When you choose to publish with PLOS, your research makes an impact. Make your work accessible to all, without restrictions, and accelerate scientific discovery with options like preprints and published peer review that make your work more Open.
- PLOS Biology
- PLOS Climate
- PLOS Complex Systems
- PLOS Computational Biology
- PLOS Digital Health
- PLOS Genetics
- PLOS Global Public Health
- PLOS Medicine
- PLOS Mental Health
- PLOS Neglected Tropical Diseases
- PLOS Pathogens
- PLOS Sustainability and Transformation
- PLOS Collections

Understanding Scientific and Research Ethics

How to pass journal ethics checks to ensure a smooth submission and publication process
Reputable journals screen for ethics at submission—and inability to pass ethics checks is one of the most common reasons for rejection. Unfortunately, once a study has begun, it’s often too late to secure the requisite ethical reviews and clearances. Learn how to prepare for publication success by ensuring your study meets all ethical requirements before work begins.
The underlying principles of scientific and research ethics
Scientific and research ethics exist to safeguard human rights, ensure that we treat animals respectfully and humanely, and protect the natural environment.
The specific details may vary widely depending on the type of research you’re conducting, but there are clear themes running through all research and reporting ethical requirements:
Documented 3rd party oversight
- Consent and anonymity
- Full transparency
If you fulfill each of these broad requirements, your manuscript should sail through any journal’s ethics check.

If your research is 100% theoretical, you might be able to skip this one. But if you work with living organisms in any capacity—whether you’re administering a survey, collecting data from medical records, culturing cells, working with zebrafish, or counting plant species in a ring—oversight and approval by an ethics committee is a prerequisite for publication. This oversight can take many different forms:
For human studies and studies using human tissue or cells, obtain approval from your institutional review board (IRB). Register clinical trials with the World Health Organization (WHO) or International Committee of Medical Journal Editors (ICMJE). For animal research consult with your institutional animal care and use committee (IACUC). Note that there may be special requirements for non-human primates, cephalopods, and other specific species, as well as for wild animals. For field studies , anthropology and paleontology , the type of permission required will depend on many factors, like the location of the study, whether the site is publicly or privately owned, possible impacts on endangered or protected species, and local permit requirements.
TIP: You’re not exempt until your committee tells you so
Even if you think your study probably doesn’t require approval, submit it to the review board anyway. Many journals won’t consider retrospective approvals. Obtaining formal approval or an exemption up front is worth it to ensure your research is eligible for publication in the future.
TIP: Keep your committee records close
Clearly label your IRB/IACUC paperwork, permit numbers, and any participant permission forms (including blank copies), and keep them in a safe place. You will need them when you submit to a journal. Providing these details proactively as part of your initial submission can minimize delays and get your manuscript through journal checks and into the hands of reviewers sooner.
Consent & anonymity
Obtaining consent from human subjects.
You may not conduct research on human beings unless the subjects understand what you are doing and agree to be a part of your study. If you work with human subjects, you must obtain informed written consent from the participants or their legal guardians.
There are many circumstances where extra care may be required in order to obtain consent. The more vulnerable the population you are working with the stricter these guidelines will be. For example, your IRB may have special requirements for working with minors, the elderly, or developmentally delayed participants. Remember that these rules may vary from country to country. Providing a link to the relevant legal reference in your area can help speed the screening and approval process.
TIP: What if you are working with a population where reading and writing aren’t common?
Alternatives to written consent (such as verbal consent or a thumbprint) are acceptable in some cases, but consent still has to be clearly documented. To ensure eligibility for publication, be sure to:
- Get IRB approval for obtaining verbal rather than written consent
- Be prepared to explain why written consent could not be obtained
- Keep a copy of the script you used to obtain this consent, and record when consent was obtained for your own records
Consent and reporting for human tissue and cell lines
Consent from the participant or their next-of-kin is also required for the use of human tissue and cell lines. This includes discarded tissue, for example the by-products of surgery.
When working with cell lines transparency and good record keeping are essential. Here are some basic guidelines to bear in mind:
- When working with established cell lines , cite the published article where the cell line was first described.
- If you’re using repository or commercial cell lines , explain exactly which ones, and provide the catalog or repository number.
- If you received a cell line from a colleague , rather than directly from a repository or company, be sure to mention it. Explain who gifted the cells and when.
- For a new cell line obtained from a colleague there may not be a published article to cite yet, but the work to generate the cell line must meet the usual requirements of consent—even if it was carried out by another research group. You’ll need to provide a copy of your colleagues’ IRB approval and details about the consent procedures in order to publish the work.
Finally, you’re obliged to keep your human subjects anonymous and to protect any identifying information in photos and raw data. Remove all names, birth dates, detailed addresses, or job information from files you plan to share. Blur faces and tattoos in any images. Details such as geography (city/country), gender, age, or profession may be shared at a generalized level and in aggregate. Read more about standards for de-identifying datasets in The BMJ .
TIP: Anonymity can be important in field work too
Be careful about revealing geographic data in fieldwork. You don’t want to tip poachers off to the location of the endangered elephant population you studied, or expose petroglyphs to vandalism.
Full Transparency
No matter the discipline, transparent reporting of methods, results, data, software and code is essential to ethical research practice. Transparency is also key to the future reproducibility of your work.
When you submit your study to a journal, you’ll be asked to provide a variety of statements certifying that you’ve obtained the appropriate permissions and clearances, and explaining how you conducted the work. You may also be asked to provide supporting documentation, including field records and raw data. Provide as much detail as you can at this stage. Clear and complete disclosure statements will minimize back-and-forth with the journal, helping your submission to clear ethics checks and move on to the assessment stage sooner.
TIP: Save that data
As you work, be sure to clearly label and organize your data files in a way that will make sense to you later. As close as you are to the work as you conduct your study, remember that two years could easily pass between capturing your data and publishing an article reporting the results. You don’t want to be stuck piecing together confusing records in order to create figures and data files for repositories.
Read our full guide to preparing data for submission .
Keep in mind that scientific and research ethics are always evolving. As laws change and as we learn more about influence, implicit bias and animal sentience, the scientific community continues to strive to elevate our research practice.
A checklist to ensure you’re ethics-check ready
Before you begin your research
Obtain approval from your IRB, IACUC or other approving body
Obtain written informed consent from human participants, guardians or next-of-kin
Obtain permits or permission from property owners, or confirm that permits are not required
Label and save all of records
As you work
Adhere strictly to the protocols approved by your committee
Clearly label your data, and store it in a way that will make sense to your future self
As you write, submit and deposit your results
Be ready to cite specific approval organizations, permit numbers, cell lines, and other details in your ethics statement and in the methods section of your manuscript
Anonymize all participant data (including human and in some cases animal or geographic data)
If a figure does include identifying information (e.g. a participant’s face) obtain special consent
The contents of the Peer Review Center are also available as a live, interactive training session, complete with slides, talking points, and activities. …
The contents of the Writing Center are also available as a live, interactive training session, complete with slides, talking points, and activities. …
There’s a lot to consider when deciding where to submit your work. Learn how to choose a journal that will help your study reach its audience, while reflecting your values as a researcher…
An Introduction to Research Ethics and Scientific Integrity
- Living reference work entry
- First Online: 30 May 2019
- Cite this living reference work entry

- Ron Iphofen 2
485 Accesses
1 Citations
This chapter outlines the aims for the handbook. A main aim is to be a first point of contact for contemporary information, issues, and challenges in the fields of research ethics and scientific integrity. It is aimed at researchers, reviewers, and policymakers to help them pursue the best ways forward in seeking ethics and integrity in all research across disciplines, methods, subjects, participants, and contexts. The authors form a global network of scholars, practitioners, and researchers with a range of experience and insights that scope a challenging field but one that is vital to the maintenance of research standards and public confidence in science. Fact-based policymaking remains under threat from political and ideological pressures. Scientists and researchers in all disciplines and professions hold a clear responsibility to protect their subjects, research participants, and society from pressures, interests, and prejudices that risk undermining the value of their work. This overview outlines how the handbook is constructed and how readers might gain from it.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.
Institutional subscriptions
Cadwalladr C (2018) Our Cambridge Analytica scoop shocked the world. But the whole truth remains elusive, The Guardian , 23rd December Accessed at: https://www.theguardian.com/uk-news/2018/dec/23/cambridge-analytica-facebook-scoop-carole-cadwalladr-shocked-world-truth-still-elusive
Cadwalladr C (2019) Cambridge Analytica a year on: ‘a lesson in institutional failure’, The Guardian, 17 March, accessed at: https://www.theguardian.com/uk-news/2019/mar/17/cambridge-analytica-year-on-lesson-in-institutional-failure-christopher-wylie
Cohen S, Young J (1973) The manufacture of news: a reader. Sage, London
Google Scholar
Collingridge D (1980) The social control of technology. Frances Pinter, London
ESOMAR (2016) https://www.esomar.org/what-we-do/code-guidelines
Evans RJ (2002) Telling lies about Hitler: the holocaust, history and the David Irving trial. Verso, London
Fuhrman J (2013) Super immunity. HarperCollins, London
Goldacre B (2001) Bad pharma. HarperCollins, London
Gross J (2018) Poland’s death camp law is designed to falsify history, Financial Times, February 6, accessed at: https://www.ft.com/content/1c183f56-0a6a-11e8-bacb-2958fde95e5e
Hutton W, Adonis A (2018) Saving Britain: how we must change to prosper in Europe. Abacus, London
Ioris AAR (2015) Cracking the nut of agribusiness and global food insecurity: in search of a critical agenda of research. Geoforum 63:1–4. https://doi.org/10.1016/j.geoforum.2015.05.004
Article Google Scholar
Iphofen R (2017) Epistemological metaphors: orders of knowledge and control in the Encyclopedist myths of cyberspace. International Journal of Humanities and Cultural Studies. (ISSN 2356-5926) 4(2):122–141. https://www.ijhcs.com/index.php/ijhcs/article/view/3128
Levitt SD, Dubner SJ (2005) Freakonomics: a rogue economist explores the hidden side of everything. Allen Lane, London
Levitt SD, Dubner SJ (2009) SuperFreakonomics: global cooling, patriotic prostitutes, and why suicide bombers should buy life insurance. Harper Collins, New York
Portes R (2017) Who needs experts? The London Business Review, 9th May. Accessed at: https://www.london.edu/faculty-and-research/lbsr/who-needs-experts
Posner M (2006) Social sciences under attack in the UK (1981–1983), La revue pour l’histoire du CNRS [], 7|2002, 20 October 2006, 09 January 2017. http://histoire-cnrs.revues.org/547 ; https://doi.org/10.4000/histoire-cnrs.547
Schrag Z (2010) Ethical Imperialism: Institutional Review Boards and the Social Sciences, 1965–2009. The John Hopkins University Press, Baltimore
Download references
Author information
Authors and affiliations.
Independent Research Consultant, Chatelaillon Plage, France
Ron Iphofen
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Ron Iphofen .
Editor information
Editors and affiliations.
Chatelaillon Plage, France
Rights and permissions
Reprints and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry.
Iphofen, R. (2019). An Introduction to Research Ethics and Scientific Integrity. In: Iphofen, R. (eds) Handbook of Research Ethics and Scientific Integrity. Springer, Cham. https://doi.org/10.1007/978-3-319-76040-7_62-1
Download citation
DOI : https://doi.org/10.1007/978-3-319-76040-7_62-1
Received : 17 March 2019
Accepted : 01 May 2019
Published : 30 May 2019
Publisher Name : Springer, Cham
Print ISBN : 978-3-319-76040-7
Online ISBN : 978-3-319-76040-7
eBook Packages : Springer Reference Religion and Philosophy Reference Module Humanities and Social Sciences Reference Module Humanities
- Publish with us
Policies and ethics
- Find a journal
- Track your research

Site Search
- How to Search
- Advisory Group
- Editorial Board
- OEC Fellows
- History and Funding
- Using OEC Materials
- Collections
- Research Ethics Resources
- Ethics Projects
- Communities of Practice
- Get Involved
- Submit Content
- Open Access Membership
- Become a Partner
Introduction: What is Research Ethics?
Research Ethics is defined here to be the ethics of the planning, conduct, and reporting of research. This introduction covers what research ethics is, its ethical distinctions, approaches to teaching research ethics, and other resources on this topic.
What is Research Ethics
Why Teach Research Ethics
Animal Subjects
Biosecurity
Collaboration
Conflicts of Interest
Data Management
Human Subjects
Peer Review
Publication
Research Misconduct
Social Responsibility
Stem Cell Research
Whistleblowing
Descriptions of educational settings , including in the classroom, and in research contexts.
Case Studies
Other Discussion Tools
Information about the history and authors of the Resources for Research Ethics Collection
What is Research Ethics?
Research Ethics is defined here to be the ethics of the planning, conduct, and reporting of research. It is clear that research ethics should include:
- Protections of human and animal subjects
However, not all researchers use human or animal subjects, nor are the ethical dimensions of research confined solely to protections for research subjects. Other ethical challenges are rooted in many dimensions of research, including the:
- Collection, use, and interpretation of research data
- Methods for reporting and reviewing research plans or findings
- Relationships among researchers with one another
- Relationships between researchers and those that will be affected by their research
- Means for responding to misunderstandings, disputes, or misconduct
- Options for promoting ethical conduct in research
The domain of research ethics is intended to include nothing less than the fostering of research that protects the interests of the public, the subjects of research, and the researchers themselves.
Ethical Distinctions
In discussing or teaching research ethics, it is important to keep some basic distinctions in mind.
- It is important not to confuse moral claims about how people ought to behave with descriptive claims about how they in fact do behave. From the fact that gift authorship or signing off on un-reviewed data may be "common practice" in some contexts, it doesn't follow that they are morally or professionally justified. Nor is morality to be confused with the moral beliefs or ethical codes that a given group or society holds (how some group thinks people should live). A belief in segregation is not morally justified simply because it is widely held by a group of people or given society. Philosophers term this distinction between prescriptive and descriptive claims the 'is-ought distinction.'
- A second important distinction is that between morality and the law. The law may or may not conform to the demands of ethics (Kagan, 1998). To take a contemporary example: many believe that the law prohibiting federally funded stem cell research is objectionable on moral (as well as scientific) grounds, i.e., that such research can save lives and prevent much human misery. History is full of examples of bad laws, that is laws now regarded as morally unjustifiable, e.g., the laws of apartheid, laws prohibiting women from voting or inter-racial couples from marrying.
- It is also helpful to distinguish between two different levels of discussion (or two different kinds of ethical questions): first-order or "ground-level" questions and second-order questions.
- First-order moral questions concern what we should do. Such questions may be very general or quite specific. One might ask whether the tradition of 'senior' authorship should be defended and preserved or, more generally, what are the principles that should go into deciding the issue of 'senior' authorship. Such questions and the substantive proposals regarding how to answer them belong to the domain of what moral philosophers call 'normative ethics.'
- Second-order moral questions concern the nature and purpose of morality itself. When someone claims that falsifying data is wrong, what exactly is the standing of this claim? What exactly does the word 'wrong' mean in the conduct of scientific research? And what are we doing when we make claims about right and wrong, scientific integrity and research misconduct? These second-order questions are quite different from the ground-level questions about how to conduct one's private or professional life raised above. They concern the nature of morality rather than its content, i.e., what acts are required, permitted or prohibited. This is the domain of what moral philosophers call 'metaethics' (Kagan, 1998).
Ethical Approaches
Each of these approaches provides moral principles and ways of thinking about the responsibilities, duties and obligations of moral life. Individually and jointly, they can provide practical guidance in ethical decision-making.
- One of the most influential and familiar approaches to ethics is deontological ethics, associated with Immanuel Kant (1742-1804). Deontological ethics hold certain acts as right or wrong in themselves, e.g., promise breaking or lying. So, for example, in the context of research, fraud, plagiarism and misrepresentation are regarded as morally wrong in themselves, not simply because they (tend to) have bad consequences. The deontological approach is generally grounded in a single fundamental principle: Act as you would wish others to act towards you OR always treat persons as an end, never as a means to an end.
- From such central principles are derived rules or guidelines for what is permitted, required and prohibited. Objections to principle-based or deontological ethics include the difficulty of applying highly general principles to specific cases, e.g.: Does treating persons as ends rule out physician-assisted suicide, or require it? Deontological ethics is generally contrasted to consequentialist ethics (Honderich, 1995).
- According to consequentialist approaches, the rightness or wrongness of an action depends solely on its consequences. One should act in such a way as to bring about the best state of affairs, where the best state of affairs may be understood in various ways, e.g., as the greatest happiness for the greatest number of people, maximizing pleasure and minimizing pain or maximizing the satisfaction of preferences. A theory such as Utilitarianism (with its roots in the work of Jeremy Bentham and John Stuart Mill) is generally taken as the paradigm example of consequentialism. Objections to consequentialist ethics tend to focus on its willingness to regard individual rights and values as "negotiable." So, for example, most people would regard murder as wrong independently of the fact that killing one person might allow several others to be saved (the infamous sacrifice of an ailing patient to provide organs for several other needy patients). Similarly, widespread moral opinion holds certain values important (integrity, justice) not only because they generally lead to good outcomes, but in and of themselves.
- Virtue ethics focuses on moral character rather than action and behavior considered in isolation. Central to this approach is the question what ought we (as individuals, as scientists, as physicians) to be rather than simply what we ought to do. The emphasis here is on inner states, that is, moral dispositions and habits such as courage or a developed sense of personal integrity. Virtue ethics can be a useful approach in the context of RCR and professional ethics, emphasizing the importance of moral virtues such as compassion, honesty, and respect. This approach has also a great deal to offer in discussions of bioethical issues where a traditional emphasis on rights and abstract principles frequently results in polarized, stalled discussions (e.g., abortion debates contrasting the rights of the mother against the rights of the fetus).
- The term 'an ethics of care' grows out of the work of Carol Gilligan, whose empirical work in moral psychology claimed to discover a "different voice," a mode of moral thinking distinct from principle-based moral thinking (e.g., the theories of Kant and Mill). An ethics of care stresses compassion and empathetic understanding, virtues Gilligan associated with traditional care-giving roles, especially those of women.
- This approach differs from traditional moral theories in two important ways. First, it assumes that it is the connections between persons, e.g., lab teams, colleagues, parents and children, student and mentor, not merely the rights and obligations of discrete individuals that matter. The moral world, on this view, is best seen not as the interaction of discrete individuals, each with his or her own interests and rights, but as an interrelated web of obligations and commitment. We interact, much of the time, not as private individuals, but as members of families, couples, institutions, research groups, a given profession and so on. Second, these human relationships, including relationships of dependency, play a crucial role on this account in determining what our moral obligations and responsibilities are. So, for example, individuals have special responsibilities to care for their children, students, patients, and research subjects.
- An ethics of care is thus particularly useful in discussing human and animal subjects research, issues of informed consent, and the treatment of vulnerable populations such as children, the infirm or the ill.
- The case study approach begins from real or hypothetical cases. Its objective is to identify the intuitively plausible principles that should be taken into account in resolving the issues at hand. The case study approach then proceeds to critically evaluate those principles. In discussing whistle-blowing, for example, a good starting point is with recent cases of research misconduct, seeking to identify and evaluate principles such as a commitment to the integrity of science, protecting privacy, or avoiding false or unsubstantiated charges. In the context of RCR instruction, case studies provide one of the most interesting and effective approaches to developing sensitivity to ethical issues and to honing ethical decision-making skills.
- Strictly speaking, casuistry is more properly understood as a method for doing ethics rather than as itself an ethical theory. However, casuistry is not wholly unconnected to ethical theory. The need for a basis upon which to evaluate competing principles, e.g., the importance of the well-being of an individual patient vs. a concern for just allocation of scarce medical resources, makes ethical theory relevant even with case study approaches.
- Applied ethics is a branch of normative ethics. It deals with practical questions particularly in relation to the professions. Perhaps the best known area of applied ethics is bioethics, which deals with ethical questions arising in medicine and the biological sciences, e.g., questions concerning the application of new areas of technology (stem cells, cloning, genetic screening, nanotechnology, etc.), end of life issues, organ transplants, and just distribution of healthcare. Training in responsible conduct of research or "research ethics" is merely one among various forms of professional ethics that have come to prominence since the 1960s. Worth noting, however, is that concern with professional ethics is not new, as ancient codes such as the Hippocratic Oath and guild standards attest (Singer, 1986).
- Adams D, Pimple KD (2005): Research Misconduct and Crime: Lessons from Criminal Science on Preventing Misconduct and Promoting Integrity. Accountability in Research 12(3):225-240.
- Anderson MS, Horn AS, Risbey KR, Ronning EA, De Vries R, Martinson BC (2007): What Do Mentoring and Training in the Responsible Conduct of Research Have To Do with Scientists' Misbehavior? Findings from a National Survey of NIH-Funded Scientists . Academic Medicine 82(9):853-860.
- Bulger RE, Heitman E (2007): Expanding Responsible Conduct of Research Instruction across the University. Academic Medicine. 82(9):876-878.
- Kalichman MW (2006): Ethics and Science: A 0.1% solution. Issues in Science and Technology 23:34-36.
- Kalichman MW (2007): Responding to Challenges in Educating for the Responsible Conduct of Research, Academic Medicine. 82(9):870-875.
- Kalichman MW, Plemmons DK (2007): Reported Goals for Responsible Conduct of Research Courses. Academic Medicine. 82(9):846-852.
- Kalichman MW (2009): Evidence-based research ethics. The American Journal of Bioethics 9(6&7): 85-87.
- Pimple KD (2002): Six Domains of Research Ethics: A Heuristic Framework for the Responsible Conduct of Research. Science and Engineering Ethics 8(2):191-205.
- Steneck NH (2006): Fostering Integrity in Research: Definitions, Current Knowledge, and Future Directions. Science and Engineering Ethics 12:53-74.
- Steneck NH, Bulger RE (2007): The History, Purpose, and Future of Instruction in the Responsible Conduct of Research. Academic Medicine. 82(9):829-834.
- Vasgird DR (2007): Prevention over Cure: The Administrative Rationale for Education in the Responsible Conduct of Research. Academic Medicine. 82(9):835-837.
- Aristotle. The Nichomachean Ethics.
- Beauchamp RL, Childress JF (2001): Principles of Biomedical Ethics, 5th edition, NY: Oxford University Press.
- Bentham, J (1781): An Introduction to the Principles of Morals and Legislation.
- Gilligan C (1993): In a Different Voice: Psychological Theory and Women's Development. Cambridge: Harvard University Press.
- Glover, Jonathan (1977): Penguin Books.
- Honderich T, ed. (1995): The Oxford Companion to Philosophy, Oxford and New York: Oxford University Press.
- Kagan S (1998): Normative Ethics. Westview Press.
- Kant I (1785): Groundwork of the Metaphysics of Morals.
- Kant I (1788): Critique of Practical Reason.
- Kant I (1797): The Metaphysics of Morals.
- Kant I (1797): On a Supposed right to Lie from Benevolent Motives.
- Kuhse H, Singer P (1999): Bioethics: An Anthology. Blackwell Publishers.
- Mill JS (1861): Utilitarianism.
- Rachels J (1999): The Elements of Moral Philosophy, 3rd edition, Boston: McGraw-Hill.
- Regan T (1993): Matters of Life and Death: New Introductory Essays in Moral Philosophy, 3rd edition. New York: McGraw-Hill. The history of ethics.
- Singer P (1993): Practical Ethics, 2nd ed. Cambridge University Press.
The Resources for Research Ethics Education site was originally developed and maintained by Dr. Michael Kalichman, Director of the Research Ethics Program at the University of California San Diego. The site was transferred to the Online Ethics Center in 2021 with the permission of the author.
Related Resources
Submit Content to the OEC Donate

This material is based upon work supported by the National Science Foundation under Award No. 2055332. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
- Original article
- Open access
- Published: 13 July 2021
Assisting you to advance with ethics in research: an introduction to ethical governance and application procedures
- Shivadas Sivasubramaniam 1 ,
- Dita Henek Dlabolová 2 ,
- Veronika Kralikova 3 &
- Zeenath Reza Khan 3
International Journal for Educational Integrity volume 17 , Article number: 14 ( 2021 ) Cite this article
17k Accesses
12 Citations
4 Altmetric
Metrics details
Ethics and ethical behaviour are the fundamental pillars of a civilised society. The focus on ethical behaviour is indispensable in certain fields such as medicine, finance, or law. In fact, ethics gets precedence with anything that would include, affect, transform, or influence upon individuals, communities or any living creatures. Many institutions within Europe have set up their own committees to focus on or approve activities that have ethical impact. In contrast, lesser-developed countries (worldwide) are trying to set up these committees to govern their academia and research. As the first European consortium established to assist academic integrity, European Network for Academic Integrity (ENAI), we felt the importance of guiding those institutions and communities that are trying to conduct research with ethical principles. We have established an ethical advisory working group within ENAI with the aim to promote ethics within curriculum, research and institutional policies. We are constantly researching available data on this subject and committed to help the academia to convey and conduct ethical behaviour. Upon preliminary review and discussion, the group found a disparity in understanding, practice and teaching approaches to ethical applications of research projects among peers. Therefore, this short paper preliminarily aims to critically review the available information on ethics, the history behind establishing ethical principles and its international guidelines to govern research.
The paper is based on the workshop conducted in the 5th International conference Plagiarism across Europe and Beyond, in Mykolas Romeris University, Lithuania in 2019. During the workshop, we have detailed a) basic needs of an ethical committee within an institution; b) a typical ethical approval process (with examples from three different universities); and c) the ways to obtain informed consent with some examples. These are summarised in this paper with some example comparisons of ethical approval processes from different universities. We believe this paper will provide guidelines on preparing and training both researchers and research students in appropriately upholding ethical practices through ethical approval processes.
Introduction
Ethics and ethical behaviour (often linked to “responsible practice”) are the fundamental pillars of a civilised society. Ethical behaviour with integrity is important to maintain academic and research activities. It affects everything we do, and gets precedence with anything that would include/affect, transform, or impact upon individuals, communities or any living creatures. In other words, ethics would help us improve our living standards (LaFollette, 2007 ). The focus on ethical behaviour is indispensable in certain fields such as medicine, finance, or law, but is also gaining recognition in all disciplines engaged in research. Therefore, institutions are expected to develop ethical guidelines in research to maintain quality, initiate/own integrity and above all be transparent to be successful by limiting any allegation of misconduct (Flite and Harman, 2013 ). This is especially true for higher education organisations that promote research and scholarly activities. Many European institutions have developed their own regulations for ethics by incorporating international codes (Getz, 1990 ). The lesser developed countries are trying to set up these committees to govern their academia and research. World Health Organization has stated that adhering to “ ethical principles … [is central and important]... in order to protect the dignity, rights and welfare of research participants ” (WHO, 2021 ). Ethical guidelines taught to students can help develop ethical researchers and members of society who uphold values of ethical principles in practice.
As the first European-wide consortium established to assist academic integrity (European Network for Academic Integrity – ENAI), we felt the importance of guiding those institutions and communities that are trying to teach, research, and include ethical principles by providing overarching understanding of ethical guidelines that may influence policy. Therefore, we set up an advisory working group within ENAI in 2018 to support matters related to ethics, ethical committees and assisting on ethics related teaching activities.
Upon preliminary review and discussion, the group found a disparity in understanding, practice and teaching approaches to ethical applications among peers. This became the premise for this research paper. We first carried out a literature survey to review and summarise existing ethical governance (with historical perspectives) and procedures that are already in place to guide researchers in different discipline areas. By doing so, we attempted to consolidate, document and provide important steps in a typical ethical application process with example procedures from different universities. Finally, we attempted to provide insights and findings from practical workshops carried out at the 5th International Conference Plagiarism across Europe and Beyond, in Mykolas Romeris University, Lithuania in 2019, focussing on:
• highlighting the basic needs of an ethical committee within an institution,
• discussing and sharing examples of a typical ethical approval process,
• providing guidelines on the ways to teach research ethics with some examples.
We believe this paper provides guidelines on preparing and training both researchers and research students in appropriately upholding ethical practices through ethical approval processes.
Background literature survey
Responsible research practice (RRP) is scrutinised by the aspects of ethical principles and professional standards (WHO’s Code of Conduct for responsible Research, 2017). The Singapore statement on research integrity (The Singapore Statement on Research integrity, 2010) has provided an internationally acceptable guidance for RRP. The statement is based on maintaining honesty, accountability, professional courtesy in all aspects of research and maintaining fairness during collaborations. In other words, it does not simply focus on the procedural part of the research, instead covers wider aspects of “integrity” beyond the operational aspects (Israel and Drenth, 2016 ).
Institutions should focus on providing ethical guidance based on principles and values reflecting upon all aspects/stages of research (from the funding application/project development stage upto or beyond project closing stage). Figure 1 summarizes the different aspects/stages of a typical research and highlights the needs of RRP in compliance with ethical governance at each stage with examples (the figure is based on Resnik, 2020 ; Žukauskas et al., 2018 ; Anderson, 2011 ; Fouka and Mantzorou, 2011 ).
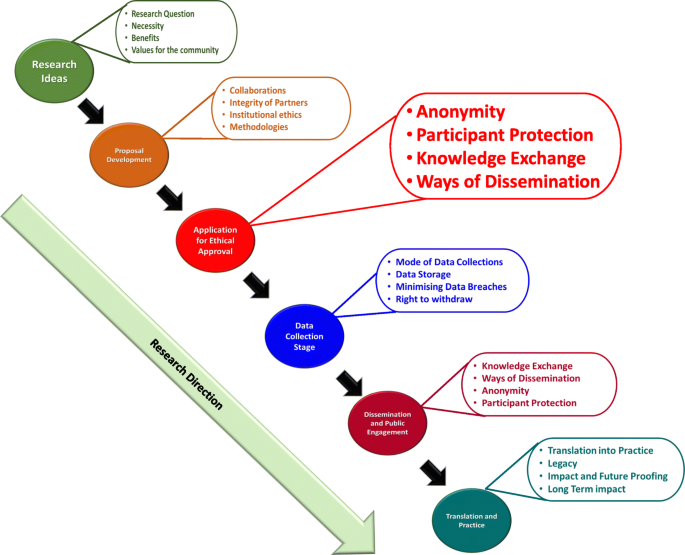
Summary of the enabling ethical governance at different stages of research. Note that it is imperative for researchers to proactively consider the ethical implications before, during and after the actual research process. The summary shows that RRP should be in line with ethical considerations even long before the ethical approval stage
Individual responsibilities to enhance RRP
As explained in Fig. 1 , a successfully governed research should consider ethics at the planning stages prior to research. Many international guidance are compatible in enforcing/recommending 14 different “responsibilities” that were first highlighted in the Singapore Statement (2010) for researchers to follow and achieve competency in RRP. In order to understand the purpose and the expectation of these ethical guidelines, we have carried out an initial literature survey on expected individual responsibilities. These are summarised in Table 1 .
By following these directives, researchers can carry out accountable research by maximising ethical self-governance whilst minimising misconducts. In our own experiences of working with many researchers, their focus usually revolves around ethical “clearance” rather than behaviour. In other words, they perceive this as a paper exercise rather than trying to “own” ethical behaviour in everything they do. Although the ethical principles and responsibilities are explicitly highlighted in the majority of international guidelines [such as UK’s Research Governance Policy (NICE, 2018 ), Australian Government’s National Statement on Ethical Conduct in Human Research (Difn website a - National Statement on Ethical Conduct in Human Research (NSECHR), 2018 ), the Singapore Statement (2010) etc.]; and the importance of holistic approach has been argued in ethical decision making, many researchers and/or institutions only focus on ethics linked to the procedural aspects.
Studies in the past have also highlighted inconsistencies in institutional guidelines pointing to the fact that these inconsistencies may hinder the predicted research progress (Desmond & Dierickx 2021 ; Alba et al., 2020 ; Dellaportas et al., 2014 ; Speight 2016 ). It may also be possible that these were and still are linked to the institutional perceptions/expectations or the pre-empting contextual conditions that are imposed by individual countries. In fact, it is interesting to note many research organisations and HE institutions establish their own policies based on these directives.
Research governance - origins, expectations and practices
Ethical governance in clinical medicine helps us by providing a structure for analysis and decision-making. By providing workable definitions of benefits and risks as well as the guidance for evaluating/balancing benefits over risks, it supports the researchers to protect the participants and the general population.
According to the definition given by National Institute of Clinical care Excellence, UK (NICE 2018 ), “ research governance can be defined as the broad range of regulations, principles and standards of good practice that ensure high quality research ”. As stated above, our literature-based research survey showed that most of the ethical definitions are basically evolved from the medical field and other disciplines have utilised these principles to develop their own ethical guidance. Interestingly, historical data show that the medical research has been “self-governed” or in other words implicated by the moral behaviour of individual researchers (Fox 2017 ; Shaw et al., 2005 ; Getz, 1990 ). For example, early human vaccination trials conducted in 1700s used the immediate family members as test subjects (Fox, 2017 ). Here the moral justification might have been the fact that the subjects who would have been at risk were either the scientists themselves or their immediate families but those who would reap the benefits from the vaccination were the general public/wider communities. However, according to the current ethical principles, this assumption is entirely not acceptable.
Historically, ambiguous decision-making and resultant incidences of research misconduct have led to the need for ethical research governance in as early as the 1940’s. For instance, the importance of an international governance was realised only after the World War II, when people were astonished to note the unethical research practices carried out by Nazi scientists. As a result of this, in 1947 the Nuremberg code was published. The code mainly focussed on the following:
Informed consent and further insisted the research involving humans should be based on prior animal work,
The anticipated benefits should outweigh the risk,
Research should be carried out only by qualified scientists must conduct research,
Avoiding physical and mental suffering and.
Avoiding human research that would result in which death or disability.
(Weindling, 2001 ).
Unfortunately, it was reported that many researchers in the USA and elsewhere considered the Nuremberg code as a document condemning the Nazi atrocities, rather than a code for ethical governance and therefore ignored these directives (Ghooi, 2011 ). It was only in 1964 that the World Medical Association published the Helsinki Declaration, which set the stage for ethical governance and the implementation of the Institutional Review Board (IRB) process (Shamoo and Irving, 1993 ). This declaration was based on Nuremberg code. In addition, the declaration also paved the way for enforcing research being conducted in accordance with these guidelines.
Incidentally, the focus on research/ethical governance gained its momentum in 1974. As a result of this, a report on ethical principles and guidelines for the protection of human subjects of research was published in 1979 (The Belmont Report, 1979 ). This report paved the way to the current forms of ethical governance in biomedical and behavioural research by providing guidance.
Since 1994, the WHO itself has been providing several guidance to health care policy-makers, researchers and other stakeholders detailing the key concepts in medical ethics. These are specific to applying ethical principles in global public health.
Likewise, World Organization for Animal Health (WOAH), and International Convention for the Protection of Animals (ICPA) provide guidance on animal welfare in research. Due to this continuous guidance, together with accepted practices, there are internationally established ethical guidelines to carry out medical research. Our literature survey further identified freely available guidance from independent organisations such as COPE (Committee of Publication Ethics) and ALLEA (All European Academics) which provide support for maintaining research ethics in other fields such as education, sociology, psychology etc. In reality, ethical governance is practiced differently in different countries. In the UK, there is a clinical excellence research governance, which oversees all NHS related medical research (Mulholland and Bell, 2005 ). Although, the governance in other disciplines is not entirely centralised, many research funding councils and organisations [such as UKRI (UK-Research and Innovation; BBSC (Biotechnology and Biological Sciences Research Council; MRC (Medical Research Council); EPSRC (Economic and Social Research Council)] provide ethical governance and expect institutional adherence and monitoring. They expect local institutional (i.e. university/institutional) research governance for day-to-day monitoring of the research conducted within the organisation and report back to these funding bodies, monthly or annually (Department of Health, 2005). Likewise, there are nationally coordinated/regulated ethics governing bodies such as the US Office for Human Research Protections (US-OHRP), National Institute of Health (NIH) and the Canadian Institutes for Health Research (CIHR) in the USA and Canada respectively (Mulholland and Bell, 2005 ). The OHRP in the USA formally reviews all research activities involving human subjects. On the other hand, in Canada, CIHR works with the Natural Sciences and Engineering Research Council (NSERC), and the Social Sciences and Humanities Research Council (SSHRC). They together have produced a Tri-Council Policy Statement (TCPS) (Stephenson et al., 2020 ) as ethical governance. All Canadian institutions are expected to adhere to this policy for conducting research. As for Australia, the research is governed by the Australian code for the responsible conduct of research (2008). It identifies the responsibilities of institutions and researchers in all areas of research. The code has been jointly developed by the National Health and Medical Research Council (NHMRC), the Australian Research Council (ARC) and Universities Australia (UA). This information is summarized in Table 2 .
Basic structure of an institutional ethical advisory committee (EAC)
The WHO published an article defining the basic concepts of an ethical advisory committee in 2009 (WHO, 2009 - see above). According to this, many countries have established research governance and monitor the ethical practice in research via national and/or regional review committees. The main aims of research ethics committees include reviewing the study proposals, trying to understand the justifications for human/animal use, weighing the merits and demerits of the usage (linking to risks vs. potential benefits) and ensuring the local, ethical guidelines are followed Difn website b - Enago academy Importance of Ethics Committees in Scholarly Research, 2020 ; Guide for Research Ethics - Council of Europe, 2014 ). Once the research has started, the committee needs to carry out periodic surveillance to ensure the institutional ethical norms are followed during and beyond the study. They may also be involved in setting up and/or reviewing the institutional policies.
For these aspects, IRB (or institutional ethical advisory committee - IEAC) is essential for local governance to enhance best practices. The advantage of an IRB/EEAC is that they understand the institutional conditions and can closely monitor the ongoing research, including any changes in research directions. On the other hand, the IRB may be overly supportive to accept applications, influenced by the local agenda for achieving research excellence, disregarding ethical issues (Kotecha et al., 2011 ; Kayser-Jones, 2003 ) or, they may be influenced by the financial interests in attracting external funding. In this respect, regional and national ethics committees are advantageous to ensure ethical practice. Due to their impartiality, they would provide greater consistency and legitimacy to the research (WHO, 2009 ). However, the ethical approval process of regional and national ethics committees would be time consuming, as they do not have the local knowledge.
As for membership in the IRBs, most of the guidelines [WHO, NICE, Council of Europe, (2012), European Commission - Facilitating Research Excellence in FP7 ( 2013 ) and OHRP] insist on having a variety of representations including experts in different fields of research, and non-experts with the understanding of local, national/international conflicts of interest. The former would be able to understand/clarify the procedural elements of the research in different fields; whilst the latter would help to make neutral and impartial decisions. These non-experts are usually not affiliated to the institution and consist of individuals representing the broader community (particularly those related to social, legal or cultural considerations). IRBs consisting of these varieties of representation would not only be in a position to understand the study procedures and their potential direct or indirect consequences for participants, but also be able to identify any community, cultural or religious implications of the study.
Understanding the subtle differences between ethics and morals
Interestingly, many ethical guidelines are based on society’s moral “beliefs” in such a way that the words “ethics”‘and “morals” are reciprocally used to define each other. However, there are several subtle differences between them and we have attempted to compare and contrast them herein. In the past, many authors have interchangeably used the words “morals”‘and “ethics”‘(Warwick, 2003 ; Kant, 2018 ; Hazard, GC (Jr)., 1994 , Larry, 1982 ). However, ethics is linked to rules governed by an external source such as codes of conduct in workplaces (Kuyare et al., 2014 ). In contrast, morals refer to an individual’s own principles regarding right and wrong. Quinn ( 2011 ) defines morality as “ rules of conduct describing what people ought and ought not to do in various situations … ” while ethics is “... the philosophical study of morality, a rational examination into people’s moral beliefs and behaviours ”. For instance, in a case of parents demanding that schools overturn a ban on use of corporal punishment of children by schools and teachers (Children’s Rights Alliance for England, 2005 ), the parents believed that teachers should assume the role of parent in schools and use corporal or physical punishment for children who misbehaved. This stemmed from their beliefs and what they felt were motivated by “beliefs of individuals or groups”. For example, recent media highlights about some parents opposing LGBT (Lesbian, Gay, Bisexual, and Transgender) education to their children (BBC News, 2019 ). One parent argued, “Teaching young children about LGBT at a very early stage is ‘morally’ wrong”. She argued “let them learn by themselves as they grow”. This behaviour is linked to and governed by the morals of an ethnic community. Thus, morals are linked to the “beliefs of individuals or group”. However, when it comes to the LGBT rights these are based on ethical principles of that society and governed by law of the land. However, the rights of children to be protected from “inhuman and degrading” treatment is based on the ethical principles of the society and governed by law of the land. Individuals, especially those who are working in medical or judicial professions have to follow an ethical code laid down by their profession, regardless of their own feelings, time or preferences. For instance, a lawyer is expected to follow the professional ethics and represent a defendant, despite the fact that his morals indicate the defendant is guilty.
In fact, we as a group could not find many scholarly articles clearly comparing or contrasting ethics with morals. However, a table presented by Surbhi ( 2015 ) (Difn website c ) tries to differentiate these two terms (see Table 3 ).
Although Table 3 gives some insight on the differences between these two terms, in practice many use these terms as loosely as possible mainly because of their ambiguity. As a group focussed on the application of these principles, we would recommend to use the term “ethics” and avoid “morals” in research and academia.
Based on the literature survey carried out, we were able to identify the following gaps:
there is some disparity in existing literature on the importance of ethical guidelines in research
there is a lack of consensus on what code of conduct should be followed, where it should be derived from and how it should be implemented
The mission of ENAI’s ethical advisory working group
The Ethical Advisory Working Group of ENAI was established in 2018 to promote ethical code of conduct/practice amongst higher educational organisations within Europe and beyond (European Network for Academic Integrity, 2018 ). We aim to provide unbiased advice and consultancy on embedding ethical principles within all types of academic, research and public engagement activities. Our main objective is to promote ethical principles and share good practice in this field. This advisory group aims to standardise ethical norms and to offer strategic support to activities including (but not exclusive to):
● rendering advice and assistance to develop institutional ethical committees and their regulations in member institutions,
● sharing good practice in research and academic ethics,
● acting as a critical guide to institutional review processes, assisting them to maintain/achieve ethical standards,
● collaborating with similar bodies in establishing collegiate partnerships to enhance awareness and practice in this field,
● providing support within and outside ENAI to develop materials to enhance teaching activities in this field,
● organising training for students and early-career researchers about ethical behaviours in form of lectures, seminars, debates and webinars,
● enhancing research and dissemination of the findings in matters and topics related to ethics.
The following sections focus on our suggestions based on collective experiences, review of literature provided in earlier sections and workshop feedback collected:
a) basic needs of an ethical committee within an institution;
b) a typical ethical approval process (with examples from three different universities); and
c) the ways to obtain informed consent with some examples. This would give advice on preparing and training both researchers and research students in appropriately upholding ethical practices through ethical approval processes.
Setting up an institutional ethical committee (ECs)
Institutional Ethical Committees (ECs) are essential to govern every aspect of the activities undertaken by that institute. With regards to higher educational organisations, this is vital to establish ethical behaviour for students and staff to impart research, education and scholarly activities (or everything) they do. These committees should be knowledgeable about international laws relating to different fields of studies (such as science, medicine, business, finance, law, and social sciences). The advantages and disadvantages of institutional, subject specific or common (statutory) ECs are summarised in Fig. 2 . Some institutions have developed individual ECs linked to specific fields (or subject areas) whilst others have one institutional committee that overlooks the entire ethical behaviour and approval process. There is no clear preference between the two as both have their own advantages and disadvantages (see Fig. 2 ). Subject specific ECs are attractive to medical, law and business provisions, as it is perceived the members within respective committees would be able to understand the subject and therefore comprehend the need of the proposed research/activity (Kadam, 2012 ; Schnyder et al., 2018 ). However, others argue, due to this “ specificity ”, the committee would fail to forecast the wider implications of that application. On the other hand, university-wide ECs would look into the wider implications. Yet they find it difficult to understand the purpose and the specific applications of that research. Not everyone understands dynamics of all types of research methodologies, data collection, etc., and therefore there might be a chance of a proposal being rejected merely because the EC could not understand the research applications (Getz, 1990 ).
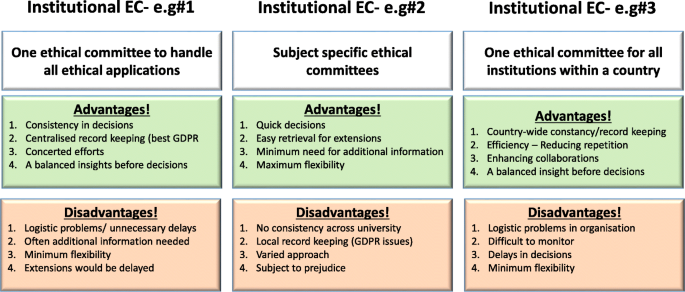
Summary of advantages and disadvantages of three different forms of ethical committees
[N/B for Fig. 2 : Examples of different types of ethical application procedures and forms used were discussed with the workshop attendees to enhance their understanding of the differences. GDPR = General Data Protection Regulation].
Although we recommend a designated EC with relevant professional, academic and ethical expertise to deal with particular types of applications, the membership (of any EC) should include some non-experts who would represent the wider community (see above). Having some non-experts in EC would not only help the researchers to consider explaining their research in layperson’s terms (by thinking outside the box) but also would ensure efficiency without compromising participants/animal safety. They may even help to address the common ethical issues outside research culture. Some UK universities usually offer this membership to a clergy, councillor or a parliamentarian who does not have any links to the institutions. Most importantly, it is vital for any EC members to undertake further training in addition to previous experience in the relevant field of research ethics.
Another issue that raises concerns is multi-centre research, involving several institutions, where institutionalised ethical approvals are needed from each partner. In some cases, such as clinical research within the UK, a common statutory EC called National Health Services (NHS) Research Ethics Committee (NREC) is in place to cover research ethics involving all partner institutions (NHS, 2018 ). The process of obtaining approval from this type of EC takes time, therefore advanced planning is needed.
Ethics approval forms and process
During the workshop, we discussed some anonymised application forms obtained from open-access sources for qualitative and quantitative research as examples. Considering research ethics, for the purpose of understanding, we arbitrarily divided this in two categories; research based on (a) quantitative and (b) qualitative methodologies. As their name suggests their research approach is extremely different from each other. The discussion elicited how ECs devise different types of ethical application form/questions. As for qualitative research, these are often conducted as “face-to-face” interviews, which would have implications on volunteer anonymity.
Furthermore, discussions posited when the interviews are replaced by on-line surveys, they have to be administered through registered university staff to maintain confidentiality. This becomes difficult when the research is a multi-centre study. These types of issues are also common in medical research regarding participants’ anonymity, confidentially, and above all their right to withdraw consent to be involved in research.
Storing and protecting data collected in the process of the study is also a point of consideration when applying for approval.
Finally, the ethical processes of invasive (involving human/animals) and non-invasive research (questionnaire based) may slightly differ from one another. Following research areas are considered as investigations that need ethical approval:
research that involves human participants (see below)
use of the ‘products’ of human participants (see below)
work that potentially impacts on humans (see below)
research that involves animals
In addition, it is important to provide a disclaimer even if an ethical approval is deemed unnecessary. Following word cloud (Fig. 3 ) shows the important variables that need to be considered at the brainstorming stage before an ethical application. It is worth noting the importance of proactive planning predicting the “unexpected” during different phases of a research project (such as planning, execution, publication, and future directions). Some applications (such as working with vulnerable individuals or children) will require safety protection clearance (such as DBS - Disclosure and Barring Service, commonly obtained from the local police). Please see section on Research involving Humans - Informed consents for further discussions.
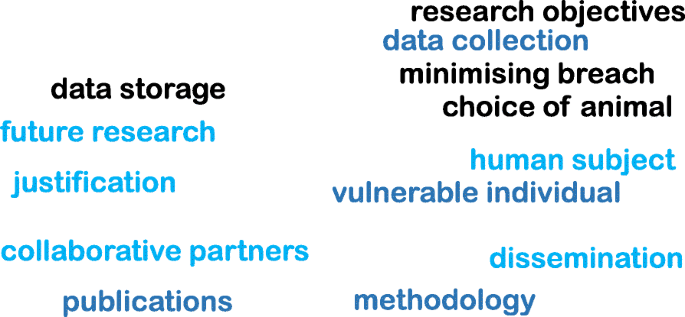
Examples of important variables that need to be considered for an ethical approval
It is also imperative to report or re-apply for ethical approval for any minor or major post-approval changes to original proposals made. In case of methodological changes, evidence of risk assessments for changes and/or COSHH (Control of Substances Hazardous to Health Regulations) should also be given. Likewise, any new collaborative partners or removal of researchers should also be notified to the IEAC.
Other findings include:
in case of complete changes in the project, the research must be stopped and new approval should be seeked,
in case of noticing any adverse effects to project participants (human or non-human), these should also be notified to the committee for appropriate clearance to continue the work, and
the completion of the project must also be notified with the indication whether the researchers may restart the project at a later stage.
Research involving humans - informed consents
While discussing research involving humans and based on literature review, findings highlight the human subjects/volunteers must willingly participate in research after being adequately informed about the project. Therefore, research involving humans and animals takes precedence in obtaining ethical clearance and its strict adherence, one of which is providing a participant information sheet/leaflet. This sheet should contain a full explanation about the research that is being carried out and be given out in lay-person’s terms in writing (Manti and Licari 2018 ; Hardicre 2014 ). Measures should also be in place to explain and clarify any doubts from the participants. In addition, there should be a clear statement on how the participants’ anonymity is protected. We provide below some example questions below to help the researchers to write this participant information sheet:
What is the purpose of the study?
Why have they been chosen?
What will happen if they take part?
What do they have to do?
What happens when the research stops?
What if something goes wrong?
What will happen to the results of the research study?
Will taking part be kept confidential?
How to handle “vulnerable” participants?
How to mitigate risks to participants?
Many institutional ethics committees expect the researchers to produce a FAQ (frequently asked questions) in addition to the information about research. Most importantly, the researchers also need to provide an informed consent form, which should be signed by each human participant. The five elements identified that are needed to be considered for an informed consent statement are summarized in Fig. 4 below (slightly modified from the Federal Policy for the Protection of Human Subjects ( 2018 ) - Diffn website c ).
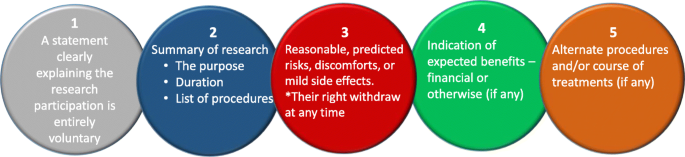
Five basic elements to consider for an informed consent [figure adapted from Diffn website c ]
The informed consent form should always contain a clause for the participant to withdraw their consent at any time. Should this happen all the data from that participant should be eliminated from the study without affecting their anonymity.
Typical research ethics approval process
In this section, we provide an example flow chart explaining how researchers may choose the appropriate application and process, as highlighted in Fig. 5 . However, it is imperative to note here that these are examples only and some institutions may have one unified application with separate sections to demarcate qualitative and quantitative research criteria.
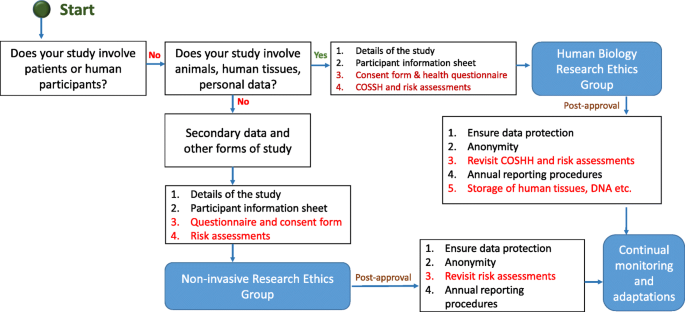
Typical ethical approval processes for quantitative and qualitative research. [N/B for Fig. 5 - This simplified flow chart shows that fundamental process for invasive and non-invasive EC application is same, the routes and the requirements for additional information are slightly different]
Once the ethical application is submitted, the EC should ensure a clear approval procedure with distinctly defined timeline. An example flow chart showing the procedure for an ethical approval was obtained from University of Leicester as open-access. This is presented in Fig. 6 . Further examples of the ethical approval process and governance were discussed in the workshop.
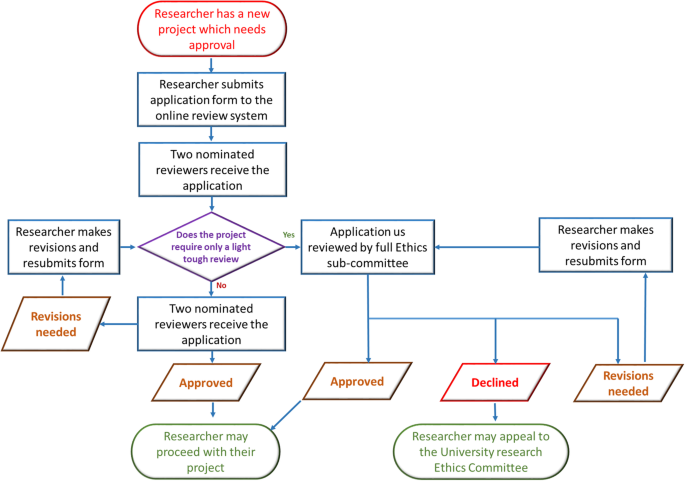
An example ethical approval procedures conducted within University of Leicester (Figure obtained from the University of Leicester research pages - Difn website d - open access)
Strategies for ethics educations for students
Student education on the importance of ethics and ethical behaviour in research and scholarly activities is extremely essential. Literature posits in the area of medical research that many universities are incorporating ethics in post-graduate degrees but when it comes to undergraduate degrees, there is less appetite to deliver modules or even lectures focussing on research ethics (Seymour et al., 2004 ; Willison and O’Regan, 2007 ). This may be due to the fact that undergraduate degree structure does not really focus on research (DePasse et al., 2016 ). However, as Orr ( 2018 ) suggested, institutions should focus more on educating all students about ethics/ethical behaviour and their importance in research, than enforcing punitive measures for unethical behaviour. Therefore, as an advisory committee, and based on our preliminary literature survey and workshop results, we strongly recommend incorporating ethical education within undergraduate curriculum. Looking at those institutions which focus on ethical education for both under-and postgraduate courses, their approaches are either (a) a lecture-based delivery, (b) case study based approach or (c) a combined delivery starting with a lecture on basic principles of ethics followed by generating a debate based discussion using interesting case studies. The combined method seems much more effective than the other two as per our findings as explained next.
As many academics who have been involved in teaching ethics and/or research ethics agree, the underlying principles of ethics is often perceived as a boring subject. Therefore, lecture-based delivery may not be suitable. On the other hand, a debate based approach, though attractive and instantly generates student interest, cannot be effective without students understanding the underlying basic principles. In addition, when selecting case studies, it would be advisable to choose cases addressing all different types of ethical dilemmas. As an advisory group within ENAI, we are in the process of collating supporting materials to help to develop institutional policies, creating advisory documents to help in obtaining ethical approvals, and teaching materials to enhance debate-based lesson plans that can be used by the member and other institutions.
Concluding remarks
In summary, our literature survey and workshop findings highlight that researchers should accept that ethics underpins everything we do, especially in research. Although ethical approval is tedious, it is an imperative process in which proactive thinking is essential to identify ethical issues that might affect the project. Our findings further lead us to state that the ethical approval process differs from institution to institution and we strongly recommend the researchers to follow the institutional guidelines and their underlying ethical principles. The ENAI workshop in Vilnius highlighted the importance of ethical governance by establishing ECs, discussed different types of ECs and procedures with some examples and highlighted the importance of student education to impart ethical culture within research communities, an area that needs further study as future scope.
Declarations
The manuscript was entirely written by the corresponding author with contributions from co-authors who have also taken part in the delivery of the workshop. Authors confirm that the data supporting the findings of this study are available within the article. We can also confirm that there are no potential competing interests with other organisations.
Availability of data and materials
Authors confirm that the data supporting the findings of this study are available within the article.
Abbreviations
ALL European academics
Australian research council
Biotechnology and biological sciences research council
Canadian institutes for health research
Committee of publication ethics
Ethical committee
European network of academic integrity
Economic and social research council
International convention for the protection of animals
institutional ethical advisory committee
Institutional review board
Immaculata university of Pennsylvania
Lesbian, gay, bisexual, and transgender
Medical research council)
National health services
National health services nih national institute of health (NIH)
National institute of clinical care excellence
National health and medical research council
Natural sciences and engineering research council
National research ethics committee
National statement on ethical conduct in human research
Responsible research practice
Social sciences and humanities research council
Tri-council policy statement
World Organization for animal health
Universities Australia
UK-research and innovation
US office for human research protections
Alba S, Lenglet A, Verdonck K, Roth J, Patil R, Mendoza W, Juvekar S, Rumisha SF (2020) Bridging research integrity and global health epidemiology (BRIDGE) guidelines: explanation and elaboration. BMJ Glob Health 5(10):e003237. https://doi.org/10.1136/bmjgh-2020-003237
Article Google Scholar
Anderson MS (2011) Research misconduct and misbehaviour. In: Bertram Gallant T (ed) Creating the ethical academy: a systems approach to understanding misconduct and empowering change in higher education. Routledge, pp 83–96
BBC News. (2019). Birmingham school LGBT LESSONS PROTEST investigated. March 8, 2019. Retrieved February 14, 2021, available online. URL: https://www.bbc.com/news/uk-england-birmingham-47498446
Children’s Rights Alliance for England. (2005). R (Williamson and others) v Secretary of State for Education and Employment. Session 2004–05. [2005] UKHL 15. Available Online. URL: http://www.crae.org.uk/media/33624/R-Williamson-and-others-v-Secretary-of-State-for-Education-and-Employment.pdf
Council of Europe. (2014). Texts of the Council of Europe on bioethical matters. Available Online. https://www.coe.int/t/dg3/healthbioethic/Texts_and_documents/INF_2014_5_vol_II_textes_%20CoE_%20bio%C3%A9thique_E%20(2).pdf
Dellaportas S, Kanapathippillai S, Khan, A and Leung, P. (2014). Ethics education in the Australian accounting curriculum: a longitudinal study examining barriers and enablers. 362–382. Available Online. URL: https://doi.org/10.1080/09639284.2014.930694 , 23, 4, 362, 382
DePasse JM, Palumbo MA, Eberson CP, Daniels AH (2016) Academic characteristics of orthopaedic surgery residency applicants from 2007 to 2014. JBJS 98(9):788–795. https://doi.org/10.2106/JBJS.15.00222
Desmond H, Dierickx K (2021) Research integrity codes of conduct in Europe: understanding the divergences. https://doi.org/10.1111/bioe.12851
Difn website a - National Statement on Ethical Conduct in Human Research (NSECHR). (2018). Available Online. URL: https://www.nhmrc.gov.au/about-us/publications/australian-code-responsible-conduct-research-2018
Difn website b - Enago academy Importance of Ethics Committees in Scholarly Research (2020, October 26). Available online. URL: https://www.enago.com/academy/importance-of-ethics-committees-in-scholarly-research/
Difn website c - Ethics vs Morals - Difference and Comparison. Retrieved July 14, 2020. Available online. URL: https://www.diffen.com/difference/Ethics_vs_Morals
Difn website d - University of Leicester. (2015). Staff ethics approval flowchart. May 1, 2015. Retrieved July 14, 2020. Available Online. URL: https://www2.le.ac.uk/institution/ethics/images/ethics-approval-flowchart/view
European Commission - Facilitating Research Excellence in FP7 (2013) https://ec.europa.eu/research/participants/data/ref/fp7/89888/ethics-for-researchers_en.pdf
European Network for Academic Integrity. (2018). Ethical advisory group. Retrieved February 14, 2021. Available online. URL: http://www.academicintegrity.eu/wp/wg-ethical/
Federal Policy for the Protection of Human Subjects. (2018). Retrieved February 14, 2021. Available Online. URL: https://www.federalregister.gov/documents/2017/01/19/2017-01058/federal-policy-for-the-protection-of-human-subjects#p-855
Flite, CA and Harman, LB. (2013). Code of ethics: principles for ethical leadership Perspect Health Inf Mana; 10(winter): 1d. PMID: 23346028
Fouka G, Mantzorou M (2011) What are the major ethical issues in conducting research? Is there a conflict between the research ethics and the nature of nursing. Health Sci J 5(1) Available Online. URL: https://www.hsj.gr/medicine/what-are-the-major-ethical-issues-in-conducting-research-is-there-a-conflict-between-the-research-ethics-and-the-nature-of-nursing.php?aid=3485
Fox G (2017) History and ethical principles. The University of Miami and the Collaborative Institutional Training Initiative (CITI) Program URL https://silo.tips/download/chapter-1-history-and-ethical-principles # (Available Online)
Getz KA (1990) International codes of conduct: An analysis of ethical reasoning. J Bus Ethics 9(7):567–577
Ghooi RB (2011) The nuremberg code–a critique. Perspect Clin Res 2(2):72–76. https://doi.org/10.4103/2229-3485.80371
Hardicre, J. (2014) Valid informed consent in research: an introduction Br J Nurs 23(11). https://doi.org/10.12968/bjon.2014.23.11.564 , 567
Hazard, GC (Jr). (1994). Law, morals, and ethics. Yale law school legal scholarship repository. Faculty Scholarship Series. Yale University. Available Online. URL: https://digitalcommons.law.yale.edu/cgi/viewcontent.cgi?referer=https://www.google.com/&httpsredir=1&article=3322&context=fss_papers
Israel, M., & Drenth, P. (2016). Research integrity: perspectives from Australia and Netherlands. In T. Bretag (Ed.), Handbook of academic integrity (pp. 789–808). Springer, Singapore. https://doi.org/10.1007/978-981-287-098-8_64
Kadam R (2012) Proactive role for ethics committees. Indian J Med Ethics 9(3):216. https://doi.org/10.20529/IJME.2012.072
Kant I (2018) The metaphysics of morals. Cambridge University Press, UK https://doi.org/10.1017/9781316091388
Kayser-Jones J (2003) Continuing to conduct research in nursing homes despite controversial findings: reflections by a research scientist. Qual Health Res 13(1):114–128. https://doi.org/10.1177/1049732302239414
Kotecha JA, Manca D, Lambert-Lanning A, Keshavjee K, Drummond N, Godwin M, Greiver M, Putnam W, Lussier M-T, Birtwhistle R (2011) Ethics and privacy issues of a practice-based surveillance system: need for a national-level institutional research ethics board and consent standards. Can Fam physician 57(10):1165–1173. https://europepmc.org/article/pmc/pmc3192088
Kuyare, MS., Taur, SR., Thatte, U. (2014). Establishing institutional ethics committees: challenges and solutions–a review of the literature. Indian J Med Ethics. https://doi.org/10.20529/IJME.2014.047
LaFollette, H. (2007). Ethics in practice (3rd edition). Blackwell
Larry RC (1982) The teaching of ethics and moral values in teaching. J High Educ 53(3):296–306. https://doi.org/10.1080/00221546.1982.11780455
Manti S, Licari A (2018) How to obtain informed consent for research. Breathe (Sheff) 14(2):145–152. https://doi.org/10.1183/20734735.001918
Mulholland MW, Bell J (2005) Research Governance and Research Funding in the USA: What the academic surgeon needs to know. J R Soc Med 98(11):496–502. https://doi.org/10.1258/jrsm.98.11.496
National Institute of Health (NIH) Ethics in Clinical Research. n.d. Available Online. URL: https://clinicalcenter.nih.gov/recruit/ethics.html
NHS (2018) Flagged Research Ethics Committees. Retrieved February 14, 2021. Available online. URL: https://www.hra.nhs.uk/about-us/committees-and-services/res-and-recs/flagged-research-ethics-committees/
NICE (2018) Research governance policy. Retrieved February 14, 2021. Available online. URL: https://www.nice.org.uk/Media/Default/About/what-we-do/science-policy-and-research/research-governance-policy.pdf
Orr, J. (2018). Developing a campus academic integrity education seminar. J Acad Ethics 16(3), 195–209. https://doi.org/10.1007/s10805-018-9304-7
Quinn, M. (2011). Introduction to Ethics. Ethics for an Information Age. 4th Ed. Ch 2. 53–108. Pearson. UK
Resnik. (2020). What is ethics in Research & why is it Important? Available Online. URL: https://www.niehs.nih.gov/research/resources/bioethics/whatis/index.cfm
Schnyder S, Starring H, Fury M, Mora A, Leonardi C, Dasa V (2018) The formation of a medical student research committee and its impact on involvement in departmental research. Med Educ Online 23(1):1. https://doi.org/10.1080/10872981.2018.1424449
Seymour E, Hunter AB, Laursen SL, DeAntoni T (2004) Establishing the benefits of research experiences for undergraduates in the sciences: first findings from a three-year study. Sci Educ 88(4):493–534. https://doi.org/10.1002/sce.10131
Shamoo AE, Irving DN (1993) Accountability in research using persons with mental illness. Account Res 3(1):1–17. https://doi.org/10.1080/08989629308573826
Shaw, S., Boynton, PM., and Greenhalgh, T. (2005). Research governance: where did it come from, what does it mean? Research governance framework for health and social care, 2nd ed. London: Department of Health. https://doi.org/10.1258/jrsm.98.11.496 , 98, 11, 496, 502
Book Google Scholar
Speight, JG. (2016) Ethics in the university |DOI: https://doi.org/10.1002/9781119346449 scrivener publishing LLC
Stephenson GK, Jones GA, Fick E, Begin-Caouette O, Taiyeb A, Metcalfe A (2020) What’s the protocol? Canadian university research ethics boards and variations in implementing tri-Council policy. Can J Higher Educ 50(1)1): 68–81
Surbhi, S. (2015). Difference between morals and ethics [weblog]. March 25, 2015. Retrieved February 14, 2021. Available Online. URL: http://keydifferences.com/difference-between-morals-and-ethics.html
The Belmont Report (1979). Ethical Principles and Guidelines for the Protection of Human Subjects of Research. The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. Retrieved February 14, 2021. Available online. URL: https://www.hhs.gov/ohrp/sites/default/files/the-belmont-report-508c_FINAL.pdf
The Singapore Statement on Research Integrity. (2020). Nicholas Steneck and Tony Mayer, Co-chairs, 2nd World Conference on Research Integrity; Melissa Anderson, Chair, Organizing Committee, 3rd World Conference on Research Integrity. Retrieved February 14, 2021. Available online. URL: https://wcrif.org/documents/327-singapore-statement-a4size/file
Warwick K (2003) Cyborg morals, cyborg values, cyborg ethics. Ethics Inf Technol 5(3):131–137. https://doi.org/10.1023/B:ETIN.0000006870.65865.cf
Weindling P (2001) The origins of informed consent: the international scientific commission on medical war crimes, and the Nuremberg code. Bull Hist Med 75(1):37–71. https://doi.org/10.1353/bhm.2001.0049
WHO. (2009). Research ethics committees Basic concepts for capacity-building. Retrieved February 14, 2021. Available online. URL: https://www.who.int/ethics/Ethics_basic_concepts_ENG.pdf
WHO. (2021). Chronological list of publications. Retrieved February 14, 2021. Available online. URL: https://www.who.int/ethics/publications/year/en/
Willison, J. and O’Regan, K. (2007). Commonly known, commonly not known, totally unknown: a framework for students becoming researchers. High Educ Res Dev 26(4). 393–409. https://doi.org/10.1080/07294360701658609
Žukauskas P, Vveinhardt J, and Andriukaitienė R. (2018). Research Ethics In book: Management Culture and Corporate Social Responsibility Eds Jolita Vveinhardt IntechOpenEditors DOI: https://doi.org/10.5772/intechopen.70629 , 2018
Download references
Acknowledgements
Authors wish to thank the organising committee of the 5th international conference named plagiarism across Europe and beyond, in Vilnius, Lithuania for accepting this paper to be presented in the conference.
Not applicable as this is an independent study, which is not funded by any internal or external bodies.
Author information
Authors and affiliations.
School of Human Sciences, University of Derby, DE22 1, Derby, GB, UK
Shivadas Sivasubramaniam
Department of Informatics, Mendel University in Brno, Zemědělská, 1665, Brno, Czechia
Dita Henek Dlabolová
Centre for Academic Integrity in the UAE, Faculty of Engineering & Information Sciences, University of Wollongong in Dubai, Dubai, UAE
Veronika Kralikova & Zeenath Reza Khan
You can also search for this author in PubMed Google Scholar
Contributions
The manuscript was entirely written by the corresponding author with contributions from co-authors who have equally contributed to presentation of this paper in the 5th international conference named plagiarism across Europe and beyond, in Vilnius, Lithuania. Authors have equally contributed for the information collection, which were then summarised as narrative explanations by the Corresponding author and Dr. Zeenath Reza Khan. Then checked and verified by Dr. Dlabolova and Ms. Králíková. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Shivadas Sivasubramaniam .
Ethics declarations
Competing interests.
We can also confirm that there are no potential competing interest with other organisations.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sivasubramaniam, S., Dlabolová, D.H., Kralikova, V. et al. Assisting you to advance with ethics in research: an introduction to ethical governance and application procedures. Int J Educ Integr 17 , 14 (2021). https://doi.org/10.1007/s40979-021-00078-6
Download citation
Received : 17 July 2020
Accepted : 25 April 2021
Published : 13 July 2021
DOI : https://doi.org/10.1007/s40979-021-00078-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Higher education
- Ethical codes
- Ethics committee
- Post-secondary education
- Institutional policies
- Research ethics
International Journal for Educational Integrity
ISSN: 1833-2595
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Afr J Emerg Med
- v.10(Suppl 2); 2020
Principles of research ethics: A research primer for low- and middle-income countries
Cindy c. bitter.
a Saint Louis University School of Medicine, Division of Emergency Medicine, St. Louis MO, USA
Annet Alenyo Ngabirano
b Aga Khan University, Kampala, Uganda
e Makerere University College of Health Sciences, Kampala, Uganda
Erin L. Simon
c Cleveland Clinic Akron General, Department of Emergency Medicine, Akron, OH, USA
f Northeast Ohio Medical University, Rootstown, OH, USA
David McD. Taylor
d University of Melbourne, Department of Medicine, Parkville, Victoria, Australia
g Austin Health, Heidelburg, Victoria, Australia
Ethical oversight in the form of review boards and research ethics committees provide protection for research subjects as well as guidance for safe conduct of studies. As the number of collaborative emergency care research studies carried out in low- and middle-income countries increases, it is crucial to have a shared understanding of how ethics should inform choice of study topic, study design, methods of obtaining consent, data management, and access to treatment after closure of the study. This paper describes the basic principles of Western research ethics – respect for persons, beneficence, and justice - and how the principles may be contextualized in different settings, by researchers of various backgrounds with different funding streams. Examples of lapses in ethical practice of research are used to highlight best practices.
African relevance
- • Low and middle income countries (LMICs) have a high burden of acute illness, but insufficient research on emergency care
- • Ethical barriers and lack of oversight impede emergency care research in LMICs
- • Ethical clinical research in LMICs builds capacity and contributes to the evidence base for emergency care
The International Federation for Emergency Medicine global health research primer
This paper forms part 7 of a series of how to papers, commissioned by the International Federation for Emergency Medicine. This primer discusses ways to ensure that research is carried out ethically. It discusses principles for researchers from the global north involved in emergency care research projects in LMICs and provides guidance for LMIC researchers who may not have experience for navigating ethical issues in research. We have also included additional tips and pitfalls that are relevant to emergency medicine researchers.
Despite a disproportionally high burden of acute illnesses in low-and middle-income countries (LMICs), there is insufficient emergency care research. In a survey carried out through the African Federation for Emergency Medicine (AFEM), 67.3% of respondents agreed with this statement [ 1 ]. Research that targets local health challenges, such as context-specific health service delivery and drug development for diseases prevalent in LMIC, has the potential to improve population health in host countries [ [2] , [3] , [4] ].
Ethical barriers and oversight have been cited as an important barrier to research in LMIC [ 5 ]. Emanuel et al. provided a framework for conducting ethical research that stressed collaborative partnerships, social value, scientific validity, fair selection of study populations, favourable risk-benefit ratio, independent review, informed consent, and respect for recruited participants and study communities [ 6 ]. In their review of the framework, Tsoka-Gwegweni and colleagues concluded that most recurring ethical issues relating to research in LMICs were informed consent, scientific validity, fair participant selection and on-going respect for participants [ 7 ]. Research priorities are often determined outside the host country, raising concerns of outsourcing risk and exploitation of uninformed populations [ 8 ].
The value of building ethical clinical research in LMICs goes beyond the specific information collected in a study. Conducting research has substantial positive impact in a community by raising local scholarly standards and encouraging independent thinking and creativity as many LMICs work towards building evidence-based emergency care systems. Each project builds confidence and skills which allows local researchers to participate fully in the scientific process which impacts the care of their local community. This may lead to intellectual and financial resources for the community which in turn encourages local empowerment and self-sufficiency for additional research.
Bioethical principles
Review of research protocols by experts not involved with the study is one way of ensuring protection for human research subjects. In the United States of America (US), such review committees are called Institutional Review Boards (IRBs). In other countries, these committees may be called Human Research Ethics Committees (HREC) or have other titles. Several articles discuss the evolution of HRECs in sub Saharan Africa [ 9 , 10 ].
Most Western scholars agree on the main principles of autonomy, beneficence, and justice for clinical ethics, with some adjustments in how these principles are applied in the research setting. However, these principles are not universally acknowledged. Some authors argue that these principles are less relevant in populations where community rather than individual values are stressed. Others argue that local development of research ethics is crucial to ensuring buy-in and avoiding bioethical imperialism [ 11 , 12 ]. Acknowledging the past abuses that took place under the guise of research, Western ethical thought now insists on voluntary consent, with special protections for traditionally vulnerable populations such as children, women, and ethnic minorities. Emergency care research, however, introduces a unique type of vulnerability in unconscious or other otherwise mentally altered patients who do not meet criteria for giving informed consent. In some Western countries like the US and the United Kingdom (UK), such patients may still be enrolled in studies through a different process called an exemption from informed consent or waiver of consent [ [13] , [14] , [15] ]. The same principle may be applied in LMICs, however, a detailed assessment of the research components necessary to guide HRECs in order to reach decisions that protect this vulnerable group from harm or unnecessary risk.

International standards
In 1964, the World Medical Association adopted the Declaration of Helsinki. This document and subsequent revisions lay out the principles of minimizing risks, informed consent, privacy, special protections for vulnerable groups, access to beneficial treatment after trial, and dissemination [ 16 ]. The first Declaration was adopted largely in response to the unethical experiments carried out by Nazi researchers during the Holocaust, in which prisoners were exposed to infectious disease, extremes of temperature, and experimental drugs without their consent, often resulting in death [ 17 ].
The Declaration is a guideline of research ethics but is not legally binding. Individual countries are responsible for implementing legislation that reflects the principles. In the United States, discovery of ongoing research abuses such as the Tuskegee syphilis study in the 1970's led to adoption of the Belmont Report [ 18 ]. The Belmont Report requires that researchers uphold three basic principles: respect for persons, beneficence, and justice. Researchers in the US must have the protocol approved by an IRB prior to enrolling subjects. IRBs are charged with operationalizing the principles outlined in the Belmont Report and subsequent regulations specified in the Common Rule [ 19 ]. Certain research may be exempted from IRB review and these typically include surveys, interviews, and research using existing specimens, records or data. The concept of “minimal risk to participants” plays a key role in allowing this exemption and should be considered when conducting research in LMIC's. “Minimal risk” is defined as that “ordinarily encountered in daily life or the performance of routine physical and psychological testing.”
These previous regulations, however, were written without consideration for Emergency Care research. Therefore in 1996, the US' Food and Drug Administration recommended procedures for enrolling patients in emergency situations into research studies through exemption of informed consent [ 20 ]. Similar procedures were adopted by the United Kingdom in 2006 [ 21 ]. This concept of “waiver of consent” in emergency care research should be considered in LMICs where contextualized processes are critical for development of sustainable emergency care systems.
Guidelines intended for global adoption often lack details required for context-specific implementation and can be slow to respond to emerging realities. Barugahare uses an analogy to jurisprudence to discuss how local HRECs can guide implementation of global ethical principles within their own contexts [ 22 ]. With that in mind, we will go through the guiding principles and discuss pitfalls in implementation.
Respect for persons
Respect for persons requires that research subjects freely participate in research after informed consent. Subjects should be counselled on the known risks of the drug or treatment being examined, the expected course of illness without intervention, and any compensation or benefit they may receive from participating. This must occur in the subjects' native language. If documents require translation from the researchers' language to the local language, the documents should be translated back to the original language and reviewed to ensure consistency with the originals. Only after the subject has shown comprehension and has had any questions answered can they be considered to have given informed consent. There is some disagreement about the need for a signature on a consent form, but researchers must treat consent as an ongoing process rather than a piece of paper. Subjects must also understand they are free to withdraw from the study at any time.
Research in the global context highlights challenges to the notion of consent. In the global north, the concept of respect for persons is often supplanted by the principle of autonomy. But many cultures outside the global north stress the importance of the family unit and communitarian values over individual decisions [ 23 ]. Community leaders and heads-of-household should be involved in the process, often before approaching individual subjects [ 24 ]. This is especially critical in emergency situations where “waiver of consent” protocols require involvement of family or communities of unconscious or otherwise incapacitated patients. Consent processes may also be challenging when approaching female subjects, who may require approval of a male head of household in addition to their own consent [ [25] , [26] , [27] ]. While 18 years is considered the age of adulthood in many Western countries, other cultures may consider younger persons to have reached maturity [ 28 , 29 ]. In one qualitative study of 15–19 year old participants in HIV research in Kenya, 50% thought parental consent should be sought, 25% felt this was not necessary, and 25% had mixed feelings [ 30 ].
Lack of education, limited health literacy, poor access to medical care, and the sense of urgency that accompanies disease outbreaks also call into question the notion of voluntary, informed consent. The Pfizer meningitis trial in Nigeria reported that 100% of patients approached were enrolled in the protocol, suggesting patients were desperate to access care [ 31 ]. All participants in a clinical trial for malaria believed procedures followed solely to meet research objectives were a required part of their individual curative treatment [ 32 ]. Some participants in an Ebola vaccine trial enrolled in hopes of accessing free healthcare for other conditions [ 33 ].
Beneficence
Beneficence requires that researchers obtain scientifically valid data with useful applications, while minimizing risks within the study protocol and protecting subjects during the trial.
Although several guidelines recommend research in LMIC focus on health conditions that contribute substantially to the local burden of disease, research objectives are set by funders and do not always meet this guideline. Research on cancer therapeutics focuses on lung and breast cancer, even when trials are performed in LMIC which have a large burden of cervical, liver and gastric cancers [ 34 ]. While some risk is unavoidable when testing new drugs, research subjects should expect benefits to accrue for their population.
Use of rigorous methodology with adequate power and appropriate control groups is essential to ethical research. Underpowered research, poorly designed studies, and falsified data will not yield valid results and are inherently unethical. The use of placebo controls has been an area of contention since early studies of maternal-fetal transmission of HIV [ 35 ]. While placebo-controlled trials are optimal from a methodological standpoint, patients should be treated with their standard local regimen if effective treatment is available. Cheah et al. provide a compelling rationale for a placebo arm in their malaria trial, as use of the placebo is equivalent to current clinical practice, thus the subjects do not incur additional risk [ 36 ]. Researchers should ensure that interventions proven successful will be sustainable in their study population [ 37 ].
Unproven interventions may be utilized during emergency and disaster situations. The World Health Organization convened a panel to help guide use of novel therapies during epidemics [ 38 ]. They stress the importance rigorous data collection to assess effectiveness and safety of such interventions, and transparency and fairness in decisions regarding access to investigational treatments.
Minimizing risk means building safety into the protocol and monitoring for adverse events throughout the trial. Adverse events may require unblinding, a report to the HREC, or cessation of the trial. Dissemination of all results, regardless of the success of the trial, is crucial to ensure future study participants are not exposed to risks of unsuccessful interventions and funding can be distributed to projects more likely to result in improved health [ 39 ].
Protection of privacy is another important principle of beneficence. Data should only be accessible to the research team. Surveys and other paper documents must be kept in a locked cabinet or drawer in a secure room. Files should be password protected. Study ID numbers should be substituted for individual identifiers. Master lists that could be used to link data back to participants should be destroyed after data is cleaned. Data must be de-identified before sharing data with anyone outside the original study team. Data-sharing is important for verification of results and avoidance of duplicate efforts, but regulations and guidelines have not kept pace with emerging technologies [ 40 , 41 ]. Privacy laws vary widely between countries, with the European Union having some of the strictest protections. Although it would seem that outside researchers would adhere to the standards they must uphold to in their own settings, this is not always the case. Medical students from the US are more likely to violate patients' privacy online during international electives than during rotations at home [ 42 ].
Justice requires that the costs and benefits of research are distributed fairly within the population. Even this statement is open to interpretation, as there are no global norms of fairness [ 43 ]. Justice is a key consideration in the research framework proposed by Pratt and Loff [ 44 ].
The global distribution of harms and benefits in research is particularly problematic in the setting of clinical trials. The cost of research is significantly lower in LMIC but there is concern that pharmaceutical companies are also benefitting from lax regulatory oversight to conduct high-risk research that would not be tolerated in the global north [ 45 ]. Millions of persons participate in clinical research each year, but the number of adverse events is difficult to determine and likely underreported [ 46 , 47 ]. Deaths in clinical trials are not distributed evenly across nations, with higher rates of morbidity and mortality in LMIC. While deaths in the global north tend to be rare and highly publicized, India had 2644 deaths during clinical trials in the years 2005–12 [ 48 ]. Laws regarding compensation for injuries sustained during research are markedly different between nations, adding another layer of disparities [ 49 ].
Compensation for participation in research is another potential pitfall. Western research ethics considers whether compensation for participation is enough to exert undue influence or coercion. Paradoxically, using this standard in LMIC research would decrease the value of compensation as smaller amounts would likely constitute a larger proportion of subjects' income [ 50 ]. Participation “gifts” of nominal value, such as soap or sugar, may carry hidden meanings in the local context and should be discussed with community leaders [ 51 ]. One proposed solution would be to consider participation as work, to be compensated according to local custom [ 52 ].
A final area of justice concerns authorship of manuscripts. There is an inherent imbalance of power when outsiders approach a community to do research. Researchers should strive to build capacity in local communities and ensure all members of the study team receive appropriate credit. The International Committee of Medical Journal Editors requires the following criteria be met to be listed as an author: contributions to study design, data acquisition, or data analysis, drafting or substantial revisions to the manuscript, final approval of submission, and agreement to be accountable for integrity of the work [ 53 ]. All persons who meet these criteria should be listed. Guest authorship has been reported as common and usually involves including superiors or subject experts that did not contribute substantially to the manuscript [ 54 ]. Some authors fear that the criteria to draft/substantially revise and approve the final document discriminates against researchers from LMIC whose native language is not commonly used for international publications [ 55 ]. Determination of authorship and order of authors may cause great angst, particularly if not done early in the process.
Emerging challenges
Data security.
Collection of genetic material, even blood samples, may jeopardize anonymity. Consensus is lacking on how best to use “big data” to improve public health while protecting individual privacy concerns.
Tips on this topic
- • Research in LMICs, contextualized to their local health systems, is necessary to ensure patients receive the most appropriate care.
- • Researchers should ensure sound methodology and data management practices in their studies.
- • Community engagement is one way of ensuring patients' values are incorporated into setting research priorities, the consent process, risk assessment, and dissemination of results.
- • HRECs have a responsibility to protect human subjects but also serve to protect researchers from allegations of improper behaviour. Ethical approval for a protocol is required before researchers approach participants and is required for publication by most journals.
Pitfalls to avoid
- • Failure to consider local norms in obtaining informed consent
- • Failure to execute the study in exact accordance with the approved study protocol and other documentation may undermine the ethical integrity of the project and violate the ethical principles of research. Any changes to approved study protocols must be reviewed and approved by the local HREC prior to adoption.
- • Inadequate procedures for confidentiality and data security
Annotated bibliography
- • Biruk [ 51 ] uses the example of soap as a gift for participation in surveys to discuss ethical compensation for participants and duties of researchers.
- • Das et al. [ 32 ] discuss participants' limited understanding of the overlap of clinical care and research in malaria studies.
- • Emanuel et al. [ 6 ] established benchmarks for ethical research in the global setting.
- • The WHO advisory panel report [ 38 ] and Ezeome and Simon [ 31 ] discuss ways of minimizing ethical pitfalls when performing research during epidemics – these articles are applicable to the discussion of experimental treatments for the ongoing COVID-19 pandemic.
- • Groves et al. [ 30 ] used qualitative methods to explore adolescents' views on research requirements for parental involvement in studies of HIV in youth.
- • Ochieng et al. [ 10 ] describe the development of research ethics within Uganda, starting with early regulations on protection of human subjects, through revisions to applicable statues, and training of local experts.
- • Tangwa [ 8 ] critiques the lack of inclusion of African researchers in the efforts surrounding HIV vaccine trials and the response to the Ebola outbreak of 2013–16.
- • World Medical Association Declaration of Helsinki [ 16 ] describes the principles that should guide research. Most recent version available at https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ .
Academic Life in Emergency Medicine: Society for Academic Emergency Medicine Research Learning Series provides helpful guidance for junior researchers in many stages of starting a research project. In particular, episode 3 deals with IRB pitfalls and episode 8 discusses waivers from informed consent.
University of Oxford Global Health podcast has an interview with Dr. Phaik Yeong Cheah on the ethics of research in vulnerable populations (Oct 5, 2015).
Authors' contribution
Authors contributed as follow to the conception or design of the work; the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content: CB contributed 50%; AAN contributed 30%; and ES and DMT contributed 10% each. All authors approved the version to be published and agreed to be accountable for all aspects of the work.
Declaration of competing interest
The authors declared no conflicts of interest.
Child Care and Early Education Research Connections
Ethics of research.
Researchers are expected to adhere to the principles of ethical research. The Belmont Report provides a broad framework for the ethics of research involving human subjects. Three basic ethical principles are identified:
Respect for persons—requires that research subjects are not coerced into participating in a study and requires the protection of research subjects who have diminished autonomy.
Beneficence—requires that research does not harm research subjects, and that researchers minimize the risks for subjects while maximizing the benefits for them.
Justice—requires that all forms of differential treatment among research subjects be justified.
Applications of these principles lead to considerations of the following:
Informed Consent Participants should give informed consent before participating in a study. In order for participants to give informed consent.
The researcher must inform the participants of the study's purpose, content, duration, and potential risks and benefits.
The researcher must inform the participants that they can stop participating in the study at any point.
In the event of survey research, the researcher must inform the participants that they do not have to answer all the survey questions.
If the participants are children under legal age, the researcher must seek consent from their parents or guardians.
Confidentiality Unless consent is given otherwise, it is absolutely imperative that researchers keep participants' identities confidential. Confidentiality means that participants cannot be identified in any way. In survey research, this includes but is not limited to making sure that participants' identifiers are not linked to their survey responses. Common identifiers include names, social security numbers, addresses, and telephone numbers. Such Personal Identifying Information or PII must be safeguarded. When analyzing data collected from small groups or samples with small n's and when reporting the findings from these analyses, the researchers must be extra mindful of not revealing participants' identities. Cell sizes with fewer than three cases should not be reported because information about the individuals in this group could be obtained by subtraction.
Anonymity Anonymity is an even stronger safeguard of participant privacy. If a researcher assures anonymity, it means that the researcher is unable to link participants' names to the information they provide.
In addition to the above principles, considerations of specific ethical issues are often required depending on the form and context of research. For example, when using administrative data, the researcher must keep in mind that there are many legal protections set by the federal and state governments that require the privacy of program applicant information. For instance, in 1977, the Privacy Protection Study Commission determined that records or information used for statistical research could not be used in an individually identifiable form and that researchers could not take any action that would affect the individual to whom the information pertains.
A main ethical issue confronting researchers engaged in participant observation research is deciding when and how to inform those being observed that they are part of a research study. In theory, a researcher should identify himself or herself as a researcher at the onset of participant observation. However, in reality this may not be feasible without inherently changing the interactions at the outset. If the researcher decides to do so, a general but forthright description of the aims of the research should be sufficient. As relationships with members deepen, any controversial aspects of the study should be revealed. A researcher must obtain informed consent from any member who agrees to a formal, in-depth interview.
Institutional Review Board (IRB) Review
In order to assure that research subject and participant rights and welfare are protected, all researchers should have their project reviewed by an IRB or comparable bodies. The National Institutes of Health supplies strict guidelines for project approval. Many of these guidelines are based on the Belmont Report .
See the following for additional information about research ethics and protecting the rights of study participants and their data:
CASRO Code of Standards and Ethics for Survey Research (PDF)
AAPOR Code of Ethics
National Center for Education Statistical Standards
Tips on Informed Consent
The content on this page was prepared by Jerry West. It was last modified March 2019.

Research Methods
- Introduction
- Key Resources
- Books, Articles & Videos
What is Research Ethics?
Research misconducts, responsible conduct of research, youtube video.
- Methods by Subject
Research ethics provides guidelines for the responsible conduct of research. In addition, it educates and monitors scientists conducting research to ensure a high ethical standard. The following is a general summary of some ethical principles:
Honestly report data, results, methods and procedures, and publication status. Do not fabricate, falsify, or misrepresent data.
Objectivity:
Strive to avoid bias in experimental design, data analysis, data interpretation, peer review, personnel decisions, grant writing, expert testimony, and other aspects of research.
Keep your promises and agreements; act with sincerity; strive for consistency of thought and action.
Carefulness:
Avoid careless errors and negligence; carefully and critically examine your own work and the work of your peers. Keep good records of research activities.
Share data, results, ideas, tools, resources. Be open to criticism and new ideas.
Respect for Intellectual Property:
Honor patents, copyrights, and other forms of intellectual property. Do not use unpublished data, methods, or results without permission. Give credit where credit is due. Never plagiarize.
Confidentiality:
Protect confidential communications, such as papers or grants submitted for publication, personnel records, trade or military secrets, and patient records.
Responsible Publication:
Publish in order to advance research and scholarship, not to advance just your own career. Avoid wasteful and duplicative publication.
Responsible Mentoring:
Help to educate, mentor, and advise students. Promote their welfare and allow them to make their own decisions.
Respect for Colleagues:
Respect your colleagues and treat them fairly.
Social Responsibility:
Strive to promote social good and prevent or mitigate social harms through research, public education, and advocacy.
Non-Discrimination:
Avoid discrimination against colleagues or students on the basis of sex, race, ethnicity, or other factors that are not related to their scientific competence and integrity.
Competence:
Maintain and improve your own professional competence and expertise through lifelong education and learning; take steps to promote competence in science as a whole.
Know and obey relevant laws and institutional and governmental policies.
Animal Care:
Show proper respect and care for animals when using them in research. Do not conduct unnecessary or poorly designed animal experiments.
Human Subjects Protection:
When conducting research on human subjects, minimize harms and risks and maximize benefits; respect human dignity, privacy, and autonomy.
Source: What is Ethics in Research & Why is it Important? U.S. National Institute of Environmental Health Sciences
- Five Principles for Research Ethics (American Psychological Association)
- Ethical Guidelines for Good Research Practice (Association of Social Anthropologists, UK)
- Australian Code for the Responsible Conduct of Research, 2018 (Australian Government)
- ESRC Framework for Research Ethics 2015 (The Economic and Social Research Council, UK)
How different aspects of your research relate to the six ethics principles set out in the ESRC Framework for Research Ethics? Click the image below to find out.
http://www.ethicsguidebook.ac.uk/EthicsPrinciples
What are research misconducts?
(a) Fabrication - making up data or results and recording or reporting them.
(b) Falsification - manipulating research materials, or changing or omitting data or results such that the research is not accurately represented in the research record.
(c) Plagiarism - the appropriation of another person's ideas, processes, results, or words without giving appropriate credit.
(d) Research misconduct does not include honest error or differences of opinion.
Source: Definition of Research Misconduct The Office of Research Integrity, U.S. Department of Health & Human Services
ORI Introduction to the Responsible Conduct of Research
Yale School of Medicine Professor Robert Levine spoke on guidelines for human subjects protection.
Video from: https://www.youtube.com/watch?v=jD-YCDE_5yw
- << Previous: Books, Articles & Videos
- Next: Methods by Subject >>
- Last Updated: Jan 17, 2024 7:25 PM
- URL: https://libguides.library.cityu.edu.hk/researchmethods
© City University of Hong Kong | Copyright | Disclaimer
How To Apply Ethics to Your Research

Applying ethics to your research is super important! It’s how you show the readers that you’ve researched morality and your participants in mind rather than going against important rules or regulations. There are many different principles you should follow to make sure your research is ethical and they’re all as equally important. Without applying ethics to your research, you could find yourself in trouble. So, if you want to find out exactly what ethics is and how to apply it to your work, keep reading!
What is research ethics?
The research ethics definition is to follow moral and professional codes throughout any research conducted, which can be in the collection of the research, the publication of the research, interviews, analysis and more. To break it down, it basically means being moral and professional throughout any research that you do. It helps to protect both yourself and your participants from harm, so it’s super important to make sure you utilise ethics to ensure you’re working in the right way.
One important thing to note is that research ethics can actually differ with the university that you attend. That’s why you must check out your universities website to find out exactly what their rules and regulations are.
So, what should you do?
Make sure you have consent, keep anonymity, keep confidentiality, maintain integrity, respect the views of others, avoid causing any harm.
Something extremely crucial is making sure that the participants don’t come to any harm during the research process. It won’t come as any surprise that this another principle regularly featured on universities websites. It’s your responsibility that no harm is caused and that can be anything like psychological harm, emotional harm to even physical harm. You need to know from the outset that your research is safe to conduct for everyone involved.
You may also like

Ethical Considerations In Psychology Research
Saul Mcleod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul Mcleod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
Learn about our Editorial Process
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
On This Page:
Ethics refers to the correct rules of conduct necessary when carrying out research. We have a moral responsibility to protect research participants from harm.
However important the issue under investigation, psychologists must remember that they have a duty to respect the rights and dignity of research participants. This means that they must abide by certain moral principles and rules of conduct.
What are Ethical Guidelines?
In Britain, ethical guidelines for research are published by the British Psychological Society, and in America, by the American Psychological Association. The purpose of these codes of conduct is to protect research participants, the reputation of psychology, and psychologists themselves.
Moral issues rarely yield a simple, unambiguous, right or wrong answer. It is, therefore, often a matter of judgment whether the research is justified or not.
For example, it might be that a study causes psychological or physical discomfort to participants; maybe they suffer pain or perhaps even come to serious harm.
On the other hand, the investigation could lead to discoveries that benefit the participants themselves or even have the potential to increase the sum of human happiness.
Rosenthal and Rosnow (1984) also discuss the potential costs of failing to carry out certain research. Who is to weigh up these costs and benefits? Who is to judge whether the ends justify the means?
Finally, if you are ever in doubt as to whether research is ethical or not, it is worthwhile remembering that if there is a conflict of interest between the participants and the researcher, it is the interests of the subjects that should take priority.
Studies must now undergo an extensive review by an institutional review board (US) or ethics committee (UK) before they are implemented. All UK research requires ethical approval by one or more of the following:
- Department Ethics Committee (DEC) : for most routine research.
- Institutional Ethics Committee (IEC) : for non-routine research.
- External Ethics Committee (EEC) : for research that s externally regulated (e.g., NHS research).
Committees review proposals to assess if the potential benefits of the research are justifiable in light of the possible risk of physical or psychological harm.
These committees may request researchers make changes to the study’s design or procedure or, in extreme cases, deny approval of the study altogether.
The British Psychological Society (BPS) and American Psychological Association (APA) have issued a code of ethics in psychology that provides guidelines for conducting research. Some of the more important ethical issues are as follows:
Informed Consent
Before the study begins, the researcher must outline to the participants what the research is about and then ask for their consent (i.e., permission) to participate.
An adult (18 years +) capable of being permitted to participate in a study can provide consent. Parents/legal guardians of minors can also provide consent to allow their children to participate in a study.
Whenever possible, investigators should obtain the consent of participants. In practice, this means it is not sufficient to get potential participants to say “Yes.”
They also need to know what it is that they agree to. In other words, the psychologist should, so far as is practicable, explain what is involved in advance and obtain the informed consent of participants.
Informed consent must be informed, voluntary, and rational. Participants must be given relevant details to make an informed decision, including the purpose, procedures, risks, and benefits. Consent must be given voluntarily without undue coercion. And participants must have the capacity to rationally weigh the decision.
Components of informed consent include clearly explaining the risks and expected benefits, addressing potential therapeutic misconceptions about experimental treatments, allowing participants to ask questions, and describing methods to minimize risks like emotional distress.
Investigators should tailor the consent language and process appropriately for the study population. Obtaining meaningful informed consent is an ethical imperative for human subjects research.
The voluntary nature of participation should not be compromised through coercion or undue influence. Inducements should be fair and not excessive/inappropriate.
However, it is not always possible to gain informed consent. Where the researcher can’t ask the actual participants, a similar group of people can be asked how they would feel about participating.
If they think it would be OK, then it can be assumed that the real participants will also find it acceptable. This is known as presumptive consent.
However, a problem with this method is that there might be a mismatch between how people think they would feel/behave and how they actually feel and behave during a study.
In order for consent to be ‘informed,’ consent forms may need to be accompanied by an information sheet for participants’ setting out information about the proposed study (in lay terms), along with details about the investigators and how they can be contacted.
Special considerations exist when obtaining consent from vulnerable populations with decisional impairments, such as psychiatric patients, intellectually disabled persons, and children/adolescents. Capacity can vary widely so should be assessed individually, but interventions to improve comprehension may help. Legally authorized representatives usually must provide consent for children.
Participants must be given information relating to the following:
- A statement that participation is voluntary and that refusal to participate will not result in any consequences or any loss of benefits that the person is otherwise entitled to receive.
- Purpose of the research.
- All foreseeable risks and discomforts to the participant (if there are any). These include not only physical injury but also possible psychological.
- Procedures involved in the research.
- Benefits of the research to society and possibly to the individual human subject.
- Length of time the subject is expected to participate.
- Person to contact for answers to questions or in the event of injury or emergency.
- Subjects” right to confidentiality and the right to withdraw from the study at any time without any consequences.
Debriefing after a study involves informing participants about the purpose, providing an opportunity to ask questions, and addressing any harm from participation. Debriefing serves an educational function and allows researchers to correct misconceptions. It is an ethical imperative.
After the research is over, the participant should be able to discuss the procedure and the findings with the psychologist. They must be given a general idea of what the researcher was investigating and why, and their part in the research should be explained.
Participants must be told if they have been deceived and given reasons why. They must be asked if they have any questions, which should be answered honestly and as fully as possible.
Debriefing should occur as soon as possible and be as full as possible; experimenters should take reasonable steps to ensure that participants understand debriefing.
“The purpose of debriefing is to remove any misconceptions and anxieties that the participants have about the research and to leave them with a sense of dignity, knowledge, and a perception of time not wasted” (Harris, 1998).
The debriefing aims to provide information and help the participant leave the experimental situation in a similar frame of mind as when he/she entered it (Aronson, 1988).
Exceptions may exist if debriefing seriously compromises study validity or causes harm itself, like negative emotions in children. Consultation with an institutional review board guides exceptions.
Debriefing indicates investigators’ commitment to participant welfare. Harms may not be raised in the debriefing itself, so responsibility continues after data collection. Following up demonstrates respect and protects persons in human subjects research.
Protection of Participants
Researchers must ensure that those participating in research will not be caused distress. They must be protected from physical and mental harm. This means you must not embarrass, frighten, offend or harm participants.
Normally, the risk of harm must be no greater than in ordinary life, i.e., participants should not be exposed to risks greater than or additional to those encountered in their normal lifestyles.
The researcher must also ensure that if vulnerable groups are to be used (elderly, disabled, children, etc.), they must receive special care. For example, if studying children, ensure their participation is brief as they get tired easily and have a limited attention span.
Researchers are not always accurately able to predict the risks of taking part in a study, and in some cases, a therapeutic debriefing may be necessary if participants have become disturbed during the research (as happened to some participants in Zimbardo’s prisoners/guards study ).
Deception research involves purposely misleading participants or withholding information that could influence their participation decision. This method is controversial because it limits informed consent and autonomy, but can provide otherwise unobtainable valuable knowledge.
Types of deception include (i) deliberate misleading, e.g. using confederates, staged manipulations in field settings, deceptive instructions; (ii) deception by omission, e.g., failure to disclose full information about the study, or creating ambiguity.
The researcher should avoid deceiving participants about the nature of the research unless there is no alternative – and even then, this would need to be judged acceptable by an independent expert. However, some types of research cannot be carried out without at least some element of deception.
For example, in Milgram’s study of obedience , the participants thought they were giving electric shocks to a learner when they answered a question wrongly. In reality, no shocks were given, and the learners were confederates of Milgram.
This is sometimes necessary to avoid demand characteristics (i.e., the clues in an experiment that lead participants to think they know what the researcher is looking for).
Another common example is when a stooge or confederate of the experimenter is used (this was the case in both the experiments carried out by Asch ).
According to ethics codes, deception must have strong scientific justification, and non-deceptive alternatives should not be feasible. Deception that causes significant harm is prohibited. Investigators should carefully weigh whether deception is necessary and ethical for their research.
However, participants must be deceived as little as possible, and any deception must not cause distress. Researchers can determine whether participants are likely distressed when deception is disclosed by consulting culturally relevant groups.
Participants should immediately be informed of the deception without compromising the study’s integrity. Reactions to learning of deception can range from understanding to anger. Debriefing should explain the scientific rationale and social benefits to minimize negative reactions.
If the participant is likely to object or be distressed once they discover the true nature of the research at debriefing, then the study is unacceptable.
If you have gained participants’ informed consent by deception, then they will have agreed to take part without actually knowing what they were consenting to. The true nature of the research should be revealed at the earliest possible opportunity or at least during debriefing.
Some researchers argue that deception can never be justified and object to this practice as it (i) violates an individual’s right to choose to participate; (ii) is a questionable basis on which to build a discipline; and (iii) leads to distrust of psychology in the community.
Confidentiality
Protecting participant confidentiality is an ethical imperative that demonstrates respect, ensures honest participation, and prevents harms like embarrassment or legal issues. Methods like data encryption, coding systems, and secure storage should match the research methodology.
Participants and the data gained from them must be kept anonymous unless they give their full consent. No names must be used in a lab report .
Researchers must clearly describe to participants the limits of confidentiality and methods to protect privacy. With internet research, threats exist like third-party data access; security measures like encryption should be explained. For non-internet research, other protections should be noted too, like coding systems and restricted data access.
High-profile data breaches have eroded public trust. Methods that minimize identifiable information can further guard confidentiality. For example, researchers can consider whether birthdates are necessary or just ages.
Generally, reducing personal details collected and limiting accessibility safeguards participants. Following strong confidentiality protections demonstrates respect for persons in human subjects research.
What do we do if we discover something that should be disclosed (e.g., a criminal act)? Researchers have no legal obligation to disclose criminal acts and must determine the most important consideration: their duty to the participant vs. their duty to the wider community.
Ultimately, decisions to disclose information must be set in the context of the research aims.
Withdrawal from an Investigation
Participants should be able to leave a study anytime if they feel uncomfortable. They should also be allowed to withdraw their data. They should be told at the start of the study that they have the right to withdraw.
They should not have pressure placed upon them to continue if they do not want to (a guideline flouted in Milgram’s research).
Participants may feel they shouldn’t withdraw as this may ‘spoil’ the study. Many participants are paid or receive course credits; they may worry they won’t get this if they withdraw.
Even at the end of the study, the participant has a final opportunity to withdraw the data they have provided for the research.
Ethical Issues in Psychology & Socially Sensitive Research
There has been an assumption over the years by many psychologists that provided they follow the BPS or APA guidelines when using human participants and that all leave in a similar state of mind to how they turned up, not having been deceived or humiliated, given a debrief, and not having had their confidentiality breached, that there are no ethical concerns with their research.
But consider the following examples:
a) Caughy et al. 1994 found that middle-class children in daycare at an early age generally score less on cognitive tests than children from similar families reared in the home.
Assuming all guidelines were followed, neither the parents nor the children participating would have been unduly affected by this research. Nobody would have been deceived, consent would have been obtained, and no harm would have been caused.
However, consider the wider implications of this study when the results are published, particularly for parents of middle-class infants who are considering placing their young children in daycare or those who recently have!
b) IQ tests administered to black Americans show that they typically score 15 points below the average white score.
When black Americans are given these tests, they presumably complete them willingly and are not harmed as individuals. However, when published, findings of this sort seek to reinforce racial stereotypes and are used to discriminate against the black population in the job market, etc.
Sieber & Stanley (1988) (the main names for Socially Sensitive Research (SSR) outline 4 groups that may be affected by psychological research: It is the first group of people that we are most concerned with!
- Members of the social group being studied, such as racial or ethnic group. For example, early research on IQ was used to discriminate against US Blacks.
- Friends and relatives of those participating in the study, particularly in case studies, where individuals may become famous or infamous. Cases that spring to mind would include Genie’s mother.
- The research team. There are examples of researchers being intimidated because of the line of research they are in.
- The institution in which the research is conducted.
salso suggest there are 4 main ethical concerns when conducting SSR:
- The research question or hypothesis.
- The treatment of individual participants.
- The institutional context.
- How the findings of the research are interpreted and applied.
Ethical Guidelines For Carrying Out SSR
Sieber and Stanley suggest the following ethical guidelines for carrying out SSR. There is some overlap between these and research on human participants in general.
Privacy : This refers to people rather than data. Asking people questions of a personal nature (e.g., about sexuality) could offend.
Confidentiality: This refers to data. Information (e.g., about H.I.V. status) leaked to others may affect the participant’s life.
Sound & valid methodology : This is even more vital when the research topic is socially sensitive. Academics can detect flaws in methods, but the lay public and the media often don’t.
When research findings are publicized, people are likely to consider them fact, and policies may be based on them. Examples are Bowlby’s maternal deprivation studies and intelligence testing.
Deception : Causing the wider public to believe something, which isn’t true by the findings, you report (e.g., that parents are responsible for how their children turn out).
Informed consent : Participants should be made aware of how participating in the research may affect them.
Justice & equitable treatment : Examples of unjust treatment are (i) publicizing an idea, which creates a prejudice against a group, & (ii) withholding a treatment, which you believe is beneficial, from some participants so that you can use them as controls.
Scientific freedom : Science should not be censored, but there should be some monitoring of sensitive research. The researcher should weigh their responsibilities against their rights to do the research.
Ownership of data : When research findings could be used to make social policies, which affect people’s lives, should they be publicly accessible? Sometimes, a party commissions research with their interests in mind (e.g., an industry, an advertising agency, a political party, or the military).
Some people argue that scientists should be compelled to disclose their results so that other scientists can re-analyze them. If this had happened in Burt’s day, there might not have been such widespread belief in the genetic transmission of intelligence. George Miller (Miller’s Magic 7) famously argued that we should give psychology away.
The values of social scientists : Psychologists can be divided into two main groups: those who advocate a humanistic approach (individuals are important and worthy of study, quality of life is important, intuition is useful) and those advocating a scientific approach (rigorous methodology, objective data).
The researcher’s values may conflict with those of the participant/institution. For example, if someone with a scientific approach was evaluating a counseling technique based on a humanistic approach, they would judge it on criteria that those giving & receiving the therapy may not consider important.
Cost/benefit analysis : It is unethical if the costs outweigh the potential/actual benefits. However, it isn’t easy to assess costs & benefits accurately & the participants themselves rarely benefit from research.
Sieber & Stanley advise that researchers should not avoid researching socially sensitive issues. Scientists have a responsibility to society to find useful knowledge.
- They need to take more care over consent, debriefing, etc. when the issue is sensitive.
- They should be aware of how their findings may be interpreted & used by others.
- They should make explicit the assumptions underlying their research so that the public can consider whether they agree with these.
- They should make the limitations of their research explicit (e.g., ‘the study was only carried out on white middle-class American male students,’ ‘the study is based on questionnaire data, which may be inaccurate,’ etc.
- They should be careful how they communicate with the media and policymakers.
- They should be aware of the balance between their obligations to participants and those to society (e.g. if the participant tells them something which they feel they should tell the police/social services).
- They should be aware of their own values and biases and those of the participants.
Arguments for SSR
- Psychologists have devised methods to resolve the issues raised.
- SSR is the most scrutinized research in psychology. Ethical committees reject more SSR than any other form of research.
- By gaining a better understanding of issues such as gender, race, and sexuality, we are able to gain greater acceptance and reduce prejudice.
- SSR has been of benefit to society, for example, EWT. This has made us aware that EWT can be flawed and should not be used without corroboration. It has also made us aware that the EWT of children is every bit as reliable as that of adults.
- Most research is still on white middle-class Americans (about 90% of research is quoted in texts!). SSR is helping to redress the balance and make us more aware of other cultures and outlooks.
Arguments against SSR
- Flawed research has been used to dictate social policy and put certain groups at a disadvantage.
- Research has been used to discriminate against groups in society, such as the sterilization of people in the USA between 1910 and 1920 because they were of low intelligence, criminal, or suffered from psychological illness.
- The guidelines used by psychologists to control SSR lack power and, as a result, are unable to prevent indefensible research from being carried out.
American Psychological Association. (2002). American Psychological Association ethical principles of psychologists and code of conduct. www.apa.org/ethics/code2002.html
Baumrind, D. (1964). Some thoughts on ethics of research: After reading Milgram’s” Behavioral study of obedience.”. American Psychologist , 19 (6), 421.
Caughy, M. O. B., DiPietro, J. A., & Strobino, D. M. (1994). Day‐care participation as a protective factor in the cognitive development of low‐income children. Child development , 65 (2), 457-471.
Harris, B. (1988). Key words: A history of debriefing in social psychology. In J. Morawski (Ed.), The rise of experimentation in American psychology (pp. 188-212). New York: Oxford University Press.
Rosenthal, R., & Rosnow, R. L. (1984). Applying Hamlet’s question to the ethical conduct of research: A conceptual addendum. American Psychologist, 39(5) , 561.
Sieber, J. E., & Stanley, B. (1988). Ethical and professional dimensions of socially sensitive research. American psychologist , 43 (1), 49.
The British Psychological Society. (2010). Code of Human Research Ethics. www.bps.org.uk/sites/default/files/documents/code_of_human_research_ethics.pdf
Further Information
- MIT Psychology Ethics Lecture Slides
BPS Documents
- Code of Ethics and Conduct (2018)
- Good Practice Guidelines for the Conduct of Psychological Research within the NHS
- Guidelines for Psychologists Working with Animals
- Guidelines for ethical practice in psychological research online
APA Documents
APA Ethical Principles of Psychologists and Code of Conduct
Responsible and Ethical Conduct of Research
Categories:
- Award Management
- Regulatory Compliance
Introduction
Responsible and Ethical Conduct of Research applies to all researchers and research staff engaged in scholarly research. While written with all researchers in mind, special consideration has been given to the needs of students and postdoctoral scholars. The education of students at all levels includes appropriate training and oversight in the responsible and ethical conduct of research. The University expects that every academic program and each research advisor/mentor will do their part to assure that students/trainees receive appropriate guidance in such areas as research integrity, data acquisition and management, authorship, research collaborations, conflicts of interest, and others as appropriate.
NIH RCR Training Requirements
The National Institutes of Health (NIH) has long required Responsible Conduct of Research (RCR) education. The requirement for RCR instruction applies to all NIH Institutional Research Training Grants, Individual Fellowship Awards, Career Development Awards (Institutional and Individual), Research Education Grants, Dissertation Research Grants, or other grant programs with a training component.
The Responsible Conduct of Research (RCR) course is designed to engage participants in productive discussions about ethical issues that are commonly encountered during their research careers. This course is required for graduate students and postdoctoral scholars who are supported by grants from the National Institutes of Health. Many departments and programs also recommend or require this course as part of their curricula.
The objectives of both courses are:
- To engage participants in case-based discussions of ethical issues commonly encountered in, and raised by, current biomedical research.
- To introduce participants to methods of analysis of ethical issues
- To introduce participants to policies and regulations relevant to the conduct of research.
There are two different RCR courses: MED 255 and MED 255C (although the latter is not currently being offered).
MED 255 is a necessary, but not always sufficient, component of training in responsible conduct of research. Please be sure to contact your funder to inquire about additional requirements beyond MED 255. In general, however, the NIH requires several components of training in the Responsible Conduct of Research (RCR) including:
The required MED 255 or 255C course (the CITI class does not fulfill this requirement)
Refresher RCR instruction at each stage of training (e.g., graduate, postdoc, etc)
Continuing informal or formal training in research ethics throughout the year.
You must include a description of a program to provide instruction in the responsible conduct of research (see regulations ). A template is available for your use as a statement in your grant application. Please be sure to supplement the template with funder-specific requirements, and address all structural components required.
NSF RECR Training Requirements
Stanford University is committed to the highest scholarly and ethical standards among its students, faculty, and staff. Education in the ethical conduct of research is a key element in fostering these standards. In accordance with the America COMPETES Act (42 USC 1862o–1) and the Creating Helpful Incentives to Produce Semiconductors and Science Act of 2022 (“CHIPS and Science Act”), The National Science Foundation (“NSF”) requires training in the Responsible and Ethical Conduct of Research (“RECR”) for all undergraduates, graduate students, postdocs, faculty, and other senior personnel who receive NSF funds in the form of support from salary and/or stipends to conduct research on NSF grants.
For undergraduate and graduate students and postdoctoral scholars, the Responsible and Ethical Conduct of Research (RECR) training requirement has been in effect since 2010 and education has been recommended for faculty and other senior personnel. For faculty and other senior personnel identified on research proposals submitted or due on or after July 31, 2023, NSF’s Proposal and Award Policies and Procedures Guide (PAPPG) requires that, at the time of proposal submission, the institution certify that it has “a plan to provide appropriate training and oversight in the responsible and ethical conduct of research to undergraduate students, graduate students, postdoctoral researchers, faculty, and other senior personnel supported by the proposed research project.”
EDUCATION REQUIREMENTS
All undergraduates, graduate students and postdoctoral scholars must complete the CITI RCR training modules relevant to their area of study within the first quarter (90 days) of support on an NSF project. The PI on an NSF award is responsible for ensuring that this training is complete.
Faculty and senior or key personnel on NSF projects must complete RECR education prior to the processing of any grant or contract award resulting from a research proposal submitted to NSF after July 1 2023.
The Education requirement can be satisfied by completing the RCR modules in CITI most relevant to their particular discipline or through other options identified below:
OPTIONS FOR RECR EDUCATION:
Stanford offers a variety of options to complete RECR education for NSF funded awards. For initial RECR training:
OPTION 1: Take the full RCR Basic Course through the Collaborative Institutional Training Initiative (CITI) CITI Program , CITI provides a web-based training in the Responsible Conduct of Research. Learners are required to complete 10 modules, with 2 optional where applicable. Use the Log-in “through my institution” option on the CITI website. Select Stanford as your institution. After logging into CITI, click on “institutional courses” and then “Add a Course” from the menu at the bottom of the screen. ” Select Question #3, RCR education. You can then select one of four programs in the following disciplines: Biomedical Sciences, Physical Science, Social and Behavioral Sciences, Humanities or Engineering.
The modules to be completed in the program include the following:
1. Introduction to RCR 2. Authorship 3. Collaborative Research 4. Conflicts of Interest and Commitment (All PIs and Key personnel satisfy this element via the COI/COC training in Stanford’s STARS system) 5. Data Management 6. Mentoring 7. Peer review 8. Research Misconduct 9. Plagiarism 10. Research, Ethics and Society 11. Mentoring and Healthy Research Environments (Refresher) 12. Human subjects (if applicable) 13. Animal subjects (if applicable)
REFRESHER COURSES If it has been more than three years since you completed this course, you are required to re-take the full RCR Refresher Course.
OPTION 2: For Graduate Students and Postdoctoral Scholars:
Enrollment in Stanford School of Medicine Course Med 255
OPTION 3: For Faculty and Key or Senior Personnel on NSF grants:
completion of the Stanford RECR course in Canvas [Under development]. NOTE: Completion of the Stanford COI/COC training in STARS fulfills the COI education requirement and completion of Stanford’s Code of Conduct training in STARS or the IDEAL program in STARS meets requirements for the educational components regarding treating peers, colleagues and students fairly and with respect.
Stanford School of Medicine also offers education relative to research integrity, replicability and reproducibility and data management via the SPORR program that can supplement the RECR education requirement. https://med.stanford.edu/sporr
FREQUENCY:
Undergraduate Students, Graduate Students and Postdoctoral Scholars must complete RECR training at “each career stage”. Faculty and key personnel on NSF grants must complete the original training prior to processing of an NSF award and thereafter, a refresher course in RECR every three years so long as they continue to be supported with NSF funding.
Individuals who have completed the CITI RCR training modules within a year of joining Stanford can utilize that prior education to meet the training requirement at Stanford. Completion of a different University’s program will not be accepted and either the Stanford or the CITI courses must be completed.
ROLES AND RESPONSIBILITIES:
The Principal Investigator is responsible for ensuring that all postdocs, graduate students, and undergraduates who receive NSF funds in the form of support from salary and/or stipends to conduct research on NSF grants receive training in RECR. In general, training should be completed within three months of when the individual begins work on the project, or the individual must provide a plan through which training will be completed within a reasonable timeframe (e.g., registering for a course in the coming semester). Where individuals are working on NSF projects for a short time, such as the summer, training should be completed before the individual’s work on the project ends.
ORA is responsible for certifying that Stanford has an RECR education plan in place and will notify the PI when an NSF award is received along with a notice for the PI and Key Personnel to complete the required RECR education. ORA will ensure faculty and key personnel have completed RECR requirements prior to processing an NSF award resulting from a proposal submitted after July 31, 2023.
The Research Policy and Integrity Office (RPI) in the Office of the Vice Provost and Dean of Research is responsible for identifying or providing educational modules to meet RECR requirements, updating training materials as needed to meet federal requirements, and monitoring ongoing completion of RECR requirements. RPI will work with ORA and University IT to identify individuals who are required to receive training and will track training completions. Reports of trainee non-compliance will be generated and shared with PIs and Departmental support for follow-up.
Questions regarding the NSF RECR requirement may be directed to the Office of Research Policy and Integrity, [email protected] . Information is also available at https://doresearch.stanford.edu/training/responsible-conduct-research
Created: 11.19.2020
Updated: 04.26.2024

Ethics and Morality
Scientists exaggerate how ethical they are in doing science, new study suggests that scientists tend to inflate their own research ethics..
Posted April 24, 2024 | Reviewed by Davia Sills
- New study finds most scientists rate themselves higher or equal to peers in their research practice ethics.
- They also rate their field as more ethical than other fields.
- One important implication is that this overconfidence may lead to ethical blindspots.
We have known for a long time that people tend to paint a rosy picture of how good they are. Now, we know that scientists are no exception, at least when it comes to conducting their own research. This is especially surprising since scientists are regularly thought to be objective.

This new discovery emerged from a massive survey of 11,050 scientific researchers in Sweden, conducted by Amanda M. Lindkvist, Lina Koppel, and Gustav Tinghög at Linköping University and published in the journal Scientific Reports. The survey was very simple, with only two questions:
Question One: In your role as a researcher, to what extent do you perceive yourself as following good research practices—compared to other researchers in your field?
Rather than allowing the survey participants to each define what “good research practice” is, the researchers gave them these criteria:
1. Tell the truth about one’s research.
2. Consciously review and report the basic premises of one’s studies.
3. Openly account for one’s methods and results.
4. Openly account for one’s commercial interests and other associations.
5. Do not make unauthorized use of others’ research results.
6. Keep one’s research organized, for example, through documentation and filing.
7. Strive to conduct one’s research without doing harm to people, animals, or the environment .
8. Be fair in one’s judgment of others’ research.
Note that many of these criteria have to do with honesty, but there are also ones on conscientiousness , non-malevolence, and fairness.
What were the results? Participants used a scale to rate themselves from 1 to 7, with 1 = Much less than other researchers, 4 = As much as other researchers, and 7 = Much more than other researchers. This is what the responses revealed:
- Forty-four percent rated themselves as more ethical in their research practices than other researchers in their field.
- Fifty-five percent rated themselves as the same as their peers.
- Not even 1 percent rated themselves as less ethical than their peers.
Of course, these results can’t reflect real life since, mathematically, there have to be more than 1 percent of scientists who are less than average in this area of their lives.
The other question that Lindkvist and his colleagues asked these scientific researchers was this:
Question Two: To what extent do you perceive researchers within your field as following good research practices—compared to researchers within other fields?
Here, too, the results were very skewed. Around 29 percent said their field followed good research practices to a greater extent than did scientists in other fields. Only 8 percent said it was the other way around.
These results should surprise us for a couple of reasons. One is that they go against the popular narrative of scientists as objective and neutral. When it comes to their own ethical behavior in conducting their research, they appear as a whole to be biased and overconfident. Another reason these results are surprising is that many scientists are likely aware of the existence of scientific research on how people, in general, tend to have an inflated view of their own virtue . So you’d expect that they would be on guard against such a tendency in their own case.
There are dangers that come with scientists having an overly positive view of their own research ethics . Lindkvist helpfully explains one of them: It “may lead researchers to underestimate the ethical implications of the decisions they make and to sometimes be blind to their own ethical failures. For example, researchers may downplay their own questionable practices but exaggerate those of other researchers, perhaps especially researchers outside their field.” Another danger that Lindkvist notes is a greater tendency to ignore warnings and ethical safeguards if they are dismissed by a scientist as applying to others but not to themselves since they think they are above average.
It would be interesting in future work to see if similar patterns emerge with researchers in other countries besides Sweden. It would also be interesting to look at researchers anonymously rating the research ethics of their colleagues in their own departments and schools.
If these results hold up, it will be important to find ways to encourage scientific researchers to correct their inflated perceptions. As Lindkvist urges, “To restore science’s credibility, we need to create incentive structures, institutions, and communities that foster ethical humility and encourage us to be our most ethical selves in an academic system that otherwise incentivizes us to be bad.”
This article also appears in Forbes .

Christian Miller, Ph.D. , is a professor of philosophy at Wake Forest University.
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- International
- New Zealand
- South Africa
- Switzerland
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Therapy Center NEW
- Diagnosis Dictionary
- Types of Therapy

Understanding what emotional intelligence looks like and the steps needed to improve it could light a path to a more emotionally adept world.
- Coronavirus Disease 2019
- Affective Forecasting
- Neuroscience
Scientists inspired the right guardrails for nuclear energy, the internet, and DNA research. Let them do the same for AI

In July 1957, 22 prominent scientists gathered quietly at a private lodge in Pugwash, a small town in Canada’s Nova Scotia province. They had answered a call to action by Albert Einstein, inviting scientists to shape guardrails that would contain the danger of nuclear weapons. The Pugwash Conference earned a Nobel Peace Prize, and more importantly, it laid the foundations for the nuclear non-proliferation treaties, which saved our world from risks of annihilation.
Today, governments and businesses are frantically searching for ways to limit the many feared perils of AI–especially those from Artificial General Intelligence (AGI), the next phase of AI evolution. AGI will perform a wide range of cognitive tasks with an efficiency and accuracy far superior to current AI systems. This next stage of A.I., often referred to by Silicon Valley enthusiasts as “God-like,” is expected to surpass human intelligence and efficiency by a substantial margin. It is rumored that an internal report on the risks of AGI may be what ignited the recent board drama at OpenAI, the maker of ChatGPT. But while the race to build AGI is still in progress, we can be certain that whoever controls it will have enormous sway on society and the economy, potentially exerting influence on the lives of humans everywhere.
In the past year, numerous and uncoordinated efforts by government and business to contain AI sprang across the world, in the U.S., China, the EU, and the U.K. Businesses have been “pleading” with governments to regulate their AI creations, whilst knowing full well that governments will never succeed in regulating effectively at the speed of A.I. evolution. The EU recently completed a multi-year effort to deliver the AI Act. However, the shifts in generative AI capabilities mean that by the time it is enacted in 2025, the new AI Act may already be outdated.
Governments are not equipped to outgallop fast-moving technologies with effective rules and policies–especially in the early hyperfast stages of development. Moreover, AI technologies have a transnational borderless reach, limiting the effectiveness of national and regional rule systems to govern them. As for businesses, they are in intense competition to dominate and profit from these technologies. In such a race, fueled by billions of investments, safety guardrails are inevitably a low priority for most businesses.
Ironically, governments and businesses are in fact the two stakeholders who are most in need of guardrails to prevent them from misusing A.I. in surveillance, warfare, and other endeavors to influence or control the public.
Who can be trusted with shaping A.I. guardrails?
A careful analysis of how prior technologies and scientific innovations were tamed in the 20 th century offers a clear answer to this dilemma. Guardrails were designed by scientists who know their own creations and understand (better than most) how they might evolve.
At Pugwash, influential scientists came together to develop strategies to mitigate the risks of nuclear weapons, significantly contributing to the formulation of arms control agreements and fostering international dialogue during the tense Cold War era.
In February 1975, at the Asilomar Conference in California, it was again scientists who met and successfully established critical guidelines for the safe and ethical research of DNA, thereby preventing potential biohazards. The Asilomar guidelines not only paved the way for responsible scientific inquiry but also informed regulatory policies worldwide. More recently, it was again the scientists and inventors of the Internet, led by Vint Cerf, who convened and shaped the framework of guardrails and protocols that made the Internet thrive globally.
All these successful precedents are proof that we need businesses and governments to first make space and let A.I. scientists shape a framework of guardrails that contain the risks without limiting the many benefits of A.I. Businesses can then implement such a framework voluntarily, and only when necessary, governments should step in to enforce the implementation by enacting policies and laws based on the scientists’ framework. This proven approach worked well for nuclear technology, DNA, and the Internet. It should be a blueprint to build safer AI.
A “Pugwash Conference for AI scientists” is therefore urgently needed. The conference should include no more than two dozen scientists, in the mold of Geoffrey Hinton who chose to quit Google in order to speak his mind on AI’s promise and perils.
Like Pugwash, the scientists should be chosen from all the key countries where advanced A.I. technologies are developing, in order to at least strive for a global consensus. Most importantly, the choice of the participants at this seminal A.I. conference must reassure the public that the conferees are shielded from special interests, geopolitical pressures, and profit-centric motives.
While hundreds of government leaders and business bosses will cozy up to discuss A.I. at multiple annual international events, thoughtful and independent A.I. scientists must urgently get together to make A.I. good for all.
Fadi Chehadé is chairman, co-founder, and managing partner of Ethos .
More must-read commentary published by Fortune :
- $122 Thai delivery and $26 to-go coffees: New wage laws meant to help gig workers are backfiring big-time
- I’m a venture capitalist, and here’s why I believe we need to guarantee everyone’s basic needs: The social floor is actually a trampoline that can propel our economy
- How to fix Boeing, according to a former Airbus technology chief
- DEI is under attack. Here’s the real reason it makes many white men uncomfortable
The opinions expressed in Fortune.com commentary pieces are solely the views of their authors and do not necessarily reflect the opinions and beliefs of Fortune .
Latest in Commentary
- 0 minutes ago

Starbucks’s case at the Supreme Court is a venti lose-lose for the company and the burgeoning unionization movement

More Americans are working past age 65—and that’s good news for employers

Burned out and underappreciated, women’s career advancement had stalled long before the anti-DEI backlash

A new Cold War is brewing at sea–and the West’s security and prosperity are at stake

The FTC noncompete ruling will narrow the gender gap in entrepreneurship
Most popular.

The meteoric rise and stunning fall of Prime, Logan Paul’s energy drink that was once resold for almost $1,500 a can: ‘A brand cannot live on hype alone’

Pottery Barn parent Williams-Sonoma fined for marketing furniture as ‘crafted’ in the U.S. when it was made in China

‘We expect Powell to make a hawkish pivot’—Fed meeting to headline busy week for global markets

The U.S. economy is actually a ‘wolf in sheep’s clothing’ as the weak GDP report masks underlying strength, Wells Fargo says

The 5 best supplements for healthy aging, according to a longevity expert

Tesla’s Elon Musk speeds past Mark Zuckerberg on the billionaires list after Meta stock plummets on its cash-sucking AI plans

IMAGES
VIDEO
COMMENTS
Education in research ethics is can help people get a better understanding of ethical standards, policies, and issues and improve ethical judgment and decision making. Many of the deviations that occur in research may occur because researchers simply do not know or have never thought seriously about some of the ethical norms of research. For ...
Revised on June 22, 2023. Ethical considerations in research are a set of principles that guide your research designs and practices. Scientists and researchers must always adhere to a certain code of conduct when collecting data from people. The goals of human research often include understanding real-life phenomena, studying effective ...
Research ethics are moral principles that need to be adhered to when conducting a research study as well as when writing a scientific article, with the prime aim of avoiding deception or intent to harm study's participants, the scientific community, and society. Practicing and adhering to research ethics is essential for personal integrity as ...
In a research application for a study on coping with undesirable social behavior at the workplace in China, the researchers planned to ask participants to complete a questionnaire which was estimated to takes up to 15 min. Participants would receive ¥8 (roughly 1 Euro) in compensation for their effort, but only once they completed the questionnaire.
Reputable journals screen for ethics at submission—and inability to pass ethics checks is one of the most common reasons for rejection. Unfortunately, once a study has begun, it's often too late to secure the requisite ethical reviews and clearances. Learn how to prepare for publication success by ensuring your study meets all ethical requirements before work begins.
Abstract. This chapter outlines the aims for the handbook. A main aim is to be a first point of contact for contemporary information, issues, and challenges in the fields of research ethics and scientific integrity. It is aimed at researchers, reviewers, and policymakers to help them pursue the best ways forward in seeking ethics and integrity ...
Research Ethics is defined here to be the ethics of the planning, conduct, and reporting of research. It is clear that research ethics should include: Protections of human and animal subjects. However, not all researchers use human or animal subjects, nor are the ethical dimensions of research confined solely to protections for research ...
Ethics and ethical behaviour are the fundamental pillars of a civilised society. The focus on ethical behaviour is indispensable in certain fields such as medicine, finance, or law. In fact, ethics gets precedence with anything that would include, affect, transform, or influence upon individuals, communities or any living creatures. Many institutions within Europe have set up their own ...
Low and middle income countries (LMICs) have a high burden of acute illness, but insufficient research on emergency care. •. Ethical barriers and lack of oversight impede emergency care research in LMICs. •. Ethical clinical research in LMICs builds capacity and contributes to the evidence base for emergency care.
Advancing psychology to benefit society and improve lives. Find resources on research misconduct, publication ethics, protecting research participants, ethics of online research, and guidance from various agencies and organizations, such as the NIH.
It is important to adhere to ethical principles in order to protect the dignity, rights and welfare of research participants. As such, all research involving human beings should be reviewed by an ethics committee to ensure that the appropriate ethical standards are being upheld. Discussion of the ethical principles of beneficence, justice and ...
Research using an experiment, a survey or an observational study can be an exciting way of investigating a topic or gathering data to help you answer a question. Anyone who carries out research must consider the ethics of what they are doing, including you as an extended project student. Research is all about exploring questions to which we do not
Researchers are expected to adhere to the principles of ethical research. The Belmont Report provides a broad framework for the ethics of research involving human subjects. Three basic ethical principles are identified: Respect for persons—requires that research subjects are not coerced into participating in a study and requires the protection of research subjects who have diminished autonomy.
Research ethics provides guidelines for the responsible conduct of research. In addition, it educates and monitors scientists conducting research to ensure a high ethical standard. The following is a general summary of some ethical principles: Honesty: Honestly report data, results, methods and procedures, and publication status.
4. Respect confidentiality and privacy. Upholding individuals' rights to confidentiality and privacy is a central tenet of every psychologist's work. However, many privacy issues are idiosyncratic to the research population, writes Susan Folkman, PhD, in "Ethics in Research with Human Participants" (APA, 2000).
The research ethics definition is to follow moral and professional codes throughout any research conducted, which can be in the collection of the research, the publication of the research, interviews, analysis and more. To break it down, it basically means being moral and professional throughout any research that you do. ...
The research team. There are examples of researchers being intimidated because of the line of research they are in. The institution in which the research is conducted. salso suggest there are 4 main ethical concerns when conducting SSR: The research question or hypothesis. The treatment of individual participants.
Continuing informal or formal training in research ethics throughout the year. You must include a description of a program to provide instruction in the responsible conduct of research (see regulations). A template is available for your use as a statement in your grant application. Please be sure to supplement the template with funder-specific ...
You may already be familiar with what research is, but what defines good research? Ethics help answer this question. Researchers rely on ethics to guide them...
Key points. New study finds most scientists rate themselves higher or equal to peers in their research practice ethics. They also rate their field as more ethical than other fields.
Centre for Digital Ethics (CEDE) Research Paper Series. Subscribe to this free journal for more curated articles on this topic FOLLOWERS. 653. PAPERS. 287. This Journal is curated by: Luciano Floridi at Yale University - Digital Ethics Center, Mariarosaria Taddeo at University of Oxford - Oxford Internet Institute. Feedback. Feedback to SSRN ...
In February 1975, at the Asilomar Conference in California, it was again scientists who met and successfully established critical guidelines for the safe and ethical research of DNA, thereby ...
The experiments were approved by the Ethics Committee of Universidade Federal do Rio Grande do Sul (CEUA − 23554). Availability of data and material The data of current study is available from the corresponding author on reasonable request.