Building greater resilience in vaccine manufacturing
Public-health crises have vaulted to the top of national agendas over the past two years. Even as COVID-19 enters the endemic stage in the United States and Europe, 1 “ When will the COVID-19 pandemic end? ,” McKinsey, July 28, 2022. the monkeypox outbreak has been declared a public-health emergency of international concern by the World Health Organization (WHO). 2 “WHO director-general declares the ongoing monkeypox outbreak a Public Health Emergency of International Concern,” WHO, July 23, 2022; note: WHO is actively working with partners and experts around the world to rename monkeypox.
Preparing for the next pandemic is a priority for many national public-health leaders and requires them to lay the groundwork to mount an effective vaccine response. COVID-19 laid bare the gaps in the global vaccine supply, 3 Celynne A. Balatbat, Victor J. Dzau, and Anaeze C. Offodile II, “Closing the global vaccine equity gap: Equitably distributed manufacturing,” Lancet , May 21, 2022, Volume 399, Number 10339. shifting expectations of what is possible in development and manufacturing and highlighting immunization as a viable path to protect populations quickly. This suggests that creating more resilient vaccine ecosystems—ones that allow a country or region to develop and trade expertise and resources and collaborate with partners to secure enough vaccine doses when they need them—is likely to play a critical role in readiness in the future. The US government, for example, recently launched an initiative aimed at ensuring that biotechnology manufacturing capabilities are available in the country. 4 The United States has launched an initiative focused on manufacturing in biotechnology. For more, see “Fact sheet: President Biden to launch a National Biotechnology and Biomanufacturing Initiative,” The White House, September 12, 2022.
There are no safe bets in vaccine development—and our analysis shows that investments have abated following the surge of capital during the initial COVID-19 crisis, in 2020–21. But the developmental paths of the major COVID-19 vaccines suggest that future vaccines can achieve a radically shortened time to market if there are many candidates and modalities to work with. Vaccine production capacity has expanded significantly, and many countries have started to reverse previous trends toward offshoring. The current challenge for public-health authorities is how to combine insights from the first two years of the pandemic with analysis and actions to produce more robust national and regional vaccine ecosystems for future pandemics and epidemics.
Decision makers could set the stage for vaccine resilience by defining what their countries and regions need; assessing the local capacity to scale production of vaccine doses; identifying gaps and weaknesses in their national and regional vaccine value chains (across multiple technology platforms, such as mRNA and viral vectors); building coordination capabilities; and identifying and sizing the strategic investments required. While the task ahead may be daunting, the payoffs for public health could be significant.

A transformed vaccine landscape
The rapid development of COVID-19 vaccines has reframed expectations for immunizations in four significant areas: time-to-market horizons, competition, production capacity, and onshoring.
Radically shorter time to market
The COVID-19 vaccine was developed in 11 months—a dramatic acceleration compared with the more typical ten years. 5 “ Fast-forward: Will the speed of COVID-19 vaccine development reset industry norms? ,” McKinsey, May 13, 2021. This swiftness showed that with the right basic research, the right investments, and concerted cross-sector collaboration, the vaccine industry can dramatically shorten the vaccine development timeline. 6 For more, see “ Fast-forward ,” May 13, 2021. This precedent has inspired some organizations to try to reduce time to market to 100 days. 7 Melanie Saville et al., “Delivering pandemic vaccines in 100 days—what will it take?,” New England Journal of Medicine , July 14, 2022, Volume 387, Number 2; “100 days,” CEPI, 2022.
Of course, maintaining robust vaccine development, let alone achieving a regulatory-appproved product in 100 days or less, requires a large and diverse portfolio of modalities and partnerships with a wide variety of producers. Existing research will likely prove critical, as will a willingness from stakeholders across sectors to make investments that may not pay off.
Technological advances and the COVID-19 pandemic have motivated start-ups and established companies alike to strive for the next innovation in vaccines.
Heightened competition
Technological advances and the COVID-19 pandemic have motivated start-ups and established companies alike to strive for the next innovation in vaccines. Consider that effective COVID-19 vaccines came from Moderna, a biotech with no prior commercial launches, and AstraZeneca, a pharmaceuticals company without a broad portfolio of vaccines.
These successes demonstrate that effective vaccines can emerge from a wide variety of modalities and producers. And indeed, more developers are pursuing vaccines. For COVID-19 alone, 276 vaccines were in active development as of July 2022, and several have now gained full regulatory approval in multiple markets. 8 COVID-19 Treatment and Vaccine Tracker, Milken Institute, May 25, 2022. The implications for new entrants are significant. McKinsey analysis shows that in 2019, the four largest vaccine manufacturers accounted for about 90 percent of the market by revenue. Following the entrance of new players into the vaccine market with the advent of multiple COVID-19 vaccines, it is likely that the top four manufacturers’ share of the market will diminish. Industry analysts predict this trend will continue over the next five years, with a variety of players capturing increasing proportions of market share. 9 Evaluate Pharma, Evaluate, May and November 2021.
Increased production capacity
For vaccines with regulatory approval, unprecedented production capacity awaits even though capital expenditures inspired by the initial COVID-19 crisis have started to wane.
By February 2021, more than $10 billion—most of it from the public sector, with contributions from global philanthropic and multilateral organizations 10 Olivier J. Wouters, “Challenges in ensuring global access to COVID-19 vaccines: Production, affordability, allocation, and deployment,” Lancet , March 31, 2021, Volume 397, Number 10278. —had been invested in production capacity for COVID-19 vaccines. This push extended the annualized global capacity to up to 24 billion doses by June 2022. 11 “Momentum of COVID-19 vaccine manufacturing scale up sufficient for step change in distribution,” International Federation of Pharmaceutical Manufacturers & Associations (IFPMA), September 7, 2021. The result has been an estimated 12.66 billion doses administered globally, 12 “Coronavirus (COVID-19) vaccinations,” Our World in Data, updated September 19, 2022. almost three times the volume of vaccines distributed in 2019. 13 “MI4A Vaccine purchase data,” WHO, 2019 data. This increase in capacity is unprecedented and may be advantageous to retain. 14 “Invest in biotech,” BioIndustry Association, August 31, 2022.
Biosecurity and the onshoring of vaccine capabilities
The COVID-19 pandemic has made vaccine manufacturing a biosecurity matter. Because infectious diseases can emerge from all parts of the globe, multiple countries, including the United States, have announced their intentions to scale their domestic vaccine production capabilities. 15 For examples, see “Government of Canada announces agreement with leading COVID-19 vaccine developer Moderna, Inc. to build mRNA vaccine facility in Canada,” Government of Canada, August 10, 2021; “Fact sheet,” September 12, 2022; “Government further boosts UK vaccine manufacturing capacity,” Government of the United Kingdom, August 3, 2020; and “Statement on the remarkable progress made by several African countries as part of the Partnerships for African Vaccine Manufacturing (PAVM),” African Union, July 13, 2021.
This is a reversal of prepandemic trends in biopharma manufacturing and requires a careful examination of the supply of vaccine inputs. 16 “Government of Canada,” August 10, 2021; “Government further boosts,” August 3, 2020. During the initial phases of the COVID-19 pandemic, supply bottlenecks for critical vaccine inputs such as bioreactor bags arose because manufacturing capabilities were concentrated among a small group of suppliers that struggled to keep up with rocketing global demand.
Potential steps toward resilient vaccine production
Developing a resilient ecosystem for vaccine production means balancing current needs, short-term pressures, and long-term objectives. National public-health leaders could start by translating vaccine-manufacturing resilience into concrete requirements and then use that information to assess local manufacturing capacity. Next, they could assess the value chain to identify strengths and weaknesses and to coordinate stakeholders throughout the ecosystem. Finally, they could evaluate the options for investment and collaboration against their ability to contribute to local vaccine manufacturing resilience.
Define vaccine resilience
As a starting point, leaders could quantify and define what a resilient in-country vaccine ecosystem should be able to accomplish. For example, decision makers could determine the number of doses needed to elicit a short-term immune response. From there, they could calculate their country’s vaccine requirements. Using the COVID-19 pandemic as a model, a hypothetical country with a population of 40 million people may need more than 80 million doses in a similar pandemic if the population followed a two-dose vaccine regimen.
But in a pandemic, timeliness is paramount. A country of 40 million people would need to distribute more than three million doses per week to achieve full vaccination within six months. With such scenarios in mind, countries could use data from the COVID-19 pandemic on the responsiveness of their vaccine distribution systems to estimate how quickly they could scale up distribution when needed.
Evaluate the local manufacturing base
To assess the local manufacturing base, decision makers may need to evaluate the ease of access to available capacity and determine the flexibility of the manufacturing base in scaling up vaccine manufacturing. Both assessments will likely require a nuanced approach because they involve analysis of the constraints on the domestic vaccine-manufacturing capacity, which comes with technological and regulatory constraints. Strong working relationships between manufacturers and sophisticated regulators will likely be key, and regulators might vary their approaches according to vaccines’ technical requirements. For instance, protein-based and attenuated-virus vaccine technologies are relatively well established, while mRNA-based vaccines saw commercial application for the first time during the COVID-19 pandemic. 17 “Who we are,” Singapore Economic Development Board, accessed September 27, 2022; “Biopharma,” IDA Ireland, accessed September 1, 2022.
High-level decision makers could assess their domestic manufacturing capabilities through the lenses of accessibility, reliability, and scalability.
An emphasis on potential constraints on the national vaccine ecosystem’s ability to scale up production may be important. Specifically, not all capacity will be accessible for a timely response, partly because some capacity cannot be shifted from other therapeutics. Reliability may also be an open question because not all capacity comes with the processes and capabilities required to ramp up rapidly. And additional time would likely be needed to accelerate production, which involves both access and technology transfer (exhibit).
After the active ingredient or drug substance is produced, the process of making the drug could encounter supply chain snags. This step involves combining the drug substance with other materials such as adjuvants (which enhance immune response to antigens) and excipients (inactive ingredients that carry the drug substance) or lipid nanoparticle compounds for mRNA vaccines, all of which have experienced an unprecedented surge in demand. 18 Eric Langer, “Fill/finish capacity use for biologics,” Pharmaceutical Technology , January 2, 2015, Volume 39, Number 1.
The final step of manufacturing, “fill and finish,” could also encounter hurdles. This step prepares the final medical products for distribution. Utilization of fill-and-finish capacity is often high, partly because not all capacity is interchangeable. The risk of bottlenecks can therefore be even greater in times of heightened demand. 19 Eric Langer, “Fill/finish capacity use for biologics,” Pharmaceutical Technology , January 2, 2015, Volume 39, Number 1.
Experience from previous epidemics suggests that leaders will need to make strategic trade-offs. As far back as 2016, in the wake of the Ebola epidemic, industry leaders recognized that there was almost no excess capacity in global vaccine manufacturing. 20 Eric Sagonowsky, “GSK chief: Outbreak prep demands more vaccine production capacity,” Fierce Pharma, December 29, 2016. While this situation improved after the initial COVID-19 crisis, 21 For one example, see “Increase in manufacturing capacity for COVID-19 vaccines from Janssen, Moderna and BioNTech/Pfizer,” European Medicines Agency, December 12, 2021. responding to future outbreaks will likely require sustained investments in preparation, as well as strategic sales and operations planning. For example, it may be important to ensure a minimum level of “warm capacity” that is consistently used and ready to be redeployed quickly. Consider how manufacturers found novel ways to release capacity during the COVID-19 pandemic, such as accelerating advance production of some medicines with long shelf lives to free up capacity for potential vaccine production. 22 Rodney Zemmel, “ The race to create a vaccine: A conversation with Frank D’Amelio ,” McKinsey, December 10, 2021.
Identify capabilities and gaps in the domestic vaccine-manufacturing ecosystem
Few countries and regions have the capacity for end-to-end vaccine manufacturing, which encompasses everything from R&D to final distribution and administration. This scarcity of global capacity suggests that many countries could create more resilient vaccine ecosystems by gaining access to more segments of the vaccine value chain and by strategically focusing on different areas.
Beyond manufacturing, countries could develop the institutional and human-capital infrastructure needed for a robust R&D and innovation pipeline as well as the capabilities to run and process large clinical trials. Both could be worthy medium-term goals.
Countries with strong institutional and intellectual infrastructure—as well as reporting capabilities—may be more responsive to new pathogens. The first coronavirus sequence, for example, was published less than a week after WHO announced the discovery of what was then understood as a new kind of pneumonia. 23 Edward C. Holmes, “Novel 2019 coronavirus genome,” Virological, January 10, 2020; “COVID-19 – China,” WHO, January 5, 2020. This kind of speed requires the capability to collect and transport samples and to sequence whole genomes. It also requires credible ways to disseminate findings to academic and research communities.
Equally important is experience in vaccine development from early-concept platforms to full candidates ready for clinical trials. Oxford’s Jenner Institute and partners, for example, had been focusing on the development of a viral vectored candidate to combat Middle East respiratory syndrome (MERS) before switching their focus to the novel coronavirus. Fostering innovation could increase the chances of having a candidate product (or forging a development collaboration with other countries) and developing and attracting key talent. The ability to recruit, scale, and process data from large clinical trials is also critical for delivering safe products.
Build capabilities to coordinate ecosystem stakeholders
Connecting the public, private, and academic sectors for collaborations is a critical enabler to ensure access to investment and develop domestic capabilities. Both the United States and the United Kingdom brought together leaders from vaccine manufacturers, industry associations, technical experts, public-sector stakeholders, and private-sector experts in adjacent areas as a response to the COVID-19 pandemic. Countries with less access to resources could consider modeling their stakeholder engagement on coalitions such as IDA Ireland and the Economic Development Board in Singapore. 24 “Who we are,” Singapore Economic Development Board, accessed September 27, 2022; “Biopharma,” IDA Ireland, accessed September 1, 2022. Of course, multiparty engagement is critical in building the vaccine value chain, even in non-pandemic times.
Building a domestic talent pipeline is also key to success. Years of global optimization in R&D and manufacturing have led to talent clustering in a handful of locations, effectively locking the rest of the world out of the vaccine ecosystem. Leaders in countries that do not have such talent pools could deploy a range of incentives to attract, develop, and retain highly skilled individuals. 25 For a consideration of developing hubs of talent relevant to the vaccine ecosystem in Africa, see Andrea Gennari, Tania Holt, Emma Jordi, and Leah Kaplow, “ Africa needs vaccines. What would it take to make them here? ,” McKinsey, April 14, 2021. In the longer term, investments and changes to education systems may be required to sustain a pipeline of workers in the vaccine ecosystem.
Connecting the public, private, and academic sectors for collaborations is a critical enabler to ensure access to investment and develop domestic capabilities.
Though vital, tech transfer could be another major obstacle for countries and regions that have historically imported vaccines instead of manufacturing them. Because some local manufacturing capabilities are necessary, these countries could focus on building the infrastructure and capabilities to meet the safety and quality requirements of products that, while potentially lifesaving, have a high bar for efficacy.
Finally, advanced capabilities in data and analytics could support the discovery and development of vaccine candidates. Sharing pathogen information such as outbreak sites and genomic sequencing requires advanced capabilities to gather and analyze data and distribute the resulting insights for the development process. Other than talent, initiatives such as the Three Million African Genomes project 26 Ambroise Wonkam, “Sequence three million genomes across Africa,” Nature , February 10, 2021. and the Fiocruz Genomics Network in Brazil can serve as models for data infrastructure.
Invest and collaborate
Since most countries cannot handle end-to-end vaccine development within their borders, investments and collaboration with other countries will likely be important.
Potential avenues to explore in investments, for example, include international development funding, philanthropic funds, public funding, and incentives. Historically, incentives such as tax credits, capital write-offs, R&D tax incentives, subsidies, and commitments to purchase set amounts of products in advance have been associated with thriving biopharma industries. Dedicated partnerships between the public and private sectors and leadership from public-sector stakeholders may help countries create deeper connections with the vaccine manufacturing industry and strengthen their vaccine ecosystems. Additionally, planning and advance commitments could help assure access to vaccines.
Partnerships among countries are also likely to play a critical role. For instance, the Access Consortium—which predates the COVID-19 pandemic and includes regulatory authorities from Australia, Canada, Singapore, Switzerland, and the United Kingdom—became a source of strategic information during the pandemic and presented an opportunity to improve operational alignment and efficiency. The member countries have agreed to align regulatory approaches and policies to coordinate efforts and resources in developing and manufacturing vaccines, a move that would benefit the members’ 150 million total residents. 27 “Access Consortium,” Health Sciences Authority, Government of Singapore, updated May 20, 2022.
Beyond sharing the load, international collaboration may make it easier to forecast demand for vaccines of all kinds by pooling data. Better forecasting can be of outsize value to low- and lower-middle-income countries, which are more likely to see changes related to demographic shifts and increasing vaccine uptake. International collaboration can also improve supply chain transparency and help ensure steady supplies, especially when it involves cooperation between public and private sectors. Finally, countries and regions that collaborate can form their own purchasing blocs, aggregating demand and gaining greater bargaining power than individual countries might have on their own.
COVID-19 highlighted a broad lack of readiness and caused major societal disruption, but national and regional leaders now have the opportunity to harness the lessons they’ve learned to build more resilient vaccine ecosystems. If effective, these ecosystems could ensure that when the next pandemic takes hold, nations will be better prepared.
Mitch Cuddihy is a consultant in McKinsey’s Dublin office; Andrea Gennari is a partner in the London office, where Tania Holt is a senior partner and Cormac O’Sullivan is an associate partner.
The authors wish to thank Tommaso Cariati, David Meredith, Tom Neuberger, and Kathrin Skiba for their contributions to this article.
Explore a career with us
Related articles.

Out of the shadows: A brighter future for pharma technical development

A data-driven approach to addressing COVID-19 vaccine uptake in Africa
Four ways to make sure your pharma manufacturing strategy delivers value
Recently Viewed
Listening...

From lab to vaccine vial: The historic manufacturing journey of Johnson & Johnson’s Janssen COVID-19 Vaccine
As the u.s. government announces an agreement between johnson & johnson and merck to collaborate on vaccine production, we trace all the steps it takes—from growing cells in a bioreactor to prepping palettes with high-tech track-and-trace technology—to develop and manufacture a vaccine during a pandemic..
- Copy link Link copied

A Lead Investigational Janssen COVID-19 Vaccine Candidate Is Announced

Global Collaborations Spur Manufacturing

Drug Substance Manufacturing Begins

It’s Time to Fill, Finish and Package the Investigational Janssen COVID-19 Vaccine Candidate

An Equitable Distribution Pledge Is Signed

Process Performance Qualification in Leiden Is Complete

The U.S. Government Announces an Agreement Between Johnson & Johnson and Merck
Stay up to date on johnson & johnson’s janssen covid-19 vaccine.

More from Johnson & Johnson

“I couldn’t speak, walk or sit.” Inside a rare autoimmune disease that attacks the muscles

5 things we now know about bladder cancer

What is IL-23?
ORIGINAL RESEARCH article
Vaccine production in africa: a feasible business model for capacity building and sustainable new vaccine introduction.

- 1 Department of Molecular and Developmental Medicine, University of Siena, Siena, Italy
- 2 GSK Vaccines, Siena, Italy
- 3 Takeda vaccines, Inc., Chicago, IL, United States
- 4 GSK Vaccines Institute for Global Health, Siena, Italy
Africa has the highest incidence of mortality caused by infectious diseases, and remarkably does not have the capacity to manufacture vaccines that are essential to reduce mortality, improving life expectancy, and promoting economic growth. GAVI has significantly helped introduction of new vaccines in Africa but its sustainability is questionable, and new vaccines introduction post-graduation is rare. Conversely, Africa with its high population and economy growth is an increasing potential market for vaccines. This study aimed to investigate how investment for vaccine production in Africa could be triggered and in which way it could be affordable to most African governments or investors. The investigation was based on a literature review and supplemented by online questionnaires directed to global vaccine stakeholders, African governments and regulatory authorities. In-depth interviews with experts in manufacturing capacity implementation and regulatory capacity building in Africa complemented the study. We also developed business plan scenarios including facility costs calculations and a possible investment plan based on expert opinions and publicly available information from pertinent sources. We saw that, governments in Africa, show interest in vaccine production establishments but only with external support for investment. The common regulatory functionality gap was the quality control laboratories to test vaccine lots before regulatory release. The global vaccine stakeholders showed less preference in investment for vaccine production establishment in Africa. The diverse political ambitions among African governments make it difficult to predict and access the market, a prerequisite for competitive production. A feasible solution could be a small production facility that would use technologies with high yield at low costs of goods to cover the regional needs. A respective antigen production facility is estimated to cost USD 25 Million, an affordable dimension for investors or interested African governments. Attractiveness for the African market is deemed to be high when targeting diseases almost exclusively for Africa (e.g., malaria or invasive non-typhoidal salmonella). With a smart 5 years tangible implementation plan, marketing agreements within existing regional collaborations and with a strong political will, an African government alone or together with an investor could convince global vaccine stakeholders and investors to support.
Introduction
Current challenges of concern for africa.
According to the Global Burden of Disease Study, published in 2016 ( 1 ), generally, infectious diseases decreased during the previous decade as a leading cause of death, and much of this decrease was driven by reductions in large contributors to global mortality, including HIV/AIDS, malaria, tuberculosis, and diarrhoeal diseases. However, in African and Asian regions, infectious diseases are still the leading cause of death, especially in children <5 years of age ( 2 ). About 44.4% of children deaths in 2016 occurred in sub-Saharan Africa, and 24·8% in South Asia ( 3 ). Children in low and middle income countries (mostly in Africa and Asia) are at much higher risk, with a 34-fold higher death rate than children in high income countries ( 2 ). Moreover, about half of the burden is contributed by diseases that seem to be almost exclusively reserved in Africa, such as malaria and invasive non-typhoidal salmonella (iNTS), also referred to as diseases of poverty ( 4 ).
Vaccination is one of the most important medical practice ever introduced, it has been essential to reduce mortality, improve life expectancy and economic growth ( 5 – 7 ). Africa is lagging behind in realizing the opportunities of reducing burden of disease by vaccination. Thanks to the Global Alliance for Vaccines and Immunization (GAVI), vaccines are becoming widely introduced ( 8 – 10 ). However, the concern is how to sustain such vaccines and whether countries would afford new vaccine introduction after graduation from GAVI ( 11 , 12 ). Even if economic cost-benefit evaluation is one of the criteria relevant for priority setting in health ( 13 ), decision on vaccine introduction for most African countries would likely depend on pure program cost ( 14 , 15 ). The more vaccines introduced in a country, the more expensive is the country's vaccination programme in terms of vaccine procurement, cold chain capacity and programmatic logistics ( 16 – 19 ). For example, Ethiopia spends USD 150 Million annually on vaccine procurement of which USD 100 Million currently come from donors ( 20 ). Eventually, upon graduation, the Ethiopian government is expected to triple its budget allocated to vaccine importation to sustain its immunization programme ( 21 ). We have seen several countries, such as India, Nigeria, and Thailand, which are now focusing on local production possibilities rather than importation of new vaccines with support from GAVI.
The biological processes with their inherent difficulty to manufacture vaccine batches with consistent characteristics and quality have been a hurdle to capacity expansion. Therefore, transfer of technologies and production processes to facilities located in Africa is a particular challenge ( 22 – 24 ). Brazil and Cuba are good learning examples for vaccine production setup by public institutions ( 25 – 28 ), while India is an example for private manufacturers ( 26 , 29 ). These countries committed to build or shape their own biopharmaceutical manufacturing capacity, initially focused on domestic needs and later expanded to supply international markets through the United Nations Children's Fund (UNICEF) and the Pan American Health Organization (PAHO). This became an important source of income despite of the technology transfer challenges ( 28 , 30 ). The likelihood of success for Africa is favored by the predicted population and thus market growth. The population of the less developed countries was projected to increase to 2.9 Billion in 2100, and four African countries, Ethiopia, the Democratic Republic of the Congo, the United Republic of Tanzania and Uganda, will be among the twenty most populous countries in the world in 2100 ( 31 ). In addition, African economy is growing at a steady rate of 5–6%, led by the East African region (EAC) ( 20 – 26 , 26 – 34 ).
Vaccine Production Concepts for Africa
According to Plotkin et al. ( 24 ) there are particular challenges involved in vaccine production, including process development, process maintenance, lead time, production facilities, equipment, life cycle management, and product portfolio management. The authors emphasized the importance of a robust and stable manufacturing process and consistent component supplies over decades to ensure long life cycle of a vaccine in a market. These areas should be carefully considered when planning vaccine production investment in Africa. Failure to manage these risks can result in costly product recalls, suspensions from the market and penalties may be assessed if a manufacturer fails to fulfill supply agreements ( 24 ). In this view, the choice of production technologies has huge impact on success of vaccine production establishment especially in the current African environment. The technologies for vaccine production, mainly the expression systems, play an important role in the cost of production, in terms of process stability and maintenance, life cycle and lead-time. There are several expression platforms, each with its yield capacity, and some are complex to develop ( 35 – 37 ). They have an important impact on cost of goods (COGs) and thus on the price for an affordable vaccine ( 37 ). Gerke et al. ( 38 ) has shown, production process for outer membrane particles from genetically modified bacteria called Generalized Modules of Membrane Antigens (GMMA), where, even a relatively small production facility (e.g., a 500 L fermenter) could produce in excess of 100,000,000 doses of vaccine per year. Such a technology would be highly favorable for African vaccine production because of its simplicity and the low production costs at a high yield.
Production can be done in either traditional fermenters so called stainless steel fermenters or single-use systems (SUS) ( 39 – 41 ). The choice of SUS or stainless steel or a mixed approach would depend on specific needs and the production scale ( 42 , 43 ), also considering regulatory requirements ( 37 , 44 , 45 ), commissioning ( 46 , 47 ) and facility maintenance. This has to be planned from the early phase of facility construction. A weak National Regulatory Authority (NRA) would create serious difficulties for the national and global business of a vaccine manufacturer. Since 2010, after the World Health Organisation (WHO) assessed NRAs in Africa ( 48 ), there have been great development in African NRAs and some NRA became fully functional, though, usually for oversight of pharmaceuticals only and not yet for biopharmaceuticals like vaccines. For vaccines, they depend on WHO pre-qualification programme (WHO-PQ) or other competent NRA licensure before local marketing authorization ( 48 ). These aspects must be keenly thought through and covered in the implementation plan of facility establishment and maintenance in Africa.
This was a review based on literature and pertinent websites, to investigate how investment for vaccine production in Africa could be triggered. The study was supplemented by online questionnaires developed in Google form and directed to specified organizations and African countries, aimed at determining their interest on vaccine manufacturing capacity implementation in Africa. The online questionnaires were structured differently according to the role of the respondent. The survey was conducted between June and September 2016 and the respondents included:
(1) Local officials of sub Saharan African countries governmental institutions (public health and economy)
(2) NRA officials of sub Saharan African countries and non-African developing countries with vaccine manufacturers
(3) Members of Developing Countries Vaccine Manufacturers (DCVMs)
(4) Global vaccine manufacturers / multinational companies (MNC)
(5) Global vaccine stakeholders (defined as officials from advanced NRAs, Independent consultants who have worked on or pioneered vaccine production or operations in developing countries, and officials from non for profit organizations that do vaccine production and development for developing countries)
(6) WHO-NRA capacity building officials
The online questionnaire was customized to each group, e.g., DCVMs' members received questions on investment costs, benefit of indigenous vaccine production, experience on possible challenges incurred during setup of their facilities, governmental incentives and regulatory capacity building. Information collected from online questionnaires was limited to accessibility of the respondents whose addresses were obtained from attendance list of international meetings, such as the African Vaccine Regulatory Forum (AVAREF), official website for a particular organization (MNC, DVCM, NRA, WHO) or through LinkedIn search. In addition, we conducted in-depth interviews with officials from the Bill and Merinda Gates Foundation (BMGF) and the African Vaccine Manufacturers Initiative (AVMI). This was to gather information on their view on investment in vaccine production, efforts already made and challenges for capacity building in Africa. Results were analyzed descriptively.
In collaboration with subject matter experts, we generated and qualified the business model on manufacturing capacity building in Africa. This included the development of high level planning scenarios for manufacturing capacity implementation, the feasibility evaluation, identification of related needs for regulatory capacity building in Africa and the description of the impact that enhanced manufacturing and regulatory capacity would have on new vaccine introduction in Africa and its sustainability.
The scientific committee of the University of Siena, Italy approved the study. Respondents were treated anonymously and were consented for their participation. Ethical review and approval was not required for this study in accordance with the local legislation and institutional guidelines.
Results From Survey Questionnaires
In total 30 responses were collected from various stakeholders including African governments (4 out of 14 contacted), African NRAs (11 out of 22 contacted), WHO (2 responses), MNC (3 out of 5 contacted), DCVM (5 out of 30 contacted), and Global vaccine stakeholders (5 out of 6 contacted). We also had two in-depth interviews, one with three officials from the AVMI and the other one with an official from BMGF.
Figure 1 shows the results summary from the questionnaires grouped into three categories namely governments in Africa, NRAs in Africa and global stakeholders (combining MNC, DVCMs, WHO, and BMGF).
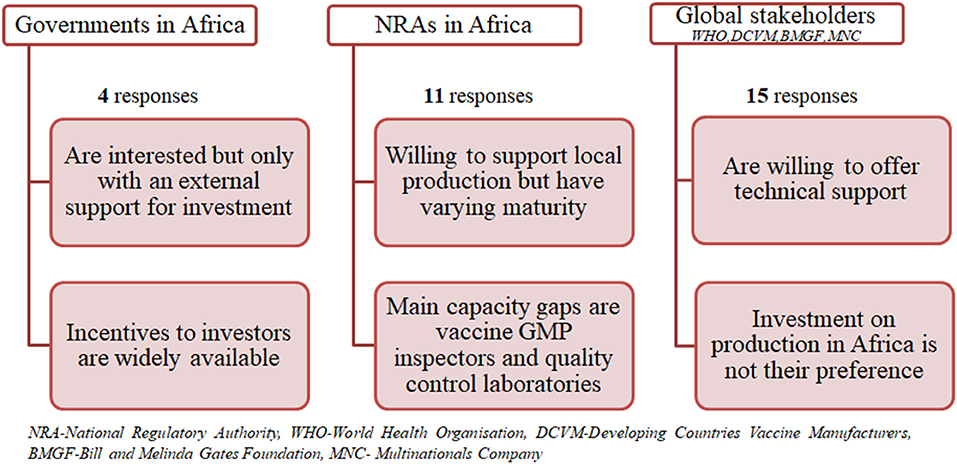
Figure 1 . Summary of results from questionnaires.
Governments Interest and Support
All responders were interested in the establishment of vaccine manufacturing capacity in their countries. However, they would do this only with an external financial and/or technical capacity building support. Thus, they are willing to support an investor at varying levels, such as land, tax incentives, infrastructure provision, and monetary support as a private public partnership. They are also willing to facilitate necessary extra capacity building in their NRA in a form of training or to support collaboration with competent authorities of other countries and the WHO. The latter would help them during an interim phase to cover all regulatory aspects around vaccine manufacturing facility establishment and at the initial stage of product life cycle. All responders expect access to a vaccine at an affordable price and establishment of employment for native experts.
Regulatory Authority Capacity Gaps and Improvements
Capacity gaps vary from country to country; most NRAs would mainly need capacity building in relation to qualification of GMP inspectors, quality control laboratories for vaccine and technical expertise to perform lot release. They would require varying time for filling capacity gaps for indigenous production and supply, depending on availability of funding, from 1 to 5 years. The NRAs are willing to collaborate and rely on WHO and other competent NRAs from other countries to cover an interim period for regulatory need of vaccine manufacturing and batch release oversight. Some NRAs already perform batch release of all imported vaccines, e.g., Zimbabwe. With regard to global vaccine supply from an African country, some have already established regional regulatory collaboration, such as the EAC's Medicines Regulation Harmonization, making it easier for them to support each other in that regard.
Developing Countries Vaccine Manufacturers' Perspective
Developing countries vaccine manufacturers are interested in seeing Africa developing their own manufacturing capacity; some would even expand their capacities to Africa in the future. However, the critical aspects for establishment of vaccine production capacity differ from country to country. Most of them are challenged by the access to expertise, source of raw materials, consumables, equipment, market access, country's import policy, and regulatory shortcomings in GMP inspection and long timelines for dossier review and approval. Other critical aspects included construction of facility, financial support, and acquisition of technology. Some government owned manufacturers would prefer such capacity to come from African government, as it is essential that a government will provide financial support for the project, construction, start-up of production, and commits to use the produced vaccine. The private manufacturers in India got supply of raw materials from the Indian government, which also provided incentives, such as sales tax, excise duty, and customs duty concessions as long as the unit was established in designated economic zones. DCVM acknowledged that indigenous vaccine production has significantly benefited their countries. The state owned manufacturers, such as Brazil base their success on their population (200 Million people with a 3 Million birth cohort) which is large enough for them to offer vaccine at a low price (similar to that of UNICEF) to their national immunization programmes. Hence their local production has provided security, avoiding shortage and strengthens their national technological capacity. Most private manufacturers in India have based their business on supplying vaccines to UNICEF and some claim to have reduced price to even half for indigenous purchase.
Experts From Multinational Companies
As personal opinions, experts from multinational companies pointed out that feasibility for facility investment in Africa depends on the novel nature of the vaccine and the epidemiology of the disease, regulations etc. If a vaccine will provide high benefit in long-term for the majority of the African countries, there might be an opportunity to evaluate further the economic value of the vaccine and public health benefit via a business case. Some experts stressed the importance of research and development (R&D) costs in addition to facility investment costs for consideration when establishing vaccine prices. They also highlighted the importance of significant incentives like benefit of conducting business in a politically stable environment, ease of conducting business activities and regulatory navigation along the development and licensure roadmap, and tax incentives in the long term. Experts suggested that directing focus on Neglected Tropical Diseases (NTD) of particular interest for Africa would possibly lead to easier market development and eventually access to GAVI market.
Global Vaccine Stakeholders
There are stakeholders who showed interest in establishment of vaccine manufacturing in Africa, reasoning that such capacity is needed in Africa, and would reduce cost of vaccines, help overcoming vaccine shortages, make countries better positioned to respond to outbreaks and would be a way for African countries to win their independence from the big pharmaceutical companies. It was suggested that, since no African country has enough inhabitants to justify the establishment of manufacturing capacities of significant size, African countries should identify a champion to build vaccine manufacturing capacities focusing on the needs of all African countries. In terms of support, most stakeholders were interested in investment at different levels. Based on their experience they believe that capital investment is likely substantial and therefore support from several NGOs, foundations, private companies will be crucial to facilitate vaccine manufacturing capacity implementation, including technology transfer and e.g., to establish advanced market commitments by securing financial resources. However, one of the experts was of the opinion that vaccines for Africa can be sourced more economically from countries like India, where a very robust vaccine industry exists.
Role of WHO for Regulatory Capacity Building
The WHO is interested in establishment of vaccine manufacturing capacity in Africa and is willing to support the NRA where such capacity is being built. Basically, a country in which manufacturing capacity is being built should have appropriate technical skills, Quality Management System, market, and political commitment. Moreover, the ability to ensure adherence to international quality standards in a sustainable manner is a critical aspect for consideration. WHO's level of support is mostly technical, they have heavily supported regulatory system development in developing countries with vaccine manufacturing capacities, such as Brazil, India, Indonesia, and Thailand, and they did this through creating institutional development plans based on NRA assessment. In order to speed up regulatory capacity building in Africa, WHO officials suggest improvement on governance in countries and training of staff. There is doubt that global vaccine supply from a country in sub Saharan Africa is feasible from regulatory perspective in near or mid-term future. In any case it will require lots of funding and support by experts to become a reality.
In-depth Interview With Experts
Experts from AVMI explained while there are several entities at various stages of vaccine production the current capacity of vaccine production in sub-Saharan Africa is mainly limited to Senegal's Institute Pasteur of Dakar (IPD), which produces yellow fever vaccine, the only WHO pre-qualified vaccine produced in Africa. Historically, most of the current vaccine facilities in Africa were government owned and competing priorities shifted the focus away from developing further capacity. However, the slow pace of progress in vaccine manufacture in Africa can be accelerated through political choice. Biovac is an example of how, with backing from the South African government and taking a reverse integration approach in building capacity across the value chain through partnering, globally recognized vaccine development and manufacturing capability can be established. It is critically important over the long term to reverse the current situation where <1% of vaccines used in Africa are made in Africa, leaving African countries vulnerable in emergency pandemic events. The same point was raised by the expert from BMGF who pointed out that commitment by African government has been a challenging as governments usually prefer cheaper vaccines from abroad over local ones, making it difficult for a manufacturer in Africa to have economies of scale to compete within the international vaccine market. It is envisaged that commercial agreement between African countries is necessary to ensure relevant local market size for return on investment. Nonetheless, governments' budget allocated to health is so limited and thus unlikely allows larger investment in vaccine manufacturing capacity building. Vaccine manufacturing remains complex and requires a highly integrated set of well-coordinated activities, including a highly skilled workforce, elaborate supply chain for many specialty reagents and consumables, exquisite quality control, and appropriately trained and staffed regulatory agencies. In view of current regulatory environment, it was recommended to first enhance pharmaceutical capacity and only in a second step (mid to long term) to build vaccine manufacturing capacity. BMGF is interested to accelerate availability of largest amount of high impact health products at best quality-price balance. Building manufacturing capacity for vaccines in Africa is not the most direct pathway to reach this objective. With regard to the technical or knowledge capacity building and the parallel development of the regulatory environment toward full functionality, BMGF collaborates strongly with the WHO.
High Level Planning for a Vaccine Manufacturing Capacity Implementation in a Green Field in Africa
With the subsequent planning scenarios African governments and investors are supported to perform their own evaluation on investment feasibility, vaccine affordability, and market access possibilities, especially for vaccines targeted mainly or almost exclusively for Africa, such as malaria or invasive non-typhoidal salmonella.
Process Conceptual Plans
In general, a facility conceptual plan requires inputs from R&D and Marketing, especially technical information available at clinical phase 2 and sales forecast based on a clinical data driven initial marketing strategy. The following three production capacity building scenarios were developed based either on typical process-yield assumptions for a recombinant protein vaccine ( 49 ) or on published data for a GMMA based vaccine, all to be available in 2025, the earliest time point for availability of a validated facility, in case planning and implementation would start in 2018. The total yearly demand for an infant or toddler vaccine at a predicted birth cohort of 50 Million in Africa by 2025 ( 50 ), is 121 Million doses under the assumption of a 2-dose schedule, immunization coverage rate of 85% and the vaccine wastage of 30% ( 51 , 52 ).
First scenario
As there is currently no concrete interest for investment, we arbitrarily selected a recombinant protein based vaccine as an example with the following assumptions: An investor in Europe or elsewhere has limited capacity (only one third) for producing and supplying a vaccine to Africa. According to the supply forecast, a yearly capacity to produce 121 Million vaccine doses will be needed by 2025. A fermenter will yield 75 mg/L of protein, if production is at a cycle time of 1 week. The resulting capacity will be ~21 Million doses per 1,000 L fermenter per year at a dose of 50 μg/0.5 ml. If an investor already produces 42 Million doses from an existing facility, which has two 1,000 L fermenters, the additional required capacity in the African country will be 80 Million doses from four production lines, each with a 1,000 L fermenter and respective purification capacity, assuming “like-for-like” technical transfer (this will create a total production capacity of 126 Million doses). The vaccine is adsorbed to aluminum hydroxide and filled into multidose vials. Respective formulation, fill and packaging capacities to produce 121 Million doses per year are required.
Second scenario
The second scenario is based on the first scenario, but here we use a single production line that results in 21 Million doses per year, sufficient to supply the EAC region. Alternatively, if production is targeted for a single country, such as Tanzania with estimated need of 7 Million doses a year, two other similar single antigen vaccines can be produced in campaign during the year.
Figure 2 shows a typical protein antigen vaccine production process to feature first and second scenarios.
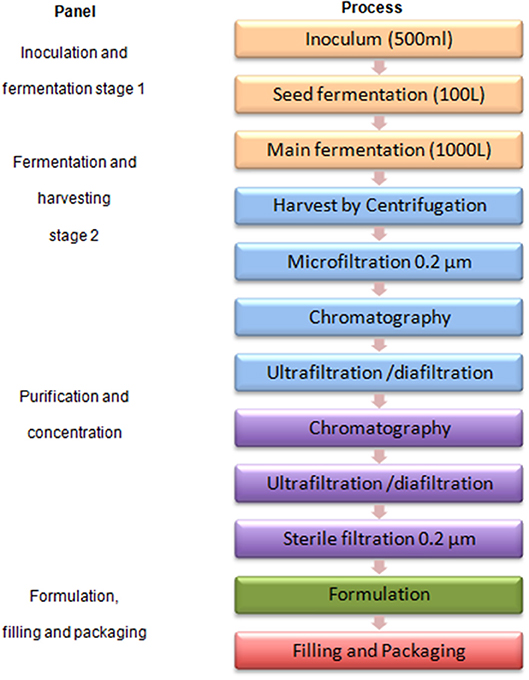
Figure 2 . A typical protein antigen vaccine production process.
Third scenario
For vaccine production using GMMA technology as described by Gerke et al. ( 38 ), a relatively small production facility (e.g., a 500 L fermenter) could produce in excess of 100 Million doses of vaccine per year ( 38 ). The production time from setting up the inoculum for fermentation to final purified GMMA could be as short as 3 days per batch. Thus, depending on the size of human dose, with this kind of technology, just a single production line with a 500 L fermenter capacity would be almost enough to cover the whole African market need in 2025, assuming a 2-dose schedule infant vaccine.
Figure 3 shows the GMMA based process flow diagram generated from process description by Gerke et al. ( 38 ).
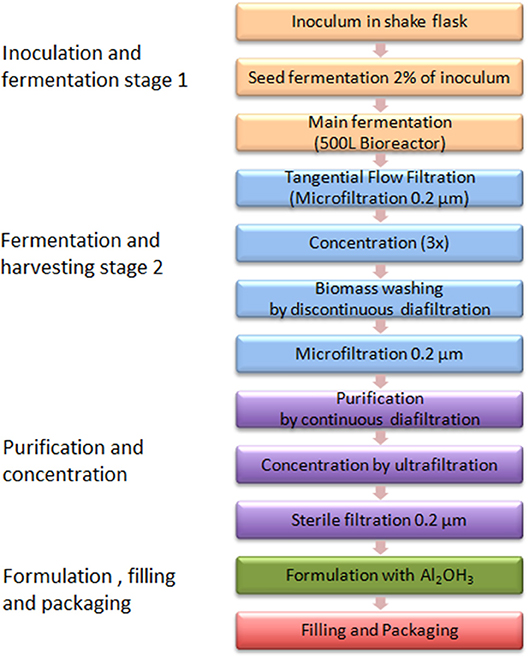
Figure 3 . A GMMA based vaccine production process.
Site Master Plan (SMP)
The SMP for all three scenarios follows the same concept and, in proportion, leads to the same cost estimates. Therefore, only the scenario for a new green-field facility, with a capacity to produce recombinant protein bulk for 84 Million doses and to formulate, fill and package 121 Million doses is planned. As described above, the fermentation technology platform is assumed to be well-known and for scenario 2 a flexible downstream capacity which has a capability to provide a range of recombinant protein vaccine products is required.
The size estimate assumptions for the SMP are developed in consideration of an “ideal site” with 75% of plant usage, providing 50% expansion capacity and production suites completely segregated with dedicated support areas and clean utilities. It is recommended that the facility location has to be well-characterized by considering aspects like land price, geographical conditions (flat, appropriate soil characteristics, easiness and security for foundation), the proximity to public water, energy supplies, sewer, waste disposal capabilities, transport connection, location in trade zones, topography of the site, type of neighbors and analysis of possible contrasts/synergies. The layout designs have to consider distances for personnel movements tailored to location of employees' parking spaces, guardhouse and canteen with respect to production buildings. They also have to describe relationships between buildings for main personnel flows, main material flow and utilities distribution requirements. This will then guide setup for construction phases.
Architectural Size Estimates
The bulk production building will accommodate four production lines with a separated purification suite. The formulation areas need to accommodate ~180 batches per year (44 cycles × 4 production lines). The filling area requires two vial filling lines using disposable flow-paths at a nominal capacity of 24,000 units/h to fill the 180 batches and the packaging area requires two lines, each with a nominal capacity of 12,000 units/h to finish the 121 Million doses. Furthermore, a warehouse, a QC and QA building with a 1:2 QA: QC ratio to accommodate laboratories and offices for 40 people and an animal house for 250 mice, 20 guinea pigs, 60 rabbits and a BSL2 testing room are required. The plan contains also a canteen with 100 seats and a building for administration that can accommodate 50 people. In addition, a guard house and parking lots are included. Space for central utilities, an electrical substation and a waste water treatment (WWT) plant to provide treatment for liquid effluents based on biological demand correspondent to sanitary streams of 1,000 people equivalent as worst case complete the dimension setting for the architectural planning.
The size of the production buildings was estimated based on a rule of thumb ( 53 ) as described in Table 1 below. For one Drug Substance (DS) production line, a process area of 3,500 sq. feet is estimated. Thus, assuming this process area is 17.5% of total area in a production building, the building for DS ptoduction with four lines will have a total process area of 14,000 sq. feet and hence, a total dimension of 80,000 sq. feet. For the second and third scenario, a single production line facility with process area of 3,500 sq. feet would require 20,000 sq. feet total building area. The areas of the other buildings are calculated following expert opinion and are shown in the Table 2 .

Table 1 . Rule of thumb for area distribution in a drug substance production building.
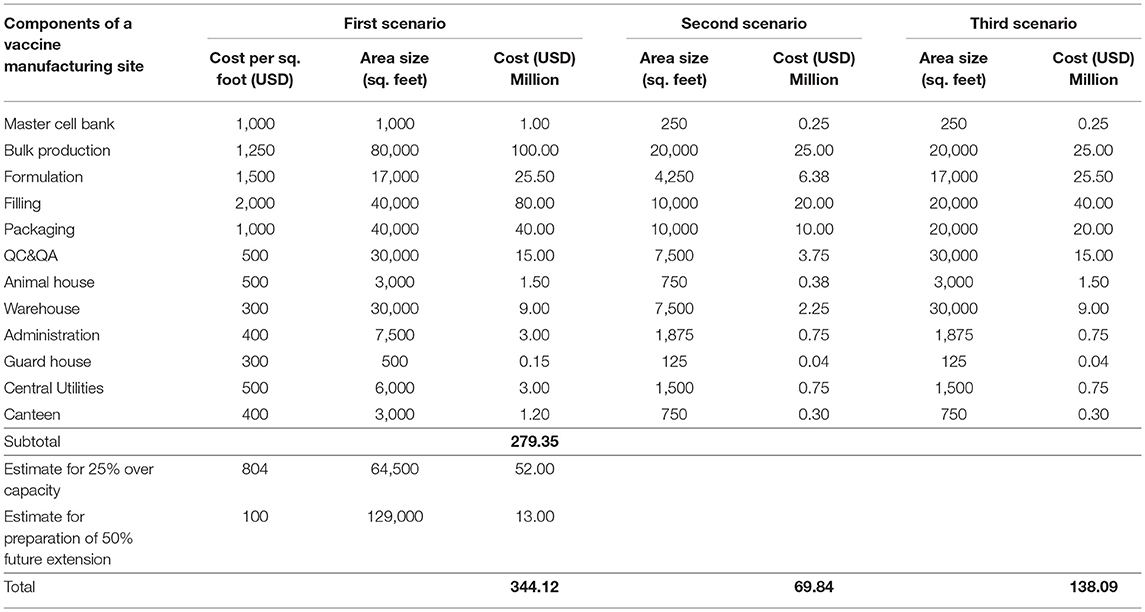
Table 2 . Size and cost estimates for vaccine manufacturing site scenarios.
Fabrication, Construction, Schedule and Validation
All aspects for fabrication, construction and validation have to be properly addressed linking to regulatory requirements ( 53 ). Therefore, the investor should carefully select experienced engineering companies and consider using pre-fabricated modules for which qualification is in part already performed when they arrive. For construction one has to decide whether to use an onsite (stick built) approach, where assembly of walls, piping, steel, etc. has to be done on site, or an offsite (modular) approach, where the facility modules are built elsewhere and then shipped to site. The other alternative is a hybrid approach where the facility shell is stick built onsite while process equipment are built as skids at the equipment vender. This would allow for concurrent facility and equipment construction. However, the choice has to be made based on costs, validations and qualification steps to be followed for the different cases.
We recommend project schedule to be divided into scope, design, procurement, construction, Installation Qualification and Operation Qualification (IQ/OQ), start-up or Process Qualification (PQ), validation, and approval. The schedule should be timed in view of the date for expected launch of product produced in the new facility. Even though, the schedule duration is typically 5 years (see Figure 4 ), for the first vaccine manufacturing capacity implementation in Africa additional preparative time should be included in the plan. Extremely important will be to build sufficient scientific, technical and regulatory expertise, e.g., by sending scientists, technicians and regulators for training to sites/countries with process and regulatory expertise. For the execution, it is important to receive the building permission early and to plan and manage the construction phases in a way to minimize construction time. It could be better to use several expert companies with sufficient staff to work in parallel on sub-areas of the overall plan for the best and efficient project execution and meanwhile using collaborating institutions for global support on capacity building in human resources, improving NRAs and other aspects of technology transfer in each step.
Figure 4 . A typical vaccine manufacturing facility building schedule.
Cost Estimates for Facility Construction
For Africa a modular type facility will be likely the best choice due to supposedly limited or lack of expertise in biopharmaceutical facility building. With the calculations following expert opinion based on a modular type facility, for which it can be assumed that costs are globally similar, we managed to come up with estimates for each of the three scenarios, which are detailed in Table 2 . It should be taken into consideration that experts' facility cost estimation in the initial planning phase usually should be within an accuracy of ±30%.
The total cost for an ideal facility according to the first scenario would be about USD 344 Million. This could be reduced to about USD 280 Million not considering over capacity and future expansions. The second scenario with a cost of about USD 70 Million has a production capacity of 21 Million doses per year sufficient for regional supply (e.g., the six countries of EAC region). The third scenario costs about USD 138 Million and is able to supply either almost the whole African market with 1 single antigen vaccine or e.g., the EAC region with three different single antigen vaccines to cover the estimated demand in a region. However, only the concrete real scenario plan can provide accurate estimates on key components of a vaccine manufacturing site.
These cost estimates do not include the costs involved in start-up or Process Qualification (PQ) and validation activities. Usually at least 3–5 product batches will be produced during PQ and validation. There are significant costs involved but, ideally, these batches can later be sold. A full cost estimate for PQ and validation requires a detailed product knowledge that enables estimation of the COGs. Each facility project should include respective costs in the overall plan. In addition, facility-running costs should be calculated as they may impact the project as idle cost of the facility, once established and not used. In case of continuous use, these costs are absorbed into the COGs. The larger or complex the facility, the higher the running costs. However, we anticipate that labor costs in Africa would be low compared to Asia and Europe ( 54 ), even though at the beginning costs may even be higher, due to the need of high cost experts from high income countries ( 24 ). The involved cost risk i.e., long burden of start-up costs or idle costs ( 24 ) can be reduced by assuring full size market access in the initial facility establishment plan.
Product Cost Calculations and Marketing
Table 3 gives special considerations for vaccine cost calculations ( 53 ). Applying the respective criteria to the GMMA based technology (third scenario) this technology ensures a competitive vaccine price likely acceptable to UNICEF and affordable to African countries. The technology results in high yield and needs only few steps for purification, labor cost is greatly reduced (few people for few process steps), materials/consumables are highly reduced and due to the high yield there is room for scale increase in reasonable dimension. The other two scenarios will have higher manufacturing costs and are likely not as favorable as a GMMA based vaccine. Concrete estimates ( 24 , 55 ) would be required to determine their competitiveness and acceptability to UNICEF and GAVI.
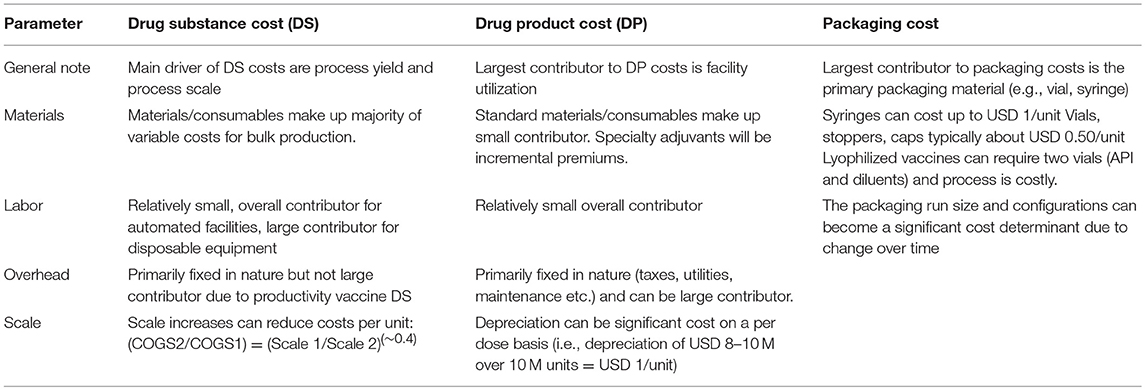
Table 3 . Typical inputs for product cost calculations.
This study was a review based on literature and pertinent websites, to investigate how investment for vaccine production in Africa could be triggered. The study was supplemented by online questionnaires developed in Google form and directed to specified organizations and African countries, aimed at determining their interest on vaccine manufacturing capacity implementation in Africa. The response rate was limited, e.g., only four out of fourteen contacted African government officials responded. The low response rate had several causes, e.g., the email address was no longer valid as contacted persons had changed tasks or government officials were not comfortable to respond on behalf of their governments. However, in total 30 responses from various organizations were obtained and in-depth interviews with experts in the field complemented the information. We are therefore confident that the results are representative.
Governments in Africa show interest in vaccine production establishments, but only with external support for investment. However, investment for vaccine production establishment in Africa is currently not the preference of global vaccine stakeholders. Therefore, African governments should change their thinking, if they want to realize vaccine manufacturing implementation in their country. The key challenge is to ensure sufficient and assured market access for potential investors. Responses to the survey questionnaire showed that investors, including global vaccine manufacturers, are attracted more by market assurance than by provision of incentives ( 56 , 57 ). Global stakeholders, such as GAVI and UNICEF can be involved to spearhead marketing aspects in the spirit of building capacity in Africa. Solutions in view of future challenges due to growth of African population and GAVI graduation will anyway be needed, and therefore, financial potential coming from projected growth of African economy could be directed into a program for self-sustainability with regard to vaccines. Interested governments can be approached for commitment to access an agreeable market through Africa's regional collaborations, such as EAC, SADC, ECOWAS, and AU, with consideration that a large enough population, like in India, China or Brazil, allows to grow due to an economy of scale. It is expected not to be easy getting neighbor countries to agree on common procurement of vaccines from an African manufacturer, but only this can guarantee a low affordable price and thus an investment with calculable risk. Disunity of African governments has delayed establishment and expansion of manufacturing capacity for both, vaccines and pharmaceuticals in Africa even though African economy and population growth were eminent and invited for investment.
Many African countries lack political will for concrete integration of pharmaceutical manufacturing development into economic development planning. However, e.g., Ethiopia has written and published a pharmaceutical manufacturing development plan ( 57 ) and the African Union Council (AUC) issued the Pharmaceutical Manufacturing Plan for Africa (PMPA) ( 58 ). Unfortunately, the plans do not show concrete political and or economical commitment to support implementation of pharmaceutical manufacturing capacity extension. The establishment of a vaccine policy by countries may assist in identifying how and when to consider local production. In particular, at the beginning, establishing local vaccine manufacturing is not necessarily cost-effective, but vaccines should not be seen purely as commodities. Factors, such as national health security should be considered as well ( 56 ). Committed governments, such as Cuba and Brazil have a requirement for public health delivery written in their constitutions ( 23 , 25 ), despite their different historical backgrounds both countries have found a way to sustain their investment in public facilities and they have become role model in the PAHO region. African countries can learn from them.
Many African NRAs have developed capacities, including capacity for GMP inspection, for pharmaceuticals rather than vaccines due to globally driven traditional ways of vaccine procurement. If a country completely procures vaccines from UNICEF, it would not be cost effective and necessary for them to repeat tests already done by another competent authority. Therefore, such a country will likely remain dormant in NRA capacity development for vaccines unless a facility is established in that country. In Cuba, Brazil and India it was the development of vaccine production that pushed the governments to improve their NRAs. Improvement of regulatory oversight would also be needed in Africa, in particular as nowadays it is expected that a vaccine manufacturing facility implementation will be accompanied and approved by a fully functional NRA. Therefore, an interested African country should prepare early on by using available technical expertise provided by WHO and other stakeholders, such as AVMI for capacity building while a facility is being built in the country. For example, local training and research institutions can establish collaboration with abroad institutions (global vaccine stakeholders) and or companies for special vaccinology courses ( 59 ) and internship programmes aiming at building local capacity in terms of human resource that will work in both, NRAs and industries, with regard to vaccines.
Businesswise, investment in manufacturing capacity for a vaccine produced in a high yield technology, such as GMMA would favor quick return of investment at an affordable vaccine price for most African countries compared to production using a low yield technology. Building up a concrete plan within a specific country in Africa could even significantly lower the cost of investment, if e.g., available capacity from adjacent pharmaceutical industry with established QA/QC, formulation and fill and finish capacity could be built into the planning (brown field). Only the DS production facility for one antigen with a capacity for the whole African market would approximately cost USD 25 Million. Such a cost is affordable for most African governments as long as they include it in their economic plan and deliver within 5 years of implementation plan schedule. In addition, there is the opportunity that facility costs are lower in Africa as it has been shown that facility cost in Europe is even twice as high as in the Asian region (Russia, China, and India) ( 60 – 64 ). The brown field also offers an opportunity for existing private pharmaceutical companies to partner with an interested African government or a private investor for vaccine production tailored to market access.
Technical transfer can either be transfer of a licensed vaccine to extend capacity of a global manufacturer (first scenario) or an early transfer during development before or after proof of clinical concept. The first scenario would likely require an intense phase of vaccine specific knowledge and know how building of African scientists and technicians before or in parallel to the facility building and support by or transfer of key personal of the global manufacturer until robust process routine is achieved. The latter concept could be in collaboration with institutes like the GSK Vaccine Institute for Global Health (GVGH), the International Vaccine Institute (IVI), the Hilleman Laboratories, the Gates Medical Research Institute (GMRI), or other similar Institutes and could use a staggered approach: Building a GMP pilot facility that could be used for early clinical development (even as contract manufacturing site) and then extended to industrial or market scale (full capacity) for vaccine roll out according to demand forecast. This would also ease the technology transfer pathway and provide an opportunity to build staggered knowledge transfer. For a high yield process like GMMA a 250 L fermentation scale could be sufficient as industrial scale and then extended with 1 or 2 further production lines to cover the full African market. This would reduce investment risk and is an opportunity for any interested African country or its institutions to collaborate with global institutions focusing on vaccine development against neglected diseases of developing countries. International funds to support Phase 3 development of candidate vaccines against neglected diseases that have the potential to save many lives of African children will likely be required ( 65 ) in addition to a funding concept for the facility construction.
The Meningitis Vaccine Project (MVP) was a collaboration of the WHO (responsible for surveillance and vaccine introduction) and PATH (responsible for product development), who partnered with Serum Institute of India Private Ltd (SIIL) and public health officials across Africa to develop an affordable, tailor-made vaccine for use against meningitis A in sub-Saharan Africa (MenAfriVac). The project was set up after African leaders called for the development of a vaccine that would eliminate group A meningitis epidemics in Africa ( 66 ). The vaccine MenAfriVac was introduced via mass vaccination campaigns in 2010 and had a dramatic impact in reducing meningitis A epidemic. The project got funds from BMGF in 2001 (USD 70 M later added USD 17 M) to fight Meningitis in the meningitis Belt of Africa. In this collaboration SIIL supplied tetanus toxoid (TT) and Synco Bio Partners BV of Netherlands supplied Meningococcal A polysaccharide (MenA-PS). The FDA-CBER did the conjugation of MenA-PS with the carrier protein (TT) before transferring the production process to Serum Institute of India Limited (SIIL). The UK National Institute for Biological Standards and Control (NIBSC) did the testing of the vaccine batches produced by SIIL. The MVP shows how local institutions collaborated with international organization for capacity building on vaccine clinical development and disease surveillance. It also shows how international stakeholders could be involved for fast vaccine development and introduction, including capacity building and technical transfer. The success of the project mainly came from a strong political will geared by meningitis disease prevalence and mortality in that region. It was a good example showing that funds for clinical development of a vaccine that is almost exclusive for Africa can be obtained. MVP lowered investment costs for the producing industry (SIIL) and SIIL also benefited from the knowledge transfer on conjugation technology as that knowledge could be used to produce other vaccines. Africa definitely needs a partnership like for the MVP; this time as a long term development and/or manufacturing partner and not just as an end user. Such a collaboration project could build African capacity to overcome other problems, such as malaria, HIV, iNTS, etc.
We recommend that African countries should not start a very big complex project plan, which costs a lot of money and require sophisticated expertise and experience in vaccine manufacture. Instead they can make small projects targeting one antigen after another and grow over time. Preference should be given to already well-researched antigens or new antigens, which can be produced with simple, straightforward processes and for which there is no patent infringement. In addition, countries should quickly utilize their available high learning institutions and biotechnology research institutes to build sufficient indigenous technical expertise (human resources) required for vaccine production in collaboration with WHO, PATH, BMGF, and other institutes, which are dedicated to development of vaccines for Developing Countries, such as IVI, Hilleman Laboratories and GVGH. There would also be the opportunity to perform contract manufacturing during clinical development with the option to partner or acquire the project/vaccine at a later stage. This would allow a smooth and low risk phasing into realization of a sustainable vaccine manufacturing capacity and new vaccine introduction in Africa.
Data Availability
The datasets generated on survey via google forms will be made available on request. Otherwise all relevant data is contained within the manuscript.
Author Contributions
GM and JA: conceptualization. GM: writing original draft. GM and JA: data curation. GM, SB, TC, and JA: formal analysis. EM: funding acquisition. GM and JA: investigation. GM, SB, TC, and JA: methodology. SB, EM, TC, and JA: resources. JA: supervision. TC and JA: validation. GM: visualization. SB, EM, TC, JA: writing review and editing. EM: project administration.
The study was funded by the University of Siena, Italy.
Conflict of Interest Statement
During the course of the study, GM spent 6 months as an intern at the GSK Vaccine Institute for Global Health, Siena, Italy in 2016. SB worked as a global operational engineer of GSK, Siena, Italy. TC worked with Takeda vaccines as a global operational engineer, and JA is the head of regulatory affairs at GSK Vaccine Institute for Global Health. TC is currently the Chief Executive Officer of CytoSen Therapeutics, Inc., North Carolina, USA.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the University of Siena, who, in collaboration with the Vaccines Academy and the GSK Vaccines Institute for Global Health, Siena, Italy, made this investigation possible and to all who provided contributions, including Dr. Rino Rappuoli (GSK Vaccines Italy). Special thanks go to the experts who participated in this study, which includes: Officials from AVMI for in-depth interview namely Mr. Patrick Tippoo, Dr. Ebrahim Mohamed, and Dr. Seanette Wilson. The official from BMGF, Dr. Rayasam Prasad for his time and contribution on in-depth interview. Officials who responded to questionnaires: Officials from multinational companies, the Global vaccine stakeholders, Independent consultants (from Switzerland and Pakistan). Officials from US FDA, Health Canada and WHO. Officials from Developing countries vaccine manufacturers (Brazil, India, Indonesia and Pakistan). Officials from Africa (Ghana, Malawi, Cameroon, Burundi, Tanzania, Kenya, Zimbabwe, The Gambia, Zambia, and Nigeria).
1. Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, Aboyans V, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2017) 390:1151–210. doi: 10.1016/S0140-6736(17)32152-9
CrossRef Full Text | Google Scholar
2. MacLennan CA, Saul A. Vaccines against poverty. Proc Natl Acad Sci USA. (2014) 111:12307–12. doi: 10.1073/pnas.1400473111
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Wang H, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, et al. Global, regional, and national under-5 mortality, adult mortality, age-specific mortality, and life expectancy, 1970–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2017) 390:1084–150. doi: 10.1016/S0140-6736(17)31833-0
4. Stevens P. Diseases of poverty and the 90/10 gap. Int Policy Netw. (2004) 16. Available online at: www.policynetwork.net
Google Scholar
5. Andre FE, Booy R, Bock HL, Clemens J, Datta SK, Lee BW, et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ. (2016) 86:140–146. doi: 10.2471/BLT.07.040089
6. Roberts L. Polio: Health Workers Scramble to Contain African Epidemic. Science. (2004) 305:24–5. doi: 10.1126/science.305.5680.24
7. Keiny MP, Girard MP. Human vaccine research and development: an overview. Vaccine. (2005) 23:5705–7. doi: 10.1016/j.vaccine.2005.07.077
8. Zuber PLF, El-ziq I, Kaddar M, Ottosen AE, Rosenbaum K, Shirey M, et al. Sustaining GAVI-supported vaccine introductions in resource-poor countries. Vaccine. (2011) 29:3149–54. doi: 10.1016/j.vaccine.2011.02.042
9. GAVI. Eligibility and Transition Policy . (2015) Available online at: http://www.gavi.org/about/governance/programme-policies/eligibility-and-transition/ (accessed September 5, 2016).
10. GAVI. Transition Process. (2016). Available online at: http://www.gavi.org/support/apply/graduating-countries/ (accessed September 6, 2016).
11. Levine OS, Hajjeh R, Wecker J, Cherian T, O'Brien KL, Knoll MD, et al. A policy framework for accelerating adoption of new vaccines. Hum Vaccin. (2010) 6:1021–4. doi: 10.4161/hv.6.12.13076
12. Sosler S, Kallenberg J, Johnson HL. Gavi's balancing act: accelerating access to vaccines while ensuring robust national decision-making for sustainable programmes. Vaccine. (2015) 33:A4–5. doi: 10.1016/j.vaccine.2014.12.051
13. Musgrove P. Public spending on health care: how are different criteria related? Health Policy. (1999) 47:207–23. doi: 10.1016/S0168-8510(99)00024-X
14. Walker DG, Hutubessy R, Beutels P. WHO Guide for standardisation of economic evaluations of immunization programmes. Vaccine. (2010) 28:2356–9. doi: 10.1016/j.vaccine.2009.06.035
15. World Bank. World Development Report 1993: Investing in Health. (1993). Available online at: http://www-wds.worldbank.org/external/default/WDSContentServer/WDSP/IB/2006/02/02/000160016_20060202161329/Rendered/PDF/351170Benefit0incidence0practitioner.pdf
16. Schutte C, Chansa C, Marinda E, Guthrie TA, Banda S, Nombewu Z, et al. Cost analysis of routine immunisation in Zambia. Vaccine. (2015) 33:A47–52. doi: 10.1016/j.vaccine.2014.12.040
17. Kaufmann JR, Miller R, Cheyne J. Vaccine supply chains need to be better funded and strengthened, or lives will be at risk. Health Aff. (2011) 30:1113–21. doi: 10.1377/hlthaff.2011.0368
18. Bell KN, Hogue CJ, Manning C, Kendal AP. Risk factors for improper vaccine storage and handling in private provider offices. Pediatrics. (2001) 107:E100. Available online at: https://pediatrics.aappublications.org/content/pediatrics/107/6/e100.full.pdf
PubMed Abstract | Google Scholar
19. Zaffran M, Vandelaer J, Kristensen D, Melgaard B, Yadav P, Antwi-agyei KO, et al. The imperative for stronger vaccine supply and logistics systems. Vaccine. (2013) 31 Suppl. 2:B73–80. doi: 10.1016/j.vaccine.2012.11.036
20. Ampofo W. Vaccine manufacturing in Africa. In: Global Vaccine and Immunisation Research Forum . Johannesburg: WHO (2016). Available online at: http://www.who.int/immunization/research/forums_and_initiatives/1_Wlliam_Ampofo_Vaccine_Manufacturing_Africa.pdf?ua=1
21. Wiysonge CS, Waggie Z, Hawkridge A, Schoub BD, Madhi SA, Rees H, et al. The Cape Town declaration on vaccines 2012: unlocking the full potential of vaccines in Africa. Vaccine J. (2016) 34:3713–14. doi: 10.1016/j.vaccine.2016.05.072
22. Bae K, Choi J, Jang Y, Ahn S, Hur B. Innovative vaccine production technologies: the evolution and value of vaccine production technologies. Arch Pharm Res. (2009) 32:465–80. doi: 10.1007/s12272-009-1400-1
23. Cortes MDLA, Cardoso D, Fitzgerald J, Difabio JL. Biologicals public vaccine manufacturing capacity in the Latin American and Caribbean region: current status and perspectives. Biologicals. (2012) 40:3–14. doi: 10.1016/j.biologicals.2011.09.013
24. Plotkin S, Robinson JM, Cunningham G, Iqbal R, Larsen S. The complexity and cost of vaccine manufacturing – an overview. Vaccine. (2017) 35:4064–71. doi: 10.1016/j.vaccine.2017.06.003
25. WHO. Cuban Experience With Local Production of Medicines, Technology Transfer and Improving Access to Health. (2015). Available online at: http://apps.who.int/medicinedocs/documents/s21938en/s21938en.pdf
26. Kaddar M, Milstien J, Schmitt S. Impact of BRICS' investment in vaccine development on the global vaccine market. Bull World Health Organ. (2014) 92:436–46. doi: 10.2471/BLT.13.133298
27. Milstien JB, Kaddar M. The role of emerging manufacturers in access to innovative vaccines of public health importance. Vaccine. (2010) 28:2115–21. doi: 10.1016/j.vaccine.2009.12.036
28. Milstien JB, Gaulé P, Kaddar M. Access to vaccine technologies in developing countries: Brazil and India. Vaccine. (2007) 25:7610–9. doi: 10.1016/j.vaccine.2007.09.007
29. GAVI Alliance. Saving Children's Lives and Protecting People's Health by Increasing Access to Immunisation in Poor Countries. (2014). Available online at: http://www.gavi.org/about/mission/ (accessed February 12, 2014).
30. Milstien J, Kaddar M. Managing the effect of TRIPS on availability of priority vaccines. Bull World Health Organ. (2006) 84:360–5. doi: 10.2471/BLT.05.028431
31. United Nations. World population prospects: the 2012 revision. Highlights and advance tables. Popul Dev Rev. (2013) 36:775–801. doi: 10.1111/j.1728-4457.2010.00357.x
32. African Economic Outlook . Regional Development and Spatial Inclusion. (2015) 38. Available online at: http://www.africaneconomicoutlook.org/fileadmin/uploads/aeo/2015/PDF_Chapters/Overview_AEO2015_EN-web.pdf
33. Jadhav SS, Gautam M, Gairola S. Emerging markets & emerging needs: developing countries vaccine manufacturers' perspective & its current status. Biologicals (2009) 37:165–8. doi: 10.1016/j.biologicals.2009.02.009
34. Ambrosetti Club. The Role of Research in Promoting Future Technologies and Innovations, and its Funding Mechanisms. (2017). Available online at: https://www.ambrosetti.eu/en/research-and-presentations/g7-report-2017/
35. Levy MS, O'Kennedy RD, Ayazi-Shamlou P, Dunnill P. Biochemical engineering approaches to the challenges of producing pure plasmid DNA. Trends Biotechnol. (2000) 18:296–305. doi: 10.1016/S0167-7799(00)01446-3
36. Josefsberg JO, Buckland B, Consulting B, Avenue J, Beach M. Vaccine process technology. Biotechnol Bioeng. (2012) 109:1443–60. doi: 10.1002/bit.24493
37. Gomez PL, Robinson JM, Rogalewicz JA. Vaccine manufacturing. In: Plotkin SA, Orenstein WA, editors. Vaccines . Elsevier Health Sciences (2012). p. 47–57.
38. Gerke C, Colucci AM, Giannelli C, Sanzone S, Vitali CG, Sollai L, et al. Production of a Shigella sonnei vaccine based on generalized modules for membrane antigens (GMMA), 1790GAHB. PLoS ONE (2015) 10:e0134478. doi: 10.1371/journal.pone.0134478
39. Levine HL, Lilja JE, Stock R, Hummel H, Jones SD. Efficient, flexible facilities for the 21st century. Bioprocess Int. (2012) 10:20–30. Available online at: https://bioprocessintl.com/manufacturing/facility-design-engineering/efficient-flexible-facilities-for-the-21st-century-337813/
40. GE Healthcare. Process economy and production capacity using single-use versus stainless steel fermentation equipment. Brochure. (2015) 29143348 A:1–12. Available online at: https://cdn.gelifesciences.com/dmm3bwsv3/AssetStream.aspx?mediaformatid=10061&destinationid=10016&assetid=17736
41. Rader RA, Langer ES. Bioprocess advances drive vaccine manufacturing in developing countries. Bioprocess Int. (2014) 12:10–5. Available online at: https://bioprocessintl.com/manufacturing/monoclonal-antibodies/bioprocess-advances-drive-vaccine-manufacturing-in-developing-countries-349767/
42. Scott C. Choosing between single-use and multiuse technologies: an interphex 2015 roundtable discussion. Bioprocess Int. (2015) 13:30–41. Available online at: https://bioprocessintl.com/2015/choosing-between-single-use-and-multiuse-technologies-an-interphex-2015-roundtable-discussion/
43. Easwaran BSS, Technology P, Since E, Easwaran SS, Manager T, Millipore M. The Pros And Cons Of Single - Use Bioreactors . (2016). p. 1–4. Available online at: http://www.pharmtech.com/pros?and?cons?single?use?bioreactors (Accessed September 14, 2016).
44. Faretra Peysson L. Tracing and control of raw materials sourcing for vaccine manufacturers. Biologicals. (2010) 38:352–3. doi: 10.1016/j.biologicals.2010.01.011
45. Rouby JC. Low levels of extraneous agents in vaccine starting materials. Biologicals (2010) 38:354–7. doi: 10.1016/j.biologicals.2010.01.004
46. Wikipedia. Project Commissioning. (2016). Available online at: https://en.wikipedia.org/wiki/Project_commissioning (accessed September 15, 2016).
47. Liesman P. Commissioning is an Effective Strategy for Evaluating Facility Performance and Maximizing Efficiencies. (2005). Available online at: http://www.facilitiesnet.com/energyefficiency/article/Commissioning-Making-it-Pay-Facilities-Management-Energy-Efficiency-Feature-3515 (accessed September 15, 2016).
48. WHO. Assessment of Medicines Regulatory Systems in Sub-Saharan African countries. Singapore: WHO (2010) pp 210. Available online at: https://www.who.int/healthsystems/Assessment26African_countries.pdf?ua=1
49. Stephenne J. Development and production aspects of a recombinant yeast-derived hepatitis B vaccine. Vaccine. (1990) 8:69–73. doi: 10.1016/0264-410X(90)90221-7
50. UNICEF. Generation 2030 Africa 2.0. (2017). Available online at: https://www.unicef.org/publications/files/Generation_2030_Africa_2.0.pdf
51. UNICEF SD. Guideline to Complete the 2017–2021 Immunization Forecast Spreadsheet. (2016). Available online at: http://www.unicef.org/supply/index_55506.html
52. WHO. Demand Forecasting for Routine Immunization. (2016). Available online at: http://apps.who.int/immunization_supply/introduction/forecasting/en/index.html (accessed September 17, 2016).
53. Carrier T. Technical Transfer, Scale up, Cost of Goods, Supply Chain and Engineering. . Siena: Module 3 Lecture Notes.
54. Jain S. A case for biopharmaceutical operations in low-cost countries to maximize return on investment. Bioprocess Int. (2013) 13:16–21. Available online at: https://bioprocessintl.com/business/case-biopharmaceutical-operations-low-cost-countries-maximize-return-investment/
55. The Bill and Mellinda Gates Foundation. Production Economics for Vaccines (2016). Available online at: https://docs.gatesfoundation.org/Documents/Production_Economics_Vaccines_2016.pdf
PubMed Abstract
56. Sampath PG, Mirza Z, Adachi K, Terry R. Local Production for Access to Medical Products: Developing a Framework to Improve Public Health (2011). p. 1–78.
57. MOH and MOI of Ethiopia. National Strategy and Plan of Action for Pharmaceutical Manufacturing Development in Ethiopia (2015–2025): Developing the Pharmaceutical Industry and Improving Access WHO (2015). Available online at: https://www.who.int/phi/publications/Ethiopia_strategy_local_poduction.pdf
58. African Union Commission. Pharmaceutical Manufacturing Plan for Africa: Business Plan . Addis Ababa: African Union and UNIDO (2012). Available online at: https://au.int/en/documents/20140320
59. Lambert PH, Podda A. Education in vaccinology: an important tool for strengthening global health. Front Immunol. (2018) 9:1134. doi: 10.3389/fimmu.2018.01134
60. Pralong A, Levine HL, Lilja J, Gaasvik ÅSA, Hummel H. Paradigm shift for vaccine manufacturing facilities: the next generation of flexible, modular facilities. Eng Life Sci. (2014) 14:244–53. doi: 10.1002/elsc.201400027
61. Analytics LP. White Paper (2015). Available online at: https://www.manufacturingchemist.com/news/article_page/From_egg_to_bioreactor_new_trends_in_vaccine_manufacture/82411 (Accessed September 17, 2016)
62. Takeda Pharmaceutical Manufacturing Plant Yaroslavl Russia (2013). Available online at: http://www.pharmaceutical-technology.com/projects/takeda-pharmaceutical-manufacturing-yaroslavl-russia/ (accessed October 3, 2016).
63. Takeda Pharmaceutical Manufacturing Plant Yaroslavl Russia (2016). Available online at: http://www.pharmaceutical-technology.com/projects/takeda-pharmaceutical-manufacturing-yaroslavl-russia/ (accessed September 28, 2016).
64. GSK's New Biopharmaceutical Plant Ulverston United Kingdom (2016). Available online at: http://www.pharmaceutical-technology.com/projects/gsks-new-biopharmaceutical-plant-ulverston/ (accessed September 28, 2016).
65. Tennant SM, MacLennan CA, Simon R, Martin LB, Khan MI. Nontyphoidal salmonella disease: current status of vaccine research and development. Vaccine. (2016) 34:2907–10. doi: 10.1016/j.vaccine.2016.03.072
66. MVP. Meningitis Vaccine Project (2017). Available online at: https://www.meningvax.org/ (accessed January 10, 2018).
Keywords: vaccine production, technologies, regulatory capacity, Africa, business model
Citation: Makenga G, Bonoli S, Montomoli E, Carrier T and Auerbach J (2019) Vaccine Production in Africa: A Feasible Business Model for Capacity Building and Sustainable New Vaccine Introduction. Front. Public Health 7:56. doi: 10.3389/fpubh.2019.00056
Received: 30 November 2018; Accepted: 25 February 2019; Published: 20 March 2019.
Reviewed by:
Copyright © 2019 Makenga, Bonoli, Montomoli, Carrier and Auerbach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Geofrey Makenga, [email protected]
† Present Address: Geofrey Makenga, National Institute for Medical Research (NIMR), Tanga, Tanzania Stefano Bonoli, Freelance Consultant, Siena, Italy Trent Carrier, CytoSen Therapeutics, Inc., Durham, NC, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

- How We're Funded
- Staff Directory
- Board of Directors
Modelling the Manufacturing Process for COVID-19 Vaccines: Our Approach
Recommended.
Researchers across the world are working flat out to develop and manufacture a vaccine for COVID-19 that can end what has been the worst pandemic in at least a century. To better understand how long it will take to develop a vaccine and manufacture it at scale, CGD is teaming up with Ariadne Labs and others to conduct expert interviews and build a tool that will generate probabilistic estimates for the likelihood of success of different types of vaccine and how long it could take to develop a vaccine, and to model how long it will take to produce this vaccine at sufficient scale to meet global demand. This will build on the work of other tools which track the stages of development of different vaccines, to estimate the overall probability of success for vaccine candidates and the time taken to reach large-scale manufacturing targets. In this blog post, we focus on the manufacturing part of this process (which we are undertaking with Bryden Wood ) and the steps we are taking to model the vaccine manufacturing timeline.
We are developing a system of interconnected models which represent global manufacturing capability from the start of clinical trials to secondary vaccine manufacture; that is, time from first human trials to finished product ready to be shipped. We will split the process of modelling vaccines research and development into three stages, as shown in figure 1: (i) modelling clinical trials until their approval, (ii) modelling the time it takes to scale up manufacturing, and (iii) modelling capacity to predict how long it will take to produce significant volumes of new vaccines.
Figure 1. Three stages of modelling the vaccine manufacturing process
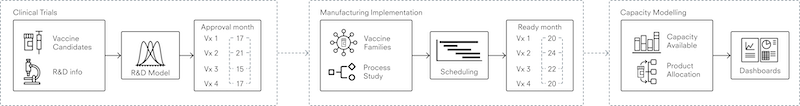
Source: Author's (Reader) own rendering

Data collection
There are currently more than 200 vaccine candidates against COVID-19, and more than 300 manufacturing facilities across the world that are capable of producing vaccines. With so many moving parts—research continuing at a speed never before seen, and large amounts of the information being politically or commercially sensitive—data collection at this scale is not an exact science. To provide a practical and realistic framework for data collection, we are grouping vaccines into seven different “platforms.” These platforms range from more traditional and established methods—such as an inactivated viral vaccine, where virus particles with no ability to produce disease are used to stimulate an immune response—to newer technologies including DNA and RNA vaccines which produce an immune response without viral particles.
Figure 2. Vaccine candidate platforms
In light of this, a model must be able to function with uncertainty and provide useful indications within practical limitations. In order to achieve this we will us use ranges and distributions for plausible estimates and put these into a Monte Carlo simulation . This is a technique that estimates the probability of different outcomes by running multiple simulations with different estimates randomly chosen from a plausible range, and then aggregating the results.
Where factual inputs exist, these go straight into the model. We are then collecting ranges or plausible estimates from a number of sources, all of which is currently underway. These include:
- expert interviews
- interviews with development and manufacturing organizations
- a survey of research groups with a vaccine candidate
- independent research on vaccine candidates
We will use this information to estimate how many doses of vaccines can be produced in a given period. We will then combine this information with WHO- published estimates of doses required to cover priority groups, support enough coverage to protect healthcare systems, reduce COVID-19- related mortality, and ensure fair access to vaccines. As and when the WHO estimates of phased global demand are updated, we will update accordingly in our model.
R&D and clinical trials
To estimate how long it will take to manufacture enough vaccines, we must first know which vaccines need to be manufactured. To estimate the probability of success for different vaccine platforms, we interviewed 17 experts with expertise in vaccine development and vaccine manufacturing. We also collected information on funding for vaccine candidates, and on plausible timelines. We then put this into a Monte Carlo simulation model, which thousands of times, with each run, vaccines randomly succeed and fail in line with their perceived probability of success. While the results of an individual run will not tell us a lot about the vaccine portfolio, by aggregating the results we should get a sense of both timelines and numbers of vaccines that could be approved.
Manufacturing and implementation
This part of the model aims to estimate the time required after a vaccine has been approved before commercial manufacture can begin. This is an important, yet perhaps publicly underdiscussed, factor in the supply of any drug. A drug substance that has been manufactured for early stage clinical trials is most often at “R&D scale,” produced in facilities with lab or small-scale equipment to supply doses in the thousands. Scaling up manufacturing to commercial scale adds complexity. Therefore, any attempt at commercial manufacture in the order of millions or billions of doses needs to consider the steps to scale up the process and adapt it for manufacture at a given location; under normal circumstances, this could take a few years.
The transition from R&D to manufacturing is typically carried out by a large multifunctional team and includes process development activities, design and construction activities, and quality assurance/regulatory activities. We have broken this transition down and simplified it to form a series of steps for any vaccine. For each step, we assigned a probabilistic distribution of durations around a value based on experience. The distributions tend to be right skewed (meaning things take longer than planned) because, unfortunately, there are constraints that stop things being done in less than a certain time but many potential delays that are without constraints. Steps can overlap; in particular, scale-up and preparation of manufacturing facilities can start earlier than normal, “at risk” (of failure of the candidate).
Strict regulations to ensure that manufacturing processes and plants meet current good manufacturing practice (CGMP) guidelines also add time to the transition from R&D to commercial manufacturing. The plant and equipment must be qualified, and processes shown to be safe and effective and licensed by regulators from all the jurisdictions where the vaccine will be used. Data for the product, process, and plant must be submitted to regulators in every jurisdiction where the vaccine will be used, and regulators vary in their requirements and, often, their areas of focus. Regulators can ask for clarifications or improvements, sometimes requiring additional data to be generated or even modifications to plant or process. There are some intellectual property (IP) issues when companies are trying to scale up manufacturing using Contract Manufacturing Organisations, a particular risk for newer companies in the business with newer manufacturing platforms, who don’t always have an established process for how to deal with IP with suppliers of clinical material (this has already been a problem for two candidates ). All of this can cause delay.
In addition to manufacturing capacity, such a large scale-up also requires sufficient quantities of auxiliary supplies, such as vials (or other primary containers), adjuvants, and in some cases, single-use bioreactors.
The overall sequence is well-understood, but there are many factors—such as type of vaccine and dosage form, manufacturing location, and supply markets—that affect how long each step takes. Through industry expert input, we are developing an initial model to represent each key stage of a vaccine’s journey from success in clinical trials to commercial manufacturing. The model incorporates possibilities that capacity already exists at sites, that existing capacity requires modification, or a new factory is required. This includes all steps of “qualification”—validation and testing processes that ensure equipment has been installed correctly and will perform as expected under real factory conditions. To account for variability, stages will be defined by ranges and distributions rather than single parameters.
Manufacturing capacity
The primary output from the overall model is an understanding of when global vaccine demand—defined here by the WHO targets—may be met. It is intended to collect global manufacturing capability for primary (manufacture of the active ingredient, for instance virus or mRNA) and secondary (manufacture of the dose form, for instance a vial of a liquid solution of the active ingredient) manufacturing, which would be used to build a picture of currently available capacity and potential future capacity for the suite of vaccine platforms and technologies. CEPI recently published an extensive survey-based assessment of available capacity This data will inform our understanding of global manufacturing capability for different vaccine platforms, and be used in the manufacturing model to enable us to calculate the timelines
Outputs from the R&D model are used a starting point, indicating when individual vaccine candidates may pass clinical trials—using a defined point in time as a reference point. Each successful vaccine will then pass through the manufacturing implementation model to determine a timeline for start of primary and secondary manufacture. Vaccines can then be allocated to available network capacity for the appropriate platform, computing the monthly dose production and cumulative dose count not only as a total, but also for each vaccine individually. This level of detail may be important given the possibility that different vaccines can be used for different populations (age ranges, for example) based on the level of immune response elicited. In comparing these production figures with the WHO targets, the time from the reference point to production milestones can be estimated.
Figure 3. Overview of the calculation stages and output of the manufacturing capacity model
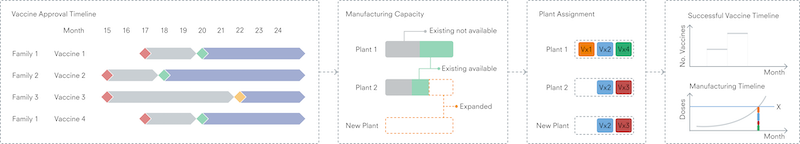
Usage and next steps
We are currently developing and testing this model based on the functionality outlined above. Once we’ve completed preliminary data collection and building the model, it will be run for parameters as defined by data collection and expert input. Our aim is to be able to give initial estimates of timelines of when vaccines would be widely available to the patient population.
We hope that with more collaboration and knowledge sharing, collective understanding of the challenges of manufacturing vaccines at scale will improve, allowing governments and institutional purchasers who are investing in vaccine development programs and manufacturing capacity to better identify potential gaps or shortcomings. Such models may also provide a picture of when a vaccine is likely to be in our hands.
CGD blog posts reflect the views of the authors, drawing on prior research and experience in their areas of expertise. CGD is a nonpartisan, independent organization and does not take institutional positions.
View the discussion thread.
More Reading
Ideas to action: independent research for global prosperity
© 2024 Center for Global Development | Privacy Notice and Cookie Policy
Sign up to get weekly development updates:
Loading metrics
Open Access
Peer-reviewed
Research Article
Expanding global vaccine manufacturing capacity: Strategic prioritization in small countries
Roles Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft
* E-mail: [email protected]
Affiliation Center for Global Health Science and Security, Department of Microbiology and Immunology, Georgetown University, Washington, District of Columbia, United States of America
Roles Investigation, Writing – original draft
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – original draft
Affiliations Department of Environmental Health and Engineering, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland, United States of America, Center for Health Security, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland, United States of America
- Sanjana Mukherjee,
- Kanika Kalra,
- Alexandra L. Phelan

- Published: June 29, 2023
- https://doi.org/10.1371/journal.pgph.0002098
- Peer Review
- Reader Comments
The COVID-19 pandemic highlighted significant gaps in equitable access to essential medical countermeasures such as vaccines. Manufacturing capacity for pandemic vaccines, therapeutics, and diagnostics is concentrated in too few countries. One of the major hurdles to equitable vaccine distribution was “vaccine nationalism”, countries hoarded vaccines to vaccinate their own populations first which significantly reduced global vaccine supply, leaving significant parts of the world vulnerable to the virus. As part of equitably building global capacity, one proposal to potentially counter vaccine nationalism is to identify small population countries with vaccine manufacturing capacity, as these countries could fulfill their domestic obligations quickly, and then contribute to global vaccine supplies. This cross-sectional study is the first to assesses global vaccine manufacturing capacity and identifies countries with small populations, in each WHO region, with the capacity and capability to manufacture vaccines using various manufacturing platforms. Twelve countries were identified to have both small populations and vaccine manufacturing capacity. 75% of these countries were in the European region; none were identified in the African Region and South-East Asia Region. Six countries have facilities producing subunit vaccines, a platform where existing facilities can be repurposed for COVID-19 vaccine production, while three countries have facilities to produce COVID-19 mRNA vaccines. Although this study identified candidate countries to serve as key vaccine manufacturing hubs for future health emergencies, regional representation is severely limited. Current negotiations to draft a Pandemic Treaty present a unique opportunity to address vaccine nationalism by building regional capacities in small population countries for vaccine research, development, and manufacturing.
Citation: Mukherjee S, Kalra K, Phelan AL (2023) Expanding global vaccine manufacturing capacity: Strategic prioritization in small countries. PLOS Glob Public Health 3(6): e0002098. https://doi.org/10.1371/journal.pgph.0002098
Editor: Madhukar Pai, McGill University, CANADA
Received: January 20, 2023; Accepted: June 1, 2023; Published: June 29, 2023
Copyright: © 2023 Mukherjee et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the manuscript and Supporting Information files.
Funding: This work was funded by the Carnegie Corporation of New York (GR-21-58414) (ALP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Manufacturing capacity for pandemic vaccines, therapeutics, and diagnostics is concentrated in too few countries. During the COVID-19 pandemic, safe and effective vaccines have been developed at a record-breaking speed. However, global accessibility and affordability of these vaccinations has been unjustly inadequate. Eight of every ten vaccination doses produced has gone to high income countries [ 1 ]. As of June 2022, more than 5∙22 billion people worldwide have received at least one dose of vaccine for COVID-19, accounting for 66∙3% of the world population [ 2 ]. However, these numbers mask the inequity when disaggregated by region or income status. Only 18% of people in low-income countries have received at least one dose, compared to 80% of people in high income countries. The disparity is even more stark when it comes to fully vaccinated populations or populations that have received booster doses. Vaccine procurement and manufacturing has been especially difficult for low- and middle-income countries (LMICs) [ 1 ]. This has been partly due to the scale of vaccine nationalism seen throughout the pandemic: in particular high-income countries using legal and policy measures, such as advance purchase agreements and export controls, to divert or prevent the global equitable distribution of vaccines. Global governance efforts to allocate global vaccine supply on public-health-need have not been successful in rectifying these disparities: the World Health Organization’s Strategy to Achieve Global COVID-19 Vaccination missed its goal of vaccinating 70% of the world’s population by mid-2022 [ 2 ], and while the COVAX facility has resulted in more than 1 billion doses shipped to over 144 countries [ 3 ], it was also vulnerable to acts of vaccine nationalism, including the Indian government halting export of vaccines, leaving the COVAX Facility without vaccines, and the global supply constraints resulting from high income countries such as US, the European Union (EU) and Canada buying up supply [ 4 – 6 ].
This situation is enabled by the fact that global vaccine manufacturing capacity remains largely in high income countries, who have benefited from imperialism and colonialism to have the resources for manufacturing capacities. This issue extends well beyond COVID-19. According to the 2022 WHO Vaccine Market Report, globally, only 10 manufacturers provide 70% of non-COVID-19 vaccine doses [ 7 ]. An estimated 55% of vaccine manufacturing capacity is in East Asia and 40% in Europe and North America. This leaves Africa and South America with less than 5% of worldwide vaccine manufacturing capacity [ 8 ]. Furthermore, current intellectual property laws magnify inequities in access to life-saving and essential medical products, discriminating against low-income economies resulting in poor health outcomes [ 9 ].
Such inequity demands reform. Global health institutions have sought to address this inequity in a variety of ways, including through WTO Members adoption of a TRIPS waiver [ 10 ], and in proposed text for technology transfer in the draft Pandemic Treaty [ 11 ], but reform must include equitably increasing global vaccine manufacturing capacity. We have a rare opportunity to “reflect, reimagine and reset the world in a more just manner” [ 12 ].
Given the urgency of the task, there are a range of strategies that have been proposed to prioritize increasing global manufacturing capacities. Regional equity is a critical consideration: ensuring appropriate geographic distribution of manufacturing supply. For instance, WHO is leading efforts to create an mRNA vaccine technology transfer hub in South Africa to increase mRNA vaccine production in LMICs [ 13 ]. One additional strategy is the prioritization of building capacity in small population countries. The goal of this strategy is to directly limit vaccine nationalism by easily fulfilling national vaccine need well below full manufacturing capacity, removing the incentive to constrain global or regional supply. Thus, this study aims to assess overall global vaccine manufacturing capacity and identify small population countries with the capacity to manufacture vaccines, potentially contributing to global vaccine supplies in future health emergencies. Furthermore, we present a thorough overview of the existing global vaccine manufacturing capacity. Although previous studies and databases have collated data on countries and companies possessing vaccine manufacturing capabilities [ 14 – 16 ], our study is pioneering in its inclusion of information pertaining to vaccine technology/platform, the various stages of vaccine production, and the history of WHO pre-qualification.
Study design
We designed a cross-sectional study to assess global vaccine manufacturing capacity as of March 1, 2022. Furthermore, we identified countries with small populations, defined as less than 15 million people, which had the capacity and capability for vaccine manufacturing, coding for different stages of the vaccine development and manufacturing pipeline ( Fig 1 ). We then identified countries with very small populations, defined as less than 5 million people, regardless of existing vaccine manufacturing capacity as potential sites for vaccine manufacturing capacity to be established. Since this study does not involve clinical data or human participants, it was not submitted for ethics review.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
Vaccine manufacturing processes are highly complex and differ based on the type of vaccine manufacturing platform used for production. The upstream processing, downstream processing and formulation steps vary significantly based on biologic-derived vaccine manufacturing processes (inactivated, live-attenuated, subunit and viral vector vaccines) and chemical-based RNA vaccine (mRNA) manufacturing processes. Fill, finish, packaging, quality assurance and quality control steps, however, occur in the same manner across different vaccine manufacturing platforms. Information for generating figure adapted from the literature [ 8 , 17 ].
https://doi.org/10.1371/journal.pgph.0002098.g001
Data collection and extraction
We conducted an online search on country vaccine manufacturing capacity including on COVID-19 vaccine development and production activity. Information was extracted from: 1) academic publications and reports, 2) websites and reports from international organizations and non-governmental organizations, 3) websites and reports from private pharmaceutical companies, academic or public institutions with vaccine manufacturing and distribution experience and, 4) local and international news media reports. The detailed methodology is provided in S1 Text and the list of variables collected for each country is available in S1 Table .
Data analysis
Data were managed using Airtable (Airtable, San Francisco, CA, USA) and MS Excel. ‘Small countries’ were defined as countries with a population less than 15 million, whereas ‘very small countries’ were defined as countries with a population of less than 5 million. A country was defined as having the capacity for vaccine production if it contained at least one documented manufacturing facility with prior/current vaccine production activity identified in our search ( S1 Data ).
We also identified countries with WHO prequalified vaccines. WHO’s vaccines prequalification is used by United Nation agencies, such as UNICEF, for the procurement of life-saving vaccines for immunization programs for a range of diseases, including influenza (seasonal and pandemic). By prequalifying vaccines, WHO applies “international standards to assess the safety, efficacy and quality of vaccines produced” [ 18 ]. To this end, WHO conducts regular manufacturing facility site inspections for good manufacturing practice (GMP). Additionally, vaccine prequalification also depends on close collaboration with a country’s National Regulatory Authority (NRA) considered “functional”- defined as having been WHO-listed as operating at a minimum of maturity level 3 [ 19 ]. NRAs conduct regulatory oversight of WHO prequalified vaccines by conducting facility inspections, evaluating vaccine clinical performance manufactured in the country and vaccine post-marketing surveillance. Manufacturers can apply for “vaccine prequalification” only if the country NRA is “functional”. While COVID-19 vaccines are not currently on WHO’s list of priority products for UN prequalification, we use this variable as a proxy to identify small population countries that can produce vaccines for global COVID-19 and other future pandemic threats immunization programs. Since these countries have functional NRAs and have manufacturing sites following GMP, COVID-19 vaccines produced in these countries can potentially be used for procurement by UN agencies and other organizations.
All statistical analyses were carried out using MS Excel. ArcGIS Pro version 2∙9 (Esri Inc, Redlands, CA, USA) was used for data visualization and the World Countries map package (Source: Esri Data and Maps) [ 20 ] was used as the base map.
Small population countries with vaccine manufacturing capacity
Our analysis identified a total of 43 countries with vaccine manufacturing capacity ( S2 Table ); 5 were in the Eastern Mediterranean Region, 17 in the European Region, 3 in the African Region, 7 in the Region of the Americas, 7 in the Western Pacific Region and 4 in the South-East Asia Region. However, when stratified by population size, only 12 countries with small populations were identified to have vaccine manufacturing capacity ( Fig 2 ). Of these 12 countries, 75% (9/12) are in the European Region and one each in the Region of Americas, Western Pacific Region, and Eastern Mediterranean Region. We did not identify any small population countries with vaccine manufacturing capacity in the African Region and South-East Asia Region.
Small population countries with vaccine manufacturing capacity (n = 12) are highlighted in blue, while those with populations >15 million are highlighted in green. Countries with unknown or absent vaccine manufacturing capacity are shaded gray. The figure also highlights a country’s capacity to manufacture vaccines using various vaccine manufacturing platforms (inactivated vaccines, live-attenuated vaccines, subunit vaccines, virus-like particle vaccines, viral-vector vaccines, and RNA based vaccines) and the vaccine manufacturing steps present (‘Bioprocessing and formulation’ capacity, ‘Fill, finish, and packaging’, ‘Unknown/Unclear’). World Countries map package (Source: Esri Data and Maps) was used as the base map [ 20 ].
https://doi.org/10.1371/journal.pgph.0002098.g002
The 12 countries identified in our analysis are Austria, Azerbaijan, Belgium, Bulgaria, Cuba, Czech Republic, Denmark, Serbia, Singapore, Sweden, Switzerland, and Tunisia. Vaccines manufactured in these countries vary widely, with 6 countries having portfolios of producing vaccines for 3 or more diseases (Belgium, Bulgaria, Cuba, Denmark, Serbia, and Sweden) ( S2 Table ). Currently, COVID-19 vaccines are manufactured in 4 countries with small populations (Belgium, Cuba, Czech Republic, and Switzerland) ( Table 1 ); a COVID-19 vaccine candidate manufactured in Austria was terminated. Two COVID-19 vaccines are currently manufactured in Belgium, including Pfizer-BioNTech BNT162b2 COVID-19 Vaccine and Oxford/AstraZeneca (ChAdOx1-S [recombinant]) COVID-19 vaccine. Belgium has both ‘Bioprocessing & Formulation’ and ‘Fill, Finish & Packaging’ capacity for manufacturing Pfizer-BioNTech COVID-19 vaccines. Additionally, manufacturing facilities in Belgium also carry out ‘Bioprocessing & Formulation’ of Oxford/AstraZeneca COVID-19 vaccines. Czech Republic and Switzerland, both in the European Region, also have facilities manufacturing COVID-19 vaccines. Cuba, present in the Region of the Americas, manufactures and produces two protein subunit COVID-19 vaccines: Soberana 2 FINLAY-FR-2 vaccine and Abdala CIGB-66 vaccine. Since both manufacturers of these Cuban vaccines operate in a “closed cycle” platform, these facilities have the capacity and capability to carry out the complete development cycle of a vaccine from research and development to production and marketing [ 21 ]. Our assessment did not identify any small population countries manufacturing COVID-19 vaccines in the other four WHO regions.
For each country, the type of COVID-19 vaccine produced, the names of manufacturing facilities, steps of vaccine production and vaccine manufacturing platform are included.
https://doi.org/10.1371/journal.pgph.0002098.t001
Vaccine manufacturing platforms in small population countries
To identify countries with the potential to repurpose existing vaccine manufacturing platforms and installed vaccine manufacturing bases for expansion of COVID-19 vaccine production, we assembled a list of all countries with vaccine manufacturing capacities and the platforms used to produce vaccines for any infectious disease ( S2 Table ). Additionally, manufacturing capacity also depends on the vaccine manufacturer’s capacity and capability to manufacture the drug substance or active pharmaceutical ingredient (Bioprocessing & Formulation) and provide fill-and-finish services ( Fig 1 ). A full list of a country’s “Bioprocessing & Formulation” and “Fill, Finish and Packaging” capacity is provided in S2 Table .
Overall, the 43 countries identified in this study had a wide range of vaccine manufacturing platforms; inactivated vaccine platforms were the most common (n = 26) followed by subunit vaccine platforms (n = 23), viral vector vaccine platforms (n = 21) and live-attenuated vaccines (n = 20). Eight countries were identified to have and/or potentially have the capacity to manufacture mRNA vaccines.
In the 12 small population countries, subunit vaccine platforms (n = 6), inactivated vaccines platforms (n = 5) and live-attenuated vaccine platforms (n = 5) are the most common ( Fig 2 ). Additionally, 25% (3/12) of the small population countries have the capacity to manufacture/potentially manufacture RNA based mRNA vaccines; these countries are Austria, Belgium, and Switzerland, all of which are present in the European Region. Belgium is the only country which has manufacturing facilities utilizing six vaccine manufacturing platforms. Manufacturers utilizing subunit vaccine platforms, the platform most commonly present in the small population countries identified in this study and which have the potential to be repurposed for COVID-19 production, are present in Belgium, Bulgaria, Cuba, Czech Republic, Serbia, and Singapore. Majority of the countries with subunit vaccine platforms are present in the European Region (4/6), along with one each in the Region of the Americas (Cuba) and the Western Pacific Region (Singapore). While Singapore produces active pharmaceutical ingredients for different vaccines using the subunit vaccine platform, it does not have fill-finish capacity and is currently not producing finished vaccines ( S2 Table ). Vaccine manufacturers utilizing viral vector vaccines are present in Belgium and Denmark, which is another potential platform that can be repurposed to expand COVID-19 vaccine production.
A list of COVID-19 vaccines, the vaccine manufacturing platforms used for their production and the countries producing these vaccines are provided in S3 Table . While most of these vaccines are RNA-based mRNA vaccines (n = 6), vaccines using subunit vaccine platforms (n = 4) and viral vector vaccine platforms (n = 4) are also common.
Countries with WHO prequalified vaccines
Overall, only 22 countries have manufacturing facilities with prior/current WHO prequalified vaccines ( Fig 3 ). 45∙4% (n = 10) of these countries are present in the European Region, 18∙2% (n = 4) are in the Region of the Americas, 18∙2% (n = 4) are in the Western Pacific Region, and 13∙6% (n = 3) are in the South-East Asia Region. Senegal is the only country in the African Region producing a prequalified vaccine (Yellow Fever vaccine). There are no countries in the Eastern Mediterranean Region producing WHO prequalified vaccines. Of the 22 countries, 5 small population countries have manufacturing facilities producing at least one prequalified vaccine—Belgium, Bulgaria, Cuba, Denmark, and Sweden ( Fig 3 ). 80% of these countries (n = 4) countries are in the European region. None of these 5 countries produce WHO prequalified influenza vaccines.
Small population countries with manufacturers producing a WHO prequalified vaccine (n = 5) are highlighted in pink, while those with populations >15 million are highlighted in blue. World Countries map package (Source: Esri Data and Maps) was used as the base map [ 20 ].
https://doi.org/10.1371/journal.pgph.0002098.g003
Very small population countries regardless of vaccine manufacturing capacity
To identify very small population countries where vaccine manufacturing capacity and drug regulatory system capacity can be built, we compiled a list of countries with populations less than 5 million. While these countries may not currently have existing vaccine manufacturing or regulatory capacity, countries from this list can be identified to potentially serve as vaccine manufacturing hubs if resources and attention are directed to initiate the development of vaccine manufacturing facilities with GMPs and establishing medical product regulatory systems. By geographically diversifying vaccine manufacturing in such countries with very small populations, security, and stability to large parts of the world can be ensured during future health emergencies.
We identified 73 countries with populations less than 5 million ( S1 Fig ). None of these countries were identified to have existing vaccine manufacturing capacity in our analysis. When stratified by WHO region, 4 countries were in the Eastern Mediterranean Region, 20 countries in the European Region, 15 countries in the African Region, 15 countries in the Region of the Americas, 16 countries in the Western Pacific Region and 3 in the South-East Asia Region.
Preempting vaccine nationalism can take direct or indirect paths. The ongoing negotiations for a Pandemic Treaty is an opportunity to directly address vaccine nationalism through legally binding obligations. This may include limiting the use of advance purchase agreements (APAs) and export controls during a pandemic, such as prohibiting their use, or imposing maximum limits for both, based on real time production capacity or public health justifications. Prohibiting the use of APAs and export controls is unlikely to be politically palatable, domestically, or internationally, while imposing maximum limits alone is unlikely to be sufficient to minimize the use of export controls or APAs. In addition, to be effective, the latter, along with the imposition of maximums, would require reporting, verification, and adjudication processes. Such a compliance mechanism may be feasible under a new international legal instrument such as the Pandemic Treaty but will require championing from negotiating Member States.
The ongoing Pandemic Treaty negotiations are also an opportunity to indirectly address vaccine nationalism by establishing legally binding obligations on States Parties to build global capacities for vaccine research, development, and manufacturing capacities. These efforts should be supported by an equity-focused and strategically informed approach to minimize the impacts of vaccine nationalism (especially in the absence of direct obligations) that may persist even when regional capacities have been developed.
One possible strategy for the short to medium term is to not only identify countries with existing manufacturing capacities that can be developed, but to map that against countries with small populations to prioritize capacity building and pre negotiated commitments. The goal of this strategy is to minimize the potential impact of vaccine nationalism on regional supply: even if a country with dedicated diagnostic, vaccine, and therapeutic production were to fulfill its domestic need first, with a small population, it will be rapidly fulfilled with minimized impact on regional distribution.
In our results, 12 countries have met the criteria of having current vaccine manufacturing capacity and populations below 15 million. However, the number and types of vaccines manufactured by these countries vary widely. For instance, while manufacturers in Belgium are producing more than 10 vaccines using six different vaccine manufacturing platforms, Tunisia, present in the Eastern Mediterranean Region, currently has the capacity to only produce ‘bacillus Calmette−Guérin’ (BCG) vaccines at the Pasteur Institute of Tunis (IP Tunis). The manufacturing hubs identified in our study are in the European Region, Region of Americas, Western Pacific Region, and Eastern Mediterranean Region. Importantly, we did not identify any small population countries with vaccine manufacturing hubs in the African Region and South-East Asia Region, highlighting the disparity in regional vaccine producers.
Since production capacity varies widely among vaccines and vaccine manufacturing platforms, the potential for capacity expansion is not spread evenly amongst global vaccine makers. RNA-based vaccines are a novel vaccine manufacturing platform and used almost exclusively to produce COVID-19 vaccines. In our study, we identified Belgium, Switzerland, and Austria as the only small population countries with capacity for production of mRNA vaccines, all three of which are in the European region. The Pfizer facility in Puurs, Belgium has the capacity to carry out both “Bioprocessing & Formulation” and “Fill, Finish and Packaging” of the Pfizer-BioNTech COVID-19 Vaccine and has the capacity to produce approximately 1∙3 billion shots annually [ 22 , 23 ]. Meanwhile, Novartis has the capacity to conduct “Fill, Finish and Packaging” of the Pfizer-BioNTech COVID-19 Vaccine at its Stein, Switzerland facility [ 24 ], and Lonza manufactures the active pharmaceutical ingredient for the Moderna mRNA-1273 vaccine at its manufacturing site in Visp, Switzerland [ 25 ]. Since RNA-based vaccine platforms are new, repurposing existing facilities may not be possible as manufacturing of the mRNA vaccine drug substance requires installation of commercial-grade mRNA and lipid nanoparticles (LNPs) GMP manufacturing capacity [ 8 ]. While industry stakeholders have estimated that vaccine manufacturing capacity building can take three-to-four years [ 8 , 26 ], efforts have been made to successfully accelerate this timeline. For instance, Pfizer established mRNA manufacturing capacity in its Puurs, Belgium and Kalamazoo, USA facilities in an estimated 100 days which included building formulation laboratories and designing industrial processes for mRNA vaccine production [ 22 ]. Additionally, in June 2021, WHO announced that it was supporting the development of an mRNA technology transfer hub in South Africa in collaboration with Biovac, Afrigen Biologics and Vaccines, a network of universities and the Africa Centres for Disease Control and Prevention (CDC) [ 27 ]. Seven months after the establishment of the mRNA hub, in January 2022, Afrigen was reported to have produced the first batch of drug product formulation for an mRNA vaccine candidate [ 28 ]. Additionally, 15 companies are currently in the process of learning to make such mRNA vaccines at Afrigen [ 29 ]; two of these institutes were identified in our study as they are present in small population countries–Torlak Institute in Serbia and Pasteur Institut Tunis in Tunisia. A report also identified more than 100 manufacturers across Asia, Africa and Latin America with the capacity to manufacture mRNA vaccines based on the manufacturers’ current capacity to manufacture sterile injectables [ 30 ]. The establishment of mRNA vaccine manufacturing hubs globally will allow for greater and diversified vaccine manufacturing capability resulting in strengthening of regional health security and responding more equitably to future pandemics.
Since many manufacturing facilities globally employ subunit and viral vector vaccine platforms for vaccine production, rapid and effective repurposing using existing installed facilities and bioreactors is possible. Indeed, estimates suggest that 1–5% of existing subunit and viral vector vaccine manufacturing capacity can be repurposed to expand the production of COVID-19 vaccines [ 8 ]. Additionally, protein subunit vaccines may have advantages over other types of vaccines including relatively inexpensive production, suitability for people with compromised immune systems, and stability at a broad range of temperatures making vaccine supply chain and logistics (vaccine storage, distribution, handling, and management) easier [ 31 – 33 ]. As shown in S3 Table , currently, at least three COVID-19 vaccines employ subunit vaccine platforms; Novavax NVX- CoV2373 protein-based vaccine is currently being manufactured in multiple countries. In our results, we identified six small population countries with the capacity to manufacture subunit vaccines—Belgium, Bulgaria, Cuba, Czech Republic, Serbia, and Singapore. Since these countries have existing subunit vaccine manufacturing capacities, facilities in these countries can be repurposed to produce subunit COVID-19 vaccines. Indeed, to develop the Soberana 2 FINLAY-FR-2 vaccine, the Finlay Institute of Vaccines in Havana, Cuba repurposed its existing ‘conjugate’ vaccine technology to produce the Soberana 2 COVID-19 vaccine and has the capacity to produce 10 million Soberana 2 doses per month [ 31 ]. Additionally, we identified two small population countries (Belgium and Denmark) with the capacity to manufacture viral vector vaccines, which was identified as another platform with the potential to be repurposed for expanding COVID-19 vaccine production. We also identified numerous small population countries with inactivated and live-attenuated vaccine platforms; however, repurposing these facilities for COVID-19 vaccine production may not be possible due to specific viral containment requirements for handling live viruses [ 8 ].
A country’s vaccine manufacturing capacity is also dependent on the steps of vaccine production available at facilities. While centralized production involves manufacturing vaccines and other health products at scale in centrally located sites, distributed manufacturing involves the production of different components of the vaccine at different geographical locations and facilities [ 34 ]. For example, while Lonza produces the active pharmaceutical ingredient for the Moderna mRNA-1273 vaccine in Visp, Switzerland, CordenPharma produces lipid nanoparticles required for the Moderna mRNA-1273 vaccine in Boulder, Colorado, USA and the final fill-finish steps are carried out by Catalent in Bloomington, Indiana, USA [ 25 ]. Such a scenario is also seen in Singapore, the only small population country in the Western Pacific Region with vaccine manufacturing capacity, where only active ingredients are manufactured and not finished vaccines [ 35 ]. However, efforts are being made to include Singapore as a producer of finished vaccines, to expand vaccine manufacturing capacity in the region. In 2020, Thermo Fisher Scientific Inc. announced plans to develop two new sterile filling lines in Singapore to expand Singapore’s fill-finish capacity for vaccines and other products [ 36 ]. Improving a region’s capacity to produce both the active ingredient for vaccines and finished vaccines is critical for responding to future pandemic threats.
Throughout the course of the COVID-19 pandemic, rapid scale up of manufacturing capacity has been observed to meet the global demand for COVID-19 vaccines. This included formation of more than 150 partnerships with contract development and manufacturing companies (CDMOs) and other vaccine manufacturers, globally [ 8 ]. Despite this, vaccine manufacturing and supply of COVID-19 vaccines remains disproportionate and inequitable. Based on our results, we have identified multiple small population countries with the capacity to manufacture different components of vaccines using various vaccine manufacturing platforms, although a majority of these are in the European region. An efficient strategy to increase COVID-19 vaccine supply is for innovator companies to enter agreements to transfer vaccine technology and knowledge to manufacturers in such small population countries.
We also identified five small population countries which have a history of manufacturing WHO prequalified vaccines, four of which are in the European Region (Belgium, Bulgaria, Denmark, and Sweden) and one in the Region of the Americas (Cuba). Vaccines manufactured in these countries can be used for procurement of vaccines by UN agencies for responding to future pandemic threats as these countries have “functional” NRAs and have manufacturing sites following GMP. The presence of a competent, effective, and transparent regulatory authority within a country is crucial to ensure access to safe and effective medical products to consumers and protect consumers from substandard or falsified medical products. Without the presence of functional NRAs, countries cannot assess whether vaccines and other health products being manufactured locally meet approved quality control standards [ 18 , 37 ]. It is reported that, of the 194 Member States, only 26% (50 countries) contain mature regulatory agencies, while 144 countries have suboptimal regulatory systems [ 38 ]. Thus, in the short to medium term, expansion of COVID-19 vaccine production should prioritize manufacturers in small population countries with functional NRAs, while long-term goals to expand regional vaccine manufacturing capacity should also include elements to strengthen regulatory capacity of countries.
In addition to the availability of vaccine manufacturing capacity in strategic regional hubs, it is crucial to bolster the vaccine input supply chain which includes raw materials necessary for production in all stages of vaccine manufacturing. Examples of such critical raw materials include syringes, glass vials, bioreactor bags, filters, and cell culture media. During the COVID-19 pandemic, numerous countries instituted export restrictions on such key raw materials, significantly disrupting global supply chains [ 39 , 40 ]. The Pandemic Treaty is an opportunity for States to also address export bans, restrictions and other trade-related measures of crucial goods required for production of essential medical countermeasures and identify countries with small populations that can produce such essential raw materials during future pandemics.
To improve access and availability of drugs, vaccines, and other medical products during health emergencies, a comprehensive policy toolkit is required that employs both long-term and short-term strategies. Lessons learned from the COVID-19 pandemic have highlighted the need to establish and strengthen national and regional vaccine manufacturing capacity in LMICs, with many countries pledging to do so. For instance, in September 2022, the African Union called for a new for a ‘New Public Health Order for Africa’ to expand “manufacturing of vaccines, diagnostics, and therapeutics to democratize access to life-saving medicines and equipment” [ 41 ]. Improving the overall vaccine manufacturing ecosystem, including establishing vaccine manufacturing hubs and strengthening medical product regulatory systems, would likely fall under long-term strategies, given the significant time and investment required to build research & development infrastructure, train workforce, and create financing arrangements. Thus, in this study, we propose that small population countries with vaccine manufacturing capacity and functional NRAs can aid in global vaccine supply by supporting vaccine procurement for facilities like the COVAX facility. Furthermore, procurement commitments by UN agencies and export of vaccines can stimulate private sector investment in vaccine and therapeutics manufacturing in the small population countries identified in this study, aiding in the establishment of a financing mechanism to bolster in-country manufacturing efforts. However, as seen during the COVID-19 pandemic, vaccine export restrictions and vaccine nationalism was a major barrier to vaccine access. Indeed, during the pandemic, the EU placed COVID-19 vaccine export restrictions and required authorization for the export of vaccines outside the EU [ 6 , 42 ]. This is particularly important, as four of the five small countries with both vaccine manufacturing capacities and functional NRAs identified in our study, are EU member states. To tackle this, policy proposals to limit the use of export restrictions during future health emergencies have been introduced, with the EU highlighting that vaccine producing countries “should be ready to export a fair share of their domestic production” [ 43 ]. Thus, the Pandemic Treaty provides a unique opportunity to negotiate such obligations from vaccine producing countries, including small vaccine manufacturing countries, to help improve vaccine access, globally.
Conclusions
The inequitable distribution of lifesaving COVID-19 vaccines has resulted in vastly different COVID-19 responses and outcomes. While many high income and upper-middle income countries have vaccinated their populations against the SARS-CoV-2 virus, many resource-constrained countries have been unable to secure vaccines for their populations and are left vulnerable to the continuing devastating impact of this disease. A pandemic treaty which addresses the issue of inequitable access to essential medical countermeasures is crucial to respond to future emerging infectious disease threats. By outlining legally binding obligations for State Parties to build global capacities for vaccine research, development, and manufacturing in strategic locations in all WHO regions, the treaty would provide an opportunity to ensure that future pandemic preparedness and response is centered on principles of equity and justice.
Supporting information
S1 text. methods..
https://doi.org/10.1371/journal.pgph.0002098.s001
S1 Fig. Countries with very small populations (< 5 million).
While these countries may not have existing vaccine manufacturing capacity or regulatory systems, countries from this list can be identified to potentially serve as vaccine manufacturing hubs, while ensuring geographical diversity. World Countries map package (Source: Esri Data and Maps) was used as the basemap [ 20 ]. The countries are: Albania, Andorra, Antigua and Barbuda, Armenia, Bahamas, Bahrain, Barbados, Belize, Bhutan, Bosnia and Herzegovina, Botswana, Brunei Darussalam, Cabo Verde, Comoros, Cook Islands, Croatia, Cyprus, Djibouti, Dominica, Equatorial Guinea, Eritrea, Estonia, Eswatini, Fiji, Gabon, Gambia, Georgia, Grenada, Guinea-Bissau, Guyana, Iceland, Ireland, Jamaica, Kiribati, Kuwait, Latvia, Lesotho, Lithuania, Luxembourg, Maldives, Malta, Marshall Islands, Mauritania, Mauritius, Federated States of Micronesia, Monaco, Mongolia, Montenegro, Namibia, Nauru, New Zealand, Niue, North Macedonia, Palau, Panama, Qatar, Republic of Moldova, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines, Samoa, San Marino, Sao Tome and Principle, Seychelles, Slovenia, Solomon Islands, Suriname, Timor-Leste, Tonga, Trinidad and Tobago, Tuvalu, Uruguay, Vanuatu.
https://doi.org/10.1371/journal.pgph.0002098.s002
S1 Table. Detailed information on variables collected per country.
https://doi.org/10.1371/journal.pgph.0002098.s003
S2 Table. Global vaccine manufacturing capacity as of March 1, 2022.
The list includes vaccine portfolios and vaccine manufacturing procedures in each country.
https://doi.org/10.1371/journal.pgph.0002098.s004
S3 Table. List of COVID-19 vaccines manufactured by the 43 countries identified in our study as of March 1, 2022.
The vaccine manufacturing platforms used for their production and the countries producing these vaccines are included in this list.
https://doi.org/10.1371/journal.pgph.0002098.s005
S1 Data. Raw dataset of countries and companies with vaccine manufaturing capacity identified in our study as of March 1, 2022.
https://doi.org/10.1371/journal.pgph.0002098.s006
- View Article
- Google Scholar
- PubMed/NCBI

OR WAIT null SECS
- Editorial Info
- Editorial Contacts
- Editorial Advisory Board
- Do Not Sell My Personal Information
- Privacy Policy
- Terms and Conditions

© 2024 MJH Life Sciences ™ and Pharmaceutical Technology . All rights reserved.
GMPs Guide COVID-19 Vaccine Manufacturing
Recent industry guidance aims to anchor rapid COVID-19 vaccine development in good manufacturing practice protocols.
Major bio/pharmaceutical companies and contract service providers leading the charge to bring COVID-19 vaccines to market are familiar with established good manufacturing practices (GMPs). To support manufacturers in the rush to develop a vaccine, FDA, in June 2020, released industry guidance (1) highlighting the development and licensure of COVID-19 vaccines.
Pharmaceutical Technology October 2020 Regulatory Sourcebook
For bio/pharmaceutical companies, quality must be more than a vague concept. Quality practices impact all aspects of drug development and manufacturing. In the Pharmaceutical Technology Regulatory Sourcebook , experts share insights and best practices on a range of quality issues including the following:
Enhancing Process Validation for Sterile Liquid and Freeze-Dried Forms: Part 1
AFI representatives of the process validation working group explore and define key elements for an enhanced approach to process validation for sterile liquid and freeze-dried forms.
Enhancing Process Validation for Solid Oral Dosage Forms: Part 2
AFI representatives of the process validation working group explore and define key elements for an enhanced approach to process validation for solid oral dosage forms.
Due Diligence Assessment of CMC Activities
For planned acquisitions or licensing, a careful analysis of CMC factors is vital to ensure no problem areas are overlooked.
Do Pharmacopoeias Inadvertently Facilitate Data Integrity Violations?
Analysts should understand how a monograph, together with the associated general notices and general chapters, relate to their responsibilities under good manufacturing practices.
Tackling Cybersecurity Challenges in Legacy Systems
Ongoing and emerging cyber-risks to legacy operational technology systems in bio/pharmaceutical manufacturing must be addressed.
Ending ‘Magical Thinking’ in Compounding
Consistent product quality requires a clear understanding of the essence of CGMPs. Experts fear that the message is not always getting through to compounders.
Viral Vector API Characterization of Product-Related Impurities
FDA’s final CMC guidance sets expectations for manufacturing and quality for human gene therapy INDs. This article reviews existing analytical applications, focusing on viral vector characterization of impurities.
Viewpoint: Precompetitive Collaboration Drives Pharma Industry Innovation
The IQ Consortium marks a decade of impactful innovation of collaboration through a network of pharmaceutical companies driving change for the bio/pharmaceutical industry.
Strong Quality Culture: A How-To for Busy Managers
Building employee participation and forming good habits contribute to a company-wide quality culture that pays off.
To read these articles, view the Pharmaceutical Technology October 2020 Regulatory Sourcebook online at www.PharmTech.com/journals/pharmaceutical-technology/pharmaceutical-technology-10-15-2020 .
For example, FDA noted in the guidance that no “accepted surrogate endpoints” to which clinical testing can aim currently exist. Without these target endpoints, there is likely no ability to form a reasonable prediction that a potential COVID-19 vaccine would demonstrate a clinical benefit. FDA has recommended that any vaccine development program should, therefore, follow a traditional route to approval, meaning the development programs should demonstrate the vaccine candidate’s safety and efficacy in protecting patients from infection by the SARS-CoV-2 virus and/or clinical disease that results from infection.
“The FDA Guidance highlights the pertinent CGMP [current good manufacturing practice] considerations for all vaccine manufacturing and serves as a reminder of statutory GMP expectations for COVID-19 vaccines specifically,” says Karolyn Gale, senior director, regulatory affairs, Emergent BioSolutions, a contract development and manufacturing organization (CDMO). The CDMO was granted a $628-million task order under an existing contract with the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health & Human Services, for the rapid, large-scale production of leading COVID-19 vaccine candidates through 2021. Emergent will manufacture vaccine drug substance at its Baltimore Bayview facility in Maryland and will manufacture drug product and conduct fill/finish operations at its facilities in Baltimore, Camden, and Rockville, MD (2). The company currently has deals with Johnson & Johnson and AstraZeneca to manufacture their respective COVID-19 vaccine candidates (3).
“As with all FDA guidance, it gives sponsors and manufacturers clearer insight into the FDA’s current thinking on a topic and how they are leveraging and/or interpreting, in this instance, standing 21 [ Code of Federal Regulations ] CFR Part 601 regulations for chemistry, manufacturing, and controls (CMC) in the COVID-19 vaccine development landscape,” says Gale.
For drug substance and drug product manufacturing, the guidance emphasizes that data be provided showing adequate control over all source material used in the manufacturing of the vaccine, including the history and qualification of cell banks and virus banks as well as the identification of all animal-derived materials that are used for cell culture and virus growth.
“It is recognized that this guidance will not cover all the considerations necessary to satisfy all statutory and regulatory requirements for licensure. It further acknowledges that in some instances, platform knowledge could be leveraged, the specifics of this potential and the science they are based upon must be considered carefully, thus highlighting the importance of early engagement with the agency further,” Gale states.
Recognizing the challenges
Three important challenges to overcome include being able to validate currently unproven platform technologies, demonstrating that the vaccine confers protection against SARS-CoV-2 infection/COVID-19 disease, and targeting an appropriate vaccine design (4).
Vaccines being developed with unvalidated, unproven platform technologies (e.g., DNA- and messenger RNA-platform based vaccines) raise uncertainties about long-term safety and the ability to confer long-term immunity against COVID-19. These newer platform technologies may offer unique advantages (e.g., accelerating timelines), but vaccines developed from them may face greater regulatory scrutiny than vaccines coming from traditional established technologies.
In designing a vaccine, developers must establish a sufficient “indicator of protection” (4) (e.g., antibody response levels) to first demonstrate that the vaccine protects against COVID-19 and take into account the SARS-CoV-2 virus’ mutation potential, which can impact the vaccine’s relevance and delay launch.
Knowledge and technology transfer
Ensuring that the overall regulatory strategy for a vaccine product is understood by a CDMO facility/partner chosen by a vaccine developer is one of the biggest regulatory challenges currently, says Gale. This transfer of knowledge and strategy is especially challenging considering the speed with which a COVID-19 vaccine is needed. “This is accomplished by ensuring the appropriate contacts are in place between the two organizations and that the lines of communication are open between the two entities that allow for exchange of pertinent information in real-time. Pertinent information includes FDA (or other health authority) feedback that informs or changes the overarching strategy for either the facility or the product development,” Gale says.
“Another challenge we face currently is pursuing FDA feedback for the facility and its capabilities while keeping the interests of more than one client and/or COVID-19 vaccine candidate in mind,” she continues. “The landscape is constantly shifting, and new information is coming in daily; however, decisions for the facility and/or process need to be made to move forward. Despite these challenges, FDA, BARDA, and innovator companies have been supportive throughout the process.”
“Manufacture of vaccines is a complex process that requires multiple unit operations to be executed in accordance with CGMPs,” Gale adds. “The development and scale up of a commercial manufacturing process takes up to several years. As we work with our partners to support their COVID products, we are accelerating these processes from years to months. The technical challenges we face relate to ensuring we have a robust and reproducible manufacturing process that results in high-quality product.”
1. FDA, Guidance for Industry: Development and Licensure of Vaccines to Prevent COVID-19 (CBER, June 2020). 2. Emergent BioSolutions, “Emergent BioSolutions Joins US Government’s Warp Speed Program in Landmark Public-Private CDMO Partnership for COVID-19 Vaccine Development and Manufacturing,” Press Release, June 1, 2020. 3. Emergent BioSolutions, “Emergent BioSolutions Reports Financial Results for Second Quarter and Six Months Ended June 30, 2020 and Revises Upward Full Year 2020 Guidance,” Press Release, July 30, 2020. 4. G. Agrawal, et al., “On Pins and Needles: Will COVID-19 Vaccines ‘Save The World’?” www.mckinsey.com, July 9, 2020.
About the author
Feliza Mirasol is the science editor for Pharmaceutical Technology .
Article Details
Pharmaceutical Technology Vol. 44, No. 11 November 2020 Pages: 50–52
When referring to this article, please cite it as F. Mirasol, “ GMPs Guide COVID-19 Vaccine Manufacturing ,” Pharmaceutical Technology 44 (11) 2020.

Related Content:

- International edition
- Australia edition
- Europe edition

US to boost vaccine manufacturing and produce at least a billion doses a year
Plan is part of effort to shore up global supply for poorer countries while ‘preparing for the next pandemic’
The Biden administration is planning to dedicate billions of dollars to build up vaccine manufacturing in the US to produce at least a billion doses each year, in an effort to shore up global Covid-19 supply for poorer countries while also pre-empting future pandemics.
As part of a public-private partnership, the government will draw on knowledge from companies that already use mRNA technology to make vaccines. Its ambitious goal is to get to a point where the US can produce at least a billion doses each year starting around mid-2022, according to the New York Times , which first reported the news.
“This is about assuring expanded capacity against Covid variants and also preparing for the next pandemic,” David Kessler , who leads vaccine distribution for the White House, told the Times.
“The goal, in the case of a future pandemic, a future virus, is to have vaccine capability within six to nine months of identification of that pandemic pathogen, and to have enough vaccines for all Americans.”
A billion vaccines could also boost availability around the world, fighting the stark inequities that have so far plagued Covid-19 vaccination campaigns.
“This effort is specifically aimed at building US domestic capacity,” Kessler said. “But that capacity is important not only for the US supply, but for global supply.”
The initiative – which Kessler said will probably cost several billion dollars – relies on funding from the $1.9tn American Rescue Plan, pandemic relief that was signed into law earlier this year. Roughly 59% of eligible people in the US are fully vaccinated and nearly 16% have received booster shots.
The White House said on Wednesday that about 10% of eligible children aged five to 11 have received a dose of the Pfizer Covid-19 vaccine since its approval for their age group two weeks ago. That equates to at least 2.6 million kids, said Jeff Zients, the White House Covid-19 coordinator. The pace of vaccination for that age bracket over the last week is more than three times faster than the rate adults were vaccinated at the start of the country’s vaccination campaign 11 months ago.
The US Food and Drug Administration (FDA) is trying to expand eligibility for boosters to all adults in the coming days, providing Americans added protection during the holiday season.
But many global citizens have yet to access a single shot, much less a full dosage or booster. Across Africa, for example, only 6% of the population has been fully vaccinated, the World Health Organization (WHO) reported in late October.
These yawning disparities have incited pushback from global health leaders, who are urging policymakers to consider the bigger picture instead of focusing on domestic safety alone.
“In the context of ongoing global vaccine supply constraints, broad-based administration of booster doses risks exacerbating inequities in vaccine access by driving up demand and diverting supply while priority populations in some countries, or in subnational settings, have not yet received a primary vaccination series,” the WHO said in a statement.
In response to these critiques, Joe Biden’s administration has committed to donating over a billion vaccines abroad, a process that’s slowly rolling out .
“I made – and I’m keeping – the promise that America will become the arsenal of vaccines as we were the arsenal of democracy during World War II,” the president said during a Covid-19 summit in September .
“To put it another way: for every one shot we’ve administered today in America, we have now committed to do three shots to the rest of the world.”
Yet critics have warned that the administration’s commitments abroad lack the urgency a deadly virus demands. Whether its new plan to increase manufacturing capacity will substantively address those concerns remains to be seen.
“Purchasing doses for donation sometime next year is helpful, but it does not meaningfully expand the global supply,” Peter Maybarduk, who works for the non-profit consumer advocacy group Public Citizen, previously told the Times. “And it is not justice.”
Associated Press contributed to this report
- Biden administration
- Coronavirus
- Vaccines and immunisation
Most viewed
clock This article was published more than 2 years ago
Biden administration will invest billions to expand coronavirus vaccine manufacturing
The Biden administration is planning to invest billions of dollars to expand U.S. manufacturing capabilities of coronavirus vaccines to increase the supply of doses for poorer nations and better prepare the country for future pandemics, the White House said Wednesday.
The White House will aim to spur the production of at least 1 billion doses a year by investing in companies that make mRNA vaccines, such as Pfizer and Moderna, and helping them expand capacity by funding facilities, equipment, staff and training. Pfizer and Moderna said Wednesday that they are reviewing the government’s proposal and while open to the idea, made no commitments to working with U.S. officials on this effort.
Tracking the coronavirus vaccine
The announcement received mixed reactions from global health activists, who lauded the investment but raised concerns about the speed of its implementation and the latitude that could be given to pharmaceutical companies. For months, the United States has been under pressure to play a larger role in sharing vaccines with the world, but one administration official, who spoke on the condition of anonymity to disclose private conversations, said some of the advocacy groups specifically lobbied an investment on the scale the United States is making.
Wednesday’s announcement marks the latest partnership between the federal government and pharmaceutical companies to bolster vaccine production during the pandemic.
“The goal is to guarantee capacity to produce approximately 100 million mRNA vaccines a month against covid or other pandemic viruses upon demand for the United States or global use,” said David Kessler, the administration’s chief science officer who oversees vaccine distribution. “We are looking to enter into a historic partnership with one or more experienced pharmaceutical partners. This partnership will be used for covid and any future pandemic viruses with the goal of having enough vaccines available within six to nine months of the identification of the virus.”
Kessler said the funds for the effort have already been allocated as part of the American Rescue Plan , the $1.9 trillion coronavirus relief package that President Biden signed into law in March.
The Biomedical Advanced Research and Development Authority (BARDA) has published a “request for information,” seeking proposals from companies that have experience using mRNA technology. BARDA, which is housed within the Department of Health and Human Services, is responsible for developing vaccines and other medical countermeasures.
“ It would combine the expertise of the U.S. government in basic scientific research with the robust ability of pharmaceutical companies to manufacture mRNA vaccines,” Jeff Zients, the White House coronavirus coordinator, said Wednesday at the White House’s coronavirus news briefing. “We hope companies step up and act quickly to take us up on this opportunity to expand production of mRNA vaccines for the current pandemic and set us up to react quickly to any future pandemic threats.”
Zients also touted the country’s effort to share vaccines globally , saying the United States has already donated 250 million doses and has committed a total of $1.1 billion. He said the United States has already donated more vaccines than all other countries combined.
Vaccine manufacturers said they were open to the Biden administration’s new plan but were also seeking further details.
In an interview Wednesday, Moderna President Stephen Hoge said that his firm was reviewing the government’s request for information.
“We haven’t talked about it, but the concepts we’re definitely supportive of and would expect to participate in,” Hoge said.
Amy Rose, a spokeswoman for Pfizer, said the company appreciates the administration’s focus on ensuring long term supply, and the company would review BARDA’s “request for information.”
“Pfizer is proud to be a strong and reliable partner to the U.S. government with vast capacity and capabilities that create solutions,” Rose said in a statement. “As we consider the White House’s proposal, we will come to the table with how we can best contribute to the ongoing global fight against the coronavirus.”
But current and former government health officials raised questions about the administration’s newest vaccine manufacturing proposal, suggesting that the White House still needed to flesh out its plan.
“How long will this take — at least nine months? Is it really necessary or will we already basically be done with the need by the time it’s online?” asked one former official who previously worked with BARDA and spoke on the condition of anonymity to discuss the government’s plan.
Since the United States started distributing vaccines, activists have criticized the Biden administration for failing to scale up domestic vaccine manufacturing capacity to boost the global supply of vaccines. Protesters have gathered outside the homes of top officials in Washington in recent weeks, including Zients and White House chief of staff Ron Klain, demanding the White House do more to share vaccines with the world. In September, activists gathered outside Klain’s house and set up a 12-foot pile of fake bones they said symbolized American inaction in combating the global coronavirus crisis.
On Wednesday, some global vaccine activists characterized the administration’s move as a half-measure.
“It’s a positive step that reflects months of activist pressure. However, it is not a substitute for distributed global manufacturing,” said Zain Rizvi, research director at Public Citizen, faulting vaccine manufacturers for not sharing more intellectual property and know-how with the developing world. “Doses are charity. Knowledge is justice.”
Rizvi also listed new questions raised by the administration’s announcement, including what levers the Biden administration is planning to use to compel more production.
“Will the companies that have not played ball for months agree to expand production?” he asked. “Who will control this new production?”
The White House’s announcement comes amid simmering tensions between the U.S. government, vaccine manufacturers and global advocacy groups. While Biden has vowed that the United States will be “the arsenal of vaccines” for the world, and pharmaceutical companies have argued that they are ramping up production to meet global needs, advocates have concerned that developing countries are being left behind. The World Health Organization warned last month that just five of Africa’s 54 nations are set to hit a year-end target of fully vaccinating 40 percent of their residents.
“The U.S. is rightly investing in manufacturing … but in order to gain the upper hand on this and future pandemics, manufacturing should be spread around the world, especially Africa, not limited to the U.S.,” Robbie Silverman, senior advocacy manager for Oxfam America, an organization focused on global inequality, said in a statement.
The United States has also faced criticism for moving forward with booster shots for Americans while many countries are struggling to provide the first round of vaccines to its citizens. The Food and Drug Administration is expected to authorize booster shots of Pfizer-BioNTech’s vaccine for all adults this week after some state officials already widened eligibility in recent days. The FDA approved booster shots for some Americans in September, but the agency is likely to broaden access as evidence shows waning effectiveness of the vaccines over time.
States circumvent federal guidelines to offer booster shots to all adults
James Krellenstein, co-founder of health equity organization PrEP4All, offered qualified support for the new proposal.
“This is the biggest investment, the biggest move we’ve seen from the Biden administration so far to increase production capability. And that’s a really welcome sign,” Krellenstein said. “But we also have a lot of concerns — in particular, the idea that we could just end up giving a bunch of money to Pfizer or Moderna … which we don’t think would be a sustainable investment in American biosecurity.”
Krellenstein also said that the federal government needed to learn from “the past 30 years of mistakes” of relying on private-sector firms that failed to deliver on promises to fight global health threats, citing the recent cancellation of a coronavirus vaccine contract with manufacturer Emergent BioSolutions as the latest example. “We need the government to realize that there has to be government control” of the manufacturing process, he said.
Krellenstein and other advocates, who first called for the U.S. government to pursue a similar proposal last year, said they were unhappy that the Biden administration had not moved faster to address global manufacturing needs.
“This problem has been really apparent from day one, the fact that we don’t have enough mRNA vaccine manufacturing capacity,” Krellenstein said. “We do need to ask the hard question — what is going at the White House?”
He added: “It’s great that this happened now. But it would be better if it happened in March or April.”
Coronavirus: What you need to know
Covid isolation guidelines: Americans who test positive for the coronavirus no longer need to routinely stay home from work and school for five days under new guidance planned by the Centers for Disease Control and Prevention. The change has raised concerns among medically vulnerable people .
New coronavirus variant: The United States is in the throes of another covid-19 uptick and coronavirus samples detected in wastewater suggests infections could be as rampant as they were last winter. JN.1, the new dominant variant , appears to be especially adept at infecting those who have been vaccinated or previously infected. Here’s how this covid surge compares with earlier spikes .
Latest coronavirus booster: The CDC recommends that anyone 6 months or older gets an updated coronavirus shot , but the vaccine rollout has seen some hiccups , especially for children . Here’s what you need to know about the latest coronavirus vaccines , including when you should get it.

- Subscribe Now (Opens in new window)
- Air Force Times (Opens in new window)
- Marine Corps Times (Opens in new window)
- Navy Times (Opens in new window)
- Pentagon & Congress
- Defense News (Opens in new window)
- Flashpoints
- Benefits Guide (Opens in new window)
- Military Pay Center
- Military Retirement
- Military Benefits
- Discount Depot
- Gear Scout (Opens in new window)
- Military Culture
- Military Fitness
- Military Movies & Video Games
- Military Sports
- Transition Guide (Opens in new window)
- Pay It Forward (Opens in new window)
- Black Military History (Opens in new window)
- Congressional Veterans Caucus (Opens in new window)
- Military Appreciation Month (Opens in new window)
- Military History
- Vietnam Vets & Rolling Thunder (Opens in new window)
- Honor the Fallen (Opens in new window)
- Hall of Valor (Opens in new window)
- Service Members of the Year (Opens in new window)
- Create an Obituary (Opens in new window)
- Medals & Misfires
- Installation Guide (Opens in new window)
- Task Force Violent
- Battle Bracket
- CFC Givers Guide
- Photo Galleries
- Early Bird Brief
- Newsletters (Opens in new window)
- Long-Term Care Partners
- Navy Federal
- Digital Edition (Opens in new window)
Health Care
Will dod need to start producing some medicines to protect troops.
Longstanding problems with drug shortages are prompting senators to seek more solutions for the military medical system, including the possibility of having the military manufacture some medications.
Senators are calling for a return of manufacturing medicines in the United States due to national security concerns over risks to the Defense Department’s pharmaceutical supply chain, and possible risks to service members and their families.
But that includes the possibility of some military manufacturing, according to Sen. Elizabeth Warren, D-Mass., chair of the Senate Armed Services subcommittee on personnel, during a hearing on April 30.
“It’s a critical national defense issue. It’s also critical to the health of our people,” Warren said. One issue is that commercial manufacturers don’t have the right incentives in place to produce many drugs in the U.S.
Additionally, she said, “we don’t even have the right information in place to require meaningful domestic manufacturing and meaningful insight into the supply chain to know we are safe in the drugs we are getting,” and their ingredients.
Warren said she plans to introduce legislation that would direct the Defense Department to manufacture drugs, devices, vaccines and other products when DOD determines there are risks of shortage or quality concerns.
“Most of the time DOD will continue to purchase drugs from the commercial drug market. But there are some instances where it makes sense for DOD to produce the medication itself, for example, when DOD is the only customer,” Warren said. DOD spends more than $5 billion a year on pharmaceuticals, she said, which is about 2% of the entire U.S. commercial pharmaceutical market.
A number of drugs that are used in the military are not commonly needed in the commercial market, defense officials testified. Some of these are drugs that are needed to fight infectious diseases and are not commercially available because there’s no market for them.
If the manufacturing challenges are too great for smaller — but needed — quantities of drugs, Warren said, the government may have to move to military manufacturing. “Otherwise, we’re just not going to get them. Or we’ll pay prices that are so outrageous that it would have been cheaper to have built [the manufacturing facilities] internally,” she said.
One example of that is the adenovirus vaccine. While adenovirus typically causes mild cold or flu-like symptoms, she said, “it is a major cause of serious respiratory illness among service members, particularly those who are in basic training.” That’s why the Walter Reed Army Institute of Research developed the adenovirus vaccine and licensed it to private industry.
But in the 1998-1999 time frame, DOD exhausted its last supply of the vaccine after the sole manufacturer decided to stop making it. At that time, DOD estimated that the lack of vaccine would lead to about 10,000 preventable infections from adenovirus, over 4,200 medical visits by recruits, and over 850 hospitalizations within a year, said Bryce H.P. Mendez, a specialist in defense health care policy for the Congressional Research Service, testifying before the panel. “To an extent, DOD did observe that,” he said.
But that’s not unique, Mendez told lawmakers, adding that the Defense Department has had challenges over many decades in getting certain medicines. Current challenges include the production of medicines to address anthrax, botulism, cholera, hemorrhagic fevers, and others, he said.
Lawmakers should consider legislation that establishes clear options for creating a government-owned facility to manufacture priority health products to meet the military’s needs, said Melissa Barber, an expert in pharmaceutical manufacturing who is a postdoctoral fellow at the Yale School of Medicine, Yale Law School and Yale Collaboration for Regulatory Rigor, Integrity and Transparency. “Such a facility would ensure reliable access to quality drugs for service members, as well as generate significant cost savings.” For example, she told lawmakers, the current contract for producing the adenovirus vaccine costs the government about $38 million a year. “That’s a lot of money to pay for a single vaccine,” she said.
Barber cited an Army report that estimated the startup costs for the government to manufacture the adenovirus vaccine would be about $100 million, with annual costs of about $10 million. DOD would break even in about three years by building and operating its own facility for producing that vaccine, she said.
Government-owned and operated facilities for manufacturing medicines is not a new concept, Barber said. The first example she’s aware of, she said, is during the Civil war, when the U.S. Army set up facilities to manufacture some needed medicines.
The Walter Reed Army Institute of Research does vaccine research and manufactures test batches, but its manufacturing is limited, and the military relies on commercial manufacturers for quantity.
Despite drug shortages, military has mostly been able to find alternative supplies
National supply chain issues are causing more drug shortages, according to reports. but the military appears okay for now., the larger problem of drug shortages.
According to the Food and Drug Administration, almost half of the drugs on DOD’s operational medicines list are in shortage, and most of these are generics, Warren said. This list includes drugs necessary for warfighting, she said. Some of those in short supply include the blood thinner heparin, the common anesthesia drug midazolam and morphine for pain management.
And many drugs, and their key ingredients, come from foreign manufacturers, including China. The Defense Department has less visibility over its operations, and thus, the safety of the drugs.
Following a congressionally-mandated requirement, DoD analyzed 12,917 specific drugs, or about 10% of the total U.S. marketplace as part of efforts to evaluate the military pharmaceutical supply chain. The medicines are identified in the FDA Essential Medicine List. Only a quarter of the drugs analyzed have domestic manufacturers.
According to DOD’s November report, 27% of the drugs analyzed are at a very high risk because they are either dependent on Chinese manufacturers using Chinese ingredients, or were derived from unknown sources.
“I don’t know anybody in their right mind who trusts anything made in China,” said Sen. Rick Scott, R-Fla., the ranking member of the panel.
“During COVID, we learned the hard way that relying on non-allied countries for our medical supply chain poses a real danger. For that reason, it is imperative that we work to ensure DOD supply chains are independent from non-allied nations for necessary pharmaceutical treatments,” Scott said. “In future contingencies, these supply chains could easily cease to exist.”
Defense officials are assessing the chain, and developing policies and procedures to enable the allocation of resources in the case of supply chain disruptions, said Dr. Lester Martinez-Lopez, assistant secretary of defense for health affairs.
When a DOD provider can’t get a critical drug because of the shortage, that provider has to look at alternatives, Martinez-Lopez said. He used the example of amoxicillin, an antibiotic made overseas, used for basic infections. “Let’s say I don’t have it. Now I have to [use] another antibiotic, at the same time I’m trying to combat resistance to antibiotics, using an antibiotic that’s not indicated for that condition. So there I lose twice. One, because I’m not giving the right antibiotic to my patient, but on top of that, I’m losing ground on my fight against antibiotic resistance.”
In other cases, such as when an epinephrine injection is not available to treat a severe allergic reaction, he said, “that can be life and death. We don’t have hours to decide what the alternate is. So that might translate into a life, right on the spot. So this creates a conundrum for all health care professionals. I don’t think it’s just us. It’s across the nation, we’re facing this.”
Questioned by Scott about buying from China, Matthew R. Beebe, director of acquisition for the Defense Logistics Agency agreed that the military shouldn’t buy from them, but said the reality is that current regulations sometimes require it. “We don’t buy from China unless it’s the only source available,” Beebe said. If the end product is available domestically or from a country that’s an ally, that’s where they buy it, he added.
“But we don’t always have visibility over the sourcing,” the ingredients used to make the medicines which are called active pharmaceutical ingredients, and the ingredients used to make those APIs, Beebe said.
About 5% of the active pharmaceutical ingredients are coming from China, said Martinez-Lopez, but officials aren’t able to determine the source of about 20% of the remainder.
The percentage of those unknown sources is “equally troubling, that I don’t even know how to characterize the risk,” Beebe said. He and the other officials said they support bringing more manufacturing back to the U.S.
Scott questioned the witnesses about why the military couldn’t just immediately stop buying any pharmaceuticals that are sourced or produced in any way in China. Because of the volume of pharmaceuticals that would fall into that category, it would mean some medicines wouldn’t be available, officials said.
Of the 60 vital medicines in the U.S. about 20% are solely sourced in China, said retired Army Col. Victor A. Suarez, founder of Blu Zone Bioscience & Supply Chain Solutions.
For many of these medicines, it’s not economically viable for companies to manufacture them here in the U.S., Suarez said. Over the last several decades, much of drug manufacturing has moved overseas. China has used its competitive advantages — such as cheap labor — to drive down prices, and that has forced some U.S. companies out of business.
And 40% of generic drugs sold in the U.S. have just one manufacturer, Warren said.
Scott asked the DOD witnesses to help craft a letter to a number of associations in the health care community, to invite them to a conference call for ideas on helping build a domestic market for pharmaceuticals. The letter would come from DOD and other government officials as well as from members of Congress, Scott said.
Karen has covered military families, quality of life and consumer issues for Military Times for more than 30 years, and is co-author of a chapter on media coverage of military families in the book "A Battle Plan for Supporting Military Families." She previously worked for newspapers in Guam, Norfolk, Jacksonville, Fla., and Athens, Ga.
In Other News
The Holocaust survivor who became a Medal of Honor recipient
Tibor rubin had a history of defying the reaper..
Impact of massive health care cyberattack on vets remains unclear
A massive cyberattack against a private health care firm in february may have exposed millions of veterans' personal medical records..
VA’s Under Secretary of Memorial Affairs to step down in late May
Matthew quinn, va's top cemeteries official, will leave the key department leadership post later this month..
Charity founder in homeless vets hoax charged with fraud, stolen valor
A new york charity official who falsely claimed that homeless vets were being evicted in favor of immigrants was charged with fraud and stolen valor..
- Share full article
Advertisement
Supported by
Global Health
Millions of Girls in Africa Will Miss HPV Shots After Merck Production Problem
The company has told countries that it can supply only 18.8 million of the 29.6 million doses it was contracted to deliver this year.

By Stephanie Nolen
Stephanie Nolen has been following efforts to bring the HPV vaccine to girls in Africa for more than a decade.
Nearly 1.5 million teenage girls in some of the world’s poorest countries will miss the chance to be protected from cervical cancer because the drugmaker Merck has said it will not be able to deliver millions of promised doses of the HPV vaccine this year.
Merck has notified Gavi, the international organization that helps low- and middle-income countries deliver lifesaving immunizations, and UNICEF, which procures the vaccines, that it will deliver only 18.8 million of the 29.6 million doses it was contracted to deliver in 2024, Gavi said.
That means that more than 10 million girls will not receive their expected HPV shots this year — and 1.5 million of them most likely will never get them because they will be too old to qualify for the vaccine in subsequent years.
Patrick Ryan, a spokesman for Merck, said the company “experienced a manufacturing disruption” that required it to hold and reinspect many doses by hand. He declined to give further details about the cause of the delay.
“We are acting with urgency and rigor to deploy additional personnel and resources to resolve this matter as soon as possible,” he said.
Mr. Ryan said that Merck would deliver the delayed doses in 2025.
He also said the company would ship 30 million doses of the vaccine to Gavi-supported countries this year. However, about a third of these are doses that were supposed to have been sent in 2023, leaving Gavi with the 10.7 million dose shortfall.
The delay is a big setback for countries that had already waited years to begin vaccinating girls against HPV, the human papillomavirus, which causes an estimated 90 percent of cervical cancers.
About 350,000 women die from cervical cancer annually, according the World Health Organization . Ninety percent of them are in low-income countries, where routine screening for the disease is rare. The vaccine offers near-total protection against HPV infection, making it the lone vaccine against cancer.
“HPV is the highest impact vaccine Gavi has: If you vaccinate 1,000 girls, you prevent 17.4 future deaths,” said Dr. Aurélia Nguyen, Gavi’s chief program officer. “If there is one vaccine that you want to get out and do well on, this is it.”
The W.H.O. recommends the vaccine for girls up to age 14. The delay means that girls in countries including Sierra Leone, Burkina Faso and Mozambique who are now 14 will no longer be eligible for vaccination when these campaigns finally start.
The HPV vaccine is a complex one to deliver , since it is associated with sexual activity, a taboo topic for teenagers in many of the cultures affected by the delay, and because it is given to children who are outside the usual age for routine immunization. Both girls and their parents must be amenable to vaccination, and that requires crafting distinct messages, delivered on different media, to drum up demand. The vaccine has to be given before girls are sexually active to be effective.
The countries affected have some of the lowest-resource health systems in the world, Dr. Nguyen said. They have invested in planning for the scrapped HPV campaign, while juggling other urgent vaccination needs such as measles or cholera, and can ill-afford the disruption, she said.
The delay will disrupt carefully laid plans to catch up on vaccinations — most of the delayed doses were bound for what’s called “multi-age cohorts,” when countries try to reach all unvaccinated girls between 9 and 14, alongside a standard immunization program for 9-year-olds, usually run in schools.
Most high-income countries routinely vaccinate both girls and boys against HPV, but the global coverage rate for the vaccine is only 20 percent.
Gavi has been trying to expand HPV vaccination for more than a decade. Many low-income countries had designed programs to begin in 2018, but Gavi could not get shots then either because it and UNICEF were competing with a global market and suppliers did not increase production to meet Gavi’s predicted demand.
The version of the Merck HPV vaccine used in the United States costs about $285. UNICEF, which typically negotiates big discounts from pharmaceutical companies, pays $3 to $5 per shot for the large volumes of vaccine it sought to procure.
“UNICEF and Gavi have struggled for years to get sufficient supply, and that was finally starting to change,” said Andrew Jones, UNICEF’s deputy director, immunization supplies.
UNICEF has contracts with other suppliers, but because the Merck product is in high demand from countries, the Gavi program is dependent on the company’s supply. That means this delay disrupts vaccination campaigns in a half-dozen countries, many of which have already had to postpone repeatedly.
“It affects countries’ confidence because for years they were told there wasn’t sufficient supply, but when finally supply opened up, they campaigned, got political buy-in, and now delivery is delayed by six or eight months,” Mr. Jones said.
Mr. Ryan of Merck said the company was committed to supporting the drive to vaccinate millions of girls in developing countries against HPV and had invested more than $2 billion in that effort.
Though Mr. Ryan said the company will deliver the delayed doses next year, Merck has yet to notify Gavi when countries can expect those deliveries, which means they cannot yet begin to plan new campaigns.
The countries that won’t get doses this year include Mozambique, Sierra Leone, Ivory Coast and Burkina Faso. They were going to do the multi-age blitz campaigns aimed at catching as many girls as possible, in addition to the routine vaccination of 9-year-olds. The routine program will continue using doses Merck has delivered.
In addition, Burundi, and, in Asia, Tajikistan, were supposed to get supplies at the end of this year to start doing both multi-age and routine vaccinations, while Cameroon and Liberia were to take delivery of shots so they could start doing multi-age vaccination early in the new year. All of those campaigns will be postponed.
The girls who won’t get vaccinated this year are some of the least likely in the world to be screened or treated for cervical cancer, said Dr. Cathy Ndiaye, the Dakar-based director of the HPV vaccine program for the health-focused nonprofit organization PATH.
“In some countries you can say, ‘OK, you weren’t vaccinated but if you have anything later on in life you can go and get treated’, but not for these girls,” Dr. Ndiaye said.
The delay also complicates the challenge of maintaining political and community support for the HPV shot, she said.
“When you have momentum you want to take advantage of that: When you manage to create demand from the community, you want to deliver, to give them what they need,” she said. “Even at the national level you have to convince them this is important, that it should be a priority because they don’t see cervical cancer, they don’t see the disease now, they say, ‘No let’s deal with polio, let’s deal with measles, that is urgent now.’”
In Mozambique, the plan was to begin the multi-age campaign in June. “There is huge demand, people are asking for it,,” said Dr. Betuel Sigaúque, who works to support routine immunization in Mozambique through JSI, a global nonprofit focused on health and education.
Merck also failed to deliver 7.7 million doses of vaccine to Ethiopia that were scheduled to arrive late last year, and now says they will arrive in June. The country had to scrap a planned school campaign set for spring. Instead, that campaign will take place later this year and will miss girls who have aged out.
Stephanie Nolen is a global health reporter for The Times. More about Stephanie Nolen
The Fight Against Cancer
We asked experts what to know about melanoma symptoms, treatment and prevention. Here’s how to avoid one of the deadliest forms of skin cancer.
Colon and rectal cancers are increasing among people younger than 50. Experts have a few ideas about why .
Should alcoholic beverages have cancer warning labels? Ireland will require them starting in 2026, and there are nascent efforts elsewhere .
Risk calculators can offer a more personalized picture of an individual patient’s breast cancer risk. But experts warn that the results need to be interpreted with the help of a doctor .
The human papillomavirus vaccine provides powerful protection against the leading cause of cervical cancer and against a strong risk factor for anal cancer. Here’s what to know about the shot .
A recent study adds to growing evidence that exercise is an important part of preventing prostate cancer , the second most common and second most fatal cancer in the United States for men.

Rockville-based Narcan maker Emergent is cutting hundreds of jobs
Jeff Clabaugh | [email protected]
May 2, 2024, 1:40 PM
- Share This:
- share on facebook
- share on threads
- share on linkedin
- share on email
Rockville-based Emergent BioSolutions is eliminating 300 jobs and will not fill 85 current job openings, as part of a broad restructuring that also includes closing manufacturing facilities, including one in the Maryland city.
Emergent also reported quarterly revenue of $300 million, almost double revenue in the same quarter a year ago, and a $9 million profit, compared to steep losses in the first quarter of 2023.
Emergent BioSolutions manufactures and sells over-the-counter opioid overdose antidote Narcan, and supplies government stockpiles with vaccines. The company says its latest reorganization would focus the company on its core products business, including Narcan and its anthrax vaccine.
Last quarter, it was awarded a procurement contract valued at up to $235.8 million to supply its anthrax vaccine, BioThrax, to the U.S. Department of Defense.
As part of its restructuring, Emergent is shutting down its Baltimore-Bayview drug manufacturing facility and its drug product facility in Rockville. It said it will concentrate manufacturing operations at sites in Winnipeg, Canada, and Lansing, Michigan.
“Today’s actions are about the future of Emergent,” said CEO Joe Papa. “We have put in place a multiyear plan to position Emergent for sustainable and long-term success, and that starts by stabilizing our operations, strengthening our balance sheet and managing our debt.”
Papa, former Bausch + Lombe CEO, was named Emergent chief executive in February.
The Food and Drug Administration approved over-the-counter sales of Emergent’s Narcan in March 2023, and it began shipping it last fall. Emergent gained rights to Narcan as part of its 2018 acquisition of Adapt Pharma.
Get breaking news and daily headlines delivered to your email inbox by signing up here .
© 2024 WTOP. All Rights Reserved. This website is not intended for users located within the European Economic Area.

Jeff Clabaugh has spent 20 years covering the Washington region's economy and financial markets for WTOP as part of a partnership with the Washington Business Journal, and officially joined the WTOP newsroom staff in January 2016.
- @wtopclabaugh
Related News

Boeing threatens to lock out its private firefighters around Seattle in a dispute over pay

Yellen says threats to democracy risk US economic growth, an indirect jab at Trump

Roofers Union DC reboot: New owners, (mostly) same vibe
Recommended.

Montgomery Co. horseback riding center announces closure in July

Young girl shot in Southeast DC

First legal DC marijuana patio to open 'any day now'
Related categories:.
Exclusive: Tesla retreats from next-generation ‘gigacasting’ manufacturing process
- Medium Text

- Company Tesla Inc Follow
- Company BYD Co Ltd Follow
BIG UPFRONT INVESTMENT
Sign up here.
Reporting by Norihiko Shirouzu in Austin, Texas, and Giulio Piovaccari in Milan Editing by Brian Thevenot and Matthew Lewis
Our Standards: The Thomson Reuters Trust Principles. New Tab , opens new tab

Business Chevron

Fed's Williams says 2% inflation target 'critical'
The U.S. central bank's 2% target for inflation is key to achieving price stability and essential for ensuring economic prosperity, New York Federal Reserve Bank President John Williams said on Friday.

Elon Musk's plan for a cheap EV seems to have hit another major snag
- Tesla has quietly backed away from plans to build EVs using a new "gigacasting" method, per Reuters.
- This manufacturing innovation was seen as a key part of Tesla's plans to produce cheaper EVs.
- It raises further doubts over Elon Musk's plans for an affordable EV.

Elon Musk's transformation of Tesla continues at pace.
The automaker has reportedly pulled back on plans to roll out a new manufacturing method for its electric cars, Reuters said — a move that casts further doubt on Elon Musk's low-cost EV ambitions.
Tesla had been developing a new "gigacasting" method that would allow it to use enormous presses to cast the underbody of an EV in a single piece. The company thought this could simplify manufacturing and cut costs.
Sources told Reuters that Tesla has backed away from these plans and decided to stick to the casting method that it used to build the Model Y and Cybertruck, with the underbody made in three separate pieces.
The gigacasting innovation was thought to be a key part of Tesla's plans to produce cheaper EVs . Last year, Tesla's top engineers told investors that the company's next generation of electric models would cost 50% less to make .
Musk has been hinting at plans to build a $25,000 electric vehicle for years. In December, he told Sandy Munro, an automotive expert, that plans for an affordable Tesla were "quite advanced."
Related stories
"The revolution in manufacturing that will be represented by that car will blow people's minds," Musk said during an interview with Munro. "It is not like any car production line that anyone's ever seen."
Reports earlier this month have suggested that the billionaire has set aside plans to build a cheaper model in favor of getting a Tesla robotaxi to market.
Musk has denied this and said on Tesla's latest earnings call that the company would accelerate the construction of cheaper EVs .
While sales of electric vehicles continue to rise in the US, hitting record levels last year, EVs remain more expensive on average than their combustion counterparts — something that has been an issue for many consumers.
This has made the race to build an affordable, mass-market electric car the crucial next frontier for automotive makers, with companies like Ford also developing plans for cheap EVs.
It's especially important for Tesla, which has come under pressure abroad from Chinese manufacturers like BYD, which offer cheaper models.
The Musk-run automaker has experienced significant upheaval in recent weeks, with Tesla conducting layoffs and being hit by a recall of nearly every Cybertruck shipped to customers .
Tesla did not immediately respond to a request for comment made outside normal working hours.
Watch: How did Tesla's bulletproof Cybertruck become so expensive and so delayed?
- Main content

IMAGES
COMMENTS
2. Vaccine manufacturing overview. Vaccine manufacture is one of the most challenging industries. Even the most basic manufacturing steps necessary to produce vaccines in a manner that is safe, effective, and consistent over the life cycle of a vaccine are difficult to execute .Outcomes can vary widely due to the nearly infinite combinations of biological variability in basic starting ...
Developing a resilient ecosystem for vaccine production means balancing current needs, short-term pressures, and long-term objectives. National public-health leaders could start by translating vaccine-manufacturing resilience into concrete requirements and then use that information to assess local manufacturing capacity.
a number of cancer vaccines in clinical stages of development (e.g. for advanced melanoma). These future developments will benefit from the rapid technological advancements made with COVID-19 vaccines, enabling faster clinical development and manufacturing scale-up ahead of commercialisation.
Drug Substance Manufacturing Begins. Johnson & Johnson began mass manufacturing its investigational Janssen COVID-19 vaccine candidate at the vaccine launch facility. In the first step of the production process, cells are grown in a bioreactor. "Then we introduce our virus seed, which is our actual drug product," Colarusso says.
The U.S. aims to lift Covid vaccine manufacturing to create a billion doses a year. The multibillion-dollar plan is intended to address immediate needs both overseas and domestically, and to ...
We have continued to find ways to scale up our manufacturing capabilities to increase global vaccine supply. This includes but isn't limited to expanding our global network to include 11 of Pfizer-owned and contractor sites, reducing the production time for a batch from 110 to 60 days, and performing ongoing stability studies, improving storage and stability to provide pharmacies and other ...
DNA. The DNA sequences are tested again, and will serve as templates for the next stage of the process. Each bottle of DNA will produce about 1.5 million doses of the vaccine. The Chesterfield ...
Vaccine worldwide. Pfizer has manufacturing and distribution sites across the world for the COVID-19 vaccine. How It Happened Pfizer's manufacturing and supply chain professionals took several steps to accelerate the scale-up and manufacturing of the vaccine to ensure it was one of the most successful manufacturing operations in history.
Manufacturing and Distributing the COVID-19 Vaccine. Pfizer consistently and diligently monitors the supply of our medicines. We operate one of the most sophisticated supply chain systems in the industry, with over 35 Pfizer-owned sites and over 300 suppliers globally, which provides capacity and redundancy as needed.
Nov. 17, 2021. WASHINGTON — The Biden administration, under pressure to increase the supply of coronavirus vaccines to poor nations, plans to spend billions of dollars to expand manufacturing ...
In-depth interviews with experts in manufacturing capacity implementation and regulatory capacity building in Africa complemented the study. We also developed business plan scenarios including facility costs calculations and a possible investment plan based on expert opinions and publicly available information from pertinent sources.
RNA-based vaccines are a novel vaccine manufacturing platform and used almost exclusively to produce COVID-19 vaccines. In our study, we identified Belgium, Switzerland, and Austria as the only small population countries with capacity for production of mRNA vac-cines, all three of which are in the European region.
According to media reports, "Moderna said it would have 20 million doses ready by the end of 2020; Pfizer said it would have about 50 million by then.". Providing COVID-19 vaccines—complex, specially manufactured and distributed products—involves significant supply chain considerations. Needed supplies may be limited in quantity and ...
Source: Author's (Reader) own rendering. Data collection. There are currently more than 200 vaccine candidates against COVID-19, and more than 300 manufacturing facilities across the world that are capable of producing vaccines. With so many moving parts—research continuing at a speed never before seen, and large amounts of the information being politically or commercially sensitive—data ...
The COVID-19 pandemic highlighted significant gaps in equitable access to essential medical countermeasures such as vaccines. Manufacturing capacity for pandemic vaccines, therapeutics, and diagnostics is concentrated in too few countries. One of the major hurdles to equitable vaccine distribution was "vaccine nationalism", countries hoarded vaccines to vaccinate their own populations ...
The United States plans to invest billions of dollars in expanding COVID-19 vaccine manufacturing capacity and make available an additional one billion doses per year, White House COVID-19 ...
Major bio/pharmaceutical companies and contract service providers leading the charge to bring COVID-19 vaccines to market are familiar with established good manufacturing practices (GMPs). In FDA's bid to support vaccine manufacturers, the agency released industry guidance in June 2020 that highlights the development and licensure protocols for COVID-19 vaccines.
The Biden administration is planning to dedicate billions of dollars to build up vaccine manufacturing in the US to produce at least a billion doses each year, in an effort to shore up global ...
The Biden administration is planning to invest billions of dollars to expand U.S. manufacturing capabilities of coronavirus vaccines to increase the supply of doses for poorer nations and better ...
Barber cited an Army report that estimated the startup costs for the government to manufacture the adenovirus vaccine would be about $100 million, with annual costs of about $10 million.
Emergent said it expects the reorganization to save it about $80 million a year, and for the restructuring plan to cost roughly $18 million to $21 million mostly in the second half of this year.
Emergent BioSolutions said on Wednesday that it would cut about 300 jobs across all areas of the company and shut down several manufacturing facilities as part of a restructuring plan.
The vaccine has to be given before girls are sexually active to be effective. Image Merck notified Gavi that it will deliver only 18.8 million of the 29.6 million doses it was contracted to ...
Rockville-based Emergent BioSolutions is eliminating 300 jobs and will not fill 85 current job openings, as part of a broad restructuring that also includes closing manufacturing facilities ...
Tesla has backed away from an ambitious plan for innovations in gigacasting, its pioneering manufacturing process, according to two sources familiar with the matter, in another sign that the ...
The automaker has reportedly pulled back on plans to roll out a new manufacturing method for its electric cars, Reuters said — a move that casts further doubt on Elon Musk's low-cost EV ambitions.