- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

U.S. Food and Drug Administration
- Search
- Menu
- Emergency Preparedness | Drugs
- Coronavirus (COVID-19) | Drugs
Ensuring the Safety of Patients in Clinical Trials Studying Investigational New Drugs to Prevent or Treat COVID-19
Fda’s role in reviewing research proposals for patient safety.
FDA has a critical role in ensuring the safety of patients in clinical trials studying drugs or biological products for the prevention or treatment of COVID-19 that require an investigational new drug (IND) application.
Many sponsors are providing clinical trial proposals to FDA for COVID-19 therapies before submitting an IND application. FDA has evaluated and responded to hundreds of these study proposals and questions from sponsors through this process. More information is available on the Coronavirus Treatment Acceleration Program (CTAP) webpage . As part of its review, FDA identifies any safety concerns that the sponsor must adequately address in the IND application before proceeding with a clinical trial.
Once a sponsor submits an IND application, FDA may determine that sufficient information has been provided to allow the trial to proceed (issuing a “Safe to Proceed” letter), or, if there is insufficient support to assure patient safety, or if there are safety concerns, FDA can issue a “clinical hold” to prevent a proposed trial from starting. This is to assure that subjects are not exposed to an unreasonable and significant risk of illness or injury. More information is available on CDER’s IND Application Procedures: Clinical Hold webpage . FDA has evaluated numerous IND applications for COVID-19 therapies and in some cases, due to patient safety concerns, issued a clinical hold to prevent a trial from proceeding.
FDA has been careful to assess whether proposed COVID-19 therapies in research proposals have potential safety concerns that might exacerbate the most serious and life-threatening symptoms of COVID-19 patients, such as respiratory adverse effects or pro-inflammatory immune response.
Examples of Patient Safety Concerns
Some examples of safety concerns identified by FDA in COVID-19 therapy proposals include:
- For example, serious pre-clinical safety signals of a drug’s toxic effect were found in animal studies at a level that patients in the trial might be exposed to.
- These safety concerns included therapies that can amplify cytokine release syndrome (a severe inflammatory response in the body that can lead to respiratory failure, organ failure and death) in a disease where patients were already at increased risk of this serious complication.
- For a new drug or biological product, insufficient information was provided about the product’s characteristics or how it is manufactured to allow FDA to assess the risks to subjects of the proposed study
Types of Research Proposals Not Allowed to Proceed
Some of the proposals that FDA has not allowed to proceed because of a lack of any plausible scientific basis for use in preventing or treating COVID-19 virus or symptoms, and which may expose the patient to significant known risks of the drug include proposed interventions with:
- Known toxic substances
- Botanical substances (e.g., plant materials, algae, mushrooms)
- Drugs or biological products approved for other indications without any plausible scientific basis for use in preventing or treating the virus or COVID-19 virus or symptoms, and which may expose the patient to significant known risks of the drug
- Research article
- Open access
- Published: 17 May 2016
Patient and public involvement in patient safety research: a workshop to review patient information, minimise psychological risk and inform research
- Dominic Furniss 1 ,
- Ioanna Iacovides 1 ,
- Imogen Lyons 1 ,
- Ann Blandford 1 &
- Bryony Dean Franklin 2 , 3
Research Involvement and Engagement volume 2 , Article number: 19 ( 2016 ) Cite this article
3526 Accesses
4 Citations
18 Altmetric
Metrics details
Plain English summary
Patient safety is a growing research area. However, although patients and the public are increasingly involved in clinical research, there is little guidance on how best to involve patients in patient safety research. Here we focus on how patients can contribute to the design of patient safety research.
We conducted a workshop with patients as part of a project exploring errors and safety in the delivery of intravenous medication (medication given via a vein). The workshop was designed to explore how best to engage with hospital inpatients about these issues, to generate research topics, and to inform researchers about patients’ experiences. Nine patients participated, each of whom had previously received intravenous medication. Participants advised against using terms such as ‘error’; they also advocated caution when using terms such as ‘safety’ when describing the study to patients as this may worry some who had not thought about these issues before. We received thorough and useful feedback on our patient information sheets to ensure they were clear and understandable to patients. Patients also shared rich experiences with us about their treatment, which emphasised the need to extend our research focus to include a wider range of factors affecting quality and safety.
Patient safety has attracted increasing attention in recent years. This paper explores patients’ contributions to informing patient safety research at an early stage, within a project on intravenous infusion errors. Currently, there is little or no guidance on how best to involve patients and the wider public in shaping patient safety research, and indeed, whether such efforts are worthwhile.
We ran a 3-hour workshop involving nine patients with experience of intravenous therapy in the hospital setting. The first part explored patients’ experiences of intravenous therapy. We derived research questions from the resulting discussion through qualitative analysis. In the second part, patients were asked for feedback on patient information sheets considering both content and clarity, and on two potential approaches to framing our patient information: one that focused on research on safety and error, the other on quality improvement.
The workshop led to a thorough review of how we should engage with patients. Importantly, there was a clear steer away from terms such as ‘error’ and ‘safety’ that could worry patients. The experiences that patients revealed were also richer than we had anticipated, revealing different conceptions of how patients related to their treatment and care, their role in safety and use of medical devices, the different levels of information they preferred, and broader factors impacting perceptions of their care.
Involving patients at an early stage in patient safety research can be of great value. Our workshop highlighted sensitivities around potentially worrying patients about risks that they might not have considered previously, and how to address these. Patient representatives also emphasised a need to expand the focus of patient safety research beyond clinicians and error, to include factors affecting perceptions of quality and safety for patients more broadly.
Peer Review reports
Patient safety and the role of patients
Patient safety is a global health issue [ 27 ]. The Institute of Medicine’s report To Err is Human estimated that between 44,000 and 98,000 Americans die each year due to medical error [ 13 ]. Even assuming the lower estimate, this suggests it is the eighth leading cause of death in America, with more people dying from medical error than motor vehicle accidents every year. Similarly grim statistics are contained within the NHS report An Organisation with a Memory, which estimates that around 1 in ten patients admitted to NHS hospitals will experience an adverse event [ 5 ]. Among these statistics are real stories of human tragedy. Increased awareness of unacceptable levels of preventable patient harm has led to global efforts to improve patient safety and increased research in this field [ 27 , 28 ].
Despite patients being the main stakeholder in their care, their potential contribution to patient safety was largely ignored until the turn of the millennium [ 26 ]. Such contributions could help with reaching an accurate diagnosis, choosing appropriate treatment plans, monitoring treatment and conditions, recognising and reporting adverse events, improving how incident reporting is handled, and putting pressure on policy makers to improve standards [ 15 , 26 ]. The patient’s potential contribution is particularly important because they and their families are the only people present along the entire continuum of care in which they engage with multiple healthcare providers from different disciplines, and sometimes from different facilities [ 27 ].
Patient participation and involvement in patient safety is sometimes seen as a single broad area [ 14 ]. However, it is important to distinguish between patients and the public participating in safety practices and involving patients in research on patient safety. For example, the former would include a patient confirming which limb a surgeon will operate on, whereas the latter would include patients advising researchers on the design of research studies. Previous research has investigated the role of patients in their own care and the co-production of patient safety in practice (e.g. [ 6 , 10 , 26 ]), but there is little published work on the patient’s role in shaping patient safety research.
The patient’s role in shaping patient safety research
With respect to the patient’s role in research activities, INVOLVE [ 11 ] outline the different types of role that patients and the public can have:
Participation - the traditional role of patients within research where they are the subject of research and provide data to be analysed.
Involvement – lay people actively working with researchers who seek help and advice on the design, management and/or conduct of research, which can also include their active involvement in data gathering and/or data analysis.
Engagement - dissemination of research findings and their implications to patients and the public.
In contrast to patient participation in their own safety, patient involvement in research focuses on helping to shape the research, e.g. prioritising research topics, informing how studies should be conducted, actually conducting data gathering and analysis, and giving advice on how to conduct engagement.
Current work on patient and public involvement (PPI) in research suggests there are few practical examples of how it can be conducted and its potential benefits [ 12 ]. Some positive PPI examples have been documented in response to threats of tokenism [ 24 ], which also include details of the ethical issues and practicalities of using community researchers and patients to gather data in hospital [ 9 , 21 ]. These studies demonstrate the value of PPI in their qualitative descriptions of context, experiential knowledge and contributions to the project [ 23 ].
Recognising the special sensitivities in patient safety research, the World Health Organization published advice on Ethical Issues in Patient Safety Research [ 28 ]. The absence of any reference to patient and public involvement (PPI) activities in this is notable. For example, they advise that a safety committee of expert clinicians could be set-up to advise on how to handle sensitive issues that arise during the conduct of the study, but they do not mention how PPI could also advise on ethical issues and how they can be addressed before the study begins. We explore the role PPI has to play in the early stages of patient safety research by 1) reviewing patient safety information and psychological risk; and 2) informing research topics and sensitizing researchers to patients’ experiences. These aims were shaped by early PPI feedback on our proposal for a project to explore errors and safety in the delivery of intravenous medication.
Reviewing psychological risk and patient safety information
When initially developing our research proposal, we sought informal feedback on our project plans from two patient representatives. One was a patient representative from an earlier related project and the other an acquaintance who had recently been an inpatient, both of whom had received intravenous therapy.
The feedback received at this stage raised potential issues about the conduct of the study. For example one of the patient representative giving feedback on our proposal said “ …for patients and relatives [the proposed project] contains too much information regarding hazards. The patients and relatives need to have complete confidence in the staff and equipment, almost blind faith in many instances ”. This caution against exposing patients to details about potential errors was in stark contrast to our desire to inform potential participants about the study in an open manner. We therefore wanted to explore how to approach patients about patient safety issues while minimising psychological risk. Psychological risk refers to the potential to have detrimental effects on the psychological state of the participant, e.g. raising concerns, anxieties and levels of stress.
The WHO [ 28 ] guidance notes: “ Psychological risks include the possibility that research participants will become emotionally distressed, fearful or anxious as a result of their participation. For example, studies that interview patients or families about harmful incidents that occurred previously or about their perceptions of the quality of care may cause them to question the quality of their medical providers and to become anxious. ” WHO ([ 28 ] p.15). To support this, Rhodes et al. [ 20 ] found that, during their research, many patients had not thought about any safety issues in depth until they were asked. One area where PPI could make a positive contribution to patient safety research is to review potential psychological risks and mitigating strategies from a patient’s perspective before the study begins. This has not been reported on before.
Typical PPI activities include reviewing patient information so it is written in plain English and more easily understood from a patient’s perspective. However, there are not many published accounts of this in practice, and no accounts of the special sensitivities associated with patient safety terminology. In their work with primary medical care patients Rhodes et al. [ 20 ] deliberately avoided framing their research questions in terms of ‘error’ and ‘harm’ and let patients introduce topics they considered relevant. However, published accounts of PPI advice in this area is lacking.
Informing research topics and sensitizing researchers to patients’ experiences
The initial informal patient feedback received on our project plans also encouraged us to investigate intravenous infusion practices from the patient’s perspective. For example, one of the patients providing feedback informed us that, “ patients have no idea of what is going on. […] I was told next to nothing about what was going on. ” We proposed to involve patients as participants in our research to investigate experiences of their intravenous infusion practices, both positive and negative. However, before doing that we wanted to use PPI to help focus our research questions and sensitize ourselves, as researchers, to patients’ experiences in this area.
Sensitizing researchers to patients’ experiences works at two levels: 1) by informing the researcher’s knowledge of an area so that they are more aware of potential issues, and 2) by informing the researcher’s emotional awareness and the experiential aspects associated with speaking to patients around the topic of research.
As part of helping to focus our research questions we were interested to hear from our PPI participants about their general experiences of receiving intravenous medication including, but not limited to, the extent to which patients were involved in intravenous infusion administration. For instance, in previous studies we had observed an indirect role for patients as a member of staff realised a mistake as they explained the intravenous medication details to a patient [ 7 ], and a more direct role as patients silenced alarms of their infusion pumps [ 8 ]. Although the role of patients in patient safety has been investigated generally (e.g. [ 3 , 4 , 6 , 10 , 14 , 17 – 19 , 22 , 26 ]) we know of no published accounts in the specific area of intravenous medication infusion.
In this paper we report on a detailed case study of a PPI workshop that has informed the design of our patient safety research. This is the first detailed published account of patient involvement in shaping patient safety research. We provide a description of our PPI activity, report experiential knowledge gained through the process, and document the PPI contribution to our project. Specifically we believe that a PPI workshop can help to review patient information and psychological risk, inform research topics and sensitize researchers to patients’ experiences.
Study setting
Our PPI workshop took place in the context of a larger project called ECLIPSE (Exploring the Current Landscape of Intravenous Infusion Practices and Error) [ 1 ]. ECLIPSE seeks to investigate different intravenous infusion practices in English hospitals and how these relate to the prevalence of error, in order to make recommendations for safer infusions.
ECLIPSE has an eight-member steering committee, one of whom is a patient representative, as well as a wider advisory group with twelve members, two of whom are patient representatives. The three phases of research in ECLIPSE, and their associated PPI activities, are shown in Table 1 . The workshop described in this paper is part of the first phase, with the workshop being viewed as a preliminary activity to inform the planned research.
Objectives of the workshop
A PPI workshop was designed to address two main objectives:
Establish how to appropriately inform and engage potential patient participants in our research.
Sensitise researchers to patients’ experiences, and shape research topics and questions related to intravenous infusions practices and safety for Phase 2 of the study.
Recruitment and sampling
Information about the focus of the workshop, date, time, location, compensation, requirements for attendance, and links to the wider project were posted on the project’s website ( http://www.eclipse.ac.uk ) and People in Research ( http://www.peopleinresearch.org ), a website that advertises PPI opportunities. We also circulated the relevant information to existing contacts. Experiencing harm was not a pre-requisite to participate; indeed we wanted ‘normal’ experiences to be the focus. Six patient representatives were recruited via these different channels. The three patient representatives on our steering committee and advisory group also participated. These patient representatives had seen our project plan and so were familiar with the high-level objectives of the research, but they had no involvement in organising the workshop and were largely unfamiliar with the researchers when it was held.
All nine attendees (2 male, 7 female; approximate age range 35–70) had first-hand experience of receiving intravenous medication in hospital, and some had additional experiences of friends and family receiving intravenous medication. One attendee was also a qualified nurse. Some patients were familiar with patient and public involvement activities, e.g. the steering committee member had experience of reviewing patient information material and someone entirely external to the project had experience of sitting on Research Ethics Committees.
The first three authors ran the workshop on university premises. Their research interests include medication safety, the psychology of human error and human factors (i.e. evaluating and designing products and systems while taking proper account of the people who use them). The 3-hour workshop was organised into five parts:
Introduction to the workshop;
Patients’ experiences with intravenous infusions (in two sub-groups)
[lunch break];
Reporting back from the two sub-groups;
Review of ECLIPSE patient information sheets and discussion about how best to engage with patient participants;
Wrap-up session.
During the introduction we outlined the scope and plan for our project, and the role of the PPI workshop within it. As researchers, we recognised the benefits of a bottom-up approach to exploring patients’ experiences in an open way - generating research topics and themes without restriction. However, we were aware that this had to be balanced with trying to remain within the remit of the project. For example, we were conscious that it could be considered unethical to create expectations about researching areas that were outside the project’s remit. Participants were therefore informed about this challenge and the project’s constraints so they could help us manage this issue.
We intentionally started by listening to patients’ experiences of receiving drugs and fluids intravenously as this would set the context for the rest of the workshop. Given the size of the workshop, i.e. nine patients and three researchers, we split the participants into two parallel groups to allow for in-depth discussion. There were four patients and one researcher in one group, and five patients and two researchers in the other. The division was made arbitrarily. Each group had a researcher with experience of facilitating focus group discussions. The lunch break was also strategically placed so that conversations could continue over lunch before reporting back to the whole group on the main topics of discussion.
In the second part of the workshop, patients were asked to provide feedback on the patient information flyer for Phase 1 of ECLIPSE and what the Phase 1 observers should say to patients (i.e. we had written a small introductory paragraph for Phase 1 observers to use about the project). We were not involving patients as participants in Phase 1 and so only needed a flyer to inform them about the study taking place on the ward. The Research Ethics Committee had already approved these. The attendees were then given two alternative patient information sheets for Phase 2, which would be used to seek informed consent for patient participation. One informed the patient about ECLIPSE and the study’s aims of reducing error, and the other was framed around quality improvement. Patients were asked about their preference and to suggest improvements. Documentation for Phase 2 had not been submitted to the Research Ethics Committee at the time of the workshop.
Ethical considerations
As this was a PPI activity to shape research, rather than an activity involving patient participation in research, NHS research ethics approval was not required. However, the UCL Research Ethics Committee granted ethical approval (UCLIC/1213/023/Staff) for audio recording of the discussions and use of this in subsequent analysis. Patient representatives were given information sheets to describe what was involved in participating in the workshop, its objectives, data protection, compensation and reimbursement. Each signed to confirm their consent. We sought ethical approval for this involvement activity for two reasons: 1) this provided confirmation that we were in line with good research practices such as fully informing the workshop participants as to what was involved; and 2) to obtain consent for audio-recording the workshop discussion and using the data in publications. A similar rationale for seeking ethical approval for a PPI activity has been reported elsewhere [ 16 ].
Data collection
All workshop discussions were audio recorded and subsequently professionally transcribed. The researchers also took notes and annotated patient information sheets to capture patients’ feedback.
Data analysis
The transcripts and notes were reviewed. Codes and themes were identified inductively using principles of thematic analysis [ 2 ]. Through carrying out the analysis in this way we were able to approach the data collected in a systematic manner, thus increasing the integrity of our analysis, which would inform our research. The resulting data set and findings could also be shared more easily across the research project team. The first author performed the main analysis. The second and third authors reviewed the transcript, codes and resulting themes to identify any themes that had been overlooked. QSR NVivo version 10 was used to facilitate this process.
The findings are presented according to our workshop objectives: 1) how to appropriately inform and engage patients in our patient safety research; and 2) sensitising researchers to patient experiences and exploring topics that will be further investigated through research in Phase 2 of the project.
Informing and engaging patient participants
Two main issues were highlighted in terms of how to inform patients about the research. The first was simplifying the patient information provided, and the second was the potential for raising concerns among patients about errors or poor care.
Feedback on phase 1 flyer: simplifying patient information
The information flyer for Phase 1 had been reviewed internally within the project team, and approved by an NHS Research Ethics Committee. However, participants expressed concerns about both the length of the flyer, and the language used to convey information about the study and taking part. They emphasised the need to reduce the burden on participants, recognising that they may be very unwell when receiving this information.
Patients drew our attention to particular words and phrases that could be simplified. For example, most patients agreed that the term ‘drip’ would be better than ‘intravenous medication’, and that asking patients about ‘using’ the pump would be more easily understood than ‘interacting’ with it. The information sheet also specified that patient names and hospital numbers would be ‘disposed of’ following data collection, which they thought was a poor choice of phrasing.
Patients wanted a shorter and simpler flyer; however, one patient who had Research Ethics Committee experience recognised that the longer format is what would be expected. The group also questioned the necessity of the legal note: “NHS Indemnity does not offer no-fault compensation i.e. for non-negligent harm, and NHS bodies are unable to agree in advance to pay compensation for non-negligent harm.” This was described as ‘gobbledygook’, but again the group agreed that this probably needed to be included if this was a standard phrase.
Feedback on phase 2 information sheets: concerns about safety and compromising care
To generate discussion about how to appropriately engage with patients in Phase 2 we provided patients with two potential versions of an information sheet. Sheet A was framed around ECLIPSE’s focus on “understanding and reducing the prevalence of medication error,” and sheet B was framed around “developing strategies to improve safety.” Sheet A was quickly dismissed as unworkable as there was broad agreement that mentioning the term ‘error’ could alarm patients. Sheet B was preferred; however, some patients were also concerned about use of the term ‘safety’:
Patient 1: “…immediately you flag up the word safety and you’ve got people worrying.”
Further discussion suggested that safety terms could be used, but they needed to be used with care. Patient 8 said they would be comforted to know that this work was going on and that safety was being checked. However, there was some recognition that while this might suit some patients, it might not be comforting to others. In summary, workshop participants were broadly supportive of the approach used in sheet B with an emphasis on quality improvement and improving safety rather than reducing error.
In addition, participants suggested that ‘poor’ should be deleted in the phrase “action will be taken if researchers have concerns about poor practice” which was initially intended to reassure patients but they thought it could raise concerns. They also did not think the phrase ‘action will be taken’ was informative and friendly. This resonates with existing research as patient do not want to engage in activities that can be seen to be ‘checking up’ because they have relationships to manage between themselves and their healthcare providers (e.g. [ 10 ]). To reassure patients who did not want to take part, the draft information sheets stated, “This would not affect the standard of your care.” However, there was some concern about how this statement could be interpreted and participants suggested improvements:
Patient 6: “The other thing that I would worry about, to a degree, is the sentence, this would not affect the standard of care you receive. […] I wouldn’t be sure whether that was a threat.
Patient 1: You have to say that, though, don’t you?
Patient 4: You could word it to say you’ll get the same care you always would.
Patient 5: Yes, it’s about reassuring people that if they drop out, they’re not – there’s not going to be any penalties for it, essentially, but it’s a horrible way of putting it. […] It’s one of those things that raises more questions than it answers.”
Some also raised concern about observers ‘checking’ their prescription as this could imply that there may be something wrong. Further, participants were keen to convey that this was a broader study across the whole ward and the hospital so patients did not feel their particular care was being singled out for any reason.
Informing research topics and sensitising researchers to patients’ experiences
Issues that emerged from patients sharing their experience of intravenous infusions have allowed us to draft a list of questions to consider for the patient interviews in Phase 2, which are broader in scope than our initial ideas and grounded in patient experiences:
What diversity is there in the way infusions are administered and what factors influence the patient’s perception of the quality of their care?
To what extent do infusion practices instil patients with confidence? Do they get the level and type of information they want? Do they understand enough about their intravenous treatment?
What issues do patients have with their intravenous medication administration and infusion pumps?
What does patient participation look like in the context of intravenous medication administration? What factors affect patient willingness to participate in safety behaviours related to their infusions? How interested are patients in their pumps? Do patients interact with their own pumps, and under what circumstances?
What information about intravenous infusions do patients think would be useful to provide other patients? What would be the best way to share this information, e.g. a leaflet?
How could intravenous infusion practice be improved from a patient’s perspective?
To demonstrate how these questions emerged from the workshop we highlight some of the topics discussed using direct quotations to preserve the patient’s voice.
Participants reported a wide diversity in the way infusions were administered and what influenced the quality of their care. For example, one patient complained about the lack of information received from some staff in the emergency department, despite asking:
Patient 7: “ I kind of found it quite impersonal, to be honest with you, the approach of the nurses. […] It wasn’t really explained. […] when I asked what was going on and why it was being flushed with water, the answer that was given was kind of grudgingly given, as if to say, well, what’s it to do with you, you know. We’re in charge here. ”
In contrast another patient felt that her care team involved her in her care, and kept her as happy and engaged as they could, which was critical to her recovery due to the extent of her illness and her extended isolation:
Patient 2: “ In fact, during my 10 months seeing only healthcare professionals was actually really interesting because we cracked a lot of jokes, [and] some people might think they were theatrical, they were kind of trivial, not important, but in fact, they’re very, very important to keep the patient in focus, especially when the patient is very ill, to keep the patient as involved as possible. ”
A different patient highlighted that the attitude of staff could reveal itself and impact their perception of care through indirect means; e.g., throwaway comments and discussions between staff, when they think patients cannot hear, can have a big impact on their confidence in their care:
Patient 6: “ There was a nurse […] post-surgery, I was in the ICU for 48 h or something and then shipped up to the ward for, I can’t remember the phrase now, but specialised nursing. And so there was two nurses there for something like 48 h constantly […]. You know, lots of drips and infusions and pain relief. And one of them, obviously, was really annoyed that, as far as she was concerned, that she had to sit in a special wing. I mean, I was fairly well conscious by this time, but still, you know, morphine going in, so you’re sort of… so a bit cloudy and a bit vague […] and you can hear all these comments. And she’s teaching another nurse and she was saying, you know, we shouldn’t be doing this. We should be somewhere else in another ward, in a main ward, not in a side room like this. This is special treatment. ”
Patients empathised with staff who often had to deal with difficult jobs in difficult circumstances, and who may be having a bad day, but they also recognised the need to be professional and thought that some staff just did not have the right attitude for the job. Patients also reported good experiences and were full of admiration for staff who contributed to their care and went out of their way to make them feel comfortable:
Patient 1: “ If you think they know what they’re doing and they really want to help you, you feel much more relaxed and much happier about them attaching things to you and pumping things into you and pumping them out. And the really good ones do explain things. [One positive experience involved a member of staff waiting with me for my chemotherapy to finish long beyond the end of his shift.] And he didn’t moan at all. We had a long chat about holidays and where he came from [and] he just made me feel that I wasn’t being a nuisance. […] And when you’re so anxious about the whole thing, it makes such a difference. ”
The provision of information can affect patient understanding. In one case a patient given a patient-controlled analgesia pump for pain relief did not know how often she could press the button and whether she could overdose. Patients also remarked on not knowing if air in the line is a problem for them to worry about.
Patient 1: “ I was going to say, you don’t know whether you should be panicking about air in the line, do you, because as a patient, people say, you can get air in and you’ll be dead, so that’s the modern view of it – that’s the modern myth. I don’t know to what extent it’s true but that’s the perception people have […] ”
Patients also did not know why pump alarms were going off.
Patient 3: “ It’s that understanding of what and why and [Patient 5] very rightly corrected me. I said pumps go off for no reason and they go off for a reason because something’s wrong. I think [there is a] difference [between] the repetitive alarms when they’ve just been silenced [and] actual error alarms. I think that’s the patient safety issue. It’s not knowing whether [staff silence the alarms because they don’t have time to attend to actual error alarms or whether the alarm is for some sort of repetitive alert.] ”
Patient 1: “if you’re a patient, you don’t speak pump beep, beep.”
Some patients would not dream of touching their pumps whereas others, particularly those in hospital for a long time, learnt how to use theirs to some degree.
Some participants expressed concern about their intravenous treatment being set up properly but felt it was hard to question processes they did not understand fully and in some cases were concerned about undermining the healthcare professional by asking questions.
Participants suggested developing a leaflet or poster or similar to improve understanding around intravenous infusions and pumps, to make people less frightened and empower patients to ask questions. Participants also raised the challenge of adapting information to the different needs of patients and their different reactions to it.
Participants were aware that the quality of staff, equipment and staffing levels would affect their care. The maintenance and availability of equipment was raised as a specific issue. Patients were aware of broken equipment being put aside, and shortages of equipment that needed to be borrowed from adjacent wards, which did not instil confidence in the equipment that they were relying on.
Patient 2 commented on how some staff treat the equipment “ what I found with the equipment is that in the case of some nurses, not all, but some nurses, they don’t care. They have no attention to detail, they don’t care about the equipment and so the equipment isn’t working very well. ”
The quality of equipment was brought up as a different issue as one participant felt that kinks in giving sets could be more prevalent or problematic with cheaper products.
Staley [ 23 ] argues that we need more details of PPI activity to understand ‘how it works’ and what value it adds. In the following sections we outline the main contributions of our PPI workshop in shaping the design of patient safety research.
The format of the workshop, which ran like a focus group, proved to be successful. Patients were able to compare and contrast their experiences as stories were shared and discussed in the group. This provided common themes as well as a rich source of variability. It may not be appropriate to discuss all patient safety topics in a group format, e.g. particularly sensitive topics might be more appropriate on a one-on-one basis. However, patients with shared experiences are likely to be able to better empathise with one another.
Reviewing potential psychological risk and mitigating strategies for patient safety research through PPI
The WHO’s [ 28 ] guidance on ethical issues for patient safety research draws attention to the potential psychological risk as participating patients could be worried about possible errors. Although the WHO [ 28 ] guidance lacks any mention of PPI activities, our workshop shows that PPI can make a positive contribution to addressing this issue in patient safety research. Patient representatives can review research plans, anticipate how patients might feel, and suggest mitigating strategies.
The initial PPI feedback we received when drafting the project proposal suggested our project could increase anxiety in patients due to the issues it raises. We therefore explored this issue in the workshop. Patients gave a clear steer away from terms such as ‘error’ and even warned that terms such as ‘safety’ should be used with care. Others have adopted similar approaches, e.g. Rhodes et al. [ 20 ] avoided the use of ‘error’ and ‘harm’ in their interviews with patients. This does not mean that we should not provide information about risks, but that we need to do so in an open and honest way that manages patient anxiety. Patients also raised other areas of concern with our patient information material that we had not foreseen, which could be used in other patient safety projects, e.g. to ensure that patients did not feel that they, or their care, were being singled out for any reason.
Simplifying patient information
Reviewing patient information material is seen as a fairly standard function of patient and public involvement; however, we have not seen many publications on this – perhaps because it is considered trivial and uninteresting. Patients thoroughly reviewed our materials and gave useful feedback on things that we simply did not see as researchers. In particular they highlighted subtle differences in the terminology used and the need to give full consideration to how patients may interpret particular expressions in the context of their care, and how this could impact on patient well being.
These recommendations have already benefited our research procedures for Phase 1 of our study (which we acted on by submitting an amendment to the research ethics committee to make changes to the patient information sheet), and will further benefit our planned research in Phase 2.
Research ethics committees and sponsors typically have standard formats and requirements for information sheets, with which at least one participant was familiar. On this occasion we did not challenge these conventions. However, future research could explore shorter, simpler forms of the information sheets, removing the legal note at the bottom of the sheet, or more radical departures from the usual requirements and expectations of the Research Ethics Committee. Since doing the PPI workshop, our funders have requested that a funding acknowledgement and NIHR and Department of Health disclaimer is added to every information sheet, which may create further challenges in creating shorter forms and reflects different stakeholders’ interests and expectations.
The research questions that emerged from the workshop reflect the themes of the discussions we had around patients’ experiences of receiving intravenous infusions. This highlighted the situated nature of experiences of receiving medication intravenously in hospital. For example, patients might want more information but might not be given it, patients might have become expert in their own condition over a long period of time and feel confident in questioning medical professionals, and patients’ experiences can be influenced by staff attitude, staffing levels, device alarms, the environment, their own illness, their treatment and personal preferences. Similar to published findings we found a strong interplay of complex factors that seemed to impact patients’ experiences of the quality and safety of their infusion treatment (e.g. [ 20 ]). Indeed, it seemed difficult for patients to disentangle quality and safety issues [ 20 ].
Aspects of what was discussed have been referred to in the patient involvement in patient safety literature. For example, the attitude of staff affecting whether patients will speak up has been previously reported [ 6 , 10 , 14 ]. Also, it was clear that staff attitude played a critical role in the patient’s broad experience, not just their experience of safety. Participants expressed concern about undermining healthcare professionals by asking questions. This resonates with previous research on speaking up about safety (e.g. [ 6 ]). Workshop participants also expressed concern about patients raising negative issues with the researchers as it could compromise their relationship with staff (e.g. [ 6 , 10 , 14 ]).
In our ongoing research we will need to consider research into patient’s involvement in detecting, preventing and recovering from error [ 25 ], and patients’ reactions to alarms [ 19 ]. However, we are not aware of specific studies looking at the patient experience of intravenous infusion practices, and so the experiences shared in this PPI exercise provide a good foundation for our research.
Challenges/limitations
The participants in this workshop described diverse experiences of different types of care as patients who had received intravenous infusions. However, we recognise that patients who take part in PPI activities may not be representative of the patient population in general. For example, one patient was a nurse and a number of participants had previous experience of involvement in research studies, research steering groups, or Research Ethics Committees in addition to their experiences as patients. Nonetheless, these patients provided rich detail about their patient experiences that contributed valuable insights, and they were able to view the problem from different angles.
Patients’ attitudes towards and expectations of healthcare services will be affected by their experience of harm. For example, a patient who has lost confidence in the healthcare service will be less likely to passively submit himself or herself to treatment. Experiencing harm was alluded to by at least one participant in the workshop, and both positive and negative experiences were reported more broadly. However, the direct relationship between experience of harm and the patient’s perspective of care was not explored in the workshop.
Furthermore, we asked patients to share their stories and experiences of intravenous infusions, and to provide feedback on the information sheets. Our data may therefore include a mixture of their own specific needs and their assumed needs of other patients. Further research could tease out any differences between these perspectives.
There is a lack of literature on PPI for shaping patient safety research, either in showing how it can be conducted or the value it can bring. We have reported on three outcomes that show a clear contribution to our patient safety project: reviewing potential psychological risk to patient participants, simplifying patient information materials, and generating topics to pursue in research. There has also been great value in sensitizing the researchers to patients’ experiences in this area before we speak to patients at their bedsides on wards. These lessons could be of broader value to researchers in patient safety. For example, high profile advice has been published on how to handle ethical issues in patient safety research [ 28 ], but this does not include any form of PPI. This case study shows how PPI activities can positively contribute to this area.
Abbreviations
National Health Service
patient and public involvement
World Health Organization
Blandford A, Furniss D, Lyons I, Chumbley G, Iacovides I, Wei L, Cox A, Mayer A, Schnock K, Bates D, Dykes P, Bell H, Franklin B. Exploring the current landscape of intravenous infusion practices and errors (ECLIPSE): protocol for a mixed methods observational study. BMJ open. 2016;6(3):e009777.
Article PubMed PubMed Central Google Scholar
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
Article Google Scholar
Davis RE, Jacklin R, Sevdalis N, Vincent CA. Patient involvement in patient safety: what factors influence patient participation and engagement? Health Expect. 2007;10(3):259–67.
Article PubMed Google Scholar
Davis RE, Sevdalis N, Vincent CA. Patient involvement in patient safety: how willing are patients to participate? BMJ Qual Saf. 2011;20(1):108–14.
Article CAS PubMed Google Scholar
Department of Health. An organisation with a memory: report of an expert group on learning chaired by the chief medical officer from adverse events in the NHS. 2000.
Google Scholar
Entwistle VA, McCaughan D, Watt IS, Birks Y, Hall J, Peat M. Speaking up about safety concerns: multi-setting qualitative study of patients’ views and experiences. Qual. Saf. Health Care. 2010;19(6)e33
Furniss D, Blandford A, Mayer A. Unremarkable errors: low-level disturbances in infusion pump use. Proc. British HCI. 2011.
Furniss D, Blandford A, Mayer A. The wrong trousers: misattributing medical device issues to the wrong part of the sociotechnical system. In: CHI 2014 workshop: HCI research in healthcare: using theory from evidence to practice. 2014.
Garfield S, Jheeta S, Jacklin A, Bischler A, Norton C, Franklin BD. Patient and public involvement in data collection for health services research: a descriptive study. Res Involvement Engagement. 2015;1(1):1–16.
Hrisos S, Thomson R. Seeing it from both sides: do approaches to involving patients in improving their safety risk damaging the trust between patients and healthcare professionals? an interview study. PLoS One. 2013;8(11):e80759.
Article CAS PubMed PubMed Central Google Scholar
INVOLVE. Briefing notes for researchers: public involvement in NHS, public health and social care. 2012. http://www.invo.org.uk/wp-content/uploads/2014/11/9938_INVOLVE_Briefing_Notes_WEB.pdf Accessed 30 Nov 2015.
Jenner MK, Gilchrist M, Baker GC. Practical considerations in improving research through public involvement. Res Involvement Engagement. 2015;1(1):1–6.
Kohn LT, Corrigan JM, Donaldson MS, editors. To err is human: Building a Safer Health System (Vol. 6). Washington DC: National Academies Press; 2000.
Longtin Y, Sax H, Leape LL, Sheridan SE, Donaldson L, Pittet D. Patient participation: current knowledge and applicability to patient safety. Mayo Clin Proc. 2010;85(1): 53–62. doi: 10.4065/mcp.2009.0248
McDonald KM, Bryce CL, Graber ML. The patient is in: patient involvement strategies for diagnostic error mitigation. BMJ qual Saf. 2013;22(2):ii33–9.
PubMed PubMed Central Google Scholar
Morgan H, Thomson G, Crossland N, Dykes F, Hoddinott P. Combining PPI with qualitative research to engage ‘harder-to-reach’ populations: service user groups as co-applicants on a platform study for a trial. Res Involvement Engagement. 2016;2(1):1.
Ocloo J, Matthews R. From tokenism to empowerment: progressing patient and public involvement in healthcare improvement. BMJ Qual Saf. 2016. doi: 10.1136/bmjqs-2015-004839
PubMed Google Scholar
Peat M, Entwistle V, Hall J, Birks Y, Golder S. Scoping review and approach to appraisal of interventions intended to involve patients in patient safety. J Health Serv Res Policy. 2010;15 suppl 1:17–25.
Randell, R. Accountability in an alarming environment. In Proceedings of the 2004 ACM conference on Computer supported cooperative work. ACM. 2004. 125–131.
Rhodes P, McDonald R, Campbell S, Daker‐White G, Sanders C. Sensemaking and the co-production of safety: a qualitative study of primary medical care patients. Sociol Health Illn. 2015;38(2):270–85. doi: 10.1111/1467-9566.12368 .
Salway S, Chowbey P, Such E, Ferguson B. Researching health inequalities with community researchers: practical, methodological and ethical challenges of an ‘inclusive’ research approach. Res Involvement Engagement. 2015;1(1):1–21.
Severinsson E, Holm A. Patients’ role in their Own safety—a systematic review of patient involvement in safety. Open J Nurs. 2015;5:642–53. doi: 10.4236/ojn.2015.57068 .
Staley K. ‘Is it worth doing?’ measuring the impact of patient and public involvement in research. Res Involvement Engagement. 2015;1(1):1–10.
Supple D, Roberts A, Hudson V, Masefield S, Fitch N, Rahmen M. From tokenism to meaningful engagement: best practices in patient involvement in an EU project. Research Involvement and Engagement, 2015;1(1)5
Unruh KT, Pratt W. Patients as actors: the patient’s role in detecting, preventing, and recovering from medical errors. Int J Med Inform. 2007;76:S236–44.
Vincent CA, Coulter A. Patient safety: what about the patient? Qual Saf Health Care. 2002;11(1):76–80.
WHO. Patients for patient safety: partnerships for safer health care. 2013.
WHO. Ethical issues in patient safety research: interpreting existing guidance. 2013.
Download references
Acknowledgements
We are grateful to all the patients who participated in the workshop, including Sylvia Bailey, Glenys Davies, Gerry Freedman, Laura Duchnicki, Fran Husson, John Trow, Carole Trow, and Carolyn Wheatley. The participants listed have chosen to be named in this section.
This work was supported by NIHR grant number 12/209/27, from the Health Services and Delivery Research (HS&DR) stream. The research was partially funded by the National Institute for Health Research (NIHR) Health Protection Research Unit (HPRU) in Healthcare Associated Infection and Antimicrobial Resistance at Imperial College London in partnership with Public Health England (PHE), and the NIHR Imperial Patient Safety Translational Research Centre. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, PHE or the Department of Health.
Author information
Authors and affiliations.
UCL Interaction Centre, University College London, Gower Street, London, UK
Dominic Furniss, Ioanna Iacovides, Imogen Lyons & Ann Blandford
Centre for Medication Safety and Service Quality, Imperial College Healthcare NHS Trust, London, UK
Bryony Dean Franklin
Research Department of Practice and Policy, UCL School of Pharmacy, Mezzanine Floor, BMA House, Tavistock Square, London, UK
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Dominic Furniss .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors’ contributions
DF and II organized the workshop. DF, II and IL led the workshop and reviewed the transcripts. DF led the writing of the paper. All authors contributed to the conception and design of the workshop and to writing the paper; all authors approved the final version.
Authors’ information
DF is a senior research associate at UCL with an interest in human factors and medical device design and use.
II is a research associate at UCL, whose research has explored learning and technology in contexts such as infusion device training.
IL is a research associate at UCL, whose research interests include the safe and effective use of medicines in hospital and community settings, and patients’ experiences of care.
AB is Professor of Human–Computer Interaction in the Department of Computer Science at UCL and Director of UCL Institute of Digital Health.
BDF is Executive Lead Pharmacist for Research at Imperial College Healthcare NHS Trust, Professor of Medication Safety at UCL School of Pharmacy and Director of the Centre for Medication Safety and Service Quality.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Furniss, D., Iacovides, I., Lyons, I. et al. Patient and public involvement in patient safety research: a workshop to review patient information, minimise psychological risk and inform research. Res Involv Engagem 2 , 19 (2016). https://doi.org/10.1186/s40900-016-0035-x
Download citation
Received : 03 December 2015
Accepted : 11 May 2016
Published : 17 May 2016
DOI : https://doi.org/10.1186/s40900-016-0035-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Patient and public involvement
- Patient safety
- Medication error
- Intravenous medication
- Health services research
Research Involvement and Engagement
ISSN: 2056-7529
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Engaging patients and stakeholders in research proposal review: the patient-centered outcomes research institute
- PMID: 25023251
- DOI: 10.7326/M13-2412
The inaugural round of merit review for the Patient-Centered Outcomes Research Institute (PCORI) in November 2012 included patients and other stakeholders, as well as scientists. This article examines relationships among scores of the 3 reviewer types, changes in scoring after in-person discussion, and the effect of inclusion of patient and stakeholder reviewers on the review process. In the first phase, 363 scientists scored 480 applications. In the second phase, 59 scientists, 21 patients, and 31 stakeholders provided a "prediscussion" score and a final "postdiscussion" score after an in-person meeting for applications. Bland-Altman plots were used to characterize levels of agreement among and within reviewer types before and after discussion. Before discussion, there was little agreement among average scores given by the 4 lead scientific reviewers and patient and stakeholder reviewers. After discussion, the 4 primary reviewers showed mild convergence in their scores, and the 21-member panel came to a much stronger agreement. Of the 25 awards with the best (and lowest) scores after phase 2, only 13 had ranked in the top 25 after the phase 1 review by scientists. Five percent of the 480 proposals submitted were funded. The authors conclude that patient and stakeholder reviewers brought different perspectives to the review process but that in-person discussion led to closer agreement among reviewer types. It is not yet known whether these conclusions are generalizable to future rounds of peer review. Future work would benefit from additional data collection for evaluation purposes and from long-term evaluation of the effect on the funded research.
PubMed Disclaimer
Similar articles
- Unique Review Criteria and Patient and Stakeholder Reviewers: Analysis of PCORI's Approach to Research Funding. Forsythe LP, Frank LB, Tafari AT, Cohen SS, Lauer M, Clauser S, Goertz C, Schrandt S. Forsythe LP, et al. Value Health. 2018 Oct;21(10):1152-1160. doi: 10.1016/j.jval.2018.03.017. Epub 2018 Jun 8. Value Health. 2018. PMID: 30314615
- Researchers, Patients, and Stakeholders Evaluating Comparative-Effectiveness Research: A Mixed-Methods Study of the PCORI Reviewer Experience. Forsythe LP, Frank LB, Hemphill R, Tafari AT, Szydlowski V, Lauer M, Goertz C, Clauser S. Forsythe LP, et al. Value Health. 2018 Oct;21(10):1161-1167. doi: 10.1016/j.jval.2018.03.018. Epub 2018 Jun 12. Value Health. 2018. PMID: 30314616
- Panel discussion does not improve reliability of peer review for medical research grant proposals. Fogelholm M, Leppinen S, Auvinen A, Raitanen J, Nuutinen A, Väänänen K. Fogelholm M, et al. J Clin Epidemiol. 2012 Jan;65(1):47-52. doi: 10.1016/j.jclinepi.2011.05.001. Epub 2011 Aug 9. J Clin Epidemiol. 2012. PMID: 21831594
- Patient engagement in the design and execution of urologic oncology research. Lee DJ, Avulova S, Conwill R, Barocas DA. Lee DJ, et al. Urol Oncol. 2017 Sep;35(9):552-558. doi: 10.1016/j.urolonc.2017.07.002. Epub 2017 Jul 26. Urol Oncol. 2017. PMID: 28755961 Review.
- Evaluating patient and stakeholder engagement in research: moving from theory to practice. Esmail L, Moore E, Rein A. Esmail L, et al. J Comp Eff Res. 2015 Mar;4(2):133-45. doi: 10.2217/cer.14.79. J Comp Eff Res. 2015. PMID: 25825842 Review.
- Patient Advocates for Clinical Research (PACER): A Step Toward Ethical, Relevant, and Truly Participatory Clinical Research in India. Bagai P, Sharma P, Ansari A, Singh N, Sharma S, Singh P, Chougule D, Singh MK, Singh G, Singh S. Bagai P, et al. Cureus. 2024 Apr 17;16(4):e58454. doi: 10.7759/cureus.58454. eCollection 2024 Apr. Cureus. 2024. PMID: 38765448 Free PMC article.
- Community members' experiences training as medical journal reviewers. Collins CC, Hood E, Jewett-Tennant J, Stange K, Sehgal AR. Collins CC, et al. Res Involv Engagem. 2023 Aug 14;9(1):66. doi: 10.1186/s40900-023-00482-x. Res Involv Engagem. 2023. PMID: 37582827 Free PMC article.
- Community Members as Reviewers of Medical Journal Manuscripts: a Randomized Controlled Trial. Huml AM, Albert JM, Beltran JM, Berg KA, Collins CC, Hood EN, Nelson LC, Perzynski AT, Stange KC, Sehgal AR. Huml AM, et al. J Gen Intern Med. 2023 May;38(6):1393-1401. doi: 10.1007/s11606-022-07802-z. Epub 2022 Sep 26. J Gen Intern Med. 2023. PMID: 36163530 Free PMC article.
- Patient involvement in priority-setting for medical research: A mini review of initiatives in the rare disease field. Katirai A, Kogetsu A, Kato K, Yamamoto B. Katirai A, et al. Front Public Health. 2022 Jul 19;10:915438. doi: 10.3389/fpubh.2022.915438. eCollection 2022. Front Public Health. 2022. PMID: 35928485 Free PMC article. Review.
- Patient involvement in clinical trials. Geißler J, Isham E, Hickey G, Ballard C, Corbett A, Lubbert C. Geißler J, et al. Commun Med (Lond). 2022 Jul 25;2:94. doi: 10.1038/s43856-022-00156-x. eCollection 2022. Commun Med (Lond). 2022. PMID: 35903184 Free PMC article.
- Search in MeSH
Related information
- Cited in Books
LinkOut - more resources
Full text sources.
- Ovid Technologies, Inc.
Other Literature Sources
- scite Smart Citations
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Advertisement
A scoping review of patient safety research carried out in the Republic of Ireland
- Review Article
- Open access
- Published: 05 February 2022
- Volume 192 , pages 1–9, ( 2023 )
Cite this article
You have full access to this open access article

- Paul O’Connor ORCID: orcid.org/0000-0001-9036-098X 1 , 2 ,
- Roisin O’Malley 1 , 2 ,
- Yazeed Kaud 1 , 4 ,
- Emily St. Pierre 3 ,
- Rosie Dunne 5 ,
- Dara Byrne 2 , 3 &
- Sinéad Lydon 2 , 3
5247 Accesses
3 Citations
14 Altmetric
Explore all metrics
Maintaining the highest levels of patient safety is a priority of healthcare organisations. However, although considerable resources are invested in improving safety, patients still suffer avoidable harm. The aims of this study are: (1) to examine the extent, range, and nature of patient safety research activities carried out in the Republic of Ireland (RoI); (2) make recommendations for future research; and (3) consider how these recommendations align with the Health Service Executive’s (HSE) patient safety strategy. A five-stage scoping review methodology was used to synthesise the published research literature on patient safety carried out in the RoI: (1) identify the research question; (2) identify relevant studies; (3) study selection; (4) chart the data; and (5) collate, summarise, and report the results. Electronic searches were conducted across five electronic databases. A total of 31 papers met the inclusion criteria. Of the 24 papers concerned with measuring and monitoring safety, 12 (50%) assessed past harm, 4 (16.7%) the reliability of safety systems, 4 (16.7%) sensitivity to operations, 9 (37.5%) anticipation and preparedness, and 2 (8.3%) integration and learning. Of the six intervention papers, three (50%) were concerned with education and training, two (33.3%) with simplification and standardisation, and one (16.7%) with checklists. One paper was concerned with identifying potential safety interventions. There is a modest, but growing, body of patient safety research conducted in the RoI. It is hoped that this review will provide direction to researchers, healthcare practitioners, and health service managers, in how to build upon existing research in order to improve patient safety.
Similar content being viewed by others
Tools for primary care patient safety: a narrative review
Contributory factors to patient safety incidents in primary care: protocol for a systematic review, top 10 interventions in paediatric patient safety.
Avoid common mistakes on your manuscript.
Introduction
A commitment to improving safe healthcare features in governmental policies worldwide. However, progress in delivering on this aspiration has been modest, with patients still suffering avoidable harm [ 1 ]. A major challenge to improving safety is the lack of high-quality information to allow healthcare organisations, teams, and individuals to evaluate how they are performing, and where there are deficits and risks [ 2 ]. This safety information is complex and multi-faceted, yet vitally important if safety is to improve [ 3 ].
In the Republic of Ireland (RoI), “maintaining the highest levels of patient safety is a fundamental priority for patients and for healthcare organisations”(p.5) [ 4 ]. The need for proactive approaches to patient safety has been identified by the Irish Health Service Executive (HSE) [ 4 ]. There is a recognition that such an approach requires high-quality data that will support learning from patient safety incidents, identification of hazards or risks, and the implementation of interventions to improve safety [ 4 ]. It is only through effective measurement and monitoring of safety (MMS) that comparisons can be made between the safety performance of different healthcare organisations, the impact of safety interventions can be assessed, and there can be a shift to a more proactive approach to safety.
In addition to efforts to improve the MMS, there is also a need to consider the effectiveness of patient safety interventions. There has been considerable investment in patient safety improvement efforts, for which there may be limited evidence of effectiveness [ 5 ]. It has been found that the majority of safety interventions tend to be person-focused (e.g. education and training), with more effective systems focused interventions far less commonplace [ 6 ]. Moreover, high-quality research on the effectiveness of safety intervention is lacking [ 5 ]. Therefore, there is a need for rigorous assessment of the effectiveness of interventions to ensure that they are having the desired effect, and the resources required to implement such interventions are justified. Crucially, given the recognised impact of context on intervention implementation and effectiveness, such assessments must be conducted within different healthcare systems and services [ 7 ].
The purpose of this scoping review is to examine the extent, range, and nature of patient research activities carried out in the RoI. Research is fundamental to improving practice, particularly within an applied science such as patient safety [ 8 ]. Accordingly, the findings from this review will be used to make recommendations for future patient safety research, and the alignment between these recommendations and the HSE patient safety strategy 2019–2024 [ 4 ] will be delineated.
This scoping review is conducted using the five-stage approach proposed by Arksey and O’Malley [ 9 ] and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist [ 10 ]. Scoping reviews provide an increasingly popular option for synthesising and mapping evidence in healthcare research [ 11 ].
Stage 1: Identify the research question
The purpose of the review was clearly defined with concept of interest (i.e. patient safety research), target population (i.e. healthcare staff and patients in secondary care), and location (i.e. RoI).
Stage 2: Identify relevant studies
Search strategy.
Electronic searches were conducted across five electronic databases in July 2021: Medline, CINAHL, Embase, PsycInfo, and Web of Science. The search strategy was finalised by a Research Librarian (RD). The search strategy comprised Medical Subject Headings terms along with free-text keywords, and was altered as necessary for the remaining databases (see Supplementary Data 1 [ 12 ] for the Medline search strategy). In addition to electronic searches, the reference lists of all studies identified as eligible for inclusion from the electronic searches were screened to identify any other potentially suitable articles.
Stage 3: Study selection
Titles and abstracts of all articles identified during the electronic searches were screened by one of three authors (ROM, YK, or ESP) in July 2021. The full-texts of articles that appeared eligible for inclusion, or articles in which the title and abstract did not provide sufficient information for the determination to be made, were reviewed in full to confirm their eligibility. For papers where inclusion was unclear, all members of the research team reviewed the paper, and decisions on eligibility were made through discussion.
Inclusion criteria
Inclusion criteria required that studies: (1) were focused on patient safety in hospitals in the RoI including, but not limited to, the measurement of safety or implementation of initiatives aimed at improving safety; (2) reported original research; (3) were published in a peer-reviewed journal; and (4) were written in English.
Exclusion criteria
Studies were excluded if they: (1) focused on patient safety in the context of patients with a particular medical condition only (e.g. patients with cancer); (2) focused on the safety of one process only (e.g. medication errors); (3) were conducted in healthcare settings other than hospitals; (4) were conducted in a country other than the RoI or a sample of countries including the RoI where RoI-specific data could not be extracted; (5) only employed one item/question relating to patient safety as part of a larger survey or assessment (i.e. studies had to use a full measure of patient safety); or (6) did not report original research. No limits were placed on the publication year.
Stage 4: Chart the data
A preliminary data charting form was developed in accordance with best practice [ 13 ], and piloted by two authors (YK, ROM). The form was used to extract data on author(s), year of publication, study location, study aim, methods, sample, intervention (if included), comparator (if included), outcome measures, and key reported outcomes. Data were extracted by three authors (ROM, YK, and ESP), with two of these authors extracting data independently for each included article. Disagreements were resolved through discussion.
Stage 5: Collate, summarise, and report the results
The characteristics of the included studies were collated and summarised across several key descriptors: location; aim; methods; sample; type and duration of intervention (if applicable); comparators (if applicable); outcome measures; and key outcomes.
Included studies were summarised according to one of two different frameworks. Studies that involved MMS were categorised using the five domains of Vincent et al. [ 3 , 14 ] MMS framework (see Table 1 ). It was possible for both studies and measures described to be categorised under more than one MMS dimension.
Studies of a safety intervention were classified using the hierarchy of intervention effectiveness framework [ 15 ] (see Table 1 ). The framework delineates interventions according to six levels of effectiveness from 1 (most effective) to 6 (least effective). The hierarchy of intervention effectiveness framework was first discussed by the Institute for Safe Medication Practices, and has since been referenced a number of patient safety organisations as an approach to guide the identification of suitable safety interventions (e.g. Incident Analysis Collaborating Parties [ 16 ], Health Information and Quality Authority [ 17 ]). The hierarchy of interventions was extended by Woods et al. [ 18 ], who added three additional levels (staff organisation, risk assessment, learning from errors, and personal initiative) as this was deemed necessary in order to appropriately classify solutions to improving clinical communication and patient safety. However, for the purposes of this scoping review, we used the original six level framework due to our focus on interventions, rather than solutions (see Table 1 ).
The categorisation of study content via these two frameworks was carried out independently by three reviewers (ROM, YK, and ESP). Where disagreements arose, the study was discussed by all members of the review team and a decision on the categorisation was made by consensus. Following completion of all data charting and coding, the meaning of the findings and their implications were appraised within the context of the broader literature in this area, and the HSE patient safety strategy [ 4 ].
A total of 6515 articles were identified from electronic database searches (see Fig. 1 ), with 170 full-texts examined and 27 papers ultimately meeting the inclusion criteria. Four additional studies were identified through reference list screening, resulting in the inclusion of 31 studies (published 2003–2021). Study characteristics are outlined in Table 2 , and a summary of the main findings from the studies is provided in Table 3 .

PRISMA flowchart of the search and screening process
Studies focused on past harm
Past harm was the most frequently assessed dimension of the MMS framework, and was measured in 12 studies (see Tables 2 and 3 , and Online Supplementary Material 2 [ 12 ]). Six studies employed surveys to measure past harm. Two of these studies used surveys to estimate the frequency of a range of adverse events [ 19 ] and to examine nurse adverse event reporting rates [ 20 ]. Of the four remaining studies that used a survey design, two examined the association of burnout with self-reported medical error and poor-quality care [ 21 , 22 ], and two studies explored nurse incident reporting [ 23 , 24 ]. Four studies measured past harm by retrospectively reviewing patient records. Two of these record reviews were undertaken as part of the Irish National Adverse Events studies [ 1 , 25 ], and examined trends in adverse event rates in the Irish healthcare system. The two remaining record reviews were conducted to estimate the economic cost of nurse-sensitive adverse events [ 26 ] and to compare the health system performance of 15 Organisation for Economic Co-operation (OECD) countries across seven patient safety indicators [ 27 ]. Furthermore, one study used a combination of survey and interview methods to examine the nature and frequency of medical error among junior doctors [ 28 ], and one study comprised a review of medico-legal claims to identify current adverse event reporting trends in Irish surgical specialties [ 29 ].
Studies focused on reliability of safety critical processes
Four studies assessed the reliability of safety critical processes (see Tables 2 and 3 , and Online Supplementary Material 2 [ 12 ]). Of the two studies that used a survey design to monitor reliability, one study employed surveys to examine the implementation of Surgical Safety Checklists (SSC) in Irish operating theatres [ 30 ] while the other study used interviews to develop a survey evaluating the attitudes of theatre staff towards a surgical checklist [ 31 ]. Two studies used patient record review methodology to assess reliability, one of which reviewed patient records to assess the prevalence of surgical checklist use in Europe [ 32 ] while the other study used hospital data to improve the international comparability of patient safety indicators [ 27 ].
Studies focused on sensitivity to operations
Four studies included a measure that assessed sensitivity to operations (see Tables 2 and 3 , and Online Supplementary Material 2 [ 12 ]). Three of these studies used surveys and asked nurses to give their ward an overall safety grade [ 19 , 20 , 33 ]. One study conducted interviews to explore aspects of safety culture that were important to the staff at the time of the interviews [ 34 ].
Studies focused on anticipation and preparedness
Almost a third of the included studies focused on anticipation and preparedness (see Tables 2 and 3 , and Online Supplementary Material 2 [ 12 ]). Five studies used surveys to assess patient safety culture. Three of these studies employed the Safety Attitudes Questionnaire (SAQ) [ 35 , 36 , 37 ], and two studies used items from other surveys [ 19 , 20 ]. Interviews and/or observations were used by three studies to investigate healthcare workers’ perceptions of the safety culture [ 34 ] and to explore how nurses promote safety in perioperative settings [ 38 , 39 ]. One study used in situ simulation to examine latent safety hazards in response to preparation for an expected COVID-19 surge [ 40 ].
Studies focused on integration and learning
Integration and learning was assessed by two studies (see Tables 2 and 3 , and Online Supplementary Material 2 [ 12 ]). McNamara and O' Donoghue [ 41 ] reviewed patient records to objectively demonstrate if a change in labour ward clinical activity occurred following serious adverse perinatal events. Jee et al. [ 40 ] identified system errors and latent safety hazards using in situ simulation and described the resulting corrective measures taken to improve their pandemic response locally.
Intervention studies
Six studies were categorised as intervention studies. Studies employed several different types of intervention of varying effectiveness (see Tables 2 and 3 , and Online Supplementary Material 2 [ 12 ]). Three studies comprised interventions that focused on improving patient safety through education and training [ 42 , 43 , 44 ]. One of these studies implemented a board game to educate junior doctors about patient safety and the importance of reporting safety concerns [ 42 ]. The second educational intervention was concerned with training aimed at improving interns’ attitudes towards, and ability to, “speak up” to senior physicians [ 43 ], and the third comprised an online patient safety education programme for junior doctors [ 44 ].
Two of the studies implemented interventions focused on improving safety through simplification and standardisation. Both of these studies involved the implementation of an incident/near miss reporting form [ 45 ] or complication proforma [ 46 ]. Finally, one study sought to improve patient safety by implementing an intervention focused on reminders, checklists, and double checks. This intervention involved the development and implementation of an adhesive surgical ward round checklist [ 47 ].
There was one study included in the review that was not concerned with MMS or constituted an intervention itself. Rather, the focus of this study was on the development of a collective leadership intervention for healthcare teams to improve team performance and patient safety culture [ 48 ].
This scoping review has demonstrated that, although overall modest in size, there is a growing body of research on patient safety in the RoI published in peer-reviewed journals—particularly in recent years. This growth is consistent with the action from the HSE patient safety strategy “to support patient safety research and publish and act on the results” (p.19]) [ 4 ]. The majority of the research on MMS in the RoI was focused on measuring past harm (particularly adverse events), and anticipation and preparedness (particularly assessments of safety culture/climate). Most of the intervention studies were concerned with education and training. We will make recommendations for areas of future research based on the findings from the scoping review, and identify how these recommendations align with relevant aims from the HSE patient safety strategy 2019–2024 [ 4 ].
The focus on adverse events as a method of measuring past harm is consistent with the substantial increase in research publications on this approach to measuring safety in healthcare [ 49 ]. Staff surveys are a commonly used source of information on adverse events. However, a survey approach is constrained by the extent to which conclusions can be drawn about adverse event prevalence. Patient record review has been considered the “gold standard” patient safety research method [ 50 ], and was used in four of the reviewed studies. Such data are useful in demonstrating the scope of the problem in the Irish healthcare system, allows for international comparisons, and for an assessment of any changes over time. However, patient record review data are limited in terms of identifying specific areas for safety improvement [ 50 , 51 ]. Therefore, there is a need for measures tailored to distinct aspects of patient harm (e.g. specific care-related injuries, missed diagnoses that lead to harm) [ 50 ]. Such data is important to address the HSE goal to “measure and monitor safety, to evaluate the effects of safety improvement initiatives, and to inform further emerging priorities”(p.19) [ 4 ]. Data on specific aspects of patient harm will allow the alignment of adverse events with failures in care, and the development and evaluation of interventions to address these issues [ 52 ].
Safety culture/climate surveys were the most frequently used approach to measuring and monitoring anticipation and preparedness. Again, this is consistent with the large amount of research devoted to these types of measures more broadly in the safety literature [ 53 , 54 ]. Safety culture/climate data is useful in identifying areas of both strength and weakness. However, it has been suggested that such survey measures may be best viewed as a trusted “wet finger” to find out which way the wind blows [ 55 ], and do not identify specific areas for improvement. To illustrate, working conditions were identified as an area for improvement across four of the included studies [ 34 – 37 ]. However, further data is required to identify the specific working conditions that should be prioritised for change. This is why, in some safety culture interventions, the survey data is used to inform discussion in qualitative safety culture workshops to identify the specific issues that need to be addressed [ 56 ]. It is recommended that future research should consider how to measure safety culture/climate in a way that is practical, sufficiently specific to identify areas for safety improvement, and can be used to measure whether improvements have occurred. This will likely require a combination of quantitative and qualitative data collection methodologies. A consideration of how to measure safety culture/climate is particularly important in the RoI as this has been identified as a specific action in the HSE patient safety strategy [ 4 ].
Compared to the MMS dimensions of past harm and anticipation and preparedness, a lower number of studies in our scoping review were concerned with MMS in the other three safety dimensions—particularly integration and learning. These proportions are similar to the findings from a systematic review of MMS in prehospital care [ 51 ]. Although the studies in our scoping review that assessed one of these three dimensions of MMS provided informative data, they were largely based upon staff survey responses. Only one study [ 41 ] utilised clinical data. It is suggested that consideration should be given to the identification of feasible methods to MMS in these three under-researched dimensions beyond that derived only from survey data. A robust safety surveillance system should comprise multiple methods and address all five MMS domains. Research is recommended to critically appraise the existing safety monitoring system in the RoI healthcare system in order to identify blind spots as well as where there may be duplication of effort. Such research is consistent with the HSE patient safety strategy aim to “further develop and enhance local and national suites of key patient safety indicators” (p. 19) [ 4 ].
Although MMS is important, what is also essential is that this data is used to identify and evaluate the effectiveness of interventions to improve patient safety and quality of care [ 52 ]. In fact, there is arguably little point in collecting safety data if it is not then used to bring about improvement. Three out of the six safety interventions identified were focused on education and training—a person-focused intervention at the lowest level of the hierarchy of intervention effectiveness [ 15 ]. Although two interventions [ 45 , 46 ] with a focus on simplification and standardisation were identified, no interventions were found at the highest two levels of the hierarchy—forcing functions, automation and computerisation. The evaluations of the interventions included in the review were positive. However, similar to the majority of assessments of patient safety interventions, the quality of the evidence of effectiveness was low, with limited evidence of an impact on patient outcomes [ 5 , 57 , 58 ]. It is recommended that future research focuses on the evaluation of more effective system-focused interventions. It is further recommended that interventions are closely aligned to appropriate, and meaningful, measures of MMS in order to support rigour in evaluation of the impact of interventions on patient safety. This alignment will be necessary to achieve the HSE patient safety aims of putting in place appropriate actions to mitigate risks to patients, prioritising specific safety improvement initiatives, and evaluating the effects of safety improvement and risk mitigation initiatives [ 4 ]. It is also suggested that the co-design approach used by Ward et al. [ 48 ] may offer a useful approach to identify specific interventions that healthcare staff believe will improve safety.
Limitations
There are a number of limitations to our scoping review. Firstly, a quality assessment was not carried out, although this absence is consistent with the majority of other scoping reviews [ 59 ]. Secondly, our scoping review provided a more descriptive summary of the literature than would be the case from a systematic review. This is a result of the goal of a scoping review to provide a map of existing research, rather than to answer a specific question [ 60 ]. Thirdly, as in any synthesis of the literature, scoping reviews are at risk for bias [ 60 ]. Fourthly, studies that focused on patient safety in the context of patients with a particular medical condition or focused on the safety of one process were excluded from our review. The rationale for this exclusion was that it would have been impossible to devise a search strategy that included every possible medical condition, and process. Therefore, we chose to take an approach that included all papers that met the inclusion criteria rather than an approach that, although broader, may have missed particular studies. Finally, we did not carry out a search of the grey literature. These searches were not carried out as there are methodological issues with including grey literature searches in systematic reviews (e.g. compromised methodological reproducibility, difficulties in interpreting these publications [ 61 ]).
There is a modest, but growing, body of patient safety research conducted in the RoI. This scoping review has demonstrated the variety of patient safety research being carried out in the RoI. It is hoped that this review will provide direction to researchers, healthcare practitioners, and health service managers, in how to build upon the existing research in order to improve patient safety and quality of care.
Rafter N, Hickey A, Conroy RM et al (2017) The Irish National Adverse Events Study (INAES): the frequency and nature of adverse events in Irish hospitals-a retrospective record review study. BMJ Qual Saf 26(2):111–119
Article Google Scholar
Dixon-Woods M, Baker R, Charles K et al (2014) Culture and behaviour in the English National Health Service: overview of lessons from a large multimethod study. BMJ Qual Saf 23(2):106–115
Vincent C, Burnett S, Carthey J (2013) The measurement and monitoring of safety: drawing together academic evidence and practical experience to produce a framework for safety measurement and monitoring. The Health Foundation, London
Google Scholar
Health Services Executive (2019) Patient safety strategy 2019–2024. Dublin: Author
Zegers M, Hesselink G, Geense W et al (2016) Evidence-based interventions to reduce adverse events in hospitals: a systematic review of systematic reviews. BMJ Open 6(9):e012555
Soong C, Shojania KG (2020) Education as a low-value improvement intervention: often necessary but rarely sufficient. BMJ Qual Saf 29:353–357
Øvretveit JC, Shekelle PG, Dy SM et al (2011) How does context affect interventions to improve patient safety? An assessment of evidence from studies of five patient safety practices and proposals for research. BMJ Qual Saf 20(7):604–610
Pronovost PJ, Goeschel CA, Marsteller JA et al (2009) Framework for patient safety research and improvement. Circ 119(2):330–337
Arksey H, O’Malley L (2005) Scoping studies: towards a methodological framework. Int J Soc Res Method 8(1):19–32
Tricco AC, Lillie E, Zarin W et al (2018) PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Annal Int Med 169(7):467–473
Levac D, Colquhoun H, O’Brien KK (2010) Scoping studies: advancing the methodology. Implement Sci 5(1):1–9
O’Connor P, O’Malley, Kaud Y et al (2022) A scoping review of patient safety research carried out in the Republic of Ireland. Zenodo. https://doi.org/10.5281/zenodo.5846748 .
Peters MD, Godfrey C, McInerney P et al (2020) Scoping reviews. In: Aromataris E, Munn Z (Eds). JBI manual for evidence synthesis (pp. 406–451). Adeleide, Austalia: JBI Global
Vincent C, Burnett S, Carthey J (2014) Safety measurement and monitoring in healthcare: a framework to guide clinical teams and healthcare organisations in maintaining safety. BMJ Qual Saf 23(8):670–677
Institute for Safe Medication Practices (1999) Medication error prevention toolbox. ISMP Med Saf Alert 4:1–2
Incident Analysis Collaborating Parties (2012) Canadian incident analysis framework. Edmont AB: Canad Patient Saf Inst
Health Information and Quality Authority (2018) Medication safety monitoring programme in public acute hospitals- an overview of findings. Dublin: Author
Woods DM, Holl JL, Angst D et al (2008) Improving clinical communication and patient safety: clinician-recommended solutions. In: Henriksen K, Battles JB, Keyes MA (eds)Advances in patient safety: new directions and alternative approaches (Vol 3: performance and tools) (pp. 1–18). Henriksen Rockville, MD: Agen Healthcare Res Qual
Aiken LH, Sloane DM, Bruyneel L et al (2013) Nurses’ reports of working conditions and hospital quality of care in 12 countries in Europe. Int J Nurs Stud 50(2):143–153
Kirwan M, Matthews A, Scott PA (2013) The impact of the work environment of nurses on patient safety outcomes: a multi-level modelling approach. Int J Nurs Stud 50(2):253–263
O’Connor P, Lydon S, O’Dea A et al (2017) A longitudinal and multicentre study of burnout and error in Irish junior doctors. Postgrad Med J 93(1105):660–664
Sulaiman CFC, Henn P, Smith S et al (2017) Burnout syndrome among non-consultant hospital doctors in Ireland: relationship with self-reported patient care. Int J Qual Saf Health 29(5):679–684
Moore L, McAuliffe E (2010) Is inadequate response to whistleblowing perpetuating a culture of silence in hospitals?. Clin Gov 15(3):166–178
Moore L, McAuliffe E (2012) To report or not to report? Why some nurses are reluctant to whistleblow. Clin Gov 17(4):332–342
Connolly W, Rafter N, Conroy RM et al (2021) The Irish National Adverse Event Study-2 (INAES-2): longitudinal trends in adverse event rates in the Irish healthcare system. BMJ Qual Saf 30(7):547–558
Murphy A, Griffiths P, Duffield C et al (2021) Estimating the economic cost of nurse sensitive adverse events amongst patients in medical and surgical settings. J Adv Nurs 77(8):3379–3388
Drosler SE, Romano PS, Tancredi DJ et al (2012) International comparability of patient safety indicators in 15 OECD member countries: a methodological approach of adjustment by secondary diagnoses. Health Serv Res 47(1):275–292
O’Connor P, Lydon S, Mongan O et al (2019) A mixed-methods examination of the nature and frequency of medical error among junior doctors. Postgrad Med J 95(1129):583–589
Breathnach O, Cousins G, Dunne D et al (2011) A review of adverse event reporting in Irish surgical specialties. Clin Risk 17(2):43–49
Nugent E, Hseino H, Ryan K et al (2013) The surgical safety checklist survey: a national perspective on patient safety. Ir J Med Sci 182(2):171–176
Article CAS Google Scholar
O’Connor P, Reddin C, O’Sullivan M et al (2013) Surgical checklists: the human factor. Pat Saf Surg 7(1):14
Jammer I, Ahmad T, Aldecoa C et al (2015) Point prevalence of surgical checklist use in Europe: relationship with hospital mortality. Br J Anaesth 114(5):801–807
Aiken LH, Sermeus W, Van den Heede K et al (2012) Patient safety, satisfaction, and quality of hospital care: cross sectional surveys of nurses and patients in 12 countries in Europe and the United States. BMJ (Clin Res Ed) 344:e1717
Gleeson LL, O’Brien GL, O’Mahony D et al (2021) Thirst for change in a challenging environment: healthcare providers’ perceptions of safety culture in a large Irish teaching hospital. Ir J Med Sci. https://doi.org/10.1007/s11845-021-02611-5
Dwyer L, Smith A, McDermott R et al (2018) Staff attitudes towards patient safety culture and working conditions in an Irish tertiary neonatal unit. Ir Med J 111(7):786
CAS Google Scholar
Gleeson LL, Tobin L, O’Brien GL et al (2020) Safety culture in a major accredited Irish university teaching hospital: a mixed methods study using the safety attitudes questionnaire. Ir J Med Sci 189(4):1171–1178
Relihan E, Glynn S, Daly D et al (2009) Measuring and benchmarking safety culture: application of the safety attitudes questionnaire to an acute medical admissions unit. Ir J Med Sci 178(4):433–439
O’Brien B, Andrews T, Savage E (2019) Nurses keeping patients safe by managing risk in perioperative settings: a classic grounded theory study. J Nurs Manag 27(7):1454–1461
O’Brien B, Andrews T, Savage E (2018) Anticipatory vigilance: a grounded theory study of minimising risk within the perioperative setting. J Clin Nurs 27(1–2):247–256
Jee M, Khamoudes D, Brennan AM et al (2020) COVID-19 outbreak response for an emergency department using in situ simulation. Cureus 12(4):e7876
McNamara K, O’Donoghue K (2019) The perceived effect of serious adverse perinatal events on clinical practice. Can it be objectively measured? Euro J Obst Gyn Repod Bio 240:267–272
Ward M, Ni She E, De Brun A et al (2019) The co-design, implementation and evaluation of a serious board game PlayDecide patient safety to educate junior doctors about patient safety and the importance of reporting safety concerns. BMC Med Ed 19(1):232
O’Connor P, Byrne D, O’Dea A et al (2013) Excuse me: teaching interns to speak up. Jt Comm J Qual Patient Saf 39(9):426–431
McCarthy SE, O’Boyle CA, O’Shaughnessy A et al (2016) Online patient safety education programme for junior doctors: is it worthwhile?. Ir J Med Sci 185(1):51–58
McElhinney J, Heffernan O (2003) Using clinical risk management as a means of enhancing patient safety: the Irish experience. Int J Health Care Qual Assur 16(2–3):90–98
McVeigh TP, Waters PS, Murphy R et al (2013) Increasing reporting of adverse events to improve the educational value of the morbidity and mortality conference. J Amer Coll Surg 216(1):50–56
Dhillon P, Murphy RKJ, Ali H et al (2011) Development of an adhesive surgical ward round checklist: a technique to improve patient safety. Ir Med J 104(10):303–305
Ward ME, De Brún A, Beirne D et al (2018) Using co-design to develop a collective leadership intervention for healthcare teams to improve safety culture. Int J Environ Res Pub Health 15(6):1182
Stelfox HT, Palmisani S, Scurlock C et al (2006) The to err is human report and the patient safety literature. BMJ Qual Saf 15(3):174–178
Shojania KG, Marang-van de Mheen PJ (2020) Identifying adverse events: reflections on an imperfect gold standard after 20 years of patient safety research. BMJ Qual Saf 29:265–270
O’Connor P, O’Malley R, Oglesby AM et al (2021) Measurement and monitoring patient safety in prehospital care: a systematic review. Int J Qual Saf Health 33(1). https://doi.org/10.1093/intqhc/mzab013
Sauro K, Ghali WA, Stelfox HT (2020) Measuring safety of healthcare: an exercise in futility?. BMJ Qual Saf 29(4):341–344
Alsalem G, Bowie P, Morrison J (2018) Assessing safety climate in acute hospital settings: a systematic review of the adequacy of the psychometric properties of survey measurement tools. BMC Health Serv Res 18(1):1–14
Waterson P, Carman E-M, Manser T et al (2019) Hospital Survey on Patient Safety Culture (HSPSC): a systematic review of the psychometric properties of 62 international studies. BMJ Open 9(9):e026896
Guldenmund FW (2007) The use of questionnaires in safety culture research–an evaluation. Saf Sci 45(6):723–743
Madden C, Lydon S, Cupples ME et al (2019) Safety in primary care (SAP-C): a randomised, controlled feasibility study in two different healthcare systems. BMC Fam Prac 20(1):22
Kirkman MA, Sevdalis N, Arora S et al (2015) The outcomes of recent patient safety education interventions for trainee physicians and medical students: a systematic review. BMJ Open 5(5):e007705
O’Dea A, O’Connor P, Keogh I (2014) A meta-analysis of the effectiveness of crew resource management training in acute care domains. Postgrad Med J 90(1070):699–708
Rumrill PD, Fitzgerald SM, Merchant WR (2010) Using scoping literature reviews as a means of understanding and interpreting existing literature. Work (Reading, Mass) 35(3):399–404
Sucharew H, Macaluso M (2019) Methods for research evidence synthesis: the scoping review approach. J Hosp Med 14(7):416–418
Mahood Q, Van Eerd D, Irvin E (2014). Searching for grey literature for systematic reviews: challenges and benefits. Res Synth Meth 5(3):221–234
Download references
Open Access funding provided by the IReL Consortium.
Author information
Authors and affiliations.
Department of General Practice, School of Medicine, National University of Ireland Galway, 1 Distillery Road, Galway, Co, Ireland
Paul O’Connor, Roisin O’Malley & Yazeed Kaud
Irish Centre for Applied Patient Safety and Simulation, National University of Ireland Galway, Galway, Ireland
Paul O’Connor, Roisin O’Malley, Dara Byrne & Sinéad Lydon
School of Medicine, National University of Ireland Galway, Galway, Ireland
Emily St. Pierre, Dara Byrne & Sinéad Lydon
Department of Public Health, Saudi Electronic University, Riyadh, Saudi Arabia
Yazeed Kaud
James Hardiman Library, National University of Ireland Galway, Galway, Ireland
Rosie Dunne
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Paul O’Connor .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
O’Connor, P., O’Malley, R., Kaud, Y. et al. A scoping review of patient safety research carried out in the Republic of Ireland. Ir J Med Sci 192 , 1–9 (2023). https://doi.org/10.1007/s11845-022-02930-1
Download citation
Received : 13 December 2021
Accepted : 19 January 2022
Published : 05 February 2022
Issue Date : February 2023
DOI : https://doi.org/10.1007/s11845-022-02930-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Patient safety
- Scoping review
- Find a journal
- Publish with us
- Track your research
- Systematic Review
- Open access
- Published: 26 April 2024
Systematic review on the frequency and quality of reporting patient and public involvement in patient safety research
- Sahar Hammoud ORCID: orcid.org/0000-0003-4682-9001 1 ,
- Laith Alsabek 1 , 2 ,
- Lisa Rogers 1 &
- Eilish McAuliffe 1
BMC Health Services Research volume 24 , Article number: 532 ( 2024 ) Cite this article
580 Accesses
Metrics details
In recent years, patient and public involvement (PPI) in research has significantly increased; however, the reporting of PPI remains poor. The Guidance for Reporting Involvement of Patients and the Public (GRIPP2) was developed to enhance the quality and consistency of PPI reporting. The objective of this systematic review is to identify the frequency and quality of PPI reporting in patient safety (PS) research using the GRIPP2 checklist.
Searches were performed in Ovid MEDLINE, EMBASE, PsycINFO, and CINAHL from 2018 to December, 2023. Studies on PPI in PS research were included. We included empirical qualitative, quantitative, mixed methods, and case studies. Only articles published in peer-reviewed journals in English were included. The quality of PPI reporting was assessed using the short form of the (GRIPP2-SF) checklist.
A total of 8561 studies were retrieved from database searches, updates, and reference checks, of which 82 met the eligibility criteria and were included in this review. Major PS topics were related to medication safety, general PS, and fall prevention. Patient representatives, advocates, patient advisory groups, patients, service users, and health consumers were the most involved. The main involvement across the studies was in commenting on or developing research materials. Only 6.1% ( n = 5) of the studies reported PPI as per the GRIPP2 checklist. Regarding the quality of reporting following the GRIPP2-SF criteria, our findings show sub-optimal reporting mainly due to failures in: critically reflecting on PPI in the study; reporting the aim of PPI in the study; and reporting the extent to which PPI influenced the study overall.
Conclusions
Our review shows a low frequency of PPI reporting in PS research using the GRIPP2 checklist. Furthermore, it reveals a sub-optimal quality in PPI reporting following GRIPP2-SF items. Researchers, funders, publishers, and journals need to promote consistent and transparent PPI reporting following internationally developed reporting guidelines such as the GRIPP2. Evidence-based guidelines for reporting PPI should be encouraged and supported as it helps future researchers to plan and report PPI more effectively.
Trial registration
The review protocol is registered with PROSPERO (CRD42023450715).
Peer Review reports
Patient safety (PS) is defined as “the absence of preventable harm to a patient and reduction of risk of unnecessary harm associated with healthcare to an acceptable minimum” [ 1 ]. It is estimated that one in 10 patients are harmed in healthcare settings due to unsafe care, resulting in over three million deaths annually [ 2 ]. More than 50% of adverse events are preventable, and half of these events are related to medications [ 3 , 4 ]. There are various types of adverse events that patients can experience such as medication errors, patient falls, healthcare-associated infections, diagnostic errors, pressure ulcers, unsafe surgical procedures, patient misidentification, and others [ 1 ].
Over the last few decades, the approach of PS management has shifted toward actively involving patients and their families in managing PS. This innovative approach has surpassed the traditional model where healthcare providers were the sole managers of PS [ 5 ]. Recent research has shown that patients have a vital role in promoting their safety and decreasing the occurrence of adverse events [ 6 ]. Hence, there is a growing recognition of patient and family involvement as a promising method to enhance PS [ 7 ]. This approach includes involving patients in PS policy development, research, and shared decision making [ 1 ].
In the last decade, research involving patients and the public has significantly increased. In the United Kingdom (U.K), the National Institute for Health Research (NIHR) has played a critical role in providing strategic and infrastructure support to integrate Public and Patient Involvement (PPI) throughout publicly funded research [ 8 ]. This has established a context where PPI is recognised as an essential element in research [ 9 ]. In Ireland, the national government agency responsible for the management and delivery of all public health and social services; the National Health Service Executive (HSE) emphasise the importance of PPI in research and provide guidance for researchers on how to involve patients and public in all parts of the research cycle and knowledge translation process [ 10 ]. Similar initiatives are also developing among other European countries, North America, and Australia. However, despite this significant expansion of PPI research, the reporting of PPI in research articles continues to be sub-optimal, inconsistent, and lacks essential information on the context, process, and impact of PPI [ 9 ]. To address this problem, the Guidance for Reporting Involvement of Patients and the Public (GRIPP) was developed in 2011 following the EQUATOR methodology to enhance the quality, consistency, and transparency of PPI reporting. Additionally, to provide guidance for researchers, patients, and the public to advance the quality of the international PPI evidence-base [ 11 ]. The first GRIPP checklist was a significant start in producing higher-quality PPI reporting; however, it was developed following a systematic review, and did not include any input from the international PPI research community. Given the importance of reaching consensus in generating current reporting guidelines, a second version of the GRIPP checklist (GRIPP2) was developed to tackle this problem by involving the international PPI community in its development [ 9 ]. There are two versions of the GRIPP2 checklist, a long form (GRIPP2-LF) for studies with PPI as the primary focus, and a short form (GRIPP2-SF) for studies with PPI as secondary or tertiary focus.
Since the publication of the GRIPP2 checklist, several systematic reviews have been conducted to assess the quality of PPI reporting on various topics. For instance, Bergin et al. in their review to investigate the nature and impact of PPI in cancer research, reported a sub-optimal quality of PPI reporting using the GRIPP2-SF, mainly due to failure to address PPI challenges [ 12 ]. Similarly, Owyang et al. in their systematic review to assess the prevalence, extent, and quality of PPI in orthopaedic practice, described a poor PPI reporting following the GRIPP2-SF checklist criteria [ 13 ]. While a few systematic reviews have been conducted to assess theories, strategies, types of interventions, and barriers and enablers of PPI in PS [ 5 , 14 , 15 , 16 ], no previous review has assessed the quality of PPI reporting in PS research. Thus, our systematic review aims to address this knowledge gap. The objective of this review is to identify the frequency PPI reporting in PS research using the GRIPP2 checklist from 2018 (the year after GRIPP2 was published) and the quality of reporting following the GRIPP2-SF. The GRIPP2 checklist was chosen as the benchmark as it is the first international, evidence-based, community consensus informed guideline for the reporting of PPI in research and more specifically in health and social care research [ 9 ]. Additionally, it is the most recent report-focused framework and the most recommended by several leading journals [ 17 ].
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to plan and report this review [ 18 ]. The review protocol was published on PROSPERO the International Database of Prospectively Registered Systematic Reviews in August 2023 (CRD42023450715).

Search strategy
For this review, we used the PICo framework to define the key elements in our research. These included articles on patients and public (P-Population) involvement (I- phenomenon of Interest) in PS (C-context). Details are presented in Table 1 . Four databases were searched including Ovid MEDLINE, EMBASE, PsycINFO, and CINAHL to identify papers on PPI in PS research. A systematic search strategy was initially developed using MEDLINE. MeSH terms and keywords relevant to specific categories (e.g., patient safety) were combined using the “OR” Boolean term (i.e. patient safety OR adverse event OR medical error OR surgical error) and categories were then combined using the “AND” Boolean term. (i.e. “patient and public involvement” AND “patient safety”). The search strategy was adapted for the other three databases. Full search strategies are provided in Supplementary file 1 . The search was conducted on July 27th, 2023, and was limited to papers published from 2018. As the GRIPP2 tool was published in 2017, this limit ensured the retrieval of relevant studies. An alert system was set on the four databases to receive all new published studies until December 2023, prior to the final analysis. The search was conducted without restrictions on study type, research design, and language. To reduce selection bias, hand searching was carried out on the reference lists of all the eligible articles in the later stages of the review. This was done by the first author. The search strategy was developed by the first author and confirmed by the research team and a Librarian. The database search was conducted by the first author.
Inclusion and exclusion criteria
Studies on PPI in PS research with a focus on health/healthcare were included in this review. We defined PPI as active involvement which is in line with the NIHR INVOLVE definition as “research being carried out ‘with’ or ‘by’ members of the public rather than ‘to’, ‘about’ or ‘for’ them” [ 19 ]. This includes any PPI including, being a co-applicant on a research project or grant application, identifying research priorities, being a member of an advisory or steering group, participating in developing research materials or giving feedback on them, conducting interviews with study participants, participating in recruitment, data collection, data analysis, drafting manuscripts and/or dissemination of results. Accordingly, we excluded studies where patients or the public were only involved as research participants.
We defined patients and public to include patients, relatives, carers, caregivers and community, which is also in line with the NIHR PPI involvement in National Health Service [ 19 ].
Patient safety included topics on medication safety, adverse events, communication, safety culture, diagnostic errors, and others. A full list of the used terms for PPI and PS is provided in Supplementary file 1 . Regarding the research type and design, we included empirical qualitative, quantitative, mixed methods, and case studies. Only articles published in peer-reviewed journals and in English were included.
Any article that did not meet the inclusion criteria was excluded. Studies not reporting outcomes were excluded. Furthermore, review papers, conference abstracts, letters to editor, commentary, viewpoints, and short communications were excluded. Finally, papers published prior to 2018 were excluded.
Study selection
The selection of eligible studies was done by the first and the second authors independently, starting with title and abstracts screening to eliminate papers that failed to meet our inclusion criteria. Then, full text screening was conducted to decide on the final included papers in this review. Covidence, an online data management system supported the review process, ensuring reviewers were blinded to each other’s decisions. Disagreements between reviewers were discussed first, in cases where the disagreement was not resolved, the fourth author was consulted.
Data extraction and analysis
A data extraction sheet was developed using excel then piloted, discussed with the research team and modified as appropriate. The following data were extracted: citation and year of publication, objective of the study, country, PS topic, design, setting, PPI participants, PPI stages (identifying research priorities, being a member of an advisory or steering group, etc.…), frequency of PPI reporting as per the GRIPP2 checklist, and the availability of a plain language summary. Additionally, data against the five items of GRIPP2-SF (aim of PPI in the study, methods used for PPI, outcomes of PPI including the results and the extent to which PPI influenced the study overall, and reflections on PPI) were extracted. To avoid multiple publication bias and missing outcomes, data extraction was done by the first and the second authors independently and then compared. Disagreements between reviewers were first discussed, and then resolved by the third and fourth authors if needed.
Quality assessment
The quality of PPI reporting was assessed using GRIPP2-SF developed by Staniszewska et al. [ 9 ] as it is developed to improve the quality, consistency, and reporting of PPI in social and healthcare research. Additionally the GRIPP2-SF is suitable for all studies regardless of whether PPI is the primary, secondary, or tertiary focus, whereas the GRIPP2-LF is not suitable for studies where PPI serves as a secondary or tertiary focus. The checklist includes five items (mentioned above) that authors should include in their studies. It is important to mention that Staniszewska et al. noted that “while GRIPP2-SF aims to guide consistent reporting, it is not possible to be prescriptive about the exact content of each item, as the current evidence-base is not advanced enough to make this possible” ([ 9 ] p5). For that reason, we had to develop criteria for scoring the five reporting items. We used three scoring as Yes, No, and partial for each of the five items of the GRIPP2-SF. Yes, was given when authors presented PPI information on the item clearly in the paper. No, when no information was provided, and partial when the information partially met the item requirement. For example, as per GRIPP2-SF authors should provide a clear description of the methods used for PPI in the study. In the example given by Staniszewska et al., information on patient/public partners and how many of them were provided, as well as the stages of the study they were involved in (i.e. refining the focus of the research questions, developing the search strategy, interpreting results). Thus, in our evaluation of the included studies, we gave a yes if information on PPI participants (i.e. patient partners, community partners, or family members etc..) and how many of them were involved was provided, and information on the stages or actions of their involvement in the study was provided. However, we gave a “partial” if information was not fully provided (i.e. information on patient/public partners and how many were involved in the study without describing in what stages or actions they were involved, and vice versa), and a “No” if no information was presented at all.
The quality of PPI reporting was done by the first and the second authors independently and then compared. Disagreements between reviewers were first discussed, and then resolved by the third and fourth author when needed.
Assessing the quality or risk of bias of the included studies was omitted, as the focus in this review was on appraising the quality of PPI reporting rather than assessing the quality of each research article.
Data synthesis
After data extraction, a table summarising the included studies was developed. Studies were compared according to the main outcomes of the review; frequency of PPI reporting following the GRIPP2 checklist and the quality of reporting as per GRIPP2-SF five items, and the availability of a plain language summary.
Search results and study selection
The database searches yielded a total of 8491 studies. First, 2496 were removed as duplicates. Then, after title and abstract screening, 5785 articles were excluded leaving 210 articles eligible for the full text review. After a careful examination, 68 of these studies were included in this review. A further 38 studies were identified from the alert system that was set on the four databases and 32 studies from the reference check of the included studies. Of these 70 articles, 56 were further excluded and 14 were added to the previous 68 included studies. Thus, 82 studies met the inclusion criteria and were included in this review. A summary of the database search results and the study selection process are presented in Fig. 1 .
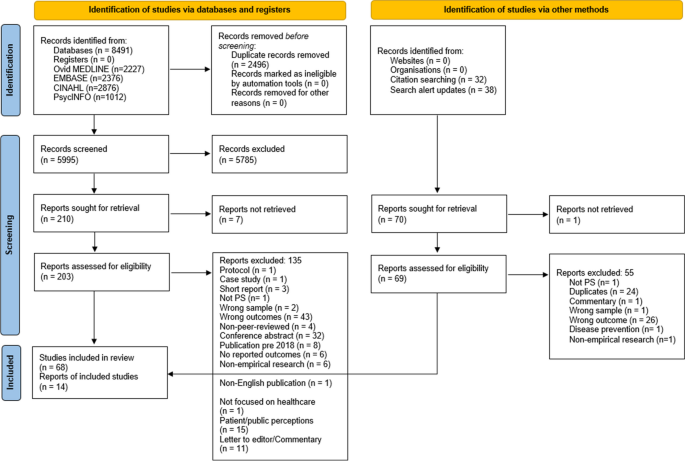
PRISMA flow diagram of the study selection process. The PRISMA flow diagram details the review search results and selection process
Overview of included studies
Details of the study characteristics including first author and year of publication, objective, country, study design, setting, PS topic, PPI participants and involvement stages are presented in Supplementary file 2 . The majority of the studies were conducted in the U.K ( n = 24) and the United States of America ( n = 18), with the remaining 39 conducted in other high income countries, the exception being one study in Haiti. A range of study designs were identified, the most common being qualitative ( n = 31), mixed methods ( n = 13), interventional ( n = 5), and quality improvement projects ( n = 4). Most PS topics concerned medication safety ( n = 17), PS in general (e.g., developing a PS survey or PS management application) ( n = 14), fall prevention ( n = 13), communication ( n = 11), and adverse events ( n = 10), with the remaining PS topics listed in Supplementary file 2 .
Patient representatives, advocates, and patient advisory groups ( n = 33) and patients, service users, and health consumers ( n = 32) were the main groups involved. The remaining, included community members/ organisations. Concerning PPI stages, the main involvement across the studies was in commenting on or developing research materials ( n = 74) including, patient leaflets, interventional tools, mobile applications, and survey instruments. Following this stage, involvement in data analysis, drafting manuscripts, and disseminating results ( n = 30), and being a member of a project advisory or steering group ( n = 18) were the most common PPI evident in included studies. Whereas the least involvement was in identifying research priorities ( n = 5), and being a co-applicant on a research project or grant application ( n = 6).
Regarding plain language summary, only one out of the 82 studies (1.22%) provided a plain language summary in their paper [ 20 ].
Frequency and quality of PPI reporting
The frequency of PPI reporting following the GRIPP2 checklist was 6.1%, where only five of the 82 included studies reported PPI in their papers following the GRIPP2 checklist. The quality of PPI reporting in those studies is presented in Table 2 . Of these five studies, one study (20%) did not report the aim of PPI in the study and one (20%) did not comment on the extent to which PPI influenced the study overall.
The quality of PPI reporting of the remaining 77 studies is presented in Table 3 . The aim of PPI in the study was reported in 62.3% of articles ( n = 48), while 3.9% ( n = 3) partially reported this. A clear description of the methods used for PPI in the study was reported in 79.2% of papers ( n = 61) and partially in 20.8% ( n = 16). Concerning the outcomes, 81.8% of papers ( n = 63) reported the results of PPI in the study, while 10.4% ( n = 8) partially did. Of the 77 studies, 68.8% ( n = 53) reported the extent to which PPI influenced the study overall and 3.9% ( n = 3) partially reported this. Finally, 57.1% ( n = 44) of papers critically reflected on the things that went well and those that did not and 2.6% ( n = 2) partially reflected on this.
Summary of main findings
This systematic review assessed the frequency of reporting PPI in PS research using the GRIPP2 checklist and quality of reporting using the GRIPP2-SF. In total, 82 studies were included in this review. Major PS topics were related to medication safety, general PS, and fall prevention. Patient representatives, advocates, patient advisory groups, patients, service users, and health consumers were the most involved. The main involvement across the studies was in commenting on or developing research materials such as educational and interventional tools, survey instruments, and applications while the least was in identifying research priorities and being a co-applicant on a research project or grant application. Thus, significant effort is still needed to involve patients and the public in the earlier stages of the research process given the fundamental impact of PS on their lives.
Overall completeness and applicability of evidence
A low frequency of reporting PPI in PS research following the GRIPP2 guidelines was revealed in this review, where only five of the 82 studies included mentioned that PPI was reported as per the GRIPP2 checklist. This is despite it being the most recent report-focused framework and the most recommended by several leading journals [ 17 ]. This was not surprising as similar results were reported in recent reviews in other healthcare topics. For instance, Musbahi et al. in their systematic review on PPI reporting in bariatric research reported that none of the 90 papers identified in their review mentioned or utilised the GRIPP2 checklist [ 102 ]. Similarly, a study on PPI in orthodontic research found that none of the 363 included articles reported PPI against the GRIPP2 checklist [ 103 ].
In relation to the quality of reporting following the GRIPP2-SF criteria, our findings show sub-optimal reporting within the 77 studies that did not use GRIPP2 as a guide/checklist to report their PPI. Similarly, Bergin et al. in their systematic review to investigate the nature and impact of PPI in cancer research concluded that substandard reporting was evident [ 12 ]. In our review, this was mainly due to failure to meet three criteria. First, the lowest percentage of reporting (57.1%, n = 44) was related to critical reflection on PPI in the study (i.e., what went well and what did not). In total, 31 studies (42.9%) did not provide any information on this, and two studies were scored as partial. The first study mentioned that only involving one patient was a limitation [ 27 ] and the other stated that including three patients in the design of the tool was a strength [ 83 ]. Both studies did not critically comment or reflect on these points so that future researchers are able to avoid such problems and enhance PPI opportunities. For instance, providing the reasons/challenges behind the exclusive inclusion of a single patient and explaining how this limits the study findings and conclusion would help future researchers to address these challenges. Likewise, commenting on why incorporating three patients in the design of the study tool could be seen as a strength would have been beneficial. This could be, fostering diverse perspectives and generating novel ideas for developing the tool. Similar to our findings, Bergin et al. in their systematic review reported that 40% of the studies failed to meet this criterion [ 12 ].
Second, only 48 out of 77 articles (62.3%) reported the aim of PPI in their study, which is unlike the results of Bergin et al. where most of the studies (93.1%) in their review met this criterion [ 12 ]. Of the 29 studies which did not meet this criterion in our review, few mentioned in their objective developing a consensus-based instrument [ 41 ], reaching a consensus on the patient-reported outcomes [ 32 ], obtaining international consensus on a set of core outcome measures [ 98 ], and facilitating a multi-stakeholder dialogue [ 71 ] yet, without indicating anything in relation to patients, patient representatives, community members, or any other PPI participants. Thus, the lack of reporting the aim of PPI was clearly evident in this review. Reporting the aim of PPI in the study is crucial for promoting transparency, methodological rigor, reproducibility, and impact assessment of the PPI.
Third, 68.8% ( n = 53) of the studies reported the extent to which PPI influenced the study overall including positive and negative effects if any. This was again similar to the findings of Bergin et al., where 38% of the studies did not meet this criterion mainly due to a failure to address PPI challenges in their respective studies [ 12 ]. Additionally, Owyang et al. in their review on the extent, and quality of PPI in orthopaedic practice, also described a poor reporting of PPI impact on research [ 13 ]. As per the GRIPP2 guidelines, both positive and negative effects of PPI on the study should be reported when applicable. Providing such information is essential as it enhances future research on PPI in terms of both practice and reporting.
Reporting a clear description of the methods used for PPI in the study was acceptable, with 79.2% of the papers meeting this criterion. Most studies provided information in the methods section of their papers on the PPI participants, their number, stages of their involvement and how they were involved. Providing clear information on the methods used for PPI is vital to give the reader a clear understanding of the steps taken to involve patients, and for other researchers to replicate these methods in future research. Additionally, reporting the results of PPI in the study was also acceptable with 81.8% of the papers reporting the outcomes of PPI in the results section. Reporting the results of PPI is important for enhancing methodological transparency, providing a more accurate interpretation for the study findings, contributing to the overall accountability and credibility of the research, and informing decision making.
Out of the 82 studies included in this review, only one study provided a plain language summary. We understand that PS research or health and medical research in general is difficult for patients and the public to understand given their diverse health literacy and educational backgrounds. However, if we expect patients and the public to be involved in research then, it is crucial to translate this research that has a huge impact on their lives into an easily accessible format. Failing to translate the benefits that such research may have on patient and public lives may result in them underestimating the value of this research and losing interest in being involved in the planning or implementation of future research [ 103 ]. Thus, providing a plain language summary for research is one way to tackle this problem. To our knowledge, only a few health and social care journals (i.e. Cochrane and BMC Research Involvement and Engagement) necessitate a plain language summary as a submission requirement. Having this as a requirement for submission is crucial in bringing the importance of this issue to researchers’ attention.
Research from recent years suggests that poor PPI reporting in articles relates to a lack of submission requirements for PPI reporting in journals and difficulties with word limits for submitted manuscripts [ 13 ]. Price et al. assessed the frequency of PPI reporting in published papers before and after the introduction of PPI reporting obligations by the British Medical Journal (BMJ) [ 104 ]. The authors identified an increase in PPI reporting in papers published by BMJ from 0.5% to 11% between the periods of 2013–2014 and 2015–2016. The study findings demonstrate the impact of journal guidelines in shaping higher quality research outputs [ 13 ]. In our review, we found a low frequency of PPI reporting in PS research using the GRIPP2 checklist, alongside sub-optimal quality of reporting following GRIPP2-SF. This could potentially be attributed to the absence of submission requirements for PPI reporting in journals following the GRIPP2 checklist, as well as challenges posed by word limits.
Strengths and limitations
This systematic review presents an overview on the frequency of PPI reporting in PS research using the GRIPP2 checklist, as well as an evaluation of the quality of reporting following the GRIPP2-SF. As the first review to focus on PS research, it provides useful knowledge on the status of PPI reporting in this field, and the extent to which researchers are adopting and adhering to PPI reporting guidelines. Despite these strengths, our review has some limitations that should be mentioned. First, only English language papers were included in this review due to being the main language of the researchers. Thus, there is a possibility that relevant articles on PPI in PS research may have been omitted. Another limitation is related to our search which was limited to papers published starting 2018 as the GRIPP2 guidelines were published in 2017. Thus it is probable that the protocols of some of these studies were developed earlier than the publication of the GRIPP2 checklist, meaning that PPI reporting following GRIPP2 was not common practice and thus not adopted by these studies. This might limit the conclusions we can draw from this review. Finally, the use of GRIPP2 to assess the quality of PPI reporting might be a limitation as usability testing has not yet been conducted to understand how the checklist works in practice with various types of research designs. However, the GRIPP2 is the first international, evidence-based, community consensus informed guideline for the reporting of PPI in health and social care research. Reflections and comments from researchers using the GRIPP2 will help improve its use in future studies.
Implications for research and practice
Lack of PPI reporting not only affects the quality of research but also implies that others cannot learn from previous research experience. Additionally, without consistent and transparent reporting it is difficult to evaluate the impact of various PPI in research [ 9 ]: “if it is not reported it cannot be assessed” ([ 105 ] p19). Enhanced PPI reporting will result in a wider range and richer high-quality evidence-based PPI research, leading to a better understanding of PPI use and effectiveness [ 103 ]. GRIPP2 reporting guidelines were developed to provide guidance for researchers, patients, and the public to enhance the quality of PPI reporting and improve the quality of the international PPI evidence-base. The guidance can be used prospectively to plan PPI or retrospectively to guide the structure or PPI reporting in research [ 9 ]. To enhance PPI reporting, we recommend the following;
Publishers and journals
First, we encourage publishers and journals to require researchers to report PPI following the GRIPP2 checklist. Utilising the short or the long version should depend on the primary focus of the study (i.e., if PPI is within the primary focus of the research then the GRIPP2-LF is recommended). Second, we recommend that journals and editorial members advise reviewers to evaluate PPI reporting within research articles following the GRIPP2 tool and make suggestions accordingly. Finally, we encourage journals to add a plain language summary as a submission requirement to increase research dissemination and improve the accessibility of research for patients and the public.
Researchers
Though there is greater evidence of PPI in research, it is still primarily the researchers that are setting the research agenda and deciding on the research questions to be addressed. Thus, significant effort is still needed to involve patients and the public in the earlier stages of the research process given the fundamental impact of PS on their lives. To enhance future PPI reporting, perhaps adding a criterion following the GRIPP2 tool to existing EQUATOR checklists for reporting research papers such as STROBE, PRISMA, CONSORT, may support higher quality research. Additionally, currently, there is no detailed explanation paper for the GRIPP2 where each criterion is explained in detail with examples. Addressing this gap would be of great benefit to guide the structure of PPI reporting and to explore the applicability of each criterion in relation to different stages of PPI in research. For instance, having a detailed explanation for each criterion across different research studies having various PPI stages would be of high value to improve future PPI reporting given the growing interest in PPI research in recent years and the relatively small PPI evidence base in health and medical research.
Funding bodies can also enhance PPI reporting by adding a requirement for researchers to report PPI following the GRIPP2 checklist. In Ireland, the National HSE has already initiated this by requiring all PPI in HSE research in Ireland to be reported following the GRIPP2 guidelines [ 10 ].
This study represents the first systematic review on the frequency and quality of PPI reporting in PS research using the GRIPP2 checklist. Most PS topics were related to medication safety, general PS, and fall prevention. The main involvement across the studies was in commenting on or developing research materials. Thus, efforts are still needed to involve patients and the public across all aspects of the research process, especially earlier stages of the research cycle. The frequency of PPI reporting following the GRIPP2 guidelines was low, and the quality of reporting following the GRIPP2-SF criteria was sub-optimal. The lowest percentages of reporting were on critically reflecting on PPI in the study so future research can learn from this experience and work to improve it, reporting the aim of the PPI in the study, and reporting the extent to which PPI influenced the study overall including positive and negative effects. Researchers, funders, publishers, journals, editorial members and reviewers have a responsibility to promote consistent and transparent PPI reporting following internationally developed reporting guidelines such as the GRIPP2. Evidence-based guidelines for reporting PPI should be supported to help future researchers plan and report PPI more effectively, which may ultimately improve the quality and relevance of research.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its Supplementary information files.
Abbreviations
- Patient safety
United Kingdom
National Institute for Health Research
Public and Patient Involvement
Health Service Executive
Guidance for Reporting Involvement of Patients and the Public
Second version of the GRIPP checklist
Long form of GRIPP2
Short form of GRIPP2
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
The International Database of Prospectively Registered Systematic Reviews
British Medical Journal
Patient saftey: World Health Organisation. 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/patient-safety . Updated 11 Sept 2023.
Slawomirski L, Klazinga N. The economics of patient safety: from analysis to action. Paris: Organisation for Economic Co-operation and Development; 2020.
Google Scholar
Panagioti M, Khan K, Keers RN, Abuzour A, Phipps D, Kontopantelis E, et al. Prevalence, severity, and nature of preventable patient harm across medical care settings: systematic review and meta-analysis. Bmj-Brit Med J. 2019;366:l4185.
Article Google Scholar
Hodkinson A, Tyler N, Ashcroft DM, Keers RN, Khan K, Phipps D, et al. Preventable medication harm across health care settings: a systematic review and meta-analysis. Bmc Medicine. 2020;18(1):313.
Article PubMed PubMed Central Google Scholar
Park M, Giap TTT. Patient and family engagement as a potential approach for improving patient safety: A systematic review. J Adv Nurs. 2020;76(1):62–80.
Article PubMed Google Scholar
Chegini Z, Janati A, Bababie J, Pouraghaei M. The role of patients in the delivery of safe care in hospital: Study protocol. J Adv Nurs. 2019;75(9):2015–23.
Chegini Z, Arab-Zozani M, Islam SMS, Tobiano G, Rahimi SA. Barriers and facilitators to patient engagement in patient safety from patients and healthcare professionals’ perspectives: A systematic review and meta-synthesis. Nurs Forum. 2021;56(4):938–49.
Going the extra mile: improving the nation’s health and wellbeing through public involvement in research. London: National Institute for Health; 2015.
Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. Bmj-Brit Med J. 2017;358:j3453.
Article CAS Google Scholar
Minogue V. Knowledge translation, dissemination, and impact: a practical guide for researchers. Guide No 8: patient and public involvement in HSE research. Ireland: Health Service Executive Research and Development; 2021.
Staniszewska S, Brett J, Mockford C, Barber R. The GRIPP checklist: Strengthening the quality of patient and public involvement reporting in research. Int J Technol Assess Health Care. 2011;27(4):391–9.
Bergin RJ, Short CE, Davis N, Marker J, Dawson MT, Milton S, et al. The nature and impact of patient and public involvement in cancer prevention, screening and early detection research: A systematic review. Prev Med. 2023;167:107412.
Owyang D, Bakhsh A, Brewer D, Boughton OR, Cobb JP. Patient and public involvement within orthopaedic research a systematic review. J Bone Joint Surg Am. 2021;103(13):e51.
Busch IM, Saxena A, Wu AW. Putting the patient in patient safety investigations: barriers and strategies for involvement. J Patient Saf. 2021;17(5):358–62.
Lee M, Lee NJ, Seo HJ, Jang H, Kim SM. Interventions to engage patients and families in patient safety: a systematic review. West J Nurs Res. 2021;43(10):972–83.
Ocloo J, Garfield S, Franklin BD, Dawson S. Exploring the theory, barriers and enablers for patient and public involvement across health, social care and patient safety: a systematic review of reviews. Health Res Policy Syst. 2021;19(1):8.
Greenhalgh T, Hinton L, Finlay T, Macfarlane A, Fahy N, Clyde B, et al. Frameworks for supporting patient and public involvement in research: Systematic review and co-design pilot. Health Expect. 2019;22(4):785–801.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Plos Medicine. 2021;18(3):372.
INVOLVE. What is public involvement in research? NIHR; 2019. Available from: https://www.invo.org.uk/find-out-more/what-is-public-involvement-in-research-2/ .
Shahid A, Sept B, Kupsch S, Brundin-Mather R, Piskulic D, Soo A, et al. Development and pilot implementation of a patient-oriented discharge summary for critically Ill patients. World J Crit Care Med. 2022;11(4):255–68.
Bisset CN, Dames N, Oliphant R, Alasadi A, Anderson D, Parson S, et al. Exploring shared surgical decision-making from the patient’s perspective: is the personality of the surgeon important? Colorectal Dis. 2020;22(12):2214–21.
Article CAS PubMed Google Scholar
Morris RL, Ruddock A, Gallacher K, Rolfe C, Giles S, Campbell S. Developing a patient safety guide for primary care: A co-design approach involving patients, carers and clinicians. Health Expect. 2021;24(1):42–52.
Tobiano G, Marshall AP, Gardiner T, Jenkinson K, Shapiro M, Ireland M. Development and psychometric testing of the patient participation in bedside handover survey. Health Expect. 2022;25(5):2492–502.
Francis-Coad J, Farlie MK, Haines T, Black L, Weselman T, Cummings P, et al. Revising and evaluating falls prevention education for older adults in hospital. Health Educ J. 2023;82(8):878–91.
Troya MI, Chew-Graham CA, Babatunde O, Bartlam B, Higginbottom A, Dikomitis L. Patient and public involvement and engagement in a doctoral research project exploring self-harm in older adults. Health Expect. 2019;22(4):617–31.
Aharaz A, Kejser CL, Poulsen MW, Jeftic S, Ulstrup-Hansen AI, Jorgensen LM, et al. Optimization of the Danish National Electronic Prescribing System to improve patient safety: Development of a user-friendly prototype of the digital platform shared medication record. Pharmacy (Basel, Switzerland). 2023;11(2):41.
PubMed Google Scholar
Aho-Glele U, Bouabida K, Kooijman A, Popescu IC, Pomey MP, Hawthornthwaite L, et al. Developing the first pan-Canadian survey on patient engagement in patient safety. BMC Health Serv Res. 2021;21(1):1099.
Albutt A, O’Hara J, Conner M, Lawton R. Involving patients in recognising clinical deterioration in hospital using the patient wellness questionnaire: A mixed-methods study. J Res Nurs. 2020;25(1):68–86.
Bell SK, Bourgeois F, DesRoches CM, Dong J, Harcourt K, Liu SK, et al. Filling a gap in safety metrics: development of a patient-centred framework to identify and categorise patient-reported breakdowns related to the diagnostic process in ambulatory care. BMJ Qual Saf. 2022;31(7):526–40.
Boet S, Etherington N, Lam S, Lê M, Proulx L, Britton M, et al. Implementation of the Operating Room Black Box research program at the Ottawa Hospital through patient, clinical, and organizational engagement: Case study. J Med Internet Res. 2021;23(3):e15443.
Carter J, Tribe RM, Shennan AH, Sandall J. Threatened preterm labour: Women’s experiences of risk and care management: A qualitative study. Midwifery. 2018;64:85–92.
Da Silva Lopes AM, Colomer-Lahiguera S, Mederos Alfonso N, Aedo-Lopez V, Spurrier-Bernard G, Tolstrup LK, et al. Patient-reported outcomes for monitoring symptomatic toxicities in cancer patients treated with immune-checkpoint inhibitors: A Delphi study. Eur J Cancer. 2021;157:225–37.
de Jong LD, Lavender AP, Wortham C, Skelton DA, Haines TP, Hill AM. Exploring purpose-designed audio-visual falls prevention messages on older people’s capability and motivation to prevent falls. Health Soc Care Community. 2019;27(4):e471–82.
Doucette L, Kiely BT, Gierisch JM, Marion E, Nadler L, Heflin MT, et al. Participatory research to improve medication reconciliation for older adults in the community. J Am Geriatr Soc. 2023;71(2):620–31.
Elrod CS, Pappa ST, Heyn PC, Wong RA. Using an academic-community partnership model to deliver evidence-based falls prevention programs in a metropolitan setting: A community case study. Front Public Health. 2023;11:1073520.
Feldman E, Pos FJ, Smeenk RJ, van der Poel H, van Leeuwen P, de Feijter JM, et al. Selecting a PRO-CTCAE-based subset for patient-reported symptom monitoring in prostate cancer patients: a modified Delphi procedure. ESMO Open. 2023;8(1):100775.
Article CAS PubMed PubMed Central Google Scholar
Francis-Coad J, Watts T, Bulsara C, Hill A-M. Designing and evaluating falls prevention education with residents and staff in aged care homes: a feasibility study. Health Educ (0965-4283). 2022;122(5):546–63.
Fuller TE, Pong DD, Piniella N, Pardo M, Bessa N, Yoon C, et al. Interactive digital health tools to engage patients and caregivers in discharge preparation: implementation study. J Med Internet Res. 2020;22(4):e15573.
Gibson B, Butler J, Schnock K, Bates D, Classen D. Design of a safety dashboard for patients. Patient Educ Couns. 2020;103(4):741–7.
Giles SJ, Lewis PJ, Phipps DL, Mann F, Avery AJ, Ashcroft DM. Capturing patients’ perspectives on medication safety: the development of a patient-centered medication safety framework. J Patient Saf. 2020;16(4):e324–39.
Gnagi R, Zuniga F, Brunkert T, Meyer-Massetti C. Development of a medication literacy assessment instrument (MELIA) for older people receiving home care. J Adv Nurs. 2022;78(12):4210–20.
Goodsmith N, Zhang L, Ong MK, Ngo VK, Miranda J, Hirsch S, et al. Implementation of a community-partnered research suicide-risk management protocol: case study from community partners in care. Psychiatr Serv (Washington, DC). 2021;72(3):281–7.
Gorman LS, Littlewood DL, Quinlivan L, Monaghan E, Smith J, Barlow S, et al. Family involvement, patient safety and suicide prevention in mental healthcare: ethnographic study. BJPsych open. 2023;9(2):e54.
Green MM, Meyer C, Hutchinson AM, Sutherland F, Lowthian JA. Co‐designing being your best program—a holistic approach to frailty in older community dwelling australians. Health Soc Care Community. 2021;30(5):e2022–32.
Guo X, Wang Y, Wang L, Yang X, Yang W, Lu Z, et al. Effect of a fall prevention strategy for the older patients: A quasi-experimental study. Nurs Open. 2023;10(2):1116–24.
Hahn-Goldberg S, Chaput A, Rosenberg-Yunger Z, Lunsky Y, Okrainec K, Guilcher S, et al. Tool development to improve medication information transfer to patients during transitions of care: A participatory action research and design thinking methodology approach. Res Social Adm Pharm. 2022;18(1):2170–7.
Harrington A, Darke H, Ennis G, Sundram S. Evaluation of an alternative model for the management of clinical risk in an adult acute psychiatric inpatient unit. Int J Ment Health Nurs. 2019;28(5):1099–109.
Harris K, Softeland E, Moi AL, Harthug S, Ravnoy M, Storesund A, et al. Development and validation of patients’ surgical safety checklist. BMC Health Serv Res. 2022;22(1):259.
Hawley-Hague H, Tacconi C, Mellone S, Martinez E, Ford C, Chiari L, et al. Smartphone apps to support falls rehabilitation exercise: app development and usability and acceptability study. JMIR Mhealth Uhealth. 2020;8(9):e15460.
Holmqvist M, Ros A, Lindenfalk B, Thor J, Johansson L. How older persons and health care professionals co-designed a medication plan prototype remotely to promote patient safety: case study. JMIR aging. 2023;6:e41950.
Jayesinghe R, Moriarty F, Khatter A, Durbaba S, Ashworth M, Redmond P. Cost outcomes of potentially inappropriate prescribing in middle-aged adults: A Delphi consensus and cross-sectional study. Br J Clin Pharmacol. 2022;88(7):3404–20.
Johannessen T, Ree E, Stromme T, Aase I, Bal R, Wiig S. Designing and pilot testing of a leadership intervention to improve quality and safety in nursing homes and home care (the SAFE-LEAD intervention). BMJ Open. 2019;9(6):e027790.
Joseph K, Newman B, Manias E, Walpola R, Seale H, Walton M, et al. Engaging with ethnic minority consumers to improve safety in cancer services: A national stakeholder analysis. Patient Educ Couns. 2022;105(8):2778–84.
Khan A, Spector ND, Baird JD, Ashland M, Starmer AJ, Rosenbluth G, et al. Patient safety after implementation of a coproduced family centered communication programme: multicenter before and after intervention study. BMJ. 2018;363:k4764.
Khazen M, Mirica M, Carlile N, Groisser A, Schiff GD. Developing a framework and electronic tool for communicating diagnostic uncertainty in primary care: a qualitative study. JAMA Network Open. 2023;6(3):e232218-e.
Knight SW, Trinkle J, Tschannen D. Hospital-to-homecare videoconference handoff: improved communication, coordination of care, and patient/family engagement. Home Healthc Now. 2019;37(4):198–207.
Lawrence V, Kimona K, Howard RJ, Serfaty MA, Wetherell JL, Livingston G, et al. Optimising the acceptability and feasibility of acceptance and commitment therapy for treatment-resistant generalised anxiety disorder in older adults. Age Ageing. 2019;48(5):741–50.
Louch G, Reynolds C, Moore S, Marsh C, Heyhoe J, Albutt A, et al. Validation of revised patient measures of safety: PMOS-30 and PMOS-10. BMJ Open. 2019;9(11):e031355.
MacDonald T, Jackson S, Charles M-C, Periel M, Jean-Baptiste M-V, Salomon A, et al. The fourth delay and community-driven solutions to reduce maternal mortality in rural Haiti: a community-based action research study. BMC Pregnancy Childbirth. 2018;18(1):254.
Mackintosh N, Sandall J, Collison C, Carter W, Harris J. Employing the arts for knowledge production and translation: Visualizing new possibilities for women speaking up about safety concerns in maternity. Health Expect. 2018;21(3):647–58.
Marchand K, Turuba R, Katan C, Brasset C, Fogarty O, Tallon C, et al. Becoming our young people’s case managers: caregivers’ experiences, needs, and ideas for improving opioid use treatments for young people using opioids. Subst Abuse Treat Prev Policy. 2022;17(1):1–15.
Mazuz K, Biswas S. Co-designing technology and aging in a service setting: Developing an interpretive framework of how to interact with older age users. Gerontechnology. 2022;21(1):1–13.
McCahon D, Duncan P, Payne R, Horwood J. Patient perceptions and experiences of medication review: qualitative study in general practice. BMC Prim Care. 2022;23(1):293.
McMullen S, Panagioti M, Planner C, Giles S, Angelakis I, Keers RN, et al. Supporting carers to improve patient safety and maintain their well-being in transitions from mental health hospitals to the community: A prioritisation nominal group technique. Health Expect. 2023;26(5):2064–74.
Morris RL, Giles S, Campbell S. Involving patients and carers in patient safety in primary care: A qualitative study of a co-designed patient safety guide. Health Expect. 2023;26(2):630–9.
Morris RL, Stocks SJ, Alam R, Taylor S, Rolfe C, Glover SW, et al. Identifying primary care patient safety research priorities in the UK: a James Lind Alliance Priority Setting Partnership. BMJ Open. 2018;8(2):e020870.
Nether KG, Thomas EJ, Khan A, Ottosen MJ, Yager L. Implementing a robust process improvement program in the neonatal intensive care unit to reduce harm. J Healthc Qual. 2022;44(1):23–30.
Powell C, Ismail H, Cleverley R, Taylor A, Breen L, Fylan B, et al. Patients as qualitative data analysts: Developing a method for a process evaluation of the “Improving the Safety and Continuity of Medicines management at care Transitions” (ISCOMAT) cluster randomised control trial. Health Expect. 2021;24(4):1254–62.
Article PubMed Central Google Scholar
Powell C, Ismail H, Davis M, Taylor A, Breen L, Fylan B, et al. Experiences of patients with heart failure with medicines at transition intervention: Findings from the process evaluation of the Improving the Safety and Continuity of Medicines management at Transitions of care (ISCOMAT) programme. Health Expect. 2022;25(5):2503–14.
Radecki B, Keen A, Miller J, McClure JK, Kara A. Innovating fall safety: engaging patients as experts. J Nurs Care Qual. 2020;35(3):220–6.
Rosgen BK, Plotnikoff KM, Krewulak KD, Shahid A, Hernandez L, Sept BG, et al. Co-development of a transitions in care bundle for patient transitions from the intensive care unit: a mixed-methods analysis of a stakeholder consensus meeting. BMC Health Serv Res. 2022;22(1):10.
Schenk EC, Bryant RA, Van Son CR, Odom-Maryon T. Developing an intervention to reduce harm in hospitalized patients: patients and families in research. J Nurs Care Qual. 2019;34(3):273–8.
Spazzapan M, Vijayakumar B, Stewart CE. A bit about me: Bedside boards to create a culture of patient-centered care in pediatric intensive care units (PICUs). J Healthc Risk Manag. 2020;39(3):11–9.
Stoll JA, Ranahan M, Richbart MT, Brennan-Taylor MK, Taylor JS, Brady L, et al. Development of video animations to encourage patient-driven deprescribing: A team alice study. Patient Educ Couns. 2021;104(11):2716–23.
Subbe CP, Tomos H, Jones GM, Barach P. Express check-in: developing a personal health record for patients admitted to hospital with medical emergencies: a mixed-method feasibility study. Int J Qual Health Care. 2021;33(3):121.
Tai D, Li E, Liu-Ambrose T, Bansback N, Sadatsafavi M, Davis JC. Patient-Reported Outcome Measures (PROMs) to support adherence to falls prevention clinic recommendations: a qualitative study. Patient Prefer Adherence. 2020;14:2105–21.
Thakur T, Chewning B, Zetes N, Lee JTY. Involving caregivers in design and assessment of opioid risk and safety communication intervention in children. Patient Educ Couns. 2021;104(10):2432–6.
Thomas J, Dahm MR, Li J, Georgiou A. Can patients contribute to enhancing the safety and effectiveness of test-result follow-up? Qualitative outcomes from a health consumer workshop. Health Expect. 2021;24(2):222–33.
Tremblay MC, Bradette-Laplante M, Witteman HO, Dogba MJ, Breault P, Paquette JS, et al. Providing culturally safe care to indigenous people living with diabetes: Identifying barriers and enablers from different perspectives. Health Expect. 2021;24(2):296–306.
Troya MI, Dikomitis L, Babatunde OO, Bartlam B, Chew-Graham CA. Understanding self-harm in older adults: A qualitative study. EClinicalMedicine. 2019;12:52–61.
Tyler N, Giles S, Daker-White G, McManus BC, Panagioti M. A patient and public involvement workshop using visual art and priority setting to provide patients with a voice to describe quality and safety concerns: Vitamin B12 deficiency and pernicious anaemia. Health Expect. 2021;24(1):87–94.
Tyler N, Planner C, Shears B, Hernan A, Panagioti M, Giles S. Developing the Resident Measure of Safety in Care Homes (RMOS): A Delphi and think aloud study. Health Expect. 2023;26(3):1149–58.
Van den Bulck SA, Vankrunkelsven P, Goderis G, Van Pottelbergh G, Swerts J, Panis K, et al. Developing quality indicators for Chronic Kidney Disease in primary care, extractable from the Electronic Medical Record. A Rand-modified Delphi method. BMC Nephrol. 2020;21(1):161.
Van Strien-Knippenberg IS, Boshuizen MCS, Determann D, de Boer JH, Damman OC. Cocreation with Dutch patients of decision-relevant information to support shared decision-making about adjuvant treatment in breast cancer care. Health Expect. 2022;25(4):1664–77.
Wilson NA, Reich AJ, Graham J, Bhatt DL, Nguyen LL, Weissman JS. Patient perspectives on the need for implanted device information: Implications for a post-procedural communication framework. Health Expect. 2021;24(4):1391–402.
Winterberg AV, Lane B, Hill LM, Varughese AM. Optimizing Pediatric Induction Experiences Using Human-centered Design. J Perianesth Nurs. 2022;37(1):48–52.
Yang R, Donaldson GW, Edelman LS, Cloyes KG, Sanders NA, Pepper GA. Fear of older adult falling questionnaire for caregivers (FOAFQ-CG): Evidence from content validity and item-response theory graded-response modelling. J Adv Nurs. 2020;76(10):2768–80.
Young A, Menon D, Street J, Al-Hertani W, Stafinski T. A checklist for managed access programmes for reimbursement co-designed by Canadian patients and caregivers. Health Expect. 2018;21(6):973–80.
Yuen EYN, Street M, Abdelrazek M, Blencowe P, Etienne G, Liskaser R, et al. Evaluating the efficacy of a digital App to enhance patient-centred nursing handover: A simulation study. J Clin Nurs. 2023;32(19–20):7626–37.
Jo S, Nabatchi T. Coproducing healthcare: individual-level impacts of engaging citizens to develop recommendations for reducing diagnostic error. Public Manag Rev. 2019;21(3):354–75.
O’Hara JK, Reynolds C, Moore S, Armitage G, Sheard L, Marsh C, et al. What can patients tell us about the quality and safety of hospital care? Findings from a UK multicentre survey study. BMJ Qual Saf. 2018;27(9):673–82.
de Jong LD, Francis-Coad J, Wortham C, Haines TP, Skelton DA, Weselman T, et al. Evaluating audio-visual falls prevention messages with community-dwelling older people using a World Cafe forum approach. BMC Geriatrics. 2019;19(1):345.
O’Donnell D, Shé ÉN, McCarthy M, Thornton S, Doran T, Smith F, et al. Enabling public, patient and practitioner involvement in co-designing frailty pathways in the acute care setting. BMC Health Serv Res. 2019;19(1):797.
Russ S, Latif Z, Hazell A, Ogunmuyiwa H, Tapper J, Wachuku-King S, et al. A Smartphone app designed to empower patients to contribute toward safer surgical care: community-based evaluation using a participatory approach. Jmir Mhealth Uhealth. 2020;8(1):e12859.
Mazuz K, Biswas S, Lindner U. Developing self-management application of fall prevention among older adults: a content and usability evaluation. Front Digital Health. 2020;2:11.
Hjelmfors L, Strömberg A, Friedrichsen M, Sandgren A, Mårtensson J, Jaarsma T. Using co-design to develop an intervention to improve communication about the heart failure trajectory and end-of-life care. Bmc Palliat Care. 2018;17:17.
Horgan S, Hegarty J, Andrews E, Hooton C, Drennan J. Impact of a quality improvement intervention on the incidence of surgical site infection in patients undergoing colorectal surgery: Pre-test-post-test design. J Clin Nurs. 2023;32(15–16):4932–46.
Tyler N, Wright N, Grundy A, Waring J. Developing a core outcome set for interventions to improve discharge from mental health inpatient services: a survey, Delphi and consensus meeting with key stakeholder groups. BMJ Open. 2020;10(5):e034215.
Ward ME, De Brún A, Beirne D, Conway C, Cunningham U, English A, et al. Using Co-Design to Develop a Collective Leadership Intervention for Healthcare Teams to Improve Safety Culture. Int J Environ Res Public Health. 2018;15(6):1182.
Berthelsen DB, Simon LS, Ioannidis JPA, Voshaar M, Richards P, Goel N, et al. Stakeholder endorsement advancing the implementation of a patient-reported domain for harms in rheumatology clinical trials: outcome of the OMERACT safety working group. Semin Arthritis Rheum. 2023;63:152288.
Okkenhaug A, Tritter JQ, Landstad BJ. Developing a research tool to detect iatrogenic adverse events in psychiatric health care by involving service users and health professionals. J Psychiatr Ment Health Nurs. 2023;00:1–12.
Musbahi A, Clyde D, Small P, Courtney M, Mahawar K, Lamb PJ, et al. A systematic review of patient and public involvement (PPI) in bariatric research trials: the need for more work. Obes Surg. 2022;32(11):3740–51.
Patel VA, Shelswell J, Hillyard N, Pavitt S, Barber SK. A study of the reporting of patient and public involvement and engagement (PPIE) in orthodontic research. J Orthod. 2021;48(1):42–51.
Price A, Schroter S, Snow R, Hicks M, Harmston R, Staniszewska S, et al. Frequency of reporting on patient and public involvement (PPI) in research studies published in a general medical journal: a descriptive study. BMJ Open. 2018;8:e020452.
Amadea T, Anne-Marie B, Louise L. A researcher’s guide to patient and public involvement. 2017.
Download references
Acknowledgements
This research is funded as part of the Collective Leadership and Safety Cultures (Co-Lead) research programme which is funded by the Irish Health Research Board, grant reference number RL-2015–1588 and the Health Service Executive. The funders had no role in the study conceptualisation, design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and affiliations.
UCD Centre for Interdisciplinary Research, Education and Innovation in Health Systems (UCD IRIS), School of Nursing, Midwifery and Health Systems, Health Sciences Centre, University College Dublin, Dublin, Ireland
Sahar Hammoud, Laith Alsabek, Lisa Rogers & Eilish McAuliffe
Department of Oral and Maxillofacial Surgery, University Hospital Galway, Galway, Ireland
Laith Alsabek
You can also search for this author in PubMed Google Scholar
Contributions
S.H and E.M.A designed the study. S.H developed the search strategies with feedback from L.A, L.R, and E.M.A. S.H conducted all searches. S.H and L.A screened the studies, extracted the data, and assessed the quality of PPI reporting. S.H analysed the data with feedback from E.M.A. S.H drafted the manuscript. All authors revised and approved the submitted manuscript. All authors agreed to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Authors’ information
Corresponding author.
Correspondence to Sahar Hammoud .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hammoud, S., Alsabek, L., Rogers, L. et al. Systematic review on the frequency and quality of reporting patient and public involvement in patient safety research. BMC Health Serv Res 24 , 532 (2024). https://doi.org/10.1186/s12913-024-11021-z
Download citation
Received : 10 January 2024
Accepted : 21 April 2024
Published : 26 April 2024
DOI : https://doi.org/10.1186/s12913-024-11021-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Patient and public involvement
- Patient participation
- Research reporting
- Research involvement
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
| Findings | Target Population | Study Design |
|---|---|---|
| Rottman et al. (1997) compared on-scene time, appropriateness of therapy, and accuracy of paramedic clinical assessments when prehospital care was provided with the use of on-line medical control (OLMC) by EMS-certified nurses from a single base station or by paramedics using chief complaint-based protocols. The use of protocols resulted in small improvements in both on-scene time and the appropriateness of therapeutic decisions, without a change in agreement between paramedic and physician [ ]. | EMS call center | Prospective cohort |
| Holstein et al. (2003) found that training of the emergency team is an effective and efficient intervention to improve quality of treatment and prognosis outcome for patients with type 1 diabetic emergencies [ ]. | Diabetic patients | Prospective population-based study |
| Watts et al. (2004) found that providers who were able to learn and implement the Brain Trauma Foundation (BTF) Guidelines and outcomes in traumatic brain injury patients were significantly improved [ ]. | Traumatic brain injury patients | Prospective observational study |
| Combes et al. (2006) determined the rate of difficult intubations and the factors associated with prehospital difficult airways when a standard protocol for sedation and intubation was applied [ ]. | Tracheal intubation patients | Observational et prospective study |
| Sasson et al. (2009) discussed the operational issues within local EMS systems that may serve as barriers or facilitators to full acceptance of national guidelines for prehospital termination of resuscitation in appropriate circumstances [ ]. | Termination of resuscitation | Qualitative and focus groups study |
| Atary et al. (2010) showed that a standardized regional acute myocardial infarction treatment protocol achieved optimal and uniformly distributed pre-hospital performance in the region ‘Hollands-Midden’, resulting in minimal time delays regardless of area of residence [ ]. | Myocardial injuries patients | Standardized pre-hospital care guidelines applied in practice |
| Rognas et al. (2013) reported a prospective quality control study of the effect on pre-hospital critical care anesthesiologists’ behavior of implementing a standard operating procedure for pre-hospital controlled ventilation [ ]. | Airway management patients | Prospective registry |
| Brandler et al. (2015) found that EMS care providers missed more than a third of stroke cases. Seizures and other atypical presentations contribute significantly to stroke misdiagnosis [ ]. | Prehospital stroke identification methods | Retrospective report |
| Osborne et al. (2015) summarized the United Kingdom (UK) Ambulance Service guidelines for the management of seizures and explored the extent to which these guidelines are evidence-based [ ]. | Management of seizures | Guidelines report |
| Kerner et al. (2017) evaluated how the use of checklists for prehospital emergency care may help to improve adherence to treatment guidelines [ ]. | Checklists in prehospital emergency care | Standard operating procedures study |
| Lenssen et al. (2017) suggested that routine, remote, physician-based, telemedically-delegated (opioid-based) analgesia in trauma and non-trauma emergencies, as applied by paramedics, shows comparable efficacy to analgesia administered by on-scene prehospital EMS physicians [ ]. | Analgesia management patients | Retrospective observational study |
| Pride et al. (2017) discussed the importance of prehospital care delivery and triage in cases of stroke with emergent large vessel occlusion (ELVO) [ ]. | Stroke patients | Guidelines report |
| Rodríguez et al. (2020) found that the use of early warning scores can help the EMS to differentiate traumatic brain injury patients with a high risk of deterioration [ ]. | Traumatic brain injury patients | Prospective cohort |
3.6. Selection of a Homogeneous Study Group
4. discussion, 4.1. what are the solutions to implement, 4.2. what are the future opportunities and perspectives.
| Findings | Target Population | Study Design |
|---|---|---|
| Liu et al. (2014) highlighted the potential for machine learning algorithms to improve the accuracy of predicting the need for life-saving interventions in trauma patients, enabling faster and more appropriate treatment for these patients. The hybrid system developed in this study may also serve as a model for integrating machine learning algorithms into clinical decision-making processes [ ]. | Analgesia management patients | Retrospective and prospective cohort study |
| Desautels et al. (2016) highlighted the potential for machine learning models to improve sepsis prediction in the ICU using minimal EHR data, which may be particularly useful in resource-limited settings. However, the study also acknowledges the limitations of using retrospective data and the need for prospective validation of the models in clinical practice [ ]. | Sepsis prediction | Retrospective study |
| Cheng et al. (2021) highlighted the potential for deep learning algorithms to assist sonographers in the detection of abdominal free fluid in Morison’s pouch during sonography in trauma, potentially enabling faster and more accurate diagnosis of abdominal trauma. However, the study also acknowledges the limitations of using retrospective data and the need for prospective validation in clinical practice [ ]. | Abdominal trauma patients | Observational study |
| Fontanellaz et al. (2021) highlighted the potential for deep learning algorithms to assist radiologists in the detection of COVID-19 using chest radiographs, potentially enabling faster and more accurate diagnosis of the disease. However, the study also acknowledges the limitations of using retrospective data and the need for prospective validation of the deep learning diagnostic support system in clinical practice [ ]. | COVID-19 patients | Retrospective study |
| Uchida et al. (2021) demonstrated the feasibility and effectiveness of using machine learning algorithms as a diagnostic support tool in the prediction of stroke probability and type at the prehospital stage, potentially leading to improved stroke care and patient outcomes [ ]. | Stroke-management patients | Retrospective and prospective cohort study |
| Shahi et al. (2021) highlighted the potential for deep learning algorithms to improve decision-making in pediatric blunt solid organ injury, enabling faster and more accurate predictions of the need for massive transfusion, need for operative management, and mortality risk. The use of deep learning algorithms in trauma care may also reduce healthcare costs and improve patient outcomes [ ]. | Pediatric blunt solid organ injury | Retrospective study |
| Chen et al. (2022) highlighted the potential for AI-assisted systems to improve prehospital care by enabling faster and more accurate detection of ST-elevation myocardial infarction, which is crucial for timely intervention and improved patient outcomes. The use of a mini-12-lead ECG device also makes the system more accessible for use in resource-limited settings [ ]. | Myocardial injury patients | Retrospective study |
5. Limitations
6. conclusions, author contributions, acknowledgments, conflicts of interest.
- Wibring, K.; Magnusson, C.; Axelsson, C.; Lundgren, P.; Herlitz, J.; Andersson Hagiwara, M. Towards definitions of time-sensitive conditions in prehospital care. Scand. J. Trauma Resusc. Emerg. Med. 2020 , 28 , 7. [ Google Scholar ] [ CrossRef ]
- World Health Organization. Classification and Minimum Standards for Emergency Medical Teams ; World Health Organization: Geneva, Switzerland, 2021. [ Google Scholar ]
- Patiño, A.M.; Chen, J.; DeVos, E.L.; Lee, J.A.; Anderson, K.; Banks, M.; Arbelaez, C. Emergency medicine around the world: Analysis of the 2019 American College of emergency physicians international Ambassador country reports. J. Am. Coll. Emerg. Physicians Open 2022 , 3 , e12681. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Moore, M.R.; Archer, M.L. Red cross and red crescent health information system (RCHIS): Functional design and usability testing protocol. Prehosp. Disaster Med. 2019 , 34 , s86–s87. [ Google Scholar ] [ CrossRef ]
- Debenham, S.; Fuller, M.; Stewart, M.; Price, R.R. Where there is no EMS: Lay providers in Emergency Medical Services care-EMS as a public health priority. Prehospital Disaster Med. 2017 , 32 , 593–595. [ Google Scholar ] [ CrossRef ]
- Martin-Gill, C.; Brown, K.M.; Cash, R.E.; Haupt, R.M.; Potts, B.T.; Richards, C.T.; Prehospital Guidelines Consortium. 2022 systematic review of evidence-based guidelines for prehospital care. Prehospital Emerg. Care 2023 , 27 , 131–143. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jobé, C.; Carron, P.N.; Métrailler, P.; Bellagamba, J.M.; Briguet, A.; Zurcher, L.; Dami, F. Introduction of Telemedicine in a Prehospital Emergency Care Setting: A Pilot Study. Int. J. Telemed. Appl. 2023 , 2023 , 1171401. [ Google Scholar ] [ CrossRef ]
- Metelmann, B.; Metelmann, C. Mobile Health Applications in Prehospital Emergency Medicine. In Mobile Health Applications for Quality Healthcare Delivery ; IGI Global: Hershey, PA, USA, 2019; pp. 117–135. [ Google Scholar ]
- Kunz, A.; Ebinger, M.; Geisler, F.; Rozanski, M.; Waldschmidt, C.; Weber, J.E.; Wendt, M.; Winter, B.; Zieschang, K.; Fiebach, J.B.; et al. Functional outcomes of pre-hospital thrombolysis in a mobile stroke treatment unit compared with conventional care: An observational registry study. Lancet Neurol. 2016 , 15 , 1035–1043. [ Google Scholar ] [ CrossRef ]
- Plummer, V.; Boyle, M. EMS systems in lower-middle income countries: A literature review. Prehospital Disaster Med. 2017 , 32 , 64–70. [ Google Scholar ]
- Sudantha, B.H.; Warnakulasooriya, K.M.H.K.; Jayasuriya, Y.P.; Ratnayaka, G.R.; Mahanama, P.K.S.; Warusavitharana, E.J.; Weerasinghe, S.N. Open-source implementation of an integrated low-cost environmental monitoring system (EMS) for developing countries. Bhumi Plan. Res. J. 2018 , 6 , 23–29. [ Google Scholar ] [ CrossRef ]
- Rathore, N.; Jain, P.K.; Parida, M. A sustainable model for emergency medical services in developing countries: A novel approach using partial outsourcing and machine learning. Risk Manag. Healthc. Policy 2022 , 15 , 193–218. [ Google Scholar ] [ CrossRef ]
- El Sayed, M.; Al Assad, R.; Abi Aad, Y.; Gharios, N.; Refaat, M.M.; Tamim, H. Measuring the impact of emergency medical services (EMS) on out-of-hospital cardiac arrest survival in a developing country: A key metric for EMS systems’ performance. Medicine 2017 , 96 , e7570. [ Google Scholar ] [ CrossRef ]
- Bhandari, D.; Yadav, N.K. Developing an integrated emergency medical services in a low-income country like Nepal: A concept paper. Int. J. Emerg. Med. 2020 , 13 , 7. [ Google Scholar ] [ CrossRef ]
- Thygerson, S.; Memmott, G.; Chaney, R. A Global Emergency: Identifying Priorities for Reforming International Emergency Medical Systems. Med. Res. Arch. 2023 , 11 , 3922. [ Google Scholar ] [ CrossRef ]
- Miskimins, R.; Pati, S.; Schreiber, M. Barriers to clinical research in trauma. Transfusion 2019 , 59 (Suppl. 1), 846–853. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cimino, J.; Braun, C. Building a competitive infrastructure to support clinical research in healthcare institution. Eur. J. Clin. Investig. 2021 , 51 , e13641. [ Google Scholar ] [ CrossRef ]
- Steg, P.G.; Bonnefoy, E.; Chabaud, S.; Lapostolle, F.; Dubien, P.Y.; Cristofini, P.; Touboul, P. Impact of time to treatment on mortality after prehospital fibrinolysis or primary angioplasty: Data from the CAPTIM randomized clinical trial. Circulation 2003 , 108 , 2851–2856. [ Google Scholar ] [ CrossRef ]
- Bernard, S.A.; Nguyen, V.; Cameron, P.; Masci, K.; Fitzgerald, M.; Cooper, D.J.; Walker, T.; Std, B.P.; Myles, P.; Murray, L.; et al. Prehospital rapid sequence intubation improves functional outcome for patients with severe traumatic brain injury: A randomized controlled trial. Ann. Surg. 2010 , 252 , 959–965. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- El-Menyar, A.; Sathian, B.; Asim, M.; Latifi, R.; Al-Thani, H. Efficacy of prehospital administration of tranexamic acid in trauma patients: A meta-analysis of the randomized controlled trials. Am. J. Emerg. Med. 2018 , 36 , 1079–1087. [ Google Scholar ] [ CrossRef ]
- Martín-Rodríguez, F.; Del Pozo Vegas, C.; Mohedano-Moriano, A.; Polonio-López, B.; Miquel, C.M.; Viñuela, A.; Fernández, C.D.; Correas, J.G.; López-Izquierdo, R.; Martín-Conty, J.L. Role of Biomarkers in the Prediction of Serious Adverse Events after Syncope in Prehospital Assessment: A Multi-Center Observational Study. J. Clin. Med. 2020 , 9 , 651. [ Google Scholar ] [ CrossRef ]
- Misra, D.P.; Ravindran, V. An overview of the functionalities of PubMed. J. R. Coll. Physicians Edinb. 2022 , 52 , 8–9. [ Google Scholar ] [ CrossRef ]
- Berkowitz, J.; Cotarelo, A.; Washko, J.; Levinsky, B. Emergency Medical Services and the Elderly Patient: Prehospital Management. In Acute Care Surgery in Geriatric Patients ; Springer International Publishing: Cham, Switzerland, 2023; pp. 107–113. [ Google Scholar ]
- Ramage, L.; McLachlan, S.; Williams, K. Determining the top research priorities in UK prehospital critical care: A modified delphi study. Emerg. Med. J. 2023 , 40 , 271–276. [ Google Scholar ] [ CrossRef ]
- Delgado, R.C.; Gonzalez, K.A.; Martinez, J.A.C.; Alvarez, T.C.; Gonzalez, P.A. Top research priorities in prehospital care in Spain. Prehospital Disaster Med. 2023 , 38 , 81–87. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ali, J.; Howard, M.R.; Stedman, M.R.; Miller, P.P.M.; Winn, J.R.; Butler, K.A.M.; Gonsalves, D.R.; Chang, H.M.; Adam, R.M.; Williams, J. The Advanced Trauma Life Support (ATLS) program improves trauma patient outcome in a developing country. J. Trauma Acute Care Surg. 1992 , 33 , 149. [ Google Scholar ] [ CrossRef ]
- Søreide, K. Three decades (1978–2008) of Advanced Trauma Life Support (ATLS™) practice revised and evidence revisited. Scand. J. Trauma Resusc. Emerg. Med. 2008 , 16 , 19. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cummins, R.O.; Eisenberg, M.S.; Hallstrom, A.P.; Litwin, P.E. Survival of out-of-hospital cardiac arrest with early initiation of cardiopulmonary resuscitation. Am. J. Emerg. Med. 1985 , 3 , 114–119. [ Google Scholar ] [ CrossRef ]
- Bickell, W.H.; Wall, M.J., Jr.; Pepe, P.E.; Martin, R.R.; Ginger, V.F.; Allen, M.K.; Mattox, K.L. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N. Engl. J. Med. 1994 , 331 , 1105–1109. [ Google Scholar ] [ CrossRef ]
- Sampalis, J.S.; Denis, R.; Lavoie, A.; Fréchette, P.; Boukas, S.; Nikolis, A.; Benoit, D.; Fleiszer, D.; Brown, R.; Churchill-Smith, M.; et al. Trauma care regionalization: A process-outcome evaluation. J. Trauma Acute Care Surg. 1999 , 46 , 565–581. [ Google Scholar ] [ CrossRef ]
- Gausche, M.; Lewis, R.J.; Stratton, S.J.; Haynes, B.E.; Gunter, C.S.; Goodrich, S.M.; Poore, P.D.; McCollough, M.D.; Henderson, D.P.; Pratt, F.D.; et al. Effect of out-of-hospital pediatric endotracheal intubation on survival and neurological outcome: A controlled clinical trial. JAMA 2000 , 283 , 783–790. [ Google Scholar ] [ CrossRef ]
- Davis, D.P.; Peay, J.; Sise, M.J.; Vilke, G.M.; Kennedy, F.; Eastman, A.B.; Brent, A.; Velky, T.; Hoyt, D.B. The impact of prehospital endotracheal intubation on outcome in moderate to severe traumatic brain injury. J. Trauma Acute Care Surg. 2005 , 58 , 933–939. [ Google Scholar ] [ CrossRef ]
- Richard, J.; Osmond, M.H.; Nesbitt, L.; Stiell, I.G. Management and outcomes of pediatric patients transported by emergency medical services in a Canadian prehospital system. Can. J. Emerg. Med. 2006 , 8 , 6–12. [ Google Scholar ] [ CrossRef ]
- Ortolani, P.; Marzocchi, A.; Marrozzini, C.; Palmerini, T.; Saia, F.; Baldazzi, F.; Silenzi, S.; Taglieri, N.; Bacchi-Reggiani, M.; Branzi, A.; et al. Usefulness of prehospital triage in patients with cardiogenic shock complicating ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Am. J. Cardiol. 2007 , 100 , 787–792. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kragh, J.F., Jr.; Walters, T.J.; Baer, D.G.; Fox, C.J.; Wade, C.E.; Salinas, J.; Holcomb, J.B. Survival with emergency tourniquet use to stop bleeding in major limb trauma. Ann. Surg. 2009 , 249 , 1–7. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Nassif, A.; Ostermayer, D.G.; Hoang, K.B.; Claiborne, M.K.; Camp, E.A.; Shah, M.I. Implementation of a prehospital protocol change for asthmatic children. Prehospital Emerg. Care 2018 , 22 , 457–465. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dracup, K.; McKinley, S.; Riegel, B.; Moser, D.K.; Meischke, H.; Doering, L.V.; Davidson, P.; Paul, S.; Baker, H.; Pelter, M. A randomized clinical trial to reduce patient prehospital delay to treatment in acute coronary syndrome. Circ. Cardiovasc. Qual. Outcomes 2009 , 2 , 524–532. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bergs, J.; Sabbe, M.; Moons, P. Prehospital stroke scales in a Belgian prehospital setting: A pilot study. Eur. J. Emerg. Med. 2010 , 17 , 2–6. [ Google Scholar ] [ CrossRef ]
- Monsieurs, K.G.; De Regge, M.; Vansteelandt, K.; De Smet, J.; Annaert, E.; Lemoyne, S.; Kalmar, A.; Calle, P.A. Excessive chest compression rate is associated with insufficient compression depth in prehospital cardiac arrest. Resuscitation 2012 , 83 , 1319–1323. [ Google Scholar ] [ CrossRef ]
- Meretoja, A.; Kaste, M. Pre-and in-hospital intersection of stroke care. Ann. N. Y. Acad. Sci. 2012 , 1268 , 145–151. [ Google Scholar ] [ CrossRef ]
- Brown, J.B.; Sperry, J.L.; Fombona, A.; Billiar, T.R.; Peitzman, A.B.; Guyette, F.X. Pre-trauma center red blood cell transfusion is associated with improved early outcomes in air medical trauma patients. J. Am. Coll. Surg. 2015 , 220 , 797–808. [ Google Scholar ] [ CrossRef ]
- Lockey, D.J.; Healey, B.; Crewdson, K.; Chalk, G.; Weaver, A.E.; Davies, G.E. Advanced airway management is necessary in prehospital trauma patients. Br. J. Anaesth. 2015 , 114 , 657–662. [ Google Scholar ] [ CrossRef ]
- Crewdson, K.; Lockey, D.J.; Røislien, J.; Lossius, H.M.; Rehn, M. The success of pre-hospital tracheal intubation by different pre-hospital providers: A systematic literature review and meta-analysis. Crit. Care 2017 , 21 , 31. [ Google Scholar ] [ CrossRef ]
- Wang, H.E.; Schmicker, R.H.; Daya, M.R.; Stephens, S.W.; Idris, A.H.; Carlson, J.N.; Colella, M.; Herren, H.; Hansen, M.; Richmond, N.; et al. Effect of a strategy of initial laryngeal tube insertion vs endotracheal intubation on 72-hour survival in adults with out-of-hospital cardiac arrest: A randomized clinical trial. JAMA 2018 , 320 , 769–778. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Guyette, F.X.; Brown, J.B.; Zenati, M.S.; Early-Young, B.J.; Adams, P.W.; Eastridge, B.J.; Nirula, R.; Vercruysse, G.; O’Keeffe, T.; Joseph, B.; et al. Tranexamic acid during prehospital transport in patients at risk for hemorrhage after injury: A double-blind, placebo-controlled, randomized clinical trial. JAMA Surg. 2021 , 156 , 11–20. [ Google Scholar ] [ CrossRef ]
- Scquizzato, T.; Imbriaco, G.; Moro, F.; Losiggio, R.; Cabrini, L.; Consolo, F.; Zangrillo, A. Non-invasive ventilation in the prehospital emergency setting: A systematic review and meta-analysis. Prehospital Emerg. Care 2023 , 27 , 566–574. [ Google Scholar ] [ CrossRef ]
- Carney, N.; Totten, A.M.; Cheney, T.; Jungbauer, R.; Neth, M.R.; Weeks, C.; Davis-O’Reilly, C.; Fu, R.; Yu, Y.; Chou, R.; et al. Prehospital airway management: A systematic review. Prehospital Emerg. Care 2021 , 26 , 716–727. [ Google Scholar ] [ CrossRef ]
- McMullan, J.; Droege, C.; Strilka, R.; Hart, K.; Lindsell, C. Intranasal ketamine as an adjunct to fentanyl for the prehospital treatment of acute traumatic pain: Design and rationale of a randomized controlled trial. Prehospital Emerg. Care 2021 , 25 , 519–529. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Helwig, S.A.; Ragoschke-Schumm, A.; Schwindling, L.; Kettner, M.; Roumia, S.; Kulikovski, J.; Keller, I.; Manitz, M.; Martens, D.; Fassbender, K.; et al. Prehospital stroke management optimized by use of clinical scoring vs mobile stroke unit for triage of patients with stroke: A randomized clinical trial. JAMA Neurol. 2019 , 76 , 1484–1492. [ Google Scholar ] [ CrossRef ]
- Ehlers, J.; Fisher, B.; Peterson, S.; Dai, M.; Larkin, A.; Bradt, L.; Mann, N.C. Description of the 2020 NEMSIS public-release research dataset. Prehospital Emerg. Care 2022 , 27 , 473–481. [ Google Scholar ] [ CrossRef ]
- Perkins, G.D.; Ji, C.; Deakin, C.D.; Quinn, T.; Nolan, J.P.; Scomparin, C.; Regan, S.; Long, J.; Slowther, A.; Lall, R.; et al. A randomized trial of epinephrine in out-of-hospital cardiac arrest. N. Engl. J. Med. 2018 , 379 , 711–721. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Delano, M.J.; Rizoli, S.B.; Rhind, S.G.; Cuschieri, J.; Junger, W.; Baker, A.J.; Bulger, E.M. Prehospital resuscitation of traumatic hemorrhagic shock with hypertonic solutions worsens hypocoagulation and hyperfibrinolysis. Shock 2015 , 44 , 25. [ Google Scholar ] [ CrossRef ]
- Sikka, N.; Margolis, G. Understanding diversity among prehospital care delivery systems around the world. Emerg. Med. Clin. 2005 , 23 , 99–114. [ Google Scholar ] [ CrossRef ]
- Bounes, V.; Dehours, E.; Houze-Cerfon, V.; Vallé, B.; Lipton, R.; Ducassé, J.L. Quality of publications in emergency medicine. Am. J. Emerg. Med. 2013 , 31 , 297–301. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Charzyńska, E.; Habibi Soola, A.; Mozaffari, N.; Mirzaei, A. Patterns of work-related stress and their predictors among emergency department nurses and emergency medical services staff in a time of crisis: A latent profile analysis. BMC Nurs. 2023 , 22 , 98. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Stratman, E.G.; Boutilier, J.J.; Albert, L.A. Uncertainty in Facility Location Models for Emergency Medical Services. In Uncertainty in Facility Location Problems ; Springer: Berlin/Heidelberg, Germany, 2023; pp. 213–250. [ Google Scholar ]
- Lerner, E.B.; Weik, T.; Edgerton, E.A. Research in prehospital care: Overcoming the barriers to success. Prehospital Emerg. Care 2016 , 20 , 448–453. [ Google Scholar ] [ CrossRef ]
- Kim, J.H. Data integration using information and communication technology for emergency medical services and systems. Clin. Exp. Emerg. Med. 2023 , 10 , 129–131. [ Google Scholar ] [ CrossRef ]
- Sandman, L.; Nordmark, A. Ethical conflicts in prehospital emergency care. Nurs. Ethics 2006 , 13 , 592–607. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Moscati, R. Protection of human subjects in prehospital research. Prehospital Emerg. Care 2002 , 6 (Suppl. 2), S18–S23. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kim, D.T.; Spivey, W.H. A retrospective analysis of institutional review board and informed consent practices in EMS research. Ann. Emerg. Med. 1994 , 23 , 70–74. [ Google Scholar ] [ CrossRef ]
- Jensen, A.S.R.; Valentin, J.B.; Mulvad, M.G.; Hagenau, V.H.V.; Skaarup, S.H.; Johnsen, S.P.; VæggemoseGude, U.; Gude, M.F. Standard vs. Targeted Oxygen Therapy Prehospitally for Chronic Obstructive Pulmonary Disease (STOP-COPD); study protocol for a randomised controlled trial. Res. Square 2023 . [ Google Scholar ]
- Saver, J.L.; Kidwell, C.; Eckstein, M.; Ovbiagele, B.; Starkman, S. Physician-investigator phone elicitation of consent in the field: A novel method to obtain explicit informed consent for prehospital clinical research. Prehospital Emerg. Care 2006 , 10 , 182–185. [ Google Scholar ] [ CrossRef ]
- Abelsson, A.; Lindwall, L. What is dignity in prehospital emergency care? Nurs. Ethics 2017 , 24 , 268–278. [ Google Scholar ] [ CrossRef ]
- Roule, V.; Ardouin, P.; Blanchart, K.; Lemaitre, A.; Wain-Hobson, J.; Legallois, D.; Beygui, F. Prehospital fibrinolysis versus primary percutaneous coronary intervention in ST-elevation myocardial infarction: A systematic review and meta-analysis of randomized controlled trials. Crit. Care 2016 , 20 , 359. [ Google Scholar ] [ CrossRef ]
- Boersma, E. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrinolysis in acute myocardial infarction patients. Eur. Heart J. 2006 , 27 , 779–788. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Van der Velden, M.W.A.; Ringburg, A.N.; Bergs, E.A.; Steyerberg, E.W.; Patka, P.; Schipper, I.B. Prehospital interventions: Time wasted or time saved? An observational cohort study of management in initial trauma care. Emerg. Med. J. 2008 , 25 , 444–449. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pedley, D.K.; Bissett, K.; Connolly, E.M.; Goodman, C.G.; Golding, I.; Pringle, T.H.; Jones, M.C. Prospective observational cohort study of time saved by prehospital thrombolysis for ST elevation myocardial infarction delivered by paramedics. BMJ 2003 , 327 , 22–26. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fladt, J.; Ospel, J.M.; Singh, N.; Saver, J.L.; Fisher, M.; Goyal, M. Optimizing Patient-Centered Stroke Care and Research in the Prehospital Setting. Stroke 2023 , 54 , 2453–2460. [ Google Scholar ] [ CrossRef ]
- Cimino, J.; Braun, C. Design a Clinical Research Protocol: Influence of Real-World Setting. Healthcare 2023 , 11 , 2254. [ Google Scholar ] [ CrossRef ]
- Jarvis, S.; Salottolo, K.; Berg, G.M.; Carrick, M.; Caiafa, R.; Hamilton, D.; Banton, K.; Lieser, M.; Bar-Or, D. Examining emergency medical services’ prehospital transport times for trauma patients during COVID-19. Am. J. Emerg. Med. 2021 , 44 , 33–37. [ Google Scholar ] [ CrossRef ]
- Sucov, A.; Verdile, V.P.; Garettson, D.; Paris, P.M. The outcome of patients refusing prehospital transportation. Prehospital Disaster Med. 1992 , 7 , 365–371. [ Google Scholar ] [ CrossRef ]
- Wallace, D.J.; Kahn, J.M.; Angus, D.C.; Martin-Gill, C.; Callaway, C.W.; Rea, T.D.; Chhatwal, J.; Kurland, K.; Seymour, C.W. Accuracy of prehospital transport time estimation. Acad. Emerg. Med. 2014 , 21 , 9–16. [ Google Scholar ] [ CrossRef ]
- Quake, S.Y.L.; Khoda, F.; Arjomandi Rad, A.; Subbiah Ponniah, H.; Vardanyan, R.; Frisoni, P.; Arjomandi Rad, H.; Brasesco, M.; Mustoe, S.; Godfrey, J.; et al. The current status and challenges of prehospital trauma care in low-and middle-income countries: A systematic review. Prehospital Emerg. Care 2023 , 1–11. [ Google Scholar ] [ CrossRef ]
- Jamshidi, H.; Jazani, R.K.; Khani Jeihooni, A.; Alibabaei, A.; Alamdari, S.; Kalyani, M.N. Facilitators and barriers to collaboration between pre-hospital emergency and emergency department in traffic accidents: A qualitative study. BMC Emerg. Med. 2023 , 23 , 58. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Drayna, P.C.; Browne, L.R.; Guse, C.E.; Brousseau, D.C.; Lerner, E.B. Prehospital pediatric care: Opportunities for training, treatment, and research. Prehospital Emerg. Care 2015 , 19 , 441–447. [ Google Scholar ] [ CrossRef ]
- Kim, H.; Kim, S.W.; Park, E.; Kim, J.H.; Chang, H. The role of fifth-generation mobile technology in prehospital emergency care: An opportunity to support paramedics. Health Policy Technol. 2020 , 9 , 109–114. [ Google Scholar ] [ CrossRef ]
- Ångerman, S.; Kirves, H.; Nurmi, J. Multifaceted implementation and sustainability of a protocol for prehospital anaesthesia: A retrospective analysis of 2115 patients from helicopter emergency medical services. Scand. J. Trauma Resusc. Emerg. Med. 2023 , 31 , 21. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- McManamny, T.; Ortega, M.; Munro, S.; Jennings, P.; Whitley, G.A. A pre-hospital mixed methods systematic review protocol. Br. Paramed. J. 2023 , 8 , 38–43. [ Google Scholar ] [ CrossRef ]
- Finn, Z.; Carter, P.; Rogers, D.; Burnett, A. Prehospital COVID-19-Related Encounters Predict Future Hospital Utilization. Prehospital Emerg. Care 2023 , 27 , 297–302. [ Google Scholar ] [ CrossRef ]
- Li, T.; Koloden, D.; Berkowitz, J.; Luo, D.; Luan, H.; Gilley, C.; Kurgansky, G.; Barbara, P. Prehospital transport and termination of resuscitation of cardiac arrest patients: A review of prehospital care protocols in the United States. Resusc. Plus 2023 , 14 , 100397. [ Google Scholar ] [ CrossRef ]
- Rottman, S.J.; Schriger, D.L.; Charlop, G.; Salas, J.H.; Lee, S. On-line medical control versus protocol-based prehospital care. Ann. Emerg. Med. 1997 , 30 , 62–68. [ Google Scholar ] [ CrossRef ]
- Holstein, A.; Plaschke, A.; Vogel, M.Y.; Egberts, E.H. Prehospital management of diabetic emergencies—A population-based intervention study. Acta Anaesthesiol. Scand. 2003 , 47 , 610–615. [ Google Scholar ] [ CrossRef ]
- Watts, D.D.; Hanfling, D.; Waller, M.A.; Gilmore, C.; Fakhry, S.M.; Trask, A.L. An evaluation of the use of guidelines in prehospital management of brain injury. Prehospital Emerg. Care 2004 , 8 , 254–261. [ Google Scholar ] [ CrossRef ]
- Combes, X.; Jabre, P.; Jbeili, C.; Leroux, B.; Bastuji-Garin, S.; Margenet, A.; Adnet, F.; Dhonneur, G. Prehospital standardization of medical airway management: Incidence and risk factors of difficult airway. Acad. Emerg. Med. 2006 , 13 , 828–834. [ Google Scholar ] [ CrossRef ]
- Sasson, C.; Forman, J.; Krass, D.; Macy, M.; Hegg, A.J.; McNally, B.F.; Kellermann, A.L. A qualitative study to understand barriers to implementation of national guidelines for prehospital termination of unsuccessful resuscitation efforts. Prehospital Emerg. Care 2010 , 14 , 250–258. [ Google Scholar ] [ CrossRef ]
- Atary, J.Z.; de Visser, M.; van den Dijk, R.; Bosch, J.; Liem, S.S.; Antoni, M.L.; Bootsma, M.; Viergever, E.P.; Kirchhof, C.J.; Padmos, I.; et al. Standardised pre-hospital care of acute myocardial infarction patients: MISSION! guidelines applied in practice. Neth. Heart J. 2010 , 18 , 408–415. [ Google Scholar ] [ CrossRef ]
- Rognås, L.; Hansen, T.M.; Kirkegaard, H.; Tønnesen, E. Standard operating procedure changed pre-hospital critical care anaesthesiologists’ behaviour: A quality control study. Scand. J. Trauma Resusc. Emerg. Med. 2013 , 21 , 84. [ Google Scholar ] [ CrossRef ]
- Brandler, E.S.; Sharma, M.; McCullough, F.; Ben-Eli, D.; Kaufman, B.; Khandelwal, P.; Helzner, E.; Sinert, R.H.; Levine, S.R. Prehospital stroke identification: Factors associated with diagnostic accuracy. J. Stroke Cerebrovasc. Dis. 2015 , 24 , 2161–2166. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Osborne, A.; Taylor, L.; Reuber, M.; Grünewald, R.A.; Parkinson, M.; Dickson, J.M. Pre-hospital care after a seizure: Evidence base and United Kingdom management guidelines. Seizure 2015 , 24 , 82–87. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kerner, T.; Schmidbauer, W.; Tietz, M.; Marung, H.; Genzwuerker, H.V. Use of checklists improves the quality and safety of prehospital emergency care. Eur. J. Emerg. Med. 2017 , 24 , 114–119. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lenssen, N.; Krockauer, A.; Beckers, S.K.; Rossaint, R.; Hirsch, F.; Brokmann, J.C.; Bergrath, S. Quality of analgesia in physician-operated telemedical prehospital emergency care is comparable to physician-based prehospital care-a retrospective longitudinal study. Sci. Rep. 2017 , 7 , 1536. [ Google Scholar ] [ CrossRef ]
- Pride, G.L.; Fraser, J.F.; Gupta, R.; Alberts, M.J.; Rutledge, J.N.; Fowler, R.; Ansari, S.; Abruzzo, T.; Albani, B.; Jayaraman, M.V.; et al. Prehospital care delivery and triage of stroke with emergent large vessel occlusion (ELVO): Report of the Standards and Guidelines Committee of the Society of Neurointerventional Surgery. J. Neurointerv. Surg. 2017 , 9 , 802–812. [ Google Scholar ] [ CrossRef ]
- Martín-Rodríguez, F.; López-Izquierdo, R.; Mohedano-Moriano, A.; Polonio-López, B.; Maestre Miquel, C.; Viñuela, A.; Durantez Fernandez, C.; Correas, J.; Marques, G.; Martín-Conty, J.L. Identification of serious adverse events in patients with traumatic brain injuries, from prehospital care to Intensive-Care Unit, using Early Warning Scores. Int. J. Environ. Res. Public Health 2020 , 17 , 1504. [ Google Scholar ] [ CrossRef ]
- Johnson, M.; O’Hara, R.; Hirst, E.; Weyman, A.; Turner, J.; Mason, S.; Siriwardena, A.N. Multiple triangulation and collaborative research using qualitative methods to explore decision making in pre-hospital emergency care. BMC Med. Res. Methodol. 2017 , 17 , 11. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Oanesa, R.D.; Su, T.W.-H.; Weissman, A. Evidence for Use of Validated Sepsis Screening Tools in the Prehospital Population: A Scoping Review. Prehospital Emerg. Care 2023 , 1–21. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tuerxun, K.; Eklund, D.; Wallgren, U.; Dannenberg, K.; Repsilber, D.; Kruse, R.; Särndahl, E.; Kurland, L. Predicting sepsis using a combination of clinical information and molecular immune markers sampled in the ambulance. Sci. Rep. 2023 , 13 , 14917. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Janerka, C.; Leslie, G.D.; Mellan, M.; Arendts, G. Prehospital telehealth for emergency care: A scoping review. Emerg. Med. Australas. 2023 , 35 , 540–552. [ Google Scholar ] [ CrossRef ]
- Jaeger, L.R.; McCartin, M.P.; Haamid, A.; Weber, J.M.; Tataris, K.L. TeleEMS: An EMS Telemedicine Pilot Program Barriers to Implementation: (Preliminary Investigation). Prehospital Emerg. Care 2023 , 1–6. [ Google Scholar ] [ CrossRef ]
- Katz, B.S.; McMullan, J.T.; Sucharew, H.; Adeoye, O.; Broderick, J.P. Design and validation of a prehospital scale to predict stroke severity: Cincinnati Prehospital Stroke Severity Scale. Stroke 2015 , 46 , 1508–1512. [ Google Scholar ] [ CrossRef ]
- Weerts, J.O.; Schier, L.; Schmidt, H.; Kreinest, M. Review of existing measurement tools to assess spinal motion during prehospital immobilization. Eur. J. Emerg. Med. 2018 , 25 , 161–168. [ Google Scholar ] [ CrossRef ]
- Wu, X.; Dunne, R.; Yu, Z.; Shi, W. STREMS: A smart real-time solution toward enhancing EMS prehospital quality. In Proceedings of the 2017 IEEE/ACM International Conference on Connected Health: Applications, Systems and Engineering Technologies (CHASE), Philadelphia, PA, USA, 17–19 July 2017; pp. 365–372. [ Google Scholar ]
- Brown, N. Should the Kendrick Extrication Device have a place in pre-hospital care? J. Paramed. Pract. 2015 , 7 , 300–304. [ Google Scholar ] [ CrossRef ]
- Ayub, E.M.; Sampayo, E.M.; Shah, M.I.; Doughty, C.B. Prehospital providers’ perceptions on providing patient and family centered care. Prehospital Emerg. Care 2017 , 21 , 233–241. [ Google Scholar ] [ CrossRef ]
- Farcas, A.M.; Zaidi, H.Q.; Wleklinski, N.P.; Tataris, K.L. Implementing a Patient Tracking System in a Large EMS System. Prehospital Emerg. Care 2022 , 26 , 305–310. [ Google Scholar ] [ CrossRef ]
- Denecke, K.; Meier, L.; Bauer, J.G.; Bender, M.; Lueg, C. Information capturing in pre-hospital emergency medical settings (EMS). Stud. Health Technol. Inform. 2020 , 270 , 613–617. [ Google Scholar ] [ PubMed ]
- Davidson, T.J.; Waxenegger, H.; Mohamed, I.; McConnell, D.S.; Sanderson, P.M. SPECTRa: An Online Tool for Simulating Prehospital Patient Care. HERD Health Environ. Res. Des. J. 2022 , 15 , 375–394. [ Google Scholar ] [ CrossRef ]
- Walsh, K.B. Non-invasive sensor technology for prehospital stroke diagnosis: Current status and future directions. Int. J. Stroke 2019 , 14 , 592–602. [ Google Scholar ] [ CrossRef ]
- Amaral, C.B.; Ralston, D.C.; Becker, T.K. Prehospital point-of-care ultrasound: A transformative technology. SAGE Open Med. 2020 , 8 , 2050312120932706. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gonzalez, M.A.; Hanna, N.; Rodrigo, M.E.; Satler, L.F.; Waksman, R. Reliability of prehospital real-time cellular video phone in assessing the simplified National Institutes of Health Stroke Scale in patients with acute stroke: A novel telemedicine technology. Stroke 2011 , 42 , 1522–1527. [ Google Scholar ] [ CrossRef ]
- Ahmad, M.; Page, M.; Goodsman, D. What is simulation-based medical education (SBME) debriefing in prehospital medicine? A qualitative, ethnographic study exploring SBME debriefing in prehospital medical education. BMC Med. Educ. 2023 , 23 , 625. [ Google Scholar ] [ CrossRef ]
- Lowrie, L.N.; Duncan, L.; Samuels, D.A.; Ablah, E.; Ofei-Dodoo, S. Prehospital Clinical Decision-Making for Medication Administration for Behavioral Emergencies. Kans. J. Med. 2023 , 16 , 189. [ Google Scholar ] [ CrossRef ]
- Kang, D.Y.; Cho, K.J.; Kwon, O.; Kwon, J.M.; Jeon, K.H.; Park, H.; Lee, Y.; Park, J.; Oh, B.H. Artificial intelligence algorithm to predict the need for critical care in prehospital emergency medical services. Scand. J. Trauma Resusc. Emerg. Med. 2020 , 28 , 17. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kim, D.; You, S.; So, S.; Lee, J.; Yook, S.; Jang, D.P.; Young Kim, I.; Park, E.; Cho, K.; Park, H.K.; et al. A data-driven artificial intelligence model for remote triage in the prehospital environment. PLoS ONE 2018 , 13 , e0206006. [ Google Scholar ] [ CrossRef ]
- Simionescu, C.; Insuratelu, M.; Herscovici, R. Prehospital cerebrovascular accident detection using artificial intelligence powered mobile devices. Procedia Comput. Sci. 2020 , 176 , 2773–2782. [ Google Scholar ] [ CrossRef ]
- Koceska, N.; Komadina, R.; Simjanoska, M.; Koteska, B.; Strahovnik, A.; Jošt, A.; Maček, R.; Madevska-Bogdanova, A.; Trajkovik, V.; Tasič, J.F.; et al. Mobile wireless monitoring system for prehospital emergency care. Euro. J. Trauma Emerg. Surg. 2020 , 46 , 1301–1308. [ Google Scholar ] [ CrossRef ]
- Spangler, D.; Hermansson, T.; Smekal, D.; Blomberg, H. A validation of machine learning-based risk scores in the prehospital setting. PLoS ONE 2019 , 14 , e0226518. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Carlson, J.N.; Das, S.; De la Torre, F.; Frisch, A.; Guyette, F.X.; Hodgins, J.K.; Yealy, D.M. A novel artificial intelligence system for endotracheal intubation. Prehospital Emerg. Care 2016 , 20 , 667–671. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hasan, M.; Bath, P.A.; Marincowitz, C.; Sutton, L.; Pilbery, R.; Hopfgartner, F.; Mazumdar, S.; Campbell, R.; Stone, T.; Goodacre, S.; et al. Pre-hospital prediction of adverse outcomes in patients with suspected COVID-19: Development, application and comparison of machine learning and deep learning methods. Comput. Biol. Med. 2022 , 151 , 106024. [ Google Scholar ] [ CrossRef ]
- Choi, A.; Kim, M.J.; Sung, J.M.; Kim, S.; Lee, J.; Hyun, H.; Kim, H.C.; Kim, J.H.; Chang, H.J. Development of Prediction Models for Acute Myocardial Infarction at Prehospital Stage with Machine Learning Based on a Nationwide Database. J. Cardiovasc. Dev. Dis. 2022 , 9 , 430. [ Google Scholar ] [ CrossRef ]
- Desai, M.D.; Tootooni, M.S.; Bobay, K.L. Can Prehospital Data Improve Early Identification of Sepsis in Emergency Department? An Integrative Review of Machine Learning Approaches. Appl. Clin. Inform. 2022 , 13 , 189–202. [ Google Scholar ] [ CrossRef ]
- Abe, D.; Inaji, M.; Hase, T.; Takahashi, S.; Sakai, R.; Ayabe, F.; Tanaka, Y.; Otomo, Y.; Maehara, T. A prehospital triage system to detect traumatic intracranial hemorrhage using machine learning algorithms. JAMA Netw. Open 2022 , 5 , e2216393. [ Google Scholar ] [ CrossRef ]
- Liu, N.T.; Holcomb, J.B.; Wade, C.E.; Batchinsky, A.I.; Cancio, L.C.; Darrah, M.I.; Salinas, J. Development and validation of a machine learning algorithm and hybrid system to predict the need for life-saving interventions in trauma patients. Med. Biol. Eng. Comput. 2014 , 52 , 193–203. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Desautels, T.; Calvert, J.; Hoffman, J.; Jay, M.; Kerem, Y.; Shieh, L.; Shimabukuro, D.; Chettipally, U.; Feldman, M.; Das, R.; et al. Prediction of sepsis in the intensive care unit with minimal electronic health record data: A machine learning approach. JMIR Med. Inform. 2016 , 4 , e5909. [ Google Scholar ] [ CrossRef ]
- Cheng, C.Y.; Chiu, I.M.; Hsu, M.Y.; Pan, H.Y.; Tsai, C.M.; Lin, C.H.R. Deep learning assisted detection of abdominal free fluid in Morison’s pouch during focused assessment with sonography in trauma. Front. Med. 2021 , 8 , 707437. [ Google Scholar ] [ CrossRef ]
- Fontanellaz, M.; Ebner, L.; Huber, A.; Peters, A.; Löbelenz, L.; Hourscht, C.; Klaus, J.; Munz, J.; Ruder, T.; Christe, A.; et al. A deep-learning diagnostic support system for the detection of COVID-19 using chest radiographs: A multireader validation study. Investig. Radiol. 2021 , 56 , 348–356. [ Google Scholar ] [ CrossRef ]
- Uchida, K.; Kouno, J.; Yoshimura, S.; Kinjo, N.; Sakakibara, F.; Araki, H.; Morimoto, T. Development of Machine Learning Models to Predict Probabilities and Types of Stroke at Prehospital Stage: The Japan Urgent Stroke Triage Score Using Machine Learning (JUST-ML). Transl. Stroke Res. 2021 , 13 , 370–381. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Shahi, N.; Shahi, A.K.; Phillips, R.; Shirek, G.; Bensard, D.; Moulton, S.L. Decision-making in pediatric blunt solid organ injury: A deep learning approach to predict massive transfusion, need for operative management, and mortality risk. J. Pediatr. Surg. 2021 , 56 , 379–384. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chen, K.W.; Wang, Y.C.; Liu, M.H.; Tsai, B.Y.; Wu, M.Y.; Hsieh, P.H.; Wei, J.-T.; Shi, E.; Shiao, Y.-T.; Chang, K.C.; et al. Artificial intelligence-assisted remote detection of ST-elevation myocardial infarction using a mini-12-lead electrocardiogram device in prehospital ambulance care. Front. Cardiovasc. Med. 2022 , 9 , 1001982. [ Google Scholar ] [ CrossRef ] [ PubMed ]
| Findings | Target Population | Study Design |
|---|---|---|
| Cummins et al. (1985) examined the records of 1297 people with witnessed out-of-hospital cardiac arrest caused by heart disease and treated by both emergency medical technicians (EMTs) and paramedics, to determine early cardiopulmonary resuscitation (CPR) improved survival [ ]. | Cardiac arrest patients | Observational |
| Bickell et al. (1994) found that prehospital intravenous fluid replacement therapy improved survival in hypotensive trauma patients, highlighting the importance of aggressive fluid resuscitation in this population [ ]. | Hypotensive trauma patients | Prospective cohort |
| Sampalis et al. (1999) showed that tertiary trauma centers and reduced prehospital times are the essential components of an efficient trauma care system [ ]. | Traumatic injury patients | Prospective cohort |
| Gausche et al. (2000) found that out-of-hospital pediatric endotracheal intubation did not improve survival or neurological outcomes compared to bag–mask ventilation, highlighting the challenges of airway management in pediatric patients [ ]. | Endotracheal intubation in pediatric patients | Controlled clinical trial |
| Davis et al. (2005) found that prehospital oxygen therapy did not improve mortality in severe traumatic brain injury patients, challenging the previous standard of care [ ]. | Brain injury patients | Retrospective registry |
| Richard et al. (2006) shed light on the management and outcomes of pediatric patients transported by EMS in a Canadian prehospital system. Through an analysis of a prospective cohort, the research provides valuable insights into the characteristics, interventions, and outcomes of pediatric patients in the prehospital setting [ ]. | Pediatric emergency medicine | Prospective cohort |
| Ortolani et al. (2007) demonstrated the significant benefit of prehospital triage in identifying patients with cardiogenic shock complicating ST-elevation myocardial infarction (STEMI) who would benefit from primary percutaneous coronary intervention (PCI). The results indicate that patients who received prehospital triage had a significantly lower mortality rate compared to those who did not undergo prehospital triage. Furthermore, the study reveals that prehospital triage was associated with a higher likelihood of achieving optimal revascularization [ ]. | Prehospital cardiogenic shock | Prospective registry |
| Kragh et al. (2009) evaluated the use of tourniquets in trauma patients in war areas. The study found that tourniquet use when shock was absent was strongly associated with saved lives, and prehospital use was also strongly associated with life-saving outcomes [ ]. | Traumatic injury patients | Prospective survey |
| Nassif et al. (2009) found that prehospital protocol change for asthmatic children is associated with shorter total hospital and total care times. This protocol change was also associated with decreased hospitalization rates and less need for critical care in those hospitalized. Further study is necessary to determine if other factors also contributed. [ ]. | Children with minor head trauma | Prospective cohort |
| Dracup et al. (2009) demonstrated the effectiveness of a targeted educational intervention in reducing prehospital delay to treatment in acute coronary syndrome (ACS) patients. The study emphasizes the importance of patient education and empowerment in promoting timely medical care-seeking behavior. The findings suggest that interventions aimed at improving symptom recognition, knowledge of ACS symptoms, and overcoming barriers can contribute to better outcomes for ACS patients by facilitating early access to appropriate treatments [ ]. | Acute coronary syndrome patients | Randomized clinical trial |
| Bergs et al. (2010) demonstrated the feasibility and reliability of prehospital stroke scales in the Belgian prehospital setting. The results indicate that EMS personnel were able to effectively administer and interpret the stroke scales, leading to accurate identification and triage of potential stroke patients. The study also reveals a high level of inter-rater reliability among EMS providers in using the stroke scales [ ]. | Stroke patients | Prospective cohort |
| Monsieurs et al. (2012) showed an association between higher compression rates and lower compression depths. Avoiding excessive compression rates may lead to more compressions of sufficient depth [ ]. | Myocardial infarction patients | Observational |
| Meretoja et al. (2012) found that the implementation of a prehospital stroke protocol by emergency medical services improved stroke outcomes, emphasizing the importance of early recognition and treatment of stroke symptoms [ ]. | Stroke patients | Observational |
| Brown et al. (2015) found that prehospital blood product transfusion improved mortality and functional outcomes in trauma patients during medical evacuation, highlighting the potential benefits of this intervention [ ]. | Traumatic injury patients | Retrospective cohort |
| Lockey et al. (2015) found that prehospital advanced life support improved outcomes for major trauma patients, highlighting the importance of early and effective interventions in this population [ ]. | Major trauma patients | Prospective observational |
| Crewdson et al. (2017) conducted a systematic review and meta-analysis and found that prehospital rapid sequence intubation was associated with improved outcomes for trauma patients, highlighting the importance of effective airway management in this population [ ]. | Injury patients | Systematic review and meta-analysis |
| Wang et al. (2018) compared the effectiveness of two methods of airway management in adults with out-of-hospital cardiac arrest: laryngeal tube (LT) and endotracheal intubation (ETI). Based on these findings, the authors concluded that initial LT insertion may be considered as an alternative to ETI for airway management in adults with out-of-hospital cardiac arrest [ ]. | Cardiac arrest patients | Randomized clinical trial |
| Guyette et al. (2021) found that prehospital administration of tranexamic acid after injury did not result in a higher incidence of thrombotic complications or adverse events. Tranexamic acid given to injured patients at risk for hemorrhage in the prehospital setting is safe and associated with survival benefit in specific subgroups of patients [ ]. | Trauma patients with hypovolemic shock | Randomized clinical trial |
| Scquizzato et al. (2023) demonstrated that adults with acute respiratory failure treated in the prehospital setting with noninvasive ventilation had a lower risk of intubation than those managed with standard oxygen therapy, with similar risk of death, intensive care admission, and length of hospital stay. [ ]. | Prehospital respiratory failure | Retrospective cohort |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Cimino, J.; Braun, C. Clinical Research in Prehospital Care: Current and Future Challenges. Clin. Pract. 2023 , 13 , 1266-1285. https://doi.org/10.3390/clinpract13050114
Cimino J, Braun C. Clinical Research in Prehospital Care: Current and Future Challenges. Clinics and Practice . 2023; 13(5):1266-1285. https://doi.org/10.3390/clinpract13050114
Cimino, Jonathan, and Claude Braun. 2023. "Clinical Research in Prehospital Care: Current and Future Challenges" Clinics and Practice 13, no. 5: 1266-1285. https://doi.org/10.3390/clinpract13050114
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- PMC10605888

Clinical Research in Prehospital Care: Current and Future Challenges
Jonathan cimino.
1 Clinical Research Unit, Fondation Hôpitaux Robert Schuman, 44 Rue d’Anvers, 1130 Luxembourg, Luxembourg
2 Hôpitaux Robert Schuman, 9 Rue Edward Steichen, 2540 Luxembourg, Luxembourg
Claude Braun
Prehospital care plays a critical role in improving patient outcomes, particularly in cases of time-sensitive emergencies such as trauma, cardiac failure, stroke, bleeding, breathing difficulties, systemic infections, etc. In recent years, there has been a growing interest in clinical research in prehospital care, and several challenges and opportunities have emerged. There is an urgent need to adapt clinical research methodology to a context of prehospital care. At the same time, there are many barriers in prehospital research due to the complex context, posing unique challenges for research, development, and evaluation. Among these, this review allows the highlighting of limited resources and infrastructure, ethical and regulatory considerations, time constraints, privacy, safety concerns, data collection and analysis, selection of a homogeneous study group, etc. The analysis of the literature also highlights solutions such as strong collaboration between emergency medical services (EMS) and hospital care, use of (mobile) health technologies and artificial intelligence, use of standardized protocols and guidelines, etc. Overall, the purpose of this narrative review is to examine the current state of clinical research in prehospital care and identify gaps in knowledge, including the challenges and opportunities for future research.
1. Introduction
Prehospital care is a crucial aspect of emergency medicine that involves providing medical assistance to patients before they arrive at a hospital or healthcare facility [ 1 ]. According to the World Health Organization (WHO), injuries and illnesses that require emergency care affect millions of people globally, with many of these incidents occurring in low- and middle-income countries [ 2 ]. Despite the importance of prehospital care, there are significant disparities in access to this care around the world. In some areas, there may be limited or no access to emergency medical services, while in others, the quality of care may be inadequate. Recently, the American College of Emergency Physicians (ACEP) International Ambassador Country Reports shed light on the varying levels of access to emergency medical services worldwide with certain observations: disparities in access, infrastructure and resource constraints, geographical and financial barriers, lack of international collaboration, etc. [ 3 ]. Despite these challenges, efforts are underway to improve access to prehospital care and reduce disparities worldwide. Organizations such as the WHO and the International Federation of Red Cross and Red Crescent Societies are working to improve training and resources for emergency medical services (EMS) personnel and expand access to emergency medical services in underserved areas [ 4 ].
Prehospital care is often the first point of contact between a patient and the healthcare system and plays a critical role in reducing mortality and morbidity associated with acute illnesses and injuries. Clinical research in prehospital care is essential to ensure that the care provided to patients in this environment is evidence-based and effective. This type of care is typically delivered by EMS personnel, who are trained to provide a range of treatments and interventions to stabilize patients and prepare them for transport to a hospital [ 5 ]. Prehospital care plays a vital role in improving patient outcomes, as early intervention can often mean the difference between life and death.
The use of evidence-based guidelines and protocols can help to improve the quality of care provided in the prehospital environment, reduce the risk of adverse events, and improve patient outcomes [ 6 ]. With an acceleration over the past decade, prehospital care has become increasingly sophisticated, with advances in technology and medical treatments allowing EMS personnel to deliver more advanced care in the field. The integration of technology in prehospital care is becoming increasingly pivotal for enhancing patient outcomes. Notably, health technologies such as telemedicine and mobile health tools—encompassing smartphones, tablets, and wearable devices—are driving this progress. These innovations hold the potential to significantly enhance communication between prehospital care providers and healthcare experts [ 7 , 8 , 9 ]. The advent of mobile technology has empowered EMS personnel to transmit real-time patient data, encompassing crucial metrics such as vital signs and electrocardiograms, to hospitals and healthcare practitioners. This facilitates hospitals in preparing for the patient’s arrival, ensuring the availability of essential resources and medical staff for immediate care. In addition, new medical treatments and procedures are now available in developed countries to EMS personnel in the field, allowing them to provide advanced care that was previously only available in a hospital setting [ 10 ]. For example, paramedics can now administer intravenous medications, perform advanced airway management or ultrasonography in life-threatening conditions, utilize new devices for rapid intraosseous access, operate analyzers of cardiac markers or electrolytes in ambulances, and even perform life-saving procedures such as needle decompression of a tension pneumothorax [ 11 , 12 , 13 , 14 ]. Overall, technology and medical advancements in prehospital care are transforming the field and improving patient outcomes.
In recent years, there has been growing interest in clinical research in prehospital care. Clinical research in prehospital care is necessary to provide healthcare professionals with evidence-based guidelines and protocols for the treatment of patients in this environment [ 15 ]. Indeed, and despite this progress, there are still significant gaps in our understanding of the best approaches to prehospital care [ 16 ]. One challenge in conducting clinical research in prehospital care is the limited availability of resources and infrastructure, ethical and regulatory considerations, time constraints, safety concerns, data collection, and the difficulty of selecting a homogeneous study group of patient [ 17 ]. Prehospital care providers often work in challenging environments, with limited resources and time constraints. This can make it difficult to conduct high-quality research in this field. Another challenge in conducting clinical research in prehospital care is the need to balance research with the provision of timely and appropriate care to patients. Prehospital care providers must always prioritize the needs of their patients, which can sometimes conflict with the needs of researchers. Despite these challenges, there have been many important advances in clinical research in prehospital care. For example, randomized controlled trials (RCTs) have been used to evaluate the effectiveness of different interventions in the prehospital environment, including advanced airway management, pain management, and the use of mechanical chest compressions in cardiac arrest [ 18 , 19 , 20 ]. In addition, observational studies have been used to identify risk factors for adverse events in the prehospital environment and to evaluate the effectiveness of prehospital care protocols and guidelines. The use of observational studies has also allowed researchers to identify gaps in the best approaches to prehospital care and to develop hypotheses for future research [ 21 ].
For the purposes of this narrative review, we conducted a search in the PubMed electronic database [ 22 ]—the most commonly used search platform for medical literature and for scientific literature published in English—up to April 2023; “prehospital care”, “prehospital research”, “emergency medical research”, “prehospital quality”, and “prehospital technology” were used as search terms. Additional references were retrieved from reviewing the references cited in the original articles. All methodological human studies were included in this review (e.g., single-center, multi-center, randomized or not, prospective or retrospective studies, etc.).
Within the scope of this review, several key factors come to the forefront for examination. These include limited resources and infrastructure, ethical and regulatory considerations, time constraints, data collection methods, privacy, and safety concerns, as well as the challenges related to selecting a homogeneous study group. Furthermore, the literature analysis underscores potential solutions to these challenges. These solutions encompass fostering robust collaboration between Emergency Medical Services (EMS) and hospital care, leveraging (mobile) health technologies and artificial intelligence, and adopting standardized protocols and guidelines. In essence, this narrative review’s overarching aim is to assess the current landscape of clinical research in prehospital care while pinpointing areas where knowledge gaps persist. Additionally, it seeks to shed light on the challenges and opportunities that will shape future research endeavors.
2. Research in Prehospital Care: State of the Art
Prehospital care is a rapidly evolving field, with ongoing research aiming to improve patient outcomes and optimize emergency medical services [ 23 ]. Current clinical research studies in prehospital care are investigating a range of topics, including airway management, hemorrhage control, pain management, and stroke care [ 24 , 25 ]. Clinical research in prehospital care aims to identify the best practices and evidence-based approaches to managing acute illnesses and injuries before the patient is transported to a hospital [ 6 ].
In the past, there was little field-focused research in the prehospital setting. However, as EMS systems became more established and technology improved, the need for evidence-based approaches to prehospital care became apparent. One of the earliest examples of clinical research in prehospital care was the development of the Advanced Trauma Life Support (ATLS) program in the 1970s [ 26 , 27 ]. The program is a systematic approach to managing trauma patients in emergency situations. It was developed by the American College of Surgeons (ACS) as a standard of care for the initial assessment and treatment of trauma patients. The ATLS program was extended to prehospital care and has since undergone several updates by promoting standardized care, data collection, quality improvement, and collaboration among healthcare professionals. Table 1 provides some examples of clinical research studies conducted in prehospital care from 1980 to 2020, with a focus on their findings and implications for practice [ 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 ]. The selection of factors was based on the available evidence and their practical implication in prehospital research. For the presentation of the findings in Table 1 , Table 2 and Table 3 , a categorization based on their relation to important research studies, evidence-based practice guidelines, and potential for artificial intelligence was applied. These studies cover a range of topics in prehospital care, including trauma management, airway management, cardiac arrest, and intravenous fluid replacement therapy. Other studies are evaluating the use of tranexamic acid and tourniquets to control hemorrhage in trauma patients, with a focus on prehospital administration and the impact on survival rates. All this research provides important insights into the best practices for prehospital care during the late 20th century.
Examples of research studies (in chronological order) from a given prehospital “potential killer” conducted in pre-hospital care.
| Findings | Target Population | Study Design |
|---|---|---|
| Cummins et al. (1985) examined the records of 1297 people with witnessed out-of-hospital cardiac arrest caused by heart disease and treated by both emergency medical technicians (EMTs) and paramedics, to determine early cardiopulmonary resuscitation (CPR) improved survival [ ]. | Cardiac arrest patients | Observational |
| Bickell et al. (1994) found that prehospital intravenous fluid replacement therapy improved survival in hypotensive trauma patients, highlighting the importance of aggressive fluid resuscitation in this population [ ]. | Hypotensive trauma patients | Prospective cohort |
| Sampalis et al. (1999) showed that tertiary trauma centers and reduced prehospital times are the essential components of an efficient trauma care system [ ]. | Traumatic injury patients | Prospective cohort |
| Gausche et al. (2000) found that out-of-hospital pediatric endotracheal intubation did not improve survival or neurological outcomes compared to bag–mask ventilation, highlighting the challenges of airway management in pediatric patients [ ]. | Endotracheal intubation in pediatric patients | Controlled clinical trial |
| Davis et al. (2005) found that prehospital oxygen therapy did not improve mortality in severe traumatic brain injury patients, challenging the previous standard of care [ ]. | Brain injury patients | Retrospective registry |
| Richard et al. (2006) shed light on the management and outcomes of pediatric patients transported by EMS in a Canadian prehospital system. Through an analysis of a prospective cohort, the research provides valuable insights into the characteristics, interventions, and outcomes of pediatric patients in the prehospital setting [ ]. | Pediatric emergency medicine | Prospective cohort |
| Ortolani et al. (2007) demonstrated the significant benefit of prehospital triage in identifying patients with cardiogenic shock complicating ST-elevation myocardial infarction (STEMI) who would benefit from primary percutaneous coronary intervention (PCI). The results indicate that patients who received prehospital triage had a significantly lower mortality rate compared to those who did not undergo prehospital triage. Furthermore, the study reveals that prehospital triage was associated with a higher likelihood of achieving optimal revascularization [ ]. | Prehospital cardiogenic shock | Prospective registry |
| Kragh et al. (2009) evaluated the use of tourniquets in trauma patients in war areas. The study found that tourniquet use when shock was absent was strongly associated with saved lives, and prehospital use was also strongly associated with life-saving outcomes [ ]. | Traumatic injury patients | Prospective survey |
| Nassif et al. (2009) found that prehospital protocol change for asthmatic children is associated with shorter total hospital and total care times. This protocol change was also associated with decreased hospitalization rates and less need for critical care in those hospitalized. Further study is necessary to determine if other factors also contributed. [ ]. | Children with minor head trauma | Prospective cohort |
| Dracup et al. (2009) demonstrated the effectiveness of a targeted educational intervention in reducing prehospital delay to treatment in acute coronary syndrome (ACS) patients. The study emphasizes the importance of patient education and empowerment in promoting timely medical care-seeking behavior. The findings suggest that interventions aimed at improving symptom recognition, knowledge of ACS symptoms, and overcoming barriers can contribute to better outcomes for ACS patients by facilitating early access to appropriate treatments [ ]. | Acute coronary syndrome patients | Randomized clinical trial |
| Bergs et al. (2010) demonstrated the feasibility and reliability of prehospital stroke scales in the Belgian prehospital setting. The results indicate that EMS personnel were able to effectively administer and interpret the stroke scales, leading to accurate identification and triage of potential stroke patients. The study also reveals a high level of inter-rater reliability among EMS providers in using the stroke scales [ ]. | Stroke patients | Prospective cohort |
| Monsieurs et al. (2012) showed an association between higher compression rates and lower compression depths. Avoiding excessive compression rates may lead to more compressions of sufficient depth [ ]. | Myocardial infarction patients | Observational |
| Meretoja et al. (2012) found that the implementation of a prehospital stroke protocol by emergency medical services improved stroke outcomes, emphasizing the importance of early recognition and treatment of stroke symptoms [ ]. | Stroke patients | Observational |
| Brown et al. (2015) found that prehospital blood product transfusion improved mortality and functional outcomes in trauma patients during medical evacuation, highlighting the potential benefits of this intervention [ ]. | Traumatic injury patients | Retrospective cohort |
| Lockey et al. (2015) found that prehospital advanced life support improved outcomes for major trauma patients, highlighting the importance of early and effective interventions in this population [ ]. | Major trauma patients | Prospective observational |
| Crewdson et al. (2017) conducted a systematic review and meta-analysis and found that prehospital rapid sequence intubation was associated with improved outcomes for trauma patients, highlighting the importance of effective airway management in this population [ ]. | Injury patients | Systematic review and meta-analysis |
| Wang et al. (2018) compared the effectiveness of two methods of airway management in adults with out-of-hospital cardiac arrest: laryngeal tube (LT) and endotracheal intubation (ETI). Based on these findings, the authors concluded that initial LT insertion may be considered as an alternative to ETI for airway management in adults with out-of-hospital cardiac arrest [ ]. | Cardiac arrest patients | Randomized clinical trial |
| Guyette et al. (2021) found that prehospital administration of tranexamic acid after injury did not result in a higher incidence of thrombotic complications or adverse events. Tranexamic acid given to injured patients at risk for hemorrhage in the prehospital setting is safe and associated with survival benefit in specific subgroups of patients [ ]. | Trauma patients with hypovolemic shock | Randomized clinical trial |
| Scquizzato et al. (2023) demonstrated that adults with acute respiratory failure treated in the prehospital setting with noninvasive ventilation had a lower risk of intubation than those managed with standard oxygen therapy, with similar risk of death, intensive care admission, and length of hospital stay. [ ]. | Prehospital respiratory failure | Retrospective cohort |
A new frontier in prehospital care is the enhancement of various aspects, such as airway management, pain control, and stroke care. In the realm of airway management, ongoing research is delving into the utilization of video laryngoscopy, supraglottic airway devices, and neuromuscular blocking agents. These investigations aim to bolster the success rate and safety of endotracheal intubation [ 47 ]. Pain management also takes center stage in prehospital care, with active studies examining the efficacy of intranasal fentanyl and ketamine in providing relief to patients experiencing acute pain [ 48 ]. Furthermore, the field of stroke care is experiencing substantial growth in research endeavors. Studies are now exploring the deployment of mobile stroke units, equipped with advanced imaging and treatment capabilities, to extend rapid and effective care to individuals exhibiting acute stroke symptoms [ 49 ].
Another milestone in the evolution of clinical research in prehospital care came from the United States and was the establishment of the National Emergency Medical Services Information System (NEMSIS) in the early 2000s. NEMSIS is a standardized data collection and reporting system (including patient demographics, clinical outcomes, and interventions) specifically designed for EMS agencies in all the United States. This collaborative effort among federal agencies, EMS stakeholders, and state EMS offices to identify trends in prehospital care and to establish a national standard for collecting and sharing EMS data [ 50 ].
In recent years, there has been an increased focus on the use of randomized controlled trials (RCTs) in prehospital care research. RCTs are considered the gold standard for evaluating the effectiveness of medical interventions, and their use in prehospital care has led to significant advancements in the field. For example, a recent RCT compared the use of prehospital epinephrine to placebo in patients with out-of-hospital cardiac arrest, and found that epinephrine improved rates of survival to hospital discharge [ 51 ]. The study was conducted as a randomized, double-blind trial involving 8014 patients in 10 different countries. The patients were randomly assigned to receive either epinephrine or a placebo during resuscitation efforts. The primary outcome measure of the study was survival to hospital discharge with a favorable neurologic outcome. Secondary outcomes included return of spontaneous circulation, survival to hospital admission, and adverse events. The study found that the rate of survival to hospital discharge with a favorable neurologic outcome was higher in the group that received epinephrine compared to the placebo group. However, the study also found that the use of epinephrine was associated with a higher rate of severe neurological impairment among survivors. The study highlights the need for further research and improved resuscitation techniques to improve patient outcomes in cardiac arrest situations.
In addition to RCTs, there has also been an increased focus on the use of observational studies in prehospital care research. Observational studies allow researchers to evaluate the effectiveness of interventions in real-world settings and can provide valuable insights into the effectiveness of interventions that may not be feasible to study using RCTs. For example, a recent observational study assessed the effects of prehospital resuscitation with hypertonic solutions on coagulation and fibrinolysis in patients with traumatic hemorrhagic shock. The study included 34 patients who received prehospital resuscitation with hypertonic saline and dextran and the study highlights the potential negative effects of prehospital resuscitation with hypertonic solutions in patients with traumatic hemorrhagic shock, particularly on coagulation and fibrinolysis, and supports the need for further research to determine optimal resuscitation strategies for these patients [ 52 ].
3. Research in Prehospital Care: Major Challenges
Conducting clinical research in prehospital care presents several unique challenges that can impede the quality and feasibility of studies in this field. From limited resources and logistical constraints to ethical considerations and patient safety concerns, prehospital research requires careful planning and execution to ensure valid and reliable results. Understanding the challenges and opportunities in prehospital clinical research is essential for advancing the field and improving patient care. In this context, Figure 1 shows how the feasibility phase is clearly the most important for any clinical researcher and refers to the necessary limited steps (scientific methodology, people management skills, ethics and regulatory compliance, financial dynamics, participant recruitment, information technology & systems, institutional commitment, how to calculate the sample size and power of the study, fixing the objectives/endpoints, etc.), how all of these are organized, and how they communicate operationally (for activities such as financing, patient recruitment, informed consent process, safety and deviation to protocol reporting, investigational medicinal product administration/destruction, staff training, etc.) to design clinical research (from observational to investigational clinical phases) within the action plan. The first questions that needs to be answered before conducting research in prehospital care can be summed up as:
- “What are the aims of the study?”, which encompasses the SMART (Specific, Measurable, Achievable, Relevant, and Time-frame) criteria ( Figure 1 ) and are linked to methodology/statistics.
- “Why, Where and How should the study be conducted?”, which encompasses the FINER (feasible, interesting, novel, ethical, relevant) criteria ( Figure 1 ) and are linked to ethical/safety criteria.

Feasibility criteria needed for the development and success of a clinical research project in prehospital care.
3.1. Limited Resources and Infrastructure
The limited resources available in prehospital care for research pose significant barriers to scientific investigations, which can result in inadequate sample sizes, incomplete data collection, and inconsistent outcomes. These challenges are primarily due to the nature of prehospital care, where medical personnel have to manage emergency situations with limited time, resources, and information [ 53 ]. One of the most significant challenges in conducting research in prehospital care is the limited availability of funding. Prehospital care is often underfunded and receives less attention compared to other areas of healthcare. The lack of funding means that there is a limited pool of resources available to support research in this field. As a result, many researchers struggle to access funding to support their investigations, which can lead to underpowered studies with limited generalizability [ 54 ]. Emergency medical services personnel are often overworked, and their primary focus is on providing immediate medical care to patients [ 55 ]. As a result, it can be challenging to recruit personnel to participate in research studies or to allocate time to collect data. Additionally, prehospital care infrastructure is often decentralized, with services provided by multiple organizations with different protocols and resources [ 56 ]. This can make it difficult to standardize data collection and analysis across different regions, leading to inconsistent outcomes. The lack of access to prehospital care data is another significant barrier to conducting research in this field [ 57 ]. Prehospital care data are often fragmented and dispersed across multiple agencies, making it difficult to collect, integrate, and analyze the data. There are also issues related to data privacy and security that can limit the sharing of data between agencies and organizations, further complicating the research process [ 58 ]. Despite these challenges, there is a growing recognition of the importance of prehospital care research in improving patient outcomes and optimizing the delivery of care.
3.2. Ethical Considerations
Conducting clinical research in the prehospital setting can be challenging due to a number of ethical considerations that need to be taken into account [ 59 , 60 ]. These include issues related to informed consent, confidentiality, privacy, and autonomy. In addition, there are unique challenges related to the prehospital setting, such as time constraints, patient acuity, and the potential for emergencies that can further complicate the ethical considerations of conducting research in this setting. One of the main ethical considerations in prehospital research is informed consent. Patients in the prehospital setting are often in a state of distress, and may not be able to provide informed consent for research participation [ 61 ]. Additionally, there may be situations where informed consent cannot be obtained in a timely manner due to the patient’s condition or the urgency of the situation [ 62 ]. In such cases, alternative methods of obtaining informed consent, such as deferred or waived consent, may need to be considered [ 63 ]. Confidentiality and privacy are also important ethical considerations in prehospital research. Patients’ medical information is sensitive and must be protected. Researchers must take steps to ensure that patients’ data are kept confidential and are not shared with unauthorized individuals. Additionally, patients’ right to privacy must be respected, and their personal information should only be collected for research purposes that are clearly defined and explained. Another important ethical consideration in prehospital research is autonomy. Patients have the right to make decisions about their own care, and their autonomy should be respected in the research process as well. Patients should be given the opportunity to decline participation in research, and their decisions should be respected without negative consequences to their care [ 64 ]. Overall, ethical considerations in prehospital care for research are essential to ensure that research is conducted in a manner that is respectful, safe, and beneficial to patients. It is important for researchers and EMS providers to be aware of these ethical considerations and to take them into account when designing and conducting research studies in the prehospital setting.
3.3. Impact of Time Constraints
Time is of the essence in prehospital care. Emergency medical responders must work quickly and efficiently to provide critical care to patients in emergency situations. Whether it is a heart attack, stroke, or traumatic injury, every second counts in providing life-saving treatment to those in need [ 65 , 66 ]. With time being such a critical factor in prehospital care, emergency medical responders must be able to work under pressure and prioritize their actions to maximize the chances of a positive outcome. In this context, understanding and effectively managing time constraints in prehospital care is essential for saving lives and improving patient outcomes.
Researchers may have a limited amount of time to collect data in prehospital care settings, such as in the case of observing emergency medical responders during real-life situations. They must be able to collect accurate and meaningful data in a short period of time while minimizing the impact on the care provided to patients [ 67 ]. Time constraints can arise due to a variety of factors, such as the need to rapidly stabilize and transport patients, the unpredictability of emergency situations, and the limited availability of EMS resources [ 68 ]. To maximize the benefits of prehospital research, it is essential to address the time constraints on study conduct, data collection, analysis, and privacy associated with conducting studies in this setting [ 69 ].
3.4. Safety Concerns
Prehospital care providers often operate in high-stress environments, where they are required to make rapid and accurate decisions to ensure the best possible outcomes for their patients. One of the most significant challenges in conducting research in prehospital care is ensuring the safety of both patients and prehospital care providers.
Due to the urgent nature of prehospital care, prehospital care providers are frequently exposed to hazardous conditions or violent incidents, which can increase the risk of injury or harm. To address these concerns, researchers must take appropriate measures to ensure the safety and well-being of all individuals involved in prehospital care research. This may involve implementing strict protocols to minimize risks and ensuring that all prehospital care providers receive proper training and education on research protocols [ 70 ]. These protocols and procedures are designed to ensure that patients receive the highest quality of care while minimizing the potential for medical errors or adverse events. These protocols can include procedures for assessing patient needs, determining the appropriate course of treatment, and transporting patients to the hospital safely [ 71 ]. The challenges and risks associated with prehospital care can range from environmental factors such as adverse weather conditions, to patient-specific factors such as the severity of the patient’s condition, the presence of comorbidities, and the patient’s age or decisions [ 72 ]. Additionally, healthcare providers must contend with transportation-related risks, such as accidents or equipment malfunctions [ 73 ].
A significant challenges is the lack of access to medical resources that are typically available in a hospital setting [ 74 ]. This means that prehospital providers must be able to make quick decisions based on the information they have available, often with limited resources at their disposal. This can lead to situations where healthcare providers must rely on their training and experience to provide care in a timely and effective manner. Another challenge in prehospital care is the need for effective communication between healthcare providers [ 75 ]. Prehospital providers must be able to communicate effectively with each other, as well as with hospital staff, to ensure that patients receive the care they need. Communication breakdowns can lead to delays in treatment, misdiagnoses, and other adverse events. Training is also an essential component of ensuring safety in prehospital care. Healthcare providers must undergo extensive training to learn how to assess patient needs, provide appropriate care, and respond to emergencies. Ongoing training and continuing education are also critical to ensuring that healthcare providers stay up-to-date with the latest techniques and best practices [ 76 ]. Another critical factor is the use of appropriate equipment and technology. Healthcare providers rely on a wide range of tools and equipment to provide care, such as defibrillators, oxygen tanks, and stretchers. Ensuring that this equipment is well maintained and functioning correctly is essential to providing safe and effective care [ 77 ].
Healthcare providers must contend with a wide range of challenges and risks when providing care outside of a hospital setting, and safety protocols and procedures are essential to mitigating these risks [ 78 ]. Effective communication, ongoing training, and the use of appropriate equipment and technology are all critical components of ensuring patient safety in prehospital care. By prioritizing safety in every aspect of prehospital care, healthcare providers can improve patient outcomes and provide the highest quality of care possible.
3.5. Data Collection and Analysis
There are several challenges associated with data collection and analysis in prehospital care for research purposes [ 79 ]. These challenges can include difficulties in obtaining informed consent from patients, the need to prioritize patient care over research data collection, and the lack of standardized data collection tools and methods. In addition, prehospital care providers often work in diverse and geographically dispersed settings, which can make it difficult to coordinate data collection efforts and ensure consistency across different study sites. Despite these challenges, there have been significant advances in the field of prehospital care research, with many studies demonstrating the potential benefits of collecting and analyzing prehospital data [ 80 ]. By examining prehospital care interventions and outcomes, researchers can identify areas for improvement, evaluate the effectiveness of new treatments, and inform evidence-based practice guidelines. To address the challenges of data collection and analysis in prehospital care research, it is essential to develop standardized data collection tools and methods that can be easily implemented across different study sites [ 81 ]. Table 2 provides some historical references of evidence-based practice guidelines and standardized protocols for prehospital care, with a focus on their findings and implications for practice [ 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 ].
Examples (in chronological order) of evidence-based practice guidelines and standardized protocols for prehospital care.
| Findings | Target Population | Study Design |
|---|---|---|
| Rottman et al. (1997) compared on-scene time, appropriateness of therapy, and accuracy of paramedic clinical assessments when prehospital care was provided with the use of on-line medical control (OLMC) by EMS-certified nurses from a single base station or by paramedics using chief complaint-based protocols. The use of protocols resulted in small improvements in both on-scene time and the appropriateness of therapeutic decisions, without a change in agreement between paramedic and physician [ ]. | EMS call center | Prospective cohort |
| Holstein et al. (2003) found that training of the emergency team is an effective and efficient intervention to improve quality of treatment and prognosis outcome for patients with type 1 diabetic emergencies [ ]. | Diabetic patients | Prospective population-based study |
| Watts et al. (2004) found that providers who were able to learn and implement the Brain Trauma Foundation (BTF) Guidelines and outcomes in traumatic brain injury patients were significantly improved [ ]. | Traumatic brain injury patients | Prospective observational study |
| Combes et al. (2006) determined the rate of difficult intubations and the factors associated with prehospital difficult airways when a standard protocol for sedation and intubation was applied [ ]. | Tracheal intubation patients | Observational et prospective study |
| Sasson et al. (2009) discussed the operational issues within local EMS systems that may serve as barriers or facilitators to full acceptance of national guidelines for prehospital termination of resuscitation in appropriate circumstances [ ]. | Termination of resuscitation | Qualitative and focus groups study |
| Atary et al. (2010) showed that a standardized regional acute myocardial infarction treatment protocol achieved optimal and uniformly distributed pre-hospital performance in the region ‘Hollands-Midden’, resulting in minimal time delays regardless of area of residence [ ]. | Myocardial injuries patients | Standardized pre-hospital care guidelines applied in practice |
| Rognas et al. (2013) reported a prospective quality control study of the effect on pre-hospital critical care anesthesiologists’ behavior of implementing a standard operating procedure for pre-hospital controlled ventilation [ ]. | Airway management patients | Prospective registry |
| Brandler et al. (2015) found that EMS care providers missed more than a third of stroke cases. Seizures and other atypical presentations contribute significantly to stroke misdiagnosis [ ]. | Prehospital stroke identification methods | Retrospective report |
| Osborne et al. (2015) summarized the United Kingdom (UK) Ambulance Service guidelines for the management of seizures and explored the extent to which these guidelines are evidence-based [ ]. | Management of seizures | Guidelines report |
| Kerner et al. (2017) evaluated how the use of checklists for prehospital emergency care may help to improve adherence to treatment guidelines [ ]. | Checklists in prehospital emergency care | Standard operating procedures study |
| Lenssen et al. (2017) suggested that routine, remote, physician-based, telemedically-delegated (opioid-based) analgesia in trauma and non-trauma emergencies, as applied by paramedics, shows comparable efficacy to analgesia administered by on-scene prehospital EMS physicians [ ]. | Analgesia management patients | Retrospective observational study |
| Pride et al. (2017) discussed the importance of prehospital care delivery and triage in cases of stroke with emergent large vessel occlusion (ELVO) [ ]. | Stroke patients | Guidelines report |
| Rodríguez et al. (2020) found that the use of early warning scores can help the EMS to differentiate traumatic brain injury patients with a high risk of deterioration [ ]. | Traumatic brain injury patients | Prospective cohort |
Finally, close collaboration between prehospital care providers, researchers, and other stakeholders is essential to ensure that data are collected and analyzed in a way that maximizes their value and potential impact on patient care [ 95 ]. Overall, data collection and analysis are critical components of prehospital care research, and the challenges associated with these activities must be carefully considered and addressed to advance the field and improve patient outcomes.
3.6. Selection of a Homogeneous Study Group
One of the crucial challenges encountered in prehospital care research is the difficulty of selecting a homogeneous study group. The unique nature of prehospital care, with its diverse patient population and varying emergency scenarios, presents researchers with numerous complexities when it comes to forming a cohesive and homogeneous study group. Paramedics and emergency medical service providers encounter an extensive range of medical conditions, injuries, and socioeconomic backgrounds among patients they treat. From traumatic injuries to cardiac arrests, respiratory distress to neurological emergencies, the diversity of cases encountered in prehospital care is vast and constantly evolving. This difficulty goes beyond its impact on the validity and generalizability of findings. It also has practical implications for the translation of research outcomes into clinical practice. Healthcare providers rely on evidence-based guidelines derived from rigorous research to inform their decision-making process in emergency situations. If the study groups lack homogeneity, the applicability and relevance of the research findings may be compromised, impeding the development of effective interventions and guidelines for prehospital care. Hence the need to better identify emergency phenotypes [ 96 , 97 ].
4. Discussion
Overall, clinical research in prehospital care is an essential component of improving the quality of care provided to patients in this environment. Despite the challenges, researchers in this field have made significant progress in identifying effective interventions and improving patient outcomes. For example, studies can help to identify best practices for responding to emergencies and treating specific conditions, as well as to develop new technologies and interventions for use in prehospital care. So, continued research and innovation will be critical to ensuring that prehospital care providers have access to evidence-based guidelines and protocols for the treatment of patients in this critical setting.
To address the challenges facing prehospital clinical research, researchers must carefully plan and execute their studies, taking into account the unique constraints and considerations of prehospital care. This may involve working closely with EMS agencies and other healthcare providers to develop study protocols and ensure that studies are conducted in a safe and ethical manner. Additionally, researchers may need to leverage new technologies and data sources to collect and analyze data from prehospital care environments ( Figure 2 ).

EMS and hospital care research activities: balance between challenges (red) and opportunities (green).
4.1. What Are the Solutions to Implement?
There are several solutions that can be implemented to improve clinical research in prehospital care ( Figure 2 ):
Collaboration: Collaboration between prehospital care providers, hospitals, and research institutions can improve the quality of research in prehospital care. This collaboration can lead to better study design, more robust data collection, and stronger analysis.
Technology: Technology can be leveraged to improve data collection and analysis. For example, the use of electronic health records (EHRs) can help standardize data collection and make it easier to share data between prehospital care providers and researchers. Additionally, the use of mobile apps and wearables can provide real-time data that can be used for research purposes.
Training: Prehospital care providers should receive training on research methods and data collection to ensure they are collecting data in a standardized and accurate manner. This training can also help providers understand the importance of research and the impact it can have on patient care.
Funding: Increased funding for prehospital care research can help support larger, more comprehensive studies. This funding can also be used to develop new technologies and research methods to improve data collection and analysis.
Ethics committees: Ethical considerations must be addressed when conducting research in prehospital care. Establishing ethics committees that review research proposals and ensure that patient privacy and safety are maintained can help improve the quality and trustworthiness of research in prehospital care.
Public awareness: Greater public awareness of the importance of prehospital care research can help increase funding and support for this area of study. It can also help improve patient participation in research studies and increase the overall impact of the research.
In practice, collaboration, technology, training, funding, ethics committees, and public awareness can all contribute to improving clinical research in prehospital care. By addressing the challenges and implementing solutions, we can improve our understanding of prehospital care and ultimately improve patient outcomes.
4.2. What Are the Future Opportunities and Perspectives?
As technology advances, new treatments and interventions emerge, and the landscape of prehospital care is continually evolving. Despite the challenges discussed earlier, there are also several opportunities and perspectives for improving clinical research in prehospital care.
First, the use of telemedicine and remote monitoring has the potential to improve data collection and analysis in prehospital care research. Telemedicine enables real-time communication between emergency medical services (EMS) providers and remote medical professionals, allowing for the exchange of vital patient information and coordination of care. Remote monitoring technologies can also collect data on patients’ vital signs and other metrics in real time, providing valuable insights into patient outcomes and treatment effectiveness. The advent of telemedicine has also played a role in the evolution of clinical research in prehospital care. Telemedicine allows EMS providers to consult with physicians and other healthcare providers in real-time, providing access to expert advice and improving patient outcomes [ 98 , 99 ]. One example of the use of telemedicine in prehospital care is the Stroke Prehospital Assessment and Treatment program, which allows EMS providers to transmit brain imaging and other data to stroke specialists in real time, improving the speed and accuracy of diagnosis and treatment. The study from Katz et al. outlines the development and validation of the Cincinnati Prehospital Stroke Severity Scale, which is a key component of this program [ 100 ]. Besides that, there are also other connected technologies that allow for improved data collection and information distribution (movement tracking devices, video and audio recording, running simulations, IT infrastructure, etc.) [ 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 ].
Second, the use of simulation-based training for EMS providers can improve the quality of care delivered in prehospital settings. Simulation-based training allows EMS providers to practice responding to a wide range of emergency scenarios in a safe and controlled environment, improving their skills and confidence in delivering effective care. Moreover, simulation-based training can help researchers evaluate the effectiveness of new interventions and treatments in prehospital care [ 111 ].
Third, the development and use of standardized protocols and guidelines for prehospital care can improve the quality and consistency of care delivered by EMS providers. Standardized protocols can also facilitate the evaluation of new treatments and interventions by providing a consistent framework for comparing outcomes across different settings and populations [ 112 ].
Finally, with the advent of artificial intelligence (AI) technologies, there is great potential to enhance prehospital care by providing faster and more accurate assessments of patient conditions, and enabling more efficient allocation of resources. Prehospital AI refers to the use of machine learning algorithms and other AI techniques to improve emergency medical care before the patient arrives at the hospital. One area where prehospital AI is being explored is in the use of predictive models to help identify patients who are at risk of deteriorating rapidly or experiencing a cardiac arrest. By analyzing patient data such as vital signs, medical history, and demographic information, these models can provide early warnings to EMS personnel, allowing them to intervene quickly and potentially prevent a critical event from occurring. Other applications of prehospital AI include automated triage, diagnosis support, and resource allocation optimization [ 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 ]. Table 3 provides a comprehensive overview of clinical research studies conducted in prehospital care using AI, with a focus on their findings and implications for practice [ 123 , 124 , 125 , 126 , 127 , 128 , 129 ].
Examples of studies (in chronological order) that demonstrate the potential for artificial intelligence to enhance prehospital care.
| Findings | Target Population | Study Design |
|---|---|---|
| Liu et al. (2014) highlighted the potential for machine learning algorithms to improve the accuracy of predicting the need for life-saving interventions in trauma patients, enabling faster and more appropriate treatment for these patients. The hybrid system developed in this study may also serve as a model for integrating machine learning algorithms into clinical decision-making processes [ ]. | Analgesia management patients | Retrospective and prospective cohort study |
| Desautels et al. (2016) highlighted the potential for machine learning models to improve sepsis prediction in the ICU using minimal EHR data, which may be particularly useful in resource-limited settings. However, the study also acknowledges the limitations of using retrospective data and the need for prospective validation of the models in clinical practice [ ]. | Sepsis prediction | Retrospective study |
| Cheng et al. (2021) highlighted the potential for deep learning algorithms to assist sonographers in the detection of abdominal free fluid in Morison’s pouch during sonography in trauma, potentially enabling faster and more accurate diagnosis of abdominal trauma. However, the study also acknowledges the limitations of using retrospective data and the need for prospective validation in clinical practice [ ]. | Abdominal trauma patients | Observational study |
| Fontanellaz et al. (2021) highlighted the potential for deep learning algorithms to assist radiologists in the detection of COVID-19 using chest radiographs, potentially enabling faster and more accurate diagnosis of the disease. However, the study also acknowledges the limitations of using retrospective data and the need for prospective validation of the deep learning diagnostic support system in clinical practice [ ]. | COVID-19 patients | Retrospective study |
| Uchida et al. (2021) demonstrated the feasibility and effectiveness of using machine learning algorithms as a diagnostic support tool in the prediction of stroke probability and type at the prehospital stage, potentially leading to improved stroke care and patient outcomes [ ]. | Stroke-management patients | Retrospective and prospective cohort study |
| Shahi et al. (2021) highlighted the potential for deep learning algorithms to improve decision-making in pediatric blunt solid organ injury, enabling faster and more accurate predictions of the need for massive transfusion, need for operative management, and mortality risk. The use of deep learning algorithms in trauma care may also reduce healthcare costs and improve patient outcomes [ ]. | Pediatric blunt solid organ injury | Retrospective study |
| Chen et al. (2022) highlighted the potential for AI-assisted systems to improve prehospital care by enabling faster and more accurate detection of ST-elevation myocardial infarction, which is crucial for timely intervention and improved patient outcomes. The use of a mini-12-lead ECG device also makes the system more accessible for use in resource-limited settings [ ]. | Myocardial injury patients | Retrospective study |
5. Limitations
The approaches cited in this article have the potential to improve patient outcomes and advance the field of prehospital care. However, as a narrative review, there are some limitations. The first is that rather than focusing on recent research in the last five years, this review has included historical and influential scientific studies that may no longer be relevant in the current setting. It is a limitation that the authors did not begin with a research question when conducting the review; therefore, there was no guide as to what information would be significant and what might be circumstantial. It is a limitation that the authors did not conduct any pooled analyses of the data from the studies summarized. Additionally, searches were only conducted in one database.
6. Conclusions
In conclusion, clinical research in prehospital care is essential for improving patient outcomes and developing evidence-based best practices. Overall, the evolution of clinical research in prehospital care has led to significant advancements in the field, improving outcomes for patients with acute medical emergencies. However, the field of prehospital care presents several challenges to clinical research, including limited resources, ethical considerations, time constraints, safety concerns, and data collection and analysis. Fortunately, there are also several opportunities and perspectives for improving clinical research in this field, including the use of telemedicine and remote monitoring, simulation-based training, standardized protocols and guidelines, and collaborations between EMS providers, hospitals, and academic institutions.
Acknowledgments
The CRU (implemented in Robert Schuman Hospital) and this work were fully developed and funded by the Foundation of Robert Schuman Hospital (FHRS). We thank Georges Heirendt, for his caring support.
Funding Statement
This research received no external funding.
Author Contributions
All authors have substantially contributed to the manuscript design and have approved this final version of the work. J.C. wrote the manuscript and designed the figures with support from C.B. C.B. provided critical feedback and helped shape the final version of the figures and manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.





IMAGES
VIDEO
COMMENTS
FDA's Role in Reviewing Research Proposals for Patient Safety. FDA has a critical role in ensuring the safety of patients in clinical trials studying drugs or biological products for the ...
Summary of the evidence on patient safety : implications for research / Edited by Ashish Jha. 1.Patient care - standards. 2.Health services research. 3.Research - trends. 4.Health priorities. 5.Medical errors. 6.Quality of health care. I.World Health Organization. II.World Alliance for Patient Safety. Research Priority Setting Working Group.
This chapter explains why clinical practice guidelines are needed to improve patient safety and how further research into safety practices can successfully influence the guideline development process. There is a description of the structured process by which guidelines that aim to increase the likelihood of a higher score are created. Proposals are made relating to (a) the live updating of ...
The Making Healthcare Safer (MHS) reports from the Agency for Healthcare Research and Quality (AHRQ) 1-3 have provided reliable information for improving the safety and quality of care for patients since 2001. The reports—providing an analysis of the evidence for various patient safety practices (PSPs)—have served as a consolidated and up-to-date source of information for multiple ...
Background and Objectives for the Systematic Review The critical systematic review and analysis of Patient Safety Practices is an expansion of earlier evidence reports and current listing of Safe Practices for Better Healthcare 2010 Update by the National Quality Forum's (NQF)1. The analysis will assess the evidence of the effectiveness of new safe practices and the adoption of scientific ...
Findings Twenty-three major patient safety topics were examined. Much of the evidence of the outcomes of unsafe care is from developed nations, where prevalence studies demonstrate that between 3% and 16% of hospitalised patients suffer harm from medical care. Data from transitional and developing countries also suggest substantial harm from ...
Patient Safety and Clinical Quality Measures. Another excellent source of information about the capacity of a hospital or other health-care organization to provide a safe environment in which to conduct clinical research is the organization's patient safety and clinical quality performance measurement program.
A multidisciplinary team developed a policy-based approach that provides guidance for using peer review protected information for safety research while maintaining peer review privilege. The approach includes project approval by an ad hoc review committee, signed confidentiality agreements by investigators and study staff, early removal of case identification numbers, standards for maintaining ...
Idea of study was to enhance understanding of the incidence of adverse events as a basis for preventing them. Data on frequency of adverse events related to inappropriate care in hospitals often comes from medical records. However, chart analyses alone may be inadequate to determine the frequency of adverse events.
Plain English summary Patient safety is a growing research area. However, although patients and the public are increasingly involved in clinical research, there is little guidance on how best to involve patients in patient safety research. Here we focus on how patients can contribute to the design of patient safety research. We conducted a workshop with patients as part of a project exploring ...
A recent report from the National Academies of Sciences, Engineering, and Medicine goes as far to claim that "the country is at a relative standstill in patient safety progress," 7 a claim supported by a recent meta-analysis indicating that as many as 1 in 20 patients continue to experience preventable harm. 8 A recent report from the U.S ...
research results. Patient safety researchers within the Patient Safety Portfolio also were recruited to complete the draft tool with their own research in mind. In line with the expert feedback, researchers also recommended adding an action planning section to help the respondent consider practical next steps to help make the plan operational.
The inaugural round of merit review for the Patient-Centered Outcomes Research Institute (PCORI) in November 2012 included patients and other stakeholders, as well as scientists. This article examines relationships among scores of the 3 reviewer types, changes in scoring after in-person discussion, and the effect of inclusion of patient and ...
Maintaining the highest levels of patient safety is a priority of healthcare organisations. However, although considerable resources are invested in improving safety, patients still suffer avoidable harm. The aims of this study are: (1) to examine the extent, range, and nature of patient safety research activities carried out in the Republic of Ireland (RoI); (2) make recommendations for ...
Background In recent years, patient and public involvement (PPI) in research has significantly increased; however, the reporting of PPI remains poor. The Guidance for Reporting Involvement of Patients and the Public (GRIPP2) was developed to enhance the quality and consistency of PPI reporting. The objective of this systematic review is to identify the frequency and quality of PPI reporting in ...
Patient safety cannot be fully achieved without healthcare worker safety and well-being. This NOFO will contribute to AHRQ's goal of reinvigorating the patient safety movement by adding fresh perspectives and insights of healthcare professionals to efforts to improve patient safety. Patient safety requires a foundation of safe and healthy ...
Why It MattersTo develop a total systems approach to advance patient safety, health care organizations must commit to fully engaging patients and families in every aspect of care at all levels. In September 2020, the Institute for Healthcare Improvement (IHI)-convened National Steering Committee for Patient Safety (NSC) announced the release of Safer Together: A National Action Plan to Advance ...
In doing so, we have not just studied patient engagement in patient safety but produced measures, reporting systems, interventions and training that have the potential to reduce harm and improve the health of patients. We established comprehensive and effective systems for involving patients and the public in codesign and coproduction of applied health research and evaluated the impact of this ...
Our national review and response work is a key part of the NHS patient safety strategy, which estimate 160 lives and £13.5 million in treatment costs are saved every year from the resulting advice, guidance and other outputs. Other parts of the strategy are also interlinked and support this work such as the roll out of the new national patient ...
Agency for Healthcare Research and Quality, Rockville, MD. All submissions will be reviewed by at least two AHRQ patient safety subject matter expert staff who will score them based on the review criteria and provide a brief comment about the submission. The scores/comments on submissions will be compiled and a ranked summary provided to AHRQ ...
The unifying theme of successful nursing research proposals is that the author(s) observed a problem, did research to make sure the observation was not personal bias, and then wrote to describe not only the problem, but a potential solution..... Writing a grant proposal can be a daunting task, but with the right guidance and information, you can create an effective proposal that will help you ...
Prehospital care plays a critical role in improving patient outcomes, particularly in cases of time-sensitive emergencies such as trauma, cardiac failure, stroke, bleeding, breathing difficulties, systemic infections, etc. In recent years, there has been a growing interest in clinical research in prehospital care, and several challenges and opportunities have emerged. There is an urgent need ...
2. Research in Prehospital Care: State of the Art. Prehospital care is a rapidly evolving field, with ongoing research aiming to improve patient outcomes and optimize emergency medical services [].Current clinical research studies in prehospital care are investigating a range of topics, including airway management, hemorrhage control, pain management, and stroke care [24,25].