
- Teesside University Student & Library Services
- Learning Hub Group

Critical Appraisal for Health Students
- Critical Appraisal of a quantitative paper
- Critical Appraisal: Help
- Critical Appraisal of a qualitative paper
- Useful resources
Appraisal of a Quantitative paper: Top tips

- Introduction
Critical appraisal of a quantitative paper (RCT)
This guide, aimed at health students, provides basic level support for appraising quantitative research papers. It's designed for students who have already attended lectures on critical appraisal. One framework for appraising quantitative research (based on reliability, internal and external validity) is provided and there is an opportunity to practise the technique on a sample article.
Please note this framework is for appraising one particular type of quantitative research a Randomised Controlled Trial (RCT) which is defined as
a trial in which participants are randomly assigned to one of two or more groups: the experimental group or groups receive the intervention or interventions being tested; the comparison group (control group) receive usual care or no treatment or a placebo. The groups are then followed up to see if there are any differences between the results. This helps in assessing the effectiveness of the intervention.(CASP, 2020)
Support materials
- Framework for reading quantitative papers (RCTs)
- Critical appraisal of a quantitative paper PowerPoint
To practise following this framework for critically appraising a quantitative article, please look at the following article:
Marrero, D.G. et al (2016) 'Comparison of commercial and self-initiated weight loss programs in people with prediabetes: a randomized control trial', AJPH Research , 106(5), pp. 949-956.
Critical Appraisal of a quantitative paper (RCT): practical example
- Internal Validity
- External Validity
- Reliability Measurement Tool
How to use this practical example
Using the framework, you can have a go at appraising a quantitative paper - we are going to look at the following article:
Marrero, d.g. et al (2016) 'comparison of commercial and self-initiated weight loss programs in people with prediabetes: a randomized control trial', ajph research , 106(5), pp. 949-956., step 1. take a quick look at the article, step 2. click on the internal validity tab above - there are questions to help you appraise the article, read the questions and look for the answers in the article. , step 3. click on each question and our answers will appear., step 4. repeat with the other aspects of external validity and reliability. , questioning the internal validity:, randomisation : how were participants allocated to each group did a randomisation process taken place, comparability of groups: how similar were the groups eg age, sex, ethnicity – is this made clear, blinding (none, single, double or triple): who was not aware of which group a patient was in (eg nobody, only patient, patient and clinician, patient, clinician and researcher) was it feasible for more blinding to have taken place , equal treatment of groups: were both groups treated in the same way , attrition : what percentage of participants dropped out did this adversely affect one group has this been evaluated, overall internal validity: does the research measure what it is supposed to be measuring, questioning the external validity:, attrition: was everyone accounted for at the end of the study was any attempt made to contact drop-outs, sampling approach: how was the sample selected was it based on probability or non-probability what was the approach (eg simple random, convenience) was this an appropriate approach, sample size (power calculation): how many participants was a sample size calculation performed did the study pass, exclusion/ inclusion criteria: were the criteria set out clearly were they based on recognised diagnostic criteria, what is the overall external validity can the results be applied to the wider population, questioning the reliability (measurement tool) internal validity:, internal consistency reliability (cronbach’s alpha). has a cronbach’s alpha score of 0.7 or above been included, test re-test reliability correlation. was the test repeated more than once were the same results received has a correlation coefficient been reported is it above 0.7 , validity of measurement tool. is it an established tool if not what has been done to check if it is reliable pilot study expert panel literature review criterion validity (test against other tools): has a criterion validity comparison been carried out was the score above 0.7, what is the overall reliability how consistent are the measurements , overall validity and reliability:, overall how valid and reliable is the paper.
- << Previous: Critical Appraisal of a qualitative paper
- Next: Useful resources >>
- Last Updated: Apr 30, 2024 4:47 PM
- URL: https://libguides.tees.ac.uk/critical_appraisal

- Critical Appraisal Tools
- Introduction
- Related Guides
- Getting Help
Critical Appraisal of Studies
Critical appraisal is the process of carefully and systematically examining research to judge its trustworthiness, and its value/relevance in a particular context by providing a framework to evaluate the research. During the critical appraisal process, researchers can:
- Decide whether studies have been undertaken in a way that makes their findings reliable as well as valid and unbiased
- Make sense of the results
- Know what these results mean in the context of the decision they are making
- Determine if the results are relevant to their patients/schoolwork/research
Burls, A. (2009). What is critical appraisal? In What Is This Series: Evidence-based medicine. Available online at What is Critical Appraisal?
Critical appraisal is included in the process of writing high quality reviews, like systematic and integrative reviews and for evaluating evidence from RCTs and other study designs. For more information on systematic reviews, check out our Systematic Review guide.
- Next: Critical Appraisal Tools >>
- Last Updated: Nov 16, 2023 1:27 PM
- URL: https://guides.library.duq.edu/critappraise
Jump to navigation
- Bahasa Indonesia
- Bahasa Malaysia

Master critical appraisal with Cochrane Evidence Essentials module 6

New online learning is now freely available about critical appraisal of rapid reviews as part of Cochrane’s flagship Evidence Essentials modules for the public.
Written from the perspective of a healthcare consumer this new module is for anyone interested in the critical appraisal of rapid systematic reviews or any review.
By the end of this module, you should be able to:
- Outline when and why a rapid review may be conducted
- Describe the differences between rapid and systematic reviews
- Understand and apply key concepts for assessing the quality of a rapid review by using the CASP tool
The learning is interactive, with quizzes, animations, and ways to check your knowledge.

This module is the latest in Cochrane’s “Evidence Essentials” that gives an introduction to Evidence Based Medicine, clinical trials, systematic reviews and how to use evidence when making decisions about your health. The modules have been visited over 97,000 times since their launch, and the first 4 modules have now been translated into Spanish, German, and Russian.

The module’s content creation has been led by Emily Clark, National Collaborating Centre for Methods and Tools at the McMaster University, School of Nursing. Emily welcomed the launch of the new module:
“I’m so excited to see the launch of the module. It builds on the work that we have been doing at McMaster to spread understanding of critical appraisal of rapid reviews amongst the wider public. We’ve used a rapid review about risk factors associated with severe COVID-19 outcomes in children 5 years and under as the basis for this learning, and we show people how to use the CASP framework to appraise a review. This new Evidence Essential model should give non-scientists a way to check the quality of a review.”

Richard Morley, Cochrane’s Consumer Engagement Officer, said:
“The Evidence Essentials puts into one exciting place information that consumers (patients, carers and the public) can use to understand about health research, and in the case of this latest module, assess the quality of a review before using it to make decisions about healthcare choices. In a world where information and misinformation are abundant, the ability to judge the evidence is vital for the public.”
There are now six interactive modules in Evidence Essentials: an introduction to Evidence-Based Medicine, Randomized Controlled Trials, Introduction to systematic reviews, Understanding and using systematic reviews; Consumer involvement in Cochrane and the latest, Critical appraisal of rapid reviews.
Modules are free to use, with a Cochrane account and are found at https://training.cochrane.org/essentials

Critical Appraisal : Critical appraisal full list of checklists and tools
- What is critical appraisal?
- Where to start
- Education and childhood studies
- Occupational Therapy
- Physiotherapy
- Interpreting statistics
- Further reading and resources
Which checklist or tool should I use?
There are hundreds of critical appraisal checklists and tools you can choose from, which can be very overwhelming. There are so many because there are many kinds of research, knowledge can be communicated in a wide range of ways, and whether something is appropriate to meet your information needs depends on your specific context.
We have asked for recommendations from lecturers in different academic departments, to give you an idea about which checklists and tools may be the most relevant for you. Please hover over the drop-down menu at the top of the page, underneath 'Critical appraisal checklists and tools' to view the individual subject pages.
Below are lists of as many critical appraisal tools and checklists as we have been able to find. These are split into health sciences and social sciences because the two areas tend to take different approaches to evaluation, for various reasons!
To see a selection of checklists more suitable for your subject, hover over the top tab of this page.
Critical appraisal checklists and tools for Health Sciences
- AACODS Checklist for appraising grey literature
- AMSTAR 2 critical appraisal tool for systematic reviews that include randomised and non-randomised studies of healthcare interventions or both
- AOTA Critically Appraised Papers American Occupational Therapy Association
- Bandolier - "Evidence based thinking about healthcare"
- BestBETS critical appraisal worksheet
- BMJ critical appraisal checklists
- CASP Critical Appraisal Skills Programme includes checklists for case control studies, clinical prediction rule, cohort studies, diagnostic studies, economic evaluation, qualitative studies, RCTs and systematic reviews
- Centre for Evidence Based Medicine (Oxford) Critical Appraisal Tools CEBM's worksheets to assess systematic reviews, diagnostic, prognosis, and RCTs
- Centre for Evidence Based Medicine (Oxford) CATmaker and EBM calculator CEBM's computer assisted critical appraisal tool CATmaker
- CEMB critical appraisal sheets (Centre for Evidence Based Medicine)
- Cochrane Assessing Risk of Bias in a Randomized Trial
- Critical appraisal: a checklist from Students for Best Evidence S4BE (student network with simple explanations of difficult concepts)
- Critical appraisal and statistical skills (Knowledge for Healthcare)
- Critical appraisal of clinical trials from Testing Treatments International
- Critical appraisal of clinical trials (Medicines Learning Portal)
- Critical appraisal of quantitative research
- Critical appraisal of a quantitative paper from Teeside University
- Critical appraisal of a qualitative paper from Teeside University
- Critical appraisal tools from the Centre for Evidence-Based Medicine
- Critical Evaluation of Research Papers – Qualitative Studies from Teeside University
- Critical Evaluation of Research Papers – RCTs/Experimental Studies from Teeside University
- Evaluation tool for mixed methods study designs
- GRADE - The Grading of Recommendations Assessment, Development and Evaluation working group guidelines and publications for grading the quality of evidence in healthcare research and policy
- HCPRDU Evaluation Tool for Mixed Methods Studies - University of Salford Health Care Practice R&D Unit
- HCPRDU Evaluation Tool for Qualitative Studies - University of Salford Health Care Practice R&D Unit
- HCPRDU Evaluation Tool for Quantitative Studies - University of Salford Health Care Practice R&D Unit
- JBI Joanna Briggs Institute critical appraisal tools checklists for Analytical cross sectional studies, case control studies, case reports, case series, cohort studies, diagnostic test accuracy, economic evaluations, prevalence studies, qualitative research, quasi-experimental (non-randomised) studies, RCTs, systematic reviews and for text and opinion
- Knowledge Translation Program - Toronto based KTP critical appraisal worksheets for systematic reviews, prognosis, diagnosis, harm and therapy
- MATT Mixed Methods Appraisal Tool
- McMaster University Evidence Based Practice Research Group quantitative and qualitative review forms
- NHLBI (National Heart, Blood and lung Institute) study quality assessment tools for case control studies, case series, controlled intervention, observational cohort and cross sectional studies, before-after (pre-post) studies with no control group, systematic reviews and meta analyses
- NICE Guidelines, The Manual Appendix H. pp9-24
- QUADAS-2 tool for evaluating risk of bias in systematic reviews from the University of Bristol
- PEDro PEDro (Physiotherapy Evidence Database) Scale - appraisal resources including a tutorial and appraisal tool
- RoB 2 A revised Cochrane risk-of-bias tool for randomized trials
- ROBINS-I Risk Of Bias In Non-Randomized Studies of Interventions
- ROBIS Risk of Bias in Systematic Reviews
- ROB-ME A tool for assessing Risk Of Bias due to Missing Evidence in a synthesis
- SIGN - Critical appraisal notes and checklists for case control studies, cohort studies, diagnostic studies, economic studies, RCTs, meta-analyses and systematic reviews
- Strength of Recommendation Taxonomy - the SORT scale for quality, quantity and consistency of evidence in individual studies or bodies of evidence
- STROBE (Strengthening the Reporting of Observational studies in Epidemiology) for cohort, case-control, and cross-sectional studies (combined), cohort, case-control, cross-sectional studies and conference abstracts
- SURE Case Controlled Studies Critical Appraisal checklist
- SURE Case Series Studies Critical Appraisal checklist
- SURE Cohort Studies Critical Appraisal checklist
- SURE Cross-sectional Studies Critical Appraisal checklist
- SURE Experimental Studies Critical Appraisal checklist
- SURE Qualitative Studies Critical Appraisal checklist
- SURE Systematic Review Critical Appraisal checklist
Critical appraisal checklists and tools for Social Sciences
- AACODS Checklist for appraising grey literature
- CRAAP test to evaluate sources of information
- Critical Appraisal of an Article on an Educational Intervention (variable study design) from the University of Glasgow
- Educational Interventions Critical Appraisal worksheet from BestBETs
- PROMPT from Open University
- PROVEN - tool to evaluate any source of information
SIFT (The Four Moves) to help students distinguish between truth and fake news
Some Guidelines for the Critical Reviewing of Conceptual Papers
- << Previous: Where to start
- Next: Business >>
- Last Updated: Apr 1, 2024 11:00 AM
- URL: https://libguides.qmu.ac.uk/critical-appraisal
Revising the JBI quantitative critical appraisal tools to improve their applicability: an overview of methods and the development process
Affiliations.
- 1 JBI, Faculty of Health and Medical Sciences, The University of Adelaide, Adelaide, SA, Australia.
- 2 Queen's Collaboration for Health Care Quality, Queen's University, Kingston, ON, Canada.
- 3 Czech National Centre for Evidence-Based Healthcare and Knowledge Translation (Cochrane Czech Republic; The Czech Republic [Middle European] Centre for Evidence-Based Healthcare: A JBI Centre of Excellence; Masaryk University GRADE Centre), Faculty of Medicine, Institute of Biostatistics and Analyses, Masaryk University, Brno, Czech Republic.
- 4 The Nottingham Centre for Evidence-Based Healthcare: A JBI Centre of Excellence, School of Medicine, University of Nottingham, Nottingham, UK.
- 5 Centre for Health Informatics, Australian Institute of Health Innovation, Macquarie University, Sydney, NSW, Australia.
- PMID: 36121230
- DOI: 10.11124/JBIES-22-00125
JBI offers a suite of critical appraisal instruments that are freely available to systematic reviewers and researchers investigating the methodological limitations of primary research studies. The JBI instruments are designed to be study-specific and are presented as questions in a checklist. The JBI instruments have existed in a checklist-style format for approximately 20 years; however, as the field of research synthesis expands, many of the tools offered by JBI have become outdated. The JBI critical appraisal tools for quantitative studies (eg, randomized controlled trials, quasi-experimental studies) must be updated to reflect the current methodologies in this field. Cognizant of this and the recent developments in risk-of-bias science, the JBI Effectiveness Methodology Group was tasked with updating the current quantitative critical appraisal instruments. This paper details the methods and rationale that the JBI Effectiveness Methodology Group followed when updating the JBI critical appraisal instruments for quantitative study designs. We detail the key changes made to the tools and highlight how these changes reflect current methodological developments in this field.
Copyright © 2023 JBI.
Publication types
- Research Support, Non-U.S. Gov't
- Research Design*
CASP Checklists
How to use our CASP Checklists
Referencing and Creative Commons
- Online Training Courses
- CASP Workshops
- What is Critical Appraisal
- Study Designs
- Useful Links
- Bibliography
- View all Tools and Resources
- Testimonials
Critical Appraisal Checklists
We offer a number of free downloadable checklists to help you more easily and accurately perform critical appraisal across a number of different study types.
The CASP checklists are easy to understand but in case you need any further guidance on how they are structured, take a look at our guide on how to use our CASP checklists .
CASP Checklist: Systematic Reviews with Meta-Analysis of Observational Studies
CASP Checklist: Systematic Reviews with Meta-Analysis of Randomised Controlled Trials (RCTs)
CASP Randomised Controlled Trial Checklist
- Print & Fill
CASP Systematic Review Checklist
CASP Qualitative Studies Checklist
CASP Cohort Study Checklist
CASP Diagnostic Study Checklist
CASP Case Control Study Checklist
CASP Economic Evaluation Checklist
CASP Clinical Prediction Rule Checklist
Checklist Archive
- CASP Randomised Controlled Trial Checklist 2018 fillable form
- CASP Randomised Controlled Trial Checklist 2018
CASP Checklist
Need more information?
- Online Learning
- Privacy Policy

Critical Appraisal Skills Programme
Critical Appraisal Skills Programme (CASP) will use the information you provide on this form to be in touch with you and to provide updates and marketing. Please let us know all the ways you would like to hear from us:
We use Mailchimp as our marketing platform. By clicking below to subscribe, you acknowledge that your information will be transferred to Mailchimp for processing. Learn more about Mailchimp's privacy practices here.
Copyright 2024 CASP UK - OAP Ltd. All rights reserved Website by Beyond Your Brand
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- Write for Us
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 21, Issue 4
- How to appraise quantitative research
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
This article has a correction. Please see:
- Correction: How to appraise quantitative research - April 01, 2019
- Xabi Cathala 1 ,
- Calvin Moorley 2
- 1 Institute of Vocational Learning , School of Health and Social Care, London South Bank University , London , UK
- 2 Nursing Research and Diversity in Care , School of Health and Social Care, London South Bank University , London , UK
- Correspondence to Mr Xabi Cathala, Institute of Vocational Learning, School of Health and Social Care, London South Bank University London UK ; cathalax{at}lsbu.ac.uk and Dr Calvin Moorley, Nursing Research and Diversity in Care, School of Health and Social Care, London South Bank University, London SE1 0AA, UK; Moorleyc{at}lsbu.ac.uk
https://doi.org/10.1136/eb-2018-102996
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Introduction
Some nurses feel that they lack the necessary skills to read a research paper and to then decide if they should implement the findings into their practice. This is particularly the case when considering the results of quantitative research, which often contains the results of statistical testing. However, nurses have a professional responsibility to critique research to improve their practice, care and patient safety. 1 This article provides a step by step guide on how to critically appraise a quantitative paper.
Title, keywords and the authors
The authors’ names may not mean much, but knowing the following will be helpful:
Their position, for example, academic, researcher or healthcare practitioner.
Their qualification, both professional, for example, a nurse or physiotherapist and academic (eg, degree, masters, doctorate).
This can indicate how the research has been conducted and the authors’ competence on the subject. Basically, do you want to read a paper on quantum physics written by a plumber?
The abstract is a resume of the article and should contain:
Introduction.
Research question/hypothesis.
Methods including sample design, tests used and the statistical analysis (of course! Remember we love numbers).
Main findings.
Conclusion.
The subheadings in the abstract will vary depending on the journal. An abstract should not usually be more than 300 words but this varies depending on specific journal requirements. If the above information is contained in the abstract, it can give you an idea about whether the study is relevant to your area of practice. However, before deciding if the results of a research paper are relevant to your practice, it is important to review the overall quality of the article. This can only be done by reading and critically appraising the entire article.
The introduction
Example: the effect of paracetamol on levels of pain.
My hypothesis is that A has an effect on B, for example, paracetamol has an effect on levels of pain.
My null hypothesis is that A has no effect on B, for example, paracetamol has no effect on pain.
My study will test the null hypothesis and if the null hypothesis is validated then the hypothesis is false (A has no effect on B). This means paracetamol has no effect on the level of pain. If the null hypothesis is rejected then the hypothesis is true (A has an effect on B). This means that paracetamol has an effect on the level of pain.
Background/literature review
The literature review should include reference to recent and relevant research in the area. It should summarise what is already known about the topic and why the research study is needed and state what the study will contribute to new knowledge. 5 The literature review should be up to date, usually 5–8 years, but it will depend on the topic and sometimes it is acceptable to include older (seminal) studies.
Methodology
In quantitative studies, the data analysis varies between studies depending on the type of design used. For example, descriptive, correlative or experimental studies all vary. A descriptive study will describe the pattern of a topic related to one or more variable. 6 A correlational study examines the link (correlation) between two variables 7 and focuses on how a variable will react to a change of another variable. In experimental studies, the researchers manipulate variables looking at outcomes 8 and the sample is commonly assigned into different groups (known as randomisation) to determine the effect (causal) of a condition (independent variable) on a certain outcome. This is a common method used in clinical trials.
There should be sufficient detail provided in the methods section for you to replicate the study (should you want to). To enable you to do this, the following sections are normally included:
Overview and rationale for the methodology.
Participants or sample.
Data collection tools.
Methods of data analysis.
Ethical issues.
Data collection should be clearly explained and the article should discuss how this process was undertaken. Data collection should be systematic, objective, precise, repeatable, valid and reliable. Any tool (eg, a questionnaire) used for data collection should have been piloted (or pretested and/or adjusted) to ensure the quality, validity and reliability of the tool. 9 The participants (the sample) and any randomisation technique used should be identified. The sample size is central in quantitative research, as the findings should be able to be generalised for the wider population. 10 The data analysis can be done manually or more complex analyses performed using computer software sometimes with advice of a statistician. From this analysis, results like mode, mean, median, p value, CI and so on are always presented in a numerical format.
The author(s) should present the results clearly. These may be presented in graphs, charts or tables alongside some text. You should perform your own critique of the data analysis process; just because a paper has been published, it does not mean it is perfect. Your findings may be different from the author’s. Through critical analysis the reader may find an error in the study process that authors have not seen or highlighted. These errors can change the study result or change a study you thought was strong to weak. To help you critique a quantitative research paper, some guidance on understanding statistical terminology is provided in table 1 .
- View inline
Some basic guidance for understanding statistics
Quantitative studies examine the relationship between variables, and the p value illustrates this objectively. 11 If the p value is less than 0.05, the null hypothesis is rejected and the hypothesis is accepted and the study will say there is a significant difference. If the p value is more than 0.05, the null hypothesis is accepted then the hypothesis is rejected. The study will say there is no significant difference. As a general rule, a p value of less than 0.05 means, the hypothesis is accepted and if it is more than 0.05 the hypothesis is rejected.
The CI is a number between 0 and 1 or is written as a per cent, demonstrating the level of confidence the reader can have in the result. 12 The CI is calculated by subtracting the p value to 1 (1–p). If there is a p value of 0.05, the CI will be 1–0.05=0.95=95%. A CI over 95% means, we can be confident the result is statistically significant. A CI below 95% means, the result is not statistically significant. The p values and CI highlight the confidence and robustness of a result.
Discussion, recommendations and conclusion
The final section of the paper is where the authors discuss their results and link them to other literature in the area (some of which may have been included in the literature review at the start of the paper). This reminds the reader of what is already known, what the study has found and what new information it adds. The discussion should demonstrate how the authors interpreted their results and how they contribute to new knowledge in the area. Implications for practice and future research should also be highlighted in this section of the paper.
A few other areas you may find helpful are:
Limitations of the study.
Conflicts of interest.
Table 2 provides a useful tool to help you apply the learning in this paper to the critiquing of quantitative research papers.
Quantitative paper appraisal checklist
- 1. ↵ Nursing and Midwifery Council , 2015 . The code: standard of conduct, performance and ethics for nurses and midwives https://www.nmc.org.uk/globalassets/sitedocuments/nmc-publications/nmc-code.pdf ( accessed 21.8.18 ).
- Gerrish K ,
- Moorley C ,
- Tunariu A , et al
- Shorten A ,
Competing interests None declared.
Patient consent Not required.
Provenance and peer review Commissioned; internally peer reviewed.
Correction notice This article has been updated since its original publication to update p values from 0.5 to 0.05 throughout.
Linked Articles
- Miscellaneous Correction: How to appraise quantitative research BMJ Publishing Group Ltd and RCN Publishing Company Ltd Evidence-Based Nursing 2019; 22 62-62 Published Online First: 31 Jan 2019. doi: 10.1136/eb-2018-102996corr1
Read the full text or download the PDF:
Occupational Therapy and Rehabilitation Sciences
- Defining the Research Question(s)
- Reference Resources
- Evidence Summaries & Clinical Guidelines
- Health Data & Statistics
- Patient & Consumer Facing Materials
- Images/Streaming Video
- Database Tutorials
- Crafting a Search
- Narrowing / Filtering a Search
- Expanding a Search
- Cited Reference Searching
- Find Grey Literature
- Save Your Searches
- Cite Sources
- Critical Appraisal
- Different Types of Literature Reviews
- Conducting & Reporting Systematic Reviews
- Finding Systematic Reviews
- Tutorials & Tools for Literature Reviews
- Mobile Apps for Health
PRISMA or Preferred Reporting Items for Systematic Reviews and Meta-Analyses is an evidence-based protocol for reporting information in systematic reviews and meta-analyses.
- The PRISMA STATEMENT , a 27-item checklist and a four-phase flow diagram to help authors improve the reporting of systematic reviews and meta-analyses.
- PRISMA also offers editable templates for the flow diagram as PDF and Word documents
Appraising the Evidence: Getting Started
To appraise the quality of evidence, it is essential understand the nature of the evidence source. Begin the appraisal process by considering these general characteristics:
- Is the source primary, secondary or tertiary? (See University of Minnesota Library - Primary, Secondary, and Tertiary Sources in the Health Sciences )
- If the source is a journal article, what kind of article is it? (A report of original research? A review article? An opinion or commentary?)
- If the source is reporting original research, what was the purpose of the research?
- What is the date of publication?
- Would the evidence presented in the source still be applicable today? (Consider: has technology changed? Have recommended best clinical practices changed? Has consensus understanding of a disease, condition, or treatment changed?)
Authority/Accuracy
- Who is the author? What are the author's credentials and qualifications and to write on the topic?
- Was the source published by a credible entity? (a scholarly journal? a popular periodical, e.g, newspaper or magazine? an association? an organization?)
- Did the source go through a peer review or editorial process before being published? (See this section of the guide for more information about locating peer reviewed articles)
Determining Study Methodology
Understanding how a study was conducted (the methodology) is fundamental for determining the level of evidence that was generated by the study, as well as assessing the quality of the evidence it generated. While some papers state explicitly in the title what kind of method was used, it is often not so straightforward. When looking at report of a study, there are a few techniques you can use to help classify the study design.
1. Notice Metadata in Database Records
In some bibliographic databases, there is information found in the Subject field, or the Publication Type field of the record that can provide information about a study's methodology. Try to locate the record for the article of interest in CINAHL, PubMed or PsycINFO and look for information describing the study (e.g., is it tagged as a "randomized controlled trial," a "case report," and "observational study", a "review" article, etc).
- A word of caution : A "review" article is not necessarily a "systematic review." Even if the title or abstract says "systematic review," carefully evaluate what type of review it is (a systematic review of interventions? a mixed methods SR? a scoping review? a narrative review?).
2. Read the Methods Section
While there may be some information in the abstract that indicates a study's design, it is often necessary to read the full methods section in order to truly understand how the study was conducted. For help understanding the major types of research methodologies within the health sciences, see:
- Understanding Research Study Designs (University of Minnesota Library)
- Study Designs (Centre for Evidence Based Medicine)
- Jeremey Howick's Introduction to Study Designs (Flow Chart) [PDF]
- Quantitative Study Designs (Deakin University Library)
- Grimes, D. A., & Schulz, K. F. (2002). An overview of clinical research: the lay of the land . Lancet (London, England) , 359 (9300), 57–61. https://doi.org/10.1016/S0140-6736(02)07283-5
- Deconstructing the Research Article (May/Jun2022; 42(3): 138-140)
- Background, Significance, and Literature Review (Jul-Aug2022; 42(4): 203-205)
- Purpose Statement, Research Questions, and Hypotheses (Sep/Oct2022; 42(5): 249-257)
- Quantitative Research Designs (Nov/Dec2022; 42(6): 303-311)
- Qualitative Research Designs (Jan/Feb2023; 43(1): 41-45)
- Non-Experimental Research Designs (Mar/Apr2023; 43(2): 99-102)
Once the study methodology is understood, a tool or checklist can be selected to appraise the quality of the evidence that was generated by that study.
Critical Appraisal Resources
In order to select a tool for critical appraisal (also known as quality assessment or "risk of bias" assessment), it is necessary to understand what methodology was used in the study. (For help understanding study design, see this section of the guide .)
The list below sets of contains critical appraisal tools and checklists, with information about what types of studies those tools are meant for. Additionally, there are links to reporting guidelines for different types of students, which can also be useful for quality assessment.
If you're new to critical appraisal, check out this helpful video overview of some of the common tools:

Checklists & Tools
The AGREE II an instrument is valid and reliable tool that can be applied to any practice guideline in any disease area and can be used by health care providers, guideline developers, researchers, decision/policy makers, and educators.
For help using the AGREE II instrument, see the AGREE II Training Tools
- AMSTAR 2 AMSTAR 2 is the revised version of the popular AMSTAR tool (a tool for critically appraising systematic reviews of RCTs). AMSTAR 2 can be used to critically appraise systematic reviews that include randomized or non-randomized studies of healthcare interventions, or both.
A collection of checklists for a number of purposes related to EBM, including finding, interpreting, and evaluating research evidence.
Found in Appendix 1 of Greenhalgh, Trisha. (2010). How to Read a Paper : The Basics of Evidence Based Medicine, 4th edition .
Systematic reviews Randomised controlled trials Qualitative research studies Economic evaluation studies Cohort studies Case control studies Diagnostic test studies
CEBM offers Critical Appraisal Sheets for:
- GRADE The GRADE working group has developed a common, sensible and transparent approach to grading quality of a body of evidence and strength of recommendations that can be drawn from randomized and non-randomized trials . GRADE is meant for use in systematic reviews and other evidence syntheses (e.g., clinical guidelines) where a recommendation impacting practice will be made.
JBI’s critical appraisal tools assist in assessing the trustworthiness, relevance and results of published papers. There are checklists available for:
- The Patient Education Materials Assessment Tool (PEMAT) and User’s Guide The Patient Education Materials Assessment Tool (PEMAT) is a systematic method to evaluate and compare the understandability and actionability of patient education materials . It is designed as a guide to help determine whether patients will be able to understand and act on information. Separate tools are available for use with print and audiovisual materials.
- MMAT (Mixed Methods Appraisal Tool) 2018 "The MMAT is a critical appraisal tool that is designed for the appraisal stage of systematic mixed studies reviews, i.e., reviews that include qualitative, quantitative and mixed methods studies. It permits to appraise the methodological quality of five categories to studies: qualitative research, randomized controlled trials, non randomized studies, quantitative descriptive studies, and mixed methods studies."
- PEDro Scale (Physiotherapy Evidence Database) The PEDro scale was developed to help users rapidly identify trials that are likely to be internally valid and have sufficient statistical information to guide clinical decision-making.
- Risk of Bias (RoB) Tools The RoB 2 tool is designed for assessing risk of bias in randomized trials , while the ROBINS-I tool is meant for assessing non-randomized studies of interventions .
- CanChild / McMaster EBP Research Group - Evidence Review Forms Evidence review forms from the McMaster University Occupational Therapy Evidence-Based Practice for appraising quantitative and qualitative evidence.
Reporting Guidelines
- CONSORT (CONsolidated Standards Of Reporting Trials) The CONSORT Statement is an evidence-based, minimum set of standards for reporting of randomized trials . It offers a standard way for authors to prepare reports of trial findings, facilitating their complete and transparent reporting, and aiding their critical appraisal and interpretation.
- TREND (Transparent Reporting of Evaluations with Nonrandomized Designs) The TREND statement has a 22-item checklist specifically developed to guide standardized reporting of non-randomized controlled trials .
RISMA is an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses . PRISMA primarily focuses on the reporting of reviews evaluating the effects of interventions, but can also be used as a basis for reporting systematic reviews with objectives other than evaluating interventions.
There are also extensions available for scoping reviews , as well as other aspects or types of systematic reviews.
- SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence) The SQUIRE guidelines provide a framework for reporting new knowledge about how to improve healthcare (i.e., quality improvement ). These guidelines are intended for reports that describe system level work to improve the quality, safety, and value of healthcare, and used methods to establish that observed outcomes were due to the intervention(s).
Searchable Registries of Appraisal Tools & Reporting Guidelines
- Equator Network: Enhancing the QUAlity and Transparency Of health Research Comprehensive searchable database of reporting guidelines for main study types and also links to other resources relevant to research reporting.
- The Registry of Methods and Tools for Evidence-Informed Decision Making The Registry of Methods and Tools for Evidence-Informed Decision Making ("the Registry") is a collection of resources to support evidence-informed decision making in practice, programs and policy. This curated, searchable resource offers a selection of methods and tools for each step in the evidence-informed decision-making process. Includes tools related to implementation science , assessing the applicability and transferability of evidence.
For a list of additional tools, as well as some commentary on their use, see:
Ma, L.-L., Wang, Y.-Y., Yang, Z.-H., Huang, D., Weng, H., & Zeng, X.-T. (2020). Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better ? Military Medical Research, 7 (1), 7. https://doi.org/10.1186/s40779-020-00238-8
Determining Level of Evidence
Determining the level of evidence for a particular study or information source depends on understanding, the nature of the research question that is being investigated and the methodology that was used to collect the evidence. See these these resources for help understanding study methodologies .
There are a number of evidence hierarchies that could be used to 'rank' evidence. Which hierarchy is applied often depends on disciplinary norms - students should refer to materials and guidance from their professors about which hierarchy is appropriate to use.
- Oxford Centre for Evidence Based Medicine - Levels of Evidence The CEBM has put together a suite of documents to enable ranking of evidence into levels. Where a study falls in the ranking depends on the methodology of the study, and what kind of question (e.g., therapy, prognosis, diagnosis) is being addressed.
- Joanna Briggs Levels of Evidence [PDF] The JBI Levels of Evidence and Grades of Recommendation are meant to be used alongside the supporting document (PDF) outlining their use.
- << Previous: Evidence-Based Practice
- Next: Literature Reviews >>
- Last Updated: May 2, 2024 10:52 AM
- URL: https://guides.nyu.edu/ot
- Research article
- Open access
- Published: 16 September 2004
A systematic review of the content of critical appraisal tools
- Persis Katrak 1 ,
- Andrea E Bialocerkowski 2 ,
- Nicola Massy-Westropp 1 ,
- VS Saravana Kumar 1 &
- Karen A Grimmer 1
BMC Medical Research Methodology volume 4 , Article number: 22 ( 2004 ) Cite this article
156k Accesses
207 Citations
14 Altmetric
Metrics details
Consumers of research (researchers, administrators, educators and clinicians) frequently use standard critical appraisal tools to evaluate the quality of published research reports. However, there is no consensus regarding the most appropriate critical appraisal tool for allied health research. We summarized the content, intent, construction and psychometric properties of published, currently available critical appraisal tools to identify common elements and their relevance to allied health research.
A systematic review was undertaken of 121 published critical appraisal tools sourced from 108 papers located on electronic databases and the Internet. The tools were classified according to the study design for which they were intended. Their items were then classified into one of 12 criteria based on their intent. Commonly occurring items were identified. The empirical basis for construction of the tool, the method by which overall quality of the study was established, the psychometric properties of the critical appraisal tools and whether guidelines were provided for their use were also recorded.
Eighty-seven percent of critical appraisal tools were specific to a research design, with most tools having been developed for experimental studies. There was considerable variability in items contained in the critical appraisal tools. Twelve percent of available tools were developed using specified empirical research. Forty-nine percent of the critical appraisal tools summarized the quality appraisal into a numeric summary score. Few critical appraisal tools had documented evidence of validity of their items, or reliability of use. Guidelines regarding administration of the tools were provided in 43% of cases.
Conclusions
There was considerable variability in intent, components, construction and psychometric properties of published critical appraisal tools for research reports. There is no "gold standard' critical appraisal tool for any study design, nor is there any widely accepted generic tool that can be applied equally well across study types. No tool was specific to allied health research requirements. Thus interpretation of critical appraisal of research reports currently needs to be considered in light of the properties and intent of the critical appraisal tool chosen for the task.
Peer Review reports
Consumers of research (clinicians, researchers, educators, administrators) frequently use standard critical appraisal tools to evaluate the quality and utility of published research reports [ 1 ]. Critical appraisal tools provide analytical evaluations of the quality of the study, in particular the methods applied to minimise biases in a research project [ 2 ]. As these factors potentially influence study results, and the way that the study findings are interpreted, this information is vital for consumers of research to ascertain whether the results of the study can be believed, and transferred appropriately into other environments, such as policy, further research studies, education or clinical practice. Hence, choosing an appropriate critical appraisal tool is an important component of evidence-based practice.
Although the importance of critical appraisal tools has been acknowledged [ 1 , 3 – 5 ] there appears to be no consensus regarding the 'gold standard' tool for any medical evidence. In addition, it seems that consumers of research are faced with a large number of critical appraisal tools from which to choose. This is evidenced by the recent report by the Agency for Health Research Quality in which 93 critical appraisal tools for quantitative studies were identified [ 6 ]. Such choice may pose problems for research consumers, as dissimilar findings may well be the result when different critical appraisal tools are used to evaluate the same research report [ 6 ].
Critical appraisal tools can be broadly classified into those that are research design-specific and those that are generic. Design-specific tools contain items that address methodological issues that are unique to the research design [ 5 , 7 ]. This precludes comparison however of the quality of different study designs [ 8 ]. To attempt to overcome this limitation, generic critical appraisal tools have been developed, in an attempt to enhance the ability of research consumers to synthesise evidence from a range of quantitative and or qualitative study designs (for instance [ 9 ]). There is no evidence that generic critical appraisal tools and design-specific tools provide a comparative evaluation of research designs.
Moreover, there appears to be little consensus regarding the most appropriate items that should be contained within any critical appraisal tool. This paper is concerned primarily with critical appraisal tools that address the unique properties of allied health care and research [ 10 ]. This approach was taken because of the unique nature of allied health contacts with patients, and because evidence-based practice is an emerging area in allied health [ 10 ]. The availability of so many critical appraisal tools (for instance [ 6 ]) may well prove daunting for allied health practitioners who are learning to critically appraise research in their area of interest. For the purposes of this evaluation, allied health is defined as encompassing "...all occasions of service to non admitted patients where services are provided at units/clinics providing treatment/counseling to patients. These include units primarily concerned with physiotherapy, speech therapy, family panning, dietary advice, optometry occupational therapy..." [ 11 ].
The unique nature of allied health practice needs to be considered in allied health research. Allied health research thus differs from most medical research, with respect to:
• the paradigm underpinning comprehensive and clinically-reasoned descriptions of diagnosis (including validity and reliability). An example of this is in research into low back pain, where instead of diagnosis being made on location and chronicity of pain (as is common) [ 12 ], it would be made on the spinal structure and the nature of the dysfunction underpinning the symptoms, which is arrived at by a staged and replicable clinical reasoning process [ 10 , 13 ].
• the frequent use of multiple interventions within the one contact with the patient (an occasion of service), each of which requires appropriate description in terms of relationship to the diagnosis, nature, intensity, frequency, type of instruction provided to the patient, and the order in which the interventions were applied [ 13 ]
• the timeframe and frequency of contact with the patient (as many allied health disciplines treat patients in episodes of care that contain multiple occasions of service, and which can span many weeks, or even years in the case of chronic problems [ 14 ])
• measures of outcome, including appropriate methods and timeframes of measuring change in impairment, function, disability and handicap that address the needs of different stakeholders (patients, therapists, funders etc) [ 10 , 12 , 13 ].
Search strategy
In supplementary data [see additional file 1 ].
Data organization and extraction
Two independent researchers (PK, NMW) participated in all aspects of this review, and they compared and discussed their findings with respect to inclusion of critical appraisal tools, their intent, components, data extraction and item classification, construction and psychometric properties. Disagreements were resolved by discussion with a third member of the team (KG).
Data extraction consisted of a four-staged process. First, identical replica critical appraisal tools were identified and removed prior to analysis. The remaining critical appraisal tools were then classified according to the study design for which they were intended to be used [ 1 , 2 ]. The scientific manner in which the tools had been constructed was classified as whether an empirical research approach has been used, and if so, which type of research had been undertaken. Finally, the items contained in each critical appraisal tool were extracted and classified into one of eleven groups, which were based on the criteria described by Clarke and Oxman [ 4 ] as:
• Study aims and justification
• Methodology used , which encompassed method of identification of relevant studies and adherence to study protocol;
• Sample selection , which ranged from inclusion and exclusion criteria, to homogeneity of groups;
• Method of randomization and allocation blinding;
• Attrition : response and drop out rates;
• Blinding of the clinician, assessor, patient and statistician as well as the method of blinding;
• Outcome measure characteristics;
• Intervention or exposure details;
• Method of data analyses ;
• Potential sources of bias ; and
• Issues of external validity , which ranged from application of evidence to other settings to the relationship between benefits, cost and harm.
An additional group, " miscellaneous ", was used to describe items that could not be classified into any of the groups listed above.
Data synthesis
Data was synthesized using MS Excel spread sheets as well as narrative format by describing the number of critical appraisal tools per study design and the type of items they contained. Descriptions were made of the method by which the overall quality of the study was determined, evidence regarding the psychometric properties of the tools (validity and reliability) and whether guidelines were provided for use of the critical appraisal tool.
One hundred and ninety-three research reports that potentially provided a description of a critical appraisal tool (or process) were identified from the search strategy. Fifty-six of these papers were unavailable for review due to outdated Internet links, or inability to source the relevant journal through Australian university and Government library databases. Of the 127 papers retrieved, 19 were excluded from this review, as they did not provide a description of the critical appraisal tool used, or were published in languages other than English. As a result, 108 papers were reviewed, which yielded 121 different critical appraisal tools [ 1 – 5 , 7 , 9 , 15 – 102 , 116 ].
Empirical basis for tool construction
We identified 14 instruments (12% all tools) which were reported as having been constructed using a specified empirical approach [ 20 , 29 , 30 , 32 , 35 , 40 , 49 , 51 , 70 – 72 , 79 , 103 , 116 ]. The empirical research reflected descriptive and/or qualitative approaches, these being critical review of existing tools [ 40 , 72 ], Delphi techniques to identify then refine data items [ 32 , 51 , 71 ], questionnaires and other forms of written surveys to identify and refine data items [ 70 , 79 , 103 ], facilitated structured consensus meetings [ 20 , 29 , 30 , 35 , 40 , 49 , 70 , 72 , 79 , 116 ], and pilot validation testing [ 20 , 40 , 72 , 103 , 116 ]. In all the studies which reported developing critical appraisal tools using a consensus approach, a range of stakeholder input was sought, reflecting researchers and clinicians in a range of health disciplines, students, educators and consumers. There were a further 31 papers which cited other studies as the source of the tool used in the review, but which provided no information on why individual items had been chosen, or whether (or how) they had been modified. Moreover, for 21 of these tools, the cited sources of the critical appraisal tool did not report the empirical basis on which the tool had been constructed.
Critical appraisal tools per study design
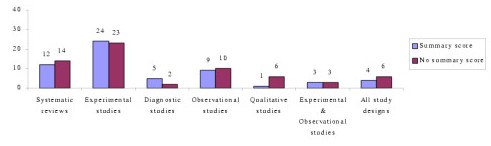
Seventy-eight percent (N = 94) of the critical appraisal tools were developed for use on primary research [ 1 – 5 , 7 , 9 , 18 , 19 , 25 – 27 , 34 , 37 – 41 ], while the remainder (N = 26) were for secondary research (systematic reviews and meta-analyses) [ 2 – 5 , 15 – 36 , 116 ]. Eighty-seven percent (N = 104) of all critical appraisal tools were design-specific [ 2 – 5 , 7 , 9 , 15 – 90 ], with over one third (N = 45) developed for experimental studies (randomized controlled trials, clinical trials) [ 2 – 4 , 25 – 27 , 34 , 37 – 73 ]. Sixteen critical appraisal tools were generic. Of these, six were developed for use on both experimental and observational studies [ 9 , 91 – 95 ], whereas 11 were purported to be useful for any qualitative and quantitative research design [ 1 , 18 , 41 , 96 – 102 , 116 ] (see Figure 1 , Table 1 ).

Number of critical appraisal tools per study design [1,2]
Critical appraisal items
One thousand, four hundred and seventy five items were extracted from these critical appraisal tools. After grouping like items together, 173 different item types were identified, with the most frequently reported items being focused towards assessing the external validity of the study (N = 35) and method of data analyses (N = 28) (Table 2 ). The most frequently reported items across all critical appraisal tools were:
Eligibility criteria (inclusion/exclusion criteria) (N = 63)
Appropriate statistical analyses (N = 47)
Random allocation of subjects (N = 43)
Consideration of outcome measures used (N = 43)
Sample size justification/power calculations (N = 39)
Study design reported (N = 36)
Assessor blinding (N = 36)
Design-specific critical appraisal tools
Systematic reviews.
Eighty-seven different items were extracted from the 26 critical appraisal tools, which were designed to evaluate the quality of systematic reviews. These critical appraisal tools frequently contained items regarding data analyses and issues of external validity (Tables 2 and 3 ).
Items assessing data analyses were focused to the methods used to summarize the results, assessment of sensitivity of results and whether heterogeneity was considered, whereas the nature of reporting of the main results, interpretation of them and their generalizability were frequently used to assess the external validity of the study findings. Moreover, systematic review critical appraisal tools tended to contain items such as identification of relevant studies, search strategy used, number of studies included and protocol adherence, that would not be relevant for other study designs. Blinding and randomisation procedures were rarely included in these critical appraisal tools.
Experimental studies
One hundred and twenty thirteen different items were extracted from the 45 experimental critical appraisal tools. These items most frequently assessed aspects of data analyses and blinding (Tables 1 and 2 ). Data analyses items were focused on whether appropriate statistical analysis was performed, whether a sample size justification or power calculation was provided and whether side effects of the intervention were recorded and analysed. Blinding was focused on whether the participant, clinician and assessor were blinded to the intervention.
Diagnostic studies
Forty-seven different items were extracted from the seven diagnostic critical appraisal tools. These items frequently addressed issues involving data analyses, external validity of results and sample selection that were specific to diagnostic studies (whether the diagnostic criteria were defined, definition of the "gold" standard, the calculation of sensitivity and specificity) (Tables 1 and 2 ).
Observational studies
Seventy-four different items were extracted from the 19 critical appraisal tools for observational studies. These items primarily focused on aspects of data analyses (see Tables 1 and 2 , such as whether confounders were considered in the analysis, whether a sample size justification or power calculation was provided and whether appropriate statistical analyses were preformed.
Qualitative studies
Thirty-six different items were extracted from the seven qualitative study critical appraisal tools. The majority of these items assessed issues regarding external validity, methods of data analyses and the aims and justification of the study (Tables 1 and 2 ). Specifically, items were focused to whether the study question was clearly stated, whether data analyses were clearly described and appropriate, and application of the study findings to the clinical setting. Qualitative critical appraisal tools did not contain items regarding sample selection, randomization, blinding, intervention or bias, perhaps because these issues are not relevant to the qualitative paradigm.
Generic critical appraisal tools
Experimental and observational studies.
Forty-two different items were extracted from the six critical appraisal tools that could be used to evaluate experimental and observational studies. These tools most frequently contained items that addressed aspects of sample selection (such as inclusion/exclusion criteria of participants, homogeneity of participants at baseline) and data analyses (such as whether appropriate statistical analyses were performed, whether a justification of the sample size or power calculation were provided).
All study designs
Seventy-eight different items were contained in the ten critical appraisal tools that could be used for all study designs (quantitative and qualitative). The majority of these items focused on whether appropriate data analyses were undertaken (such as whether confounders were considered in the analysis, whether a sample size justification or power calculation was provided and whether appropriate statistical analyses were preformed) and external validity issues (generalization of results to the population, value of the research findings) (see Tables 1 and 2 ).
Allied health critical appraisal tools
We found no critical appraisal instrument specific to allied health research, despite finding at least seven critical appraisal instruments associated with allied health topics (mostly physiotherapy management of orthopedic conditions) [ 37 , 39 , 52 , 58 , 59 , 65 ]. One critical appraisal development group proposed two instruments [ 9 ], specific to quantitative and qualitative research respectively. The core elements of allied health research quality (specific diagnosis criteria, intervention descriptions, nature of patient contact and appropriate outcome measures) were not addressed in any one tool sourced for this evaluation. We identified 152 different ways of considering quality reporting of outcome measures in the 121 critical appraisal tools, and 81 ways of considering description of interventions. Very few tools which were not specifically targeted to diagnostic studies (less than 10% of the remaining tools) addressed diagnostic criteria. The critical appraisal instrument that seemed most related to allied health research quality [ 39 ] sought comprehensive evaluation of elements of intervention and outcome, however this instrument was relevant only to physiotherapeutic orthopedic experimental research.
Overall study quality
Forty-nine percent (N = 58) of critical appraisal tools summarised the results of the quality appraisal into a single numeric summary score [ 5 , 7 , 15 – 25 , 37 – 59 , 74 – 77 , 80 – 83 , 87 , 91 – 93 , 96 , 97 ] (Figure 2 ). This was achieved by one of two methods:
Number of critical appraisal tools with, and without, summary quality scores
An equal weighting system, where one point was allocated to each item fulfilled; or
A weighted system, where fulfilled items were allocated various points depending on their perceived importance.
However, there was no justification provided for any of the scoring systems used. In the remaining critical appraisal tools (N = 62), a single numerical summary score was not provided [ 1 – 4 , 9 , 25 – 36 , 60 – 73 , 78 , 79 , 84 – 90 , 94 , 95 , 98 – 102 ]. This left the research consumer to summarize the results of the appraisal in a narrative manner, without the assistance of a standard approach.
Psychometric properties of critical appraisal tools
Few critical appraisal tools had documented evidence of their validity and reliability. Face validity was established in nine critical appraisal tools, seven of which were developed for use on experimental studies [ 38 , 40 , 45 , 49 , 51 , 63 , 70 ] and two for systematic reviews [ 32 , 103 ]. Intra-rater reliability was established for only one critical appraisal tool as part of its empirical development process [ 40 ], whereas inter-rater reliability was reported for two systematic review tools [ 20 , 36 ] (for one of these as part of the developmental process [ 20 ]) and seven experimental critical appraisal tools [ 38 , 40 , 45 , 51 , 55 , 56 , 63 ] (for two of these as part of the developmental process [ 40 , 51 ]).
Critical appraisal tool guidelines
Forty-three percent (N = 52) of critical appraisal tools had guidelines that informed the user of the interpretation of each item contained within them (Table 2 ). These guidelines were most frequently in the form of a handbook or published paper (N = 31) [ 2 , 4 , 9 , 15 , 20 , 25 , 28 , 29 , 31 , 36 , 37 , 41 , 50 , 64 – 67 , 69 , 80 , 84 – 87 , 89 , 90 , 95 , 100 , 116 ], whereas in 14 critical appraisal tools explanations accompanied each item [ 16 , 26 , 27 , 40 , 49 , 51 , 57 , 59 , 79 , 83 , 91 , 102 ].
Our search strategy identified a large number of published critical appraisal tools that are currently available to critically appraise research reports. There was a distinct lack of information on tool development processes in most cases. Many of the tools were reported to be modifications of other published tools, or reflected specialty concerns in specific clinical or research areas, without attempts to justify inclusion criteria. Less than 10 of these tools were relevant to evaluation of the quality of allied health research, and none of these were based on an empirical research approach. We are concerned that although our search was systematic and extensive [ 104 , 105 ], our broad key words and our lack of ready access to 29% of potentially useful papers (N = 56) potentially constrained us from identifying all published critical appraisal tools. However, consumers of research seeking critical appraisal instruments are not likely to seek instruments from outdated Internet links and unobtainable journals, thus we believe that we identified the most readily available instruments. Thus, despite the limitations on sourcing all possible tools, we believe that this paper presents a useful synthesis of the readily available critical appraisal tools.
The majority of the critical appraisal tools were developed for a specific research design (87%), with most designed for use on experimental studies (38% of all critical appraisal tools sourced). This finding is not surprising as, according to the medical model, experimental studies sit at or near the top of the hierarchy of evidence [ 2 , 8 ]. In recent years, allied health researchers have strived to apply the medical model of research to their own discipline by conducting experimental research, often by using the randomized controlled trial design [ 106 ]. This trend may be the reason for the development of experimental critical appraisal tools reported in allied health-specific research topics [ 37 , 39 , 52 , 58 , 59 , 65 ].
We also found a considerable number of critical appraisal tools for systematic reviews (N = 26), which reflects the trend to synthesize research evidence to make it relevant for clinicians [ 105 , 107 ]. Systematic review critical appraisal tools contained unique items (such as identification of relevant studies, search strategy used, number of studies included, protocol adherence) compared with tools used for primary studies, a reflection of the secondary nature of data synthesis and analysis.
In contrast, we identified very few qualitative study critical appraisal tools, despite the presence of many journal-specific guidelines that outline important methodological aspects required in a manuscript submitted for publication [ 108 – 110 ]. This finding may reflect the more traditional, quantitative focus of allied health research [ 111 ]. Alternatively, qualitative researchers may view the robustness of their research findings in different terms compared with quantitative researchers [ 112 , 113 ]. Hence the use of critical appraisal tools may be less appropriate for the qualitative paradigm. This requires further consideration.
Of the small number of generic critical appraisal tools, we found few that could be usefully applied (to any health research, and specifically to the allied health literature), because of the generalist nature of their items, variable interpretation (and applicability) of items across research designs, and/or lack of summary scores. Whilst these types of tools potentially facilitate the synthesis of evidence across allied health research designs for clinicians, their lack of specificity in asking the 'hard' questions about research quality related to research design also potentially precludes their adoption for allied health evidence-based practice. At present, the gold standard study design when synthesizing evidence is the randomized controlled trial [ 4 ], which underpins our finding that experimental critical appraisal tools predominated in the allied health literature [ 37 , 39 , 52 , 58 , 59 , 65 ]. However, as more systematic literature reviews are undertaken on allied health topics, it may become more accepted that evidence in the form of other research design types requires acknowledgement, evaluation and synthesis. This may result in the development of more appropriate and clinically useful allied health critical appraisal tools.
A major finding of our study was the volume and variation in available critical appraisal tools. We found no gold standard critical appraisal tool for any type of study design. Therefore, consumers of research are faced with frustrating decisions when attempting to select the most appropriate tool for their needs. Variable quality evaluations may be produced when different critical appraisal tools are used on the same literature [ 6 ]. Thus, interpretation of critical analysis must be carefully considered in light of the critical appraisal tool used.
The variability in the content of critical appraisal tools could be accounted for by the lack of any empirical basis of tool construction, established validity of item construction, and the lack of a gold standard against which to compare new critical tools. As such, consumers of research cannot be certain that the content of published critical appraisal tools reflect the most important aspects of the quality of studies that they assess [ 114 ]. Moreover, there was little evidence of intra- or inter-rater reliability of the critical appraisal tools. Coupled with the lack of protocols for use, this may mean that critical appraisers could interpret instrument items in different ways over repeated occasions of use. This may produce variable results [123].
Based on the findings of this evaluation, we recommend that consumers of research should carefully select critical appraisal tools for their needs. The selected tools should have published evidence of the empirical basis for their construction, validity of items and reliability of interpretation, as well as guidelines for use, so that the tools can be applied and interpreted in a standardized manner. Our findings highlight the need for consensus to be reached regarding the important and core items for critical appraisal tools that will produce a more standardized environment for critical appraisal of research evidence. As a consequence, allied health research will specifically benefit from having critical appraisal tools that reflect best practice research approaches which embed specific research requirements of allied health disciplines.
National Health and Medical Research Council: How to Review the Evidence: Systematic Identification and Review of the Scientific Literature. Canberra. 2000
Google Scholar
National Health and Medical Research Council: How to Use the Evidence: Assessment and Application of Scientific Evidence. Canberra. 2000
Joanna Briggs Institute. [ http://www.joannabriggs.edu.au ]
Clarke M, Oxman AD: Cochrane Reviewer's Handbook 4.2.0. 2003, Oxford: The Cochrane Collaboration
Crombie IK: The Pocket Guide to Critical Appraisal: A Handbook for Health Care Professionals. 1996, London: BMJ Publishing Group
Agency for Healthcare Research and Quality: Systems to Rate the Strength of Scientific Evidence. Evidence Report/Technology Assessment No. 47, Publication No. 02-E016. Rockville. 2002
Elwood JM: Critical Appraisal of Epidemiological Studies and Clinical Trials. 1998, Oxford: Oxford University Press, 2
Sackett DL, Richardson WS, Rosenberg W, Haynes RB: Evidence Based Medicine. How to Practice and Teach EBM. 2000, London: Churchill Livingstone
Critical literature reviews. [ http://www.cotfcanada.org/cotf_critical.htm ]
Bialocerkowski AE, Grimmer KA, Milanese SF, Kumar S: Application of current research evidence to clinical physiotherapy practice. J Allied Health Res Dec.
The National Health Data Dictionary – Version 10. http://www.aihw.gov.au/publications/hwi/nhdd12/nhdd12-v1.pdf and http://www.aihw.gov.au/publications/hwi/nhdd12/nhdd12-v2.pdf
Grimmer K, Bowman P, Roper J: Episodes of allied health outpatient care: an investigation of service delivery in acute public hospital settings. Disability and Rehabilitation. 2000, 22 (1/2): 80-87.
CAS PubMed Google Scholar
Grimmer K, Milanese S, Bialocerkowski A: Clinical guidelines for low back pain: A physiotherapy perspective. Physiotherapy Canada. 2003, 55 (4): 1-9.
Grimmer KA, Milanese S, Bialocerkowski AE, Kumar S: Producing and implementing evidence in clinical practice: the therapies' dilemma. Physiotherapy. 2004,
Greenhalgh T: How to read a paper: papers that summarize other papers (systematic reviews and meta-analysis). BMJ. 1997, 315: 672-675.
CAS PubMed PubMed Central Google Scholar
Auperin A, Pignon J, Poynard T: Review article: critical review of meta-analysis of randomised clinical trials in hepatogastroenterology. Alimentary Pharmacol Therapeutics. 1997, 11: 215-225. 10.1046/j.1365-2036.1997.131302000.x.
CAS Google Scholar
Barnes DE, Bero LA: Why review articles on the health effects of passive smoking reach different conclusions. J Am Med Assoc. 1998, 279: 1566-1570. 10.1001/jama.279.19.1566.
Beck CT: Use of meta-analysis as a teaching strategy in nursing research courses. J Nurs Educat. 1997, 36: 87-90.
Carruthers SG, Larochelle P, Haynes RB, Petrasovits A, Schiffrin EL: Report of the Canadian Hypertension Society Consensus Conference: 1. Introduction. Can Med Assoc J. 1993, 149: 289-293.
Oxman AD, Guyatt GH, Singer J, Goldsmith CH, Hutchinson BG, Milner RA, Streiner DL: Agreement among reviewers of review articles. J Clin Epidemiol. 1991, 44: 91-98. 10.1016/0895-4356(91)90205-N.
Sacks HS, Reitman D, Pagano D, Kupelnick B: Meta-analysis: an update. Mount Sinai Journal of Medicine. 1996, 63: 216-224.
Smith AF: An analysis of review articles published in four anaesthesia journals. Can J Anaesth. 1997, 44: 405-409.
L'Abbe KA, Detsky AS, O'Rourke K: Meta-analysis in clinical research. Ann Intern Med. 1987, 107: 224-233.
PubMed Google Scholar
Mulrow CD, Antonio S: The medical review article: state of the science. Ann Intern Med. 1987, 106: 485-488.
Continuing Professional Development: A Manual for SIGN Guideline Developers. [ http://www.sign.ac.uk ]
Learning and Development Public Health Resources Unit. [ http://www.phru.nhs.uk/ ]
FOCUS Critical Appraisal Tool. [ http://www.focusproject.org.uk ]
Cook DJ, Sackett DL, Spitzer WO: Methodologic guidelines for systematic reviews of randomized control trials in health care from the Potsdam Consultation on meta-analysis. J Clin Epidemiol. 1995, 48: 167-171. 10.1016/0895-4356(94)00172-M.
Cranney A, Tugwell P, Shea B, Wells G: Implications of OMERACT outcomes in arthritis and osteoporosis for Cochrane metaanalysis. J Rheumatol. 1997, 24: 1206-1207.
Guyatt GH, Sackett DL, Sinclair JC, Hoyward R, Cook DJ, Cook RJ: User's guide to the medical literature. IX. A method for grading health care recommendations. J Am Med Assoc. 1995, 274: 1800-1804. 10.1001/jama.274.22.1800.
Gyorkos TW, Tannenbaum TN, Abrahamowicz M, Oxman AD, Scott EAF, Milson ME, Rasooli Iris, Frank JW, Riben PD, Mathias RG: An approach to the development of practice guidelines for community health interventions. Can J Public Health. 1994, 85: S8-13.
Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF: Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999, 354: 1896-1900. 10.1016/S0140-6736(99)04149-5.
Oxman AD, Cook DJ, Guyatt GH: Users' guides to the medical literature. VI. How to use an overview. Evidence-Based Medicine Working Group. J Am Med Assoc. 1994, 272: 1367-1371. 10.1001/jama.272.17.1367.
Pogue J, Yusuf S: Overcoming the limitations of current meta-analysis of randomised controlled trials. Lancet. 1998, 351: 47-52. 10.1016/S0140-6736(97)08461-4.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB: Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. J Am Med Assoc. 2000, 283: 2008-2012. 10.1001/jama.283.15.2008.
Irwig L, Tosteson AN, Gatsonis C, Lau J, Colditz G, Chalmers TC, Mostellar F: Guidelines for meta-analyses evaluating diagnostic tests. Ann Intern Med. 1994, 120: 667-676.
Moseley AM, Herbert RD, Sherrington C, Maher CG: Evidence for physiotherapy practice: A survey of the Physiotherapy Evidence Database. Physiotherapy Evidence Database (PEDro). Australian Journal of Physiotherapy. 2002, 48: 43-50.
Cho MK, Bero LA: Instruments for assessing the quality of drug studies published in the medical literature. J Am Med Assoc. 1994, 272: 101-104. 10.1001/jama.272.2.101.
De Vet HCW, De Bie RA, Van der Heijden GJ, Verhagen AP, Sijpkes P, Kipschild PG: Systematic reviews on the basis of methodological criteria. Physiotherapy. 1997, 83: 284-289.
Downs SH, Black N: The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998, 52: 377-384.
Evans M, Pollock AV: A score system for evaluating random control clinical trials of prophylaxis of abdominal surgical wound infection. Br J Surg. 1985, 72: 256-260.
Fahey T, Hyde C, Milne R, Thorogood M: The type and quality of randomized controlled trials (RCTs) published in UK public health journals. J Public Health Med. 1995, 17: 469-474.
Gotzsche PC: Methodology and overt and hidden bias in reports of 196 double-blind trials of nonsteroidal antiinflammatory drugs in rheumatoid arthritis. Control Clin Trials. 1989, 10: 31-56. 10.1016/0197-2456(89)90017-2.
Imperiale TF, McCullough AJ: Do corticosteroids reduce mortality from alcoholic hepatitis? A meta-analysis of the randomized trials. Ann Int Med. 1990, 113: 299-307.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ: Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Control Clin Trials. 1996, 17: 1-12. 10.1016/0197-2456(95)00134-4.
Khan KS, Daya S, Collins JA, Walter SD: Empirical evidence of bias in infertility research: overestimation of treatment effect in crossover trials using pregnancy as the outcome measure. Fertil Steril. 1996, 65: 939-945.
Kleijnen J, Knipschild P, ter Riet G: Clinical trials of homoeopathy. BMJ. 1991, 302: 316-323.
Liberati A, Himel HN, Chalmers TC: A quality assessment of randomized control trials of primary treatment of breast cancer. J Clin Oncol. 1986, 4: 942-951.
Moher D, Schulz KF, Altman DG, for the CONSORT Group: The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. J Am Med Assoc. 2001, 285: 1987-1991. 10.1001/jama.285.15.1987.
Reisch JS, Tyson JE, Mize SG: Aid to the evaluation of therapeutic studies. Pediatrics. 1989, 84: 815-827.
Sindhu F, Carpenter L, Seers K: Development of a tool to rate the quality assessment of randomized controlled trials using a Delphi technique. J Advanced Nurs. 1997, 25: 1262-1268. 10.1046/j.1365-2648.1997.19970251262.x.
Van der Heijden GJ, Van der Windt DA, Kleijnen J, Koes BW, Bouter LM: Steroid injections for shoulder disorders: a systematic review of randomized clinical trials. Br J Gen Pract. 1996, 46: 309-316.
Van Tulder MW, Koes BW, Bouter LM: Conservative treatment of acute and chronic nonspecific low back pain. A systematic review of randomized controlled trials of the most common interventions. Spine. 1997, 22: 2128-2156. 10.1097/00007632-199709150-00012.
Garbutt JC, West SL, Carey TS, Lohr KN, Crews FT: Pharmacotherapy for Alcohol Dependence. Evidence Report/Technology Assessment No. 3, AHCPR Publication No. 99-E004. Rockville. 1999
Oremus M, Wolfson C, Perrault A, Demers L, Momoli F, Moride Y: Interarter reliability of the modified Jadad quality scale for systematic reviews of Alzheimer's disease drug trials. Dement Geriatr Cognit Disord. 2001, 12: 232-236. 10.1159/000051263.
Clark O, Castro AA, Filho JV, Djubelgovic B: Interrater agreement of Jadad's scale. Annual Cochrane Colloqium Abstracts. 2001, [ http://www.biomedcentral.com/abstracts/COCHRANE/1/op031 ]October Lyon
Jonas W, Anderson RL, Crawford CC, Lyons JS: A systematic review of the quality of homeopathic clinical trials. BMC Alternative Medicine. 2001, 1: 12-10.1186/1472-6882-1-12.
Van Tulder M, Malmivaara A, Esmail R, Koes B: Exercises therapy for low back pain: a systematic review within the framework of the Cochrane Collaboration back review group. Spine. 2000, 25: 2784-2796. 10.1097/00007632-200011010-00011.
Van Tulder MW, Ostelo R, Vlaeyen JWS, Linton SJ, Morley SJ, Assendelft WJJ: Behavioral treatment for chronic low back pain: a systematic review within the framework of the cochrane back. Spine. 2000, 25: 2688-2699. 10.1097/00007632-200010150-00024.
Aronson N, Seidenfeld J, Samson DJ, Aronson N, Albertson PC, Bayoumi AM, Bennett C, Brown A, Garber ABA, Gere M, Hasselblad V, Wilt T, Ziegler MPHK, Pharm D: Relative Effectiveness and Cost Effectiveness of Methods of Androgen Suppression in the Treatment of Advanced Prostate Cancer. Evidence Report/Technology Assessment No. 4, AHCPR Publication No.99-E0012. Rockville. 1999
Chalmers TC, Smith H, Blackburn B, Silverman B, Schroeder B, Reitman D, Ambroz A: A method for assessing the quality of a randomized control trial. Control Clin Trials. 1981, 2: 31-49. 10.1016/0197-2456(81)90056-8.
der Simonian R, Charette LJ, McPeek B, Mosteller F: Reporting on methods in clinical trials. New Eng J Med. 1982, 306: 1332-1337.
Detsky AS, Naylor CD, O'Rourke K, McGeer AJ, L'Abbe KA: Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol. 1992, 45: 255-265. 10.1016/0895-4356(92)90085-2.
Goudas L, Carr DB, Bloch R, Balk E, Ioannidis JPA, Terrin MN: Management of Cancer Pain. Evidence Report/Technology Assessment No. 35 (Contract 290-97-0019 to the New England Medical Center), AHCPR Publication No. 99-E004. Rockville. 2000
Guyatt GH, Sackett DL, Cook DJ: Users' guides to the medical literature. II. How to use an article about therapy or prevention. A. Are the results of the study valid? Evidence-Based Medicine Working Group. J Am Med Assoc. 1993, 270: 2598-2601. 10.1001/jama.270.21.2598.
Khan KS, Ter Riet G, Glanville J, Sowden AJ, Kleijnen J: Undertaking Systematic Reviews of Research on Effectiveness: Centre of Reviews and Dissemination's Guidance for Carrying Out or Commissioning Reviews: York. 2000
McNamara R, Bass EB, Marlene R, Miller J: Management of New Onset Atrial Fibrillation. Evidence Report/Technology Assessment No.12, AHRQ Publication No. 01-E026. Rockville. 2001
Prendiville W, Elbourne D, Chalmers I: The effects of routine oxytocic administration in the management of the third stage of labour: an overview of the evidence from controlled trials. Br J Obstet Gynae Col. 1988, 95: 3-16.
Schulz KF, Chalmers I, Hayes RJ, Altman DG: Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. J Am Med Assoc. 1995, 273: 408-412. 10.1001/jama.273.5.408.
The Standards of Reporting Trials Group: A proposal for structured reporting of randomized controlled trials. J Am Med Assoc. 1994, 272: 1926-1931. 10.1001/jama.272.24.1926.
Verhagen AP, de Vet HC, de Bie RA, Kessels AGH, Boers M, Bouter LM, Knipschild PG: The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998, 51: 1235-1241. 10.1016/S0895-4356(98)00131-0.
Zaza S, Wright-De Aguero LK, Briss PA, Truman BI, Hopkins DP, Hennessy MH, Sosin DM, Anderson L, Carande-Kullis VG, Teutsch SM, Pappaioanou M: Data collection instrument and procedure for systematic reviews in the guide to community preventive services. Task force on community preventive services. Am J Prevent Med. 2000, 18: 44-74. 10.1016/S0749-3797(99)00122-1.
Haynes BB, Wilczynski N, McKibbon A, Walker CJ, Sinclair J: Developing optimal search strategies for detecting clinically sound studies in MEDLINE. J Am Informatics Assoc. 1994, 1: 447-458.
Greenhalgh T: How to read a paper: papers that report diagnostic or screening tests. BMJ. 1997, 315: 540-543.
Arroll B, Schechter MT, Sheps SB: The assessment of diagnostic tests: a comparison of medical literature in 1982 and 1985. J Gen Int Med. 1988, 3: 443-447.
Lijmer JG, Mol BW, Heisterkamp S, Bonsel GJ, Prins MH, van der Meulen JH, Bossuyt PM: Empirical evidence of design-related bias in studies of diagnostic tests. J Am Med Assoc. 1999, 282: 1061-1066. 10.1001/jama.282.11.1061.
Sheps SB, Schechter MT: The assessment of diagnostic tests. A survey of current medical research. J Am Med Assoc. 1984, 252: 2418-2422. 10.1001/jama.252.17.2418.
McCrory DC, Matchar DB, Bastian L, Dutta S, Hasselblad V, Hickey J, Myers MSE, Nanda K: Evaluation of Cervical Cytology. Evidence Report/Technology Assessment No. 5, AHCPR Publication No.99-E010. Rockville. 1999
Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, DeVet HCW: Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Clin Chem. 2003, 49: 1-6. 10.1373/49.1.1.
Greenhalgh T: How to Read a Paper: Assessing the methodological quality of published papers. BMJ. 1997, 315: 305-308.
Angelillo I, Villari P: Residential exposure to electromagnetic fields and childhood leukaemia: a meta-analysis. Bull World Health Org. 1999, 77: 906-915.
Ariens G, Mechelen W, Bongers P, Bouter L, Van der Wal G: Physical risk factors for neck pain. Scand J Work Environ Health. 2000, 26: 7-19.
Hoogendoorn WE, van Poppel MN, Bongers PM, Koes BW, Bouter LM: Physical load during work and leisure time as risk factors for back pain. Scand J Work Environ Health. 1999, 25: 387-403.
Laupacis A, Wells G, Richardson WS, Tugwell P: Users' guides to the medical literature. V. How to use an article about prognosis. Evidence-Based Medicine Working Group. J Am Med Assoc. 1994, 272: 234-237. 10.1001/jama.272.3.234.
Levine M, Walter S, Lee H, Haines T, Holbrook A, Moyer V: Users' guides to the medical literature. IV. How to use an article about harm. Evidence-Based Medicine Working Group. J Am Med Assoc. 1994, 271: 1615-1619. 10.1001/jama.271.20.1615.
Carey TS, Boden SD: A critical guide to case series reports. Spine. 2003, 28: 1631-1634. 10.1097/00007632-200308010-00001.
Greenhalgh T, Taylor R: How to read a paper: papers that go beyond numbers (qualitative research). BMJ. 1997, 315: 740-743.
Hoddinott P, Pill R: A review of recently published qualitative research in general practice. More methodological questions than answers?. Fam Pract. 1997, 14: 313-319. 10.1093/fampra/14.4.313.
Mays N, Pope C: Quality research in health care: Assessing quality in qualitative research. BMJ. 2000, 320: 50-52. 10.1136/bmj.320.7226.50.
Mays N, Pope C: Rigour and qualitative research. BMJ. 1995, 311: 109-112.
Colditz GA, Miller JN, Mosteller F: How study design affects outcomes in comparisons of therapy. I: Medical. Stats Med. 1989, 8: 441-454.
Turlik MA, Kushner D: Levels of evidence of articles in podiatric medical journals. J Am Pod Med Assoc. 2000, 90: 300-302.
Borghouts JAJ, Koes BW, Bouter LM: The clinical course and prognostic factors of non-specific neck pain: a systematic review. Pain. 1998, 77: 1-13. 10.1016/S0304-3959(98)00058-X.
Spitzer WO, Lawrence V, Dales R, Hill G, Archer MC, Clark P, Abenhaim L, Hardy J, Sampalis J, Pinfold SP, Morgan PP: Links between passive smoking and disease: a best-evidence synthesis. A report of the working group on passive smoking. Clin Invest Med. 1990, 13: 17-46.
Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F: Systematic reviews of trials and other studies. Health Tech Assess. 1998, 2: 1-276.
Chestnut RM, Carney N, Maynard H, Patterson P, Mann NC, Helfand M: Rehabilitation for Traumatic Brain Injury. Evidence Report/Technology Assessment No. 2, Agency for Health Care Research and Quality Publication No. 99-E006. Rockville. 1999
Lohr KN, Carey TS: Assessing best evidence: issues in grading the quality of studies for systematic reviews. Joint Commission J Qual Improvement. 1999, 25: 470-479.
Greer N, Mosser G, Logan G, Halaas GW: A practical approach to evidence grading. Joint Commission J Qual Improvement. 2000, 26: 700-712.
Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, Atkins D: Current methods of the U.S. Preventive Services Task Force: a review of the process. Am J Prevent Med. 2001, 20: 21-35. 10.1016/S0749-3797(01)00261-6.
Anonymous: How to read clinical journals: IV. To determine etiology or causation. Can Med Assoc J. 1981, 124: 985-990.
Whitten PS, Mair FS, Haycox A, May CR, Williams TL, Hellmich S: Systematic review of cost effectiveness studies of telemedicine interventions. BMJ. 2002, 324: 1434-1437. 10.1136/bmj.324.7351.1434.
PubMed PubMed Central Google Scholar
Forrest JL, Miller SA: Evidence-based decision making in action: Part 2-evaluating and applying the clinical evidence. J Contemp Dental Pract. 2002, 4: 42-52.
Oxman AD, Guyatt GH: Validation of an index of the quality of review articles. J Clin Epidemiol. 1991, 44: 1271-1278. 10.1016/0895-4356(91)90160-B.
Jones T, Evans D: Conducting a systematic review. Aust Crit Care. 2000, 13: 66-71.
Papadopoulos M, Rheeder P: How to do a systematic literature review. South African J Physiother. 2000, 56: 3-6.
Selker LG: Clinical research in Allied Health. J Allied Health. 1994, 23: 201-228.
Stevens KR: Systematic reviews: the heart of evidence-based practice. AACN Clin Issues. 2001, 12: 529-538.
Devers KJ, Frankel RM: Getting qualitative research published. Ed Health. 2001, 14: 109-117. 10.1080/13576280010021888.
Canadian Journal of Public Health: Review guidelines for qualitative research papers submitted for consideration to the Canadian Journal of Public Health. Can J Pub Health. 2000, 91: I2-
Malterud K: Shared understanding of the qualitative research process: guidelines for the medical researcher. Fam Pract. 1993, 10: 201-206.
Higgs J, Titchen A: Research and knowledge. Physiotherapy. 1998, 84: 72-80.
Maggs-Rapport F: Best research practice: in pursuit of methodological rigour. J Advan Nurs. 2001, 35: 373-383. 10.1046/j.1365-2648.2001.01853.x.
Cutcliffe JR, McKenna HP: Establishing the credibility of qualitative research findings: the plot thickens. J Advan Nurs. 1999, 30: 374-380. 10.1046/j.1365-2648.1999.01090.x.
Andresen EM: Criteria for assessing the tools of disability outcomes research. Arch Phys Med Rehab. 2000, 81: S15-S20. 10.1053/apmr.2000.20619.
Beatie P: Measurement of health outcomes in the clinical setting: applications to physiotherapy. Phys Theory Pract. 2001, 17: 173-185. 10.1080/095939801317077632.
Charnock DF, (Ed): The DISCERN Handbook: Quality criteria for consumer health information on treatment choices. 1998, Radcliffe Medical Press
Pre-publication history
The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2288/4/22/prepub
Download references
Author information
Authors and affiliations.
Centre for Allied Health Evidence: A Collaborating Centre of the Joanna Briggs Institute, City East Campus, University of South Australia, North Terrace, Adelaide, 5000, Australia
Persis Katrak, Nicola Massy-Westropp, VS Saravana Kumar & Karen A Grimmer
School of Physiotherapy, The University of Melbourne, Melbourne, 3010, Australia
Andrea E Bialocerkowski
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Karen A Grimmer .
Additional information
Competing interests.
No competing interests.
Authors' contributions
PK Sourced critical appraisal tools
Categorized the content and psychometric properties of critical appraisal tools
AEB Synthesis of findings
Drafted manuscript
NMW Sourced critical appraisal tools
VSK Sourced critical appraisal tools
KAG Study conception and design
Assisted with critiquing critical appraisal tools and categorization of the content and psychometric properties of critical appraisal tools
Drafted and reviewed manuscript
Addressed reviewer's comments and re-submitted the article
Electronic supplementary material
Additional file 1: search strategy. (doc 30 kb), authors’ original submitted files for images.
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2, authors’ original file for figure 3, authors’ original file for figure 4, authors’ original file for figure 5, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Katrak, P., Bialocerkowski, A.E., Massy-Westropp, N. et al. A systematic review of the content of critical appraisal tools. BMC Med Res Methodol 4 , 22 (2004). https://doi.org/10.1186/1471-2288-4-22
Download citation
Received : 10 May 2004
Accepted : 16 September 2004
Published : 16 September 2004
DOI : https://doi.org/10.1186/1471-2288-4-22
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Research Consumer
- Empirical Basis
- Ally Health Literature
- Critical Appraisal Tool
- Sample Size Justification
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]

Library Services
UCL LIBRARY SERVICES
- Guides and databases
- Library skills
LibrarySkills@UCL for NHS staff
- Critical Appraisal of a quantitative study (RCT)
- Library skills for NHS staff
- Accessing resources
- Evidence-based resources
- Bibliographic databases
- Developing your search
- Reviewing and refining your search
- Health management information
- Critical appraisal of a qualitative study
- Critical appraisal of a systematic review
- Referencing basics
- Referencing software
- Referencing styles
- Publishing and sharing research outputs
- Publishing a protocol for your systematic or scoping review
- Communicating and disseminating research
- Help and training
Critical appraisal of a quantitative study (RCT)
The following video (5 mins, 36 secs.) helps to clarify the process of critical appraisal, how to systematically examine research, e.g. using checklists; the variety of tools /checklists available, and guidance on identifying the type of research you are faced with (so you can select the most appropriate appraisal tool).
Critical appraisal of an RCT: introduction to use of CASP checklists
The following video (4 min. 58 sec.) introduces the use of CASP checklists, specifically for critical appraisal of a randomised controlled trial (RCT) study paper; how the checklist is structured, and how to effectively use it.
Webinar recording of critical appraisal of an RCT
The following video is a recording of a webinar, with facilitator and participants using a CASP checklist, to critically appraise a randomised controlled trial paper, and determine whether it constitutes good practice.
'Focus on' videos
The following videos (all approx. 2-7 mins.) focus on a particular aspects of critical appraisal methodology for quantitative studies.
- << Previous: Evaluating information & critical appraisal
- Next: Critical appraisal of a qualitative study >>
- Last Updated: Apr 11, 2024 9:57 AM
- URL: https://library-guides.ucl.ac.uk/nhs

IMAGES
VIDEO
COMMENTS
JBI's Evidence Synthesis Critical Appraisal Tools Assist in Assessing the Trustworthiness, ... "Revising the JBI quantitative critical appraisal tools to improve their applicability: An overview of methods and the development process" ... Munn Z, Porritt K. Qualitative research synthesis: methodological guidance for systematic reviewers ...
More. Critical appraisal tools and reporting guidelines are the two most important instruments available to researchers and practitioners involved in research, evidence-based practice, and policymaking. Each of these instruments has unique characteristics, and both instruments play an essential role in evidence-based practice and decision-making.
ChapterPDF Available. Critical Appraisal of Quantitative Research. June 2018. DOI: 10.1007/978-981-10-2779-6_120-2. In book: Handbook of Research Methods in Health Social Sciences (pp.1-23 ...
Critical appraisal of a quantitative paper PowerPoint To practise following this framework for critically appraising a quantitative article, please look at the following article: Marrero, D.G. et al (2016) 'Comparison of commercial and self-initiated weight loss programs in people with prediabetes: a randomized control trial', AJPH Research ...
For example, in quantitative research a critical appraisal checklist assists a reviewer in assessing each study according to the same (pre-determined) criteria; that is, checklists help standardize the process, if not the outcome (they are navigational tools, not anchors, Booth, Citation 2007). Also, if the checklist has been through a rigorous ...
Critical Appraisal of Studies. Critical appraisal is the process of carefully and systematically examining research to judge its trustworthiness, and its value/relevance in a particular context by providing a framework to evaluate the research. During the critical appraisal process, researchers can: Decide whether studies have been undertaken ...
Abstract. Healthcare professionals are often expected to critically appraise research evidence in order to make recommendations for practice and policy development. Here we describe the Critical Appraisal Toolkit (CAT) currently used by the Public Health Agency of Canada. The CAT consists of: algorithms to identify the type of study design ...
Abstract. Critical appraisal skills are important for anyone wishing to make informed decisions or improve the quality of healthcare delivery. A good critical appraisal provides information regarding the believability and usefulness of a particular study. However, the appraisal process is often overlooked, and critically appraising quantitative ...
A research study can be at risk of bias in its conduct, analysis and interpretation of its data. The assessment of a study's risk of bias is a fundamental step in the systematic review of quantitative evidence. ... The previous iterations of the JBI critical appraisal tools for quantitative study designs did not always ask questions that were ...
JBI instruments have existed in a checklist-style format for approximately 20 years; however, as the field of research synthesis expands, many of the tools offered by JBI have become outdated. The JBI critical appraisal tools for quantitative studies (eg, randomized controlled trials, quasi-experimental studies) must be updated to reflect the current methodologies in this field. Cognizant of ...
There are now six interactive modules in Evidence Essentials: an introduction to Evidence-Based Medicine, Randomized Controlled Trials, Introduction to systematic reviews, Understanding and using systematic reviews; Consumer involvement in Cochrane and the latest, Critical appraisal of rapid reviews. Modules are free to use, with a Cochrane ...
The inclusion and consistent use of critical appraisal tools may be realized with the development of critical appraisal criteria for the variety of different mixed-method approaches. The continued cataloging of current appraisal tools and evaluating the depth and breadth of coverage may be a useful starting point. 9, 12, 23, 24, 30, 31
There are hundreds of critical appraisal checklists and tools you can choose from, which can be very overwhelming. There are so many because there are many kinds of research, knowledge can be communicated in a wide range of ways, and whether something is appropriate to meet your information needs depends on your specific context.
The JBI instruments have existed in a checklist-style format for approximately 20 years; however, as the field of research synthesis expands, many of the tools offered by JBI have become outdated. The JBI critical appraisal tools for quantitative studies (eg, randomized controlled trials, quasi-experimental studies) must be updated to reflect ...
Critical appraisal is the assessment of research studies' worth to clinical practice. Critical appraisal—the heart of evidence-based practice—involves four phases: rapid critical appraisal, evaluation, synthesis, and recommendation. This article reviews each phase and provides examples, tips, and caveats to help evidence appraisers ...
Critical appraisal is the course of action for watchfully and systematically examining research to assess its reliability, value and relevance in order to direct professionals in their vital clinical decision making [ 1 ]. Critical appraisal is essential to: Continuing Professional Development (CPD).
Critical Appraisal is the process of carefully and systematically examining research to judge its trustworthiness, and its value and relevance in a particular context. It is an essential skill for evidence-based medicine because it allows people to find and use research evidence reliably and efficiently. Learn more about what critical appraisal ...
Critical Appraisal Checklists. We offer a number of free downloadable checklists to help you more easily and accurately perform critical appraisal across a number of different study types. The CASP checklists are easy to understand but in case you need any further guidance on how they are structured, take a look at our guide on how to use our ...
This is evidenced by the recent report by the Agency for Health Research Quality in which 93 critical appraisal tools for quantitative studies were identified . Such choice may pose problems for research consumers, as dissimilar findings may well be the result when different critical appraisal tools are used to evaluate the same research report ...
Title, keywords and the authors. The title of a paper should be clear and give a good idea of the subject area. The title should not normally exceed 15 words 2 and should attract the attention of the reader. 3 The next step is to review the key words. These should provide information on both the ideas or concepts discussed in the paper and the ...
"The MMAT is a critical appraisal tool that is designed for the appraisal stage of systematic mixed studies reviews, i.e., reviews that include qualitative, quantitative and mixed methods studies. It permits to appraise the methodological quality of five categories to studies: qualitative research, randomized controlled trials, non randomized ...
Consumers of research (researchers, administrators, educators and clinicians) frequently use standard critical appraisal tools to evaluate the quality of published research reports. However, there is no consensus regarding the most appropriate critical appraisal tool for allied health research. We summarized the content, intent, construction and psychometric properties of published, currently ...
The following video (5 mins, 36 secs.) helps to clarify the process of critical appraisal, how to systematically examine research, e.g. using checklists; the variety of tools /checklists available, and guidance on identifying the type of research you are faced with (so you can select the most appropriate appraisal tool).
Methods for the synthesis of primary qualitative research predate formalised systematic reviews of quantitative research. 4 Nevertheless, ... Our novel question is comparable to questions in the JBI critical appraisal tool. 48 Five of 10 questions in the JBI tool prompt the reviewer to consider the congruity between the research methodology and ...