- DOI: 10.22533/at.ed.1594532406062
- Corpus ID: 270546638

BRONCHIAL ASTHMA: A REVIEW OF SYMPTOMS AND EMERGENCY CONDUCTS
- Eduardo de Pádua Scarpellini , João Victor Ribeiro
- Published in International Journal of… 7 June 2024
Related Papers
Showing 1 through 3 of 0 Related Papers
- Open access
- Published: 13 August 2021
Biological therapy for severe asthma
- Silvano Dragonieri ORCID: orcid.org/0000-0003-1563-6864 1 &
- Giovanna Elisiana Carpagnano 1
Asthma Research and Practice volume 7 , Article number: 12 ( 2021 ) Cite this article
12k Accesses
21 Citations
3 Altmetric
Metrics details
Around 5–10% of the total asthmatic population suffer from severe or uncontrolled asthma, which is associated with increased mortality and hospitalization, increased health care burden and worse quality of life. In the last few years, new drugs have been launched and several asthma phenotypes according to definite biomarkers have been identified. In particular, therapy with biologics has revolutionized the management and the treatment of severe asthma, showing high therapeutic efficacy associated with significant clinical benefits. To date, four types of biologics are licensed for severe asthma, i.e. omalizumab (anti-immunoglobulin E) antibody, mepolizumab and reslizumab (anti-interleukin [IL]-5antibody), benralizumab (anti-IL-5 receptor a antibody) and dupilumab (anti-IL-4 receptor alpha antibody). The aim of this article was to review the biologic therapies currently available for the treatment of severe asthma, in order to help physicians to choose the most suitable biologic agent for their asthmatic patients.
Since the beginning of this millennium, asthma assessment and management have been revolutionized. While some new therapeutic approaches have been suggested for mild asthmatics, the most relevant changes have occurred in severe asthma. Severe asthma accounts for the 5–10% of the global asthma population, with 3 to 5% being uncontrolled despite adherence to therapy and proper use of inhalers [ 1 ]. These subjects cannot achieve symptoms control despite maximal therapy with inhaled corticosteroids (ICS) and, quite often, maintenance oral corticosteroids (OCS) are necessary in an endeavor to avoid life-threatening exacerbations [ 2 ]. Although OCS courses remain essential for the management of acute exacerbations, their recurrent or continuous usage is associated with several complications, such as an increased risk of developing osteoporotic fractures and pneumonia [ 3 ]. Moreover, other conditions including cardiovascular and cerebrovascular events, renal dysfunction, diabetes mellitus type 2, humor alterations, obesity and sleep apneas are known to be associated with systemic corticosteroid exposure [ 3 ]. Additionally, many patients remain poorly controlled and show recurrent exacerbations despite a strict adherence to therapy [ 4 ].
The recent advances in our knowledge of the etiopathological mechanisms of different phenotypes and endotypes of severe asthma gave us very innovative therapies, such as biological drugs for severe asthma. These medications are mostly directed against molecules involved in the type 2 inflammatory pathway, thus modifying the natural course of the disease by reducing airways inflammation without the collateral damage associated with corticosteroids. Based on the above, the aim of this article was to review the biologic therapies currently available for the treatment of severe asthma, in order to help physicians to choose the most suitable biologic agent for their asthmatic patients.
Licensed medications for severe asthma
To date, there are five biologic molecules officially approved for use in selected severe asthmatic patients. The first of these is omalizumab, an anti-IgE monoclonal antibody acting through various mechanisms on allergic pathways (Table 1 ). Three more biologics for asthma, belonging to a different class, have been approved, i.e. mepolizumab, reslizumab and benralizumab. They all target the interleukin-5 (IL-5) pathway with the first two targeting the interleukin itself and the last one its receptor. Finally, dupilumab is a monoclonal antibody against the receptor of interleukin-4 (IL-4) which blocks the signaling pathways of IL-4 and IL-13.
BIOLOGICS TARGETING IgE
Omalizumab was the first targeted biologic therapy developed and licensed for severe asthma, being approved by the Food and Drugs Administration in 2003 [ 5 ]. It is a recombinant monoclonal Antibody which binds to IgE, thereby lowering blood IgE levels of up to 99% [ 6 ]. Moreover, It decreases expression of IgE receptor FCRI on inflammatory cells such as mast cells and basophils, thus helping to both mitigate the allergic response and strengthen the antiviral immune response, finally leading to prevent asthma exacerbations [ 7 ]. Omalizumab is approved in adults and children above 6 years old with IgE-driven moderate-to-severe persistent allergic asthma which remains uncontrolled despite GINA step 4/5 treatment, high levels of blood IgE, and documented sensitization to a perennial allergen [ 8 ]. Its dosage varies according to patient’s bodyweight and circulating IgE levels and it is administered subcutaneously every 14 or 28 days [ 9 ]. Although not necessary from a safety point of view, it is advisable to re-evaluate patients after the initial 16 weeks of treatment to assess the drug efficacy before continuing with omalizumab therapy [ 8 ].
The efficacy and safety of omalizumab are nowadays unquestionably recognized, with numerous studies demonstrating that this biological is generally well-tolerated, with no serious adverse effects reported [ 10 , 11 , 12 , 13 , 14 , 15 ]. Common side effects include injection site or diffuse rash, fever, nose bleeding, joint pain, gastro-intestinal disturbances, headache, dizziness and cold symptoms [ 10 , 11 , 12 , 13 , 14 , 15 ]. A Cochrane systematic review assessing 25 randomized controlled trials in patients with allergic asthma showed the efficacy of omalizumab in reducing asthma exacerbations, hospitalizations, and inhaled corticosteroid dosage [ 10 , 15 , 16 , 17 , 18 , 19 ].
During the last few years, a number of biomarkers for monitoring the efficacy of omalizumab therapy have been proposed, including total and antigen-specific IgE, blood eosinophil count and exhaled nitric oxide (FeNO) [ 20 , 21 ]. Surprisingly, total IgE did not appear to be a reliable predictor of response to omalizumab therapy, evidencing that our knowledge on this field is still limited [ 21 ]. Peripheral blood eosinophil count ≥300 cells/mL are linked to higher asthma severity and to a better response to omalizumab [ 22 , 23 ]. Furthermore, patients under omalizumab with higher blood eosinophil count have a higher chance to suffer from asthma exacerbations in case of omalizumab discontinuation [ 24 ]. Regarding FeNO, elevated values at baseline correlated with a better response to omalizumab with regard to exacerbations decrease [ 20 , 25 ]. Likewise, elevated levels of FeNO after suspension of long-term therapy with omalizumab may be a predictor of successive exacerbations [ 24 ].
Biologics targeting IL-5
IL-5 is a well-known regulator of the activation, differentiation, effector function, migration and survival and effector function of eosinophils [ 26 ]. Eosinophil levels associated with symptoms of asthma correlate with disease severity and increase the risk of asthma exacerbations, evidencing that this granulocyte type plays a key role in the pathophysiololgy of asthma [ 26 ]. Currently, licensed biologics against IL-5 pathways are mepolizumab, reslizumab, and benralizumab.
MEPOLIZUMAB
Mepolizumab is a monoclonal antibody directed against IL-5 which has been approved as an add-on treatment for patients ≥6 years old in Europe and for patients ≥12 years old in the USA. Mepolizumab was the first anti-IL-5 antibody approved for the treatment of severe asthma by the Food and Drugs Administration in 2015. Eligible subjects are those with severe eosinophilic asthma that remains uncontrolled despite GINA step 4/5 therapy, with blood eosinophil count of ≥150 cells/μl during the first administration or ≥ 300 cells/μl in the previous year and with at least 2 asthma exacerbations requiring systemic steroid course in the past year [ 27 , 28 ]. Mepolizumab is administered by a subcutaneous injection at a fixed dose of 100 mg every 28 days.
Several studies evaluating mepolizumab for uncontrolled eosinophilic asthma showed a markedly reduction with regard to number of exacerbations, systemic corticosteroid usage, emergency room accesses and hospital admissions, and a concurrent improvement of asthma controls and lung function parameters [ 29 , 30 , 31 , 32 , 33 ].
Furthermore, a number of studies revealed that mepolizumab has a positive long-term safety profile [ 34 , 35 , 36 ]. No reports of mepolizumab-associated anaphylaxis reactions were documented, as well as parasitic infections [ 34 , 35 , 36 ]. Common side effects include headache, injection site reaction, fatigue, flu symptoms, urinary tract infection, abdominal pain, itching, eczema, and muscle spasms [ 34 , 35 , 36 ].
Additionally, numerous investigations highlighted that the most important markers of response prediction to mepolizumab are the rate of previous exacerbation and baseline peripheral blood eosinophil count [ 29 , 32 , 37 , 38 , 39 ]. Indeed, a better clinical efficacy is directly proportional to a higher eosinophil count and to a higher rate of exacerbations [ 29 , 32 , 37 , 38 , 39 ]. Interestingly, mepolizumab effectiveness was not related to baseline IgE and to atopy [ 40 , 41 ] and earlier treatment with omalizumab is not a predictor for mepolizumab efficacy [ 42 , 43 , 44 ].
There is a lack of consensus about the duration of treatment before evaluating the effectiveness of mepolizumab. Actually, the GINA statement suggests that a 4-month trial may be adequate [ 8 ], whereas the NICE guidelines recommend that mepolizumab should not be discontinued before 12 months of therapy and that drug-responsiveness should be assessed every year [ 45 ].
Reslizumab is monoclonal antibody approved in 2016, which binds with high-affinity to IL-5 [ 46 ]. By an analogous mechanism of action to mepolizumab, reslizumab lowers circulating blood eosinophil levels [ 47 ]. It has been approved for patients ≥18 years old with severe eosinophilic asthma which remains uncontrolled despite therapy with high-doses of ICS plus another inhaler. Reslizumab is indicated in patients with ≥400 eosinophils/μl and history of asthma exacerbations in the previous 12 months [ 48 , 49 ]. Reslizumab is administered intravenously every 28 days at a weight-based dose of 3 mg/kg.
Similarly to mepolizumab, studies assessing reslizumab have shown a decreased number of asthma exacerbations and improved asthma control and lung function parameters in subjects with high blood eosinophil levels [ 47 , 50 ].
The safety profile of reslizumab has been evaluated for up to 24 months, revealing minor adverse effects without any reports of parasitic and opportunistic infections [ 51 ]. Most frequent side effects include cough, dizziness, itching, skin rash and fatigue [ 51 ].
However, despite its proven excellent clinical efficacy, intravenous formulation has a significant impact on the ease of administration compared to mepolizumab and/or benralizumab. Studies using reslizumab showed unsatisfactory results, without significant improvements in terms of acute exacerbations reduction or OCS lowering [ 52 ].
BENRALIZUMAB
Benralizumab is a monoclonal antibody approved in 2017 and directed against IL-5 receptor a (IL-5Ra) which induces eosinophil apoptosis via the antibody-dependent cell-mediated cytotoxicity (ADCC) involving natural killer cells, leading to peripheral blood eosinophil depletion [ 53 , 54 ]. Benralizumab acts like a competitive inhibitor to IL-5, binding with higher affinity to the a-subunit of IL-5Ra, which is expressed on mature (and precursors) eosinophils and basophils [ 55 ].
This biologic drug is licensed as an add-on treatment for uncontrolled severe eosinophilic asthma in patients ≥18 years with ≥300 blood eosinophils/μl [ 56 , 57 ]. A 30 mg dose of benralizumab is injected subcutaneously every 28 days for the first 3 administrations and afterwards every 56 days.
Large studies evaluating benralizumab in patients with moderate to severe asthma have shown a decrease in exacerbations number, improved lung function, and reduced use of OCS [ 53 , 54 , 58 ]. Combined analysis of these investigation have revealed that the best predictors of response to benralizumab are adult-onset asthma, more than 3 exacerbations in the previous year, nasal polyposis and pre-bronchodilator FVC < 65% of predicted [ 53 , 54 , 58 ].. The most common adverse effect were fever after the first injection, headache and pharyngitis [ 53 , 54 , 58 ].
Interestingly, based on its mechanism, benralizumab almost completely depletes blood eosinophils within 24 h of administration and a total depletion of airway eosinophils compared to that caused by mepolizumab [ 59 , 60 ]. Likewise, nasal eosinophils were totally suppressed after 6 months of therapy with benralizumab [ 61 ].
Recently, some concerns have been raised about the theoretical risks following an eosinophil depletion, especially with respect to host defense. However, these warnings were not confirmed, since it appears that there is adequate redundancy within human immune apparatus, which is not impaired by eosinophils depletion [ 62 ].
Biologics targeting IL-4 and IL-13
IL-4 and IL-13 are two interleukins which regulate and drive Type-2 inflammation. IL-4 increases the Th-2 cell population and B-cell isotype rearrangement of IgE as well as promoting eosinophilic transmigration through endothelium, whereas IL-13 plays an important role in asthma by promoting airway hyperresponsiveness, mucus secretion and airway remodeling [ 63 , 64 ]. Thus far, the only licensed drug acting on the two aforementioned ILs is dupilumab.
Dupilumab is a monoclonal antibody approved in 2018 which binds to the IL-4 receptor alpha-subunit, mutual to IL-4 and IL-13 receptors and inhibits both IL-4 and IL-13 pathways. Dupilumab is licensed as an add-on maintenance therapy in asthmatic patients GINA step 4/5 ≥ 12 years with type 2 inflammation characterized by increased blood eosinophils and/or raised FeNO. Dupilumab is administered subcutaneously at a starting dose of two injections of 200 mg each (total 400 mg), followed by one injection of 200 mg every 14 days, or at a starting dose of 600 mg (two injections of 300 mg each) followed by 300 mg every 14 days. The latter regimen is recommended for asthmatic subjects strictly dependent from OCS or with atopic dermatitis [ 65 ]. Dupilumab is also indicated for moderate to severe atopic dermatitis and for nasal polyposis.
A number of studies have demonstrated that therapy with dupilumab in severe asthmatics lowers the number of asthma exacerbations, improves lung function parameters and asthma control test scores, and lowers the use of OCS, irrespective of peripheral blood eosinophil count [ 66 , 67 , 68 , 69 ]. Indeed, a transitory increase of blood eosinophilia at the beginning of treatment with dupilumab has been observed although it may be due to blocked migration into tissues rather than hyperproduction [ 69 ]. Furthermore, reduced levels of T2 inflammation markers, including FeNO, serum levels of eotaxin-3, periostin and thymus and activation regulated chemokine (TARC) and total IgE, may serve as parameters for monitoring the efficacy of therapy with dupilumab [ 66 , 67 , 68 , 69 ]. The most common adverse reactions were injection site reactions, various types of infections, conjunctivitis and related conditions [ 66 , 67 , 68 , 69 ].
Biologics under development
Research for next-generation biologics is ongoing. Currently, other effector molecules are under the spotlight as new targets for perspective biological therapies, particularly the so-called alarmins [ 70 ]. These molecules are released by the airway epithelium against the harmful actions of germs, pollutants, allergens and cigarette smoke.
Tezepelumab is a human monoclonal antibody which binds to thymic stromal lymphopoietin (TSLP), an epithelium-derived alarmin that plays a relevant role in the pathogenesis of asthma, being an upstream effector T2-high pathobiologic pathways [ 71 , 72 , 73 ]. With the presence of tezepelumab, TLSP cannot bind to its receptor [ 74 ] hence inhibiting downstream signaling. A number of phase 2 and 3 trials have clearly shown that patients with severe uncontrolled asthma who received tezepelumab had fewer exacerbations and better lung function, asthma control, and health-related quality of life than those who received placebo [ 75 , 76 ]. Concerning its safety profile, neither investigational tezepelumab-related anaphylactic reactions nor the detection of neutralizing antibodies were reported [ 75 , 76 ]. To date, license application for tezepelumab has been accepted and granted Priority Review for the treatment of asthma from the US Food and Drug Administration, whose regulatory decision is expected during the first quarter of 2022.
Ipetekimab is a monoclonal antibody targeting IL-33, another alarmin which associates with TSLP leading to an activation of T2-high inflammatory pathway in asthma [ 77 ]. Phase 2 studies with this biologic are ongoing, however preliminary results did not show adequate efficacy in severe asthmatics when associated with dupilumab or vs dupilumab alone [ 70 ].
Moreover, Tralokinumab and lebrokizumab are monoclonal antibodies both targeting IL-13 alone with disappointing results of phase 3 studies in terms of exacerbations reduction and OCS sparing in severe asthmatics [ 78 ].
Finally, regarding Th2-low asthma, mainly characterized by a neutrophilic airways inflammation, efforts are focusing on its pathogenic cascade involving cytokines such as IL-1beta, IL-17 and IL-23. Several monoclonal antibodies against the aforementioned interleukins such as canakinumab (anti IL-1beta), brodalumab (anti IL-17 receptor) and risankizumab (anti IL-23) are under evaluation with phase 1–2 trials showing controversial results [ 79 , 80 , 81 ].
Which biologic should I choose for my asthmatic patient?
When choosing a biologic medication for their patients with severe uncontrolled asthma, clinicians should always take into account the asthma endotype, clinical biomarkers, and patient-focused aspects (Fig 1 ).
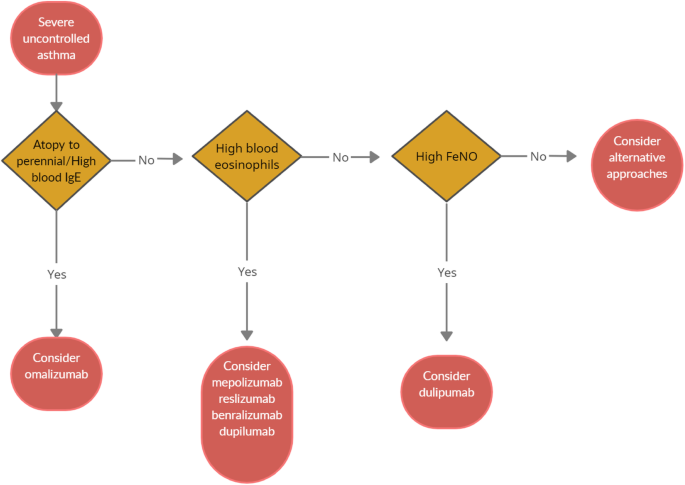
Algorithm for Selecting Ideal Biologic Treatment for severe uncontrolled asthma
Omalizumab should always be the first biological option in allergic non-eosinophilic severe asthmatics, with high levels of blood IgE, and with at least a documented positivity to a perennial aeroallergen. Contrariwise, patients with a non-allergic eosinophilic phenotype should be treated with an anti-IL-5 biological drug. Finally, anti- IL-4/IL-13 should be reserved to patients with severe eosinophilic type 2 asthma OCS dependent [ 8 ].
Given to the a lack of comparison studies, to date there are no recommendations about the selection of appropriate anti IL-5 biologic drug among those available. Hence, the choice is empirical and possibly shared between physician and patient.
According to GINA guidelines, a (at least) 4-month trial should be carried to evaluate asthma control. In the event of poor asthma control, a switch to a different biological treatment can be attempted if the patient meets the eligibility criteria.
Nevertheless, the right time and the right modality of switching from one biologic to another and the treatment time are still unknown. Large studies focused on biological drug switch in patients with severe asthma are ongoing and will help physicians to ease therapeutic strategies.
Conclusions
Severe asthma accounts for a small proportion of total asthma cases, but impose a heavy burden on health care system. Recent revelations of the T2 inflammatory pathways and the development of monoclonal antibodies acting on the T2 cascade has completely revolutionized the management of severe asthma, by introducing new, life-improving treatment options for this class of patients. This paves the way for a biomarker-driven personalized medicine. Strictly following GINA recommendations, the categorization of T2 molecular targets has allowed the identification of patients with severe asthma who would likely respond to specific biological molecules. However, the most suitable biological option for severe asthmatics with overlapping phenotypes is still unclear, thus requiring further discriminatory and predicting biomarkers which may allow a better patient selection.
Availability of data and materials
Not applicable.
Abbreviations
interleukin
inhaled corticosteroids
oral corticosteroids
immunoglobulin E
fractional exhaled nitric oxide
forced vital capacity
Hekking PPW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. https://doi.org/10.1016/j.jaci.2014.08.042 .
Article PubMed Google Scholar
Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–73. https://doi.org/10.1183/09031936.00202013 .
Article CAS PubMed Google Scholar
Price DB, Trudo F, Voorham J, Xu X, Kerkhof M, Ling Zhi Jie J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;11:193–204. https://doi.org/10.2147/JAA.S176026 .
Article CAS PubMed PubMed Central Google Scholar
Sulaiman I, Greene G, MacHale E, Seheult J, Mokoka M, D’Arcy S, et al. A randomised clinical trial of feedback on inhaler adherence and technique in patients with severe uncontrolled asthma. Eur Respir J. 2018;51(1):1701126. https://doi.org/10.1183/13993003.01126-2017 .
Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113(1):101–8. https://doi.org/10.1016/j.jaci.2003.10.041 .
Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev 2014 https://doi.org/10.1002/14651858 . CD003559.pub4.
Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ, Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136(6):1476–85. https://doi.org/10.1016/j.jaci.2015.09.008 .
Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2021. https://ginasthma.org/ .
European Medicines Agency. EMEA/H/C/000606. 2014. www.ema.europa.eu/en/documents/overview/xolair-epar-summary-public_en.pdf . Accessed 30 May 2021.
Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2):184–90. https://doi.org/10.1067/mai.2001.117880 .
Rodrigo GJ, Neffen H, Castro-Rodriguez JA. Efficacy and safety of subcutaneous omalizumab vs placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest. 2011;139:28e35.
Article Google Scholar
Alhossan A, Lee CS, MacDonald K, Abraham I. “Real-life” effectiveness studies of omalizumab in adult patients with severe allergic asthma: meta-analysis. J Allergy Clin Immunol Pract. 2017;5(5):1362–70. https://doi.org/10.1016/j.jaip.2017.02.002 .
Ohta K, Miyamoto T, Amagasaki T, Yamamoto M, Study G. Efficacy and safety of omalizumab in an Asian population with moderate-to-severe persistent asthma. Respirology. 2009;14(8):1156–65. https://doi.org/10.1111/j.1440-1843.2009.01633.x .
Adachi M, Kozawa M, Yoshisue H, Lee Milligan K, Nagasaki M, Sasajima T, et al. Real-world safety and efficacy of omalizumab in patients with severe allergic asthma: a long-term post-marketing study in Japan. Respir Med. 2018;141:56–63. https://doi.org/10.1016/j.rmed.2018.06.021 .
Ledford D, Busse W, Trzaskoma B, Omachi TA, Rosen K, Chipps BE, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140(1):162–9. https://doi.org/10.1016/j.jaci.2016.08.054 .
Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev 2014:CD003559.
[Holgate ST, Chuchalin AG, Hebert J, Lotvall J, Persson GB, Chung KF, et al. Efficacy and safety of a recombinant antiimmunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy 2004;34:632–638.
Soler M, Matz J, Townley R, Buhl R, O’Brien J, Fox H, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18(2):254–61. https://doi.org/10.1183/09031936.01.00092101 .
Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364(11):1005–15. https://doi.org/10.1056/NEJMoa1009705 .
Hanania NA, Wenzel S, Rosen K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187(8):804–11. https://doi.org/10.1164/rccm.201208-1414OC .
Tabatabaian F, Ledford DK. Omalizumab for severe asthma: toward personalized treatment based on biomarker profile and clinical history. J Asthma Allergy. 2018;11:53–61. https://doi.org/10.2147/JAA.S107982 .
Casale TB, Chipps BE, Rosen K, Trzaskoma B, Haselkorn T, Omachi TA, et al. Response to omalizumab using patient enrichment criteria from trials of novel biologics in asthma. Allergy. 2018;73(2):490–7. https://doi.org/10.1111/all.13302 .
Busse W, Spector S, Rosen K, Wang Y, Alpan O. High eosinophil count: a potential biomarker for assessing successful omalizumab treatment effects. J Allergy Clin Immunol. 2013;132(2):485–6. https://doi.org/10.1016/j.jaci.2013.02.032 .
Ledford D, Busse W, Trzaskoma B, Omachi TA, Rosen K, Chipps BE, et al. A randomized multicenter study evaluating Xolair persistence of response after longterm therapy. J Allergy Clin Immunol. 2017;140(1):162–9. https://doi.org/10.1016/j.jaci.2016.08.054 .
Mansur AH, Srivastava S, Mitchell V, Sullivan J, Kasujee I. Longterm clinical outcomes of omalizumab therapy in severe allergic asthma: study of efficacy and safety. Respir Med. 2017;124:36–43. https://doi.org/10.1016/j.rmed.2017.01.008 .
Akdis CA, Arkwright PD, Bruggen MC, Busse W, Gadina M, Guttman-Yassky E, et al. Type 2 immunity in the skin and lungs. Allergy. 2020;75(7):1582–605. https://doi.org/10.1111/all.14318 .
US Food and Drug Administration. NUCALA (mepolizumab) for injection, for subcutaneoususe.2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125526s004lbl.pdf . .
European Medicines Agency. Nucala. EMEA/H/C/003860-N/0027. 2015. https://www.ema.europa.eu/en/documents/product-information/nucala-eparproduct-information_en.pdf . Accessed 1 Jun 2021.
Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–207. https://doi.org/10.1056/NEJMoa1403290 .
Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973–84. https://doi.org/10.1056/NEJMoa0808991 .
Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360(10):985–93. https://doi.org/10.1056/NEJMoa0805435 .
Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–9. https://doi.org/10.1016/S0140-6736(12)60988-X .
Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–97. https://doi.org/10.1056/NEJMoa1403291 .
Lugogo N, Domingo C, Chanez P, Leigh R, Gilson MJ, Price RG, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38(9):2058–70. https://doi.org/10.1016/j.clinthera.2016.07.010 .
Khatri S, Moore W, Gibson PG, Leigh R, Bourdin A, Maspero J, et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2018;143:1742–51.
Khurana S, Brusselle GG, Bel EH, FitzGerald JM, Masoli M, Korn S, et al. Long-term safety and clinical benefit of mepolizumab in patients with the most severe eosinophilic asthma: the COSMEX study. Clin Ther. 2019;41(10):2041–56. https://doi.org/10.1016/j.clinthera.2019.07.007 .
Ortega HG, Yancey SW, Mayer B, Gunsoy NB, Keene ON, Bleecker ER, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4(7):549–56. https://doi.org/10.1016/S2213-2600(16)30031-5 .
Ortega H, Li H, Suruki R, Albers F, Gordon D, Yancey S: Cluster analysis and characterization of response to mepolizumab. A step closer to personalized medicine for patients with severe asthma. Ann Am Thorac Soc 2014;11:1011–1017, 7, DOI: https://doi.org/10.1513/AnnalsATS.201312-454OC .
Katz LE, Gleich GJ, Hartley BF, Yancey SW, Ortega HG. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc. 2014;11(4):531–6. https://doi.org/10.1513/AnnalsATS.201310-354OC .
Ortega H, Chupp G, Bardin P, Bourdin A, Garcia G, Hartley B, et al. The role of mepolizumab in atopic and nonatopic severe asthma with persistent eosinophilia. Eur Respir J. 2014;44(1):239–41. https://doi.org/10.1183/09031936.00220413 .
Prazma CM, Wenzel S, Barnes N, Douglass JA, Hartley BF, Ortega H. Characterisation of an OCS-dependent severe asthma population treated with mepolizumab. Thorax. 2014;69(12):1141–2. https://doi.org/10.1136/thoraxjnl-2014-205581 .
Magnan A, Bourdin A, Prazma CM, Albers FC, Price RG, Yancey SW, et al. Treatment response with mepolizumab in severe eosinophilic asthma patients with previous omalizumab treatment. Allergy. 2016;71(9):1335–44. https://doi.org/10.1111/all.12914 .
Galkin D, Liu MC, Chipps BE, Chapman KR, Munoz X, Angel Bergna M, et al. Efficacy and safety of mepolizumab in uncontrolled patients with severe eosinophilic asthma following a switch from omalizumab (OSMO Study): exacerbation and safety outcomes. J Allergy Clin Immunol. 2018;141(2):AB409. https://doi.org/10.1016/j.jaci.2017.12.965 .
Chapman KR, Albers FC, Chipps B, Munoz X, Devouassoux G, Bergna M, et al. The clinical benefit of mepolizumab replacing omalizumab in uncontrolled severe eosinophilic asthma. Allergy Eur J Allergy Clin Immunol. 2019;74(9):1716–26. https://doi.org/10.1111/all.13850 .
Article CAS Google Scholar
National Institute for Health and Care Excellence (NICE). Mepolizumab for treating severe refractory eosinophilic asthma. 2017. http://www.nice.org.uk/guidance/ta431 . Accessed 1 Jun 2021.
Egan R, Athwal D, Bodmer M, Carter J, Chapman R, Choua CC, et al. Effect of Sch 55700, a humanized monoclonal antibody to human interleukin-5, on eosinophilic responses and bronchial hyperreactivity. Arzneimittelforschung. 2011;49:779–90.
Corren J, Weinstein S, Janka L, Zangrilli J, Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma. Chest. 2016;150(4):799–810. https://doi.org/10.1016/j.chest.2016.03.018 .
US Food and Drug Administration. CINQAIR (reslizumab) injection, for intravenous use. ReferenceID:3906489.2016. www.accessdata.fda.gov/drugsatfda_docs/label/2016/761033lbl.pdf . .
European Medicines Agency. EMEA/H/C/003912.2016. www.ema.europa.eu/en/documents/overview/cinqaero-epar-summarypublic_en.pdf . Accessed 3 Jun 2021.
Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355–66. https://doi.org/10.1016/S2213-2600(15)00042-9 .
Murphy K, Jacobs J, Bjermer L, Fahrenholz JM, Shalit Y, Garin M, et al. Long-term safety and efficacy of reslizumab in patients with eosinophilic asthma. J Allergy Clin Immunol. 2017;5:1572–81.
Bernstein JA, Virchow JC, Murphy K, Maspero JF, Jacobs J, Adir Y, et al. Effect of fixed-dose subcutaneous reslizumab on asthma exacerbations in patients with severe uncontrolled asthma and corticosteroid sparing in patients with oral corticosteroid- dependent asthma: results from two phase 3, randomised, double-blind, placebo. Lancet Respir Med. 2020;8(5):461–74. https://doi.org/10.1016/S2213-2600(19)30372-8 .
FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–41. https://doi.org/10.1016/S0140-6736(16)31322-8 .
Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–58. https://doi.org/10.1056/NEJMoa1703501 .
Ghazi A, Trikha A, Calhoun WJ. Benralizumab – a humanized mAb to IL-5Ra with enhanced antibody-dependent cell-mediated cytotoxicity – a novel approach for the treatment of asthma. Expert Opin Biol Ther. 2012;12(1):113–8. https://doi.org/10.1517/14712598.2012.642359 .
US Food and Drug Administration. FASENRA (benralizumab) injection, for subcutaneous use. ReferenceID:4181236.2019. www.accessdata.fda.gov/drugsatfda_docs/label/2017/761070s000lbl.pdf . .
European Medicines Agency. EMEA/H/C/4433. 2019. www.ema.europa.eu/en/documents/overview/fasenra-epar-medicineoverview_en.pdf . Accessed 3 Jun 2021.
Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–27. https://doi.org/10.1016/S0140-6736(16)31324-1 .
Laviolette M, Gossage DL, Gauvreau G, Leigh R, Olivenstein R, Katial R, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. 2013;132(5):1086–96. https://doi.org/10.1016/j.jaci.2013.05.020 .
Roxas C, Fernandes M, Green L, D’Ancona G, Kavanagh J, Kent B, et al. A comparison of the clinical response to mepolizumab and benralizumab at 4 weeks. Thorax. 2018;73:A50.
Google Scholar
Buonamico E, Dragonieri S, Sciancalepore PI, Carratù P, Carpagnano GE, Resta O, et al. Assessment of eosinophilic nasal inflammation in patients with severe asthma and nasal polyposis before and after six months of therapy with Benralizumab. J Biol Regul Homeost Agents. 2020;34(6):2353–7. https://doi.org/10.23812/20-323-L .
Jackson DJ, Korn S, Mathur SK, Barker P, Meka VG, Martin UJ, et al. Safety of eosinophil-depleting therapy for severe, eosinophilic asthma: focus on benralizumab. Drug Saf. 2020;43(5):409–25. https://doi.org/10.1007/s40264-020-00926-3 .
Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity. 2019;50(4):975–91. https://doi.org/10.1016/j.immuni.2019.03.018 .
Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol. 2015;15(5):271–82. https://doi.org/10.1038/nri3831 .
European Medicines Agency. Dupinex: EMEA/H/C/004390. 2018. https://www.ema.europa.eu/en/documents/product-information/dupixent-epar-productinformation_en.pdf . Accessed 4 Jun 2021.
Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-tohigh-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388(10039):31–44. https://doi.org/10.1016/S0140-6736(16)30307-5 .
Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–96. https://doi.org/10.1056/NEJMoa1804092 .
Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and safety of dupilumab in glucocorticoid dependent severe asthma. N Engl J Med. 2018;378(26):2475–85. https://doi.org/10.1056/NEJMoa1804093 .
Huang J, Pansare M. New treatments for asthma. Pediatr Clin. 2019;66(5):925–39. https://doi.org/10.1016/j.pcl.2019.06.001 .
Porsbjerg CM, Sverrild A, Lloyd CM, Menzies-Gow AN, Bel EH. Anti-alarmins in asthma: targeting the airway epithelium with next-generation biologics. Eur Respir J. 2020;56(5):2000260. https://doi.org/10.1183/13993003.00260-2020 .
Harada M, Hirota T, Jodo AI, Hitomi Y, Sakashita M, Tsunoda T, et al. Thymic stromal lymphopoietin gene promoter polymorphisms are associated with susceptibility to bronchial asthma. Am J Respir Cell Mol Biol. 2011;44(6):787–93. https://doi.org/10.1165/rcmb.2009-0418OC .
Li Y, Wang W, Lv Z, Li Y, Chen Y, Huang K, et al. Elevated expression of IL-33 and TSLP in the airways of human asthmatics in vivo: a potential biomarker of severe refractory disease. J Immunol. 2018;200(7):2253–62. https://doi.org/10.4049/jimmunol.1701455 .
He JQ, Hallstrand TS, Knight D, Chan-Yeung M, Sandford A, Tripp B, et al. A thymic stromal lymphopoietin gene variant is associated with asthma and airway hyperresponsiveness. J Allergy Clin Immunol. 2009;124(2):222–9. https://doi.org/10.1016/j.jaci.2009.04.018 .
Verstraete K, Peelman F, Braun H, Lopez J, Van Rompaey D, Dansercoer A, et al. Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. NatCommun. 2017;8:14937.
CAS Google Scholar
Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377(10):936–46. https://doi.org/10.1056/NEJMoa1704064 .
Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384(19):1800–9. https://doi.org/10.1056/NEJMoa2034975 .
Murakami-Satsutani N, Ito T, Nakanishi T, Inagaki N, Tanaka A, Vien PTX, et al. IL-33 promotes the induction and maintenance of Th2 immune responses by enhancing the function of OX40 ligand. Allergol Int. 2014;63(3):443–55. https://doi.org/10.2332/allergolint.13-OA-0672 .
Busse WW, Brusselle GG, Korn S, Kuna P, Magnan A, Cohen D, et al. Tralokinumab did not demonstrate oral corticosteroid-sparing effects in severe asthma. Eur Respir J. 2019;53(2):1800948. https://doi.org/10.1183/13993003.00948-2018 .
Nair P, Prabhavalkar KS. Neutrophilic asthma and potentially related target therapies. Curr Drug Targets. 2020;21(4):374–88. https://doi.org/10.2174/1389450120666191011162526 .
Kalchiem-Dekel O, Yao X, Levine SJ. Meeting the Challenge of Identifying New Treatments for Type 2-Low Neutrophilic Asthma. Chest;15:26–33.
Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188(11):1294–302. https://doi.org/10.1164/rccm.201212-2318OC .
Download references
Acknowledgements
Authors did not receive any funding for the current review.
Author information
Authors and affiliations.
Department of Respiratory Diseases, University of Bari “Aldo Moro”, Piazza Giulio Cesare 11, 70124, Bari, Italy
Silvano Dragonieri & Giovanna Elisiana Carpagnano
You can also search for this author in PubMed Google Scholar
Contributions
SD and GEC equally contributed in writing the current review. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Silvano Dragonieri .
Ethics declarations
Ethics approval and consent to participate.
Not required.
Consent for publication
Obtained from all authors.
Competing interests
None of the authors have conflicts to disclose.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Dragonieri, S., Carpagnano, G.E. Biological therapy for severe asthma. asthma res and pract 7 , 12 (2021). https://doi.org/10.1186/s40733-021-00078-w
Download citation
Received : 29 June 2021
Accepted : 02 August 2021
Published : 13 August 2021
DOI : https://doi.org/10.1186/s40733-021-00078-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Severe asthma
- Biological therapy
Asthma Research and Practice
ISSN: 2054-7064
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Asthma, bronchial hyperresponsiveness, allergy and lung function development until early adulthood: A systematic literature review
Affiliations.
- 1 Department of Pediatric Pulmonology and Pediatric Allergology, Beatrix Children's Hospital, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
- 2 Groningen Research Institute for Asthma and COPD (GRIAC), University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
- 3 Department of Epidemiology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
- PMID: 33835532
- PMCID: PMC8453965
- DOI: 10.1111/pai.13516
Background: It is unclear in which periods of life lung function deficits develop, and whether these are affected by risk factors such as asthma, bronchial hyper-responsiveness (BHR) and allergic comorbidity. The goal of this systematic review was to identify temporal associations of asthma, BHR and allergic comorbidity with large and small lung function development from birth until peak function in early adulthood.
Methods: We searched MEDLINE, EMBASE, Web of Science and CINAHL for papers published before 01.01.2020 on risk factors and lung function measurements of large and small airways. Studies were required to report lung function at any time point or interval from birth until peak lung function (age 21-26) and include at least one candidate risk factor.
Results: Of the 45 papers identified, 44 investigated cohorts and one was a clinical trial with follow-up. Asthma, wheezing, BHR and allergic sensitization early in life and to multiple allergens were associated with a lower lung function growth of large and small airways during early childhood compared with the control populations. Lung function development after childhood in subjects with asthma or persistent wheeze, although continuing to grow at a lower level, largely tracked parallel to non-affected individuals until peak function was attained.
Clinical implications and future research: Deficits in lung function growth develop in early childhood, and children with asthma, BHR and early-life IgE (poly)sensitization are at risk. This period is possibly a critical window of opportunity to identify at-risk subjects and provide treatment aimed at preventing long-term sequelae of lung function.
Keywords: allergy; asthma; bronchial hyperresponsiveness; growth; lung function; small airways.
© 2021 The Authors. Pediatric Allergy and Immunology published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
PubMed Disclaimer
Lung function growth from childhood…
Lung function growth from childhood to adulthood. The green line represents normal lung…
Preferred Reporting Items for Systematic…
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram
Similar articles
- [Standard technical specifications for methacholine chloride (Methacholine) bronchial challenge test (2023)]. Pulmonary Function and Clinical Respiratory Physiology Committee of Chinese Association of Chest Physicians; Chinese Thoracic Society; Pulmonary Function Group of Respiratory Branch of Chinese Geriatric Society. Pulmonary Function and Clinical Respiratory Physiology Committee of Chinese Association of Chest Physicians, et al. Zhonghua Jie He He Hu Xi Za Zhi. 2024 Feb 12;47(2):101-119. doi: 10.3760/cma.j.cn112147-20231019-00247. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38309959 Chinese.
- Clinical phenotypes of bronchial hyperresponsiveness in school-aged children. Lee E, Kim YH, Cho HJ, Yoon J, Jung S, Yang SI, Kim HY, Kwon JW, Seo JH, Kim HB, Lee SY, Hong SJ. Lee E, et al. Ann Allergy Asthma Immunol. 2018 Oct;121(4):434-443.e2. doi: 10.1016/j.anai.2018.05.033. Epub 2018 Jun 8. Ann Allergy Asthma Immunol. 2018. PMID: 29886267
- Low-grade disease activity in early life precedes childhood asthma and allergy. Chawes BL. Chawes BL. Dan Med J. 2016 Aug;63(8):B5272. Dan Med J. 2016. PMID: 27477800 Review.
- Epidemiology of asthma and recurrent wheeze in childhood. Wright AL. Wright AL. Clin Rev Allergy Immunol. 2002 Feb;22(1):33-44. doi: 10.1007/s12016-002-0004-z. Clin Rev Allergy Immunol. 2002. PMID: 11803801 Review.
- Asymptomatic bronchial hyperresponsiveness in adolescents and young adults. Kolnaar BG, Folgering H, van den Hoogen HJ, van Weel C. Kolnaar BG, et al. Eur Respir J. 1997 Jan;10(1):44-50. doi: 10.1183/09031936.97.10010044. Eur Respir J. 1997. PMID: 9032490
- Elevated Saliva Pepsin Concentration as a Risk Factor for Asthma in Children with Allergic Rhinitis: A Preliminary Study. Sui H, Shen H, Zhang C, Wang M, Zhen Z, Zhang J. Sui H, et al. J Asthma Allergy. 2024 Apr 22;17:391-397. doi: 10.2147/JAA.S447145. eCollection 2024. J Asthma Allergy. 2024. PMID: 38681237 Free PMC article.
- Clinical implications of airway obstruction with normal or low FEV 1 in childhood and adolescence. Koefoed HJL, Wang G, Gehring U, Ekstrom S, Kull I, Vermeulen R, Boer JMA, Bergstrom A, Koppelman GH, Melén E, Vonk JM, Hallberg J. Koefoed HJL, et al. Thorax. 2024 May 20;79(6):573-580. doi: 10.1136/thorax-2023-220952. Thorax. 2024. PMID: 38514183 Free PMC article.
- ERS International Congress 2023: highlights from the Paediatrics Assembly. Vijverberg SJH, Kampouras A, Nayir Büyükşahin H, Makrinioti H, Petrarca L, Schmidt M, Schreck LD, Urbantat RM, Beydon N, Goutaki M, Lavizzari A, Proesmans M, Schramm D, Stahl M, Zacharasiewicz A, Moeller A, Pijnenburg MW. Vijverberg SJH, et al. ERJ Open Res. 2024 Feb 26;10(1):00853-2023. doi: 10.1183/23120541.00853-2023. eCollection 2024 Jan. ERJ Open Res. 2024. PMID: 38410713 Free PMC article.
- Early Prediction of Asthma. Romero-Tapia SJ, Becerril-Negrete JR, Castro-Rodriguez JA, Del-Río-Navarro BE. Romero-Tapia SJ, et al. J Clin Med. 2023 Aug 20;12(16):5404. doi: 10.3390/jcm12165404. J Clin Med. 2023. PMID: 37629446 Free PMC article. Review.
- The role of small airway function parameters in preschool asthmatic children. Yi L, Zhao Y, Guo Z, Li Q, Zhang G, Tian X, Xu X, Luo Z. Yi L, et al. BMC Pulm Med. 2023 Jun 20;23(1):219. doi: 10.1186/s12890-023-02515-3. BMC Pulm Med. 2023. PMID: 37340433 Free PMC article.
- Quanjer PH, Stanojevic S, Cole TJ, et al. Multi‐ethnic reference values for spirometry for the 3–95‐yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324‐1343. - PMC - PubMed
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1(6077):1645‐1648. - PMC - PubMed
- McGeachie MJ, Yates KP, Zhou X, et al. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med. 2016;374(19):1842‐1852. - PMC - PubMed
- Sears MR, Greene JM, Willan AR, et al. A longitudinal, population‐based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349(15):1414‐1422. - PubMed
- Morgan WJ, Stern DA, Sherrill DL, et al. Outcome of asthma and wheezing in the first 6 years of life: follow‐up through adolescence. Am J Respir Crit Care Med. 2005;172(10):1253‐1258. - PMC - PubMed
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Europe PubMed Central
- Ovid Technologies, Inc.
- PubMed Central
Other Literature Sources
- The Lens - Patent Citations
- scite Smart Citations
- Genetic Alliance
- MedlinePlus Consumer Health Information
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu
- Subscribe SciFeed
- Recommended Articles
- PubMed/Medline
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
The role of histamine in the pathophysiology of asthma and the clinical efficacy of antihistamines in asthma therapy.

1. Histamine Receptors in the Lung
2. histamine and mast cells in asthma, 3. histamine in the pathophysiology of asthma, 4. histamine transport in the pathophysiology of asthma, 5. h1ra in asthma therapy, 6. the immunological roles of histamine in allergic reactions, 7. a new insight on the immunological pathway in asthma, 8. a new aspect of h1ra and h4ra in asthma therapy, author contributions, acknowledgments, conflicts of interest.
- Jones, J.V. The nature of the pulmonary receptors excited by antihistamines. Br. J. Pharmacol. Chemother. 1952 , 7 , 450–454. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tucker, A.; Weir, E.K.; Reeves, J.T.; Grover, R.F. Histamine H1- and H2-receptors in pulmonary and systemic vasculature of the dog. Am. J. Physiol. 1975 , 229 , 1008–1013. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Ichinose, M.; Barnes, P.J. Inhibitory histamine H3-receptors on cholinergic nerves in human airways. Eur. J. Pharmacol. 1989 , 163 , 383–386. [ Google Scholar ] [ CrossRef ]
- Kay, L.J.; Suvarna, S.K.; Peachell, P.T. Histamine H4 receptor mediates chemotaxis of human lung mast cells. Eur. J. Pharmacol. 2018 , 837 , 38–44. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Curry, J.J. The effect of antihistamine substances and other drugs on histamine bronchoconstriction in asthmatic subjects. J. Clin. Investig. 1946 , 25 , 792–799. [ Google Scholar ] [ CrossRef ]
- Drazen, J.M.; Schneider, M.W. Comparative responses of tracheal spirals and parenchymal strips to histamine and carbachol in vitro. J. Clin. Investig. 1978 , 61 , 1441–1447. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ahmed, T.; Mirbahar, K.B.; Oliver, W., Jr.; Eyre, P.; Wanner, A. Characterization of H1- and H2-receptor function in pulmonary and systemic circulations of sheep. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1982 , 53 , 175–184. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Nadel, J.A.; Davis, B.; Phipps, R.J. Control of mucus secretion and ion transport in airways. Annu. Rev. Physiol. 1979 , 41 , 369–381. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- White, M.V. The role of histamine in allergic diseases. J. Allergy Clin. Immunol. 1990 , 86 , 599–605. [ Google Scholar ] [ CrossRef ]
- Casale, T.B.; Wood, D.; Richerson, H.B.; Trapp, S.; Metzger, W.J.; Zavala, D.; Hunninghake, G.W. Elevated bronchoalveolar lavage fluid histamine levels in allergic asthmatics are associated with methacholine bronchial hyperresponsiveness. J. Clin. Investig. 1987 , 79 , 1197–1203. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tomioka, M.; Ida, S.; Shindoh, Y.; Ishihara, T.; Takishima, T. Mast cells in bronchoalveolar lumen of patients with bronchial asthma. Am. Rev. Respir. Dis. 1984 , 129 , 1000–1005. [ Google Scholar ]
- Dale, H.H.; Laidlaw, P.P. Histamine shock. J. Physiol. 1919 , 52 , 355–390. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Ishizaka, T.; Ishizaka, K.; Tomioka, H. Release of histamine and slow reacting substance of anaphylaxis (SRS-A) by IgE-anti-IgE reactions on monkey mast cells. J. Immunol. 1972 , 108 , 513–520. [ Google Scholar ]
- Curry, J.J. The action of histamine on the respiratory tract in normal and asthmatic subjects. J. Clin. Investig. 1946 , 25 , 785–791. [ Google Scholar ] [ CrossRef ]
- Hogg, J.C.; Paré, P.D.; Boucher, R.C.; Michoud, M.C. The pathophysiology of asthma. Can. Med. Assoc. J. 1979 , 121 , 409–414. [ Google Scholar ]
- Rafferty, P.; Beasley, R.; Holgate, S.T. The contribution of histamine to immediate bronchoconstriction provoked by inhaled allergen and adenosine 5′ monophosphate in atopic asthma. Am. Rev. Respir. Dis. 1987 , 136 , 369–373. [ Google Scholar ] [ CrossRef ]
- Yamauchi, K. Regulation of gene expression of l -histidine decarboxylase and histamine N -methyl-transferase, and its relevance to the pathogenesis of bronchial asthma. Nihon Rinsho 1996 , 54 , 377–388. [ Google Scholar ]
- Sekizawa, K.; Nakazawa, H.; Morikawa, M.; Yamauchi, K.; Maeyama, K.; Watanabe, T.; Sasaki, H. Histamine N -methyltransferase inhibitor potentiates histamine- and antigen-induced airway microvascular leakage in guinea pigs. J. Allergy Clin. Immunol. 1995 , 96 , 910–916. [ Google Scholar ] [ CrossRef ]
- Yamauchi, K.; Sekizawa, K.; Suzuki, H.; Nakazawa, H.; Ohkawara, Y.; Katayose, D.; Ohtsu, H.; Tamura, G.; Shibahara, S.; Takemura, M.; et al. Structure and function of human histamine N -methyltransferase: Critical enzyme in histamine metabolism in airway. Am. J. Physiol. 1994 , 267 , L342–L349. [ Google Scholar ] [ CrossRef ]
- Anvari, S.; Vyhlidal, C.A.; Dai, H.; Jones, B.L. Genetic Variation along the Histamine Pathway in Children with Allergic versus Nonallergic Asthma. Am. J. Respir. Cell Mol. Biol. 2015 , 53 , 802–809. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The Role of Histamine and histamine receptors in Mast cell-mediated allergy and inflammation: The Hunt for New therapeutic Targets. Front. Immunol. 2018 , 9 , 1–9. [ Google Scholar ] [ CrossRef ]
- Ohtsu, H. Progress in allergy signal research on mast cells: The role of histamine in immunological and cardiovascular disease and the transporting system of histamine in the cell. J. Pharmacol. Sci. 2008 , 106 , 347–353. [ Google Scholar ] [ CrossRef ]
- Ogasawara, M.; Yamauchi, K.; Satoh, Y.; Yamaji, R.; Inui, K.; Jonker, J.W.; Schinkel, A.H.; Maeyama, K. Recent advances in Molecular Pharmacology of the histamine systems: Organic cation Transporters as a Histamine transporter and Histamine Metabolism. J. Pharmacol. Sci. 2006 , 101 , 24–30. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Koepsell, H.; Endou, H. The SLC22 drug transporter family. Pflugers Arch. 2004 , 447 , 666–676. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yoshikawa, T.; Yanai, K. Histamine Clearance through Polyspecific Transporters in the Brain. Handb. Exp. Pharmacol. 2017 , 241 , 173–187. [ Google Scholar ] [ PubMed ]
- Zwart, R.; Verhaagh, S.; Buitelaar, M.; Popp-Snijders, C.; Barlow, D.P. Impaired activity of the extraneuronal monoamine transporter system known as uptake-2 in Orct3/Slc22a3-deficient mice. Mol. Cell Biol. 2001 , 21 , 4188–4196. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhu, P.; Hata, R.; Ogasawara, M.; Cao, F.; Kameda, K.; Yamauchi, K.; Schinkel, A.H.; Maeyama, K.; Sakanaka, M. Targeted disruption of organic cation transporter 3(Oct3) ameliorates ischemic brain damage through modulating histamine and regulatory T cells. J. Cereb. Blood Flow Metab. 2012 , 32 , 1897–1908. [ Google Scholar ] [ CrossRef ]
- Yamauchi, K.; Shikanai, T.; Nakamura, Y.; Kobayashi, H.; Ogasawara, M.; Maeyama, K. Roles of histamine in the pathogenesis of bronchial asthma and reevaluation of the clinical usefulness of antihistamines. Yakugaku Zasshi 2011 , 131 , 185–191. [ Google Scholar ] [ CrossRef ]
- Sakata, T.; Anzai, N.; Kimura, T.; Miura, D.; Fukutomi, T.; Takeda, M.; Sakurai, H.; Endou, H. Functional analysis of human Organic cation Transporter OCT3(SLC22A3) polymorphisms. J. Pharmacol. Sci. 2010 , 113 , 263–266. [ Google Scholar ] [ CrossRef ]
- Lee, N.; Hebert, M.F.; Wagner, D.J.; Easterling, T.R.; Liang, C.J.; Rice, K.; Wang, J. Organic Cation Transporter 3 facilitates Fetal Exposure to Metformin during Pregnancy. Mol. Pharmacol. 2018 , 94 , 1125–1131. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mahrooz, A.; Alizadeh, A.; Hashemi-Soteh, M.B.; Ghaffari-Cherati, M.; Hosseyni-Talei, S.R. Polymorphic Variants rs3088442 and rs2292334 in the Organic cation Transporter 3(OCT3) gene and Susceptibility Against Type 2 Diabetes: Role of their Interaction. Arch. Med. Res. 2017 , 48 , 162–168. [ Google Scholar ] [ CrossRef ]
- Hosseyni-Talei, S.R.; Mahrooz, A.; Hashemi-Soteh, M.B.; Ghaffari-Cherati, M.; Alizadeh, A. Association between the synonymous variant organic cation transporter 3(OCT3)-1233>A and the glycemic response following metformin therapy in patients with type 2 diabetes. Iran. J. Basic Med. Sci. 2017 , 20 , 250–255. [ Google Scholar ]
- Ghaffari-Cherati, M.; Mahrooz, A.; Hashemi-Soteh, M.B.; Hosseyni-Talei, S.R.; Alizadeh, A.; Nakhaei, S.M. Allele frequency and genotype distribution of a common variant in the 3′-untranslated region of the SLC22A3 gene in patients with type 2 diabetes: Association with response to metformin. J. Res. Med. Sci. 2016 , 21 , 92. [ Google Scholar ] [ CrossRef ]
- Zaharenko, L.; Kalnina, I.; Geldnere, K.; Konrade, I.; Grinberga, S.; Židzik, J.; Javorský, M.; Lejnieks, A.; Nikitina-Zake, L.; Fridmanis, D.; et al. Single nucleotide polymorphisms in the intergenic region between metformin transporter OCT2 and OCT3 coding genes are associated with short-term response to metformin monotherapy in type 2 diabetes mellitus patients. Eur. J. Endocrinol. 2016 , 175 , 531–540. [ Google Scholar ] [ CrossRef ]
- Chen, E.C.; Liang, X.; Yee, S.W.; Geier, E.G.; Stocker, S.L.; Chen, L.; Giacomini, K.M. Targeted disruption of organic cation transporter 3 attenuates the pharmacological response to metformin. Mol. Pharmacol. 2015 , 88 , 75–83. [ Google Scholar ] [ CrossRef ]
- Chen, L.; Hong, C.; Chen, E.C.; Yee, S.W.; Xu, L.; Almof, E.U.; Wen, C.; Fujii, K.; Johns, S.J.; Stryke, D.; et al. Genetics and epigenetic regulation of the organic cation transporter 3, SLC22A3. Pharm. J. 2013 , 13 , 110–120. [ Google Scholar ] [ CrossRef ]
- Aoyama, N.; Takahashi, N.; Kitaichi, K.; Ishihara, R.; Saito, S.; Maeno, N.; Ji, X.; Takagi, K.; Sekina, Y.; Iyo, M.; et al. Association between gene polymorphisms of SLA22A3 and methamphetamine use disorder. Alchol. Clin. Exp. Res. 2006 , 30 , 1644–1649. [ Google Scholar ] [ CrossRef ]
- Ferstl, R.; Frei, R.; Barcik, W.; Sciavi, E.; Wanke, K.; Ziegler, M.; Rodriguez-Perez, N.; Groeger, D.; Konieczna, P.; Zeiter, S.; et al. Histamine receptor 2 modified iNKT cell activity within the inflamed lung. Allergy 2017 , 72 , 1925–1935. [ Google Scholar ] [ CrossRef ]
- Schneider, E.; Machavoine, F.; Plěau, J.M.; Berton, A.F.; Thurmond, R.L.; Otsu, H.; Watanabe, T.; Schinkel, A.H.; Dy, M. Organic cation transporter 3 modulates murine basophil functions by controlling intracellular histamine levels. J. Exp. Med. 2005 , 202 , 387–393. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Herxheimer, H. Antihistamines in bronchial asthma. Proc. R. Soc. Med. 1949 , 42 , 615–629. [ Google Scholar ] [ CrossRef ]
- Okubo, K.; Kurono, Y.; Ichimura, K.; Enomoto, T.; Okamoto, Y.; Kawauchi, H.; Suzaki, H.; Fujieda, S.; Masuyama, K. Japanese Society of Allergology. Japanese guidelines for allergic rhinitis 2017. Allergol. Int. 2017 , 66 , 205–219. [ Google Scholar ] [ CrossRef ]
- Wheatley, L.M.; Togias, A. Clinical practice. Allergic rhinitis. N. Engl. J. Med. 2015 , 372 , 456–463. [ Google Scholar ] [ CrossRef ]
- Zuberbier, T.; Aberer, W.; Asero, R. The EAACI/GA 2 LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy 2018 , 73 , 1393–1414. [ Google Scholar ] [ CrossRef ]
- The Global Initiative for Asthma; Global Strategy for Asthma Management and Prevention (2018 Update). Available online: https://ginasthma.org/about-us/ (accessed on 3 February 2019).
- Roquet, A.; Dahlén, B.; Kumlin, M.; Ihre, E.; Anstrén, G.; Binks, S.; Dahlén, S.E. Combined antagonism of leukotrienes and histamine produces predominant inhibition of allergen-induced early and late phase airway obstruction in asthmatics. Am. J. Respir. Crit. Care Med. 1997 , 155 , 1856–1863. [ Google Scholar ] [ CrossRef ]
- Jutel, M.; Watanabe, T.; Klunker, S.; Akdis, M.; Thomet, O.A.; Malolepszy, J.; Zak-Nejmark, T.; Koga, R.; Kobayashi, T.; Blaser, K.; et al. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature 2001 , 413 , 420–425. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Del Prete, G.F.; De Carli, M.; Mastromauro, C. Purified protein derivate of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profiles of cytokine production. J. Clin. Investig. 1991 , 88 , 344–350. [ Google Scholar ] [ CrossRef ]
- Robinson, D.S.; Hamid, Q.; Ying, S.; Tsicopoulos, A.; Barkans, J.; Bentley, A.M.; Corrigan, C.; Durham, S.R.; Kay, A.B. Predominant Th2-like bronchoalveolar T lymphocyte population in atopic asthma. N. Engl. J. Med. 1992 , 326 , 298–304. [ Google Scholar ] [ CrossRef ]
- Ohtsu, H.; Tanaka, S.; Terui, T.; Hori, Y.; Makabe-Kobayashi, Y.; Pejler, G.; Tchougounova, E.; Hellman, L.; Gertsenstein, M.; Hirasawa, N.; et al. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 2001 , 502 , 53–56. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Koarai, A.; Ichinose, M.; Ishigaki-Suzuki, S.; Yamagata, S.; Sugiura, H.; Sakurai, E.; Makabe-Kobayashi, Y.; Kuramasu, A.; Watanabe, T.; Shirato, K.; et al. Disruption of L-histidine decarboxylase reduces airway eosinophilia but not hyperresponsiveness. Am. J. Respir. Crit. Care Med. 2003 , 167 , 758–763. [ Google Scholar ] [ CrossRef ]
- O’Reilly, M.; Alpert, R.; Jenkinson, S.; Gladue, R.P.; Foo, S.; Trim, S.; Peter, B.; Trevethick, M.; Fidock, M. Identification of a histamine H4 receptor on human eosinophils—Role in eosinophil chemotaxis. J. Recept. Signal Transduct. Res. 2002 , 22 , 431–448. [ Google Scholar ] [ CrossRef ]
- Makabe-Kobayashi, Y.; Hori, Y.; Adachi, T.; Ishigaki-Suzuki, S.; Kikuchi, Y.; Kagaya, Y.; Shirato, K.; Nagy, A.; Ujike, A.; Takai, T.; et al. The control effect of histamine on body temperature and respiratory function in IgE-dependent systemic anaphylaxis. J. Allergy Clin. Immunol. 2002 , 110 , 298–303. [ Google Scholar ] [ CrossRef ]
- Yamauchi, K.; Piao, H.M.; Nakadate, T.; Shikanai, T.; Nakamura, Y.; Ito, H.; Mouri, T.; Kobayashi, H.; Maesawa, C.; Sawai, T.; et al. Enhanced goblet cell hyperplasia in HDC knockout mice with allergic airway inflammation. Allergol. Int. 2009 , 58 , 125–134. [ Google Scholar ]
- Nakanishi, A.; Morita, S.; Iwashita HSagiya, Y.; Ashida, Y.; Shirafuji, H.; Fujisawa, Y.; Nishimura, O.; Fujino, M. Role of gob-5 in mucus overproduction and airway hyperresponsiveness in asthma. Proc. Natl. Acad. Sci. USA 2001 , 98 , 5175–5180. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Gosset, P.; Tsicopoulos, A.; Wallaert, B.; Joseph, M.; Capron, A.; Tonnel, A.B. Tumor necrosis factor-α and interleukin-6 production by human mononuclear phagocytes from allergic asthmatics after IgEdependent stimulation. Am. Rev. Respir. Dis. 1992 , 146 , 768–774. [ Google Scholar ] [ CrossRef ]
- Busse, P.J.; Zhang, T.F.; Srivastava KLin, B.P.; Schofield, B.; Sealfon, S.C.; Li, X.M. Chronic exposure to TNF-alpha increases airway mucus gene expression in vivo. J. Allergy Clin. Immunol. 2005 , 116 , 1256–1263. [ Google Scholar ] [ CrossRef ]
- Hirose, K.; Iwata, A.; Tamachi, T.; Nakajima, H. Allergic airway inflammation: Key players beyond the Th2 cell pathway. Immunol. Rev. 2017 , 278 , 145–161. [ Google Scholar ] [ CrossRef ]
- Moldaver, D.M.; Larché, M.; Rudulier, C.D. An Update on Lymphocyte Subtypes in Asthma and Airway Disease. Chest 2017 , 151 , 1122–1130. [ Google Scholar ] [ CrossRef ]
- Li, B.W.; Hendriks, R.W. Group 2 innate lymphoid cells in lung inflammation. Immunology 2013 , 140 , 281–287. [ Google Scholar ] [ CrossRef ]
- Barlow, J.L.; McKenzie, A.N. Type-2 innate lymphoid cells in human allergic disease. Curr. Opin. Allergy Clin. Immunol. 2014 , 14 , 397–403. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Becker, A.B.; Abrams, E.M. Asthma guidelines: The Global Initiative for Asthma in relation to national guidelines. Curr. Opin. Allergy Clin. Immunol. 2017 , 17 , 99–103. [ Google Scholar ] [ CrossRef ]
- Ichinose, M.; Sugiura, H.; Nagase, H.; Yamaguchi, M.; Inoue, H.; Sagara, H.; Tamaoki, J.; Tohda, Y.; Munakata, M.; Yamauchi, K.; et al. Japanese Society of Allergology Japanese guidelines for adult asthma 2017. Allergol. Int. 2017 , 66 , 163–189. [ Google Scholar ] [ CrossRef ]
- Busse, W.; Corren, J.; Lanier, B.Q.; McAlary, M.; Fowler-Taylor, A.; Cioppa, G.D.; van As, A.; Gupta, N. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J. Allergy Clin. Immunol. 2001 , 108 , 184–190. [ Google Scholar ] [ CrossRef ]
- Yamauchi, K.; Tamura, G.; Akasaka, T.; Chiba, T.; Honda, K.; Kishi, M.; Kobayashi, H.; Kuronuma, T.; Matsubara, A.; Morikawa, T.; et al. Analysis of the comorbidity of bronchial asthma and allergic rhinitis by questionnaire in 10,009 patients. Allergol. Int. 2009 , 58 , 55–61. [ Google Scholar ] [ CrossRef ]
- Buckland, K.F.; Williams, T.J.; Conroy, D.M. Histamine induces cytoskeletal changes in human eosinophils via the H(4) receptor. Br. J. Pharmacol. 2003 , 140 , 1117–1127. [ Google Scholar ] [ CrossRef ]
- Neumann, D.; Beermann, S.; Seifert, R. Does the histamine H4 receptor have a pro- or anti-inflammatory role in murine bronchial asthma? Pharmacology 2010 , 85 , 217–223. [ Google Scholar ] [ CrossRef ]
- Hofstra, C.L.; Desai, P.J.; Thurmond, R.L.; Fung-Leung, W.P. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J. Pharmacol. Exp. Ther. 2003 , 305 , 1212–1221. [ Google Scholar ] [ CrossRef ]
- Dunford, P.J.; O’Donnell, N.; Riley, J.P.; Williams, K.N.; Karlsson, L.; Thurmond, R.L. The Histamine H4 Receptor Mediates Allergic Airway Inflammation by Regulating the Activation of CD4+ T Cells. J. Immunol. 2006 , 176 , 7062–7070. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Cowden, J.M.; Riley, J.P.; Ma, J.Y.; Thurmond, R.L.; Dunford, P.J. Histamine H4 receptor antagonism diminishes existing airway inflammation and dysfunction via modulation of Th2 cytokines. Respir. Res. 2010 , 11 , 86. [ Google Scholar ] [ CrossRef ]
- Thurmond, R.L.; Chen, B.; Dunford, P.J.; Greenspan, A.J.; Karlsson, L.; La, D.; Ward, P.; Xu, X.L. Clinical and preclinical characterization of the histamine H(4) receptor antagonist JNJ-39758979. J. Pharmacol. Exp. Ther. 2014 , 349 , 176–184. [ Google Scholar ] [ CrossRef ]
- Kollmeier, A.P.; Barnathan, E.S.; O’Brien, C.; Chen, B.; Xia, Y.K.; Zhou, B.; Loza, M.J.; Silkoff, P.E.; Ge, M.; Thurmond, R.L. A phase 2a study of toreforant, a histamine H4 receptor antagonist, in eosinophilic asthma. Ann. Allergy Asthma Immunol. 2018 , 121 , 568–574. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| Step 1 | Step 2 | Step 3 | Step 4 | Step 5 | |
|---|---|---|---|---|---|
| Controllers | Low dose ICS | Low dose ICS/LABA | Med/high ICS/LABA | Add-on Tiotropium, Anti-IgE, Anti-IL-5 | |
| Other controllers options | ICS (low dose) | LTRA Low dose Theophylline | Med/high dose ICS; low dose ICS + LTRA (or + Theophylline) | Add Tiotropium Med/high dose ICS; low dose ICS + LTRA (or + Theophylline) | Add low dose OCS |
| Relievers | SABA | SABA | SABA or low dose ICS/formoterol | ||
| Number | Yes | No | n.d. | |
|---|---|---|---|---|
| Adult asthma | 2781 | 1693 (60.8%) * | 1044 (37.5%) | 44 (1.6%) |
| Child asthma | 3283 | 2238 (68.2%) ** | 1035 (31.5%) | 10 (0.3%) |
| Allergic rhinitis (AR) | 3945 | 1935(49.0%) | 2010 (51.0%) | – |
Share and Cite
Yamauchi, K.; Ogasawara, M. The Role of Histamine in the Pathophysiology of Asthma and the Clinical Efficacy of Antihistamines in Asthma Therapy. Int. J. Mol. Sci. 2019 , 20 , 1733. https://doi.org/10.3390/ijms20071733
Yamauchi K, Ogasawara M. The Role of Histamine in the Pathophysiology of Asthma and the Clinical Efficacy of Antihistamines in Asthma Therapy. International Journal of Molecular Sciences . 2019; 20(7):1733. https://doi.org/10.3390/ijms20071733
Yamauchi, Kohei, and Masahito Ogasawara. 2019. "The Role of Histamine in the Pathophysiology of Asthma and the Clinical Efficacy of Antihistamines in Asthma Therapy" International Journal of Molecular Sciences 20, no. 7: 1733. https://doi.org/10.3390/ijms20071733
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
- Open access
- Published: 15 August 2020
Treatment strategies for asthma: reshaping the concept of asthma management
- Alberto Papi 1 , 7 ,
- Francesco Blasi 2 , 3 ,
- Giorgio Walter Canonica 4 ,
- Luca Morandi 1 , 7 ,
- Luca Richeldi 5 &
- Andrea Rossi 6
Allergy, Asthma & Clinical Immunology volume 16 , Article number: 75 ( 2020 ) Cite this article
49k Accesses
60 Citations
4 Altmetric
Metrics details
Asthma is a common chronic disease characterized by episodic or persistent respiratory symptoms and airflow limitation. Asthma treatment is based on a stepwise and control-based approach that involves an iterative cycle of assessment, adjustment of the treatment and review of the response aimed to minimize symptom burden and risk of exacerbations. Anti-inflammatory treatment is the mainstay of asthma management. In this review we will discuss the rationale and barriers to the treatment of asthma that may result in poor outcomes. The benefits of currently available treatments and the possible strategies to overcome the barriers that limit the achievement of asthma control in real-life conditions and how these led to the GINA 2019 guidelines for asthma treatment and prevention will also be discussed.
Asthma, a major global health problem affecting as many as 235 million people worldwide [ 1 ], is a common, non-communicable, and variable chronic disease that can result in episodic or persistent respiratory symptoms (e.g. shortness of breath, wheezing, chest tightness, cough) and airflow limitation, the latter being due to bronchoconstriction, airway wall thickening, and increased mucus.
The pathophysiology of the disease is complex and heterogeneous, involving various host-environment interactions occurring at various scales, from genes to organ [ 2 ].
Asthma is a chronic disease requiring ongoing and comprehensive treatment aimed to reduce the symptom burden (i.e. good symptom control while maintaining normal activity levels), and minimize the risk of adverse events such as exacerbations, fixed airflow limitation and treatment side effects [ 3 , 4 ].
Asthma treatment is based on a stepwise approach. The management of the patient is control-based; that is, it involves an iterative cycle of assessment (e.g. symptoms, risk factors, etc.), adjustment of treatment (i.e. pharmacological, non-pharmacological and treatment of modifiable risk factors) and review of the response (e.g. symptoms, side effects, exacerbations, etc.). Patients’ preferences should be taken into account and effective asthma management should be the result of a partnership between the health care provider and the person with asthma, particularly when considering that patients and clinicians might aim for different goals [ 4 ].
This review will discuss the rationale and barriers to the treatment of asthma, that may result in poor patient outcomes. The benefits of currently available treatments and the possible strategies to overcome the barriers that limit the achievement of asthma control in real-life situations will also be discussed.
The treatment of asthma: where are we? Evolution of a concept
Asthma control medications reduce airway inflammation and help to prevent asthma symptoms; among these, inhaled corticosteroids (ICS) are the mainstay in the treatment of asthma, whereas quick-relief (reliever) or rescue medicines quickly ease symptoms that may arise acutely. Among these, short-acting beta-agonists (SABAs) rapidly reduce airway bronchoconstriction (causing relaxation of airway smooth muscles).
National and international guidelines have recommended SABAs as first-line treatment for patients with mild asthma, since the Global Initiative for Asthma guidelines (GINA) were first published in 1995, adopting an approach aimed to control the symptoms rather than the underlying condition; a SABA has been the recommended rescue medication for rapid symptom relief. This approach stems from the dated idea that asthma symptoms are related to bronchial smooth muscle contraction (bronchoconstriction) rather than a condition concomitantly caused by airway inflammation. In 2019, the GINA guidelines review (GINA 2019) [ 4 ] introduced substantial changes overcoming some of the limitations and “weaknesses” of the previously proposed stepwise approach to adjusting asthma treatment for individual patients. The concept of an anti-inflammatory reliever has been adopted at all degrees of severity as a crucial component in the management of the disease, increasing the efficacy of the treatment while lowering SABA risks associated with patients’ tendency to rely or over-rely on the as-needed medication.
Until 2017, the GINA strategy proposed a pharmacological approach based on a controller treatment (an anti-inflammatory, the pillar of asthma treatment), with a SABA as an additional rescue intervention. The reliever, a short-acting bronc hodilator, was merely an addendum , a medication to be used in case the real treatment (the controller) failed to maintain disease control: SABAs effectively induce rapid symptom relief but are ineffective on the underlying inflammatory process. Based on the requirement to achieve control, the intensity of the controller treatment was related to the severity of the disease, varying from low-dose ICS to combination low-dose ICS/long-acting beta-agonist (LABA), medium-dose ICS/LABA, up to high-dose ICS/LABA, as preferred controller choice, with a SABA as the rescue medication. As a result, milder patients were left without any anti-inflammatory treatment and could only rely on SABA rescue treatment.
Poor adherence to therapy is a major limitation of a treatment strategy based on the early introduction of the regular use of controller therapy [ 5 ]. Indeed, a number of surveys have highlighted a common pattern in the use of inhaled medication [ 6 ], in which treatment is administered only when asthma symptoms occur; in the absence of symptoms, treatment is avoided as patients perceive it as unnecessary. When symptoms worsen, patients prefer to use reliever therapies, which may result in the overuse of SABAs [ 7 ]. Indirect evidence suggests that the overuse of beta-agonists alone is associated with increased risk of death from asthma [ 8 ].
In patients with mild persistent disease, low-dose ICS decreases the risk of severe exacerbations leading to hospitalization and improves asthma control [ 9 ]. When low-dose ICS are ineffective in controlling the disease (Step 3 of the stepwise approach), a combination of low-dose ICS with LABA maintenance was the recommended first-choice treatment, plus as-needed SABA [ 3 , 10 ]. Alternatively, the combination low-dose ICS/LABA (formoterol) was to be used as single maintenance and reliever treatment (SMART). The SMART strategy containing the rapid-acting formoterol was recommended throughout GINA Steps 3 to 5 based on solid clinical-data evidence [ 3 ].
The addition of a LABA to ICS treatment reduces both severe and mild asthma exacerbation rates, as shown in the one-year, randomized, double-blind, parallel-group FACET study [ 11 ]. This study focused on patients with persistent asthma symptoms despite receiving ICS and investigated the efficacy of the addition of formoterol to two dose levels of budesonide (100 and 400 µg bid ) in decreasing the incidence of both severe and mild asthma exacerbations. Adding formoterol decreased the incidence of both severe and mild asthma exacerbations, independent of ICS dose. Severe and mild exacerbation rates were reduced by 26% and 40%, respectively, with the addition of formoterol to the lower dose of budesonide; the corresponding reductions were 63% and 62%, respectively, when formoterol was added to budesonide at the higher dose.
The efficacy of the ICS/LABA combination was confirmed in the post hoc analysis of the FACET study, in which patients were exposed to a combination of formoterol and low-dose budesonide [ 12 ]. However, such high levels of asthma control are not achieved in real life [ 5 ]. An explanation for this is that asthma is a variable condition and this variability might include the exposure of patients to factors which may cause a transient steroid insensitivity in the inflammatory process. This, in turn, may lead to an uncontrolled inflammatory response and to exacerbations, despite optimal controller treatment. A typical example of this mechanism is given by viral infections, the most frequent triggers of asthma exacerbations. Rhinoviruses, the most common viruses found in patients with asthma exacerbations, interfere with the mechanism of action of corticosteroids making the anti-inflammatory treatment transiently ineffective. A transient increase in the anti-inflammatory dose would overcome the trigger-induced anti-inflammatory resistance, avoiding uncontrolled inflammation leading to an exacerbation episode [ 13 , 14 , 15 ].
Indeed, symptoms are associated with worsening inflammation and not only with bronchoconstriction. Romagnoli et al. showed that inflammation, as evidenced by sputum eosinophilia and eosinophilic markers, is associated with symptomatic asthma [ 16 ]. A transient escalation of the ICS dose would prevent loss of control over inflammation and decrease the risk of progression toward an acute episode. In real life, when experiencing a deterioration of asthma control, patients self-treat by substantially increasing their SABA medication (Fig. 1 ); it is only subsequently that they (modestly) increase the maintenance treatment [ 17 ].
Mean use of SABA at different stages of asthma worsening. Patients have been grouped according to maintenance therapy shown in the legend. From [ 17 ], modified
As bronchodilators, SABAs do not control the underlying inflammation associated with increased symptoms. The “as required” use of SABAs is not the most effective therapeutic option in controlling a worsening of inflammation, as signaled by the occurrence of symptoms; instead, an anti-inflammatory therapy included in the rescue medication along with a rapid-acting bronchodilator could provide both rapid symptom relief and control over the underlying inflammation. Thus, there is a need for a paradigm shift, a new therapeutic approach based on the rescue use of an inhaled rapid-acting beta-agonist combined with an ICS: an anti-inflammatory reliever strategy [ 18 ].
The symptoms of an exacerbation episode, as reported by Tattersfield and colleagues in their extension of the FACET study, increase gradually before the peak of the exacerbation (Fig. 2 ); and the best marker of worsening asthma is the increased use of rescue beta-agonist treatment that follows exactly the pattern of worsening symptomatology [ 19 ]. When an ICS is administered with the rescue bronchodilator, the patient would receive anti-inflammatory therapy when it is required; that is, when the inflammation is uncontrolled, thus increasing the efficiency of the anti-inflammatory treatment.
(From [ 19 ])
Percent variation in symptoms, rescue beta-agonist use and peak expiratory flow (PEF) during an exacerbation. In order to allow comparison over time, data have been standardized (Day-14 = 0%; maximum change = 100%)
Barriers and paradoxes of asthma management
A number of barriers and controversies in the pharmacological treatment of asthma have prevented the achievement of effective disease management [ 20 ]. O’Byrne and colleagues described several such controversies in a commentary published in 2017, including: (1) the recommendation in Step 1 of earlier guidelines for SABA bronchodilator use alone, despite asthma being a chronic inflammatory condition; and (2) the autonomy given to patients over perception of need and disease control at Step 1, as opposed to the recommendation of a fixed-dose approach with treatment-step increase, regardless of the level of symptoms [ 20 ]. Other controversies outlined were: (3) a difficulty for patients in understanding the recommendation to minimize SABA use at Step 2 and switch to a fixed-dose ICS regimen, when they perceive SABA use as more effective; (4) apparent conflicting safety messages within the guidelines that patient-administered SABA monotherapy is safe, but patient-administered LABA monotherapy is not; and (5) a discrepancy as to patients’ understanding of “controlled asthma” and their symptom frequency, impact and severity [ 20 ].
Controversies (1) and (2) can both establish an early over-dependence on SABAs. Indeed, asthma patients freely use (and possibly overuse) SABAs as rescue medication. UK registry data have recently suggested SABA overuse or overreliance may be linked to asthma-related deaths: among 165 patients on short-acting relievers at the time of death, 56%, 39%, and 4% had been prescribed > 6, > 12, and > 50 SABA inhalers respectively in the previous year [ 21 ]. Registry studies have shown the number of SABA canisters used per year to be directly related to the risk of death in patients with asthma. Conversely, the number of ICS canisters used per year is inversely related to the rate of death from asthma, when compared with non-users of ICS [ 8 , 22 ]. Furthermore, low-dose ICS used regularly are associated with a decreased risk of asthma death, with discontinuation of these agents possibly detrimental [ 22 ].
Other barriers to asthma pharmacotherapy have included the suggestion that prolonged treatment with LABAs may mask airway inflammation or promote tolerance to their effects. Investigating this, Pauwels and colleagues found that in patients with asthma symptoms that were persistent despite taking inhaled glucocorticoids, the addition of regular treatment with formoterol to budesonide for a 12-month period did not decrease asthma control, and improved asthma symptoms and lung function [ 11 ].
Treatment strategies across all levels of asthma severity
Focusing on risk reduction, the 2014 update of the GINA guidelines recommended as-needed SABA for Step 1 of the stepwise treatment approach, with low-dose ICS maintenance therapy as an alternative approach for long-term anti-inflammatory treatment [ 23 ]. Such a strategy was only supported by the evidence from a post hoc efficacy analysis of the START study in patients with recently diagnosed mild asthma [ 24 ]. The authors showed that low-dose budesonide reduced the decline of lung-function over 3 years and consistently reduced severe exacerbations, regardless of symptom frequency at baseline, even in subjects with symptoms below the then-threshold of eligibility for ICS [ 24 ]. However, as for all post hoc analyses, the study by Reddel and colleagues does not provide conclusive evidence and, even so, their results could have questionable clinical significance for the management of patients with early mild asthma. To be effective, this approach would require patients to be compliant to regular twice-daily ICS for 10 years to have the number of exacerbations reduce by one. In real life, it is highly unlikely that patients with mild asthma would adhere to such a regular regimen [ 25 ].
The 2016 update to the GINA guidelines lowered the threshold for the use of low-dose ICS (GINA Step 2) to two episodes of asthma symptoms per month (in the absence of any supportive evidence for the previous cut-off). The objective was to effectively increase the asthma population eligible to receive regular ICS treatment and reduce the population treated with a SABA only, given the lack of robust evidence of the latter’s efficacy and safety and the fact that asthma is a variable condition characterized by acute exacerbations [ 26 ]. Similarly, UK authorities recommended low-dose ICS treatment in mild asthma, even for patients with suspected asthma, rather than treatment with a SABA alone [ 10 ]. However, these patients are unlikely to have good adherence to the regular use of an ICS. It is well known that poor adherence to treatment is a major problem in asthma management, even for patients with severe asthma. In their prospective study of 2004, Krishnan and colleagues evaluated the adherence to ICS and oral corticosteroids (OCS) in a cohort of patients hospitalized for asthma exacerbations [ 27 ]. The trend in the data showed that adherence to ICS and OCS treatment in patients dropped rapidly to reach nearly 50% within 7 days of hospital discharge, with the rate of OCS discontinuation per day nearly double the rate of ICS discontinuation per day (− 5.2% vs. − 2.7%; p < 0.0001 respectively, Fig. 3 ), thus showing that even after a severe event, patients’ adherence to treatment is suboptimal [ 27 ].

(From [ 27 ])
Use of inhaled (ICS) and oral (OCS) corticosteroids in patients after hospital discharge among high-risk adult patients with asthma. The corticosteroid use was monitored electronically. Error bars represent the standard errors of the measured ICS and OCS use
Guidelines set criteria with the aim of achieving optimal control of asthma; however, the attitude of patients towards asthma management is suboptimal. Partridge and colleagues were the first in 2006 to evaluate the level of asthma control and the attitude of patients towards asthma management. Patients self-managed their condition using their medication as and when they felt the need, and adjusted their treatment by increasing their intake of SABA, aiming for an immediate relief from symptoms [ 17 ]. The authors concluded that the adoption of a patient-centered approach in asthma management could be advantageous to improve asthma control.
The concomitant administration of an as-needed bronchodilator and ICS would provide rapid relief while administering anti-inflammatory therapy. This concept is not new: in the maintenance and reliever approach, patients are treated with ICS/formoterol (fast-acting, long-acting bronchodilator) combinations for both maintenance and reliever therapy. An effective example of this therapeutic approach is provided in the SMILE study in which symptomatic patients with moderate to severe asthma and treated with budesonide/formoterol as maintenance therapy were exposed to three different as-needed options: SABA (terbutaline), rapid-onset LABA (formoterol) and a combination of LABA and ICS (budesonide/formoterol) [ 28 ]. When compared with formoterol, budesonide/formoterol as reliever therapy significantly reduced the risk of severe exacerbations, indicating the efficacy of ICS as rescue medication and the importance of the as-needed use of the anti-inflammatory reliever.
The combination of an ICS and a LABA (budesonide/formoterol) in one inhaler for both maintenance and reliever therapy is even more effective than higher doses of maintenance ICS and LABA, as evidenced by Kuna and colleagues and Bousquet and colleagues (Fig. 4 ) [ 29 , 30 ].
(Data from [ 29 , 30 ])
Comparison between the improvements in daily asthma control resulting from the use of budesonide/formoterol maintenance and reliever therapy vs. higher dose of ICS/LABA + SABAZ and steroid load for the two regimens
The effects of single maintenance and reliever therapy versus ICS with or without LABA (controller therapy) and SABA (reliever therapy) have been recently addressed in the meta-analysis by Sobieraj and colleagues, who analysed 16 randomized clinical trials involving patients with persistent asthma [ 31 ]. The systematic review supported the use of single maintenance and reliever therapy, which reduces the risk of exacerbations requiring systemic corticosteroids and/or hospitalization when compared with various strategies using SABA as rescue medication [ 31 ].
This concept was applied to mild asthma by the BEST study group, who were the first to challenge the regular use of ICS. A pilot study by Papi and colleagues evaluated the efficacy of the symptom-driven use of beclomethasone dipropionate plus albuterol in a single inhaler versus maintenance with inhaled beclomethasone and as-needed albuterol. In this six-month, double-blind, double-dummy, randomized, parallel-group trial, 455 patients with mild asthma were randomized to one of four treatment groups: an as-needed combination therapy of placebo bid plus 250 μg of beclomethasone and 100 μg of albuterol in a single inhaler; an as-needed albuterol combination therapy consisting of placebo bid plus 100 μg of albuterol; regular beclomethasone therapy, comprising beclomethasone 250 μg bid and 100 μg albuterol as needed); and regular combination therapy with beclomethasone 250 μg and albuterol 100 μg in a single inhaler bid plus albuterol 100 μg as needed.
The rescue use of beclomethasone/albuterol in a single inhaler was as efficacious as the regular use of inhaled beclomethasone (250 μg bid ) and it was associated with a lower 6-month cumulative dose of the ICS [ 32 ].
The time to first exacerbation differed significantly among groups ( p = 0.003), with the shortest in the as-needed albuterol and placebo group (Fig. 5 ). Figure 5 also shows equivalence between the as-needed combination therapy and the regular beclomethasone therapy. However, these results were not conclusive since the study was not powered to evaluate the effect of the treatment on exacerbations. In conclusion, as suggested by the study findings, mild asthma patients may require the use of an as-needed ICS and an inhaled bronchodilator rather than a regular treatment with ICS [ 32 ].
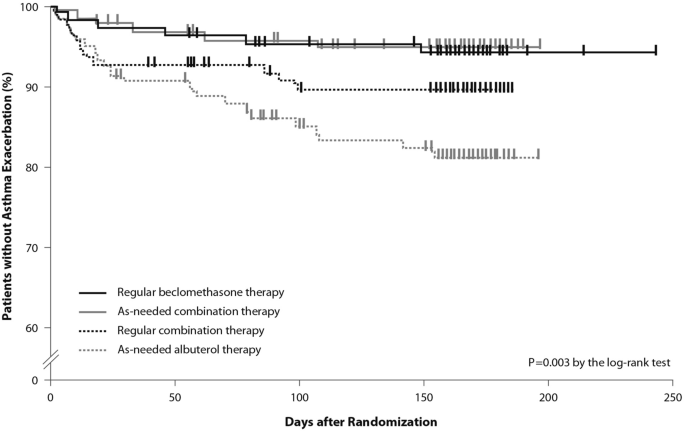
(From [ 32 ])
Kaplan Meier analysis of the time to first exacerbation (modified intention-to-treat population). First asthma exacerbations are shown as thick marks. As-needed albuterol therapy = placebo bid plus 100 μg of albuterol as needed; regular combination therapy = 250 μg of beclomethasone and 100 μg of albuterol in a single inhaler bid plus 100 μg of albuterol as needed; regular beclomethasone therapy = 250 μg of beclomethasone bid and 100 μg of albuterol as needed; as-needed combination therapy = placebo bid plus 250 μg of beclomethasone and 100 μg of albuterol in a single inhaler as needed

Moving forward: a new approach to the management of asthma patients
Nearly a decade after the publication of the BEST study in 2007, the use of this alternative therapeutic strategy was addressed in the SYGMA 1 and SYGMA 2 trials. These double-blind, randomized, parallel-group, 52-week phase III trials evaluated the efficacy of as-needed use of combination formoterol (LABA) and the ICS budesonide as an anti-inflammatory reliever in patients requiring GINA Step 2 treatment, with the current reliever therapy (e.g. as-needed SABA) or with low-dose maintenance ICS (inhaled budesonide bid ) plus as-needed SABA, administered as regular controller therapy [ 33 , 34 ].
The SYGMA 1 trial, which enrolled 3849 patients, aimed to demonstrate the superiority of the as-needed use of the combination budesonide/formoterol over as-needed terbutaline, as measured by the electronically-recorded proportion of weeks with well-controlled asthma [ 34 ]. The more pragmatic SYGMA 2 trial enrolled 4215 patients with the aim to demonstrate that the budesonide/formoterol combination is non-inferior to budesonide plus as-needed terbutaline in reducing the relative rate of annual severe asthma exacerbations [ 33 ]. Both trials met their primary efficacy outcomes. In particular, as-needed budesonide/formoterol was superior to as-needed SABA in controlling asthma symptoms (34.4% versus 31.1%) and preventing exacerbations, achieving a 64% reduction in exacerbations. In both trials, budesonide/formoterol as-needed was similar to budesonide maintenance bid at preventing severe exacerbations, with a substantial reduction of the inhaled steroid load over the study period (83% in the SYGMA 1 trial and 75% in the SYGMA 2 trial). The time to first exacerbation did not differ significantly between the two regimens; however, budesonide/formoterol was superior to SABA in prolonging the time to first severe exacerbation [ 33 , 34 ].
The double-blind, placebo-controlled design of the SYGMA trials does not fully address the advantages of anti-inflammatory reliever strategy in patients who often rely on SABAs for symptom relief, so to what extent the study findings could apply to real-life practice settings was unclear.
These limitations were overcome by the results of the Novel START study, an open-label, randomized, parallel-group, controlled trial designed to reflect real-world practice, which demonstrated the effectiveness in mild asthma of budesonide/formoterol as an anti-inflammatory reliever therapy [ 35 ].
In real-world practice, mild asthma patients are treated with an as-needed SABA reliever or with daily low-dose ICS maintenance therapy plus a SABA reliever. In the Novel START study, 668 patients with mild asthma were randomized to receive either as-needed albuterol 100 µg, two inhalations (SABA reliever as a continuation of the Step 1 treatment according to the 2017 GINA guidelines), budesonide 200 µg (ICS maintenance treatment) plus as-needed albuterol (Step 2 therapy of the GINA 2017 guidelines), or 200 µg/6 µg budesonide/formoterol as anti-inflammatory reliever therapy taken as-needed for a 52-week study period.
In this study, the rate of asthma exacerbations for budesonide/formoterol was lower compared with albuterol (51%) and similar to the twice-daily maintenance budesonide plus albuterol, despite a 52% reduction in the mean steroid dose with the single combination inhaler treatment [ 35 ]. In addition, severe exacerbation rate was lower with budesonide/formoterol as compared with as-needed albuterol and regular twice-daily budesonide. These data support the findings of the SYGMA 1 and 2 trials, highlighting the need for a critical re-examination of current clinical practice. Along with the results of the SYGMA trials, they provide convincing evidence of the advantages of the anti-inflammatory reliever strategy, particularly in real-life settings.
The SYGMA 1, SYGMA 2 and the novel START studies complete the picture of the treatment strategies for asthma at any degree of severity, including mild asthma. A growing body of evidence shows that an anti-inflammatory reliever strategy, when compared with all other strategies with SABA reliever, consistently reduces the rate of exacerbations across all levels of asthma severity (Fig. 6 ) [ 28 , 29 , 34 , 36 , 37 , 38 , 39 ].
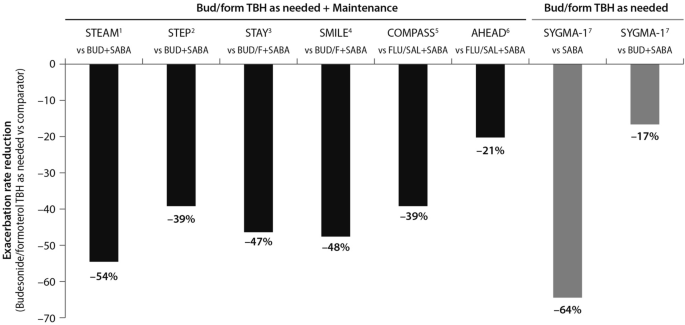
(Data source: [ 39 ])
Risk reduction of severe asthma attack of anti-inflammatory reliever versus SABA across all levels of asthma severity. Bud = budesonide; form = formoterol; TBH = turbohaler. Data from: 1: [ 36 ]; 2: [ 37 ]; 3: [ 38 ]; 4: [ 28 ]; 5: [ 29 ]; 6: [ 30 ]; 7: [ 34 ]
This evidence set the ground (Fig. 7 ) for the release of the 2019 GINA strategy updates. The document provides a consistent approach towards the management of the disease and aims to avoid the overreliance and overuse of SABAs, even in the early course of the disease. The 2019 GINA has introduced key changes in the treatment of mild asthma: for safety reasons, asthmatic adults and adolescents should receive ICS-containing controller treatment instead of the SABA-only treatment, which is no longer recommended.
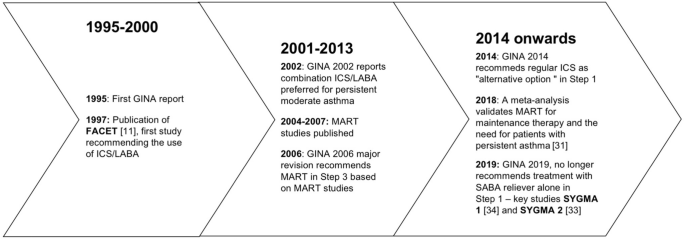
Timeline of key randomized controlled trials and meta-analyses providing the supporting evidence base leading to the Global Initiative for Asthma (GINA) 2019 guidelines. GINA global initiative for asthma, MART maintenance and reliever therapy, SMART single inhaler maintenance and reliever therapy
In Step 1 of the stepwise approach to adjusting asthma treatment, the preferred controller option for patients with fewer than two symptoms/month and no exacerbation risk factors is low-dose ICS/formoterol as needed. This strategy is indirectly supported by the results of the SYGMA 1 study which evaluated the efficacy and safety of budesonide/formoterol as needed, compared with as-needed terbutaline and budesonide bid plus as-needed terbutaline (see above). In patients with mild asthma, the use of an ICS/LABA (budesonide/formoterol) combination as needed provided superior symptom control to as-needed SABA, resulting in a 64% lower rate of exacerbations (p = 0.07) with a lower steroid dose (17% of the budesonide maintenance dose) [ 34 ]. The changes extend to the other controller options as well. In the 2017 GINA guidelines, the preferred treatment was as-needed SABA with the option to consider adding a regular low-dose ICS to the reliever. In order to overcome the poor adherence with the ICS regimen, and with the aim to reduce the risk of severe exacerbations, the 2019 GINA document recommends taking low-dose ICS whenever SABA is taken, with the daily ICS option no longer listed.
Previous studies including the TREXA study in children and adolescents [ 40 ], the BASALT study [ 41 ] and research conducted by the BEST study group [ 32 ] have already added to the evidence that a low-dose ICS with a bronchodilator is an effective strategy for symptom control in patients with mild asthma. A recently published study in African-American children with mild asthma found that the use of as-needed ICS with SABA provides similar asthma control, exacerbation rates and lung function measures at 1 year, compared with daily ICS controller therapy [ 42 ], adding support to TREXA findings that in children with well controlled, mild asthma, ICS used as rescue medication with SABA may be an efficacious step-down strategy [ 40 ].
In Step 2 of the stepwise approach, there are now two preferred controller options: (a) a daily low-dose ICS plus an as-needed SABA; and (b) as-needed low-dose ICS/formoterol. Recommendation (a) is supported by a large body of evidence from randomized controlled trials and observations showing a substantial reduction of exacerbation, hospitalization, and death with regular low-dose ICS [ 7 , 8 , 9 , 24 , 43 ], whereas recommendation (b) stems from evidence on the reduction or non-inferiority for severe exacerbations when as-needed low-dose ICS/formoterol is compared with regular ICS [ 33 , 34 ].
The new GINA document also suggests low-dose ICS is taken whenever SABA is taken, either as separate inhalers or in combination. This recommendation is supported by studies showing reduced exacerbation rates compared with taking a SABA only [ 32 , 40 ], or similar rates compared with regular ICS [ 32 , 40 , 41 ]. Low-dose theophylline, suggested as an alternative controller in the 2017 GINA guidelines, is no longer recommended.
Airway inflammation is present in the majority of patients with asthma, and although patients with mild asthma may have only infrequent symptoms, they face ongoing chronic inflammation of the lower airways and risk acute exacerbations. The GINA 2019 strategy recognizes the importance of reducing the risk of asthma exacerbations, even in patients with mild asthma (Steps 1 and 2) [ 4 ]. In this regard, the new recommendations note that SABA alone for symptomatic treatment is non-protective against severe exacerbation and may actually increase exacerbation risk if used regularly or frequently [ 4 ].
The reluctance by patients to regularly use an ICS controller means they may instead try and manage their asthma symptoms by increasing their SABA reliever use. This can result in SABA overuse and increased prescribing, and increased risk of exacerbations.
As part of the global SABINA (SABA use IN Asthma) observational study programme, a UK study examined primary care records to describe the pattern of SABA and ICS use over a 10-year period in 373,256 patients with mild asthma [ 44 ]. Results showed that year-to-year SABA prescribing was more variable than that of ICS indicating that, in response to fluctuations in asthma symptom control, SABA use was increased in preference to ICS use. Furthermore, more than 33% of patients were prescribed SABA inhalers at a level equivalent to around ≥ 3 puffs per week which, according to GINA, suggests inadequate asthma control.
The problem of SABA overuse is further highlighted by two studies [ 45 , 46 ], also as part of the SABINA programme. These analysed data from 365,324 patients in a Swedish cohort prescribed two medications for obstructive lung disease in any 12-month period (HERA).
The first study identified SABA overuse (defined as ≥ 3 SABA canisters a year) in 30% of patients, irrespective of their ICS use; 21% of patients were collecting 3–5 canisters annually, 7% were collecting 6–10, and 2% more than 11 [ 45 ]. Those patients who were overusing SABA had significantly more asthma exacerbations relative to those using < 3 canisters (20.0 versus 12.5 per 100 patient years; relative risk 1.60, 95% CI 1.57–1.63, p < 0.001). Moreover, patients overusing SABA and whose asthma was more severe (GINA Steps 3 and 4) had greater exacerbation risk compared with overusing patients whose asthma was milder (GINA Steps 1 and 2).
The second study found those patients using three or more SABA reliever canisters a year had an increased all-cause mortality risk relative to patients using fewer SABA canisters: hazard ratios after adjustment were 1.26 (95% CI 1.14–1.39) for 3–5 canisters annually, 1.67 (1.49–1.87) for 6–10 canisters, and 2.35 (2.02–2.72) for > 11 canisters, relative to patients collecting < 3 canisters annually [ 46 ].
The recently published PRACTICAL study lends further support to as-needed low-dose ICS/formoterol as an alternative option to daily low-dose ICS plus as-needed SABA, outlined in Step 2 of the guidelines [ 47 ]. In their one-year, open-label, multicentre, randomized, superiority trial in 890 patients with mild to moderate asthma, Hardy and colleagues found that the rate of severe exacerbations per patient per year (the primary outcome) was lower in patients who received as-needed budesonide/formoterol than in patients who received controller budesonide plus as-needed terbutaline (relative rate 0.69, 95% CI 0.48–1.00; p < 0.05). Indeed, they suggest that of these two treatment options, as-needed low-dose ICS/formoterol may be preferred over controller low-dose ICS plus as-needed SABA for the prevention of severe exacerbations in this patient population.
Step 3 recommendations have been left unchanged from 2017, whereas Step 4 treatment has changed from recommending medium/high-dose ICS/LABA [ 3 ] to medium-dose ICS/LABA; the high-dose recommendation has been escalated to Step 5. Patients who have asthma that remains uncontrolled after Step 4 treatment should be referred for phenotypic assessment with or without add-on therapy.
To summarise, the use of ICS medications is of paramount importance for optimal asthma control. The onset and increase of symptoms are indicative of a worsening inflammation leading to severe exacerbations, the risk of which is reduced by a maintenance plus as-needed ICS/LABA combination therapy. The inhaled ICS/bronchodilator combination is as effective as the regular use of inhaled steroids.
The efficacy of anti-inflammatory reliever therapy (budesonide/formoterol) versus current standard-of-care therapies in mild asthma (e.g. reliever therapy with a SABA as needed and regular maintenance controller therapy plus a SABA as-needed) has been evaluated in two randomized, phase III trials which confirmed that, with respect to as-needed SABA, the anti-inflammatory reliever as needed is superior in controlling asthma and reduces exacerbation rates, exposing the patients to a substantially lower glucocorticoid dose.
Conclusions
A growing body of evidence shows that anti-inflammatory reliever strategy is more effective than other strategies with SABA reliever in controlling asthma and reducing exacerbations across all levels of asthma severity. A budesonide/formoterol therapy exposes asthma patients to a substantially lower glucocorticoid dose while cutting the need for adherence to scheduled therapy.
Availability of data and materials
Not applicable.
Abbreviations
Global Initiative for Asthma
Inhaled corticosteroids
Long-acting beta-agonists
Oral corticosteroids
Short-acting beta-agonists
Single inhaler maintenance and reliever treatment
World Health Organization. Asthma. 2017. https://www.who.int/news-room/fact-sheets/detail/asthma . Accessed 9 April 2019.
Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(10122):783–800.
Article PubMed Google Scholar
Global Initiative for Asthma. Global strategy for asthma management and prevention, 2017. http://www.ginasthma.org . Accessed 1 June 2019.
Global Initiative for Asthma. Pocket guide for asthma management and prevention. 2019, pp. 1–32. www.ginasthma.org . Accessed 1 June 2019.
Rabe KF, Vermeire PA, Soriano JB, Maier WC. Clinical management of asthma in 1999: the Asthma Insights and Reality in Europe (AIRE) study. Eur Respir J. 2000;16(5):802–7.
Article CAS PubMed Google Scholar
Price D, Fletcher M, Molen V. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24:14009.
Article PubMed Central PubMed Google Scholar
Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax. 2002;57(10):880–4.
Article CAS PubMed Central PubMed Google Scholar
Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. New Engl J Med. 2000;343(5):332–6.
Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, et al. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet. 2003;361(9363):1071–6.
Healthcare Improvement Scotland and British Thoracic Society. SIGN 153: British guideline on the management of asthma. 2016. https://www.sign.ac.uk/sign-153-british-guideline-on-the-management-of-asthma.html .
Pauwels RA, Löfdahl CG, Postma DS, Tattersfield AE, O’Byrne P, Barnes PJ, Ullman A. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med. 1997;337(20):1405–11.
O’Byrne PM, Naya IP, Kallen A, Postma DS, Barnes PJ. Increasing doses of inhaled corticosteroids compared to adding long-acting inhaled β2-agonists in achieving asthma control. Chest. 2008;134(6):1192–9.
Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310(6989):1225–9.
Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, Johnston SL. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359(9309):831–4.
Papi A, Contoli M, Adcock IM, Bellettato C, Padovani A, Casolari P, et al. Rhinovirus infection causes steroid resistance in airway epithelium through nuclear factor κb and c-Jun N-terminal kinase activation. J Allergy Clin Immunol. 2013;132(5):1075–85.
Romagnoli M, Vachier I, Tarodo de la Fuente P, Meziane H, Chavis C, Bousquet J, et al. Eosinophilic inflammation in sputum of poorly controlled asthmatics. Eur Respir J. 2002;20(6):1370–7.
Partridge MR, van der Molen T, Myrseth SE, Busse WW. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulm Med. 2006;6:13.
Papi A, Caramori G, Adcock IM, Barnes PJ. Rescue treatment in asthma. More than as-needed bronchodilation. Chest. 2009;135(6):1628–33.
PubMed Google Scholar
Tattersfield AE, Postma DS, Barnes PJ, Svensson K, Bauer CA, Byrne PM, et al. Exacerbations of asthma: a descriptive study of 425 severe exacerbations The FACET International Study Group. Am J Respir Crit Care Med. 1999;160(2):594–9.
O’Byrne PM, Jenkins C, Bateman ED. The paradoxes of asthma management: time for a new approach? Eur Respir J. 2017. https://doi.org/10.1183/13993003.01103-2017 .
Royal College of Physicians. Why asthma still kills: the National Review of Asthma Deaths (NRAD). Confidential Enquiry report. London: RCP; 2014.
Google Scholar
Suissa S, Ernst P, Boivin JF, Horwitz RI, Habbick B, Cockroft D, et al. A cohort analysis of excess mortality in asthma and the use of inhaled β-agonists. Am J Respir Crit Care Med. 1994;149(3 Pt 1):604–10.
Global Initiative for Asthma. Global strategy for asthma management and prevention, 2014. https://ginasthma.org/wp-content/uploads/2019/01/2014-GINA.pdf .
Reddel HK, Busse WW, Pedersen S, Tan WC, Chen YZ, Jorup C, et al. Should recommendations about starting inhaled corticosteroid treatment for mild asthma be based on symptom frequency: a post hoc efficacy analysis of the START study. Lancet. 2017;389(10065):157–66.
Papi A, Fabbri LM. Management of patients with early mild asthma and infrequent symptoms. Lancet. 2017;389(10065):129–30.
Global Initiative for Asthma. Global strategy for asthma management and prevention, 2016. https://ginasthma.org/wp-content/uploads/2016/04/GINA-Appendix-2016-final.pdf .
Krishnan JA, Riekert KA, McCoy JV, Stewart DY, Schmidt S, Chanmugam A, et al. Corticosteroid use after hospital discharge among high-risk adults with asthma. Am J Respir Crit Care Med. 2004;170(12):1281–5.
Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo UG. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double-blind study. Lancet. 2006;368(9537):744–53.
Kuna P, Peters MJ, Manjra AI, Jorup C, Naya IP, Martínez-Jimenez NE, Buhl R. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract. 2007;61(5):725–36.
Bousquet J, Boulet LP, Peters MJ, Magnussen H, Quiralte J, Martinez-Aguilar NE, Carlsheimer A. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high-dose salmeterol/fluticasone. Respir Med. 2007;101(12):2437–46.
Sobieraj DM, Weeda ER, Nguyen E, Coleman CI, White CM, Lazarus SC, et al. Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma a systematic review and meta-analysis. JAMA. 2018;319(14):1485–96.
Papi A, Canonica GW, Maestrelli P, Paggiaro P, Olivieri D, Pozzi E, BEST Study Group, et al. Rescue use of beclomethasone and albuterol in a single inhaler for mild asthma. New Engl J Med. 2007;356(20):2040–52.
Bateman ED, Reddel HK, O’Byrne PM, Barnes PJ, Zhong N, Keen C, et al. As-needed budesonide–formoterol versus maintenance budesonide in mild asthma. New Engl J Med. 2018;378(20):1877–87.
O’Byrne PM, FitzGerald JM, Bateman ED, Barnes PJ, Zhong N, Keen C, et al. Inhaled combined budesonide-formoterol as needed in mild asthma. New Engl J Med. 2018;378(20):1865–76.
Beasley R, Holliday M, Reddel HK, Braithwaite I, Ebmeier S, Hancox RJ, Novel START Study Team, et al. Controlled trial of budesonide-formoterol as needed for mild asthma. New Engl J Med. 2019;380(21):2020–30.
Rabe K, Pizzichini E, Ställberg B, Romero S, Balanzat AM, Atienza T, et al. Budesonide/formoterol in a single inhaler for maintenance and relief in mild-to-moderate asthma: a randomized, double-blind trial. Chest. 2006;129(2):246–56.
Scicchitano R, Aalbers R, Ukena D, Manjra A, Fouquert L, Centanni S, et al. Efficacy and safety of budesonide/formoterol single inhaler therapy versus a higher dose of budesonide in moderate to severe asthma. Curr Med Res Opin. 2004;20(9):1403–18.
O’Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med. 2005;171(2):129–36.
Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G, et al. After asthma: redefining airways diseases. Lancet. 2017;391(10118):350–400.
Martinez FD, Chinchilli VM, Morgan WJ, Boehmer SJ, Lemanske RF Jr, Mauger DT, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377(9766):650–7.
Calhoun WJ, Ameredes BT, King TS, Icitovic N, Bleecker ER, Castro M, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA. 2012;308(10):987–97.
Sumino K, Bacharier LB, Taylor J, et al. A pragmatic trial of symptom-based inhaled corticosteroid use in African-American children with mild asthma. J Allergy Clin Immunol Pract. 2020;8(176–85):e2.
Byrne PM, Barnes PJ, Rodriguez-Roisin R, Runnerstrom E, Sandstrom T, Svensson K, Tattersfield A. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med. 2001;164(8):1392–7.
Article Google Scholar
Bloom C, Quint J, Cabrera C. SABA and ICS prescriptions among mild asthma patients in UK primary care. Poster presented at the European Respiratory Society International Congress; 2019 Sep 28–Oct 2; Madrid, Spain.
Janson C, Nwaru B, Hasvold P, Wicklund F, Telg G, Ekstrom M. Use of short-acting beta-2 agonists (SABA) and exacerbations in a nationwide Swedish asthma cohort (HERA). Poster presented at the European Respiratory Society International Congress; 2019 Sep 28–Oct 2; Madrid, Spain.
Janson C, Nwaru B, Hasvold P, Wicklund F, Telg G, Ekstrom M. SABA overuse and risk of mortality in a nationwide Swedish asthma cohort (HERA). Late Breaker abstract at the European Respiratory Society International Congress; 2019 Sep 28–Oct 2; Madrid, Spain.
Hardy J, Baggott C, Fingleton J, Reddel HK, Hancox RJ, Harwood M, et al. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet. 2019;394(10202):919–28.
Download references
Acknowledgements
The Authors thank Maurizio Tarzia and Gayle Robins, independent medical writers who provided editorial assistance on behalf of Springer Healthcare Communications. The editorial assistance was funded by AstraZeneca.
No funding was received for this study. The editorial assistance was funded by AstraZeneca.
Author information
Authors and affiliations.
Section of Cardiorespiratory and Internal Medicine, Department of Morphology, Surgery and Experimental Medicine, University of Ferrara, Ferrara, Italy
Alberto Papi & Luca Morandi
Internal Medicine Department, Respiratory Unit and Adult Cystic Fibrosis Center, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy
Francesco Blasi
Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy
Personalized Medicine Asthma & Allergy Clinic, Humanitas University & Istituto Clinico Humanitas, Milan, Italy
Giorgio Walter Canonica
Università Cattolica del Sacro Cuore, Fondazione Policlinico A. Gemelli IRCCS, Rome, Italy
Luca Richeldi
Respiratory Section, Department of Medicine, University of Verona, Verona, Italy
Andrea Rossi
Respiratory Unit, Emergency Department, University Hospital S. Anna, Via Aldo Moro 8, 44124, Ferrara, Italy
You can also search for this author in PubMed Google Scholar
Contributions
AP, FB, GWC, LM, LR and AR contributed to writing. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Alberto Papi .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
AP reports grants, personal fees, non-financial support and payment for advisory board membership, consultancy, payment for lectures, grants for research, and travel expenses reimbursement from Chiesi, AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Mundipharma and Teva, and personal fees and non-financial support from Menarini, Novartis, Zambon and Sanofi.
FB reports having received in the last three years research grants as well as lecture or advisory board fees from: Alk-Abelló, AstraZeneca, Boehringer Ingelheim, Chiesi, Guidotti, Glaxo Smith Kline, Grifols, Menarini, Novartis, Sanofi, Valeas, Zambon.
GWC reports having received in the last 3 years research grants as well as lecture or advisory board fees from: A. Menarini, Alk-Abelló, AstraZeneca-Medimmune, Boehringer Ingelheim, Chiesi Farmaceutici, Genentech, Guidotti-Malesci, Glaxo Smith Kline, Hal Allergy, Merck Sharp & Dohme, Mundipharma, Novartis, Orion, Sanofi-Aventis, Sanofi Genzyme/Regeneron, Stallergenes-Greers, UCB Pharma, Uriach Pharma, Valeas.
LR Receipt of grants/research supports: Roche, Boehringer Ingelheim.
Receipt of honoraria or consultation fees: Boehringer Ingelheim, Roche, Biogen, FibroGen,
Sanofi-Aventis, Anthera, Promedior, ImmuneWorks, Asahi-Kasei, Bayer, Celgene, RespiVant,
Nitto, Bristol Myers Squibb, Prometic, Pliant Therapeutics, Toray, Global Blood Therapeutics,
Zambon, Veracyte, Acceleron, CSL Behring.
LM and AR reports no conflicts of interest in the last 3 years.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Papi, A., Blasi, F., Canonica, G.W. et al. Treatment strategies for asthma: reshaping the concept of asthma management. Allergy Asthma Clin Immunol 16 , 75 (2020). https://doi.org/10.1186/s13223-020-00472-8
Download citation
Received : 18 March 2020
Accepted : 05 August 2020
Published : 15 August 2020
DOI : https://doi.org/10.1186/s13223-020-00472-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Anti-inflammatory treatment
- Disease control
- Patient outcomes
Allergy, Asthma & Clinical Immunology
ISSN: 1710-1492
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Treatments for poorly...
Treatments for poorly controlled asthma
- Related content
- Peer review
- Orla O’Carroll , respiratory fellow 1 ,
- Cormac McCarthy , consultant respiratory physician 1 2 ,
- Marcus W Butler , consultant respiratory physician 1 2
- 1 Department of Respiratory and Sleep Medicine, St Vincent’s University Hospital, Dublin, Republic of Ireland
- 2 School of Medicine, University College Dublin, Dublin, Republic of Ireland
- Correspondence to M W Butler marcus.butler{at}ucd.ie
What you need to know
In a patient with worsening symptoms of asthma, confirm the diagnosis and address possible causes of worsening, including treatment adherence, comorbidities, and environmental factors
Combined inhaled corticosteroids with long acting β agonists (LABA) as single maintenance and reliever therapies are associated with reduced exacerbations and improved asthma related quality of life compared with traditional dual maintenance and reliever therapies
LABA monotherapy is not recommended owing to increased risk of exacerbations and asthma related death
Written asthma plans given to patients as part of a supported self-management strategy help reduce hospitalisation and emergency department attendances
A 35 year old man with a prior diagnosis of mild asthma presents to his general practitioner with a three month history of intermittently waking at night with cough and shortness of breath. He works as a chef and has found it difficult to complete a full shift during this time. He has been prescribed beclometasone 200 μg twice a day for a long time and asks if there is a need to change his treatment in light of his new persistent symptoms.
Poorly controlled or moderate asthma occurs when patients experience either daily symptoms of asthma, nocturnal awakenings, more than two exacerbations per year, or some limitation of their daily activities. 1 2 With frequent alterations of guidelines, and continual development of new medications and delivery devices, choosing a suitable treatment strategy can pose a challenge in clinical practice. In this article, we present the latest guidance on drugs used in primary care for poorly controlled asthma in adults.
What treatments are available for poorly controlled asthma?
Ics/laba combination.
Inhaled corticosteroids (ICS, eg, beclometasone dipropionate, budesonide, fluticasone furoate, etc) and long acting β agonists (LABA, eg, formoterol, salmeterol, vilanterol, etc) are the mainstay of treatment for poorly controlled or moderate asthma ( table 1 ). ICS suppress airway inflammation leading to reduced bronchial hyperresponsiveness. LABAs act on bronchial smooth muscle β adrenoceptors and cause bronchodilation. ICS and LABA are typically prescribed as a single fixed dose combination inhaler rather than in separate inhalers for better efficacy, safety, adherence, and convenience. 1
Global Initiative for Asthma treatment ladder 1
- View inline
National and international guidelines—Global Initiative for Asthma, 1 National Institute for Health and Care Excellence (NICE), 3 British Thoracic Society/Scottish Intercollegiate Guidelines Network, 4 and the National Asthma Education and Prevention Program (American) 5 —suggest two options for commencing ICS and LABA treatments in moderate persistent asthma. The Single Maintenance and Reliever Therapy (SMART or MART) protocol recommends ICS and a fast acting LABA as both maintenance and rescue treatment for relief from symptoms (for example, low dose budesonide-formoterol or beclomethasone-formoterol). 1 Alternatively, ICS plus LABA (ICS/LABA) can be combined with a short acting β agonist (SABA) as needed as reliever treatment.
Montelukast
The guidelines recommend montelukast in patients whose symptoms are not well controlled despite adequate dosing of ICS/LABA treatments and good adherence. 1 2 3 5 Montelukast is a leukotriene receptor antagonist (LTRA). It blocks the production of cysteinyl leukotrienes which are potent broncho-constricting and proinflammatory mediators. This results in enhanced bronchodilation and reduced airway mucus production. 6
Newer treatments
Patients whose asthma is severe and poorly controlled with the above drugs can be considered for newer add-on treatments. These include long acting muscarinic antagonists (LAMAs), azithromycin, and biological therapies. 1 2 7 8 9 They are usually prescribed in specialist settings after careful evaluation of a patient’s symptoms, and consideration of risks and benefits.
How well do they work?
Two Cochrane reviews published in 2013 compared budesonide and formoterol as maintenance and reliever therapy (MART) with inhaled corticosteroids alone or as combination therapy for maintenance with any reliever treatment for chronic asthma. 10 11 SMART was associated with reduced exacerbation rates requiring hospitalisation or emergency department visits (odds ratio (OR) 0.72, 95% confidence interval (CI) 0.57 to 0.90; I 2 =0%, P=0.66), oral corticosteroids (OR 0.75, 95% CI 0.65 to 0.87; I 2 =0%, P=0.82) and lower overall ICS total daily dose. 11 The strength of evidence that SMART reduces hospitalisations or emergency department visits is weak (one fewer per 100 treated than in the control group, 95% CI, 0 to 2 fewer). 10 11 Two fewer people needed a course of oral steroids per 100 treated (95% CI 1 to 3 fewer) with SMART compared with alternative regimens. Two randomised controlled trials (RCTs) published in 2013 showed similar results with use of the SMART regimen. 12 13 Most studies are industry sponsored, and do not include children and adolescents. A post-hoc analysis of industry sponsored trials reported similar efficacy and safety for SMART regimens in adolescents (12-17 years). 14 Few studies have directly compared the efficacy of various ICS preparations and have shown comparable effects. 15
Very limited evidence is available for montelukast in patients with poorly controlled asthma. An open label industry sponsored trial (1681 participants) reported that addition of oral montelukast to ICS or ICS+LABA improved asthma control and asthma related quality of life over six months in adults with insufficiently controlled asthma. 16 LTRAs have been shown to decrease sputum eosinophilia, 17 fraction of exhaled nitric oxide, 18 airway inflammation, 19 and bronchial hyperresponsiveness, 20 but the clinical relevance of these outcomes is not established. LTRAs may have a role in managing exercise induced asthma, 21 22 aspirin exacerbated respiratory disease (which includes non-steroidal anti-inflammatory drug intolerance), 23 and in patients with asthma and elevated body mass index, 24 but this evidence comes from small single studies.
What are the harms?
Few studies assess the risk of systemic adverse effects associated with the use of ICS in asthma. A Cochrane review found that the rate of serious adverse events (eg, hospitalisation, intensive care admission, intubation) was 23 per 1000 patients in a pooled analysis of patients taking ICS plus salmeterol compared with 21 per 1000 patients taking ICS alone. 25 ICS is generally considered safe at low to medium doses (<1000 µg beclomethasone dipropionate equivalent per day). 26 Local side effects include dysphonia and oral candidiasis. These can be mitigated with careful attention to oral or throat hygiene, good inhaler technique, and the preferential use of spacer devices or valved holding chambers to reduce oropharyngeal deposition.
Higher doses of ICS are associated with systemic side effects; in particular, adrenal insufficiency. About 6.8% of patients on ICS experience adrenal insufficiency, according to a systematic review. 27 These doses are not routinely used for treatment of mild to moderate asthma but some patients may be on higher cumulative doses before treatment is escalated in response to persistent symptoms. NICE recommends that patients on high dose ICS (>1000 µg/day beclomethasone dipropionate or 1600 µg/day for budesonide or equivalent) carry a steroid warning card. 3
LABA monotherapy is associated with an increased risk of severe asthma attacks leading to hospitalisation and asthma related death, 28 29 30 and is discouraged by all major asthma guidelines. 1 3 4 5 LABAs are combined with ICS in all fixed dose combination inhalers to prevent substitution of ICS maintenance therapy with LABA monotherapy. 25 31
Montelukast can have neuropsychiatric adverse effects including nightmares, aggression, and depression. 32 A case series reported development of eosinophilic granulomatosis with polyangiitis with use of LTRAs, sometimes linked to weaning of corticosteroids, but causation has not been established. 33
How are they given and monitored?
Confirm the diagnosis of asthma in any patient with worsening symptoms, ideally by observing for reversible airflow limitation. Table 2 lists aspects to cover on initial assessment and potential causes of reduced asthma control to address before considering a change in medication. 7 8 9 34 35
Care of a patient with worsening symptoms 1
If a change in medication is indicated, prescribe ICS/LABA combinations at the lowest dose of ICS possible to achieve symptomatic control. The aims of treatment are to minimise disruptive symptoms, improve the patient’s asthma related quality of life, and prevent exacerbations and worsening. Most inhaled corticosteroids are prescribed for use twice daily. Ciclesonide is a once daily preparation associated with lower incidence of oral candidiasis compared with fluticasone at equivalent doses. 36 Fluticasone furoate is a once daily preparation available in some countries, including the US and Japan, as a standalone ICS and is more widely available as a combination ICS/LABA (vilanterol) inhaler preparation. Arrange a regular review to assess symptom control. 1
Choosing the appropriate inhaler device for a patient is important to optimise dosing schedule, adherence to therapy, and avoidance of critical errors in inhaler technique. 37 When switching from single to combination inhalers, ensure that the equivalent ICS dosing is not lower than the patient’s current treatment.
With SMART regimens daily dosing varies in response to symptoms. Guidelines recommend a maximum safe daily dose. 1 Dosing is capped at 72 μg metered dose for budesonide-formoterol and 48 μg metered dose for beclomethasone-formoterol. Provide patients with a written action plan tailored to the specific ICS prescribed, outlining how to change their dosing schedule in response to symptom triggers including exacerbation. Self-management involves patient education reinforced by an asthma action plan and supported by regular review. A meta-review (27 systematic reviews and 13 RCTs) showed that supported self-management for asthma can reduce hospitalisations, emergency department attendances, and unscheduled consultations in specialist and primary care. 38
Given the uncertain benefits of montelukast and potential adverse reactions, discuss the risks and benefits with your patient before commencing this medication.
How cost effective are they?
Few studies have compared cost effectiveness of individual ICS. A review article suggested that fluticasone propionate was associated with a marginal cost benefit over budesonide, but this effect was not seen at higher doses of ICS. 39 An analysis of cost effectiveness in the UK and other European countries in 2006 found that SMART is more cost effective compared with the alternative dosing strategy of LABA/ICS plus SABA reliever. 40 The current availability of generic preparations suitable for use in SMART protocols will have reduced the costs further.
The cost of inhaler devices can differ and can sometimes be a barrier to compliance. Discuss with your patient the expected costs when choosing a suitable inhaler device. Sustainability is another factor in inhaler choice. Metered dose inhalers (MDIs) contain hydrofluorocarbons, which are potent greenhouse gases that contribute significantly to the carbon footprint. A simple switch from MDI to equivalent dry powder inhaler (DPI) could reduce the yearly carbon footprint of an asthmatic patient by a similar amount as would be achieved by switching to a plant based diet (approximately 455 CO 2 equivalent).
Tips for patients
Always take your asthma medication as prescribed
Your doctor may provide written advice (asthma action plan) on how to change how often you take your inhaler if you develop symptoms or feel an asthma attack coming on. It’s important to recognise changes in your symptoms so that you can follow this asthma plan correctly
Peak flow meters can be useful when changing your inhaler frequency according to your asthma plan
Inhalers can sometimes be difficult to use. If you have any doubt about how to take your inhaler, contact your pharmacist or GP
Some inhalers can cause fungal infections in the mouth (white coating of your mouth or tongue) and this may require medication to treat. Report this to your doctor
Rinse your mouth after taking your inhaler if it contains an inhaled steroid. This can prevent fungal mouth infections from developing
Education into practice
How would you assess an asthmatic patient with worsening symptoms to determine if therapy needs to be escalated?
How do you assess inhaler technique in your consultation with asthmatic patients?
How would you explain the SMART regimen to your patients?
How would you facilitate self-management at your practice at an institutional level for people with asthma, ideally in a way that is regularly audited to facilitate ongoing service improvement?
How patients were involved in the creation of this article
We asked three patients who attend our asthma service to review a draft of this manuscript and to help develop the “tips for patients” box. They highlighted the importance of understanding symptom control and knowing when to seek help from their practitioner for troublesome symptoms.
This is one of a series of occasional articles on therapeutics for common or serious conditions, covering new drugs and old drugs with important new indications or concerns. To suggest a topic, please email us at [email protected]
Advisers to this series are Robin Ferner, honorary professor of clinical pharmacology, University of Birmingham and Birmingham City Hospital, and Patricia McGettigan, clinical senior lecturer in clinical pharmacology, Queen Mary's University, London
Competing interests: Competing interests The BMJ has judged that there are no disqualifying financial ties to commercial companies. The authors declare the following other interests: none.
Further details of The BMJ policy on financial interests are here: https://www.bmj.com/about-bmj/resources-authors/forms-policies-and-checklists/declaration-competing-interests
Provenance and peer review: commissioned, based on an idea from the authors.
- ↵ Global Initiative for Asthma (GINA). 2020 GINA Report, Global strategy for asthma management and prevention. 2020.
- Williams SG ,
- Schmidt DK ,
- National Asthma Education and Prevention Program
- ↵ National Institute for Health and Care Excellence. 2018 exceptional surveillance of asthma: diagnosis, monitoring and chronic asthma management (NICE guideline NG80). 2018. https://www.nice.org.uk/guidance/ng80/resources/2018-exceptional-surveillance-of-asthma-diagnosis-monitoring-and-chronic-asthma-management-nice-guideline-ng80-6599448397/chapter/Surveillance-decision?tab=evidence
- Du Rand IA ,
- Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC) ,
- Cloutier MM ,
- Baptist AP ,
- Vandemheen KL ,
- FitzGerald JM ,
- Canadian Respiratory Research Network
- Boulet LP ,
- Reddel HK ,
- Heaney LG ,
- Robinson DS
- Mindus SM ,
- Corradi M ,
- Pigeon-Francisco C ,
- Pilcher J ,
- Pritchard A ,
- SMART Study Group
- Lythgoe D ,
- Beasley R ,
- Maijers I ,
- Weatherall M ,
- Virchow JC ,
- Ljungblad L ,
- Mitfessel H ,
- MONICA study group
- Pizzichini E ,
- Sandrini A ,
- Ferreira IM ,
- Gutierrez C ,
- Jardim JR ,
- Ramsay CF ,
- Sullivan P ,
- Gizycki M ,
- Currie GP ,
- Lipworth BJ
- Raissy HH ,
- Harkins M ,
- Dahlén SE ,
- Malmström K ,
- Nizankowska E ,
- Peters-Golden M ,
- Hustad CM ,
- Schmidt S ,
- Griffiths C ,
- Broersen LH ,
- Pereira AM ,
- Jørgensen JO ,
- Nelson HS ,
- Bleecker ER ,
- Yancey SW ,
- Dorinsky PM ,
- Wieland LS ,
- Oleszczuk M ,
- Bateman ED ,
- Caplan AL ,
- Haarman MG ,
- van Hunsel F ,
- de Vries TW
- Schroeder JW ,
- Losappio LM ,
- Ställberg B ,
- Menzies-Gow A ,
- Manning P ,
- Gibson PG ,
- Lasserson TJ
- Kocks JWH ,
- Chrystyn H ,
- van der Palen J ,
- Pinnock H ,
- Panagioti M ,
- PRISMS and RECURSIVE groups
- Stempel DA ,
- Stanford RH ,
- Thwaites R ,
- Johansson G ,
- Andreasson EB ,
- Larsson PE ,
- Vogelmeier CF
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Mediators Inflamm
- v.2016; 2016

Herbal Medicines for Asthmatic Inflammation: From Basic Researches to Clinical Applications
1 Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310009, China
Nan-Xia Xuan
Song-min ying, zhi-hua chen, hua-hao shen.
2 State Key Laboratory of Respiratory Diseases, Guangzhou 510120, China
Asthma is one of the most common chronic inflammatory disorders, associated with reversible airflow obstruction, airway hyperresponsiveness, and airway remodeling. This disease has a significant impact on individuals, their families, and society. Standardized therapeutics such as inhaled corticosteroid in combination with long acting β 2 agonist have been applied for asthma control; however, complementary and alternative medicines, especially herbal medicines, are still widely used all over the world. A growing body of literature suggests that various herbals or related products might be effective in inhibiting asthmatic inflammation. In this review, we summarize recent advances about the mechanistic studies of herbal medicines on allergic airway inflammation in animal models and their potential application into clinic for asthma control.
1. Introduction
Asthma is a chronic inflammatory disease characterized by reversible airway obstruction, airway hyperresponsiveness (AHR), infiltration of inflammatory cells, mucus hypersecretion, and airway remodeling [ 1 ]. It affects 300 million individuals worldwide, with the prevalence ranging from 1% to 18% of the population in different countries [ 2 ]. A variety of immune cells, structure cells in the lung, cytokines, chemokines, adhesion molecules, and signaling pathways contributes to the asthmatic pathogenesis. Although standardized therapeutics such as inhaled corticosteroid (ICS) in combination with long acting β 2 agonist (LABA) have been used to control asthma symptoms, complementary and alternative medicine (CAM) is still common all over the world. A survey involved 7685 individuals aged 55 or older with current asthma was performed recently in USA, and it showed that CAM use in the older adult asthmatic population was frequent, with nearly 40% using some type of CAM [ 3 ]. Another survey about traditional Chinese medicine (TCM) for pediatric asthma in Taiwan showed that 57.95% ( N = 26 585) of the investigated children had used TCM [ 4 ].
Based on the facts that TCM or CAM is widely used in asthma control, increasing basic or clinical studies have been conducted to investigate the molecular mechanisms or clinical applications of herbal medicines for asthma therapy. Given the fact that asthma pathogenesis is complex, the roles and effective targets of these herbal products in asthma therapy are also very complicated.
In general, basic researches are all trying to separate effective monomer from herbs or herbal formula for asthma study, while most clinical researches still only focus on the efficacy for patients using an intact traditional formula. We will illuminate the major achievements of them, respectively, in this review.
2. Basic Researches
Basic researches on herbal asthma therapy can be summarized into nine aspects according to the mechanism summarized below. Those researches that only reported some T helper 1 (Th1) cell or T helper 2 (Th2) cell cytokines, for example, Interleukin-4 (IL-4), IL-5, IL-13, and interferon- γ (IFN- γ ), altered after herbs intervention without any further mechanistic studies will not be expatiated in our paper, as they can be affected by many factors.
2.1. Targeting the Th1/Th2 Imbalance
It is generally accepted that Th1/Th2 imbalance is responsible for the development of allergic asthma. Th1 cells secrete IFN- γ , IL-12, and tumor necrosis factor- β (TNF- β ), whereas Th2 cells secrete IL-4, IL-5, and IL-13 [ 5 ]. IL-4 together with IL-13 causes isotype class-switching of B cells towards Immunoglobulin-E (IgE) synthesis, which can bind to high-affinity receptors on mast cells and basophils, and leads to subsequent activation of these cells. IL-5 activates eosinophils and attracts them to the lung, where they secrete numerous inflammatory cytokines and chemokines. IL-13 also directly affects the airway epithelium, including increases in goblet cell differentiation, activation of fibroblasts, and bronchial hyperresponsiveness [ 5 – 7 ]. These cytokines may also in turn affect the Th1/Th2 balance [ 5 , 8 ]. It has been acknowledged that two transcription factors, that is, T-bet and GATA-3, are responsible for Th1/Th2 balance. Further, GATA-3 and T-bet can be influenced by IL-12, IFN- γ , or IL-4 via the signal transducers and activators of transcription (STAT), that is, STAT4, STAT1, and STAT6, respectively [ 5 ].
Extractives from Astragalus , Panax ginseng , Saururus chinensis , Psoralea , and Ligustrazine [ 9 ] were reported a similar mechanism of decreasing the ratio of GATA3/T-bet expression level. Typically, Jin et al. [ 10 ] investigated the effects of the boiling water extract of Psoralea fructus (PF) and psoralen, an active ingredient of PF, on Th2 clone (D10.G.4.1) cells in vitro and in vivo , and interpreted their effect as suppressing GATA-3 protein expression. Similarly, Chen et al. [ 11 ] found that a single compound, Bavachinin, isolated from PF decreased the GATA-3 function by reducing the stability of GATA-3 mRNA and further suggested that Bavachinin may suppress the binding or coactivating function but not expression of pSTAT6. In their further study [ 12 ], two new derivatives of Bavachinin with a better water solubility were investigated, and one of these two derivatives not only inhibited GATA-3 mRNA production but also increased T-bet mRNA production. However, clinical research for Psoralea fructus in treating asthma is lacking. Only few case reports [ 13 ] involving Psoralea fructus related recipe in Chinese can be found but provided limited evidence.
Efficacy on STAT6 was reported in extracts from Scutellaria baicalensis [ 14 ] and Cnidii monnieri [ 15 ]. Chiu et al. [ 15 ] explored the effects of Osthol ( Cnidii monnieri fructus extract) on epithelial cells using human bronchial epithelial cells (BEAS-2B) in vitro . Their research demonstrated that Osthol suppressed IL-4-induced eotaxin (a key mediator in allergic diseases with eosinophilic infiltration) in epithelial cells via inhibition of STAT6 expression.
Besides, without further mechanism revealed, efficacy on Th1/Th2 cytokines was also observed in taraxasterol (isolated from Taraxacum officinale ) [ 16 ], Duchesnea chrysantha ethanol extract [ 17 ], Echinacea purpurea complex [ 18 ], matrine (isolated from Sophora flavescens ) [ 19 ], Zingiber officinale (both ethanol and aqueous extracts) [ 20 ], Actinidia polygama fructus extract [ 21 ], and formulas like Sam So Eum [ 22 ] and Qu Feng Xuan Bi [ 23 ].
2.2. Effect on MAPK and NF- κ B Signaling Pathways
Mitogen-activated protein kinases (MAPKs), which comprise three major subgroups, that is, extracellular signal-related kinase 1/2 (ERK1/2), p38, and c-Jun N-terminal kinase 1/2 (JNK1/2), play critical roles in the activation of inflammatory cells [ 24 ]. Nuclear factor kappa B (NF- κ B) is an important transcription factor involved in the expression of various proinflammatory genes. Increased activation of NF- κ B has been observed in the lungs after allergen challenge and in airway epithelial cells and macrophages from asthmatic patients [ 25 ]. Many studies have reported that allergic asthma could be improved by regulating the activation of MAPK and NF- κ B signaling pathways [ 26 , 27 ].
Herbs that were extracted targeting on MAPKs include Scutellaria baicalensis [ 28 , 29 ], Panax ginseng [ 30 ], Saururus chinensis [ 31 ], Artemisia annua [ 32 ], Magnoliae flos [ 33 , 34 ], and Crocus sativus [ 35 ]. While NF- κ B inhibitors can be found in Scutellaria baicalensis [ 36 ], Astragalus [ 37 , 38 ], Saururus chinensis [ 31 ], Astilbe chinensis [ 39 ], Artemisia annua [ 32 ], and Garlic [ 40 ]. Although they performed similar mechanisms, minor differences could be noticed. Some extracts like dihydroartemisinin [ 32 ] (isolated from Artemisia annua ) and meso-Dihydroguaiaretic acid [ 31 ] (isolated from Saururus chinensis ) act as inhibitors of MAPKs and NF- κ B meanwhile, whereas some extracts isolated from the same herb may inhibit either MAPKs or NF- κ B, respectively, for example, Oroxylin A [ 36 ] and Baicalin [ 28 , 29 ] (both isolated from Scutellaria baicalensis ). Details can be found in Table 1 .
Details of basic researches included in the paper.
| Herbs | Material | Extracts | Asthmatic model | Route | Effective dose | Mechanism | |
|---|---|---|---|---|---|---|---|
| Category | Details | ||||||
| Root | Skullcapflavone II [ ] | OVA-induced Balb/c mice | Oral | 10, 30 mg/kg/d 7 d | TGF- 1/Smad | Reduced TGF- 1 in BLAF, elevated Smad7, and suppressed Smad2/3 expression | |
| Root | Baicalein [ ] | OVA-induced Balb/c mice | i.p. | 10 mg/kg/d 6 d | AAMP | Reduced 12/15-LOX activity | |
| NA | Oroxylin A [ , ] | OVA-induced Balb/c mice | Oral | 15, 30, and 60 mg/kg/d 3 d | NF- B | Suppressed NF- B activation | |
| Specific IgE induced rat RBL-2H3 mast cells | 10, 30 M 30 min | Mast cells | Inhibited degranulation of mast cells | ||||
| Root | Wogonin [ ] | OVA-induced Balb/c mice | Oral | 10, 30 mg/kg/d 3 d | STAT6 | Suppressed OVA-induced STAT6 activation | |
| IL-4 induced BEAS-2B cells | 10, 30, and 50 M 4 h | STAT6 | Suppressed IL-4-induced eotaxin-3 expression via suppressing JAK1 and STAT6 activation | ||||
| Root | Baicalin [ , ] | OVA-induced Balb/c mice | Oral | 25, 50, and 100 mg/kg/d 4 w | MAPK | Inhibited RASM cell proliferation and migration by suppressing MAPK signal pathway | |
| Airway smooth muscle cells from SD rats (RASM) | 10, 25, and 100 nM 1 h | ||||||
| NA | Aqueous extract [ ] | OVA-induced C57BL/6 mice | Oral | 3 g/kg/2 d 9 d | GATA3/T-bet | Decreased the ratio of the GATA3/T-bet mRNA levels | |
| Root | Astragaloside IV [ , – ] | OVA-induced Balb/c mice | Oral | 50, 150 mg/kg/d 4 w | GATA3/T-bet | Decreased the ratio of the GATA3/T-bet expression level | |
| OVA-induced Balb/c mice | Oral | 50, 150 mg/kg/d 4 w | — | Increased IFN- level | |||
| OVA-induced Balb/c mice | Oral | 20, 40 mg/kg/d 4 w | Foxp3/ROR t | Increased CD4+CD25+Foxp3+ Tregs and enhanced Foxp3 mRNA expression | |||
| OVA-induced Balb/c mice | Oral | 50 mg/kg/d 8 w | TGF- 1/Smad | (1) Reduced TGF- 1 expression | |||
| NF- B | (2) Inhibited TSLP expression | ||||||
| NA | Aqueous extract [ ] | OVA-induced SD rats | Oral | 5, 10 g/kg/d 4 w | Foxp3/ROR t | Increased CD4+CD25+Foxp3+ Tregs and enhanced Foxp3+ mRNA expression | |
| NA | Aqueous extract [ ] | OVA-induced C57BL/6 mice | i.p. | 10 g/kg/d 4 w | — | Increased Th1/Th2 cytokines' ratio | |
| NA | Formononetin & calycosin [ ] | OVA-induced Balb/c mice | Oral | 0.5 g/kg/2 d 4 w | NF- B | Suppressed NF- B activation | |
| — | Astragali-Cordyceps suspensions [ ] | OVA-induced C57BL/6 mice | Oral | 6.5 g/kg/d 4 w | TGF- 1/Smad | Reduced TGF- 1 and elevated Smad7 expression in lung tissue | |
| Root | Aqueous extract [ ] | OVA-induced C57BL/6 mice | i.p. | 20 mg/kg/d 3 d | MAPK | Inhibited CD40/CD40L ligation and MAPK signal pathway | |
| Leaves | Purified aqueous extract (RG-II) [ ] | OVA-induced Balb/c mice | i.p. | 20, 100 mg/kg/d 3 d | GATA3/T-bet | Decreased the ratio of the GATA3/T-bet expression level | |
| Root | Aqueous extract (CVT-E002) [ ] | OVA-induced Balb/c mice | Oral | 200 mg/kg/d 7 d | Foxp3/ROR t | Increased Tregs function and IL-10 level in BALF | |
| Root | Ginsan [ ] | OVA-induced Balb/c mice | i.p. | 100 mg/kg/2 d 4 w | AAMP | Upregulated COX-1 and COX-2 expression, leading to the increase of PGE2 in BALF | |
| Root | Saucerneol D [ ] | OVA-induced Balb/c mice | Oral | 20, 40 mg/kg/d 3 d | Antioxidant | Upregulated the expression of HO-1 | |
| Aerial parts | A subfraction of ethanol extract [ ] | RAW264.7 cells derived from BALB/c mice | 5, 50 g /mL 2 h | Antioxidant | Upregulated the expression of HO-1 | ||
| Aerial parts | Sauchinone [ , ] | RAW264.8 cells derived from BALB/c mice | 2.5, 5, and 10 g /mL 2 h | Antioxidant | Upregulated the expression of HO-1 | ||
| OVA-induced Balb/c mice | i.p. | 10, 100 mg/kg/2 d 5 d | GATA3/T-bet | Suppressed GATA-3 activity | |||
| Root | Meso-Dihydroguaiaretic acid [ ] | OVA-induced Balb/c mice | Oral | 10, 30 mg/kg 2 w | NF- B & MAPK | Inhibited Th2 inflammation via inhibiting NF- B and MAPK | |
| Aerial parts | 70% ethanol extract [ ] | Bone marrow-derived mast cells from Balb/c mice | 0.8–50 g /mL 30 min | AAMP | Inhibited LTC4 and PGD2 level | ||
| NA | 70% ethanol extract [ ] | OVA-induced Balb/c mice | Oral | 2, 10, and 20 g/kg/d 10 d | — | Elevated the ratio of Th1/Th2 | |
| AAMP | Decreased the cysLT1 mRNA levels | ||||||
| Aerial parts | Ethanol extract [ ] | OVA-induced Balb/c mice | Oral | 100 mg/kg/d 6 d | — | (1) Inhibit increases in IgE, IL-4, and IL-5 in BALF and lung tissue | |
| Antioxidant | (2) Reduced the levels of ROS in BALF | ||||||
| Fructus | Aqueous extract [ ] | OVA-induced Balb/c mice | Oral | 10 g/kg/d 4 w | — | Inhibited the upregulation of IL-4 and IL-13 levels in BALF | |
| Fructus | Psoralen [ ] | ConA stimulated D10.G4.1 cells | 0.08 mM 2 h | GATA3/T-bet | Suppressed the upregulation of IL-4, IL-5, IL-13, and GATA-3 protein expression | ||
| Fructus | Bavachinin [ ] | OVA-induced Balb/c mice | Oral | 50 mg/kg/d 7 d | — | Suppressed IL-4, IL-5, and IL-13 in lung tissue and serum levels of IL-4, IgE | |
| ConA, IL-2, IL-4 stimulated 4GET mice spleen cells | 0.01 mM | GATA3/T-bet | Suppressed GATA-3 mRNA levels | ||||
| NA | Astilbic acid [ ] | OVA-induced Balb/c mice | Oral | 30 mg/kg/d 3 d | NF- B | Suppressed NF- B activation | |
| Flower | Crocetin [ ] | OVA-induced C57BL/6 mice | Intranasal | 3 g /d 1 w | Foxp3/ROR t | Increased Foxp3 through TIPE2 to activate Tregs | |
| Flower | Crocin [ ] | OVA-induced Balb/c mice | Oral | 100 mg/kg/d 5 d | MAPK | Inhibited the expression of lung eotaxin, p-ERK, p-JNK, and p-p38 level | |
| Flower | Safranal [ ] (A review) | — | — | — | Relaxing ASM | Antihistamine and anticholinergic and 2-adrenoreceptors stimulation and Ca2+ signaling blocking | |
| NA | Dihydroartemisinin [ ] | OVA-induced Balb/c mice | Oral | 30 mg/kg/d 3 d | NF- B & MAPK | Inhibited Th2 inflammation via inhibiting NF- B and MAPK | |
| NA | Artesunate [ ] | OVA-induced Balb/c mice | i.p. | 30 mg/kg/d | Antioxidant | Suppressed prooxidants and restoring expression of antioxidants via activation of Nrf-2 | |
| Leaves | Purified aqueous extract (AIP1) [ ] | OVA-induced Balb/c mice | i.p. | 5 mg/kg 6 times in 14 d | Dendritic cell | Reduced levels of MHC II in dendritic cells | |
| NA | Ligustrazine [ ] | OVA-induced C57BL/6 mice | i.p. | 80 mg/kg/d 3 d | GATA3/T-bet | (1) Decreased the ratio of the GATA3/T-bet expression level | |
| Foxp3/ROR t | (2) Increased the ratio of Foxp3/ROR t | ||||||
| Whole | Aqueous extract [ ] | OVA-induced Balb/c mice | NA | 0.5, 1 g/kg/d 7 d | Foxp3/ROR t | Inhibited the decrease of Tregs in BALF | |
| NA | 80% ethanol extract [ ] | OVA-induced Balb/c mice | Oral | 500 mg/kg/d 4 w | — | (1) Inhibit increases in OVA-specific IgE and IL-5 in BALF | |
| Antioxidant | (2) Reduced the levels of ROS in BALF | ||||||
| Leaves | Bakkenolide B [ ] | Rat RBL-2H3 mast cells & C57BL/6 mouse peritoneal macrophages | 1–10 g /mL 1 h | Mast cells | Inhibited degranulation in mast cells and suppressed iNOS in macrophages | ||
| Leaves | Petatewalide B [ ] | 10, 30 g /mL 1 h | |||||
| Fructus | Osthol [ ] | IL-4/TNF- induced BEAS-2B cells | 1–10 M 2 h | STAT6 | Suppressed IL-4-induced eotaxin expression via suppressing STAT6 activation | ||
| Leaves | Fargesin and epimagnolin [ ] | A549 human alveolar epithelial cells | 3.1–100 g /mL | MAPK | Modulated NO synthesis via inhibiting ERK in human respiratory epithelial cells | ||
| NA | Esculentoside A (EsA) [ ] | OVA-induced Balb/c mice | i.p. | 15 mg/kg/d 4 d | Antioxidant | Reduced the levels of ROS in BALF | |
| A549 human alveolar epithelial cells | 10, 20 mg/L 6 h | Antioxidant | Nrf-2 activator. Upregulated the expression of HO-1 | ||||
| Oil | Diallyl-disulfide (DADS) [ ] | OVA-induced Balb/c mice | Oral | 30 mg/kg/d 3 d | Antioxidant | (1) Reduced the levels of ROS in BALF | |
| NF- B | (2) Suppressed NF- B activation | ||||||
| RAW264.7 murine macrophage cell | 62.5–500 ng/mL 1 h | Antioxidant | Nrf-2 activator. Upregulated the expression of HO-1 | ||||
| NA | Trifolirhizin [ ] | Tracheal rings of OVA-induced Balb/c mice | 6 g /mL | Relaxing ASM | Inhibiting acetylcholine mediated ASM contraction | ||
| Root | 70% methanol extract [ ] | Lung slices of Balb/c mice | 0.3, 1 mg/mL | Relaxing ASM | Inhibiting acetylcholine mediated ASM contraction via blocking Ca2+ channels | ||
| Root | 7-Oxo-sandaracopimaric acid [ ] | OVA-induced guinea pigs | Oral | 25–100 mg/kg 3 in 24 h | AAMP | Inhibiting phospholipase A2 (PLA2) eosinophil peroxidase (EPO) activity in BALF | |
| Lam. | Seed | -Sitosterol [ ] | OVA-induced guinea pigs | Oral | 2.5 mg/kg/d 12 d | — | (1) Decreased the levels of TNF- , IL-4, and IL-5 in BALF and serum |
| Antihistamine | (2) Antihistamine | ||||||
| — | Aqueous extract [ ] | OVA-induced Balb/c mice | Oral | 100, 200 mg/kg/d 6 d | Antioxidant | Upregulated the expression of HO-1 | |
| NA | Eucalyptol (1.8-cineole) [ ] | Monocytes from patients with asthma | 200 mg/d 3 d | AAMP | Inhibit LTB4 and PGE2 | ||
i.p. = intraperitoneal injection. AAMP = arachidonic acid metabolism pathway. TIPE2 = TNF- α -induced protein 8-like 2. NA = not available.
2.3. Targeting the Treg/Th17 Cells
T-regulatory cells (Tregs) are a heterogeneous group of cells that play a central role in maintaining the homeostasis of pulmonary immunity by establishing immune tolerance to nonharmful antigens or suppressing effector T cell immunity. The specification of Treg subset is driven by transcription factor forkhead box P3 (Foxp3) [ 5 , 75 – 78 ]. Th17 cells are key players in chronic lung inflammation, including asthma. Steroid-resistant asthma and neutrophil-mediated asthma have been proved to be related to Th17 cells. IL-17 also directly affects the airway smooth muscle by inducing allergen-induced airway hyperresponsiveness [ 79 – 82 ]. ROR γ t is found to be the transcription factor related to Th17, which is required to activate IL-17 production in Th17 cells [ 5 ]. Increased expressions of IL-17A and IL-17F have been shown in lung tissue of asthma patients [ 6 ]. Thus, herbal treatments targeting Foxp3 and ROR γ t have been revealed gradually.
Extracts from Astragalus [ 46 , 48 ], Panax ginseng [ 52 ], Crocus sativus [ 60 ], Ligustrazine [ 9 ], and Anoectochilus formosanus [ 64 ] were observed increasing Tregs and enhancing Foxp3 mRNA expression. In particular, ligustrazine, isolated from Ligustrazine ( Chuan Qiong ), was reported modulating the expression of not only T-bet/Gata-3 but also Foxp3/ROR γ t [ 9 ]. Ji et al. [ 9 ] noticed that ligustrazine reduced not only eosinophils but neutrophils in the BALF of asthmatic mouse model, implying that it could have potential for use in alleviation of neutrophilic asthma.
Besides, there were more studies about herbal treatments in other Th17-related inflammatory diseases, such as Andrographis paniculata , Scutellaria baicalensis , Tripterygium wilfordii, and Wedelia chinensis [ 33 , 83 – 85 ]. However, whether these herbs work similarly in asthma needs further investigations.
2.4. Effect on Lung Dendritic Cells (DCs)
Dendritic Cells (DCs) participated not only in the differentiation of T helper cells but also in IL-12 production and CD8+ T cell stimulation via antigen uptake. Two subsets of blood DCs, that is, myeloid and plasmacytoid DCs, were identified based on the expression of CD11c [ 5 ]. Most CD11c+ myeloid DCs in the lung are immature, which express relatively low levels of major histocompatibility complex (MHC) class II, and have a high capacity of antigen uptake but poor T cell stimulating activity [ 5 ]. Thus, inhibiting functional differentiation of pulmonary immature DC to mature DC may be a strategy to restrict the activation of T cells.
Lee et al. [ 63 ] discussed the effect of Artemisia iwayomogi polysaccharide-1 (AIP1) on DC functions. They observed significantly reduced levels of MHC II in DCs of the AIP1 treated group, suggesting that AIP1 could reduce the expression of MHC II molecules on pulmonary DC. They also reported that AIP1 diminished the allergenic T cell stimulating ability of DCs derived from bone marrow in another study. These data suggested that AIP1 could inhibit functional differentiation of pulmonary DCs in vivo .
2.5. Effect on Mast Cell Degranulation
Mast cell degranulation, which can be triggered by antigen-mediated cross-linking of IgE bound to Fc ε R1 surface receptors or changes in the surrounding local tissue environment, plays an important role in asthmatic response. As a result, many of the mediators that are stored or newly synthesized by the mast cells are released attracting leukocytes (eosinophils, basophils, Th2 lymphocytes, and neutrophils) to the inflammatory site and amplify the inflammatory response [ 86 ]. Hence, inhibiting mast cell degranulation will be helpful for asthma treating.
We gathered three extracts that associated with this process: Oroxylin A [ 43 ], Bakkenolide B [ 66 ], and Petatewalide B [ 67 ]. The first one is isolated from Scutellaria baicalensis while the next two are from Petasites japonicus . It is worth mentioning that both Bakkenolide B and Petatewalide B do not inhibit antigen-induced Ca2+ increases in mast cells, which suggested that Bakkenolide B/Petatewalide B induced inhibition of degranulation seems not to be mediated via the inhibition of Ca2+ channel or Ca2+ increase in mast cells. As for Oroxylin A, no detailed mechanisms were provided to expand the phenomenon of inhibiting mast cell degranulation. Further mechanistic investigations on these extracts are necessary.
2.6. Effect on Oxidative Stress
Oxidative stress plays an important role in the pathogenesis of most airway diseases, particularly when inflammation is prominent. Recently, heme oxygenase-1 (HO-1) was shown to be induced in the airways of patients with asthma. As a natural antioxidant defense, HO-1 exerts cytoprotective reactions against oxidative cell injury. Greater HO-1 expression may mitigate asthma symptoms and suppressed IL-13-induced goblet cell hyperplasia and MUC5AC production [ 87 – 89 ]. Hence, targeting on HO-1 or its transcription factor nuclear factor E2-related factor 2 (Nrf-2) [ 90 ] is a considerable strategy for asthma control.
To date, a variety of HO-1 activator can be extracted from Saururus chinensis [ 54 , 55 ], Phytolacca esculenta [ 68 ], Garlic [ 40 ], and soshiho-tang [ 73 ]. Among them, diallyl-disulfide (isolated from Garlic ) [ 40 ] and esculentoside A (isolated from Phytolacca esculenta ) [ 68 ] have been further proved as Nrf-2 activators. Beyond these, artesunate from Artemisia annua was also observed suppressing prooxidants and restoring expression of antioxidants via activation of Nrf-2 [ 62 ].
There are also some studies that only showed a reduced level of oxidative stress marker reactive oxygen species (ROS) when extracts like ethanol extracts of Mentha [ 59 ] and Petasites japonicus [ 65 ] intervened. The mechanisms by which they reduce ROS level need further study.
2.7. Effect on Relaxing Airway Smooth Muscle
Airway contraction is an important feature of asthma, and recent strategies to relax airway smooth muscle include antihistamine and anticholinergic and β 2-adrenoreceptors stimulation and Ca2+ signaling blocking [ 61 ].
Mokhtari-Zaer et al. [ 61 ] summarized the Crocus sativus 's (saffron's) effect on relaxing airway smooth muscle in a review. Aqueous-ethanolic extract of Crocus sativus and safranal were mentioned in their article and showed multiple effects including antihistamine, anticholinergic, and β 2-adrenoreceptors stimulation according to four published studies.
Yang et al. [ 69 ] identified that trifolirhizin, a flavonoid compound isolated from Sophora flavescens , was responsible for inhibiting acetylcholine induced airway smooth muscle (ASM) contraction independent of β 2-adrenoceptors.
Aqueous methanolic extract from Zingiber officinale (ginger) was also reported having effect on acetylcholine induced airway contraction. Ghayur et al. [ 70 ] indicated that its effects were associated with Ca2+ signaling, possibly via blocking Ca2+ channels on plasma membrane.
2.8. Effect on Airway Remodeling
It is believed that airway smooth muscle (ASM) cell proliferation and migration play important roles in airway remodeling. Both platelet-derived growth factor (PDGF) and transforming growth factor- β (TGF- β ) are reported to be related to airway remodeling [ 91 ]. Recently, TGF- β 1/Smad signal pathway was found to be one of the important mechanisms for signal conduction in asthma airway remodeling [ 92 ].
Astragaloside IV and Skullcapflavone II, extracts from Astragalus and Scutellaria baicalensis, respectively, were reported to attenuate the allergen-induced airway remodeling in mice, likely through inhibition of TGF- β 1 [ 37 , 41 ]. Further research was performed by Jang et al. [ 41 ] and indicated that Skullcapflavone II elevated Smad7 and suppressed Smad2/3 expression, which was responsible for TGF- β 1 inhibition. As for Astragalus, our group researched several formulas using Astragalus as key component acting on TGF- β 1/Smad signal pathway. The one Astragali-Cordyceps Mixtura [ 50 ] decreased TGF- β 1 expression and recovered Smad7 protein expression, and the other one Astragali radix Antiasthmatic Decoction (AAD) [ 93 ] was also found to improve the symptoms of allergic airway remodeling through inhibition of Th2 cytokines and TGF- β 1. In a recent study, we also noticed that Suhuang antitussive capsule, a traditional Chinese medication, significantly attenuated the allergen-induced AHR, inflammation, and remodeling in mice, likely through inhibition of IL-13 and TGF- β 1 [ 94 ].
2.9. Effect on the Arachidonic Acid Metabolism Pathway (AAMP)
Arachidonic acid (AA) from the diet or after synthesis is stored in membrane phospholipids and is liberated under appropriate stimulatory conditions by the enzyme phospholipase A2 (PLA2). Arachidonic acid is then metabolized by three main classes of enzymes (cyclooxygenases (COX), lipoxygenases (LOX), and p450 epoxygenases) and all products of these three pathways like prostaglandin E2 (PGE2), prostaglandin D2 (PGD2), leukotrienes (LTs), and so forth are related to inflammatory and anaphylactic reaction. To be specific, PGE2 has been thought of as a potent proinflammatory mediator and has many beneficial functions in the lung tissues, such as the inhibition of inflammatory cell recruitment, reduction of leukotrienes and PGD2, and decrease of Th2 differentiation, thereby modulating inflammation and tissue repair. LTs (LTB4, LTC4, LTD4, etc.) are also thought to be important mediators of airway inflammation and airway obstruction in asthma. LTB4 can act as a neutrophil chemoattractant [ 53 , 95 , 96 ]. Thus, strategies targeting AA metabolism are effective in many inflammatory diseases.
Extracts from Scutellaria baicalensis [ 42 ], Panax ginseng [ 53 ], Saururus chinensis [ 57 ], Sceptridium ternatum [ 58 ], Aralia cordata [ 71 ], and eucalyptol (1.8-cineole) [ 74 ] have been found targeting different steps of the arachidonic acid metabolism pathway (AAMP). Details can be checked in Table 1 .
Particularly, there are 4 herbs we would like to review individually as follows for their multiple function of antiasthma and popularity in basic researches.
Scutellaria baicalensis is a multifunctional traditional herb. Its extracts include Skullcapflavone II, Baicalein, Oroxylin A, wogonin, and Baicalin, which showed different but maybe cooperative functions in treating asthma. All of them have been proved in animal experiment or in vitro but not in human as mentioned before. And their results only showed simplex function on some cytokines or genes related to asthma. Whether they can benefit the whole interaction when asthma occurred is still unclear. For example, Jang et al.'s experiment [ 41 ] indicated Skullcapflavone II's function on TGF- β 1/Smad signaling pathways. They observed a decreased level of TGF- β 1 in BALF, elevated Smad7 expression, and suppressed Smad2/3. However, as a pleiotropic and multifunctional growth factor, TGF- β 1 also exerts immunosuppressive effects on asthma progression, and therapies targeting on TGF- β 1 are still controversial, although it has been expatiated that TGF- β 1 is responsible for airway remodeling [ 97 ].
Another multifunctional herb with relative sufficient studies is Astragalus membranaceus. We have showed above that its extract has been reported acting on GATA3/T-bet and TGF- β 1/Smad and NF- κ B signal pathway and Tregs within last few years. Among them, Astragaloside IV should be underlined here for the fact that it nearly performed all effects that Astragalus membranaceus possessed for treating asthma. However, it is a pity that although abundant studies were performed on Astragaloside IV, no clinical research on it is available to date.
Extracts (RG-II, CVT-E002, and ginsan) from Panax were also reported acting on different pathways involving GATA3/T-bet, MAPK, and Tregs and arachidonic acid metabolism pathway in animal models or in vitro . But whether these results can be applied to human is undetermined. It is worth mentioning that CVT-E002 has been well proved to reduce respiratory infection in patients with chronic lymphocytic leukemia and prevent acute respiratory illness in institutionalized older adults [ 98 , 99 ]. It may partly reflect its immunoregulation function on human. Researches on its efficacy on asthma patients are desired.
Saururus chinensis (SC) is an effective antioxidant. Three of its extracts (saucerneol D [ 54 ], a subfraction of its ethanol extract, and sauchinone [ 55 ]) showed similar effect of antioxidant through upregulating the expression of HO-1. Among them sauchinone also suppressed GATA-3 activity [ 56 ]. Recently, a novel extract of SC named meso-Dihydroguaiaretic acid was expounded by Song and his colleagues [ 31 ]. It exhibited a protective effect on allergic airway inflammation through inhibiting the Th2 inflammation attributed to its inhibition of the NF- κ B and MAPK. Besides, its ethanol extract's action on arachidonic acid metabolism pathway was reported in this year [ 57 ]. Antiasthmatic effect for SC extracts is drawing wide attention in the last few years.
3. Clinical Studies
The clinical research of herbal therapy application in asthma is limited. Three meta-analysis or systematic reviews were published successively in 2007, 2008, and 2010 [ 100 – 102 ]. However, all of them could not get sufficient evidence to make recommendations for herbal treatment for asthma after comprehensive analysis of the efficacy and safety. Arnold et al. [ 100 ] evaluated effects of herbal medicines on lung function, reduction in use of corticosteroids, symptom scores, physical sign scores, use of reliever medications, health related quality of life, and adverse effects comparing with placebo, involving 21 different herbs or herbal formulas. Although a few of them had some effects on relief of symptoms, only boswellic acids (isolated from Boswellia ) were reported to exert a relatively comprehensive effect on lung function, while the effects of other herbs were limited or inexact. In Clark et al.'s study [ 102 ], Mai-Men-Dong-Tang, Pycnogenol, Jia-Wei-Si-Jun-Zi-Tang, and Tylophora indica also showed potential to improve lung function. Moreover, 1.8-cineol (eucalyptol) was observed to reduce the use of corticosteroids and corticosteroid reduction tolerance (<7.5 mg) in both of their studies [ 100 , 102 ].
In the last five years, some new clinical trials on herbal treatment emerged but most of them still focus on the efficacy for patients using an intact traditional or modified formula. The application of monomer extracts in clinic usually acts as adjuvant of standard asthma therapy according to the recent studies. In a noncomparative, multicenter trial [ 103 ], 148 patients with mild asthma taking ICS received NDC-052 (an extract from Magnoliae flos ) for eight weeks. Their results showed that add-on NDC-052 besides ICS therapy had benefits in both ΔPEFR and asthma symptoms. Last year, a review by Ammon [ 104 ] showed multiple effects of Boswellia serrata extracts on immune system modulation in basic research, including inhibiting activation of NF κ B, mast cell stabilisation, and antioxidant and inhibitory action on 5-LOX. However, related clinical research in the past five years can only be found for reducing the need for inhalation therapy with ICS + LABAs [ 105 ]. Although its significant effect on lung function had been analyzed by Arnold et al. as mentioned before [ 100 ], further research is still lacking.
Regarding intact traditional or modified formula for asthma reported recently, there are three types of research interests in the past five years.
First, herbal formula is singly used to relieve asthmatic symptom. These studies paid attention to the syndrome scores and frequency of asthma acute attack although these formulas may have limited efficacy on asthma process or lung function improvement. Geng et al. [ 106 ] performed a randomized, single-blind, placebo controlled trial (sample size = 60) on intermittent asthmatic children aged 2 to 5 years. Their modified formula contained Radix Astragali Mongolici 10 g, Rhizoma Polygonati Odorati 10 g, Fructus Ligustri Lucidi 10 g, Fructus Psoraleae 10 g, Radix Pseudostellariae 3 g, Fructus Schisandrae Chinensis 3 g, Fructus Jujubae 10 g, Concha Ostreae 10 g, and Endoconcha Sepiellae 10 g. Their results showed that the formula reduced the number of intermittent asthma attacks, decreased the syndrome scores, and reduced the airway resistance in the children. Homogeneously, another formula, “Zhisou Powder,” is also observed to decrease the syndrome score and cough score of cough variant asthma, but it has no effect on airway responsiveness [ 107 ].
Second, maybe the most popular direction is usage of herbal formula as add-on therapy of standard medication to relieve asthmatic symptom and recurrence rate or strengthen the effect of standard medication. A randomized controlled research on 143 patients with moderate-severe asthma was performed by Tang and colleagues [ 108 ]. They applied their formula (containing 21 herbs and excipients) as add-on therapy of standard medication and showed decrease of exacerbation frequency and improvement of related syndrome scores, for example, asthma control test (ACT) score. Lin et al. [ 109 ] studied the effect of Astragalus plus hormone treatment in 90 asthmatic children. They showed that the effective rate of the Astragalus plus hormone group is significantly higher compared to using Astragalus or hormone only. The levels of PEFR and IFN- γ significantly increased and IL-4 obviously decreased in their effective cases. Besides, in another study, benefit of “chaipo granule” combined with routine treatment on refractory asthma was also discovered. “Chaipo granule” can act as a synergist of routine treatment according to their results [ 110 ]. Similar strengthening effect was also reported in the formula “Yupingfeng powder” [ 111 ].
Third, study in this research interests is according to a traditional administration method in China. Chinese called it “Jiu” (moxibustion), which uses herbs burning at the related acupoint of patients. Peoples today modified the method and are using percutaneous absorption herbal patch to treat asthma. Typically, Chen et al. [ 112 ] compared their modified percutaneous absorption herbal patch with salmeterol fluticasone inhalation for asthma of paracmasis. Their results showed a significant improvement in clinical symptom scores.
4. Conclusion Remarks
After all the above, it can be noticed that herbal therapy mainly applied to mild asthma or acted as adjuvant of standard asthma therapy in clinical researches. It seems to become a trend. It might be a good way to use complementary and alternative medicine (CAM) to help control symptom and reduce the drug dose for those patients receiving standard medication, as it has been proved by several researches [ 100 – 105 , 108 ]. However, compared with the prosperous basic researches, clinical researches on asthma herbal therapy are relatively poor. It may be because many herbal extracts targeting on one or two factors of asthma may not be that meaningful when applied in clinic, as asthma is a disease with complex and multiple mechanisms. On the other hand, as the herbs were usually administrated as formula, simultaneously using extracts from different herbs with different mechanisms can also be a good research direction. It can be expected that they act synergetically and perform significant benefit on asthma when used simultaneously. Administrating purified extracts synergetically will have more precision than using intact formula directly.
Despite the remarkable achievements about herbal treatment for asthma during the past several years, several points should be addressed or be kept in mind in future studies. (1) Quality control of herbal medicines is always problematic, due to lack of standard procedures to make herb extractions, decoctions, or formula. Herbal patent drugs are generally of better quality, but there are few ones available for asthma researches. (2) Apparently, there is an urgent need for translational studies to transfer the current achievements on herbal medicines from animal works to clinical trials and finally to develop new clinical therapy. Current clinical trials for herbal medicines are limited, and thus more and large scaled, multicentre ones are extremely needed. Fortunately, well-designed and well-performed clinical trials for herbal medicines were appreciated by world-class journals [ 113 ], encouraging similar studies in asthma. (3) For clinical studies, the usage of herbal medicines for stable asthma is recommended. Asthma research in animal models usually cannot mimic the distinction between stable asthma and exacerbation. Herbal medicines might help to control stable asthma, while they may have limited effects during asthma exacerbations, since the herbs generally take longer time to exert therapeutic effects. (4) Clinical studies of herbal medicines in asthma control should target two major different goals. One is to take herbal medicines as a sole asthma control strategy, and the other is to use them as an add-on therapy for standard strategies, that is, to enhance the efficacy of or to reduce the usage of ICS. (5) Most of the current researches focused only on the eosinophilic phenotype of asthma, and few researches addressed other phenotypes like neutrophilic asthma, as the latter is usually severe asthma and is hard to control by current approaches. Thus, it is encouraged to conduct studies, both basic and clinical, to investigate the possible roles of herbal medicines in control of severe or neutrophilic asthma.
Nevertheless, herbal medicines for asthma hold out a cheerful prospect, which will eventually help to reduce the morbidity and mortality and increase the control levels of asthma worldwide.
Acknowledgments
This work was supported by the Key Project to Hua-Hao Shen (81130001) and the General Project to Zhi-Hua Chen (81370142) from NSFC, the Key Science-Technology Innovation Team of Zhejiang Province (2011R50016), and the program for a Key Site of the National Clinical Research Center for Respiratory Disease, Hangzhou, Zhejiang 310058, China.
Competing Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.

IMAGES
VIDEO
COMMENTS
Results/Discussion. Bronchial asthma is a chronic inflammatory disease of the airways characterized by bronchial hyperreactivity and a variable degree of airway obstruction. It is diagnosed on the basis of the clinical history, physical examination, and pulmonary function tests, including reversibility testing and measurement of bronchial ...
To The Editor, The Global Initiative for Asthma (GINA) 2021 update was published on the 28th of April, 2021. [1]. There are significant changes, including treatment of mild asthma, the role of ...
Background. Asthma, a major global health problem affecting as many as 235 million people worldwide [], is a common, non-communicable, and variable chronic disease that can result in episodic or persistent respiratory symptoms (e.g. shortness of breath, wheezing, chest tightness, cough) and airflow limitation, the latter being due to bronchoconstriction, airway wall thickening, and increased ...
Bronchial asthma is heterogeneous pulmonary disorder characterized by recurrent episodes of cough, breathlessness and wheezing, which may resolve spontaneously or after the use of bronchodilator medication 1.The global prevalence of asthma is anticipated to be approximately 4.5 per cent 2,3.There are about 334 million patients with asthma affecting all age groups, across the world 4.
Abstract. Asthma is the most common, long-lasting inflammatory airway disease that affects more than 10% of the world population. It is characterized by bronchial narrowing, airway hyperresponsiveness, vasodilatation, airway edema, and stimulation of sensory nerve endings that lead to recurring events of breathlessness, wheezing, chest tightness, and coughing.
Abstract. In this review, we discuss recent publications on asthma and review the studies that have reported on the different aspects of the prevalence, risk factors and prevention, mechanisms, diagnosis, and treatment of asthma. Many risk and protective factors and molecular mechanisms are involved in the development of asthma.
Asthma is one of the most common chronic non-communicable diseases worldwide and is characterised by variable airflow obstruction, causing dyspnoea and wheezing. Highly effective therapies are available; asthma morbidity and mortality have vastly improved in the past 15 years, and most patients can attain good asthma control. However, undertreatment is still common, and improving patient and ...
42. monophonic expiratory wheeze 43 percent. We conclude that: (1) a history of wheeze, a prior clinical diagnosis of asthma, and expiratory wheezing on physical examination are much less reliable than MIC in predicting the presence or absence of asthma; (2) these parameters cannot be used as reliable epidemiologic markers for asthma; and (3 ...
Semantic Scholar extracted view of "BRONCHIAL ASTHMA: A REVIEW OF SYMPTOMS AND EMERGENCY CONDUCTS" by Eduardo de Pádua Scarpellini et al. ... Search 219,326,018 papers from all fields of science. Search. Sign In Create Free Account. DOI: 10.22533/at.ed.1594532406062; ... AI-powered research tool for scientific literature, based at the Allen ...
Our 60-year follow-up study of adults with a history of severe childhood asthma revealed that nine out of ten still had current asthma. Persistent asthma was associated with lower lung function and higher levels of type 2 inflammatory biomarkers compared to those with asthma remission. ... bronchial provocation, and bronchodilator reversibility ...
Abstract. Importance: Asthma affects about 7.5% of the adult population. Evidence-based diagnosis, monitoring, and treatment can improve functioning and quality of life in adult patients with asthma. Observations: Asthma is a heterogeneous clinical syndrome primarily affecting the lower respiratory tract, characterized by episodic or persistent ...
The prevalence of asthma in adults in the United States is approximately 7.7%. 1 It is one of the most common chronic, noncommunicable diseases in the country and worldwide. 1,2 Among U.S. adults ...
The FASEB Journal publishes multidisciplinary basic and translational research covering biology and biomedical sciences at every level of organization. Abstract Asthma is a chronic pulmonary disease with the worldwide prevalence. The structural alterations of airway walls, termed as "airway remodeling", are documented as the core ...
Around 5-10% of the total asthmatic population suffer from severe or uncontrolled asthma, which is associated with increased mortality and hospitalization, increased health care burden and worse quality of life. In the last few years, new drugs have been launched and several asthma phenotypes according to definite biomarkers have been identified. In particular, therapy with biologics has ...
Results: Of the 45 papers identified, 44 investigated cohorts and one was a clinical trial with follow-up. Asthma, wheezing, BHR and allergic sensitization early in life and to multiple allergens were associated with a lower lung function growth of large and small airways during early childhood compared with the control populations.
(1) Background: Asthma is a syndrome found in both adults and children, characterized by airflow obstruction caused by the inflammation of the airways. In recent years, an increasing number of studies have found that lipid metabolism influences both the development and symptomatology of asthma. Lipid metabolism plays an important role both in the occurrence of exacerbations and in the ...
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... Bronchial asthma and allergic ...
Various types of asthma have been reported, such as allergic, non-allergic, late-onset, and asthma with persistent airflow limitation [1]. Globally, the prevalence ranges from 1 to 18%,and it renders an immense economic burden on society [1, 2]. According to WHO, 262 million people were affected by asthma in 2019, with a death estimate of 4,55,000.
Asthma is a common chronic disease characterized by episodic or persistent respiratory symptoms and airflow limitation. Asthma treatment is based on a stepwise and control-based approach that involves an iterative cycle of assessment, adjustment of the treatment and review of the response aimed to minimize symptom burden and risk of exacerbations. Anti-inflammatory treatment is the mainstay of ...
Introduction. Asthma is the most common chronic respiratory disease affecting millions of people of all ages across the globe (Citation 1-6).The average global prevalence ranges between 5-10% (Citation 2).Traditionally, asthma diagnosis was based on the history and the response to a trial of various treatments, but emerging evidence shows that under the umbrella of asthma, several subtypes ...
Background. Asthma remains the most common chronic respiratory disease in Canada, affecting approximately 10% of the population [].It is also the most common chronic disease of childhood [].Although asthma is often believed to be a disorder localized to the lungs, current evidence indicates that it may represent a component of systemic airway disease involving the entire respiratory tract, and ...
Inhaled corticosteroids (ICS, eg, beclometasone dipropionate, budesonide, fluticasone furoate, etc) and long acting β agonists (LABA, eg, formoterol, salmeterol, vilanterol, etc) are the mainstay of treatment for poorly controlled or moderate asthma ( table 1 ). ICS suppress airway inflammation leading to reduced bronchial hyperresponsiveness.
100. Prospective Study of Bronchial Asthma. Abstract. Bronchial asthma is an atopic disease characterized by chronic airway inflammation and hyper-responsiveness. Severe. acute asthma is a medical ...
1. Introduction. Asthma is a chronic inflammatory disorder associated with variable airflow obstruction and bronchial hyper-responsiveness; presenting with recurrent episodes of wheeze, cough, shortness of breath, and chest tightness [1].Asthma has been characterized by increased responsiveness of the trachea-bronchial tree to a multiplicity of stimuli [2], increased infiltration of various ...
We have summarized the evidence on asthma trends, environmental determinants, and long-term impacts while comparing these epidemiological features across childhood asthma and adult asthma. While asthma incidence and prevalence are higher in children, morbidity, and mortality are higher in adults. Childhood asthma is more common in boys while ...
This paper is mainly paying attention on the Bronchial Asthma (BA), It is a general continual provocative illness of the airways of lungs characterized by variables and persistent symptoms ...
2. Basic Researches. Basic researches on herbal asthma therapy can be summarized into nine aspects according to the mechanism summarized below. Those researches that only reported some T helper 1 (Th1) cell or T helper 2 (Th2) cell cytokines, for example, Interleukin-4 (IL-4), IL-5, IL-13, and interferon-γ (IFN-γ), altered after herbs intervention without any further mechanistic studies will ...