If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.

Unit 8: Acids, bases, and solutions
About this unit.
This unit is part of the Chemistry library. Browse videos, articles, and exercises by topic.
Acids, bases, and pH
- Arrhenius acids and bases (Opens a modal)
- pH, pOH, and the pH scale (Opens a modal)
- Brønsted-Lowry acids and bases (Opens a modal)
- Brønsted–Lowry acids and bases (Opens a modal)
- Autoionization of water (Opens a modal)
- Water autoionization and Kw (Opens a modal)
- Definition of pH (Opens a modal)
- Strong acid solutions (Opens a modal)
- Strong base solutions (Opens a modal)
- Acid strength, anion size, and bond energy (Opens a modal)
- Identifying weak acids and strong acids 7 questions Practice
- Identifying weak bases and strong bases 7 questions Practice
- Introduction to acid–base reactions 4 questions Practice
Acid-base equilibria
- Weak acid-base equilibria (Opens a modal)
- Conjugate acid–base pairs (Opens a modal)
- Relationship between Ka and Kb (Opens a modal)
- Ka and acid strength (Opens a modal)
- Weak acid equilibrium (Opens a modal)
- Weak base equilibrium (Opens a modal)
- Acid–base properties of salts (Opens a modal)
- pH of salt solutions (Opens a modal)
Buffer solutions
- Introduction to buffers (Opens a modal)
- Properties of buffers (Opens a modal)
- Henderson–Hasselbalch equation (Opens a modal)
- Common ion effect and buffers (Opens a modal)
- Buffer solution pH calculations (Opens a modal)
- Methods for preparing buffers (Opens a modal)
- pH and pKa relationship for buffers (Opens a modal)
- Buffer capacity (Opens a modal)
- Acid–base titrations (Opens a modal)
- Worked example: Determining solute concentration by acid–base titration (Opens a modal)
- Titration of a strong acid with a strong base (Opens a modal)
- Titration of a strong acid with a strong base (continued) (Opens a modal)
- Titration of a weak acid with a strong base (Opens a modal)
- Titration of a weak acid with a strong base (continued) (Opens a modal)
- Titration of a weak base with a strong acid (Opens a modal)
- Titration of a weak base with a strong acid (continued) (Opens a modal)
- 2015 AP Chemistry free response 3b (Opens a modal)
- 2015 AP Chemistry free response 3c (Opens a modal)
- 2015 AP Chemistry free response 3d (Opens a modal)
- 2015 AP Chemistry free response 3e (Opens a modal)
- 2015 AP Chemistry free response 3f (Opens a modal)
- Titration curves and acid-base indicators (Opens a modal)
- Redox titrations (Opens a modal)
- Introduction to titration 4 questions Practice
Solubility equilibria
- Dissolution and precipitation (Opens a modal)
- Common polyatomic ions (Opens a modal)
- Introduction to solubility equilibria (Opens a modal)
- Worked example: Calculating solubility from Kₛₚ (Opens a modal)
- 2015 AP Chemistry free response 4 (Opens a modal)
- The common-ion effect (Opens a modal)
- pH and solubility (Opens a modal)
- Solubility and complex ion formation (Opens a modal)

Acid value: Definition, Principle, Procedure, Formula, and 2 Reliable Uses
Pratiksha chaudhary.
- July 5, 2023
Table of Contents
Acid value (AV), also known as acid number , neutralization number , or acidity, is a quantity used in chemistry to express how acidic a certain chemical compound is. It’s the amount of base typically potassium hydroxide (KOH) expressed in milligrams that is necessary to neutralize the acidic components in 1 gram of a sample.
What is Acid value?
The acid value is a metric for the quantity of carboxylic acid groups (C(=O)OH) in a chemical compound, such a fatty acid, or in a blend of chemical compounds. It is a measurement of the amount of free fatty acids (FFAs) in a material. A common approach involves dissolving a known quantity of sample in an organic solvent (typically isopropanol) and titrating it with a solution of alcoholic potassium hydroxide (KOH) at a known concentration using phenolphthalein as a color indicator.
Principle of Acid value
The oil or fat in an alcoholic media is directly titrated against a standard potassium hydroxide or sodium hydroxide solution to ascertain its acid value.
RCOOH + KOH = RCOO – K + + H 2 O
Chemical required
- Balance machine
- measuring cyclinder
- conical flask
Solution required
- Phenolphthalein indicator
Procedure to Estimate acid value
Following are the procedure to estimate the acid value of given sample:
- First of all 0.1 g- 0.3 g of fat sample is taken in 100 mL of Erlenmeyer flask.
- And 10 mL of n-hexane is added and along with 1-2 drops of indicator.
- Then the solution is titrated against 0.02 N KOH solution. The end point is reached when pink colour persist for 30 seconds.
- The blank test is also carry out without taking fat sample.
- Then calculation is carried out.
Formula to calculate acid value
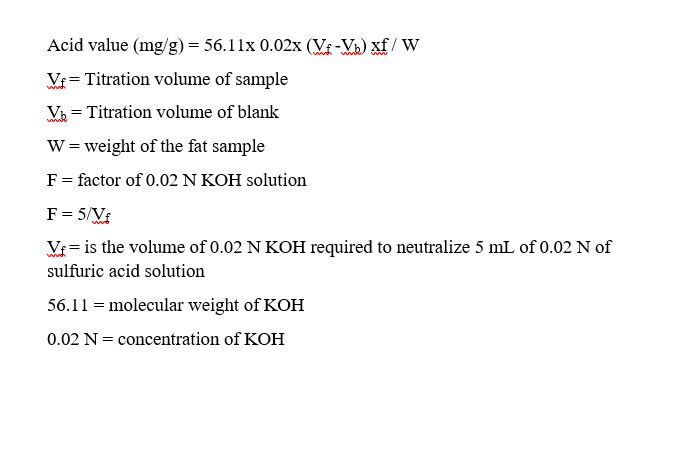
Reliable application
- The amount of rancidity of the provided fat is indicated by the acid value. Rancified oils provide high acid levels. Rancidification is the breakdown of fats and other lipids by oxidation and/or hydrolysis.
- Acid Value is a crucial sign of the quality of vegetable oil. The amount of potassium hydroxide (KOH, in milligrams) required to neutralize the free fatty acids present in 1 g of oil is the acid value.
Iodine value Vs Acid value
The amount of iodine in grams that is consumed by 100 grams of a chemical compound is known as the iodine value (IV; often referred to as the iodine absorption value, iodine number, or iodine index ) in chemistry. When assessing the level of unsaturation in fats, oils, and waxes, iodine values are frequently utilized. Unsaturation in fatty acids mostly manifests as double bonds that are highly reactive to halogens, in this example, iodine.
Acid value (AV), also known as acid number, neutralization number, or acidity, is a quantity used in chemistry to express how acidic a certain chemical compound is. It’s the amount of base—typically potassium hydroxide (KOH)—expressed in milligrams that is necessary to neutralize the acidic components in 1 gram of a sample.
Acid value of some oils
The acid value of some oils :
| 1 | maize | 0.223 |
| 2 | soya | 0.60 |
| 3 | virgin oil | 6.6 |
| 4 | used frying oil | 31 |
| 5 | canola | 0.071 |
| 6 | bee’s wax | 17-36 |
What does the acid value indicates?
The amount of potassium hydroxide milligrams needed to neutralize the free fatty acids found in one gram of fat is known as the acid value. Given that free fatty acids are often produced during the breakdown of triglycerides, it is a relative indicator of rancidity.
What does high acid value indicates?
The quality of the oil decreases with increasing acid value and free fatty acid level. As triglycerides break down with time into fatty acids and glycerol, the acid value of an oil also rises with age.
Which has lowest acid value?
Canola has lowest acid value.
Which has highest acid value?
The highest acid value is of virgin olive oil.
What is acid value?
The amount of potassium hydroxide needed to neutralize the free fatty acids in 1.0 g of fat or oil is known as the acid value.
Kardash, E. and Tur’yan, Y. I. 2005. Acid Value Determination in Vegetable Oils by Indirect Titration in Aqueous-alcohol Media. Croat. Chem. Acta 78:1:99-103. www.unctad.org/infocomm/angla…c/Cxs_033e.pdf
Share this to:
- Tags: acid number , ACID value , acid value of oils , application of acid value , estimation of acid value , formula to calculate acid value , high acid value , high acid value indicates , low acid value , neutralization number , principle of acid value
You may also like to read:
Ribozymes: Catalytic Activity of RNA
Satellitism Test: Principle, Reagents, Protocol, And Reliable Application
MacConkey Agar: Definition, Composition, Recipe, And Reliable Application

Mannitol Salt Agar: Definition, Principle, Recipe, Reliable Application
Urease Test: Principle, Protocol, Results, And Reliable Uses
LAP Test: Principle, Protocol, Results, and Reliable Uses
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Top Universities in the USA for Undergraduate and Graduate Chemistry Courses
Preparation of Phthalimide from Phthalic acid by two-step synthesis: Useful Lab Report
Feel Good Hormones: Dopamine, Oxytocin, Serotonin, and Endorphin
Invisible ink: Chemistry, Properties, and 3 Reliable Application
Pyrrole Disorder: Symptoms, Causes, and Treatment
The Chemistry of Mehendi: Composition, Side effects, and Reliable Application
How to Balance Redox Equations Using a Redox Reaction Calculator
Sugar Vs Jaggery: Differences, Calories And Many more
Centrifugation: Definition, Principle, Types, and 3 Reliable Application
Eppendorf Tube: Definition, Types, and Reliable Uses
Litmus Paper: Definition, Chemistry, Test, and 4 important Applications
Our mission is to provide free, world-class Chemisry Notes to Students, anywhere in the world.
Chemist Notes | Chemistry Notes for All | 2024 - Copyright©️ ChemistNotes.com

- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center
- Introduction & Top Questions
Basic structure
Biosynthesis and degradation.
- Chemical structure
- Biological structures
- Denaturation
- Ultraviolet absorption
- Methylation
- Supercoiling
- Sequence determination
- Messenger RNA (mRNA)
- Ribosomal RNA (rRNA)
- Transfer RNA (tRNA)
- Antisense RNAs
- Viral genomes
- RNA editing
- Basic mechanisms
- Enzymes of replication
- General recombination
- Site-specific recombination
- Transcription
- Translation

What nitrogen-containing bases occur in nucleic acids?
- Who discovered the structure of DNA?

nucleic acid
Our editors will review what you’ve submitted and determine whether to revise the article.
- The Rockefeller University - Nucleic Acid Background
- Michigan State University - Department of Chemistry - Nucleic Acids
- Khan Academy - Nucleic acids
- Thompson Rivers University Pressbooks - Nucleotides and Nucleic Acids
- Roger Williams University Open Publishing - Nucleotides and Nucleic Acids
- University of Hawaii Pressbooks - Biology - Nucleic Acids
- Chemistry LibreTexts - Nucleic Acid
- National Center for Biotechnology Information - PubMed Central - Understanding biochemistry: structure and function of nucleic acids
- Nature - The current landscape of nucleic acid therapeutics
- Table Of Contents

What are nucleic acids?
Nucleic acids are naturally occurring chemical compounds that serve as the primary information-carrying molecules in cells. They play an especially important role in directing protein synthesis. The two main classes of nucleic acids are deoxyribonucleic acid ( DNA ) and ribonucleic acid ( RNA ).
What is the basic structure of a nucleic acid?
Nucleic acids are long chainlike molecules composed of a series of nearly identical building blocks called nucleotides . Each nucleotide consists of a nitrogen-containing aromatic base attached to a pentose (five-carbon) sugar, which is in turn attached to a phosphate group.
Each nucleic acid contains four of five possible nitrogen-containing bases: adenine (A), guanine (G), cytosine (C), thymine (T), and uracil (U). A and G are categorized as purines, and C, T, and U are called pyrimidines. All nucleic acids contain the bases A, C, and G; T, however, is found only in DNA, while U is found in RNA.
When were nucleic acids discovered?
Nucleic acids were discovered in 1869 by Swiss biochemist Friedrich Miescher .
nucleic acid , naturally occurring chemical compound that serves as the main information-carrying molecule of the cell and that directs the process of protein synthesis, thereby determining the inherited characteristics of every living thing. Nucleic acids are further defined by their ability to be broken down to yield phosphoric acid , sugars , and a mixture of organic bases ( purines and pyrimidines ).
The two main classes of nucleic acids are deoxyribonucleic acid ( DNA ) and ribonucleic acid ( RNA ). DNA is the master blueprint for life and constitutes the genetic material in all free-living organisms and most viruses . RNA is the genetic material of certain viruses, but it is also found in all living cells, where it plays an important role in certain processes, such as the making of proteins.
Nucleotides : building blocks of nucleic acids
Nucleic acids are polynucleotides—that is, long chainlike molecules composed of a series of nearly identical building blocks called nucleotides . Each nucleotide consists of a nitrogen -containing aromatic base attached to a pentose (five- carbon ) sugar , which is in turn attached to a phosphate group.
Each nucleic acid contains four of five possible nitrogen-containing bases : adenine (A), guanine (G), cytosine (C), thymine (T), and uracil (U). A and G are categorized as purines , and C, T, and U are collectively called pyrimidines . All nucleic acids contain the bases A, C, and G; T, however, is found only in DNA, while U is found in RNA.
The pentose sugar in DNA ( 2′-deoxyribose ) differs from the sugar in RNA (ribose) by the absence of a hydroxyl group (―OH) on the 2′ carbon of the sugar ring. Without an attached phosphate group, the sugar attached to one of the bases is known as a nucleoside . The phosphate group connects successive sugar residues by bridging the 5′-hydroxyl group on one sugar to the 3′-hydroxyl group of the next sugar in the chain. These nucleoside linkages are called phosphodiester bonds and are the same in RNA and DNA.
Nucleotides are synthesized from readily available precursors in the cell. The ribose phosphate portion of both purine and pyrimidine nucleotides is synthesized from glucose via the pentose phosphate pathway. The six- atom pyrimidine ring is synthesized first and subsequently attached to the ribose phosphate. The two rings in purines are synthesized while attached to the ribose phosphate during the assembly of adenine or guanine nucleosides. In both cases the end product is a nucleotide carrying a phosphate attached to the 5′ carbon on the sugar. Finally, a specialized enzyme called a kinase adds two phosphate groups using adenosine triphosphate (ATP) as the phosphate donor to form ribonucleoside triphosphate, the immediate precursor of RNA. For DNA, the 2′-hydroxyl group is removed from the ribonucleoside diphosphate to give deoxyribonucleoside diphosphate. An additional phosphate group from ATP is then added by another kinase to form a deoxyribonucleoside triphosphate, the immediate precursor of DNA.
During normal cell metabolism, RNA is constantly being made and broken down. The purine and pyrimidine residues are reused by several salvage pathways to make more genetic material. Purine is salvaged in the form of the corresponding nucleotide, whereas pyrimidine is salvaged as the nucleoside.
- Engineering Mathematics
- Discrete Mathematics
- Operating System
- Computer Networks
- Digital Logic and Design
- C Programming
- Data Structures
- Theory of Computation
- Compiler Design
- Computer Org and Architecture
- DBMS Tutorial – Learn Database Management System
Basic of DBMS
- Introduction of DBMS (Database Management System) - Set 1
- History of DBMS
- Advantages of Database Management System
- Disadvantages of DBMS
- Application of DBMS
- Need for DBMS
- DBMS Architecture 1-level, 2-Level, 3-Level
- Difference between File System and DBMS
Entity Relationship Model
- Introduction of ER Model
- Structural Constraints of Relationships in ER Model
- Difference between entity, entity set and entity type
- Difference between Strong and Weak Entity
- Generalization, Specialization and Aggregation in ER Model
- Recursive Relationships in ER diagrams
Relational Model
- Introduction of Relational Model and Codd Rules in DBMS
- Types of Keys in Relational Model (Candidate, Super, Primary, Alternate and Foreign)
- Anomalies in Relational Model
- Mapping from ER Model to Relational Model
- Strategies for Schema design in DBMS
Relational Algebra
- Introduction of Relational Algebra in DBMS
- Basic Operators in Relational Algebra
- Extended Operators in Relational Algebra
- SQL Joins (Inner, Left, Right and Full Join)
- Join operation Vs Nested query in DBMS
- Tuple Relational Calculus (TRC) in DBMS
- Domain Relational Calculus in DBMS
Functional Dependencies
- Functional Dependency and Attribute Closure
- Armstrong's Axioms in Functional Dependency in DBMS
- Equivalence of Functional Dependencies
- Canonical Cover of Functional Dependencies in DBMS

Normalisation
- Introduction of Database Normalization
- Normal Forms in DBMS
- First Normal Form (1NF)
- Second Normal Form (2NF)
- Boyce-Codd Normal Form (BCNF)
- Introduction of 4th and 5th Normal Form in DBMS
- The Problem of Redundancy in Database
- Database Management System | Dependency Preserving Decomposition
- Lossless Decomposition in DBMS
- Lossless Join and Dependency Preserving Decomposition
- Denormalization in Databases
Transactions and Concurrency Control
- Concurrency Control in DBMS
ACID Properties in DBMS
- Implementation of Locking in DBMS
- Lock Based Concurrency Control Protocol in DBMS
- Graph Based Concurrency Control Protocol in DBMS
- Two Phase Locking Protocol
- Multiple Granularity Locking in DBMS
- Polygraph to check View Serializability in DBMS
- Log based Recovery in DBMS
- Timestamp based Concurrency Control
- Dirty Read in SQL
- Types of Schedules in DBMS
- Conflict Serializability in DBMS
- Condition of schedules to View-equivalent
- Recoverability in DBMS
- Precedence Graph for Testing Conflict Serializability in DBMS
- Database Recovery Techniques in DBMS
- Starvation in DBMS
- Deadlock in DBMS
- Types of Schedules based Recoverability in DBMS
- Why recovery is needed in DBMS
Indexing, B and B+ trees
- Indexing in Databases - Set 1
- Introduction of B-Tree
- Insert Operation in B-Tree
- Delete Operation in B-Tree
- Introduction of B+ Tree
- Bitmap Indexing in DBMS
- Inverted Index
- Difference between Inverted Index and Forward Index
- SQL Queries on Clustered and Non-Clustered Indexes
File organization
- File Organization in DBMS - Set 1
- File Organization in DBMS | Set 2
- File Organization in DBMS | Set 3
DBMS Interview questions and Last minute notes
- Last Minute Notes - DBMS
- Commonly asked DBMS interview questions
- Commonly asked DBMS interview questions | Set 2
DBMS GATE Previous Year Questions
- Database Management System - GATE CSE Previous Year Questions
- Database Management Systems | Set 2
- Database Management Systems | Set 3
- Database Management Systems | Set 4
- Database Management Systems | Set 5
- Database Management Systems | Set 6
- Database Management Systems | Set 7
- Database Management Systems | Set 8
A transaction is a single logical unit of work that accesses and possibly modifies the contents of a database. Transactions access data using read and write operations. In order to maintain consistency in a database, before and after the transaction, certain properties are followed. These are called ACID properties.
For those looking to master these concepts and excel in exams like GATE , our GATE course offers an in-depth exploration of database management systems. We cover everything from the basics to advanced topics, ensuring a thorough understanding that is essential for high scores and practical application
By this, we mean that either the entire transaction takes place at once or doesn’t happen at all. There is no midway i.e. transactions do not occur partially. Each transaction is considered as one unit and either runs to completion or is not executed at all. It involves the following two operations. — Abort : If a transaction aborts, changes made to the database are not visible. — Commit : If a transaction commits, changes made are visible. Atomicity is also known as the ‘All or nothing rule’.
Consider the following transaction T consisting of T1 and T2 : Transfer of 100 from account X to account Y .
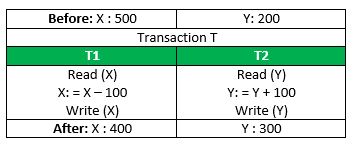
If the transaction fails after completion of T1 but before completion of T2 .( say, after write(X) but before write(Y) ), then the amount has been deducted from X but not added to Y . This results in an inconsistent database state. Therefore, the transaction must be executed in its entirety in order to ensure the correctness of the database state.
Consistency:
This means that integrity constraints must be maintained so that the database is consistent before and after the transaction. It refers to the correctness of a database. Referring to the example above, The total amount before and after the transaction must be maintained. Total before T occurs = 500 + 200 = 700 . Total after T occurs = 400 + 300 = 700 . Therefore, the database is consistent . Inconsistency occurs in case T1 completes but T2 fails. As a result, T is incomplete.
This property ensures that multiple transactions can occur concurrently without leading to the inconsistency of the database state. Transactions occur independently without interference. Changes occurring in a particular transaction will not be visible to any other transaction until that particular change in that transaction is written to memory or has been committed. This property ensures that the execution of transactions concurrently will result in a state that is equivalent to a state achieved these were executed serially in some order. Let X = 500, Y = 500. Consider two transactions T and T”.
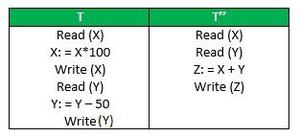
Suppose T has been executed till Read (Y) and then T’’ starts. As a result, interleaving of operations takes place due to which T’’ reads the correct value of X but the incorrect value of Y and sum computed by T’’: (X+Y = 50, 000+500=50, 500) is thus not consistent with the sum at end of the transaction: T: (X+Y = 50, 000 + 450 = 50, 450) . This results in database inconsistency, due to a loss of 50 units. Hence, transactions must take place in isolation and changes should be visible only after they have been made to the main memory.
Durability:
This property ensures that once the transaction has completed execution, the updates and modifications to the database are stored in and written to disk and they persist even if a system failure occurs. These updates now become permanent and are stored in non-volatile memory. The effects of the transaction, thus, are never lost.
Some important points:
| Atomicity | Transaction Manager |
| Consistency | Application programmer |
| Isolation | Concurrency Control Manager |
| Durability | Recovery Manager |
The ACID properties, in totality, provide a mechanism to ensure the correctness and consistency of a database in a way such that each transaction is a group of operations that acts as a single unit, produces consistent results, acts in isolation from other operations, and updates that it makes are durably stored.
ACID properties are the four key characteristics that define the reliability and consistency of a transaction in a Database Management System (DBMS). The acronym ACID stands for Atomicity, Consistency, Isolation, and Durability. Here is a brief description of each of these properties:
- Atomicity: Atomicity ensures that a transaction is treated as a single, indivisible unit of work. Either all the operations within the transaction are completed successfully, or none of them are. If any part of the transaction fails, the entire transaction is rolled back to its original state, ensuring data consistency and integrity.
- Consistency: Consistency ensures that a transaction takes the database from one consistent state to another consistent state. The database is in a consistent state both before and after the transaction is executed. Constraints, such as unique keys and foreign keys, must be maintained to ensure data consistency.
- Isolation: Isolation ensures that multiple transactions can execute concurrently without interfering with each other. Each transaction must be isolated from other transactions until it is completed. This isolation prevents dirty reads, non-repeatable reads, and phantom reads.
- Durability: Durability ensures that once a transaction is committed, its changes are permanent and will survive any subsequent system failures. The transaction’s changes are saved to the database permanently, and even if the system crashes, the changes remain intact and can be recovered.
Overall, ACID properties provide a framework for ensuring data consistency, integrity, and reliability in DBMS. They ensure that transactions are executed in a reliable and consistent manner, even in the presence of system failures, network issues, or other problems. These properties make DBMS a reliable and efficient tool for managing data in modern organizations.
Advantages of ACID Properties in DBMS:
- Data Consistency: ACID properties ensure that the data remains consistent and accurate after any transaction execution.
- Data Integrity: ACID properties maintain the integrity of the data by ensuring that any changes to the database are permanent and cannot be lost.
- Concurrency Control: ACID properties help to manage multiple transactions occurring concurrently by preventing interference between them.
- Recovery: ACID properties ensure that in case of any failure or crash, the system can recover the data up to the point of failure or crash.
Disadvantages of ACID Properties in DBMS:
- Performance: The ACID properties can cause a performance overhead in the system, as they require additional processing to ensure data consistency and integrity.
- Scalability: The ACID properties may cause scalability issues in large distributed systems where multiple transactions occur concurrently.
- Complexity: Implementing the ACID properties can increase the complexity of the system and require significant expertise and resources. Overall, the advantages of ACID properties in DBMS outweigh the disadvantages. They provide a reliable and consistent approach to data
- management, ensuring data integrity, accuracy, and reliability. However, in some cases, the overhead of implementing ACID properties can cause performance and scalability issues. Therefore, it’s important to balance the benefits of ACID properties against the specific needs and requirements of the system.
Please Login to comment...
Similar reads.
- Computer Subject
Improve your Coding Skills with Practice
What kind of Experience do you want to share?
Pardon Our Interruption
As you were browsing something about your browser made us think you were a bot. There are a few reasons this might happen:
- You've disabled JavaScript in your web browser.
- You're a power user moving through this website with super-human speed.
- You've disabled cookies in your web browser.
- A third-party browser plugin, such as Ghostery or NoScript, is preventing JavaScript from running. Additional information is available in this support article .
To regain access, please make sure that cookies and JavaScript are enabled before reloading the page.

Snapsolve any problem by taking a picture. Try it in the Numerade app?
- Solving Nursing Assignments: Body Fluid and Acid-Base Balance Explained
Understanding Body Fluids and Their Distribution

Solving nursing assignments on body fluid and acid-base balance is fundamental to providing high-quality patient care. This topic requires a deep understanding of fluid distribution, the crucial roles of electrolytes, and the intricate mechanisms that regulate pH levels in the body. Successfully tackling these assignments not only helps you grasp essential physiological concepts but also prepares you for real-world nursing challenges. By methodically approaching these assignments, you can ensure a comprehensive response that demonstrates your knowledge and clinical skills. This guide will equip you with the necessary tools and strategies to effectively complete your nursing assignment on body fluid and acid-base balance, covering key aspects from fluid distribution and electrolyte functions to regulatory mechanisms and nursing interventions.
Body fluids are divided into two main types:
- Intracellular Fluid (ICF): Found within the cells, constituting about two-thirds of the body’s total fluid.
- Extracellular Fluid (ECF): Located outside the cells, comprising intravascular (plasma) and interstitial (tissue fluid) fluids.

Water, making up 45% to 75% of the body’s total weight, is the largest single constituent of the body. Its balance is essential for physiological functions.
Electrolytes and Their Physiological Functions
Electrolytes are minerals that dissolve in body fluids to form ions. They play critical roles in:
- Neuromuscular Irritability: Necessary for muscle contraction and nerve transmission.
- Osmolarity Regulation: Maintaining the concentration of solutes in body fluids.
- Acid-Base Balance: Regulating the pH of body fluids.
- Fluid Distribution: Managing the distribution of fluids between different compartments.
Key electrolytes include sodium, potassium, calcium, and magnesium. The body regulates fluid balance through fluid and food intake, and excretion via skin, lungs, gastrointestinal tract, and kidneys.
Hormonal Regulation of Fluid Balance
When an ECF volume deficit occurs, hormones such as aldosterone and antidiuretic hormone (ADH) play vital roles in restoring the ECF volume. Sodium is the primary electrolyte that promotes water retention, crucial for maintaining fluid balance.
Acid-Base Balance Mechanisms
Acid-base balance refers to the homeostasis of hydrogen ion concentration in body fluids:
- Acidosis: Occurs when the pH value falls below 7.35 due to an increase in hydrogen ions.
- Alkalosis: Occurs when the pH value rises above 7.45 due to a decrease in hydrogen ions.
The body has three main control systems to regulate acid-base balance:
- Buffer Systems: Quickly neutralize excess acids or bases.
- Respiratory Regulation: Adjusts the pH by controlling CO2 levels through breathing.
- Renal Control: Regulates hydrogen ion concentration by excreting or retaining bicarbonate.
Impact of Electrolyte Imbalance
Disturbances in one electrolyte often affect others and can alter blood pH. For instance, slight changes in extracellular potassium can have serious effects on physiological functions, highlighting the importance of maintaining balance.
Nursing Interventions and Client Safety
Nursing interventions aim to resolve alterations in fluid balance based on client safety and standards of care. Consider the following steps in your assignments:
1. Assessment:
- Collect the client's health history, focusing on fluid intake, output, and recent weight changes.
- Conduct a physical assessment to check for signs of dehydration, such as dry mucous membranes, decreased skin turgor, and hypotension.
- Analyze biochemical data, including electrolyte levels and blood urea nitrogen (BUN) to creatinine ratio.
2. Interventions:
- Fluid Replacement: Administer oral or intravenous fluids as prescribed, ensuring adequate hydration.
- Electrolyte Management: Monitor and replace electrolytes as needed, particularly sodium and potassium.
- Dietary Advice: Encourage the client to consume a balanced diet with adequate fluid intake.
- Environment: Maintain a comfortable and safe environment to prevent overheating and encourage fluid intake.
- Education: Educate the client and family about the importance of maintaining hydration and recognizing early signs of dehydration.
3. Monitoring:
- Regularly check vital signs, including blood pressure and heart rate.
- Measure and record intake and output accurately.
- Monitor for signs of fluid overload or deficit, adjusting the treatment plan as necessary.
4. Evaluation:
- Evaluate the client’s progress by assessing weight, intake and output, vital signs, and laboratory results.
- Adjust nursing interventions based on ongoing assessments and client response to treatment.
Detailed Approach to Solving Assignments
When solving medical-surgical assignments on body fluid and acid-base balance, it's crucial to follow a structured and detailed approach. This involves understanding key concepts, conducting thorough assessments, implementing appropriate interventions, and continuously monitoring and evaluating patient outcomes. By doing so, you ensure comprehensive and effective care in your assignments. When tasked with solving nursing assignments on body fluid and acid-base balance, follow these structured steps:
Step 1: Define Key Concepts:
Defining key concepts related to body fluid and acid-base balance is essential when tackling nursing assignments. Here’s a detailed breakdown:
1. Body Fluid Compartments:
- Intracellular Fluid (ICF): Fluid inside cells, accounting for approximately 60% of total body water.
- Extracellular Fluid (ECF): Fluid outside cells, comprising intravascular (blood plasma) and interstitial fluids.
2. Electrolyte Functions:
- Sodium (Na+): Primary cation in ECF, crucial for fluid balance and neuromuscular function.
- Potassium (K+): Predominantly intracellular, essential for nerve conduction and muscle function.
- Chloride (Cl-): Major anion in ECF, maintaining osmotic pressure and acid-base balance.
- Bicarbonate (HCO3-): Key buffer in blood plasma, regulating pH by neutralizing acids.
3. Mechanisms of Acid-Base Balance:
- Buffer Systems: Bicarbonate, protein, and phosphate buffers stabilize pH by absorbing excess hydrogen ions (H+).
- Respiratory Regulation: Lungs adjust pH by altering CO2 levels; hypoventilation retains CO2, causing acidosis, while hyperventilation reduces CO2, causing alkalosis.
- Renal Control: Kidneys regulate pH by excreting or reabsorbing bicarbonate and hydrogen ions based on body needs.
4. Clinical Significance:
- Fluid Imbalances: Includes dehydration (ECF deficit) and fluid overload (ECF excess), affecting cardiovascular and renal function.
- Electrolyte Imbalances: Disorders such as hyponatremia (low sodium) or hyperkalemia (high potassium) can disrupt nerve and muscle function.
- Acid-Base Disorders: Acidosis (low pH) or alkalosis (high pH) disrupts enzyme function and cellular metabolism.
Understanding these foundational concepts equips nurses to analyze scenarios accurately and formulate appropriate interventions to maintain or restore balance in clinical practice and academic assignments.
Step 2: Analyze Assignment Requirements
Analyzing assignment requirements is crucial to effectively address the specific tasks related to body fluid and acid-base balance. Here’s how to approach it:
1. Review Assignment Prompt:
- Carefully read through the assignment prompt to identify the specific questions or tasks related to fluid and acid-base balance.
- Highlight key words or phrases that indicate the focus areas, such as "evaluate," "describe," "compare," or "analyze."
2. Identify Case Studies or Scenarios:
- Determine if the assignment includes case studies or scenarios that require application of fluid and acid-base balance concepts.
- Note any patient data provided, such as laboratory results, vital signs, or medical histories, that will inform your analysis.
3. Understand Learning Objectives:
- Refer to the course syllabus or learning objectives to align your analysis with the expected outcomes for the assignment.
- Clarify whether the assignment aims to assess your ability to apply theoretical knowledge, critical thinking skills, or both.
4. Note Specific Requirements:
- Pay attention to formatting requirements, citation styles, and word count limitations specified in the assignment instructions.
- Ensure you address all components of the assignment prompt, including any sub-questions or parts that require separate responses.
5. Consider Audience and Context:
- Reflect on the intended audience for your assignment (e.g., instructor, peers) and adjust your approach accordingly.
- Consider the clinical context or setting implied by the assignment to tailor your analysis and recommendations appropriately.
6. Plan Your Approach:
- Outline a structured approach to address each requirement, organizing your response logically with clear headings and subheadings.
- Allocate time for research, analysis, and drafting to meet assignment deadlines effectively.
By carefully analyzing the assignment requirements, you set a solid foundation for developing a focused and comprehensive response that demonstrates your understanding and application of body fluid and acid-base balance concepts in nursing practice.
Step 3: Apply Theoretical Knowledge
Applying theoretical knowledge is essential when addressing medical-surgical nursing assignments focused on body fluid and acid-base balance. Here’s a structured approach:
1. Conceptual Understanding:
- Begin by revisiting the theoretical concepts related to body fluid compartments, electrolyte functions, and acid-base balance that you defined earlier.
- Ensure clarity on how these concepts interrelate and their significance in maintaining homeostasis.
2. Case Study Analysis:
- If the assignment includes a case study or scenario, apply your theoretical knowledge to analyze patient data.
- Interpret laboratory values, such as electrolyte levels and arterial blood gases, to assess the patient's fluid and acid-base status.
3. Identify Imbalances:
- Identify any fluid or electrolyte imbalances indicated in the case study, such as dehydration, hypokalemia, metabolic acidosis, etc.
- Consider the implications of these imbalances on the patient's health and nursing care priorities.
4. Formulate Nursing Diagnoses:
- Based on your analysis, formulate relevant nursing diagnoses related to fluid and acid-base balance.
- Use standardized nursing language (e.g., NANDA-I) to articulate these diagnoses accurately.
5. Develop Interventions:
- Propose nursing interventions aimed at addressing identified imbalances and promoting optimal fluid and electrolyte status.
- Tailor interventions to the specific needs of the patient, considering factors such as age, comorbidities, and treatment preferences.
6. Provide Evidence-Based Rationale:
- Justify your chosen interventions with evidence-based rationale supported by current literature and clinical guidelines.
- Reference peer-reviewed sources to strengthen the credibility of your recommendations.
7. Consider Ethical and Cultural Factors:
- Reflect on ethical considerations, such as patient autonomy and informed consent, when planning interventions.
- Consider cultural factors that may influence fluid intake preferences or attitudes towards healthcare practices.
By systematically applying theoretical knowledge to analyze case studies and formulate nursing interventions, you demonstrate your ability to integrate classroom learning with clinical reasoning in addressing complex nursing assignments. This approach ensures a thorough and evidence-based response that meets the academic requirements and prepares you for practical nursing challenges.
Step 4: Formulate Nursing Interventions
Formulating nursing interventions is crucial when solving medical-surgical nursing assignments on body fluid and acid-base balance. Follow these steps to develop effective interventions:
1. Assessment-Based Interventions:
- Base interventions on the assessment findings related to fluid and electrolyte imbalances identified in the assignment scenario.
- Prioritize interventions according to the urgency and severity of the imbalance, focusing on patient safety and physiological stability.
2. Fluid Management:
- Implement strategies to manage fluid intake and output based on patient needs and clinical indications.
- Adjust fluid administration according to fluid balance assessments and prescribed therapies, such as intravenous fluids or oral rehydration solutions.
3. Electrolyte Replacement:
- Prescribe electrolyte replacement therapies as indicated by laboratory results and clinical symptoms.
- Monitor electrolyte levels closely and adjust replacement therapies to maintain optimal balance and prevent complications.
4. Monitoring and Assessment:
- Establish a plan for ongoing monitoring of fluid status, electrolyte levels, and acid-base balance.
- Use assessment tools and techniques, such as daily weights, intake-output records, and laboratory tests, to track patient responses to interventions.
5. Patient Education:
- Educate patients and caregivers about the importance of fluid intake, dietary modifications, and adherence to prescribed therapies.
- Provide clear instructions on recognizing signs of fluid or electrolyte imbalances and when to seek medical assistance.
6. Collaborative Care:
- Collaborate with interprofessional team members, such as physicians, pharmacists, and dietitians, to coordinate comprehensive care.
- Communicate effectively to ensure continuity of care and shared decision-making regarding patient management.
7. Documentation and Evaluation:
- Document nursing interventions, patient responses, and outcomes accurately and comprehensively.
- Evaluate the effectiveness of interventions based on predefined goals and modify the plan of care as needed to achieve desired outcomes.
By systematically formulating nursing interventions based on assessment data and evidence-based practices, you demonstrate clinical reasoning skills and readiness to address complex challenges in nursing practice. This approach ensures a structured and patient-centered response that aligns with academic expectations and enhances your ability to provide quality care.
Step 5: Provide Rationale
When formulating nursing interventions for assignments on body fluid and acid-base balance, it's crucial to justify your choices with clear rationale. Here’s how to provide a strong rationale:
1. Evidence-Based Practice:
- Support your interventions with evidence from current nursing literature, clinical practice guidelines, and relevant research studies.
- Cite sources that demonstrate the effectiveness and safety of the interventions chosen.
2. Pathophysiological Understanding:
- Explain how each intervention addresses the underlying pathophysiology of the fluid or electrolyte imbalance.
- Link physiological principles to the expected outcomes of the interventions.
3. Patient-Specific Factors:
- Consider individual patient factors, such as age, comorbidities, and preferences, when justifying interventions.
- Tailor your rationale to reflect how patient-specific needs influence the choice of interventions.
4. Risk-Benefit Analysis:
- Evaluate the potential risks and benefits associated with each intervention.
- Discuss how the chosen interventions maximize benefits while minimizing potential adverse effects.
5. Nursing Standards and Protocols:
- Align interventions with established nursing standards, protocols, and best practices.
- Highlight compliance with institutional policies and procedures to ensure safe and effective care delivery.
6. Clinical Decision-Making:
- Describe the thought process that led to selecting specific interventions over alternative options.
- Justify why the chosen interventions are most appropriate given the patient's clinical presentation and overall care plan.
7. Holistic Patient Care:
- Emphasize the holistic approach to patient care by considering the impact of interventions on the patient’s overall well-being.
- Address psychosocial, cultural, and ethical considerations that may influence the implementation of interventions.
By providing a thorough and evidence-based rationale for your nursing interventions, you demonstrate critical thinking skills and a comprehensive understanding of nursing practice. This approach strengthens your academic assignments and prepares you to deliver quality patient care in clinical settings.
Maintaining body fluid and acid-base balance is critical for health promotion and effective patient care. As you do your nursing assignment on this topic, understanding the intricate details of fluid types, electrolyte functions, and regulatory mechanisms is essential. This guide has provided a structured approach to tackling such assignments, ensuring a thorough and well-rounded response. By following the outlined steps, you can enhance your comprehension of these vital concepts and improve your ability to apply them in clinical practice. Solving these assignments not only solidifies your theoretical knowledge but also hones your practical nursing skills, ultimately contributing to your growth as a competent and confident nursing professional.
Post a comment...
Solving nursing assignments: body fluid and acid-base balance explained submit your assignment, attached files.
| File | Actions |
|---|
- Implementing Joint Venture Management
How Ownership Definition Assignment Rules Are Applied to Joint Venture Transactions
Before you create ownership definition assignment rules for a joint venture, it's important to understand how assignment rules are applied to joint venture transactions.
- Determine which transactions are eligible for processing. Only transactions dated within the specified period are eligible.
- Identify the assignment rules to process for a joint venture. Only assignment rules with an effective date range that overlaps the dates for the selected period are processed.
The following illustration shows how assignment rules A, B, and C, but not D, will be processed for Period Jan 21. This is because the dates for the period are 1/1/21 through 1/31/21, and the dates for assignment rules A, B, and C overlap these dates. The dates for assignment rule D don't coincide with the dates for the period.

The assignment rule is applied to all transactions that match the account and project information specified in the assignment rule.
The process ignores transactions to which an ownership definition or direct billed stakeholder has already been applied.
Check for Conflicts
You need to make sure that there are no conflicts or redundancies within the assignment rules that you create for a joint venture. You want to avoid setting up two different assignment rules for the same account in a joint venture, with each assignment rule having a different ownership definition or direct billed stakeholder, for example:
- An assignment rule to assign an ownership definition to transactions in a joint venture account set.
- An assignment rule to assign a direct billed stakeholder to transactions in one of the accounts specified in the account set in the preceding assignment rule.
After the process applies one of the assignment rules to transactions, the other assignment rule can't be used for the same transactions because an assignment already exists.
To avoid this and other potential conflicts, it's recommended that you use a spreadsheet to plan or map out all the accounts with joint venture transactions that you need to create assignment rules for. If applicable, include project details if you're creating assignment rules for the distribution of project-related transactions. Identify the ownership definition or direct billed stakeholder for each assignment rule and the sequence or order in which you want to process each assignment rule. After reviewing the assignment rules and ensuring that there aren't conflicts, gaps, or redundancies, create the assignment rules in Oracle Joint Venture Management.
Unassigned Joint Venture Transactions
After running the process to identify joint venture transactions and apply assignment rules, it's possible to have unassigned joint venture transactions in the Joint Venture Transactions work area. This occurs when neither an ownership definition nor a direct billed stakeholder has been associated with a joint venture transaction. Unassigned transactions can't be distributed. However, you can manually assign an ownership definition or direct billed stakeholder to these transactions in the Joint Venture Transactions work area.
Harris' border work was on 'root causes' of migration; she wasn't in charge | Fact check

The claim: Kamala Harris was 'put in charge of the border'
A July 21 Instagram post ( direct link , archive link ) by Donald Trump Jr. blames Vice President Kamala Harris for the country's immigration problems.
"She was put in charge of the border and we saw the worst invasion of illegals in our history!!!" reads part of the post, which is a screenshot of a post from X, formerly Twitter.
Similar posts on Threads have described Harris as the Biden administration's "border czar."
The Instagram post was liked more than 200,000 times in a day.
More from the Fact-Check Team: How we pick and research claims | Email newsletter | Facebook page
Our rating: False
The post exaggerates the vice president's role in addressing migration at the southern border. Harris was never put in charge of the border or made "border czar," immigration experts said. President Joe Biden tasked Harris with leading the administration's diplomatic efforts addressing the "root causes" of migration in El Salvador, Guatemala and Honduras.
Harris led effort addressing 'root causes' of migration in Central America
Early in his presidency, Biden tasked Harris with addressing the “root causes” of migration in Central America. The assignment came out of an executive order Biden issued in February 2021 that sought to reduce migration from the Northern Triangle countries of El Salvador, Guatemala and Honduras, where gang violence, trafficking networks and economic insecurity have caused people to flee.
But the vice president’s role was more limited than being put in charge of the southern border, or being named a so-called “border czar,” immigration experts said.
"VP Harris was never made the border czar or charged with managing the border," Andrew Salee , president of the Migration Policy Institute , said in an email. "That role has always been held by the secretary of Homeland Security . She was asked to be the chief diplomatic officer with Central American countries at a time when most of the increase in unauthorized immigration was coming from three countries in Central America and to help lead a private investment strategy in the region."
Homeland Security Secretary Alejandro Mayorkas himself noted the different responsibilities between himself and Harris in June 2021 comments at the El Paso, Texas, border.
"The vice president is leading our nation’s efforts to address the root causes – that fundamental question of why people leave their homes," Mayorkas said. "And it is my responsibility as the secretary of Homeland Security to address the security and management of our border."
In March 2021, Biden announced Harris would lead the administration's diplomatic efforts with the Northern Triangle countries to stem migration to the U.S. southern border and work with these nations to enhance migration enforcement at their borders. Harris said at the time that the administration "must address the root causes that – that cause people to make the trek, as the president has described, to come here."
Aaron Reichlin-Melnick , policy director at the American Immigration Council , said the "root causes" work Harris took on is distinct from border policy because it focuses on different problems and targets.
"Border policy focuses on individuals who have already made the decision to leave home and have made it to the U.S.-Mexico border and aims to either prevent them or to quickly process them for humanitarian relief or deportation once they cross," Reichlin-Melnick said in an email. "By contrast, 'root causes' policy focuses on individuals who have not left their homes yet, and aims to convince them to stay in their home countries either through economic development – which discourages migration for economic opportunities – or through reduction of violence and persecution that forces people to seek protection elsewhere."
The White House released the administration's " Root Causes Strategy " in July 2021. Its implementation was ongoing as of March when the vice president and the Partnership for Central America , a non-governmental organization, jointly announced $1 billion in new private-sector commitments to address the underlying conditions leading to migration in Guatemala, El Salvador and Honduras. The public-private partnership has generated more than $5.2 billion since May 2021 , the White House said.
Fact check : Joe Biden dropped out of presidential race but is finishing term
Elina Treyger , a senior political scientist at the RAND Corporation whose research includes migration and immigration enforcement, also said Harris' diplomatic role with the Central American countries "is in no way a 'border czar'-like position." Treyger said border policy involves many other issues such as enforcement policies, how to process migrants expressing fear of prosecution or torture and how to allocate resources at the border.
U.S. Border Patrol encounters with migrants at the southern border have soared under the Biden administration . Illegal crossings at the U.S.-Mexico border hit a record high of 2.2 million in 2022, and the number of people taken into custody by U.S. Border Patrol has reached the highest levels in the agency's history under Biden, the Washington Post reported .
After a bipartisan border security bill failed to advance in Congress, Biden issued a directive in June to turn away migrants who do not enter the country through legal ports of entry when the number of crossings is high.
Trump, the son of former President Donald Trump, did not immediately respond to a request for comment.
Our fact-check sources:
- Aaron Reichlin-Melnick , July 22, Email exchange with USA TODAY
- Andrew Salee , July 22, Email exchange with USA TODAY
- Elina Treyger , July 22, Email Exchange with USA TODAY
- White House, Feb. 2, 2021, Executive Order on Creating a Comprehensive Regional Framework to Address the Causes of Migration, to Manage Migration Throughout North and Central America, and to Provide Safe and Orderly Processing of Asylum Seekers at the United States Border
- White House, Feb. 6, 2023, FACT SHEET: Vice President Harris Announces Public-Private Partnership Has Generated More than $4.2 Billion in Private Sector Commitments for Northern Central America
- White House, March 24, 2021, Remarks by President Biden and Vice President Harris in a Meeting on Immigration
- White House, June 25, 2021, Remarks by Vice President Harris, Secretary of Homeland Security Mayorkas, Chairman Durbin, and Representative Escobar in Press Gaggle
- White House, July 29, 2021, FACT SHEET: Strategy to Address the Root Causes of Migration in Central America
- White House, March 25, FACT SHEET: Vice President Harris Announces Public-Private Partnership Has Generated More Than $5.2 Billion in Private Sector Commitments for Northern Central America
- White House, July 2021, U.S. Strategy for Addressing the Root Causes of Migration in Central America
- Department of State, Aug. 1, 2023, Central America Forward
- The Washington Post, Feb. 11, Trump vs. Biden on immigration: 12 charts comparing U.S. border security
- U.S. Embassy in Honduras, March 25, FACT SHEET: UPDATE ON THE U.S. STRATEGY FOR ADDRESSING THE ROOT CAUSES OF MIGRATION IN CENTRAL AMERICA
- USA TODAY, July 17, Border security takes center stage at RNC. Here's the actual data under Trump, Biden
Thank you for supporting our journalism. You can subscribe to our print edition, ad-free app or e-newspaper here .
USA TODAY is a verified signatory of the International Fact-Checking Network, which requires a demonstrated commitment to nonpartisanship, fairness and transparency. Our fact-check work is supported in part by a grant from Meta .
Fatty Acids: Definition,Types and FAQs
Table of Contents
Fatty Acid Meaning and Definition
Types of Fatty Acids
Even and odd chain fatty acids, saturated and unsaturated fatty acids, length of fatty acids, properties of fatty acids, circulation of fatty acids, frequently asked questions, fatty acid – meaning and definition.
In biochemistry, fatty acids can be defined as carboxylic acids with long aliphatic chains that can either be branched or unbranched. The fatty acids that occur naturally, possess carbon atoms in even numbers and are usually unbranched. Fatty acids are major components of lipids ; they exist in three main forms of esters: phospholipids, triglycerides and cholesteryl esters.
Fatty acids are classified based on their number of carbons, length and saturation.
Most naturally occuring fatty acids have an even number of carbons in their aliphatic chain. Example: oleic acid (18), stearic acid (18).
However, some fatty acids also have an odd number of carbons in their chain. They are known as odd-chain fatty acids (OCFA). Example: heptadecanoic and pentadecanoic acid that are found in dairy products.
The biosynthesis of odd chain fatty acids is a little more complex than the even chain fatty acids.
The acids that have no double bond (C=C) in their aliphatic chain are known as saturated fatty acids . The chemical formula of saturated fatty acids can be written as CH 3 (CH 2 ) n COOH. Below is a table of common saturated fatty acids with their formula.
| Caprylic Acid | CH (CH ) COOH |
|---|---|
| Capric Acid | CH (CH ) COOH |
| Lauric Acid | CH (CH ) COOH |
| Myristic Acid | CH (CH ) COOH |
| Palmitic Acid | CH (CH ) COOH |
| Stearic Acid | CH (CH ) COOH |
The unsaturated fatty acids possess at least one double bond in their aliphatic chain. The double bond in the molecule can generate two isomers for unsaturated fatty acids: cis and trans configurations.
The cis isomers have the two hydrogen atoms placed adjacently to the double bond on the same side of the aliphatic chain. The double bond gives rigidity to the molecule and the cis conformation limits the conformational freedom of the fatty acid. The more number of cis bonds found in a fatty acid, the less flexible and curved it becomes in conformation. Example: oleic and linoleic acid.
The trans isomers, conversely, have the two hydrogen atoms placed on opposite sides of the aliphatic chain. The trans configuration does not bend the structure of the molecule as seen in cis isomers, but remains straight like saturated fatty acids.
Most naturally occuring unsaturated fatty acids possess a cis configuration whereas most trans fats are a result of human processing, they do not occur naturally.
- Fatty acids with aliphatic chains of five or lesser carbons are called short-chain fatty acids (SCFA). Example: butyric acid
- Fatty acids with aliphatic chains of 6 to 12 carbons are called medium-chain fatty acids (MCFA). Example: capric acid
- Fatty acids with aliphatic chains of 13 to 21 carbons are called long-chain fatty acids (LCFA). Example: oleic acid
- Fatty acids with aliphatic chains of 22 or more carbons are called very long chain fatty acids (VLCFA). Example: lignoceric acid
- Acidity: Fatty acids have similar acidities. As the chain length of a fatty acid increases, their solubility in water decreases, posing no or little effect on the pH of the aqueous solution. Example: Nonanoic acid (C9) has a pK a of 4.96 whereas acetic acid (C2) has a pK a of 4.76.
- Hydrogenation: Unsaturated fatty acids are prone to get rancid (autoxidation or hydrolysis of fats when exposed to air). Therefore the unsaturated fatty acids undergo hydrogenation to minimise this problem.
- Autoxidation: Unsaturated fatty acids undergo a chemical change in the presence of air and trace metals called autoxidation. Treatment with chelating agents can prevent this action as they remove the metal catalysts.
- Ozonolysis: Unsaturated fatty acids have high chances to get degraded by ozone.
- Digestion and Intake: SCFA and MCFA are directly absorbed in our blood by the intestinal capillaries and travel via the hepatic portal vein, similar to other absorbed nutrients. LCFA, however, is not absorbed directly into the blood. They are absorbed in the villi of the intestine to form triglycerides . The triglycerides get coated with cholesterol and proteins to form chylomicrons. The chylomicrons are transported to a location near the heart via the lymphatic duct where they are either stored or broken down for energy.
- Metabolism: Fatty acids are broken down via beta-oxidation and citric acid cycle in mitochondria into CO 2 and water. After oxidative phosphorylation they release energy in the form of ATP.
Visit BYJU’S Biology for more updates.
- Fatty acid synthesis
- Importance of Fats – Types of Fats, Sources and Its Benefits
- Difference between Fats and Oils
- Difference between Lipids and Fats
What are essential fatty acids? Give examples.
What is the function of a fatty acid.
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

IMAGES
VIDEO
COMMENTS
Definition of pH (Opens a modal) Strong acid solutions (Opens a modal) Strong base solutions (Opens a modal) Acid strength, anion size, and bond energy (Opens a modal) ... Determining solute concentration by acid-base titration (Opens a modal) Titration of a strong acid with a strong base (Opens a modal) Titration of a strong acid with a ...
acid, any substance that in water solution tastes sour, changes the colour of certain indicators (e.g., reddens blue litmus paper), reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and promotes certain chemical reactions (acid catalysis). Examples of acids include the inorganic substances known as the ...
The sodium hydroxide, calcium carbonate and potassium oxide are examples of bases. A base is a substance that reacts with hydrogen ions and can neutralize the acid. Most bases are minerals which form water and salts by reacting with acids. Bases include the metal oxides, hydroxides, and carbonates. Q8.
The acid that conducts electricity strongly is a strong acid, and the acid that conducts electricity weakly is a weak acid. Chemical Properties of Acid and Bases 1. Reactions of Acids and Bases with Metals. When a metal reacts with an acid, it generally displaces hydrogen from the acids. This leads to the evolution of hydrogen gas. The metals ...
Study with Quizlet and memorize flashcards containing terms like According to the Arrhenius definitions of acids and bases, choose the bases from the list of acids and bases. Check all that apply., Consider this reaction: NH4+ + HPO42− → NH3 + H2PO4− Which is the Bronsted-Lowry acid ?, Consider this reaction: NH4+ + HPO42− → NH3 + H2PO4− Which is the conjugate base? and more.
Identify a water molecules and click on it. Choose any of the molecules that are floating. Identify the acid and click on it. Red one on the left (hydrochloric acid) What was transferred from the hydrochloric acid to the water molecule? H+. Based on what you have seen, which is the best definition for an acid?
Salts. Salt is the product formed from the neutralisation reaction of acids and bases. In the reaction between hydrochloric acid and sodium hydroxide, the salt formed is sodium chloride. HCl+N aOH→N aCl+H2O. Salt can be acidic, basic or neutral in nature. To know more about Salts, visit here.
The acid value is a metric for the quantity of carboxylic acid groups (C (=O)OH) in a chemical compound, such a fatty acid, or in a blend of chemical compounds. It is a measurement of the amount of free fatty acids (FFAs) in a material. A common approach involves dissolving a known quantity of sample in an organic solvent (typically isopropanol ...
Study with Quizlet and memorize flashcards containing terms like Which of the following would most likely act as a Bronsted-Lowry acid? (A)OH- (B)HCN (C)CCl4 (D)Mg(OH)+, Which best describes the definition of Lewis acids and bases? (A)a general definition based on electron structure (B)a general definition based on hydrogen ion concentration (C)a specific definition based on a compound's ...
Acids and Bases Assignment Questions 9-10 refer to the following scenario of an acid-base titration. An unknown strong acid was measured out to 50.0 mL and was placed in a beaker. This unknown analyte was titrated with a 0.100 M NaOH solution. The data for the titration curve of mL of titrant vs pH was collected and shown below.
nucleic acid, naturally occurring chemical compound that serves as the main information-carrying molecule of the cell and that directs the process of protein synthesis, thereby determining the inherited characteristics of every living thing. Nucleic acids are further defined by their ability to be broken down to yield phosphoric acid, sugars ...
The ACID properties, in totality, provide a mechanism to ensure the correctness and consistency of a database in a way such that each transaction is a group of operations that acts as a single unit, produces consistent results, acts in isolation from other operations, and updates that it makes are durably stored.. ACID properties are the four key characteristics that define the reliability and ...
The concept of conjugate acid and base came from Bronsted -Lowry acid base theory. The conjugate acid and conjugate base forms as a pair. They differ only by a proton. Conjugate acid is a compound formed from a base by accepting a proton. B + H + → BH + BH + is the conjugate acid of B. Conjugate base is a compound formed from an acid by ...
Acid rain is any form of rain that is more acidic than normal (with a pH lower then 5.6). Pure water has a pH of 7, normal rainfall has a pH of a bit less than 7, but acid rain can have a pH of about 5.0-5.5, and even in the 4 range in the northeastern United States.
Predict the products of the following acid-base reaction. Express your answer as part of a chemical equation. Identify all of the phases in your answer. A + NH3(aq) â†' NH4+(aq) + A-(aq) A chemical reaction does not occur for this question. Submit Request Answer. Part B. Predict the products of the following acid-base reaction.
In an account set for an ownership definition assignment rule, you have the following options for the setup: Select a joint venture and the ledger associated with the joint venture. This enables you to set up an account set to use with a specific joint venture. In this scenario, the primary segment values that you specified in the joint venture ...
A pH scale is a tool for measuring acids and bases. The scale ranges from 0-14. Litmus paper is an indicator used to tell if a substance is an acid or a base. The colour of the paper matches up with the numbers on the pH scale to indicate what kind of substance is being tested. For example, Vinegar is an acid and measures 2.4 on the pH scale.
For Process Ownership Definition Assignment Rules, select Yes. For Bypass Default Ownership Definition Assignment: Select No if you want the process to assign the default ownership definition defined in the joint venture definition to any transactions not assigned with an assignment rule. Select Yes to ignore the default ownership definition ...
Allocate time for research, analysis, and drafting to meet assignment deadlines effectively. By carefully analyzing the assignment requirements, you set a solid foundation for developing a focused and comprehensive response that demonstrates your understanding and application of body fluid and acid-base balance concepts in nursing practice.
Identify the ownership definition or direct billed stakeholder for each assignment rule and the sequence or order in which you want to process each assignment rule. After reviewing the assignment rules and ensuring that there aren't conflicts, gaps, or redundancies, create the assignment rules in Oracle Joint Venture Management.
Immigration levels from Guatemala, Honduras, and El Salvador have gone down in the years since Harris' assignment began, with data from U.S. Customs and Border Protection ...
For many, the suggestion that Harris could somehow be unqualified because of her race, traded on familiar racist tropes about women of color in the workplace.
Acid rain is made up of highly acidic water droplets due to air emissions, most specifically the disproportionate levels of sulphur and nitrogen emitted by vehicles and manufacturing processes. It is often called acid rain as this concept contains many types of acidic precipitation. The acidic deposition takes place in two ways: wet and dry.
"Brat" is less a noun than it is a concept, though it is also a thing. The title of Charli XCX's sixth studio album, she envisions a "brat" as someone who has a "pack of cigs, a Bic ...
The assignment came out of an executive order Biden issued in February 2021 that sought to reduce migration from the Northern Triangle countries of El Salvador, Guatemala and Honduras, ...
From endless memes to MTA announcements and even political campaigns, the past weeks have become awash with lurid green. But why?
Fatty Acid - Meaning and Definition. In biochemistry, fatty acids can be defined as carboxylic acids with long aliphatic chains that can either be branched or unbranched. The fatty acids that occur naturally, possess carbon atoms in even numbers and are usually unbranched. Fatty acids are major components of lipids; they exist in three main ...