Advertisement

An Overview of Bacteria-Mediated Heavy Metal Bioremediation Strategies
- Review Article
- Published: 06 July 2023
- Volume 196 , pages 1712–1751, ( 2024 )
Cite this article

- Rima Roy 1 na1 ,
- Saikat Samanta 1 na1 ,
- Soumya Pandit 2 ,
- Tahseena Naaz 2 ,
- Srijoni Banerjee 1 ,
- Janhvi Mishra Rawat 3 ,
- Kundan Kumar Chaubey 4 &
- Rudra P. Saha ORCID: orcid.org/0000-0002-1043-3323 1
2046 Accesses
4 Citations
Explore all metrics
Contamination-free groundwater is considered a good source of potable water. Even in the twenty-first century, over 90 percent of the population is reliant on groundwater resources for their lives. Groundwater influences the economical state, industrial development, ecological system, and agricultural and global health conditions worldwide. However, different natural and artificial processes are gradually polluting groundwater and drinking water systems throughout the world. Toxic metalloids are one of the major sources that pollute the water system. In this review work, we have collected and analyzed information on metal-resistant bacteria along with their genetic information and remediation mechanisms of twenty different metal ions [arsenic (As), mercury (Hg), lead (Pb), chromium (Cr), iron (Fe), copper (Cu), cadmium (Cd), palladium (Pd), zinc (Zn), cobalt (Co), antimony (Sb), gold (Au), silver (Ag), platinum (Pt), selenium (Se), manganese (Mn), molybdenum (Mo), nickel (Ni), tungsten (W), and uranium (U)]. We have surveyed the scientific information available on bacteria-mediated bioremediation of various metals and presented the data with responsible genes and proteins that contribute to bioremediation, bioaccumulation, and biosorption mechanisms. Knowledge of the genes responsible and self-defense mechanisms of diverse metal-resistance bacteria would help us to engineer processes involving multi-metal-resistant bacteria that may reduce metal toxicity in the environment.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or Ebook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Deciphering the Key Factors for Heavy Metal Resistance in Gram-Negative Bacteria

Detoxification of Heavy Metals Using Marine Metal Resistant Bacteria: A New Method for the Bioremediation of Contaminated Alkaline Environments

Recent Trends in Bioremediation of Heavy Metals: Challenges and Perspectives
Abbreviations.
Total dissolved solids
Total suspended solids
Central Bureau of Health Intelligence
Ministry of Health
N,N′-dicyclohexylcarbodiimide
2,4-Dinitrophenol
Minimum inhibitory concentration
International Agency for the Research on Cancer
Fienen, M. N., & Arshad, M. (2016). The international scale of the groundwater issue. In A. J. Jakeman, O. Barreteau, R. J. Hunt, J. D. Rinaudo, & A. Ross (Eds.), Integrated groundwater management. Springer.
Google Scholar
Edokpayi, J. N., Rogawski, E. T., Kahler, D. M., Hill, C. L., Reynolds, C., Nyathi, E., Smith, J. A., Odiyo, J. O., Samie, A., Bessong, P., & Dillingham, R. (2018). Challenges to sustainable safe drinking water: A case study of water quality and use across seasons in rural communities in Limpopo province, South Africa. Water, 10 , 159.
Article PubMed PubMed Central Google Scholar
USGS Science for a changing world. (2015). Water use . US: USGS Water Science School. https://www.usgs.gov/special-topic/water-science-school/science . Accessed 23 May 2022.
Margat, J., & van der Gun, J. (2013). Groundwater around the world: A geographic synopsis (1st ed.). London.
Book Google Scholar
Jinwal, U. K., Abisambra, J. F., Zhang, J., Dharia, S., O’Leary, J. C., Patel, T., Braswell, K., Jani, T., Gestwicki, J. E., & Dickey, C. A. (2012). Cdc37/Hsp90 protein complex disruption triggers an autophagic clearance cascade for TDP-43 protein. Journal of Biological Chemistry, 287 (29), 24814–24820.
Article CAS PubMed PubMed Central Google Scholar
Fernández-Luqueño, F., López-Valdez, F., Gamero-Melo, P., Suárez, S. L., Aguilera-González, E. N., Martínez, A. I., Guillermo, M. S. G., Hernández-Martínez, G., Herrera-Mendoza, R., Garz, M. A. Á., & Pérez-Velázquez, I. R. (2013). Heavy metal pollution in drinking water - A global risk for human health: A review. African Journal of Environmental Science and Technology, 7 (7), 567–584.
Vetrimurugan, E., Brindha, K., Elango, L., & Ndwandwe, O. M. (2017). Human exposure risk to heavy metals through groundwater used for drinking in an intensively irrigated river delta. Springer Applied Water Science, 7 , 3267–3280.
Article ADS CAS Google Scholar
Jamir, T. T., Devi, W. B., Singh, U. I., & Singh, R. K. B. (2011). Lead, iron and manganese contamination in spring, pond and well water in Nagaland, one of the Seven North-Eastern states of India: A future danger. Journal of Chemical and Pharmaceutical Research, 3 (3), 403–411.
CAS Google Scholar
Khlifi, R., & Hamza-Chaffai, A. (2010). Head and neck cancer due to heavy metal exposure via tobacco smoking and professional exposure: A review. Toxicology and Applied Pharmacology, 248 , 71–88.
Article CAS PubMed Google Scholar
Flora, S. J. S., Saxena, G., Gautam, P., Kaur, P., & Gill, K. D. (2007). Lead induced oxidative stress and alterations in biogenic amines in different rat brain regions and their response to combined administration of DMSA and MiADMSA. Chemico-Biological Interactions, 170 , 209–220.
Hawkes, J. S. (1997). What is “heavy metal.” Journal of Chemical Education, 74 , 1374.
Priyalaxmi, R., Murugan, A., Raja, P., & Raj, K. D. (2014). Bioremediation of cadmium by Bacillus safensis (JX126862), a marine bacterium isolated from mangrove sediments. International Journal of Current Microbiology and Applied Sciences, 3 (12), 326–335.
Wuana, R. A., & Okieimen, F. E. (2011). Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecology, 2011 , 402647.
Article Google Scholar
Briffa, J., Sinagra, E., & Blundell, R. (2020). Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon, 6 (9), e04691.
Kumar, C. (2017). Contaminated water kills 1 every 4 hours . The Times of India. webpage: https://timesofindia.indiatimes.com/city/bengaluru/contaminated-water-kills-1-every-4-hours/articleshow/62286683.cms . Accessed 23 May 2022.
Roy, N. A., Bak, J. H., Akrami, A., Brody, C. D., & Pillow, J. W. (2018). Efficient inference for time-varying behavior during learning. Advances in Neural Information Processing Systems, 31 , 5696–5706.
Tarfeen, N., Nisa, K. I., Hamid, B., Bashir, Z., Yatoo, A. M., Dar, M. A., Mohiddin, F. A., Amin, Z., Ahmad, R. A., & Sayyed, R. Z. (2022). Microbial remediation: A promising tool for reclamation of contaminated sites with special emphasis on heavy metal and pesticide pollution: A review. Processes, 10 , 1358.
Article CAS Google Scholar
Lahiri, D., Nag, M., Dey, A., Sarkar, T., Joshi, S., Pandit, S., Das, A. P., Pati, S., Pattanaik, S., Tilak, V. K., & Ray, R. R. (2022). Biofilm mediated degradation of petroleum products. Geomicrobiology Journal, 39 (3–5), 389–398.
Ianeva, O. D. (2009). Mechanisms of bacteria resistance to heavy metals. Mikrobiolohichnyĭ zhurnal, 71 (6), 54–65.
CAS PubMed Google Scholar
Megharaj, M., Venkateswarlu, K., & Naidu, R. (2014). Bioremediation. In P. Wexler (Ed.), Encyclopedia of Toxicology (3rd ed., pp. 485–489). Academic Press. https://doi.org/10.1016/B978-0-12-386454-3.01001-0
Sonawdekar, S. (2012). Bioremediation: a boon to hydrocarbon degradation. International Journal of Environmental Sciences, 2 , 2408–2424.
Hlihor, R. M., Apostol, L. C., & Gavrilescu, M. (2017). Environmental bioremediation by biosorption and bioaccumulation: principles and applications. In N. Anjum, S. Gill, & N. Tuteja (Eds.), Enhancing cleanup of environmental pollutants. Springer.
Azubuike, C. C., Chikere, C., B., & Okpokwasili, G., C. (2016). Bioremediation techniques classification based on site of application: principles, advantages, limitations and prospects. World Journal of Microbiology and Biotechnology, 32 (11), 180. https://doi.org/10.1007/s11274-016-2137-x
Dias, R. L., Ruberto, L., Calabro, A., Balbo, A. L., Del Panno, M. T., & Mac Cormack, W. P. (2015). Hydrocarbon removal and bacterial community structure in on-site biostimulated biopile systems designed for bioremediation of diesel-contaminated Antarctic soil. Polar Biol, 38 , 677–687.
Gomez, F., & Sartaj, M. (2014). Optimization of field scale biopiles for bioremediation of petroleum hydrocarbon contaminated soil at low temperature conditions by response surface methodology (RSM). International Biodeterioration and Biodegradation, 89 , 103–109.
Whelan, M. J., Coulon, F., Hince, G., Rayner, J., McWatters, R., Spedding, T., & Snape, I. (2015). Fate and transport of petroleum hydrocarbons in engineered biopiles in polar regions. Chemosphere, 131 , 232–240.
Article ADS CAS PubMed Google Scholar
Chemlal, R., Abdi, N., Lounici, H., Drouiche, N., Pauss, A., & Mameri, N. (2013). Modeling and qualitative study of diesel biodegradation using biopile process in sandy soil. International Biodeterioration and Biodegradation, 78 , 43–48.
Akbari, A., & Ghoshal, S. (2014). Pilot-scale bioremediation of a petroleum hydrocarbon-contaminated clayey soil from a sub-Arctic site. Journal of Hazardous Materials, 280 , 595–602.
Aislabie, J., Saul, D. J., & Foght, J. M. (2006). Bioremediation of hydrocarbon-contaminated polar soils. Extremophiles, 10 , 171–179.
Sanscartier, D., Zeeb, B., Koch, I., & Reimer, K. (2009). Bioremediation of diesel-contaminated soil by heated and humidified biopile system in cold climates. Cold Regions Science and Technology, 55 , 167–173.
Barr, D. (2002). Biological methods for assessment and remediation of contaminated land: Case studies . Construction Industry Research and Information Association.
Coulon, F., Al Awadi, M., Cowie, W., Mardlin, D., Pollard, S., Cunningham, C., Risdon, G., Arthur, P., Semple, K. T., & Paton, G. I. (2010). When is soil remediated? Comparison of biopiled and windrowed soils contaminated with bunker-fuel in a full-scale trial. Environmental Pollution, 158 , 3032–3040.
Hobson, A. M., Frederickson, J., & Dise, N. B. (2005). CH 4 and N 2 O from mechanically turned windrow and vermicomposting systems following in-vessel pre-treatment. Waste Management, 25 , 345–352.
Philp, J. C., & Atlas, R. M. (2005). Bioremediation of contaminated soils and aquifers. In R. M. Atlas & J. C. Philp (Eds.), Bioremediation: applied microbial solutions for real-world environmental cleanup (pp. 139–236). American Society for Microbiology (ASM) Press.
Höhener, P., & Ponsin, V. (2014). In situ vadose zone bioremediation. Current Opinion in Biotechnology, 27 , 1–7.
Article PubMed Google Scholar
Gidarakos, E., & Aivalioti, M. (2007). Large scale and long term application of bioslurping: The case of a Greek petroleum refinery site. Journal of Hazardous Materials, 149 , 574–581.
Meagher, R. B. (2000). Phytoremediation of toxic elemental organic pollutants. Current Opinion in Plant Biology, 3 , 153–162.
Kuiper, I., Lagendijk, E. L., Bloemberg, G. V., & Lugtenberg, B. J. J. (2004). Rhizoremediation: a beneficial plant-microbe interaction. Molecular Plant-Microbe Interactions, 7 , 6–15.
Igiri, B. E., Okoduwa, S. I. R., Idoko, G. O., Akabuogu, E. P., Adeyi, A. O., & Ejiogu, I. K. (2018). Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: a review. J Toxicol, 2018 , 2568038.
Saha, R. P., Samanta, S., Patra, S., Sarkar, D., Saha, A., & Singh, M. K. (2017). Metal homeostasis in bacteria: the role of ArsR–SmtB family of transcriptional repressors in combating varying metal concentrations in the environment. BioMetals, 30 (4), 459–503.
Banerjee, A., Tabassum, S. K., Debnath, D., Hazra, D., & Pal, R. (2022). A review on detoxification and bioremediation of antimony by bacterial species. Acta Scientific Microbiology, 5 (9), 56–72.
Sun, W., Sun, X., Häggblom, M. M., Kolton, M., Lan, L., Li, B., Dong, Y., Xu, R., & Li, F. (2021). Identification of antimonate reducing bacteria and their potential metabolic traits by the combination of stable isotope probing and metagenomic-pangenomic analysis. Environmental Science and Technology, 55 (20), 13902–13912.
Mateos, L. M., Ordóñez, E., Letek, M., & Gil, J. A. (2006). Corynebacterium glutamicum as a model bacterium for the bioremediation of arsenic. International Microbiology, 9 (3), 207–215.
Ojuederie, O. B., & Babalola, O. O. (2017). Microbial and plant-assisted bioremediation of heavy metal polluted environments: a review. International Journal of Environmental Research and Public Health, 14 (12), 1504.
Verma, S., & Kuila, A. (2019). Bioremediation of heavy metals by microbial process. Environmental Technology & Innovation, 14 , 100369.
Crupper, S. S., Worrell, V., Stewart, G. C., & Iandolo, J. J. (1999). Cloning and expression of cadD , a new cadmium resistance gene of Staphylococcus aureus . Journal of Bacteriology, 181 (13), 4071–4075.
Das, S., Dash, H. R., & Chakraborty, J. (2016). Genetic basis and importance of metal resistant genes in bacteria for bioremediation of contaminated environments with toxic metal pollutants. Applied Microbiology and Biotechnology, 100 (7), 2967–2984.
Cheung, K., & Gu, J. (2007). Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: A review. International Biodeterioration & Biodegradation, 59 (1), 8–15.
Dulay, H., Tabares, M., Kashefi, K., & Reguera, G. (2020). Cobalt resistance via detoxification and mineralization in the iron-reducing bacterium Geobacter sulfurreducens . Frontiers in Microbiology, 11 , 600463.
Wang, L., Yan, L., Ye, L., Chen, J., Li, Y., Zhang, Q., & Jing, C. (2022). Identification and Characterization of a Au(III) Reductase from Erwinia sp. IMH. JACS Au, 2 (6), 1435–1442.
Choi, M. H., Jeong, S. W., Shim, H. E., Yun, S. J., Mushtaq, S., Choi, D. S., Jang, B. S., Yang, J. E., Choi, Y. J., & Jeon, J. (2017). Efficient bioremediation of radioactive iodine using biogenic gold nanomaterial-containing radiation-resistant bacterium, Deinococcus radiodurans R1. Chemical Communications (Cambridge, England), 53 (28), 3937–3940.
Bradley, J. M., Svistunenko, D. A., Wilson, M. T., Hemmings, A. M., Moore, G. R., & Le Brun, N. E. (2020). Bacterial iron detoxification at the molecular level. Journal of Biological Chemistry, 295 (51), 17602–17623.
Barboza, N. R., Guerra-Sá, R., & Leão, V. A. (2016). Mechanisms of manganese bioremediation by microbes: An overview. Journal of Chemical Technology and Biotechnology, 91 , 2733–2739.
Purkan, P., Nurmalyya, S., & Hadi, S. (2016). Resistance level of Pseudomonas stutzeri against mercury and its ability in production of mercury reductase enzyme. Molekul, 11 (2), 230–238.
Figueiredo, N. L., Canário, J., O’Driscoll, N. J., Duarte, A., & Carvalho, C. (2016). Aerobic mercury-resistant bacteria alter mercury speciation and retention in the Tagus Estuary (Portugal). Ecotoxicology and Environmental Safety, 124 , 60–67.
Yakasai, H. M., Rahman, M. F., Manogaran, M., Yasid, N. A., Syed, M. A., Shamaan, N. A., & Shukor, M. Y. (2021). Microbiological reduction of molybdenum to molybdenum blue as a sustainable remediation tool for molybdenum: A comprehensive review. International Journal of Environmental Research and Public Health, 18 (11), 5731.
Malunga, K. B., & Chirwa, E. M. N. (2019). Recovery of Palladium (II) by biodeposition using a pure culture and a mixed culture. Chemical Engineering Transactions, 74 , 1519–1524.
Capeness, M. J., Edmundson, M. C., & Horsfall, L. E. (2015). Nickel and platinum group metal nanoparticle production by Desulfovibrio alaskensis G20. New Biotechnology, 32 (6), 727–731.
Eswayah, A. S., Smith, T. J., & Gardiner, P. H. (2016). Microbial transformations of selenium species of relevance to bioremediation. Applied and Environment Microbiology, 82 (16), 4848–4859.
Silver, S. (2003). Bacterial silver resistance: Molecular biology and uses and misuses of silver compounds. FEMS Microbiology Reviews, 27 (2–3), 341–353.
Lin, I.W.-S., Lok, C.-N., & Che, C.-M. (2014). Biosynthesis of silver nanoparticles from silver(I) reduction by the periplasmic nitrate reductase c-type cytochrome subunit NapC in a silver-resistant E. coli . Chemical Science, 5 , 3144–3150.
Shanware, A. S., & Phadtare, P. (2014). Tungsten toxicity in soil and biological role of tungsten in bacteria. Indian Journal of Science, 10 (24), 2014.
Kletzin, A., & Adams, M. W. (1996). Tungsten in biological systems. FEMS Microbiology Reviews, 18 (1), 5–63.
You, W., Peng, W., Tian, Z., & Zheng, M. (2021). Uranium bioremediation with U(VI)-reducing bacteria. Science of the Total Environment, 798 , 149107.
Newsome, L., Morris, K., & Lloyd, J. R. (2014). The biogeochemistry and bioremediation of uranium and other priority radionuclides. Chemical Geology, 363 , 164–184.
Choudhury, R., & Srivastava, S. (2001). Zinc resistance mechanisms in bacteria. Current Science, 81 (7), 768–775.
Luo, S., Xiao, X., Xi, Q., Wan, Y., Chen, L., Zeng, G., Liu, C., Guo, H., & Chen, J. (2011). Enhancement of cadmium bioremediation by endophytic bacterium Bacillus sp. L14 using industrially used metabolic inhibitors (DCC or DNP). Journal of Hazardous Materials, 190 (1–3), 1079–1082.
Loni, P. C., Wu, M., Wang, W., Wang, H., Ma, L., Liu, C., Song, Y., & Tuovinen, H. O. (2020). Mechanism of microbial dissolution and oxidation of antimony in stibnite under ambient conditions. J Hazard Mater, 385 , 121561.
Das, P., Sinha, S., & Mukherjee, S. K. (2014). Nickel bioremediation potential of Bacillus thuringiensis KUNi1 and some environmental factors in nickel removal. Bioremediation Journal, 18 (2), 169–177.
Shukor, M. Y., Rahman, M. F., Suhaili, Z., Shamaan, N. A., & Syed, M. A. (2009). Bacterial reduction of hexavalent molybdenum to molybdenum blue. World Journal of Microbiology & Biotechnology, 25 , 1225–1234.
Satyapal, G. K., Mishra, S. K., Srivastava, A., Ranjan, R. K., Prakash, K., Haque, R., & Kumar, N. (2018). Possible bioremediation of arsenic toxicity by isolating indigenous bacteria from the middle Gangetic plain of Bihar, India. Biotechnology Reports (Amsterdam, Netherlands), 17 , 117–125.
PubMed Google Scholar
Palanivel, T. M., Sivakumar, N., Al-Ansari, A., & Victor, R. (2020). Bioremediation of copper by active cells of Pseudomonas stutzeri LA3 isolated from an abandoned copper mine soil. Journal of Environmental Management, 253 , 109706.
Guo, H., Luo, S., Chen, L., Xiao, X., Xi, Q., Wei, W., Zeng, G., Liu, C., Wan, Y., Chen, J., & He, Y. (2010). Bioremediation of heavy metals by growing hyperaccumulaor endophytic bacterium Bacillus sp. L14. Bioresource Technology, 101 (22), 8599–8605.
Lengke, M. F., Fleet, M. E., & Southam, G. (2006). Bioaccumulation of gold by filamentous cyanobacteria between 25 and 200°C. Geomicrobiology Journal, 23 (8), 591–597.
de Vargas, I., Macaskie, L. E., & Guibal, E. (2004). Biosorption of palladium and platinum by sulfate-reducing bacteria. Journal of Chemical Technology and Biotechnology, 79 , 49–56.
Blindauer, C. A., Harrison, M. D., Robinson, A. K., Parkinson, J. A., Bowness, P. W., Sadler, P. J., & Robinson, N. J. (2002). Multiple bacteria encode metallothioneins and SmtA-like zinc fingers. Molecular Microbiology, 45 (5), 1421–1432.
Ewart, D. K., & Hughes, M. N. (1991). The extraction of metals from ores using bacteria. Advances in Inorganic Chemistry, 36 , 103–135.
Bahar, M. M., Megharaj, M., & Naidu, R. (2012). Arsenic bioremediation potential of a new arsenite-oxidizing bacterium Stenotrophomonas sp MM-7 isolated from soil. Biodegradation, 23 (6), 803–12.
Tan, L. C., Nancharaiah, Y. V., van Hullebusch, E. D., & Lens, P. N. L. (2016). Selenium: Environmental significance, pollution, and biological treatment technologies. Biotechnology Advances, 34 (5), 886–907.
Li, J., Wang, Q., Zhang, S., Qin, D., & Wang, G. (2013). Phylogenetic and genome analyses of antimony-oxidizing bacteria isolated from antimony mined soil. International biodeterioration & biodegradation, 76 , 76–80.
Sun, W., Xiao, E., Dong, Y., Tang, S., Krumins, V., Ning, Z., Sun, M., Zhao, Y., Wu, S., & Xiao, T. (2016). Profiling microbial community in a watershed heavily contaminated by an active antimony (Sb) mine in Southwest China. Science of the Total Environment, 550 , 297–308.
Nguyen, V. K., Choi, W., Yu, J., & Lee, T.-H. (2017). Microbial oxidation of antimonite and arsenite by bacteria isolated from antimony-contaminated soils. International Journal of Hydrogen Energy, 42 (45), 27832–27842.
Gu, J., Sunahara, G., Duran, R., Yao, J., Cui, Y., Tang, C., Li, H., & Mihucz, V. G. (2019). Sb(III)-resistance mechanisms of a novel bacterium from non-ferrous metal tailings. Ecotoxicology and Environmental Safety, 186 , 109773.
Nguyen, V. K., Park, Y., & Lee, T. (2019). Microbial antimonate reduction with a solid-state electrode as the sole electron donor: A novel approach for antimony bioremediation. Journal of Hazardous Materials, 377 , 179–185.
Sun, X., Li, B., Han, F., Xiao, E., Wang, Q., Xiao, T., & Sun, W. (2019). Vegetation type impacts microbial interaction with antimony contaminants in a mining-contaminated soil environment. Environmental Pollution, 252 (Pt B), 1872–1881.
Wu, B., Wang, Z., Zhao, Y., Gu, Y., Wang, Y., Yu, J., & Xu, H. (2019). The performance of biochar-microbe multiple biochemical material on bioremediation and soil micro-ecology in the cadmium aged soil. Science of the Total Environment, 686 , 719–728.
Lovley, D. R., & Coates, J. D. (1997). Bioremediation of metal contamination. Current Opinion in Biotechnology, 8 (3), 285–289.
Páez-Espino, D., Tamames, J., de Lorenzo, V., & Cánovas, D. (2009). Microbial responses to environmental arsenic. BioMetals, 22 (1), 117–130.
Banerjee, S., Datta, S., Chattyopadhyay, D., & Sarkar, P. (2011). Arsenic accumulating and transforming bacteria isolated from contaminated soil for potential use in bioremediation. Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances & Environmental Engineering, 46 (14), 1736–1747.
Kumari, N., Rana, A., & Jagadevan, S. (2019). Arsenite biotransformation by Rhodococcus sp.: Characterization, optimization using response surface methodology and mechanistic studies. Science of the Total Environment, 687 , 577–589.
Ali Khan, M. W., & Ahmad, M. (2006). Detoxification and bioremediation potential of a Pseudomonas fluorescens isolate against the major Indian water pollutants. Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances & Environmental Engineering, 41 (4), 659–674.
Ike, A., Sriprang, R., Ono, H., Murooka, Y., & Yamashita, M. (2007). Bioremediation of cadmium contaminated soil using symbiosis between leguminous plant and recombinant rhizobia with the MTL4 and the PCS genes. Chemosphere, 66 (9), 1670–1676.
Juwarkar, A. A., Nair, A., Dubey, K. V., Singh, S. K., & Devotta, S. (2007). Biosurfactant technology for remediation of cadmium and lead contaminated soils. Chemosphere, 68 (10), 1996–2002.
Bai, H. J., Zhang, Z. M., Yang, G. E., & Li, B. Z. (2008). Bioremediation of cadmium by growing Rhodobacter sphaeroides : Kinetic characteristic and mechanism studies. Bioresource Technology, 99 (16), 7716–7722.
Rajbanshi, A. (2009). Study on heavy metal resistant bacteria in Guheswori sewage treatment plant. Our Nature, 6 (1), 52–57.
Rani, A., Souche, Y. S., & Goel, R. (2009). Comparative assessment of in situ bioremediation potential of cadmium resistant acidophilic Pseudomonas putida 62BN and alkalophilic Pseudomonas monteilli 97AN strains on soybean. International Biodeterioration & Biodegradation, 63 (1), 62–66.
Sinha, S., & Mukherjee, S. K. (2009). Pseudomonas aeruginosa KUCD1, a possible candidate for cadmium bioremediation. Brazilian Journal of Microbiology, 40 (3), 655–662.
Zahoor, A., & Rehman, A. (2009). Isolation of Cr(VI) reducing bacteria from industrial effluents and their potential use in bioremediation of chromium containing wastewater. Journal of Environmental Sciences (China), 21 (6), 814–820.
Siripornadulsil, S., & Siripornadulsil, W. (2013). Cadmium-tolerant bacteria reduce the uptake of cadmium in rice: Potential for microbial bioremediation. Ecotoxicology and Environmental Safety, 94 , 94–103.
Sen, S. K., Raut, S., Dora, T. K., & Mohapatra, P. K. (2014). Contribution of hot spring bacterial consortium in cadmium and lead bioremediation through quadratic programming model. Journal of Hazardous Materials, 265 , 47–60.
Kumar, M., Kumar, V., Varma, A., Prasad, R., Sharma, A. K., Pal, A., Arshi, A., & Singh, J. (2016). An efficient approach towards the bioremediation of copper, cobalt and nickel contaminated field samples. Journal of Soils and Sediments, 16 (8), 2118–2127.
Shen, Y., Zhu, W., Li, H., Ho, S. H., Chen, J., Xie, Y., & Shi, X. (2018). Enhancing cadmium bioremediation by a complex of water-hyacinth derived pellets immobilized with Chlorella sp. Bioresource Technology, 257 , 157–163.
Massoud, R., Hadiani, M. R., Hamzehlou, P., & Khosravi-Darani, K. (2019). Bioremediation of heavy metals in food industry: Application of Saccharomyces cerevisiae . Electronic Journal of Biotechnology, 37 , 56–60.
Ma, H., Wei, M., Wang, Z., Hou, S., Li, X., & Xu, H. (2020). Bioremediation of cadmium polluted soil using a novel cadmium immobilizing plant growth promotion strain Bacillus sp. TZ5 loaded on biochar. Journal of Hazardous Materials, 388 , 122065.
Shi, Z., Zhang, Z., Yuan, M., Wang, S., Yang, M., Yao, Q., Ba, W., Zhao, J., & Xie, B. (2020). Characterization of a high cadmium accumulating soil bacterium, Cupriavidus sp. WS2. Chemosphere, 247 , 125834.
Akhtar, M. S., Chali, B., & Azam, T. (2013). Bioremediation of arsenic and lead by plants and microbes from contaminated soil. Research in Plant Sciences, 1 (3), 68–73.
Krumholz, L. R., Elias, D. A., & Suflita, J. M. (2003). Immobilization of cobalt by sulfate-reducing bacteria in subsurface sediments. Geomicrobiology Journal, 20 (1), 61–72.
Hau, H. H., Gilbert, A., Coursolle, D., & Gralnick, J. A. (2008). Mechanism and consequences of anaerobic respiration of cobalt by Shewanella oneidensis strain MR-1. Applied and Environment Microbiology, 74 (22), 6880–6886.
Tajer-Mohammad-Ghazvini, P., Kasra-Kermanshahi, R., Nozad-Golikand, A., Sadeghizadeh, M., Ghorbanzadeh-Mashkani, S., & Dabbagh, R. (2016). Cobalt separation by Alphaproteobacterium MTB-KTN90: Magnetotactic bacteria in bioremediation. Bioprocess and Biosystems Engineering, 39 (12), 1899–1911.
Shakibaie, M. R., Khosravan, A., Frahmand, A., & Zare, S. (2008). Application of metal resistant bacteria by mutational enhancment technique for bioremediation of copper and zinc from industrial wastes. Iran Journal of Environmental Health Science and Engineering, 5 (4), 251–256.
Sun, L. N., Zhang, Y. F., He, L. Y., Chen, Z. J., Wang, Q. Y., Qian, M., & Sheng, X. F. (2010). Genetic diversity and characterization of heavy metal-resistant-endophytic bacteria from two copper-tolerant plant species on copper mine wasteland. Bioresource Technology, 101 (2), 501–509.
Ghosh, A., & Saha, P. D. (2013). Optimization of copper bioremediation by Stenotrophomonas maltophilia PD2. Journal of Environmental Chemical Engineering, 1 (3), 159–163.
Islam, F., Yasmeen, T., Ali, Q., Mubin, M., Ali, S., Arif, M. S., Hussain, S., Riaz, M., & Abbas, F. (2016). Copper-resistant bacteria reduces oxidative stress and uptake of copper in lentil plants: Potential for bacterial bioremediation. Environmental Science and Pollution Research International, 23 (1), 220–233.
Cornu, J. Y., Huguenot, D., Jézéquel, K., Lollier, M., & Lebeau, T. (2017). Bioremediation of copper-contaminated soils by bacteria. World Journal of Microbiology & Biotechnology, 33 (2), 26.
Reith, F., Etschmann, B., Grosse, C., Moors, H., Benotmane, M. A., Monsieurs, P., Grass, G., Doonan, C., Vogt, S., Lai, B., Martinez-Criado, G., George, G. N., Nies, D. H., Mergeay, M., Pring, A., Southam, G., & Brugger, J. (2009). Mechanisms of gold biomineralization in the bacterium Cupriavidus metallidurans. Proceedings of the National Academy of Sciences, 106 (42), 17757–17762.
Fashola, M. O., Ngole-Jeme, V. M., & Babalola, O. O. (2016). Heavy metal pollution from gold mines: Environmental effects and bacterial strategies for resistance. International Journal of Environmental Research and Public Health, 13 (11), 1047.
Rea, M. A., Zammit, C. M., & Reith, F. (2016). Bacterial biofilms on gold grains-implications for geomicrobial transformations of gold. FEMS Microbiology Ecology, 92 (6), fiw082.
Barkay, T., & Schaefer, J. (2001). Metal and radionuclide bioremediation: Issues, considerations and potentials. Current Opinion in Microbiology, 4 (3), 318–323.
Gadd, G. M. (2004). Microbial influence on metal mobility and application for bioremediation. Geoderma, 122 (2–4), 109–119.
Chen, W. B. (2011). The study of bioremediation on heavy metal of cultured seawater by Sphingomonas sp. XJ2 Immobilized Sphingomonas Strain. Advanced Materials Research, 347–353 , 1436–1441. https://doi.org/10.4028/www.scientific.net/amr.347-353.1436
Naik, M. M., & Dubey, S. K. (2013). Lead resistant bacteria: Lead resistance mechanisms, their applications in lead bioremediation and biomonitoring. Ecotoxicology and Environmental Safety, 98 , 1–7.
Gupta, M. K., Kumari, K., Shrivastava, A., & Gauri, S. (2014). Bioremediation of heavy metal polluted environment using resistant bacteria. Journal of Environmental Research and Development, 8 (4), 883–889.
Coelho, L. M., Rezende, H. C., Coelho, L. M., de Sousa, P. A. R., Melo, D. F. O., & Coelho, N. M. M. (2015). Bioremediation of polluted waters using microorganisms. Advances in Bioremediation of Wastewater and Polluted Soil . https://doi.org/10.5772/60770
Nascimento, A. M., & Chartone-Souza, E. (2003). Operon mer: bacterial resistance to mercury and potential for bioremediation of contaminated environments. Genetics and Molecular Research, 2 (1), 92–101.
Wagner-Döbler, I. (2003). Pilot plant for bioremediation of mercury-containing industrial wastewater. Applied Microbiology and Biotechnology, 62 (2–3), 124–133.
Shukor, M. Y., Rahman, M. F., Suhaili, Z., Shamann, N. A., & Syed, M. A. (2010). Hexavalent molybdenum reduction to Mo-blue by Acinetobacter calcoaceticus . Folia Microbiologica, 55 , 137–143.
Baxter-Plant, V. S., Mikheenko, I. P., & Macaskie, L. E. (2003). Sulphate-reducing bacteria, palladium and the reductive dehalogenation of chlorinated aromatic compounds. Biodegradation, 14 (2), 83–90.
Lesniewska, B. A., Messerschmidt, J., Jakubowski, N., & Hulanicki, A. (2004). Bioaccumulation of platinum group elements and characterization of their species in Lolium multiflorum by size-exclusion chromatography coupled with ICP-MS. Science of the Total Environment, 322 (1–3), 95–108.
Zimmermann, S., Messerschmidt, J., von Bohlen, A., & Sures, B. (2005). Uptake and bioaccumulation of platinum group metals (Pd, Pt, Rh) from automobile catalytic converter materials by the zebra mussel ( Dreissena polymorpha ). Environmental Research, 98 (2), 203–209.
Pollmann, K., Raff, J., Merroun, M., Fahmy, K., & Selenska-Pobell, S. (2006). Metal binding by bacteria from uranium mining waste piles and its technological applications. Biotechnology Advances, 24 (1), 58–68.
Hennebel, T., Van Nevel, S., Verschuere, S., De Corte, S., De Gusseme, B., Cuvelier, C., Fitts, J. P., van der Lelie, D., Boon, N., & Verstraete, W. (2011). Palladium nanoparticles produced by fermentatively cultivated bacteria as catalyst for diatrizoate removal with biogenic hydrogen. Applied Microbiology and Biotechnology, 91 (5), 1435–1445.
Veltz, I., Arsac, F., Biagianti-Risbourg, S., Habets, F., Lechenault, H., & Vernet, G. (1996). Effects of platinum (Pt4+) on Lumbriculus variegatus Müller (Annelida, Oligochaetae): acute toxicity and bioaccumulation. Archives of Environmental Contamination and Toxicology, 31 (1), 63–67.
Losi, M. E., & Frankenberger, W. T. (1997). Reduction of selenium oxyanions by Enterobacter cloacae SLD1a-1: isolation and growth of the bacterium and its expulsion of selenium particles. Applied and Environment Microbiology, 63 (8), 3079–3084.
Bledsoe, T. L., Cantafio, A. W., & Macy, J. M. (1999). Fermented whey-an inexpensive feed source for a laboratory-scale selenium-bioremediation reactor system inoculated with Thauera selenatis . Applied Microbiology and Biotechnology, 51 (5), 682–685.
Kashiwa, M., Nishimoto, S., Takahashi, K., Ike, M., & Fujita, M. (2000). Factors affecting soluble selenium removal by a selenate-reducing bacterium Bacillus sp. SF-1. Journal of Bioscience and Bioengineering, 89 (6), 528–533.
Ranjard, L., Prigent-Combaret, C., Nazaret, S., & Cournoyer, B. (2002). Methylation of inorganic and organic selenium by the bacterial thiopurine methyltransferase. Journal of Bacteriology, 184 (11), 3146–3149.
Siddique, T., Zhang, Y., Okeke, B. C., & Frankenberger, W. T., Jr. (2006). Characterization of sediment bacteria involved in selenium reduction. Bioresource Technology, 97 (8), 1041–1049.
Charley, R. C., & Bull, A. T. (1979). Bioaccumulation of silver by a multispecies community of bacteria. Archives of Microbiology, 123 (3), 239–244.
Slawson, R. M., Lee, H., & Trevors, J. T. (1990). Bacterial interactions with silver. Biology of Metals, 3 (3–4), 151–154.
Ibrahim, Z., Ahmad, W. A., & Baba, A. B. (2001). Bioaccumulation of silver and the isolation of metal-binding protein from P. diminuta . Brazilian Archives of Biology and Technology, 44 (3), 223–225.
Ganorkar, R., Jadeja, N. B., Shanware, A., & Ingle, A. B. (2022). Characterisation of novel microbial strains Proteus mirabilis and Bordetella avium for heavy metal bioremediation and dye degradation. Archives of Microbiology, 204 (5), 262.
Radhika, V., Subramanian, S., & Natarajan, K. A. (2006). Bioremediation of zinc using Desulfotomaculum nigrificans : Bioprecipitation and characterization studies. Water Research, 40 (19), 3628–3636.
Natarajan, K. A. (2008). Microbial aspects of acid mine drainage and its bioremediation. Transactions of Nonferrous Metals Society of China, 18 (6), 1352–1360.
Biondo, R., da Silva, F. A., Vicente, E. J., Souza Sarkis, J. E., & Schenberg, A. C. (2012). Synthetic phytochelatin surface display in Cupriavidus metallidurans CH34 for enhanced metals bioremediation. Environmental Science and Technology, 46 (15), 8325–8332.
Peng, W., Li, X., Song, J., Jiang, W., Liu, Y., & Fan, W. (2018). Bioremediation of cadmium- and zinc-contaminated soil using Rhodobacter sphaeroides . Chemosphere, 197 , 33–41.
Tumanyan, A. F., Seliverstova, A. P., & Zaitseva, N. A. (2020). Effect of heavy metals on ecosystems. Chemistry and Technology of Fuels and Oils, 56 , 390–394.
Ashraf, R., & Ali, T. A. (2007). Effect of heavy metals on soil microbial community and mung beans seed germination. Pakistan Journals of Botany, 39 (2), 629–636.
Bhattacharyya, P., Chakrabarti, K., Chakraborty, A., Tripathy, S., & Powell, M. A. (2008). Fractionation and bioavailability of Pb in municipal solid waste compost and Pb uptake by rice straw and grain under the submerged condition in amended soil. Geosciences Journal, 12 (1), 41–45.
Jordao, C. P., Nascentes, C. C., Cecon, P. R., Fontes, R. L. F., & Pereira, J. L. (2006). Heavy metal availability in soil amended with composted urban solid wastes. Environmental Monitoring and Assessment, 112 , 309–326.
Singh, J. (2011). Effects of heavy metals on soil, plants, human health and aquatic life. International Journal of Research in Chemistry and Environment, 1 (2), 15–21.
Hinojosa, M. B., Carreira, J. A., Ruız, R. G., & Dick, R. P. (2004). Soil moisture pre-treatment effects on enzyme activities as indicators of heavy metal contaminated and reclaimed soils. Soil Biology & Biochemistry, 36 , 1559–1568.
Yao, H., Xu, J., & Huang, C. (2003). Substrate utilization pattern, biomass and activity of microbial communities in a sequence of heavy metalpolluted paddy soils. Geoderma, 115 , 139–148.
Speira, T. W., Kettlesb, H. A., Percivalc, H. J., & Parshotam, A. (1999). Is soil acidification the cause of biochemical responses when soils are amended with heavy metal salts? Soil Biology and Biochemistry, 31 , 1953–1961.
Shun-hong, H., Bing, P., Zhi-hui, Y., Li-yuan, C., & Li-cheng, Z. (2009). Chromium accumulation, microorganism population and enzyme activities in soils around chromium-containing slag heap of steel alloy factory. Transactions of Nonferrous Metals Society of China, 19 , 241–248.
Mora, A. P., Calvo, J. J. O., Cabrera, F., & Madejon, E. (2005). Changes in enzyme activities and microbial biomass after ‘“in situ”’ remediation of a heavy metal-contaminated soil. Applied Soil Ecology, 28 , 125–137.
Wang, Y. P., Shi, J. Y., Wang, H., Li, Q., Chen, X. C., & Chen, Y. X. (2007). The influence of soil heavy metals pollution on soil microbial biomass, enzyme activity, and community composition near a copper smelters. Ecotoxicology and Environmental Safety, 67 , 75–81.
Garrido, S., Campo, G. M. D., Esteller, M. V., Vaca, R., & Lugo, J. (2002). Heavy metals in soil treated with sewage sludge composting, their effect on yield and uptake of broad bean seeds ( Vicia faba L.). Water, Air, and Soil Pollution, 166 , 303–319.
Article ADS Google Scholar
Rascio, N., & Izzo, F. N. (2011). Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Science, 180 , 169–181.
Sharma, R. K., Agrawal, M., & Marshall, F. (2007). Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicology and Environmental Safety, 66 , 258–266.
Guala, S. D., Vega, F. A., & Covelo, E. F. (2010). The dynamics of heavy metals in plant–soil interactions. Ecological Modelling, 221 , 1148–1152.
Woo, S., Yum, S., Park, H. S., Lee, T. K., & Ryu, J. C. (2009). Effects of heavy metals on antioxidants and stress-responsive gene expression in Javanese medaka ( Oryzias javanicus ). Comparative Biochemistry and Physiology, Part C, 149 , 289–299.
Ayandiran, T. A., Fawole, O. O., Adewoye, S. O., & Ogundiran, M. A. (2009). Bioconcentration of metals in the body muscle and gut of Clarias gariepinus exposed to sublethal concentrations of soap and detergent effluent. Journal of Cell and Animal Biology, 3 (8), 113–118.
Peng, K., Luo, C., Luo, L., Li, X., & Shena, Z. (2008). Bioaccumulation of heavy metals by the aquatic plants Potamogeton pectinatus L. and Potamogeton malaianus Miq. and their potential use for contamination indicators and in wastewater treatment. Scince of the Total Environment, 392 , 22–29.
Gurrieri, J. T. (1998). Distribution of metals in water and sediment and effects on aquatic biota in the upper Stillwater River basin, Montana. Journal of Geochemical Exploration, 64 , 83–100.
Soliman, Z. I. (2006). A study of heavy metals pollution in some aquatic organisms in Suez Canal in port-said harbour. Journal of Applied Sciences Research, 2 (10), 657–663.
Iwasaki, Y., Kagaya, T., Miyamoto, K., & Matsuda, H. (2002). Environmental effects of heavy metals on riverine benthic macroinvertebrate assemblages with reference to potential food availability for drift-feeding fishes. Toxicology and Chemistry, 28 (2), 354–363.
Abernathy, C. O., Liu, Y. P., Longfellow, D., Aposhian, H. V., Beck, B., Fowler, B., Goyer, R., Menzer, R., Rossman, T., Thompson, C., & Waalkes, R. (1999). Arsenic: health effects, mechanisms of actions and research issues. Environmental Health Perspectives, 107 , 593–597.
Caserta, D., Graziano, A., Monte, G. L., Bordi, G., & Moscarini, M. (2013). Heavy metals and placental fetal-maternal barrier: A mini-review on the major concerns. European Review for Medical and Pharmacological Sciences, 17 , 2198–2206.
Lenntech Company Websites Lenntech BV, Delfgauw. (1998–2019). Water treatment solutions . The Netherlands. https://www.lenntech.com/periodic/elements/cr.htm . Accessed 23 May 2022.
Smith, A. H., Lingas, E. O., & Rahman, M. (2000). Contamination of drinking-water by arsenic in Bangladesh: A public health emergency. Bulletin of the World Health Organization, 78 (9), 1093–1103.
CAS PubMed PubMed Central Google Scholar
Mazumder, G. (2008). Chronic arsenic toxicity & human health. Indian Journal of Medical Research, 128 (4), 436–447.
Bernard, A. (2008). Cadmium & its adverse effects on human health. Indian Journal of Medical Research, 128 (4), 557–564.
Nelson, R. L. (1992). Dietary iron and colorectal cancer risk. Free Radical Biology & Medicine, 12 (2), 161–168.
Bhasin, G., Kauser, H., & Athar, M. (2002). Iron augments stage-I and stage-II tumour promotion in murine skin. Cancer Letters, 183 (2), 113–122.
Martin, S., & Griswold, W. (2009). Human health effects of heavy metals. Environmental Science and Technology Briefs for Citizens, 15 , 1–6.
Papanikolaou, N. C., Hatzidaki, E. G., Belivanis, S., Tzanakakis, G. N., & Tsatsakis, A. M. (2005). Lead toxicity update. A brief review. Medical Science Monitor, 11 (10), RA329.
Taylor, M. P., Winder, C., & Lanphear, B. P. (2012). Eliminating childhood lead toxicity in Australia: a call to lower the intervention level. MJA, 197 (9), 493.
Teo, J., Goh, K., Ahuja, A., Ng, H., & Poon, W. (1997). Intracranial vascular calcifications, glioblastoma multiforme, and lead poisoning. AJNR, 18 , 576–579.
Alina, M., Azrina, A., Mohd Yunus, A. S., Mohd Zakiuddin, S., Mohd Izuan Effendi, H., & Muhammad Rizal, R. (2012). Heavy metals (mercury, arsenic, cadmium, platinum) in selected marine fish and shellfish along the straits of Malacca. International Food Research Journal, 19 (1), 135–140.
Agency for Toxic Substances and Disease Registry (ATSDR). (2013). Toxicological profile for uranium . Atlanta: U.S. Department of Health and Human Services, Public Health Service. Website: https://www.atsdr.cdc.gov/phs/phs.asp?id=438&tid=77 . Accessed 23 May 2022.
Plum, L. M., Rink, L., & Haase, H. (2010). The essential toxin: impact of zinc on human health. International Journal of Environmental Research and Public Health, 7 (4), 1342–1365.
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K., & Sutton, D. J. (2014). Heavy metals toxicity and the environment. EXS, 101 , 133–164.
Banu, R. J., & Subramanian, L. (2001). Biomanagement of paper mill sludge using anindigenous ( Lampito mauritii ) and two exotic ( Eudrilus eugineae and Eisenia fetida ) earthworms. Journal of Environmental Biology, 22 (3), 181–185.
Patrick, L. (2002). Mercury toxicity and antioxidants: part 1: role of glutathione and alpha-lipoic acid in the treatment of mercury toxicity. Alternative Medicine Review, 7 (6), 456–471.
Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B., & Beeregowda, K. N. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary Toxicology, 7 (2), 60–72.
Albretsen, J. (2006). The toxicity of iron, an essential element. Veterinary Medicine, 101 , 82–90.
Stohs, S. J., & Bagchi, D. (1995). Oxidative mechanisms in the toxicity of metal ions. Free Radical Biology & Medicine, 18 (2), 321–336.
Grazuleviciene, R., Nadisauskiene, R., Buinauskiene, J., & Grazulevicius, T. (2009). Effects of elevated levels of manganese and iron in drinking water on birth outcomes. Polish Journal of Environmental Studies, 18 (5), 819–825.
Kaul, B., Sandhu, R. S., Depratt, C., & Reyes, F. (1999). Follow-up screening of lead-poisoned children near an auto battery recycling plant, Haina, Dominican Republic. Environmental Health Perspectives, 107 (11), 917–920.
Bechara, E. J., Medeiros, M. H., Monteiro, H. P., Hermes-Lima, M., Pereira, B., & Demasi, M. (1993). A free radical hypothesis of lead poisoning and inborn porphyrias associated with 5-aminolevulinic acid overload. Quimica Nova, 16 , 385–392.
Waalkes, M. P., Hiwan, B. A., Ward, J. M., Devor, D. E., & Goyer, R. A. (1995). Renal tubular tumors and a typical hepper plasics in B6C3F, mice exposed to lead acetate during gestation and lactation occur with minimal chronic nephropathy. Cancer Research, 55 , 5265–5271.
Yang, J. L., Wang, L. C., Chamg, C. Y., & Liu, T. Y. (1999). Singlet oxygen is the major species participating in the induction of DNA strand breakage and 8-hydrocy-deoxyguanosine adduct by lead acetate. Environmental and Molecular Mutagenesis, 33 , 194–201.
Mathew, B. B., Tiwari, A., & Jatawa, S. K. (2011). Free radicals and antioxidants: A review. Journal of Pharmacy Research, 4 (12), 4340–4343.
Wadhwa, N., Mathew, B. B., Jatawa, S., & Tiwari, A. (2012). Lipid peroxidation: Mechanism, models and significance. International Journal of Current Science, 3 , 29–38.
Valko, M. M. H. C. M., Morris, H., & Cronin, M. T. D. (2005). Metals, toxicity and oxidative stress. Current Medicinal Chemistry, 12 (10), 1161–1208.
Lash, L. H., Putt, D. A., Hueni, S. E., Payton, S. G., & Zwicki, J. (2007). Interactive toxicity of inorganic mercury and trichloroethylene in rat and human proximal tubules (Effects of apoptosis, necrosis, and glutathione status). Toxicology and Applied Pharmacology, 221 (3), 349–362.
Lund, B. O., Miller, D. M., & Woods, J. S. (1991). Mercury induced H 2 O 2 formation and lipid peroxidation in vitro in rat kidney mitochondria. Biochemical Pharmacology, 42 , S181–S187.
Palmeira, C. M., & Madeira, V. M. C. (1997). Mercuric chloride toxicity in rat liver mitochondria and isolated hepatocytes. Environmental Toxicology and Pharmacology, 3 , 229–235.
Dixit, R., & Malaviya, W. D. (2015). Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability, 7 (2), 2189–2212.
Wu, G., Kang, H., Zhang, X., Shao, H., Chu, L., & Ruan, C. (2010). A critical review on the bio-removal of hazardous heavy metals from contaminated soils: issues, progress, eco-environmental concerns and opportunities. Journal of Hazardous Materials, 174 (1–3), 1–8.
Yang, T., Chen, M., & Wang, J. (2015). Genetic and chemical modification of cells for selective separation and analysis of heavy metals of biological or environmental significance. Trends in Analytical Chemistry, 66 , 90–102.
Ramasamy, K., Kamaludeen, S., & Parwin, B. (2006). Bioremediation of metals microbial processes and techniques. In S. N. Singh & R. D. Tripathi (Eds.), Environmental bioremediation technologies (pp. 173–187). Springer Publication.
Gavrilescu, M. (2004). Removal of heavy metals from the environment by biosorption. Engineering in Life Sciences, 4 (3), 219–232.
Vijayaraghavan, K., & Yun, Y. S. (2008). Bacterial biosorbents and biosorption. Biotechnology Advances, 26 (3), 266–291.
Godlewska-Żyłkiewicz, B. (2006). Microorganisms in inorganic chemical analysis. Analytical and Bioanalytical Chemistry, 384 (1), 114–123.
Cha, J. S., & Cooksey, D. A. (1991). Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proceedings of the National Academy of Sciences of the United States of America, 88 (20), 8915–8919.
Article ADS CAS PubMed PubMed Central Google Scholar
Telwell, C., Robinson, N. J., & Turner-Cavet, J. S. (1998). An SmtB-like repressor from Synechocystis PCC 6803 regulate a zinc exporter. Proceedings of the National Academy of Sciences of the United States of America, 95 (18), 10728–10733.
Teitzel, G. M., & Parsek, M. R. (2003). Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa . Applied and Environmental Microbiology, 69 (4), 2313–2320.
Jyoti, B., & Harsh, K. S. N. (2014). Utilizing Aspergillus niger for bioremediation of tannery effluent. Octa Journal of Environmental Research, 2 (1), 77–81.
Viti, C., Pace, A., & Giovannetti, L. (2003). Characterization of Cr(VI)-resistant bacteria isolated from chromium-contaminated soil by tannery activity. Current Microbiology, 46 (1), 1–5.
Barkay, T., Miller, S. M., & Summers, A. O. (2003). Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiology Reviews, 27 (2–3), 355–384.
Jasu, A., & Ray, R. R. (2021). Biofilm mediated strategies to mitigate heavy metal pollution: a critical review in metal bioremediation. Biocatalysis and Agricultural Biotechnology, 37 , 102183.
Download references
This work was supported by an Early Career Research grant (ECR/2016/001598) to Dr. Rudra P. Saha from DST-SERB, India.
Author information
Rima Roy and Saikat Samanta contributed equally to this work.
Authors and Affiliations
Department of Biotechnology, School of Life Science & Biotechnology, Adamas University, Kolkata, 700126, India
Rima Roy, Saikat Samanta, Srijoni Banerjee & Rudra P. Saha
Department of Life Sciences, School of Basic Sciences and Research, Sharda University, Greater Noida, 201306, India
Soumya Pandit & Tahseena Naaz
Department of Life Sciences, Graphic Era Deemed to Be University, Dehradun, 248002, Uttarakhand, India
Janhvi Mishra Rawat
Division of Research and Innovation, School of Applied and Life Sciences, Uttaranchal University, Dehradun, Uttarakhand, 248007, India
Kundan Kumar Chaubey
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Rima Roy or Rudra P. Saha .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Roy, R., Samanta, S., Pandit, S. et al. An Overview of Bacteria-Mediated Heavy Metal Bioremediation Strategies. Appl Biochem Biotechnol 196 , 1712–1751 (2024). https://doi.org/10.1007/s12010-023-04614-7
Download citation
Accepted : 19 June 2023
Published : 06 July 2023
Issue Date : March 2024
DOI : https://doi.org/10.1007/s12010-023-04614-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Bioremediation
- Heavy metal toxicity
- Microorganisms
- Bioaccumulation
- Bioabsorption
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 27 February 2023
Microbial bioremediation as a tool for the removal of heavy metals
- Mohamed I. Abo-Alkasem ORCID: orcid.org/0000-0002-0077-6621 1 ,
- Ne’mat H. Hassan 2 &
- Mostafa Mostafa Abo Elsoud 3
Bulletin of the National Research Centre volume 47 , Article number: 31 ( 2023 ) Cite this article
13k Accesses
14 Citations
Metrics details
The demand for designing a new technology that can emphasize the complete removal of heavy metals increased as a result of the industrial revolution. Bioremediation was found to have a potent impact on the degradation of organic and inorganic environmental pollutants.
Bioremediation is a multidisciplinary technology that possesses safe, efficient, and low-cost characteristics. Also, one of the important features of bioremediation technology is the in-situ treatment which reduces the possibility of transmitting the contaminants to another site. The application of genetic engineering, to engineer a microorganism to acquire the ability to remove different types of heavy metals at a time or to generate a transgenic plant, is considered one of the new promising bioremediation approaches.
Short conclusion
Removal of heavy metal pollution still represents a big challenge for ecologists that’s why this review shed some light on bioremediation technology; its importance, mechanism of action, and prospects.
The world accelerated industrial revolution and the uses of natural resources during metal mining and industry have a great impact on the environment due to heavy metal pollution. Today, one of the most destructive effects facing the world is the contamination with heavy metals, which reaches the air, soil, and water (Asha and Sandeep 2013 ; Raghunandan et al. 2014 , 2018 ). Although trace concentrations of some metals have a vital effect on the health of living organisms, high levels of heavy metals represent toxic effects too (Ahemad 2019 ; Ahuti 2015 ). Also, heavy metals can hardly be degraded in the soil, so their complete detoxification represents a challenge to scientists. Despite the efforts spent to tackle the environmental pollution issue, the world still suffers from the hazardous effects of heavy metals, and so a new technology should be discovered to contain the disaster of heavy metal contamination, one of which is the bioremediation (Raghunandan et al. 2014 , 2018 ).
Several methods have been accomplished to remediate heavy metals pollution, among them Physico-chemical (conventional) methods such as ion exchange, redox, electrochemical techniques, membrane filtration, and precipitation (Nissim et al. 2018 ; Qasem et al. 2021 ). The disadvantages of the conventional methods are the inability of these methods to detoxify heavy metals permanently (Sun et al. 2020 ), in addition to the cost-effectiveness and the hazardous by-products produced by the elimination process. However, the conventional method is considered effective for large areas contaminated with small amounts of heavy metals and for highly polluted local areas (Huët and Puchooa 2017 ). Consequently, building a new technology that emphasizes the complete removal of heavy metals represents a challenge for scientists. Interestingly, microbial remediation of heavy metal has a far-reaching progressive prospect among the decontamination methods. Microorganisms especially soil microbes can tolerate high levels of heavy metals, some microorganisms need certain types of metals as a micronutrient (i.e., Fe 3+ is essentially utilized by all bacteria while Fe 2+ is significant for anaerobic bacteria) to perform their metabolic activities (Ahemad 2019 ). The bioremediation process could be conducted Ex-situ by transferring the contaminated area to be treated or even in situ by delivering the biological source to the polluted land (Shannon and Unterman 1993 ; Naz et al. 2005 ). Most microorganisms follow two common mechanisms in the bioremediation process; metal sequestering or immobilization and enhancement of solubility properties of the metal, other organisms oxidize or reduce the heavy metals to a less toxic form (Donald 2013 ). The bioremediation process also could be accomplished in aerobic and anaerobic environments; however, the aerobic environment was found to be more efficient and faster than anaerobic conditions.
Definition of heavy metals
These can be defined as the elements that have a density higher than 5 g/cm 3 , also the metals or metalloids which have an atomic mass greater than 4000 kg m −3 or 5 times larger than water are considered heavy metals (Paschoalini and Bazzoli 2021 ). A lot of elements fall into this class however, only a few metals (arsenic (As), cadmium (Cd), chromium (Cr), cobalt (Co), copper (Cu), lead (Pb), mercury (Hg), nickel (Ni), tin (Sn), vanadium (V) and zinc (Zn).) commonly existed in the contaminated air, water, and soil. These metals could be found in many forms; insoluble such as carbonate, oxides, silicate, and sulfides, or soluble such as salt forms (Arfala et al. 2018 ), also, heavy metals when persisted in their ionic state (e.g., Cd +2 , Pb +2 , Hg +2 , As +3 ) represent the most toxic form as they combined with bio-molecules and for a complex harder to be dissociated (Duruibe et al. 2007 ). Recently, researchers paid great attention to studying the diffusion phenomenon and mobility through soil layers and in aquifers (Cuevas et al. 2012 ). According to the European Environment Agency reports, industrial process and product use, energy production and distribution, and energy use in industry are the most contributed sectors in the emission of Cd, Hg, and Pb as represented in Fig. 1 . However, road transportation, commercial, institutional, and households have a significant contribution in Pb emission (EEA 2019).
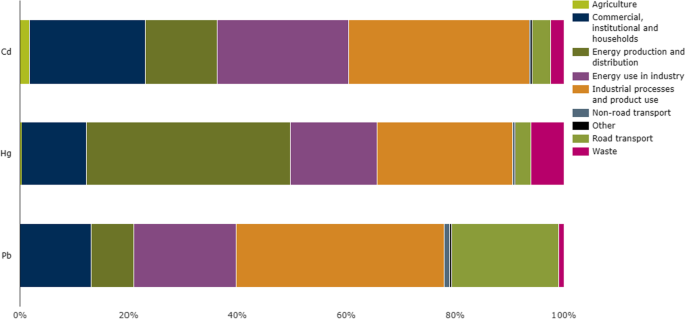
Effect of different life sectors on the emission of Cd, Hg, and Pb in the environment (EEA, 2019 )
Effect of heavy metals on living organisms
Heavy metals with trace concentrations are considered micronutrients that are essential and have nutritional value for some metabolic processes of living cells (Ray and Ray 2009 ), however, elevated levels may have an adverse impact on the health of aquatic and terrestrial living organisms and the environment as they cause dangerous morbidity and mortality (Wang et al. 2006 ; Ray and Ray 2009 ). Heavy metals could be transported to the living cells through the air, water, and food chains and consequently, they alter the physical and chemical properties of the transported material. Heavy metal pollution affects the ecosystem balance by reducing the microbial population of the soil which participates in decomposing the organic material used in crops growing, and so they indirectly affect the food chain of other living organisms, thereby, the world health organization (WHO) and the United States Environmental Protection Agency (USEPA) assigned the acceptable limit for different heavy metals in water as represented in Table 1 . Some metals can destruct living cells directly such as mercury, cadmium, lead, and chromium others have indirect effects such as zinc a corrosive material, and arsenic which pollute catalysts (Hogan 2010 ).
Effect of heavy metals on human
Heavy metals exert their effects by interfering with the function of the organs, however, some of these metals are useful at low concentrations such as arsenic, copper, nickel, iron, etc. (Ray and Ray 2009 ), however, at a high concentration, these metals become cytotoxic as well as carcinogenic for the cells, especially after long term exposure (Jaishankar et al. 2014 ; Valko et al. 2016 ).
Malfunction of human organs is the predominant phenomenon of infected bodies, Zinc for example causes severe gastrointestinal, kidney, brain, respiratory, and heart damage (Hrynkiewicz and Baum 2014 ). Cadmium has the same effect in addition to hypophosphatemia and causes damage to the central nervous system (Hrynkiewicz and Baum 2014 ). Arsenic and mercury damage the liver, the heart, and the central nervous system and cause hypophosphatemia and cancer (Tamele and Vázquez Loureiro 2020 ). Lead which is commonly introduced to the environment in different forms such as mining, lead smelting, ceramic and glass industries, ammunition, storage battery, and tetraethyl-lead manufacturing (Held and Don 2000 ) has a destructive effect on the liver, the heart, and the central nervous system and cause hypophosphatemia, cancer, and anemia (Koning et al. 2001 ; Iranzo et al. 2001 ; Hrynkiewicz and Baum 2014 ). A disastrous disease has already emerged due to heavy metal pollution such as “Itai Itai” in Japan as a result of Cd pollution (Gautam et al . 2015 ), “Arsenecosis” in Bangladesh due to As, and “Minimata” in Japan due to Hg (Volesky 1990 ).
Effect of heavy metals on plants
Physiological dysfunction and malnutrition are the most important disorders that affect plant growth due to excessive concentration of heavy metal pollution, also the disturbance in the ecological balance between plants and microorganisms has a great impact on crops. Malfunctions of the vital physiological processes such as Photosynthesis, and respiration may lead to the degradation of the major organelles following plant death (Glombitza and Reichel 2013 ). As a consequence of the excessive intake of heavy metals by plants, human and animal health will be affected (Babak et al . 2013 ).
Toxicity of heavy metals to the microorganisms
Heavy metals also have a great impact on the growth of microorganisms depending on the type and concentration of the polluted source. Different mechanisms were found to be involved in the toxicity of heavy metals such as dysfunction of enzymatic reactions, production of reactive oxygen species (ROS) which function as soluble electron carries, induction of oxidative damage that may cause changes in DNA and protein formation (Gauthier et al. 2014 ; Hildebrandt et al. 2007 ). Also, heavy metal toxicity affects the transcription and translation of DNA by charging the phosphate group negatively using electrostatic interaction which may cause mutagenesis (Genchi et al. 2020 ), causing acute hurt to the cell membrane and cytoplasmic molecules. Hence, exposure to heavy metals can affect both morphological, biochemical, and physiological properties (Frimmel 2003 ; Fashola et al. 2016 ).
Principles of the bioremediation process
Bioremediation can be defined as the use of biological diversity, directly or indirectly, to convert toxic pollutants into a harmless form (Asha and Sandeep 2013 ), so bioremediation is a holistic approach that includes plant, fungi, bacteria, actinomycetes, and algae all of them could be used as a biological agent to detoxify heavy metals. Two different strategies are utilized to remediate toxic pollutants; in-situ, where the process of decontamination occurred at the contaminated place itself by bringing the biological agent to the site of contamination or promoting the indigenous organisms to deal with contaminants by facilitating the suitable condition for their propagation. The second one is ex-situ , by which the contaminated place is transferred away to another site to be processed (Kumar et al. 2011a , b ; Kumar et al. 2016 ; Raghunandan et al. 2014 , 2018 ). There are many mechanisms by which the organism can manipulate the detoxification process, however, the utilization of the toxic metal by the microorganism as a source of nutrition is the main concept (Sun et al. 2020 ). So, microbial bioremediation is considered a multidisciplinary field that required more research and investigations.
Types of bioremediations
Bioremediation is classified, according to the site at which the bioremediation process occurred, into two different strategies:
- In-situ bioremediation
This strategy corresponded with treating the polluted surfaces where they are located, this strategy depends on detoxifying the dissolved and sorbed pollutants directly by the microorganism, it can be applied in groundwater, unsaturated and saturated soils, also it is considered an efficient method to remediate organic chemicals in contrary to ex-situ strategy (Brar et al. 2006 ), also in-situ bioremediation expanded to treat inorganic and toxic metals. Moreover, the application of microorganisms that have a chemotactic ability to facilitate moving into the contaminated areas and hence the degradation of harmful compounds will be safer (Kulshreshtha et al. 2014 ). Furthermore, stimulating the reduction of heavy metals at the place minimizes the chance of contaminant transportation downgradient. A challenging issue facing in-situ bioremediation is the selection of one organism or a consortium of organisms that has the potential ability to detoxify the targeted metals. In lab-scale, it was found that Fe 3+ and sulfate-reducing microorganisms have the enzymatic ability to biodegrade some heavy metals such as U(VI), Tc (VIII), Cr (VI), and Co (III) (Gorby et al. 1998 ; Tebo and Obraztsova 1998 ; Lloyd et al. 2000 ). Also, species of Geobacteraceae were found to be a dominant group during the stimulation process for reducing Fe 3+ , also, the members of this group were detected in the stimulation process to reduce U(VI) of contaminated Aquifer. So, the Geobacteraceae group was considered to play an important role in stabilizing contaminants and reducing metals within subsurface environments (He et al. 2019 ). The following are some techniques used for “in-situ” bioremediation:
Biosparging
Biosparging system is Constructed by injecting the air through a pipe below the water table which enhances the growth of indigenous microbes due to elevated oxygen concentration (Jain et al. 2012 ). Also, it differs from bioventing in mixing the soil and the groundwater by injecting the air in the saturated area, which allows the movement of volatile organic compounds upward to the unsaturated area this process is affected by the biodegradability of the contaminants and soil characteristics. This system possesses low construction cost and flexibility in adapting the design (Atlas and Philp 2005 ).
Bioventing is a system that stimulates the existing soil microorganisms to degrade the source of pollution via injecting a limited amount of oxygen that sustains microbial activity (Jain et al. 2012 ). Injection of air is conducted in the unsaturated area in addition to supplementing it with nutrients and moisture (Philp and Atlas 2005). Bioveting could be more efficient in anaerobic biodegradation, also mixing nitrogen with oxygen will increase the potency of chlorinating remediation (Mihopoulos et al. 2000 , 2002 ; Shah et al. 2001 ).
Bioaugmentation
Bioaugmentation is the application of outsourcing microbial strains that naturally occurred or are genetically engineered to decontaminate polluted soil or water. Treatment usually utilizes a consortium of microorganisms that produce all the required enzymes and degradative pathways. Bioaugmentation is used to treat municipal wastewater, soil, and groundwater polluted with chlorinated ethenes which are degraded to nontoxic ethylene and chloride (Jain et al. 2012 ).
Intrinsic bioremediation
Intrinsic bioremediation is defined as the stimulation of naturally occurring organisms by providing nutritional materials and oxygen to remediate heavy metals without attribution of any engineering steps (Riseh et al. 2022 ).
Engineered bioremediation
Engineered bioremediation is the adaptation of physicochemical conditions to enhance the propagation of introduced microorganisms to accelerate the bioremediation process.
Advantage of in-situ bioremediation
Cost-effectiveness of in-situ bioremediation
It can be used to treat large contaminated areas which could reach inaccessible regions.
Treating a wide variety of wastes, it may be used the decontaminate organic and inorganic wastes.
In-situ bioremediation is faster than the pump-and-treat method.
Challenges facing in-situ bioremediations
Limitations in depending on indigenous microorganisms as their metabolic activity could be inhibited by high levels of heavy metals.
Some pollutants may be bio-transformed due to microbial metabolic activity to an intermediate which could be more toxic and mobile than the original form.
In-situ bioremediation could be inappropriate in treating some contaminants such as recalcitrant.
In-situ bioremediation is most suitable for low-level scenarios of pollution (Kulshreshtha et al. 2014 ).
- Ex-situ bioremediation
The core concept of this strategy is to treat the contaminated site by the excavation of soil to enhance microbial degradation. Five techniques were used in this strategy.
Slurry-phase
This technique relies on excavating the contaminated soil and mixing it with water and transporting the mixture to a bioreactor, followed by stone and rubble removal. The amount of water depends on the pollutant's type and concentration, the soil's nature, and the biodegradation rate. This process is followed by the separation of the soil by filtration or centrifugation, the soil is dried and retransferred to its original location, and the fluids are submitted to a further treatment step (EPA 2003 ).
Solid-phase
This technique involves three steps: excavation of the soil, followed by putting the soil into piles, the soil may contain municipal, agricultural, and organic wastes, followed by stimulation of the biodegradation process by supplying oxygen through a network of pipes to enhance microbial respiration and subsequently microbial activity. Solid-phase bioremediation requires a large space and a long time to be completed (Hyman and Dupont 2001 ).
Landfarming
This technique relies on the stimulation of indigenous organisms spread over the surface by supplementing the excavated soil with suitable nutrients and minerals, the excavated soil should be periodically tilled to stimulate the biodegradation process.
Soil biopiles
This technique is almost similar to landfarming bioremediation except in using above-ground piles and perforated pipes to inject air through the soil (Verma 2022 ). Application of this technique is interestingly valuable because of its low cost and full control of nutritional feed, aeration, and temperature (Whelan et al. 2015 ), also it’s the technique of choice in treating contaminated sites of extreme environments and in treating low molecular weight compounds by limiting volatilization (Gomez and Sartaj 2014 ).
Composting bioremediation
Composting bioremediation is quite similar to landfarming bioremediation in excavating the contaminated soil to the surface and stimulating the indigenous microorganisms through feeding of nutrients and injecting air but differs in supplementing the soil with a bulk of additives such as corncobs, straw, and hay, this additive helps in oxygen distribution through the soil, maintaining the moisture content constant and turning frequency, however, application of composting process for biodegradation of volatile pollutants is not favorable because of the periodic turning during the process (Hobson et al. 2005 ).
Advantage of ex-situ bioremediation
Adequate control of the biodegradation process.
Suitability to detoxify a wide variety of contaminants.
Reduction of time required to complete the treatment process.
Challenges facing ex-situ bioremediation
Limitation of ex-situ bioremediation to biodegrade chlorinated hydrocarbons.
Some types of soils required further processing such as non-permeable soils.
Bioremediation mechanism of action
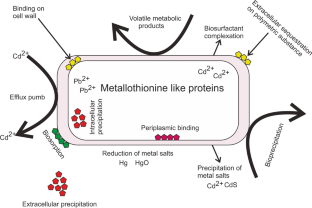
Due to the ubiquitous nature of microorganisms, they play a crucial role in the bioremediation of heavy metals, they can interact with heavy metals using different mechanisms to survive the toxicity of the metals. The two main concepts by which the organism can deal with contaminants are using the contaminant as a source of nutrition and protecting the organism itself (defense mechanism) from the toxic effect ( Alvarez et al. 2017 ). As illustrated in Fig. 2 , the microorganism reacts with the environmental contaminants using direct or indirect mechanisms some of which are biosorption and biotransformation (Tang et al. 2021 ).
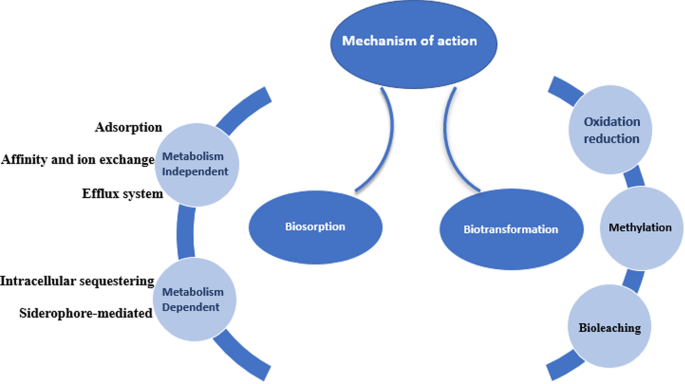
Diagram showing different mechanisms of bioremediation action
- Biosorption
It’s a mechanism by which the organism binds with the metal to form a complex that possesses a nontoxic feature. Certain criteria should be considered and investigated to achieve a potential biosorption mechanism; nature of the biosorbent, sorption capacity, kinetics of sorption, regeneration ability of the sorbent, percentage of metal recovery, cost-effectiveness of biosorption process, and separation flexibility of the biosorbent-metal complex (Bae et al. 2001 , 2002 , 2003 ). Two main categories are involved in the bioremediation process using the biosorption mechanism.
Metabolism-independent biosorption
This type of biosorption depends on the physical and chemical properties of the cell whether it was a live cell or a dead cell, this category involves the following:
Adsorption, also called extracellular sequestration, relies on the affinity between cellular components of the periplasm and the metal ion. Extracellular polymeric substance (EPS) associated with bacterial cell wall plays a significant role in metal adsorption. EPS is composed of polysaccharides, mucopolysaccharides, and proteins. It also contains a lot of functional groups (hydroxyl, carboxyl, amine, and phosphoric groups) that facilitate heavy metal sequestering (Guine et al . 2006 ).
Affinity and ion exchange by which the biosorbent (cellular component) binds with the metal ion, Cunninghamella were found to have a promising binding ability to heavy metals released textile wastewater (Tigini et al. 2010 ), also Saccharomyces cerevisiae can degrade Cd(II) and Zn(II) using the ion-exchange method.
Efflux system as a type of extracellular sequestration is one of the most important methods by which the organism can defend against the toxic effect of heavy metals by forming an outer protective material and ejecting the metal ion out of the cytoplasm to the periplasmic region (Dixit et al. 2015 ). Ma et al. ( 2016 ) reported that transformation and efflux are the basic methods usually used in bacterial resistance to heavy metals.
Metabolism-dependent biosorption
This mechanism is associated with the metabolic activity of a viable microorganism contrary to metabolism-independent biosorption.
Intracellular sequestering (Bioaccumulation), is a process by which the complex form of cell-metal occurred inside the cytoplasm (Ramasamy and Banu 2007 ), as reported by (Abo-Alkasem et al. 2022a ) and illustrated in Fig. 3 , examination of Salipaludibacillus agaradhaerens strain NRC-R cells using the Transmission Electron Microscope (TEM) showed the accumulation of chromium inside the cell which also confirmed by EDX analysis, accumulation of metals conducted by attaching with the cell surface follows slow penetration to periplasm to the cell cytoplasm by a process that looks like nutrient uptake (Mishra and Malik 2013 ), it was reported that cysteine-rich protein plays an important role in sequestering Zn, Cd, and Cu in cadmium-tolerant Pseudomonas putida, also, glutathione helps in sequestering Cd by Rhizobium leguminosarum. Fungi also play a vital role in inorganic metal elimination using their rigid cell wall which works as a ligand in the decontamination process.
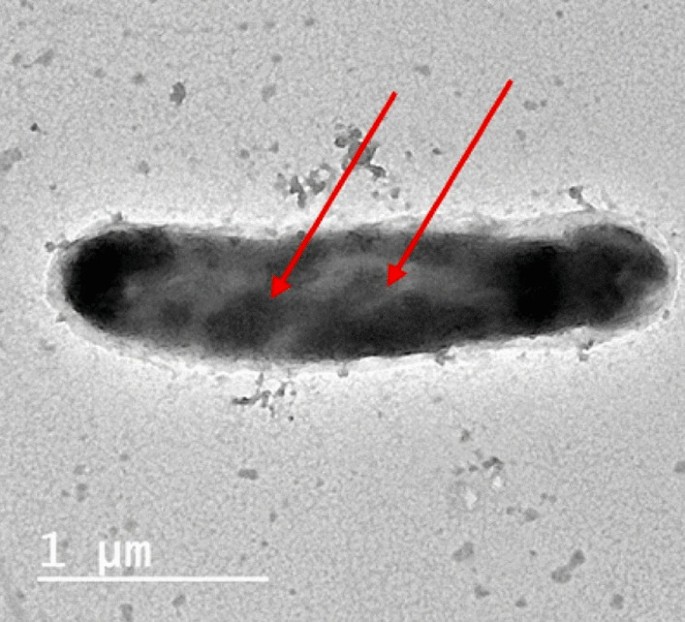
TEM image of the cells grown in the presence of Cr (VI) (Abo-Alkasem et al. 2022a )
Siderophore-mediated biosorption, also called a chelating agent, in aerobic soils some microorganisms produce siderophores that mediate the ability of the microorganisms to utilize low water-soluble metals using an energy-dependent process (John et al. 2001 ). Microbacterium flavescens was found to use siderophore to uptake their nutritional requirements of iron, also the organism uses the siderophore desferrioxamine-(DF) to bind with uranium, plutonium, and iron.
- Biotransformation
Biotransformation relies on the cellular metabolic activity of the microorganism through the redox mechanism, reduction of metals by changing the oxidation number of the metal is common in nature, such as the reduction of chromium (Abo-Alkasem et al. 2022a , b ), selenium (Lloyd et al. 2001 ), uranium (Chang et al. 2001 ) and mercury (Brim et al. 2000 ).
Oxidation and reduction mechanisms
The mechanism by which the microorganism works as an oxidizing agent by releasing electrons that react with the anions in the contaminated soil is the same mechanism utilized to decontaminate organic compounds under anaerobic conditions (Lovley and Phillips 1988 ). However, it was found that the presence of iron (III) stimulates the degradation process (Spormann and Widdel 2000 ). The reduction could be occurred directly using a bioreactor, (pump and treat) or after the excavation of soils, inoculated with the appropriate microbial consortium, or indirectly using sulfate-reducing bacteria which plays an important role in the ecological balance directly by sulfate reduction or indirectly by the formation of biofilms (Abo Elsoud and Abo-Alkasem 2022 ). The indirect mechanism is more favorable due to its cost-effectiveness and eco-friendly method (Asha and Sandeep 2013 ). Decontamination of uranium by Desulfosphorosinus spp. And Closteridium spp is an applicable example of utilizing sulfate-reducing bacteria (Prasad and Freitas 2003 ).
Methylation of metals (volatilization)
Volatilization of metal by microbial methylation plays a significant role in metal remediation, for instance, some Pseudomonas spp., Escherichia spp., Clostridium spp . , and Bacillus spp . can convert Hg (II), Se, As, and Pb to a gaseous methylated form (Ramasamy and Banu 2007 ).
Bioleaching
Bioleaching is the secretion of low molecular weight compounds that aid the transformation of a toxic form of metals to a nontoxic form by dissolution or precipitation mechanisms, (Chanmugathas and Bollag 1988 ) reported that leaching of Cd is promoted by the secretion of organic acids by some microorganisms, also the production of inorganic phosphate by Citrobacter organism leads to precipitation of metal phosphate coat.
Plant-microbial remediation
Rhizoremediation is the association of microorganisms with plants to improve the potential of the bioremediation process and it now plays a crucial role in environmental bioremediation due to cost-effectiveness and outstanding efficiency (Nie et al. 2011 ; Marihal and Jagadeesh 2013 ; Prabha et al. 2017 ).
The capability of microorganisms to develop a symbiotic relationship enhances the biodegradability of different types of contaminants (Kumar et al. 2017 ). The predominant type of organisms associated with the plant-microorganism relationships is mycorrhizal fungi which can bio-sorb heavy metals (Bojorquez and Voltolina 2016 ). The potentiality of rhizoremediation was reported by Joner and Leyva ( 1997 ) who found that mycorrhizal plants when subjected to soil contaminated with Cd 2+ 1, 10, and 100 mg/kg, Cd uptake of mycorrhizal was higher than non-mycorrhizal plants by 90%, 127%, and 131% respectively. The mechanisms utilized in rhizoremediation are mainly through the activation of metal phosphates, acidification, production of organic acids, chelating agents, and ion carriers.
Microorganisms responsible for bioremediation
In nature, the presence of microorganisms guarantees the retrieval of ecological balance and the removal of contaminants that hinder biological life. The use of microorganisms for the removal of contaminants from the environment is described as "Bioremediation". The concept of environmental remediation using microorganisms was first registered as a patent in 1981 for the degradation of petroleum oil by Pseudomonas putida (Prescott et al. 2002 ; Glazer and Nikaido 2007 ). Bioremediation aims to stimulate microbial metabolic activity, with nutrients or other chemical agents, to be able to remove, destroy, or neutralize the effect of these contaminants. The microorganisms used for bioremediation should not only be able to tolerate a wide concentration range of the contaminant(s) but also be physiologically active. Once favorable conditions are obtained, the metabolic activity and growth rate of these microorganisms reach alarming levels as well as the bioremediation process. Many theories have been illustrated for the mechanism of microbial tolerance to heavy metals. These theories include the accumulation and formation of non-toxic complexes with the metal ions inside the cells, the efflux of toxic metals outside the cell, biotransformation of the toxic metal into a less toxic form, or methylation and/or de-methylation.
In nature, the type of micro-flora (microbial consortium) is a significant factor affecting the tolerance and rate of heavy metal bioremediation depending on the gene and metabolic diversity (Juwarkar et al. 2010 ). Two types of microorganisms are used for heavy metal bioremediation based on their sources: indigenous (microorganisms present in the site of contamination and have bioremediation capability) and extraneous (microorganisms introduced into the site of contamination and have bioremediation capability), Table 2 summarizes some of the organisms used in the bioremediation process and their target pollutants. The utilization of indigenous microorganisms excludes the need for continuous monitoring according to Asha and Sandeep ( 2013 ). After the bioremediation process, the soil and/or water retrieve their ability to be reused in various activities.
It was reported that many microorganisms including bacteria, Actinomycetes, fungi, yeast, and algae can remediate heavy metals from soil and water:
Endophytic bacteria and Plant Growth Promoting Rhizobacteria (PGPR) are the most common bacterial strains associated with heavy metal bioremediation.
The endophytic bacteria colonize the sub-epidermal layer of the plant tissues (Schulz and Boyle 2006 ). The presence of endophytic bacteria helps the protection of the plant cells from heavy metals stress conditions (Ryan et al. 2008 ). They diminish or remove the phytotoxicity of the heavy metals by altering their phyto-availability (Weyens et al. 2009 ; Ma et al. 2011 ) such as some species of Pseudomonas, Bacillus, and Rahnella that showed high resistance to Pb, Mn, and Cd (Luo et al. 2012 ; Yuan et al. 2014 ; Babu et al. 2015 ).
On the other hand, PGPR comprises a group of free-living, symbiotic, or endophytic bacteria (Glick 2012 ). For example, Bacillus, Enterobacter, Erwinia, Flavobacterium, Klebsiella, Gluconacetobacter, and Pseudomonas (Nadeem et al. 2010 ) can mitigate the toxicity of heavy metals, improve plant growth in heavy metal-contaminated soils (Seth 2012 ) and produce phytohormones and siderophores and help phosphate solubilization (Ullah et al. 2015 ).
Actinobacteria
In addition to their well-known ability to utilize complex organic matter as a carbon and energy source (Kieser et al. 2000 ), Actinobacteria, such as Amycolatopsis , Corynebacterium, Rhodococcus, and Streptomyces , can tolerate and remediate heavy metals, such as Hg(II), Co(II), Cd(II), Cr(VI), Zn(II) and Ni(II) (Oyetibo et al. 2010 ; Alvarez et al. 2017 ).
Some fungal strains have been reported to possess metal chelating and sequestrating systems that increase their heavy metal tolerance and biotransformation into a less toxic form such as Allescheriella, Pleurotus, Phlebia, and Stachybotrys (D’Annibale et al. 2007 ). The hyphal and high biomass growth adds an advantage to this type of microorganism as it allows simple harvest along with the attached heavy metals (Aly et al. 2011 ). Aspergillus, Penicillium, Cephalosporium, and Rhizopus are the most studied fungal genera for their potential activity in the removal of heavy metals, such as Pb 2+ and Zn 2+ , from aqueous solutions and soils (Volesky and Holan 1995 ; Huang and Huang 1996 ; Tunali et al. 2006 ; Akar et al. 2007 ).
Factors affecting the bioremediation process
To confine the biodegradation potential on selecting the most appropriate method, mechanism, and technique without paying attention to the factors that may affect the utilized application, limit the efficiency of the bioremediation process. A lot of factors could exhibit significant effects on the bioremediation process, for instance, metal ion concentration, valance state and chemical forms of the metal, the bioavailability of the metal, redox potential, availability of low molecular weight organic acids, and environmental factors such as temperature and pH (Bandowe et al. 2014 ).
Substrate concentration
To establish the process of bioremediation, bio-sorbent accumulation features should be quantified, two models could be used; the Langmuir model mainly defines adsorption by assuming an adsorbate behaves of the single-layer (Acar and Malkoc 2004 ), and the Freundlich model which mainly estimates the adsorption equilibrium (Febrianto et al. 2009 ). However, the main concept is that the adsorption efficiency increases with the increment of heavy metal concentration until a certain value.
Type of the substrate
The efficiency of the adsorption mechanism is affected by the type of soil, the type of heavy metal, and the type of soil additives. since the adsorption between the soil and heavy metals may lower the mobility of heavy metals and hence reduce microbial adsorption (Hu et al. 2010 ). Also, soil additives have a significant effect on heavy metals removal, Tyagi et al. ( 2014 ) found that increasing FeSO 4 .7H 2 O higher than 20 gm/l has an adverse effect on the leaching rate of Cu and Zn.
The potential of hydrogen (pH) plays a vital role in both microbial activity and metal characteristics. Growing of microorganisms in unfavorable pH may affect the enzyme activity thereby lowering the rate of microbial metabolism, also, the charge of the microorganism surface will be changed that affects the binding capacity between the adsorbent and heavy metals (Bandowe et al. 2014 ; Galiulin and Galiulina 2008 ). Furthermore, changes in pH value may alter metal mobility and hydration as the metals tend to be free ionic at acidic pH (Bandowe et al. 2014 ; Dermont et al. 2008 ). According to (Rodríguez-Tirado et al. 2012 ; Wierzba 2015 ), the adsorption capacity of Pb 2+ and Zn 2+ increased by raising the pH value to 5.5, however, an observed decrease in the removal of metals was recorded upon increasing pH value over (5.5).
Temperature
Temperature is an important parameter in adjusting the optimum conditions for microbial growth, metabolism, and enzyme activity (Fang et al. 2011 ), increasing temperature affects the diffusion of metals across different layers and also, increases the bioavailability of metals. However, the optimum biodegradation temperature differs according to the type of metal, for instance, the biodegradation of Cd 2+ by Bacillus jeotgali was the highest at 35 °C, however, it was 30 °C for Zn 2+ biodegradation (Chanmugathas and Bollag 1988 ).
Role of biotechnology in the bioremediation process
Biotechnology is the discipline of using the engineering of scientific principles to improve the efficiency of organisms to serve humans and remediate the environmental toxic substance (McHughen 2016 ), by using genetic engineering, one of the biotechnology approaches, a single organism can be engineered to produce all the needed enzymes or to utilize all the degradative pathways for bioremediation process (Dangi et al. 2019 ). The purpose of utilizing genetic tools is to enhance efficiency and reduce the cost and time of the bioremediation process.
Degradation of Polychlorinated biphenyls (PCBs) is controlled by to group of genes that were found in the genetic material of two different organisms, thereby, using genetic engineering for achieving recombination between Pseudomonas pseudoalcaligenes KF707 and Burkholderia cepacia LB400 bph genes may enhance the degradation rate of PCBs and stimulate the remediation of toluene and benzene (Seeger et al. 2010 ), also the application of DNA probes helps in accelerating the process of the isolation and identification of a particular strain from a mixed population (Dua et al. 2002 ). Another example of using biotechnology is the fusion between metallothionein (MT) isolated from rats, IgA protease protein isolated from Neisseria gonorrhoeae, and the fusion vehicle lpp-ompA to provide the bacterial cell wall with metal ion-binding polypeptides (Bae et al. 2000 and Valls et al. 2000 ).
Another discipline of biotechnology involves the use of transgenic plants in the bioremediation process, this could be conducted by transferring a desirable gene from different sources (other plants, microorganisms, or even animals) to improve the ability of the plant to remove the toxic pollutant (Truu et al. 2015 ) this process of transmission increases the phytoremediation ability of the plant (Dixit et al. 2015 ).
Immobilized microorganism technology
Immobilization is one utilized technique in bioremediation, it possesses stability of the biological cell, also the immobilized cell did not compete with indigenous organisms, therefore it is considered eco-friendly and has high degradation efficiency (lone et al. 2008 ).
Advantage of bioremediation
A natural bioprocess is characterized by a safe effect on the environment which makes it globally accepted as a technique for treating wastes.
The consumed energy is lower than the technologies.
Cost-effectiveness is one of the most bioremediation features.
Several types of pollutants could be eliminated at the same time.
Minimize the risk of transferring the contamination from one site to another.
Disadvantages of bioremediation
Several factors could affect the efficiency of the bioremediation process.
Elimination of toxic metals to be achieved could take a lot of time.
Limited to those contaminates that can be biodegradable.
Biodegradation capacity and efficiency cannot be predicted because of dealing with a live organism (Zeyaullah et al. 2009 ).
Conclusions
Great efforts were spent during the last few decades to address the problem of heavy metal pollution by developing new strategies to fix this issue, however, the application of bioremediation techniques still represents the most favorable strategy due to the cost-effectiveness and safety impacts of bioremediation techniques on the environment and also due to the variability of bioremediation mechanisms which makes these techniques applicable and affordable, this article enumerates different types of bioremediation and the advantage and disadvantage of these types also the suitability of these types to different environments and conditions moreover, the article summarizes some of the mechanisms of action of different bioremediation techniques in addition to the microorganisms that play an important role and the factors that may affect the bioremediation process and how the newly developed technologies can improve the bioremediation techniques to be more efficient.
Availability of data and materials
Not applicable.
Abbreviations
Deoxyribonucleic acid
Desferrioxamine
Extracellular polymeric substance
Plant Growth Promoting Rhizobacteria
Polychlorinated biphenyls
Reactive oxygen species
The United States Environmental Protection Agency
World Health Organization
Abo Elsoud MM, Abo-Alkasem MI (2022) Environmental sulfate-reducing microorganisms. In: Ahamed MI, Prasad R (eds) Application of Microbes in environmental and microbial biotechnology. Environmental and Microbial Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-16-2225-0_23
Chapter Google Scholar
Abo-Alkasem MI, Maany DA, El-Abd MA, Ibrahim AS (2022a) Bioreduction of hexavalent chromium by a novel haloalkaliphilic Salipaludibacillus agaradhaerens strain NRC-R isolated from hypersaline soda lakes. 3 Biotech 12(1):1–15
Article Google Scholar
Abo-Alkasem M, El-Abd M, Maany D, Ibrahim A (2022b) Hexavalent Chromium Reduction by a Potent Novel Haloalkaliphilic Nesterenkonia sp strain NRC-Y Isolated from Hypersaline Soda Lakes. Egypt J Chem. https://doi.org/10.21608/ejchem.2022.136986.6039
Acar FN, Malkoc E (2004) The removal of chromium (VI) from aqueous solutions by Fagus orientalis L. Biores Technol 94(1):13–15
Article CAS Google Scholar
Achal V, Kumari D, Pan X (2011) Bioremediation of chromium-contaminated soil by a brown-rot fungus. Gloeophyllum Sepiarium Res J Microbiol 6(2):166
CAS Google Scholar
Ahemad M (2019) Remediation of metalliferous soils through the heavy metal resistant plant growth promoting bacteria: paradigms and prospects. Arab J Chem 12(7):1365–1377
Ahuti S (2015) Industrial growth and environmental degradation. Int Educ Res J 1(5):5–7
Google Scholar
Akar T, Tunali S, Çabuk A (2007) Study on the characterization of lead (II) biosorption by fungus Aspergillus parasiticus . Appl Biochem Biotechnol 136(3):389–405
Article CAS PubMed Google Scholar
Al-Garni SM, Ghanem KM, Ibrahim AS (2010) Biosorption of mercury by capsulated and slime layerforming Gram-ve bacilli from an aqueous solution. Afr J Biotech 9(38):6413–6421
Alvarez A, Saez JM, Costa JSD, Colin VL, Fuentes MS, Cuozzo SA, Amoroso MJ (2017) Actinobacteria: current research and perspectives for bioremediation of pesticides and heavy metals. Chemosphere 166:41–62
Article ADS CAS PubMed Google Scholar
Aly AH, Debbab A, Proksch P (2011) Fungal endophytes: unique plant inhabitants with great promises. Appl Microbiol Biotechnol 90(6):1829–1845
Arfala Y, Douch J, Assabbane A, Kaaouachi K, Tian H, Hamdani M (2018) Assessment of heavy metals released into the air from the cement kilns co-burning waste: case of Oujda cement manufacturing (Northeast Morocco). Sustain Environ Res 28(6):363–373
Asha LP, Sandeep RS (2013) Review on bioremediation-potential tool for removing environmental pollution. Int J Basic Appl Chem Sci 3(3):21–33
Ashokkumar P, Loashini VM, Bhavya V (2017) Effect of pH, Temperature and biomass on biosorption of heavy metals by Sphaerotilus natans. Int J Microbiol Mycol 6(1):32–38
Atlas RM, Philp J (2005) Bioremediation. Applied microbial solutions for real-world environmental cleanup. ASM press
Book Google Scholar
Babák L, Šupinova P, Zichova M, Burdychova R, Vítová E (2013) Biosorption of Cu, Zn and Pb by thermophilic bacteria–effect of biomass concentration on biosorption capacity. Acta Universitatis Agriculturae Et Silviculturae Mendelianae Brunensis 60(5):9–18
Babu AG, Shea PJ, Sudhakar D, Jung IB, Oh BT (2015) Potential use of Pseudomonas koreensis AGB-1 in association with Miscanthus sinensis to remediate heavy metal (loid)-contaminated mining site soil. J Environ Manage 151:160–166
Bae W, Chen W, Mulchandani A, Mehra RK (2000) Enhanced bioaccumulation of heavy metals by bacterial cells displaying synthetic phytochelatins. Biotechnol Bioeng 70(5):518–524
Bae W, Mehra RK, Mulchandani A, Chen W (2001) Genetic engineering of Escherichia coli for enhanced uptake and bioaccumulation of mercury. Appl Environ Microbiol 67(11):5335–5338
Article ADS CAS PubMed PubMed Central Google Scholar
Bae W, Mulchandani A, Chen W (2002) Cell surface display of synthetic phytochelatins using ice nucleation protein for enhanced heavy metal bioaccumulation. J Inorg Biochem 88(2):223–227
Bae W, Wu CH, Kostal J, Mulchandani A, Chen W (2003) Enhanced mercury biosorption by bacterial cells with surface-displayed MerR. Appl Environ Microbiol 69(6):3176–3180
Bandowe BAM, Bigalke M, Boamah L, Nyarko E, Saalia FK, Wilcke W (2014) Polycyclic aromatic compounds (PAHs and oxygenated PAHs) and trace metals in fish species from Ghana (West Africa): bioaccumulation and health risk assessment. Environ Int 65:135–146
Benazir JF, Suganthi R, Rajvel D, Pooja MP, Mathithumilan B (2010) Bioremediation of chromium in tannery effluent by microbial consortia. Afr J Biotech 9(21):3140–3143
Bhattacharya A, Gupta A (2013) Evaluation of Acinetobacter sp. B9 for Cr (VI) resistance and detoxification with potential application in bioremediation of heavy-metals-rich industrial wastewater. Environ Sci Pollut Res 20(9):6628–6637
Bhattacharya A, Gupta A, Kaur A, Malik D (2015) Simultaneous bioremediation of phenol and Cr (VI) from tannery wastewater using bacterial consortium. Int J Appl Sci Biotechnol 3(1):50–55
Bissen M, Frimmel FH (2003) Arsenic—a review: Part I: occurrence, toxicity, speciation, mobility. Acta Hydrochim Hydrobiol 31(1):9–18
Bojórquez C, Espericueta MGF, Voltolina D (2016) Removal of cadmium and lead by adapted strains of Pseudomonas aeruginosa and Enterobacter cloacae . Revista Internacional De Contaminación Ambiental 32(4):407–412
Bondarenko O, Rõlova T, Kahru A, Ivask A (2008) Bioavailability of Cd, Zn and Hg in soil to nine recombinant luminescent metal sensor bacteria. Sensors 8(11):6899–6923
Brar SK, Verma M, Surampalli RY, Misra K, Tyagi RD, Meunier N, Blais JF (2006) Bioremediation of hazardous wastes—a review. Pract Period Hazard Toxic Radioact Waste Manag 10(2):59–72
Brim H, McFarlan SC, Fredrickson JK, Minton KW, Zhai M, Wackett LP, Daly MJ (2000) Engineering Deinococcus radiodurans for metal remediation in radioactive mixed waste environments. Nat Biotechnol 18(1):85–90
Chang YJ, Peacock AD, Long PE, Stephen JR, McKinley JP, Macnaughton SJ, White DC (2001) Diversity and characterization of sulfate-reducing bacteria in groundwater at a uranium mill tailings site. Appl Environ Microbiol 67(7):3149–3160
Chanmugathas P, Bollag JM (1988) A column study of the biological mobilization and speciation of cadmium in soil. Arch Environ Contam Toxicol 17(2):229–237
Chaturvedi MK (2011) Studies on chromate removal by chromium-resistant Bacillus sp. isolated from tannery effluent. J Environ Prot 2(01):76
Cuevas J, Ruiz AI, de Soto IS, Sevilla T, Procopio JR, Da Silva P, Leguey S (2012) The performance of natural clay as a barrier to the diffusion of municipal solid waste landfill leachates. J Environ Manag 95:S175–S181
D’Annibale A, Leonardi V, Federici E, Baldi F, Zecchini F, Petruccioli M (2007) Leaching and microbial treatment of a soil contaminated by sulphide ore ashes and aromatic hydrocarbons. Appl Microbiol Biotechnol 74(5):1135–1144
Article PubMed Google Scholar
Dangi AK, Sharma B, Hill RT, Shukla P (2019) Bioremediation through microbes: systems biology and metabolic engineering approach. Crit Rev Biotechnol 39(1):79–98
De J, Ramaiah N, Vardanyan L (2008) Detoxification of toxic heavy metals by marine bacteria highly resistant to mercury. Mar Biotechnol 10(4):471–477
Deng X, Yi XE, Liu G (2007) Cadmium removal from aqueous solution by gene-modified Escherichia coli JM109. J Hazard Mater 139(2):340–344
Dermont G, Bergeron M, Mercier G, Richer-Laflèche M (2008) Soil washing for metal removal: a review of physical/chemical technologies and field applications. J Hazard Mater 152(1):1–31
Dixit R, Malaviya D, Pandiyan K, Singh UB, Sahu A, Shukla R, Paul D (2015) Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability 7(2):2189–2212
Dua M, Singh A, Sethunathan N, Johri A (2002) Biotechnology and bioremediation: successes and limitations. Appl Microbiol Biotechnol 59(2–3):143–152
CAS PubMed Google Scholar
Duruibe JO, Ogwuegbu MOC, Egwurugwu JN (2007) Heavy metal pollution and human biotoxic effects. Int J Phys Sci 2(5):112–118
EEA (2019) National emissions reported to the Convention on Long-range Transboundary Air Pollution (LRTAP Convention) provided by European Environment Agency (EEA). https://www.eea.europa.eu/data-andmaps/indicators/eea32-heavy-metal-hm-emissions-1/assessment-10
EPA (2003) EPA protocol for the review of existing national primary drinking water regulations, united states environmental protection agency office of water office of ground water and drinking water standards and risk management division targeting and analysis branch1200 pennsylvania avenue, NW (4607M) Washington, DC 20460
Fang L, Zhou C, Cai P, Chen W, Rong X, Dai K, Huang Q (2011) Binding characteristics of copper and cadmium by cyanobacterium Spirulina platensis . J Hazard Mater 190(1–3):810–815
Fashola MO, Ngole-Jeme VM, Babalola OO (2016) Heavy metal pollution from gold mines: environmental effects and bacterial strategies for resistance. Int J Environ Res Public Health 13(11):1047
Article PubMed PubMed Central Google Scholar
Favero N, Costa P, Massimino ML (1991) In vitro uptake of cadmium by basidiomycetes Pleurotus ostreatus . Biotech Lett 13(10):701–704
Febrianto J, Kosasih AN, Sunarso J, Ju YH, Indraswati N, Ismadji S (2009) Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J Hazard Mater 162(2–3):616–645
Gabriel J, Kofroňová O, Rychlovský P, Krenželok M (1996) Accumulation and effect of cadmium in the wood-rotting basidiomycete Daedalea quercina . Bull Environ Contam Toxicol 57(3):383–390
Gabriel J, Mokrejš M, Bílý J, Rychlovský P (1994) Accumulation of heavy metals by some wood-rotting fungi. Folia Microbiol 39(2):115–118
Galiulin RV, Galiulina RA (2008) Removing heavy metals from soil with plants. Her Russ Acad Sci 78(2):141–143
Gautam RK, Soni S, Chattopadhyaya MC (2015) Functionalized magnetic nanoparticles for environmental remediation. In: Handbook of research on diverse applications of nanotechnology in biomedicine, chemistry, and engineering. IGI Global, pp 518–551
Gauthier PT, Norwood WP, Prepas EE, Pyle GG (2014) Metal–PAH mixtures in the aquatic environment: a review of co-toxic mechanisms leading to more-than-additive outcomes. Aquat Toxicol 154:253–269
Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A (2020) The effects of cadmium toxicity. Int J Environ Res Public Health 17(11):3782
Article CAS PubMed PubMed Central Google Scholar
Glazer AN, Nikaido H (2007) Microbial biotechnology: fundamentals of applied microbiology. Cambridge University Press
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012.
Glombitza F, Reichel S (2013) Metal-containing residues from industry and in the environment: Geobiotechnological urban mining. In: Geobiotechnology I. Springer, Berlin, Heidelberg, pp 49–107
Goher ME, Abdel-Satar AM, Ali MH, Hussian AE, Napiórkowska-Krzebietke A (2016) Biosorption of some toxic metals from aqueous solution using non-living algal cells of Chlorella vulgaris . J Elementol 21(3)
Gomaa ABM, Al-Hazmi RH, Al-Fassi FA, Al-Garhi TF (2016) Bio-remediation of Cd-contaminated soil cultivated with Faba bean via application of Alcaligenes faecalis Rhizobacterium. Rev Res J 5(6):1–7
Gomaa AM, Al-Fassi FA, Al-Kenawy Z, Al-Gharbawi HT (2012) Role of Azospirillum and Rhizobium in bio-remediating Cd and Zn polluted soil cultivated with wheat plant. Aust J Basic Appl Sci 6(10):550–556
Gomez F, Sartaj M (2014) Optimization of field scale biopiles for bioremediation of petroleum hydrocarbon contaminated soil at low temperature conditions by response surface methodology (RSM). Int Biodeterior Biodegrad 89:103–109
Gorby YA, Caccavo F, Bolton H (1998) Microbial reduction of cobaltIIIEDTA-in the presence and absence of manganese (IV) oxide. Environ Sci Technol 32(2):244–250
Article ADS CAS Google Scholar
Guiné V, Spadini L, Sarret G, Muris M, Delolme C, Gaudet JP, Martins JMF (2006) Zinc sorption to three gram-negative bacteria: combined titration, modeling, and EXAFS study. Environ Sci Technol 40(6):1806–1813
Article ADS PubMed Google Scholar
He Y, Gong Y, Su Y, Zhang Y, Zhou X (2019) Bioremediation of Cr (VI) contaminated groundwater by Geobacter sulfurreducens: environmental factors and electron transfer flow studies. Chemosphere 221:793–801
Held T, Don H (2000) In situ remediation. Biotechnology 11b:350–370
Hildebrandt U, Regvar M, Bothe H (2007) Arbuscular mycorrhiza and heavy metal tolerance. Phytochemistry 68(1):139–146
Hobson AM, Frederickson J, Dise NB (2005) CH4 and N2O from mechanically turned windrow and vermicomposting systems following in-vessel pre-treatment. Waste Manage 25(4):345–352
Hogan CM (2010) Heavy metal. Encyclopedia of Earth. National Council for Science and the Environment. Eds E. Monosson & C. Cleveland, Washington DC.
Hrynkiewicz K, Baum C (2014) Application of microorganisms in bioremediation of environment from heavy metals. In: Environmental deterioration and human health. Springer, Dordrecht, pp 215–227
Hu N, Luo Y, Song J, Wu L, Zhang H (2010) Influences of soil organic matter, pH and temperature on Pb sorption by four soils in Yangtze River Delta. Acta Pedol Sin 47(2):246–252
Huang C, Huang CP (1996) Application of Aspergillus oryze and Rhizopus oryzae for Cu (II) removal. Water Res 30(9):1985–1990
Huët MAL, Puchooa D (2017) Bioremediation of heavy metals from aquatic environment through microbial processes: a potential role for probiotics. J Appl Biol Biotechnol 5(6):14–23
Hyma M, Dupont RR (2001) Groundwater and soil remediation: process design and cost estimating of proven technologies. American Society of Civil Engineers, Chicago
Iqbal M, Edyvean RGJ (2004) Biosorption of lead, copper and zinc ions on loofa sponge immobilized biomass of Phanerochaete chrysosporium . Miner Eng 17(2):217–223
Iranzo M, Sainz-Padro I, Boluda R, Sanchez J, Mormeneo S (2001) The use of microorganisms in environmental engineering. Ann Microbiol 51:135–143
Ivask A, Dubourguier HC, Põllumaa L, Kahru A (2011) Bioavailability of Cd in 110 polluted top soils to recombinant bioluminescent sensor bacteria: effect of soil particulate matter. J Soils Sedim 11(2):231–237
Jafari SA, Cheraghi S, Mirbakhsh M, Mirza R, Maryamabadi A (2015) Employing response surface methodology for optimization of mercury bioremediation by Vibrio parahaemolyticus PG02 in coastal sediments of Bushehr, Iran. CLEAN-Soil Air Water 43(1):118–126
Jain AN, Udayashankara TH, Lokesh KS (2012) Review on bioremediation of heavy metals with microbial isolates and amendments on soil residue. Int J Sci Res 6:2319–7064
Jaishankar M, Mathew BB, Shah MS, Murthy TPK, Gowda KRS (2014) Biosorption of few heavy metal ions using agricultural wastes. J Environ Pollut Hum Health 2(1):1–6
Javaid AMNA, Bajwa R, Manzoor T (2011) Biosorption of heavy metals by pretreated biomass of Aspergillus niger . Pak J Bot 43(1):419–425
John SG, Ruggiero CE, Hersman LE, Tung CS, Neu MP (2001) Siderophore mediated plutonium accumulation by Microbacterium flavescens (JG-9). Environ Sci Technol 35(14):2942–2948
Joner EJ, Leyval C (1997) Uptake of 109Cd by roots and hyphae of a Glomus mosseae / Trifolium subterraneum mycorrhiza from soil amended with high and low concentrations of cadmium. New Phytol 135(2):353–360
Juwarkar AA, Singh SK, Mudhoo A (2010) A comprehensive overview of elements in bioremediation. Rev Environ Sci Bio/technol 9(3):215–288
Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA (2000) Practical streptomyces genetics, vol 291. John Innes Foundation, Norwich
Kim IH, Choi JH, Joo JO, Kim YK, Choi JW, Oh BK (2015) Development of a microbe-zeolite carrier for the effective elimination of heavy metals from seawater. J Microbiol Biotechnol 25(9):1542–1546
Koning M, Hupe K, Stegmann R (2001) Thermal processes, scrubbing/extraction, bioremediation and disposal. Biotechnology Set 304–317
Kulshreshtha A, Agrawal R, Barar M, Saxena S (2014) A review on bioremediation of heavy metals in contaminated water. IOSR J Environ Sci Toxicol Food Technol 8(7):44–50
Kumar A, Bisht BS, Joshi VD, Dhewa T (2011a) Review on bioremediation of polluted environment: a management tool. Int J Environ Sci 1(6):1079–1093
Kumar A, Chanderman A, Makolomakwa M, Perumal K, Singh S (2016) Microbial production of phytases for combating environmental phosphate pollution and other diverse applications. Crit Rev Environ Sci Technol 46(6):556–591
Kumar R, Bhatia D, Singh R, Rani S, Bishnoi NR (2011b) Sorption of heavy metals from electroplating effluent using immobilized biomass Trichoderma viride in a continuous packed-bed column. Int Biodeterior Biodegrad 65(8):1133–1139
Kumar SS, Kadier A, Malyan SK, Ahmad A, Bishnoi NR (2017) Phytoremediation and rhizoremediation: uptake, mobilization and sequestration of heavy metals by plants. In: Plant-microbe interactions in agro-ecological perspectives. Springer, Singapore, pp 367–394
Kumaran NS, Sundaramanicam A, Bragadeeswaran S (2011) Adsorption studies on heavy metals by isolated cyanobacterial strain (nostoc sp.) from uppanar estuarine water, southeast coast of India. J Appl Sci Res 7(11):1609–1615
Lloyd JR, Mabbett AN, Williams DR, Macaskie LE (2001) Metal reduction by sulphate-reducing bacteria: physiological diversity and metal specificity. Hydrometallurgy 59(2–3):327–337
Lloyd JR, Sole VA, Van Praagh CVG, Lovley DR (2000) Direct and Fe (II)-mediated reduction of technetium by Fe (III)-reducing bacteria. Appl Environ Microbiol 66(9):3743–3749
Lone MI, He ZL, Stoffella PJ, Yang XE (2008) Phytoremediation of heavy metal polluted soils and water: progresses and perspectives. J Zhejiang Univ Sci B 9(3):210–220
Lovley DR, Phillips EJ (1988) Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol 54(6):1472–1480
Luo S, Xu T, Chen L, Chen J, Rao C, Xiao X, Liu Y (2012) Endophyte-assisted promotion of biomass production and metal-uptake of energy crop sweet sorghum by plant-growth-promoting endophyte Bacillus sp. SLS18. Appl Microbiol Biotechnol 93(4):1745–1753
Ma Y, Prasad MNV, Rajkumar M, Freitas H (2011) Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29(2):248–258
Ma Y, Oliveira RS, Freitas H, Zhang C (2016) Biochemical and molecular mechanisms of plant-microbe-metal interactions: relevance for phytoremediation. Front Plant Sci 7:918
Mane PC, Bhosle AB (2012) Bioremoval of Some Metals by Living Algae Spirogyra sp. and Spirullina sp . from aqueous solution.
Marihal AK, Jagadeesh KS (2013) Plant–microbe interaction: a potential tool for enhanced bioremediation. In: Plant microbe symbiosis: fundamentals and advances. Springer, New Delhi, pp 395–410
Marzan LW, Hossain M, Mina SA, Akter Y, Chowdhury AMA (2017) Isolation and biochemical characterization of heavy-metal resistant bacteria from tannery effluent in Chittagong city, Bangladesh: Bioremediation viewpoint. Egypt J Aquatic Res 43(1):65–74
McDonald J, Gaston L, Elbana T, Andres K, Cranfield E (2013) Dimoxystrobin sorption and degradation in sandy loam soil: impact of different landscape positions. Soil Sci 178(12):662–670
McHughen A (2016) A critical assessment of regulatory triggers for products of biotechnology: Product vs process. GM Crops Food 7(3–4):125–158
Mihopoulos PG, Sayles GD, Suidan MT, Shah J, Bishop DF (2000) Vapor phase treatment of PCE in a soil column by lab-scale anaerobic bioventing. Water Res 34(12):3231–3237
Mihopoulos PG, Suidan MT, Sayles GD, Kaskassian S (2002) Numerical modeling of oxygen exclusion experiments of anaerobic bioventing. J Contam Hydrol 58(3–4):209–220
Mishra A, Malik A (2013) Recent advances in microbial metal bioaccumulation. Crit Rev Environ Sci Technol 43(11):1162–1222
Muneer B, Iqbal MJ, Shakoori FR, Shakoori AR (2013) Tolerance and biosorption of mercury by microbial consortia: potential use in bioremediation of wastewater. Pak J Zool 45(1):247–254
Nadeem SM, Zahir ZA, Naveed M, Asghar HN, Arshad M (2010) Rhizobacteria capable of producing ACC-deaminase may mitigate salt stress in wheat. Soil Sci Soc Am J 74(2):533–542
Nayak AK, Panda SS, Basu A, Dhal NK (2018) Enhancement of toxic Cr (VI), Fe, and other heavy metals phytoremediation by the synergistic combination of native Bacillus cereus strain and Vetiveria zizanioides L. Int J Phytorem 20(7):682–691
Naz N, Young HK, Ahmed N, Gadd GM (2005) Cadmium accumulation and DNA homology with metal resistance genes in sulfate-reducing bacteria. Appl Environ Microbiol 71(8):4610–4618
Nie M, Wang Y, Yu J, Xiao M, Jiang L, Yang J, Li B (2011) Understanding plant-microbe interactions for phytoremediation of petroleum-polluted soil. PLoS ONE 6(3):e17961
Nissim WG, Palm E, Mancuso S, Azzarello E (2018) Trace element phytoextraction from contaminated soil: a case study under Mediterranean climate. Environ Sci Pollut Res 25(9):9114–9131
Oyetibo GO, Ilori MO, Adebusoye SA, Obayori OS, Amund OO (2010) Bacteria with dual resistance to elevated concentrations of heavy metals and antibiotics in Nigerian contaminated systems. Environ Monit Assess 168(1–4):305–314
Parvathi K, Nagendran R, Nareshkumar R (2007) Effect of pH on chromium biosorption by chemically treated Saccharomyces
Paschoalini AL, Bazzoli N (2021) Heavy metals affecting Neotropical freshwater fish: a review of the last 10 years of research. Aquat Toxicol 237:105906
Pepper IL, Gerba CP, Gentry TJ, Maier RM (eds) (2011) Environmental microbiology. Academic press
Philip L, Iyengar L, Venkobachar C (2000) Site of interaction of copper on Bacillus polymyxa . Water Air Soil Pollut 119(1–4):11–21
Prabha R, Singh DP, Verma MK (2017) Microbial interactions and perspectives for bioremediation of pesticides in the soils. In: Plant-microbe interactions in agro-ecological perspectives. Springer, Singapore, pp 649–671
Prescott LM, Harley JP, Klein DA (2002) Microbiology. 5th International Edition
Qasem NA, Mohammed RH, Lawal DU (2021) Removal of heavy metal ions from wastewater: a comprehensive and critical review. Npj Clean Water 4(1):36
Raghunandan K, Kumar A, Kumar S, Permaul K, Singh S (2018) Production of gellan gum, an exopolysaccharide, from biodiesel-derived waste glycerol by Sphingomonas spp. 3 Biotech 8(1):71
Raghunandan K, Mchunu S, Kumar A, Kumar KS, Govender A, Permaul K, Singh S (2014) Biodegradation of glycerol using bacterial isolates from soil under aerobic conditions. J Environ Sci Health Part A 49(1):85–92
Rajendran P, Muthukrishnan J, Gunasekaran P (2003) Microbes in heavy metal remediation.
Ramasamy K, Banu SP (2007) Bioremediation of metals: microbial processes and techniques. In: Environmental bioremediation technologies. Springer, Berlin, Heidelberg, pp 173–187
Ray SA, Ray MK (2009) Bioremediation of heavy metal toxicity-with special reference to chromium. Al Ameen J Med Sci 2(2):57–63
Riseh RS, Vazvani MG, Hajabdollahi N, Thakur VK (2022) Bioremediation of heavy metals by Rhizobacteria. Appl Biochem Biotechnol 1–23
Rodríguez-Tirado V, Green-Ruiz C, Gómez-Gil B (2012) Cu and Pb biosorption on Bacillus thioparans strain U3 in aqueous solution: Kinetic and equilibrium studies. Chem Eng J 181:352–359
Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN (2008) Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett 278(1):1–9
Salehizadeh H, Shojaosadati SA (2003) Removal of metal ions from aqueous solution by polysaccharide produced from Bacillus firmus . Water Res 37(17):4231–4235
Sar P, D’Souza SF (2001) Biosorptive uranium uptake by a Pseudomonas strain: characterization and equilibrium studies. J Chem Technol Biotechnol 76(12):1286–1294
Saranya K, Sundaramanickam A, Shekhar S, Swaminathan S, Balasubramanian T (2017) Bioremediation of mercury by Vibrio fluvialis screened from industrial effluents. BioMed Res Int 2017
Schulz B, Boyle C (2006) What are endophytes?. In: Microbial root endophytes. Springer, Berlin, Heidelberg, pp 1–13
Seeger M, Hernández M, Méndez V, Ponce B, Córdova M, González M (2010) Bacterial degradation and bioremediation of chlorinated herbicides and biphenyls. J Soil Sci Plant Nutr 10(3):320–332
Seema S, Alok A (2012) Hexavalent chromium reduction in tannery effluent by bacterial species isolated from tannery effluent contaminated soil. J Environ Sci Technol 5(3):142–154
Seth CS (2012) A review on mechanisms of plant tolerance and role of transgenic plants in environmental clean-up. Bot Rev 78(1):32–62
Shah JK, Sayles GD, Suidan MT, Mihopoulos P, Kaskassian S (2001) Anaerobic bioventing of unsaturated zone contaminated with DDT and DNT. Water Sci Technol 43(2):35–42
Shannon MJ, Unterman R (1993) Evaluating bioremediation: distinguishing fact from fiction. Annu Rev Microbiol 47(1):715–736
Sharma PK, Balkwill DL, Frenkel A, Vairavamurthy MA (2000) A new Klebsiella planticola strain (Cd-1) grows anaerobically at high cadmium concentrations and precipitates cadmium sulfide. Appl Environ Microbiol 66(7):3083–3087
Shipra J, Dikshit SN, Pandey G (2011) Comparative study of agitation rate and stationary phase for the removal of Cu 2+ by A. Lentulus. Int J Pharma Bio Sci 2(3)
Singh N, Verma T, Gaur R (2013) Detoxification of hexavalent chromium by an indigenous facultative anaerobic Bacillus cereus strain isolated from tannery effluent. Afr J Biotechnol 12(10)
Spormann AM, Widdel F (2000) Metabolism of alkylbenzenes, alkanes, and other hydrocarbons in anaerobic bacteria. Biodegradation 11(2–3):85–105
Sun X, Meng J, Huo S, Zhu J, Zheng S (2020) Remediation of heavy metal pollution in soil by microbial immobilization with carbon microspheres. Int J Environ Sci Dev 11(1)
Tamele IJ, Vázquez Loureiro P (2020) Lead, mercury and cadmium in fish and shellfish from the Indian Ocean and Red Sea (African Countries): public health challenges. J Mar Sci Eng 8(5):344
Tan H, Champion JT, Artiola JF, Brusseau ML, Miller RM (1994) Complexation of cadmium by a rhamnolipid biosurfactant. Environ Sci Technol 28(13):2402–2406
Tang X, Huang Y, Li Y, Wang L, Pei X, Zhou D, Hughes SS (2021) Study on detoxification and removal mechanisms of hexavalent chromium by microorganisms. Ecotoxicol Environ Saf 208:111699
Taştan BE, Ertuğrul S, Dönmez G (2010) Effective bioremoval of reactive dye and heavy metals by Aspergillus versicolor . Biores Technol 101(3):870–876
Tebo BM, Obraztsova AY (1998) Sulfate-reducing bacterium grows with Cr (VI), U (VI), Mn (IV), and Fe (III) as electron acceptors. FEMS Microbiol Lett 162(1):193–199
Tigini V, Prigione V, Giansanti P, Mangiavillano A, Pannocchia A, Varese GC (2010) Fungal biosorption, an innovative treatment for the decolourisation and detoxification of textile effluents. Water 2(3):550–565
Truu J, Truu M, Espenberg M, Nõlvak H, Juhanson J (2015) Phytoremediation and plant-assisted bioremediation in soil and treatment wetlands: a review. Open Biotechnol J 9(1)
Tunali S, Çabuk A, Akar T (2006) Removal of lead and copper ions from aqueous solutions by bacterial strain isolated from soil. Chem Eng J 115(3):203–211
Tyagi S, Kumar V, Singh J, Teotia P, Bisht S, Sharma S (2014) Bioremediation of pulp and paper mill effluent by dominant aboriginal microbes and their consortium. Int J Environ Res 8(3):561–568
Ullah A, Mushtaq H, Ali H, Munis MFH, Javed MT, Chaudhary HJ (2015) Diazotrophs-assisted phytoremediation of heavy metals: a novel approach. Environ Sci Pollut Res 22(4):2505–2514
Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K (2016) Redox-and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol 90(1):1–37
Valls M, Atrian S, de Lorenzo V, Fernández LA (2000) Engineering a mouse metallothionein on the cell surface of Ralstonia eutropha CH34 for immobilization of heavy metals in soil. Nat Biotechnol 18(6):661–665
Vara Prasad MN, de Oliveira Freitas HM (2003) Metal hyperaccumulation in plants: biodiversity prospecting for phytoremediation technology. Electron J Biotechnol 6(3):285–321
Verma A (2022) Bioremediation techniques for soil pollution: an introduction. Biodegrad Technol Organ Inorgan Pollut. https://doi.org/10.5772/intechopen.99028
Vinopal S, Ruml T, Kotrba P (2007) Biosorption of Cd 2+ and Zn 2+ by cell surface-engineered Saccharomyces cerevisiae . Int Biodeterior Biodegrad 60(2):96–102
Volesky B (1990) Removal and recovery of heavy metals by biosorption. Biosorption of Heavy Metals 7–43
Volesky B, Holan ZR (1995) Biosorption of heavy metals. Biotechnol Prog 11(3):235–250
Wang D, Li H, Wei Z, Wang X, Hu F (2006) Effect of earthworms on the phytoremediation of zinc-polluted soil by ryegrass and Indian mustard. Biol Fertil Soils 43(1):120–123
Weyens N, van der Lelie D, Taghavi S, Newman L, Vangronsveld J (2009) Exploiting plant–microbe partnerships to improve biomass production and remediation. Trends Biotechnol 27(10):591–598
Whelan MJ, Coulon F, Hince G, Rayner J, McWatters R, Spedding T, Snape I (2015) Fate and transport of petroleum hydrocarbons in engineered biopiles in polar regions. Chemosphere 131:232–240
Wierzba S (2015) Biosorption of lead (II), zinc (II) and nickel (II) from industrial wastewater by Stenotrophomonas maltophilia and Bacillus subtilis . Pol J Chem Technol 17(1):79–87
Wu MC, Hou CY, Jiang CM, Wang YT, Wang CY, Chen HH, Chang HM (2007) A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chem 101(4):1753–1758
Yan G, Viraraghavan T (2001) Heavy metal removal in a biosorption column by immobilized M. rouxii biomass. Bioresour Technol 78(3):243–249
Yuan M, He H, Xiao L, Zhong T, Liu H, Li S, Jing Y (2014) Enhancement of Cd phytoextraction by two Amaranthus species with endophytic Rahnella sp. JN27. Chemosphere 103:99–104
Zakaria ZA, Zakaria Z, Surif S, Ahmad WA (2007) Hexavalent chromium reduction by Acinetobacter haemolyticus isolated from heavy-metal contaminated wastewater. J Hazard Mater 146(1–2):30–38
Zeyaullah MD, Atif M, Islam B, Abdelkafe AS, Sultan P, ElSaady MA, Ali A (2009) Bioremediation: a tool for environmental cleaning. Afr J Microbiol Res 3(6):310–314
Download references
Acknowledgements
The authors would like to acknowledge the support of all members of the Department of Chemistry of Natural and Microbial Products and the Department of Microbial Biotechnology at the National Research Centre.
No funding was obtained for this study.
Author information
Authors and affiliations.
Department of Chemistry of Natural and Microbial Products, Pharmaceutical and Drug Industries Research Institute, National Research Centre, Giza, Egypt
Mohamed I. Abo-Alkasem
Egyptian Organization for Standards and Quality, Cairo, Egypt
Ne’mat H. Hassan
Department of Microbial Biotechnology, Genetic Engineering and Biotechnology Research Institute, National Research Centre, Giza, Egypt
Mostafa Mostafa Abo Elsoud
You can also search for this author in PubMed Google Scholar
Contributions
All the authors participate in writing, editing, and revising the manuscript. All authors have read and approved the manuscript.
Corresponding author
Correspondence to Mohamed I. Abo-Alkasem .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Abo-Alkasem, M.I., Hassan, N.H. & Abo Elsoud, M.M. Microbial bioremediation as a tool for the removal of heavy metals. Bull Natl Res Cent 47 , 31 (2023). https://doi.org/10.1186/s42269-023-01006-z
Download citation
Received : 13 October 2022
Accepted : 19 February 2023
Published : 27 February 2023
DOI : https://doi.org/10.1186/s42269-023-01006-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Bioremediation
REVIEW article
Microbial interventions in bioremediation of heavy metal contaminants in agroecosystem.

- 1 Cell and Molecular Biology Laboratory, Department of Zoology (DST-FIST Sponsored), Soban Singh Jeena University Campus, Almora, India
- 2 Department of Biotechnology, Sir J C Bose Technical Campus, Kumaun University, Bhimtal, India
- 3 Department of Zoology, Kumaun University, Nainital, India
- 4 Department of Agricultural and Biological Engineering, PurdueUniversity, West Lafayette, IN, United States
Soil naturally comprises heavy metals but due to the rapid industrialization and anthropogenic events such as uncontrolled use of agrochemicals their concentration is heightened up to a large extent across the world. Heavy metals are non-biodegradable and persistent in nature thereby disrupting the environment and causing huge health threats to humans. Exploiting microorganisms for the removal of heavy metal is a promising approach to combat these adverse consequences. The microbial remediation is very crucial to prevent the leaching of heavy metal or mobilization into the ecosystem, as well as to make heavy metal extraction simpler. In this scenario, technological breakthroughs in microbes-based heavy metals have pushed bioremediation as a promising alternative to standard approaches. So, to counteract the deleterious effects of these toxic metals, some microorganisms have evolved different mechanisms of detoxification. This review aims to scrutinize the routes that are responsible for the heavy metal(loid)s contamination of agricultural land, provides a vital assessment of microorganism bioremediation capability. We have summarized various processes of heavy metal bioremediation, such as biosorption, bioleaching, biomineralization, biotransformation, and intracellular accumulation, as well as the use of genetically modified microbes and immobilized microbial cells for heavy metal removal.
Introduction
Contamination of heavy metals (HMs) has widely spread all over the world and therefore is a primary matter of concern as it poses threat to animals, plants as well as humans and disturbs the environment. HMs, like other metals and metalloids, are present in the earth’s crust, however, the recalcitrant nature of HMs makes them resistant to degradation. Bioaccumulation of HMs and metalloids via different sources like air, water, causes them to infiltrate plants, animals, and humans, as well as the advancement of the food chain over time ( Briffa et al., 2020 ). Several natural and man-made processes could release these HMs into the environment ( Dembitsky and Rezanka, 2003 ). Due to the growing usage of agrochemicals and inorganic fertilizers, modern agricultural methods have resulted in agricultural pollution, resulting in the ecosystem and environmental destruction ( Malik et al., 2017 ). HMs are also introduced into agricultural systems through the use of sewage sludge and organic waste manure, industrial wastes, and wastewater irrigation ( Srivastava et al., 2016 ; Sharma et al., 2017 ) ( Figure 1 ). Extraction of HMs from their ores occurs during the processing of minerals and throughout this process, some portions are left out in the open and get relocated to different places due to flood and wind thus causing serious environmental hazards. The essential nourishment of food crops is the soil and therefore agrarian soil is of huge concern owing to its linkage with the production of food, which could affect the health of living organisms. Despite being part of the soil, HMs cause serious harm to the soil as well as plants in their concentrated form. Thus, they are considered to be hazardous ( Osmani et al., 2015 ). HMs are responsible for not only changing the composition of soil but also forming the basis of stress in the plants resulting in the failure of the crop. Biological molecules like lipids, nucleic acids, proteins, and enzymes get damaged due to the production of free radicals by the HMs thus increasing intracellularly the reactive oxygen species (ROS) levels thereby leading to oxidative stress. The failure in all of these biological substances creates several physiological issues, including, cell damage, DNA damage, and enzyme inhibition, all of which can lead to the plant’s death ( Wu et al., 2016 ). HM pollution in modern agriculture has become a severe challenge in most emerging and underdeveloped countries due to a variety of social-economical, scientific, and developmental difficulties. Discovering environmentally safe, long-term solutions to the HM contamination problem is a serious task. Currently, the use of microorganisms or functional biocatalysts in the remediation of soil contaminated with HMs entails the integration of genomes, transcriptomics, proteomics, signaling systems, and synthetic biology knowledge ( Hemmat-Jou et al., 2018 ). These strategies offer a new vista in biotechnology, allowing for the creation of a complex biological system to produce a better microbial system capable of combating HMs contamination ( Sayqal and Ahmed, 2021 ). HMs affect the soil microbiology and modify rhizospheric connections between plants and microorganisms, influencing soil characteristics, plant growth, vegetation type, and agricultural land production, among other things. Different microbial communities with specific metabolic capacities reside in the soil, e.g., organic substances are formed by certain microorganisms while interacting with toxic metals whereas some other assists in the formation of natural nanoparticles thus reducing HMs ( Wang and Chen, 2006 ). Owing to their high surface area to volume ratio, which is associated directly with their increased reactivity, nanotechnology-based materials have also been explored for HMs micro-remediation ( Vijayaraj et al., 2019 ). The approaches and successes of biotechnological applications for environmental protection, decontamination, and the elimination of HMs and metalloids have thus been covered in this study.
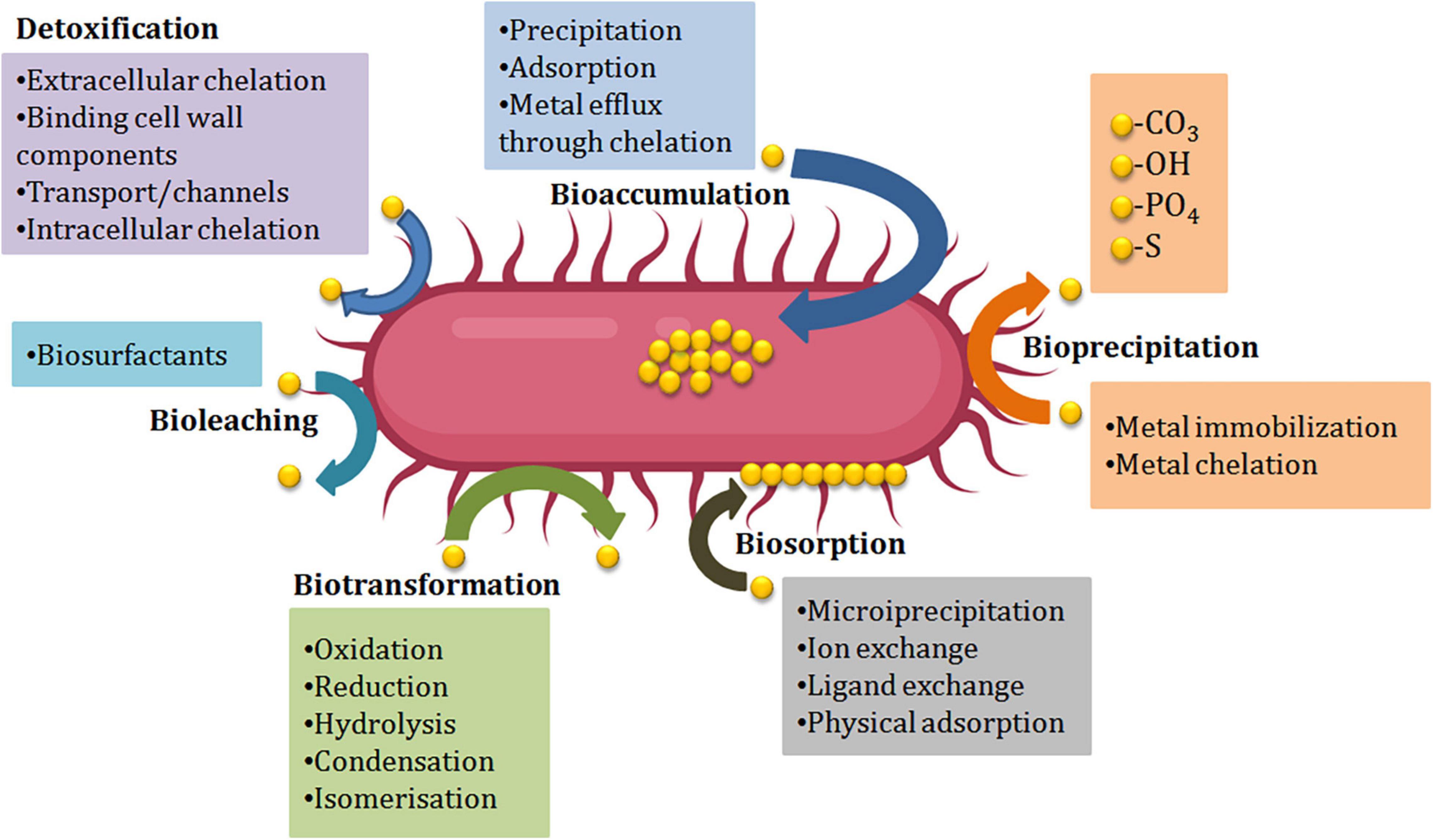
Figure 1. The primary sources and effects of heavy metal exposure at various trophic levels.
The advancement of biotechnological applications and strategies for environmental protection, detoxification, and the removal of HMs and metalloids are the subject of this review. The goal of this review is to compile a list of key findings on HM contamination in modern agriculture, as well as to sketch a probable research roadmap for the future. The review explored the depth information about the mechanism and impacts of the HMs in microbial systems.
Heavy Metal Pollution in Agroecosystem: Consequences and Plant Responses
Effect on soil health, fertility, and microbial dynamics.
Soil biology plays a vital part in maintaining healthy soil quality, which is crucial for sustainable agriculture. The anthropogenic activities are central in contaminating soil with HMs, e.g., industrial, mining, and agricultural operations as the s present in mining waste, sewage sludge, inorganic fertilizers, and pesticides, tend to disturb soil microbes by percolating into the soil environment ( Gupta et al., 2010 ; Tóth et al., 2016 ; Sharma et al., 2017 ). With rising amounts of HM pollution, microbial viability declines. Yuan et al. (2015) showed that microbial survivability was found to be negatively correlated with prolonged Pb exposure. According to de Quadros et al. (2016) , coal mining operations; result in a decrease in microbial biomass, abundance, and variability. Nayak et al. (2015) reported that up to 40 and 100% fly ash amendments resulted in better microbial population dynamics with increased concentrations of Zn, Fe, Cu, Mn, Cd, and Cr in agricultural soils. Total microbial activity, as determined by the fluoresceindiacetate (FDA) test, and denitrifiers, on the contrary, exhibited an increasing tendency of up to 40% fly ash addition. The application of fly ash, on the other hand, reduced the activity of both acid and alkaline phosphatase. Various types of HM toxicity and their harmful effects on soil, plants, and humans are presented in Table 1 .
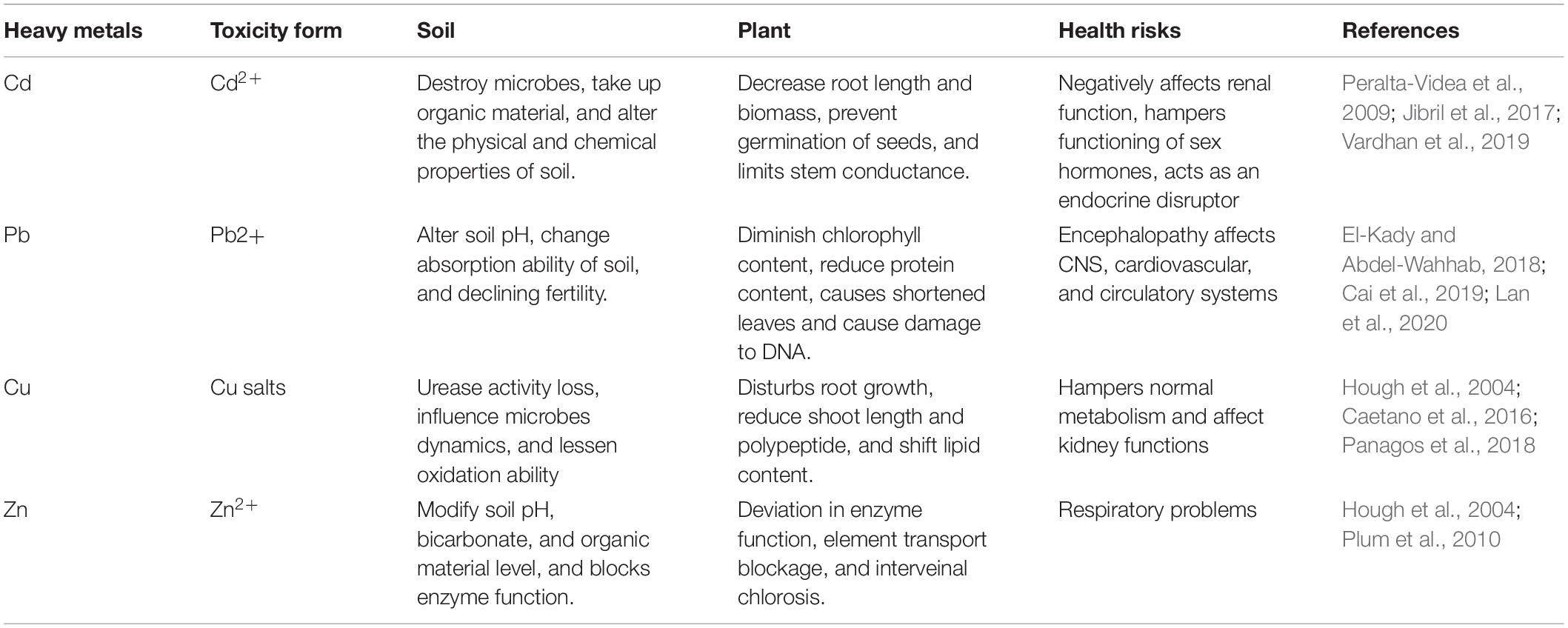
Table 1. Various types of heavy metal toxicity and their harmful effects on soil, plants, and humans.
Effect on Soil Microbial Functions and Processes
Due to HM toxicity, litter breakdown is slowed, resulting in an uneven litter deposit on the soil ( Illmer and Schinner, 1991 ; Giller et al., 1998 ; Marschner and Kalbitz, 2003 ). Kozlov and Zvereva (2015) investigated the breakdown rate of mountain birch ( Betula pubescens ssp. czerepanovii ) leaves in a significantly contaminated industrial setting close to the nickel-copper smelter in Monchegorsk. During 2 years of exposure, there was a substantial reduction of 49% in the relative weight of native leaves compared to the loss observed in the unpolluted forest. Furthermore, anthropogenic HM contamination has been found in a number of studies to have a negative impact on stream litter decomposition ( Carlisle and Clements, 2005 ; Hogsden and Harding, 2012 ; Ferreira et al., 2016 ). In both ecological toxicology and ecological tracking investigations, the rate of soil organic carbon mineralization has been routinely utilized as a test for metal toxicity ( Giller et al., 1998 ). Carbon mineralization may be measured using the soil respiration rate. A negative association was found between soil microbial respiration and HM concentration by Nwuche and Ugoji (2008) . From an average rate of 2.51–2.56 g of C/g at the start of the trial, the rate of soil microbial respiration was lowered to 0.98, 1.08, and 1.61 g of C/g in the Cu: Zn, Cu, and Zn treated soils, respectively. Because of differences in the experimental designs, fluctuations in soil characteristics, and substrate concentrations, HM exposure can either stimulate or impede N-mineralization. HM pollution disrupts nitrogen transformation pathways, which ultimately affects N-mineralization ( Dai et al., 2004 ; Vásquez-Murrieta et al., 2006 ; Hamsa et al., 2017 ). HM pollution has a similar impact on both N mineralization as well as nitrification i.e., both processes tend to decrease with an increasing amount of HM pollutants ( deCatanzaro and Hutchinson, 1985 ). Furthermore, nitrification is more susceptible to HM contamination than N mineralization ( Rother et al., 1982 ; Bewley and Stotzky, 1983 ).

Impact on Soil Enzymes
Metal composition, pH of the soil, organic matter, and clay content are important factors regulating the biological availability of metals in the soil. HMs affect soil enzymatic activity such as alkaline phosphatase, arylsulfatase, β-glucosidase, cellulase, dehydrogenase, invertase, protease, and urease ( Oliveira and Pampulha, 2006 ; Burges et al., 2015 ; Xian et al., 2015 ). Pan and Yu (2011) reported that HMs (Cd or/and Pb) reduce the activity of soil enzymes such as acid phosphatase, dehydrogenase, and urease, as well as the soil microbial community. Some researchers investigated the combined impact of HMs and soil characteristics on soil functions and concluded that arylsulfatase is the most sensitive soil enzyme that might be utilized as a marker of soil toxicity ( Xian et al., 2015 ).
Heavy Metals Responses in Plant System
Heavy metal contamination is a modern ecological issue that pollutes air, water, and soil. This not only results in significant crop yield losses but also raises health risks. In order to mitigate the damage caused by HM contamination, the antioxidative machinery of plants gets triggered.
Oxidative Stress and Reactive Oxygen Species
“Reactive oxygen species (ROS)” are reactive chemical species produced from molecular oxygen. Various diverse ROS are present momentarily among all aerobes which include: (a) oxygen-derived non-radicals viz. singlet oxygen ( 1/2 O 2 ), organic hydroperoxide (ROOH), hydrogen peroxide (H 2 O 2 ); and (b) oxygen-derived free radicals viz. superoxide anion (O 2 – ), alkoxyl (RO⋅) radicals, peroxyl (RO 2 ⋅), and hydroxyl (HO⋅) ( Circu and Aw, 2010 ; Shahid et al., 2014 ; Tamás et al., 2017 ). Plants tend to produce more ROS, after exposure to HMs as they can disrupt the electron transport chain of the mitochondrial and chloroplast membrane. The increased load of ROS interrupts the redox balance of the cell by causing plasma membrane damage and ion leakage ( Dingjan et al., 2016 ; Anjum et al., 2017 ), lipid peroxidation, and the disintegration of cellular macromolecules ( Carrasco-Gil et al., 2012 ; Chen et al., 2012 ; Venkatachalam et al., 2017 ). The increased amount of Cr in two maize genotypes reduced the content of soluble protein and elevated the level of phenol, proline, and other soluble sugars ( Anjum et al., 2017 ).
Genotoxicity
The pathways regulating metal-induced genotoxicity are intricate and are understudied ( Cuypers et al., 2011 ). However, it is clear that HM-induced genotoxicity/DNA damage happens indirectly via the generation of ROS during oxidative stress ( Barbosa et al., 2010 ; Shahid et al., 2014 ; Aslam et al., 2017 ). HM-induced nucleic acid impairments have previously been identified in plants such as Helianthus annuus ( Chakravarty and Srivastava, 1992 ), Vicia faba ( Pourrut et al., 2011 ; Arya et al., 2013 ; Arya and Mukherjee, 2014 ), Solanum tuberosum , and Nicotiana tabacum ( Gichner et al., 2006 ), and Allium cepa ( Arya et al., 2013 ; Arya and Mukherjee, 2014 ; Qin et al., 2015 ). The oxidation state of HM, its amount, and duration of exposure greatly affects the genotoxic response of any plant ( Aslam et al., 2017 ). The extremely reactive species among ROS is the hydroxyl radical (OH⋅), which can react to and damage all of the DNA molecule’s components ( Jones et al., 2011 ). When ROS react with DNA, it can cause deletion, and modification of nitrogenous bases, breakage of strands, damage to cross-links, and generation of nucleotide dimmers ( Gastaldo et al., 2008 ). When Pb and Cd react with DNA, Yang et al. (1999) detected the formation of 8-hydroxydeoxyguanosine (8-OHdG) adducts, which resulted in the breaking of the strand. Further, Hirata et al. (2011) reported Cr and As-triggered translesion DNA synthesis as a consequence of 8-OHdG production. Several recent studies were made on the genotoxic effects of copper and lead ( Qin et al., 2015 ; Silva et al., 2017 ; Venkatachalam et al., 2017 ).
Interference With Signaling Pathways
Deregulation of signaling pathways mediated by HM interactions is the main cause behind HM toxicity by influencing G-proteins, growth factor receptors, and receptor tyrosine kinases ( Harris and Shi, 2003 ). HMs also boost H 2 O 2 production in plants by increasing the synthesis of salicylic acid (SA), jasmonic acid (JA), and ethylene (ET), which interferes with the cell signaling mechanism ( Maksymiec, 2007 ; Schellingen et al., 2014 ; van de Poel et al., 2015 ). Plants exposed to As, have higher levels of JA, which stimulates the expression of several signaling and stress-response genes such as MAPK, CDC25, and genes regulating glutathione metabolism ( Thapa et al., 2012 ; Islam et al., 2015 ).
Physiological and Biochemical Response
The anti-oxidative enzymatic machinery of plants such as ascorbate peroxidase (APX), catalase (CAT), glutathione (GSH), glutathione reductase (GR), guaiacol peroxidase (GPX), peroxidase (POX), and superoxide dismutase (SOD) plays a critical role in neutralizing extra ROS ( Zhang and Klessig, 2001 ; Venkatachalam et al., 2017 ). Increased MDA generation due to enhanced ROS in the cell was observed when two mangrove plants were exposed to HMs ( Zhang and Gu, 2007 ). Similarly, superoxide dismutase (SOD) level was dramatically increased in leaves and roots at low metal concentrations, but dropped drastically at greater concentrations, suggesting a reduction in SOD scavenging ability. Abelmoschus esculentus plant grown on sewage sludge reflected an initial increase in chlorophyll content but fall sharply in later stages. The apparent decrease in chlorophyll content might be due to the accumulation of HMs in plants at later stages ( Singh and Agrawal, 2008 ). Similar findings were made in Vigna radiata ( Singh and Agrawal, 2010 ) and Oryza sativa ( Singh and Agrawal, 2010 ).
Microbial Resistance to Heavy Metals and Their Mechanisms
During stress situations developed by HMs, microorganisms either dies of the toxicity caused by the metal or thrive the situation by evolving mechanism of resistance against metals. For the selection of potent bioremediation agents, microorganisms should develop the mechanism of resistance against the toxicity of metals. Different resistance mechanisms developed by microorganisms like Extracellular barriers, extracellular and intracellular sequestration, active transport of metal ions, and enzymatic detoxification are discussed below ( Figure 2 ). Barriers like cell walls, plasma membrane, and other structures present at the surface like EPS, biofilms restrict the passage of HMs into the cells of bacteria. Microbes’ cell surfaces have a variety of characteristics that prevent metal ions from entering by adsorbing them on their surface and functioning as barriers. E.g., a study by Kumar et al. (2014) showed that isolates of fungi and bacteria can cause biosorption of HMs like copper, lead, and chromium. Tolerance against numerous HMs like copper, zinc, iron, nickel, lead, and cadmium was shown by Cellulosimicrobium sp. Chemisorption sites were involved in this resistance mechanism ( Bhati et al., 2019 ). The Biofilms produced by microbes are made up of extracellular polymers that are capable of accumulating metal ions and therefore protect the cells present inside them. The tolerance against lead, zinc, and copper ions has been displayed by the biofilm of Pseudomonas aeruginosa ( Teitzel and Parsek, 2003 ). The efficiency to eliminate metal was enhanced from 91.71 to 95.35% by the biofilm of Rhodotorula mucilaginosa ( Grujić et al., 2017 ). In addition to biofilms and cell walls, EPS has also been shown to be a barrier against metals, e.g., Adsorption of lead ions was reported in P. aeruginosa , Acinetobacter junii L. Pb1, and Azotobacter chroococcum XU1 ( Bramhachari et al., 2007 ; Rasulov et al., 2013 ; Kushwaha et al., 2017 ).
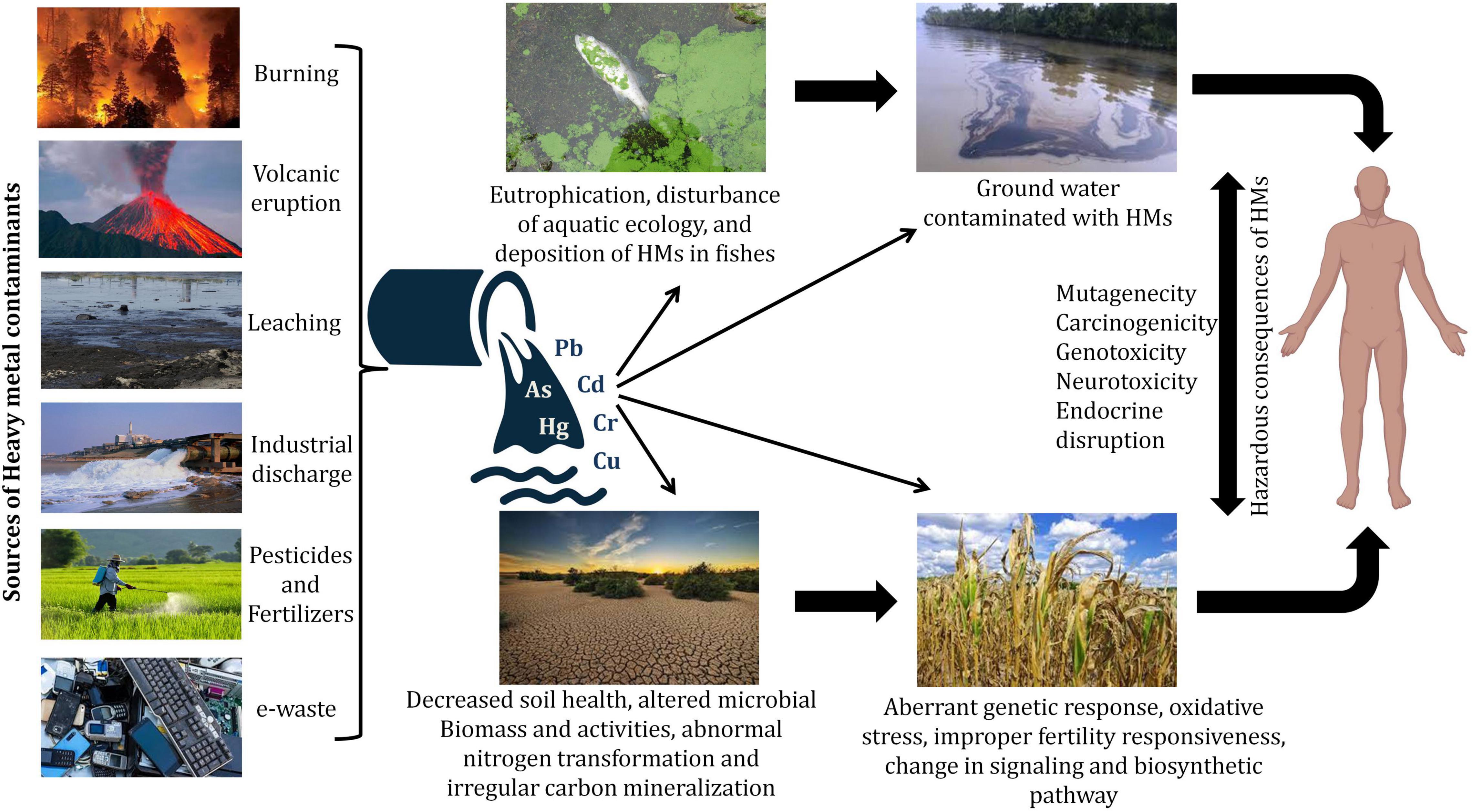
Figure 2. Microbe-mediated environmental remediation of heavy metals.
Numerous proteins and metabolic products are found in the cell membrane of the microbes that are capable of making complex structures (chelation) with the metal ions. Extracellular sequestration can be defined as the complexation of metal ions as insoluble compounds or metal ions accumulation by the components of the cell in the periplasm. Copper-inducible proteins CopA, CopB (periplasmic proteins), and CopC (outer membrane protein) are produced by copper-resistant Pseudomonas syringae strains that are responsible for the binding of microbial colonies and copper ions. Zinc ions can pass from the cytoplasm and get accumulated into the periplasm of the Synechocystis PCC 6803 strain via the efflux mechanism ( Thelwell et al., 1998 ). Hazardous metals can be reduced by iron and sulfur-reducing bacteria like Desulfuromonas spp. and Geobacter spp. into less or non-hazardous metals. An obligate anaerobe, G. metallireducens , can reduce manganese (Mn) from poisonous Mn (IV) to Mn (II) and uranium (U) from toxic U(VI) to U(II) (IV) ( Gavrilescu, 2004 ). In intracellular sequestration, metal ions are complexed by distinct compounds in the cell cytoplasm. The interaction of metals with the ligands presents in the surface, followed by sluggish transport into the cell, can result in a high concentration of metals within the cells of microorganisms. The ability to accumulate metals intracellularly by bacterial cells has been used in a variety of applications, most notably in waste treatment. With the help of low molecular weight proteins that were rich in cysteine, a cadmium-tolerant Pseudomonas putida strain was able to sequester copper, cadmium, and zinc ions intracellularly ( Higham et al., 1986 ). In Rhizobium leguminosarum cells, glutathione was also found to be involved in the sequestration of cadmium ions intracellularly ( Lima et al., 2006 ). Lipids, chitin, mineral ions, nitrogen-bearing polysaccharide, polyphosphates, and proteins make up the firm cell wall of fungi. The accumulation of metals by numerous fungi into their spores and mycelium helps in decontaminating metal ions by energy uptake, intracellular and extracellular precipitation, and valence exchange. Another strategy to protect against HM stress is to transport HM ions out from the intracellular environment, which can happen through efflux mechanisms that can effectively regulate intracellular HM ion concentrations ( Remenar et al., 2018 ). Efflux systems have been discovered in a variety of microbes, particularly those isolated from metal contaminated surroundings. Metal exporting proteins, such as ABC transporters, P-type efflux ATPase, cation diffusion facilitator, and proton-cation antiporters are widely distributed in the cell membrane to achieve HM ion efflux. For the export of Cu (II), Cd (II), and Zn (II), Gram-positive bacteria utilize P-type efflux ATPase. With the help of ATPase, an exporting protein on the cell membrane regulates arsenite outflow ( Yang et al., 2012 ; Soto et al., 2019 ). ABC transporters which also called traffic ATPases can assist microorganisms to survive the stress caused due to HMs by mediating membrane translocation of HM ions ( Al-Gheethi et al., 2015 ; Lerebours et al., 2016 ; Zammit et al., 2016 ).
The resistance to HMs ions in microbes is also contributed by the enzymes that biologically transform or chemically modify the HM ions from highly hazardous form to less toxic form ( Liu et al., 2017 ). HM ions’ toxicity can be effectively reduced by changing their redox state via reduction or oxidation reactions ( Giovanella et al., 2016 ). Detoxification enzymes can influence this defensive mechanism, which is also controlled by microbe resistance genes. Through mercuric ion reductase, bacteria like Bacillus sp. display resistance to mercury ions ( Noroozi et al., 2017 ). Mercuric reductase transforms the mercuric ion into metallic mercury, which is then discharged into the environment via the cell membrane ( Zhang et al., 2012 ). To reduce toxicity, bacteria such as Micrococcus sp. and Acinetobacter sp. can oxidize hazardous as (III) into less soluble and non-toxic As (V) ( Nagvenkar and Ramaiah, 2010 ).
Microbial Mechanism Involved in Heavy Metal Bioremediation
For the elimination of HMs from the polluted sites, bioremediation methods are employed ( Pratush et al., 2018 ). Usually, these methods involve the absorption/adsorption of toxic metallic ions, and this alleviates the related side effects ( Njoku et al., 2020 ). Different natural resources like wood bark/dust, coconut husk/shells, agro wastes, microorganisms, seaweeds, seeds, discarded coffee beans, and aquatic plants, etc. are being used constantly to reduce the number of HM ions from the place of their origin, in which microbes (algae, fungi, bacteria, yeasts, etc.) play a considerable role ( Mudila et al., 2019 ). Microorganisms change the HMs’ ionic state that influences the solubility, bioavailability, and movement in the soil as well as in the aquatic surroundings ( Ayangbenro and Babalola, 2017 ). Mobilization or immobilization of HMs aids microbial remediation, which is then proceeded by oxidation-reduction, chelation, modification of the metallic complex, and biomethylation ( Pratush et al., 2018 ). The enzymatic catalysis by microbes solubilizes the metals with higher oxidation state to lower oxidation state, for instance, Thiobacillus ferrooxidans and T . thiooxidans are responsible for the enzymatic oxidation of Uranium ( Cumberland et al., 2016 ). The isolation of microorganisms responsible for the degradation of HMs could occur from aerobic as well as anaerobic locations. However, in comparison to anaerobic microorganisms, aerobic microbes are more willing for bioremediation ( Azubuike et al., 2016 ). Microorganisms carry out the transportation of HMs utilizing membrane-linked transport mechanisms and transform them into non-hazardous forms ( Igiri et al., 2018 ). Microorganisms use processes like biosorption, bioaccumulation, biotransformation, and bioleaching to stay alive in a metal-polluted environment ( Figure 2 and Table 2 ). These techniques have been used in clean-up processes ( Gadd, 2000 ; Lin and Lin, 2005 ).
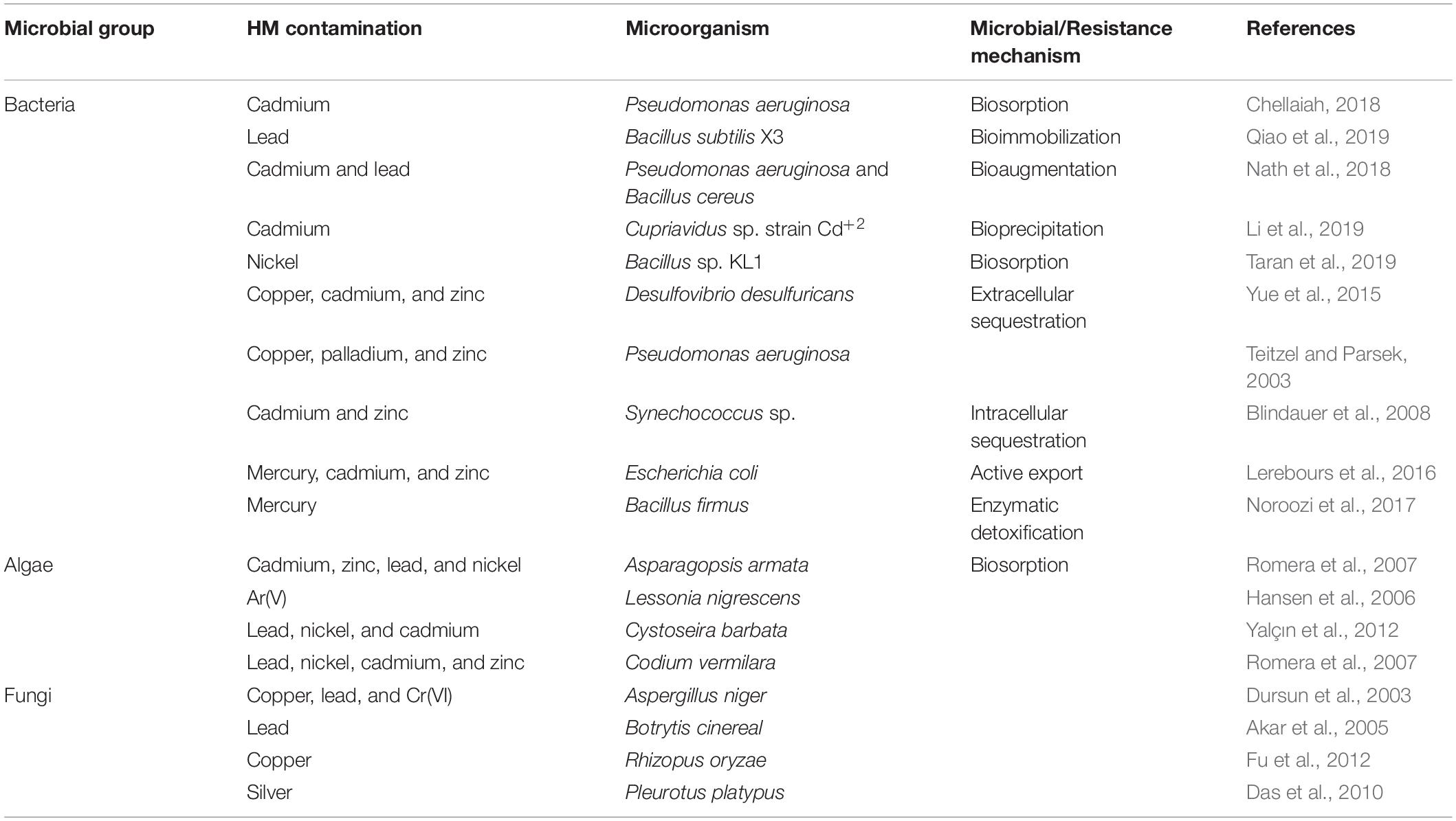
Table 2. Microbe-mediated remediation and resistance mechanism of heavy metals.
Bioaccumulation and Biosorption
Both bioaccumulation and biosorption are the processes utilizing which microbes or biomass gets bound to the HMs and pollutants from the surroundings and concentrates them ( Joutey et al., 2015 ). However, the working manner of the processes differs. Biosorption is a process in which microorganisms use their cellular structure to capture HM ions, which they then sorb onto the cell wall’s binding sites ( Malik, 2004 ). This is a passive uptake process and does not depend upon the metabolic cycle. Two common methods for the bioremediation of HMs are adsorption and absorption onto the cell surface of microbes. Adsorption differs from absorption in that it involves the dissolution or permeation of a fluid (the absorbate) by a liquid or solid (the absorbent) ( Jovancicevic et al., 1986 ). Adsorption, on the other hand, is a surface occurrence, whereas absorption affects the full volume of the substance. Various living creatures have been shown to be possible bio sorbents e.g., bacteria like Magnetospirillum gryphiswaldense, Bacillus subtilis , algae-like marine microalgae, and Chaetomorphalinum , fungi like Rhizopus arrhizus and yeast-like Saccharomyces cerevisiae ( Romera et al., 2006 ; Wang and Chen, 2009 ; Zhou et al., 2012 ). Bacteria, on the other hand, are regarded as the most exceptional biosorbents among all other creatures due to their high surface-to-volume ratios and numerous chemosorption active sites in their cell wall, such as teichoic acid ( Beveridge, 1989 ). Dead bacterial strains have also been considered as promising biosorbents, with biosorption abilities that exceed those of living cells of the same strain. 13–20% increased capacity for the biosorption of chromium ions was shown by dead Bacillus sphaericus as compared to the cell of its living strain ( Velásquez and Dussan, 2009 ). However, unlike biosorption, bioaccumulation by microbes is metabolically active and relies upon the import-storage system. In this system, HM ions are transported through the lipid bilayer of the cell membrane into the intracellular spaces or cytoplasm with the help of transporter proteins. This is known as active uptake or bioaccumulation. Endocytosis, ion channels, carrier-mediated transport, complex permeation, and lipid permeation are all involved in HM bioaccumulation in the bacterial membrane ( Ahemad, 2012 ; Geva et al., 2016 ; Shahpiri and Mohammadzadeh, 2018 ). Bioaccumulation studies of various metals like lead, nickel, silver, mercury, and cadmium have been reported by Ahemad (2012) . The study of cadmium by Rani and Goel (2009) discovered periplasmic and intracellular metal accumulation by P. putida 62 BN, and it was performed using transmission electron microscopy. The growing cells of Bacillus cereus M116 were shown to accumulate about 20% nickel (II) intracellularly, as reported by Naskar et al. (2020) . Lead and chromium accumulation by Aspergillus niger and Monodictys pelagic was reported by Sher and Rehman (2019) . In the process of bioleaching, metal oxides and sulfides from ores deposits and secondary wastes are solubilized by different microorganisms like fungi and bacteria ( Mishra et al., 2005 ; Jafari et al., 2019 ). Following solubilization, purification is achieved with the help of appropriate methods like ion exchange, selective precipitation, adsorption, and membrane separation ( Rohwerder et al., 2003 ).
Bioleaching
Bioleaching is performed by an extensive range of microbes and among them, acidophiles are the prominent ones. Acidophiles are chemolithotrophs that oxidize Fe (II) to Fe (III) and/or reduce sulfur to sulfuric acid and flourish in low pH environments, particularly 2.0 or below. Sulfuric acid produces ferric ions and protons, which solubilize metal sulfides and oxides from ores ( Srichandan et al., 2014 ), aiding extraction of metal by segregating metals in the solid phase from the more water-soluble phase. Bioleaching, which uses microorganisms as reduction agents, can also be used to extract and recover heavy metals ( Wang and Zhao, 2009 ). The ability of microorganisms to convert the solid chemical within contaminated soil into a soluble substance that can be removed and recovered determines the efficacy of the recovery process. Due to metal resources being non–renewable, recovering metal from industrial waste water may be a viable option for ensuring heavy metal supply ( Atkinson et al., 1998 ). Bioremediation has been offered by a number of researchers as a way to recover raw materials from effluent ( Pollmann et al., 2006 ; Gadd, 2010 ). Using an Annona squamosa -based absorbent with 0.1 M HCl, Cd(II) recovery up to 98.7% was achieved ( Isaac and Sivakumar, 2013 ). Using Pseudomonas aeruginosa biomass with 0.1 M HCl, a Cd(II) recovery up to 82% was achieved. Using volcanic rock matrix-immobilized P. putida cells with surface-displayed cyanobacterial metallothioneins at pH 2.35 ( Ni et al., 2012 ), 100% Cu(II) recovery was reported. Using activated sludge at pH 1.0 resulted in a 100% recovery of Cu(II) ( Hammaini et al., 2007 ). The introduction of an indigenous strain Enterobacter sp. J1 resulted in a Cu and Pb recovery of over 90% at pH 2 ( Lu et al., 2006 ).
Biotransformation
Biotransformation is a process in which structurally a chemical compound is altered, thereby relatively a more polar molecular is synthesized ( Asha and Vidyavathi, 2009 ; Pervaiz et al., 2013 ). In other words, this contact of metal and microorganisms causes toxic metals and organic compounds to get altered to a comparatively less hazardous form. The development of this mechanism in the microorganisms causes them to acclimatize the environmental changes. Microbial transformations can be attained through the production of new carbon bonds, isomerization, introducing functional groups, oxidation, reduction, condensation, hydrolysis, methylation, and demethylation. Transformation of metals by application of microbes has been reported. Micrococcus sp. and Acinetobacter sp. oxidize hazardous As (III) into less soluble and non-toxic As(III) and reduce its toxicity ( Nagvenkar and Ramaiah, 2010 ). Thatoi et al. (2014) reported that Cr (VI)-tolerant Bacillus sp. SFC 500-1E through NADH-dependent reductase has been shown to lessen the hazardous Cr (VI) to less toxic Cr (III).
Influence of Environmental Change on the Remediation of Heavy Metal Contaminants
The pH is important for microbial biosorption, and the optimal pH varies depending on the microbe. Firstly, pH influences the enzymatic activity in bacteria, altering the rate of HM microbial metabolism ( Morton-Bermea et al., 2002 ). Secondly, pH alters the microorganism’s surface charge, affecting its ability to adsorb HM ions ( Galiulin and Galiulina, 2008 ). Besides this, pH has an impact on the hydration and movement of a variety of metal ions in the soil ( Dermont et al., 2008 ). Both Rodríguez-Tirado et al. (2012) and Wierzba (2015) found that the rate of HMs removal by microbe upsurges with an increase in pH across a certain range, but after the pH climbs to a specific level, the elimination rate begins to decline. According to a study the ideal pH range for most bacteria, is 5.5–6.5, however there are exceptions ( Wang et al., 2001 ). For instance, Bacillus jeotgali thrives at a pH of 7. This could be because as the pH rises over a certain point, some metal ions form hydroxide precipitates, which are less prone to microbial adsorption ( Hu et al., 2010 ; Rodríguez-Tirado et al., 2012 ). Furthermore, the optimal pH for aerobic microbes and anaerobic microorganisms may differ.
The rate of absorption of HM is mostly influenced by ambient temperature, which impacts the growth and multiplication of microorganisms ( Fang et al., 2011 ). Various bacteria have different optimal temperatures ( Acar and Malkoc, 2004 ) for example, Acidianus brierleyi and Sulfolobussolfa-tataricus are very thermophilic bacteria, while, Thiobacillus acidophilus , Thiobacillus tepidarius , and Thiobacillus ferrooxidans are medium temperature bacteria.
When it comes to understanding substrate species, there are three things to keep in mind: HM ions, soil additives, and the type of soil. HM adsorption characteristics on different soils might be quite varied. According to a study, beach tidal soil (Freundlich adsorption constant K = 93.79) has a larger adsorption capacity than black soil ( K = 16.41), which is higher than yellow mud ( K = 1.17), and that the mean desorption rate of soil is Lithic Ochri-Aquic Cambosols in ascending order (0.67%), Fe-accumulic Gleyic Stagnic Anthrosols (3.62%), and Endogleyic Fe-accumulic Stagnic Anthrosols (35.85%) ( Chen et al., 1997 ). Clearly, soil’s adsorption rate and retention of HM ions result in poor mobility of HM ion, making microbial adsorption difficult to achieve ( Hu et al., 2010 ). HM ion species influence HM elimination by changing microbial generation time. The generation period of Thiobacillus ferrooxidans on sulfur as a substrate is about 10–25 h, which is significantly longer than the 6.5–15 h generation time on Fe. Moreover, the existence of metal ions in the soil affects microbial enrichment ( Kapoor and Viraraghavan, 1997 ). Individual bioavailability of Pb2+, Cd2+, and Zn2+ in the soil is often more than that of several metal ions, according to Park et al. (2016) . The adsorption of Cd2+ alone is 11.2 mg/g. Its adsorption is reduced to 3.15 mg/g in the presence of Zn2+ and Pb2+, with similar results for Zn2+ and Pb2+, displaying the reduction from 19.5 and 2.25 to 8.08 and 0.915 mg/g, respectively. Soil additions can considerably boost microbial removal of HMs, and the concentration of additives can have varied impacts on HM ion leaching rates. Tyagi et al. (2014) found that adding 20 g/L FeSO4.7H2O to a solution increased the leaching rate of Zn and Cu by 2 and 1.9 times, respectively, but not when the concentration was larger than 20 g/L. The adsorption rate of microorganisms is also affected by the concentration of HM ions. To estimate the quantification of accumulative properties of a bio-sorbent, adequate assessment is required in general ( Cervantes et al., 2001 ). The Langmuir model, whose parameters are interpretable and primarily explains the adsorption of a single-layer surface, is one of the most commonly used equations to describe the features and another Freundlich model, is mostly employed to the adsorption equilibrium of the adsorption surface equation ( Febrianto et al., 2009 ). Despite the fact that the Freundlich model is simpler, it grows unbounded, hence the Langmuir model has been more extensively employed than the Freundlich model until recently. The Langmuir model was utilized by some researchers to investigate the influence of HM concentration ( Ehrlich, 1997 ; Brunetti et al., 2012 ). They discovered that depending on the microorganisms and HM ions investigated, the concentrations of HM ions with the highest adsorption rates change. Though, the trend, which is consistent across all examples, implies that adsorption increases to a certain point and then remains constant as HM ion concentrations rise.
Modern Approaches in Microbe-Intervened Biotechnologies
Rhizoremediation: the phyto-microbial remediation system.
Rhizoremediation combines two methods for cleansing polluted substrates: phytoremediation and bioaugmentation. Rhizoremediation is the process of using microorganisms found in the rhizosphere of plants that are involved in the phytoremediation process. Application of plants and plant growth-promoting bacteria (PGPB) is being assessed as an effective and environmentally acceptable way for soil renewal and HMs elimination, among the several integrated techniques ( Ali et al., 2013 ; Sati et al., 2022 ). Many microorganisms in the rhizosphere, such as mycorrhizal fungi and other rhizospheric organisms, can help plants absorb or adsorb HMs ( Bojórquez et al., 2016 ). Joner and Leyval (1997) found that mycorrhizal plants uptake 90, 127, and 131% more Cd than non-mycorrhizal plants, when the concentration of Cd 2+ in the soil is 1, 10, and 100 mg/kg, respectively. Bissonnette et al. (2010) displayed that mycorrhiza inoculation improves the ability to absorb Cu2+, Cd2+, and Zn2+. Mycorrhizal fungi possess mycelia that grow into the soil, thereby increasing the surface area of plant roots ( Trellu et al., 2016 ). For metal extraction with plants, PGPR including Azospirillum, Alcaligenes, Agrobacterium, Arthrobacter, Burkholderia, Bacillus, Pseudomonas, Rhizobium , and Serratia , are commonly utilized ( Carlot et al., 2002 ; Glick, 2003 ). Metal transformation, immobilization, chelation, or solubilization is aided by the production of exopolysaccharides by PGPB, like oxidases, reductases, siderophores, and organic acids which promotes phytoremediation of HMs. PGPB reduces the pH of the soil by producing organic acids, which aids in the removal of HM ions. Metal resistant siderophore-producing bacteria found near the rhizosphere supply nutrients to the plants namely iron, perhaps reducing the negative consequences of metal contamination ( Dimkpa et al., 2008 ; Sinha and Mukherjee, 2008 ). Siderophore is also responsible for the formation of stable complexes with radionuclides and metals concerning environment like Cd, Ga, Al, Cu, Zn, In, and Pb ( Neubauer et al., 2000 ; Rajkumar et al., 2010 ). The synergistic effects of bioaugmentation and phytoremediation leading to rhizoremediation may overcome the difficulties that arise when both processes are employed distinctly. Moreover, the remediation of HM with the help of higher plants has also been reported. Wang et al. (2021) also found that planting Salix in Cd-polluted soil improved the diversity of beneficial microbes, such as the bacteria genera Arthrobacter and Bacillus. Anaeromyxobacter, Novosphingobium, Niabella, Niastella, Flavobacterium, Thermomonas, Lysobacter, Pedomicrobium, Solitalea, Devosia, Flavisolibacter, Mesorhizobium, Nitrospira, Rmlibacter , and Rubrivivax. Phyllobacterium and mycorrhizal genera of fungi include Amanita, Cryptococcus, Conocytes, Actinomucor, Ramicandelaber, Spizellomyces, Xylaria, Rhodotorula, Umbilicaria, Sporobolomyces, Tilletiopsis, Claroideoglomus , and Cirrenaliain plant rhizosphere.
Genetically Engineered Organisms and Modern Molecular Biology
Bioremediation using microorganisms can degrade and dissipate chemicals of complex substances, making it a long-term solution for reducing HMs contamination in soil ( Mosa et al., 2016 ; Bhatt et al., 2020a , b ). Recent advances in genetic engineering, as well as the adequacy of genetically engineered microorganisms/biocatalysts for the restoration of the environment, have shown that they are more capable than natural microbes, particularly for the removal of persistent compounds under natural environments ( de Lorenzo, 2009 ; Bhatt et al., 2020c ). By the application of various genetic and metabolic engineering approaches, the genetic material of microbes is modified, and engineered microorganisms are produced which are more efficient thus resulting in enhanced bioremediation. Single-gene editing, pathway construction, and change of existing gene sequences i.e., both coding as well as controlling sequences are included in the aspects of engineering, with a focus on the modification of rate-limiting stages of the metabolic processes ( Diep et al., 2018 ). HMs such as Fe, Cd, As, Cu, Hg, and Ni can now be eliminated with the help of engineered bacteria ( D’Souza, 2001 ; Verma and Singh, 2005 ; Azad et al., 2014 ). The rate of degradation, on the other hand, is determined by the catalytic efficiency of enzymes present in the cells or those stimulated to a specific substrate ( Kang, 2014 ). Using recombinant DNA technology, foreign genes from another creature of the same or other species are put into the genome of genetically engineered microorganisms (GEMs). The utilization of genetically modified Pseudomonas putida and Escherichia coli strain M109 harboring the merA gene to successfully remove Hg from polluted soils and sediments has been reported ( Chen and Wilson, 1997 ; Barkay et al., 2003 ; Deckwer et al., 2004 ). Azad et al. (2014) provided a thorough evaluation of the application of genetically modified bacteria and plants in the bioremediation of HMs and other organic pollutant-contaminated environments. According to a study, the addition of the mer operon from Escherichia coli , which codes for the reduction of Hg2+, into the genetically modified bacterium Deinococcus geothemalis provides the ability to microorganism to lessen the Hg pollution at high temperatures by mer genes ( Dixit et al., 2015 ). Cupriavidus metallidurans strain MSR33, which was genetically engineered with a pTP6 plasmid that provided genes merB and merG which regulate the biodegradation of Hg as well as the production of merB and merA, i.e., organomercurial lyase protein and mercuric reductase, was able to reduce Hg contamination from polluted sites ( Rojas et al., 2011 ; Dixit et al., 2015 ). The insertion of novel genes into Pseudomonas cultures using the pMR68 plasmid has also resulted in Hg resistance ( Sone et al., 2013 ). Specific genes in n-alkane-degrading microbes, such as alkB, alkB1, alkB2, alkM, aromatic hydrocarbons: xylE, and polycyclic aromatic hydrocarbons: nidA, ndoB, are frequently found on plasmids that allow horizontal gene transfer and are employed as markers to identify microbial biodegradation ( Wolejko et al., 2016 ). Microbial membrane transporters can be genetically modified to improve the bioremediation of HMs in the environment. Transporters and binding mechanisms play crucial roles in this context of HMs remediation ( Manoj et al., 2020 ). Channels, secondary carriers, and primary active transporters are the three principal types of transporters that are usually emphasized. Their location is in the inner lipid membrane such as Fps, Mer T/P, and GlpF in channel transporters; Hxt7, NixA, and Pho84 in secondary carriers; and cdtB/Ip_3327, MntA, TcHMA3, and CopA in primary active transporters. Some of them, such as the porin channels transporters, may also be found in the outer lipid membrane ( Jain et al., 2011 ). As soon as HMs comes inside the cell, numerous phytochelatins, metallothioneins, and polyphosphates work together for the sequestration of the HMs and changing microorganisms’ HM import-storage systems could boost their ability to extract HMs from water and soil ( Diep et al., 2018 ). Thus, in the fight against harmful compounds in the environment, the use of GEMs to speed up the restoration process is crucial. For the successful implementation of GEMs for bioremediation in adverse environmental conditions, the preservation of recombinant bacterial population in the soil is essential, with appropriate environmental conditions prepared and the recombinant bacteria should be capable to endure antagonism from native bacterial species ( Dixit et al., 2015 ). Consequently, further novel molecular approaches for the screening and isolation of microbes for HM bioremediation should be investigated. Multi-omics comprising genomics, metagenomics, metabolomics, proteomics and transcriptomics, and computational biology techniques have been successfully employed in gene mining that supports system biology research of microorganisms at the genetic level concerning bioremediation of HMs ( Subashchandrabose et al., 2018 ; Pande et al., 2020 ; Sayqal and Ahmed, 2021 ). Novel genes implicated in the biodegradation processes of several HM contaminants have been discovered because of high-throughput and next-generation sequencing. New technologies involve gene-editing tools like CRISPR-Cas which possesses the ability to enhance the process of bioremediation by engineering microorganisms with genes engaged in the degradation of, particularly recalcitrant substances. When compared to conventional low-throughput ZFNs and TALENs, CRISPR might be utilized to transmit a preferred set of instructions into the genome of microbe in a straightforward manner because it is a programmable, next-generation approach for high-throughput genetic manipulation ( Miglani, 2017 ). A CRISPR segment is likely for the bioremediation by the application of gRNA-guided dCas9 to control the expression of a gene. As a result, fusing transcription factors with dCas9 can either suppress boost or suppress RNA polymerase transcription, which can cause either upregulation or downregulation of the gene expression or a set of genes of interest. Although CRISPER-based approaches can be employed on a variety of mycobacteria and fungi, further applied research in the area of microbe-based removal of HMs from the environment is needed ( Shapiro et al., 2018 ). With the help of multi-omics, biotechnology has developed a large number of strains. The following are some examples Arthrobacter, Chlorella ( Gong et al., 2018 ), Stenotrophomonas maltophilia ( Cho et al., 2018 ), Rhodococcus wratislaviensis, Mycobacterium ( Gołêbiewski et al., 2014 ), Alcaligenes eutrophus, Pseudomonas putida ( Cycon and Piotrowska-Seget, 2016 ), Cyanobacterium synechocystis , Saccharomyces cerevisiae , Populus sp. ( Cai et al., 2019 ), Candida pelliculosa strain S-02, Streptomyces aureus strain HPS-0, Aspergillus niger ( Kumar et al., 2018 ), Sphingomonas sp., and Pseudomonas putida strain KT2440 ( Liu et al., 2019 ).
Nanotechnology in Microbial Bioremediation
With the application of chemical or biological methods, several types of nanoparticles have been effectively produced and studied for bioremediation of HMs, over the last decade ( Baragaño et al., 2020 ). The advantages of nano-biosorbents with an ultrafine arrangement and a great surface area include (1) enhancing chemical activity and capacity of adsorption, (2) boosting surface binding energy, and (3) lowering internal diffusion resistance ( Khati et al., 2017 ). As a result, nano-biosorbents could be used as a replacement for traditional biosorbents ( Abdi and Kazemi, 2015 ; Alviz-Gazitua et al., 2019 ). Latest advancements in the nanobiosorption model have resulted in a number of sophisticated ways that improve the complete efficiency of a conventional biosorption process while also ensuring its economic viability ( Devatha et al., 2018 ). Different functional groups, such as –NH 2 , –COOH, and –OH, are intrinsically present in nanoparticles, and tailoring the appropriate functional groups by activating physically/chemically or by modifying surface has proven to improve elimination of HMs. Bacterial strains can also produce nanoparticles that can aid in the bioremediation of HMs ( Arshad et al., 2019 ). Nanomaterials are combined with microbes to improve reduction of HMs, which makes them more effective as compared to their independent application. Factors in determining the interaction between nanomaterials and microbes include (1) the chemical properties of the nanomaterial, its particle size, coating characteristics, and shape, (2) the chemical properties of the nanomaterial, along with the shape, size of the particle, and its coating characteristics, (3) method of metabolism, (4) the nanomaterial’s crystalline phase, (5) the extent of contamination and, lastly (6) the resistance of nanomaterials to the hazardous contaminant and the prevalent ecological conditions. Microbial biostimulation, bioaccumulation, and biotransformation activities are enhanced by nanocomposites, which increase absorption, adsorption, and the number of chemical processes for the reduction of HMs ( Tan et al., 2018 ). Microbes are trapped within nanomaterials to create a nanocomposite; for example, immobilization of gram-negative Halomonas sp. within polyvinyl pyrrolidone-coated iron oxide nanoparticles was confirmed to eliminate Pb (II) and Cd (II) ( Cao et al., 2020 ). On the other hand, the microbe can function as a nanoparticle synthesizer, a method known as green synthesis. Though separating or recovering HMs from nanomaterials, is a time-consuming/laborious technique and hence magnetic nanoparticles have gained considerable attention in recent years, wherein surface amendment, coating of iron/iron oxides, and encapsulation focused for simple separation or retrieval of HMs.
Throughout the world, HM contamination causes severe environmental problems. In this review, several technical strategies i.e., microbe-based as well as hybrid have been discussed, that are currently being employed to mitigate HM contamination in soils and other contaminated surroundings. Because of the contribution in the regulation of biogeochemical cycles that influence climate, soil structure, and fertility, the environmental microbiome is thought to play a critical role. Microbe-mediated bioremediation should be given high attention from a practical standpoint since microbes have a variety of natural roles and mechanisms that considers them a great candidate for the clean-up of the polluted site, management of wastes, and sustainable agriculture. Although microbes are being employed to improve the effectiveness of HM removal from the soil, there is still room for improvement.
Directions to the Future Research
To create approaches that support better tolerance of HMs in microbes, more emphasis should be placed on understanding the physical, chemical, and biological characteristics of microbes in the prevalence of HMs in soil, water, and gaseous surroundings ( Njoku et al., 2020 ). Furthermore, the application of additives in bioremediation, such as surfactants, might expand the region of the interphase between microorganisms and pollutants, pushing microbes beyond their bioremediation limits. Recently, yeast has been genetically modified to have plant-like properties and to act as hyperaccumulators of several HMs in the aqueous environment ( Sun et al., 2020 ). Other bacteria could be developed in the same way to help in clean-up of HMs. More emphasis should be paid to algae in future study studies, as it is considered an effective microbe for the sorption of HMs from the soil. Because of the better genetic abilities and tolerance to HMs, omics-based techniques are advantageous for the production of improved industrial strains that are tolerant to the prevalent environmental surroundings ( Hemmat-Jou et al., 2018 ). Furthermore, as mentioned in this study, the application of nano- and nano (bio)technologies has enormous ability to promote the use of microbial technologies to deal with HMs pollution. When nanotechnology and microbe-based technology are coupled in environmental restoration procedures, the nanoparticles will promote the elimination of greater pollutant loads, reducing the toxicity-based inhibition of the contaminant on the microbe ( Ma and Zhang, 2008 ). As a result, combining various traditional procedures and current technology could be a potential choice if they could improve relevant material qualities and speed up the restoration process. All life forms and the natural ecosystem are in danger from pollution caused by HMs in soil, water, and agrarian land. Sustainable policies have been developed and revised regularly; nevertheless, awareness of the negative effects, as well as knowledge of how to reduce HMs contaminantion in the soil, should be expanded.
Author Contributions
VP conceptualized and wrote the manuscript. SP wrote the manuscript and made the diagrams. DS wrote the manuscript. PB and MS supervised, helped in writing, reviewing, and editing the manuscript. All authors contributed to the article and approved the submitted version.
We are thankful to the Department of Zoology, Soban Singh Jeena University, Almora (Uttarakhand), India, and DST FIST grant SR/FST/LS-I/2018/131 for providing the facility for this work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdi, O., and Kazemi, M. (2015). A review study of biosorption of heavy metals and comparison between different biosorbents. J. Mater Environ. Sci. 6, 1386–1399.
Google Scholar
Acar, F. N., and Malkoc, E. (2004). The removal of chromium (VI) from aqueous solutions by Fagus orientalis L. Bioresour. Technol. 94, 13–15. doi: 10.1016/j.biortech.2003.10.032
PubMed Abstract | CrossRef Full Text | Google Scholar
Ahemad, M. (2012). Implications of bacterial resistance against heavy metals in bioremediation: a review. J. Inst. Integr. Omics Appl. Biotechnol. IIOAB 3, 39–46.
Akar, T., Tunali, S., and Kiran, I. (2005). Botrytis cinerea as a new fungal biosorbent for removal of Pb (II) from aqueous solutions. Biochem. Eng. J. 25, 227–235. doi: 10.1016/j.bej.2005.05.006
CrossRef Full Text | Google Scholar
Al-Gheethi, A. A. S., Lalung, J., Noman, E. A., Bala, J. D., and Norli, I. (2015). Removal of heavy metals and antibiotics from treated sewage effluent by bacteria. Clean Technol. Environ. Policy 17, 2101–2123. doi: 10.1007/s10098-015-0968-z
Ali, H., Khan, E., and Sajad, M. A. (2013). Phytoremediation of heavy metals—concepts and applications. Chemosphere 91, 869–881. doi: 10.1016/J.CHEMOSPHERE.2013.01.075
Alviz-Gazitua, P., Fuentes-Alburquenque, S., Rojas, L. A., Turner, R. J., Guiliani, N., and Seeger, M. (2019). The response of Cupriavidus metallidurans CH34 to cadmium involves inhibition of the initiation of biofilm formation, decrease in intracellular c-di-GMP levels, and a novel metal regulated phosphodiesterase. Front. Microbiol. 10:1499. doi: 10.3389/fmicb.2019.01499
Anjum, S. A., Ashraf, U., Imran, K., Tanveer, M., Shahid, M., Shakoor, A., et al. (2017). Phyto-toxicity of chromium in maize: oxidative damage, osmolyte accumulation, anti-oxidative defense and chromium uptake. Pedosphere 27, 262–273. doi: 10.1016/s1002-0160(17)60315-1
Arshad, F., Selvaraj, M., Zain, J., Banat, F., and Haija, M. A. (2019). Polyethylenimine modified graphene oxide hydrogel composite as an efficient adsorbent for heavy metal ions. Separat. Purif. Technol. 209, 870–880. doi: 10.1016/j.seppur.2018.06.035
Arya, S. K., and Mukherjee, A. (2014). Sensitivity of Allium cepa and Vicia faba towards cadmium toxicity. J. Soil Sci. Plant Nutr. 14, 447–458.
Arya, S. K., Basu, A., and Mukherjee, A. (2013). Lead induced genotoxicity and cytotoxicity in root cells of Allium cepa and Vicia faba . Nucleus 56, 183–189. doi: 10.1007/s13237-013-0099-z
Asha, S., and Vidyavathi, M. (2009). Cunninghamella–a microbial model for drug metabolism studies–a review. Biotechnol. Adv. 27, 16–29. doi: 10.1016/j.biotechadv.2008.07.005
Aslam, R., Bhat, T. M., Choudhary, S., and Ansari, M. Y. K. (2017). An overview on genotoxicity of heavy metal in a spice crop ( Capsicum annuum L.) in respect to cyto-morphological behaviour. Caryologia 70, 42–47. doi: 10.1080/00087114.2016.1258884
Atkinson, B. W., Bux, F., and Kasan, H. C. (1998). Considerations for application of biosorption technology to remediate metal-contaminated industrial effluents. Water SA 24, 129–135.
Ayangbenro, A. S., and Babalola, O. O. (2017). A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int. J. Environ. Res. Public Health 14:94. doi: 10.3390/ijerph14010094
Azad, M. A. K., Amin, L., and Sidik, N. M. (2014). Genetically engineered organisms for bioremediation of pollutants in contaminated sites. Chin. Sci. Bull. 59, 703–714. doi: 10.1007/s11434-013-0058-8
Azubuike, C. C., Chikere, C. B., and Okpokwasili, G. C. (2016). Bioremediation techniques–classification based on site of application: principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 32:180. doi: 10.1007/s11274-016-2137-x
Baragaño, D., Forján, R., Welte, L., and Gallego, J. L. R. (2020). Nanoremediation of As and metals polluted soils by means of graphene oxide nanoparticles. Sci. Rep. 10:1896. doi: 10.1038/s41598-020-58852-4
Barbosa, J. S., Cabral, T. M., Ferreira, D. N., Agnez-Lima, L. F., and Batistuzzo de Medeiros, S. R. (2010). Genotoxicity assessment in aquatic environment impacted by the presence of heavy metals. Ecotoxicol. Environ. Saf. 73, 320–325. doi: 10.1016/j.ecoenv.2009.10.008
Barkay, T., Miller, S. M., and Summers, A. O. (2003). Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiol. Rev. 27, 355–384. doi: 10.1016/S0168-6445(03)00046-9
Beveridge, T. J. (1989). Role of cellular design in bacterial metal accumulation and mineralization. Annu. Rev. Microbiol. 43, 147–171. doi: 10.1146/annurev.mi.43.100189.001051
Bewley, R. J. F., and Stotzky, G. (1983). Effects of cadmium and zinc on microbial activity in soil; influence of clay minerals. Part I: metals added individually. Sci. Total Environ. 31, 41–55. doi: 10.1016/0048-9697(83)90055-4
Bhati, T., Gupta, R., Yadav, N., Singh, R., Fuloria, A., Waziri, A., et al. (2019). Assessment of bioremediation potential of Cellulosimicrobium sp. for treatment of multiple heavy metals. Microbiol. Biotechnol. Lett. 47, 269–277. doi: 10.4014/mbl.1808.08006
Bhatt, P., Bhatt, K., Huang, Y., Lin, Z., and Chen, S. (2020a). Esterase is a powerful tool for the biodegradation of pyrethroid insecticides. Chemosphere 244:125507. doi: 10.1016/J.CHEMOSPHERE.2019.125507
Bhatt, P., Huang, Y., Zhang, W., Sharma, A., and Chen, S. (2020b). Enhanced cypermethrin degradation kinetics and metabolic pathway in Bacillus thuringiensis strain SG4. Microorganisms 8:223. doi: 10.3390/microorganisms8020223
Bhatt, P., Sethi, K., Gangola, S., Bhandari, G., Verma, A., Adnan, M., et al. (2020c). Modeling and simulation of atrazine biodegradation in bacteria and its effect in other living systems. J. Biomol. Struct. Dyn. 40, 3285–3295. doi: 10.1080/07391102.2020.1846623
Bissonnette, L., St-Arnaud, M., and Labrecque, M. (2010). Phytoextraction of heavy metals by two Salicaceae clones in symbiosis with arbuscular mycorrhizal fungi during the second year of a field trial. Plant Soil 332, 55–67. doi: 10.1007/s11104-009-0273-x
Blindauer, C., Harrison, M., Parkinson, J., Robinson, N., and Sadler, P. (2008). Isostructural replacement of zinc by cadmium in bacterial metallothionein. Metal Ions Biol. Med. 10, 167–173. doi: 10.1016/0300-483x(92)90110-z
Bojórquez, C., Frías Espericueta, M. G., and Voltolina, D. (2016). Removal of cadmium and lead by adapted strains of Pseudomonas aeruginosa and Enterobacter cloacae . Rev. Int. Contaminación Ambiental 32, 407–412. doi: 10.20937/rica.2016.32.04.04
Bramhachari, P. V., Kishor, P. B. K., Ramadevi, R., Rao, B. R., and Dubey, S. K. (2007). Isolation and characterization of mucous exopolysaccharide (EPS) produced by Vibrio furnissii strain VB0S3. Microbiol. Biotechnol. 17, 44–51.
PubMed Abstract | Google Scholar
Briffa, J., Sinagra, E., and Blundell, R. (2020). Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6:e04691. doi: 10.1016/j.heliyon.2020.e04691
Brunetti, G., Farrag, K., Soler-Rovira, P., Ferrara, M., Nigro, F., and Senesi, N. (2012). The effect of compost and Bacillus licheniformis on the phytoextraction of Cr, Cu, Pb and Zn by three brassicaceae species from contaminated soils in the Apulia region, Southern Italy. Geoderma 170, 322–330. doi: 10.1016/j.geoderma.2011.11.029
Burges, A., Epelde, L., and Garbisu, C. (2015). Impact of repeated single-metal and multi-metal pollution events on soil quality. Chemosphere 120, 8–15. doi: 10.1016/j.chemosphere.2014.05.037
Caetano, A. L., Marques, C. R., Gonçalves, F., da Silva, E. F., and Pereira, R. (2016). Copper toxicity in a natural reference soil: ecotoxicological data for the derivation of preliminary soil screening values. Ecotoxicology 25, 163–177. doi: 10.1007/s10646-015-1577-7
Cai, P., Gao, J., and Zhou, Y. (2019). CRISPR-mediated genome editing in non-conventional yeasts for biotechnological applications. Microb. Cell Fact. 18:63. doi: 10.1186/s12934-019-1112-2
Cao, X., Alabresm, A., Chen, Y. P., Decho, A. W., and Lead, J. (2020). Improved metal remediation using a combined bacterial and nanoscience approach. Sci. Total Environ. 704:135378. doi: 10.1016/j.scitotenv.2019.135378
Carlisle, D. M., and Clements, W. H. (2005). Leaf litter breakdown, microbial respiration and shredder production in metal-polluted streams. Freshwater Biol. 50, 380–390. doi: 10.1111/j.1365-2427.2004.01323.x
Carlot, M., Giacomini, A., and Casella, S. (2002). Aspects of plant-microbe interactions in heavy metal polluted soil. Acta Biotechnol. 22, 13–20. doi: 10.1002/1521-3846(200205)22:1/2<13::aid-abio13>3.0.co;2-9
Carrasco-Gil, S., Estebaranz-Yubero, M., Medel-Cuesta, D., Millán, R., and Hernández, L. E. (2012). Influence of nitrate fertilization on Hg uptake and oxidative stress parameters in alfalfa plants cultivated in a Hg-polluted soil. Environ. Exp. Bot. 75, 16–24. doi: 10.1016/j.envexpbot.2011.08.013
Cervantes, C., Campos-García, J., Devars, S., Gutiérrez-Corona, F., Loza-Tavera, H., Torres-Guzmán, J. C., et al. (2001). Interactions of chromium with microorganisms and plants. FEMS Microbiol. Rev. 25, 335–347. doi: 10.1111/j.1574-6976.2001.tb00581.x
Chakravarty, B., and Srivastava, S. (1992). Toxicity of some heavy metals in vivo and in vitro in Helianthus annuus . Mutat. Res. Lett. 283, 287–294. doi: 10.1016/0165-7992(92)90061-L
Chellaiah, E. R. (2018). Cadmium (heavy metals) bioremediation by Pseudomonas aeruginosa : a minireview. Appl. Water Sci. 8, 1–10. doi: 10.9734/ajsspn/2017/36868
Chen, L., Hu, J. Y., and Wang, S. Q. (2012). The role of antioxidants in photoprotection: a critical review. J. Am. Acad. Dermatol. 67, 1013–1024. doi: 10.1016/j.jaad.2012.02.009
Chen, S., and Wilson, D. B. (1997). Genetic engineering of bacteria and their potential for Hg 2+ bioremediation. Biodegradation 8, 97–103. doi: 10.1023/A:1008233704719
Chen, T. B., Wong, J. W. C., Zhou, H. Y., and Wong, M. H. (1997). Assessment of trace metal distribution and contamination in surface soils of Hong Kong. Environ. Pollut. 96, 61–68. doi: 10.1016/s0269-7491(97)00003-1
Cho, S., Shin, J., and Cho, B.-K. (2018). Applications of CRISPR/Cas system to bacterial metabolic engineering. Int. J. Mol. Sci. 19:1089. doi: 10.3390/ijms19041089
Circu, M. L., and Aw, T. Y. (2010). Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 48, 749–762. doi: 10.1016/j.freeradbiomed.2009.12.022
Cumberland, S. A., Douglas, G., Grice, K., and Moreau, J. W. (2016). Uranium mobility in organic matter-rich sediments: a review of geological and geochemical processes. Earth Sci. Rev. 159, 160–185. doi: 10.1016/j.earscirev.2016.05.010
Cuypers, A., Karen, S., Jos, R., Kelly, O., Els, K., Tony, R., et al. (2011). The cellular redox state as a modulator in cadmium and copper responses in Arabidopsis thaliana seedlings. J. Plant Physiol. 168, 309–316. doi: 10.1016/j.jplph.2010.07.010
Cycon, M., and Piotrowska-Seget, Z. (2016). Pyrethroid-degrading microorganisms and their potential for the bioremediation of contaminated soils: a review. Front. Microbiol. 7:1463. doi: 10.3389/fmicb.2016.01463
D’Souza, S. F. (2001). Microbial biosensors. Biosens. Bioelectron. 16, 337–353. doi: 10.1016/S0956-5663(01)00125-7
Dai, J., Becquer, T., Rouiller, J. H., Reversat, G., Bernhard-Reversat, F., and Lavelle, P. (2004). Influence of heavy metals on C and N mineralisation and microbial biomass in Zn-, Pb-, Cu-, and Cd-contaminated soils. Appl. Ecol. 25, 99–109. doi: 10.1016/j.apsoil.2003.09.003
Das, D., Das, N., and Mathew, L. (2010). Kinetics, equilibrium and thermodynamic studies on biosorption of Ag (I) from aqueous solution by macrofungus Pleurotus platypus . J. Hazard. Mater. 184, 765–774. doi: 10.1016/j.jhazmat.2010.08.105
de Lorenzo, V. (2009). Recombinant bacteria for environmental release: what went wrong and what we have learnt from it. Clin. Microbiol. Infect. 15, 63–65. doi: 10.1111/j.1469-0691.2008.02683.x
de Quadros, P. D., Zhalnina, K., Davis-Richardson, A. G., Drew, J. C., Menezes, F. B., Flávio, A., et al. (2016). Coal mining practices reduce the microbial biomass, richness and diversity of soil. Appl. Soil Ecol. 98, 195–203. doi: 10.1016/j.apsoil.2015.10.016
deCatanzaro, J. B., and Hutchinson, T. C. (1985). Effects of nickel addition on nitrogen mineralization, nitrification, and nitrogen leaching in some boreal forest soils. Water Air Soil Pollut. 24, 153–164.
Deckwer, W.-D., Becker, F. U., Ledakowicz, S., and Wagner-Döbler, I. (2004). Microbial removal of ionic mercury in a three-phase fluidized bed reactor. Environ. Sci. Technol. 38, 1858–1865. doi: 10.1021/es0300517
Dembitsky, V. M., and Rezanka, T. (2003). Natural occurrence of arseno compounds in plants, lichens, fungi, algal species, and microorganisms. Plant Sci. 165, 1177–1192. doi: 10.1016/j.plantsci.2003.08.007
Dermont, G., Bergeron, M., Mercier, G., and Richer-Laflèche, M. (2008). Soil washing for metal removal: a review of physical/chemical technologies and field applications. J. Hazard. Mater. 152, 1–31. doi: 10.1016/j.jhazmat.2007.10.043
Devatha, C. P., Jagadeesh, K., and Patil, M. (2018). Effect of green synthesized iron nanoparticles by Azardirachta indica in different proportions on antibacterial activity. Environ. Nanotechnol. Monit. Manag. 9, 85–94. doi: 10.1016/j.enmm.2017.11.007
Diep, P., Mahadevan, R., and Yakunin, A. F. (2018). Heavy metal removal by bioaccumulation using genetically engineered microorganisms. Front. Bioeng. Biotechnol. 6:157. doi: 10.3389/fbioe.2018.00157
Dimkpa, C. O., Svatoš, A., Dabrowska, P., Schmidt, A., Boland, W., and Kothe, E. (2008). Involvement of siderophores in the reduction of metal-induced inhibition of auxin synthesis in Streptomyces spp. Chemosphere 74, 19–25.
Dingjan, I., Verboogen, D. R., Paardekooper, L. M., Revelo, N. H., Sittig, S. P., Visser, L. J., et al. (2016). Lipid peroxidation causes endosomal antigen release for cross-presentation. Sci. Rep. 6:22064. doi: 10.1038/srep22064
Dixit, R., Malaviya, D., Pandiyan, K., Singh, U. B., Sahu, A., Shukla, R., et al. (2015). Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability 7, 2189–2212. doi: 10.3390/su7022189
Dursun, A. Y., Uslu, G., Cuci, Y., and Aksu, Z. (2003). Bioaccumulation of copper (II), lead (II) and chromium (VI) by growing Aspergillus niger . Process Biochem. 38, 1647–1651. doi: 10.1016/s0032-9592(02)00075-4
Ehrlich, H. L. (1997). Microbes and metals. Appl. Microbiol. Biotechnol. 48, 687–692.
El-Kady, A. A., and Abdel-Wahhab, M. A. (2018). Occurrence of trace metals in foodstuffs and their health impact. Trends Food Sci. Technol. 75, 36–45. doi: 10.1016/j.tifs.2018.03.001
Fang, L., Zhou, C., Cai, P., Chen, W., Rong, X., Dai, K., et al. (2011). Binding characteristics of copper and cadmium by cyanobacterium Spirulina platensis . J. Hazard. Mater. 190, 810–815. doi: 10.1016/j.jhazmat.2011.03.122
Febrianto, J., Kosasih, A. N., Sunarso, J., Ju, Y.-H., Indraswati, N., and Ismadji, S. (2009). Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J. Hazard. Mater. 162, 616–645. doi: 10.1016/j.jhazmat.2008.06.042
Ferreira, V., Koricheva, J., Duarte, S., Niyogi, D. K., and Guérold, F. (2016). Effects of anthropogenic heavy metal contamination on litter decomposition in streams – a meta-analysis. Environ. Pollut. 210, 261–270. doi: 10.1016/j.envpol.2015.12.060
Fu, Y.-Q., Li, S., Zhu, H.-Y., Jiang, R., and Yin, L.-F. (2012). Biosorption of copper (II) from aqueous solution by mycelial pellets of Rhizopus oryzae . Afr. J. biotechnol. 11, 1403–1411.
Gadd, G. M. (2000). Bioremedial potential of microbial mechanisms of metal mobilization and immobilization. Curr. Opin. Biotechnol. 11, 271–279. doi: 10.1016/s0958-1669(00)00095-1
Gadd, G. M. (2010). Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology 156, 609–643. doi: 10.1099/mic.0.037143-0
Galiulin, R. V., and Galiulina, R. A. (2008). Removing heavy metals from soil with plants. Herald Russ. Acad. Sci. 78, 141–143. doi: 10.1134/s1019331608020044
Gastaldo, J., Viau, M., Bouchot, M., Joubert, A., Charvet, A.-M., and Foray, N. (2008). Induction and repair rate of DNA damage: a unified model for describing effects of external and internal irradiation and contamination with heavy metals. J. Theor. Biol. 251, 68–81. doi: 10.1016/j.jtbi.2007.10.034
Gavrilescu, M. (2004). Removal of heavy metals from the environment by biosorption. Eng. Life Sci. 4, 219–232. doi: 10.1002/elsc.200420026
Geva, P., Kahta, R., Nakonechny, F., Aronov, S., and Nisnevitch, M. (2016). Increased copper bioremediation ability of new transgenic and adapted Saccharomyces cerevisiae strains. Environ. Sci. Pollut. Res. 23, 19613–19625. doi: 10.1007/s11356-016-7157-4
Gichner, T., Patková, Z., Száková, J., and Demnerová, K. (2006). Toxicity and DNA damage in tobacco and potato plants growing on soil polluted with heavy metals. Ecotoxicol. Environ. Saf. 65, 420–426. doi: 10.1016/j.ecoenv.2005.08.006
Giller, K. E., Witter, E., and Mcgrath, S. P. (1998). Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biol. Biochem 30, 1389–1414. doi: 10.1016/s0038-0717(97)00270-8
Giovanella, P., Cabral, L., Bento, F. M., Gianello, C., and Camargo, F. A. O. (2016). Mercury (II) removal by resistant bacterial isolates and mercuric (II) reductase activity in a new strain of Pseudomonas sp. B50A. New Biotechnol. 33, 216–223. doi: 10.1016/j.nbt.2015.05.006
Glick, B. R. (2003). Phytoremediation: synergistic use of plants and bacteria to clean up the environment. Biotechnol. Adv. 21, 383–393. doi: 10.1016/s0734-9750(03)00055-7
Gołêbiewski, M., Deja-Sikora, E., Cichosz, M., Tretyn, A., and Wróbel, B. (2014). 16S rDNA pyrosequencing analysis of bacterial community in heavy metals polluted soils. Microb. Ecol. 67, 635–647. doi: 10.1007/s00248-013-0344-7
Gong, T., Xu, X., Dang, Y., Kong, A., Wu, Y., Liang, P., et al. (2018). An engineered Pseudomonas putida can simultaneously degrade organophosphates, pyrethroids and carbamates. Sci. Total Environ. 628–629, 1258–1265. doi: 10.1016/j.scitotenv.2018.02.143
Grujić, S., Vasić, S., Radojević, I., Čomić, L., and Ostojić, A. (2017). Comparison of the Rhodotorula mucilaginosa biofilm and planktonic culture on heavy metal susceptibility and removal potential. Water Air Soil Pollut. 228, 73.
Gupta, A. K., Singh, R. P., Singh, A., and Ibrahim, M. H. (2010). “Effects of heavy metal and metalloid contamination on the soil microbial response: an overview,” in Microbial Ecology of Tropical Soils , eds A. S. F. de Araújo and M. D. V. B. Figueiredo (Hauppauge, NY: Nova Science Publisher), 1–16. doi: 10.1002/ieam.1513
Hammaini, A., González, F., Ballester, A., Blázquez, M. L., and Munoz, J. A. (2007). Biosorption of heavy metals by activated sludge and their desorption characteristics. J. Environ. Manag. 84, 419–426. doi: 10.1016/j.jenvman.2006.06.015
Hamsa, N., Yogesh, G. S., Koushik, U., and Patil, L. (2017). Nitrogen transformation in soil: effect of heavy metals. Int. J. Curr. Microbiol. Appl. Sci 6, 816–832. doi: 10.20546/ijcmas.2017.605.092
Hansen, H. K., Ribeiro, A., and Mateus, E. (2006). Biosorption of arsenic (V) with Lessonia nigrescens . Miner. Eng. 19, 486–490. doi: 10.1016/j.mineng.2005.08.018
Harris, G. K., and Shi, X. (2003). Signaling by carcinogenic metals and metal-induced reactive oxygen species. Mutat. Res. 533, 183–200. doi: 10.1016/j.mrfmmm.2003.08.025
Hemmat-Jou, M. H., Safari-Sinegani, A. A., Mirzaie-Asl, A., and Tahmourespour, A. (2018). Analysis of microbial communities in heavy metals-contaminated soils using the metagenomic approach. Ecotoxicology 27, 1281–1291. doi: 10.1007/s10646-018-1981-x
Higham, D. P., Sadler, P. J., and Scawen, M. D. (1986). Cadmium-binding proteins in Pseudomonas putida: pseudothioneins. Environ. Health Perspect. 65, 5–11. doi: 10.1289/ehp.86655
Hirata, A., Corcoran, G. B., and Hirata, F. (2011). Carcinogenic heavy metals, As 3+ and Cr 6+ , increase affinity of nuclear mono-ubiquitinated annexin A1 for DNA containing 8-oxo-guanosine, and promote translesion DNA synthesis. Toxicol. Appl. Pharmacol. 252, 159–164. doi: 10.1016/j.taap.2011.01.022
Hough, R. L., Breward, N., Young, S. D., Crout, N. M., Tye, A. M., Moir, A. M., et al. (2004). Assessing potential risk of heavy metal exposure from consumption of home produced vegetables by urban populations. Environ. Health Perspect. 112, 215–221. doi: 10.1289/ehp.5589
Hogsden, K. L., and Harding, J. S. (2012). Consequences of acid mine drainage for the structure and function of benthic stream communities: a review. Freshw. Sci. 31, 108–120. doi: 10.1899/11-091.1
Hu, N., Luo, Y., Song, J., Wu, L., and Zhang, H. (2010). Influences of soil organic matter, pH and temperature on Pb sorption by four soils in Yangtze River Delta. Acta Pedol. Sin. 47, 246–252.
Igiri, B. E., Okoduwa, S. I. R., Idoko, G. O., Akabuogu, E. P., Adeyi, A. O., and Ejiogu, I. K. (2018). Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: a review. J. Toxicol. 2018:2568038. doi: 10.1155/2018/2568038
Illmer, P., and Schinner, F. (1991). Effects of lime and nutrient salts on the microbiological activities of forest soils. Biol. Fertil. Soils 11, 261–266. doi: 10.1007/bf00335845
Isaac, C. P. J., and Sivakumar, A. (2013). Removal of lead and cadmium ions from water using Annona squamosa shell: kinetic and equilibrium studies. Desalination Water Treat. 51, 7700–7709. doi: 10.1080/19443994.2013.778218
Islam, E., Khan, M. T., and Irem, S. (2015). Biochemical mechanisms of signaling: perspectives in plants under arsenic stress. Ecotoxicol. Environ. Saf. 114, 126–133. doi: 10.1016/j.ecoenv.2015.01.017
Jafari, M., Abdollahi, H., Shafaei, S. Z., Gharabaghi, M., Jafari, H., Akcil, A., et al. (2019). Acidophilic bioleaching: a review on the process and effect of organic–inorganic reagents and materials on its efficiency. Miner. Process. Extract. Metall. Rev. 40, 87–107. doi: 10.1080/08827508.2018.1481063
Jain, P. K., Gupta, V. K., Bajpai, V., Lowry, M., and Jaroli, D. P. (2011). “GMO’s: perspective of bioremediation,” in Recent Advances in Environmental Biotechnology , eds P. K. Jain, V. K. Gupta, and V. Bajpai (Saarbrücken: LAP Lambert Academic Publishing AG and Co. KG), 6–23.
Jibril, S. A., Hassan, S. A., Ishak, C. F., and Megat Wahab, P. E. (2017). Cadmium toxicity affects phytochemicals and nutrient elements composition of lettuce ( Lactuca sativa L.). Adv. Agric. 2017:1236830. doi: 10.1155/2017/1236830
Joner, E. J., and Leyval, C. (1997). Uptake of 109Cd by roots and hyphae of a Glomus mosseae / Trifolium subterraneum mycorrhiza from soil amended with high and low concentrations of cadmium. New Phytol. 135, 353–360. doi: 10.1046/j.1469-8137.1997.00633.x
Jones, G. C., Corin, K. C., van Hille, R. P., and Harrison, S. T. (2011). The generation of toxic reactive oxygen species (ROS) from mechanically activated sulphide concentrates and its effect on thermophilic bioleaching. Miner. Eng. 241, 1198–1208. doi: 10.1016/j.mineng.2011.05.016
Joutey, N. T., Sayel, H., Bahafid, W., and El Ghachtouli, N. (2015). Mechanisms of hexavalent chromium resistance and removal by microorganisms. Rev. Environ. Contam. Toxicol. 233, 45–69. doi: 10.1007/978-3-319-10479-9_2
Jovancicevic, V., Bockris, J., Carbajal, J. L., Zelenay, P., and Mizuno, T. (1986). Adsorption and absorption of chloride ions on passive iron systems. J. Electrochem. Soc. 133:2219. doi: 10.1149/1.2108377
Kang, J. W. (2014). Removing environmental organic pollutants with bioremediation and phytoremediation. Biotechnol. Lett. 36, 1129–1139. doi: 10.1007/s10529-014-1466-9
Kapoor, A., and Viraraghavan, T. (1997). Heavy metal biosorption sites in Aspergillus niger . Bioresour. Technol. 61, 221–227. doi: 10.1016/s0960-8524(97)00055-2
Khati, P., Sharma, A., Gangola, S., Kumar, R., Bhatt, P., and Kumar, G. (2017). Impact of agri-usable nanocompounds on soil microbial activity: an indicator of soil health. CLEAN Soil Air Water 45:1600458. doi: 10.1002/clen.201600458
Kozlov, M. V., and Zvereva, E. L. (2015). Decomposition of birch leaves in heavily polluted industrial barrens: relative importance of leaf quality and site of exposure. Environ. Sci. Pollut. Res. 22, 9943–9950. doi: 10.1007/s11356-015-4165-8
Kumar, R., Sharma, A. K., Singh, P., Dhir, B., and Mehta, D. (2014). Potential of some fungal and bacterial species in bioremediation of heavy metals. J. Nucl. Phys. Mater. Sci. Radiat. Appl. 1, 213–223.
Kumar, S., Kaushik, G., Dar, M. A., Nimesh, S., Lopez-Chuken, U. J., and Villarreal-Chiu, J. F. (2018). Microbial degradation of organophosphate pesticides: a review. Pedosphere 28, 190–208. doi: 10.1016/S1002-0160(18)60017-7
Kushwaha, A., Rani, R., Kumar, S., Thomas, T., David, A. A., and Ahmed, M. (2017). A new insight to adsorption and accumulation of high lead concentration by exopolymer and whole cells of lead-resistant bacterium Acinetobacter junii L. Pb1 isolated from coal mine dump. Environ. Sci. Pollut. Res. 24, 10652–10661. doi: 10.1007/s11356-017-8752-8
Lan, M. M., Liu, C., Liu, S. J., Qiu, R. L., and Tang, Y. T. (2020). Phytostabilization of cd and Pb in highly polluted farmland soils using ramie and amendments. Int. J. Environ. Res. Public Health 17:1661. doi: 10.3390/ijerph17051661
Lerebours, A., To, V. V., and Bourdineaud, J. (2016). Danio rerio ABC transporter genes abcb3 and abcb7 play a protecting role against metal contamination. J. Appl. Toxicol. 36, 1551–1557. doi: 10.1002/jat.3313
Li, F., Zheng, Y., Tian, J., Ge, F., Liu, X., Tang, Y., et al. (2019). Cupriavidus sp. strain Cd02-mediated pH increase favoring bioprecipitation of Cd 2+ in medium and reduction of cadmium bioavailability in paddy soil. Ecotoxicol. Environ. Saf. 184:109655.
Lima, A. I. G., Corticeiro, S. C., Figueira, E. M., and de, A. P. (2006). Glutathione-mediated cadmium sequestration in Rhizobium leguminosarum . Enzyme Microb. Technol. 39, 763–769. doi: 10.1016/j.enzmictec.2005.12.009
Lin, C.-C., and Lin, H.-L. (2005). Remediation of soil contaminated with the heavy metal (Cd 2+ ). J. Hazard. Mater. 122, 7–15.
Liu, S.-H., Zeng, G.-M., Niu, Q.-Y., Liu, Y., Zhou, L., Jiang, L.-H., et al. (2017). Bioremediation mechanisms of combined pollution of PAHs and heavy metals by bacteria and fungi: a mini review. Bioresour. Technol. 224, 25–33. doi: 10.1016/j.biortech.2016.11.095
Liu, X.-X., Hu, X., Cao, Y., Pang, W.-J., Huang, J.-Y., Guo, P., et al. (2019). Biodegradation of phenanthrene and heavy metal removal by acid-tolerant Burkholderia fungorum FM-2. Front. Microbiol. 10:408. doi: 10.3389/fmicb.2019.00408
Lu, W.-B., Shi, J.-J., Wang, C.-H., and Chang, J.-S. (2006). Biosorption of lead, copper and cadmium by an indigenous isolate Enterobacter sp. J1 possessing high heavy-metal resistance. J. Hazard. Mater. 134, 80–86. doi: 10.1016/j.jhazmat.2005.10.036
Ma, L., and Zhang, W. (2008). Enhanced biological treatment of industrial wastewater with bimetallic zero-valent iron. Environ. Sci. Technol. 42, 5384–5389. doi: 10.1021/es801743s
Maksymiec, W. (2007). Signaling responses in plants to heavy metal stress. Acta Physiol. Plant. 29:177. doi: 10.1007/s11738-007-0036-3
Malik, A. (2004). Metal bioremediation through growing cells. Environ. Int. 30, 261–278. doi: 10.1016/j.envint.2003.08.001
Malik, Z., Ahmad, M., Abassi, G. H., Dawood, M., Hussain, A., and Jamil, M. (2017). “Agrochemicals and soil microbes: interaction for soil health,” in Xenobiotics in the Soil Environment , eds M. Hashmi, V. Kumar, and A. Varma (Cham: Springer), 139–152. doi: 10.1007/978-3-319-47744-2_11
Manoj, S. R., Karthik, C., Kadirvelu, K., Arulselvi, P. I., Shanmugasundaram, T., Bruno, B., et al. (2020). Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: a review. J. Environ. Manag. 254, 109779. doi: 10.1016/j.jenvman.2019.109779
Marschner, B., and Kalbitz, K. (2003). Controls of bioavailability and biodegradability of dissolved organic matter in soils. Geoderma 113, 211–235. doi: 10.1016/s0016-7061(02)00362-2
Miglani, G. S. (2017). Genome editing in crop improvement: present scenario and future prospects. J. Crop Improv. 31, 453–559. doi: 10.1080/15427528.2017.1333192
Mishra, D., Kim, D.-J., Ahn, J.-G., and Rhee, Y.-H. (2005). Bioleaching: a microbial process of metal recovery; a review. Metals Mater. Int. 11, 249–256. doi: 10.1007/bf03027450
Morton-Bermea, O., Álvarez, E. H., Gaso, I., and Segovia, N. (2002). Heavy metal concentrations in surface soils from Mexico City. Bull. Environ. Contam. Toxicol. 68, 383–388. doi: 10.1007/s001280265
Mosa, K. A., Saadoun, I., Kumar, K., Helmy, M., and Dhankher, O. P. (2016). Potential biotechnological strategies for the cleanup of heavy metals and metalloids. Front. Plant Sci. 7:303. doi: 10.3389/fpls.2016.00303
Mudila, H., Prasher, P., Kumar, M., Kapoor, H., Kumar, A., Zaidi, M. G. H., et al. (2019). An insight into cadmium poisoning and its removal from aqueous sources by graphene adsorbents. Int. J. Environ. Health Res. 29, 1–21. doi: 10.1080/09603123.2018.1506568
Nagvenkar, G. S., and Ramaiah, N. (2010). Arsenite tolerance and biotransformation potential in estuarine bacteria. Ecotoxicology 19, 604–613. doi: 10.1007/s10646-009-0429-8
Naskar, A., Majumder, R., and Goswami, M. (2020). Bioaccumulation of Ni (II) on growing cells of Bacillus sp.: response surface modeling and mechanistic insight. Environ. Technol. Innov. 20:101057. doi: 10.1016/j.eti.2020.101057
Nath, S., Deb, B., and Sharma, I. (2018). Isolation of toxic metal-tolerant bacteria from soil and examination of their bioaugmentation potentiality by pot studies in cadmium- and lead-contaminated soil. Int. Microbiol. 21, 35–45. doi: 10.1007/s10123-018-0003-4
Nayak, A. K., Raja, R., Rao, K. S., Shukla, A. K., Mohanty, S., Shahid, M., et al. (2015). Effect of fly ash application on soil microbial response and heavy metal accumulation in soil and rice plant. Ecotoxicol. Environ. Saf. 114, 257–262. doi: 10.1016/j.ecoenv.2014.03.033
Neubauer, U., Furrer, G., Kayser, A., and Schulin, R. (2000). Siderophores, NTA, and citrate: potential soil amendments to enhance heavy metal mobility in phytoremediation. Int. J. Phytoremediation 2, 353–368. doi: 10.1080/15226510008500044
Ni, H., Xiong, Z., Ye, T., Zhang, Z., Ma, X., and Li, L. (2012). Biosorption of copper (II) from aqueous solutions using volcanic rock matrix-immobilized Pseudomonas putida cells with surface-displayed cyanobacterial metallothioneins. Chem. Eng. J. 204, 264–271. doi: 10.1016/j.cej.2012.05.029
Njoku, K. L., Asunmo, M. O., Ude, E. O., Adesuyi, A. A., and Oyelami, A. O. (2020). The molecular study of microbial and functional diversity of resistant microbes in heavy metal contaminated soil. Environ. Technol. Innov. 17:100606. doi: 10.1016/j.eti.2020.100606
Noroozi, M., Amoozegar, M. A., Pourbabaee, A. A., Naghavi, N. S., and Nourmohammadi, Z. (2017). Isolation and characterization of mercuric reductase by newly isolated halophilic bacterium, Bacillus firmus MN8. Glob. J. Environ. Sci. Manag. 3:427.
Nwuche, C. O., and Ugoji, E. O. (2008). Effects of heavy metal pollution on the soil microbial activity. Int. J. Environ. Sci. Technol. 5, 409–414.
Oliveira, A., and Pampulha, M. E. (2006). Effects of long-term heavy metal contamination on soil microbial characteristics. J. Biosci. Bioeng. 102, 157–161. doi: 10.1263/jbb.102.157
Osmani, M., Bani, A., and Hoxha, B. (2015). Heavy metals and ni phytoextractionin in the metallurgical area soils in Elbasan. Albanian J. Agric. Sci. 14:414.
Pan, J., and Yu, L. (2011). Effects of Cd or/and Pb on soil enzyme activities and microbial community structure. Ecol. Eng. 37, 1889–1894. doi: 10.1016/j.ecoleng.2011.07.002
Panagos, P., Ballabio, C., Lugato, E., Jones, A., Borrelli, P., Scarpa, S., et al. (2018). Potential sources of anthropogenic copper inputs to European agricultural soils. Sustainability 10:2380. doi: 10.3390/su10072380
Pande, V., Pandey, S., Sati, D., Pande, V., and Samant, M. (2020). Bioremediation: an emerging effective approach towards environment restoration. Environ. Sustain. 3, 91–103. doi: 10.1007/s42398-020-00099-w
Park, J. H., Lee, S.-J., Lee, M.-E., and Chung, J. W. (2016). Comparison of heavy metal immobilization in contaminated soils amended with peat moss and peat moss-derived biochar. Environ. Sci. Process. Impacts 18, 514–520. doi: 10.1039/c6em00098c
Peralta-Videa, J. R., Lopez, M. L., Narayan, M., Saupe, G., and Gardea-Torresdey, J. (2009). The biochemistry of environmental heavy metal uptake by plants: implications for the food chain. Int. J. Biochem. Cell Biol. 41, 1665–1677. doi: 10.1016/j.biocel.2009.03.005
Pervaiz, I., Ahmad, S., Madni, M. A., Ahmad, H., and Khaliq, F. H. (2013). Microbial biotransformation: a tool for drug designing. Appl. Biochem. Microbiol. 49, 437–450. doi: 10.7868/s0555109913050097
Plum, L. M., Rink, L., and Haase, H. (2010). The essential toxin: impact of zinc on human health. Int. J. Environ. Res. Public Health 7, 1342–1365. doi: 10.3390/ijerph7041342
Pollmann, K., Raff, J., Merroun, M., Fahmy, K., and Selenska-Pobell, S. (2006). Metal binding by bacteria from uranium mining waste piles and its technological applications. Biotechnol. Adv. 24, 58–68. doi: 10.1016/j.biotechadv.2005.06.002
Pourrut, B., Jean, S., Silvestre, J., and Pinelli, E. (2011). Lead-induced DNA damage in Vicia faba root cells: potential involvement of oxidative stress. Mutat. Res. 726, 123–128. doi: 10.1016/j.mrgentox.2011.09.001
Pratush, A., Kumar, A., and Hu, Z. (2018). Adverse effect of heavy metals (As, Pb, Hg, and Cr) on health and their bioremediation strategies: a review. Int. Microbiol. 21, 97–106. doi: 10.1007/s10123-018-0012-3
Qiao, W., Zhang, Y., Xia, H., Luo, Y., Liu, S., Wang, S., et al. (2019). Bioimmobilization of lead by Bacillus subtilis X3 biomass isolated from lead mine soil under promotion of multiple adsorption mechanisms. R. Soc. Open Sci. 6:181701. doi: 10.1098/rsos.181701
Qin, R., Wang, C., Chen, D., Björn, L. O., and Li, S. (2015). Copper-induced root growth inhibition of Allium cepa var. agrogarum L. involves disturbances in cell division and DNA damage. Environ. Toxicol. Chem. 34, 1045–1055. doi: 10.1002/etc.2884
Rajkumar, M., Ae, N., Prasad, M. N. V., and Freitas, H. (2010). Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 28, 142–149. doi: 10.1016/j.tibtech.2009.12.002
Rani, A., and Goel, R. (2009). “Strategies for crop improvement in contaminated soils using metal-tolerant bioinoculants,” in Microbial Strategies for Crop Improvement , eds M. Khan, A. Zaidi, and J. Musarrat (Berlin: Springer), 85–104. doi: 10.1007/978-3-642-01979-1_5
Rasulov, B. A., Yili, A., and Aisa, H. A. (2013). Biosorption of metal ions by exopolysaccharide produced by Azotobacter chroococcum XU1. J. Environ. Protect. 4:36802.
Remenar, M., Kamlarova, A., Harichova, J., Zámocký, M., and Ferianc, P. (2018). The heavy-metal resistance determinant of newly isolated bacterium from a nickel-contaminated soil in Southwest Slovakia. Pol. J. Microbiol. 67:191. doi: 10.21307/pjm-2018-022
Rodríguez-Tirado, V., Green-Ruiz, C., and Gómez-Gil, B. (2012). Cu and Pb biosorption on Bacillus thioparans strain U3 in aqueous solution: Kinetic and equilibrium studies. Chem. Eng. J. 181, 352–359. doi: 10.1016/j.cej.2011.11.091
Rohwerder, T., Gehrke, T., Kinzler, K., and Sand, W. (2003). Bioleaching review part A. Appl. Microbiol. Biotechnol. 63, 239–248. doi: 10.1007/s00253-003-1448-7
Rojas, L. A., Yáñez, C., González, M., Lobos, S., Smalla, K., and Seeger, M. (2011). Characterization of the metabolically modified heavy metal-resistant Cupriavidus metallidurans strain MSR33 generated for mercury bioremediation. PLoS One 6:e17555. doi: 10.1371/journal.pone.0017555
Romera, E., Gonzalez, F., Ballester, A., Blázquez, M. L., and Munoz, J. A. (2006). Biosorption with algae: a statistical review. Crit. Rev. Biotechnol. 26, 223–235. doi: 10.1080/07388550600972153
Romera, E., González, F., Ballester, A., Blázquez, M. L., and Munoz, J. A. (2007). Comparative study of biosorption of heavy metals using different types of algae. Bioresour. Technol. 98, 3344–3353. doi: 10.1016/j.biortech.2006.09.026
Rother, J. A., Millbank, J. W., and Thornton, I. (1982). Seasonal fluctuations in nitrogen fixation (acetylene reduction) by free-living bacteria in soils contaminated with cadmium, lead and zinc. J. Soil Sci. 33, 101–113. doi: 10.1111/j.1365-2389.1982.tb01751.x
Sati, D., Pande, V., Pandey, S. C., and Samant, M. (2022). Recent advances in PGPR and molecular mechanisms involved in drought stress resistance. J. Soil Sci. Plant Nutr. doi: 10.1007/s42729-021-00724-5
Sayqal, A., and Ahmed, O. B. (2021). Advances in heavy metal bioremediation: an overview. Appl. Bionics Biomech. 2021:1609149. doi: 10.1155/2021/1609149
Schellingen, K., van der Straeten, D., Vandenbussche, F., Prinsen, E., Remans, T., Vangronsveld, J., et al. (2014). Cadmium-induced ethylene production and responses in Arabidopsis thaliana rely on ACS2 and ACS6 gene expression. BMC Plant Biol. 14:214. doi: 10.1186/s12870-014-0214-6
Shahid, M., Pourrut, B., Dumat, C., Nadeem, M., Aslam, M., and Pinelli, E. (2014). Heavy-metal-induced reactive oxygen species: phytotoxicity and physicochemical changes in plants. Rev. Environ. Contam. Toxicol. 232, 1–44. doi: 10.1007/978-3-319-06746-9_1
Shahpiri, A., and Mohammadzadeh, A. (2018). Mercury removal by engineered Escherichia coli cells expressing different rice metallothionein isoforms. Ann. Microbiol. 68, 145–152. doi: 10.1007/s13213-018-1326-2
Shapiro, R. S., Chavez, A., and Collins, J. J. (2018). CRISPR-based genomic tools for the manipulation of genetically intractable microorganisms. Nat. Rev. Microbiol. 16, 333–339. doi: 10.1038/s41579-018-0002-7
Sharma, B., Sarkar, A., Singh, P., and Singh, R. P. (2017). Agricultural utilization of biosolids: a review on potential effects on soil and plant grown. Waste Manag. 64, 117–132. doi: 10.1016/j.wasman.2017.03.002
Sher, S., and Rehman, A. (2019). Use of heavy metals resistant bacteria—a strategy for arsenic bioremediation. Appl. Microbiol. Biotechnol. 103, 6007–6021. doi: 10.1007/s00253-019-09933-6
Silva, S., Silva, P., Oliveira, H., Gaivão, I., Matos, M., Pinto-Carnide, O., et al. (2017). Pb low doses induced genotoxicity in Lactuca sativa plants. Plant Physiol. Biochem. 112, 109–116. doi: 10.1016/j.plaphy.2016.12.026
Singh, R. P., and Agrawal, M. (2008). Potential benefits and risks of land application of sewage sludge. Waste Manag. 28, 347–358. doi: 10.1016/j.wasman.2006.12.010
Singh, R. P., and Agrawal, M. (2010). Variations in heavy metal accumulation, growth and yield of rice plants grown at different sewage sludge amendment rates. Ecotoxicol. Environ. Saf. 73, 632–641. doi: 10.1016/j.ecoenv.2010.01.020
Sinha, S., and Mukherjee, S. K. (2008). Cadmium–induced siderophore production by a high Cd-resistant bacterial strain relieved Cd toxicity in plants through root colonization. Curr. Microbiol. 56, 55–60. doi: 10.1007/s00284-007-9038-z
Sone, Y., Mochizuki, Y., Koizawa, K., Nakamura, R., Pan-Hou, H., Itoh, T., et al. (2013). Mercurial-resistance determinants in Pseudomonas strain K-62 plasmid pMR68. AMB Express 3:41. doi: 10.1186/2191-0855-3-41
Soto, D. F., Recalde, A., Orell, A., Albers, S.-V., Paradela, A., Navarro, C. A., et al. (2019). Global effect of the lack of inorganic polyphosphate in the extremophilic archaeon Sulfolobus solfataricus : a proteomic approach. J. Proteomics 191, 143–152. doi: 10.1016/j.jprot.2018.02.024
Srichandan, H., Pathak, A., Singh, S., Blight, K., Kim, D.-J., and Lee, S. W. (2014). Sequential leaching of metals from spent refinery catalyst in bioleaching–bioleaching and bioleaching–chemical leaching reactor: comparative study. Hydrometallurgy 150, 130–143. doi: 10.1016/j.hydromet.2014.09.019
Srivastava, V., de Araujo, A. S. F., Vaish, B., Bartelt-Hunt, S., Singh, P., and Singh, R. P. (2016). Biological response of using municipal solid waste compost in agriculture as fertilizer supplement. Rev. Environ. Sci. Biotechnol. 15, 677–696. doi: 10.1007/s11157-016-9407-9
Subashchandrabose, S. R., Venkateswarlu, K., Krishnan, K., Naidu, R., Lockington, R., and Megharaj, M. (2018). Rhodococcus wratislaviensis strain 9: an efficient p-nitrophenol degrader with a great potential for bioremediation. J. Hazard. Mater. 347, 176–183. doi: 10.1016/j.jhazmat.2017.12.063
Sun, G. L., Reynolds, E. E., and Belcher, A. M. (2020). Using yeast to sustainably remediate and extract heavy metals from waste waters. Nat. Sustain. 3, 303–311. doi: 10.1038/s41893-020-0478-9
Tamás, L., Mistrík, I., and Zelinová, V. (2017). Heavy metal-induced reactive oxygen species and cell death in barley root tip. Environ. Exp. Bot. 140, 34–40. doi: 10.1016/j.envexpbot.2017.05.016
Tan, W., Peralta-Videa, J. R., and Gardea-Torresdey, J. L. (2018). Interaction of titanium dioxide nanoparticles with soil components and plants: current knowledge and future research needs–a critical review. Environ. Sci. Nano 5, 257–278. doi: 10.1039/c7en00985b
Taran, M., Fateh, R., Rezaei, S., and Gholi, M. K. (2019). Isolation of arsenic accumulating bacteria from garbage leachates for possible application in bioremediation. Iran. J. Microbiol. 11:60.
Teitzel, G. M., and Parsek, M. R. (2003). Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa . Appl. Environ. Microbiol. 69, 2313–2320. doi: 10.1128/AEM.69.4.2313-2320.2003
Thapa, G., Sadhukhan, A., Panda, S. K., and Sahoo, L. (2012). Molecular mechanistic model of plant heavy metal tolerance. Biometals 25, 489–505. doi: 10.1007/s10534-012-9541-y
Thatoi, H., Das, S., Mishra, J., Rath, B. P., and Das, N. (2014). Bacterial chromate reductase, a potential enzyme for bioremediation of hexavalent chromium: a review. J. Environ. Manag. 146, 383–399. doi: 10.1016/j.jenvman.2014.07.014
Thelwell, C., Robinson, N. J., and Turner-Cavet, J. S. (1998). An SmtB-like repressor from Synechocystis PCC 6803 regulates a zinc exporter. Proc. Natl. Acad. Sci. 95, 10728–10733. doi: 10.1073/pnas.95.18.10728
Tóth, G., Hermann, T., da Silva, M. R., and Montanarella, L. (2016). Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 88, 299–309. doi: 10.1016/j.envint.2015.12.017
Trellu, C., Mousset, E., Pechaud, Y., Huguenot, D., van Hullebusch, E. D., Esposito, G., et al. (2016). Removal of hydrophobic organic pollutants from soil washing/flushing solutions: a critical review. J. Hazard. Mater. 306, 149–174. doi: 10.1016/j.jhazmat.2015.12.008
Tyagi, S., Kumar, V., Singh, J., Teotia, P., Bisht, S., and Sharma, S. (2014). Bioremediation of pulp and paper mill effluent by dominant aboriginal microbes and their consortium. Int. J. Environ. Res. 8, 561–568.
van de Poel, B., Smet, D., and van der Straeten, D. (2015). Ethylene and hormonal cross talk in vegetative growth and development. Plant Physiol. 169, 61–72. doi: 10.1104/pp.15.00724
Vardhan, K. H., Kumar, P. S., and Panda, R. C. (2019). A review on heavy metal pollution, toxicity and remedial measures: current trends and future perspectives. J. Mol. Liq. 290:111197. doi: 10.1016/j.molliq.2019.111197
Vásquez-Murrieta, M. S., Migueles-Garduño, I., Franco-Hernández, O., Govaerts, B., and Dendooven, L. (2006). C and N mineralization and microbial biomass in heavy-metal contaminated soil. Eur. J. Soil Biol. 42, 89–98. doi: 10.1016/j.ejsobi.2005.10.002
Velásquez, L., and Dussan, J. (2009). Biosorption and bioaccumulation of heavy metals on dead and living biomass of Bacillus sphaericus . J. Hazard. Mater. 167, 713–716. doi: 10.1016/j.jhazmat.2009.01.044
Venkatachalam, P., Jayalakshmi, N., Geetha, N., Sahi, S. V., Sharma, N. C., Rene, E. R., et al. (2017). Accumulation efficiency, genotoxicity and antioxidant defense mechanisms in medicinal plant Acalypha indica L. under lead stress. Chemosphere 171, 544–553. doi: 10.1016/j.chemosphere.2016.12.092
Verma, N., and Singh, M. (2005). Biosensors for heavy metals. Biometals 18, 121–129. doi: 10.1007/s10534-004-5787-3
Vijayaraj, A. S., Mohandass, C., and Joshi, D. (2019). Microremediation of tannery wastewater by siderophore producing marine bacteria. Environ. Technol. 41, 3619–3632. doi: 10.1080/09593330.2019.1615995
Wang, G., Zhang, Q., Du, W., Ai, F., Yin, Y., Ji, R., et al. (2021). Microbial communities in the rhizosphere of different willow genotypes affect phytoremediation potential in Cd contaminated soil. Sci. Total Environ. 769:145224. doi: 10.1016/j.scitotenv.2021.145224
Wang, J., and Chen, C. (2006). Biosorption of heavy metals by Saccharomyces cerevisiae : a review. Biotechnol. Adv. 24, 427–451. doi: 10.1016/J.BIOTECHADV.2006.03.001
Wang, J., and Chen, C. (2009). Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 27, 195–226. doi: 10.1016/j.biotechadv.2008.11.002
Wang, S., and Zhao, X. (2009). On the potential of biological treatment for arsenic contaminated soils and groundwater. J. Environ. Manag. 90, 2367–2376. doi: 10.1016/j.jenvman.2009.02.001
Wang, Y., Guo, J., and Liu, R. (2001). Biosorption of heavy metals by bacteria isolated from activated sludge. Huan Jing Ke Xue 22, 72–75.
Wierzba, S. (2015). Biosorption of lead (II), zinc (II) and nickel (II) from industrial wastewater by Stenotrophomonas maltophilia and Bacillus subtilis . Pol. J. Chem. Technol. 17, 79–87. doi: 10.1515/pjct-2015-0012
Wolejko, E., Wydro, U., and Loboda, T. (2016). The ways to increase efficiency of soil bioremediation. Ecol. Chem. Eng. 23:155. doi: 10.1515/eces-2016-0011
Wu, X., Cobbina, S. J., Mao, G., Xu, H., Zhang, Z., and Yang, L. (2016). A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. 23, 8244–8259. doi: 10.1007/s11356-016-6333-x
Xian, Y., Wang, M., and Chen, W. (2015). Quantitative assessment on soil enzyme activities of heavy metal contaminated soils with various soil properties. Chemosphere 139, 604–608. doi: 10.1016/j.chemosphere.2014.12.060
Yalçın, S., Sezer, S., and Apak, R. (2012). Characterization and lead (II), cadmium (II), nickel (II) biosorption of dried marine brown macro algae Cystoseira barbata . Environ. Sci. Pollut. Res. 19, 3118–3125. doi: 10.1007/s11356-012-0807-2
Yang, H.-C., Fu, H.-L., Lin, Y.-F., and Rosen, B. P. (2012). Pathways of arsenic uptake and efflux. Curr. Top. Membr. 69, 325–358. doi: 10.1016/b978-0-12-394390-3.00012-4
Yang, J., Wang, L., Chang, C., and Liu, T. (1999). Singlet oxygen is the major species participating in the induction of DNA strand breakage and 8-hydroxydeoxyguanosine adduct by lead acetate. Environ. Mol. Mutagen. 33, 194–201. doi: 10.1002/(sici)1098-2280(1999)33:3<194::aid-em3>3.0.co;2-o
Yuan, L., Zhi, W., Liu, Y., Karyala, S., Vikesland, P. J., Chen, X., et al. (2015). Lead toxicity to the performance, viability, and community composition of activated sludge microorganisms. Environ. Sci. Technol. 49, 824–830. doi: 10.1021/es504207c
Yue, Z.-B., Li, Q., Li, C., Chen, T., and Wang, J. (2015). Component analysis and heavy metal adsorption ability of extracellular polymeric substances (EPS) from sulfate reducing bacteria. Bioresour. Technol. 194, 399–402. doi: 10.1016/j.biortech.2015.07.042
Zammit, C. M., Weiland, F., Brugger, J., Wade, B., Winderbaum, L. J., Nies, D. H., et al. (2016). Proteomic responses to gold (III)-toxicity in the bacterium Cupriavidus metallidurans CH34. Metallomics 8, 1204–1216. doi: 10.1039/c6mt00142d
Zhang, S., and Klessig, D. F. (2001). MAPK cascades in plant defense signaling. Trends Plant Sci. 6, 520–527. doi: 10.1016/s1360-1385(01)02103-3
Zhang, W., Chen, L., and Liu, D. (2012). Characterization of a marine-isolated mercury-resistant Pseudomonas putida strain SP1 and its potential application in marine mercury reduction. Appl. Microbiol. Biotechnol. 93, 1305–1314. doi: 10.1007/s00253-011-3454-5
Zhang, W.-M., and Gu, S.-F. (2007). Catalytic effect of activated carbon on bioleaching of low-grade primary copper sulflde ores. Trans. Nonferrous Metals Soc. China 17, 1123–1127. doi: 10.1016/s1003-6326(07)60236-2
Zhou, W., Zhang, Y., Ding, X., Liu, Y., Shen, F., Zhang, X., et al. (2012). Magnetotactic bacteria: promising biosorbents for heavy metals. Appl. Microbiol. Biotechnol. 95, 1097–1104. doi: 10.1007/s00253-012-4245-3
Keywords : heavy metals, bioremediation, biosorption, biotransformation, bioleaching
Citation: Pande V, Pandey SC, Sati D, Bhatt P and Samant M (2022) Microbial Interventions in Bioremediation of Heavy Metal Contaminants in Agroecosystem. Front. Microbiol. 13:824084. doi: 10.3389/fmicb.2022.824084
Received: 28 November 2021; Accepted: 31 March 2022; Published: 06 May 2022.
Reviewed by:
Copyright © 2022 Pande, Pandey, Sati, Bhatt and Samant. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pankaj Bhatt, [email protected] ; Mukesh Samant, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Recent Strategies for Bioremediation of Emerging Pollutants: A Review for a Green and Sustainable Environment
1 Department of Microbiology, Punjab Agriculture University, Ludhiana 141001, India
Diksha Garg
Banjagere veerabhadrappa thirumalesh.
2 Microbial Processes and Technology Division, CSIR-National Institute for Interdisciplinary Science and Technology, Thiruvananthapuram 695019, India
Minaxi Sharma
3 Laboratoire de Chimie Verte et Produits Biobasés, Département Agro Bioscience et Chimie, Haute Ecole Provinciale de Hainaut-Condorcet, 11 Rue de la Sucrerie, 7800 Ath, Belgium
Kandi Sridhar
4 UMR1253, Science et Technologie du Lait et de l’œuf, INRAE, L’Institut Agro Rennes-Angers, 65 Rue de Saint Brieuc, F-35042 Rennes, France
Baskaran Stephen Inbaraj
5 Department of Food Science, Fu Jen Catholic University, New Taipei City 24205, Taiwan
Manikant Tripathi
6 Biotechnology Program, Dr. Rammanohar Lohia Avadh University, Ayodhya 224001, India
Associated Data
Data that support these findings available within the article.
Environmental pollution brought on by xenobiotics and other related recalcitrant compounds have recently been identified as a major risk to both human health and the natural environment. Due to their toxicity and non-biodegradability, a wide range of pollutants, such as heavy metals, polychlorinated biphenyls, plastics, and various agrochemicals are present in the environment. Bioremediation is an effective cleaning technique for removing toxic waste from polluted environments that is gaining popularity. Various microorganisms, including aerobes and anaerobes, are used in bioremediation to treat contaminated sites. Microorganisms play a major role in bioremediation, given that it is a process in which hazardous wastes and pollutants are eliminated, degraded, detoxified, and immobilized. Pollutants are degraded and converted to less toxic forms, which is a primary goal of bioremediation. Ex situ or in situ bioremediation can be used, depending on a variety of factors, such as cost, pollutant types, and concentration. As a result, a suitable bioremediation method has been chosen. This review focuses on the most recent developments in bioremediation techniques, how microorganisms break down different pollutants, and what the future holds for bioremediation in order to reduce the amount of pollution in the world.
1. Introduction
Pollution of the environment, freshwater, and topsoil has evolved from global industrialization. Water quality has worsened as a result of human activity, such as due to mining and ultimate removal of toxic metal effluents from steel mills, battery companies, and electricity generation, posing major environmental concerns. Effluents like petroleum, polythenes, and trace metals harm the environment. Heavy metals are pollutants that exist in nature in the Earth’s crust and are difficult to decompose. They exist as ores in rocks and are recovered as minerals. High-level exposures can release heavy metals into the environment. Once in the environment, they remain toxic for much longer [ 1 ]. Many of these pollutants are mutagenic to both humans along with their surroundings. Absorbing heavy metals accumulates in the brain, liver, and kidney. Other effects on animals include cancer, nervous system damage, stunted growth, and even death [ 2 ]. Heavy metals in soils reduce food quality and quantity by inhibiting nutrient absorption, plant growth, and physiological metabolic processes. Metal-contaminated soils are being remedied using chemical, biological, and physical methods. However, physicochemical methods produce a lot of waste and pollution, so they are not valued [ 3 ]. Bioremediation is a cost-effective and practical solution for removing environmental contaminants [ 4 ]. Plant growth promotion, insect control, soil conservation, nutrient recycling, and pollutant reduction are all key functions of soil microorganisms [ 5 ]. Bioremediation has come a long way in terms of efficiency, cost, and social acceptability [ 6 ]. Bioremediation research has largely focused on bacterial processes, which have numerous applications. Archaea are known to play a role in bioremediation in many applications where bacteria are involved. Many hostile situations have degraded, requiring bioremediation. Microbes can also assist in the elimination of pollutants from hyperthermal, acidic, hypersaline, or basic industrial waste [ 7 , 8 ]. Recent research suggests that using more than one living organism will improve the efficiency and results, and allow for greater microbial diversity in bioremediation [ 8 , 9 ]. Many researchers employed bioremediation technology for the removal of organic and inorganic pollutants [ 10 , 11 , 12 ]. In a study, bioremediation technology was used for the treatment of various pollutants, including organophosphate pesticides such as chlorpyrifos, methyl parathion, and profenofos, by Aspergillus sydowii , and chloramphenicol by endophytic fungi, respectively [ 13 , 14 ]. In another study, Cymbella sp. has been shown to detoxify naproxen-polluted water with an efficiency of 97.1% [ 15 ].
A bioremediation approach requires the use of microbial enzymes to break down hydrocarbons into less harmful compounds. The widespread use of genetically-modified microorganisms that can also help to eliminate petroleum, naphthalene, toluene, benzene, and other xenobiotic chemicals is now being studied [ 16 ]. Several factors, such as temperature of the surrounding environment, aerobic or anaerobic conditions, and nutrient availability, all influence bioremediation for better outcomes. Emerging environmental pollutants, such as persistent organic compounds, heavy metals, toxins, and air pollutants that are of synthetic or natural origin, reach ecosystems mainly through anthropogenic activities and pose adverse threats to lifeforms like plants, animals, and humans [ 17 ]. One of the most economical and environmentally favorable biotechnological innovations is bioremediation. Waste management mainly relies on bioremediation. It can remove persistent organic pollutants, which are hard to breakdown and are thought to be heterologous biological substances. This review addresses the recent approaches and updated information of bioremediation strategies for eco-friendly detoxification and the effective degradation of various organic and inorganic contaminants to control environmental pollution.
2. Microorganisms Used in Bioremediation
Biological equilibrium is maintained in part by the contribution of microorganisms to nutritional chains. Bioremediation is the process of using bacteria, algae, fungi, and yeast to remove contaminated materials from the environment [ 18 ]. In the presence of hazardous compounds or any waste stream, microbes can grow at temperatures as low as −196 degrees Fahrenheit and as high as 1200 degrees Fahrenheit. The adaptability and biological systems of microbes make them an ideal choice for remediation [ 19 ]. Carbon is the most important nutrient for microorganisms. Microbes from a variety of environments were used to perform bioremediation. Achromobacter , Alcaligenes , Xanthobacter , Arthrobacter , Pseudomonas , Bacillus , Mycobacterium , Corynebacterium , Flavobacterium , Nitrosomonas , and other microorganisms [ 9 ] are examples of microbes.
2.1. Aerobic
Several microorganisms have the ability to bioremediate different types of environmental pollutants under aerobic conditions. Bacillus , Pseudomonas , Sphingomonas , Flavobacterium , Nocardia , Rhodococcus , and Mycobacterium are aerobic bacteria that can degrade a variety of complex organic compounds [ 20 ]. Pesticides, alkane hydrocarbons, and polyaromatic compounds have been shown to be degraded by these microbes. Several of these microorganisms make use of these contaminants as a source of carbon and energy [ 21 ]. In the aerobic bioremediation process, oxygen is the limiting factor for the growth of microorganisms.
2.2. Anaerobic
Amphibious bacteria that degrade and convert pollutants to fewer toxic forms are becoming increasingly popular for the bioremediation of polychlorinated biphenyls, chlorine compounds, and the chlorinated solvents, trichlorethylene and chloroform [ 22 ]. Several bacteria, such as Pseudomonas , Aeromonas, and sulfate-reducing bacteria, have been used in the bioremediation process under anaerobic conditions. Garg and Tripathi [ 23 ] reported microbial discoloration of azo dyes under different environmental situations. Azo dyes can decompose anaerobically through reduction reactions using electrons produced by the oxidation of the organic substrate(s). Due to such controlled dye decolorization events, microbe electrochemical properties would have a major impact on the effectiveness of color removal. Dyes were anaerobically decolored for industrial activities to progressively acquire such time-variant decolorized-metabolites (DMs). However, external augmentation of DMs gathered under certain conditions was carried out for improved research so that a precise system can be used [ 24 ].
3. Factors Affecting Microbial Bioremediation
Bioremediation is the process of using microorganisms such as bacteria, algae, fungi, and plants to break down, change, remove, immobilize, or detoxify various physical and chemical pollutants in the environment. Microorganisms’ enzymatic metabolic pathways speed up biochemical reactions that break down pollutants [ 25 , 26 ]. In order for microorganisms to combat pollutants, they must come into contact with compounds that provide them with the energy and nutrients they need to multiply. There are several factors such as physical, chemical, biological, soil-type, carbon and nitrogen source, type of microorganisms—i.e., single or consortium—and others that affect the process of bioremediation [ 27 ]. Microbial consortiums often have both multifunctionality and resistance because different species work together to use all substrates in the best way possible, thereby increasing the bioremediation efficiency compared to single microorganism [ 28 ]. In a study, carbon is one of the most important nutrients that help in situ bioremediation by increasing the metabolic activity of natural microbial communities and speeding up the bioremediation process to break down existing pollutants. Bioremediation may use organic carbon more than any other additive. In an anaerobic environment, many microorganisms can ferment organic carbon and make hydrogen gas [ 29 ]. In a study, bioremediation was found to be significantly affected by soil types, and the removal efficiency of pollutants varied in sandy soil and clay soil, respectively [ 30 ]. For bioremediation to be a success, it must be able to access existing microorganisms as well as the environment’s physicochemical characteristics ( Table 1 ). The microbial population responsible for degrading pollutants, the accessibility of contaminants, and the following factors are taken into consideration.
Critical factors for microbial bioremediation.
| Factors | Remarks | References |
|---|---|---|
| Biological factors | Soil microorganisms compete for carbon sources, or bacteriophages and protozoa prey on each other, all of which can affect organic compound degradation. Derivatization rates are influenced by contaminants and catalyst levels. Expressed enzymes can speed up or slow contaminant degradation. Enzymes must also be involved in contaminant metabolism to have an affinity for the contaminant and availability. The major biological factors: interaction (competition, predation, and succession), population size, and composition. | [ , ] |
| Oxygen | Biodegradation rates can be improved by using organisms that don’t require oxygen. Anaerobic decomposition occurs as most living organisms need oxygen to survive. In most cases, hydrocarbon metabolism can be boosted by the addition of oxygen. | [ ] |
| Moisture content | Microorganisms require a sufficient amount of water to achieve their growth goals. When the soil is too wet, the biodegradation agents don’t work as well. | [ ] |
| Nutrients | Nutrients can influence microbial growth and reproduction, as well as biodegradation rate and effectiveness. Optimizing the bacterial C:N:P ratio can improve biodegradation efficiency, especially when essential nutrients like N and P are supplied. Carbon, phosphorous, and nitrogen are just a few of the nutrients microorganisms need to survive. In low concentrations, hydrocarbon degradation is also limited. Adding nutrients to cold environments can increase microorganisms’ metabolic activity and thus the biodegradation rate. Aquatic biodegradation is limited by nutrient availability. Oil-eating microbes require nutrients to thrive. These essential nutrients are found in small amounts in nature. | [ , ] |
| Temperature | The most important physical factor influencing microorganism survival and hydrocarbon composition is temperature. In cold climates like the Arctic, natural oil degradation is slow, putting more pressure on microbes to clean up spilled oil. Here, the sub-zero water freezes the microbial transport channels, rendering them unable to perform their metabolic functions.Temperature affects the metabolic turnover of enzymes involved in degradation. Also, each compound’s degradation requires a specific temperature. Temperature affects microbial physiological properties and thus speeds up or slows down bioremediation. Increased microbial activity occurs at higher temperatures. It started to drop suddenly as the temperature increased or decreased, and theneventually stopped. | [ , ] |
| pH | A compound’s acidity, alkalinity, and basicity affect microbial metabolism and the removal process. Microbial growth can be predicted by the soil’s pH. Even minor pH shifts have a significant impact on metabolic processes. | [ ] |
| Sitecharacterization and selection | Before proposing a bioremediation remedy, it is necessary to conduct adequate remedial investigation work to characterize the extent of the contamination. Site selection procedures include determining the horizontal and vertical extent of contamination, defining parameters and sampling locations, and describing sampling and analysis methods. | [ ] |
| Metal ions | Metals are essential for bacteria and fungi, but excessive amounts inhibit cell metabolism. Degradation rates are influenced by metal compounds on both a direct and indirect basis. | [ ] |
| Microorganisms | High concentrations of some toxic compounds can harm microorganisms and slow decontamination process. Toxicity varies with the toxicant, concentration, and microorganisms exposed. | [ ] |
4. Principle of Bioremediation
When organic wastes are biologically degraded under controlled conditions, “bioremediation” is the term used to describe this process. Using bioremediation, harmful substances can be degraded or detoxified by providing the organisms with the nutrients and other chemicals they need to function optimally. Enzymes play a critical role in every stage of the metabolic process [ 24 , 43 ]. It is part of the family of oxidoreductases, lyases, transferases, and hydrolases. Non-specific and specific substrate affinities allow many enzymes to degrade a wide range of substrates. There must be enzymatic action on the pollutants in order for bioremediation to be successful. In order to speed up microbial growth and degradation, environmental parameters must often be manipulated during bioremediation [ 38 , 43 ]. This is because bioremediation only works when the environment is right for microbes to grow and move around.
Living organisms and fertilizers can aid in the process of bioremediation, which occurs naturally and is encouraged. Biodegradation is a key component of bioremediation technology. It’s the process of converting harmful organic pollutants like carbon dioxide and water into non-toxic or naturally-occurring inorganic compounds that are safe for use by humans, plants, animals, and aquatic life [ 44 ].
5. Types of Bioremediations
Bioremediation can be used in a plethora of ways, and some of the most commonly used methods are presented here ( Figure 1 ).

Diverse bioremediation techniques.
5.1. Biopile
In bioremediation, aeration and nutrient supplementation are used to enhance microbial metabolic activities in the piled-up polluted soil above ground. Aeration, nutrients, irrigation, leachate collection, and treatment bed systems are all included in this procedure. When it comes to ex situ biodegradation, this method is becoming increasingly popular because of its cost-effectiveness and useful features, such as pH and nutrient control. Using the biopile to clean up polluted cold environments and treat low-molecular-weight volatile pollutants is an option [ 15 , 45 ]. The biopile’s adaptability allows for a reduction in remediation time by increasing microbial activity and contaminant availability while also increasing biodegradation rate. When warm air is introduced into the biopile system to provide air and heat simultaneously, bioremediation is improved. The biopile’s remediation process has been helped by the addition of bulking agents like straw, sawdust, or wood chips. To replenish the air supply to polluted piled soil in biopiles, ex situ bioremediation techniques such as land farming, biosparging, and bioventing can be applied [ 46 ]. However, these techniques are expensive to implement and require a power supply at remote locations. Bioremediation may be slowed down by extreme air temperatures that dry soil and make it more likely to be vaporized than to be broken down by living organisms [ 47 ]. Bio-available organic carbon (BOC) plays an important role in bioremediation through the biopile method. Petroleum contaminated soil has been bioremediated using mesophillic conditions (30 °C–40 °C) and a low aeration rate for the removal of total petroleum hydrocarbon (TPH) using alpha , beta , and gamma proteobacteria [ 48 ]. Biopile systems have also been utilized for treating the diesel contaminated soil of the sub-Antarctic region. A total of 93% of the total petroleum hydrocarbon (TPH) was removed using the biopile system within one year [ 49 ].
5.2. Windrows
Windrows boosts bioremediation by enhancing the biodegradation processes of native and transitory hydrocarbon plastic found in the contaminated soils when spinning the heaped contaminated soils. The aeration, mineralization, and biotransformation of toxic soil can be performed through acclimation, biological treatment, and mineralization [ 50 ], can speed up bioremediation. The biopile approach can remove more hydrocarbons from soil than windrow treatment [ 15 , 51 ], which was more efficient in terms of hydrocarbon removal. The periodic rotation connected with windrow remediation is not a better selection approach for the bioremediation of soil affected by harmful volatile chemicals. Windrow treatment is a source of greenhouse gas (CH 4 ) due to the anaerobic system generated inside the heaped contaminated soil [ 52 ]. The windrow method of has been applied for the bioremediation of the Gurugram–Faridabad dumpsite in Bandhwari, India by forming terraces and windrows and utilizing bio-culture, and the results showed a decrease in the garbage [ 53 ].
5.3. Land Farming
Land farming is the most significant and simple bioremediation method because of its low operating costs and lack of specialized equipment [ 54 ]. Ex situ bioremediation is the most common method, but it can also occur with in situ bioremediation. The reason for this is the location of the treatment. It is common practice in land farming to remove and till polluted soils on a regular basis, and the location of treatment dictates the type of bioremediation. On-site treatment is classified as in situ , whereas ex situ bioremediation approaches are used for the treatment of the contaminated soil [ 55 ]. Extracted contaminated soils are usually placed on a permanent layer of substrate well above Earth’s surface to permit native microorganisms to aerobically degrade contaminants [ 56 ]. Land bioremediation of polluted soil using land farming bioremediation technology is a reasonably simple process that takes little capital, has little ecological footprint, and uses very little energy [ 57 ].
5.4. Bioreactor
Following a series of biological reactions, bioreactors transform raw materials into specific products. Bioremediation thrives in a bioreactor, which provides the ideal conditions for growth [ 58 ]. The remediation samples are placed in a bioreactor. There are a number of advantages to using a bioreactor to treat contaminated soil as opposed to ex situ bioremediation methods. An efficient bioremediation process based on bioreactors that can precisely regulate pH, agitation, temperature, aeration, substrate concentration, and inoculum concentration significantly reduce the time required for bioremediation [ 59 ]. Biological reactions can take place when the bioreactor can be controlled and manipulated. Given their adaptability, bioreactor designs are able to maximize microbial degradation while abiotic losses are kept to a minimum.
In Situ Bioremediation Techniques
These methods entail cleaning up polluted substances right where they were created. It does not necessitate any digging or disturbance of the surrounding soil. These techniques ought to be more cost-effective in comparison to the ex situ bioremediation techniques. Bioventing, phytoremediation, and biosparging are examples of in situ bioremediation techniques that can be improved, while intrinsic bioremediation and natural attenuation are examples of in situ bioremediation techniques that cannot be improved [ 60 ]. In situ bioremediation approaches have effectively treated chlorine, paints, toxic metals, and hydrocarbon-contaminated areas [ 61 ]. The practice of in situ bioremediation can be categorized into two distinct types: intrinsic and engineered.
- (a) Intrinsic in situ bioremediation:
Natural reduction is another term for in situ bioremediation. Intrinsic bioremediation utilizes polluted sites in a non-invasive manner (human intervention) [ 62 ]. The goal of this procedure is to stimulate an already existing microbial population. The biodegradation of polluting constituents, including those that are recalcitrant, is based on aerobic and anaerobic processes in microorganisms. It costs less because there isn’t a lot of force behind this technique [ 63 ]. Intrinsic in situ bioremediation can be performed using anaerobic reductive dechlorination, aerobic treatment, amendment delivery, biosparging, and bioslurping [ 64 ]. Using a stimulation–optimization approach that is powered by machine learning and particle swarm optimization (ELM–PSO) techniques, in situ bioremediation has been used as a method for the biological treatment of clogged groundwater [ 65 ]. This technique was implemented through the use of in situ bioremediation. This results in cheaper technology for the pumping system and requires less capital for the whole process. The concentration of contaminants was reduced from 40 ppm to 5 ppm (within permissible range) in 3 years using in situ bioremediation. In situ remediation has also been explored for the decontamination of Cr (VI) found in shallow unsaturated soil. Microorganisms possess the capability to survive under high concentrations of Cr (VI) in the soil and their sub-cellular machinery was utilized to interact with heavy metals. Microbial inoculants can be utilized for the in situ treatment of heavy metals [ 66 ]. Cr (VI) interacts with Fe (II) ions also through the redox reactions, and the release of iron in soluble forms promotes the reductive reactions [ 67 ].
- (b) Engineered in-situ bioremediation
In the second method, a specific microorganism is brought into the area of contamination to clean it up. In situ bioremediation is a technique that employs microorganisms that have undergone genetic engineering in order to hasten the decomposition process. This is accomplished by enhancing the physicochemical conditions that foster the growth of microorganisms [ 68 ].
5.5. Bioventing
Bioventing is a technique that uses controlled airflow to increase the activity of indigenous microbes for bioremediation by delivering oxygen to the unsaturated zone. The bioremediation process is aided by the addition of nutrients and moisture during the bioventing process. This will lead to the microbial transformation of pollutants into harmless substances. Other in situ bioremediation methods have flocked to this one in recent years [ 69 ]. Bioventing is a technique that helps in stimulating the indigenous microflora through ample amounts of aeration to enhance the biodegradation ability of the various microbes and promote decontamination of the heavy metal pollutants by precipitation [ 70 ].
5.6. Bioslurping
A direct oxygen supply and stimulation of contaminant biodegradation are used in conjunction with vacuum-assisted pumping, bioventing, and soil vapour extraction (SVE) in order to reach soil and groundwater levels for restoration [ 71 ]. This approach can be used to recover unsaturated and saturated zones as well as light non-aqueous phase liquids (LNAPLs). This technology can be used to remediate soils contaminated with flammable and moderately-flammable organic substances. Liquid is drawn from the free product layer by means of a “slurp” that spreads into the layer. LNAPLs are lifted to the surface by the pumping machine, where they are separated from the surrounding air and water [ 72 ]. To reduce microbial activity, soil moisture is used in this technique to reduce air permeability and oxygen transfer rate. Given that it uses less groundwater, this method saves money on storage, disposal, and treatment, even though it’s not ideal for remediation in low-permeable soils. Bioslurping requires 25 feet of digging below the ground surface and then the contaminants floating on the water can be removed. It combines both the approaches of bioventing, which utilize aerobic bioremediation of contaminated soil in situ. Free product is recovered by a vacuum-enhanced system that utilizes LNAPLs from the capillary fringe [ 73 ]. Free product is “slurped” up the bioslurping tube into a trap or oil–water separator for further treatment after the bioslurping tube is vacuumed. When the LNAPL is removed, the height of the LNAPL drops, which encourages the flow of LNAPL from distant locations into the bioslurping well. The bioslurping tube starts to remove vapours from the unsaturated zone when the fluid level in the bioslurping well decreases as a result of the vacuum extraction of LNAPL. This vapour extraction encourages soil gas movement, which in turn boosts aerobic biodegradation and aeration [ 74 ].
5.7. Biosparging
Air is introduced into the soil’s core, just like bioventing, to encourage microbiological activity, which in turn removes pollutants from polluted sites. As an alternative to conventional biodegradation methods, bioventing involves injecting air into a saturated zone in order to encourage the movement of flammable organic chemicals upward to an unsaturated zone nearby [ 75 ]. The success of biosparging is dependent on soil porosity and contaminant biodegradability. When it comes to bioventing and soil vapour extraction (SVE), in situ air sparging (IAS) uses high air-flow rates to volatilize contaminants, while biosparging encourages microbial degradation [ 76 ]. It is common practice to use biosparging to remove diesel and kerosene from water supplies. In order to hasten the biodegradation processes, oxygen is supplied into microorganisms during enhanced bioremediation [ 77 ]. The removal of organic pollutants (BTEX) can be accomplished using a variety of technologies, including adsorption, microbial degradation, biosparging, PRBs, and the use of modified or synthesized zeolites. However, there aren’t many investigations on readily available, inexpensive materials like natural zeolite for BTEX adsorption [ 78 ].
5.8. Phytoremediation
Contaminated soils can be cleaned up using phytoremediation. In contaminated areas, this method uses plant interactions at the physical, biological, chemical, biochemical, and microbiological levels to reduce pollutant toxicity. Depending on the quantity and form of the pollutant, phytoremediation employs a variety of processes [ 79 ]. Extraction, sequestration, and transformation are common methods for removing pollutants like heavy metals. When using plants like willow or alfalfa, the decay, immobilization, rhizoremediation, and evaporation of organic contaminants such as oils and chloro-compounds is feasible [ 80 ]. Tap root system or fibrous root system, penetration, toxicity levels, adaptability to the harsh environmental conditions of the contaminants, plant annual growth, supervision, and, notably, the time needed to reach standard of cleanliness are all important factors in plants that serve as phytoremediators. The plant must also be disease and insect resistant [ 81 ]. An important part of phytoremediation is removing pollutants from the roots and shoots. The movement of water and nutrients is also dependent on transpiration and partitioning [ 82 ]. When it comes to contaminants and plant nature, it is possible to alter this process. Phytoremediation can be accomplished with the help of the majority of the plants present at a polluted site. In polluted environments, native plants can be bioaugmented by natural or anthropogenic plants, or a combination of both. Phytomining, the process of extracting precious metals from polluted sites with plants, is one of them [ 83 ].
Numerous plants (over 300) are better candidates for phytoremediation because they ideally absorb Cu, Zn, and Ni. Phytostabilization, sometimes referred to as in situ inactivation or immobilisation of heavy metals, reduces their bioavailability and prevents their off-site transfer. At the plant roots, it absorbs metals and restores them. Several species, notably Acanthus ilicifolius and Virola surinamensis , are capable of Cd photostability. Cinnamomum camphora , Osmanthus fragrans , Euonymus japonicus , Ligustrum vicaryi , and Loropetalum chinense are five decorative plants chosen for their capacity to phytostabilize Cd [ 84 ]. Water from various places that has been contaminated with metal can be successfully treated using bacterially-aided phytoremediation. The phytoremediation method of metal reduction in wastewater utilising plants can be used by coalitions of growth-promoting rhizobacteria, degrading bacteria, as well as endophytic bacteria [ 85 ]. There are a few limitations to bioremediation techniques, as presented in Table 2 .
Limitations of various bioremediation techniques.
| Methods | Limitation | Reference |
|---|---|---|
| Biopile | The extent of weathering can change the chemical make-up by making the materials more hydrophobic, which limits the potential of the biopiling method for biodegradation. | [ ] |
| Windrows | The major limitation in studying windrows is probably knowing where and when they will emerge. Although it is possible to forecast some sub-mesoscale convergences, it is still difficult to predict where and when litter windrows would form because of the additional uncertainty brought on by the dependency on litter loading. | [ ] |
| Land Farming | This method has the drawback that the objectives specified in the constraint set must be strictly upheld; if they are not, the issue will appear to be insurmountable. Fresh organic waste can be troublesome since it can occasionally lead to anoxic conditions, which are hazardous to plant development. To preserve the quality of pre-existing soils, it is advisable to refrain from adding more organic material over years. | [ ] |
| Bioreactor | The primary limitation to employing membrane bioreactors (MBR) at such high concentrations of mixed liquid suspended solids (MLSS) appears to be very low to zero oxygen transfer efficiency reported when using traditional diffused aeration systems (such as fine and coarse bubble diffusers). This suggests that a deeper understanding is required of the constraints imposed by traditional bubble diffusers (measured in terms of the alpha factor) under that specific combination of operational parameters (high MLSS). | [ ] |
| Intrinsic bioremediation | The site has to have very permeable soil for bioremediation, which is the main limitation of bioremediation. | [ ] |
| Bioventing | This technique’s disadvantage is that it only works at the deepest levels of the contaminated soil ecosystem. | [ ] |
| Phytoremediation | Phytoremediation, such as phytoextraction and rhizodegradation, is used to remediate the polluted soil in the superficial layers of the soil. This approach could be time-consuming and may not be able to eliminate all the contaminants. | [ ] |
6. Bioremediation of Various Pollutants
6.1. bioremediation for organic pollutants.
Organic compounds (OCs) such as biocides and flame retardants have been widely used and are now considered a threat to nearly all forms of life on the planet because of the widespread and massive use of these chemicals in the environment. Most OCs, such as polychlorinated biphenyls (PCBs), polybrominated biphenyl ethers (PBEs), and polycyclic aromatic hydrocarbons (PAHs), can be degraded in the environment by microbes. Biodegradation is the process by which microbes break down organic compounds into less toxic or entirely non-toxic residues [ 91 ]. In order to obtain organic carbons and energy, the microbes consume the organic substrate. Isolated from other microbes, an individual microbial species usually does not degrade any organic substrate [ 92 ] and does well in a community. As a result of community microbe interactions, resistance, chemical-degrading ability, and tolerance are all conferred by the exchange of genetic information among microbial species. Many OC-degrading microorganisms are misidentified due to a lack of internationally agreed-upon methods and protocols for microbial identification [ 93 ]. This underlines the significance of studies into microbial consortiums using metagenomics tools and conventional genetic engineering protocols. Bacteria and other microorganisms have the ability to degrade a wide range of organic compounds, depending on the chromosomal genes, as well as the extracellular enzymatic activity (in the case of bacteria) (fungal degradation process). The varying environmental conditions that affect the microbe growth pattern further complicate these processes [ 94 ].
A successfully bioengineered microbe requires the identification of the relevant species and strains for each substrate. A viable alternative to the recombinant degradation of resistant organic compounds is biodegradation by microbes using readily-available organic carbon and energy sources in the surrounding environment. Microbes use the fluctuation in chemical gradients in their environment to determine the most favourable conditions for growth. This allows them to thrive in an optimal environment [ 95 ]. Microbial consortia and microbial fuel cells (MFCs and bioreactors) are two new developments in microbiological bioremediation that are being used to degrade recalcitrant organic compounds. Toxic organics can be remedied more effectively using fungi rather than bacteria because the latter cannot grow at high concentrations of toxic organics [ 96 ]. For example, the enzymes, laccase (LAC), lignin peroxidase (Lip), and manganese decarboxylase (MDA), are active in the metabolism of lignocellulosic compounds by the white rot fungus Phanerochaete chrysosporium [ 97 ].
6.2. Bioremediation for Inorganic Pollutants
Toxic heavy metals and their compounds resulting from mining, power plants, metallurgy, and chemical manufacturing processes are among the most common inorganic contaminants [ 98 ]. One of the main concerns of environmentalists is toxic elemental pollution because the disposal of toxic metals to soils and waters on or below the surface causes unacceptable health risks [ 99 ]. Microbes cannot degrade metal ions; it is essential to know that they are only capable of changing the oxidation states of the metals to stabilize them [ 100 ]. They can metabolize and detoxify metals like any other nutrient in the cells. Several microorganisms have been reported for the bioremediation of organic and inorganic pollutants ( Table 3 ). Microbes that release chelating agents and acids, as well as those that alter physicochemical properties such as redox potential in their environment can cause significant changes in the environment by increasing the bioavailability of metal ions [ 101 ]. Physical adsorption, biosorption, and ion complexation are the first steps in the interaction between metals and microbial cells [ 102 ]. Enzymes for oxidation, methylation, reduction, precipitation, and dealkylation are involved in the biochemical transformation of metal ions by microorganisms. The adaptability of microbes to heavy metals, such as iron, zinc, chrome, magnesium, mercury, and barium in textile waste, was demonstrated in the multidrug-resistant Pseudomonas aeruginosa T-3 isolate from tannery effluent [ 67 , 83 ]. This shows that microbes can adapt to changing environmental conditions. A plasmid-encoded copper and cadmium metal resistance gene in the bacteria, Pseudomonas putida PhCN, has also been discovered [ 103 ]. Plasmid-encoded biochemical information and genetic engineering techniques were used to create recombinant Escherichia coli that expresses the metallothionein gene ( Neurospora crasa ) for Cd uptake, resulting in significantly faster Cd uptake than the donor microbe [ 104 ]. A poly-histidyl peptide was introduced into Staphylococcus xylosus and Staphylococcus carnosus that encoded genes that allowed these microbes to bind nickel [ 105 ].
Potentially hazardous organic and inorganic pollutants and their degrading microbes (bacteria, fungi, and algae).
| Substrate | Compound | Microorganisms | References |
|---|---|---|---|
| Organic substrate | Chlorobenzenes | (GJ31) | [ ] |
| N, N-dimethyl-pphenylenediamine | (RS-13) | [ ] | |
| Polycyclic aromatic hydrocarbons | sp., | [ ] | |
| Remazol Black B | [ ] | ||
| Sulfonate benzene | (S2) | [ ] | |
| 4,4 dibromodiphenyl ether | [ ] | ||
| Aromatic hydrocarbons | sp., sp., sp. and sp. | [ ] | |
| Phenol | , , , and | [ ] | |
| Toluene and its derivatives | (F1), | [ ] | |
| Methyl parathion and chlorpyrifos | sp., sp., and sp. | [ ] | |
| Endosulfan | , | [ ] | |
| Azo dyes effluents | , , and | [ ] | |
| Vat dyes | , , , and | [ ] | |
| Oil-based based paints | strain NAP1, NAP2, NAP4 | [ ] | |
| Crude oil | , , , and | [ ] | |
| Diesel oil | , , , and | [ ] | |
| Oils | , , and sp. | [ ] | |
| Inorganic substrate | Heavy metals, mercury nickel and lead | and, | [ ] |
| Cr | [ ] | ||
| Cobalt, chromium, copper, and lead | CBAM5 | [ ] | |
| Cadmium | , sp., , sp., sp. and sp. | [ ] | |
| Uranium, copper, nickel, chromium | , sp. | [ ] | |
| Lead, chromium, and cadmium | sp., | [ ] | |
| Hg | , , , | [ ] | |
| Cr O | spp. sp. (HD-104) | [ ] | |
| Cr | sp., and | [ ] | |
| Pb | , , and | [ ] |
7. Recent Advancement and Challenges in Bioremediation
7.1. bioinformatics approaches in bioremediation.
When it comes to waste management, bioremediation is a useful technique that can be used to remove waste from contaminated areas and sites. It is particularly concerned with the utilization of organisms to consume or neutralize pollutants [ 20 ]. Using data from various biological databases, such as databases of chemical structure and composition, RNA/protein expression, organic compounds, catalytic enzymes, microbial degradation pathways, and comparative genomics to interpret the underlying degradation mechanism carried out by a particular organism for a specific pollutant is the goal of bioremediation [ 133 ]. A variety of bioinformatics tools are used to interpret all of these sources in order to study bioremediation in order to develop more effective environmental cleaning technology. There has been a scarcity of data on the factors that control the growth and metabolism of microbes with bioremediation potential, which has resulted in a limited number of bioremediation applications [ 134 ]. These microorganisms with bioremediation capabilities have been profiled and their mineralization pathways and mechanisms have been mapped out using bioinformatics [ 135 ]. The use of proteomic approaches such as two-dimensional polyacrylamide gel electrophoresis, microarrays, and mass spectrometry is also critical in the investigation of bioremediation methods and technologies. It significantly improves the structural characterization of microbial proteins that have contaminant-degradable properties, according to the researchers [ 135 ]. The structural characterization of microbial proteins capable of degrading contaminants has greatly improved. Research in this field crosses the boundaries between computer science and biology. For example, computers are used to store, manipulate, and retrieve information linked to the DNA, RNA, and proteins of the genome [ 133 , 135 ].
7.1.1. Bioremediation Tools Based on Omics
Bioremediation studies can benefit from the use of genomics, transcriptomics, metabolomics, and proteomics. Given its ability to correlate DNA sequences with the abundance of metabolites, proteins, and mRNA, this technology aids in the in situ bioremediation process’s evaluation [ 136 , 137 ].
7.1.2. Genomics
There is a new field in genomics for the study of bioremediation microbes. This strategy is based on microbes’ ability to fully analyze their genetic information within the cell. Bioremediation uses a wide variety of microorganisms [ 138 ]. To better understand the biodegradation process, genomic tools such as PCR, analysis of isotope distribution, DNA hybridization, molecular connectivity, metabolic footprinting, and metabolic engineering are used. For genotypic fingerprinting, a variety of PCR-based techniques are available, including amplified fragment length polymorphisms (AFLP), amplified ribosomal DNA restriction analysis (ARDRA), automated ribosomal intergenic spacer analysis (ARISA), terminal-restriction fragment length polymorphism (T-RFLP), randomly amplified polymorphic DNA analysis (RAPD), single strand conformation polymorphism (SSCP), and length heterogeneity [ 139 ]. When it comes to studying soil microbial communities, RAPD can be utilized for assessing inherently related bacterial species, constructing functional structural models, and generating genetic fingerprints [ 140 ]. In microbial communities, LH-PCR may be used to detect natural length variations of various SSU rRNA genes. Multiple taxonomic groups of microbes can be profiled simultaneously using T-RFLP [ 141 ]. Research into how soil microbes interact with natural factors can also make use of a combination of molecular tools, such as genetic fingerprinting, microradiography, FISH, stable isotope probing, and quantitative PCR. A PCR-based quantitative analysis of soil microbial communities can be used to determine the abundance and appearance of taxonomic and operational gene markers in the soil. Techniques for analysing a person’s DNA use amplified PCR products as a starting point for the direct analysis of specific molecular biomarker genes [ 142 ]. In order to better understand the relationship between diverse microbial communities, cluster-assisted analysis, which compares fingerprints from different samples, could be used.
7.1.3. Transcriptomics and Metatranscriptomics
The transcriptome is a crucial link between cellular phenotype, interactome, genome, and proteome because it represents the set of genes that are being transcribed at a specific time and condition. The ability to control gene expression is critical to adapting to changes in the environment and thus ensuring survival. Transcriptomics provides a comprehensive view of this process across the entire human genome. In transcriptomics, DNA microarray analysis is a powerful tool for determining mRNA expression levels [ 143 ]. To perform a transcriptomic analysis, one must first isolate and enrich the total mRNA, then synthesize cDNA, and then sequence the cDNA transcriptome. Using a DNA microarray as a transcriptomics tool, almost every gene in an organism’s mRNA expression can be examined and studied [ 144 ]. The study of transcriptional mRNA profiles, also known as transcriptomics or metatranscriptomics, is critical for gaining functional insights into the activities of environmental microbial communities [ 145 ]. Syntrophism between microbes and complementary metabolic pathways can be discovered using metagenomics and genome binning as well as metatranscriptomics during the entire biodegradation process [ 146 ]. Metatranscriptomics is a way to look at gene expression that can be used by researchers [ 147 ].
7.1.4. Proteomics and Metabolomics
In contrast to metabolomics, which focuses on the total metabolites produced by an organism in a given period of time or environment, proteomics focuses on the total proteins expressed in a cell at a given location and time [ 148 ]. The analysis of protein abundance and changes in composition, as well as the identification of key microbe-related proteins, has been accomplished using proteomics [ 149 ]. In comparison to genomics, the functional analysis of microbial communities is more useful and holds more promise. There are two primary ways in which metabolomics studies can be used to analyze biological systems. It is not necessary to have any prior knowledge of the metabolic pathways of the biological system in order to conduct the first type of study. By employing this strategy, there are numerous metabolites in the sample that can be identified and recovered, which generates enormous amounts of data that can be used to establish the interconnectedness of various samples in metabolic pathways. Another option is to conduct a targeted study to identify specific metabolic pathways or metabolites based on prior research [ 150 ]. Metabolite profiling, foot printing, and target analysis are just some of the many tools in the toolbox of microbial metabolomics that can be used to identify and quantify the myriad of cellular byproducts present in living organisms [ 151 ]. Data from both the proteome and metabolome will be useful for cell-free bioremediation.
7.2. Bioremediation Using Nanotechnological Methods
A nanometer is the smallest unit of measurement used in nanotechnology. Many toxic substances can be removed with their help because of their unique abilities against various recalcitrant contaminants. Technology such as water treatment has been given a new perspective by nanotechnology. Techniques that are good for the environment can now be categorized as nanofiltration [ 151 ].
7.2.1. Microbe and Nanotechnology
When using effective microbes (EM) technology, wastewater can be treated with effective microbes, and the water can then be used for irrigation [ 152 ]. For water purification, nanotechnology and EM technology can be helpful. Innumerable and all-pervasive environmental issues arise from the presence of recalcitrant organic pollutants like polycyclic aromatic hydrocarbons (PAHs) with multiple benzene rings. Polycyclic aromatic hydrocarbons (PAHs) are mutagenic and non-biodegradable [ 153 ]. In a study, Ramos et al. [ 154 ] synthesized silver nanoparticles using whole cells of the fungi Trichoderma spp. for its application.
7.2.2. Engineered Polymeric Nanoparticles for Hydrophobic Contaminant Bioremediation
Soil sorption of organic pollutants, such as petroleum hydrocarbons and PAHs, reduces their solubility and mobility, which in turn reduces their environmental impact. The phenanthrene solubility and phenanthrene release from contaminated aquifer material are both improved by polymeric nano-network particles [ 155 ]. Precursor chains of poly-(ethylene) glycol-modified urethane acrylate (PMUA) are used to create polymeric nanoparticles. PMUA nanoparticles are designed to maintain their properties in the presence of a diverse range of bacterial populations [ 156 ].
7.3. Genetic and Metabolic Engineering
“Gene editing” refers to scientific technical developments that enable rational genetically-created fragments at genome level to provide exact addition, deletion, or substitution of pieces of DNA molecules. Transcription activators are utilized in a variety of gene editing methods, including TALENs, ZFNs, and CRISPRs, which are widely used in research. CRISPR-Cas has been dubbed the most efficient and straightforward gene editing tool [ 157 ]. A DNA-binding element in TALEN is complementary to the sequence of the host DNA. When TALEN attaches to DNA and exposes sticky ends for stabilization, it creates double-stranded breaks (DSBs). ZFNs also have a DNA-binding domain made up of 30 amino acids. At the target location of the host DNA, the Fok1 cleavage domain causes DSBs. A novel perspective on composite endonuclease comprising TALENs and ZFN nucleases was required to solve molecular problems [ 158 , 159 ]. Two of the CRISPR-Cas system’s unique properties are sequence similarity complementarity and simultaneous gene editing [ 160 , 161 ]. The bacteria, Streptococcus pyogenes, provides this unique ability as a sort of virus resistance. In the CRISPR-Cas system, guide RNA connects crisper-derived RNA (crRNA) and trans-acting antisense RNA (trcRNA). The Cas9 enzyme is able to carry out the requisite DSB when gRNA recognizes the target DNA sequence. These gene editing tools’ knock-in and knock-out effects are being analyzed for usage in bioremediation investigations [ 161 ]. In model organisms like Pseudomonas and Escherichia coli , the CRISPR-Cas system has been widely accepted by researchers [ 138 ]. In non-model species (such as Rhodococcus ruber TH, Achromobacter sp. HZ01, and Comamonas testosteroni ), the area of bioremediation is also exploring new insights into CRISPR toolkits and the synthesis of gRNA for the production of remediation-specific genes [ 162 ].
Pollutant-tolerant bacteria are the greatest choices for genetic manipulation and biochemical pathways since they are accustomed to tolerating and storing a variety of toxic, refractory, and non-degradable xenobiotic compounds under harsh circumstances. Furthermore, recognizing biochemical functions is critical for analyzing microbiological bioremediation, such as the bioremediation of harmful pollutants through the production of haloalkane dehalogenases and the disposal of pyrethrins from land through the anaerobic decomposition pathway of fenpropathrin studied in Bacillus sp. DG-02 [ 163 ]. The bioremediation process can be improved by metabolic engineering, which alters the existing pathway. The likelihood of obtaining recombinant enzymes increases significantly when using a genetic approach. Some extracellular enzymes have been found to play a role in enzymatic bioremediation, according to some studies. PAHs are degraded by LiPs ((lignin peroxidase) from P. chrysosporium that encode hemoproteins [ 164 ]. Even though contaminants can be consumed by microbes as substrates or intermediates in biological pathways, incomplete or partial degradation leads to simpler, non-toxic degradable compounds [ 136 ]. For example, LiP can degrade benzopyrene into three quinine compounds, namely 1,6-quinol, 3,6-quinine, and 6,12-quinine [ 165 ]. MnP (Mn (II) peroxidase) can also oxidise organic compounds in the presence of MnP (Manganese peroxidase) [ 166 ]. As well, laccase, glutathione S transferase, and cytochrome P 450 are involved in the biodegradation of recalcitrant compounds [ 167 ]. It has been shown that the immobilization of enzymes significantly increases enzyme stability, activity, and stability. Enzymatic bioremediation is a simple, environmentally-friendly, and fast method for removing and degrading persistent xenobiotic compounds by microorganisms [ 134 , 145 ]. Enzyme-producing microorganisms have been isolated and characterized with the limitation of low productivity. Insecticides’ main ingredients, organophosphates (OP) and organochlorines (OC), are found in agricultural soil and run-off into waterways.
Genetically-engineered microorganisms have demonstrated successful bioremediation of hexachlorocyclohexane and methyl parathion [ 135 , 146 ]. Genetically-modified P. putida KT2440 was used in organophosphate and pyrethroid bioremediation experiments [ 168 ]. The degradation and catabolism of a variety of persistent compounds has been documented since the advent of metabolic engineering. Sphingobium japonicum and Pseudomonas sp. WBC-3 showed bioremediation of methyl parathion and -hexachlorocyclohexane degradation pathways [ 169 ]. When three enzymes from two different microorganisms are combined in E. coli , a persistent fumigant called 1-, 2-, 3-trichloropropane is released into the environment via heterologous catabolism [ 137 , 148 ]. To do this, microbes can be used to turn persistent compounds into minerals [ 49 ].
7.4. Designing the Synthetic Microbial Communities
Synthetic biology advancements have had a significant impact on environmental issues in recent years. Toxic compounds, pesticides, and xenobiotics can be removed from the environment by using genetically-modified organisms (GMOs). Natural microbial communities must be understood in order to create a synthetic one [ 170 ]. Identifying which species are participating in bioremediation is difficult in a natural community. Through the use of a synthetic microbial community, the development of an artificial microbiome with functionally specific species is possible. Model systems for studying functional and structural characteristics can be found in these communities. Synthetic communities were formed by the co-culturing of two distinct microorganisms under precisely defined conditions, which were based on their interactions and functions [ 171 ]. The community’s dynamics and structure are determined by these variables; it is based on the discovery of bacterial processes and behaviors. Metabolism drives these patterns of microbial interaction, which in turn facilitates communication within communities [ 172 ]. Interactions between two microbial populations are social in nature (such as mutualism, competition, and cooperation). Cooperation is said to be a key factor in community structure and operation. Cooperation in community dynamics is influenced by the creation of synthetic communities [ 173 ] and it was found that modifying environmental conditions, such as deleting genes, could be used to engineer cooperation between two microbial strains. In addition to this, the synthetic community’s engineered microbial species have been examined for other patterns of interaction. Bioremediation strategies frequently make use of this type of engineered interaction [ 174 ]. It is possible to sustain the existence of microorganisms in a large population by using synthetic biology.
8. Advantages and Disadvantages
Due to the harm that pollutants exert on both humans and other living things, environmental pollution is a serious public health problem. The complete elimination of contaminants via chemical and physical methods of remediation is costly [ 175 ]. Additionally, both approaches may result in increased pollution and site disruption, which could have a detrimental effect on nearby humans and other biota. As a result, remediation techniques using chemicals and physical means are not regarded as eco-sustainable. Contrary to these techniques, bioremediation is the suggested solution to remove various persistent contaminants by relying on biological processes (mediated by various types of living organisms). However, all bioremediation techniques have their own advantages and disadvantages ( Figure 2 ) because they have their own specific applications [ 176 , 177 ].

Advantages and disadvantages of bioremediation.
9. Future Perspectives and Conclusions
Omics has gained prominence in the field of microbial remediation of the pulp and paper industry, textile industry, food industry, dairy industry, wood industry, fisheries, water and soil treatment industry, solid waste remediation, heavy metal pollution remediation, and hydrocarbon remediation. In order to better understand degradative pathways, bioremediation data must be mined, and new algorithms can be used to fit these data into simulation and numerical modeling with ease along with data assemblage, repositioning, exploration, and transmission, which necessitate standard protocols. Bioremediation processes may be better understood if new biomarkers are studied. Combining all the omics data with genetically-engineered tools could provide a comprehensive picture of the microbial remediation process. The role of phytoremediation in reducing environmental pollution can also be studied. The phytoremediation process has a number of advantages over other remediation strategies, including lower costs, greater public acceptance, and increased pollution degradation capacity [ 178 ]. Groundwater and air pollution, along with toxic waste generation as a by-product of semiconductor manufacturing, are problems for the environment; some examples include glycol ethers, hydrochloric acid (HCl), xylene, hydrogen fluoride (HF), and methanol [ 179 ]. In the case of pharmaceuticals that are designed to be long-lasting or even non-degradable, they pose a unique threat to the environment. The pharmaceutical pollutants are environmentally persistent substances. Trace amounts of pharmaceutical ingredients, such as birth control pills, anti-epileptics, pain relievers, and antidepressant medications, are found in many urban and rural sources of groundwater [ 180 ]. While operating, solar power generation facilities produce less greenhouse gas emissions, including air pollutants such as carbon monoxide, volatile organic compounds, nitrogen oxides, and carbon dioxide, than conventional fossil fuel-based power generation facilities [ 181 ]. Genetically-engineered plants can be able to bioremediate specific pollutants through discovered metabolic processes, enzymes, genes, or operons [ 182 ]. Although genomics, metabolomics, and proteomics in bioremediation aid in the exploration of possible solutions to specific pollutants, identifying and comparing gene and protein sequences that are effective at removing contaminants is the next step in bioremediation research. GMOs can clean up a wide range of waste effluents and polluted land [ 183 ]. When used in conjunction with other physical and chemical methods, bioremediation can provide a comprehensive approach toward removing pollution from the environment. Since it appears to be a long-term solution, there is a need for additional research in this area.
Acknowledgments
The author M.T. grateful to the Biotechnology Program, Department of Biochemistry, Dr. Rammanohar Lohia Avadh University, Ayodhya, India, for providing us with the research environment.
Funding Statement
This research received no external funding.
Author Contributions
Conceptualization, M.T., M.S., K.S. and B.S.I.; Writing—original draft preparation, S.B., D.G. and B.V.T.; Writing—review and editing, M.S., M.T., K.S. and B.V.T.; visualization, S.B. and D.G.; supervision, M.T., M.S., K.S. and B.S.I. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- PubMed/Medline
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Bioremediation of heavy metals by the genus bacillus.

1. Introduction
2. scale of heavy metal contamination, 3. impacts of heavy metal pollution on environment and human health, 4. heavy metal bioremediation strategies detected in bacillus, 4.1. biosorption, 4.2. bioremediation by extracellular polymeric substances (eps), 4.3. bioaccumulation, 4.4. bioprecipitation, 4.5. biological removal of heavy metals using plant growth-promoting bacteria, 5. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest.
- Cegiełkowska, W.; Michalska-Kacymirow, M.; Wierzbicka, M. Heavy metals in the environment. In Ecotoxicology. Plants, Soils, Metals [Ekotoksykologia. Rośliny, Gleby, Metale] , 1st ed.; Wierzbicka, M., Ed.; Warsaw University Press: Warsaw, Poland, 2015; pp. 22–23, 31–36. ISBN 978-83-235-1854-9. [ Google Scholar ]
- Gholizadeh, M.; Hu, X. Removal of heavy metals from soil with biochar composite: A critical review of the mechanism. J. Environ. Chem. Eng. 2021 , 9 , 105830. [ Google Scholar ] [ CrossRef ]
- Kacprzak, M.; Fijałkowski, K. Trace elements and heavy metals. Occurrence, toxicity. In Potential of Plants to Clean Up the Environment [Fitoremediacja. Potencjał Roślin do Oczyszczania Środowiska] , 1st ed.; Charitonow, E., Ed.; Scientific Publishers PWN: Warsaw, Poland, 2020; pp. 6–11. ISBN 978-830-121-170-7. [ Google Scholar ]
- Ociepa-Kubicka, A.; Ociepa, E. Toxic effects of heavy metals on plants, animals and humans. Inż. Ochr. Śr. 2012 , 15 , 169–180. Available online: https://www.infona.pl/resource/bwmeta1.element.baztech-article-LODD-0002-0015/tab/summary (accessed on 11 March 2015).
- Rostański, A.; Cabala, J.; Slota, M. Environmental exposure to heavy metals. In Ecotoxicology. Plants, Soils, Metals [Ekotoksykologia. Rośliny, Gleby, Metale] , 1st ed.; Wierzbicka, M., Ed.; Warsaw University Press: Warsaw, Poland, 2015; pp. 522–541. ISBN 978-83-235-1854-9. [ Google Scholar ]
- Kumar, A.; Kumar, A.; Cabral-Pinto, M.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead toxicity: Health hazards, influence on food chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 2020 , 17 , 2179. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Liu, X.; Shunbin, G.; Shiyan, Y.; Jinsong, D. Heavy metals in soil-vegetable system around E-waste site and the health risk assessment. Sci. Total Environ. 2021 , 779 , 146438. [ Google Scholar ] [ CrossRef ]
- Pham, V.H.T.; Kim, J.; Chang, S.; Chung, W. Bacterial biosorbents, an efficient heavy metals green clean-up strategy: Prospects, challenges, and opportunities. Microorganisms 2022 , 10 , 610. [ Google Scholar ] [ CrossRef ]
- Dell’Anno, F.; Brunet, C.; van Zyl, L.J.; Trindade, M.; Golyshin, P.N.; Dell’Anno, A.; Ianora, A.; Sansone, C. Degradation of hydrocarbons and heavy metal reduction by marine bacteria in highly contaminated sediments. Microorganisms 2020 , 8 , 1402. [ Google Scholar ] [ CrossRef ]
- United Nations Home Page. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 25 September 2015).
- Wang, L.; Rinklebe, J.; Tack, F.M.; Hou, D. A review of green remediation strategies for heavy metal contaminated soil. Soil Use Manag. 2021 , 37 , 936–963. [ Google Scholar ] [ CrossRef ]
- Shamim, S. Biosorption of Heavy Metals ; IntechOpen: London, UK, 2018. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Das, A.; Osborne, J.W. Bioremediation of heavy metals. In Nanotechnology, Food Security and Water Treatment. Environmental Chemistry for a Sustainable World , 1st ed.; Gothandam, K.M., Ranjan, S., Dasgupta, N., Ramalingam, C., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2018; Volume 9, pp. 277–311. ISBN 978-331-970-165-3. [ Google Scholar ]
- Guo, H.; Hanjun, S.; Chen, L.; Xiao, X.; Xi, Q.; Wei, W.; Zeng, G.; Liu, C.; Wan, Y.; Chen, J.; et al. Bioremediation of heavy metals by growing hyperaccumulator endophytic bacterium Bacillus sp. L14. Bioresour. Technol. 2010 , 101 , 8599–8605. [ Google Scholar ] [ CrossRef ]
- Sadowska, A.; Obidoska, G.; Rumowska, M. Heavy metals. In Ecotoxicology. Toxic Environmental Agents and Methods of Their Detection [Ekotoksykologia. Toksyczne Czynniki Środowiskowe i Metody ich Wykrywania] ; SGGW Publishing: Warsaw, Poland, 2000; pp. 54–57. ISBN 837-244-171-5. [ Google Scholar ]
- Niklińska, M.; Stefanowicz, A.M. Soil microorganisms in metal-bearing areas. In Ecotoxicology. Plants, Soils, Metals [Ekotoksykologia. Rośliny, Gleby, Metale] , 1st ed.; Wierzbicka, M., Ed.; Warsaw University Press: Warsaw, Poland, 2015; p. 210. ISBN 978-83-235-1854-9. [ Google Scholar ]
- Dadrasnia, A.; Chuan Wei, K.S.; Shahsavari, N.; Azirun, M.S.; Ismail, S. Biosorption potential of Bacillus salmalaya strain 139SI for removal of Cr (VI) from aqueous solution. Int. J. Environ. Res. 2015 , 12 , 15321–15338. [ Google Scholar ] [ CrossRef ]
- Ontañon, O.M.; Fernandez, M.; Agostini, E.; González, P.S. Identification of the main mechanisms involved in the tolerance and bioremediation of Cr (VI) by Bacillus sp. SFC 500-1E. Environ. Sci. Pollut. Res. 2018 , 25 , 16111–16120. [ Google Scholar ] [ CrossRef ]
- Heidari, P.; Panico, A. Sorption mechanism and optimization study for the bioremediation of Pb (II) and Cd (II) contamination by two novel isolated strains Q3 and Q5 of Bacillus sp. Int. J. Environ. Res. Public Health 2020 , 17 , 4059. [ Google Scholar ] [ CrossRef ]
- Alotaibi, B.S.; Khan, M.; Shamim, S. Unraveling the underlying heavy metal detoxification mechanisms of Bacillus species. Microorganisms 2021 , 9 , 1628. [ Google Scholar ] [ CrossRef ]
- Babar, Z.; Khan, M.; Chotana, G.A.; Murtaza, G.; Shamim, S. Evaluation of the potential role of Bacillus altitudinis MT422188 in nickel bioremediation from contaminated industrial effluents. Sustainability 2021 , 13 , 7353. [ Google Scholar ] [ CrossRef ]
- Alou, M.T.; Rathored, J.; Khelaifia, S.; Michelle, C.; Brah, S.; Diallo, B.A.; Raoult, D.; Lagier, J.-C. Bacillus rubiinfantis sp. nov. strain mt2T, a new bacterial species isolated from human gut. New Microbes New Infect. 2015 , 8 , 51–60. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Kotb, E. Purification and partial characterization of serine fibrinolytic enzyme from Bacillus megaterium KSK-07 isolated from kishk, a traditional Egyptian fermented food. Appl. Biochem. Microbiol. 2015 , 51 , 34–43. [ Google Scholar ] [ CrossRef ]
- Mingmongkolchai, S.; Panbangred, W. Bacillus probiotics: An alternative to antibiotics for livestock production. J. Appl. Microbiol. 2018 , 124 , 1334–1346. [ Google Scholar ] [ CrossRef ]
- Qin, Y.; Angelini, L.L.; Chai, Y. Bacillus subtilis cell differentiation, biofilm formation and environmental prevalence. Microorganisms 2022 , 10 , 1108. [ Google Scholar ] [ CrossRef ]
- Zocca, V.F.B.; Corrêa, G.G.; Lins, M.R.D.C.R.; de Jesus, V.N.; Tavares, L.F.; Amorim, L.A.D.S.; Kundlatsch, G.E.; Pedrolli, D.B. The CRISPR toolbox for the gram-positive model bacterium Bacillus subtilis . Crit. Rev. Biotechnol. 2022 , 42 , 813–826. [ Google Scholar ] [ CrossRef ]
- Ikram, M.; Naeem, M.; Zahoor, M.; Hanafiah, M.M.; Oyekanmi, A.A.; Islam, N.U.; Ullah, M.; Mahnashi, M.H.; Ali, A.A.; Jalal, N.A.; et al. Bacillus subtilis : As an efficient bacterial strain for the reclamation of water loaded with textile azo dye, orange II. Int. J. Mol. Sci. 2022 , 23 , 10637. [ Google Scholar ] [ CrossRef ]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as potential probiotics: Status, concerns, and future perspectives. Front. Microbiol. 2017 , 8 , 1490. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017 , 31 , 446–459. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018 , 285 , 44–55. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kuebutornye, F.K.; Abarike, E.D.; Lu, Y. A review on the application of Bacillus as probiotics in aquaculture. Fish. Shellfish. Immunol. 2019 , 87 , 820–828. [ Google Scholar ] [ CrossRef ]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 2020 , 8 , 1037. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ngalimat, M.S.; Yahaya, R.S.R.; Baharudin, M.M.A.-A.; Yaminudin, S.M.; Karim, M.; Ahmad, S.A.; Sabri, S. A review on the biotechnological applications of the operational group Bacillus amyloliquefaciens . Microorganisms 2021 , 9 , 614. [ Google Scholar ] [ CrossRef ]
- Tsotetsi, T.; Nephali, L.; Malebe, M.; Tugizimana, F. Bacillus for plant growth promotion and stress resilience: What have we learned? Plants 2022 , 11 , 2482. [ Google Scholar ] [ CrossRef ]
- Tran, C.; Cock, I.E.; Chen, X.; Feng, Y. Antimicrobial Bacillus: Metabolites and their mode of action. Antibiotics 2022 , 11 , 88. [ Google Scholar ] [ CrossRef ]
- Tirloni, E.; Stella, S.; Celandroni, F.; Mazzantini, D.; Bernardi, C.; Ghelardi, E. Bacillus cereus in dairy products and production plants. Foods 2022 , 11 , 2572. [ Google Scholar ] [ CrossRef ]
- Liu, Y.; Zhang, Q.; Qi, X.; Gao, H.; Wang, M.; Guan, H.; Yu, B. Metabolic engineering of Bacillus subtilis for riboflavin production: A review. Microorganisms 2023 , 11 , 164. [ Google Scholar ] [ CrossRef ]
- Hodgson, E. Introduction to toxicology. In A Textbook of Modern Toxicology , 4th ed.; Hodgson, E., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 3–14. ISBN 978-0-470-46206-5. [ Google Scholar ]
- European Environment Agency Home Page. Heavy Metal Emissions in Europe. Available online: https://www.eea.europa.eu/data-and-maps/indicators/eea32-heavy-metal-hm-emissions-1/assessment-10 (accessed on 4 September 2019).
- European Environment Agency Home Page. Industrial Pollutant Releases to Air in Europe. Available online: https://www.eea.europa.eu/data-and-maps/indicators/industrial-pollution-in-europe-3/assessment (accessed on 13 September 2019).
- Mohammadi, A.A.; Zarei, A.; Esmaeilzadeh, M.; Taghavi, M.; Yousefi, M.; Yousefi, Z.; Sedighi, F.; Javan, S. Assessment of heavy metal pollution and human health risks assessment in soils around an industrial zone in Neyshabur, Iran. Biol. Trace Elem. Res. 2020 , 195 , 343–352. [ Google Scholar ] [ CrossRef ]
- Sun, Y.; Li, H.; Guo, G.; Semple, K.T.; Jones, K.C. Soil contamination in China: Current priorities, defining background levels and standards for heavy metals. J. Environ. Manag. 2019 , 251 , 109512. [ Google Scholar ] [ CrossRef ]
- Barsova, N.; Yakimenko, O.; Tolpeshta, I.; Motuzova, G. Current state and dynamics of heavy metal soil pollution in Russian Federation–A review. Environ. Pollut. 2019 , 249 , 200–207. [ Google Scholar ] [ CrossRef ]
- Ramon, F.; Lull, C. Legal measures to prevent and manage soil contamination and to increase food safety for consumer health: The case of Spain. Environ. Pollut. 2019 , 250 , 883–891. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Diarra, I.; Prasad, S. The current state of heavy metal pollution in Pacific Island Countries: A review. Appl. Spectrosc. Rev. 2020 , 56 , 27–51. [ Google Scholar ] [ CrossRef ]
- Solademi, F.; Thompson, S. Spatial analysis of heavy metal emissions in residential, commercial and industrial areas adjacent to a scrap metal shredder in Winnipeg, Canada. J. Geosci. Environ. Prot. 2020 , 8 , 359–386. [ Google Scholar ] [ CrossRef ]
- Kabata-Pendias, A.; Pendias, H. Biogeochemistry of Trace Elements [Biogeochemia Pierwiastków Śladowych] , 2nd ed.; Rajpert, M., Ed.; Scientific Publishers PWN: Warsaw, Poland, 1999; ISBN 83-01128-23-2. [ Google Scholar ]
- Sher, S.; Abdul, R. Use of heavy metals resistant bacteria-a strategy for arsenic bioremediation. Appl. Microbiol. Biotechnol. 2019 , 103 , 6007–6021. [ Google Scholar ] [ CrossRef ]
- Wierzbicka, M. Plant defense against heavy metals. In Ecotoxicology. Plants, Soils, Metals [Ekotoksykologia. Rośliny, Gleby, Metale] , 1st ed.; Wierzbicka, M., Ed.; Warsaw University Press: Warsaw, Poland, 2015; p. 83. ISBN 978-83-235-1854-9. [ Google Scholar ]
- Seńczuk, W. Modern Toxicology [Toksykologia Współczesna] , 2nd ed.; Seńczuk, W., Ed.; PZWL: Warsaw, Poland, 2012; ISBN 978-83-200-6099-7. [ Google Scholar ]
- Kozłowska, A.; Mikołajczyk, A.; Boroń, M.; Kasperczyk, S.; Pawlas, N. Effects of lead exposure on the concentration of cadmium, selenium and values of morphology in the blood. Environ. Med. 2015 , 18 , 17–25. [ Google Scholar ]
- International Agency for Research on Cancer Home Page. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 7 September 2022).
- Sharma, B.; Shukla, P. Lead bioaccumulation mediated by Bacillus cereus BPS-9 from an industrial waste contaminated site encoding heavy metal resistant genes and their transporters. J. Hazard. Mater. 2021 , 401 , 123285. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pande, V.; Pandey, S.C.; Sati, D.; Bhatt, P.; Samant, M. Microbial interventions in bioremediation of heavy metal contaminants in agroecosystem. Front. Microbiol. 2022 , 13 , 824084. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Shao, W.; Li, M.; Teng, Z.; Qiu, B.; Huo, Y.; Zhang, K. Effects of Pb (II) and Cr (VI) stress on phosphate-solubilizing bacteria ( Bacillus sp. strain MRP-3): Oxidative stress and bioaccumulation potential. Int. J. Environ. Res. Public Health. 2019 , 16 , 2172. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Ahemad, M. Remediation of metalliferous soils through the heavy metal resistant plant growth promoting bacteria: Paradigms and prospects. Arab. J. Chem. 2019 , 12 , 1365–1377. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Zabochnicka-Świątek, M.; Krzywonos, M. Potentials of biosorption and bioaccumulation processes for heavy metal removal. Pol. J. Environ. Stud. 2014 , 23 , 551–561. [ Google Scholar ]
- Tiquia-Arashiro, S.M. Lead absorption mechanisms in bacteria as strategies for lead bioremediation. Appl. Microbiol. Biotechnol. 2018 , 102 , 5437–5444. [ Google Scholar ] [ CrossRef ]
- Babák, L.; Šupinova, P.; Zichova, M.; Burdychova, R.; Vitova, E. Biosorption of Cu, Zn and Pb by thermophilic bacteria–effect of biomass concentration on biosorption capacity. Acta Univ. Agric. Silvic. Mendel. Brun. 2012 , 60 , 9–18. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Das, P.; Sinha, S.; Mukherjee, S.K. Nickel bioremediation potential of Bacillus thuringiensis KUNi1 and some environmental factors in nickel removal. Bioremediat. J. 2014 , 18 , 169–177. [ Google Scholar ] [ CrossRef ]
- Khan, M.; Ijaz, M.; Chotana, G.A.; Murtaza, G.; Malik, A.; Shamim, S. Bacillus altitudinis MT422188: A potential agent for zinc bioremediation. Bioremediat. J. 2022 , 26 , 228–248. [ Google Scholar ] [ CrossRef ]
- Chen, X.; Lin, H.; Dong, Y.; Li, B.; Liu, C.; Yin, T. Mechanisms underlying enhanced bioremediation of sulfamethoxazole and zinc (II) by Bacillus sp. SDB4 immobilized on biochar. J. Clean. Prod. 2020 , 370 , 133483. [ Google Scholar ] [ CrossRef ]
- Oves, M.; Khanm, M.S.; Zaidim, A.; Ahmadm, E. Soil contamination, nutritive value and human health risk assessment of heavy metals: An overview. In Toxicity of Heavy Metals to Legumes and Bioremediation , 1st ed.; Zaidi, A., Wani, P., Khan, M., Eds.; Springer: Vienna, Austria, 2012; pp. 1–27. ISBN 978-3-7091-0729-4. [ Google Scholar ]
- Sinha, A.; Pant, K.K.; Khare, S.K. Studies on mercury bioremediation by alginate immobilized mercury tolerant Bacillus cereus cells. Int. Biodeterior. Biodegrad. 2012 , 2071 , 1–8. [ Google Scholar ] [ CrossRef ]
- Chen, Z.; Pan, X.; Chen, H.; Lin, Z.; Guan, X. Investigation of lead (II) uptake by Bacillus thuringiensis 016. World J. Microbiol. Biotechnol. 2015 , 31 , 1729–1736. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Joo, J.H.; Hassan, S.H.; Oh, S.E. Comparative study of biosorption of Zn2+ by Pseudomonas aeruginosa and Bacillus cereus . Int. Biodeter. Biodegrad. 2010 , 64 , 734–741. [ Google Scholar ] [ CrossRef ]
- Giri, A.; Patel, R.K.; Mahapatra, S.S.; Mishra, P.C. Biosorption of arsenic (III) from aqueous solution by living cells of Bacillus cereus . Environ. Sci. Pollut. Res. 2013 , 20 , 1281–1291. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Altowayti, W.A.H.; Algaifi, H.A.; Bakar, S.A.; Shahir, S. The adsorptive removal of As (III) using biomass of arsenic resistant Bacillus thuringiensis strain WS3: Characteristics and modelling studies. Ecotoxicol. Environ. Saf. 2019 , 172 , 176–185. [ Google Scholar ] [ CrossRef ]
- Kumawat, T.K.; Kumawat, V.; Sharma, S.; Kandwani, N.; Biyani, M. Applications of EPS in environmental bioremediations. In Microbial Exopolysaccharides as Novel and Significant Biomaterials , 1st ed.; Nadda, A.K., Sajna, K.V., Sharma, S., Eds.; Springer: Cham, Switzerland, 2021; pp. 285–302. ISBN 978-3-030-75288-0. [ Google Scholar ]
- Pal, A.; Paul, A.K. Microbial extracellular polymeric substances: Central elements in heavy metal bioremediation. Indian J. Microbiol. 2008 , 48 , 49–64. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Salehizadeh, H.; Shojaosadati, S.A. Removal of metal ions from aqueous solution by polysaccharide produced from Bacillus firmus . Water Res. 2003 , 37 , 4231–4235. [ Google Scholar ] [ CrossRef ]
- Krishnamurthy, M.; Uthaya, C.J.; Thangavel, M.; Annadurai, V.; Rajendran, R.; Gurusamy, A. Optimization, compositional analysis, and characterization of exopolysaccharides produced by multi-metal resistant Bacillus cereus KMS3-1. Carbohydr. Polym. 2020 , 227 , 115369. [ Google Scholar ] [ CrossRef ]
- Kalpana, R.; Angelaalincy, M.J.; Kamatchirajan, B.V.; Vasantha, V.S.; Ashokkumar, B.; Ganesh, V.; Varalakshmi, P. Exopolysaccharide from Bacillus cereus VK1: Enhancement, characterization and its potential application in heavy metal removal. Colloids Surf. B Biointerfaces 2018 , 171 , 327–334. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Morillo Pérez, J.A.; García-Ribera, R.; Quesada, T.; Aguilera, M.; Ramos-Cormenzana, A.; Monteoliva-Sánchez, M. Biosorption of heavy metals by the exopolysaccharide produced by Paenibacillus jamilae . World J. Microbiol. Biotechnol. 2008 , 24 , 2699–2704. [ Google Scholar ] [ CrossRef ]
- Issazadeh, K.; Jahanpour, N.; Pourghorbanali, F.; Raeisi, G.; Faekhondeh, J. Heavy metals resistance by bacterial strains. Ann. Biol. Res. 2013 , 4 , 60–63. [ Google Scholar ]
- Vijayaraghavan, K.; Yun, Y.S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008 , 26 , 266–291. [ Google Scholar ] [ CrossRef ]
- Srinath, T.; Verma, T.; Ramteke, P.W.; Garg, S.K. Chromium (VI) biosorption and bioaccumulation by chromate resistant bacteria. Chemosphere 2002 , 48 , 427–435. [ Google Scholar ] [ CrossRef ]
- Hamer, D.H. Metallothioneins. Annu. Rev. Biochem. 1986 , 55 , 913–951. [ Google Scholar ] [ CrossRef ]
- Blindauer, C.A.; Harrison, M.D.; Robinson, A.K.; Parkinson, J.A.; Bowness, P.W.; Sadler, P.J.; Robinson, N.J. Multiple bacteria encode metallothione in sand SmtA-like fingers. Mol. Microbiol. 2002 , 45 , 1421–1432. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Liu, T.; Nakashima, S.; Hirose, K.; Uemura, Y.; Shibasaka, M.; Katsuhara, M.; Kasamo, K. A metallothionein and CPx-ATPase handle heavy-metal tolerance in the filamentous cyanobacteria Oscillatoria brevis . FEBS Lett. 2003 , 542 , 159–163. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Das, S.; Dash, H.R.; Chakraborty, J. Genetic basis and importance of metal resistant genes in bacteria for bioremediation of contaminated environments with toxic metal pollutants. Appl. Microbiol. Biotechnol. 2016 , 100 , 2967–2984. [ Google Scholar ] [ CrossRef ]
- Yang, H.C.; Fu, H.L.; Lin, Y.F.; Rosen, B.P. Pathways of arsenic uptake and efflux. Curr. Top. Membr. 2012 , 69 , 325–358. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Borremans, B.; Hobman, J.L.; Provoost, A.; Brown, N.L.; van Der Lelie, D. Cloning and functional analysis of the pbr lead resistance determinant of Ralstonia metallidurans CH34. J. Bacteriol. 2001 , 183 , 5651–5658. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Yu, P.; Yuan, J.; Deng, X.; Ma, M.; Zhang, H. Subcellular targeting of bacterial CusF enhances Cu accumulation and alters root to shoot Cu translocation in Arabidopsis . Plant Cell Physiol. 2014 , 55 , 1568–1581. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Kiyono, M.; Oka, Y.; Sone, Y.; Nakamura, R.; Sato, M.H.; Sakabe, K.; Pan-Hou, H. Bacterial heavy metal transporter MerC increases mercury accumulation in Arabidopsis thaliana . Biochem. Eng. J. 2013 , 71 , 19–24. [ Google Scholar ] [ CrossRef ]
- Murthy, S.; Geetha, B.; Sarangi, S.K. Effect of lead on metallothionein concentration in lead-resistant bacteria Bacillus cereus isolated from industrial effluent. Afr. J. Biotechnol. 2011 , 10 , 15966–15972. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Huang, F.; Wang, Z.H.; Cai, Y.X.; Chen, S.H.; Tian, J.H.; Cai, K.Z. Heavy metal bioaccumulation and cation release by growing Bacillus cereus RC-1 under culture conditions. Ecotoxicol. Environ. Saf. 2018 , 157 , 216–226. [ Google Scholar ] [ CrossRef ]
- Belapurkar, P.; Goyal, P.; Kar, A. In vitro evaluation of bioremediation capacity of a commercial probiotic, Bacillus coagulans , for chromium (VI) and lead (II) toxicity. J. Pharm. Bioallied Sci. 2016 , 8 , 272–276. [ Google Scholar ] [ CrossRef ]
- Singh, N.; Gupta, S.; Marwa, N.; Pandey, V.; Verma, P.C.; Rathaur, S.; Singh, N. Arsenic mediated modifications in Bacillus aryabhattai and their biotechnological application for arsenic bioremediation. Chemosphere 2016 , 164 , 524–534. [ Google Scholar ] [ CrossRef ]
- Naskar, A.; Majumder, R.; Goswami, M. Bioaccumulation of Ni (II) on growing cells of Bacillus sp.: Response surface modeling and mechanistic insight. nviron. Technol. Innov. 2020 , 20 , 101057. [ Google Scholar ] [ CrossRef ]
- Kaksonen, A.H.; Puhakka, J.A. Sulfate reduction-based bioprocesses for the treatment of acid mine drainage and the recovery of metals. Eng. Life Sci. 2007 , 7 , 541–564. [ Google Scholar ] [ CrossRef ]
- De, J.; Ramaiah, N.; Vardanyan, L. Detoxification of toxic heavy metals by marine bacteria highly resistant to mercury. Mar. Biotechnol. 2008 , 10 , 471–477. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, F.; Wang, W.; Li, C.; Zhu, R.; Ge, F.; Zheng, Y.; Tang, Y. Self-mediated pH changes in culture medium affecting biosorption and biomineralization of Cd(2+) by Bacillus cereus Cd01. J. Hazard. Mater. 2018 , 358 , 178–186. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Molokwane, P.E.; Meli, C.K.; Chirwa, E.M. Chromium (VI) reduction in activated sludge bacteria exposed to high chromium loading: Brits culture (South Africa). Water Sci. Technol. 2008 , 58 , 399–405. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Xiao, R.; Liu, X.; Ali, A.; Chen, A.; Zhang, M.; Li, R.; Chang, H.; Zhang, Z. Bioremediation of Cd-spiked soil using earthworms ( Eisenia fetida ): Enhancement with biochar and bacillus megatherium application. Chemosphere 2021 , 264 , 128517. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, Q.; Xing, Y.; Huang, B.; Chen, X.; Ji, L.; Fu, X.; Li, T.; Wang, J.; Chen, G.; Zhang, Q. Rhizospheric mechanisms of Bacillus subtilis bioaugmentation-assisted phytostabilization of cadmium-contaminated soil. Sci. Total Environ. 2022 , 825 , 154136. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chen, Z.; Zheng, Y.; Ding, C.; Ren, X.; Yuan, J.; Sun, F.; Li, Y. Integrated metagenomics and molecular ecological network analysis of bacterial community composition during the phytoremediation of cadmium-contaminated soils by bioenergy crops. Ecotoxicol. Environ. Saf. 2017 , 145 , 111–118. [ Google Scholar ] [ CrossRef ]
- Jing, R.; Kjellerup, B.V. Biogeochemical cycling of metals impacting by microbial mobilization and immobilization. J. Environ. Sci. 2018 , 66 , 146–154. [ Google Scholar ] [ CrossRef ]
- Ashraf, M.A.; Hussain, I.; Rasheed, R.; Iqbal, M.; Riaz, M.; Arif, M.S. Advances in microbe-assisted reclamation of heavy metal contaminated soils over the last decade: A review. J. Environ. Manag. 2017 , 198 , 132–143. [ Google Scholar ] [ CrossRef ]
- Ren, X.-M.; Guo, S.-J.; Tian, W.; Chen, Y.; Han, H.; Chen, E.; Li, B.-L.; Li, Y.-Y.; Chen, Z.-J. Effects of Plant Growth-Promoting Bacteria (PGPB) inoculation on the growth, antioxidant activity, Cu uptake, and bacterial community structure of rape ( Brassica napus L.) grown in Cu-contaminated agricultural soil. Front. Microbiol. 2019 , 10 , 1455. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Pandey, S.; Ghosh, P.K.; Ghosh, S.; De, T.K.; Maiti, T.K. Role of heavy metal resistant Ochrobactrum sp. and Bacillus spp. strains in bioremediation of a rice cultivar and their PGPR like activities. J. Microbiol. 2013 , 51 , 11–17. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pishchik, V.N.; Filippova, P.S.; Mirskaya, G.V.; Khomyakov, Y.V.; Vertebny, V.E.; Dubovitskaya, V.I.; Ostankova, Y.V.; Semenov, A.V.; Chakrabarty, D.; Zuev, E.V.; et al. Epiphytic PGPB Bacillus megaterium AFI1 and Paenibacillus nicotianae AFI2 improve wheat growth and antioxidant status under Ni Stress. Plants 2021 , 10 , 2334. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Luo, S.L.; Chen, L.; Chen, J.L.; Xiao, X.; Xu, T.Y.; Wan, Y.; Rao, C.; Liu, C.B.; Liu, Y.T.; Lai, C.; et al. Analysis and characterization of cultivable heavy metal-resistant bacterial endophytes isolated from Cd-hyperaccumulator Solanum nigrum L. and their potential use for phytoremediation. Chemosphere 2011 , 85 , 1130–1138. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Schwartz, A.R.; Ortiz, I.; Maymon, M.; Herbold, C.W.; Fujishige, N.A.; Vijanderan, J.A.; Villella, W.; Hanamoto, K.; Diener, A.; Sanders, E.R.; et al. Bacillus simplex —A little known PGPB with anti-fungal activity—Alters pea legume root architecture and nodule morphology when coinoculated with Rhizobium leguminosarum bv. viciae. Agronomy 2013 , 3 , 595–620. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Hiller, J.; Napora, A.; Grobelak, A. Growth promotion by PGBP bacteria. In Innovations in Technological Processes [Innowacje w Procesach Technologicznych] , 1st ed.; Bajdur, W.M., Ed.; Wydawnictwo Wydziału Zarządzania Politechniki Częstochowskiej: Częstochowa, Poland, 2016; pp. 74–83. ISBN 978-83-65179-72-2. [ Google Scholar ]
- Hong, S.; Kim, T.Y.; Won, S.-J.; Moon, J.-H.; Ajuna, H.B.; Kim, K.Y.; Ahn, Y.S. Control of fungal diseases and fruit yield improvement of strawberry using Bacillus velezensis CE 100. Microorganisms 2022 , 10 , 365. [ Google Scholar ] [ CrossRef ]
- Mirskaya, G.V.; Khomyakov, Y.V.; Rushina, N.A.; Vertebny, V.E.; Chizhevskaya, E.P.; Chebotar, V.K.; Chesnokov, Y.V.; Pishchik, V.N. Plant development of early-maturing spring wheat ( Triticum aestivum L.) under inoculation with Bacillus sp. V2026. Plants 2022 , 11 , 1817. [ Google Scholar ] [ CrossRef ]
- Mukhtar, T.; Rehman, S.U.; Smith, D.; Sultan, T.; Seleiman, M.F.; Alsadon, A.A.; Amna; Ali, S.; Chaudhary, H.J.; Solieman, T.H.I.; et al. Mitigation of heat stress in Solanum lycopersicum L. by ACC-deaminase and exopolysaccharide producing Bacillus cereus: Effects on biochemical profiling. Sustainability 2020 , 12 , 2159. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant growth enhancement using rhizospheric halotolerant phosphate solubilizing bacterium Bacillus licheniformis QA1 and Enterobacter asburiae QF11 isolated from Chenopodium quinoa Willd. Microorganisms 2020 , 8 , 948. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Nithyapriya, S.; Lalitha, S.; Sayyed, R.Z.; Reddy, M.S.; Dailin, D.J.; El Enshasy, H.A.; Luh Suriani, N.; Herlambang, S. Production, purification, and characterization of bacillibactin siderophore of Bacillus subtilis and its application for improvement in plant growth and oil content in sesame. Sustainability 2021 , 13 , 5394. [ Google Scholar ] [ CrossRef ]
- Dobrzyński, J.; Wierzchowski, P.S.; Stępień, W.; Górska, E.B. The reaction of cellulolytic and potentially cellulolytic spore-forming bacteria to various types of crop management and farmyard manure fertilization in bulk soil. Agronomy 2021 , 11 , 772. [ Google Scholar ] [ CrossRef ]
- Dobrzyński, J.; Jakubowska, Z.; Dybek, B. Potential of Bacillus pumilus to directly promote plant growth. Front. Microbiol. 2022 , 13 , 1069053. [ Google Scholar ] [ CrossRef ]
- Khan, N.; Maymon, M.; Hirsch, A.M. Combating Fusarium infection using Bacillus -based antimicrobials. Microorganisms 2017 , 5 , 75. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Sibponkrung, S.; Kondo, T.; Tanaka, K.; Tittabutr, P.; Boonkerd, N.; Yoshida, K.-I.; Teaumroong, N. Co-inoculation of Bacillus velezensis strain S141 and Bradyrhizobium strains promotes nodule growth and nitrogen fixation. Microorganisms 2020 , 8 , 678. [ Google Scholar ] [ CrossRef ]
- Kwon, J.-H.; Won, S.-J.; Moon, J.-H.; Lee, U.; Park, Y.-S.; Maung, C.E.H.; Ajuna, H.B.; Ahn, Y.S. Bacillus licheniformis PR2 controls fungal diseases and increases production of jujube fruit under field conditions. Horticulturae 2021 , 7 , 49. [ Google Scholar ] [ CrossRef ]
- Dobrzyński, J.; Wróbel, B.; Górska, E.B. Cellulolytic properties of a potentially lignocellulose-degrading Bacillus sp. 8E1A strain isolated from bulk soil. Agronomy 2022 , 12 , 665. [ Google Scholar ] [ CrossRef ]
- Chiboub, M.; Jebara, S.H.; Saadani, O.; Fatnassi, I.C.; Abdelkerim, S.; Jebara, M. Physiological responses and antioxidant enzyme changes in Sulla coronaria inoculated by cadmium resistant bacteria. J. Plant Res. 2018 , 131 , 99–110. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rizvi, A.; Khan, M.S. Heavy metal-induced oxidative damage and root morphology alterations of maize ( Zea mays L.) plants and stress mitigation by metal tolerant nitrogen-fixing Azotobacter chroococcum . Ecotoxicol. Environ. Saf. 2018 , 157 , 9–20. [ Google Scholar ] [ CrossRef ]
- Alka, S.; Shahir, S.; Ibrahim, N.; Chai, T.T.; Mohd Bahari, Z.; Abd Manan, F. The role of plant growth promoting bacteria on arsenic removal: A review of existing perspectives. Environ. Technol. Innov. 2020 , 17 , 100602. [ Google Scholar ] [ CrossRef ]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation–A review. Earth Sci. Rev. 2017 , 171 , 621–645. [ Google Scholar ] [ CrossRef ]
- Kong, Z.; Glick, B.R. The role of plant growth-promoting bacteria in metal phytoremediation. Adv. Microb. Physiol. 2017 , 71 , 97–132. [ Google Scholar ] [ CrossRef ]
- Wu, J.; Kamal, N.; Hao, H.; Qian, C.; Liu, Z.; Shao, Y.; Zhong, X.; Xu, B. Endophytic Bacillus megaterium BM18-2 mutated for cadmium accumulation and improving plant growth in Hybrid Pennisetum. Biotechnol. Rep. 2019 , 24 , e00374. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020 , 11 , 359. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Burd, G.I.; Dixon, D.G.; Glick, B.R. A plant growth promoting bacterium that decreases nickel toxicity in seedlings. Appl. Environ. Microbiol. 1998 , 64 , 3663–3668. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Glick, B.R.; Penrose, D.M.; Li, J. A model for lowering of plant ethylene concentrations by plant-growth-promoting bacteria. J. Theor. Biol. 1998 , 190 , 63–68. [ Google Scholar ] [ CrossRef ]
- Hussein, K.A.; Joo, J.H. Potential of siderophore production by bacteria isolated from heavy metal: Polluted and rhizosphere soils. Curr. Microbiol. 2014 , 68 , 717–723. [ Google Scholar ] [ CrossRef ]
- Huo, Y.; Kang, J.P.; Ahn, J.C.; Kim, Y.J.; Piao, C.H.; Yang, D.U.; Yang, D.C. Siderophore-producing rhizobacteria reduce heavy metal-induced oxidative stress in Panax ginseng Meyer. J. Ginseng Res. 2021 , 45 , 218–227. [ Google Scholar ] [ CrossRef ]
- Caracciolo, A.B.; Terenzi, V. Rhizosphere microbial communities and heavy metals. Microorganisms 2021 , 9 , 1462. [ Google Scholar ] [ CrossRef ]
- Rajkumar, M.; Ae, N.; Prasad, M.N.V.; Freitas, H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010 , 28 , 142–149. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Schalk, I.J.; Hannauer, M.; Braud, A. New roles for bacterial siderophores in metal transport and tolerance. Environ. Microbiol. 2011 , 13 , 2844–2854. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Roskova, Z.; Skarohlid, R.; McGachy, L. Siderophores: An alternative bioremediation strategy? Sci. Total Environ. 2022 , 819 , 153144. [ Google Scholar ] [ CrossRef ]
- Zaidi, S.; Usmani, S.; Singh, B.R.; Musarrat, J. Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea . Chemosphere 2006 , 64 , 991–997. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010 , 28 , 367–374. [ Google Scholar ] [ CrossRef ]
- Sessitsch, A.; Kuffner, M.; Kidd, P.; Vangronsveld, J.; Wenzel, W.W.; Fallmann, K.; Puschenreiter, M. The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol. Biochem. 2013 , 60 , 182–194. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- He, L.Y.; Chen, Z.J.; Ren, G.D.; Zhang, Y.F.; Qian, M.; Sheng, X.F. Increased cadmium and lead uptake of a cadmium hyperaccumulator tomato by cadmium-resistant bacteria. Ecotoxicol. Environ. Saf. 2009 , 72 , 1343–1348. [ Google Scholar ] [ CrossRef ]
- Abou-Shanab, R.A.; Ghanem, K.; Ghanem, N.; Al-Kolaibe, A. The role of bacteria on heavy-metal extraction and uptake by plants growing on multi-metal-contaminated soils. World J. Microbiol. Biotechnol. 2008 , 24 , 253–262. [ Google Scholar ] [ CrossRef ]
- Saran, A.; Imperato, V.; Fernandez, L.; Gkorezis, P.; D′Haen, J.; Merini, L.J.; Vangronsveld, J.; Thijs, S. Phytostabilization of polluted military soil supported by bioaugmentation with PGP-trace element tolerant bacteria isolated from Helianthus petiolaris. Agronomy 2020 , 10 , 204. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Al-Dhabi, N.A.; Esmail, G.A.; Mohammed Ghilan, A.-K.; Valan Arasu, M. Optimizing the management of cadmium bioremediation capacity of metal-resistant Pseudomonas sp. strain Al-Dhabi-126 isolated from the industrial city of Saudi Arabian Environment. Int. J. Environ. Res. Public Health 2019 , 16 , 4788. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Malekzadeh, E.; Alikhani, H.A.; Savaghebi-Firoozabadi, G.R.; Zarei, M. Bioremediation of cadmium-contaminated soil through cultivation of maize inoculated with plant growth–promoting rhizobacteria. Bioremed. J. 2012 , 16 , 204–211. [ Google Scholar ] [ CrossRef ]
- Pinter, M.I.F.; Salomon, M.V.; Berli, F.; Gil, R.; Bottini, R.; Piccoli, P. Plant growth promoting rhizobacteria alleviate stress by AsIII in grapevine. Agric. Ecosyst. Environ. 2018 , 267 , 100–108. [ Google Scholar ] [ CrossRef ]
- He, C.Q.; Tan, G.; Liang, X.; Du, W.; Chen, Y.; Zhi, G.; Zhu, Y. Effect of Zn-tolerant bacterial strains on growth and Zn accumulation in Orychophragmus violaceus . Appl. Soil Ecol. 2010 , 44 , 1–5. [ Google Scholar ] [ CrossRef ]
- Ma, Y.; Rajkumar, M.; Freitas, H. Improvement of plant growth and nickel uptake by nickel resistant-plant-growth promoting bacteria. J. Hazard. Mater. 2009 , 66 , 1154–1161. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Rajkumar, M.; Nagendran, R.; Lee, K.J.; Lee, W.H.; Kim, S.Z. Influence of plant growth promoting bacteria and Cr6+ on the growth of Indian mustard. Chemosphere 2006 , 62 , 741–748. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sheng, X.; He, L.; Wang, Q.; Ye, H.; Jiang, C. Effects of inoculation of biosurfactant-producing Bacillus sp. J119 on plant growth and cadmium uptake in a cadmium-amended soil. J. Hazard. Mater. 2008 , 155 , 17–22. [ Google Scholar ] [ CrossRef ] [ PubMed ]
Click here to enlarge figure
| Strains | Plant | Bioremediated Metal | PGP Traits | PGP Effects | References |
|---|---|---|---|---|---|
| Bacillus sp. RJ16 | Solanum lycopersicum | Cd and Pb | IAA, siderophores and ACC deaminase | Stimulatation of tomato root growth | He et al. [ ] |
| Bacillus cereus SRA10 | Brassica juncea | Ni | IAA, siderophores | Overall plant growth promotion | Ma et al. [ ] |
| Bacillus sp. Ba32 | Brassica juncea | Cr | Siderophores | Increase in root and shoot length | Rajkumar et al. [ ] |
| Bacillus proteolyticus ST89 | Helianthus annuus | Cd and Pb | IAA | Increase in biomass production | Saran et al. [ ] |
| Bacillusparamycoides ST9 | Helianthus annuus | Cd and Pb | ⎼ | Increase in shoot biomass production | Saran, et al. [ ] |
| Bacillus sp. J119 | Brassica napus Huiyou-50, Zea mays Denhai-11, Sorghum bicolor × Sorghum sudanense, Lycopersicon esculentum Shanghai-906 | Cd, Pb, Zn and Cu | IAA | Increase in stem length | Sheng et al. [ ] |
| Bacillus subtilis SJ-101 | Brassica juncea | Ni | IAA | Increase in growth of above-ground tissue and root | Zaidi et al. [ ] |
| Bacillus megaterium BM18-2 | Pennisetum americanum × Pennisetum purpureum Schumach | Cd | IAA | Increase in shoot and root length | Wu et al. [ ] |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Wróbel, M.; Śliwakowski, W.; Kowalczyk, P.; Kramkowski, K.; Dobrzyński, J. Bioremediation of Heavy Metals by the Genus Bacillus. Int. J. Environ. Res. Public Health 2023 , 20 , 4964. https://doi.org/10.3390/ijerph20064964
Wróbel M, Śliwakowski W, Kowalczyk P, Kramkowski K, Dobrzyński J. Bioremediation of Heavy Metals by the Genus Bacillus. International Journal of Environmental Research and Public Health . 2023; 20(6):4964. https://doi.org/10.3390/ijerph20064964
Wróbel, Monika, Wojciech Śliwakowski, Paweł Kowalczyk, Karol Kramkowski, and Jakub Dobrzyński. 2023. "Bioremediation of Heavy Metals by the Genus Bacillus" International Journal of Environmental Research and Public Health 20, no. 6: 4964. https://doi.org/10.3390/ijerph20064964
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
- DOI: 10.1021/acsnano.4c02493
- Corpus ID: 270763005
Engineered Cyanobacteria-Based Living Materials for Bioremediation of Heavy Metals Both In Vitro and In Vivo.
- Tao Sun , Huaishu Huo , +8 authors Lei Chen
- Published in ACS Nano 27 June 2024
- Environmental Science, Biology
42 References
Fungal symbionts impact cyanobacterial biofilm durability and photosynthetic efficiency, solar-driven waste-to-chemical conversion by wastewater-derived semiconductor biohybrids, toxicity of cadmium on dynamic human gut microbiome cultures and the protective effect of cadmium-tolerant bacteria autochthonous to the gut., evaluation of cd2+ stress on synechocystis sp. pcc6803 based on single-cell elemental accumulation and algal toxicological response., hydrogel-encapsulated engineered microbial consortium as a photoautotrophic "living material" for promoting skin wound healing., an integrated approach for the phycoremediation of pb(ii) and the production of biofertilizer using nitrogen-fixing cyanobacteria., photoresponsive hydrogel‐coated upconversion cyanobacteria nanocapsules for myocardial infarction prevention and treatment, longitudinal physiological and transcriptomic analyses reveal the short term and long term response of synechocystis sp. pcc6803 to cadmium stress., microalgae-based oral microcarriers for gut microbiota homeostasis and intestinal protection in cancer radiotherapy, machine learning-informed and synthetic biology-enabled semi-continuous algal cultivation to unleash renewable fuel productivity, related papers.
Showing 1 through 3 of 0 Related Papers

IMAGES
VIDEO
COMMENTS
HM contamination, caused by human activities like mining and industrial production, damages the environment and poses health risks in Fig. 1.These metals are non-degradable and can negatively affect plant metabolism and human health, with lead, mercury, and arsenic being particularly toxic [4].Exposure to low levels of heavy metals can lead to long-term health issues, especially in children.
Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustainability . 2015;7:2189- 2212.
Bioremediation is an effective and eco-friendly approach that can reduce heavy metal contamination in the environment. Bioremediation agents include bacteria of the genus Bacillus, among others. The best-described species in terms of the bioremediation potential of Bacillus spp. Are B. subtilis, B. cereus, or B. thuringiensis.
Advanced methods of heavy metal remediation include physicochemical and biological methods; the latter can be further classified into in situ and ex situ bioremediation. The in situ process includes bioventing, biosparging, biostimulation, bioaugmentation, and phytoremediation. Ex situ bioremediation includes land farming, composting, biopiles ...
Influence of Environmental Change on the Remediation of Heavy Metal Contaminants. The pH is important for microbial biosorption, and the optimal pH varies depending on the microbe. Firstly, pH influences the enzymatic activity in bacteria, altering the rate of HM microbial metabolism ( Morton-Bermea et al., 2002 ).
The papers published from 2018 to 2022 on bioremediation of heavy metals from water with specified bacterial strains and mechanism were compiled and tabulated. The practical applications of bacteria mediated heavy metal bioremediation is provided, mentioning the bacterial species involved and its removal efficiency. 2. Mechanism of bioremediation
Bioremediation is an inventive and optimistic technology which is applicable for the retrieval and reduction of heavy metals in water and polluted lands. Microorganism plays an essential part in bioremediation of heavy metals. By using genetic engineering, genetically modified organisms can be generated which can likely reduce different types ...
Cadmium stress protein. Exposure of bacteria to heavy metals leads to altered expression of genes involved in metal transport as well as other stress responses such as heat shock and oxidative stress (Blom et al. 1992).In general, exposure of bacteria to low levels of one stress can induce a consequent increase in resistance to the same (adaptive) or unrelated (cross-protection) stress ...
Feature papers represent the most advanced research with significant potential for high impact in the field. ... Irawati, W.; Parhusip, A.J.N.; Sopiah, N.; Tnunay, J.A. The Role of Heavy Metals-Resistant Bacteria Acinetobacter sp. in Copper Phytoremediation ... 2023. "Microbial-Based Heavy Metal Bioremediation: Toxicity and Eco-Friendly ...
PDF | On Mar 26, 2021, Amanso Tayang and others published Microbial Bioremediation of Heavy Metals | Find, read and cite all the research you need on ResearchGate
Heavy elements accumulate rapidly in the soil due to industrial activities and the industrial revolution, which significantly impact the morphology, physiology, and yield of crops. Heavy metal contamination will eventually affect the plant tolerance threshold and cause changes in the plant genome and genetic structure. Changes in the plant genome lead to changes in encoded proteins and protein ...
Turkish Science and Technology Publishing (TURSTEP) Bioremediation of Heavy Metals by Use of Bacteria. Orcan Demircan 1,a,*, A bdulrezzak Memon 2,b. 1 Department of Molecular Biology and Genetics ...
In the present article, we have gathered information on several metal-resistant bacteria and their mechanisms of action. We have curated a data set of toxic heavy metals such as arsenic, chromium, mercury, lead, iron, copper, cadmium, zinc, cobalt, antimony, selenium, manganese, molybdenum, nickel, tungsten, and uranium, as well as a few less toxic heavy metals such as platinum, gold, silver ...
Background The demand for designing a new technology that can emphasize the complete removal of heavy metals increased as a result of the industrial revolution. Bioremediation was found to have a potent impact on the degradation of organic and inorganic environmental pollutants. Main body Bioremediation is a multidisciplinary technology that possesses safe, efficient, and low-cost ...
Microbial remediation presents new techniques for addressing the problem of heavy metal pollution in the environment, and it has become a focus of new research and development in bioremediation technology. This review addresses microbes used as a tool for bioremediation of heavy metals from the environment. 2.
Heavy-metal contaminants are one of the most relevant problems of contemporary agriculture. High toxicity and the ability to accumulate in soils and crops pose a serious threat to food security. To solve this problem, it is necessary to accelerate the pace of restoration of disturbed agricultural lands. Bioremediation is an effective treatment for agricultural soil pollution. It relies on the ...
Heavy metal bioremediation in the form of metallic nanoparticles with the help of bacteria and the use of genetically modified microorganisms as a part of the bioremediation process have also been reported. 99-101. Intracellular sequestration is the concentration of metal ions within the microbial cells.
The papers published from 2018 to 2022 on bioremediation of heavy metals from water with specified bacterial strains and mechanism were compiled and tabulated. The practical applications of bacteria mediated heavy metal bioremediation is provided, mentioning the bacterial species involved and its removal efficiency.
The pollution caused by heavy metals (HMs) represents a global concern due to their serious environmental threat. Photosynthetic cyanobacteria have a natural niche and the ability to remediate HMs such as cadmium. However, their practical application is hindered by a low tolerance to HMs and issues related to recycling. In response to these challenges, this study focuses on the development and ...
Due to metal resources being non-renewable, recovering metal from industrial waste water may be a viable option for ensuring heavy metal supply (Atkinson et al., 1998). Bioremediation has been offered by a number of researchers as a way to recover raw materials from effluent ( Pollmann et al., 2006 ; Gadd, 2010 ).
Bioremediation research has largely focused on bacterial processes, which have numerous applications. ... Hoboken, NJ, USA: 2021. Bioremediation of heavy metals and other toxic substances by microorganisms; pp. 285-329. ... Das S. Biosorption and removal of toxic heavy metals by metal tolerating bacteria for bioremediation of metal ...
@article{Ketaubon2024EnhancingHM, title={Enhancing heavy metal phytoremediation in landfill soil by Chrysopogon zizanioides (L.) Roberty through the application of bacterial-biochar pellets}, author={Patipat Ketaubon and Naritsorn Ritthikasem and Pantaree Tanheng and Benjaphorn Prapagdee}, journal={Environmental Technology \& Innovation ...
Abstract. Significant amounts of heavy metals are being added. into the soil and effluents each and every day due to. enhanced industrialization and other anthropocentric. activities. Many heavy ...
The removal of heavy metals by the bacterial mixtures was assessed using a 2 mM mixture of heavy metals, and the remediation potentials of the bacterial mixtures were observed to vary between 5.56% and 98.25% (Fig. 5 B). Pb, Cd, and Cu concentrations were reduced to 0.06, 19.34, and 137.50 mg/L in about 48 h, respectively. This amounted to ...
Environmental contamination with heavy metals is one of the major problems caused by human activity. Bioremediation is an effective and eco-friendly approach that can reduce heavy metal contamination in the environment. Bioremediation agents include bacteria of the genus Bacillus, among others. The best-described species in terms of the bioremediation potential of Bacillus spp.
DOI: 10.1021/acsnano.4c02493 Corpus ID: 270763005; Engineered Cyanobacteria-Based Living Materials for Bioremediation of Heavy Metals Both In Vitro and In Vivo. @article{Sun2024EngineeredCL, title={Engineered Cyanobacteria-Based Living Materials for Bioremediation of Heavy Metals Both In Vitro and In Vivo.}, author={Tao Sun and Huaishu Huo and Yingying Zhang and Yaru Xie and Yize Li and ...
Finally, based on the review of literature, this paper presents the emerging strategies to overcome the low adsorption capacity of LAB. ... and provides theoretical support for the practical application of LAB bioremediation of HMs. Keywords: Lactic acid bacteria; Heavy metals; Bioremediation; Adsorption; Removal mechanism 1 Introduction Metal ...