Cell Phones and Cancer Risk
Why has there been concern that cell phones may cause cancer.
There are two main reasons why people are concerned that cell (or mobile) phones might have the potential to cause certain types of cancer or other health problems: Cell phones emit radiation (in the form of radiofrequency radiation , or radio waves ), and cell phone use is widespread. Even a small increase in cancer risk from cell phones would be of concern given how many people use them.
Brain and central nervous system cancers have been of particular concern because hand-held phones are used close to the head and because ionizing radiation—a higher energy form of radiation than what cell phones emit—has been found to cause some brain cancers. Many different kinds of studies have been carried out to try to investigate whether cell phone use is dangerous to human health.
However, the evidence to date suggests that cell phone use does not cause brain or other kinds of cancer in humans.

Is the radiation from cell phones harmful?
Cell phones emit radiation in the radiofrequency region of the electromagnetic spectrum . Second-, third-, and fourth-generation cell phones (2G, 3G, 4G) emit radiofrequency in the frequency range of 0.7–2.7 GHz. Fifth-generation (5G) cell phones are anticipated to use the frequency spectrum up to 80 GHz.
These frequencies all fall in the nonionizing range of the spectrum, which is low frequency and low energy. The energy is too low to damage DNA. By contrast, ionizing radiation , which includes x-rays , radon , and cosmic rays, is high frequency and high energy. Energy from ionizing radiation can damage DNA. DNA damage can cause changes to genes that may increase the risk of cancer.
The NCI fact sheet Electromagnetic Fields and Cancer lists sources of radiofrequency radiation . More information about ionizing radiation can be found on the Radiation page.
The human body does absorb energy from devices that emit radiofrequency radiation. The only consistently recognized biological effect of radiofrequency radiation absorption in humans that the general public might encounter is heating to the area of the body where a cell phone is held (e.g., the ear and head). However, that heating is not sufficient to measurably increase core body temperature. There are no other clearly established dangerous health effects on the human body from radiofrequency radiation.
Has the incidence of brain and central nervous system cancers changed during the time cell phone use increased?
No. Investigators have studied whether the incidence of brain or other central nervous system cancers (that is, the number of new cases of these cancers diagnosed each year) has changed during the time that cell phone use increased dramatically. These studies found:
- stable incidence rates for adult gliomas in the United States ( 1 ), Nordic countries ( 2 ) and Australia ( 3 ) during the past several decades
- stable incidence rates for pediatric brain tumors in the United States during 1993–2013 ( 4 )
- stable incidence rates for acoustic neuroma ( 5 ), which are nonmalignant tumors , and meningioma ( 6 ), which are usually nonmalignant, among US adults since 2009
In addition, studies using cancer incidence data have tested different scenarios (simulations) determining whether the incidence trends are in line with various levels of risk as reported in studies of cell phone use and brain tumors between 1979 and 2008 ( 7 , 8 ). These simulations showed that many risk changes reported in case–control studies were not consistent with incidence data, implying that biases and errors in the study may have distorted the findings.
Because these studies examine cancer incidence trends over time in populations rather than comparing risk in people who do and don’t use cell phones, their ability to observe potential small differences in risk among heavy users or susceptible populations is limited. Observational/epidemiologic studies—including case–control and cohort studies (described below)—are designed to measure individual exposure to cell phone radiation and ascertain specific health outcomes.
How is radiofrequency radiation exposure measured in studies of groups of people?
Epidemiologic studies use information from several sources, including questionnaires and data from cell phone service providers, to estimate radiofrequency radiation exposure in groups of people. Direct measurements are not yet possible outside of a laboratory setting. Estimates from studies reported to date take into account the following:
- How regularly study participants use cell phones (the number of calls per week or month)
- The age and the year when study participants first used a cell phone and the age and the year of last use (allows calculation of the duration of use and time since the start of use)
- The average number of cell phone calls per day, week, or month (frequency)
- The average length of a typical cell phone call
- The total hours of lifetime use, calculated from the length of typical call times, the frequency of use, and the duration of use
What has research shown about the link between cell phone use and cancer risk?
Researchers have carried out several types of population studies to investigate the possibility of a relationship between cell phone use and the risk of tumors, both malignant (cancerous) and nonmalignant (not cancer). Epidemiologic studies (also called observational studies ) are research studies in which investigators observe groups of individuals (populations) and collect information about them but do not try to change anything about the groups.
Two main types of epidemiologic studies— cohort studies and case–control studies —have been used to examine associations between cell phone use and cancer risk. In a case–control study, cell phone use is compared between people who have tumors and people who don’t. In a cohort study, a large group of people who do not have cancer at the beginning of the study is followed over time and tumor development in people who did and didn’t use cell phones is compared. Cohort studies are limited by the fact that they may only be able to look at cell phone subscribers, who are not necessarily the cell phone users.
The tumors that have been investigated in epidemiologic studies include malignant brain tumors, such as gliomas , as well as nonmalignant tumors, such as acoustic neuroma (tumors in the cells of the nerve responsible for hearing that are also known as vestibular schwannomas), meningiomas (usually nonmalignant tumors in the membranes that cover and protect the brain and spinal cord ), parotid gland tumors (tumors in the salivary glands ), skin cancer, and thyroid gland tumors.
Four large epidemiologic studies have examined the possible association between cell phone use and cancer: Interphone, a case–control study, and three cohort studies, the Danish Study, the Million Women Study, and the Cohort Study on Mobile Phones and Health (COSMOS). The findings of these studies are mixed, but overall, they do not show an association between cell phone use and cancer ( 9 – 23 ).
Interphone Case–Control Study
How the study was done: This is the largest case–control study of cell phone use and the risk of head and neck tumors. It was conducted by a consortium of researchers from 13 countries. The data came from questionnaires that were completed by study participants in Europe, Israel, Canada, Australia, New Zealand, and Japan.
What the study showed: Most published analyses from this study have shown no increases overall in brain or other central nervous system cancers (glioma and meningioma) related to higher amounts of cell phone use. One analysis showed a statistically significant , although small, increase in the risk of glioma among study participants who spent the most total time on cell phone calls. However, for a variety of reasons the researchers considered this finding inconclusive ( 11 – 13 ).
An analysis of data from all 13 countries reported a statistically significant association between intracranial distribution of tumors within the brain and self-reported location of the phone ( 14 ). However, the authors of this study noted that it is not possible to draw firm conclusions about cause and effect based on their findings.
An analysis of data from five Northern European countries showed an increased risk of acoustic neuroma in those who had used a cell phone for 10 or more years ( 15 ).
In subsequent analyses of Interphone data, investigators investigated whether tumors were more likely to form in areas of the brain with the highest exposure. One analysis showed no relationship between tumor location and level of radiation ( 16 ). However, another found evidence that glioma and, to a lesser extent, meningioma were more likely to develop where exposure was highest ( 17 ).
Danish Cohort Study
How the study was done: This cohort study linked billing information from more than 358,000 cell phone subscribers with brain tumor incidence data from the Danish Cancer Registry.
What the study showed: No association was observed between cell phone use and the incidence of glioma, meningioma, or acoustic neuroma, even among people who had been cell phone subscribers for 13 or more years ( 18 – 20 ).
Million Women Cohort Study
How the study was done: This prospective cohort study conducted in the United Kingdom used data obtained from questionnaires that were completed by study participants.
What the study showed: Self-reported cell phone use was not associated with an increased risk of glioma, meningioma, or non-central nervous system tumors. Although the original published findings reported an association with an increased risk of acoustic neuroma ( 21 ), it was not observed with additional years of follow-up of the cohort ( 22) .
Cohort Study of Mobile Phones and Health (COSMOS)
How the study was done: This large prospective cohort study conducted in Denmark, Finland, Sweden, the Netherlands, and the United Kingdom used data on health, lifestyle, and current and past cell phone use obtained from a questionnaire completed by participants when they joined the study. That information was supplemented with cancer occurrence data obtained from linkage to national cancer registries and cell phone records obtained from mobile network operators.
What the study showed: Among 264,574 participants with a median follow-up of just over 7 years, the cumulative amount of mobile phone call-time was not associated with the risk of developing glioma, meningioma, or acoustic neuroma ( 23 ). No associations with cancer risk were seen in the heaviest mobile phone users or among among those with the longest history of mobile phone use (15 or more years).
Other Epidemiologic Studies
In addition to these four large studies, other, smaller epidemiologic studies have looked for associations between cell phone use and individual cancers in both adults and children. These include:
- Two NCI-sponsored case–control studies, each conducted in multiple US academic medical centers or hospitals between 1994 and 1998 that used data from questionnaires ( 24) or computer-assisted personal interviews ( 25 ). Neither study showed a relationship between cell phone use and the risk of glioma, meningioma, or acoustic neuroma in adults.
- The CERENAT study, another case–control study conducted in multiple areas in France from 2004 to 2006 using data collected in face-to-face interviews using standardized questionnaires ( 26 ). This study found no association for either gliomas or meningiomas when comparing adults who were regular cell phone users with non-users. However, the heaviest users had significantly increased risks of both gliomas and meningiomas.
- A pooled analysis of two case–control studies conducted in Sweden that reported statistically significant trends of increasing brain cancer risk for the total amount of cell phone use and the years of use among people who began using cell phones before age 20 ( 27 ).
- Another case–control study in Sweden, part of the Interphone pooled studies, did not find an increased risk of brain cancer among long-term cell phone users between the ages of 20 and 69 ( 28 ).
- The CEFALO study, an international case–control study of children diagnosed with brain cancer between ages 7 and 19, found no relationship between their cell phone use and risk for brain cancer ( 29 ).
- The MOBI-Kids study, a large international case–control study of young people ages 10 to 24 years diagnosed with brain tumors, found no evidence of an association between wireless phone use and the risk of brain tumors ( 30 ).
- A population-based case–control study conducted in Connecticut found no association between cell phone use and the risk of thyroid cancer ( 31 ).
What are the findings from studies of the human body?
Researchers have carried out several kinds of studies to investigate possible effects of cell phone use on the human body. In 2011, two small studies were published that examined brain glucose metabolism in people after they had used cell phones. The results were inconsistent. One study showed increased glucose metabolism in the region of the brain close to the antenna compared with tissues on the opposite side of the brain ( 32 ); the other study ( 33 ) found reduced glucose metabolism on the side of the brain where the phone was used.
The authors of these studies noted that the results were preliminary and that possible health outcomes from changes in glucose metabolism in humans were unknown. Such inconsistent findings are not uncommon in experimental studies of the physiological effects of radiofrequency electromagnetic radiation in people ( 11 ). Some factors that can contribute to inconsistencies across such studies include assumptions used to estimate doses, failure to consider temperature effects, and investigators not being blinded to exposure status.
Another study investigated blood flow in the brain of people exposed to radiofrequency radiation from cell phones and found no evidence of an effect on blood flow in the brain ( 34 ).
What are the findings from experiments in laboratory animals?
Early studies involving laboratory animals showed no evidence that radiofrequency radiation increased cancer risk or enhanced the cancer-causing effects of known chemical carcinogens ( 35 – 38 ).
Because of inconsistent findings from epidemiologic studies in humans and the lack of clear data from previous experimental studies in animals, in 1999 the Food and Drug Administration (FDA) nominated radiofrequency radiation exposure associated with cell phone exposures for study in animal models by the US National Toxicology Program (NTP). NTP is an interagency program that coordinates toxicology research and testing across the US Department of Health and Human Services and is headquartered at the National Institute of Environmental Health Sciences, part of NIH.
The NTP studied radiofrequency radiation (2G and 3G frequencies) in rats and mice ( 39 , 40 ). This large project was conducted in highly specialized labs. The rodents experienced whole-body exposures of 3, 6, or 9 watts per kilogram of body weight for 5 or 7 days per week for 18 hours per day in cycles of 10 minutes on, 10 minutes off. A research overview of the rodent studies , with links to the peer-review summary, is available on the NTP website. The primary outcomes observed were a small number of cancers of Schwann cells in the heart and non-cancerous changes ( hyperplasia ) in the same tissues for male rats, but not female rats, nor in mice overall.
These experimental findings raise new questions because cancers in the heart are extremely rare in humans. Schwann cells of the heart in rodents are similar to the kind of cells in humans that give rise to acoustic neuromas (also known as vestibular schwannomas), which some studies have suggested are increased in people who reported the heaviest use of cell phones. The NTP plans to continue to study radiofrequency exposure in animal models to provide insights into the biological changes that might explain the outcomes observed in their study.
Another animal study, in which rats were exposed 7 days per week for 19 hours per day to radiofrequency radiation at 0.001, 0.03, and 0.1 watts per kilogram of body weight was reported by investigators at the Italian Ramazzini Institute ( 41 ). Among the rats with the highest exposure levels, the researchers noted an increase in heart schwannomas in male rats and nonmalignant Schwann cell growth in the heart in male and female rats. However, key details necessary for interpretation of the results were missing: exposure methods, other standard operating procedures, and nutritional/feeding aspects. The gaps in the report from the study raise questions that have not been resolved.
ICNIRP (an independent nonprofit organization that provides scientific advice and guidance on the health and environmental effects of nonionizing radiation) critically evaluated both studies. It concluded that both followed good laboratory practice, including using more animals than earlier research and exposing the animals to radiofrequency radiation throughout their lifetimes. However, it also identified what it considered major weaknesses in how the studies were conducted and statistically analyzed and concluded that these limitations prevent drawing conclusions about the ability of radiofrequency exposures to cause cancer ( 42 ).
Why are the findings from different studies of cell phone use and cancer risk inconsistent?
A few studies have shown some evidence of statistical association of cell phone use and brain tumor risks in humans, but most studies have found no association. Reasons for these discrepancies include the following:
- Recall bias , which can occur when data about prior habits and exposures are collected from study participants using questionnaires administered after diagnosis of a disease in some of the participants. Study participants who have brain tumors, for example, may remember their cell phone use differently from individuals without brain tumors.
- Inaccurate reporting , which can happen when people say that something has happened more often or less often than it actually did. For example, people may not remember how much they used cell phones in a given time period.
- Morbidity and mortality among study participants who have brain cancer. Gliomas are particularly difficult to study because of their high death rate and the short survival of people who develop these tumors. Patients who survive initial treatment are often impaired, which may affect their responses to questions.
- Participation bias , which can happen when people who are diagnosed with brain tumors are more likely than healthy people (known as controls) to enroll in a research study.
- Changing technology. Older studies evaluated radiofrequency radiation exposure from analog cell phones. Today, cell phones use digital technology, which operates at a different frequency and a lower power level than analog phones, and cellular technology continues to change ( 43 ).
- Exposure assessment limitations. Different studies measure exposure differently, which makes it difficult to compare the results of different studies ( 44 ). Investigations of sources and levels of exposure, particularly in children, are ongoing ( 45 ).
- Insufficient follow-up of highly exposed populations. It may take a very long time to develop symptoms after exposure to radiofrequency radiation, and current studies may not yet have followed participants long enough.
- Inadequate statistical power and methods to detect very small risks or risks that affect small subgroups of people specifically
- Chance as an explanation of apparent effects may not have been considered.
What are other possible health effects from cell phone use?
The most consistent health risk associated with cell phone use is distracted driving and vehicle accidents ( 46 , 47 ). Several other potential health effects have been reported with cell phone use. Neurologic effects are of particular concern in young persons. However, studies of memory, learning, and cognitive function have generally produced inconsistent results ( 48 – 51 ).
What have expert organizations said about the cancer risk from cell phone use?
In 2011, the International Agency for Research on Cancer (IARC) , a component of the World Health Organization, appointed an expert working group to review all available evidence on the use of cell phones. The working group classified cell phone use as “possibly carcinogenic to humans,” based on limited evidence from human studies, limited evidence from studies of radiofrequency radiation and cancer in rodents, and inconsistent evidence from mechanistic studies ( 11 ).
The working group indicated that, although the human studies were susceptible to bias, the findings could not be dismissed as reflecting bias alone, and that a causal interpretation could not be excluded. The working group noted that any interpretation of the evidence should also consider that the observed associations could reflect chance, bias, or confounding variables rather than an underlying causal effect. In addition, the working group stated that the investigation of brain cancer risk associated with cell phone use poses complex research challenges.
The American Cancer Society’s cell phones page states “It is not clear at this time that RF (radiofrequency) waves from cell phones cause dangerous health effects in people, but studies now being done should give a clearer picture of the possible health effects in the future.”
The National Institute of Environmental Health Sciences (NIEHS) states that the weight of the current scientific evidence has not conclusively linked cell phone use with any adverse health problems, but more research is needed.
The US Food and Drug Administration (FDA) notes that studies reporting biological changes associated with radiofrequency radiation have failed to be replicated and that the majority of human epidemiologic studies have failed to show a relationship between exposure to radiofrequency radiation from cell phones and health problems. FDA, which originally nominated this exposure for review by the NTP in 1999, issued a statement on the draft NTP reports released in February 2018, saying “based on this current information, we believe the current safety limits for cell phones are acceptable for protecting the public health.” FDA and the Federal Communications Commission (FCC) share responsibility for regulating cell phone technologies.
The US Centers for Disease Control and Prevention (CDC) states that no scientific evidence definitively answers whether cell phone use causes cancer.
The Federal Communications Commission (FCC) concludes that currently no scientific evidence establishes a definite link between wireless device use and cancer or other illnesses.
In 2015, the European Commission Scientific Committee on Emerging and Newly Identified Health Risks concluded that, overall, the epidemiologic studies on cell phone radiofrequency electromagnetic radiation exposure do not show an increased risk of brain tumors or of other cancers of the head and neck region ( 9 ). The committee also stated that epidemiologic studies do not indicate increased risk for other malignant diseases, including childhood cancer ( 9 ).
Has radiofrequency radiation from cell phone use been associated with cancer risk in children?
There are theoretical considerations as to why the potential health effects of cell phone use should be investigated separately in children. Their nervous systems are still developing and, therefore, more vulnerable to factors that may cause cancer. Their heads are smaller than those of adults and consequently have a greater proportional exposure to radiation emitted by cell phones. And, children have the potential of accumulating more years of cell phone exposure than adults.
Thus far, the data from studies of children with cancer do not suggest that children are at increased risk of developing cancer from cell phone use. The first published analysis came from a large case–control study called CEFALO, which was conducted in Europe. The study included 352 children who were diagnosed with brain tumors between 2004 and 2008 at the ages of 7 to 19 years. They were matched by age, sex, and geographical region with 646 young people randomly selected from population registries. Researchers did not find an association between cell phone use and brain tumor risk by amount of use or by the location of the tumor ( 29 ).
The largest case–control study among children, a 14-country study known as MOBI-Kids, included 899 young people ages 10 to 24 years who were diagnosed with brain tumors between 2010 and 2015. They were matched by sex, age, and region with 1,910 young people who were undergoing surgery for appendicitis. Researchers found no evidence of an association between wireless phone use and brain tumors in young people ( 30 ).
Which US federal agencies have a role in evaluating the effects of or regulating cell phones?
The National Institutes of Health (NIH), including the National Cancer Institute (NCI), conducts research on cell phone use and the risks of cancer and other diseases.
FDA and FCC share regulatory responsibilities for cell phones. FDA is responsible for testing and evaluating electronic product radiation and providing information for the public about the radiofrequency energy emitted by cell phones. FCC sets limits on the emissions of radiofrequency energy by cell phones and similar wireless products.
Where can I find more information about radiofrequency radiation from my cell phone?
The dose of the energy that people absorb from any source of radiation is estimated using a measure called the specific absorption rate (SAR), which is expressed in watts per kilogram of body weight ( 52 ). The SAR decreases very quickly as the distance to the exposure source increases. For cell phone users who hold their phones next to their head during voice calls, the highest exposure is to the brain, acoustic nerve, salivary gland, and thyroid.
The FCC provides information about the SAR of cell phones produced and marketed within the previous 1 to 2 years. Consumers can access this information using the phone’s FCC ID number, which is usually located on the case of the phone, and the FCC’s ID search form . SARs for older phones can be found by checking the phone settings or by contacting the manufacturer.
What can cell phone users do to reduce their exposure to radiofrequency radiation?
FDA has suggested some steps that concerned cell phone users can take to reduce their exposure to radiofrequency radiation :
- Reduce the amount of time spent using your cell phone.
- Use speaker mode, head phones, or ear buds to place more distance between your head and the cell phone.
- Avoid making calls when the signal is weak as this causes cell phones to boost RF transmission power.
- Consider texting rather than talking, but don’t text while you are driving.
Use of wired or wireless headsets reduces the amount of radiofrequency radiation exposure to the head because the phone is not placed against the head ( 53 ). Exposures decline dramatically when cell phones are used hands-free. For example, wireless (Bluetooth) devices (such as headphones and earbuds) use short-range signals that typically transmit radiofrequency waves at power levels 10–400 times lower than cell phones ( 54 ).
- Cancer Topics
- Research Branches
- Research Teams
- Knowledge Transfer
- Research Project Websites
- International Research Collaborations
- Useful Links
- Press Releases
- Featured News
- Videos and Podcasts
- Infographics and Photos
- Questions and Answers
- Publications
- Scientific Meetings and Lectures
- IARC Seminar Series
- IARC/NCI Tumour Seminars
- Medals of Honour
- Professional Staff
- General service Staff
- Talent Pools
- Visiting Scientist and Postdoctoral Opportunities
- Postdoctoral Fellowships
- Call for Tenders
- Office of the Director
- Organization and Management
- Supporters and Friends
- IARC Newsletter
- Visitor Information
- Terms of use
- Privacy Policy
- iarc newsletter
Mobile phone use and brain tumour risk – COSMOS, a prospective cohort study
Researchers from the International Agency for Research on Cancer (IARC) and partners have delivered the most recent results of the Cohort Study of Mobile Phone Use and Health (COSMOS) project, which investigates the potential long-term health effects related to the use of wireless communication technologies. The latest findings of this prospective cohort study indicate that people with the most total hours of mobile phone calls do not have a higher risk of developing a brain tumour compared with light users of mobile phones.
The COSMOS project includes data related to more than 250 000 users of mobile phones, many of whom had 15 or more years of regular mobile phone use before being enrolled in the study. The participants answered detailed questions about their mobile phone use and were then followed up through cancer registries for a median of more than 7 years to record any newly diagnosed brain tumours (glioma, meningioma, or acoustic neuroma).
The occurrence of brain tumours among the 10% of participants with the largest total number of hours of mobile phone calls during their lifetime did not differ from the occurrence in participants who used mobile phones significantly less. These findings suggest that mobile phone use is not associated with increased risk of developing these tumours.
The COSMOS project is a joint project of Karolinska Institutet (Sweden), Imperial College London (United Kingdom; joint lead), the Danish Cancer Institute (Denmark), the Institute for Risk Assessment Sciences of Utrecht University (The Netherlands), Tampere University (Finland), and IARC.
Feychting M, Schüz J, Toledano MB, Vermeulen R, Auvinen A, Poulsen AH, et al. Mobile phone use and brain tumour risk – COSMOS, a prospective cohort study Environ Int , Published online 2 March 2024; https://doi.org/10.1016/j.envint.2024.108552
Read the article
Published in section: IARC News
Publication date: 11 March, 2024, 8:54
Direct link: https://www.iarc.who.int/news-events/mobile-phone-use-and-brain-tumour-risk-cosmos-a-prospective-cohort-study/

Moskowitz: Cellphone radiation is harmful, but few want to believe it
The telecommunications industry insists cellphone technology is safe. But the director of UC Berkeley’s Center for Family and Community Health is determined to prove it wrong.
By Anne Brice

July 1, 2021

The vast majority of American adults — 97% — own a cellphone of some kind, according to the Pew Research Center . (Photo by Susanne Nilsson via Flickr)
For more than a decade, Joel Moskowitz , a researcher in the School of Public Health at UC Berkeley and director of Berkeley’s Center for Family and Community Health, has been on a quest to prove that radiation from cellphones is unsafe. But, he said, most people don’t want to hear it.
“People are addicted to their smartphones,” said Moskowitz. “We use them for everything now, and, in many ways, we need them to function in our daily lives. I think the idea that they’re potentially harming our health is too much for some people.”
Since cellphones first came onto the market in 1983, they have gone from clunky devices with bad reception to today’s sleek, multifunction smartphones. And although cellphones are now used by nearly all American adults , considerable research suggests that long-term use poses health risks from the radiation they emit, said Moskowitz.

Joel Moskowitz is a researcher in the School of Public Health and director of the Center for Family and Community Health at UC Berkeley. (School of Public Health photo)
“Cellphones, cell towers and other wireless devices are regulated by most governments,” said Moskowitz. “Our government, however, stopped funding research on the health effects of radiofrequency radiation in the 1990s.”
Since then, he said, research has shown significant adverse biologic and health effects — including brain cancer — associated with the use of cellphones and other wireless devices. And now, he said, with the fifth generation of cellular technology, known as 5G, there is an even bigger reason for concern .
Berkeley News spoke with Moskowitz about the health risks of cellphone radiation, why the topic is so controversial and what we can expect with the rollout of 5G.
Berkeley News: I think we should address upfront is how controversial this research is. Some scientists have said that these findings are without basis and that there isn’t enough evidence that cellphone radiation is harmful to our health. How do you respond to that?
Joel Moskowitz: Well, first of all, few scientists in this country can speak knowledgeably about the health effects of wireless technology. So, I’m not surprised that people are skeptical, but that doesn’t mean the findings aren’t valid.
A big reason there isn’t more research about the health risks of radiofrequency radiation exposure is because the U.S. government stopped funding this research in the 1990s, with the exception of a $30 million rodent study published in 2018 by the National Institute of Environmental Health Sciences’ National Toxicology Program, which found “clear evidence” of carcinogenicity from cellphone radiation.
In 1996, the Federal Communications Commission, or FCC, adopted exposure guidelines that limited the intensity of exposure to radiofrequency radiation. These guidelines were designed to prevent significant heating of tissue from short-term exposure to radiofrequency radiation, not to protect us from the effects of long-term exposure to low levels of modulated, or pulsed, radiofrequency radiation, which is produced by cellphones, cordless phones and other wireless devices, including Wi-Fi. Yet, the preponderance of research published since 1990 finds adverse biologic and health effects from long-term exposure to radiofrequency radiation, including DNA damage.
More than 250 scientists, who have published over 2,000 papers and letters in professional journals on the biologic and health effects of non-ionizing electromagnetic fields produced by wireless devices, including cellphones, have signed the International EMF Scientist Appeal , which calls for health warnings and stronger exposure limits. So, there are many scientists who agree that this radiation is harmful to our health.
I first heard you speak about the health risks of cellphone radiation at Berkeley in 2019, but you’ve been doing this research since 2009. What led you to pursue this research?
I got into this field by accident, actually. During the past 40 years, the bulk of my research has been focused on tobacco-related disease prevention. I first became interested in cellphone radiation in 2008, when Dr. Seung-Kwon Myung, a physician scientist with the National Cancer Center of South Korea, came to spend a year at the Center for Family and Community Health. He was involved in our smoking cessation projects, and we worked with him and his colleagues on two reviews of the literature, one of which addressed the tumor risk from cellphone use.
At that time, I was skeptical that cellphone radiation could be harmful. However, since I was dubious that cellphone radiation could cause cancer, I immersed myself in the literature regarding the biological effects of low-intensity microwave radiation, emitted by cellphones and other wireless devices.
After reading many animal toxicology studies that found that this radiation could increase oxidative stress — free radicals, stress proteins and DNA damage — I became increasingly convinced that what we were observing in our review of human studies was indeed a real risk.
While Myung and his colleagues were visiting the Center for Family and Community Health, you reviewed case-control studies examining the association between mobile phone use and tumor risk. What did you find?
Our 2009 review , published in the Journal of Clinical Oncology , found that heavy cellphone use was associated with increased brain cancer incidence, especially in studies that used higher quality methods and studies that had no telecommunications industry funding.
Last year, we updated our review , published in the International Journal of Environmental Research and Public Health , based on a meta-analysis of 46 case-control studies — twice as many studies as we used for our 2009 review — and obtained similar findings. Our main takeaway from the current review is that approximately 1,000 hours of lifetime cellphone use, or about 17 minutes per day over a 10-year period, is associated with a statistically significant 60% increase in brain cancer.
Why did the government stop funding this kind of research?
The telecommunications industry has almost complete control of the FCC, according to Captured Agency , a monograph written by journalist Norm Alster during his 2014-15 fellowship at Harvard University’s Center for Ethics. There’s a revolving door between the membership of the FCC and high-level people within the telecom industry that’s been going on for a couple of decades now.
The industry spends about $100 million a year lobbying Congress. The CTIA , which is the major telecom lobbying group, spends $12.5 million per year on 70 lobbyists. According to one of their spokespersons, lobbyists meet roughly 500 times a year with the FCC to lobby on various issues. The industry as a whole spends $132 million a year on lobbying and provides $18 million in political contributions to members of Congress and others at the federal level.
The telecom industry’s influence over the FCC, as you describe, reminds me of the tobacco industry and the advertising power it had in downplaying the risks of smoking cigarettes.
Yes, there are strong parallels between what the telecom industry has done and what the tobacco industry has done, in terms of marketing and controlling messaging to the public. In the 1940s, tobacco companies hired doctors and dentists to endorse their products to reduce public health concerns about smoking risks. The CTIA currently uses a nuclear physicist from academia to assure policymakers that microwave radiation is safe. The telecom industry not only uses the tobacco industry playbook, it is more economically and politically powerful than Big Tobacco ever was. This year, the telecom industry will spend over $18 billion advertising cellular technology worldwide.
You mentioned that cellphones and other wireless devices use modulated, or pulsed, radiofrequency radiation. Can you explain how cellphones and other wireless devices work, and how the radiation they emit is different from radiation from other household appliances, like a microwave?
Basically, when you make a call, you’ve got a radio and a transmitter. It transmits a signal to the nearest cell tower. Each cell tower has a geographic cell, so to speak, in which it can communicate with cellphones within that geographic region or cell.
Then, that cell tower communicates with a switching station, which then searches for whom you’re trying to call, and it connects through a copper cable or fiber optics or, in many cases, a wireless connection through microwave radiation with the wireless access point. Then, that access point either communicates directly through copper wires through a landline or, if you’re calling another cellphone, it will send a signal to a cell tower within the cell of the receiver and so forth.
The difference is the kind of microwave radiation each device emits. With regard to cellphones and Wi-Fi and Bluetooth, there is an information-gathering component. The waves are modulated and pulsed in a very different manner than your microwave oven.
What, specifically, are some of the health effects associated with long-term exposure to low-level modulated radiofrequency radiation emitted from wireless devices?
Many biologists and electromagnetic field scientists believe the modulation of wireless devices makes the energy more biologically active, which interferes with our cellular mechanisms, opening up calcium channels, for example, and allowing calcium to flow into the cell and into the mitochondria within the cell, interfering with our natural cellular processes and leading to the creation of stress proteins and free radicals and, possibly, DNA damage. And, in other cases, it may lead to cell death.
In 2001, based upon the biologic and human epidemiologic research, low-frequency fields were classified as “possibly carcinogenic” by the International Agency for Research on Cancer (IARC) of the World Health Organization. In 2011, the IARC classified radiofrequency radiation as “possibly carcinogenic to humans,” based upon studies of cellphone radiation and brain tumor risk in humans. Currently, we have considerably more evidence that would warrant a stronger classification.
Most recently, on March 1, 2021, a report was released by the former director of the National Center for Environmental Health at the Centers for Disease Control and Prevention , which concluded that there is a “high probability” that radiofrequency radiation emitted by cellphones causes gliomas and acoustic neuromas, two types of brain tumors.
Let’s talk about the fifth generation of cellphone technology, known as 5G, which is already available in limited areas across the U.S. What does this mean for cellphone users and what changes will come with it?
For the first time, in addition to microwaves, this technology will employ millimeter waves, which are much higher frequency than the microwaves used by 3G and 4G. Millimeter waves can’t travel very far, and they’re blocked by fog or rain, trees and building materials, so the industry estimates that it’ll need 800,000 new cell antenna sites.
Each of these sites may have cell antennas from various cellphone providers, and each of these antennas may have microarrays consisting of dozens or even perhaps hundreds of little antennas. In the next few years in the U.S., we will see deployed roughly 2.5 times more antenna sites than in current use unless wireless safety advocates and their representatives in Congress or the judicial system put a halt to this.
How are millimeter waves different from microwaves, in terms of how they affect our bodies and the environment?
Millimeter wave radiation is largely absorbed in the skin, the sweat glands, the peripheral nerves, the eyes and the testes, based upon the body of research that’s been done on millimeter waves . In addition, this radiation may cause hypersensitivity and biochemical alterations in the immune and circulatory systems — the heart, the liver, kidneys and brain.
Millimeter waves can also harm insects and promote the growth of drug-resistant pathogens, so it’s likely to have some widespread environmental effects for the microenvironments around these cell antenna sites.
What are some simple things that each of us can do to reduce the risk of harm from radiation from cellphones and other wireless devices?
First, minimize your use of cellphones or cordless phones — use a landline whenever possible. If you do use a cellphone, turn off the Wi-Fi and Bluetooth if you’re not using them. However, when near a Wi-Fi router, you would be better off using your cellphone on Wi-Fi and turning off the cellular because this will likely result in less radiation exposure than using the cellular network.
Second, distance is your friend. Keeping your cellphone 10 inches away from your body, as compared to one-tenth of an inch, results in a 10,000-fold reduction in exposure. So, keep your phone away from your head and body. Store your phone in a purse or backpack. If you have to put it in your pocket, put it on airplane mode. Text, use wired headphones or speakerphone for calls. Don’t sleep with it next to your head — turn it off or put it in another room.
Third, use your phone only when the signal is strong. Cellphones are programmed to increase radiation when the signal is poor, that is when one or two bars are displayed on your phone. For example, don’t use your phone in an elevator or in a car, as metal structures interfere with the signal.
Also, I encourage people to learn more about the 150-plus local groups affiliated with Americans for Responsible Technology , which are working to educate policymakers, urging them to adopt cell tower regulations and exposure limits that fully protect us and the environment from the harm caused by wireless radiation.
For safety tips on how to reduce exposure to wireless radiation from the California Department of Public Health and other organizations, Moskowitz recommends readers visit his website, saferemr.com , Physicians for Safe Technology and the Environmental Health Trust .
Mobile phone use and risk of brain tumours: a systematic review of association between study quality, source of funding, and research outcomes
Affiliations.
- 1 Department of Community Medicine, Postgraduate Institute of Medical Sciences, Rohtak, 124001, India, Haryana. [email protected].
- 2 Department of Neurology, All India Institute of Medical Sciences, Ansari Nagar East, AIIMS Campus, New Delhi, 110029, India.
- PMID: 28213724
- DOI: 10.1007/s10072-017-2850-8
Mobile phones emit electromagnetic radiations that are classified as possibly carcinogenic to humans. Evidence for increased risk for brain tumours accumulated in parallel by epidemiologic investigations remains controversial. This paper aims to investigate whether methodological quality of studies and source of funding can explain the variation in results. PubMed and Cochrane CENTRAL searches were conducted from 1966 to December 2016, which was supplemented with relevant articles identified in the references. Twenty-two case control studies were included for systematic review. Meta-analysis of 14 case-control studies showed practically no increase in risk of brain tumour [OR 1.03 (95% CI 0.92-1.14)]. However, for mobile phone use of 10 years or longer (or >1640 h), the overall result of the meta-analysis showed a significant 1.33 times increase in risk. The summary estimate of government funded as well as phone industry funded studies showed 1.07 times increase in odds which was not significant, while mixed funded studies did not show any increase in risk of brain tumour. Metaregression analysis indicated that the association was significantly associated with methodological study quality (p < 0.019, 95% CI 0.009-0.09). Relationship between source of funding and log OR for each study was not statistically significant (p < 0.32, 95% CI 0.036-0.010). We found evidence linking mobile phone use and risk of brain tumours especially in long-term users (≥10 years). Studies with higher quality showed a trend towards high risk of brain tumour, while lower quality showed a trend towards lower risk/protection.
Keywords: Brain tumour; Meta-analysis; Metaregression; Mobile phones; Risk.
Publication types
- Systematic Review
- Brain Neoplasms / epidemiology*
- Brain Neoplasms / etiology
- Cell Phone / trends
- Cell Phone Use / adverse effects*
- Cell Phone Use / statistics & numerical data*
- Databases, Bibliographic / statistics & numerical data

Image credit: Shutterstock
No increased risk of brain tumours for mobile phone users, new study finds
Longstanding fears that using mobile phones may increase the risk of developing a brain tumour have been reignited recently by the launch of 5G (fifth generation) mobile wireless technologies. Mobile phones emit radiofrequency waves which, if absorbed by tissues, can cause heating and damage.
Since mobile phones are held close to the head, the radiofrequency waves they emit penetrate into the brain, with the temporal and parietal lobes being most exposed. This has led to concern that mobile phone users may be at an increased risk of developing brain tumours, with the International Agency for Research on Cancer (IARC) classifying radiofrequency waves as ‘possibly carcinogenic.’ However, most of the studies that have investigated this question to date have been retrospective studies in which individuals report mobile phone use after a diagnosis of cancer, meaning that the results may be biased.
Today, researchers from Oxford Population Health and IARC have reported the results of a large UK prospective study (a study in which participants are enrolled before they develop the disease(s) in question) to investigate the association between mobile phone use and brain tumour risk. The results are published in the Journal of the National Cancer Institute .
The researchers used data from the UK Million Women Study: an ongoing study which recruited one in four of all UK women born between 1935 and 1950. Around 776,000 participants completed questionnaires about their mobile phone usage in 2001; around half of these were surveyed again in 2011. The participants were then followed up for an average of 14 years through linkage to their NHS records.
Mobile phone use was examined in relation to the risk of various specific types of brain tumour: glioma (a tumour of the nervous system); acoustic neuroma (a tumour of the nerve connecting the brain and inner ear); meningioma (a tumour of the membrane surrounding the brain); and pituitary gland tumours. The researchers also investigated whether mobile phone use was associated with the risk of eye tumours.
Key findings:
• By 2011, almost 75% of women aged between 60 and 64 years used a mobile phone, and just below 50% of those aged between 75 and 79 years
• Over the 14 year follow-up period, 3,268 (0.42%) of the women developed a brain tumour
• There was no significant difference in the risk of developing a brain tumour between those who had never used a mobile phone, and mobile phone users. These included tumours in the temporal and parietal lobes, which are the most exposed parts of the brain
• There was also no difference in the risk of developing glioma, acoustic neuroma, meningioma, pituitary tumours or eye tumours
• There was no increase in the risk of developing any of these types of tumour for those who used a mobile phone daily, spoke for at least 20 minutes a week and/or had used a mobile phone for over 10 years
• The incidence of right-sided and left-sided tumours was similar in mobile phone users, even though mobile phone use tends to be considerably greater on the right than the left side
Co-investigator Kirstin Pirie from Oxford Population Health’s Cancer Epidemiology Unit said: ‘These results support the accumulating evidence that mobile phone use under usual conditions does not increase brain tumour risk.’
Although the findings are reassuring, it remains unclear whether the risks associated with mobile phone use are different in those who use mobile phones considerably more than was typical of women in this cohort. In this study, only 18% of phone-users reported talking on a mobile phone for 30 minutes or more each week. Those who use mobile phones for long durations can reduce their exposure to radiofrequency waves by using hands-free kits or loudspeakers.
The study did not include children or adolescents, but researchers elsewhere have investigated the association between mobile phone use and brain tumour risk in these groups, not finding any association.
Lead investigator Joachim Schüz from IARC said: ‘Mobile technologies are improving all the time, so that the more recent generations emit substantially lower output power. Nevertheless, given the lack of evidence for heavy users, advising mobile phone users to reduce unnecessary exposures remains a good precautionary approach.’
The study is published in Journal of the National Cancer Institute .
The study was funded by the UK Medical Research Council and Cancer Research UK.
DISCOVER MORE
- Support Oxford's research
- Partner with Oxford on research
- Study at Oxford
- Research jobs at Oxford
You can view all news or browse by category
No link found between brain cancer and cell phone use, experts say
November 9, 2022 – Concerns about a possible link between cell phones and brain cancer —a hot topic in the news 5 to 10 years ago—have all but disappeared in recent years. Experts say this is simply because a number of large studies have found no real evidence of such a link.
“I think that we can make a policy or public health decision that cell phones don’t cause cancer based on the summary of the evidence,” said Harvard T.H. Chan School of Public Health’s Timothy Rebbeck in an October 20, 2022 MedPage Today article. Rebbeck, Vincent L. Gregory, Jr. Professor of Cancer Prevention and director of the Zhu Family Center for Global Cancer Prevention , acknowledged that there are limitations with large human studies, but said that experts view the issue regarding cell phones and brain cancer as largely settled. “My take on it is, when you get to a certain level of human data … the risk that might be conferred by cell phone use is so small,” he said.
For those interested in reducing their cancer risk, it’s important to focus on proven risks, such as smoking , Rebbeck said. He encouraged people to take preventive measures such as cancer screenings and vaccinations . “If you’re worried about cancer, there are many other things that you can do to reduce your cancer risk that are real,” he said.
Read the MedPage Today article: Should We Still Be Concerned About Cell Phones and Brain Cancer?
Cancer FactFinder
Ad-free. Influence-free. Powered by consumers.
The payment for your account couldn't be processed or you've canceled your account with us.
We don’t recognize that sign in. Your username maybe be your email address. Passwords are 6-20 characters with at least one number and letter.
We still don’t recognize that sign in. Retrieve your username. Reset your password.
Forgot your username or password ?
Don’t have an account?
- Account Settings
- My Benefits
- My Products
- Donate Donate
Save products you love, products you own and much more!
Other Membership Benefits:
Suggested Searches
- Become a Member
Car Ratings & Reviews
2024 Top Picks
Car Buying & Pricing
Which Car Brands Make the Best Vehicles?
Tires, Maintenance & Repair
Car Reliability Guide
Key Topics & News
Listen to the Talking Cars Podcast
Home & Garden
Bed & Bath
Top Picks From CR
Best Mattresses
Lawn & Garden
TOP PICKS FROM CR
Best Lawn Mowers and Tractors
Home Improvement
Home Improvement Essential
Best Wood Stains
Home Safety & Security
HOME SAFETY
Best DIY Home Security Systems
REPAIR OR REPLACE?
What to Do With a Broken Appliance
Small Appliances
Best Small Kitchen Appliances
Laundry & Cleaning
Best Washing Machines
Heating, Cooling & Air
Most Reliable Central Air-Conditioning Systems
Electronics
Home Entertainment
FIND YOUR NEW TV
Home Office
Cheapest Printers for Ink Costs
Smartphones & Wearables
BEST SMARTPHONES
Find the Right Phone for You
Digital Security & Privacy
MEMBER BENEFIT
CR Security Planner
Take Action

Does Cell Phone Use Cause Brain Cancer? What the New Study Means For You
Groundbreaking study reveals the strongest link yet between cell phone radiation and cancer. important advice for all consumers., sharing is nice.
We respect your privacy . All email addresses you provide will be used just for sending this story.
The results of a new study by the National Toxicology Program—the largest and most expensive study of its kind—show a link between cell phone radiation and cancer in rats.
For many people, these findings likely raise questions and concerns about the safety of devices that we now carry with us nearly all the time.
Consumer Reports health and safety experts, who have long been concerned about the potential risks of cell phones and urged precautions when using them, say the new study supports that caution.
"Consumers don't need to stop using their phones," says Michael Hansen, Ph.D., a senior scientist with Consumer Reports who has studied this issue for years. "But there are some simple, common-sense steps you can and should take to reduce your exposure."
Specifically, Consumer Reports recommends that you:
- Try to keep the cell phone away from your head and body. Keeping it an arm's distance away significantly reduces exposure to the low-level radiation it emits. This is particularly important when the cellular signal is weak—when your phone has only one bar, for example—because phones may increase their power then to compensate.
- Text or video call when possible, because this allows you to hold the phone farther from your body.
- When speaking, use the speakerphone on your device or a hands-free headset .
- Don't stow your phone in your pants or shirt pocket. Instead, carry it in a bag or use a belt clip.
Below, answers to other basic questions about the study and what it means for you and your family.
So What Did This New Study Find?
The study found that male rats had a higher incidence of two kinds of tumors when exposed to the same type of radiation emitted by cell phones.
The results are not conclusive, and the overall relevance to human cell phone use is something that's "not currently completely worked out," said John Bucher, Ph.D., associate director of the NTP, part of the National Institutes of Health.
But the new report adds weight to human epidemiological studies that have previously raised similar concerns, and when combined with those earlier studies, is poised to force a reconsidering among federal agencies of the potential risks posed by cell phones. "In my experience," Bucher said, "the people who have reviewed our findings agree with the findings."
A spokesman for CTIA, a trade group for the wireless industry, says "Numerous international and U.S. organizations, including the U.S. Food and Drug Administration, World Health Organization, and American Cancer Society, have determined that the already existing body of peer-reviewed and published studies shows that there are no established health effects from radio frequency signals used in cellphones."
Why Should I Be Worried About a Study Using Rats?
Animal studies are actually the gold standard for determining cancer risk, for several reasons.
For one, it is unethical to expose humans to suspected carcinogens in a lab setting.
Second, studies in animals such as rats and mice can be completed much more quickly than they can be in humans, simply because their lifespans are so much shorter than ours. For example, the new NIH study involved exposing the rodents to cell phone radiation for just two years.
Finally, animal studies can validate results of previous observational studies in humans. Those studies, which track large groups of people over time, can look for associations between how many hours people said they used cell phones every day and the incidence of cancers in those people, but they can't prove a cause and effect relationship. Laboratory studies in rats, showing that exposure to cell phone radiation can cause cancers compared to a similar non-exposed group of rats, give credence to the results of observational human studies, and point strongly to cause and effect.
What Do Studies in Humans Show?
The current animal studies are worrisome precisely because they do line up with the results of some previous observational studies in humans.
Last year, Consumer Reports reviewed that research , focusing on five large population studies that investigated that question. Together the studies included more than a million people worldwide, comparing cell phone users with nonusers.
Three of the studies—one from Sweden, another from France, and a third that combined data from 13 countries—suggest a connection between heavy cell phone use and gliomas, the same kind of tumors detected in the new NIH study. Those tumors are usually cancerous and often deadly.
One of those studies also hinted at a link between cell phones and acoustic neuromas (noncancerous tumors); that kind of tumor is related to the second cancer detected in the current study, malignant schwannoma of the heart.
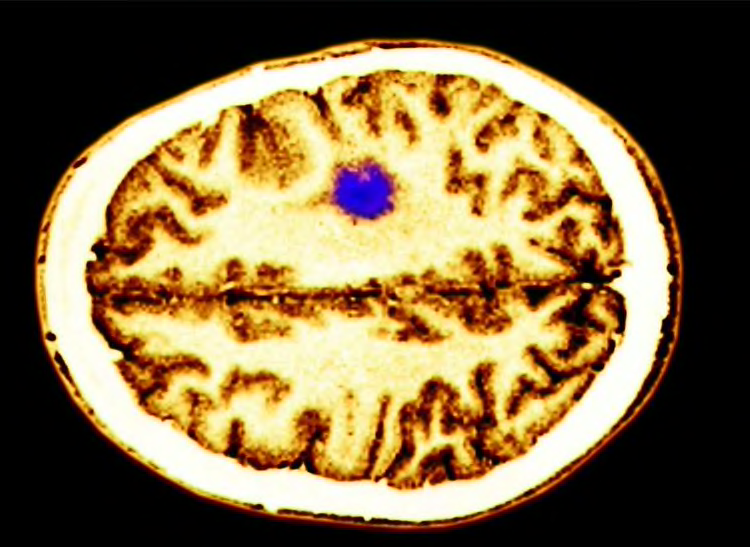
How Might Cell Phone Radiation Cause Cancer?
Scientists previously thought that the radiation from cell phones might damage cells by heating human tissue. At high power levels radiofrequency waves—the kind emitted by cell phones—can heat up water molecules. Since human tissue is mostly water, scientists hypothesized that those waves might cause damage by heating.
The Federal Communications Commission's cell phone emission test—which all cell phones must pass before being allowed on the market—is based on that principle.
But in 2011, scientists at the NIH found that low level radiation, held close to the head, could alter brain cells without raising body temperatures. Likewise, in 2015, German researchers reported that the same type of radiation emitted by cell phones could promote the growth of brain tumors in mice without raising body temperatures.
The NTP study controlled for heating effects by making sure that the body temperatures of exposed rats did not increase by more than 1° C (1.9° F), suggesting that the cancers were triggered by some other mechanism.
Read our previous coverage about the potential dangers of radiation from cell phones and CT scans and X-rays.
How Well Does the NTP Study Mimic Current Cell Phone Usage?
The study used specially designed chambers that allowed researchers to expose rodents to standardized doses of radiation. The rodents were exposed for nine hours total each day, at intervals of 10 minutes on, 10 minutes off, for two years.
The radiation frequencies and signal modulation used were the same used by 2G (GSM or CDMA) phones, which were standard when the study began. Newer cell phones use 3G (such as UMTS or CDMA-2000) or 4G (LTE), which may have lower power outputs and different signal modulation.
"These changes may be a critical difference in whether there is a hazard today," says Consumer Reports' Hansen. "But the study raises enough concern with the older technologies that we recommend an additional study be done with current technology."
The rodents were exposed over their entire bodies. While that's obviously different than the way humans use cell phones, the rodent results are still revealing, Hansen says.
"The reason we see schwannomas in the heart here, and not the auditory system, could be due to the fact that in rodents the heart is closer to the surface of the body," he says. "What's more important is that the cell type found in the heart in the NTP study is the same as in some brain tumors found in several human epidemiology studies."
What Does Consumer Reports Think the Government and Industry Should Do Now?
The substantial questions and concerns raised by this and previous research regarding cell phones and cancer requires swift and decisive action by the government and industry. Specifically, Consumer Reports believes that:
- The National Institutes of Health should commission another animal study using current cell phone technology to determine if it poses the same risks as found in this new study.
- The Federal Communications Commission should update its requirements for testing the effect of cell phone radiation on human heads. The agency's current test is based on the devices' possible effect on large adults, though research suggests that children's thinner skulls mean they may absorb more radiation. The FCC should develop new tests that take into account the potential increased vulnerability of children.
- The Food and Drug Administration and the FCC should determine whether the maximum specific absorption rate of 1.6 W/kg over a gram of tissue is an adequate maximum limit of radiation from cell phones.
- The Centers for Disease Control and Prevention should repost it's advice on the potential hazard of cell phone radiation and cautionary advice that was taken down in August 2014.
- Cell phone manufacturers should prominently display advice on steps that cell phone users can take to reduce exposure to cell phone radiation.

Jeneen Interlandi
I'm a scientist-turned-journalist, covering the intersection of science, policy, and consumer health. I have an abiding passion for good storytelling and verifiable data. I live in Manhattan with my husband and our cat. When I'm not working, I love museums, parks, and visiting my people in New Jersey. Follow me on Twitter (@JInterlandi).
More From Consumer Reports

Be the first to comment
March 29, 2018
New Studies Link Cell Phone Radiation with Cancer
Researchers call for greater caution, but skeptics say the evidence from rat studies is not convincing
By Charles Schmidt

Getty Images
Does cell phone radiation cause cancer? New studies show a correlation in lab rats, but the evidence may not resolve ongoing debates over causality or whether any effects arise in people.
The ionizing radiation given off by sources such as x-ray machines and the sun boosts cancer risk by shredding molecules in the body. But the non-ionizing radio-frequency (RF) radiation that cell phones and other wireless devices emit has just one known biological effect: an ability to heat tissue by exciting its molecules.
Still, evidence advanced by the studies shows prolonged exposure to even very low levels of RF radiation, perhaps by mechanisms other than heating that remain unknown, makes rats uniquely prone to a rare tumor called a schwannoma, which affects a type of neuron (or nerve cell) called a Schwann cell.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
The studies are notable for their sizes. Researchers at the National Toxicology Program, a federal interagency group under the National Institutes of Health, tested 3,000 rats and mice of both sexes for two years—the largest investigation of RF radiation and cancer in rodents ever undertaken in the U.S. European investigators at the Ramazzini Institute in Italy were similarly ambitious; in their recent study they investigated RF effects in nearly 2,500 rats from the fetal stage until death.
Also noteworthy is that the studies evaluated radiation exposures in different ways. The NTP looked at “near-field” exposures, which approximate how people are dosed while using cell phones. Ramazzini researchers looked at “far-field” exposures, which approximate the wireless RF radiation that bombards us from sources all around us, including wireless devices such as tablet and laptop computers. Yet they generated comparable results: Male rats in both studies (but not mice or female animals) developed schwannomas of the heart at statistically higher rates than control animals that were not exposed.
Taken together, the findings “confirm that RF radiation exposure has biological effects” in rats, some of them “relevant to carcinogenesis,” says Jon Samet, a professor of preventive medicine and dean of the Colorado School of Public Health, who did not participate in either study. Samet, however, cautioned the jury is still out as to whether wireless technology is similarly risky to people. Indeed, heart schwannomas are exceedingly rare in humans; only a handful of cases have ever been documented in the medical literature.
When turned on, cell phones and other wireless devices emit RF radiation continually, even if they are not being actively used, because they are always communicating with cell towers. The dose intensity tails off with increasing distance from the body, and reaches a maximum when the devices are used next to the head during phone calls or in front of the body during texting or tweeting.
Launched at the U.S. Food and Drug Administration’s request 10 years ago, the NTP study dosed rats and mice of both sexes with RF radiation at either 1.5, 3 or 6 watts of radiation per kilogram of body weight, or W/kg. The lowest dose is about the same as the Federal Communications Commission’s limit for public exposure from cell phones, which is 1.6 watts W/kg. The animals were exposed nine hours a day for two years (about the average life span for a rat), and the exposures were cranked up steadily as the animals grew, so the absorbed doses per unit body weight remained constant over time.
Initially leaked in 2016 , results from that $25-million study provided the most compelling evidence yet that RF energy may be linked to cancer in lab rodents. The strongest finding connected RF with heart schwannomas in male rats, but the researchers also reported elevated rates of lymphoma as well as cancers affecting the prostate, skin, lung, liver and brain in the exposed animals. Rates for those cancers increased as the doses got higher but the evidence linking them with cell phone radiation specifically was weak by comparison, and the researchers could not rule out that they might have increased for reasons other than RF exposure. Paradoxically, the radiation-treated animals also lived longer than the nonexposed controls. The study results were reviewed by a panel of outside experts during a three-day meeting that ended on March 28. They concluded there was "clear evidence" linking RF radiation with heart schwannomas and "some evidence" linking it to gliomas of the brain. It is now up to the NTP to either accept or reject the reviewer's conclusions. A final report is expected within several months.
Limited to rats only, the Ramazzini study tested three doses expressed as the amount of radiation striking the animal’s bodies: either 5, 25 or 50 volts per meter. The exposure measures therefore differed from the absorbed doses calculated during the NTP study. But the Ramazzini scientists also converted their measures to W/kg, to show how the doses compared with RF limits for cell phones and cell towers set by the FCC and the International Commission on Non-Ionizing Radiation Protection; they ranged down to a 1,000 times lower. The exposures began when the rats were fetuses and continued for 19 hours a day until the animals died from natural causes.
As in the NTP study, Ramazzini investigators detected statistically elevated rates of heart schwannomas in male rats at the highest dose. They also had weaker findings linking RF exposure to cancer of glial cells in the brain, which were limited to females. Ronald Melnick, a retired NTP toxicologist who designed the NTP study, says a measure of consistency between the two studies is important, because “reproducibility in science increases our confidence in the observed results.”
Just why Schwann and glial cells appear to be targets of cell phone radiation is not clear. David Carpenter, a physician who directs the Institute for Health and the Environment at the University at Albany, S.U.N.Y., explained the purpose of these cells is to insulate nerve fibers throughout the body. These are electrical systems, so that may be some sort of factor, he wrote in an e-mail. “But this is only speculation.”
A few epidemiology studies have reported higher rates of tumors inside the skull among people who use cell phones heavily for 10 years or more. Of particular concern are benign Schwann cell tumors called acoustic neuromas, which affect nerve cells connecting the inner ear with structures inside the brain. These growths can in some instances progress to malignant cancer with time. But other studies have found no evidence of acoustic neuromas or brain tumors in heavy cell phone users.
Samet adds a major challenge now would be to draw a biologically relevant connection between acoustic neuromas and other glial tumors in the brains of humans with Schwann tumors in rat hearts. “The mechanism is uncertain,” he says. “There’s a lot of information we still need to fill in.”
Since 2011 RF radiation has been classified as a Group 2B “possible” human carcinogen by the International Agency on Cancer (IARC), an agency of the World Health Organization. Based on the new animal findings, and limited epidemiological evidence linking heavy and prolonged cell phone use with brain gliomas in humans, Fiorella Belpoggi, director of research at the Ramazzini Institute and the study’s lead author, says IARC should consider changing the RF radiation designation to a “probable” human carcinogen. Even if the hazard is low, billions of people are exposed, she says, alluding to the estimated number of wireless subscriptions worldwide. Véronique Terrasse, an IARC spokesperson, says a reevaluation may occur after the NTP delivers its final report.
Stephen Chanock, who directs the Division of Cancer Epidemiology and Genetics at the National Cancer Institute, remains skeptical, however. Cancer monitoring by the institute and other organizations has yet to show increasing numbers of brain tumors in the general population, he says. Tracking of benign brain tumors, such as acoustic neuromas, was initiated in 2004 by investigators at the institute’s Surveillance, Epidemiology and End Results program, which monitors and publishes statistics on cancer incidence rates. According to Chanock’s spokesperson, the acoustic neuroma data “haven’t accumulated to the point that we can say something meaningful about them.”
Asked if brain cancer’s long latency might explain why higher rates in the population have not appeared yet, Chanock says, “Cell phones have been around a long time. We are by no means dismissing the evidence, and the Ramazzini study raises interesting questions. But it has to be factored in with other reports, and this is still work in progress.”
Epidemiology studies investigating cell phone use patterns with human cancer risk have produced inconsistent results. Some studies enrolled people who already had tumors with suspected links to RF radiation, such as gliomas, acoustic neuromas and salivary gland tumors. Researchers compared the self-reported cell phone use habits of the cancer patients with those of other people who did not have the same diseases. Other studies enrolled people while they were still healthy, and then followed them over time to see if new cancer diagnoses tracked with how they used cell phones. All the epidemiology studies, however, have troubling limitations, including that enrolled subjects often do not report their cell phone use habits accurately on questionnaires.
In a February 2 statement, Jeffrey Shuren, director of the FDA’s Center for Devices and Radiological Health, wrote that despite the NTP study’s results, the combined evidence on RF exposure and human cancer—which by now amounts to hundreds of studies—has “given us confidence that the current safety limits for cell phone radiation remain acceptable for protecting the public health.” Chonock says that for him, evidence from the Ramazzini study does not alter that conclusion. “We continue to agree with the FDA statement,” he says.
Cell phones and cancer: New UC Berkeley study suggests cell phones sharply increase tumor risk

File of cell phone user. New UC Berkeley research draws link between cell phone use and increase risk of tumors. (Photo by Justin Sullivan/Getty Images).
BERKELEY, Calif. - New UC Berkeley research draws a strong link between cell phone radiation and tumors, particularly in the brain.
Researchers took a comprehensive look at statistical findings from 46 different studies around the globe and found that the use of a cell phone for more than 1,000 hours, or about 17 minutes a day over a ten year period, increased the risk of tumors by 60 percent.
Researchers also pointed to findings that showed cell phone use for 10 or more years doubled the risk of brain tumors.
Joel Moskowitz, director of the Center for Family and Community Health with the UC Berkeley School of Public Health conducted the research in partnership with Korea’s National Cancer Center, and Seoul National University. Their analysis took a comprehensive look at statistical findings from case control studies from 16 countries including the U.S., Sweden, United Kingdom, Japan, Korea, and New Zealand.
"Cell phone use highlights a host of public health issues and it has received little attention in the scientific community, unfortunately," said Moskowitz.
Cell phone use has increasingly become part of people’s daily lives, especially with the emergence of smartphones. Recent figures from the Pew Research Center showed that 97% of Americans now own a cell phone of some kind.
This, as more and more people have become dependent on their mobile phones as an integral mode of communication. In fact, an increasing number of people have ditched their landlines at home, relying on their cell phone as their sole device for telephone communication.
Figures from the Center for Disease Control and Prevention's National Center for Health Statistics found 61.8% of adults have decided to go wireless-only.
With the increased use of mobile devices, the research has been vast on their potential link to cancer. The findings have varied and at times been controversial.
Many studies looking into the health risks of cell phone use have been funded or partially funded by the cellular phone industry, which critics argue can skew research results.
"Moskowitz emphasized that these studies have been controversial as it is a highly sensitive political topic with significant economic ramifications for a powerful industry," Berkeley Public Health noted.
The position held by federal regulators point to a lack of evidence showing a direct link.
"To date, there is no consistent or credible scientific evidence of health problems caused by the exposure to radio frequency energy emitted by cell phones," the Food and Drug Administration stated on its website.
The FDA also said that the Federal Communications Commission has set a limit on radio frequency energy that "remains acceptable for protecting the public health."
SEE ALSO: San Jose neighbors oppose 5G cell equipment installed feet from homes
UC Berkeley researchers noted that in 2017, California regulators alerted the public of potential health risks related to cell phone use, although some felt the warning did not go far enough.
In its alert, the California Department of Public Health said, "Although the science is still evolving, some laboratory experiments and human health studies have suggested the possibility that long-term, high use of cell phones may be linked to certain types of cancer and other health effects."
The agency also provided advice on how to reduce exposure, including keeping phones away from your body and carrying devices in a backpack, briefcase, or purse. Health experts said cell phones should not be held in a pocket, bra, or belt holster, as a phone’s antenna tries to stay connected with a cell tower whenever it’s on, emitting radio frequency (RF) energy even when not in use.

A view of cellular communication towers in Emeryville, California. (Photo by Justin Sullivan/Getty Images)
Experts also suggested when not in use, putting the phone in airplane mode, which turns off cellular, Wi-Fi, and Bluetooth.
When on a call, experts advised avoid holding the phone up to your head and instead use the speaker feature or a headset.
Experts also said you should reduce or avoid use of your phone when there’s only one or two bars displayed showing the strength of connectivity. "Cell phones put out more RF energy to connect with cell towers when the signal is weak," health officials noted.
That’s also true when using a mobile device in a fast-moving car, bus, or train because the phone emits more RF energy to maintain connections to avoid dropping calls as it switches connections from cell tower to cell tower.
Ultimately, when it comes to cell phones, "distance is your friend," Moskowitz said. "Keeping your cellphone 10 inches away from your body, as compared to one-tenth of an inch, results in a 10,000-fold reduction in exposure. So, keep your phone away from your head and body," he advised.
SIGN UP FOR THE KTVU NEWSLETTER
Mobile phones have been around for decades, becoming widely accessible to the mainstream public in the 1980's. And as more people spend more time on the devices, researchers warned that could increase the risk of health problems related to their use. The study called for further in-depth research using exact data on the time spent on cell phones to confirm the latest findings.
Moskowitz, who has been researching and writing about the dangers of radiation from cell phones and cell towers for more than a decade, said publication of his findings have consistently led to increased calls for continued research. "…as soon as those stories went public in the media," he said, "I was contacted from survivors of cell phone radiation begging me to stay on this topic."
This latest study has been published in the International Journal of Environmental Research and Public Health .
- Search Menu
- Sign in through your institution
- Advance articles
- Author Guidelines
- Submission Site
- Self-Archiving Policy
- Why publish with us?
- About Psychoradiology
- About West China Hospital
- Editorial Board
- Advertising & Corporate Services
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Smartphone (over-)use and brain structure (structural mri studies), smartphone (over-)use and functional mri, summary of the current status and a roadmap for future research, conclusions, conflict of interest, acknowledgments and funding.
- < Previous
Neuroimaging the effects of smartphone (over-)use on brain function and structure—a review on the current state of MRI-based findings and a roadmap for future research
- Article contents
- Figures & tables
- Supplementary Data
Christian Montag, Benjamin Becker, Neuroimaging the effects of smartphone (over-)use on brain function and structure—a review on the current state of MRI-based findings and a roadmap for future research, Psychoradiology , Volume 3, 2023, kkad001, https://doi.org/10.1093/psyrad/kkad001
- Permissions Icon Permissions
The smartphone represents a transformative device that dramatically changed our daily lives, including how we communicate, work, entertain ourselves, and navigate through unknown territory. Given its ubiquitous availability and impact on nearly every aspect of our lives, debates on the potential impact of smartphone (over-)use on the brain and whether smartphone use can be “addictive” have increased over the last years. Several studies have used magnetic resonance imaging to characterize associations between individual differences in excessive smartphone use and variations in brain structure or function. Therefore, it is an opportune time to summarize and critically reflect on the available studies. Following this overview, we present a roadmap for future research to improve our understanding of how excessive smartphone use can affect the brain, mental health, and cognitive and affective functions.
At the time of writing, more than six billion smartphone subscriptions have been estimated for the year 2022 (Statista, 2022 ). This tremendously high number reflects that over the last 15 years—since the inception of the iPhone in 2007 (Macedonia, 2007 )—a global mobile digital revolution happened leading to ubiquitously and permanently available smartphone technologies around the world. Smartphones enable us to find our way in unknown territory, to initiate and maintain social communications, to join and provide content for social networks, and find information on whatever we are interested in as long a network signal is available. Due to the numerous applications in occupational and recreational contexts the smartphone has attracted many users around the globe. The gigantic rise of social media over the last 15 years is highly interwoven with the digital mobile revolution (Jurgenson, 2012 ; Korolija, 2020 ).
Adverse consequences of excessive smartphone use
Despite the many obvious improvements in our daily life by the smartphone, the scientific and public debates have drawn attention toward potential detrimental effects of smartphone (over-)use on different levels ranging from the societal to the individual level. Initial evidence suggests negative effects of excessive smartphone use on cognitive and affective domains, such that excessive smartphone use has being linked to lower productivity (Duke & Montag, 2017 ), lower learning outcomes/academic achievements (Sapci et al ., 2021 ; Sunday et al ., 2021 ), and elevated levels of negative emotionality (Elhai et al ., 2019 ; Montag et al ., 2016 ). Moreover, being distracted by the smartphone in the traffic represents a considerable danger on the road (Jannusch et al ., 2021 ; Rosenthal et al ., 2022 ). Although the cross-sectional nature of many of the available studies does not allow to make causal interpretations with respect to the associations—as it is, for example, also conceivable that higher negative emotionality renders individuals at an increased risk to develop escalating smartphone use—the reported associations between excessive smartphone use and adverse outcomes led to scientific and societal debates around the globe. Most of the research endeavors to date have been trying to understand how the digital revolution and the ubiquitous use of digital platforms may affect mental health, daily life functioning, social interactions, and also the brain (Firth et al ., 2019 ; Montag & Diefenbach, 2018 ). In the context of smartphone use, much research has focused in recent years on the question of how (excessive) smartphone use is linked to altered cognition (Liebherr et al ., 2020 ) and whether excessive smartphone use resembles an addictive behavior.
Debate on the medium versus function of smartphones
One critical debate concerns the differentiation of the medium versus function in the discussion on “smartphone addiction.” From the perspective of substance addiction, it would clearly be misleading to refer to being addicted to bottles, but reasonably the content of the bottle matters (Kuss & Griffiths, 2017 ; Panova & Carbonell, 2018 ). Applying this example to the area of excessive smartphone use or “smartphone addiction,” one would need to disentangle which specific contents the smartphone transmits and ultimately which specific needs these contents fulfill. Initial evidence suggests that drivers of excessive smartphone use likely are social media applications (Montag, 2021 ; Rozgonjuk et al ., 2020 ; Sha et al ., 2019 ) promoted through highly immersive app designs (Montag, Lachmann, et al ., 2019 ). Of note, many social media apps need to be considered in the context of the so-called data business model, which aims to prolong online time to enlarge the digital footprints of their users (Montag & Hegelich, 2020 ). Other drivers of excessive smartphone use might be video games on the phone (Leung et al ., 2020 ) and other functions such as unregulated access to work-related e-mails (Sadeghi et al ., 2022 ), which in turn might lower well-being (Kushlev & Dunn, 2015 ). In the context of ongoing debates about the conceptualization of excessive and potentially harmful smartphone use a very recent systematic meta-analysis reported global prevalence rates as high as 26.99% (Meng et al ., 2022 ) and another work mentioned an increase of “smartphone addiction” around the world (Olson et al ., 2022 ). Interestingly, the work by Meng et al . ( 2022 ) observed that prevalence rates of smartphone addiction are particularly high among adults (26.84%), followed by adolescents (21.62%), and children (15.19%). Males and females did not differ significantly. For a review on antecedents and consequences of smartphone addiction, see a recent overview by Busch & McCarthy ( 2021 ).
Is the actual nature of excessive smartphone use “addictive”?
In addition to the need for a more detailed examination with respect to which specific contents on smartphones drive excessive use, a large and growing number of studies (primarily employing self-report questionnaires) has examined excessive smartphone behavior within an addiction framework. Within the addiction framework, one would hypothesize that excessive—and ultimately problematic—use of the smartphone would be characterized by symptoms such as loss of control and preoccupation with use, reflected in, for example, unsuccessful attempts to reduce use or continued use despite negative consequences, as well as social problems and functional impairments in daily life—which represent key diagnostic symptoms of both substance and behavioral addictions (e.g. see also the new Gaming Disorder criteria by the WHO; Montag, Schivinski et al ., 2021 ). Of note, the duration of use, so spending a considerable amount of time on the smartphone, is not a distinct criterion per se. On the one hand, not everyone who spends much time on the smartphone shows problematic smartphone use behavior (for instance, the time could reflect constant job-related smartphone use); however, on the other hand, those using the smartphone in an “addictive” way will inherently spend much of their time on the smartphone. The most prominent self-report measures to assess problematic use of the smartphone are currently perhaps Kwon's Smartphone Addiction Scale (SAS) (Kwon et al ., 2013 ) and the Korean Smartphone Addiction Proneness Scale (SAPS) (D. Kim et al ., 2014 ). This notion is also supported by their use in many of the brain imaging studies discussed in the present review.
While the introduced scales and several of the current studies refer to smartphone addiction some researchers argue that the term “addiction” should not be simply transferred into the context of smartphone use (Panova & Carbonell, 2018 ) or propose that the term should be abandoned (Carbonell et al ., 2022 ) “to refocus clinical and research efforts on real disorders” (p. 2). Further, many researchers refer in the context of smartphone addiction research to problematic smartphone use as a more neutral term (Elhai et al ., 2017 ; Fischer-Grote et al ., 2019 ), while others prefer terms such as Smartphone Use Disorder (SmUD) to align the terminology with substance addictions and addictive behaviors in the ICD-11 of the World Health Organization (Gao, Jia, et al ., 2020 ; Gao, Sun, et al ., 2020 ). We currently consider the term SmUD as most suitable as it aligns well with the WHO terminology of Gaming Disorder in the realm of addictive behaviors (Marengo et al ., 2020 ; Montag et al ., 2021 ), but explicitly state that SmUD (or the other terms used) are not officially recognized at the moment and in many studies at best tendencies toward SmUD are investigated. Moreover, we have iterated already on the many problem areas when using terms such as smartphone addiction or SmUD. It is important to not overpathologize everyday behavior, and this is what needs to be kept in mind in this research field (Billieux et al ., 2015 ) until evidence from different areas comes up to understand the actual nature of excessive behavior (Brand et al ., 2020 )—here, excessive smartphone use. A final thought on the terminology: the umbrella term “Smartphone Use Disorder”—instead of focusing exclusively on excessive use of single app groups such as game apps or social-media apps—might also be relevant, when people show problematic use behavior in several categories on their phones. In this case, the SmUD terminology can summarize the problematic behavior being observed across several apps on the smartphone.
Although research on the detrimental effects of smartphone (over-)use or SmUD has only recently begun, initial studies have employed neuroimaging techniques, in particular magnetic resonance imaging (MRI), to explore associations between excessive smartphone use and individual variations in brain structure and function. These variations may reflect adaptations that neurally underlie excessive use or mediate the association between problematic smartphone use and affective, cognitive, and behavioral dysregulations. The initial yet rapidly growing literature has used different MRI-based approaches to examine variations in the structural organization of the brain using e.g. voxel-based morphometry (VBM) or diffusion tensor imaging (DTI), the functional organization of the brain using resting state functional MRI (fMRI) techniques, and functional alterations during cognitive and affective processes, using task-based fMRI (Ahn et al ., 2021 ; Arató et al ., 2023 ; Cho et al ., 2021 ; Choi et al ., 2021 ; Chun et al ., 2017 , 2018 ; Han & Kim, 2022 ; Hirjak et al ., 2022 ; Horvath et al ., 2020 ; Hu et al ., 2017 ; Kwon et al ., 2022 ; Lee et al ., 2019 ; D. Liu et al ., 2022 ; Lou et al ., 2019 ; Paik et al ., 2019 ; Pyeon et al ., 2021 ; Rashid et al ., 2021 ; Schmitgen et al ., 2020 , 2022 ; Tymofiyeva et al ., 2020 ; Wang et al ., 2016 ; Yoo et al ., 2021 ; Zou et al ., 2021 , 2022 ). Please note that the papers cited here will be reviewed in the next section of the present work (for a summary, see Tables 1 and 2 ; see also an overview in Fig. 1 ).

Structural and functional MRI studies on SmUD tendencies (also known as smartphone addiction or problematic smartphone use) as reviewed in the present work.
Findings from structural MRI studies on smartphone (over-)use (in alphabetic order following the surname of the first author); gray shaded boxes represent DTI studies, the Wang et al . ( 2016 ) and Zou et al . ( 2021 ) studies in the table include both a VBM and DTI approach.
GMV = gray matter volume, SPA = smartphone addiction, PSU = problematic smartphone use (smartphone use disorder tendencies), SPAI = Smartphone Addiction Inventory, TBSS = tract-based spatial statistics, SAS-SV = SAS-Short Version, MPAI = Mobile Phone Addiction Index, MPD = Mobile Phone Dependent group.
Findings from functional MRI studies on smartphone (over-)use (in alphabetic order following the surname of the first author); gray colored parts of the table represent task-based fMRI studies, the remaining studies represent resting state fMRI studies.
Meanwhile >20 studies on MRI-neuroimaging and excessive smartphone use or SmUD have been published and the number has strongly increased since 2020. Within this context, it is an opportune time for a review that (i) critically reflects on where the field stands and how strong the evidence is for smartphone-use associated brain changes, and (ii) provides a roadmap that outlines critical issues in the field and next steps that can help to shed light on the cognitive, affective, and neurobiological basis of smartphone (over-)use.
Neuroimaging of SmUD
The last few years have seen a strong increase in studies investigating individual differences in SmUD and associated brain variations by means of MRI. As depicted in Fig. 1 , the studies encompass structural MRI focusing on use-associated variations in gray and white matter as well as functional MRI studies examining associations with the intrinsic functional organization of the brain or during engagement in cognitive and affective tasks.
Associations between SmUD and the structural organization of the brain have been examined on the level of gray and white matter. Further, different methodological strategies including the use of individual differences association designs (e.g. examining linear relationships between the level of SmUD and brain structural variations) as well as between group designs aiming to examine brain structural differences between groups of individuals with high and low SmUD levels have been implemented. Differences in the gray matter organization of the brain are commonly examined by means of voxel based morphometry of T1 images [Ashburner & Friston ( 2000 ); for recent methodological aspects see the work by Zhou et al . ( 2022 ) and for information on cortical thickness or cortical folding patterns the work by Chen et al . ( 2013 ); additional insights can be derived from the work by Jiang et al . ( 2022 )]. Investigations on the level of the white matter tract organization are for instance examined using DTI (and the application of tract based spatial statistics: Bach et al ., 2014 ). While structural brain imaging provides insights into the structural brain architecture, fMRI is applied to study the intrinsic functional organization of the brain (resting state fMRI) or the engagement of specific brain regions during cognitive or affective task paradigms (task-based fMRI). To better understand individual differences in SmUD tendencies both task-based fMRI and resting state fMRI have been applied. During task-based fMRI studies, the individuals engage into specific cognitive or affective processes of interest, e.g. viewing a smartphone stimulus that can trigger "cue-reactivity." Cue-reactivity is a process during which a stimulus that is frequently paired with the addictive substance or the addictive behavior gains strong incentive salience (e.g. Yu et al ., 2020 ; X. Zhou et al ., 2019 ). In contrast, resting state fMRI aims to gain insights into the intrinsic functional architecture of the brain ("at rest") while the participants do not engage in a specific mental operation ("do not think of something in particular") (Gonzalez-Castillo et al ., 2021 ; Markett et al ., 2018 ).
The structural and functional MRI-approaches have been extensively used—either separately or in combination—to determine the brain basis of substance-related and (established) behavioral addictions (for quantitative and qualitative reviews, see also Klugah-Brown et al ., 2021 ; Taebi et al ., 2022 ; Tolomeo & Yu, 2022 ; and Zilverstand et al ., 2018 for examples). The combination of the different imaging approaches can allow a more holistic evaluation at different levels and allow to examine different research questions with respect to potential addiction-related changes. The present review aims to provide a brief overview on the smartphone-(over-)use MRI literature, and is divided in both structural and functional MRI sections summarizing the results of the current literature (Fig. 1 ). Next, the review presents a roadmap for future studies in the field of SmUD and associated brain changes.
As becomes apparent in Table 1 , several studies examined differences in gray matter brain volumes in the context of SmUD tendencies. Overall deriving a consistent picture of SmUD tendency associated variations in brain structure is currently limited by the use of varying SmUD assessments in these studies. Moreover, differences in MRI analysis strategy may further limit direct comparisons. In this context, recent studies have shown that the specific brain structural variations that are identified may strongly depend on the choice of processing pipeline (see Zhou et al ., 2022 for a methodological evaluation of the effects of choice of processing pipeline on brain structural analyses). Moreover, some of the identified studies focused in their analysis on specific hypothesis-driven brain regions such as the brain stem (Cho et al ., 2021 ) or specifically on striatal morphology (Yoo et al ., 2021 ), whereas other studies used whole-brain analytic approaches (Horvath et al ., 2020 ; Rashid et al ., 2021 ). Further complicating matters is the use of different rigor in the brain analysis and in the level of description of the analyses used, such that for several studies the exact multiple comparisons approach used remains unclear.
An initial overview of the reported associations between brain volume and SmUD tendencies mostly suggests an association of inverse nature (such that higher SmUD tendencies associated with lower volumes in specific brain regions, e.g. Wang et al ., 2016 ; Zou et al ., 2021 ). Accordingly, between-group designs comparing participants with high versus low SmUD often revealed decreased regional brain volumes in the group of excessive smartphone users compared to control individuals (e.g. Lee et al ., 2019 ; Yoo et al ., 2021 ). With respect to the volumetric gray matter approach, studies reported lower gray matter volume in regions such as the anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), fusiform gyrus, parahippocampal regions, and the striatum (caudate). These regions partly resemble regions that have been identified in previous works examining brain volumetric alterations in substance and behavioral addictions (e.g. see the following studies: Klugah-Brown et al ., 2021 ; Koester et al ., 2012 ; Qin et al ., 2020 ; Yu et al ., 2022 ; Zhang et al ., 2021 ). However, none of the regions consistently replicated across SmUD studies and, together with the lack of standardized SmUD assessments and brain structural analyses strategies, the previous studies currently do not allow to draw clear conclusions with respect to the specific regions that might show brain structural alterations associated with excessive smartphone use. While the lack of consistently reported regions and the methodological limitations do not allow a clear interpretation of the underlying brain structural variations, previous studies in behavioral addictions have associated volumetric decreases in some of the mentioned regions with, for example, the severity of problematic online gaming or social media engagement (Montag et al ., 2018 ; F. Zhou et al ., 2019 ) or higher impulsivity in cocaine dependent individuals (Moreno-López et al ., 2012 ), which may suggest an association with key features of addiction. For details on the specific regions reported in the SmUD studies, please see also Table 1 .
Although the reviewed number of structural studies - from our perspective - is currently too small and heterogenous to support an overarching picture, we further refer to studies that examined either white matter tract integrity (Hu et al ., 2017 ; Tymofiyeva et al ., 2020 ; Zou et al ., 2021 ) or variations in cortical folding (Hirjak et al ., 2022 ) in the context of SmUD tendencies.
Table 2 shows studies investigating the neural correlates of SmUD tendencies using fMRI. Most previous studies employed a resting state fMRI approach examining associations between SmUD tendencies and the intrinsic functional organization of the brain (Ahn et al ., 2021 ; Chun et al ., 2018 ; Horvath et al ., 2020 ; Kwon et al ., 2022 ; D. Liu et al ., 2022 ; Lou et al ., 2019 ; Paik et al ., 2019 ; Pyeon et al ., 2021 ; Rashid et al ., 2021 ; Schmitgen et al ., 2022 ; Zou et al ., 2022 ). A direct comparison between the studies is hindered by differences in the methodological approaches, ranging from different preprocessing methods to different network analytic strategies. The studies reported potential associations between SmUD tendencies and variations in the intrinsic architecture of a number of brain systems and large-scale networks, including for instance altered connectivity of striatal, limbic, and frontal regions (e.g. Pyeon et al ., 2021 ; Zou et al ., 2022 ; Chun et al ., 2018 ; Paik et al ., 2019 ), as well as altered functional interaction between and within large scale networks including the default mode network and the salience network (Ahn et al ., 2021 ; Kwon et al ., 2022 ). While the different approaches employed in these studies and the methodological limitations prevent clear conclusions at the present stage, the identified pathways partly overlap with the intrinsic pathways and networks that have been identified in substance and behavioral addictions (Zhou et al ., 2018 ; Taebi et al ., 2022 ; Tolomeo & Yu, 2022 ; Yan et al ., 2021 ; Zimmermann et al ., 2018 ). Within the context of the previous literature on the role of dysregulations in the intrinsic functional organization of the brain in addiction, it is conceivable that alterations in specific systems may promote different symptomatic features. Alterations in salience and executive control systems may, for instance, underly dysregulations in several affective and cognitive domains, while alterations in the striato-frontal organization may reflect the development of compulsive behavior and alterations in the default mode network may promote dysfunctional self-related decision-making (Taebi et al ., 2022 ; Tolomeo & Yu, 2022 ; Yan et al ., 2021 ; Yu et al ., 2022 ; Zhang & Volkow, 2019 ; X. Zhou et al ., 2019 ; Zimmermann et al ., 2018 ). However, clear conclusions with respect to consistent and robust effects of excessive smartphone use or SmUD on the functional architecture of the brain remain to be determined.
Five studies investigated the neural basis of altered cognitive and affective behaviors related to SmUD tendencies by means of task-based functional MRI (Arató et al ., 2023 ; Choi et al ., 2021 ; Chun et al ., 2017 ; Han & Kim, 2022 ; Schmitgen et al ., 2020 ). An early study by Chun et al . ( 2017 ) examined facial emotion processing alterations in participants with high SmUD versus participants with low SmUD and found that the high SmUD group displayed decreased activiation in the dorsolateral prefrontal cortex and dorsal ACC during the presentation of angry faces. Schmitgen et al . ( 2020 ) used a cue reactivity paradigm during which participants were presented with smartphone or neutral images and reported group differences between participants with high and low SmUD tendencies in several regions including anterior cingulate, medial prefrontal, and temporal regions. Choi et al . ( 2021 ) used a modified version of a cognitive conflict task and reported that participants with high SmUD exhibited lower task performance accompanied by enhanced recruitment of fronto-parietal regions. Han and Kim ( 2022 ) used a modified oddball task in participants with high versus low risk for SmUD and observed attention filtering impairments and a lower engagement of the frontopolar cortex in participants at high risk for SmUD. The most current study by Arató et al . ( 2023 ) applied a facial emotion recognition paradigm, where higher scores in the smartphone application-based addiction scale were associated with higher functional connectivity among brain regions related to emotional/cognitive control. While these findings may reflect that (social) cognitive and addiction-related changes in SmUD are accompanied by changes in corresponding brain systems, the low number of available studies and some other methodological limitations, such as the comparably small samples and lack of replication designs, do not allow to draw conclusions at present with respect to robust task-based brain functional alterations related to excessive smartphone use. Please note that two studies are not listed in Table 2 as they did not investigate SmUD tendencies, but either directly investigated links between objective smartphone use measures and resting state fMRI (Huckins et al ., 2019 ) or applied a general screen time self-report measure/time spent on reading to investigate functional connectivity in children (Horowitz-Kraus & Hutton, 2018 ).
From the literature review, it becomes apparent that although the smartphone technology has now been available for over 15 years and the detrimental consequences of excessive smartphone use have been increasingly debated, the present knowledge about how smartphone use affects our neurobiology or is linked to variations in brain structure and function still is very limited. The available literature—although growing—does currently not allow us to draw firm conclusions with respect to potential effects of excessive smartphone use on the brain. This is partly because no consensus exists on which inventories to best use to assess smartphone (over-)use and a lack of studies including “objective” tracked smartphone data in the available MRI literature (see exceptions in studies such as those by Huckins et al ., 2019 and Montag et al ., 2017 ). While the conventional (neuroimaging) studies in this field employ a combination of self-report data for determining the severity or the extent of smartphone (over-)use with respect to symptoms or actual duration of use, “objective” in this context refers to tracked behavior on smartphones providing quantifiable and precise data of actual use, including, for example, how often a person checks the phone, what apps are used in particular, etc. (for a tracking app, see Montag, Baumeister, et al ., 2019 ). This approach is often described as digital phenotyping or mobile sensing in the literature (Baumeister & Montag, 2023 ). In this context, it is of importance to mention that SmUD tendencies not necessarily need to strongly overlap with time spent on the phone [“not everyone spending much time on the phone is addicted”; see also lack of association between fear of missing out (FOMO) with actual phone behavior (Rozgonjuk et al ., 2021 )]. With respect to the design of the studies, several of the studies cited here are underpowered for determining robust brain alterations in SmUD and some studies do not adhere to multiple correction procedures, which are the current standard in the field. Finally, the predominance of retrospective cross-sectional study designs limits the conclusions that can be drawn with respect to disentangling predisposing brain variations from effects that are directly linked to smartphone (over-)usage.
Nevertheless, the available literature suggests a potential association between smartphone (over-)use and variations in brain structure and function that may mediate cognitive and behavioral changes, as well as detrimental effects on mental health and probably even addictive usage. Going forward, it will be essential to apply strategies and experimental designs that have been evaluated in the context of other mental disorders to disentangle the potential impact of different factors and thus to describe potential smartphone (over-)use associated brain changes. In contrast to other fields of research on the neurobiological basis of addiction, i.e. substance addiction, animal models for SmUD have not been developed and it will be challenging—or even impossible—to develop corresponding mechanistic animal models. Within this context, neuroimaging potential brain changes in humans will be even more important as a strategy to determine the underlying neurobiological and potentially neuropathological pathways. Based on the present overview, we outline the following key questions and strategies as a roadmap on the way forward to determining brain changes associated with smartphone (over-)use.
SmUD (or smartphone addiction/problematic smartphone use) consists of many symptoms such as loss of control, functional impairments due to excessive use of the smartphone, and preoccupation with the smartphone, etc. Therefore, it is of importance to not only understand how overall SmUD scores are linked to brain structure and function, but also the different symptoms/facets. Initial studies have begun to determine separable and common brain alterations associated with different facets of general internet gaming behavior (e.g. Yu et al ., 2022 ). Within the SmUD context similar approaches may allow to better describe associations between specific symptomatic and behavioral dysregulations and associated brain changes. Moreover, we mention that several studies are hard to compare because different inventories to assess SmUD have been applied, as no agreement exists regarding a conceptual framework (but see Billieux's framework to understand problematic smartphone use; Billieux, 2012 ). Beyond this, it will be vital to disentangle brain changes that are specifically associated with SmUD and to separate these from other psychological processes that might be associated with or even inherently linked with SmUD. The construct fear of missing out (FOMO) for instance has gained increasing interest in the field of digital addictions (Elhai, Yang, Montag, et al ., 2020 ; Elhai, Yang, Rozgonjuk, et al ., 2020 ) and has been associated with individual variations in brain morphology (Wang et al ., 2022 ). Moreover, it will be critical to further separate brain changes related to specific or unspecific pathology relevant domains such as depression and anxiety—which have been related to brain structural and functional variations (X. Liu et al ., 2021 , 2022 ; Serra-Blasco et al ., 2021 ; Wise et al ., 2017 )—from variations that specifically associate with SmUD.
The literature search demonstrated (see also Fig. 1 ), that only few studies applied task-based fMRI methods in the field of SmUD. While resting state and brain structural approaches may allow to determine variations in the intrinsic architecture of the brain, task-based fMRI studies will further allow to determine the neural alterations that underlie dysregulations in domains that have been found to be disrupted across other addictive disorders. Promising underlying domains in this respect may be to examine whether: (a) smartphone-associated stimuli have gained an increased salience or even engage habit and compulsive use associated circuits during cue reactivity paradigms (for substance related addictions, see e.g. Vollstädt-Klein et al ., 2010 ; X. Zhou et al ., 2019 ; for behavioral addictions, see e.g. L. Liu et al ., 2017 ; for SmUD see Schmitgen et al ., 2020 ); (b) whether cognitive functions, in particular executive functions and the underlying fronto-parietal networks, show alterations in SmUD (for studies in other addictions please see Klugah-Brown et al ., 2021 ; Zheng et al ., 2019 ); and whether brain systems involved in (c) emotion and stress reactivity; or (d) natural reward processing are affected by SmUD (for studies in other addictions see e.g. Luijten et al ., 2017 ; J. Zhang et al ., 2020 ; Zhao et al., 2020 ).
Aside from self-reported SmUD tendencies, more studies need to correlate objective tracked smartphone use with brain data to add a further data layer to the neuroscientific study on smartphone use (Montag, Elhai, et al ., 2021b ). Meanwhile, it became clear that humans have problems in correctly assessing their technology use, in particular regarding the quantity of technology use (Parry et al ., 2021 ).
To our knowledge no study in the field investigated potential changes of the brain due to smartphone use in term of structure and function with repeated MRI measures. This will be of particular importance within prospective longitudinal designs that hold the promise to separate predisposing brain alterations that render participants at an increased risk of developing SmUD from effects that are rather a consequence of escalating smartphone use or develop in association with the transition to addictive use (for prospective longitudinal designs in substance addiction research, see also the following studies: Becker et al ., 2013 , 2015 ; Jager et al ., 2007 ). The implementation of prospective longitudinal designs would allow to draw stronger conclusions with respect to whether and how the smartphone technology affects human neurobiology (for comparable approaches in the field of behavioral addictions, see also previous studies: Gleich et al ., 2017 ; Kühn et al ., 2018 ; Yu et al ., 2020 ; Zhou et al ., 2019 ).
The scientific works available in the field usually study the different MRI sources in an independent fashion, hence they correlate the smartphone behavior or SmUD scores with the brain data without shedding light on what differences in structure mean for functionality of the brain when studying smartphone use. Bringing these different brain sources together in a meaningful fashion would open interesting research avenues.
As already mentioned, to understand how smartphone (over-)use affects human neurobiology, a closer look needs to be taken on what smartphone applications humans use in what intensity and in what context. A taxonomy of different smartphone use patterns will be needed to be taken into account to better grasp the nature of smartphone (over-)use and potential brain changes (Marengo et al ., 2021 ; Montag et al ., 2021 ). Generalized views on overusing the smartphone might be helpful to get a bird's eye view on the topic, but consuming different contents might lead to different results when one is trying to understand the neurobiology of smartphone (over-)use. See also exemplary research in related areas investigating general social media (over-)use, specific social media (over-)use or e-mail (over-)use (He et al ., 2017 ; Lee et al ., 2021 ; Montag et al ., 2017 , 2018 ; Nasser et al ., 2020 ; Sadeghi et al ., 2022 ; Sherman et al ., 2016 ; Turel et al ., 2014 , 2018 ), which at best would directly be also put in the context of smartphone (over-)use research (seldom done at the moment). We also mention highly interesting work investigating smartphone touchscreen use and the brain (Balerna & Ghosh, 2018 ; Gindrat et al ., 2015 ).
Finally, the present review showed that most studies (to our knowledge) focused on the study of SmUD or related topics by means of MRI. There is so much else to be studied in the context of smartphone use—which likely will result in the study of so called digital biomarkers (Montag, Elhai, et al ., 2021a ). By this, we mean that the digital footprints left on smartphones (and other devices of the Internet of Things) can help us to get insights into the neurobiology of a person (Montag, Elhai, et al ., 2021b ). Given that the smartphone is our companion with whom we interact in many everyday life situations, it is not surprising that the smartphone can provide a detailled characterization of behavioral, cognitive and affective domains and this could inform not only psychological but also neuroscientific approaches.
An increasing number of studies suggests sex differences in the brain correlates of addiction (see e.g. Grace et al ., 2021 ) and going forward it will be important to explore potential differences in the effects of smartphone (over-)use in men and women. Moreover, it will be important to determine the effects of smartphone (over-)use on the brain over the life span, it is e.g. conceivable that in particular developing brains are more sensitive to the impact of excessive usage (but see interesting opposing prevalence numbers as mentioned above; Meng et al ., 2022 ).
In terms of a general challenge of the MRI-based research field it will be vital to better address and enhance the replicability of MRI research (see e.g. Klugah-Brown et al . ( 2022 ) for an example of replicable brain structural markers in behavioral addictions), employing designs that extend the view of the traditional case-control designs in neuroimaging of mental disorders (Etkin, 2019 ), and implement transparent data and code sharing as well as preregistration of studies that allow an a priori specification of brain-based hypotheses (see also Nichols et al ., 2017 ; Poldrack et al ., 2017 ).
The present paper provides an initial overview on research examining potential brain changes related to smartphone (over-)use from studies applying MRI techniques (studies are of cross-sectional nature though). While many studies observed functional and structural differences and associations with SmUD in brain systems spanning cortical and subcortical regions involved in reward/motivational processes, affective and cognitive domains, and the development of addictive behavior, the current evidence remains patchy and overarching neuroscientific frameworks uniquely touching on smartphone (over-)use are lacking. The studies do not allow us to determine whether the observed brain characteristics are a result of a certain kind of smartphone use or merely represent a predisposition to use the smartphone in a certain kind of way. Many study findings are based on small study populations. In this context, prospective longitudinal designs and replication studies are needed soon to determine the direction of the associations and the robustness of the findings. Finally, MRI, although being a powerful tool to understand the human brain, comes with technical limitations—among others a limited temporal resolution. Therefore, multimodal assessments that integrate the advantages of different brain imaging methods are warranted. Within this context, we shortly hint toward already existing literature investigating the present smartphone use complex with means of electroencephalography (S.-K. Kim et al ., 2015 ; Weon, 2017 ), fNIRS (Li et al ., 2022 ; Xiang et al ., 2023 ) as well as positron emission tomography (Westbrook et al ., 2021 ). The combination of advanced prospective study designs with different neuroscientific techniques (also hormones and genetics) can promote a better and more complete understanding of the neurobiological changes related to smartphone (over-)use.
The author B.B. is editorial-board member of Psychoradiology . He was blinded from the review process and making decisions on the manuscript.
C.M. reports no conflict of interest. Nevertheless, for reasons of transparency, C.M. mentions that he has received (to Ulm University and earlier University of Bonn) grants from agencies such as the German Research Foundation (DFG). C.M. has performed grant reviews for several agencies; has edited journal sections and articles; has given academic lectures in clinical or scientific venues or companies; and has generated books or book chapters for publishers of mental health texts. For some of these activities he received royalties, but never from the gaming or social media industry. C.M. was part of a discussion circle (Digitalität und Verantwortung: https://about.fb.com/de/news/h/gespraechskreis-digitalitaet-und-verantwortung/ ) debating ethical questions linked to social media, digitalization, and society/democracy at Meta. In this context, he received no salary for his activities. C.M. currently functions as independent scientist on the scientific advisory board of the Nymphenburg group (Munich, Germany). This activity is financially compensated. Further, he is on the scientific advisory board of Applied Cognition (Redwood City, CA, USA), an activity that is also compensated.
The present study was partly supported by the China Brain Project (MOST2030, grant no. 2022ZD0208500), National Natural Science Foundation of China (grant no. NSFC 82271583; 32250610208) and the National Key Research and Development Program of China (grant no. 2018YFA0701400).
Ahn J , Lee D , Namkoong K et al. ( 2021 ) Altered functional connectivity of the salience network in problematic smartphone users . Front Psychiatry . 12 : 636730 .
Google Scholar
Arató Á , Nagy S , Perlaki G et al. ( 2023 ) Emotional face expression recognition in problematic internet use and excessive smartphone use: task-based fMRI study . Sci Rep . 13 : 354 .
Ashburner J , Friston KJ ( 2000 ) Voxel-based morphometry—the methods . Neuroimage . 11 : 805 – 21 .
Bach M , Laun FB , Leemans A et al. ( 2014 ) Methodological considerations on tract-based spatial statistics (TBSS) . Neuroimage . 100 : 358 – 69 .
Balerna M , Ghosh A ( 2018 ) The details of past actions on a smartphone touchscreen are reflected by intrinsic sensorimotor dynamics . NPJ Digital Med . 1 : Article 1 .
Baumeister H , Montag C ( 2023 ) Digital phenotyping and mobile sensing in psychoinformatics—a rapidly evolving interdisciplinary research endeavor . In Montag C. , Baumeister H. (Eds.), Digital Phenotyping and Mobile Sensing: New Developments in Psychoinformatics (pp. 1 – 9 .). Springer International Publishing .
Google Preview
Becker B , Wagner D , Koester P et al. ( 2013 ) Memory-related hippocampal functioning in ecstasy and amphetamine users: a prospective fMRI study . Psychopharmacology . 225 : 923 – 34 .
Becker B , Wagner D , Koester P et al. ( 2015 ) Smaller amygdala and medial prefrontal cortex predict escalating stimulant use . Brain . 138 : 2074 – 86 .
Billieux J ( 2012 ) Problematic use of the mobile phone: a literature review and a pathways model . Curr Psychiatry Rev . 8 : 299 – 307 .
Billieux J , Schimmenti A , Khazaal Y et al. ( 2015 ) Are we overpathologizing everyday life? A tenable blueprint for behavioral addiction research . J Behav Addict . 4 : 119 – 23 .
Brand M , Rumpf H-J , Demetrovics Z et al. ( 2020 ) Which conditions should be considered as disorders in the International Classification of Diseases (ICD-11) designation of “other specified disorders due to addictive behaviors”? . J Behav Addict . 11 : 150 – 9 .
Busch PA , McCarthy S ( 2021 ) Antecedents and consequences of problematic smartphone use: a systematic literature review of an emerging research area . Comput Hum Behav . 114 : 106414 .
Carbonell X , Panova T , Carmona A ( 2022 ) Commentary: editorial: significant influencing factors and effective interventions of mobile phone addiction . Front Psychol . 13 : 957163 .
Chen H , Zhang T , Guo L et al. ( 2013 ) Coevolution of gyral folding and structural connection patterns in primate brains . Cereb Cortex . 23 : 1208 – 17 .
Cho IH , Yoo JH , Chun J-W et al. ( 2021 ) Reduced volume of a brainstem substructure in adolescents with problematic smartphone use . J Korean Acad Child Adolesc Psychiatry . 32 : 137 – 43 .
Choi J , Cho H , Choi J-S et al. ( 2021 ) The neural basis underlying impaired attentional control in problematic smartphone users . Translational Psychiatry . 11 : 129 .
Chun J-W , Choi J , Cho H et al. ( 2018 ) Role of frontostriatal connectivity in adolescents with excessive smartphone use . Front Psychiatry . 9 : 437 .
Chun J-W , Choi J , Kim J-Y et al. ( 2017 ) Altered brain activity and the effect of personality traits in excessive smartphone use during facial emotion processing . Sci Rep . 7 : 12156 .
Duke É , Montag C ( 2017 ) Smartphone addiction, daily interruptions and self-reported productivity . Addict Behav Rep . 6 : 90 – 5 .
Elhai JD , Dvorak RD , Levine JC et al. ( 2017 ) Problematic smartphone use: a conceptual overview and systematic review of relations with anxiety and depression psychopathology . J Affect Disord . 207 : 251 – 9 .
Elhai JD , Yang H , Montag C ( 2019 ) Cognitive- and emotion-related dysfunctional coping processes: transdiagnostic mechanisms explaining depression and Anxiety's relations with problematic smartphone use . Curr Addict Rep . 6 : 410 – 7 .
Elhai JD , Yang H , Montag C et al. ( 2020 ) Fear of missing out (FOMO): overview, theoretical underpinnings, and literature review on relations with severity of negative affectivity and problematic technology use . Braz J Psychiatry, AHEAD . 43 : 203 – 9 .
Elhai JD , Yang H , Rozgonjuk D et al. ( 2020 ) Using machine learning to model problematic smartphone use severity: the significant role of fear of missing out . Addict Behav . 103 : 106261 .
Etkin A ( 2019 ) A reckoning and research agenda for neuroimaging in psychiatry . Am J Psychiatry . 176 : 507 – 11 .
Firth J , Torous J , Stubbs B et al. ( 2019 ) The “online brain”: how the internet may be changing our cognition . World Psychiatry . 18 : 119 – 29 .
Fischer-Grote L , Kothgassner OD , Felnhofer A ( 2019 ) Risk factors for problematic smartphone use in children and adolescents: a review of existing literature . Neuropsychiatrie . 33 : 179 – 90 .
Gao Q , Jia G , Fu E et al. ( 2020 ) A configurational investigation of smartphone use disorder among adolescents in three educational levels . Addict Behav . 103 : 106231 .
Gao Q , Sun R , Fu E et al. ( 2020 ) Parent–child relationship and smartphone use disorder among Chinese adolescents: the mediating role of quality of life and the moderating role of educational level . Addict Behav . 101 : 106065 .
Gindrat A-D , Chytiris M , Balerna M et al. ( 2015 ) Use-dependent cortical processing from fingertips in touchscreen phone users . Curr Biol . 25 : 109 – 16 .
Gleich T , Lorenz RC , Gallinat J et al. ( 2017 ) Functional changes in the reward circuit in response to gaming-related cues after training with a commercial video game . Neuroimage . 152 : 467 – 75 .
Gonzalez-Castillo J , Kam JWY , Hoy CW et al. ( 2021 ) How to interpret resting-state fMRI: ask your participants . J Neurosci . 41 : 1130 – 41 .
Grace S , Rossetti MG , Allen N et al. ( 2021 ) Sex differences in the neuroanatomy of alcohol dependence: hippocampus and amygdala subregions in a sample of 966 people from the ENIGMA Addiction Working Group . Transl Psychiatry . 11 : 156 .
Han SW , Kim CH ( 2022 ) Neurocognitive mechanisms underlying internet/smartphone addiction: a preliminary fMRI study . Tomography . 8 : 1781 – 90 .
He Q , Turel O , Brevers D et al. ( 2017 ) Excess social media use in normal populations is associated with amygdala-striatal but not with prefrontal morphology . Psychiatry Res Neuroimaging . 269 : 31 – 5 .
Hirjak D , Henemann GM , Schmitgen MM et al. ( 2022 ) Cortical surface variation in individuals with excessive smartphone use . Dev Neurobiol . 82 : 277 – 87 .
Horowitz-Kraus T , Hutton JS ( 2018 ) Brain connectivity in children is increased by the time they spend reading books and decreased by the length of exposure to screen-based media . Acta Paediatr . 107 : 685 – 93 .
Horvath J , Mundinger C , Schmitgen MM et al. ( 2020 ) Structural and functional correlates of smartphone addiction . Addict Behav . 105 : 106334 .
Hu Y , Long X , Lyu H et al. ( 2017 ) Alterations in white matter integrity in young adults with smartphone dependence . Front Hum Neurosci . 11 : 532 .
Huckins JF , daSilva AW , Wang R et al. ( 2019 ) Fusing mobile phone sensing and brain imaging to assess depression in college students . Front Neurosci . 13 : 248 .
Jager G , de Win MM , Vervaeke HK et al. ( 2007 ) Incidental use of ecstasy: no evidence for harmful effects on cognitive brain function in a prospective fMRI study . Psychopharmacology . 193 : 403 – 14 .
Jannusch T , Shannon D , Völler M et al. ( 2021 ) Smartphone use while driving: an investigation of young novice driver (YND) behaviour . Transp Res F Traffic Psychol Behav . 77 : 209 – 20 .
Jiang M , Chen Y , Yan J et al. ( 2022 ) Anatomy-guided spatio-temporal graph convolutional networks (AG-STGCNs) for modeling functional connectivity between gyri and sulci across multiple task domains . IEEE Trans Neural Network Learning Syst , 1 – 11 . PP .
Jurgenson N ( 2012 ) When atoms meet bits: social Media, the mobile web and augmented revolution . Future Internet . 4 : Article 1 .
Kim D , Lee Y , Lee J et al. ( 2014 ) Development of Korean smartphone addiction proneness scale for youth . PLoS ONE . 9 : e97920 .
Kim S-K , Kim S-Y , Kang H-B ( 2015 ) Comparison of EEG during watching emotional videos according to the degree of smartphone addiction . J Korea Multimedia Soc . 18 : 599 – 609 .
Klugah-Brown B , Zhou X , Pradhan BK et al. ( 2021 ) Common neurofunctional dysregulations characterize obsessive–compulsive, substance use, and gaming disorders—An activation likelihood meta-analysis of functional imaging studies . Addict Biol . 26 : e12997 .
Klugah-Brown B , Zhou X , Wang L et al. ( 2022 ) Associations between levels of Internet Gaming Disorder symptoms and striatal morphology replicate and may mediate the effects on elevated social anxiety . Psychoradiology . 2 : 207 – 15 .
Koester P , Tittgemeyer M , Wagner D et al. ( 2012 ) Cortical thinning in amphetamine-type stimulant users . Neuroscience . 221 : 182 – 92 .
Korolija D ( 2020 ) Why iPhones and Social Media Go Hand in Hand in the United States . https://streetsignals.com/iphone-social-media-united-states/ (accessed on 24th February 2023) .
Kühn S , Kugler D , Schmalen K et al. ( 2018 ) The myth of blunted gamers: no evidence for desensitization in empathy for pain after a violent video game intervention in a longitudinal fMRI study on non-gamers . Neurosignals . 26 : 22 – 30 .
Kushlev K , Dunn EW ( 2015 ) Checking email less frequently reduces stress . Comput Hum Behav . 43 : 220 – 8 .
Kuss DJ , Griffiths MD ( 2017 ) Social networking sites and addiction: ten lessons learned . Int J Environ Res Public Health . 14 : Article 3 .
Kwon M , Jung Y-C , Lee D et al. ( 2022 ) Altered resting-state functional connectivity of the dorsal anterior cingulate cortex with intrinsic brain networks in male problematic smartphone users . Front Psychiatry . 13 : 1008557 .
Kwon M , Kim D-J , Cho H et al. ( 2013 ) The Smartphone Addiction Scale: development and validation of a short version for adolescents . PLoS ONE . 8 : e83558 .
Lee D , Lee J , Namkoong K et al. ( 2021 ) Altered functional connectivity of the dorsal attention network among problematic social network users . Addict Behav . 116 : 106823 .
Lee D , Namkoong K , Lee J et al. ( 2019 ) Lateral orbitofrontal gray matter abnormalities in subjects with problematic smartphone use . J Behav Addict . 8 : 404 – 11 .
Leung H , Pakpour AH , Strong C et al. ( 2020 ) Measurement invariance across young adults from Hong Kong and Taiwan among three internet-related addiction scales: bergen Social Media Addiction Scale (BSMAS), Smartphone application-based Addiction Scale (SABAS), and Internet Gaming Disorder Scale-Short Form (IGDS-SF9) (Study Part A) . Addict Behav . 101 : 105969 .
Li X , Li Y , Wang X et al. ( 2022 ) Reduced brain activity and functional connectivity during creative idea generation in individuals with smartphone addiction . Soc Cogn Affect Neurosci . 18 : nsac052 . https://doi.org/10.1093/scan/nsac052
Liebherr M , Schubert P , Antons S et al. ( 2020 ) Smartphones and attention, curse or blessing? - A review on the effects of smartphone usage on attention, inhibition, and working memory . Comput Hum Behav Rep . 1 : 100005 .
Liu D , Liu X , Long Y et al. ( 2022 ) Problematic smartphone use is associated with differences in static and dynamic brain functional connectivity in young adults . Front Neurosci . 16 : 1010488 .
Liu L , Yip SW , Zhang J-T et al. ( 2017 ) Activation of the ventral and dorsal striatum during cue reactivity in Internet gaming disorder . Addict Biol . 22 : 791 – 801 .
Liu X , Klugah-Brown B , Zhang R et al. ( 2022 ) Pathological fear, anxiety and negative affect exhibit distinct neurostructural signatures: evidence from psychiatric neuroimaging meta-analysis . Transl Psychiatry . 12 : 405 .
Liu X , Lai H , Li J et al. ( 2021 ) Gray matter structures associated with neuroticism: a meta-analysis of whole-brain voxel-based morphometry studies . Hum Brain Mapp . 42 : 2706 – 21 .
Lou J-H , Zhang Z-Z , Guan M et al. ( 2019 ) Altered default-mode network functional connectivity in college students with mobile phone addiction . Int J Clin Exp Med . 12 : 1877 – 87 .
Luijten M , Schellekens AF , Kühn S et al. ( 2017 ) Disruption of reward processing in addiction: an image-based meta-analysis of functional magnetic resonance imaging studies . JAMA Psychiatry . 74 : 387 – 98 .
Macedonia M ( 2007 ) IPhones target the tech elite . Computer . 40 : 94 – 5 .
Marengo D , Sariyska R , Schmitt HS et al. ( 2021 ) Objective recordings of smartphone and instant messaging and social network app usage are associated with self-reported tendencies towards smartphone use disorder: the distinctive role of image-based apps (Preprint) . J Med Internet Res . 9 : e27093 .
Marengo D , Sindermann C , Häckel D et al. ( 2020 ) The association between the Big five personality traits and smartphone use disorder: a meta-analysis . J Behav Addict . 9 : 534 – 50 .
Markett S , Montag C , Reuter M ( 2018 ) Network neuroscience and personality . Personal Neurosci . 1 : E14 .
Meng S-Q , Cheng J-L , Li Y-Y et al. ( 2022 ) Global prevalence of digital addiction in general population: a systematic review and meta-analysis . Clin Psychol Rev . 92 : 102128 .
Montag C ( 2021 ) Du gehörst uns! Die psychologischen Strategien von Facebook, TikTok, Snapchat & Co—Und wie wir uns vor der großen Manipulation schützen . Blessing .
Montag C , Baumeister H , Kannen C et al. ( 2019 ) Concept, possibilities and pilot-testing of a new smartphone application for the social and life sciences to study Human behavior including validation data from personality psychology . J—Multidisciplinary Sci J . 2 : Article 2 .
Montag C , Diefenbach S ( 2018 ) Towards Homo Digitalis: important research issues for psychology and the neurosciences at the dawn of the Internet of Things and the digital society . Sustainability . 10 : Article 2 .
Montag C , Elhai JD , Dagum P . ( 2021a ) On blurry boundaries when defining digital biomarkers: how much biology needs to be in a digital biomarker? . Front Psychiatry . 12 : 1690 .
Montag C , Elhai JD , Dagum P . ( 2021b ) Show me your smartphone… and then I will show you your brain structure and brain function . Hum Behav Emerg Technol . 3 : 891 – 7 .
Montag C , Hegelich S ( 2020 ) Understanding detrimental aspects of social Media use: will the real culprits please stand up? . Front Sociol . 5 : 599270 .
Montag C , Lachmann B , Herrlich M et al. ( 2019 ) Addictive features of social media/messenger platforms and freemium games against the background of psychological and economic theories . Int J Environ Res Public Health . 16 : Article 14 .
Montag C , Markowetz A , Blaszkiewicz K et al. ( 2017 ) Facebook usage on smartphones and gray matter volume of the nucleus accumbens . Behav Brain Res . 329 : 221 – 8 .
Montag C , Schivinski B , Pontes H ( 2021 ) Is the proposed distinction of gaming disorder into a predominantly online vs. offline form meaningful? Empirical evidence from a large German speaking gamer sample . Addict Behav Rep . 14 : 100391 .
Montag C , Sindermann C , Becker B et al. ( 2016 ) An affective neuroscience framework for the molecular study of internet addiction . Front Psychol . 7 : 1906 .
Montag C , Wegmann E , Sariyska R et al. ( 2021 ) How to overcome taxonomical problems in the study of internet use disorders and what to do with “smartphone addiction”? . J Behav Addict . 9 : 908 – 14 .
Montag C , Zhao Z , Sindermann C et al. ( 2018 ) Internet Communication Disorder and the structure of the human brain: initial insights on WeChat addiction . Sci Rep . 8 : 2155 .
Moreno-López L , Catena A , Fernández-Serrano MJ et al. ( 2012 ) Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals . Drug Alcohol Depend . 125 : 208 – 14 .
Nasser NS , Sharifat H , Rashid AA et al. ( 2020 ) Cue-reactivity among young adults with problematic Instagram use in response to Instagram-themed risky behavior cues: a pilot fMRI study . Front Psychol . 11 : 556060 .
Nichols TE , Das S , Eickhoff SB et al. ( 2017 ) Best practices in data analysis and sharing in neuroimaging using MRI . Nat Neurosci . 20 : 299 – 303 .
Olson JA , Sandra DA , Colucci ÉS et al. ( 2022 ) Smartphone addiction is increasing across the world: a meta-analysis of 24 countries . Comput Hum Behav . 129 : 107138 .
Paik S-H , Park C , Kim J-Y et al. ( 2019 ) Prolonged bedtime smartphone use is associated with altered resting-state functional connectivity of the insula in adult smartphone users . Front Psychiatry . 10 : 516 .
Panova T , Carbonell X ( 2018 ) Is smartphone addiction really an addiction? . J Behav Addict . 7 : 252 – 9 .
Parry DA , Davidson BI , Sewall CJR et al. ( 2021 ) A systematic review and meta-analysis of discrepancies between logged and self-reported digital media use . Nat Hum Behav . 5 : 1535 – 47 .
Poldrack RA , Baker CI , Durnez J et al. ( 2017 ) Scanning the horizon: towards transparent and reproducible neuroimaging research . Nat Rev Neurosci . 18 : 115 – 26 .
Pyeon A , Choi J , Cho H et al. ( 2021 ) Altered connectivity in the right inferior frontal gyrus associated with self-control in adolescents exhibiting problematic smartphone use: a fMRI study . J Behav Addict . 10 : 1048 – 60 .
Qin K , Zhang F , Chen T et al. ( 2020 ) Shared gray matter alterations in individuals with diverse behavioral addictions: a voxel-wise meta-analysis . J Behav Addict . 9 : 44 – 57 .
Rashid AA , Suppiah S , Nasser NS et al. ( 2021 ) The neurobiology of smartphone addiction in emerging adults evaluated using brain morphometry and resting-state functional MRI . Neurosci Res Notes . 4 : Article 4 .
Rosenthal SR , Li Y , Wensley IA et al. ( 2022 ) Smartphone addiction and traffic accidents: the moderating role of texting while driving . J Technol Behav Sci . 7 : 406 – 13 .
Rozgonjuk D , Elhai JD , Sapci O et al. ( 2021 ) Discrepancies between self-reports and behavior: fear of missing out (FoMO), self-reported problematic smartphone use severity, and objectively measured smartphone use . Digital Psychology . 2 : Article 2 .
Rozgonjuk D , Sindermann C , Elhai JD et al. ( 2020 ) Associations between symptoms of problematic smartphone, Facebook, WhatsApp, and Instagram use: an item-level exploratory graph analysis perspective . J Behav Addict . 9 : 686 – 97 .
Sadeghi S , Takeuchi H , Shalani B et al. ( 2022 ) Brain anatomy alterations and mental health challenges correlate to email addiction tendency . Brain Sci . 12 : Article 10 .
Sapci O , Elhai JD , Amialchuk A et al. ( 2021 ) The relationship between smartphone use and students` academic performance . Learn Individ Differ . 89 : 102035 .
Schmitgen MM , Horvath J , Mundinger C et al. ( 2020 ) Neural correlates of cue reactivity in individuals with smartphone addiction . Addict Behav . 108 : 106422 .
Schmitgen MM , Wolf ND , Sambataro F et al. ( 2022 ) Aberrant intrinsic neural network strength in individuals with “smartphone addiction”: an MRI data fusion study . Brain Behav . 12 : e2739 .
Serra-Blasco M , Radua J , Soriano-Mas C et al. ( 2021 ) Structural brain correlates in major depression, anxiety disorders and post-traumatic stress disorder: a voxel-based morphometry meta-analysis . Neurosci Biobehav Rev . 129 : 269 – 81 .
Sha P , Sariyska R , Riedl R et al. ( 2019 ) Linking internet communication and smartphone use disorder by taking a closer look at the Facebook and WhatsApp applications . Addict Behav Rep . 9 : 100148 .
Sherman LE , Payton AA , Hernandez LM et al. ( 2016 ) The power of the like in adolescence: effects of peer influence on neural and behavioral responses to social media . Psychol Sci . 27 : 1027 – 35 .
Statista ( 2023 ) Number of smartphone subscriptions worldwide from 2016 to 2021, with forecasts from 2022 to 2027 . Statista . https://www.statista.com/statistics/330695/number-of-smartphone-users-worldwide/ (accessed on 24th February 2023) .
Sunday OJ , Adesope OO , Maarhuis PL ( 2021 ) The effects of smartphone addiction on learning: a meta-analysis . Comput Hum Behav Rep . 4 : 100114 .
Taebi A , Becker B , Klugah-Brown B et al. ( 2022 ) Shared network-level functional alterations across substance use disorders: a multi-level kernel density meta-analysis of resting-state functional connectivity studies . Addict Biol . 27 : e13200 .
Tolomeo S , Yu R ( 2022 ) Brain network dysfunctions in addiction: a meta-analysis of resting-state functional connectivity . Transl Psychiatry . 12 : 41 .
Turel O , He Q , Brevers D et al. ( 2018 ) Delay discounting mediates the association between posterior insular cortex volume and social media addiction symptoms . Cogn Affect Behav Neurosci . 18 : 694 – 704 .
Turel O , He Q , Xue G et al. ( 2014 ) Examination of neural systems sub-serving facebook “addiction” . Psychol Rep . 115 : 675 – 95 .
Tymofiyeva O , Yuan JP , Kidambi R et al. ( 2020 ) Neural correlates of smartphone dependence in adolescents . Front Hum Neurosci . 14 : 564629 .
Vollstädt-Klein S , Wichert S , Rabinstein J et al. ( 2010 ) Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum . Addiction . 105 : 1741 – 9 .
Wang L , Zhou X , Song X et al. ( 2022 ) Fear of missing out (FOMO) associates with reduced cortical thickness in core regions of the posterior default mode network and higher levels of problematic smartphone and social media use . preprint ( BioRxiv.org ; doi: 10.1101/2022.10.24.513508) .
Wang Y , Zou Z , Song H et al. ( 2016 ) Altered gray matter volume and white matter integrity in college students with mobile phone dependence . Front Psychol . 7 : 597 .
Weon H-W ( 2017 ) Comparison of QEEG between EEG asymmetry and coherehnce with elderly people according to smart_phone game addiction tendency . J Korea Academia-Industrial Coop Soc . 18 : 644 – 52 . https://www.koreascience.or.kr/article/JAKO201734964190221.page
Westbrook A , Ghosh A , van den Bosch R et al. ( 2021 ) Striatal dopamine synthesis capacity reflects smartphone social activity . IScience . 24 : 102497 .
Wise T , Radua J , Via E et al. ( 2017 ) Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis . Mol Psychiatry . 22 : 1455 – 63 .
Xiang M-Q , Lin L , Song Y-T et al. ( 2023 ) Reduced left dorsolateral prefrontal activationin problematic smartphone users during the Stroop task: An fNIRS study . Front Psychiatry . 13 : 1097375 .
Yan H , Li Q , Yu K et al. ( 2021 ) Large-scale network dysfunction in youths with internet gaming disorder: a meta-analysis of resting-state functional connectivity studies . Prog Neuropsychopharmacol Biol Psychiatry . 109 : 110242 .
Yoo JH , Chun J-W , Choi MR et al. ( 2021 ) Caudate nucleus volume mediates the link between glutamatergic neurotransmission and problematic smartphone use in youth . J Behav Addict . 10 : 338 – 46 .
Yu F , Li J , Xu L et al. ( 2022 ) Opposing associations of Internet Use Disorder symptom domains with structural and functional organization of the striatum: a dimensional neuroimaging approach . J Behav Addict . 1 : 1068 – 79 .
Yu F , Sariyska R , Lachmann B et al. ( 2020 ) Convergent cross-sectional and longitudinal evidence for gaming-cue specific posterior parietal dysregulations in early stages of internet gaming disorder . Addict Biol . 26 : e12933 .
Zhang J , Dong H , Zhao Z et al. ( 2020 ) Altered neural processing of negative stimuli in people with internet gaming disorder: fMRI evidence from the comparison with recreational game users . J Affect Disord . 264 : 324 – 32 .
Zhang M , Gao X , Yang Z et al. ( 2021 ) Shared gray matter alterations in subtypes of addiction: a voxel-wise meta-analysis . Psychopharmacology . 238 : 2365 – 79 .
Zhang R , Volkow ND ( 2019 ) Brain default-mode network dysfunction in addiction . Neuroimage . 200 : 313 – 31 .
Zhao W , Zimmermann K , Zhou X et al. ( 2020 ) Impaired cognitive performance under psychosocial stress in cannabis-dependent men is associated with attenuated precuneus activity . J Psychiatry Neurosci . 45 , 88 – 97 .
Zheng H , Hu Y , Wang Z et al. ( 2019 ) Meta-analyses of the functional neural alterations in subjects with internet gaming disorder: similarities and differences across different paradigms . Prog Neuropsychopharmacol Biol Psychiatry . 94 : 109656 .
Zhou F , Montag C , Sariyska R et al. ( 2019 ) Orbitofrontal gray matter deficits as marker of internet gaming disorder: converging evidence from a cross-sectional and prospective longitudinal design . Addict Biol . 24 : 100 – 9 .
Zhou F , Zimmermann K , Xin F et al. ( 2018 ) Shifted balance of dorsal versus ventral striatal communication with frontal reward and regulatory regions in cannabis-dependent males . Hum Brain Mapp . 39 : 5062 – 73 .
Zhou X , Wu R , Zeng Y et al. ( 2022 ) Choice of voxel-based morphometry processing pipeline drives variability in the location of neuroanatomical brain markers . Commun Biol . 5 : 913 .
Zhou X , Zimmermann K , Xin F et al. ( 2019 ) Cue reactivity in the ventral striatum characterizes heavy cannabis use, whereas reactivity in the dorsal striatum mediates dependent use . Biol Psychiatry Cogn Neurosci Neuroimaging . 4 : 751 – 62 .
Zilverstand A , Huang AS , Alia-Klein N et al. ( 2018 ) Neuroimaging impaired response inhibition and salience attribution in human drug addiction: a systematic review . Neuron . 98 : 886 – 903 .
Zimmermann K , Yao S , Heinz M et al. ( 2018 ) Altered orbitofrontal activity and dorsal striatal connectivity during emotion processing in dependent marijuana users after 28 days of abstinence . Psychopharmacology . 235 : 849 – 59 .
Zou L , Wu X , Tao S et al. ( 2021 ) Anterior cingulate gyrus acts as a moderator of the relationship between problematic mobile phone use and depressive symptoms in college students . Soc Cogn Affect Neurosci . 16 : 484 – 91 .
Zou L , Wu X , Tao S et al. ( 2022 ) Functional connectivity between the parahippocampal gyrus and the middle temporal gyrus moderates the relationship between problematic mobile phone use and depressive symptoms: evidence from a longitudinal study . J Behav Addict . 11 : 40 – 8 .
- neuroimaging
- magnetic resonance imaging
- mental health
- cell phones
Email alerts
Citing articles via.
- Advertising and Corporate Services
- Journals Career Network

Affiliations
- Online ISSN 2634-4416
- Copyright © 2024 West China School of Medicine/West China Hospital (WCSM/WCH) of Sichuan University
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

Clear as a bell

Women who follow Mediterranean diet live longer

Harvard-led study IDs statin that may block pathway to some cancers
‘Dramatic’ inroads against aggressive brain cancer
Cutting-edge therapy shrinks tumors in early glioblastoma trial
Haley Bridger
Mass General Communications
A collaborative project to bring the promise of cell therapy to patients with a deadly form of brain cancer has shown dramatic results among the first patients to receive the novel treatment.
In a paper published Wednesday in The New England Journal of Medicine, researchers from Mass General Cancer Center shared the results for the first three patient cases from a Phase 1 clinical trial evaluating a new approach to CAR-T therapy for glioblastoma.
Just days after a single treatment, patients experienced dramatic reductions in their tumors, with one patient achieving near-complete tumor regression. In time, the researchers observed tumor progression in these patients, but given the strategy’s promising preliminary results, the team will pursue strategies to extend the durability of response.
Left: MRI in Participant 3 before infusion. Right: After infusion on day five.
Image courtesy of The New England Journal of Medicine
“This is a story of bench-to-bedside therapy, with a novel cell therapy designed in the laboratories of Massachusetts General Hospital and translated for patient use within five years, to meet an urgent need,” said co-author Bryan Choi , a neurosurgeon at Harvard-affiliated Mass General and an assistant professor at Harvard Medical School. “The CAR-T platform has revolutionized how we think about treating patients with cancer, but solid tumors like glioblastoma have remained challenging to treat because not all cancer cells are exactly alike and cells within the tumor vary. Our approach combines two forms of therapy, allowing us to treat glioblastoma in a broader, potentially more effective way.”
The new approach is a result of years of collaboration and innovation springing from the lab of Marcela Maus , director of the Cellular Immunotherapy Program and an associate professor at the Medical School. Maus’ lab has set up a team of collaborating scientists and expert personnel to rapidly bring next-generation genetically modified T cells from the bench to clinical trials in patients with cancer.
“We’ve made an investment in developing the team to enable translation of our innovations in immunotherapy from our lab to the clinic, to transform care for patients with cancer,” said Maus. “These results are exciting, but they are also just the beginning — they tell us that we are on the right track in pursuing a therapy that has the potential to change the outlook for this intractable disease. We haven’t cured patients yet, but that is our audacious goal.”
CAR-T (chimeric antigen receptor T-cell) therapy works by using a patient’s own cells to fight cancer — it is known as the most personalized way to treat the disease. A patient’s cells are extracted, modified to produce proteins on their surface called chimeric antigen receptors, and then injected back into the body to target the tumor directly. Cells used in this study were manufactured by the Connell and O’Reilly Families Cell Manipulation Core Facility of the Dana-Farber/Harvard Cancer Center.
CAR-T therapies have been approved for the treatment of blood cancers, but the therapy’s use for solid tumors is limited. Solid tumors contain mixed populations of cells, allowing some malignant cells to continue to evade the immune system’s detection even after treatment with CAR-T. Maus’ team is working to overcome this challenge by combining two previously separate strategies: CAR-T and bispecific antibodies, known as T-cell engaging antibody molecules. The version of CAR-TEAM for glioblastoma is designed to be directly injected into a patient’s brain.
In the new study, the three patients’ T cells were collected and transformed into the new version of CAR-TEAM cells, which were then infused back into each patient. Patients were monitored for toxicity throughout the duration of the study. All patients had been treated with standard-of-care radiation and temozolomide chemotherapy and were enrolled in the trial after disease recurrence.
- A 74-year-old man had his tumor regress rapidly but transiently after a single infusion of the new CAR-TEAM cells.
- A 72-year-old man was treated with a single infusion of CAR-TEAM cells. Two days after receiving the cells, an MRI showed a decrease in the tumor’s size by 18 percent. By day 69, the tumor had decreased by 60 percent, and the response was sustained for more than six months.
- A 57-year-old woman was treated with CAR-TEAM cells. An MRI five days after the infusion showed near-complete tumor regression.
The authors note that despite the remarkable responses among the first three patients, they observed eventual tumor progression in all the cases, though in one case, there was no progression for over six months. Progression corresponded in part with the limited persistence of the CAR-TEAM cells over the weeks following infusion. As a next step, the team is considering serial infusions or preconditioning with chemotherapy to prolong the response.
“We report a dramatic and rapid response in these three patients. Our work to date shows signs that we are making progress, but there is more to do,” said co-author Elizabeth Gerstner, a Mass General neuro-oncologist.
In addition to Choi, Maus, and Gerstner, other authors are Matthew J. Frigault, Mark B. Leick. Christopher W. Mount, Leonora Balaj, Sarah Nikiforow, Bob S. Carter, William T. Curry, and Kathleen Gallagher.
The study was supported in part by the National Gene Vector Biorepository at Indiana University, which is funded under a National Cancer Institute contract.
Share this article
You might like.
New successful, expanded trial of groundbreaking therapy for genetic deafness suggests it may be available relatively soon

Large study shows benefits against cancer, cardiovascular mortality, also identifies likely biological drivers of better health

Cholesterol-lowering drug suppresses chronic inflammation that creates dangerous cascade
When should Harvard speak out?
Institutional Voice Working Group provides a roadmap in new report
- Português Br
- Journalist Pass
8 innovations in neuroscience and brain research at Mayo Clinic
Mayo Clinic Staff
Share this:

The brain is a critical, complex organ and intricate diseases affect it. Mayo Clinic researchers are leading discoveries into many conditions, including cancer, Alzheimer's disease and other forms of dementia , as well as how the brain fundamentally works. Eight research advancements led by neuroscience experts include:

Researchers discover new molecular drug targets for progressive neurological disorder
Progressive supranuclear palsy (PSP) is an uncurable brain disorder marked by walking and balance difficulties. Its symptoms mimic Parkinson's disease and dementia. Mayo researchers and collaborators have outlined new therapeutic targets that may lead to future treatments for PSP as well as Alzheimer's disease and related disorders.
"This research enhances our understanding of progressive supranuclear palsy and other related, incurable neurological disorders," says the study's senior author, Nilufer Ertekin-Taner, M.D., Ph.D., a Mayo Clinic neurologist and neuroscientist. "Moving forward, we can target these specific genes or others that are biologically related to them to develop a potential treatment for this untreatable disease."
Mapping cell behaviors in high-grade glioma to improve treatment
High-grade gliomas are cancerous tumors that spread quickly in the brain or spinal cord. Mayo Clinic researchers found invasive brain tumor margins of high-grade glioma contain biologically distinct genetic and molecular alterations that indicate aggressive behavior and disease recurrence. They also found that MRI techniques, such as dynamic susceptibility contrast and diffusion tensor imaging, can help distinguish between the genetic and molecular alterations of invasive tumors, which is important for clinically characterizing areas that are difficult to surgically biopsy.
"We need to understand what is driving tumor progression," says lead author Leland Hu, M.D. , a neuroradiologist at Mayo Clinic. "Our results demonstrate an expanded role of advanced MRI for clinical decision-making for high-grade glioma."
Researchers identify new criteria to detect rapidly progressive dementia
Rapidly progressive dementia (RPD) is caused by several disorders that quickly impair intellectual functioning and interfere with normal activities and relationships. If patients' symptoms appear suddenly causing rapid decline, a physician may diagnose RPD. These patients can progress from initial symptoms of dementia to complete incapacitation, requiring full-time care, in less than two years. Mayo Clinic researchers have identified new scoring criteria allowing for the detection of treatable forms of RPD with reasonably high confidence during a patient's first clinical visit. This scoring criteria may allow physicians to substantially reduce the time it takes to begin treatment.
"Many conditions that cause rapidly progressive dementia can be treated and even reversed. We found that more than half of the patients in our study with rapidly progressive dementia had a treatable underlying condition. We may be able to identify many of these patients early in the symptomatic course by intentionally searching for key clinical symptoms and exam findings and integrating these with results of a brain MRI and spinal tap," says the study's senior author, Gregg Day, M.D. , a clinical researcher at Mayo Clinic.

Global consortium to study Pick’s disease, rare form of early-onset dementia
Pick's disease , a neurodegenerative disease of unknown genetic origin, is a rare type of frontotemporal dementia that affects people under the age of 65. The condition causes changes in personality, behavior and sometimes language impairment. In patients with the disease, tau proteins build up and form abnormal clumps called Pick bodies, which restrict nutrients to the brain and cause neurodegeneration. Researchers at Mayo Clinic and collaborators worldwide have established the Pick's Disease International Consortium to study a specific MAPT gene variation known as MAPT H2 that makes the tau protein and acts as a driver of disease. They investigated a connection between the gene and disease risk, age at onset and duration of Pick's disease. "We found that the MAPT H2 genetic variant is associated with an increased risk of Pick's disease in people of European descent," says Owen Ross, Ph.D. , a Mayo Clinic neuroscientist and senior author of the paper. "We were only able to determine that because of the global consortium, which greatly increased the sample size of pathology cases to study Pick's disease."

Moments of clarity in the fog of dementia
Researchers define lucid episodes as unexpected, spontaneous, meaningful and relevant communication from a person who is assumed to have permanently lost the capacity for coherent interactions, either verbally or through gestures and actions. A study surveyed family caregivers of people living with dementia and asked them about witnessing lucid episodes.
"We have found in our research and stories from caregivers that these kinds of episodes change how they interact with and support their loved ones — usually for the better," says lead author Joan Griffin, Ph.D. "These episodes can serve as reminders that caregiving is challenging, but we can always try to care with a little more humanity and grace."
Untangling the threads of early-onset dementia
Changes in personality, behavior and language are hallmarks of frontotemporal dementia (FTD) , the most common form of dementia in patients under the age of 65. New research provides insight into the role a specific gene and the protein it produces play in the development and progression of FTD, which is associated with degeneration of the frontal and temporal lobes of the brain. The researchers think the key may lie in the formation of fibrils, or tiny fiber-like structures produced by part of this protein, that sometimes get tangled up in the brain.
"We also think that these fibrils could one day serve as biomarkers to help clinicians determine FTD prognosis or severity, " says Jordan Marks, an M.D.–Ph.D. student with the Mayo Clinic Graduate School of Biomedical Sciences .
Mayo Clinic researchers' new tool links Alzheimer's disease types to rate of cognitive decline
Through a new corticolimbic index tool that identifies changes in specific areas of the brain, Mayo Clinic researchers discovered a series of brain changes characterized by unique clinical features and immune cell behaviors for Alzheimer's disease , a leading cause of dementia .
"By combining our expertise in the fields of neuropathology, biostatistics, neuroscience, neuroimaging and neurology to address Alzheimer's disease from all angles, we've made significant strides in understanding how it affects the brain," says Melissa E. Murray, Ph.D. , a translational neuropathologist at Mayo Clinic. "The corticolimbic index is a score that could encourage a paradigm shift toward understanding the individuality of this complex disease and broaden our perspective. This study marks a significant step toward personalized care, offering hope for more effective future therapies."
New research platform assesses brain cancer mutations during surgery
Brain cancer is difficult to treat when it starts growing, and a prevalent type, known as a glioma , has a poor five-year survival rate. Mayo Clinic researchers report on a new surgical platform used during surgery that informs critical decision-making about tumor treatment within minutes. Time is of the utmost importance when dealing with aggressive malignant tumors.
The researchers say that, in addition to enabling real-time diagnosis, the platform allows surgeons to determine a patient's prognosis and perform tumor resection to improve patient outcomes.
“We will be able to bring the fight against cancer to the operating room, before chemotherapy and radiation treatments begin, and before the disease has progressed and invaded further," says the study's senior author, Alfredo Quiñones-Hinojosa, M.D.
- Comprehensive testing leads to targeted treatment for rare autoimmune encephalitis antibody Mayo Clinic Minute: Types of brain tumors and treatments
Related Articles

News Center
New glioblastoma treatment reaches human brain tumor and helps immune cells recognize cancer cells, novel immunotherapy is first-in-human treatment for this brain cancer.

In a major advance for the treatment of the deadly brain cancer glioblastoma, Northwestern Medicine scientists used ultrasound technology to penetrate the blood-brain barrier and provide a small dose of a chemotherapy and immunotherapy drug cocktail. The study, published in Nature Communications, found that this treatment boosted the immune system’s recognition of the cancer cells and could lead to a new treatment approach.
The scientists made several breakthroughs reported in a new study, including showing for the first time that a skull-implantable ultrasound device can enhance the penetration of the chemotherapy drug doxorubicin and immune checkpoint blockade antibodies — a novel immunotherapy treatment combination — into the human brain. The device produces microbubbles that temporarily open the blood-brain barrier, allowing the immunotherapy to enter the brain.
The scientists also showed for the first time that a small dose of doxorubicin (smaller than the dose used for traditional chemotherapy regimens) delivered with the immune checkpoint antibodies can boost the recognition of malignant glioblastoma cells by the immune system and reinvigorate the lymphocytes (immune cells) that are in charge of attacking the cancer cells.
An immune checkpoint blockade antibody blocks the deactivation of the immune system by the cancer cells. The immune system has built-in brakes — called immune checkpoints — so it doesn’t overdo it and injure the body when attacking cancer and infections. Glioblastoma evolves to activate the brakes, and therefore, the immune system (i.e., lymphocytes) won’t attack it.
In addition to the tumor cells, glioblastoma contains other cell populations called macrophages and microglia. These are the most abundant components of the tumor microenvironment and the cells that glioblastoma modulates to inhibit lymphocytes. The study showed that the chemo and antibody cocktail altered these cells, enabling the lymphocytes to recognize and kill the cancer cells.
“This is the first report in humans where an ultrasound device has been used to deliver drugs and antibodies to glioblastoma to change the immune system, so it can recognize and attack the brain cancer,” said co-corresponding author Adam Sonabend, MD , associate professor of Neurological Surgery and a Northwestern Medicine neurosurgeon. “This could be a major advance for the treatment of glioblastoma, which has been a frustratingly difficult cancer to treat, in part due to poor penetration of circulating drugs and antibodies into the brain.”
The study was conducted in four patients who had advanced progression of their tumors. They had already been treated with conventional chemotherapy for their tumors as well as an experimental treatment in a clinical trial, but both times, the tumors returned.
“This is a great example of translational bench-to-bedside-back-to-bench research, which sets an exceptional scenario to learn about the ability of the immune system to kill brain tumors in real-time upon treatment,” said co-corresponding author Catalina Lee-Chang, PhD , assistant professor of Neurological Surgery. “Given the lack of effective immune response against these deadly tumors, these findings encourage us to envision a potential new treatment approach.”
Clinical trial launched with new treatment
These new findings are the basis for a novel clinical trial that was just launched at Northwestern using ultrasound to deliver immunotherapy for glioblastoma. The trial will initially enroll 10 participants to determine the safety of the treatment, followed by 15 additional to measure whether the treatment can prolong survival.
Previous large clinical trials have failed to show that this type of immunotherapy can prolong survival in glioblastoma patients. Sonabend, however, believes that by enhancing the delivery of these antibodies and drugs into the brain and relying on biomarkers that indicate which tumors are most susceptible to immunotherapy, this treatment might be shown to be effective for some glioblastoma patients.
“Here we show in a small cohort of patients that when you use this technology, you can enhance the delivery of the chemotherapy and the antibodies, and change the tumor’s microenvironment, so the immune system can recognize the tumor,” Sonabend said.
Sonabend and Lee-Chang are members of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University and the Malnati Brain Tumor Institute . Sonaband is also director of translational neuro-oncology at Feinberg.
Other Northwestern authors include first author Víctor A. Arrieta, PhD; Andrew Gould; Kwang-Soo Kim, PhD; Karl J. Habashy, PhD; Crismita Dmello, PhD , research assistant professor of Neurological Surgery; Gustavo I. Vázquez-Cervantes, PhD; Irina Palacín-Aliana, PhD; Graysen McManus; Christina Amidei, PhD, research assistant professor of Neurological Surgery; Cristal G. Gomez, MS; Silpol Dhiantravan; Li Chen, PhD; Daniel Y. Zhang; Ruth Saganty; Meghan E. Cholak; Surya Pandey, PhD , research assistant professor of Medicine in the Division of Hematology and Oncology ; Matthew McCord, PhD; Kathleen McCortney, MS; Rachel Ward, RN; Bin Zhang, MD, PhD ; the Johanna Dobe Professor of Cancer Immunology in the Department of Medicine; Jason M. Miska, PhD , assistant professor of Neurological Surgery; Maciej S. Lesniak, MD , the chair and Michael J. Marchese Professor of Neurosurgery; Craig M. Horbinski, MD, PhD , the Director of Neuropathology in the Department of Pathology ; Rimas V. Lukas, MD , associate professor of Neurology in the Division of Neuro-oncology ; and Roger Stupp, MD , chief of Neuro-oncology in the Department of Neurology and the Paul C. Bucy Professor of Neurological Surgery.
The research was supported in part by the National Cancer Institute grants 1R01NS110703-01A1, 1U19CA264338-01 and 1R01CA245969-01A1 of the National Institutes of Health, grant P50CA221747 SPORE for Translational Approaches to Brain Cancer and the Moceri Family Foundation and the Panattoni family. Sonabend and Stupp have received funding support for research from Agenus, BMS, and Carthera. Sonabend, Arrieta, Kim, Amidei, and Stupp are co-authors of an IP filed by Northwestern University related to the content of this manuscript. (PCT/US2023/034299). Sonabend has served as a consultant for Carthera and EnClear Therapies. Stupp has acted or is acting as a scientific advisor or has served on advisory boards for the following companies: Alpheus Medical, AstraZeneca, Boston Scientific, Carthera, Celularity, GT Medical, Insightec, Lockwood (BlackDiamond), Northwest Biotherapeutics, Novocure, Inc., Syneos Health (Boston Biomedical), TriAct Therapeutics, Varian Medical Systems. Other co-authors C. Desseaux, G. Bouchoux, and M. Canney are employees and hold an ownership interest in Carthera. M. Canney, G. Bouchoux, and A. Carpentier have patents related to the ultrasound technology described herein. Stupp is an advisory member and consultant for Carthera. Carpentier is a consultant for Carthera. Lee-Chang is a co-founder and consultant for Sera BioPharma.
Related Posts
Utilizing electronic alerts to help control blood pressure, mesulam center celebrates 30th annual alzheimer day, foltz named new director of driskill graduate program.
Comments are closed.
Type above and press Enter to search. Press Esc to cancel.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
June 6, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
New glioblastoma treatment reaches human brain tumor and helps immune cells recognize cancer cells
by Northwestern University
In a major advance for the treatment of the deadly brain cancer glioblastoma, Northwestern Medicine scientists have used ultrasound technology to penetrate the blood-brain barrier and provide a small dose of a chemotherapy and immunotherapy drug cocktail. The study found that this treatment boosted the immune system's recognition of the cancer cells and could lead to a new treatment approach.
The scientists made several breakthroughs reported in a new study published in Nature Communications .
Scientists showed for the first time that a skull-implantable ultrasound device can enhance the penetration of the chemotherapy drug doxorubicin and immune checkpoint blockade antibodies—a novel immunotherapy treatment combination—into the human brain. The device produces microbubbles that temporarily open the blood-brain barrier , allowing the immunotherapy to enter the brain.
The scientists also showed for the first time that a small dose of doxorubicin (smaller than the dose used for traditional chemotherapy regimens) delivered with the immune checkpoint antibodies can boost the recognition of malignant glioblastoma cells by the immune system and reinvigorate the lymphocytes ( immune cells ) that are in charge of attacking the cancer cells .
An immune checkpoint blockade antibody blocks the deactivation of the immune system by the cancer cells. The immune system has built-in brakes—called immune checkpoints—so that it doesn't overdo it and injure the body when attacking cancer and infections. Glioblastoma evolves to activate the brakes, and therefore, the immune system (i.e., lymphocytes) won't attack it.
In addition to the tumor cells , glioblastoma contains other cell populations called macrophages and microglia. These are the most abundant components of the tumor microenvironment and the cells that glioblastoma modulates to inhibit lymphocytes. The study showed that the chemo and antibody cocktail altered these cells, enabling the lymphocytes to recognize and kill the cancer cells.
"This is the first report in humans where an ultrasound device has been used to deliver drugs and antibodies to glioblastoma to change the immune system, so it can recognize and attack the brain cancer," said co-corresponding author Dr. Adam Sonabend, associate professor of neurological surgery at Northwestern University Feinberg School of Medicine and a Northwestern Medicine neurosurgeon. "This could be a major advance for the treatment of glioblastoma, which has been a frustratingly difficult cancer to treat, in part due to poor penetration of circulating drugs and antibodies into the brain."
The study was conducted on four patients who had advanced progression of their tumors. They had already been treated with conventional chemotherapy for their tumors as well as an experimental treatment in a clinical trial, but both times, the tumors returned.
"This is a great example of translational bench-to-bedside-back-to-bench research, which sets an exceptional scenario to learn about the ability of the immune system to kill brain tumors in real-time upon treatment," said co-corresponding author Catalina Lee-Chang, assistant professor of neurological surgery at Northwestern University Feinberg School of Medicine. "Given the lack of effective immune response against these deadly tumors, these findings encourage us to envision a potential new treatment approach."
Clinical trial launched with new treatment
These new findings are the basis for a novel clinical trial that was just launched at Northwestern using ultrasound to deliver immunotherapy for glioblastoma. The trial will initially enroll 10 participants to determine the safety of the treatment, followed by 15 additional to measure whether the treatment can prolong survival.
Previous large clinical trials have failed to show that this type of immunotherapy can prolong survival in glioblastoma patients. Sonabend, however, believes that by enhancing the delivery of these antibodies and drugs into the brain and relying on biomarkers that indicate which tumors are most susceptible to immunotherapy, this treatment might be shown to be effective for some glioblastoma patients.
"Here we show in a small cohort of patients that when you use this technology, you can enhance the delivery of the chemotherapy and the antibodies, and change the tumor's microenvironment, so the immune system can recognize the tumor," Sonabend said.
Explore further
Feedback to editors
Metabolic parameters found to be similar in children born via frozen versus fresh embryo transfer, research shows
14 hours ago
Researchers identify key differences in inner workings of immune cells
Researchers detail molecular pathway that impacts pancreatic cancer progression and treatment response
Epstein-Barr virus and brain cross-reactivity: Possible mechanism for multiple sclerosis detected
Precision laser surgery cuts focal epileptic seizure spread
15 hours ago

Researchers find flavor restrictions affect tobacco buyers differently depending on socioeconomic status

Younger children are more commonly diagnosed with ADHD than their older classmates, says new study
Researchers say AI blood test provides a reliable way to identify lung cancer
16 hours ago

New combination therapy shows promise for bladder cancer patients unresponsive to standard treatment

What toilet paper and game shows can teach us about the spread of epidemics
Related stories.

Which glioblastoma patients will respond to immunotherapy? A biomarker may hold answers
Nov 29, 2021

Study identifies enzyme that helps tumors evade the immune system
Apr 12, 2023
Study could help explain why certain brain tumors don't respond well to immunotherapy
Sep 1, 2023
Developing new nanoparticle treatments for brain tumors
Jun 7, 2023

Genetically engineering a treatment for incurable brain tumors
Apr 22, 2024
Chemotherapy drug reaches brain in humans for first time
May 2, 2023
Recommended for you

'Artificial lymph node' used to treat cancer in mice
Study uncovers how cancer stem cells spread and resist treatment
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Environ Health Perspect
- v.116(10); 2008 Oct

Cancer: Strong Signal for Cell Phone Effects
With 3 billion cell phone users worldwide and more than 260 million in the United States alone—among them 46% of U.S. children aged 8–12, according to Nielsen Mobile figures released 10 September 2008—human exposure to low-energy radiation in the 800- to 2,000-megahertz range is at an all-time high. The most recent attempt to systematically review the epidemiologic evidence for increased risk of brain tumors related to cell phone use indicates that repercussions from this global experiment are coming to light. In a meta-analysis published in the May 2008 issue of the International Journal of Oncology , Swedish researchers found significant associations between long-term cell phone use and brain tumor risk.
“We found that cell phone use is linked to gliomas [malignant brain tumors] and acoustic neuromas [benign tumors of the brain’s auditory nerve] and are showing up after only ten years,” says lead author Lennart Hardell, an oncologist and cancer epidemiologist at University Hospital in Örebro, Sweden. Specifically, for studies that included at least 10 years of exposure, there was a doubling in the risk of gliomas for ipsilateral (same-side) but not contralateral (opposite-side) exposures to the head (as reflected by which hand the subject typically used to hold his/her cell phone). A 2.4-fold increase in risk was seen for acoustic neuromas due to ipsilateral exposures, whereas no increased risk occurred for meningiomas (tumors that occur in the membranes covering the brain and spinal cord).
“Clearly we need more studies of long-term cell phone usage to better assess the cancer risks,” says coauthor Michael Carlberg. Cell phones have been in mainstream usage for only a decade or so, and yet radiation-induced brain tumors normally take about 10–15 years to develop, according to the American Cancer Society.
Hardell’s research team was itself the source of several studies included in the meta-analysis. In the October 2006 issue of the World Journal of Surgical Oncology , the investigators reported a 70% increased risk of grade III–IV astrocytomas (highly aggressive brain tumors) for analog cell phone users. This same study found a nearly 4-fold increase in risk for acoustic neuromas after 15 years of exposure to analog cell phones. Notably, there was no increased risk for testicular cancer, B-cell lymphoma, or salivary gland tumors, suggesting that the findings were not due to observational or recall bias, as such bias should have existed for all tumor types.
To address whether their earlier studies may have skewed the conclusions of their 2008 meta-analysis, the team omitted their own studies from the analysis and still found significantly increased risk for gliomas and nonsignificantly increased risk for acoustic neuromas (50% and 210% increases, respectively) for ipsilateral exposures. “We are now seeing a consistent pattern of increased risk for glioma and acoustic neuroma,” says coauthor Kjell Hansson Mild, a radiation physicist at Umeå University, Sweden. “Not only our own studies are showing this but also all other studies that have included at least ten years as a latency period.”
Emerging evidence suggests that children may be more vulnerable to the potential carcinogenic effects of cell phones and other microwave-emitting technologies. “Concerns about children’s potential vulnerability to RF [radiofrequency] fields have been raised because of the potentially greater susceptibility of their developing nervous systems,” says Leeka Kheifets, an epidemiology professor at the University of California, Los Angeles, and former director of the Electric Power Research Institute EMF research program. “In addition, their brain tissue is more conductive, RF penetration is greater relative to head size, and they will have a longer lifetime of exposure [although the degree of risk for any carcinogen will be primarily determined by the exact timing and magnitude of exposure].”
The importance of a thinner skull and differing dielectric properties is confirmed by a study in the 7 June 2008 issue of Physics in Medicine and Biology showing that a child’s brain absorbs up to twice as much RF as an adult brain. Children today will experience a longer period of exposure because they start using cell phones at an earlier age, according to Hardell; this might be important, because cumulative dose seems to have a strong influence on increased risk of brain tumors. Kheifets adds, however, that “data are lacking on effects of exposures on brain tumors in children . . . [and] other health effects need to be looked at as well.”
The wireless industry takes a cautious view of the research. “The weight of the scientific evidence and the conclusions of a large number of expert scientific reviews show that wireless phones do not pose a health risk,” says Joseph Farren, assistant vice president for public affairs with CTIA–The Wireless Association. “The industry supports continued research as technology continues to evolve, but wishes to stress the fact that there is a consensus among leading health organizations regarding published scientific research showing no reason for concern.”
Hardell concedes it is too soon to determine a safe limit for cell phone use. “Can we say that a ten-minute call is equal to ten one-minute calls?” he asks. “Until we answer such questions, we cannot establish a new limit or even state which parameters or units help define that limit. Nonetheless, since we do see an increased risk of brain tumors, it is necessary to apply the precautionary principle in this situation, especially for long-range exposures that are likely to affect children in particular.” In practice, this might involve limiting children’s use of cell phones and using speaker phones to minimize direct exposure to the head.

In July 2008 market research firm MultiMedia Intelligence reported that more than 16 million U.S. teens use cell phones.
- Introduction
- Conclusions
- Article Information
Findings shown for intracranial progression-free survival (PFS) (A), PFS (B), and overall survival (OS) (C). Chemotherapy comprised pemetrexed, 500 mg/m 2 , combined with cisplatin, 75 mg/m 2 , or nedaplatin, 80 mg/m 2 , in a 4-week cycle for 4 to 6 cycles, followed by pemetrexed, 500 mg/m 2 , as maintenance every 4 weeks. P values were calculated using a stratified log-rank test. HR indicates hazard ratio.
Hazard ratios (HRs) and corresponding 95% CIs were evaluated using Cox proportional hazards regression model. Chemotherapy comprised pemetrexed, 500 mg/m 2 , combined with cisplatin, 75 mg/m 2 , or nedaplatin, 80 mg/m 2 , in a 4-week cycle for 4 to 6 cycles, followed by pemetrexed, 500 mg/m 2 , as maintenance every 4 weeks. ECOG indicates Eastern Cooperative Oncology Group; Del, deletion; and EGFR , epidermal growth factor receptor.
Trial Protocol
eTable 1. Disease Progressive Patterns
eTable 2. Adverse Events in the Intention-to-Treat Population
eTable 3. Summary of Drug-Related Adverse Events in Intention-to-Treat Population
eTable 4. Summary of Postprogression Treatments
eFigure 1. Flowchart of the Trial
eFigure 2. Subgroup Analyses for Progression-Free Survival
eFigure 3. Best Percentage Change From Baseline in Target Lesion Size in the Intention-to-Treat Population
eFigure 4. Percentage of EGFR Thr790Met Mutation After First-Line Treatment Progression in the Gefitinib Plus Chemotherapy Group and Gefitinib Group
eFigure 5. Kaplan-Meier Curves for PFS of Subsequent Third-Generation TKIs in Gefitinib Plus Chemotherapy Group and Gefitinib Group
eFigure 6. Kaplan-Meier Curves for Overall Survival According to Third-Generation TKIs and Brain Radiotherapy in All Treatment Courses
eFigure 7. Kaplan-Meier Curves for Overall Survival Incorporating the Subsequent Third-Generation TKIs and Brain Radiotherapy Upon Progression
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Hou X , Li M , Wu G, et al. Gefitinib Plus Chemotherapy vs Gefitinib Alone in Untreated EGFR -Mutant Non–Small Cell Lung Cancer in Patients With Brain Metastases : The GAP BRAIN Open-Label, Randomized, Multicenter, Phase 3 Study . JAMA Netw Open. 2023;6(2):e2255050. doi:10.1001/jamanetworkopen.2022.55050
Manage citations:
© 2024
- Permissions
Gefitinib Plus Chemotherapy vs Gefitinib Alone in Untreated EGFR -Mutant Non–Small Cell Lung Cancer in Patients With Brain Metastases : The GAP BRAIN Open-Label, Randomized, Multicenter, Phase 3 Study
- 1 State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Department of Medical Oncology, Sun Yat-Sen University Cancer Center, Guangzhou, China
- 2 Cancer Center, Department of Medical Oncology, Meizhou People’s Hospital, Meizhou, China
- 3 Department of Head and Neck/Thoracic Medical Oncology, the First People's Hospital of Foshan, Foshan, China
- 4 Department of Respiratory and Critical Care Medicine, Chronic Airways Diseases Laboratory, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 5 Department of Oncology, Southern Theater Air Force Hospital, Guangzhou, China
- 6 Dongguan Institute of Clinical Cancer Research, Department of Medical Oncology, Southern Medical University-affiliated Dongguan People's Hospital, Dongguan, China
- 7 State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Disease, the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
- 8 State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Department of Statistics, Sun Yat-Sen University Cancer Center, Guangzhou, China
Question What are the efficacy and safety of gefitinib plus chemotherapy in patients with untreated epidermal growth factor receptor ( EGFR ) mutated non–small cell lung cancer (NSCLC) brain metastases?
Findings In this randomized clinical trial including 161 patients with untreated EGFR -mutant NSCLC brain metastases, gefitinib plus chemotherapy significantly improved intracranial progression-free survival, overall progression-free survival, and overall survival than gefitinib alone, with manageable adverse events. Gefitinib plus chemotherapy also had better intracranial, extracranial, and overall response rates than gefitinib alone.
Meaning The findings of this randomized clinical trial suggest that gefitinib plus chemotherapy may be a viable first-line treatment for patients with brain metastases associated with EGFR -mutant NSCLC.
Importance Use of tyrosine kinase inhibitors (TKIs) is the standard therapy for epidermal growth factor receptor ( EGFR )–mutated non–small cell lung cancer (NSCLC) with brain metastases. Several studies have shown that adding chemotherapy to EGFR-TKIs could improve progression-free survival (PFS) in patients with EGFR -mutant advanced NSCLC; however, the efficacy of these agents in patients with brain metastases remains unclear.
Objective To investigate the efficacy and safety of gefitinib plus chemotherapy (pemetrexed with platinum) compared with gefitinib alone in patients with untreated EGFR- mutant NSCLC brain metastases.
Design, Setting, and Participants This open-label prospective, multicenter, phase 3 randomized clinical trial was conducted in 6 centers in China from January 13, 2016, to August 27, 2021. The median follow-up time was 21.1 months (IQR, 13.5-31.8 months). Patients with untreated confirmed brain metastases and EGFR -sensitive mutated NSCLC were enrolled.
Interventions The eligible patients were randomly assigned (1:1) to receive gefitinib plus chemotherapy or gefitinib alone.
Main Outcomes and Measures The primary end point was intracranial PFS; secondary end points included PFS, overall survival (OS), intracranial objective response rate, overall objective response rate, and safety. Intention-to-treat analysis was performed.
Results A total of 161 patients (87 [54.0%] women; mean [SD] age, 55 [9.8] years; range, 26-80 years) were enrolled and randomized to receive gefitinib (n = 81) or gefitinib plus chemotherapy (n = 80). The median intracranial PFS was 15.6 months (95% CI, 14.3-16.9 months) in the gefitinib plus chemotherapy group vs 9.1 months (95% CI, 8.0-10.2 months) in the gefitinib group (hazard ratio, 0.36; 95% CI, 0.25-0.53; P < .001). Similarly, the median PFS was significantly longer with gefitinib plus chemotherapy than gefitinib alone (16.3; 95% CI, 14.4-18.2 months vs 9.5; 95% CI, 8.3-10.8 months; P < .001). Gefitinib plus chemotherapy had a better intracranial objective response rate (85.0%; 95% CI, 77.0%-93.0% vs 63.0%; 95% CI, 52.2%-73.7%; P = .002) and overall objective response rate (80.0%; 95% CI, 71.0%-89.0% vs 64.2%; 95% CI, 53.5%-74.9%; P = .03) than gefitinib alone. At data cutoff, the median OS was also significantly longer in the gefitinib plus chemotherapy group vs the gefitinib group (35.0 vs 28.9 months; hazard ratio, 0.65; 95% CI, 0.43-0.99; P = .04). Grade 3 or worse adverse events were more common with gefitinib plus chemotherapy, most of which were manageable.
Conclusions and Relevance In this randomized clinical trial, gefitinib plus chemotherapy significantly improved intracranial PFS, PFS, and OS compared with gefitinib alone in patients with untreated EGFR- mutant NSCLC brain metastases and could be an optional first-line treatment for these patients.
Trial Registration ClinicalTrials.gov Identifier: NCT01951469
Brain metastases occur in approximately 30% to 40% of patients with non–small cell lung cancer (NSCLC) during the course of the disease and 20% to 25% of patients with advanced NSCLC have brain metastasis at the initial diagnosis. 1 Patients with epidermal growth factor receptor ( EGFR )-mutant NSCLC were more prone to the development of brain metastases, with an approximate frequency of 44% to 63% during the treatment course, 2 - 4 which is higher than in patients with EGFR wild-type. Improving the treatment outcome of patients with brain metastases became the key point of management of treatment for patients with EGFR -mutant NSCLC.
Historically, brain metastases were treated with surgical resection, radiotherapy, and antitumor agents, either alone or in combination. For patients with metastatic NSCLC harboring EGFR mutation, EGFR tyrosine kinase inhibitors (TKIs) have been the standard first-line treatment, 5 , 6 and accumulating evidence suggests EGFR-TKIs also exhibit efficacy on intracranial lesions. 7 - 9 A phase 3 study (BRAIN) demonstrated that patients who received the first-generation EGFR-TKI icotinib have significantly longer intracranial progression-free survival (PFS) and fewer adverse events than those who received whole-brain irradiation plus chemotherapy, indicating the EGFR-TKIs were better first-line treatment in patients with EGFR- mutant NSCLC brain metastases. 10
Although superior efficacy of EGFR-TKIs was shown, resistance to treatment with the first-generation EGFR-TKIs developed, and their median PFS is approximately 8 to 12 months. Several strategies have been explored to improve PFS and overcome resistance, including use of next-generation EGFR-TKIs and EGFR-TKI combination treatment. The third-generation EGFR-TKI osimertinib prolonged PFS to 18 months as first-line treatment and to approximately 15 months in a subgroup of patients with brain metastases. The vascular endothelial growth factor signaling pathway is a candidate target for combination therapy; however, the efficacy of vascular endothelial growth factor treatment in patients with brain metastasis is controversial. 11 , 12 Treatment with EGFR-TKIs combined with chemotherapy was another strategy. In early clinical trials, adding EGFR-TKIs to chemotherapy showed no significant improvement of PFS, partially because patients were not selected for EGFR -sensitive mutation. 13 - 15 However, clinical trials showed that the combination of chemotherapy and gefitinib could significantly improve PFS in patients with EGFR -mutant NSCLC, which makes a case for revisiting the combination therapy strategy. 16 - 18 The NEJ009 Study demonstrated superior PFS benefit in a subgroup analysis of patients with brain metastases, 17 supplying a promising strategy for these patients. However, the statistical deficiency and sample size of subgroup analysis limited the generalization of the conclusion, and prospective randomized trials for patients with EGFR -mutant brain metastases are urgently required. Herein, we report the results of a phase 3 trial that compared gefitinib plus pemetrexed with platinum (chemotherapy) with gefitinib alone for the first-line treatment in patients with asymptomatic EGFR -mutant NSCLC brain metastases.
This was an open-label, parallel, phase 3 randomized clinical trial (GAP BRAIN) conducted in 6 centers in China. The main eligibility criteria included histologically or cytologically confirmed NSCLC with EGFR -sensitive mutation (exon 19 deletion or exon 21 L858R mutation); confirmed brain metastases noted on enhanced brain magnetic resonance imaging; asymptomatic brain metastases; at least 3 intracranial metastatic lesions or patients with 1 to 2 intracranial lesions who are not suitable for localized treatment or refused to receive localized treatment for intracranial metastatic lesions; at least 1 intracranial evaluable lesion, which was defined as lesions with the longest diameter of greater than 5 mm according to modified Response Evaluation Criteria in Solid Tumours [RECIST] 1.1 guidelines (based on 1 mm for magnetic resonance imaging scan slices) 19 , 20 ; treatment naive; aged 18 to 80 years; Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; adequate organ function; and a life expectancy of 12 weeks or more. The main exclusion criteria were previous systemic or localized therapy; obvious central nervous system symptoms and lack of response to treatment of dehydration; radiologically or pathologically confirmed leptomeningeal metastases; history of interstitial lung disease, radiation-associated pneumonitis that required corticosteroid treatment, or any evidence of clinical active interstitial lung disease; concomitant serious systemic disorders; and any second primary malignant disease within 5 years. This study was performed according to the Declaration of Helsinki, 21 and also reported following the Consolidated Standards of Reporting Trials ( CONSORT ) reporting guideline. The study protocol was approved by the ethics committees of Sun Yat-Sen University Cancer Center and other participating centers. The study protocol and statistical analysis plan are available in Supplement 1 . All patients provided written informed consent; participants did not receive financial compensation.
The eligible patients were randomly assigned (1:1) to receive gefitinib plus pemetrexed with platinum chemotherapy or gefitinib alone. Random assignment was performed using a computer-generated randomization sequence. The Clinical Trials Center of Sun Yat-Sen University Cancer Center generated the randomization sequence, confirmed participant eligibility, assigned the eligible patients to trial groups, and notified investigators of treatment allocation for each patient. Patients and investigators were not blinded to treatment allocation.
Patients assigned to the gefitinib-alone group received gefitinib, 250 mg, once daily; patients assigned to the gefitinib plus chemotherapy group received gefitinib, 250 mg, once daily with chemotherapy (pemetrexed, 500 mg/m 2 , combined with cisplatin, 75 mg/m 2 , or nedaplatin, 80 mg/m 2 , in a 4-week cycle for 4 to 6 cycles, followed by pemetrexed, 500 mg/m 2 , as maintenance every 4 weeks). Patients continued treatment until disease progression, development of unacceptable adverse events, or any cause of death. Patients were allowed to receive granulocyte colony-stimulating factor, antiemetics, and other supportive treatment. After disease progression, subsequent treatment was at the discretion of the physician.
Tumor evaluation for intracranial and extracranial lesions was independently assessed by investigators. The number of intracranial target lesions was extended to 5 lesions, as well as 5 extracranial target lesions according to modified RECIST 1.1 guidelines. Tumor assessments were performed within the 3 weeks before enrollment, then every 8 weeks (enhanced computed tomography scans for extracranial lesions and enhanced magnetic resonance imaging for intracranial lesions) until disease progression was noted. Physical and laboratory examinations were performed within 7 days before enrollment, every 4 weeks during treatment, and at the time of disease progression. After disease progression, follow-up for survival analysis was performed every 3 months. All adverse events were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. 22
The primary end point was intracranial PFS, defined as time from randomization to intracranial disease progression or death. The secondary end points included PFS, defined as time from randomization to overall disease (both intracranial and extracranial) progression or death; overall survival (OS), defined as time from randomization to death from any cause; intracranial objective response rate, defined as proportion of patients with complete or partial response of intracranial lesions; objective response rate, defined as proportion of patients with complete or partial response of overall lesions; and safety.
We hypothesized that median intracranial PFS be prolonged from 8 months in the gefitinib-alone group to 12 months in the gefitinib plus chemotherapy group. Assuming a hazard ratio (HR) of 0.67 for gefitinib plus chemotherapy vs gefitinib alone, with 80% power and 1-sided α value of 10%, our estimated sample size was 160 patients.
Intention-to-treat analysis was performed. Intracranial PFS, PFS, and OS were estimated using Kaplan-Meier curves, and differences between groups were compared with a stratified log-rank test. Hazard ratios and 95% CIs were evaluated with Cox proportional hazards regression models. Tumor overall objective response rate, disease control rate, and incidence of adverse events were compared with the Fisher exact test between the 2 treatment groups. All end points are reported as 2-sided P values in this study, with a significance threshold of .05. All statistical analyses were performed using R software, version 4.0.5 (R Foundation for Statistical Computing).
From January 13, 2016, to August 27, 2021, a total of 161 patients (87 [54.0%] women, 74 [46.0%] men; mean [SD] age, 55 [9.8] years; range, 26-80 years) were enrolled and randomized to receive gefitinib (n = 81) or gefitinib plus chemotherapy (n = 80) (eFigure 1 in Supplement 2 ). All randomized patients received at least 1 dose of study drugs and were included in efficacy and safety analyses. The baseline characteristics were balanced between the 2 groups ( Table 1 ). Most patients were nonsmokers and had lung adenocarcinoma. Regarding EGFR mutation type, 85 patients (52.8%) had exon 19 deletions, 70 (43.5%) had exon 21 L858R mutations, and 6 (3.7%) had uncommon EGFR mutations. In addition, 37 patients (45.7%) in the gefitinib group and 47 patients (58.7%) in the gefitinib plus chemotherapy group had baseline next-generation sequencing data with TP53 mutation type. At the data cutoff, the median follow-up time was 21.1 months (IQR, 13.5-31.8 months); 18 patients in the gefitinib plus chemotherapy group and 10 patients in the gefitinib group were still receiving study treatment.
At the data cutoff, 51 patients (63.8%) in the gefitinib plus chemotherapy group and 65 patients (80.2%) in the gefitinib group had confirmed intracranial disease progression. The median intracranial PFS was 15.6 months (95% CI, 14.3-16.9 months) in the gefitinib plus chemotherapy group vs 9.1 months (95% CI, 8.0-10.2 months) in the gefitinib group (HR, 0.36; 95% CI, 0.25-0.53; P < .001) ( Figure 1 A). In subgroup analysis based on baseline characteristics, intracranial PFS favored gefitinib plus chemotherapy over gefitinib in most subgroups ( Figure 2 ), whereas the benefit was not statistically significant in the subgroup of patients with TP53 wild-type. Similarly, the gefitinib plus chemotherapy group achieved a better intracranial objective response rate than the gefitinib group (85.0%, 95% CI, 77.0%-93.0% vs 63.0%; 95% CI, 52.2%-73.7%; P = .002); odds ratios are given in Table 2 . The maximum tumor change from baseline in intracranial tumors is shown in eFigure 3 in Supplement 2 .
At disease progression, 50 patients had intracranial lesions progression only, 18 patients had extracranial lesions progression only, and 63 patients had simultaneous intracranial and extracranial lesions progression. Patients’ progressive patterns between the 2 groups is noted in eTable 1 in Supplement 2 .
At the data cutoff, 50 patients (62.5%) in the gefitinib plus chemotherapy group and 60 patients (74.1%) in the gefitinib group had systemic disease progression. The median PFS was 16.3 months (95% CI, 14.4-18.2 months) in the gefitinib plus chemotherapy group vs 9.5 months (95% CI, 8.3-10.8 months) in the gefitinib group (HR, 0.39; 95% CI, 0.27-0.58; P < .001) ( Figure 1 B). The overall objective response rate was significantly higher in the gefitinib plus chemotherapy group than in the gefitinib group (80.0%; 95% CI, 71.0%-89.0% vs 64.2%; 95% CI, 53.5%-74.9%; P = .03) ( Table 2 ); subgroup analyses also demonstrated gefitinib plus chemotherapy obtained PFS benefit in most subgroups (eFigure 2 in Supplement 2 ).
At the data cutoff, 59.0% of patients (41 patients in the gefitinib plus chemotherapy group and 54 patients in the gefitinib group) had died. The median OS was significantly longer in the gefitinib plus chemotherapy group than in the gefitinib group (35.0; 95% CI, 28.3-41.7 vs 28.9; 95% CI, 23.2-34.5 months; HR, 0.65; 95% CI, 0.43-0.99; P = .04) ( Figure 1 C). The 3-year OS rate was 48.8% (95% CI, 37.6%-59.9%) in the gefitinib plus chemotherapy group and 24.1% (95% CI, 14.9%-33.9%) in the gefitinib group ( P = .002). In subgroup analyses, the OS benefit of gefitinib plus chemotherapy was noted in subgroups of patients with EGFR 19del mutation, nonsmokers, good performance status, small intracranial lesions (largest diameter of intracranial lesion <20 mm), and patients with extracranial metastases ( Figure 3 ).
All 80 patients in the gefitinib plus chemotherapy group and 75 (92.6%) of the 81 patients in the gefitinib group experienced at least 1 drug-related adverse event. Of these, 32 patients (40.0%) in the gefitinib plus chemotherapy group and 17 patients (21.0%) in the gefitinib group reported grade 3 or worse adverse events (eTable 2 in Supplement 2 ). The most common grade 3 or worse adverse event was alanine aminotransferase level increase in both the gefitinib plus chemotherapy (9 [11.3%]) and gefitinib (12 [14.8%]) group. Ten patients (12.5%) in the gefitinib plus chemotherapy group and 7 patients (8.6%) in the gefitinib group experienced treatment interruption due to adverse events. One death due to pneumonitis occurred in the gefitinib plus chemotherapy group that was considered treatment related. No treatment-related deaths occurred in the gefitinib group (eTable 3 in Supplement 2 ).
In total, 55 patients (68.8%) in the gefitinib plus chemotherapy group and 64 patients (79.0%) in the gefitinib group received at least 1 subsequent therapy after progression; details of the postprogression therapy are listed in eTable 4 in Supplement 2 . At disease progression, EGFR Thr790Met was detected in 39 patients in the gefitinib plus chemotherapy group and 38 patients in the gefitinib group. Thirty-one of 77 patients (40.3%) overall developed EGFR T790M mutation (12 of 39 [30.8%] in the gefitinib plus chemotherapy group vs 19 of 38 [50.0%] in the gefitinib group), without a significant difference in this limited sample size ( P = .11) (eFigure 4 in Supplement 2 ). Regarding second-line treatment, 6 patients in the gefitinib plus chemotherapy group and 8 patients in the gefitinib group received salvage brain radiotherapy (BRT), 32 patients in the gefitinib plus chemotherapy group and 31 patients in the gefitinib group received subsequent third-generation EGFR-TKIs as second-line treatment (per patient request in some who were EGFR T790M-negative). The PFS on subsequent third-generation EGFR-TKI use was not significantly different between the gefitinib plus chemotherapy vs gefitinib groups (7.7 vs 8.5 months; P = .75) (eFigure 5 in Supplement 2 ). Considering all subsequent treatment, 86 patients received third-generation EGFR-TKIs and 44 patients received BRT as second-line or further treatment. Patients who received third-generation EGFR-TKIs or BRT had longer OS than those who did not (eFigure 6 in Supplement 2 ). The median OS were 35.2 months in patients who received both TKIs and BRT, 28.8 months in those who received third-generation TKIs only, 22.8 months in those who received BRT only, and 16.4 months for patients who received neither third-generation TKIs nor BRT ( P < .001) (eFigure 7 in Supplement 2 ).
In this phase 3 randomized clinical trial, our results revealed that gefitinib plus chemotherapy significantly improved intracranial PFS, PFS, and OS in patients with untreated NSCLC EGFR mutation and asymptomatic brain metastases. To our knowledge, this is the first randomized clinical trial to compare the intracranial efficacy and safety of gefitinib plus chemotherapy with gefitinib as first-line treatment in EGFR -mutant NSCLC with brain metastases.
Recently, EGFR-TKI combination therapy has shown superior efficacy than EGFR-TKI treatment alone in the NEJ009. 17 Consistent with subgroup analysis of brain metastases in that study, our data showed that gefitinib plus chemotherapy has a similar magnitude of intracranial PFS benefit with overall PFS in patients with untreated brain metastases. Also, with all patients who had evaluable intracranial lesions, gefitinib plus chemotherapy showed better intracranial, extracranial, and overall response rates than gefitinib alone.
The third-generation EGFR-TKI osimertinib has demonstrated longer PFS and OS and better central nervous system permeability than first-generation EGFR-TKIs in the FLAURA study. 23 , 24 At the start of our trial, the FLAURA study had not reported results, and first-generation EGFR-TKIs were still the standard first-line treatment for advanced EGFR -mutant NSCLC. However, for Asian patients and patients with brain metastases, the OS benefit was less with osimertinib compared with standard EGFR-TKI treatment in the global FLAURA and FLAURA China studies. 25 , 26 Due to a complicated resistance mechanism, there are no standard targeted treatment options after disease progression while patients receive osimertinib—more than two-thirds of the patients could only receive cytotoxic chemotherapy and immunotherapy, which provided little benefit. 24 In our study, the median intracranial PFS (15.6 months) and overall PFS (16.3 months) in the gefitinib plus chemotherapy group were numerically comparable with the PFS of osimertinib as first-line treatment in patients with brain metastases (15.2 months). 25 Thus, the combination treatments of first-generation EGFR-TKIs and chemotherapy is still an attractive option to improve outcomes, especially for patients in good condition.
In the NEJ009 study, individual OS data on a subgroup with brain metastases were not reported, and the OS benefit with gefitinib plus chemotherapy was not significant, with a limited sample size. 17 In our study, the median OS and 3-year OS rates were significantly longer in the gefitinib plus chemotherapy group than in the gefitinib group, supporting the finding that gefitinib plus chemotherapy was a promising optional first-line treatment in Chinese patients with EGFR mutation and brain metastases.
For the mechanism of acquired resistance to EGFR-TKI combination therapy, the NEJ026 study reported that erlotinib combined with bevacizumab had a similar percentage of EGFR T790M mutation with erlotinib alone at progression. 27 In our study, the EGFR T790M mutation rate was numerically lower in the gefitinib plus chemotherapy group than in the gefitinib group, although with no statistically significant difference due to the limited sample size. In addition, the PFS on subsequent treatment with third-generation TKIs was not statistically significantly different between the 2 groups. Therefore, the effect of EGFR-TKI combination therapy on the subsequent treatment needs further investigation in a larger sample size.
Radiotherapy has been the traditional method for localized management of brain metastases. The optimal sequence or combination of BRT and targeted therapy in EGFR -mutant NSCLC with brain metastases remains controversial. A multicenter retrospective study reported that up-front stereotactic radiosurgery followed by EGFR-TKI treatment had the longest OS compared with whole-brain radiotherapy followed by EGFR-TKIs, and up-front EGFR-TKI treatment. 28 However, other studies found whole-brain radiotherapy combined with EGFR-TKIs did not prolong the OS more than EGFR-TKIs alone in patients with EGFR -mutation with brain metastases. 29 , 30 In the present study, we found patients with larger intracranial lesions benefited less from gefitinib plus chemotherapy than those with a better prognosis. The results were consistent with those noted in a practice setting presented in a retrospective study, 28 which tended to administer up-front BRT to patients with larger intracranial lesions. In our study, considering the further-line treatment upon progression, patients who received both BRT and third-generation TKIs had the longest OS, followed by those who received third-generation TKIs only and those who received BRT only. Although there was a limited sample size, the results of our study suggest that administration of next-generation central nervous system–penetrant TKIs as subsequent treatment is associated with longer survival and may contribute more to OS than BRT in EGFR- mutant NSCLC with brain metastases. Previous studies also reported that receiving third-generation EGFR -TKIs in all treatment courses is associated with longer survival in advanced EGFR -mutated NSCLC. 17 , 31
The genomic context of the driver EGFR mutation plays a role in target therapy resistance and prior studies analyzing the impact of comutations have identified worse outcomes associated with alterations in other genes, the most important of which is TP53 , 32 - 36 and thus provided the rationale for investigating treatment intensification with chemotherapy in patients with TP53 mutation. Zhao et al 12 reported that EGFR -mutant NSCLC with concomitant TP53 mutation favored gefitinib plus apatinib (an oral vascular EGFR -2 TKI) than gefitinib plus placebo. In our study, subgroup analysis of patients with next-generation sequencing data demonstrated that those with concomitant TP53 mutation benefit more from gefitinib combination therapy. Although the limited sample size of subgroup analysis for both trials necessitates cautious interpretation of the findings and restricts their generalization, the promising efficacy of combination therapy in EGFR -mutant NSCLC with concomitant TP53 mutation warrants further verification in prospective randomized clinical trials.
This study has several limitations. First, the study was not blinded and an independent radiology review committee was not established, which may lead to bias. Second, the subsequent treatment after disease progression was not uniform, and treatment was at the discretion of the physician according to progressive patterns, patients’ symptoms, and ECOG status.
In this randomized clinical trial, gefitinib plus chemotherapy significantly improved intracranial PFS, PFS, and OS compared with gefitinib alone in asymptomatic patients with untreated EGFR -mutant NSCLC brain metastases, with manageable adverse events. Combination gefitinib and chemotherapy could be an optional first-line treatment for this patient population.
Accepted for Publication: November 28, 2022.
Published: February 8, 2023. doi:10.1001/jamanetworkopen.2022.55050
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Hou X et al. JAMA Network Open .
Corresponding Author: Likun Chen, MD, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Department of Medical Oncology, Sun Yat-Sen University Cancer Center, 651 Dongfeng E Rd, Yuexiu District, Guangzhou 510060, China ( [email protected] ).
Author Contributions: Dr Chen had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The following authors contributed equally to this work and share first authorship: Dr Hou, Dr Li, Mr Wu, and Dr Feng.
Concept and design: Hou, Li, Liu, L. Chen.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Hou, Li, Feng, G. Jiang, J. Chen, You.
Critical revision of the manuscript for important intellectual content: Hou, Li, Wu, Feng, Su, H. Jiang, G. Jiang, Zhang, Liu, L. Chen.
Statistical analysis: Hou, Li, Feng, G. Jiang, You, Liu.
Obtained funding: L. Chen.
Administrative, technical, or material support: Hou, Li, Feng, Su, H. Jiang, G. Jiang, Zhang.
Supervision: Hou, L. Chen.
Other: J. Chen.
Conflict of Interest Disclosures: None reported.
Funding/Support: This work was supported by the National Natural Science Foundation of China (grant 82072559), the Natural Science Foundation of Guangdong Province (grant 2022A1515012582), the Sun Yat-sen University Young Teacher Plan (grant 19ykpy179), and the Guangzhou Science and Technology Program (grant) 202002020074).
Role of the Funder/Sponsor: The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 3 .
Additional Contributions: We thank the patients and family members who gave their consent on presenting the data in this study, as well as the investigators and research staff involved in this study.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

IMAGES
VIDEO
COMMENTS
No. Investigators have studied whether the incidence of brain or other central nervous system cancers (that is, the number of new cases of these cancers diagnosed each year) has changed during the time that cell phone use increased dramatically. These studies found: stable incidence rates for adult gliomas in the United States (), Nordic countries and Australia during the past several decades
Mobile phone use and brain tumour risk - COSMOS, a prospective cohort study. Researchers from the International Agency for Research on Cancer (IARC) and partners have delivered the most recent results of the Cohort Study of Mobile Phone Use and Health (COSMOS) project, which investigates the potential long-term health effects related to the use of wireless communication technologies.
Since then, he said, research has shown significant adverse biologic and health effects — including brain cancer — associated with the use of cellphones and other wireless devices. And now, he said, with the fifth generation of cellular technology, known as 5G, there is an even bigger reason for concern .
The most recent update of the study followed people through 2007. Cell phone use, even for more than 13 years, was not linked with an increased risk of brain tumors, salivary gland tumors, or cancer overall, nor was there a link with any brain tumor subtypes or with tumors in any location within the brain.
The primary outcome was the risk of tumors by cellular phone use, which was measured by pooling each odds ratio (OR) and its 95% confidence interval (CI). In a meta-analysis of 46 case-control studies, compared with never or rarely having used a cellular phone, regular use was not associated with tumor risk in the random-effects meta-analysis.
Each new generation of mobile phone technology has triggered discussions about potential carcinogenicity from exposure to radiofrequency electromagnetic fields (RF-EMF). Available evidence has been insufficient to conclude about long-term and heavy mobile phone use, limited by differential recall and selection bias, or crude exposure assessment.
The relationship between cell phones and brain tumors is just such a puzzle. Cell phones have been in use for a scant 40 years, exploding in prevalence from zero to billions of users ( 1 ). If their use causes cancer, we need to know. We need to solve this puzzle.
During 14 years follow-up of 776 156 women who completed the 2001 questionnaire, a total of 3268 incident brain tumors were registered. Adjusted relative risks for ever vs never cellular telephone use were 0.97 (95% confidence interval = 0.90 to 1.04) for all brain tumors, 0.89 (95% confidence interval = 0.80 to 0.99) for glioma, and not ...
Mobile phones emit electromagnetic radiations that are classified as possibly carcinogenic to humans. Evidence for increased risk for brain tumours accumulated in parallel by epidemiologic investigations remains controversial. This paper aims to investigate whether methodological quality of studies …
If cell phone use does, in fact, triple the odds of getting cancer, these stats would suggest that over 60 years a man's risk of developing a brain tumor from cell phone use increases from 0.206 ...
The researchers also investigated whether mobile phone use was associated with the risk of eye tumours. Key findings: • By 2011, almost 75% of women aged between 60 and 64 years used a mobile phone, and just below 50% of those aged between 75 and 79 years. • Over the 14 year follow-up period, 3,268 (0.42%) of the women developed a brain tumour.
November 9, 2022 - Concerns about a possible link between cell phones and brain cancer—a hot topic in the news 5 to 10 years ago—have all but disappeared in recent years. Experts say this is simply because a number of large studies have found no real evidence of such a link.
The argument that cell phones cause cancer lacks biological plausibility because the energy contained in the waves is too low to cause damage. To the surprise and alarm of many, the investigators found what they called "clear evidence" that cell phone radiation could cause a type of nerve tissue cancer called a malignant schwannoma in rats ...
We examined the use of cellular telephones in a case-control study of intracranial tumors of the nervous system conducted between 1994 and 1998. We enrolled 782 patients through hospitals in ...
Groundbreaking study reveals the strongest link yet between cell phone radiation and cancer. Important advice for all consumers. By Jeneen Interlandi. May 27, 2016. The results of a new study by ...
Based on the new animal findings, and limited epidemiological evidence linking heavy and prolonged cell phone use with brain gliomas in humans, Fiorella Belpoggi, director of research at the ...
The American Cancer Society (ACS) does not have any official position or statement on whether or not radiofrequency (RF) radiation from cell phones, cell phone towers, or other sources is a cause of cancer. ACS generally looks to other expert organizations to determine if something causes cancer (that is, if it is a carcinogen), including ...
BERKELEY, Calif. - New UC Berkeley research draws a strong link between cell phone radiation and tumors, particularly in the brain. Researchers took a comprehensive look at statistical findings ...
Other drivers of excessive smartphone use might be video games on the phone (Leung et al., 2020) ... The present paper provides an initial overview on research examining potential brain changes related to smartphone (over-)use from studies applying MRI techniques (studies are of cross-sectional nature though). ...
That radiation is what might link cell phones to cancer. ... and 2016 suggested that people who'd used cell phones for 10 years or longer had a higher risk of brain tumors. 2018 trend research ...
March 14, 2024 5 min read. A collaborative project to bring the promise of cell therapy to patients with a deadly form of brain cancer has shown dramatic results among the first patients to receive the novel treatment. In a paper published Wednesday in The New England Journal of Medicine, researchers from Mass General Cancer Center shared the ...
June 3, 2024. The brain is a critical, complex organ and intricate diseases affect it. Mayo Clinic researchers are leading discoveries into many conditions, including cancer, Alzheimer's disease and other forms of dementia, as well as how the brain fundamentally works. Eight research advancements led by neuroscience experts include:
The research was supported in part by the National Cancer Institute grants 1R01NS110703-01A1, 1U19CA264338-01 and 1R01CA245969-01A1 of the National Institutes of Health, grant P50CA221747 SPORE for Translational Approaches to Brain Cancer and the Moceri Family Foundation and the Panattoni family.
Dr. Quiñones-Hinojosa is also director of the Brain Tumor Stem Cell Research Laboratory. In the study, researchers were able to diagnose IDH gene mutations with 100% accuracy.
The study found that this treatment boosted the immune system's recognition of the cancer cells and could lead to a new treatment approach. The scientists made several breakthroughs reported in a ...
Cell phones have been in mainstream usage for only a decade or so, and yet radiation-induced brain tumors normally take about 10-15 years to develop, according to the American Cancer Society. Hardell's research team was itself the source of several studies included in the meta-analysis. In the October 2006 issue of the World Journal of ...
The breakthrough now will be tested in a Phase 1 pediatric clinical trial for brain cancer. Reported May 1 in the journal Cell, the discovery represents a potential new way to recruit the immune system to fight notoriously treatment-resistant cancers using an iteration of mRNA technology and lipid nanoparticles, similar to COVID-19 vaccines ...
Our scientists pursue every aspect of cancer research—from exploring the biology of genes and cells, to developing immune-based treatments, uncovering the causes of metastasis, and more. ... Drug Combination Shows Promise in Lung Cancer That Has Spread to the Brain and Spinal Cord. Many non-small cell lung cancers are driven by mutations in a ...
Key Points. Question What are the efficacy and safety of gefitinib plus chemotherapy in patients with untreated epidermal growth factor receptor (EGFR) mutated non-small cell lung cancer (NSCLC) brain metastases?. Findings In this randomized clinical trial including 161 patients with untreated EGFR-mutant NSCLC brain metastases, gefitinib plus chemotherapy significantly improved intracranial ...