Case Study: Managing Severe Asthma in an Adult
—he follows his treatment plan, but this 40-year-old male athlete has asthma that is not well-controlled. what’s the next step.
By Kirstin Bass, MD, PhD Reviewed by Michael E. Wechsler, MD, MMSc
This case presents a patient with poorly controlled asthma that remains refractory to treatment despite use of standard-of-care therapeutic options. For patients such as this, one needs to embark on an extensive work-up to confirm the diagnosis, assess for comorbidities, and finally, to consider different therapeutic options.

Case presentation and patient history
Mr. T is a 40-year-old recreational athlete with a medical history significant for asthma, for which he has been using an albuterol rescue inhaler approximately 3 times per week for the past year. During this time, he has also been waking up with asthma symptoms approximately twice a month, and has had three unscheduled asthma visits for mild flares. Based on the National Asthma Education and Prevention Program guidelines , Mr. T has asthma that is not well controlled. 1
As a result of these symptoms, spirometry was performed revealing a forced expiratory volume in the first second (FEV1) of 78% predicted. Mr. T then was prescribed treatment with a low-dose corticosteroid, fluticasone 44 mcg at two puffs twice per day. However, he remained symptomatic and continued to use his rescue inhaler 3 times per week. Therefore, he was switched to a combination inhaled steroid and long-acting beta-agonist (LABA) (fluticasone propionate 250 mcg and salmeterol 50 mcg, one puff twice a day) by his primary care doctor.
Initial pulmonary assessment Even with this step up in his medication, Mr. T continued to be symptomatic and require rescue inhaler use. Therefore, he was referred to a pulmonologist, who performed the initial work-up shown here:
- Spirometry, pre-albuterol: FEV1 79%, post-albuterol: 12% improvement
- Methacholine challenge: PC 20 : 1.0 mg/mL
- Chest X-ray: Within normal limits
Continued pulmonary assessment His dose of inhaled corticosteroid (ICS) and LABA was increased to fluticasone 500 mcg/salmeterol 50 mcg, one puff twice daily. However, he continued to have symptoms and returned to the pulmonologist for further work-up, shown here:
- Chest computed tomography (CT): Normal lung parenchyma with no scarring or bronchiectasis
- Sinus CT: Mild mucosal thickening
- Complete blood count (CBC): Within normal limits, white blood cells (WBC) 10.0 K/mcL, 3% eosinophils
- Immunoglobulin E (IgE): 25 IU/mL
- Allergy-skin test: Positive for dust, trees
- Exhaled NO: Fractional exhaled nitric oxide (FeNO) 53 parts per billion (pbb)
Assessment for comorbidities contributing to asthma symptoms After this work-up, tiotropium was added to his medication regimen. However, he remained symptomatic and had two more flares over the next 3 months. He was assessed for comorbid conditions that might be affecting his symptoms, and results showed:
- Esophagram/barium swallow: Negative
- Esophageal manometry: Negative
- Esophageal impedance: Within normal limits
- ECG: Within normal limits
- Genetic testing: Negative for cystic fibrosis, alpha1 anti-trypsin deficiency
The ear, nose, and throat specialist to whom he was referred recommended only nasal inhaled steroids for his mild sinus disease and noted that he had a normal vocal cord evaluation.
Following this extensive work-up that transpired over the course of a year, Mr. T continued to have symptoms. He returned to the pulmonologist to discuss further treatment options for his refractory asthma.
Diagnosis Mr. T has refractory asthma. Work-up for this condition should include consideration of other causes for the symptoms, including allergies, gastroesophageal reflux disease, cardiac disease, sinus disease, vocal cord dysfunction, or genetic diseases, such as cystic fibrosis or alpha1 antitrypsin deficiency, as was performed for Mr. T by his pulmonary team.
Treatment options When a patient has refractory asthma, treatment options to consider include anticholinergics (tiotropium, aclidinium), leukotriene modifiers (montelukast, zafirlukast), theophylline, anti-immunoglobulin E (IgE) antibody therapy with omalizumab, antibiotics, bronchial thermoplasty, or enrollment in a clinical trial evaluating the use of agents that modulate the cell signaling and immunologic responses seen in asthma.
Treatment outcome Mr. T underwent bronchial thermoplasty for his asthma. One year after the procedure, he reports feeling great. He has not taken systemic steroids for the past year, and his asthma remains controlled on a moderate dose of ICS and a LABA. He has also been able to resume exercising on a regular basis.
Approximately 10% to 15% of asthma patients have severe asthma refractory to the commonly available medications. 2 One key aspect of care for this patient population is a careful workup to exclude other comorbidities that could be contributing to their symptoms. Following this, there are several treatment options to consider, as in recent years there have been several advances in the development of asthma therapeutics. 2
Treatment options for refractory asthma There are a number of currently approved therapies for severe, refractory asthma. In addition to therapy with ICS or combination therapies with ICS and LABAs, leukotriene antagonists have good efficacy in asthma, especially in patients with prominent allergic or exercise symptoms. 2 The anticholinergics, such as tiotropium, which was approved for asthma in 2015, enhance bronchodilation and are useful adjuncts to ICS. 3-5 Omalizumab is a monoclonal antibody against IgE recommended for use in severe treatment-refractory allergic asthma in patients with atopy. 2 A nonmedication therapeutic option to consider is bronchial thermoplasty, a bronchoscopic procedure that uses thermal energy to disrupt bronchial smooth muscle. 6,7
Personalizing treatment for each patient It is important to personalize treatment based on individual characteristics or phenotypes that predict the patient's likely response to treatment, as well as the patient's preferences and practical issues, such as adherence and cost. 8
In this case, tiotropium had already been added to Mr. T's medications and his symptoms continued. Although addition of a leukotriene modifier was an option for him, he did not wish to add another medication to his care regimen. Omalizumab was not added partly for this reason, and also because of his low IgE level. As his bronchoscopy was negative, it was determined that a course of antibiotics would not be an effective treatment option for this patient. While vitamin D insufficiency has been associated with adverse outcomes in asthma, T's vitamin D level was tested and found to be sufficient.
We discussed the possibility of Mr. T's enrollment in a clinical trial. However, because this did not guarantee placement within a treatment arm and thus there was the possibility of receiving placebo, he opted to undergo bronchial thermoplasty.
Bronchial thermoplasty Bronchial thermoplasty is effective for many patients with severe persistent asthma, such as Mr. T. This procedure may provide additional benefits to, but does not replace, standard asthma medications. During the procedure, thermal energy is delivered to the airways via a bronchoscope to reduce excess airway smooth muscle and limit its ability to constrict the airways. It is an outpatient procedure performed over three sessions by a trained physician. 9
The effects of bronchial thermoplasty have been studied in several trials. The first large-scale multicenter randomized controlled study was the Asthma Intervention Research (AIR) Trial , which enrolled patients with moderate to severe asthma. 10 In this trial, patients who underwent the procedure had a significant improvement in asthma symptoms as measured by symptom-free days and scores on asthma control and quality of life questionnaires, as well as reductions in mild exacerbations and increases in morning peak expiratory flow. 10 Shortly after the AIR trial, the Research in Severe Asthma (RISA) trial was conducted to evaluate bronchial thermoplasty in patients with more severe, symptomatic asthma. 11 In this population, bronchial thermoplasty resulted in a transient worsening of asthma symptoms, with a higher rate of hospitalizations during the treatment period. 11 Hospitalization rate equalized between the treatment and control groups in the posttreatment period, however, and the treatment group showed significant improvements in rescue medication use, prebronchodilator forced expiratory volume in the first second (FEV1) % predicted, and asthma control questionnaire scores. 11
The AIR-2 trial followed, which was a multicenter, randomized, double-blind, sham-controlled study of 288 patients with severe asthma. 6 Similar to the RISA trial, patients in the treatment arm of this trial experienced an increase in adverse respiratory effects during the treatment period, the most common being airway irritation (including wheezing, chest discomfort, cough, and chest pain) and upper respiratory tract infections. 6
The majority of adverse effects occurred within 1 day of the procedure and resolved within 7 days. 6 In this study, bronchial thermoplasty was found to significantly improve quality of life, as well as reduce the rate of severe exacerbations by 32%. 6 Patients who underwent the procedure also reported fewer adverse respiratory effects, fewer days lost from work, school, or other activities due to asthma, and an 84% risk reduction in emergency department visits. 6
Long-term (5-year) follow-up studies have been conducted for patients in both the AIR and the AIR-2 trials. In patients who underwent bronchial thermoplasty in either study, the rate of adverse respiratory effects remained stable in years 2 to 5 following the procedure, with no increase in hospitalizations or emergency department visits. 7,12 Additionally, FEV1 remained stable throughout the 5-year follow-up period. 7,12 This finding was maintained in patients enrolled in the AIR-2 trial despite decreased use of daily ICS. 7
Bronchial thermoplasty is an important addition to the asthma treatment armamentarium. 7 This treatment is currently approved for individuals with severe persistent asthma who remain uncontrolled despite the use of an ICS and LABA. Several clinical trials with long-term follow-up have now demonstrated its safety and ability to improve quality of life in patients with severe asthma, such as Mr. T.
Severe asthma can be a challenge to manage. Patients with this condition require an extensive workup, but there are several treatments currently available to help manage these patients, and new treatments are continuing to emerge. Managing severe asthma thus requires knowledge of the options available as well as consideration of a patient's personal situation-both in terms of disease phenotype and individual preference. In this case, the patient expressed a strong desire to not add any additional medications to his asthma regimen, which explained the rationale for choosing to treat with bronchial thermoplasty. Personalized treatment necessitates exploring which of the available or emerging options is best for each individual patient.
Published: April 16, 2018
- 1. National Asthma Education and Prevention Program: Asthma Care Quick Reference.
- 2. Olin JT, Wechsler ME. Asthma: pathogenesis and novel drugs for treatment. BMJ . 2014;349:g5517.
- 3. Boehringer Ingelheim. Asthma: U.S. FDA approves new indication for SPIRIVA Respimat [press release]. September 16, 2015.
- 4. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med . 2010;363:1715-1726.
- 5. Kerstjens HA, Engel M, Dahl R. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med . 2012;367:1198-1207.
- 6. Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med . 2010;181:116-124.
- 7. Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol . 2013;132:1295-1302.
- 8. Global Initiative for Asthma: Pocket Guide for Asthma Management and Prevention (for Adults and Children Older than 5 Years).
- 10. Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med . 2007;356:1327-1337.
- 11. Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med . 2007;176:1185-1191.
- 12. Thomson NC, Rubin AS, Niven RM, et al. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med . 2011;11:8.

Treatable traits and future exacerbation risk in severe asthma, baker’s asthma, the long-term trajectory of mild asthma, age, gender, & systemic corticosteroid comorbidities, ask the expert: william busse, md, challenges the current definition of the atopic march, considering the curveballs in asthma treatment, do mucus plugs play a bigger role in chronic severe asthma than previously thought, an emerging subtype of copd is associated with early respiratory disease.

- Search Menu
- Advance Articles
- Supplements
- Author Guidelines
- Submission Site
- Open Access
- Self-Archiving Policy
- Advertising & Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- Journals Career Network
- About Paediatrics & Child Health
- About The Canadian Paediatric Society
- Editorial Board
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Case 1 diagnosis: allergy bullying, clinical pearls.
- < Previous
Case 1: A 12-year-old girl with food allergies and an acute asthma exacerbation
- Article contents
- Figures & tables
- Supplementary Data
Lopamudra Das, Michelle GK Ward, Case 1: A 12-year-old girl with food allergies and an acute asthma exacerbation, Paediatrics & Child Health , Volume 19, Issue 2, February 2014, Pages 69–70, https://doi.org/10.1093/pch/19.2.69
- Permissions Icon Permissions
A 12-year-old girl with a history of asthma presented to the emergency department with a three-day history of increased work of breathing, cough and wheezing. She reported no clear trigger for her respiratory symptoms, although she had noted some symptoms of a mild upper respiratory tract infection. With this episode, the patient had been using a short-acting bronchodilator more frequently than she had in the past, without the expected resolution of symptoms.
On the day of presentation, the patient awoke feeling ‘suffocated’ and her mother noted her lips to be blue. In the emergency department, her oxygen saturation was 85% and her respiratory rate was 40 breaths/min. She had significantly increased work of breathing and poor air entry bilaterally to both lung bases, with wheezing in the upper lung zones. She was treated with salbutamol/ipratropium and received intravenous steroids and magnesium sulfate. Her chest x-ray showed hyperinflation and no focal findings.
Her medical history revealed that she was followed by a respirologist for her asthma, had good medication adherence and had not experienced a significant exacerbation for six months. She also had a history of wheezing, dyspnea and pruritis with exposure to peanuts, chickpeas and lentils; she had been prescribed an injectible epinephrine device for this. However, her device had expired at the time of presentation. In the past, her wheezing episodes had been seasonal and related to exposure to grass and pollens; this presentation occurred during the winter. Further history revealed the probable cause of her presentation.
Although reluctant to disclose the information, our patient later revealed that she had been experiencing significant bullying at school, which was primarily related to her food allergies. Three days before her admission, classmates had smeared peanut butter on one of her schoolbooks. She developed pruritis immediately after opening the book and she started wheezing and coughing later that day. This event followed several months of being taunted with peanut products at school. The patient was experiencing low mood and reported new symptoms of anxiety related to school. The review of systems was otherwise negative, with no substance use.
The patient's asthma exacerbation resolved with conventional asthma treatment. Her pulmonary function tests were nonconcerning (forced expiratory volume in 1 s 94% and 99% of predicted) after her recovery. The trigger for her asthma exacerbation was likely multifactorial, related to exposure to the food allergen as well as the upper respiratory infection. A psychologist was consulted to assess the symptoms of anxiety and depression that had occurred as a result of the bullying. During the hospitalization, the medical team contacted the patient's school to provide education on allergy bullying, treatment of severe allergic reactions and its potential for life-threatening reactions with exposure to allergens. The medical team also recommended community resources for further education of students and staff about allergy bullying and its prevention.
Allergy bullying is a form of bullying with potentially severe medical outcomes. In recent years, it has gained increasing notoriety in schools and in the media. Population-based studies have shown that 20% to 35% of children with allergies experience bullying. In many cases (31% in one recent study [ 1 ]), this bullying is related directly to the food allergy. From a medical perspective, there are little published data regarding allergy bullying, and many health care providers may not be aware of the issue.
Allergy bullying can include teasing a child about their allergy, throwing food at a child, or even forcing them to touch or eat allergenic foods. Most episodes of allergy bullying occur at school, and can include episodes perpetrated by teachers and/or staff ( 2 ).
Allergy bullying can lead to allergic reactions, which may be mild or severe (eg, urticaria, wheezing, anaphylaxis), but may also lead to negative emotional consequences (sadness, depression) ( 2 ) and an overall decrease in quality of life measures ( 1 ). Adolescents commonly resist using medical devices, such as injectible epinephrine devices, and bullying may be a contributing factor for this ( 3 ). Attempting to conceal symptoms in a bullying situation may place children at risk for a worse outcome.
Physicians can play a key role in detecting allergy bullying and its health consequences. In many cases, children have not discussed this issue with their parents ( 1 ). Given the prevalence of bullying, its potential to lead to severe harm, including death, and the lack of awareness of this issue, clinicians should specifically ask about bullying in all children and teens with allergies. Physicians can also work with families and schools to support these children, educate their peers and school staff, and help prevent negative health outcomes from allergy bullying.
Online resources
www.anaphylaxis.ca − A national charity that aims to inform, support, educate and advocate for the needs of individuals and families living with anaphylaxis, and to support and participate in research. This website includes education modules for schools and links to local support groups throughout Canada.
www.whyriskit.ca/pages/en/live/bullying.php − A website for teenagers with food allergies; includes a segment that addresses food bullying.
www.foodallergy.org − Contains numerous resources for children and their families, including a significant discussion on bullying and ways to prevent it.
Allergy bullying is common but is often unrecognized as a factor in clinical presentations of allergic reactions.
Physicians should make a point of asking about bullying in patients with allergies and become familiar with resources for dealing with allergy bullying.
Physicians can play roles as advocates, educators and collaborators with the school system to help make the school environment safer for children with allergies who may be at risk for allergy bullying.
Google Scholar
Email alerts
Citing articles via, looking for your next opportunity.
- About Paediatrics & Child Health
- Recommend to Your Librarian
- Advertising and Corporate Services
Affiliations
- Online ISSN 1918-1485
- Print ISSN 1205-7088
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Case report
- Open access
- Published: 21 February 2018
Pediatric severe asthma: a case series report and perspectives on anti-IgE treatment
- Virginia Mirra 1 ,
- Silvia Montella 1 &
- Francesca Santamaria 1
BMC Pediatrics volume 18 , Article number: 73 ( 2018 ) Cite this article
11k Accesses
11 Citations
12 Altmetric
Metrics details
The primary goal of asthma management is to achieve disease control for reducing the risk of future exacerbations and progressive loss of lung function. Asthma not responding to treatment may result in significant morbidity. In many children with uncontrolled symptoms, the diagnosis of asthma may be wrong or adherence to treatment may be poor. It is then crucial to distinguish these cases from the truly “severe therapy-resistant” asthmatics by a proper filtering process. Herein we report on four cases diagnosed as difficult asthma, detail the workup that resulted in the ultimate diagnosis, and provide the process that led to the prescription of omalizumab.
Case presentation
All children had been initially referred because of asthma not responding to long-term treatment with high-dose inhaled steroids, long-acting β 2 -agonists and leukotriene receptor antagonists. Definitive diagnosis was severe asthma. Three out four patients were treated with omalizumab, which improved asthma control and patients’ quality of life. We reviewed the current literature on the diagnostic approach to the disease and on the comorbidities associated with difficult asthma and presented the perspectives on omalizumab treatment in children and adolescents. Based on the evidence from the literature review, we also proposed an algorithm for the diagnosis of pediatric difficult-to-treat and severe asthma.
Conclusions
The management of asthma is becoming much more patient-specific, as more and more is learned about the biology behind the development and progression of asthma. The addition of omalizumab, the first targeted biological treatment approved for asthma, has led to renewed optimism in the management of children and adolescents with atopic severe asthma.
Peer Review reports
Children with poor asthma control have an increased risk of severe exacerbations and progressive loss of lung function, which results in the relevant use of health resources and impaired quality of life (QoL) [ 1 ]. Therefore, the primary goal of asthma management at all ages is to achieve disease control [ 2 , 3 , 4 ].
According to recent international guidelines, patients with uncontrolled asthma require a prolonged maintenance treatment with high-dose inhaled corticosteroids (ICS) in association with a long-acting β 2 -agonist (LABA) plus oral leukotriene receptor antagonist (LTRA) (Table 1 ) [ 5 ].
Nevertheless, in the presence of persistent lack of control, reversible factors such as adherence to treatment or inhalation technique should be first checked for, and diseases that can masquerade as asthma should be promptly excluded. Finally, additional strategies, in particular anti-immunoglobulin E (anti-IgE) treatment (omalizumab), are suggested for patients with moderate or severe allergic asthma that remains uncontrolled in Step 4 [ 5 ].
Herein, we reviewed the demographics, clinical presentation and treatment of four patients with uncontrolled severe asthma from our institution in order to explain why we decided to prescribe omalizumab. We also provided a review of the current literature that focuses on recent advances in the diagnosis of pediatric difficult asthma and the associated comorbidities, and summarizes the perspectives on anti-IgE treatment in children and adolescents.
Case presentations
Table 2 summarizes the clinical characteristics and the triggers/comorbidities of the cases at referral to our Institution. Unfortunately, data on psychological factors, sleep apnea, and hyperventilation syndrome were not available in any case. Clinical, lung function and airway inflammation findings at baseline and after 12 months of follow-up are reported in Table 3 . In the description of our cases, we used the terminology recommended by the ERS/ATS guidelines on severe asthma [ 6 ].
A full-term male had severe preschool wheezing and, since age 3, recurrent, severe asthma exacerbations with frequent hospital admissions. At age 11, severe asthma was diagnosed. Sensitization to multiple inhalant allergens (i.e., house dust mites, dog dander, Graminaceae pollen mix, and Parietaria judaica ) and high serum IgE levels (1548 KU/l) were found. Body mass index (BMI) was within normal range. Combined treatment with increasing doses of ICS (fluticasone, up to 1000 μg/day) in association with LABA (salmeterol, 100 μg/day) plus LTRA (montelukast, 5 mg/day) has been administered over 2 years. Nevertheless, persistent symptoms and monthly hospital admissions due to asthma exacerbations despite correct inhaler technique and good adherence were reported. Parents refused to perform any test to exclude gastroesophageal reflux (GER) as comorbidity [ 6 ]. However, an ex-juvantibus 2-month-course with omeprazole was added to asthma treatment [ 7 ], but poor control persisted. Anterior rhinoscopy revealed rhinosinusitis that was treated with nasal steroids for six months [ 8 ], but asthma symptoms were unmodified. Treatment with omalizumab was added at age 12. Reduced hospital admissions for asthma exacerbations, no further need for systemic steroids, and improved QoL score (from 2.0 up to 6.7 out of a maximum of 7 points) were documented over the following months. Unfortunately, after one year of treatment, adherence to omalizumab decreased because of family complaints, and eventually parents withdrew their informed consent and discontinued omalizumab. Currently, by age 17, treatment includes inhaled salmeterol/fluticasone (100 μg/500 μg∙day -1 , respectively) plus oral montelukast (10 mg/day). Satisfactory symptom control is reported, with no asthma exacerbations.
A full-term male, who had a recurrent severe preschool wheezing, at 6 years of age developed exercise-induced asthma. At age 10, severe asthma was diagnosed. High serum IgE levels (1300 KU/l) and skin prick tests positive to house dust mites were found. Despite a 3-year treatment with progressively increasing doses of inhaled fluticasone (up to 1000 μg/day) combined with salmeterol (100 μg/day) and oral montelukast (5 mg/day), monthly hospital admissions with systemic steroids use were reported. At age 13, a 24-h esophageal impedance/pH study demonstrated the presence of acid and non-acid GER [ 7 ]. Esomeprazole was added to asthma medications, but with an incomplete clinical benefit for respiratory symptoms. Esomeprazole was withdrawn after 3 months, and parents refused to re-test for GER. As respiratory symptoms persisted uncontrolled despite treatment, severe asthma was definitively diagnosed [ 6 ]. BMI was within the normal range and anterior rhinoscopy excluded rhinosinusitis. Inhaler technique and adherence were good; thus we considered the anti-IgE treatment option [ 9 ]. Subcutaneous omalizumab was started, with fast improvement of both symptoms and QoL score (from 3.9 up to 6.5). Seventeen months later, the dose of ICS had been gradually tapered and oral montelukast definitely discontinued. Currently, at age 14, treatment includes the combined administration of bimonthly subcutaneous omalizumab and of daily inhaled salmeterol/fluticasone (50 μg/100 μg∙day - 1 , respectively). Asthma control is satisfactory and no side effects are reported. Omalizumab has been continuously administered for 2.6 years and is still ongoing.
A full-term male had severe preschool wheezing and, since age 3, recurrent, severe asthma exacerbations with acute respiratory failure that frequently required intensive care unit (ICU) admission. At age 6, sensitization to multiple perennial inhalant (i.e., house dust mites, dog and cat danders, Alternaria alternata , Graminaceae pollen mix, Artemisia vulgaris , Parietaria judaica , and Olea europaea pollen) and food allergens (i.e., egg, milk, and peanut) was diagnosed. Serum IgE levels were 2219 KU/l. Weight and height were appropriate for age and sex. The patient has been treated over 3 years with a combined scheme of high-dose inhaled fluticasone (up to 1000 μg/day) plus salmeterol (100 μg/day) and oral montelukast (5 mg/day), with correct inhaler technique and good adherence. Despite this, monthly hospital admissions with systemic steroids use were recorded. Rhinosinusitis and GER were excluded on the basis of appropriate testing; thus treatment with omalizumab was started when the patient was 9 years old. At age 11, adherence to treatment is satisfactory, with no side effects. More importantly, reduced hospital admissions for asthma exacerbations, no further need for systemic steroids, and improved QoL score (from 6.4 to 6.8) were reported. Finally, progressive step-down of anti-asthma treatment was started, and at present (by 11.5 years) inhaled fluticasone (200 μg/day) plus bimonthly subcutaneous omalizumab provide good control of symptoms. Omalizumab has been continuously administered for 2.6 years and is still ongoing.
A full-term male had severe preschool wheezing and, since age 4, recurrent, severe asthma exacerbations with frequent hospital admissions. At age 8, multiple perennial inhalants and food sensitization (i.e., house dust mites, dog dander, Graminaceae pollen mix, Olea europaea pollen, tomatoes, beans, shrimps, and peas) and high serum IgE levels (1166 KU/l) were found. The patient has been treated over 5 years with inhaled fluticasone (up to 1000 μg/day) in association with salmeterol (100 μg/day) and oral montelukast (5 mg/day). Despite this, monthly hospital admissions with systemic steroids need were recorded. After checking the inhaler technique and adherence to treatment, comorbidities including obesity, rhinosinusitis and GER were excluded. Omalizumab was proposed, but parents refused it. By 13.6 years, despite a treatment including the association of inhaled salmeterol/fluticasone (100 μg/1000 μg∙day − 1 , respectively) plus oral montelukast (10 mg/day), monthly exacerbations requiring systemic steroids are reported.
Discussion and conclusions
Most children and adolescents with asthma respond well to inhaled short-acting beta 2 -agonists (SABA) on demand if symptoms are intermittent, or to low dose controller drugs plus as-needed SABA if the risk of exacerbations increases [ 1 ]. Nevertheless, a proportion of patients is referred to specialists because this strategy is not working and asthma is persistently uncontrolled [ 4 ]. For these children, assessment is primarily aimed at investigating the reasons for poor control. Indeed, when the child is initially referred, before the label of “severe, therapy-resistant asthma” (i.e., not responding to treatment even when factors as exposure to allergens and tobacco smoke have been considered) is assigned, three main categories need to be identified: 1) “not asthma at all”, in which response to treatment is suboptimal because the diagnosis is wrong; 2) “asthma plus ”, when asthma is mild but exacerbated by one or more comorbidities; and 3) “difficult-to-treat asthma”, when asthma is uncontrolled because of potentially reversible factors [ 10 ].
The reported cases highlight some aspects of the disease process that may expand the diagnosis and improve patients’ care. At our institution, the severe asthma program includes a multidisciplinary approach with consultations by gastroenterologists as well as ear, nose and throat experts. Recently, sleep medicine experts joined this multidisciplinary team; thus, unfortunately, sleep-disordered breathing (SDB) could not be excluded at the time of our patients’ assessment. Inhalation technique is periodically evaluated by nurses or doctors in each patient. Unfortunately, in Italy an individual prescription database is not available and thus we cannot assess patients’ use of medication. In two cases, the filtering process eventually identified GER and rhinosinusitis, but poor control of asthma persisted even after comorbidities were treated. In all subjects, inhaler skills, treatment adherence, and environmental exposure to indoor/outdoor allergens as well as to second- and third-hand smoke were excluded as cause of lack of control. Eventually, three out of four patients started anti-IgE treatment; asthma control was obtained and maintenance drugs were progressively reduced. In the case that refused omalizumab therapy, pulmonary function, clinical features and controller treatment including high-dose ICS were unchanged.
Previous studies have highlighted an association between increasing asthma severity in children and reduced QoL [ 11 , 12 , 13 ]. Uncontrolled asthma symptoms not only affect children physically, but can impair them socially, emotionally, and educationally [ 13 ]. In line with previous observations, 3 out 4 of our cases had poor QoL, assessed by a standardized questionnaire [ 14 ]. It is well known that improving QoL in difficult asthma is not an easy task, despite a variety of treatments aimed at achieving control [ 12 ], and much more remains to be done to address the problem. Nevertheless, 2 of our 3 cases showed a remarkable improvement of QoL after one year of treatment with omalizumab.
Reduction in forced expiratory volume in the first second (FEV 1 ) is often used to define childhood asthma severity in treatment guidelines and clinical studies [ 5 , 11 , 15 ]. Nevertheless, children with severe asthma often have a normal FEV 1 that does not improve after bronchodilators, indicating that spirometry may be a poor predictor of asthma severity in childhood [ 6 , 16 , 17 ]. Actually, children with a normal FEV 1 , both before and after β 2 -agonist, may show a bronchodilator response in terms of forced expiratory flow between 25% and 75% (FEF 25–75 ) [ 18 ]. However, the utility of FEF 25–75 in the assessment or treatment of severe asthma is currently unknown. Interestingly, all the reported cases showed normal or slightly reduced values of FEV 1 but severe impairment of FEF 25–75 . Two cases showed a bronchodilator response in terms of FEV 1 (subjects 3 and 4), while 3 patients had a significant increase of FEF 25–75 (cases 1, 3 and 4). Unfortunately, we could not provide the results of bronchodilator response during or after the treatment with omalizumab in any case.
Available literature on the diagnostic approach to difficult asthma in children offers a number of reviews which basically summarize the steps needed to fill the gap between a generic diagnosis of “difficult asthma” and more specific labels (i.e., “severe” asthma, “difficult-to-treat” asthma, or even different diagnoses) [ 3 , 5 , 6 , 8 , 10 , 19 , 20 , 21 ]. So far, few original articles and case reports have been published, probably due to the peculiarity of the issue, which makes retrospective discussion of cases easier than the design of a prospective clinical study [ 4 , 22 , 23 , 24 , 25 , 26 ]. Available knowledge mainly derives from the experience of specialized centers.
The evaluation of a child referred for uncontrolled asthma should start with a careful history focused on typical respiratory symptoms and on the definition of possible triggers. In the “severe asthma” process, it is crucial for clinicians to maintain a high degree of skepticism about the ultimate diagnosis, particularly in the presence of relevant discrepancies between history, physical features and lung function, as many conditions may be misdiagnosed as asthma. In order to simplify this process, herein we propose an algorithm for the diagnosis of difficult-to-treat and severe asthma (Fig. 1 ). Confirmation of the diagnosis through a detailed clinical and laboratory re-evaluation is important because in 12–50% of cases assumed to have severe asthma this might not be the correct diagnosis [ 10 ]. Several documents have indicated the main steps of the process that should be followed in children with uncontrolled asthma [ 3 , 8 , 10 ]. The translation of these procedures into real life practice may deeply change from one subject to another due to the variability of individual patients’ history and clinical features, which will often lead the diagnostic investigations towards the most likely reason for uncontrolled asthma. For children with apparently severe asthma, the first step is to confirm the diagnosis and, before proceeding to broader investigations, to verify that the poor control is not simply determined by poor adherence to treatment, inadequate inhaler skills and/or environmental exposure to triggers. A nurse-led assessment, including a home visit, despite not being applicable in all settings, may be useful for identifying potentially modifiable factors in uncontrolled pediatric asthma [ 27 ].
A practical algorithm for the diagnosis of difficult-to-treat and severe asthma. ICS, inhaled corticosteroids; OCS, oral corticosteroids
A number of comorbidities have been increasingly recognized as factors that may impact asthma clinical expression and control in childhood [ 10 , 28 ]. Children with uncontrolled disease should be investigated for GER, rhinosinusitis, dysfunctional breathing and/or vocal cord dysfunction, obstructive sleep apnea, obesity, psychological factors, smoke exposure, hormonal influences, and ongoing drugs [ 3 , 6 , 8 , 20 ]. Indeed, the exact role played by comorbidities in pediatric asthma control is still debated [ 28 ]. The most impressive example is GER. Several pediatric documents recommend assessing for GER because reflux may be a contributing factor to problematic or difficult asthma [ 7 , 29 ]. Nevertheless, GER treatment might not be effective for severe asthma [ 30 , 31 ], as confirmed by current cases 1 and 2. There is an established evidence that chronic rhinosinusitis is associated with more severe asthma in children [ 32 , 33 , 34 ]. Therefore, examination of upper airways and ad hoc treatment if rhinosinusitis is evident are recommended in children with severe asthma [ 3 , 8 , 35 ]. However, intranasal steroids for rhinitis resulted in a small reduction of asthma risk in school-aged children [ 36 ], and actual placebo-controlled studies on the effect of treatment of rhinosinusitis on asthma control in children are lacking [ 10 , 37 ].
Dysfunctional breathing, including hyperventilation and vocal cord dysfunction, is associated with poorer asthma control in children [ 8 , 10 , 38 , 39 ]. Unfortunately, there is scarce literature on the effect of its treatment on the control of severe asthma in children [ 40 ]. SDB ranging from primary snoring to obstructive sleep apnea syndrome is very common in children [ 41 ], and an increased prevalence of SDB together with increasing asthma severity has been reported [ 42 ]. Interestingly, GER may also be worsened by recurrent episodes of upper airway obstruction associated with SDB, and this may further trigger bronchial obstruction. Asthma guidelines recommend the assessment of SDB through nocturnal polysomnography in poorly controlled asthmatics, particularly if they are also obese [ 5 ]. There are no studies examining whether pediatric asthma improves after SDB has been treated, for example, with nasal steroids, adenotonsillectomy, continuous positive airway pressure or weight reduction if the child is also obese [ 43 ]. The parallel increase in obesity and asthma suggests that the two conditions are linked and that they can aggravate each other [ 44 , 45 ], even though the exact mechanisms that underlie this association remain unclear [ 46 ]. Indeed, other coexisting comorbidities such as SDB or GER may play a confounding role in the development of the interactions between obesity and the airways [ 47 , 48 ]. Obesity is associated with increased markers of inflammation in serum and adipose tissue and yet decreased airway inflammation in obese people with asthma [ 49 ]. Several interventions, including behavioral and weight reduction programs or bariatric surgery, may result in improved asthma control, quality of life and lung function in adult obese asthmatics [ 50 ]. Although reports of adolescent bariatric surgery demonstrate a significant body weight decrease, this approach is not widely available and there are no published reports on its effect on pediatric severe asthma control [ 51 ]. Finally, although it is still unclear whether food allergy is causative or shares a common pathway with difficult asthma, it might explain the loss of asthma control at least in some children and thus be considered as a comorbid condition [ 10 , 16 , 52 ].
In conclusion, establishing the impact of comorbidities on asthma control may be cumbersome, and an ex-juvantibus treatment is sometimes necessary to assess their role. Comorbid conditions can also worsen each other, and symptoms arising from some of them may mimic asthma [ 6 ]. Although the ability to improve pediatric severe asthma by treating comorbidities remains unconfirmed, they should be treated appropriately [ 9 ].
The vast majority of asthmatic children exhibit a mild or at most a moderate disease that can be fully controlled with low-to-medium dose ICS associated or not with other controllers [ 5 , 6 ]. However, a subset of asthmatics remains difficult-to-treat [ 5 , 6 ]. With the advent of biologics, these severe steroid-dependent asthmatics have alternative options for treatment, as steroid-related adverse events are common in severe asthma [ 53 ]. Omalizumab, an anti-IgE monoclonal antibody, is the only biologic therapy recommended in children with moderate-to-severe asthma by the recent guidelines [ 5 , 6 ]. In Italy, this treatment is fully covered by the National Health System. Therefore, there is no influence by any funding on treatment decisions. It was approved by the US (Food and Drug Administration) in 2003 and by the European Union (European Medicines Agency) in 2005 as an add-on treatment for patients aged > 12 years with severe persistent allergic asthma and who have a positive skin test or in-vitro reactivity to a perennial aeroallergen, FEV 1 < 80% predicted, frequent daytime symptoms or nighttime awakenings, and multiple documented severe asthma exacerbations despite daily ICS plus a LABA [ 54 , 55 ]. In 2009, it also received approval in Europe for treating patients aged 6–12 years. Figure 2 illustrates current indications for treatment with omalizumab in children and adolescents with severe asthma.
Indications for omalizumab in children and adolescents with severe asthma
IgE antibodies, Th 2 -derived cytokines and eosinophils play a major role in the development of chronic airway inflammation in asthmatic subjects [ 56 ]. Once released from plasma cells, IgE binds principally to the high-affinity IgE receptor (FcεRI) on mast cells, triggering different effector responses, including the release of mediators leading to allergic inflammatory reactions [ 56 ]. The activation of the allergic cascade by IgE, under constant allergen stimulation, leads to the establishment of chronic allergic inflammation in the airways of asthmatic patients, with IgE being a key element of the vicious circle that maintains it. Cytokines produced during the late phase and subsequent chronic inflammation stage have been directly associated with the induction of airway remodelling, indirectly implicating IgE in the process [ 56 ]. At present, omalizumab is the only commercially available recombinant humanized anti-IgE monoclonal antibody that specifically binds serum free IgE at its CH 3 domain, in the proximity of the binding site for FcεRI, thus preventing IgE from interacting with its receptor on mast cells, basophils, antigen-presenting cells and other inflammatory cells [ 57 ]. The rapid reduction of free IgE levels leads to a downregulation of the FcεRI expression on inflammatory cells and an interruption of the allergic cascade, which results in the reduction of peripheral and bronchial tissue eosinophilia and of levels of granulocyte macrophage colony stimulating factor, interleukin (IL)-2, IL-4, IL-5, and IL-13 [ 58 ]. Moreover, basophils have a relevant role in the initiation and progression of allergic inflammation, suggesting that they may represent a viable therapeutic target. Indeed, in children with severe asthma, it has been reported that omalizumab therapy is associated with a significant reduction in circulating basophil numbers, a finding that is concurrent with improved clinical outcomes [ 59 ]. This finding supports a mechanistic link between IgE levels and circulating basophil populations, and may provide new insights into one mechanism by which omalizumab improves asthma symptoms.
Several clinical controlled and real-life studies of adults with severe, inadequately controlled allergic asthma have demonstrated the efficacy and safety of omalizumab in reducing asthma-related symptoms, corticosteroid use, exacerbation rates, and healthcare resource utilization, and in improving QoL and lung function [ 60 , 61 , 62 , 63 ]. Fewer studies have been published in children. In two double-blind, randomized, placebo-controlled trials (RCTs) of children aged 6 to 12 years with moderate-to-severe allergic asthma, treatment with omalizumab reduced the requirement for ICS and protected against disease exacerbations, but there was little change in asthma symptom scores or spirometry [ 9 , 64 ]. These findings were confirmed and extended in older children [ 65 , 66 , 67 ].
The results of the ICATA study, a multicenter RCT of 419 inner-city children, adolescents and young adults with persistent allergic asthma, showed that, compared to placebo, omalizumab reduces the number of days with asthma symptoms and the proportion of participants with at least one exacerbation by approximately 25% and 19%, respectively ( p < 0.001), thus reducing the need for asthmatic symptom controllers [ 68 ]. Another multicenter RCT of inner-city children and adolescents showed that the addition of omalizumab to ongoing guidelines-based care before patients return to school reduces fall asthma exacerbations (odds ratio, 0.48), particularly in subjects with a recent exacerbation [ 69 ]. Moreover, in a real-life study of 104 children and adolescents with severe allergic refractory asthma followed over 1 year, treatment with omalizumab resulted in good asthma control in 67% of the cases ( p < 0.001), while FEV 1 improved by 4.9% ( p = 0.02) and exacerbation rates and healthcare utilisation decreased approximately by 30% ( p < 0.001) [ 70 ]. The same authors also showed that, after two years of treatment, exacerbation rate and healthcare utilisation were further decreased by 83% and 100%, respectively, while level of asthma control, steroid use and lung function remained unchanged [ 71 ].
A systematic review of pediatric RCTs pooled the data of 1381 children and adolescents with moderate-to-severe allergic asthma in order to establish the efficacy of omalizumab as an add-on therapy [ 72 ]. During the stable-steroid phase, omalizumab decreased the number of patients with at least one exacerbation (risk ratio, 0.69; p < 0.001), the mean number of asthma exacerbations per patient (risk ratio, 0.35; p < 0.001), and the asthma symptom score (mean difference, 0.12; p = 0.005) when compared to placebo. During the steroid reduction phase, omalizumab further reduced the number of patients with at least one exacerbation (risk ratio, 0.48; p < 0.001) and the mean number of asthma exacerbations per patient (mean difference, 0.12; p < 0.05).
Given the cost of omalizumab, many authors have argued for the importance of identifying specific asthma populations who will have significant benefit from it [ 68 , 73 , 74 ]. In the ICATA study, baseline predictors of good response to treatment were sensitization and exposure to cockroach allergen, sensitization to house dust mite allergens, a serum IgE level of more than 100 IU per milliliter, a BMI of 25 or more, and a history of at least one unscheduled medical visit in the previous year [ 68 ].
Several studies have assessed the long-term safety of omalizumab in children and adults. A pooled analysis of 67 RCTs conducted over 2 decades on 4254 children and adults treated with omalizumab showed no association between omalizumab treatment and risk of malignancy [ 75 ]. In an RCT evaluating 225 school-aged children, omalizumab was well tolerated, there were no serious adverse events, and the frequency and types of all adverse events were similar to the placebo group [ 9 ]. These results have been further confirmed by a recent systematic review of RCTs that concluded that treatment with omalizumab does not result in increased risk of malignancy or hypersensitivity reactions [ 72 ].
While the rationale for long-term treatment with omalizumab is supported by pharmacokinetic-pharmacodynamic models [ 76 ], the duration of treatment is still under discussion. Results from published studies suggest that omalizumab should be continued for > 1 year [ 77 , 78 ]. In a retrospective study of adults and children with uncontrolled severe asthma treated with omalizumab, the response to treatment was ‘excellent’ in 52.5% of patients, particularly in the subgroup of children aged 6 to 11 years [ 77 ]. After the discontinuation of treatment, loss of asthma control was documented in 69.2% of the patients who had received omalizumab for < 1 year, 59.1% of the subjects treated for 1–2 years, and 46.1% of the cases treated for > 2 years. Time to loss of control was shorter in younger children and longer in patients with an ‘excellent’ response compared with patients with a ‘good’ response. No early loss of control (within 6 months) was observed among patients with > 3.5 years of continuous treatment with omalizumab. Finally, 20% of patients in whom omalizumab was re-prescribed because of loss of control did not respond to the treatment anymore [ 77 ]. Despite these encouraging findings, the impact of omalizumab on the natural history of severe asthma in children deserves to be further investigated by long-term studies that will also define the criteria and timing for discontinuing the treatment.
It is well known that asthma pharmacotherapy is effective in controlling symptoms and bronchial inflammation, but cannot affect the underlying immune response, thus leading to the possibility of symptom reappearance after its discontinuation [ 79 ]. In this scenario, allergen-specific immunotherapy (AIT) has been proposed as the only therapeutic method that can modulate the underlying immune pathophysiology in allergic asthma [ 80 ].
AIT is currently indicated in children and adults with mild-moderate allergic asthma that is completely or partially controlled by pharmacotherapy and with the evidence of a clear relationship between symptoms and exposure to a specific allergen [ 81 , 82 , 83 , 84 ]. However, according to recent guidelines, the efficacy of AIT in asthmatic subjects is limited, and its potential benefits must be weighed against the risk of side effects and the inconvenience and costs of the prolonged therapy [ 5 ]. Moreover, severe or uncontrolled asthma (regardless of its severity) is a major independent risk factor for non-fatal or even fatal adverse reactions, thus representing a contraindication for AIT [ 85 , 86 , 87 ]. Finally, children with severe asthma are often sensitized to multiple allergens, thus making AIT prescription even more complicated [ 88 ].
In subjects with uncontrolled and/or severe allergic asthma, a combination of omalizumab and AIT has been proposed [ 88 ]. Surprisingly, only a few studies have addressed this issue [ 89 , 90 , 91 , 92 ]. However, pre-treatment with omalizumab seems to improve the efficacy and tolerability of subcutaneous AIT in children and adults with severe allergic asthma both during omalizumab treatment and after its discontinuation [ 89 , 91 , 92 ]. Omalizumab has also been successfully used as a supplementary treatment to AIT in order to improve asthma control in children ≥6 years with severe persistent allergic asthma [ 90 ]. Given the scarcity of studies on AIT plus omalizumab in children with severe allergic asthma, further research is warranted to assess risks and benefits of the combined treatment.
Children with severe asthma require a detailed and individualized approach including re-assessment for differential diagnoses, comorbidities and contributory factors, environmental triggers, lung function and inflammation, adherence and response to therapy, and QoL. Treatment of pediatric severe asthma still relies on the maximal optimal use of corticosteroids, bronchodilators and other controllers recommended for moderate-to-severe disease. However, the management of asthma is becoming much more patient-specific, as more and more is learned about the biology behind the development and progression of asthma.
In the current paper, we described the characteristics of four children with severe asthma in whom omalizumab was prescribed. A review of the relevant literature on the topic was also performed. Finally, we provided an algorithm for the diagnosis of difficult-to-treat and severe asthma in children and adolescents, based on the evidence from the literature review. As all algorithms, it is not meant to replace clinical judgment, but it should drive physicians to adopt a systematic approach towards difficult and severe asthma and provide a useful guide to the clinician.
The addition of omalizumab, the first targeted biological treatment approved for asthma, has led to renewed optimism of outcome improvements in patients with allergic severe asthma. As severe asthma is a heterogeneous condition consisting of different phenotypes, the future of asthma management will likely involve phenotypic and potentially even genotypic characterization in selected cases in order to determine appropriate therapy and thus to provide the highest possible benefit, especially if specific responder phenotypes can be identified and selected for this highly specific treatment.
Abbreviations
Anti-immunoglobulin E
Body mass index
IgE receptor
Forced expiratory flow between 25% and 75%
Forced expiratory volume in the first second
Gastroesophageal reflux
Inhaled corticosteroids
Intensive care unit
Interleukin
Long-acting β 2 -agonist
Oral leukotriene receptor antagonist
Quality of life
Randomized controlled trials
Short-acting β 2 -agonists
Sleep-disordered breathing
O'Byrne PM, Pedersen S, Schatz M, Thoren A, Ekholm E, Carlsson LG, et al. The poorly explored impact of uncontrolled asthma. Chest. 2013;143:511–3.
Article PubMed Google Scholar
National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): guidelines for the diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–8.
Article Google Scholar
Hedlin G. Management of severe asthma in childhood-state of the art and novel perspectives. Pediatr Allergy Immunol. 2014;25:111–21.
Konradsen JR, Nordlund B, Lidegran M, Pedroletti C, Grönlund H, van Hage M, et al. Problematic severe asthma: a proposed approach to identifying children who are severely resistant to therapy. Pediatr Allergy Immunol. 2011;22:9–18.
Global Initiative for Asthma Report. Global strategy for asthma management and prevention (updated 2016). https://www.ginasthma.org . Accessed 07 June 2017.
Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–53.
Article CAS PubMed Google Scholar
Vandenplas Y, Rudolph CD, Di Lorenzo C, Hassall E, Liptak G, Mazur L, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the north American Society for Pediatric Gastroenterology, Hepatology, and nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49:498–507.
Lødrup Carlsen KC, Hedlin G, Bush A, Wennergren G, de Benedictis FM, De Jongste JC, et al. Assessment of problematic severe asthma in children. Eur Respir J. 2011;37:432–40.
Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab). Pediatrics. 2001;108:E36.
Bush A, Saglani S. Management of severe asthma in children. Lancet. 2010;376:814–5.
Article CAS PubMed PubMed Central Google Scholar
Lang A, Mowinckel P, Sachs-Olsen C, Riiser A, Lunde J, Carlsen KH, et al. Asthma severity in childhood, untangling clinical phenotypes. Pediatr Allergy Immunol. 2010;21:945–53.
Nordlund B, Konradsen JR, Pedroletti C, Kull I, Hedlin G. The clinical benefit of evaluating health-related quality-of-life in children with problematic severe asthma. Acta Paediatr. 2011;100:1454–60.
Dean BB, Calimlim BC, Sacco P, Aguilar D, Maykut R, Tinkelman D. Uncontrolled asthma: assessing quality of life and productivity of children and their caregivers using a cross-sectional internet-based survey. Health Qual Life Outcomes. 2010;8:6.
Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in children with asthma. Qual Life Res. 1996;5:35–46.
British Thoracic Society. Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma, 2014. https://www.brit-thoracic.org.uk/guidelines-and-quality-standards/asthma-guideline . Accessed 13 Apr 2016.
Montella S, Baraldi E, Cazzato S, Aralla R, Berardi M, Brunetti LM, et al. Severe asthma features in children: a case-control online survey. Ital J Pediatr. 2016;42:9.
Article PubMed PubMed Central Google Scholar
Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG, National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. Features of severe asthma in school-age children: Atopy and increased exhaled nitric oxide. J Allergy Clin Immunol. 2006;118:1218–25.
Simon MR, Chinchilli VM, Phillips BR, Sorkness CA, Lemanske RF Jr, Szefler SJ, et al. Forced expiratory flow between 25% and 75% of vital capacity and FEV1/forced vital capacity ratio in relation to clinical and physiological parameters in asthmatic children with normal FEV1 values. J Allergy Clin Immunol. 2010;126:527–34.
Hedlin G, Bush A, Lødrup Carlsen K, Wennergren G, De Benedictis FM, Melén E, et al. Problematic severe asthma in children, not one problem but many: a GA2LEN initiative. Eur Respir J. 2010;36:196–201.
Fitzpatrick AM, Teague WG. Severe asthma in children: insights from the National Heart, Lung, and Blood Institute's severe asthma research program. Pediatr Allergy Immunol Pulmonol. 2010;23:131–8.
Konradsen JR, Caffrey Osvald E, Hedlin G. Update on the current methods for the diagnosis and treatment of severe childhood asthma. Expert Rev Respir Med. 2015;9:769–77.
Lang AM, Konradsen J, Carlsen KH, Sachs-Olsen C, Mowinckel P, Hedlin G, et al. Identifying problematic severe asthma in the individual child—does lung function matter? Acta Paediatr. 2010;99:404–10.
Rao DR, Gaffin JM, Baxi SN, Sheehan WJ, Hoffman EB, Phipatanakul WJ. The utility of forced expiratory flow between 25% and 75% of vital capacity in predicting childhood asthma morbidity and severity. Asthma. 2012;49:586–92.
Eid N, Yandell B, Howell L, Eddy M, Sheikh S. Can peak expiratory flow predict airflow obstruction in children with asthma? Pediatrics. 2000;105:354–8.
Cicutto LC, Chapman KR, Chamberlain D, Downey GP. Difficult asthma: consider all of the possibilities. Can Respir J. 2000;7:415–8.
Wener RR, Bel EH. Severe refractory asthma: an update. Eur Respir Rev. 2013;22:227–35.
Bracken M, Fleming L, Hall P, et al. The importance of nurse-led home visits in the assessment of children with problematic asthma. Arch Dis Child. 2009;94:780–4.
De Groot EP, Kreggemeijer WJ, Brand PL. Getting the basics right resolves most cases of uncontrolled and problematic asthma. Acta Paediatr. 2015;104:916–21.
Grimaldi-Bensouda L, Zureik M, Aubier M, Humbert M, Levy J, Benichou J, et al. Does omalizumab make a difference to the real-life treatment of asthma exacerbations? Results from a large cohort of patients with severe uncontrolled asthma. Chest. 2013;143:398–405.
American Lung Association Asthma Clinical Research Centers, Mastronarde JG, Anthonisen NR, Castro M, Holbrook JT, Leone FT, et al. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487–9.
Article PubMed Central Google Scholar
Writing Committee for the American Lung Association Asthma Clinical Research Centers, Holbrook JT, Wise RA, Gold BD, Blake K, Brown ED, et al. Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. JAMA 2012;307:373-381.
Wright AL, Holberg CJ, Martinez FD, Halonen M, Morgan W, Taussig LM. Epidemiology of physician-diagnosed allergic rhinitis in childhood. Pediatrics. 1994;94:895–901.
CAS PubMed Google Scholar
De Groot EP, Nijkamp A, Duiverman EJ, Brand PL. Allergic rhinitis is associated with poor asthma control in children with asthma. Thorax. 2012;67:582–7.
Rotiroti G, Roberts G, Scadding GK. Rhinitis in children: common clinical presentations and differential diagnoses. Pediatr Allergy Immunol. 2015;26:103–10.
Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA). 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63:S8–160.
Deliu M, Belgrave D, Simpson A, Murray CS, Kerry G, Custovic A. Impact of rhinitis on asthma severity in school-age children. Allergy. 2014;69:1515–21.
Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466–76.
Weinberger M, Abu-Hasan M. Pseudo-asthma: when cough, wheezing, and dyspnea are not asthma. Pediatrics. 2007;120:855–64.
De Groot EP, Duiverman EJ, Brand PL. Dysfunctional breathing in children with asthma: a rare but relevant comorbidity. Eur Respir J. 2013;41:1068–73.
Barker NJ, Jones M, O'Connell NE, Everard ML. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in children. Cochrane Database Syst Rev. 2013;12:CD010376.
Google Scholar
Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome, American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:704–12.
Goldstein NA, Aronin C, Kantrowitz B, Hershcopf R, Fishkin S, Lee H, Weaver DE, et al. The prevalence of sleep-disordered breathing in children with asthma and its behavioral effects. Pediatr Pulmonol. 2015;50:1128–36.
Ross KR, Storfer-Isser A, Hart MA, Kibler AM, Rueschman M, Rosen CL, et al. Sleep-disordered breathing is associated with asthma severity in children. J Pediatr. 2012;160:736–42.
Santamaria F, Montella S, Greco L, Valerio G, Franzese A, Maniscalco M, et al. Obesity duration is associated to pulmonary function impairment in obese subjects. Obesity (Silver Spring). 2011;19:1623–8.
Sivapalan P, Diamant Z, Ulrik CS. Obesity and asthma: current knowledge and future needs. Curr Opin Pulm Med. 2015;21:80–5.
Rasmussen F, Hancox RJ. Mechanisms of obesity in asthma. Curr Opin Allergy Clin Immunol. 2014;14:35–43.
Santamaria F, Montella S, Pietrobelli A. Obesity and pulmonary disease: unanswered questions. Obes Rev. 2012;13:822–33.
Lang JE, Hossain J, Holbrook JT, Teague WG, Gold BD, Wise RA, et al. Gastro-oesophageal reflux and worse asthma control in obese children: a case of symptom misattribution? Thorax. 2016;71:238–46.
Santamaria F, Montella S, De Stefano S, Sperlì F, Barbarano F, Valerio G. Relationship between exhaled nitric oxide and body mass index in children and adolescents. J Allergy Clin Immunol. 2005;116:1163–4.
Van Huisstede A, Rudolphus A, Castro Cabezas M, Biter LU, van de Geijn GJ, Taube C, et al. Effect of bariatric surgery on asthma control, lung function and bronchial and systemic inflammation in morbidly obese subjects with asthma. Thorax. 2015;70:659–67.
Katzmarzyk PT, Bouchard C. Where is the beef? Waist circumference is more highly correlated with BMI and total body fat than with abdominal visceral fat in children. Int J Obes. 2014;38:753–4.
Article CAS Google Scholar
De Groot EP, Duiverman EJ, Brand PL. Comorbidities of asthma during childhood: possibly important, yet poorly studied. Eur Respir J. 2010;36:671–8.
Sweeney J, Patterson CC, Menzies-Gow A, Niven RM, Mansur AH, Bucknall C, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the optimum patient care research database and the British thoracic difficult asthma registry. Thorax. 2016; https://doi.org/10.1136/thoraxjnl-2015-207630 .
Federal Drug Administration Advisory for Omalizumab. Available at: https://wayback.archive-it.org/7993/20170111075347/ . http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/default.htm . Accessed 4 Feb 2018.
European Medicines Agency: assessment report for Xolair. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000606/human_med_001162.jsp&mid=WC0b01ac058001d124 . Accessed 7 June 2017.
Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet. 2015;386:1086–96.
Jensen RK, Plum M, Tjerrild L, Jakob T, Spillner E, Andersen GR. Structure of the omalizumab Fab. Acta Crystallogr F Struct Biol Commun. 2015;71:419–26.
Holgate S, Smith N, Massanari M, Jimenez P. Effects of omalizumab on markers of inflammation in patients with allergic asthma. Allergy. 2009;64:1728–36.
Hill DA, Siracusa MC, Ruymann KR, Tait Wojno ED, Artis D, Spergel JM. Omalizumab therapy is associated with reduced circulating basophil populations in asthmatic children. Allergy. 2014;69:674–7.
Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60:309–16.
Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;1:CD003559.
Lai T, Wang S, Xu Z, Zhang C, Zhao Y, Hu Y, Cao C, et al. Long-term efficacy and safety of omalizumab in patients with persistent uncontrolled allergic asthma: a systematic review and meta-analysis. Sci Rep. 2015;5:8191.
Abraham I, Alhossan A, Lee CS, Kutbi H, MacDonald K. “real-life” effectiveness studies of omalizumab in adult patients with severe allergic asthma: systematic review. Allergy. 2015; https://doi.org/10.1111/all.12815 .
Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol. 2009;124:1210–6.
Solèr M, Matz J, Townley R, Buhl R, O'Brien J, Fox H, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–61.
Holgate ST. Cytokine and anti-cytokine therapy for the treatment of asthma and allergic disease. Cytokine. 2004;28:152–7.
Odajima H, Ebisawa M, Nagakura T, Fujisawa T, Akasawa A, Ito K, et al. Omalizumab in Japanese children with severe allergic asthma uncontrolled with standard therapy. Allergol Int. 2015;64:364–70.
Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–15.
Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ Jr, Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136:1476–85.
Deschildre A, Marguet C, Salleron J, Pin I, Rittié JL, Derelle J, et al. Add-on omalizumab in children with severe allergic asthma: a 1-year real life survey. Eur Respir J. 2013;42:1224–33.
Deschildre A, Marguet C, Langlois C, Pin I, Rittié JL, Derelle J, et al. Real-life long-term omalizumab therapy in children with severe allergic asthma. Eur Respir J. 2015;46:856–9.
Rodrigo GJ, Neffen H. Systematic review on the use of omalizumab for the treatment of asthmatic children and adolescents. Pediatr Allergy Immunol. 2015;26:551–6.
Oba Y, Salzman GA. Cost-effectiveness analysis of omalizumab in adults and adolescents with moderate-to-severe allergic asthma. J Allergy Clin Immunol. 2004;114:265–9.
Campbell JD, Spackman DE, Sullivan SD. The costs and consequences of omalizumab in uncontrolled asthma from a USA payer perspective. Allergy. 2010;65:1141–8.
Busse W, Buhl R, Fernandez Vidaurre C, Blogg M, Zhu J, Eisner MD, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. 2012;129:983–9.
Lowe PJ, Renard D. Omalizumab decreases IgE production in patients with allergic (IgE-mediated) asthma; PKPD analysis of a biomarker, total IgE. Br J Clin Pharmacol. 2011;72:306–10.
Molimard M, Mala L, Bourdeix I, Le Gros V. Observational study in severe asthmatic patients after discontinuation of omalizumab for good asthma control. Respir Med. 2014;108:571–6.
Busse WW, Trzaskoma B, Omachi TA, Canvin J, Rosen K, Chipps BE, et al. Evaluating Xolair persistency of response after long-term therapy (XPORT). Am J Respir Crit Care Med. 2014;189:A6576.
Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–97.
Akdis CA. Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med. 2012;18:736–49.
National Heart, Lung, and Blood Institute. Expert panel report 3: Guidelines for the diagnosis and management of asthma—full report 2007. Available at: https://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln.pdf . Accessed 4 Feb 2018.
Joint Task Force on Practice Parameters, American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology, Joint Council of Allergy, Asthma and Immunolgy. Allergen immunotherapy: a practice parameter second update. J Allergy Clin Immunol. 2007;120:S25–85.
Zuberbier T, Bachert C, Bousquet PJ, Passalacqua G, Walter Canonica G, Merk H, et al. GA(2) LEN/EAACI pocket guide for allergen-specific immunotherapy for allergic rhinitis and asthma. Allergy. 2010;65:1525–30.
Pajno GB, Bernardini R, Peroni D, Arasi S, Martelli A, Landi M, et al. Clinical practice recommendations for allergen-specific immunotherapy in children: the Italian consensus report. Ital J Pediatr. 2017;43:13.
Pitsios C, Demoly P, Bilo MB, Gerth van Wijk R, Pfaar O, Sturm GJ, et al. Clinical contraindications to allergen immunotherapy: an EAACI position paper. Allergy. 2015;70:897–909.
Tsabouri S, Mavroudi A, Feketea G, Guibas GV. Subcutaneous and sublingual immunotherapy in allergic asthma in children. Front Pediatr. 2017;5:82.
Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556–68.
Hedlin G, van Hage M. The role of immunotherapy in the management of childhood asthma. Ther Adv Respir Dis. 2012;6:137–46.
Lambert N, Guiddir T, Amat F, Just J. Pre-treatment by omalizumab allows allergen immunotherapy in children and young adults with severe allergic asthma. Pediatr Allergy Immunol. 2014;25:829–32.
Kopp MV, Hamelmann E, Zielen S, Kamin W, Bergmann K-C, Sieder C. Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and co-morbid seasonal allergic asthma. Clin Exp Allergy. 2009;39:271–9.
Massanari M, Nelson H, Casale T, Busse W, Kianifard F, Geba GP. Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. J Allergy Clin Immunol. 2010;125:383–9.
Stelmach I, Kaczmarek-Woźniak J, Majak P, Olszowiec-Chlebna M, Jerzynska J. Efficacy and safety of high-doses sublingual immunotherapy in ultra-rush scheme in children allergic to grass pollen. Clin Exp Allergy. 2009;39:401–8.
Download references
Acknowledgements
The authors gratefully thank Dr. Marco Maglione for his contribution in the clinical assessment of the described cases. Medical writing assistance was provided by Stephen Walters on behalf of City Hills Proofreading.
No funding was secured for this study.
Availability of data and materials
All relevant data and materials are published in the manuscript.
Author information
Authors and affiliations.
Department of Translational Medical Sciences, Federico II University, Via Sergio Pansini 5, 80131, Naples, Italy
Virginia Mirra, Silvia Montella & Francesca Santamaria
You can also search for this author in PubMed Google Scholar
Contributions
VM, SM and FS, authors of the current manuscript, declare that they have participated sufficiently in the work to take public responsibility for appropriate portions of the content. VM and SM carried out the initial investigations, drafted the initial manuscript, revised the manuscript, and approved the final manuscript as submitted. FS conceptualized and designed the study, and critically reviewed and approved the final manuscript as submitted. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Francesca Santamaria .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the ethics committee “Carlo Romano”, Federico II University, Naples, Italy. Children’s parents/legal guardians gave informed written consent to participate. The description of our cases adheres to the CARE standards of reporting checklist.
Consent for publication
Children’s parents/legal guardians provided informed written consent for the case report to be published.
Competing interests
The authors declare that they have no competing interests to disclose. Authors have no financial relationships relevant to this article to disclose.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Mirra, V., Montella, S. & Santamaria, F. Pediatric severe asthma: a case series report and perspectives on anti-IgE treatment. BMC Pediatr 18 , 73 (2018). https://doi.org/10.1186/s12887-018-1019-9
Download citation
Received : 24 May 2016
Accepted : 29 January 2018
Published : 21 February 2018
DOI : https://doi.org/10.1186/s12887-018-1019-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Severe asthma
- Adolescents
- Asthma exacerbations
BMC Pediatrics
ISSN: 1471-2431
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Join our Mailing List
Working locally in primary care and collaborating globally to improve respiratory health
Clinical case study - asthma, clinical case study - asthma, resource information.
- Disease management
- Open access
- Published: 03 April 2020
Determinants of Acute Asthma Attack among adult asthmatic patients visiting hospitals of Tigray, Ethiopia, 2019: case control study
- Melaku Negash 1 ,
- Hagos Tsegabrhan 2 ,
- Teklit Meles 3 ,
- Degena Bahrey Tadesse 1 ,
- Gebreamlak Gidey 4 ,
- Yemane Berhane 5 ,
- Kibrom Berhanu 6 &
- Tsgalem Haylemaryam 7
Asthma Research and Practice volume 6 , Article number: 1 ( 2020 ) Cite this article
4879 Accesses
3 Citations
3 Altmetric
Metrics details
Introduction
Acute asthma attack is one of the most common causes of visits to hospital emergency departments in all age groups of the population and accounts for the greater part of healthcare burden from the disease. Despite, Acute asthma attack is an important public health problem that affects not only the patients, but also to the family, health professionals, health care institutions and development of the nation, little is known about the risk factors of acute asthma attack.
Therefore, this study is aimed to investigate the determinants of acute asthma attack among.
The aim of this study was to assess the determinant factors of acute asthma attack among adult asthmatic patients visiting general hospitals of central zone, Tigray, Ethiopia, 2019.
Hospital based unmatched case control study design was conducted in general hospitals of central zone of Tigray, Ethiopia 2019. Data were collected using pretested interviewer administered questionnaire. A total of 289 study subjects (96 cases &193 controls) were selected by systematic random sampling. Data were entered to Epi data version 3.1 then exported to SPSS version 23 for analysis. Bivariate logistic regression was employed to examine the statistical association between dependent and independent variables. Variables with p value < 0.25 in binary logistic regression were entered to multivariable logistic regression model and variables with p value < 0.05 was taken as significant determinants of the outcome variable.
A total of 96 adult asthmatic patients who have acute asthma attack (cases) and 193 adult asthmatic patients without attack (controls)) with 100% response rate were participated in this study. Upper Respiratory tract Infection [AOR = 6.835,95% CI = 3.285,14.222], Season [AOR =2.204,95% CI = 1.011,4.805] kitchen smoke [AOR = 2.307,95%CI1.010,5.272]& sleep apnea [AOR = 9.254, 5%CI =3.563,25.460] were significantly associated with acute asthma exacerbation.
Asthma is a long-term inflammatory disease of the respiratory system which is characterized by wheezing, shortness of breath, chest tightness. Globally it affects approximately 300 million people and is estimated to rise to 400 million by 2025 globally [ 1 , 2 ]. And it is ranked 16th among the leading causes of disability and 28th among the leading causes of burden of disease, as measured by disability adjusted life years (DALYs) [ 3 ].
According to Croatian medical journal 2013, an estimate of asthma prevalence in Africa, was 49.7 million in the age of < 15 years (13.9%), < 45 years 102.9 million (13.8%), and in total population 119.3 million (12.8%) in 2010 [ 4 ].
Asthma exacerbation is defined as a worsening of shortness of breath, cough, wheezing, or chest tightness. If not treated immediately there will be increase in flow resistance causing increased work of breathing, gas exchange inefficiency, respiratory muscle tiredness and finally hypercapnic and hypoxemic respiratory failure [ 5 ]. This implies that acute asthma attack is a significant public health problem that affects patients with their parents or families and the community through labor and school loss, frequent emergency clinic visits, a poor quality of life hospitalizations and finally death [ 6 ]. According to Centers for Disease Control and prevention (CDC) report, More than 11 million people reported having an acute asthma attack [ 7 ].
Despite, in Ethiopia little is known about how risk factors are associated with exacerbation, according to asthma severity and the relative importance of the risk factors. This may be the reason for no policy and strategy to ascertain and acting out of effective intervention in order to reduce the burden of acute asthma attack [ 8 ]. Therefore, this study is aimed to full fill this gap.
Study setting and study design
Hospital based unmatched case control study was conducted in the selected general Hospitals of Central zone of Tigray from November 2018 to July 2019.
Study population and sample size determination
Source population.
All adult asthmatic patients visited to emergency unit who have acute asthma attack.
All adult patients diagnosed as asthma but without acute asthmatic attack who visited the OPD and the regular follow-up unit during the data collection period.
Study population
All selected adult asthmatic patients visited to emergency unit who have acute asthma attack during the data collection period.
All selected adult patients diagnosed as asthma but without acute asthmatic attack who visited the OPD and the regular follow-up unit during the data collection period.
Eligibility criteria
Inclusion criteria.
Adult asthmatic patients who have acute asthma attack during the data collection period.
Adult asthmatic patient without acute asthma attack during the data collection period.
Exclusion criteria
Patients with any history of pulmonary embolism, chronic obstructive pulmonary disease, active pulmonary TB, known congestive heart failure and known mechanical obstruction.
Sample size determination
Sample size was calculated from Previous study conducted in Uganda [ 9 ],using Epi info version 7. sample size was determined based on the assumption of confidence level = 95%; Power = 80%; Odds ratio = 2.132 with case to control ratio = 1:2, proportion of among controls 37.2%, proportion of among cases = 55.8%.
Therefore, the required sample size for cases was =92 where as for the controls =183 and the overall sample size was = 275 then after adding 5% non-response rate, the total sample size was 289. Finally, a sample size for cases was 96 and for controls 193.
Sampling technique and procedure
The total sample size was allocated to each hospital proportionally based on the number of patients who attend in the selected hospitals. A total number of 585(case 165, control.420) patients attended at the selected Hospitals with in 2 months of the previous year (April 1 to May 30–2018). Systematic random sampling method was applied in each hospital to select 289 participants.
Study Variables
Dependent variable.
Acute asthma attack.
Independent variables
Socio-demographic variables.
Age, Gender, Marital status, Residence, Educational level, Employment status and Occupational status.
Behavioral factors
Exercise, vigorous activity Smoking cigarette.
Environmental factors
Humidity, Kitchen smoke, dust, Season.
Medical and Clinical characteristics
URTI, Sleep apnea, Missing follow-up / appointments,
Operational definitions
Those who present with cough, wheezing and difficulty of breathing and diagnosed asthma by physician [ 10 ].
Acute Asthma Attack
Those who present with worsening of wheezing, shortness of breath, cough, chest tightness and diagnosed as acute asthma attack by physician [ 10 ].
Smoker:( daily smoker and non-daily smoker) those who currently smokes or those who quit smoking less than 1 year before the assessment [ 10 ].
Passive smoker: Smoke inhaled involuntarily by non-smokers [ 11 ].
Nonsmoker: Respondents who report never smoke those who quit smoking greater than 1 year before the assessment.
Vigorous activity: participants doing activity more than 10 min continuously, that increases breathing, like carrying or lifting heavy loads, digging or construction work, cutting fire wood [ 11 ].
Data collection tool
Structured questionnaire was used to collect the data which was adapted from different literatures [ 9 , 12 , 13 , 14 ]. The questionnaire contains four parts: socio-demographic, environmental factors, behavioral factors, and Medical &Clinical characteristics.
Data collection procedures
Data were collected from cases and controls using structured questionnaire and checklists through face-to-face interview and from patients chart review respectively.
Twelve BSc nurses as data collectors and three senior nurse supervisors were recruited for the data collection, Then data from cases were collected after they take all the necessary medical care and they recover from their attack whereas from the controls data were collected after they have completed their assessment by physician and at the last record reviews from their chart. Participants were identified as having upper respiratory tract infection and Obstructive sleep apnea from their medical charts which was diagnosed by senior physicians. This is to mean that, it was just suspected clinically by the time of the acute event. The reason we obeyed to use clinically diagnosis for obstructive sleep apnea is that, there is no accesses of modern diagnostic modality like polysomnography in the study area which was Tigray regional state not only in the study area but also in the country Ethiopia as a whole. The evaluation protocol that we use were a single evaluation visit for each case and even those who have follow-up and developed acute asthma attack were included .
Data quality control techniques
Data quality was ensured by training of data collectors and supervisors before data collection period. 5% of the questionnaire was pre-tested in Shire Hospital which was not included in the actual data collection. Based on the findings of the pre-test, questionnaire was modified. The filled questionnaire was checked for completeness and accuracy by data collectors, supervisors and principal investigator each day.. The questionnaire was translated into Tigrigna language for better understanding to both the data collectors and respondents and then back translated into English by another expert to ensure accuracy and consistency.
Data analysis procedures
Data were entered in to Epi data version 3.1 and analyzed using SPSS version 23.0. The degree of association between independent and dependent variables were assessed using adjusted odds ratio with 95% confidence interval. Variables < 0.25 p -value in binary logistic regression were entered to multivariable logistic regression model to control the potential confounding variables. Variables with p-value less than 0.05 in multivariable logistic regression model were taken as significantly associated factors. Variance inflation factor (VIF) was used to assess Multicollinearity between the independent variables. Hosmer and Lemeshow goodness fit model were used to check model fitness.
Ethical consideration
Ethical clearance was obtained from Mekelle University College of health sciences institutional review board (IRB). A subsequent permission was also obtained from Tigray teaching hospitals. Respondents were informed about the purpose of the study and the interview was conducted after receiving the written consent from participants. Confidentiality of the data/information was secured and was not used for other purposes.
Sociodemographic characteristic of study participants
Among the participants, 67.7% (65) of the cases and 60.6% (117) of the controls were females. The median ages of participants were 43 years with interquartile range (IQR) of 26.5 years among cases and 43 median ages with interquartile range (IQR) of 22 for control.
The educational status, one third 33.3% (32) of the cases and 24.9% (48) of the controls were collage and above, where as 14.6% (14) of the cases and 16.6% (32) of the controls were unable to read and write. The majority of the cases 63.5% (61) and 60.1% (116) of the controls were married (Table 1 ).
Behavioral characteristics of study participants
Among the participants, 2.1% (2) of the cases and 1.1% (6) of the controls were smokers.in parallel with this 3.1% of the cases and 4.7% of the control were passive smokers. Regarding vigorous activity 37.5% (36) of the cases and 23.8% (46) of the controls were do vigorous activity. Majority of the participants 72.9% (70) of the cases and 58% (112) of the controls were doing exercise.
Medical & clinical characteristics of study participants
Among the participants, 44.8% (43) of the cases and 13.5% (26) of the controls had Upper Respiratory Tract Infections (URTI) and 29.2% (28) of the cases and few of the controls 5.2% (10) had obstructive sleep apnea.
Among the participants, 31.3% (30) of the cases and 20.7% (40) of the controls had Missing follow up.
Environmental characteristics of study participants
Regarding the seasons of a year, spring season (April, May, June) were the season with high percentage 37.7% (109) of acute asthma attack than the autumn season. Majority of the participants 79.5% (230) were open their window/door while they were cooking. Concerning the kitchen of the participants 32.3% (31) of the cases and 20.2% (39) of the control’s kitchen have no kitchen smoke (chimney) (Table 2 ).
Unmatched case control study with 96 cases and 193 controls was conducted to show the determinants of acute asthma attack among adult asthmatic patients visiting general hospitals of central zone, Tigray, Ethiopia.
Having URTI increases the occurrence of acute asthma attack 6.8 times [AOR = 6.835,95% CI = 3.285,14.222] than those who have not upper respiratory tract infection (URTI) (Table 3 ).
This is consistent with the studies conducted in Gondar, Uganda and Ireland [ 9 , 12 , 15 ].
The association might be due to the mechanism of airway inflammation,mucus hyper secretion, and bronchial hyper responsiveness [ 16 ]. In contrast to this study upper respiratory tract infections was no risk factor for acute asthma exacerbation on the study conduct in Pretoria and New Zealand [ 14 , 17 ]. This difference might be due to difference in health care seeking behavior of the participants in this study.
This study revealed that, sleep apnea was strongly associated with the occurrence of acute asthma exacerbation. Those who have sleep apnea are 9.5 times more likely to run in to acute asthma exacerbation than those who have not sleep apnea [AOR = 9.524, 95% CI = 3.563, 25.460].
This findings is comparable with a study done in Gondar and USA [ 12 , 18 ].
The possible reason is the fact that sleep apnea lead to the worsening of asthma control in patients with concomitant sleep apnea secondary to bronchoconstriction as a result of increase vagal tone while sleeping [ 19 ].
The result of this study shows that the odds of having acute asthma in Spring season was 2.2 times higher than the odds of having acute asthma attack in the autumn season [AOR = 2.204,95% CI = 1.011,4.805]. This is consistent with a study conducted in Canada in which spring season was triggering factor for asthma exacerbation [ 20 ]. Seasonal variation is the risk factors for acute asthma attack especially pollens appearing seasons like spring season exacerbates acute asthma attack. This may be due to the reason that during the spring, tree pollen, mold spores and grass have the power to inflame and narrow the air passages of people who have asthma [ 21 ].
The result of this study was different from a study conducted in Spain which was resulting winter season as higher risk of developing acute asthma attack [ 22 ]. The difference could be arisen from seasonal variation between the study areas, due to the influence of temperature and humidity.
In this study, Kitchen smoke (chimney) is highly associated with risk of acute asthma exacerbation.
Those who have no kitchen smoke in their kitchen were 2.3 times at risk to develop acute asthma exacerbation [AOR = 2.307,95%CI = 1.010,5.2725] than those who have kitchen smoke. This finding is comparable with the study conducted in India [ 13 ]. This is due to the fact that kitchen smoke (chimney) is a way that helps in removing the smokes and fumes from the kitchen and making it clean and smoke free which result in reduction of indoor air pollution and prevents acute asthma exacerbation [ 23 ]. Inhaling harmful smoke can inflame lungs and airway, causing them to swell and block oxygen. This can lead to acute asthma exacerbation [ 24 ]
In this study the determinant factors of acute asthma attack were spring season, presence of upper respiratory tract infection (URTI), having no Kitchen smoke in their kitchen and having obstructive sleep apnea.
Limitations
The diagnosis of respiratory tract infections and sleep apnea was empirical (without laboratory) and all measures used were based on self-reporting, this might end up with social desirability bias. This study may have recall bias, since some of the information was based on the recall of the study participants. Unavailability of studies on acute asthma exacerbation.
Availability of data and materials
The datasets used and analyzed during the current study are presented within the manuscript and available from the corresponding author on reasonable request.
Abbreviations
Adjusted Odds Ratio
Confidence Interval
Crude Odds Ratio
Central Statistical Agency
Interquartile Range
National Health Interview Survey
Out Patient Department
Tigray Region Health Development Agency
Upper Respiratory Tract Infection
Variance Inflation Factor
Adams, JY., Sutter, M.E. & Albertson, T.E. The Patient with Asthma in the Emergency Department. Clinic Rev Alleg Immunol 43, 14-29 (2012). https://doi.org/10.1007/s12016-011-8273-z .
Shah R , Saltoun CA . Chapter 14: Acute severe asthma (status asthmaticus). Allergy and Asthma Proceedings, 2012; 33(Supplement 1):S47-S50. Acute severe asthma. InAllergy and Asthma proceedings 2012 (Vol. 33, No. 3, p. 47). OceanSide Publications..
The Global Asthma Report 2018. Auckland, New Zealand: Global Asthma Network, 2018.
Adeloye D, Chan KY, Rudan I, Campbell H. An estimate of asthma prevalence in Africa: a systematic analysis. Croat Med J. 2013;54(6):519–31.
Article Google Scholar
Park HW, Tantisira KG. Genetic signatures of asthma exacerbation. Allergy, Asthma Immunol Res. 2017;9(3):191–9.
Article CAS Google Scholar
Stewart WF, Ricci JA, Chee E, Morganstein D. Lost productive work time costs from health conditions in the United States: results from the American Productivity Audit. J Occup Environ Med. 2003;45(12):1234–46.
CDC , National Health Interview Survey (NHIS) 2014.
Google Scholar
Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: Origin, effect and prevention. J Allergy Clin Immunol. 2011;128:1165–74.
Sanya RE, Kirenga BJ, Worodria W, Okot-Nwang M. Risk factors for asthma exacerbation in patients presenting to an emergency unit of a national referral hospital in Kampala, Uganda. Afr Health Sci. 2014;14(3):707–15.
Riley L, Gouda H, Cowan M. Noncommunicable Diseases Progress Monitor, 2017: World Health Organization; 2017.
Ethiopia steps report on risk factors for Chronic Non Communicable Diseases and prevalence of selected NCDs. Ethiopia public Health institute. 2016 . .
Belachew SA, Erku DA, Yimenu DK, Gebresillassie BM. Assessment of predictors for acute asthma attack in asthmatic patients visiting an Ethiopian hospital: are the potential factors still a threat? Asthma Res Pract. 2018;4(1):8.
Sharma GL, Choudhary GS. Assessment of Risk Factors for Acute Asthma Attack in Asthmatic Patients: A Hospital Based Study. Int Arch BioMed Clin Res. 2018;4(4):46–8.
Geyser M, Rheeder P. Risk factors precipitating exacerbations in adult asthma patients presenting at Kalafong Hospital, Pretoria. S Afr Fam Pract. 2008;50(4):67–e.
Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 2003;307(6910):982–6.
Fraenkel DJ, Bardin PG, Sanderson G, et al. Lower airways inflammation during rhinovirus colds in normal and in asthmatic subjects. Am J Respir Crit Care Med. 2009;151(3):879–86.
Kolbe J, Fergusson W, Vamos M, Garrett J. Case-control study of severe life-threatening asthma (SLTA) in adults. Thorax. 2002;57(4):317–22.
De-Lei K, Zheng Q, Hui S, Hong Y. Association of Obstructive Sleep Apnea with Asthma exacerbation; 2017.
Alkhalil M, Schulman E, Getsy J. Obstructive sleep apnea syndrome and asthma: what are the links? J Clin Sleep Med. 2009;5(01):71–8.
Tarlo S, Broder I, Corey P, et al. A case-control study of the role of cold symptoms and other historical triggering factors in asthma exacerbations. Can Respir J. 2000;7(1):42–8.
Surrena H, editor. Handbook for Brunner and Suddarth’s textbook of medical-surgical nursing. Lippincott Williams & Wilkins; 2010.
Pola-Bibian B, et al. Asthma exacerbations in a tertiary hospital: clinical features, triggers, and risk factors for hospitalization. J Investig Allergol Clin Immunol. 2016: 0 . https://doi.org/10.18176/jiaci.0128 .
Eisner M, et al. Exposure to indoor combustion and adult asthma outcomes: environmental tobacco smoke, gas stoves, and woodsmoke. Thorax. 2002;57(11):973–8.
Smeltzer SC, Bare BG, Hinkele JL, Cheever KH. Brunner and Suddath’s Text Book of Medical Surgical 2010. Wolters Kluwer Health:Lippincott Williams & Wilkins. Nursing, vol. 1. 12th ed. p. 622.
Download references
Acknowledgments
Authors thanks to public general hospitals of central zone Tigray, Ethiopia for their co-operation, to data collectors, supervisors, for the health staffs of the hospitals and to the study participants for their valuable information.
Not applicable.
Author information
Authors and affiliations.
Department of adult health nursing ,school of Nursing, Aksum University, Aksum, Ethiopia
Melaku Negash & Degena Bahrey Tadesse
Department of Psychiatric, Mekelle University, Mekelle, Ethiopia
Hagos Tsegabrhan
Adwa General Hospital, Adwa, Ethiopia
Teklit Meles
Department of midwifery, Aksum University, Aksum, Ethiopia
Gebreamlak Gidey
college of medicine and health science, Adigrat university, Adigrat, Ethiopia
Yemane Berhane
Maternity and reproductive health nursing, Mekelle University, Mekelle, Ethiopia
Kibrom Berhanu
Department of Emergency and critical care nursing, Mekelle University, Mekelle, Ethiopia
Tsgalem Haylemaryam
You can also search for this author in PubMed Google Scholar
Contributions
MN: was made substantially contributions to conceived and designed the study, analysis the data, methodology, data interpretation and wrote the final manuscript.TM, DB, GG,YB, had equally contributed to analysis and interpretation of the data. Whereas HT, TH and KB substantial contribution in reviewing overall the study in analysis, interpretation of data, have drafted the manuscript and substantively revised the work. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Melaku Negash .
Ethics declarations
Ethics approval and consent to participate.
Ethical clearance was obtained from Mekelle University College of health sciences institutional review board (IRB). Official supportive letters were obtained from Regional Health Bureau (TRHB) and central zone health office. Respondents were informed about the purpose of the study and the interview was conducted after receiving the written consent from participants. The right of participants to withdraw from the study at any time, without any precondition were secured and participants were informed. Confidentiality of the data/information was secured and was not used for other purposes. No personal identifiers was used on the questionnaire. To maintain confidentiality, data collector was recruited from the study unit.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1..
Annex I: English version structured interview questionnaire.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Negash, M., Tsegabrhan, H., Meles, T. et al. Determinants of Acute Asthma Attack among adult asthmatic patients visiting hospitals of Tigray, Ethiopia, 2019: case control study. asthma res and pract 6 , 1 (2020). https://doi.org/10.1186/s40733-020-00054-w
Download citation
Received : 07 December 2019
Accepted : 17 March 2020
Published : 03 April 2020
DOI : https://doi.org/10.1186/s40733-020-00054-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Acute asthma attack
- Determinants
Asthma Research and Practice
ISSN: 2054-7064
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 26 January 2024
The association between asthma and atrial fibrillation: systematic review and meta-analysis
- Beatriz Nogueira-Garcia 1 ,
- Mariana Alves 2 , 3 , 4 ,
- Fausto J. Pinto 1 , 5 &
- Daniel Caldeira 1 , 3 , 5 , 6
Scientific Reports volume 14 , Article number: 2241 ( 2024 ) Cite this article
1216 Accesses
Metrics details
- Medical research
- Risk factors
Respiratory disease and atrial fibrillation (AF) frequent coexist, but the risk of AF among asthma patients is less characterized. Growing evidence suggest that AF shares with asthma a systemic inflammation background and asthma treatments, such as beta agonists, have been associated with increased risk of cardiac arrhythmias. The aim of this systematic review was to assess the risk of AF in patients with asthma in observational studies. We search for longitudinal studies reporting AF outcome in asthma and control patients through MEDLINE, Cochrane Central Register of Controlled Trials and EMBASE. Pooled estimates of odds ratios (ORs) and 95% confidence intervals (CIs) were derived by random effects meta-analysis. Heterogeneity was assessed using the I2 test. The risk of bias of individual studies was evaluated using the ROBINS-E tool. The study protocol was registered at PROSPERO: CRD42020215707. Seven cohort/nested case–control studies with 1 405 508 individuals were included. The mean follow-up time was 9 years, ranging from 1 to 15 years. Asthma was associated with a higher risk of AF (OR 1.15. 95% CI 1.01–1.29). High heterogeneity (I 2 = 81%) and overall “serious” risk of bias, lead to a very low confidence in in this result. Asthma was associated with an increased risk of AF. However, the high risk of bias and high heterogeneity reduces the robustness of these results, calling for further high-quality data.
Similar content being viewed by others

Long COVID: major findings, mechanisms and recommendations
Hannah E. Davis, Lisa McCorkell, … Eric J. Topol

Key recommendations for primary care from the 2022 Global Initiative for Asthma (GINA) update
Mark L. Levy, Leonard B. Bacharier, … Helen K. Reddel

Cardiovascular disease and cancer: shared risk factors and mechanisms
Nicholas S. Wilcox, Uri Amit, … Bonnie Ky
Introduction
Atrial fibrillation (AF) is a supraventricular arrhythmia characterized by uncoordinated atrial electrical activation. It is the most common sustained arrhythmia worldwide and is associated with significant patients’ mortality and impacts quality of life 1 , 2 . However, AF can be silent, and the first clinical manifestation may be a stroke if this arrhythmia is not diagnosed before 3 , 4 . Therefore, it is important to recognize which are the clusters of patients that are more prone to develop AF to plan screening strategies.
Respiratory diseases and AF frequently coexist but the relationship with asthma is not well characterized 5 , 6 . Proposed mechanisms include an inflammatory pathway, particularly in obstructive sleep apnea which is one of the respiratory diseases most commonly associated with AF. Other respiratory diseases such as asthma are starting to show an association with AF 7 . It is now recognized that pathophysiology of AF is extremely heterogeneous 8 , 9 , sharing with asthma an inflammatory pathway. Systematic inflammation may conduct to atrial electrophysiology and structural remodelling, leading to increase vulnerability to AF 10 . Leukotrienes—inflammatory mediators produced in leukocytes—have systematic effects and their receptors are highly expressed in the heart 11 , 12 . Furthermore, asthma treatments, such as beta-2 adrenergic agonists, and airway obstruction with hypoxemia have been associated with increased risk of cardiac arrhythmias 13 , 14 , but some doubts still exist regarding this link.
The aim of the present systematic review was to evaluate the available evidence regarding the risk of atrial fibrillation in patients with asthma.
We performed a systematic review and meta-analysis following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) 15 and “The Meta-analysis of Observational Studies in Epidemiology” 16 recommendations. The study protocol was registered with PROSPERO (CRD42020215707).
Data sources and search strategy
Potentially eligible studies were identified through an electronic search of bibliographic databases from inception to December 2020 (MEDLINE through PubMed, Cochrane Central Register of Controlled Trials and EMBASE). The search methods used are summarized in Supplementary Table 1 . Additionally, we searched for relevant data by checking the reference lists of included studies. No dates or language restrictions were applied.
Eligibility criteria
For the purposes of our systematic review all longitudinal (prospective or retrospective) studies reporting atrial fibrillation outcome in both patients with asthma—defined by clinical signs/symptoms and/or functional tests, administrative codes or as defined by the physician/investigator—and matched controls were considered eligible.
Study selection and data extraction
Two authors (BG and MA) independently screened the title and abstracts of the citations retrieved in the electronic database search 17 . The full-text reports of all potentially relevant studies were obtained and the authors independently selected studies to be included in the review according to the predefined inclusion criteria. Doubts and disagreements were resolved by consensus. Reasons for the exclusion of articles were recorded.
Whenever available, data extracted included: study design, location, period of study, patient and control population characteristics, outcomes of interest and the adjustments of estimates.
Atrial fibrillation was the primary outcome. It was defined as a supraventricular tachyarrhythmia with uncoordinated atrial electrical activation and consequently ineffective atrial contraction, with abnormal ECG activity (absence of P waves and irregular R–R intervals) 18 . Diagnosis made by the patient’s physician or corresponding administrative code were also acceptable for the definition of the patient’s condition.

Study-level risk of bias and meta-biases
Each study was evaluated independently by two authors (BG and MA) in each of the domains of bias contained in the ROBINS-E tool, accordingly to the algorithm 19 . Then, the overall risk of bias judgement was performed. Publication bias was evaluated through funnel plot evaluation and Egger test.
Assessment of confidence in the cumulative evidence
The evaluation of primary outcomes was performed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework regarding the study design, study quality, consistency, and directness 20 . The pooled evidence was then classified as having very low, low, moderate, or high confidence.
Data synthesis
We used STATA 17.0 to derive forest plots and to perform pooled analysis and related tests. Random-effects meta-analysis was performed with the DerSimonian-Laird model to estimate pooled odds ratio (OR) and 95% confidence intervals (95% CIs). Heterogeneity was defined as p value < 0,10 in the Chi-square test and the magnitude was reported through the I 2 metric and was considered substantial if I 2 > 50% 21 . Meta-regression was performed to evaluate the impact of age and follow-up.
Included studies
The search of the electronic databases yielded 292 studies after removal of duplicates. From those, 266 were excludes after titles and abstracts screening and full-text assessment was performed for 26 articles. Following our inclusion and exclusion criteria, we were able to include 7 studies for analysis (Fig. 1 ). The main reasons for excluding studies were lack of control group and no assessment of the outcome of interest.
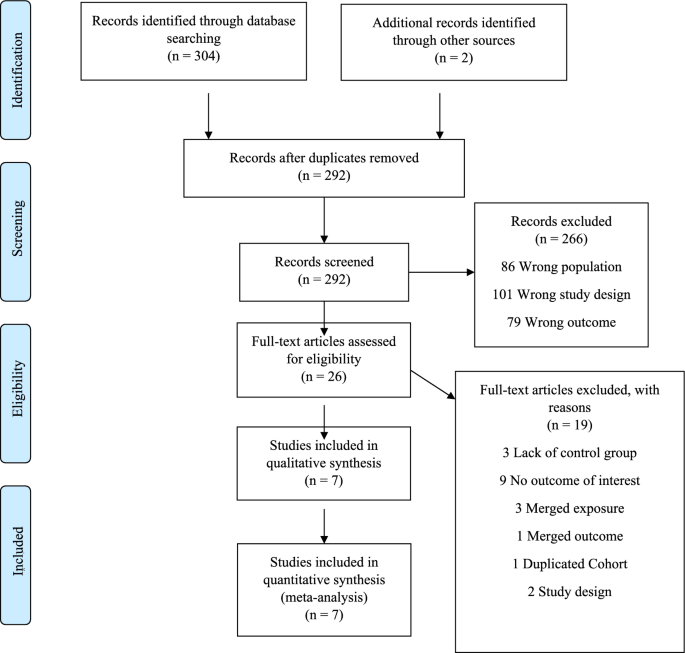
Flow diagram showing the study selection process.
Description of the studies
All studies had a prospective/retrospective cohort design 7 , 22 , 23 , 24 , 25 , 26 , 27 . Four studies were conducted in Europe, two in America and one in Asia. The mean follow-up time was 9 years, ranging from 1 to 15 years. Overall, 1,405,508 patients were included. Table 1 shows the main study characteristics.
Many studies did not report previous cardiovascular risk factors of the participants included (Supplementary Table 2 ). Asthma diagnosis methods were essentially based on codification systems and patient report. Almost all did not mention asthma severity and the type of medications for asthma control (Supplementary Table 3 ). Outcome adjustment for cardiovascular risk factors was present in only few studies (Supplementary Table 4 ).
Risk of bias
The risk of bias is serious mainly due to significant bias in the measurement outcome domain (Table 2 ). Diagnosis method of asthma vary from self-reported to detection through codification systems. In terms of AF diagnostic, it was also verified an important heterogeneity, being often based on codification systems but in some studies an ECG or Holter monitoring was required. Bias in selection of participants was classified as serious in two studies—Carter et al. 22 and Marín-Pérez et al. 28 —since the participants are part of a hospitalized and heart failure population, respectively, which can influence the risk and enhance AF diagnosis. On the other hand, in these two studies the risk in measurement outcome was accepted as being low once this kind of populations usually performed ECG regularly.
Outcome: risk of atrial fibrillation
In our meta-analysis asthma was associated with a higher risk of AF (OR 1.15. 95% CI 1.01–1.29) (Fig. 2 ). The analysis showed very high statistical heterogeneity (I 2 = 81%). A sensitivity analysis evaluating the result of the meta-analysis with exclusion of each single study was performed and the outcome remain consistent regarding the direction and magnitude of the effect, however the significance was lost with the exclusion of the studies Cepelis et al., Chan et al. and Martin-Pérez et al. which can be considered a robustness indicator (Fig. 3 ). Subgroup analysis according to the evaluation of risk of bias was also performed and the results between low and moderate or serious risk of bias regarding different domains, such as selection bias, classification of interventions and measurement outcome, were quite consistent (Fig. 4 ).
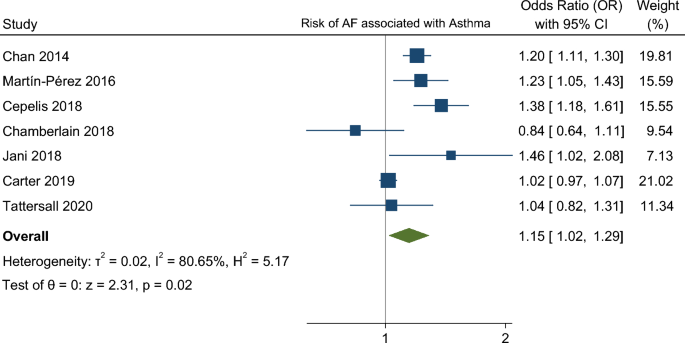
Forest plot for risk of atrial fibrillation associated with asthma.
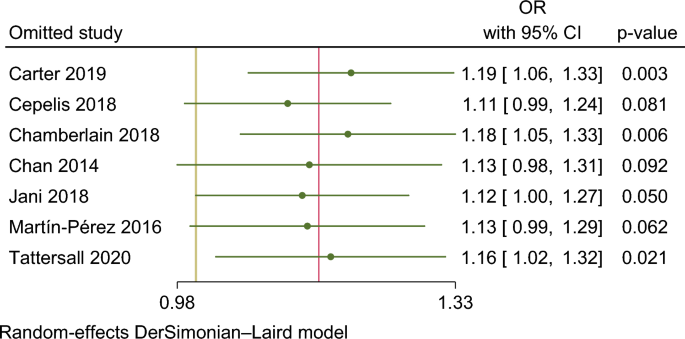
Sensitivity analysis evaluating the result of the meta-analysis with exclusion of each single study (leave-one-out analysis).
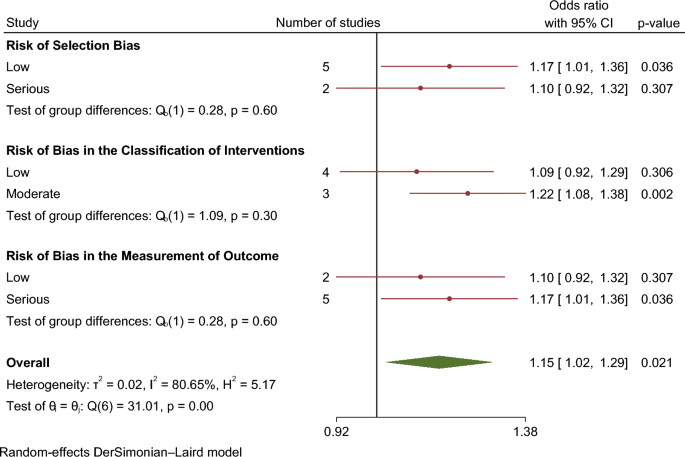
Results of subgroup analysis according to the evaluation of risk of bias.
Meta regression results analyzing the effect of age and follow in the results did not find any significant estimates ( p = 0.326 and p = 0.587, respectively) (Supplementary Fig. 1 ). Only few studies reported data according to asthma severity and asthma treatment.
The evaluation of the funnel plot and Egger regression test ( p = 0.89) result did suggest the existence of publication bias (Supplementary Fig. 2 ).
Assessment of confidence in cumulative evidence
Applying GRADE criteria, the confidence in the evidence is very low, being the downgrading reasons mainly due to risk of bias and imprecision—Supplementary Table 5 .
Our analysis showed that asthma was significantly associated with a higher risk of AF, however with very low certainty according to GRADE criteria.
It is now recognized that pathophysiology of AF is complex and probably in association with systemic disease 9 , 29 . The initiation and maintenance of this supraventricular arrhythmia can be the result of the interaction between a trigger and the substrate, induced by an electrical and structural remodeling 8 . Emerging evidence suggest mechanisms that goes beyond the atrium, sharing with asthma an inflammatory pathway. Inflammation represents a trigger of AF and is also implicated in its perpetuation 10 . Different inflammatory cytokines mediate function of ion channels and atrial remodelling 30 , 31 . Important mediators of inflammation such as C reactive protein and interleukin-6, have been found to be high in patients with AF, and even their influence on the success of the AF ablation have been shown 32 , 33 .
Furthermore, asthma treatments, such as beta 2-agonists and corticosteroid therapy, in different ways can modulate the risk of cardiac arrythmias 34 . The beta 2-adrenergic receptor agonists, such as salbutamol or formoterol, have a positive chronotropic effect and also decrease the atrioventricular nodal, atrial, and ventricular refractoriness which can enhance the risk of both supraventricular and ventricular arrhythmias 35 . In the other hand, corticosteroids were found to be beneficial for the prevention of atrial fibrillation occurrence in patients undergoing cardiac surgery and AF ablation procedures, probably due to its anti-inflammatory activity 36 , 37 .
There is suggestion that the severity of asthma as a surrogate of inflammation and beta2 adrenergic agonists use can influence the incidence of AF. However, most of the studies included in our analysis did not report patients’ asthma severity or medication used. In the few studies reporting such data there was a higher risk for AF in participants with uncontrolled asthma (HR 1.74 [95%CI 1.26–2.42]) (Cepelis et al. 25 study); persistent asthma, defined as asthmatics requiring controller medications, but not intermittent asthma was associated with a 1.5-fold increase in risk of AF in Tattersall et al. 7 ; in Chan et al. 24 bronchodilator therapy—corticosteroid and non-corticosteroid—was associated with an increased risk for AF (OR 2.13; 95% CI 1.226–3.701, p = 0.007 and OR 2.849; 95% CI 2.48–3.273, P < 0.001, respectively). The association between AF and the use of inhaled corticosteroids is still unclear and a possible explanation for these results is the relationship between these drugs use and asthma severity.
The strength of association between asthma and AF might be weaker due to the statistical and clinical heterogeneity among the included studies as well as the risk of bias which preclude robust conclusion. More evidence is needed to establish robustly this relationship, which can be of tremendous value concerning identification of individuals at higher risk in the community and, therefore, improving prevention and screening programmes for early AF detection and stroke prevention. These studies should be able to provide further evidence about the magnitude of the relationship between asthma and drugs used in the AF outcome.
Limitations
Our systematic review with meta-analysis has some limitations. The diagnosis of asthma was heterogenous in the included studies, varying from self-reported and codification systems and atrial fibrillation assessment was also variable (ECG or Holter monitoring and mainly based on codification system and patients’ reports) which represents a potential source of bias. The period of follow-up ranged from 1 to 15 years (mean follow-up time 7 years), which can influence the main outcome. One of the major drawbacks of most of the studies as well of this systematic review is that most of risk factors for AF were not adequately characterized which underpowers further exploratory analyses that were not performed due to lack of data (Supplementary Table 2 ).
The low confidence in the generated evidence was recognized by the GRADE assessment where the risk of bias due to confounding and risk the of bias due to the assessment and measurement of outcomes have an important role, and should be acknowledged in the planning of future studies.
Nevertheless, most of the outcomes were adjusted at least to some of these potential confounders, such as smoking, alcohol consumption, diabetes, lipid profile, arterial hypertension, obesity, and other cardiovascular diseases (Supplementary Table 4 ).
The risk of AF associated with asthma patients was significantly increased. However, more evidence is necessary to support these association and clarify etiologic mechanisms. Identifying individuals at higher risk of developing AF could facilitate screening programmes and earlier diagnose. Based on our data it is wise to recommend special attention in clinical evaluation of asthma patients, including a systematic revision of symptoms like palpitations, dyspnea and fatigue, as well as opportunistic evaluation of the pulse regularity, to prompt ECG recording (e.g. 12-lead ECG or Holter monitoring).
Data availability
The datasets generated and/or analysed during the current study are public. However, any data can be provided from the authors upon reasonable request by contacting Prof. Daniel Caldeira ([email protected]), the corresponding author.
Hindricks, G. et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 42 , 373–498 (2020).
Article Google Scholar
Thrall, G., Lane, D., Carroll, D. & Lip, G. Y. H. Quality of life in patients with atrial fibrillation: A systematic review. Am. J. Med. 119 , 448-e1 (2006).
Article PubMed Google Scholar
Sgreccia, D. et al. Comparing outcomes in asymptomatic and symptomatic atrial fibrillation: A systematic review and meta-analysis of 81,462 patients. J. Clin. Med. 10 , 3979 (2021).
Article PubMed PubMed Central Google Scholar
Boriani, G. et al. Asymptomatic atrial fibrillation: Clinical correlates, management, and outcomes in the EORP-AF pilot general registry. Am. J. Med. 128 (5), 509-518.e2 (2015).
Romiti, G. F. et al. Prevalence, management and impact of chronic obstructive pulmonary disease in atrial fibrillation: A systematic review and meta-analysis of 4,200,000 patients. Eur. Heart J. 42 , 3555–3557 (2021).
Huang, Q. et al. Risk factors for new-onset atrial fibrillation in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. PeerJ 2 , 8 (2020).
Google Scholar
Tattersall, M. C. et al. Persistent asthma is associated with increased risk for incident atrial fibrillation in the MESA. Circ. Arrhythm. Electrophysiol. 13 , 121–130 (2020).
Wijesurendra, R. S. & Casadei, B. Mechanisms of atrial fibrillation. Heart 105 (24), 1860–1867 (2019).
Article CAS PubMed Google Scholar
Wijesurendra, R. S. & Casadei, B. Atrial fibrillation: Effects beyond the atrium?. Cardiovasc. Res. 105 (3), 238–247 (2015).
Article CAS PubMed PubMed Central Google Scholar
Hu, Y. F., Chen, Y. J., Lin, Y. J. & Chen, S. A. Inflammation and the pathogenesis of atrial fibrillation. Nat. Rev. Cardiol. [Internet] 12 (4), 230–243 (2015).
Sanak, M. et al. Pharmacological inhibition of leukotriene biosynthesis: Effects on the heart conductance. J. Physiol. Pharmacol. 61 (1), 53–58 (2010).
CAS PubMed Google Scholar
Sokołowska, B. et al. Influence of leukotriene biosynthesis inhibition on heart rate in patients with atrial fibrillation. Int. J. Cardiol. 145 (3), 625–626 (2010).
Salpeter, S. R., Ormiston, T. M. & Salpeter, E. E. Cardiovascular effects of β-agonists in patients with asthma and COPD: A meta-analysis. Chest [Internet] 125 (6), 2309–2321 (2004).
Hyunjae, K. et al. The relationship between chronic atrial fibrillation and reduced pulmonary function in cases of preserved left ventricular systolic function. Korean Circ. J. 39 (9), 372–377 (2009).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 15 , 339 (2009).
Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting—Meta-analysis of observational studies in epidemiology (MOOSE) Group B. JAMA Neurol. 283 , 2008–2012 (2000).
CAS Google Scholar
Ouzzani, M., Hammady, H., Fedorowicz, Z. & Elmagarmid, A. Rayyan: A web and mobile app for systematic reviews. Syst. Rev. 5 (1), 210 (2016).
Hindricks, G. et al. ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020 , 1–126 (2020).
Sterne, J. A. et al. The Risk of bias in non-randomized studies-of Interventions (ROBINS-I) assessment tool ROBINS-I tool (Stage I): At protocol stage. BMJ 355 , 1–22 (2016).
Guyatt, G. H. et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J. Clin. Epidemiol. 64 (4), 407–415 (2011).
Higgins, J. P. T. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21 (11), 1539–1558 (2002).
Carter, P. et al. Association of cardiovascular disease with respiratory disease. J. Am. Coll. Cardiol. 73 (17), 2166–2177 (2019).
Chamberlain, A. M. et al. Multimorbidity and the risk of hospitalization and death in atrial fibrillation: A population-based study. Am. Heart J. 1 (185), 74–84 (2017).
Chan, W. L. et al. The association of asthma and atrial fibrillation: A nationwide population-based nested case-control study. Int. J. Cardiol. 176 (2), 464–469 (2014).
Cepelis, A. et al. Associations of asthma and asthma control with atrial fibrillation risk results from the Nord-Trøndelag health study (HUNT). JAMA Cardiol. 3 (8), 721–728 (2018).
Yang, K. P., Chao, T. F., Huang, C. C. & Leu, H. B. Asthma and atrial fibrillation: A nationwide population-based study. In: Heart Rhythm S186–S215 (Elsevier, 2011).
Jani, B. D. et al. Multimorbidity and co-morbidity in atrial fibrillation and effects on survival: Findings from UK Biobank cohort. Europace 20 (FI3), f329–f336 (2018).
Martin-Perez, M., Ruigomez, A., Michel, A. & Garcia Rodriguez, L. A. Incidence and risk factors for atrial fibrillation in patients with newly diagnosed heart failure. J. Cardiovasc. Med. 17 (8), 608–615 (2016).
Article CAS Google Scholar
Ball, J., Carrington, M. J., McMurray, J. J. V. & Stewart, S. Atrial fibrillation: Profile and burden of an evolving epidemic in the 21st century. Int. J. Cardiol. 167 , 1807–1824 (2013).
Liew, R. et al. Role of tumor necrosis factor-α in the pathogenesis of atrial fibrosis and development of an arrhythmogenic substrate. Circ. J. 77 (5), 1171–1179 (2013).
Wakili, R., Voigt, N., Kääb, S., Dobrev, D. & Nattel, S. Recent advances in the molecular pathophysiology of atrial fibrillation. J. Clin. Investig. 121 , 2955–2968 (2011).
Jiang, H., Wang, W., Wang, C., Xie, X. & Hou, Y. Associationofpre-ablation levelofpotential blood markers with atrial fibrillation recurrence after catheter ablation: A meta-analysis. Europace 19 (3), 392–400 (2017).
PubMed Google Scholar
da Silva, R. M. F. L. Influence of inflammation and atherosclerosis in atrial fibrillation. Curr. Atheroscler. Rep. 19 , 1 (2017).
Warnier, M. J. et al. Cardiac arrhythmias in adult patients with asthma. J. Asthma 49 (9), 942–946 (2012).
Kallergis, E. M. et al. Acute electrophysiologic effects of inhaled salbutamol in humans. Chest 127 (6), 2057–2063 (2005).
Liu, L. et al. Effects of corticosteroids on new-onset atrial fibrillation after cardiac surgery A meta-analysis of randomized controlled trials. Medicine https://doi.org/10.1097/MD.0000000000025130 (2021).
de Vecchis, R. Promising effects of moderate-dose corticosteroid therapy in the blanking period for prevention of atrial fibrillation (AF) recurrences in patients undergoing AF ablation. Eur. J. Clin. Pharmacol. 75 , 1179–80 (2019).
Download references
Acknowledgements
This work was supported by national funds, Fundação para a Ciência e a Tecnologia, reference number UIDB/00306/2020.
Author information
Authors and affiliations.
Serviço de Cardiologia, Hospital Universitário de Santa Maria – CHULN, Lisbon, Portugal
Beatriz Nogueira-Garcia, Fausto J. Pinto & Daniel Caldeira
Serviço de Medicina III, Hospital Pulido Valente, CHLN, Lisbon, Portugal
Mariana Alves
Laboratório de Farmacologia Clínica e Terapêutica, Faculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal
Mariana Alves & Daniel Caldeira
Instituto de Medicina Molecular, Faculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal
Centro Cardiovascular da Universidade de Lisboa – CCUL (CCUL@RISE), CAML, Faculdade de Medicina, Universidade de Lisboa, Av. Prof. Egas Moniz, 1649-028, Lisbon, Portugal
Fausto J. Pinto & Daniel Caldeira
Centro de Estudos de Medicina Baseada na Evidência (CEMBE), Faculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal
Daniel Caldeira
You can also search for this author in PubMed Google Scholar
Contributions
B.G. and M.A. wrote the main manuscript. M.A. and D.C. performed statistical analysis and prepare figures. D.C. and F.J.P. contributed to manuscript discussion. All authors reviewed the manuscript.
Corresponding author
Correspondence to Daniel Caldeira .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information 1., supplementary information 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Nogueira-Garcia, B., Alves, M., Pinto, F.J. et al. The association between asthma and atrial fibrillation: systematic review and meta-analysis. Sci Rep 14 , 2241 (2024). https://doi.org/10.1038/s41598-023-50466-w
Download citation
Received : 02 January 2023
Accepted : 20 December 2023
Published : 26 January 2024
DOI : https://doi.org/10.1038/s41598-023-50466-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Open access
- Published: 18 April 2024
A survey of severe asthma in Canada: results from the CASCADE practice reflective program
- Krystelle Godbout 1 ,
- Harold Kim 2 , 3 ,
- Irvin Mayers 4 ,
- James Paterson 5 &
- Charles K. N. Chan ORCID: orcid.org/0000-0002-7772-706X 6
Allergy, Asthma & Clinical Immunology volume 20 , Article number: 31 ( 2024 ) Cite this article
118 Accesses
1 Altmetric
Metrics details
Since the last guidance was published by the Canadian Thoracic Society, there have been several advances in the clinical management of severe asthma. To gain a better understanding of the current standards of care and treatment patterns of patients, the CASCADE practice reflective program was established to conduct a real-world analysis of severe asthma management among specialists in Canada with a goal of identifying areas of opportunity to enhance patient management and outcomes.
The CASCADE program was a two-part practice reflective and assessment program delivered through an on-line portal for selected specialists (Respirologists and Allergists) in Canada. The program consisted of a one-time overview survey of physician practice to establish overall practice parameters, followed by a review of at least 5 severe asthma patients to establish the current landscape of severe asthma management.
The program collected practice overview surveys from 78 specialists (52 Respirologists, 24 Allergists, and 2 General practice physicians with an interest in respiratory disease) in 8 provinces. Practices included a variety of types in both large metropolitan centres and smaller regional settings. There were 503 patients reviewed and included in the program. Most (65%) patients were currently using a biologic treatment, 30% were biologic naive, and 5% had used a biologic treatment in the past. Most patients (53%) were reported to have mixed allergic and eosinophilic phenotypes, despite a perception that allergic, eosinophilic and mixed phenotypes were evenly balanced in the physician practice. Overall, patients currently treated with biologic agents had parameters suggesting higher control and were more satisfied with treatment. However, there was less than optimal treatment satisfaction for more than half of all patients, particularly for those patients not treated with a biologic agent.
Conclusions
Phenotyping is hampered by poor availability for several assessments, and the full range of treatments are not currently fully utilized, partly due to physician familiarity with the agents and partly due to prescribing restrictions. Even when treated with biologic agents, patient satisfaction can still be improved.
Asthma is estimated to affect ~ 11% of the Canadian population, or nearly 250,000 Canadians [ 1 ] with long-standing estimates of ~ 5–10% of asthma patients with severe disease. It is a complex, heterogenous, and dynamic disease that is classified by treatment measures necessary for management: Severe asthma is defined by the Canadian Thoracic Society (and GINA) as requiring a high-dose inhaled corticosteroid and a second controller (or oral steroids) for six months or longer to maintain symptom control (or who do not achieve control with treatment) [ 2 , 3 ]. In patients who remain uncontrolled despite optimized inhaled therapies, eligible patients may be considered for treatment escalation to biologic therapy. While clinical trials support the efficacy and safety profile of marketed biologics in Canada, real-world clinical experience is variable owing to the complexity and heterogeneity of disease and limitations related to the different clinical practice environments.
Since the publication of the last guidance document by the Canadian Thoracic Society in 2017 for the management of severe asthma, there have been major changes in the available treatment options, including the addition of a number of biologic agents, most often targeting individual downstream pathways or end products of type 2 inflammation [ 4 ] but also non-type 2 inflammatory pathways [ 5 ]. While severe asthma patients are typically managed primarily by primary care providers, those providers may not be qualified or comfortable to prescribe advanced therapies, so advanced management of patients with severe asthma frequently falls to specialists, despite the often slow referral of those patients to specialist care [ 6 ]. Effective management of asthma, including appropriate use of pharmaceutical agents, is an important consideration in the reduction of negative outcomes associated with inadequate asthma management. To improve the management of patients with severe asthma, identification of factors that hinder the timely use of advanced therapies in specialist care could aid in the development of practices to overcome these barriers.
To better understand the current standards of care and treatment patterns in the context of the growing complexity of severe asthma and expanding therapeutic options, we sought to evaluate current management practices in severe asthma through a practice reflective exercise conducted with specialist physicians treating severe asthma. We conducted an analysis of real-world severe asthma management among practicing specialists (Allergists and Respirologists) across Canada to gain insights into current management practices of physicians treating severe asthma. The overall purpose was to provide a current snapshot of management practices with the goal of identifying areas of opportunity to enhance optimal patient management and outcomes.
The Canadian Asthma Specialists Collection and Discussion of Patient Experience (CASCADE) program was developed by a faculty consisting of Allergists and Respirologists from across Canada, with extensive experience in severe asthma. Program development was supported by the program sponsor and facilitated by an independent agency responsible for implementing the program. CASCADE was reviewed and accredited by an independent central research ethics board. The program was conducted from January to May 2023, available to physicians across Canada. The program was open to Canadian Allergists, Pneumologists/Respirologists or general practice physicians with a high proportion of patients with severe asthma in their clinical practice and who are authorized to prescribe biologic medications. Physicians were invited to participate after they were identified through routine interactions with representatives of the program sponsor.
The program consisted of two separate, physician completed surveys administered through a custom online portal. The first survey to be completed, consisting of 18 questions (see Additional file 1 : Appendix 1), assessed the physician demographics (practice size, number of severe asthma patients, specialization, etc.) as well as their perceptions of their current practice (number of patients, distribution of phenotypes, percentage poorly controlled, etc.) and their general management of patients with severe asthma. The second survey was completed multiple times: once per severe asthma patient reviewed in the program that the physician had recently assessed in their clinical practice. Participating physicians were asked to review at least 5 patients using a survey designed to elicit their current choices for patient management and to provide a comparison to their overall perceptions of their own practice.
The patients that participating physicians were asked to assess were those who were aged 12 years or more, were diagnosed with severe asthma, had received high dose inhaled corticosteroid and at least one other maintenance medication. To try to reflect clinical practice, there were no specific exclusions for patients beyond age and diagnosis, however, physicians were encouraged to include patients that they had recently had a clinical visit with to reflect their most recent practice habits. All patient data was collected anonymously.
The overall goal of the program was to provide physicians with insights into their own practice, comparing their perceptions with their current, day-to-day practice habits, as well as allowing a comparison to the practice habits of their peers after aggregation of survey data through the program. To achieve this, the program was designed with a plan to aggregate all patient and physician surveys nationally, as well as aggregating the data provided for the patients collected by each individual physician. Summary statistics for each of the responses for each of the questions were prepared, and qualitative comparisons between individual physicians and national data were carried out, as well as between their perceptions and the patient-to-patient reality of their own practices.
Program delivery was facilitated using an independent organization with funding from the study sponsor. All editorial and data control resided with the steering committee. Ethical review and approval of the program was obtained from Center for IRB Intelligence/Advarra.
Overall, 503 patients were reviewed by physicians at 69 sites across Canada for the CASCADE program. A total of 78 physicians submitted the practice profile overview survey (9 physicians submitted the practice overview but did not review any patients for the program). Response rate for the program was high: 99 physicians were invited to participate, and 80% provided data for the program.
No specific strategy was employed for the selection of physicians to participate beyond ensuring representation from across Canada. In Canada, only specialists, specifically qualified internists or specifically qualified primary care physicians are authorized to prescribe biologics for asthma; all participating physicians were authorized to prescribed biologics. The majority (67%) of physicians were respirologists, with 2.6% of physicians reporting “other” specializations (internal medicine / general practice). The remaining (31%) reported allergy as a specialization; the ratio of respirologists to allergists in the program is similar to the national ratio. Physicians represented a broad distribution of experience and practice types from both larger metropolitan centres as well as smaller, regional urban centres, representing both academic and community practices. Physicians from all regions in Canada (Atlantic Provinces, Quebec, Ontario, Prairies, and the West), with the exception of the three territories, participated in the program. The program was available in both English (87% of physicians) and French (13% of physicians). Physician demographics are summarized in Table 1 .
A total of 69 physicians reviewed at least one patient case that was included in the program. Of those, physicians reviewed an average of 7.3 patient cases (median 6) for the program. Patients included in the program had a median age of 55 years and physicians reported a median age of diagnosis of severe asthma of 35 years (Table 2 ). Comparison of patient age and patient age at diagnosis showed that nearly 1/3 (32%) of patients were diagnosed with severe asthma over 20 years prior, with nearly one in four (24%) reporting diagnosis in the last 2–5 years. Most patients (60%) were female.
Asthma control
Physicians estimated that, on average, 31% of severe asthma patients in their practices were not controlled, with half of the physicians estimating that only ~ 25% or fewer of their patients are not well-controlled. Overall control was not reviewed in the program for the individual patient, however, a number of Canadian Thoracic Society control criteria were reviewed.
About 47% of patients reported 2 or more exacerbations requiring oral corticosteroids in the past 12 months and 14% of patients required hospitalization due to exacerbation in the past 12 months (Table 3 ). Overall, half (50%) of assessed patients experienced either 2 or more exacerbations or hospitalization in the past year (Table 3 ). The Asthma Control Questionnaire (ACQ) results were available for 40% of patients. In those patients with an available result, the mean score was 2.0, with 58% reporting a value of 1.5 or larger, the level considered to indicate poor asthma control [ 7 , 8 ]. A further 19% of patients had results between 0.75 and 1.5, considered to be questionable control. While only 6% of patients had a sputum eosinophil test reported, 63% of those had sputum eosinophils above 3% and 38% with levels of 10% or greater. Taken together, 58% of patients reviewed in this program had at least one indicator (exacerbations, hospitalization, ACQ, sputum eosinophils) of poor asthma control.
Other disease assessments
Despite the central importance of spirometry testing for diagnosis of asthma, FEV 1 was not available for all patients reviewed. It was reported for 92% of all patients (79% in allergist practices, 98% in respirology practices). Median FEV 1 was reported to be 72.9% of predicted, with a median volume of 2.1 L (Fig. 1 , Table 3 ). Skin prick test results were also not completed for all patients, with results available for 97% of patients reviewed in allergist practices, compared to only 61% in respirology practices (72% total). In patients with a skin prick test, 82% were reported as positive. The specific panel for skin prick testing that was used was not asked, however, it is likely that most include both seasonal and perennial allergens. Blood eosinophil count was available for 96% of patents reviewed, with a mean of 436 cells/uL. More than three quarters (76%) of patients with an available result had an eosinophil count of 150 cells/uL or more and 56% had greater than 300 cells/µL (Fig. 2 , Table 3 ). FeNO testing was available for 20% of the patient assessments, with 63% of available results being 20 parts per billion or greater.
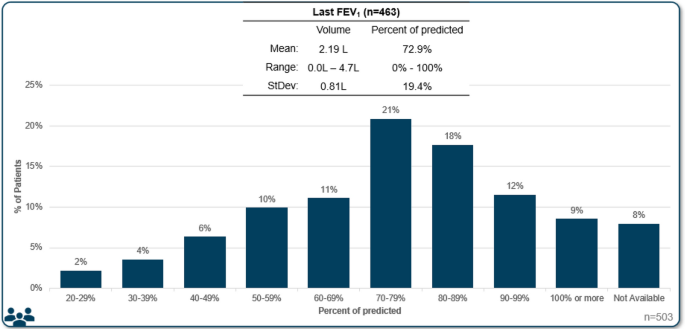
FEV 1 results
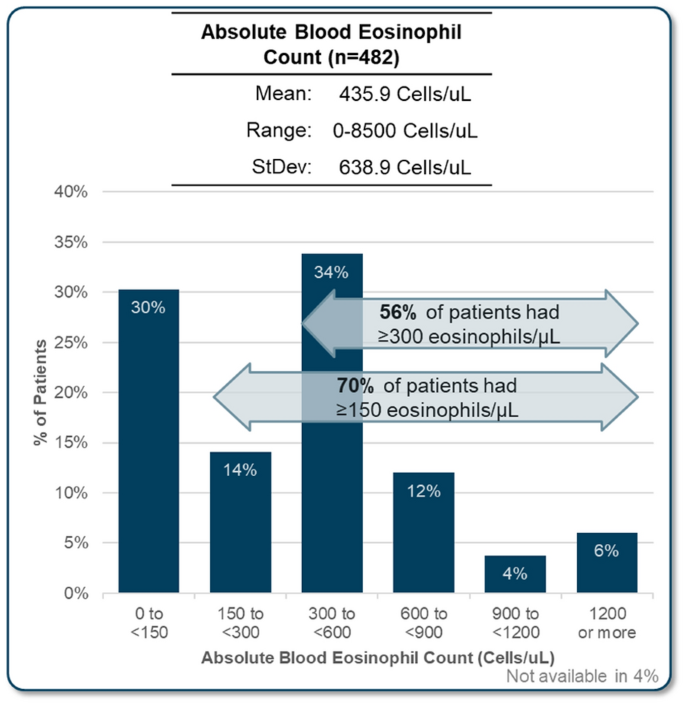
Most recent absolute blood eosinophil count for patients reviewed in the program
Biologic use
Nearly two thirds (65%) of patients assessed in the program were currently using a biologic; a further 5% had used a biologic in the past but were not currently using one (Table 3 ). The remaining 30% of patients had never used a biologic, a proportion similar to the physician’s reported perception of overall biologic use in their practice. Most (64%) physicians estimated that more than 61% of their patients were treated with biologic.
Control is impacted by biologic use. In patients that were currently using biologics, 54% of patients had at least one criterion suggesting poor asthma control (exacerbations, hospitalization, sputum eosinophils, or ACQ result), compared to 73% of patients who used biologics in the past and 66% of patients that never used biologics.
For those patients that had never used a biologic, eligibility was the most frequently cited factor for not using a biologic therapy (in 43% of patients). Other factors included patient reluctance in 18% of cases and 18% of reviews mentioned recent diagnosis or referral. Financial cost or reimbursement was a minor factor (7%). Physician perception was that reimbursement was the major barrier to biologic use (56% of physicians), with eligibility or patient not being a candidate cited as the major barrier by 23% of physicians. Patient reluctance was perceived by 19% of physicians as the major barrier. In the province of Quebec, which has a different reimbursement and eligibility framework, physicians perceived that eligibility or candidacy for biologic was the major barrier (57% of physicians) with patient reluctance and reimbursement cited by 21% of physicians as the major barrier. Review of Quebec patients not currently using a biologic revealed that eligibility was the primary barrier in 65% of patients, patient reluctance in 13% and other factors in 22%.
Disease phenotype
Physicians were asked to estimate the proportion of each asthma phenotype (mixed, eosinophilic, allergic, non-type 2, or other) in the severe asthma patients in their practice and were then asked the phenotype for each patient they included in the program. Definitions of each phenotype were not defined; physicians provided responses based on the definitions they apply to their own practice as this also reflects their clinical decision making. Overall, the estimated proportion of patients with each phenotype in the practice (Fig. 3 a) did not match with the phenotype of the patients reviewed in the program (Fig. 3 b, Table 3 ). Physicians perceived that a majority (33%) were eosinophilic, followed by allergic (26%), mixed (26%) and T2 low (18%) in their overall practice. Contrary to physician perception, review of the patients selected for the program revealed the mixed phenotype was actually the most predominant phenotype (53% of patients), followed by eosinophilic (25%), allergic (12%) and Type 2 low (9%).
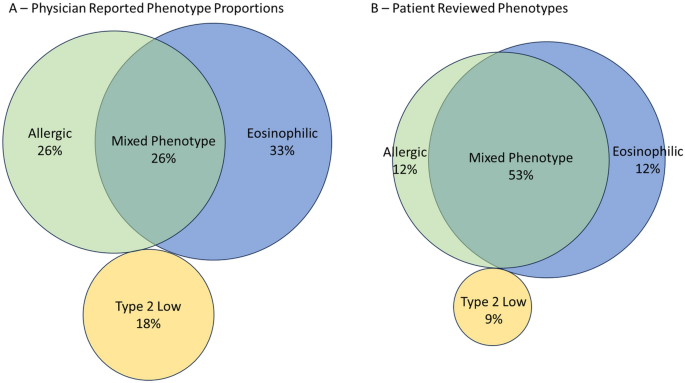
Venn diagrams of physician mean estimated phenotypic representations in practice ( A ) (n = 78) and the reported phenotypes of patients reviewed in the program ( B ) (n = 503). Mean proportion for each phenotype is reported
Treatment according to phenotype
Physicians were questioned about their general treatment of choice for severe asthma in patients with certain phenotypes. Overall, most physicians indicated preferring an anti-IgE for allergic asthma (59% of physicians), an anti-IL5 for eosinophilic asthma (87%), and an anti-TSLP for Type 2 Low asthma (68%) (Fig. 4 ). For patients with mixed phenotype, physicians were divided between anti-IL4 (27%), anti-IL5 (37%) and anti-TSLP (26%). When compared to biologic use in patients reviewed, the pattern was maintained (Fig. 4 ). Most patients with an allergic phenotype were treated with an anti-IgE (53%), patients with eosinophilic asthma were treated with an anti-IL5 (61%), patients with mixed phenotypes were treated with a variety of agents, however, anti-IL5 agents were most frequently used (40%). Anti-TSLP and anti-IL5 were the most frequently used biologics for patients with type 2 low asthma (14% and 9%, respectively); however, most (70%) type 2 low phenotype patients were not treated with biologic agents. Reasons cited for the selected agents were most frequently biomarker with another factor for allergic or mixed phenotypes, or biomarker alone for eosinophilic phenotype.
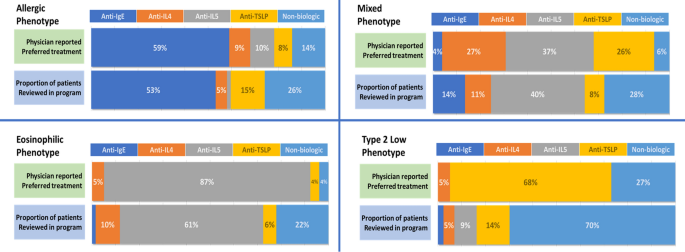
Physician reported preferred treatments (n = 78) for each severe asthma phenotype compared to patient prescribed (n = 503) treatment in the program. Physician preferences is the proportion of the number of physicians selecting each treatment as their preference compared with the entire group. Recorded treatment for patients assessed with allergic phenotype (n = 62), eosinophilic (n = 127), mixed (n = 265) or type 2 low (n = 42) were assigned to one of the four classes of biologic agents or to a treatment regimen that did not include a biologic
Treatment goals and satisfaction
When asked to select and rank the top five treatment goals for severe asthma patients in general, reduction in OCS use was the goal appearing most frequently in the top five (selected as a treatment goal by 77% of physicians) (Fig. 5 A). However, exacerbation reduction was identified by physicians as the goal with the highest priority (reduce to less than 1 per year ranked first in 31% and reduce by 50% or more in 27%).
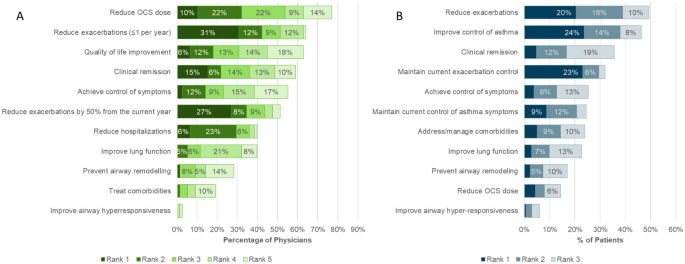
Treatment Goals for severe asthma. General overall treatment goals ( A ) and specific treatment goals for each patients assessed in the program ( B )
Physicians selected and ranked the top three treatment goals for each patient entered in the program. Exacerbation control or reduction and symptom control were most selected (Fig. 5 B). Reduction in OCS was infrequently selected as a treatment goal for patient, suggesting that OCS use was already limited in these patients.
The majority of physicians (55%) agreed that reduction in symptoms of airway hyperresponsiveness should be considered a goal of therapy in severe asthma. Despite this, physicians rarely included it as one of the top 5 goals of therapy in general (3% of physicians), and it was not a prominent goal of therapy for the patients reviewed in the program, even though nearly two thirds of patients reported symptoms consistent with AHR.
In general, physicians considered that a mean estimate of 47% of severe asthma patients in their practices were achieving all their treatment goals (Fig. 6 ). However, in specific patient assessments, physicians were fully satisfied with their patient’s treatment in only 37% of cases and thought that 44% of their patients were fully satisfied and meeting their treatment goals. In all cases, the majority of patients were not meeting all their treatment goals. When considering patient treatment with biologic, physicians were much more satisfied, with treatment for patients currently using a biologic when compared with patients who used a biologic in the past or who had never used a biologic (Fig. 7 ).
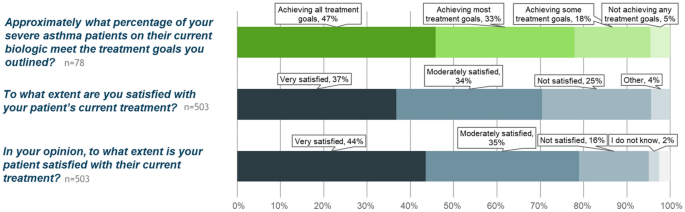
Treatment goals and satisfaction. Overall, physicians perceive that nearly half of their patients are meeting all treatment goals (top panel, green). In reality, physicians are fully satisfied and consider that their patients are meeting all goals in 37% (middle panel) and feel that patients are slightly more satisfied (bottom panel)
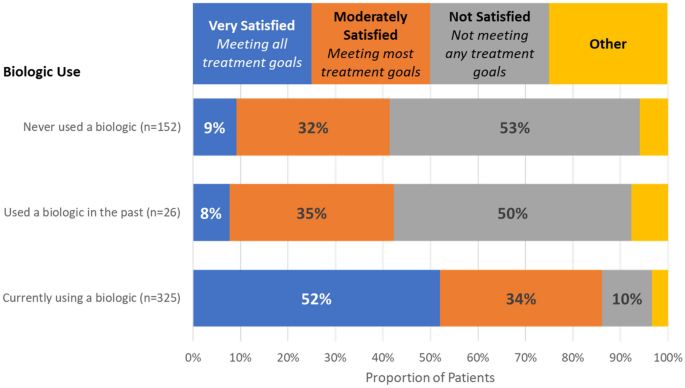
Physician satisfaction with treatment for assessed patients, based on biologic use
The overall objective of the CASCADE program was to allow physicians to assess their perceptions of their own clinical practice and then compare those perceptions with their clinical management of their own patients as well with the perception and clinical practice of their peers from across Canada. As part of the program, a series of meetings between the physicians who participated in the program were held, moderated by the program faculty, to identify barriers and gaps in patient management and to develop strategies to improve patient outcomes. The consensuses from these meetings will be published subsequently.
The physician practices included in the program were not specifically selected using any specific characteristic but through normal interactions with representatives of the sponsor based on an expressed interest in participation; sponsor representatives did not approach all physicians but did invite physicians with a wide variety of practice patterns. The practice profile data that were collected in the program support that the practices represented a diverse variety of clinical practices. Geospatial analysis of a variety of population characteristics from Canadian Census data (data not shown) suggested diverse patient populations for urban–rural ratio, large metropolitan centres versus smaller regional centres, a range of reported incomes and ethnic composition. Physician provided data further supports the diversity of the clinical populations: for example, physicians reported a varied proportion of children, youth or adult patients, with some practices reported a high number of children with severe asthma (up to 40%) and others reported no children (Table 1 ). Likewise, the number of patients seen in a typical week, practice type (academic versus community) and other data are consistent with a varied selection of physicians.
The program did not collect data on the total number of patients with any condition in the practice, nor did the program ask clinicians to specific any specific clinical interest. However, the program did ask physicians to estimate the proportion of their severe asthma patients who were adult, adolescent, or pediatric. It should be noted that this does not represent the distribution of patients that were included in the patient reviews as data collection was limited to adolescent and adult patients only. Overall, in these practices, an average estimate of 91% of severe asthma patients were adult (over the age of 18 years); however, there was variation between practices with some reporting higher estimates of younger patients. Considering the patients that were actually reviewed for this program, 97.4% where adult (over the age of 18 years), with only 2.6% adolescence patients.
This is the first Canadian assessment of specialist management of patients with severe asthma, particularly focusing on the use of biologic add-on therapies. Physician management of mild to moderate asthma [ 9 ] and in moderate to severe asthma [ 10 ], has been assessed following the release of GINA recommendations in 2021 [ 11 ], however, these were not focused on specialist care for severe asthma and biologic use. Perception of asthma control and management of patients from the Carenity asthma community was assessed in a study including 200 patients from 7 countries. However, this may not fully reflect Canadian patients as few patients from Canada were included [ 12 ].
Several key observations arose from this program. It identified that there is a difference in perception of the distribution of asthma phenotypes in specialist practice and the phenotype reported for individual patients, with patients of mixed phenotype representing a majority of patients (53%), while they are perceived to represent a much lower proportion (26%). The reason for this difference is unclear but may reflect either a selection of patients of mixed phenotype for inclusion in the program or an incorrect perception of the composition of their practice. The demographics of the patients included in this program were however similar to recent clinical trials in severe asthma (e.g., ASTHMA QUEST [ 13 ], NAVIGATOR [ 14 ], and others) suggesting that the included patients are representative of a severe asthma population.
Asthma phenotyping is considered an essential step in management of severe asthma [ 2 ], and is included in both the Asthma Canada patient charter [ 15 , 16 ] and in the recommendations from the Canadian Delphi consensus for severe asthma [ 17 ]. However, the identification of phenotypes is complicated by the complexity of the underlying disease immunopathology, with current biomarkers representing an incomplete surrogate measure of underlying processes. Assessment of the biomarker data that was collected during the patient reviews shows that 83% of the patients had at least one biomarker associated with type 2 inflammation (FeNO above 20ppb, blood eosinophils 300 cells/µL or greater, sputum eosinophils 3% or greater, IgE of 30 or greater); when using a lower eosinophils threshold of 150cells/uL, the number of patients with at least one biomarker increases to 89%. This suggests that type 2 inflammation is the predominant mechanism for asthma pathogenesis in severe asthma and a common finding [ 18 ]. However, in this program, this may be due to a selection bias of the participating physicians who may have chosen patients on biologics as they are more easily identified but are also more likely to have a type 2 phenotype. Highly variable and mixed biomarkers are a reflect of real-world patient populations when compared with selected clinical trial populations. Physicians may perceive a limited number of biomarker driven phenotypes, but, in reality, the majority of patients will likely have characteristics found in many phenotypes. Analysis of the International Severe Asthma Registry identified that the majority of patients in the registry had multiple elevated biomarkers, with 59% having 2 or more [ 19 ]. An issue for effectively phenotyping asthma that has been confirmed in this study is poor access to specialized testing such as sputum analysis and FeNO testing [ 3 , 17 ]. While FeNO was reported in about one quarter of the patients (24%), sputum eosinophils were infrequently available (6%). The expertise currently required to perform induced sputum analysis prevent its widespread use but point-of-care alternatives are being develop and may assist in future patient assessment [ 20 ].
In the Canadian context, there are a number of drivers that may impact specific assessments of asthma severity and phenotype. Typically, FeNO testing is not reimbursed by provincial medical systems and patients may not wish to pay for testing. FEV 1 testing is essential, but in certain conditions it may also not be reimbursed (for example, when performed by allergists in Quebec). The underuse of testing for asthma represents a specific concern for ensuring that patients are offered the most effective treatment for their severe asthma.
A high proportion of patients in this study were experienced with biologic (65% currently using a biologic and 5% used a biologic in the past) which is consistent with studies performed in other jurisdictions. In the US, the CHRONICLE study identified that 66% of severe asthma subspecialist-treated US adults where using biologics [ 21 ]. However, data for proportion of patients using biologic treatments is scarce, and none could be identified for Canada. The data collected here offers an estimate of biologic use in Canada, but this must be tempered by the realization that the patients reviewed in this program were not rigorously selected and may therefore represent either an over- or under-representation of patients with severe asthma treated with biologics.
While biologic use was similar in Quebec versus the rest of Canada, there were different perceived and reported burdens for using biologics in the different jurisdictions. In Quebec, there was less consideration of cost or access for the use of biologics, but eligibility was a major barrier; in other provinces, the concern over cost and access was greater, although on the individual patient basis, that was not a limiting factor.
This program sought to assess treatment satisfaction and treatment goals, a different perspective from disease control that is assessed in clinical trials. It is clear that, for this group of specialists, there is a specific concern for overuse of systemic corticosteroids and the adverse outcomes associated with them [ 22 ]. These specialists, as a group, perceived that limiting OCS use was an important goal for patients with severe asthma, in general. However, when assessing patients on an individual basis, reduction in the OCS dose was infrequently cited, suggesting that this goal was already achieved for most patients entered in the program. While symptom and disease control is a well-established benefit of biologics in clinical trials, in real-world clinical settings, patient response to a specific biologic may be suboptimal and clinicians should be willing to explore other biologic therapies in their patients.
The program represents a point in time assessment of the patients included by the physician. It was clear that while patients using biologics were less likely to have frequent exacerbations (41% vs 57% of patients never using a biologic), the outcomes over time cannot be assessed. However, physicians indicated their intention to start a biologic for about half of the patients that were not currently using a biologic, most likely to address the high frequency of exacerbations. Indeed, 47% of the patients included in the program reported 2 or more exacerbations in the past year, a proportion that increased to 84% in those prescribed a biologic after the visit with the healthcare provider. Similarly, 68% of patients who were reported to be switching to a different biologic had experienced 2 or more exacerbations. It is however unclear if the starting or switching of biologic were triggered by these exacerbations or other factors of the clinical evaluation.
In the patients included in this program, when an ACQ value was available, it was generally indicating poor control. Over three quarters of patients had ACQ values that suggested poor or borderline control of asthma symptoms. However, it is understood, particularly by specialists treating severe asthma, that ACQ assessment may also capture non-asthma respiratory symptoms, leading to higher ACQ scores than those from asthma alone [ 23 ]. In some cases, the treating physician may consider that a patient is well controlled by current treatment despite an elevated ACQ value, particularly in those cases where the value is only slightly elevated. Hence, the high proportion of patient with ACQ above 1.5 in the program may be an overestimation of the real proportion of patients with symptomatic asthma.
Symptoms of airway hyperresponsiveness (AHR) were noted in the majority of patients assessed in this program. Furthermore, physicians reported that management of AHR should be considered a general goal of treatment. However, even though AHR is an important characteristic of the disease that contributes to disease pathology, this appears to be under-appreciated or disregarded in the individual patient assessments. This may be due to a relative lack of data regarding the impact of biologic treatment on AHR and a need to further investigate how to target this trait in clinical practice [ 24 ].
Patients were not deemed fully satisfied with their treatments, including those that were treated with biologics. This may reflect a biologic use that in poorly tailored to the patient’s disease. Biologic selection is an art based on the available evidence and the reimbursement requirements. However, the levels of individual biomarkers don’t always predict the response to the biologic treatment, and patients with variable levels may have stronger or weaker response [ 3 ]. Physicians should be cognizant of this factor and accept that patients with complex diseases may not respond to treatment in expected ways. Compounding this, biologics that have been commercially available for a longer time may be more frequently used, either because patients have been on these therapies for several years and are sufficiently satisfied or because physician are most comfortable with prescribing a drug they are familiar with. At the time of data collection for this program, tezepelumab had been available for prescription for less than one year, accounting for the limited use in this patient population.
While patients were not universally satisfied with their current biologic treatment, it was apparent from the program that patients currently using biologics were deemed much more satisfied with their treatment than patients who were not currently using a biologic (see Fig. 7 ). This suggests that biologics do improve patient goals. Due to limited sample size, it is unclear why the patients who used biologics in the past were no longer using them, but if those patients were achieving similar satisfaction when using a biologic, it is a wonder that they are not agitating for that treatment again.
In this program, more than 20% of patients reviewed (of any phenotype) where not using biologics, with 70% of patients with type 2 low phenotype not treated with biologics. These patients experienced much poorer satisfaction with treatment than those patients that were treated with biologics. In half of these cases, the major barrier for patients to biologic access was eligibility—likely referring to the specific criteria required for biologic prescription (exacerbations, biomarkers, OCS, etc.). The results from this program clearly show that patients not eligible to current biologics have an unmet need that should be addressed in future biologic development. Biologics effective in several phenotypes or in non-exacerbating patients may provide an avenue for these patients.
Limitations
The CASCADE program was not comprehensive in the selection of practices to be included. Practice selection could be skewed by bias of both the recruiter and the physicians as not all physicians who were invited to participate in the program chose to do so. Further, the patient selection was not random or sequential and no specific criteria were given to the participating physicians to guide their selection. Selection of patients was rather left to the discretion of the physicians and their personal biases may have intruded, leading to a population that do not fully represent the makeup of patients in the practice. Because of these limitations, the findings of this program should be extended to other practices with care and these results should only be considered qualitatively.
The practice reflective program was carried out in the context of the Canadian single payer health care system and negotiated drug reimbursement criteria by Health Technology Access (HTA) organizations. We believe these approaches to align more closely to European Union models and less to the United States models of care. We hope to see similar programs carried out in other jurisdictions such as the European Union, the United States of America, or Asian Pacific countries.
Despite guidelines advocating for phenotype specific biologic therapies, treatment patterns of biologics use in Canada do not always align well to patient phenotype. The majority of severe asthmatics in Canada present with a mixed phenotype yet are most commonly treated with biologics targeting individual downstream pathways of inflammation. The introduction of broader spectrum biologics and those acting farther upstream in the inflammatory pathway is relatively recent and experience with these agents is therefore more limited. In those patients with mixed phenotypes and multiple overlapping pathways of inflammation currently treated with narrow target biologics and who may not be achieving all their treatment goals, an upstream approach to biologic therapy may be advantageous in controlling more aspects of the underlying mechanism of disease. Based on this practice reflective program, it is clear that a significant proportion of patients are not meeting treatment goals on their current treatment regimen and changes to that regimen should be investigated. We hope that the results from this study can help guide specialists across Canada in further refining their approach in utilizing the right biologic for the right severe asthma patient to achieve optimal outcomes.
Data availability
The aggregate dataset used and analysed in during the current study are available from the corresponding author on reasonable request. Individual data are not publicly available to ensure anonymity of the individual patients reviewed for the program is maintained.
Abbreviations
Asthma control questionnaire
Airway Hyperresponsiveness
Exhaled nitric oxide test
Forced expiratory volume in 1 s
Global initiative for asthma
Immunoglobin E
Interleukin 4
Interleukin 5
Parts per billion
Oral corticosteroids
Standard deviation
Thymic stromal lymphopoietin
Public Health Agency of Canada. Asthma and Chronic Obstructive Pulmonary Disease (COPD) in Canada, 2018 [Internet]. 2018 [cited 2023 Oct 22]. Available from: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/asthma-chronic-obstructive-pulmonary-disease-canada-2018.html .
Global Initiative for Asthma. Difficult-to-treat and Severe Asthma in Adolescent and Adult Patients [Internet]. 2023 [cited 2023 Oct 22]. Available from: https://ginasthma.org/wp-content/uploads/2023/09/GINA-Severe-Asthma-Guide-2023-WEB-WMS.pdf .
FitzGerald JM, Lemiere C, Lougheed MD, Ducharme FM, Dell SD, Ramsey C, et al. Recognition and management of severe asthma: A Canadian Thoracic Society position statement. Can J Respir Crit Care Sleep Med. 2017;1(4):199–221.
Google Scholar
Dorscheid DR, Lee JK, Ramesh W, Greenwald M, Del Carpio J. Guidance for administering biologics for severe asthma and allergic conditions. Can Respir J. 2022;2022:9355606.
Article PubMed PubMed Central Google Scholar
Ando K, Fukuda Y, Tanaka A, Sagara H. Comparative efficacy and safety of tezepelumab and other biologics in patients with inadequately controlled asthma according to thresholds of type 2 inflammatory biomarkers: a systematic review and network meta-analysis. Cells. 2022;11(5):819.
Article CAS PubMed PubMed Central Google Scholar
Husereau D, Goodfield J, Leigh R, Borrelli R, Cloutier M, Gendron A. Severe, eosinophilic asthma in primary care in Canada: a longitudinal study of the clinical burden and economic impact based on linked electronic medical record data. Allergy Asthma Clin Immunol. 2018;14(1):15.
Olaguibel JM, Quirce S, Juliá B, Fernández C, Fortuna AM, Molina J, et al. Measurement of asthma control according to Global Initiative for Asthma guidelines: a comparison with the Asthma Control Questionnaire. Respir Res. 2012;13(1):50.
Juniper EF, Bousquet J, Abetz L, Bateman ED, GOAL Committee. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100(4):616–21.
Article PubMed Google Scholar
Chapman KR, An L, Bosnic-Anticevich S, Campomanes CM, Espinosa J, Jain P, et al. Asthma patients’ and physicians’ perspectives on the burden and management of asthma. Respir Med. 2021;186: 106524.
Chapman KR, Canonica GW, Lavoie KL, Nenasheva N, Garcia G, Bosnic-Anticevich S, et al. Patients’ and physicians’ perspectives on the burden and management of asthma: Results from the APPaRENT 2 study. Respir Med. 2022;201: 106948.
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2021 [Internet]. 2021 [cited 2023 Oct 22]. Available from: https://ginasthma.org/wp-content/uploads/2023/04/GINA-Main-Report-2021-V2-WMSA.pdf .
George M, Graff C, Bombezin-Domino A, Pain E. Patients with severe uncontrolled asthma: perception of asthma control and its management. Pulm Ther. 2022;8(2):209–23.
Bleecker ER, Panettieri RA, Lugogo NL, Corren J, Daizadeh N, Jacob-Nara JA, et al. Dupilumab efficacy in patients with type 2 asthma with and without elevated blood neutrophils. J Immunol Res. 2023;2023:9943584.
Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. Tezepelumab in adults and adolescents with severe, Uncontrolled Asthma. N Engl J Med. 2021;384(19):1800–9.
Article CAS PubMed Google Scholar
Asthma Canada. Severe Asthma Patient Charter [Internet]. Asthma Canada. [cited 2023 Oct 22]. Available from: https://asthma.ca/get-help/severe-asthma/severe-asthma-patient-charter/ .
Menzies-Gow A, Canonica GW, Winders TA, Correia de Sousa J, Upham JW, Fink-Wagner AH. A charter to improve patient care in severe asthma. Adv Ther. 2018;35(10):1485–96.
Godbout K, Bhutani M, Connors L, Chan CKN, Connors C, Dorscheid D, et al. Recommendations from a Canadian Delphi consensus study on best practice for optimal referral and appropriate management of severe asthma. Allergy Asthma Clin Immunol. 2023;19(1):12.
Custovic A, Siddiqui S, Saglani S. Considering biomarkers in asthma disease severity. J Allergy Clin Immunol. 2022;149(2):480–7.
Denton E, Price DB, Tran TN, Canonica GW, Menzies-Gow A, FitzGerald JM, et al. Cluster analysis of inflammatory biomarker expression in the international severe asthma registry. J Allergy Clin Immunol Pract. 2021;9(7):2680-2688.e7.
Ali MM, Mukherjee M, Radford K, Patel Z, Capretta A, Nair P, et al. A rapid sputum-based lateral flow assay for airway eosinophilia using an RNA-cleaving DNAzyme selected for eosinophil peroxidase. Angew Chem Int Ed Engl. 2023;62(38): e202307451.
Panettieri RA, Ledford DK, Chipps BE, Soong W, Lugogo N, Carr W, et al. Biologic use and outcomes among adults with severe asthma treated by US subspecialists. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. 2022;129(4):467-474.e3.
Article CAS Google Scholar
Bleecker ER, Al-Ahmad M, Bjermer L, Caminati M, Canonica GW, Kaplan A, et al. Systemic corticosteroids in asthma: a call to action from World Allergy Organization and Respiratory Effectiveness Group. World Allergy Organ J. 2022;15(12): 100726.
Tomita K, Sano H, Chiba Y, Sato R, Sano A, Nishiyama O, et al. A scoring algorithm for predicting the presence of adult asthma: a prospective derivation study. Prim Care Respir J J Gen Pract Airw Group. 2013;22(1):51–8.
Article Google Scholar
Chan R, Lipworth B. Efficacy of biologic therapy on airway hyperresponsiveness in asthma. Ann Allergy Asthma Immunol. 2023;131(1):37–41.
Download references
Acknowledgements
AstraZeneca Canada Ltd and Amgen Canada Initiated and fully supported the practice reflective program including the initial selection faculty. On Behalf of AstraZeneca Canada Ltd., Andrew Foster and Ruth Rezai supported the project and provided editorial assistance by way of a factual accuracy check for the final manuscript. The authors would like to acknowledge and mourn the tragic loss of life caused by the conflicts in Ukraine and Israel and Palestine and hope that these conflicts will swiftly come to an end.
Funding of this project was by AstraZeneca Canada Ltd and Amgen Canada.
Author information
Authors and affiliations.
Quebec Heart and Lung Institute, Laval University, Quebec City, Canada
Krystelle Godbout
Division of Clinical Immunology and Allergy, Department of Medicine, Western University, London, ON, Canada
Department of Medicine, McMaster University, Hamilton, ON, Canada
Division of Pulmonary Medicine, Department of Medicine, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, AB, Canada
Irvin Mayers
Scientific Insights Consulting Group Inc., Mississauga, ON, Canada
James Paterson
Faculty of Medicine, University of Toronto, Toronto, ON, Canada
Charles K. N. Chan
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the development of the program, analysis and discussion of the results, and read and approved the final manuscript. JP compiled the manuscript and managed the submission. The authors would like to acknowledge and thank all the physicians who submitted data to the program: Shawn Aaron, Sam Agaybi, Reza Alizadehfar, Syed Anees, Tanweer Azher, Meyer Balter, Zenon Belak, Steven Bencze, Celine Bergeron, Marc Bibeau, Jean-Nicolas Boursiquot, Michael Braganza, Stephen Chao, Pascale Clark, Maxime Cormier, Christopher Davis, Maya De Zoysa, Christine Drapeau, Hannah Elfassy, Anne Ellis, Michael Fein, David Fishbein, George Fox, Winder Gill, Ann Gordon, Wendy Gould, Allen Greenwald, Mark Greenwald, Brian Grondin-Beaudoin, Dennis Gurwitz, Jacques Hébert, Victor Hoffstein, David Huang, Eissa Islam, Vipul Jain, Amin Kanani, Alan Kaplan, Irfan Khan, Patrick Killorn, Philippe Lachapelle, Gina Lacuesta, Pierre Landry, Jamie Le, Jean Gregoire Leduc, Jason Lee, Richard Leigh, Shun Chi Ryan Lo, Masoud Mahdavian, Raymond Mak, Andrew McIvor, Lundy McKibbin, Lyle Melenka, Michael Mitar, Mike Nicholson, Andrew O'Keefe, Bonavuth Pek, Erika Penz, Siobhan Perkins, Hoang (Tano) Pham, George Philteos, Yannick Poulin, Parisa Rahimi, Ellie Rivest-Abel, Mathieu Saint Pierre, Kevin Sanders, Richard Tan, Collin Terpstra, Olga Tourin, Madeleine Warwick, Susan Waserman, Benson Wong, Sophia Xu, Cory Yamashita, Harold Zackon, Nan Zhao.
Corresponding author
Correspondence to Charles K. N. Chan .
Ethics declarations
Ethical approval and consent to participate.
Ethics review for the conduct of this program was provided by Adverra central review board (Protocol 69571)
Consent for publication
Not applicable.
Competing interests
KG reports grants and/or personal fees from AstraZeneca, Covis, GSK, Novartis, Sanofi, TEVA, Valeo, Boehringer Ingelheim, and Regeneron. HK reports lecturing or attending advisory boards for ALK, AstraZeneca, Bausch Health, CSL Behring, GSK, Miravo, Novartis, Medexus, Pfizer, Sanofi, Takeda, and Valeopharma. HK is the Editor-in-Chief of Allergy, Asthma and Clinical Immunology as well as Associate Professor at Western University and Assistant Clinical Professor at McMaster University. IM reports [to be provided]. JP reports consulting fees from AstraZeneca, Ipsen, Edwards Lifesciences, RedNucleus; CKNC reports financial relationships (grants or research support, speakers fees, consulting fees) with AstraZeneca, Boehringer Ingelheim, CHIEF, Cipher, COVIS, E & Y, GSK, HRH, IC-EBM, MD-Briefcase, MeduCom, Merck, Novartis, Regeneron, Sanofi-Genzyme and acts as an inspector for CPSO.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix s1.
. Survey Questionnaires.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Godbout, K., Kim, H., Mayers, I. et al. A survey of severe asthma in Canada: results from the CASCADE practice reflective program. Allergy Asthma Clin Immunol 20 , 31 (2024). https://doi.org/10.1186/s13223-024-00891-x
Download citation
Received : 13 December 2023
Accepted : 02 April 2024
Published : 18 April 2024
DOI : https://doi.org/10.1186/s13223-024-00891-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Severe asthma
- Biological products
- Treatment goals
- Treatment satisfaction
- Asthma phenotype
- Standard of care
Allergy, Asthma & Clinical Immunology
ISSN: 1710-1492
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Isolated respiratory tract microorganisms and clinical characteristics in asthma exacerbation of obese patients: a multicenter study
Affiliations.
- 1 Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Ewha Womans University Mokdong Hospital, Ewha Womans University College of Medicine, 1071 Anyangcheon-Ro, Yangcheon-gu, 07985, Seoul, Republic of Korea.
- 2 Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Republic of Korea.
- 3 Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea.
- 4 Division of Pulmonology and Critical Care Medicine, Department of Internal Medicine, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Republic of Korea.
- 5 Division of Pulmonology, Allergy and Critical Care Medicine, Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Republic of Korea.
- 6 Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Ewha Womans University Seoul Hospital, Ewha Womans University College of Medicine, Seoul, Republic of Korea.
- 7 Division of Respiratory Medicine and Allergy, Department of Internal Medicine, Research Center for Pulmonary Disorders, Jeonbuk National University Medical School, Jeonju, Republic of Korea.
- 8 Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Ewha Womans University Mokdong Hospital, Ewha Womans University College of Medicine, 1071 Anyangcheon-Ro, Yangcheon-gu, 07985, Seoul, Republic of Korea. [email protected].
- PMID: 38308277
- PMCID: PMC10837954
- DOI: 10.1186/s12890-024-02880-7
Background: Viral infection is a risk factor for asthma exacerbation (AE). However, bacterial infections related to AE in adults are poorly known. On the other hand, obese patients with asthma have their own clinical and biological characteristics compared with non-obese patients.
Methods: We investigated the differences in isolated pathogens for AE between obese and non-obese patients with asthma. We included 407 patients with AE from 24 medical centers in Korea. Microorganisms isolated from culture, RT-PCR or serologic tests using lower respiratory tract specimens were retrospectively investigated.
Results: A total of 171 obese and 236 non-obese patients with asthma were included for analysis. Compared to non-obese patients, obese patients were associated with women (77.2% vs. 63.6%), never smoker (82.5% vs. 73.9%), shorter duration of asthma (7.9 ± 8.4 vs. 10.5 ± 10.1 years), less history of pulmonary tuberculosis (8.8% vs. 17.4%), and more comorbidity of allergic rhinitis (48.5% vs. 0.8%). Viral and/or bacterial infections were detected in 205 patients (50.4%) with AE. The numbers of patients with viral only, bacterial only, or both infections were 119, 49, and 37, respectively. The most commonly isolated bacterium was Streptococcus pneumoniae, followed by Pseudomonas aeruginosa and Chlamydia pneumoniae. Obese patients showed a lower incidence of Chlamydia pneumoniae infection. In the non-obese group, bacterial infection, especially Chlamydia pneumoniae infection, was significantly associated with the duration of systemic corticosteroid use (13.6 ± 19.8 vs. 9.7 ± 6.7 days, p = 0.049).
Conclusion: Bacterial infection was associated with a longer period of corticosteroid use in the non-obese group. Acute Chlamydia pneumoniae infection was less associated with obese patients with AE. Further well-designed studies are needed to evaluate microorganisms and the efficacy of antibiotics in patients with AE.
Keywords: Asthma; Bacteria; Exacerbation; Obesity; Virus.
© 2024. The Author(s).
Publication types
- Multicenter Study
- Adrenal Cortex Hormones
- Asthma* / complications
- Asthma* / epidemiology
- Bacterial Infections* / complications
- Bacterial Infections* / epidemiology
- Chlamydophila Infections* / complications
- Obesity / complications
- Obesity / epidemiology
- Respiratory System
- Respiratory Tract Infections* / complications
- Respiratory Tract Infections* / epidemiology
- Respiratory Tract Infections* / microbiology
- Retrospective Studies
Association of Interleukins 17A and 17F Gene Polymorphism with Asthma: A Case Control Study in North Indian Population
- ORIGINAL RESEARCH ARTICLE
- Published: 12 April 2024
Cite this article
- Rashmi Pandey 1 nAff2 &
- Ved Prakash 1
Explore all metrics
The number of cases of asthma patients has been on rise globally due to several factors. The available scanty information concerning attributes of certain genes in asthma’s pathophysiology is a blockade in its early diagnosis and treatment. The objective of present study was to investigate roles of IL-17A and IL-17F in occurrence and severity of asthma in North Indian population. This study involved 150 cases with asthmatics and 150 healthy controls. Asthmatic patients were confirmed by Spirometry and other clinical parameters. The genotype frequencies observed for all genes in controls were in Hardy Weinberg Equilibrium (HWE). The results suggested that the asthmatics of the same height and age had lower weight and higher smoking rate in comparison to controls. The asthmatics displayed cough, breathlessness, congestion, disturbed sleep, headache, and wheezing. These parameters were found to be significantly elevated in asthmatics. Excepting Hb, levels of blood eosinophils, AEC, TLC and serum IgE were higher in cases than controls. The lower serum IL-17F and IL-17A levels were recorded in asthmatics. In IL-17F, rs 2397084 and rs763780 mutants and variant genotypes were found to be increased in cases than in controls. In rs 2397084 CC, CT + CC was significantly higher in cases. Also, rs763780 mutant and variant genotypes were significantly higher in cases. In contrast, IL-17A rs2275913 showed hetero GA variant GA + AA significantly higher in cases. The rs3748067 indicated variant GA + AA to be non-significantly higher in cases. The RFLP analysis of certain regions in IL17F and IL17A genes and increased IL-17A and IgE levels in the blood of asthmatics have generated evidences to indicate that these markers might be playing key roles in onset and severity of asthma. These indices may therefore be exploited for timely diagnosis and adequate management of asthma.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
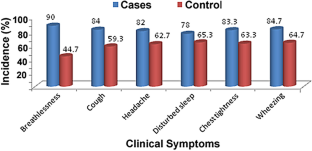
Similar content being viewed by others

IL-10 and IL-17F Promoter Single Nucleotide Polymorphism and Asthma: A Case-Control Study in South India
Sareh Raeiszadeh Jahromi, P. A. Mahesh, … Nallur. B. Ramachandra
Relationship between asthma and IL-17 gene polymorphism in a Turkish population
Gülbahar Darılmaz Yüce, Tuba Erdoğan, … Gülay Güleç Ceylan

Association of Interleukin-12A rs568408 with Susceptibility to Asthma in Taiwan
Te-Chun Shen, Chia-Wen Tsai, … Da-Tian Bau
Borak J, Lefkowitz RY. Bronchial hyperresponsiveness. Occupat Med. 2016;66(2):95–105.
Article CAS Google Scholar
Vince N, Limou S, Daya M, Mori W, Rafaels N, Geffard E, et al. Association of HLADRB1*09:01 with tIgE levels among African-ancestry individuals with asthma. J Allergy Clin Immunol. 2020;146:147–55.
Article CAS PubMed Google Scholar
Mukhopadhyay S, Sypek J, Tavendale R, Gartner U, Winter J, Li W, et al. Matrix metalloproteinase-12 is a therapeutic target for asthma in children and young adults. J Allergy Clin Immunol. 2010;126(70–6): e16.
Google Scholar
Kynyk JA, Mastronarde JG, McCallister JW. Asthma, the sex difference. Curr Opin Pulm Med. 2011;17:6–11.
Article PubMed Google Scholar
El-Shal AS, Shalaby SM, Abdel-Nour HM, Sarham WM, Gehad MH, Yousif YM. Impact of cytokines genes polymorphisms and their serum levels on childhood asthma in Egyptian population. Cytokine. 2022;157:155933.
Yüce GD, Erdoğan T, Bozkurt B, Toprak U, Ceylan GG. Relationship between asthma and IL-17 gene polymorphism in a Turkish population. Irish J Med Sci. 2022;192:269–75.
Barnes PJ. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat Rev Drug Disc. 2013;12:543–59.
Article Google Scholar
Luckheeram RV, Zhou R, Verma AD, Xia B. CD4 + T cells: differentiation and functions. Clin Dev Immunol. 2012;2012:12.
Peck A, Mellins ED. Precarious balance: Th17 cells in host defense. Infect Immunity. 2010;78(1):32–8.
Luo W, Hu J, Xu W, Dong J. Distinct spatial and temporal roles for Th1, Th2 and Th17 cells in asthma. Front Immunol. 2022;13:974066.
Article CAS PubMed PubMed Central Google Scholar
Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2017;8:218–30.
Shean JA, John FA. TH17 cells in asthma and inflammation. Biochim Biophys Acta. 2011;1810:1066–79.
Pierre MK, Vijay KK. Mechanisms of disease interleukin-17 and type 17 helper T cells. The New Eng J Med. 2009;361(9):888–98.
Vazqquez-Tello A, Halwani R, Li R, Nadigel J, Bar-Or A, et al. IL-17A and IL-17F expression in B lymphocytes. Int Arch All Immunol. 2012;157:406–16.
Liu T, Yang L, Lv X, Zuo C, Jia C, Yang Z, et al. Cumulative evidence for associations between genetic variants in interleukin 17 family gene and risk of human diseases. Front Immunol. 2022;13:1008184.
Hashim MH, Al-Rikabi HR. Sequencing of the IL-17 gene polymorphism in asthmatic patients in Thi Qar province. NeuroQuantology. 2022;20(8):1790–8.
Lindén A, Dahlén B. Interleukin-17 cytokine signalling in patients with asthma. Eur Resp J. 2014;44:1319–31.
Ota K, Kawaguchi M, Matsukura S, Kurokawa M, Kokubu F, Fujita J, et al. Potential involvement of IL-17F in asthma. J Immunol Res. 2014;2014:602846.
Article PubMed PubMed Central Google Scholar
Trevor JL, Deshane JS. Refractory asthma: mechanisms, target, and therapy. Allergy. 2014;69(7):817–27.
Pawlik A, Kotrych D, Malinowski D, Dzeidziejko V, Czerewaty M, Safranow K. IL17A and IL17F gene polymorphisms in patients with rheumatoid arthritis. BMC Musculoskelet Disord. 2016;17:208.
Omraninava M, Eslami MM, Aslani S, Razi B, Ilmani D, Feyzinia S. Interleukin 13 gene polymorphism and susceptibility to asthma: a meta-regression and meta-analysis. Eur Ann Allergy Clin Immunol. 2020;54(4):150–67.
Shi F, Zhang Y, Qiu C. Gene polymorphisms in asthma: a narrative review. Ann Transl Med. 2022;10(12):711.
Doe C, Bafadhel M, Siddiqui S, Desai D, Mistry V, Rugman P, et al. Expression of the T helper 17-associated cytokines IL17A and IL-17F in asthma and COPD. Chest J. 2010;138(5):1140–7.
Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, et al. IL-17A produced by alphabeta T cells drives airway hyperresponsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nature Med. 2012;18(4):547–54.
Arand M, Mühlbauer R, Hengstler J, Jager E, Fuchs J, Winkler L, et al. A multiplex polymerase chain reaction protocol for the simultaneous analysis of the glutathione S- transferase GSTM1 and GSTT1 polymorphism. Anal Biochem. 1996;236(1):184–6.
Wright CM, Larsen JE, Colosimo ML, Barr JJ, Chen L, McLachlan RE, et al. Genetic association study of CYP1A1 polymorphisms identifies risk haplotypes in nonsmall cell lung cancer. Eur Resp J. 2010;35:152–9.
Chang YB, Kaidarova Z, Hindes D, Hindes D, Bravo M, Kiely N, et al. Seroprevalence and demographic determinants of human T-lymphotropic virus type 1 and 2 infections among first-time blood donors—United States, 2000–2009. The J Inf Dis. 2014;209(4):523–31.
Sutter CH, Qian Z, Hong YP, Mammen JS, Strickland P, Sutter T. Genotyping human cytochrome: P450 1B1 variants. Methods Enzymol. 2002;357:53–8.
Weng Y, Fei B, He P, Cai M. Glutathione-S-transferase T1 polymorphism is associated with esophageal cancer risk in Chinese han population. Asian Pacific J Cancer Prev. 2012;13(9):4403–7.
Vidal F, Lorenzo A, Auguet T. Genetic polymorphisms of ADH2, ADH3, CYP4502E1 Dra-I and Pst-I, and ALDH2 in Spanish men: lack of association with alcoholism and alcoholic liver disease. J Hepatol. 2004;41(5):744–50.
Alyasin S, Karimi MH, Amin R, Babaei M, Darouger S. Interleukin-17 gene expression and serum levels in children with severe asthma. Iran J Immunol. 2013;10(3):177–85.
CAS PubMed Google Scholar
Castro-Rodriguez JA, Saglani S, Rodriguez-Martinez CE, Oyarzun MA, Louis Fleming L, Bush A. The relationship between inflammation and remodeling in childhood asthma: a systematic review. Ped Pulmonol. 2018;53(6):824–35.
Prakash YS, Halayko AJ, Gosens R, Panettieri RA Jr, Camoretti-Mercado B, Penn RB, et al. An official american thoracic society research statement: current challenges facing research and therapeutic advances in airway remodeling. Am J Resp Critical Care Med. 2017;195(2):e4–19.
Hatta M, Surachmanto EE, Islam AA, Wahid S. Expression of mRNA IL-17F and sIL-17F in atopic asthma patients. BMC Res Notes. 2017;10:202.
Mowahedi M, Samet M, Zare F, Samadi M. Serum levels of IL-17A increase in asthma but don’t correlate with serum level of IgE and asthma severity. Int J Med Lab. 2015;2(1):25–33.
CAS Google Scholar
IwakuraY NS, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev. 2008;226:57–79.
Romero Palacios PJ. Asthma and tobacco smoke. Arch Bronconeumol. 2004;40:414–8.
Gomez M, Vollmer WM, Caceres ME, Jossen R, Baena-Cagnani CE. Adolescent smokers are at greater risk for current asthma and rhinitis. Int J Tuberc Lung Dis. 2009;13:1023–8.
Yoo S, Kim HB, Lee SY, Kim BS, Kim JH, Yu J, et al. Effect of active smoking on asthma symptoms, pulmonary function, and BHR in adolescents. Pediatr Pulmonol. 2009;44:954–61.
Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2:372–7.
Floreani AA, Rennard SI. The role of cigarette smoke in the pathogenesis of asthma and as a trigger for acute symptoms. Curr Opin Pulm Med. 1999;5:38–46.
Tilp C, Bucher H, Haas H, Duechs MJ, Wex E, Erb KJ. Effects of conventional tobacco smoke and nicotine-free cigarette smoke on airway inflammation, airway remodelling and lung function in a triple allergen model of severe asthma. Clin Exp Allergy. 2016;46:957–72.
Mortaz E, Folkerts G, Engels F, Nijkamp FP, Redegeld FA. Cigarette smoke suppresses in vitro allergic activation of mouse mast cells. Clin Exp Allergy. 2009;39:679–87.
Ganta S, Komaravalli PL, Ahmad S, Gaddam SL. Influence of genetic variants and mRNA expression of interleukin IL17A gene in asthma susceptibility. Gene. 2023;854:147119.
Shaban SA, Brakhas SA, Ad’hiah AH. Association of interleukin-17A genetic polymorphisms with risk of asthma: a case-control study in Iraqi patients. Meta Gene. 2021;29: 100935.
Bazzi MD, Sultan MA, Al Tassan N, et al. Interleukin 17A and F and asthma in Saudi Arabia: gene polymorphisms and protein levels. J Invest Aller Clin Immunol. 2011;21(7):551–5.
Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205(5):1063–75.
Download references
Acknowledgements
RP thanks to UPCST-Lucknow, UP for providing a grant to support this research work, KGMU-Lucknow and CSIR-IITR Lucknow for providing research facilities for carrying out experiments.
RP thanks to UPCST-Lucknow, UP, India for financial support in the form of a research grant for carrying out the study.
Author information
Rashmi Pandey
Present address: Department of Biochemistry, Autonomous State Medical College, Hardoi, 241001, Uttar Pradesh, India
Authors and Affiliations
Department of Pulmonary and Critical Care Medicine, King George’s Medical University, Lucknow, 226003, Uttar Pradesh, India
Rashmi Pandey & Ved Prakash
You can also search for this author in PubMed Google Scholar
Contributions
VP designed the research project, provided guidance, and supported the research. RP conducted the experiments, arranged the data, statistically analyzed the data and prepared the manuscript.
Corresponding author
Correspondence to Rashmi Pandey .
Ethics declarations
Conflicts of interests.
The authors declare that they do not have any conflict of interest.
Informed Consent
Informed consent was sought from the subjects participating in this study.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Pandey, R., Prakash, V. Association of Interleukins 17A and 17F Gene Polymorphism with Asthma: A Case Control Study in North Indian Population. Ind J Clin Biochem (2024). https://doi.org/10.1007/s12291-024-01202-2
Download citation
Received : 31 December 2023
Accepted : 19 February 2024
Published : 12 April 2024
DOI : https://doi.org/10.1007/s12291-024-01202-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Asthmatic patients
- North Indian Population
- Polymorphism
- Find a journal
- Publish with us
- Track your research
Advertisement
Sponsored by AngioDynamics, Inc
A Novel Way to Treat Patients With Radial Access
Two peripheral artery disease case examples highlight the utility of Auryon XL, a radial-length laser atherectomy device.
By Ankur Lodha, MD
View PDF Reprints
The Auryon Atherectomy System (AngioDynamics, Inc.) is a 355-nm, solid-state, short-pulse laser that transmits energy to the diseased artery at preset fluency levels of 50 and 60 mJ/mm 2 ( Figure 1 ). The system works over 0.014-inch wires, and there are four available catheter sizes. On January 23, 2024, AngioDynamics, Inc. announced FDA 510(k) clearance of the Auryon XL Catheter, a 225-cm radial access catheter, for use with the Auryon Atherectomy System in the treatment of peripheral artery disease (PAD).

Figure 1. The Auryon laser console.
This article presents two of the first cases performed utilizing the Auryon XL Catheter.
CASE STUDY 1
Patient presentation.
A man in his mid 60s presented with a history of hypertension, dyslipidemia, congestive heart failure, former smoker, alcohol abuse, type 2 diabetes mellitus, end-stage renal disease on hemodialysis, and cerebrovascular accident. He had been treated for PAD in the past. On his left side, he underwent atherectomy and balloon angioplasty of the superficial femoral artery (SFA) and anterior tibial artery in late summer 2022. He also received treatment on his right side, with angioplasty of his right anterior tibial artery and peroneal artery 1 month later and atherectomy and angioplasty of his right SFA the following month. Two years later in early 2024, he presented to our clinic with Rutherford class 4 rest pain of his left leg and was scheduled for angiography.
Procedural Overview
Via ultrasound, access was achieved to the right radial artery with a 5-F, 11-cm sheath and exchanged for a 6-F, 150-cm Sublime Radial sheath (Surmodics, Inc.). A 5-F, 125-cm pigtail catheter was inserted, and angiography was performed. This revealed a 90% stenosis of the left anterior tibial artery and a 90% ostial stenosis of the left posterior tibial artery ( Figure 2A and 2B ). The decision was made to intervene, and a 1.5-mm-diameter Auryon XL Catheter was introduced over a 0.014-inch, 450-cm hydrophilic-coated wire; the posterior tibial artery was treated with one pass at a fluency of 50 mJ/mm 2 . Angioplasty was performed of the posterior tibial artery with a 3- X 120-mm Sublime balloon (Surmodics, Inc.) inflated to 6 atm for 1 minute. Next, the focus shifted to the anterior tibial artery, which was again treated with one pass of atherectomy using the Auryon laser. Angioplasty of the anterior tibial artery was performed using a 3- X 120-mm Sublime balloon, inflated to 6 atm for 1 minute, followed by a 2- X 220-mm Sublime balloon inflated to 6 atm for 1 minute. Posttreatment angiography revealed resolution of the stenoses in the posterior tibial artery and anterior tibial artery with brisk inline flow to the foot ( Figure 2C and 2D ). All catheters and sheaths were then removed, and a TR Band (Terumo Interventional Systems) was applied to the radial access site.

Figure 2. Pretreatment angiogram showing 90% stenosis of the left anterior tibial artery and a 90% ostial stenosis of the left posterior tibial artery (A, B). Posttreatment angiogram showing successful revascularization of the anterior and posterior tibial artery (C). Below-the-knee distal runoff showing straight line flow into the foot (D).
CASE STUDY 2
A woman in her mid 80s presented with a history of dyslipidemia, breast cancer, and active smoking. She presented to our clinic with bilateral lifestyle-limiting claudication, and an arterial duplex ultrasound revealed a > 75% stenosis of the right SFA with monophasic waveforms. Additionally, her right-sided ankle-brachial index was 0.56, consistent with moderate PAD. The patient was scheduled for lower extremity angiography.
Access was obtained using ultrasound through the left radial artery using a 5-F, 11-cm sheath. This was exchanged for a 5-F, 120-cm Sublime Radial sheath, and a 5-F pigtail catheter was used for diagnostic angiography. This demonstrated a 99% critical diffuse stenosis of the right SFA ( Figure 3A ). The decision was made to intervene, and a 1.5-mm Auryon XL Catheter was advanced over a 0.014-inch, 450-cm hydrophilic-coated wire through the lesion twice at a fluency of 50 mJ/mm 2 . Next, a 4- X 150-mm Sublime balloon was inserted, and angioplasty was performed at 12 atm for 1 minute. Postprocedure angiography revealed marked improvement in the SFA stenosis ( Figure 3B ), with no distal embolus noted below the knee ( Figure 3C ). All catheters and sheaths were then removed, and a TR Band was applied to the radial access site.

Figure 3. Preprocedure angiography demonstrating 99% critical diffuse stenosis of the right SFA (A). Posttreatment angiography demonstrating resolution of the stenosis (B). Posttreatment angiography of the below-the-knee vessels showing no evidence of distal embolus (C).
Auryon XL is the first radial-length laser atherectomy device. It has allowed us to perform peripheral atherectomy procedures safely via radial access, when previously, the only option for these cases would have been groin or pedal access. In my practice, radial access is the default as it decreases groin complication and improves patient safety. The Auryon XL Catheter, with its innovative design and ease of use brings significant advancements to radial procedures as the first nonorbital atherectomy device available in radial length—setting a new standard for laser atherectomy technology.
For risk info, visit auryon-system.com/risk-information/

Ankur Lodha, MD Cardiovascular Institute of the South Lafayette, Louisiana Disclosures: Paid consultant to AngioDynamics, Inc.
Pelvic Venous Disorder: A Mystery in Missed Diagnosis and Treatment
With Thekla Bacharach, MD
left-arrow Created with Sketch. Previous Article
The Next Wave of Intravascular Lithotripsy: A Panel Interview on Advances in Treating Calcific PAD
With Arthur Lee, MD; Amir Kaki, MD; Miguel Montero-Baker, MD; Venkatesh Ramaiah, MD, FACS; and Peter Schneider, MD
Next Article right-arrow Created with Sketch.

Navigating Complex Cases
Illustrative tutorials of key decisions across a variety of challenging scenarios.
Related Articles
Indications for Use
A Meeting of Radial Revolutionaries
Drivers of Radial-to-Peripheral Adoption in Real-World Practice
Most Read Articles
Serration Technology: Achieving Optimal Lumen Gain in Patients With CLTI
With Christopher J. Grilli, DO, and Michael C. Siah, MD
Pioneering Neurovascular Office-Based Labs: Enhancing Patient Satisfaction and Accessibility With Advanced Biplane Systems
With Andrey Belayev, MD, and Jacob Rodman, CEO
Directions in Venous Care
By Karem Harth, MD, MHS, RPVI, and Marie Josee van Rijn, MD, PhD
Practical Lessons From PRESERVE
Pelvic Venous Disorders: Practice Realities
With Karem Harth, MD, MHS, RPVI; Aleksandra Jaworucka-Kaczorowska, MD, PhD; Ronald S. Winokur, MD, FSIR, RPVI
© 2024 Bryn Mawr Communications II, LLC. All Rights Reserved • Privacy Policy
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Allergy Rhinol (Providence)
- v.2(2); Apr-Jun 2011
A case of uncontrolled asthma
Ömür aydin.
From the 1 Department of Chest Diseases, Division of Immunology and Allergy,
Cabir Yüksel
2 Department of Thoracic Surgery,
Aylin Okçu Heper
3 Department of Pathology, Ankara University School of Medicine, Ankara, Turkey
Șevket Kavukc̦u
Zeynep misirligil.
A 48-year-old female patient with uncontrolled severe asthma was referred to our hospital for anti-IgE therapy. She was suffering with persistent wheezing and dyspnea after a severe asthma attack that had taken place 5 months previously. Her asthma had not been controlled with adequate asthma treatment, including budesonide at 320 μg + formoterol at 9 μg b.i.d. combination, montelukast at 10 mg/day, and oral steroids (30–40 mg/day of prednisolone), during this period. She was hospitalized for evaluation for anti-IgE therapy. Chest radiography revealed a left-sided hilar opacity. Fiberoptic bronchoscopy was performed and showed an endobronchial lesion obstructing the left lower bronchus lumen. Computed tomography also revealed a nodular lesion at the same location. The patient underwent left lower lobectomy and mediastinal lymph node dissection. Pathological examination concluded the diagnosis of typical carcinoid tumor. After surgery, her symptoms disappeared and she has had no recurrence. In conclusion, a diagnosis of severe asthma requires confirmation of asthma. Uncontrolled symptoms that linger despite aggressive therapy warrant evaluation to rule out other etiologies, such as a carcinoid tumor, before selecting new treatment options.
CASE PRESENTATION
A 48-year-old white woman, a housewife, was admitted to our tertiary clinic complaining of wheezing and dyspnea. She had been diagnosed with asthma 12 years previously and was well controlled using budesonide at 160 μg + formoterol at 4.5 μg b.i.d. combination therapy until 5 months before her visit to our clinic. She had had a severe asthma attack at that time, during which her wheezing was not well correlated with physical exercise and had persisted for several months. She was treated unsuccessfully with budesonide at 320 μg + formoterol at 9 μg b.i.d. combination, montelukast at 10 mg/day, and oral steroids (30–40 mg/day of prednisolone) during that period, and because her asthma had failed to come back under control, was referred to our clinic and hospitalized for evaluation for anti-IgE therapy. Her medical history was significant for appendectomy and hemorrhoidectomy. She was taking thyroid hormone for Hashimoto's thyroiditis and calcium tablets for osteoporosis.
Her vitals were stable with a heart rate of 76 bpm, a temperature of 36.5°C, blood pressure of 110/70 mmHg, and respiratory rate of 18/min on physical examination. Her examination was normal with the exception of decreased auscultation in the left lung. Her routine blood count was hematocrit, 38.2%; leukocyte, 9300; and erythrocyte sedimentation rate 13, mm/hr. Spirometry showed an obstructive pattern (forced expiratory volume in 1 second [FEV 1 ], 2.20 L [82%]; forced vital capacity [FVC], 3.45 L [110%]; FEV 1 /FVC, 60%). We were unable to show spirometric reversibility but were able to learn that during a previous hospitalization at another clinic, she had had a reversible airway obstruction. (prebronchodilator FEV 1 , 1.70 L [64%]; postbronchodilator FEV 1 , 2.01 L [75%]; reversibility, 17%). Her skin-prick test was positive for house-dust mites. Total IgE level was 115 kU/L. All data about the patient seemed to indicate that she could be a candidate for anti-IgE therapy. Chest radiography revealed a left-sided hilar opacity. For further evaluation, computerized tomography was performed and showed a 15-mm nodular lesion located in the left lower lobe bronchus ( Fig. 1 ). These radiological findings changed our management plan and diagnosis from asthma to a chest mass. A fiberoptic bronchoscopy was performed, which revealed an endobronchial lesion obstructing the left lower bronchus lumen ( Fig. 2 ). Biopsy was not performed because the lesion was highly vascularized and there was a risk of bleeding. Bronchial lavage fluid was removed from the left bronchus. Cytological examination of the lavage fluid was normal. The patient was transferred to the thoracic surgery ward for surgical treatment. She underwent left lower lobectomy and mediastinal lymph node dissection.

Thorax CT scan of the patient.

Bronchoscopic imaging of the carcinoid tumor.
Histopathological evaluation revealed an intrabronchial tumor, made up of monotonous cells with oval or round, finely granular nuclei and eosinophilic cytoplasm. No mitotic figures or necrosis was detected. The stroma was vascular and scant. Focal tumoral invasion of the lung parenchyma through the bronchial wall was also noted. Immunohistochemical staining indicated epithelial and neuroendocrine differentiation of the tumor cells with cytoplasmic positivity of pancytokeratin, chromogranin A, synaptophysin, and CD56. These findings established the diagnosis of a typical carcinoid (TC) tumor ( Figs. 3 and and4). 4 ). The dissected peribronchial and regional lymph nodes showed no metastasis.

The tumor made up of uniform polygonal cells with finely granular chromatin in round nuclei and moderate amount of eosinophilic cytoplasm. There were no nuclear atypia, mitosis and necrosis, H&Ex400.

The cytoplasmic positivity of chromogranin-A in tumor cells, Chromogranin-Ax400.
After surgical resection, she was asymptomatic with budesonide at 160 μg + formoterol at 4.5 μg combination therapy and had a better pulmonary function (FEV 1 , 2.53 L [95%], FVC, 4.29 L [138%]; FEV 1 /FVC, 59%). Eight months after the operation, she had another asthma attack. She was hospitalized for asthma treatment and further evaluation of recurrent tumor. There was the presence of reversible airway obstruction, particularly in the small airways, on spirometric evaluation (FEV 1 , 2.24 L [85%] with 10% reversibility and forced expiratory flow at 25–75%, 1.63 L [49%] with 17% reversibility). Computerized tomography of the thorax, abdomen, and pelvis revealed no pathological finding. Bronchoscopy was performed and cytological examination of the lavage fluid result was normal. She had no recurrence for 2 years and her asthma is presently well controlled.
Today, achieving asthma control is indicated as the main goal of asthma management by international guidelines. Although most asthma patients can be treated and controlled with inhaled steroids, some patients remain uncontrolled despite adequate asthma therapy. In our country, nearly one-half of patients with asthma were found uncontrolled in a multicenter survey. 1 A systematic review should be conducted during the management of uncontrolled asthmatic patients, and it is imperative that this include first reconfirming that a diagnosis of asthma is appropriate and then evaluating for other coexisting diseases that may influence one's asthma control. Here, we report a case of uncontrolled asthma that was, after further evaluation, revealed to be a carcinoid tumor.
Pulmonary carcinoid tumors are the most frequently encountered benign tumors of the tracheobronchial tree and constitute 2–5% of all lung cancers. 2 , 3 TCs and atypical carcinoids (ACs) are subgroups of neuroendocrine tumors that are determined as low-grade and intermediate-grade tumors according to biological aggressiveness, respectively. TCs account for 90% of all carcinoids and 80% show up in a peripheral location. 4 Although TCs are low-grade tumors, regional lymph node metastasis can be seen in 10–23% of cases; this rate, however, is 40–50% for ACs. 5 This accounts for the higher 5-year survival rates seen in TCs when compared with ACs. 5 – 7
The most common symptoms of pulmonary carcinoid tumors are hemoptysis (caused by high vascularization), lower respiratory tract infections, cough, wheezing, and shortness of breath. 8 , 9 Some patients may be asymptomatic. There is usually a time gap from the onset of symptoms until diagnosis, and patients are often misdiagnosed with asthma. 6 , 10 – 13 There are a limited number of cases diagnosed as carcinoid tumor who had also received a true diagnosis of coexisting asthma. The patient we present here had already received a diagnosis of asthma proven by reversible bronchial obstruction, and it was for this reason that her symptoms of dyspnea and wheezing were first attributed to asthma. The differential diagnosis was expanded after her poor response to standard therapy; thus, it is not surprising that a further treatment choice of anti-IgE was considered for this patient.
Anti-IgE (omalizumab) is an approved treatment for patients with severe asthma that acts on decreasing serum IgE levels. Several published studies have documented the effectiveness of this molecule in effectively treating asthma. We have been prescribing anti-IgE therapy in our tertiary clinic since 2006. In light of our experience, we believe that several factors impact a good response to anti-IgE treatment. First, proper determination of the correct indications for medicine use is vital, closely followed by the proper selection of patients. The most important issue, in our opinion, in achieving this is confirming diagnosis and excluding comorbid diseases. Therefore, the patient described in this study was evaluated accordingly. Clinical symptoms and reversible airway obstruction in spirometry led us to believe her asthma diagnosis was valid initially even though another disease state did in fact exist. Also, because an asthma attack occurred 8 months after the surgery we were convinced that she did have real asthma, retrospectively. In the literature, the associated factors with worsening asthma control included poor adherence, rhinitis, gastroesophageal reflux disease, nasal polyps, vocal cord dysfunction, bronchiectasis, allergic bronchopulmonary aspergillosis, Churg-Strauss syndrome, drugs, airway malignancy, respiratory tract infections, and thyrotoxicosis. 14 – 16 Our patient had already been evaluated for upper airway disease and gastroesophageal reflux disease by an ear–nose–throat physician and a gastroenterologist, respectively, and no pathology was determined at the first hospital to which she was admitted. During the hospitalization period, she was adherent to her asthma therapy. There were no other diagnostic criteria supporting allergic bronchopulmonary aspergillosis and Churg-Strauss syndrome. She was not taking any kind of medication ( e.g. , β-blocker, angiotensin-converting enzyme inhibitor, or nonsteroidal anti-inflammatory drug) that could exacerbate asthma. No clinical or laboratory finding of thyrotoxicosis or infection was present. We decided to make the differential diagnosis of a possible chest mass based on the left hilar opacity observable from chest radiography. In the light of computerized tomography, we performed a fiberoptic bronchoscopy and made the diagnosis of carcinoid tumor by bronchoscopic biopsy specimen.
Pulmonary carcinoids are generally located centrally in the main or lobar bronchi. 17 , 18 Available specimens for pathological examination can generally be provided from fiberoptic bronchoscopy and histopathological diagnosis is easily achieved. In this case, the tumor was located in the left lower bronchus and could easily be seen during fiberoptic bronchoscopic examination. A biopsy specimen was not taken because carcinoid tumors are highly vascularized and there is a risk for hemorrhage in nearly one-fourth of cases. 4 , 19 Furthermore, some authors advise against performing biopsies with flexible bronchoscopes. 20
Because treatment options differ according to tumor type, determining a tumor's histological type is important. In this case, the microscopic, morphological, and immunohistochemical features were characteristic for pulmonary carcinoid tumor. Pulmonary carcinoid tumors are divided into low-grade TCs and intermediate-grade ACs based on histopathological criteria. A typical pulmonary carcinoid tumor shows no focal necrosis and rare mitosis whereas an atypical pulmonary carcinoid tumor shows either focal necrosis or mitosis numbering between 2 and 10/mm 2 . 21 , 22 In our case, the absence of mitosis and necrosis with the characteristic morphological and immunohistochemical features were compatible with a low-grade typical pulmonary carcinoid tumor.
Surgery is the main choice for treatment of carcinoid tumors. In general, radical excision with detailed lymph node sampling is recommended. 8 In patients with a centrally located typical pulmonary carcinoid, bronchial sleeve resection or sleeve lobectomy is preferred. Despite its having a low recurrence rate, peripherally located TCs should be thought of as low-malignant tumors and resected anatomically. A more extensive surgical approach is recommended in AC tumors. 18 Our patient was treated with left lower lobectomy and mediastinal lymph node dissection and had experienced no recurrence for 30 months.
This case is an example of the importance of making a good differential diagnosis and confirming a diagnosis of asthma. Asthma unresponsive to treatment should alert clinicians to the possibility of differential diagnoses of other reasons for airway obstruction. Consequently, we strongly support the view that diagnosis confirmation is essential in patients with uncontrolled asthma before trying more expensive treatments.
The authors have no conflicts of interest to declare pertaining to this article

COMMENTS
Case presentation and patient history. Mr. T is a 40-year-old recreational athlete with a medical history significant for asthma, for which he has been using an albuterol rescue inhaler ...
The patient's asthma control was assessed and management optimised in two structured reviews. ... Effective implementation of this recommendation for the lady in this case study is considered ...
The patient is a 60-year-old white female presenting to the emergency department with acute onset shortness of breath. Symptoms began approximately 2 days before and had progressively worsened with no associated, aggravating, or relieving factors noted. She had similar symptoms approximately 1 year ago with an acute, chronic obstructive pulmonary disease (COPD) exacerbation requiring ...
Dr. Wechsler: This case highlights the problem of asthma-associated morbidity and mortality, with more than 4000 deaths from asthma occurring annually in the United States. 1 This patient's ...
CASE REPORT. In January 2020, a 53-year-old gentleman with a background of asthma on long-term low dose inhaled corticosteroid inhaler had an acute exacerbation of his asthma in February 2020 triggered by a viral upper respiratory tract infection and acute sinusitis and was managed with bronchodilator nebulization and a 7-day course of oral prednisone 30 mg daily.
CASE 1 DIAGNOSIS: ALLERGY BULLYING ... The patient's asthma exacerbation resolved with conventional asthma treatment. Her pulmonary function tests were nonconcerning (forced expiratory volume in 1 s 94% and 99% of predicted) after her recovery. ... Population-based studies have shown that 20% to 35% of children with allergies experience ...
In a study involving patients who were assigned to receive maintenance therapy with budesonide-formoterol, there were fewer occurrences of severe asthma exacerbations among patients assigned to ...
Children with poor asthma control have an increased risk of severe exacerbations and progressive loss of lung function, which results in the relevant use of health resources and impaired quality of life (QoL) [].Therefore, the primary goal of asthma management at all ages is to achieve disease control [2,3,4].According to recent international guidelines, patients with uncontrolled asthma ...
Clinical presentation (recurrent, episodic attacks of wheezing, cough, dyspnea, itchy red eyes, nasal discharge, stuffiness, and chest tightness), and PFT findings (FEV1/FVC of 0.65, FEV1 is 60% of predictive and post-bronchodilator therapy the FEV1 increases to 74% of predictive) are consistent with the diagnosis of asthma.
Current studies show that Sevoflurane has significant bronchodilator properties and is an effective treatment option for severe acute asthma before rescue therapies [9]. The beneficial effect of Sevoflurane in our case is supported by alveolar unit and distal airway dilation, which reduce distortion of the surrounding parenchyma and amount of ...
Quick-Relief Medication for All Patients: SABA as needed for symptoms. Intensity of treatment depends on severity of symptoms: up to 3 treatments at 20-minute intervals as needed. Short course of systemic oral corticosteroids may be needed. Caution: Increasing of b-agonist or use >2x/week for symptoms control indicates.
Resources for patients about pulmonary rehabilitation; Resources for professionals about pulmonary rehabilitation; COPD Magazine; Diagnosis; Respiratory infections; Tobacco dependence; ... Clinical case study - asthma . 2019 . Clinical Case Study - Asthma. pdf. Clinical Case Study - Asthma. 6.34 MB. Resource information. Respiratory conditions ...
Purpose: To review the pathophysiology of asthma, present a case study, and provide management strategies for treating this common, yet complex disorder in children and adults. Data sources: Selected clinical guidelines, clinical articles, and research studies. Conclusions: Asthma is a chronic inflammatory airway disorder with acute exacerbations that currently affects approximately 14 million ...
In a prospective study 7 of 18 patients with acute exacerbation of asthma, attending the emergency department, the change in lactate levels after administering 1200 µg of inhaled salbutamol over 120 min was assessed. Fifty per cent of patients had increased lactate levels of over 2.5 mmol/L, and were over 4 mmol/L in four of these.
BACKGROUND Severe life threatening asthma (SLTA) is important in its own right and as a proxy for asthma death. In order to target hospital based intervention strategies to those most likely to benefit, risk factors for SLTA among those admitted to hospital need to be identified. A case-control study was undertaken to determine whether, in comparison with patients admitted to hospital with ...
There has been concern in recent years regarding rising rates of asthma morbidity and mortality ().The reasons for this are unclear. Case-control studies using retrospectively collected data have suggested that excess use of short-acting β-agonists (4, 5) and underuse of inhaled corticosteroids may be associated with this phenomenon, although more recent data suggest that patterns of β ...
The aim of this study was to assess the determinant factors of acute asthma attack among adult asthmatic patients visiting general hospitals of central zone, Tigray, Ethiopia, 2019. Hospital based unmatched case control study design was conducted in general hospitals of central zone of Tigray, Ethiopia 2019.
Seven cohort/nested case-control studies with 1 405 508 individuals were included. ... The aim of this systematic review was to assess the risk of AF in patients with asthma in observational ...
Methods: A case-control study to determine the level of the serum trace elements with 838 subjects (n = 242 asthma patients, n = 140 asthmatic obese, n = 185 obese patients, and n = 271 controls) between the ages of 20 and 60 years was carried out. Asthma was diagnosed based on the clinical examination and pulmonary function tests.
Asthma is estimated to affect ~ 11% of the Canadian population, or nearly 250,000 Canadians [] with long-standing estimates of ~ 5-10% of asthma patients with severe disease.It is a complex, heterogenous, and dynamic disease that is classified by treatment measures necessary for management: Severe asthma is defined by the Canadian Thoracic Society (and GINA) as requiring a high-dose inhaled ...
Isolated respiratory tract microorganisms and clinical characteristics in asthma exacerbation of obese patients: a multicenter study BMC Pulm Med. 2024 Feb 2 ... Results: A total of 171 obese and 236 non-obese patients with asthma were included for analysis. Compared to non-obese patients, obese patients were associated with women (77.2% vs. 63 ...
Case Presentation. History of Present Illness: A 33-year-old white female presents after admission to the general medical/surgical hospital ward with a chief complaint of shortness of breath on exertion. She reports that she was seen for similar symptoms previously at her primary care physician's office six months ago.
The number of cases of asthma patients has been on rise globally due to several factors. The available scanty information concerning attributes of certain genes in asthma's pathophysiology is a blockade in its early diagnosis and treatment. The objective of present study was to investigate roles of IL-17A and IL-17F in occurrence and severity of asthma in North Indian population. This study ...
CASE STUDY 2 Patient Presentation. A woman in her mid 80s presented with a history of dyslipidemia, breast cancer, and active smoking. She presented to our clinic with bilateral lifestyle-limiting claudication, and an arterial duplex ultrasound revealed a > 75% stenosis of the right SFA with monophasic waveforms. Additionally, her right-sided ...
CDSS has traditionally been seen as technology to support clinicians, but can also promote patient‐focussed practice 37 through the involvement of patients in decision‐making about their health and well‐being. 38 , 39 In this study, participants liked the idea of visualizing the probability of asthma, seeing simulations of lung physiology ...
The diagnosis was confirmed postmortem as sporadic CJD with homozygous methionine at codon 129 (sCJDMM1). The patient's history, including a similar case in his social group, suggests a possible novel animal-to-human transmission of CWD. Based on non-human primate and mouse models, cross-species transmission of CJD is plausible.
A 48-year-old female patient with uncontrolled severe asthma was referred to our hospital for anti-IgE therapy. She was suffering with persistent wheezing and dyspnea after a severe asthma attack that had taken place 5 months previously. Her asthma had not been controlled with adequate asthma treatment, including budesonide at 320 μg ...