An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.


StatPearls [Internet].
Emily Buck ; Nancy A. Finnigan .
Affiliations
Last Update: July 31, 2023 .
- Continuing Education Activity
Malaria is a parasitic infection transmitted by the Anopheles mosquito that leads to acute life-threatening disease and poses a significant global health threat. Two billion people risk contracting malaria annually, including those in 90 endemic countries and 125 million travelers. The Plasmodium parasite has a multistage lifecycle, which leads to characteristic cyclical fevers. With timely treatment, most people experience rapid resolution of symptoms; however, significant complications may occur, including cerebral malaria, severe malarial anemia, coma, or death. This activity reviews the epidemiology, presentation, and complications of Plasmodium malaria and the role of the interprofessional team in evaluating and managing patients with this life-threatening infection.
- Review the epidemiology of malaria infection.
- Describe the pathophysiology of malaria infection.
- Summarize the pharmacologic treatment strategies for malaria infection.
- Outline the importance of collaboration amongst an interprofessional team to improve outcomes for patients receiving malaria treatment.
- Introduction
Malaria is a parasitic infection transmitted by the Anopheles mosquito that leads to acute life-threatening disease and poses a significant global health threat. Two billion people risk contracting malaria annually, including those in 90 endemic countries and 125 million travelers, and 1.5 to 2.7 million people die in a year. [1] The Plasmodium parasite has a multistage lifecycle, which leads to characteristic cyclical fevers. With timely treatment, most people experience rapid resolution of symptoms; however, significant complications may occur, including cerebral malaria, severe malarial anemia, coma, or death. Preferred antimalarial therapeutic and chemoprophylactic regimens get dictated by species, geography, susceptibility, and patient demographics. Latent or reactivating infections may be reported years following exposure.
The incubation period, and therefore time to symptom development, varies by species: 8 to 11 days for P. falciparum , 8 to 17 days for P. vivax , 10 to 17 days for P. ovale , 18 to 40 days for P. malariae (though possibly up to several years), and 9 to 12 days for P. knowlesi . [1] The periodicity of the Plasmodium lifecycle creates the classic "malarial paroxysm" of rigors, followed by several hours of fever, followed by diaphoresis, and a drop to normal body temperature ( P. vivax infection establishes a 48-hour cycle), though this is less commonly seen today due to rapid identification and treatment. [1]
- Epidemiology
Forty percent of the global population resides in or visits malaria-endemic regions annually. [1] P. falciparum is present in Western and sub-Saharan Africa and displays the highest morbidity and mortality of the Plasmodia species. [2] P. vivax is present in South Asia, the Western Pacific, and Central America. [2] P. ovale and P. malariae are present in Sub-Saharan Africa. [2] P. knowlesi is present in Southeast Asia. [2] As many as 500 million malaria cases occur annually, with 1.5 to 2.7 million deaths. [1] Ninety percent of fatalities occur in Africa. [1] Those at highest risk include children under age 5, pregnant women, and disease naïve populations, including refugees in Central and Eastern Africa, nonimmune civilian and military travelers, and immigrants returning to their place of origin. [2]
Of the 125 million travelers who visit endemic locations each year, 10000 to 30000 develop malaria, and 1% of these will die from complications of their disease. [2] [3] Rising average global temperatures and changes in weather patterns are projected to expand the burden of malaria; a rise of 3 degrees Celsius is postulated to increase malaria incidence by 50 to 80 million. [1]
- Pathophysiology
Five Plasmodium species possess the ability to infect humans: P. falciparum, P. ovale, P. vivax, P. malariae , and P. knowlesi . [2] The female Anopheles mosquito ingests gametes during a blood meal, which form sporozoites that replicate in the gut. [1] During subsequent bloodmeals, saliva containing sporozoites gets released into a human host's bloodstream. [1] Within 60 minutes, sporozoites reach the liver, invade hepatocytes, and then rapidly divide, forming merozoites. In an active infection, organisms reenter the bloodstream and invade erythrocytes. [1] [4] Within erythrocytes, Plasmodia consume hemoglobin and develop from immature trophozoites (ring stage) to either mature trophozoites or gametocytes (CDC Malaria 2019). Mature trophozoites replicate, forming schizonts, disrupting erythrocyte cell membrane integrity, and leading to capillary endothelial adherence and cell lysis. [1]
Free heme is released into the peripheral blood, which stimulates endothelial activation. [5] [6] Untreated malaria lasts 2 to 24 months. [1] P. vivax and P. ovale infections may display "dormant schizogony," where inactive intrahepatic parasites (hypnozoites) remain until reactivation months to years in the future. [1] Although hypnozoite parasites do not routinely develop in the liver in the setting of P. falciparum and P. malariae infection, there are few reports of resurgent P. falciparum infection years after initial exposure. [7]
Pathogenesis stems from toxin-induced IFN-gamma and TNF-alpha secretion. [8] The innate immune response is dominated by monocyte and macrophage phagocytosis within the splenic red pulp. Adaptive immunity develops by IFN-gamma and TNF-alpha-induced class switching of CD4-positive lymphocytes. [4] TNF also suppresses hematopoiesis, which contributes to anemia. The liver and spleen enlarge, causing massive splenomegaly. [8]
Low arginine, low nitric oxide, and elevated arginase activity have been observed in severe malaria in peripheral blood. [9] Studies have shown that the parasite's arginase enzyme may contribute to low arginine in severely ill patients, thus reducing nitric oxide production. Low nitric may lead to subsequent pulmonary hypertension and myocardial wall stress in children. Therefore, peripheral arginine or inhaled nitric oxide are possible treatment options. [10]
Parasitemia dictates symptom onset and severity: symptoms typically develop with 0.002% parasitemia in naïve patients and 0.2% parasitemia in previously exposed patients. [1] Severe infection usually exhibits parasitemia of 5%. [1] [4]
- Histopathology
Intracellular digestion of hemoglobin by parasites forms hemozoin and makes the membrane less deformable, which results in hemolysis or splenic clearance.
- History and Physical
In taking a history, it is essential to inquire about the location of residence, recent travel and use of chemoprophylaxis, exposures (including sick contacts, fresh water, caves, farm/wild animals, insects/arthropods), HIV status, history of current or recent pregnancy, history of G6PD deficiency, history of sickle cell disease, history of anemia, history of blood or other cancers, and history of prior malarial infections (including successful or failed treatments).
Fever is the dominant symptom of malaria—fever, especially for seven or more days, in a patient residing in or with recent travel to an endemic region is highly suspicious and should prompt evaluation. [3] Adults may exhibit headaches, malaise, weakness, gastrointestinal distress, upper respiratory symptoms, and muscle aches; severe cases may include jaundice, confusion, seizures, and dark urine. [2] [1] Children are more likely to present with non-specific or gastrointestinal symptoms such as fever, lethargy, malaise, nausea, vomiting, abdominal cramps, and somnolence. [2] They are more likely to develop hepatomegaly, splenomegaly, and severe anemia without major organ dysfunction than adults. In the case of severe malaria, they present with more frequent seizures (60 to 80%), hypoglycemia, and concomitant sepsis but are less likely to develop pulmonary edema and renal failure than adults. [11] [2]
Pregnant Women
The clinical features of infection in pregnancy vary from asymptomatic to severe, depending on the degree of (incomplete) immunity that a woman had acquired by the time she got pregnant. In semi-immune pregnant women, only a few infections result in fever or other symptoms. [12] Malaria in pregnancy has a devastating effect on maternal health and has been associated with increased infant mortality due to low birth weight caused by either intrauterine growth restriction or preterm labor, or both. [12] P. falciparum infections are associated with complications such as maternal anemia, low birth weight, miscarriage, stillbirths, and congenital malaria. [13] [12] It is more likely for a pregnant woman in the second or third trimester to develop severe malaria with complications such as hypoglycemia and pulmonary edema compared to non-pregnant adults. [14]
Initial evaluation of undifferentiated fever in stable patients with possible malaria exposure includes a complete blood count, comprehensive metabolic panel, coagulation panel, blood culture, urinalysis, chest radiograph, and thick and thin blood smears. In patients with altered mental status, when cerebral malaria is suspected, a lactate level, arterial blood gas, and lumbar puncture may also be indicated. [2]
In patients with malaria, complete blood count reveals thrombocytopenia in 60-70% of all cases and varying degrees of anemia in 29% of adults and 78% of children. [2] Anemia is more severe in P. falciparum due to invasion of all aged erythrocytes and capillary and splenic erythrocyte sequestration secondary to decreased flexibility and cytoadherence. [1] Anemia is typically moderate with P. vivax and P. malariae due to preferential invasion of reticulocytes and older erythrocytes, respectively. [1] A comprehensive metabolic panel may reveal hepatocellular injury secondary to parasitic invasion, indirect hyperbilirubinemia due to hemolysis, electrolyte abnormalities secondary to the release of intracellular contents, concomitant dehydration, and kidney injury secondary to glomerular damage. [2] The coagulation panel may reveal coagulopathy concerning bleeding risk in patients with severe thrombocytopenia or liver dysfunction. Urinalysis may show proteinuria indicative of nephrotic syndrome. [1]
The gold standard for malaria diagnosis is a microscopic evaluation of Giemsa-stained thick and thin smears of a free-flowing venipuncture blood specimen. [2] [1] Examination with oil immersion must be completed at 100-times and 1000-times magnification to avoid missing low-level parasitemia or "delicate ring forms." [1] The extent of parasitemia is estimated by the number of organisms per high-powered field. [1] Varying microscopic appearance of infected erythrocytes guides speciation:
- The ring stage in P. falciparum appears as a "purple spot with a thin ring;" in P. vivax as a "purple spot with a deformed body;" in P. ovale as a "ring with a large purple spot;" in P. malariae as a "purple spot with a thick body;" and in P. knowlesi as a "purple spot (or spots) with an amorphous thick ring." [15]
- The trophozoite stage in P. falciparum appears as "a bigger spot [growing] around a smaller spot;" in P. vivax as "a misshapen circle which contains an extended spot;" in P. ovale as "an oval circle (sometimes with small corners) which contains a purple spot with undefined shapes;" in P. malariae as "basket or band-shaped [without a] spot;" and in P. knowlesi as a "purple branched spot." [15]
- The schizont stage in P. falciparum is not established; in P. vivax, it appears as "not defined purple spots inside a circle;" in P. ovale as "more than one spot inside an oval circle (sometimes with small corners);" in P. malariae as "diffuse purple spots around a darker spot;" and in P. knowlesi as "defined purple spots [that are] easy to count." [15]
- The gametocyte stage in P. falciparum appears as "banana [or] sausage-shaped;" in P. vivax as an "extended, big spot;" in P. ovale as a "row of accumulated spots;" in P. malariae as a "big stained spot which almost fills[s] the circle;" and in P. knowlesi as a "big spot which contains small spots." [15]
An initial negative smear does not rule out malaria, as infected erythrocytes may become intravascularly sequestered; if clinical suspicion of malaria is high, smears require repetition in 12 and 24 hours. [2] The malarial pigment in monocytes and neutrophils may also manifest on the blood smear, particularly in patients with cerebral malaria. [1]
Other diagnostic modalities include rapid diagnostic testing (RDT), microhematocrit centrifugation, and polymerase chain reaction (PCR). RDTs detecting parasitic antigens histidine-rich-protein-2, lactate dehydrogenase, and aldolase are increasingly being utilized to diagnose P. falciparum infection. [2] [16] Sensitivities approach 100%, though microscopy is still a recommendation at the time of presentation and 12 and 24 hours. Limitations of RDTs include the detection of P. falciparum species only, the inability to quantify parasitic burden, and false-positive results occurring weeks after infection due to persistent blood antigens. [2] Microhematocrit centrifugation isolates infected erythrocytes, then binds to acridine in the collection tube, causing the fluorescence of parasites. [1] PCR is useful in low-level parasitemia detection and speciation.
- Treatment / Management
Treatment for patients diagnosed with malaria includes schizonticidal medications, supportive care, and hospitalization for high-risk patients. Naïve adult and pediatric patients receiving active antimalarial treatment should remain inpatient for at least 24 hours to ensure adequate and correctly timed medication dosing and to trend parasitemia to evaluate treatment response. Higher initial parasitemia and poor downtrend are associated with fluid imbalance, renal dysfunction, and respiratory distress syndrome. [2] Unstable patients, particularly those with cerebral malaria or significant respiratory sequelae, require intensive care. [2]
Treatment involves combination therapy targeting both the hepatic and erythrocytic forms. [17] The chief antimalarials are chloroquine, hydroxychloroquine, primaquine, artemisinin-based combination therapy (ACT), and atovaquone-proguanil. Chloroquine and hydroxychloroquine are synthetic forms of quinine. [18] [19] They disrupt the erythrocytic stage by interfering with parasitic hemoglobin metabolism and increasing intracellular pH. [18] [19] They generally require two days of treatment, allowing for better tolerance and shorter admissions. [2] However, chloroquine may enhance gametogenesis, contributing to resistance, which is a concern, particularly in South Asia. [17] Primaquine is a hypnozointocidal agent added for P. vivax or P. ovale infection for the eradication of liver parasites and the prevention of dormancy and relapse. [2] [20]
Primaquine is contraindicated in pregnant and G6PD deficient patients due to fetal teratogenicity and hemolytic reaction (will see bite cells and Heinz bodies on blood smear), respectively. [3] Artemisinins are active against all parasite lifecycle stages. [2] Atovaquone targets the cellular electron transport chain inhibiting ATP production; proguanil enhances atovaquone’s effect by sensitizing parasitic mitochondria. [21] Atovaquone-proguanil is active against the erythrocytic and extraerythrocytic forms. [17] [21]
Per the 2019 CDC Guidelines below, appropriate treatment depends on the Plasmodium species, clinical stability, age of the patient, and regional antimalarial susceptibility:
- Uncomplicated P. falciparum, P. malariae or P. knowlesi infections in chloroquine-sensitive regions are treated with a chloroquine phosphate 600 mg (pediatric: 10 mg/kg) loading dose, followed by 300 mg (pediatric: 5 mg/kg) at 6, 24, 48 hours; or a hydroxychloroquine 620 mg (pediatric: 10 mg/kg) loading dose, followed by 310 mg (pediatric: 5 mg/kg) at 6, 24, and 48 hours.
- Uncomplicated P. falciparum infections in chloroquine-resistant or unknown regions are treated with atovaquone-proguanil 250 mg/100 mg 4 tabs (pediatric: varied weight-based dosing, 6.5 mg/25 mg tabs) daily for 4 days; or artemether-lumefantrine 20 mg/120 mg 4 tabs (pediatric: varied weight-based tabs) at initial dose, then 8 hours later, then twice daily for 2 days; or quinine sulfate 542 mg (pediatric: 8.3 mg/kg) three times daily for 3 days (7 days if in Southeast Asia) plus either doxycycline 100 mg daily for 7 days (pediatrics 2.2 mg/kg every 12 hours), or tetracycline 250 mg daily for 7 days (pediatric: 25 mg/kg/day divided four times daily for 7 days), or clindamycin 20 mg/kg/day divided three times daily for 7 days (pediatric: same); or mefloquine 684 mg (pediatric: 13.7 mg/kg) loading dose followed by 456 mg (pediatric: 9.1 mg/kg) every 6 to 12 hours for total of 1250 mg (pediatric total: 25 mg/kg).
- Uncomplicated P. vivax or P. ovale infections in chloroquine-sensitive regions receive treatment with chloroquine phosphate or hydroxychloroquine as per above, plus either primaquine phosphate 30 mg (pediatric: 0.5 mg/kg) daily for 14 days or tafenoquine 300 mg once (same in children older than 16 years).
- Uncomplicated P. vivax infections in chloroquine-resistant regions (Indonesia, Papua New Guinea) get treated with quinine sulfate as per above plus either doxycycline, primaquine, or tafenoquine as per above; or atovaquone-proguanil as per above plus either primaquine or tafenoquine; or mefloquine as per above plus either primaquine or tafenoquine as per above.
- Uncomplicated infections with any species in pregnant women in chloroquine-sensitive regions require treatment with chloroquine or hydroxychloroquine as per above.
- Uncomplicated infections with any species in pregnant women in chloroquine-resistant regions are treated with quinine sulfate as per above plus either clindamycin or mefloquine as per above in the first, second, or third trimesters; or artemether-lumefantrine as per above in only the second and third trimesters.
- Severe malaria infection in unstable, non-pregnant patients in all regions includes IV artesunate 2.4 mg/kg (pediatric: children greater than 20 kg receive 2.4 mg/kg, children less than 20 kg receive 3.0 mg/kg) at 0, 12, 24, and 48 hours and either artemether-lumefantrine, atovaquone-proguanil, doxycycline, or mefloquine as per above.
- Differential Diagnosis
The differential for undifferentiated fever is extremely broad and varies based on geographic location and age. In a 2017 review of fever in returning travelers, 77% had protozoal malaria, 18% had a bacterial enteric fever ( Salmonella enterica, typhi, or paratyphi ), and 5% had another infection. In patients presenting with fever and significant somnolence or seizures, viral or bacterial meningitis or meningoencephalitis must remain on the differential and prompt consideration of lumbar puncture. [2] [22] Viral etiologies include avian influenza, Middle East respiratory syndrome coronavirus, hemorrhagic fever (Ebola virus, Lassa fever, Marburg hemorrhagic fever, Crimean-Congo hemorrhagic fever), yellow fever, dengue, Japanese encephalitis, Rift Valley fever, hepatitis virus (A or B), viral gastroenteritis, and rabies. [22] Bacterial etiologies include anthrax, epidemic typhus, ehrlichiosis, leptospirosis, melioidosis, murine (endemic) typhus, spotted fever group rickettsioses, Q fever, and Yersinia pestis. [22] [2]
The differential in children varies by region, with the most likely etiology being a viral or bacterial infection. In a 2014 study of febrile children in a tropical region, 10.5% were diagnosed with malaria, 62% were diagnosed with a respiratory infection, 13.3% with a systemic bacterial infection (usually staphylococcus or streptococcus bacteremia), and 10.3% with gastroenteritis (viral or bacterial). [23] Urinary tract infection and typhoid may also be considerations. Meningitis must be ruled out in somnolent children. [23]
- Treatment Planning
Artemether 20 mg/ lumefantrine 120 mg Artemether 40 mg/ lumefantrine 240 mg
Table 1. Artemisinin combination therapy (ACT) regimens for treatment of uncomplicated Plasmodium falciparum malaria in nonpregnant adults and children
Quinine: 542 mg base (= 650 mg salt) three times daily for a weekClindamycin: 20 mg base/kg/day (up to 1.8 grams) divided three times daily for a week OR Artemisinin combination therapy can be used as an alternate therapy in the first trimester if the above (more...)
Table 2. Oral regimens for treatment of Plasmodium falciparum malaria in pregnant women regions with c hloroquine-resistant P. falciparum infection.
00 hours: 600 mg base (= 1000 mg salt) 06 hours: 300 mg base (= 500 mg salt)
Table 3. Oral regimens for treatment of Plasmodium falciparum malaria in pregnant women in regions with c hloroquine-sensitive P. falciparum infection
The duration of untreated infection and time to relapse vary by location and species. P. falciparum and P. ovale infections last 2 to 3 weeks and may relapse 6 to 18 months later, usually from a new primary infection. [1] P. vivax infection lasts 3 to 8 weeks and may relapse months to up to 5 years later. [1] P. malariae infection lasts 3 to 24 weeks and may relapse up to 20 years later. [1]
Relapse is a case of recurrent symptoms months to years after the resolution of erythrocytic organisms due to reinfection or hypnozoite activation. [2] [1] Recrudescence is defined as recurrent symptoms within days to weeks of acute illness due to remaining parasitemia after ineffective or incomplete treatment or failed host immune response, more commonly in P. falciparum . [2] [1] Appropriate, complete treatment usually results in a full resolution of symptoms.
The two main determinants reflecting the outcome for both adults and children were the level of consciousness assessed by coma scales and the degree of metabolic acidosis, assessed clinically by breathing pattern or, more precisely, with measurement of bicarbonate, base deficit, and plasma lactate. [32] While the general mortality of treated severe malaria is between 10 to 20%, the mortality in pregnant women reaches approximately 50%. [14]
- Complications
The significant complications of malaria are cerebral malaria, severe malarial anemia, and nephrotic syndrome (NS).
Cerebral malaria accounts for 80% of fatal malaria cases, most often occurring with P. falciparum infection. [1] It presents as slow-onset altered mental status, violent behavior, headache, and extremely high fever (up to 42 degrees C), followed by coma, metabolic acidosis, hypoglycemia, and possibly seizures and death. [1] [4] It most commonly affects children under age 5, with a case fatality rate of 18%. [33] Pathogenesis involves malarial rosettes (one infected erythrocyte surrounded by three uninfected erythrocytes), causing cerebral sequestration and vasodilation, as well as excessive oxygen free radicals, IFN-gamma, and TNF-alpha leading to an extreme inflammatory response. [1] [4] [33] This leads to congestion, decreased perfusion, endothelial activation, impairment of the blood-brain barrier, and cerebral edema, which increases brain volume. [33]
Increased brain volume is the major contributor to mortality in cerebral malaria. In a 2015 study of Malawian children with cerebral malaria, 84% of those who died had severely increased brain volume on MRI; children who survived showed lower initial brain volume or a downtrend over time. [33]
Severe malarial anemia stems from TNF-alpha-mediated mechanisms involving both increased destruction and decreased production of erythrocytes, including cell lysis as parasites replicate and exit erythrocytes, splenic removal and autoimmune lysis of immune-marked erythrocytes, poor iron incorporation into new heme molecules, and bone marrow suppression during severe infection leading to decreased production. [1] [4] Blackwater fever is severe anemia with hemoglobinuria and renal failure in the context of "massive intravascular hemolysis" in the setting of repeat P. falciparum infections treated with chronic quinine; it is rare and thought to be associated with G6PD deficiency. [34]
Nephrotic syndrome occurs secondary to glomerular antigen-antibody complex deposition and presents similarly to membranoproliferative glomerulonephritis with proteinuria and decreased renal function, which may lead to renal failure. Nephrotic syndrome is common in P. malariae and P. knowlesi , possible in P. vivax , and rare in P. falciparum and P. ovale infections. [1]
Additional complications include:
- Bilious remittent fever presents with abdominal pain and persistent vomiting that may lead to severe dehydration, jaundice, and dark urine.
- Algid malaria is an adrenal insufficiency due to parasitic congestion and subsequent necrosis of the adrenal glands.
- Acute respiratory distress syndrome, circulatory collapse, disseminated intravascular coagulation, pulmonary edema, coma, and death. [1]
Malaria infection during pregnancy may result in low birth weight or fetal demise. [1]
- Consultations
Recommended consultations for non-infectious disease experts in the management or prevention of malaria include infectious disease and preventive or travel medicine.
- Deterrence and Patient Education
The recommendation is that patients schedule a pre-travel appointment with a preventive medicine or infectious disease physician for education regarding malaria deterrence. Malaria prevention centers around vector control and chemoprophylaxis while exposed to mosquito-ridden environments.
Vector control is the prevention of mosquito bites by way of insecticide-impregnated bed nets, permethrin treatment of clothing, and DEET application to the skin. [3] The three main prophylactic agents for Plasmodium falciparum are atovaquone-proguanil, doxycycline, and mefloquine. Atovaquone-proguanil is taken once daily during and one week after travel to an endemic region; it suppresses the hepatic stage and does not have approval for pregnancy. [2] Doxycycline is taken once daily during and one month after travel; it suppresses the blood stage. [2] Doxycycline has the added benefit of prophylaxis against Rickettsial disease, Q fever, leptospirosis, and travelers’ diarrhea; however, it may cause gastrointestinal distress, photosensitivity, and increased risk of candida infection. Mefloquine is taken once weekly during and one month after travel; it suppresses the blood stage. [2] It has the benefit of safety in the second and third trimester of pregnancy; however, it has a far higher risk of neuropsychiatric side effects. [2] The US military primarily utilizes doxycycline if susceptibilities are equal. [2] For pregnant women in the first trimester or breastfeeding women, chloroquine or mefloquine prophylaxis are preferable; data regarding the safety of atovaquone-proguanil prophylaxis in pregnancy is limited. [35]
- Enhancing Healthcare Team Outcomes
The timely care of patients diagnosed with malaria and clinically relevant research regarding advancing diagnostic techniques and treatment requires interprofessional teamwork and communication between clinicians, infectious disease experts, pharmacists, nurses, and global health professionals.
Any clinician treating malaria will initiate treatment as outlined above. Still, it is good policy to include an infectious disease specialist and involve an infectious disease board-certified pharmacist, who can also examine the regimen and agents chosen, as well as verify dosing and drug interactions. A nurse with infectious disease specialty training can also help by answering patient questions, serving as a bridge to the treating clinician, and monitoring treatment progress and potential adverse drug reactions. All team members must keep accurate and updated records, so everyone involved in treatment has the same information on the patient's case. If there are any concerns, each team member must be free to communicate with other team members so that appropriate interventions can be started or therapeutic modifications can be implemented. This collaborative interprofessional approach can optimize outcomes for malaria patients. [Level 5]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Life Cycle of the Malaria Parasite Contributed by Wikimedia Commons, National Institutes of Health (NIH) (Public Domain)
Aedes species mosquito Image courtesy of S Bhimji MD
Blood smear malaria Image courtesy S Bhimji MD
Table 1 - Diagnostic criteria for severe P.falciparum malaria. Contributed by Lara Zekar, MD
Disclosure: Emily Buck declares no relevant financial relationships with ineligible companies.
Disclosure: Nancy Finnigan declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Buck E, Finnigan NA. Malaria. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. [Cochrane Database Syst Rev. 2022] Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Malaria Surveillance - United States, 2016. [MMWR Surveill Summ. 2019] Malaria Surveillance - United States, 2016. Mace KE, Arguin PM, Lucchi NW, Tan KR. MMWR Surveill Summ. 2019 May 17; 68(5):1-35. Epub 2019 May 17.
- Malaria surveillance--United States, 2010. [MMWR Surveill Summ. 2012] Malaria surveillance--United States, 2010. Mali S, Kachur SP, Arguin PM, Division of Parasitic Diseases and Malaria, Center for Global Health, Centers for Disease Control and Prevention (CDC). MMWR Surveill Summ. 2012 Mar 2; 61(2):1-17.
- Review [The reemergence of malaria in Israel?]. [Harefuah. 2004] Review [The reemergence of malaria in Israel?]. Anis E, Pener H, Goldmann D, Leventhal A. Harefuah. 2004 Nov; 143(11):815-9, 838, 837.
- Review Diagnosis and Treatment of the Febrile Child. [Reproductive, Maternal, Newbor...] Review Diagnosis and Treatment of the Febrile Child. Herlihy JM, D’Acremont V, Hay Burgess DC, Hamer DH. Reproductive, Maternal, Newborn, and Child Health: Disease Control Priorities, Third Edition (Volume 2). 2016 Apr 5
Recent Activity
- Malaria - StatPearls Malaria - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
How climate change will affect malaria transmission
A new model for predicting the effects of climate change on malaria transmission in Africa could lead to more targeted interventions to control the disease according to a new study.
Previous methods have used rainfall totals to indicate the presence of surface water suitable for breeding mosquitoes, but the research led by the University of Leeds used several climatic and hydrological models to include real-world processes of evaporation, infiltration and flow through rivers.
This groundbreaking approach has created a more in-depth picture of malaria-friendly conditions on the African continent.
It has also highlighted the role of waterways such as the Zambezi River in the spread of the disease with almost four times the population estimated to live in areas suitable for malaria for up to nine months of the year than was previously thought.
The research entitled "Future malaria environmental suitability in Africa is sensitive to hydrology" was funded by the Natural Environment Research Council and is published today (9 May 2024) in the journal Science .
Dr Mark Smith an Associate Professor in Water Research in the Leeds' School of Geography and lead author of the study said: "This will give us a more physically realistic estimate of where in Africa is going to become better or worse for malaria.
"And as increasingly detailed estimates of water flows become available, we can use this understanding to direct prioritisation and tailoring of malaria interventions in a more targeted and informed way. This is really useful given the scarce health resources that are often available."
Malaria is a climate-sensitive vector-borne disease that caused 608,000 deaths among 249 million cases in 2022.
95% of global cases are reported in Africa but reductions in cases there have slowed or even reversed in recent years, attributed in part to a stall in investments in global responses to malaria control.
The researchers predict that the hot and dry conditions brought about by climate change will lead to an overall decrease in areas suitable for malaria transmission from 2025 onwards.
The new hydrology-driven approach also shows that changes in malaria suitability are seen in different places and are more sensitive to future greenhouse gas emissions than previously thought.
For example, projected reductions in malaria suitability across West Africa are more extensive than rainfall-based models suggested, stretching as far east as South Sudan, whereas projected increases in South Africa are now seen to follow watercourses such as the Orange River.
Co-author of the study Professor Chris Thomas from the University of Lincoln said: "The key advancement is that these models factor in that not all water stays where it rains, and this means breeding conditions suitable for malaria mosquitoes too can be more widespread -- especially along major river floodplains in the arid, savannah regions typical of many regions in Africa.
"What is surprising in the new modelling is the sensitivity of season length to climate change -- this can have dramatic effects on the amount of disease transmitted."
Simon Gosling, Professor of Climate Risks & Environmental Modelling at the University of Nottingham, co-authored the study and helped to coordinate the water modelling experiments used in the research. He said: "Our study highlights the complex way that surface water flows change the risk of malaria transmission across Africa, made possible thanks to a major research programme conducted by the global hydrological modelling community to compile and make available estimates of climate change impacts on water flows across the planet.
"Although an overall reduction in future risk of malaria might sound like good news, it comes at a cost of reduced water availability and a greater risk of another significant disease, dengue."
The researchers hope that further advances in their modelling will allow for even finer details of waterbody dynamics which could help to inform national malaria control strategies.
Dr Smith added: "We're getting to the point soon where we use globally available data to not only say where the possible habitats are, but also which species of mosquitoes are likely to breed where, and that would allow people to really target their interventions against these insects."
- Infectious Diseases
- Pests and Parasites
- Environmental Issues
- Environmental Awareness
- Global climate model
- Climate model
- Infiltration (hydrology)
- Pest (animal)
- IPCC Report on Climate Change - 2007
- Consensus of scientists regarding global warming
- Climate engineering
Story Source:
Materials provided by University of Leeds . Note: Content may be edited for style and length.
Journal Reference :
- Mark W. Smith, Thomas Willis, Elizabeth Mroz, William H. M. James, Megan J. Klaar, Simon N. Gosling, Christopher J. Thomas. Future malaria environmental suitability in Africa is sensitive to hydrology . Science , 2024; 384 (6696): 697 DOI: 10.1126/science.adk8755
Cite This Page :
Explore More
- Controlling Shape-Shifting Soft Robots
- Brain Flexibility for a Complex World
- ONe Nova to Rule Them All
- AI Systems Are Skilled at Manipulating Humans
- Planet Glows With Molten Lava
- A Fragment of Human Brain, Mapped
- Symbiosis Solves Long-Standing Marine Mystery
- Surprising Common Ideas in Environmental ...
- 2D All-Organic Perovskites: 2D Electronics
- Generative AI That Imitates Human Motion
Trending Topics
Strange & offbeat.
Notes from the Field : Increases in Imported Malaria Cases — Three Southern U.S. Border Jurisdictions, 2023
Weekly / May 9, 2024 / 73(18);417–419
Cedar L. Mitchell, PhD 1 ,2 ,3 ; Audrey Kennar, MSPH 1 ,4 ,5 ; Yvonne Vasquez 6 ; Kaitlyn Noris 2 ; Thomas Williamson, MPH 3 ; Andrea Mannell, MPH 2 ; Anissa Taylor, MPH 2 ; Irene Ruberto, PhD 3 ; Theresa A. Cullen, MD 2 ; Mariana Singletary, MD 2 ; Seema Shah, MD 5 ; Hector Ocaranza, MD 6 ; Alfonso Rodriguez Lainz, PhD, DVM 4 ; Kimberly E. Mace, PhD 7 ( View author affiliations )
What is already known about this topic?
Approximately 2,000 malaria cases are imported into the United States annually, mostly among U.S. residents with recent travel to areas with endemic malaria.
What is added by this report?
In 2023, reports of imported malaria in three U.S. southern border jurisdictions increased from cases reported in 2022. Enhanced case investigations documenting traveler residency indicated higher percentages of malaria infections among new arrivals to the United States who traveled through at least one country with endemic malaria, including crossing land borders.
What are the implications for public health practice?
Outreach and education about malaria should be provided to local health care professionals and new arrivals, including migrants, with travel through areas with endemic malaria, to facilitate identification of cases, initiation of prompt treatment, and reduction in morbidity.
- Article PDF
- Full Issue PDF
Introduction
Malaria is a severe and potentially fatal mosquitoborne disease caused by infection with Plasmodium spp. parasites. Although malaria is no longer endemic in the United States, imported infections are reported annually; the primary risk group has been U.S. residents traveling to areas where malaria is endemic ( 1 ). In 2023, sporadic locally acquired mosquito-transmitted malaria cases were reported in several U.S. states ( 2 , 3 ). This report describes increases in imported malaria cases in 2023 compared with 2022 in three public health jurisdictions along the U.S. southern border.
Investigation and Outcomes
During January–December 2023, a total of 68 imported malaria cases were identified from reportable disease surveillance systems in Pima, Arizona (18), San Diego, California (27), and El Paso, Texas (23), compared with 28 cases in 2022 (three in Pima, 12 in San Diego, and 13 in El Paso) ( Table ). Because malaria case counts were higher than expected, enhanced case investigations were initiated. Malaria cases were defined according to CDC case definitions.* To describe imported malaria cases in these three jurisdictions, this report summarized patient travel and illness characteristics by U.S. residence status. New arrivals were non–U.S.-born persons who had arrived in the United States within the preceding 6 months and were classified into the following three subgroups: 1) newly arrived refugees (i.e., officially admitted to the United States as part of the U.S. Refugee Admissions Program), 2) other new arrivals (including asylum seekers and other migrants), and 3) persons whose immigration status was unknown. Among jurisdictions, differences were identified in epidemiologic investigation protocols for patients without a local address and whether they were included in local surveillance case counts. This activity was reviewed by CDC, deemed not research, and was conducted consistent with applicable federal law and CDC policy. †
Among the 68 imported malaria cases identified in 2023, 15 (22%) occurred among U.S. residents, two (3%) among newly arrived refugees, 49 (72%) among other newly arrived migrants (i.e., asylum seekers and other migrants), and two (3%) among travelers with unknown immigration status. The local public health jurisdictions attempted an interview with 61 (90%) patients. Among the 68 patients with malaria, 33 (49%) met residence criteria for inclusion in local surveillance case counts (i.e., the 15 U.S. residents, two newly arrived refugees, and 16 [33%] of the 49 other newly arrived migrants). The U.S. residents and refugees traveled directly from another country with endemic malaria to the United States. Among the 49 other newly arrived migrants, 46 (94%) had traveled through one or more countries with endemic malaria, including the country of origin (complex travel). The median travel duration was 29 days (range = 8–85 days), and 36 (73%) persons reported having traversed land borders. Overall, 63 (91%) patients with malaria were hospitalized; no deaths were reported. Nearly one third (21; 31%) of patients with malaria experienced severe disease ( 1 ), of which Plasmodium vivax was reported among 11 (52%), P. falciparum among six (29%), and another or unknown Plasmodium spp. parasite among four patients. Severe malaria was more common among other newly arrived migrants (18 of 49; 37%) than among U.S. residents (one of 15; 7%).
Preliminary Conclusions and Actions
Imported malaria in three U.S. southern border jurisdictions increased in 2023, particularly among new arrivals to the United States with recent, complex transit through at least one country with endemic malaria. During the same period, entry of asylum seekers and other migrants into the United States across the southern land border increased. § In light of the different jurisdictional protocols used in case investigations, implementation of classifications and consistent investigation and reporting protocols for non-U.S. residents could facilitate better characterization of malaria incidence among new arrival subgroups in different jurisdictions. ¶
New arrivals to the United States with complex travel through areas with endemic malaria are potentially at higher risk for malaria and, for reasons not fully understood, for more severe illness. Health care professionals should obtain a complete travel history, consider malaria among symptomatic patients with recent travel through areas where malaria is endemic, and initiate prompt testing and, if indicated, treatment.** Outreach and education about malaria directed to local health care professionals and to new arrivals with recent travel in areas with endemic malaria are crucial because prompt care seeking, diagnosis, and treatment of malaria will reduce morbidity in this population.
Corresponding author: Cedar L. Mitchell, [email protected] .
1 Epidemic Intelligence Service, CDC; 2 Pima County Health Department, Tucson, Arizona; 3 Arizona Department of Health Services; 4 Division of Global Migration Health, National Center for Emerging and Zoonotic Infectious Diseases, CDC; 5 County of San Diego Health and Human Services Agency, San Diego, California; 6 City of El Paso Department of Public Health, El Paso, Texas; 7 Division of Parasitic Diseases and Malaria, National Center for Emerging and Zoonotic Infectious Diseases, CDC.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
* https://ndc.services.cdc.gov/case-definitions/malaria-2014/
† 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.
§ An increase in the entry of asylum seekers and other migrants across the U.S. southern border was identified using annual numbers of persons with credible fear who were released with a notice to appear for immigration court or paroled into the United States as a proxy for asylum seekers and other migrants. Data are publicly available from 2022 and 2023 annual U.S. Customs and Border Protection Southwest border reports. https://www.cbp.gov/newsroom/stats/custody-and-transfer-statistics
¶ https://cdn.ymaws.com/www.cste.org/resource/resmgr/PS/03-ID-10revised.pdf
** https://www.cdc.gov/malaria/diagnosis_treatment/clinicians1.html
- Mace KE, Lucchi NW, Tan KR. Malaria surveillance—United States, 2018. MMWR Surveill Summ 2022;71(No. SS-8):1–35. https://doi.org/10.15585/mmwr.ss7108a1 PMID:36048717
- Blackburn D, Drennon M, Broussard K, et al. Outbreak of locally acquired mosquito-transmitted (autochthonous) malaria—Florida and Texas, May–July 2023. MMWR Morb Mortal Wkly Rep 2023;72:973–8. https://doi.org/10.15585/mmwr.mm7236a1 PMID:37676839
- Duwell M, DeVita T, Torpey D, et al. Notes from the field: locally acquired mosquito-transmitted (autochthonous) Plasmodium falciparum malaria—National Capital Region, Maryland, August 2023. MMWR Morb Mortal Wkly Rep 2023;72:1123–5. https://doi.org/10.15585/mmwr.mm7241a3 PMID:37824424
* Jurisdictions included Pima, Arizona; San Diego, California; and El Paso, Texas. † During 2022, a total of 28 imported malaria cases were reported from these three jurisdictions, including 15 (54%) among U.S. residents, zero among newly arrived refugees, 11 (39%) among other new arrivals, and two (7%) among persons with an unknown immigration status. § Refugees from areas in sub-Saharan Africa with endemic malaria receive presumptive treatment for malaria during their predeparture health assessment. https://www.cdc.gov/immigrantrefugeehealth/guidelines/overseas-guidelines.html ¶ Asylum seekers and other migrants. ** Includes one short-term traveler to the United States and one patient without enough information to determine their status. †† Case investigation protocols differed among jurisdictions. Some protocols required interviews for all reported patients, whereas others only required interviews for patients with a local residential address. Reasons for an incomplete case investigation included inability to contact the patient, and loss to follow-up because of missing or incorrect patient contact information or no response. §§ Inclusion criteria for local surveillance counts differed among jurisdictions. Some jurisdictions did not include patients who were missing a residential address or whose address was outside the local jurisdiction, regardless of case investigation status. ¶¶ Region of travel origin for new arrivals or region of destination for U.S. residents. Regions included the following countries of travel origin: Africa: Angola, Côte d’Ivoire, Ethiopia, Guinea, Mauritania, Nigeria, Senegal, Sudan, The Gambia, and Uganda; Asia: Afghanistan and China; Central America: Nicaragua and Panama; South America: Colombia, Ecuador, and Venezuela. CDC provides information about areas with endemic malaria. https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/malaria *** Date of care and diagnosis based on care received at a U.S. health care facility. ††† According to the CDC case definition for severe malaria, which includes laboratory confirmation with neurologic symptoms, acute kidney injury, severe anemia (hemoglobin <7g/dL), acute respiratory distress syndrome, or ≥5% parasitemia; treatment for severe malaria (i.e., artesunate or exchange transfusion); or death. https://doi.org/10.15585/mmwr.ss7108a1
Suggested citation for this article: Mitchell CL, Kennar A, Vasquez Y, et al. Notes from the Field: Increases in Imported Malaria Cases — Three Southern U.S. Border Jurisdictions, 2023. MMWR Morb Mortal Wkly Rep 2024;73:417–419. DOI: http://dx.doi.org/10.15585/mmwr.mm7318a2 .
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services. Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services. References to non-CDC sites on the Internet are provided as a service to MMWR readers and do not constitute or imply endorsement of these organizations or their programs by CDC or the U.S. Department of Health and Human Services. CDC is not responsible for the content of pages found at these sites. URL addresses listed in MMWR were current as of the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version ( https://www.cdc.gov/mmwr ) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
share this!
May 2, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Deeper understanding of malaria parasite sexual development unlocks opportunities to block disease spread
by Wellcome Trust Sanger Institute
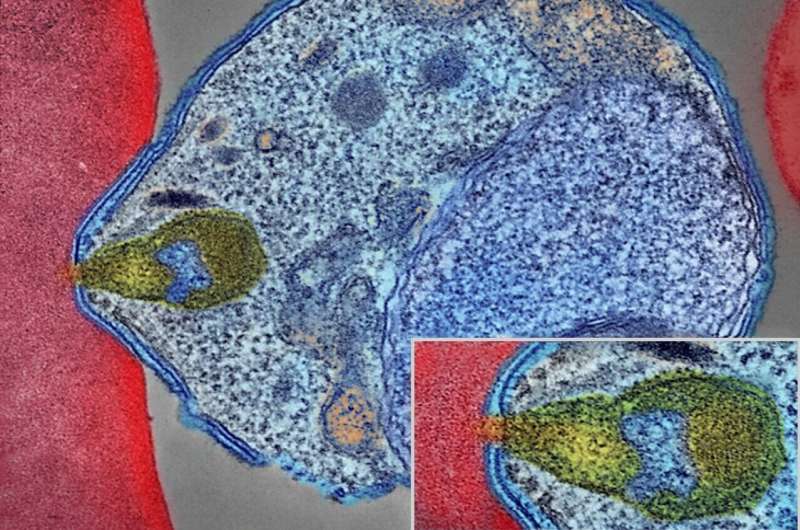
For the first time, the developmental stages of the deadliest human malaria parasite have been mapped in high resolution, allowing researchers to understand this ever-adapting adversary in more detail than previously possible.
The study, published in Science , details the critical developmental stages of the malaria parasite, Plasmodium falciparum, using single-cell RNA sequencing. This gives detailed information on the life stages of this parasite as it matures, changing from an asexual state to a sexual state, which is necessary before the parasite can be transmitted to mosquitoes.
The research from the Wellcome Sanger Institute, the Malaria Research and Training Center (MRTC) in Mali, and other collaborators, adds to the freely available Malaria Cell Atlas. The Atlas provides information for researchers worldwide to investigate and generate tools to track the disease.
The novel insights accessible through the Malaria Cell Atlas can also help identify new ways to block the parasite's development, including through new drugs or vaccines that can prevent transmission.
Malaria is a life-threatening disease with an estimated 249 million cases and 608,000 deaths globally in 2022. It is caused by the Plasmodium parasite, with P. falciparum being the deadliest type of this parasite and the most prevalent on the African continent.
P. falciparum is a single-celled parasite that evolves quickly, making it difficult to develop long-lasting and effective diagnostics, drugs and vaccines to protect against it. Malaria parasites have a huge amount of genetic diversity and people are frequently infected with multiple different parasite strains. In Mali, around 80 percent of people infected with malaria carry multiple genetically distinct parasite strains.
Malaria parasites are found in either an asexual or sexually developed form in the human host. Asexual replication in humans is what causes the symptoms of malaria, but to transmit, parasites have to develop and become either a male or female reproductive cell, known as a gametocyte.
Sexual commitment and development are controlled by transcription factors , which are proteins that regulate gene activity. The mature sexual forms of the parasite circulate in the bloodstream until they are taken up by mosquitoes.
In the latest research, from the Wellcome Sanger Institute and the MRTC in Mali researchers used both long-read and short-read single-cell RNA sequencing to map the sexual development stages of P. falciparum in the laboratory. This allowed them to track the gene expression levels and highlight which genes are involved in each stage of the process.
The team then applied this approach to parasites from blood samples collected from four people naturally infected with malaria in Mali. This is the first time that these technologies have been applied to real-time infection strains at such a high resolution.
By comparing the laboratory data with the natural infection data, the researchers found parasite cell types not previously seen in laboratory strains, highlighting the importance of real-world data.
The team compared different natural P. falciparum strains within each donor to identify genes of interest.
Some of the genes that were overexpressed in particular strains in the sexual development stages are involved in the survival of the parasite in the mosquito, including one that plays a role in dampening mosquito immunity. The next step will be to assess the impact these genes have on transmission.
Jesse Rop, co-first author from the Wellcome Sanger Institute, said, "This is the first time that we have been able to map the sexual development stages of malaria parasites in both laboratory and natural strains, allowing us to gain deeper insight into the similarities and differences. Our research uncovered new biology present in the naturally occurring strains that are not seen in laboratory strains, improving our understanding of how malaria develops and spreads."
Dr. Sunil Dogga, co-first author from the Wellcome Sanger Institute, said, "Our research adds to the growing Malaria Cell Atlas, giving a high-quality, open-access genomic resource for researchers worldwide. This high-resolution atlas can be used by scientists to gain a clear understanding of the genes they are investigating, combine research efforts, and help us more effectively prevent, control, and treat malaria. Working together as a scientific community is the only way we are going to successfully control and treat malaria."
Professor Abdoulaye Djimdé, co-author from the Malaria Research and Training Centre, University of Bamako, Mali, and Honorary Faculty at the Wellcome Sanger Institute, said, "Malaria has a huge global impact, affecting millions of people each year, and attempts to control and treat the disease are quickly overcome by the parasite. Understanding more about the parasite's life cycle, the genes involved, and the factors that control these, can be vital to ongoing malaria research. Our research highlights key points in the sexual development of the parasite, which if targeted in future drug development could break the cycle of transmission and help minimize the spread."
Dr. Mara Lawniczak, senior author from the Wellcome Sanger Institute, said, "This new focus of the Malaria Cell Atlas project on natural infections coincides with malaria vaccines being used for the first time and a continued rise of drug resistance. Single-cell RNA sequencing gives us a window into parasite gene usage that is not possible with any other approach, while also providing a much clearer understanding of just how genetically diverse parasites are, even within the same person. The Malaria Cell Atlas is a resource we hope will be increasingly useful on the path to malaria elimination."
Journal information: Science
Provided by Wellcome Trust Sanger Institute
Explore further
Feedback to editors

Solar storm puts on brilliant light show across the globe, but no serious problems reported
5 hours ago

Study discovers cellular activity that hints recycling is in our DNA
6 hours ago

Weaker ocean currents lead to decline in nutrients for North Atlantic ocean life during prehistoric climate change

Research explores ways to mitigate the environmental toxicity of ubiquitous silver nanoparticles

AI may be to blame for our failure to make contact with alien civilizations
9 hours ago

Saturday Citations: Dietary habits of humans; dietary habits of supermassive black holes; saving endangered bilbies
12 hours ago

Scientists unlock key to breeding 'carbon gobbling' plants with a major appetite
May 10, 2024

Clues from deep magma reservoirs could improve volcanic eruption forecasts

Study shows AI conversational agents can help reduce interethnic prejudice during online interactions

NASA's Chandra notices the galactic center is venting
Relevant physicsforums posts, who chooses official designations for individual dolphins, such as fb15, f153, f286.
May 9, 2024
Is it usual for vaccine injection site to hurt again during infection?
May 8, 2024
The Cass Report (UK)
May 1, 2024
Is 5 milliamps at 240 volts dangerous?
Apr 29, 2024
Major Evolution in Action
Apr 22, 2024
If theres a 15% probability each month of getting a woman pregnant...
Apr 19, 2024
More from Biology and Medical
Related Stories
Malaria cell atlas launched: parasite development mapped in unprecedented detail.
Mar 27, 2018

Map of malaria behavior set to revolutionize research
Aug 22, 2019

Blocking fertilization of parasite-causing malaria opens new doors in eradication efforts
Feb 27, 2024

For a new approach to fighting malaria, study focuses on special RNA molecules in human malaria parasite
Aug 28, 2023

Atlas of malaria parasite gene activity provides new targets for drugs and vaccines
May 27, 2021

Deadliest human malaria parasite reveals the genomic chinks in its armor
May 3, 2018
Recommended for you

New research shows microevolution can be used to predict how evolution works on much longer timescales

GoT-ChA: New tool reveals how gene mutations affect cells
Researchers shed new light on carboxysomes in key discovery that could boost photosynthesis

Researchers reveal new cellular mechanical transducer

Scientists link oocyte-specific histone H1FOO to better iPS cell generation
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.

E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- News & Views
- Published: 06 May 2024
Ecotoxicology in malaria vector control
- Andrew Forbes ORCID: orcid.org/0000-0001-9992-0699 1
Nature Sustainability ( 2024 ) Cite this article
32 Accesses
Metrics details
- Ecological epidemiology
Environmental health is an under-studied aspect of the One Health approach, despite being equally important to human, animal and plant health. Now, a study, aiming to redress this imbalance, shows the potential ecotoxicological effects of treating cattle with insecticide to control mosquitoes that spread malaria.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
111,21 € per year
only 9,27 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Heinrich, A. P. et al. Nat. Sustain. https://doi.org/10.1038/s41893-024-01332-8 (2024).
Article Google Scholar
Carpenter, S. R. Ecology 77 , 677–680 (1996).
Krüger, K. & Scholtz, C. H. Acta Oecol. 19 , 425–438 (1998).
Kryger, U., Deschodt, C. & Scholtz, C. H. Agric. Ecosyst. Environ. 105 , 649–656 (2005).
Article CAS Google Scholar
Hanski, I. & Cambefort, Y. (eds) Dung Beetle Ecology (Princeton Univ. Press, 1991).
Campbell, W. C. (ed.) Ivermectin and Abamectin (Springer, 1989).
Download references
Author information
Authors and affiliations.
School of Biodiversity, One Health and Veterinary Medicine, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, UK
Andrew Forbes
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Andrew Forbes .
Ethics declarations
Competing interests.
The author declares no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Forbes, A. Ecotoxicology in malaria vector control. Nat Sustain (2024). https://doi.org/10.1038/s41893-024-01342-6
Download citation
Published : 06 May 2024
DOI : https://doi.org/10.1038/s41893-024-01342-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Malaria cases in Texas and Florida are the first US spread in 20 years, CDC says

The United States has seen five cases of malaria spread by mosquitos in the past two months − the first time there has been local spread in 20 years − prompting authorities to issue a public health alert warning doctors, public health authorities and the public about the risk.
Four cases were identified in southwest Florida and one in southern Texas, the Centers for Disease Control and Prevention said. The five cases are the first in 20 years to be caught locally in the United States.
"Malaria is a medical emergency and should be treated accordingly," the CDC said. "Patients suspected of having malaria should be urgently evaluated in a facility that is able to provide rapid diagnosis and treatment, within 24 hours of presentation."
Malaria is a serious disease transmitted through the bite of an infective female anopheline mosquito, according to the CDC. Although malaria can be fatal, the CDC said, illness and death from the disease can usually be prevented.
There is no evidence the five cases in the two states are related, the CDC said. The four cases in Florida were identified in Sarasota County, and the Florida Department of Health issued a statewide mosquito-borne illness advisory Monday.
Only one case was identified in a Texas resident who spent time working outdoors in Cameron County, according to the Texas Department of State Health Services .
Both departments in Florida and Texas said public health authorities were monitoring local mosquito populations and surveilling their regions for other cases. The Florida Department of Health said it was also working to control the mosquito population in Sarasota County.
The CDC said all five patients have been treated and were improving. Cases of locally acquired malaria have not occurred in the United States since 2003, when eight cases were identified in Palm Beach County, Florida.
Malaria cases are rare in US
Even with the five identified cases, the CDC said, the risk of catching malaria in the United States "remains extremely low."
But the health agency warned that female anopheline mosquitoes can be found throughout many regions in the country and can spread malaria if they feed on a person already infected with the disease.
"The risk is higher in areas where local climatic conditions allow the Anopheles mosquito to survive during most of or the entire year and where travelers from malaria-endemic areas are found," the CDC said.
More than 240 million cases of malaria occur each year worldwide, and 95% of cases are in Africa, according to the CDC. And a majority of cases in the United States are from people who travel from countries with malaria transmission.
Before the COVID-19 pandemic, the CDC said, there were about 2,000 cases of mostly travel-related malaria in the United States each year, and about 300 people experienced severe disease.
Although rare, malaria can also spread through blood transfusions, organ transplants, unsafe needle-sharing practice and from mother to fetus, according to the CDC.
The CDC warned that more people could bring the disease into the United States with summer international travel increasing to pre-pandemic levels and advised people to use bug spray during the warmer months.
Symptoms of malaria include fever, chills, headache, muscle aches and fatigue. People may also experience nausea, vomiting and diarrhea. Though symptoms generally start about 10 days to four weeks after infection, people may feel sick as late as a year after infection.
Experts report increase in 'mosquito days'
The number of "mosquito days," or periods where mosquitoes thrive in warm and humid weather, has increased in more than 170 U.S. locations over the past several decades, according to a report in May 2023 from the nonprofit climate science research organization Climate Central.
According to the report, a mosquito day has an average relative humidity of 42% or higher in addition to daily temperatures of 50 to 95 degrees. From 1979 to 2022, the report said, 173 U.S. locations saw annual mosquito days increase by 16 days on average.
The report warned that as the climate warms, especially during the spring and fall, many regions are becoming "more hospitable to mosquitoes," which allow the flying insects to arrive earlier and survive later into the year.
More mosquitoes also means a possible increase in health risks. "More mosquito days mean more opportunities for mosquitoes to bite people and potentially transmit disease," the report said.
How to get rid of mosquitoes
Mosquitoes flock toward dark, humid places like under the sink, in showers, closets and laundry rooms, and under furniture, according to the CDC. Once they’re inside, they may start laying eggs in your home.
The first step you can take to minimize mosquitoes in or around your home is to check for and eliminate any standing water. One of the most common examples are trays under potted plants to catch excess water, said Elmer Gray , a public health extension specialist at the University of Georgia.
“If you have house plants on your deck and you have mosquitoes on your deck, you might be growing them right there,” Gray said.
Check your house and yard for areas that might be gathering water. That could be old tires collecting rainwater, dog dishes left outside, tree holes, rain barrels, gutters or garbage cans.
Contributing: Clare Mulroy
- Open access
- Published: 30 April 2024
Exploring the role of spending on malaria incidence in Uganda using the auto-regressive distributed lag approach
- Jemimah Katushabe 1 ,
- John Bosco Nnyanzi 1 &
- Gertrude Sebunya Muwanga 1
Malaria Journal volume 23 , Article number: 129 ( 2024 ) Cite this article
241 Accesses
1 Altmetric
Metrics details
Malaria has remained a persistent global health problem. Despite multiple government and donor initiatives to eradicate malaria and its detrimental effects on Uganda's health outcomes, the incidence of malaria is worrying as it appears higher than the average of 219 cases per 1000 for sub-Saharan Africa for the period 2017–2018. This study investigated the effect of public and private healthcare spending on the incidence of malaria in Uganda.
Employing time series data spanning over 20 years from the first quarter of 2000 to the last quarter of 2019, the study builds a model based on the Grossman framework for analysing demand for health. The estimation technique used was the ARDL approach that takes into account reverse causality and incidental relationships. Prior to the adoption of the technique, a bounds test was performed to determine whether the variables contained in the model have a long-term relationship. Several diagnostic tests for serial correlation, functional normality, and heteroskedastic specification error were carried out to verify the ARDL model's goodness of fit. Additionally, the cumulative sum of recursive (CUSUM) and cumulative sum of squares of recursive residuals (CUSUMSQ) were used to test model stability.
The results indicate that in the long run, an increase in public spending of one percent significantly reduces malaria incidence by 0.196 at the 10 percent level of significance. On the other hand, there is no significant evidence of private health expenditure's effect on malaria incidence. However, in the short run, public spending reduces malaria incidence by a smaller magnitude of 0.158 percent relative to the long-run. Still, private expenditure is found to exhibit no significant effect. Additional findings point to the importance of GDP per capita and urban population growth in reducing malaria incidence, whereas female unemployment, income inequality, as well as female-headed household. In the short run, however, the female-headed households and urban population growth are found to significantly reduce malaria incidence while an improvement in regulatory quality decreases malaria incidence by 0.129 percent.
Conclusions
There is need for further government interventions to reduce malaria incidence in the country via budget allocation, as well as the strengthening of programmes to raise household income to support private health spending, in addition to the development of strategies to promote well-planned and organized urban centres.
Malaria has remained a persistent global health problem. Children and pregnant women are particularly at risk for malaria, making it a major global public health concern. In 87 countries where malaria is endemic, there were 229 million estimated cases in 2019, down from 238 million in 2000. There were 218 million estimated malaria cases worldwide in 2015, according to the baseline data for the Global Technical Strategy for Malaria (GTSM) 2016–2030 of the World Health Organization (WHO) [ 1 ]. Global malaria incidence decreased from 80 cases per 1000 people at risk in 2000 to 58 cases in 2015 and 57 cases in 2019. Global malaria case incidence decreased by 27 percent between 2000 and 2015, and by less than two percent between 2015 and 2019, showing a slowing of the decline's pace since 2015 [ 2 ]. The 2022 WHO World Malaria Report further puts the global malaria death to about 619,000 in 2021, which reflects a 9 percent increase from the 568,000 deaths recorded before the pandemic struck. Similarly, worrisome is that malaria cases reached 247 million in 2021 compared to 245 million in 2020 and 232 million in 2019 [ 3 ].
Understanding the drivers of malaria incidence can be handy in guiding policies aimed at reducing malaria. This is the main objective of the current study. The focus is on Uganda as a country with one of the highest global burden of malaria cases with over 90% of the population at risk, but also a country where malaria remains the leading cause of death, especially in children. According to the WHO [ 3 ], there were an estimated 13 million malaria cases and over 19,600 estimated deaths in the country in 2021 alone. Existing data further shows that the disease causes immense detrimental health effects and is responsible for 30 to 50% of outpatient visits and 15 to 20% of hospital admissions [ 4 ]. In addition to its considerable impact on morbidity and mortality, the economic sector has not been spared in terms of unimaginable economic loss that has averaged over $500 million during the last decade.
Therefore, malaria continues to rank among Uganda's most critical diseases. Pregnant women and children under five are disproportionately impacted. According to hospital data [ 5 ], malaria is thought to be the cause of 30 to 50 percent of outpatient visits, 15 to 20 percent of hospitalizations, and 9 to 14 percent of inpatient mortality. Uganda has the third largest global burden of malaria cases, that is, five percent of the 229 million cases worldwide, and the eighth highest level of deaths, that is, three percent of the anticipated 405,000 malaria-related deaths worldwide [ 1 ].
Specifically, the objective is first to determine if government expenditure on health results in low malaria incidence in Uganda. Secondly, the study determines if private expenditure on health results in low malaria incidence in Uganda. Despite multiple government and donor initiatives to eradicate malaria and its detrimental effects on Uganda's health outcomes, the incidence of malaria is worrying as it appears higher than the average of 219 cases per 1000 for sub-Saharan Africa for the period 2017–2018, for example. In fact, Uganda was estimated to have the 3rd highest number of Plasmodium falciparum malaria cases globally in 2018, with incidence rates of greater than 250 cases per 1000 population at risk within a perennial transmission setting [ 6 ]. According to the 2018 WHO report [ 7 ], Uganda is one of the 15 nations that account for 80 percent of the global malaria burden and one of the five that account for nearly half of all malaria cases worldwide. Figure 1 shows malaria prevalence map of Uganda for the period 2018–2019. The map below shows the percentage of children 6 to 59 months of age tested using microscopy who are positive for malaria.
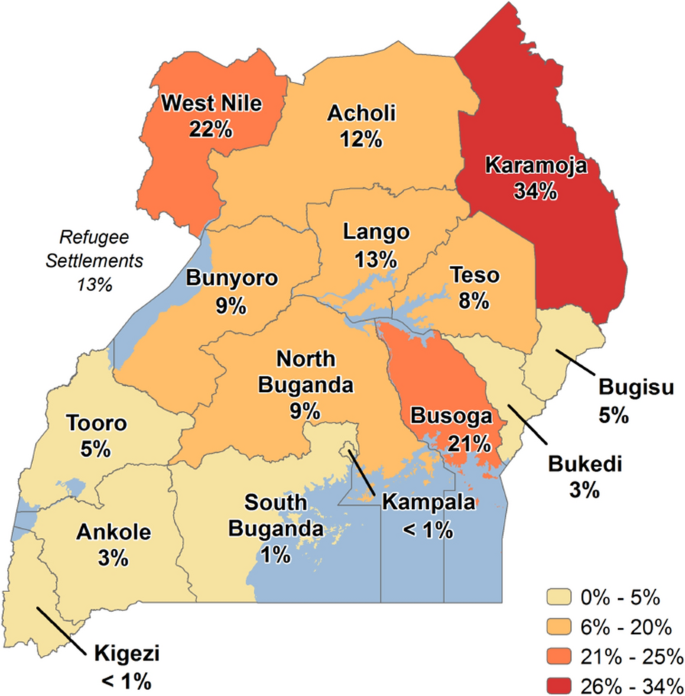
Source: Malaria Indicator Survey 2018–2019 [ 12 ]
Malaria Prevalence Map—Uganda.
As argued elsewhere [ 6 ], given the increasing progress in international efforts to reduce malaria transmission, it is increasingly important to track changes in malaria incidence rather than prevalence. Note that while malaria prevalence reflects the number of existing cases of a disease, malaria incidence reflects the number of new cases of the disease and can be reported as a risk or as an incidence rate. Given the nature of the research question to answer in the current study, as earlier explained, the focus is on incidence, to trace the effect of expenditure on new cases within a specific period. Understanding the drivers of malaria incidence can be handy in guiding policies aimed at reducing malaria cases in Uganda. The purpose of the study is to ascertain how public and private spending affects the incidence of malaria in Uganda to be able to inform policy formulation and implementation.
From the empirical arena, certainly much has been documented albeit with emphasis on the impact of public and private health expenditure on health outcomes, including inter alia life expectancy, infant mortality, under-five mortality, or a combination of these factors. One important note from these existing works of literature is that the focus has mainly been on other countries rather than Uganda, but also most importantly, there is scanty literature that considers malaria incidence despite its importance in understanding the dynamics of malaria. Also, a critical methodological issue not given much attention in the previous literature is endogeneity. One related study [ 8 ] examines the impact of private and governmental health spending on health indicators (viz. Life expectancy, infant mortality, and the under-five mortality rate inter alia) in 105 countries with moderate- and high-income levels, using the Generalized Least Squares (GLS) technique. The findings showed that public health spending increased health status and had a significant impact on health indicators across all groups. The study found no evidence of the role of private health spending on the selected outcomes.
On the other hand, a study focusing on Nigeria, and utilizing the ordinary least square (OLS) approach, find that while private health spending, the number of doctors, and life expectancy had negative relationships with neonatal mortality rate, child mortality rate, and infant mortality, respectively, government health expenditure per capita had a positive relationship with each of these variables [ 9 ]. Similarly, a positive relationship between public health expenditure and life expectancy is reported in a recent study on Ghana during the period 1980 to 2014, draw its findings based on the ordinary least squares (OLS) and two-stage least squares (2SLS) estimators and observes that raising public health spending by 10 percent prevented 0.102 to 4.4 infant and under-five deaths for every 1000 live births while boosting life expectancy by 0.77 to 47 days each year [ 10 ]. The latter findings are not much different from a related study employed the Kenyan household data supplemented with county-level data [ 11 ].
The study findings based on the ARDL model, which takes care of endogeneity, indicate that in the long run, an increase in public spending of one percent significantly reduces malaria incidence by 0.196 at the 10 percent level of significance. An increase in private health expenditure does not significantly influence malaria incidence at the 10 percent level of significance, but at the 15 percent level of significance, a one percent increase would lead to 0.018 percent decrease in malaria incidence. However, in the short run, public spending reduces malaria incidence by 0.158 percent while private expenditure has no impact.
Narrative history of malaria health expenditures in Uganda
Uganda's overall public health spending has been rising, but it still falls short of the 15 percent Abuja target and the five percent WHO Commission on Macro Economics target. Similarly, with the current level of health expenditure, Uganda is still far from achieving the 2030 Sustainable Development Goal (SDG) aim to "end the epidemics of AIDS, TB, malaria, and neglected tropical diseases; tackle hepatitis, water-borne infections, and other communicable diseases”. The clear observation is that in general, over the years, the nation continues to be heavily dependent on aid from outside, which accounts for around 45 percent of health spending and raises worries about sustainability. The National Health Accounts (NHAs) for 2018 showed that donor financing for the malaria programme was falling, but there is no evidence that the government is substituting its funds for those of the donors. On the other hand, out-of-pocket health expenses account for 37% of total household consumption spending, much beyond the threshold of 20 percent for catastrophic household consumption spending that is often advised [ 7 ].
Specifically, while government funding for malaria programming increased from FY2010/11 to FY2014/15, it decreased in absolute terms in FY 2015/16, from USD 30.6 million (UGX 102,900 million) in FY2014/15 to USD 29 million (UGX 97,400 million). However, it increased as a percentage of the government budget for the corresponding fiscal years, from 21.36 to 21.94 percent. The Global Fund and the U.S. President's Malaria Initiative Uganda (PMI), which increased their disbursement for malaria programmes in Uganda from approximately USD 71.3 million (UGX 239.6 billion) in 2014 to approximately USD 125 million (UGX 420 billion) in 2016, are the two main external malaria funders [ 7 ]. Despite the claimed increase in donor financing, households continue to make a sizable financial contribution to the fight against malaria in Uganda, endangering their ability to get effective treatment.
To lower the incidence of malaria, the government is stepping up its control efforts, such as increasing the use of indoor residual insecticide spraying (IRS), long-lasting insecticidal nets (LLINs), and artemisinin-based combination therapy (ACT). Despite this, there are still many cases of malaria. For example, in 2019, there were 263 cases per 1000 persons annually, down from 283 in 2016 [ 2 ]. When compared to the target reduction of 50 percent in 2025, this is a pitiful 7.2 percent drop [ 12 ]. Available statistics also indicate that in 2020, Uganda had the 3rd highest global burden of malaria cases and deaths (5.4%). This qualified the country to have the 5th highest proportion of malaria cases in East and Southern Africa (23.2%), increasing expenditures to curb the same notwithstanding. This raises the question of whether increasing public and private expenditure on health effectively leads to decreases in malaria incidence.
Data type and source
The study used quarterly time series secondary data for the period 2000:1 to 2019:4. The variables included malaria incidence, public health expenditure, private health expenditure, GDP per capita growth, female unemployment, poverty proportion, urban population growth, female-headed households, foreign aid per-capita, female literacy rate, GINI index, regulatory quality, corruption control, and government effectiveness. Data for all variables was obtained from World Development Indicators [ 13 ].
Model specification
The study employed the Grossman [ 14 ] framework for analysing demand for health. The emphasis is on investment in human capital (health and education) for better health outcomes and economic growth. According to the framework, a theoretical health production function can be specified as:
where H is a measure of individual health output and X is a vector of individual inputs to the health production function F. The elements of the vector include nutrient intake, income, consumption of public goods, education, time devoted to health-related procedures, initial individual endowments like genetic make-up, and community endowments such as the environment.
Although Grossman designed this theoretical model for the analysis of health production at the micro level, several studies including the above-mentioned, have tried to employ his specification at the macroeconomic level. To switch from micro to macro analysis, without losing the theoretical ground, the elements of the vector X were represented by per capita variables and regrouped into sub-sectoral vectors of economic, social, and environmental factors as:
where \(y\) a vector of per capita economic variables is, \(s\) is a vector of per capita social variables, and \(v\) is a vector of per capita environmental factors. In its scalar form, Eq. ( 4 ) can be rewritten as
where h is the individual’s health status, \(\left({y}_{1},{y}_{2},\dots , {y}_{n}\right)=y\) ; \(\left({s}_{1},{s}_{2},\dots , {s}_{m}\right)=s\) ; \(\left({v}_{1},{v}_{2},\dots , {v}_{l}\right)=v\) , while \(n\) , \(m\) , and \(l\) are the number of variables in each sub-group, respectively.
The theoretical model presented above can be modified to health production functions for estimating the relationship between health expenditure and malaria incidence. Due to the existing cultural and environmental conditions of Uganda, and the availability of continuous, reliable, and sufficient data on the variables; the variables representing economic factors are limited to include GDP per capita growth \(({y}_{1})\) ; domestic general government health expenditure per capita \(({y}_{2})\) ; domestic private health expenditure per-capita \(({y}_{3})\) ; female unemployment rate \(({y}_{4})\) ; proportion of population pushed below poverty line \(({y}_{5})\) ; foreign aid per-capita \(({y}_{6})\) ; and GINI index ( \({y}_{7}\) ). The variables representing social factors are limited to education proxied by school enrolment at the primary of female as percentage of gross enrolment \(({s}_{1})\) ; female-headed households \(({s}_{2})\) ; regulatory quality \(({s}_{3})\) ; control of corruption \(({s}_{4})\) ; and government effectiveness \(({s}_{5})\) . The variables representing environmental factors include urbanization proxied by urban population growth \(({v}_{1})\) ; and use of insecticide-treated bed nets \(({v}_{2})\) ; and individual’s health status proxied by malaria incidence \((h)\) . The relationship can be expressed as follows.
where, \({mi}_{t}\) is the malaria incidence at time \(t\) , \(j=1,...,N\) is the number of economic, social and environmental control variables. According to the WHO [ 15 ], malaria incidence refers to the number of new malaria cases per 1000 population at risk per year. In turn, the population at risk is defined as the population living in areas where malaria transmission occurs. \({puhe}_{t}\) represents public expenditure on health at time \(t\) and \({pvhe}_{t}\) represents private health expenditure at time \(t\) and these two are the variables of prime interest. \({Z}_{t}\) is a vector representing economic, social and environmental control variables, and \({\delta }_{j}\) is a vector of coefficients for economic, social, and environmental variables, while \({\varepsilon }_{t}\) and \({\beta }_{0}\) are the error term and intercept, respectively.
The variables in Eq. ( 6 ) were transformed using the natural logarithmic form of the series to capture the elasticities while removing any skewness in the data. The non-linear and non-monotonic link between the independent variables and dependent variables is captured by the model's logarithmic transformation. Heteroscedasticity is also decreased by log transformation. Additionally, the transformation of the variables implies that the coefficients of the variables are read as measuring elasticities. So, the logarithmic transformation results in:
It is paramount to do unit root tests of all the variables in the model before estimating the model to avoid the problem of spurious results which emanate from estimation using non- stationary time series. The study employs both Augmented Dickey Fuller (ADF) [ 16 ] and Phillips Perron (PP) [ 17 ] tests on each of the variables included in the model to ascertain whether they are stationary or non-stationary and to indicate their order of integration [ 18 ].
Estimation technique
The results from the unit root tests, as later shown in Table 5 , are indicative of a mixture of integration of I (0) and I (1), suggesting the use of the Pesaran and Shin technique [ 19 ]. Specifically, the Bound Testing Cointegration method using the ARDL methodology was used in this investigation to determine if a long run equilibrium relationship exists among the variables. Nevertheless, before estimating the ARDL model, the best lag needs to be determined. The Akaike Information Criterion (AIC) with minimum value or the Schwarz Bayesian Information Criterion (SBIC) might be used for this. The ARDL model used in this study is chosen by SBIC because according to Pesaran and Smith [ 20 ] a model selected by SBIC is a more parsimonious model which helps in saving degrees of freedom especially in studies with small sample size like the current study.
Before the ARDL model is estimated, a bounds test must be performed to determine whether the variables contained in the model have a long-term relationship. The following is the ARDL estimate model:
where ∆ = First-difference operator; \({\alpha }_{0}\) = the intercept, \(i\) = lag length, \({\varepsilon }_{t}\) = error term, \(j=1,...,N\) is the number of economic, social and environmental control variables. The Wald test (F-statistics) is employed to determine whether there is a long-term link between the variables in the model. The alternative hypothesis for the test asserts that cointegration among the variables in the model does exist, contrary to the null hypothesis, which argues that cointegration among the variables in the mode does not exist. The following is a statement of the null and alternative hypotheses:
The calculated F-statistic is compared to the critical F-values given by Pesaran, Shin, and Smith [ 21 ], where two sets of critical values, that is, lower bound critical value and upper bound critical value, for a particular significance level are provided. For all classes of the variables, such as strictly I (0), strictly I (1), or mutually cointegrated, they yield boundaries critical values.
Based on an F-statistic bound table and the following criteria, cointegration is concluded. The null hypothesis is rejected, implying that there is cointegration between the variables if the estimated F-statistic is greater than the higher critical value. Note that it cannot be concluded that there is no cointegration if the estimated F-statistic is less than the lower bound critical value. The test is inconclusive if the estimated F-statistic is between the lower and upper boundaries, and a determination of the existence of a long-term association requires information about the order of integration.
A diagnostic test for serial correlation, functional normality, and heteroskedastic specification error were carried out to verify the ARDL model's goodness of fit. The cumulative sum of recursive (CUSUM) and cumulative sum of squares of recursive residuals (CUSUMSQ) were used to test model stability.
The ARDL model's ECM equation for this investigation is as follows:
where ECT is the error correction term, \(\mu_{t}\) is the error term.
\(lnmi\) is the logged annual incidence of malaria cases per 1000 at-risk populations. In this study, it served as the dependent variable. Public health expenditure per capita ( \(lnpuhe\) ) in logs, is the amount the government spends on healthcare expressed in current international US dollars at purchasing power parity. \(lnpuhe\) and \(lnmi\) are expected to be negatively correlated, a priori. \(lnpvhe\) stands for current private health expenditures per capita expressed in US current international dollars at purchasing power parity and is in log form. \(lnpvhe\) is included as a separate determinant variable because public allocations do not account for private health spending, even though they make up a sizable share of all SSA health expenditures. \(lnpvhe\) affects \(lnmi\) via augmenting \(lnpuhe\) , making necessary medications affordable, and people using dietary supplements. The expected sign is negative. Table 1 provides the variable description and source of data.
The variables contained in the vector \(Z_{t}\) arise from literature and include; GDP per-capita growth ( \(ggdppgr\) ), female unemployment ( \(lnue\_fem\) ), poverty proportion ( \(lnpov\_prop\) ), urban population growth ( \(urbanpopn\_gr\) ), female-headed households ( \(lnfemhh\) ), foreign aid per-capita ( \(lnodapc\) ), female literacy rate ( \(litracy\_fem\) ), GINI index ( \(lngini\) ), regulatory quality ( \(rqlty\) ), corruption control ( \(ccorr\) ) and government effectiveness ( \(geff\) ). The rationale for including these variables can be found in the next section.
\(ECT_{t - 1}\) is the lagged error correction term of the residual from the cointegrating regression equation. It captures the adjustment toward the long-run equilibrium. The coefficient \({\gamma }_{4}\) represents the proportion of disequilibrium in malaria incidence in one period corrected in the next period. That is, the speed of adjustment, and is expected to have negative sign.
Summary statistics, rationale for variable inclusion and the pairwise correlation matrix
The fundamental descriptive statistics for all variables by the original data are shown in Table 2 . The lowest malaria incidence was 250.62 in the third quarter of 2015, and the highest was 509.68 in the third quarter of 2001, indicating a wide range in malaria incidence within the sample. It was also discovered a lot of heterogeneity in the health-care spending proxies. With a mean of $16.66, public health expenditures ranged from $12.006 (second quarter of 2000) to $22.918 (fourth quarter of 2010). With a mean of $42.57, private health expenditures ranged from $18.89 (first quarter of 2004) to $59.945 (third quarter of 2013). The lowest percentage population increase in urban centres (5.763 percent) occurred in the first quarter of 2000, while the highest occurred in the third quarter of 2017 (6.257 percent). The summary statistics show reduced levels of skewness and kurtosis, indicating that data normality is not a problem. The minor discrepancies between the mean and median values of these variables support this, implying that the data has a high level of consistency.
The inclusion of GDP per-capita growth as a measure of an increase in average income per person in a specific country serves as a control variable for utilization of healthcare services since income is an influencing factor in health-seeking behaviour. A priori, it would be expected that this variable to be negatively correlated with malaria incidence rates, based on the argument that as real per capita increases, one would expect the standards of living of the people to improve. Similarly, the female unemployment rate (the proportion of the female population ages 15–64 that is not economically active) was included to capture the effect of female unemployment on home health production for family members or how their absence in the labor market denies supplementing family income to command more health inputs to help improve the health of their families [ 22 ]. The expected sign is positive. Additionally, poverty proportion quantifies the percentage of the population that out-of-pocket medical expenses cause to fall below the $1.90 ($2011 PPP) poverty threshold. This variable is included because people with different socioeconomic statuses experience illness burden, coverage, and impact of public health interventions differently. To be more specific, the poorest of the poor are disproportionately affected by malaria [ 23 ]. Moreover, as the latter authors state, in developing nations, the poorest populations frequently reside in the most isolated places and are ostracized socially or culturally. Therefore, a priori, the variable is expected to be positively correlated with malaria incidence rates.
The other variable appearing in the analysis is urban population growth (i.e. urban population growth as a percentage of total population growth). For, as some authors have argued [ 24 ], urbanization rate may improve the health status since health facilities in urban areas are more cost-effective. Moreover, it is avered that in Africa, malaria transmission is comparatively higher among the rural setting than urban areas which may be because of the higher vector density, lower housing quality, and the poor drainage systems in rural settings [ 25 ]. Therefore, a prior expected sign for this variable is negative. Also included is the female-headed household measured as percent of households with a female head. This is meant to illustrate how gender roles affect how households control malaria. Recently, it has been argued that female-headed families are more likely to report adopting preventative measures against malaria, such as dousing the home in pesticide, draining stagnant puddles, and maintaining a clear environment [ 26 ]. However, this may not always be true. The expected sign of the coefficient is therefore uncertain. Foreign aid is another control factor included in our model, measured as the official development assistance provided per person in current US dollars. The rationale for its inclusion is because, as earlier presented, external donors provided the vast majority (95%) of financing for malaria prevention, control, and treatment. A negative sign is expected.
Similarly, the study includes female literacy (the ratio of female to total primary school enrollment, regardless of age, to the population of the age group that officially corresponds to the level of education shown) because it captures the efficiency with which health is produced, and reduces the malaria incidence rates [ 27 ]. This is because in most developing countries, women play an important role in family health such as sleeping under mosquito-treated nets and contributing to malaria eradication programmes [ 24 ], and therefore, women’s ability to make informed health decisions for the family could be enhanced by their literacy level since educated mothers are more likely to be aware and assimilate information about the health needs of the family [ 28 ]. An a priori expectation is that female literacy is negatively related to malaria incidence. Income inequality, as measured by the GINI coefficient, is also included in the analysis as it is alleged that higher-income households are more likely than lower-income households to undertake malaria prevention measures [ 29 ]. As such, it is hypothesized that the variable would possess a positive sign. The data source for all the aforementioned variables is the World Bank World Development Indicators [ 13 ].
However, institutional variables, with data sourced from the World Bank World Governance Indicators [ 13 ] are also included. Specifically, regulatory quality is included because poor-quality and counterfeit pharmaceuticals pose a serious risk to the public's health by directly raising the likelihood of treatment failure, the development of antimicrobial resistance, morbidity, death, and healthcare costs. When a socially disadvantaged group or groups within a population are unable to reach their optimal level of health, health inequalities result. For instance, when those with lower socioeconomic statuses are more likely to suffer from poor health outcomes, it leads to an unequal distribution of health that may be traced back to a specific social situation. Moreover, it has been argued that the burden of malaria falls disproportionately on children, the poor, and rural communities in low- and middle-income countries [ 30 ]. The regulatory quality is expected to be negatively correlated with malaria incidence rates.
The second institutional variable is the Control of Corruption that tracks the misuse of public funds allocated for the fight against malaria for personal gain. In essence, corruption depletes resources, making it difficult to provide for the needs of the community, such as access to clean water, good health care, and education [ 31 ]. A priori, corruption control is expected to be negatively correlated with malaria incidence rates. Finally, government effectiveness is another institutional factor included in our analysis as it is likely that in developing nations the government plays a crucial role in the provision of high-quality healthcare, implying that government efficiency is critical. For, a government's capacity to develop and carry out sound policies and provide for the common good is measured by its effectiveness. Moreover, as elsewhere suggested [ 32 ], the policies that result from competent government management include inter alia health insurance, free prenatal care, and good road infrastructure. As a result, the incidence of malaria is directly correlated with government effectiveness. A negative correlation between the two variables is expected.
The check for regressors’ correlation (to exclude strongly correlated variables) revealed no issues (correlation coefficients were found to be below 0.8, as suggested by econometric studies). The correlation coefficients between the independent variables are shown in Table 3 . Multicollinearity was unlikely to be a problem in the estimations, as the coefficients were relatively low. As a result, no explanatory variables were removed from the study.
Unit root tests results
The results of the stationarity tests based on the Augmented Dickey-Fuller (ADF) and Phillips-Peron (PP) unit root tests are shown in Table 4 . The stationarity tests show that at 95 percent level of confidence, the data are not stationary with the exception of GDP per-capita growth, the percentage of people living in poverty, the growth of the urban population, and female headed households which are stationary at the level. Thus, the variables are either I (0) or I (1).
Bounds test results
Based on the bounds test, when the computed F-statistic is greater than the upper bound, I (1), the null hypothesis that no cointegration exists between the series is rejected. However, if the F-statistic is less than the lower bound, I (0), the null hypothesis that there is no cointegration between the series is accepted. Otherwise, if the F-statistic falls between I (0) and I (1), our inference would be inconclusive. The findings across all models show that for I (0) regressors, the null hypothesis of no cointegration is rejected at one percent. Additionally, for I (1) regressors, all models show that the null hypothesis is rejected at a one percent level. It can therefore be concluded that there is a long run relationship at the one percent significance level except Model 3 for which it exists at the five percent significant level. The results of the bounds test are presented in Table 5 .
ARDL model estimation results
Effect of public and private expenditure in the longrun and shortrun.
In Table 6 , Model 1, (the preferred model), the main finding is that in the long run, increasing public spending by one percent would cause malaria incidence to reduce by 0.196 percent. This result is statistically significant at five percent level. Also, in terms of magnitude, public expenditure on health yields greater effect in terms of reducing the malaria incidence compared to private spending. Inferentially, government health spending, in contrast to findings recorded elsewhere [ 33 ], is crucial for lowering the incidence of malaria in Uganda. These results are in line with past empirical findings [ 8 , 32 , 34 ], inter alia] . It can however be noted that private health expenditure does not significantly influence malaria incidence in the long run. This could point to the possibility that many Ugandans suffering from malaria turn to using local herbs rather than spend money on malaria treatment. Nevertheless, the private health expenditure effect on malaria incidence is, as expected, negative, although it was not statistically significant [coefficient = − 0.018, p-value = − 0.18]. By implication, the possibility for the private spending increase to reduce malaria incidence exists.
On the other hand, the lack of statistical significance could point to the ineffectiveness of the private individuals to take care of themselves when it comes to malaria treatment. To support this argument, recent authors [ 33 ] have found out that high out-of-pocket expenses act as a barrier to reducing malaria incidence. This is probably because in Africa, out-of-pocket expense percentage attributed to self-care in terms of disease fight is low. This leads to limiting access to prevention and treatment measures, delayed diagnosis and inadequate treatment. It is not uncommon to find some attributing their sickness to witchcraft. As a result, there is increased malaria transmission and higher incidence rates. Moreover, many Africans suffer from financial constraints that hinder preventive measures such as buying insecticide-treated bed nets and antimalarial medication. When one has nothing to eat, it is very difficult to think of buying a mosquito net or even going in for expensive malaria treatment. The most vulnerable are the poor folks normally living in SSA.
Based on the results presented in Table 7 , the short-run malaria incidence effect of public health expenditure remains highly significant at a one percent conventional level, though the coefficient is lower in magnitude. Precisely, increasing public health expenditure by one percent would yield a reduction of about 0.158 percent in malaria incidence in the short-run. Note, however, that at the first lag, the effect turns significantly positive. Nevertheless, the latter finding is not uncommon in literature as can be traced in an earlier study [ 35 ] to determine whether health spending reduces malaria cases in Kwara State of Nigeria. Private health expenditure having zero lags did not appear in the short run which shows that it has no impact on malaria incidence in the short run. This may be because the private expenditures are undertaken for treatment purposes other than prevention purposes in the short-run. However, treating malaria in the short run eliminates P. falciparum that causes malaria in the population thereby reducing the malaria incidence in the long-run.
Additional findings
The long-term impact of the GDP per capita on malaria incidence is also demonstrated by the results. GDP per capita coefficient is negative and significant at one percent level in the long run. This suggests that a one percent rise in GDP per capita results in a decrease in the incidence of malaria by 0.464. This finding is consistent with an earlier study [ 36 ] where it is asserted that economic growth may lessen malaria if it made more resources available for the disease's prevention. This is conceivable given the high direct expenses associated with treating and preventing malaria. An increase in household income might make it possible for them to spend more on mosquito control measures including providing bed nets, using insect repellents, and emptying wetlands and canals. Increased income would also enable households to spend more on medical care, including medication, travel, professional fees, and lodging at a hospital.
Higher incomes at the national level would make it possible to build public health facilities for the treatment of malaria patients and local initiatives to lessen the number of mosquitoes in a region. Additionally, increasing income is linked to the movement of workers to cities, which may reduce the percentage of the population that lives in rural areas with high rates of malaria. Considering all of these factors while holding constant variables like geography and temperature, it can be anticipated that wealthy countries will have lower malaria rates [ 36 ].
The study also found that in the long run, malaria incidence would increase when the fraction of female unemployment rises. The coefficient of female unemployment is statistically significant at one percent conventional level. Malaria incidence would increase by 0.186 percent as the percentage of female unemployment rises by one percent. On the other hand, increasing urbanization appears good news for the control of malaria. According to the results in Tables 7 and 8 , the relevant coefficient is significant at one percent and displays a negative relationship between urbanization and malaria incidence both in the long run and short run. Specifically, a one percent growth in urban population would reduce malaria incidence by 0.619 percent and 0.809 in the short run and long run, respectively. Similarly, recent studies [ 37 , 38 ] noted that infection probability declined with increasing urbanization, suggesting this be due to the reduction of suitable breeding grounds for malaria vectors through the reduction of vegetative cover, water surfaces, and other natural surfaces with building structures and other paved surfaces as well as through pollution of available breeding sites.
The current study also found that long-term malaria incidence would be impacted by income inequality. The GINI coefficient is positive and significant at a five percent level in the long run. This means that malaria incidence would increase by 0.960 percent as income inequality rises by one percent. This result is consistent with a recent study [ 39 ] where it is contended that the long-term effects of inequality become apparent when a high growth rate for vulnerable people with low income is observed. This indicates that rising economic disparity would increase the number of persons who find it difficult to afford both personal protection against malaria and its treatment because this category includes the lowest-income earners.
Relatedly, an increase in the number of female-headed households would lead to an increase in malaria incidence in the long run and short run. Malaria incidence will rise by 0.924 percent in the short term for every one percent increase in the proportion of female-headed households in the short run. When the proportion of households with a female head rises by one percent, malaria incidence will rise by 1.947 percent over the long term. This may be the case since female headed households are among the most vulnerable groups in society and are more likely to live in poverty, which makes it difficult for them to prevent malaria. Regulatory quality would impact malaria incidence negatively, though the impact is not significant.
The ECT coefficient is -0.166, and is significant at one percent level of significance. This demonstrates that any divergence from the long-term equilibrium between the variables and the incidence of malaria may be corrected and regained on average every quarter at a rate of 0.166 percent.
Diagnostic tests results
In Table 8 , a summary of the diagnostic tests that were used to identify model flaws is presented. There was no serial correlation in the models, according to the findings of the Breusch-Godfrey LM Test (BG test) and the Durbin-Watson test. There was no heteroscedasticity issue in the models, as demonstrated by the White test technique and the LM test for ARCH. The anticipated Ramsey reset tests have shown that the estimated models' functional form was correct and they were free of the omitted variables issue. In a similar manner, the results of the CUSUM and CUSUMSQ tests (not shown here due to space but available on request) indicated the model structures were stable. The Jarque–Bera test indicated that the models were normal, except model 3, thus model 1 was the most appropriate model.
Concluding remarks
Utilizing quarterly data from 2000Q1 to 2019Q4, the study's primary goal was to examine the short- and long-term relationships between malaria incidence, health spending indicators, and the economic and policy control variables in Uganda. The estimated ARDL models indicate that both public and private health spending have a significant impact on malaria incidence. The incidence of malaria is also greatly impacted by growth in GDP per capita. Additionally, while urban population growth, and an increase in proportion of the population falling in poverty may help to reduce the incidence of malaria in the long and short term, respectively, other factors such as an increase in households headed by women, and widening income inequality are likely to increase it. One other important finding is that female literacy appear to increase malaria incidence in the short run, while regulatory quality reduces it.
It is recommended that the Ugandan government implement new policies to boost health spending and strengthen the programmes being implemented to combat malaria, as well as strengthening the existing undertakings such as the President's Malaria Initiative, Home Based Management, the Health Sector Strategic and Investment Plan, the Against Malaria Foundation, Roll Back Malaria, and the Uganda Malaria Reduction Strategic Plan. Also, there is need to design interventions to ensure that a large percentage of the poorest are using effective treatment, insecticide-treated bed nets (ITNs). Similarly, policymakers are urged to devise strategies for enhancing the standard of regulation. For instance, efforts to strengthen and broaden the reach of the Uganda National Bureau of Standards (UNBS) should be well supported to increase their capacity to enforce quality medical and health-related supplies. On the other hand, the development of suitable infrastructural facilities, such as drainage systems that ensure there is no breeding ground for disease vectors, should be backed by policies or master plans given the evident role of urbanization in malaria incidence in Uganda. The study concurs with previous authors [ 40 ] that if Uganda is to eliminate malaria there is a need to ensure access to malaria prevention, diagnosis, and treatment as part of universal health coverage, through increased budget allocation.
In the process of investigation, some areas albeit outside the scope of the current study but which deserve attention for future studies were observed. For example, a similar analysis would be carried out for the entire East African region using panel data and using simultaneous equation modelling to discover and estimate the effects that are not discernible in pure cross-sections or time series. Additionally, the use of household-level models with survey data, once available, would be another future area of study to estimate the proportion of new money spent on malaria prevention and treatment, and the success of expenditures made to combat malaria. A detailed venture in these areas would provide a critical contribution to policy in kicking malaria out of the region.
Availability of data and materials
The study uses quarterly data on several variables spanning a period 2000:1 to 2019:4 for Uganda. All data is available from the relevant sources, including the World Bank World Development Indicators and World Bank Governance Indicators, accessible at https://data.worldbank.org
Abbreviations
Augmented Dickey-Fuller
Akaike Information Criterion
Acquired Immune-Deficiency Syndrome
Auto-regressive distributed lags
Global fund to fight HIV, tuberculosis and malaria
Global technical strategy for malaria
Home based management of fever
Health Sector Strategic and Investment Plan
Insecticide-treated nets
Ministry of Health
National Malaria Control Programme
Sustainable development goal
Sub-Saharan Africa
World Bank Development Indicators
World Bank Governance Indicators
World Health Organization
WHO. World Malaria Report 2019. Geneva: World Health Organization; 2019.
WHO. World Malaria Report 2020. Geneva: World Health Organization; 2020.
WHO. World Malaria Report 2022. Geneva: World Health Organization; 2022.
Namuganga JF, Briggs J, Roh ME, Okiring J, Kisambira Y, Sserwanga A, et al. Impact of COVID-19 on routine malaria indicators in rural Uganda: an interrupted time series analysis. Malar J. 2021;20:475.
Article CAS PubMed PubMed Central Google Scholar
Ministry of Health. National Malaria Control Programme. Uganda, Kampala, 2018.
Kigozi SP, Kigozi RN, Sebuguzi CM, Cano J, Rutazaana D, Opigo J, et al. Spatial-temporal patterns of malaria incidence in Uganda using HMIS data from 2015 to 2019. BMC Public Health. 2020;20:1913.
Article PubMed PubMed Central Google Scholar
WHO. Country cooperation strategy at a glance: Uganda. Geneva, World Health Organization; 2018.
Rezapour A, Mousavi A, Lotfi F, Movahed MS, Alipour S. The effects of health expenditure on health outcomes based on the classification of public health expenditure: a panel data approach. Shiraz E-Med J. 2019;20:1–7.
Article Google Scholar
Adewumi SB, Acca YA, Afolayan O. Government health expenditure and health outcomes in Nigeria: the challenge to underdeveloped economy. Int J Res Innovation Soc Sci. 2018;2:463–71.
Google Scholar
Boachie MK, Ramu K, Polajeva T. Public health expenditures and health outcomes: new evidence from Ghana. Economies. 2018;6:1–25.
Muthaka DI. Health expenditures and child mortality: evidence from Kenya. Doctoral dissertation. University of Nairobi, Kenya; 2013.
Ministry of Health. The Uganda Malaria Reduction and Elimination Strategic Plan 2021–2025. Kampala, Uganda; 2020.
WDI. World Bank World Development Indicators. World Bank; 2020.
Grossman M. The demand for health—a theoretical and empirical investigation. New York: National Bureau of Economic Research; 1972.
WHO. World Malaria Report 2023. Geneva: World Health Organization; 2023.
Dickey DA, Fuller WA. Likelihood ratio statistics for autoregressive time series with a unit root. Econometrica. 1981;49:1057–72.
Phillips PCB, Perron P. Testing for a unit root in time series regression. Biometrika. 1988;75:335–434.
Emeka N, Aham KU. Autoregressive Distributed Lag (ARDL) cointegration technique: application and interpretation. J Stat Econom Methods. 2016;5:63–91.
Pesaran MH, Shin Y. An Autoregressive Distributed Lag Modelling Approach to Cointegration Analysis. In: Strom S. (Ed.). Chapt. 11: Econometrics and Economic Theory in the 20th Century: The Ragnar Frisch Centennial Symposium, Cambridge University Press, Cambridge, 1999;371–413.
Pesaran MH, Smith RJ. Structural analysis of cointegration VARS. J Econ Surv. 1998;12:471–505.
Pesaran H, Shin Y, Smith RJ. Bounds testing approaches to the analysis of level relationships. J Appl Econ. 2001;16:289–326.
Boachie MK, Ramu K. Effect of public health expenditure on health status in Ghana. Int J Health. 2016;4:1–25.
Barat LM, Palmer N, Basu S, Worrall E, Hanson K, Mills A. Do malaria control interventions reach the poor? A view through the equity lens. J Trop Med. 2004;71:174–8.
Fayissa B, Gutema P. The determinants of health status in sub-Saharan Africa. Am Econ. 2005;49:60–6.
Sultana M, Sheikh N, Mahumud RA, Jahir T, Islam Z, Sarker AR. Prevalence and associated determinants of malaria parasites among Kenyan children. Trop Med Health. 2017;45:25.
Iddrisu D, Moyer CA. Using the Ghana malaria indicator survey to understand the difference between female and male-headed households and their prevention and testing for malaria among children under 5. Malar J. 2022;21:112.
Okunade AA. Analysis and implications of the determinants of healthcare expenditure in African countries. Health Care Manag Sci. 2005;8:267–76.
Article PubMed Google Scholar
Currie J, Moretti E. Mother’s education and the intergenerational transmission of human capital: evidence from college openings. Q J Econ. 2003;118:1495–532.
Pellegrini L, Tasciotti L. The electrification–malaria nexus: the case of rural Uganda. Eur J Dev Res. 2016;28:521–35.
Evans DR, Higgins CR, Laing SK, Awor P, Ozawa S. Poor-quality antimalarials further health inequities in Uganda. Health Policy Plan. 2019;34:iii36–47.
Siverson RM, Johnson RA. Politics and parasites: the contribution of corruption to human misery. Int Stud Q. 2014;58:199–206.
Ashiabi N, Nketiah-Amponsah E, Senadza B. The effect of health expenditure on selected maternal and child health outcomes in sub-Saharan Africa. Int J Soc Econ. 2016;43:1386–99.
Eboh A, Adebayo AO. Addressing malaria incidence in Africa through health care expenditure and access to basic sanitation services. Discov Health Syst. 2023;2:1–12.
Arthur E, Oaikhenan HE. The effects of health expenditure on health outcomes in sub-Saharan Africa (SSA). Afr Dev Rev. 2017;29:524–36.
Olalekan MS, Nurudeen AS. Malaria burden and the effectiveness of malaria control measures in Nigeria: a case study of Asa Local Government Area of Kwara State. J Econ Sustain Dev. 2013;4:295–308.
Datta SC, Reimer JJ. Malaria and economic development. Rev Dev Econ. 2013;17:1–15.
Kabaria CW, Gilbert M, Noor AM, Snow RW, Linard C. the impact of urbanization and population density on childhood Plasmodium falciparum parasite prevalence rates in Africa. Malar J. 2017;16:49.
Larson PS, Eisenberg JN, Berrocal VJ, Mathanga DP, Wilson ML. An urban-to-rural continuum of malaria risk: new analytic approaches characterize patterns in Malawi. Malar J. 2021;20:418.
Bala S, Gimba B. Global sensitivity analysis to study the impacts of bed-nets, drug treatment, and their efficacies on a two-strain malaria model. Math Comput Appl. 2019;24:1–32.
Yeka A, Gasasira A, Mpimbaza A, Achan J, Nankabirwa J, Nsobya S, et al. Malaria in Uganda: challenges to control on the long road to elimination: I. Epidemiology and current control efforts. Acta Trop. 2012;121:184–219.
Download references
Not applicable.
Author information
Authors and affiliations.
School of Economics, Makerere University, Kampala, Uganda
Jemimah Katushabe, John Bosco Nnyanzi & Gertrude Sebunya Muwanga
You can also search for this author in PubMed Google Scholar
Contributions
JK initiated the idea, developed it and wrote the draft manuscript. JBN contributed to the analysis and discussion, editing, additions to literature review and to the final text. GM worked on the editing, problem strengthening and language issues. All authors reviewed the manuscript.
Corresponding author
Correspondence to John Bosco Nnyanzi .
Ethics declarations
Ethics approval and consent to participate, competing interests.
The authors of this manuscript declare no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Katushabe, J., Nnyanzi, J.B. & Muwanga, G.S. Exploring the role of spending on malaria incidence in Uganda using the auto-regressive distributed lag approach. Malar J 23 , 129 (2024). https://doi.org/10.1186/s12936-024-04929-8
Download citation
Received : 05 March 2023
Accepted : 04 April 2024
Published : 30 April 2024
DOI : https://doi.org/10.1186/s12936-024-04929-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Malaria Incidence
- Health expenditure
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]
- Share full article
Advertisement
Supported by
Environmental Changes Are Fueling Human, Animal and Plant Diseases, Study Finds
Biodiversity loss, global warming, pollution and the spread of invasive species are making infectious diseases more dangerous to organisms around the world.

By Emily Anthes
Several large-scale, human-driven changes to the planet — including climate change, the loss of biodiversity and the spread of invasive species — are making infectious diseases more dangerous to people, animals and plants, according to a new study.
Scientists have documented these effects before in more targeted studies that have focused on specific diseases and ecosystems. For instance, they have found that a warming climate may be helping malaria expand in Africa and that a decline in wildlife diversity may be boosting Lyme disease cases in North America.
But the new research, a meta-analysis of nearly 1,000 previous studies, suggests that these patterns are relatively consistent around the globe and across the tree of life.
“It’s a big step forward in the science,” said Colin Carlson, a biologist at Georgetown University, who was not an author of the new analysis. “This paper is one of the strongest pieces of evidence that I think has been published that shows how important it is health systems start getting ready to exist in a world with climate change, with biodiversity loss.”
In what is likely to come as a more surprising finding, the researchers also found that urbanization decreased the risk of infectious disease.
The new analysis, which was published in Nature on Wednesday, focused on five “global change drivers” that are altering ecosystems across the planet: biodiversity change, climate change, chemical pollution, the introduction of nonnative species and habitat loss or change.
The researchers compiled data from scientific papers that examined how at least one of these factors affected various infectious-disease outcomes, such as severity or prevalence. The final data set included nearly 3,000 observations on disease risks for humans, animals and plants on every continent except for Antarctica.
The researchers found that, across the board, four of the five trends they studied — biodiversity change, the introduction of new species, climate change and chemical pollution — tended to increase disease risk.
“It means that we’re likely picking up general biological patterns,” said Jason Rohr, an infectious disease ecologist at the University of Notre Dame and senior author of the study. “It suggests that there are similar sorts of mechanisms and processes that are likely occurring in plants, animals and humans.”
The loss of biodiversity played an especially large role in driving up disease risk, the researchers found. Many scientists have posited that biodiversity can protect against disease through a phenomenon known as the dilution effect.
The theory holds that parasites and pathogens, which rely on having abundant hosts in order to survive, will evolve to favor species that are common, rather than those that are rare, Dr. Rohr said. And as biodiversity declines, rare species tend to disappear first. “That means that the species that remain are the competent ones, the ones that are really good at transmitting disease,” he said.
Lyme disease is one oft-cited example. White-footed mice, which are the primary reservoir for the disease, have become more dominant on the landscape, as other rarer mammals have disappeared, Dr. Rohr said. That shift may partly explain why Lyme disease rates have risen in the United States. (The extent to which the dilution effect contributes to Lyme disease risk has been the subject of debate, and other factors, including climate change, are likely to be at play as well.)
Other environmental changes could amplify disease risks in a wide variety of ways. For instance, introduced species can bring new pathogens with them, and chemical pollution can stress organisms’ immune systems. Climate change can alter animal movements and habitats, bringing new species into contact and allowing them to swap pathogens .
Notably, the fifth global environmental change that the researchers studied — habitat loss or change — appeared to reduce disease risk. At first glance, the findings might appear to be at odds with previous studies, which have shown that deforestation can increase the risk of diseases ranging from malaria to Ebola. But the overall trend toward reduced risk was driven by one specific type of habitat change: increasing urbanization.
The reason may be that urban areas often have better sanitation and public health infrastructure than rural ones — or simply because there are fewer plants and animals to serve as disease hosts in urban areas. The lack of plant and animal life is “not a good thing,” Dr. Carlson said. “And it also doesn’t mean that the animals that are in the cities are healthier.”
And the new study does not negate the idea that forest loss can fuel disease; instead, deforestation increases risk in some circumstances and reduces it in others, Dr. Rohr said.
Indeed, although this kind of meta-analysis is valuable for revealing broad patterns, it can obscure some of the nuances and exceptions that are important for managing specific diseases and ecosystems, Dr. Carlson noted.
Moreover, most of the studies included in the analysis examined just a single global change drive. But, in the real world, organisms are contending with many of these stressors simultaneously. “The next step is to better understand the connections among them,” Dr. Rohr said.
Emily Anthes is a science reporter, writing primarily about animal health and science. She also covered the coronavirus pandemic. More about Emily Anthes
Explore the Animal Kingdom
A selection of quirky, intriguing and surprising discoveries about animal life..
Indigenous rangers in Australia’s Western Desert got a rare close-up with the northern marsupial mole , which is tiny, light-colored and blind, and almost never comes to the surface.
For the first time, scientists observed an orangutan, a primate, in the wild treating a wound with a plant that has medicinal properties.
A new study resets the timing for the emergence of bioluminescence back to millions of years earlier than previously thought.
Scientists are making computer models to better understand how cicadas emerge collectively after more than a decade underground .
New research questions the long-held theory that reintroduction of Yellowstone’s wolves caused a trophic cascade , spawning renewal of vegetation and spurring biodiversity.
To protect Australia’s iconic animals, scientists are experimenting with vaccine implants , probiotics, tree-planting drones and solar-powered tracking tags.

IMAGES
COMMENTS
Latest malaria report. The 2023 World malaria report delves into the nexus between climate change and malaria. Changes in temperature, humidity and rainfall can influence the behaviour and survival of the malaria-carrying Anopheles mosquito. Extreme weather events, such as heatwaves and flooding, can also directly impact transmission and ...
The estimated number of global malaria cases in 2022 exceeded pre-COVID-19 pandemic levels in 2019, according to WHO's 2023 World malaria report. Several threats to the malaria global response are highlighted in the report, including climate change. The report, an annual assessment of global trends in malaria control and elimination, noted ...
In 2019, before the pandemic struck, the number of deaths stood at 568 000. Malaria cases continued to rise between 2020 and 2021, but at a slower rate than in the period 2019 to 2020. The global tally of malaria cases reached 247 million in 2021 compared to 245 million in 2020 and 232 million in 2019. 8 December 2022.
Introduction. Recent World Health Organization (WHO) data provide insight into the evolving epidemiology of malaria globally. According to the World Malaria Report 2022, the number of total malaria cases increased again in 2021 (to 247 million), although case incidence remained stable following an increase from the preceding year [1••].Similarly, deaths due to malaria increased in 2020 ...
6. Investments in malaria programmes and research 44 6.1 Funding trends for malaria control and elimination 45 6.2 Global trends in real GDP growth and out-of-pocket health expenditure 54 6.3 Investments in malaria-related R&D 58 7. Distribution and coverage of malaria prevention, diagnosis and treatment 60 7.1 Distribution and coverage of ITNs 60
The World malaria report 2022 provides comprehensive data and analysis on the global, regional and country-level progress and challenges in the fight against malaria. It covers topics such as antimalarial drug resistance, urban malaria, malaria vaccine and humanitarian emergencies. Download the full report in PDF format from iris.who.int.
Research Open Access 02 May 2024 Scientific Reports Volume: 14, P: 9816 Estimating the effects of temperature on transmission of the human malaria parasite, Plasmodium falciparum
1. Introduction. Malaria affected an estimated 219 million people causing 435,000 deaths in 2017 globally. This burden of morbidity and mortality is a result of more than a century of global effort and research aimed at improving the prevention, diagnosis, and treatment of malaria [].Malaria is the most common disease in Africa and some countries in Asia with the highest number of indigenous ...
In 2020, malaria was estimated to have resulted in 627,000 deaths and 241 million cases, with 77% of deaths in children <5 years of age 1. Overall, 90% of malaria cases and deaths are reported in ...
DOI: 10.1056/NEJMp2216703. In this documentary video from the New England Journal of Medicine, physicians and scientists from across the world discuss the epidemiology of malaria and outline key ...
Malaria vaccine introduction in endemic countries is a game-changing milestone in the fight against the disease. This article examines the inequity in the global pharmaceutical research, development, manufactu... Floriano Amimo. Malaria Journal 2024 23 :136. Perspective Published on: 6 May 2024.
The World Malaria Report, released in December 2021, reflects the unique challenges currently facing the global malaria community. The report showed the devastating toll of malaria, with an estimated 627,000 people losing their lives to the disease in 2020. The improved methodological approach used for calculating cause of death for young children revealed a systematic underestimation of ...
Malaria is a life-threatening febrile illness caused by Plasmodium spp. parasites that are transmitted to humans by infected female Anopheles mosquitoes. According to WHO, 241 million cases ...
Malaria Journal, in partnership with Research Square, is offering In Review. Authors choosing this free optional service will be able to: ... Track their manuscript - including seeing when reviewers are invited, and when reports are received; See what the Malaria Journal In Review platform looks like. Aims and Scope.
Malaria is a mosquito-borne infectious disease of humans and other animals caused by. protists (a type of microorganism) of the genus Plasmodium. It begins with a bite from an. infected female ...
Results General Surveillance. CDC received 1,823 reports of confirmed malaria cases among persons tested in the United States with onset of symptoms in 2018 ().These cases represented a 15.6% relative decrease from 2017 (n = 2,161), which had the most reported confirmed cases since 1971 when there were 3,180 cases (51) ().Of the 1,823 cases reported in 2018, a total of 1,788 (98.1%) were ...
PDF | Malaria is a global public health burden with an estimated 229 million cases reported worldwide in 2019. About 94% of the reported cases were... | Find, read and cite all the research you ...
Malaria is a parasitic infection transmitted by the Anopheles mosquito that leads to acute life-threatening disease and poses a significant global health threat. Two billion people risk contracting malaria annually, including those in 90 endemic countries and 125 million travelers, and 1.5 to 2.7 million people die in a year.[1] The Plasmodium parasite has a multistage lifecycle, which leads ...
This year's report includes, for the first time, a dedicated chapter focused on the intersection between climate change and malaria. As described in the report, climate change is one of many threats to the global response to malaria. Millions of people continue to miss out on the services they need to prevent, detect, and treat the disease.
The research entitled "Future malaria environmental suitability in Africa is sensitive to hydrology" was funded by the Natural Environment Research Council and is published today (9 May 2024) in ...
Introduction. Malaria is a severe and potentially fatal mosquitoborne disease caused by infection with Plasmodium spp. parasites.Although malaria is no longer endemic in the United States, imported infections are reported annually; the primary risk group has been U.S. residents traveling to areas where malaria is endemic (1).In 2023, sporadic locally acquired mosquito-transmitted malaria cases ...
The research from the Wellcome Sanger Institute, the Malaria Research and Training Center (MRTC) in Mali, and other collaborators, adds to the freely available Malaria Cell Atlas.
Malaria, transmitted by mosquitoes, remains a scourge of humans in sub-Saharan Africa, where control rests on the targeted use of insecticides sprayed in and around dwellings and on mosquito nets.
The CDC issued a public health alert Monday warning the public about acquired cases of malaria reported in the United States, the first in 20 years. ... according to a report in May 2023 from the ...
The 2022 WHO World Malaria Report further puts the global malaria death to about 619,000 in 2021, which reflects a 9 percent increase from the 568,000 deaths recorded before the pandemic struck. ... Given the nature of the research question to answer in the current study, as earlier explained, the focus is on incidence, to trace the effect of ...
Biodiversity loss, global warming, pollution and the spread of invasive species are making infectious diseases more dangerous to organisms around the world.