- Open access
- Published: 14 September 2022

Effectiveness of early essential newborn care on breastfeeding and maternal outcomes: a nonrandomized controlled study
- Chuanya Huang 1 , 2 na1 ,
- Lei Hu 1 , 2 na1 ,
- Yonghong Wang 2 , 3 &
- Biru Luo 2 , 3
BMC Pregnancy and Childbirth volume 22 , Article number: 707 ( 2022 ) Cite this article
7713 Accesses
7 Citations
Metrics details
Breastfeeding and maternal health play crucial roles in improving newborn health, which is closely related to the development of families and society. Early essential newborn care, which emphasizes early exclusive breastfeeding and skin-to-skin contact, is recommended by the World Health Organization. This study aimed to explore the association of early essential newborn care with breastfeeding and maternal outcomes.
A nonrandomized controlled study was carried out from May 2020 to January 2021 in a tertiary hospital in Chengdu city, China. Pregnant women were recruited from the maternity ward before they gave birth. Early essential newborn care was performed for 91 mother-newborn pairs after birth in the intervention group, while routine birth care was performed for 91 mother-newborn pairs in the control group. Data on breastfeeding and maternal outcomes were collected pre-test and post-test and were recorded by trained data collectors and retrieved from hospital case record files.
Compared with the control group, the intervention group had a higher incidence of early breastfeeding initiation, an earlier initiation and longer duration for the first breastfeeding, a higher incidence of successful first breastfeeding, more exclusive breastfeeding at hospital discharge, higher maternal breastfeeding self-efficacy, a shorter duration of the third stage of labour, lower postpartum blood loss, and lower scores of maternal pain and anxiety postpartum; the differences were statistically significant ( p < 0.05).
The implementation of high-quality early essential newborn care can help mothers initiate early breastfeeding, improve exclusive breastfeeding rates at hospital discharge, enhance breastfeeding self-efficacy, promote the woman’s recovery from labour, and reduce maternal anxiety and pain in the postpartum period. High-quality early essential newborn care is recommended to policymakers and medical professionals to improve breastfeeding and maternal outcomes.
Trial registration
Chinese Clinical Trial Registry, Retrospective Registration (27/7/2021), registration number: ChiCTR2100049231.
Peer Review reports
Breastfeeding is the ideal method for infant feeding. It is estimated that if the breastfeeding rate were to increase to 50% worldwide, the deaths of approximately 823,000 under-five children can be avoided every year [ 1 ]. Early postnatal breastfeeding behaviour is associated with long-term breastfeeding [ 2 ]. To improve the breastfeeding rate, the World Health Organization (WHO) has recommended skin-to-skin contact between mothers and newborn infants immediately after birth and breastfeeding during the first hour after birth [ 3 ]. Studies have shown that breastfeeding within the first hour after birth can improve exclusive breastfeeding rates at 6 weeks, 10 weeks, and 6 months postpartum [ 4 , 5 , 6 ] and that mothers who breastfeed early have a higher acceptance of exclusive breastfeeding [ 7 ]. Compared with newborn infants who initiated breastfeeding at 2–23 h and 24–96 h after birth, newborn infants who initiated breastfeeding within the first hour after birth had lower neonatal mortality [ 8 ].
Previous studies indicated that many medical professionals, especially in the West Pacific region, often implemented outdated and harmful practices during and after birth, such as unnecessary suctioning, delayed early skin-to-skin contact between the mother and the newborn infant, as well as umbilical cord cutting immediately after birth [ 9 ]. These outdated practices lead to an increase in the risk of neonatal morbidity and mortality [ 10 ]. To improve the quality of newborn care, the Action Plan for Healthy Newborn Infants in the Western Pacific Region (2014–2020) was issued by the WHO Western Pacific Regional Office (WHO/WPRO) [ 11 ]. This plan aimed to give every newborn a healthy start and implement early essential newborn care (EENC) for all newborn infants. EENC contains evidence-based interventions that are simple, that are low-cost and that do not require expensive technologies. The central element of EENC is immediate skin-to-skin contact between the mother and newborn infant after birth for at least 90 min and initiation of exclusive breastfeeding when cues occur (such as drooling, tonguing, rooting, and hand biting). Additionally, midwives should appropriately delay clamping and cutting of the cord and other routine care. These practices can ensure that most newborn infants complete the first breastfeeding during the period of skin-to-skin contact and improve the early breastfeeding initiation rate, as well as strengthen the rooting reflex of the newborn infant [ 12 ]. Furthermore, implementing EENC may also have positive effects on mothers because skin-to-skin contact between mothers and newborn infants can reduce maternal pain, depression and anxiety, accelerate placental detachment, reduce postpartum haemorrhage, and promote uterine involution by promoting the secretion of oxytocin [ 13 ].
EENC was introduced to China in 2016 and had been implemented in 112 medical institutions by 2017 [ 14 ]. Yang et al. surveyed the medical institutions of four provinces that implemented EENC in China and showed that only 36.2% of the newborn infants had skin-to-skin contact with their mothers, the rate of the duration of skin-to-skin contact over 90 min was 19.7%, and the breastfeeding rate and exclusive breastfeeding rate before discharge were 76.5% and 32.2%, respectively [ 15 ]. The findings of the study by Yang et al. indicated that EENC was not fully implemented in line with the WHO guidelines in these medical institutions. Xu et al. pointed out that there were many obstacles to implementing EENC in China hospital policies, including insufficient awareness of medical professionals, shortage of human resources, and little clinical evidence about EENC in China [ 16 ]. Previous studies have explored the benefits of skin-to-skin contact and timed clamping for newborn infants separately [ 13 , 17 ]. However, the EENC is an intervention package; thus, the effect of EENC should be regarded as a general effect on mothers and newborn infants. In addition, most published studies have focused on the effect of implementing EENC on improving newborn outcomes, while few studies have explored the benefits of EENC for breastfeeding and maternal outcomes. This study aimed to fill this research gap, explore the effect of implementing high-quality EENC on breastfeeding and maternal outcomes, and provide more clinical evidence for improving the health of newborn infants and mothers in the West Pacific Region.
The definitions of certain terminology used in this paper are as follows: early breastfeeding initiation, defined as the initiation of first breastfeeding within the first hour after birth; successful first breastfeeding, defined as the score of first breastfeeding assessed by the 4-item Infant Breast Feeding Assessment Tool (IBFAT) [ 18 ] is between 10 and 12; exclusive breastfeeding, defined as only breast milk given to the newborn infant without any liquid or solid food; mixed feeding, defined as breastfeeding combined with artificial feeding of the newborn infant; and artificial feeding, defined as feeding newborn infants with foods other than breast milk, such as formula milk.
Study design and setting
This study was a nonrandomized controlled study and was carried out from May 2020 to January 2021 in a tertiary hospital in Chengdu city, Sichuan Province, China. This hospital is one of the largest women and children’s hospitals in Sichuan Province and has two labour wards with identical health facilities and similar human resources in different hospital areas. These two wards had not implemented EENC or received any EENC coaching before this study, and they were assigned randomly to be the intervention group and control group. Each pregnant woman chose the labour ward in which she preferred to give birth at her antenatal visit in the hospital, and her selection depended entirely on her preferences. However, we began participant recruitment when the woman was awaiting delivery in the maternity ward; thus, the participants were not assigned to each group randomly.
The current study was a part of a larger trial. Because of the limitations to the length of an article, this paper focuses only on breastfeeding and maternal outcomes.
Participants
Participants in this study comprised women and their newborn infants. Pregnant women were recruited from the maternity ward when they were admitted to hospitals for await delivery and with no signs of labour. Pregnant women who met the following inclusion criteria were considered eligible and were invited by the researchers to participate in this study: (1) aged over 18 years, (2) gestational age between 37 and 42 weeks, (3) singleton pregnancy, (4) vaginal delivery, (5) no severe pregnancy complications and/or underlying disease, and (6) no medical indications against breastfeeding. If the woman was transferred from vaginal delivery to caesarean section or the newborn infant had an abnormal birthweight (< 2500 g or > 4000 g), deformities or needed to be transferred to the neonatal intensive care unit (NICU) immediately after birth, the mother and infant were excluded from the study. Written informed consent was obtained from all participants. Ethical approval was received from the hospital ethics review board.
Interventions
Participants in the intervention group received the EENC interventions after birth from midwives in the intervention group, while participants in the control group received routine birth care from midwives in the control group. These interventions delivered in the delivery room. The midwives in the intervention group received 5-month training sessions from national and provincial facilitators, following the guidelines formulated by the WHO [ 19 ]. After 5-month training sessions, a pilot study on 18 mother-newborn pairs was conducted in October 2020 to ensure that every midwife could implement the EENC correctly. The formal interventions were performed from November 2020 to January 2021.
EENC interventions include (1) drying the newborn infant immediately and thoroughly within five seconds after birth, (2) immediate skin-to-skin contact within the first minute and lasting for at least 90 min, (3) exclusive breastfeeding, (4) appropriately timed clamping and cutting of the cord, and (5) other routine care – eye care, vitamin K, immunizations, weighing and examinations [ 11 ]. The duration of implementing EENC was between 90 to 120 min.
The sequence of routine birth care in this hospital was (1) drying of the newborn infant, (2) placement of the newborn infant on a heated table to keep warm for 20 min, during which the umbilical cord is clamped and the weight and length are measured, (3) vaccination, (4) skin-to-skin contact between the mother and newborn infant, and (5) exclusive breastfeeding after the third stage of labour. The duration of implementing routine birth care was between 90 to 120 min.
For both groups, the same postnatal care and education were delivered to mothers by midwives, including the contents of breastfeeding, diet, physical activity, safety, urine output, and stool output.
This study aimed to explore the effect of implementing EENC on breastfeeding and maternal outcomes. We hypothesized that implementing EENC could improve the breastfeeding outcomes and help mothers recover from delivery, especially for the incidence of early breastfeeding initiation.
Measures and data collection
Variables collected at baseline for women included age, educational level, height, weight, gestational age, previous obstetric history, anxiety, and nipple pattern. Variables for newborn infants included sex, length, and birthweight. Among these variables, the anxiety of women was assessed by the Chinese version of the strait form of the State-Trait Anxiety Inventory (STAI-S), which was developed by Spielberger in 1970 and was introduced to China in 1988 [ 20 ]. The STAI-S has 20 self-report items and items are scored on a four-point Likert scale of 1 (not at all) to 4 (severe), with the scores summated to derive a total score ranging from 20 to 40 points. The Cronbach’s α of the Chinese version of STAI-S was 0.91. Higher STAI-S scores indicate severer anxiety. In addition, the nipple pattern was classified into three types, namely, normal, flat, and inverted patterns, and assessed by two female data collectors.
The primary outcome of the current study was the incidence of early breastfeeding initiation. If the first breastfeeding was initiated successfully within the first hour after birth, the early breastfeeding initiation was considered and would be recorded by the data collectors.
The second outcome of this study consisted of some breastfeeding-related outcomes and maternal outcomes; namely, the time of rooting reflex occurrence, the initiation time and duration of first breastfeeding, the number of successful first breastfeeding, the time when formula milk is first served, the total amount of formula milk given before discharge, the number of breastfeeding within the first day after birth, the feeding pattern before discharge, the duration of the third stage of labour, the postpartum blood loss within 2 h after birth, and the pain and anxiety of the woman after birth. Data on the duration of the third stage of labour and the postpartum blood loss within 2 h after birth were retrieved from hospital case record files. The woman’s pain was evaluated by means of the Visual Analogue Scale (VAS) [ 21 ] at 30 min, 60 min, and 120 min after birth. Anxiety was evaluated by the state form of the State-Trait Anxiety Inventory (STAI-S) [ 22 ] at 120 min after birth. Other variables were recorded by data collectors. Additionally, the 4-item Infant Breast Feeding Assessment Tool (IBFAT) [ 18 ] was used to assess the success of the first breastfeeding by data collectors. The total score of IBFAT ranges from 0 to 12, with 10–12 being the scores for vigorous and effective breastfeeding. The Breastfeeding Self-efficacy Scale Short Form (BSES-SF) [ 23 ] was used to assess the confidence of women to breastfeed before discharge from the hospital, with a higher BSES-SE score indicating stronger breastfeeding self-efficacy.
The data collectors were all women with medical educational background. Before the study, data collectors received the methods for collecting data by researchers. They were permitted to enter the ward to collect data by both the participants and the ethnic committee of hospitals.
Sample size
PASS version 15.0 was used to calculate the sample size. We estimated the sample size based on the primary outcome of this study, which is the incidence of early breastfeeding initiation. The results of the pilot study showed that the incidence of early breastfeeding initiation were 77.8% and 44.4% in the intervention group and the control group, respectively. Hence, a sample size of 100 participants would be required ( α = 0.05, β = 0.1) [ 10 ]. Considering that the drop-out rate was 10%, the minimum sample size needed was 110 participants, with 55 participants in each group. To reduce sampling error [ 24 ], we include all pregnant women who met the inclusion criteria in the study during the recruitment phase.
The current study was a single-blinded trial. It was impossible to blind the midwives and data collectors in the delivery rooms because midwives were responsible for implementing EENC or routine birth care and data collectors were responsible for assessing and recording. Hence, only participants were blinded.
Statistical methods
SPSS version 25.0 was used to analyse the data. The smallest unit that is being analyzed to assess intervention effects was the group. The mean ± standard deviation (SD) and median (interquartile range, IQR) were used to describe continuous data, and t test and Mann–Whitney U test were used to identify the differences. The number (n) and percentage (%) were used to describe categorical data, and the chi-square test and Fisher’s exact test were used to identify differences.
Baseline information of participants
In total, 203 pregnant women were recruited for this study from November 2020 to January 2021, with 102 included in the intervention group and 101 in the control group. Figure 1 shows the flow of participants through each stage of the study. Ultimately, there were 91 mother-newborn pairs in the intervention group and 91 mother-newborn pairs in the control group. Table 1 shows the basic information of all participants. There were no significant differences between the two groups regarding the baseline information.
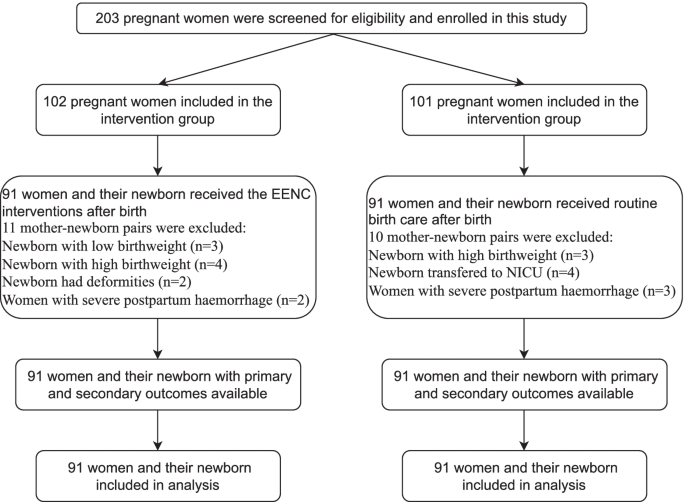
Flow of participants through each stage of the study
Breastfeeding within 2 h after birth in the two groups
The incidence of early breastfeeding initiation in the intervention group was higher than that in the control group ( n = 69 vs. n = 39, p < 0.001). The first breastfeeding in the intervention group started earlier (48.02 ± 16.30 min vs. 66.97 ± 35.41 min, p < 0.001) and lasted longer (34.98 ± 15.02 min vs. 22.30 ± 11.70 min, p < 0.001) than that in the control group. Additionally, the mean IBFAT scores of the first breastfeeding were higher (10.05 ± 2.17 vs. 8.68 ± 2.04, p < 0.001). Furthermore, more successful first breastfeeding ( n = 83 vs. n = 68, p = 0.003) were observed in the intervention group. However, there was no significant difference in the time of rooting reflex occurrence. (Table 2 ).
Breastfeeding before discharge in the two groups
The median time at which the formula milk was first served in the intervention group was later than that in the control group (4 h vs. 2 h, p < 0.001), and the median amount of formula milk given to babies before discharge was higher in the control group than in the intervention group (70 ml vs. 90 ml, p < 0.001). The number of breastfeeding within 24 h after birth in the intervention group was greater than that in the control group ( n = 7 vs. n = 5, p < 0.001). Regarding the feeding pattern, the number of exclusive breastfeeding in the intervention group was greater than that in the control group ( n = 67 vs. n = 40), with less mixed breastfeeding ( n = 24 vs. n = 47) and artificial breastfeeding ( n = 0 vs. n = 4). The women in the intervention group had higher breastfeeding self-efficacy assessed by the BSES-SF (55.78 ± 8.96 vs. 46.74 ± 10.08, p = 0.024). (Table 3 ).
Duration of third stage of labour, postpartum blood loss, pain and anxiety of women
Compared to those in the control group, the duration of the third stage of labour was shorter (5.25 ± 5.66 min vs. 6.10 ± 2.92 min, p < 0.001), and the amount of postpartum blood loss within 2 h after birth was lower (234.64 ± 63.65 ml vs. 281.37 ± 72.29 ml, p < 0.001) in the intervention group. The mean VAS (at 30 min, 1 h, and 2 h) and STAI-S scores in the control group were higher than those in the intervention group, which indicated that pain and anxiety were more severe in the control group (Table 4 ).
This study compared the effect of EENC and routine birth care on breastfeeding and maternal outcomes in a tertiary hospital in China. The results showed that EENC can improve the early breastfeeding initiation, establish correct breastfeeding behaviour, increase the self-efficacy in breastfeeding among mothers and help them recover from childbirth.
Although there were more primiparous women in the intervention group, our results showed that the breastfeeding outcomes in the intervention group were better than that in the control group. Some studies showed that women who have breastfed previously have better breastfeeding outcomes than primiparous women [ 25 , 26 ]. However, the study by Anette et al. showed that parity cannot affect the duration of exclusive breastfeeding or any breastfeeding, but early first breastfeeding can lead to a positive impact [ 27 ]. Similarly, previous studies also pointed out that although inverted or flat nipples would hinder breastfeeding [ 28 ], if the babies can be breastfed early, they are more likely to attach and can be fed well in the postnatal period. In this study, although more women with inverted and flat nipples were in the intervention group, the breastfeeding outcomes were still better than that in the control group. For primiparous women and women with flatted or inverted nipples, EENC may therefore also be recommended as it can improve breastfeeding outcomes.
The findings of this study indicated that the intervention group had a higher incidence of early breastfeeding initiation, earlier initiation and longer duration of first breastfeeding, and a higher IBFAT score for first breastfeeding. Similar findings have been reported in studies by Aiping G et al. and Min et al. [ 12 , 29 ]. Early breastfeeding is an important factor for constructing correct breastfeeding behaviour. The WHO proposed Protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services in 2017 [ 30 ], which emphasized that medical institutions should provide all feasible support to help women initiate early breastfeeding. Previous studies also showed that women who initiated breastfeeding within the first hour after birth had a higher acceptance of breastfeeding, which is especially important for improving the exclusive breastfeeding rates up to 6 months postpartum [ 5 , 6 ]. EENC interventions contain a long duration of skin-to-skin contact, which is a key factor in ensuring the success of early breastfeeding. The study by Mahmood et al. showed that newborn infants with successful skin-to-skin contact can initiate first breastfeeding 62 min earlier than newborn infants with routine birth care, and the success rate of first breastfeeding increased by 26.3% [ 31 ]. In addition, EENC recommends that midwives assist women in initiating first breastfeeding when the newborn infants experience a rooting reflex and active breast-seeking action, which is in line with newborn infants’ instincts and can avoid excessive intervention in breastfeeding. Therefore, implementing EENC could increase the rate of early breastfeeding initiation and successful first breastfeeding.
In the current study, the rates of exclusive breastfeeding and the breastfeeding self-efficacy of women at hospital discharge in the intervention group were higher than those in the control group, which were impacted mainly by the early skin-to-skin contact between mothers and newborn infants and successful first breastfeeding. The study by Almqvist et al. showed that the issues of breastfeeding encountered by women in the early postpartum period were the main reason they gave up exclusive breastfeeding [ 32 ]. Success in the first breastfeeding means that the issues in the process of breastfeeding will be partly solved with the help of health professionals. Therefore, the women in the intervention group could gain confidence and skills from the experience of success in the first breastfeeding, which would in turn motivate them to perform exclusive breastfeeding [ 33 ]. The WHO recommends that unless there are medical indications, the staff of medical institutions should dissuade women and their families from providing any food other than breast milk to their infants. However, in the clinical practice setting, the phenomenon of mothers or family members feeding infants with formula milk or other food is unavoidable even though breast milk is sufficient because midwives and nurses cannot help every woman solve the problems encountered in breastfeeding due to the demands of their work. The study by Raghavan et al. showed that formula milk given to babies on the first day emerged as the only independent predictor of failure to continue exclusive breastfeeding at 6 weeks after birth (OR 2.96; 95% CI 1.09–8.06) [ 5 ]. In this study, newborn infants in the intervention group were given formula milk for the first time approximately 2 h later than those in the control group, and the total amount of formula milk added before discharge was also lower, which indicated that the construction of correct breastfeeding behaviour within the first hour after birth can reduce the use of unnecessary formula milk to some extent. Additionally, babies in the intervention group had more breastfeeding times within 24 h postpartum on the first day postpartum than those in the control group, indicating that the implementation of EENC can help women breastfeed correctly and have higher breastfeeding self-efficacy, which is conducive to the growth and development of newborn infants [ 34 ].
Our findings also showed that the EENC can help women recover from labour. The women in the intervention group had a shorter duration of the third stage of labour and lower postpartum blood loss, which is in line with the study by Yuan et al. [ 35 ]. During skin-to-skin contact, sucking from newborn infants can stimulate the nerve endings of the maternal nipple and then promote the synthesis and secretion of oxytocin [ 36 ]. Oxytocin can stimulate uterine contraction directly, reduce the interference of oxidative stress on uterine contraction, and finally reduce postpartum blood loss [ 37 , 38 ]. In addition, placing the newborn infant on the mother’s breast and abdomen plays a similar role to massage, which can also promote the contraction of the uterus [ 13 ]. Furthermore, lower levels of postnatal anxiety and pain among mothers were observed in the intervention group, which may be related to the secretion of oxytocin and the joy of successful breastfeeding. Previous studies indicated that oxytocin can increase the threshold of maternal pain perception [ 39 ] and alleviate maternal anxiety [ 40 , 41 ].
This study systematically explored the effects of EENC on breastfeeding and maternal outcomes and provided more evidence for the implementation of EENC in the future. However, this study also has some shortcomings. First, the design of this study is quasi-experimental. Due to hospital policies and funding limits, the participants could not be randomly assigned to two groups. However, because the intervention and control measures are implemented in two wards of the same hospital, which have similar human resources and facilities and are far away from each other, contamination and bias were excluded as much as possible. Second, the results of pain and anxiety were self-reported variables, so self-report bias cannot be avoided. Third, follow-up in this study lasted until the mother was discharged from the hospital, so a longer-term follow-up study to clarify the long-term effect can be considered in the future. Last, although the sample size had been previously calculated, this study was conducted only in a tertiary hospital, so the generalization of the results is limited. Large-sample and multicentre randomized controlled trials are necessary to further clarify the effect of EENC.
The implementation of EENC is associated with better breastfeeding and maternal outcomes, which can not only improve the early initiation of breastfeeding and exclusive breastfeeding rate but also relieve the anxiety and pain of the mother and increase her confidence in breastfeeding at hospital discharge. Hence, it is strongly recommended that policymakers and medical professionals implement EENC in clinical practice to improve the outcomes of both women and infants.
Availability of data and materials
All raw data generated or analyzed during this study are available from the corresponding author upon reasonable request.
Abbreviations
Early Essential Newborn Care
Inter Quartile Range
World Health Organization
Standard deviation
Neonatal intensive care unit
Western Pacific Regional Office
Confidence interval
Infant Breast Feeding Assessment Tool
Breastfeeding Self-efficacy Scale Short Form
Visual Analogue Scale
The state form of State-Trait Anxiety Inventory
Victora CG, Bahl R, Barros AJ, França GV, Horton S, Krasevec J, Murch S, Sankar MJ, Walker N, Rollins NC. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. The Lancet. 2016;387(10017):475–90.
Article Google Scholar
Kronborg H, Vaeth M, Olsen J, Iversen L, Harder I. Effect of early postnatal breastfeeding support: a cluster-randomized community based trial. Acta Paediatr. 2007;96(7):1064–70.
Article CAS Google Scholar
World Health Organization: Implementation guidance: protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services: the revised baby-friendly hospital initiative. 2018.
Arusei RJ, Ettyang GA, Esamai F. Feeding patterns and growth of term infants in Eldoret. Kenya Food nutrition bulletin. 2011;32(4):307–14.
Raghavan V, Bharti B, Kumar P, Mukhopadhyay K, Dhaliwal L. First hour initiation of breastfeeding and exclusive breastfeeding at six weeks: prevalence and predictors in a tertiary care setting. The Indian Journal of Pediatrics. 2014;81(8):743–50.
Meshram I, Laxmaiah A, Venkaiah K, Brahmam G. Impact of feeding and breastfeeding practices on the nutritional status of infants in a district of Andhra Pradesh, India. Natl Med J India. 2012;25(4):201.
CAS PubMed Google Scholar
Kavle JA, LaCroix E, Dau H, Engmann C. Addressing barriers to exclusive breast-feeding in low-and middle-income countries: a systematic review and programmatic implications. Public Health Nutr. 2017;20(17):3120–34.
Group NS. Timing of initiation, patterns of breastfeeding, and infant survival: prospective analysis of pooled data from three randomised trials. Lancet Glob Health. 2016;4(4):e266–75.
Sobel HL, Silvestre MAA, Mantaring JBV III, Oliveros YE, Nyunt-U S. Immediate newborn care practices delay thermoregulation and breastfeeding initiation. Acta Paediatr. 2011;100(8):1127–33.
Cohen J. A power primer. Psychol Bull. 1992;112(1):155.
World Health Organization. Regional Office for the Western Pacific: Action plan for healthy newborn infants in the Western Pacific region (2014–2020). Geneva: World Health Organization; 2014.
Google Scholar
Min C, Zhenfang L, Aihua W, Xiaolian Z, Yuanyuan Z, Xuelian W: Effects of early essential newborn care on the onset of lactogenesis among primiparaes. Chinese Journal of Child Health Care 2019.
Moore ER, Bergman N, Anderson GC, Medley N: Early skin‐to‐skin contact for mothers and their healthy newborn infants. Cochrane database of systematic Reviews 2016(11).
Wang CR, Li XY, Zhang L, Wu LM, Tan L, Yuan F, Guo Y, Williams S, Xu T. Early essential newborn care is associated with increased breastfeeding: a quasi-experimental study from Sichuan Province of Western China. Int Breastfeed J. 2020;15(1):99.
Jin Y, Xing L, Xiayun L, Yan W, Yue X, Yingpeng Q, Liwei S, Lai W, Hongyu L, Min Z. Current status of early basic neonatal health care services in 4 provinces and county-level medical and health care institutions in western my country. Maternal and Child Health Care of China. 2019;34(10):2178–82.
Tao X. Early essential newborn care: priority interventions to end preventable neonatal death. Chinese Journal of Preventive Medicine. 2021;54(5):498–502.
Rabe H, Reynolds GJ, Diaz‐Rosello JL: Early versus delayed umbilical cord clamping in preterm infants. Cochrane Database of Systematic Reviews 2004(4).
Matthews MK. Developing an instrument to assess infant breastfeeding behaviour in the early neonatal period. Midwifery. 1988;4(4):154–65.
WHO Regional Office for the Western Pacific. Coaching for the First Embrace: Facilitator’s Guide (Early Essential Newborn Care) Module 2 (A WPRO Publication). Geneva: World Health Organization; 2016.
Spielberger CD: State‐Trait anxiety inventory. The Corsini encyclopedia of psychology 2010:1–1.
Crichton N. Visual analogue scale (VAS). J Clin Nurs. 2001;10(5):706–706.
Spielberger CD: State-trait anxiety inventory for adults. 1983.
Dennis CL. The breastfeeding self-efficacy scale: Psychometric assessment of the short form. Journal of Obstetric, Gynecologic, Neonatal Nursing. 2003;32(6):734–44.
Sedgwick P: What is sampling error? BMJ 2012, 344.
Hackman NM, Schaefer EW, Beiler JS, Rose CM, Paul IM. Breastfeeding outcome comparison by parity. Breastfeed Med. 2015;10(3):156–62.
Koskinen KS, Aho AL, Hannula L, Kaunonen MJM. Maternity hospital practices and breast feeding self-efficacy in Finnish primiparous and multiparous women during the immediate postpartum period. 2014;30(4):464–70.
Ekström A, Widström A-M, Nissen E. Duration of Breastfeeding in Swedish Primiparous and Multiparous Women. J Hum Lact. 2003;19(2):172–8.
Chakrabarti K, Basu S. Management of Flat or Inverted Nipples with Simple Rubber Bands. Breastfeed Med. 2011;6(4):215–9.
Aiping G, Jiejing T, Xiaodan C, Yeping W, Hehe W, Yangyang L. Effects of early basic health care for newborns on newborns and their mothers. Chinese Journal Woman and Child Health Research. 2019;30(5):4.
World Health Organization: Guideline: protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services: World Health Organization; 2017.
Mahmood I, Jamal M, Khan N. Effect of mother-infant early skin-to-skin contact on breastfeeding status: a randomized controlled trial. J Coll Physicians Surg Pak. 2011;21(10):601–5.
PubMed Google Scholar
Almqvist-Tangen G, Bergman S, Dahlgren J, Roswall J, Alm B. Factors associated with discontinuation of breastfeeding before 1 month of age. Acta Paediatr. 2012;101(1):55–60.
Araban M, Karimian Z, Kakolaki ZK, McQueen KA, Dennis C-L. Randomized controlled trial of a prenatal breastfeeding self-efficacy intervention in primiparous women in Iran. J Obstet Gynecol Neonatal Nurs. 2018;47(2):173–83.
Chipojola R, Chiu H-Y, Huda MH, Lin Y-M, Kuo S-Y. Effectiveness of theory-based educational interventions on breastfeeding self-efficacy and exclusive breastfeeding: A systematic review and meta-analysis. Int J Nurs Stud. 2020;109: 103675.
Li Y, Lin Z, Ling Y, Yunyun C: Effect of the early essential newborn care on the early prognosis of the newborn and the rehabilitation of the parturient. Maternal and Child Health Care of China 2020.
Saxton A, Fahy K, Hastie C. Effects of skin-to-skin contact and breastfeeding at birth on the incidence of PPH: a physiologically based theory. Women and Birth. 2014;27(4):250–3.
Marín Gabriel M, Llana Martín I, López Escobar A, Fernández Villalba E, Romero Blanco I, Touza Pol P. Randomized controlled trial of early skin-to-skin contact: effects on the mother and the newborn. Acta Paediatr. 2010;99(11):1630–4.
Velandia M: Parent-infant skin-to-skin contact studies: Parent-infant interaction and oxytocin levels during skin-to-skin contact after Cesarean section and mother-infant skin-to-skin contact as treatment for breastfeeding problems: Inst för kvinnors och barns hälsa/Dept of Women's and Children's Health; 2012.
Tracy LM, Georgiou-Karistianis N, Gibson SJ, Giummarra MJ. Oxytocin and the modulation of pain experience: implications for chronic pain management. Neuroscience Biobehavioral Reviews. 2015;55:53–67.
Handlin L, Jonas W, Petersson M, Ejdebäck M, Ransjö-Arvidson A-B, Nissen E, Uvnäs-Moberg K. Effects of sucking and skin-to-skin contact on maternal ACTH and cortisol levels during the second day postpartum—influence of epidural analgesia and oxytocin in the perinatal period. Breastfeed Med. 2009;4(4):207–20.
Uvnäs-Moberg K, Arn I, Magnusson D. The psychobiology of emotion: the role of the oxytocinergic system. Int J Behav Med. 2005;12(2):59–65.
Download references
Acknowledgements
The authors would like to express their sincere appreciation to all the midwives and participants who contributed to this study.
Author information
Chuanya Huang and Lei Hu contributed equally to this work.
Authors and Affiliations
West China School of Nursing, Sichuan University/Department of Nursing, West China Second University Hospital, Sichuan University, Chengdu, 610000, China
Chuanya Huang & Lei Hu
Key Laboratory of Birth Defects and Related Diseases of Women and Children, Ministry of Education, West China Second University Hospital, Sichuan University, Chengdu, 610000, China
Chuanya Huang, Lei Hu, Yonghong Wang & Biru Luo
Department of Nursing, West China Second University Hospital, Sichuan University/West China School of Nursing, Sichuan University, Chengdu, 610000, China
Yonghong Wang & Biru Luo
You can also search for this author in PubMed Google Scholar
Contributions
HCY, WYH and LBR designed and conducted the research study. HCY and HL wrote the original manuscript and conceptualized the analysis. HCY and LBR performed the analysis. HL, WYH, LBR and HL, reviewed and contributed to the final draft. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Yonghong Wang or Biru Luo .
Ethics declarations
Ethics approval and consent to participate.
The study was part of a larger research trail that was conducted in Chengdu, Sichuan, China. This study was approved by the Ethics Committee of West China Second University Hospital, Sichuan University, and the ethics approval number is 2020 (144). The date of approval was 21 September 2020. All methods were performed in accordance with the relevant guidelines and regulations. Informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Huang, C., Hu, L., Wang, Y. et al. Effectiveness of early essential newborn care on breastfeeding and maternal outcomes: a nonrandomized controlled study. BMC Pregnancy Childbirth 22 , 707 (2022). https://doi.org/10.1186/s12884-022-05037-8
Download citation
Received : 28 February 2022
Accepted : 09 September 2022
Published : 14 September 2022
DOI : https://doi.org/10.1186/s12884-022-05037-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Early essential newborn care
- Breastfeeding
BMC Pregnancy and Childbirth
ISSN: 1471-2393
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 12 February 2022
Preparing newborn screening for the future: a collaborative stakeholder engagement exploring challenges and opportunities to modernizing the newborn screening system
- Sara M. Andrews 1 na1 ,
- Katherine Ackerman Porter 1 na1 ,
- Donald B. Bailey Jr 1 &
- Holly L. Peay 1
BMC Pediatrics volume 22 , Article number: 90 ( 2022 ) Cite this article
5084 Accesses
15 Citations
21 Altmetric
Metrics details
Background and objectives
Projections that 60 transformative cell and gene therapies could be approved by the U.S. Food and Drug Administration (FDA) within 10 years underscore an urgent need to modernize the newborn screening (NBS) system. This study convened expert stakeholders to assess challenges to the NBS system and propose solutions for its modernization.
NBS stakeholders (researchers, clinicians, state NBS leaders, advocates, industry professionals, and current/former advisory committee members) participated in one of five mixed-stakeholder panel discussions. Prior to panels, participants completed a survey in which they reviewed and ranked NBS challenges generated from relevant literature. During panels, participants deliberated on challenges and explored potential solutions. Pre-panel survey data were analyzed descriptively. Data from panel discussions were analyzed using a rapid qualitative analysis.
Median scores of the ranked challenges (1 = most important) reveal the top three most important barriers to address: critical missing data for NBS decision-making (Median = 2), burden on state NBS laboratories (Median = 3), and the amount of time required for state-level implementation of screening for new conditions (Median = 4). Panel discussions were rooted in recurring themes: the infant’s well-being should be the focal point; the transformative therapy pipeline, although undeniably positive for individuals with rare diseases, is a threat to NBS capacity; decisions about modernizing NBS should be evidence-based; additional financial support is required but not sufficient for modernization; and modernization will require participation of multiple NBS stakeholders. This final overarching theme is reported in depth, including expertise, coordination, and collaboration challenges facing NBS and novel approaches to oversight, partnership, and coordination that were suggested by participants.
Conclusions
This study engaged representatives from multiple stakeholder groups to generate potential solutions to challenges facing NBS in the United States. These solutions provide a rich starting point for policy makers and other stakeholders who desire to maximize the impact of new transformative therapies for babies, families, and society.
Peer Review reports
Public health decision-making is inevitably complicated because of the involvement of multiple stakeholders, such as federal, state, and local government, researchers, health care providers, advocates, and the public at large. In the United States, history has demonstrated the challenges of unifying and mobilizing stakeholders when a public health system needs to adapt to external forces such as advances in technology or the onset of new infectious diseases [ 1 , 2 ]. Newborn Screening (NBS) presents a classic example of a complex public health system needing to adapt to best serve its intended beneficiaries: babies and families. Over the past 50 years, NBS has saved or improved the lives of countless babies by identifying rare, but serious medical conditions presymptomatically and referring them for immediate treatment. However, the system in its current state is unprepared to adapt to an approaching opportunity and challenge: the advent of a growing number of transformative therapies targeting rare conditions. The number of conditions that are likely to be candidates for NBS once they have an associated therapy threatens to overwhelm the system unless stakeholder partnerships are leveraged and processes are changed.
NBS in the United States requires coordination between state and federal governments, which increases the complexity of implementation and change. The federal government has oversight over the national Recommended Uniform Screening Panel (RUSP), and the process of adding a condition to the RUSP involves contributions of both government and nongovernment stakeholders. Conditions are recommended for the RUSP by the Advisory Committee on Heritable Disorders in Newborns and Children (ACHDNC), a committee consisting of 15 voting members appointed by the Secretary of Health and Human services (10 individuals with relevant expertise and 5 individuals who represent federal agencies that fund or support aspects of NBS, such as Centers for Disease Control and Prevention [CDC]) and additional representatives from organizations that intersect with infant and child health (e.g., American Academy of Pediatrics). At the state level, legislatures, departments of public health, NBS laboratories, and advisory committees are key players in decisions about adding RUSP conditions to each state’s screening panel, and screening and follow-up procedures for each condition vary by state.
Other NBS stakeholders include rare disease advocates and policymakers, who worked together to enact the federal legislation that made NBS a public health program and who continue to advocate to fund and enhance the program on federal and state levels. Families and advocacy groups also play an important role in advocating for conditions to be added to the RUSP and state NBS panels. Additionally, prior to implementing NBS for new conditions, there is a need for researchers to collect natural history data, academic and industry sponsors to develop and test treatments, and private companies and public institutions to develop diagnostic tests and screening protocols. Finally, health care providers have the critical role of performing diagnostic testing, communicating with families about their child’s condition, connecting babies to treatment, and providing long-term follow-up services.
Despite successful collaboration of these stakeholders to build and sustain NBS, the system now faces a disruptive, albeit positive, force: the transformative therapy pipeline for rare conditions. At least 60 cell and gene therapies are projected to be approved by the Food and Drug Administration (FDA) by 2030, and FDA recently reported more than 1000 Investigational New Drugs (INDs) applications for cell and gene therapy treatments are currently on file [ 3 , 4 ]. NBS could ensure timely access to these treatments. However, the need for federal approval and state implementation of screening for so many conditions would almost certainly overwhelm the current system. Collecting sufficient evidence to meet the criteria for RUSP approval [ 5 ] can take years. And once a condition is added to the RUSP, there is wide variability in how long it takes each state to approve a condition for its panel and allocate funding for implementation. For example, X-linked adrenoleukodystrophy was nominated to be considered for the RUSP in 2012 and added to the RUSP in 2016 [ 6 ], but currently only 23 states screen for the condition [ 7 ]. Thus, it can take years to achieve nationwide screening for even one condition. The degree to which this problem could be exacerbated by the transformative therapy pipeline is sobering and underscores the critical need to engage all NBS stakeholders in modernizing the system.
In this study we convened a series of multistakeholder panels to engage NBS experts and advocates in collectively prioritizing challenges to NBS modernization and proposing solutions to enable realizing the potential of transformative therapies within the NBS system.
We employed a multistakeholder expert panel approach to address three aims:
Aim 1. Prioritize and explore the most impactful barriers to realizing the potential of transformative therapies within the NBS system.
Aim 2. Generate potential solutions to barriers of NBS modernization.
Aim 3. Rate the feasibility, acceptability, and sustainability of implementation of solutions identified across the panels.
The first two aims were addressed through multistakeholder panel discussions described herein. The third aim was addressed by a follow-up survey completed by panel participants, which is reported elsewhere [ 8 ].
Study design
We modeled our approach after several methodologies that have implemented group discussion among heterogeneous stakeholders for brainstorming or problem-solving [ 9 , 10 , 11 , 12 , 13 ]. Focus groups, which are traditionally and intentionally composed of individuals with similar experiences, are an established method for eliciting stakeholder perspectives [ 14 ]. However, when the objective is systems change, discourse between stakeholders with diverse priorities and experiences can result in a more holistic understanding of the breadth of a problem and the generation of more viable solutions [ 15 ]. Accordingly, we selected mixed-stakeholder discourse as the preferred methodology to explore barriers and solutions to modernizing the NBS system. Participants in each of our panels represented five NBS stakeholder groups. During the panel sessions we did not aim to achieve thematic saturation or consensus, but instead to facilitate initial cross-stakeholder consideration of challenges and brainstorm solutions, with the expectation that ongoing engagement of a larger number of stakeholders would be needed to refine solutions.
Research activities were framed around this transformative therapy scenario: It is 2030—ten years from today. Thirty or more new transformative gene or cell therapies have been approved by the FDA to treat monogenic, non-oncology rare disorders. Please consider the following assumptions. These assumptions may not reflect future reality but will be useful to frame our upcoming group discussion.
Each treats a different genetic disorder.
Each has a valid screening assay that is not prohibitively costly.
The therapies are curative or significantly disease modifying if given early in life, but much less or not effective if given later.
The longer-term risks and duration of efficacy are unknown.
Assume that the cost of the therapies will be completely covered by payers ( e.g. , insurance, Medicaid).
Recruitment
NBS experts (i.e., individuals highly experienced with some aspect of NBS) were nominated by a consortium of funders and RTI researchers and invited to participate. We aimed to recruit approximately 50 participants, representing 10 individuals from each of 5 stakeholder groups:
NBS researchers or clinicians
State NBS directors or program leaders
Representatives of patient advocacy organizations
Representatives of pharmaceutical or diagnostic companies
Current and former members of federal or state advisory committees
Prior to initiating data collection, the investigators conducted a literature scan to identify and summarize challenges to NBS that are reflected in published literature; these are available as Additional File 1 . Data collection was conducted between December 2020 and January 2021 and included three phases.
Pre-panel survey
An online survey included basic demographic questions and a rating activity where participants indicated their familiarity with and expertise in various aspects of NBS. Additionally, participants were asked to rank a list of NBS challenges stemming from the summarization of the published literature. Participants were asked to indicate which challenges were the most important to address to achieve NBS for 30 new disorders in 10 years, based on the context of the transformative therapy scenario (Additional File 1 ).
Multistakeholder expert panels
We then convened five mixed-stakeholder expert panels of 7–10 participants per panel. Each 90-min panel discussion was conducted virtually using Zoom, a web conferencing platform. Audio and video were recorded. Investigators used a semistructured interview guide to address the following:
Exploration of each panel’s prioritized challenges to NBS modernization. Investigators displayed the results of the pre-panel ranking of challenges for each of the panels (i.e., each panel viewed their own panel members’ aggregated ranking results). The moderator asked participants to elaborate on the most highly prioritized challenges and to make a case for any challenges that were missing from the list.
Solutions . Participants were guided to explore potential solutions to the most highly prioritized challenges and to consider the acceptability, feasibility, and sustainability of those solutions based their own expertise, priorities, and experience with NBS.
Post-panel survey
Approximately one month after each panel discussion, participants were asked to respond to a second online survey, reported in a separate article.
Participants were asked to draw on all of their NBS experiences and perspectives during their participation (i.e., not attempt to reflect the experiences or attitudes of one stakeholder group). Each participant was offered a $100 gift card for participation. Some participants declined the incentive. This study was determined to be exempt by the RTI International Institutional Review Board (IRB).
Pre-panel survey data were analyzed using descriptive statistics. Multistakeholder panel discussions were analyzed qualitatively by investigators who moderated and took notes during all five panels (HLP and SMA, respectively) and a third analyst (KAP). The investigators, all of whom have expertise in qualitative methods, conducted a rapid assessment process, which is a team-based qualitative inquiry that uses triangulation and iterative data analysis to quickly develop an understanding of the data [ 16 ]. Rapid qualitative analyses are increasingly used for health services and implementation research in which there is a need to quickly, but rigorously, synthesize findings for use in policy and practice decision-making [ 17 , 18 , 19 , 20 ]. Investigators implemented the rapid assessment using a matrix-based approach that included audio-recordings and detailed notes from each panel discussion [ 17 , 21 , 22 , 23 ]. Specifically, recordings and notes were used to develop structured summaries of each panel. Preliminary coding of the structured summaries was completed using codes derived from the moderator guide. The third analyst reviewed transcripts to quality check the summaries and the preliminary coding, transferred summaries into a data matrix organized by the challenges and solutions proposed by each panel, and incorporated supplementary notes and quotes. Using a consensus process, the analysis team reviewed the matrix to refine the coding into meaningful categories and compare challenge and solution themes across panels [ 17 , 24 ]. The study Principal Investigator (DB) reviewed the analysis and provided an additional expert perspective on the interpretation of the summary findings.
Here we describe primary themes that emerged; this report is not inclusive of all challenges and solutions that were discussed.
Participants
Forty-two experts consented to participate. Participant demographics and self-identified stakeholder group are reported in Table 1 . Stakeholders’ ages ranged from between 35 years and 75 years or older. Most stakeholders (71%) had a doctorate degree. All were from the United States or Canada.
Ranking of challenges in pre-panel survey
Aggregated results of the ranking activity, across all panels, are reported in Table 2 . A median ranking score was calculated for each of the challenges, with lower score indicating higher importance of the challenge for addressing the transformative therapy scenario. Of the nine challenges, critical missing data for NBS decision-making was the highest ranked challenge (Median = 2, range 1–9), followed by burden on state NBS laboratories (Median = 3, range 1–8) and the amount of time required for state-level implementation of screening for new conditions (Median = 4, range 1–7).
Overarching themes
Although all panel discussions were framed around the same hypothetical scenario, the content of discussions varied by nature of being prompted based on highly ranked challenges from the pre-panel survey, facilitated by a semi-structured guide, and largely driven by participants. Across all panel discussions, stakeholders expressed shared attitudes and beliefs around which they framed their discussion of NBS’s challenges and solutions. Overarching themes and exemplary quotes can be found in Table 3 .
The infant’s well-being should be the focal point for the NBS system as new solutions are developed and implemented
Stakeholders reinforced their shared desire to connect babies to life-saving treatments and shared frustration at current and potential future barriers to this objective (Table 3 , Quote 3.1.).
The transformative therapy pipeline is a threat to NBS system capacity, which already suffers from inefficiencies and delays because of burden on federal and state systems
Stakeholders acknowledged that the time-consuming nature of evidence review by the ACHDNC and implementation of new conditions by states is not scalable in the context of the transformative therapy pipeline (Table 3 , Quotes 3.2.a. and 3.2.b.).
Decisions about how to modernize the NBS system should be evidence-based
Stakeholders valued evidence-based decision-making. They emphasized that critical data are missing in the current system and that these missing data further threaten the ability of NBS to adapt to anticipated therapeutic advances. Stakeholders thus underscored the importance of solutions that result in data generation using standardized approaches. They also endorsed ongoing assessment of the evidence generated by such approaches, and evidence-based refinements or revisions to the system (Table 3 , Quote 3.3.).
Additional financial support is required but is not sufficient for successful NBS modernization
Stakeholders reinforced the vital need to provide sufficient financial support at all levels of the system: to allow for critical data to be collected and analyzed; to make it feasible for the ACHDNC to more rapidly review a larger number of conditions; for states to implement screening; and for states to support and improve their follow-up programs. But while financial resources are critically needed, the stakeholders agreed that an infusion of funding would not address NBS challenges unless implemented along with other changes to the system (Table 3 , Quote 3.4.).
Successful modernization will require the participation and coordination of multiple stakeholders and organizations in the development, implementation, and ongoing evaluation of new solutions
Participant discussion revealed a shared understanding that NBS is a multifaceted system that requires engagement and collaboration among stakeholders with differing motivations and norms, including federal agencies, researchers, policymakers, treatment facilities, state NBS laboratory and follow-up teams, patient advocates, and the public. Successful NBS requires a broad range of expertise, and diverse stakeholders must be part of planning, implementation, and evaluation of new approaches to modernize NBS modernization (Table 3 , Quote 3.5.).
This final overarching theme of collaboration, coordination and expertise-sharing was reflected in much of the panel deliberations, as described below. Exemplary quotes that address the theme of expertise and coordination challenges can be found in Table 4 .
Expertise and coordination challenges
State/federal coordination challenges.
As a national public health service, NBS requires leadership from the federal level and coordination across 50 states and territories. Stakeholders described the RUSP as an “unfunded mandate,” underscoring the challenge of federally initiated policies driving decision-making for state implementation, but without accompanying financial support or policies to standardize implementation and follow-up. For each condition added to the RUSP, individual state laboratories must expend time and resources on preparatory activities such as verifying screening methodologies and developing standard operating procedures (SOPs) for screening and follow up before implementing a screening approach (Table 4 , quote 4.1.).
As a result, there can be wide variability in the rollout of screening for new conditions across states and the possibility that babies in neighboring states will be screened for a different set of conditions. Longitudinal data, particularly around child outcomes after follow-up, are often missing because of the variability in state data collection requirements and lack of coordination between the medical specialists who provide treatment and the state follow-up program. Stakeholders also described lack of federal guidance and coordination as a contributor to gaps in follow-up data and lack of standardized follow-up practices across states. Finally, stakeholders acknowledged that there is often a lack of coordination and communication between federal agencies that collect data and make decisions regarding the funding and implementation of NBS, such as FDA, CDC, and the Health Resources & Services Administration.
Expertise-related implementation challenges
A successful NBS program requires a broad range of expertise. Stakeholders described how screening, confirmatory testing, and follow-up have become more complex with the advent of new testing methodologies and technology advances (e.g., molecular techniques for confirmatory testing, genotype-specific treatments), causing a need for expanding expertise and a more specialized workforce (Table 4 , Quote 4.2.). Additionally, as NBS expands, follow-up personnel and institutions that provide treatment and management require additional expertise in many rare conditions and across all organ systems.
Simultaneously, NBS has been impacted by staffing issues associated with high turnover rates at state laboratories, in some cases attributed to lower salaries as state employees compared to academic or industry laboratories. NBS is also one of many competing public health priorities that state laboratories are managing. Highly competent laboratory staff and follow-up personnel are necessary for the advent of 30 new conditions being added to the RUSP, and stakeholders doubted states’ ability to recruit and maintain the requisite staffing and expertise to implement so many conditions.
Public education and awareness challenges
NBS stakeholders also described challenges related to public awareness and education. Stakeholders acknowledged NBS as a highly impactful public health initiative but recognized that the impact and benefits of NBS are not widely known by the public or by state legislature . Furthermore, the public perception of NBS has been threatened by recent lawsuits and legislative battles over privacy and allowable uses of dried blood spots collected for NBS (Table 4 , Quote 4.3.).
Stakeholders described a need to improve public knowledge of NBS and build appreciation for the benefits and impact of NBS. There was an emphasis on expanding conversations beyond the typical stakeholders and reaching those who have the greatest stake in NBS: families.
Novel approaches to oversight, partnership, and collaboration
Stakeholders’ proposed solutions to these challenges led to discussions of intersecting topics, including expertise-sharing, capacity-building, and good communication, addressing a broader theme of leveraging stakeholder collaboration to modernize NBS. Additional concepts explored improved integration of federal, state, and nongovernment systems. Exemplary quotes addressing the theme of novel approaches to oversight, partnership, and collaboration can be found in Table 5 .
Expand collaborative pilot studies to test implementation of screening
Stakeholders proposed expanding pilot studies as a way to collect data or increase state laboratory capacity before adding new conditions to states’ panels, which may improve the feasibility of screening for multiple new conditions at once. One proposed model was to support several large or diverse states in conducting collaborative, multi-state pilot studies to obtain missing data on a new condition, or set of conditions, to support evidence-based decision-making prior to adding conditions to the RUSP. This model would leverage cross-state collaboration and information-sharing and likely involvement of federal and academic partners (Table 5 , Quote 5.1.).
Another proposed model was for individual states to offer screening for conditions soon to be added to the RUSP, prior to adding them to the state’s screening panel, through a consented research study similar to existing pilot studies such as Early Check (North Carolina) or ScreenPlus (New York) [ 25 , 26 ]. This model would particularly leverage collaboration between state and academic partners to provide access to screening for some babies prior to full state implementation. Collection of critical missing data would be an important objective, with an understanding that it would be more challenging to achieve representative participation for research that requires parental permission (i.e., not opt-out) for newborn participation.
Use of either of these pilot study models may allow for new academic/public health partnerships to emerge while generating critical data and providing opportunities for states to use research funding (e.g., from NIH) to expand their expertise, obtain necessary equipment, develop and evaluate screening and confirmatory testing SOPs, and test follow-up procedures during pilot implementation.
Develop expertise-sharing models
Stakeholders proposed ways in which some screening methodologies could be moved out of state laboratories to locations with the necessary expertise to conduct screening or second-tier testing. These approaches would maintain state oversight and encourage resource sharing while alleviating implementation burdens on state laboratories.
One proposed solution was the creation of regional NBS laboratories with expertise and capacity in particular methodologies. This model, in which multiple states would send blood spots to the regional laboratory, was proposed to either span all of NBS or to be used for laboratory methodologies that require technological expertise not available in most states. This model would allow states to specialize in some conditions and associated laboratory methodologies and become a regional laboratory for other states while outsourcing other conditions for which they have insufficient equipment or expertise. This regionalization approach was anticipated to potentially streamline processes while reducing overall costs and burden associated with NBS in each state (Table 5 , Quote 5.2.a.).
Another proposed solution was to create or expand collaboration between state NBS programs and universities and academic medical centers. For example, states could outsource screening for certain conditions to university laboratories, and NBS could become more closely aligned with academic medical centers such that states draw from screening, confirmatory testing, interpretation, data analysis and reporting, and follow-up expertise of academic partners rather than bringing new expertise into state laboratories (Table 5 , Quote 5.2.b.). These expertise-sharing solutions may increase state-level resources and capacity and support systematic data collection and analysis, especially as screening and confirmation approaches become more complex.
Develop a public-private partnership to increase resources and reduce burden on the NBS system
Stakeholders described the creation of a consortium of industry, state and federal government, academic partners, and other stakeholders as a possible facilitator of other proposed solutions (e.g., expansion of pilot studies and expertise-sharing models). They suggested that a public-private partnership could provide scientific leadership, oversight, and funding for NBS implementation, ongoing data collection, and reporting of outcomes (Table 5 , Quote 5.3.). For example, private companies involved in therapeutics could fund pilot studies or aspects of screening implementation and in turn be benefited by funding associated with identification of babies who need the treatment they have developed. Private funding could support academic-state partnership models such as regional laboratories or conducting NBS screening and follow-up through academic medical centers, either as the sole funder or supplemented by federal funding streams. Short- and long-term follow-up could also be enhanced by such partnerships, for example by incorporating private genetic counseling organizations into NBS.
Other innovative solutions
Such partnerships may provide the resources and expertise needed to pilot even more radical innovations to the system in response to the rapid development of new treatment approaches for rare conditions. One example was the idea of collaborative pilot studies where industry professionals, academic institutions, and states provide screening for a condition with the goal of offering the parents of affected newborns immediate enrollment of their baby in clinical trials. Another example was the idea that conditions would be automatically added to state NBS at the time of new treatment approval by FDA, either as a pilot or as part of the full state panel. Finally, some stakeholders suggested a “conditional RUSP,” where conditions would have a much lower bar for RUSP approval, with the expectation that data would be obtained during implementation and regularly reviewed to determine whether the condition should remain on the RUSP (Table 5 , Quote 5.4.),
Improve education and public opinion about NBS
Stakeholders suggested opportunities to increase awareness and educate the public about NBS through collaboration of parents/patient advocates, federal and state programs, and other stakeholders. Some recommended reframing rare diseases identified through NBS as a collective public health burden, rather than each new NBS condition presenting a standalone example when it comes to justifying costs or benefits of screening (Table 5 , Quote 5.5.a.).
Stakeholders also suggested that promoting the benefits of NBS through a public relations campaign could prove beneficial for advocacy and “making a case” for screening to state legislators (Table 5 , Quote 5.5.b.). Such a campaign could incorporate the data generated as part of other solutions proposed by stakeholders, such as data on family perspectives of NBS and results of cost-benefit analyses.
NBS is at a critical inflection point. Although the NBS system has been successful at achieving its goals, obvious challenges and inefficiencies will cause major hurdles and delays when lifesaving therapies become available for numerous disorders that are not yet part of the RUSP. The findings from our multistakeholder panels provide important input to inform efforts to modernize NBS in the United States. Participants identified multifaceted challenges that have been previously reported, particularly vital missing data [ 6 , 27 , 28 , 29 ], inadequate resources [ 30 , 31 , 32 , 33 ], and processes that are ill-equipped for rapid or large-scale change [ 30 , 31 , 33 , 34 ]. Although many of the challenges to NBS are exacerbated by insufficient funding [ 28 , 30 , 32 , 33 ], our results suggest that NBS modernization will require systemwide change that includes, but extends beyond, strategic financial investments to support RUSP approval and screening implementation.
Developing and implementing acceptable and efficacious solutions will require new and strengthened collaborations and capacity-building that cannot easily be achieved through the existing NBS system. It is vital that multiple stakeholders are active in developing solutions, and that those stakeholders remain engaged as solutions are implemented and evaluated [ 35 ]. Innovations must work within existing federal and state policies or be addressed through legislative changes. The federal government is unable to mandate state public health practice (with a few notable exceptions), as evidenced by the RUSP being a recommended rather than mandated panel, with states having agency in determining their own screening practices. To that end, participants described innovative ways to leverage strengths, expertise, and resources of stakeholder groups to benefit the overall system.
Participants envisioned both practical and innovative approaches to future NBS. Some of these require only modest change and could be achieved through active collaboration and support of existing NBS state, federal, and academic partners. One example is developing new approaches to regional expertise and expertise sharing. This would allow states to identify their priorities for internal capacity building and share that expertise with others in the region, while relying on reciprocal screening arrangements with other laboratories. The expansion of pilot studies is another approach to develop new collaborations that would result in important data while allowing states an opportunity to implement screening for conditions that are not yet on the RUSP. However, maximizing impact will require addressing multiple limitations, such as the lack of required, standardized collection of natural history and outcomes data that can be used to inform future NBS decision making. It was suggested that more standardized data collection could be facilitated by cross-agency coordination on the federal level.
Other approaches suggested by participants, such as implementing a public-private partnership, have been considered in the NBS context [ 33 ] and could dramatically change the way NBS is led, funded, and conducted in the United States if implemented on a larger scale. Such an approach brings exciting opportunities for innovation but also potential conflicts of interest that must be carefully examined and mitigated. NBS stakeholders’ focal point on the infant’s well-being provides a guiding principle against which to deliberate and negotiate on conflicts of interest, in that conflicts that potentially negatively impact the infant and family should be considered differently than those that do not.
The focus on enhancing infant well-being also provides stakeholders with a shared framework to evaluate the potential benefits and harms of other proposed (and yet to be proposed) innovations to NBS. For example, the intriguing concept of linking NBS pilots to the drug development process highlights competing potential benefits and harms to infants and their families. Newborn identification makes trial participation available as an option for more parents of newborns with rare conditions, which may provide hope and a potential for a better outcome for the child. Additionally, conducting NBS pilots to identify children who can be recruited to ongoing clinical trials would very likely speed up the drug development process, since recruitment of infants with rare disorders is time consuming and costly for trial sponsors [ 25 , 36 ]. This could result in faster access to lifesaving and approved therapies for children around the world. And yet it should not be assumed that trial participation will be acceptable and appealing for all parents, and moreover that all children will meet inclusion criteria or that all families will have the resources (such as parent time, ability to miss work, the ability to travel to sites) necessary to make trial participation possible. Most important is the need to avoid therapeutic misconception (i.e., a failure to appreciate that the purpose of clinical research is to produce generalizable knowledge, regardless of the potential for individual benefit) in the NBS system [ 37 ]. Clinical trials do not provide treatments to affected children, but rather test an experimental drug to determine whether it is safe and whether it works.
Another innovative solution was the concept of conditional RUSP approval, whereby conditions could be approved using a less stringent set of criteria but then periodically be reevaluated based on emerging data from state implementation. Such an approach permits the implementation of a “learning system” that could support infant well-being through enhanced access to disease modifying therapies in the presymptomatic or early symptom stage. And yet conditional approval based on a lower threshold of evidence could result in challenges such as unacceptably high false positive or false negative rates. It could lead to increased uncertainty about which infants need treatment, and when (i.e., based on insufficient natural history data and unexpected disorder heterogeneity), potentially exposing infants to unneeded treatment-related risk and parents to anxiety and burden. Finally, it may be challenging for states to remove a condition from the state’s panel once screening for that condition begins, even if emerging evidence leads to the conditional approval being revoked. Stakeholders across the system should be engaged in this type of deliberation to weigh the potential benefits, harms, and limitations of approaches to NBS modernization.
The findings generated by our multistakeholder panels are an important first step in support of system change to pave the way for next generation NBS. While our study was focused on the NBS system in the United States, the findings will have some applicability to NBS in other countries. The themes that emerged do not reflect consensus among stakeholders; rather, we present concepts that emerged from their discourse on the future of NBS in the context of the transformative therapy scenario. It was not feasible to compare themes by stakeholder group because many participants had current or past experiences in more than one stakeholder group. It will be important for future research and engagement efforts to include stakeholders with broad expertise and experiences to develop detailed implementation objectives and procedures for NBS in the United States. Additional efforts should examine the applicability of the findings to non-U.S. countries.
Availability of data and materials
Because of the nature of this work, the small sample, and assurances of confidentiality provided to participants in the consent form, transcripts of panel discussions cannot be shared. We can provide aggregated, de-identified ranking results from each of the five pre-panel surveys upon request.
Abbreviations
Advisory Committee on Heritable Disorders in Newborns and Children
Centers for Disease Control and Prevention
U.S. Food and Drug Administration
Investigational New Drug
Institutional Review Board
newborn screening
Recommended Uniform Screening Panel
standard operating procedure
Safran C, Bloomrosen M, Hammond WE, Labkoff S, Markel-Fox S, Tang PC, et al. Toward a national framework for the secondary use of health data: an American medical informatics association white paper. J Am Med Inform Assoc. 2007;14(1):1–9.
Article Google Scholar
Schuchat A, Team CC-R. Public health response to the initiation and spread of pandemic COVID-19 in the United States, February 24-April 21, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(18):551–6.
Article CAS Google Scholar
MIT NEWDIGS FoCUS Project. Updated projection of US durable cell and gene therapies product-indication approvals based on December 2019 development pipeline: Ongoing pipeline modeling by the MIT NEWDIGS FoCUS team projects 10X growth from July 2020 levels, but with large uncertainties. MIT Center for Biomedical Innovation; 2020. Report No.: 2020F207-v051.
Bryan WW. FDA/CBER Office of Tissues and Advanced Therapies (OTAT) selected topics. American Society of Gene & Cell Therapy (ASGCT) Liaison Meeting; 2020.
Kemper AR, Green NS, Calonge N, Lam WK, Comeau AM, Goldenberg AJ, et al. Decision-making process for conditions nominated to the recommended uniform screening panel: statement of the US Department of Health and Human Services Secretary's advisory committee on heritable disorders in newborns and children. Genet Med. 2014;16(2):183–7.
Kemper AR, Brosco J, Comeau AM, Green NS, Grosse SD, Jones E, et al. Newborn screening for X-linked adrenoleukodystrophy: evidence summary and advisory committee recommendation. Genet Med. 2017;19(1):121–6.
ALD Alliance. Newborn screening 2021 [Available from: https://www.aldalliance.org/newborn-screening.html .]
Bailey DB, Porter KA, Andrews SM, Raspa M, Gwaltney AY, Peay HL. Expert Evaluation of Strategies to Modernize Newborn Screening in the United States. JAMA Netw Open. 2021;4(12):e2140998. https://doi.org/10.1001/jamanetworkopen.2021.40998
Torok RD, Li JS, Kannankeril PJ, Atz AM, Bishai R, Bolotin E, et al. Recommendations to enhance pediatric cardiovascular drug development: report of a multi-stakeholder think tank. J Am Heart Assoc. 2018;7(4).
Aziz PF, Berger S, Kowey P, Krucoff M, Lopez-Anderson M, Michelson E, et al. The second annual think tank on prevention of sudden cardiac death in the Young: developing a rational, reliable, and sustainable national health care resource. A report from the cardiac safety research consortium. Am Heart J. 2018;202:104–8.
Morain SR, Whicher DM, Kass NE, Faden RR. Deliberative engagement methods for patient-centered outcomes research. Patient. 2017;10(5):545–52.
Bentley C, Peacock S, Abelson J, Burgess MM, Demers-Payette O, Longstaff H, et al. Addressing the affordability of cancer drugs: using deliberative public engagement to inform health policy. Health Res Policy Syst. 2019;17(1):17.
O'Sullivan TL, Corneil W, Kuziemsky CE, Toal-Sullivan D. Use of the structured interview matrix to enhance community resilience through collaboration and inclusive engagement. Syst Res Behav Sci. 2014;32(6):616–28.
Grønkjær M, Curtis T, De Crespigny C, Delmar C. Analysing group interaction in focus group research: impact on content and the role of the moderator. Qualitative Studies. 1970;2(1):16–30.
Ladeji EO. Multi-stakeholder engagement in health services research. J Comp Eff Res. 2018;7(6):517–21.
Beebe J. Rapid assessment process: an introduction. 1st ed. Walnut Creek, CA: AltaMira Press; 2001.
Google Scholar
Hamilton A, editor Qualitative methods in rapid turnaround health services research. VA HSR&D Cyberseminar Spotlight on Women’s Health; 2013; Online.
Koenig CJ, Abraham T, Zamora KA, Hill C, Kelly PA, Uddo M, et al. Pre-implementation strategies to adapt and implement a veteran peer coaching intervention to improve mental health treatment engagement among rural veterans. J Rural Health. 2016;32(4):418–28.
Zuchowski JL, Chrystal JG, Hamilton AB, Patton EW, Zephyrin LC, Yano EM, et al. Coordinating care across health care systems for veterans with gynecologic malignancies: a qualitative analysis. Med Care 2017;55 Suppl 7 Suppl 1:S53-S60.
Moreau JL, Cordasco KM, Young AS, Oishi SM, Rose DE, Canelo I, et al. The use of telemental health to meet the mental health needs of women using Department of Veterans Affairs Services. Womens Health Issues. 2018;28(2):181–7.
Abraham TH, Finley EP, Drummond KL, Haro EK, Hamilton AB, Townsend JC, et al. A method for developing trustworthiness and preserving richness of qualitative data during team-based analysis of large data sets. Am J Eval. 2021;42(1):139–56.
Halcomb EJ, Davidson PM. Is verbatim transcription of interview data always necessary? Appl Nurs Res. 2006;19(1):38–42.
Vindrola-Padros C, Johnson GA. Rapid techniques in qualitative research: a critical review of the literature. Qual Health Res. 2020;30(10):1596–604.
Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855–66.
Bailey DB Jr, Gehtland LM, Lewis MA, Peay H, Raspa M, Shone SM, et al. Early check: translational science at the intersection of public health and newborn screening. BMC Pediatr. 2019;19(1):238.
Wasserstein M, Caggana M, Gelb MH, Goldenberg A, Kelly N, Matern D, et al. ScreenPlus: a comprehensive, dynamic, multi-disorder newborn screening pilot program. Mol Genet Metab 2020;129(2).
Goldenberg AJ, Comeau AM, Grosse SD, Tanksley S, Prosser LA, Ojodu J, et al. Evaluating harms in the assessment of net benefit: a framework for newborn screening condition review. Matern Child Health J. 2016;20(3):693–700.
Prosser LA, Grosse SD, Kemper AR, Tarini BA, Perrin JM. Decision analysis, economic evaluation, and newborn screening: challenges and opportunities. Genet Med. 2013;14(8):703–12.
Riley C, Wheeler A. Assessing the fragile X syndrome newborn screening landscape. Pediatrics. 2017;139(Suppl 3):S207–s15.
(CDC) CfDCaP. Assessment of current practices and feasibility of routine screening for critical congenital heart defects - Georgia, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(15):288–91.
Kellar-Guenther Y, McKasson S, Hale K, Singh S, Sontag MK, Ojodu J. Implementing statewide newborn screening for new disorders: U.S. Program Experiences. International Journal of Neonatal Screening. 2020;6(2):35.
Peterson C, Grosse SD, Glidewell J, Garg LF, Van Naarden BK, Knapp MM, et al. A public health economic assessment of hospitals' cost to screen newborns for critical congenital heart disease. Public Health Rep. 2014;129(1):86–93.
Mann S. Insights in public health: newborn screening saves babies using public/private partnerships. Hawai'i journal of medicine & public health : a journal of Asia Pacific Medicine & Public Health. 2015;74(12):415–8.
Downing GJ, Zuckerman AE, Coon C, Lloyd-Puryear MA. Enhancing the quality and efficiency of newborn screening programs through the use of health information technology. Semin Perinatol. 2010;34(2):156–62.
Bailey DB Jr, Zimmerman SJ. The future of newborn screening: why and how partnerships will be needed for success. N C Med J. 2019;80(1):28–31.
PubMed Google Scholar
Park CH, Winglee M, Kwan J, Andrews L, Hudak ML. Comparison of recruitment strategy outcomes in the National Children's study. Pediatrics. 2017;140(2).
Henderson GE, Churchill LR, Davis AM, Easter MM, Grady C, Joffe S, et al. Clinical trials and medical care: defining the therapeutic misconception. PLoS Med. 2007;4(11):e324.
Download references
Acknowledgements
We are grateful to the study participants who contributed time and expertise to inform the study findings. We would like to recognize the support of Drs. Melissa Raspa and Angela Gwaltney of RTI International for their input in the development of the study and analysis of findings, respectively, and of Ms. Christine Hill of RTI International for her support in coordinating study logistics. We also thank the Consortium of Funders: Orchard Therapeutics, Sarepta Therapeutics, Travere Therapeutics, BioMarin, and the EveryLife Foundation for Rare Diseases.
The study was funded by a Consortium consisting of Orchard Therapeutics, Sarepta Therapeutics, Travere, BioMarin, and the EveryLife Foundation.
Author information
Sara M. Andrews and Katherine Ackerman Porter contributed equally to this work.
Authors and Affiliations
RTI International, 3040 E Cornwallis Rd. Research, Triangle Park, NC, 27709, USA
Sara M. Andrews, Katherine Ackerman Porter, Donald B. Bailey Jr & Holly L. Peay
You can also search for this author in PubMed Google Scholar
Contributions
DBB and HLP contributed to the acquisition of this work. HLP led conception and design of the reported aspects of the study, with significant contributions from KAP and SMA. HLP and SMA collected study data. KAP led rapid qualitative data analysis, with contributions from HLP and SMA (quality check) and DBB (interpretation). SMA and KAP contributed equally to leading the writing of the manuscript. All authors contributed to writing the manuscript and have approved the final text. DBB is the principal investigator and oversaw all aspects of the research.
Although input was obtained from the Consortium of funders at each stage of the research process, the research team at RTI International (inclusive of all authors) was fully responsible for final decisions about study methodology and the content of this manuscript.
Corresponding author
Correspondence to Holly L. Peay .
Ethics declarations
Ethics approval and consent to participate.
The RTI International IRB exempted this study from IRB review (STUDY00021366) under category 2 (Tests, Surveys, Interviews, Public Observation), criteria 2ii (Any disclosure of the human participants’ responses outside the research would not reasonably place the participants at risk of criminal or civil liability or be damaging to the participants’ financial standing, employability, educational advancement, or reputation). This study is not under federal oversight; thus, the IRB used 45 CFR 46 as the ethical framework to guide the review. This study was conducted in accordance with the Declaration of Helsinki. Informed consent to participate was obtained from all participants.
Consent for publication
Informed consent to participate and publish these data was obtained from all participants. No identifying information is included in the manuscript.
Competing interests
In addition to funding for this project, SMA, KAP, DBB, and HLP disclose research funding from Janssen Pharmaceuticals and Asuragen, each of which support projects funded through RTI International.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:.
Hypothetical scenario and challenges from the literature
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Andrews, S.M., Porter, K.A., Bailey, D.B. et al. Preparing newborn screening for the future: a collaborative stakeholder engagement exploring challenges and opportunities to modernizing the newborn screening system. BMC Pediatr 22 , 90 (2022). https://doi.org/10.1186/s12887-021-03035-x
Download citation
Received : 30 September 2021
Accepted : 19 November 2021
Published : 12 February 2022
DOI : https://doi.org/10.1186/s12887-021-03035-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Newborn screening
- Stakeholder engagement
- Public health policy
- Transformative therapies
BMC Pediatrics
ISSN: 1471-2431
- Submission enquiries: [email protected]
- General enquiries: [email protected]
April 9, 2024
COVID Vaccination during Pregnancy Protects Newborn Babies
Studies show that vaccination against COVID during pregnancy provides a powerful safeguard for vulnerable infants too young to receive the vaccine on their own
By Shannon Hall

Stock photo. For illustrative purposes only.
ArtistGNDphotography/Getty Images
When Emily Kara was 34 weeks pregnant, she received an additional COVID vaccine. She did not technically qualify for one. She had received her latest dose merely five months earlier, and her midwife even advised against another shot. But Kara (who asked to go by her middle name out of concern for her privacy) was determined. She had read multiple studies that strongly suggested a maternal COVID vaccine would pass along antibodies to her baby girl and protect her after she was born, when she was vulnerable to SARS-CoV-2 (the virus that causes COVID) and too young to receive the vaccine herself.
So Kara received an extra shot. And she is incredibly thankful that she did. “It gives me peace of mind,” says Kara, whose baby is now nine months old and has not tested positive for COVID.
The first wave of COVID vaccine trials that began in 2020 excluded pregnant people—leaving expectant parents in the dark as to the vaccine’s safety for themselves and their child. But now that millions of pregnant people have received the vaccines, the data are solid. Not only do they show that the vaccines are safe and effective during pregnancy, but a growing consensus is also emerging that vaccinating a pregnant person against COVID can protect their newborn at a time when their little one’s immune system is not mature enough to mount its own defense. Some studies even suggest that the protection lasts until roughly six months of age, when infants are old enough to receive their own vaccine.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
The science is relatively simple: when a COVID vaccine is given during pregnancy, the parent’s immune system develops antibodies against a protein in SARS-CoV-2 that then cross the placenta to the fetus and thus protect the newborn. This is why pregnant people have long been advised to receive the flu shot and the Tdap (tetanus, diphtheria and pertussis) vaccine. And one vaccine—the respiratory syncytial virus (RSV) vaccine approved last year —was even developed specifically to be given during pregnancy to protect the baby after birth. “That is something that we really ought to be leveraging for COVID as well,” says David Kimberlin, a pediatric infectious disease specialist at the University of Alabama at Birmingham. “I think the data are clear.”
A study published in March in Pediatrics from the National Institute of Allergy and Infectious Diseases (NIAID) found that mothers who received an mRNA-based COVID vaccine during pregnancy protected their infant against symptomatic COVID infection for at least six months after birth. Last fall the U.S. Centers for Disease Control and Prevention similarly noted that infants born to women who had received a COVID vaccine—be it the primary series or a booster—at any point during pregnancy had a decreased risk of COVID hospitalization compared with infants who were born to women who had never received a COVID vaccine. And a study published in Nature Medicine in March 2023 found that newborns born to mothers who were vaccinated with a third (booster) dose were half as likely to be hospitalized for COVID as newborns born to mothers who had received the primary series and were eligible for a third dose during pregnancy but had not received it.
This protection is great news given COVID’s risk to newborn babies. “One of the facts that gets lost in the general public is that in the pediatric population, COVID is most severe among young infants , resulting in the highest rates of hospitalization and death in this young age group,” says Cristina Cardemil of the NIAID, who led the Pediatrics investigation. The hospitalization rate in babies under six months increased during the Omicron period and rivals that of adults aged 65-74. Not only have these infants never encountered these infectious diseases but they also have small airways and become dehydrated easily. “They’re doubly at risk for being vulnerable to a number of infectious diseases,” Cardemil says.

Credit: Amanda Montañez; Source: “Effectiveness of BNT162b2 Vaccination during Pregnancy in Preventing Hospitalization for Severe Acute Respiratory Syndrome Coronavirus 2 in Infants,” by Dana Danino et al., in Journal of Pediatrics , Vol. 254; March 2023 ( data )
Now expectant parents have a tool to shield their baby. Multiple studies show similar findings , and many suggest that a COVID booster during the second or third trimester confers the best protection. The Nature Medicine study authors write: “We anticipate that future guidelines will adopt recommendations for routine COVID booster vaccination during the third trimester, aiming to reduce early infant morbidity, similar to recommendations for pertussis and influenza prevention.”
And yet that is not the case. The CDC currently recommends that everyone, pregnant people included, receive the most recent version of the COVID vaccine, but it does not recommend an additional booster to ensure vaccination during pregnancy or point toward a specific administration time. For example, a pregnant person could receive a COVID vaccine in the fall before conceiving and deliver a baby before the next fall vaccine is released—thus missing out on the benefits that an extra vaccine dose confers. The World Health Organization does recommend a single additional dose of the COVID vaccine during pregnancy, but this guideline seems to be the exception. In January, for example, Canada’s National Advisory Committee on Immunization provided guidance on who should get an additional spring booster, and pregnant people were not mentioned. The same was true for the U.K.’s spring booster campaign. (Guidelines from both countries note that the vaccine is safe and effective during pregnancy.)
The issue, experts say, is COVID fatigue. Governing agencies must make recommendations based on what is actually feasible, and an extra booster might be a hard sell when so few pregnant people are up to date on their COVID vaccine in the first place. In the U.S., for example, a mere 13 percent of pregnant people aged 18 to 49 have received the updated 2023–2024 COVID booster. “People are very lackadaisical about it,” says Laura Riley, chair of obstetrics and gynecology at Weill Cornell Medicine in New York City. “And I’m in a place where people get vaccinated.” So the CDC has streamlined the most important message: vaccination protects against disease.
In response to a request for comment, a spokesperson from the CDC said: “Available data show the vaccines for all eligible people—including pregnant people—continue to be strongly protective against severe illness and death.” For that reason, the agency recommends that pregnant people stay up to date on their vaccines, but it will continue to review available evidence on whether additional or differently timed doses might be needed.
Yet many experts argue that the recommendation does a disservice to pregnant people, who are at heightened risk from the disease, and their newborn. “The politicization of vaccines has led to this vaccine not being utilized as much as it should be,” says Sallie Permar, chair of pediatrics at Weill Cornell Medicine and pediatrician in chief at NewYork-Presbyterian Komansky Children’s Hospital. She argues that the COVID vaccine clearly falls into the same category as the flu and Tdap vaccines, whose “safety records and the benefits to both mom and baby have just been universally awesome.”
Kimberlin agrees and is hopeful that the tides will soon shift toward stronger recommendations and a higher vaccine uptake during pregnancy. “This is a very easy way to keep your baby safe,” he says. “And it absolutely should be recommended vigorously.”
Disclaimer » Advertising
- HealthyChildren.org

- Previous Article
- Next Article
Milk Production and Transfer
Neonatal weight and output assessment, glucose stabilization, hyperbilirubinemia, immune development and the microbiome, supplementation, health system interventions: the baby-friendly hospital initiative, limitations and implications for future research, conclusions, acknowledgment, evidence-based updates on the first week of exclusive breastfeeding among infants ≥35 weeks.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- CME Quiz Close Quiz
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Lori Feldman-Winter , Ann Kellams , Sigal Peter-Wohl , Julie Scott Taylor , Kimberly G. Lee , Mary J. Terrell , Lawrence Noble , Angela R. Maynor , Joan Younger Meek , Alison M. Stuebe; Evidence-Based Updates on the First Week of Exclusive Breastfeeding Among Infants ≥35 Weeks. Pediatrics April 2020; 145 (4): e20183696. 10.1542/peds.2018-3696
Download citation file:
- Ris (Zotero)
- Reference Manager
The nutritional and immunologic properties of human milk, along with clear evidence of dose-dependent optimal health outcomes for both mothers and infants, provide a compelling rationale to support exclusive breastfeeding. US women increasingly intend to breastfeed exclusively for 6 months. Because establishing lactation can be challenging, exclusivity is often compromised in hopes of preventing feeding-related neonatal complications, potentially affecting the continuation and duration of breastfeeding. Risk factors for impaired lactogenesis are identifiable and common. Clinicians must be able to recognize normative patterns of exclusive breastfeeding in the first week while proactively identifying potential challenges. In this review, we provide new evidence from the past 10 years on the following topics relevant to exclusive breastfeeding: milk production and transfer, neonatal weight and output assessment, management of glucose and bilirubin, immune development and the microbiome, supplementation, and health system factors. We focus on the early days of exclusive breastfeeding in healthy newborns ≥35 weeks’ gestation managed in the routine postpartum unit. With this evidence-based clinical review, we provide detailed guidance in identifying medical indications for early supplementation and can inform best practices for both birthing facilities and providers.
Exclusive breastfeeding significantly improves maternal and child health. Although US pediatricians’ recommendations are increasingly aligned with American Academy of Pediatrics (AAP) policies, their optimism about the potential for breastfeeding success has declined. 1 To maintain familiarity with the benefits of breastfeeding and the skills necessary to promote this positive health intervention, providers caring for neonates and/or new mothers need updated evidence-based information and tools to assess and manage breastfeeding.
In this review, we provide new evidence from the past 10 years on the following topics relevant to exclusive breastfeeding: milk production and transfer, neonatal weight and output assessment, glucose stabilization, hyperbilirubinemia, immune development and the microbiome, supplementation, and health system interventions. We focus on the early days of exclusive breastfeeding in healthy newborns ≥35 weeks’ gestation managed in the routine postpartum unit. 2 – 6 Tables 1 through 3 and Fig 1 provide summaries based on evidence and authors’ recommendations to provide concise and clear bullets on optimal management. The search strategy and tables of evidence for milk production and transfer, neonatal weight and output assessment, management of glucose, and hyperbilirubinemia are summarized in the Supplemental Information .
Breastfeeding Assessment During the First Postnatal Week
—, not applicable.
Mother, Infant, and Systems-Level Risk Factors for Breastfeeding Difficulties
Adapted from Evans A, Marinelli KA, Taylor JS; Academy of Breastfeeding Medicine. ABM clinical protocol #2: guidelines for hospital discharge of the breastfeeding term newborn and mother: “The going home protocol,” revised 2014. Breastfeed Med . 2014;9(1):4.
Risk Factors for Hypoglycemia
Adapted from Thornton PS, Stanley CA, De Leon DD, et al; Pediatric Endocrine Society. Recommendations from the Pediatric Endocrine Society for evaluation and management of persistent hypoglycemia in neonates, infants, and children. J Pediatr . 2015;167(2):241 and Adamkin DH; Committee on Fetus and Newborn. Postnatal glucose homeostasis in late-preterm and term infants. Pediatrics . 2011;127(3):576.

Supplementation decision algorithm.
Three stages of milk production, lactogenesis I to III, are defined on the basis of volume and composition of milk. For volume, Fig 2 shows estimated daily milk production. 16 In relation to composition, human milk changes dramatically over the first week of lactation. Colostrum, which is produced during the initial stage of lactation (lactogenesis I) in the first days after birth, contains more protein than mature milk. This highly dense early milk has a high concentration of immunoglobulins, activated macrophages, lymphocytes, neutrophils, and growth factors with essential roles in development of gut-associated lymphoid tissue. 17 As milk volume increases (lactogenesis II), sodium concentration and the sodium/potassium ratio decline rapidly with increased secretory activity of the lactocytes and closure of tight cellular junctions. 18 Production of fat-rich, higher-calorie mature milk typically occurs by ∼10 days post partum (lactogenesis III).

Milk volume estimated by breast milk transfer over the first 6 days in vaginal and cesarean births. *Adjusted difference P < .05. Adapted from Evans KC, Evans RG, Royal R, Esterman AJ, James SL. Effect of caesarean section on breast milk transfer to the normal term newborn over the first week of life. Arch Dis Child Fetal Neonatal Ed . 2003;88(5):F382.
Most, but not all, women experience lactogenesis II, referred to as “milk coming in,” by 72 hours post partum. In the Infant Feeding Practices Survey II, 19% of multiparous women and 35% of primiparous women reported milk coming in on day 4 or later. 19 Reasons for delayed lactogenesis II include primiparity, cesarean delivery, and BMI > 27. 20 – 22 Conditions associated with obesity, such as advanced maternal age (possibly related to reduced fertility associated with obesity-variant polycystic ovarian syndrome) and excessive gestational weight gain, may also lead to a delay. 23 , 24 Delayed lactogenesis II is associated with neonatal weight loss >10%. 20
Occasionally, a woman does not experience lactogenesis II and only produces small volumes of milk (prevalence 5%–8%). 19 , 25 The differential diagnosis includes breast pathology, previous breast surgery (with damage to ducts or augmentation for hypoglandular breasts), developmental anomalies of the breast tissue, hormonal disruptions (such as retained placental fragments and pituitary insufficiency, including Sheehan’s syndrome, hypothyroidism, polycystic ovarian syndrome, or theca-lutein ovarian cysts), and toxins (such as excessive tobacco exposure). 26 Occasionally, strategies described here to improve milk production and transfer are not effective, and long-term supplementation with either donor milk or infant formula is medically necessary.
Milk expression is safely and effectively achieved by both manual and mechanical methods and can be used to maintain milk supply in the event of separation from the infant. 27 Hand expression also facilitates milk transfer for the infant learning to breastfeed; both latch and an effective suckling pattern are key. Among mothers of term infants who were feeding poorly, those randomly assigned to hand expression versus electric pumps were more likely to still be breastfeeding at 2 months (96.1% vs 72.7%; P = .02). 28 Infrequent or inadequate signaling due to ineffective or infrequent breastfeeding or milk expression may trigger the autocrine-paracrine mechanisms of halting milk production and dismantling the mammary gland architecture. 29 Milk removal, either via direct breastfeeding or expression, is essential for continuation of milk production.
Some women experience engorgement with lactogenesis II. There is limited evidence regarding the optimal management of engorgement. However, because severe engorgement can impede infant removal of milk, breastfeeding mothers should learn hand expression and reverse pressure softening, which is positive pressure to the central subareolar region, 30 before discharge from maternity care. 31 , 32 If a mother is unable to hand express or her infant is unable to latch, she may require a breast massage 33 and/or use of an electric breast pump.
The components of a comprehensive breastfeeding assessment are described in Table 1 . 12 , 34 It is important to note that a mother’s pumped milk volume may be an inaccurate estimate of milk transfer because transfer also depends on the infant’s capabilities. Associated risk factors for suboptimal milk transfer are listed in Table 2 .
Painful latching deserves special attention as a contributor to low supply, impaired milk transfer, and early cessation of breastfeeding. 35 In an ultrasound study in which breastfeeding mothers with nipple pain were compared with those without, nipple pain was associated with abnormal infant tongue movement, restricted nipple expansion, and lower rates of milk transfer. 36 In a retrospective audit of an Australian breastfeeding center, 36% of visits were for nipple pain. 37 A US study revealed that nipple pain and trauma were among the most frequently cited reasons for early weaning. In a study of >1600 women with singleton births, ∼10% had nipple pain that persisted at postpartum day 7; 72% was attributed to inappropriate positioning and latching, 23% to tongue-tie in the infant, and 4% to oversupply. Women who received treatment recovered within 1 to 2 weeks, and 6-week exclusive breastfeeding rates were no different from those of mothers without nipple pain. 38 Although high-quality randomized controlled trials (RCTs) are needed, frenotomy has been shown to reduce maternal nipple pain in infants with congenital ankyloglossia. 39 There is no evidence that any one topical treatment is superior 40 ; the mainstay of management for nipple pain and fissuring is assistance with positioning and latching. 41
Healthy newborns experience physiologic weight loss after birth, 42 , 43 which, in the exclusively breastfed infant, typically plateaus as the mother’s milk transitions from lactogenesis I to lactogenesis II. The addition of infant formula, either as a supplement or in the form of exclusive formula feeding, is associated with rapid weight gain. This nonphysiologic weight trajectory is associated with childhood obesity. 44 Exclusive direct breastfeeding is inversely associated with the velocity of weight gain throughout the first year of life. 45 In one prospective cohort study of >300 newborns, weight gain >100 g during the first week after birth was independently associated with overweight status at age 2 (adjusted odds ratio [aOR] 2.3; 95% confidence interval [CI] 1.1 to 4.8). 44
Early infant weight loss should be evaluated in the context of the clinical status of the infant and the mother. Nomograms for newborn weight have been developed by using data from >100 000 healthy, exclusively breastfed infants in California. 46 Individual infant weights can be plotted against these nomograms by using the Newborn Early Weight Tool (NEWT) ( https://www.newbornweight.org ). Weight loss trajectory over time, combined with clinical information, provides a robust context for evidence-based decision-making. 47 Weight loss in the >75th percentile on NEWT nomograms for mode of delivery and infant age should prompt a thorough evaluation.
A term newborn’s weight is 75% water, compared with 60% for an adult. Urine output is usually low in the first 1 to 2 days after birth, after which a physiologic diuresis and loss of up to 7% to 10% of birth weight occurs. 48 , 49 Insufficient milk production and/or transfer in the exclusively breastfed newborn can contribute to excessive weight loss in the first few days of life. Low milk supply, often exacerbated by poor feeding or difficulty in suckling, correlates with elevated milk sodium levels. 50 Exclusively breastfed infants, especially those born via cesarean delivery, are at increased risk for greater weight loss, dehydration, and hypernatremia. 51 , 52 In a systematic review of hypernatremia among breastfed infants, significant risk factors included weight loss >10%, cesarean delivery, primiparity, breast anomalies, reported breastfeeding problems, excessive prepregnancy maternal weight, delayed first breastfeeding, lack of previous breastfeeding experience, and low maternal education. 53 Prevention strategies included daily weights coupled with lactation support during the first 4 to 5 days after birth.
Early weight loss nomograms for exclusively breastfed newborns can help identify those infants at risk for hypernatremic dehydration (HD), 54 , 55 a rare condition characterized by lethargy, restlessness, hyperreflexia, spasticity, hyperthermia, and seizures, with an estimated incidence of 20 to 70 per 100 000 births and up to 223 per 100 000 births among primiparous mothers. 56 Use of charts for weight loss with SD scores specifically to detect HD, combined with a policy of weight checks on days 2, 4, and 7 of life, had high sensitivity (97%) and specificity (98.5%) to detect HD. 47 However, given the low incidence of HD, the positive predictive value (PPV) of repeated weight checks alone was only 4.4%. 56
Importantly, elimination patterns during the first 2 days of life are neither sensitive nor specific as measures of infant intake. 49 Infants may be voiding and stooling despite insufficient intake or, more commonly, have decreased voiding and stooling compared with exclusively formula-fed infants despite adequate intake. In a cohort study of 313 infants, the frequency of urination and stooling was significantly decreased among exclusively breastfed infants compared with exclusively formula-fed infants during the first 3 days of life then rose and significantly surpassed that of exclusively formula-fed infants by day 6 of life. 49 Another prospective cohort study of 280 mother-infant pairs examined elimination patterns in relation to excessive weight loss (>10%) between 72 and 96 hours after birth. 48 The strongest association with weight loss >10% was with <4 stools after 72 hours or maternal perception of delayed lactogenesis II. Although term and late-preterm infants generally pass meconium within 48 hours (76%–83% in a study of 198 infants), delayed passage of meconium can be a marker for insufficient milk intake. 57 Correlations between infants’ intake and elimination are more reliable after the first 3 days (lactogenesis II).
To prepare for transitional energy needs, the third-trimester fetus stores glycogen, manufactures catecholamines, and deposits brown fat. Healthy newborns use these stores to maintain thermoregulation and meet their energy needs through metabolism of brown fat and the release of counterregulatory hormones such as glucagon, epinephrine, cortisol, and growth hormone. Combined with declining insulin secretion, these hormones mobilize glucose and alternative fuels, such as lactate and ketone bodies, to support organ functions. 58 , 59
Because oral intake is not the main energy source for healthy term neonates in the first days after birth, physiologic volumes of colostrum (16 kcal/oz) are sufficient to meet metabolic demands. As glycogen stores are depleted, coinciding with the transition from colostrum to mature milk, newborns transition from a catabolic state to reliance on enteral feeds, with approximately half of the caloric content derived from fat. 60
After placental detachment, neonatal glucose levels reach a physiologic nadir in the first hours after birth and then typically rise to adult levels a few days later. The threshold for neonatal glucose that is associated with neurotoxicity is unclear; a 2008 National Institutes of Health workshop concluded that “there is no evidence-based study to identify any specific plasma glucose concentration (or range of glucose values) to define pathologic hypoglycemia.” 61 In one cohort study, treatment of asymptomatic newborn hypoglycemia to maintain blood glucose levels >47 mg/dL had no effect on cognitive performance at 2 years; however, at 4.5 years, there were dose-dependent concerns regarding visual motor and executive function, with the highest risk in children exposed to severe (<36 mg/dL),and recurrent (≥3 episodes) hypoglycemia. 62 , 63
In the first hours after birth, healthy term neonates compensate for relatively low glucose levels by decreasing insulin production and increasing glycolysis, gluconeogenesis, and ketone production. Among at-risk newborns, early skin-to-skin care plus early feeding and blood glucose assessment at 90 minutes supports glucose homeostasis and is associated with decreased risk of hypoglycemia and NICU admission. 64 In a Cochrane review, early skin-to-skin contact increased glucose levels by 10.49 (95% CI 8.39 to 12.59) mg/dL or 0.6 (0.5 to 0.7) mmol/L. 65 Conversely, practices that separate the mother and infant and delay the first feeding increase hypoglycemia risk.
Glucose monitoring is recommended for infants with risk factors ( Table 3 ) and for any infant who exhibits symptoms of hypoglycemia. 66 Because operational thresholds for treating hypoglycemia and target glucose levels are not defined, clinical recommendations vary. Infants who require early or more frequent feedings should be supported to breastfeed and/or receive expressed milk. Authors of multiple studies confirm the benefits of using glucose gel rather than formula as an initial treatment of low glucose levels, and this practice has become increasingly commonplace. 67 – 73 Some institutions use pasteurized donor human milk (PDHM) as a treatment of hypoglycemia; however, there are, as yet, no published studies describing outcomes of this practice. The option of antenatal milk expression for lower-risk women with preexisting or gestational diabetes may also be considered because this technique may preserve exclusive breastfeeding without adversely affecting perinatal outcomes. 74 Infants requiring intravenous glucose should breastfeed, when able, during the therapy.
Persistent or late-onset hypoglycemia (>48 hours after birth) can occur in the setting of congenital endocrine disorders or, more commonly, perinatal stress due to birth asphyxia, intrauterine growth restriction, maternal preeclampsia, 75 or persistent problems establishing breastfeeding. 76 Infants with these risk factors may be more vulnerable to insufficient feeding, so skilled assessment is essential.
Management of hyperbilirubinemia in the exclusively breastfed newborn depends on whether the excess in bilirubin is pathologic or physiologic. Neonatal bilirubin levels rise after birth because of physiologic immaturity of glucuronyl transferase, which is exaggerated with each decreasing week of gestational age. Exclusively breastfed infants have higher serum bilirubin levels than formula-fed infants, possibly because of differences in fluid intake and bilirubin excretion and increased enterohepatic resorption of bilirubin. 77 Some individuals may also have a genetic predisposition to higher bilirubin levels. 78 , 79 Bilirubin is an antioxidant, and it has been hypothesized that moderate increases in bilirubin levels may be protective for the transition to extrauterine life. 77 , 80
In contrast, pathologic hyperbilirubinemia resulting from insufficient breastfeeding, sometimes referred to as breastfeeding jaundice, is better defined as suboptimal intake jaundice. 77 In the United States and Canada, it is recommended that all neonates undergo bilirubin risk screening at least once before hospital discharge. 81 The Academy of Breastfeeding Medicine and the AAP advise the use of Bhutani curves to assess risk and need for treatment of hyperbilirubinemia; clinical tools are available on mobile device applications. 77 , 81 , 82 This approach has led to a decrease in severe pathologic hyperbilirubinemia 83 ; however, concerns for overtreatment and the potential harm of phototherapy have arisen recently. 84 Using subthreshold bilirubin levels to initiate phototherapy as a mechanism to prevent readmission is not recommended because this approach increases length of stay and results in many infants receiving unnecessary treatment to reduce each case of readmission. 85
Breastfed infants with hyperbilirubinemia require assessment of milk production and transfer, feeding frequency, and neonatal weight loss. 86 – 91 If there is pathologic hyperbilirubinemia, and infant intake at the breast is sufficient, exclusive breastfeeding should be continued while the infant receives phototherapy. Although supplementation with infant formula may decrease the bilirubin level and risk of readmission for phototherapy, 85 it will also interfere with the establishment and continuation of breastfeeding. 92 If intake at the breast is insufficient and supplementation is medically necessary, expressed maternal milk is preferred. Despite the current lack of data on its benefits in reducing hyperbilirubinemia in term infants, the use of PDHM to preserve exclusive human-milk feeding is increasing. 93
Phototherapy for neonatal jaundice and concerns about insufficient milk can be anxiety provoking for parents, even in a supportive environment, and can be disruptive to successful breastfeeding. 94 Practices to minimize mother-infant separation, including providing phototherapy in the same room and maintaining safe skin-to-skin care with the infant’s mother, also promote exclusive breastfeeding. 95
Early colostrum and exclusive breastfeeding establish an optimal and intact immune system. Unlike infant formula, human milk has a dynamic composition of both macro- and micronutrients that varies within a feed, diurnally, and over the course of lactation. Protective proteins abound in human milk, including lactoferrin, secretory immunoglobulin A, transforming growth factor-β, and α-lactalbumin. These factors promote development of the infant’s immune system. 96 Additionally, lactoferrin has unique antibacterial properties important in the prevention of sepsis. Unique nonnutritive oligosaccharides that are specific to the mother-infant pair’s shared environment and exposures prevent binding of pathogenic bacteria and promote a healthy microbiome in the gut. 97 Differences in immune cell distributions based on neonatal diet can be detected through 6 months of age, with natural killer cells most significantly affected. 98
During vaginal birth, the newborn’s intestine and mucosal surfaces are colonized with maternal microbes that act synergistically with bioactive factors in mother’s milk to establish a robust lymphoid follicle replete with a healthy balance of T helper cells. 99 , 100 Surgical delivery is associated with aberrant colonization, which may lead to differences in the mother’s milk microbiome 101 only partially restored by vaginal secretions. 102 Formula supplementation may effect the most change in the newborn’s microbiome 103 , 104 and immune development. These basic science findings are supported by clinical studies.
Given the multiple mechanisms through which exclusive human milk impacts gut development, formula supplementation should always be avoided when the mother’s own milk is available. Although an exploratory study of early limited supplementation with extensively hydrolyzed formula followed by a return to exclusive breastfeeding did not reveal differences in the developing microbiome ( N = 15), 105 a longitudinal study among infants exclusively breastfeeding at 3 months ( N = 579) revealed alterations in the microbiome among infants exposed to formula as neonates ( n = 179). 106 Just as antimicrobial stewardship requires appropriate use of antibiotics, 107 supplementation stewardship requires judicious use of formula when medically indicated.
A systematic review of healthy, term, breastfed newborns revealed no benefit from routine supplementation with foods or fluids in the early postpartum period. 108 These findings are consistent with consensus recommendations for exclusive breastfeeding for the first 6 months, followed by continued breastfeeding with the addition of complementary foods until at least 12 months of age. 2 , 109 – 111 Early introduction of supplemental formula is associated with a greater than twofold increase in risk of early cessation of breastfeeding even when controlling for confounding variables. 112 – 114 Among almost 1500 women in the Infant Feeding Practices Study II, only early exclusive breastfeeding remained significant for achieving intended breastfeeding duration (aOR 2.3; 95% CI 1.8 to 3.1) after adjustment for relevant hospital practices. 113 This finding may be due in part to the supply and demand nature of milk production and the role of suckling, oxytocin release, and milk removal in establishing lactation.
If supplemental feeds are medically indicated, they should be accompanied by manual or mechanical milk expression, recognizing that direct breastfeeding usually provides more complete milk removal. 115 In a pilot RCT ( N = 40), early limited formula supplementation for infants with ≥5% weight loss increased exclusive breastfeeding at 3 months post partum. 116 In a subsequent larger study ( N = 164), early limited supplementation did not affect overall breastfeeding at 1 or 6 months but slightly increased rates of formula use at 1 month (36.7% vs 22.4%; P = .08), 105 decreased breastfeeding at 12 months (30% vs 48%; risk difference −18% [CI −34% to −3%]), and shortened the time to breastfeeding cessation (hazard ratio 0.65; 95% CI 0.43 to 0.97). 117
Because evidence continues to accrue that supplementation in the first days after birth has major health risks, 103 , 106 judicious use of supplementation is a critical goal, with a return to exclusivity whenever possible. If supplementation is indicated ( Fig 1 ), options in order of preference are (1) expressed milk from the infant’s own mother, 4 (2) PDHM, and (3) commercial infant formulas. The potential risks and benefits of these options should be considered in the context of the infant’s age, the volume required, and the impact on the establishment of breastfeeding. 4
Methods of supplemental feeding include spoon or cup feeds, supplemental nursing systems, syringe feeds, and paced bottle feeds. Methods should be tailored to staff training and family preferences. 7 Among late-preterm newborns, there is evidence that some may be more susceptible to feeding problems when supplemented via a bottle; in an RCT in which the 2 methods were compared, cup feeding was associated with a longer duration of exclusive breastfeeding compared with bottle-feeding. 118 Among term newborns, the manner in which supplementation is delivered, whether a bottle or alternative devices, has no apparent impact on continuation of breastfeeding. 119 If the supplement is the mother’s own expressed milk, avoidance of bottles and nipples may preserve a longer duration of breastfeeding, especially among late-preterm newborns. 120
To ensure milk removal, which is key to establishing a milk supply, a mother should be assisted to express milk each time her infant is supplemented, even if the infant is also “practicing” at the breast. 4 “Hands on” pumping, combining breast massage with pumping, has been shown to increase milk production in mothers of preterm infants who are hospitalized. 121
Physiologic early infant feeding is facilitated by keeping mothers close to their infants, beginning with skin-to-skin care immediately after birth and continuing with 24-hour rooming-in and feeding on cue. These are core practices of the recently updated World Health Organization’s Ten Steps to Successful Breastfeeding of the Baby-Friendly Hospital Initiative (BFHI). 7 Feeding on cue or “responsive feeding” is associated with more frequent breastfeeding throughout the day, more exclusive breastfeeding up to 6 months and beyond, 122 – 124 and decreased likelihood of abnormal rapid weight gain in infancy. 125
Several major health organizations, including the US Preventive Services Task Force and the Agency for Healthcare Research and Quality, have generated systematic reviews and quality improvement (QI) reports that demonstrate the positive impact of the BFHI on breastfeeding outcomes. 10 , 13 , 14 Implementation of maternity care practices aligned with any component of the BFHI is associated with improved in-hospital and postdischarge breastfeeding rates. 11 , 13 , 126 Best Fed Beginnings increased exclusive breastfeeding initiation from 39% to 61% ( t = 9.72; P < .001) at 89 hospitals over 2 years. 127 The Community and Hospitals Advancing Maternity Care Practices initiative reported that the BFHI helped to reduce racial disparities in breastfeeding in southern US states. 128
Since the initial implementation of the BFHI, safety concerns have emerged, including case reports of inadvertent bed-sharing, suffocation, falls, and increased risk of neonatal jaundice. 3 , 129 In this context, the World Health Organization conducted an extensive evidence-based review. 7 , 130 Key differences in the revised Ten Steps include highlighting the Code of Marketing of Breastmilk Substitutes, the need to collect ongoing data, a focus on safety and surveillance (especially as it relates to skin-to-skin care and rooming-in), and acknowledgment that there is insufficient evidence to limit pacifiers and other artificial nipples.
Step 10 of the BFHI requires a direct connection between the delivery hospital and the community for ongoing support. Referral for outpatient support as well as provision of contact information for those who can manage breastfeeding problems is paramount.
Given the importance of exclusive breastfeeding for maternal and child health, both intent and initiation are increasing. However, maternal conditions linked with delayed lactogenesis, such as advanced maternal age, obesity, and fertility treatment, are increasingly common. Priority research areas to help families meet their breastfeeding goals include accurate identification of women with risk factors for delay or absence of lactogenesis, more sensitive methods of identifying at-risk newborns, and exploration of the implications of early limited formula supplementation on infant outcomes such as ontogeny of the immune system and the microbiome, maternal self-efficacy, and continued breastfeeding.
Health care professionals’ support is critical for families to meet their infant feeding goals and achieve optimal health outcomes. All physicians who care for new mothers and infants need skills to evaluate early breastfeeding, perform maternal and infant risk stratification, understand the range of potential interventions in the context of the risk/benefit ratio of supplementation, and ensure appropriate follow-up.
Most mothers can produce adequate colostrum and mature milk, and most newborns are able to breastfeed exclusively. Nevertheless, conditions that require medical supplementation are common and important to recognize. The decision to supplement with infant formula requires thoughtful analysis of the risks and benefits, with consideration of the family’s informed choice. Early-term and late-preterm newborns are at a higher risk of complications. Therefore, more careful monitoring, detailed assessments, and case-based interventions are warranted. Further research is needed to identify the best methods to support exclusive breastfeeding in high-risk populations.
We thank Delali Lougou for organizing the articles used in this article to provide the original framework for the authors’ review.
Drs Feldman-Winter, Kellams, and Stuebe conceptualized and designed the review of the literature, conducted the literature review and analysis, drafted the initial manuscript, and reviewed and revised the manuscript; Dr Peter-Wohl made substantial contributions to the acquisition of data and to the analysis and interpretation of data, drafted the article, and revised it critically for important intellectual content; Dr Taylor made substantial contributions to conception and design and made critical revisions; Drs Lee and Terrell made substantial contributions to the design and to the acquisition of data and made critical revisions for important intellectual content; Drs Meek and Noble and Ms Maynor made substantial contributions to the conception, design, and analysis and interpretation of data and revised the article critically for important intellectual content; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FUNDING: No external funding.
American Academy of Pediatrics
adjusted odds ratio
Baby-Friendly Hospital Initiative
confidence interval
hypernatremic dehydration
Newborn Early Weight Tool
pasteurized donor human milk
positive predictive value
quality improvement
randomized controlled trial
Competing Interests
Supplementary data.
Advertising Disclaimer »

Citing articles via
Email alerts.

Affiliations
- Editorial Board
- Editorial Policies
- Journal Blogs
- Pediatrics On Call
- Online ISSN 1098-4275
- Print ISSN 0031-4005
- Pediatrics Open Science
- Hospital Pediatrics
- Pediatrics in Review
- AAP Grand Rounds
- Latest News
- Pediatric Care Online
- Red Book Online
- Pediatric Patient Education
- AAP Toolkits
- AAP Pediatric Coding Newsletter
First 1,000 Days Knowledge Center
Institutions/librarians, group practices, licensing/permissions, integrations, advertising.
- Privacy Statement | Accessibility Statement | Terms of Use | Support Center | Contact Us
- © Copyright American Academy of Pediatrics
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Share full article
Advertisement
Supported by
Large Scientific Review Confirms the Benefits of Physical Touch
Premature babies especially benefited from skin-to-skin contact, and women tended to respond more strongly than men did.

By Joanne Silberner
A hug, a handshake, a therapeutic massage. A newborn lying on a mother’s bare chest.
Physical touch can buoy well-being and lessen pain, depression and anxiety, according to a large new analysis of published research released on Monday in the journal Nature Human Behaviour.
Researchers from Germany and the Netherlands systematically reviewed years of research on touch, strokes, hugs and rubs. They also combined data from 137 studies, which included nearly 13,000 adults, children and infants. Each study compared individuals who had been physically touched in some way over the course of an experiment — or had touched an object like a fuzzy stuffed toy — to similar individuals who had not.
For example, one study showed that daily 20-minute gentle massages for six weeks in older people with dementia decreased aggressiveness and reduced the levels of a stress marker in the blood. Another found that massages boosted the mood of breast cancer patients. One study even showed that healthy young adults who caressed a robotic baby seal were happier, and felt less pain from a mild heat stimulus, than those who read an article about an astronomer.
Positive effects were particularly noticeable in premature babies, who “massively improve” with skin-to-skin contact, said Frédéric Michon, a researcher at the Netherlands Institute for Neuroscience and one of the study’s authors.
“There have been a lot of claims that touch is good, touch is healthy, touch is something that we all need,” said Rebecca Boehme, a neuroscientist at Linkoping University in Sweden, who reviewed the study for the journal. “But actually, nobody had looked at it from this broad, bird’s eye perspective.”
The analysis revealed some interesting and sometimes mysterious patterns. Among adults, sick people showed greater mental health benefits from touch than healthy people did. Who was doing the touching — a familiar person or a health care worker — didn’t matter. But the source of the touch did matter to newborns.
“One very intriguing finding that needs further support is that newborn babies benefit more from their parents’ touch than from a stranger’s touch,” said Ville Harjunen, a researcher at the University of Helsinki in Finland, who also reviewed the study for the journal. Babies’ preference for their parents could be related to smell, he speculated, or to the differences in the way parents hold them.
Women seem to benefit more from touch than men, which may be a cultural effect, Dr. Michon said. The frequency of the touch also mattered: A massage once every two years isn’t going to do much.
Several studies included in the review looked at what happened during the height of the Covid pandemic, when people were isolated and had less physical contact with others. “They found correlations during Covid times between touch deprivation and health aspects like depression and anxiety,” Dr. Michon said.
Touching the head appears to have more of a beneficial effect than touching the torso, some studies found. Dr. Michon couldn’t explain that finding, but thought it could have to do with the greater number of nerve endings on the face and scalp.
Another mystery: Studies of people in South America tended to show stronger health benefits of touch than did those studies that looked at people in North America or Europe. Dr. Michon said that culture may somehow play a role. But Dr. Boehme said the studies showing the differences between countries were too small to be definitive. “I think the mechanism behind this is biological,” she said. “I think that’s hard-wired and will be the same for all of us.”
In 2023, Jeeva Sankar, a pediatrics researcher at All India Institute of Medical Sciences, and a colleague published a rigorous review of skin-to-skin care for newborns. The analysis concluded that touch therapy for preterm or low-birth-weight infants should start as soon as possible and last eight hours or more, a recommendation that the World Health Organization adopted. Dr. Sankar said the new review was important because touch is often neglected in modern medical care, but it was too broad. He would have liked it to focus more on how various forms of touch could be integrated in medical care.
Dr. Michon stressed that the types of touch considered in these studies were positive experiences to which the volunteers agreed. “If someone doesn’t feel a touch as being pleasant, it’s likely going to stress them out,” he said.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
March 21, 2023
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Newborn babies' brains reveal new insights in child development
by Emma Robinson, King's College London
Researchers publishing in Nature Human Behaviour , looked at how different (asymmetrical) the left and right side of babies' brains were in terms of their shape and patterns of brain activity to establish a benchmark for future studies to investigate how asymmetries relate to brain disorders.
Previous research has shown that people with and without mental health conditions have different brain asymmetries . There is a limited understanding of how asymmetric babies' brains are at birth, and since babies act differently from adults, and cannot do tasks during MRI scans, this study aimed to understand how asymmetric the brain is at birth, if at all.
For this study, the researchers investigated asymmetries in 442 healthy term-born neonate babies (newborns in the first month after birth ) using MRI images that showed the function and structure of the brain, from the Developing Human Connectome Project . These differences were compared against similar patterns taken from 103 preterm neonates scanned at the same age, as well as 1,110 healthy young adults from the Human Connectome Project .
This study found the presence of many brain asymmetries in the areas responsible for language and vision tasks like face recognition. Moreover, we found that brain asymmetries in babies born early were the same as those in babies born on time. This is significant, because being born early affects nearly all other aspects of brain development except brain asymmetry, said Logan Williams, Ph.D. student from the School of Biomedical Engineering & Imaging Sciences.
He added, "Our findings suggest that brain asymmetries are hard-wired and are very important for future brain development."
The study found that neonatal babies' brains are very asymmetric and show similar patterns to adults. This finding will act as a baseline for future studies that explore brain symmetry and allow scientists to interpret studies where they are investigating changes of brain asymmetry in relation to certain health conditions.
Explore further
Feedback to editors
Study reveals potential to reverse lung fibrosis using the body's own healing technique
2 hours ago
Researchers discover cell 'crosstalk' that triggers cancer cachexia

Study improves understanding of effects of household air pollution during pregnancy

Wearable sensors for Parkinson's can improve with machine learning, data from healthy adults
3 hours ago

New insights on B cells: Researchers explore building better antibodies and curbing autoimmune diseases

Grieving pet owners comforted by 'supernatural' interactions

Study shows AI improves accuracy of skin cancer diagnoses

Cell's 'garbage disposal' may have another role: Helping neurons near skin sense the environment

Chlamydia vaccine shows promise in early trial

Researchers find no link between COVID-19 virus and development of asthma in children
4 hours ago
Related Stories

Breast milk boosts premature babies' brain development, suggests study
Feb 28, 2023

Premature birth associated with 'profound reduction' in brain connections
Jul 27, 2021

How left brain asymmetry is related to reading ability
Apr 5, 2022
Premature birth linked to older 'brain age' in adult life
Sep 26, 2017

Brain scans may indicate clues to later problems
Feb 1, 2017
Baby brain scans made available online to advance research
Sep 23, 2019
Recommended for you

Study finds that dopamine projections to the amygdala contribute to encoding identity-specific reward memories
9 hours ago

First clinical trial of vosoritide for children with hypochondroplasia shows increased growth
5 hours ago
How the inflamed brain becomes disconnected after a stroke
6 hours ago

Two key brain systems are central to psychosis, study finds
20 hours ago
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
- Mission, Facts and Figures
- Deans, Chairs and Staff
- Leadership Council
- Dean in the News
- Get Involved
- DEIB Mission
- Message from DEIB Associate Dean
- News and Media
- Reading Lists
- The Yale and Slavery Research Project
- Photo Gallery
- Winslow Medal
- Coat of Arms & Mace
- $50 Million Challenge
- For Pandemic Prevention and Global Health
- For Understanding the Health Impacts of Climate Change
- For Health Equity and Justice
- For Powering Health Solutions through Data Science
- For Future Leaders
- For Faculty Leaders
- For Transformational Efforts
- An abiding love for Yale turns into a lasting gift – in 15 minutes
- Endowed Professorship Created at Critical Time for Yale School of Public Health
- Brotherly encouragement spurs gift to support students
- Prestipino creates opportunities for YSPH students, now and later
- Alumna gives back to the school that “opened doors” in male-dominated field
- For Public Health, a Broad Mission and a Way to Amplify Impact
- Couple Endows Scholarship to Put Dreams in Reach for YSPH Students
- A Match Made at YSPH
- A HAPPY Meeting of Public Health and the Arts
- Generous Gift Bolsters Diversity & Inclusion
- Alumni Donations Aid Record Number of YSPH Students
- YSPH’s Rapid Response Fund Needs Donations – Rapidly
- Podiatric Medicine and Orthopedics as Public Health Prevention
- Investing in Future Public Health Leaders
- Support for Veterans and Midcareer Students
- Donor Eases Burden for Policy Students
- A Personal Inspiration for Support of Cancer Research
- Reducing the Burden of Student Debt
- Learning About Global Health Through Global Travel
- A Meeting in Dubai, and a Donation to the School
- Rapid Response Fund
- Planned Giving
- Testimonials
- Faculty, Postdoc Jobs
- For the Media
- Issues List
- PDF Issues for Download
- Editorial Style Guide
- Social Media
- Accreditation
- Faculty Directory by Name
- Career Achievement Awards
- Annual Research Awards
- Teaching Spotlights
- Biostatistics
- Chronic Disease Epidemiology
- Climate Change and Health Concentration
- Environmental Health Sciences
- Epidemiology of Microbial Diseases
- Global Health
- Health Policy and Management
- Maternal and Child Health Promotion Track
- Public Health Modeling Concentration
- Regulatory Affairs Track
- Social & Behavioral Sciences
- U.S. Health Justice Concentration
- Events and Contact
- What Does it Take to be a Successful YSPH Student?
- How to Apply and FAQs
- Incoming Student Gateway
- Traveling to Yale
- Meet Students and Alumni
- Past Internship Spotlights
- YSPH in Video
- Student-run Organizations
- MS and PhD Student Leaders
- Staff Spotlights
- Life in New Haven
- Libraries at Yale
- The MPH Internship Experience
- Practicum Course Offerings
- Summer Funding and Fellowships
- Downs Fellowship Committee
- Stolwijk Fellowship
- Climate Change and Health
- Career Management Center
- What You Can Do with a Yale MPH
- MPH Career Outcomes
- MS Career Outcomes
- PhD Career Outcomes
- Employer Recruiting
- Tuition and Expenses
- External Funding and Scholarships
- External Fellowships for PhD Candidates
- Alumni Spotlights
- Bulldog Perks
- Stay Involved
- Board of Directors
- Emerging Majority Affairs Committee
- Award Nomination Form
- Board Nomination Form
- Alumni Engagement Plus
- Mentorship Program
- The Mentoring Process
- For Mentors
- For Students
- Recent Graduate Program
- Transcript and Verification Requests
- Applied Practice and Student Research
- Competencies and Career Paths
- Applied Practice and Internships
- Student Research
- Seminar and Events
- Competencies and Career paths
- Why the YSPH Executive MPH
- Message from the Program Director
- Two-year Hybrid MPH Schedule
- The Faculty
- Student Profiles
- Newsletter Articles
- Approved Electives
- Physicians Associates Program
- Joint Degrees with International Partners
- MS in Biostatistics Standard Pathway
- MS Implementation and Prevention Science Methods Pathway
- MS Data Sciences Pathway
- Internships and Student Research
- Competencies
- Degree Requirements - Quantitative Specialization
- Degree Requirements - Clinical Specialization
- Degree Requirements- PhD Biostatistics Standard Pathway
- Degree Requirements- PhD Biostatistics Implementation and Prevention Science Methods Pathway
- Meet PhD Students in Biostatistics
- Meet PhD Students in CDE
- Degree Requirements and Timeline
- Meet PhD Students in EHS
- Meet PhD Students in EMD
- Meet PhD Students in HPM
- Degree Requirements - PhD in Social and Behavioral Sciences
- Degree Requirements - PhD SBS Program Maternal and Child Health Promotion
- Meet PhD Students in SBS
- Differences between MPH and MS degrees
- Academic Calendar
- Translational Alcohol Research Program
- Molecular Virology/Epidemiology Training Program (MoVE-Kaz)
- For Public Health Practitioners and Workforce Development
- Course Description
- Instructors
- Registration
- Coursera Offerings
- Non-degree Students
- International Initiatives & Partnerships
- NIH-funded Summer Research Experience in Environmental Health (SREEH)
- Summer International Program in Environmental Health Sciences (SIPEHS)
- 2022 Student Awards
- APHA Annual Meeting & Expo
- National Public Health Week (NPHW)
- Leaders in Public Health
- YSPH Dean's Lectures
- The Role of Data in Public Health Equity & Innovation Conference
- Innovating for the Public Good
- Practice- and community-based research and initiatives
- Practice and community-based research and initiatives
- Activist in Residence Program
- Publications
- Health Care Systems and Policy
- Heart Disease and Stroke
- SalivaDirect™
- COVID Net- Emerging Infections Program
- Panels, Seminars and Workshops (Recordings)
- Public Health Modeling Unit Projects
- Rapid Response Fund Projects
- HIV-AIDS-TB
- The Lancet 2023 Series on Breastfeeding
- 'Omics
- News in Biostatistics
- Biostatistics Overview
- Seminars and Events
- Seminar Recordings
- Statistical Genetics/Genomics, Spatial Statistics and Modeling
- Causal Inference, Observational Studies and Implementation Science Methodology
- Health Informatics, Data Science and Reproducibility
- Clinical Trials and Outcomes
- Machine Learning and High Dimensional Data Analysis
- News in CDE
- Nutrition, Diabetes, Obesity
- Maternal and Child Health
- Outcomes Research
- Health Disparities
- Women's Health
- News in EHS
- EHS Seminar Recordings
- Climate change and energy impacts on health
- Developmental origins of health and disease
- Environmental justice and health disparities
- Enviromental related health outcomes
- Green chemistry solutions
- Novel approaches to assess environmental exposures and early markers of effect
- 1,4 Dioxane
- Reproducibility
- Tissue Imaging Mass Spectrometry
- Alcohol and Cancer
- Olive Oil and Health
- News in EMD
- Antimicrobial Resistance
- Applied Public Health and Implementation Science
- Emerging Infections and Climate Change
- Global Health/Tropical Diseases
- HIV and Sexually Transmitted Infections
- Marginalized Population Health & Equity
- Pathogen Genomics, Diagnostics, and Molecular Epidemiology
- Vector-borne and Zoonotic Diseases
- Disease Areas
- EMD Research Day
- News in HPM
- Health Systems Reform
- Quality, Efficiency and Equity of Healthcare
- Substance Abuse and Mental Health
- Modeling: Policy, Operations and Disease
- Pharmaceuticals, Vaccines and Medical Devices
- Health and Wellbeing
- News in SBS
- Aging Health
- Community Engagement
- Health Equity
- Mental Health
- Reproductive Health
- Sexuality and Health
- Nutrition, Exercise
- Stigma Prevention
- Community Partners
- For Public Health Practitioners
- Reports and Publications
- Fellows Stipend Application
- Agency Application
- Past Fellows
- PHFP in the News
- Frequently Asked Questions
- International Activity
- Research Publications
- Grant Listings
- Modeling Analyses
- 3 Essential Questions Series
INFORMATION FOR
- Prospective Students
- Current Students
Yale Research Team Awarded $4 Million Grant to Evaluate New Immunizations for Infant RSV
A multidisciplinary team of Yale scientists has received a $4 million federal grant to study the effectiveness of a new vaccine and monoclonal antibody shot designed to prevent respiratory syncytial virus (RSV) in infants.
The five-year grant from the National Institutes of Health will allow the researchers to investigate the interventions’:
- overall effectiveness
- durability in providing immunity
- effectiveness against different virus lineages
- effectiveness across age groups
Globally, RSV is second only to malaria as the leading cause of infant death. It is estimated that over 100,000 children under 5 die from RSV annually, half of them infants less than 6 months of age. In the United States, RSV is associated with 1.5 million annual medical encounters in children less than 5 years old and is the leading cause of hospitalization among infants under 1 year. It is the most common form of bronchitis and pneumonia among infants.
After years of trials and study, the U.S. Food and Drug Administration and the U.S. Centers for Disease Control and Prevention approved the use of two new tools to help protect infants from RSV in 2023. Nirsevimab , a long-acting monoclonal antibody, is the first drug of its kind to be used as part of a routine immunization program for infants. Abrysvo is the first vaccine to be specifically labeled for use in pregnant women to protect their infants from RSV disease
“The … introduction of new immunoprophylactic agents offers unique opportunities to confront the challenge of RSV in infants,” the researchers said in their project summary. “As is the case with any new vaccine, it will be important to conduct studies during the early phases of implementing these new immunization strategies to answer many unanswered questions surrounding their risks and benefits in real-world settings.”
Data collected in the study will inform health officials and policymakers on the optimal use of the new RSV preventative strategies and will help build public confidence in the immunization program, the researchers said.
The research team includes specialists in a variety of disciplines — vaccinology, clinical epidemiology, pediatric infectious diseases, viral genomics, bioinformatics, and translational immunology. A distinct feature of the project is that it will utilize a “vaccinomics” framework to study interactions between the virus, the vaccines, and the mother/child immune system. The researchers believe this approach will generate novel mechanistic data that will advance our understanding of the various factors that may contribute to diminished or maladaptive vaccine responses.
Carlos R. Oliveira, MD, PhD , an attending physician and specialist in pediatric infectious diseases at Yale New Haven’s Children Hospital, is principal investigator. Oliveira is an assistant professor of pediatrics (infectious diseases and global health), of biostatistics (health informatics), and of biomedical informatics & data science at both Yale School of Medicine (YSM) and the Yale School of Public Health (YSPH).
YSPH co-investigators on the project are Professor of Epidemiology of Microbial Diseases Linda Niccolai, PhD , Associate Professor of Epidemiology of Microbial Diseases Nathan Grubaugh, PhD, and Associate Professor of Epidemiology of Microbial Diseases Daniel Weinberger, PhD . Eugene Shapiro, MD , professor of pediatrics, of epidemiology, and of investigative medicine at both YSM and YSPH, is also a co-investigator along with Paul Aronson, MD , associate professor of pediatrics (emergency medicine), and Carrie Lucas, PhD , associate professor of immunobiology, both of YSM.
With the NIH funding, the research team plans to conduct a large-scale case-controlled study using data collected from an estimated 3,750 children one year of age or younger who receive care for acute respiratory illness at inpatient and outpatient clinical sites of the Yale New Haven Health System, the largest and most comprehensive health care system in Connecticut.
Data will be collected from multiple sources including health records, interviews, immunization registries, and population surveys. Investigators will also conduct genetic characterization of all RSV viruses identified in the study, monitor the genetic diversity of the virus over time, and quantify the relative effectiveness of the immunizations against various viral lineages.
- Vaccination
Featured in this article
- Carlos R Oliveira, MD, PhD Assistant Professor of Pediatrics (Infectious Diseases & Global Health), of Biostatistics (Health Informatics), and of Biomedical Informatics and Data Science; Director, Pediatric AIDS, and Congenital Infectious Diseases; Co-Lead of Yale Network of Vaccine Initiatives, Yale Institute for Global Health
- Linda Niccolai, PhD Associate Dean for Academic Affairs and Professor of Epidemiology (Microbial Diseases); Affiliated Faculty, Yale Institute for Global Health; Director, HPV Working Group at Yale; Director, CT Emerging Infections Program at Yale, Epidemiology of Microbial Diseases
- Nathan Grubaugh, PhD Associate Professor of Epidemiology (Microbial Diseases); Affiliated Faculty, Yale Institute for Global Health
- Eugene Shapiro, MD Professor of Pediatrics (General Pediatrics) and of Epidemiology (Microbial Diseases); Vice Chair for Research, Department of Pediatrics, Pediatrics; Deputy Director, Investigative Medicine PhD Program, Investigative Medicine Program; Co-Director of Education, Yale Center for Clinical Investigation, YCCI Senior Leadership; Affiliated Faculty, Yale Institute for Global Health
- Paul Aronson, MD, MHS Associate Professor of Pediatrics (Emergency Medicine); Deputy Director, Pediatric Residency Program; Director, Pediatric Emergency Medicine Elective, Pediatrics
- Carrie L Lucas, PhD Associate Professor of Immunobiology
Premium Content
Just one pregnancy can add months to your biological age
A landmark new study confirms that growing a human being in nine months takes a toll—and multiple pregnancies can have a cumulative effect.

Surprising no one who has ever been pregnant, scientists have found that growing a human being from scratch makes your body “older."
New research suggests that a single pregnancy can add between two to 14 months to your biological age.
“Pregnancy has a cost that appears to be detectable even" as early as your 20s, says study leader Calen Ryan , a human biologist at Columbia University’s school of public health in New York City.
It’s a “landmark study” that reaffirms what women already know—pregnancy takes a tremendous toll on the body, says Yousin Suh , a Columbia University professor who researches how pregnancy affects aging and wasn’t involved in the study, published April 8 in Proceedings of the National Academy of Sciences .
Your chronological age—or the number of trips you’ve made around the sun—may be different than your biological age, which is how old your cells and organs seem based on their biochemistry.
Ryan studies the reasons why our bodies may age faster or slower than we expect them too, and a lot of that comes down to epigenetics, or how and when our bodies decide to turn genes on and off. (Read how scientists are finally studying women's bodies—and what they're learning.)
Certain life events—including major illnesses, trauma, or periods of intense stress —seem to cause “jumps” in epigenetic age as the body redirects energy and resources toward coping with these challenges.
And since there are few biological functions more arduous than growing an entire person in just nine months, the recent study confirms the scientists’ suspicion that pregnancy—particularly multiple pregnancies—come at a cost to biological age.
Your epigenetic clock
If our genome is an instruction manual, the epigenome is a complex system of bookmarks, highlights, and underlines that tells our cells which genes to read and when. This often happens through methylation, a process by which tiny chemical tags called methyl groups attach to a section of DNA .
Which genes need to be active changes constantly in response to our environment and experiences, so those methyl groups need frequent moving and replacing. Yet as we age, this maintenance machinery appears to start making errors, causing methylations to accumulate in some places and disappear in others. (Read how influencing your genes could help you live longer.)
By taking a blood sample and tallying methyl bookmarks in key locations along the genetic code, scientists can calculate a person’s epigenetic age via a suite of algorithms called “clocks.” These clocks predict your risk of death and health complications, but less known is how fertility impacts your biological age .
To learn more, Ryan and his colleagues turned to a long-running study on intergenerational health in the Philippines . In 2005, they analyzed blood samples from 825 women participants between the ages of 20 and 22. (Learn about simple innovations that could help millions of pregnant women.)
The scientists identified a striking difference—the number of epigenetic changes in their DNA revealed that women who had been pregnant were between four and 14 months were biologically older than their peers who hadn’t, even after controlling for factors such as income level and smoking habits.
A cumulative effect
Despite being close in age, the women in the study were already on very different fertility trajectories—some had never been pregnant, some reported one or more previous pregnancies, and some were pregnant at the time the samples were collected.
You May Also Like

Scientists are finally studying women’s bodies. This is what we’re learning.
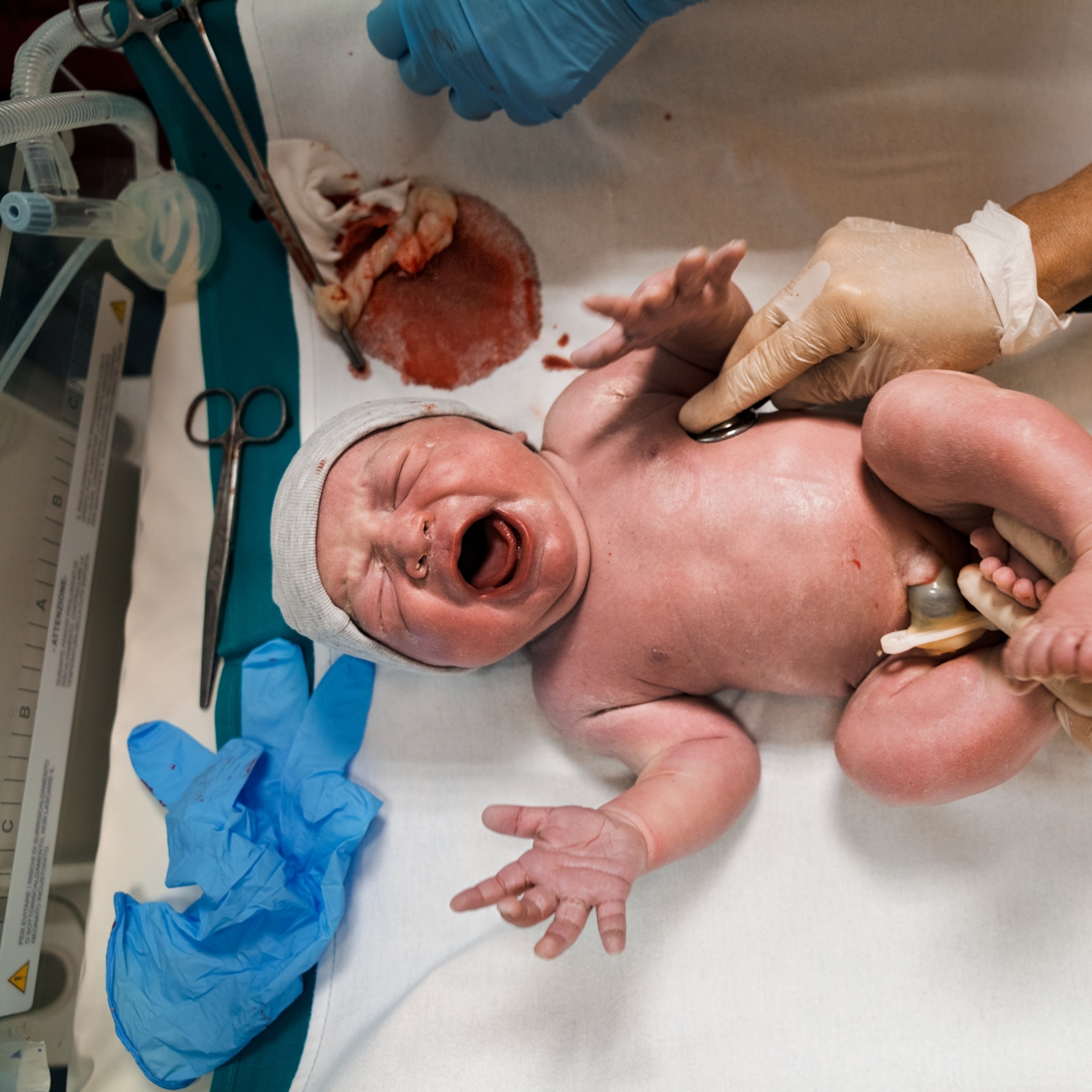
Can aging be cured? Scientists are giving it a try

It's not your life span you need to worry about. It's your health span.
That raised a crucial question: Did multiple pregnancies create a cumulative effect of aging, with each additional pregnancy further raising the mother’s epigenetic age?
Using the first blood samples as a baseline, the researchers collected new samples from 331 of the same women while they were pregnant between four and nine years later. (Learn how babies develop in the womb.)
By comparing the two snapshots of each woman’s epigenetic age, Ryan and his team calculated the impact of each additional pregnancy during the intervening years.
“Women who had more pregnancies during that time had more change in epigenetic aging,” Ryan says, with each pregnancy tacking on two to three months to the parent’s biological age.
Suh, who studies the cost of reproduction on the human body, says Ryan’s findings represent an important advancement in our understanding of how multiple pregnancies affect biological age, as the bulk of existing research has examined just one pregnancy.
The new research, she says, squares with what we know about high birthrates—that experiencing many pregnancies can lead to a shorter life span and higher risk of cardiovascular disease.

Reason for optimism
But would-be parents shouldn’t despair, Suh and Ryan agree—it’s not certain that a slightly higher epigenetic age during your childbearing years will lead to complications decades down the road.
In fact, some research suggests there may be a “sweet spot” for fertility, Suh says. For instance, one or two pregnancies may be better than none in some cases, as pregnancy is linked to lower risks of certain cancers and having at least one child is associated with a slightly longer life expectancy .
As scientists learn more about aging and fertility, “we can work towards identifying people who might be at higher risk,” Ryan adds, and come up with strategies to lessen the negative impacts of pregnancy.
Recent studies indicate the epigenetic cost of pregnancy may differ by country and culture, suggesting that parental support and access to healthcare may play a significant role—improving these could soften pregnancy’s blow to epigenetic age.
Suh adds more research will be needed to untangle the impact of child- rearing from childbirth on epigenetic age, as well as investigate whether the burden of pregnancy is greater when parents are older than those in the study.
While it may feel like common knowledge that pregnancy ages you, it’s a relatively new concept in the scientific literature—and Suh says that research like Ryan’s is long overdue.
“I’m so encouraged that this kind of study is now being done,” she adds.
Related Topics
- FAMILY LIFE

What can your DNA say about your risk of opioid addiction?

IVF revolutionized fertility. Will these new methods do the same?

What is our fear of aging doing to our kids’ mental health?

Twins can become ‘unidentical’—and more fascinating twin facts

This is one of the leading causes of maternal mortality. A new test could change that.
- Environment
- Paid Content
History & Culture
- History & Culture
- History Magazine
- Gory Details
- 2023 in Review
- Mind, Body, Wonder
- Terms of Use
- Privacy Policy
- Your US State Privacy Rights
- Children's Online Privacy Policy
- Interest-Based Ads
- About Nielsen Measurement
- Do Not Sell or Share My Personal Information
- Nat Geo Home
- Attend a Live Event
- Book a Trip
- Inspire Your Kids
- Shop Nat Geo
- Visit the D.C. Museum
- Learn About Our Impact
- Support Our Mission
- Advertise With Us
- Customer Service
- Renew Subscription
- Manage Your Subscription
- Work at Nat Geo
- Sign Up for Our Newsletters
- Contribute to Protect the Planet
Copyright © 1996-2015 National Geographic Society Copyright © 2015-2024 National Geographic Partners, LLC. All rights reserved
ORIGINAL RESEARCH article
The interactive effect between economic uncertainty and life history strategy on corrupt intentions: a life history theory approach.

- 1 Fudan University, Shanghai, Shanghai Municipality, China
- 2 Department of Psychology, Fudan University, Shanghai, China
The final, formatted version of the article will be published soon.
Select one of your emails
You have multiple emails registered with Frontiers:
Notify me on publication
Please enter your email address:
If you already have an account, please login
You don't have a Frontiers account ? You can register here
Why do some people show more corruption when facing uncertain environment?The present study aimed to give a plausible answer from an evolutionary perspective: this might be rooted in people's different life history strategies (slow vs. fast). The present study measured the participants' corrupt intentions by a hypothetical scenario and primed the feeling of economic environmental uncertainty by requiring the participants to read economic uncertainty (vs. neutral) materials. It is revealed that the participants with fast life history strategies had stronger corrupt intentions after reading materials about economic uncertainty than reading neutral materials. In addition, the desire for power mediated the interactive effect between life history strategy and economic uncertainty on corrupt intentions for fast life history strategists. This finding was discussed for its theoretical and practical implications from the perspective of life history theory.
Keywords: Economic uncertainty, life history strategy, Desire for power, corrupt intentions, evolutionary psychology perspective
Received: 25 Dec 2023; Accepted: 11 Apr 2024.
Copyright: © 2024 Sai and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Lei Zhu, Department of Psychology, Fudan University, Shanghai, China
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Pediatr Rev
Respiratory Distress in the Newborn
Suzanne reuter.
* Department of Neonatal-Perinatal Medicine, Sanford School of Medicine–University of South Dakota, Sanford Children’s Specialty Clinic, Sioux Falls, SD.
Chuanpit Moser
† Department of Pediatric Pulmonology, Sanford School of Medicine–University of South Dakota, Sanford Children’s Specialty Clinic, Sioux Falls, SD.
Michelle Baack
‡ Sanford Children’s Health Research Center, Sioux Falls, SD.
Educational Gap
Respiratory distress is common, affecting up to 7% of all term newborns, ( 1 ) and is increasingly common in even modest prematurity. Preventive and therapeutic measures for some of the most common underlying causes are well studied and when implemented can reduce the burden of disease. ( 2 )( 3 )( 4 )( 5 )( 6 )( 7 )( 8 ) Failure to readily recognize symptoms and treat the underlying cause of respiratory distress in the newborn can lead to short- and long-term complications, including chronic lung disease, respiratory failure, and even death.
After completing this article, the reader should be able to:
- Use a physiologic approach to understand and differentially diagnose the most common causes of respiratory distress in the newborn infant.
- Distinguish pulmonary disease from airway, cardiovascular, and other systemic causes of respiratory distress in the newborn.
- Appreciate the risks associated with late preterm (34–36 weeks’ gestation) and early term (37–38 weeks’ gestation) deliveries, especially by caesarean section.
- Recognize clinical symptoms and radiographic patterns that reflect transient tachypnea of the newborn (TTN), neonatal pneumonia, respiratory distress syndrome (RDS), and meconium aspiration syndrome (MAS).
- Identify the short- and long-term complications associated with common neonatal respiratory disorders, including pneumothorax, persistent pulmonary hypertension of the newborn, and chronic lung disease.
- Understand management strategies for TTN, pneumonia, RDS, and MAS.
- Implement up-to-date recommendations for the prevention of neonatal pneumonia, RDS, and MAS.
Introduction
Respiratory distress is one of the most common reasons an infant is admitted to the neonatal intensive care unit. ( 1 ) Fifteen percent of term infants and 29% of late preterm infants admitted to the neonatal intensive care unit develop significant respiratory morbidity; this is even higher for infants born before 34 weeks’ gestation. ( 2 ) Certain risk factors increase the likelihood of neonatal respiratory disease. These factors include prematurity, meconium-stained amniotic fluid (MSAF), caesarian section delivery, gestational diabetes, maternal chorioamnionitis, or prenatal ultrasonographic findings, such as oligohydramnios or structural lung abnormalities. ( 2 )( 9 )( 10 )( 11 )( 12 )( 13 )( 14 ) However, predicting which infants will become symptomatic is not always possible before birth. Regardless of the cause, if not recognized and managed quickly, respiratory distress can escalate to respiratory failure and cardiopulmonary arrest. Therefore, it is imperative that any health care practitioner caring for newborn infants can readily recognize the signs and symptoms of respiratory distress, differentiate various causes, and initiate management strategies to prevent significant complications or death.
Definition, Signs, Symptoms
Respiratory distress in the newborn is recognized as one or more signs of increased work of breathing, such as tachypnea, nasal flaring, chest retractions, or grunting. ( 1 )( 15 ) Normally, the newborn’s respiratory rate is 30 to 60 breaths per minute. Tachypnea is defined as a respiratory rate greater than 60 breaths per minute. ( 15 ) Tachypnea is a compensatory mechanism for hypercarbia, hypoxemia, or acidosis (both metabolic and respiratory), ( 16 ) making it a common but nonspecific finding in a large variety of respiratory, cardiovascular, metabolic, or systemic diseases. Pulmonary disease may incite tachypnea, especially in neonates. The natural elastic property of the lungs is to deflate. When balanced by the outward recoil of the chest wall, functional residual capacity (FRC) occurs at the end of expiration to prevent alveoli from collapsing. The newborn chest wall, composed primarily of cartilage, is more pliable, predisposing neonatal lungs to pulmonary atelectasis and decreased FRC. ( 16 )( 17 )( 18 ) Pulmonary compliance refers to a given change in volume (ΔVolume) for every given change in pressure (ΔPressure), essentially the ability of the alveoli to fill with air under a set pressure. If lung compliance is decreased, such as with transient tachypnea of the newborn (TTN), respiratory distress syndrome (RDS), pneumonia, or pulmonary edema, there is a decrease in tidal volume. To achieve sufficient minute ventilation, the respiratory rate must increase. Hypoxemia further increases tachypnea. ( 16 )( 18 ) Therefore, affected newborns present with marked tachypnea. Because tachypnea is a nonspecific symptom, additional clinical findings aid in narrowing the cause to a respiratory disorder.
Increased work of breathing results from mismatched pulmonary mechanics from increased airway resistance (ΔPressure/Volumetric Flow), decreased lung compliance (ΔVolume/ ΔPressure), or both. Airway resistance increases when there is obstruction of air flow. The critical importance of airway radius is indicated in the equation R = V(8lη/πr(4)), where R is resistance, V is flow, l is length, η is viscosity, and r is radius. ( 19 ) If the airway radius is halved, resistance increases 16-fold. Nasal flaring is a compensatory symptom that increases upper airway diameter and reduces resistance and work of breathing. Retractions, evident by the use of accessory muscles in the neck, rib cage, sternum, or abdomen, occur when lung compliance is poor or airway resistance is high. Noisy breathing may indicate increased airway resistance, and the type of noise auscultated may help localize airway obstruction ( Table 1 ). Stertor is a sonorous snoring sound heard over extrathoracic airways that indicates nasopharyngeal obstruction. Stridor is a high-pitched, monophonic breath sound that indicates obstruction at the larynx, glottis, or subglottic area. Wheezing may also be high pitched but is typically polyphonic, is heard on expiration, and indicates tracheobronchial obstruction. Grunting is an expiratory sound caused by sudden closure of the glottis during expiration in an attempt to maintain FRC and prevent alveolar atelectasis. Because lung compliance is worse at very low or very high FRC, achieving and maintaining physiologic FRC is essential in the management of respiratory disorders with poor compliance, such as RDS or TTN. On the other end of the spectrum, meconium aspiration syndrome (MAS) is an example of lower airway obstruction with air trapping. These newborns often have high lung volumes, which adversely affects their lung compliance. Regardless of the cause, it is vital to recognize symptoms and act quickly. If the newborn cannot sustain the extra work of breathing to meet its respiratory needs, respiratory failure follows. This failure may manifest as impaired oxygenation (cyanosis) or ventilation (respiratory acidosis). Without prompt intervention, respiratory arrest is imminent.
Noisy Breathing Characteristics in Term Infants
FRC=functional residual capacity; MAS=meconium aspiration syndrome; RDS=respiratory distress syndrome; TTN=transient tachypnea of the newborn.
Pathogenesis
The causes of respiratory distress in a newborn are diverse and multisystemic. Pulmonary causes may be related to alterations during normal lung development or transition to extrauterine life. Normal lung development occurs in 5 phases ( 20 ) ( Table 2 ). Respiratory disease may result from developmental abnormalities that occur before or after birth. Early developmental malformations include tracheoesophageal fistula, bronchopulmonary sequestration (abnormal mass of pulmonary tissue not connected to the tracheobronchial tree), and bronchogenic cysts (abnormal branching of the tracheobronchial tree). Later in gestation, parenchymal lung malformations, including congenital cystic adenomatoid malformation or pulmonary hypoplasia from congenital diaphragmatic hernia or severe oligohydramnios, may develop. More common respiratory diseases, such as TTN, RDS, neonatal pneumonia, MAS, and persistent pulmonary hypertension of the newborn (PPHN), result from complications during the prenatal to postnatal transition period. Although mature alveoli are present at 36 weeks’ gestation, a great deal of alveolar septation and microvascular maturation occur postnatally. The lungs are not fully developed until ages 2 to 5 years. ( 20 )( 21 ) Therefore, developmental lung disease can also occur after birth. Bronchopulmonary dysplasia (BPD), for example, is a significant lung disease that complicates prematurity due to arrested alveolarization in developing lungs exposed to mechanical ventilation, oxygen, and other inflammatory mediators before normal development is complete. As defined by an ongoing oxygen requirement at 36 weeks’ adjusted gestational age, BPD affects up to 32% of premature infants and 50% of very low-birth-weight infants. ( 22 )
Developmental Stages of Lung Development and Respiratory Disease Pathogenesis
BPD=bronchopulmonary dysplasia; MAS=meconium aspiration syndrome; PPHN=persistent pulmonary hypertension of the newborn; RDS=respiratory distress syndrome; TTN=transient tachypnea of the newborn;
Differential Diagnosis
The underlying cause of respiratory distress in a newborn varies and does not always lie within the lungs ( 15 ) ( Table 3 ). Thus, after initial resuscitation and stabilization, it is important to use a detailed history, physical examination, and radiographic and laboratory findings to determine a more specific diagnosis and appropriately tailor management. A thorough history may guide in identifying risk factors associated with common causes of neonatal respiratory distress ( Table 4 ). A detailed physical examination should focus beyond the lungs to identify nonpulmonary causes, such as airway obstruction, abnormalities of the chest wall, cardiovascular disease, or neuromuscular disease, that may initially present as respiratory distress in a newborn. Radiographic findings can identify diaphragmatic paralysis, congenital pulmonary malformations, and intrathoracic space–occupying lesions, such as pneumothorax, mediastinal mass, and congenital diaphragmatic hernia, that can compromise lung expansion. Significant tachypnea without increased work of breathing should prompt additional laboratory investigation to identify metabolic acidosis or sepsis. Hypoglycemia, hypomagnesemia, and hematologic abnormalities may result in a depressed ventilatory drive or impaired oxygen transport to the peripheral tissues, so laboratory evaluation should also be considered with these clinical findings. Hypermagnesemia may contribute to respiratory distress and affect a newborn’s capacity to respond to resuscitation due to hypotonia and a depressed respiratory drive or even apnea.
Differential Diagnosis of Respiratory Distress in the Newborn
BPD=bronchopulmonary dysplasia; MAS=meconium aspiration syndrome; PPHN=persistent pulmonary hypertension of the newborn; RDS=respiratory distress syndrome; TTN=transient tachypnea of the newborn.
Perinatal History Associated With Common Respiratory Diseases in the Newborn Infant
MAS=meconium aspiration syndrome; MSAF=meconium-stained amniotic fluid; PROM=prolonged rupture of membranes; RDS=respiratory distress syndrome; TTN=transient tachypnea of the newborn.
Cardiovascular disease may be difficult to distinguish from pulmonary causes of respiratory distress ( Table 5 ). Most congenital heart defects present with cyanosis, tachypnea, or respiratory distress from cardiac failure. Timing may be an important clue to differentiation because very few congenital heart defects present immediately after birth; more often they present several hours to days after delivery as the ductus arteriosus closes. ( 2 ) Table 5 aids in this differentiation.
Differentiation of Cyanotic Heart Disease From Pulmonary Disease Among Infants in Respiratory Distress a
MSAF=meconium-stained amniotic fluid; PPHN=persistent pulmonary hypertension of the newborn; RDS=respiratory distress syndrome; TTN=transient tachypnea of the newborn.
Pulmonary hypertension should be considered in any infant with respiratory distress and cyanosis. This condition results when there is a failure to transition from in utero to postnatal pulmonary circulation after delivery. Pulmonary vascular resistance remains high, resulting in cyanosis from impaired pulmonary blood flow and right-to-left shunting of blood across the foramen ovale and ductus arteriosus. Shunting further contributes to systemic hypoxemia and metabolic acidemia—both of which contribute to ongoing increased pulmonary vascular resistance. PPHN may be primary or secondary to respiratory disease, particularly congenital diaphragmatic hernia, MAS, or RDS. When PPHN occurs without concurrent pulmonary disease, differentiating from cyanotic heart disease is difficult. The response to ventilation with 100% oxygen (hyperoxia test) can help distinguish the 2 conditions. In some neonates with PPHN, the Pa o 2 will increase to above 100 mm Hg, whereas it will not increase above 45 mm Hg in infants with cyanotic heart defects that have circulatory mixing. ( 5 )( 23 )
Common Case Scenarios
Four case scenarios are highlighted to help in identifying the most common causes of respiratory distress in the newborn followed by discussion about the pathophysiology, risk factors, prevention, and management strategies for each disorder.
A 3.2-kg female infant is delivered by caesarean section at 38 weeks’ gestational age without a trial of labor. Her Apgar scores are 9 and 9 at 1 and 5 minutes, respectively. She develops tachypnea and subcostal retractions with nasal flaring at 1 hour of life. Temperature is 97.9°F (36.6°C), pulse is 165 beats per minute, and respiratory rate is 74 breaths per minute. Aside from increased work of breathing, her physical examination findings are normal. The chest radiograph is shown in Figure 1 . She requires supplemental oxygen via nasal cannula with a fraction of inspired oxygen (Fi o 2 ) of 0.3 for 36 hours. She then weans to room air. Her respiratory rate is 35 breaths per minute, and she has no increased work of breathing.

Case 1: Transient tachypnea of the newborn is characterized by streaky, pulmonary interstitial markings and fluid in the fissure apparent on chest radiograph. Case 2: Neonatal pneumonia with bilateral opacities, air bronchograms, and pleural effusions is apparent. Case 3: Respiratory distress syndrome is characterized by diffuse, bilateral, ground glass fields with air bronchograms secondary to diffuse atelectasis. Case 4: Meconium aspiration syndrome causes a chemical pneumonitis, partial airway obstruction, and a localized surfactant inactivation that leads to areas of hyperinflation mixed with diffuse, patchy infiltrates radiographically.
Transient Tachypnea of the Newborn
TTN, also known as retained fetal lung fluid syndrome, presents with early respiratory distress in term and late-preterm infants. TTN is a frequent cause of respiratory distress in newborns and is caused by impaired fetal lung fluid clearance. Normally in utero, the fetal airspaces and air sacs are fluid filled. For effective gas exchange to occur after birth, this fluid must be cleared from the alveolar airspaces. Late in gestation and before birth, the chloride and fluid-secreting channels in the lung epithelium are reversed so that fluid absorption predominates and fluid is removed from the lungs. This process is enhanced by labor, so that delivery before labor onset increases the risk of retained fetal lung fluid. ( 20 ) Factors that increase the clearance of lung fluid include antenatal corticosteroids, fetal thorax compression with uterine contractions, and a release of fetal adrenaline in labor, which enhances uptake of lung fluids. ( 24 )
Infants with TTN usually present with tachypnea and increased work of breathing, which persists for 24 to 72 hours. Chest radiographs reveal excess diffuse parenchymal infiltrates due to fluid in the interstitium, fluid in the interlobar fissure, and occasionally pleural effusions ( Figure 1 ). Management is supportive. Infants may require supplemental oxygen, and frequently the distending forces of continuous positive airway pressure (CPAP) are necessary to assist in maintaining alveolar integrity and driving fluid into circulation. Blood gases often reveal a mild respiratory acidosis and hypoxemia. The course of TTN is self-limited and does not usually require mechanical ventilation.
Preventive measures may include avoiding elective caesarean section before the onset of labor in infants younger than 39 weeks’ gestation. This is because the most common risk factors for TTN include delivery before 39 weeks’ gestation, ( 1 )( 2 )( 3 )( 9 )( 25 )( 26 ) precipitous delivery, fetal distress, maternal sedation, and maternal diabetes. Although it is well known that premature infants have a higher risk of respiratory problems, the consequences of early-term delivery (37–38 weeks’ gestation) are underrecognized. Early-term infants have an increased risk of requiring respiratory support, mechanical ventilation, and neonatal service; delivery by caesarean section in this population is common and further increases risk. ( 25 ) In addition, a single course of antenatal glucocorticoids (2 doses of betamethasone) at least 48 hours before an elective term caesarean delivery decreases respiratory morbidity among infants. ( 27 ) On the basis of multiple cohort studies and expert opinion, we recommend a careful consideration about elective delivery before spontaneous onset of labor at less than 39 weeks’ gestation and encourage pediatricians to be aware of the increased risk of respiratory morbidity in late preterm and early-term newborns. ( 1 )( 2 )( 3 )( 9 )( 25 )( 26 )
A 2.9-kg male infant is born by vaginal delivery at 39 weeks’ gestational age after rupture of membranes for 22 hours. Apgar scores are 8 and 8 at 1 and 5 minutes, respectively. He requires an Fi o 2 of 0.4 in the delivery room. He is tachypneic and has acrocyanosis. There are coarse rales noted bilaterally. Temperature is 98.6°F (37°C), pulse is 144 beats per minute, and respiratory rate is 65 breaths per minute. Despite being given CPAP, his grunting and tachypnea worsen, and he requires intubation and ventilation for progressive increased work of breathing, respiratory acidosis, and oxygen requirement during the next 6 hours. The chest radiograph is shown in Figure 1 .
Neonatal Pneumonia
Respiratory infections in the newborn may be bacterial, viral, fungal, spirochetal, or protozoan in origin. Infants may acquire pneumonia transplacentally, through infected amniotic fluid, via colonization at the time of birth, or nosocomially. ( 20 ) Perinatal pneumonia is the most common form of neonatal pneumonia and is acquired at birth. Group B streptococcus (GBS) is the most common organism that affects term infants. ( 28 )( 29 ) Congenital pneumonia occurs when the causative organism is passed transplacentally to the fetus. The most common pathogens are rubella, cytomegalovirus, adenovirus, enteroviruses, mumps, Toxoplasma gondii, Treponema pallidum, Mycobacterium tuberculosis, Listeria monocytogenes, varicella zoster, and human immunodeficiency virus. ( 30 ) Immaturity of the infant’s immune system and the pulmonary anatomical and physiologic features make the newborn at higher risk of infection. The underdeveloped respiratory cilia and the decreased number of pulmonary macrophages result in decreased clearance of pathogens from the respiratory system. Newborns also have diminished cellular and humoral immune function, which is even more pronounced in the premature infant. ( 28 )
Risk factors for perinatal pneumonia include prolonged rupture of membranes (PROM), maternal infection, and prematurity. ( 1 ) Infants present with increased work of breathing and oxygen requirement. Chest radiography often reveals diffuse parenchymal infiltrates with air bronchograms or lobar consolidation. Pleural effusions may also be seen. In contrast to older infants and children, neonatal pneumonia is part of a generalized sepsis illness; thus, obtaining blood and cerebrospinal fluid cultures and initiating broad-spectrum antibiotic therapy is recommended for any symptomatic infant. ( 31 )( 32 )
In the newborn with early-onset pneumonia or sepsis, a combination of penicillin and an aminoglycoside are the preferred initial treatment. ( 31 ) For infants who have been hospitalized in a neonatal intensive care unit for more than 4 days, organisms such as methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis require vancomycin therapy. Infants who develop pneumonia in the nursery or at home are likely to have infections caused by respiratory viruses (adenovirus, respiratory syncytial virus, and influenza virus), gram-positive bacteria (streptococcal species and S aureus ), and gram-negative enteric bacteria ( Klebsiella , Proteus , Pseudomonas aeruginosa , Serratia marcescens , and Escherichia coli ). ( 30 ) Infants with pneumonia caused by Chlamydia trachomatis present later in the newborn period (4–12 weeks of age) with a staccato cough but no wheezing or fever. ( 33 ) Chlamydial conjunctivitis may also be present (5 to 14 days after birth). Chest radiography reveals diffuse bilateral infiltrates, and a complete blood cell count with a differential reveals eosinophilia. Treatment of chlamydial pneumonia or conjunctivitis (even without pneumonia) requires systemic macrolide antibiotic therapy and ophthalmologic follow-up. Regardless of the causal organism, newborns with pneumonia require supportive care in addition to antibiotics. Many infants will require not only supplemental oxygen but also CPAP and mechanical ventilation. Other supportive measures include intravenous nutrition and vasopressors for cardiovascular support. PPHN is a common complication of neonatal pneumonia.
On the basis of strong evidence, prevention of neonatal pneumonia and its complications focuses on maternal GBS screening, intrapartum antibiotic prophylaxis, and appropriate follow-up of newborns at high risk after delivery. ( 4 )( 31 )( 32 )( 34 ) Anyone caring for newborns should be able to recognize at-risk infants and whether appropriate intrapartum antibiotic prophylaxis has been administered. They must also know which infants require additional screening, observation, and antibiotic initiation after birth. Guidelines have been established by the Centers for Disease Control and Prevention and endorsed by the American Academy of Pediatrics and the American College of Obstetrics and Gynecology for best practice management of at-risk infants. ( 4 ) Infants who require additional attention include those born to mothers who are GBS carriers (culture or polymerase chain reaction positive), those with a history of GBS bacteruria, those affected by GBS or with an unknown GBS status but who were delivered at less than 37 weeks’ gestation, those with PROM of 18 hours or long, or those with intrapartum fever (≥100.4°F [38°C]). ( 4 )( 31 ) The preferred intrapartum antibiotic for these situations is intravenous penicillin (5 million units followed by 2.5 million to 3.0 million units every 4 hours) administered at least 4 hours before delivery; cefazolin may be used for penicillin-allergic women who are at low risk for anaphylaxis. ( 4 )( 31 ) For severely penicillin-allergic women, clindamycin culture sensitivity should be performed, and if mother’s strain is sensitive (75% of cases), clindamycin should be used. Vancomycin is reserved for severely allergic women with resistant strains. ( 4 )( 31 ) In addition to intrapartum antibiotic prophylaxis, promising GBS vaccines are in clinical trials ( 35 ) and may be widely accepted by patients ( 36 ) but are not yet ready for general use.
Since widespread implementation of maternal GBS screening and intrapartum antibiotic prophylaxis administration, the incidence of early-onset GBS infection has decreased from 1.8 cases per 1,000 to 0.3 case per 1,000 live births. ( 31 )( 32 ) However, cases and deaths continue to occur with GBS as the leading offender. ( 31 )( 34 )( 35 ) Most of the term infants affected are born to mothers without or with an unknown GBS status but who had PROM or fever and did not receive antibiotic administration during labor. ( 34 ) Others are born to women who received inadequate prophylaxis (<4 hours before delivery or macrolide antibiotic use). ( 31 ) Many missed opportunities for prevention increase the burden of disease. ( 29 )
Thus, it is imperative to appropriately manage any newborn with the aforementioned risk factors cautiously after birth. According to updated 2010 guidelines, any infant who develops signs or symptoms of illness requires a full diagnostic evaluation (including blood and spinal fluid cultures) and antibiotic initiation. ( 4 )( 31 )( 32 ) If maternal chorioamnionitis is suspected but the infant has no signs or symptoms of disease, a limited evaluation (blood culture and complete blood cell count), along with antibiotic therapy initiation for at least 48 hours, is recommended. ( 4 )( 31 )( 32 ) Asymptomatic, at-risk infants, who did not receive adequate antibiotic prophylaxis, require a limited evaluation and observation for 48 hours, but antibiotic initiation is not necessary unless clinical suspicion arises. ( 4 )( 31 )( 32 ) Asymptomatic, at-risk infants who received adequate intrapartum antibiotic prophylaxis should be observed for 48 hours. Adherence to these guidelines will decrease the incidence of neonatal pneumonia and allow for early detection and treatment that may prevent life-threatening complications, such as PPHN or death.
A 1.5-kg male is delivered via vaginal delivery because of preterm labor at 33 weeks’ gestation. Apgar scores are 7 and 8 at 1 and 5 minutes, respectively. The infant is cyanotic and requires CPAP immediately after delivery. He has subcostal retractions, grunting, and nasal flaring. Auscultation reveals decreased air entry in the lung fields throughout. Temperature is 98.2°F (36.8°C), pulse is 175 beats per minute, and respiratory rate is 70 breaths per minute. He requires an Fi o 2 of 0.4. His chest radiograph is shown in Figure 1 .
Respiratory Distress Syndrome
RDS, also known as hyaline membrane disease, is a common cause of respiratory disease in the premature infant. RDS is also seen in infants whose mothers have diabetes in pregnancy. RDS is caused by a deficiency of alveolar surfactant, which increases surface tension in alveoli, resulting in microatelectasis and low lung volumes. Surfactant deficiency appears as diffuse fine granular infiltrates on radiograph ( Figure 1 ). Pulmonary edema plays a central role in the pathogenesis of RDS and contributes to the development of air bronchograms. Excess lung fluid is attributed to epithelial injury in the airways, decreased concentration of sodium-absorbing channels in the lung epithelium, and a relative oliguria in the first 2 days after birth in premature infants. ( 37 ) Infants typically improve on onset of diuresis by the fourth day after birth.
Infants with RDS typically present within the first several hours of life, often immediately after delivery. Clinically, infants have marked respiratory distress with tachypnea, nasal flaring, grunting, and subcostal, intercostal, and/or suprasternal retractions. Grunting occurs when an infant attempts to maintain an adequate FRC in the face of poorly compliant lungs by partial glottic closure. As the infant prolongs the expiratory phase against this partially closed glottis, there is a prolonged and increased residual volume that maintains the airway opening and also an audible expiratory sound. Infants with RDS have cyanosis and require supplemental oxygen. Mild cases of RDS may respond to the distending pressures of CPAP, but more severe cases require endotracheal intubation and administration of exogenous surfactant into the lungs. Currently, there are no universal guidelines that dictate if and when to administer exogenous surfactant. Some institutions advocate administration of prophylactic surfactant in the first 2 hours of life for all premature infants younger than 30 weeks’ gestation. Others begin with noninvasive ventilation (CPAP) and reserve intubation and surfactant administration only for infants who require more than 35% to 45% oxygen concentration to maintain an arterial PaO2 greater than 50 mm Hg. In determining a management strategy, it is important to consider the administration of antenatal corticosteroids, the clinical presentation, radiographic findings, and the infant’s oxygen requirements. ( 38 )
The course of RDS is self-limited and typically improves by age 3 to 4 days in correlation with the aforementioned diuresis phase and as the infant begins to produce endogenous surfactant. ( 20 ) Use of mechanical ventilation before this is supportive and should proceed with caution to avoid ventilator-induced lung injury. Infants who do not improve with surfactant administration should be evaluated for the presence of a patent ductus arteriosus or other congenital heart disease. The infant who initially improves with administration of surfactant and subsequently deteriorates should also be evaluated for nosocomial pneumonia. ( 20 ) On admission, it is appropriate to initiate antibiotic therapy in the newborn with RDS because pneumonia may present clinically in the same manner and findings on chest radiographs can be indistinguishable from RDS.
Preventing premature birth will lower the incidence of RDS. However, attempts to prevent premature births have been largely unsuccessful, with the rate of premature births still 11.5% of all births in 2012. To benefit those infants who will deliver prematurely, multiple randomized clinical trials strongly support the use of maternal antenatal corticosteroids. Two doses of betamethasone significantly reduce the incidence of RDS, intraventricular hemorrhage, and mortality in infants age 23 to 29 weeks’ gestation. ( 5 )( 39 )( 40 )
A 4.4-kg female infant is delivered via caesarean section at 41 weeks’ gestational age because of presumed large for gestational age status. The amniotic fluid is stained with thick meconium. She is limp and cyanotic at birth with minimal respiratory effort. Apgar scores are 2 and 7 at 1 and 5 minutes, respectively. Temperature is 99°F (37.2°C), pulse is 177 beats per minute, and respiratory rate is 80 breaths per minute. Physical examination findings are significant for marked increased work of breathing with nasal flaring, subcostal and suprasternal retractions, a barrel-shaped chest, and coarse rhonchi in bilateral lung fields. Her chest radiograph is shown in Figure 1 .
Meconium Aspiration Syndrome
MSAF occurs when the fetus passes meconium before birth. Infants born through MSAF are at risk for aspiration of meconium in utero or immediately after birth. Any infant who is born through MSAF and develops respiratory distress after delivery, which cannot be attributed to another cause, is diagnosed as having MAS.
Meconium is composed of lanugo, bile, vernix, pancreatic enzymes, desquamated epithelia, amniotic fluid, and mucus. Meconium is present in the gastrointestinal tract as early as 16 weeks’ gestation but is not present in the lower descending colon until 34 weeks’ gestation; therefore, MSAF is seldom seen in infants younger than 37 weeks’ gestation. ( 41 ) In the compromised fetus, hypoxia or acidosis may result in a peristaltic wave and relaxation of the anal sphincter, resulting in meconium passage in utero. Aspiration may occur in utero or immediately after birth as the compromised fetus gasps.
Meconium is toxic to the newborn lung, causing inflammation and epithelial injury as it migrates distally. The pH of meconium is 7.1 to 7.2. The acidity causes airway inflammation and a chemical pneumonitis with release of cytokines. ( 41 ) As meconium reaches the small airways, partial obstruction occurs, which results in air trapping and hyperaeration. The typical chest radiograph initially appears streaky with diffuse parenchymal infiltrates. In time, lungs become hyperinflated with patchy areas of atelectasis and infiltrate amid alveolar distension ( Figure 1 ). Surfactant is inactivated by the bile acids in meconium, resulting in localized atelectasis, so alternatively, radiographs may resemble those of RDS with low lung volumes. Although air leak syndromes may occur with other respiratory diseases of the newborn, pneumomediastinum, pneumothorax, and PPHN are common in MAS ( Figure 2 ).

Common complications of meconium aspiration syndrome include pneumothorax (left upper) and persistent pulmonary hypertension of the newborn (right upper) characterized by cyanosis with normal lung fields and decreased pulmonary vascular markings.
Management is directed at strategies to support the infant. Supplemental oxygen is required, and CPAP and mechanical ventilation may also be considered in severe cases. Replacement with exogenous surfactant is common practice and reduces the need for extracorporal membrane oxygenation (ECMO) and the risk of pneumothorax. ( 42 ) Because MAS results in a ventilation-perfusion mismatch whereby ventilated alveolar units are not perfused by pulmonary blood vessels, severe hypoxemia may result and further increases pulmonary vascular resistance. Echocardiography helps confirm PPHN by revealing ventricular septal wall flattening, tricuspid regurgitation, and right-to-left shunting at the patent ductus arteriosus. Inhaled nitric oxide is a selective pulmonary vasodilator without systemic effects. It is often used with high-frequency ventilation in severe cases of MAS to maintain adequate oxygenation and ventilation and reduce the need for ECMO. Initiation of broad-spectrum antibiotic therapy is appropriate because meconium is a growth medium for gram-negative organisms. Residual pulmonary compromise is common after MAS. As many as 50% of affected infants are diagnosed as having reactive airway disease during their first 6 months of life, and persistent pulmonary insufficiency is seen in children as old as 8 years. ( 43 )
Because of the significant morbidity associated with MAS, preventive measures are important. Historically, oropharyngeal and nasopharyngeal suctioning was performed on the meconium-stained infant after delivery of the head but before delivery of the shoulders and was initially thought to be an effective preventive measure. ( 44 ) However, a large, multicenter randomized controlled trial in 2004 found that this practice does not prevent MAS or decrease the need for mechanical ventilation or hospital length of stay. ( 45 ) Consequently, routine suctioning on the perineum is no longer indicated. Endotracheal suctioning immediately after birth was also a routine practice for all meconium-stained infants until a large randomized controlled trial found that intubating and suctioning vigorous infants born through MSAF had no benefit and increased the rate of complications. ( 46 ) This finding has been confirmed by additional, well-designed studies, ( 47 ) prompting a change in practice guidelines in 2000. Current evidence still supports immediate endotracheal suctioning of the depressed infant as defined by a low heart rate (<100 beats per minute), poor muscle tone, and no spontaneous respiratory effort. ( 8 ) Intubation and suctioning the vigorous, spontaneously breathing infant is not recommended. ( 8 )( 47 )( 48 )
Approximately 13% of all live births are through MSAF. Although the number of cases has decreased during the past decade, 4% to 5% of these will develop MAS. ( 30 )( 41 ) Previously, many postterm infants (≥42 weeks’ gestation) developed MAS. However, a recent meta-analysis provides evidence that induction of labor at 41 weeks’ gestation reduces the risk of MAS and perinatal death without increasing the risk of caesarean section. ( 7 ) Therefore, many obstetricians do not allow pregnancies to advance beyond 41 weeks’ gestation. In addition, advances in fetal heart rate monitoring have identified compromised fetuses, allowing for timely obstetric intervention that may help prevent in utero aspiration of meconium. Amnioinfusion or transcervical infusion of saline into the amniotic cavity has been proposed as a practice to decrease the incidence of MAS. Although amnioinfusion is beneficial for the distressed fetus with oligohydramnios, best evidence does not indicate a reduced risk of moderate to severe MAS or perinatal death. ( 49 )
Learning to readily recognize respiratory distress in the newborn and understanding physiologic abnormalities associated with each of the various causes will guide optimal management. Although decreasing the incidence through preventive measures is ideal, early recognition and treatment of the common neonatal respiratory diseases will decrease both short- and long-term complications and related mortality of at-risk infants.
- Respiratory distress presents as tachypnea, nasal flaring, retractions, and grunting and may progress to respiratory failure if not readily recognized and managed.
- Causes of respiratory distress vary and may not lie within the lung. A thorough history, physical examination, and radiographic and laboratory findings will aid in the differential diagnosis. Common causes include transient tachypnea of the newborn, neonatal pneumonia, respiratory distress syndrome (RDS), and meconium aspiration syndrome (MAS).
- Strong evidence reveals an inverse relationship between gestational age and respiratory morbidity. ( 1 )( 2 )( 9 )( 25 )( 26 ) Expert opinion recommends careful consideration about elective delivery without labor at less than 39 weeks’ gestation.
- Extensive evidence, including randomized control trials, cohort studies, and expert opinion, supports maternal group B streptococcus screening, intrapartum antibiotic prophylaxis, and appropriate follow-up of high-risk newborns according to guidelines established by the Centers for Disease Control and Prevention. ( 4 )( 29 )( 31 )( 32 )( 34 ) Following these best-practice strategies is effective in preventing neonatal pneumonia and its complications. ( 31 )( 32 )( 34 )
- On the basis of strong evidence, including randomized control trials and Cochrane Reviews, administration of antenatal corticosteroids ( 5 ) and postnatal surfactant ( 6 ) decrease respiratory morbidity associated with RDS.
- Trends in perinatal management strategies to prevent MAS have changed. There is strong evidence that amnioinfusion, ( 49 ) oropharyngeal and nasopharyngeal suctioning at the perineum, ( 45 ) or intubation and endotracheal suctioning of vigorous infants ( 46 )( 47 ) do not decrease MAS or its complications. Some research and expert opinion supports endotracheal suctioning of nonvigorous meconium-stained infants ( 8 ) and induction of labor at 41 weeks’ gestation ( 7 ) to prevent MAS.
AUTHOR DISCLOSURES
Drs Reuter, Moser, and Baack have disclosed no financial relationships relevant to this article. This commentary does not contain information about unapproved/investigative commercial products or devices.
- Skip to main content
- Keyboard shortcuts for audio player

- LISTEN & FOLLOW
- Apple Podcasts
- Google Podcasts
- Amazon Music
- Amazon Alexa
Your support helps make our show possible and unlocks access to our sponsor-free feed.
Watch your garden glow with new genetically modified bioluminescent petunias
Sasa Woodruff

A long exposure photo of Firefly petunias, which are genetically modified to produce their own light through bioluminescence Sasa Woodruff/Boise State Public Radio hide caption
A long exposure photo of Firefly petunias, which are genetically modified to produce their own light through bioluminescence
Keith Wood, Ph.D. spent most of his career in pharmaceutical research in molecular and chemical biology, using his work with bioluminescence to understand how molecules interacted with diseases. His work started as a graduate student when the team he was on inserted a firefly gene into a tobacco plant.
It was a small plant and couldn't sustain light without the addition of a substrate. It wasn't something a consumer would buy, but it was good for understanding pathways within an organism.
Now, about 40 years after that first plant, Wood and his company in Ketchum, Light Bio, are marketing a garden petunia with a twist: it glows in the dark.
View this post on Instagram A post shared by Alexandra L. Woodruff (@trowelandfork)
"People don't think about science as just bringing joy to our lives," Wood said, "We thought we could do something really special here. We could create a kind of decorative plant that was really just enjoyment, just bringing a kind of magic into our lives."

Scientist Keith Wood stands in his Ketchum home with a photo of a tobacco plant modified with a firefly gene Sasa Woodruff/Boise State Public Radio hide caption
Scientist Keith Wood stands in his Ketchum home with a photo of a tobacco plant modified with a firefly gene
The petunia with bright, white flowers looks like something you'd buy in spring at a garden nursery. But, when the lights are turned out, the petals slowly start lighting up with a greenish, white glow. The plant is always glowing, it's just our eyes that need to adjust to see the light. The newest buds are the brightest and punctuate the glowing flowers.
"That's why we call it the Firefly Petunia. Because these bright buds resemble fireflies sitting on top of the plant.," Wood explained.
And despite its name, this plant doesn't have any firefly genes, rather four genes from a bioluminescent mushroom and a fifth from a fungi.
"The first gene takes a metabolite and turns it into an intermediate," Wood explained, "The second gene takes the intermediate and turns it into the actual fuel for the bioluminescence. The third gene is what actually makes the light. And then the last gene takes the product from the light reaction and recycles it back to the starting point."
This cycle is self-sustaining, which means it shines brightly and doesn't need an extra chemical like the tobacco plant did to light up.
"The [firefly] gene was functional, but it didn't connect seamlessly into the natural metabolic processes," Wood said.
"You've got glow, but it was a weak glow. Not satisfying at all."
Petunia approval paperwork
It took about 10 years to go from development to approval from the U.S. Department of Agriculture last fall.
The plants went on sale online in February and the first ones were shipped out this week.
Diane Blazek, the executive director of the National Garden Bureau, an educational nonprofit, says customers are always looking for the next new plant and petunias are a guaranteed bestseller.
"Grandma grew petunias, but oh, look, now I've got a petunia that glows in the dark. So, this is really cool," Blazek said.

The Firefly Petunia emanates light because it's been modified with genes from a bioluminescent mushroom Sasa Woodruff/Boise State Public Radio hide caption
The Firefly Petunia emanates light because it's been modified with genes from a bioluminescent mushroom
She doesn't think that the fact that it's genetically modified will affect customers buying it because there's a precedent.
Seven years ago, an orange petunia modified with a maize gene showed up in gardens and nurseries in Europe and the U.S. The plant was never supposed to leave a closed lab but somehow ended up in lots of gardens. Regulators eventually asked people to destroy the plants and seeds.
"Overwhelmingly, the response was, wait a minute, it's a petunia. We're not eating it. The orange gene came from maize. Why? Why can't we plant this?" Blazek remembered.
Eventually, regulators approved the plants in the U.S.
Chris Beytes, at Ball Publishing, who oversees several horticulture publications, said the Firefly Petunia could open up gardening to new customers.
"If you buy your first plant because it glows in the dark or it's dyed pink, your second and third and 100th plant may be the traditional stuff. You never know," Beytes said. "Anything that creates excitement around flowers and plants. I'm all for it."
The Firefly Petunia may not have practical implications for things like drug advances or crop production, but for Wood this petunia is transcendent.
"There's something magical about seeing this living presence, this glowing vitality coming from a living plant that in person gives a kind of magical experience that you just can't see in a photograph.
And this summer, that magic could be sitting on the patio watching your garden glow from the light of a petunia.
An Ozempic baby boom? Some GLP-1 users report unexpected pregnancies.
Across social media, women who have used Ozempic or similar medications for diabetes or weight loss are reporting an unexpected side effect — surprise pregnancies.
The Facebook group “I got pregnant on Ozempic,” has more than 500 members. Numerous posts on Reddit and TikTok discuss unplanned pregnancies while on Ozempic and similar drugs which can spur significant weight loss by curbing appetite and slowing the digestive process. The drugs are known as “Glucagon-like peptide 1” or GLP-1 drugs.
The reports of an Ozempic baby boom are anecdotal, and it’s not known how widespread the phenomenon is. Experts say significant weight loss can affect fertility. Others speculate that the GLP-1 drugs could interfere with the absorption of oral contraceptives, causing birth control failures.
“I got pregnant on a GLP-1,” posted Deb Oliviara, 32, on her @Dkalsolive TikTok account, which has 36,000 followers. She had noted in another video that she’d previously suffered two miscarriages and a stillbirth.
Oliviara, who lives in Michigan, said in a direct message that she had been using Ozempic for three months before getting pregnant. “I was three weeks along when I found out,” Oliviara said. “I am now 3 months pregnant, and baby is doing amazing.”
“My little Mounjaro baby is almost 6 months old after trying for over 10 years with PCOS!” another woman commented on the post, referring to polycystic ovary syndrome , a hormonal health condition that is a leading cause of infertility.
Paige Burnham, 29, who lives in Louisville, had lost about 80 pounds while using Ozempic, also known as semaglutide, for Type 2 diabetes when she began feeling nauseous on a trip to Disney World. She assumed the symptom was due to the drug. “My most typical Ozempic side effect was nausea,” she said.
But she learned the symptom was actually morning sickness due to pregnancy — a surprise since she and her partner had tried for four years to conceive. She stopped taking Ozempic and gave birth to a healthy baby boy, Creed, in March 2023.
A lack of research on pregnancy and GLP-1 drugs
Little is known about the effects of Ozempic and similar drugs on women who want to get pregnant or who become pregnant while taking the drugs because they were specifically excluded from early clinical trials of the drug.
A spokesman for Novo Nordisk, which makes Ozempic and Wegovy, said the company is collecting data to evaluate the safety of becoming pregnant while using Wegovy, the version of semaglutide approved for weight loss.
“Pregnancy or intention to become pregnant were exclusion criteria in our trials with semaglutide in both obesity and type 2 diabetes,” the company said in a statement.
Get Well+Being tips straight to your inbox

Eli Lilly, maker of the GLP-1 drugs Mounjaro and Zepbound, did not respond to requests for comment.
The biggest concern among women who become pregnant using a GLP-1 is whether the drug poses a risk to the fetus. While women like Burnham and Oliviara have posted reassuring stories of delivering healthy babies, doctors say it’s important to use backup birth control and stop the drug immediately if you become pregnant.
A Novo Nordisk spokesman said in a statement that there isn’t enough available data to know if the drug poses a risk for birth defects, miscarriage or other adverse events related to pregnancy. Based on animal reproduction studies for Wegovy, the company said there “may be potential risks to the fetus from exposure to semaglutide during pregnancy.”
The company recommends stopping Wegovy at least two months before a planned pregnancy.
According to Ozempic’s prescribing information , pregnant rats administered Ozempic showed fetal structural abnormalities, fetal growth problems and embryonic mortality. In rabbits and cynomolgus monkeys, there were early pregnancy losses or structural abnormalities as well as marked maternal body weight loss.
Controlling diabetes is important for a healthy pregnancy, and experts say patients taking Ozempic for diabetes should discuss the risks and benefits with their doctor.
Why drugs like Ozempic might affect pregnancy risk
While it’s unclear whether women taking a GLP-1 have a higher risk of unplanned pregnancies, doctors say there are a few explanations why some women are getting pregnant while using the drugs.
Weight loss can have an effect on ovulation and fertility, said Lora Shahine , a reproductive endocrinologist with a fertility practice in Seattle and Bellevue, Wash.
“I think that with weight loss and balancing of hormones and improved insulin resistance, the hormonal access clicks back in, and all of a sudden they start ovulating again — they might not have been ovulating for years,” said Shahine, who is also an associate clinical professor at the University of Washington.
Stephanie Fein , an internist in Los Angeles who specializes in helping women lose weight for their fertility, said that losing just 5 to 10 percent of body weight can help someone conceive. “No one knows exactly the reason,” she said. “Fat is hormonally active. We know it has effects on estrogen, and it will impact ovulation and possibly egg development.”
The drugs also may interfere with oral contraceptives in some patients, doctors say. The GLP-1 drugs help people lose weight by slowing gastric emptying, curbing hunger and leaving people feel full sooner. It may be that the GLP-1 drugs also affect the absorption of oral contraceptives, said William Dietz, physician and chair of the STOP Obesity Alliance at the Milken Institute School of Public Health at George Washington University. “This may mean that birth control medications are metabolized or ineffective,” he said.
Dietz said most experts recommend discontinuing GLP-1 medications when pregnancy is detected. “I don’t think we know the impact of these drugs on fetal development,” he added.
Shahine recommends that women using oral contraceptives who are taking a GLP-1 drug use a second form of birth control. The drugs also aren’t recommended for mothers who are breastfeeding. Animal studies have shown semaglutide is present in the milk of lactating rats treated with the drug.
After Burnham stopped breastfeeding, she resumed taking Ozempic. Because of her past struggles with infertility, she doesn’t want to take birth control, although she said she is concerned about getting pregnant too soon. “I’m not ready yet,” she said.
Amy Klein is the author of “The Trying Game: Get Through Fertility Treatment and Get Pregnant without Losing Your Mind.”
Read more from Well+Being
Well+Being shares news and advice for living well every day. Sign up for our newsletter to get tips directly in your inbox.
Are you taking your meds wrong ? Many patients make these common mistakes.
Centenarians give their advice about everything.
The wall sit is a simple exercise that can lower your blood pressure.
Tart cherries — more specifically, tart cherry juice — may help with inflammation and pain.
Do you self-sabotage ? Here’s how to stop.


IMAGES
COMMENTS
Background Breastfeeding and maternal health play crucial roles in improving newborn health, which is closely related to the development of families and society. Early essential newborn care, which emphasizes early exclusive breastfeeding and skin-to-skin contact, is recommended by the World Health Organization. This study aimed to explore the association of early essential newborn care with ...
Research articles were excluded if: (1) the measures of social interactions were based solely on self- or diagnostic reports; (2) brain measure was collected during the interaction, incl. hyperscanning studies; (3) the design was an intervention study; (4) the article was a review paper, a protocol or a book chapter; (5) the paper did not have ...
In all cases except for newborns to 3 months, the latest NSF recommendations increased by at least 1 hour from the preceding ... we limited results to original research articles investigating infant sleep during the first 2 years of life in relation to cognitive development and physical growth outcomes both during the first 2 years of life or ...
This manuscript highlights recent advances in newborn research, programming, policy, and funding, and highlights key opportunities to bend the curve on advancing neonatal health globally.
Newborn Research Center and Neonatal Services, The Royal Women's Hospital, Melbourne, VIC, Australia. Shiraz Badurdeen & Peter Davis. Division of Neonatology, Department of Paediatrics, Leiden ...
Existing research has primarily focused on newborns with low birthweight or illness [Citation 3]. There is little insight into ENC practices for healthy newborns in the literature, and evidence is based on direct observations and data extracted from medical files [Citation 11-15].
We measured prenatally French-exposed newborns' (n = 49, age: 2.39 days; range 1 to 5 days; 19 girls) neural activity using electroencephalography (EEG) over 10 frontal, temporal, and central electrode sites while infants were at rest in their hospital bassinets (Fig. 1, A and B).We first measured resting state activity for 3 min (silence 1). Then, infants heard speech in three different ...
Exclusive breastfeeding, both through the newborn visits and through the 6-month visit, was a significant negative predictor for having at least one in-person sick visit in the first 6 months of ...
Projections that 60 transformative cell and gene therapies could be approved by the U.S. Food and Drug Administration (FDA) within 10 years underscore an urgent need to modernize the newborn screening (NBS) system. This study convened expert stakeholders to assess challenges to the NBS system and propose solutions for its modernization. NBS stakeholders (researchers, clinicians, state NBS ...
The science is relatively simple: when a COVID vaccine is given during pregnancy, the parent's immune system develops antibodies against a protein in SARS-CoV-2 that then cross the placenta to ...
Abstract. Infections take their greatest toll in early life necessitating robust approaches to protect the very young. Here, we review the rationale, current state, and future research directions for one such approach: neonatal immunization. Challenges to neonatal immunization include natural concern about safety as well as a distinct neonatal ...
Methods should be tailored to staff training and family preferences. 7 Among late-preterm newborns, there is evidence that some may be more susceptible to feeding problems when supplemented via a bottle; in an RCT in which the 2 methods were compared, cup feeding was associated with a longer duration of exclusive breastfeeding compared with ...
Research has improved our ability to identify which infants are at high-risk of developmental delay and/or impairments, and there is mounting evidence that early interventions can improve outcomes of these infants. ... With colleagues he instigated a clinical trial of intensive care in babies born 1000-1500 g birthweight.
Luke Sharrett for The New York Times. By Joanne Silberner. April 8, 2024. A hug, a handshake, a therapeutic massage. A newborn lying on a mother's bare chest. Physical touch can buoy well-being ...
Previous research has shown that people with and without mental health conditions have different brain asymmetries.There is a limited understanding of how asymmetric babies' brains are at birth ...
Newborns. Newborns, or neonates, are infants who are in their first 28 days of life. Health research focused on newborns is particularly vital as this period of life is associated with significant transitions and can be a critical period for development. Research into newborn health covers a multitude of areas, including prenatal and perinatal ...
AWHONN 1800 M Street, NW, Suite 740 South, Washington, DC 20036, (800) 673-8499. Approved by the AWHONN Board of Directors, May 2005. Revised and reaffirmed November 2010. Revised and approved June 2022. Last published in Journal of Obstetric,Gynecologic, & Neonatal Nursing ( AWHONN, 2016 ).
Skin-to-skin contact is holding the unclothed newborn on the mother's or caretaker's bare chest, usually in an upright position. Skin-to-skin contact has become an expectation of care for newborns following vaginal birth, and many facilities have also adopted policies for implementing immediate or early STS after cesarean birth (Brimdyr et al., 2020). The initial benefits of immediate STS ...
1) Improving clinical care for newborns. The two key research priorities identified by respondents for improving clinical care for newborns were: 1) implementation of existing evidence into nursing practice (translational research); and 2) training and educational needs of neonatal nurses, midwives and health workers.
The purpose of this project was to align with the Association of Women's Health, Obstetric and Neonatal Nurses' (AWHONN's) evidence-based clinical practice guideline, Neonatal Skin Care, in the use of swaddled immersion tub bathing.The goal is for newborns and caregivers to experience the benefits when switching from sponge to swaddled immersion bathing while overcoming challenges and ...
With the NIH funding, the research team plans to conduct a large-scale case-controlled study using data collected from an estimated 3,750 children one year of age or younger who receive care for acute respiratory illness at inpatient and outpatient clinical sites of the Yale New Haven Health System, the largest and most comprehensive health ...
Research Articles | March 12 2024. Serum Calcium Normal Range in 1,000 Term Newborns ... [2.5 and 97.5 percentiles], with a mean of 2.45 mmol/L. Serum calcium was similar between babies born from vitamin D-supplemented mothers and those born from non-supplemented ones. In univariate and multivariable analyses, we demonstrated the importance of ...
Genetic testing in the neonatal ICU. First-line whole-genome sequencing may lead to more-focused clinical management for infants with a suspected genetic disease. Karen O'Leary. Research ...
April 08, 2024. Surprising no one who has ever been pregnant, scientists have found that growing a human being from scratch makes your body "older." New research suggests that a single pregnancy ...
St. Jude Children's Research Hospital. St. Jude Children's Research Hospital is leading the way the world understands, treats and cures childhood cancer, sickle cell disease, and other life-threatening disorders. It is the only National Cancer Institute-designated Comprehensive Cancer Center devoted solely to children. Treatments developed at St. Jude have helped push the overall childhood ...
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Definition, Signs, Symptoms. Respiratory distress in the newborn is recognized as one or more signs of increased work of breathing, such as tachypnea, nasal flaring, chest retractions, or grunting. ( 1 ) ( 15) Normally, the newborn's respiratory rate is 30 to 60 breaths per minute. Tachypnea is defined as a respiratory rate greater than 60 ...
A long exposure photo of Firefly petunias, which are genetically modified to produce their own light through bioluminescence. Keith Wood, Ph.D. spent most of his career in pharmaceutical research ...
April 5, 2024 at 12:14 p.m. EDT. Newborn babies sleeping in hospital nursery. (Getty Images) Across social media, women who have used Ozempic or similar medications for diabetes or weight loss are ...