- Reference Manager
- Simple TEXT file

People also looked at
Mini review article, past, present, and future of dna typing for analyzing human and non-human forensic samples.

- 1 Department of Biological Sciences, Florida International University, Miami, FL, United States
- 2 International Forensic Research Institute, Florida International University, Miami, FL, United States
Forensic DNA analysis has vastly evolved since the first forensic samples were evaluated by restriction fragment length polymorphism (RFLP). Methodologies advanced from gel electrophoresis techniques to capillary electrophoresis and now to next generation sequencing (NGS). Capillary electrophoresis was and still is the standard method used in forensic analysis. However, dependent upon the information needed, there are several different techniques that can be used to type a DNA fragment. Short tandem repeat (STR) fragment analysis, Sanger sequencing, SNapShot, and capillary electrophoresis-single strand conformation polymorphism (CE-SSCP) are a few of the techniques that have been used for the genetic analysis of DNA samples. NGS is the newest and most revolutionary technology and has the potential to be the next standard for genetic analysis. This review briefly encompasses many of the techniques and applications that have been utilized for the analysis of human and nonhuman DNA samples.
Introduction
Forensic genetics applies genetic tools and scientific methodology to solve criminal and civil litigations ( Editorial, 2007 ). Locard’s Exchange Principle states that every contact leaves a trace, making any evidence a key component in forensic analysis. Biological evidence can comprise of cellular material or cell-free DNA from crime scenes, and as technologies improved, genetic methodologies were expanded to include human and non-human forensic analyses. Although these methodologies can be used for any genome, the prevalence of databases and standard guidelines has allowed human DNA typing to become the gold standard. This review will discuss the historical progression of DNA analysis techniques, strengths and limitations, and their possible forensic applications applied to human and non-human genetics.
Methodologies to Detect Genetic Differences in Humans Is the “Gold Standard”
“dna fingerprinting”: the beginning of human forensic dna typing.
“DNA fingerprinting” was serendipitously discovered in 1984 ( Jeffreys, 2013 ). What they found propelled DNA “fingerprinting,” or DNA typing, to the forefront in legal cases to become the “gold standard” for forensic genetics in a court of law. Jeffreys first used restriction enzymes to fragment DNA, a method in which restriction endonucleases (RE) enzymes fragment the genomic DNA, producing restriction fragment length polymorphisms (RFLP) patterns. Since each RE recognizes specific DNA sequences to enzymatically cut the DNA, then inherent differences between gene sequences, due to evolutionary changes, will produce different fragment lengths. If the enzyme site is present in one individual but has changed in a different individual, the fragment lengths, once separated and visualized, will differ. While this technique was useful for some studies, Jeffreys did not find it useful for his particular genetic studies. Subsequently when working with the myoglobin gene in seals, he discovered that a short section of that gene – a minisatellite – was conserved and when isolated and cloned could be used to detect inherited genetic lineages as well as individualize a subject. Fragment length separation by electrophoresis, followed by transfer to Southern blot membranes, hybridized with a specific or non-specific complementary isotopic DNA probe, allowed for DNA fragments visualization ( Jeffreys et al., 1985b ). Upon careful analysis, Jeffreys determined that the fragments represented different combinations of DNA repetitive elements, unique to each individual, and could be used to better identify individuals or kinship lineages ( Jeffreys et al., 1985b ). Jeffreys’ technology was used in several subsequent paternity, immigration, and forensic genetics cases ( Gill et al., 1985 ; Jeffreys et al., 1985a ; Evans, 2007 ). This was just the beginning of a whole new era in DNA typing.
Restriction Fragment Length Polymorphism (RFLP) Analysis: The Past
After Jeffreys’ discoveries, many DNA analyses methods involving electrophoretic fragment separation were discovered. Many were based on RFLP principles ( Botstein et al., 1980 ), e.g., amplified fragment length polymorphism (AFLP) ( Vos et al., 1995 ), and terminal restriction fragment length polymorphism (TRFLP) ( Liu et al., 1997 ). Others like length heterogeneity- polymerase chain reaction (LH-PCR) ( Suzuki et al., 1998 ) were based on intrinsic insertions and deletions of bases within specific genetic markers. Sanger sequencing ( Sanger and Coulson, 1975 ), and single-strand conformational polymorphism (SSCP) analysis ( Orita et al., 1989 ), while separated by electrophoresis, are theoretically based on single base sequence changes rather than insertions, deletions or RE site differences. While Jeffrey’s DNA fingerprinting method provided a very high power of discrimination, the main limitations were it was very time-consuming and required at least 10–25 ng of DNA to be successful ( Wyman and White, 1980 ). With these limitations, RFLP was not always feasible for forensic cases.
Short Tandem Repeat (STR) Analysis: The Present
The polymerase chain reaction (PCR) was discovered by Kary Mullis in 1985 and helped transform all DNA analyses ( Mullis et al., 1986 ). The current standard for human DNA typing is short tandem repeat (STR) analysis ( McCord et al., 2019 ). This method amplifies highly polymorphic, repetitive DNA regions by PCR and separates them by amplicon length using capillary electrophoresis. These inheritable markers are a series of 2–7 bases tandemly repeated at a specific locus, often in non-coding genetic regions. Forensic STRs are commonly tetranucleotide repeats ( Goodwin et al., 2011 ), chosen because of their technical robustness and high variation among individuals ( Kim et al., 2015 ). The combined DNA index system (CODIS) uses 20 core STR loci, expanded in 2017, and several commercial kits are available that contain these STRs ( Oostdik et al., 2014 ; Ludeman et al., 2018 ). After amplification, different fluorochromes on each primer set allow for visualization of STRs after deconvolution, creating a STR profile consisting of a combination of genotypes ( Gill et al., 2015 ). This method has become the gold standard for human forensics. Its greatest strength is the standardization of loci used by all laboratories and an extremely large searchable database of genetic profiles. However, some limitations and challenges are faced when dealing with highly degraded or low template DNA samples. To overcome these technical challenges, standardized mini-STR kits have been developed which use shorter versions of the core STRs and can be used in the same manner for forensic cases ( Butler et al., 2007 ; Constantinescu et al., 2012 ). Keep in mind, DNA typing of humans – a single species – is the gold standard because of (a) the concerted scientific effort to standardize loci to analyze, (b) the development of commercial kits that can produce the same results regardless of instrumentation or laboratory performing the work, (c) a compatible and very large database that provides allelic frequencies for all sub-populations of humans, (d) standardized statistical methods used to report the results and (e) many court cases that have accepted human DNA typing evidence in a court of law – setting the precedent for future cases to use DNA typing results.
Methodologies to Detect Genetic Differences in Non-Humans: Past and Present
Amplified fragment length polymorphism (aflp) analysis.
It was not long before scientists realized that non-human DNA could provide informative genetic evidence in forensic cases. Applications include bioterrorism, wildlife crimes, human identification through skin microorganisms, and so much more ( Arenas et al., 2017 ). Since large quantities of biological materials are frequently not found at crime scenes, successful RFLP analyses were unlikely. Combining restriction enzymes and PCR technology, a process known as AFLP analysis ( Vos et al., 1995 ), became a method for DNA fingerprinting using minute amounts of unknown sourced DNA. REs digest genomic DNA, then ligation of a constructed adapter sequence to the ends of all fragments allows the annealing of primers designed to recognize the adaptor sequences. Subsequent amplification generates many amplicons ranging in length when separated and visualized in an electropherogram or on a gel ( Vos et al., 1995 ; Butler, 2012 ). AFLP markers for plant forensic DNA typing have been used because it provides high discrimination, requires only small amounts of DNA and the method is reproducible, all forensically important characteristics ( Datwyler and Weiblen, 2006 ). For example, since most cannabis is clonally propagated, subsequent generations will have identical genetic profiles as seen with AFLP ( Miller Coyle et al., 2003 ), providing useful intelligence links back to the source population. But there are significant variation between cultivars and within populations, so not having a standard database representing the species’ diversity for statistical comparisons greatly limits the method’s applicability. Another forensic example of its use is differentiating between marijuana and hemp, two morphologically and genetically similar plants, one an illicit drug while the other is not. In this study, three populations of hemp and one population of marijuana were analyzed with AFLP producing 18 bands that were specific to hemp samples. Additionally, 51.9% of molecular variance occurred within populations indicating these polymorphisms were useful for forensic individualization ( Datwyler and Weiblen, 2006 ).
Terminal Restriction Fragment Length Polymorphism (TRFLP) Analysis
As a result of the anthrax letter attacks of 2001, microbial forensics came to the forefront ( Schmedes et al., 2016 ), a discipline that combines multiple scientific specialties – microbiology, genetics, forensic science, and analytical chemistry. One method used to compare microbial communities is TRFLP ( Liu et al., 1997 ; Osborn et al., 2000 ; Butler, 2012 ). With this method, the DNA is amplified using “universal,” highly conserved primer sequences shared across all organisms of interest, i.e., the 16S rRNA genes in bacteria and Archaea, and then uses REs to fragment the PCR products ( Table 1 ). Separated by capillary electrophoresis, only the fluorescently tagged terminal restricted fragments are visualized ( Mrkonjic Fuka et al., 2007 ), reducing the profile complexity and providing high discrimination. TRFLP has been used to characterize complex microbial communities for forensic applications by linking the similarity of the amplicon patterns generated from the intrinsic soil communities to the evidence from a crime scene ( Meyers and Foran, 2008 ; Habtom et al., 2017 ). This method does provide a distinct pattern reflective of the microbial community, useful for forensic genetics but the method does not provide any sequence information. Another limitation is no standardization of which primer pairs or REs are used, making direct comparisons between studies difficult. This lack of standardization also hinders the development of a database for species identification. Additionally, the method is time-consuming due to the additional step of restriction digestion and the possibility of incomplete enzymatic digestion can complicate the interpretation of results ( Osborn et al., 2000 ; Moreno et al., 2006 ).
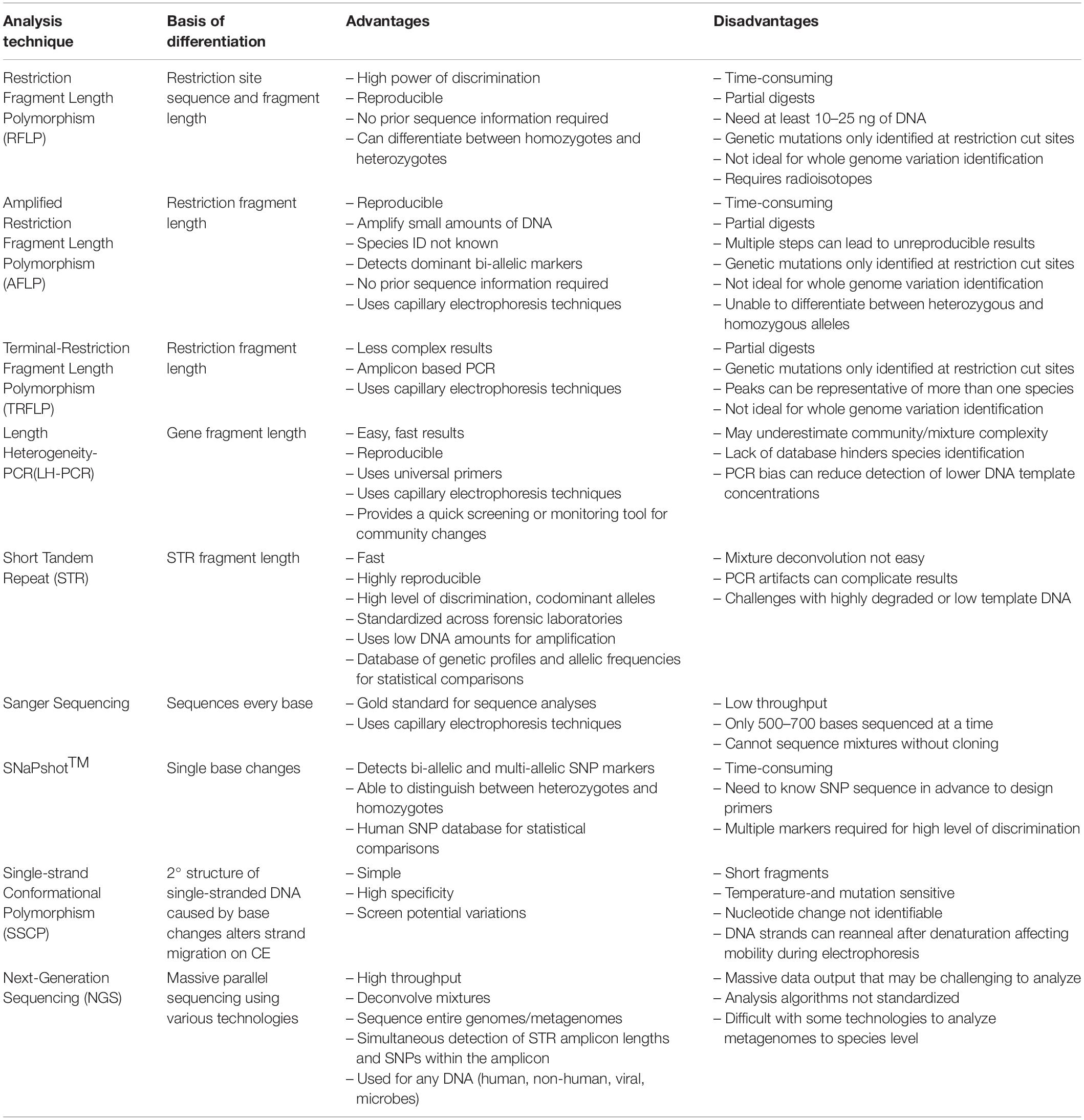
Table 1. The basis of differentiation, advantages, and disadvantages of past and current technologies.
Length Heterogeneity-Polymerase Chain Reaction (LH-PCR)
Another methodology has been used to characterize microbial communities is length heterogeneity- polymerase chain reaction (LH-PCR) ( Suzuki et al., 1998 ). Universal primers complementary to highly conserved domains within genomes are used to amplify hypervariable sequences within specific sequence domains. The 16S/18S rRNA genes, the chloroplast genes or Internal Transcribed Spacer (ITS) regions are commonly used. This technique is based on the natural sequence length variation due to insertions and deletions of bases that occur within a domain ( Moreno et al., 2006 ). It has been used to characterize microbial communities for forensic soil applications where a correlation between geographic location and microbial profiles has proven to be more discriminating than elemental soil analysis ( Moreno et al., 2006 , 2011 ; Damaso et al., 2018 ). With LH-PCR, metagenomic DNA extracted from the soil is amplified using fluorescently labeled universal primers with amplicon peaks within the electropherogram representing the minimum diversity within the community. However, specific sequence information is not known as many peaks of the same size could represent more than one species, thereby masking the community’s actual taxonomic diversity. A recent study showed the intrinsic diversity of a microbial mat, masked by LH-PCR, could be further resolved by the inherent sequence differences using capillary electrophoresis-single strand conformational polymorphism (CE-SSCP) analysis ( Damaso et al., 2014 ) and confirmed by sequencing. The advantage of LH-PCR is it is a fast and reproducible method that can correlate geographical areas to microbial patterns with bioinformatics ( Damaso et al., 2018 ); but a soil database would need to be developed to be useful beyond specific geographical areas.
Methodologies to Detect Intersequence Variation: The Past and Present
Sanger sequencing and single nucleotide polymorphism (snp) variation.
The basis of genomic differentiation is the intrinsic order of base pairs within a region that can be evaluated by sequencing. Sanger sequencing has been the gold standard since the 1970s ( Sanger and Coulson, 1975 ). Sanger sequencing was termed the gold standard because of the ability for single base pair resolution allowing for full sequence information to be determined. Robust and extensive databases are also readily available for comparison, i.e., GenBank, to identify an organism. However, it does have some limitations such as the short length (<500–700 bp) and it cannot sequence mixtures of organisms, for example, without cloning, so it would not be useful for sequencing complex microbial communities without intense time, effort and cost.
Other approaches use the ability to identify intrinsic single base sequence variation using single nucleotide polymorphisms (SNPs) within four forensically relevant SNP classes: identity-testing, ancestry informative, phenotype informative, and lineage informative. SNPs are particularly useful when typing degraded DNA or increasing the amount of genetic information retrieved from a sample ( Budowle and van Daal, 2008 ; Goodwin et al., 2011 ). SNaPshot TM is a commercially available SNP kit that can identify known SNPs using single base extension (SBE) technology ( Daniel et al., 2015 ; Fondevila et al., 2017 ). Wildlife forensics has used SNaPshot TM to identify endangered or trafficked species that are illegally poached to support criminal prosecutions. Elephant species identification from ivory and ivory products ( Kitpipit et al., 2017 ) or differentiating wolf species from dog subspecies ( Jiang et al., 2020 ) are both examples of SNaPshot TM assays developed for wildlife forensics. By using species-specific SNPs, the samples could be identified. But yet again, the limitation becomes the need for species-specific reference databases and the monumental task of developing a robust database for each species. Human SNPs databases with allele frequencies, as seen in dbSNP, however, are available making their forensic application more feasible in some cases.
Next-Generation Sequencing: The Present
Massively parallel sequencing (MPS) or next-generation sequencing (NGS) allows for mixtures of genomes of any species to be sequenced in one analysis ( Ansorge, 2009 ). This technology can sequence thousands of genomic regions simultaneously, allowing for whole-genome, metagenomic sequencing or targeted amplicon sequencing ( Gettings et al., 2016 ). Various NGS technologies are available each using slightly different technologies to sequence DNA ( Heather and Chain, 2016 ). Verogen has developed kits explicitly for human forensic genomics using Illumina’s MiSeq FGx system ( Guo et al., 2017 ; Moreno et al., 2018 ). The FBI recently approved DNA profiles generated by Verogen forensic technology to be uploaded into the National DNA Index System (NDIS) ( SWGDAM, 2019 ), making it the first NGS technology approved for NDIS.
Short tandem repeat mixture deconvolution, degraded, low template samples, and even microbial community samples are just a few of the potential NGS applications for forensic genomics and metagenomics ( Borsting and Morling, 2015 ). In human STR analyses, the greatest challenge is mixture deconvolution. NGS technology presents an increased power of discrimination of STR alleles using the intrinsic SNPs genetic microhaplotypes – a combination of 2–4 closely linked SNPs within an allele ( Kidd et al., 2014 ; Pang et al., 2020 ). However, the acceptance of analyses programs to deconvolve mixtures has not been standardized to the same level as it has for STRs.
Microbes are the first responders to changes in any environment because they are rapidly affected by the availability of nutrients and their intrinsic habitats. This makes them excellent indicators for studies investigating post-mortem interval (PMI) or as an indicator of soil geographical provenance ( Giampaoli et al., 2014 ; Finley et al., 2015 ). In decaying organisms, shifts in epinecrotic communities or the thanatomicrobiome are becoming increasingly critical components in investigating PMI ( Javan et al., 2016 ). Sequencing of the thanatomicrobiome revealed the Clostridium spp. varied during different stages human decomposition, the “Postmortem Clostridium Effect” (PCE), providing a time signature of the thanatomicrobiome, which could only have been uncovered through NGS ( Javan et al., 2017 ). However, the lack of consensus in analyses techniques must be addressed before NGS methodologies can be introduced into the justice system ( Table 1 ).
Future Directions and Concluding Remarks
Forensic DNA typing has progressed quickly within a short timeframe ( Figure 1 ), which can be attributed to the many advancements in molecular biology technologies. As these techniques advance, forensic scientists will analyze more atypical forms of evidence to answer questions deemed unresolvable with traditional DNA analyses. For example, epigenetics and DNA methylation markers have been proposed to estimate age, determine the tissue type, and even differentiate between monozygotic twins ( Vidaki and Kayser, 2018 ). However, since epigenetic patterns are also influenced by environmental factors, they can be dynamic, and a number of confounding factors have the potential to affect predictions and must be taken into account when preparing prediction models (i.e., age estimation). Additionally, phenotype informative SNPs across the genome can infer physical characteristics like eye, hair, and skin color, even age, from an unknown source of DNA retrieved from a crime scene. But this technology could pose an “implicit bias” toward minorities, especially in “societies where racism and xenophobia are now on the rise” ( Schneider et al., 2019 ) if not ethically and judicially implemented. With the increased sensitivity of NGS, low biomass samples from environmental DNA (eDNA) – DNA from soil, water, air – can complement and enhance intelligence gathering or provenance in criminal cases. Pollen and dust are two types of eDNA recently explored for their future forensic potential ( Alotaibi et al., 2020 ; Young and Linacre, 2021 ). However, if used in criminal investigations where the eDNA collected has had interaction with other environments, there must be some protocol or quality control established to account for variability that is likely to occur. This makes the prudent validation of this type of DNA analysis, essential. Limitations also arise due to lack of a database for comparison of samples and statistical analyses to evaluate the strength of a match like in the analysis of human STR profiles.
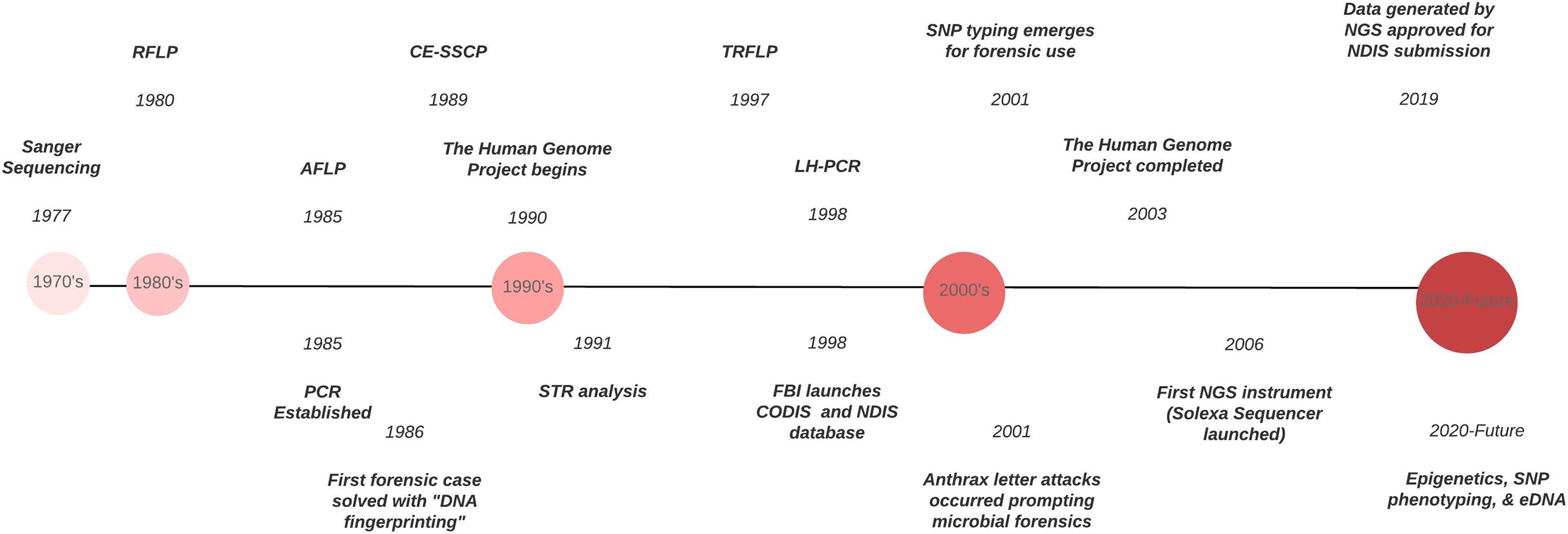
Figure 1. Timeline of the evolution of DNA typing technologies from the 1970’s to the present.
DNA has long been the gold standard in human forensic analysis because of the standardization of DNA markers, databases and statistical analyses. It has laid the foundation for these promising new technologies that will significantly enhance intelligence gathering and species identification – human and non-human – in forensic cases. In order for these methodologies to be useful in criminal investigations, they must adhere to the legal standards such as the Frye or Daubert Standards which determines if an expert testimony or evidence is admissible in court. A method can be deemed acceptable if it follows forensic guidelines set by organizations such as NIST’s Organization Scientific Area Committees (OSAC), Society for Wildlife Forensic Sciences (SWFS), Scientific Working Group on DNA Analysis Methods (SWGDAM), and the International Society for Forensic Genetics (ISFG) ( Linacre et al., 2011 ) just to name a few. These committees provide the guidelines for validation, interpretation, and quality assurance, all necessary components for DNA analysis. The US Fish and Wildlife forensic laboratory has standardized protocols for crimes against federally endangered or threatened species 1 . However, the more common limiting factors in the development of standard guidelines of non-human forensic genetic analyses across different state laboratories are the lack of consensus in methodologies, supporting allelic databases and standardized statistical analyses. Addressing those issues could lay the foundation for non-human analyses to be on par with human analyses.
Author Contributions
DJ designed and wrote the manuscript. DM edited and contributed to the writing of the manuscript. Both authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to acknowledge the invitation by the editors to contribute to this special edition. DJ was supported by the Florida Education Fund’s McKnight Doctoral Fellowship.
- ^ https://www.fws.gov/lab/about.php
Alotaibi, S. S., Sayed, S. M., Alosaimi, M., Alharthi, R., Banjar, A., Abdulqader, N., et al. (2020). Pollen molecular biology: Applications in the forensic palynology and future prospects: A review. Saudi J. Biol. Sci. 27, 1185–1190. doi: 10.1016/j.sjbs.2020.02.019
PubMed Abstract | CrossRef Full Text | Google Scholar
Ansorge, W. J. (2009). Next-generation DNA sequencing techniques. N. Biotechnol. 25, 195–203. doi: 10.1016/j.nbt.2008.12.009
Arenas, M., Pereira, F., Oliveira, M., Pinto, N., Lopes, A. M., Gomes, V., et al. (2017). Forensic genetics and genomics: Much more than just a human affair. PLoS Genet. 13:e1006960. doi: 10.1371/journal.pgen.1006960
Borsting, C., and Morling, N. (2015). Next generation sequencing and its applications in forensic genetics. Forensic Sci. Int. Genet. 18, 78–89. doi: 10.1016/j.fsigen.2015.02.002
Botstein, D., White, R. L., Skolnick, M., and Davis, R. W. (1980). Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 32, 314–331.
Google Scholar
Budowle, B., and van Daal, A. (2008). Forensically relevant SNP classes. Biotechniques 60:610. doi: 10.2144/000112806
Butler, J. M. (2012). “Non-human DNA,” in Advanced Topics in Forensic DNA Typing , ed. J. M. Butler (San Diego: Academic Press), 473–495.
Butler, J. M., Coble, M. D., and Vallone, P. M. (2007). STRs vs. SNPs: thoughts on the future of forensic DNA testing. Forensic Sci. Med. Pathol. 3, 200–205. doi: 10.1007/s12024-007-0018-1
Constantinescu, C. M., Barbarii, L. E., Iancu, C. B., Constantinescu, A., Iancu, D., and Girbea, G. (2012). Challenging DNA samples solved with MiniSTR analysis. Brief overview. Rom. J. Leg. Med. 20, 51–56. doi: 10.4323/rjlm.2012.51
CrossRef Full Text | Google Scholar
Damaso, N., Martin, L., Kushwaha, P., and Mills, D. (2014). F-108 polymer and capillary electrophoresis easily resolves complex environmental DNA mixtures and SNPs. Electrophoresis 35, 3208–3211. doi: 10.1002/elps.201400069
Damaso, N., Mendel, J., Mendoza, M., von Wettberg, E. J., Narasimhan, G., and Mills, D. (2018). Bioinformatics Approach to Assess the Biogeographical Patterns of Soil Communities: The Utility for Soil Provenance. J. Forensic. Sci. 63, 1033–1042. doi: 10.1111/1556-4029.13741
Daniel, R., Santos, C., Phillips, C., Fondevila, M., van Oorschot, R. A., Carracedo, A., et al. (2015). A SNaPshot of next generation sequencing for forensic SNP analysis. Forensic Sci. Int. Genet. 14, 50–60. doi: 10.1016/j.fsigen.2014.08.013
Datwyler, S. L., and Weiblen, G. D. (2006). Genetic variation in hemp and marijuana (Cannabis sativa L.) according to amplified fragment length polymorphisms. J. Forensic Sci. 51, 371–375. doi: 10.1111/j.1556-4029.2006.00061.x
Editorial. (2007). Launching Forensic Science International daughter journal in 2007: Forensic Science International: Genetics. Forensic Sci. Int. Genet. 1, 1–2. doi: 10.1016/j.fsigen.2006.10.001
Evans, C. (2007). The Casebook of Forensic Detection: How Science Solved 100 of the World’s Most Baffling Crimes. New York, NY: Berkley Books.
Finley, S. J., Benbow, M. E., and Javan, G. T. (2015). Potential applications of soil microbial ecology and next-generation sequencing in criminal investigations. Appl. Soil. Ecol. 88, 69–78. doi: 10.1016/j.apsoil.2015.01.001
Fondevila, M., Borsting, C., Phillips, C., de la Puente, M., Consortium, E. N., Carracedo, A., et al. (2017). Forensic SNP genotyping with SNaPshot: Technical considerations for the development and optimization of multiplexed SNP assays. Forensic Sci. Rev. 29, 57–76.
Gettings, K. B., Kiesler, K. M., Faith, S. A., Montano, E., Baker, C. H., Young, B. A., et al. (2016). Sequence variation of 22 autosomal STR loci detected by next generation sequencing. Forensic Sci. Int. Genet. 21, 15–21. doi: 10.1016/j.fsigen.2015.11.005
Giampaoli, S., Berti, A., Di Maggio, R. M., Pilli, E., Valentini, A., Valeriani, F., et al. (2014). The environmental biological signature: NGS profiling for forensic comparison of soils. Forensic Sci. Int. 240, 41–47. doi: 10.1016/j.forsciint.2014.02.028
Gill, P., Haned, H., Bleka, O., Hansson, O., Dorum, G., and Egeland, T. (2015). Genotyping and interpretation of STR-DNA: Low-template, mixtures and database matches-Twenty years of research and development. Forensic Sci. Int. Genet. 18, 100–117. doi: 10.1016/j.fsigen.2015.03.014
Gill, P., Jeffreys, A. J., and Werrett, D. J. (1985). Forensic application of DNA ‘fingerprints’. Nature 318, 577–579. doi: 10.1038/318577a0
Goodwin, W., Linacre, A., and Hadi, S. (2011). “An introduction to forensic genetics.” 2nd ed. West Sussex, UK: Wiley-Blackwell, 53–62.
Guo, F., Yu, J., Zhang, L., and Li, J. (2017). Massively parallel sequencing of forensic STRs and SNPs using the Illumina((R)) ForenSeq DNA Signature Prep Kit on the MiSeq FGx Forensic Genomics System. Forensic Sci. Int. Genet. 31, 135–148. doi: 10.1016/j.fsigen.2017.09.003
Habtom, H., Demaneche, S., Dawson, L., Azulay, C., Matan, O., Robe, P., et al. (2017). Soil characterisation by bacterial community analysis for forensic applications: A quantitative comparison of environmental technologies. Forensic Sci. Int. Genet. 26, 21–29. doi: 10.1016/j.fsigen.2016.10.005
Heather, J. M., and Chain, B. (2016). The sequence of sequencers: The history of sequencing DNA. Genomics 107, 1–8. doi: 10.1016/j.ygeno.2015.11.003
Javan, G. T., Finley, S. J., Abidin, Z., and Mulle, J. G. (2016). The Thanatomicrobiome: A Missing Piece of the Microbial Puzzle of Death. Front. Microbiol. 7:225. doi: 10.3389/fmicb.2016.00225
Javan, G. T., Finley, S. J., Smith, T., Miller, J., and Wilkinson, J. E. (2017). Cadaver Thanatomicrobiome Signatures: The Ubiquitous Nature of Clostridium Species in Human Decomposition. Front. Microbiol. 8:2096. doi: 10.3389/fmicb.2017.02096
Jeffreys, A. J. (2013). The man behind the DNA fingerprints: an interview with Professor Sir Alec Jeffreys. Investig. Genet. 4:21. doi: 10.1186/2041-2223-4-21
Jeffreys, A. J., Brookfield, J. F., and Semeonoff, R. (1985a). Positive identification of an immigration test-case using human DNA fingerprints. Nature 317, 818–819. doi: 10.1038/317818a0
Jeffreys, A. J., Wilson, V., and Thein, S. L. (1985b). Hypervariable ‘minisatellite’ regions in human DNA. Nature 314, 67–73. doi: 10.1038/314067a0
Jiang, H. H., Li, B., Ma, Y., Bai, S. Y., Dahmer, T. D., Linacre, A., et al. (2020). Forensic validation of a panel of 12 SNPs for identification of Mongolian wolf and dog. Sci. Rep. 10:13249. doi: 10.1038/s41598-020-70225-5
Kidd, K. K., Pakstis, A. J., Speed, W. C., Lagace, R., Chang, J., Wootton, S., et al. (2014). Current sequencing technology makes microhaplotypes a powerful new type of genetic marker for forensics. Forensic Sci. Int. Genet. 12, 215–224. doi: 10.1016/j.fsigen.2014.06.014
Kim, Y. T., Heo, H. Y., Oh, S. H., Lee, S. H., Kim, D. H., and Seo, T. S. (2015). Microchip-based forensic short tandem repeat genotyping. Electrophoresis 36, 1728–1737. doi: 10.1002/elps.201400477
Kitpipit, T., Thongjued, K., Penchart, K., Ouithavon, K., and Chotigeat, W. (2017). Mini-SNaPshot multiplex assays authenticate elephant ivory and simultaneously identify the species origin. Forensic Sci. Int. Genet. 27, 106–115. doi: 10.1016/j.fsigen.2016.12.007
Linacre, A., Gusmão, L., Hecht, W., Hellmann, A. P., Mayr, W. R., Parson, W., et al. (2011). ISFG: Recommendations regarding the use of non-human (animal) DNA in forensic genetic investigations. Forensic Sci. Int. Genet. 5, 501–505. doi: 10.1016/j.fsigen.2010.10.017
Liu, W. T., Marsh, T. L., Cheng, H., and Forney, L. J. (1997). Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl. Environ. Microbiol. 63, 4516–4522. doi: 10.1128/AEM.63.11.4516-4522.1997
Ludeman, M. J., Zhong, C., Mulero, J. J., Lagace, R. E., Hennessy, L. K., Short, M. L., et al. (2018). Developmental validation of GlobalFiler PCR amplification kit: a 6-dye multiplex assay designed for amplification of casework samples. Int. J. Legal. Med. 132, 1555–1573. doi: 10.1007/s00414-018-1817-5
McCord, B. R., Gauthier, Q., Cho, S., Roig, M. N., Gibson-Daw, G. C., Young, B., et al. (2019). Forensic DNA Analysis. Anal. Chem. 91, 673–688. doi: 10.1021/acs.analchem.8b05318
Meyers, M. S., and Foran, D. R. (2008). Spatial and temporal influences on bacterial profiling of forensic soil samples. J. Forensic Sci. 53, 652–660. doi: 10.1111/j.1556-4029.2008.00728.x
Miller Coyle, H., Palmbach, T., Juliano, N., Ladd, C., and Lee, H. C. (2003). An overview of DNA methods for the identification and individualization of marijuana. Croat Med. J. 44, 315–321.
Moreno, L. I., Galusha, M. B., and Just, R. (2018). A closer look at Verogen’s Forenseq DNA Signature Prep kit autosomal and Y-STR data for streamlined analysis of routine reference samples. Electrophoresis 39, 2685–2693. doi: 10.1002/elps.201800087
Moreno, L. I., Mills, D. K., Entry, J., Sautter, R. T., and Mathee, K. (2006). Microbial metagenome profiling using amplicon length heterogeneity-polymerase chain reaction proves more effective than elemental analysis in discriminating soil specimens. J. Forensic Sci. 51, 1315–1322. doi: 10.1111/j.1556-4029.2006.00264.x
Moreno, L. I., Mills, D., Fetscher, J., John-Williams, K., Meadows-Jantz, L., and McCord, B. (2011). The application of amplicon length heterogeneity PCR (LH-PCR) for monitoring the dynamics of soil microbial communities associated with cadaver decomposition. J. Microbiol. Methods 84, 388–393. doi: 10.1016/j.mimet.2010.11.023
Mrkonjic Fuka, M., Gesche Braker, S. H., and Philippot, L. (2007). “Molecular Tools to Assess the Diversity and Density of Denitrifying Bacteria in Their Habitats,” in Biology of the Nitrogen Cycle , eds H. Bothe, S. J. Ferguson, and W. E. Newton (Amsterdam: Elsevier), 313–330.
Mullis, K., Faloona, F., Scharf, S., Saiki, R., Horn, G., and Erlich, H. (1986). Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb. Symp. Quant. Biol. 1, 263–273. doi: 10.1101/sqb.1986.051.01.032
Oostdik, K., Lenz, K., Nye, J., Schelling, K., Yet, D., Bruski, S., et al. (2014). Developmental validation of the PowerPlex((R)) Fusion System for analysis of casework and reference samples: A 24-locus multiplex for new database standards. Forensic Sci. Int. Genet. 12, 69–76. doi: 10.1016/j.fsigen.2014.04.013
Orita, M., Iwahana, H., Kanazawa, H., Hayashi, K., and Sekiya, T. (1989). Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc. Natl. Acad. Sci. U S A 86, 2766–2770.
Osborn, A. M., Moore, E. R., and Timmis, K. N. (2000). An evaluation of terminal-restriction fragment length polymorphism (T-RFLP) analysis for the study of microbial community structure and dynamics. Environ. Microbiol. 2, 39–50. doi: 10.1046/j.1462-2920.2000.00081.x
Pang, J. B., Rao, M., Chen, Q. F., Ji, A. Q., Zhang, C., Kang, K. L., et al. (2020). A 124-plex Microhaplotype Panel Based on Next-generation Sequencing Developed for Forensic Applications. Sci. Rep. 10:1945. doi: 10.1038/s41598-020-58980-x
Sanger, F., and Coulson, A. R. (1975). A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J. Mol. Biol. 94, 441–448. doi: 10.1016/0022-2836(75)90213-2
Schmedes, S. E., Sajantila, A., and Budowle, B. (2016). Expansion of Microbial Forensics. J. Clin. Microbiol. 54, 1964–1974. doi: 10.1128/JCM.00046-16
Schneider, P. M., Prainsack, B., and Kayser, M. (2019). The Use of Forensic DNA Phenotyping in Predicting Appearance and Biogeographic Ancestry. Dtsch Arztebl. Int. 52, 873–880. doi: 10.3238/arztebl.2019.0873
Suzuki, M., Rappe, M. S., and Giovannoni, S. J. (1998). Kinetic bias in estimates of coastal picoplankton community structure obtained by measurements of small-subunit rRNA gene PCR amplicon length heterogeneity. Appl. Environ. Microbiol. 64, 4522–4529.
SWGDAM (2019). Addendum to SWGDAM Autosomal Interpretation Guidelines for NGS.Swgdam. Available online at: https://www.swgdam.org/publications
Vidaki, A., and Kayser, M. (2018). Recent progress, methods and perspectives in forensic epigenetics. Forensic Sci. Int. Genet. 37, 180–195. doi: 10.1016/j.fsigen.2018.08.008
Vos, P., Hogers, R., Bleeker, M., Reijans, M., van de Lee, T., Hornes, M., et al. (1995). AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 23, 4407–4414. doi: 10.1093/nar/23.21.4407
Wyman, A. R., and White, R. (1980). A highly polymorphic locus in human DNA. Proc. Natl. Acad. Sci. U S A 77, 6754–6758. doi: 10.1073/pnas.77.11.6754
Young, J. M., and Linacre, A. (2021). Massively parallel sequencing is unlocking the potential of environmental trace evidence. Forensic Sci. Int. Genet. 50:102393. doi: 10.1016/j.fsigen.2020.102393
Keywords : forensic genetics, DNA typing, metabarcoding, soil, microbes, minisatellites, next-generation sequencing
Citation: Jordan D and Mills D (2021) Past, Present, and Future of DNA Typing for Analyzing Human and Non-Human Forensic Samples. Front. Ecol. Evol. 9:646130. doi: 10.3389/fevo.2021.646130
Received: 25 December 2020; Accepted: 02 March 2021; Published: 22 March 2021.
Reviewed by:
Copyright © 2021 Jordan and Mills. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: DeEtta Mills, [email protected]
This article is part of the Research Topic
Life and Death: New Perspectives and Applications in Forensic Science

- © 2018
DNA Fingerprinting: Advancements and Future Endeavors
- Hirak Ranjan Dash 0 ,
- Pankaj Shrivastava 1 ,
- Braja Kishore Mohapatra 2 ,
- Surajit Das 3
DNA Fingerprinting Unit, State Forensic Science Laboratory, Sagar, India
You can also search for this editor in PubMed Google Scholar
Department of Biology and DNA Fingerprinting Unit, Central Forensic Science Laboratory, New Delhi, India
Department of life science, national institute of technology, rourkela, india.
- Inclusion of Real Case Studies related to DNA Fingerprinting
- Advanced tools and techniques of DNA Fingerprinting along with the hands on notes
- Use of real-time images for easy understanding
22k Accesses
32 Citations
11 Altmetric
- Table of contents
About this book
Editors and affiliations, about the editors, bibliographic information.
- Publish with us
Buying options
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Other ways to access
This is a preview of subscription content, log in via an institution to check for access.
Table of contents (18 chapters)
Front matter, basics of dna fingerprinting: tools and techniques, dna fingerprinting: discovery, advancements, and milestones.
- Jahangir Imam, Romana Reyaz, Ajay Kumar Rana, Vrijesh Kumar Yadav
DNA Fingerprinting Techniques for Forensic Application: Past, Present, and Future
- Nisha Bara, Ramkishan Kumawat, Jahangir Imam
Techniques Involved in DNA Fingerprinting: Isolation, Quantification, PCR, Genotyping, and Analysis
Braja Kishore Mohapatra
STR Typing and Available Kits
- Pankaj Shrivastava, Hirak Ranjan Dash, R. K. Kumawat, Ankit Srivastava, Jahangir Imam
Applications of DNA Fingerprinting
Application of dna fingerprinting and wildlife forensics.
- Sandeep Kumar Gupta
Species Characterisation from Hair of Protected Mammals: Comparison of Molecular Methods
- Vivek Sahajpal, S. P. Goyal
Molecular Basis of Identification Through DNA Fingerprinting in Humans
- Moumita Sinha, I. Arjun Rao, Mitashree Mitra
Genetic Fingerprinting for Human Diseases: Applications and Implications
- Inusha Panigrahi
Molecular Diagnosis of Enteric Bacterial Pathogens
- Amita Shrivastava, Pradeep K. Singhal, Pankaj Shrivastava
Application of DNA Fingerprinting: DNA and Human Trafficking
- Maria Jesus Alvarez-Cubero, Maria Saiz, Luis Javier Martinez-Gonzalez, Juan Carlos Alvarez, Jose Antonio Lorente
Three Decades of DNA Evidence: Judicial Perspective and Future Challenges in India
- G. K. Goswami, Siddhartha Goswami
DNA Fingerprinting: Case Studies
Fundamentals of autosomal str typing for forensic applications: case studies.
- Hirak R. Dash, Neha Rawat, Sonia Kakkar, Arun Kumar Swain
Y-Chromosomal STR Typing and Case Studies
- Jahangir Imam, Ajay Kumar Rana, Romana Reyaz
Applications of the Mitochondrion in Forensic DNA Typing
- Ranyelle Reid
Future of DNA Fingerprinting
Future of dna fingerprinting: application of ngs in forensic science.
- Jahangir Imam, Pankaj Shrivastava, Shivani Dixit, Amita Shrivastava
This book describes the basics and various applications of DNA fingerprinting, including in actual case studies. The book is divided in four modules; Module 1: Basics of DNA Fingerprinting, Module 2: Applications of DNA Fingerprinting, Module 3: DNA Fingerprinting: Case Studies, and Module 4: Future of DNA Fingerprinting. Each module consists of 4 to 5 chapters, written by reputed researchers, academics and forensic scientists from around the globe. The respective chapters cover e.g. related fields, the tools and techniques used, various genotyping kits, real-world case studies, ancient DNA and wild life forensics, molecular diagnosis of human diseases, legal aspects, microbial forensics and the economics of the DNA fingerprinting technique.
- DNA Fingerprinting
- Forensic Science
- Wile life Forensics
- Microbial Forensics
- Legal issue
Hirak Ranjan Dash, Pankaj Shrivastava
Surajit Das
Dr. Hirak Ranjan Dash completed his Ph.D. at the Department of Life Science, National Institute of Technology, Rourkela, India and is currently working as a Scientific Officer (DNA) at the Forensic Science Laboratory, Madhya Pradesh, India. He received his M.Sc. in Microbiology from Orissa University of Agriculture and Technology, Odisha, India. His research interests include forensic microbiology, thanatomicrobiome analysis, molecular microbiology, environmental microbiology, DNA fingerprinting, microbial phylogeny, genetic manipulation of bacterial systems and microbial diversity. He has developed a number of microbial techniques for the assessment of mercury pollution in marine environments, and has successfully constructed a transgenic marine bacterium for enhanced utilization in mercury removal by simultaneous mercury volatilization and sequestration. He has written 3 books and published 28 research papers, 11 book chaptersand 12 conference proceedings.
Dr. Pankaj Shrivastava received his Ph.D. in Microbiology from Rani Durgawati University, Jabalpur. He is presently serving as a Scientific Officer at the DNA Fingerprinting Unit, Forensic Science Laboratory, Madhya Pradesh, India. He has more than 10 years of experience in examining a variety of criminal cases using DNA fingerprinting. The central theme of his research is the DNA analysis of caste and tribal populations of different parts of India, along with the development of new methodologies for improved forensic DNA typing. To date he has published 11 books and 61 scientific articles in reputed international journals. He is visiting faculty of the National Police Academy, Hyderabad, National Institute of Criminology and Forensic Science, Govt. of India, Delhi and the Central Police Academy, Bhopal, along with many Central and State Universities of India. He is a recipient of the Pt. Govind Vallabh Pant Samman Award from the Ministry of Home, Govt. of India, the Anusrijan Samman Award from AISECT University, Bhopal, the Dr. Lalji Singh Memorial Award and the FICCI Smart Policing Award for the development of a direct protocol in forensic DNA typing.
Dr. Braja Mohapatra completed his Ph.D. at Utkal University, Bhubaneswar. He is presently serving as a Senior Scientific Officer and Head of the Department, Biology and DNA Profiling Unit, Central Forensic Science Laboratory (CBI), New Delhi, India. He has more than 10 years of experience in examining various criminal cases using DNA fingerprinting. His research interests include the interpretation of DNA profiles in mixed samples, touch DNA, and population genetics. He has 13 peer-reviewed publications in reputed national and international journals to his credit. He is a recipient of the meritorious service award in Forensic Science. He has solved various high-profile cases through DNA fingerprinting, both in India and the Republic of Seychelles.
Dr. Surajit Das is an Associate Professor at the Department of Life Science, National Institute of Technology, Rourkela, India. He received his Ph.D. in Marine Biology from the Centre of Advanced Study in Marine Biology, Annamalai University, Tamil Nadu, India for his research work on marine microbiology. He has been awarded an Endeavour Research Fellowship by the Australian Government to carry out postdoctoral research at the University of Tasmania. As group leader of the Laboratory of Environmental Microbiology and Ecology (LEnME), he is currently conducting research on the biofilm-based bioremediation of PAHs and heavy metals using marine bacteria; nanoparticle-based drug delivery and nano-bioremediation; and metagenomic approaches for exploring the diversity of immunoglobulins in the Indian Major Carps, supported by research grants from the Ministry of Science and Technology, Indian Council of Agricultural Research, Ministry of Environment, Forest and Climate Change, and the Government of India. He is an Academic Editor for PLOS One and an Associate Editor (Ecological and Evolutionary Microbiology) for BMC Microbiology.
Book Title : DNA Fingerprinting: Advancements and Future Endeavors
Editors : Hirak Ranjan Dash, Pankaj Shrivastava, Braja Kishore Mohapatra, Surajit Das
DOI : https://doi.org/10.1007/978-981-13-1583-1
Publisher : Springer Singapore
eBook Packages : Biomedical and Life Sciences , Biomedical and Life Sciences (R0)
Copyright Information : Springer Nature Singapore Pte Ltd. 2018
Hardcover ISBN : 978-981-13-1582-4 Published: 12 November 2018
Softcover ISBN : 978-981-13-4651-4 Published: 29 December 2018
eBook ISBN : 978-981-13-1583-1 Published: 01 November 2018
Edition Number : 1
Number of Pages : X, 325
Number of Illustrations : 16 b/w illustrations, 49 illustrations in colour
Topics : Forensic Science , Molecular Medicine , Animal Genetics and Genomics , Microbial Genetics and Genomics
Policies and ethics
- Find a journal
- Track your research
- Open access
- Published: 18 November 2013
DNA fingerprinting in anthropological genetics: past, present, future
- Michael H Crawford 1 &
- Kristine G Beaty 1
Investigative Genetics volume 4 , Article number: 23 ( 2013 ) Cite this article
14k Accesses
5 Citations
24 Altmetric
Metrics details
In 1985, Sir Alec Jeffreys developed the variable-number tandem repeat method used to identify individuals and giving researchers the first DNA fingerprints. These initial methods were used in anthropological genetics, a field that uses a comparative approach to answer questions about human history, including the discernment of the origin of Native American populations and the discrimination of clan affiliation from individuals in Siberia. The technological and methodological advances since this time have led to the use of many more markers, including restriction fragment length polymorphisms, Y chromosomal and autosomal short tandem repeats, single nucleotide polymorphisms, and direct sequencing not only to identify individuals, but to examine frequencies and distributions of markers (or “prints”) of entire populations. In the field of anthropological genetics these markers have been used to reconstruct evolutionary history and answer questions concerning human origins and diaspora, migration, and the effects of admixture and adaptation to different environments, as well as susceptibility and resistance to disease. This review discusses the evolution of DNA markers since their application by Sir Alec Jeffreys and their applications in anthropological genetics.
Introduction
Anthropological genetics is a synthetic field that examines evolutionary theory of interest to anthropologists while applying genetic methodologies [ 1 ]. This intimate relationship between genetics and anthropology was first characterized in 1973, in a volume entitled Methods and Theories of Anthropological Genetics [ 2 ]. This initial synthesis was followed by three volumes on Current Developments in Anthropological Genetics [ 3 – 5 ]. The far-reaching impact of the molecular revolution on the field of anthropological genetics in the 1980s and 1990s was assessed by a volume entitled Anthropological Genetics: Theory, Methods and Applications [ 6 ]. The field of anthropological genetics utilizes a comparative approach on small, isolated populations and topics such as human variation, evolutionary theory, reconstruction of the human diaspora (out-of-Africa), genetic epidemiology, and forensic sciences [ 7 ]. Anthropological geneticists (particularly from the Department of Genetics, Texas Biomedical Research Institute) have been successful in mapping quantitative trait loci involved in biological pathways of diseases such as diabetes mellitus, cancers, obesity, osteoporosis, and coronary heart disease [ 8 ]. Schanfield has reviewed the prominent role of anthropological genetics in cases of legal interest, using classic genetic markers and molecular methods [ 9 ]. See the thematic review of the application of DNA fingerprints to forensic sciences in this special issue of Investigative Genetics . In population studies, genetic markers have been defined as “discrete, segregating genetic traits which can be used to characterize populations by virtue of their presence, absence, or high frequency in some populations and low frequencies in others” [ 10 ]; in a sense, a combination of these markers can be used as a “fingerprint” of a population. Although this definition was first applied to blood groups and protein variation, any segregating regions of DNA, present in some populations but absent or infrequent in others, may be termed genetic markers. Thus, variable-number tandem repeats (VNTRs), short tandem repeats (STRs), mitochondrial DNA haplogroups, Y-specific non-recombining region (NRY) haplotypes, and single nucleotide polymorphisms (SNPs) have been used as “genetic markers” to document population history and to assess the actions of the forces of evolution. This thematic review focuses on the application of a variety of genetic markers (from VNTRs to STRs to SNPs) to the resolution of several evolutionary controversies. Examples of the application of these DNA fingerprints (genetic markers) to evolutionary questions come primarily from studies conducted by researchers of the Laboratory of Biological Anthropology at the University of Kansas, and provides a more “personalized view” of anthropological genetics that has built upon the work that Sir Alec Jeffrey began over 35 years ago.
Review and discussion
- DNA fingerprints
In 1985, Alec Jeffreys and his colleagues developed a method using VNTRs or minisatellites of DNA to identify specific individuals for forensic purposes and parenthood determination [ 11 ]. These DNA fingerprints are specific to an individual (or to a set of monozygotic twins) with 1 in 30 billion chances that the identical patterns will be encountered in an unrelated individual. Southern blot methodology was utilized to identify specific loci and alleles from a multitude of DNA fragments. This method involved cutting intact DNA with a sequence specific restriction enzyme, followed by separation of fragments using electrophoresis, transferring these fragments onto a nitrocellulose membrane, and hybridizing the fragments with specific probes labeled by radioactive isotopes or biotin. Numerous minisatellite loci were considered simultaneously, which increased the observable variation but made it difficult to discern specific alleles. A series of fragments of various lengths were digitized and grouped into size bins and the frequencies of fragments within these bins were calculated for each population. Because of the time-consuming nature of this methodology and the ambiguity associated with whether fragments within bins were specific alleles, this Southern blot method was eventually supplanted by PCR-based assays [ 12 ]. The PCR methodology is less expensive, more sensitive, less time consuming and amplifies the specific regions of DNA, using multiplexes and “cocktails” containing thermostable DNA polymerase.
Anthropological genetic applications of DNA fingerprints
During the late 1980s and early 1990s, frequency distributions of VNTRs were used as genetic markers to discriminate between ethnically defined populations [ 13 – 15 ]. In addition, because of the non-coding nature of VNTRs, high mutation rates, and high genetic diversity, McComb et al. applied VNTR restriction fragment length polymorphism distributions to questions concerning the peopling of the Americas and the characterization of the genetic structure of indigenous Siberian populations [ 16 – 18 ]. Data assessing morphological traits and classic genetic markers suggested a Siberian origin of Native American populations, but until 1989, DNA samples from Siberian indigenous groups were not available to western scientists to verify this origin. Field investigations in Siberia were made possible by the breakup of the Soviet Union and “perestroika” (rebuilding). During the summers of 1989–1993, an international team of researchers from the University of Kansas and the Russian Academy of Sciences, funded by the NSF, collected blood samples from volunteers in two adjacent Evenki reindeer herding brigades (Surinda and Poligus), a small Ket fishing/hunting village on the Yenesei River (Sulamai), and a cattle-herding village from Gorno-Altai (Mendur-Sokhon). In 2002, DNA samples were collected from Even, Koryak, and Aleut communities of Kamchatka and Bering Island. DNA was extracted at the Laboratory of Biological Anthropology, University of Kansas, and analyzed using Southern blots to assign DNA fragments into length bins through digital comparisons with sizing ladders. All statistical analyses were based on a conservative standard error of ± 2%. Intergroup variation was tested for statistical significance using the Kolmogorov-Smirnov test with Bonferroni correction for multiple comparisons ( P = 0.05). Siberian populations clustered with the Native American groups were statistically significantly different from European and African Americans [ 17 ] (Figure 1 ). In addition to DNA fingerprints, mtDNA analyses of the same DNA samples demonstrated that Siberian and Native American populations shared the founding haplotypes A, B, C, and D [ 19 ]. Phillips-Krawczak et al. later identified the presence of a Siberian X haplogroup in the Kizhi population of Gorno Altai [ 20 ]. Non-recombining Y chromosome markers further verified the Siberian origins of Native Americans [ 21 ].
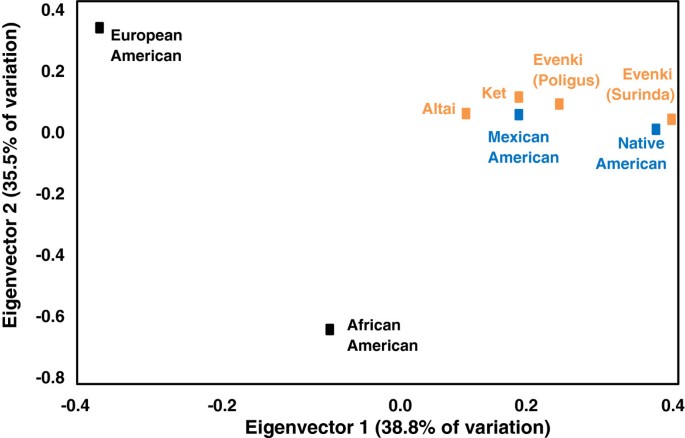
Least square reduction of an R-matrix plot based on allelic frequencies from 5 VNTR loci ( D7S104 , D11S129 , D18S17 , D20S15 , and D21S112 ). Figure adapted from McComb et al. [ 17 ].
Crawford et al. also utilized VNTR loci to determine clan affiliation in the Kizhi pastoral community of Mendur-Sokhon, Gorno Altai region of Southern Siberia [ 22 ]. A sample of Altai Kizhi were characterized for three VNTR loci ( D7S104 , D11S129 and D18S17 ) and linear discriminant function analysis was used to classify unknown individuals to a specific clan. The Kizhi community contained three major clans, Irkit, Todosh, and Kipchak, and other smaller clans. Linear discriminant function correctly classified 72% of all unknowns entered into the analysis. The highest correct classification occurred when 80% of the research subjects were placed in the Todosh clan, followed by 75% correct classification of individuals assigned to the Irkit clan, and 60% into the Kipchak clan. Those Kizhi individuals who were not affiliated with the Irkit, Todosh, or Kipchak were assigned randomly to a fourth group. If all of the clan assignments were random in regards to the VNTR loci, individuals would have been correctly assigned 25% of the time, while the unassigned individuals were classified into that category 29% of the time. These data suggest that VNTR markers have detected genetic similarities within each clan that permit a high probability of correct assignment of each individual to a correct clan (Table 1 ).
Microsatellites (STRs)
Technological advances have allowed for more efficient means of investigating the genetic makeup of individuals with the use of DNA fingerprints such as STRs. In anthropological genetics, these markers have been used as ancestry-informative markers to reconstruct the human diaspora and to interpret the evolutionary history of human populations to answer questions of population origins, migration, and admixture. STRs, also known as microsatellites, are sequences of 2 to 6 base pairs (bp) repeated in a region of DNA from 3 to 100 times. Variant alleles usually result from slipped strand mispairing during DNA replication. In this review, we focus on the anthropological genetic questions that have been investigated during the last decade using STRs. STR variation can be examined in a number of different ways to test hypotheses concerning anthropological genetics. The following examples demonstrate the usefulness of STRs in answering evolutionary questions, such as (1) Are the Basque inhabitants of Spain and France remnants of the Paleolithic populations of Europe prior to the expansion of agriculture and Indo-European languages from the Middle East, circa 10,000 years B.P.? Are they Iberian groups that have been geographically isolated from their neighbors or are they related to distant populations from North Africa or the Caucasus? (2) How much gene flow did the populations of the Aleutian Islands experience from Russian, English, and/or Scandinavian sources? (3) Can a single ubiquitous STR allele ( D9S1120 9 RA ) reveal the number of migrations that have occurred from Siberia into the Americas?
STRs and Basque origins
Are the Basque populations remnants of the Paleolithic settlers of Europe and/or do they show affinities to populations of the Caucasus or North Africa? Most of the early molecular genetic studies of Basque populations were based primarily on small samples of school children or adults from urban sites, with some admixture with the surrounding Spanish communities [ 23 ]. The Vizcaya Province sample (68 unrelated volunteers) revealed, on the basis of 13 autosomal STR loci, that the Basques are outliers relative to neighboring Spanish and the more distant North African populations. Young et al. characterized a total of 404 DNA samples for nine autosomal STR loci collected from rural villages and towns of four Basque Provinces [ 24 ]. Multidimensional scaling based on Shriver’s D sw distance matrix did not support the hypothesis of a recent common ancestry between the Basques and populations from the Caucasus or North Africa [ 25 ]. STR, mtDNA, and NRY genetic markers indicate that the Basques are distinct from the surrounding Spanish populations but also differ from the inhabitants of the Caucasus and North Africa. The most parsimonious explanation for the distribution of the genetic markers is that the contemporary Basques are descendants of the earliest Paleolithic migrants into Europe. However, recent analyses of ancient DNA from early Neolithic farmers and hunter-gatherers suggest that the maternal genetic contribution of farmers coming from the Middle East is higher than previously suspected [ 26 , 27 ].
Aleutian island admixture
Estimates of gene flow and admixture in human populations may vary depending on which specific genetic markers are used to characterize the populations. If the indigenous Aleutian island populations are characterized solely by mitochondrial DNA haplogroups shown in Figure 2 , only the native haplogroups A (shown in blue) and D (shown in orange) are observed [ 28 ]. Based solely on these data, one might conclude that there was no gene flow from Russian, English, or Scandinavian populations into the Aleutian Islands. However, morphologically, the Aleuts appear to be highly admixed. In Figure 3 , NRY haplotypes based on SNPs indicate that only 15% of the Y chromosomes from male participants of the Aleutian archipelago were either Q* or Q3 (shown in light orange and orange), considered Native American paternal lineages [ 29 ]. Thus, 85% of the Y chromosomes of the Aleutian Islands inhabitants are of European origin, primarily R1b (dark green) or R1a (dark blue), depending on whether the samples are from the western or eastern islands [ 30 ]. The calculation of admixture (using the program Admix 3.1) based on nine autosomal STR loci revealed that approximately 40% of the genes in the Bering gene pool were of Russian origin while 60% were Aleut. Genetic markers that recombine, such as STRs, provide a more accurate assessment of the total contents of an admixed gene pool in human populations, but fail to detect gender-specific patterns of gene flow.
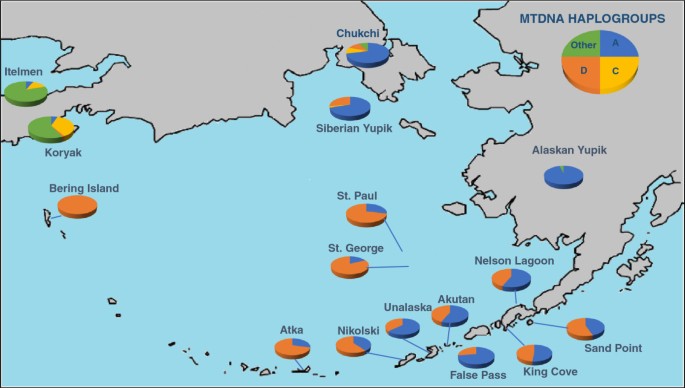
Frequency of mtDNA haplogroups present in the Aleutian Islands determined by restriction fragment length polymorphisms and hypervariable segment-1 sequences, adapted from Crawford et al. [ 28 ] . Only haplgroups A (shown in blue) and D (shown in orange) are present in the Aleutian Islands, whereas haplogroup C (shown in yellow) and other mtDNA haplgroups (shown in green) are found on the Alaskan mainland and Siberia.
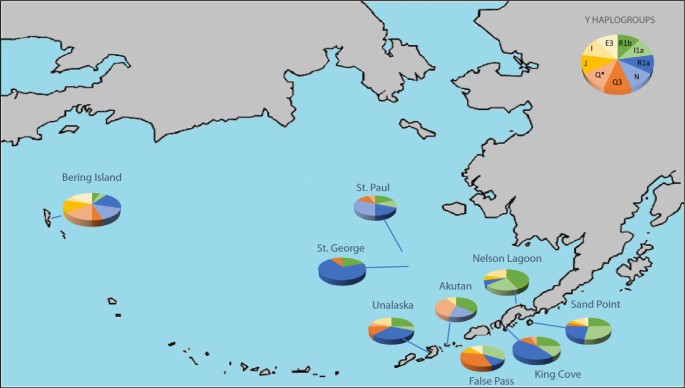
Frequency of Y haplogroups present in the Aleutian Islands determined by SNPs and STR haplotypes, adapted from Crawford et al. with data from Rubicz et al. [ 28 , 29 ] . Haplogroups shown in green represent haplogroups found in high frequencies in Western Europe, haplogroups shown in blue are found in high frequencies in Russia, and haplogroups in orange are believed to be native to Aleuts.
Private STR alleles and migration into the Americas
The frequencies of private STR alleles and their ubiquitous distributions can provide invaluable information concerning the evolutionary history of populations. Schroeder et al. described a private STR allele ( D9S1120 9 RA ), which is ubiquitous in the Americas but present in only two indigenous Siberian populations, Koryaks and Chukchi, both groups located proximally to the former location of the land bridge, Beringia (Figure 4 ) [ 31 ]. While this private allele, shown in orange, is frequent in the Americas and in two Siberian populations, it is absent in Europe, Africa, Australia, Oceania, and most of Asia. The most parsimonious explanation for the geographic distribution of this private allele is that an ancestral Siberian population migrated across the Bering land bridge in a single wave. This single migration theory is based on the assumptions that all copies of the 9-bp allele are identical by descent and not influenced by selection. Schroeder et al. tested these underlying assumptions by examining the haplotypic background in the vicinity of D9S1120 [ 32 ]. They observed that 91% of these chromosomes share the same 76.26 kb haplotype that they termed “American Modal Haplotype”. Schroeder et al. suggest that the high frequency and widespread distribution of the 9-repeat alleles are unlikely to be the result of natural selection [ 32 ]. They conclude that all contemporary Native Americans and Western Beringians can trace their ancestry to a single founding population.
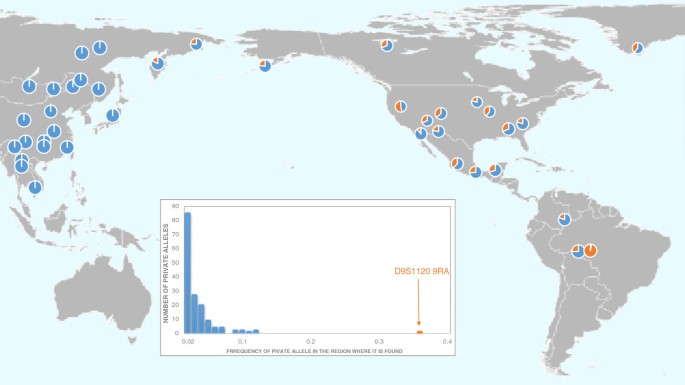
Distribution of the D9S1120 9 RA allele shown in orange. Redrawn following Schroeder et al. [ 32 ].
Recent analyses of genome-wide SNP data suggest multiple waves of migration from Siberia to the Americas [ 33 ]. The exact number of migrations is difficult to assess because of the few North American populations analyzed in this sample (n = 4). Reich et al. conclude that three migrations occurred (the same number postulated by Greenberg et al. [ 34 ]), consisting of Amerinds (earliest migrants), NaDene (Canada and SW United States), and Aleut/Eskimo (last arrivals) [ 33 ]. How can these differing conclusions be reconciled? One possible scenario is that multiple migration waves came from the same Beringian source population. Among Siberian populations, only the Altai share all of the founding mtDNA haplogroups A, B, C, D, and X. Yet, these Central Siberian groups are located more than 1,000 miles from Beringia with numerous genetically distinct populations located between the Altai and the region closest to Beringia, Chukotka. Does the Altai region share common ancestry with the populations that founded the Americas? An alternative explanation is that the multiple migrations were followed by extensive gene flow between the migrant groups, thus, spreading the private allele. A third possible explanation is that the STR mutation occurred on the land bridge, followed by gene flow into both the Americas and Siberia. This ubiquitous mutation is found in all Native populations of the Americas tested to date, but in only two contemporary Siberian groups, Chukchi, located on Chukotka, which is adjacent to Beringia and Koryaks, located south of Chukotka on the Kamchatkan peninsula (Figure 3 ).
DNA sequencing and the reconstruction of evolutionary history
In the late 1970s and early 1980s, DNA sequencing, which allows for direct identification of individual or population fingerprints, was a costly and time-consuming methodology inappropriate for use in population genetics due to the required sample size. As a result, most of the early sequencing in anthropological genetics was focused on hypervariable segment-1 of mtDNA, a non-coding region that contains considerable genetic variation, approximately 400 bp in length. However, vast expanses of genomic DNA were useful for determining the structure and function of specific genes. High throughput DNA sequencing methodologies and machines have made large samples from specific populations economically feasible, with a cost, projected by the National Human Genome Research Center, of $1,000 per genome within the next year [ 35 ]. Sequencing human genomes yields large numbers of SNPs that can be considered equivalent to fingerprints or genetic markers.
One application of whole genome sequencing is its application to questions of admixture and gene flow. Measures of admixture and gene flow were initially based on estimates of the frequencies of marker genes in parental populations and compared to frequencies in the admixed groups. Earliest attempts to ascertain the proportions of African and European genes in African Americans depended on frequencies of Rhesus blood group R o in an African American population and compared to estimated frequencies of these alleles in West Africa and Europe [ 36 ]. This proportion of admixture (m) was computed using the Bernstein (1931) formula:
where, q h is the frequency of the allele in the admixed population; q 1 and q 2 are frequencies of the same allele in the parental populations [ 37 ].
In the 1960s and 1970s, Bernstein’s method for estimating admixture for populations with two ancestral groups was expanded for populations with three or more parental groups using parental frequencies and maximum likelihood, true least squares, or multiple regression statistical approaches [ 38 ]. All of these approaches suffered from the same weaknesses, i.e., the parental frequencies were rough estimates from populations dating back centuries before.
Halder et al. developed a panel of ancestry informative markers (AIMs) consisting of SNPs for estimating individual bio-geographical ancestry and admixture. These are genetic loci with large frequency differences between ancestral populations allowing them to act as “prints” or marks of a specific population [ 39 ]. They initially employed 176 autosomal AIMs from four continents, namely Europeans, West Africans, Indigenous Americans, and East Asians. This approach for estimating admixture based on AIM SNPs was effectively applied to two Mexican American samples from San Antonio, Texas, to determine if their genetic structures were equivalent [ 40 ]. A total of 706 participants from the San Antonio Family Diabetes Study (SAFDS) were compared to 586 males from the San Antonio Center for Biomarkers of Risk of Prostate Cancer (SABOR) using 64 ancestry informative markers. Significant genetic differences in population structure were observed in the ancestral proportions of the two samples of Mexican Americans from San Antonio. The SAFDS sample exhibited 50.2 ± 0.6% European admixture, while the SABOR sample had 58.9 ± 0.7%. Similar differences were observed using this method for estimating Native American proportions, SAFDS 46.4 ± 0.6% versus SABOR 38.2 ± 0.7%. The West African admixture was estimated at 3.1 ± 0.2% for the SAFDS sample and 2.9 ± 0.2% for the SABOR Mexican American samples from San Antonio. These AIM (SNP) methodologies are considerably more robust and provide more informative estimates of admixture than standard genetic markers, mtDNA, or NRY haplotypes in subpopulations.
Because of high throughput sequencing and the characterization of entire genomes, Johnson et al. have been able to reconstruct the history of admixed populations using DNA recombination to parse out the more specific geographical sources of the parental populations [ 41 ]. The shorter chromosomal segments reflect a longer evolutionary history because they have had more time to recombine with unrelated DNA; the longer chromosomal segments reflect a more recent admixture. By comparing DNA segments from one ancestral population (either European, African, or Native American) with admixed groups, greater accuracy can be obtained about the origin of the parental groups and the sizes of the source of the gene flow. They found that the European contribution to the Latino population came from Spain and Portugal and had a low genetic diversity, indicating that few individuals contributed to the admixed population [ 42 ].
Among the projects underway to better understand genome wide diversity is the 1000 Genomes Project, which is currently sequencing 2,500 genomes from individuals from all over the world in an attempt to reveal the extent of the diversity contained in the human species and determine how this genetic diversity translates into specific phenotypes [ 43 ]. This project has identified several hundred thousand SNPs that vary in allelic frequencies by population, exposing potential variants that will allow us to better define and reconstruct the human diaspora, provide a better understanding of ancestry at both the individual and population level, and allow us to better tell the story of both ancient and recent admixture. These data will initiate a new era of anthropological genetics and will further shift the definition of what constitutes a genetic marker or DNA fingerprint.
Ancient DNA (whole genome)
The last decade has also seen an emergence of technology that has allowed for investigation of ancient genomes beyond mtDNA, traditionally a focus in ancient molecular studies because of the abundance of mitochondria in skeletal remains. These advances have included the sequencing of entire genomes of ancient remains of Neandertals and a hominin group from Siberia, called Denisovans, that were identified by their unique genetic characteristics [ 44 , 45 ]. These studies have shown that we shared a common ancestor with Neandertals and Denisovans some 800,000 years ago [ 45 ], that Neandertals have contributed more genes to non-African populations than African populations [ 46 ], and that Denisovans have contributed to the genomes of Melanesians, Australian aborigines, and Southeast Asians [ 45 ]. Studies of both groups of ancient hominins have also unraveled functional genes. For example, Neandertal remains from various sites indicate the presence of type O blood [ 47 ], alleles that may be associated with red hair and fair skin [ 48 ], and the ability to taste the bitter chemical phenylthiocarbamide [ 49 ]. Genetic variants of the Denisovan individual suggest the presence of dark skin, hair, and eyes [ 45 ]. These advances have allowed us to look further back into our evolutionary history and allow us to better refine our knowledge of how, when and why we have come to be.
In anthropology, whole genome studies of ancient individuals have also been used to answer questions regarding the peopling of the Americas. A human hair tuft, excavated in 1986 at Qeqertasussuk, a Saqqaq archeological site from West Greenland, was rediscovered in a museum in Copenhagen. Because of the permafrost conditions, there was excellent preservation of both mitochondrial and genomic DNA. The whole mtDNA genome was first sequenced from this Paleo-Eskimo, dating back 4,000 to 5,000 years B.P. [ 50 ]. The mtDNA haplogroup (D2a1) detected in this Paleo-Eskimo is distinct from modern Native Americans and Neo-Eskimos but is identical to the haplogroup observed in contemporary Aleuts of the Archipelago [ 50 ]. This analysis raised questions about a potential early migration of Siberians who expanded into Greenland prior to the later Thule Eskimo expansion.
Rasmussen et al. sequenced the whole genome of the Paleo-Eskimo and recovered 353,151 high confidence SNPs [ 51 ]. This Saqqaq genome clusters with Asian populations instead of the contemporary Eskimo or Native American populations. The maternal discontinuity first described by Gilbert et al. was further verified through whole genomic sequencing [ 50 ].
Because of the identification of the vast array of SNPs in the Saqqaq genome, it was possible to identify the functional SNPs in this 4,000 year old Paleo-Eskimo. Rasmussen et al. utilized the observed SNPs to reconstruct the following phenotypes of Saqqaq man: blood group subtype A1 , Q1 NRY haplogroup, brown eyes, non-European light skin, increased risk of baldness, higher body mass index, dry cerumen, shovel-shaped incisors, and a metabolism that was adapted to a cold environment [ 51 ]. These phenotypes were deduced from their associations to SNPs, such as a single base deletion in a transferase gene that results in an additional domain at the carboxyl terminal and an A1 phenotype [ 52 ]. Similarly, the presence of a non-synonymous variant (C/C) in the TP53 on chromosome 17, suggested that Saqqaq man possessed a more active form of p53 by coding for an Arg variant which is related to the more effective regulation of metabolism in cold climates [ 53 ]. Similar functional associations may yield future information about the evolution of complex diseases and the genetic predispositions for chronic conditions, such as heart disease or breast cancer, in contemporary and ancient populations.
With the rapid changes in technology and data analyses, DNA genetic markers will play a significant role in future anthropological genetics. Whole genome sequencing is going to become cheaper and faster. The main hurdle for scientists will be the analysis of immense data sets (millions of nucleotides) that are being generated by massive sequencing programs. Within anthropological genetics, these developments are going to mean improvements in the use of molecular data in forensics (with less reliance on more subjective morphological techniques), genetic epidemiology, and population genetics. Greater emphasis can then be placed on unraveling the cultural and environmental factors that shape the expression of our genomes.
Anthropological geneticists investigating disease associations and adaptation have long worked toward uncovering the genetic variation that leads to disease and disease susceptibility. These attempts have, over the past decade, generally been performed using genome wide association studies that have identified some common variants that can lead to, or provide protection from, pathology. However, many of these diseases and disorders may be caused by rare variants that do not give a strong enough signal for identification (see Gibson, 2012 for a review [ 54 ]). The 1000 Genomes Project may rectify some of these shortcomings as it aims to identify variants that are found at a frequency of 1% compared to the frequency of common variants used in genome-wide association studies that are found at roughly 5%. Furthermore, whole genome sequencing will reveal rare variants that lie farther from the block of linkage disequilibrium that may also influence the disease pathway. These data will only expand as more studies involve the use of whole genome sequences towards a better understanding of disease.
Future studies of admixed populations will be based on whole genomic sequencing, the effects of recombination, linkage disequilibrium and the use of panels of ancestry informative markers. In the past, the effects of natural selection on admixture estimates could only be examined using imprecise approaches such as the examination, locus by locus, of deviations from expectation under a specific gene flow model. Through the use of whole genomic sequencing, regions of the genome can be examined for the signature of selection in both modern and ancient populations. In addition, rare alleles found only in specific groups should allow for a more detailed picture of human history and better define the complicated ways in which humans interact with one another and the environment.
In the 1980s, Sir Alec Jeffreys first pioneered DNA fingerprints as a means of identifying individuals. Since that time many more genetic markers and polymorphisms have been developed to identify unknown individuals of forensic interest. Now, an individual’s entire genome can be considered a DNA fingerprint, but its size, and the computational power necessary for analysis, makes its use in forensics inefficient and costly. The changing technology has resulted in the discovery of many more genetic markers (mtDNA, NRY, autosomal STRs, and SNPs) that are better suited for forensic and anthropological analyses, as well as cheaper and faster ways of achieving these analyses.
The future application of genetic markers (DNA fingerprints) is wide open and the next decade of research will lead to a better understanding of the origins and evolution of our species. It is unclear how far back in time studies of ancient DNA will take us, but these new methodologies will provide anthropologists with a refined story of human history, unraveling the complexities of human migration, admixture, and the successful and unsuccessful ways in which hominin genomes were selected by their environment. We are in the initial stages of personalized medicine in which our familial genomic endowment will determine specific treatments. We envisage a future where genetic information, a fingerprint of an individual’s genome, will be readily available and utilized for the assessment of ancestry, health risks and the treatment of disease, and crimes will be solved by comparisons of DNA from individuals of interest in particular cases with huge DNA data bases. When Sir Alec Jeffreys first began his work using fingerprints to identify individuals for forensic purposes, it opened a door to research that has allowed a better understanding of who we are both as individuals and as a species.
Abbreviations
Ancestry informative markers
Y-specific non-recombining region
San Antonio center for biomarkers of risk of prostate cancer
San Antonio family diabetes study
Single nucleotide polymorphisms
Short tandem repeats
Variable-number tandem repeats.
Crawford MH: Foundations of anthropological genetics. Anthropological Genetics: Theory, Methods and Applications. Edited by: Crawford MH. 2007, New York: Cambridge University Press, 1-16.
Google Scholar
Current Developments in Anthropological Genetics. Edited by: Crawford MH, Workman PL. 1973, Albuquerque: University of New Mexico Press
Current Developments in Anthropological Genetics. Vol. 1. Theory and Methods. Edited by: Mielke JH, Crawford MH. 1980, New York: Plenum Press
Current Developments in Anthropological Genetics. Vol. 2. Theory and Methods. Edited by: Crawford MH, Mielke JH. 1982, New York: Plenum Press
Current Developments in Anthropological Genetics Vol. 3. Black Caribs, A Case Study in Biocultural Adaptation. Edited by: Crawford MH. 1984, New York: Plenum Press
Anthropological Genetics: Theory, Methods and Applications. Edited by: Crawford MH. 2007, Cambridge: Cambridge University Press
Crawford MH: Anthropological genetics in the 21 st century: introduction. Hum Biol. 2000, 72 (1): 3-13.
CAS PubMed Google Scholar
Blangero J, Williams JT, Almasy L, Williams-Blangero S: Mapping genes influencing human quantitative trait variation. Anthropological Genetics. Edited by: Crawford MH. 2007, New York: Cambridge University Press, 306-334.
Schanfield M: Applications of molecular genetics to forensic sciences. Anthropological Genetics: Theory, Methods and Applications. Edited by: Crawford MH. 2007, New York: Cambridge University Press, 235-276.
Crawford MH: The use of genetic markers of the blood in the study of the evolution of human populations. Methods and Theories of Anthropological Genetics. Edited by: Crawford MH, Workman PL. 1973, Albuquerque: University of New Mexico Press, 19-38.
Jeffreys AJ, Wilson V, Thein SL: Individual-specific “finger-prints” of human DNA. Nature. 1985, 316: 76-
Article CAS PubMed Google Scholar
Mullis KB, Faloona FA, Scharf SJ, Saiki RK, Horn GT, Erlich HA: Specific enzymatic amplification of DNA in vitro : the polymerase chain reaction. Cold Spring Harb Symp Quant Biol. 1986, 51: 263-273.
Balazs I, Baird M, Clyne M, Meade E: Human population genetic studies of five hypervariable DNA loci. Am J Hum Genet. 1989, 39: 182-190.
Chakraborty R, Deka R, Jin L, Budowle B: Allele sharing at six VNTR loci and genetic distances among three ethnically defined human populations. Am J Hum Biol. 1992, 4: 387-397.
Article Google Scholar
Deka R, Deroo S, Yu LM, Ferrell RE: Variable number of tandem repeat (VNTR) polymorphism at locus D17S5 (YNZ22) in four ethnically defined human populations. Hum Genet. 1992, 90: 86-90.
McComb J, Blagitko N, Comuzzie A, Leonard WR, Sukernik RI, Schanfield MS, Crawford MH: VNTR variation in Siberian indigenous populations. Hum Biol. 1995, 67 (2): 217-229.
McComb J, Crawford MH, Osipova L, Karaphet T, Posukh O, Schanfield MS: DNA inter-populational variation in Siberian indigenous populations: the Mountain Altai. Am J Hum Biol. 1996, 8 (5): 599-608.
McComb J, Crawford MH, Leonard WR, Osipova L, Schanfield MS: Applications of DNA fingerprints for the study of genetic structure of human populations. Genomes of Plants and Animals: 21st Stadler Genetics Symposium. Edited by: Gustafson JP, Flavell RB. 1996, New York: Plenum Press, 31-46.
Chapter Google Scholar
Torroni A, Sukernik RI, Schurr TG, Starikovskaya YB, Cabell MF, Crawford MH, Comuzzie AG, Wallace DG: Mitochondrial DNA variation of aboriginal Siberians reveals distinct genetic affinities with Native Americans. Am J Hum Genet. 1993, 53 (3): 563-590.
PubMed Central CAS PubMed Google Scholar
Phillips-Krawczak C, Devor E, Zlojutro M, Crawford MH: mtDNA variation in the Kizhi population of Gorno Altai: a comparative study. Hum Biol. 2006, 78 (4): 477-494.
Article PubMed Google Scholar
Santos FR, Pandya A, Tyler-Smith C, Pena SDJ, Schanfield MS, Crawford MH, Mitchell RJ: The Central Siberian origin of Native American Y chromosomes. Am J Hum Genet. 1998, 64 (2): 619-628.
Crawford MH, McComb J, Mitchell RJ: Genetic structure of pastoral populations of Siberia: the evenki of Central Siberia and the Kizhi of Gorno Altai. Human Biology of the Pastoral Populations. Edited by: Leonard WR, Crawford MH. 2002, New York: Cambridge University Press, 10-49.
Zlojutro M, Gonzalez Apraiz A, Roy R, Crawford MH: Autosomal STR variation in a Basque population: Vizcaya Province. Hum Biol. 2006, 78 (5): 599-618.
Young KL, Sun G, Deka R, Crawford MH: Autosomal short tandem repeat genetic variation of the Basques in Spain. Croat Med J. 2011, 52 (3): 372-383.
Article PubMed Central PubMed Google Scholar
Shriver MD, Boerwinkle E, Deka R, Ferrell RE, Chakraborty R: A novel measure of genetic distance for highly polymorphic tandem repeat loci. Mol Biol Evol. 1995, 12 (5): 914-920.
Skoglund P, Malmstrom H, Raghavan M, Stora J, Hall P, Willerslev E, Gilbert MTP, Gotherstrom A, Jakobsson M: Origins and genetic legacy of Neolithic farmers and hunter-gatherers in Europe. Science. 2012, 336 (6080): 466-469.
Sanchez-Quinto F, Schroeder H, Ramirez O, Avila-Acros MC, Pybus M, Olalde I, Velazquez AMV, Marcos MEP, Encinas JMV, Bertranpetit J, Orlando L, Gilbert MTP, Lalueza-Fox C: Genomic affinities of two 7,000-year-old Iberian hunter-gatherers. Curr Biol. 2012, 22 (16): 1494-1499.
Crawford MH: Genetic structure of circumpolar populations: a synthesis. Am J Hum Biol. 2007, 19 (2): 203-217.
Rubicz R, Zlojutro M, Sun G, Spitsyn V, Deka R, Young K, Crawford MH: Genetic architecture of a small, recently aggregated Aleut population: Bering Island. Hum Biol. 2010, 82 (506): 719-736.
PubMed Google Scholar
Crawford MH, Rubicz RC, Zlojutro M: Origins of Aleuts and the genetic structure of populations of the archipelago: molecular and archaeological perspectives. Hum Biol. 2010, 82 (5–6): 695-717.
Schroeder KB, Schurr TG, Long JC, Rosenberg NA, Crawford MH, Tarskaia LA, Osipova LP, Zhadanov SI, Smith DG: A private allele ubiquitous in the Americas. Biol Lett. 2007, 3 (2): 218-223.
Article PubMed Central CAS PubMed Google Scholar
Schroeder KB, Jakobsson M, Crawford MH, Schurr TG, Conrad DF, Titotadeo R, Osipova LP, Tarskaia LA, Zhadanov SI, Wall JD, Pritchard JK, Malhi R, Smith DG, Rosenberg NA: Haplotypic background of a private allele at high frequency in the Americas. Mol Biol Evol. 2009, 26 (5): 995-1016.
Reich D, Patterson N, Campbell D, Tandon A, Mazieres S, Ray N, Parra MV, Rojas W, Duque C, Mesa N, García LF, Triana O, Blair S, Maestre A, Dib JC, Bravi CM, Bailliet G, Corach D, Hünemeier T, Bortolini MC, Salzano FM, Petzl-Erler ML, Acuña-Alonzo V, Aguilar-Salinas C, Canizales-Quinteros S, Tusié-Luna T, Riba L, Rodríguez-Cruz M, Lopez-Alarcón M, Coral-Vazquez R: Reconstructing Native American population history. Nature. 2012, 488 (7441): 370-374.
Greenberg JH, Turner CG, Zegura SL: The settlement of the Americas: a comparison of the linguistic, dental and genetic evidence. Curr Anthropol. 1986, 27: 477-497.
National Human Genome Research Institute: Concept papers for two new DNA sequencing technology development programs. http://www.genome.gov/11008124#al-4 ,
Glass B, Li CC: The dynamics of racial intermixture—an analysis based on the American Negro. Am J Hum Genet. 1953, 5 (1): 1-20.
Bernstein F: Die geographische Verteilung der Blutgruppen und ihre anthropologische Bedeutung. 1931, Comitato Italiano: Poligrafico dello Stato Roma, 227-243.
Crawford MH, Workman PL, McLean C, Lees FC: Admixture estimates and selection in Tlaxcala. The Tlaxcaltecans: Prehistory, Demography, Morphology, and Genetics, Series 7. Edited by: Crawford MH. 1976, Lawrence: University of Kansas Anthropology, 161-168.
Halder I, Shriver M, Thomas M, Fernandez JR, Frudakis T: A panel of ancestry informative markers for estimating individual biogeographical ancestry and admixture from four continents: utility and applications. Hum Mutat. 2008, 29: 648-658.
Beuten J, Halder I, Fowler SP, Goring HH, Duggirala R, Arya R, Thompson IM, Leach R, Lehman D: Wide disparity in genetic admixture among Mexican Americans from San Antonio, TX. Ann Hum Genet. 2011, 75: 529-538.
Johnson NA, Coram MA, Shriver MD, Romieu I, Barsh GS, London SJ, Tang H: Ancestral components of admixed genomes in a Mexican cohort. PLoS. 2011, 12: e 1002410-
Pennisi E: In Latino genomes, a rich source of history. Science. 2013, 340: 910-911.
CAS Google Scholar
The 1000 Genomes Project Consortium: A map of human genome variation from population-scale sequencing. Nature. 2010, 467: 1061-1073.
Article PubMed Central Google Scholar
Green RE, Krause J, Briggs AW, Maricic T, Stenzel U, Kircher M, Patterson N, Li H, Zhai W, Fritz MH, Hansen NF, Durand EY, Malaspinas A, Jensen JD, Marques-Bonet T, Alkan C, Prufer K, Meyer M, Burbano HA, Good JM, Schultz R, Aximu-Petri A, Butthof A, Hober B, Hoffner B, Sijegemund M, Weihmann A, Nusbaum C, Lander ES, Russ C: A draft sequence of the Neandertal genome. Science. 2010, 328: 710-722.
Meyer M, Kircher M, Gansauge M, Li H, Racimo F, Mallick S, Schraiber JG, Jay F, Prufer K, de Filippo C, Sudmant PH, Alkan C, Fu Q, Do R, Rohland N, Tandon A, Siebauer M, Green RE, Bryc K, Briggs AW, Stenzel U, Dabney J, Shendure J, Kitzman J, Hammer MF, Shunkov MV, Derevianko AP, Patterson N, Andres AM, Eichler EE: A high-coverage genome sequence from an archaic Denisovan individual. Science. 2012, 338: 222-226.
Sankararaman S, Patterson N, Li H, Paabo S, Reich D: The date of interbreeding between Neandertals and modern humans. PLoS Genet. 2012, 8 (10): e1002947-
Lalueza-Fox C, Gigli E, de la Rasilla M, Fortea J, Rosas A, Bertranpetit J, Krause J: Genetic characterization of the ABO blood group in Neandertals. BMC Evol Biol. 2008, 8: 342-
Lalueza-Fox C, Rompler H, Caramelli D, Straubert C, Hughes D, Rohland N, Pilli E, Longo L, Condemi S, de la Rasilla M, Fortea J, Rosas A, Stoneking M, Schoneberg T, Bertranpetit J, Hofreiter M: A melanocortin 1 receptor allele suggests varying pigmentation among Neandertals. Science. 2007, 318 (5855): 1453-1455.
Lalueza-Fox C, Gigli E, de la Rasilla M, Fortea J, Rosas A: Bitter taste perception in Neanderthals through the analysis of the TAS2R38 gene. Biol Lett. 2009, 5 (6): 809-811.
Gilbert MTP, Kivisild T, Gronnow B, Andersen PK, Metspalu E, Reilda M, Tamm E, Axelsson E, Gotherstrom A, Campos PF, Rasmussen M, Metspalu M, Higham TFG, Schwenninger JL, Nathan R, De Hoog C, Koch A, Moller LN, Andrease C, Meldgaard M, Villems R, Bendixen C, Willerslev E: Paleo-Eskimo mtDNA genome reveals matrilineal discontinuity in Greenland. Science. 2008, 320 (5884): 1787-1789.
Rasmussen M, Li Y, Lindgreen S, Pedersen JS, Albrechtsen A, Moltke I, Metspalu M, Metspalu E, Kivisild T, Gupta R, Bertalan M, Nielsen K, Gilbert MTP, Wang Y, Raghavan M, Campos PF, Kamp HM, Wilson AS, Gledhill A, Tridico S, Bunce M, Lorenzen ED, Binladen J, Guo X, Zhao J, Zhang X, Zhang H, Li Z, Chen M, Orlando L: Ancient human genome sequence of an Extinct Paleo-Eskimo. Nature. 2010, 463 (11): 757-762.
Yamamoto F, McNeill PD, Hakomori S: Human histo-blood group A2 transferase coded by A2 allele, one of the a subtypes, is characterized by a single base deletion in the coding sequence which results in an additional domain at the carboxyl terminal. Biochem Biophys Res Commun. 1992, 187: 366-374.
Hong S, Tan S, Zhong H, Hu W, Levine A, Xiao C, Peng Y, Qi X, Shou W, Ma RZ, Li Y, Su B, Lu X: Winter temperature and UV are tightly linked to genetic changes in the p53 tumor suppressor pathways in Eastern Asia. Am J Hum Genet. 2009, 84 (4): 534-541.
Gibson G: Rare and common variants: twenty arguments. Nature. 2012, 13: 135-145.
Download references
Author information
Authors and affiliations.
Laboratory of Biological Anthropology, Department of Anthropology, University of Kansas, 1415 Jayhawk Blvd., 622 Fraser Hall, 66045, Lawrence, KS, USA
Michael H Crawford & Kristine G Beaty
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Michael H Crawford .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors’ contributions
MHC and KGB participated in the writing of the manuscript. Both authors read and approved the final manuscript.

Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2, authors’ original file for figure 3, authors’ original file for figure 4, rights and permissions.
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Crawford, M.H., Beaty, K.G. DNA fingerprinting in anthropological genetics: past, present, future. Investig Genet 4 , 23 (2013). https://doi.org/10.1186/2041-2223-4-23
Download citation
Received : 03 September 2013
Accepted : 03 September 2013
Published : 18 November 2013
DOI : https://doi.org/10.1186/2041-2223-4-23
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Anthropological genetics
- Variable-number tandem repeats
- Y chromosomal and autosomal short tandem repeats
Investigative Genetics
ISSN: 2041-2223
- Submission enquiries: [email protected]
- General enquiries: [email protected]

DNA Fingerprinting: A Milestone in Forensic Science
* corresponding author(s):.
The successful interpretation of DNA in forensic science is contingent upon the significance of the evidence found at a crime scene and the availability of suitable reference samples. However, it's important to acknowledge that errors and uncertainties are inherent aspects of DNA fingerprinting. Ongoing efforts are dedicated to enhancing the accuracy and reliability of results in this field. DNA has become a ubiquitous tool, serving as a kind of genetic identification card in many countries.
DNA fingerprinting remains a promising technique for forensic scientists, offering a powerful means to investigate individuals. This paper aims to provide a comprehensive and scientific perspective on the challenges associated with DNA profiling within forensic laboratories. While it briefly outlines the objectives and techniques of DNA profiling, the primary focus lies in DNA fingerprinting.
One of the fascinating aspects of DNA fingerprinting is its ability to identify non-coding regions of the genome, which are unique to every living organism. This innovation has played a pivotal role in forensic science, aiding in the exoneration of innocent individuals. Additionally, this article sheds light on the evolving landscape of DNA fingerprinting technology in recent years and highlights real-world cases where various iterations of this technology have successfully been employed to solve criminal investigations in research laboratories.
DNA profiling; DNA typing; Genomic finger-printing; RFLP; VNTR’s
- Lorem ipsum dolor sit amet, adipiscing elitazaa.
- Lorem ipsum dolor sit amet, consectetur adipiscing elitazaa.
- Lorem ipsum dolor sit amet, consectetur elitazaa.
- Lorem ipsum dolor sit, adipiscing elitazaa.
Introduction
IN 1886 in a study Sherlock Holmes shouted“I’ve found it! I’ve found it”, running towards Watson In 1886, a pivotal moment occurred in scientific history when Sherlock Holmes exclaimed, "I've found it! I've found it," rushing towards Watson with a test tube in hand, declaring, "I have found a re-agent which is precipitated by hemoglobin, and by nothing else" [1]. This exclamation echoed across England, much like the famous Eureka shout did in 1984 when Alec Jeffreys, a researcher at the University of Leicester in the UK, made a groundbreaking discovery. Jeffreys unveiled innovative variables and heritable patterns within repetitive DNA, analyzed using multi-locus probes. Unlike Sherlock Holmes, Jeffreys didn't name the method after himself but rather coined it 'DNA fingerprinting' [2]. Jeffreys' work focused on genes and aimed to establish genetic connections between individuals for purposes such as determining paternity and resolving immigration disputes.
This revolutionary technology allowed for the verification of whether two samples originated from the same person. Every individual on Earth possesses a unique molecular identity encoded within the sequence of their DNA, which is inherited from their biological parents and remains consistent in every cell of their body. This technique, known as DNA fingerprinting, can definitively establish an individual's parentage [3].
Forensic genetic fingerprinting, as defined by Jeffreys and Wilson [4], involves comparing the nucleated cells of an individual's DNA with biological samples collected from a crime scene or with another person's DNA for identification or exclusion.
DNA fingerprinting is a distinctive form of identification that relies on shreds of evidence. One of the earliest criminal cases solved using DNA analysis involved Colin Pitchfork, who had committed rapes and murders in 1983 and 1986, targeting two young girls, Lynda and Dawn. Investigators collected semen samples and analyzed them in a forensic laboratory. The DNA evidence conclusively proved Pitchfork's guilt, leading to his admission of the crimes and subsequent arrest [5].
The 1990s marked a golden era for technological advancements and discoveries, greatly benefiting forensic science. DNA fingerprinting emerged as a powerful tool for investigating criminal cases and played a crucial role in court decisions. DNA typing, a laboratory procedure that detects variations in DNA, proved invaluable for victim identification in both small and large-scale disasters.
DNA fingerprinting techniques can be categorized into PCR-based and non-PCR-based methods. PCR-based methods, such as STR (Short Tandem Repeat) analysis, are faster and more sensitive, while non-PCR methods like RFLP (Restriction Fragment Length Polymorphism) are slower and require larger DNA samples.DNA profiling typically uses a set of multi-allelic STR markers. Different countries and regions have established standard sets of markers for criminal databases, such as the EU standard set of 12 STR markers and the US CODIS standard of 13 markers.
- Uniqueness and probability
The uniqueness of each person's DNA, except for identical twins, allows for extremely low probabilities of two individuals having identical DNA profiles. This statistical uniqueness makes DNA evidence highly reliable in criminal investigations.
- DNA databases
Many countries maintain DNA databases like CODIS (Combined DNA Index System) to store DNA profiles from crime scenes and individuals. These databases help match DNA evidence to known individuals, aiding investigations.
- Historical background
The Federal Bureau of Investigation's article, published in 2008, represents a crucial milestone in understanding the statistical aspects of DNA profiling. It likely contributed to the standardization and improvement of DNA profiling techniques, emphasizing its growing significance in forensic science. Lutz Roewer, a forensic expert, published an article in 2013 that not only delves into the history of DNA fingerprinting but also provides insights into the modern technologies and techniques employed in this field. This article likely offered an updated perspective on the state of DNA fingerprinting. The National Institute of Standards and Technology (NIST) is a prominent research center that significantly contributed to the enhancement of DNA fingerprinting in 2016. NIST's involvement indicates ongoing efforts to improve the accuracy and reliability of DNA profiling methods. The international article published in 2017 explores the applications of DNA fingerprinting in forensic investigations. It likely highlights the expanding use of DNA profiling techniques in solving crimes and identifying individuals. Anam Hameed's research review in 2019 focuses on the issues related to DNA fingerprinting. This review may address various challenges, controversies, and ethical concerns associated with the use of DNA profiling in forensic science.
Currently, there is broad recognition of the advancements in scientific understanding related to the composition of the Y chromosome. This progress has been significantly bolstered by the introduction of highly sensitive panels that encompass up to 27 Short Tandem Repeats (STRs), incorporating markers that undergo rapid mutations. To calculate the probability of matches between Y-STR or mtDNA profiles, the prevalent counting method is typically employed [6]. However, ensuring accurate outcomes demands the existence of extensive, well-represented databases, rigorously verified for quality, containing haplotypes sampled from diverse reference populations. This necessity arises because individual allele frequencies in Y-STR and mtDNA profiles exhibit a distinct pattern of variation compared to independently obtained autosomal STRs.
In the traditional methodology of DNA fingerprinting, radio-labeled DNA samples containing minisatellite or oligonucleotide sequences undergo hybridization with DNA that has undergone processing using restriction enzymes. Following this, the fragments are separated through agarose electrophoresis and then immobilized-either through the Southern blotting method or, in the case of oligonucleotide tests, directly within a dried gel. Subsequently, the radio-labeled probes bind to various minisatellites or oligonucleotide sequences within genomic DNA. These sequences are found within restriction fragments of varying sizes, attributed to differences in the number of repeat units. After the removal of excess probes, visualization of these variable components is made possible through exposure to X-ray film (autoradiography), facilitating the comparison of profiles among individuals [7].
In the contemporary context, forensic DNA technology holds a profound influence over countless lives across the globe. The widespread acceptance of this technology is notably evident in its pivotal role in identifying survivors of significant events, including the 9/11 terrorist attacks, natural disasters like Hurricane Katrina [8], as well as the resolution of conflicts such as those in the former Yugoslavia [9] and Argentina [10,11]. Despite its portrayal as a definitive solution by both the general public and law enforcement personnel clad in white suits, the reality introduces nuanced questions. The advantages of forensic DNA fingerprinting must be considered in light of the associated social and ethical costs. Consequently, the legal community grapples with the dilemma of how DNA fingerprinting technology should evolve.
An increasing consensus suggests that DNA sequencing is poised to replace part-length analysis soon, and this viewpoint is substantiated by compelling arguments. The emergence of Next Generation Sequencing (NGS) technologies has significantly broadened the scope of forensic data collection and has enabled rapid, cost-effective analysis, considering the extensive pool of potentially informative DNA loci [12,13].
Nonetheless, the progression of forensic science is not without its share of challenges and controversies. Public perception often diverges from the realities of DNA profiling. A significant drawback lies in the potential invasion of personal privacy, as an individual's DNA reveals substantial information regarding their physical characteristics and health. This sensitivity necessitates meticulous handling, as individuals often harbor reservations about divulging their complete genetic information due to concerns related to privacy [14,15].
Moreover, the lack of standardization in DNA profiling protocols among various laboratories has contributed to inconsistencies in results obtained from identical samples submitted to different facilities. Furthermore, environmental factors such as humidity, temperature fluctuations, bacterial contamination, and exposure to UV radiation have been demonstrated to exert significant influences on the integrity of DNA profiles. Adverse conditions, including high humidity levels, can lead to oxidative damage and hydrolytic cleavage of DNA bonds, ultimately culminating in the degradation of genetic material [10,16,17].
DNA evidence is a powerful tool that not only helps us understand the genetic makeup of living organisms but also plays a crucial role in forensic science. In every cell of an individual's body, their unique DNA profile serves as an immutable identifier. This genetic distinctiveness becomes especially valuable in cases such as paternity tests, where the relationship between individuals can be accurately determined. Furthermore, in the field of forensic science, DNA fingerprinting has emerged as an indispensable technique. By analyzing DNA samples collected at crime scenes, law enforcement can identify and apprehend suspects, shedding light on the circumstances of criminal activities. Criminology benefits significantly from DNA analysis, contributing to the precision of criminal investigations and ensuring more accurate outcomes in the pursuit of justice. Ongoing research continues to refine DNA fingerprinting methods, promising even greater precision and broader applications in the future.
Acknowledgment
I would like to thank Allah Almighty for giving me strength and all blessings, my parents, and to my teachers, whose guidance encouraged and motivated me to have confidence in my abilities. And also, to my friends who always support me.
- Balding D (2013) Evaluation of mixed-source, low-template DNA profiles in forensic science. Journal of PubMed 110: 12241-12246.
- Jobling M, Gill P (2004) Encoded evidence: DNA in forensic analysis. Journal of Nature Reviews Genetics 5: 739-751 .
- Reilly P (2001) Legal, and public policy issues in DNA forensics. Journal of Nature Reviews Genetics 2: 313-317 .
- Jeffreys A, Wilson V (1985) Hypervariable ’minisatellite’ regions in human DNA. Journal of Nature 314: 67-73 .
- Wong Z, Wilson V, Patel I, Povey S, Jeffreys AJ (1987) Characterization of a panel of highly variable minisatellites cloned from human DNA. Journal of Annals of Human Genetics 51: 269-288 .
- Roewer L (2000) A new method for the evaluation of matches in non-recombining genomes: Application to Y-chromosomal short tandem repeat (STR) haplotypes in European males. Journal of ELSEVIER 114: 31-43 .
- Schafer R, Zischler H, Birsner U, Becker A, Epplen JT (1988) Epplen JT: Optimized oligonucleotide probes for DNA fingerprinting. Electrophoresis 9: 369-374 .
- Dolan SM, Saraiya DS, Donkervoort S, Rogel K, Lieber C, et al. (2009) The emerging role of genetics professionals in forensic kinship DNA identification after a mass fatality: Lessons learned from Hurricane Katrina volunteers. Journal of Genetics in Medicine 11: 414-417 .
- Huffine E, Crews J, Kennedy B, Bomberger K, Zinbo A (2001) Mass identification of persons missing from the break-up of the former Yugoslavia: structure, function, and role of the International Commission on Missing Persons. Journal of PubMed 42: 271-275 .
- Corach D, Corach D, Sala A, Penacino G, Iannucci N, et al. (1997) Additional approaches to DNA typing of skeletal remains: The search for ''missing'' persons killed during the last dictatorship in Argentina. Journal of PubMed 18:1608-1612 .
- Godard B, Schmidtke J, Cassiman JJ, Ayme S (2003) Data storage and DNA banking for biomedical research: Informed consent, confidentiality, quality issues, ownership, return of benefits. A professional perspective. European Journal of Human Genetics 11: 88-122.
- Christensen AM, Crowder CM, Ousley SD, Houck MM (2014) Error and it’s Meaning in Forensic Science. Journal of Forensic Sciences 59: 123-126 .
- Gill P, Fereday L, Morling N, Schneider PM (2006) The evolution of DNA databases- Recommendations for new European STR loci. Journal of ELSEVIER 156: 242-244.
- Hameed A (2019) Evaluation Issues with DNA Fingerprinting in Forensic Lab: A Review. Journal of Medical Research and Case Reports :1
- Jeffreys AJ, Wilson V (1985) Individual-specific “fingerprints” of Human DNA. Journal of Nature 314: 67-74.
- Jeffreys AJ, Brookfield JF, Semeonoff R (1985) Positive identification of an immigration test-case using human DNA fingerprints. Journal of Nature 317: 818-819 .
- Roewer L (2013) DNA fingerprinting in forensics: Past, present, future. Investigative Genetics 4: 1 .
Citation: Shahid S, Farrukh F (2023) DNA Fingerprinting: A Milestone in Forensic Science. Forensic Leg Investig Sci 9: 084.
Copyright: © 2023 Samya Shahid, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

Forensic Science DNA Profiling Finger Printing Legal Science Criminal Investigation
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 19 May 2020
DNA fingerprinting: an effective tool for taxonomic identification of precious corals in jewelry
- Bertalan Lendvay 1 , 2 ,
- Laurent E. Cartier 2 , 3 ,
- Mario Gysi 1 ,
- Joana B. Meyer 4 ,
- Michael S. Krzemnicki 2 ,
- Adelgunde Kratzer 1 &
- Nadja V. Morf 1
Scientific Reports volume 10 , Article number: 8287 ( 2020 ) Cite this article
6530 Accesses
6 Citations
21 Altmetric
Metrics details
- Biological techniques
- Marine biology
- Phylogenetics
Precious coral species have been used to produce jewelry and ornaments since antiquity. Due to the high value and demand for corals, some coral beds have been heavily fished over past centuries. Fishing and international trade regulations were put in place to regulate fishing practices in recent decades. To this date, the control of precious coral exploitation and enforcement of trade rules have been somewhat impaired by the fact that different species of worked coral samples can be extremely difficult to distinguish, even for trained experts. Here, we developed methods to use DNA recovered from precious coral samples worked for jewelry to identify their species. We evaluated purity and quantity of DNA extracted using five different techniques. Then, a minimally invasive sampling protocol was tested, which allowed genetic analysis without compromising the value of the worked coral objects.The best performing DNA extraction technique applies decalcification of the skeletal material with EDTA in the presence of laurylsarcosyl and proteinase, and purification of the DNA with a commercial silica membrane. This method yielded pure DNA in all cases using 100 mg coral material and in over half of the cases when using “quasi non-destructive” sampling with sampled material amounts as low as 2.3 mg. Sequence data of the recovered DNA gave an indication that the range of precious coral species present in the trade is broader than previously anticipated.
Similar content being viewed by others

A remarkable assemblage of petroglyphs and dinosaur footprints in Northeast Brazil
Leonardo P. Troiano, Heloísa B. dos Santos, … Aline M. Ghilardi
Archaeological and molecular evidence for ancient chickens in Central Asia
Carli Peters, Kristine K. Richter, … Robert N. Spengler III

Lineage dynamics of the endosymbiotic cell type in the soft coral Xenia
Minjie Hu, Xiaobin Zheng, … Yixian Zheng
Introduction
Precious corals are among the most appreciated and oldest known gems. They are valued for their color, texture and workability (polishing, carving), and have thus been collected and used for adornment for millennia 1 , 2 , 3 . Growing demand, particularly in Asia in recent years, has led to an increase in prices of precious corals used in jewelry 4 , 5 , 6 .
The most valuable precious coral species belong to the Coralliidae family within the Octocorallia subclass of the Anthozoa. The precious coral material used for jewelry is the worked (i.e. cut, carved and polished) hard coral skeletal axis, which is a biogenic material created by a biomineralization process 7 . In this process, closely packed magnesium-rich calcite crystals are secreted by coral polyps (1–2 mm in size) to build up a skeleton over decades. The polyps can thrive on the surface of the skeleton as colonies connected and surrounded by a 0.5–1 mm thick surface tissue (coenenchyme) 8 . The Coral Commission of The World Jewellery Confederation (CIBJO) lists eight Coralliidae species as significant in the precious coral jewelry industry 9 , 10 . Precious coral products are sold worldwide, with production centers located in Italy, Japan and Taiwan and large-scale trade of raw material between these areas 5 , 6 , 11 .
Until recent decades, the populations of these highly coveted marine animals experienced exploitation in boom and bust cycles where the discovery of precious coral beds led to rushes by coral fishers and these beds were exploited as long as it remained economically feasible 12 , 13 . Local and international regulations were put in place to control both fishing and international trade of precious corals, among which four Pacific species were listed in Appendix III of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) at the request of China 4 , 13 , 14 , 15 (Table 1 ). It has been reported that traders may often not be aware of the origin and species of their coral jewelry products 4 , 6 . At the same time, consumers and jewelers increasingly request specific information about precious corals, particularly their geographic origin and species, mainly due to the perceptions of value that different types of coral have in the market and possible sustainability considerations 16 .
Therefore, accurate taxonomic identification of precious coral products is of paramount importance for both efficient enforcement of precious coral trade regulations and for the jewelry industry. However, species of polished corals can be extremely difficult to distinguish even for trained experts based on morphological characteristics, and proper analytical tools to conclusively identify the species of worked precious corals are still lacking 6 , 12 , 16 , 17 .
The various analytical methods tested to distinguish precious coral species based on skeletal material were either unable to provide clear-cut distinction among the different coral species (e.g. trace element analysis, such as X-ray fluorescence spectroscopy, LA-ICP-MS and EMPA 18 ; and Raman spectroscopy 19 ), or were not improved to become a standardized and easy-to-use tool (such as immunolabeling 20 ). As a novel approach, Cartier, et al . 21 recently proposed DNA analysis to distinguish species, assuming that coral DNA molecules can be trapped in the organic material or adhered to the CaCO 3 crystals during the formation of the skeleton.
Genetic analyses have become a powerful analytical tool to elucidate the species identity and trace the geographic origin of various valuable artefacts of biogenic origin. These include processed products of tortoise shell 22 , snake skin 23 , fur 24 , 25 , ivory 26 , 27 or tiger bones 28 . Of greatest relevance to this present study, Meyer, et al . 29 reported quasi-nondestructive species identification of pearls based on DNA analysis, where so little amount of pearl material was used for the analyses that the market value of the pearl was not compromised. Particular biogenic materials require specific DNA extraction methods, moreover, we anticipate that DNA preserved in precious coral skeletons to be present in very small amounts and highly fragmented due to the lengthy skeleton-formation process and the degradation of the DNA after the death or the coral 30 , 31 , 32 , 33 . A further challenge of using DNA to distinguish Coralliidae species may arise from the exceptionally slow evolution of the Octocorallia mitochondrial genomes, which causes different species to be genetically highly similar 34 , 35 , 36 , 37 , 38 .
In the present proof of concept study, we aim to explore whether precious coral skeleton fragments cut, carved and polished for jewelry can be taxonomically identified through genetic analysis. We compare five different DNA extraction methods to find the method producing the highest purity and quantity of DNA. We then apply the most successful DNA extraction technique using a minimally destructive sampling method and amplify and sequence the recovered DNA to taxonomically identify the coral samples. We demonstrate that genetic analysis of gem-quality precious corals is a promising method to assess the identity of their species.
Comparison of DNA retrieved from worked precious corals with five extraction methods
Using a set of 25 worked coral samples, we evaluated which one of five candidate DNA extraction protocols is most suited to retrieve DNA from worked precious coral samples. Each of the five tested methods (abbreviated as “W”, “F”, “B”, “E”, “Y”) have earlier proven to be useful in extracting DNA from biomineralized material 29 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 . DNA was extracted from each of the 25 worked coral skeletal samples with all five techniques, and DNA purity and quantity were assessed using real-time quantitative PCR (qPCR) technology.
To test DNA extract purity, we assessed PCR inhibition with qPCR using an internal amplification control molecule. Three extraction methods, “F”, “E” and “Y”, resulted in DNA with no detectable PCR inhibition effect from any of the tested 25 samples (Fig. 1 , Supplementary Results S1 ). In contrast, a PCR inhibition effect was observed in 15 out of 25 samples extracted with the “B” method. Of these, complete inhibition of the PCR was observed in one case. Inhibition was also detected in three DNA extracts produced with the “W” method. Of these, no PCR product was observed at all in one sample.
Results of the DNA extract purity and quantity measurement experiment and taxonomic identification of 25 worked precious coral samples. Five methods were used to extract DNA from equal amounts of material from each sample. PCR inhibition measurement and absolute template quantification was performed with quantitative real-time PCR. Two short mitochondrial DNA fragments were sequenced and each specimen was taxonomically assigned. Note that identifications as Corallium japonicum , Pleurocorallium elatius or P. konojoi were possible based on the combination of genetic and morphological assessments.
Absolute quantity of the DNA obtained with the five extraction techniques was tested using qPCR with a standard curve from a dilution series of a standard template DNA molecule with known concentrations. Throughout these analyses, the average qPCR efficiency was 88.5% (± 3.6% standard deviation) and the coefficient of determination for the calibration curve was R 2 = 0.9947 (± 0.0035 standard deviation).
The five extraction methods yielded highly varying amounts of DNA (Fig. 1 , Supplementary Results S1 .). Methods “E” and “Y” both yielded PCR amplifications for all 25 samples. Method “W” yielded PCR product for 13 samples, while methods “F” and “B” both yielded PCR product for 21 samples. Overall, there was concordance among the amplification results; the 13 samples that amplified with method “W” also amplified with methods “F” and “B”, and the latter two methods amplified DNA of the very same 21 samples. Strong significant correlation was found between the copy numbers obtained from the same coral items with the “E” and “Y” methods (r = 0.97, t = 19.223, df = 23, p < 0.001). DNA yield was higher with method “Y” than with method “E” (595 versus 944 molecules per mg coral sample with “E” and “Y”, respectively; paired t-test: t = −2.8832, df = 24, p = 0.008). Focusing on the best performing “Y” method, DNA concentrations ranged between three orders of magnitude: three samples had over 10 3 DNA copies in each mg of coral skeleton material. In five other samples this value was below 10 (Fig. 1 ).
DNA extraction with “quasi non-destructive” sampling of worked precious coral samples
In the previous experiment, 25 samples were completely pulverized and five DNA extractions were carried out with different methods from each. The aim was to select the most suitable technique for extracting DNA from worked coral samples. In the current experiment, the best performing DNA extraction technique was used with “quasi non-destructive” sampling of worked corals. We developed a “quasi non-destructive” technique to take material for analysis from the worked corals with minimal weight loss and virtually invisible effects of the sampling (Fig. 2 ). A new set of 25 worked coral samples were sampled in this manner; removed material amounts ranged from 2.3 mg to 13.1 mg and were 7.9 mg on average. Modifications were applied to the lysis step of the “Y” extraction method compared to the original protocol, which resulted in an essentially complete dissolution of the coral powder. This allowed the amount of DNA that remained trapped in the undissolved powder to be kept to a minimum. Out of the 25 “quasi non-destructively” sampled worked coral objects, 16 gave qPCR amplicons at least twice (Fig. 3 , Supplementary Results S1 ). Another two samples produced amplification only once and were omitted from further analyses. DNA copy numbers calculated per mg of coral sample were in the same range as in the case of the extractions carried out from ca. 100 mg material using the “Y” method. However, the presence of unsuccessful amplifications and lower average copy number (160 DNA copies) recovered per mg of coral skeletal material indicates that DNA recovery from low amount samples is less effective than from standard material amount, despite the amendments made in the DNA extraction protocol.
“Quasi non-destructive” sampling of worked coral skeletons. ( a ) Widening the inner surface of the existing drill-hole in a bead. ( b ) Sampling the back side of a cabochon item.
Results of DNA quantity measurement and taxonomic identification of 25 worked precious corals sampled by the minimally invasive technique. Absolute template quantification was performed with quantitative real-time PCR. Two short mitochondrial DNA fragments were sequenced and each specimen was taxonomically assigned. Note that identifications as Corallium japonicum , Pleurocorallium elatius or P. konojoi were possible based on the combination of genetic and morphological assessments.
Taxonomic assignment of worked precious corals
We sequenced amplicons of the large ribosomal RNA gene subunit (LR) and the putative mismatch repair protein (MSH) fragments originating from a total of 41 worked coral skeletal samples using massively parallel sequencing. In our entire DNA sequence dataset, the sequence of altogether three OTUs were highly divergent from any of the Coralliidae LR or MSH reference sequences. NCBI BLAST search did not find any sequence entries in the NCBI database with higher than 95% sequence similarity for any of these sequences.
The lengths of the concatenated LR and MSH sequences were between 264 base-pairs (bp) and 290 bp long per coral sample (Supplementary Results S2 ). Bayesian phylogenetic analysis identified 10 samples (11, 14, 19, 22, 23, 31, 34, 38, 41, 45) as Corallium rubrum , of which nine had sequences identical to either of two the reference C. rubrum sequences, and one (11) had a single variable site (Fig. 4 ). Six samples (9, 17, 20, 21, 28, 35) were identical with reference samples of Corallium japonicum , but also with the reference samples of C . nix and C . tortuosum .
Majority-rule Bayesian phylogenetic tree constructed from combined mitochondrial LR and MSH region DNA sequence data of worked precious corals and reference samples. Posterior probability value is displayed after each tree node.
Three samples (6, 15, 24) formed a polytomic clade with Hemicorallium reference sequences. Two of these (6, 15) had sequences identical to Hemicorallium laauense , but also to samples of H . abyssale , H . bathyrubrum , H . ducale and H . imperiale . The third sample (24) was one bp different from these sequences. Seven samples (3, 5, 10, 13, 37, 40, 47) with identical sequences appeared as an unresolved clade basal to the formerly mentioned samples. These had identical sequences with H . abyssale , H . ducale and H . imperiale .
Six samples (4, 7, 8, 12, 16, 39) had identical sequences with Pleurocorallium carusrubrum , P . elatius and P . konojoi reference samples. Two samples (18, 46) formed a sister clade to the former group with the posterior probability value 1. Finally, seven identical samples (1, 2, 25, 33, 42, 44, 50) were same as sequences of Pleurocorallium niveum . These were grouped together as an unresolved tree branch.
Technical advancements and the growing body of reference DNA data have made genetic analyses a powerful tool to combat poaching, illegal trading and mislabeling of animal products 49 . Application of genetic barcoding was suggested by Ledoux, et al . 50 as a forensic tool to identify species of corals. Acknowledging that the discriminatory power of standard species barcoding markers (e.g. the cytochrome c oxidase subunit I gene) is poor to distinguish the closely related precious coral species, these authors suggested development of custom designed species identification markers. Moreover, if the aim is to distinguish coral skeletal samples, then the high portion of fragmented DNA will call these markers to be as short as possible. A further challenge is if sampling of the coral sample is to be done with minimal material loss. As a consequence, the chosen DNA extraction method has to be capable of recovering DNA from a small sample amount.
In our quest to find an optimal method to recover DNA from worked coral samples, we tested the performance of five DNA extraction methods, each on equal amounts of coral material from the same set of 25 worked coral samples. We found two methods, protocol “E” and “Y” that yielded DNA that was successfully amplified and sequenced from all of the 25 tested corals. Methods “E” and “Y” are two similar techniques developed for the extraction of DNA from ancient eggshells and ancient bones. They only slightly differ in their lysis buffer ingredients and the type of DNA-binding silica column used for the purification of the recovered DNA molecules 45 , 46 . These methods produced similar amounts of DNA, however method “Y” produced slightly higher DNA yield, particularly in the samples that had <50 DNA copies per mg of coral powder. The three other tested DNA extraction methods did not result in amplifiable DNA from all samples, which may be due to their inability to recover DNA coupled with PCR-inhibitory effect of co-extracted substances, which was detected in some extracts, PCR inhibition was not detected in any extracts produced with methods “W”, “E” and “Y”. By using these methods, PCR inhibition seems to be overcome in precious corals, unlike in other types of corals, where it led to technical challenges 51 .
DNA concentration of the extracts differed largely; while in certain samples <10 copies per mg of material was recovered, in some others this reached up to the order of magnitude of 10 3 copies per mg of material. The large variation in DNA preservation of the samples may be determined by several factors; the age of the coral when fished, whether the coral was fished dead or alive 30 , 31 , 32 and the time since the coral was fished 6 . However, without specific knowledge about the age of the samples this remains hypothetical.
Our test to choose the best DNA extraction protocol from potential methods was based on 100 mg of coral skeleton material, which is a standard amount used for extracting DNA from pulverized material with the applied protocols. The essence of precious material testing would be to use as little material as possible, ideally using a “quasi non-destructive” sampling method. This means that the sampling area is not visible and the sampling does not cause significant weight loss of the coral object. Worked coral samples can be separated into two main types; the ones that have a hole drilled through the item (generally those that are strung as beads for bracelets or necklaces) and the ones that do not have a hole, instead generally have flat reverse or bottom sides (those that are mounted to a frame and used as pendants, i.e. cabochons, or the carved figures used as ornaments). We performed “quasi non-destructive” sampling using a drill with a 0.8 mm diameter diamond engraver head taking care not to heat up the sampled object (no hard pressing of the drill and regular pauses to let the drill head cool down). With careful handling, it was possible to take sample material by slightly widening the internal surface of the ca. 1 mm wide drill-holes, which ensures that this material extraction is invisible by eye upon subsequent inspection of the sample. From the cabochons, a thin layer was removed from the reverse side; therefore the visible front side remains unaffected by the sampling. Assuming approximately 3.8 kg/dm 3 density of the precious corals 9 , the removed 2.3–13.1 (on average 7.9) mg powder per sample corresponds to a 0.7–3.5 (2.1) mm 3 volume loss of the items.
We were able to repeatedly produce PCR products for 16 out of the 25 “quasi non-destructively” sampled worked coral samples. We could not determine a threshold for the minimum amount of material necessary for successful genetic testing; the two samples processed with the lowest weight of coral powder, 2.3 mg and 2.6 mg, respectively, both produced results. Although it was not possible to genetically analyze all samples with the minimally destructive method, there might be a good chance that when analyzing several samples from a batch of samples, at least some will produce results.
We expected that all of the DNA sequences we generated will be identical with at least one reference sequence of the eight species listed by CIBJO as relevant in the jewelry industry 9 . However, against our expectations, we found a much higher diversity within our samples, with several of our sequences not grouping together with any of the reference sequences of the eight species. Hence, we performed an other phylogenetic analysis with a more extended reference sample set. The results of this analysis show that samples could clearly be identified as Corallium rubrum . The samples grouping together with Corallium japonicum also grouped together with two other species, C . nix and C . tortuosum , which, however, have white and pink color, respectively, unlike the dark red color of C . japonicum 44 , 52 . Hence, we can confidently identify these red corals as C . japonicum based on the combination of genetic and morphological characteristics.
Samples that grouped together with the Hemicorallium references all had identical sequences with multiple Hemicorallium species. As a consequence, these samples could be identified only to the genus level as Hemicorallium . A part of these samples (i.e. 3, 5, 10, 13, 37, 40, 47) did not cluster with the three reportedly fished Hemicorallium species ( H . laauense , H regale , H . sulcatum ), but instead had identical sequences to other species ( H . abyssale , H . ducale and H . imperiale ) that all occur around the Hawaii islands, a historically important fishing area 44 , 45 , 47 . This result strongly suggests that H . laauense , H regale and H . sulcatum are not the only Hemicorallium species present in the jewelry trade.
Some samples had identical sequences as the three genetically and morphologically, very similar species, Pleurocorallium , P . carusrubrum, P . elatius and P . konojoi 47 , 53 . Of these species, the latter two are well known in the jewelry industry, while the former is a recently described species known from a single area of the West Pacific 53 . To distinguish these species, the coloration of the skeletal axis may provide a partial solution. In particular, the color of P . carusrubrum is red, P. elatius varies from pale to dark pink, while P. konojoi is always pure white 4 , 6 , 9 , 10 , 17 . Consequently, our specimens identified as one of these species with pink shading may be identified as P. elatius , while our samples with white color are determined as P . konojoi .
Of our multiple samples within the Pleurocorallium clade that did not group together with the species traditionally accepted as being present in the coral trade ( P . elatius , P . konojoi and P . secundum ), two samples (18, 46) formed an individual clade and were identified to the genus level as Pleurocorallium . DNA sequences of the other samples were all identical with the sequences of the Pleurocorallium niveum samples. This species was described from waters surrounding the Hawaii islands, which was a historically important coral fishing area 54 , 55 . The 41 samples that we managed to genetically analyze from 50 samples of a single collection is not representative enough to be able to draw conclusions about the entire jewelry industry, but it indicates that there may be more species present in the trade than the eight precious coral species commonly listed as part of the jewelry industry (cf 9 , 10 , 16 .). This is conceivable, if we consider that in the Pacific Ocean different precious coral species may co-occur and coral fishing does not seek to individually separate them based on species. The presence of more than the previously anticipated eight species also implies that accurate species identification in all cases will only be possible using markers that can differentiate among all species within the Coralliidae family.
Conclusions
This study is a proof of concept demonstrating that genetic analysis can be an effective tool to taxonomically identify precious corals worked for jewelry. We demonstrated that while 100 mg coral skeletal material is sufficient for successful DNA extraction in all cases, DNA sequencing and taxonomic assignment were possible with minute amounts of “quasi non-destructive” samples in more than half of the cases. Among the worked precious corals examined in this study, DNA sequence analyses revealed several samples very likely belonging to precious coral species previously not considered to be present in the jewelry industry. Future research should focus on broadening the reference data by sequencing multiple specimens for each species identified by experts in order to substantiate their intra- and interspecific genetic diversity. Additionally, the development of more specific markers will allow for the identification of coral samples with higher accuracy. These will be essential steps in developing genetic tests that can become a reliable and standardized method to promote transparency, traceability and sustainable use of precious corals in the jewelry industry.
Materials and Methods
Studied species.
The precious corals relevant to the high-end jewelry industry are Octocorallid Anthozoans that belong to the Alcyonacea order and Coralliidae family. Recent phylogenetic studies confirmed the existence of three genera in the family; Corallium , Hemicorallium and Pleurocorallium 56 , 57 . Of the eight species listed by CIBJO as significant in the precious coral industry, a single, Corallium rubrum , is distributed in the Mediterranean Sea and has been fished since antiquity 6 . Four other species, Corallium japonicum , Hemicorallium sulcatum , Pleurocorallium elatius and Pleurocorallium konojoi have been fished in the Western Pacific ocean since the early 19 th century 12 . The remaining three species, Hemicorallium laauense , Hemicorallium regale and Pleurocorallium secundum were discovered on seamounts surrounding the Hawaii archipelago and were fished in large quantities during the second half of the 20 th century 58 . Distribution, CITES listing and trade names of the eight precious coral species relevant to the jewelry industry are summarized in Table 1 , while further details on their distribution, taxonomy, harvesting and conservation are available in Cannas, et al . 59 .
Genetic markers used in the study
We expected that the DNA extracted from the coral skeletal samples would be highly degraded. Therefore, we used markers developed on the mitochondrial genome, which is present in each cell in multiple copies and offers the best chances of achieving positive results for fragmented DNA. Octocoral mitochondrial genomes have an exceptionally low rate of evolution and standard taxonomic markers are unable to distinguish closely related species 34 , 38 , 60 . Hence, we designed primers for two genetic markers with the criteria that the resulting amplicon sequences are short enough to be suitable for degraded DNA and highly variable in order to maximize our ability to identify the precious coral species to the lowest possible taxonomic level. We expected each analyzed sample to originate from one of the eight precious coral species listed by CIBJO, thus chose our markers with the aim that they should be capable of distinguishing these eight species. The two mitochondrial markers were developed based on DNA sequence data of Tu, et al . 57 , which is the most detailed study on precious coral phylogeny to date. Marker selection and procedures for designing PCR primers are detailed in Supplementary Methods S3 .
Following examination of the phylogenetic resolution of multiple short mitochondrial genome fragments, we developed two sets of primers for the large ribosomal RNA gene subunit (LR gene, LR-F 5′TTCATCACAGTGAGGGTTTGT3′ and LR-R 5′TGCAAAGAAGGAGAACAAAAGG3′) and the putative mismatch repair protein (MSH gene, MSH-F 5′CGAAAGCGGATAAAAGCTACC3′ and MSH-R 5′CCTCACTGTCAGGCTAATGAG3′), respectively. The LR marker was used for the assessment of DNA purity and DNA quantification. Phylogenetic analysis using the combined LR and MSH markers showed that these two short markers were able to reconstruct the phylogenetic relationships obtained by much longer sequences, and they allowed the distinction of each of the eight precious coral species from each other, except for Pleurocorallium elatius and P. konojoi , It is not possible to conclusively distinguish these two species based on the data of Tu, et al . 57 (Supplementary Methods S3 ).
Comparison of DNA purity and quantity extracted with different methods
Dna extraction.
All laboratory work was carried out at the Forensic Genetics department of the Zurich Institute of Forensic Medicine, University of Zurich, in the laboratory facility dedicated to human and animal forensic casework. We strictly adhered to the ISO 17025 guidelines throughout the laboratory workflow with stringent rules to avoid contamination and authenticate our results (Supplementary Methods S4 ). Precious coral samples used in this study originated from the collection of the Swiss Gemmological Institute SSEF, Basel, Switzerland.
Twenty-five worked coral samples were selected for the experiment (named samples 1–25, Supplementary Table S5). The samples were cleaned as described in Supplementary Methods S4 and crushed in a metal mortar with a metal pistil to produce crude coral powder, which was then transferred to a porcelain mortar and ground to fine powder. The coral skeleton powder was divided into five aliquots of equal weight, 100 mg ± 1 mg in general, except for four samples that had less available powder (Supplementary Table S5 ). The powder aliquots were used to extract DNA using five different extraction methods, which have proven to be effective in successfully recovering DNA from biomineralized material (Table 2 ). For each method, we followed the protocols cited in Table 2 . All DNA extracts were eluted in 100 µl and stored at −20 °C.
Assessment of the purity of the DNA extracts
We used qPCR to compare the purity of the DNA extracts produced from worked precious coral samples with five different extraction protocols. DNA purity was measured by testing the PCR inhibiting effect of the coral extracts during amplification of an internal positive control DNA fragment. We used 10 3 copies of a synthetic oligonucleotide (gBlocks Gene Fragments; International DNA Technologies, Coralville, IA, USA 61 ) as internal amplification control (IAC, Supplementary Methods S6 ). The 197 bp sequence of the IAC matched 151 bp of the C. rubrum LR gene fragment (with manual introduction of five unique mismatches for contamination detection purpose) flanked by potato-specific sequences as primer sites following Nolan, et al . 62 .
Following optimization (see Supplementary Methods S6 ), reactions were conducted in 20 µl volumes containing 1 × PowerUp SYBR Green Master Mix (Thermo Fisher), 1 µl of both 15 uM concentration primers, 10 3 copies of the AIC in 3 µl and 3 µl coral DNA extract. Alongside the samples containing coral DNA extracts, we run three positive standard reactions that did not contain coral DNA. Following the manufacturer’s recommendation, reactions commenced with 50 °C for 2 minutes, which was followed by initial denaturation at 95 °C for 2 minutes and 50 cycles of denaturation at 95 °C for 15 seconds, primer annealing at 60 °C for 15 seconds and elongation at 72 °C for 1 minute. A melting-curve analysis was performed at the end of the reaction by heating the PCR products from 60 °C to 95 °C with 1% ramping speed. Each coral extract was run in triplicates on an ABI 7500 qPCR instrument (Thermo Fisher).
The quantification cycle (Cq) value of each reaction containing coral DNA extract was compared to the average Cq value of the three positive standard reactions and then the three Cq shift values of each sample were averaged. The intensity of PCR inhibition in each reaction was determined as follows: we considered inhibition to be present if there was a 0.5< cycle Cq shift compared to the positive standard Cq. Four categories of PCR inhibition were considered: 0.5–1, 1–2, 2<cycle shifts and complete inhibition in case at least one out of the three reactions produced no PCR product.
Absolute DNA quantification of the coral DNA
Absolute quantification of the coral LR gene fragment was conducted by qPCR of the coral DNA using a calibration curve prepared as a series of standard reactions with a known template DNA amount. The standards contained seven different 10-fold diluted template inputs (10 7 –10 1 copies) of a GBlocks synthetic oligonucleotides of the 154 bp long sequence of the LR gene fragment characteristic to C. rubrum (with manual introduction of three unique mismatches for the purpose of contamination detection) flanked by the LR primer sequences (Supplementary Methods S6 ). Following optimization of the reaction setup (Supporting Methods S6 ), reactions were carried out in 20 µl volumes containing 1 × PowerUp SYBR Green Master Mix (Thermo Fisher), 1 µl of both 15 µM concentration primers and 3 µl coral DNA extract. The cycling conditions were identical to those of the DNA extract purity test.
For each sample, PCR was considered successful if at least two reactions of the triplicates amplified. The Ct values were averaged for each sample and the mean Ct values were transformed to number of DNA molecules per mg of coral sample based on the volume of the DNA template in the PCR reaction, the DNA extract elution volume and the amount of coral powder used for the DNA extraction. We compared the DNA quantities gained with the extraction methods for which DNA was successfully amplified for all 25 samples with a correlation test and paired t-test in R 63 .
“Quasi non-destructive” sampling, DNA extraction and quantification
We define “quasi non-destructive” sampling as taking material for analysis from the worked objects without compromising its market value. A new set of 25 worked coral samples were selected from the SSEF coral collection for this experiment (named samples 26–50, Supplementary Table S7 ), and each was thoroughly cleaned as described in Supporting Methods S4 . Two main types of samples were sampled differently: (i) beads with drill-holes: the inner surface of the drill-hole was carefully widened (Fig. 2a ); (ii) worked items with no existing drill-hole: a small layer of the surface of the back side of a cabochon was removed (Fig. 2b ). We used 0.8 mm diameter diamond engraver bit heads attached to a Dremel 4000–4 rotary tool (Dremel, Racine, WI, USA). The rotation speed was set to 10,000 rpm and the extracted coral powder was left to drop in 1.5 ml collection tubes.
DNA was extracted from the quasi non-destructively sampled drill-powder of the 25 samples with the “Y” method. The material amount obtained by the “quasi non-destructive” sampling was far lower than the 100 mg used in the experiment comparing extraction methods, therefore we slightly modified the “Y” protocol to accommodate it to the low material amount. In particular, 200 µl lysis buffer was added to the coral powder and incubated at 56 °C for one hour with mixing, then another 100 µl lysis buffer was added. The lysis-mixture was incubated again with mixing at 56 °C for one hour and then at 37 °C for an additional 65 hours. In the next step, the lysate was mixed with 450 µl 1 × TE buffer and 3750 µl PB buffer (Qiagen) and the entire volume of the mixture was centrifuged through a MinElute (Qiagen) column. The column was washed with PE buffer and the DNA was eluted in 35 µl EB buffer (Qiagen).
Taxonomic identification
Dna amplification and sequencing.
We sequenced PCR products of DNA samples extracted with the “Y” method. For the LR fragment, qPCR products generated for the DNA quantity assessment were sequenced: from each sample one of the triplicate qPCR was selected for sequencing. The MSH region was amplified and sequenced for altogether 41 DNA samples: all 25 DNA samples from the DNA extraction test and those 16 DNA extracts from the “quasi non-destructive” sampling that gave amplification products for the LR region. The MSH was amplified in singlicate for each sample with identical reaction setup and cycling conditions as described above for the LR region.
The 16S and MSH PCR products were purified with the AMPure bead system (Beckman Coulter, Brea, CA, USA) and quantified with a Qubit 4 Fluorimeter (Thermo Fisher). The two amplicons of each DNA sample were pooled with equimolar concentrations, and sequencing libraries were constructed with the Ion Plus Fragment Library Kit (Thermo Fisher) according to the vendor’s protocol. The libraries were quantified with the Ion Library TaqMan Quantitation Kit (Thermo Fisher) and all samples were pooled with equimolar concentrations. Sequencing was carried out on an Ion S5 (Thermo Fisher) instrument at the Zurich Institute of Forensic Medicine, University of Zurich.
Analysis of the amplicon sequence data
Raw DNA sequence read data was exported to fastq files according to sequencing barcodes with the FileExporter plugin of the Torrent Suite software version 5.10. Primer sequences were removed from the end of the sequences of each fastq file using the cutadapt algorithm 64 implemented on the Galaxy server 65 . Trimmed sequences were quality-filtered using Usearch 66 with a maximum expected error threshold of 100 and clustered into operational taxonomic units (OTUs) with Uparse 67 at 97% minimal identity threshold and minimal OTU size of 10 sequence reads, as default settings. In some cases, these settings were slightly modified for more relaxed quality filtering and clustering to allow OTU creation for samples with lower quality sequence reads. Sequences of the resulting LR and MSH OTUs were aligned and the alignments were concatenated in Geneious version 11.1.5 ( https://www.geneious.com ). Our concatenated LR-MSH sequence alignment was added to the LR-MSH alignment of reference samples of the eight precious coral species listed in Table 1 . The taxonomic identity of our sequences was determined by constructing a Bayesian phylogenetic tree as described in Supporting Methods S2 . We noticed that several of the DNA sequences obtained from the coral samples were not identical with any of the reference sequences of the eight precious coral species described to be found in the international trade. We therefore performed an additional phylogenetic analysis with identical settings, which included the orthologous LR-MSH DNA sequences of all Coralliidae specimens from Tu, et al . 47 that were identified to the species level (Supplementary Table S8 ).
Data availability
Raw DNA sequence data generated for this study are deposited in the NCBI Sequence Read Archive under submission number SUB6412194. Data used for the analyses is available as Supplementary Information.
Fürst, S. et al . Raman investigations to identify Corallium rubrum in Iron Age jewelry and ornaments. Minerals 6 , 56 (2016).
Article CAS Google Scholar
Moradi, Z. The role of coral in art and architecture. An overview . International Journal of Aquatic Biology 4 , 125–142 (2016).
Google Scholar
Skeates, R. Mediterranean coral: its use and exchange in and around the alpine region during the later Neolithic and copper age. Oxford Journal of Archaeology 12 , 281–292 (1993).
Article Google Scholar
Shiraishi, H. Seeing red. Precious coral trade in East Asia. (TRAFFIC Office Japan) (2018).
Chang, S.-K. Precious corals become more precious in the northwestern pacific: Urgent need for integrated policy. Marine Policy 52 , 103–107 (2015).
Torntore, S. J. Precious corals in a global marketplace. in Proceedings of the first international workshop on Corallium science, management, and trade. (ed AW Bruckner & GG Roberts) 34–58 (NOAA Technical Memorandum NMFS-OPR-43 and CRCP-8) (2009).
Perrin, J. et al . Block-by-block and layer-by-layer growth modes in coral skeletons. American Mineralogist 100 , 681–695 (2015).
Article ADS Google Scholar
Nonaka, M., Muzik, K. & Iwasaki, N. Descriptions of two new species and designation of three neotypes of Japanese Coralliidae from recently discovered specimens that were collected by Kishinouye, and the introduction of a statistical approach to sclerite abundance and size. Zootaxa 3428 , 1–67 (2012).
CIBJO. The coral book. (Coral Commission of The World Jewellery Confederation) (2015).
CIBJO. Coral guide for customes. Classification & identification of coral materials. (The World Jewellery Confederation) (2017).
Cattaneo-Vietti, R. et al . An overexploited Italian treasure: past and present distribution and exploitation of the precious red coral Corallium rubrum (L., 1758) (Cnidaria: Anthozoa). Italian Journal of Zoology 83 , 443–455 (2016).
Tsounis, G. et al . The exploitation and conservation of precious corals. Vol. 48 (CRC Press) (2010).
Bruckner, A. W. Advances in management of precious corals in the family Corallidae: are new measures adequate? Current Opinion in Environmental Sustainability 7 , 1–8 (2014).
CITES. Convention on International Trade in Endangered Species of Wild Fauna and Flora. Checklist of CITES Species. www.checklist.cites.org Accessed: 04.07.2019 (2019).
Cau, A., Cannas, R., Sacco, F. & Follesa, M. Adaptive management plan for red coral ( Corallium rubrum ) in the GFCM competence area. (University of Cagliari) (2013).
de Carvalho, R. G. Precious corals. InColor. A publication of the International Colored Gemstone Association 37 , 70–78 (2018).
Cooper, E. W., Torntore, S. J., Leung, A. S., Shadbolt, T. & Dawe, C. Guide to the identification of precious and semi-precious corals in commercial trade. (TRAFFIC North America and WWF-Canada) (2011).
Hasegawa, H., Rahman, M. A., Luan, N. T., Maki, T. & Iwasaki, N. Trace elements in Corallium spp. as indicators for origin and habitat. Journal of Experimental Marine Biology and Ecology 414 , 1–5 (2012).
Macchia, M., Resta, V., Quarta, G. & Calcagnile, L. Precious coral non-destructive characterization by Raman and XRF spectroscopy. X-Ray Spectrometry 45 , 281–287 (2016).
Article ADS CAS Google Scholar
Debreuil, J. et al . Specific organic matrix characteristics in skeletons of Corallium species. Marine Biology 158 , 2765–2774 (2011).
Cartier, L. E., Krzemnicki, M. S., Lendvay, B. & Meyer, J. B. DNA fingerprinting of pearls, corals and ivory: a brief review of applications in Gemmology. Journal of Gemmology 36 , 152–160 (2018).
Foran, D. R. & Ray, R. L. Mitochondrial DNA profiling of illegal tortoiseshell products derived from hawksbill sea turtles. Journal of Forensic Sciences 61 , 1062–1066 (2016).
Article CAS PubMed Google Scholar
Dubey, B., Meganathan, P. & Haque, I. DNA mini-barcoding: an approach for forensic identification of some endangered Indian snake species. Forensic Science International: Genetics 5 , 181–184 (2011).
Pilli, E. et al . Pet fur or fake fur? A forensic approach. Investigative Genetics 5 , 7 (2014).
Article CAS PubMed PubMed Central Google Scholar
Janjua, S., Fakhar-I-Abbas, William, K., Malik, I. U. & Mehr, J. DNA Mini-barcoding for wildlife trade control: a case study on identification of highly processed animal materials. Mitochondrial DNA Part A 28 , 544–546 (2017).
Kitpipit, T., Thongjued, K., Penchart, K., Ouithavon, K. & Chotigeat, W. Mini-SNaPshot multiplex assays authenticate elephant ivory and simultaneously identify the species origin. Forensic Science International: Genetics 27 , 106–115 (2017).
Winters, M. et al . Isolation of DNA from small amounts of elephant ivory: Sampling the cementum with total demineralization extraction. Forensic Science International 288 , 131–139 (2018).
Kitpipit, T., Tobe, S. S., Kitchener, A. C., Gill, P. & Linacre, A. The development and validation of a single SNaPshot multiplex for tiger species and subspecies identification—Implications for forensic purposes. Forensic Science International: Genetics 6 , 250–257 (2012).
Meyer, J. B. et al . DNA fingerprinting of pearls to determine their origins. PloS One 8 , e75606 (2013).
Article ADS CAS PubMed PubMed Central Google Scholar
Chen, C.-S. Management of the precious coral fishery in Taiwan: progress and perspectives. Marine Policy 36 , 623–629 (2012).
Huang, M.-H. & Ou, C.-H. Precious coral fisheries management in Taiwan—Past, present & future. Marine Policy 34 , 1002–1009 (2010).
Okumura, T. 14 C dating of precious corals in Kochi for understanding the fishing field formation processes. in International precious coral conference (Kochi, Japan) (2018).
Iwasaki, N. Precious coral fishery in Japanese history since World War II: issues and visions for sustainable use of resources. in The academic pilgrimage to sustainable social development. Vol. 1 225-258 (Rissho University) (2018).
Shearer, T., Van Oppen, M., Romano, S. & Wörheide, G. Slow mitochondrial DNA sequence evolution in the Anthozoa (Cnidaria). Molecular Ecology 11 , 2475–2487 (2002).
Uda, K. et al . Complete mitochondrial genomes of the Japanese pink coral ( Corallium elatius ) and the Mediterranean red coral ( Corallium rubrum ): a reevaluation of the phylogeny of the family Coralliidae based on molecular data. Comparative Biochemistry and Physiology, Part D 8 , 209–219 (2013).
CAS PubMed Google Scholar
Takata, K. et al . Multiplexed ISSR genotyping by sequencing distinguishes two precious coral species (Anthozoa: Octocorallia: Coralliidae) that share a mitochondrial haplotype. PeerJ 7 , e7769 (2019).
Article PubMed PubMed Central Google Scholar
McFadden, C. S. et al . Limitations of mitochondrial gene barcoding in Octocorallia. Molecular Ecology Resources 11 , 19–31 (2011).
Hellberg, M. E. No variation and low synonymous substitution rates in coral mtDNA despite high nuclear variation. BMC Evolutionary Biology 6 , 24 (2006).
Article PubMed PubMed Central CAS Google Scholar
Chatters, J. C. et al . Late Pleistocene human skeleton and mtDNA link Paleoamericans and modern Native Americans. Science 344 , 750–754 (2014).
Article ADS CAS PubMed Google Scholar
Villanea, F. A., Parent, C. E. & Kemp, B. M. Reviving Galápagos snails: Ancient DNA extraction and amplification from shells of probably extinct endemic land snails. Journal of Molluscan Studies 82 , 449–456 (2016).
Stray, J. et al . Extraction of high quality DNA from biological materials and calcified tissues. Forensic Science International: Genetics Supplement Series 2 , 159–160 (2009).
ADS Google Scholar
Hasap, L. et al . Comparison of two DNA extraction methods: PrepFiler® BTA and modified PCI-silica based for DNA analysis from bone. Forensic Science International: Genetics Supplement Series 7 , 669–670 (2019).
Oskam, C. L. et al . Fossil avian eggshell preserves ancient DNA. Proceedings of the Royal Society of London B: Biological Sciences 277 , 1991–2000 (2010).
Huynen, L., Gill, B. J., Millar, C. D. & Lambert, D. M. Ancient DNA reveals extreme egg morphology and nesting behavior in New Zealand’s extinct moa. Proceedings of the National Academy of Sciences 107 , 16201–16206 (2010).
Oskam, C. L. & Bunce, M. DNA extraction from fossil eggshell. in Ancient DNA. Methods and protocols. (eds Beth Shapiro & Michael Hofreiter) 65-70 (Springer) (2012).
Gamba, C. et al . Comparing the performance of three ancient DNA extraction methods for high‐throughput sequencing. Molecular Ecology Resources 16 , 459–469 (2016).
Der Sarkissian, C. et al . Ancient DNA analysis identifies marine mollusc shells as new metagenomic archives of the past. Molecular Ecology Resources 17 , 835–853 (2017).
Der Sarkissian, C. et al . Unveiling the ecological applications of ancient DNA from mollusk shells. Frontiers in Ecology and Evolution 8 , 37 (2020).
Iyengar, A. Forensic DNA analysis for animal protection and biodiversity conservation: a review. Journal for Nature Conservation 22 , 195–205 (2014).
Ledoux, J.-B. et al . Molecular forensics into the sea: how molecular markers can help to struggle against poaching and illegal trade in precious corals? in The cnidaria, past, present and future (eds Stefano Goffredo & Zvy Dubinsky) 729-745 (Springer (2016).
Weber, L., DeForce, E. & Apprill, A. Optimization of DNA extraction for advancing coral microbiota investigations. Microbiome 5 , 18 (2017).
Bayer, F. M. Three new species of precious coral (Anthozoa: Gorgonacea, genus Corallium ) from Pacific waters. Proceedings of the Biological Society of Washington 109 , 205–228 (1996).
Tu, T.-H., Dai, C.-F. & Jeng, M.-S. Precious corals (Octocorallia: Coralliidae) from the northern West Pacific region with descriptions of two new species. Zootaxa 3395 , 1–17 (2012).
Parrish, F., Baco, A., Kelley, C. & Reiswig, H. State of deep‐sea coral and sponge ecosystems of the U.S. Pacific Islands Region. in The state of deep-sea coral and sponge ecosystems of the United States. NOAA Technical Memorandum NMFS‐OHC-4 (eds Thomas F Hourigan, Peter J Etnoyer, & Stephen Douglas Cairns) Chapter 7, 40 p. (US Department of Commerce, National Oceanic and Atmospheric Administration) (2017).
Parrish, F. A., Baco-Taylor, A., Kelley, C., Cairns, S. D. & Hourigan, T. F. Deep-sea coral taxa in the Hawaiian Archipelago and other U.S. Pacific Islands: depth and geographical distribution (Online resource: https://deepseacoraldata.noaa.gov ) (2017).
Ardila, N. E., Giribet, G. & Sánchez, J. A. A time-calibrated molecular phylogeny of the precious corals: reconciling discrepancies in the taxonomic classification and insights into their evolutionary history. BMC Evolutionary Biology 12 , 246 (2012).
Tu, T.-H., Dai, C.-F. & Jeng, M.-S. Phylogeny and systematics of deep-sea precious corals (Anthozoa: Octocorallia: Coralliidae). Molecular Phylogenetics and Evolution 84 , 173–184 (2015).
Article PubMed Google Scholar
Grigg, R. W. The precious corals. Fishery management plan of the Western Pacific Regional Fishery Management Council. (2010).
Cannas, R., Follesa, M., Cau, A., Cau, A. & Friedman, K. Global report on the biology, fishery and trade of precious corals. (FAO Fisheries and Aquaculture) (2019).
Bilewitch, J. P. & Degnan, S. M. A unique horizontal gene transfer event has provided the octocoral mitochondrial genome with an active mismatch repair gene that has potential for an unusual self-contained function. BMC Evolutionary Biology 11 , 228 (2011).
Conte, J., Potoczniak, M. J. & Tobe, S. S. Using synthetic oligonucleotides as standards in probe-based qPCR. BioTechniques 64 , 177–179 (2018).
Nolan, T., Hands, R. E., Ogunkolade, W. & Bustin, S. A. SPUD: a quantitative PCR assay for the detection of inhibitors in nucleic acid preparations. Analytical Biochemistry 351 , 308–310 (2006).
R Core Development Team. (ed R Foundation for Statistical Computing) (2013).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. Journal 17 , 10–12 (2011).
Afgan, E. et al . The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Research 46 , W537–W544 (2018).
Edgar, R. C. & Flyvbjerg, H. Error filtering, pair assembly and error correction for next-generation sequencing reads. Bioinformatics 31 , 3476–3482 (2015).
Edgar, R. C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature Methods 10 , 996 (2013).
Liverino, V. Citing environmental responsibility, CIBJO Coral Commission seeks to find common ground with scientific community. in The World Jewellery Confederation Congress (Bangkok, Thailand) 4 p. (2017).
Download references
Acknowledgements
Enzo Liverino Srl (Torre del Greco, Italy) provided some of the precious coral material for the SSEF coral collection, which we used in this study. This study benefited largely from discussions with Dr. Nozomu Iwasaki (Rissho University, Japan).
Author information
Authors and affiliations.
Zurich Institute of Forensic Medicine, University of Zurich, Winterthurerstrasse 190/52, CH-8057, Zurich, Switzerland
Bertalan Lendvay, Mario Gysi, Adelgunde Kratzer & Nadja V. Morf
Swiss Gemmological Institute SSEF, Aeschengraben 26, CH-4051, Basel, Switzerland
Bertalan Lendvay, Laurent E. Cartier & Michael S. Krzemnicki
Institute of Earth Sciences, University of Lausanne, Géopolis, CH-1015, Lausanne, Switzerland
Laurent E. Cartier
Federal Office for the Environment FOEN, Worblentalstrasse 68, CH-3063, Ittigen, Switzerland
Joana B. Meyer
You can also search for this author in PubMed Google Scholar
Contributions
B.L., A.K., M.S.K., L.E.C. and N.V.M. conceived the study. M.S.K. and L.E.C. provided the coral samples. J.B.M. conducted preliminary DNA extraction and sequence analysis. B.L., N.V.M. and M.G. performed the laboratory work and analyzed the data. B.L. and L.E.C. wrote the manuscript with support from the other co‐authors.
Corresponding author
Correspondence to Bertalan Lendvay .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information., supplementary information2., supplementary information3., supplementary information4., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Lendvay, B., Cartier, L.E., Gysi, M. et al. DNA fingerprinting: an effective tool for taxonomic identification of precious corals in jewelry. Sci Rep 10 , 8287 (2020). https://doi.org/10.1038/s41598-020-64582-4
Download citation
Received : 05 August 2019
Accepted : 17 April 2020
Published : 19 May 2020
DOI : https://doi.org/10.1038/s41598-020-64582-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Mitochondrial genes as strong molecular markers for species identification.
- Zahra Elyasigorji
- Mehrnaz Izadpanah
- Maryam Zare
The Nucleus (2023)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Mol Sci

Touch DNA Sampling Methods: Efficacy Evaluation and Systematic Review
Associated data.
Not applicable.
Collection and interpretation of “touch DNA” from crime scenes represent crucial steps during criminal investigations, with clear consequences in courtrooms. Although the main aspects of this type of evidence have been extensively studied, some controversial issues remain. For instance, there is no conclusive evidence indicating which sampling method results in the highest rate of biological material recovery. Thus, this study aimed to describe the actual considerations on touch DNA and to compare three different sampling procedures, which were “single-swab”, “double-swab”, and “other methods” (i.e., cutting out, adhesive tape, FTA ® paper scraping), based on the experimental results published in the recent literature. The data analysis performed shows the higher efficiency of the single-swab method in DNA recovery in a wide variety of experimental settings. On the contrary, the double-swab technique and other methods do not seem to improve recovery rates. Despite the apparent discrepancy with previous research, these results underline certain limitations inherent to the sampling procedures investigated. The application of this information to forensic investigations and laboratories could improve operative standard procedures and enhance this almost fundamental investigative tool’s probative value.
1. Introduction
When approaching a crime scene, given the limited availability of biological evidence, it is essential to choose the best forensic approach to collect DNA evidence in order to achieve as much information as possible. Among many possibilities, recovering DNA from different biological materials left behind by criminals and matching them to suspects has become increasingly relevant, giving an effective tool to investigators and courts. Moreover, in recent years, scientific improvements in recovery, extraction, amplification, and analysis led to obtaining informative profiles even from extremely limited traces [ 1 , 2 , 3 , 4 , 5 , 6 ]. In this scenario, the capacity to interpret DNA deposited through handling items (“touch DNA”) becomes a necessary tool in most forensic genetic laboratories, even if some challenges remain.
“Touch DNA” can be defined as DNA transferred from a person to an object via contact with the object itself. In the literature, this form of evidence has also been called “contact DNA”, “trace DNA”, or “transfer DNA”. The nature of this type of genetic material is still the subject of ongoing scientific debate, which expresses the lack of knowledge in the present forensic field. While many studies support DNA deposited by touch came from shed keratinocytes [ 7 , 8 ], several papers offer a wider perspective, identifying multiple sources as complete or partial skin cells, nucleated epithelial cells from other fluids or body parts in contact with one’s hands (i.e., saliva, sebum, sweat), or cell-free DNA, either endogenous or transferred onto the contact region from the abovementioned fluids [ 9 , 10 ]. In particular, cell-free DNA has been proven to be a reliable source of genetic material, often generating higher yields than its cellular counterpart [ 11 ] although considerable doubt remains about its origin; it is still unclear whether cell-free DNA is derived directly from body fluids or whether it is released after cellular degradation following touch deposition. Reports of fragmented DNA traces deposited from freshly washed hands suggest that DNA alteration begins within the organism [ 12 ].
However, touch DNA samples are generally known to contain low levels of DNA [ 13 ] and the presence of degraded genetic material, regardless of its origin, makes genotype detection challenging [ 14 , 15 , 16 , 17 , 18 , 19 , 20 ].
Degraded DNA is not the only component of touch deposits that can compromise forensic profiling. The presence of small amounts of genetic material available, sometimes even below the minimum thresholds of modern highly sensitive commercial STR kits, is another phenomenon commonly found in contact samples. In this contingency, PCR amplification can miss the detection of short DNA fragments even when the procedure is implemented with additional cycles to maximize the results. These evident limitations suggest the occurrence of stochastic effects related to sampling techniques rather than mere analytical defects [ 21 , 22 ] and precisely describe the so-called Low Template DNA (LT-DNA) or Low Copy Number DNA (LCN-DNA). In Figure 1 we describe methods used to enhance LT-DNA extraction, amplification, and sequencing.

DNA analysis workflow and improvement for low template DNA. In sample collection, the correct swab should be chosen, and, in particular, collection through a single swab should be performed on non-porous surfaces; the use of tape lifting is a preferred option for porous surfaces. Moreover, in this step, the moistening agent is also of fundamental importance to improve the final results (Step 1). Other possible solutions to improve the DNA analysis of low template DNA consist of the concentration of the DNA after its extraction or in the use of reducing agent lysis buffer with a prolonged time of incubation to increase, in both cases, the concentration of the final extracted DNA in the reaction volume (Step 2). The following step of DNA amplification may be modified in different ways to improve the DNA analysis in the case of low-template DNA. It is possible to increase the number of PCR cycles, decrease the PCR reaction volume to further concentrate the amount of DNA, or perform an additional purification step of the amplicons (Step 3). Eventually, it is possible to also intervene in the last step of fragments sequencing by increasing the time and the tension for the injection of the DNA fragments into the sequencer (Step 4).
Many factors can affect the quantity and the success of recovering the genetic material, schematically grouped into three categories of variables influencing sample generation, deposition, and analysis.
The concept of good or bad shedder status, primarily introduced in 1999 [ 23 ], is a person’s propensity to deposit a high or low amount of DNA on a touched object, respectively. According to the current notions, this ability varies greatly between individuals or in the same person under distinct conditions [ 24 ]. Although biological and genetic factors affecting this status are largely unknown, age, sex, and certain activities (i.e., touching DNA-free objects, wearing gloves, rubbing fingers on body parts) seem to influence the deposited traces. Generally, men shed more DNA than women, especially younger males compared to older ones (the trend was not investigated in females) and washing hands can reduce the available quantity [ 25 , 26 ]. In contrast, physical activities involving sweating leads to an increase in DNA transfer [ 27 ]. Closely related to this subject, body location impact results too, for example, sebaceous skin areas (vs. non-sebaceous), the dominant hand (vs. non-dominant), and fingertips (vs. palms) potentially facilitate DNA deposits [ 28 ].
Biological evidence can be virtually left behind everywhere during criminal activities, i.e., from wooden murder weapons to metallic handle doors. Considering this, in daily forensic practice, different material compositions had to be investigated, with variable results. Several authors have reported increased sloughed epithelial cells on rough and porous substrates, while non-porous substrates adhere to genetic material less readily [ 9 , 29 ]. Thus, fabrics and cotton appear to be better DNA collectors than plastic or glass surfaces and it has been proven more difficult to consistently recover touch DNA from metal surfaces [ 30 ]. The manner and duration of contact also influence the amount of genetic material transferred. It has been demonstrated that DNA deposits increase when pressure or friction are involved [ 28 ], directly proportional to the intensity applied [ 31 ]. Instead, the influence of time in the resulting amount of DNA on handling/wearing items remains controversial. While recent studies propose a linear correlation between variables [ 32 ], previous papers excluded any linkage, suggesting the origin of traces in a single transfer step upon initial contact [ 33 ]. Additionally, the possible interactions between other investigative methods, such as dactyloscopic enhancement methods, bloodstain enhancement methods, and DNA typing techniques, cannot be excluded [ 34 , 35 , 36 ].
Since each operative step expresses great availability in devices and techniques as well as in the manner of recovering, processing, and analysing samples, results from DNA analysis may be influenced by the combination between the singular forensic approach to the crime scene and following laboratory procedures [ 37 , 38 ]. Considered from a methodological perspective, the collection of touch DNA traces may involve the use of various sampling devices, such as swabs, adhesive tapes, or directly examining the evidence, in whole or in part. Considering their cost-effectiveness and minimal training requirements, the use of swabs is one of the most versatile and widely used methods. They can be applied dry or moistened with several agents and in varied materials. For example, standard cotton swabs are traditionally preferred for the collection of biological fluids and, notwithstanding further research, showed a tendency for the organic residue to get entrapped within cotton fibres, reducing sample availability [ 39 , 40 ]. When trace DNA is expected to be recovered, the double-swab technique [ 38 , 41 ] can be implemented. It consists of a wet swab and a second dry one sequentially applied onto the surface of interest, aimed at maximising recovery. Although the efficiency of this method has not been fully discussed, it is usually exploited to improve the collection of cellular material [ 42 ]. When other procedures are employed, effective alternatives are represented by “cutting out” the sampling area of soft tissues or the adhesive tape lifting the solid surface. The last sampling method is quick and straightforward, and tapes with better adhesion have been reported to produce a higher yield of trace DNA than swabbing, although the stickiness, rigidity, and size of the tape make the interpretation of the results more difficult [ 43 , 44 , 45 , 46 ].
Laboratory methods employed also affect the success of touch DNA analysis. Once recovered, standard workflows for processing touch DNA evidence first of all involves DNA extraction, for which a multitude of approaches exists, and then DNA quantification is conducted [ 47 ], which is critical to determine the quantity and quality of DNA extracted. This process is fundamental to decide the downstream genotyping methods to use and the proportion of the initial amount of evidence to submit to possible destructive analysis, thus, achieving a more informed interpretation of further analytical results [ 48 ]. However, the DNA extraction and quantification processes both result in the loss of a portion of the original sample and increase the probability of introducing exogenous DNA [ 49 ]. The amplification phase frequently implies the use of one of the commercially available kits most commonly used for criminal cases [ 50 , 51 ].
As can be inferred from the above, numerous factors influence touch DNA’s effectiveness as a forensic tool. Thus, we present here a brief review regarding the current state of knowledge on touch DNA analysis, with a particular focus on the impact the sampling techniques have on the results. The present paper evaluates several experimental settings in which different sampling methods have been used to provide valuable guidance in selecting the most appropriate collecting technique in relation to operative conditions. We believe it is necessary to enhance each analytical phase of the investigation in order to maximise the chance of finding useful profiles at crime scenes.
2. Materials and Methods
This review was performed in accordance with the Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) Guidelines [ 52 ].
In December 2021, a systematic literature review was performed by selecting papers from the Pubmed Database, according to the query “touch DNA”. The search terms were intentionally kept generic to include the highest number of potentially interesting works. A total of 997 articles were identified. Different inclusion criteria were then applied using specific PubMed filters to start the screening process: (1) English or Italian language; (2) availability of abstract and full text. Duplicates were manually removed. The screening process was conducted by the selection of titles and abstracts, and, when necessary, the evaluation of the full text. In cases of doubt, the consensus opinions of the research supervisors were solicited.
After title and abstract evaluation, a total of 136 manuscripts were considered. In the last phase, articles were selected when results were expressed in the form of STR alleles number (Group 1), informative profiles (Group 2), and percentage or DNA quantities (Group 3) to allow the comparison even between different experimental settings. Eventually, a total of 60 studies were carefully chosen.
The PRISMA flow chart in Figure 2 summarises the study screening and selection process as described above.

Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) 2020 flow diagram. A total of 60 studies were included in our systematic review.
3.1. STR Alleles and Informative Profiles
Based on the assumption that each article is composed of several separate tests, the experimental settings were highlighted (i.e., the number of samples collected, the recovery method, the extraction process, and the amplification procedure) to help distinguish the individual trials. Then, each trial’s results, represented by the mean number of STR alleles obtained, was converted into a percentage, compared to the specific amplification kit used, and classified as “low” or “high” if it was less than or greater than 66%, respectively. Similarly, the mean percentage of informative profiles was categorized as “low” or “high” with the same distinctive values.
We eventually individuated 9 articles (15% of the total) in which the results were expressed as STR alleles obtained (papers shown in Table 1 ). Figure 3 displays the variables “low” and “high” grouped by three types of sampling methods (single-swabbing, double-swabbing, and other methods).

Variables “low” and “high” grouped by sampling methods for Group 1. With 36.2%, single-swabbing obtains the greatest “high” value, followed by double-swabbing (29.7%), and other methods (14.3%).
Papers categorized in Group 1. Features displayed are authors and publication year, number (n°) of samples collected, sampling methods implemented, important findings, and remarks highlighted.
Likewise, 14 papers (23.4%) selected stated their results in the form of informative profiles (articles in Table 2 ). In Figure 4 , we categorised the variables “low” and “high”, in percentage by the same previous sampling method type (single-swabbing, double-swabbing, and others).

Variables “low” and “high” grouped by sampling methods for Group 2. Other methods collected the worst “high” value with 50%. Double-swabbing and single swabbing obtained 52.8% and 72%, respectively.
Papers categorized in Group 2. Features displayed are authors and publication year, number (n°) of samples collected, sampling methods implemented, important findings, and remarks highlighted.
3.2. DNA Quantitation
The last group of papers consisted of 43 articles where the authors published their results as DNA quantities, which represents 66.7% of the total. To be able to compare different findings, we identified two sub-groups: experiments where DNA concentration (Group 3a, with 17 articles) was declared, and trials where DNA quantity was indicated in absolute value (Group 3b, with 26 articles). Table 3 and Table 4 report the selection of the respective papers.
Papers categorized in Group 3a. Features displayed are authors and publication year, number (n°) of samples collected, sampling methods implemented, important findings, and remarks highlighted. N.A. not assigned.
Papers categorized in Group 3b. Features displayed are authors and publication year, number (n°) of samples collected, sampling methods implemented, important findings, and remarks highlighted. N.A. not assigned.
As for previous result types, we set cut-offs to classify the efficacy of different sampling methods. When the mean DNA concentration reported was under or above 0.1 ng/uL, a “low” or “high” value was assigned, respectively; the same variables were attributed when mean DNA quantity resulted in less than or greater than 1 ng. Figure 5 and Figure 6 show the values, in percentage, grouped by sampling methods (single-swabbing, double-swabbing, and other methods).

Variables “low” and “high” grouped by sampling methods for Group 3a. “Low” value represents the totality of results collected for double-swabbing. Single-swabbing and other methods obtained 67.1% and 52.9%, respectively.

Variables “low” and “high” grouped by sampling methods for Group 3b. Single-swabbing appears to be the most efficient technique, with a “high” value equal to 80%. Other methods and double-swabbing collected 68.2% and 51.8%, respectively.
4. Discussion
The collection and analysis of touch DNA, especially when low amounts of genetic material are expected, can be challenging yet extremely precious for investigations. Touch DNA testing is limited by the difficulty of obtaining not only sufficient quality DNA to generate a complete profile, but also sufficient material to allow re-testing. Hence, optimising the procedures is fundamental even to improving the STR typing success rate. Moreover, studies investigating touch DNA often implement wide variability among experimental settings, with few papers examining the topic transversally. This analysis was designed to operate a literature review on touch DNA, with a focus on the comparison between the efficacy of different sampling methods. Since there is significant variability in the way results are presented and on what kind of data the comparison of touch DNA scenarios is based, we evaluate the performance of three collecting technique categories (single-swabbing vs. double-swabbing vs. other methods) by analysing the mean number of STR alleles, the percentages of informative profiles, and the quantity of touch DNA obtained. This variability in results can partially be explained by the fact that there is currently no consensus regarding which aspects of analysis are most suitable for comparing DNA traces [ 28 ]. DNA quantities seem ineffective, from an investigative standpoint, as they do not correlate with profile quality and do not contain any information about the presence of more than one contributor. However, they can provide an insight into the efficacy of procedures, the aim of the present study, and assist in the interpretation of research findings [ 102 ]. On the other hand, some experimental studies evaluate outcomes by analysing profile compositions. This sub-group was also considered to provide a broader perspective on the topic.
4.1. Single-Swabbing
In general, swabbing appears to be the most common procedure used, with other methods being applied depending on the setting. A large majority of the trials (72.6%) were conducted using a swabbing technique, as compared to only 27.4% of experiments that applied alternative approaches. From the examination of the results, single-swabbing emerges as an effective sampling technique, with the greatest percentage of “high” efficiency in Group 1 (36.2%), Group 2 (72%), and Group 3b (80%). In Group 3a (32.9%), however, its effectiveness appears as the second-best value. A possible explanation for the current considerations could be its extreme versatility. Swabs vary in several ways, such as the material from which they are made, their thickness and length, how tightly they are wound and/or articulated with the swab shaft, the shape and design of the storage/transport tubes, and the inclusion of or not of features that help to preserve the DNA, such as vents for improved air-drying, desiccants, or antimicrobial chemicals [ 103 ]. To maximise the chance of obtaining an informative DNA profile, swabs can be moistened with fluids such as sterile water and laboratory or commercial detergents [ 104 ]. Thus, crime scene officers have the possibility to adapt the most efficient combination, both regarding the substrate from which the sample is being collected and the type of biological material.
4.2. Double-Swabbing
Scrubbing an area with multiple swabs (and the co-extraction of these tools) has been promoted to enhance the overall recovery of trace DNA. It has now become a common practice, since some evidence stated a single moist cotton swab picks up less than half of the available sample [ 105 ]. In the present work, we found a controversial performance of the technique, as it did not achieve the best result in any of the groups considered. All the experiments in Group 3a produced a low value of DNA traces. Given the limitations of the present statistical analysis, it seems to be in direct contradiction to previous works showing that this procedure is recommended and improves the quality of the resulting DNA profiles [ 38 , 41 , 103 ]. Actually, De Bruin et al. [ 106 ], in comparing the double-swab method versus stubbing (an adapted tape-lifting technique) for collecting offender epithelial material, underline its slightly better performance despite not being as easy a procedure. Moreover, Vickar et al. [ 107 ] found that M-Vac ® (Microbial Vacuum), an industrial device initially developed to sample food for potential pathogens, was better performing than double-swabbing for touch DNA collection on brick surfaces, even if it collected less DNA on non-porous tiles. As it is evident, the double-swab method does have limitations, particularly when used on certain substrates that can be found at crime scenes. According to this, the present considerations cannot exclude the possible influence of the adequacy with which the sampling procedure has been implemented in each trial. Under non-optimal experimental conditions, the double-swab technique not only yields less DNA than alternative methods, but it also damages the surface of items [ 44 ]. The success rate of obtaining a DNA profile from contact traces is largely dependent upon the selection of the appropriate recovery method for biological material and how it is applied.
4.3. Other Methods
In this last group, several procedures have been proposed in the literature. Overall, this category results in the most effective tests in Group 3a (47.1%) and the second-best in Group 2 (50%) and Group 3b (68.2%). In Group 1, this category collects the worst rate, with “low” efficacy (85.7%). The most frequently used sampling method examined in the present group is the so-called tapelifting, which consists in repeatedly pressing the adhesive part of a strip against the material surface of interest. Many other studies have already investigated its efficiency. Barash et al. [ 108 ] found that the tape collection of biological material simplifies sampling, is non-destructive, and is also highly effective in genotyping DNA from many previously untested items left at crime scenes. Another work evaluates nine collection methods in sampling touch DNA from human skin following skin-to-skin contact in mock assault scenarios [ 53 ]. The results express that the different tools did not have a distinct impact on the STR recovery even if adhesive tape seemed to be the least adequate for this purpose as it achieved the lowest DNA collection. Surprisingly, FTA paper scraping was employed in several experiments, while just a few papers exist in the forensic literature. It employs a novel approach based on Whatman FTA cards ® that was used to collect touch DNA from the steering wheel surface in one case study [ 109 ]. Based on Kirgiz et al.’s work [ 56 ], FTA paper scraping seemed to yield significantly more DNA when compared to double-swabbing and tapelifting. The authors also provide some possible explanations for these concerns. In particular, FTA paper chemical composition allows greater preservation and release of DNA, a larger sampling area than swabs and a slower drying process. The “cutting out” technique is another procedure engaged in the considered articles. Despite some critical constraints, such as the material on which it is implemented (not every surface can be cut out) and its irreversibility, it has been reported to achieve the best results in DNA recovery in comparison with adhesive tape and dry swabbing [ 42 ]. Despite the limitations of a global consideration, these alternative collection procedures seem to be available in limited experimental groups, as evidenced by the low number of trials. These restrictions may also account for the unsatisfactory outcomes of the present paper regarding the efficacy of the treatment. It is likely that challenging scenarios requiring unconventional approaches may produce low-quality DNA samples because of the intrinsic complexity rather than the ineffectiveness of the recovery methods.
From our perspective, single-swabbing appears as an effective first-level technique, due to its versatility, cost-effectiveness, and ease of use. Virtually, this tool can be applied to every type of solid surface, with different biological matrices and high efficiency, as our study suggests. In the case of a limited number of evident traces, this collecting method may be preceded by visualisation techniques or by moistening the device to enhance the recovery success. When operative settings are particularly challenging, i.e., insufficient availability of samples or dryness of specimen, double-swabbing may be implemented as a second-level technique. However, the surface material needs to be carefully chosen, as the procedure has shown low efficacy when applied to porous patterns. Lastly, alternative methods represent dynamic forensic tools that may be used as third-level procedures in certain circumstances. In particular, the use of tapelifting is limited by a subsequently more complex extraction process and low performance on the human skin surface. FTA paper scraper seems to be a promising collecting method, which undoubtedly requires further investigations into its recovery rate on different materials. When touch DNA samples need to be recovered from soft tissue with great availability of evidence, direct cutting appears as a valid solution, even compared to traditional swabbing.
In conclusion, evident limitations underline our review, which are intrinsically related to the difficulty of the subject matter. Firstly, as a complete and systematic review requires, we consider an extensive temporal range to collect a significant number of experiments. Nonetheless, the number of articles taken into consideration may still be insufficient. Unfortunately, results from older studies must be treated with caution when compared to more recent publications. This is because the sensitivity of detecting traces of DNA has increased appreciably in recent years, potentially adulterating the final reflections. Secondly, besides sample collection, DNA profiling success is dependent on extraction technique, quantification method, and amplification procedures. These considerations are certainly complicated by inter-laboratory and inter-individual differences regarding profile assessment and internal standard practices. Since it is not feasible to consider every contribution, we assume each trial has been conducted according to the most appropriate, yet internationally validated, available procedures. There is no doubt that further analysis of touch DNA variables influencing outcomes will contribute to shedding light on a still-controversial topic.
5. Conclusions
The collection of useful touch DNA evidence cannot prescind the selection of an appropriate sampling method. While the current scientific opinion on the topic remains questioned, this review contributes to the debate by offering an updated perspective on the actual state of the art. While single-swabbing appears more efficient than alternative methods, double-swabbing does not improve touch DNA collections in advance. Less common sampling procedures such as FTA paper scraping, cutting out or adhesive tape-lifting require pre-operative considerations to maximise their unquestioned efficacy. The present paper also highlights some intrinsic limitations, such as the inevitable impact of numerous variables on outcomes. Among these, the site on which biological material sampling is conducted and the type of traces recovered result as the most significant. Different settings require different devices to obtain the highest profiles from touch DNA samples. This information, along with future considerations, will contribute to enhancing the forensic ability to produce interpretable DNA profiles during investigations, even when minimal biological traces are available, with potential benefits to the criminal justice process.
According to the studies examined in this review, it is nowadays possible to obtain satisfactory results from the analysis of LCN-DNA, depending on the recovery technique used. However, almost all articles revealed that further research is needed on the impact of using different methodologies to collect samples to determine the most effective collection method. More comprehensive knowledge of detecting a profile based on the type of object and its history, identifying the most appropriate area(s) to target for DNA sampling, and the impact of additional factors, such as duration, frequency, and manner of contact, is required. Additionally, further research regarding the mechanisms of DNA shedding status, including the differences between sexes, the effects of activities performed before deposition, as well as other factors that may affect the amount of DNA deposited, is highly desirable for the forensic discipline. Being able to know, harmonise, and improve these aspects would definitely strengthen the value of DNA evidence in courtrooms.
Funding Statement
No funding was received to assist with the preparation of this manuscript. All authors certify that they have no affiliations with or involvement in any organisation or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Author Contributions
Conceptualization, L.C.; methodology, P.T. and E.M.; investigation, E.M.; data curation, E.M.; writing—original draft, E.M.; writing—review and editing, P.T., B.M., L.C. and A.D.; resources, B.M.; formal Analysis, A.D. and E.M.; supervision, L.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

COMMENTS
The period in the 1990s was the golden research age of DNA fingerprinting succeeded by two decades of engineering, implementation, and high-throughput application. ... Schmitter H, Schneider PM. A brief history of the formation of DNA databases in forensic science within Europe. Forensic Sci Int. 2001; 119:225-231. doi: 10.1016/S0379-0738(00 ...
Forensic DNA analysis has vastly evolved since the first forensic samples were evaluated by restriction fragment length polymorphism (RFLP). Methodologies advanced from gel electrophoresis techniques to capillary electrophoresis and now to next generation sequencing (NGS). Capillary electrophoresis was and still is the standard method used in forensic analysis. However, dependent upon the ...
Abstract. The Forensic world has been using fingerprints as the standard for identifying people for more than a century.Even after the discovery of DNA Profiling, science of fingerprint is still ...
DNA profiling ( DNA fingerprinting) is a technique employed by forensic scientists to assist in the identification. of individuals b y their resp ective DNA pr ofiles. DNA profiling shou ld not be ...
DNA fingerprinting, since its discovery around two and half decades back, has taken a great leap in its advancement and made the justice delivery system more efficient and accurate in the investigation of criminal and civil cases [30,31,30].This is much like a valuable armory in the hands of judiciary which aids in the conviction of the guilty as well as exoneration of the innocent [].
DNA fingerprinting is a revolutionary technique that enables scientists to match minute tissue samples and facilitates scientific studies on the composition, Reproduction, and evolution of animal ...
He has written 3 books and published 28 research papers, 11 book chaptersand 12 conference proceedings. ... He has more than 10 years of experience in examining various criminal cases using DNA fingerprinting. His research interests include the interpretation of DNA profiles in mixed samples, touch DNA, and population genetics. He has 13 peer ...
In 1962, Thomas Kuhn famously argued that the progress of scientific knowledge results from periodic 'paradigm shifts' during a period of crisis in which new ideas dramatically change the status quo. Although this is generally true, Alec Jeffreys' identification of hypervariable repeat motifs in the human beta-globin gene, and the subsequent development of a technology known now as ...
DNA fingerprinting, one of the great discoveries of the late 20th century, has revolutionized forensic investigations. This review briefly recapitulates 30 years of progress in forensic DNA analysis which helps to convict criminals, exonerate the wrongly accused, and identify victims of crime, disasters, and war. Current standard methods based on short tandem repeats (STRs) as well as lineage ...
In 1985, Sir Alec Jeffreys developed the variable-number tandem repeat method used to identify individuals and giving researchers the first DNA fingerprints. These initial methods were used in anthropological genetics, a field that uses a comparative approach to answer questions about human history, including the discernment of the origin of Native American populations and the discrimination ...
Lastly, the review of the potential contribution of forensic DNA analysis suggests that the effectiveness of investigations may be enhanced via a combination of forensic DNA analysis and other biometrics or evidence types. This integrated/synergistic approach should be explored in future research to maximize the significance of DNA analysis.
Forensic genetic fingerprinting, as defined by Jeffreys and Wilson [4], involves comparing the nucleated cells of an individual's DNA with biological samples collected from a crime scene or with another person's DNA for identification or exclusion. DNA fingerprinting is a distinctive form of identification that relies on shreds of evidence.
The procedure for creating a DNA fingerprint consists of first obtaining a sample of cells, such as skin, hair, or blood cells, which contain DNA. The DNA is extracted from the cells and purified. In Jeffreys's original approach, which was based on restriction fragment length polymorphism (RFLP) technology, the DNA was then cut at specific points along the strand with proteins known as ...
Abstract and Figures. DNA fingerprinting, one of the great discoveries of the late 20th century, has revolutionized forensic investigations. This review briefly recapitulates 30 years of progress ...
This method yielded pure DNA in all cases using 100 mg coral material and in over half of the cases when using "quasi non-destructive" sampling with sampled material amounts as low as 2.3 mg ...
Abstract DNA is present in most of the cells in our body, which is unique in each and every. individual, and we leave a trail of it everywhere we go. This has become an advantage. for for ensic ...
This review paper covers the forensic-relevant literature in biological sciences from 2019 to 2022 as a part of the 20th INTERPOL International Forensic Science Managers Symposium. Topics reviewed include rapid DNA testing, using law enforcement DNA databases plus investigative genetic genealogy DNA databases along with privacy/ethical issues ...
Thus, this study aimed to describe the actual considerations on touch DNA and to compare three different sampling procedures, which were "single-swab", "double-swab", and "other methods" (i.e., cutting out, adhesive tape, FTA ® paper scraping), based on the experimental results published in the recent literature.
DNA recovery methods from fingerprints on papers are a specific area of interest to law enforcement personnel. Recovery methods, such as swabbing of surfaces, are destructive to fingerprints, so ...