50+ Remarkable Chemistry Project Topics for BSC Students: Chemical Kinetics

- Post author By admin
- October 6, 2023
Explore a comprehensive list of chemistry project topics for BSC students. Enhance your knowledge and excel in your academic pursuits.
Welcome to the captivating world of chemistry! For Bachelor of Science (BSC) students, the journey through the diverse landscapes of chemical science is an exciting adventure.
Central to this journey are chemistry projects—opportunities for hands-on exploration, experimentation, and discovery.
Yet, the secret to a truly rewarding project lies in the choice of the right topic—one that not only aligns with academic goals but also stirs up genuine curiosity and enthusiasm.
In this article, we’re about to embark on an inspiring quest through a specially curated list of chemistry project topics, tailor-made for BSC students like you.
These topics promise not only to enhance your academic journey but also to kindle your passion for the captivating world of chemistry.
So, let’s dive in and explore the boundless possibilities and wonders that await in the realm of chemistry projects!
Table of Contents

What is Chemistry Project Topics ?
Chemistry, often dubbed the central science, has its fingerprints on virtually every facet of our lives. It’s the hidden force behind the scents we love, the reactions that fuel our cars, and even the medicines that keep us healthy.
Now, suppose this: BSC students are at the forefront of this captivating science, armed with a unique chance to dive headfirst into its various branches through project work.
These projects aren’t just your run-of-the-mill assignments; they’re like scientific adventures.
They do much more than boost your knowledge; they’re contributions to the grand tapestry of scientific discovery. So, imagine being part of this world, where you not only learn but also shape the future of chemistry!
The Importance of Choosing the Right Chemistry Project
Have a close look at the importance of choosing the right chemistry project:-
Personal Engagement
A well-suited project captures your interest and keeps you engaged throughout, making your academic journey more enjoyable.
It should align with your coursework and academic goals, ensuring that your efforts contribute meaningfully to your education.
Contribution
Choosing the right project means you’re not just benefiting yourself; you’re also adding to the body of scientific knowledge and benefiting the broader scientific community.
Skill Development
The right project challenges you, helping you acquire and refine valuable skills essential for your academic and professional growth.
When you’re passionate about your project, it transforms the work into a thrilling journey filled with curiosity, discovery, and enthusiasm.
In summary, the importance of selecting the right chemistry project goes beyond academics; it influences your engagement, relevance, contribution, skill development, and passion, enriching your scientific experience and personal growth.
Chemistry Project Topics for BSC Students
Here are Chemistry Project Topics for BSC Students:-
Organic Chemistry Projects
- Synthesis of Aspirin: Investigate the synthesis process, purity, and properties of this widely used pain reliever.
- Extraction of Natural Pigments: Explore the extraction of pigments from various plants and assess their applications in dyes and cosmetics.
- Analysis of Essential Oils: Analyze the chemical composition of essential oils from different sources and study their potential medicinal properties.
- Green Chemistry: Investigate environmentally friendly synthesis methods and processes in organic chemistry.
- Organic Synthesis of Pharmaceuticals: Design and synthesize organic compounds with potential pharmaceutical applications.
- Study of Aromatic Compounds: Explore the properties and reactions of aromatic compounds, such as benzene and its derivatives.
- Polymer Chemistry: Investigate the synthesis and properties of polymers, including their applications in various industries.
- Organic Chemistry of Natural Products: Analyze the chemical makeup of natural products like alkaloids, terpenes, and flavonoids.
- Organometallic Chemistry: Study the bonding and reactivity of compounds containing metal-carbon bonds.
- Organic Photochemistry: Explore the effects of light on organic compounds and their photochemical reactions.
Inorganic Chemistry Projects
- Synthesis of Metal Complexes: Investigate the preparation and characterization of metal complexes with ligands of varying structures.
- Coordination Chemistry: Explore the coordination behavior of transition metal ions with different ligands.
- Inorganic Synthesis of Nanoparticles: Synthesize and characterize metal or metal oxide nanoparticles with potential applications in catalysis or nanotechnology.
- Study of Lanthanides and Actinides: Investigate the properties and applications of lanthanide and actinide series elements.
- Inorganic Reaction Mechanisms: Analyze the reaction mechanisms of various inorganic reactions, such as redox reactions or ligand substitution reactions.
- Organometallic Synthesis: Study the synthesis and reactivity of organometallic compounds containing metal-carbon bonds.
- Bioinorganic Chemistry: Explore the role of metal ions in biological systems and their significance in biochemical processes.
- Main Group Chemistry: Investigate the chemistry of main group elements and their compounds.
- Inorganic Synthesis of Coordination Polymers: Synthesize and characterize coordination polymers with unique structures and properties.
- Supramolecular Chemistry: Study non-covalent interactions in inorganic chemistry, such as host-guest complexes and molecular recognition.
Physical Chemistry Projects
- Chemical Kinetics: Investigate the rate of chemical reactions under different conditions and analyze reaction mechanisms.
- Electrochemistry: Explore the principles of electrochemical cells, study electrode processes, and investigate applications in energy storage.
- Thermodynamics of Reactions: Study the thermodynamic parameters of chemical reactions, including enthalpy, entropy, and Gibbs free energy.
- Quantum Chemistry: Apply quantum mechanical principles to predict molecular structures and electronic properties of chemical compounds.
- Statistical Mechanics: Explore the statistical behavior of particles in systems, including the Boltzmann distribution and partition functions.
- Surface Chemistry: Investigate the physical and chemical properties of surfaces and interfaces, including adsorption and catalysis.
- Chemical Thermodynamics: Study the thermodynamic properties of chemical systems and phase equilibria.
- Spectroscopy and Molecular Structure: Analyze the interaction of matter with electromagnetic radiation and determine molecular structures.
- Chemical Equilibrium: Investigate chemical equilibrium and the factors that influence it in various chemical reactions.
- Photochemistry: Explore the effects of light on chemical reactions, including photochemical mechanisms and applications.
These diverse project topics encompass a wide range of subfields within chemistry, offering BSC students opportunities for hands-on exploration and research in their chosen area of interest.
How to Select the Ideal Chemistry Project Topic?
Selecting the ideal chemistry project topic is a crucial step that can significantly impact your academic journey and research experience. Here’s a guide on how to make the right choice:
Personal Interest
Start by considering your personal interests within the field of chemistry. What topics or areas intrigue you the most? Projects aligned with your passions are more likely to keep you motivated and engaged throughout.
Academic Alignment
Ensure that the chosen topic aligns with your coursework and academic goals. It should complement your studies and contribute to your overall understanding of chemistry.
Research Existing Knowledge
Before finalizing a topic, research existing literature and studies in that area. Understanding what has already been explored can help you identify gaps in knowledge or areas where further investigation is needed.
Consult with Professors
Seek guidance from your professors or mentors. They can provide valuable insights into potential project topics, offer suggestions, and help you refine your ideas.
Available Resources
Consider the resources available to you, including laboratory equipment, chemicals, and access to research materials. Ensure that your chosen project is feasible within your academic environment.
Scope and Complexity
Assess the scope and complexity of the project. It should be challenging enough to stimulate your intellectual growth but not so complex that it becomes unmanageable.
Relevance and Impact
Think about the broader relevance and potential impact of your project. How does it contribute to the field of chemistry or address real-world issues? Projects with practical applications or scientific significance can be particularly rewarding.
Feasibility
Evaluate the feasibility of your project in terms of time, budget, and available support. Ensure that you have a clear plan for conducting experiments and gathering data.
Ethical Considerations
Be aware of any ethical considerations related to your project, especially if it involves human subjects, animals, or hazardous materials. Ensure that your research adheres to ethical guidelines.
Flexibility
Keep some degree of flexibility in your project plan. Research may take unexpected turns, and being adaptable can help you navigate challenges and make the most of unexpected discoveries.
Passion and Curiosity
Choose a topic that genuinely excites your curiosity. A project driven by passion often leads to more enthusiastic and successful research.
Peer Feedback
Discuss your ideas with peers or fellow students. Their perspectives and feedback can offer valuable insights and help you refine your project concept.
By carefully considering these factors and conducting thorough research, you can select an ideal chemistry project topic that not only aligns with your interests and academic goals but also offers a rewarding and enriching research experience.
Tips for Successful Project Execution
Have a close look at the tips for successful project execution:-
Detailed Planning
Start with a well-structured project plan. Define your objectives, set clear goals, and create a timeline outlining each phase of your project.
Research Extensively
Before conducting experiments, thoroughly research the relevant literature to understand existing knowledge and methodologies related to your topic.
Prioritize safety at all times. Familiarize yourself with safety protocols, wear appropriate protective gear, and handle chemicals and equipment with care.
Experimental Design
Design your experiments carefully, considering variables, controls, and potential sources of error. Consult with professors or advisors for input on your experimental setup.
Data Collection
Maintain accurate and organized records of your experiments, including measurements, observations, and any unexpected results.
Analytical Tools
Utilize appropriate analytical tools and techniques for data analysis. This may involve statistical analysis, spectroscopy, chromatography, or other methods depending on your project.
Troubleshooting
Be prepared to encounter challenges during experiments. Develop problem-solving skills and seek guidance from mentors or colleagues when needed.
Regular Updates
Keep your professors or advisors informed of your progress. Regular meetings can provide valuable feedback and help you stay on track.
Documentation
Create a detailed laboratory notebook or digital records that document your procedures, results, and any modifications made during the project.
Data Interpretation
Analyze your data critically and draw meaningful conclusions. Discuss your findings with mentors and peers to gain different perspectives.
Adaptability
Be flexible in your approach. If your initial experiments do not yield the expected results, be open to adjusting your methods or hypotheses.
Time Management
Manage your time effectively to meet project milestones and deadlines. Avoid procrastination and allocate sufficient time for analysis and report writing.
Communication Skills
Develop strong communication skills to convey your research findings clearly and effectively, both in written reports and oral presentations.
Collaboration
Collaborate with colleagues or fellow students when applicable. Sharing ideas and resources can enhance the quality of your research.
Continuous Learning
Stay updated with the latest developments in your field through scientific journals, conferences, and discussions with experts.
Ethical Conduct
Adhere to ethical guidelines and principles in your research. Ensure that your work is conducted with integrity and honesty.
Feedback Incorporation
Embrace constructive feedback from mentors, peers, or reviewers, and use it to improve your project and research skills.
Celebrate Milestones
Acknowledge and celebrate your achievements and milestones throughout the project. It can boost motivation and morale.
Stay Organized
Maintain a well-organized workspace and records. A tidy and systematic approach can save time and prevent errors.
Reflect and Learn
After completing your project, reflect on your experiences and lessons learned. Consider how you can apply these insights to future research endeavors.
By following these tips and maintaining a dedicated and systematic approach, you can enhance the chances of successful project execution in the field of chemistry.
| : |
Benefits of Chemistry Projects for BSC Students
Certainly, here are the benefits of chemistry projects for BSC (Bachelor of Science) students:
Hands-On Experience
Chemistry projects provide students with practical, hands-on experience in conducting experiments, handling chemicals, and using laboratory equipment. This experience is invaluable for future careers in science.
Deeper Understanding
Engaging in research projects allows students to delve deeper into specific areas of chemistry, gaining a more profound understanding of concepts and theories.
Problem-Solving Skills
Projects often involve troubleshooting and problem-solving, honing students’ critical thinking and analytical skills . They learn to overcome challenges and adapt their approaches.
BSC students acquire a wide range of laboratory and research skills, including data collection, analysis, and interpretation. These skills are transferable and valuable in various scientific fields.
Research Ethics
Students learn about research ethics, including responsible conduct and the importance of integrity in scientific inquiry.
Scientific Method
Projects follow the scientific method, teaching students how to formulate hypotheses, design experiments, and draw conclusions based on evidence.
Encouragement to explore unique topics fosters creativity and innovation. Students may discover new approaches or solutions to existing problems.
Interdisciplinary Learning
Chemistry projects often intersect with other scientific disciplines, providing opportunities for interdisciplinary learning and collaboration.
Publication and Presentation
Successful projects can lead to publications or presentations at conferences, enhancing students’ academic and professional portfolios.
Career Preparation
The skills and experiences gained from chemistry projects prepare students for careers in research, academia, industry, or healthcare.
Increased Confidence
Completing a project independently or as part of a team boosts students’ confidence in their abilities to tackle complex scientific challenges.
Projects often involve interaction with professors, mentors, and peers, helping students build a professional network within the scientific community.
Resume Enhancement
A well-executed project can serve as a strong addition to a student’s resume or graduate school application, setting them apart from their peers.
Real-World Applications
Many chemistry projects have real-world applications, allowing students to see the practical relevance of their studies.
Contributions to Knowledge
Students may make meaningful contributions to the field of chemistry by generating new data, theories, or insights.
Personal Fulfillment
Successfully completing a challenging project can provide a sense of personal fulfillment and accomplishment.
Preparation for Advanced Degrees
For those considering postgraduate studies, chemistry projects provide valuable research experience and strengthen applications for advanced degrees.
Critical Evaluation
Students learn to critically evaluate existing literature and research, improving their ability to assess scientific claims and findings.
Teamwork and Leadership
Collaborative projects enhance teamwork and leadership skills, important attributes for any career path.
Life-Long Learning: Engaging in research projects fosters a love for learning and encourages students to continue exploring and discovering throughout their careers.
What is the best topic for chemistry project?
Selecting the right chemistry project topic is crucial for a successful project. The ideal topic should align with your interests, offer access to ample research materials, and be suitable for your skill level and experience.
Here are some ideas to consider for chemistry projects:
Chemical Composition Analysis
Investigate the chemical composition of a commonly used household product. This can provide insights into the ingredients and their properties.
Factors Affecting Chemical Reactions
Explore how various factors, such as temperature or pH levels, impact a chemical reaction. This research can reveal the variables influencing reaction outcomes.
Innovative Compound Synthesis
Develop a novel method for synthesizing a chemical compound. This project offers an opportunity to innovate and create something new.
Material Properties Study
Study the properties of a recently discovered material. This can involve characterizing its physical, chemical, and structural attributes.
Experimental Hypothesis Testing
Design and conduct an experiment to test a scientific hypothesis related to chemistry. This approach allows you to apply the scientific method.
If you find yourself unsure about the right topic, consider seeking suggestions from your teacher or browsing the internet for a wealth of chemistry project ideas.
Remember, the key is to choose a topic that sparks your curiosity and aligns with your abilities, ensuring a rewarding and successful project.
What are hot topics in chemistry?
In the realm of chemistry, 2023 brings forth some scintillating and cutting-edge areas of research:
Sustainable Chemistry
With a laser focus on eco-friendliness, sustainable chemistry aims to birth cleaner chemical processes and products. Think novel catalysts for green energy, inventive techniques for recycling and waste reduction, and biodegradable, non-toxic materials.
Materials Science
This arena is all about crafting and scrutinizing new materials, from polymers to metals, ceramics, and composites. Researchers are fashioning materials for advanced batteries, solar cells, medical devices, and robust, lightweight structural applications.
Biochemistry
At the intersection of chemistry and life itself, biochemistry explores the intricate chemistry of living organisms.
Dive into the study of proteins and enzymes, the development of groundbreaking drugs and therapies, and the engineering of microorganisms to yield valuable products.
Quantum Chemistry
The captivating fusion of quantum mechanics and chemistry gives birth to groundbreaking methods for simulating and predicting molecular properties. Think about the design and synthesis of new materials and the rise of quantum computing.
Artificial Intelligence (AI)
AI’s infusion into the chemistry landscape is revolutionary. It’s shaping the development of next-gen drugs that are both potent and gentle, as well as the creation of robust, lightweight materials.
Moreover, AI is predicting chemical reaction outcomes, optimizing processes, and pushing the boundaries of innovation.
These are just a glimpse into the dynamic world of chemistry research in 2023. It’s a vast and swiftly evolving domain, teeming with opportunities for groundbreaking discoveries and scientific progress.
What is an example of a chemistry topic?
A chemistry topic worth exploring is the impact of temperature on chemical reaction rates. This intriguing area can be probed through experimentation.
Imagine having two identical sets of reactants, each subjected to different temperatures, with the reaction rate meticulously measured at each temperature point.
The data collected can then be plotted on a graph, revealing the relationship between reaction rate and temperature.
This graphical representation can unveil critical insights, including the activation energy of the reaction and how the reaction rate fluctuates at varying temperatures.
Another captivating chemistry topic involves the synthesis of aspirin, a widely used pain reliever. Aspirin can be created through the reaction of acetic anhydride and salicylic acid.
Delving into this process entails carefully combining the two reactants in precise proportions and subjecting them to specific conditions.
The resulting product can then undergo purification and rigorous analysis to ascertain its purity and identity.
These examples merely scratch the surface of the diverse world of chemistry topics. The field encompasses an array of areas ripe for exploration, such as:
- Unraveling the mysteries of matter’s structure and properties.
- Exploring the intricacies of chemical bonding.
- Unearthing the mechanisms behind chemical reactions.
- Probing the fascinating realms of thermodynamics and kinetics.
- Delving into the electrifying world of electrochemistry.
- Mastering the art of analytical chemistry.
- Navigating the intricate pathways of organic and inorganic chemistry.
- Investigating the physical forces that drive chemical phenomena.
- Exploring the chemistry of life itself through biochemistry.
The specific chemistry topic you choose to explore should align with your interests and objectives. If you’re keen on delving deeper into a particular facet of chemistry, consider perusing research papers, articles, and discussions on the subject.
Engaging with your teacher or a knowledgeable chemistry professor can also provide valuable guidance and suggestions.
Which is the best project in MSC chemistry?
Selecting the perfect M.Sc. chemistry project is a crucial step in your academic journey. It should both captivate your interest and pose a satisfying challenge.
Equally important is the feasibility of completing the project within the confines of your program’s time constraints.
Consider these ideas for M.Sc. chemistry projects:
Embark on the creation of a groundbreaking method for synthesizing a chemical compound, pushing the boundaries of chemical innovation.
Material Exploration
Dive into the study of a novel material’s properties, shedding light on its characteristics and potential applications.
Design and execute experiments aimed at testing scientific hypotheses, employing meticulous methods and precise data analysis.
Factors Shaping Reactions
Investigate the intricate dance of different factors, such as temperature or pH levels, on the outcomes of chemical reactions, revealing the secrets of chemical kinetics.
Complex Sample Analysis
Analyze the intricate chemical composition of complex samples like plant extracts or biological fluids, offering insights into the mysteries of nature.
Analytical Advancements
Pave the way for cutting-edge analytical methods capable of detecting or quantifying specific chemical compounds with precision.
Therapeutic Innovation
Design and synthesize a new pharmaceutical or therapeutic agent, potentially impacting healthcare and medicine.
Molecular Insights
Delve deep into the molecular mechanisms underlying biological processes like photosynthesis or cell signaling, unraveling nature’s secrets.
Computational Chemistry
Forge new frontiers in computational chemistry by developing methods to predict the properties of molecules or materials.
Environmental Impact Assessment
Scrutinize the environmental consequences of chemicals or chemical processes, contributing to sustainability efforts.
Champion sustainability by crafting novel chemical processes or products that are gentle on the planet.
If you find yourself uncertain about the ideal topic, engage in discussions with your advisor or other seasoned professors within your department.
They possess valuable insights and can help pinpoint a project that aligns seamlessly with your interests and expertise.
Once you’ve chosen your focus, meticulously craft a research plan. Outline your research question, delineate the research methods, establish a timeline for completion, and identify necessary resources, including equipment, materials, and potential funding.
With your advisor’s approval, embark on your project, keeping detailed records of your work and maintaining regular communication with your mentor.
Upon project completion, compile your findings into a comprehensive thesis or dissertation. Additionally, consider presenting your research at seminars or conferences, sharing your discoveries with the scientific community.
Undertaking an M.Sc. chemistry project is a formidable yet gratifying endeavor. It’s an opportunity to cultivate new skills, conduct independent research, and contribute meaningfully to the realm of chemistry.
In wrapping up, the world of chemistry is like an endless playground for BSC students, filled with intriguing possibilities waiting to be explored.
Think of it as your chance to embark on a captivating adventure where every project is a new chapter in your scientific journey.
Choosing the right topic is your compass, guiding you toward a project that not only aligns with your interests but also fuels your academic ambitions. Remember, it’s not just an academic checkbox; it’s your gateway to an exhilarating exploration.
As you dive into your chosen project, consider it a rendezvous with curiosity, a chance to develop invaluable skills, and an opportunity to contribute your unique brushstroke to the canvas of scientific knowledge.
Throughout this adventure, you’ll navigate the twists and turns of experimentation, data analysis, and the thrill of discovery. Your dedication and inquisitiveness will be your trusty companions on this scientific quest.
In the grand scheme of things, every chemistry project is a stepping stone towards a deeper comprehension of the marvelous world of molecules and reactions.
It’s your invitation to join a community of scientists, explorers of the unknown, and seekers of truth.
So, as you venture forth into your chemistry project as a BSC student, do so with a heart full of excitement and a mind buzzing with questions.
Your journey promises not only academic growth but also the potential to make your mark on the ever-evolving landscape of scientific understanding. Enjoy the ride!
Frequently Asked Questions
How do i choose the best chemistry project topic for me.
Consider your interests, available resources, and relevance to your coursework.
Can I collaborate with professors on a project?
Yes, collaborating with professors can provide valuable guidance and resources.
What are the key skills I can gain from a chemistry project?
Skills include research, experimentation, data analysis, and critical thinking.
Are there any online resources for chemistry project ideas?
Yes, various websites and academic journals offer project ideas.
Where can I find more information on project execution and methodology?
University libraries and online databases are excellent sources for project guidance.
- australia (2)
- duolingo (13)
- Education (283)
- General (78)
- How To (16)
- IELTS (127)
- Latest Updates (162)
- Malta Visa (6)
- Permanent residency (1)
- Programming (31)
- Scholarship (1)
- Sponsored (4)
- Study Abroad (187)
- Technology (12)
- work permit (8)
Recent Posts

BSc 1st Year Chemistry Notes PDF

Are you taking a chemistry class? Chemistry may be challenging, but there are many things you can do to help yourself succeed. Chemistry is one of those classes you either love or dread. At the high school level, chemistry is usually not a required course – it’s an elective. However, most prominent colleges require all undergraduate students to take at least one chemistry course as a necessity for graduation. If you plan on pursuing a career in medicine, engineering, or a field of natural science, then you’re likely going to be expected to take at least one chemistry course before you graduate.
Chemistry is a tough subject for most people, but it doesn’t have to be. The number one reason people struggle with chemistry is that they don’t approach it the right way and they don’t have access to the right notes and resources. Putting this into consideration, here on knowdemia, we have made BSc 1st year organic chemistry notes , BSc 1st year chemistry notes pdf , BSc chemistry practical notes part 1, BSc 1st year chemistry notes PDF , BSc 1st year physical chemistry notes , and other materials to help you pass chemistry.
Below we’ll explore good chemistry notes for BSc 1st year 2022, which will help you to study and learn chemistry.
Table of Contents
Inorganic Chemistry Syllabus
| 1. Atomic Structure | 1. Chemical Bonding |
| 2. Periodic Properties | 2. Ionic Solids |
| 3. Redox Reaction I | 3. s-Block Elements |
| 4. Chemical Bonding | 4. p-Block Elements |
| 5. Metallurgical Processes |
Organic Chemistry Syllabus
| 1. Structure & Bonding | 1. Alkenes, Cycloalkenes, Dienes & Alkynes |
| 2. Mechanism of Organic Reactions | 2. Arenes & Aromaticity |
| 3. Stereochemistry of Organic Compounds | 3. Alkyl & Aryl Halides |
| 4. Alkanes & Cycloalkanes |
Physical Chemistry Syllabus
| 1. Gaseous State | 1. Chemical Kinetics & Catalysis |
| 2. Liquid State | 2. Thermodynamics I |
| 3. Solid State | |
| 4. Colloidal State |
BSc 1st Year Chemistry Notes Download (PDF)
Click on the below links to download notes.
Inorganic Chemistry Notes:
| 1. Atomic Structure | |
| 2. Periodic Properties | |
| 3. Redox Reaction I | |
| 4. Chemical Bonding | |
| 5. Ionic Solids | Download |
| 6. s-Block Elements | |
| 7. p-Block Elements | |
| 8. Metallurgical Processes |
Organic Chemistry Notes:
| 1. Structure & Bonding | |
| 2. Mechanism of Organic Reactions | |
| 3. Stereochemistry of Organic Compounds | |
| 4. Alkanes & Cycloalkanes | |
| 5. Alkenes, Cycloalkenes, Dienes & Alkynes | |
| 6. Arenes & Aromaticity | |
| 7. Alkyl & Aryl Halides |
Physical Chemistry Notes:
| 1. Gaseous State | |
| 2. Liquid State | |
| 3. Solid State | |
| 4. Colloidal State | |
| 5. Chemical Kinetics & Catalysis | |
| 6. Thermodynamics I |
Atomic Structure & Chemical Bonding Notes pdf bsc 1st year
Atomic structure and chemical bonding notes pdf.
Free Atomic Structure and Chemical Bonding notes pdf are provided here for Atomic Structure and Chemical Bonding students so that they can prepare and score high marks in their Atomic Structure and Chemical Bonding exam.
In these free Atomic Structure and Chemical Bonding notes pdf, we will study the atom, which is a necessary pre-requisite in understanding the nature of chemical bonding in compounds. It provides basic knowledge about ionic, covalent, and metallic bonding and explains that chemical bonding is best regarded as a continuum between the three cases. It discusses the periodicity in properties with reference to the s and p block, which is necessary for understanding their group chemistry.
We have provided complete Atomic Structure and Chemical Bonding handwritten notes pdf for any university student of BCA, MCA, B.Sc, B.Tech, M.Tech branch to enhance more knowledge about the subject and to score better marks in their Atomic Structure and Chemical Bonding exam.
Free Atomic Structure and Chemical Bonding notes pdf are very useful for Atomic Structure and Chemical Bonding students in enhancing their preparation and improving their chances of success in Atomic Structure and Chemical Bonding exam.
These free Atomic Structure and Chemical Bonding pdf notes will help students tremendously in their preparation for Atomic Structure and Chemical Bonding exam. Please help your friends in scoring good marks by sharing these free Atomic Structure and Chemical Bonding handwritten notes pdf from below links:
Topics in our Atomic Structure and Chemical Bonding Notes PDF
The topics we will cover in these Atomic Structure & Chemical Bonding Notes PDF will be taken from the following list:
Atomic Structure: Recapitulation of Bohr’s theory, its limitations, and the atomic spectrum of hydrogen atom. Wave mechanics: de Broglie equation, Heisenberg’s Uncertainty Principle, and its significance. Schrödinger’s wave equation, the significance of ψ and ψ2 . Quantum mechanical treatment of H- atom, Quantum numbers, and their significance. Normalized and orthogonal wave functions. Sign of wave functions. Radial and angular wave functions for hydrogen atom. Radial and angular distribution curves. Shapes of s , p , and d o rbitals, Relative energies of orbitals. Pauli’s Exclusion Principle, Hund’s rule of maximum spin multiplicity, Aufbau principle, and its limitations.
Periodicity of Elements: Brief discussion of the following properties of the elements, with reference to s- & p -block and the trends shown: (a) Effective nuclear charge, shielding or screening effect, Slater rules, variation of effective nuclear charge in periodic table. (b) Atomic and ionic radii (c) Ionization enthalpy, Successive ionization enthalpies, and factors affecting ionization enthalpy and trends in groups and periods. (d) Electron gain enthalpy and trends in groups and periods. (e) Electronegativity, Pauling’s/ Allred Rochow’s scales. Variation of electronegativity with bond order, partial charge, hybridization, group electronegativity.
Ionic bond: General characteristics, types of ions, size effects, radius ratio rule and its limitations. Packing of ions in crystals. Born-Landé equation with derivation and importance of Kapustinskii expression for lattice energy. Madelung constant, Born-Haber cycle and its application, Solvation energy. Covalent character in ionic compounds, polarizing power and polarizability. Fajan’s rules and consequences of polarization.
Covalent bond : Valence Bond theory ( Heitler-London approach). Energetics of hybridization, equivalent and non-equivalent hybrid orbitals. Bent’s rule, Resonance and resonance energy. Ionic character in covalent compounds: Bond moment and dipole moment. Percentage ionic character from dipole moment and electronegativity difference. Molecular orbital theory. Molecular orbital diagrams of diatomic and simple polyatomic molecules N2, O2, C2, B2, F2, CO, NO, and their ions; HCl (idea of s-p mixing and orbital interaction to be given).
VSEPR Theory : Lewis structure, Valence shell electron pair repulsion theory (VSEPR), shapes of the following simple molecules and ions containing lone pairs and bond pairs of electrons: H2O, NH3, PCl3, PCl5, SF6, ClF3, I3- , BrF2+ , PCl6- ,ICl2- ICl4- , and SO4 2-.
Metallic Bond: Qualitative idea of valence bond and band theories. Semiconductors and insulators, defects in solids.
Weak Chemical Forces: v an der Waals forces, ion-dipole forces, dipole-dipole interactions, induced dipole interaction, Hydrogen bonding (theories of hydrogen bonding, valence bond treatment). Effects of weak chemical forces, melting and boiling points, solubility, energetics of dissolution process.
Atomic Structure and Chemical Bonding Notes PDF FREE Download
Atomic Structure and Chemical Bonding students can easily make use of all these complete Atomic Structure and Chemical Bonding notes pdf by downloading them from below links:
Atomic Structure and Chemical Bonding Notes pdf Source: nptel.ac.in

Atomic Structure Chemistry Notes pdf for b.sc 1st year Source: egyankosh.ac.in
Atomic Structure Chemistry Notes pdf for b.sc 1st year Source: nios.ac.in

Atomic structure bsc 1st year notes pdf Source: nptel.ac.in

Atomic structure notes pdf b.sc 1st year Source: vssdcollege.ac.in
atomic structure bsc 1st year notes pdf Source: bits-pilani.ac.in

Atomic structure and bonding bsc 1st year notes pdf Source: vssut.ac.in
How to Download FREE Atomic Structure and Chemical Bonding Notes PDF?
Atomic Structure and Chemical Bonding students can easily download free Atomic Structure and Chemical Bonding notes pdf by following the below steps:
- Visit TutorialsDuniya.com to download free Atomic Structure and Chemical Bonding notes pdf
- Select ‘College Notes’ and then select ‘Chemistry Course’
- Select ‘Atomic Structure and Chemical Bonding Notes’
- Now, you can easily view or download free Atomic Structure and Chemical Bonding handwritten notes pdf
Benefits of FREE Atomic Structure and Chemical Bonding Notes PDF
Free Atomic Structure and Chemical Bonding notes pdf provide learners with a flexible and efficient way to study and reference Atomic Structure and Chemical Bonding concepts. Benefits of these complete free Atomic Structure and Chemical Bonding pdf notes are given below:
- Accessibility: These free Atomic Structure and Chemical Bonding handwritten notes pdf files can be easily accessed on various devices that makes it convenient for students to study Atomic Structure and Chemical Bonding wherever they are.
- Printable: These Atomic Structure and Chemical Bonding free notes pdf can be printed that allows learners to have physical copies of their Atomic Structure and Chemical Bonding notes for their reference and offline reading.
- Structured content: These free Atomic Structure and Chemical Bonding notes pdf are well-organized with headings, bullet points and formatting that make complex topics easier to follow and understand.
- Self-Paced Learning: Free Atomic Structure and Chemical Bonding handwritten notes pdf offers many advantages for both beginners and experienced students that make it a valuable resource for self-paced learning and reference.
- Visual Elements: These free Atomic Structure and Chemical Bonding pdf notes include diagrams, charts and illustrations to help students visualize complex concepts in an easier way.
We hope our free Atomic Structure and Chemical Bonding notes pdf has helped you and please share these Atomic Structure and Chemical Bonding handwritten notes free pdf with your friends as well 🙏
Download FREE Study Material App for school and college students for FREE high-quality educational resources such as notes, books, tutorials, projects and question papers.
If you have any questions feel free to reach us at [email protected] and we will get back to you at the earliest.
TutorialsDuniya.com wishes you Happy Learning! 🙂
Chemistry Notes
- Analytical Clinical Biochemistry Notes
- Atomic Structure & Chemical Bonding Notes
- Basic Analytical Chemistry Notes
- Biomolecules Notes
- Business Skills for Chemists Notes
- Coordination Chemistry Notes
- Conductance & Chemical Kinetics Notes
- Chemical Thermodynamics and its Applications Notes
- Chemical Technology & Society Notes
- Cheminformatics Notes
- Chemistry of Cosmetics & Perfumes Notes
- Fuel Chemistry Notes
- Green Methods in Chemistry Notes
- Halogenated Hydrocarbons and Oxygen Containing Functional Groups Notes
- Intellectual Property Rights Notes
- IT Skills for Chemists Notes
- Organic Chemistry Basics and Hydrocarbons Notes
- Organometallic Chemistry & Bioinorganic Chemistry Notes
- Pesticide Chemistry Notes
- Pharmaceutical Chemistry Notes
- Phase Equilibria and Electrochemical Cells Notes
- Polynuclear Hydrocarbons, Heterocyclic Chemistry, Alkaloids and Terpenes Notes
- Quantum Chemistry & Spectroscopy Notes
- S-block and P-block Elements Notes
- Spectroscopy & Applied Organic Chemistry Notes
- States of Matter & Ionic Equilibrium Notes
Atomic Structure and Chemical Bonding Notes FAQs
Q: Where can I get complete Atomic Structure and Chemical Bonding Notes pdf FREE Download?
A: TutorialsDuniya.com have provided complete Atomic Structure and Chemical Bonding free Notes pdf so that students can easily download and score good marks in your Atomic Structure and Chemical Bonding exam.
Q: How to download Atomic Structure and Chemical Bonding notes pdf?
A: Atomic Structure and Chemical Bonding students can easily make use of all these complete free Atomic Structure and Chemical Bonding pdf notes by downloading them from TutorialsDuniya.com
Software Engineering Projects with Source & Documentation

You will always find the updated list of top and best free Software Engineering projects with source code in an easy and quick way. Our Free Software Engineering projects list has projects for beginners, intermediates as well as experts to learn in 2023.
URL: https://www.tutorialsduniya.com/software-engineering-projects-pdf/
Author: Delhi University

- [email protected]
- Physics 101
- Chemistry 101
Calculus(Math) 101
- Analytical Geometry(Math) 102
Statistics 101
- Zoology 101
Geology 101
Microbiology 101.
- Scientific Communication 101
- Physics -201
- Chemistry - 201
Differential Equation - Math 202
- Linear Algebra - Math 201
- Statistics 201
- Zoology 201
- Geology 201
- Microbiology 201
- Applied Statistics 201
- Physics 301
- Space Science 305
- Chemistry 301
- Analytical Chemistry 305
Real Analysis 302
- Computer Programming 301
- Descrete Mathematics 304
- Zoology 301
- Natural Resource Management 303
- Evolution and Aromatic Plants 303
- Geology 301
- Geomorphology 303
- Microbiology 301
- Pharmaceutical Microbiology & Quality Management 303
- Research Methodology
- Chemistry Volume II
- Chemistry Volume III
- Design of Experiment
- Statistical Modeling
- Exploration Geology
- Mining Geology
- Engineering Geology
- Hydrogeology
- Computational Course
- Class 10(SEE)
- Driving License
- Entrance Preperation
- Loksewa Preperation
- Sikshak Sewa Preperation
- All courses
- Course bundles
- BSc 1st Year
- BSc 2nd Year
- BSc 3rd Year
- BSc 4th Year
- Find a tutor
This website uses cookies to personalize content and analyse traffic in order to offer you a better experience. Cookie policy
Nepal's no. 1 e learning platform
Explore thousands of courses for you at lowest price ever !

Expert instruction
Find the right course for you
14 Online courses
Explore a variety of fresh topics
Lifetime access
Learn on your schedule
Top courses
These are the most popular courses among listen courses learners worldwide

Applied Statistics BSc 2nd Year - Full Course & Free Notes
(1 Reviews)

In this Course, You will get Complete Lectures of BSc 2nd Year Applied Statistics along with Live Sessions, Chapterwise PDF notes, and other study materials.
53:59:44 Hours
Last updated Mon, 10-Jun-2024
- Expand your understanding of Statistics Learn about Mean Median Mode along with Probability Test Hypothesis with Different Testing Methods Clear the Concepts and do good on the Exam

BSc 1st Year Physics - Full Course & Free Notes
(0 Reviews)
In this Course, You will get Complete Lectures of BSc 1st Year Physics along with Live Sessions, Practical Exam Help, Chapterwise PDF notes, and other study materials.
38:51:09 Hours
Last updated Mon, 17-Jun-2024

BSc 1st Year Chemistry Full Course & Free Notes
You will get Complete Lectures of BSc 1st Year Chemistry along with Live Sessions, Practical Exam Help, Chapterwise PDF notes, and other study materials.
28:08:56 Hours
Last updated Fri, 21-Jun-2024

BSc 1st Year Math- Analytical Geometry Full Course & Free Notes
In this Course, You will get Complete Lectures of BSc 1st Year Analytical Geometry along with Live Sessions, Chapterwise PDF notes, and other study materials.
35:21:28 Hours

BSc 1st Year Math - Calculus Full Course & Free Notes
In this Course, You will get Complete Lectures of BSc 1st Year Calculus along with Live Sessions, Chapterwise PDF notes, and other study materials.
30:18:52 Hours

BSc 1st Year Zoology - Full Course & Free Notes
In this Course, You will get Complete Lectures of BSc 1st Year Microbiology along with Live Sessions, Practical Exam Help, Chapterwise PDF notes, and other study materials.
09:10:05 Hours
Last updated Fri, 31-May-2024

BSc 1st Year Botany - Full Course & Free Notes
You will get Complete Lectures of BSc 1st Year Botany along with Live Sessions, Practical Exam Help, Chapterwise PDF notes, and other study materials.
04:40:46 Hours

BSc 1st Year Statistics - Full Course & Free Notes
In this Course, You will get Complete Lectures of BSc 1st Year Geology along with Live Sessions, Practical Exam Help, Chapterwise PDF notes, and other study materials.
01:23:13 Hours

BSc 1st Year Geology - Full Course and Free Notes
In this Course, You will get Complete Lectures of BSc 1st Year Geology along with Live Sessions, Chapterwise PDF notes, and other study materials.
04:29:53 Hours

BSc 2nd Year Maths (Differential Equation) - Full Course & Free Notes
In this Course, You will get Complete Lectures of BSc 2nd Year Maths (Differential Equation), Live Sessions, Chapterwise PDF notes, and other study materials.
00:53:00 Hours
Last updated Thu, 06-Jun-2024
- Expand your understanding of Derivatives
- Learn about the order, Degree, and Linearity of Differential Equations
- Solving Differential Equations by Various Methods
- Clear the Concepts and do your best on the Exam
Top categories
Top 10 latest courses.
These are the most latest courses among listen courses learners worldwide

Research Methodology BSc 3rd Year - Full Course & Free Notes
In this Course You will get Complete Lectures on BSc 3rd Year Research Methodology along with Live Sessions, Chapterwise PDF notes and other study materials.
39:09:10 Hours
Last updated Thu, 20-Jun-2024
- BSc 3rd Year Course Structure
- BSc 3rd Year Research Methodology Syllabus
- Expand your understanding of Research & Statistics
- Learn about Research Phenomenon
- Clear the Concepts and do good on Exam

BSc 3rd Year Real Analysis - Full Course and Free Notes
In this Course, You will get Complete Lectures of Real Analysis BSc 3rd Year along with Live Sessions, Chapterwise PDF notes and other study materials.
39:12:54 Hours
Last updated Wed, 19-Jun-2024
- Course Structure of BSc 3rd Year
- Course Syllabus of BSc 3rd Year Real Analysis
- Expand your understanding of Calculus
- Learn about Real Numbers, Functions, Limits and more

BSc 1st Year Microbiology - Full Course & Free Notes
14:32:25 Hours
Our expert instructor
They efficiently serve large number of students on our platform

Test Teacher

Sushmita Shrestha

Santosh Subedi

Nabaraj Khanal

Manoj Acharya

Palpali Bharat

Hamro Master
Frequently asked questions.
Have something to know? Check here if you have any questions about us.
How Can I Access the Courses ?
After Purchasing the Course You will get enrolled and can access lecture notes & other study materials from the My Courses Section.
What's Included on the Course ? Do I get Live Classes & Notes ?
YES! After enrollment you will get: Complete Recorded Sessions of Each Chapter, Each Chapter Notes, Study Materials, Practical Exam Help & Live Sessions when it Starts.
Do I have to Pay extra For Notes & Live Classes after Enrolling on Recorded Sessions?
NO! all of the Resources are included on Our Single Package

Can I Access all Materials till my Exam ?
YES! All of the Resources are Available Till Exam.
How to Join Live Classes?
To Join Live Classes, There will be an Option for Live Classes on the Mobile App and the Website Learning page. we will also add you to Our Dedicated Paid Group where links get distributed.
Visit our latest blogs

BSc 2nd Year Notes
Methods of data summarization - applied statistics free notes.
Explore Complete Notes of Methods of Data Summarization, Applied Statistics Chapter 1, BSc 2nd Year ...
Sun, 02 Jun 2024

Applied Statistics BSc 2nd Year Complete Free Notes
Explore Complete Notes of Applied Statistics BSc 2nd Year on Hamromaster Platform. BSc 2nd Year Appl...

Tips & Tricks
Tu bsc entrance 2080 exam - complete guidelines.
Explore the TU BSc Entrance 2080 Exam Guidelines, which include an entrance notice, criteria, entran...
Fri, 31 May 2024
Learn New skills when and where you like.
Discover a world of learning opportunities through our upcoming courses, where industry experts.

Join now to start learning
Learn from our quality instructors!
Become a new instructor
Teach thousands of students and earn money!

Hamromaster is your beloved learning platform where you will get Class 10, Class 11, Class 12, BSC, BBS, BBA, Languages & Training Classes.
Useful links
- Become an instructor
- Privacy policy
- Terms and condition
- Refund policy
Subscribe to our newsletter
- © 2024 hamromaster | all rights reserved
Are you sure ?
B.Sc. Chemistry First Semester (I) Class Notes

Inorganic Chemistry Notes :
Inorganic Chemistry Units (Sem I & Sem II) | Download Links |
| 1. Atomic Structure |
|
| 2. Periodic Properties |
|
| 3. Redox Reaction I |
|
| 4. Chemical Bonding |
|
| 5. Ionic Solids | Download |
| 6. s-Block Elements |
|
| 7. p-Block Elements |
|
| 8. Metallurgical Processes |
|
Organic Chemistry Notes:
Organic Chemistry Units (Sem I & Sem II) | Download Links |
| 1. Structure & Bonding |
|
| 2. Mechanism of Organic Reactions |
|
| 3. Stereochemistry of Organic Compounds |
|
| 4. Alkanes & Cycloalkanes |
|
| 5. Alkenes, Cycloalkenes, Dienes & Alkynes |
|
| 6. Arenes & Aromaticity |
|
| 7. Alkyl & Aryl Halides |
|
Physical Chemistry Notes:
Physical Chemistry Units (Sem I & Sem II) | Download Links |
| 1. Gaseous State |
|
| 2. Liquid State |
|
| 3. Solid State |
|
| 4. Colloidal State |
|
| 5. Chemical Kinetics & Catalysis |
|
| 6. Thermodynamics I |
|
Post a Comment
Contact form.

- _9th Class Chemistry Notes
- _10th Class Chemistry Notes
- _11th Class Chemistry Notes
- _12th Class Chemistry Notes
- _BSc Chemistry Notes
- _MSc Chemistry Notes
- _Spectroscopy
- 🛒 Shop 🛍️
- _Chemistry Podcast
- _Science & Chemistry Quiz
- _Symbol and Formula
- _Chemistry Infographics
- _Chemistry Videos
- _Chemistry Terms
- _Download PDF Notes
- _Chemistry and Pharma Jobs
- _Famous scientists and their inventions
- _Foods for Good Health
- _Chemistry Lab Experiments
- _Financial Education
- _Science & Chemistry Web Stories
- _Interesting Facts of Elements
BSc1Year Atomic Structure
Atomic structure, atomic structure in chemistry, philosopher scientists and their ideas about matter & atom, 1. democritus.
| (or ). | |
2. Aristotle
| was made of four elements namely: | |
3. John Dalton
- In the nineteenth century John Dalton (1766–1844), marks the beginning of the progress of modern atomic theory.
- John Dalton challenges the Aristotelian theory. Dalton revived and revised Democritus’s ideas based on the results of scientific research he conducted.
- The ideas of Democritus’s and Dalton’s were similar. Dalton carried out a number of experiments that allowed him to refine and support his hypotheses.
- Dalton carried out numerous chemicals reactions where he was able to determine the mass ratios of different elements involves in the chemical reactions.
- The outcomes of his research are known as Dalton’s atomic theory, which he proposed in 1803.
- Based on Dalton’s atomic theory , John Dalton calculated the first relative weights of atoms.
- He assessed the atomic weights of some elements according to the mass ratios in which they combined; with the hydrogen atom taken as unity.

Dalton’s Atomic Theory
- Matter consists of atoms, which cannot be divided into simpler element. Therefore atoms can neither be created nor destroyed.
- Atoms of one element cannot be transformed into other atoms of an element. In chemical reactions, the atoms of an element can combine with same or other atoms of an element to form new substances.
- Atoms of an element have mass, physical and chemical properties and are different from atoms of any other element.
- Compounds are formed from a chemical reaction of a specific ratio of atoms of different elements.
How the Theory Explains the Mass Laws
Mass conservation, definite composition, multiple proportions, nuclear atom model.
- In 1855, Sir William Crookes carried out a series on experiment to investigate the behavior of heated metals in a vacuum. The experiments showed that a heated cathode produced radiation, which could make substances to emit light.
- The radiation emitted from the cathode is called cathode rays.
- According to some research, it was known that cathode rays could be deflected by a magnetic field and electric field, and they carried a negative charge (the cathode is the negatively charged electrode and because the beam originated from the cathode, it must therefore be negatively charged).
- Subscribe Chemistry Notes Info for more chemistry notes related to atomic structure and follow us on Facebook Page of ChemistryNotesInfo .
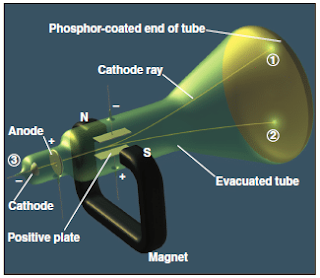
The cathode-ray tube
- In 1897, Joseph J. Thomson demonstrated that both the beam and the charged particles could be bent by an electrical field is applied perpendicular to the path of the beam, as shown in Figure 5 .
- Thomson used the cathode-ray tube to show the deflection of electron by an applied electric field.
- When the cathode is heated, cathode rays is produced which travel along the tube and hit the phosphor-coated end of the tube and emit a glowing spot of light (Figure 5).
- The rays produced at the negative electrode (cathode) and moved to the positive electrode (anode).
- It was concluded that cathode rays consist of negatively charged particles found in all matter.
- By varying the electric field strength and measuring the angle of deflection, Thomson was able to determine the charge-to-mass ( e / m ) ratio of the particles, which are known as electrons .
- Thomson measured the e / m ratio as −1.76 × 108 C/g.
- According to Thomson, the e / m ratio is larger than the one expected compared to the atomic weights of the lightest of atoms. Therefore this indicates that the negatively charged electrons must be much smaller in size than a typical atom.
- As a result of his experiment, Thomson proposed the plum pudding model of the atom, where the atom consisted of one or more of these tiny electrons distributed in a sea of positive charge.
Millikan’s oil-drop experiment for measuring an electron’s charge
Mass and charge of the electron.
- In 1897, the British physicist J. J. Thomson (1856–1940) measured the ratio of the mass of a cathode ray particle to its charge.
- By comparing this value with the mass/charge ratio for the lightest charged particle in solution, he estimated that the cathode ray particle weighed less than as much as hydrogen , the lightest atom.
- In 1909, the American researcher Robert Millikan designed an experiment that measured the charge on the electron.
- He did so by observing the movement of oil droplets in an apparatus that contained electrically charged plates and an x-ray source as shown in Figure 6 .
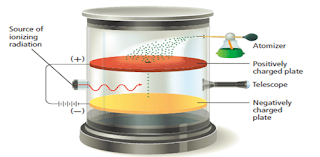
- This experiment also enabled the calculation of the mass of the electron based from its mass: charge ration obtained from J.J Thomson experiment.
- Millikan measured the charge on a great number of tiny oil drops which had been charged.
- An x-ray radiation is used to remove electrons from the gas molecules present in the chamber.
- The oil drops pick up the electrons present in the chamber, making them to become negatively charged.
- A small number of the oil drops sprayed into the box above the positively charged plate pass through the hole.
- When there is no electric field between the plates, the drops fall slowly with a steady velocity.
- An individual drop carrying a charge may be brought to rest by applying a voltage across the plates so that its weight ( acting downwards) is exactly balanced by the electrostatic force in it ( acting upwards).
- Millikan varies the voltage so that the oil droplet suspend in the air.
- Then he measured it total charge, by measuring the voltage needed to bring the drop to rest and the rate at which it falls when there is no voltage between the plates.
- Millikan was able to determine that each of the charged particles was some integral multiple of the electronic charge, which he determined to be −1.592 × 10−19 C, a measurement that is fairly close to the modern value for the charge on an electron (−1.60217733 × 10−19 C).
- As a drop could only pick up only whole number of electrons, this indicated that the charge on an individual electron must be -1.6 x 10-19.
- Therefore the value of electron’s charge is -1.602×10-19 C (C stands for coulomb, the SI unit of charge).
- Therefore the mass of the electron m e could be calculated using the electron’s mass; charge ration and the value for the electron’s charge.
The plum pudding model
- Thomson proposed the plum pudding model of the atom, where the atom consisted of one or more of these tiny electrons distributed in a sea of positive charge.
- Thomson thought that the positive charge necessary to counterbalance the negative charges of electrons in a neutral atom was in the form of a cloud.
- According to this model, an atom is spherical in shape and it consist of positive and negative charges equally distributed as shown in Figure 7 .

- In 1911, Ernest Rutherford (1871–1937) began to study how positively charged alpha particles interacted with solid matter.
- Rutherford carried out an experiment using a thin gold metal sheet to see if alpha particles would be deflected as they passed through the sheet.
Rutherford’s experiment
- The Rutherford’s gold fold experiment consists of a source of alpha particles, a thin gold sheet and a fluorescent screen. The screen consists of a phosphorescent coating of zinc-sulphide on its interior surface. The screen is circular in shape. The metal foil was mounted at the centre of the apparatus as shown in Figure 10 .
- When an energetic alpha particle struck the phosphorescent screen, a flash of light would be observed. By noting where the flashes occurred, the scientists could determine if the atoms in the gold foil deflected the alpha particles.
- Based on the plum pudding model, where the electrons were evenly dispersed in a sphere of positive charge, Rutherford expected that the heavier alpha particles should be able to pass through the atom with little or no deflections
- The diagram below ( Figure 8 )shows the results Rutherford expected from the experiment.

Rutherford’s model of the atom
- Rutherford demonstrated that the plum pudding model proposed by Thomson was false.
- Based on some calculations, they showed that the diameter of the nucleus was about five orders of magnitude smaller than that of the atom.
- Using this model, Rutherford showed that the nucleus of an atom is of the order of 10 -14 m across compared with the size of an atom which is of the order of 10 -10 m.
- The large angles of scattering for a very small number of particles led Rutherford to propose that the majority of the mass of the atom was concentrated in a minute positively charged region, around which the electrons in the atom circulated.
- When an alpha particle came very close to the nucleus, Rutherford reasoned, the electrostatic repulsion between the two would be sufficient to repel the alpha particle and so produce the large angle of scattering.
- Since the nucleus was small, this scattering would occur for only the few particles which approached the nucleus sufficiently closely.
- Rutherford’s nuclear atomic model is shown in the Figure 10.
Rutherford’s model of the atom conclusion
- Therefore they concluded that matter is mostly empty space, with the very lightweight electrons orbiting around an incredibly dense and positively charged nucleus.
- He also concluded that almost all of the positive charges were contained in a tiny, dense region in the centre of the atom, which he called the nucleus.
- The negatively charged electrons are held within the atom by their attraction to the positively charged nucleus.
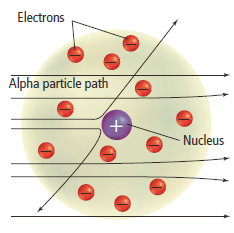
Summary of Rutherford’s experiment
| Observation | Conclusion |
| • Most alpha particles went straight through | • The atom contains large empty space |
| • Some particles were deflected. • Very few bounced back. | • Nucleus (positively charged center). • All mass of atom resides in the nucleus. • Size of nucleus is very small. • Electrons revolve in the empty space. |
| • Atom is electrically neutral | • Number of protons=Number of electrons |
The proton and the neutron
- By 1920, Rutherford had refined the concept of the nucleus and concluded that the nucleus contained positively charged particles called protons.
- A proton is a subatomic particle carrying a charge of 1+.
- In 1932, James Chadwick (1891–1974), showed that the nucleus also contained another subatomic neutral particle, called the neutron.
- A neutron is a subatomic particle that has a mass nearly equal to that of a proton, but it carries no electric charge.
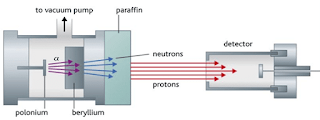
- Chadwick’s experiment consists of bombarding a beryllium plate with alpha particle. An uncharged radiation is produced on the opposite side of the sheet. Therefore, he used a solid material containing many hydrogen atoms (paraffin wax) in the path of this radiation caused protons to be knocked out of the wax. He showed that the unknown radiation must consist of uncharged particles with a mass similar to that of the proton.
The Atomic Theory
Structure of the atom.
- An atom is an electrically neutral, spherical entity composed of a positively charged central nucleus surrounded by one or more negatively charged electrons.
- All atoms are made up of the three fundamental subatomic particles—the electron, the proton, and the neutron.
- The electrons move rapidly within the available volume, held there by the attraction of the positively charged nucleus.
- An atom’s diameter (≈1×10 -10 m) is about 20,000 times the diameter of its nucleus (≈5×10 -15 m).
- The nucleus, which is composed of neutral neutrons and positively charged protons, contains all of an atom’s positive charge and more than 99.97% of its mass.

- An atomic nucleus consists of protons and neutrons (the only exception is the simplest hydrogen nucleus, which is a single proton).
- The proton (p + ) has a positive charge, and the neutron (n 0 ) has no charge; thus, the positive charge of the nucleus results from its protons.
- The magnitudes of the charges possessed by a proton and by an electron (e – ) are equal, but the signs of the charges are opposite.
- Since an atom is electrically neutral, the number of protons in the nucleus of an atom must be exactly equal to the number of electrons.
- The proton number of an atom is also known as the atomic number Z . This represents the number of protons in the nucleus.
- All carbon atoms ( Z= 6) have 6 protons, all oxygen atoms ( Z= 8) have 8 protons, and all uranium atoms ( Z= 92) have 92 protons. There are currently 117 known elements, of which 90 occur in nature and 27 have been synthesized by nuclear scientists.
Atomic number
- The mass number (or nucleon number) A is the number of protons plus the number of neutrons in the nucleus of an atom.
- Thus, a carbon atom with 6 protons and 6 neutrons in its nucleus has a mass number of 12, and a uranium atom with 92 protons and 146 neutrons in its nucleus has a mass number of 238.
Mass number
- An atom with Z protons and N neutrons in represented as shown in Figure 13. The atomic symbol X is based on the element Latin, Greek or English name.
- For example, C for carbon, S for sulfur, and Na for sodium (Latin natrium ). Often written with the symbol are the atomic number ( Z ) as a left sub script and the mass number ( A ) as a left super script.

- Since the mass number is the sum of protons and neutrons, the number of neutrons ( N ) equals the mass number minus the atomic number:
| | = 12 (mass number) = 6 (atomic number) = 12-6 =6 (number of neutrons) |
| | = 16 (mass number) = 8 (atomic number) = 18-8= 8 (number of neutrons) |
| | = 35 (mass number) = 17 (atomic number) = 37-17 =18 (number of neutrons) |
- Isotopes are atoms with the same proton number but different nucleon numbers. They have the same electron arrangement and , therefore, the same chemical properties
- For example, all carbon atoms ( Z =6) have 6 protons and 6 electrons, but only 98.89% of naturally occurring carbon atoms have 6 neutrons ( A= 12). A small percentage (1.11%) have 7 neutrons ( A= 13), and even fewer (less than 0.01%) have 8 ( A= 14). These are carbon’s three naturally occurring isotopes: 12 C, 13 C, and 14 C.
- The chemical properties of an element are primarily determined by the number of electrons, so all isotopes of an element have nearly identical chemical behaviour, even though they have different masses.
Calculation of the atomic mass of chlorine
- To calculate the weighted average atomic mass of chlorine, you first need to calculate the mass contribution of each isotope.

Atomic Masses of the Elements
- The mass of an atom is measured relative to the mass of an atomic standard. The modern standard is the carbon-12 atom, whose mass is defined as exactly 12 atomic mass units. Thus, the atomic mass unit (amu) is 1/12th the mass of a carbon-12 atom.
- Based on this standard, the 1 H atom has a mass of 1.008 amu; in other words, a 12 C atom has almost 12 times the mass of an 1 H atom.
- The other unit for atomic mass is dalton (Da). Therefore one 12 C atom has a mass of 12 daltons (12 Da, or 12 amu).
- The atomic mass unit is a unit of relative mass, but it has an absolute mass of 1.66054×10 -24 g.
Masses of Subatomic Particles
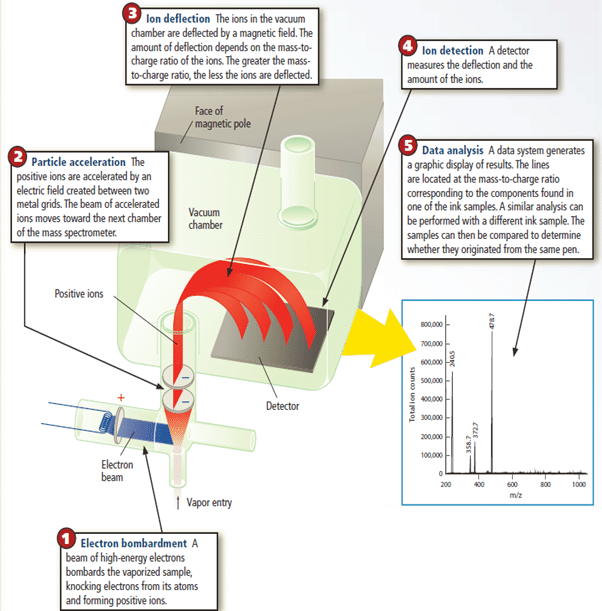
Mass Spectrometry

How the Mass Spectrometer Works
Unstable Nuclei and Radioactive Decay
Radioactivity, nuclear reactions.
- Substances that emitted radiation spontaneously in a process is called radioactivity.
- Radiation s are rays and particles emitted by the radioactive material.
- A nuclear reaction involves a change in the nuclide.
- A nuclear reaction results in the formation of new kinds of atoms.
- Radioactive atoms produce radiation because their nuclei are unstable.
Radioactive decay
- Unstable nuclei disintegrate (break up) and lose energy by emitting radiation such as alpha, beta or gamma radiation. The disintegrated is radioactive decay.
- Unstable atoms disintegrate to form stable atoms, often of a different element.
Types of Radiation
- There are three different types of radiation based on their electric charge.
- An experiment was carried out to investigate the effect of electric fields and magnetic on radiation.
- By directing radiation from a radioactive source between two electrically charged plates, As shown in the Figure 17, radiation were deflected toward the negative plate, the positive plate, or not at all.
Alpha radiation
- Alpha particles having a positive charge deflected toward the negatively charged plate were named alpha radiation.
- An alpha particle contains two protons and two neutrons, and thus has a 2+ charge, which explains why alpha particles are attracted to the negatively charged plate as shown in Figure 17.
- An alpha particle is equivalent to a helium-4 nucleus and is represented by α .
- The alpha decay of radioactive radium-226 into radon-222 is shown below.

- The new element, radon (Rn), is created as a result of the alpha decay of the unstable radium-226 nucleus.
- The type of equation shown above is known as a nuclear equation. It shows the atomic numbers and mass numbers of the particles involved. The mass number is conserved in nuclear equations.
Beta radiation
- Beta particles having a negative charge deflected toward the positively charged plate were named beta radiation as shown in Figure 17.
- This radiation consists of fast-moving beta particles.
- Beta particle is an electron with a 1- charge and it represented by the symbol β or e – .
- An example of beta decay of carbon-14 into nitrogen-14 is shown below.

Gamma radiation
- Gamma rays are undeflected since they are electromagnetic and it possessed a high-energy radiation.
- Gamma rays have no mass and are denoted by the symbol γ .
- Gamma ray are neutral, gamma rays are not deflected by electric or magnetic fields.
- They usually accompany alpha and beta radiation, and they account for most of the energy lost during radioactive decays.
- An example gamma rays is shown below:
- Gamma rays are mass less; the emission of gamma rays by themselves cannot result in the formation of a new atom.
Nuclear Stability – Atomic Structure Notes
- The primary factor in determining an atom’s stability is its ratio of neutrons to protons. Atoms that contain either too many or too few neutrons are unstable and lose energy through radioactive decay to form a stable nucleus.
- They emit alpha and beta particles and these emissions affect the neutron-to-proton ratio of the newly created nucleus.
- Radioactive atoms undergo enough radioactive decay to form stable, non-radioactive atoms.
Buy Atomic Structure Books
- Reactions of Aromatic Compounds (Part 2) – BSc Chemistry Notes
- Electrophilic Aromatic Substitution – Reaction of Aromatic Compounds (Part 1)
- Solid State Chemistry – BSc Chemistry Notes
- MSc Chemistry Notes Iron Metalloporphyrin Complexes in Bioinorganic Molecules
- Zinc Metalloenzymes BSc Chemistry Notes
- B.Sc. Chemistry Notes - Atomic Structure
- University Chemistry Notes Thermodynamics
- Graduation Chemistry Notes - Electromagnetic spectrum UV and Visible Spectroscopy
- College Chemistry of Elements of First Transition Series
You might like
Our website uses cookies to improve your experience. Privacy Policy WhatsApp
121 Interesting Chemistry Project Topics for BSC Students

In the world of academia, a Bachelor of Science (BSC) in Chemistry offers a fascinating journey into the realm of molecules, reactions, and scientific discoveries. As a chemistry student, one of the most exciting aspects of your academic journey is the opportunity to undertake unique and engaging chemistry projects.
However, chemistry project topics for bsc students not only deepen your understanding of the subject but also provide valuable hands-on experience. To make your life easier, we’ve compiled a comprehensive list of simple chemistry project topics tailored specifically for BSC students.
| And if you ever need assistance or guidance with your projects, consider exploring our services to excel in your academic pursuits. |
Why Are Chemistry Projects Important?
Table of Contents
Here are some reasons for importance of Chemistry Project topics for BSC students:
1. Application of Knowledge: Chemistry projects allow students to apply theoretical knowledge gained in the classroom to real-world scenarios. This hands-on experience helps bridge the gap between theory and practice.
2. Skill Development: Students develop essential laboratory and analytical skills, including precision, observation, data analysis, and problem-solving. These skills are valuable in both academic and professional settings.
3. Research Skills: Conducting chemistry projects fosters research skills, encouraging students to explore existing literature, design experiments, and draw meaningful conclusions.
4. Critical Thinking: Projects challenge students to think critically, make connections between concepts, and formulate hypotheses. This cultivates their ability to solve complex problems.
5. Collaboration: Group projects promote teamwork and communication, skills that are essential in any scientific career.
6. Innovation: Students have the opportunity to create something new, whether it’s a novel experiment, a chemical compound, or a solution to a real-world problem.
How to Choose the Right Chemistry Project?
Selecting the right chemistry project is crucial for a successful and fulfilling experience. Here are some steps to help you choose the right chemistry project topics for BSc students:
1. Identify Your Interests
Consider your personal interests and passions within the field of chemistry. Choosing a project that aligns with your interests will keep you motivated throughout the process.
2. Review Coursework
Review your coursework to identify topics that you have found particularly intriguing or challenging. Choosing a project related to these topics will allow you to deepen your understanding.
3. Consult with Professors
Talk to your professors or academic advisors for guidance. They can provide insights into potential projects and may even suggest specific topics or research areas.
4. Assess Resources
Consider the resources available to you, including laboratory equipment, materials, and time. Ensure that your chosen project is feasible given your resources.
5. Set Clear Objectives
Define clear objectives and goals for your project. What do you hope to achieve, and what questions do you want to answer? Having a clear focus will guide your research.
6. Plan Your Timeline
Create a timeline for your project that includes milestones and deadlines. This will help you stay organized and on track throughout the project.
7. Seek Collaboration
If possible, consider collaborating with peers or professors on your project. Collaborative projects often yield valuable insights and support.
8. Consider Ethical and Safety Issues
Ensure that your project adheres to ethical guidelines and safety protocols. Discuss these aspects with your advisors and prioritize safety at all times.
9. Stay Flexible
Be prepared to adapt and make adjustments as needed during the project. Research can be unpredictable, so flexibility is key to success.
10. Enjoy the Process
Lastly, choose a project that excites you and that you’re passionate about. Enjoying the process will make your project more rewarding.
List of Simple Chemistry Project Topics for BSC Students
Now, let’s explore a wide range of chemistry project topics for BSC students. These topics cover various aspects of chemistry to cater to different interests and specialties.
Organic Chemistry
Discover some chemistry project topics in organic chemistry for students:
1. Synthesis of Biodegradable Plastics from Renewable Resources
2. Investigating the Chemical Properties of Essential Oils
3. Analysis of Organic Pollutants in Water Samples
4. Isolation and Characterization of Natural Products from Plants
5. Study of Green Chemistry Reactions for Sustainable Synthesis
6. Development of New Organic Reactions for Drug Synthesis
7. Investigation of the Chemistry of Perfumes and Fragrances
8. Synthesis and Characterization of Nanoparticles for Drug Delivery
9. Analysis of Food Additives and Their Effects on Health
10. Extraction and Analysis of Natural Dyes from Plants
11. Study of the Synthesis and Applications of Biocompatible Polymers
Inorganic Chemistry
Here are some chemistry project topics for bsc students in inorganic chemistry:
1. Synthesis and Characterization of Metal-Organic Frameworks (MOFs)
2. Study of the Properties of Transition Metal Complexes
3. Investigation of Lanthanide Chemistry and Applications
4. Analysis of Heavy Metal Contamination in Soil and Water
5. Synthesis and Applications of Inorganic Nanomaterials
6. Study of the Chemistry of Coordination Compounds
7. Exploration of Rare Earth Element Chemistry
8. Analysis of Inorganic Pollutants in Environmental Samples
9. Synthesis of Zeolites for Catalysis and Adsorption
10. Investigation of Supramolecular Chemistry in Inorganic Systems
11. Study of Metal-Catalyzed C-C Bond Formation Reactions
Analytical Chemistry
Let’s dive into some chemistry project topics in analytical chemistry for students:
1. Development of Electrochemical Sensors for Environmental Monitoring
2. Analysis of Pharmaceuticals in Water and Wastewater
3. Study of Spectroscopic Techniques for Chemical Analysis
4. Investigation of Food Adulteration using Analytical Methods
5. Analysis of Trace Metals in Biological Samples
6. Development of Chromatographic Methods for Drug Analysis
7. Study of Mass Spectrometry in Proteomics and Metabolomics
8. Analysis of Pesticide Residues in Agricultural Products
9. Investigation of Forensic Chemistry Techniques for Crime Scene Analysis
10. Development of Biosensors for Healthcare Applications
11. Study of Gas Chromatography-Mass Spectrometry (GC-MS) in Environmental Analysis
Physical Chemistry
Let’s explore some chemistry project topics for bsc students in physical chemistry:
1. Study of Phase Equilibria and Phase Diagrams
2. Investigation of Reaction Kinetics and Mechanisms
3. Analysis of Thermodynamic Properties of Materials
4. Study of Quantum Mechanics and Molecular Structure
5. Investigation of Electrochemistry and Batteries
6. Analysis of Surface Chemistry and Interfaces
7. Study of Nuclear Chemistry and Radioactive Decay
8. Investigation of Chemical Thermodynamics in Biological Systems
9. Analysis of Non-Newtonian Fluids and Rheology
10. Study of Computational Chemistry and Molecular Modeling
11. Study of Photochemistry and Photophysics of Organic Compounds
Biochemistry
Here are some chemistry project topics in biochemistry for bsc students
1. Investigation of Enzyme Kinetics and Inhibition
2. Analysis of Protein Structure and Function
3. Study of Metabolic Pathways and Bioenergetics
4. Investigation of DNA Damage and Repair Mechanisms
5. Analysis of Lipid Metabolism and Lipoproteins
6. Study of Hormone Chemistry and Signaling Pathways
7. Investigation of Antioxidants and Free Radical Chemistry
8. Analysis of Enzyme Catalysis in Biotechnology
9. Study of Bioinformatics and Genomic Analysis
10. Investigation of the Chemistry of Neurotransmitters
11. Analysis of Protein Folding and Misfolding in Disease Mechanisms
Environmental Chemistry
Let’s explore easy chemistry project topics for bsc students in environmental chemistry:
1. Analysis of Air Pollution and Atmospheric Chemistry
2. Study of Water Treatment and Purification Techniques
3. Investigation of Soil Chemistry and Nutrient Cycling
4. Analysis of Pollutants in Marine Ecosystems
5. Study of Green Chemistry Principles for Environmental Sustainability
6. Investigation of Chemical Contaminants in Aquatic Systems
7. Analysis of Heavy Metal Accumulation in Plants
8. Study of Environmental Impact Assessments and Risk Assessment
9. Investigation of Sustainable Energy Sources and Fuel Cells
10. Analysis of Microplastics in the Environment
11. Study of the Impact of Ocean Acidification on Marine Life.
Materials Chemistry
Discover some best chemistry project topics in materials chemistry for students:
1. Synthesis and Characterization of Nanomaterials for Energy Storage
2. Study of Polymer Chemistry and Polymerization Reactions
3. Investigation of Smart Materials and Their Applications
4. Analysis of Nanocomposites for Advanced Materials
5. Study of Liquid Crystals and Liquid Crystal Displays (LCDs)
6. Investigation of Nanoparticles in Drug Delivery
7. Analysis of Superconductors and High-Temperature Superconductivity
8. Study of Photovoltaic Materials for Solar Cells
9. Investigation of Magnetic Materials and Their Properties
10. Analysis of Nanomaterials in Biomedical Applications
11. Synthesis and Applications of Carbon Nanotubes in Nanocomposites
Food Chemistry
Let’s dive into the simple chemistry project topics for bsc students in food chemistry:
1. Study of Food Additives and Their Effects on Health
2. Analysis of Food Packaging Materials and Their Properties
3. Investigation of Food Preservation Techniques
4. Study of Flavors and Aromas in Food
5. Analysis of Food Contamination and Foodborne Pathogens
6. Investigation of Food Chemistry in Cooking and Culinary Arts
7. Analysis of Antioxidants in Food and Their Health Benefits
8. Study of Food Dye Chemistry and Food Coloring Agents
9. Investigation of Fermentation Processes in Food Production
10. Analysis of Nutritional Chemistry and Food Labeling
11. Study of the Maillard Reaction and Its Role in Food Flavor Development
Pharmaceutical Chemistry
Here are some chemistry project topics in pharmaceutical chemistry for students
1. Synthesis and Characterization of Drug Compounds
2. Study of Drug Delivery Systems and Formulations
3. Investigation of Medicinal Chemistry and Drug Design
4. Analysis of Natural Products in Drug Discovery
5. Study of Pharmacokinetics and Pharmacodynamics
6. Investigation of Drug Interactions and Drug-Drug Interactions
7. Analysis of Drug Stability and Degradation
8. Study of Quality Control and Assurance in Pharmaceutical Industry
9. Investigation of Clinical Pharmacology and Drug Testing
10. Analysis of Nanomedicine and Nanoparticles in Drug Delivery
11. Study of Drug-Excipient Compatibility in Pharmaceutical Formulations
Green Chemistry
Let’s explore easy chemistry project topics for bsc students in green chemistry:
1. Investigation of Sustainable Synthesis and Green Solvents
2. Analysis of Renewable Energy Sources and Their Chemistry
3. Study of Biodegradable Polymers and Materials
4. Investigation of Green Chemistry in Agriculture and Pest Control
5. Analysis of Sustainable Chemical Processes in Industry
6. Study of Green Chemistry in Water Treatment
7. Investigation of Green Chemistry Education and Outreach
8. Analysis of Green Chemistry Metrics and Assessment
9. Study of Green Chemistry Innovations and Future Prospects
10. Investigation of Eco-Friendly Chemical Reactions and Catalysis
11. Study of Sustainable Synthesis of Bio-Based Fuels and Chemicals
Miscellaneous
Discover some chemistry project topics for bsc students:
1. Study of Chemistry of Art Conservation and Restoration
2. Analysis of Chemistry in Cosmetics and Personal Care Products
3. Investigation of Chemistry in Space Exploration and Astrochemistry
4. Study of Forensic Chemistry and Crime Scene Analysis
5. Investigation of Chemistry of Pharmaceuticals in the Environment
6. Analysis of Chemistry of Gemstones and Precious Metals
7. Study of Chemistry of Paints, Pigments, and Dyes
8. Investigation of Chemistry of Perfumes and Fragrances
9. Analysis of Chemistry in Sports and Performance Enhancement
10. Study of Chemistry in Music and Musical Instruments
11. Study of Chemical Reactions in Brewing and Fermentation Processes
Chemistry projects are essential for BSC students as they offer a myriad of benefits, from applying theoretical knowledge to developing critical thinking and problem-solving skills. When choosing the right project, consider your interests, available resources, and clear objectives. The list of interesting chemistry project topics for bsc students provided covers a wide spectrum of areas within the field, catering to various interests and specialties. Whether you choose to delve into organic chemistry, environmental chemistry, or any other category, remember to enjoy the journey of exploration and discovery that a chemistry project offers.
Related Posts

Science Fair Project Ideas For 6th Graders
When it comes to Science Fair Project Ideas For 6th Graders, the possibilities are endless! These projects not only help students develop essential skills, such…

Java Project Ideas for Beginners
Java is one of the most popular programming languages. It is used for many applications, from laptops to data centers, gaming consoles, scientific supercomputers, and…

- Study Abroad Get upto 50% discount on Visa Fees
- Top Universities & Colleges
- Abroad Exams
- Top Courses
- Read College Reviews
- Admission Alerts 2024
- Education Loan
- Institute (Counselling, Coaching and More)
- Ask a Question
- College Predictor
- Test Series
- Practice Questions
- Course Finder
- Scholarship
- All Courses
- B.Sc (Nursing)
BSc 1st Year Syllabus, Subjects, Electives, Books, Skills, Colleges

Waqar Niyazi
Content Curator
BSc course is a 3-year undergraduate program that helps students to get a holistic and theoretical understanding of subjects. BSc Subjects are divided into 6 semesters. The BSc Course syllabus is a mix of theoretical, practical as well as research-based subjects.
BSc Syllabus depends on the specialization selected by the student. There are many specialisations available for students such as BSc Nursing, BSc Agriculture, BSc Computer Science, BSc Biotechnology, BSc IT, BSc Microbiology, BSc Psychology, BSc Physics, BSc Zoology etc. Students are taught through various methods such as theory, laboratory, project, internships etc.
|
|
Table of Contents
BSc Specializations List
Bsc books in 1st year, bsc 1st year electives, bsc 1st year syllabus.
4.1 BSc Nursing First Year Syllabus
4.2 BSc Agriculture First Year Syllabus
4.3 BSc Computer Science First Year Syllabus
4.4 BSc Biotechnology First Year Syllabus
4.5 BSc IT First Year Syllabus
4.6 BSc Microbiology First Year Syllabus
4.7 BSc Psychology First Year Syllabus
4.8 BSc Forensic sciences First Year Syllabus
4.9 BSc Chemistry First Year Syllabus
4.10 BSc Physics First Year Syllabus
4.11 BSc Nautical Science First Year Syllabus
4.12 BSc Forestry First Year Syllabus
4.13 BSc Mathematics First Year Syllabus
4.14 BSc Zoology First Year Syllabus
4.15 BSc Botany First Year Syllabus
4.16 BSc Optometry First Year Syllabus
4.17 BSc Statistics First Year Syllabus
4.18 BSc Food Technology First Year Syllabus
Why Study BSc?
Bsc skills required, top bsc colleges, bsc 1st year: faqs.
The specialisation courses that are offered in BSc are:
- BSc Nursing - BSc Nursing course is a bachelor's degree program that trains students in areas of critical care and incorporates values necessary to become nurses and midwives. The course has 6 months of mandatory internship.
- BSc Agriculture - This course aims to provide training to implement modern agricultural techniques and technologies in real-world scenarios. The course primarily focuses on research and practices in agricultural science, dealing with disciplines such as Genetics and Plant Breeding, Agricultural Microbiology, Soil Science, Plant Pathology, etc.
- BSc Computer Science - The 3 years BSc Computer Science course allows the candidates to learn about the different architectures related to computer networking, operating system, and programming languages. The course can also be pursued as a replacement for BTech in Computer Science .
- BSc Biotechnology - BSc Biotechnology provides students with good insights into modern biology. This course provides training to students in the area of Molecular Biology, Genetic Medicine and Biotechnology.
- BSc IT - BSc IT provides a detailed study of software testing, software engineering, databases programming, software development, web designing, computer networks and computer systems.
- BSc Microbiology - BSc Microbiology provides students with detailed knowledge of microorganisms and their effect on the human body in the course. There is a great focus on the microorganisms that cause diseases in humans in the course.
- BSc Psychology - BSc Psychology course focuses on the study of the mind, emotions, social development and the individuality of a human being. It provides fundamental knowledge of the human brain and its reactions.
- BSc Forensic sciences - BSc Forensic Science is a 3-year graduate course that involves the application of scientific knowledge for crime investigation and it mainly focuses on crime-laboratory-based professions.
- BSc Chemistry - Candidates will get an understanding of all the basic concepts that they have studied in school with an in-depth knowledge of those topics and an understanding of new dimensions of chemistry as well. The course deals with different aspects of Chemistry including organic chemistry and inorganic chemistry.
- BSc Physics - BSc physics is a three-year undergraduate course that deals with the fundamentals of physics and teaches graduates essential topics such as quantum mechanics, Electromagnetism, optics, calculus semiconductors, waves, statistics, etc.
- BSc Nautical science - BSc Nautical science covers the concept of nautical motion, navigation and operational process that are required in the development, management, operation, and maintenance of marine vessels
- BSc Forestry - BSc Forestry gives the students practical knowledge of healthy growth and maintenance of forested land, woodlands and parks. The forestry program trains students in managing forests, growing new plantations, maintaining old plantations and other natural resources.
- BSc Mathematics - B.Sc Mathematics is a 3-year duration undergraduate course that deals with the discipline of mathematics along with the analysis of numbers. It also includes mathematical structure, transition, and space.
The important books for BSc 1st year are
| Specialisation | Books Name | Author |
|---|---|---|
| BSc IT | Computer Organization & Architecture –Designing & Performance | William Stallings, Prentice Hall of India. |
| Digital Electronics-An Introduction to Theory and Practice | William H.Gothmann, Prentice Hall of India | |
| Discrete Mathematics and its Application | Kenneth H.Rosen | |
| Discrete Mathematics, | Schaum Series | |
| Object Oriented Programming | Robert Lafore | |
| Programming in C | Schaum Series | |
| Software Engineering- A Practitioners Approach | R. Pressman, McGraw Hill | |
| . An Integrated Approach to Software Engineering | Pankaj Jalote, Narosa | |
| Operating Systems with case Studies | Achyut S Godbole,TMG. | |
| The Unix Programming Environment | Pike rob & Kerningham Brain. | |
| Database Systems and Concepts | Henry F. Korth | |
| Principles of Database System | Ullman, Galgotia Publication | |
| BSc Food technology | Functioning in English Book I & II | Dr. P. Bhaskaran, Emerald Publishers, 2018 |
| Environmental Chemistry, 2nd edition, Prentice-Hall of India, New Delhi. | Banerjee SK (2005) | |
| A Text Book of Environmental Chemistry and Pollution Control, S. Chand & Company, New Delhi | Dara SS (2005) | |
| Statistical Methods | S.P. Gupta, Sultan 2000 | |
| Operations Research | Hira and Gupta, S. Chand, 2015 | |
| Advanced Accountancy | T.S. Grewell, 2016. | |
| Food Analysis: Theory and Practice. | Y. Pomeranz& C.E. Meloan, Chapman and Hall, 2004. | |
| Introduction to Food Analysis | S. Suzanne Nielsen, West Lafayette, IN 47907-2009, USA,n 2009. | |
| Food science, New Age International Publishers (India), 2003 | B.Srilakshmi | |
| Foods : Facts and Principles - New Age Publishers, 2004 | N Shakuntalamanay, M. ShadaksharaSwamy | |
| Lehninger’s Principles of Biochemistry | David L. Nelson and Michael M. Cox, Macmillan Worth publisher, 2000. | |
| Biochemistry, 4th Edition, WH. Freeman and co., 2000. | LubertStryer | |
| Harper’s Biochemistry, 2003. | Murray, R.K., Granner, B.K., Mayes, P.A., Rodwell, V.W., | |
| BSc Agriculture | A General Text Book of Entomology | A.D. Imms |
| Text Book of Agricultural Entomology | P.M..Srivastava and Ashok Kumar | |
| BSc Computer Science | Fundamentals of Database Systems 6th Edition, Pearson Education, 2010. | R. Elmasri, S.B. Navathe, |
| Database Management Systems 3rd Edition, McGraw-Hill, 2002. | R. Ramakrishanan, J. Gehrke | |
| Operating Systems Concepts, 8th Edition, John Wiley Publications 2008. | A Silberschatz, P.B. Galvin, G. Gagne, | |
| BSc Biotechnology | Cell Biology and Genetics | By P.K. Gupta |
| Cell and Molecular Biology | By De Robertis | |
| Genetics | By Gardner (Macmillan Press) | |
| BSc Psychology | Organizational Behaviour | Newstrom JW |
| Psychology Foundation | B.T. Basavanthappa | |
| Educational Psychology | J.W.Santrock | |
| Theory and Practice of Counselling and Psychotherapy | G.Corey | |
| BSc Forensic sciences | Inorganic Chemistry | Shriver and Atkins |
| Mycobacteria Protocols | Tanya Parish and Amanda Claire Brown | |
| Molecular Diagnostics | George P. Patrinos | |
| Physical Evidence in Forensic Science | Henry C. | |
| Spoken English | Alison Reid | |
| BSc Chemistry | Inorganic Chemistry | J.E.Huheey |
| A Text Book of Organic chemistry | I L Finar Vol I | |
| A textbook of qualitative inorganic analysis | A.I. Vogel | |
| Advanced Inorganic Chemistry Vol-I | Satyaprakash, Tuli, Basu and Madan | |
| Principles of physical chemistry | Prutton and Marron | |
| BSc Physics | Elements of properties of matter | – D.S. Mathur – S. Chand & Co., 2004. |
| Properties of matter | Brijlal and Subramanian S. Chand & Co., 2006. | |
| Mechanics | D.S.Mathur, S.Chand& Co., 2ndEdition (2001) | |
| Properties of Matter | R.Murugeshan, S. Chand & Co., New Delhi (2001) |
Elective subjects are crucial in adding valuable knowledge to students that are recent and modern. Students get to know about topics that are happening in the world right now. All BSc Specialisations do not have electives in the first year.
| BSc Computer Science First Year Elective Subjects | |
|---|---|
| Computer Graphics | Resource Management Techniques |
| E-Commerce | Cloud Computing |
| Security in Information Technology | Security in Information Technology |
| Computer Networks | Software Engineering and testing |
| BSc Microbiology First Year Elective Subjects | |
| Yoga and Meditation | Industrial and Organizational Psychology |
| NSS | Right to Information and Good Governance |
| Introduction to Information Technology | - |
| BSc Statistics First Year Elective Subjects | |
| Actuarial Statistics Bio-Statistics | Data Analytics with R Reliability Analysis |
| Operations Research | - |
See Also : Computer Operator Courses
BSc consists of various specialisation such as BSc Nursing, BSc Agriculture, BSc Computer Science, BSc Biotechnology, BSc IT, BSc Microbiology, BSc Psychology etc. The BSc 1st year syllabus for specialisation courses are listed below for proper understanding:
BSc Nursing First Year Syllabus
The syllabus for BSc Nursing is listed below:
| First Year | |
|---|---|
| English | Nursing Foundations |
| Anatomy | Psychology |
| Physiology | Microbiology |
| Nutrition | Introduction to computers |
| Biochemistry | Regional Language |
| Co-curricular activities | Library Work /Self Study |
BSc Agriculture First Year Syllabus
The syllabus for BSc Computer Agriculture is listed below:
| Semester I | Semester II |
|---|---|
| Principles of Agronomy and Agricultural Heritage | Fundamentals of Agricultural Meteorology |
| Agricultural Microbiology | Fundamentals of Biochemistry |
| Fundamentals of Information Technology | Crop Physiology |
| Applied Mathematics | Principles of Genetics |
| Principles of Analytical Chemistry | Dimensions of Agricultural Extension |
| Introduction to Agricultural Botany | Principles of Agricultural Economics |
| English for effective communication | Fundamentals of Horticulture |
| National Service Scheme / National Cadet Corps | Principles of Environmental Sciences |
| Physical Education | Principles of food science and processing |
BSc Computer Science First Year Syllabus
The syllabus for BSc Computer Science is listed below:
| First Semester | Second Semester |
|---|---|
| Foundation in English | Foundation in English |
| Allied Mathematics - I | C Programming |
| Problem Solving | Digital Electronics |
| Fundamentals of Computing | Principles of Programming Languages |
| Office Automation Laboratory | C Programming Laboratory |
BSc Biotechnology First Year Syllabus
The syllabus for BSc Biotechnology is listed below:
| Semester I | Semester II |
|---|---|
| Animal Science | Introduction to Microbiology |
| Plant Science | Biochemistry I |
| Foundations of Chemistry & Biochemistry | Cell Biology |
| Basics of Computer | Genetics |
| Animal Science Lab | Introduction to Practical Microbiology |
| Plant Science Lab | Biochemistry I Lab |
| Foundations of Chemistry & Biochemistry Lab | Cell Biology Lab |
| Computer Fundamentals & C Programming Lab | Genetics Lab |
| Elective | Elective |
| Elective | Elective |
BSc IT First Year Syllabus
The syllabus for BSc IT is listed below :
| Semester I | Semester II |
|---|---|
| Communication-I | Communication II |
| Programming in C | Basic Mathematics - II |
| Human Value & Professional Ethics | OOPS using C++ |
| Mathematics- I | Digital Circuits and Logic Designs |
| Information Technology | Environmental Science |
| Software Lab-I (Programming in C) | Software Lab- III (OOPS using C++) |
| Software Lab-II (Information Technology) | Hardware Lab-1 (Digital Circuits and Logic Designs) |
BSc Microbiology First Year Syllabus
The syllabus for BSc Microbiology is listed below :
| Semester - I | Semester - II |
|---|---|
| Introduction to Microbiology and Microbial Diversity | Microbial Systematics |
| Introduction to Biotechnology | Industrial Biotechnology |
| Biochemistry -1 | Biochemistry -2 |
| English -1/Hindi-1/Kannada-1 | English-2/ Hindi-2/Kannada-2 |
| Constitution of India | - |
BSc Psychology First Year Syllabus
The syllabus for BSc Psychology is listed below :
| First Semester | Second Semester |
|---|---|
| Tamil/French/Hindi | Tamil/French/Hindi |
| English | English |
| Introduction to Psychology | Social Psychology |
| General Psychology | Physiological Psychology |
| Biological Basis of Behavior | Experimental Psychology |
BSc Forensic Sciences First Year Syllabus
The syllabus for Bachelor of Science in Forensic Science is listed below :
| Semester - I | Semester II |
|---|---|
| Basic of Forensic Science | Basic of Forensic Science |
| Basic of Forensic Chemistry | Basic of Forensic Chemistry |
| Basic of Forensic Physics | Basic of Forensic Physics |
| Basic of Forensic Biology | Basic of Forensic Biology |
| Basic of Forensic Psychology | Basic of Forensic Psychology |
| Basic of Digital and Cyber Forensics | Basic of Digital and Cyber Forensics |
| Communication skill/Criminology | Communication skill/Criminology |
| Indian penal code | Indian penal code |
BSc Chemistry First Year Syllabus
The syllabus for BSc Chemistry is listed below :
| First Year | Second Year |
|---|---|
| Tamil–I | Tamil –II |
| Hindi –I | Hindi –II |
| French –I | French –II |
| English –I | English –II |
| Structure and Bonding in Chemistry | Thermodynamics and Solutions |
| Basic Concepts of Organic Chemistry | Inorganic Qualitative Analysis – II |
| Inorganic Qualitative Analysis – I | Allied Mathematics – II |
| Allied Mathematics – I | Basic Computer Skills |
| Soft Skills | Quantitative Attitude and Logical Reasoning –I |
| - | NSS |
| - | NCC |
| - | NSO |
| - | Yoga |
See Also:
BSc Physics First Year Syllabus
The syllabus for BSc Physics is listed below :
| Semester - I | Semester - II |
|---|---|
| English I | English II |
| English II/ Common Course I | English III/ Common Course II |
| Second Language I | Second Language II |
| Methodology and Perspectives of Physics | Mechanics and Properties of Matter |
| Complementary I: Mathematics I | Complementary I: Mathematics II |
| Complementary II: Chemistry I | Complementary II: Chemistry II |
| Core Practical I:Mechanicsand Properties of Matter | Core Practical I :Mechanics and Properties of Matter |
| Complementary II Practical I | Complementary II Practical I |
See Also :
BSc Nautical Science First Year Syllabus
The syllabus for BSc Nautical Science is listed below :
| Semester - I | Semester - II |
|---|---|
| English & Communication Skills | Applied Mathematics Paper – II |
| Applied Mathematics Paper – I | Nautical Physics & Electronics Paper –II |
| Nautical Physics & Electronics Paper – | Voyage Planning & Collision Prevention Paper – I |
| Principles of Navigation Paper – I | Cargo Work & Marine Communication Paper – I |
| Ship Operation Technology Paper – I | Environmental Science Paper – I |
| Naval Architecture Paper – I | Marine Engineering & Control System Paper – I |
| Practical Navigation Paper – I | Chart Work Paper – I |
See Also: Diploma in Nautical Science
BSc Forestry First Year Syllabus
The syllabus for BSc Forestry is listed below :
| Semester - I | Semester - II |
|---|---|
| Principles of Silviculture | Dendrology |
| Fundamentals of Forest Pathology | Practices of Silviculture |
| Fundamentals of Meteorology | Production Technology of Field Crops |
| Forest Botany and Ethnobotany | Principles of Agricultural Economics |
| Principles of Analytical Chemistry | Forest Surveying |
| English for Effective Communication | Fundamentals of Biochemistry |
| Applied Mathematics | Crop Physiology |
| Fundamentals of Information Technology | Principles of Genetics |
| Physical Education | Study Tour to Southern Tamil Nadu |
| National Service Scheme (NSS) | Physical Education (contd.) |
| - | National Service Scheme (NSS) (contd.) |
See Also: Forestry Courses
BSc Mathematics First Year Syllabus
The syllabus for BSc Mathematics is listed below :
| Semester - I | Semester - II |
|---|---|
| Tamil/Other Languages | Tamil/Other Languages |
| English | English |
| Algebra | Calculus |
| Trigonometry | Analytical Geometry of three dimensions |
| (to choose 1 out of 4) III ALLIED Paper (For non practical Allied subjects only) (or) | (to choose 1 out of 4) (For non practical Allied subjects only) (or) |
| (to choose 1 out of 4) (Theory) III Paper -(For practical Allied subjects) | (to choose 1 out of 4) (For practical Allied Subjects) |
| Environmental Studies | Value Education |
| Soft Skill |
BSc Zoology First Year Syllabus
The syllabus for BSc Zoology is listed below :
| Semester 1 | Semester 2 |
|---|---|
| LanguageT/H/F Paper I | English |
| English Paper I/ Functional English | Core Paper II Chordata |
| Core Paper I-Invertebrata | Core Zoology Practicals - I |
| Allied Chemistry Paper - II | |
| Allied chemistry Practical | |
| Basic Tamil II **/ Advanced Tamil II ** /Self Study Online Courses | |
| Personality development program | |
| General Awareness | |
| Effective English Communication |
See Also : Zoology Courses
BSc Botany First Year Syllabus
The syllabus for BSc Botany is listed below :
| Semester - I | Semester - II |
|---|---|
| Fine-tune Your English | Issues that Matter |
| Pearls from the Deep | Savouring the Classics |
| Additional Language | Additional Language |
| Prose and One Act Plays | Short stories and Novel |
| Kathasahithyam | Kavitha |
| Poetry/ Grammar & History of Syriac Language & Literature | Poetry/ Grammar & History of Syriac Literature |
| Methodology of science and an introduction to botany | Microbiology, mycology and plant pathology |
| Methodology of science and an introduction to botany | Microbiology, mycology and plant pathology |
| Non chordate diversity | Chordate Diversity |
| Basic Theoretical and Analytical Chemistry | Chordate Diversity |
| Volumetric analysis | Basic Organic Chemistry |
| - | Volumetric analysis |
See Also : Botany Courses
BSc Optometry First Year Syllabus
The syllabus for BSc Optometry is listed below :
| First Year | |
|---|---|
| General Anatomy & General physiology | Computer Basics & Computer Programming |
| Basic Biochemistry & Nutrition | Functional English& Communications |
| Ocular Anatomy ,ocular Physiology& biochemistry | Mathematics |
| Physical & Geometric Optics & principles of lighting | Kannada |
| - | Basic Accountancy |
See Also : Computer Application Courses
BSc Statistics First Year Syllabus
The syllabus for BSc Statistics is listed below :
| Semester - I | Semester - II |
|---|---|
| Statistical Methods-I | Statistical Methods-II |
| Probability Theory | Probability Distributions |
| Practicals | Practicals |
See Also: Statistics Courses
BSc Food Technology First Year Syllabus
The syllabus for BSc Food Technology is listed below:
| Semester - I | Semester - II |
|---|---|
| English-I | English-II |
| Applied Chemistry | Food Analysis Techniques |
| Business Mathematics | Principles of Food Science |
| Environmental Chemistry | Introduction to Biochemistry |
| Applied Chemistry Lab | Food Chemistry Lab-II |
| Food Chemistry Lab-I | Biochemistry Lab |
There are great opportunities available for BSc graduates.BSc degree courses help students with building strong fundamentals in science-based courses. The benefits of pursuing a BSc degree are listed below:
- Extensive Job profiles – The job profiles for BSc candidates include scientists, Research analysts, chemists, Consultants, Clinical research managers, and lecturers in an academic institution
- Respected Professions - Some of the most respected and popular professions in the country like doctor and engineering require students to have a B.Sc. degree. If students want to become a doctor, they need to be extremely good in PCB (physics, chemistry, and biology), subjects. Students who want to become an engineer, they need to be good in PCM (physics, chemistry and mathematics) subjects. Professions like scientists and lawyers (science-related specializations) also need to have a B.Sc. degree.
- Job opportunities in research and development fields - A BSc degree can open floodgates of job opportunities in the field of science and R&D sector.
- Diverse job sectors – BSc degree provides students with the option to work in various sectors such as Hospitals, Chemical industries, Pharmaceutical companies, Forensic crime research, Diagnostic laboratories, Geological survey departments, and many others.
- Scholarships – Sometimes, scholarships are given to bright students who want to pursue higher education but require financial aid. Non-governmental and government organizations provide these merit and need-based scholarships
Students must have great skills to excel in their careers. The important BSc skills that students require are listed below:
- Artificial Intelligence - Students must understand the ideas that give rise to modern technologies like natural language processing (NLP), computer vision, speech generation, biometrics, and chatbots. Check: AI Courses
- Analytical Reasoning - Analytical skills are the abilities that allow students to collect, organize, visualize, and assimilate data. They help students to see patterns, draw conclusions, and find solutions that can boost their productivity and the company's bottom-line performance. Check : Data Analytics Courses
- People Management - Patience, Great communication skills, Flexible to change, Ability to listen, and Empathy are some of the important skills required to manage any team efficiently and for great career growth. Check : Management Courses
- Excel - Students must know how to perform basic calculations with formulas and functions, professionally format spreadsheets, and create visualizations of data through charts and graphs, the ability to produce graphs and tables, use spreadsheets efficiently etc
- Programming - With the advancement of technology, learning how to program is a must for students. It is important for them to learn how to develop software, web applications, and websites. The most common languages that are in demand are Python , C++, and JavaScript .
- Cloud computing - Cloud Computing skills are in demand and Students can work as a cloud developer, cloud administrator, and cloud architect. Knowledge of the cloud platforms such as AWS, Google Cloud, Microsoft Azure, and Oracle can be useful for students.
- Machine learning - Machine Learning is a skill that is useful for programmers and data professionals. It is a subset of artificial intelligence which has become one of the most prominent skills to learn in the technology sphere. Students can start learning basic skills through various online machine-learning courses.
The Top colleges for BSc are:
| College Name | Fees |
|---|---|
| INR 42,835 | |
| INR 20,460 | |
| INR 91,000 | |
| INR 20,670 | |
| INR 89,500 | |
| INR 19,800 | |
| INR 96,161 | |
| INR 24,515 | |
| INR 49,500 | |
| INR 14,595 | |
| INR 14,610 | |
| INR 14,555 | |
| INR 13,345 | |
| INR 19,345 |
See Also : BSc Full Form
Ques. What are the subjects in plain BSc 1st year?
Ans. The subjects in BSc Syllabus are Maths, Zoology, Physics, Chemistry, Botany, Home Science, Statistics, Psychology, Nutrition, Forestry, Computer Science, Microbiology, Genetics, Agriculture etc.
Ques. Does BSc have a scope?
Ans. BSc degree has excellent career opportunities. Students can find jobs in the field of management, law, engineering, and science.
Ques. What is the future after BSc?
Ans. Students can opt for courses such as MBA , MSc , BEd, LLM or up and coming specialisations like Machine Learning and Artificial Intelligence after the BSc course.
Ques. Can I do MBA after my BSc?
Ans. Yes, students can pursue MBA after BSc with any specialisation and the basic eligibility criteria for an MBA is a bachelor's degree with minimum marks specified by the university. For some colleges, Students might be required to provide their GMAT scores and work experience is also preferred.
Ques. Can a BSc student do MCA?
Ans. Yes, Students can pursue an MCA after completing their BSc in Computer Science degree. MCA is an excellent and standard program. MCA course will expand student's understanding of the fundamental ideas of Computer Science and better job opportunities.
Ques. Can I do MTech after BSc?
Ans. BSc graduates often opt for MTech courses in their relevant fields in order to gain technical knowledge, skill and experience in that subject which would help them in their career prospects and research opportunities.
Ques. Which is best MBA or MCA after BSc?
Ans. The choice between an MBA and MCA course is dependent upon the career goals and preferences of a candidate. MBA is a better course for candidates who are interested in leadership roles while MCA is more suitable for candidates who want technical roles.
Ques. What are the specialisation in BSc?
Ans. The specialisation courses that are offered in BSc are:
- BSc Nursing
- BSc Agriculture
- BSc Computer Science
- BSc Biotechnology
- BSc IT
- BSc Microbiology
- BSc Psychology
- BSc Forensic sciences
- BSc Chemistry
- BSc Physics
- BSc Nautical science
- BSc Forestry
- BSc Mathematics
- BSc Zoology
- BSc Biology
- BSc Optometry
- BSc Statistics
- BSc Food technology
Ques. Is a BSc student eligible for NEET?
Answer. Yes, students can apply for the NEET entrance exam after their B.Sc.
Bachelor of Science [B.Sc] : 32 answered questions
Ques. what happens in bsc ignou practicals.
● Top Answer By Shruti Singh on 13 Oct 22
Ques. Does DU Offer B Tech Courses? What are the names of colleges under DU offering B Tech?
● Top Answer By Tanya Mehra on 06 Oct 22
Ques. What is the scope after DU B.Sc Hons in Computer Science?
● Top Answer By Tejaswini Jha on 06 Oct 22
Ques. Is IGNOU good for B.Sc?
● Top Answer By Shivangi Guha on 07 Apr 22
Ques. What if I miss the IGNOU BSC practical exam?
● Top Answer By Tanvi Agarwal on 31 Oct 22
Ques. How do I crack the BHU entrance exam on the first attempt for a B.Sc?
● Top Answer By Aditya Gupta on 18 Nov 22
Ques. What are the future prospects of getting a BSc degree from DU? What are the best colleges in DU?
● Top Answer By Prashant Mathur on 12 Oct 22
Ques. What is the procedure to calculate the percentage for B.Sc Honours from Patna University? Does it involve only the marks of honours papers or are papers of qualifying nature only involved?
● Top Answer By Shreya Rai, on 11 Nov 21
Ques. Which is better for a B.Sc. Deshbandhu College or ANDC College?
● Top Answer By Josephine Austin on 22 Jul 22
Ques. Is a BSc (general) distance learning IGNOU student eligible for giving the UPSC-CSE, IFoS, IIT-JAM and CAT?
● Top Answer By Deepak Kumar on 01 Nov 22
Bachelor of Science [B.Sc] + Bachelor of Education [B.Ed]
Bachelor of science [b.sc] (physics), bachelor of science [b.sc] (chemistry), bachelor of science [b.sc] (computer science), bachelor of science [b.sc] (mathematics), bachelor of science [b.sc] (zoology), bachelor of arts [ba], bachelor of science [b.sc] (forensic sciences), bachelor of science [b.sc] (optometry), master of science [m.sc] (botany), bachelor of science [b.sc] (information technology), bachelor of science [b.sc] (agriculture), bachelor of science [b.sc] colleges in india.

Amity University
![chemistry assignment topics for bsc 1st year english Jamia Millia Islamia University-[JMI]](https://images.collegedunia.com/public/college_data/images/appImage/25460_JMI_APP.jpg?h=150&w=320&mode=stretch)
Jamia Millia Islamia University-[JMI]
![chemistry assignment topics for bsc 1st year english Acharya Nagarjuna University - [ANU]](https://images.collegedunia.com/public/college_data/images/appImage/25344_ANU_APP.jpg?h=150&w=320&mode=stretch)
Acharya Nagarjuna University - [ANU]

St Joseph's University

Shoolini University

Ramnarain Ruia Autonomous College
![chemistry assignment topics for bsc 1st year english Savitribai Phule Pune University - [SPPU]](https://images.collegedunia.com/public/college_data/images/appImage/1504593097ccccccccccccaaaaaaaaaaammmmmmmmmm.png?h=150&w=320&mode=stretch)
Savitribai Phule Pune University - [SPPU]
![chemistry assignment topics for bsc 1st year english Jai Hind College - [JHC]](https://images.collegedunia.com/public/college_data/images/appImage/2100_Capture cvr pic edt.png?h=150&w=320&mode=stretch)
Jai Hind College - [JHC]
Subscribe to our news letter.


Liquid State B.Sc. 1st Year Notes
Liquid state notes, trouton's rule:, trouton's rule download pdf, which of the following molecules does not follow trouton's rule.
A. C 6 H 6 B. C 6 H 12 C. CCl 4 D. NH 3
The liquid that deviates from the Trouton’s rule is
A. Hydrochloric acid B. Sulphuric acid C. Phosphoric acid D. Acetic acid
Molar Volume:
Molar volume of any substance is the volume occupied by one mole of the substance. This is easily determined by dividing the molecular mass by the density of the compound (i.e V=M/D). According to Avogadro's hypothesis, the molar volume of all gases at S.T.P. is 22.4L. It is the volume so expressed in ml. or c.c.. If molecular weight and density of a substance are known, molar volume can easily be calculated.
Kopp's Rule:
Kopp in 1842 state that the molar volume of a liquid at its boiling point is equal to the sum of the atomic volume of its constituent atoms. This is known as Kopp’s rule. According to Kopp's rule it has been found that the molar volumes of two members of a homologous series of organic liquids differ by about 22 mL, for each CH 2 group Kopp calculated the volume equivalent of each element by a simple arithmetic means.
Macleod in 1923 gave the following relation between the surface tension (γ) and density (D) for a normal liquid- C=γ 1/4 /(D −d) where d is vapour density of the liquid at given temperature and C is constant. In 1924, Sugden modified the above equation as- γ 1/4 /(D −d)=MC=P where M is the molecular weight of the liquid and P is the parachor. At ordinary temperature, d is very small in comparision to D then- M γ 1/4 /D=P If γ=1 at a particular temperature, then- M/D=P Thus, at a particular temperature, the molar volume of a liquid having surface tension unity is called Parachor . If two liquids having the same surface tension are taken whose molecular weights are M 1 and M 2 and their densities are D 1 and D 2 respectively, then-
M 1 γ 1/4 /D 1 =P 1 and M 2 γ 1/4 /D 2 =P 2 P 1 /P 2 =(M 1 /D 1 )/ (M 2 /D 2 ) So, the ratio of parachors of two liquids having the same surface tension is equal to the ratio of molar volumes. Parachor is both an additive and constitutive property.
Parachor Value of some Elements and Groups:
| Element | P Value | Group | P Value |
|---|---|---|---|
| C | 8.6 | C=O | 44.4 |
| H | 15.7 | OH | 30.2 |
| N | 12.5 | COOH | 73.7 |
| O | 19.8 | NO | 73.8 |
| Cl | 55.2 | Double Bond | 19.9 |
| Br | 68.8 | Triple Bond | 40.6 |
| I | 90.3 | Six Membered Ring | 1.4 |
Application of Parachor:
Structure-1 6C=6 X 8.6=51.6 4H=4 X 15.7=62.8 2O=2 X 19.8=39.6 Four double bonds=4 X 19.9=79.6 one 6 membered ring=1 X 1.4=1.4 So, Calculated Parachor=235 Structure-2 6C=6 X 8.6=51.6 4H=4 X 15.7=62.8 2O=2 X 19.8=39.6 Three double bonds=3 X 19.9=59.7 Two 6 membered ring=2 X 1.4=2.8 So, Calculated Parachor=216.5 Since the experimental Parachor is 236.8. Hence structure-1 is correct for QUINONE.
It was introduced by Newton Friend in 1943. Rheochor is a constant obtained by multiplying molar volume and eighth root of the co-efficient of viscosity. It is denoted by letter Capital 'R'. R=( M/D ) X η 1/8 If η=1 then- M/D=R So, we can say that Rheochor is the molar volume of the liquid at the temperature at which its viscosity is unity. Like Parachor, Rheochor is both additive and constitutive. However it has not proved of much use in solving structural problems.
Surface Tension
A molecule lying inside (bulk) the liquid is surrounded by other molecules and so is attracted equally in all directions. Thus, the resultant force of attraction acting on the molecule is zero. However, A molecule lying at the surface of liquid is attracted by liquid molecules from the bulk of the liquid and feel inward pull. As a result of this inward pull on all molecules lying at the surface, the surface behave as if it were under tension and the surface of the liquid tends to the smallest possible area for a given volume of the liquid. This gives the lowest energy state of the liquid. Surface tension of a liquid is defined as the force in dyne acting at right angles to the surface along one cm length of the liquid surface. unit of surface tension is dyne per centimetre or Newton per metre.
Surface tension is a property that arises due to the intermolecular forces of attraction among the liquid molecules. Greater the intermolecular force of attraction, higher is the surface tension of the liquid. Surface tension of liquid generally decreases with increase of temperature and becomes zero at the critical temperature. Surface tension decreases with increase in temperature is due to on increasing the temperature, the kinetic energy of the molecules increases ,and, therefore the intermolecular attraction decreases.
Surface Energy
The work in ergs required to be done to increase or extend the surface area by one square centimeter is called surface energy. The unit of surface energy is ergs per square centimeter or joules per square meter.
What is the Effect of Temperature on Surface Tension ?
The surface tension of liquid is generally decreases with increase of temperature and becomes zero at critical temperature. The decrease in surface tension with increase of temperature is due to the fact that with increase of temperature, the kinetic energy of the molecules increases and hence intermolecular attraction decreases.
Viscosity is a property of liquid which resist to flow. Due to viscosity some liquid flow slowly and some liquid flow quickly. Viscosity is nothing but internal reistance to flow possessed by liquid. Liquids which flow slowly, have high internal resistance which is due to strong intermolecular forces says more viscous or are of high viscosity. However, Liquids which flow rapidly have low internal resistance which is due to weak intermolecular forces says less viscous or are of low viscosity. Greater are the intermolecular forces, higher is the viscosity of the liquid. Viscosity decreases with increasing the temperature. Kinetic energy increases on increasing temperature and so intermolecular force of attraction decreases, consequently, viscosity decreases and liquid flow quickly.
We know that liquid flow in layers in a tube. Liquid that contact in the surface of tube is almost stationary. As we move from the surface towards the centre of the tube, the velocity of the liquid layers keeps on increasing till it is maximum at the centre. The force of friction F between two layers each having area A cm 2 , separated by a distance dx cm , and having a velocity difference of dv cm/sec ,is given by- F ∝ A ( dv / dx ) F = η A ( dv/dx) where η is coefficient of viscosity. dv / dx is viscosity gradient If dx = 1cm, A = 1cm 2 and dV = 1cm/sec F = η Coefficient of viscosity may be defined as the force of friction required to maintain a velocity difference of 1 cm/sec between two parallel layers, 1 cm apart and each having an area of 1 sq cm. The unit of viscosity are dynes sec cm -2 .This is also called 1 Poise.
What is the Effect of Temperature on Viscosity ?
The viscosity of a liquid is generally decreases with rise in temperature. This decrease is about 2% per degree rise of temperature in many cases. This has been explained in terms of Hole Theory of liquids. The variation of viscosity (η) with temperature can be expressed by the following relationship- η = Ae Ea/RT -----[Equation-1] where A is constant and Ea is called the activation energy for viscous flow. Taking natural log on both sides, we get-
The gas viscosity (η) will increase with temperature. According to the kinetic theory of gases, viscosity should be proportional to the square root of the absolute temperature, in practice, it increases more rapidly.
What is the Effect of Pressure on Viscosity ?
On increasing pressure, viscosity of liquids increases. This is due to fact that decrease in the number of holes as the pressure increases. Therefore it becomes more difficult for liquid molecules to move around and thus it becomes more difficult for them to flow.
Contact form


BSc 1st Year English Books: Free Download [2024 Updated]
BSc 1st Year English Books PDF
In today’s digital world, English is a necessity in almost every field.
If you are a BSc 1st year student with English as a subject, then it is essential to develop a strong command of the English language to excel in academic pursuits and future career opportunities.
Through this post, we aim to provide you with updated free download links for the English books for BSc 1st year students.
BSc 1st Year English Syllabus
The BSc 1st year English course covers a wide range of topics designed to enhance language skills, critical thinking, and communication abilities.
The syllabus includes areas such as grammar, vocabulary building, reading comprehension, writing skills, and spoken English.
Each topic holds great importance, as they form the foundation for effective communication and academic success.
The syllabus of English for BSc 1st year is divided into two semesters: Semester I & Semester II. Each semester comprises one to two books.
You can view the list of subjects in the below table:
| Semester I | Semester II |
|---|---|
| 1. British Poetry and Drama: 17th and 18th Century | 1. Indian Writing in English |
| 2. English Communication (L1) | 2. British Literature: 18th Century |
| 3. English Communication (L2) |
How to download the BSc English Syllabus of my University/College?
To download the syllabus of your university, follow these simple steps:
1. Visit the official website of your university.
2. Navigate to the “Faculty” section or a similar section that houses academic resources.
3. Look for the syllabus section or a dedicated page for course materials.
4. Locate the relevant department or program corresponding to your field of study.
5. Within the department or program page, you should find a list of available syllabi for different courses.
6. Click on the desired course or program to access the syllabus document.
7. Download the syllabus to your device or print it for reference.
Additionally, it is advisable to connect with your teachers or professors for any specific or updated information regarding the syllabus.
They can provide you with detailed insights, answer your questions, and guide you through the course requirements.
Remember, the official website of your university is the most reliable source for accessing the syllabus. Stay proactive in staying up-to-date with the syllabi for your courses to ensure a successful academic journey.
Also, please note that some universities use the same English syllabus for all UG programs. So in that case you might find “ BA/B.Com English Syllabus ”, instead of BSc.
Related: Download BA English Books (All Semesters)
BSc 1st Year English Books Download PDF
In the last section, we have already discussed all the subjects when it comes to BSc with English. Now, following this section, you will be able to find download links for BSc 1st year english books in pdf format.
But, before you download these books please keep in mind that Fullonstudy does not hold the copyright for the books mentioned in this post. These books are the intellectual property of various websites (source at the end of this post). The purpose of sharing these books is solely to assist and support students in their academic pursuits. However, Fullonstudy cannot guarantee the accuracy or completeness of the content provided. It is advisable to cross-reference information and exercise caution while using these resources. Fullonstudy shall not be held liable for any errors, omissions, or consequences arising from the use of these books.
All the books along with their download links are shared in the below table. You can simply click on the download link & proceed with the download process.
| Book Name | Download Link |
|---|---|
| British Poetry and Drama: 17th and 18th Century | |
| English Communication (L1) | |
| Indian Writing in English | |
| British Literature: 18th Century | |
| English Communication (L2) |
Further, if you still can’t download these books using these download links, then you can follow a brief tutorial discussed in the next section.
Step-by-step guide to download BSc 1st Year English Books
1. First click the links shared in the last section. Let’s say we have to download “ British Poetry & Drama (17th & 18th century) ”, so we can simply click on the first download link.
2. After clicking the download link, the pdf will be directly downloaded on your computer. In case if you are using a smartphone, you have to use a pdf viewer like Adobe, WPS Office, etc.
Best English Books for BSc 1st Year
To assist you in your English language journey, we have compiled a list of recommended books that align with the BSc 1st year English syllabus.
These books have been carefully selected for their comprehensive coverage of the topics and their ability to engage and enhance your language skills.
Here are three books that we highly recommend:
1. The Best Guide British Poetry and Drama (17th-18th Century)
It is an exceptional literary resource that delves into the rich heritage of British poetry and drama from the 17th to the 18th century.
This comprehensive guide offers readers a profound analysis and insightful exploration of the literary masterpieces that emerged during this remarkable era.
Whether you are a student of literature, an avid reader, or simply an enthusiast of classical English literature, “The Best Guide British Poetry and Drama” is an indispensable companion.
Its engaging narrative and profound insights will captivate readers, enabling them to appreciate the depth and significance of these influential works.
This book serves as an invaluable tool for academic study, providing comprehensive coverage of major poets like John Donne, Alexander Pope, John Milton, and William Wordsworth, as well as iconic playwrights such as William Shakespeare and John Dryden.
| |
2. A Guidebook of Indian Writing in English
This book is an indispensable literary companion that takes readers on a captivating journey through the rich and diverse landscape of Indian literature in the English language.
This comprehensive guide offers a deep exploration of the vibrant tapestry of Indian writing, showcasing the incredible contributions made by Indian authors to the world of literature.
This guidebook goes beyond a mere listing of authors and their works; it delves into the historical, social, and cultural contexts that have influenced the development of Indian literature in English. The author’s expertise and meticulous research bring to life the complexities and nuances of this literary tradition, shedding light on the themes, techniques, and narrative styles employed by Indian writers.
There are three major English subjects in the syllabus of BSc 1st year namely: British Literature, Indian Writing & English Communication.
For Indian Writing, you can go with “A guidebook of Indian Writing – by Elit Publications”.
In order to download the syllabus of your university, you can visit the official website of your university & under Faculty section, download the syllabus. Further, you can also connect with your teachers for the same.
To download BSc 1st year English books in pdf format, you can simply visit our website fullonstudy.com & search for the same.
Mastering the English language is an integral part of your BSc 1st year journey and future professional success.
By utilizing the recommended English books and exploring supplementary reading materials, you will enhance your language skills, broaden your knowledge, and gain a competitive edge.
I hope following this post, you will be able to download BSc 1st year English books in pdf format. In case you have any issue, simply leave a comment or email us ( [email protected] ) & our team will reach out to you asap!
- https://ddceutkal.ac.in/Download_Course_Materials_UG.asp
- https://egyankosh.ac.in/handle/123456789/25364
- https://www.ugc.gov.in/pdfnews/5430486_B.A.-Hons-English.pdf
- http://www.lalgolacollege.org/syllabus/engg.pdf
Fullonstudy
Related posts.

BSc 1st Year Chemistry Notes 2024 (Download PDF)

IGNOU Study Material 2024: Free Download PDF (eGyanKosh)

BSc Botany Syllabus: 1st, 2nd & 3rd Year (PDF) – 2024 Edition

First Semester General Chemistry Notes (Sections 1-11)
Purchase 1st semester notes.
To purchase and instantly download ALL 211 pages of "First Semester Notes - Sections 1-11," simply follow the link below.
1st Semester of "GENERAL CHEMISTRY NOTES"
- Section 1 - Section 11 (211 pages)
- Amounts to Only $4.54 per Section When You Buy the Entire Semester (Save 56% per Section)
- Immediate Download!
Get the Notes! - $49.99
The First Semester of General Chemistry Notes is 211 pages in length (Section 1 through Section 11) and covers ALL lecture notes and topics discussed in the 1st semester of your general chemistry lecture course.
For the detailed contents of each "Section", use the Main Menu and navigate to the GENERAL CHEMISTRY NOTES+ tab.
SECTION 1 – Foundations of Chemistry - 19 pages. SECTION 2 – Atom, Molecules, and Ions - 21 pages. SECTION 3 – Chemical Quantities and Stoichiometry - 16 pages. SECTION 4 – Types of Chemical Reactions and Solution Stoichiometry - 26 pages. SECTION 5 – Gases - 18 pages. SECTION 6 – Thermochemistry - 17 pages. SECTION 7 – The Quantum Mechanical View of the Atom, and Periodicity - 19 pages. SECTION 8 – Chemical Bonding - 28 pages. SECTION 9 – Covalent Bonding and Molecular Orbitals - 16 pages. SECTION 10 – Liquids, Solids, and Intermolecular Forces - 18 pages. SECTION 11 – Solutions and Their Properties - 13 pages.
Get the Notes! - $49.99
- choosing a selection results in a full page refresh

COMMENTS
BSc 1st year consists of two semesters, i.e. Semester I and Semester II. In this post, I have provided the download links of Chemistry notes for BSc Sem I as well as Sem II. In our recent post, we have also shared physics & botany notes for BSc 1st year. This is all about our previous similar posts for BSc 1st Year Students.
Physical Chemistry Projects. Chemical Kinetics: Investigate the rate of chemical reactions under different conditions and analyze reaction mechanisms. Electrochemistry: Explore the principles of electrochemical cells, study electrode processes, and investigate applications in energy storage.
Putting this into consideration, here on knowdemia, we have made BSc 1st year organic chemistry notes, BSc 1st year chemistry notes pdf, BSc chemistry practical notes part 1, BSc 1st year chemistry notes PDF, BSc 1st year physical chemistry notes, and other materials to help you pass chemistry. Below we'll explore good chemistry notes for BSc ...
Here, b is a constant. Having taken into account the corrections for pressure and volume is Ideal Gas Equation becomes-. (P + an 2 /V 2) (V − nb) = nRT ----- (equation-1) (equation-1) is known as van der Waals equation. In this equation n is number of moles of the gas.
For Bsc 1st year studentsAn organic reaction mechanism is a complete, step-by-step account of how a reaction of organic compounds takes place.The description...
The paired electrons of the valence shell does not take part in the bond formation. 4. A covalent bond is formed by the overlapping of two half filled valence atomic orbitals of two different atoms. 5. The electrons in the overlapping orbitals get paired and confined between the nuclei of two atoms. 6.
CBCS B.Sc. 2nd Semester Admission is going on, Class Start: 11th January @7.30AM. Access comprehensive B.Sc. 1st Semester Chemistry notes, previous year questions, syllabus, and eBooks for upcoming exams. In this page i will provide you complete notes of B.Sc. 1st year and also 1st semester including chapters- Gaseous State, Liquid State, Phase ...
In this video we have discussed Atomic Structure B.SC 1st Year Chemistry Most Important Topics For Exam 2021📝Download B.SC 1st Year Complete Notes and Previ...
In these free Atomic Structure and Chemical Bonding notes pdf, we will study the atom, which is a necessary pre-requisite in understanding the nature of chemical bonding in compounds. It provides basic knowledge about ionic, covalent, and metallic bonding and explains that chemical bonding is best regarded as a continuum between the three cases.
BSc chemistry books for 1st, 2nd & 3rd-year students in pdf format is available for free to download. For each year we have provided chemistry textbooks for Inorganic, Organic & Physical Chemistry. ... Let us now move to our main topic, i.e. BSc Chemistry books. ... BSc 1st Year English Books: Free Download [2024 Updated] BSc 1st Year Physics ...
Some of BSc 1st year important questions in Chemistry (question bank) in pdf format are provided below, which can be used to prepare for the exam. In the BSc examination, most of the questions asked are from the theory section. As per the syllabus of chemistry, you should make notes. Pro Tip: Leave all the topics which are not in the syllabus.
Gaseous State - BSc 1st Year Chemistry Notes Read More Details. By Dipendra Chaulagain 08 Nov, 2023. Alkene - BSc 1st Year Chemistry Notes pdf Read More Details. ... Notes, Assignments & Quizzes; Friendly Environments & Teachers; Quality at Friendly Budget; Explore Our Courses. Online Class Interface. Our Masters Meet Our Expert Team.
Home BSC NOTES B.Sc. Chemistry First Semester (I) Class Notes ... GATE, TIFR, BARC, ISRO Previous Year Papers and their solutions, free video lectures, Best Books Recommendations for preparation for the CSIR-UGC NET, GATE and Other Competitive Exams to the ASPIRANTS who want to pursue their career in the field of Chemistry. @IndianChemistry
The modern standard is the carbon-12 atom, whose mass is defined as exactly 12 atomic mass units. Thus, the atomic mass unit (amu) is 1/12th the mass of a carbon-12 atom. Based on this standard, the 1 H atom has a mass of 1.008 amu; in other words, a 12 C atom has almost 12 times the mass of an 1 H atom.
Here are some chemistry project topics in biochemistry for bsc students. 1. Investigation of Enzyme Kinetics and Inhibition. 2. Analysis of Protein Structure and Function. 3. Study of Metabolic Pathways and Bioenergetics. 4. Investigation of DNA Damage and Repair Mechanisms.
The emission or absorption of radiation by the atom takes place when an electron jumps from one stationary orbit to another. 6. The radiation is emitted or absorbed as a single quantum (photon) whose energy h𝜈 is equal to the difference in energy ΔE of the electron in the two orbits involved. Thus, h𝜈 = ΔE.
Students also viewed. BSC Chemistry 1st Year UNIT 1 Atomic Structure Part 2. Topic Rice Grain Milling. 4.3 Selective Dissemination OF Information. 3.2.3 Reference Service Types AND Functions. Capital budgeting. Capital budgeting decision criteria.
5. Investigating Environmental Chemistry. Environmental chemistry projects are particularly relevant in today's world. BSc students can focus on topics like water quality, air pollution, or soil ...
The 1st year of BSc Chemistry contains very few electives. Some of the electives are Chemical Energetics, Equilibria & Functional Organic Chemistry. The top skills required for BSc Chemistry are Patience, determination, Flexibility, Scientific and numerical skills, Excellent analytical skills, etc. Mendham.
4.9 BSc Chemistry First Year Syllabus. 4.10 BSc Physics First Year Syllabus. 4.11 BSc Nautical Science First Year Syllabus. 4.12 BSc Forestry First Year Syllabus. 4.13 BSc Mathematics First Year Syllabus. 4.14 BSc Zoology First Year Syllabus. 4.15 BSc Botany First Year Syllabus. 4.16 BSc Optometry First Year Syllabus.
Macleod in 1923 gave the following relation between the surface tension (γ) and density (D) for a normal liquid-. C=γ 1/4 / (D −d) where d is vapour density of the liquid at given temperature and C is constant. In 1924, Sugden modified the above equation as-. γ 1/4 / (D −d)=MC=P.
The syllabus of English for BSc 1st year is divided into two semesters: Semester I & Semester II. Each semester comprises one to two books. You can view the list of subjects in the below table: Semester I. Semester II. 1. British Poetry and Drama: 17th and 18th Century. 1. Indian Writing in English.
Get the Notes! - $49.99. The First Semester of General Chemistry Notes is 211 pages in length (Section 1 through Section 11) and covers ALL lecture notes and topics discussed in the 1st semester of your general chemistry lecture course. For the detailed contents of each "Section", use the Main Menu and navigate to the GENERAL CHEMISTRY NOTES+ tab.