An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.


USAID awards $45 million to African scientists developing HIV vaccine
January/february 2024 | volume 23 number 1.
By Susan Scutti

The South African Medical Research Council (SAMRC) received a grant worth more than $45 million to implement the HIV Vaccine Innovation, Science, and Technology Acceleration in Africa (HIV-VISTA) program. SAMRC President and CEO and former Fogarty trainee, Dr. Glenda Gray guided the application process for this grant from the U.S. Agency for International Development (USAID).
To compete for funding, Gray established the BRILLIANT consortium (BRinging Innovation to cLinical and Laboratory research to end HIV In Africa through New vaccine Technology). BRILLIANT, which includes scientists from eight African countries, will design and implement early-stage clinical trials of HIV vaccine immunogens and conduct laboratory analyses and epidemiological studies.
The consortium plans to test HIV vaccine concepts and advance the most promising candidates while working with existing scientific talent and community engagement channels in sub-Saharan Africa (SSA). As part of its SSA-led HIV vaccine effort, consortium members plan to partner with policymakers, affected communities (including people living with HIV/AIDS), advocates, and communicators. A complementary goal of the consortium is to strengthen systems of collaboration throughout SSA while increasing career opportunities for promising regional scientists. One such scientist is Dr. Sophia Osawe, who has been working at the Institute for Human Virology in Nigeria (IHVN) for the past 16 years.
Developing skills while developing a vaccine
In her role at IHVN, Osawe, a former Fogarty Global Health Fellow who recently defended her PhD in medical microbiology and immunology, writes, implements, and manages grants, and coordinates researchers and study participants. Osawe sees BRILLIANT as an opportunity “to develop more of my skills in the lab and in immunology, interact with other early career researchers, and just continue improving my ability to write grants and help implement studies in my community.”
To date, a vaccine to sustainably control the HIV pandemic has been elusive. UNAIDS estimates that 1.3 million people became newly infected in 2022, with almost two-thirds of these new infections occurring in SSA. And while women and girls accounted for 50% of new infections globally, they accounted for 63% of all new infections in SSA. Already, there are twice as many young SSA women as men between the ages of 15 and 24 living with HIV, and they struggle to access treatment and prevention due to stigma and policies. BRILLIANT sees communities as a central part of its effort to design a pivotal HIV vaccine, and so it includes community representatives in all its decision-making bodies.
In the absence of a vaccine, scientists have made significant progress combating HIV and achieving some control over the epidemic. New biomedical tools, such as pre-exposure prophylaxis, offer promise for continuing to reduce HIV incidence. Still, a safe, effective and affordable HIV vaccine could enable a more profound, rapid, and cost-effective rate of reduction. Experts believe it is crucial that HIV vaccine candidates be designed to neutralize virus types prevalent in the SSA region, where most new infections occur.
Osawe said, “Nigeria is the only West African country involved in the consortium, so we’re very excited. Hopefully we will be a site chosen to implement a HIV vaccine trial in our population.” Through IHVN, she’s worked with colleagues in other countries in the past. One project she helped implement in Nigeria and South Africa focused on mothers living with HIV and their infants. The differences across communities, cohorts and operational systems taught both teams more about recruitment and retention strategies. Osawe noted that the Nigerian mothers were less likely to drop out of the study compared to the South African mothers, partly because they were more often married and so found participation, including traveling to the study site, easier. To boost retention of the South African mothers, the teams shared their experiences and retention strategies.
“I've grown over the years interacting with and learning from the different groups that we work with, so it's very important to have that mix,” said Osawe. “We learn from each other how to improve what we do to help our communities."

Lessons learned from regional burden
Over the past two decades, SAMRC has conducted essential research on HIV and related conditions. South African scientists collaborating with SAMRC are ranked among the world’s best in all aspects of the HIV response, including prevention of HIV infection from mother to child, the development of newer and safer drug regimens, and the health service delivery of antiretroviral treatment and preventions. USAID’s investment, then, is not just a financial contribution, it's a beacon of hope for HIV vaccine discovery in Africa, stated Gray in a press release.
Dr. Linda-Gail Bekker, a BRILLIANT scientist and a former Fogarty trainee who is now director and CEO of the Desmond Tutu HIV Centre and Health Foundation, commented, “Africa has borne an enormous burden of the HIV epidemic, and it is fitting that our continent plays a significant role in bringing an effective, affordable and accessible HIV vaccine to the world.”
Fogarty's Impact
The BRILLIANT consortium also boasts three other former Fogarty trainees and grant recipients: Osawe’s colleague at IHVN, Dr. Evaezi Okpokoro; Dr. Penny Moore of the National Institute for Communicable Diseases/University of Witwatersrand, South Africa; and Dr. Wilbert Mbuya of the National Institute for Medical Research, Tanzania. All are well acquainted with the challenges of conducting research in low- and middle-income countries.
As a SSA researcher, Osawe contends with many difficulties, she told Fogarty, including traveling to hard-to-reach regions—because of a lack of road infrastructure, political volatility or regional crises—and providing additional safety measures for refrigerated samples in areas where power outages can occur. Still, she said, “I love what I do. When I was little, I was very curious about my surroundings, I was very curious about how people fell ill. Now, I'm able to contribute my own part to help my community. Overall, it has been fulfilling. And it's just important to be fulfilled in life.”
More Information
- SAMRC awarded a multi-million rand grant from USAID to develop and test novel HIV vaccines in Africa , South African Medical Research Council news release, September 21, 2023
- BRILLIANT Consortium
- Related articles:
- Preventing mother-to-child HIV transmission in South Africa: Q and A with Dr Glenda Gray
- Q and A with former Fogarty trainee, Dr Linda-Gail Bekker, President of the International AIDS Society
Updated February 14, 2024
To view Adobe PDF files, download current, free accessible plug-ins from Adobe's website .
Related World Regions / Countries
- Sub-Saharan Africa
Related Global Health Research Topics
- Open access
- Published: 06 November 2021
Meeting report: South African Medical Research Council Standard of Care in Clinical Research in Low- And Middle-Income Settings Summit, November 2017
- Maurine D. Miner ORCID: orcid.org/0000-0002-4637-1283 1 ,
- Linda-Gail Bekker 2 ,
- Tamara Kredo 3 , 4 ,
- Niresh Bhagwandin 3 ,
- Lawrence Corey 1 , 5 &
- Glenda E. Gray 1 , 3
Trials volume 22 , Article number: 778 ( 2021 ) Cite this article
1044 Accesses
2 Citations
Metrics details
A cornerstone of HIV prevention clinical trials is providing a combination prevention package to all trial participants. The elements included in that standard of care (SoC) package evolve as new prevention modalities are developed. Pre-exposure prophylaxis (PrEP) was recommended by the World Health Organization for persons at high risk of acquiring HIV, but not all countries immediately adopted those recommendations. The South African Medical Research Council (SAMRC) convened a summit to discuss issues relating to SoC and PrEP in HIV prevention clinical trials taking place in lower- to middle-income countries (LMIC). Policymakers, regulators, ethicists, experts in law, researchers, representatives of advocacy groups, and the HIV Vaccine Trials Network (HVTN) presented a framework within which SoC principles could be articulated. A group of subject matter experts presented on the regulatory, ethical, scientific, and historic framework of SoC in clinical trials, focusing on PrEP in South Africa. Summit participants discussed how and when to include new HIV treatment and prevention practices into existing clinical guidelines and trial protocols, as well as the opportunities for and challenges to scaling up interventions. The summit addressed challenges to PrEP provision, such as inconsistent efficacy amongst different populations and various biological, virological, and immunological explanations for this heterogeneity. Advocates and community members propagated the urgent need for accessible interventions that could avert HIV infection. The meeting recommended supporting access to PrEP in HIV prevention trials by (1) developing PrEP access plans for HIV vaccine trials, (2) creating a PrEP fund that would supply PrEP to sites conducting HIV prevention trials via a central procurement mechanism, and (3) supporting the safety monitoring of PrEP. This report summarizes the presentations and discussions from the summit in order to highlight the importance of SoC in HIV prevention clinical trials.
Peer Review reports
Introduction
HIV prevention has evolved tremendously over the last decade. Traditionally, antiretroviral therapy (ART) was prescribed to people living with HIV (PLWH) to slow the progression of AIDS when CD4+ T-cell counts dipped below 200 cells per microliter. As ART reduces viral load, it was hypothesized to work as ‘treatment as prevention’ (TasP) to inhibit sexual transmission. In a trial of serodiscordant couples that compared early ART administration (irrespective of CD4 counts) to delayed, the early treatment reduced the risk of sexual HIV transmission to their uninfected partner by over 90% [ 1 , 2 ]. This study, conducted by the HIV Prevention Trials Network (HPTN) HPTN 052, has since been joined by other trials that have shown undetectable viral loads prevent transmission and solidified the use of ART for TasP [ 3 , 4 ].
The ‘inverse’ of HPTN 052 was investigating the efficacy of ART when administered to the HIV-uninfected partner in a serodiscordant relationship, a concept of pre-exposure prophylaxis (PrEP). Several randomized controlled trials (RCTs) comparing different combinations of oral tenofovir disoproxil fumarate (TDF) and TDF/emtricitabine (TDF/FTC, brand name Truvada) in serodiscordant couples have shown that PrEP can reduce HIV acquisition, although to different extents based on the population [ 5 , 6 , 7 ]. Prevention efficacy is robust in men, but contradictory results have been reported in women [ 5 , 6 , 7 , 8 , 9 ].
A cornerstone of HIV prevention clinical trials is providing a combination prevention package (e.g. risk reduction counselling, free condoms, diagnosis, and treatment for STIs) to all trial participants. The package provision is part of an obligation to minimize the participants’ risk of acquiring HIV, as assumption of risk can be underestimated in RCT participants [ 10 , 11 ]. As new prevention modalities emerge, the prevention packages are adapted accordingly to provide the best care to participants whilst considering cost, population impact/efficacy, government and other funders’ roles, and clinical science integrity. In 2010, after iPrEx results showed PrEP efficacy in men who have sex with men (MSM) and transgender women, the HIV Vaccine Trials Network (HVTN) held a series of consultations with advocates, ethicists, and other stakeholders; surveys of participants; and discussions with protocol team leadership and research site investigators to evaluate the pros and cons of potential approaches to PrEP access in an ongoing HIV vaccine efficacy trial (HVTN 505) [ 12 ]. The group considered three options (ranging from providing information on PrEP to providing PrEP itself) and four main issues: researcher obligation to participants and their communities, effects on study design, health policy recommendations, and stakeholder opinions. The consensus was to provide PrEP information and referrals for PrEP access. For the latter, Gilead donated Truvada for any interested participant and the HVTN coordinated a contract with a mail-order pharmacy to ease access. As HVTN 505 was conducted only in the United States (US), ethical issues about differential health resources and regulatory standards in other countries were not addressed. Following this prevention package amendment, in 2012, PrEP was approved for use in populations at high risk of HIV infection in the US by the Food and Drug Administration [ 13 ].
In 2015, the World Health Organization (WHO) made a recommendation for the inclusion of oral PrEP as part of a combination HIV prevention package for people at substantial risk of HIV infection [ 14 ]. In 2017, TDF/FTC was licensed for use as PrEP in 17 countries and was included in the WHO Essential Medicines List (EML). At that time, although PrEP was approved in South Africa, its availability was limited to the National Department of Health (NDoH)-sanctioned sub-populations (specifically MSM and sex workers) and research-led demonstration projects. Unfortunately, this precluded heterosexual girls and women access to PrEP. Due to the high prevalence of HIV in women in South Africa, up to 24% in some areas [ 15 ], this population makes up a large proportion of participants in HIV prevention clinical trials conducted there. A substantial gap existed between participants in HIV prevention trials and local access to comprehensive prevention packages. The South African Medical Research Council (SAMRC) has a mandate to address issues that could potentially impact the conduct of health research, including clinical trials. As such, the SAMRC together with the HVTN convened a summit in Cape Town in 2017 to address PrEP access as a case study on the standard of care (SoC) in clinical trials in lower- and middle-income countries (LMIC). Whilst the summit focused on PrEP provision in South Africa, the issues raised are applicable to other forms of SoC across LMICs. This article summarizes the viewpoints and discussions that took place in November 2017 at the summit.
As a result of the lack of PrEP licensure for those at high risk of HIV acquisition in South Africa, the SAMRC reached out to stakeholders in communities, government, academia, and pharmaceutical companies to discuss issues concerning PrEP provision as SoC in HIV prevention trials. The 2-day summit began with presentations from experts on ethics, regulatory processes, clinical studies, basic science, and community engagement, followed by a day of panel discussions with the goal of providing a comprehensive and thought-out plan on PrEP provision for HIV prevention trials in-country. The overall objective of the meeting was to engage regulatory, legal, and ethical frameworks, whilst considering perspectives of government, community organizations and advocates, and the funders of research to review the principles behind setting SoC for prevention clinical trials in LMIC to address the PrEP gap. There were 18 presentations on the first day (Supplemental Table 1 ) and three panel discussions on day 2. Both days were recorded and transcribed. This report summarizes the main themes from the summit, which resulted in a recommendation and Executive Statement from the SAMRC [ 16 ]. An Epilogue provides an update of what has happened in this space since the summit took place in 2017.
Objectives:
To outline what a SoC is and whether PrEP falls into this category for HIV prevention clinical trials in South Africa
To present context of PrEP efficacy in South African females
To discuss the approaches of how to provide PrEP as a SoC in HIV prevention trials in South Africa
Themes of the summit
Use of local and international guidelines to define soc in efficacy trials.
A modern definition of SoC is ‘that which a minimally competent physician in the same field would do under similar circumstances’ [ 17 ]. In the context of HIV vaccine RCT, SoC includes a basic prevention package that every participant has access to regardless of whether they receive vaccine or placebo. Local regulatory bodies ensure clinical trials include SoC and adhere to guidelines such as Sections 21 and 19 of the Medicines and Related Substances Act and General Regulations [ 18 ], the Nuremberg Code, Declaration of Helsinki and the Belmont Report [ 19 , 20 , 21 ], and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) on Good Clinical Practice (GCP) [ 22 , 23 ]. GCP requires clinical trial SoC to include informed consent, information about adverse outcomes, potential for post-trial treatment, and adverse drug event reporting. The South African Bill of Rights discusses the right to health in Section 27 of the Constitution: ‘The state has to take reasonable legislative measures within its available resources.’ The WHO, US Centers for Disease Control and Prevention (CDC), South African NDoH, and South African HIV Clinicians Society all have HIV prevention/treatment trial guidelines available. The purpose of these international guidelines on SoC is to promote consistency and ensure equity in clinical trials globally.
However, the definition of SoC can vary depending on context or location. PrEP licensure for ‘those at risk of HIV’ is such an example. Apart from a universal SoC, an international consensus standard considers SoC factors such as cost, the balance between desirable and undesirable effects, and acceptability to those who will receive treatment [ 24 , 25 , 26 ]. The SAMRC has funded the South African Guidelines Excellence ( SAGE ) project, which is a multi-partner, 5-goal project to develop and implement primary care guidelines in South Africa [ 27 ]. SAGE applies stakeholder expertise to efficiently identify and address resource limitations in SoC across the diverse health care system of South Africa. The SAGE project is in the midst of evaluating PrEP use for women in South Africa.
In 1996, the NDoH implemented the South African Standard Treatment Guidelines and Essential Medicines List (STG/EML), which determines access to and availability of medicines in the public sector, to ensure SoC for its citizens. Four criteria must be met for a medication to be approved to the EML: (1) public health need; (2) safety, efficacy, and quality; (3) pharma-economics/cost; and (4) practice considerations. In 2017, PrEP was not part of the EML although 26 countries had adopted the WHO recommendations for its use [ 28 ].
Historical precedent for implementing new SoC for HIV prevention in South Africa
South Africa has, in the past, adopted international SoC guidelines for the prevention of mother-to-child transmission (PMTCT) of HIV. In 2002, the non-governmental organization Treatment Action Campaign (TAC) filed a lawsuit against the South African government over its limited ‘duty of care’ distribution of the antiretroviral drug nevirapine [ 29 ]. The government at the time denied scientific evidence surrounding HIV/AIDS and the Ministry of Health was opposed to any form of nevirapine rollout. TAC argued these limitations put PLWH and their newborns at a substantial health risk, violating their constitutional rights. The court ruled in favour of TAC that the government’s limited supply was unconstitutional as it denied nevirapine administration in the public health system where there was capacity and its use was medically indicated [ 30 , 31 ].
In 2013, the Joint United Nations Programme on HIV/AIDS (UNAIDS) developed a new SoC for PMTCT of HIV. The new SoC, called Option B+, was to replace the previously used Options A and B that involved different ART regimens [ 32 ]. There was a large local debate after the international community proposed that South Africa implement Option B+, as the country was doing reasonably well with Options A/B. As it turned out, it was the right decision to move to Option B+; South Africa lowered the MTCT rate from 8% in 2009 to 2% in 2015 [ 33 ]. South African researchers used local data to make decisions based on the international guidance. This exemplifies how global decision-making can affect local SoC guidelines in South Africa.
Implications of conflicting PrEP efficacy data for South African women
As stated before, SoC can vary based on context or region. The economic strength/status and medical infrastructure of a nation, city, or village can profoundly impact the type of health care its citizens receive. South Africa is one of the most unequal societies in the world, with a Gini coefficient of 0.63 [ 34 ] and an annual health expenditure of 8.2% gross national product [ 35 ]. There were an estimated 7.7 million PLWH in 2017, equating to an overall prevalence of ~ 14% [ 15 ]. However, heterosexual females are one of the most at-risk groups with an estimated prevalence of 24%. This is in contrast to, for example, the US where MSM and transgender women are at the highest risk (~ 12% prevalence) [ 36 , 37 ].
In fact, the discrepancy in PrEP efficacy between MSM and heterosexual women is a major factor in its omission from the South African EML. A systematic review of oral PrEP RCT across a range of populations and settings concluded that it was effective in reducing HIV infection risk across gender, PrEP regimen, dosing, and mode of acquisition [ 38 ], and numerous studies have shown high efficacy of oral PrEP in MSM populations [ 6 , 39 , 40 , 41 ]. Table 1 outlines the five efficacy trials of oral PrEP that enrolled women to date: Partners PrEP, Bangkok TDF, FEM-PrEP, VOICE, and TDF2. As depicted in the table, efficacy in heterosexual women is inconsistent. Numerous confounders have been suggested for the discrepancy, including biological factors (e.g. viral subtype, vaginal microenvironment) and behavioural factors (e.g. drug adherence, relationship status). In fact, a meta-analysis of these trials found that adherence to PrEP correlated with efficacy [ 43 ], and unfortunately, adherence has been lower for women in LMIC than MSM in high-income countries. Further data will be required to refine the estimates of PrEP efficacy in southern African women to aid in appropriate messaging, characterizing the impact of PrEP at a population level and within HIV prevention efficacy trials and understanding conditions that might compromise efficacy [ 43 ]. Despite these unknowns, the South African AIDS Committee guidelines included the provision of daily PrEP for high-risk populations in 2016 [ 44 ].
Ethics committees responsible for evaluating SoC in clinical trials face a dilemma when there is a lack of consensus in the scientific community. In 2005, a prominent South African ethicist encouraged researchers that, ‘Contributing to sustainable improvements in health by progressively ratcheting the SoC upwards for research participants and their communities is an ethical obligation of those resource-rich countries who sponsor and implement research in poorer ones’ [ 45 ]. In 2016, the US-based ethicist Jeremy Sugarman referred to what is now highly quoted as the ‘rebuttable presumption’ [ 46 ]. According to Sugarman, the onus was placed on researchers to justify not offering PrEP to participants in HIV prevention trials. But, as stated before, this is not a simple issue when local governments do not provide that SoC to everyone in the target community.
The National PrEP Technical Working Group and PrEP availability in South Africa
In October 2015, the NDoH convened a meeting to consider the soon-to-be-released WHO guidelines recommending PrEP use for all populations at substantial risk of HIV. At that meeting, the National PrEP Technical Working Group was formed, which has been an important vehicle for guiding PrEP introduction into South Africa. Shortly thereafter, the Medicines Control Council licensed TDF/FTC for PrEP use and, by June 2016, the beginnings of a publicly funded programme began where PrEP was provided to sex workers as part of the National Sex Worker Program. It was one of the first nationally funded PrEP programmes in Africa and was lauded by UNAIDS [ 47 ]. Between 2016 and 2017, publicly funded programmes provided nearly 3000 PrEP initiations at 17 sites across South Africa. A key take-home from the National Sex Worker Program data was that initially uptake was quite low, partly because prior to licensure, sex workers were sceptical as to the motives of including them in a programme. But with increasing awareness of PrEP and perhaps expansion of access to other populations, hesitancy seemed to decrease.
Since the kick-off of the National Sex Worker Program, the National PrEP Technical Working Group began implementing demonstration projects for adolescent girls and young women. Those projects have evaluated a number of strategies, including sex-positive materials targeted at young people, fixed facilities and mobile delivery models, drug-level feedback counselling, use of social clubs, and integration with services such as gender-based violence prevention. The demonstration projects have indicated that PrEP uptake, which varies across regions from 36 to 98%, increases when it is part of a broader prevention package that includes peer support, mobile services, and convenient operating times, or as part of a sexual reproductive health package. The working group has also investigated ways to reduce the burden of repeated visits for PrEP, particularly because the goal is to provide a convenient service for healthy populations. Finally, it is critical to train health care workers and ensure their positive attitudes in prescribing PrEP.
It is predicted that PrEP uptake will be variable and likely evolve over time. Clinical trialists should agree on an adequate package for adherence support and recognize that patterns of PrEP use will most likely vary over the course of a trial.
Designing clinical trials in the era of PrEP
Offering PrEP to vaccine or placebo recipients in an HIV vaccine clinical trial will not truly disturb the ability to answer the question at hand: whether the new vaccine prevents HIV acquisition. This is true for most HIV vaccine studies, as the two modalities typically have different mechanisms of action. It would, however, have an impact. If participants use PrEP effectively, HIV incidence would decrease and therefore affect the statistical power of the primary objective (i.e. vaccine efficacy). For example, the HIV vaccine trial HVTN 505 increased its sample size from 1350 to 2500 participants to accommodate for PrEP use [ 48 ]. As such, consideration of PrEP provision must be addressed and analysed by statisticians early during the trial design process so that the scientific validity of the study is not compromised.
Another issue to consider is how PrEP provision is paid for. Most HIV prevention trials conducted in South Africa are sponsored by the US government (via the NIH), which stipulates research funds cannot be used for medication and SoC procedures, including lab work. The HVTN has previously raised philanthropic dollars to provide TDF/FTC through an online pharmacy when a participant received a prescription from their physician. This preserved community equity as well as lessened the potential burden on CRS staff of medical care that could detract from the necessary documentation and effort required to perform the trial. Typical vaccine efficacy trials last 5 years, and because NIH-sponsored trials are reliant on governmental budgeting, there is never a guarantee that grant funding will continue. In this system, an organization such as the HVTN can only guarantee PrEP access for the life of the trial. Ideally, local governments in the region a trial is conducted will step up at that point to provide post-trial access.
Representatives from pharmaceutical companies sponsoring clinical trials have acknowledged the importance of providing post-trial access to interventions that work. As these companies are themselves benefitting from the participants in a trial, the argument could be made that the companies owe the participants. There is precedent for including language in trial protocols that the sponsor will continue to provide access to a medication until it is accessible to study participants elsewhere. This post-trial access, some argue, is the industry’s responsibility. As PrEP could offer a population-level reduction in HIV prevalence, post-trial access would also provide benefit to local communities where the trials are conducted.
Community voices should be considered when implementing PrEP access in clinical trials
The benefits of community engagement and collaboration between clinical trial networks and community advocates are well documented [ 49 , 50 , 51 ]. Community Advisory Board (CAB) members are integral colleagues in the operations of trials conducted by the US NIH-funded HIV/AIDS networks, such as the HVTN. CABs help ensure that SoC given to clinical trial participants are ethical, scientifically valid, and developed with sincere collaboration with communities and advocates. In regard to HIV prevention trials conducted in South Africa, community stakeholder involvement in decision-making on PrEP provision is essential to ensuring protocols are acceptable to trial participants. As such, advocates and community representatives should be engaged in the design of the PrEP plans. Communities should feel ‘ownership’ rather than ‘buy-in.’ It is also important there be a mechanism for advocates and community members to monitor and inform SoC evolution so that the care is accountable, transparent, and client centred.
As there are approximately 2000 new HIV infections in young women in South Africa each week, it is imperative that if an effective agent against HIV acquisition exists, its use should not be put on hold. Clinical trial participants will need choices for HIV prevention; a one-size-fits-all approach will not likely make a significant dent in acquisition rates. PrEP should be provided to participants who choose to take it either through easily accessible health care organizations/clinics in the region or by the clinical trial sites themselves. In addition, the success of PEPFAR in ART provision in Africa can be viewed as a model for government action to drastically increase access to PrEP [ 52 ].
Conclusions
The topic of how to use PrEP ethically, safely, and effectively has been a key consideration in the planning of prevention trials planned for South Africa. The recommendations of the Ministry of Health, South African ethics boards, research teams, and the vulnerable communities should be heeded in clinical trials conducted in any LMIC. Consensus across all these stakeholders is crucial for deciding how and when to provide access to modalities that have not yet fully bloomed in-country.
During the SoC summit discussions, it became clear that HIV prevention researchers should move towards making PrEP available as part of the HIV prevention package for study participants of a clinical trial. South African investigators and collaborators, including the NIH’s two largest clinical trial networks working in South Africa (HVTN and HPTN), proposed to work together as standard of prevention services evolve in southern Africa. The scientific validity of a clinical trial must be considered in decisions made regarding PrEP access. In addition, it is crucial that investigators engage their government bodies or working groups to motivate the support of PrEP demonstration projects close to research sites, not only for PrEP access, but to also provide HIV testing and PrEP-related safety monitoring.
This summit demonstrated that considerations of SoC in a clinical trial setting are complex. These complexities are dynamic and contextual nuances may vary from site to site. Both local and international guidelines can inform the review and the need to modify the standard of prevention to ensure LMIC clinical trials are performed with the highest ethical standards.
Following the summit, the SAMRC, with additional support from the Fred Hutchinson Cancer Research Center (parent institution of the HVTN) and industry partners, set up a PrEP fund for drug acquisition and laboratory monitoring for HIV prevention study participants across sub-Saharan Africa. This enabled the SAMRC to purchase PrEP at state tender prices and utilize pre-existing negotiated laboratory contracts and within-study systems to extend available PrEP funds. PrEP drug provision and related essential laboratory monitoring support were subsequently initiated for interested and eligible HVTN efficacy trial participants at the 24 clinical trial sites conducting trials in sub-Saharan Africa (HVTN 702 [ ClinicalTrials.gov : NCT02968849), HVTN 703/HPTN 081 [ ClinicalTrials.gov : NCT02568215], and HVTN 705/HPX2008 [ ClinicalTrials.gov : NCT03060629]). PrEP was offered at each instance of risk reduction counselling along with other prevention methods provided during participant study visits and during the informed consent process. Where possible, sites could offer PrEP from their own pharmacies and staff were trained to prescribe and manage PrEP initiation and follow-up. Each clinical site received an up-to-date list of pharmacies and clinics that stocked and prescribed PrEP in their areas. PrEP training for site staff was offered periodically to enable PrEP prescribing and management. The more recent HPX3002/HVTN 706 (Mosaico) trial conducted amongst MSM and transgender individuals in the Americas and Europe offered a slightly different approach to PrEP administration to ensure all participants could receive access to the highest standard of prevention. The trial was designed to give volunteers three options regarding PrEP: (1) participants already on PrEP or who wished to take PrEP will not be included in the study, but referred to PrEP resources in their community if requested; (2) participants who decide to take PrEP after receiving vaccination can remain in the study; and (3) participants not wanting to take PrEP can be included in the study [ 53 ].
Availability of data and materials
Abbreviations.
South African Medical Research Council
- Standard of care
Lower- and middle-income countries
World Health Organization
Tenofovir disoproxil fumarate/emtricitabine
Essential Medicines List
- Pre-exposure prophylaxis
National Department of Health
Men who have sex with men
HIV Vaccine Trials Network
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
Good Clinical Practice
Randomized controlled trial
Prevention of mother-to-child transmission
Antiretroviral therapy
Community Advisory Board
Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375(9):830–9. https://doi.org/10.1056/NEJMoa1600693 .
Article CAS PubMed PubMed Central Google Scholar
Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. https://doi.org/10.1056/NEJMoa1105243 .
Bavinton BR, Pinto AN, Phanuphak N, Grinsztejn B, Prestage GP, Zablotska-Manos IB, et al. Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet HIV. 2018;5(8):e438–e47. https://doi.org/10.1016/S2352-3018(18)30132-2 .
Article PubMed Google Scholar
Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, et al. Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. JAMA. 2016;316(2):171–81. https://doi.org/10.1001/jama.2016.5148 .
Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. https://doi.org/10.1056/NEJMoa1108524 .
Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99. https://doi.org/10.1056/NEJMoa1011205 .
Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–34. https://doi.org/10.1056/NEJMoa1110711 .
Article CAS PubMed Google Scholar
Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–18. https://doi.org/10.1056/NEJMoa1402269 .
Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–22. https://doi.org/10.1056/NEJMoa1202614 .
Chesney MA, Chambers DB, Kahn JO. Risk behavior for HIV infection in participants in preventive HIV vaccine trials: a cautionary note. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16(4):266–71. https://doi.org/10.1097/00042560-199712010-00007 .
Ndebele PM, Wassenaar D, Munalula E, Masiye F. Improving understanding of clinical trial procedures among low literacy populations: an intervention within a microbicide trial in Malawi. BMC Med Ethics. 2012;13(1):29. https://doi.org/10.1186/1472-6939-13-29 .
Article PubMed PubMed Central Google Scholar
Dawson L, Garner S, Anude C, Ndebele P, Karuna S, Holt R, et al. Testing the waters: ethical considerations for including PrEP in a phase IIb HIV vaccine efficacy trial. Clin Trials. 2015;12(4):394–402. https://doi.org/10.1177/1740774515579165 .
U.S. Food and Drug Administration approves Gilead’s Truvada® for reducing the risk of acquiring HIV [press release]. https://www.gilead.com/news-and-press/press-room/press-releases/2012/7/us-food-and-drug-administration-approves-gileads-truvada-for-reducing-the-risk-of-acquiring-hiv: Gilead Sciences, Inc.2012.
World Health Organization. WHO expands recommendation on oral preexposure prophylaxis of HIV infection (PrEP). Geneva, Switzerland: World Health Organization; 2015.
Google Scholar
UNAIDS. Aidsinfo [Available from: https://aidsinfo.unaids.org/ .
South African Medical Research Council. Executive Summary of the Summit on the Standard of Care in Clinical Trials in Low-Middle Income Settings. Cape Town, South Africa 2017.
Moffett P, Moore G. The standard of care: legal history and definitions: the bad and good news. West J Emerg Med. 2011;12(1):109–12.
PubMed PubMed Central Google Scholar
South African Health Products Regulatory Authority. Medicines and Related Substances Act No. 101 of 1965. 1965. https://www.sahpra.org.za/documents/abdb0bc7MedicinesandRelatedSubstancesAct101of1965,asatMay2017.pdf . Accessed 1 Apr 2021.
Annas GJ, Grodin MA. The Nazi Doctors and the Nuremberg Code: human rights in human experimentation. New York: Oxford University Press; 1992.
National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont Report. 1979. https://www.hhs.gov/ohrp/sites/default/files/the-belmont-report-508c_FINAL.pdf . Accessed 1 Apr 2021.
World Medical Association. Wma Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects. Helsinki, Finland; 1964. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ . Accessed 1 Apr 2021.
INTERNATIONAL COUNCIL FOR HARMONISATION OF TECHNICAL REQUIREMENTS FOR PHARMACEUTICALS FOR HUMAN USE (ICH). Integrated addendum to ICH E6(R1): guideline for good clinical practice E6(R2). https://www.ich.org/page/efficacy-guidelines#6-2; 2016.
UNAIDS A. Good participatory practice: guidelines for biomedical HIV prevention trials. Geneva; 2011.
Institute of Medicine (US) Committee on Standards for Developing Trustworthy Clinical Practice Guidelines. Clinical practice guidelines we can trust. Graham R, Mancher M, Wolman D, Greenfield S, Steinberg E, editors. Washington, DC: National Academies Press; 2011.
Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2. https://doi.org/10.1016/j.jclinepi.2010.09.011 .
Emanuel EJ, Wendler D, Killen J, Grady C. What makes clinical research in developing countries ethical? The benchmarks of ethical research. J Infect Dis. 2004;189(5):930–7. https://doi.org/10.1086/381709 .
Kredo T, Machingaidze S, Louw Q, Young T, Grimmer K. South African Guideline Excellence (SAGE): what’s in a name? S Afr Med J. 2015;106(1):18–20. https://doi.org/10.7196/SAMJ.2016.v106i1.10286 .
WHO. Global data shows increasing PrEP use and widespread adoption of WHO PrEP recommendations. https://www.who.int/news-room/feature-stories/detail/global-data-shows-increasing-prep-use-and-widespread-adoption-of-who-prep-recommendations; 2021.
Annas GJ. The right to health and the nevirapine case in South Africa. N Engl J Med. 2003;348(8):750–4. https://doi.org/10.1056/NEJMlim022737 .
Treatment Action Campaign V. Minister of Health, High Court of South Africa, Transvaal Provincial Div., 2002 (4) Bclr 356(T). 2002. https://www.globalhealthrights.org/wp-content/uploads/2013/01/HC-2001-Treatment-Action-Campaign-v.-Minister-of-Health-No-1.pdf . Accessed 1 Apr 2021.
Minister of Health and Others V Treatment Action Campaign and Others (No 2) (Cct8/02) [2002] Zacc 15; 2002 (5) Sa 721; 2002 (10) Bclr 1033 (5 July 2002), 2002. http://www.saflii.org/za/cases/ZACC/2002/15.html . Accessed 1 Apr 2021.
World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2013. https://www.sm.ee/sites/default/files/content-editors/eesmargid_ja_tegevused/Tervis/Ravimid/who_guidelines_june_2013.pdf . Accessed 1 Apr 2021.
UNAIDS. 2016 Global Plan Country Fact Sheet: South Africa Geneva, Switzerland: UNAIDS; 2016 [Available from: https://www.unaids.org/sites/default/files/media/documents/UNAIDS_GlobalplanCountryfactsheet_sa_en.pdf .
World Bank. Gini Index (World Bank Estimate) [Available from: https://data.worldbank.org/indicator/si.pov.gini .
World Health Organization. Current health expenditure (CHE) as percentage of gross domestic product (GDP) (%) [Available from: http://apps.who.int/gho/data/node.main.GHEDCHEGDPSHA2011?lang = en.
HIV.gov . U.S. statistics [Available from: https://www.hiv.gov/hiv-basics/overview/data-and-trends/statistics .
CDC. HIV in the United States and dependent areas. https://www.cdc.gov/hiv/pdf/statistics/overview/cdc-hiv-us-ataglance.pdf: Centers for Disease Control and Prevention; 2021.
Fonner VA, Dalglish SL, Kennedy CE, Baggaley R, O'Reilly KR, Koechlin FM, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30(12):1973–83. https://doi.org/10.1097/QAD.0000000000001145 .
Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–9. https://doi.org/10.1016/S1473-3099(14)70847-3 .
McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. https://doi.org/10.1016/S0140-6736(15)00056-2 .
Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373(23):2237–46. https://doi.org/10.1056/NEJMoa1506273 .
Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–90. https://doi.org/10.1016/S0140-6736(13)61127-7 .
Janes H, Corey L, Ramjee G, Carpp LN, Lombard C, Cohen MS, et al. Weighing the evidence of efficacy of oral PrEP for HIV prevention in women in Southern Africa. AIDS Res Hum Retroviruses. 2018;34(8):645–56. https://doi.org/10.1089/aid.2018.0031 .
Bekker LG, Rebe K, Venter F, Maartens G, Moorhouse M, Conradie F, et al. Southern African guidelines on the safe use of pre-exposure prophylaxis in persons at risk of acquiring HIV-1 infection. South Afr J HIV Med. 2016;17(1):455. https://doi.org/10.4102/sajhivmed.v17i1.455 .
Shapiro K, Benatar SR. HIV prevention research and global inequality: steps towards improved standards of care. J Med Ethics. 2005;31(1):39–47. https://doi.org/10.1136/jme.2004.008102 .
Sugarman J. Ethical considerations regarding oral preexposure prophylaxis in HIV prevention trials. Curr Opin HIV AIDS. 2016;11(1):109–15. https://doi.org/10.1097/COH.0000000000000214 .
UNAIDS welcomes South Africa’s groundbreaking national sex worker HIV plan [press release]. Geneva, Switzerland, 11 March 2016 2016.
Hammer SM, Sobieszczyk ME, Janes H, Karuna ST, Mulligan MJ, Grove D, et al. Efficacy trial of a DNA/Rad5 HIV-1 preventive vaccine. N Engl J Med. 2013;369(22):2083–92. https://doi.org/10.1056/NEJMoa1310566 .
Broder GB, Lucas JP, Davis J, Wallace SE, Luthuli N, Baepanye K, et al. Standardized metrics can reveal region-specific opportunities in community engagement to aid recruitment in HIV prevention trials. PLoS One. 2020;15(9):e0239276. https://doi.org/10.1371/journal.pone.0239276 .
Holzer JK, Ellis L, Merritt MW. Why we need community engagement in medical research. J Investig Med. 2014;62(6):851–5. https://doi.org/10.1097/JIM.0000000000000097 .
King KF, Kolopack P, Merritt MW, Lavery JV. Community engagement and the human infrastructure of global health research. BMC Med Ethics. 2014;15(1):84. https://doi.org/10.1186/1472-6939-15-84 .
Beyrer C, Bekker LG, Pozniak A, Barre-Sinoussi F. Pre-exposure prophylaxis works—it’s time to deliver. Lancet. 2015;385(9977):1482–4. https://doi.org/10.1016/S0140-6736(15)60724-3 .
NIH and partners to launch HIV vaccine efficacy trial in the Americas and Europe [press release]. https://www.nih.gov/news-events/news-releases/nih-partners-launch-hiv-vaccine-efficacy-trial-americas-europe , July 15, 2019 2019.
Download references
Acknowledgements
We would like to thank all contributors to the 2017 Standard of Care Summit, including the following presenters and panel members: Joey Gouws, Gavin Steel, Anne Pope, Paul Ruff, Yogan Pillay, Jerome Singh, Michael Chirenje, Keymanthri Moodley, Quarraisha Abdool Karim, Gita Ramjee, Sinead Delaney, Vuyokazi Gonyela, Maureen Luba, Helen Rees, Charles Wiysonge, Holly Janes, Deborah Donnell, Carl Dieffenbach, Steven Smith, Frank Tomaka, Laura Trivino, Marc Blockman, Nércia Langa Mandlhate, Gugu Mahlangu, Kathy Mngadi, Ntando Yola, Steven Wakefield, Julia Hill, Tian Johnson, Rachel Jewkes, Carl Lombard, and Yvette Raphael. We also thank all participants of the summit for their insight and discussion and those who helped with planning.
The summit was supported by the South African Medical Research Council and HIV Vaccine Trials Network (by the National Institutes of Health, National Institute of Allergy and Infectious Diseases award UM1 AI068614). TK received funding from The Social Impact Bond (Global Fund).
Author information
Authors and affiliations.
HVTN, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue North, Mail-stop E3-300, Seattle, WA, 98109, USA
Maurine D. Miner, Lawrence Corey & Glenda E. Gray
Desmond Tutu HIV Centre, University of Cape Town, P.O. Box 13801, Mowbray, Cape Town, 7705, South Africa
Linda-Gail Bekker
South African Medical Research Council, Francie van Zijl Drive, Parowvallei, Cape Town, PO Box 19070, Tygerberg, 7505, South Africa
Tamara Kredo, Niresh Bhagwandin & Glenda E. Gray
Division of Clinical Pharmacology, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University, Private Bag X1, Matieland, Stellenbosch, 7602, South Africa
Tamara Kredo
Department of Medicine and Laboratory Medicine, University of Washington, 1959 NE Pacific St, Seattle, WA, 98195, USA
Lawrence Corey
You can also search for this author in PubMed Google Scholar
Contributions
MDM wrote the first draft and edited the manuscript; LGB contributed to summit organization and edited the manuscript; TK contributed to summit organization and edited the manuscript; NB contributed to summit organization and edited the manuscript; LC contributed to summit organization and edited the manuscript; GEG contributed to summit organization and edited the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Correspondence to Glenda E. Gray .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
TK is a member of the National Essential Medicines List Committee and a Guideline Methodologist for WHO. All other authors declare no conflicts of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: supplement table 1..
Summit presentations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Miner, M.D., Bekker, LG., Kredo, T. et al. Meeting report: South African Medical Research Council Standard of Care in Clinical Research in Low- And Middle-Income Settings Summit, November 2017. Trials 22 , 778 (2021). https://doi.org/10.1186/s13063-021-05754-z
Download citation
Received : 17 May 2021
Accepted : 19 October 2021
Published : 06 November 2021
DOI : https://doi.org/10.1186/s13063-021-05754-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- South Africa
- Clinical trials
ISSN: 1745-6215
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: April 1999
The South African Medical Research Council: Africanizing health research
- Malegapuru William Makgoba 1
Nature Medicine volume 5 , pages 367–370 ( 1999 ) Cite this article
128 Accesses
1 Citations
Metrics details
The South African Medical Research Council (MRC) is one of South Africa's eight statutory science councils and the country's premier biomedical science research institution. Malegapuru W. Makgoba, appointed in January as the organization's first black African president, discusses the MRC's history, transformation and vision for the future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Author information
Authors and affiliations.
President, South African Medical Research Council, P.O. Box 19070, Tygerberg, 7505, South Africa
Malegapuru William Makgoba
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Makgoba, M. The South African Medical Research Council: Africanizing health research . Nat Med 5 , 367–370 (1999). https://doi.org/10.1038/7351
Download citation
Issue Date : April 1999
DOI : https://doi.org/10.1038/7351
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
You are using an outdated browser. Please upgrade your browser to improve your experience.


The South African Medical Research Council (SAMRC)
African health solutions for global impact.
Thanks to its ethos of change being inevitable but growth intentional, the South African Medical Research Council has a five-decade history and a long-term future mandate.

The South African Medical Research Council (SAMRC) was established in 1969 to conduct and fund health research, health innovation, development, and research translation. The SAMRC focuses on the top 10 causes of mortality, morbidity, disability, and associated risk factors. The scope of research includes laboratory investigations, preclinical and clinical research, and public health studies.
As part of the GloPID-R Network, the SAMRC is well positioned to enable research collaboration and excellence among scientists on the African continent to respond to global health challenges. For example, the SAMRC is funding health research to develop a rapid point-of-care (POC) diagnostic assay for Ebola virus infection. The development of POC tests would be a medical innovation that would vastly improve the rapid detection of infected patients, clinical decisions, and far more efficient containment measures and patient management. This technology, if successful, would serve as the blueprint for other infection control measures and tools of international public health threats.
Research and innovation in a time of COVID-19
Humanity lives in a constant state of flux with the COVID-19 epidemic as a stark reminder of threats to human health. The SAMRC has been responsive to change, leading research and dialogue on COVID-19 and investing over ZAR100 million (over €5.5 million), with the South African Department of Science and Innovation (DSI), into COVID-19 research and innovation.
As the fight against COVID-19 intensifies, the SAMRC is at the forefront when it comes to public health research. Through its intramural research units, the SAMRC is engaged in a broad spectrum of studies looking into health impacts of COVID-19 across research streams, from gender-based violence (GBV) and impact on substance use, to studies on prevalence, clinical characteristics, and immunologic responses and outcomes of children with suspected or confirmed COVID-19, among other studies.
COVID-19 vaccines, finding solutions to save lives
Since SARS-CoV-2 was identified in Wuhan, China in December 2019, there have been tremendous global efforts to find multiple vaccine candidates to protect against infection and subsequent development of COVID-19 disease. Now the biggest vaccination campaign in history is underway. More than 80 million doses in 59 countries have been administered to date.
Even if early results have found some vaccines to be safe and effective, continuing to conduct trials may bring further benefits for society. The SAMRC and DSI provided ZAR10 million (over €562 000) funding into the first South African Covid-19 vaccine trial; the South African Ox1Cov-19 Vaccine VIDA-Trial announced in June 2020.
The ENSEMBLE trial, a Phase 3 efficacy vaccine trial with the Janssen Pharmaceutical Companies of Johnson & Johnson, is underway. The SAMRC is participating in the trial as part of the COVID-19 Prevention Network funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the U.S. National Institutes of Health. Leading the trial are two respected scientists, Professors Glenda Gray, President and CEO of the SAMRC and Linda-Gail Bekker, Deputy Director of the Desmond Tutu HIV Centre.
South African Vaccine Rollout Programme
Rapid rollout of COVID-19 vaccination will fast-track the return to normality, and South Africa is making every effort to secure enough vaccines to attain herd immunity in the country. South Africa is part of COVAX, the vaccines’ pillar of the access to COVID-19 tools (ACT) Accelerator, co-led by the Global Alliance for Vaccines and Immunisation (Gavi), the Coalition for Epidemic Preparedness Innovations (CEPI), and WHO. In addition to the COVAX facility, South Africa will procure additional vaccines through the African Union (which is chaired by the President of South Africa) and bilateral negotiations with pharmaceutical companies.
However, vaccine hesitancy poses a real challenge to the country’s vaccination efforts. The SAMRC is investigating the scale and determinants of vaccine hesitancy in South Africa so that tailored and targeted strategies can be developed to address it. This would eventually enhance confidence in, and increase demand for, COVID-19 vaccination in South Africa. The SAMRC is also working with the DSI and the African Alliance to implement a communications and public engagement strategy to counter vaccine misinformation.
Genomics and personalised medicine
Genomics research offers a unique opportunity to leapfrog technologies for a better understanding of factors that impact on the health of South Africans and inform strategies to improve their response to diseases.
In July 2019, the SAMRC launched a genomics research centre in partnership with the Beijing Genomics Institute. The Centre conducts genomics research to address the growing disease burden of South Africa and builds towards a future where 4IR is a major component in African healthcare.
More than 50 whole genome experiments have been conducted, and the SAMRC – together with the DSI – has made a number of awards to help in understanding the basis of treatment failure for non-communicable disease treatments in Africa; and setting up a pilot project around HIV elite controllers, where genetics are believed to be a major contributing factor in disease management.
Even in this state of flux, the SAMRC remains responsive to change, continuing its journey of growth and innovation.
The SAMRC is engaged in a wide spectrum of research areas related to COVID- 19.
More information
Phone: +27 21 938 0911 E-mail: [email protected] Website: www.samrc.ac.za

The GloPID-R Secretariat is a project which receives funding from the European Union’s Horizon Europe research and innovation programme under grant agreement No 101094188.
Privacy Preference Center

87% of people murdered in South Africa are men: Study

- Graphic depiction of a murder investigation
- Image Credits :
- 8 December 2023, 20:35 [SAST]
A study by the South African Medical Research Council (MRC) has revealed that 87% of people murdered in South Africa are men. The study is aimed at challenging the idea that men are invulnerable.
Professor Richard Matzopoulos, researcher at the Burden of Disease Unit at the council, says this is the first study on male murders in South Africa, as previous studies focused mainly on femicide.
“There are gender norms around violence, and why perpetrators for example will exert different types of violence against men, and against women. So, it’s no surprise that women in South Africa are subjected to enormous amounts of abuse and sexual abuse, primarily affecting women rather than men. But the levels of physical violence against men, that’s also a gender dynamic that we need to unpack and understand.”
Matzopoulos says this is not exclusive to adult males.
“It’s not just adults, we even see that amongst young children from the ages of zero – to 4 years old. We see that boys, 80% are more likely to be murdered than girls.”
View all posts
Privacy Overview
About project MIND
Project MIND is a collaborative research study between the South African Medical Research Council (SAMRC), the University of Cape Town, Oxford University, and the Western Cape Department of Health (WCDoH). The purpose of this study is to develop two collaborative care models for mental health and chronic disease care and to test which of these models is the most effective for improving mental health and chronic disease outcomes.
HEALTH STATUS OF SOUTH AFRICA
South Africa is faced with many health challenges. Some of these challenges include a high prevalence of:

Communicable diseases
such as HIV
Non-communicable diseases
such as diabetes
Common mental disorders
such as depression and alcohol abuse
Many people who live with a chronic disease also suffer from a common mental disorder. Unfortunately, these mental disorders often go undetected and untreated. When common mental disorders remain untreated, it can affect a person’s adherence and response to treatment for chronic diseases such HIV and diabetes. Therefore, treating common mental disorders are important for improving treatment outcomes of chronic disease services. Project MIND aims to address this health challenge by Strengthening South Africa's health system through integrating treatment for mental illness into chronic disease care.
engage with us
Strengthening South Africa's health system through integrating treatment for mental illness into chronic disease care.
Western Cape Health Indaba
Twitter Feeds

Funding opportunity: South Africa-UK health research collaboration: non-communicable diseases
MRC and SAMRC invite health research applications in the focus area of non–communicable diseases (NCDs), including:
- mental health
- cardiovascular diseases
- chronic lung diseases
- chronic kidney disease
- chronic liver disease (non-infectious aetiology)
You must be:
- a South Africa-UK collaboration and can additionally include African collaborators
- submitted to SAMRC by the South African lead investigator, on behalf of the consortium
- be official development assistance-eligible and focused on research of primary relevance to South Africa or low and middle-income countries
Up to 70 million South African Rand (approximately £3 million) is available for the focus area, with an expectation that we will fund several three-year projects per topic.
See the full funding opportunity details of the scheme and apply on the SAMRC website .
Contact details
Get help with developing your proposal.
For help and advice on costings and writing your proposal please contact your research office in the first instance, allowing sufficient time for your organisation’s submission process.
Ask about this funding opportunity
This funding opportunity is led by SAMRC please direct any requests for information and questions or queries on this by emailing [email protected]
Aaron Holiday, International Programme Manager (MRC lead)
Email: [email protected]
This is the website for UKRI: our seven research councils, Research England and Innovate UK. Let us know if you have feedback or would like to help improve our online products and services .

Banner image
Research capacity development.
The SAMRC's Division of Research Capacity Development (RCD) is a well-established research funding division that runs streamlined funding programmes to support the growth of health research capacity, by offering multiple strategic capacity development grants in the national research priorities and directly contributing to National Targets for research capacity development as well as transformation.

The strategic goal # 4 of the SAMRC is “building capacity for the long-term sustainability of the South African health research”. This is the priority goal for SAMRC to attain health research transformation and fully achieve the other four strategic goals.
The RCD Division runs two funding portfolios namely: the Research Grants Portfolio (covering 60% of the RCD budget) and the Scholarship Portfolio (covering 40% of RCD budget). Over the years the SAMRC RCD funding has been a great opportunity for funding early to mid-career researchers and postgraduate students, mainly PhDs at universities and research institutions with a particular focus on historically disadvantaged individuals as well as institutions previously constrained by inadequate access to resources to strengthen capacity development and ensure the ability to produce scientific knowledge for the advancement of health. All RCD funding processes follow well accepted standard grant management procedures from request for applications to the award processes. The applicants must be eligible for the programmes they are interested in.
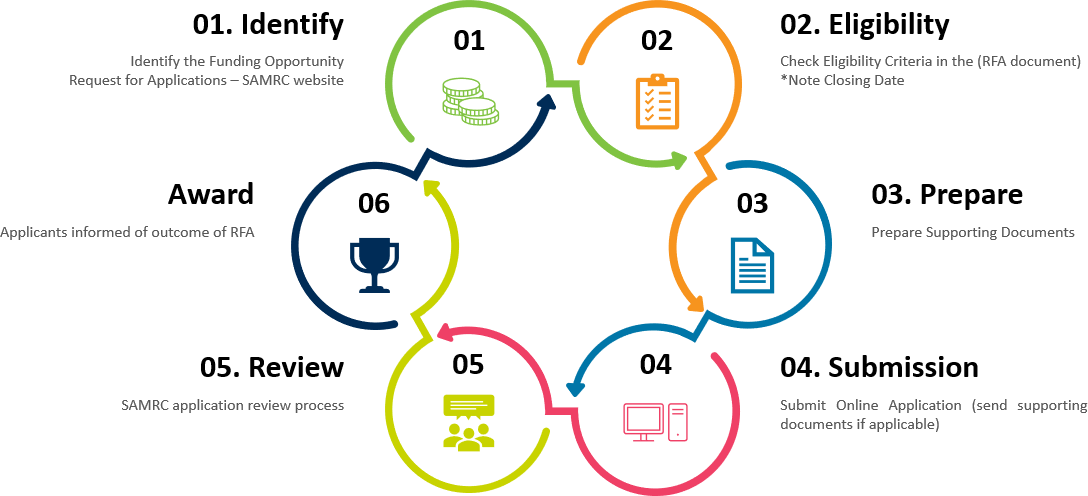
Application Process
The applications for SAMRC RCD funding undergo a thorough, transparent and fair review process. The candidates are selected based on the eligibility criteria of each request for applications (RFA). SAMRC RCD funding process consists of multiple stages. Below are the main steps to guide you through the application process.
Enter your search term
*Limited to most recent 250 articles Use advanced search to set an earlier date range
Sponsored by
Saving articles
Articles can be saved for quick future reference. This is a subscriber benefit. If you are already a subscriber, please log in to save this article. If you are not a subscriber, click on the View Subscription Options button to subscribe.
Article Saved
Contact us at [email protected]
Forgot Password
Please enter the email address that you used to subscribe on Engineering News. Your password will be sent to this address.
Content Restricted
This content is only available to subscribers
REAL ECONOMY NEWS
sponsored by
- LATEST NEWS
- LOADSHEDDING
- MULTIMEDIA LATEST VIDEOS REAL ECONOMY REPORTS SECOND TAKE AUDIO ARTICLES CREAMER MEDIA ON SAFM
- SECTORS AGRICULTURE AUTOMOTIVE CHEMICALS CONSTRUCTION DEFENCE & AEROSPACE ECONOMY ELECTRICITY ENERGY ENVIRONMENTAL MANUFACTURING METALS MINING RENEWABLE ENERGY SERVICES TECHNOLOGY & COMMUNICATIONS TRADE TRANSPORT & LOGISTICS WATER
- SPONSORED POSTS
- ANNOUNCEMENTS
- BUSINESS THOUGHT LEADERSHIP
- MINING WEEKLY
- SHOWROOM PLUS
- PRODUCT PORTAL
- MADE IN SOUTH AFRICA
- PRESS OFFICE
- COMPANY PROFILES
- VIRTUAL SHOWROOMS
- CREAMER MEDIA
- BACK COPIES
- BUSINESS LEADER
- SUPPLEMENTS
- FEATURES LIBRARY
- RESEARCH REPORTS
- PROJECT BROWSER
Article Enquiry
South Africa's science, innovation dept launches circular minerals, metals initiative
Email This Article
separate emails by commas, maximum limit of 4 addresses
DSI technology innovation deputy director general Mmboneni Muofhe
Photo by Creamer Media

As a magazine-and-online subscriber to Creamer Media's Engineering News & Mining Weekly , you are entitled to one free research report of your choice . You would have received a promotional code at the time of your subscription. Have this code ready and click here . At the time of check-out, please enter your promotional code to download your free report. Email [email protected] if you have forgotten your promotional code. If you have previously accessed your free report, you can purchase additional Research Reports by clicking on the “Buy Report” button on this page. The most cost-effective way to access all our Research Reports is by subscribing to Creamer Media's Research Channel Africa - you can upgrade your subscription now at this link .
The most cost-effective way to access all our Research Reports is by subscribing to Creamer Media's Research Channel Africa - you can upgrade your subscription now at this link . For a full list of Research Channel Africa benefits, click here
If you are not a subscriber, you can either buy the individual research report by clicking on the ‘Buy Report’ button, or you can subscribe and, not only gain access to your one free report, but also enjoy all other subscriber benefits , including 1) an electronic archive of back issues of the weekly news magazine; 2) access to an industrial and mining projects browser; 3) access to a database of published articles; and 4) the ability to save articles for future reference. At the time of your subscription, Creamer Media’s subscriptions department will be in contact with you to ensure that you receive a copy of your preferred Research Report. The most cost-effective way to access all our Research Reports is by subscribing to Creamer Media's Research Channel Africa - you can upgrade your subscription now at this link .
If you are a Creamer Media subscriber, click here to log in.
11th April 2024
By: Darren Parker
Creamer Media Contributing Editor Online
Font size: - +

South Africa's Department of Science and Innovation (DSI), through the Council for Scientific and Industrial Research (CSIR), has launched the first of three new government-led collaborative circular economy initiatives focusing on priority economic sectors that were identified by government in the science, technology and innovation (STI) 2022 to 2032 decadal plan.
The first of the three initiatives, the South African Circular Minerals and Metals Initiative (SACMMI), was launched in Pretoria, on April 11. The other two, the South African Circular Agricultural Initiative (SACAI) and the South African Circular Manufacturing Initiative (SACMI), will follow.
The mandate of these initiatives is to drive sector-specific circular economy STI to support the development of these sectors and to fast-track the uptake of circular interventions.
“The circular economy is not a nice to have. It's also not an environmental agenda. It's a social and economic and an environmental imperative for every country as we face growing resource scarcity,” DSI and CSIR circular innovation South Africa manager Professor Linda Godfrey explained.
She revealed that the SACMMI was aimed at creating an opportunity to embed circular economy STI within the National System of Innovation (NSI), as well as to build local and international STI partnerships. Ultimately, the goal is to provide real benefits to the local mining sector.
She noted that the DSI would publish calls for expressions of interest to host the SACAI and the SACMI, respectively.
Godfrey emphasised that the circular economy was not about waste management, but rather about sustainable resource management in support of development. She said that a circular economy transition was an economic, social and environmental imperative for every country globally, and that South Africa’s universities and science councils would be crucial to evidencing this transition. They would also play a central role in derisking circular economy solutions and developing new innovative circular economy solutions.
“We look forward to collaborating with public- and private-sector partners, and driving impact through these new circular economy initiatives,” she said.
Godfrey said government recognised the circular economy as a new source of economic growth for a re-industrialised and modernised economy, with a strong role for STI.
She explained that investment in STI for a circular economy would provide the means to unlock technology opportunities through technology development, localisation and adaptation. It would also help evidence decision-making in both the public and private sectors, and drive policy development and implementation.
Such investment would also support businesses to derisk and scale interventions, thereby bringing academia closer to business, especially small to medium-sized enterprises.
“The circular economy is not new to South Africa. We've been doing a lot of these things for many years. We just never called it circularity. We haven't yet achieved the scale for meaningful impact. How do we assist in fast-tracking the adoption and scaling of these interventions?” Godfrey said.
“I honestly do believe that the circular economy provides an entirely different future or trajectory in terms of our growth as an African continent. With that comes the exciting opportunity for small businesses. I don't even think we've scratched the surface in terms of those opportunities for the continent,” Godfrey said.
Mandela Mining Precinct (MMP) executive director Julie Courtnage said the SACMMI provided an opportunity to build a national system of innovation and capability, to directly support industry and other sector stakeholders in the adoption and acceleration of circular practices and technologies.
She noted that collaboration and partnerships would be instrumental to the success of this initiative. So far, the MMP and CSIR were fully on board, with talks being entered into with Mintek, the University of Cape Town, and the Council for Geoscience to also come on board.
"There is no way that we can do this alone. Radical collaboration is the only way forward. I use the term radical because we talk collaboration and so often, we find ourselves hamstrung by protectionism, by fear, or by ego. I really hope that as a precinct, we can help bridge some of those perceived barriers that exist mostly in our minds and not in reality."
Courtnage explained that one of the first activities that would need to take place following the SACMMI launch would be a stakeholder workshop to be hosted midyear to develop a national circular minerals and metals research agenda. This would be in response to the STI decadal plan and would also serve as a substrategy to the DSI’s STI for a Circular Economy Strategy.
This workshop would need to address what circularity means for minerals and metals availability, extraction and use in South Africa, as well as explore where the knowledge or research gaps are to determine research priorities. Critically, the key stakeholders in each focus area need to be identified, from research collaborators to partners and decision-makers.
The aim of this workshop would be to produce a national STI substrategy on circularity in minerals and metals in South Africa and to build a network of STI partnerships.
Courtnage added that the critical minerals space was vital within the circularity agenda, as circularity was a vital consideration in the competitiveness of the sector.
“Therefore, we have just signed off on a critical minerals competitive assessment project that is going to be undertaken as one of MMP's strategic initiatives this year. It is being coordinated by the CSIR mining cluster,” she said.
DSI technology innovation deputy director-general Mmboneni Muofhe voiced government’s support.
“We are committed in ensuring that the circular economy is indeed becoming mainstreamed in whatever we promote in terms of research but also in terms of implementation. That is why we are making sure that all sectors, including the private sector, government and civil society, are really part of this process,” he said.
Edited by Chanel de Bruyn Creamer Media Senior Deputy Editor Online
Research Reports

Latest Multimedia

Latest News

ESAB South Arica, the leading supplier of high-end welding and cutting products to the Southern African industrial market is based in...

For over 60 years, VEGA has provided industry-leading products for the measurement of level, density, weight and pressure. As the inventor of the...
sponsored by

Press Office
Announcements
Subscribe to improve your user experience...
Option 1 (equivalent of R125 a month):
Receive a weekly copy of Creamer Media's Engineering News & Mining Weekly magazine (print copy for those in South Africa and e-magazine for those outside of South Africa) Receive daily email newsletters Access to full search results Access archive of magazine back copies Access to Projects in Progress Access to ONE Research Report of your choice in PDF format
Option 2 (equivalent of R375 a month):
All benefits from Option 1 PLUS Access to Creamer Media's Research Channel Africa for ALL Research Reports, in PDF format, on various industrial and mining sectors including Electricity; Water; Energy Transition; Hydrogen; Roads, Rail and Ports; Coal; Gold; Platinum; Battery Metals; etc.
Already a subscriber?
Forgotten your password?
MAGAZINE & ONLINE
R1500 (equivalent of R125 a month)
Receive weekly copy of Creamer Media's Engineering News & Mining Weekly magazine (print copy for those in South Africa and e-magazine for those outside of South Africa)
Access to full search results
Access archive of magazine back copies
Access to Projects in Progress
Access to ONE Research Report of your choice in PDF format
RESEARCH CHANNEL AFRICA
R4500 (equivalent of R375 a month)
All benefits from Option 1
Electricity
Energy Transition
Roads, Rail and Ports
Battery Metals
CORPORATE PACKAGES
Discounted prices based on volume
Receive all benefits from Option 1 or Option 2 delivered to numerous people at your company
Intranet integration access to all in your organisation

Watch CBS News
Why is looking at a solar eclipse dangerous without special glasses? Eye doctors explain.
By Sara Moniuszko
Edited By Allison Elyse Gualtieri
Updated on: April 8, 2024 / 8:54 AM EDT / CBS News
The solar eclipse will be visible for millions of Americans on April 8, 2024, making many excited to see it — but how you watch it matters, since it can be dangerous for your eyes.
A solar eclipse occurs when the moon passes between the sun and Earth, blocking the sun's light . When the moon blocks some of the sun, it's a partial solar eclipse, but when moon lines up with the sun, blocking all of its light, a total solar eclipse occurs, NASA explains . Either way, you need eye protection when viewing.
"The solar eclipse will be beautiful, so I hope that everyone experiences it — but they need to experience it in the right way," said Dr. Jason P. Brinton, an ophthalmologist and medical director at Brinton Vision in St. Louis.
Here's what to know to stay safe.
Why is looking at a solar eclipse dangerous?
Looking at the sun — even when it's partially covered like during an eclipse — can cause eye damage.
There is no safe dose of solar ultraviolet rays or infrared radiation, said Dr. Yehia Hashad , an ophthalmologist, retinal specialist and the chief medical officer at eye health company Bausch + Lomb.
"A very small dose could cause harm to some people," he said. "That's why we say the partial eclipse could also be damaging. And that's why we protect our eyes with the partial as well as with the full sun."
Some say that during a total eclipse, it's safe to view the brief period time when the moon completely blocks the sun without eye protection. But experts warn against it.
"Totality of the eclipse lasts only about 1 to 3 minutes based on geographic location, and bright sunlight suddenly can appear as the moon continues to move," notes an eclipse viewing guide published in JAMA , adding, "even a few seconds of viewing the sun during an eclipse" can temporarily or permanently damage your vision.
Do I need special glasses for eclipse viewing?
Yes. Eclipse glasses are needed to protect your eyes if you want to look at the eclipse.
Regular sunglasses aren't protective enough for eclipse viewing — even if you stack more than one.
"There's no amount of sunglasses that people can put on that will make up for the filtering that the ISO standard filters and the eclipse glasses provide," Brinton said.
You also shouldn't look at the eclipse through a camera lens, phone, binoculars or telescope, according to NASA, even while wearing eclipse glasses. The solar rays can burn through the lens and cause serious eye injury.
Eclipse glasses must comply with the ISO 12312-2 international safety standard , according to NASA, and should have an "ISO" label printed on them to show they comply. The American Astronomical Society has a list of approved solar viewers.
Can't find these, or they're sold out near you? You can also make homemade viewers , which allow you to observe the eclipse indirectly — just don't accidentally look at the sun while using one.
How to keep kids safe during the solar eclipse
Since this eclipse is expected to occur around the time of dismissal for many schools across the country, it may be tempting for students to view it without the proper safety precautions while getting to and from their buses. That's why some school districts are canceling classes early so kids can enjoy the event safely with their families.
Dr. Avnish Deobhakta, vitreoretinal surgeon at New York Eye and Ear Infirmary at Mount Sinai, said parents should also be careful because it can be difficult for children to listen or keep solar eclipse glasses on.
"You want to actually, in my opinion, kind of avoid them even looking at the eclipse, if possible," he said. "Never look directly at the sun, always wear the right eclipse sunglasses if you are going to look at the sun and make sure that those are coming from a reliable source."
Brinton recommends everyone starts their eclipse "viewing" early, by looking at professional photos and videos of an eclipse online or visiting a local planetarium.
That way, you "have an idea of what to expect," he said.
He also recommends the foundation Prevent Blindness , which has resources for families about eclipse safety.
What happens if you look at a solar eclipse without eclipse glasses?
While your eyes likely won't hurt in the moment if you look at the eclipse without protection, due to lowered brightness and where damage occurs in the eye, beware: The rays can still cause damage .
The harm may not be apparent immediately. Sometimes trouble starts to appear one to a few days following the event. It could affect just one or both eyes.
And while some will regain normal visual function, sometimes the damage is permanent.
"Often there will be some recovery of the vision in the first few months after it, but sometimes there is no recovery and sometimes there's a degree to which it is permanent," Brinton said.
How long do you have to look at the eclipse to damage your eyes?
Any amount of time looking at the eclipse without protection is too long, experts say.
"If someone briefly looks at the eclipse, if it's extremely brief, in some cases there won't be damage. But damage can happen even within a fraction of a second in some cases," Brinton said. He said he's had patients who have suffered from solar retinopathy, the official name for the condition.
Deobhakta treated a patient who watched the 2017 solar eclipse for 20 seconds without proper eye protection. She now has permanent damage in the shape of a crescent that interferes with her vision.
"The crescent that is burned into the retina, the patient sees as black in her visual field," he said. "The visual deficit that she has will never go away."
How to know if you've damaged your eyes from looking at the eclipse
Signs and symptoms of eye damage following an eclipse viewing include headaches, blurred vision, dark spots, changes to how you see color, lines and shapes.
Unfortunately, there isn't a treatment for solar retinopathy.
"Seeing an eye care professional to solidify the diagnosis and for education I think is reasonable," Brinton said, but added, "right now there is nothing that we do for this. Just wait and give it time and the body does tend to heal up a measure of it."
Sara Moniuszko is a health and lifestyle reporter at CBSNews.com. Previously, she wrote for USA Today, where she was selected to help launch the newspaper's wellness vertical. She now covers breaking and trending news for CBS News' HealthWatch.
More from CBS News

Couple gets engaged on flight to see total solar eclipse

How to find the best tax relief company

Arizona's abortion ban likely to cause people to travel to states it's still legal

Inflation's rising. Here's how debt relief can help.

IMAGES
VIDEO
COMMENTS
On 7 March 2024, the South African Medical Council held its 10th scientific merit awards to honour some of the best scientific research in South Africa especially in health sciences. Through these prestigious awards, the SAMRC aims to acknowledge outstandin. The SAMRC is pleased to announce that it has awarded a R21 million grant to the South ...
News Articles Dr Ebrahim Samodien Science Writer & Editor Corporate & Marketing Communications Tel: +27 21 938 0294 E-mail: [email protected]. ... The South African Medical Research Council recognizes the catastrophic and persisting consequences of colonialism and apartheid, including land dispossession and the intentional imposition ...
Her focus will be to research HIV vaccines and other areas of vaccinology. Professor Glenda Gray has stepped down as the president and CEO of the South African Medical Research Council (SAMRC), where she served for two terms. The SAMRC said on Monday that, after her 10-year tenure, Gray would take up another role within the SAMRC next year.
The South African Medical Research Council (SAMRC) received a grant worth more than $45 million to implement the HIV Vaccine Innovation, Science, and Technology Acceleration in Africa (HIV-VISTA) program. SAMRC President and CEO and former Fogarty trainee, Dr. Glenda Gray guided the application process for this grant from the U.S. Agency for ...
A study conducted by the SA Medical Research Council showed a range of top risk factors for disease and death in South Africa.; Unsafe sex, alcohol consumption and interpersonal violence are some of the factors, according to the study. It found alcohol abuse has widespread effects on health and contributes to more than 200 health conditions.
Professor Glenda Gray is the first female President and CEO of the South African Medical Research Council (SAMRC). She was the Chair of the Research Committee on COVID-19, bringing together scientific evidence and experience to the Minister of Health and the National Coronavirus Command Council. Gray spearheads the SAMRC funding broadly and for ...
22, May 2020. Cape Town | The South African Medical Research Council (SAMRC) supports the National Department of Health in our vision of a "long and healthy life for all South Africans.". Globally, we face the greatest test since World War II, the COVID-19 pandemic which has already claimed thousands of lives and caused a worldwide ...
Sharon Seretlo. The South African Medical Research Council says it is seeing an increase in Covid-19 samples at wastewater treatment plants in parts of the Western Cape. The National Institute for Communicable Diseases weekly testing report shows there has been an increase in the number of people testing positive for Covid-19.
SURVEILLANCE & RESEARCH PROGRAMME The South African Medical Research Council's Wastewater Surveillance and Research Programme (SAMRC WSARP) has been tracking SARS-CoV-2 viral RNA in wastewater across 80+ wastewater treatment plants in the Western Cape, Eastern Cape, Limpopo, Gauteng, Free State and KwaZulu-Natal provinces of South Africa.
The SAMRC is leading South Africa's research and innovation response to COVID-19. "Throughout the pandemic, the South African Medical Research Council has been actively funding, conducting and facilitating research and development," said Dr Michelle Mulder, Executive Director Grants, Innovation and Product Development (GIPD) of the SAMRC.
The South African Medical Research Council (SAMRC) has a mandate to address issues that could potentially impact the conduct of health research, including clinical trials. As such, the SAMRC together with the HVTN convened a summit in Cape Town in 2017 to address PrEP access as a case study on the standard of care (SoC) in clinical trials in ...
The European African Personalized Medicine Consortium is a project funded by the Horizon 2020 program and the European Union. Much time has been spent evaluating Africa's research and innovation ...
The South African Medical Research Council (MRC) was established in 1969 through an Act of Parliament. Its mission was to promote, coordinate and support medical research on a national level.
The South African Medical Research Council has launched a dashboard that tracks Covid-19 through wastewater. Dr Rabia Johnson from BRIP said they were working with various higher education institutions where students and staff from partner institutions were being trained on laboratory methods, with the focus being on Under-Resourced Institutions.
South African Medical Research Council | 90,639 followers on LinkedIn. Advancing Life | Our Vision Building a healthy nation through research and innovation. Our mission To improve the nation's health and quality of life through promoting and conducting relevant and responsive health research. About Us The South African Medical Research Council (SAMRC) was established in 1969 with the aim ...
Thanks to its ethos of change being inevitable but growth intentional, the South African Medical Research Council has a five-decade history and a long-term future mandate. The South African Medical Research Council (SAMRC) was established in 1969 to conduct and fund health research, health innovation, development, and research translation.
A study by the South African Medical Research Council (MRC) has revealed that 87% of people murdered in South Africa are men. The study is aimed at challenging the idea that men are invulnerable. Professor Richard Matzopoulos, researcher at the Burden of Disease Unit at the council, says this is the first study on male murders in South Africa ...
Project MIND is a collaborative research study between the South African Medical Research Council (SAMRC), the University of Cape Town, Oxford University, and the Western Cape Department of Health (WCDoH). The purpose of this study is to develop two collaborative care models for mental health and chronic disease care and to test which of these ...
News and events; Who we are; Our councils; Search: Apply for funding. Funding finder; How to apply for research and innovation funding ... South African Medical Research Council (SAMRC) Funding type: Grant. Total fund: £3,000,000. Maximum award: £600,000. Publication date: 12 February 2024. Opening date: 12 February 2024.
The South African Medical Research Council (SAMRC) was established in 1969 with the aim to deliver on a mandate to promote the improvement of the health and the quality of life of the population ...
The SAMRC's Division of Research Capacity Development (RCD) is a well-established research funding division that runs streamlined funding programmes to support the growth of health research capacity, by offering multiple strategic capacity development grants in the national research priorities and directly contributing to National Targets for research capacity development as well as ...
South Africa's Department of Science and innovation (DSI), through the Council for Scientific and Industrial Research (CSIR), has launched the first of three new government-led collaborative ...
Why looking directly at a solar eclipse is so dangerous for your eyes 01:41. The solar eclipse will be visible for millions of Americans on April 8, 2024, making many excited to see it — but how ...