If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.

AP®︎/College Biology
Course: ap®︎/college biology > unit 6.
- Introduction to genetic engineering
Intro to biotechnology
- DNA cloning and recombinant DNA
- Overview: DNA cloning
- Polymerase chain reaction (PCR)
- Gel electrophoresis
- DNA sequencing
- Applications of DNA technologies
- Biotechnology
Key points:
- Biotechnology is the use of an organism, or a component of an organism or other biological system, to make a product or process.
- Many forms of modern biotechnology rely on DNA technology.
- DNA technology is the sequencing, analysis, and cutting-and-pasting of DNA.
- Common forms of DNA technology include DNA sequencing , polymerase chain reaction , DNA cloning , and gel electrophoresis .
- Biotechnology inventions can raise new practical concerns and ethical questions that must be addressed with informed input from all of society.
Introduction
What is biotechnology.
- Beer brewing . In beer brewing, tiny fungi (yeasts) are introduced into a solution of malted barley sugar, which they busily metabolize through a process called fermentation. The by-product of the fermentation is the alcohol that’s found in beer. Here, we see an organism – the yeast – being used to make a product for human consumption.
- Penicillin. The antibiotic penicillin is generated by certain molds. To make small amounts of penicillin for use in early clinical trials, researchers had to grow up to 500 liters of “mold juice” a week 1 . The process has since been improved for industrial production, with use of higher-producing mold strains and better culture conditions to increase yield 2 . Here, we see an organism (mold) being used to make a product for human use – in this case, an antibiotic to treat bacterial infections.
- Gene therapy. Gene therapy is an emerging technique used to treat genetic disorders that are caused by a nonfunctional gene. It works by delivering the “missing” gene’s DNA to the cells of the body. For instance, in the genetic disorder cystic fibrosis, people lack function of a gene for a chloride channel produced in the lungs. In a recent gene therapy clinical trial, a copy of the functional gene was inserted into a circular DNA molecule called a plasmid and delivered to patients’ lung cells in spheres of membrane (in the form of a spray) 3 . In this example, biological components from different sources (a gene from humans, a plasmid originally from bacteria) were combined to make a new product that helped preserve lung function in cystic fibrosis patients.
What is DNA technology?
Examples of dna technologies.
- DNA cloning. In DNA cloning , researchers “clone” – make many copies of – a DNA fragment of interest, such as a gene. In many cases, DNA cloning involves inserting a target gene into a circular DNA molecule called a plasmid. The plasmid can be replicated in bacteria, making many copies of the gene of interest. In some cases, the gene is also expressed in the bacteria, making a protein (such as the insulin used by diabetics). Insertion of a gene into a plasmid.
- Polymerase chain reaction (PCR). Polymerase chain reaction is another widely used DNA manipulation technique, one with applications in almost every area of modern biology. PCR reactions produce many copies of a target DNA sequence starting from a piece of template DNA. This technique can be used to make many copies of DNA that is present in trace amounts (e.g., in a droplet of blood at a crime scene).
- Gel electrophoresis. Gel electrophoresis is a technique used to visualize (directly see) DNA fragments. For instance, researchers can analyze the results of a PCR reaction by examining the DNA fragments it produces on a gel. Gel electrophoresis separates DNA fragments based on their size, and the fragments are stained with a dye so the researcher can see them. DNA fragments migrate through the gel from the negative to the positive electrode. After the gel has run, the fragments are separated by size, with the smallest ones near the bottom (positive electrode) and the largest ones near the top (negative electrode). Based on similar diagram in Reece et al. 5
- DNA sequencing. DNA sequencing involves determining the sequence of nucleotide bases (As, Ts, Cs, and Gs) in a DNA molecule. In some cases, just one piece of DNA is sequenced at a time, while in other cases, a large collection of DNA fragments (such as those from an entire genome) may be sequenced as a group. What is a genome? A genome refers to all of an organism's DNA. In eukaryotes, which have a nucleus in their cells to hold their DNA, the word genome is usually used for the nuclear genome (DNA found in the nucleus), excluding the DNA found in organelles such as chloroplasts or mitochondria.
Biotechnology raises new ethical questions
- Some of these relate to privacy and non-discrimination. For instance should your health insurance company be able to charge you more if you have a gene variant that makes you likely to develop a disease? How would you feel if your school or employer had access to your genome?
- Other questions relate to the safety, health effects, or ecological impacts of biotechnologies. For example, crops genetically engineered to make their own insecticide reduce the need for chemical spraying, but also raise concerns about plants escaping into the wild or interbreeding with local populations (potentially causing unintended ecological consequences).
- Biotechnology may provide knowledge that creates hard dilemmas for individuals. For example, a couple may learn via prenatal testing that their fetus has a genetic disorder. Similarly, a person who has her genome sequenced for the sake of curiosity may learn that she is going to develop an incurable, late-onset genetic disease, such as Huntington's.
Educate yourself and share your perspective
Works cited:.
- American Chemical Society. (2016). Discovery and development of penicillin. In Chemical landmarks . Retrieved from http://www.acs.org/content/acs/en/education/whatischemistry/landmarks/flemingpenicillin.html .
- Meštrović, T. and Chow, S. (2015, April 29). Penicillin production. In News medical . Retrieved from http://www.news-medical.net/health/Penicillin-Production.aspx .
- Alton, E. W. F. W., Armstrong, D. K., Ashby, D., Bayfield, K. J., Bilton, Diana, Bloomfield, E. V., ... Wolstenholme-Hogg, P. (2015). Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: A randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respiratory Medicine , 3 (9), 684-691. http://dx.doi.org/10.1016/S2213-2600(15)00245-3 .
- Reece, J. B., Urry, L. A., Cain, M. L., Wasserman, S. A., Minorsky, P. V., and Jackson, R. B. (2011). The DNA toolbox. In Campbell biology (10th ed., pp. 408-409). San Francisco, CA: Pearson.
- Reece, J. B., Taylor, M. R., Simon, E. J., and Dickey, J. L. (2012). Figure 12.13. Gel electrophoresis of DNA. In Campbell biology: Concepts & connections (7th ed., p. 243).
Additional references:
Want to join the conversation.
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page


- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

1.1: Introduction to Biotechnology
- Last updated
- Save as PDF
- Page ID 39470

- Jack O'Grady
- Austin Community College
What is Biotechnology?
According to the United Nations Convention on Biological Diversity, biotechnology is “any technological application that uses biological systems, living organisms, or derivatives thereof, to make or modify products or processes for a specific use." The concept of “specific use” typically involves a commercial application or benefit to humanity. Genetic engineering, artificial selection, antibiotic production, and cell culture are current topics of study in biotechnology. However, humans were using microbes to create useful products long before Karl Ereky, a Hungarian engineer, coined the term biotechnology. Some of the products of this early biotechnology are as familiar as cheese, wine, yogurt, and beer, which employ microbes, such as yeast, a fungus (Figure \(\PageIndex{1}\)).
Early Biotechnology
Cheese production began around 4,000 to 7,000 years ago when humans began to breed animals and process their milk. Fermentation, in this case, preserves nutrients: Milk will spoil relatively quickly, but when processed like cheese, it is more stable. As for beer, the oldest records of brewing are about 6,000 years old and were an integral part of the Sumerian culture. Evidence indicates that the Sumerians discovered fermentation by chance. Wine has been produced for about 4,500 years, and evidence suggests that cultured milk products, like yogurt, have existed for at least 4,000 years.
In the early twentieth century, scientists gained a greater understanding of microbiology and explored ways of manufacturing specific products. In 1917, Chaim Weizmann first used a pure microbiological culture in an industrial process that of manufacturing corn starch using Clostridium acetobutylicum to produce acetone, which was used to manufacture explosives during World War I. Shortly after that, in 1928, Alexander Fleming discovered the mold Penicillium. His work led to the purification of the antibiotic compound formed by the mold by Howard Florey, Ernst Boris Chain, and Norman Heatley – to form what we today know as penicillin. In 1940, penicillin became available for medicinal use to treat bacterial infections in humans.
The New Biotechnology
The field of modern biotechnology is generally thought of as having been born in 1971 when Paul Berg's experiments in gene splicing had early success. Herbert W. Boyer Stanley N. Cohen significantly advanced the new technology in 1972 by transferring genetic material into a bacterium, such that the imported material would be reproduced, giving birth to the field of recombinant DNA technology. The commercial viability of a biotechnology industry was significantly expanded on June 16, 1980, when the United States Supreme Court ruled that a genetically modified microorganism could be patented. Technology breakthroughs since the 1980s, such as Polymerase Chain Reaction, Sanger Sequencing, Whole Genome Sequencing, and more recently, CRISPR have brought forth a new age of Biotechnology and products.
New approaches to Biotechnology: Watch this video!: youtu.be/V0rIP_u1JPQ

Test Your Knowledge!
- In your own words, define biotechnology
- Can you think of a biotechnology product that has improved your life? What makes it a biotechnology product?
Browse Course Material
Course info, instructors.
- Prof. Christopher Burge
- Prof. David Sabatini
- Dr. Marilee Ogren-Balkema
- Dr. Alice Rushforth
Departments
As taught in.
- Biotechnology
- Molecular Biology
Learning Resource Types
Experimental molecular biology: biotechnology ii, scientific comm..
This course includes significant instruction in scientific communications. During the term, Dr. Marilee Ogren-Balkema presents ten lectures on a range of reading, presentation and writing topics.
Background reading
Gopen, George D., and Judith A. Swan. “ The Science of Scientific Writing .” The American Scientist 78 (1990): 550-558.
Lectures on Scientific Communications
1: Basic Scientific Communication ( PDF )
2: How to Review the Literature ( PDF )
3: How To Write a Research Proposal ( PDF )
4: Preparing Effective Oral Presentations ( PDF )
5: How to Write a Mini Literature Review ( PDF )
6: How to Write a Research Paper I: Illustrations ( PDF - 1.2 MB )
7: How to Write a Research Paper II: Results Section ( PDF )
8: How to Write a Research Paper III: Methods Section ( PDF )
9: How to Write a Research Paper IV: Introduction and Discussion ( PDF )
10: How to Write a Research Paper V: Title and Abstract ( PDF )

You are leaving MIT OpenCourseWare

Basic and Applied Aspects of Biotechnology pp 1–21 Cite as
An Introduction to Biotechnology
- Varsha Gupta 5 ,
- Manjistha Sengupta 6 ,
- Jaya Prakash 7 &
- Baishnab Charan Tripathy 8
- First Online: 23 October 2016
9986 Accesses
11 Citations
14 Altmetric
Biotechnology is multidisciplinary field which has major impact on our lives. The technology is known since years which involves working with cells or cell-derived molecules for various applications. It has wide range of uses and is termed “technology of hope” which impact human health, well being of other life forms and our environment. It has revolutionized diagnostics and therapeutics; however, the major challenges to the human beings have been threats posed by deadly virus infections as avian flu, Chikungunya, Ebola, Influenza A, SARS, West Nile, and the latest Zika virus. Personalized medicine is increasingly recognized in healthcare system. In this chapter, the readers would understand the applications of biotechnology in human health care system. It has also impacted the environment which is loaded by toxic compounds due to human industrialization and urbanization. Bioremediation process utilizes use of natural or recombinant organisms for the cleanup of environmental toxic pollutants. The development of insect and pest resistant crops and herbicide tolerant crops has greatly reduced the environmental load of toxic insecticides and pesticides. The increase in crop productivity for solving world food and feed problem is addressed in agricultural biotechnology. The technological advancements have focused on development of alternate, renewable, and sustainable energy sources for production of biofuels. Marine biotechnology explores the products which can be obtained from aquatic organisms. As with every research area, the field of biotechnology is associated with many ethical issues and unseen fears. These are important in defining laws governing the feasibility and approval for the conduct of particular research.
- Stem Cell Research
- Itaconic Acid
- Levulinic Acid
- Salmon Calcitonin
- Agricultural Biotechnology
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
You have full access to this open access chapter, Download chapter PDF
1.1 Introduction
The term “biotechnology ” was coined by a Hungarian engineer Karl Ereky, in 1919, to refer to the science and methods that permit products to be produced from raw materials with the aid of living organisms. Biotechnology is a diverse field which involves either working with living cells or using molecules derived from them for applications oriented toward human welfare using varied types of tools and technologies. It is an amalgamation of biological science with engineering whereby living organisms or cells or parts are used for production of products and services. The main subfields of biotechnology are medical (red) biotechnology, agricultural (green) biotechnology, industrial (white) biotechnology, marine (blue) biotechnology, food biotechnology, and environmental biotechnology (Fig. 1.1 .). In this chapter the readers will understand the potential applications of biotechnology in several fields like production of medicines; diagnostics; therapeutics like monoclonal antibodies, stem cells, and gene therapy ; agricultural biotechnology ; pollution control (bioremediation ); industrial and marine biotechnology ; and biomaterials , as well as the ethical and safety issues associated with some of the products.
Major applications of biotechnology in different areas and some of their important products
The biotechnology came into being centuries ago when plants and animals began to be selectively bred and microorganisms were used to make beer, wine, cheese, and bread. However, the field gradually evolved, and presently it is the use or manipulation of living organisms to produce beneficiary substances which may have medical, agricultural, and/or industrial utilization. Conventional biotechnology is referred to as the technique that makes use of living organism for specific purposes as bread/cheese making, whereas modern biotechnology deals with the technique that makes use of cellular molecules like DNA, monoclonal antibodies, biologics, etc. Before we go into technical advances of DNA and thus recombinant DNA technology , let us have the basic understanding about DNA and its function.
The foundation of biotechnology was laid down after the discovery of structure of DNA in the early 1950s. The hereditary material is deoxyribonucleic acid (DNA) which contains all the information that dictates each and every step of an individual’s life. The DNA consists of deoxyribose sugar, phosphate, and four nitrogenous bases (adenine, guanine, cytosine, and thymine). The base and sugar collectively form nucleoside, while base, sugar, and phosphate form nucleotide (Fig. 1.2 ). These are arranged in particular orientation on DNA called order or sequence and contain information to express them in the form of protein. DNA has double helical structure, with two strands being complimentary and antiparallel to each other, in which A on one strand base pairs with T and G base pairs with C with two and three bonds, respectively. DNA is the long but compact molecule which is nicely packaged in our nucleus. The DNA is capable of making more copies like itself with the information present in it, as order or sequence of bases. This is called DNA replication. When the cell divides into two, the DNA also replicates and divides equally into two. The process of DNA replication is shown in Fig. 1.3 , highlighting important steps.
The double helical structure of DNA where both strands are running in opposite direction. Elongation of the chain occurs due to formation of phosphodiester bond between phosphate at 5′ and hydroxyl group of sugar at 3′ of the adjacent sugar of the nucleotide in 5–3′ direction. The sugar is attached to the base. Bases are of four kinds: adenine ( A ), guanine ( G ) (purines), thymine ( T ), and cytosine ( C ) (pyrimidines). Adenine base pairs with two hydrogen bonds with thymine on the opposite antiparallel strand and guanine base pairs with three hydrogen bonds with cytosine present on the opposite antiparallel strand
The process of DNA replication. The DNA is densely packed and packaged in the chromosomes. The process requires the action of several factors and enzymes. DNA helicase unwinds the double helix. Topoisomerase relaxes DNA from its super coiled nature. Single-strand binding proteins bind to single-stranded open DNA and prevent its reannealing and maintains strand separation. DNA polymerase is an enzyme which builds a new complimentary DNA strand and has proofreading activity. DNA clamp is a protein which prevents dissociation of DNA polymerase. Primase provides a short RNA sequence for DNA polymerase to begin synthesis. DNA ligase reanneals and joins the Okazaki fragments of the lagging strand. DNA duplication follows semiconservative replication, where each strand serves as template which leads to the production of two complimentary strands. In the newly formed DNA, one strand is old and the other one is new (semiconservative replication). DNA polymerase can extend existing short DNA or RNA strand which is paired to template strand and is called primer. Primer is required as DNA polymerase cannot start the synthesis directly. DNA polymerase is capable of proofreading, that is, correction of wrongly incorporated nucleotide. One strand is replicated continuously with single primer, and it is called as leading strand. Other strand is discontinuous and requires the addition of several primers. The extension is done in the form of short fragments called as Okazaki fragments. The gaps are sealed by DNA ligase. Replication always occurs in 5′–3′ direction
DNA contains whole information for the working of the cell. The part of the DNA which has information to dictate the biosynthesis of a polypeptide is called a “gene.” The arrangement or order of nucleotides determines the kind of proteins which we produce. Each gene is responsible for coding a functional polypeptide. The genes have the information to make a complimentary copy of mRNA. The information of DNA which makes RNA in turn helps cells to incorporate amino acids according to arrangement of letters for making many kinds of proteins. These letters are transcribed into mRNA in the form of triplet codon, where each codon specifies one particular amino acid. The polypeptide is thus made by adding respective amino acids according to the instructions present on RNA. Therefore, the arrangement of four bases (adenine, guanine, cytosine, and thymine) dictates the information to add any of the 20 amino acids to make all the proteins in all the living organisms. Few genes need to be expressed continuously, as their products are required by the cell, and these are known as “constitutive genes.” They are responsible for basic housekeeping functions of the cells. However, depending upon the physiological demand and cell’s requirement at a particular time, some genes are active and some are inactive, that is, they do not code for any protein. The information contained in the DNA is used to make mRNA in the process of “transcription ” (factors shown in Table 1.1 ). The information of mRNA is used in the process of “translation ” for production of protein. Transcription occurs in the nucleus and translation in the cytoplasm of the cell. In translation several initiation factors help in the assembly of mRNA with 40S ribosome and prevent binding of both ribosomal subunits; they also associate with cap and poly(A) tail. Several elongation factors play an important role in chain elongation. Though each cell of the body has the same genetic makeup, but each is specialized to perform unique functions, the activation and expression of genes is different in each cell. Thus, one type of cells can express some of its genes at one time and may not express the same genes some other time. This is called “temporal regulation” of the gene. In the body different cells express different genes and thus different proteins. For example, liver cell and lymphocyte, would express different genes. This is known as spatial regulation of the gene. Therefore, in the cells of the body, the activation of genes is under spatial regulation (cells present at different locations and different organs produce different proteins) and temporal regulation (same cells produce different proteins at different times). The proteins are formed by the information contained in the DNA and perform a variety of cellular functions. The proteins may be structural (responsible for cell shape and size), or they may be functional like enzymes, signaling intermediates, regulatory proteins, and defense system proteins. However, any kind of genetic defect results in defective protein or alters protein folding which can compromise the functioning of the body and is responsible for the diseases. Figure 1.4 shows the outline of the process of transcription and translation with important steps.
It shows the process of transcription and translation. Transcription occurs in the nucleus and requires the usage of three polymerase enzymes. RNApol I for rRNA, pol II for mRNA, and pol III for both rRNA and tRNA. RNApol II initiates the process by associating itself with seven transcription factors, TFIIA, TFIIB, TFIID, TFIIE, TFIIH, and TFIIJ. After the synthesis, preRNA transcript undergoes processing to form mRNA by removal of introns by splicing and polyadenylation and capping. Protein synthesis is initiated by formation of ribosome and initiator tRNA complex to initiation codon (AUG) of mRNA and involves 11 factors. Chain elongation occurs after sequential addition of amino acids by formation of peptide bonds. Then polypeptide can fold or conjugate itself to other biomolecules and may undergo posttranslational modifications as glycosylation or phosphorylation to perform its biological functions
The biotechnological tools are employed toward modification of the gene for gain of function or loss of function of the protein. The technique of removing, adding, or modifying genes in the genome or chromosomes of an organism to bring about the changes in the protein information is called genetic engineering or recombinant DNA technology (Fig. 1.5 ). DNA recombination made possible the sequencing of the human genome and laid the foundation for the nascent fields of bioinformatics, nanomedicine, and individualized therapy. Multicellular organisms like cows, goats, sheep, rats , corn, potato, and tobacco plants have been genetically engineered to produce substances medically useful to humans. Genetic engineering has revolutionized medicine, enabling mass production of safe, pure, more effective versions of biochemicals that the human body produces naturally [ 20 – 22 ].
The process of recombinant DNA technology . The gene of interest from human nucleus is isolated and cloned in a plasmid vector. The gene is ligated with the help of DNA ligase. The vector is transformed into a bacterial host. After appropriate selections, the gene is amplified when bacteria multiply or the gene can be sequenced or the gene can be expressed to produce protein
The technological advancements have resulted in (1) many biopharmaceuticals and vaccines, (2) new and specific ways to diagnose, (3) increasing the productivity and introduction of quality traits in agricultural crops, (4) the ways to tackle the pollutants efficiently for sustainable environmental practices, (5) helped the forensic experts by DNA fingerprinting and profiling, (6) fermentation technology for production of industrially important products. The list is very long with tremendous advancements and products which have boosted the economy of biotechnology sector worldwide [ 16 ]. The biotechnology industry and the products are regulated by various government organizations such as the US Food and Drug Administration (FDA), the Environmental Protection Agency (EPA), and the US Department of Agriculture (USDA).
1.2 Medical Biotechnology
This field of biotechnology has many applications and is involved in production of recombinant pharmaceuticals, tissue engineering products, regenerative medicines such as stem cell and gene therapy , and many more biotechnology products for better human life (Fig. 1.6 ). Biotechnological tools produce purified bio-therapeutic agents on industrial scales. These include both novel agents and agents formerly available only in small quantities. Crude vaccines were used in antiquity in China, India, and Persia. For example, vaccination with scabs that contained the smallpox virus was a practice in the East for centuries. In 1798 English country doctor Edward Jenner demonstrated that inoculation with pus from sores due to infection by a related cowpox virus could prevent smallpox far less dangerously. It marked the beginning of vaccination . Humans have been benefited incalculably from the implementation of vaccination programs.
Various applications of medical biotechnology
Tremendous progress has been made since the early recombinant DNA technology (RDT) experiments from which the lively—and highly profitable—biotechnology industry emerged. RDT has fomented multiple revolutions in medicine. Safe and improved drugs, accelerated drug discovery , better diagnostic and quick methods for detecting an infection either active or latent, development of new and safe vaccines, and completely novel classes of therapeutics and other medical applications are added feathers in its cap. The technology has revolutionized understanding of diseases as diverse as cystic fibrosis and cancer. Pharmaceutical biotechnology being one of the important sectors involves using animals or hybrids of tumor cells or leukocytes or cells (prokaryotic , mammalian) to produce safer, more efficacious, and cost-effective versions of conventionally produced biopharmaceuticals . The launch of the new biopharmaceutical or drug requires screening and development. Mice were widely used as research animals for screening. However, in the wake of animal protection, animal cell culture offers accurate and inexpensive source of cells for drug screening and efficacy testing. Pharmaceutical biotechnology’s greatest potential lies in gene therapy and stem cell-based therapy. The underlying cause of defect of many inherited diseases is now located and characterized. Gene therapy is the insertion of the functional gene in place of defective gene into cells to prevent, control, or cure disease. It also involves addition of genes for pro-drug or cytokines to eliminate or suppress the growth of the tumors in cancer treatment.
But the progress so far is viewed by many scientists as only a beginning. They believe that, in the not-so-distant future, the refinement of “targeted therapies” should dramatically improve drug safety and efficacy. The development of predictive technologies may lead to a new era in disease prevention, particularly in some of the world’s rapidly developing economies. Yet the risks cannot be ignored as new developments and discoveries pose new questions, particularly in areas as gene therapy , the ethics of stem cell research, and the misuse of genomic information.
Many bio-therapeutic agents in clinical use are biotech pharmaceuticals. Insulin was among the earliest recombinant drugs. Canadian physiologists Frederick Banting and Charles Best discovered and isolated insulin in 1921. In that time many patients diagnosed with diabetes died within a few years. In the mid-1960s, several groups reported synthesizing the hormone .
The first “bioengineered” drug, a recombinant form of human insulin , was approved by the US Food and Drug Administration (FDA) in 1982. Until then, insulin was obtained from a limited supply of beef or pork pancreas tissue. By inserting the human gene for insulin into bacteria, scientists were able to achieve lifesaving insulin production in large quantities. In the near future, patients with diabetes may be able to inhale insulin , eliminating the need for injections. Inhaled insulin products like Exubera® were approved by the USFDA; however, it was pulled out and other products like Technosphere® insulin (Afrezza®) are under investigation. They may provide relief from prandial insulin . Afrezza consists of a pre-meal insulin powder loaded into a cartridge for oral inhalation.
Technosphere technology: The technology allows administration of therapeutics through pulmonary route which otherwise were required to be given as injections. These formulations have broad spectrum of physicochemical characteristics and are prepared with a diverse assortment of drugs with protein or small molecule which may be hydrobhobic or hydrophilic or anionic or cationic in nature. The technology can have its applicability not only through pulmonary route but also for other routes of administration including local lung delivery.
The first recombinant vaccine, approved in 1986, was produced by cloning a gene fragment from the hepatitis B virus into yeast (Merck’s Recombivax HB). The fragment was translated by the yeast’s genetic machinery into an antigenic protein. This was present on the surface of the virus that stimulates the immune response. This avoided the need to extract the antigen from the serum of people infected with hepatitis B.
The Food and Drug administration (FDA) approved more biotech drugs in 1997 than in the previous several years combined. The FDA has approved many recombinant drugs for human health conditions. These include AIDS , anemia, cancers (Kaposi’s sarcoma, leukemia, and colorectal, kidney, and ovarian cancers), certain circulatory problems, certain hereditary disorders (cystic fibrosis, familial hypercholesterolemia, Gaucher’s disease, hemophilia A, severe combined immunodeficiency disease , and Turner’s syndrome), diabetic foot ulcers, diphtheria , genital warts, hepatitis B, hepatitis C, human growth hormone deficiency, and multiple sclerosis. Today there are more than 100 recombinant drugs and vaccines. Because of their efficiency, safety, and relatively low cost, molecular diagnostic tests and recombinant vaccines may have particular relevance for combating long-standing diseases of developing countries, including leishmaniasis (a tropical infection causing fever and lesions) and malaria .
Stem cell research is very promising and holds tremendous potential to treat neurodegenerative disorders, spinal cord injuries, and other conditions related to organ or tissue loss.
DNA analysis is another powerful technique which compares DNA pattern [ 14 ] after performing RFLP and probing it by minisatellite repeat sequence between two or more individuals. Its modification, DNA profiling (process of matching the DNA profiles for STS markers in two or more individuals; see chapter 18), which utilizes multilocus PCR analysis of DNA of suspect and victims, is very powerful and is useful in criminal investigation, paternity disputes, and so many other legal issues. The analysis is very useful in criminal investigations and involves evaluation of DNA from samples of the hair , body fluids, or skin at a crime scene and comparison of these with those obtained from the suspects.
1.2.1 Improved Diagnostic and Therapeutic Capabilities
The sequencing of the human genome in 2003, has given scientists an incredibly rich “parts list” with which to better understand why and how disease happens. It has given added power to gene expression profiling, a method of monitoring expression of thousands of genes simultaneously on a glass slide called a microarray . This technique can predict the aggressiveness of cancer.
The development of monoclonal antibodies in 1975 led to a medical revolution. The body normally produces a wide range of antibodies—the immune system proteins—that defend our body and eliminate microorganisms and other foreign invaders. By fusing antibody-producing cells with myeloma cells, scientists were able to generate antibodies that would, like “magic bullets,” bind with specific targets including unique markers, called antigenic determinants (epitopes ), on the surfaces of inflammatory cells. When tagged with radioisotopes or other contrast agents, monoclonal antibodies can help in detecting the location of cancer cells, thereby improving the precision of surgery and radiation therapy and showing—within 48 h—whether a tumor is responding to chemotherapy.
The polymerase chain reaction , a method for amplifying tiny bits of DNA first described in the mid-1980s, has been crucial to the development of blood tests that can quickly determine exposure to the human immunodeficiency virus (HIV) . Genetic testing currently is available for many rare monogenic disorders, such as hemophilia, Duchenne muscular dystrophy, sickle cell anemia, thalassemia, etc.
Another rapidly developing field is proteomics, which deals with analysis and identification of proteins. The analysis is done by two-dimensional gel electrophoresis of the sample and then performing mass spectrometric analysis for each individual protein. The technique may be helpful in detecting the disease-associated protein in the biological sample. They may indicate early signs of disease, even before symptoms appear. One such marker is C-reactive protein, an indicator of inflammatory changes in blood vessel walls that presage atherosclerosis.
Nanomedicine is a rapidly moving field. Scientists are developing a wide variety of nanoparticles and nanodevices, scarcely a millionth of an inch in diameter, to improve detection of cancer, boost immune responses, repair damaged tissue, and thwart atherosclerosis. The FDA has approved a paclitaxel albumin-stabilized nanoparticle formulation (Abraxane® for injectable suspension, made by Abraxis BioScience) for the treatment of metastatic adenocarcinoma of the pancreas. Nanoparticles are being explored in heart patients in the USA as a way to keep their heart arteries open following angioplasty.
Therapeutic proteins are those, which can replace the patients naturally occurring proteins, when levels of the natural proteins are low or absent due to the disease. High-throughput screening, conducted with sophisticated robotic and computer technologies, enables scientists to test tens of thousands of small molecules in a single day for their ability to bind to or modulate the activity of a “target,” such as a receptor for a neurotransmitter in the brain. The goal is to improve the speed and accuracy of therapeutic protein or potential drug discovery while lowering the cost and improving the safety of pharmaceuticals that make it to market.
Many of the molecules utilized for detection also have therapeutic potential too, for example, monoclonal antibodies. The monoclonal antibodies are approved for the treatment of many diseases as cancer, multiple sclerosis, and rheumatoid arthritis. They are currently being tested in patients as potential treatments for asthma, Crohn’s disease, and muscular dystrophy. As the antibodies may be efficiently targeted against a particular cell surface marker, thus they are used to deliver a lethal dose of toxic drug to cancer cells, avoiding collateral damage to nearby normal tissues.
1.3 Agricultural Biotechnology
The man has made tremendous progress in crop improvement in terms of yield; still the ultimate production of crop is less than their full genetic potential. There are many reasons like environmental stresses (weather condition as rain, cold, frost), diseases, pests, and many other factors which reduce the ultimate desired yield affecting crop productivity. The efforts are going on to design crops which may be grown irrespective of their season or can be grown in frost or drought conditions for maximum utilization of land, which would ultimately affect crop productivity [ 24 ]. Agricultural biotechnology aims to introduce sustainable agricultural practices with best yield potential and minimal adverse effects on environment (Fig. 1.7 ). For example, combating pests was a major challenge. Thus, the gene from bacterium Bacillus thuringiensis , the Bt gene, that functions as insect-resistant gene when inserted into crop plants like cotton, corn, and soybean helps prevent the invasion of pathogen , and the tool is called integrated pest management . This management is helpful in reducing usage of potentially dangerous pesticides on the crop. Not only the minimal or low usage of pesticides is beneficial for the crop but also the load of the polluting pesticides on environment is greatly reduced [ 24 ].
Various applications of agricultural biotechnology
Resistance to Infectious Agents Through Genetic Engineering
The gene comes from the soil bacterium Bacillus thuringiensis .
The gene produces crystal proteins called Cry proteins. More than 100 different variants of the Bt toxins have been identified which have different specificity to target insect lepidoptera. For eg., CryIa for butterflies and CRYIII for beetles.
These Cry proteins are toxic to larvae of insects like tobacco budworm, armyworm, and beetles.
The Cry proteins exist as an inactive protoxins.
These are converted into active toxin in alkaline pH of the gut upon solubilization when ingested by the insect.
After the toxin is activated, it binds to the surface of epithelial cells of midgut and creates pores causing swelling and lysis of cells leading to the death of the insect (larva).
The genes (cry genes) encoding this protein are isolated from the bacterium and incorporated into several crop plants like cotton, tomato, corn, rice, and soybean.
The proteins encoded by the following cry genes control the pest given against them:
Cry I Ac and cry II Ab control cotton bollworms.
Cry I Ab controls corn borer.
Cry III Ab controls Colorado potato beetle.
Cry III Bb controls corn rootworm.
Protection against nematodes
A nematode Meloidogyne incognita infects tobacco plants and reduces their yield.
The specific genes (in the form of cDNA) from the parasite are introduced into the plant using Agrobacterium -mediated transformation.
The genes are introduced in such a way that both sense/coding RNA and antisense RNA (complimentary to the sense/coding RNA) are produced.
Since these two RNAs are complementary, they form a double-stranded RNA (ds RNA).
This neutralizes the specific RNA of the nematode, by a process called RNA – interference.
As a result, the parasite cannot multiply in the transgenic host, and the transgenic plant is protected from the pest.
These resistant crops result in reduced application of pesticides. The yield is high without the pathogen infestations and insecticides. This also helps to reduce load of these toxic chemicals in the environment.
The transformation techniques and their applications are being utilized to develop rice, cassava, and tomato, free of viral diseases by “International Laboratory for Tropical Agricultural Biotechnology” (ILTAB). ILTAB in 1995 reported the first transfer of a resistance gene from a wild-type species of rice to a susceptible cultivated rice variety. The transferred gene expressed and imparted resistance to crop-destroying bacterium Xanthomonas oryzae . The resistant gene was transferred into susceptible rice varieties that are cultivated on more than 24 million hectares around the world [ 6 ].
The recombinant DNA technology reduces the time between the identification of a particular gene to its application for betterment of crops by skipping the labor-intensive and time-consuming conventional breeding [ 3 ]. For example, the alteration of known gene in plant for the improvement of yield or tolerance to adverse environmental conditions or resistance to insect in one generation and its inheritance to further generations. Plant cell and tissue culture techniques are one of the applications where virus-free plants can be grown and multiplied irrespective of their season on large scale (micropropogation), raising haploids, or embryo rescue . It also opens an opportunity to cross two manipulated varieties or two incompatible varieties (protoplast culture) for obtaining best variety for cultivation.
With the help of technology, new, improved, and safe agricultural products may emerge which would be helpful for maintaining contamination-free environment. Biotechnology has the potential to produce:
High crop yields [ 4 ]
Crops are engineered to have desirable nutrients and better taste (e.g., tomatoes and other edible crops with increased levels of vitamin C, vitamin E, and/or beta-carotene protect against the risk of some prevalent chronic diseases and rice with increased iron levels protects against anemia)
Insect- and disease-resistant plants
Genetic modification avoids nonselective changes
Longer shelf life of fruits and vegetables
The potential of biotechnology may contribute to increasing agricultural, food, and feed production, protecting the environment, mitigating pollution , sustaining agricultural practices, and improving human and animal health. Some agricultural crops as corn and marine organisms can be potential alternative for biofuel production. The by-products of the process may be processed to produce other chemical feedstocks for various products. It is estimated that the world’s chemical and fuel demand could be supplied by such renewable resources in the first half of the next century [ 5 ].
1.3.1 Food Biotechnology
Food biotechnology is an emerging field, which can increase the production of food, improving its nutritional content and improving the taste of the food. The food is safe and beneficial as it needs fewer pesticides and insecticides. The technology aims to produce foods which have more flavors, contain more vitamins and minerals, and absorb less fat when cooked. Food biotechnology may remove allergens and toxic components from foods, for their better utility [ 6 , 7 ].
1.4 Environmental Biotechnology
Environmental biotechnology grossly deals with maintenance of environment, which is pollution-free, the water is contamination-free, and the atmosphere is free of toxic gases. Thus, it deals with waste treatment, monitoring of environmental changes, and pollution prevention. Bioremediation in which utilization of higher living organisms (plants: phytoremediation ) or certain microbial species for decontamination or conversion of harmful products is done is the main application of environmental biotechnology. The enzyme bioreactors are also being developed which would pretreat some industrial and food waste components and allow their removal through the sewage system rather than through solid waste disposal mechanisms. The production of biofuel from waste can solve the fuel crisis (biogas). Microbes may be engineered to produce enzymes required for conversion of plant and vegetable materials into building blocks for biodegradable plastics. In some cases, the by-products of the pollution-fighting microorganisms are themselves useful. For example, methane can be derived from a form of bacteria that degrades sulfur liquor, a waste product of paper manufacturing. This methane thus obtained is used as a fuel or in other industrial processes. Insect- and pest-resistant crops have reduced the use and environmental load of insecticides and pesticides. Insect-protected crops allow for less potential exposure of farmers and groundwater to chemical residues while providing farmers with season-long control.
1.5 Industrial Biotechnology
The utilization of biotechnological tools (bioprocessing) for the manufacturing of biotechnology-derived products (fuels, plastics, enzymes, chemicals, and many more compounds) on industrial scale is industrial biotechnology. The aim is to develop newer industrial manufacturing processes and products, which are economical and better than preexisting ones with minimal environmental impact. In industrial biotechnology, (1) microorganisms are being explored for producing material goods like fermentation products as cheese; (2) biorefineries where oils, sugars, and biomass may be converted into biofuels , bioplastics, and biopolymers; (3) and value-added chemicals from biomass. The utilization of modern techniques can improve the efficiency and reduces the environmental impacts of industrial processes like textile, paper, pulp, and chemical manufacturing. For example, development and usage of biocatalysts, such as enzymes, to synthesize chemicals and development of antibiotics and better tasting liquors and their usage in food industry have provided safe and effective processing for sustainable productions. Biotechnological tools in the textile industry are utilized for the finishing of fabrics and garments. Biotechnology also produces spider silk and biotech-derived cotton that is warmer and stronger and has improved dye uptake and retention, enhanced absorbency, and wrinkle and shrink resistance.
Biofuels may be derived from photosynthetic organisms, which capture solar energy, transform it in other products like carbohydrates and oils, and store them. Different plants can be used for fuel production:
Bioethanol can be obtained from sugar (as sugarcane or sugar beet) or starch (like corn or maize). These are fermented to produce ethanol, a liquid fuel commonly used for transportation.
Biodiesel can be obtained from natural oils from plants like oil palm, soybean, or algae. They can be burned directly in a diesel engine or a furnace, or blended with petroleum, to produce fuels such as biodiesel.
Wood and its by-products can be converted into liquid biofuels , such as methanol or ethanol, or into wood gas. Wood can also be burned as solid fuel, like the irewood.
In these kinds of biological reaction, there are many renewable chemicals of economic importance coproduced as side streams of bioenergy and biofuels as levulinic acid, itaconic acid, and sorbitol. These have tremendous economic potential and their fruitful usage would depend upon the collaboration for research and development between the government and the private sector.
1.5.1 Enzyme Production
The enzymes have big commercial and industrial significance. They have wide applications in food industry, leather industry, pharmaceuticals, chemicals, detergents, and research. In detergents the alkaline protease, subtilisin (from Bacillus subtilis ), was used by Novo Industries, Denmark. The production of enzymes is an important industrial application with world market of approximately 5 billion dollars. The enzymes can be obtained from animals, plants, or microorganisms. The production from microorganisms is preferred as they are easy to maintain in culture with simple media requirements and easy scale-up. The important enzymes for the industrial applications are in food industry, human application, and research. A few animal enzymes are also important as a group of proteolytic enzymes, for example, plasminogen activators, which act on inactive plasminogen and activate it to plasmin, which destroys fibrin network of blood clot. Some of the plasminogen activators are urokinase and tissue plasminogen activators (t-PA) . Urokinase (from urine) is difficult to obtain in ample quantity; thus, t-PA is obtained from cells grown in culture medium. Streptokinase (bacterial enzyme) is also a plasminogen activator but is nonspecific and immunogenic.
Enzyme engineering is also being tried where modifications of specific amino acid residue are done for improving the enzyme properties. One of the enzymes chymosin (rennin) coagulates milk for cheese manufacturing.
The enzymes can be produced by culturing cells, growing them with appropriate substrates in culture conditions. After optimum time the enzymes may be obtained by cell disruption (enzymatic/freeze–thaw/osmotic shock) followed by preparative steps (centrifugation, filtration), purification, and analysis . The product is then packaged and ultimately launched in the market.
After their production, they can be immobilized on large range of materials (agar, cellulose, porous glass, or porous alumina) for subsequent reuse. Some of the important industrial enzymes are α-amylase (used for starch hydrolysis), amyloglucosidase (dextrin hydrolysis), β-galactosidase (lactose hydrolysis), aminoacylase (hydrolysis of acylated L-amino acids), glucose oxidase (oxidation of glucose), and luciferase (bioluminescence). Some of the medically important enzymes are urokinase and t-PA for blood clot removal and L-asparaginase for removal of L-asparagine essential for tumor growth and thus used for cancer chemotherapy in leukemia.
1.5.2 Exploring Algae for Production of Biofuels
The energy requirement of present population is increasing and gradually fossil fuels are rapidly depleting. Thus, renewable energy sources like solar energy and wind-, hydro-, and biomass-based energy are being explored worldwide. One of the feedstocks may be microalgae, which are fast-growing, photosynthetic organisms requiring carbon dioxide, some nutrients, and water for its growth. They produce large amount of lipids and carbohydrates, which can be processed into different biofuels and commercially important coproducts. The production of biofuels using algal biomass is advantageous as they (1) can grow throughout the year and thus their productivity is higher than other oil seed crops, (2) have high tolerance to high carbon dioxide content, (3) utilize less water, (4) do not require herbicides or pesticides with high growth potential (waste water can be utilized for algal cultivation), (5) can sustain harsh atmospheric conditions, and (6) do not interfere with productivity of conventional crops as they do not require agricultural land. The production of various biofuels from algae is schematically represented in Fig. 1.8 .
Different biofuel productions by using microalgae. The algae use sunlight, CO2, water, and some nutrients
Algae can serve as potential source for biofuel production; however, biomass production is low. The production has certain limitations, as cultivation cost is high with requirement of high energy [ 1 ].
1.6 Marine or Aquatic Biotechnology
Marine or aquatic biotechnology also referred to as “blue biotechnology” deals with exploring and utilizing the marine resources of the world. Aquatic or marine life has been intriguing and a source of livelihood for many since years. As major part of earth is acquired by water, thus nearly 75–80 % types of life forms exist in oceans and aquatic systems. It studies the wide diversity found in the structure and physiology of marine organisms. They are unique in their own ways and lack their equivalent on land. These organisms have been explored and utilized for numerous applications as searching new treatment for cancer or exploring other marine resources, because of which the field is gradually gaining momentum and economic opportunities [ 19 ]. The global economic benefits are estimated to be very high. The field aims to:
Fulfill the increasing food supply needs
Identify and isolate important compounds which may benefit health of humans
Manipulate the existing traits in sea animals for their improvement
Protect marine ecosystem and gain knowledge about the geochemical processes occurring in oceans
Some of the major applications are discussed:
Aquaculture: Aquaculture refers to the growth of aquatic organisms in culture condition for commercial purposes. These animals may be shellfish, finfish, and many others. Mariculture refers to the cultivation of marine animals. Their main applications are in food, food ingredients, pharmaceuticals, and fuels, the products are in high demand, and various industries are in aquaculture business, for example, crawfish farming (Louisiana), catfish industry (Alabama and Mississippi Delta), and trout farming (Idaho and West Virginia).
Biotechnology discoveries and products.
Transgenic species of salmon with growth hormone gene has accelerated growth of salmons.
Molt-inhibiting hormone (MIH) from blue crabs leads to soft-shelled crab.
Antifreeze proteins : A novel protein antifreeze protein (AFP) was identified. AFPs were isolated from Northern cod (bottom-dwelling fish) living at the Eastern Canada coast and teleosts living in extremely cold weather of Antarctica. AFPs have been isolated from Osmerus mordax (smelt), Clupea harengus (herring), Pleuronectes americanus (winter flounder), and many others. Due to antifreeze properties (lowering the minimal freezing temperature by 2–3 °C), the gene has potential for raising plants which are cold tolerant (e.g., tomatoes).
Green fluorescent protein : A much popular green fluorescent protein (GFP) was obtained from jellyfish Aequorea victoria . It can fluoresce and thus glow in the dark. Many marine microorganisms have bioluminescent capability. GFP is widely used as reporter gene in experiments related to gene cloning , expression, and transgenics. A transgenic strain of zebra fish in the name of GloFish was created by Yorktown Industries, Texas, in 2004. This was with red fluorescent protein gene obtained from sea anemones, and it was the first genetically modified pet animal in the market.
Medicinal applications : For osteoporosis, salmon calcitonin (calcitonin is thyroid hormone promoting calcium uptake and bone calcification) with 20 times higher bioactivity is available as injection and nasal spray.
Hydroxyapatite ( HA ): Obtained from coral reefs and is an important component of bone and cartilage matrix. Its implants are prepared by Interpore Internationals which may be used for filling gaps in fractured bones.
Byssal fibers : Are protein-rich superadhesive which have elastic properties obtained from mussels ( Mytilus edulis ). Their isolation would not be very economical, but they can have wide applications in surgical sutures, artificial tendons, and ligament grafts.
Many anti-inflammatory, analgesic, anticancerous compounds have been identified from sea organisms which can have tremendous potential for human health.
Tetrodotoxin (TTX) is the most toxic poison (10,000 times more lethal than cyanide) produced by Japanese pufferfish or blowfish ( Fugu rubripes ). TTX is being used to study and understand its effect on sodium channels which can help guide the development of drugs with anesthetic and analgesic properties.
Other Products
Taq polymerase from Thermus aquaticus which is used in PCR reactions and obtained from hot spring Archaea.
Collagenase (protease) obtained from Vibrio is used in tissue engineering and culturing.
1.7 Transgenic Animals and Plants
In the early 1980s, inserting DNA from humans into mice and other animals became possible. The animals and plants which have foreign gene in each of their cells are referred to as transgenic organisms and the inserted gene as transgene. Expression of human genes in these transgenic animals can be useful in studies, as models for the development of diabetes , atherosclerosis, and Alzheimer’s disease. They also can generate large quantities of potentially therapeutic human proteins. Transgenic plants also offer many economic, safe, and practical solutions for production of variety of biopharmaceuticals . The plants have been engineered to produce many blood products (human serum albumin, cytokines), human growth hormone , recombinant antibodies, and subunit vaccines.
The usage of transgenic plants for the production of recombinant pharmaceuticals might open new avenues in biotechnology. As plants can be grown inexpensively with minimal complicated requirements, thus they may have tremendous therapeutic potential. The plants have been engineered to produce more nutrients or better shelf life. The transgenic plants have been created which have genes for insect resistance (Bt cotton, soybean, corn). Now billion acres of land is used for cultivation of genetically engineered crops of cotton, corn, and soybean as they have higher yield and are pest resistant. However, due to social, ethical, and biosafety issues, they have received acceptance as well as rejections at many places and health and environment-related concerns in many parts of the world [ 8 ].
1.8 Response to Antibiotic Resistance
Antibiotics are one of the broadly used therapeutic molecules produced by certain classes of microorganisms (bacteria and fungi) which can be used in diverse clinical situations to eliminate bacteria, improve symptoms, and prevent number of infections. Antibiotics have various other applications apart from clinical aspects. They can be used for the treatment of tumors and treatment of meat, in cattles and livestocks, in basic biotechnological work. However, their effectiveness is a matter of concern as bacteria which are continuously exposed to certain antibiotics might become resistant to it due to accumulation of mutations. These days antibiotic-resistant bacteria have increased and some of them have developed multiple drug resistance. Thus, it has become very difficult to initiate therapy in diseases like tuberculosis and leprosy . Biotechnology is solving the urgent and growing problem of antibiotic resistance. With the help of bioinformatics—powerful computer programs capable of analyzing billions of bits of genomic sequence data—scientists are cracking the genetic codes of bacteria and discovering “weak spots” vulnerable to attack by compounds identified via high-throughput screening. This kind of work led in 2000 to the approval of Zyvox (linezolid), an antibiotic to reach the market in 35 years.
Lytic bacteriophage viruses that infect and kill bacteria may be another way to counter resistance. First used to treat infection in the 1920s, “phage therapy” was largely eclipsed by the development of antibiotics. However, researchers in the former Soviet Republic of Georgia reported that a biodegradable polymer impregnated with bacteriophages and the antibiotic Cipro successfully healed wounds infected with a drug-resistant bacterium.
After exposure of strontium-90, three Georgian lumberjacks from village Lia had systemic effects, and two of them developed severe local radiation injuries which subsequently became infected with Staphylococcus aureus . Upon hospitalization, the patients were treated with various medications, including antibiotics and topical ointments; however, wound healing was only moderately successful, and their S. aureus infection could not be eliminated. Approximately 1 month after hospitalization, treatment with PhagoBioDerm (a wound-healing preparation consisting of a biodegradable polymer impregnated with ciprofloxacin and bacteriophages ) was initiated. Purulent drainage stopped within 2–7 days. Clinical improvement was associated with rapid (7 days) elimination of the etiologic agent, and a strain of S. aureus responsible for infection was resistant to many antibiotics (including ciprofloxacin) but was susceptible to the bacteriophages contained in the PhagoBioDerm preparation [ 11 ].
1.9 The Challenges for the Technology
1.9.1 gene therapy.
Some biotech approaches to better health have proven to be more challenging than others. An example is gene transfer, where the defective gene is replaced with a normally functioning one. The normal gene is delivered to target tissues in most cases by virus that is genetically altered to render it harmless. The first ex vivo gene transfer experiment, conducted in 1990 at the National Institutes of Health (NIH), on Ashanti DeSilva who was suffering from severe combined immunodeficiency (SCID) helped boost her immune response and successfully corrected an enzyme deficiency. However, treatment was required every few months. However, 9 years later, a major setback occurred in gene therapy trial after the death of 18-year-old Jesse Gelsinger suffering from ornithine transcarbamylase (OTC) deficiency due to intense inflammatory responses followed by gene therapy treatment. There were some positive experiences and some setbacks from gene therapy trials leading to stricter safety requirements in clinical trials.
1.9.2 Designer Babies
The fancy term designer baby was invented by media. Many people in society prefer embryos with better traits, intellect, and intelligence. They want to select embryo post germline engineering. This technique is still in infancy but is capable of creating lot of differences in the society thus requires appropriate guidelines.
1.9.3 Genetically Modified Food
Genetically modified crops harboring genes for insect resistance were grown on billion of acres of land. These crops became very popular due to high yield and pest resistance. However, some of the pests gradually developed resistance for a few of these transgenic crops posing resistant pest threat. The other technologies as “traitor” and “terminator” technologies pose serious risk on crop biodiversity and would impart negative characters in the crop (they were not release d due to public outcry).
1.9.4 Pharmacogenomics
S cientists do not believe they will find a single gene for every disease. As a result, they are studying relationships between genes and probing populations for variations in the genetic code, called single nucleotide polymorphisms, or SNPs , that may increase one’s risk for a particular disease or determine one’s response to a given medication. This powerful ability to assign risk and response to genetic variations is fueling the movement toward “individualized medicine.” The goal is prevention, earlier diagnosis, and more effective therapy by prescribing interventions that match patients’ particular genetic characteristics.
1.9.5 Tissue Engineering
Tissue engineering is one of the emerging fields with tremendous potential to supply replacement tissue and organ option for many diseases. Lot is achieved, lot more need to be achieved.
1.10 Ethical Issues
The pursuit of cutting-edge research “brings us closer to our ultimate goal of eliminating disability and disease through the best care which modern medicine can provide.” Understanding of the genetics of heart disease and cancer will aid the development of screening tools and interventions that can help prevent the spread of these devastating disorders into the world’s most rapidly developing economies.
Biotechnology is a neutral tool; nevertheless, its capabilities raise troubling ethical questions. Should prospective parents be allowed to “engineer” the physical characteristics of their embryos? Should science tinker with the human germ line, or would that alter in profound and irrevocable ways what it means to be human?
More immediately, shouldn’t researchers apply biotechnology—if they can—to eliminate health disparities among racial and ethnic groups? While genetic variation is one of many factors contributing to differences in health outcome (others include environment, socioeconomic status, health-care access, stress , and behavior), the growing ability to mine DNA databases from diverse populations should enable scientists to parse the roles these and other factors play.
Biotechnology along with supportive health-care infrastructure can solve complicated health problems. Accessibility to the new screening tests, vaccines, and medications and cultural, economic, and political barriers to change must be overcome. Research must include more people from disadvantaged groups, which will require overcoming long-held concerns, some of them have had about medical science.
Biotechnology has been a significant force which has improved the quality of lives and has incalculably benefitted human beings. However, technology does have prospects of doing harm also due to unanticipated consequences. Each technology is subjected to ethical assessment and requires a different ethical approach. Obviously the changes are necessary as technology can have major impact on the world; thus, a righteous approach should be followed. There is uncertainty in predicting consequences, as this powerful technology has potential to manipulate humans themselves. Ethical concerns are even more important as the future of humanity can change which require careful attention and consideration. Therefore, wisdom is required to articulate our responsibilities toward environment, animals, nature, and ourselves for the coming future generations. We need to differentiate what is important technologically rather that what technology can do. For an imperative question, that is, whether this can be achieved, the research must answer “Why should it be achieved”? Who would it benefit?
1.11 Issues Related to Safety
As the new GM crops are entering the market, the issue regarding their consumption, whether they are safe, without any risk, is one of the important concerns [ 2 ]. Though the results related to safety and usage are well reported (as compared to conventional crops), unknown fear from these products makes them non acceptable at many places [ 20 ].
As insect- and pest-resistant varieties are being prepared and used as Bt genes in corn and cotton crops, there exists a risk of development of resistance insect population. Another important factor is that these resistant crops may harm other species like birds and butterfly.
The development of more weeds may occur as cross-pollination might result in production of weeds with herbicide resistance which would be difficult to control.
The gene transfers might cross the natural species boundary and affect biological diversity.
The judgment of their usage would depend upon the clear understanding of risks associated with safety of these products in determining the impact of these on environment, other crops, and other animal species.
1.12 Future of the Technology
With the understanding of science, we should understand that genetic transfers have been occurring in animals and plant systems; thus, the risk of the biotechnology-derived products is similar as conventional crops [ 12 ].
The biotechnology products would be acceptable to many if they are beneficial and safe. People are willing to buy crops free of pesticides and insecticides. Nowadays people are also accepting crops grown without the usage of chemical fertilizers or pesticides, which are high in nutritive values.
The labeling of the product is also an ethical issue as some believe that labeling any product as biotechnology product might be taken by consumer as warning signs; however, others believe that labeling should be done as consumer has every right to know what he is consuming [ 9 ]. The products may be acceptable if consumers can accept the food derived from biotechnology weighing all pros and cons and, if the price is right, has more nutritive values, is good in taste, and is safe to consume [ 10 ].
Biotechnology is at the crossroads in terms of fears and thus public acceptance [ 15 ]. Surprisingly the therapeutic products are all accepted and find major place in biopharmaceutical industry, but food crops are still facing problems in worldwide acceptance. The future of the world food supply depends upon how well scientists, government, and the food industry are able to communicate with consumers about the benefits and safety of the technology [ 13 , 16 ]. Several major initiatives are under way to strengthen the regulatory process and to communicate more effectively with consumers by conducting educational programs [ 18 , 23 ].
1.13 Chapter End Summary
The advantages of biotechnology are so broad that it is finding its place in virtually every industry. It has applications in areas as diverse as pharmaceuticals, diagnostics, textiles, aquaculture, forestry, chemicals, household products, environmental cleanup, food processing, and forensics to name a few.
Biotechnology is enabling these industries to make new or better products, often with greater speed, efficiency, and flexibility.
With the applications of recombinant DNA technology , more safer and therapeutic drugs are produced. These recombinant products do not elicit unwanted immunological response which is observed when the product is obtained from other live or dead sources. Many of these therapeutics are approved for human usage, and many of them are in the phase of development.
Immunological and DNA-based techniques like PCR (polymerase chain reaction) are used for early diagnosis of disorders. PCR and NAAT with microarray can be utilized for the diagnosis of many diseases, and it can detect mutations in gene.
The technology holds promise through stem cell research and gene therapy and holds applications in forensic medicine.
The technique may be helpful in developing useful and beneficial plants. It overcomes the limitations of traditional plant breeding . The techniques of plant tissue culture , transgenics, and marker-assisted selections are largely used for selecting better yielding varieties and imparting quality traits in plants.
It is also helpful in maintaining environment by bioremediation and other treatment. The areas where it finds applications are:
Food industries. Production of single-cell protein, spirulina, enzymes, and solid-state fermentations
Increase and improvement of agricultural production
Production of therapeutic pharmaceuticals
Production of vaccines and monoclonal antibodies
Cultivation of virus for vaccine production
Multiple Choice Questions
Which abiotic stress can be tolerated by genetically modified crops?
All of the above
The golden rice is a crop having high nutritive value in:
Vitamin D and calcium
Bt toxin gene which encodes cry protein is:
The first recombinant product to reach the market was:
Growth hormone
Tissue plasminogen activator
Factor VIII
Biotechnology deals with:
Genetically modifying organism
Production of therapeutics
Production of better diagnosis
Green revolution is:
Increase in yield of crops
Improved crop varieties
Lesser fertilizers and agrochemicals
All of these
Insecticidal protein cry does not kill bacillus because:
It is resistant to it.
The toxin is enclosed in vesicle.
The toxin is present in inactive form.
None of these.
DNA defects may be solved by:
Gene therapy
Replacement protein therapy
Stem cell therapy
The use of insect resistant crop would be:
The productivity would improve.
The usage of chemical agent would be reduced.
The environment and crop would be insecticide free.
All of the above.
Bioremediation can be helpful in:
Detoxifying waste material
Burying waste material
Burning waste material
None of these
Which of the following statements are true?
(1) In all the cells of our body, all the genes are active.
(2) In different cells of our body, different genes are active.
(3) Gene expression is spatially and temporally regulated.
All 1, 2, and 3 are correct.
1 and 2 are correct.
1 and 3 are correct.
2 and 3 are correct.
In a classic experiment, Dr. Edward Jenner demonstrated that:
Inoculation with monoclonal antibody was able to prevent small pox.
Inoculation with pus from sores due to cowpox could prevent small pox.
Attenuated vaccine was able to prevent small pox.
None of the above.
1. (c); 2. (a); 3. (c); 4. (d); 5. (d); 6. (d); 7. (c); 8. (a); 9. (d); 10. (a); 11. (d); 12. (b)
Review Questions
Q1. What are cry proteins? What is their importance?
Q2. Give some applications of biotechnology in agriculture.
Q3. What is your opinion about labeling of biotechnology-based food product as rDNA technology derived product?
Q4. What are applications of biotechnology in maintaining environment?
Q5. What is medical biotechnology?
Q6. What are the challenges faced by biotechnology industry?
Q7. What do you think about GM crops?
Behera et al. (2015) Scope of algae as third generation biofuels. Front Bioeng Biotechnol 2: doi: 10.3389/fbioe.2014.00090
Bruhn CM (1992) Consumer concerns and educational strategies: focus on biotechnology. Food Technol 46:80–102
Google Scholar
Council for Agricultural Science and Technology: “Applications of Biotechnology to Crops: Benefits and Risks”, Issue Paper, Number 12, Dec. 1999
Definition of Biotechnology-Economic Research Service at United States Department of Agriculture
Erickson B, Nelson JE, Winters P (2012) Perspective on opportunities in industrial biotechnology in renewable chemicals. Biotechnol J 7:176–185
Article CAS Google Scholar
Food biotechnology-benefits for the developing countries. http://ificinfo.health.org/insight/janfeb99/foodbiotechnology.htm
Food biotechnology: health and harvest for our times. http://ificinfo.health.org/brochure/biobroch.htm
Genetically engineered foods, fears and facts: FDA. Consumer 27(1). January/February 1993, pp. 11–14. http://www.fda.gov/fdac/100_toc.html
Hoban T, Kendall P (1994) Consumer attitudes about food biotechnology: final project report. North Carolina Cooperative Extension Service, North Carolina State University, Raleigh
IFIC Foundation- Americans remain positive on food biotechnology. http://ificinfo.health.org/press/positivebio.htm
Jikia et al (2005) The use of a novel biodegradable preparation capable of the sustained release of bacteriophages and ciprofloxacin, in the complex treatment of multidrug-resistant Staphylococcus aureus-infected local radiation injuries caused by exposure to Sr90. Clin Exp Dermatol 30:23–6
Myths and facts about food biotechnology, food insight, September/October 1999, pp. 2–3
National and international policy making in biotechnology. http://www.biotechknowledge.com/showlib.php3?194
New York Times Editorial, titled: “Food….people who would have the most to lose”, 19 Nov 1999
North Carolina Biotechnology Center. “About Biotech”. http://www.ncbiotech.org/aboutbt/main.cfm
Principles of biotechnology. http://www.nal.usda.gov/bic/Education_res/iastate.info/bio1.html
Public perceptions of biotechnology. A summary of research by Dr. Thomas Hoban at North Carolina State University. http://www4.ncsu.edu/~hobantj/biotech.htm
The Council for Biotechnology Information. http://www.whybiotech.com
Thieman WJ, Palladino MA (2004) Introduction to biotechnology, 2nd edn. Pearson Publications, USA
U.S. Food and Drug Administration Center for food Safety and Applied Nutrition: October-November 1997. http://vm.cfsan.fda.gov/~tdms/pubalgry.html
U.S. Food and Drug Administration Center for food Safety and Applied Nutrition: Q & A Sheet: June 1992. http://vm.cfsan.fda.gov/~lrd/bioqa.html
United States Department of Agriculture “Agricultural Biotechnology Concepts and Definitions”. http://www.biotechknowledge.com/showlib.php3?1739
van Beuzekom B, Arundel A (2006) OECD biotechnology statistics 2006. OECD, Paris
What the experts say about food biotechnology http://ificinfo.health.org/foodbiotech/whatexpertssay.htm
Some Related Resources
http://ificinfo.health.org/backgrnd/BKGR14.htm
http://www.bio.org/aboutbio/guide1.html
http://www.bio.org/aboutbio/guide2000/guide00_toc.html
http://www.bio.org/aboutbio/guide3.html
http://www.bio.org/aboutbio/guide4.html
http://www.dec.ny.gov/energy/44157.html
http://www.ers.usda.gov/whatsnew/issues/biotech/define.htm
http://www.nal.usda.gov/bic/bio21
http://www.nature.com/nbt/press_release/nbt1199.html
www.angelfire.com/scary/intern/links.html
www.bio-link.org/library.htm
www.biospace.com
www.dnai.org
www.fiercebiotech.com
www.iastate.edu
www.icgeb.trieste.it
www.ncbi.nlm.nih.gov
Download references
Author information
Authors and affiliations.
Institute of Biosciences and Biotechnology, Chhatrapati Shahu Ji Maharaj University, Kanpur, UP, India
Varsha Gupta
George Washington University, Washington DC, USA
Manjistha Sengupta
Orthopaedics Unit, Community Health Centre, Kanpur, UP, India
Jaya Prakash
School of Life sciences, Jawaharlal Nehru University, New Delhi, India
Baishnab Charan Tripathy
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
Copyright information
© 2017 Springer Science+Business Media Singapore
About this chapter
Cite this chapter.
Gupta, V., Sengupta, M., Prakash, J., Tripathy, B.C. (2017). An Introduction to Biotechnology. In: Basic and Applied Aspects of Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-10-0875-7_1
Download citation
DOI : https://doi.org/10.1007/978-981-10-0875-7_1
Published : 23 October 2016
Publisher Name : Springer, Singapore
Print ISBN : 978-981-10-0873-3
Online ISBN : 978-981-10-0875-7
eBook Packages : Earth and Environmental Science Earth and Environmental Science (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Plant biotechnology articles from across Nature Portfolio
Plant biotechnology can be defined as the introduction of desirable traits into plants through genetic modification.
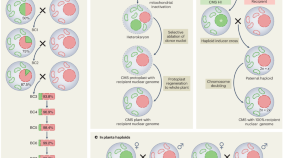
Haploids fast-track hybrid plant breeding
Two studies report the use of paternal haploids to enable one-step transfer of cytoplasmic male sterility in maize and broccoli, which resolves a key technical bottleneck in hybrid crop breeding.
- Ravi Maruthachalam
Related Subjects
- Agricultural genetics
- Field trials
- Molecular engineering in plants
Latest Research and Reviews
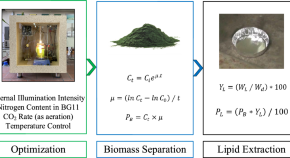
Lipids productivity of cyanobacterium Anabaena vaginicola in an internally illuminated photobioreactor using LED bar lights
- Hootan Goldoost
- Farzaneh Vahabzadeh
- Narges Fallah

Dynamics of starch formation and gene expression during grain filling and its possible influence on grain quality
- Sanjeeva Rao Durbha
- N. Siromani
- R. M. Sundaram
Cyto-swapping in maize by haploid induction with a cenh3 mutant
An efficient method of cyto-swapping by haploid induction using a CENH3 mutation is reported in maize, to convert commercial germplasm to cytoplasmic male sterility for hybrid seed production.
- Esteban Bortiri
- Rebecca Selby
- Tim Kelliher
One-step creation of CMS lines using a BoCENH3 -based haploid induction system in Brassica crop
Han et al. develop BoCENH3 mutants which trigger paternal haploid induction in Brassica oleracea . On the basis of this haploid inducer line, a workable system is proposed for transferring cytoplasmic male sterility to broccoli inbred lines.
- Fengqing Han
- Xiaoli Zhang
- Zhansheng Li
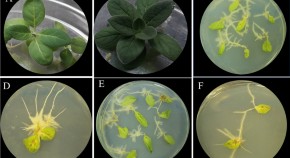
Optimization of induction and hairy root culture establishment in two mullein species, Verbascum erianthum and Verbascum stachydiforme
- Soniya Amini
- Mohammad Fattahi
- Hossein Nazemiyeh

Design, construction and field testing of a manually feeding semiautomatic sugarcane dud chipper
- Abdallah Elshawadfy Elwakeel
- Saher M. A. Mohamed
- Zaher Mundher Yaseen
News and Comment
Feeding the future global population.
Climate change is exacerbating challenges both for global food production and from its environmental impacts. Sustainable and socially responsible solutions for future world-wide food security are urgently needed.
Novel gene for herbicide resistance
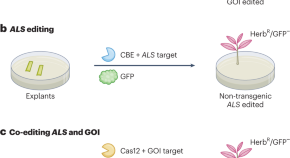
Blueprint for non-transgenic edited plants
A robust strategy to obtain edited crops without integration of a transgene is developed based on co-editing the ALS gene and a gene of interest.
- Jean-Luc Gallois
- Fabien Nogué
A new chance for genome editing in Europe
- Hervé Vanderschuren
- Patience Chatukuta
- Devang Mehta
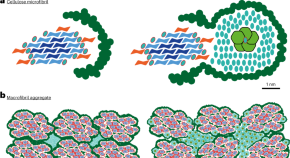
Callose integration into secondary cell walls modifies woody biomass ultrastructure and accessibility
Previous genetic engineering of plant secondary cell walls targeted its core polymers to facilitate their extractability. The ectopic introduction of the polymer callose into poplar wood secondary cell walls modifies the ultrastructure of cellulose microfibril aggregates and suggests new avenues when considering biomass genetic engineering.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection

Animal tissue culture principles and applications
1 Department of Plant Pathology, Institute of Plant Breeding Genetics & Genomics, Center for Applied Genetic Technologies, University of Georgia, Athens, GA, United States
Megha Verma
2 College of Arts and Sciences, St. Louis, MO, United States
Anchal Singh
3 Department of Biochemistry, Institute of Science, Banaras Hindu University, Varanasi, UP, India
Animal cell culture technology in today’s scenario has become indispensable in the field of life sciences, which provides a basis to study regulation, proliferation, and differentiation and to perform genetic manipulation. It requires specific technical skills to carry out successfully. This chapter describes the essential techniques of animal cell culture as well as its applications.
What you can expect to know
This chapter describes the basics of animal cell culture along with the most recent applications. The primary aim is to progressively guide students through fundamental areas and to demonstrate an understanding of basic concepts of cell culture as well as how to perform cell cultures and handle cell lines. This chapter gives insights into types of cell culture, culture media and use of serum, viability assays, and the translational significance of cell culture.
History and methods
Introduction.
Cell culture is the process by which human, animal, or insect cells are grown in a favorable artificial environment. The cells may be derived from multicellular eukaryotes, already established cell lines or established cell strains. In the mid-1900s, animal cell culture became a common laboratory technique, but the concept of maintaining live cell lines separated from their original tissue source was discovered in the 19th century. Animal cell culture is now one of the major tools used in the life sciences in areas of research that have a potential for economic value and commercialization. The development of basic culture media has enabled scientists to work with a wide variety of cells under controlled conditions; this has played an important role in advancing our understanding of cell growth and differentiation, identification of growth factors, and understanding of mechanisms underlying the normal functions of various cell types. New technologies have also been applied to investigate high cell density bioreactor and culture conditions.
Many products of biotechnology (such as viral vaccines) are fundamentally dependent on mass culturing of animal cell lines. Although many simpler proteins are being produced using rDNA in bacterial cultures, more complex proteins that are glycosylated (carbohydrate-modified) currently have to be made in animal cells. At present, cell culture research is aimed at investigating the influence of culture conditions on viability, productivity, and the constancy of post-translational modifications such as glycosylation, which are important for the biological activity of recombinant proteins. Biologicals produced by recombinant DNA (rDNA) technology in animal cell cultures include anticancer agents, enzymes, immunobiologicals [interleukins, lymphokines, monoclonal antibodies (mABs)], and hormones.
Animal cell culture has found use in diverse areas, from basic to advanced research. It has provided a model system for various research efforts:
- 1. The study of basic cell biology, cell cycle mechanisms, specialized cell function, cell–cell and cell–matrix interactions.
- 2. Toxicity testing to study the effects of new drugs.
- 3. Gene therapy for replacing nonfunctional genes with functional gene-carrying cells.
- 4. The characterization of cancer cells, the role of various chemicals, viruses, and radiation in cancer cells.
- 5. Production of vaccines, mABs, and pharmaceutical drugs.
- 6. Production of viruses for use in vaccine production (e.g., chicken pox, polio, rabies, hepatitis B, and measles).
Today, mammalian cell culture is a prerequisite for manufacturing biological therapeutics such as hormones, antibodies, interferons, clotting factors, and vaccines.
Development of animal cell culture
The first mammalian cell cultures date back to the early 20th century. The cultures were originally created to study the development of cell cultures and normal physiological events such as nerve development. Ross Harrison in 1907 showed the first nerve fiber growth in vitro. However, it was in the 1950s that animal cell culture was performed at an industrial scale. It was with major epidemics of polio in the 1940s and 1950s and the accompanying requirement for viral vaccines that the need for cell cultures on a large scale became apparent. The polio vaccine from a de-activated virus became one of the first commercial products developed from cultured animal cells ( Table 14.1 ).
Milestones in cell cultures and microfluidics.
Basic concept of cell culture
Tissue culture is in vitro maintenance and propagation of isolated cells tissues or organs in an appropriate artificial environment. Many animal cells can be induced to grow outside of their organ or tissue of origin under defined conditions when supplemented with a medium containing nutrients and growth factors. For in vitro growth of cells, the culture conditions may not mimic in vivo conditions with respect to temperature, pH, CO 2 , O 2 , osmolality, and nutrition. In addition, the cultured cells require sterile conditions along with a steady supply of nutrients for growth and sophisticated incubation conditions. An important factor influencing the growth of cells in culture medium is the medium itself. At present, animal cells are cultured in natural media or artificial media depending on the needs of the experiment. The culture medium is the most important and essential step in animal tissue culture. This depends on the type of cells that need to be cultured for the purpose of cell growth differentiation or production of designed pharmaceutical products. In addition, serum-containing and serum-free media are now available that offer a varying degree of advantage to the cell culture. Sterile conditions are important in the development of cell lines.
Cells from a wide range of different tissues and organisms are now grown in the lab. Earlier, the major purpose of cell culture was to study the growth, the requirements for growth, the cell cycle, and the cell itself. At present, homogenous cultures obtained from primary cell cultures are useful tools to study the origin and biology of the cells. Organotypic and histotypic cultures that mimic the respective organs/tissues have been useful for the production of artificial tissues.
How are cell cultures obtained?
There are three methods commonly used to initiate a culture from animals.
Organ culture
Whole organs from embryos or partial adult organs are used to initiate organ culture in vitro. These cells in the organ culture maintain their differentiated character, their functional activity, and also retain their in vivo architecture. They do not grow rapidly, and cell proliferation is limited to the periphery of the explant. As these cultures cannot be propagated for long periods, a fresh explanation is required for every experiment that leads to interexperimental variation in terms of reproducibility and homogeneity. Organ culture is useful for studying functional properties of cells (production of hormones) and for examining the effects of external agents (such as drugs and other micro or macro molecules) and products on other organs that are anatomically placed apart in vivo.
Primary explant culture
Fragments exercised from animal tissue may be maintained in a number of different ways. The tissue adheres to the surface aided by an extracellular matrix (ECM) constituent, such as collagen or a plasma clot, and it can even happen spontaneously. This gives rise to cells migrating from the periphery of the explant. This culture is known as a primary explant, and migrating cells are known as outgrowth. This has been used to analyze the growth characteristics of cancer cells in comparison to their normal counterparts, especially with reference to altered growth patterns and cell morphology.
Cell culture
This is the most commonly used method of tissue culture and is generated by collecting the cells growing out of explants or dispersed cell suspensions (floating free in culture medium). Cells obtained either by enzymatic treatment or by mechanical means are cultured as adherent monolayers on solid substrate.
Cell culture is of three types: (1) precursor cell culture, which is undifferentiated cells committed to differentiate; (2) differentiated cell culture, which is completely differentiated cells that have lost the capacity to further differentiate; and (3) stem cell culture, which is undifferentiated cells that go on to develop into any type of cell.
Cells with a defined cell type and characteristics are selected from a culture by cloning or by other methods; this cell line becomes a cell strain.
Monolayer cultures
The monolayer culture is an anchorage-dependent culture of usually one cell in thickness with a continuous layer of cells at the bottom of the culture vessel.
Suspension cultures
Some of the cells are nonadhesive and can be mechanically kept in suspension, unlike most cells that grow as monolayers (e.g., cells of leukemia). This offers numerous advantages in the propagation of cells.

Cell passage and use of trypsin
Passaging is the process of subculturing cells in order to produce a large number of cells from pre-existing ones. Subculturing produces a more homogeneous cell line and avoids the senescence associated with prolonged high cell density. Splitting cells involves transferring a small number of cells into each new vessel. After subculturing, cells may be propagated, characterized, and stored. Adherent cell cultures need to be detached from the surface of the tissue culture flasks or dishes using proteins. Proteins secreted by the cells form a tight bridge between the cell and the surface. A mixture of trypsin-EDTA is used to break proteins at specific places. Trypsin is either protein-degrading or proteolytic; it hydrolyzes pepsin-digested peptides by hydrolysis of peptide bonds. EDTA sequesters certain metal ions that can inhibit trypsin activity, and thus enhances the efficacy of trypsin. The trypsinization process and procedure to remove adherent cells is given in Flowchart 14.1 .

Trypsinization of adherent cells.
Quantitation
Quantitation is carried out to characterize cell growth and to establish reproducible culture conditions.
Hemocytometer
Cell counts are important for monitoring growth rates as well as for setting up new cultures with known cell numbers. The most widely used type of counting chamber is called a hemocytometer. It is used to estimate cell number. The concentration of cells in suspension is determined by placing the cells in an optically clear chamber under a microscope. The cell number within a defined area of known depth is counted, and the cell concentration is determined from the count.
Electronic counting
For high-throughput work, electronic cell counters are used to determine the concentration of each sample.
Other quantitation
In some cases, the DNA content or the protein concentration needs to be determined instead of the number of cells.
Reconstruction of three-dimensional structures
Cells propagated as a cell suspension or monolayer offer many advantages but lack the potential for cell-to-cell interaction and cell–matrix interaction seen in organ cultures. For this reason, many culture methods that start with a dispersed population of cells encourage the arrangement of these cells into organ-like structures. These types of cultures can be divided into two basic types.
Histotypic culture
Cell–cell interactions similar to tissue-like densities can be attained by the use of an appropriate ECM and soluble factors and by growing cell cultures to high cell densities. This can be achieved by (a) growing cells in a relatively large reservoir with adequate medium fitted with a filter where the cells are crowded; (b) growing the cells at high concentrations on agar or agarose or as stirred aggregates (spheroids); and (c) growing cells on the outer surface of hollow fibers where the cells are seeded on the outer surface and medium is pumped through the fibers from a reservoir.
Organotypic culture
To simulate heterotypic cell interactions in addition to homotypic cell interactions, cells of differentiated lineages are re-combined. Co-culturing of epithelial and fibroblast cell clones from the mammary gland allows the cells to differentiate functionality under the correct hormonal environment, thus producing milk proteins.
Types of cell culture
Primary cell culture.
These cells are obtained directly from tissues and organs by mechanical or chemical disintegration or by enzymatic digestion. These cells are induced to grow in suitable glass or plastic containers with complex media. These cultures usually have a low growth rate and are heterogeneous; however, they are still preferred over cell lines as these are more representative of the cell types in the tissues from which they are derived. The morphological structure of cells in culture is of various types: (1) epithelium type, which are polygonal in shape and appear flattened as they are attached to a substrate and form a continuous thin layer (i.e., monolayer on solid surfaces); (2) epitheloid type, which have a round outline and do not form sheets like epithelial cells and do not attach to the substrate; (3) fibroblast type, which are angular in shape and elongated and form an open network of cells rather than tightly packed cells, are bipolar or multipolar, and attach to the substrate; and (4) connective tissue type, which are derived from fibrous tissue, cartilage, and bone, and are characterized by a large amount of fibrous and amorphous extracellular materials.
Advantages and disadvantages of primary cell culture
These cultures represent the best experimental models for in vivo studies. They share the same karyotype as the parent and express characteristics that are not seen in cultured cells. However, they are difficult to obtain and have limited lifespans. Potential contamination by viruses and bacteria is also a major disadvantage.
Depending on the kind of cells in culture, the primary cell culture can also be divided into two types.
Anchorage-dependent/adherent cells
These cells require a stable nontoxic and biologically inert surface for attachment and growth and are difficult to grow as cell suspensions. Mouse fibroblast STO cells are anchorage cells.
Anchorage-independent/suspension cells
These cells do not require a solid surface for attachment or growth. Cells can be grown continuously in liquid media. The source of cells is the governing factor for suspension cells. Blood cells are vascular in nature and are suspended in plasma and these cells can be very easily established in suspension cultures.
Secondary cell culture
When primary cell cultures are passaged or subcultured and grown for a long period of time in fresh medium, they form secondary cultures and are long-lasting (unlike cells of primary cell cultures) due to the availability of fresh nutrients at regular intervals. The passaging or subculturing is carried out by enzymatic digestion of adherent cells. This is followed by washing and re-suspending of the required amount of cells in appropriate volumes of growth media. Secondary cell cultures are preferred as these are easy to grow and are readily available; they have been useful in virological, immunological, and toxicological research.
Advantages and disadvantages of secondary cell culture
This type of culture is useful for obtaining a large population of similar cells and can be transformed to grow indefinitely. These cell cultures maintain their cellular characteristics. The major disadvantage of this system is that the cells have a tendency to differentiate over a period of time in culture and generate aberrant cells.
The primary culture, when subcultured, becomes a cell line or cell strain that can be finite or continuous, depending on its lifespan in culture. They are grouped into two types on the basis of the lifespan of the culture.
Finite cell lines
Cell lines with a limited number of cell generations and growth are called finite cell lines. The cells are slow growing (24–96 hours). These cells are characterized by anchorage dependence and density limitation.
Indefinite cell lines
Cell lines obtained from in vitro transformed cell lines or cancerous cells are indefinite cell lines and can be grown in monolayer or suspension form. These cells divide rapidly with a generation time of 12–14 hours and have a potential to be subcultured indefinitely. The cell lines may exhibit aneuploidy ( Bhat, 2011 ) or heteroploidy due to an altered chromosome number. Immortalized cell lines are transformed cells with altered growth properties. HeLa cells are an example of an immortal cell line. These are human epithelial cells obtained from fatal cervical carcinoma transformed by human papilloma virus 18 (HPV18). Indefinite cell lines are easy to manipulate and maintain. However, these cell lines have a tendency to change over a period of time.
Commonly used cell lines
Nowadays, for the production of biologically active substances on an industrial scale, a mammalian cell culture is a prerequisite. With advancements in animal cell culture technology, a number of cell lines have evolved and are used for vaccine production, therapeutic proteins, pharmaceutical agents, and anticancerous agents. For the production of cell lines, human, animal, or insect cells may be used. Cell lines that are able to grow in suspension are preferred as they have a faster growth rate. Chinese hamster ovary (CHO) is the most commonly used mammalian cell line.
When selecting a cell line, a number of general parameters must be considered, such as growth characteristics, population doubling time, saturation density, plating efficiency, growth fraction, and the ability to grow in suspension. Table 14.2 shows some of the commonly used cell lines.
Commonly used cell lines and their origins.
Advantages of continuous cell lines
- 1. Continuous cell lines show faster cell growth and achieve higher cell densities in culture.
- 2. Serum-free and protein-free media for widely used cell lines may be available in the market.
- 3. The cell lines have a potential to be cultured in suspension in large-scale bioreactors.
The major disadvantages of these cultures are chromosomal instability, phenotypic variation in relation to the donor tissue, and a change in specific and characteristic tissue markers ( Freshney, 1994 ).
Growth cycle
The cells in the culture show a characteristic growth pattern, lag phase, exponential or log phase, followed by a plateau phase. The population doubling time of the cells can be calculated during the log phase and plateau phase. This is critical and can be used to quantify the response of the cells to different culture conditions for changes in nutrient concentration and effects of hormonal or toxic components. The population doubling time describes the cell division rates within the culture and is influenced by nongrowing and dying cells.
Phases of the growth cycle
The population doubling time, lag time, and saturation density of a particular cell line can be established and characterized for a particular cell type. A growth curve consists of a normal culture and can be divided into a lag phase, log phase, and plateau phase.
This is the initial growth phase of the subculture and re-seeding during which the cell population takes time to recover. The cell number remains relatively constant prior to rapid growth. During this phase, the cell replaces elements of the glycocalyx lost during trypsinization, attaches to the substrate, and spreads out. During the spreading process, the cytoskeleton reappears; its reappearance is probably an integral part of the process.
This is a period of exponential increase in cell number and growth of the cell population due to continuous division. The length of the log phase depends on the initial seeding density, the growth rate of the cells, and the density at which cell proliferation is inhibited by density. This phase represents the most reproducible form of the culture as the growth fraction and viability is high (usually 90%–100%), and the population is at its most uniform. However, the cell culture may not be synchronized, and the cells can be randomly distributed in the cell cycle.
Plateau phase
The culture becomes confluent at the end of the log phase as growth rates during this phase are reduced, and cell proliferation can cease in some cases due to exhaustion. The cells are in contact with surrounding cells, and the growth surface is occupied. At this stage, the culture enters the stationary phase and the growth fraction falls to between 0% and 10%. Also, the constitution and charge of the cell surface may be changed, and there may be a relative increase in the synthesis of specialized versus structural proteins.
Monitoring cell growth
The animal cell culture can be grown for a wide variety of cell-based assays to investigate morphology, protein expression, cell growth, differentiation, apoptosis, and toxicity in different environments. Product yields can be increased if monitoring of cell growth is managed properly. A number of factors affect the maximum growth of cells in a batch reactor. Regular observation of cells in culture helps monitor cell health and the stage of growth; small changes in pH, temperature, humidity, O 2 , CO 2 , dissolved nutrients, etc., could have an impact on cell growth. Monitoring the rate of growth continuously also provides a record that the cells have reached their maximum density within a given time frame.
Characteristics of cell cultures
Animal cell cultures show specific characteristics and differ from microbial cultures. The important characteristics of the animal cell are slow growth rate, requirement of solid substrata for anchorage-dependent cells, lack of a cell wall (which leads to fragility), and sensitivity to physiochemical conditions such as pH, CO 2 levels, etc. Some of the fundamental bioprocess variables are as follows:
Temperature
Temperature is one of the most fundamental variables as it directly interferes with the growth and production processes. On a small scale, thermostatically controlled incubators can be used to control temperature. However, cell cultures grown on a large scale in bioreactors require more sensitive control of temperature. Different bioreactors use different methods to maintain the temperature of the cell culture. Temperature in a bioreactor is maintained by a heat blanket and water jacket with a temperature sensor.
pH of the culture medium can be controlled by adding alkali (NaOH, KOH) or acid (HCl) solution. Addition of CO 2 gas to the bioreactor, buffering with sodium bicarbonate, or use of naturally buffering solutes help maintain the pH of the culture. A silver chloride electrochemical-type pH electrode is the most commonly used electrode in the bioreactor.
Dissolved oxygen is the most fundamental variable that needs to be continuously supplied to the cell culture medium. It is consumed with a carbon source in aerobic cultures ( Moore et al., 1995 ). Diffusion through a liquid surface or membranes is one of the methods for providing dissolved oxygen to the medium.
Cell viability
The number of viable cells in the culture provides an accurate indication of the health of the cell culture ( Stacey and Davis, 2007 ). Trypan blue and erythrosin B determine cell viability through the loss of cellular membrane integrity. Both these dyes are unable to penetrate the cell membrane when the membrane is intact, but are taken up and retained by dead cells (which lack an intact membrane). Erythrosin B stain is preferred over Trypan blue as it generates more accurate results with fewer false negatives and false positives.
Cytotoxicity
The toxic chemicals in the culture medium affect the basic functions of cells. The cytotoxicity effect can lead to the death of the cells or alterations in their metabolism. Methods to access viable cell number and cell proliferation rapidly and accurately is the important requirement in many experimental situations that involve in vitro and in vivo studies. The cell number determination can be useful for determining the growth factor activity, concentration of toxic compound, drug screening, duration of exposure, change in colony size, carcinogenic effects of chemical compounds, and effects of solvents (such as ethanol, propylene, etc.).
The assays to measure viable cells (viability assays) are as follows:
- 1. [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT)/MTS/resazurin assay.
- 2. Protease marker assay.
- 3. ATP assay.
The MTT assay allows simple, accurate, and reliable counting of metabolically active cells based on the conversion of pale yellow tetrazolium MTT. Nicotinamide adenine dinucleotide in metabolically active viable cells reduces tetrazolium compounds into brightly colored formazan products or reduces resazurin into fluorescent resorufin ( Fig. 14.1 ). MTT and resazurin assays are widely used, as they are inexpensive and can be used with all cell types. The protease marker assay utilizes the cell-permeant protease substrate glycylphenylalanyl-aminofluorocoumarin (GF-AFC). The substrate, which lacks an aminoterminal blocking moiety, is processed by aminopeptidases within the cytoplasm to release AFC. The amount of AFC released is proportional to the viable cell number. This assay has better sensitivity than resuzurin and the cells remain viable; thus, multiplexing is possible. The ATP assay is the most sensitive cell viability assay. It is measured using the beetle luciferase reaction to generate light. The MTT assay and procedure is given in Flowchart 14.2 .

Schematic summary of biochemical events in different viability assays.

Assays to detect dead cells are as follows:
- 1. Lactate dehydrogenase (LDH) release.
- 2. Protease release.
- 3. DNA staining.
The viable cells in culture have intact outer membranes. Loss of membrane integrity defines a “dead” cell. The dead cells can be detected by measuring the activity of marker enzymes that leak out of dead cells into the culture medium or by staining the cytoplasmic or nuclear content by vital dyes that can only enter dead cells. LDH is an enzyme that is present in all cell types. It catalyzes the oxidation of lactate to pyruvate in the presence of co-enzyme NAD + . In the damaged cells, LDH is rapidly released. The amount of released LDH is used to assess cell death ( Fig. 14.2 ). This assay is widely used but has limited sensitivity as half-life of LDH at 37 °C is 9 hours.

Principle of the LDH release assay.
The protease release assay is based on the intracellular release of proteases from the dead/compromised cell into the culture medium. The released proteases cleave the substrate to liberate aminoluciferin, which serves as a substrate for luciferase ( Fig. 14.3 ) and leads to the production of a “glowtype” signal ( Cho et al., 2008 ).

Principle of the luminescent protease release assay.
Hayflick’s phenomenon
Hayflick limit or Hayflick’s phenomena is defined as the number of times a normal cell population divides before entering the senescence phase. Macfarlane Burnet coined the term “c limit” in 1974. Hayflick and Moorhead (1961) demonstrated that a population of normal human fetal cells divide in culture between 40 and 60 times before stopping. There appears to be a correlation between the maximum number of passages and aging. This phenomenon is related to telomere length. Repeated mitosis leads to shortening of the telomeres on the DNA of the cell. Telomere shortening in humans eventually makes cell division impossible, and correlates with aging. This explains the decrease in passaging of cells harvested from older individuals.
Culture media
One of the most important factors in animal cell culture is the medium composition. In vitro growth and maintenance of animal cells require appropriate nutritional, hormonal, and stromal factors that resemble their milieu in vivo as closely as possible. Important environmental factors are the medium in which the cells are surrounded, the substratum upon which the cells grow, temperature, oxygen and carbon dioxide concentration, pH, and osmolality. In addition, the cell requires chemical substances that cannot be synthesized by the cells themselves. Any successful medium is composed of isotonic, low-molecular-weight compounds known as basal medium and provides inorganic salts, an energy source, amino acids, and various supplements.
Basic components in culture media
The 10 basic components that make up most of the animal cell culture media are as follows: inorganic salts (Ca 2+ , Mg 2+ , Na + , K + ), nitrogen source (amino acids), energy sources (glucose, fructose), vitamins, fat and fat soluble component (fatty acids, cholesterols), nucleic acid precursors, growth factors and hormones, antibiotics, pH and buffering systems, and oxygen and carbon dioxide concentrations.
Complete formulation of media that supports growth and maintenance of a mammalian cell culture is very complex. For this reason, the first culture medium used for cell culture was based on biological fluids such as plasma, lymph serum, and embryonic extracts. The nutritional requirements of cells can vary at different stages of the culture cycle. Different cell types have highly specific requirements, and the most suitable medium for each cell type must be determined experimentally. Media may be classified into two categories: (1) natural media and (2) artificial media.
Natural media
Natural media consist of naturally occurring biological fluids sufficient for the growth and proliferation of animals cells and tissues. This media useful for promoting cell growth are of the following three types:
- 1. Coagulant or clots: Plasma separated from heparinized blood from chickens or other animals is commercially available in the form of liquid plasma.
- 2. Biological fluids: This includes body fluids such as plasma, serum lymph, amniotic fluid, pleural fluid, insect hemolymph, and fetal calf serum. These fluids are used as cell culture media after testing for toxicity and sterility.
- 3. Tissue extract: Extracts of liver, spleen, bone marrow, and leucocytes are used as cell culture media. Chicken embryo extract is the most common tissue extract used in some culture media.
Artificial media
The media contains partly or fully defined components that are prepared artificially by adding several nutrients (organic and inorganic). It contains a balanced salt solution with specific pH and osmotic pressure designed for immediate survival of cells. Artificial media supplemented with serum or with suitable formulations of organic compounds supports prolonged survival of the cell culture.
The artificial media may be grouped into the following four classes: serum-containing media, serum-free media, chemically defined media, and protein-free media.
The clear yellowish fluid obtained after fibrin and cells are removed from blood is known as serum. It is an undefined media supplement of extremely complex mixture of small and large molecules and contains amino acids, growth factors, vitamins, proteins, hormones, lipids, and minerals, among other components ( Table 14.3 ).
Serum components, their composition, and role in animal cell culture.
Advantages of serum in cell culture medium
- 1. It has basic nutrients present either in soluble or in protein-bound form.
- 2. It provides several hormones such as insulin and transferrin. Insulin is essential for the growth of nearly all cells in culture and transferrin acts as an iron binder.
- 3. It contains numerous growth factors such as platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-B), epidermal growth factor (EGF), and chondronectin. These factors stimulate cell growth and support specialized functions of cells.
- 4. It supplies protein, which helps in the attachment of cells to the culture surface (e.g., fibronectin).
- 5. It provides binding proteins such as albumin and transferrin, which helps transport molecules in cells.
- 6. It provides minerals such as Ca 2+ , Mg 2+ , Fe 2+ , K + Na + , Zn 2+ , etc., which promote cell attachment.
- 7. It increases the viscosity of the medium, which provides protection against mechanical damage during agitation and aeration of suspension cultures.
- 8. It provides appropriate osmotic pressure.
Disadvantages of serum-containing medium
- 1. Expensive: Fetal calf serum is expensive and difficult to obtain in large quantities.
- 2. Variation: Batch-to-batch variation occurs in serum, and there is no uniformity in composition of serum. This can affect growth and yields and can give inconsistent results.
- 3. Contamination: Serum medium carries a high risk of contamination with virus, fungi, and mycoplasma.
- 4. Cytotoxic and inhibiting factors: The serum itself may be cytotoxic and may contain inhibiting factors, which in turn may inhibit cultured cell growth and proliferation. The enzyme polyamine oxidase in serum reacts with polyamines such as spermine and spermidine to form cytotoxic polyamino-aldehyde.
- 5. Downstream processing: The presence of serum in culture media may interfere with isolation and purification of culture products. Additional steps may be required to isolate cell culture products.
Serum-free media
The use of serum in culture media presents a safety hazard and source of unwanted contamination for the production of biopharmaceuticals. As a number of cell lines can be grown in serum-free media supplemented with certain components of bovine fetal serum, the development of this type of medium with a defined composition has intensified in the last few decades. Eagle (1959) developed a “minimal essential medium” composed of balanced salts, glucose, amino acids, and vitamins. In the last 50 years, considerable work has been carried out to develop more efficient culture media to meet the specific requirements of specific cell lines.
Advantages of serum-free culture media
- 1. Serum-free media are simplified, and the composition is better defined.
- 2. They can be designed specifically for a cell type. It is possible to create different media and to switch from growth-enhancing media to differentiation-inducing media by altering the combination and types of growth factors and inducers.
- 3. They decrease variability from batch to batch and improve reproduction between cultures.
- 4. Downstream processing of products from cell cultures in serum-free media is easier.
- 5. They reduce the risk of microbial contamination (mycoplasma, viruses, and prions).
- 6. Serum-free media are easily available and ready to use. They are also cost-effective when compared with serum-containing media.
Disadvantages of serum-free media
- 1. Growth rate and saturation density attained are lower than those compared to serum-containing media.
- 2. Serum-free media prove to be more expensive as supplementing with hormone and growth factors increases the cost enormously.
- 3. Different media are required for different cell types as each species has its own characteristic requirements.
- 4. Critical control of pH and temperature and ultra-purity of reagent and water are required as compared to serum-containing media.
Chemically defined media
These media contain pure inorganic and organic constituents along with protein additions like EGFs, insulin, vitamins, amino acids, fatty acids, and cholesterol.
Protein-free media
These media contain nonprotein constituents necessary for the cell culture. The formulations of DME, MEM, RPMI-1640, ProCHO TM, and CDM-HD are examples of protein-free media. They promote superior cell growth and facilitate downstream purification of expressed products.
Characterization of cell lines
The characterization of cell lines is important to ensure the quality of cell-derived biopharmaceutical products. It helps in determining the cell source with regard to its identity and the presence of other cell lines, molecular contaminants, and endogenous agents. The characterization of mammalian cell lines is species-specific and can vary depending on the history of the cell line and type of media components used for culturing.
Mammalian cell line characterization can be done in four ways:
- 1. Identity testing.
- 2. Purity testing.
- 3. Stability testing.
- 4. Virological safety testing.
Identity testing
Identity testing can be carried out by isoenzyme analysis. The banding pattern of the intracellular enzyme (which is species-specific) can be determined by using agarose gels. DNA fingerprinting and karyotyping, and DNA and RNA sequencing are alternative methods to identity testing.
Karyotyping
Karyotyping is important as it determines any gross chromosomal changes in the cell line. The growth conditions and subculturing of a cell line may lead to alteration in the karyotype; for example, HeLa cells were the first human epithelial cancer cell line established in long-term culture, and they have a hyper-triploid chromosome number (3n1).
Purity testing
Bacterial and fungal contamination of cell lines occurs due to impure techniques and source material. The occurrence of contaminants can be tested by a direct inoculation method on two different media. Mycoplasma infection is the contamination of cell cultures/cell lines with mycoplasmas, and it represents a serious problem. Detection by microscopy is not adequate and requires additional testing by fluorescent staining PCR, ELISA assay, autoradiography, immune-staining, or microbiological assay.
Stability testing
Characterization and testing of cell substrate (cell line derived from human or animal source) is one of the most important components in the control of biological products. It helps to confirm the identity, purity, and suitability of the cell substrate for manufacturing use. The substrate stability should be examined at a minimum of two time points during cultivation for production. In addition, genetic stability can be tested by genomic or transcript sequencing, restriction map analysis, and copy number determination (FDA guidelines, 2012).
Viral testing assays
Virus testing of cell substrate should be designed to detect a spectrum of viruses. Appropriate screening tests should be carried out based on the cultivation history of cell lines. The development of characteristic cytopathogenic effect (CPE) provides an early indication of viral contamination. Some of the viruses of special concern in cell production work are human immunodeficiency virus, human papilloma virus, hepatitis virus, human herpes virus, hantavirus, simian virus, sendai virus, and bovine viral diarrhea virus. For detection of viruses causing immunodeficiency diseases and hepatitis, detection of sequences by PCR testing is adequate. Cells exposed to be serum or bovine serum albumin require a bovine virus test. Some of the viral testing assays are XC plaque assays, S+L-focus assay, reverse transcription assay. XC plaque assay is utilized to detect infectious ecotropic murine retroviruses. S+L-focus assay is used to test cells for the presence of infectious xenotropic and amphotropic murine retroviruses that are capable of interacting with both murine and nonmurine cells. Real-time (RT) assays such as real-time fluorescent product-enhanced reverse transcriptase (FPERT) assay and quantitative real-time for fluorescent product-enhanced reverse transcript (QPERT) assay detect the conversion of an RNA template to cDNA due to the presence of the RT template when retrovirus infection is present in the cell line.
Advantages of animal cell culture
- 1. Physiochemical and physiological condition: Role and effect of pH, temperature, O 2 /CO 2 concentration, and osmotic pressure of the culture media can be altered to study their effects on the cell culture ( Freshney, 2010 ).
- 2. Metabolism of cell: To study cell metabolism and investigate the physiology and biochemistry of cells.
- 3. Cytotoxic assay: Effect of various compounds or drugs on specific cell types such as liver cells can be studied.
- 4. Homogenous cultures: These cultures help study the biology and origin of the cells.
- 5. Valuable biological data from large-scale cell cultures: Specific proteins can be synthesized in large quantities from genetically modified cells in large-scale cultures.
- 6. Consistency of results: Reproducibility of the results that can be obtained by the use of a single type/clonal population.
- 7. Identification of cell type: Specific cell types can be detected by the presence of markers such as molecules or by karyotyping.
- 8. Ethics: Ethical, moral, and legal questions for utilizing animals in experiments can be avoided.
Disadvantages of animal cell culture
- 1. Expenditure and expertise: This is a specialized technique that requires aseptic conditions, trained personnel, and costly equipment.
- 2. Dedifferentiation: Cell characteristics can change after a period of continuous growth of cells in cultures, leading to differentiated properties compared to the original strain.
- 3. Low amount of product: The miniscule amount of mAB and recombinant protein produced followed by downstream processing for extracting pure products increases expenses tremendously.
- 4. Contamination: Mycoplasma and viral infection are difficult to detect and are highly contagious.
- 5. Instability: Aneuploidy chromosomal constitution in continuous cell lines leads to instability.
In addition, this system cannot replace the complex live animal for testing the response of chemicals or the impact of vaccines or toxins.
Ethical issues
Despite considerable progress in the development of cell culture techniques, the potential biohazards of working with animal and human tissues presents a number of ethical problems, including issues of procurement, handling, and ultimate use of material. In most countries, biomedical research is strictly regulated. Legislation varies considerably in different countries. Research ethics committees, animal ethics committees for animal-based research, and institutional research boards for human subjects have a major role in research governance.
Some guidelines for the use of experimental or donor animals include assurances of proper conditions for housing animals and minimal pain or discomfort to any animal that is put to death or operated upon. These guidelines apply to higher vertebrates and not to lower vertebrates such as fish or other invertebrates.
Use of fetal bovine serum in animal culture of media
Fetal bovine serum (FBS)-supplemented media are commonly used in animal cell cultures. In recent years, FBS production methods have come under scrutiny because of animal welfare concerns. FBS is harvested from bovine fetuses taken from pregnant cows during slaughter. The common method of harvesting the fetus is by cardiac puncture without any anesthesia. This practice of harvesting FBS is inhumane as it exposes the fetus to pain and/or discomfort. In addition to moral concerns, numerous scientific and technical problems exist with regard to the use of FBS in cell culture. Efforts are now being made to reduce the use of FBS and replace it with synthetic alternatives.
In the case of human tissues, some considerations that need to be addressed are as follows ( Freshney, 2011 ):
- 1. Consent: Patient’s and/or relative’s approval of tissue use.
- 2. Project summary: Explanation of the project, including the purpose, outcome, and medical benefits of the research.
- 3. Permission requests: Paperwork regarding possible use of the tissues.
- 4. Ownership: Establishment of ownership with regard to cell lines and their derivatives.
- 5. Patent issues: Commercial use of the tissues.
Translational significance
In biomedical research, the use of animal and human cell cultures has become beneficial for diverse applications. It provides indispensable tools for producing a number of products, including biopharmaceuticals, mABs, and products for gene therapy. In addition, animal cell cultures provide adequate test systems for studying biochemical pathways, intra- and intercellular responses, pathological mechanisms, and virus production. Some of the applications of animal cell culture are discussed below.
Antiviral vaccines
Animal cell culture technology has played an important role in the development of viral vaccine production. The establishment of cell culture technology in the 1950s and the consequent replacement of live animals for the development of antigens have led to considerable progress in bioprocess technology. With the advent of DNA technology, molecular manipulation of viruses has led to the development of a recombinant vaccine against hepatitis B virus (HBV) and several others potential vaccines that are in the final phase of clinical trials. Table 14.4 lists recombinant hepatitis B vaccines in eukaryotic cells.
Recombinant hepatitis B vaccines in eukaryotic cells.
Viral particles production by cell culture
Viral particle production by cell culture differs from the production of molecules such as proteins, enzymes, and toxins by bacteria or animal cells. The product formation may not be related to the development or growth of a cell and may occur through secondary metabolic pathways, unlike virus production, which does not result from secondary metabolic pathway. Virus production occurs after the viral infection directs cell machinery to perform viral particle production.
Two stages are involved in viral production:
- 1. Cell culture system: This requires the development of an efficient system for conversion of the culture medium substrate in the cell mass.
Cell lines used for viral vaccine production.
Production of virus-like particles
Most of the existing classical vaccines for viral disease are either altered or chemically inactive live viruses. However, incomplete inactivation of a virus or reversion of an attenuated strain can risk infection in vaccinated individuals. Viruses with segmented genomes with a high degree of genetic exchange can undergo re-assortment or recombination of genetic material with viruses of different serotypes in the vaccinated host, which can result in the production of new variants of the virus. Moreover, some live virus vaccines are teratogenic; for example, Smithburn neurotropic strain (SNS) ( Smithburn, 1949 ) and MP12-attenuated ( Caplen et al., 1985 ) vaccine strains of the Rift Valley fever virus. A new type of vaccine that does not present the typical side effects of an attenuated or inactivated viral vaccine has been made possible with the development of rDNA technology. Virus-like particles (VLPs) are highly effective as they mimic the overall structure of the virus; however, these particles lack the infectious genetic material. Capsid proteins can aggregate to form core-like particles in the absence of nucleic acids. These spontaneously assembled particles are structurally similar to authenticate viruses and are able to stimulate B-cell-mediated immune responses. In addition, VLPs stimulate a CD4-proliferative response and cytotoxic T-lymphocyte response ( Jeoung et al., 2011 ).
VLPs resemble and mimic virus structure and are able to elicit a strong immune response without causing harm. The major advantage of VLPs is their simplicity and nonpathogenic nature. They are replication-deficient as they lack any viral genetic information, thus eliminating the need for inactivation of the virus. This is important as inactivation treatments lead to epitope modifications ( Cruz et al., 2002 ). As the structural morphology of VLPs is similar to the virus, the conformational epitopes presented to the immune system are the same as for the native virus particles. The immune response/antibody reactivity in the case of VLPs is significantly improved as VLPs present conformation epitopes more similar to the native virus. VLPs also induce a strong B-cell response. For broader and more efficient protection, it is possible to adapt one or more antigens to the multimeric protein structure. Another advantage offered by VLPs is that they significantly reduce vaccine costs as these can elicit a protective response at lower doses of antigen.
Vaccines based on virus-like particles
The FDA has approved VLP-based vaccines for HBV and HPV. The HBV vaccine was approved in 1986 and the HPV one in 2006 ( Justin et al., 2011 ). To generate immunogenic VLPs, the S gene is cloned and expressed in a eukaryotic expression host such as yeast or mammalian cells (e.g., CHO cell line). The mammalian cell culture allows easy recovery because the cells are able to secrete the antigen HBsAg. The two companies producing CHO-based vaccines are the French-based Pasteur-Merieux Aventis (Gene Hevac B) and the Israeli-based SciGen (Sci-B-Vac). The Gene Hevac B vaccine contains the HBsAg S protein and M protein, whereas Sci-B-Vac contains the M and L proteins.
Human papilloma virus vaccine
Viruses of the Papillomaviridae family are known to induce lesions and warts and also cause cervical cancer. Fifteen strains of Papillomaviridae are known to cause cervical cancer. HPV-16 is considered a high-risk HPV type as the risk of cancer may be higher than for other high-risk HPV types. The two virally encoded proteins of HPV are L1 and L2. L1 is the main capsid protein that forms the outer shell of the virus. L2 is found in the interior of the viral particle and is less abundant. The recombinant L1 VLP is able to induce neutralizing antibodies in animals. Gardasil (the first HPV vaccine) was approved by the FDA in 2006. This vaccine is manufactured by Merck and Co., Inc. Ceravarix, another HPV vaccine (manufactured by Glaxo Smithkline), was approved by the FDA in 2009. It uses the Trichoplusia ni (Hi-5) insect cell line infected with L1 recombinant baculovirus ( Jiang et al., 1998 , Wang et al., 2000 ).
A number other VLP-based vaccines are in clinical trials. These include the anti-influenza A M2-HBcAg VLP vaccine ( Clarke et al., 1987 ), two antimalarial vaccine nicotine-Qβ VLPs ( Maurer et al., 2005 ), and an anti-AngIIQβ VLP. The VLP production in mammalian cell lines and Baculo cell lines of viruses infecting humans and other animals is summarized in Table 14.6 .
VLP production in mammalian and baculo cell lines of viruses infecting humans and other animals.
AAV, adeno-associated virus; rBVs , recombinant baculoviruses; ZIKV , Zika virus.
Recombinant therapeutic proteins
Proteins play a major role in carrying out biochemical reactions, transporting small molecules within a cell or from one organ to another, formation of receptors and channels in membranes, and providing frameworks for scaffolding. The number of functionally distinct proteins in humans far exceeds the number of genes as a result of post-translational modifications. These modifications include glycosylation, phosphorylation, ubiquitination, nitrosylation, methylation, acetylation, and lipidation. The changes in protein structure as a result of mutation or other abnormalities often lead to a disease condition. Protein therapeutics offer tremendous opportunities for alleviating disease. The first therapeutic from recombinant mammalian cells was human tissue plasminogen, which obtained market approval in 1986. At present, 60%–70% of all the recombinant therapeutic proteins are produced in mammalian cells.
Main therapeutic proteins
The main therapeutic proteins can be divided into seven groups ( Walsh, 2003 ):
- 1. Cytokines
- 2. Hematopoietic growth factors
- 3. Growth factors
- 4. Hormones
- 5. Blood products
- 7. Antibodies
Most of the proteins have complex structures and undergo chemical modification to insure full biological activity. Protein post-translation modifications (PTM) can happen in several ways. The most widely recognized form of PTM is glycosylation, which involves extensive sequence processing and trimming in the Golgi apparatus and endoplasmic reticulum. Eukaryotic cells are capable of carrying out this type of modification and are thus preferred in biopharmaceutical processes. Hamster, baby hamster kidney (BHK), and CHO cells are often the host cells of choice as glycosylation patterns generated from these cells are more similar to human patterns. Table 14.7 lists various therapeutic proteins produced in animal cell lines.
Various therapeutic proteins produced in animal cell lines.
Cytokines are proteins of the immune system that play a central role in immune response. Cytokines are produced as a result of immune stimulus by various white blood cells. Interferons (IFNs) were the first family of cytokines to be discovered and used as biopharmaceuticals.
Applications of interferons
IFNα is used for treatment of hepatitis, and more recently has been approved for leukemia and other types of cancers. IFNβ is used for treatment of multiple sclerosis and is marketed under the names Avonex, Belaseron, and Rebif. IFNγ is used for the treatment of chronic granulomatous disease. Interleukin is another kind of cytokine that helps regulate cell growth, differentiation, and motility and is used as a biopharmaceutical. The recombinant form of IL-2 is used for the treatment of renal cell carcinoma.
Growth factors
Growth factors are proteins that bind to receptors on the surface of cells to activate the cells for proliferation and or differentiation. The different types of growth factors are TGF, insulin-like growth factor, and (EGF. The primary sources of PDGF are platelets, endothelial cells, and the placenta. Two isoforms of this protein are present in the human body and both of these have one glycosylation site and three disulfide bonds. Examples of growth factors used as biopharmaceuticals are the following:
- 1. Osigraft/Eptotermin alfa (bone morphogenetic protein) is used for the treatment of tibia fractures, is grown commercially in CHO cells, and was first approved in 2001 in Europe.
- 2. InductOS/Dibotermin (bone morphogenetic protein) is used for tibia fractures and in spinal surgery; it is also commercially grown in CHO cells. This product was first approved in Europe in 2002.
Insulin, glucagon, gonadotropins, and growth hormones are the most well-known therapeutic hormones. The first biopharmaceuticals that obtained approval by regulatory agencies were insulin and recombinant human growth hormones. These were produced in microbial cells. The commercial recombinant forms of the gonadotropin family of hormones are Gonal-F, Luveris, Puregon, and Ovitrelle. All these are produced using CHO cells and are used for treating female infertility.
Therapeutic enzymes
A number of recombinant therapeutic enzymes are expressed in mammalian cells. Tissue plasminogen activator (tPA) is a thrombolytic agent involved in dissolving blood clots. Recombinant tPA is commercially is known as Alteplase and Tenectplase, which are used for the treatment of acute myocardial infraction.
Fabry disease, a genetic metabolic disorder, is characterized by a lack of enzyme α-galactosidase A. Fabrazyme (approved in 2001) is a recombinant α-galactosidase A and is produced by genetically modified CHO cells.
Blood coagulation factors
Hemophilia A is caused by the lack of blood-clotting factor VIII, hemophilia B is caused by deficiency of factor IX, and hemophilia C by lack of factor XI. Factor VIII and IX are proteins. The first recombinant factor VII products were Recombinate and Kogenate, which were expressed in CHO and BKH cells, respectively. Recombinant factor FIX is commercially sold as BeneFIX and is produced in recombinant CHO cells.
Therapeutic antibodies are used in the treatment of cancer, cardiovascular disease, infections, and autoimmune diseases. In 2004, the antibody Avasin (Bevacizeimab) was approved for the treatment of metastatic colorectal cancer. This antibody acts as an inhibitor of vascular endothelial growth factor. Zenapax, another commercially available antibody, is used during prophylaxis for preventing the rejection of transplanted organs. This is commercially grown in the NSO cell line and was approved for human use in 1997.
Gene therapy
Importance of cell culture in gene therapy.
Gene therapy involves the insertion, removal, or alteration of a therapeutic or working gene copy to cure a disease or defect or to slow the progression of a disease, thereby improving the quality of life. The human genome map was the first major step toward a new way of addressing human health and illness. Gene therapy holds great promise, however, the task of transferring genetic material into the cell remains an enormous technical challenge and requires ex vivo cell cultivation and adaptation from the lab to a clinically relevant state. The development of animal cell culture technology is imperative for advances in gene therapy.
Monogenic diseases caused by single gene defects (such as cystic fibrosis, hemophilia, muscular dystrophy, and sickle cell anemia) are the primary targets of human gene therapy.
The first step in gene therapy is to identify the faulty gene. This is followed by gene isolation and generation of a construct for correct expression. Integration of the gene followed by delivery of the genetic material in vivo or ex vivo is crucial to the success of gene therapy. In in vivo therapy, the genetic material is introduced directly into the individual at a specific site, and in ex vivo treatment, the target cells are treated outside the patient’s body. These cells are then expanded and transferred back to the individual at a specific site. The ex vivo technique involves gene therapy in the cultured cells, which are expanded and subsequently transferred to the targeted tissue.
Clinical correlation
A number of clinical studies and trials for gene therapy have already been approved and are being conducted worldwide. From 1989 up to the present, about 500 clinical studies have been reported; 70% of these studies are intended for cancer treatment.
The first product designed for gene therapy was Gendicine, a medication produced by Shenzhen Sibiono Genetech, China. Gendicine is used for head and neck carcinoma treatment. The tumor 4 suppressing gene p53 in recombinant adenovirus expresses protein p53, which leads to tumor control and elimination. SBN-cel is a cell line that was subcloned from the human embryonic kidney (HEK) cell line 293 and has been used for the production of Gendicine.
Biopesticides
In recent years, biopesticides have gained importance due to increased concerns about agrochemicals and their residues in the environment and food. Biopesticides provide an effective means for the control of insects and plant disease, and they are environmentally safe. The biological control of insect pests by another living organism (in order to suppress the use of pesticides) is an age-old practice. Presently, a number of biological controls are being used as biopesticides. With the high cost of chemical-based pesticides and the development of resistance to multiple chemical pesticides, baculoviruses are one of the most promising biocontrols for insect pests and have been increasingly used effectively against caterpillars worldwide. However, the major impediment in the development of baculoviruses as biopesticides is the high cost and small volumes of in vitro methods. Development of an in vitro production process for large quantities of baculoviruses at comparable costs to chemical pesticides will help provide insect control that is safe, efficacious, cost-effective, and environmentally safe.
Baculovirus production in animal cell culture
A number of factors are important for a successful commercial production of bioinsecticides:
- 1. Large-scale production of viruses at competitive costs.
- 2. Economic production of viruses (i.e., low cost for the media and running the culture).
- 3. Effective cell line with high virus per cell productivity.
- 4. With passage of the virus into cells, there is a loss of virulence and an increased risk of mutant formation; this should be avoided.
- 5. The quality of the polyhedral produced in the cell culture should be comparable to those obtained from caterpillars.
The insect baculovirus cell system offers a number of advantages. It produces recombinant proteins that are functional and are immunologically active, as it is able to make post-translational modifications. The recombinant system uses a powerful promoter polyhedron.
Cell lines for biopesticide production
The most commonly used cell lines in biopesticide production are the Sf21 and Sf9 cell lines, which are derived from ovarian tissues of the fall army worm ( Spodoptera frugiperda ). Sf9 cells show a faster growth rate and higher cell density than Sf21 cells and are preferred. High Five cell lines (designated BTI-Tn-5BI-4) established from Trichoplusia ni embryonic tissue are also being used.
Viral mutant formation in cell culture
The continuous culturing of cells for virus production leads to virus instability and the so-called passage effect. This can result in a decrease of virulence and polyhedral production and a variety of mutations. All these changes affect commercial production in vitro. Two types of mutations are commonly seen in continuous passaging of cell cultures for viral productions: (1) defective infective particles (DIPs) and (2) few polyhedral (Fp) mutations.
Fp mutations are characterized by (1) reduced polyhedral, (2) enhanced production of BV, and (3) lack of occluded virions in polyhedra. All these factors reduce the infectivity of the target pest.
Spontaneously generated Fp mutants have been reported in AcMNPV ( Autographa California nucleopolyhedroviruses) ( Wood, 1980 ), Galleria mellonella nucleopolyhedroviruses (GmMNPV) ( Fraser and Hink, 1982 ), and Helicoverpa armigera nucleopolyhedroviruses (HaSNPV) ( Chankraborty and Reid, 1999 ).
DIP mutations are the formation of DIPs. They occur due to serial passaging for long periods, which results in a decrease in the filtering of infectious virus. DIPs have been reported in a number of animal virus systems and in baculovirus systems. DIP formation can be avoided by low multiplicity of infection. This minimizes the probability of the defective virus entering the cell with an intact helper virion.
Monoclonal antibodies
The majority of antibodies available on the market today are produced in animal cell cultures ( Van Dijk and Van de Winkle, 2001 ). Animal cells are preferred because they are capable of glycosylation and structural conformation, which is essential for a drug to be productive. Hybridoma technology has been the most widely used method for small- and large-scale production of mABs. However, these antibodies have limited therapeutic applications since they produce an adverse immune response on repeated use.
A number of cell lines are now being used for the production of recombinant antibodies. The CHO lines are the most commonly used. Other cell lines used are marine myelomas NSO, Sp 2/0, HEK-93, and BHK.
A number of factors influence the production of mABs. For a high concentration of mAB production, the cell line should have high productivity. For high protein productivity, it is important that the selected cell line be productive in order to avoid large reaction volumes and the high cost of protein purification. Cell lines with the capacity to grow without anchorage offer an advantage in terms of scaling up the process; it is much simpler than with those designed for the growth culture of anchorage-dependent cells. Sp2/0 and NSO cell lines can grow naturally in suspension; other cells such as CHO and BHK can be easily adapted to this form of cultivation.
Stem cells are unspecified cells that have the potential to differentiate into other kinds of cells or tissues and become specialized cells. The two characteristics that define stem cells are their ability of self-regenerate and to differentiate into any other cells or tissues. These cells have the capability to renew themselves to form cells of more specialized function. In recent years, stem cell research has been hailed as a major breakthrough in the field of medicine. This property of turning a cell into any other specialized function cell has made researchers believe that stem cells could be utilized to make fully functional, healthy organs to replace damaged or diseased organs.
Culturing embryonic stem cells in the laboratory
Human embryonic stem cells (hESCs) are grown on nutrient broth. These cells are traditionally cultured on mouse embryonic fibroblast feeder layers, which allows continuous growth in an undifferentiated stage. The mouse cells at the bottom of the culture dish provide a sticky surface to which the cells can attach. In addition, the feeder cells release nutrients into the culture medium. Researchers have now devised animal-free culture systems for hESCs and have used human embryonic fibroblasts and adult fallopian tube epithelial cells as feeder layers (in addition to serum-free mediums).
More recently, methods to subculture embryonic cells without the feeder layer have been developed. Martigel from BD Biosciences has been used to coat the culture plate ( Hassan et al., 2012 ) for effective attachment and differentiation of both normal and transformed anchorage-dependent epithelioid and other cell types. This is a gelatinous protein mixture isolated from mouse tumor cells.
Microfluidics three-dimensional culture
A major milestone in the biological sciences was the establishment of the tissue culture technique that can both maintain and propagate the growth of living cells under sterile in vitro conditions. Traditional cell cultures, which are two-dimensional (2D), are grown as monolayer cultures on a flat and rigid surface. Since their development, several advancements have been made to improve cell culture media as well as the biological materials used for culturing. The improvements have proven valuable for cell-based study due to their amalgamation of various modern analytical techniques, such as fluorescence, electrochemistry, and mass spectroscopy. 2D cell culture does not provide an adequate in vivo environment, where other cells surround the cells in a three-dimensional (3D) ECM ( Edmondson et al., 2014 ). Cells under in vivo conditions both produce and continuously consume oxygen nutrients and other molecules, and such dynamic distributions are not mimicked in conventional 2D cell cultures. Moreover, 2D cell cultures fail to recapitulate the highly complex 3D environment, function, and physiology of living tissues, the multitudinous regulatory interactions from surrounding tissue cells, the ECM, and other systemic factors that lead to nonpredictive data of an in vivo response ( Li et al., 2012 ). The limitations of 2D cell culture systems have recently become more evident. Recent standard protocol advances in the fields of quantitative and system biology and imaging technology have allowed analysis of individual cells and observation of live individual cells growing in a natural physiological 3D environment. Cells cultured in a 3D model system more closely mimic in vivo conditions. Thus, unlike 2D cell cultures, which can sometimes cause misleading and nonpredictive data of in vivo responses, 3D systems are realistic for translating study findings. Compared to the 2D cell culture system, the 3D cell culture system provides a physiologically relevant and closer biomimetic environment, promotes better cell differentiation, and improves cell function ( Edmondson et al., 2014 ). The 3D culture system holds great promise for applications in various fields, such as cancer cell biology, stem cell research, drug discovery, and various cell-based analyses and devices. While this culturing model offers state-of-the-art technology for facilitating drug development and numerous other applications, several hurdles remain before a universal, standardized, and validated system can be established ( Sung et al., 2014 ). Recent developments in the transition from 2D to 3D cell cultures indicate promising applications for many industries; however, the cost of automation and easy-to-use readout systems are still key concerns.
The 3D cell culture system has provided a powerful tool that mimics a highly complex and dynamic in vivo environment, and it has gained greater momentum with the integration of microfluidic technology. Microfluidics is a technology characterized by the manipulation of fluids at the micron-scale for the improvement of diagnostics and cell culture research. It uses microfluidic devices to manipulate fluids in the small capillaries or microchannels. Microfluidics is a science of manipulating, mixing, monitoring, and analyzing minute volumes of fluids or gases on the surfaces of chips and microfluidic chips. This technology is ideal because it recreates the microenvironment of the vasculature and has become a powerful tool in cell culture research. It encompasses knowledge of the biological sciences, chemistry, physics, and engineering applications ( Xu and Attinger, 2008 ). The microfluidic 3D cell culture model also allows precise spatial control over the gradients and medium exchange. It not only mimics but also promotes several biologically relevant functions not seen in the 2D cell culture. Furthermore, it has been increasingly used to generate high-throughput cell culture models and has shown considerable promise for improving diagnostics and biological research ( El-Ali et al., 2006 ).
Notably, microfluidic cell cultures are potential candidates for next generation cell analysis systems. Several 3D-based cell culture approaches have been created to provide a better biomimetic microenvironment for cells than those of 2D cultures. In addition, crucial liquid handling steps, including cell loading, nutrient supply, and waste removal—under physiologically relevant conditions—can be performed with real-time microscopy ( Xu et al., 2014 ). Numerous microfluidic devices have been developed to not only provide nutrients and oxygen continuously for cell proliferation but also to investigate several characteristics of a dynamic 3D cell culture, such as differences in concentration, temperature gradients, and shear force conditions on cell transport and cultivation. Numerous microfluidic platforms for 3D cell culturing have been developed and based on the substrates used for microdevice fabrication, including glass/silicon-based, polymer-based, and paper-based platforms. Polydimethylsiloxane (PDMS)-based microdevices are the predominant form of microfluidic 3D cell culture systems because they are economical and allow permeability of O2, which is vital in cell proliferation. To provide an in vivo-like environment that resembles living tissues, several natural polymers, such as collagen, fibrin, and agarose, have been used to fabricate microfluidic devices ( Li et al., 2012 ).
Applications
Microfluidics technology has emerged as a viable and robust platform for tissue engineering—a multidisciplinary field aimed at replacing and repairing damaged and diseased tissues and/or organs and developing in vitro models to mimic physiological conditions. Successful clinical applications include the development of organ-on-a-chip technology—a microfluidic perfusion device for regenerative medicine—and a chip-based platform for the culture of cells and toxicological studies.
Organ-on-a-chip technology
Scientists currently rely on in vitro cell culture platforms and in vivo animal models to study biological processes and develop therapeutic strategies, although informative have significant shortcomings ( Ziółkowska et al., 2011 ). In vitro platforms may not simulate the intricate cell–cell and cell–matrix interactions that are vital to regulating cell behavior in vivo ( Guillouzo & Guguen-Guillouzo, 2008 ). Organ-on-a-chip devices could offer biological relevance and be a requisite for high-throughput applications. An organ-on-a-chip is a microfluidic cell culture device comprising a microchip with continuously perfused chambers that are infused with living cells that are arranged to mimic the 3D tissue microenvironment and physiology ( Ghaemmaghami et al., 2012 ). These chips have the potential to significantly impact drug discovery and toxicity testing ( Ghaemmaghami et al., 2012 ). The simplest functional unit of organ-on-a-chip devices consists of a single, perfused microfluidic chamber that is composed of a single type of cultured cell. These systems are utilized for studying organ-specific responses, chemical responses, such as drugs or toxins, and physical stimuli. In a complex system, two or more independently perfused parallel microchannels are connected by porous membranes to recreate interfaces between different tissues.
Tissue models on a chip
Numerous tissue models have been developed to mimic in vivo biological processes. On-chip tissue models include those for the liver, kidney, lungs, intestines, muscle, fat, and blood vessels as well as models of tumors.
Liver-on-a-chip
Various chemicals and drugs, when administered over a long period, result in adverse effects and acute liver toxicity, known as hepatotoxicity ( Gershell & Atkins, 2003 ). In vitro models used for identifying drug-induced liver toxicity have drastically limited utility. Therefore, efficient and reliable tools for testing liver toxicity are required. Microfluidics devices for liver tissue and cells that can maintain metabolic activity and can be used for drug discovery and toxicity studies have shown great potential for solving this problem.
Bioreactors with a perfused multiwell plate device were developed by Domansky et al. (2010) to recapitulate both the physiological and mechanical microenvironments of hepatocytes that can support both growth and functional integrity for up to 1 week. Khetani and Bhatia (2008) developed microscale cultures of human liver cells in a multiwell micropatterned co-culture system that can maintain phenotypic functions of liver cells for up to several weeks.
Tumor-on-a-chip
A significant challenge for cancer research is the early detection and development of in vitro strategies for studying the role of drug-carrier design in tumor transport and therapies for targeting rapidly dividing cancer cells while leaving normal, healthy cells untouched. The microfluidics tumor-on-a-chip platform can be used for detecting circulating tumor cells (CTCs) in blood flow, which may lead to early diagnosis of cancer ( Millner et al., 2013 ). A variety of designs for studying the microenvironment of microfluidic devices that culture solid and liquid tumors were reviewed by Young (2013) . Tatosian and Shuler (2009) developed a novel microfluidic system to study the multidrug resistance of cancer cells to chemotherapeutic combinations. Jang et al. (2011) fabricated a microfluidic device with an active injection system that produced 64 of 100 combinations of different chemical solutions at various concentrations and stored them in isolated chambers. To optimize system parameters for varied types of cancer cells while requiring minute amounts of reagents and cells, Jedrych et al. (2011) generated a microfluidics system for photodynamic therapy-based measurements. This system allows light-induced photosensitizers to be delivered to the carcinoma cells, which—on reaction with oxygen—produce a chemical toxin that is lethal to tumor cells.
World Wide Web resources
http://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm202439.pdf
http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm205541.htm
The Food and Drug Administration (FDA or USFDA) protects and promotes public health through the regulation of all foods (except meats and poultry), the nation’s blood supply, and other biologics (such as vaccines and transplant tissues). Drugs must be tested, manufactured, and labeled according to FDA standards before they can be sold or prescribed.
http://www.promega.com/~/media/files/products%20and%20services/na/webinars/mechanism%20of%20toxicitywebinar2.pdf?la=en
Promega manufactures enzymes and other products for biotechnology and molecular biology.
http://www.who.int/biologicals/publications/trs/areas/vaccines/cells/WHO_TRS_878_A1Animalcells.pdf
The World Health Organization (WHO) is a specialized agency that is concerned with international public health. It is affiliated with the United Nations and headquartered in Geneva, Switzerland. WHO ensures that more people, especially those living in dire poverty, have access to equitable, affordable care, so that they can lead healthy, happy, and productive lives.
http://amgenscholars.com/images/uploads/contentImages/biotechnology-timeline.pdf
Amgen Scholars provides hundreds of undergraduate students with the opportunity to engage in a hands-on summer research experience at some of the world’s leading institutions.
http://monographs.iarc.fr/ENG/Monographs/vol90/mono90-6.pdf
The IARC monographs identify environmental factors that can increase the risk of human cancer. These include chemicals, complex mixtures, occupational exposures, physical agents, biological agents, and lifestyle factors.
http://www.iptonline.com/articles/public/IPTFIVE76NP.pdf
IPTonline publishes “The Pharmaceutical Technology Journal,” which is designed to provide information on the latest ideas, cutting-edge technologies, and innovations shaping the future of pharmaceutical research, development, and manufacturing.
http://www.aceabio.com/UserFiles/doc/literature/xcell_appnotes/RTCA_AppNote07_ACEA_LoRes.pdf
ACEA Biosciences, Inc. (ACEA) is a privately owned biotechnology company. ACEA’s mission is to transform cell-based assays by providing innovative and cutting-edge products and solutions to the research and drug discovery community.
- Aucoin M.G., Jacob D., Chahal P.S., Meghrous J., Bernier A., Kamen A.A. Virus-like particle and viral vector production using the baculovirus expression vector system/insect cell system. Methods Mol. Biol. 2007; 388 :281–296. [ PubMed ] [ Google Scholar ]
- Bhat S.M. Animal Cell Culture Concept and Application. Alpha Science International Limited; Oxford: 2011. [ Google Scholar ]
- Caplen H., Peters B.J., Bishop D.H.L. Mutagen-directed attenuation of Rift Valley fever virus as a method for vaccine development. J. Gen. Virol. 1985; 66 :2271–2277. [ PubMed ] [ Google Scholar ]
- Chakraborty S., Reid S. Serial passage of a Helicoverpa armigera nucleopolyhedrovirus in Helicoverpa zea cell cultures. J. Invertebr. Pathol. 1999; 73 :303–308. [ PubMed ] [ Google Scholar ]
- Chen T.H., Liu W.C., Chen I.C., Liu C.C., Huang M.H., Jan J.T. Recombinant hemagglutinin produced from Chinese Hamster Ovary (CHO) stable cell clones and a PELC/CpG combination adjuvant for H7N9 subunit vaccine development. Vaccine. 2019; 37 (47):6933–6941. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cho M.H., Niles A., Huang R., Inglese J., Austin C.P., Riss T. A bioluminescent cytotoxicity assay for assessment of membrane integrity using a proteolytic biomarker. Toxicol. Vitro. 2008; 22 :1099–1106. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Clarke B.E., Newton S.E., Carroll A.R., Francis M.J., Appleyard G., Syred A.D. Improved immunogenicity of a peptide epitope after fusion to hepatitis B core protein. Nature. 1987; 330 :381–384. [ PubMed ] [ Google Scholar ]
- Cruz P.E., Maranga L., Carrondo M.J.T. Integrated process optimisation: lessons from retrovirus and virus like production. J. Biotechnol. 2002; 99 :199–214. [ PubMed ] [ Google Scholar ]
- Domansky K., Inman W., Serdy J., Dash A., Lim M.H., Griffith L.G. Perfused multiwell plate for 3D liver tissue engineering. Lab. a chip. 2010; 10 (1):51–58. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Eagle H. Amino acid metabolism in mammalian cell cultures. Science. 1959; 130 :432–437. [ PubMed ] [ Google Scholar ]
- Edmondson R., Broglie J.J., Adcock A.F., Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay. drug. Dev. Technol. 2014; 12 (4):207–218. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- El-Ali J., Sorger P.K., Jensen K.F. Cells on chips. Nature. 2006; 442 (7101):403–411. FDA guidelines. ( http://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm202439.pdf2012 . [ PubMed ] [ Google Scholar ]
- Fraser M.J., Hink W.F. The isolation and characterization of the MP and FP plaque variants of Galleria mellonella nuclear polyhedrosis virus. Virology. 1982; 117 :366–378. [ PubMed ] [ Google Scholar ]
- Freshney R.I. Culture of Animal Cells: A Manual of Basic Technique. 3rd ed. Wiley-Liss Inc; New York: 1994. [ Google Scholar ]
- Freshney R.I. Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications. Wiley, John & Sons, Inc; New Jersey: 2010. [ Google Scholar ]
- Freshney R.I. Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications. Wiley, John & Sons, Inc; New Jersey: 2011. [ Google Scholar ]
- Gershell L.J., Atkins J.H. A brief history of novel drug discovery technologies. Nat. Rev. Drug. Discovery. 2003; 2 (4):321–327. [ PubMed ] [ Google Scholar ]
- Ghaemmaghami A.M., Hancock M.J., Harrington H., Kaji H., Khademhosseini A. Biomimetic tissues on a chip for drug discovery. Drug. Discov. Today. 2012; 17 (3):173–181. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Guillouzo A., Guguen-Guillouzo C. Evolving concepts in liver tissue modeling and implications for in vitro toxicology. Expert. Opin. drug. Metab. Toxicol. 2008; 4 (10):1279–1294. [ PubMed ] [ Google Scholar ]
- Hassan F., Ren D., Zhang W., Gu X.-X. Role of c-Jun N-terminal protein kinase 1/2 (JNK1/2) in macrophage-mediated MMP-9 production in response to Moraxella catarrhalis lipooligosaccharide (LOS) PLoS ONE. 2012; 7 :e37912. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hayflick L., Moorhead P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961; 3 :585–621. [ PubMed ] [ Google Scholar ]
- Ho Y., Lin P.H., Liu C.Y., Lee S.P., Chao Y.C. Assembly of human severe acute respiratory syndrome coronavirus-like particles. Biochemical and biophysical research communications. 2004; 318 (4):833–838. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jang Y.H., Hancock M.J., Kim S.B., Selimović Š., Sim W.Y., Bae H. An integrated microfluidic device for two-dimensional combinatorial dilution. Lab. a Chip. 2011; 11 (19):3277–3286. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jedrych E., Pawlicka Z., Chudy M., Dybko A., Brzozka Z. Evaluation of photodynamic therapy (PDT) procedures using microfluidic system. Analytica Chim. acta. 2011; 683 (2):149–155. [ PubMed ] [ Google Scholar ]
- Jeoung H.Y., Lee W.H., Jeong W., Shin B.H., Choi H.W., Lee H.S. Immunogenicity and safety of the virus-like particle of the porcine encephalomyocarditis virus in pig. Virology J. 2011; 8 :170. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jiang B., Barniak V., Smith R.P., Sharma R., Corsaro B., Hu B. Synthesis of rotavirus-like particles in insect cells: comparative and quantitative analysis. Biotechnol. Bioeng. 1998; 60 :369–374. [ PubMed ] [ Google Scholar ]
- Justin C., Masum H., Perampaladas K., Heys J., Singer P.A. Indian vaccine innovation: the case of Shantha Biotechnics. Globalization Health. 2011; 7 :9. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Khetani S.R., Bhatia S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008; 26 (1):120–126. [ PubMed ] [ Google Scholar ]
- Li, X.J., Valadez, A.V., Zuo, P., & Nie, Z. (2012). Microfluidic 3D cell culture: potential application for tissue-based bioassays. [ PMC free article ] [ PubMed ]
- Licata J.M., Johnson R.F., Han Z., Harty R.N. Contribution of Ebola virus glycoprotein, nucleoprotein, and VP24 to budding of VP40 virus-like particles. Journal of virology. 2004; 78 (14):7344–7351. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Maurer P., Jennings G.T., Willers J., Rohner F., Lindman Y., Roubicek K. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and phase I safety and immunogenicity. Eur. J. Immunology. 2005; 35 :2031–2040. [ PubMed ] [ Google Scholar ]
- Mello I.M.V.G., Meneghesso da Conceicao M., Jorge S.A.C., Cruz P.E., Alves P.M.M., Carrondo M.J.T. Viral vaccines: concepts, principles, and bioprocesses. In: Castilho L.R., Moraes A.M., Augusto E.F.P., editors. Animal Cell Technology: From Biopharmaceuticals to Gene Therapy. Taylor & Francis Group; New York: 2008. pp. 435–458. [ Google Scholar ]
- Millner L.M., Linder M.W., Valdes R. Circulating tumor cells: a review of present methods and the need to identify heterogeneous phenotypes. Ann. Clin. Lab. Sci. 2013; 43 (3):295–304. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Moore A., Donahue C.J., Hooley J., Stocks D.L., Bauer K.D., Mather J.P. Apoptosis in CHO cell batch cultures: examination by flow cytometry. Cytotechnology. 1995; 17 :1–11. [ PubMed ] [ Google Scholar ]
- Mortola E., Roy P. Efficient assembly and release of SARS coronavirus-like particles by a heterologous expression system. FEBS Lett. 2004; 576 :174–178. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Parez N., Garbarg-Chenon A., Fourgeux C., Le Deist F., Servant-Delmas A., Charpilienne A. The VP6 protein of rotavirus interacts with a large fraction of human naive B cells via surface immunoglobulins. Journal of virology. 2004; 78 (22):12489–12496. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Smithburn K.C. Rift Valley fever: the neurotropic adaption of virus and experimental use of this modified virus as a vaccine. Br. J. Exp. Pathol. 1949; 30 :1–16. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stacey G.N., Davis J. Medicines from Animal Cell Culture. John Wiley & Sons; Chichester: 2007. [ Google Scholar ]
- Sung J.H., Srinivasan B., Esch M.B., McLamb W.T., Bernabini C., Shuler M.L. Using physiologically-based pharmacokinetic-guided “body-on-a-chip” systems to predict mammalian response to drug and chemical exposure. Exp. Biol. Med. 2014 1535370214529397. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Swenson D.L., Warfield K.L., Negley D.L., Schmaljohn A., Aman M.J., Bavari S. Virus-like particles exhibit potential as a pan-filovirus vaccine for both Ebola and Marburg viral infections. Vaccine. 2005; 23 (23):3033–3042. [ PubMed ] [ Google Scholar ]
- Tatosian D.A., Shuler M.L. A novel system for evaluation of drug mixtures for potential efficacy in treating multidrug resistant cancers. Biotechnol. Bioeng. 2009; 103 (1):187–198. [ PubMed ] [ Google Scholar ]
- van Dijk M.A., van de Winkel J.G.J. Human antibodies as next generation therapeutics. Curr. Opin. Chem. Biol. 2001; 5 :368–374. [ PubMed ] [ Google Scholar ]
- Walsh G. Biopharmaceuticals – Biochemistry and Biotechnology. 2nd ed. John Wiley and Sons; Chichester: 2003. [ Google Scholar ]
- Wang C., Zheng X., Gai W., Zhao Y., Wang H. MERS-CoV virus-like particles produced in insect cells induce specific humoural and cellular imminity in rhesus macaques. Oncotarget. 2017; 8 (8):12686. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang M.Y., Kuo Y.Y., Lee M.S., Doong S.R., Ho J.Y., Lee L.H. Self-assembly of the infectious bursal disease virus capsid protein, rVP2, expressed in insect cells and purification of immunogenic chimeric rVP2H particles by immobilized metal-ion affinity chromatography. Biotechnol. Bioeng. 2000; 67 :104–111. [ PubMed ] [ Google Scholar ]
- Wood H.A. Isolation and replication of an occlusion body-deficient mutant of the Autographa californica nuclear polyhedrosis virus. Virology. 1980; 105 :338–344. [ PubMed ] [ Google Scholar ]
- Xu J., Attinger D. Drop on demand in a microfluidic chip. J. Micromech. Microeng. 2008; 18 (6):065020. [ Google Scholar ]
- Xu X., Farach-Carson M.C., Jia X. Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnol. Adv. 2014; 32 (7):1256–1268. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yamshcikov G.V., Ritter G.D., Vey M., Compans R.W. Assembly of SIV virus-like particles containing envelope proteins using a baculovirus expression system. Virology. 1995; 214 (1):50–58. [ PubMed ] [ Google Scholar ]
- Young E.W. Cells, tissues, and organs on chips: challenges and opportunities for the cancer tumor microenvironment. Integr. Biol. 2013; 5 (9):1096–1109. [ PubMed ] [ Google Scholar ]
- Ziółkowska K., Kwapiszewski R., Brzózka Z. Microfluidic devices as tools for mimicking the in vivo environment. N. J. Chem. 2011; 35 (5):979–990. [ Google Scholar ]
Further reading
- Castilho L.R., Moraes A.M., Augusto E.F.P. Animal Cell Technology. Taylor & Francis Group; New York: 2008. From Biopharmaceuticals to Gene Therapy. [ Google Scholar ]
- Freshney R.I. Culture of Animal Cells: A Manual of Basic Techniques and Specialized Applications. 7 th Edition. Wiley Blackwell; USA: 2015. [ Google Scholar ]
- Kolesnikova S., Moiseeva I. Prospects for the use of animal cell cultures in screening of pharmaceutical substances. J. Phys.: Conf. Ser. 2017; 784 :012028. doi: 10.1088/1742-6596/784/1/012028. [ CrossRef ] [ Google Scholar ]
- Luo Tao. Microfluidic single-cell manipulation and analysis: methods and applications. Micromachines. 2019; 10 (2):104. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Naskalska A., Dabrowska A., Szczepanski A., Milewska A., Jasik K.P., Pyrc K. Membrane protein of human coronavirus nl63 is responsible for interaction with the adhesion receptor. J Virology. 2019; 93 (19):e00355–19. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Polat A.N., Özlü N. Towards single-cell LC-MS phosphoproteomics. Analyst. 2014; 139 :4733–4749. [ PubMed ] [ Google Scholar ]
- Stecey G.N., Davis J. Medicines from Animal Cell Culture. John Wiley & Sons; Chichester : 2007. [ Google Scholar ]
- Verma A.S., Singh A. Animal Biotechnology: Models in Discovery and Translation. 1st Edition. Acadamic Press; USA: 2014. [ Google Scholar ]
- Yu F., Hunziker W., Choudhury D. Engineering microfluidic organoid-on-a-chip platforms. Micromachines. 2019; 10 (3):165. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Abbreviations
Long answer questions.
- 1. What are the components of serum and how do they help the cell culture?
- 2. What is the role of media in animal cell culture?
- 3. What are the advantages and limitations of animal tissue culture?
- 4. How can cell viability and cytotoxicity be tested in cell culture?
- 5. What is the role of cell culture in gene therapy and viral vaccines?
- 6. How can microfluidics revolutionize animal tissue culture?
Short answer questions
- 1. What is the Hayflick effect?
- 2. What is the source of cells for primary monolayer cell culture?
- 3. Serum is one of the basic components of cell culture media (true/false)?
- 4. What was the first recombinant human protein?
- 5. What are the different phases of the growth curve?
- 6. Is the VLP-based HPV vaccine approved by the FDA?
Answers to short answer questions
- 1. Limited replication capacity of cells in culture medium.
- 2. Organ/tissue of live animal.
- 4. Somatostatin.
- 5. Lag phase, log phase, and plateau phase.
- 6. Yes, Gardasil (the first HPV vaccine) was approved by the FDA in 2006.
Yes/no type questions
- 1. Are cells obtained directly from organs and tissues in primary cell culture?
- 2. Is secondary culture used for studying transformed cells?
- 3. Is identity testing a way to determine purity of culture?
- 4. Is IFN-α used for the treatment of multiple sclerosis?
- 5. Is Bevacizumab approved for the treatment of colorectal cancer?
- 6. Does passage effect leads to an increase in the virulence of cultured viruses?
- 7. Do stem cells can not differentiate into other kinds of cells?
- 8. Microfluidic devices provide nutrients and oxygen for cell proliferation.
- 9. Living cells are used in organ-on-a-chip microfluidic cell culture.
- 10. Can embryonic cells be cultured without any feeder layer?
Answers to yes/no type questions
- 1. Yes—Mechanical, chemical, or enzymatic disintegration of tissues and organs is required in primary cell culture.
- 2. Yes—Secondary cultures are used in the study of transformed cells as these cultures maintain their cellular characteristics.
- 3. No—For testing the purity, one should use fluorescent staining PCR or ELISA.
- 4. No—IFN-β is used in the treatment of multiple sclerosis.
- 5. Yes—It is an inhibitor of vascular endothelial growth factor.
- 6. No—Passage effect leads to viral instability.
- 7. No—Stem cells can differentiate into other kind of cell types.
- 8. Yes—Microfluidic devices also help in investigating characteristics of 3D cell culture.
- 9. Yes—Chambers of organ-on-a-chip devices are continuously infused with living cells.
- 10. Yes—Martigel from BD biosciences can be used to coat the culture plate.

VIDEO
COMMENTS
Pharmaceutical biotechnology's greatest potential lies in gene therapy and stem cell-based therapy. The underlying cause of defect of many inherited diseases is now located and characterized. Gene therapy is the insertion of the functional gene in place of defective gene into cells to prevent, control, or cure disease.
Biotechnology is a technology of hope, with the potential to improve human health, raise living standards, and protect our planet. The aim of this paper is to provide a comprehensive overview of ...
Biotechnology is the use of an organism, or a component of an organism or other biological system, to make a product or process. Many forms of modern biotechnology rely on DNA technology. DNA technology is the sequencing, analysis, and cutting-and-pasting of DNA. Common forms of DNA technology include DNA sequencing, polymerase chain reaction ...
According to the United Nations Convention on Biological Diversity, biotechnology is "any technological application that uses biological systems, living organisms, or derivatives thereof, to make or modify products or processes for a specific use." The concept of "specific use" typically involves a commercial application or benefit to ...
Introduction. Biotechnology research covers a broad range of topics. As defined in the scopes of the journals Current Research in Biotechnology and Current Opinion in Biotechnology, ... (2018 journal impact factor of 2.03; publishing original full-length research papers, reviews, and Letters to the Editor) ...
INTRODUCTION TO BIOTECHNOLOGY - article which is on history of BIOTECHNOLOGY, MODERN BIOTECHNOLOGY, its scope and fields. Discover the world's research 25+ million members
The Introduction and Discussion are like bookends around your Methods and Results. They integrate your original findings with the literature and current knowledge. Context, Justification, and Focus. addressed in the Introduction are echoed in the Discussion section: Focus = Summary of findings.
The first molecular and cellular tools of modern biotechnology emerged in the 1960s and '70s. A fledgling "biotech" industry began to coalesce in the mid- to late 1970s. Modern biotechnology stands in contrast to older forms of "biotechnology," which emerged thousands of years ago, when humans began to domesticate plants and animals.
Read the latest Research articles from Nature Biotechnology. ... Research Note (8) Research Paper (510) Resource (72) Roundtable (3) Speakers (61) Technical Report (92) Technologies (11)
2000. Synthesis and amplification of DNA in a test tube by Har gobind Khorana and Kary mullis, respectively (Verma et al., 2011). Completion of rough copy of human genome by Celeria Genomics and Human Genome Project (Verma et al., 2011). Kenya's first biotech crop, a virus-resistant sweet potato, was field-tested (Colwell, 2020). Sir Ian Wilmut cloned an adult sheep and named it "Dolly ...
Medicine and medical technology are developing rapidly in the UK. Biotechnology industry revenue is expected to increase by 6.7% from 2020 to 2021 to 13.8 billion euros. After Britain's exit from the European Union, the government has a focus on the science and research industries to drive the economy.
Biotechnology is a broad discipline in which biological processes, organisms, cells or cellular components are exploited to develop new technologies. New tools and products developed by ...
6: How to Write a Research Paper I: Illustrations (PDF - 1.2 MB) 7: How to Write a Research Paper II: Results Section . 8: How to Write a Research Paper III: Methods Section . 9: How to Write a Research Paper IV: Introduction and Discussion . 10: How to Write a Research Paper V: Title and Abstract
Abstract. This article surveys the rise of biotechnology since the closing decades of the twentieth century, examining both scientific developments and social and institutional change. As an area ...
Introduction. Products from natural sources are being used from centuries [1-3].Processing the natural products to get significant benefits have been the priority in every era of science [4-7].Biotechnology is an advanced, yet developed, technology that develops or modifies a product for some applied purpose utilizing living organisms and/or substances from these.
Abstract. Biotechnology is multidisciplinary field which has major impact on our lives. The technology is known since years which involves working with cells or cell-derived molecules for various applications. It has wide range of uses and is termed "technology of hope" which impact human health, well being of other life forms and our ...
The purpose of this chapter is to provide a brief overview of the spectrum of applications of plant biotechnology that are in current use or are under development in research labs around the world. Plant biotechnology, in the sense of the application of recombinant DNA techniques to crop improvement, or the production of valuable molecules in ...
Biotechnology derives from the Greek words - bios - life, technos - technology and logos - language, proof - that is biotechnology deals with the technical usage of living organisms for various purposes such as food, medicine, pharmaceuticals, recycling. Nowadays we deal with various colours or categories namely 10 introduced (red ...
Key Issues in Biotechnology 3 INTRODUCTION Biotechnology is a collective term for a group of technologies that use biological matter or processes to generate new and useful products and processes. As such, it ranges in complexity and maturity from ancient brewing and bread-making techniques to genetic modification through
1. Introduction. There is no precise definition of artificial intelligence (AI) so far, but in general it refers to the ability of any machines which can simulate the intelligences of higher organisms. The field of AI has important roots in almost every branch of research including philosophy, mathematics, computing, psychology and biology . An ...
This paper further examines the potential impacts, constraints, and adoption of open source for agricultural biotechnology. The paper concludes with a summary of issues arising from adopting the ...
Plant biotechnology can be defined as the introduction of desirable traits into plants through genetic modification. ... Research Open Access 01 Mar 2024 Nature Communications.
Introduction. Cell culture is the process by which human, animal, or insect cells are grown in a favorable artificial environment. ... Animal cell culture is now one of the major tools used in the life sciences in areas of research that have a potential for economic value and commercialization. The development of basic culture media has enabled ...