

How to Read a Scholarly Article
- Introduction
Article Text
- References/Works Cited
- 2. Sections of a Scholarly Article: Humanities Article
Sections of a Scholarly Journal Article About Scientific Research
Let's look at the different parts of a scholarly article that presents scientific research:
- Brief description of the article
- You can read this to decide whether you want to read the entire article.

Introduction:
- Description of the problem, or the research question, and why this study is being done
- Sometimes includes a short literature review

- The main part of an article is its body text.
- This is where the author analyzes the argument, research question, or problem. This section also includes analysis and criticism.
- The author may use headings to divide this part of the article into sections.
Scientific research articles may include these sections:
- Literature review (Discussion of other sources, such as books and articles, that informed the author(s) of this article)
- Methods (Description of the way the research study was set up and how data was collected)
- Results (Presentation of the research study results)
- Discussion (Discussion of whether the results of the study answer the research question)
You may see some of these same sections in articles that present humanities scholarship.
Conclusion:
- Wraps up the article.
- This section isn't always labeled.
- Description of how this article or research study contributes to or builds on the previous research of other scholars.
- Also includes ideas for future research others might do on this topic.

References/Works Cited:
List of resources (books, articles, etc.) cited in this article.

This example uses pages from this article: Sampson, L., Ettman, C., Abdalla, S., Colyer, E., Dukes, K., Lane, K., & Galea, S. (2021). Financial hardship and health risk behavior during COVID-19 in a large US national sample of women. SSM - Population Health, 13, 100734–100734 . https://doi.org/10.1016/j.ssmph.2021.100734
- << Previous: Home
- Next: 2. Sections of a Scholarly Article: Humanities Article >>
- Last Updated: Nov 28, 2023 2:22 PM
- URL: https://libguides.sjf.edu/scholarly-article

- Duke NetID Login
- 919.660.1100
- Duke Health Badge: 24-hour access
- Accounts & Access
- Databases, Journals & Books
- Request & Reserve
- Training & Consulting
- Request Articles & Books
- Renew Online
- Reserve Spaces
- Reserve a Locker
- Study & Meeting Rooms
- Course Reserves
- Digital Health Device Collection
- Pay Fines/Fees
- Recommend a Purchase
- Access From Off Campus
- Building Access
- Computers & Equipment
- Wifi Access
- My Accounts
- Mobile Apps
- Known Access Issues
- Report an Access Issue
- All Databases
- Article Databases
- Basic Sciences
- Clinical Sciences
- Dissertations & Theses
- Drugs, Chemicals & Toxicology
- Grants & Funding
- Interprofessional Education
- Non-Medical Databases
- Search for E-Journals
- Search for Print & E-Journals
- Search for E-Books
- Search for Print & E-Books
- E-Book Collections
- Biostatistics
- Global Health
- MBS Program
- Medical Students
- MMCi Program
- Occupational Therapy
- Path Asst Program
- Physical Therapy
- Researchers
- Community Partners
Conducting Research
- Archival & Historical Research
- Black History at Duke Health
- Data Analytics & Viz Software
- Data: Find and Share
- Evidence-Based Practice
- NIH Public Access Policy Compliance
- Publication Metrics
- Qualitative Research
- Searching Animal Alternatives
- Systematic Reviews
- Test Instruments
Using Databases
- JCR Impact Factors
- Web of Science
Finding & Accessing
- COVID-19: Core Clinical Resources
- Health Literacy
- Health Statistics & Data
- Library Orientation
Writing & Citing
- Creating Links
- Getting Published
- Reference Mgmt
- Scientific Writing
Meet a Librarian
- Request a Consultation
- Find Your Liaisons
- Register for a Class
- Request a Class
- Self-Paced Learning
Search Services
- Literature Search
- Systematic Review
- Animal Alternatives (IACUC)
- Research Impact
Citation Mgmt
- Other Software
Scholarly Communications
- About Scholarly Communications
- Publish Your Work
- Measure Your Research Impact
- Engage in Open Science
- Libraries and Publishers
- Directions & Maps
- Floor Plans
Library Updates
- Annual Snapshot
- Conference Presentations
- Contact Information
- Gifts & Donations
Scientific Writing: Sections of a Paper
- Sections of a Paper
- Common Grammar Mistakes Explained
- Citing Sources
Introduction
- Materials & Methods
Typically scientific journal articles have the following sections:
Materials & Methods
References used:
Kotsis, S.V. and Chung, K.C. (2010) A Guide for Writing in the Scientific Forum. Plastic and Reconstructive Surgery. 126(5):1763-71. PubMed ID: 21042135
Van Way, C.W. (2007) Writing a Scientific Paper. Nutrition in Clinical Practice. 22: 663-40. PubMed ID: 1804295
What to include:
- Background/Objectives: include the hypothesis
- Methods: Briefly explain the type of study, sample/population size and description, the design, and any particular techniques for data collection and analysis
- Results: Essential data, including statistically significant data (use # & %)
- Conclusions: Summarize interpretations of results and explain if hypothesis was supported or rejected
- Be concise!
- Emphasize the methods and results
- Do not copy the introduction
- Only include data that is included in the paper
- Write the abstract last
- Avoid jargon and ambiguity
- Should stand-alone
Additional resources: Fisher, W. E. (2005) Abstract Writing. Journal of Surgical Research. 128(2):162-4. PubMed ID: 16165161 Peh, W.C. and Ng, K.H. (2008) Abstract and keywords. Singapore Medical Journal. 49(9): 664-6. PubMed ID: 18830537
- How does your study fit into what has been done
- Explain evidence using limited # of references
- Why is it important
- How does it relate to previous research
- State hypothesis at the end
- Use present tense
- Be succinct
- Clearly state objectives
- Explain important work done
Additional resources: Annesley, T. M. (2010) "It was a cold and rainy night": set the scene with a good introduction. Clinical Chemistry. 56(5):708-13. PubMed ID: 20207764 Peh, W.C. and Ng, K.H. (2008) Writing the introduction. Singapore Medical Journal. 49(10):756-8. PubMed ID: 18946606
- What was done
- Include characteristics
- Describe recruitment, participation, withdrawal, etc.
- Type of study (RCT, cohort, case-controlled, etc.)
- Equipment used
- Measurements made
- Usually the final paragraph
- Include enough details so others can duplicate study
- Use past tense
- Be direct and precise
- Include any preliminary results
- Ask for help from a statistician to write description of statistical analysis
- Be systematic
Additional resources: Lallet, R. H. (2004) How to write the methods section of a research paper. Respiratory Care. 49(10): 1229-32. PubMed ID: 15447808 Ng, K.H. and Peh, W.C. (2008) Writing the materials and methods. Singapore Medical Journal. 49(11): 856-9. PubMed ID: 19037549
- Describe study sample demographics
- Include statistical significance and the statistical test used
- Use tables and figures when appropriate
- Present in a logical sequence
- Facts only - no citations or interpretations
- Should stand alone (not need written descriptions to be understood)
- Include title, legend, and axes labels
- Include raw numbers with percentages
- General phrases (significance, show trend, etc. should be used with caution)
- Data is plural ("Our data are" is correct, "Our data is" is in-correct)
Additional resources: Ng, K.H and Peh, W.C. (2008) Writing the results. Singapore Medical Journal. 49(12):967-9. PubMed ID: 19122944 Streiner, D.L. (2007) A shortcut to rejection: how not to write the results section of a paper. Canadian Journal of Psychiatry. 52(6):385-9. PubMed ID: 17696025
- Did you reject your null hypothesis?
- Include a focused review of literature in relation to results
- Explain meaning of statistical findings
- Explain importance/relevance
- Include all possible explanations
- Discuss possible limitations of study
- Suggest future work that could be done
- Use past tense to describe your study and present tense to describe established knowledge from literature
- Don't criticize other studies, contrast it with your work
- Don't make conclusions not supported by your results
- Stay focused and concise
- Include key, relevant references
- It is considered good manners to include an acknowledgements section
Additional resources: Annesley, T. M. (2010) The discussion section: your closing argument. Clinical Chemistry. 56(11):1671-4. PubMed ID: 20833779 Ng, K.H. and Peh, W.C. (2009) Writing the discussion. Singapore Medical Journal. 50(5):458-61. PubMed ID: 19495512
Tables & Figures: Durbin, C. G. (2004) Effective use of tables and figures in abstracts, presentations, and papers. Respiratory Care. 49(10): 1233-7. PubMed ID: 15447809 Ng, K. H. and Peh, W.C.G. (2009) Preparing effective tables. Singapore Medical Journal. (50)2: 117-9. PubMed ID: 19296024
Statistics: Ng, K. H. and Peh, W.C.G. (2009) Presenting the statistical results. Singapore Medical Journal. (50)1: 11-4. PubMed ID: 19224078
References: Peh, W.C.G. and Ng, K. H. (2009) Preparing the references. Singapore Medical Journal. (50)7: 11-4. PubMed ID: 19644619
Additional Resources
- More from Elsevier Elsevier's Research Academy is an online tutorial to help with writing books, journals, and grants. It also includes information on citing sources, peer reviewing, and ethics in publishing
- Research4Life Training Portal Research4Life provides downloadable instruction materials, including modules on authorship skills as well as other research related skills.
- Coursera: Science Writing Coursera provides a wide variety of online courses for continuing education. You can search around for various courses on scientific writing or academic writing, and they're available to audit for free.

- << Previous: Lit Review
- Next: Grammar/Language >>
- Last Updated: Mar 20, 2024 2:34 PM
- URL: https://guides.mclibrary.duke.edu/scientificwriting
- Duke Health
- Duke University
- Duke Libraries
- Medical Center Archives
- Duke Directory
- Seeley G. Mudd Building
- 10 Searle Drive
- [email protected]
Writing the Five Principal Sections: Abstract, Introduction, Methods, Results and Discussion
- First Online: 12 October 2013
Cite this chapter

- Karen Englander 2
Part of the book series: SpringerBriefs in Education ((BRIEFSEDUCAT))
4595 Accesses
The five-part structure of research papers (Introduction, Method, Results and Discussion plus Abstract) serves as the conceptual basis for the content. The structure can be considered an “hourglass” or a “conversation” with predictable elements. Each section of the paper has its own common internal structure and linguistic features. The structures and features are explained with examples from published papers in a range of scientific disciplines. Exceptions to the four-part structure are also discussed.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Cargill, M., & O’Connor, P. (2009). Writing scientific research articles . West Sussex: Wiley-Blackwell.
Google Scholar
Cooper, E. A., Piazza, E. A., & Banks, M. S. (2012). The perceptual basis of common photographic practice. Journal of Vision, 12 (5), 1–14.
Article Google Scholar
Esquer Mendez, J. L., Hernández Rodríguez, M., & Bückle Ramirez, L. F. (2010). Thermal tolerance and compatibility zones as a tool to establish the optimum culture condition of the halibut Paralichthys californicus (Ayres, 1859). Aquaculture Research, 41 (7), 1015–1021.
Glassman-Deal, H. (2010). Science research writing: For non-native speakers of English . London: Imperial College Press.
Latour, B., & Woolgar, S. (1986). Laboratory life: The social construction of scientific facts . Princeton: Princeton University Press.
Penrose, A. M., & Katz, S. B. (2001). Writing in the sciences: Exploring conventions of scientific discourse . New York: Longman.
Pérez-Flores, M. A., Antonio-Carpio, R. G., Enrique Gómez-Treviño, E., & Ferguson, I. (2011). 3D inversion of control source LIN electromagnetic data. Unpublished manuscript.
Rodríguez-Santiago, M. A., & Rosales-Casián, J. A. (2011). Parasite structure of the Ocean Whitefish Caulolatilus princeps from Baja California, México (East Pacific). Helgoland Marine Research, 65 (2), 197–202.
Swales, J. (1990). Genre analysis: English in academic and research settings . Cambridge: Cambridge University Press.
Swales, J. M., & Feak, C. (2004). Academic writing for graduate students: Essential tasks and skills (2nd ed.). Ann Arbor: University of Michigan Press.
Download references
Author information
Authors and affiliations.
Department of Languages, Literatures and Linguistics, York University, Toronto, ON, Canada
Karen Englander
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Karen Englander .
Rights and permissions
Reprints and permissions
Copyright information
© 2014 The Author(s)
About this chapter
Englander, K. (2014). Writing the Five Principal Sections: Abstract, Introduction, Methods, Results and Discussion. In: Writing and Publishing Science Research Papers in English. SpringerBriefs in Education. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7714-9_8
Download citation
DOI : https://doi.org/10.1007/978-94-007-7714-9_8
Published : 12 October 2013
Publisher Name : Springer, Dordrecht
Print ISBN : 978-94-007-7713-2
Online ISBN : 978-94-007-7714-9
eBook Packages : Humanities, Social Sciences and Law Education (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Library Instruction
Structure of typical research article.
The basic structure of a typical research paper includes Introduction, Methods, Results, and Discussion. Each section addresses a different objective.
- the problem they intend to address -- in other words, the research question -- in the Introduction ;
- what they did to answer the question in Methodology ;
- what they observed in Results ; and
- what they think the results mean in Discussion .
A substantial study will sometimes include a literature review section which discusses previous works on the topic. The basic structure is outlined below:
- Author and author's professional affiliation is identified
- Introduction
- Literature review section (a discussion about what other scholars have written on the topic)
- Methodology section (methods of data gathering are explained)
- Discussion section
- Conclusions
- Reference list with citations (sources of information used in the article)
Thanks for helping us improve csumb.edu. Spot a broken link, typo, or didn't find something where you expected to? Let us know. We'll use your feedback to improve this page, and the site overall.

Journal Article Basics
- What, Why, & Where
- Peer Review
What is an abstract?
Publication, introduction, charts, graphs, etc., article text, methods or methodology.
- Identification
- Reading an Article
- Types of Articles
Knowing about the different sections of a scholarly article and the type of information presented in each section, will make it easier to understand what the article is about. Also, reading specific parts or sections of an article can help save you time as you decide whether an article is relevant.

- Anatomy of a Scholarly Article [NCSU Interactive Tutorial] Excellent interactive tool for learning about the sections of a scholarly article.
The title of a scholarly article is generally (but not always) an extremely brief summary of the article's contents. It will usually contain technical terms related to the research presented.
Authors and their credentials will be provided in a scholarly article. Credentials may appear with the authors' names, as in this example, or they may appear as a footnote or an endnote to the article. The authors' credentials are provided to establish the authority of the authors, and also to provide a point of contact for the research presented in the article. For this reason, authors' e-mail addresses are usually provided in recent articles.
On the first page of an article you will usually find the journal title, volume/issue numbers, if applicable, and page numbers of the article. This information is necessary for you to write a citation of the article for your paper.
The information is not always neatly outlined at the bottom of the first page; it may be spread across the header and footer of the first page, or across the headers or footers of opposite pages, and for some online versions of articles, it may not be present at all.
The abstract is a brief summary of the contents of the article, usually under 250 words. It will contain a description of the problem and problem setting; an outline of the study, experiment, or argument; and a summary of the conclusions or findings. It is provided so that readers examining the article can decide quickly whether the article meets their needs.
The introduction to a scholarly article describes the topic or problem the authors researched. The authors will present the thesis of their argument or the goal of their research. The introduction may also discuss the relevance or importance of the research question.
An overview of related research and findings, called a literature review, may appear in the introduction, though the literature review may be in its own section.
Scholarly articles frequently contain charts, graphs, equations, and statistical data related to the research. Pictures are rare unless they relate directly to the research presented in the article.
The body of an article is usually presented in sections, including an introduction , a literature review , one or more sections describing and analyzing the argument, experiment or study.
Scientific research articles typically include separate sections addressing the methods and results of the experiment, and a discussion of the research findings.
Articles typically close with a conclusion summarizing the findings.
The parts of the article may or may not be labeled, and two or more sections may be combined in a single part of the text. The text itself is typically highly technical, and assumes a familiarity with the topic. Jargon, abbreviations, and technical terms are used without definition.
The methods section of a scholarly article generally outlines the experimental design, the materials, and the methods (procedures) of the experiment.
The results section of a scholarly article is generally devoted to discussing the type of analysis conducted regarding the data as well as the results.
A scholarly article will end with a conclusion, where the authors summarize the results of their research. The authors may also discuss how their findings relate to other scholarship, or encourage other researchers to extend or follow up on their work.
The discussion of a scholarly article generally includes a description of how the study contributes to the existing body of research, an analysis of the research questions and hypotheses, and a discussion of the research in connection to the real world.
Most scholarly articles contain many references to publications by other authors. You will find these references scattered throughout the text of the article, as footnotes at the bottom of the page, or endnotes at the end of the article.
Most papers provide a list of references at the end of the paper. Each reference listed there corresponds to one of the citations provided in the body of the paper. You can use this list of references to find additional scholarly articles and books on your topic.
- << Previous: Peer Review
- Next: Identification >>
- Last Updated: Feb 16, 2024 2:37 PM
- URL: https://libguides.pittcc.edu/journal-articles

The Sections of a Research Article
If you’ve ever read or written almost any type of academic document, you might have noticed that they start with introductions and end with conclusions. However, research articles – as a genre – have other consistent sections as well. The complete list of sections for research articles include the following:
- Introduction
- Discussion/Conclusion
A common acronym for teaching the sections of a research article is IMRD/C. In this book, we will focus heavily on helping you understand each of those IMRD/C sections’ various pieces, including their communicative goals and strategies you can use to achieve those goals. We will also use a visual of an hourglass to demonstrate this IMRD/C organizational structure.
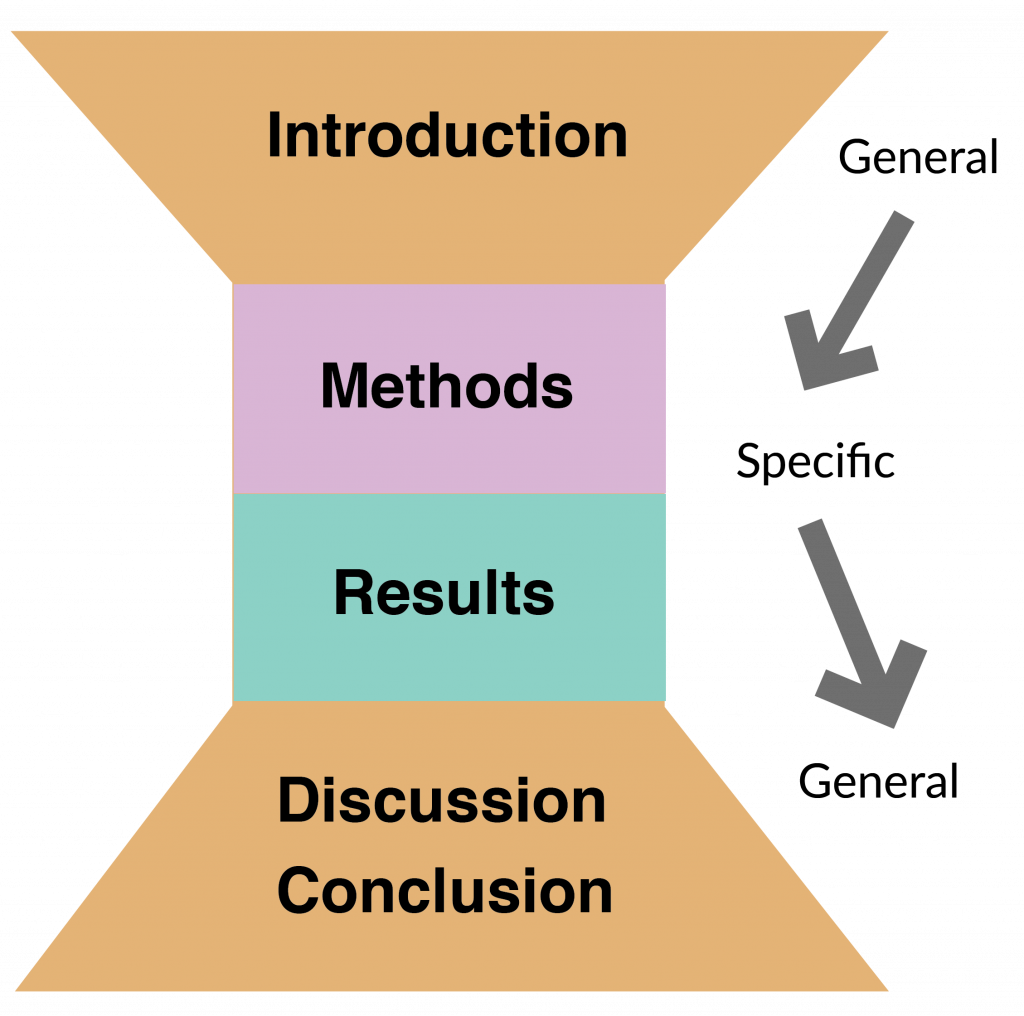
We hope that this graphic along with the explanations and examples in Chapters 3-6 will allow you to deepen your understanding of research writing and become a more successful author.
Preparing to Publish Copyright © 2023 by Sarah Huffman; Elena Cotos; and Kimberly Becker is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License , except where otherwise noted.
- UNC Libraries
- HSL Subject Research
- Structure of Scholarly Articles and Peer Review
- Structure of a Biomedical Research Article
Structure of Scholarly Articles and Peer Review: Structure of a Biomedical Research Article
Created by health science librarians.

Title, Authors, Sources of Support and Acknowledgments
Structured abstract, introduction, results and discussion, international committee of medical journal editors (icmje).
- Compare Types of Journals
- Peer Review
Medical research articles tend to be structured in similar ways. This standard structure helps assure that research is reported with the information readers need to critically appraise the research process and results.
This guide to the structure of a biomedical research article was informed by the description of standard manuscript sections found in the International Committee of Medical Journal Editors (ICMJE) Recommendations chapter on Manuscript Preparation: Preparing for Submission .
If you are writing an article for submisson to a particular journal be sure to obtain that journal's instructions for authors for specific guidelines.
Example Article: Lyons EJ, Tate DF, Ward DS, Wang X. Energy intake and expenditure during sedentary screen time and motion-controlled video gaming. Am J Clin Nutr. 2012 Aug;96(2):234-9. doi: 10.3945/ajcn.111.028423. Epub 2012 Jul 3. PubMed PMID: 22760571; PubMed Central PMCID: PMC3396440. (Free full text available)
Article title: Should provide a succinct description of the purpose of the article using words that will help it be accurately retrieved by search engines.

Author information: Includes the author names and the institution(s) where each author was affiliated at the time the research was conducted. Full contact information is provided for the corresponding author.
Source(s) of support: Specific information about grant funding or source of equipment, drugs, etc. obtained to support the research.

Acknowledgments: This section may found at the end of the article and is used to name people who contributed to the paper, but not fully enough to be named as an author. It may also include more information about the authors' specific roles.

The structure of quantitative research articles is derived from the scientifc process and includes sections covering introduction, methods, results, and discussion (IMRaD). The actual labels for the various parts may vary between journals.
Abstract: A structured abstract reports a summary of each of the IMRaD sections. Enough information should be included to provide the purpose of the research; an outline of methods used; results with data; and conclusions that highlight the findings.

The introduction provides background information about what is known from previous related research, citing the relevant studies, and points out the gap in previous research that is being addressed by the new study. Often, many of the references cited in a paper are in the introduction. The purpose of the research should be clearly stated in this section.
The sample paper's introduction links television watching to increased energy intake and obesity, notes that several studies have shown a similar link with video gaming, and states no study was identified that compared television and video gaming. Eighteen of the thirty-one references used in the paper are cited in the introduction. The final paragraph of the introduction has two sentences that clearly state the purpose and the hypothesized expected outcome of the study.
The methods section clearly explains how the study was conducted. The ICMJE recommends that this section include information about how participants were selected, detailed demographics about who the participants were, and explanations of why any particular populations were included or excluded from the study. The details of how the study was conducted should be described with enough detail that the study could be replicated. Selected statistical methods should be reported in enough detail that readers can evaluate their appropriateness to the data being gathered.
The sample paper’s methods section includes subsections covering: recruitment; procedures used for each study subgroup (TV, VG, motion-controlled VG); what snacks and beverages were used and how they were made available; how energy intake and energy expenditure were measured; how the data was analyzed and the specific statistical analysis and secondary analysis that was used.
The results section reports the data gathered and the statistical analysis of the data. Tables and / or graphs are often used to clearly and compactly present the data.
The results section of the sample paper has two subsections and two tables. One subsection and related table shows the analysis of participant characteristics, The other subsection and table covers the analysis of energy intake, expenditure, and surplus.
In order to critically appraise the quality of the study you need to be able to understand the statistical analysis of the data. Two articles that help with this task are:
- Greenhalgh Trisha. How to read a paper: Statistics for the non-statistician. I: Different types of data need different statistical tests BMJ 1997; 315:364
- Greenhalgh Trisha. How to read a paper: Statistics for the non-statistician. II: “Significant” relations and their pitfalls BMJ 1997; 315:422
Another aid to critically reading a paper is to see if it has been included and evaluated in a systematic review. Try searching for the article you are reading in Google Scholar and seeing if the cited references include a systematic review. The sample paper was critically reviewed in:
- Marsh S, Ni Mhurchu C, Maddison R. The non-advertising effects of screen-based sedentary activities on acute eating behaviours in children, adolescents, and young adults. A systematic review. Appetite. 2013 Dec;71:259-73. doi: 10.1016/j.appet.2013.08.017. Epub 2013 Aug 31. PubMed Abstract . Full-text for UNC-CH .
The discussion section clearly states the primary findings of the study, poses explanations for the findings and any conclusions that can be drawn from them. It may also include the author’s assessment of limitations in the research as conducted and suggestions for further research that is needed.
The structure of biomedical research articles has been standardized across different journals at least in part due to the work of the International Committee of Medical Journal Editors. This group first published the Uniform Requirements for Manuscripts Submitted to Biomedical Journals in 1978.
The Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly work in Medical Journals (2013) is the most recent update of ICMJE's work.
- Next: Compare Types of Journals >>
- Last Updated: Aug 14, 2023 12:15 PM
- URL: https://guides.lib.unc.edu/scholarly-articles
Search & Find
- E-Research by Discipline
- More Search & Find
Places & Spaces
- Places to Study
- Book a Study Room
- Printers, Scanners, & Computers
- More Places & Spaces
- Borrowing & Circulation
- Request a Title for Purchase
- Schedule Instruction Session
- More Services
Support & Guides
- Course Reserves
- Research Guides
- Citing & Writing
- More Support & Guides
- Mission Statement
- Diversity Statement
- Staff Directory
- Job Opportunities
- Give to the Libraries
- News & Exhibits
- Reckoning Initiative
- More About Us
- Search This Site
- Privacy Policy
- Accessibility
- Give Us Your Feedback
- 208 Raleigh Street CB #3916
- Chapel Hill, NC 27515-8890
- 919-962-1053
- Search This Site All UCSD Sites Faculty/Staff Search Term
- Contact & Directions
- Climate Statement
- Cognitive Behavioral Neuroscience
- Cognitive Psychology
- Developmental Psychology
- Social Psychology
- Adjunct Faculty
- Non-Senate Instructors
- Researchers
- Psychology Grads
- Affiliated Grads
- New and Prospective Students
- Honors Program
- Experiential Learning
- Programs & Events
- Psi Chi / Psychology Club
- Prospective PhD Students
- Current PhD Students
- Area Brown Bags
- Colloquium Series
- Anderson Distinguished Lecture Series
- Speaker Videos
- Undergraduate Program
- Academic and Writing Resources
Writing Research Papers
- Research Paper Structure
Whether you are writing a B.S. Degree Research Paper or completing a research report for a Psychology course, it is highly likely that you will need to organize your research paper in accordance with American Psychological Association (APA) guidelines. Here we discuss the structure of research papers according to APA style.
Major Sections of a Research Paper in APA Style
A complete research paper in APA style that is reporting on experimental research will typically contain a Title page, Abstract, Introduction, Methods, Results, Discussion, and References sections. 1 Many will also contain Figures and Tables and some will have an Appendix or Appendices. These sections are detailed as follows (for a more in-depth guide, please refer to " How to Write a Research Paper in APA Style ”, a comprehensive guide developed by Prof. Emma Geller). 2
What is this paper called and who wrote it? – the first page of the paper; this includes the name of the paper, a “running head”, authors, and institutional affiliation of the authors. The institutional affiliation is usually listed in an Author Note that is placed towards the bottom of the title page. In some cases, the Author Note also contains an acknowledgment of any funding support and of any individuals that assisted with the research project.
One-paragraph summary of the entire study – typically no more than 250 words in length (and in many cases it is well shorter than that), the Abstract provides an overview of the study.
Introduction
What is the topic and why is it worth studying? – the first major section of text in the paper, the Introduction commonly describes the topic under investigation, summarizes or discusses relevant prior research (for related details, please see the Writing Literature Reviews section of this website), identifies unresolved issues that the current research will address, and provides an overview of the research that is to be described in greater detail in the sections to follow.
What did you do? – a section which details how the research was performed. It typically features a description of the participants/subjects that were involved, the study design, the materials that were used, and the study procedure. If there were multiple experiments, then each experiment may require a separate Methods section. A rule of thumb is that the Methods section should be sufficiently detailed for another researcher to duplicate your research.
What did you find? – a section which describes the data that was collected and the results of any statistical tests that were performed. It may also be prefaced by a description of the analysis procedure that was used. If there were multiple experiments, then each experiment may require a separate Results section.
What is the significance of your results? – the final major section of text in the paper. The Discussion commonly features a summary of the results that were obtained in the study, describes how those results address the topic under investigation and/or the issues that the research was designed to address, and may expand upon the implications of those findings. Limitations and directions for future research are also commonly addressed.
List of articles and any books cited – an alphabetized list of the sources that are cited in the paper (by last name of the first author of each source). Each reference should follow specific APA guidelines regarding author names, dates, article titles, journal titles, journal volume numbers, page numbers, book publishers, publisher locations, websites, and so on (for more information, please see the Citing References in APA Style page of this website).
Tables and Figures
Graphs and data (optional in some cases) – depending on the type of research being performed, there may be Tables and/or Figures (however, in some cases, there may be neither). In APA style, each Table and each Figure is placed on a separate page and all Tables and Figures are included after the References. Tables are included first, followed by Figures. However, for some journals and undergraduate research papers (such as the B.S. Research Paper or Honors Thesis), Tables and Figures may be embedded in the text (depending on the instructor’s or editor’s policies; for more details, see "Deviations from APA Style" below).
Supplementary information (optional) – in some cases, additional information that is not critical to understanding the research paper, such as a list of experiment stimuli, details of a secondary analysis, or programming code, is provided. This is often placed in an Appendix.
Variations of Research Papers in APA Style
Although the major sections described above are common to most research papers written in APA style, there are variations on that pattern. These variations include:
- Literature reviews – when a paper is reviewing prior published research and not presenting new empirical research itself (such as in a review article, and particularly a qualitative review), then the authors may forgo any Methods and Results sections. Instead, there is a different structure such as an Introduction section followed by sections for each of the different aspects of the body of research being reviewed, and then perhaps a Discussion section.
- Multi-experiment papers – when there are multiple experiments, it is common to follow the Introduction with an Experiment 1 section, itself containing Methods, Results, and Discussion subsections. Then there is an Experiment 2 section with a similar structure, an Experiment 3 section with a similar structure, and so on until all experiments are covered. Towards the end of the paper there is a General Discussion section followed by References. Additionally, in multi-experiment papers, it is common for the Results and Discussion subsections for individual experiments to be combined into single “Results and Discussion” sections.
Departures from APA Style
In some cases, official APA style might not be followed (however, be sure to check with your editor, instructor, or other sources before deviating from standards of the Publication Manual of the American Psychological Association). Such deviations may include:
- Placement of Tables and Figures – in some cases, to make reading through the paper easier, Tables and/or Figures are embedded in the text (for example, having a bar graph placed in the relevant Results section). The embedding of Tables and/or Figures in the text is one of the most common deviations from APA style (and is commonly allowed in B.S. Degree Research Papers and Honors Theses; however you should check with your instructor, supervisor, or editor first).
- Incomplete research – sometimes a B.S. Degree Research Paper in this department is written about research that is currently being planned or is in progress. In those circumstances, sometimes only an Introduction and Methods section, followed by References, is included (that is, in cases where the research itself has not formally begun). In other cases, preliminary results are presented and noted as such in the Results section (such as in cases where the study is underway but not complete), and the Discussion section includes caveats about the in-progress nature of the research. Again, you should check with your instructor, supervisor, or editor first.
- Class assignments – in some classes in this department, an assignment must be written in APA style but is not exactly a traditional research paper (for instance, a student asked to write about an article that they read, and to write that report in APA style). In that case, the structure of the paper might approximate the typical sections of a research paper in APA style, but not entirely. You should check with your instructor for further guidelines.
Workshops and Downloadable Resources
- For in-person discussion of the process of writing research papers, please consider attending this department’s “Writing Research Papers” workshop (for dates and times, please check the undergraduate workshops calendar).
Downloadable Resources
- How to Write APA Style Research Papers (a comprehensive guide) [ PDF ]
- Tips for Writing APA Style Research Papers (a brief summary) [ PDF ]
- Example APA Style Research Paper (for B.S. Degree – empirical research) [ PDF ]
- Example APA Style Research Paper (for B.S. Degree – literature review) [ PDF ]
Further Resources
How-To Videos
- Writing Research Paper Videos
APA Journal Article Reporting Guidelines
- Appelbaum, M., Cooper, H., Kline, R. B., Mayo-Wilson, E., Nezu, A. M., & Rao, S. M. (2018). Journal article reporting standards for quantitative research in psychology: The APA Publications and Communications Board task force report . American Psychologist , 73 (1), 3.
- Levitt, H. M., Bamberg, M., Creswell, J. W., Frost, D. M., Josselson, R., & Suárez-Orozco, C. (2018). Journal article reporting standards for qualitative primary, qualitative meta-analytic, and mixed methods research in psychology: The APA Publications and Communications Board task force report . American Psychologist , 73 (1), 26.
External Resources
- Formatting APA Style Papers in Microsoft Word
- How to Write an APA Style Research Paper from Hamilton University
- WikiHow Guide to Writing APA Research Papers
- Sample APA Formatted Paper with Comments
- Sample APA Formatted Paper
- Tips for Writing a Paper in APA Style
1 VandenBos, G. R. (Ed). (2010). Publication manual of the American Psychological Association (6th ed.) (pp. 41-60). Washington, DC: American Psychological Association.
2 geller, e. (2018). how to write an apa-style research report . [instructional materials]. , prepared by s. c. pan for ucsd psychology.
Back to top
- Formatting Research Papers
- Using Databases and Finding References
- What Types of References Are Appropriate?
- Evaluating References and Taking Notes
- Citing References
- Writing a Literature Review
- Writing Process and Revising
- Improving Scientific Writing
- Academic Integrity and Avoiding Plagiarism
- Writing Research Papers Videos
- Research Guides

Reading for Research: Social Sciences
Structure of a research article.
- Structural Read
Guide Acknowledgements
How to Read a Scholarly Article from the Howard Tilton Memorial Library at Tulane University
Strategic Reading for Research from the Howard Tilton Memorial Library at Tulane University
Bridging the Gap between Faculty Expectation and the Student Experience: Teaching Students toAnnotate and Synthesize Sources
Librarian for Sociology, Environmental Sociology, MHS and Public Policy Studies

Academic writing has features that vary only slightly across the different disciplines. Knowing these elements and the purpose of each serves help you to read and understand academic texts efficiently and effectively, and then apply what you read to your paper or project.
Social Science (and Science) original research articles generally follow IMRD: Introduction- Methods-Results-Discussion
Introduction
- Introduces topic of article
- Presents the "Research Gap"/Statement of Problem article will address
- How research presented in the article will solve the problem presented in research gap.
- Literature Review. presenting and evaluating previous scholarship on a topic. Sometimes, this is separate section of the article.
Method & Results
- How research was done, including analysis and measurements.
- Sometimes labeled as "Research Design"
- What answers were found
- Interpretation of Results (What Does It Mean? Why is it important?)
- Implications for the Field, how the study contributes to the existing field of knowledge
- Suggestions for further research
- Sometimes called Conclusion
You might also see IBC: Introduction - Body - Conclusion
- Identify the subject
- State the thesis
- Describe why thesis is important to the field (this may be in the form of a literature review or general prose)
Body
- Presents Evidence/Counter Evidence
- Integrate other writings (i.e. evidence) to support argument
- Discuss why others may disagree (counter-evidence) and why argument is still valid
- Summary of argument
- Evaluation of argument by pointing out its implications and/or limitations
- Anticipate and address possible counter-claims
- Suggest future directions of research
- Next: Structural Read >>
- Last Updated: Jan 19, 2024 10:44 AM
- URL: https://researchguides.library.vanderbilt.edu/readingforresearch

- SpringerLink shop
Structuring your manuscript
Once you have completed your experiments it is time write it up into a coherent and concise paper which tells the story of your research. Researchers are busy people and so it is imperative that research articles are quick and easy to read. For this reason papers generally follow a standard structure which allows readers to easily find the information they are looking for. In the next part of the course we will discuss the standard structure and what to include in each section.
Overview of IMRaD structure
IMRaD refers to the standard structure of the body of research manuscripts (after the Title and Abstract):
- I ntroduction
- M aterials and Methods
- D iscussion and Conclusions
Not all journals use these section titles in this order, but most published articles have a structure similar to IMRaD. This standard structure:
- Gives a logical flow to the content
- Makes journal manuscripts consistent and easy to read
- Provides a “map” so that readers can quickly find content of interest in any manuscript
- Reminds authors what content should be included in an article
Provides all content needed for the work to be replicated and reproduced Although the sections of the journal manuscript are published in the order: Title, Abstract, Introduction, Materials and Methods, Results, Discussion, and Conclusion, this is not the best order for writing the sections of a manuscript. One recommended strategy is to write your manuscript in the following order:
1. Materials and Methods
These can be written first, as you are doing your experiments and collecting the results.
3. Introduction
4. Discussion
5. Conclusion
Write these sections next, once you have had a chance to analyse your results, have a sense of their impact and have decided on the journal you think best suits the work
7. Abstract
Write your Title and Abstract last as these are based on all the other sections.
Following this order will help you write a logical and consistent manuscript.
Use the different sections of a manuscript to ‘tell a story’ about your research and its implications.
Back │ Next

Reading a Scholarly Article
- Parts of a Scholarly Article
- How to Read a Scholarly Article
- Additional Library Tutorials This link opens in a new window
Need Help? Ask Us!
Sources consulted.
Anatomy of a Scholarly Article: NCSU Libraries . (2009, July 13). https://www.lib.ncsu.edu/tutorials/scholarly-articles/
Evelyn, S. (2021). LibGuides: Evaluating Information: How to Read a Scholarly Article . https://libguides.brown.edu/evaluate/ Read
Rempel, H. (2021). LibGuides: FW 107: Orientation to Fisheries and Wildlife: 5. The Anatomy of a Scholarly Article . https://guides.library.oregonstate.edu/ c.php?g=286038&p=1905154
- Next: How to Read a Scholarly Article >>
- Last Updated: Feb 26, 2024 12:42 PM
- URL: https://libguides.wku.edu/reading_scholarly_articles
How to Read an Academic Journal Article
- What Is in an Academic Journal
- Anatomy of a Journal Article
- How to Read an Article
Anatomy of a Primary/Original Journal Article
Nearly all primary source journal articles (reporting the results of authors' own research) in the sciences, and most in the social sciences, follow the standard format below. Arts and humanities articles are more variable in specific formats, but in general they are at least roughly similar; even if they are not divided and labeled in the same manner, they will address these same points:
Introduction – What question was asked? Methods – How was it answered? Results – What was the answer? Conclusion – What does the answer mean?
Knowing the structure of a typical article helps you quickly skim to find and focus on what you need at different stages in your research process, which can save you a lot of time and effort.
For an example, let's take a detailed look at this article on college students and sleep .

Journal Title - The title of the journal that published the article, as well as the volume and issue number and date of publication.
Article Title and Authors - The title is usually a concise statement of the topic of the article, and often contains key technical or specific terms. The authors' institutional affiliations are often listed, sometimes including contact information.
Article Info - There is often a section saying when the article was initially submitted, then resubmitted and accepted after revisions. Sometimes it includes a list of keywords the author selected to describe the article and make it findable via search engines.
Abstract - A brief summation of the article, usually including the purpose of the study, main findings, conclusions, and significance of the results.
Introduction - Overview of the contents of the article, explaining the purpose of the study (e.g., the hypothesis being tested, or the question the study seeks to answer) and putting it in context of other research done. Often this will include a literature review - a summary of other work done on the topic, to which this article is adding new findings - but sometimes a literature review is in a section of its own.

Methods - The specific methodologies – study design, procedures, measurement instruments, data collection, etc. – used to conduct the study.

Results - Detailed outcomes of the study, often including charts, graphs, or tables of data.

Discussion of results – Analysis and interpretation of the results, explaining what conclusions can be drawn (e.g., was the hypothesis confirmed, or were the study’s questions answered). This section also places the results in the wider research context outlined in the introduction, and addresses implications for future research.

Limitations and Questions - This could be integrated into the Discussion or Conclusion, a subsection of Discussion (as in this example) or Conclusion, or a separate section. This addresses what the results do not and cannot say, and points to remaining questions unanswered or new questions raised by the findings.

Conclusion - A concise summary of the main findings and significance of the study.
References - A list of all the publications and other sources cited in the article.
Acknowledgments, Appendices, etc. - More recent articles usually disclose sources of funding or possible conflicts of interest (funding or affiliations that may influence the author’s interpretations). Some related data, images, etc., may be included in an appendix.
- << Previous: What Is in an Academic Journal
- Next: How to Read an Article >>
- Last Updated: Oct 27, 2023 11:59 AM
- URL: https://libguides.wesleyan.edu/readanarticle
- Systematic Review
- Open access
- Published: 26 April 2024
Systematic review on the frequency and quality of reporting patient and public involvement in patient safety research
- Sahar Hammoud ORCID: orcid.org/0000-0003-4682-9001 1 ,
- Laith Alsabek 1 , 2 ,
- Lisa Rogers 1 &
- Eilish McAuliffe 1
BMC Health Services Research volume 24 , Article number: 532 ( 2024 ) Cite this article
212 Accesses
Metrics details
In recent years, patient and public involvement (PPI) in research has significantly increased; however, the reporting of PPI remains poor. The Guidance for Reporting Involvement of Patients and the Public (GRIPP2) was developed to enhance the quality and consistency of PPI reporting. The objective of this systematic review is to identify the frequency and quality of PPI reporting in patient safety (PS) research using the GRIPP2 checklist.
Searches were performed in Ovid MEDLINE, EMBASE, PsycINFO, and CINAHL from 2018 to December, 2023. Studies on PPI in PS research were included. We included empirical qualitative, quantitative, mixed methods, and case studies. Only articles published in peer-reviewed journals in English were included. The quality of PPI reporting was assessed using the short form of the (GRIPP2-SF) checklist.
A total of 8561 studies were retrieved from database searches, updates, and reference checks, of which 82 met the eligibility criteria and were included in this review. Major PS topics were related to medication safety, general PS, and fall prevention. Patient representatives, advocates, patient advisory groups, patients, service users, and health consumers were the most involved. The main involvement across the studies was in commenting on or developing research materials. Only 6.1% ( n = 5) of the studies reported PPI as per the GRIPP2 checklist. Regarding the quality of reporting following the GRIPP2-SF criteria, our findings show sub-optimal reporting mainly due to failures in: critically reflecting on PPI in the study; reporting the aim of PPI in the study; and reporting the extent to which PPI influenced the study overall.
Conclusions
Our review shows a low frequency of PPI reporting in PS research using the GRIPP2 checklist. Furthermore, it reveals a sub-optimal quality in PPI reporting following GRIPP2-SF items. Researchers, funders, publishers, and journals need to promote consistent and transparent PPI reporting following internationally developed reporting guidelines such as the GRIPP2. Evidence-based guidelines for reporting PPI should be encouraged and supported as it helps future researchers to plan and report PPI more effectively.
Trial registration
The review protocol is registered with PROSPERO (CRD42023450715).
Peer Review reports
Patient safety (PS) is defined as “the absence of preventable harm to a patient and reduction of risk of unnecessary harm associated with healthcare to an acceptable minimum” [ 1 ]. It is estimated that one in 10 patients are harmed in healthcare settings due to unsafe care, resulting in over three million deaths annually [ 2 ]. More than 50% of adverse events are preventable, and half of these events are related to medications [ 3 , 4 ]. There are various types of adverse events that patients can experience such as medication errors, patient falls, healthcare-associated infections, diagnostic errors, pressure ulcers, unsafe surgical procedures, patient misidentification, and others [ 1 ].
Over the last few decades, the approach of PS management has shifted toward actively involving patients and their families in managing PS. This innovative approach has surpassed the traditional model where healthcare providers were the sole managers of PS [ 5 ]. Recent research has shown that patients have a vital role in promoting their safety and decreasing the occurrence of adverse events [ 6 ]. Hence, there is a growing recognition of patient and family involvement as a promising method to enhance PS [ 7 ]. This approach includes involving patients in PS policy development, research, and shared decision making [ 1 ].
In the last decade, research involving patients and the public has significantly increased. In the United Kingdom (U.K), the National Institute for Health Research (NIHR) has played a critical role in providing strategic and infrastructure support to integrate Public and Patient Involvement (PPI) throughout publicly funded research [ 8 ]. This has established a context where PPI is recognised as an essential element in research [ 9 ]. In Ireland, the national government agency responsible for the management and delivery of all public health and social services; the National Health Service Executive (HSE) emphasise the importance of PPI in research and provide guidance for researchers on how to involve patients and public in all parts of the research cycle and knowledge translation process [ 10 ]. Similar initiatives are also developing among other European countries, North America, and Australia. However, despite this significant expansion of PPI research, the reporting of PPI in research articles continues to be sub-optimal, inconsistent, and lacks essential information on the context, process, and impact of PPI [ 9 ]. To address this problem, the Guidance for Reporting Involvement of Patients and the Public (GRIPP) was developed in 2011 following the EQUATOR methodology to enhance the quality, consistency, and transparency of PPI reporting. Additionally, to provide guidance for researchers, patients, and the public to advance the quality of the international PPI evidence-base [ 11 ]. The first GRIPP checklist was a significant start in producing higher-quality PPI reporting; however, it was developed following a systematic review, and did not include any input from the international PPI research community. Given the importance of reaching consensus in generating current reporting guidelines, a second version of the GRIPP checklist (GRIPP2) was developed to tackle this problem by involving the international PPI community in its development [ 9 ]. There are two versions of the GRIPP2 checklist, a long form (GRIPP2-LF) for studies with PPI as the primary focus, and a short form (GRIPP2-SF) for studies with PPI as secondary or tertiary focus.
Since the publication of the GRIPP2 checklist, several systematic reviews have been conducted to assess the quality of PPI reporting on various topics. For instance, Bergin et al. in their review to investigate the nature and impact of PPI in cancer research, reported a sub-optimal quality of PPI reporting using the GRIPP2-SF, mainly due to failure to address PPI challenges [ 12 ]. Similarly, Owyang et al. in their systematic review to assess the prevalence, extent, and quality of PPI in orthopaedic practice, described a poor PPI reporting following the GRIPP2-SF checklist criteria [ 13 ]. While a few systematic reviews have been conducted to assess theories, strategies, types of interventions, and barriers and enablers of PPI in PS [ 5 , 14 , 15 , 16 ], no previous review has assessed the quality of PPI reporting in PS research. Thus, our systematic review aims to address this knowledge gap. The objective of this review is to identify the frequency PPI reporting in PS research using the GRIPP2 checklist from 2018 (the year after GRIPP2 was published) and the quality of reporting following the GRIPP2-SF. The GRIPP2 checklist was chosen as the benchmark as it is the first international, evidence-based, community consensus informed guideline for the reporting of PPI in research and more specifically in health and social care research [ 9 ]. Additionally, it is the most recent report-focused framework and the most recommended by several leading journals [ 17 ].
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to plan and report this review [ 18 ]. The review protocol was published on PROSPERO the International Database of Prospectively Registered Systematic Reviews in August 2023 (CRD42023450715).
Search strategy
For this review, we used the PICo framework to define the key elements in our research. These included articles on patients and public (P-Population) involvement (I- phenomenon of Interest) in PS (C-context). Details are presented in Table 1 . Four databases were searched including Ovid MEDLINE, EMBASE, PsycINFO, and CINAHL to identify papers on PPI in PS research. A systematic search strategy was initially developed using MEDLINE. MeSH terms and keywords relevant to specific categories (e.g., patient safety) were combined using the “OR” Boolean term (i.e. patient safety OR adverse event OR medical error OR surgical error) and categories were then combined using the “AND” Boolean term. (i.e. “patient and public involvement” AND “patient safety”). The search strategy was adapted for the other three databases. Full search strategies are provided in Supplementary file 1 . The search was conducted on July 27th, 2023, and was limited to papers published from 2018. As the GRIPP2 tool was published in 2017, this limit ensured the retrieval of relevant studies. An alert system was set on the four databases to receive all new published studies until December 2023, prior to the final analysis. The search was conducted without restrictions on study type, research design, and language. To reduce selection bias, hand searching was carried out on the reference lists of all the eligible articles in the later stages of the review. This was done by the first author. The search strategy was developed by the first author and confirmed by the research team and a Librarian. The database search was conducted by the first author.
Inclusion and exclusion criteria
Studies on PPI in PS research with a focus on health/healthcare were included in this review. We defined PPI as active involvement which is in line with the NIHR INVOLVE definition as “research being carried out ‘with’ or ‘by’ members of the public rather than ‘to’, ‘about’ or ‘for’ them” [ 19 ]. This includes any PPI including, being a co-applicant on a research project or grant application, identifying research priorities, being a member of an advisory or steering group, participating in developing research materials or giving feedback on them, conducting interviews with study participants, participating in recruitment, data collection, data analysis, drafting manuscripts and/or dissemination of results. Accordingly, we excluded studies where patients or the public were only involved as research participants.
We defined patients and public to include patients, relatives, carers, caregivers and community, which is also in line with the NIHR PPI involvement in National Health Service [ 19 ].
Patient safety included topics on medication safety, adverse events, communication, safety culture, diagnostic errors, and others. A full list of the used terms for PPI and PS is provided in Supplementary file 1 . Regarding the research type and design, we included empirical qualitative, quantitative, mixed methods, and case studies. Only articles published in peer-reviewed journals and in English were included.
Any article that did not meet the inclusion criteria was excluded. Studies not reporting outcomes were excluded. Furthermore, review papers, conference abstracts, letters to editor, commentary, viewpoints, and short communications were excluded. Finally, papers published prior to 2018 were excluded.
Study selection
The selection of eligible studies was done by the first and the second authors independently, starting with title and abstracts screening to eliminate papers that failed to meet our inclusion criteria. Then, full text screening was conducted to decide on the final included papers in this review. Covidence, an online data management system supported the review process, ensuring reviewers were blinded to each other’s decisions. Disagreements between reviewers were discussed first, in cases where the disagreement was not resolved, the fourth author was consulted.
Data extraction and analysis
A data extraction sheet was developed using excel then piloted, discussed with the research team and modified as appropriate. The following data were extracted: citation and year of publication, objective of the study, country, PS topic, design, setting, PPI participants, PPI stages (identifying research priorities, being a member of an advisory or steering group, etc.…), frequency of PPI reporting as per the GRIPP2 checklist, and the availability of a plain language summary. Additionally, data against the five items of GRIPP2-SF (aim of PPI in the study, methods used for PPI, outcomes of PPI including the results and the extent to which PPI influenced the study overall, and reflections on PPI) were extracted. To avoid multiple publication bias and missing outcomes, data extraction was done by the first and the second authors independently and then compared. Disagreements between reviewers were first discussed, and then resolved by the third and fourth authors if needed.
Quality assessment
The quality of PPI reporting was assessed using GRIPP2-SF developed by Staniszewska et al. [ 9 ] as it is developed to improve the quality, consistency, and reporting of PPI in social and healthcare research. Additionally the GRIPP2-SF is suitable for all studies regardless of whether PPI is the primary, secondary, or tertiary focus, whereas the GRIPP2-LF is not suitable for studies where PPI serves as a secondary or tertiary focus. The checklist includes five items (mentioned above) that authors should include in their studies. It is important to mention that Staniszewska et al. noted that “while GRIPP2-SF aims to guide consistent reporting, it is not possible to be prescriptive about the exact content of each item, as the current evidence-base is not advanced enough to make this possible” ([ 9 ] p5). For that reason, we had to develop criteria for scoring the five reporting items. We used three scoring as Yes, No, and partial for each of the five items of the GRIPP2-SF. Yes, was given when authors presented PPI information on the item clearly in the paper. No, when no information was provided, and partial when the information partially met the item requirement. For example, as per GRIPP2-SF authors should provide a clear description of the methods used for PPI in the study. In the example given by Staniszewska et al., information on patient/public partners and how many of them were provided, as well as the stages of the study they were involved in (i.e. refining the focus of the research questions, developing the search strategy, interpreting results). Thus, in our evaluation of the included studies, we gave a yes if information on PPI participants (i.e. patient partners, community partners, or family members etc..) and how many of them were involved was provided, and information on the stages or actions of their involvement in the study was provided. However, we gave a “partial” if information was not fully provided (i.e. information on patient/public partners and how many were involved in the study without describing in what stages or actions they were involved, and vice versa), and a “No” if no information was presented at all.
The quality of PPI reporting was done by the first and the second authors independently and then compared. Disagreements between reviewers were first discussed, and then resolved by the third and fourth author when needed.
Assessing the quality or risk of bias of the included studies was omitted, as the focus in this review was on appraising the quality of PPI reporting rather than assessing the quality of each research article.
Data synthesis
After data extraction, a table summarising the included studies was developed. Studies were compared according to the main outcomes of the review; frequency of PPI reporting following the GRIPP2 checklist and the quality of reporting as per GRIPP2-SF five items, and the availability of a plain language summary.
Search results and study selection
The database searches yielded a total of 8491 studies. First, 2496 were removed as duplicates. Then, after title and abstract screening, 5785 articles were excluded leaving 210 articles eligible for the full text review. After a careful examination, 68 of these studies were included in this review. A further 38 studies were identified from the alert system that was set on the four databases and 32 studies from the reference check of the included studies. Of these 70 articles, 56 were further excluded and 14 were added to the previous 68 included studies. Thus, 82 studies met the inclusion criteria and were included in this review. A summary of the database search results and the study selection process are presented in Fig. 1 .
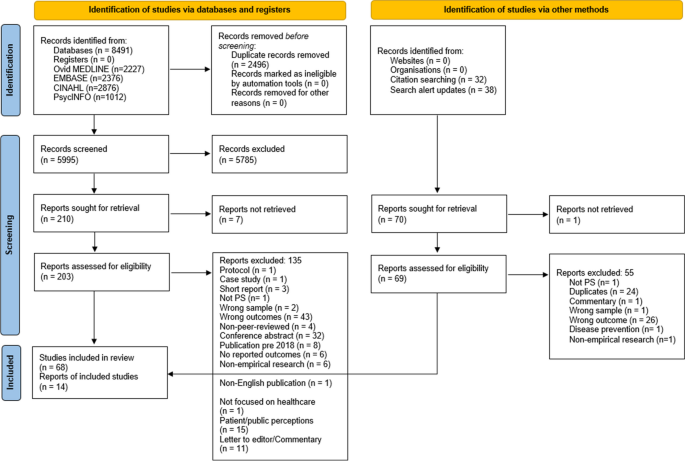
PRISMA flow diagram of the study selection process. The PRISMA flow diagram details the review search results and selection process
Overview of included studies
Details of the study characteristics including first author and year of publication, objective, country, study design, setting, PS topic, PPI participants and involvement stages are presented in Supplementary file 2 . The majority of the studies were conducted in the U.K ( n = 24) and the United States of America ( n = 18), with the remaining 39 conducted in other high income countries, the exception being one study in Haiti. A range of study designs were identified, the most common being qualitative ( n = 31), mixed methods ( n = 13), interventional ( n = 5), and quality improvement projects ( n = 4). Most PS topics concerned medication safety ( n = 17), PS in general (e.g., developing a PS survey or PS management application) ( n = 14), fall prevention ( n = 13), communication ( n = 11), and adverse events ( n = 10), with the remaining PS topics listed in Supplementary file 2 .
Patient representatives, advocates, and patient advisory groups ( n = 33) and patients, service users, and health consumers ( n = 32) were the main groups involved. The remaining, included community members/ organisations. Concerning PPI stages, the main involvement across the studies was in commenting on or developing research materials ( n = 74) including, patient leaflets, interventional tools, mobile applications, and survey instruments. Following this stage, involvement in data analysis, drafting manuscripts, and disseminating results ( n = 30), and being a member of a project advisory or steering group ( n = 18) were the most common PPI evident in included studies. Whereas the least involvement was in identifying research priorities ( n = 5), and being a co-applicant on a research project or grant application ( n = 6).
Regarding plain language summary, only one out of the 82 studies (1.22%) provided a plain language summary in their paper [ 20 ].
Frequency and quality of PPI reporting
The frequency of PPI reporting following the GRIPP2 checklist was 6.1%, where only five of the 82 included studies reported PPI in their papers following the GRIPP2 checklist. The quality of PPI reporting in those studies is presented in Table 2 . Of these five studies, one study (20%) did not report the aim of PPI in the study and one (20%) did not comment on the extent to which PPI influenced the study overall.
The quality of PPI reporting of the remaining 77 studies is presented in Table 3 . The aim of PPI in the study was reported in 62.3% of articles ( n = 48), while 3.9% ( n = 3) partially reported this. A clear description of the methods used for PPI in the study was reported in 79.2% of papers ( n = 61) and partially in 20.8% ( n = 16). Concerning the outcomes, 81.8% of papers ( n = 63) reported the results of PPI in the study, while 10.4% ( n = 8) partially did. Of the 77 studies, 68.8% ( n = 53) reported the extent to which PPI influenced the study overall and 3.9% ( n = 3) partially reported this. Finally, 57.1% ( n = 44) of papers critically reflected on the things that went well and those that did not and 2.6% ( n = 2) partially reflected on this.
Summary of main findings
This systematic review assessed the frequency of reporting PPI in PS research using the GRIPP2 checklist and quality of reporting using the GRIPP2-SF. In total, 82 studies were included in this review. Major PS topics were related to medication safety, general PS, and fall prevention. Patient representatives, advocates, patient advisory groups, patients, service users, and health consumers were the most involved. The main involvement across the studies was in commenting on or developing research materials such as educational and interventional tools, survey instruments, and applications while the least was in identifying research priorities and being a co-applicant on a research project or grant application. Thus, significant effort is still needed to involve patients and the public in the earlier stages of the research process given the fundamental impact of PS on their lives.
Overall completeness and applicability of evidence
A low frequency of reporting PPI in PS research following the GRIPP2 guidelines was revealed in this review, where only five of the 82 studies included mentioned that PPI was reported as per the GRIPP2 checklist. This is despite it being the most recent report-focused framework and the most recommended by several leading journals [ 17 ]. This was not surprising as similar results were reported in recent reviews in other healthcare topics. For instance, Musbahi et al. in their systematic review on PPI reporting in bariatric research reported that none of the 90 papers identified in their review mentioned or utilised the GRIPP2 checklist [ 102 ]. Similarly, a study on PPI in orthodontic research found that none of the 363 included articles reported PPI against the GRIPP2 checklist [ 103 ].
In relation to the quality of reporting following the GRIPP2-SF criteria, our findings show sub-optimal reporting within the 77 studies that did not use GRIPP2 as a guide/checklist to report their PPI. Similarly, Bergin et al. in their systematic review to investigate the nature and impact of PPI in cancer research concluded that substandard reporting was evident [ 12 ]. In our review, this was mainly due to failure to meet three criteria. First, the lowest percentage of reporting (57.1%, n = 44) was related to critical reflection on PPI in the study (i.e., what went well and what did not). In total, 31 studies (42.9%) did not provide any information on this, and two studies were scored as partial. The first study mentioned that only involving one patient was a limitation [ 27 ] and the other stated that including three patients in the design of the tool was a strength [ 83 ]. Both studies did not critically comment or reflect on these points so that future researchers are able to avoid such problems and enhance PPI opportunities. For instance, providing the reasons/challenges behind the exclusive inclusion of a single patient and explaining how this limits the study findings and conclusion would help future researchers to address these challenges. Likewise, commenting on why incorporating three patients in the design of the study tool could be seen as a strength would have been beneficial. This could be, fostering diverse perspectives and generating novel ideas for developing the tool. Similar to our findings, Bergin et al. in their systematic review reported that 40% of the studies failed to meet this criterion [ 12 ].
Second, only 48 out of 77 articles (62.3%) reported the aim of PPI in their study, which is unlike the results of Bergin et al. where most of the studies (93.1%) in their review met this criterion [ 12 ]. Of the 29 studies which did not meet this criterion in our review, few mentioned in their objective developing a consensus-based instrument [ 41 ], reaching a consensus on the patient-reported outcomes [ 32 ], obtaining international consensus on a set of core outcome measures [ 98 ], and facilitating a multi-stakeholder dialogue [ 71 ] yet, without indicating anything in relation to patients, patient representatives, community members, or any other PPI participants. Thus, the lack of reporting the aim of PPI was clearly evident in this review. Reporting the aim of PPI in the study is crucial for promoting transparency, methodological rigor, reproducibility, and impact assessment of the PPI.
Third, 68.8% ( n = 53) of the studies reported the extent to which PPI influenced the study overall including positive and negative effects if any. This was again similar to the findings of Bergin et al., where 38% of the studies did not meet this criterion mainly due to a failure to address PPI challenges in their respective studies [ 12 ]. Additionally, Owyang et al. in their review on the extent, and quality of PPI in orthopaedic practice, also described a poor reporting of PPI impact on research [ 13 ]. As per the GRIPP2 guidelines, both positive and negative effects of PPI on the study should be reported when applicable. Providing such information is essential as it enhances future research on PPI in terms of both practice and reporting.
Reporting a clear description of the methods used for PPI in the study was acceptable, with 79.2% of the papers meeting this criterion. Most studies provided information in the methods section of their papers on the PPI participants, their number, stages of their involvement and how they were involved. Providing clear information on the methods used for PPI is vital to give the reader a clear understanding of the steps taken to involve patients, and for other researchers to replicate these methods in future research. Additionally, reporting the results of PPI in the study was also acceptable with 81.8% of the papers reporting the outcomes of PPI in the results section. Reporting the results of PPI is important for enhancing methodological transparency, providing a more accurate interpretation for the study findings, contributing to the overall accountability and credibility of the research, and informing decision making.
Out of the 82 studies included in this review, only one study provided a plain language summary. We understand that PS research or health and medical research in general is difficult for patients and the public to understand given their diverse health literacy and educational backgrounds. However, if we expect patients and the public to be involved in research then, it is crucial to translate this research that has a huge impact on their lives into an easily accessible format. Failing to translate the benefits that such research may have on patient and public lives may result in them underestimating the value of this research and losing interest in being involved in the planning or implementation of future research [ 103 ]. Thus, providing a plain language summary for research is one way to tackle this problem. To our knowledge, only a few health and social care journals (i.e. Cochrane and BMC Research Involvement and Engagement) necessitate a plain language summary as a submission requirement. Having this as a requirement for submission is crucial in bringing the importance of this issue to researchers’ attention.
Research from recent years suggests that poor PPI reporting in articles relates to a lack of submission requirements for PPI reporting in journals and difficulties with word limits for submitted manuscripts [ 13 ]. Price et al. assessed the frequency of PPI reporting in published papers before and after the introduction of PPI reporting obligations by the British Medical Journal (BMJ) [ 104 ]. The authors identified an increase in PPI reporting in papers published by BMJ from 0.5% to 11% between the periods of 2013–2014 and 2015–2016. The study findings demonstrate the impact of journal guidelines in shaping higher quality research outputs [ 13 ]. In our review, we found a low frequency of PPI reporting in PS research using the GRIPP2 checklist, alongside sub-optimal quality of reporting following GRIPP2-SF. This could potentially be attributed to the absence of submission requirements for PPI reporting in journals following the GRIPP2 checklist, as well as challenges posed by word limits.
Strengths and limitations
This systematic review presents an overview on the frequency of PPI reporting in PS research using the GRIPP2 checklist, as well as an evaluation of the quality of reporting following the GRIPP2-SF. As the first review to focus on PS research, it provides useful knowledge on the status of PPI reporting in this field, and the extent to which researchers are adopting and adhering to PPI reporting guidelines. Despite these strengths, our review has some limitations that should be mentioned. First, only English language papers were included in this review due to being the main language of the researchers. Thus, there is a possibility that relevant articles on PPI in PS research may have been omitted. Another limitation is related to our search which was limited to papers published starting 2018 as the GRIPP2 guidelines were published in 2017. Thus it is probable that the protocols of some of these studies were developed earlier than the publication of the GRIPP2 checklist, meaning that PPI reporting following GRIPP2 was not common practice and thus not adopted by these studies. This might limit the conclusions we can draw from this review. Finally, the use of GRIPP2 to assess the quality of PPI reporting might be a limitation as usability testing has not yet been conducted to understand how the checklist works in practice with various types of research designs. However, the GRIPP2 is the first international, evidence-based, community consensus informed guideline for the reporting of PPI in health and social care research. Reflections and comments from researchers using the GRIPP2 will help improve its use in future studies.
Implications for research and practice
Lack of PPI reporting not only affects the quality of research but also implies that others cannot learn from previous research experience. Additionally, without consistent and transparent reporting it is difficult to evaluate the impact of various PPI in research [ 9 ]: “if it is not reported it cannot be assessed” ([ 105 ] p19). Enhanced PPI reporting will result in a wider range and richer high-quality evidence-based PPI research, leading to a better understanding of PPI use and effectiveness [ 103 ]. GRIPP2 reporting guidelines were developed to provide guidance for researchers, patients, and the public to enhance the quality of PPI reporting and improve the quality of the international PPI evidence-base. The guidance can be used prospectively to plan PPI or retrospectively to guide the structure or PPI reporting in research [ 9 ]. To enhance PPI reporting, we recommend the following;
Publishers and journals
First, we encourage publishers and journals to require researchers to report PPI following the GRIPP2 checklist. Utilising the short or the long version should depend on the primary focus of the study (i.e., if PPI is within the primary focus of the research then the GRIPP2-LF is recommended). Second, we recommend that journals and editorial members advise reviewers to evaluate PPI reporting within research articles following the GRIPP2 tool and make suggestions accordingly. Finally, we encourage journals to add a plain language summary as a submission requirement to increase research dissemination and improve the accessibility of research for patients and the public.
Researchers
Though there is greater evidence of PPI in research, it is still primarily the researchers that are setting the research agenda and deciding on the research questions to be addressed. Thus, significant effort is still needed to involve patients and the public in the earlier stages of the research process given the fundamental impact of PS on their lives. To enhance future PPI reporting, perhaps adding a criterion following the GRIPP2 tool to existing EQUATOR checklists for reporting research papers such as STROBE, PRISMA, CONSORT, may support higher quality research. Additionally, currently, there is no detailed explanation paper for the GRIPP2 where each criterion is explained in detail with examples. Addressing this gap would be of great benefit to guide the structure of PPI reporting and to explore the applicability of each criterion in relation to different stages of PPI in research. For instance, having a detailed explanation for each criterion across different research studies having various PPI stages would be of high value to improve future PPI reporting given the growing interest in PPI research in recent years and the relatively small PPI evidence base in health and medical research.
Funding bodies can also enhance PPI reporting by adding a requirement for researchers to report PPI following the GRIPP2 checklist. In Ireland, the National HSE has already initiated this by requiring all PPI in HSE research in Ireland to be reported following the GRIPP2 guidelines [ 10 ].
This study represents the first systematic review on the frequency and quality of PPI reporting in PS research using the GRIPP2 checklist. Most PS topics were related to medication safety, general PS, and fall prevention. The main involvement across the studies was in commenting on or developing research materials. Thus, efforts are still needed to involve patients and the public across all aspects of the research process, especially earlier stages of the research cycle. The frequency of PPI reporting following the GRIPP2 guidelines was low, and the quality of reporting following the GRIPP2-SF criteria was sub-optimal. The lowest percentages of reporting were on critically reflecting on PPI in the study so future research can learn from this experience and work to improve it, reporting the aim of the PPI in the study, and reporting the extent to which PPI influenced the study overall including positive and negative effects. Researchers, funders, publishers, journals, editorial members and reviewers have a responsibility to promote consistent and transparent PPI reporting following internationally developed reporting guidelines such as the GRIPP2. Evidence-based guidelines for reporting PPI should be supported to help future researchers plan and report PPI more effectively, which may ultimately improve the quality and relevance of research.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its Supplementary information files.
Abbreviations
- Patient safety
United Kingdom
National Institute for Health Research
Public and Patient Involvement
Health Service Executive
Guidance for Reporting Involvement of Patients and the Public
Second version of the GRIPP checklist
Long form of GRIPP2
Short form of GRIPP2
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
The International Database of Prospectively Registered Systematic Reviews
British Medical Journal
Patient saftey: World Health Organisation. 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/patient-safety . Updated 11 Sept 2023.
Slawomirski L, Klazinga N. The economics of patient safety: from analysis to action. Paris: Organisation for Economic Co-operation and Development; 2020.
Google Scholar
Panagioti M, Khan K, Keers RN, Abuzour A, Phipps D, Kontopantelis E, et al. Prevalence, severity, and nature of preventable patient harm across medical care settings: systematic review and meta-analysis. Bmj-Brit Med J. 2019;366:l4185.
Article Google Scholar
Hodkinson A, Tyler N, Ashcroft DM, Keers RN, Khan K, Phipps D, et al. Preventable medication harm across health care settings: a systematic review and meta-analysis. Bmc Medicine. 2020;18(1):313.
Article PubMed PubMed Central Google Scholar
Park M, Giap TTT. Patient and family engagement as a potential approach for improving patient safety: A systematic review. J Adv Nurs. 2020;76(1):62–80.
Article PubMed Google Scholar
Chegini Z, Janati A, Bababie J, Pouraghaei M. The role of patients in the delivery of safe care in hospital: Study protocol. J Adv Nurs. 2019;75(9):2015–23.
Chegini Z, Arab-Zozani M, Islam SMS, Tobiano G, Rahimi SA. Barriers and facilitators to patient engagement in patient safety from patients and healthcare professionals’ perspectives: A systematic review and meta-synthesis. Nurs Forum. 2021;56(4):938–49.
Going the extra mile: improving the nation’s health and wellbeing through public involvement in research. London: National Institute for Health; 2015.
Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. Bmj-Brit Med J. 2017;358:j3453.
Article CAS Google Scholar
Minogue V. Knowledge translation, dissemination, and impact: a practical guide for researchers. Guide No 8: patient and public involvement in HSE research. Ireland: Health Service Executive Research and Development; 2021.
Staniszewska S, Brett J, Mockford C, Barber R. The GRIPP checklist: Strengthening the quality of patient and public involvement reporting in research. Int J Technol Assess Health Care. 2011;27(4):391–9.
Bergin RJ, Short CE, Davis N, Marker J, Dawson MT, Milton S, et al. The nature and impact of patient and public involvement in cancer prevention, screening and early detection research: A systematic review. Prev Med. 2023;167:107412.
Owyang D, Bakhsh A, Brewer D, Boughton OR, Cobb JP. Patient and public involvement within orthopaedic research a systematic review. J Bone Joint Surg Am. 2021;103(13):e51.
Busch IM, Saxena A, Wu AW. Putting the patient in patient safety investigations: barriers and strategies for involvement. J Patient Saf. 2021;17(5):358–62.
Lee M, Lee NJ, Seo HJ, Jang H, Kim SM. Interventions to engage patients and families in patient safety: a systematic review. West J Nurs Res. 2021;43(10):972–83.
Ocloo J, Garfield S, Franklin BD, Dawson S. Exploring the theory, barriers and enablers for patient and public involvement across health, social care and patient safety: a systematic review of reviews. Health Res Policy Syst. 2021;19(1):8.
Greenhalgh T, Hinton L, Finlay T, Macfarlane A, Fahy N, Clyde B, et al. Frameworks for supporting patient and public involvement in research: Systematic review and co-design pilot. Health Expect. 2019;22(4):785–801.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Plos Medicine. 2021;18(3):372.
INVOLVE. What is public involvement in research? NIHR; 2019. Available from: https://www.invo.org.uk/find-out-more/what-is-public-involvement-in-research-2/ .
Shahid A, Sept B, Kupsch S, Brundin-Mather R, Piskulic D, Soo A, et al. Development and pilot implementation of a patient-oriented discharge summary for critically Ill patients. World J Crit Care Med. 2022;11(4):255–68.
Bisset CN, Dames N, Oliphant R, Alasadi A, Anderson D, Parson S, et al. Exploring shared surgical decision-making from the patient’s perspective: is the personality of the surgeon important? Colorectal Dis. 2020;22(12):2214–21.
Article CAS PubMed Google Scholar
Morris RL, Ruddock A, Gallacher K, Rolfe C, Giles S, Campbell S. Developing a patient safety guide for primary care: A co-design approach involving patients, carers and clinicians. Health Expect. 2021;24(1):42–52.
Tobiano G, Marshall AP, Gardiner T, Jenkinson K, Shapiro M, Ireland M. Development and psychometric testing of the patient participation in bedside handover survey. Health Expect. 2022;25(5):2492–502.
Francis-Coad J, Farlie MK, Haines T, Black L, Weselman T, Cummings P, et al. Revising and evaluating falls prevention education for older adults in hospital. Health Educ J. 2023;82(8):878–91.
Troya MI, Chew-Graham CA, Babatunde O, Bartlam B, Higginbottom A, Dikomitis L. Patient and public involvement and engagement in a doctoral research project exploring self-harm in older adults. Health Expect. 2019;22(4):617–31.
Aharaz A, Kejser CL, Poulsen MW, Jeftic S, Ulstrup-Hansen AI, Jorgensen LM, et al. Optimization of the Danish National Electronic Prescribing System to improve patient safety: Development of a user-friendly prototype of the digital platform shared medication record. Pharmacy (Basel, Switzerland). 2023;11(2):41.
PubMed Google Scholar
Aho-Glele U, Bouabida K, Kooijman A, Popescu IC, Pomey MP, Hawthornthwaite L, et al. Developing the first pan-Canadian survey on patient engagement in patient safety. BMC Health Serv Res. 2021;21(1):1099.
Albutt A, O’Hara J, Conner M, Lawton R. Involving patients in recognising clinical deterioration in hospital using the patient wellness questionnaire: A mixed-methods study. J Res Nurs. 2020;25(1):68–86.
Bell SK, Bourgeois F, DesRoches CM, Dong J, Harcourt K, Liu SK, et al. Filling a gap in safety metrics: development of a patient-centred framework to identify and categorise patient-reported breakdowns related to the diagnostic process in ambulatory care. BMJ Qual Saf. 2022;31(7):526–40.
Boet S, Etherington N, Lam S, Lê M, Proulx L, Britton M, et al. Implementation of the Operating Room Black Box research program at the Ottawa Hospital through patient, clinical, and organizational engagement: Case study. J Med Internet Res. 2021;23(3):e15443.
Carter J, Tribe RM, Shennan AH, Sandall J. Threatened preterm labour: Women’s experiences of risk and care management: A qualitative study. Midwifery. 2018;64:85–92.
Da Silva Lopes AM, Colomer-Lahiguera S, Mederos Alfonso N, Aedo-Lopez V, Spurrier-Bernard G, Tolstrup LK, et al. Patient-reported outcomes for monitoring symptomatic toxicities in cancer patients treated with immune-checkpoint inhibitors: A Delphi study. Eur J Cancer. 2021;157:225–37.
de Jong LD, Lavender AP, Wortham C, Skelton DA, Haines TP, Hill AM. Exploring purpose-designed audio-visual falls prevention messages on older people’s capability and motivation to prevent falls. Health Soc Care Community. 2019;27(4):e471–82.
Doucette L, Kiely BT, Gierisch JM, Marion E, Nadler L, Heflin MT, et al. Participatory research to improve medication reconciliation for older adults in the community. J Am Geriatr Soc. 2023;71(2):620–31.
Elrod CS, Pappa ST, Heyn PC, Wong RA. Using an academic-community partnership model to deliver evidence-based falls prevention programs in a metropolitan setting: A community case study. Front Public Health. 2023;11:1073520.
Feldman E, Pos FJ, Smeenk RJ, van der Poel H, van Leeuwen P, de Feijter JM, et al. Selecting a PRO-CTCAE-based subset for patient-reported symptom monitoring in prostate cancer patients: a modified Delphi procedure. ESMO Open. 2023;8(1):100775.
Article CAS PubMed PubMed Central Google Scholar
Francis-Coad J, Watts T, Bulsara C, Hill A-M. Designing and evaluating falls prevention education with residents and staff in aged care homes: a feasibility study. Health Educ (0965-4283). 2022;122(5):546–63.
Fuller TE, Pong DD, Piniella N, Pardo M, Bessa N, Yoon C, et al. Interactive digital health tools to engage patients and caregivers in discharge preparation: implementation study. J Med Internet Res. 2020;22(4):e15573.
Gibson B, Butler J, Schnock K, Bates D, Classen D. Design of a safety dashboard for patients. Patient Educ Couns. 2020;103(4):741–7.
Giles SJ, Lewis PJ, Phipps DL, Mann F, Avery AJ, Ashcroft DM. Capturing patients’ perspectives on medication safety: the development of a patient-centered medication safety framework. J Patient Saf. 2020;16(4):e324–39.
Gnagi R, Zuniga F, Brunkert T, Meyer-Massetti C. Development of a medication literacy assessment instrument (MELIA) for older people receiving home care. J Adv Nurs. 2022;78(12):4210–20.
Goodsmith N, Zhang L, Ong MK, Ngo VK, Miranda J, Hirsch S, et al. Implementation of a community-partnered research suicide-risk management protocol: case study from community partners in care. Psychiatr Serv (Washington, DC). 2021;72(3):281–7.
Gorman LS, Littlewood DL, Quinlivan L, Monaghan E, Smith J, Barlow S, et al. Family involvement, patient safety and suicide prevention in mental healthcare: ethnographic study. BJPsych open. 2023;9(2):e54.
Green MM, Meyer C, Hutchinson AM, Sutherland F, Lowthian JA. Co‐designing being your best program—a holistic approach to frailty in older community dwelling australians. Health Soc Care Community. 2021;30(5):e2022–32.
Guo X, Wang Y, Wang L, Yang X, Yang W, Lu Z, et al. Effect of a fall prevention strategy for the older patients: A quasi-experimental study. Nurs Open. 2023;10(2):1116–24.
Hahn-Goldberg S, Chaput A, Rosenberg-Yunger Z, Lunsky Y, Okrainec K, Guilcher S, et al. Tool development to improve medication information transfer to patients during transitions of care: A participatory action research and design thinking methodology approach. Res Social Adm Pharm. 2022;18(1):2170–7.
Harrington A, Darke H, Ennis G, Sundram S. Evaluation of an alternative model for the management of clinical risk in an adult acute psychiatric inpatient unit. Int J Ment Health Nurs. 2019;28(5):1099–109.
Harris K, Softeland E, Moi AL, Harthug S, Ravnoy M, Storesund A, et al. Development and validation of patients’ surgical safety checklist. BMC Health Serv Res. 2022;22(1):259.
Hawley-Hague H, Tacconi C, Mellone S, Martinez E, Ford C, Chiari L, et al. Smartphone apps to support falls rehabilitation exercise: app development and usability and acceptability study. JMIR Mhealth Uhealth. 2020;8(9):e15460.
Holmqvist M, Ros A, Lindenfalk B, Thor J, Johansson L. How older persons and health care professionals co-designed a medication plan prototype remotely to promote patient safety: case study. JMIR aging. 2023;6:e41950.
Jayesinghe R, Moriarty F, Khatter A, Durbaba S, Ashworth M, Redmond P. Cost outcomes of potentially inappropriate prescribing in middle-aged adults: A Delphi consensus and cross-sectional study. Br J Clin Pharmacol. 2022;88(7):3404–20.
Johannessen T, Ree E, Stromme T, Aase I, Bal R, Wiig S. Designing and pilot testing of a leadership intervention to improve quality and safety in nursing homes and home care (the SAFE-LEAD intervention). BMJ Open. 2019;9(6):e027790.
Joseph K, Newman B, Manias E, Walpola R, Seale H, Walton M, et al. Engaging with ethnic minority consumers to improve safety in cancer services: A national stakeholder analysis. Patient Educ Couns. 2022;105(8):2778–84.
Khan A, Spector ND, Baird JD, Ashland M, Starmer AJ, Rosenbluth G, et al. Patient safety after implementation of a coproduced family centered communication programme: multicenter before and after intervention study. BMJ. 2018;363:k4764.
Khazen M, Mirica M, Carlile N, Groisser A, Schiff GD. Developing a framework and electronic tool for communicating diagnostic uncertainty in primary care: a qualitative study. JAMA Network Open. 2023;6(3):e232218-e.
Knight SW, Trinkle J, Tschannen D. Hospital-to-homecare videoconference handoff: improved communication, coordination of care, and patient/family engagement. Home Healthc Now. 2019;37(4):198–207.
Lawrence V, Kimona K, Howard RJ, Serfaty MA, Wetherell JL, Livingston G, et al. Optimising the acceptability and feasibility of acceptance and commitment therapy for treatment-resistant generalised anxiety disorder in older adults. Age Ageing. 2019;48(5):741–50.
Louch G, Reynolds C, Moore S, Marsh C, Heyhoe J, Albutt A, et al. Validation of revised patient measures of safety: PMOS-30 and PMOS-10. BMJ Open. 2019;9(11):e031355.
MacDonald T, Jackson S, Charles M-C, Periel M, Jean-Baptiste M-V, Salomon A, et al. The fourth delay and community-driven solutions to reduce maternal mortality in rural Haiti: a community-based action research study. BMC Pregnancy Childbirth. 2018;18(1):254.
Mackintosh N, Sandall J, Collison C, Carter W, Harris J. Employing the arts for knowledge production and translation: Visualizing new possibilities for women speaking up about safety concerns in maternity. Health Expect. 2018;21(3):647–58.
Marchand K, Turuba R, Katan C, Brasset C, Fogarty O, Tallon C, et al. Becoming our young people’s case managers: caregivers’ experiences, needs, and ideas for improving opioid use treatments for young people using opioids. Subst Abuse Treat Prev Policy. 2022;17(1):1–15.
Mazuz K, Biswas S. Co-designing technology and aging in a service setting: Developing an interpretive framework of how to interact with older age users. Gerontechnology. 2022;21(1):1–13.
McCahon D, Duncan P, Payne R, Horwood J. Patient perceptions and experiences of medication review: qualitative study in general practice. BMC Prim Care. 2022;23(1):293.
McMullen S, Panagioti M, Planner C, Giles S, Angelakis I, Keers RN, et al. Supporting carers to improve patient safety and maintain their well-being in transitions from mental health hospitals to the community: A prioritisation nominal group technique. Health Expect. 2023;26(5):2064–74.
Morris RL, Giles S, Campbell S. Involving patients and carers in patient safety in primary care: A qualitative study of a co-designed patient safety guide. Health Expect. 2023;26(2):630–9.
Morris RL, Stocks SJ, Alam R, Taylor S, Rolfe C, Glover SW, et al. Identifying primary care patient safety research priorities in the UK: a James Lind Alliance Priority Setting Partnership. BMJ Open. 2018;8(2):e020870.
Nether KG, Thomas EJ, Khan A, Ottosen MJ, Yager L. Implementing a robust process improvement program in the neonatal intensive care unit to reduce harm. J Healthc Qual. 2022;44(1):23–30.
Powell C, Ismail H, Cleverley R, Taylor A, Breen L, Fylan B, et al. Patients as qualitative data analysts: Developing a method for a process evaluation of the “Improving the Safety and Continuity of Medicines management at care Transitions” (ISCOMAT) cluster randomised control trial. Health Expect. 2021;24(4):1254–62.
Article PubMed Central Google Scholar
Powell C, Ismail H, Davis M, Taylor A, Breen L, Fylan B, et al. Experiences of patients with heart failure with medicines at transition intervention: Findings from the process evaluation of the Improving the Safety and Continuity of Medicines management at Transitions of care (ISCOMAT) programme. Health Expect. 2022;25(5):2503–14.
Radecki B, Keen A, Miller J, McClure JK, Kara A. Innovating fall safety: engaging patients as experts. J Nurs Care Qual. 2020;35(3):220–6.
Rosgen BK, Plotnikoff KM, Krewulak KD, Shahid A, Hernandez L, Sept BG, et al. Co-development of a transitions in care bundle for patient transitions from the intensive care unit: a mixed-methods analysis of a stakeholder consensus meeting. BMC Health Serv Res. 2022;22(1):10.
Schenk EC, Bryant RA, Van Son CR, Odom-Maryon T. Developing an intervention to reduce harm in hospitalized patients: patients and families in research. J Nurs Care Qual. 2019;34(3):273–8.
Spazzapan M, Vijayakumar B, Stewart CE. A bit about me: Bedside boards to create a culture of patient-centered care in pediatric intensive care units (PICUs). J Healthc Risk Manag. 2020;39(3):11–9.
Stoll JA, Ranahan M, Richbart MT, Brennan-Taylor MK, Taylor JS, Brady L, et al. Development of video animations to encourage patient-driven deprescribing: A team alice study. Patient Educ Couns. 2021;104(11):2716–23.
Subbe CP, Tomos H, Jones GM, Barach P. Express check-in: developing a personal health record for patients admitted to hospital with medical emergencies: a mixed-method feasibility study. Int J Qual Health Care. 2021;33(3):121.
Tai D, Li E, Liu-Ambrose T, Bansback N, Sadatsafavi M, Davis JC. Patient-Reported Outcome Measures (PROMs) to support adherence to falls prevention clinic recommendations: a qualitative study. Patient Prefer Adherence. 2020;14:2105–21.
Thakur T, Chewning B, Zetes N, Lee JTY. Involving caregivers in design and assessment of opioid risk and safety communication intervention in children. Patient Educ Couns. 2021;104(10):2432–6.
Thomas J, Dahm MR, Li J, Georgiou A. Can patients contribute to enhancing the safety and effectiveness of test-result follow-up? Qualitative outcomes from a health consumer workshop. Health Expect. 2021;24(2):222–33.
Tremblay MC, Bradette-Laplante M, Witteman HO, Dogba MJ, Breault P, Paquette JS, et al. Providing culturally safe care to indigenous people living with diabetes: Identifying barriers and enablers from different perspectives. Health Expect. 2021;24(2):296–306.
Troya MI, Dikomitis L, Babatunde OO, Bartlam B, Chew-Graham CA. Understanding self-harm in older adults: A qualitative study. EClinicalMedicine. 2019;12:52–61.
Tyler N, Giles S, Daker-White G, McManus BC, Panagioti M. A patient and public involvement workshop using visual art and priority setting to provide patients with a voice to describe quality and safety concerns: Vitamin B12 deficiency and pernicious anaemia. Health Expect. 2021;24(1):87–94.
Tyler N, Planner C, Shears B, Hernan A, Panagioti M, Giles S. Developing the Resident Measure of Safety in Care Homes (RMOS): A Delphi and think aloud study. Health Expect. 2023;26(3):1149–58.
Van den Bulck SA, Vankrunkelsven P, Goderis G, Van Pottelbergh G, Swerts J, Panis K, et al. Developing quality indicators for Chronic Kidney Disease in primary care, extractable from the Electronic Medical Record. A Rand-modified Delphi method. BMC Nephrol. 2020;21(1):161.
Van Strien-Knippenberg IS, Boshuizen MCS, Determann D, de Boer JH, Damman OC. Cocreation with Dutch patients of decision-relevant information to support shared decision-making about adjuvant treatment in breast cancer care. Health Expect. 2022;25(4):1664–77.
Wilson NA, Reich AJ, Graham J, Bhatt DL, Nguyen LL, Weissman JS. Patient perspectives on the need for implanted device information: Implications for a post-procedural communication framework. Health Expect. 2021;24(4):1391–402.
Winterberg AV, Lane B, Hill LM, Varughese AM. Optimizing Pediatric Induction Experiences Using Human-centered Design. J Perianesth Nurs. 2022;37(1):48–52.
Yang R, Donaldson GW, Edelman LS, Cloyes KG, Sanders NA, Pepper GA. Fear of older adult falling questionnaire for caregivers (FOAFQ-CG): Evidence from content validity and item-response theory graded-response modelling. J Adv Nurs. 2020;76(10):2768–80.
Young A, Menon D, Street J, Al-Hertani W, Stafinski T. A checklist for managed access programmes for reimbursement co-designed by Canadian patients and caregivers. Health Expect. 2018;21(6):973–80.
Yuen EYN, Street M, Abdelrazek M, Blencowe P, Etienne G, Liskaser R, et al. Evaluating the efficacy of a digital App to enhance patient-centred nursing handover: A simulation study. J Clin Nurs. 2023;32(19–20):7626–37.
Jo S, Nabatchi T. Coproducing healthcare: individual-level impacts of engaging citizens to develop recommendations for reducing diagnostic error. Public Manag Rev. 2019;21(3):354–75.
O’Hara JK, Reynolds C, Moore S, Armitage G, Sheard L, Marsh C, et al. What can patients tell us about the quality and safety of hospital care? Findings from a UK multicentre survey study. BMJ Qual Saf. 2018;27(9):673–82.
de Jong LD, Francis-Coad J, Wortham C, Haines TP, Skelton DA, Weselman T, et al. Evaluating audio-visual falls prevention messages with community-dwelling older people using a World Cafe forum approach. BMC Geriatrics. 2019;19(1):345.
O’Donnell D, Shé ÉN, McCarthy M, Thornton S, Doran T, Smith F, et al. Enabling public, patient and practitioner involvement in co-designing frailty pathways in the acute care setting. BMC Health Serv Res. 2019;19(1):797.
Russ S, Latif Z, Hazell A, Ogunmuyiwa H, Tapper J, Wachuku-King S, et al. A Smartphone app designed to empower patients to contribute toward safer surgical care: community-based evaluation using a participatory approach. Jmir Mhealth Uhealth. 2020;8(1):e12859.
Mazuz K, Biswas S, Lindner U. Developing self-management application of fall prevention among older adults: a content and usability evaluation. Front Digital Health. 2020;2:11.
Hjelmfors L, Strömberg A, Friedrichsen M, Sandgren A, Mårtensson J, Jaarsma T. Using co-design to develop an intervention to improve communication about the heart failure trajectory and end-of-life care. Bmc Palliat Care. 2018;17:17.
Horgan S, Hegarty J, Andrews E, Hooton C, Drennan J. Impact of a quality improvement intervention on the incidence of surgical site infection in patients undergoing colorectal surgery: Pre-test-post-test design. J Clin Nurs. 2023;32(15–16):4932–46.
Tyler N, Wright N, Grundy A, Waring J. Developing a core outcome set for interventions to improve discharge from mental health inpatient services: a survey, Delphi and consensus meeting with key stakeholder groups. BMJ Open. 2020;10(5):e034215.
Ward ME, De Brún A, Beirne D, Conway C, Cunningham U, English A, et al. Using Co-Design to Develop a Collective Leadership Intervention for Healthcare Teams to Improve Safety Culture. Int J Environ Res Public Health. 2018;15(6):1182.
Berthelsen DB, Simon LS, Ioannidis JPA, Voshaar M, Richards P, Goel N, et al. Stakeholder endorsement advancing the implementation of a patient-reported domain for harms in rheumatology clinical trials: outcome of the OMERACT safety working group. Semin Arthritis Rheum. 2023;63:152288.
Okkenhaug A, Tritter JQ, Landstad BJ. Developing a research tool to detect iatrogenic adverse events in psychiatric health care by involving service users and health professionals. J Psychiatr Ment Health Nurs. 2023;00:1–12.
Musbahi A, Clyde D, Small P, Courtney M, Mahawar K, Lamb PJ, et al. A systematic review of patient and public involvement (PPI) in bariatric research trials: the need for more work. Obes Surg. 2022;32(11):3740–51.
Patel VA, Shelswell J, Hillyard N, Pavitt S, Barber SK. A study of the reporting of patient and public involvement and engagement (PPIE) in orthodontic research. J Orthod. 2021;48(1):42–51.
Price A, Schroter S, Snow R, Hicks M, Harmston R, Staniszewska S, et al. Frequency of reporting on patient and public involvement (PPI) in research studies published in a general medical journal: a descriptive study. BMJ Open. 2018;8:e020452.
Amadea T, Anne-Marie B, Louise L. A researcher’s guide to patient and public involvement. 2017.
Download references
Acknowledgements
This research is funded as part of the Collective Leadership and Safety Cultures (Co-Lead) research programme which is funded by the Irish Health Research Board, grant reference number RL-2015–1588 and the Health Service Executive. The funders had no role in the study conceptualisation, design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and affiliations.
UCD Centre for Interdisciplinary Research, Education and Innovation in Health Systems (UCD IRIS), School of Nursing, Midwifery and Health Systems, Health Sciences Centre, University College Dublin, Dublin, Ireland
Sahar Hammoud, Laith Alsabek, Lisa Rogers & Eilish McAuliffe
Department of Oral and Maxillofacial Surgery, University Hospital Galway, Galway, Ireland
Laith Alsabek
You can also search for this author in PubMed Google Scholar
Contributions
S.H and E.M.A designed the study. S.H developed the search strategies with feedback from L.A, L.R, and E.M.A. S.H conducted all searches. S.H and L.A screened the studies, extracted the data, and assessed the quality of PPI reporting. S.H analysed the data with feedback from E.M.A. S.H drafted the manuscript. All authors revised and approved the submitted manuscript. All authors agreed to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Authors’ information
Corresponding author.
Correspondence to Sahar Hammoud .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hammoud, S., Alsabek, L., Rogers, L. et al. Systematic review on the frequency and quality of reporting patient and public involvement in patient safety research. BMC Health Serv Res 24 , 532 (2024). https://doi.org/10.1186/s12913-024-11021-z
Download citation
Received : 10 January 2024
Accepted : 21 April 2024
Published : 26 April 2024
DOI : https://doi.org/10.1186/s12913-024-11021-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Patient and public involvement
- Patient participation
- Research reporting
- Research involvement
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
- Submit a Manuscript
- Advanced search

American Journal of Neuroradiology
Advanced Search
Assessing the Performance of Artificial Intelligence Models: Insights from the American Society of Functional Neuroradiology Artificial Intelligence Competition
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- Info & Metrics
BACKGROUND AND PURPOSE: Artificial intelligence (AI) models in radiology are frequently developed and validated using datasets from a single institution and are rarely tested on independent, external datasets, raising questions about their generalizability and applicability in clinical practice. The American Society of Functional Neuroradiology (ASFNR) organized a multi-center AI competition to evaluate the proficiency of developed models in identifying various pathologies on NCCT, assessing age-based normality and estimating medical urgency.
MATERIALS AND METHODS: In total, 1201 anonymized, full-head NCCT clinical scans from five institutions were pooled to form the dataset. The dataset encompassed normal studies as well as pathologies including acute ischemic stroke, intracranial hemorrhage, traumatic brain injury, and mass effect (detection of these-task 1). NCCTs were also assessed to determine if findings were consistent with expected brain changes for the patient's age (task 2: age-based normality assessment) and to identify any abnormalities requiring immediate medical attention (task 3: evaluation of findings for urgent intervention). Five neuroradiologists labeled each NCCT, with consensus interpretations serving as the ground truth. The competition was announced online, inviting academic institutions and companies. Independent central analysis assessed each model's performance. Accuracy, sensitivity, specificity, positive and negative predictive values, and receiver operating characteristic (ROC) curves were generated for each AI model, along with the area under the ROC curve (AUROC).
RESULTS: 1177 studies were processed by four teams. The median age of patients was 62, with an interquartile range of 33. 19 teams from various academic institutions registered for the competition. Of these, four teams submitted their final results. No commercial entities participated in the competition. For task 1, AUROCs ranged from 0.49 to 0.59. For task 2, two teams completed the task with AUROC values of 0.57 and 0.52. For task 3, teams had little to no agreement with the ground truth.
CONCLUSIONS: To assess the performance of AI models in real-world clinical scenarios, we analyzed their performance in the ASFNR AI Competition. The first ASFNR Competition underscored the gap between expectation and reality; the models largely fell short in their assessments. As the integration of AI tools into clinical workflows increases, neuroradiologists must carefully recognize the capabilities, constraints, and consistency of these technologies. Before institutions adopt these algorithms, thorough validation is essential to ensure acceptable levels of performance in clinical settings.
ABBREVIATIONS: AI = artificial intelligence; ASFNR = American Society of Functional Neuroradiology; AUROC = area under the receiver operating characteristic curve; DICOM = Digital Imaging and Communications in Medicine; GEE = generalized estimation equation; IQR = interquartile range; NPV = negative predictive value; PPV = positive predictive value; ROC = receiver operating characteristic; TBI = traumatic brain injury.
Adam Flanders is a member of the Board of Directors at RSNA and one of the investigators at the Medical Imaging and Data Resource Center. The other authors declare no conflicts of interest related to the content of this article.
- © 2024 by American Journal of Neuroradiology
Thank you for your interest in spreading the word on American Journal of Neuroradiology.
NOTE: We only request your email address so that the person you are recommending the page to knows that you wanted them to see it, and that it is not junk mail. We do not capture any email address.
Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager

- Tweet Widget
- Facebook Like
- Google Plus One
Jump to section
Related articles.
- No related articles found.
- Google Scholar
Cited By...
- No citing articles found.
This article has not yet been cited by articles in journals that are participating in Crossref Cited-by Linking.
More in this TOC Section
- Implementation of a Survey Spine Magnetic Resonance Imaging Protocol for Cord Compression in the Emergency Department: Experience at a Level-1 Trauma Center
- Efficacy Assessment of Cerebral Perfusion Augmentation Through Functional Connectivity in an Acute Canine Stroke Model
Similar Articles

IMAGES
VIDEO
COMMENTS
Article Text. The main part of an article is its body text. This is where the author analyzes the argument, research question, or problem. This section also includes analysis and criticism. The author may use headings to divide this part of the article into sections. Scientific research articles may include these sections:
Overview. Typically scientific journal articles have the following sections: References used: Kotsis, S.V. and Chung, K.C. (2010) A Guide for Writing in the Scientific Forum. Plastic and Reconstructive Surgery. 126 (5):1763-71. PubMed ID: 21042135. Van Way, C.W. (2007) Writing a Scientific Paper. Nutrition in Clinical Practice. 22: 663-40.
The middle sections of the research paper continue the narrow focus of the specific research being reported in the Methods and Results. This is pictured as the narrow central area of the hourglass. The Discussion usually begins with comments about the specific research and progressively connects the findings to the wider literature in the field.
Your article should thus contain (in this order) an introduction, a methods section, a results section and a discussion. Added to this will be the abstract, which is more or less a summary of these main sections, and of course, the title. At the end, there must be a list of bibliographic references, the tables, and the legends to any figures.
The basic structure of a typical research paper includes Introduction, Methods, Results, and Discussion. Each section addresses a different objective. what they think the results mean in Discussion. A substantial study will sometimes include a literature review section which discusses previous works on the topic.
journal than you can from readers of a popular magazine. Use a 'hook' to capture readers' interest. The hook refers to the first sentence of your introduction, which is what you'll use to spark curiosity and entice readers to carry on. It should be concise and relatively simple. For example:
This article provides an overview of writing for publication in peer-reviewed journals. While the main focus is on writing a research article, it also provides guidance on factors influencing journal selection, including journal scope, intended audience for the findings, open access requirements, and journal citation metrics.
It is a full-length document on original research. A scholarly article generally consists of the background of a research topic, its study design and methodology, the results of the study, and then its conclusion. The scholarly articles or publications used to inform the research are listed at the end of the article as its references or works ...
The body of an article is usually presented in sections, including an introduction, a literature review, one or more sections describing and analyzing the argument, experiment or study. Scientific research articles typically include separate sections addressing the methods and results of the experiment, and a discussion of the research findings ...
However, research articles - as a genre - have other consistent sections as well. The complete list of sections for research articles include the following: Introduction. Methods. Results. Discussion/Conclusion. A common acronym for teaching the sections of a research article is IMRD/C. In this book, we will focus heavily on helping you ...
3.2 Components of a scientific paper. Nearly all journal articles are divided into the following major sections: abstract, introduction, methods, results, discussion, and references. Usually the sections are labeled as such, although often the introduction (and sometimes the abstract) is not labeled. Sometimes alternative section titles are used.
The structure of quantitative research articles is derived from the scientifc process and includes sections covering introduction, methods, results, and discussion (IMRaD). The actual labels for the various parts may vary between journals. Abstract: A structured abstract reports a summary of each of the IMRaD sections. Enough information should ...
A complete research paper in APA style that is reporting on experimental research will typically contain a Title page, Abstract, Introduction, Methods, Results, Discussion, and References sections. 1 Many will also contain Figures and Tables and some will have an Appendix or Appendices. These sections are detailed as follows (for a more in ...
Knowing these elements and the purpose of each serves help you to read and understand academic texts efficiently and effectively, and then apply what you read to your paper or project. Social Science (and Science) original research articles generally follow IMRD: Introduction- Methods-Results-Discussion. Introduction. Introduces topic of article.
This standard structure: Although the sections of the journal manuscript are published in the order: Title, Abstract, Introduction, Materials and Methods, Results, Discussion, and Conclusion, this is not the best order for writing the sections of a manuscript. One recommended strategy is to write your manuscript in the following order:
Journal Article. Scientific journal articles share a common anatomy, or structure. Each part of an article serves a purpose, and if you know the purpose, you can become more eficient at reading and understanding articles. Instead of reading from beginning to end, consult targeted sections according to the kind of information you need to learn ...
This guide discusses the parts of a scholarly article and provides tips on how to read one. ... Western Kentucky University Libraries; Research Guides; Reading a Scholarly Article; Parts of a Scholarly Article; Enter ... Orientation to Fisheries and Wildlife: 5. The Anatomy of a Scholarly Article. https://guides.library.oregonstate.edu/ c.php?g ...
Abstract. The purpose of your abstract is to express the key points of your research, clearly and concisely. An abstract must always be well considered, as it is the primary element of your work that readers will come across. An abstract should be a short paragraph (around 300 words) that summarizes the findings of your journal article.
Abstract. An article primarily includes the following sections: introduction, materials and methods, results, discussion, and conclusion. Before writing the introduction, the main steps, the heading and the familiarity level of the readers should be considered. Writing should begin when the experimental system and the equipment are available.
There are five essential components of an original/research article. Abstract/Summary. Introduction. Material & Method. Result. Discussion. These can be remembered with the help of the acronym, IMRAD that stands for : = I = Introduction, = M = Material & Method = R = Result, = A = And = D = Discussion. Key words are included with structured ...
The main components of the American Psychological Association's research style are: Title: a description of what the article is about. Abstract : a brief synopsis of the article.
The First Page . Journal Title - The title of the journal that published the article, as well as the volume and issue number and date of publication.. Article Title and Authors - The title is usually a concise statement of the topic of the article, and often contains key technical or specific terms. The authors' institutional affiliations are often listed, sometimes including contact information.
2. 1. Main Types of Research Papers. Research papers are broadly divided into empirical and theoretical papers. Empirical Research Paper. • The author reports on his or her own study. • The ...
Superagers are elderly individuals with the memory ability of people 30 years younger and provide evidence that age-related cognitive decline is not inevitable. In a sample of 64 superagers (mean age 81.9; 59% women) and 55 typical older adults (mean age 82.4; 64% women) from the Vallecas Project, we studied, cross-sectionally and longitudinally over 5 years with yearly follow-ups, the global ...
Background In recent years, patient and public involvement (PPI) in research has significantly increased; however, the reporting of PPI remains poor. The Guidance for Reporting Involvement of Patients and the Public (GRIPP2) was developed to enhance the quality and consistency of PPI reporting. The objective of this systematic review is to identify the frequency and quality of PPI reporting in ...
Star Scientist's Claim of 'Reverse Aging' Draws Hail of Criticism Other longevity researchers rebuke Harvard geneticist; 'The selling is a step too far'
Humans need social closeness to prosper. There is evidence that empathy can induce social closeness. However, it remains unclear how empathy-related social closeness is formed and how stable it is as time passes. We applied an acquisition-extinction paradigm combined with computational modelling and fMRI, to investigate the formation and stability of empathy-related social closeness. Female ...
BACKGROUND AND PURPOSE: Artificial intelligence (AI) models in radiology are frequently developed and validated using datasets from a single institution and are rarely tested on independent, external datasets, raising questions about their generalizability and applicability in clinical practice. The American Society of Functional Neuroradiology (ASFNR) organized a multi-center AI competition ...
Human neuroimaging studies of episodic memory retrieval routinely observe the engagement of specific cortical regions beyond the medial temporal lobe. Of these, medial parietal cortex (MPC) is of particular interest given its distinct functional characteristics during different retrieval tasks. Specifically, while recognition and autobiographical recall tasks are both used to probe episodic ...
Abstract. Background: Breast cancer (BCa) is a major challenge for women's health worldwide. Ferroptosis is closely related to tumorigenesis and cancer progression. However, the prognostic value of ferroptosis-related genes in BCa remains unclear, and more accurate prognostic models are urgently needed. Methods: Gene expression profiles and clinical information of BCa patients were collected ...