January 28, 2015

Genetic Memory: How We Know Things We Never Learned
I met my first savant 52 years ago and have been intrigued with that remarkable condition ever since. One of the most striking and consistent things in the many savants I have seen is that that they clearly know things they never learned.
By Darold Treffert
This article was published in Scientific American’s former blog network and reflects the views of the author, not necessarily those of Scientific American
Leslie Lemke is a musical virtuoso even though he has never had a music lesson in his life. Like “Blind Tom” Wiggins a century before him, his musical genius erupted so early and spontaneously as an infant that it could not possibly have been learned. It came ‘factory installed’. In both cases professional musicians witnessed and confirmed that Lemke and Wiggins somehow, even in the absence of formal training, had innate access to what can be called “the rules” or vast syntax of music.
A picture of Leslie Lemke and the author published in the Chicago Tribune’s Sunday magazine in 1988.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Alonzo Clemons has never had an art lesson in his life. As an infant, after a head injury, he began to sculpt with whatever was handy–Crisco or whatever–and now is a celebrated sculptor who can mold a perfect specimen of any animal with clay in an hour or less after only a single glance at the animal itself–every muscle and tendon perfectly positioned. He has had no formal training.
To explain the savant, who has innate access to the vast syntax and rules of art, mathematics, music and even language, in the absence of any formal training and in the presence of major disability, “genetic memory,” it seems to me, must exist along with the more commonly recognized cognitive/semantic and procedural/habit memory circuits.
Genetic memory, simply put, is complex abilities and actual sophisticated knowledge inherited along with other more typical and commonly accepted physical and behavioral characteristics. In savants the music, art or mathematical “chip” comes factory installed. In addition to the examples mentioned above, I describe others in my book, Islands of Genius: The Bountiful Mind of the Autistic, Acquired and Sudden Savant .
Genetic memory is not an entirely new concept. In 1940, A.A. Brill quoted Dr. William Carpenter who, in comparing math prodigy Zerah Colburn’s calculating powers to Mozart’s mastery of musical composition, wrote the following:
“In each of the foregoing cases, then, we have a peculiar example of the possession of an extraordinary congenital aptitude for certain mental activity, which showed itself at so early a period as to exclude the notion that it could have been acquired by the experience of the individual. To such congenital gifts we give the name of intuitions: it can scarcely be questioned that like the instincts of the lower animals, they are the expressions of constitutional tendencies embodied in the organism of the individuals who manifest them.”
Carl Jung used the term “collective unconscious” to define his even broader concept of inherited traits, intuitions and collective wisdom of the past.
Wilder Penfield in his pioneering 1978 book, Mystery of the Mind , also referred to three types of memory. “Animals,” he wrote, “particularly show evidence of what might be called racial memory” (this would be the equivalent of genetic memory). He lists the second type of memory as that associated with “conditioned reflexes” and a third type as “experiential”. The two latter types would be consistent with the terminology commonly applied to “habit or procedural” memory and “cognitive or semantic” memory.
In his 1998 book, The Mind’s Past , Michael Gazzaniga wrote:
“The baby does not learn trigonometry, but knows it; does not learn how to distinguish figure from ground, but knows it; does not need to learn, but knows, that when one object with mass hits another, it will move the object … The vast human cerebral cortex is chock full of specialized systems ready, willing and able to be used for specific tasks. Moreover, the brain is built under tight genetic control … As soon as the brain is built, it starts to express what it knows, what it comes with from the factory. And the brain comes loaded. The number of special devices that are in place and active is staggering. Everything from perceptual phenomena to intuitive physics to social exchange rules comes with the brain. These things are not learned; they are innately structured. Each device solves a different problem … the multitude of devices we have for doing what we do are factory installed; by the time we know about an action, the devices have already performed it.”
Steven Pinker’s 2003 book, The Blank Slate: The Modern Denial of Human Nature , refutes the “blank slate” theories of human development. Brian Butterworth, in his 1999 book, What Counts: How Every Brain is Hardwired for Math , points out that babies have many specialized innate abilities, including numerical ones that he attributes to a “number module” encoded in the human genome from ancestors 30,000 years ago.
Marshall Nivenberg, from the National Heart Institute, provided insight into the actual DNA/RNA mechanics of this innate knowledge in an article titled “Genetic Memory” published in 1968 in JAMA.
Whether called genetic, ancestral or racial memory, or intuitions or congenital gifts, the concept of a genetic transmission of sophisticated knowledge well beyond instincts, is necessary to explain how prodigious savants can know things they never learned.
We tend to think of ourselves as being born with a magnificent and intricate piece of organic machinery (“hardware”) we call the brain, along with a massive but blank hard drive (memory). What we become, it is commonly believed, is an accumulation and culmination of our continuous learning and life experiences, which are added one by one to memory. But the prodigious savant apparently comes already programmed with a vast amount of innate skill -and knowledge in his or her area of expertise–factory-installed “software” one might say–which accounts for the extraordinary abilities over which the savant innately shows mastery in the face of often massive cognitive and other learning handicaps. It is an area of memory function worthy of much more exploration and study.
Indeed recent cases of “acquired savants” or “accidental genius” have convinced me that we all have such factory-installed software. I discussed some of those cases in detail in the August issue of Scientific American under the title “Accidental Genius” . In short, certain persons, after head injury or disease, show explosive and sometimes prodigious musical, art or mathematical ability, which lies dormant until released by a process of recruitment of still intact and uninjured brain areas, rewiring to those newly recruited areas and releasing the until then latent capacity contained therein.
Finally, the animal kingdom provides ample examples of complex inherited capacities beyond physical characteristics. Monarch butterflies each year make a 2,500-mile journey from Canada to a small plot of land in Mexico where they winter. In spring they begin the long journey back north, but it takes three generations to do so. So no butterfly making the return journey has flown that entire route before. How do they “know” a route they never learned? It has to be an inherited GPS-like software, not a learned route.
Oscine birds such as such as sparrows, thrushes and warblers learn their songs from listening to others. Suboscine species, such as flycatchers and their relatives, in contrast, inherit all the genetic instructions they need for these complex arias. Even if raised in sound-proof isolation, the suboscine birds can give the usual call for their species with no formal training or learning. There are so many more examples from the animal kingdom in which very complex traits, behaviors and skills are inherited and innate. We call those instincts in animals, but we haven’t applied this concept to the complex inherited skills and knowledge in humans.
Some will argue that what the prodigious savant inherits is the “proclivity” or scaffolding on which learning can be applied unusually rapidly, rather than knowledge itself, and like the oscine bird species, they learn from others. My position is that the prodigious savant is a convincing example of suboscine genetic inheritance of the actual instructions and knowledge that precedes learning. That is not to say such inherited skills (nature) cannot be cultivated, enhanced and improved (nurture). They can be. But I agree with Dr. William Carpenter that savants demonstrate a “congenital aptitude for certain mental activity, which showed itself at so early a period as to exclude the notion that it could have been acquired by the experience of the individual”.
I call that genetic memory, and I propose that it exists in all of us. The challenge is how to tap that dormant capacity non-intrusively and without a brain injury or similar incident.
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
Memory-making involves extensive DNA breaking
Press contact :.
Previous image Next image
The urgency to remember a dangerous experience requires the brain to make a series of potentially dangerous moves: Neurons and other brain cells snap open their DNA in numerous locations — more than previously realized , according to a new study — to provide quick access to genetic instructions for the mechanisms of memory storage.
The extent of these DNA double-strand breaks (DSBs) in multiple key brain regions is surprising and concerning, says study senior author Li-Huei Tsai , Picower Professor of Neuroscience at MIT and director of The Picower Institute for Learning and Memory, because while the breaks are routinely repaired, that process may become more flawed and fragile with age. Tsai’s lab has shown that lingering DSBs are associated with neurodegeneration and cognitive decline and that repair mechanisms can falter .
“We wanted to understand exactly how widespread and extensive this natural activity is in the brain upon memory formation because that can give us insight into how genomic instability could undermine brain health down the road,” says Tsai, who is also a professor in the Department of Brain and Cognitive Sciences and a leader of MIT’s Aging Brain Initiative . “Clearly, memory formation is an urgent priority for healthy brain function, but these new results showing that several types of brain cells break their DNA in so many places to quickly express genes is still striking.”
Tracking breaks
In 2015, Tsai’s lab provided the first demonstration that neuronal activity caused DSBs and that they induced rapid gene expression. But those findings, mostly made in lab preparations of neurons, did not capture the full extent of the activity in the context of memory formation in a behaving animal, and did not investigate what happened in cells other than neurons.
In the new study published July 1 in PLOS ONE , lead author and former graduate student Ryan Stott and co-author and former research technician Oleg Kritsky sought to investigate the full landscape of DSB activity in learning and memory. To do so, they gave mice little electrical zaps to the feet when they entered a box, to condition a fear memory of that context. They then used several methods to assess DSBs and gene expression in the brains of the mice over the next half-hour, particularly among a variety of cell types in the prefrontal cortex and hippocampus, two regions essential for the formation and storage of conditioned fear memories. They also made measurements in the brains of mice that did not experience the foot shock to establish a baseline of activity for comparison.
The creation of a fear memory doubled the number of DSBs among neurons in the hippocampus and the prefrontal cortex, affecting more than 300 genes in each region. Among 206 affected genes common to both regions, the researchers then looked at what those genes do. Many were associated with the function of the connections neurons make with each other, called synapses. This makes sense because learning arises when neurons change their connections (a phenomenon called “synaptic plasticity”) and memories are formed when groups of neurons connect together into ensembles called engrams.
“Many genes essential for neuronal function and memory formation, and significantly more of them than expected based on previous observations in cultured neurons … are potentially hotspots of DSB formation,” the authors wrote in the study.
In another analysis, the researchers confirmed through measurements of RNA that the increase in DSBs indeed correlated closely with increased transcription and expression of affected genes, including ones affecting synapse function, as quickly as 10-30 minutes after the foot shock exposure.
“Overall, we find transcriptional changes are more strongly associated with [DSBs] in the brain than anticipated,” they wrote. “Previously we observed 20 gene-associated [DSB] loci following stimulation of cultured neurons, while in the hippocampus and prefrontal cortex we see more than 100-150 gene associated [DSB] loci that are transcriptionally induced.”
Snapping with stress
In the analysis of gene expression, the neuroscientists looked at not only neurons but also non-neuronal brain cells, or glia, and found that they also showed changes in expression of hundreds of genes after fear conditioning. Glia called astrocytes are known to be involved in fear learning, for instance, and they showed significant DSB and gene expression changes after fear conditioning.
Among the most important functions of genes associated with fear conditioning-related DSBs in glia was the response to hormones. The researchers therefore looked to see which hormones might be particularly involved and discovered that it was glutocortocoids, which are secreted in response to stress. Sure enough, the study data showed that in glia, many of the DSBs that occurred following fear conditioning occurred at genomic sites related to glutocortocoid receptors. Further tests revealed that directly stimulating those hormone receptors could trigger the same DSBs that fear conditioning did and that blocking the receptors could prevent transcription of key genes after fear conditioning.
Tsai says the finding that glia are so deeply involved in establishing memories from fear conditioning is an important surprise of the new study.
“The ability of glia to mount a robust transcriptional response to glutocorticoids suggest that glia may have a much larger role to play in the response to stress and its impact on the brain during learning than previously appreciated,” she and her co-authors wrote.
Damage and danger?
More research will have to be done to prove that the DSBs required for forming and storing fear memories are a threat to later brain health, but the new study only adds to evidence that it may be the case, the authors say.
“Overall we have identified sites of DSBs at genes important for neuronal and glial functions, suggesting that impaired DNA repair of these recurrent DNA breaks which are generated as part of brain activity could result in genomic instability that contribute to aging and disease in the brain,” they wrote.
The National Institutes of Health, The Glenn Foundation for Medical Research, and the JPB Foundation provided funding for the research.
Share this news article on:
Related links.
- Li-Huei Tsai
- The Picower Institute for Learning and Memory
- Department of Brain and Cognitive Sciences
Related Topics
- Brain and cognitive sciences
- Picower Institute
- Neuroscience
- Alzheimer's
Related Articles
Neuroscientists discover a molecular mechanism that allows memories to form
Study finds that aging neurons accumulate DNA damage
DNA breakage underlies both learning, age-related damage
Previous item Next item
More MIT News

MIT’s Master of Applied Science in Data, Economics, and Design of Policy program adds a public policy track
Read full story →

Astronomers spot a giant planet that is as light as cotton candy

Using ideas from game theory to improve the reliability of language models
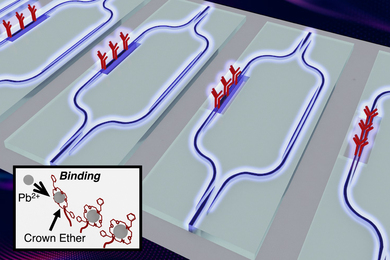
Scientists develop an affordable sensor for lead contamination

MIT researchers discover the universe’s oldest stars in our own galactic backyard

Four from MIT named 2024 Knight-Hennessy Scholars
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram

- May 14, 2024 | Do You Add Salt to Your Food? It Could Be Increasing Your Risk of Stomach Cancer by 40%
- May 14, 2024 | Tree Rings Reveal 2023 Was the Hottest Summer in 2,000 Years
- May 14, 2024 | Astonishing Discovery – Researchers Discover Life 13 Feet Below Earth’s Most Inhospitable Desert
- May 14, 2024 | Ludington’s Liquid Power: One of the Largest Batteries in the World
- May 14, 2024 | Galactic Rings of Power: Astronomers Uncover Massive Magnetic Toroids in the Milky Way Halo
Inside the Cell’s Identity Crisis: MIT’s Revolutionary Genetic “Memories” Discovery
By Anne Trafton, Massachusetts Institute of Technology January 6, 2024
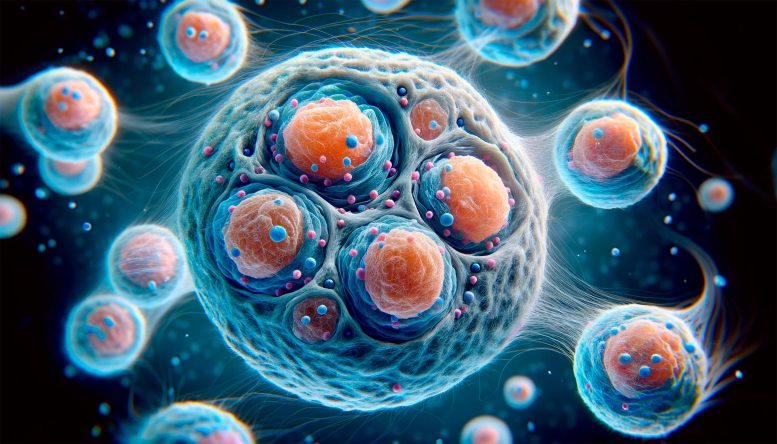
MIT researchers propose a theoretical model explaining how cells maintain their identity over generations. The model suggests that a cell’s 3D genome structure guides the restoration of epigenetic marks lost during cell division. This mechanism enables cells to remember their specific type, with implications for understanding diseases and aging processes. Credit: SciTechDaily.com
MIT study suggests 3D folding of the genome is key to cells’ ability to store and pass on “memories” of which genes they should express.
Every cell in the human body contains the same genetic instructions, encoded in its DNA . However, out of about 30,000 genes, each cell expresses only those genes that it needs to become a nerve cell, immune cell, or any of the other hundreds of cell types in the body.
Each cell’s fate is largely determined by chemical modifications to the proteins that decorate its DNA; these modifications in turn control which genes get turned on or off. When cells copy their DNA to divide, however, they lose half of these modifications, leaving the question: How do cells maintain the memory of what kind of cell they are supposed to be?
Genome Folding and Cellular Memory
A new MIT study proposes a theoretical model that helps explain how these memories are passed from generation to generation when cells divide. The research team suggests that within each cell’s nucleus, the 3D folding of its genome determines which parts of the genome will be marked by these chemical modifications. After a cell copies its DNA, the marks are partially lost, but the 3D folding allows the cell to easily restore the chemical marks needed to maintain its identity. And each time a cell divides, chemical marks allow a cell to restore its 3D folding of its genome. This way, by juggling the memory between 3D folding and the marks, the memory can be preserved over hundreds of cell divisions.
“A key aspect of how cell types differ is that different genes are turned on or off. It’s very difficult to transform one cell type to another because these states are very committed,” says Jeremy Owen PhD ’22, the lead author of the study. “What we have done in this work is develop a simple model that highlights qualitative features of the chemical systems inside cells and how they need to work in order to make memories of gene expression stable.”
Leonid Mirny, a professor in MIT’s Institute for Medical Engineering and Science and the Department of Physics, is the senior author of the paper, which was published recently in the journal Science . Dino Osmanović, a former postdoctoral fellow at MIT’s Center for the Physics of Living Systems, is also an author of the study.
Maintaining Epigenetic Memory
Within the cell nucleus, DNA is wrapped around proteins called histones, forming a densely packed structure known as chromatin. Histones can display a variety of modifications that help control which genes are expressed in a given cell. These modifications generate “epigenetic memory,” which helps a cell to maintain its cell type. However, how this memory is passed on to daughter cells is somewhat of a mystery.
Previous work by Mirny’s lab has shown that the 3D structure of chromosomes is, to a great extent, determined by these epigenetic modifications, or marks. In particular, they found that certain chromatin regions, with marks telling cells not to read a particular segment of DNA, attract each other and form dense clumps called heterochromatin, which are difficult for the cell to access.
In their new study, Mirny and his colleagues wanted to answer the question of how those epigenetic marks are maintained from generation to generation. They developed a computational model of a polymer with a few marked regions, and saw that these marked regions collapse into each other, forming a dense clump. Then they studied how these marks are lost and gained.
When a cell copies its DNA to divide it between two daughter cells, each copy gets about half of the epigenetic marks. The cell then needs to restore the lost marks before the DNA is passed to the daughter cells, and the way chromosomes were folded serves as a blueprint for where these remaining marks should go.
These modifications are added by specialized enzymes known as “reader-writer” enzymes. Each of these enzymes is specific for a certain mark, and once they “read” existing marks, they “write” additional marks at nearby locations. If the chromatin is already folded into a 3D shape, marks will accumulate in regions that already had modifications inherited from the parent cell.
“There are several lines of evidence that suggest that the spreading can happen in 3D, meaning if there are two parts that are near each other in space, even if they’re not adjacent along the DNA, then spreading can happen from one to another,” Owen says. “That is how the 3D structure can influence the spreading of these marks.”
This process is analogous to the spread of infectious disease, as the more contacts that a chromatin region has with other regions, the more likely it is to be modified, just as an individual is more likely to become infected as their number of contacts increases. In this analogy, dense regions of marked chromatin are like cities where people have many social interactions, while the rest of the genome is comparable to sparsely populated rural areas.
“That essentially means that the marks will be spreading in the dense region and will be very sparse anywhere outside it,” Mirny says.
Epigenetic Memory and Information Processing
The new model also suggests possible parallels between epigenetic memories stored in a folded polymer and memories stored in a neural network, he adds. Folding of marked regions can be thought of as analogous to the strong connections formed between neurons that fire together in a neural network.
“Broadly this suggests that akin to the way neural networks are able to do very complex information processing, the epigenetic memory mechanism we described may be able to process information, not only store it,” he says.
“One beautiful aspect of the work is how it offers and explores connections with ideas from the seemingly very distant corners of science, including spreading of infections (to describe formation of new chemical marks in the 3D vicinity of the existing one), associative memory in model neural networks, and protein folding,” says Alexander Grosberg, a professor of physics at New York University , who was not involved in the research.
Epigenetic Erosion
While this model appeared to offer a good explanation for how epigenetic memory can be maintained, the researchers found that eventually, reader-writer enzyme activity would lead to the entire genome being covered in epigenetic modifications. When they altered the model to make the enzyme weaker, it didn’t cover enough of the genome and memories were lost in a few cell generations.
To get the model to more accurately account for the preservation of epigenetic marks, the researchers added another element: limiting the amount of reader-writer enzyme available. They found that if the amount of enzyme was kept between 0.1 and 1 percent of the number of histones (a percentage based on estimates of the actual abundance of these enzymes), their model cells could accurately maintain their epigenetic memory for up to hundreds of generations, depending on the complexity of the epigenetic pattern.
It is already known that cells begin to lose their epigenetic memory as they age, and the researchers now plan to study whether the process they described in this paper might play a role in epigenetic erosion and loss of cell identity. They also plan to model a disease called progeria, in which cells have a genetic mutation that leads to loss of heterochromatin. People with this disease experience accelerated aging.
“The mechanistic link between these mutations and the epigenetic changes that eventually happen is not well understood,” Owen says. “It would be great to use a model like ours where there are dynamic marks, together with polymer dynamics, to try and explain that.”
The researchers also hope to work with collaborators to experimentally test some of the predictions of their model, which could be done, for example, by altering the level of reader-writer enzymes in living cells and measuring the effect on epigenetic memory.
Reference: “Design principles of 3D epigenetic memory systems” by Jeremy A. Owen, Dino Osmanović and Leonid Mirny, 17 November 2023, Science . DOI: 10.1126/science.adg3053
The research was funded by the National Human Genome Research Institute, the National Institute of General Medical Sciences, and the National Science Foundation.
More on SciTechDaily

NASA to Practice Artemis Moonwalking, Roving Operations in Arizona Desert

The Unseen Spark: How Ancient Microorganisms Helped Cause Massive Volcanic Events

Martian Meteorite Almost 4 Billion Years Younger Than Previously Thought

Turning Diamond Into Metal – For Improved Solar Cells, LEDs, and Power Electronics

Near-Earth Asteroid 2014 JO25 Set to Fly Past Earth on April 19
Microscopic Arms Race: Solving the Mystery Behind Bacteria’s Extensive Weaponry
How Artificial Intelligence Found the Words To Kill Cancer Cells

Megalodon Was No Cold-Blooded Killer – And That Spelled Its Doom
Be the first to comment on "inside the cell’s identity crisis: mit’s revolutionary genetic “memories” discovery", leave a comment cancel reply.
Email address is optional. If provided, your email will not be published or shared.
Save my name, email, and website in this browser for the next time I comment.
- Boise State News
- Share on Facebook
- Share on Twitter
- Share on LinkedIn
- Share through Email
- Health, Science and Technology
“Data is in our DNA”: Researchers advance DNA as a memory material
The color of a person’s eyes, the thickness of a potato’s skin, and the shape of a flower: what do these seemingly disparate elements have in common? They are all shaped by DNA. Deoxyribonucleic acid (DNA) encodes and carries the genetic instructions that shape life. But what if it could encode more than genetic information, such as digital archival data?
It might sound like something out of a science fiction movie, but in a novel study published in Nature Communications, members of Boise State’s Nucleic Acid Memory Institute revealed that the future of digital memory storage may be found in utilizing the programmable qualities of chemically synthesized DNA.
“We are simply taking advantage of the information density and programmability of DNA as a memory material,” explained Micron School of Materials Science and Engineering professor Will Hughes.

This innovation, dubbed Nucleic Acid Memory (NAM), harnesses the quaternary code of DNA (adenine, guanine, cytosine, thymine) to reliably store information well beyond the predicted lifetimes of traditional memory materials that make up hard-drives, solid-state drives, and magnetic tape.
Led by Micron School of Materials Science and Engineering professor Hughes, and research scientist George Dickinson, the team was able to encode digital information into DNA, read it back using an optical microscope, and perform error correction on the data to ensure the integrity of the information. Additionally, this technique did not require sequencing technology, which historically has been necessary to read DNA information. The team’s research was published on April 22 in Nature Communications and can be viewed at: https://www.nature.com/articles/s41467-021-22277-y
Support for this research came in 2018 from federal, state and industry agencies, including $2 million awarded by the Higher Education Research Council (HERC) Idaho Global Entrepreneurial Mission (IGEM), and $1.5 million jointly from the National Science Foundation and the Semiconductor Research Corporation.
Because the work lives at the intersection of materials science, computer science, bioengineering, and electrical engineering, a multidisciplinary team of faculty, staff, and students at Boise State pulled together to conduct this cutting-edge research. Hughes said that while the researchers each come from very different areas and fields of study, it is in fact the composition of these individuals and their skills that make this research possible.
The interdisciplinary research team included the expertise of faculty, post-doctoral research scientists and graduate students from across Boise State, including: Hughes, George Dickinson, William Clay, Luca Piantanida, Christopher Green, Chad Watson, Elton Graugnard, and Reza Zadegan of the Micron School of Materials Science and Engineering; Golam Mortuza and Tim Andersen of the Department of Computer Science; Eric Hayden of the Department of Biological Sciences; and Wan Kuang of the Department of Electrical and Computer Engineering.
“We always think of DNA as just something that can store genetic information, but we know so much about the molecular biology of DNA now that we can manipulate it, and instead of using it to store genetic information, you can use it as a building material,” said Dickinson.
This research could not come at a more pressing time. From entertainment streaming services to entire nations’ government archives, the globe’s ever-increasing digital information demands continue to rise. The data industry’s current standard for storage is flash memory, and it relies upon a natural resource: silicon. However, there is a problem.
Global data memory and storage demands are estimated to exceed the projected silicon supply by 2040. DNA is an important potential alternative to silicon-based memory because its volumetric density is 1000 times greater and its energy of operation is 100 million times less than flash memory. While the team does not anticipate DNA memory storage will completely replace flash/electronic memory, it will play an important role in widening the conversation as to possible new materials and methods.
“We may be running out of silicon 20 years from now if we don’t create new technology, and start looking for different ways to store information,” said Victor Zhirnov, chief scientist at Semiconductor Research Corporation in North Carolina. “Information and data storage is one of the major drivers of our economic and social well-being.”
Fortunately, as Zhirnov points out, DNA storage methods such as those being tested in Boise State’s Nucleic Acid Memory Institute could be the answer to the world’s future information storage insecurity.
In this research, the team began by using a long strand of DNA acquired from a bacteriophage (a kind of virus that parasitizes a bacterium) as a scaffold. The team then programmed the strand to fold itself into a ‘DNA breadboard’ using a process called DNA origami. The folding process was supported by the inclusion of short DNA strands called staple strands. Once folded, some of the staple strands extended from the DNA breadboard at particular spots that encoded digital information.
A breadboard is simply a surface that holds electronic components, such as chips and transistors, in a place where they can be built and tested together to develop prototypes. DNA can be used this way too and connected using their four quaternary bases.
Next, the team encoded a message into these DNA breadboards: ‘Data is in our DNA!\n’. Using a super-resolution microscopy technique called DNA-Points Accumulation for Imaging in Nanoscale Topography (DNA-PAINT), the team was able to read back that message optically in binary code.
Additionally, the team used error-correction algorithms that were able to compensate for defects in the synthesis and reading process, making it possible to fully recover the encoded message.
This research builds upon earlier work conducted by Ralf Jungmann (a professor at Ludwig Maximilian University of Munich), and could make it possible to encode and store data in DNA, making it accessible tens or hundreds of thousands of years later, unlike modern memory storage.
As a steppingstone towards this goal, the team believes the research could prove to be valuable in applications that demand a small amount of data to be encoded into an exceedingly small package.
While archival storage is an aspirational goal for the field, “digital Nucleic Acid Memory is attractive for applications that demand a limited amount of data to have high information density, redundancy, and copy number such as encrypted barcodes,” said Hughes.
Office of Communications and Marketing

Mice Inherit Specific Memories, Because Epigenetics?
Two weeks ago I wrote about some tantalizing research coming out of the Society for Neuroscience meeting in San Diego. Brian Dias , a postdoctoral fellow in Kerry Ressler’s lab at Emory University, had reported that mice inherit specific smell memories from their fathers — even when the offspring have never experienced that smell before, and even when they’ve never met their father. What’s more, their children are born with the same specific memory.
This was a big, surprising claim, causing many genetics experts to do a double-take, as I discovered from a subsequent flurry of Tweets . “Crazy Lamarkian shit,” quipped Laura Hercher (@laurahercher), referring to Lamarckian inheritance, the largely discredited theory that says an organism can pass down learned behaviors or traits to its offspring. “My instinct is deep skepticism, but will have to wait for paper to come out,” wrote Kevin Mitchell (@WiringTheBrain). “If true, would be revolutionary.”
The paper is out today in Nature Neuroscience , showing what I reported before as well as the beginnings of an epigenetic explanation. (Epigenetics usually refers to chemical changes that affect gene expression without altering the DNA code).
Having the data in hand allowed me to fill in the backstory of the research, as well as gather more informed reactions from experts in neuroscience and in genetics. I’ve gone into a lot of detail below, but here’s the bottom line: The behavioral results are surprising, solid, and will certainly inspire further studies by many other research groups. The epigenetic data seems gauzy by comparison, with some experts saying it’s thin-but-useful and others finding it full of holes.
So what is the surprising part, again?
If you’ve followed science news over the past decade then you’ve probably heard about epigenetics, a field that’s caught fire in the minds of scientists and the public, and understandably so. Epigenetic studies have shown that changes in an organism’s external environment — its life experiences and even its choices, if you want to get hyperbolic — can influence the expression of its otherwise inflexible DNA code. Epigenetics, in other words, is enticing because it offers a resolution to the tedious, perennial debates of nurture versus nature.
But some scientists dispute the notion that epigenetic changes have much influence on behavior (see this Nature feature this Nature feature this Nature feature for a great overview of the debate). Even more controversial is the idea that epigenetic changes can be passed down from one generation to the next, effectively giving parents a way to prime their children for a specific environment. The key question isn’t whether this so-called ‘transgenerational epigenetic inheritance’ happens — it does — but rather how it happens (and how frequently, and in what contexts and species).
For Hungry Minds
That’s what Dias and Ressler wanted to investigate. Trouble is, environmental influences such as stress are notoriously difficult to measure. So the researchers focused on the mouse olfactory system, the oft-studied and well-mapped brain circuits that process smell. “We thought it would give us a molecular foothold into how transgenerational inheritance might occur,” Dias says.
The researchers made mice afraid of a fruity odor, called acetophenone, by pairing it with a mild shock to the foot. In a study published a few years ago, Ressler had shown that this type of fear learning is specific: Mice trained to fear one particular smell show an increased startle to that odor but not others. What’s more, this fear learning changes the organization of neurons in the animal’s nose, leading to more cells that are sensitive to that particular smell.
Ten days after this fear training, Dias allowed the animals to mate. And that’s where the crazy begins. The offspring (known as the F1 generation) show an increased startle to the fruity smell even when they have never encountered the smell before, and thus have no obvious reason to be sensitive to it. And their reaction is specific: They do not startle to another odor called propanol. Craziest of all, their offspring (the F2 generation) show the same increased sensitivity to acetophenone.
The scientists then looked at the F1 and F2 animals’ brains. When the grandparent generation is trained to fear acetophenone, the F1 and F2 generations’ noses end up with more “M71 neurons,” which contain a receptor that detects acetophenone. Their brains also have larger “M71 glomeruli,” a region of the olfactory bulb that responds to this smell.
“When Brian came in with the first set of data, we both just couldn’t believe it,” Ressler recalls. “I was like, ‘Well, it must just be random, let’s do it again.’ And then it just kept working. We do a lot of behavior [experiments], but being able to see structural change that correlates with behavior is really pretty astounding.”
Still, those experiments couldn’t rule out some kind of social, rather than biological transmission. Perhaps fathers exposed to the fear training treated their children differently. Or maybe mothers, sensing something odd in their mate’s behavior, treated their children differently.
To control for these possibilities, the researchers performed an in vitro fertilization (IVF) experiment in which they trained male animals to fear acetophenone and then 10 days later harvested the animals’ sperm. They sent the sperm to another lab across campus where it was used to artificially inseminate female mice. Then the researchers looked at the brains of the offspring. They had larger M71 glomeruli, just as before. (The researchers couldn’t perform behavioral tests on these animals because of laboratory regulations about animal quarantine.)
“For me it clicked when we did the IVF,” Dias says. “When the brain anatomy persisted, that to me emphasized that it’s not really a social transmission. It’s inherited.”
Other researchers also seem convinced. “It is high time public health researchers took human transgenerational responses seriously,” says Marcus Pembrey, emeritus professor of paediatric genetics at University College London, who has been championing the idea of epigenetic inheritance for over a decade. “I suspect we will not understand the rise in neuropsychiatric disorders or obesity, diabetes and metabolic disruptions generally without taking a multigenerational approach,” he says.
In an interesting historical aside, Pembrey also notes that the new study echoes an experiment that Ivan Pavlov did * 90 years ago, in which he trained mice to associate food with the sound of a bell. Pavlov “reported that successive generations took fewer and fewer training sessions before they would search for food on hearing a bell even when food was absent,” Pembrey says. Nevertheless, the idea that experience could be biologically inherited fell out of favor in the 20th century. “If alive today, Pavlov would have been delighted by the Dias and Ressler paper, first as a vindication of his own experiment and results, and second by the amazing experimental tools available to the modern scientist.”
Neuroscientists, too, are enthusiastic about what these results might mean for understanding the brain.
“To my knowledge this is the first example, in any animal, of epigenetic transmission of a simple memory for a specific perceptual stimulus,” says Tomás Ryan , a research fellow at MIT who studies how memories form in the brain. “The broader implications for the neuroscience of memory and to evolutionary biology in general could be paradigm shifting and unprecedented.”
There are still some unanswered questions, Ryan notes. For example, the researchers didn’t do a control experiment where the F0 animals are exposed to the fruity odor without the shock. So it’s unclear whether the “memory” they’re transmitting to their offspring is a fear memory, per se, or rather an increased sensitivity to an odor. This is an important distinction, because the brain uses many brain circuits outside of the olfactory bulb to encode fear memories. It’s difficult to imagine how that kind of complicated brain imprint might get passed down to the next generation.
Ressler and Dias agree, and for that reason were careful not to refer to the transmitted information as a fear memory. “I don’t know if it’s a memory,” Dias says. “It’s a sensitivity, for now.”
What’s that got to do with epigenetics?
So let’s call it a sensitivity. How could a smell sensitivity, formed in an adult animal’s olfactory bulb in its brain, possibly be transmitted to its gonads and passed on to future generations?
The researchers are nowhere near being able to answer that question, but they have some data that points to epigenetics.
There are several types of epigenetic modifications. One of the best understood is DNA methylation. There are millions of spots along the mouse genome (and the human genome), called CpG sites, where methyl groups can attach and affect the expression of nearby genes. Typically, methylation dials down gene expression.
Dias and Ressler sent sperm samples of mice that had been fear-conditioned to either acetophenone or propanol to a private company, called Active Motif , which specializes in methylation analyses. The company’s researchers (who were blinded to which samples were which) mapped out the sperm methylation patterns near two olfactory genes: Olfr151, which codes for the M71 receptor that’s sensitive to acetophenone, and Olfr6, which codes for another odor receptor that is not sensitive to either odor.
It turns out that Olfr151, but not the other gene, is significantly less methylated in sperm from animals trained to fear acetophenone than in sperm from those trained to fear propanol. Because less methylation usually means a boost in gene expression, this could plausibly explain why these animals have more M71 receptors in their brains, the researchers say.
What’s more, the same under-methylation shows up in the sperm of F1 animals whose fathers had been trained to fear acetophenone.
“It’s a very precise signal,” Ressler says. “The convergence of this data, we think, shows that this is a really profound and robust phenomenon.”
Others, though, find a number of flaws in this epigenetic explanation.
Timothy Bestor , professor of genetics and development at Columbia University, points out that methylated CpG sites only affect gene expression when they are located in the so-called gene promoter, a region about 500 bases upstream of the gene. But the Olfr151 gene doesn’t have any CpG sites in its promoter.
That means the differences in methylation reported in the paper must have occurred within the body of the gene itself. “And methylation in the gene body is common to all genes whether they’re expressed or not,” Bestor says. “I don’t see any way by which that gene could be directly regulated by methylation.”
But what would explain the methylation differences between the trained animals and controls? They’re pretty subtle, he says, and “could easily be a statistical fluke.”
Bestor was skeptical from the outset, based on the mechanics of the reproductive system alone. “There’s a real problem in how the signal could reach the germ cells,” he says.
For one thing, the seminiferous tubules, where sperm is made inside of the testes, don’t have any nerves. “So there’s no way the central nervous system could affect germ cell development.” What’s more, he says it’s not likely that acetophenone would be able to cross the blood-testis barrier , the sheet of cells that separates the seminiferous tubules from the blood.
By this point in my conversation with Bestor, I was starting to feel a bit defensive on behalf of epigenetics and all of its wonder. “Are you saying you think epigenetic inheritance is a bunch of bologna?” I asked helplessly.
“No,” he said, laughing. “It’s just not as dynamic as people think.”

What’s next?
A good next step in resolving these pesky mechanistic questions would be to use chromatography to see whether odorant molecules like acetophenone actually get into the animals’ bloodstream, Dias says. “The technology is surely there, and I think we are going to go down those routes.”
First, though, Dias and Ressler are working on another behavioral experiment. They want to know: If the F0 mice un-learn the fear of acetophenone (which can be done by repeated exposures to the smell without a shock) and then reproduce, will their children still have an increased sensitivity to it?
“We have no idea yet,” says Ressler, a practicing psychiatrist who has long been interested in the effects of post-traumatic stress disorder (PTSD). “But we think this would have tremendous implications for the treatment of adults [with PTSD] before they have children.”
It will take a lot more work before scientists come close to understanding how these data relate to human anxiety disorders. So what, after all of these words, should we take away from this study now?
Hell if I know. Here’s the most rational and conservative appraisal I can muster: Our bodies are constantly adapting to a changing world. We have many ways of helping our children make that unpredictable world slightly more predictable, and some of those ways seem to be hidden in our genome.
Anne Ferguson-Smith , a geneticist at the University of Cambridge, put it more succinctly. The study, she says, “potentially adds to the growing list of compelling models telling us that something is going on that facilitates transmission of environmentally induced traits.”
Scientists, I have to assume, will be furiously working on what that something is for many decades to come. And I’ll be following along, or trying to, with awe.
*Update, 12/1/13, 2:35pm: It seems that that Pavlov experiment may have been retracted in 1927, though I don’t know anything about that beyond what is stated here .
Style note: A few paragraphs of this post were adapted from my earlier post on this research, published November 15 .
- Environment
- Perpetual Planet
History & Culture
- History & Culture
- History Magazine
- Mind, Body, Wonder
- Paid Content
- Terms of Use
- Privacy Policy
- Your US State Privacy Rights
- Children's Online Privacy Policy
- Interest-Based Ads
- About Nielsen Measurement
- Do Not Sell or Share My Personal Information
- Nat Geo Home
- Attend a Live Event
- Book a Trip
- Inspire Your Kids
- Shop Nat Geo
- Visit the D.C. Museum
- Learn About Our Impact
- Support Our Mission
- Advertise With Us
- Customer Service
- Renew Subscription
- Manage Your Subscription
- Work at Nat Geo
- Sign Up for Our Newsletters
- Contribute to Protect the Planet
Copyright © 1996-2015 National Geographic Society Copyright © 2015-2024 National Geographic Partners, LLC. All rights reserved
Gene linked to learning difficulties has direct impact on learning and memory
A gene previously linked to intellectual disability has been found to regulate learning and memory in mice.
The gene, called KDM5B has previously been linked to some intellectual disability disorders and autism. In the general population, some variants are also associated with reduced brain function, although not sufficient to cause an overt disability or behavioural symptoms.
Now, researchers at King's College London, the University of Exeter and the University of California Irvine have found that reduced function of the gene in the brain results in loss of learning ability and memory and a reduction in the brain's ability to strengthen connections between neurons, which is key in the formation of memories.
The team's new mouse study, published in the Journal of Neuroscience describes how mice bred without a fully functional KDM5B gene have worse learning and memory abilities. In order to rule out the possibility that the effect may have been caused by an impact on brain development, the researchers also reduced the amount of this gene in a separate group of adult mice, in the hippocampus, a brain region responsible for memory. They found that reduced gene function resulted in epileptic seizures in some mice and a deterioration of their learning and memory. Laboratory experiments suggested that the strengthening of connections between neurons during memory formation was reduced.
Professor Albert Basson, whose research group began the work at King's College London and has since moved to the University of Exeter, said: "Memory and the ability to learn are fundamental to our intellectual potential, yet we still have a lot to learn about the underpinning mechanisms. For more than a decade, the KDM5B gene has been linked to autism and some forms of intellectual disability, but a mutation in this gene alone is not always sufficient to cause these conditions, so it hasn't been studied in detail. Our work shows that KDM5B is important for learning and memory and provides new insight into the fundamental mechanisms of memory and learning, which is crucial on the pathway to finding new ways to improve these functions."
KDM5B can modify the structure of the genetic material in our cells which determines whether genes necessary for brain development or function are expressed at the correct amount at the right time.
Dr Leticia Peres-Sisquez who performed the research at King's College London, said: "We set out to investigate whether KDM5B's ability to modify genetic material has a direct impact on learning and memory. We've discovered that the gene has a direct impact on learning and memory -- which is distinct from any effect during brain development. This gene will now be of much greater interest to researchers on the quest for new treatments for conditions including autism, and other intellectual disability disorders."
The research was funded by the Medical Research Council and the National Institutes of Aging, with support from Wellcome.
The study is entitled 'The intellectual disability risk gene Kdm5b regulates long term memory consolidation in the hippocampus', published in the Journal of Neuroscience .
- Gene Therapy
- Birth Defects
- Diseases and Conditions
- Medical Topics
- Nervous System
- Children's Health
- Brain Tumor
- Computational neuroscience
- House mouse
- Tumor suppressor gene
Story Source:
Materials provided by University of Exeter . Original written by Louise Vennells. Note: Content may be edited for style and length.
Journal Reference :
- Leticia Perez-Sisques, Shail Bhatt, Rugile Matuleviciute, Talia Gileadi, Eniko Kramar, Andrew Graham, Franklin G. Garcia, Ashley Keiser, Dina P. Matheos, James A. Cain, Alan M. Pittman, Laura C. Andreae, Cathy Fernandes, Marcelo A. Wood, K. Peter Giese, M. Albert Basson. The intellectual disability risk geneKdm5bregulates long term memory consolidation in the hippocampus . The Journal of Neuroscience , 2024; e1544232024 DOI: 10.1523/JNEUROSCI.1544-23.2024
Cite This Page :
Explore More
- Universe's Oldest Stars in Our Galactic Backyard
- Polygenic Embryo Screening for IVF: Opinions
- VR With Cinematoghraphics More Engaging
- 2023 Was the Hottest Summer in 2000 Years
- Fastest Rate of CO2 Rise Over Last 50,000 Years
- Like Dad and Like Mum...all in One Plant
- What Makes a Memory? Did Your Brain Work Hard?
- Plant Virus Treatment for Metastatic Cancers
- Controlling Shape-Shifting Soft Robots
- Brain Flexibility for a Complex World
Trending Topics
Strange & offbeat.
- Election 2024
- Entertainment
- Newsletters
- Photography
- Personal Finance
- AP Investigations
- AP Buyline Personal Finance
- AP Buyline Shopping
- Press Releases
- Israel-Hamas War
- Russia-Ukraine War
- Global elections
- Asia Pacific
- Latin America
- Middle East
- Election Results
- Delegate Tracker
- AP & Elections
- Auto Racing
- 2024 Paris Olympic Games
- Movie reviews
- Book reviews
- Personal finance
- Financial Markets
- Business Highlights
- Financial wellness
- Artificial Intelligence
- Social Media
A gene long thought to just raise the risk for Alzheimer’s may cause some cases
FILE - A section of a human brain with Alzheimer’s disease is displayed at the Museum of Neuroanatomy at the University at Buffalo, in Buffalo, N.Y., Oct. 7, 2003. A long-feared gene appears to do more than raise people’s risk of Alzheimer’s: Inheriting two copies can cause the mind-robbing disease, according to research published in the journal Nature Medicine on Monday, May 6, 2024. (AP Photo/David Duprey, File)
- Copy Link copied
WASHINGTON (AP) — For the first time, researchers have identified a genetic form of late-in-life Alzheimer’s disease — in people who inherit two copies of a worrisome gene.
Scientists have long known a gene called APOE4 is one of many things that can increase people’s risk for Alzheimer’s, including simply getting older. The vast majority of Alzheimer’s cases occur after age 65. But research published Monday suggests that for people who carry not one but two copies of the gene, it’s more than a risk factor, it’s an underlying cause of the mind-robbing disease.
The findings mark a distinction with “profound implications,” said Dr. Juan Fortea, who led the study the Sant Pau Research Institute in Barcelona, Spain.
Among them: Symptoms can begin seven to 10 years sooner than in other older adults who develop Alzheimer’s.
An estimated 15% of Alzheimer’s patients carry two copies of APOE4, meaning those cases “can be tracked back to a cause and the cause is in the genes,” Fortea said. Until now, genetic forms of Alzheimer’s were thought to be only types that strike at much younger ages and account for less than 1% of all cases.
Scientists say the research makes it critical to develop treatments that target the APOE4 gene. Some doctors won’t offer the only drug that has been shown to modestly slow the disease, Leqembi, to people with the gene pair because they’re especially prone to a dangerous side effect, said Dr. Reisa Sperling, a study coauthor at Harvard-affiliated Brigham and Women’s Hospital in Boston.
Sperling hunts ways to prevent or at least delay Alzheimer’s and “this data for me says wow, what an important group to be able to go after before they become symptomatic.”
But the news doesn’t mean people should race for a gene test. “It’s important not to scare everyone who has a family history” of Alzheimer’s because this gene duo isn’t behind most cases, she told The Associated Press.
HOW DO GENETICS AFFECT ALZHEIMER’S?
More than 6 million Americans, and millions more worldwide, have Alzheimer’s. A handful of genes are known to cause rare “early-onset” forms, mutations passed through families that trigger symptoms unusually young, by age 50. Some cases also are linked to Down syndrome.
But Alzheimer’s most commonly strikes after 65, especially in the late 70s to 80s, and the APOE gene – which also affects how the body handles fats -- was long known to play some role. There are three main varieties. Most people carry the APOE3 variant that appears to neither increase nor decrease Alzheimer’s risk. Some carry APOE2, which provides some protection against Alzheimer’s.
APOE4 has long been labeled the biggest genetic risk factor for late-in-life Alzheimer’s, with two copies risker than one. About 2% of the global population is estimated to have inherited a copy from each parent.
RESEARCH POINTS TO A CAUSE FOR A SUBSET OF ALZHEIMER’S
To better understand the gene’s role, Fortea’s team used data from 3,297 brains donated for research and from over 10,000 people in U.S. and European Alzheimer’s studies. They examined symptoms and early hallmarks of Alzheimer’s such as sticky amyloid in the brain.
People with two APOE4 copies were accumulating more amyloid at age 55 than those with just one copy or the “neutral” APOE3 gene variety, they reported in the journal Nature Medicine. By age 65, brain scans showed significant plaque buildup in nearly three-quarters of those double carriers – who also were more likely to have initial Alzheimer’s symptoms around that age rather than in the 70s or 80s.
Fortea said the disease’s underlying biology was remarkably similar to young inherited types.
It appears more like “a familial form of Alzheimer’s,” said Dr. Eliezer Masliah of the National Institute on Aging. “It is not just a risk factor.”
Importantly, not everyone with two APOE4 genes develops Alzheimer’s symptoms and researchers need to learn why, Sperling cautioned.
“It’s not quite destiny,” she said.
HOW THE NEW FINDINGS MAY AFFECT ALZHEIMER’S RESEARCH AND TREATMENT
The drug Leqembi works by clearing away some sticky amyloid but Sperling said it’s not clear if carriers of two APOE4 genes benefit because they have such a high risk of a side effect from the drug – dangerous brain swelling and bleeding. One research question is whether they’d do better starting such drugs sooner than other people.
Masliah said other research aims to develop gene therapy or drugs to specifically target APOE4. He said it’s also crucial to understand APOE4’s effects in diverse populations since it’s been studied mostly in white people of European ancestry.
As for gene tests, for now they’re typically used only to evaluate if someone’s a candidate for Leqembi or for people enrolling in Alzheimer’s research – especially studies of possible ways to prevent the disease. Sperling said the people most likely to carry two APOE4 genes had parents who both got Alzheimer’s relatively early, in their 60s rather than 80s.
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group. The AP is solely responsible for all content.
A gene long thought to just raise the risk for Alzheimer's may cause some cases
For the first time, researchers have identified a genetic form of late-in-life Alzheimer’s disease
WASHINGTON — For the first time, researchers have identified a genetic form of late-in-life Alzheimer’s disease — in people who inherit two copies of a worrisome gene.
Scientists have long known a gene called APOE4 is one of many things that can increase people’s risk for Alzheimer’s, including simply getting older. The vast majority of Alzheimer’s cases occur after age 65. But research published Monday suggests that for people who carry not one but two copies of the gene, it’s more than a risk factor, it’s an underlying cause of the mind-robbing disease.

- Share full article
Advertisement
Supported by
Study Suggests Genetics as a Cause, Not Just a Risk, for Some Alzheimer’s
People with two copies of the gene variant APOE4 are almost certain to get Alzheimer’s, say researchers, who proposed a framework under which such patients could be diagnosed years before symptoms.

By Pam Belluck
Scientists are proposing a new way of understanding the genetics of Alzheimer’s that would mean that up to a fifth of patients would be considered to have a genetically caused form of the disease.
Currently, the vast majority of Alzheimer’s cases do not have a clearly identified cause. The new designation, proposed in a study published Monday, could broaden the scope of efforts to develop treatments, including gene therapy, and affect the design of clinical trials.
It could also mean that hundreds of thousands of people in the United States alone could, if they chose, receive a diagnosis of Alzheimer’s before developing any symptoms of cognitive decline, although there currently are no treatments for people at that stage.
The new classification would make this type of Alzheimer’s one of the most common genetic disorders in the world, medical experts said.
“This reconceptualization that we’re proposing affects not a small minority of people,” said Dr. Juan Fortea, an author of the study and the director of the Sant Pau Memory Unit in Barcelona, Spain. “Sometimes we say that we don’t know the cause of Alzheimer’s disease,” but, he said, this would mean that about 15 to 20 percent of cases “can be tracked back to a cause, and the cause is in the genes.”
The idea involves a gene variant called APOE4. Scientists have long known that inheriting one copy of the variant increases the risk of developing Alzheimer’s, and that people with two copies, inherited from each parent, have vastly increased risk.
The new study , published in the journal Nature Medicine, analyzed data from over 500 people with two copies of APOE4, a significantly larger pool than in previous studies. The researchers found that almost all of those patients developed the biological pathology of Alzheimer’s, and the authors say that two copies of APOE4 should now be considered a cause of Alzheimer’s — not simply a risk factor.
The patients also developed Alzheimer’s pathology relatively young, the study found. By age 55, over 95 percent had biological markers associated with the disease. By 65, almost all had abnormal levels of a protein called amyloid that forms plaques in the brain, a hallmark of Alzheimer’s. And many started developing symptoms of cognitive decline at age 65, younger than most people without the APOE4 variant.
“The critical thing is that these individuals are often symptomatic 10 years earlier than other forms of Alzheimer’s disease,” said Dr. Reisa Sperling, a neurologist at Mass General Brigham in Boston and an author of the study.
She added, “By the time they are picked up and clinically diagnosed, because they’re often younger, they have more pathology.”
People with two copies, known as APOE4 homozygotes, make up 2 to 3 percent of the general population, but are an estimated 15 to 20 percent of people with Alzheimer’s dementia, experts said. People with one copy make up about 15 to 25 percent of the general population, and about 50 percent of Alzheimer’s dementia patients.
The most common variant is called APOE3, which seems to have a neutral effect on Alzheimer’s risk. About 75 percent of the general population has one copy of APOE3, and more than half of the general population has two copies.
Alzheimer’s experts not involved in the study said classifying the two-copy condition as genetically determined Alzheimer’s could have significant implications, including encouraging drug development beyond the field’s recent major focus on treatments that target and reduce amyloid.
Dr. Samuel Gandy, an Alzheimer’s researcher at Mount Sinai in New York, who was not involved in the study, said that patients with two copies of APOE4 faced much higher safety risks from anti-amyloid drugs.
When the Food and Drug Administration approved the anti-amyloid drug Leqembi last year, it required a black-box warning on the label saying that the medication can cause “serious and life-threatening events” such as swelling and bleeding in the brain, especially for people with two copies of APOE4. Some treatment centers decided not to offer Leqembi, an intravenous infusion, to such patients.
Dr. Gandy and other experts said that classifying these patients as having a distinct genetic form of Alzheimer’s would galvanize interest in developing drugs that are safe and effective for them and add urgency to current efforts to prevent cognitive decline in people who do not yet have symptoms.
“Rather than say we have nothing for you, let’s look for a trial,” Dr. Gandy said, adding that such patients should be included in trials at younger ages, given how early their pathology starts.
Besides trying to develop drugs, some researchers are exploring gene editing to transform APOE4 into a variant called APOE2, which appears to protect against Alzheimer’s. Another gene-therapy approach being studied involves injecting APOE2 into patients’ brains.
The new study had some limitations, including a lack of diversity that might make the findings less generalizable. Most patients in the study had European ancestry. While two copies of APOE4 also greatly increase Alzheimer’s risk in other ethnicities, the risk levels differ, said Dr. Michael Greicius, a neurologist at Stanford University School of Medicine who was not involved in the research.
“One important argument against their interpretation is that the risk of Alzheimer’s disease in APOE4 homozygotes varies substantially across different genetic ancestries,” said Dr. Greicius, who cowrote a study that found that white people with two copies of APOE4 had 13 times the risk of white people with two copies of APOE3, while Black people with two copies of APOE4 had 6.5 times the risk of Black people with two copies of APOE3.
“This has critical implications when counseling patients about their ancestry-informed genetic risk for Alzheimer’s disease,” he said, “and it also speaks to some yet-to-be-discovered genetics and biology that presumably drive this massive difference in risk.”
Under the current genetic understanding of Alzheimer’s, less than 2 percent of cases are considered genetically caused. Some of those patients inherited a mutation in one of three genes and can develop symptoms as early as their 30s or 40s. Others are people with Down syndrome, who have three copies of a chromosome containing a protein that often leads to what is called Down syndrome-associated Alzheimer’s disease .
Dr. Sperling said the genetic alterations in those cases are believed to fuel buildup of amyloid, while APOE4 is believed to interfere with clearing amyloid buildup.
Under the researchers’ proposal, having one copy of APOE4 would continue to be considered a risk factor, not enough to cause Alzheimer’s, Dr. Fortea said. It is unusual for diseases to follow that genetic pattern, called “semidominance,” with two copies of a variant causing the disease, but one copy only increasing risk, experts said.
The new recommendation will prompt questions about whether people should get tested to determine if they have the APOE4 variant.
Dr. Greicius said that until there were treatments for people with two copies of APOE4 or trials of therapies to prevent them from developing dementia, “My recommendation is if you don’t have symptoms, you should definitely not figure out your APOE status.”
He added, “It will only cause grief at this point.”
Finding ways to help these patients cannot come soon enough, Dr. Sperling said, adding, “These individuals are desperate, they’ve seen it in both of their parents often and really need therapies.”
Pam Belluck is a health and science reporter, covering a range of subjects, including reproductive health, long Covid, brain science, neurological disorders, mental health and genetics. More about Pam Belluck
The Fight Against Alzheimer’s Disease
Alzheimer’s is the most common form of dementia, but much remains unknown about this daunting disease..
How is Alzheimer’s diagnosed? What causes Alzheimer’s? We answered some common questions .
A study suggests that genetics can be a cause of Alzheimer’s , not just a risk, raising the prospect of diagnosis years before symptoms appear.
Determining whether someone has Alzheimer’s usually requires an extended diagnostic process . But new criteria could lead to a diagnosis on the basis of a simple blood test .
The F.D.A. has given full approval to the Alzheimer’s drug Leqembi. Here is what to know about i t.
Alzheimer’s can make communicating difficult. We asked experts for tips on how to talk to someone with the disease .
Some cases of Alzheimer's caused by two copies of a single gene
For the first time, researchers have identified a genetic form of late-in-life Alzheimer’s disease — in people who inherit two copies of a worrisome gene.
Scientists have long known a gene called APOE4 is one of many things that can increase people’s risk for Alzheimer’s, including simply getting older. The vast majority of Alzheimer’s cases occur after age 65. But research published Monday suggests that for people who carry not one but two copies of the gene, it’s more than a risk factor, it’s an underlying cause of the mind-robbing disease.
The findings mark a distinction with “profound implications,” said Dr. Juan Fortea, who led the study the Sant Pau Research Institute in Barcelona, Spain.
Among them: Symptoms can begin seven to 10 years sooner than in other older adults who develop Alzheimer’s.
An estimated 15% of Alzheimer’s patients carry two copies of APOE4, meaning those cases “can be tracked back to a cause and the cause is in the genes,” Fortea said. Until now, genetic forms of Alzheimer’s were thought to be only types that strike at much younger ages and account for less than 1% of all cases.
Scientists say the research makes it critical to develop treatments that target the APOE4 gene. Some doctors won’t offer the only drug that has been shown to modestly slow the disease, Leqembi , to people with the gene pair because they’re especially prone to a dangerous side effect, said Dr. Reisa Sperling, a study coauthor at Harvard-affiliated Brigham and Women’s Hospital in Boston.
Sperling hunts ways to prevent or at least delay Alzheimer’s and “this data for me says wow, what an important group to be able to go after before they become symptomatic.”
But the news doesn’t mean people should race for a gene test.
“It’s important not to scare everyone who has a family history” of Alzheimer’s because this gene duo isn’t behind most cases, she told The Associated Press.
How do genetics affects Alzheimer's?
More than 6 million Americans, and millions more worldwide, have Alzheimer’s. A handful of genes are known to cause rare “early-onset” forms, mutations passed through families that trigger symptoms unusually young, by age 50. Some cases also are linked to Down syndrome.
Latest news on Alzheimer's disease
- A type of belly fat may be linked to increased risk of Alzheimer's.
- In an unusual move, FDA postpones decision on Lilly's Alzheimer's drug.
- Decades-old HGH treatments linked to five cases of early Alzheimer's.
But Alzheimer’s most commonly strikes after 65, especially in the late 70s to 80s, and the APOE gene — which also affects how the body handles fats -- was long known to play some role. There are three main varieties. Most people carry the APOE3 variant that appears to neither increase nor decrease Alzheimer’s risk. Some carry APOE2, which provides some protection against Alzheimer’s.
APOE4 has long been labeled the biggest genetic risk factor for late-in-life Alzheimer’s, with two copies risker than one. About 2% of the global population is estimated to have inherited a copy from each parent.
Pointing to a cause for a subset of Alzheimer's
To better understand the gene’s role, Fortea’s team used data from 3,297 brains donated for research and from over 10,000 people in U.S. and European Alzheimer’s studies. They examined symptoms and early hallmarks of Alzheimer’s such as sticky amyloid in the brain.
People with two APOE4 copies were accumulating more amyloid at age 55 than those with just one copy or the “neutral” APOE3 gene variety, they reported in the journal Nature Medicine. By age 65, brain scans showed significant plaque buildup in nearly three-quarters of those double carriers — who also were more likely to have initial Alzheimer’s symptoms around that age rather than in the 70s or 80s.
Fortea said the disease’s underlying biology was remarkably similar to young inherited types.
It appears more like “a familial form of Alzheimer’s,” said Dr. Eliezer Masliah of the National Institute on Aging. “It is not just a risk factor.”
Importantly, not everyone with two APOE4 genes develops Alzheimer’s symptoms and researchers need to learn why, Sperling cautioned.
“It’s not quite destiny,” she said.
How new findings may affect research
The drug Leqembi works by clearing away some sticky amyloid but Sperling said it’s not clear if carriers of two APOE4 genes benefit because they have such a high risk of a side effect from the drug — dangerous brain swelling and bleeding. One research question is whether they’d do better starting such drugs sooner than other people.
Masliah said other research aims to develop gene therapy or drugs to specifically target APOE4. He said it’s also crucial to understand APOE4’s effects in diverse populations since it’s been studied mostly in white people of European ancestry.
As for gene tests, for now they’re typically used only to evaluate if someone’s a candidate for Leqembi or for people enrolling in Alzheimer’s research — especially studies of possible ways to prevent the disease. Sperling said the people most likely to carry two APOE4 genes had parents who both got Alzheimer’s relatively early, in their 60s rather than their 80s.
The Associated Press
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Epigenetic memory articles from across Nature Portfolio
The epigenetic memory of a cell defines the set of modifications to the cell's deoxyribonucleic acid (DNA) that do not alter the DNA sequence, and have been inherited from the cell from which it descends. Such modifications can alter gene expression and therefore the properties and behaviour of the cell.
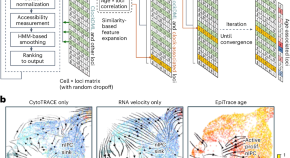
Decoding cell replicational age from single-cell ATAC-seq data
The replicational age of single cells provides a temporal reference for tracking cell fate transition trajectories. The computational framework EpiTrace measures cell age using single-cell ATAC-seq data, specifically by considering chromatin accessibility at clock-like genomic loci, enabling the reconstruction of the history of developmental and pathological processes.
Latest Research and Reviews
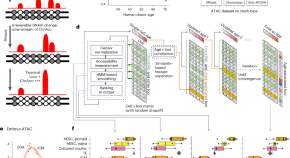
Tracking single-cell evolution using clock-like chromatin accessibility loci
EpiTrace infers cell mitotic age and evolution from single-cell ATAC-seq data.

Paternal microbiome perturbations impact offspring fitness
Disturbances in the gut microbiota of male mice manifest as fitness defects in their offspring by affecting plancenta function, revealing a paternal gut–germline axis.
- Ayele Argaw-Denboba
- Thomas S. B. Schmidt
- Jamie A. Hackett
Transient loss of Polycomb components induces an epigenetic cancer fate
A transient perturbation of transcriptional silencing mediated by Polycomb proteins is sufficient to induce an epigenetic cancer cell fate in Drosophila in the absence of driver mutations.
- V. Loubiere
Protein restriction during pregnancy alters Cdkn1c silencing, dopamine circuitry and offspring behaviour without changing expression of key neuronal marker genes
- Chiara Prodani
- Elaine E. Irvine
- Amanda G. Fisher
Paternal dietary macronutrient balance and energy intake drive metabolic and behavioral differences among offspring
The dietary factors causing varying intergenerational responses are not fully identified. Here, the authors show that the relative proportion of protein, fats, and carbohydrates in paternal diets before conception differentially influences the phenotype of the next-generation offspring on energy metabolism and behaviour.
- Angela Jane Crean
- Alistair McNair Senior
- Stephen James Simpson
Expression levels and DNA methylation profiles of the growth gene SHOX in cartilage tissues and chondrocytes
- Atsushi Hattori
- Atsuhito Seki
- Maki Fukami
News and Comment
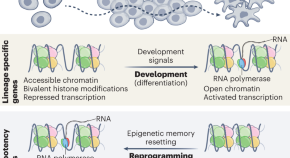
Defining the chromatin signature of pluripotency
The interplay between DNA and its associated proteins has a crucial role in regulating gene expression and determining cellular identity. Here we revisit an earlier Nature Cell Biology study that established the chromatin signature associated with pluripotency.
- Nathalie Beaujean
Epigenetic memory
In a recent Developmental Cell paper, Falvo et al. establish a role for epigenetic memory of inflammatory injury in promoting pancreatic tumorigenesis.
- Gabrielle Brewer
Safeguarding transcriptional memory: a mitotic bookmarking role for chromatin remodelers
- Godwin Sokpor
- Huu Phuc Nguyen
Distal memory in wound healing and cancer
Skin wounds induce an epigenetic memory that accelerates the healing of subsequent injuries. The underlying mechanism is now shown to involve epigenetic chromatin modifications in stem cells from a field of distal hair follicles that surround the injury. Importantly, this mechanism also results in a predisposition for skin cancer development.
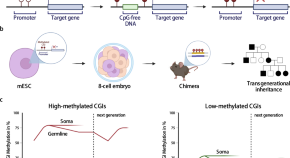
Transgenerational inheritance of engineered cytosine methylation in mice
- Maximilian M. L. Knott
- Oded Rechavi
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Study finds possible 'distinct genetic form' of Alzheimer's disease
Almost all individuals with two copies of the APOE4 gene develop signs of Alzheimer's disease, the study found.
Researchers have found that people who have two copies of a specific gene almost all develop signs of Alzheimer's disease, which could represent a distinct genetic form of the condition.
While scientists knew the gene APOE4 was linked to an increased risk for Alzheimer's, a new study suggests that for people carrying two copies of the gene, it's an underlying cause of it.
Published in Nature Medicine, the study also found that individuals with two copies of the gene develop the disease earlier than people with other variants of the APOE gene.
Alzheimer’s disease is the most common form of dementia and impacts an estimated 7.8 million people in the European Union. Its hallmark symptoms include a decline in memory function and thinking skills.
The findings mark a distinction with “profound implications,” said Dr Juan Fortea, lead author of the study from the Sant Pau Research Institute in Barcelona, Spain.
An estimated 15 per cent of Alzheimer’s patients carry two copies of the gene, meaning those cases “can be tracked back to a cause and the cause is in the genes,” Fortea said.
"This gene has been known for over 30 years and it was known to be associated with a higher risk of developing Alzheimer's disease," he added in a statement.
"But now we know that virtually all individuals with this duplicated gene develop Alzheimer's biology. This is important because they represent between 2 and 3 per cent of the population".
Scientists say the findings mean it is critical to develop treatments that target the APOE4 gene.
- Eating meat and processed foods daily linked to Alzheimer's disease
How are genetics related to Alzheimer's?
A handful of genes are known to cause rare “early-onset” forms of Alzheimer's, mutations passed through families that trigger symptoms unusually young, such as by age 50. Some cases also are linked to Down syndrome.
But Alzheimer’s most commonly strikes after 65, especially in the late 70s to 80s, and the APOE gene, which also affects how the body handles fats, was long known to play some role.
There are three main varieties. Most people carry the APOE3 variant that appears to neither increase nor decrease Alzheimer’s risk. Some carry APOE2, which provides some protection against Alzheimer’s.
APOE4 has long been labelled the biggest genetic risk factor for late-in-life Alzheimer’s, with two copies risker than one.
- Alzheimer’s disease was passed between humans in rare, obsolete medical procedure, study finds
'Familial form of Alzheimer's'
Fortea's team used data from 3,297 brains donated for research and from over 10,000 people in US and European Alzheimer’s studies.
They examined symptoms and early hallmarks of Alzheimer’s such as sticky amyloid in the brain.
People with two APOE4 copies were accumulating more amyloid at age 55 than those with just one copy or the “neutral” APOE3 gene variety, they said in the study.
By age 65, brain scans showed significant plaque buildup in nearly three-quarters of those double carriers, who also were more likely to have initial Alzheimer’s symptoms around that age rather than in the 70s or 80s.
Fortea said the disease's underlying biology was remarkably similar to young inherited types.
It appears more like “a familial form of Alzheimer’s,” said Dr Eliezer Masliah of the National Institute on Aging. “It is not just a risk factor".
Masliah said other research aims to develop gene therapy or drugs to specifically target APOE4.
He said it's also crucial to understand APOE4’s effects in diverse populations since it’s been studied mostly in white people of European ancestry.
But study co-author Dr Reisa Sperling, at Harvard-affiliated Brigham and Women’s Hospital in Boston, said the news doesn't mean people should race for a gene test.
"It’s important not to scare everyone who has a family history" of Alzheimer’s because this gene duo isn’t behind most cases, she told The Associated Press.
You might also like

Could a smartphone app help to detect early signs of dementia?

Human brains are increasing in size. Could this reduce dementia risk?

ADHD could be linked to higher risk of dementia, study finds

IMAGES
VIDEO
COMMENTS
Genetic memory is not an entirely new concept. In 1940, A.A. Brill quoted Dr. William Carpenter who, in comparing math prodigy Zerah Colburn's calculating powers to Mozart's mastery of musical ...
Memories are made by breaking DNA — and fixing it. Nerve cells form long-term memories with the help of an inflammatory response, study in mice finds. By. Max Kozlov. Neurons (shown here in a ...
The urgency to remember a dangerous experience requires the brain to make a series of potentially dangerous moves: Neurons and other brain cells snap open their DNA in numerous locations — more than previously realized, according to a new study — to provide quick access to genetic instructions for the mechanisms of memory storage.. The extent of these DNA double-strand breaks (DSBs) in ...
In psychology, genetic memory is a theorized phenomenon in which certain kinds of memories could be inherited, being present at birth in the absence of any associated sensory experience, and that such memories could be incorporated into the genome over long spans of time.. While theories about the inheritance of specific memories have been thoroughly disproven, some researchers have theorized ...
Further research on the evolution of memory focusing on a genomics approach may help understand humans and their evolution. ... (2013) Drosophila memory research through four eras. genetic, molecular biology, neuroanatomy, and systems neuroscience. Handbook Behav Neurosci 22, 359. [Google Scholar] 81.
MIT study suggests 3D folding of the genome is key to cells' ability to store and pass on "memories" of which genes they should express.. Every cell in the human body contains the same genetic instructions, encoded in its DNA.However, out of about 30,000 genes, each cell expresses only those genes that it needs to become a nerve cell, immune cell, or any of the other hundreds of cell ...
Here, we summarise and critique the last 10 years or so of molecular genetic (DNA-based) research on intelligence, including the discovery of genetic loci associated with intelligence, DNA-based ...
Genetic variability of memory performance is explained by differences in the brain's thalamus. An innovative approach has been used to link genetics to behaviour in mice. The analysis reveals ...
A memory is created when a significant signal is received from the environment and then activates groups of neurons in the brain. Next, genes called 'immediate-early genes' are activated in the neurons, and go on to produce a range of proteins that change the strength of the synapses that connect neurons with each other ( Sheng and ...
In contrast, "memory" now is used to refer to storage of information in general, including in DNA, digital information storage, and neuro-chemical processes. Today, science has moved far beyond a popular understanding of memory as fixed, subjective, and personal. In the extended definition, it is simply the capacity to store and retrieve ...
New research has uncovered the genetic basis for different functions of memory. Combining human transcriptome data with functional neuroimaging, an international team of scientists from Singapore ...
Global data memory and storage demands are estimated to exceed the projected silicon supply by 2040. DNA is an important potential alternative to silicon-based memory because its volumetric density is 1000 times greater and its energy of operation is 100 million times less than flash memory. While the team does not anticipate DNA memory storage ...
Researchers shed new light on the molecular and genetic basis of long-term memory formation in the brain. A new study reveals a single stimulation to the synapses of hippocampal neurons triggered numerous cycles where the memory-coding Arc gene produced mRNA molecules that were then translated into synapse-strengthening Arc proteins. From the findings, researchers determined a novel feedback ...
Featured Genetics Neuroscience. ·May 8, 2024. Summary: Researchers identified a critical role for the gene KDM5B in learning and memory. Their study demonstrates that mice with reduced function of this gene exhibit significant deficits in memory and the ability to learn. Experiments showed that KDM5B is essential for the strengthening of ...
Lasting cellular memories. Dorothy Clyde. Nature Reviews Genetics 21 , 578-579 ( 2020) Cite this article. 1269 Accesses. 1 Altmetric. Metrics. Gene expression can vary greatly among cells in ...
Epigenetic memory in genetic and epigenetic diseases. It has become increasingly clear that defects in epigenetic factors and epigenetic changes could be either causative or act as modifiers in various diseases including cancer (Figure 4). The number of diseases that can be somehow attributable to epigenetic deregulation has been climbing ...
What's more, their children are born with the same specific memory. This was a big, surprising claim, causing many genetics experts to do a double-take, as I discovered from a subsequent flurry ...
Dr Leticia Peres-Sisquez who performed the research at King's College London, said: "We set out to investigate whether KDM5B's ability to modify genetic material has a direct impact on learning ...
For the first time, researchers have identified a genetic form of late-in-life Alzheimer's disease. Most cases of the mind-robbing disease occur after age 65. ... RESEARCH POINTS TO A CAUSE FOR A SUBSET OF ALZHEIMER'S. To better understand the gene's role, Fortea's team used data from 3,297 brains donated for research and from over 10,000 ...
A long-feared gene appears to do more than raise people's risk of Alzheimer's: Inheriting two copies can cause the mind-robbing disease, according to research published in the journal Nature ...
The university's Memory Optimization Lab creates an automated method for selecting helpful memory cues during recall difficulties. A computer model developed by Rutgers University-New Brunswick researchers may have cracked the code on helpful memory cues, similar to how recounting shared experiences with friends can trigger memory recall, according to a study published in Psychological ...
The basic concept of using DNA for data storage can be dated back to the mid-1960s, when Norbert Wiener and Mikhail Neiman discussed 'genetic memory' ideas 8,9.However, DNA sequencing and ...
People with two copies of the gene variant APOE4 are almost certain to get Alzheimer's, say researchers, who proposed a framework under which such patients could be diagnosed years before symptoms.
Each of these heritable components has the potential to uniquely contribute to the working memory deficits observed in genetic disorders, including 22q deletion syndrome, fragile X syndrome, phenylketonuria (PKU), and schizophrenia. ... The breadth of this research has provided a number of potential mechanisms by which a WMem deficit may arise ...
Alzheimer's disease may be inherited more often than previously known, according to a new study that paints a clearer picture of a gene long known to be linked to the common form of dementia.
To better understand the gene's role, Fortea's team used data from 3,297 brains donated for research and from over 10,000 people in U.S. and European Alzheimer's studies.
Epigenetic memory articles from across Nature Portfolio ... Research Highlights 15 Jan 2024 Nature Reviews ... Research Highlights 28 Feb 2023 Nature Reviews Genetics. Volume: 24, P: 209. All News ...
Most research has been with visual WM, and all of the research on WM in humans discussed below concerns visual WM. ... Any function can be lost through genetic changes if the changes are not selected against in the situation of the given organism. Or a function may be lost during one or more of its life stages if the loss is not selected ...
'Familial form of Alzheimer's' Fortea's team used data from 3,297 brains donated for research and from over 10,000 people in US and European Alzheimer's studies.