transport to the nucleus remains low under basal conditions. This is due to its degradation through association with Kelch-like ECH-associated protein 1 (KEAP1), which facilitates its degradation by the 26S proteasome. Boosting NRF2 synthesis represents a therapeutic antioxidant approach. (2) Upon exposure to electrophiles, KEAP1 is alkylated and loses its ability to cause degradation of NRF2. Using non-toxic electrophiles to alkylate KEAP1 represents another major therapeutic approach. For KEAP1, SH is the thiol form and SX denotes the adduct formed with the electrophile (X). (3) In a parallel pathway glycogen synthase kinase 3β (GSK3β) phosphorylates NRF2, which with β-transducin repeat-containing protein (βTrCP) is degraded by the proteasome, a process that is inhibited by oxidative inactivation of GSK3β. The interaction of NRF2 and βTrCP is disrupted owing to oxidant-mediated inhibition of GSK3β and the phosphorylation of NRF2 at the Deh6 domain. Inhibiting GSK3β is another potential therapeutic approach to modulate NRF2 signalling. (4) Oxidation-induced KEAP1 degradation also occurs through p62-mediated sequestration of KEAP1 and autophagy, a process initiated by phosphorylation of p62 via TANK-binding kinase 1 (TBK1) and mechanistic target of rapamycin complex 1 (mTORC1). p62 therefore provides another potential therapeutic target. Newly synthesized NRF2 that escapes degradation is translocated into the nucleus where it binds to EpRE sequences in the promoters of antioxidant genes and increases their expression. NRF2 activity is also positively regulated through NRF2 phosphorylation by protein kinase C (PKC) 269 and its interaction with other proteins such as p21 (ref. 270 ) and BRCA1 (ref. 271 ). (5) In the nucleus, BACH1 negatively regulates NRF2 activity by competing to form heterodimers with small Maf (sMaf) or Jun proteins and binding to the electrophile response element (EpRE) 272 , 273 , 274 . Thus, compounds that inhibit BACH1 offer an alternative therapeutic approach for increasing expression of some NRF2-regulated genes. Other negative regulators of NRF2, which represent potential therapeutic targets include HRD1, CRIF1, progerin and microRNA for NRF2 (ref. 99 ).
Extracts from tea, cocoa and many dietary vegetables and fruits including broccoli, broccoli sprouts, grape seeds and turmeric can activate NRF2 signalling and induce antioxidant enzymes 184 , 185 , and some of these are in clinical trials for disease treatment and/or prevention. For example, 11 clinical trials for turmeric extract and 55 clinical trials for broccoli or broccoli sprout supplement have been completed or are in an active phase for various conditions including COPD, osteoarthritis, joint stiffness and diabetic nephropathy ( www.clinicaltrials.gov ). Yagishita et al. 186 summarized the current progress on broccoli/broccoli sprout including the formulation, bioavailability, efficacy and doses for clinical trials. In general, some beneficial effects, including a boost of antioxidant capacity, were observed in the clinical trials, but more effort is required to develop and validate biomarkers of pharmacodynamic action in humans. As pointed out above, an increase in antioxidant defence may be limited in disease treatment or prevention if oxidative stress has only a secondary role in the pathology. The underlying mechanism of the antioxidant properties of these dietary supplements, often the coumarins and polyphenols present in vegetables and fruits, relies upon their oxidation to electrophilic quinones that form adducts with KEAP1 cysteines 6 .
The effectiveness of many of these NRF2 activators in inducing antioxidant enzymes and in alleviating oxidative damage has been confirmed in non-human animal studies, and there have been significant advances in drug development based on the mechanism of NRF2 activation and antioxidant induction. Several dietary NRF2 activators, including curcumin, sulforaphane and resveratrol, have been developed as daily supplements, while some NRF2 activators are in clinical trials for disease treatment 187 . Selected electrophilic NRF2 activators and the related clinical trials have previously been summarized 187 . It is noted that these NRF2 activators may have multiple functions such as anti-inflammatory effects 188 , 189 , 190 , some of which are not dependent on NRF2 activation. Table 2 lists the total number of clinical trials of selected dietary NRF2 activators and indicates those that are based on NRF2 activation and/or antioxidant potential. For clarification, it is still possible that some of the agents for which a study of NRF2 activation is not indicated do in fact activate NRF2 even though that was not examined.
Challenges facing therapeutic NRF2 activation
There are several concerns and challenges associated with the therapeutic use of NRF2 activators 191 , 192 . The first is related to low effective biological concentration, as most NRF2 activators are electrophilic and are metabolized quickly so that their bioavailability in distal organs may be low. However, some evidence suggests that the Michael adducts of nucleophiles (including the cysteines of KEAP1) with some electrophiles, such as cyanoenones, are reversible 193 and this may significantly increase the bioavailability and concentration of these electrophiles in vivo. This concept was demonstrated by a synthesized cyanoenone compound TBE31 that had a 10-h half-life in the blood 194 and markedly increased NRF2 activity in vivo at nanomolar concentrations 195 . It remains unclear whether this reversibility of the covalent adducts also occurs with other electrophiles, especially natural compounds such as sulforaphane and curcumin. In addition, there is controversy regarding the effectiveness of oral sulforaphane to induce antioxidant expression in clinical trials, with both increased antioxidant expression 196 and no effect 197 being reported. In general, more clinical trial data on NRF2 and antioxidant induction in target organs are needed to further assess the efficacy of these NRF2 activators.
Another key concern is the risk of nonspecific effects. Besides activating NRF2 and inducing antioxidant enzymes, some NRF2 activators may act on other signalling pathways and disrupt related biological processes. For example, sulforaphane can suppress the inflammatory response through inhibition of NF-κB 188 and inflammasome activation 198 , and cause cell cycle arrest by inhibiting the PI3K–AKT and MAPK–ERK pathways 199 . Most of these nonspecific effects have been investigated in in vitro cell studies with >10 μM sulforaphane, a concentration that is less likely to be reached in vivo. Understanding the NRF2-independent effects is important in elucidating the mechanism of the beneficial and therapeutic effects, although for most NRF2 activators this has not been thoroughly studied, especially with regard to their in vivo dose dependency.
Another aspect of nonspecificity is that the effect on NRF2 activation and antioxidant induction is not restricted to a specific cell or organ, and may therefore result in systemic side effects. For example, some evidence suggests that although NRF2 activation could prevent the initiation of cancer, it can, however, promote cancer development 200 , 201 , 202 . Cell studies showed that higher NRF2 activity and antioxidant capacity can also contribute to the resistance to chemotherapeutic drugs 203 , 204 , 205 , 206 , as reviewed by others 207 , 208 , 209 . Current evidence is insufficient to draw a definitive conclusion and more systemic in vivo studies are needed to elucidate the role of NRF2 in promoting carcinogenesis and causing resistance to chemotherapies. If increased NRF2 activity does promote tumour growth and/or increase chemoresistance, the systemic administration of NRF2 activators should be avoided, at least in susceptible subjects including cancer patients under chemotherapy. Other side effects of long-term NRF2 activation are less reported. Several strategies have been proposed to avoid systemic side effects, including the development of non-electrophilic drugs and drugs that only become active in loci that exhibit oxidative stress 192 .
NADPH oxidase inhibition
NOXs are important in redox signalling as the source of O 2 • − and H 2 O 2 and in the killing of microorganisms, but excessive activation of NOXs can result in damage to normal tissue. There are two types of agent that inhibit NOXs, those that inhibit the enzymatic activity and those that prevent the assembly of the NOX2 enzyme, which is a multiprotein complex. Of the first type, diphenyleneiodonium (DPI) is commonly used in research studies but is a nonspecific inhibitor of flavoproteins as well as an inhibitor of iodide transport 210 . Several agents claimed to be NOX inhibitors, including ebselen, CYR5099, apocynin and GKT137831, some of which show promise in non-human animal models and clinical trials, exhibited effects that were not due to NOX inhibition 211 . Nonetheless, the potential value of inhibition of NOX1, NOX2 and NOX4 has been demonstrated in non-human animal models using genetic deletion 212 , and a search for low-molecular-weight NOX inhibitors continues.
Small peptides that inhibit the assembly of the NOX complexes have therapeutic potential 213 . Although these small peptides would be more specific to the different NOXs than active site inhibitors, none has advanced to clinical trials. A third potential approach is interference with the synthesis of the components of the NOX complexes; however, this too has not yet reached clinical trials.
Mitochondrial antioxidant defence
Leaks of electrons from the respiratory chain results in the production of O 2 • − . Although inhibiting O 2 • − production by either elevating uncoupling proteins or inhibiting the flow of electrons into the chain is possible, the consequences for ATP production make these approaches difficult. Yet, this strategy has been proposed for preventing hyperglycaemic damage in diabetes 214 . One drug, OP2113, which can be used in humans, has been proposed as a specific inhibitor of complex I O 2 • − production that does not interfere with ATP production 215 . However, this agent has not yet been investigated in clinical trials.
As discussed above, increasing SOD2 increases the production of H 2 O 2 in mitochondria by pulling reaction 1 (QH • − + O 2 ↔ Q + O 2 • − ) (Box 1 ) forward by dismutation of O 2 • − . Thus, SOD mimics that enter mitochondria would be expected to increase the rate of production of H 2 O 2 . However, as these agents also possess catalase activity, they appear to add protection 216 , likely by preventing formation of OONO − and protecting iron–sulfur proteins. Ebselen can also enter mitochondria but may produce unexpected toxicity 217 .
The large negative inner mitochondrial membrane potential makes it possible to target antioxidants and antioxidant mimics to these organelles by attaching a lipophilic cation to them 218 . This is an area of research that is still under development but basically uses the same principles of antioxidant defence as described in other sections of this Review.
Dietary antioxidants
The most widely used and studied dietary antioxidants are l -ascorbic acid (vitamin C) and α-tocopherol (vitamin E). Other dietary nutrients, including selenium, riboflavin and metals, are essential cofactors for antioxidant enzymes, and their adequate supply is essential for the inducers of these enzymes to reach their most effective levels, but discussion of them here is beyond the scope of this Review. Vitamin C is a water-soluble vitamin that cannot be synthesized by the human body and must be provided as an essential dietary component. Vitamin C is required for the biosynthesis of collagen, protein and several other biological molecules 219 . Vitamin C is also an important antioxidant 220 , by providing an electron to neutralize free radicals. Vitamin E, which is lipid soluble, localizes to the plasma membrane and has roles in many biological processes. Almost 100 years after its discovery, the functions and mechanism of action of vitamin E still remain of great interest. Nonetheless, the importance of the antioxidant function of vitamin E has been demonstrated by many studies 221 , 222 , 223 , especially under conditions of oxidative stress or deficiency of other antioxidants 223 , 224 . Vitamin E reduces peroxyl radicals and forms tocopheroxyl radical, which is subsequently reduced by vitamin C. Thus, vitamin E helps to maintain the integrity of long-chain polyunsaturated fatty acids in the membranes and thereby regulates the bioactivity and signalling related to membrane lipids.
For healthy individuals, sufficient levels of vitamins C and E are provided by normal dietary intake and deficiency rarely occurs. Under some extreme conditions such as malnutrition or imbalanced nutrition and diseases 225 , 226 , however, dietary supplementation of vitamins C and E is necessary. As vitamins C and E function as antioxidants, there has been great interest in investigating their therapeutic potential. Many studies and clinical trials have found that vitamins C and E have beneficial effects in reducing various diseases, many of which likely involve oxidative stress, including cancers, cardiovascular diseases and cataracts 227 . But the evidence is inconsistent, as an almost equal number of studies show no significant effect. It was assumed that both vitamin C and vitamin E have low toxicity and were not believed to cause serious adverse effects at much higher intake than needed for their function as vitamins. However, several non-human animal studies showed that antioxidant supplements, including NAC, vitamin E and the soluble vitamin E analogue Trolox, promoted cancer development and metastasis, for example, lung, melanoma and intestinal tumours in mouse models 228 , 229 , 230 . The potential effect of antioxidants on cancer promotion, including the aforementioned NRF2 activators, raises significant concerns regarding the use of antioxidant supplements, and novel strategies are needed to resolve the double-edged effect of antioxidants.
Inhibition of aberrant redox signalling
In the early years of research in redox biology the emphasis was almost entirely on damage caused by oxidants. Although studies demonstrated that the addition of non-lethal doses of H 2 O 2 or other oxidants was able to stimulate signalling pathways, it was not until the mid-1990s that NF-κB activation by endogenous generation of H 2 O 2 was first observed 231 . By the late 1990s, Lambeth and coworkers 232 had described the seven-member NOX family and began to implicate them in cell signalling pathways. Redox signalling is now the major focus of the field, although extensive coverage of the topic is beyond the scope of this article. Readers are referred to specific reviews in this area 4 , 233 . Nonetheless, as described earlier, H 2 O 2 is the major second messenger in redox signalling and like other second messengers, dysregulation of its production can result in aberrant signalling 233 . Prevention of dysregulation is tricky because attempts to inhibit the generation of oxidants by NOX proteins or mitochondria, as described in earlier sections, may interfere with physiologically important signalling including the regulation of leukotriene and prostaglandin production, which require a low level of H 2 O 2 or lipid hydroperoxides 234 .
A more successful approach may be interference with specific redox signalling that is initiated by toxic stimuli. Here, we provide one example to illustrate this approach 235 . Air pollution contains particles of enormously variable composition and includes silicates with iron on their surface. Activation of NF-κB signalling in macrophages by these particles could be inhibited with a SOD and/or catalase mimic, but also by interfering in the signalling pathway initiated by the iron-mediated lipid peroxidation that caused lipid raft disruption and signalling through phosphocholine-specific phospholipase C (PC-PLC) activation. An inhibitor of that enzyme, tricyclodecan-9-yl xanthate (D609), which was unsuccessfully tried as an anticancer agent, stopped particle-induced NF-κB-dependent cytokine production. D609 is an example of an agent that is not an antioxidant but inhibits oxidant-induced aberrant signalling. Interestingly, D609 interferes with the PC-PLC pathway when initiated by endotoxin 236 , which does not involve redox signalling. There are countless agents that have similar potential to inhibit aberrant signalling although they are not specific to redox-mediated signalling.
Challenges and limitations in targeting oxidative stress
Oxidative stress is a component of the underlying pathology of many diseases and toxicities, and the antioxidant defences and strategies that have been presented above offer some important opportunities for preventing or reducing pathology. Nonetheless, there are several limitations that challenge our ability to therapeutically apply antioxidant strategies.
Pathological role of oxidative stress
The effectiveness of antioxidant defences is limited by the extent to which oxidative stress plays a role in the pathology. When oxidative stress is a secondary contributor to disease, which is more often the case than it being the primary cause, preventing oxidative stress may not have a major impact on disease progression. Indeed, this is one of the major causes of antioxidants exerting little to no effect on pathology, even when they clearly increase antioxidant defence and decrease markers of oxidative stress. This limitation is perhaps the most significant factor that is often overlooked when considering antioxidant defences in clinical trials. The challenge here is to determine to what extent antioxidant strategies may be developed to ameliorate some symptoms if not the underlying cause of the disease. The commercialization of products containing small molecules that are chemical antioxidants but do not function as such in vivo, will ultimately fail to show significant benefit beyond what the antioxidant enzyme-inducing small molecules present in an adequate diet can achieve. This disappointment will add to the challenge of developing and gaining public acceptance of truly effective therapeutics.
Scavenging by small molecules
The negligible effect of scavenging by small molecules represents a key limitation in antioxidant defence. The claim that an antioxidant is a •OH scavenger is meaningless, as almost all molecules react with •OH at about the same rate. Thus, the only defence against •OH is to prevent its formation, and the most effective way to achieve that is H 2 O 2 elimination. For O 2 • − , scavenging inside the cell is in competition with the already ubiquitous and high activity of SOD, which catalyses reaction 3 (2O 2 • − + 2H + → H 2 O 2 + O 2 ) (Box 1 ), with a rate constant that is at least 10 5 times higher than most of the reactions of O 2 • − except that with •NO 237 . Similarly, the presence of the 15 enzymes that remove H 2 O 2 in reactions 4–6 (2H 2 O 2 → 2H 2 O + O 2 ; H 2 O 2 + 2Trx(SH) 2 → TrxS 2 + 2H 2 O; H 2 O 2 + 2GSH → GSSG + 2H 2 O) (Box 1 ) would outcompete most agents that are used intracellularly. Thus, kinetic considerations essentially rule out scavenging as an effective antioxidant defence within cells 6 . However, outside cells, SOD and catalase mimics that have relatively high kinetic rate constants compared with non-enzymatic reactions of O 2 • − and H 2 O 2 may be effective. Although not as efficient as the endogenous SOD and catalase, the rate constants for the mimics are approximately 10 5 times higher than those of most protein cysteines. SOD mimics can accumulate at high concentrations in the mitochondrial matrix by attachment of a lipophilic cationic group and can be effective in that microenvironment 106 , where it has been demonstrated that the overexpression of endogenous SOD2 increases H 2 O 2 production 238 . However, the long-term effects of the non-physiological increase in mitochondrial SOD activity is unknown.
Vitamin E is the one exception to the limitation of small molecule scavenging by dietary antioxidants because of its relatively rapid rate of reaction with lipid hydroperoxyl radicals as well as its concentration in membranes. Nonetheless, antioxidant therapies that appeared to work in cell culture or in non-human animal models have often failed to achieve significant effects in human trials. A primary reason for this discrepancy is the enormous difference in the ratio of exogenous agents in vitro versus in vivo 6 . In non-human animal models, lab chow is deficient in vitamin E and selenium 239 , which sets up a system in which antioxidants work by restoring redox homeostasis, thereby acting more like vitamins preventing a deficiency than like a drug. Interestingly, mito-Q, made by the attachment of a lipophilic cationic group to ubiquinone, can accumulate in mitochondria and act in a similar manner to vitamin E in that domain 240 . However, the long-term effects of the non-physiological increase in ubiquinone is not yet understood.
Achieving effective in vivo concentrations
Another concern is that compounds that induce antioxidant defences may not be able to reach effective concentrations in vivo, although this may be overcome with cyanoenones 194 . When adequate levels of NRF2 activators are supplied by good nutrition, supplemental NRF2 activators would not provide an advantage. In addition, if oxidative stress occurs in patients, NRF2 is usually already activated to a certain degree and the potential for further induction is limited. As a good diet would be expected for patients in clinical trials, and oxidative stress is frequently seen in patients, the lack of an increase in protection may be due to the existing effects of dietary NRF2 inducers and a lower potential for NRF2 activation. Perhaps the use of NRF2 activators should therefore be considered as similar to that of vitamins that are inadequate in the diet of a significant number of individuals and in patients who have difficulty consuming food.
As we age, the ability of electrophiles to induce NRF2-dependent expression of antioxidant enzymes declines 241 . Silencing BACH1 reverses this effect in human primary bronchial epithelial cells for some NRF2-regulated genes 242 , suggesting that BACH1 inhibition has potential in antioxidant therapy, particularly in older patients. However, as older people exhibit an increased risk of cancer, activating NRF2 in this group may be deleterious. Although NRF2 activation has long been associated with chemoprevention 243 , a downside of NRF2 activation is the protection of cancer cells against oxidative damage, which helps cancer progression 200 , 201 , 202 . However, in mice, silencing of BACH1 does not appear to increase p53-driven tumorigenesis 244 . It is hoped that more studies will further clarify the issue of cancer promotion associated with NRF2, and that additional means of increasing antioxidant defences will be found to benefit older people without adverse effects.
As oxidative stress is a component of many diseases, the development of effective antioxidant therapies is an important goal. Although using small molecules has been largely disappointing, hope lies in the realization that the rationale underlying their use was based on misconceptions that can be overcome. Increased awareness of the fact that, although the goal of antioxidant defence must be to prevent the formation of •OH and ONOO − by decreasing their precursors H 2 O 2 and O 2 • − , H 2 O 2 is also essential in physiological signalling, will lead to more nuanced approaches to antioxidant defence. In addition, the limitations highlighted in this Review — including consideration of whether oxidative stress plays a primary or secondary role in the pathology, the negligible effect of scavenging by almost all small molecules, difficulty in achieving effective in vivo concentrations and the declining ability to increase NRF2 activation in ageing — must be considered to both avoid unnecessary disappointment and set obtainable goals.
There is promise in agents that scavenge O 2 • − and H 2 O 2 in intracellular spaces and the mitochondrial matrix. SOD, and SOD–catalase and GPX mimics, appear to be effective, with some agents currently in clinical trials. Maintaining GSH, the substrate for GPXs, can be achieved using precursors including NAC and GSH esters. Indeed, NAC is already in human use for the treatment of some toxicities and diseases, although no clinical trials of GSH esters appear to be currently active. In addition to the mimics of antioxidant enzymes and GSH, another major strategy is increasing the synthesis of the endogenous antioxidant enzymes and de novo synthesis of GSH through NRF2 signalling in cells 99 . We expect that all these approaches will contribute to advancing antioxidant therapeutics and hope that this Review will encourage and inform a rational approach to that worthwhile endeavour.
Change history
13 july 2021.
A Correction to this paper has been published: https://doi.org/10.1038/s41573-021-00267-5
Sies, H. Oxidative Stress (ed. Sies, H.) 1–8 (Academic Press, 1985). This article introduces the concept of oxidative stress .
Flohé, L. Looking back at the early stages of redox biology. Antioxidants 9 , 1254 (2020). This article provides a history of oxidative stress from a current perspective .
Article PubMed Central Google Scholar
Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 11 , 613–619 (2017). This article explains the central role of hydrogen peroxide in redox signalling .
Article CAS PubMed PubMed Central Google Scholar
Sies, H., Berndt, C. & Jones, D. P. Oxidative stress. Annu. Rev. Biochem. 86 , 715–748 (2017).
Article CAS PubMed Google Scholar
Valko, M. et al. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 39 , 44–84 (2007). This article provides a review of redox signalling in disease .
Forman, H. J., Davies, K. J. & Ursini, F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 66 , 24–35 (2014). This article provides a review of the mechanisms of nutritional antioxidants .
Sies, H. Strategies of antioxidant defense. Eur. J. Biochem. 215 , 213–219 (1993).
Forman, H. J., Maiorino, M. & Ursini, F. Signaling functions of reactive oxygen species. Biochemistry 49 , 835–842 (2010). This article explains why hydrogen peroxide is the principal redox second messenger .
Evans, J. L., Goldfine, I. D., Maddux, B. A. & Grodsky, G. M. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr. Rev. 23 , 599–622 (2002). This article examines the links between oxidative stress and type 2 diabetes mellitus .
Liochev, S. I. & Fridovich, I. The role of O2 .- in the production of HO·: in vitro and in vivo. Free Radic. Biol. Med. 16 , 29–33 (1994).
Castro, L., Tortora, V., Mansilla, S. & Radi, R. Aconitases: non-redox iron-sulfur proteins sensitive to reactive species. Acc. Chem. Res. 52 , 2609–2619 (2019).
Zhang, H. & Forman, H. J. 4-hydroxynonenal-mediated signaling and aging. Free Radic. Biol. Med. 111 , 219–225 (2017).
Giuranno, L., Ient, J., De Ruysscher, D. & Vooijs, M. A. Radiation-induced lung injury (RILI). Front. Oncol. 9 , 877 (2019).
Article PubMed PubMed Central Google Scholar
Barnes, P. J. Oxidative stress-based therapeutics in COPD. Redox Biol. 33 , 101544 (2020). This article examines the current state of redox-based therapy in COPD .
Fleckenstein, K. et al. Temporal onset of hypoxia and oxidative stress after pulmonary irradiation. Int. J. Radiat. Oncol. Biol. Phys. 68 , 196–204 (2007).
Rappold, P. M. et al. Paraquat neurotoxicity is mediated by the dopamine transporter and organic cation transporter-3. Proc. Natl Acad. Sci. USA 108 , 20766–20771 (2011).
Bus, J. S. & Gibson, J. E. Paraquat: model for oxidant-initiated toxicity. Env. Health Perspect. 55 , 37–46 (1984).
Article CAS Google Scholar
Glavind, J., Hartmann, S., Clemmesen, J., Jessen, K. E. & Dam, H. Studies on the role of lipoperoxides in human pathology. II. The presence of peroxidized lipids in the atherosclerotic aorta. Acta Pathol. Microbiol. Scand. 30 , 1–6 (1952).
Suarna, C., Dean, R. T., May, J. & Stocker, R. Human atherosclerotic plaque contains both oxidized lipids and relatively large amounts of alpha-tocopherol and ascorbate. Arterioscler. Thromb. Vasc. Biol. 15 , 1616–1624 (1995).
Salomon, R. G. et al. HNE-derived 2-pentylpyrroles are generated during oxidation of LDL, are more prevalent in blood plasma from patients with renal disease or atherosclerosis, and are present in atherosclerotic plaques. Chem. Res. Toxicol. 13 , 557–564 (2000).
Gniwotta, C., Morrow, J. D., Roberts, L. J. 2nd & Kuhn, H. Prostaglandin F2-like compounds, F2-isoprostanes, are present in increased amounts in human atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 17 , 3236–3241 (1997).
Yang, X. et al. Oxidative stress-mediated atherosclerosis: mechanisms and therapies. Front. Physiol. 8 , 600 (2017). This article examines the current state of redox-based therapy in atherosclerosis .
Pryor, W. A., Dooley, M. M. & Church, D. F. Human alpha-1-proteinase inhibitor is inactivated by exposure to sidestream cigarette smoke. Toxicol. Lett. 28 , 65–70 (1985).
Montuschi, P. et al. Exhaled 8-isoprostane as an in vivo biomarker of lung oxidative stress in patients with COPD and healthy smokers. Am. J. Respir. Crit. Care Med. 162 , 1175–1177 (2000).
Rahman, I. et al. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 166 , 490–495 (2002).
Article PubMed Google Scholar
Igishi, T. et al. Elevated urinary 8-hydroxydeoxyguanosine, a biomarker of oxidative stress, and lack of association with antioxidant vitamins in chronic obstructive pulmonary disease. Respirology 8 , 455–460 (2003).
Psathakis, K. et al. Exhaled markers of oxidative stress in idiopathic pulmonary fibrosis. Eur. J. Clin. Invest. 36 , 362–367 (2006).
Montuschi, P. et al. 8-Isoprostane as a biomarker of oxidative stress in interstitial lung diseases. Am. J. Respir. Crit. Care Med. 158 , 1524–1527 (1998).
Lenz, A. G., Costabel, U. & Maier, K. L. Oxidized BAL fluid proteins in patients with interstitial lung diseases. Eur. Respir. J. 9 , 307–312 (1996).
Tsubouchi, K. et al. Involvement of GPx4-regulated lipid peroxidation in idiopathic pulmonary fibrosis pathogenesis. J. Immunol. 203 , 2076–2087 (2019).
Malli, F. et al. 8-Isoprostane levels in serum and bronchoalveolar lavage in idiopathic pulmonary fibrosis and sarcoidosis. Food Chem. Toxicol. 61 , 160–163 (2013).
Cantin, A. M., Hubbard, R. C. & Crystal, R. G. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am. Rev. Respir. Dis. 139 , 370–372 (1989).
Ble-Castillo, J. L. et al. Effect of alpha-tocopherol on the metabolic control and oxidative stress in female type 2 diabetics. Biomed. Pharmacother. 59 , 290–295 (2005).
Schuliga, M. et al. Mitochondrial dysfunction contributes to the senescent phenotype of IPF lung fibroblasts. J. Cell Mol. Med. 22 , 5847–5861 (2018).
Liu, R. M. et al. Transforming growth factor beta suppresses glutamate-cysteine ligase gene expression and induces oxidative stress in a lung fibrosis model. Free Radic. Biol. Med. 53 , 554–563 (2012).
Kliment, C. R. & Oury, T. D. Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Free Radic. Biol. Med. 49 , 707–717 (2010).
Phan, T. H. G. et al. Emerging cellular and molecular determinants of idiopathic pulmonary fibrosis. Cell Mol. Life Sci. 78 , 2031–2057 (2020).
Montezano, A. C. & Touyz, R. M. Reactive oxygen species, vascular Noxs, and hypertension: focus on translational and clinical research. Antioxid. Redox Signal. 20 , 164–182 (2014). This article examines the role of NOXs in disease .
Lacy, F., Kailasam, M. T., O’Connor, D. T., Schmid-Schonbein, G. W. & Parmer, R. J. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension 36 , 878–884 (2000).
Redon, J. et al. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension 41 , 1096–1101 (2003).
Rodrigo, R., Bachler, J. P., Araya, J., Prat, H. & Passalacqua, W. Relationship between (Na + K)-ATPase activity, lipid peroxidation and fatty acid profile in erythrocytes of hypertensive and normotensive subjects. Mol. Cell Biochem. 303 , 73–81 (2007).
Touyz, R. M. et al. Oxidative stress: a unifying paradigm in hypertension. Can. J. Cardiol. 36 , 659–670 (2020).
Oguntibeju, O. O. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 11 , 45–63 (2019).
CAS PubMed PubMed Central Google Scholar
Girona, J. et al. Oxidized to non-oxidized lipoprotein ratios are associated with arteriosclerosis and the metabolic syndrome in diabetic patients. Nutr. Metab. Cardiovasc. Dis. 18 , 380–387 (2008).
Al-Aubaidy, H. A. & Jelinek, H. F. Oxidative DNA damage and obesity in type 2 diabetes mellitus. Eur. J. Endocrinol. 164 , 899–904 (2011).
Gopaul, N. K. et al. Plasma 8-epi-PGF2 alpha levels are elevated in individuals with non-insulin dependent diabetes mellitus. FEBS Lett. 368 , 225–229 (1995).
Pandey, K. B., Mishra, N. & Rizvi, S. I. Protein oxidation biomarkers in plasma of type 2 diabetic patients. Clin. Biochem. 43 , 508–511 (2010).
Niwa, T., Naito, C., Mawjood, A. H. & Imai, K. Increased glutathionyl hemoglobin in diabetes mellitus and hyperlipidemia demonstrated by liquid chromatography/electrospray ionization-mass spectrometry. Clin. Chem. 46 , 82–88 (2000).
Davi, G. et al. In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation 99 , 224–229 (1999).
Nishikawa, T. & Araki, E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox Signal. 9 , 343–353 (2007).
Gray, S. P. et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 127 , 1888–1902 (2013).
Butterfield, D. A. & Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 20 , 148–160 (2019). This article examines the role of oxidative stress in Alzheimer disease .
Montine, T. J. et al. Increased CSF F2-isoprostane concentration in probable AD. Neurology 52 , 562–565 (1999).
Pratico, D., V, M. Y. L., Trojanowski, J. Q., Rokach, J. & Fitzgerald, G. A. Increased F2-isoprostanes in Alzheimer’s disease: evidence for enhanced lipid peroxidation in vivo. FASEB J. 12 , 1777–1783 (1998).
Lovell, M. A., Xie, C. & Markesbery, W. R. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiol. Aging 22 , 187–194 (2001).
Lovell, M. A., Ehmann, W. D., Mattson, M. P. & Markesbery, W. R. Elevated 4-hydroxynonenal in vertricular fluid in Alzheimer’s disease. Neurobiol. Aging 18 , 457–461 (1997).
Perluigi, M. et al. Redox proteomics identification of 4-hydroxynonenal-modified brain proteins in Alzheimer’s disease: role of lipid peroxidation in Alzheimer’s disease pathogenesis. Proteom. Clin. Appl. 3 , 682–693 (2009).
Ansari, M. A. & Scheff, S. W. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J. Neuropathol. Exp. Neurol. 69 , 155–167 (2010).
Wang, J., Xiong, S., Xie, C., Markesbery, W. R. & Lovell, M. A. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer’s disease. J. Neurochem. 93 , 953–962 (2005).
Butterfield, D. A. et al. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer’s disease. Neurobiol. Dis. 22 , 223–232 (2006).
Butterfield, D. A., Hensley, K., Harris, M., Mattson, M. & Carney, J. β-Amyloid peptide free radical fragments initiate synaptosomal lipoperoxidation in a sequence-specific fashion: implications to Alzheimer’s disease. Biochem. Biophys. Res. Commun. 200 , 710–715 (1994).
Simpson, D. S. A. & Oliver, P. L. ROS generation in microglia: understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 9 , 743 (2020).
Article CAS PubMed Central Google Scholar
Smith, M. A., Harris, P. L., Sayre, L. M. & Perry, G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl Acad. Sci. USA 94 , 9866–9868 (1997).
Swerdlow, R. H. et al. Cybrids in Alzheimer’s disease: a cellular model of the disease? Neurology 49 , 918–925 (1997).
Hayes, J. D., Dinkova-Kostova, A. T. & Tew, K. D. Oxidative stress in cancer. Cancer Cell 38 , 167–197 (2020).
Conklin, K. A. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr. Cancer Ther. 3 , 294–300 (2004).
Raimondi, V., Ciccarese, F. & Ciminale, V. Oncogenic pathways and the electron transport chain: a dangeROS liaison. Br. J. Cancer 122 , 168–181 (2020).
Graham, K. A. et al. NADPH oxidase 4 is an oncoprotein localized to mitochondria. Cancer Biol. Ther. 10 , 223–231 (2010).
Jiang, W. G., Douglas-Jones, A. G. & Mansel, R. E. Aberrant expression of 5-lipoxygenase-activating protein (5-LOXAP) has prognostic and survival significance in patients with breast cancer. Prostaglandins Leukot. Essent. Fat. Acids 74 , 125–134 (2006).
Chan, H. P., Tran, V., Lewis, C. & Thomas, P. S. Elevated levels of oxidative stress markers in exhaled breath condensate. J. Thorac. Oncol. 4 , 172–178 (2009).
Matsui, A. et al. Increased formation of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, in human breast cancer tissue and its relationship to GSTP1 and COMT genotypes. Cancer Lett. 151 , 87–95 (2000).
Ohtake, S. et al. Oxidative stress marker 8-hydroxyguanosine is more highly expressed in prostate cancer than in benign prostatic hyperplasia. Mol. Clin. Oncol. 9 , 302–304 (2018).
An, A. R. et al. Association between expression of 8-OHdG and cigarette smoking in non-small cell lung cancer. J. Pathol. Transl. Med. 53 , 217–224 (2019).
Chakraborty, R. K. & Burns, B. Systemic Inflammatory Response Syndrome (StatPearls 2020).
Ware, L. B., Fessel, J. P., May, A. K. & Roberts, L. J.2nd Plasma biomarkers of oxidant stress and development of organ failure in severe sepsis. Shock 36 , 12–17 (2011).
Alonso de Vega, J. M., Diaz, J., Serrano, E. & Carbonell, L. F. Oxidative stress in critically ill patients with systemic inflammatory response syndrome. Crit. Care Med. 30 , 1782–1786 (2002).
Bahar, I., Elay, G., Baskol, G., Sungur, M. & Donmez-Altuntas, H. Increased DNA damage and increased apoptosis and necrosis in patients with severe sepsis and septic shock. J. Crit. Care 43 , 271–275 (2018).
Carpenter, C. T., Price, P. V. & Christman, B. W. Exhaled breath condensate isoprostanes are elevated in patients with acute lung injury or ARDS. Chest 114 , 1653–1659 (1998).
Sittipunt, C. et al. Nitric oxide and nitrotyrosine in the lungs of patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 163 , 503–510 (2001).
Webber, R. J., Sweet, R. M. & Webber, D. S. Inducible nitric oxide synthase in circulating microvesicles: discovery, evolution, and evidence as a novel biomarker and the probable causative agent for sepsis. J. Appl. Lab. Med. 3 , 698–711 (2019).
Joseph, L. C. et al. Inhibition of NADPH oxidase 2 (NOX2) prevents sepsis-induced cardiomyopathy by improving calcium handling and mitochondrial function. JCI Insight 2 , e94248 (2017).
Galley, H. F., Davies, M. J. & Webster, N. R. Xanthine oxidase activity and free radical generation in patients with sepsis syndrome. Crit. Care Med. 24 , 1649–1653 (1996).
Crouser, E. D., Julian, M. W., Blaho, D. V. & Pfeiffer, D. R. Endotoxin-induced mitochondrial damage correlates with impaired respiratory activity. Crit. Care Med. 30 , 276–284 (2002).
Schorah, C. J. et al. Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Am. J. Clin. Nutr. 63 , 760–765 (1996).
Takeda, K. et al. Plasma lipid peroxides and alpha-tocopherol in critically ill patients. Crit. Care Med. 12 , 957–959 (1984).
Lyons, J. et al. Cysteine metabolism and whole blood glutathione synthesis in septic pediatric patients. Crit. Care Med. 29 , 870–877 (2001).
Granger, D. N. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am. J. Physiol. 255 , H1269–H1275 (1988). This article documents the sources of oxidative stress in IRI .
CAS PubMed Google Scholar
Matsushima, S., Tsutsui, H. & Sadoshima, J. Physiological and pathological functions of NADPH oxidases during myocardial ischemia-reperfusion. Trends Cardiovasc. Med. 24 , 202–205 (2014).
Duilio, C. et al. Neutrophils are primary source of O2 radicals during reperfusion after prolonged myocardial ischemia. Am. J. Physiol. Heart Circ. Physiol. 280 , H2649–H2657 (2001).
Delanty, N. et al. 8-epi PGF2 alpha generation during coronary reperfusion. A potential quantitative marker of oxidant stress in vivo. Circulation 95 , 2492–2499 (1997).
Reilly, M. P. et al. Increased formation of the isoprostanes IPF2alpha-I and 8-epi-prostaglandin F2alpha in acute coronary angioplasty: evidence for oxidant stress during coronary reperfusion in humans. Circulation 96 , 3314–3320 (1997).
Seet, R. C. et al. Oxidative damage in ischemic stroke revealed using multiple biomarkers. Stroke 42 , 2326–2329 (2011).
Nagayoshi, Y. et al. Urinary 8-hydroxy-2′-deoxyguanosine levels increase after reperfusion in acute myocardial infarction and may predict subsequent cardiac events. Am. J. Cardiol. 95 , 514–517 (2005).
Gong, P., Stewart, D., Hu, B., Vinson, C. & Alam, J. Multiple basic-leucine zipper proteins regulate induction of the mouse heme oxygenase-1 gene by arsenite. Arch. Biochem. Biophys. 405 , 265–274 (2002).
Kronke, G. et al. Expression of heme oxygenase-1 in human vascular cells is regulated by peroxisome proliferator-activated receptors. Arterioscler. Thromb. Vasc. Biol. 27 , 1276–1282 (2007).
Peng, Z. et al. Inhibitor of kappaB kinase beta regulates redox homeostasis by controlling the constitutive levels of glutathione. Mol. Pharmacol. 77 , 784–792 (2010).
Rojo, A. I., Salinas, M., Martin, D., Perona, R. & Cuadrado, A. Regulation of Cu/Zn-superoxide dismutase expression via the phosphatidylinositol 3 kinase/Akt pathway and nuclear factor-kappaB. J. Neurosci. 24 , 7324–7334 (2004).
Mulcahy, R. T. & Gipp, J. J. Identification of a putative antioxidant response element in the 5′-flanking region of the human g-glutamylcycteine synthetase heavy subunit gene. Biochem. Biophys. Res. Commun. 209 , 227–233 (1995). This article describes an essential role for NRF2 in GSH biosynthesis .
Cuadrado, A. et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 18 , 295–317 (2019). This article provides a comprehensive review of NRF2 as a target for therapy .
Moncada, S., Palmer, R. M. J. & Higgs, E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 43 , 109–142 (1991). This article provides a review of the primary role of nitric oxide in physiology and disease .
Ursini, F., Maiorino, M. & Forman, H. J. Redox homeostasis: the Golden Mean of healthy living. Redox Biol. 8 , 205–215 (2016). This article examines the relationship of redox homeostasis to disease .
Pickering, A. M., Linder, R. A., Zhang, H., Forman, H. J. & Davies, K. J. Nrf2-dependent induction of proteasome and Pa28alphabeta regulator are required for adaptation to oxidative stress. J. Biol. Chem. 287 , 10021–10031 (2012).
Chowdhury, I. et al. Oxidant stress stimulates expression of the human peroxiredoxin 6 gene by a transcriptional mechanism involving an antioxidant response element. Free Radic. Biol. Med. 46 , 146–153 (2009).
Rusyn, I. et al. Expression of base excision DNA repair genes is a sensitive biomarker for in vivo detection of chemical-induced chronic oxidative stress: identification of the molecular source of radicals responsible for DNA damage by peroxisome proliferators. Cancer Res. 64 , 1050–1057 (2004).
McCord, J. M. & Fridovich, I. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244 , 6049–6055 (1969).
Batinic-Haberle, I., Reboucas, J. S. & Spasojevic, I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid. Redox Signal. 13 , 877–918 (2010).
Bonetta, R. Potential therapeutic applications of MnSODs and SOD-mimetics. Chemistry 24 , 5032–5041 (2018).
Faraggi, M., Peretz, P. & Weinraub, D. Chemical properties of water-soluble porphyrins. 4. The reaction of a ‘picket-fence-like’ iron (III) complex with the superoxide oxygen couple. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 49 , 951–968 (1986).
Pasternack, R. F., Banth, A., Pasternack, J. M. & Johnson, C. S. Catalysis of the disproportionation of superoxide by metalloporphyrins. J. Inorg. Biochem. 15 , 261–267 (1981).
Peretz, P., Solomon, D., Weinraub, D. & Faraggi, M. Chemical properties of water-soluble porphyrins 3. The reaction of superoxide radicals with some metalloporphyrins. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 42 , 449–456 (1982).
Batinić-Haberle, I. et al. Relationship among redox potentials, proton dissociation constants of pyrrolic nitrogens, and in vivo and in vitro superoxide dismutating activities of manganese( iii ) and iron( iii ) water-soluble porphyrins. Inorg. Chem. 38 , 12 (1999).
Article Google Scholar
Jaramillo, M. C., Briehl, M. M., Batinic-Haberle, I. & Tome, M. E. Manganese ( iii ) meso-tetrakis N-ethylpyridinium-2-yl porphyrin acts as a pro-oxidant to inhibit electron transport chain proteins, modulate bioenergetics, and enhance the response to chemotherapy in lymphoma cells. Free Radic. Biol. Med. 83 , 89–100 (2015).
Ferrer-Sueta, G. et al. Reactions of manganese porphyrins with peroxynitrite and carbonate radical anion. J. Biol. Chem. 278 , 27432–27438 (2003).
Batinic-Haberle, I., Tovmasyan, A. & Spasojevic, I. An educational overview of the chemistry, biochemistry and therapeutic aspects of Mn porphyrins—from superoxide dismutation to H 2 O 2 -driven pathways. Redox Biol. 5 , 43–65 (2015). This article examines the potential use of SOD mimics in therapy .
Rawal, M. et al. Manganoporphyrins increase ascorbate-induced cytotoxicity by enhancing H 2 O 2 generation. Cancer Res. 73 , 5232–5241 (2013).
Batinic-Haberle, I., Spasojevic, I. & Fridovich, I. Tetrahydrobiopterin rapidly reduces the SOD mimic Mn( iii ) ortho-tetrakis(N-ethylpyridinium-2-yl)porphyrin. Free Radic. Biol. Med. 37 , 367–374 (2004).
Batinic-Haberle, I. et al. Design of Mn porphyrins for treating oxidative stress injuries and their redox-based regulation of cellular transcriptional activities. Amino Acids 42 , 95–113 (2012).
Dorai, T. et al. Amelioration of renal ischemia-reperfusion injury with a novel protective cocktail. J. Urol. 186 , 2448–2454 (2011).
Huber, W. Orgotein–(bovine Cu-Zn superoxide dismutase), an anti-inflammatory protein drug: discovery, toxicology and pharmacology. Eur. J. Rheumatol. Inflamm. 4 , 173–182 (1981).
Menander-Huber, K. B., Edsmyr, F. & Huber, W. Orgotein (superoxide dismutase): a drug for the amelioration of radiation-induced side effects. A double-blind, placebo-controlled study in patients with bladder tumours. Urol. Res. 6 , 255–257 (1978).
Sanchiz, F. et al. Prevention of radioinduced cystitis by orgotein: a randomized study. Anticancer. Res. 16 , 2025–2028 (1996).
Nielsen, O. S. et al. Orgotein in radiation treatment of bladder cancer. A report on allergic reactions and lack of radioprotective effect. Acta Oncol. 26 , 101–104 (1987).
Mackensen, G. B. et al. Neuroprotection from delayed postischemic administration of a metalloporphyrin catalytic antioxidant. J. Neurosci. 21 , 4582–4592 (2001).
Gauter-Fleckenstein, B. et al. Comparison of two Mn porphyrin-based mimics of superoxide dismutase in pulmonary radioprotection. Free Radic. Biol. Med. 44 , 982–989 (2008).
Rabbani, Z. N. et al. Antiangiogenic action of redox-modulating Mn( iii ) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin, MnTE-2-PyP(5+), via suppression of oxidative stress in a mouse model of breast tumor. Free Radic. Biol. Med. 47 , 992–1004 (2009).
Moeller, B. J., Cao, Y., Li, C. Y. & Dewhirst, M. W. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell 5 , 429–441 (2004).
Piganelli, J. D. et al. A metalloporphyrin-based superoxide dismutase mimic inhibits adoptive transfer of autoimmune diabetes by a diabetogenic T-cell clone. Diabetes 51 , 347–355 (2002).
Ganesh, D. et al. Impact of superoxide dismutase mimetic AEOL 10150 on the endothelin system of Fischer 344 rats. PLoS ONE 11 , e0151810 (2016).
Benatar, M. Lost in translation: treatment trials in the SOD1 mouse and in human ALS. Neurobiol. Dis. 26 , 1–13 (2007).
Aston, K. et al. Computer-aided design (CAD) of Mn( ii ) complexes: superoxide dismutase mimetics with catalytic activity exceeding the native enzyme. Inorg. Chem. 40 , 1779–1789 (2001).
Heer, C. D. et al. Superoxide dismutase mimetic GC4419 enhances the oxidation of pharmacological ascorbate and its anticancer effects in an H 2 O 2 -dependent manner. Antioxidants 7 , 18 (2018).
Salvemini, D. et al. Pharmacological manipulation of the inflammatory cascade by the superoxide dismutase mimetic, M40403. Br. J. Pharmacol. 132 , 815–827 (2001).
Salvemini, D. et al. Amelioration of joint disease in a rat model of collagen-induced arthritis by M40403, a superoxide dismutase mimetic. Arthritis Rheum. 44 , 2909–2921 (2001).
Masini, E. et al. Protective effects of M40403, a selective superoxide dismutase mimetic, in myocardial ischaemia and reperfusion injury in vivo. Br. J. Pharmacol. 136 , 905–917 (2002).
Anderson, C. M. et al. Phase 1b/2a trial of the superoxide dismutase mimetic GC4419 to reduce chemoradiotherapy-induced oral mucositis in patients with oral cavity or oropharyngeal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 100 , 427–435 (2018).
Doctrow, S. R. et al. Salen-manganese complexes: combined superoxide dismutase/catalase mimics with broad pharmacological efficacy. Adv. Pharmacol. 38 , 247–269 (1997).
McDonald, M. C. et al. A superoxide dismutase mimetic with catalase activity (EUK-8) reduces the organ injury in endotoxic shock. Eur. J. Pharmacol. 466 , 181–189 (2003). This article examines the advantage of having catalase activity in SOD mimics .
Xu, Y., Armstrong, S. J., Arenas, I. A., Pehowich, D. J. & Davidge, S. T. Cardioprotection by chronic estrogen or superoxide dismutase mimetic treatment in the aged female rat. Am. J. Physiol. Heart Circ. Physiol. 287 , H165–H171 (2004).
van Empel, V. P. et al. EUK-8, a superoxide dismutase and catalase mimetic, reduces cardiac oxidative stress and ameliorates pressure overload-induced heart failure in the harlequin mouse mutant. J. Am. Coll. Cardiol. 48 , 824–832 (2006).
Izumi, M., McDonald, M. C., Sharpe, M. A., Chatterjee, P. K. & Thiemermann, C. Superoxide dismutase mimetics with catalase activity reduce the organ injury in hemorrhagic shock. Shock 18 , 230–235 (2002).
Jung, C. et al. Synthetic superoxide dismutase/catalase mimetics reduce oxidative stress and prolong survival in a mouse amyotrophic lateral sclerosis model. Neurosci. Lett. 304 , 157–160 (2001).
Chatterjee, P. K. et al. EUK-134 reduces renal dysfunction and injury caused by oxidative and nitrosative stress of the kidney. Am. J. Nephrol. 24 , 165–177 (2004).
Baker, K. et al. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J. Pharmacol. Exp. Ther. 284 , 215–221 (1998).
Langan, A. R., Khan, M. A., Yeung, I. W., Van Dyk, J. & Hill, R. P. Partial volume rat lung irradiation: the protective/mitigating effects of Eukarion-189, a superoxide dismutase-catalase mimetic. Radiother. Oncol. 79 , 231–238 (2006).
Liu, Z. et al. Frequency modulation of synchronized Ca 2+ spikes in cultured hippocampal networks through G-protein-coupled receptors. J. Neurosci. 23 , 4156–4163 (2003).
Himori, K. et al. Superoxide dismutase/catalase mimetic EUK-134 prevents diaphragm muscle weakness in monocrotalin-induced pulmonary hypertension. PLoS ONE 12 , e0169146 (2017).
Zhang, H. J., Doctrow, S. R., Oberley, L. W. & Kregel, K. C. Chronic antioxidant enzyme mimetic treatment differentially modulates hyperthermia-induced liver HSP70 expression with aging. J. Appl. Physiol. 100 , 1385–1391 (2006).
Day, B. J. Catalase and glutathione peroxidase mimics. Biochem. Pharmacol. 77 , 285–296 (2009).
Sies, H. Ebselen, a selenoorganic compound as glutathione peroxidase mimic. Free. Radic. Biol. Med. 14 , 313–323 (1993).
Nakamura, Y. et al. Ebselen, a glutathione peroxidase mimetic seleno-organic compound, as a multifunctional antioxidant. Implication for inflammation-associated carcinogenesis. J. Biol. Chem. 277 , 2687–2694 (2002).
Kil, J., Pierce, C., Tran, H., Gu, R. & Lynch, E. D. Ebselen treatment reduces noise induced hearing loss via the mimicry and induction of glutathione peroxidase. Hear. Res. 226 , 44–51 (2007).
Garland, M. et al. The clinical drug ebselen attenuates inflammation and promotes microbiome recovery in mice after antibiotic treatment for CDI. Cell Rep. Med. 1 , 100005 (2020).
Singh, N. et al. Effect of the putative lithium mimetic ebselen on brain myo-inositol, sleep, and emotional processing in humans. Neuropsychopharmacology 41 , 1768–1778 (2016).
Ogawa, A. et al. Ebselen in acute middle cerebral artery occlusion: a placebo-controlled, double-blind clinical trial. Cerebrovasc. Dis. 9 , 112–118 (1999).
Saito, I. et al. Neuroprotective effect of an antioxidant, ebselen, in patients with delayed neurological deficits after aneurysmal subarachnoid hemorrhage. Neurosurgery 42 , 269–277; discussion 277–278 (1998).
Yamaguchi, T. et al. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Stroke 29 , 12–17 (1998).
d’Alessio, P., Moutet, M., Coudrier, E., Darquenne, S. & Chaudiere, J. ICAM-1 and VCAM-1 expression induced by TNF-alpha are inhibited by a glutathione peroxidase mimic. Free Radic. Biol. Med. 24 , 979–987 (1998).
Castagne, V. & Clarke, P. G. Neuroprotective effects of a new glutathione peroxidase mimetic on neurons of the chick embryo’s retina. J. Neurosci. Res. 59 , 497–503 (2000).
Blum, S. et al. Haptoglobin genotype determines myocardial infarct size in diabetic mice. J. Am. Coll. Cardiol. 49 , 82–87 (2007).
Puntarulo, S. & Cederbaum, A. I. Comparison of the ability of ferric complexes to catalyze microsomal chemiluminescence, lipid peroxidation, and hydroxyl radical generation. Arch. Biochem. Biophys. 264 , 482–491 (1988).
Brittenham, G. M. et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N. Engl. J. Med. 331 , 567–573 (1994).
Raftos, J. E., Whillier, S., Chapman, B. E. & Kuchel, P. W. Kinetics of uptake and deacetylation of N-acetylcysteine by human erythrocytes. Int. J. Biochem. Cell Biol. 39 , 1698–1706 (2007).
Rushworth, G. F. & Megson, I. L. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 141 , 150–159 (2014).
Smilkstein, M. J., Knapp, G. L., Kulig, K. W. & Rumack, B. H. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N. Engl. J. Med. 319 , 1557–1562 (1988).
Conrad, C. et al. Long-term treatment with oral N-acetylcysteine: affects lung function but not sputum inflammation in cystic fibrosis subjects. A phase II randomized placebo-controlled trial. J. Cyst. Fibros. 14 , 219–227 (2015).
Xu, R., Tao, A., Bai, Y., Deng, Y. & Chen, G. Effectiveness of N-acetylcysteine for the prevention of contrast-induced nephropathy: a systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 5 , e003968 (2016).
Wendel, A. & Cikryt, P. The level and half-life of glutathione in human plasma. FEBS Lett. 120 , 209–211 (1980).
Anderson, M. E. & Meister, A. Glutathione monoesters. Anal. Biochem. 183 , 16–20 (1989).
Levy, E. J., Anderson, M. E. & Meister, A. Transport of glutathione diethyl ester into human cells. Proc. Natl Acad. Sci. USA 90 , 9171–9175 (1993). This article demonstrates that glutathione diethyl ester is highly effective as a delivery agent for GSH in human cells to decrease oxidative stress .
Puri, R. N. & Meister, A. Transport of glutathione, as gamma-glutamylcysteinylglycyl ester, into liver and kidney. Proc. Natl Acad. Sci. USA 80 , 5258–5260 (1983).
Wellner, V. P., Anderson, M. E., Puri, R. N., Jensen, G. L. & Meister, A. Radioprotection by glutathione ester: transport of glutathione ester into human lymphoid cells and fibroblasts. Proc. Natl Acad. Sci. USA 81 , 4732–4735 (1984).
Tsan, M. F., White, J. E. & Rosano, C. L. Modulation of endothelial GSH concentrations: effect of exogenous GSH and GSH monoethyl ester. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 66 , 1029–1034 (1989).
CAS Google Scholar
Grattagliano, I., Vendemiale, G. & Lauterburg, B. H. Reperfusion injury of the liver: role of mitochondria and protection by glutathione ester. J. Surg. Res. 86 , 2–8 (1999).
Anderson, M. F., Nilsson, M., Eriksson, P. S. & Sims, N. R. Glutathione monoethyl ester provides neuroprotection in a rat model of stroke. Neurosci. Lett. 354 , 163–165 (2004).
Chen, T. S., Richie, J. P., Nagasawa, H. T. & Lang, C. A. Glutathione monoethyl ester protects against glutathione deficiencies due to aging and acetaminophen in mice. Mech. Ageing Dev. 120 , 127–139 (2000).
Anderson, M. E., Powrie, F., Puri, R. N. & Meister, A. Glutathione monoethyl ester: preparation, uptake by tissues, and conversion to glutathione. Arch. Biochem. Biophys. 239 , 538–548 (1985).
Zeevalk, G. D., Manzino, L., Sonsalla, P. K. & Bernard, L. P. Characterization of intracellular elevation of glutathione (GSH) with glutathione monoethyl ester and GSH in brain and neuronal cultures: relevance to Parkinson’s disease. Exp. Neurol. 203 , 512–520 (2007).
Howden, R. Nrf2 and cardiovascular defense. Oxid. Med. Cell Longev. 2013 , 104308 (2013).
Gan, L. & Johnson, J. A. Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochim. Biophys. Acta 1842 , 1208–1218 (2014).
Boutten, A., Goven, D., Artaud-Macari, E. & Bonay, M. Protective role of Nrf2 in the lungs against oxidative airway diseases. Med. Sci. 27 , 966–972 (2011).
Google Scholar
McMahon, M., Thomas, N., Itoh, K., Yamamoto, M. & Hayes, J. D. Dimerization of substrate adaptors can facilitate Cullin-mediated ubiquitylation of proteins by a “tethering” mechanism: a two-site interaction model for the Nrf2-Keap1 complex. J. Biol. Chem. 281 , 24756–24768 (2006).
Seo, H. Y. et al. Kahweol activates the Nrf2/HO-1 pathway by decreasing Keap1 expression independently of p62 and autophagy pathways. PLoS ONE 15 , e0240478 (2020).
Lee, D. H. et al. The hypertension drug, verapamil, activates Nrf2 by promoting p62-dependent autophagic Keap1 degradation and prevents acetaminophen-induced cytotoxicity. BMB Rep. 50 , 91–96 (2017).
Li, J. et al. Green tea extract provides extensive Nrf2-independent protection against lipid accumulation and NFkappaB pro-inflammatory responses during nonalcoholic steatohepatitis in mice fed a high-fat diet. Mol. Nutr. Food Res. 60 , 858–870 (2016).
Pandurangan, A. K., Saadatdoust, Z., Esa, N. M., Hamzah, H. & Ismail, A. Dietary cocoa protects against colitis-associated cancer by activating the Nrf2/Keap1 pathway. Biofactors 41 , 1–14 (2015).
Yagishita, Y., Fahey, J. W., Dinkova-Kostova, A. T. & Kensler, T. W. Broccoli or sulforaphane: is it the source or dose that matters? Molecules 24 , 3593 (2019). This article evaluates the current knowledge regarding bioavailability and efficacy of glucoraphanin and sulforaphane in terms of dose and route of administration .
Robledinos-Anton, N., Fernandez-Gines, R., Manda, G. & Cuadrado, A. Activators and inhibitors of NRF2: a review of their potential for clinical development. Oxid. Med. Cell Longev. 2019 , 9372182 (2019). This article reviewed electrophilic and non-electrophilic NRF2 activators in clinical trials for various chronic diseases including cancer .
Kwon, J. S. et al. Sulforaphane inhibits restenosis by suppressing inflammation and the proliferation of vascular smooth muscle cells. Atherosclerosis 225 , 41–49 (2012).
Shimizu, K. et al. Anti-inflammatory action of curcumin and its use in the treatment of lifestyle-related diseases. Eur. Cardiol. 14 , 117–122 (2019).
de Sa Coutinho, D., Pacheco, M. T., Frozza, R. L. & Bernardi, A. Anti-inflammatory effects of resveratrol: mechanistic insights. Int. J. Mol. Sci. 19 , 1812 (2018).
Jiang, Z. Y., Lu, M. C. & You, Q. D. Discovery and development of Kelch-like ECH-associated protein 1. Nuclear factor erythroid 2-related factor 2 (KEAP1:NRF2) protein-protein interaction inhibitors: achievements, challenges, and future directions. J. Med. Chem. 59 , 10837–10858 (2016).
Satoh, T. & Lipton, S. Recent advances in understanding NRF2 as a druggable target: development of pro-electrophilic and non-covalent NRF2 activators to overcome systemic side effects of electrophilic drugs like dimethyl fumarate. F1000Res 6 , 2138 (2017). This article evaluates NRF2 activators designed to avoid the systemic side effects caused by electrophilic activators .
Couch, R. D. et al. Studies on the reactivity of CDDO, a promising new chemopreventive and chemotherapeutic agent: implications for a molecular mechanism of action. Bioorg. Med. Chem. Lett. 15 , 2215–2219 (2005). This article demonstrates that conjugation of electrophilic cyanoenone compounds and KEAP1 is selective and reversable .
Kostov, R. V. et al. Pharmacokinetics and pharmacodynamics of orally administered acetylenic tricyclic bis(cyanoenone), a highly potent Nrf2 activator with a reversible covalent mode of action. Biochem. Biophys. Res. Commun. 465 , 402–407 (2015). This article demonstrates that oral administration of acetylenic tricyclic bis(cyanoenone), a NRF2 activator, has a reversible covalent mode of action in various tissues in C57BL/6 mice .
Dinkova-Kostova, A. T. et al. An exceptionally potent inducer of cytoprotective enzymes: elucidation of the structural features that determine inducer potency and reactivity with Keap1. J. Biol. Chem. 285 , 33747–33755 (2010).
Sedlak, T. W. et al. Sulforaphane augments glutathione and influences brain metabolites in human subjects: a clinical pilot study. Mol. Neuropsychiatry 3 , 214–222 (2018).
PubMed PubMed Central Google Scholar
Wise, R. A. et al. Lack of effect of oral sulforaphane administration on Nrf2 expression in COPD: a randomized, double-blind, placebo controlled trial. PLoS ONE 11 , e0163716 (2016).
Greaney, A. J., Maier, N. K., Leppla, S. H. & Moayeri, M. Sulforaphane inhibits multiple inflammasomes through an Nrf2-independent mechanism. J. Leukoc. Biol. 99 , 189–199 (2016).
Roy, S. K., Srivastava, R. K. & Shankar, S. Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. J. Mol. Signal. 5 , 10 (2010).
Satoh, H., Moriguchi, T., Takai, J., Ebina, M. & Yamamoto, M. Nrf2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesis. Cancer Res. 73 , 4158–4168 (2013). This article demonstrates that NRF2-deficient mice exhibited an increase in tumour foci after urethane induction but a reduction in tumours with more malignant characteristics .
Wiel, C. et al. BACH1 stabilization by antioxidants stimulates lung cancer metastasis. Cell 178 , 330–345 e322 (2019). This article demonstrates that long-term supplementation with N -acetylcysteine and vitamin E promoted KRAS-driven lung cancer metastasis, and NRF2 inhibitor BACH1 stimulated glycolysis-dependent lung cancer metastasis in a mouse model .
Tao, S., Rojo de la Vega, M., Chapman, E., Ooi, A. & Zhang, D. D. The effects of NRF2 modulation on the initiation and progression of chemically and genetically induced lung cancer. Mol. Carcinog. 57 , 182–192 (2018). This article demonstrates that sulforaphane prevented the initiation of vinyl carbamate-induced lung cancer in mouse models but promoted the progression of pre-existing tumours .
Shibata, T. et al. Genetic alteration of Keap1 confers constitutive Nrf2 activation and resistance to chemotherapy in gallbladder cancer. Gastroenterology 135 , 1358–1368 (2008).
Homma, S. et al. Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin. Cancer Res. 15 , 3423–3432 (2009).
Jiang, T. et al. High levels of Nrf2 determine chemoresistance in type II endometrial cancer. Cancer Res. 70 , 5486–5496 (2010).
Roh, J. L., Kim, E. H., Jang, H. & Shin, D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 11 , 254–262 (2017).
Sporn, M. B. & Liby, K. T. NRF2 and cancer: the good, the bad and the importance of context. Nat. Rev. Cancer 12 , 564–571 (2012).
Milkovic, L., Zarkovic, N. & Saso, L. Controversy about pharmacological modulation of Nrf2 for cancer therapy. Redox Biol. 12 , 727–732 (2017).
Wu, S., Lu, H. & Bai, Y. Nrf2 in cancers: a double-edged sword. Cancer Med. 8 , 2252–2267 (2019).
Massart, C. et al. Diphenyleneiodonium, an inhibitor of NOXes and DUOXes, is also an iodide-specific transporter. FEBS Open Bio. 4 , 55–59 (2013).
Augsburger, F. et al. Pharmacological characterization of the seven human NOX isoforms and their inhibitors. Redox Biol. 26 , 101272 (2019).
Teixeira, G. et al. Therapeutic potential of NADPH oxidase 1/4 inhibitors. Br. J. Pharmacol. 174 , 1647–1669 (2017).
Cifuentes-Pagano, M. E., Meijles, D. N. & Pagano, P. J. Nox inhibitors & therapies: rational design of peptidic and small molecule inhibitors. Curr. Pharm. Des. 21 , 6023–6035 (2015).
Nishikawa, T. et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404 , 787–790 (2000). This article demonstrates that prevention of mitochondrial superoxide production blocked oxidative stress and signal transduction .
Detaille, D., Pasdois, P., Semont, A., Dos Santos, P. & Diolez, P. An old medicine as a new drug to prevent mitochondrial complex I from producing oxygen radicals. PLoS ONE 14 , e0216385 (2019).
Craven, R. SOD mimetics to the rescue. Nat. Rev. Neurosci. 2 , 858–858 (2001).
Pavon, N., Correa, F., Buelna-Chontal, M., Hernandez-Esquivel, L. & Chavez, E. Ebselen induces mitochondrial permeability transition because of its interaction with adenine nucleotide translocase. Life Sci. 139 , 108–113 (2015).
Murphy, M. P. Targeting lipophilic cations to mitochondria. Biochim. Biophys. Acta 1777 , 1028–1031 (2008).
Li, Y. & Schellhorn, H. E. New developments and novel therapeutic perspectives for vitamin C. J. Nutr. 137 , 2171–2184 (2007).
Frei, B., England, L. & Ames, B. N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc. Natl Acad. Sci. USA 86 , 6377–6381 (1989).
Buettner, G. R. The pecking order of free radicals and antioxidants: lipid peroxidation, α-tocopherol, and ascorbate. Arch. Biochem. Biophys. 300 , 535–543 (1993).
Bruno, R. S. et al. Faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Radic. Biol. Med. 40 , 689–697 (2006).
Hill, K. E. et al. Combined deficiency of vitamins E and C causes paralysis and death in guinea pigs. Am. J. Clin. Nutr. 77 , 1484–1488 (2003).
Traber, M. G. & Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 43 , 4–15 (2007).
Maxfield, L. & Crane, J. S. Vitamin C Deficiency. StatPearls [online] https://www.ncbi.nlm.nih.gov/books/NBK493187/ (updated 2 Jul 2020).
Traber, M. G. Vitamin E inadequacy in humans: causes and consequences. Adv. Nutr. 5 , 503–514 (2014).
Fang, Y. Z., Yang, S. & Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 18 , 872–879 (2002).
Sayin, V. I. et al. Antioxidants accelerate lung cancer progression in mice. Sci. Transl Med. 6 , 221ra215 (2014). This article demonstrates that N -acetylcysteine and vitamin E markedly increased tumour progression and reduced survival in mouse models of lung cancer .
Le Gal, K. et al. Antioxidants can increase melanoma metastasis in mice. Sci. Transl Med. 7 , 308re308 (2015).
Zou, Z. V. et al. Antioxidants promote intestinal tumor progression in mice. Antioxidants 10 , 241 (2021).
Kaul, N. & Forman, H. J. Activation of NF kappa B by the respiratory burst of macrophages. Free Radic. Biol. Med. 21 , 401–405 (1996). This is probably the first article to demonstrate stimulation of cell signalling by endogenous generation of H 2 O 2 through activation of NOX .
Lambeth, J. D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4 , 181–189 (2004). This article reviews the NOX family of enzymes .
Forman, H. J., Ursini, F. & Maiorino, M. An overview of mechanisms of redox signaling. J. Mol. Cell. Cardiol. 73 , 2–9 (2014).
Marshall, P. J., Kulmacz, R. J. & Lands, W. E. M. in Hydroperoxides, Free Radicals and Prostaglandin Synthesis (eds Bors, W., Saran, M. & Tait, D) 299–304 (Walter de Gruyter, 1984).
Premasekharan, G. et al. Iron-mediated lipid peroxidation and lipid raft disruption in low-dose silica-induced macrophage cytokine production. Free Radic. Biol. Med. 51 , 1184–1194 (2011).
Monick, M. M. et al. A phosphatidylcholine-specific phospholipase C regulates activation of p42/44 mitogen-activated protein kinases in lipopolysaccharide- stimulated human alveolar macrophages. J. Immunol. 162 , 3005–3012 (1999).
Huie, R. E. & Padmaja, S. The reaction of NO with superoxide. Free Radic. Res. Commun. 18 , 195–199 (1993).
Buettner, G. R., Ng, C. F., Wang, M., Rodgers, V. G. & Schafer, F. Q. A new paradigm: manganese superoxide dismutase influences the production of H 2 O 2 in cells and thereby their biological state. Free Radic. Biol. Med. 41 , 1338–1350 (2006).
Farnsworth, C. C., Stone, W. L. & Dratz, E. A. Effects of vitamin E and selenium deficiency on the fatty acid composition of rat retinal tissues. Biochim. Biophys. Acta 552 , 281–293 (1979).
Smith, R. A. et al. Mitochondria-targeted antioxidants in the treatment of disease. Ann. NY Acad. Sci. 1147 , 105–111 (2008).
Zhang, H., Davies, K. J. & Forman, H. J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 88 , 314–336 (2015). This article reviews the evidence that NRF2 activation declines with ageing .
Zhang, H., Zhou, L., Davies, K. J. A. & Forman, H. J. Silencing Bach1 alters aging-related changes in the expression of Nrf2-regulated genes in primary human bronchial epithelial cells. Arch. Biochem. Biophys. 672 , 108074 (2019). This article demonstrates that inhibition of BACH1 partially reversed the decline of NRF2 activation in ageing .
Surh, Y. J., Kundu, J. K., Na, H. K. & Lee, J. S. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. J. Nutr. 135 , 2993S–3001S (2005). This article describes how NRF2 and other transcription factors are targets for protective phytochemicals .
Ota, K., Brydun, A., Itoh-Nakadai, A., Sun, J. & Igarashi, K. Bach1 deficiency and accompanying overexpression of heme oxygenase-1 do not influence aging or tumorigenesis in mice. Oxid. Med. Cell Longev. 2014 , 757901 (2014). This article demonstrates the effects of Bach1 knockout on the transcriptome, p53 −/− induced tumorigenesis and oxidative damage in a mouse model .
Sharpley, A. L. et al. A phase 2a randomised, double-blind, placebo-controlled, parallel-group, add-on clinical trial of ebselen (SPI-1005) as a novel treatment for mania or hypomania. Psychopharmacology 237 , 3773–3782 (2020).
Masaki, C. et al. Effects of the potential lithium-mimetic, ebselen, on impulsivity and emotional processing. Psychopharmacology 233 , 2655–2661 (2016).
Bowler, R. P. et al. A catalytic antioxidant (AEOL 10150) attenuates expression of inflammatory genes in stroke. Free Radic. Biol. Med. 33 , 1141–1152 (2002).
Rabbani, Z. N. et al. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int. J. Radiat. Oncol. Biol. Phys. 67 , 573–580 (2007).
Bianca, R. et al. Superoxide dismutase mimetic with catalase activity, EUK-134, attenuates the multiple organ injury and dysfunction caused by endotoxin in the rat. Med. Sci. Monit. 8 , BR1–BR7 (2002).
PubMed Google Scholar
Liu, H. et al. Biomarker exploration in human peripheral blood mononuclear cells for monitoring sulforaphane treatment responses in autism spectrum disorder. Sci. Rep. 10 , 5822 (2020).
Singh, K. et al. Sulforaphane treatment of autism spectrum disorder (ASD). Proc. Natl Acad. Sci. USA 111 , 15550–15555 (2014).
Ungvari, Z. et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am. J. Physiol. Heart Circ. Physiol. 299 , H18–H24 (2010).
Tanigawa, S., Fujii, M. & Hou, D. X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free. Radic. Biol. Med. 42 , 1690–1703 (2007).
Balogun, E. et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 371 , 887–895 (2003).
Pergola, P. E. et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med. 365 , 327–336 (2011).
Cleasby, A. et al. Structure of the BTB domain of Keap1 and its interaction with the triterpenoid antagonist CDDO. PLoS ONE 9 , e98896 (2014).
Dayalan Naidu, S. et al. C151 in KEAP1 is the main cysteine sensor for the cyanoenone class of NRF2 activators, irrespective of molecular size or shape. Sci. Rep. 8 , 8037 (2018).
Lynch, D. R. et al. Safety, pharmacodynamics, and potential benefit of omaveloxolone in Friedreich ataxia. Ann. Clin. Transl. Neurol. 6 , 15–26 (2019).
Madsen, K. L. et al. Safety and efficacy of omaveloxolone in patients with mitochondrial myopathy: MOTOR trial. Neurology 94 , e687–e698 (2020).
Probst, B. L. et al. RTA 408, a novel synthetic triterpenoid with broad anticancer and anti-inflammatory activity. PLoS ONE 10 , e0122942 (2015).
Reisman, S. A., Lee, C. Y., Meyer, C. J., Proksch, J. W. & Ward, K. W. Topical application of the synthetic triterpenoid RTA 408 activates Nrf2 and induces cytoprotective genes in rat skin. Arch. Dermatol. Res. 306 , 447–454 (2014).
Fox, R. J. et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N. Engl. J. Med. 367 , 1087–1097 (2012).
Gold, R. et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl. J. Med. 367 , 1098–1107 (2012).
Brennan, M. S. et al. Dimethyl fumarate and monoethyl fumarate exhibit differential effects on KEAP1, NRF2 activation, and glutathione depletion in vitro. PLoS ONE 10 , e0120254 (2015).
Ramos-Gomez, M. et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl Acad. Sci. USA 98 , 3410–3415 (2001).
Kansanen, E. et al. Nrf2-dependent and -independent responses to nitro-fatty acids in human endothelial cells: identification of heat shock response as the major pathway activated by nitro-oleic acid. J. Biol. Chem. 284 , 33233–33241 (2009).
Seo, J. Y. et al. Andrographolide activates Keap1/Nrf2/ARE/HO-1 pathway in HT22 cells and suppresses microglial activation by Abeta42 through Nrf2-related inflammatory response. Mediators Inflamm. 2017 , 5906189 (2017).
Okada, K. et al. Ursodeoxycholic acid stimulates Nrf2-mediated hepatocellular transport, detoxification, and antioxidative stress systems in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 295 , G735–G747 (2008).
Huang, H. C., Nguyen, T. & Pickett, C. B. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 277 , 42769–42774 (2002).
Chen, W. et al. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol. Cell 34 , 663–673 (2009).
Gorrini, C. et al. BRCA1 interacts with Nrf2 to regulate antioxidant signaling and cell survival. J. Exp. Med. 210 , 1529–1544 (2013).
Shan, Y., Lambrecht, R. W., Donohue, S. E. & Bonkovsky, H. L. Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. FASEB J. 20 , 2651–2653 (2006).
Chapple, S. J. et al. Bach1 differentially regulates distinct Nrf2-dependent genes in human venous and coronary artery endothelial cells adapted to physiological oxygen levels. Free Radic. Biol. Med. 92 , 152–162 (2016).
Zhang, X. et al. Bach1: function, regulation, and involvement in disease. Oxid. Med. Cell Longev. 2018 , 1347969 (2018).
Chen, F., Haigh, S., Barman, S. & Fulton, D. J. From form to function: the role of Nox4 in the cardiovascular system. Front. Physiol. 3 , 412 (2012).
Halliwell, B. & Gutteridge, J. M. C. Free Radicals in Biology and Medicine (Clarendon Press, 1989).
Boveris, A., Cadenas, E. & Stoppani, A. O. M. Role of ubiquinone in the mitochondrial generation of hydrogen peroxide. Biochem. J. 156 , 435–435 (1976).
Babior, B. M., Kipnes, R. S. & Curnutte, J. T. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Invest. 52 , 741–741 (1973).
Nisimoto, Y., Diebold, B. A., Cosentino-Gomes, D. & Lambeth, J. D. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry 53 , 5111–5120 (2014).
Koppenol, W. H. The centennial of the Fenton reaction. Free Radic. Biol. Med. 15 , 645–651 (1993).
Pattison, D. I., Davies, M. J. & Hawkins, C. L. Reactions and reactivity of myeloperoxidase-derived oxidants: differential biological effects of hypochlorous and hypothiocyanous acids. Free Radic. Res. 46 , 975–995 (2012).
Davies, K. J. A. Adaptive homeostasis. Mol. Asp. Med. 49 , 1–7 (2016). This article describes adaptive homeostasis, the endogenous defence that antioxidant therapies are intended to mimic .
Moi, P., Chan, K., Asunis, I., Cao, A. & Kan, Y. W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl Acad. Sci. USA 91 , 9926–9930 (1994).
Venugopal, R. & Jaiswal, A. K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. J. Clin. Invest. 93 , 14960–14965 (1996).
Mann, G. E. & Forman, H. J. Introduction to special issue on ‘Nrf2 regulated redox signaling and metabolism in physiology and medicine’. Free Radic. Biol. Med. 88 , 91–92 (2015). This article introduces an important group of reviews on NRF2 signalling .
Ushida, Y. & Talalay, P. Sulforaphane accelerates acetaldehyde metabolism by inducing aldehyde dehydrogenases: relevance to ethanol intolerance. Alcohol Alcohol. 48 , 526–534 (2013).
Kobayashi, E. H. et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 7 , 11624 (2016).
Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 53 , 401–426 (2013).
Thimmulappa, R. K. et al. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Invest. 116 , 984–995 (2006).
Kobayashi, A. et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 24 , 7130–7139 (2004).
Chowdhry, S. et al. Nrf2 is controlled by two distinct beta-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene 32 , 3765–3781 (2013).
Komatsu, M. et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 12 , 213–223 (2010). This article demonstrates that overproduction of p62 or a deficiency in autophagy competed with NRF2–KEAP1 interaction and activated NRF2 .
Sun, J. et al. Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network. Proc. Natl Acad. Sci. USA 101 , 1461–1466 (2004).
Warnatz, H. J. et al. The BTB and CNC homology 1 (BACH1) target genes are involved in the oxidative stress response and in control of the cell cycle. J. Biol. Chem. 286 , 23521–23532 (2011).
Reichard, J. F., Motz, G. T. & Puga, A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic Acids Res. 35 , 7074–7086 (2007).
Download references
Acknowledgements
The authors thank their many colleagues with whom conversations and collaborations concerning antioxidants have occurred over decades. The authors’ work in this area was supported by several past NIH grants and currently by P01 AG055367.
Author information
Authors and affiliations.
University of California Merced, Merced, CA, USA
Henry Jay Forman
Leonard Davis School of Gerontology, University of Southern California, Los Angeles, CA, USA
Henry Jay Forman & Hongqiao Zhang
You can also search for this author in PubMed Google Scholar
Contributions
Both authors were involved in researching data, discussion of content, writing the article and reviewing/editing the manuscript before submission.
Corresponding author
Correspondence to Henry Jay Forman .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Imbalance between generation of oxidants and the ability to prevent oxidative damage favouring the latter process.
Signal transduction in which oxidants act as second messengers.
Prevention or repair of oxidative damage.
Strictly, enzymes that remove oxidants; broadly, enzymes that contribute to the prevention or repair of oxidative damage. The broader definition is used in this Review.
Nuclear factor E2-related factor 2, which coordinates both the baseline and stress-inducible activation of a great many antioxidant enzymes.
Treatment with agents that enhance antioxidant defence.
Inflammation of the lungs caused by irritation of lung tissue, disease, infection, radiation therapy or allergy.
Cessation followed by restoration of blood flow.
A mechanism through which unnecessary or damaged cellular components are degraded.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Forman, H.J., Zhang, H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov 20 , 689–709 (2021). https://doi.org/10.1038/s41573-021-00233-1
Download citation
Accepted : 12 May 2021
Published : 30 June 2021
Issue Date : September 2021
DOI : https://doi.org/10.1038/s41573-021-00233-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Real-time imaging of mitochondrial redox reveals increased mitochondrial oxidative stress associated with amyloid β aggregates in vivo in a mouse model of alzheimer’s disease.
- Maria Calvo-Rodriguez
- Elizabeth K. Kharitonova
- Brian J. Bacskai
Molecular Neurodegeneration (2024)
Total iridoid glycoside extract of Lamiophlomis rotata (Benth) Kudo accelerates diabetic wound healing by the NRF2/COX2 axis
- Xiaoyu Geng
Chinese Medicine (2024)
Proanthocyanidins supplemented diet alter anti-aging-markers and improved lifespan in Drosophila melanogaster model
- Mohammed Sani Jaafaru
- Suleiman Alhaji Muhammad
- Ahmad Faizal Abdull Razis
Beni-Suef University Journal of Basic and Applied Sciences (2024)
Bio-nanoparticles loaded with synovial-derived exosomes ameliorate osteoarthritis progression by modifying the oxidative microenvironment
- Mingming Liu
Journal of Nanobiotechnology (2024)
Prognostic value of oxidative stress-related genes in colorectal cancer and its correlation with tumor immunity
- Leilei Yang
- Chengfeng Fang
- Shenkang Zhou
BMC Genomics (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
- Case report
- Open access
- Published: 25 May 2024
Wandering spleen presenting in the form of right sided pelvic mass and pain in a patient with AD-PCKD: a case report and review of the literature
- Yitagesu aberra shibiru ORCID: orcid.org/0000-0003-3645-9115 1 ,
- Sahlu wondimu 1 &
- Wassie almaw 1
Journal of Medical Case Reports volume 18 , Article number: 259 ( 2024 ) Cite this article
355 Accesses
2 Altmetric
Metrics details
Wandering spleen is a rare clinical entity in which the spleen is hypermobile and migrate from its normal left hypochondriac position to any other abdominal or pelvic position as a result of absent or abnormal laxity of the suspensory ligaments (Puranik in Gastroenterol Rep 5:241, 2015, Evangelos in Am J Case Rep. 21, 2020) which in turn is due to either congenital laxity or precipitated by trauma, pregnancy, or connective tissue disorder (Puranik in Gastroenterol Rep 5:241, 2015, Jawad in Cureus 15, 2023). It may be asymptomatic and accidentally discovered for imaging done for other reasons or cause symptoms as a result of torsion of its pedicle and infarction or compression on adjacent viscera on its new position. It needs to be surgically treated upon discovery either by splenopexy or splectomy based on whether the spleen is mobile or not.
Case presentation
We present a case of 39 years old female Ethiopian patient who presented to us complaining constant lower abdominal pain especially on the right side associated with swelling of one year which got worse over the preceding few months of her presentation to our facility. She is primiparous with delivery by C/section and a known case of HIV infection on HAART. Physical examination revealed a right lower quadrant well defined, fairly mobile and slightly tender swelling. Hematologic investigations are unremarkable. Imaging with abdominopelvic U/S and CT-scan showed a predominantly cystic, hypo attenuating right sided pelvic mass with narrow elongated attachment to pancreatic tail and absent spleen in its normal position. CT also showed multiple different sized purely cystic lesions all over both kidneys and the pancreas compatible with AD polycystic kidney and pancreatic disease.
With a diagnosis of wandering possibly infarcted spleen, she underwent laparotomy, the finding being a fully infarcted spleen located on the right half of the upper pelvis with twisted pedicle and dense adhesions to the adjacent distal ileum and colon. Release of adhesions and splenectomy was done. Her post-operative course was uneventful.
Wandering spleen is a rare clinical condition that needs to be included in the list of differential diagnosis in patients presenting with lower abdominal and pelvic masses. As we have learnt from our case, a high index of suspicion is required to detect it early and intervene by doing splenopexy and thereby avoiding splenectomy and its related complications.
Peer Review reports
Introduction
Wandering spleen is a rare clinical entity characterized by hypermobility of the spleen as a result of absence or abnormal laxity of its suspensory ligaments which in turn can be congenital or precipitated by a number of risk factors like repeated pregnancy, trauma, surgery or connective tissue disorder. The spleen therefore migrates from its normal left hypochondriac position, to other parts of the peritoneal cavity especially the pelvis [ 3 ]. Since the first case report in 1667, there have been less than 600 cases reported in the literature so far [ 1 , 3 ].
Wandering spleen can have different clinical presentations ranging from asymptomatic incidental finding on imaging to features of acute abdomen as a result of complete torsion of the pedicle and total infarction of the spleen or complete obstruction of adjacent hollow viscus due to pressure effect. Less dramatic presentation includes chronic lower abdominal pain, swelling and symptoms of partial obstruction of bowel especially of the colon [ 3 , 4 , 5 , 6 ].
Diagnosis is confirmed by imaging usually abdominal ultrasound or CT which reveals that the spleen is absent from its normal anatomical position but seen somewhere else in the new location within the peritoneal cavity [ 3 , 9 , 10 ]. Once diagnosed, surgical intervention is required either by splenopexy or splenectomy depending on the viability of the organ [ 3 , 5 ] and can be done laparoscopically or by laparotomy.
Owing to its rarity, a high index of suspicion is required and this condition should always be considered as a possible differential diagnosis in patients presenting with lower abdominal swelling and pain. We present this case to share our experience in diagnosing and managing such a rare pathology and once again bring it to the attention of fellow clinicians handling this sort of abdominal conditions.
Case summary
Our patient is a 39 years old female Primi-para Ethiopian, who presented with lower abdominal dull aching pain of one-year duration which got worse over the last few months associated with right lower abdominal swelling, easy fatigability, LGIF, loss of appetite and weight. She is a known case of RVI on HAART for the past 18yrs and hypertensive for the last 8 years for which she was taking enalapril and atenolol. Her only child was delivered by C/section 10 years ago.
On examination , she looked chronically sick with her vitals in the normal range. The abdomen was flat with a lower midline surgical scar and a visible round mass on the right paraumblical and lower quadrant areas. The mass was well defined, smooth surfaced, slightly tender and mobile (Fig. 1 —black arrow).
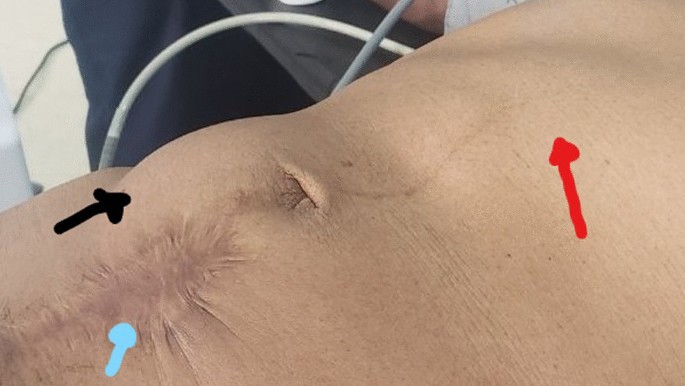
Black arrow shows the splenic mass, red arrow shows the stomach, cyan arrow shows previous CS scar
Her hematologic tests revealed WBC of 8.7 × 103, Hgb of 12.3 and PLT count of 544 × 10 3 . Serum electrolyte and liver function tests were all in the normal range. Creatinine was 1.4 mg/dl.
Abdominal ultrasound
Multiple bilateral renal, liver and pancreatic cysts. An ehcocomplex mainly hypoechoic, 13 cmx8cm well defined right sided abdomino-pelvic mass, with absent color Doppler flow. Spleen was not visualized in its normal anatomic site.
Contrast enhanced abdomino-pelvic CT
Described the mass as a hypoattenuating, well circumscribed lesion with no contrast enhancement located at right abdomino pelvic cavity (Fig. 2 ). Its long torsed pedicle could be traced to the region of the tail of the pancreas and the spleen was missing from its normal location. (Fig. 3 ) Majority of the renal parenchyma is almost replaced with different sized cystic lesions with imperceptible wall causing bilateral renal enlargement. (Fig. 3 ) The liver and the pancreas too is filled with similar cysts. The portal vein were not visualized and replaced by periportal enlarged collateral vessels. (Figs. 3 , 4 ).
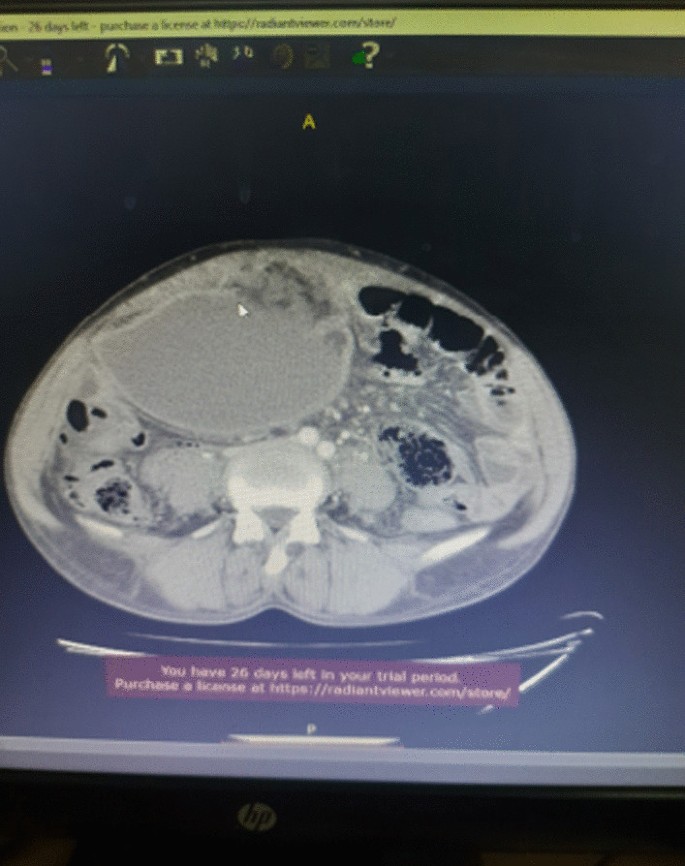
Infarcted spleen
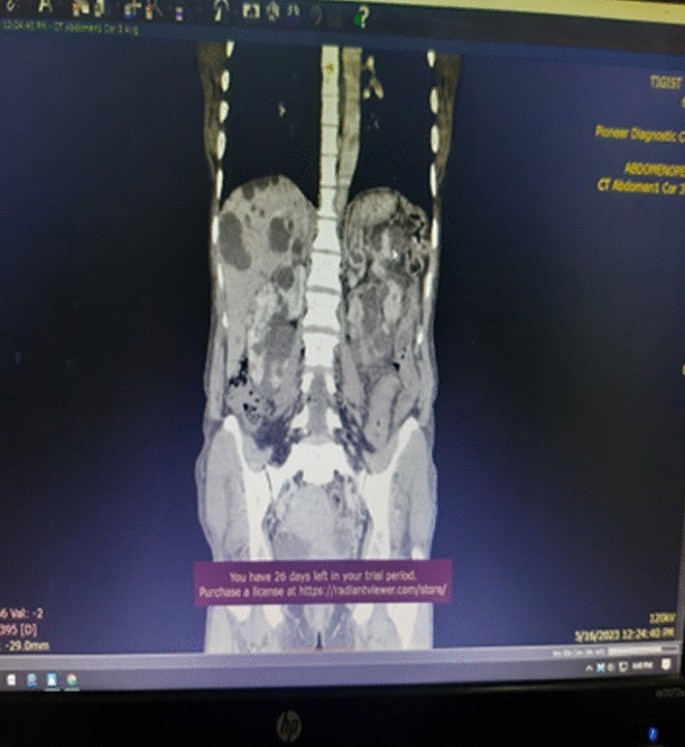
Absent spleen in the splenic fossa
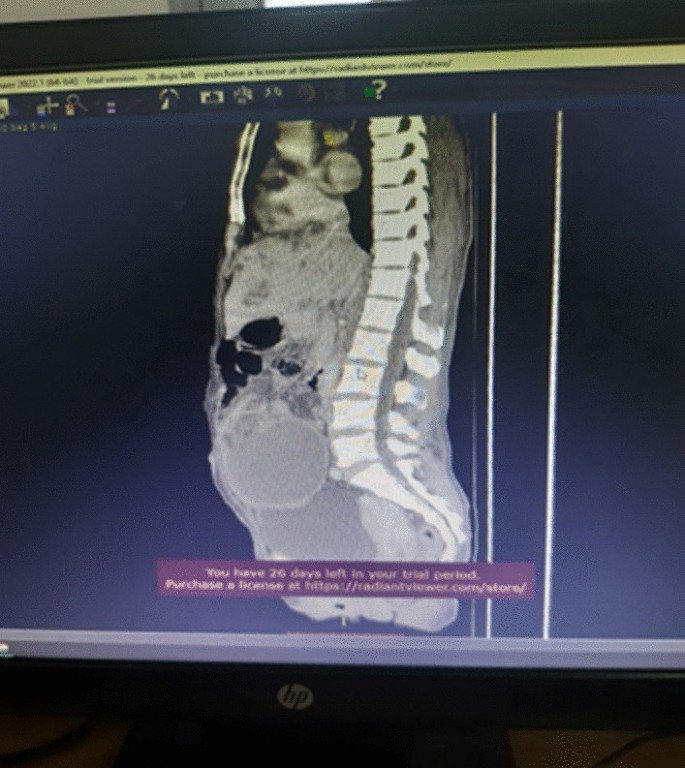
Spleen seen in the abdomino-pelvic cavity
With a diagnosis of wandering spleen located in the right abdomino pelvic region with torsion of the pedicle and infarction, she was admitted and underwent laparotomy. Intraoperatively, dense adhesion encountered between the anterior abdominal wall, omentum, the wandering spleen and small bowel. The spleen was whitish, distended and grossly infarcted with its long stalk torsed > 360°. (Fig. 5 ) Adhesions were gently released and splenectomy done. The splenic mass was sent for biopsy.
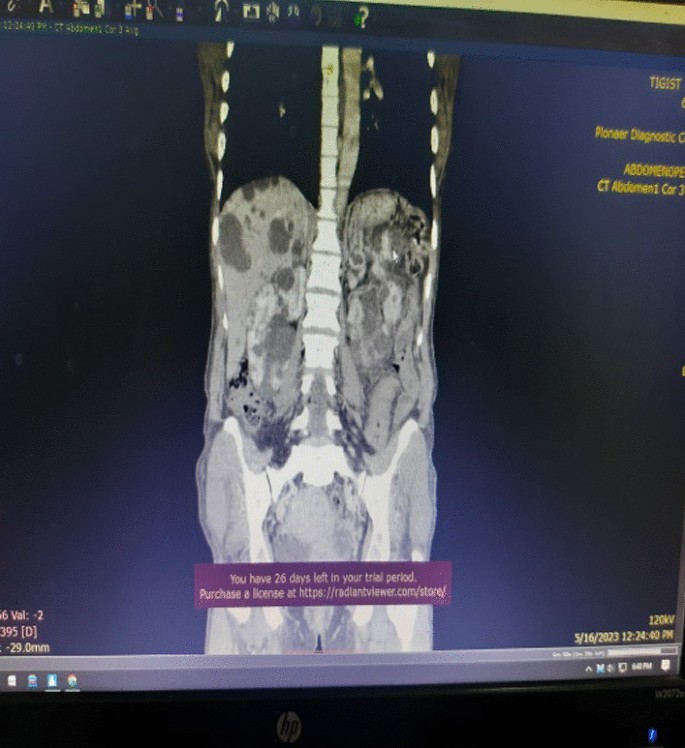
The intra-op picture of our patient upon exploration
She was discharged on the 3rd postoperative day and her post-operative course was uneventful. She was seen after a month on follow up clinic with no report of complication. Her biopsy result showed splenic tissue. She got her pentavelant vaccine on the third week.
Wandering spleen is a rare clinical entity characterized by splenic hypermobility from its left hypochondriac position to any other abdominal or pelvic position caused by absent or abnormal laxity of the suspensory ligaments [ 1 , 2 ].
The first case of wandering spleen was reported by Von Horne in 1667. So far less than 600 cases are reported world wide [ 1 , 3 ].
Anatomically a normal spleen is found in the left hypochondriac region suspended by ligaments to the stomach, kidney, pancreas, colon and left hemi-diaphram by the gastrosplenic, splenorenal, pancreaticosplenic, splenocolic, splenophreni ligaments and presplenic folds [ 1 ]. Our patient presented with RLQ palpable abdominal mass which is against the commonest presentation being in the LLQ of the abdomen (Fig. 1 ).
It could result from either a developmental failure of the embryonic septum transversum to fuse properly with the posterior abdominal wall which results in absent/lax ligaments [ 4 ] or from acquired conditions that result in lax suspensory ligaments as in pregnancy or connective tissue disorders [ 3 ]. The spleen is found in any quadrant of the abdomen or the pelvis though mostly in the left quadrants attached only by a long and loose vascular pedicle. Our patient presented with RLQ mass.
It is mostly seen in multiparous women [ 4 ] though the incidence is found to be nearly equal in both sexes in the prepubertal age group [ 3 ]. Our patient was a Para 1 mother and presented with 01 year history of abdominal pain which got worse in the past 06 months. Otherwise she had no any other pressure symptoms. She had visible umbilical area mass which was mobile up on examination
Wandering spleen can have different presentation ranging from asymptomatic incidental finding on imaging or upon surgical exploration for other surgical conditions to a presentation that mimics acute abdomen [ 3 , 5 ]. Mostly it presents as an on and of type acute/ subacute non-specific abdominal pain due to torsion and spontaneous de-torsion of the loose splenic pedicle [ 3 , 4 ]. This chronic torsion results in congestion and splenomegaly [ 3 , 5 ]. Hence patients could have palpable mobile mass [ 6 ] which is the typical presentation of this patient. The other presentations are usually related to the mass effect of the enlarged spleen and patients could present with GOO, bowel obstruction, pancreatitis and urinary symptoms [ 3 , 6 ].
In some cases it is reported to be associated with some other disorders like gastric volvulus [ 7 ] and distal pancreatic volvulus [ 8 ].
Ultrasound is one of the imaging modalities to investigate patients whom we suspect had wandering spleen. It usually shows absent spleen in the splenic fossa and a comma shaped spleen in the abdomen or pelvis [ 9 ]. Doppler study might help us see the vascular condition and ads up to a better preoperative plan. CT scan shows absence of the spleen in the left upper quadrant, ovoid or comma-shaped abdominal mass, enlarged spleen, a whirled appearance of non-enhancing splenic vessels and signs of splenic hypo-perfusion: homogenous or heterogeneous decreased enhancement depending on the degree of infarction [ 3 , 9 , 10 ].
Our patient was scanned with US and showed 13*8 cm large midline abdomino-pelvic well defined oval mass which was predominantly solid with areas of cystic component with absent color Doppler flow. Otherwise the spleen was not visualized in the splenic fossa. Bilateral kidney and liver has multiple different sized cystic lesions. With this image Abdomino-pelvic CT was done and shows spleen is located in the lower abdomen and appears to have torsed vascular pedicle and the whole splenic parenchyma is hypodense and no enhancement seen. Majority of the renal parenchyma is almost replaced with different sized cystic lesions with imperceptible wall causing bilateral renal enlargement. The whole liver is filled with cystic lesions with imperceptible wall. The portal veins were not visualized and replaced by periportal enlarged collateral vessels (Figs. 6 , 7 ).
Usually surgical management is the rule once a patient is diagnosed with wandering spleen [ 3 , 5 ]. Most patients; 65% as reported in some studies will have torsion of the vascular pedicle at some point of their life [ 5 , 6 ]. Hence splenopexy or splenectomy shall be considered when a wandering spleen is found incidentally up on surgical exploration for some other purposes [ 6 ]. Complicated wandering spleen like infarcted, signs of hypersplenism, huge in size and splenic vein thrombosis needs splenectomy while others can be managed with splenopexy [ 3 , 5 , 6 ]. Nowadays though laparoscopic technique is the gold standard, open technique can be used for splenopexy and splenectomy [ 3 , 5 ].
Partial infraction of a wandering spleen might necessitate partial splenectomy and splenopexy or splenectomy and splenic implantation [ 6 , 11 ].
The spleen might get fixed by different methods [ 8 , 9 ].
Simple splenic fixation involves simple tacking the splenic capsule to the peritoneum
Retroperitoneal pouch splenopexy- Tissue [ 11 , 12 ]/Mesh splenopexy (sandwich technique) [ 13 ].
Omental and peritoneal pouch splenic fixation [ 14 ].
In our case, Spleen was absent from the normal anatomic splenic fossa and the spleen in the abdomino-pelvic area looks infarcted. Hence she was managed with splenectomy and the patient was extubated on table and having a stable postoperative course .
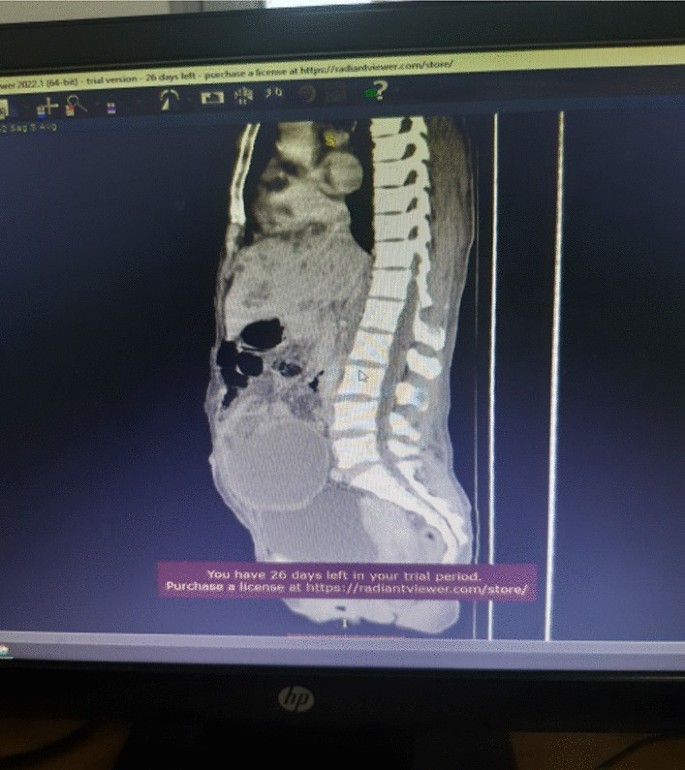
Wandering spleen is a rare form of splenic pathology. Such a rare pathology presents commonly as an acute torsion with infarction. Spleen in the RLQ with chronic torsion and infarction is a very rare presentation for wandering spleen. In addition there is no report of such a presentation in a patient with AD-PCKD.
Recommendation
We recommend Clinicians to consider wandering spleen in their differential diagnosis in a patient presenting with RLQ abdominal mass and chronic abdominal pain.
Availability of supporting data
Data related with this case report is available at Addis ababa university, Tikur Ambesa Tertiary Hospital.
Abbreviations
Autosomal dominant polycystic kidney disease
Blood pressure
Low grade intermittent fever
High active anti-retroviral therapy
Right lower quadrant
Retro viral infection
Hypertension
White blood cell count
Puranik AK, et al . Wandering spleen: a surgical enigma. Gastroenterol Rep. 2015;5:241.
Google Scholar
Evangelos K, et al . Wandering spleen volvulus: a case report and literature review of this diagnostic challenge. Am J Case Rep. 2020;21: e925301.
Jawad M. Wandering spleen: a rare case from the emergency department. Cureus. 2023;15(1): e33246.
PubMed PubMed Central Google Scholar
Ayaz UY, et al . Wandering spleen in a child with symptoms of acute abdomen. Med Ultrasonogr. 2012;14:64.
Masroor M, Sarwari MA. Torsion of the wandering spleen as an abdominal emergency: a case report. BMC Surg. 2021;21:289.
Article PubMed PubMed Central Google Scholar
Blouhos K, et al . Ectopic spleen: an easily identifiable but commonly undiagnosed entity until manifestation of complications. Int J Surg Case Rep. 2014;8:451–4.
Article Google Scholar
Uc A. Gastric volvulus and wandering spleen. Am J Gastroenrterol. 1998;93:1146–8.
Article CAS Google Scholar
Alqadi GO, Saxena AK. Is laparoscopic approach for wandering spleen in children an option? J Min Access Surg. 2019;15:93.
Awan M, et al . Torsion of wandering spleen treated by laparoscopic splenopexy. Int J Surgery Case Rep. 2019;62:58.
Taori K, et al . Wandering spleen with torsion of vascular pedicle: early diagnosis with multiplaner reformation technique of multislice spiral CT. Abdom Imaging. 2004;29:09428925.
Fonseca AZ, et al . Torsion of a wandering spleen treated with partial splenectomy and splenopexy. J Emerg Med. 2013;44:e33.
Article PubMed Google Scholar
Seashore JH, McIntosh S. Elective splenopexy for wandering spleen. J Pediatr Surg. 1990;25:270–2.
Article CAS PubMed Google Scholar
Soleimani M. Surgical treatment of patients with wandering spleen: report of six cases with a review of the literature. Surg Today. 2007;37:261.
Peitgen K. Laparoscopic splenopexy by peritoneal and omental pouch construction for intermittent splenic torsion (“wandering spleen”). Surg Endosc. 2001;15:413.
Download references
Acknowledgements
We would like to thank the managing team of this patient including all the ward staffs who played a great role in the peri-operative management of this patient. We also appreciate the support of our consultants, residents and member of the department of surgery and HPB unit. Our kind gratitude goes to the family of this patient for their unreserved support in post-operative period that helped for the fast recovery of this patient
Funding is not applicable.
Author information
Authors and affiliations.
Addis Ababa University, Addis Ababa, Ethiopia
Yitagesu aberra shibiru, Sahlu wondimu & Wassie almaw
You can also search for this author in PubMed Google Scholar
Contributions
Dr. Yitagesu Aberra, Main author of this case report, is an HPB surgery fellow in the department of surgery, college of health science, Addis Ababa University who was the leading surgeon in the management of this patient. Dr. Sahlu Wendimu is an HPB surgery subspecialist and Assistant professor of General Surgery who was the consultant in duty during the management of this patient. Dr.Wassie Almaw is a 2nd year pediatric surgery resident attaching at HPB surgery unit who took part in the management of this patient.
Corresponding author
Correspondence to Yitagesu aberra shibiru .
Ethics declarations
Ethics approval and consent to participate.
Ethical clearance is not applicable but we took oral and written consent from the patient for case presentation and publication.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
There is no competing interest in this case presentation.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
shibiru, Y.a., wondimu, S. & almaw, W. Wandering spleen presenting in the form of right sided pelvic mass and pain in a patient with AD-PCKD: a case report and review of the literature. J Med Case Reports 18 , 259 (2024). https://doi.org/10.1186/s13256-024-04580-6
Download citation
Received : 02 December 2023
Accepted : 02 May 2024
Published : 25 May 2024
DOI : https://doi.org/10.1186/s13256-024-04580-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Wandering spleen
- Splenic torsion
- Splenic infarction
- Splenectomy
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Prognostic Value of Stress Perfusion Cardiac MRI in Cardiovascular Disease: A Systematic Review and Meta-Analysis of the Effects of the Scanner, Stress Agent, and Analysis Technique
Affiliation.
- 1 From the Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China (Q.F.); Department of Radiology, Cambridge Biomedical Campus, University of Cambridge, Box 219, Level 5, Cambridge CB2 0QQ, England (Q.F., J.R.W.M.); Departments of Radiology (Q.F., J.R.W.M., S.A.) and Cardiology (S.P.H., G.A.), Royal Papworth Hospital, Cambridge, England; and School of Medicine & Population Health and INSIGNEO, Institute for In Silico Medicine, University of Sheffield, Sheffield, England (S.A.).
- PMID: 38814186
- DOI: 10.1148/ryct.230382
Purpose To perform a systematic review and meta-analysis to assess the prognostic value of stress perfusion cardiac MRI in predicting cardiovascular outcomes. Materials and Methods A systematic literature search from the inception of PubMed, Embase, Web of Science, and China National Knowledge Infrastructure until January 2023 was performed for articles that reported the prognosis of stress perfusion cardiac MRI in predicting cardiovascular outcomes. The quality of included studies was assessed using the Quality in Prognosis Studies tool. Reported hazard ratios (HRs) of univariable regression analyses with 95% CIs were pooled. Comparisons were performed across different analysis techniques (qualitative, semiquantitative, and fully quantitative), magnetic field strengths (1.5 T vs 3 T), and stress agents (dobutamine, adenosine, and dipyridamole). Results Thirty-eight studies with 58 774 patients with a mean follow-up time of 53 months were included. There were 1.9 all-cause deaths and 3.5 major adverse cardiovascular events (MACE) per 100 patient-years. Stress-inducible ischemia was associated with a higher risk of all-cause mortality (HR: 2.55 [95% CI: 1.89, 3.43]) and MACE (HR: 3.90 [95% CI: 2.69, 5.66]). For MACE, pooled HRs of qualitative, semiquantitative, and fully quantitative methods were 4.56 (95% CI: 2.88, 7.22), 3.22 (95% CI: 1.60, 6.48), and 1.78 (95% CI: 1.39, 2.28), respectively. For all-cause mortality, there was no evidence of a difference between qualitative and fully quantitative methods ( P = .79). Abnormal stress perfusion cardiac MRI findings remained prognostic when subgrouped based on underlying disease, stress agent, and field strength, with HRs of 3.54, 2.20, and 3.38, respectively, for all-cause mortality and 3.98, 3.56, and 4.21, respectively, for MACE. There was no evidence of subgroup differences in prognosis between field strengths or stress agents. There was significant heterogeneity in effect size for MACE outcomes in the subgroups assessing qualitative versus quantitative stress perfusion analysis, underlying disease, and field strength. Conclusion Stress perfusion cardiac MRI is valuable for predicting cardiovascular outcomes, regardless of the analysis method, stress agent, or magnetic field strength used. Keywords: MR-Perfusion, MRI, Cardiac, Meta-Analysis, Stress Perfusion, Cardiac MR, Cardiovascular Disease, Prognosis, Quantitative © RSNA, 2024 Supplemental material is available for this article.
Keywords: Cardiac; Cardiac MR; Cardiovascular Disease; MR-Perfusion; MRI; Meta-Analysis; Prognosis; Quantitative; Stress Perfusion.
Publication types
- Systematic Review
- Meta-Analysis
- Cardiovascular Diseases* / diagnostic imaging
- Exercise Test / methods
- Magnetic Resonance Imaging / methods
- Myocardial Perfusion Imaging / methods

IMAGES
VIDEO
COMMENTS
Based on the type, timing and severity of the applied stimulus, stress can exert various actions on the body ranging from alterations in homeostasis to life-threatening effects and death. In many cases, the pathophysiological complications of disease arise from stress and the subjects exposed to stress, e.g. those that work or live in stressful ...
Physiological responses to stress are thought to increase the risk of cardiovascular disease via haemodynamic, vascular and immune perturbations. In this Review, Vaccarino and Bremner focus on ...
The cumulative science linking stress to negative health outcomes is vast. Stress can affect health directly, through autonomic and neuroendocrine responses, but also indirectly, through changes in health behaviors. In this review, we present a brief overview of (a) why we should be interested in stress in the context of health; (b) the stress response and allostatic load; (c) some of the key ...
In this Review, Kivimäki and Steptoe assess the current evidence on the association between stress and cardiovascular disease, covering the multiple roles of stress in the development and ...
Psychological stress is generally accepted to be associated with an increased risk of cardiovascular disease (CVD), but results have varied in terms of how stress is measured and the strength of ...
Chronic stress, a validated health risk factor, remains an ambiguous construct in spite of years of research. We propose that chronic stress is best understood as a series of acute stress responses, and that these responses become maladaptive when they occur frequently or are of long duration.
Introduction. Understanding the influence of stress on health presents an ongoing challenge due to the complex nature of stress and the behavioural, endocrine and neural systems involved (Finch et al., Citation 2019; O'Connor et al., Citation 2021).Previous research has shown that high levels of stress have been directly linked with greater risk of a range of diseases and health conditions ...
Stress was associated with increased consumption of unhealthy foods (Hedges' g=0.116) but decreased consumption of healthy foods (Hedges' g=−0.111). Only one significant moderator (restraint on stress-unhealthy eating) was identified. This meta-analysis identified the magnitude of the effect of stress on eating behaviour outcomes.
In particular, occupational stress as a predictor of heart disease is a trending topic in the literature as evidenced by the articles with the most lifetime citations . Similarly, the articles with the fourth and fifth highest annual citations both examined heart disease as an outcome of occupational stress . These two findings suggest that ...
visits. Some of the health issues linked to stress include cardiovascul ar disease, obesity, diabetes, depression, anxiety, immun e system suppression, head aches, back and neck pai n, and sleep ...
Potential mechanisms by which work‐related stress may lead to coronary heart disease. Work stress has also been associated with increased levels of epinephrine and long‐term sympathetic activation, 20 which may have a ... Radoje D. Capecitabine cardiotoxicity: case reports and literature review. Hepatogastroenterology. 2008; 55:1249-1256 ...
Life stress is a central construct in many models of human health and disease. The present article reviews research on stress and health, with a focus on (a) how life stress has been conceptualized and measured over time, (b) recent evidence linking stress and disease, and (c) mechanisms that might underlie these effects. Emerging from this ...
The purpose of this Review is to summarize the evidence for an association between stress and cardiovascular disease focusing particularly on CHD and, to a lesser extent, stroke. The primary focus ...
Purpose of Review Gut microbiota contribute to several physiological processes in the host. The composition of the gut microbiome is associated with different neurological and neurodevelopmental diseases. In psychiatric disease, stress may be a major factor leading to gut microbiota alterations. Depressive disorders are the most prevalent mental health issues worldwide and patients often ...
Objective The objective of this scoping review is to review the research evidence regarding the impact of perinatal maternal stress on the maternal and infant gut and human milk microbiomes. Introduction Perinatal stress which refers to psychological stress experienced by individuals during pregnancy and the postpartum period is emerging as a public health concern. Early exposure of infants to ...
The rising prevalence of cardiovascular disease underscores the growing significance of heart failure (HF). Pathophysiological insights into HF highlight the dysregulation of the autonomic nervous system (ANS), characterized by sympathetic overactivity and diminished vagal tone, impacting cardiovascular function. Heart rate recovery (HRR), a metric measuring the heart's ability to return to ...
The plant hormone jasmonic acid plays an important role in plant growth and development, participating in many physiological processes, such as plant disease resistance, stress resistance, organ development, root growth, and flowering. With the improvement in living standards, people have higher requirements regarding the quality of vegetables. However, during the growth process of vegetables ...
Objective: This systematic review identified instruments quantitatively assessing psychosocial adaptation and outcomes in families of children with congenital heart disease (CHD) and evaluated instrument psychometrics. Methods: Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and a prospectively registered protocol, electronic databases (CINAHL, Embase ...
Nurses' turnover intention has been used in the nursing literature with different ... work environment. The number of critical cases, the uncertainty about the disease, and the frequency of death from the disease impose psychological stress on nurses ... J. 2016. EBOOK: How to do a systematic literature review in nursing: A step-by-step guide
Some studies have found that the pathological formation of kidney stones is closely related to injury and inflammatory response. Behaviors such as dietary composition, physical activity, obesity and smoking can all affect the body's oxidative stress levels. In order to evaluate the effects of various diets and lifestyles on the body's oxidative and antioxidant systems, an oxidative balance ...
Oxidative stress is a component of many diseases, including atherosclerosis, chronic obstructive pulmonary disease, Alzheimer disease and cancer. Although numerous small molecules evaluated as ...
Wandering spleen is a rare clinical entity in which the spleen is hypermobile and migrate from its normal left hypochondriac position to any other abdominal or pelvic position as a result of absent or abnormal laxity of the suspensory ligaments (Puranik in Gastroenterol Rep 5:241, 2015, Evangelos in Am J Case Rep. 21, 2020) which in turn is due to either congenital laxity or precipitated by ...
Purpose To perform a systematic review and meta-analysis to assess the prognostic value of stress perfusion cardiac MRI in predicting cardiovascular outcomes. Materials and Methods A systematic literature search from the inception of PubMed, Embase, Web of Science, and China National Knowledge Infra …