HSC Chemistry Assignment Solution 2021 | 1st Week


HSC Chemistry Assignment Syllabus 2021
The syllabus is an essential part of the HSC assignment. Students must know the syllabus properly to complete their homework. A huge numbered of students suffered from COVID-19 for almost one year. To consider the student’s situation, the prime education minister Dipu Moni arranged this Assignment system that students can come back to their studies. Here check the clear image for the HSC Chemistry Assignment Syllabus for the 1st week.
HSC Chemistry Assignment Solution 2021
Above, we have discussed the syllabus of the HSC assignment. Now we will talk in detail about the complete solution to this assignment. HSC Chemistry is the first step of Higher Secondary sectors in Bangladesh. Because of Coronavirus, all the students in this class are at home. The Bangladesh education system’s authority arranged these assignment systems to increase activity and bring back to study. The Chemistry Assignments of HSC for the 1st Week are already finished. Now 2nd and 1st-week HSC Chemistry assignments are running in different Colleges. Now Check the HSC Chemistry Assignment Solution 2021 for the 1st Week.

Thanks for being here. If you need anything then comment below or Join our Facebook Group: EducationBD . Check BCS Result Here.
About The Author
Hello This is Astafar. I am a student now and I am a professional Blogger. I have basic knowledge of Web design, Graphic design, SEO, Social Media Marketing etc.
More From Educationbd

HSC Chemistry Assignment 2021 & 2022
HSC Chemistry 1st, 3rd, 4th, 5th, 6th & 7th Week Assignment (2021 & 2022) Answer (এইচএসসি রসায়ন প্রথম, তৃতীয়, চতুর্থ, পঞ্চম, ষষ্ঠ ও সপ্তম সপ্তাহের এসাইনমেন্ট এর সমাধান) is available here. Do you need answers of HSC Chemistry 1st, 3rd, 4th, 5th, 6th & 7th week questions? We have answered all the questions of HSC Chemistry Assignment for 1st, 3rd, 4th, 5th, 6th & 7th week. This Rosayon solution of the 1st, 3rd, 4th, 5th, 6th & 7th week will be very useful for the students of HSC. So read the full post to get Chemistry solution for 1st, 3rd, 4th, 5th, 6th & 7th week of 11th & 12th class.
HSC Chemistry Assignment
A total of ten chemistry assignments will be published for the 2021 HSC candidates. Chemistry is five for the first paper and chemistry five for the second paper. We know that the Chemistry 1st week, 2nd week, 3rd week, 4th week, 5th week, 6th week and 7th week assignments of HSC candidates have been published. So the Chemistry 1st week assignment has been published. Today I will talk about the solution of HSC Chemistry Assignment. How do HSC candidates write the answers for Chemistry all week assignments?
HSC candidates who have chemistry must read our article carefully. Because today we will give instructions on the solution of the Chemistry all week assignment. We will also prepare a sample answer sheet of the Chemistry all week assignment and provide it on our website. If you want, you can download the sample chemistry assignment answer sheet of chemistry Assignment from our website.
HSC Chemistry 7th Week Assignment 2021
HSC 2021 7th week Chemistry Assignment has been published. This is the fifth determinant of chemistry. So HSC 2021 candidates have experience in answering chemistry assignments. Selected from the third chapter of HSC 2021 7th week Chemistry Assignment. The title of the third chapter is Periodic Religion and Chemical Bonding of Elements. HSC 2021 Orbital Hybridization and Chemical Bonding as Work of 7th week Chemistry Assignment. Students will be imparted knowledge about orbital and chemical bonds through the Chemistry Assignment of HSC 2021 7th week. Today we will discuss how to create answers to the HSC 2021 7th week Chemistry Assignment. So all the students who want to download the answers of HSC 2021 7th week Chemistry Assignment follow us.

অ্যাসাইনমেন্ট : অর্বিটালের সংকরণ ও রাসায়নিক বন্ধন ।
There are several guidelines for preparing answers to HSC 2021 7th week Chemistry Assignment. The answers to the HSC 2021 7th week chemistry assignment need to be prepared by following the instructions correctly. Below are the topics that must be mentioned in the answer sheet of HSC 2021 7th week Chemistry Assignment. The answer sheet must specify the classification of covalent bonds on the basis of orbital overlap. The effect of the elements present in the compound on the solubility of the compound has to be explained. Lastly, the answer sheet of the HSC 2021 7th week Chemistry Assignment will explain the intermolecular force of attraction between water molecules in different physical states.
HSC Chemistry 7th Week Assignment 2021 Answer

HSC Chemistry 6th Week Assignment 2022
The 6th week chemistry assignment of 2022 HSC candidates has been published. So all the students who could not complete the chemistry assignment of HSC 2022 6th week follow us. Because, in the next step we will discuss the answer of the HSC 2022 6th week chemistry assignment. Students who want to know how to answer the HSC 2022 6th week chemistry assignment should read this article carefully.

অ্যাসাইনমেন্ট : পরমাণুর অভ্যন্তরে ইলেকট্রনের অবস্থান এবং পারমাণবিক বর্ণালীর উৎস ।
ষষ্ঠ সপ্তাহের এই রসায়ন অ্যাসাইনমেন্টটি ২০২২ সালের এইচএসসি পরিক্ষার্থীদের (মানে বর্তমান প্রথম বর্ষের) জন্য। যারা ২০২১ সালে এইচএসসি পরীক্ষা দিবে, তাদের জন্য সকল সপ্তাহের রসায়ন এসাইনমেন্ট নিচে দেওয়া আছে।
HSC 2022 The task of the 6th week chemistry assignment is to describe the position of the electrons inside the atom and the source of the atomic spectrum. That is, when an atom breaks down, we get electrons. Explain how electrons are located between atoms. Also need to calculate the atomic spectrum source. The above topics have been given in the Chemistry Assignment of the 6th week of HSC 2022 as an assignment.
HSC Chemistry 6th Week Assignment 2022 Answer

Read More About HSC Rosayon 6th Week Assignment
A number of issues have come up through the chemistry assignment of the 6th week of HSC 2022. The subjects must be learned by the students. It will not be possible to answer the HSC 2022 6th week chemistry assignment without gaining knowledge about the above. So here are the things you need to know to answer the HSC 2022 6th week chemistry assignment. Rutherford and Bohr’s model comparison of atoms. Also explain quantum numbers, different layers and electron capacitance. Need to know about the energy and shape of the layer on Quantum. Also need to gain knowledge about the electromagnetic spectrum. Lastly, the spectrum of hydrogen atoms should be described according to the Bohr atom model with examples.
Several instructions have been given to answer the Chemistry Assignment of HSC 2022 6th week. There are also some additional guidelines for students who want to get the highest marks from the HSC 2022 6th week chemistry assignment. If you can answer the HSC 2022 6th week chemistry assignment by following our instructions, you will definitely be able to achieve very good results. Below are the things that need to be mentioned in the answer sheet to get good results from the HSC 2022 6th week chemistry assignment.
Policies related to electron configuration should be mentioned in the answer sheet. It is also necessary to determine the electron holding capacity of energies from quantum numbers. The description and shape of the sub-levels need to be explained. Lastly, the source of the atomic spectrum needs to be explained. The results will be better if the above issues can be mentioned in the answer sheet of HSC 2022 6th week chemistry assignment.
HSC Chemistry 5th Week Assignment 2021

অ্যাসাইনমেন্ট: জৈব যৌগে বন্ধন বিভাজন এবং অ্যারােমেটিক যৌগের প্রস্তুতি ও বিক্রিয়া ।
HSC Chemistry 5th Week Assignment 2021 Answer

HSC Chemistry 4th Week Assignment 2021
HSC candidates have Chemistry as their 4th Week assignment. Selected from the fourth chapter of the HSC 4th Week chemistry assignment. The title of the fourth chapter is Periodic Religion and Chemical Bonding of Elements. So the question will be about the chemical bond from the HSC 4th Week chemistry assignment. Today I will talk about how to answer the HSC 4th Week chemistry assignment. So all the students need the answer to the assignment in HSC 4th Week chemistry they follow us.
The HSC 4th Week chemistry assignment has a purpose. We know that it has not been possible to teach HSC 2021 candidates for a long time. The HSC candidates of 2021 have been given 4th Week assignments to keep the teaching process in Chemistry active. So all the teachers and HSC candidates should try to keep the chemistry assignment process active to keep the educational activities active. Classification of elements and periodic religion should be mentioned as the work of the HSC 4th Week chemistry assignment.

অ্যাসাইনমেন্ট: মৌলসমূহের শ্রেণিবিভাগ ও পর্যায়বৃত্ত ধর্ম।
HSC Chemistry All the subjects that need to be learned in order to solve the 4th Week assignment are called the subject matter of the assignment. Also, all the knowledge that will be gained by solving today’s assignment will be taught in the HSC Chemistry 4th Week assignment. So there is a lot to learn from today’s assignment. The subjects that can be learned from the 4th Week assignment of HSC Chemistry are given below.
To divide elements into different classes based on electron configuration. Also be able to explain the periodicity of the different religions of the elements. Also be able to describe the effect of different regulators on ionization energy, electron energy, electronegativity.
We will discuss the questions that need to be answered as part of the HSC 4th Week chemistry assignment. Before answering the 4th Week chemistry assignment, you have to look at all the questions that have been asked as the assignment work. This means that you have to know the work of the HSC 4th Week chemistry assignment well and answer the assignment later.
HSC Chemistry 4th Week Assignment 2021 Answer

A number of guidelines have been provided for correctly answering the Chemistry Assignment for the 4th Week of HSC 2021. We will also provide some additional guidelines for achieving excellent results from the HSC 4th Week chemistry assignment. The HSC 4th Week chemistry assignment must be answered following all the instructions and the subjects must present the answer sheet.
The classification of the elements of the periodic table on the basis of electron configuration has to be described. It is also necessary to explain the change in the ionization energy of the elements of the same stage in the periodic table. The change in the electron addiction of the elements in the same class of the periodic table has to be explained. It is also necessary to describe the effects of various diseases on the electronegativity of the element.
HSC Chemistry 3rd Week Assignment 2021
Chemistry 3rd week assignments have been published for the 2021 HSC candidates. So today’s HSC Chemistry 3rd week assignment will be applicable for all the students who will participate in the HSC exam in 2021. An important subject in chemistry. Chemistry is an essential subject for science students. So the chemistry 3rd week assignment is crucial. The HSC Chemistry 3rd week assignment is taken from the first chapter: Environmental Chemistry.

অ্যাসাইনমেন্টঃ গ্যাসের ধর্ম এবং আদর্শ ও বাস্তব গ্যাস
HSC Chemistry 3rd week assignment has been asked to analyze the nature and standard and real gas. Gas is a very important substance in our life. We know that there are three types of matter on earth. Of these, the biological or gaseous matter is one of the most important. So today from the HSC Chemistry 3rd week assignment we will learn about the religion of gas. I will also be able to gain knowledge about the religion of gas and real gas as an assignment. In the next step, we will discuss all the instructional topics from the HSC Chemistry3rd week assignment. I will also discuss all the things you need to know to write the solution or answer for the HSC Chemistry 3rd week assignment.
HSC Chemistry 3rd Week Assignment 2021 Answer

HSC 2021 Chemistry 2nd Paper Assignment Lesson
Now we will discuss the content of the HSC Chemistry Second Paper Assignment. It is possible to gain detailed knowledge about gas and the nature of gas from the chapter on environmental chemistry. Because this chapter discusses gas and the religion of gas in detail. In order to solve the Chemistry II paper 3rd week assignment, it is important to acquire knowledge about several subjects. First of all, you need to get the right idea about the content of the assignment. It is not possible to solve any assignment without a proper idea about the content of the assignment. So first of all you have to gain the right idea or knowledge of the assignment content. Below are the things you need to know to write the answer sheet of the Chemistry 3rd week assignment.
First of all you need to know the explanation of Boyle, Charles, Gay-Lusak formula and Boyle and Charles combination formula. Also, learn how to interpret the ideal gas equation from the Boyle, Charles, Gay-Lusak and Avogadro formulas. Knowledge of how to determine the total pressure of a gas mixture from Dalton’s partial pressure formula, partial pressure formula. Also, one has to know how to interpret kinetic energy on the basis of the recognition of the dynamics of gas.
What is an ideal gas and real gas and what is the difference between them? The answer to this question must be well known. The last thing you need to know is how to explain the ideal behavior of real gases. So in order to solve the HSC Chemistry 3rd week assignment, you must first know the above issues. Otherwise, it will not be possible to achieve good results from the chemistry 3rd week assignment.
HSC Rosayon 3rd Week Assignment Solution 2021
HSC examinees have been given seven days to solve the Chemistry 3rd week assignment. This means that the solution of the HSC Chemistry 3rd week assignment has to be completed within one week. But most science students have not yet completed the solution of the chemistry 3rd week assignment. Because they don’t have enough ideas about how to solve the assignment. Now I will discuss how to solve the HSC Chemistry 3rd week assignment.
2021 HSC examinees have been given several instructions to solve the chemistry 3rd week assignment. We will also provide some additional guidelines so that HSC candidates can get good marks in Chemistry 3rd week assignments. It will be the job of the examinees to follow our instructions well. We have to solve the HSC Chemistry 3rd week assignment by following our instructions. So below are the instructions on how to solve the HSC Chemistry 3rd week assignment.
In order to solve the HSC Chemistry 3rd week assignment, several subjects must be added to the answer sheet. For example, the absolute zero temperature and the absolute temperature scale must be properly explained from Charles’s formula. In this regard, a proper explanation must be given in the answer sheet. Acknowledgments must be made and calculations of momentum must be presented appropriately. That is, the correct calculations of the pressure force must be shown in the answer sheet. You also need to make the handwriting beautiful to achieve good results. And the cover page of the assignment needs to be neatly attached.
HSC Chemistry 1st Week Assignment 2021
The first chapter of the Chemistry 1st week assignment is taken from Qualitative Chemistry. This chapter discusses atoms, electron configurations, energy levels, and various atomic models. So if you can complete the Chemistry 1st week assignment properly, it will be possible to gain knowledge about the above topics. Chemistry has been asked to model the atom and arrange the electrons as part of the Chemistry 1st week’s assignment.

অ্যাসাইনমেন্ট ১: পরমাণুর মডেল ও ইলেকট্রন বিন্যাস।
So chemistry needs to present atomic models as a solution to the Chemistry 1st week assignment and explain the electron configuration with examples. In the next step, we will discuss how to write the answer for the HSC Chemistry 1st week assignment. So if you want to know the solution to the Chemistry 1st week assignment, follow the following articles.
HSC Chemistry Assignment Answer 2021
Get HSC exam 2021 Chemistry assignment answer in an Image or PDF file.

Get: HSC Assignment Answer
HSC Chemistry First Paper Assignment Lesson
The first letter assignment is to show the model of the atom and the arrangement of electrons. In order to solve the assignment, we need to acquire knowledge on several issues. That means we need to know about the contents of today’s assignment. It will not be possible to write the solution or answer of the HSC Chemistry Assignment without knowing the content of the assignment. So first of all you have to know all the things that you need to be aware of to solve the assignment. Those issues are given below.
As we know, there are different models of atoms. Among these are the Rutherford atomic model and the Bohr atomic model. It is also necessary to gain knowledge on how to compare these two atomic models. Then we have to read the details about quantum numbers. Because chemistry has to write about quantum numbers when solving the first letter assignment. It is also necessary to know the technique of determining the electron potential at different energy levels of an atom from quantum numbers. Without knowledge of the above topics, it is not possible to solve or answer the Chemistry 1st week assignment. So, first of all, you have to know the contents of the Chemistry 1st week assignment.
How to Solve the Chemistry 1st Assignment?
Today we will discuss the strategy of solving the Chemistry 1st week assignment of HSC candidates. Chemistry is instructed to solve the 1st week assignment. Nevertheless, I will provide some additional instructions to achieve the best results from the Chemistry 1st week assignment. So that HSC candidates can collect some extra marks. So below are the issues that need to be presented in the answer sheet for writing the answer to the Chemistry 1st week assignment.
First of all Bohr’s atomic model must be described with limitations. Then write four quantum numbers and describe them with proper significance. Determine the total number of orbitals of the energy level and present an accurate calculation of the electron holding capacity. The Aufbau principle and the ghost principle need to be mentioned. The electron configuration of K and cr should be shown. So the above issues must be mentioned in the answer sheet of the Chemistry 1st week assignment. Those who want to get good marks on an assignment in the HSC exam, please write the solution of the assignment by following the above instructions.
We have shared HSC Chemistry 1st, 3rd, 4th, 5th, 6th & 7th Week Assignment Answer. As an HSC student, you have gotten very help from our answer. In the future, we will share more assignment answers like HSC Chemistry 1st, 3rd, 4th, 5th, 6th & 7th Week Assignment.
Facebook Page
Facebook Group
Earn Money By Writing Article
Recent Posts

SSC Result 2024 Marksheet With Subject Wise Number

Gold Price In Bangladesh Today – 1 Vori 18K, 21K, 22K Sonar Dam (সোনা)

Eye Lens Price in Bangladesh Today – চোখের লেন্সের দাম

Nagaland State Lottery Sambad Today Result 24 April 2024

Birth Registration Online Copy Download 2024 (PDF)

4 Pics 1 Word Answer Today – April 24 2024

Dhaka Stock Exchange Latest Share Prices – ঢাকা স্টক এক্সচেঞ্জ

Grit TV Schedule Today – What’s On Grit TV Tonight

NYT Crossword Answers & Clues Today New York Times, April 24, 2024

Binance CRYPTO WODL Answers Today 24 April 2024
Privacy Policy

HSC Chemistry Assignment Answer 2021 Pdf 7th Week

In this post about HSC Chemistry Assignment Answer 2021 PDF 7th and other Week. Now posted by this web portal HSC 2021 Chemistry Answer 2021. We also found hsc science groups assignment has been assigned for another week. Also, the Bangladesh education ministry declared 2021 HSC Assignment will be held on only groups subject. For this 15th week will be taken the assignment, Among that Chemistry has 2nd, 4th, 6th, 7th, 9th, 12th, 13th, and 15th-week assignments. A total of 10 assignments have to be completed in Chemistry 2021 HSC Assignment.
You know hsc groups subject was part. The Final-year exam is held on 1st paper and the final exam will be held on the 1st and 2nd papers together. Now HSC Chemistry Assignment solution has two Parts. Students will be prepared Chemistry 1st Paper Assignment Answer for 5 weeks and Chemistry 2nd Paper Assignment Solution will be prepared for another 5 weeks.
HSC Chemistry Assignment Answer 2021
HSC Chemistry assignment Answer will be created on the student’s own. This time Students will be created a total of 10 Chemistry Assignment questions and answer for Chemistry 1st Paper and Chemistry second paper. Each week a paper on Chemistry will be selected for the answer. By this post, we have given all paper assignment answer which your need. 1st paper and 2nd paper both answer you can be found by this post. We also given 1st week to 15th-week answers will be found on this page. So visit our page regularly and get more and more new week assignment answers for the 2021 HSC Chemistry Assignment.
hsc 7th week chemistry Assignment Answer 2021

HSC Chemistry Assignment Answer 2021 5th Week

Now publish 4th Week HSC Chemistry Assignment. We Found the hsc chemistry 4th week assignment. Same time we have given here HSC 4th Week Assignment Answer on this page. If you want to download the 4th week chemistry assignment answer can be here.
HSC 2021 7th week Chemistry Assignment Answer
HSC Physics Assignment Answer Chemistry 7 week Solution Given below
You Searching now hsc chemistry assignment, HSC 2021 Chemistry answer, Chemistry assignment answer 2021, Chemistry assignment answer pdf, Hope; you can find the top or 2nd page of our Testresultbd.com website and get your necessary information by this page. Authority has been published HSC Science Group Chemistry Subject Assignment. we will provide you the Assignment task for each subject. and You Can Check the Following Subject Solution from Our Website.
Another Subject Science Groups Full Assignment Answer ✓ Physics ✓ Biology/ ✓ Higher Math
How to Download HSC Chemistry Assignment Answer 2021 (7th Week)
2021 HSC Exam Chemistry assignment answer has been assigned. Now we are given the correct answer for the HSC Chemistry Assignment 1st week. Another week’s assignment chapter of the first paper is scheduled to solve the weekly assignment. All assignment answers will solve the student’s own. If The student will complete the task assigned for the assignment by reading and practicing the vector chapter of their second chapter well. You can get an idea by looking at the answer we have prepared for the assignment. Or you can match ours with the Chemistry assignment answer 2021 you created.
Another Subjet Answer Physics Biology/ Higher Math
=7th Week Chemistry Assignment Answer 2021
Dear HSC Examiner, now we found here 1st Week Chemistry Assignment Answer. Download this assignment answer and take which answer your need.
Take the HSC 7th Week Chemistry Assignment answer pdf now online. HSC Exam Chemistry assignment answer has been assigned. Now we are given the correct answer for the HSC Chemistry Assignment 3rd week. Another week’s assignment chapter of the first paper is scheduled to solve the weekly assignment. All assignment answers will solve the student’s own.
Dear students, now we have given you the HSC Chemistry assignment related to all info on this page. If you need any help for the assignment exam 2021 you can here. Now we have given you daily assignments updated timely. If need any help you can info our comment box as soon as we will try your question answer by this page. For more information visit dshe.gov.bd Official website.
Test Result BD
HSC Chemistry Module 6 Acid-base Reactions: A Comprehensive Guide
Acid-base reactions are a pivotal topic in HSC Chemistry, particularly in Module 6. This guide aims to provide students with essential insights and study tips to excel in this area, emphasising the critical aspects of the curriculum and practical applications. Let's dive into some key strategies you can utilise to master Acid-Base Reactions in Module 6.
Revise Module 5: Equilibrium
Developing a strong grasp of equilibr ium concepts from Module 5 is crucial for the learning of module 6. Before studying acid-base chemistry, make sure to revisit and familiarise yourself particularly with the concepts of equilibrium, Le Chatelier's Principle, and also how to use ICE tables (Initial, Change, Equilibrium) for calculating equilibrium constants.
Since the same principles of equilibrium reactions apply to acid-base reactions, becoming familiar with these module 5 concepts will help you to better understanding their applications in module 6 and help you grasp new concepts regarding acids and bases more easily.
Equilibrium knowledge is necessary in the following Module 6 topics:
- Ionisation of weak acids and bases
- Any calculations involving weak acids and bases
- Auto-ionisation of water
- Acidic, basic and amphiprotic salts
Learn Beyond Just the Definitions of the Acid-Base Models

Many students view the section on acid-base models as the content-heavy part of Module 6, and there is no denying that. There are definitely features of Arrhenius and Brønsted-Lowry models that you will need to commit to memory, along with chemical equations to support each feature.
But while it is important to understand the advantages of the Arrhenius and Bronsted-Lowry models, don't neglect learning about their limitations. Recognising the limitations of existing models explains why newer models are developed, offering a deeper understanding of acid-base chemistry's evolution.
To assist you with better familiarising yourself with acid-base models, we recommend regularly recalling and revisiting your notes on these models. Giving yourself an opportunity to practise common exam questions regarding these models well before assessment time will be beneficial as it will give you more time to understand other topics in acid-base chemistry.
Familiarise Yourself with Common Acid Names
While the task of memorising all the various acid names can be daunting, regular exposure and practice will make it easier. When you begin learning module 6, don't spend excessive time on memorising all the nomenclature of acids .
Instead prioritise learning the names, properties and structures of commonly encountered acids, especially in experiments and theoretical problems.
- Common strong acids: hydrochloric acid, nitric acid, sulfuric acid
- Common weak acids: acetic acid, citric acid, phosphoric acid
For less common acids and bases, you should learn their names simply as you encounter them and via exposure by reviewing content which mentions them.
Another strategy that you can utilise is to commit to memory all the examples of strong acids. This is because there are far more examples of weak acids which means that if you know the common examples of strong acids, you can assume most of the acids are weak in nature.
Understanding Strength Vs Concentration of Acids & Bases
It is a good habit to always ask yourself whether an acid ( or a base) is strong or weak; this applies to experiments, theory, and calculation questions. Having the ability to differentiation between strong and weak acids is essential in aiding you in various aspects of acid-base chemistry from titration to theoretical understanding.
Calculating the pH of a strong acid or base solution for example, is a more straightforward calculation than calculating the pH of a weak acid or base solution. This is because the latter requires you to utilise ICE tables (remember – Revise module 5: Equilibrium) beyond simply substituting numbers into the pH formula. In this case. understanding WHY we perform these calculations differently, requires you to first recognise that the acid is weak, and is difficult to achieve simply through rote learning.
Another example of a concept which requires a deep understanding of strong and weak acids/bases is seen in the enthalpy change of neutralisation. Neutralisation between a strong acid and strong base will yield a greater magnitude of enthalpy change than if the reaction involves a weak acid or base. Again, you should understand WHY there is a difference rather than simply rote learning
Titration, Titration, Titration...

Titration is the ultimate boss of acid-base chemistry, and it's a topic that students fear the most.
Many HSC Chemistry students find titration difficult because:
- it requires understanding of acid-base knowledge from previous inquiry questions and syllabus dot points – overcome this by studying ahead of your school's schedule
- they underestimate the theory behind titration – prepare your understanding of titration and its processes ahead of your practical classes to fully benefit from these experiments.
- they were unprepared and unfocused when learning titration for the first time
- they have not spent enough time on the theory which in turn makes the practical element of titration more difficult than it supposed to be – make time to revisit this topic well before assessment time.
Being aware of the above reasons will also help you avoid being in those situations, and be better prepared for this topic in the later parts of Module 6.
With titrations often being a significant part of practical assessments and depth studies, comprehending the theory and practice, including choosing the correct reagent for equipment washing, is vital.
- Share Share on Facebook
Leave a comment
Please note, comments must be approved before they are published
- choosing a selection results in a full page refresh
- press the space key then arrow keys to make a selection
HSC Chemistry Assignment Answer 7th Week Question
HSC 2021 Chemistry Assignment Answer 7th week PDF for HSC Candidates hsc Assignment 2021 on 8th September 2021 published at dshe.gov.bd website. HSC exam batch 2021 assignment is about to be published and the HSC Chemistry Assignment 2021 Answer available on our resultbd24.com website. HSC chemistry Assignment Answer 2021 of 7th week question every science group students they download her current week assignment on here.
Table of Contents
HSC 2021 Chemistry Assignment Answer
DSHE published the HSC Chemistry Assignment 2021 Answer of hsc exam 2021. HSC 7th week Physics assignment question Download 7th week question. HSC Chemistry Assignment started so all board students submission to college within last date. Download 5th week question answer chemistry 2021 hsc assignment.
Download 7th week question answer
HSC Chemistry assignment waiting for published 7th Week assignments. You want to get HSC Physics Assignment questions and answers. Chemistry Assignment HSC Class question pdf download. You want to get HSC Chemistry Assignment questions and answers to keep reading below.
HSC 2021 Chemistry Assignment 7th Week Answer
Every students of science group they download her 7th week question and answer so students visit on dshe gov bd and download 7th week question.
- Academic Level: HSC
- Subject: Chemistry
- Group: Science
- Exam Year: 2021
- Assignment No: 5
Question Question: অর্বিটালের সংকরণ ও রাসায়নিক বন্ধন
5th week answer
See here HSC Class Chemistry Assignment Answer, Pdf question solution; HSC Chemistry Assignment submit Date 2021 and Download link on this page. Check assignment Date Of School 2021. So all Applicants continue to read our article and know all details.
HSC Chemistry Assignment Answer 2021 4th Week
HSC Assignment 2021 PDF Science group of students assignment are Chemistry.
HSC Chemistry 1st Paper Assignment Answer (4th week) 2021 answer is given here.
Assignment Work: মৌলসমূহের শ্রেণিবিভাগ ও পর্যায়বৃত্ত ধর্ম
HSC Chemistry Assignment for HSC Exam 2021 all Board. This year the newly started Assignment answer 2021 all board on their website. Every students HSC they download her 3rd week and 1st week question of hsc exam 2021 with answer sheet. Students chemistry assignment submission to college within few days.
HSC 2021 Chemistry Assignment Answer so now every students download 1st week chemistry assignment question solved.
HSC Chemistry Assignment Answer 2021 3rd week
The first chapter has been assigned as the assignment of the second paper of Chemistry. Chemistry 2nd Paper is scheduled for 3rd week of HSC Chemistry Assignment 2021 Answer.
hsc chemistry 2nd paper 3rd week assignment answer 2021, hsc পরিক্ষার্থী ২০২১ এর রসায়ন ২য় পত্র
অ্যাসাইনমেন্ট : গ্যাসের ধর্ম এবং আদর্শ ও বাস্তব গ্যাস
অ্যাসাইনমেন্ট নং ২ (দ্বিতীয় পত্র) পরিবেশ রসায়ন
HSC 1st week question solution image and pdf file upload so science group students download her chemistry assignment ans.
HSC Chemistry 1st & 3rd Week Assignment Answer 2021
For the Students of Higher secondary school (HSC) a new assignment has been published. Now, Intermediate students collect the assignment & submission to college. HSC Assignment Chemistry subject subjects For the HSC Students total 30 weeks assignment for the according dshe routine.
HSC inter 2nd year chemistry assignment solution given on here. Every students they download her 1st week assignment answer on here.
PDF Download
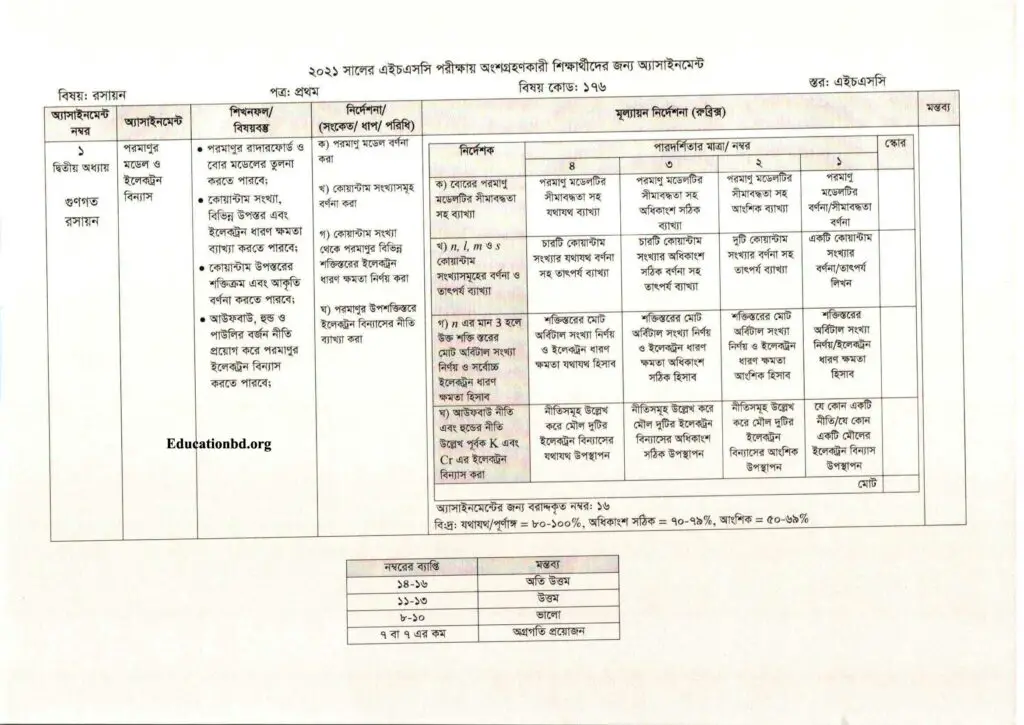
- অ্যাসাইনমেন্ট নং ১
- দ্বিতীয় অধ্যায়: গুণগত রসায়ন
- অ্যাসাইনমেন্ট উত্তর: পরমাণুর মডেল ও ইলেকট্রন বিন্যাস
- পরমাণুর রাদারফোর্ড ও বোর মডেলে তুলনা করতে পারবে
- কোয়ান্টাম সংখ্যা, বিভিন্ন উপস্তর এবং ইলেকট্রন ধারণ ক্ষমতা খা করতে পারবে।
- কোয়ান্টাম উপস্তরের শক্তিক্রম এবং আকৃতির বর্ণনা করতে পারবে।
- আউফবাউ, হুন্ড, পাউলির বর্জন নীতি প্রয়োগ করে পরমাণুর ইলেকট্রন বিন্যাস করতে পারবে।
Instruction
- ক) পরমাণু মডেল বর্ণনা করা
- খ) কোয়ান্টাম সংখ্যা সমূহ বর্ণনা করা
- গ) কোয়ান্টাম সংখ্যা থেকে পরমাণুর বিভিন্ন শক্তিস্তরের ইলেকট্রন ধারণ ক্ষমতা নির্ণয় করা ।
- ঘ) পরমাণুর উপশক্তিস্তরে ইলেকট্রন বিন্যাসের নীতি ব্যাখ্যা কর।
Assignment question image:
HSC Chemistry Assignment Answer 2021
HSC Chemistry 3rd Week Assignment 2021 The 3rd-week Chemistry assignment question has been published.
Every students of HSC they download her HSC Chemistry Assignment Answer 2021. DSHE 4th week chemistry assignment published for hsc candidates 2021. HSC Chemistry 2nd paper and 1st paper question pdf download answer/solution’s download.
HOW I GET THE ANSWER OF THIS ASSIGNMENT.
wait for we are working for solution.
Where are chemistry 2nd assignment ans
Now you can found 2nd week ans
HSC Chemistry Board Paper Solution 2020
HSC Chemistry paper board Solution 2020 of class 12 with complete explanation.
HSC Board Chemistry Question paper 2020 Click to refer
Important Questions for Chemistry Old Syllabus Repeater students 2020 Click to refer
Select and write the correct answer of the following : – [10M]
- Terylene (Correct answer)
- H2O (Correct Answer)
- +6 to +3 (Correct Answer)
- [ZN(NH 3 ) 4 ] 2+
- [Fe(CN 6 )] 4-
- [Fe(CN 6 )] 3- (Correct Answer)
- [Co(NH 3 ) 6 ] 3+
- Finkelstein reaction
- Swartz reaction (Correct Answer)
- Willianson’s Synthesis
- Wurtz Reaction
- 3 (Correct Answer)
- Hydroxyl Amine
- Bromine water
- Dilute nitric acid
- Acetic anhydride (Correct Answer)
- Penicillin (Correct answer)
- 2 (Correct answer)
- +4.988kJ (Correct Answer)
Related Posts HSC 2020 Physics Board Paper Solution Read more
Q.2) Answer the Following questions: — [8M]
a). What is the Concentration of dissolved oxygen at 50 0 C under pressure of one atmospheric if partial pressure of oxygen at 50 0 C is 0.14 atm. (Henry’s law constant for oxygen = 1.3 * 10 -3 mol dm – 3 atm -1 )
Answer: 1.82 × 10 -4 mol dm -3 .
b). Write the Structural formulae of the alcohol that results when acetaldehyde is reacted with CH3MgBr in the presence of dry ether and the product is hydrolysed?
Answer: isopropyl alcohol/2-hydroxypropane
c). Write balanced chemical reaction for preparation of acetic anhydride using acetic acid ?
d). Write the chemical reaction involved in the formation of ethyl amine using acetal doxime.
e). What is electrometallurgy?
Answer: Electrometallurgy is a method that uses electrical energy goes into electrolytic reduction of molten(fused) metallic compounds to produce metals.
SECTION – B (HSC Chemistry Board Paper Solution 2020)
Q3. State and explain Hess’s law of constant heat summation.
Answer: The law states that the change in enthalpy for a reaction is the same whether the raction takes place in one or series of steps./ The enthalpy change for a chemical reaction is the same regardless of the path by which reaction occurs.
The conversion of A ->C can directly takes place in one step
A –> C ΔH0 = ΔH1
The reaction can also takes place in two different steps
Step 1 A -> B ΔH0 = ΔH2
Step 2 B -> C ΔH0 = ΔH3
Overall A -> C ΔH0 = ΔH2 + ΔH
Q5). Distinguish between order and molecularity of a reaction.
Q6). Write two uses of each of the follwing
Answer: Uses of Helium-
i) It is used for filling balloons and air ships as it is light and non combustible.
ii) It is used in producing inert in metallurgical operations and welding of metals.
Neon Uses-:
i) It is also used in television sets, spark plug, warning signals etc. ii) It is also used in safety devices, electrical instruments like voltage stabilizer and rectifier’s
Q7). Write the name and chemical formulae of one ore of zinc.? Define Quaternary ammonium salt.
Answer: Zinc belnde ZnS, calamine ZnCO 3 , Zincite ZnO, willemite ZnSO 4 .
Tetraalkylammonium halide are called quaternary ammonium salt.
The property or phenomenon of certain organic substances to rotate the plane of plane polarized light towards right(clockwise) or left(anti-clockwise) through a certain angle is called optical activity.
Number of optical isomer of glucose is 2 4 = 16. As glucose has 4 asymmetric carbon atom.
As per the reaction above
Sulphuric acid regenerated in the second step is reused in the first step. Thus the small amount of sulphuric acid converts a large amount of ethyl alcohol into diethyl ether and the process becomes continuous. Hence it is called a continuous etherification process.
Hormones are chemicals released by the endocrine glands to control and regulate the activity of certain cells and organs.
. Silver- Metallic solid
P 4 –Molecular solid
Diamond-Covalent solid ,
NaCl – Ionic solid
Molality-The number of moles of solute dissolved in 1 kg of a solvent is known as molarity.
Osmotic pressure- The excess of pressure on the side of the solution that stops the net flow of solvent into solution through a semipermeable membrane is called osmotic pressure.
SECTION – C
Attempt any eight questions of the following-
Remaining updating soon in short while….
for solution of full MCQs you can watch
https://www.youtube.com/watch?v=uhX70jFkaZg
For answer the following you can watch
https://www.youtube.com/watch?v=EI1W4BSRF44&t=4s
Related Posts HSC Maharashtra Board New Syllabus 2020 Read more
Chapter Wise Weightage marks distribution Class 12 HSC Science Maharashtra Board all Subjects click to read
Physics question paper July 2022 Class 12 Maharashtra State Board Read MOre
Maths question paper March 2022 Class 12 Maharashtra State Board Read MOre
Class 12 HSC Chemistry question paper 2022 Maharashtra State Board Read More
Chemistry subject Viva questions class 12 Read More
Hsc biology practical exam paper pattern 2022-23 Read More
Physics Practical Exam Paper Pattern Read More
IT Sample Model Paper 2021 HSC Maharashtra Board Read More
HSC IT SOP(Skill Oriented Practical Solutions) Class 12 Read More
13 thoughts on “HSC Chemistry Board Paper Solution 2020”
Pingback: HSC Physics board paper solution 2020 | Class 12
Pingback: Reduced Syllabus for HSC Science Maharashtra Board | Techniyojan
Pingback: hsc MH board question papers with answers pdf 2020 | Techniyojan
Pingback: HSC Electronics Board Question Paper 2020 class 12 | Techniyojan
Pingback: 11th bifocal Electronics Syllabus Maharashtra Board | Techniyojan
Pingback: new paper pattern of hsc 2020 : hsc maharashtra board new paper pattern 2020 | Techniyojan
Can you give chem importance also
yes for old syllabus or new syllabus you are expecting..student pls let me know
Pingback: class 11-12 chemistry practical viva questions with answers | Techniyojan
Pingback: HSC 2020 Chemistry Board Question Paper | Techniyojan
Pingback: HSC Electronics class 12th Syllabus maharashtra board | Techniyojan
Pingback: HSC 2020 Geography Board Question Paper | Techniyojan
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

- Switch skin

HSC Chemistry Question Solution PDF (1st & 2nd Paper) – All Board
All education board HSC Chemistry 1st & 2nd paper MCQ question answer 2023 PDF. HSC chemistry question solution 2023 is available on our website. In this article will provide your the accurate question solve of HSC chemistry and other related information.
HSC Chemistry Question Solution PDF (1st & 2nd Paper)
Are you a student preparing for the HSC Chemistry exam in 2023? Are you in search of accurate solutions to the HSC Chemistry questions to boost your preparation? Look no further! In this article, we are delighted to provide you with the most accurate and reliable HSC Chemistry 1st and 2nd paper question solution 2023, along with other essential information to help you succeed in your exams.
HSC Chemistry 2nd Paper Question Answer 2023
The MCQ section of Chemistry 2nd Paper holds immense significance for candidates in the HSC Science Group. In this section, there are 30 MCQ questions, and candidates are required to answer all of them. With recent changes in question patterns due to the shortened syllabus, candidates may wonder how to access comprehensive solutions for the MCQ section of HSC Chemistry 2nd Paper Question solve.
Dhaka Board HSC Chemistry 2nd Paper MCQ Question Solve

Dinajpur Board Question Solution

আরও পড়ুন: একাদশ-দ্বাদশ শ্রেণীর সকল বই ডাউনলোড করুন
Barisal Board Question Solution

Mymensingh Board Question Solve

Chattogram Board Question Solution

HSC Chemistry 1st Paper Question Answer 2023
Chattogram board hsc mcq chemistry 1st paper question solve.

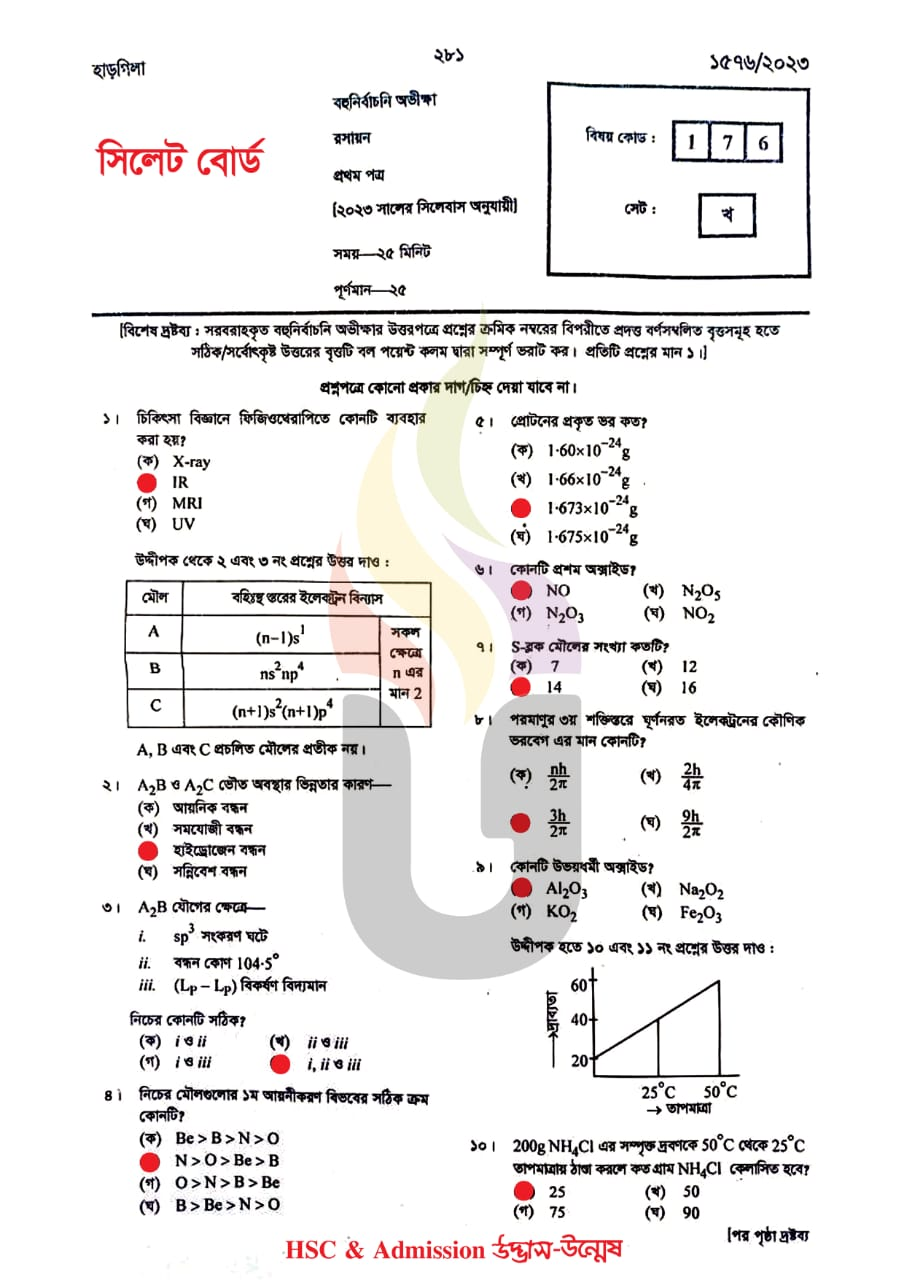
Sylhet Board HSC Chemistry 1st Paper Question Solve
These solutions are crucial as they provide correct answers, enabling candidates to cross-reference their responses. We want to reassure HSC Science Group students that there’s no need to worry. We have meticulously solved the MCQ questions for all boards offering the HSC Chemistry 2nd Paper exam, ensuring the utmost accuracy in our question solution.
We have provided the HSC Chemistry 1st Paper MCQ Question Solution for 2023, ensuring high accuracy. However, we encourage you to cross-verify the answers with your textbooks for added confidence. It’s worth mentioning that all the questions in the HSC Chemistry 1st Paper are drawn from a total of 9 chapters.
Related Articles

46 BCS Preliminary Question Solution 2024 (With PDF)

GST A Unit Question Solution 2024 PDF – Science Unit
3rd phase primary question solution 2024 pdf (29 march 2024), primary assistant teacher question solution 2024 (with answer pdf), leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Module 7: Organic Chemistry
This is one of the hardest modules, and both Chemistry in Focus and Pearson Chemistry 12 overlook significant syllabus points. If parts of this module don’t make sense, we also recommend the CrashCourse Organic Chemistry Series.
Introduction
- All life on Earth is based on compounds of Carbon, usually in an aqueous environment
- When scientist refer to “organic compounds”, it usually means compounds of carbon (excluding simpler compounds like CO A 2 , CO , CS A 2 , e t c . )
- Because Carbon is the first element with 4 valence electrons, it can easily covalently bond with many other atoms at once, meaning it can form lots of different compounds
- In module 1, the concept of Carbon allotropes, molecules of Carbon with have different chemical properties, was explored
- Module 7 looks at carbon compounds involving other elements (mostly Hydrogen, Oxygen, Nitrogen, and More Carbon™)
- Just as Modules 5 and 6 were basically harder versions of module 2, this is basically the next level of module 1, with a bit of modules 3 and 4 sprinkled in. If you need to revise these, check out our Preliminary Chemistry Course .
Structure of an Organic Molecule
- The Backbone: the longest continuous chain of connected carbon molecules in the molecule
- The Functional Group: an offshoot from the backbone which gives the molecule distinct properties, also known as side groups
- The Hydrogens: Hydrogen is used to fill all of the leftover Carbon valence electrons
Types of Organic Carbon Molecules
- Organic carbon compounds fall into two main categories: cyclic and acyclic (also known as closed and open chain). Each type has subcategories within themselves, but for the main categories, seeing an example Lewis diagram of each type gives away where the name comes from:
- For now, we’ll just look at chains, but don’t worry, it’s still going to hurt your brain.
Types of Organic Chains
Single bonds (alkanes).
- Alkanes are organic carbon molecules where Carbon atoms in the backbone are only connected via single bonds
- Alkanes have the general form of C A n H A 2 n + 2 , and are therefore a homologous series
- Alkanes are known as “saturated compounds”, and have an open, chain-like structure
Branched Alkanes
- Branched alkanes are alkane compounts with Alkyl side groups:
Remembering the names is difficult sometimes, but you might have noticed that they actually correspond to the number of Carbon atoms there are in the skeleton:
We’ll look at naming organic compounds later on. Remember that you can always jump around the post as needed.
Naming Branched Chain Alkanes
Find the longest continuous chain of carbon, and assign the parent name based on this number
Find whatever groups that are not part of the longest continuous chain. Name these as prefixes and put
them in alphabetical order .
Assign numbers to groups by counting from the closest end of the chain
- There are 6 carbons in the main chain, with the largest bond being a single bond, so the ending is -hexane
- The side chain has 1 carbon and 3 hydrogen, so methyl- is added (methylhexane)
- The side chain is at position 3, so the compound is 3-methylhexane
This system works for all 3 types of open-chain organic molecules, just switch out -ane for the correct ending.
Double bonds (alkenes).
- Alkenes are organic compounds where the Backbone carbon has double bonds
- Alkenes can also have single bonds in the backbone, but there must be at least 1 double bond (BUT NO TRIPLE BONDS!!!)
- Alkenes are known as “unsaturated compounds”, and are more reactive than alkanes as a result of the double bond
Variants of Alkenes
- The location of double bonds can be different, even if the empirical formula is the same
- For example, Butanol has 2 variants:
Triple Bonds (Alkynes)
- Alkynes are the last variant of open-chain compounds, and have at least one triple bond in their backbone
- Alkynes can also have single and double bonds in the backbone, but must have at least 1 triple bond
- Alkynes are also “unsaturated compounds”, and are the most reactive organic group
- The names of alkynes ends with -yne, for example propyne:
Physical Properties
Properties of homologous series.
Members of the same homologous series have:
- A similar structure and the same general formula and functional group (each member of a homologous series differs by a −𝐶𝐻2 − unit from the previous member)
- A pattern to their physical properties
- Similar chemical properties
Melting Points and Boiling Points
- The melting and boiling points are measures of the thermal energy required to overcome intermolecular forces .
As ↑ intermolecular forces, ↑ E H e a t , ∴ ↑ MP and ↑ BP
The packing of molecules also affects the boiling and melting point.Packing depends on molecular shape. Molecules that are small, symmetrical or unbranched tend to be able to pack more closely together. This results in stronger intermolecular forces .
The effect of packing on intermolecular forces strength is more significant for molecules in solid states (melting point).
Melting Points and Boiling Points of Alkanes
Alkane molecules are nonpolar, the only intermolecular force is dispersion forces . As the length of the carbon chain increases, the overall force of attraction between molecules also increase. (Dispersion forces is proportional to molar mass). Because boiling and point is determined by the strength of intermolecular forces, boiling point increases as alkane chain length increases.
Molecular shapes also influence the strength of dispersion forces. Straight-chained alkanes are able to fit together more closely and tend to have higher boiling points than their corresponding chain isomers.
Melting point is affected by the strength of the dispersion forces, size and shape of the molecule.
Melting points of hydrocarbons follow the same general patterns as boiling points, with a few exceptions. The melting points of straight-chain hydrocarbons increase as the number of carbon increases. However, there are deviations in this trend, relating to whether the molecules have an even or odd number of carbon atoms .
Chains with even numbers of carbon atoms pack slightly more efficiently in the solid state than chains with odd numbers . The more efficient packing requires more energy to melt the compound resulting in a higher melting point.
That took longer than it should have, all because we wanted a scaleable graph. Is that too much to ask, internet?
Melting Points and Boiling Points of Alkenes and Alkynes
Alkenes and alkynes, like alkanes, are nonpolar hydrocarbons.
Their molecules are also nonpolar so the forces of attraction between them are only weak dispersion forces.
Members of these homologous series have relatively low boiling points similar to those observed for alkanes with the same number of carbon atoms.
As with alkanes, the boiling points of alkenes and alkynes increase with molecular size as the strength of dispersion forces between molecules increase.
The boiling points of alkenes are slightly lower than alkanes with the same number of carbon atoms.
Alkynes have higher boiling points than both alkenes and alkanes that have the same number of carbon atoms. This is due to the increase packing because of its linear shape.
Since alkenes have lower molecular mass compared to alkanes with the same number of carbon atoms, the strength of the dispersion forces is weaker. Hence the boiling points are lower.
Alkenes, alkynes and haloalkanes follow the similar pattern to alkanes.
As the length of the carbon chain increases, the melting point increases.
The alkenes ethene, propene and butene are all gases at room temperature, alkenes with 5-14 carbons are liquid, and longer-chained molecules are solid.
Alkynes follow the same general trend for melting points seen in alkanes and alkenes.
However, the position of the triple bond can greatly affect the melting points as the shape of the molecule changes.
The solubility of a substance depends on the strength of the InterMolecular Forces within the solute and within the solvent (cohesive forces), in comparison to the IMF between the solute and solvent (adhesive forces).
The generalisation is that polar compounds tend to only dissolve well in polar compounds and non-polar compounds only dissolve in non-polar compounds .
Hydrocarbons are soluble in each other as well as in non-polar organic compounds such as benzene, diethyl ether and carbon tetrachloride (tetrachloromethane).
This is because the cohesive forces within the solvent are also weak dispersion forces that are similar in strength to the dispersion forces within hydrocarbons .
- Density is the measure of mass per unit volume g / m L or g / c m 3
- Alkanes cannot mix with water → If you try, the alkane will float on top of the water
- This is because all alkanes have a lower density than water
Volatility is the ability of a liquid (or solid) to escape and form a vapour . ↑ BP→↑ IMF Strength→↑ Volatility Volatility is measured by vapour pressure , which is a measure of concentration in the gas phase above the liquid. It is constant at a constant temperature .
Hydrocarbons are non-polar and hence have dispersion forces as their only intermolecular force . Since the intermolecular forces are relatively weak , their bonds are easily overcome and hence the BP is relatively low . Therefore, hydrocarbons will be volatile.
↑ Molecular Mass → ↑ Strength of dispersion forces → ↑ 𝐸h𝑒𝑎𝑡 → ↓ Volatility at a constant temperature.
Viscosity refers to a substance’s resistance to fluid flow. Liquids with a relatively high resistance to flow have high viscosity. For a substance to flow, particles must flow over each other. Viscosity depends on:
Strength of intermolecular forces. ↑ IMF→↑ Viscosity
Size of molecules. ↑ Size→↑ Viscosity
Temperature. ↑ Temperature→↓ Viscosity
Viscosity decreases with increasing temperature . At higher temperatures, molecules have greater kinetic energy . Thus, the molecules move around more which increases the space between molecules. This causes the intermolecular forces to be weaker and hence viscosity decreases .
Functional Groups
A functional group is an atom or group of atoms which give a compound some characteristic physical and chemical property .
A homologous series is a family of organic compounds with the same general formula or functional groups with similar chemical properties .
Alcohols (Alkanols)
Alcohols are formed when one of the hydrogens on the end of the chain has been replaced with an -OH group
- Find the longest continuous chain of carbon that contains the functional group
- Replace the ane/ene/yne with -ol
- Name the substituents as prefixes in alphabetical order
- Number the chain so that the functional group has the lowest possible number
Alcohols can also be classified according to the number of carbon atoms attached to the carbon bearing the -OH group.
- A primary (1°) alcohol is one in which the carbon bearing the -OH group is bonded to one other carbon atom.
- A secondary (2°) alcohol is one in which the carbon bearing the -OH group is bonded to two other carbon atoms.
- A tertiary (3°) alcohol is one in which the carbon bearing the -OH group is bonded to three other carbon atoms.
Aldehydes and Ketones (Carbonyl Group)
A carbonyl functional group consists of carbon attached to an oxygen atom by a double bond . The difference between aldehydes ( alkanals ) and ketones ( alkanone ) is the position of the carbonyl group along the carbon chain:
- If the carbonyl group is on a terminal carbon (at the end of a chain), it is called an aldehyde , which is given the suffix “ -al ”.
- If the carbonyl group is in the middle of the carbon chain, it is called a ketone , which is given the suffix “ - one” .
- When the aldehyde is the suffix , the aldehyde carbon is assigned the number 1. Since it is always carbon 1, “1” (terminal carbon) is omitted from the name. (“-al”) e.g. 2-methylbutanal
- When the ketone is the suffix , the carbon chain is numbered so that the ketone is assigned the lowest number . (“-one”) e.g. Pentan-3-one.
Carboxylic Acids (Alkanoic Acids)
Carboxylic acids contain the carbonyl group, C=O, connected to a hydroxyl group. The carboxyl group is abbreviated as -COOH, and is given by the suffix “ -oic acid ”.
Since the carboxylic acid uses up 3 bonds on the carbon atom, it must be situated at the end of the chain (terminal carbon).
Unfortunately, the carboxylic acids have different names each. However, some of them should be familiar.
Amines and Amides
- Amines and Amides both contain nitrogen
- Amines contain NH A 2 , and use the suffix “-amine” or the prefix “amino-”
- Amides are carboxylic acids where the OH group has been replaced with an amine. They use the suffix “ amide ”.
- When they are quoted as the prefix, the carbonyl and the amine are named separately (using the prefixes “oxo” and “amino” respectively).
Halogenated Organic Compounds
- Halogenated organic compounds have had hydrogen atoms replaced with a halogen (group 17 element)
- e.g. di fluoro pentane
Naming Priorities of Function Groups
The highest priority functional group takes the suffix, and the other functional groups are placed earlier in the name.
Aldehyde > Ketone > Alcohol > Alkyne = Alkene
Structural Isomers
Structural isomers are molecules that have the same molecular formula, but their atoms are arranged in different ways, giving rise to different structural formulae .
Although isomers have the same molecular formula, they are different compounds with different chemical and physical properties , as well as different names.
Chain Isomers
Chain isomers involve rearrangement of the carbons in the backbone, resulting in a different number of carbons in the longest chain or different branching in the carbon chain.
Example: Hexane
- An alkane with the molecular formula C A 6 H A 14 has 5 chain isomers
- Each isomer has the same molecular formula, but a different name
Position Isomers
Position isomers occur when molecules have the same carbon chain, but the functional group is at a different location .
Position isomers only exist for molecules that contain functional groups , where the chain is long enough for the functional group to occupy different positions. (Eg. Not possible for ethene or ethane).
Example: Butene
Functional group isomers.
Functional group isomers result when the atoms in the molecules are arrange in different ways that lead to the isomers having different functional groups .
Example: C A 3 H A 6 O A 2
- Propanoic Acid and 1-hydroxypropan-2-one are functional group isomers, with the same molecular formula ( C A 3 H A 6 O A 2 ) , but different arrangements of atoms:
- Propanoic Acid contains a carboxyl functional group, while 1-hydroxypropan-2-one contains hydroxyl and carbonyl functional groups

Hydrocarbon Reactions
Combustion reactions.
This is what chemistry is all about, isn’t it? Setting things on fire and seeing what happens.
- All combustion reactions are exothermic . It may be complete or incomplete.
- For complete combustion to occur, excess oxygen must be readily available, and the products are always carbon dioxide and water.
C A 8 H A 18 ( l ) + 25 2 O A 2 ( g ) ⟶ 8 CO A 2 ( g ) + 9 H A 2 O ( l )
Incomplete combustion occurs when there is insufficient oxygen . The products are water and three different oxidations of carbon, soot, carbon monoxide and carbon dioxide .
Incomplete combustion results in less energy being produce per mole of fuel combustion, making it less efficient .
This is due to the reduction of C=O bonds being formed. The formation of C=O releases a large amount of energy.
Alkenes and alkynes tend to burn with a more sooty flame compared to alkanes due to the higher percentage of carbon atoms . Some of the carbon may not combine with oxygen.
Standard molar heat of combustion is the energy released . Therefore, it is positive .
Standard enthalpy of combustion is always negative as combustion is exothermic. It is the change in enthalpy .
Reactions of Unsaturated Hydrocarbons
Stability of carbon bonds.
- The bond energies dictate the overall reactivity of hydrocarbon compounds. Subsequent carbon-carbon bonds in a multiple bond are less stable and weaker than the original single covalent bond.
- This means that double and triple bonds are highly reactive and can break open more easily and allow atoms to join (saturate).
- This makes the alkenes and alkynes highly reactive compared to alkanes.
- Alkenes and alkynes are able to react with a number of chemical reactions called addition reactions
Addition Reactions of Alkenes - Hydrogenation
- Alkenes react with hydrogen gas in the presence of a metal catalyst to form a saturated alkane.
General Formula: Alkene + H A 2 ( g ) → Ni Saturated Alkane
Addition Reactions of Alkenes - Bromination and Chlorination
- Bromine and chlorine add to almost all alkenes very rapidly at STP, creating a compound that has 2 bromines/chlorines on adjacent carbons
- This occurs spontaneously at STP, and can be used as an indicator for alkenes and alkynes
Addition of HX (Hydrohalogenation)
- Any of the hydrogen halides (HF, HCl, HBr and HI) can add to the double bond of an alkane to give the corresponding alkyl halide.
- The double bond is converted into a single bond.
Predicting a Major Product
When an asymmetric reagent, such as HBr is added to an asymmetric alkene, more of one isomer is produced than the other.
The predominant isomer is called the major product and the other isomer(s) is called the minor product(s) .
In some reactions, only the major product will be formed.
The major product obtained from an addition reaction can be predicted using Markovnikov’s rule .
In addition, for reactions involving unsymmetrical alkenes, the hydrogen atom will predominantly bond to the carbon atom bearing the greater number of hydrogen atoms .
Addition of Water (Hydration)
- Water alone does not react with alkenes, but if an aqueous acid catalyst (eg. Dilute H2SO4) is added and the mixture is heated, water adds to the C=C double bond to give an alkanol.
Addition Reactions of Alkynes
Alkynes undergo addition reactions in a similar fashion to alkenes.
- One of the three bonds in the triple bond is broken, and two new bonds form. The original triple bond is converted into a double bond.
- The second addition reaction can be stopped by controlling equivalents of reagent used or by using a specialised reagent.
Hydration of Alkynes
- Alkynes do not react with water with only an acid catalyst. A mercury(II) catalyst, such as mercury(II) sulfate , must also be present.
- A carbonyl (ketone or aldehyde) is produced instead of an alcohol. The alcohol forms as an intermediate which quickly rearranges to form the carbonyl.
Substitution Reactions of Alkanes
- A substitution reaction occurs when an atom or functional group in a molecule is replaced or substituted by another atom or group.
- Alkanes are far less reactive than alkanes and alkynes as C-C single bonds are relatively strong .
- However, their hydrogen atoms can be substituted by halogens .
- These reactions do not occur spontaneously at RTP.
- Substitution of alkanes can only be carried out with chlorine and bromine (fluorine reacts too explosively, and iodine does not react) and required energy in the form of ultraviolet (UV) radiation .
CH A 4 ( g ) + Cl A 2 ( g ) → UV Light CH A 3 Cl ( g ) + HCl ( g )
Physical Properties of Alkanols
In case you forgot, alkanols and alcohols are the same thing, its just that the names tend to be -nol, its just easier to remember if we consistently use Alkanol (i.e. Alkane + nol)
Intermolecular and Intramolecular Bonding Forces of Alkanols
- Alkanols contain highly electronegative oxygen atoms, creating a polar bond in the molecule.
- The hydrocarbon end of the molecule repels water.
- This end is attracted to water.
- As the chain length of an alcohol increases, the strength of the dispersion force increases . However, the extent of hydrogen bonding does not change.
- The strength of the hydrogen bonding depends primarily on the molecule’s shape and the number of hydrogen bond donors and acceptors available.
- Thus the length of the hydrocarbon chain influences the properties within the homologous series of alcohols, and the hydroxyl group influences the differences in properties between alcohols and other homologous series.
Melting and Boiling Points of Alkanols
- ↑ Chain length→↑Molecular mass→↑ Strength of dispersion forces→↑BP and ↑ MP.
- However, the shape of ethanol and methanol allow them to pack more closely together than propan-1-ol, thus resulting in stronger intermolecular forces and a higher MP.
- The position of the hydroxyl group within the molecule can also affect the melting and boiling point of alcohols
- For example, butan-2-ol has a lower boiling point than butan-1-ol
- Secondary and tertiary alkanols have lower boiling points than their primary isomers
- The dispersion forces will be the same for all three isomers
- However, hydrogen bonding is weaker for secondary and tertiary alkanols, as the alkyl group adjacent to the OH group hinders the OH group’s ability to get close to another molecule, restricting their ability to form strong hydrogen bonds
- As a result, the lower boiling points arise from weaker hydrogen bonding in secondary and tertiary alkanols.
Solubility in Water
Small alcohols dissolve well in water.
However, solubility in water decreases with increasing carbon chain length .
The solubility of alcohols in water is dictated by size because the opposing effects of the polar and non-polar portions of the molecule.
The polar hydroxyl group is hydrophilic (“water loving”) and the non-polar hydrocarbon chain is hydrophobic (“water-hating”)
Water molecules cannot solvate the large non-polar carbon chains in long alcohols.
The -OH group can form hydrogen bonds with water, allowing solvation of this portion of the molecule.
However, to solvate the large non-polar carbon chain, many strong hydrogen bonds between water
molecules need to be broken.
Since the alkyl chain has no strong attraction to water, these cohesive hydrogen bonds cannot be broken .
Thus, the alkyl end remains unsolvated, and solubility in water is drastically decreased.
A very general rule for solubility in water, if it has less than a 4:1 carbon:oxygen ratio, it is soluble.
Small alcohols are good solvents for dissolving both polar and non-polar substances due to the presence of both polar and non-polar areas in the molecule.
The polar hydroxyl group can form polar interactions such as ion-dipole, hydrogen bonding and dipole- dipole forces with other polar and ionic substances
The non-polar hydrocarbon chain can form dispersion forces with other non-polar substances.
Solubility in Organic Solvents
Alcohols become more soluble in non-polar organic solvents such as hexane, benzene and toluene ( C A 7 H A 8 ) , as the length of the carbon chain increases .
- The large alkyl chain can form strong dispersion forces with other non-polar substances. These are strong enough to disrupt hydrogen bonds holding the alcohol together.
- Therefore, the alcohol molecules separate and disperse throughout the solvent.
Reactions of Alkanols
Dehydration reactions.
When alcohols are heated with concentrated sulfuric or phosphoric acid as a catalyst, an OH and a H atom on the adjacent carbon will be eliminated from the alcohol to give an alkene and water.
CH A 3 CH A 2 OH → 180 ∘ C H A 2 SO A 4 CH A 2 CH A 2 + H A 2 O Ethanol in Sulfuric acid at 180 ∘ C → Ethylene+Water
- Tertiary alcohols are the most reactive and always react the fastest. Dehydration occurs readily at room temperature.
- Primary and secondary alcohols require higher temperatures. Primary alcohols are less reactive and react slower than secondary alcohols.
Substitution Reactions of Haloalkanes
- Alkanols undergo substitution in the presence of a hydrogen halide to form the corresponding Alkyl Halide and Water.
- The trend in reactivity is ths same as for dehydration
- For primary alcohols, larger halides react faster (chlorine is slowest, iodine is fastest)
Oxidization
- Alcohols can be oxidised with strong oxidizing agents such as acidified solutions of permanganate ( MnO A 4 A − ) or dichromate ( Cr A 2 O A 7 A 2 − ) to give carbonyl compounds
- A change in oxidization states can be seen for the carbon atoms
Oxidisation of Primary Alcohols
Primary alcohols are oxidised into carboxylic acids .
The oxidation of alcohols is driven by a simultaneous reduction reaction, usually of inorganic reagents . Either acidified permanganate (MnO4- purple in colour) or dichromate (Cr2O72- orange in colour) can be used as the oxidant.
The oxidation occurs stepwise: the alcohol is first oxidised to the aldehyde , which is then oxidised into the carboxylic acid .
Aldehydes are very reactive and the second oxidation generally occurs too rapidly for it to be separated practically.
- MnO A 4 A − goes from purple to colorless
- Cr A 2 O A 7 A 2 − goes from orange to green
Oxidisation of Secondary Alcohols
Secondary alcohols can be oxidised to produce ketones .
Either acidified permanganate (MnO4- purple in colour) or dichromate (Cr2O72- orange in colour) can be used as the oxidant.
Since there are no hydrogen atoms attached to the ketone carbon, no further oxidation can occur.
Secondary alcohols are generally less reactive than primary alcohols , thus require higher temperatures and longer reaction times to be oxidised.
Oxidisation of Tertiary Alcohols
- Tertiary alcohols cannot be oxidized to form a carbonyl compound.
- The carbon bearing the hydroxide group has no hydrogen atoms, and therefore cannot be oxidised.
Combustion of Alcohols
- All alcohols can readily combust, either completely or incompletely.
- Because they are oxygenated, they are prone to complete combustion, and the reaction is highly exothermic as a result of the oxygen and hydrogen combustion reaction which occurs.
Production of Alkanols
Why is there so much about alcohols in this module???
Production by Hydration (Production by Addition)
- Aqueous catalysts such as dilute sulfuric or phosphoric acid allow water to react with alkenes.
- HO is added to the C=C double bond, forming an alkanol
- For example:
C A 2 H A 4 + H A 2 O → 300 ∘ C H A 3 PO A 4 Catalyst C A 2 H A 5 OH
Production by Substitution
- Alkanols can also be produced from the substitution of haloalkanes.
RX + OH A − → 100 ∘ C H A 2 O ROH + X A − Halocarbon + Hydroxide Ion → Alcohol + Halide Ion
This reaction occurs by heating the haloalkane with a solution of sodium hydroxide or potassium hydroxide .
The hydroxide ion from the aqueous base replaces the halogen atom to generate an alcohol and halide salt.
X: Cl, Br, I. Fluoroalkanes will not react as the C-F bond requires too much energy to break.
This reaction occurs due to the highly polarised carbon-halogen bond, which produces a partial positive charge on the carbon atom.
- The partially positive carbon atom can be easily “attacked” by a negatively charged hydroxide ion.
- This results in the formation of a covalent carbon-oxygen bond. In the process, the negative charge is donated to the electronegative halogen atom, which leaves as a halide ion.
The rate of this reaction is dependent on the type of haloalkane and the halogen atom that leaves the molecule.
Haloalkanes can be categorised as primary, secondary and tertiary. The reactivity is highest for primary, followed by secondary and then tertiary.
This is because the presence of alkyl groups greatly hinders the ability of the hydroxide ion to approach the partially positive carbon and thus slows the reaction.
The type of halogen atom leaving the molecule also has an effect on the rate. The reaction occurs the fastest with iodide, followed by bromide and then chloride.
This is because the carbon-halogen bonds are of different strengths.
The lower the bond energy, the easier it is to break the bond.
It is also possible for haloalkanes to undergo substitution reactions with water to form alcohols. This reaction occurs much more slowly , and the reactivity is highest for tertiary .
Production by Fermentation
- Fermentation is a process that involves the conversion of carbohydrates into simple alcohols by the action of enzymes.
- This is a natural process used by microorganisms to extract energy.
- Carbohydrates have the molecular formula of C A x ( H A 2 O ) A y .
- Carbohydrates are abundant in plant material.
- They are also called saccharides.
- The simplest carbohydrates are monosaccharides.
- They are the building blocks of more complex carbohydrates, such as disaccharides like sucrose and polysaccharides like cellulose.
- The fermentation of monosaccharides , such as glucose and fructose is the simplest form of fermentation which process ethanol and carbon dioxide .
C A 6 H A 12 O A 6 ( aq ) → Yeast 2 Ch A 3 CH A 2 OH ( aq ) + 2 CO A 2 ( g )
- This process relies on the presence of zymase , an enzyme found in yeast.
Conditions for Fermentation
- Zymase is present – found in yeast
- Warm temperatures (30 − 40°C but depends on the yeast strain)
- Anaerobic environment (oxygen limited environment)
- Aqueous solution of sugar
- Fermentation of monosaccharides must be catalysed by zymase. Since zymase is a biological catalyst, it is sensitive to temperature.
- Yeast can produce ethanol to concentrations up to only about 15% v/v . This is due to ethanol being toxic and around 15% v/v, yeast will start to die.
- To produce higher alcohol contents, it is necessary to distill the liquid
- If the aqueous mixture from a fermentation process is subjected to fractional distillation, 95% ethanol can be obtained.
- To obtain 100% ethanol, more elaborate procedures such as molecular sieving are required.
Environmental Impacts of Hydrocarbons
Sources of hydrocarbons.
The primary source of hydrocarbons is from petroleum . Petroleum is a mixture of hundreds and thousands of different alkanes, ranging from methane up to alkanes with 40 or more carbons.
The mixture of gases found in petroleum is called natural gas and the mixture of liquid components is called crude oil .
Petroleum is found within pores of rocks deep in the ground.
The complex mixture is separated into fractions according to their boiling points using fractional distillation .
The petroleum is heated to about 400°C to produce hot liquid/vapour mixture that enters the fractioning tower.
Inside the tower are horizontal trays, each which contains many bubble caps upon which alkanes condense.
Fractions which have lower boiling points will rise higher in the column before condensing.
Fractions which have higher boiling points will not rise as high and will condense towards the bottom of the column.
Differences in physical and chemical properties of each petroleum fraction mean that they are suitable for different purposes.
Generally, light fractions (LPG, petrol, naphtha) are more useful and are in higher demand than heavy fractions (heavy fuel oil, lubricating oil, wax and asphalt).
Some of the longer alkanes are further processed through cracking , which involves heating alkanes to high temperatures in the absence of oxygen.
This causes them to split and form shorter , more useful alkanes as well as alkenes.
A zeolite catalyst, which consists of Al, Si and O, may be employed to allow this reaction to be carried at lower temperatures.
Uses of Hydrocarbons
- The major use of petroleum is transport. Hydrocarbons are excellent fuels and the combustion of hydrocarbons is the primary source of energy production globally.
- Unsaturated hydrocarbons are highly reactive and can undergo addition reactions.
- This makes them extremely important as raw materials for the production of other organic chemicals, such as haloalkanes and alcohols, and commercially valuable goods such as plastic.
Issues with using Petroleum
Petroleum deposits in the ground are formed by the burial and decomposition of prehistoric living organisms over millions of years . Thus, petroleum is a finite and non-renewable resource.
As the world’s crude oil diminishes, there will be enormous negative economic and sociocultural consequences . Such as the instability of world markets and increase costs of goods.
Another huge problem that arises is that the combustion of petroleum releases huge amounts of carbon dioxide into the atmosphere.
Carbon dioxide is a greenhouse gas , so it absorbs infrared radiation from the atmosphere and keeps our Earth warm.
The extra carbon dioxide produces through combustion is a major contributor to the enhanced greenhouse effect which causes global warming .
The consequences for global warming include rising sea levels which in the long term will result in land loss and flooding, more frequent and intense extreme weather events , warming of the oceans and disruptions to the feeding behaviour of wildlife.
The higher concentrations of carbon dioxide has also resulted in the acidifications of oceans which is threatening the survival of aquatic life.
Alternative Fuel Sources
- A possible solution to the reliance on non-renewable crude oil is to use biofuels .
- Biofuels are fuels derived from biomass , which is biological material from living or recently living organisms such as wood, crops, wet waste and animal waste.
- Bioethanol is ethanol produced from the fermentation of monosaccharides such as glucose and fructose .
- Monosaccharides are the building blocks of more complex carbohydrates which make up plant material.
- Sucrose based feedstock (eg. Sugarcane and fruits)
- Starch based feedstock (eg. Grains like wheat and corn)
- Cellulose based feedstock (eg. Wood residues)
- Sucrose ( C A 11 H A 22 O A 11 ) and starch are readily hydrolysed into monosaccharidesduring the fermentation process, as yeast contains the necessary enzymes required to catalyse the breakdown.
- Hydrolysis: C A 11 H A 22 O A 11 ( aq ) + H A 2 O ( l ) ⟶ 2 C A 6 H A 12 O A 6 ( aq )
- Fermentation: C A 6 H A 12 O A 6 ( aq ) → Yeast 2 C A 2 H A 5 OH ( aq ) + 2 CO A 2 ( g )
- Cellulose is difficult to break down to its component sugars as the enzyme cellulase is not readily available for industrial use.
- Bioethanol has been developed as a substitute for petroleum-based ethanol and as an alternative to petrol. It has the potential to be used as standalone fuel to completely replace petrol but is usually used as an additive to petrol.
- In Australia, most cars post-1986 can use up to 10% ethanol (E10 fuel) .
- Biogas consists of mixtures of gases , such as methane , carbon monoxide and hydrogen , released from the natural breakdown of organic matter by anaerobic bacteria.
- The organic matter is sourced from natural wastes from agriculture and households, such as manure , human sewage, food processing wastes and crop wastes .
- To produce biogas, the waste is placed in a large enclosed tank , called the digester , containing anaerobic bacteria . The gas released from the decay is collected by gas outlets.
- The biogas collected can be combusted as a fuel to generate electricity or to heat boilers from industrial processes and for cooking and heating water in homes.
- The biofuel production represents a carbon cycle , where plants absorb carbon dioxide during growth, recycling the carbon dioxide released during combustion.
- The use of biogas also helps reduce the enhanced greenhouse effect as methane is a greenhouse gas that has a larger effect than carbon dioxide.
- By collecting it and using it to produce electricity, less is released into the atmosphere.
- Another environmental benefit is that bioethanol and biodiesel are cleaner fuels than petroleum fuels .
- They are oxygenated, so complete combustion is more likely to occur and they do not contain sulfur impurities.
- They are non-toxic and biodegradable , thus do not pose as severe a threat to the environment in the event of a spill.
Obstacles in the Production of Biofuels
In Australia, biomass sources currently being used are waste residues .
- Bioethanol is made from sugar cane molasses (waste) and waste from starch and red sorghum production.
- Biogas is generated from the treatment of waste water
- Biodiesel is produced from waste vegetable oil from restaurants and industrial food producers
However, this only produces a small percentage of Australia’s fuel needs. It is not possible to manufacture enough biofuel from these sources to replace all petroleum fuels used today.
Large amounts of fertile land would be required to grow the crops.
This requires clearing of forests and bushland which will contribute to an increase in carbon dioxide levels in the atmosphere and to habitat loss .
Fertile land is also limited thus it competes with food crops , resulting in increase in food prices.
Intensive farming can lead to land degradation and erosion .
A large amount of fertiliser would be required to replace the nutrients taken from the ground.
A large amount of water would be required.
There is also a large amount of energy required for harvesting of crops and processing biofuels, energy that is currently derived from fossil fuels.
Large scale commercial agriculture leads to the reduction of biodiversity due to the loss of important
organic matter from that crop.
The potential of biofuels lies in making it financially viable compared to conventional petroleum-based fuels.
Organic Acids and Bases
Organic acids are acids that have a carbon-based structure. The most common type of carboxylic acids are alkanoic acids. They occur abundantly in nature.
- Propanoic acid is found in cheese
- Methanoic acid is produce in ant venom
- Lactic acid is in milk
- Citric acid is in citric fruits
All carboxylic acids are weak acids thus they will partially ionise in water to produce hydrogen or hydronium ions.
Each carboxylic acid group is monoprotic.
The covalent bond between oxygen and hydrogen is highly polar . The bonding electrons are strongly
attracted to oxygen so the hydrogen can be drawn easily by a base , leaving behind a negative charge on the oxygen.
If more than one carboxylic acid group is present in the molecule, these acids will be polyprotic . For
example, citric acid is triprotic.
The strength of a carboxylic acid is influenced by the length and substitution of the hydrocarbon chain.
- As the chain length increases , the strength of the acid decreases .
- This is because the alkyl groups are capable of donating electron density . Carbon is more electronegative, so it pulls electron density towards itself and away from hydrogen .
- The additional electron density allows carbon to “donate” additional negative charge to neighbouring carbon atoms.
- Substituting a highly electronegative atom , such as a halogen, onto the hydrocarbon chain increases the strength of the acid .
- The strong electron-withdrawing power of the substituent helps weaken the oxygen- hydrogen bond. This makes it easier for the hydrogen to be dissociated.
- As the number of electronegative atoms increases , so does the strength of the acid .
- Fluorine has the biggest electron-withdrawing effect , then chlorine, bromine and iodine.
Amides (Weak BL Bases)
- Organic bases are organic compounds that are characterised by the presence of an atom with a lone pair of electrons that can accept an H A +
- Nitrogen containing compounds such as amines are the most common organic bases.
- Many amine bases exist in nature.
- The most important being the four nitrogenous DNA bases: adenine, cytosine, guanine and thymine, and the 20 natural amino acids used to make proteins in living organisms.
- Amino acids are molecules that contain both a carboxylic acid and a basic amine group.
- Simpler amines are made by substitution reactions of haloalkanes with ammonia.
- Amines act as a base in an analogous manner to ammonia. The lone pair of electrons on the nitrogen can accept a proton, forming an ammonium ion . Like ammonia, they are weak bases .
- ↑ Chain length→↑ Partial negative→↑ H+ acceptor→↑ Base strength
- Alkyl groups are capable of donating electron density .
- This results in a build-up of partial negative charge on the electronegative nitrogen atom , allowing it to pick up H **+** more readily and also stabilise the positive charge on the ammonium ion.
- The longer the chain, the greater the electron density donate.
- Amides are also nitrogen containing compounds with a lone pair of electrons.
- Amides are neutral compounds.
- The presence of the highly electronegative oxygen atom in the C=O group pulls electron density away from the nitrogen atom, which makes it more difficult to accept a H+ and stabilise a positive charge if nitrogen was to accept it.
Reactions of Carboxylic Acids and Amines
- Carboxylic acid + reactive metal → salt + hydrogen gas
- Carboxylic acid + metal hydroxide/oxide → salt + water
- Carboxylic acid + metal carbonate/bicarbonate → salt + carbon dioxide + water
- Amine + acid → ammonium salt
- When carboxylic acids react with amines , the product formed will depend on the conditions.
- At low temperatures , a proton transfer (neutralisation reaction) proceeds between the carboxylic acid and amine, forming a carboxylate ion (acid) and an alkyl (base).
- At higher temperatures or in the presence of a suitable catalyst, an amide is produced with the elimination of water (condemnation reaction), which forms the OH group on the acid and a hydrogen from the amine.
- Condensation reaction: Where two or more molecules combine to form a larger molecule with the simultaneous elimination of a small molecule such as water or methanol.
Physical Properties of Carboxylic Acids, Amines, and Amides
Boiling point.
- Carboxylic acids, amines and amides are all polar molecules . They can form hydrogen bonds with other molecules.
- ↑ Alkyl chain→↑ MM→↑ Dispersion forces→↑ BP Amides do not exhibit a linear relationship between boiling point and molecular weight.
- This is because the hydrogen bonding exhibited by the amides is extensive and are more complex.
- Amides exhibit the highest boiling points compared to carboxylic acids and amines.
- Carboxylic acids have higher boiling points than amines .
- Carboxylic acids and amines have less atoms that can form hydrogen bonds.
- Each carboxylic acid molecule can form two hydrogen bonds with another acid molecule through the double bonded oxygen and OH group, forming a dimer (existing in pairs).
- Each amine molecule can only form one hydrogen bond with another amine molecule .
- Amines and amides can be classified into primary, secondary and tertiary depending on the number of alkyl groups attached to the nitrogen .
- Primary amines have the highest boiling points, followed by secondary and then tertiary amines. The same trend occurs with amides.
- Small amines, amides and carboxylic acids dissolve completely in water. However, solubility decreases as the hydrocarbon chain increases.
- ↑ Carbon chain length→↑ Non-polar nature of compound→↑ Dispersion forces domination→Water cannot solvate the long hydrocarbon chain due to cohesive bonds→↓ Solubility
- Similar to alcohols, the trend is reversed for their solubility in organic solvents .
- Esters are organic compounds with the functional group COO A −
- Esters are formed from the reaction of a carboxylic acid and an alcohol.
- The OH group on the acid is replaced with an OR group from the alcohol. This reaction is called esterification (a condensation reaction).
- The reaction is extremely slow.
- Concentrated H A 2 SO A 4 (dehydrating agent) is used to remove the water and catalyse the reaction.
- Becaise they are all liquids, K e q = [ H A 2 O ] [ Ester ] [ Alkanoic Acid ] [ Alkanol ]
- Esters are everywhere in nature and are used in a number of industrial applications .
- Short chain esters are known for their distinctive, fruit like odours and many occur naturally in fruits and the essential oils for plants.
- Due to their pleasant odours, they are commonly used as flavouring (banana lollies) agents in processed foods, as well as fragrances in perfumes and cosmetics.
- Fats and oils are also naturally occurring triesters derived from glycerol and fatty acids .
Nomenclature of Esters
- The alkanol is changed to “alkyl” and is the first word of the esters name
- The alkanoic acid becomes the alkanoate and is the second word of the esters name
Esterification
To increase rate of reaction:
- ↑ Temperature
- ↑ Acid catalyst
To increase yield:
- Remove water
- Concentrated H A 2 SO A 4 (catalyst and dehydrating agent)
- Esterification must be carried out under reflux .
- Reflux is a technique that involves heating a reaction mixture in a vessel fitted with a cooling condenser so that the volatile reactants and products are returned to the reaction mixture without any loss.
Structure of Soaps
Soaps are surfactants which, when dissolve in water, help to remove dirt, oil and foreign matter from surfaces.
Soaps are salts of fatty acids:
- Fatty acids are carboxylic acids with long hydrocarbon chains (10+)
- The salt of a fatty acid consists of a negatively charged carboxylate ion (called the head ) with a long hydrocarbon chain (called the tail ) and a positively charged ion.
In water, the sodium or potassium ions float free and do not play a part in the cleaning actions of soaps.
It is the negatively charged fatty acid ion which is responsible for the cleaning action.
- The charged head is hydrophilic (water attracting) due to its polar nature
- The tail is hydrophobic (water repelling) due to its non-polar nature
Saponification
- Soaps are produced from the hydrolysis of fats and oils (lipids) in a basic solution such as NaOH.
- Fats and oils are known as fatty esters , triglycerides or triesters .
- When esters are heated in the presence of a strong base such as NaOH or KOH, the ester is broken down to give the alcohol and a carboxylate ion .
- This reaction is known as saponification . It is a type of hydrolysis reaction, involving the breaking of a chemical bond by the addition of water
How Soaps Work
- Soap is a surfactant ( surf ace act ive a ge nt s)
- This means it functions by reducing the surface tension of water and binding to grease and dirt to emulsifies them.
- The hydrophobic part of the soap molecule is long, non-polar hydrocarbon chain . It is strongly repelled by water molecules.
- When soap molecules are added into water, they form an oriented monolayer (with tails sticking out of the water) at the surface in order to satisfy the interaction of both the hydrophobic and hydrophilic portions of the soap molecule.
- This effectively breaks the hydrogen bonding between molecules of water and thus reduces the surface tension of water.
- The components self-assemble into the most stable arrangement , which consists of spherical structures with the carboxylate groups forming a negatively-charged spherical surface , with the hydrocarbon chains inside the core of the sphere.
- The spheres are called micelles . The hydrophobic tails are shielded from the water by the polar heads, which minimises the repulsive forces in the system.
1. Dissolution
Soap molecules must be first dissolve in water. The hydrophilic head of a soap ion interacts with water molecules via ion-dipole interactions and hydrogen bonding .
2. Adsorption
Grease and oil consists of non-polar molecules.
The hydrophobic tails of the soap ions dissolve in the grease due to dispersion forces and orientates themselves.
Surfactant molecules continue to absorb into the grease, decreasing the surface tension of water at the interface between the grease and water.
The hydrophilic heads interacting with water via ion-dipole forces effectively pull the grease off the surface.
3. Emulsification
With agitation , the grease layer breaks into smaller, spherical droplets (micelles) , with the hydrophilic surfactant head groups interacting with the water via ion-dipole forces , and the hydrophobic surfactant tails adsorbed into the grease. This forms a dispersion of grease droplets in water (an emulsion ).
The negative charged heads on the soap repel each other, preventing the grease and dirt from joining together and keeping them dispersed throughout the solution. Therefore, the grease and oil can be simply rinsed away, leaving a clean surface.
Synthetic Detergents
Problems with soaps.
- The soap ion must be dissolved in water to have a cleaning effect
- As salts of weak acids, in low pH, soaps are converted into uncharged fatty acids
CH A 3 ( CH A 2 ) A 16 COONa ( aq ) + HCl ( aq ) ⟶ CH A 3 ( CH A 2 ) A 16 COOH ( s ) + NaCl ( aq ) Soluble fatty acid ion + acid → Insoluble fatty acid molecule + ionic salt
- Soaps can also form insoluble salts (scum) in hard water (water with high concentration of divalent metal ions such as Ca A 2 + and Mg A 2 + )
- All such synthetic surfactants are known as detergents
- They have a similar structure to soaps, with a long non-polar hydrocarbon tail and a polar head. However, they vary in the structure of the polar head group .
Anionic Detergent
- All anionic detergents have a negatively charged polar head.
- The main structural difference between anionic detergents and soap is the presence of a sulfate ( R − SO A 2 − O A − ) or sulfonate group ( R − O − SO A 2 − O A − ) instead of a carboxylate ( R − COO A − ) .
Effectiveness in Hard or Acidic Water
- Anionic detergents don’t form insoluble precipitates .
- They form salts that are all soluble.
- However, the effectiveness is still reduced in hard and acidic water .
- The positive ions in hard water/acidic water will attract the negative head of the detergent.
- Phosphate is an example of a builder that can be added to soften the water.
Cationic Detergents
- All cationic detergents have a positively charged polar head . The positive head is usually a quaternary ammonium ion . They are also known as fatty amine salts .
- Generally cationic detergents are not very good cleaning agents due to the strong attraction of cationic detergents to negatively charged surfaces (Most surfaces are negatively charged).
- Many fabrics acquired acquire a negative charge when they become wet.
- Cationic detergents are used in fabric softeners and hair conditioners . The strong attraction of cationic detergents to negatively charged surfaces can be detrimental in other situations.\
- Cationic detergents are particularly toxic to microorganisms .
- They are attracted to the negative surface of bacteria and damage or kill bacteria that are involved in their decomposition.
- They therefore have very low biodegradability .
Non-Ionic Detergents
- All non-ionic detergents have uncharged polar heads . The polar heads consists of polar groups such as ethoxylates .
- Although these surfactants are uncharged , the polar head groups are still attracted to the highly polar water molecules forming numerous hydrogen bonds.
- Non-ionic detergents don’t foam as much as other detergents, hence they can be used in dishwashers.
Environmental Impacts of Surfactants
- Detergents are synthetic , whereas soaps are made from naturally occurring biological materials (fats and oils). Thus detergents are less biodegradable .
- The enhanced stability of detergents means they persist in the environment, causing damage to the mucus membrane in wildlife and resulting in excessive frothing in the water ways.
- This leads to less sunlight penetration.
- Toxic to aquatic life.
- Anionic detergents often contain builders such as sodium triphosphate ( Na A 5 P A 3 O A 10 ) .
- The builders react with minerals in hard water and form soluble molecules.
- High levels of phosphates entering the rivers and waterways can lead to eutrophication (turning a lake into swamp, algae, etc.)
A polymer is a long chain molecule made up of repeating units, called monomers joined by covalent bonds.
The process of linking monomer units is called polymerisation .
Polymers may be natural or synthetic:
- Natural polymers are made by living organisms. Eg. Hair, starch, cellulose, DNA and silk
- Synthetic polymers are manufactured. Eg. Plastics like polyethylene, polyvinyl chloride and nylon.
Addition Polymers
- Addition polymers are polymers made by adding unsaturated molecules to each other, without the elimination of any atoms .
- Additional polymerisation is a type of addition reaction, in which one of the bonds in the C=C double bond is broken to form two new single bonds.
- A simple way of representing polymers is by writing the repeat units in square brackets followed by the subscript n where n is the number of monomer units in the polymer.
- Addition polymers are all synthetic , they do not exist in nature.
Condensation Polymers
- Condensation polymers are polymers formed through the condensation reaction of difunctional monomers with the elimination of a small molecule such as water or methanol in the process.
- Monomers used to synthesise condensation polymers usually contain groups such as alcohol, carboxylic and amine.
- Condensation polymers are found everywhere in nature.
- Polysaccharides such as cellulose and starch are condensation polymers made from glucose monomers.
- Polyesters are condensation polymers in which the repeating units are joined by ester links.
- Polymers made from two different monomers are called copolymers .
- Polyamides are condensation polymers in which the repeating units are joined by amide links .
- The nylon class refers to polyamides that have linear carbon chains in the repeating units.
Properties of Polymers
- Melting point (softening point)
- Mechanical strength
- Flexibility.
- Molecular weight or chain length
- Extent of chain branching
- Presence of side groups (eg. -OH)
Chain Length
- Polymers are extremely large covalent molecules. The dominant intermolecular force is dispersion.
- The length of a polymer (and its molecular weight) depends on the number of monomers the polymer contains.
- The melting point , rigidity and hardness of a polymer increases with an increase in chain length .
Chain Branching
- Polymers are able to form branched and unbranched chains.
- Unbranched chains are able to pack more closely in an orderly fashion , forming a rigid crystalline solid.
- Branched polymer chains are unable to align with each other, forming an amorphous solid that has weak intermolecular forces between the chain.
Crystalline and Amorphous Solids
If the polymer chains have few branches, as in the case with HDPE , the molecules can sometimes line up in a regular arrangement, creating crystalline regions . The regular arrangements brings the polymer chains closer together. The IMF between closely packed chains are stronger, and the presence of crystalline regions strengthens the material overall.
Crystalline regions in a polymer prevents the transmission of light through the material, making it appear cloudy or opaque.
Amorphous region will form where the polymer chains are randomly tangled and unable to pack very closely. In some polymer materials, the entire solid is amorphous. Amorphous polymers are usually more flexible and weaker and are often transparent . ( LDPE ) Increasing the percentage crystallinity of a material makes it stronger and less flexible. This also makes the material less transparent because crystalline regions scatter light . There are more crystalline regions in unbranched polymers.
There are crystalline and amorphous regions in all polymers.
Side Groups
- Side groups make a material more rigid and brittle, resulting in harder polymers.
Cross-Linking
- Polymer chains are held together by intermolecular forces or they can be linked by covalent bonds called cross-links to form a large extended network.
- Polymers with only intermolecular forces between their chains are called thermoplastic polymers.
- They soften when heated as the intermolecular forces are relatively weak and easily broken to allow the chains to move between one another.
- This property allows polymers to be remoulded.
- Polymers with cross-links are called thermosetting polymers.
- Since covalent bonds are very string, cross links limit movement between polymer chains, making the polymer more rigid, hard and heat resistant .
- These polymers cannot be remoulded (like XLDPE).
Types of Polymers
Polyethylene.
- Polyethylene (polyethene) is the most popular plastic in the world. It has a very simple structure.
- Ethene is an unsaturated molecule because of a double carbon-carbon bond .
- When ethene polymerases, the double bond breaks and new covalent bond are formed between carbon atoms on nearby monomers.
- The polyethene formed does not contain any double bonds.
- Ethene is one of the most simple and versatile monomers .
- It is easily able to undergo addition polymerisation.
LDPE (Low-Density Polyethylene)
- LDPE is produced under high temperatures and pressures .
- Under these harsh conditions, the polymer is formed too rapidly for the molecules to be neat and symmetrical.
- The products usually contain too many small chains (branches) that divide off the main polymer.
- The molecules in the polymer cannot pack closely together thus reducing the dispersion forces.
- The arrangement of the polymer molecules can be described as disordered or non-crystalline.
Properties of LDPE
Flexible, chemically inert, good elongation
Low melting point/thermoplastic
Lightweight, good puncture resistance
Used in: Milk carton lining, bowls, flexible water pipes, bottles
HDPE (High-Density Polyethylene)
- Highly specialised transition metal catalyst, known as Ziegler-Natta catalysts are used to avoid the need for high pressure.
- Due to the polymer being produced under a lower pressure, the conditions are milder and there are fewer branches.
- The lack of branches allows the molecules to pack together tightly increasing the density and the hardness of the polymer formed.
- The arrangement of the polymer molecules is more ordered, resulting in crystalline sections .
- Used in: Food packaging, dustbins, crates, drums, water pipes
Polyvinyl Chloride (PVC)
- PVC (polychloroethene) is made out of vinyl chloride monomers.
- The chlorine atoms introduce dipoles into the long molecules.
- This increases the IMF between molecules, which leads to a higher melting point .
- A PVC item burning in a flame will not continue to burn when it is removed from the flame.
- It is used in products such as conveyor belts, cordial bottles, water pipes and the covering of electrical wires.
- Pure PVC is very hard and brittle .
- Additives are incorporated into PVC to improve its flexibility, thermal stability and UV stability.
- In a fire, PVC decomposes to form toxic and corrosive hydrogen chloride.
Polystyrene
- First commercial production by IG Farben; used in disposable household products, plastic model kits, laboratory containers, insulation and packaging.
- Benzene rings are covalently bonded to every second carbon atom in the polymer chain.
- This causes polystyrene to be a hard but quite brittle plastic with a low density .
- It is used to make food containers, picnic sets, refrigerator parts, and CD and DVD cases.
- Polystyrene is made from styrene (ethylbenzene monomers).
- Polystyrene (polyethylbenzene) is commonly manufactured as a foam .
- Foamed polymers are formed by blowing a gas through melted polymer materials, making them 95-98% air by volume.
- Foaming can drastically change the physical properties of a polymer material.
- Polystyrene foam is produced by introducing pentane into melted polystyrene beads.
- The beads swell up to produce the lightweight, insulating, shock-absorbing foam that is commonly used for takeaway hot drink containers, bean bag beans, packaging materials and safety helmet linings.
- Once polystyrene has been converted to a foam, it is difficult to recycle.
Polytetrafluoroethylene (PTFE)
- Polytetrafluoroethene is used in cookware fabrics, wiper blades, nail polish, industrial coatings, also known as Teflon, or Fluon.
- Made out of tetrafluoroethene monomers.
- Tetrafluoroethene is formed when all the hydrogen atoms in ethene are replaced by highly electronegative fluorine atoms.
- It has quite exceptional properties that are very different from those of polyethene. It can be used to make non-stick frying pans, medical implants, gears and clothing.
- The electronegative fluorine atoms reduce the strength of intermolecular bonds with other substances.
Properties of PTFE
- Heat resistant
- Chemical resistant
- Good mechanical properties
- Low friction coefficient
- Flame resistant
- High melting point
Polyethylene Terephthalate (PET)
- Polyesters are a class of polymers that are formed through the process of condensation polymerisation.
- Polyesters are formed by combining monomers that contain carboxylic acid and hydroxyl functional groups.
- They are typically formed by reacting a dicarboxylic acid monomer with a diol monomer.
- This is the most often polymer used to make polyester fabric.
- PET is synthesised by reacting benzene-1,4-dioic acid monomers with ethane-1,2-diol monomers.
- PET has a range of uses including recyclable drink bottles and food packaging.
- PET is a strong material because the ester groups are polar, so that there are dipole-dipole attractions between the polymer chains.
- Benzene rings make it stiff and strong, resistant to deformation.
- Nylon stockings created shopping frenzy in the USA in the 1940s.
- It is also used in clothing, parachutes, kitchen utensils, toothbrushes, fishing lines, guitar strings, seatbelts.
- Nylon is formed when a monomer containing an anime group on each end reacts with a monomer with a carboxyl group on each end, a polyamide can form.
- The term ‘nylon’ refers to the group of polyamides , in which the monomers are linear carbon chains.
- A common example is nylon-6,6 which is named so because the dicarboxylic acid monomer has a chain of 6 carbons and the diamine monomer also has a chain of 6 carbon atoms.
- Nylon can be easily drawn into fibres that have high tensile strength.
- These fibres are used to produce strong lightweight material for clothes.
A few years after the release of nylon, a movie called “The Man in the White Suit” came out, portraying the tale of a scientist who had invented an invincible fabric, so stain resistant it couldn’t even be dyed (hence white suit). The product is an instant hit until labour unions and corporate overlords realise that as soon as everyone has the amount they need, they will stop buying more (because it’s invincible), and so they order him to make it weaker. This is almost identical to the invention of nylon, which replaced more fragile fabrics like silk. Over the last few decades, nylon has been weakened intentionally by manufacturers so that it wears out faster than it naturally would, a technique known as “planned obsolescence”. You can read more about it here.
And that’s it! The longest module is over!
Last updated on November 28, 2021
Chemistry Module 7 Notes
DOWNLOAD THE RESOURCE
Resource Description
Basically a quick but good review over mod 7. There may be some typos in the document lol
Report a problem
Popular HSC Resources
- Speech on George Orwell ‘1984’ – Human Experiences
- How To Survive the HSC
- One Night the Moon – Analysis (Video)
- 2020 – Physics – PHS (Trial Paper)
- Business Studies Influences on HR (Quiz)
- Sci Ext – Portfolio Pack
- 2020 – Science Ext – Exam Choice (Trial Paper)
- Domino’s Marketing Case Study
Become a Hero
Easily become a resource hero by simply helping out HSC students. Just by donating your resources to our library!
What are you waiting for, lets Ace the HSC together!
Join our Email List
No account needed.
Get the latest HSC updates.
All you need is an email address.

- University Admission
- School Admission
- Admission Result
- HSC Result 2023
- NU Honours Admission Result 2024
- Primary Result 3rd Phase 2024 PDF Download
- HSC Routine 2024
- NU Admission Result 2024
HSC Assignment 2021 Chemistry Answer (All Weeks)
Hsc 2021 chemistry assignment answer 7th week.

There are total 10 HSC 2021 Chemistry Assignment Answer has to be prepared. Science group students have to create 10 assignment solutions in 10 weeks. 5 of them have to be made for the Chemistry 1st paper. And 5 should be made for the Chemistry 2nd paper.
- Read more about HSC 2021 Chemistry Assignment Answer 7th week

- TERMS AND CONDITION
- Privacy Policy
- TELETALK APPLY
HSC Chemistry Assignment 2022 Solution – 12th Week
HSC Chemistry had to be submitted HSC Chemistry Assignment Answer 2022 for 12th weeks. There authority has been assigned hsc Chemistry Assignment 12th week. We also posted and you can get your twelve class Chemistry assignment 2022 pictures with pdf files at chakrirkhobor.net. HSC Chemistry Assignment is also scheduled for 9th and12th and more week 2022. So get HSC Chemistry Assignment questions and answers to keep reading below.
HSC Chemistry Assignment Answer 2022 – 12th Week
HSC Chemistry assignments start in June and end in December 2022. All Education Board start all subject Assignment and will end on maybe December 2022. Students can also check their HSC Chemistry 1st paper assignments question solve on these websites. You can get the first Chemistry Assignment all Board. This year newly start Assignment answer 2022 all board on their website.
How to do Class 12 Chemistry Assignment Answer 2022?
On the off chance that you don’t think about the HSC Class Chemistry Assignment process, this area will improve your insight in a matter of seconds. It would be ideal if you’re Assignment and ensure you are following the procedure appropriately. Here you go.
Are you Download this Chemistry Assignment?
The more precise your task arrangement is, the more stamps you are probably going to get pdf download and Hsc Chemistry Assignment Solution. Most of the Assignment finder many time fine Chemistry Assignment Twelve Class pdf download. Like different classes, the position will uncover fourth week class 12 Chemistry Assignment Answer.

How to install Chemistry Assignment ?
We have talked about the full task class HSC Chemistry for every one of you. We trust you didn’t have any trouble in downloading any of the gave tasks. You can gather class HSC Chemistry Assignment arrangement from this post. All time visit our site and Stay with us, You will get your answer. I will compose the 1st week class 12 Chemistry assignment task answers to the inquiries referenced in the inquiry concerning a help individual.
Chemistry Assignment Solution Download 2022
HSC Education Board has recently released the notification regarding the HSC Assignment Exam solution 2022 exam dates check on the official Website. HSC Chemistry Assignment 2022 PDF Download. I realize who lost his employment because of lockdown. Nonetheless, since the understudies need to offer their own input, they need to reply.
How to get 12 Chemistry paper Assignment Answer?
All education Board facilities get HSC Chemistry Answer. Well, it’s quite obvious most of the students in Chemistry are belongs from all area, they may not have the facility of Computer, Laptop or Android MOBILE, if you are not able to check your Assignment 12, then you can get your College.
When 12 Class Chemistry Assignment will be Start 2022?
To whom it may concern & get bet Class Twelve class Chemistry Assignment of All board and you try to see here. You cannot know to check and follow these rules.
Twelve class Chemistry Assignment answer- 12th Week
Finally, you can understand get the total subject 12 Class Chemistry Assignment all board 2022. In conclusion, Every Assignment seeker knows chakrirkhobor.net published all type of Assignment with an answer pdf file. This Notice also found on my website. So you have to connect for their address. In conclusion, for the next update about update assignment Notice. Finally 12th Week 12 Class Chemistry Assignment etc. So stay with us.
Recommended For You

SSC Assignment Solution for SSC Exam 2024- 1st Week

Madrasha Board Class 9 Assignment Answer 2024 – All Subject

Madrasha Board Class 8 Assignment Answer 2024 – All Subject

Dakhil Class 7 Assignment Answer 2024 – All Subject

7 Class Assignment With Solution 2024 – 1st, 2nd, 3rd, 4th week

7 (Seven) Class ICT Assignment Solution PDF 2022- 6th Week
About the Author: Desk Job

IMAGES
VIDEO
COMMENTS
The Bangladesh education system's authority arranged these assignment systems to increase activity and bring back to study. The Chemistry Assignments of HSC for the 1st Week are already finished. Now 2nd and 1st-week HSC Chemistry assignments are running in different Colleges. Now Check the HSC Chemistry Assignment Solution 2021 for the 1st Week.
A total of ten chemistry assignments will be published for the 2021 HSC candidates. Chemistry is five for the first paper and chemistry five for the second paper. We know that the Chemistry 1st week, 2nd week, 3rd week, 4th week, 5th week, 6th week and 7th week assignments of HSC candidates have been published.
There are total 10 HSC 2021 Chemistry Assignment Answer has to be prepared. Science group students have to create 10 assignment solutions in 10 weeks. 5 of them have to be made for the Chemistry 1st paper. And 5 should be made for the Chemistry 2nd paper. The Directorate of Secondary and Higher Education will publish assignment questions for 1st paper and 2nd paper continuously.
Now posted by this web portal HSC 2021 Chemistry Answer 2021. We also found hsc science groups assignment has been assigned for another week. Also, the Bangladesh education ministry declared 2021 HSC Assignment will be held on only groups subject. For this 15th week will be taken the assignment, Among that Chemistry has 2nd, 4th, 6th, 7th, 9th ...
January 21, 2024. Acid-base reactions are a pivotal topic in HSC Chemistry, particularly in Module 6. This guide aims to provide students with essential insights and study tips to excel in this area, emphasising the critical aspects of the curriculum and practical applications. Let's dive into some key strategies you can utilise to master Acid ...
Find the Study Notes you need. Our extensive library of handy and helpful HSC Chemistry resources including past papers with worked solutions, study guides, study notes, essays written by students, assignments and many more, to help you prepare for the HSC. browse Study NOTES.
HSC Chemistry 1st & 3rd Week Assignment Answer 2021. For the Students of Higher secondary school (HSC) a new assignment has been published. Now, Intermediate students collect the assignment & submission to college.
Studying HSC Chemistry in 12 - New South Wales Higher School Certificate? On Studocu you will find 169 practice materials, 117 study notes, 36 assignments and much. ... Assignments. Date Rating. year. Ratings. Account for a variety of KSP Values for chloride compounds. 2 pages 2023/2024 100% (3) 2023/2024 100% (3)
There are total 10 HSC 2021 Chemistry Assignment Answer has to be prepared. Science group students have to create 10 assignment solutions in 10 weeks. 5 of them have to be made for the Chemistry 1st paper. And 5 should be made for the Chemistry 2nd paper. HSC Chemistry Assignment 2021 Solution has been Published for 1st, 3rd, 4th, 5th, 7th, 8th ...
HSC Assignment 2021 PDF 1st Week has been Published. Download Group based subject Assignment for HSC 1st week Assignment 2021 Answer of Science, Humanities and Business Studies Groups. ... There are total 10 HSC 2021 Chemistry Assignment Answer has to be prepared. Science group students have to create 10 assignment solutions in 10 weeks. 5 of ...
HSC Chemistry paper board Solution 2020 of class 12 with complete explanation. HSC Board Chemistry Question paper 2020 Click to refer. Important Questions for Chemistry Old Syllabus Repeater students 2020 Click to refer. Select and write the correct answer of the following : - [10M] Identify synthetic polymer amongst the following : Linen. Jute.
All the content you need to revise for HSC Year 12 Chemistry, delivered by an expert presenter from our April Lectures.Access the slides: https://atarnotes.m...
We have meticulously solved the MCQ questions for all boards offering the HSC Chemistry 2nd Paper exam, ensuring the utmost accuracy in our question solution. We have provided the HSC Chemistry 1st Paper MCQ Question Solution for 2023, ensuring high accuracy. However, we encourage you to cross-verify the answers with your textbooks for added ...
hsc chemistry assignment 2 - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or read online for free. Scribd is the world's largest social reading and publishing site.
The HSC Assignment 2022 Chemistry Subject has been assigned for the 6th, 9th, 12th, 14th, 16th, 20th, 23rd, 25th, 26th week. A total of 10 assignments have to be prepared for chemistry. Chemistry 1 Paper Assignment
In this video we are going to discuss HSC 2022 Assignment Chemistry Answer 12th Week. We discuss HSC 2022 Assignment Answer 12th Week. We hope you get help i...
HSC Chemistry Assignment Answer for 3rd week of HSC-2021 candidate . The department further says that the learning outcomes achieved through assignments or assignments will be assessed. Assignments have been prepared keeping in view the weekly student assessment as per the rearranged syllabus.
The soap ion must be dissolved in water to have a cleaning effect. As salts of weak acids, in low pH, soaps are converted into uncharged fatty acids. CH A 3 ( CH A 2) A 16 COONa ( aq) + HCl ( aq) CH A 3 ( CH A 2) A 16 COOH ( s) + NaCl ( aq) Soluble fatty acid ion + acid → Insoluble fatty acid molecule + ionic salt.
Find the HSC Resources you need. Our extensive library of handy and helpful HSC Chemistry resources including past papers with worked solutions, study guides, study notes, essays written by students, assignments and many more, to help you prepare for the HSC. browse hsc resources.
Chemistry Module 7 Notes. Basically a quick but good review over mod 7. There may be some typos in the document lol. Download this Notes document for HSC - Chemistry. Find free HSC resources like study notes, essays, past papers, assignment, case studies & ...
HSC Chemistry 7.0 is not only a process flowsheet simulation software program but it also contains 22 other useful calculation modules and 12 databases with an extensive number of thermochemical, heat transfer and mineralogical data on the same CD. HSC 7 has several new features, for example: 1. Flowsheet Module
HSC 2021 Chemistry Assignment Answer 7th week. Submitted by Result BD on 11 September, 2021 - 21:50. There are total 10 HSC 2021 Chemistry Assignment Answer has to be prepared. Science group students have to create 10 assignment solutions in 10 weeks. 5 of them have to be made for the Chemistry 1st paper. And 5 should be made for the Chemistry ...
HSC Chemistry Assignment is also scheduled for 9th and12th and more week 2022. So get HSC Chemistry Assignment questions and answers to keep reading below. HSC Chemistry Assignment Answer 2022 - 12th Week. HSC Chemistry assignments start in June and end in December 2022. All Education Board start all subject Assignment and will end on maybe ...