- Open access
- Published: 13 August 2021

Biological therapy for severe asthma
- Silvano Dragonieri ORCID: orcid.org/0000-0003-1563-6864 1 &
- Giovanna Elisiana Carpagnano 1
Asthma Research and Practice volume 7 , Article number: 12 ( 2021 ) Cite this article
12k Accesses
19 Citations
3 Altmetric
Metrics details
Around 5–10% of the total asthmatic population suffer from severe or uncontrolled asthma, which is associated with increased mortality and hospitalization, increased health care burden and worse quality of life. In the last few years, new drugs have been launched and several asthma phenotypes according to definite biomarkers have been identified. In particular, therapy with biologics has revolutionized the management and the treatment of severe asthma, showing high therapeutic efficacy associated with significant clinical benefits. To date, four types of biologics are licensed for severe asthma, i.e. omalizumab (anti-immunoglobulin E) antibody, mepolizumab and reslizumab (anti-interleukin [IL]-5antibody), benralizumab (anti-IL-5 receptor a antibody) and dupilumab (anti-IL-4 receptor alpha antibody). The aim of this article was to review the biologic therapies currently available for the treatment of severe asthma, in order to help physicians to choose the most suitable biologic agent for their asthmatic patients.
Since the beginning of this millennium, asthma assessment and management have been revolutionized. While some new therapeutic approaches have been suggested for mild asthmatics, the most relevant changes have occurred in severe asthma. Severe asthma accounts for the 5–10% of the global asthma population, with 3 to 5% being uncontrolled despite adherence to therapy and proper use of inhalers [ 1 ]. These subjects cannot achieve symptoms control despite maximal therapy with inhaled corticosteroids (ICS) and, quite often, maintenance oral corticosteroids (OCS) are necessary in an endeavor to avoid life-threatening exacerbations [ 2 ]. Although OCS courses remain essential for the management of acute exacerbations, their recurrent or continuous usage is associated with several complications, such as an increased risk of developing osteoporotic fractures and pneumonia [ 3 ]. Moreover, other conditions including cardiovascular and cerebrovascular events, renal dysfunction, diabetes mellitus type 2, humor alterations, obesity and sleep apneas are known to be associated with systemic corticosteroid exposure [ 3 ]. Additionally, many patients remain poorly controlled and show recurrent exacerbations despite a strict adherence to therapy [ 4 ].
The recent advances in our knowledge of the etiopathological mechanisms of different phenotypes and endotypes of severe asthma gave us very innovative therapies, such as biological drugs for severe asthma. These medications are mostly directed against molecules involved in the type 2 inflammatory pathway, thus modifying the natural course of the disease by reducing airways inflammation without the collateral damage associated with corticosteroids. Based on the above, the aim of this article was to review the biologic therapies currently available for the treatment of severe asthma, in order to help physicians to choose the most suitable biologic agent for their asthmatic patients.
Licensed medications for severe asthma
To date, there are five biologic molecules officially approved for use in selected severe asthmatic patients. The first of these is omalizumab, an anti-IgE monoclonal antibody acting through various mechanisms on allergic pathways (Table 1 ). Three more biologics for asthma, belonging to a different class, have been approved, i.e. mepolizumab, reslizumab and benralizumab. They all target the interleukin-5 (IL-5) pathway with the first two targeting the interleukin itself and the last one its receptor. Finally, dupilumab is a monoclonal antibody against the receptor of interleukin-4 (IL-4) which blocks the signaling pathways of IL-4 and IL-13.
BIOLOGICS TARGETING IgE
Omalizumab was the first targeted biologic therapy developed and licensed for severe asthma, being approved by the Food and Drugs Administration in 2003 [ 5 ]. It is a recombinant monoclonal Antibody which binds to IgE, thereby lowering blood IgE levels of up to 99% [ 6 ]. Moreover, It decreases expression of IgE receptor FCRI on inflammatory cells such as mast cells and basophils, thus helping to both mitigate the allergic response and strengthen the antiviral immune response, finally leading to prevent asthma exacerbations [ 7 ]. Omalizumab is approved in adults and children above 6 years old with IgE-driven moderate-to-severe persistent allergic asthma which remains uncontrolled despite GINA step 4/5 treatment, high levels of blood IgE, and documented sensitization to a perennial allergen [ 8 ]. Its dosage varies according to patient’s bodyweight and circulating IgE levels and it is administered subcutaneously every 14 or 28 days [ 9 ]. Although not necessary from a safety point of view, it is advisable to re-evaluate patients after the initial 16 weeks of treatment to assess the drug efficacy before continuing with omalizumab therapy [ 8 ].
The efficacy and safety of omalizumab are nowadays unquestionably recognized, with numerous studies demonstrating that this biological is generally well-tolerated, with no serious adverse effects reported [ 10 , 11 , 12 , 13 , 14 , 15 ]. Common side effects include injection site or diffuse rash, fever, nose bleeding, joint pain, gastro-intestinal disturbances, headache, dizziness and cold symptoms [ 10 , 11 , 12 , 13 , 14 , 15 ]. A Cochrane systematic review assessing 25 randomized controlled trials in patients with allergic asthma showed the efficacy of omalizumab in reducing asthma exacerbations, hospitalizations, and inhaled corticosteroid dosage [ 10 , 15 , 16 , 17 , 18 , 19 ].
During the last few years, a number of biomarkers for monitoring the efficacy of omalizumab therapy have been proposed, including total and antigen-specific IgE, blood eosinophil count and exhaled nitric oxide (FeNO) [ 20 , 21 ]. Surprisingly, total IgE did not appear to be a reliable predictor of response to omalizumab therapy, evidencing that our knowledge on this field is still limited [ 21 ]. Peripheral blood eosinophil count ≥300 cells/mL are linked to higher asthma severity and to a better response to omalizumab [ 22 , 23 ]. Furthermore, patients under omalizumab with higher blood eosinophil count have a higher chance to suffer from asthma exacerbations in case of omalizumab discontinuation [ 24 ]. Regarding FeNO, elevated values at baseline correlated with a better response to omalizumab with regard to exacerbations decrease [ 20 , 25 ]. Likewise, elevated levels of FeNO after suspension of long-term therapy with omalizumab may be a predictor of successive exacerbations [ 24 ].
Biologics targeting IL-5
IL-5 is a well-known regulator of the activation, differentiation, effector function, migration and survival and effector function of eosinophils [ 26 ]. Eosinophil levels associated with symptoms of asthma correlate with disease severity and increase the risk of asthma exacerbations, evidencing that this granulocyte type plays a key role in the pathophysiololgy of asthma [ 26 ]. Currently, licensed biologics against IL-5 pathways are mepolizumab, reslizumab, and benralizumab.
MEPOLIZUMAB
Mepolizumab is a monoclonal antibody directed against IL-5 which has been approved as an add-on treatment for patients ≥6 years old in Europe and for patients ≥12 years old in the USA. Mepolizumab was the first anti-IL-5 antibody approved for the treatment of severe asthma by the Food and Drugs Administration in 2015. Eligible subjects are those with severe eosinophilic asthma that remains uncontrolled despite GINA step 4/5 therapy, with blood eosinophil count of ≥150 cells/μl during the first administration or ≥ 300 cells/μl in the previous year and with at least 2 asthma exacerbations requiring systemic steroid course in the past year [ 27 , 28 ]. Mepolizumab is administered by a subcutaneous injection at a fixed dose of 100 mg every 28 days.
Several studies evaluating mepolizumab for uncontrolled eosinophilic asthma showed a markedly reduction with regard to number of exacerbations, systemic corticosteroid usage, emergency room accesses and hospital admissions, and a concurrent improvement of asthma controls and lung function parameters [ 29 , 30 , 31 , 32 , 33 ].
Furthermore, a number of studies revealed that mepolizumab has a positive long-term safety profile [ 34 , 35 , 36 ]. No reports of mepolizumab-associated anaphylaxis reactions were documented, as well as parasitic infections [ 34 , 35 , 36 ]. Common side effects include headache, injection site reaction, fatigue, flu symptoms, urinary tract infection, abdominal pain, itching, eczema, and muscle spasms [ 34 , 35 , 36 ].
Additionally, numerous investigations highlighted that the most important markers of response prediction to mepolizumab are the rate of previous exacerbation and baseline peripheral blood eosinophil count [ 29 , 32 , 37 , 38 , 39 ]. Indeed, a better clinical efficacy is directly proportional to a higher eosinophil count and to a higher rate of exacerbations [ 29 , 32 , 37 , 38 , 39 ]. Interestingly, mepolizumab effectiveness was not related to baseline IgE and to atopy [ 40 , 41 ] and earlier treatment with omalizumab is not a predictor for mepolizumab efficacy [ 42 , 43 , 44 ].
There is a lack of consensus about the duration of treatment before evaluating the effectiveness of mepolizumab. Actually, the GINA statement suggests that a 4-month trial may be adequate [ 8 ], whereas the NICE guidelines recommend that mepolizumab should not be discontinued before 12 months of therapy and that drug-responsiveness should be assessed every year [ 45 ].
Reslizumab is monoclonal antibody approved in 2016, which binds with high-affinity to IL-5 [ 46 ]. By an analogous mechanism of action to mepolizumab, reslizumab lowers circulating blood eosinophil levels [ 47 ]. It has been approved for patients ≥18 years old with severe eosinophilic asthma which remains uncontrolled despite therapy with high-doses of ICS plus another inhaler. Reslizumab is indicated in patients with ≥400 eosinophils/μl and history of asthma exacerbations in the previous 12 months [ 48 , 49 ]. Reslizumab is administered intravenously every 28 days at a weight-based dose of 3 mg/kg.
Similarly to mepolizumab, studies assessing reslizumab have shown a decreased number of asthma exacerbations and improved asthma control and lung function parameters in subjects with high blood eosinophil levels [ 47 , 50 ].
The safety profile of reslizumab has been evaluated for up to 24 months, revealing minor adverse effects without any reports of parasitic and opportunistic infections [ 51 ]. Most frequent side effects include cough, dizziness, itching, skin rash and fatigue [ 51 ].
However, despite its proven excellent clinical efficacy, intravenous formulation has a significant impact on the ease of administration compared to mepolizumab and/or benralizumab. Studies using reslizumab showed unsatisfactory results, without significant improvements in terms of acute exacerbations reduction or OCS lowering [ 52 ].
BENRALIZUMAB
Benralizumab is a monoclonal antibody approved in 2017 and directed against IL-5 receptor a (IL-5Ra) which induces eosinophil apoptosis via the antibody-dependent cell-mediated cytotoxicity (ADCC) involving natural killer cells, leading to peripheral blood eosinophil depletion [ 53 , 54 ]. Benralizumab acts like a competitive inhibitor to IL-5, binding with higher affinity to the a-subunit of IL-5Ra, which is expressed on mature (and precursors) eosinophils and basophils [ 55 ].
This biologic drug is licensed as an add-on treatment for uncontrolled severe eosinophilic asthma in patients ≥18 years with ≥300 blood eosinophils/μl [ 56 , 57 ]. A 30 mg dose of benralizumab is injected subcutaneously every 28 days for the first 3 administrations and afterwards every 56 days.
Large studies evaluating benralizumab in patients with moderate to severe asthma have shown a decrease in exacerbations number, improved lung function, and reduced use of OCS [ 53 , 54 , 58 ]. Combined analysis of these investigation have revealed that the best predictors of response to benralizumab are adult-onset asthma, more than 3 exacerbations in the previous year, nasal polyposis and pre-bronchodilator FVC < 65% of predicted [ 53 , 54 , 58 ].. The most common adverse effect were fever after the first injection, headache and pharyngitis [ 53 , 54 , 58 ].
Interestingly, based on its mechanism, benralizumab almost completely depletes blood eosinophils within 24 h of administration and a total depletion of airway eosinophils compared to that caused by mepolizumab [ 59 , 60 ]. Likewise, nasal eosinophils were totally suppressed after 6 months of therapy with benralizumab [ 61 ].
Recently, some concerns have been raised about the theoretical risks following an eosinophil depletion, especially with respect to host defense. However, these warnings were not confirmed, since it appears that there is adequate redundancy within human immune apparatus, which is not impaired by eosinophils depletion [ 62 ].
Biologics targeting IL-4 and IL-13
IL-4 and IL-13 are two interleukins which regulate and drive Type-2 inflammation. IL-4 increases the Th-2 cell population and B-cell isotype rearrangement of IgE as well as promoting eosinophilic transmigration through endothelium, whereas IL-13 plays an important role in asthma by promoting airway hyperresponsiveness, mucus secretion and airway remodeling [ 63 , 64 ]. Thus far, the only licensed drug acting on the two aforementioned ILs is dupilumab.
Dupilumab is a monoclonal antibody approved in 2018 which binds to the IL-4 receptor alpha-subunit, mutual to IL-4 and IL-13 receptors and inhibits both IL-4 and IL-13 pathways. Dupilumab is licensed as an add-on maintenance therapy in asthmatic patients GINA step 4/5 ≥ 12 years with type 2 inflammation characterized by increased blood eosinophils and/or raised FeNO. Dupilumab is administered subcutaneously at a starting dose of two injections of 200 mg each (total 400 mg), followed by one injection of 200 mg every 14 days, or at a starting dose of 600 mg (two injections of 300 mg each) followed by 300 mg every 14 days. The latter regimen is recommended for asthmatic subjects strictly dependent from OCS or with atopic dermatitis [ 65 ]. Dupilumab is also indicated for moderate to severe atopic dermatitis and for nasal polyposis.
A number of studies have demonstrated that therapy with dupilumab in severe asthmatics lowers the number of asthma exacerbations, improves lung function parameters and asthma control test scores, and lowers the use of OCS, irrespective of peripheral blood eosinophil count [ 66 , 67 , 68 , 69 ]. Indeed, a transitory increase of blood eosinophilia at the beginning of treatment with dupilumab has been observed although it may be due to blocked migration into tissues rather than hyperproduction [ 69 ]. Furthermore, reduced levels of T2 inflammation markers, including FeNO, serum levels of eotaxin-3, periostin and thymus and activation regulated chemokine (TARC) and total IgE, may serve as parameters for monitoring the efficacy of therapy with dupilumab [ 66 , 67 , 68 , 69 ]. The most common adverse reactions were injection site reactions, various types of infections, conjunctivitis and related conditions [ 66 , 67 , 68 , 69 ].
Biologics under development
Research for next-generation biologics is ongoing. Currently, other effector molecules are under the spotlight as new targets for perspective biological therapies, particularly the so-called alarmins [ 70 ]. These molecules are released by the airway epithelium against the harmful actions of germs, pollutants, allergens and cigarette smoke.
Tezepelumab is a human monoclonal antibody which binds to thymic stromal lymphopoietin (TSLP), an epithelium-derived alarmin that plays a relevant role in the pathogenesis of asthma, being an upstream effector T2-high pathobiologic pathways [ 71 , 72 , 73 ]. With the presence of tezepelumab, TLSP cannot bind to its receptor [ 74 ] hence inhibiting downstream signaling. A number of phase 2 and 3 trials have clearly shown that patients with severe uncontrolled asthma who received tezepelumab had fewer exacerbations and better lung function, asthma control, and health-related quality of life than those who received placebo [ 75 , 76 ]. Concerning its safety profile, neither investigational tezepelumab-related anaphylactic reactions nor the detection of neutralizing antibodies were reported [ 75 , 76 ]. To date, license application for tezepelumab has been accepted and granted Priority Review for the treatment of asthma from the US Food and Drug Administration, whose regulatory decision is expected during the first quarter of 2022.
Ipetekimab is a monoclonal antibody targeting IL-33, another alarmin which associates with TSLP leading to an activation of T2-high inflammatory pathway in asthma [ 77 ]. Phase 2 studies with this biologic are ongoing, however preliminary results did not show adequate efficacy in severe asthmatics when associated with dupilumab or vs dupilumab alone [ 70 ].
Moreover, Tralokinumab and lebrokizumab are monoclonal antibodies both targeting IL-13 alone with disappointing results of phase 3 studies in terms of exacerbations reduction and OCS sparing in severe asthmatics [ 78 ].
Finally, regarding Th2-low asthma, mainly characterized by a neutrophilic airways inflammation, efforts are focusing on its pathogenic cascade involving cytokines such as IL-1beta, IL-17 and IL-23. Several monoclonal antibodies against the aforementioned interleukins such as canakinumab (anti IL-1beta), brodalumab (anti IL-17 receptor) and risankizumab (anti IL-23) are under evaluation with phase 1–2 trials showing controversial results [ 79 , 80 , 81 ].
Which biologic should I choose for my asthmatic patient?
When choosing a biologic medication for their patients with severe uncontrolled asthma, clinicians should always take into account the asthma endotype, clinical biomarkers, and patient-focused aspects (Fig 1 ).
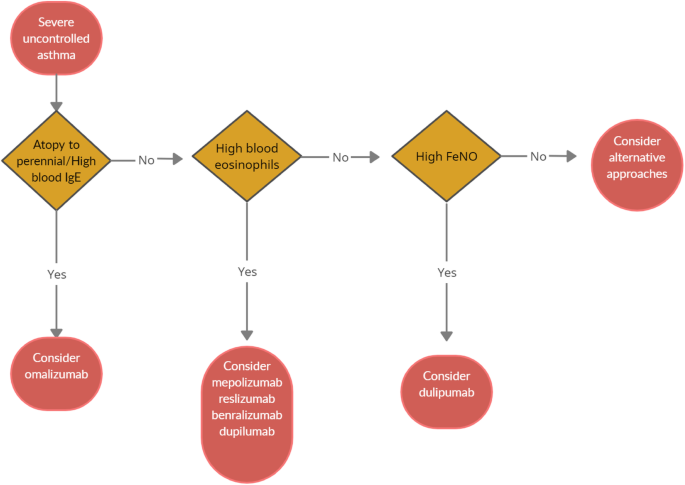
Algorithm for Selecting Ideal Biologic Treatment for severe uncontrolled asthma
Omalizumab should always be the first biological option in allergic non-eosinophilic severe asthmatics, with high levels of blood IgE, and with at least a documented positivity to a perennial aeroallergen. Contrariwise, patients with a non-allergic eosinophilic phenotype should be treated with an anti-IL-5 biological drug. Finally, anti- IL-4/IL-13 should be reserved to patients with severe eosinophilic type 2 asthma OCS dependent [ 8 ].
Given to the a lack of comparison studies, to date there are no recommendations about the selection of appropriate anti IL-5 biologic drug among those available. Hence, the choice is empirical and possibly shared between physician and patient.
According to GINA guidelines, a (at least) 4-month trial should be carried to evaluate asthma control. In the event of poor asthma control, a switch to a different biological treatment can be attempted if the patient meets the eligibility criteria.
Nevertheless, the right time and the right modality of switching from one biologic to another and the treatment time are still unknown. Large studies focused on biological drug switch in patients with severe asthma are ongoing and will help physicians to ease therapeutic strategies.
Conclusions
Severe asthma accounts for a small proportion of total asthma cases, but impose a heavy burden on health care system. Recent revelations of the T2 inflammatory pathways and the development of monoclonal antibodies acting on the T2 cascade has completely revolutionized the management of severe asthma, by introducing new, life-improving treatment options for this class of patients. This paves the way for a biomarker-driven personalized medicine. Strictly following GINA recommendations, the categorization of T2 molecular targets has allowed the identification of patients with severe asthma who would likely respond to specific biological molecules. However, the most suitable biological option for severe asthmatics with overlapping phenotypes is still unclear, thus requiring further discriminatory and predicting biomarkers which may allow a better patient selection.
Availability of data and materials
Not applicable.
Abbreviations
interleukin
inhaled corticosteroids
oral corticosteroids
immunoglobulin E
fractional exhaled nitric oxide
forced vital capacity
Hekking PPW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. https://doi.org/10.1016/j.jaci.2014.08.042 .
Article PubMed Google Scholar
Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–73. https://doi.org/10.1183/09031936.00202013 .
Article CAS PubMed Google Scholar
Price DB, Trudo F, Voorham J, Xu X, Kerkhof M, Ling Zhi Jie J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;11:193–204. https://doi.org/10.2147/JAA.S176026 .
Article CAS PubMed PubMed Central Google Scholar
Sulaiman I, Greene G, MacHale E, Seheult J, Mokoka M, D’Arcy S, et al. A randomised clinical trial of feedback on inhaler adherence and technique in patients with severe uncontrolled asthma. Eur Respir J. 2018;51(1):1701126. https://doi.org/10.1183/13993003.01126-2017 .
Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113(1):101–8. https://doi.org/10.1016/j.jaci.2003.10.041 .
Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev 2014 https://doi.org/10.1002/14651858 . CD003559.pub4.
Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ, Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136(6):1476–85. https://doi.org/10.1016/j.jaci.2015.09.008 .
Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2021. https://ginasthma.org/ .
European Medicines Agency. EMEA/H/C/000606. 2014. www.ema.europa.eu/en/documents/overview/xolair-epar-summary-public_en.pdf . Accessed 30 May 2021.
Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2):184–90. https://doi.org/10.1067/mai.2001.117880 .
Rodrigo GJ, Neffen H, Castro-Rodriguez JA. Efficacy and safety of subcutaneous omalizumab vs placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest. 2011;139:28e35.
Article Google Scholar
Alhossan A, Lee CS, MacDonald K, Abraham I. “Real-life” effectiveness studies of omalizumab in adult patients with severe allergic asthma: meta-analysis. J Allergy Clin Immunol Pract. 2017;5(5):1362–70. https://doi.org/10.1016/j.jaip.2017.02.002 .
Ohta K, Miyamoto T, Amagasaki T, Yamamoto M, Study G. Efficacy and safety of omalizumab in an Asian population with moderate-to-severe persistent asthma. Respirology. 2009;14(8):1156–65. https://doi.org/10.1111/j.1440-1843.2009.01633.x .
Adachi M, Kozawa M, Yoshisue H, Lee Milligan K, Nagasaki M, Sasajima T, et al. Real-world safety and efficacy of omalizumab in patients with severe allergic asthma: a long-term post-marketing study in Japan. Respir Med. 2018;141:56–63. https://doi.org/10.1016/j.rmed.2018.06.021 .
Ledford D, Busse W, Trzaskoma B, Omachi TA, Rosen K, Chipps BE, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140(1):162–9. https://doi.org/10.1016/j.jaci.2016.08.054 .
Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev 2014:CD003559.
[Holgate ST, Chuchalin AG, Hebert J, Lotvall J, Persson GB, Chung KF, et al. Efficacy and safety of a recombinant antiimmunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy 2004;34:632–638.
Soler M, Matz J, Townley R, Buhl R, O’Brien J, Fox H, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18(2):254–61. https://doi.org/10.1183/09031936.01.00092101 .
Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364(11):1005–15. https://doi.org/10.1056/NEJMoa1009705 .
Hanania NA, Wenzel S, Rosen K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187(8):804–11. https://doi.org/10.1164/rccm.201208-1414OC .
Tabatabaian F, Ledford DK. Omalizumab for severe asthma: toward personalized treatment based on biomarker profile and clinical history. J Asthma Allergy. 2018;11:53–61. https://doi.org/10.2147/JAA.S107982 .
Casale TB, Chipps BE, Rosen K, Trzaskoma B, Haselkorn T, Omachi TA, et al. Response to omalizumab using patient enrichment criteria from trials of novel biologics in asthma. Allergy. 2018;73(2):490–7. https://doi.org/10.1111/all.13302 .
Busse W, Spector S, Rosen K, Wang Y, Alpan O. High eosinophil count: a potential biomarker for assessing successful omalizumab treatment effects. J Allergy Clin Immunol. 2013;132(2):485–6. https://doi.org/10.1016/j.jaci.2013.02.032 .
Ledford D, Busse W, Trzaskoma B, Omachi TA, Rosen K, Chipps BE, et al. A randomized multicenter study evaluating Xolair persistence of response after longterm therapy. J Allergy Clin Immunol. 2017;140(1):162–9. https://doi.org/10.1016/j.jaci.2016.08.054 .
Mansur AH, Srivastava S, Mitchell V, Sullivan J, Kasujee I. Longterm clinical outcomes of omalizumab therapy in severe allergic asthma: study of efficacy and safety. Respir Med. 2017;124:36–43. https://doi.org/10.1016/j.rmed.2017.01.008 .
Akdis CA, Arkwright PD, Bruggen MC, Busse W, Gadina M, Guttman-Yassky E, et al. Type 2 immunity in the skin and lungs. Allergy. 2020;75(7):1582–605. https://doi.org/10.1111/all.14318 .
US Food and Drug Administration. NUCALA (mepolizumab) for injection, for subcutaneoususe.2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125526s004lbl.pdf . .
European Medicines Agency. Nucala. EMEA/H/C/003860-N/0027. 2015. https://www.ema.europa.eu/en/documents/product-information/nucala-eparproduct-information_en.pdf . Accessed 1 Jun 2021.
Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–207. https://doi.org/10.1056/NEJMoa1403290 .
Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973–84. https://doi.org/10.1056/NEJMoa0808991 .
Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360(10):985–93. https://doi.org/10.1056/NEJMoa0805435 .
Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–9. https://doi.org/10.1016/S0140-6736(12)60988-X .
Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–97. https://doi.org/10.1056/NEJMoa1403291 .
Lugogo N, Domingo C, Chanez P, Leigh R, Gilson MJ, Price RG, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38(9):2058–70. https://doi.org/10.1016/j.clinthera.2016.07.010 .
Khatri S, Moore W, Gibson PG, Leigh R, Bourdin A, Maspero J, et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2018;143:1742–51.
Khurana S, Brusselle GG, Bel EH, FitzGerald JM, Masoli M, Korn S, et al. Long-term safety and clinical benefit of mepolizumab in patients with the most severe eosinophilic asthma: the COSMEX study. Clin Ther. 2019;41(10):2041–56. https://doi.org/10.1016/j.clinthera.2019.07.007 .
Ortega HG, Yancey SW, Mayer B, Gunsoy NB, Keene ON, Bleecker ER, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4(7):549–56. https://doi.org/10.1016/S2213-2600(16)30031-5 .
Ortega H, Li H, Suruki R, Albers F, Gordon D, Yancey S: Cluster analysis and characterization of response to mepolizumab. A step closer to personalized medicine for patients with severe asthma. Ann Am Thorac Soc 2014;11:1011–1017, 7, DOI: https://doi.org/10.1513/AnnalsATS.201312-454OC .
Katz LE, Gleich GJ, Hartley BF, Yancey SW, Ortega HG. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc. 2014;11(4):531–6. https://doi.org/10.1513/AnnalsATS.201310-354OC .
Ortega H, Chupp G, Bardin P, Bourdin A, Garcia G, Hartley B, et al. The role of mepolizumab in atopic and nonatopic severe asthma with persistent eosinophilia. Eur Respir J. 2014;44(1):239–41. https://doi.org/10.1183/09031936.00220413 .
Prazma CM, Wenzel S, Barnes N, Douglass JA, Hartley BF, Ortega H. Characterisation of an OCS-dependent severe asthma population treated with mepolizumab. Thorax. 2014;69(12):1141–2. https://doi.org/10.1136/thoraxjnl-2014-205581 .
Magnan A, Bourdin A, Prazma CM, Albers FC, Price RG, Yancey SW, et al. Treatment response with mepolizumab in severe eosinophilic asthma patients with previous omalizumab treatment. Allergy. 2016;71(9):1335–44. https://doi.org/10.1111/all.12914 .
Galkin D, Liu MC, Chipps BE, Chapman KR, Munoz X, Angel Bergna M, et al. Efficacy and safety of mepolizumab in uncontrolled patients with severe eosinophilic asthma following a switch from omalizumab (OSMO Study): exacerbation and safety outcomes. J Allergy Clin Immunol. 2018;141(2):AB409. https://doi.org/10.1016/j.jaci.2017.12.965 .
Chapman KR, Albers FC, Chipps B, Munoz X, Devouassoux G, Bergna M, et al. The clinical benefit of mepolizumab replacing omalizumab in uncontrolled severe eosinophilic asthma. Allergy Eur J Allergy Clin Immunol. 2019;74(9):1716–26. https://doi.org/10.1111/all.13850 .
Article CAS Google Scholar
National Institute for Health and Care Excellence (NICE). Mepolizumab for treating severe refractory eosinophilic asthma. 2017. http://www.nice.org.uk/guidance/ta431 . Accessed 1 Jun 2021.
Egan R, Athwal D, Bodmer M, Carter J, Chapman R, Choua CC, et al. Effect of Sch 55700, a humanized monoclonal antibody to human interleukin-5, on eosinophilic responses and bronchial hyperreactivity. Arzneimittelforschung. 2011;49:779–90.
Corren J, Weinstein S, Janka L, Zangrilli J, Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma. Chest. 2016;150(4):799–810. https://doi.org/10.1016/j.chest.2016.03.018 .
US Food and Drug Administration. CINQAIR (reslizumab) injection, for intravenous use. ReferenceID:3906489.2016. www.accessdata.fda.gov/drugsatfda_docs/label/2016/761033lbl.pdf . .
European Medicines Agency. EMEA/H/C/003912.2016. www.ema.europa.eu/en/documents/overview/cinqaero-epar-summarypublic_en.pdf . Accessed 3 Jun 2021.
Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355–66. https://doi.org/10.1016/S2213-2600(15)00042-9 .
Murphy K, Jacobs J, Bjermer L, Fahrenholz JM, Shalit Y, Garin M, et al. Long-term safety and efficacy of reslizumab in patients with eosinophilic asthma. J Allergy Clin Immunol. 2017;5:1572–81.
Bernstein JA, Virchow JC, Murphy K, Maspero JF, Jacobs J, Adir Y, et al. Effect of fixed-dose subcutaneous reslizumab on asthma exacerbations in patients with severe uncontrolled asthma and corticosteroid sparing in patients with oral corticosteroid- dependent asthma: results from two phase 3, randomised, double-blind, placebo. Lancet Respir Med. 2020;8(5):461–74. https://doi.org/10.1016/S2213-2600(19)30372-8 .
FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–41. https://doi.org/10.1016/S0140-6736(16)31322-8 .
Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–58. https://doi.org/10.1056/NEJMoa1703501 .
Ghazi A, Trikha A, Calhoun WJ. Benralizumab – a humanized mAb to IL-5Ra with enhanced antibody-dependent cell-mediated cytotoxicity – a novel approach for the treatment of asthma. Expert Opin Biol Ther. 2012;12(1):113–8. https://doi.org/10.1517/14712598.2012.642359 .
US Food and Drug Administration. FASENRA (benralizumab) injection, for subcutaneous use. ReferenceID:4181236.2019. www.accessdata.fda.gov/drugsatfda_docs/label/2017/761070s000lbl.pdf . .
European Medicines Agency. EMEA/H/C/4433. 2019. www.ema.europa.eu/en/documents/overview/fasenra-epar-medicineoverview_en.pdf . Accessed 3 Jun 2021.
Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–27. https://doi.org/10.1016/S0140-6736(16)31324-1 .
Laviolette M, Gossage DL, Gauvreau G, Leigh R, Olivenstein R, Katial R, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. 2013;132(5):1086–96. https://doi.org/10.1016/j.jaci.2013.05.020 .
Roxas C, Fernandes M, Green L, D’Ancona G, Kavanagh J, Kent B, et al. A comparison of the clinical response to mepolizumab and benralizumab at 4 weeks. Thorax. 2018;73:A50.
Google Scholar
Buonamico E, Dragonieri S, Sciancalepore PI, Carratù P, Carpagnano GE, Resta O, et al. Assessment of eosinophilic nasal inflammation in patients with severe asthma and nasal polyposis before and after six months of therapy with Benralizumab. J Biol Regul Homeost Agents. 2020;34(6):2353–7. https://doi.org/10.23812/20-323-L .
Jackson DJ, Korn S, Mathur SK, Barker P, Meka VG, Martin UJ, et al. Safety of eosinophil-depleting therapy for severe, eosinophilic asthma: focus on benralizumab. Drug Saf. 2020;43(5):409–25. https://doi.org/10.1007/s40264-020-00926-3 .
Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity. 2019;50(4):975–91. https://doi.org/10.1016/j.immuni.2019.03.018 .
Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol. 2015;15(5):271–82. https://doi.org/10.1038/nri3831 .
European Medicines Agency. Dupinex: EMEA/H/C/004390. 2018. https://www.ema.europa.eu/en/documents/product-information/dupixent-epar-productinformation_en.pdf . Accessed 4 Jun 2021.
Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-tohigh-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388(10039):31–44. https://doi.org/10.1016/S0140-6736(16)30307-5 .
Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–96. https://doi.org/10.1056/NEJMoa1804092 .
Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and safety of dupilumab in glucocorticoid dependent severe asthma. N Engl J Med. 2018;378(26):2475–85. https://doi.org/10.1056/NEJMoa1804093 .
Huang J, Pansare M. New treatments for asthma. Pediatr Clin. 2019;66(5):925–39. https://doi.org/10.1016/j.pcl.2019.06.001 .
Porsbjerg CM, Sverrild A, Lloyd CM, Menzies-Gow AN, Bel EH. Anti-alarmins in asthma: targeting the airway epithelium with next-generation biologics. Eur Respir J. 2020;56(5):2000260. https://doi.org/10.1183/13993003.00260-2020 .
Harada M, Hirota T, Jodo AI, Hitomi Y, Sakashita M, Tsunoda T, et al. Thymic stromal lymphopoietin gene promoter polymorphisms are associated with susceptibility to bronchial asthma. Am J Respir Cell Mol Biol. 2011;44(6):787–93. https://doi.org/10.1165/rcmb.2009-0418OC .
Li Y, Wang W, Lv Z, Li Y, Chen Y, Huang K, et al. Elevated expression of IL-33 and TSLP in the airways of human asthmatics in vivo: a potential biomarker of severe refractory disease. J Immunol. 2018;200(7):2253–62. https://doi.org/10.4049/jimmunol.1701455 .
He JQ, Hallstrand TS, Knight D, Chan-Yeung M, Sandford A, Tripp B, et al. A thymic stromal lymphopoietin gene variant is associated with asthma and airway hyperresponsiveness. J Allergy Clin Immunol. 2009;124(2):222–9. https://doi.org/10.1016/j.jaci.2009.04.018 .
Verstraete K, Peelman F, Braun H, Lopez J, Van Rompaey D, Dansercoer A, et al. Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. NatCommun. 2017;8:14937.
CAS Google Scholar
Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377(10):936–46. https://doi.org/10.1056/NEJMoa1704064 .
Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384(19):1800–9. https://doi.org/10.1056/NEJMoa2034975 .
Murakami-Satsutani N, Ito T, Nakanishi T, Inagaki N, Tanaka A, Vien PTX, et al. IL-33 promotes the induction and maintenance of Th2 immune responses by enhancing the function of OX40 ligand. Allergol Int. 2014;63(3):443–55. https://doi.org/10.2332/allergolint.13-OA-0672 .
Busse WW, Brusselle GG, Korn S, Kuna P, Magnan A, Cohen D, et al. Tralokinumab did not demonstrate oral corticosteroid-sparing effects in severe asthma. Eur Respir J. 2019;53(2):1800948. https://doi.org/10.1183/13993003.00948-2018 .
Nair P, Prabhavalkar KS. Neutrophilic asthma and potentially related target therapies. Curr Drug Targets. 2020;21(4):374–88. https://doi.org/10.2174/1389450120666191011162526 .
Kalchiem-Dekel O, Yao X, Levine SJ. Meeting the Challenge of Identifying New Treatments for Type 2-Low Neutrophilic Asthma. Chest;15:26–33.
Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188(11):1294–302. https://doi.org/10.1164/rccm.201212-2318OC .
Download references
Acknowledgements
Authors did not receive any funding for the current review.
Author information
Authors and affiliations.
Department of Respiratory Diseases, University of Bari “Aldo Moro”, Piazza Giulio Cesare 11, 70124, Bari, Italy
Silvano Dragonieri & Giovanna Elisiana Carpagnano
You can also search for this author in PubMed Google Scholar
Contributions
SD and GEC equally contributed in writing the current review. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Silvano Dragonieri .
Ethics declarations
Ethics approval and consent to participate.
Not required.
Consent for publication
Obtained from all authors.
Competing interests
None of the authors have conflicts to disclose.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Dragonieri, S., Carpagnano, G.E. Biological therapy for severe asthma. asthma res and pract 7 , 12 (2021). https://doi.org/10.1186/s40733-021-00078-w
Download citation
Received : 29 June 2021
Accepted : 02 August 2021
Published : 13 August 2021
DOI : https://doi.org/10.1186/s40733-021-00078-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Severe asthma
- Biological therapy
Asthma Research and Practice
ISSN: 2054-7064
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Open access
- Published: 28 July 2023
Machine learning for prediction of asthma exacerbations among asthmatic patients: a systematic review and meta-analysis
- Shiqiu Xiong 1 , 2 ,
- Wei Chen 1 ,
- Xinyu Jia 1 ,
- Yang Jia 3 &
- Chuanhe Liu 1 , 2
BMC Pulmonary Medicine volume 23 , Article number: 278 ( 2023 ) Cite this article
2775 Accesses
1 Citations
1 Altmetric
Metrics details
Asthma exacerbations reduce the patient’s quality of life and are also responsible for significant disease burdens and economic costs. Machine learning (ML)-based prediction models have been increasingly developed to predict asthma exacerbations in recent years. This systematic review and meta-analysis aimed to identify the prediction performance of ML-based prediction models for asthma exacerbations and address the uncertainty of whether modern ML methods could become an alternative option to predict asthma exacerbations.
PubMed, Cochrane Library, EMBASE, and Web of Science were searched for studies published up to December 15, 2022. Studies that applied ML methods to develop prediction models for asthma exacerbations among asthmatic patients older than five years and were published in English were eligible. The prediction model risk of bias assessment tool (PROBAST) was utilized to estimate the risk of bias and the applicability of included studies. Stata software (version 15.0) was used for the random effects meta-analysis of performance measures. Subgroup analyses stratified by ML methods, sample size, age groups, and outcome definitions were conducted.
Eleven studies, including 23 prediction models, were identified. Most of the studies were published in recent three years. Logistic regression, boosting, and random forest were the most used ML methods. The most common important predictors were systemic steroid use, short-acting beta2-agonists, emergency department visit, age, and exacerbation history. The overall pooled area under the curve of the receiver operating characteristics (AUROC) of 11 studies (23 prediction models) was 0.80 (95% CI 0.77–0.83). Subgroup analysis based on different ML models showed that boosting method achieved the best performance, with an overall pooled AUROC of 0.84 (95% CI 0.81–0.87).
This study identified that ML was the potential tool to achieve great performance in predicting asthma exacerbations. However, the methodology within these models was heterogeneous. Future studies should focus on improving the generalization ability and practicability, thus driving the application of these models in clinical practice.
Peer Review reports
Asthma is a chronic heterogeneous disease affecting approximately 241 million people worldwide [ 1 ]. Despite many effective medicines available, a proportion of asthmatic patients have uncontrolled asthma and asthma exacerbations [ 2 , 3 ]. Asthma exacerbations are characterized by progressive deterioration of asthma-related symptoms and lung function, resulting in a poor quality of life [ 4 , 5 ]. Severe asthma exacerbations are also responsible for decreased lung function, hospitalization, and even death, thus leading to disease and economic burdens [ 6 , 7 ]. Early recognition and timely intervention are the best strategies to prevent severe asthma exacerbations. Therefore, identifying patients at high risk of asthma exacerbations is crucial.
According to a systematic review including ten prediction models for asthma exacerbations, the best prediction performance was achieved by logistic regression (LR) with a c-statistic of 0.80 [ 8 ]. However, this systematic review did not include models based on modern machine learning (ML) algorithms, such as random forest (RF), neural network (NN), boosting algorithms, and support vector machine (SVM). ML has become a popular method for developing prediction models in the medical field due to its ability to process complex, massive health data [ 9 ]. Many studies developing prediction models for asthma exacerbations based on ML methods have been published, especially in recent years [ 10 , 11 ]. However, few systematic reviews were conducted to evaluate these existing ML models. Therefore, we perform a systematic review and meta-analysis to estimate the prediction performance of ML-based prediction models for asthma exacerbations and identify whether modern ML methods could become an alternative option to prediction.
Search protocol
We conducted this systematic review in accordance with Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies (The PRISMA-DTA Statement). The protocol of this systematic review was registered and published on PROSPERO (reference number CRD42022380059).
Search strategy
PubMed, Cochrane Library, EMBASE, and Web of Science were searched for relevant literature published from the earliest available online date up to December 15, 2022. Our search strategies used controlled terms and free-text terms to search for studies of the ML approach and asthma exacerbations. Details of the search strategy are given in Additional file 1 . We also checked reference lists of previous systematic reviews for potentially relevant papers.
Eligibility criteria and study selection
All search records were exported from the four databases and imported to EndNote 20 (Clarivate), a reference management tool, for compiling and duplication checking. After removing the duplications, two reviewers (SQ, X and XY, J) independently screened the titles and abstracts to select the studies based on inclusion criteria. Subsequently, they screened the full texts to select eligible studies. Any discrepancies were resolved by a third reviewer (W, C).
All studies should fulfill the criteria as follows:
Studies must be published in English.
Focused on participants aged five years and older with pre-existing asthma diagnoses.
Utilized machine learning algorithms to generate prediction models.
Aimed to predict patients who would suffer asthma exacerbations in the future.
Evaluated the prediction performance of models on a validation dataset.
Provided a clear description of ML methods and input features (predictors).
Provided the performance metrics regarding sensitivity and specificity.
We did not limit the type of publication and study designs.
Data extraction
Two reviewers (SQ, X and Y, J) independently read the full texts of eligible studies and extracted data elements, including (1) the paper source, (2) study information, (3) prediction models, (4) performance measures, (5) population characteristics, and (6) outcomes. Full details of data extraction are provided in Additional file 2 . We defined asthma exacerbations in accordance with an Official American Thoracic Society/European Respiratory Society (ATS/ERS) Statement [ 12 ]. Briefly, severe asthma exacerbation should include (1) at least three days of systemic corticosteroid treatment or (2) a hospitalization/emergency department visit for asthma requiring systemic corticosteroids. Moderate asthma exacerbation should include (1) at least two days of symptoms and lung function deterioration, requiring increasing bronchodilator use, or (2) visits for asthma not requiring systemic corticosteroids intervention. Using available statistics in the manuscripts, we manually calculated parameters not reported (e.g., the number of positive cases). We also emailed the corresponding author(s) for missing data.
Quality and bias assessment
There are no widely accepted tools for assessing the quality of machine learning-based research in medical fields. In 2019, Wolff et al. [ 13 ] developed the prediction model risk of bias assessment tool (PROBAST), which could assess the risk of bias (ROB) and the applicability of prediction model studies. For ROB assessment, PROBAST includes four domains: participants, predictors, outcomes, and analysis. Each domain contains 2 to 9 signaling questions that facilitate this domain’s ROB assessment (low, high, or unclear). The overall ROB assessment for a study is “low,” “high,” or “unclear,” based on each domain’s ROB classification. The first three domains with review questions are also used for applicability judgment (low, high, or unclear concern). This paper used the PROBAST to assess the ROB and applicability of included studies. Two authors (SQ, X and CH, L) independently assessed eligible studies, and any disagreements were resolved by discussion.
Data analysis
We narratively described these included studies, such as distribution of publication year, population characteristics, popular machine learning methods, validation methods, and important features. For studies that were able to calculate the number of true positive cases, true negative cases, false positive cases, and false negative cases on the validation dataset, the overall pooled area under the curve of the receiver operating characteristics (AUROC), sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) were estimated using random effects meta-analysis. I 2 was used to describe the percentage of the variability in effect estimates due to heterogeneity.
A small sample size causes the risk of overfitting, which may lead to poor generalization of prediction models. Subgroup analysis was stratified by sample size (< 10000 participants/ > 10000 participants). In addition, we performed a subgroup analysis of ML methods (LR, boosting, and RF), age groups (children only, children and adults, and adults only), and different outcome definitions. Univariate and multivariate random-effects meta-regression for sample size, ML methods, age groups, outcome definitions, and publication year was performed to explore the source of heterogeneity. For clarity, we referred to factors used for model development as “predictors” and the factors used for meta-regression at study level as “variables”. Sensitivity analyses were conducted to examine the robustness of the result by excluding each study. Deeks’ funnel plot was applied to test publication bias. We conducted all our analyses using Stata software (version 15.0). We used the MADIS module for pooling performance measures and the “metareg” macro for conducting the meta-regression analysis. The commands used in the analysis are provided in Additional file 2 .
Study selection
A total of 10434 papers were identified from four databases (PubMed (2013), Cochrane library (193), Web of Science (4085), Embase (4083)) (see Additional file 1 ). After excluding 4210 duplicates, we browsed titles and abstracts of the remaining 6224 papers resulting in 109 papers that might be eligible based on the pre-defined selection criteria. Then, we screened these papers’ full texts and supplementary materials and included 11 papers for synthesis (Fig. 1 ). Two studies included participants without age limitation, but only a tiny proportion of participants in these two studies were aged younger than five years old [ 14 , 15 ].
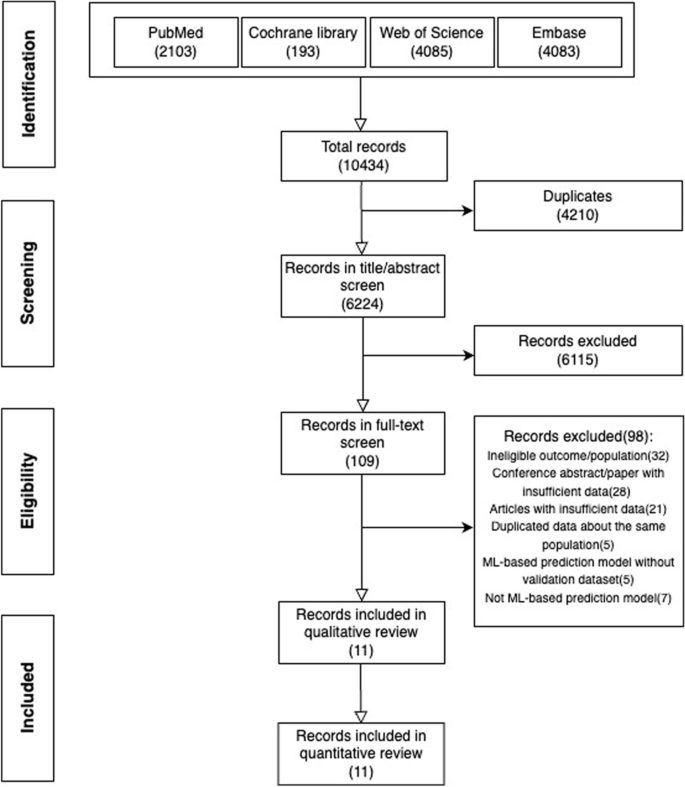
PRISMA flow diagram describing the selection process of articles
Study characteristics
The publication year of these papers ranged from 1999 to 2022, and more than half of them (6/11) were published in recent three years. Ten studies were retrospective, and the remaining one was prospective. The minimum and maximum number of included participants for prediction model development were 94 and 782762, respectively. The proportion of outcome events ranged from 0.2% to 32.8% (Table 1 ).
Most studies (9/11) included asthmatic participants regardless of asthma severity, control levels, or treatment. Only two studies mentioned additional criteria, such as participants with mild-moderate asthma [ 19 , 24 ] and stable asthma [ 24 ] (see Additional file 3 ). Prediction windows also varied from several days to 4 years, with seven studies setting the prediction window within one year (Table 1 ). For outcome events (see Additional file 3 ), nine studies defined asthma exacerbations as asthma-related hospitalization or emergency department visit according to the asthma-related diagnosis code [ 14 , 15 , 16 , 17 , 18 , 21 ], medical records [ 22 , 23 ], or questionnaires [ 19 ]. Two studies used the definitions in accordance with the ATS/ERS recommendation [ 20 , 24 ].
ML algorithms and validation methods
Eleven studies developed a total of 23 ML-based prediction models. The most popular ML algorithm was LR, followed by RF, XGBoost, and LGBoost (Fig. 2 a). Validation methods were used in 6 studies, such as cross-validation [ 15 , 19 , 24 ], bagging [ 20 ], and split-sample validation [ 16 ] (Table 1 ). For the generalization test, ten studies used external validation. One study split a single dataset into a training dataset and a test dataset and used the latter to assess the generalization ability of prediction models. We also included more detailed descriptions of the dataset and the validation method in Additional file 4 for better clarity.

a Distribution of machine learning algorithms. b Important features among included studies
Predictors in ML models
A wide range of predictors was used in these studies, such as demographic factors, clinical-related factors, and socioeconomic factors. Clinical-related factors ( n = 11) and demographic factors ( n = 7) were used most in the final models, followed by social-economic factors ( n = 3) (see Additional file 5 ). The number of predictors in best prediction models ranged from 1 to 221. Most studies that applied LR and classification and regression trees (CART) to develop prediction models had a relatively minor number of predictors. The number of predictors in models based on boosting and RF was much higher (Table 1 ). All studies reported the predictors' contributions or odds ratios (only in LR). Among these important predictors, systemic steroids use, short-acting beta2-agonists, and emergency department visit were the most common predictors, followed by age, asthma diagnosis number, and exacerbation history (Fig. 2 b, Additional file 5 ).
Risk of bias and applicability
The overall quality assessment (ROB and applicability) based on PROBAST is shown in Table 1 . Additional file 6 provides judgment details of each study. The overall bias of all studies was rated as high risk. For participants, eight studies were at high ROB mainly due to retrospective design and asthma definition that was based on asthma-related medicine use and doctors’ diagnosis. The bias of predictors mainly results from subjective predictors (such as self-report symptoms), auxiliary examinations from different medical institutions, and comorbidities. These factors were difficult to be defined consistently. The definition of asthma exacerbations given by the ATS/ERS statement is widely accepted [ 12 ]. Studies in which the outcome was not in accordance with ATS/ERS statement were rated as high risk of bias. All studies had a high risk of bias in the “analysis” domain.
For applicability assessment, one study was judged as low concerns, and the remains were rated as high concerns. Two studies included asthmatic participants with mild to moderate asthma [ 19 , 24 ], thus might reduce the generalizability and applicability. Six studies were assessed as having high concerns in the “predictors” domain. The applicability would reduce when predictors were challenging to be defined similarly. As for the outcome, studies (10/11) would receive a rating of high concern if they did not focus on moderate to severe asthma exacerbations defined by the ATS/ERS statement.
- Meta-analysis
The discrimination ability of ML-based models was various. AUROC was reported in 21 models, the best prediction performance of asthma exacerbations ranged from 0.59 to 0.90. The specificity and sensitivity based on different cut-off points were reported in all included studies, with the range of 0.54–0.93 and 0.25–0.88, respectively. Negative predictive value ( n = 4), positive predictive value ( n = 4), and accuracy ( n = 4) of prediction models in several studies were also reported (Table 1 ).
We included 11 studies (23 models) with sufficient data and pooled performance measures of these studies in a random effects meta-analysis (see Additional file 7 ). The pooled AUROC for predicting asthma exacerbations was 0.80 (95% CI 0.76–0.83), indicating a good discrimination ability (Fig. 3 ). The pooled sensitivity and specificity were 0.61 (95% CI 0.53–0.69, I 2 = 98.71, P < 0.01) and 0.82 (95% CI 0.77–0.86, I 2 = 99.95, P < 0.01), respectively (Fig. 4 ). Other values were as follows: PLR 3.33 (95% CI 2.73–4.07, I 2 = 99.58, P < 0.01), NLR 0.47 (95% CI 0.39–0.57, I 2 = 98.89, P < 0.01), and DOR 7.02 (95% CI 5.20–9.47, I 2 = 100.00, P < 0.01) (see Additional file 8 ).
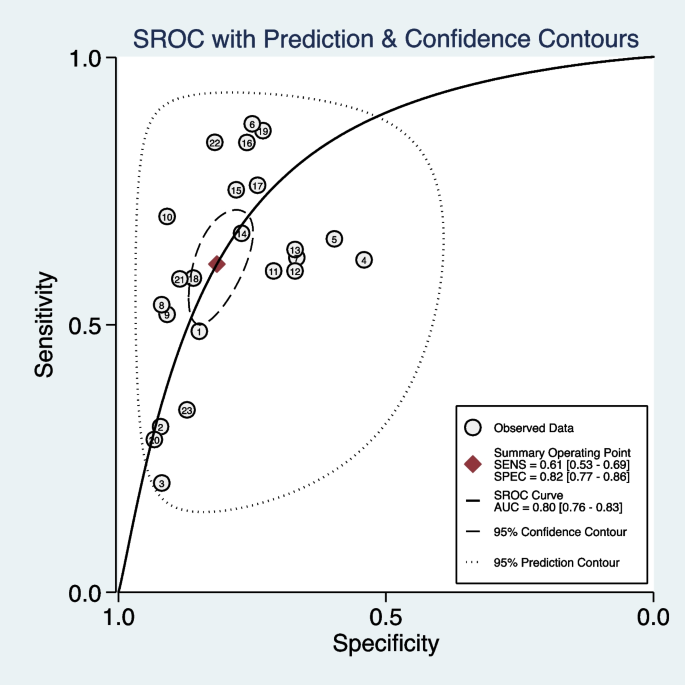
The overall pooled AUROC of machine learning prediction models

The overall pooled sensitivity ( a ) and specificity ( b ) of machine learning prediction models
We also performed subgroup analysis stratified by ML methods, sample size, age group, and outcome definitions. As shown in Table 2 , the overall pooled AUROC of boosting-based prediction models (0.84) was the highest, followed by studies using LR (0.77) and RF (0.75) (Table 2 , Fig. 5 ). DOR, another measure of overall diagnostic ability, was also highest in studies applying boosting method (11.86). In studies with a large sample size (> 10000), the pooled AUROC and DOR were relatively high, with the number of 0.82 and 8.62, respectively (Table 2 , Fig. 6 ). We classified outcome events as either emergency department visit/hospitalization (ED/HP) or in accordance with ATS/ERS statement (AE) definitions and performed subgroup analysis. The pooled AUROC in the two groups were similar, and the diagnostic odds ratio (DOR) was 7.58 for the ED/HP group and 6.01 for the AE group (Table 2 , Fig. 7 ). Studies involving participants with children and adults had the highest pooled AUROC (0.88) and DOR (9.49) (Table 2 , Fig. 8 ). Forest plots were shown in Additional file 9 .
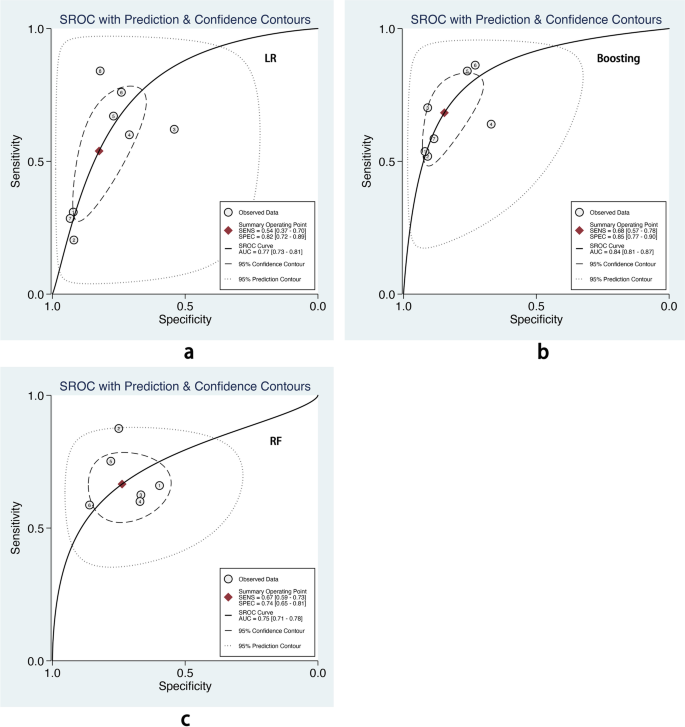
The overall pooled AUROC of machine learning prediction models stratified by logistic regression ( a ), boosting ( b ), and random forest ( c ) methods
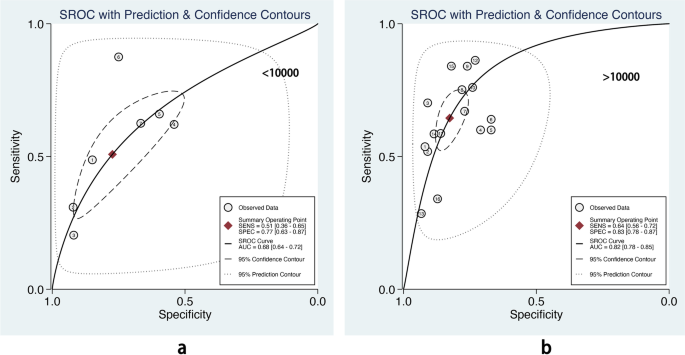
The overall pooled AUROC of machine learning prediction models stratified by different sample sizes. a Sample size < 10000. b Sample size > 10000
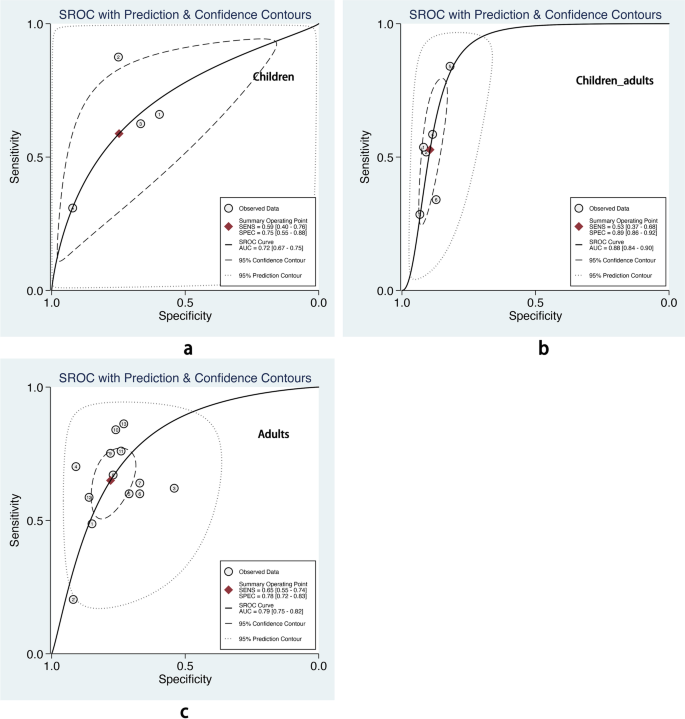
The overall pooled AUROC of machine learning prediction models stratified by different age groups. a Children. b Children and adults. c Adults

The overall pooled AUROC of machine learning prediction models stratified by different outcome events. a Emergency department visits/ hospitalization for asthma. b Asthma exacerbation definition in accordance with ATS/ERS statement
We perform the meta-regression analysis of the logit transformation of DOR due to the high level of heterogeneity. Univariate meta-regression analysis indicated that sample size and publication year contributed to the prediction power. However, only the coefficient of outcome definitions reached statistical significance in the multivariate model (Table 3 ). We included the outcome variable in the meta-regression analysis. The adjusted R-squared improved from 18.72% to 39.61%, and the Tau2 decreased from 0.4198 to 0.3118, indicating that the outcome variable could explain 25.7% heterogeneity.
Publication bias and sensitivity analysis
Deeks’ funnel plot was applied to test publication bias. As shown in Fig. 9 , the funnel plot was symmetrical, indicating no publication bias ( P = 0.29). Sensitivity analysis showed exclusion of any study did not affect the pooled estimations, suggesting the stability of the meta-analysis (see Additional file 10 ).
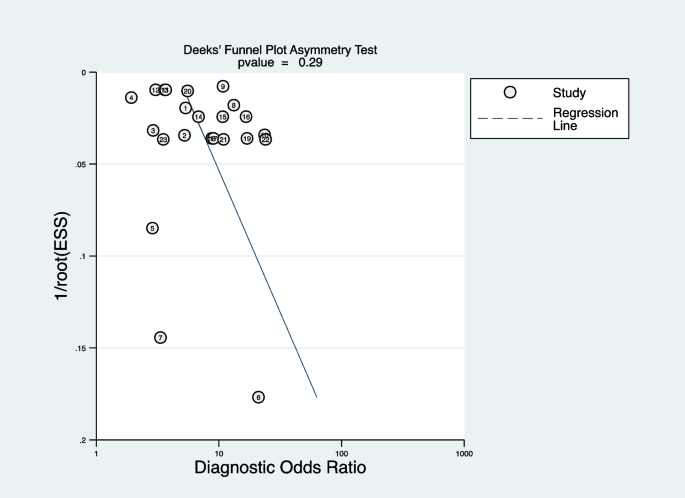
Deeks’ funnel plot of publication bias
Principal findings
This study systematically reviewed the ML-based prediction models for asthma exacerbations, which have not been discussed before. Eleven studies generated 23 ML prediction models, which were various in study design, data source, participants, outcome definitions, and ML algorithms. 6/11 studies were published in the recent three years, indicating a popular trend in applying ML algorithms in asthma. The overall pooled AUROC (0.8, 95% CI 0.76–0.83) and DOR (7.02, 95% CI 5.20–9.47) indicated that ML-based prediction models for asthma exacerbation could achieve good discrimination. ML prediction models could forecast patients at high risk of exacerbation from several days to years, helping identify patients needing closer management.
LR, boosting, and RF are the top three popular algorithms for asthma exacerbation prediction. According to the subgroup analysis, boosting-based prediction models had the highest pooled AUROC with a pooled AUROC of 0.84(95% CI 0.81–0.87), and the confidence interval of pooled AUROC was non-overlap with LR (0.77, 95% CI 0.73–0.81) and RF (0.75, 95% CI 0.71–0.78). Bridge et al. [ 8 ] conducted a systemic review and found that LR had a higher pooled c-statistic than optimal action points and CART in predicting asthma exacerbations. However, the authors did not include other ML methods. In this article, we found that boosting could also achieve good performance. It was potentially an alternative method in asthma exacerbation prediction, and more candidate models developed by ML should be tested.
The sample size is crucial for model performance. Compared with robust techniques like LR and CART, modern ML methods need higher times of events per variable to achieve stable performance [ 25 ]. Our subgroup analysis also showed that compared with prediction models with a smaller sample size (< 10000 participants), models developed in a big sample size (> 10000 participants) showed relatively high pooled AUROC (0.82, 95% CI 0.78–0.85 vs. 0.68, 95% CI 0.64–0.72) in the test dataset. This suggests that ML methods would be preferable for prediction models only if a large dataset is available [ 25 ].
As for predictors, the most important features were systemic steroids, short-acting beta2-agonists, age, ED visit, asthma diagnosis number, exacerbation history, race, BMI, duration, blood eosinophils, and smoking. Most of these factors were consistent with the risk factor identified in GINA ( https://ginasthma.org/wp-content/uploads/2021/04/GINA-2021-Main-Report_FINAL_21_04_28-WMS.pdf ) and previous studies [ 26 , 27 ]. Other biomarkers, such as volatile organic compounds and single nucleotide polymorphisms were also used as input features to predict asthma exacerbations [ 19 , 20 ]. However, these studies were performed with a small sample size of participants resulting in a high risk of overfitting. In addition, these factors require advanced equipment, limiting application in practice. Socioeconomic factors were included in only three studies but were identified as insignificant. Environmental factors, such as air pollutants, are also crucial for asthma exacerbation [ 28 ]. However, none of these studies focus on environmental factors.
Strengths and limitations
This study has several strengths. Firstly, we described included studies in detail and used logical methodology, which could provide a clear understanding of ML models in asthma exacerbation prediction. Additionally, the number of models allows us to conduct a meta-analysis of performance measures and compare different ML algorithms.
Despite the excellent prediction power of ML-based models confirmed in this study, several limitations are also identified. The main limitation was heterogeneity within studies. The difference in sample sizes, participants, feature selection, and prediction windows might affect the prediction ability of each model. Thus, the results analyzed in this study should be applied prudently. In addition, we did not include papers published in non-English, and we might not include all ML-based prediction models in the field of asthma exacerbations.
Future direction
ML methods are a potential way to achieve excellent performance in asthma exacerbation prediction, and more ML methods should be tested in the future. Although many models were developed, few of them were applied in practice. Therefore, improving the generalizability of prediction models in large separate datasets is crucial. Practicability is another critical factor. Simple models with a few predictors and using predictors that are easy to access could improve prediction models' practicability. Moreover, bundling ML algorithms to software or system would benefit in translating research into practice applications. Besides, randomized control studies are warranted to evaluate whether these models could benefit asthmatic patients by preventing asthma exacerbations.
Early identification of asthmatic patients at high risk of asthma exacerbations guides physicians to take closer management and timely intervention. This study showed that ML could achieve great performance in predicting asthma exacerbations. Future studies should focus on improving models' generalizability and practicability, thus driving the application of these models in clinical practice.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
Official American thoracic society/European respiratory society
Classification and regression trees
Diagnostic odds ratio
Global initiative for asthma
Light gradient boosting machine
Logistic regression
Mchine learning
Negative likelihood ratio
Neural network
Positive likelihood ratio
The prediction model risk of bias assessment tool
Random forest
Risk of bias
Support vector machine
Stern J, Pier J, Litonjua AA. Asthma epidemiology and risk factors. Semin Immunopathol. 2020;42:5–15.
Article PubMed Google Scholar
Bergmann KC, Skowasch D, Timmermann H, Lindner R, Virchow JC, Schmidt O, et al. Prevalence of patients with uncontrolled asthma despite NVL/GINA Step 4/5 treatment in Germany. J Asthma Allergy. 2022;15:897–906.
Article PubMed PubMed Central Google Scholar
Nagase H, Adachi M, Matsunaga K, Yoshida A, Okoba T, Hayashi N, et al. Prevalence, disease burden, and treatment reality of patients with severe, uncontrolled asthma in Japan. Allergol Int. 2020;69:53–60.
Loymans RJ, Ter Riet G, Sterk PJ. Definitions of asthma exacerbations. Curr Opin Allergy Clin Immunol. 2011;11:181–6.
Luskin AT, Chipps BE, Rasouliyan L, Miller DP, Haselkorn T, Dorenbaum A. Impact of asthma exacerbations and asthma triggers on asthma-related quality of life in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol Pract. 2014;2:544-52.e1-2.
O’Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW, START Investigators Group. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179(1):19–24. Epub 2008 Oct 31. Erratum in: Am J Respir Crit Care Med. 2010;182(7):983-984.
Zeiger RS, Schatz M, Dalal AA, Qian L, Chen W, Ngor EW, et al. Utilization and costs of severe uncontrolled asthma in a managed-care setting. J Allergy Clin Immunol Pract. 2016;4:120-129.e3.
Bridge J, Blakey JD, Bonnett LJ. A systematic review of methodology used in the development of prediction models for future asthma exacerbation. BMC Med Res Methodol. 2020;20:22.
Beam AL, Kohane IS. Big data and machine learning in health care. JAMA. 2018;319:1317–8.
Tsang KCH, Pinnock H, Wilson AM, Shah SA. Application of machine learning algorithms for asthma management with mHealth: a clinical review. J Asthma Allergy. 2022;15:855–73.
Feng Y, Wang Y, Zeng C, Mao H. Artificial intelligence and machine learning in chronic airway diseases: focus on asthma and chronic obstructive pulmonary disease. Int J Med Sci. 2021;18:2871–89.
Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99.
Moons KGM, Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med. 2019;170:W1–33.
Luo G, Nau CL, Crawford WW, Schatz M, Zeiger RS, Rozema E, et al. Developing a predictive model for asthma-related hospital encounters in patients with asthma in a large, integrated health care system: secondary analysis. JMIR Med Inform. 2020;8:e22689.
Luo G, He S, Stone BL, Nkoy FL, Johnson MD. Developing a model to predict hospital encounters for asthma in asthmatic patients: secondary analysis. JMIR Med Inform. 2020;8:e16080.
Lieu TA, Capra AM, Quesenberry CP, Mendoza GR, Mazar M. Computer-based models to identify high-risk adults with asthma: is the glass half empty of half full? J Asthma. 1999;36:359–70.
Article CAS PubMed Google Scholar
Schatz M, Nakahiro R, Jones CH, Roth RM, Joshua A, Petitti D. Asthma population management: development and validation of a practical 3-level risk stratification scheme. Am J Manag Care. 2004;10:25–32.
PubMed Google Scholar
Schatz M, Zeiger RS, Vollmer WM, Mosen D, Apter AJ, Stibolt TB, et al. Development and validation of a medication intensity scale derived from computerized pharmacy data that predicts emergency hospital utilization for persistent asthma. Am J Manag Care. 2006;12:478–84.
Xu M, Tantisira KG, Wu A, Litonjua AA, Chu JH, Himes BE, et al. Genome Wide Association Study to predict severe asthma exacerbations in children using random forests classifiers. BMC Med Genet. 2011;12:90.
van Vliet D, Smolinska A, Jöbsis Q, Rosias P, Muris J, Dallinga J, Dompeling E, van Schooten FJ. Can exhaled volatile organic compounds predict asthma exacerbations in children? J Breath Res. 2017;11:016016.
Tong Y, Messinger AI, Wilcox AB, Mooney SD, Davidson GH, Suri P, Luo G. Forecasting future asthma hospital encounters of patients with asthma in an academic health care system: predictive model development and secondary analysis study. J Med Internet Res. 2021;23:e22796.
Zein JG, Wu CP, Attaway AH, Zhang P, Nazha A. Novel machine learning can predict acute asthma exacerbation. Chest. 2021;159:1747–57.
Article CAS PubMed PubMed Central Google Scholar
Noble M, Burden A, Stirling S, Clark AB, Musgrave S, Alsallakh MA, et al. Predicting asthma-related crisis events using routine electronic healthcare data: a quantitative database analysis study. Br J Gen Pract. 2021;71:e948–57.
de Hond AAH, Kant IMJ, Honkoop PJ, Smith AD, Steyerberg EW, Sont JK. Machine learning did not beat logistic regression in time series prediction for severe asthma exacerbations. Sci Rep. 2022;12:20363.
van der Ploeg T, Austin PC, Steyerberg EW. Modern modeling techniques are data hungry: a simulation study for predicting dichotomous endpoints. BMC Med Res Methodol. 2014;14:137.
DiMango E, Rogers L, Reibman J, Gerald LB, Brown M, Sugar EA, et al. Risk factors for asthma exacerbation and treatment failure in adults and adolescents with well-controlled asthma during continuation and step-down therapy. Ann Am Thorac Soc. 2018;15:955–61.
McDowell PJ, Busby J, Hanratty CE, Djukanovic R, Woodcock A, Walker S, et al. Exacerbation profile and risk factors in a type-2-low enriched severe asthma cohort: a clinical trial to assess asthma exacerbation phenotypes. Am J Respir Crit Care Med. 2022;206:545–53.
Wang M, Li H, Huang S, Qian Y, Steenland K, Xie Y, Papatheodorou S, Shi L. Short-term exposure to nitrogen dioxide and mortality: a systematic review and meta-analysis. Environ Res. 2021;202:111766.
Download references
Acknowledgements
We would like to thank Yang Xie for obtaining the full text of included papers.
Not applicable.
Author information
Authors and affiliations.
Department of Allergy, Center for Asthma Prevention and Lung Function Laboratory, Children’s Hospital of Capital Institute of Pediatrics, Beijing, 100020, China
Shiqiu Xiong, Wei Chen, Xinyu Jia & Chuanhe Liu
Graduate School, Peking Union Medical College, Beijing, 100730, China
Shiqiu Xiong & Chuanhe Liu
Department of Pediatrics, The Second Xiangya Hospital, Central South University, Changsha, 410011, Hunan, China
You can also search for this author in PubMed Google Scholar
Contributions
SQX drafted this manuscript; SQX, WC, and XYJ conducted the research search, duplication checking, and eligible studies selection. SQX and YJ performed the data extraction and statistical analysis. SQX and CHL performed the quality and bias assessment. CHL revised this manuscript. All authors approved the final version of this paper.
Corresponding authors
Correspondence to Shiqiu Xiong or Chuanhe Liu .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
All authors declared that they had no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:.
Search term and results.
Additional file 2:
Additional file 3:.
Definitions of participants and outcomes of included studies.
Additional file 4:
The explanation of dataset split and validation methods.
Additional file 5:
Features and most important features in prediction models.
Additional file 6:
Risk of bias and applicability assessment based on PROBAST tools.
Additional file 7:
11 studies included in the meta-analysis.
Additional file 8:
The overall pooled positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio of 23 machine learning prediction models.
Additional file 9:
Forest plots of performance measures in subgroup analysis.
Additional file 10:
The influence of each model for the outcome of meta-analysis
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Xiong, S., Chen, W., Jia, X. et al. Machine learning for prediction of asthma exacerbations among asthmatic patients: a systematic review and meta-analysis. BMC Pulm Med 23 , 278 (2023). https://doi.org/10.1186/s12890-023-02570-w
Download citation
Received : 04 April 2023
Accepted : 19 July 2023
Published : 28 July 2023
DOI : https://doi.org/10.1186/s12890-023-02570-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Exacerbation
- Prediction model
- Machine learning
- Systematic review
BMC Pulmonary Medicine
ISSN: 1471-2466
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 15 August 2020
Treatment strategies for asthma: reshaping the concept of asthma management
- Alberto Papi 1 , 7 ,
- Francesco Blasi 2 , 3 ,
- Giorgio Walter Canonica 4 ,
- Luca Morandi 1 , 7 ,
- Luca Richeldi 5 &
- Andrea Rossi 6
Allergy, Asthma & Clinical Immunology volume 16 , Article number: 75 ( 2020 ) Cite this article
45k Accesses
54 Citations
4 Altmetric
Metrics details
Asthma is a common chronic disease characterized by episodic or persistent respiratory symptoms and airflow limitation. Asthma treatment is based on a stepwise and control-based approach that involves an iterative cycle of assessment, adjustment of the treatment and review of the response aimed to minimize symptom burden and risk of exacerbations. Anti-inflammatory treatment is the mainstay of asthma management. In this review we will discuss the rationale and barriers to the treatment of asthma that may result in poor outcomes. The benefits of currently available treatments and the possible strategies to overcome the barriers that limit the achievement of asthma control in real-life conditions and how these led to the GINA 2019 guidelines for asthma treatment and prevention will also be discussed.
Asthma, a major global health problem affecting as many as 235 million people worldwide [ 1 ], is a common, non-communicable, and variable chronic disease that can result in episodic or persistent respiratory symptoms (e.g. shortness of breath, wheezing, chest tightness, cough) and airflow limitation, the latter being due to bronchoconstriction, airway wall thickening, and increased mucus.
The pathophysiology of the disease is complex and heterogeneous, involving various host-environment interactions occurring at various scales, from genes to organ [ 2 ].
Asthma is a chronic disease requiring ongoing and comprehensive treatment aimed to reduce the symptom burden (i.e. good symptom control while maintaining normal activity levels), and minimize the risk of adverse events such as exacerbations, fixed airflow limitation and treatment side effects [ 3 , 4 ].
Asthma treatment is based on a stepwise approach. The management of the patient is control-based; that is, it involves an iterative cycle of assessment (e.g. symptoms, risk factors, etc.), adjustment of treatment (i.e. pharmacological, non-pharmacological and treatment of modifiable risk factors) and review of the response (e.g. symptoms, side effects, exacerbations, etc.). Patients’ preferences should be taken into account and effective asthma management should be the result of a partnership between the health care provider and the person with asthma, particularly when considering that patients and clinicians might aim for different goals [ 4 ].
This review will discuss the rationale and barriers to the treatment of asthma, that may result in poor patient outcomes. The benefits of currently available treatments and the possible strategies to overcome the barriers that limit the achievement of asthma control in real-life situations will also be discussed.
The treatment of asthma: where are we? Evolution of a concept
Asthma control medications reduce airway inflammation and help to prevent asthma symptoms; among these, inhaled corticosteroids (ICS) are the mainstay in the treatment of asthma, whereas quick-relief (reliever) or rescue medicines quickly ease symptoms that may arise acutely. Among these, short-acting beta-agonists (SABAs) rapidly reduce airway bronchoconstriction (causing relaxation of airway smooth muscles).
National and international guidelines have recommended SABAs as first-line treatment for patients with mild asthma, since the Global Initiative for Asthma guidelines (GINA) were first published in 1995, adopting an approach aimed to control the symptoms rather than the underlying condition; a SABA has been the recommended rescue medication for rapid symptom relief. This approach stems from the dated idea that asthma symptoms are related to bronchial smooth muscle contraction (bronchoconstriction) rather than a condition concomitantly caused by airway inflammation. In 2019, the GINA guidelines review (GINA 2019) [ 4 ] introduced substantial changes overcoming some of the limitations and “weaknesses” of the previously proposed stepwise approach to adjusting asthma treatment for individual patients. The concept of an anti-inflammatory reliever has been adopted at all degrees of severity as a crucial component in the management of the disease, increasing the efficacy of the treatment while lowering SABA risks associated with patients’ tendency to rely or over-rely on the as-needed medication.
Until 2017, the GINA strategy proposed a pharmacological approach based on a controller treatment (an anti-inflammatory, the pillar of asthma treatment), with a SABA as an additional rescue intervention. The reliever, a short-acting bronc hodilator, was merely an addendum , a medication to be used in case the real treatment (the controller) failed to maintain disease control: SABAs effectively induce rapid symptom relief but are ineffective on the underlying inflammatory process. Based on the requirement to achieve control, the intensity of the controller treatment was related to the severity of the disease, varying from low-dose ICS to combination low-dose ICS/long-acting beta-agonist (LABA), medium-dose ICS/LABA, up to high-dose ICS/LABA, as preferred controller choice, with a SABA as the rescue medication. As a result, milder patients were left without any anti-inflammatory treatment and could only rely on SABA rescue treatment.
Poor adherence to therapy is a major limitation of a treatment strategy based on the early introduction of the regular use of controller therapy [ 5 ]. Indeed, a number of surveys have highlighted a common pattern in the use of inhaled medication [ 6 ], in which treatment is administered only when asthma symptoms occur; in the absence of symptoms, treatment is avoided as patients perceive it as unnecessary. When symptoms worsen, patients prefer to use reliever therapies, which may result in the overuse of SABAs [ 7 ]. Indirect evidence suggests that the overuse of beta-agonists alone is associated with increased risk of death from asthma [ 8 ].
In patients with mild persistent disease, low-dose ICS decreases the risk of severe exacerbations leading to hospitalization and improves asthma control [ 9 ]. When low-dose ICS are ineffective in controlling the disease (Step 3 of the stepwise approach), a combination of low-dose ICS with LABA maintenance was the recommended first-choice treatment, plus as-needed SABA [ 3 , 10 ]. Alternatively, the combination low-dose ICS/LABA (formoterol) was to be used as single maintenance and reliever treatment (SMART). The SMART strategy containing the rapid-acting formoterol was recommended throughout GINA Steps 3 to 5 based on solid clinical-data evidence [ 3 ].
The addition of a LABA to ICS treatment reduces both severe and mild asthma exacerbation rates, as shown in the one-year, randomized, double-blind, parallel-group FACET study [ 11 ]. This study focused on patients with persistent asthma symptoms despite receiving ICS and investigated the efficacy of the addition of formoterol to two dose levels of budesonide (100 and 400 µg bid ) in decreasing the incidence of both severe and mild asthma exacerbations. Adding formoterol decreased the incidence of both severe and mild asthma exacerbations, independent of ICS dose. Severe and mild exacerbation rates were reduced by 26% and 40%, respectively, with the addition of formoterol to the lower dose of budesonide; the corresponding reductions were 63% and 62%, respectively, when formoterol was added to budesonide at the higher dose.
The efficacy of the ICS/LABA combination was confirmed in the post hoc analysis of the FACET study, in which patients were exposed to a combination of formoterol and low-dose budesonide [ 12 ]. However, such high levels of asthma control are not achieved in real life [ 5 ]. An explanation for this is that asthma is a variable condition and this variability might include the exposure of patients to factors which may cause a transient steroid insensitivity in the inflammatory process. This, in turn, may lead to an uncontrolled inflammatory response and to exacerbations, despite optimal controller treatment. A typical example of this mechanism is given by viral infections, the most frequent triggers of asthma exacerbations. Rhinoviruses, the most common viruses found in patients with asthma exacerbations, interfere with the mechanism of action of corticosteroids making the anti-inflammatory treatment transiently ineffective. A transient increase in the anti-inflammatory dose would overcome the trigger-induced anti-inflammatory resistance, avoiding uncontrolled inflammation leading to an exacerbation episode [ 13 , 14 , 15 ].
Indeed, symptoms are associated with worsening inflammation and not only with bronchoconstriction. Romagnoli et al. showed that inflammation, as evidenced by sputum eosinophilia and eosinophilic markers, is associated with symptomatic asthma [ 16 ]. A transient escalation of the ICS dose would prevent loss of control over inflammation and decrease the risk of progression toward an acute episode. In real life, when experiencing a deterioration of asthma control, patients self-treat by substantially increasing their SABA medication (Fig. 1 ); it is only subsequently that they (modestly) increase the maintenance treatment [ 17 ].
Mean use of SABA at different stages of asthma worsening. Patients have been grouped according to maintenance therapy shown in the legend. From [ 17 ], modified
As bronchodilators, SABAs do not control the underlying inflammation associated with increased symptoms. The “as required” use of SABAs is not the most effective therapeutic option in controlling a worsening of inflammation, as signaled by the occurrence of symptoms; instead, an anti-inflammatory therapy included in the rescue medication along with a rapid-acting bronchodilator could provide both rapid symptom relief and control over the underlying inflammation. Thus, there is a need for a paradigm shift, a new therapeutic approach based on the rescue use of an inhaled rapid-acting beta-agonist combined with an ICS: an anti-inflammatory reliever strategy [ 18 ].
The symptoms of an exacerbation episode, as reported by Tattersfield and colleagues in their extension of the FACET study, increase gradually before the peak of the exacerbation (Fig. 2 ); and the best marker of worsening asthma is the increased use of rescue beta-agonist treatment that follows exactly the pattern of worsening symptomatology [ 19 ]. When an ICS is administered with the rescue bronchodilator, the patient would receive anti-inflammatory therapy when it is required; that is, when the inflammation is uncontrolled, thus increasing the efficiency of the anti-inflammatory treatment.
(From [ 19 ])
Percent variation in symptoms, rescue beta-agonist use and peak expiratory flow (PEF) during an exacerbation. In order to allow comparison over time, data have been standardized (Day-14 = 0%; maximum change = 100%)
Barriers and paradoxes of asthma management
A number of barriers and controversies in the pharmacological treatment of asthma have prevented the achievement of effective disease management [ 20 ]. O’Byrne and colleagues described several such controversies in a commentary published in 2017, including: (1) the recommendation in Step 1 of earlier guidelines for SABA bronchodilator use alone, despite asthma being a chronic inflammatory condition; and (2) the autonomy given to patients over perception of need and disease control at Step 1, as opposed to the recommendation of a fixed-dose approach with treatment-step increase, regardless of the level of symptoms [ 20 ]. Other controversies outlined were: (3) a difficulty for patients in understanding the recommendation to minimize SABA use at Step 2 and switch to a fixed-dose ICS regimen, when they perceive SABA use as more effective; (4) apparent conflicting safety messages within the guidelines that patient-administered SABA monotherapy is safe, but patient-administered LABA monotherapy is not; and (5) a discrepancy as to patients’ understanding of “controlled asthma” and their symptom frequency, impact and severity [ 20 ].
Controversies (1) and (2) can both establish an early over-dependence on SABAs. Indeed, asthma patients freely use (and possibly overuse) SABAs as rescue medication. UK registry data have recently suggested SABA overuse or overreliance may be linked to asthma-related deaths: among 165 patients on short-acting relievers at the time of death, 56%, 39%, and 4% had been prescribed > 6, > 12, and > 50 SABA inhalers respectively in the previous year [ 21 ]. Registry studies have shown the number of SABA canisters used per year to be directly related to the risk of death in patients with asthma. Conversely, the number of ICS canisters used per year is inversely related to the rate of death from asthma, when compared with non-users of ICS [ 8 , 22 ]. Furthermore, low-dose ICS used regularly are associated with a decreased risk of asthma death, with discontinuation of these agents possibly detrimental [ 22 ].
Other barriers to asthma pharmacotherapy have included the suggestion that prolonged treatment with LABAs may mask airway inflammation or promote tolerance to their effects. Investigating this, Pauwels and colleagues found that in patients with asthma symptoms that were persistent despite taking inhaled glucocorticoids, the addition of regular treatment with formoterol to budesonide for a 12-month period did not decrease asthma control, and improved asthma symptoms and lung function [ 11 ].
Treatment strategies across all levels of asthma severity
Focusing on risk reduction, the 2014 update of the GINA guidelines recommended as-needed SABA for Step 1 of the stepwise treatment approach, with low-dose ICS maintenance therapy as an alternative approach for long-term anti-inflammatory treatment [ 23 ]. Such a strategy was only supported by the evidence from a post hoc efficacy analysis of the START study in patients with recently diagnosed mild asthma [ 24 ]. The authors showed that low-dose budesonide reduced the decline of lung-function over 3 years and consistently reduced severe exacerbations, regardless of symptom frequency at baseline, even in subjects with symptoms below the then-threshold of eligibility for ICS [ 24 ]. However, as for all post hoc analyses, the study by Reddel and colleagues does not provide conclusive evidence and, even so, their results could have questionable clinical significance for the management of patients with early mild asthma. To be effective, this approach would require patients to be compliant to regular twice-daily ICS for 10 years to have the number of exacerbations reduce by one. In real life, it is highly unlikely that patients with mild asthma would adhere to such a regular regimen [ 25 ].
The 2016 update to the GINA guidelines lowered the threshold for the use of low-dose ICS (GINA Step 2) to two episodes of asthma symptoms per month (in the absence of any supportive evidence for the previous cut-off). The objective was to effectively increase the asthma population eligible to receive regular ICS treatment and reduce the population treated with a SABA only, given the lack of robust evidence of the latter’s efficacy and safety and the fact that asthma is a variable condition characterized by acute exacerbations [ 26 ]. Similarly, UK authorities recommended low-dose ICS treatment in mild asthma, even for patients with suspected asthma, rather than treatment with a SABA alone [ 10 ]. However, these patients are unlikely to have good adherence to the regular use of an ICS. It is well known that poor adherence to treatment is a major problem in asthma management, even for patients with severe asthma. In their prospective study of 2004, Krishnan and colleagues evaluated the adherence to ICS and oral corticosteroids (OCS) in a cohort of patients hospitalized for asthma exacerbations [ 27 ]. The trend in the data showed that adherence to ICS and OCS treatment in patients dropped rapidly to reach nearly 50% within 7 days of hospital discharge, with the rate of OCS discontinuation per day nearly double the rate of ICS discontinuation per day (− 5.2% vs. − 2.7%; p < 0.0001 respectively, Fig. 3 ), thus showing that even after a severe event, patients’ adherence to treatment is suboptimal [ 27 ].

(From [ 27 ])
Use of inhaled (ICS) and oral (OCS) corticosteroids in patients after hospital discharge among high-risk adult patients with asthma. The corticosteroid use was monitored electronically. Error bars represent the standard errors of the measured ICS and OCS use
Guidelines set criteria with the aim of achieving optimal control of asthma; however, the attitude of patients towards asthma management is suboptimal. Partridge and colleagues were the first in 2006 to evaluate the level of asthma control and the attitude of patients towards asthma management. Patients self-managed their condition using their medication as and when they felt the need, and adjusted their treatment by increasing their intake of SABA, aiming for an immediate relief from symptoms [ 17 ]. The authors concluded that the adoption of a patient-centered approach in asthma management could be advantageous to improve asthma control.
The concomitant administration of an as-needed bronchodilator and ICS would provide rapid relief while administering anti-inflammatory therapy. This concept is not new: in the maintenance and reliever approach, patients are treated with ICS/formoterol (fast-acting, long-acting bronchodilator) combinations for both maintenance and reliever therapy. An effective example of this therapeutic approach is provided in the SMILE study in which symptomatic patients with moderate to severe asthma and treated with budesonide/formoterol as maintenance therapy were exposed to three different as-needed options: SABA (terbutaline), rapid-onset LABA (formoterol) and a combination of LABA and ICS (budesonide/formoterol) [ 28 ]. When compared with formoterol, budesonide/formoterol as reliever therapy significantly reduced the risk of severe exacerbations, indicating the efficacy of ICS as rescue medication and the importance of the as-needed use of the anti-inflammatory reliever.
The combination of an ICS and a LABA (budesonide/formoterol) in one inhaler for both maintenance and reliever therapy is even more effective than higher doses of maintenance ICS and LABA, as evidenced by Kuna and colleagues and Bousquet and colleagues (Fig. 4 ) [ 29 , 30 ].
(Data from [ 29 , 30 ])
Comparison between the improvements in daily asthma control resulting from the use of budesonide/formoterol maintenance and reliever therapy vs. higher dose of ICS/LABA + SABAZ and steroid load for the two regimens
The effects of single maintenance and reliever therapy versus ICS with or without LABA (controller therapy) and SABA (reliever therapy) have been recently addressed in the meta-analysis by Sobieraj and colleagues, who analysed 16 randomized clinical trials involving patients with persistent asthma [ 31 ]. The systematic review supported the use of single maintenance and reliever therapy, which reduces the risk of exacerbations requiring systemic corticosteroids and/or hospitalization when compared with various strategies using SABA as rescue medication [ 31 ].
This concept was applied to mild asthma by the BEST study group, who were the first to challenge the regular use of ICS. A pilot study by Papi and colleagues evaluated the efficacy of the symptom-driven use of beclomethasone dipropionate plus albuterol in a single inhaler versus maintenance with inhaled beclomethasone and as-needed albuterol. In this six-month, double-blind, double-dummy, randomized, parallel-group trial, 455 patients with mild asthma were randomized to one of four treatment groups: an as-needed combination therapy of placebo bid plus 250 μg of beclomethasone and 100 μg of albuterol in a single inhaler; an as-needed albuterol combination therapy consisting of placebo bid plus 100 μg of albuterol; regular beclomethasone therapy, comprising beclomethasone 250 μg bid and 100 μg albuterol as needed); and regular combination therapy with beclomethasone 250 μg and albuterol 100 μg in a single inhaler bid plus albuterol 100 μg as needed.
The rescue use of beclomethasone/albuterol in a single inhaler was as efficacious as the regular use of inhaled beclomethasone (250 μg bid ) and it was associated with a lower 6-month cumulative dose of the ICS [ 32 ].
The time to first exacerbation differed significantly among groups ( p = 0.003), with the shortest in the as-needed albuterol and placebo group (Fig. 5 ). Figure 5 also shows equivalence between the as-needed combination therapy and the regular beclomethasone therapy. However, these results were not conclusive since the study was not powered to evaluate the effect of the treatment on exacerbations. In conclusion, as suggested by the study findings, mild asthma patients may require the use of an as-needed ICS and an inhaled bronchodilator rather than a regular treatment with ICS [ 32 ].
(From [ 32 ])
Kaplan Meier analysis of the time to first exacerbation (modified intention-to-treat population). First asthma exacerbations are shown as thick marks. As-needed albuterol therapy = placebo bid plus 100 μg of albuterol as needed; regular combination therapy = 250 μg of beclomethasone and 100 μg of albuterol in a single inhaler bid plus 100 μg of albuterol as needed; regular beclomethasone therapy = 250 μg of beclomethasone bid and 100 μg of albuterol as needed; as-needed combination therapy = placebo bid plus 250 μg of beclomethasone and 100 μg of albuterol in a single inhaler as needed
Moving forward: a new approach to the management of asthma patients
Nearly a decade after the publication of the BEST study in 2007, the use of this alternative therapeutic strategy was addressed in the SYGMA 1 and SYGMA 2 trials. These double-blind, randomized, parallel-group, 52-week phase III trials evaluated the efficacy of as-needed use of combination formoterol (LABA) and the ICS budesonide as an anti-inflammatory reliever in patients requiring GINA Step 2 treatment, with the current reliever therapy (e.g. as-needed SABA) or with low-dose maintenance ICS (inhaled budesonide bid ) plus as-needed SABA, administered as regular controller therapy [ 33 , 34 ].
The SYGMA 1 trial, which enrolled 3849 patients, aimed to demonstrate the superiority of the as-needed use of the combination budesonide/formoterol over as-needed terbutaline, as measured by the electronically-recorded proportion of weeks with well-controlled asthma [ 34 ]. The more pragmatic SYGMA 2 trial enrolled 4215 patients with the aim to demonstrate that the budesonide/formoterol combination is non-inferior to budesonide plus as-needed terbutaline in reducing the relative rate of annual severe asthma exacerbations [ 33 ]. Both trials met their primary efficacy outcomes. In particular, as-needed budesonide/formoterol was superior to as-needed SABA in controlling asthma symptoms (34.4% versus 31.1%) and preventing exacerbations, achieving a 64% reduction in exacerbations. In both trials, budesonide/formoterol as-needed was similar to budesonide maintenance bid at preventing severe exacerbations, with a substantial reduction of the inhaled steroid load over the study period (83% in the SYGMA 1 trial and 75% in the SYGMA 2 trial). The time to first exacerbation did not differ significantly between the two regimens; however, budesonide/formoterol was superior to SABA in prolonging the time to first severe exacerbation [ 33 , 34 ].
The double-blind, placebo-controlled design of the SYGMA trials does not fully address the advantages of anti-inflammatory reliever strategy in patients who often rely on SABAs for symptom relief, so to what extent the study findings could apply to real-life practice settings was unclear.
These limitations were overcome by the results of the Novel START study, an open-label, randomized, parallel-group, controlled trial designed to reflect real-world practice, which demonstrated the effectiveness in mild asthma of budesonide/formoterol as an anti-inflammatory reliever therapy [ 35 ].
In real-world practice, mild asthma patients are treated with an as-needed SABA reliever or with daily low-dose ICS maintenance therapy plus a SABA reliever. In the Novel START study, 668 patients with mild asthma were randomized to receive either as-needed albuterol 100 µg, two inhalations (SABA reliever as a continuation of the Step 1 treatment according to the 2017 GINA guidelines), budesonide 200 µg (ICS maintenance treatment) plus as-needed albuterol (Step 2 therapy of the GINA 2017 guidelines), or 200 µg/6 µg budesonide/formoterol as anti-inflammatory reliever therapy taken as-needed for a 52-week study period.
In this study, the rate of asthma exacerbations for budesonide/formoterol was lower compared with albuterol (51%) and similar to the twice-daily maintenance budesonide plus albuterol, despite a 52% reduction in the mean steroid dose with the single combination inhaler treatment [ 35 ]. In addition, severe exacerbation rate was lower with budesonide/formoterol as compared with as-needed albuterol and regular twice-daily budesonide. These data support the findings of the SYGMA 1 and 2 trials, highlighting the need for a critical re-examination of current clinical practice. Along with the results of the SYGMA trials, they provide convincing evidence of the advantages of the anti-inflammatory reliever strategy, particularly in real-life settings.
The SYGMA 1, SYGMA 2 and the novel START studies complete the picture of the treatment strategies for asthma at any degree of severity, including mild asthma. A growing body of evidence shows that an anti-inflammatory reliever strategy, when compared with all other strategies with SABA reliever, consistently reduces the rate of exacerbations across all levels of asthma severity (Fig. 6 ) [ 28 , 29 , 34 , 36 , 37 , 38 , 39 ].
(Data source: [ 39 ])
Risk reduction of severe asthma attack of anti-inflammatory reliever versus SABA across all levels of asthma severity. Bud = budesonide; form = formoterol; TBH = turbohaler. Data from: 1: [ 36 ]; 2: [ 37 ]; 3: [ 38 ]; 4: [ 28 ]; 5: [ 29 ]; 6: [ 30 ]; 7: [ 34 ]
This evidence set the ground (Fig. 7 ) for the release of the 2019 GINA strategy updates. The document provides a consistent approach towards the management of the disease and aims to avoid the overreliance and overuse of SABAs, even in the early course of the disease. The 2019 GINA has introduced key changes in the treatment of mild asthma: for safety reasons, asthmatic adults and adolescents should receive ICS-containing controller treatment instead of the SABA-only treatment, which is no longer recommended.
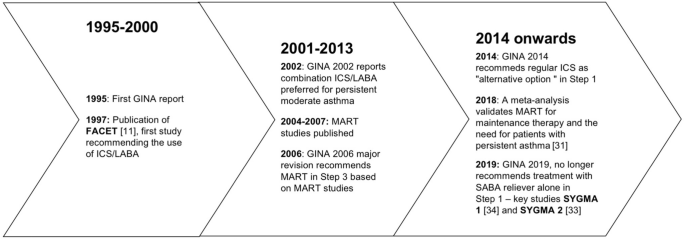
Timeline of key randomized controlled trials and meta-analyses providing the supporting evidence base leading to the Global Initiative for Asthma (GINA) 2019 guidelines. GINA global initiative for asthma, MART maintenance and reliever therapy, SMART single inhaler maintenance and reliever therapy
In Step 1 of the stepwise approach to adjusting asthma treatment, the preferred controller option for patients with fewer than two symptoms/month and no exacerbation risk factors is low-dose ICS/formoterol as needed. This strategy is indirectly supported by the results of the SYGMA 1 study which evaluated the efficacy and safety of budesonide/formoterol as needed, compared with as-needed terbutaline and budesonide bid plus as-needed terbutaline (see above). In patients with mild asthma, the use of an ICS/LABA (budesonide/formoterol) combination as needed provided superior symptom control to as-needed SABA, resulting in a 64% lower rate of exacerbations (p = 0.07) with a lower steroid dose (17% of the budesonide maintenance dose) [ 34 ]. The changes extend to the other controller options as well. In the 2017 GINA guidelines, the preferred treatment was as-needed SABA with the option to consider adding a regular low-dose ICS to the reliever. In order to overcome the poor adherence with the ICS regimen, and with the aim to reduce the risk of severe exacerbations, the 2019 GINA document recommends taking low-dose ICS whenever SABA is taken, with the daily ICS option no longer listed.
Previous studies including the TREXA study in children and adolescents [ 40 ], the BASALT study [ 41 ] and research conducted by the BEST study group [ 32 ] have already added to the evidence that a low-dose ICS with a bronchodilator is an effective strategy for symptom control in patients with mild asthma. A recently published study in African-American children with mild asthma found that the use of as-needed ICS with SABA provides similar asthma control, exacerbation rates and lung function measures at 1 year, compared with daily ICS controller therapy [ 42 ], adding support to TREXA findings that in children with well controlled, mild asthma, ICS used as rescue medication with SABA may be an efficacious step-down strategy [ 40 ].
In Step 2 of the stepwise approach, there are now two preferred controller options: (a) a daily low-dose ICS plus an as-needed SABA; and (b) as-needed low-dose ICS/formoterol. Recommendation (a) is supported by a large body of evidence from randomized controlled trials and observations showing a substantial reduction of exacerbation, hospitalization, and death with regular low-dose ICS [ 7 , 8 , 9 , 24 , 43 ], whereas recommendation (b) stems from evidence on the reduction or non-inferiority for severe exacerbations when as-needed low-dose ICS/formoterol is compared with regular ICS [ 33 , 34 ].
The new GINA document also suggests low-dose ICS is taken whenever SABA is taken, either as separate inhalers or in combination. This recommendation is supported by studies showing reduced exacerbation rates compared with taking a SABA only [ 32 , 40 ], or similar rates compared with regular ICS [ 32 , 40 , 41 ]. Low-dose theophylline, suggested as an alternative controller in the 2017 GINA guidelines, is no longer recommended.
Airway inflammation is present in the majority of patients with asthma, and although patients with mild asthma may have only infrequent symptoms, they face ongoing chronic inflammation of the lower airways and risk acute exacerbations. The GINA 2019 strategy recognizes the importance of reducing the risk of asthma exacerbations, even in patients with mild asthma (Steps 1 and 2) [ 4 ]. In this regard, the new recommendations note that SABA alone for symptomatic treatment is non-protective against severe exacerbation and may actually increase exacerbation risk if used regularly or frequently [ 4 ].
The reluctance by patients to regularly use an ICS controller means they may instead try and manage their asthma symptoms by increasing their SABA reliever use. This can result in SABA overuse and increased prescribing, and increased risk of exacerbations.
As part of the global SABINA (SABA use IN Asthma) observational study programme, a UK study examined primary care records to describe the pattern of SABA and ICS use over a 10-year period in 373,256 patients with mild asthma [ 44 ]. Results showed that year-to-year SABA prescribing was more variable than that of ICS indicating that, in response to fluctuations in asthma symptom control, SABA use was increased in preference to ICS use. Furthermore, more than 33% of patients were prescribed SABA inhalers at a level equivalent to around ≥ 3 puffs per week which, according to GINA, suggests inadequate asthma control.
The problem of SABA overuse is further highlighted by two studies [ 45 , 46 ], also as part of the SABINA programme. These analysed data from 365,324 patients in a Swedish cohort prescribed two medications for obstructive lung disease in any 12-month period (HERA).
The first study identified SABA overuse (defined as ≥ 3 SABA canisters a year) in 30% of patients, irrespective of their ICS use; 21% of patients were collecting 3–5 canisters annually, 7% were collecting 6–10, and 2% more than 11 [ 45 ]. Those patients who were overusing SABA had significantly more asthma exacerbations relative to those using < 3 canisters (20.0 versus 12.5 per 100 patient years; relative risk 1.60, 95% CI 1.57–1.63, p < 0.001). Moreover, patients overusing SABA and whose asthma was more severe (GINA Steps 3 and 4) had greater exacerbation risk compared with overusing patients whose asthma was milder (GINA Steps 1 and 2).
The second study found those patients using three or more SABA reliever canisters a year had an increased all-cause mortality risk relative to patients using fewer SABA canisters: hazard ratios after adjustment were 1.26 (95% CI 1.14–1.39) for 3–5 canisters annually, 1.67 (1.49–1.87) for 6–10 canisters, and 2.35 (2.02–2.72) for > 11 canisters, relative to patients collecting < 3 canisters annually [ 46 ].
The recently published PRACTICAL study lends further support to as-needed low-dose ICS/formoterol as an alternative option to daily low-dose ICS plus as-needed SABA, outlined in Step 2 of the guidelines [ 47 ]. In their one-year, open-label, multicentre, randomized, superiority trial in 890 patients with mild to moderate asthma, Hardy and colleagues found that the rate of severe exacerbations per patient per year (the primary outcome) was lower in patients who received as-needed budesonide/formoterol than in patients who received controller budesonide plus as-needed terbutaline (relative rate 0.69, 95% CI 0.48–1.00; p < 0.05). Indeed, they suggest that of these two treatment options, as-needed low-dose ICS/formoterol may be preferred over controller low-dose ICS plus as-needed SABA for the prevention of severe exacerbations in this patient population.
Step 3 recommendations have been left unchanged from 2017, whereas Step 4 treatment has changed from recommending medium/high-dose ICS/LABA [ 3 ] to medium-dose ICS/LABA; the high-dose recommendation has been escalated to Step 5. Patients who have asthma that remains uncontrolled after Step 4 treatment should be referred for phenotypic assessment with or without add-on therapy.
To summarise, the use of ICS medications is of paramount importance for optimal asthma control. The onset and increase of symptoms are indicative of a worsening inflammation leading to severe exacerbations, the risk of which is reduced by a maintenance plus as-needed ICS/LABA combination therapy. The inhaled ICS/bronchodilator combination is as effective as the regular use of inhaled steroids.
The efficacy of anti-inflammatory reliever therapy (budesonide/formoterol) versus current standard-of-care therapies in mild asthma (e.g. reliever therapy with a SABA as needed and regular maintenance controller therapy plus a SABA as-needed) has been evaluated in two randomized, phase III trials which confirmed that, with respect to as-needed SABA, the anti-inflammatory reliever as needed is superior in controlling asthma and reduces exacerbation rates, exposing the patients to a substantially lower glucocorticoid dose.
Conclusions
A growing body of evidence shows that anti-inflammatory reliever strategy is more effective than other strategies with SABA reliever in controlling asthma and reducing exacerbations across all levels of asthma severity. A budesonide/formoterol therapy exposes asthma patients to a substantially lower glucocorticoid dose while cutting the need for adherence to scheduled therapy.
Availability of data and materials
Not applicable.

Abbreviations
Global Initiative for Asthma
Inhaled corticosteroids
Long-acting beta-agonists
Oral corticosteroids
Short-acting beta-agonists
Single inhaler maintenance and reliever treatment
World Health Organization. Asthma. 2017. https://www.who.int/news-room/fact-sheets/detail/asthma . Accessed 9 April 2019.
Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(10122):783–800.
Article PubMed Google Scholar
Global Initiative for Asthma. Global strategy for asthma management and prevention, 2017. http://www.ginasthma.org . Accessed 1 June 2019.
Global Initiative for Asthma. Pocket guide for asthma management and prevention. 2019, pp. 1–32. www.ginasthma.org . Accessed 1 June 2019.
Rabe KF, Vermeire PA, Soriano JB, Maier WC. Clinical management of asthma in 1999: the Asthma Insights and Reality in Europe (AIRE) study. Eur Respir J. 2000;16(5):802–7.
Article CAS PubMed Google Scholar
Price D, Fletcher M, Molen V. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24:14009.
Article PubMed Central PubMed Google Scholar
Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax. 2002;57(10):880–4.
Article CAS PubMed Central PubMed Google Scholar
Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. New Engl J Med. 2000;343(5):332–6.
Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, et al. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet. 2003;361(9363):1071–6.
Healthcare Improvement Scotland and British Thoracic Society. SIGN 153: British guideline on the management of asthma. 2016. https://www.sign.ac.uk/sign-153-british-guideline-on-the-management-of-asthma.html .
Pauwels RA, Löfdahl CG, Postma DS, Tattersfield AE, O’Byrne P, Barnes PJ, Ullman A. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med. 1997;337(20):1405–11.
O’Byrne PM, Naya IP, Kallen A, Postma DS, Barnes PJ. Increasing doses of inhaled corticosteroids compared to adding long-acting inhaled β2-agonists in achieving asthma control. Chest. 2008;134(6):1192–9.
Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310(6989):1225–9.
Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, Johnston SL. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359(9309):831–4.
Papi A, Contoli M, Adcock IM, Bellettato C, Padovani A, Casolari P, et al. Rhinovirus infection causes steroid resistance in airway epithelium through nuclear factor κb and c-Jun N-terminal kinase activation. J Allergy Clin Immunol. 2013;132(5):1075–85.
Romagnoli M, Vachier I, Tarodo de la Fuente P, Meziane H, Chavis C, Bousquet J, et al. Eosinophilic inflammation in sputum of poorly controlled asthmatics. Eur Respir J. 2002;20(6):1370–7.
Partridge MR, van der Molen T, Myrseth SE, Busse WW. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulm Med. 2006;6:13.
Papi A, Caramori G, Adcock IM, Barnes PJ. Rescue treatment in asthma. More than as-needed bronchodilation. Chest. 2009;135(6):1628–33.
PubMed Google Scholar
Tattersfield AE, Postma DS, Barnes PJ, Svensson K, Bauer CA, Byrne PM, et al. Exacerbations of asthma: a descriptive study of 425 severe exacerbations The FACET International Study Group. Am J Respir Crit Care Med. 1999;160(2):594–9.
O’Byrne PM, Jenkins C, Bateman ED. The paradoxes of asthma management: time for a new approach? Eur Respir J. 2017. https://doi.org/10.1183/13993003.01103-2017 .
Royal College of Physicians. Why asthma still kills: the National Review of Asthma Deaths (NRAD). Confidential Enquiry report. London: RCP; 2014.
Google Scholar
Suissa S, Ernst P, Boivin JF, Horwitz RI, Habbick B, Cockroft D, et al. A cohort analysis of excess mortality in asthma and the use of inhaled β-agonists. Am J Respir Crit Care Med. 1994;149(3 Pt 1):604–10.
Global Initiative for Asthma. Global strategy for asthma management and prevention, 2014. https://ginasthma.org/wp-content/uploads/2019/01/2014-GINA.pdf .
Reddel HK, Busse WW, Pedersen S, Tan WC, Chen YZ, Jorup C, et al. Should recommendations about starting inhaled corticosteroid treatment for mild asthma be based on symptom frequency: a post hoc efficacy analysis of the START study. Lancet. 2017;389(10065):157–66.
Papi A, Fabbri LM. Management of patients with early mild asthma and infrequent symptoms. Lancet. 2017;389(10065):129–30.
Global Initiative for Asthma. Global strategy for asthma management and prevention, 2016. https://ginasthma.org/wp-content/uploads/2016/04/GINA-Appendix-2016-final.pdf .
Krishnan JA, Riekert KA, McCoy JV, Stewart DY, Schmidt S, Chanmugam A, et al. Corticosteroid use after hospital discharge among high-risk adults with asthma. Am J Respir Crit Care Med. 2004;170(12):1281–5.
Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo UG. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double-blind study. Lancet. 2006;368(9537):744–53.
Kuna P, Peters MJ, Manjra AI, Jorup C, Naya IP, Martínez-Jimenez NE, Buhl R. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract. 2007;61(5):725–36.
Bousquet J, Boulet LP, Peters MJ, Magnussen H, Quiralte J, Martinez-Aguilar NE, Carlsheimer A. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high-dose salmeterol/fluticasone. Respir Med. 2007;101(12):2437–46.
Sobieraj DM, Weeda ER, Nguyen E, Coleman CI, White CM, Lazarus SC, et al. Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma a systematic review and meta-analysis. JAMA. 2018;319(14):1485–96.
Papi A, Canonica GW, Maestrelli P, Paggiaro P, Olivieri D, Pozzi E, BEST Study Group, et al. Rescue use of beclomethasone and albuterol in a single inhaler for mild asthma. New Engl J Med. 2007;356(20):2040–52.
Bateman ED, Reddel HK, O’Byrne PM, Barnes PJ, Zhong N, Keen C, et al. As-needed budesonide–formoterol versus maintenance budesonide in mild asthma. New Engl J Med. 2018;378(20):1877–87.
O’Byrne PM, FitzGerald JM, Bateman ED, Barnes PJ, Zhong N, Keen C, et al. Inhaled combined budesonide-formoterol as needed in mild asthma. New Engl J Med. 2018;378(20):1865–76.
Beasley R, Holliday M, Reddel HK, Braithwaite I, Ebmeier S, Hancox RJ, Novel START Study Team, et al. Controlled trial of budesonide-formoterol as needed for mild asthma. New Engl J Med. 2019;380(21):2020–30.
Rabe K, Pizzichini E, Ställberg B, Romero S, Balanzat AM, Atienza T, et al. Budesonide/formoterol in a single inhaler for maintenance and relief in mild-to-moderate asthma: a randomized, double-blind trial. Chest. 2006;129(2):246–56.
Scicchitano R, Aalbers R, Ukena D, Manjra A, Fouquert L, Centanni S, et al. Efficacy and safety of budesonide/formoterol single inhaler therapy versus a higher dose of budesonide in moderate to severe asthma. Curr Med Res Opin. 2004;20(9):1403–18.
O’Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med. 2005;171(2):129–36.
Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G, et al. After asthma: redefining airways diseases. Lancet. 2017;391(10118):350–400.
Martinez FD, Chinchilli VM, Morgan WJ, Boehmer SJ, Lemanske RF Jr, Mauger DT, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377(9766):650–7.
Calhoun WJ, Ameredes BT, King TS, Icitovic N, Bleecker ER, Castro M, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA. 2012;308(10):987–97.
Sumino K, Bacharier LB, Taylor J, et al. A pragmatic trial of symptom-based inhaled corticosteroid use in African-American children with mild asthma. J Allergy Clin Immunol Pract. 2020;8(176–85):e2.
Byrne PM, Barnes PJ, Rodriguez-Roisin R, Runnerstrom E, Sandstrom T, Svensson K, Tattersfield A. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med. 2001;164(8):1392–7.
Article Google Scholar
Bloom C, Quint J, Cabrera C. SABA and ICS prescriptions among mild asthma patients in UK primary care. Poster presented at the European Respiratory Society International Congress; 2019 Sep 28–Oct 2; Madrid, Spain.
Janson C, Nwaru B, Hasvold P, Wicklund F, Telg G, Ekstrom M. Use of short-acting beta-2 agonists (SABA) and exacerbations in a nationwide Swedish asthma cohort (HERA). Poster presented at the European Respiratory Society International Congress; 2019 Sep 28–Oct 2; Madrid, Spain.
Janson C, Nwaru B, Hasvold P, Wicklund F, Telg G, Ekstrom M. SABA overuse and risk of mortality in a nationwide Swedish asthma cohort (HERA). Late Breaker abstract at the European Respiratory Society International Congress; 2019 Sep 28–Oct 2; Madrid, Spain.
Hardy J, Baggott C, Fingleton J, Reddel HK, Hancox RJ, Harwood M, et al. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet. 2019;394(10202):919–28.
Download references
Acknowledgements
The Authors thank Maurizio Tarzia and Gayle Robins, independent medical writers who provided editorial assistance on behalf of Springer Healthcare Communications. The editorial assistance was funded by AstraZeneca.
No funding was received for this study. The editorial assistance was funded by AstraZeneca.
Author information
Authors and affiliations.
Section of Cardiorespiratory and Internal Medicine, Department of Morphology, Surgery and Experimental Medicine, University of Ferrara, Ferrara, Italy
Alberto Papi & Luca Morandi
Internal Medicine Department, Respiratory Unit and Adult Cystic Fibrosis Center, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy
Francesco Blasi
Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy
Personalized Medicine Asthma & Allergy Clinic, Humanitas University & Istituto Clinico Humanitas, Milan, Italy
Giorgio Walter Canonica
Università Cattolica del Sacro Cuore, Fondazione Policlinico A. Gemelli IRCCS, Rome, Italy
Luca Richeldi
Respiratory Section, Department of Medicine, University of Verona, Verona, Italy
Andrea Rossi
Respiratory Unit, Emergency Department, University Hospital S. Anna, Via Aldo Moro 8, 44124, Ferrara, Italy
You can also search for this author in PubMed Google Scholar
Contributions
AP, FB, GWC, LM, LR and AR contributed to writing. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Alberto Papi .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
AP reports grants, personal fees, non-financial support and payment for advisory board membership, consultancy, payment for lectures, grants for research, and travel expenses reimbursement from Chiesi, AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Mundipharma and Teva, and personal fees and non-financial support from Menarini, Novartis, Zambon and Sanofi.
FB reports having received in the last three years research grants as well as lecture or advisory board fees from: Alk-Abelló, AstraZeneca, Boehringer Ingelheim, Chiesi, Guidotti, Glaxo Smith Kline, Grifols, Menarini, Novartis, Sanofi, Valeas, Zambon.
GWC reports having received in the last 3 years research grants as well as lecture or advisory board fees from: A. Menarini, Alk-Abelló, AstraZeneca-Medimmune, Boehringer Ingelheim, Chiesi Farmaceutici, Genentech, Guidotti-Malesci, Glaxo Smith Kline, Hal Allergy, Merck Sharp & Dohme, Mundipharma, Novartis, Orion, Sanofi-Aventis, Sanofi Genzyme/Regeneron, Stallergenes-Greers, UCB Pharma, Uriach Pharma, Valeas.
LR Receipt of grants/research supports: Roche, Boehringer Ingelheim.
Receipt of honoraria or consultation fees: Boehringer Ingelheim, Roche, Biogen, FibroGen,
Sanofi-Aventis, Anthera, Promedior, ImmuneWorks, Asahi-Kasei, Bayer, Celgene, RespiVant,
Nitto, Bristol Myers Squibb, Prometic, Pliant Therapeutics, Toray, Global Blood Therapeutics,
Zambon, Veracyte, Acceleron, CSL Behring.
LM and AR reports no conflicts of interest in the last 3 years.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Papi, A., Blasi, F., Canonica, G.W. et al. Treatment strategies for asthma: reshaping the concept of asthma management. Allergy Asthma Clin Immunol 16 , 75 (2020). https://doi.org/10.1186/s13223-020-00472-8
Download citation
Received : 18 March 2020
Accepted : 05 August 2020
Published : 15 August 2020
DOI : https://doi.org/10.1186/s13223-020-00472-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Anti-inflammatory treatment
- Disease control
- Patient outcomes
Allergy, Asthma & Clinical Immunology
ISSN: 1710-1492
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Discovery of how limiting damage from an asthma attack could stop disease
Scientists at King's College London have discovered a new cause for asthma that sparks hope for treatment that could prevent the life-threatening disease.
Most current asthma treatments stem from the idea that it is an inflammatory disease. Yet, the life-threatening feature of asthma is the attack or the constriction of airways, making breathing difficult. The new study, published today in Science , shows for the first time that many features of an asthma attack -- inflammation, mucus secretion, and damage to the airway barrier that prevents infections -- result from this mechanical constriction in a mouse model.
The findings suggest that blocking a process that normally causes epithelial cell death could prevent the damage, inflammation, and mucus that result from an asthma attack.
Professor Jody Rosenblatt from King's College London said: "Our discovery is the culmination of more than ten years work. As cell biologists who watch processes, we could see that the physical constriction of an asthma attack causes widespread destruction of the airway barrier. Without this barrier, asthma sufferers are far more likely to get long-term inflammation, wound healing, and infections that cause more attacks. By understanding this fundamental mechanism, we are now in a better position to prevent all these events."
In the UK, 5.4 million people have asthma and can suffer from symptoms such as wheezing, coughing, feeling breathlessness and a tight chest. Triggers such as pollen or dust can make asthma symptoms worse and can lead to a life-threatening asthma attack.
Despite the disease commonality, the causes of asthma are still not understood. Current medications treat the consequences of an asthma attack by opening the airways, calming inflammation, and breaking up the sticky mucus which clogs the airway, which help control asthma, but do not prevent it.
The answer to stopping asthma symptoms may lie in cell extrusion, a process the researchers discovered that drives most epithelial cell death. Scientists used mouse lung models and human airway tissue to discover that when the airways contract, known as bronchoconstriction, the epithelial cells that line the airway get squeezed out to later die.
Because bronchoconstriction causes so many cell extrusions, it damages the airway barrier which causes inflammation and excess mucus.
In previous studies, the scientists found that the chemical compound gadolinium can block extrusion. In this study, they found it could work in mice to prevent the excess extrusion that causes damage and inflammation after an asthma attack. The authors note that gadolinium has not been tested in humans and has not been deemed to be safe or efficacious.
Professor Rosenblatt said: "This constriction and destruction of the airways causes the post-attack inflammation and excess mucus secretion that makes it difficult for people with asthma to breathe.
"Current therapies do not prevent this destruction -- an inhaler such as Albuterol opens the airways, which is critical to breathing but, dishearteningly, we found it does not prevent the damage and the symptoms that follow an attack. Fortunately, we found that we can use an inexpensive compound, gadolinium which is frequently used for MRI imaging, to stop the airway damage in mice models as well as the ensuing inflammation and mucus secretion. Preventing this damage could then prevent the build-up of musculature that cause future attacks."
Professor Chris Brightling from the University of Leicester and one of the co-authors of the study said: "In the last decade there has been tremendous progress in therapies for asthma particularly directed towards airway inflammation. However, there remains ongoing symptoms and attacks in many people with asthma. This study identifies a new process known as epithelial extrusion whereby damage to the lining of the airway occurs as a consequence of mechanical constriction and can drive many of the key features of asthma. Better understanding of this process is likely to lead to new therapies for asthma."
Dr Samantha Walker, Director of Research and Innovation at Asthma + Lung UK, said: "Only two per cent of public health funding is allocated to developing new treatments for the 12 million people living with lung conditions in the UK so new research that can help in the treatment or prevention of asthma is good news.
"This research using an experimental mouse model shows that constricting the airways leads to damage to the lung lining and inflammation, like that seen in asthma. It is this constriction and resulting damage that makes it difficult for people with asthma to breathe.
"Current medications for asthma work by treating the inflammation, but this isn't effective for everyone. Treatments aim to prevent future asthma attacks and improve asthma control by taking inhalers every day, but we know that ~31 per cent of people with asthma don't have treatment options that work for them, putting them at risk of potentially life-threatening asthma attacks.
"This discovery opens important new doors to explore possible new treatment options desperately needed for people with asthma rather than focusing solely on inflammation."
The discovery of the mechanics behind cell extrusion could underlie other inflammatory diseases that also feature constriction such as cramping of the gut and inflammatory bowel disease.
The paper is in collaboration with the University of Leicester and funded by Wellcome, Howard Hughes Medical Institute and the American Asthma Foundation.
- Chronic Illness
- Diseases and Conditions
- Healthy Aging
- Heart Disease
- Hodgkin's lymphoma
- Hormone replacement therapy
- Dehydration
- Palliative care
- Rocky Mountain spotted fever
- Physical trauma
- Breast cancer
Story Source:
Materials provided by King's College London . Note: Content may be edited for style and length.
Journal Reference :
- Dustin C. Bagley, Tobias Russell, Elena Ortiz-Zapater, Sally Stinson, Kristina Fox, Polly F. Redd, Merry Joseph, Cassandra Deering-Rice, Christopher Reilly, Maddy Parsons, Christopher Brightling, Jody Rosenblatt. Bronchoconstriction damages airway epithelia by crowding-induced excess cell extrusion . Science , 2024; 384 (6691): 66 DOI: 10.1126/science.adk2758
Cite This Page :
Explore More
- Stellar Winds of Three Sun-Like Stars Detected
- Fences Causing Genetic Problems for Mammals
- Ozone Removes Mating Barriers Between Fly ...
- Parkinson's: New Theory On Origins and Spread
- Clash of Stars Solves Stellar Mystery
- Secure Quantum Computing at Home
- Ocean Currents: Collapse of Antarctic Ice ...
- Pacific Cities Much Older Than Previously ...
- The Milky Way in Ancient Egyptian Mythology
- Physical Activity Best in the Evening
Trending Topics
Strange & offbeat.
New cause of asthma lung damage revealed
In a lab study, scientists have pinpointed a potential way to "break the inflammatory cycle" of asthma.

Scientists may have uncovered an overlooked factor in why asthma attacks happen, and they say it could open up a whole new avenue for treatments.
In a laboratory study in mice and human tissues, the researchers revealed how asthma attacks kill cells in the airways of the lungs . They found that when the airways constrict during an asthma attack, the thin layer of cells that line these passageways — called epithelial cells — becomes too crowded, causing some cells to be squeezed out of the tissue and die.
As a result, this protective barrier in the lungs becomes damaged, triggering inflammation and mucus secretion that blocks the airways and hinders breathing, according to the new research, published April 4 in the journal Science .
"Without this barrier, asthma sufferers are far more likely to get long-term inflammation, wound healing, and infections that cause more attacks," Jody Rosenblatt , co-senior study author and a professor of cell biology at King's College London, said in a statement . That's partly because, without the barrier, allergens and irritants can reach places in the lungs that they might not otherwise be able to get to.
Related: Asthma drug omalizumab approved for severe food allergies
In their experiments, the researchers also found ways of stopping this chain reaction and keeping cells in their place in the lung tissue. This treatment approach "may have the capacity to break the inflammatory cycle and potentially revolutionize how asthma is treated," Dr. Jeffrey Drazen and Jeffrey Fredberg of the Harvard School of Public Health wrote in a commentary of the study .
Current treatments for asthma manage only its symptoms. For instance, the drug albuterol opens up the airways during an attack, while inhaled corticosteroids calm inflammation to reduce the chances of having an attack. The drugs don't prevent attacks by addressing their underlying causes.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Having a better understanding of how asthma attacks happen could lead to new therapies for the disease, Chris Brightling , co-senior study author and a professor of respiratory medicine at the University of Leicester in the U.K., said in the statement.
To gain this understanding, Brightling and colleagues mimicked asthma attacks in the lab by treating lung tissue from mice with a chemical that triggers constriction in muscle cells that line the airways. Under the microscope, they saw that this constriction causes epithelial cells to be squeezed out of place and die, and this triggered the characteristic inflammation and mucus secretion that occurs during asthma attacks.
These features were also seen in tissue samples from human patients with asthma who had been treated with corticosteroids. The finding reinforced the idea that cell squeezing underlies the pathology of the disease.
In previous work , the team discovered that a colorless fluid called gadolinium , which is normally used to improve the clarity of magnetic resonance imaging (MRI) scans, could stop epithelial cells from being squished out of place.
In the new study, they found gadolinium was able to prevent epithelial cell damage, inflammation and mucus secretion in the mouse lung tissue, suggesting that it could be a new treatment option for asthma in people.
However, more research is needed to translate these findings into new-and-improved asthma treatments for the many people with the disorder.
Around 1 in 12 people in the U.S. have asthma. Common symptoms of the chronic condition include coughing, chest tightness, wheezing and shortness of breath. Asthma attacks occur when these symptoms suddenly worsen or become severe, which can be life-threatening.
— How can air purifiers help with asthma?
— China's respiratory outbreak is 'expected' and not caused by new virus
— A potentially deadly, irreversible lung disease is striking workers who make popular quartz countertops
In addition, this newfound knowledge could also one day lead to new treatments for other inflammatory diseases that may be partly caused by excessive muscle constriction, such as irritable bowel syndrome (IBS) or inflammatory bowel disease (IBD), the authors wrote in the paper.
Drazen and Fredberg agreed, writing that "such a mechanism helps to paint a more complete picture of asthma pathobiology and may be relevant to other conditions, such as irritable bowel syndrome, in which epithelial cells are subject to disruptive mechanical forces."
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun ? Send us your questions about how the human body works to [email protected] with the subject line "Health Desk Q," and you may see your question answered on the website!

Emily is a health news writer based in London, United Kingdom. She holds a bachelor's degree in biology from Durham University and a master's degree in clinical and therapeutic neuroscience from Oxford University. She has worked in science communication, medical writing and as a local news reporter while undertaking journalism training. In 2018, she was named one of MHP Communications' 30 journalists to watch under 30. ( [email protected] )
Man in critical condition after catching deadly 'B virus' from wild monkeys in Hong Kong
Bite from toilet rat hospitalizes man in Canada
Underwater mountain range off Easter Island hosts creatures unknown to science, expedition reveals
Most Popular
By Elise Poore April 10, 2024
By Harry Baker April 10, 2024
By Tom Metcalfe April 10, 2024
By Sascha Pare April 10, 2024
By Peter Ray Allison April 10, 2024
By Tom Metcalfe April 09, 2024
By Rebecca Sohn April 09, 2024
By Stephanie Pappas April 09, 2024
By Samantha Mathewson April 09, 2024
By Nicoletta Lanese April 09, 2024
By Sascha Pare April 09, 2024
- 2 Giant coyote killed in southern Michigan turns out to be a gray wolf — despite the species vanishing from region 100 years ago
- 3 When is the next total solar eclipse after 2024 in North America?
- 4 Neolithic women in Europe were tied up and buried alive in ritual sacrifices, study suggests
- 5 James Webb telescope confirms there is something seriously wrong with our understanding of the universe
- 2 No, you didn't see a solar flare during the total eclipse — but you may have seen something just as special
- 3 Pet fox with 'deep relationship with the hunter-gatherer society' buried 1,500 years ago in Argentina
- 4 Eclipse from space: See the moon's shadow race across North America at 1,500 mph in epic satellite footage
- 5 Superfast drone fitted with new 'rotating detonation rocket engine' approaches the speed of sound
- OU Homepage
- The University of Oklahoma
OU Research Contributes to National Conversation on Neuropsychiatric Side Effects in Children Taking Asthma Drug

Study sheds light on effectiveness of black box warning for Singulair
TULSA, OKLA. – A University of Oklahoma study about a “black box warning” for the asthma drug Singulair continues to influence a national conversation about the medication and its reported neuropsychiatric side effects in children and adolescents. The U.S. Food and Drug Administration assigns black box warnings, sometimes called boxed warnings, as the highest safety-related consumer warning the organization assigns to medications, intended to bring attention to the risks of taking the medication.
In 1998, the FDA approved Singulair, known as montelukast in its generic form, for treating asthma and hay fever. It became a frequently prescribed drug for children struggling with asthma and allergies because it is available in a cherry-flavored chewable pill and may decrease the need for steroids or daily inhaler use. However, in the 26 years since its approval, concerned parents have sounded the alarm about dramatic and sometimes deadly mental health changes they have seen in their children, prompting the FDA to issue a black box warning in 2020.
In their study, researchers at OU-TU School of Community Medicine on the University of Oklahoma’s Tulsa campus sought to understand whether Singulair’s reported negative side effects – including depression, aggression and suicidal thoughts – decreased after the black box warning. Researchers analyzed adverse events reported to the FDA two years before and two years after the warning was issued. In children ages one to 10, reports about most harmful side effects decreased after the warning was issued. However, for youth ages 11 to 17, the outcome was mixed. Reports about side effects actually increased for five of the eight mental health symptoms. Overall, prescriptions for Singulair have decreased only slightly since the black box warning was issued.
In February, the New York attorney general cited the OU findings among other research in a letter to the FDA urging the agency to sound a new, louder alarm about the negative side effects of Singulair in children. Families, too, continue to push for more restrictions on the drug.
A limitation of the study is that the black box warning was issued at the beginning of the COVID-19 pandemic, which had its own effect on the mental health of youth. But for OU researchers, their study
underscores the importance of conversations between doctors and families, said the study’s lead author, Samer Abdelkader, D.O., a pediatrics resident physician in the OU-TU School of Community Medicine.
“As a clinician interested in public health, I hope we can maximize the intent of these warnings and mitigate potential negative impacts on our patients,” Abdelkader said. “I think we can enhance our patient care with better conversations about the benefits and risks of this medication and come to a more informed decision on whether this is the right treatment for each patient.”
Asthma is the most common chronic disease in children, and hay fever (also known as allergic rhinitis) affects the lives of one in five young people, according to the Centers for Disease Control and Prevention. Singulair treats the inflammation and airway swelling that can be dangerous in both conditions. The FDA approved the drug for treating asthma in children as young as 12 months old and for allergies in babies as young as six months old. It is prescribed to millions of children and adolescents each year.
Parents were the first to raise awareness about mental changes they saw in their children who were taking Singulair. Aggression, anxiety, depression, hyperactivity, sleep problems and suicidal thinking were among the symptoms, and several high-profile suicides brought further attention to the drug’s reported problems. Without parents’ advocacy, issues with the drug likely would not have come to light as soon, said Amy Hendrix-Dicken, a senior research assistant and co-author of the paper.
“Parents are the greatest partners in providing health care to children,” she said. “Parents know their kids; our health care providers see them for 15 minutes, and there’s only so much you can glean from a 15-minute visit. We encourage families and patients to speak up, and if they feel like their provider isn’t listening, to find someone who will.”
New information like the black box warning can take a long time to make its way into doctors’ offices large and small, and federal agencies may communicate potential drug problems in differing ways. But health care providers should take the initiative to learn everything they can about the drugs they prescribe, the study’s authors said.
“I no longer say that a side effect cannot be from a medication,” said study co-author and pharmacist Michelle Condren, a professor and vice chair of research in the OU Department of Pediatrics. “I may say, ‘I haven’t seen this side effect before’ or ‘Let me look in the medical literature,’ but I’ll never say that a side effect isn’t possible. This has changed the way I communicate with patients and families. We never want to discount someone who is concerned that a medication could be causing a side effect, but to partner with them to figure things out.”
About the Project
The research team’s study was published in The Journal of Pediatric Pharmacology and Therapeutics and can be accessed at https://doi.org/10.5863/1551-6776-28.8.704.
About the University of Oklahoma
Founded in 1890, the University of Oklahoma is a public research university located in Norman, Oklahoma. As the state’s flagship university, OU serves the educational, cultural, economic and health care needs of the state, region and nation. OU was named the state’s highest-ranking university in U.S. News & World Report’s most recent Best Colleges list . For more information about the university, visit ou.edu .
More Research News
Researchers discover cell ‘crosstalk’ that triggers cancer cachexia.
New research from the University of Oklahoma reveals a previously unknown chain of events sparking the development of cancer cachexia, a debilitating muscle-wasting condition that almost always occurs in people diagnosed with pancreatic cancer. The research, led by Min Li, Ph.D., a professor in the OU College of Medicine, is published in the journal Cancer Cell.

OU Researcher Receives $3.1M Grant for Clean Hydrogen Technologies
Hanping Ding, Ph.D., an assistant professor in the School of Aerospace and Mechanical Engineering at the University of Oklahoma, has been awarded a $3.1 million grant from the Hydrogen and Fuel Cell Technologies Office in the Department of Energy through the Bipartisan Infrastructure Law to further research in clean hydrogen production. The funding is part of a $750 million effort in President Biden’s Investing in American agenda.

Genetic Testing May Provide Improved Medication Therapy Safety and Efficacy for Pediatric Cystic Fibrosis Patients
Second-year University of Oklahoma pediatric resident Dr. Caroline Thompson has a professional and personal connection to cystic fibrosis patient care for children. A childhood friend had the disease and made a lasting impression on Thompson. Now, Thompson has received the Cystic Fibrosis Foundation’s Medical Resident Research Award for a pilot study evaluating pharmacogenomic-directed therapy for pediatric patients at the Oklahoma Cystic Fibrosis Center Tulsa.

More OU News

- Accessibility
- Sustainability
- OU Job Search
- Legal Notices
- Resources and Offices
- OU Report It!
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Springer Nature - PMC COVID-19 Collection

Asthma: Pathophysiology and Diagnosis
Susie yim yeh.
Harvard Program in Pulmonary and Critical Care Medicine, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Boston, MA 02215 USA
Although asthma is a common disorder affecting approximately 7.8% of the United States population (Schiller et al. 2006) or 23 million Americans, the pathogenesis of this disease remains to be fully elucidated. Extensive research over the last few decades has yielded a better understanding of asthma. We know that the basic features of asthma include episodic airways inflammation, airways hyperresponsiveness, and mucous hypersecretion. Although we understand the basic clinical features of asthma, the links between symptoms, physical signs, and underlying pathophysiological mechanisms are still being delineated. Asthma is a heterogeneous disease process with varying phenotypes and presentations. In this chapter, we will briefly explore some major theories of asthma pathogenesis, both new and old. We will also explore how understanding the pathophysiology of asthma can help us to understand the symptoms and presentation of asthma, as well as the best strategies for diagnosing this disease.
Introduction
Although asthma is a common disorder affecting approximately 7.8% of the United States population (Schiller et al. 2006 ) or 23 million Americans, the pathogenesis of this disease remains to be fully elucidated. Extensive research over the last few decades has yielded a better understanding of asthma. We know that the basic features of asthma include episodic airways inflammation, airways hyperresponsiveness, and mucous hypersecretion. Although we understand the basic clinical features of asthma, the links between symptoms, physical signs, and underlying pathophysiological mechanisms are still being delineated. Asthma is a heterogeneous disease process with varying phenotypes and presentations. In this chapter, we will briefly explore some major theories of asthma pathogenesis, both new and old. We will also explore how understanding the pathophysiology of asthma can help us to understand the symptoms and presentation of asthma, as well as the best strategies for diagnosing this disease.
Pathology and Histology
What do the lungs look like in asthma.
The autopsies of patients who have died of asthma gave researchers the first clues as to the possible etiology of this disease. Although there have been many advances in the treatment of asthma, death from this airways disease is still an unfortunate outcome in a minority of patients. Before describing the abnormal features of the asthmatic airway, we must first briefly describe the basic features of the normal airway.
Asthma is thought to be a disease of the small airways. If one thinks of the lungs as a series of tubes that continues to divide, the tubes get smaller and smaller until they end in small air sacks (called alveoli), where the exchange of gas occurs. The characteristics of the larger tubes as compared to the smaller tubes are very different. In the lung, the larger tubes such as the trachea and main bronchus are supported by both cartilaginous rings and smooth muscle. However, as the tubes get smaller, these cartilaginous rings disappear and only a layer of smooth muscle remains (Fig. 2.1 ). These smaller tubes are called bronchi and bronchioles. Without the support of cartilage, when smooth muscle contracts, the airways become increasingly narrow. Smooth muscle surrounds other tubular structures in the human body, such as arteries, where smooth muscle contraction dictates the flow of blood to vital organs. Similarly, in the lungs, contraction of smooth muscle in the bronchioles determines the air flow. The cross-sectional area of all the bronchioles is much larger than the cross-sectional area of the biggest airway. Therefore, contraction of smooth muscle can greatly increase airway resistance and diminish the flow of air into the lungs by decreasing size of the small airways.

The basic structure of the respiratory bronchiole. The bronchiole differs from the larger airways in that it is surrounded only by smooth muscle without cartilage support. Also note the cells lining the airways consist of mucous secreting cells
The cells that line the respiratory tract are known as the respiratory epithelium . These cells vary in appearance and function. Some cells have hair-like structures (cilia), while other cells produce mucous. Beneath these cells lie connective tissue and more glands that secrete mucous. In the trachea, cartilage, and smooth muscle is present beneath these glands. As the airways become increasingly smaller, the amount of cartilage starts to decrease and smooth muscle becomes more prominent. In the smallest airways, such as the bronchioles, there is no longer any cartilage. The connective tissue and glands decrease and smooth muscle lies beneath the respiratory epithelium. There are also many small blood vessels that lie beneath the airway supplying nutrients to both the respiratory epithelium and smooth muscle cells. In asthma, these blood vessels can become leaky, allowing the infiltration of inflammatory cells and fluid, which can cause edema.
Asthma, at its core, is an inflammatory disease. In response to a variety of stimuli, some in the environment such as allergens, and some reflecting changes within the body as occurs with exercise, a cascade of reactions that we characterize as inflammation is triggered. Autopsies of patients with fatal asthma have shown many derangements consistent with inflammation in the structure of the airways. In addition, mucous plugs fill the airways. The cells that produce mucus appear larger and are more numerous than in patients without asthma. The bronchioles also appear edematous with an increased number of inflammatory cells (such as eosinophils, neutrophils, and mast cells) that infiltrate the airways. The connective tissue is thickened and the respiratory epithelium is denuded. In addition, the amount of smooth muscle that surrounds the airways is increased (Fig. 2.2 ); whether this is due to muscle contraction and hypertrophy or is another process secondary to inflammation is still up for debate. It was thought that these dramatic changes were specific to patients with fatal asthma; bronchoscopic biopsies of patients with mild asthma, however, have demonstrated some of the same features. Although these findings can be patchy, biopsies of patients with mild to moderate asthma have shown a significant amount of inflammation as demonstrated by denuded epithelium, thickened basement membrane, and infiltration of inflammatory cells including mast cells, lymphocytes, and eosinophils (Busse and Lemanske 2001 ).

Schematic of the respiratory bronchiole during an asthma attack. The airway is lined by the respiratory epithelium which is made of ciliated and mucous producing cells. These mucous producing cells increase mucous production. Mucous then plugs up the airway, making it harder for the asthmatic to breathe. Underneath the respiratory epithelium lies a layer of smooth muscle. When the smooth muscle contracts, the airway becomes smaller, decreasing airflow
Another hallmark of asthma is that it represents a potentially reversible disease process. Between asthma attacks or during mild attacks, the airways can appear normal (Barrios et al. 2006 ). If asthma continues to progress, however, these changes become more permanent. This process is termed airway remodeling , and is thought to be due to persistent airway inflammation (Holgate et al. 1999 ). Patients with airway remodeling have thickened airway walls, with an increase in the amount of tissue directly under the respiratory epithelium, and larger smooth muscle mass (Busse and Lemanske 2001 ). Once remodeling has occurred, the medications used to reverse obstruction of the airways become less effective and symptoms may be more chronic.
Pathogenesis
As mentioned earlier, the three basic features of asthma are airways inflammation, airways hyperresponsiveness, and mucous hypersecretion. These three features lead to bronchoconstriction and airflow obstruction, which manifest as wheezing and dyspnea in the patient with asthma. The challenge for most researchers has been to uncover triggers of airways inflammation in the patient with asthma. Several theories have emerged such as the TH2 hypothesis, the Hygiene hypothesis, the infectious causes hypothesis, and the Dutch hypothesis. What these theories have in common is that in the susceptible individual, there is an exuberant immune response after exposure to a substance whether it be an allergen, a virus, or something else. This increased immune response leads to airways inflammation and bronchoconstriction. Why this occurs is still debatable.
Allergy and the Immune System
Many researchers have tried to identify the main causes of airways inflammation. Abnormalities of the immune system, which protects our bodies from infection, have been thought to be major contributors to the development of asthma. More specifically, allergic responses have been considered to be the main determinants of the asthma phenotype. Extensive research over the years, however, has shown that there are different phenotypes of asthma and not all are mediated by allergies. Even so, we will first explore the allergy-driven TH2 hypothesis before describing some of the other theories of asthma pathogenesis.
The immune system is an intricate and complicated structure, the details of which are too complex to explore here. However, to understand asthma, one must have some understanding of how the immune system works. In order to fight infection, the human body has developed a complex system to identify foreign intruders and to “remember” them in case of further invasions. This is called the adaptive immune response . That way, the body can be ready immediately for the next attack. Yet to exist in this world, the immune system cannot recognize everything foreign as being dangerous or else we would not be able to smell flowers or eat food without coughing, sneezing, or developing fevers. Life would be unbearable. The immune system, therefore, has developed a way to distinguish between benign and malignant foreign particles or antigens . There are times, however, for unclear reasons, when the immune system recognizes benign antigens such as dust, animal dander, or food as being “dangerous.” When this occurs, we say that the person has an “allergy.” When the human body develops allergies, the bronchospasm, cough, and wheeze that develop is an exaggerated response to a benign particle.
What are the steps involved from being exposed to a piece of dust to developing wheezing? It is clear that this process does not happen to everyone and that only susceptible individuals have this problem. Over the last several decades there have been several basic immune mechanisms described including antibody-mediated and cell-mediated immunity, that are thought to be responsible for airways inflammation and obstruction in response to an allergic stimulus.
Antibody-Mediated Immunity
One of the most important immune cells is called the lymphocyte . These cells are the building blocks of the immune system. There are two types of lymphocytes, the B-cell and the T-cell . When activated, some B-cells differentiate into plasma cells which then produce antibodies that are released in the blood. When an antibody recognizes a foreign pathogen or antigen , the antibody attaches to the antigen and neutralizes it. In allergic diseases, a benign particle, or allergen , acts as an antigen. Another immune cell called the macrophage , then recognizes the antibody–antigen or antibody–allergen complex, absorbs the complex and destroys it. The human body makes several different types of antibodies that have slightly different functions. They are subdivided in to five classes of isotypes called IgA, IgM, IgG, IgD, and IgE. The antibody most important to asthma is the IgE isotype. IgE differs from the other isotypes in that instead of circulating freely in the blood and extracellular fluid, IgE is bound to mast cells . Mast cells reside in the airways and are loaded with enzymes that are released once the mast cell is stimulated by the IgE–allergen complex. In developing countries, the IgE-mediated immune response is important in fighting and killing parasites. However, in developed countries, IgE-mediated immune responses are most responsible for allergic reactions.
The immediate hypersensitivity response is IgE-mediated and is one of the most important causes of asthma. When IgE recognizes an antigen (or in this case allergen ), a cascade of events occur that cause the degranulation and release of toxic inflammatory molecules from these mast cells (including proteolytic enzymes and histamine), which were meant to destroy foreign intruders (Wills-Karp 1999 ). Even when no such intruders are present, these toxic molecules cause the airways to become inflamed. The toxic molecules attract more immune cells to the area, thereby worsening the inflammation. Blood vessels become engorged and leaky, thereby allowing cells to migrate out of the blood stream and into the tissues. In asthma, the mast cells attract white blood cells called eosinophils to the area. They also initiate the production of inflammatory chemicals, leukotrienes , that are important in asthma. Leukotrienes have been implicated in inducing airway hyperresponsiveness, eosinophilia, and mucous hypersecretion (Bochner and Busse 2005 ).
Asthmatics typically have two phases during an asthma attack, the early and late response. It is thought that when the allergen activates IgE and mast cells, the histamine, leukotrienes, and cytokines released cause immediate constriction of smooth muscles that can resolve in approximately 1 h. However, 4–6 h later, another bout of airways obstruction can occur. This late reaction is thought to be due to different cytokines that are being released by the mast cells, eosinophils, macrophages, and lymphocytes (Busse and Lemanske 2001 ). The late response is responsible for prolonged asthma attacks.
Cell-Mediated Immunity
The T-cell differs from B-cells in the kind of antigen to which they respond. B-cell antibodies identify whole molecules. T-cells, on the other hand, do not rely on antibodies but rather develop receptors that recognize small pieces of a molecule. This makes it easier to recognize and destroy very small particles such as viruses. T-cells also differ from B-cells in their diversity. There are several different types of T-cells called cytotoxic T-cells, type 1 helper T-cell (TH1 cells) and the type 2 helper T-cell (TH2 cells). The roles of all these different T-cell types are too involved to explain here. In general the TH1 and TH2 cells differ in the types of immune reactions that they promote. In asthma, the TH2 cells often recognize the same allergens as B-cell antibodies do and help to activate the B-cell. The cytokines that the TH2 cell secretes to “help” the B-cell often contribute to the development of airways inflammation in the patient with asthma.
The TH2 cell does not rely on IgE antibodies but instead recognizes the allergen directly through its own receptor. The TH2 cell then activates and releases the cytokines to attract and activate more immune cells. This process was discovered when it was recognized that antibody-deficient mice (who do not make IgE molecules) were able to develop asthma (Corry et al. 1998 ). In this scenario, when TH2 cells are activated, the release of cytokines act directly on airway smooth muscle to induce airway bronchospasm (Corry et al. 1998 ; Wills-Karp et al. 1998 ). These cytokines also increase mucous secretions, airway inflammation and eosinophilia in the same way that leukotrienes do, but through a different mechanism.
To further complicate matters, the cytokines (such as IL-4) released by the TH2 cells also contribute indirectly to the immediate hypersensitivity response. IL4 is a key player in mast cell maturation (Madden et al. 1991 ), IgE secretion (Finkelman et al. 1988 ) and eosinophil recruitment to the lung (Corry et al. 1998 ). These immune responses, therefore, potentiate each other, showing how asthma can be the result of several different simultaneous processes.
TH2 Hypothesis
The observation that TH1 and TH2 cells promote different types of immunity generated the idea that perhaps one type of immunity is dominant in a particular individual. Specifically, that in one person, the TH1 cell-mediated immunity could be more active than the TH2 cell-mediated immunity. Because TH2 cell-mediated immunity has been associated with allergen-induced inflammation, it was thought that individuals who had predominantly TH2 cell-mediated immunity would be more prone to asthma and allergy. This is the basis for the TH2 hypothesis.
Further research has suggested that TH1 and TH2 cells regulate each other. For example if the TH2 cell is more active, it will release chemicals to suppress the TH1 cell and vice versa. When tested in the lab, chemicals from TH1 cells were found to decrease production of TH2 cells (Scott 1991 ). The question then becomes, what determines which TH cell mediated immunity dominates in an individual?
Hygiene Hypothesis
As the TH2 hypothesis gained popularity, the idea that the environment may determine which TH response dominates in a particular individual began to emerge. Exposures to certain pathogens or allergens at a young age (or even during the neonatal period) could determine if a person would have a TH1 or a TH2-mediated immunity (Table 2.1 ). Furthermore, if a person had a predominantly TH2-mediated immunity, then that person would be more susceptible to allergic diseases and/or asthma. This hypothesis has been dubbed the “hygiene hypothesis.” However, the idea that immunity is either TH1 or TH2 mediated is too simplistic as evidence has shown there is a complicated interaction between these two that is still being explored. That being said, we will explore briefly the hygiene hypothesis and the rationale behind this intriguing idea.
Factors promoting TH1 and TH2 phenotype
Asthma is more common in Western countries (The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee 1998 ), suggesting there may be an environmental reason for the increased prevalence of the disease in these areas. The term “hygiene hypothesis” alludes to the idea that perhaps it is the decreased exposure to infections and allergens in the Western world that promotes TH2-mediated immunity. Furthermore, use of antibiotics has been associated with increased risk of asthma perhaps by decreasing exposure to infections that would promote the TH1 mediated immunity (Cohet et al. 2004 ; Droste et al. 2000 ). Interestingly, asthma is more prevalent in urban settings when compared to rural or farm settings (von Mutius 2000 ). Intense epidemiological research has looked at why this may be true. Several studies have looked at how exposure to endotoxin early in life could affect development of wheezing and asthma. Endotoxin, a component of (gram-negative) bacterial cell walls, can induce inflammation and cause bronchoconstriction when inhaled by asthmatics (Michel et al. 1989 ). Interestingly, endotoxin promotes TH1-mediated response and has been found to increase production of TH1-related cytokines (D’Andrea et al. 1992 ; Gereda et al. 2000 ; Lapa e Silva et al. 2000 ; Le et al. 1986 ). It appears that endotoxin is more abundant in farm settings, likely due to increased exposure to livestock, than in nonfarm settings (von Mutius et al. 2000 ). Although in decreased quantities, endotoxin can also be found in common household dust. Researchers have asked whether it is the exposure to endotoxin that predicts the development of asthma thereby explaining the differences in asthma prevalence between urban and rural/farm settings.
Litonjua et al. ( 2002 ) studied children from Boston, MA who were less than 5 years old. The results of this study, which was conducted over 4 years, showed that children who were exposed to higher endotoxin levels initially had increased wheezing during the first year of life. However, as the children became older, they had a progressive decline in wheezing. By age 5–9 years, children who had higher endotoxin exposure had less wheezing when compared to children who had lower endotoxin exposure. This paradoxical relationship whereby increased endotoxin exposure increases risk of wheezing early in life, but decreases risk of wheezing later in life, suggests that exposure to endotoxin may have “protective” effects. By enhancing TH1-mediated immunity, endotoxin exposure may decrease the development of asthma and/or allergy in susceptible individuals.
When studying other exposures that may enhance the TH1-mediated response it has been shown that previous exposure to Mycobacterium tuberculosis , hepatitis A, Toxoplasma gondii , Herpes Simplex 1 and the common cold have been associated with decreased risk of allergy or asthma. Viruses and bacteria activate cell-mediated immunity (TH1 response). The German Multicenter Allergy Study studied children from birth to 7 years of age and found that children who had more colds with a runny nose had less wheezing (Illi et al. 2001 ). Similarly, the Tucson Children’s Respiratory Study, which followed children from birth, found that children who had more siblings or attended daycare from an early age were more likely to have wheezing at age 2 but increasingly less likely to have asthma as they became older (at age 6, 8, 11, and 13) (Ball et al. 2000 ).
The hygiene hypothesis, however, has remained very controversial. As stated earlier, the simple TH1 versus TH2 model does not hold true in many instances. For example, in rural Africa where parasitic diseases are common, infection with certain parasites ( Shistosoma species) (van den Biggelaar et al. 2000 ) and Ascaris hook-worm (Scrivener et al. 2001 ) was associated with decreased prevalence of asthma and allergy. Parasitic diseases activate the TH2 response and require IgE to fight off these infections. Therefore, one might think that factors favoring the TH2 phenotype would increase the incidence of asthma and allergy. However, on closer inspection, it is thought that another factor may be “bypassing” the TH2 response in parasitic diseases. Another group of T-cells called regulatory T-cells that produce a cytokine called Interleukin-10 (IL-10), may be increasingly active during parasitic infections. It is thought that these regulatory T-cells can override the TH2 response. In a mouse experiment, injection with IL-10 producing T-cells decreased the allergic response in these animals (Cottrez et al. 2000 ). In other experiments, IL-10 in combination with IL-4 caused B lymphocytes to produce IgG instead of IgE (Jeannin et al. 1998 ). This line of research is promising in further clarifying the immune responses that are contributing to the asthma and allergy phenotype.
Lastly, the hygiene hypothesis does not explain the cause-effect relationships that occur later in life. In other words, once an individual has established an allergic response, repeated exposures do not decrease this response. In the endotoxin example, individuals with established asthma have increased airways inflammation, bronchoconstriction, and susceptibility to viral illnesses when exposed to endotoxin (Reed and Milton 2001 ). Endotoxin exposure is a common cause of asthma in the workplace and repeated exposures in asthmatic individuals leads to chronic bronchitis and emphysema (Reed and Milton 2001 ). Instead of mitigating the allergic reaction, repeated exposures to endotoxin in the person who already has asthma causes worsening disease. This example suggests that the hygiene hypothesis may only be relevant early in life and cannot be extrapolated to the adult setting.
Viral/Bacterial Infections
Since the 1970s there has been a well-established relationship between asthma and respiratory tract infections (Blasi et al. 2001 ). Many patients with asthma have worsening of their symptoms in the setting of a respiratory infection. In children, studies have shown up to 45% of asthma exacerbations are related to respiratory infections (Mertsola et al. 1991 ). Likewise, in adults, up to 37% of asthma exacerbations were associated with respiratory infections (Teichtahl et al. 1997 ). However, whether these infections are involved in the etiology of asthma or the progression of disease has remained unclear. There is also interest in whether respiratory infections play a significant role in the TH1/TH2 or hygiene hypotheses as well. Although more commonly associated with viruses, several specific bacteria such as Chlamydia pneumoniae and Mycoplasma pneumoniae have been increasingly associated with asthma.
C. pneumoniae and M. pneumoniae are two common bacterial respiratory infections and are typically associated with pneumonia. C. pneumoniae is different from most other bacteria in that it must invade cells, such as respiratory epithelial cells and macrophages, in order to replicate. However, because C. pneumoniae does not have to destroy the cell that it invades; it can persist as a latent infection by allowing infected cells to proliferate. Latent infections can be quiescent without causing symptoms. If triggered, however, they can erupt into an acute infection. Cold sores, for example, are due to latent infection with Herpes Simplex virus that develops into an acute infection from time to time. C. pneumoniae has been implicated in acute exacerbations of asthma (Allegra et al. 1994 ) and chronic asthma (Black et al. 2000 ).
C. pneumoniae has been associated with asthma since the 1970s. Since then, efforts to quantify this association have been attempted. Several studies have measured antibodies against C. pneumoniae in the blood of asthma patients and found an increase in certain types of antibodies (IgA) to C. pneumoniae when compared to controls (Berkovich et al. 1970 ; Gencay et al. 2001 ; Huhti et al. 1974 ). Antibody studies, however, are difficult to interpret since the presence of antibodies does not confirm whether an infection is past, latent, or acute. It has been suggested that chronic infection with C. pneumoniae is more prevalent in asthmatics (Biscione et al. 2004 ; Gencay et al. 2001 ). When using methods to directly test for presence of the bacteria in nasal aspirates of asthmatics and their non-asthmatic spouses over a 2 month period, it was found that 22% of the asthmatics and 9% of the spouses had presence of the organism at least once during the study period (Biscione et al. 2004 ). However, it was still unclear as to whether the increase in positive tests for C. pneumoniae in asthmatics truly represents active infection or colonization.
It is logical to ask that if C. pneumoniae infection is associated with asthma, then does treatment with antibiotics improve symptoms and outcome? Unfortunately the results have been mixed. Several studies have shown that treatment of asthmatics with antibiotics for 6–8 weeks have shown decreases in eosinophil counts (Amayasu et al. 2000 ) and improvements in peak expiratory flows (PEF) (Black et al. 2001 ) but that the effect on pulmonary function tests were modest at best. Most recently, a double-blinded, randomized, placebo-controlled trial attempted to more accurately assess the effect of antibiotics (against C. pneumoniae and M. pneumoniae ) in the setting of an acute asthma exacerbation (Johnston et al. 2006 ). Patients with an acute asthma exacerbation were randomized to take placebo or an antibiotic for 10 days in addition to regular asthma treatment. Although asthma symptoms improved in the antibiotic group, there was no difference in PEF. Interestingly, 61% of the subjects studied had evidence of infection from either C. pneumoniae or M. pneumoniae or both but unfortunately there was no correlation between antibiotic response and history of infection in this study.
Results have been similar with M. pneumoniae , a common bacteria responsible for “atypical” or “walking” pneumonia. Like C. pneumoniae , it has been implicated in the etiology, progression, and clinical course of asthma, but treatment with antibiotics has not yielded significant improvements. It is the smallest free-living organism and is different from other bacteria in that it does not have a cell wall. It infects the respiratory epithelium and disables the ciliated cells responsible for clearing mucus and foreign particles from the airways. Like C. pneumoniae , M pneumoniae can persist as a chronic infection. Although it does not enter cells like C. pneumoniae , it can burrow between cells, evading host defenses and establishing residence in the airways.
Despite unclear results in small clinical trials assessing the effectiveness of treatment with antibiotics in asthmatics, there is still much interest in what role these bacterial infections play in the development of asthma. As discussed earlier, the hygiene hypothesis suggests that exposures to certain infections early in life may induce a TH1-mediated immunity resulting in a decreased propensity for asthma. However, once the allergic phenotype is established, recurrent exposures to a pathogen or allergen exacerbates the disease. Researchers looked at this phenomenon in an allergic-asthma mouse model. Chu and colleagues exposed mice to M. pneumoniae at different times and observed the response (Chu et al. 2003 ). When they infected the mice before exposure to an allergen, the mice had significantly less bronchial hyperresponsiveness, and lung inflammation and had increased production of cytokines associated with the TH1 response. Conversely, when they infected the mice after exposure to an allergen, they developed increased bronchial hyperresponsiveness, and lung inflammation, and produced cytokines associated with the TH2 response (IL-4). This line of research is very interesting and suggests that both C. pneumoniae and M. pneumoniae may have varying importance in the development and progression of asthma based on when the infection occurs.
Although we have been discussing the role of bacterial infections in asthma pathogenesis, viral infections have been implicated in the etiology of asthma as well. Viral infections during infancy have been associated with the development of asthma. This has been most convincing in studies of Respiratory Syncytial Virus (RSV). Most children are infected with RSV by 2 years of age (Simoes 1999 ) and many are hospitalized. RSV can cause respiratory distress, wheezing, and fever. It can cause a “bronchiolitis,” inflammation of the respiratory bronchioles described earlier. Many have observed that children who suffered from RSV bronchiolitis as an infant had a higher propensity to wheeze for years after the infection (Stein et al. 1999 ). Sigurs and colleagues (2005) studied a group of children who were hospitalized with RSV bronchiolitis as infants (<1 year old). They compared this group, which was followed until age 13, to a group of children who had never been hospitalized with RSV bronchiolitis. These researchers found that children in the RSV group had increased wheezing and airways obstruction. What was also interesting was that these children had increased allergies to common inhaled allergens. This research suggests there may be a relationship between early RSV infection and the development of asthma and allergies later on in life. However, other studies have shown that infants infected with RSV “outgrew” their wheezing and did not go on to develop asthma in adolescence (Taussig et al. 2003 ). Whether RSV is merely a risk factor for asthma or is a causative agent in asthma pathogenesis remains unclear.
In addition to being implicated in asthma pathogenesis, viral infections are commonly associated with asthma exacerbations (Venarske et al. 2006 ). Often, when a person develops an asthma attack, there is usually an inciting factor or “trigger” associated with the attack. For many asthmatics, the common cold can precipitate an attack. In fact, viruses have been associated with up to 85% of asthma exacerbations in children (Johnston et al. 1995 ) and 60% of exacerbations in adults (Nicholson et al. 1993 ). It has been shown that during times when viral syndromes are “going around” there are increased admissions to area hospitals with asthma exacerbations (Johnston et al. 1996 ).
The reason why upper respiratory viruses have been associated with asthma exacerbations, however, has remained unclear. Rhinovirus , one of the viruses responsible for the common cold, has been most frequently associated with asthma exacerbations. One study found that infection with rhinovirus was associated with an increase in asthma-related hospitalizations (Venarske et al. 2006 ). Some have suggested that viruses may potentiate the inflammatory response to allergens causing bronchospasm and airways obstruction in asthma patients (Busse and Lemanske 2001 ; Calhoun et al. 1991 ). Others have proposed that asthma may cause abnormalities in the immune system that makes it harder to fight viral infections in the airway (Papi and Johnston 1999 ). The role of rhinovirus and other viral illnesses (such as influenza, parainfluenza, and coronavirus ) in causing or contributing to asthma exacerbations needs to be further clarified.
Dutch Hypothesis
Before delving into what the Dutch Hypothesis is, we must first briefly explain the differences between asthma and chronic obstructive pulmonary disease (COPD). As we have been discussing, asthma is characterized by reversible airflow obstruction, airways hyperresponsiveness, and increased mucous secretion. Typically, asthma does not cause progressive loss of lung function and the lung parenchyma itself remains intact. Usually, asthma presents in childhood or young adulthood. On the other hand, COPD, a term used to describe chronic bronchitis, emphysema, and a variety of less common conditions such as bronchiectasis, is commonly associated with smoking and presents in older adulthood. Even though COPD is also characterized by airflow obstruction, it is usually irreversible or only partially reversible. There is also progressive loss of lung function. Asthma and COPD are commonly thought of as distinctly different diseases. Asthma has been described as an inflammatory airway disease mediated by a dysregulated immune response (as described by the TH2 hypothesis). COPD, on the other hand, is thought to occur when destructive enzymes damage the lung in response to some inflammatory stimulus (i.e., cigarette smoke).
The Dutch Hypothesis was first proposed in the 1960s and is one of the older but still relevant theories on asthma/COPD pathogenesis. During that time, tuberculosis was the most common respiratory illness but as effective treatment for tuberculosis became available, Drs. Orie and Sluiter began to notice that obstructive lung diseases were very common with similar characteristics in both younger and older patients (Postma and Boezen 2004 ). They proposed, in the first Bronchitis Symposium held in Groningen, Netherlands, that obstructive airways diseases such as asthma, chronic bronchitis, and emphysema should be considered not as different diseases but as different manifestations of one disease entity, which they called chronic nonspecific lung disease (CNSLD) (Postma and Boezen 2004 ). They hypothesized that both genetic and environmental factors contribute to the pathogenesis of CNSLD and that it is the interaction between these two that determines what phenotype a person will develop. One example of an interaction between a person and his/her environment is smoking. Tobacco smoke has been highly associated with COPD. However, only 10% of smokers get COPD suggesting that there is a genetic propensity for a person to develop COPD in response to cigarette smoke. There has also been an association between passive smoke exposure and the development of asthma in children. According to the Dutch hypothesis, the time of tobacco smoke exposure, whether in childhood or adulthood, and the type of exposure, passive or active, determines if a person with genetic susceptibility develops the asthma or COPD phenotype.
The Dutch hypothesis, as it is now known as, has been controversial. Efforts to try and test this hypothesis have been flawed as study designs do not lend to testing a process that spans a lifetime. Also, current studies of asthma and COPD have had strict inclusion criteria that try to eliminate subjects who have aspects of both, which limits our ability to determine if the pathogenesis of the two is similar. Over the years, however, there has been some evidence to support the Dutch hypothesis. Clinically, there are populations of asthma patients who have loss of lung function similar to COPD (Jeffery 2000 ; Ulrik et al. 1995 ). Similarly, there are patients with COPD who have reversible airflow obstruction (Bousquet et al. 1996 ). These observations suggest that there is considerable overlap between asthma and COPD.
Other observations have contributed to the blurring between asthma and COPD. There is evidence to suggest that both conditions are secondary to lung inflammation. In the past, different types of inflammation were described in patients with asthma and COPD. In asthma, it was thought that the inflammatory process was confined to the airway and that in COPD, the inflammatory process was confined to the lung parenchyma. However, there have been some studies that have shown that there are inflammatory cells, such as eosinophils and neutrophils, within the lung tissue in some subjects with asthma (Kraft et al. 1996 ; Wenzel et al. 1999 ). Additionally, biopsies of COPD subjects have shown high numbers of eosinophils in the airways especially during acute exacerbations (Saetta et al. 1994 ). Asthma and COPD share histologic features suggesting that there is substantial overlap between these two disease processes.
There have been several other features of both diseases that suggest a common pathogenesis. For example, the airways of asthma and COPD patients are similar. Both have an increase in mucous secreting cells lining the airways. Increases in smooth muscle surround the airways, however, was thought to be unique to asthma. Recent studies have shown that there is also an increase in smooth muscle among COPD patients as well (Jeffery 2000 ). Finally, changes in the lung parenchyma itself have shown some similarity among asthma and COPD subjects. Typically, as already mentioned, asthma is thought to be strictly an airways disease that does not affect the lung parenchyma or alveoli. However, the destructive enzymes found in COPD lungs have also been found in biopsies of asthma lungs as well (Atkinson and Senior 2003 ; Bousquet et al. 1996 ).
Studies are ongoing to further assess whether asthma and COPD are two distinct diseases or different presentations of the same disease. Over the years, the popularity of the Dutch Hypothesis has waxed and waned. However, there has been growing scientific evidence to support this hypothesis, which highlights that asthma is indeed a complex and heterogeneous disease process.
Asthma Subtypes
Although the majority of asthma is initially triggered by allergies, there are several different phenotypes of asthma that have different characteristics from the common allergy-induced asthma. “Intrinsic asthma,” aspirin-induced asthma (AIA), and exercise-induced asthma (EIA) are a few asthma subtypes that have unique characteristics not readily associated with allergens. There are, however, several other subtypes of asthma, such as gastroesophageal reflux associated asthma, obesity-related asthma, menstrual cycle-related asthma, and nocturnal asthma that are also included in the asthma syndromes, but will not be discussed here.
Intrinsic Asthma
The term “intrinsic asthma” has been used to describe patients who suffer from asthma but do not have typical features of atopy or allergies. This is in contrast to the allergy-induced (“extrinsic”) asthma we have been discussing. Patients with “intrinsic” asthma do not have allergies, family histories of atopy, abnormal serum IgE levels, or hypersensitivity reactions to skin prick-tests. The clinical course of patients with intrinsic asthma differs as well. Usually, patients with intrinsic asthma tend to be older, have later onset of asthma, and more severe disease (Ulrik et al. 1995 ). For many years, it was believed that “intrinsic” asthma represented a different pathological process leading to asthma and that the distinction between “intrinsic” and “extrinsic” (allergy-induced) asthma was very apparent. More recently, however, the differences between “intrinsic” and “extrinsic” asthma have become less clear. In one study, lung biopsies of patients with extrinsic asthma were compared to patients with intrinsic asthma (Humbert et al. 1996 ). Both had similar inflammatory cells and cytokines present, suggesting that a similar process was occurring in both forms of asthma regardless of whether the patient had allergies or not. These findings have prompted researchers to view “intrinsic” asthma differently. Instead of thinking of “intrinsic” asthma as being different from “extrinsic” asthma, there may only be differences in the triggers leading to the same causative pathways for asthma (Humbert et al. 1999 ). For example, some have suggested that intrinsic asthma may be a form of autoimmunity, triggered by a respiratory viral illness. In other words, antibodies made to the initial viral illness may now be initiating a cascade of inflammation leading to asthma (Humbert et al. 1999 ). Others believe that patients with “intrinsic” asthma are actually allergic to something that researchers have not yet been able to identify (Humbert et al. 1999 ). The pathogenesis of “intrinsic” asthma has not been elucidated and may reflect a heterogeneous process rather than a single disease entity. Although similarities between “intrinsic” and “extrinsic” asthma exist, the concept that asthma does not necessarily represent a solely allergy-related disease is important and speaks to the complexity of asthma.
Aspirin-Induced Asthma
One could consider AIA a kind of “intrinsic” asthma for which we know the trigger. Aspirin is one of the most widely taken medications in the world. In the United States alone, over 80 billion tablets per year are consumed. As such, the recognition of AIA is important as AIA may represent 10–20% of the asthma population (Sturtevant 1999 ). The AIA syndrome usually includes a triad of symptoms: nasal polyps and nasal congestion, sinusitis, and asthma with chronic symptoms. Patients with AIA often have chronic severe asthma with acute symptoms triggered after ingestion of aspirin or a similar drug (such as ibuprofen). Many times, symptoms can begin within 3 h after ingestion of aspirin with a profuse runny nose, swollen eyes, and flushing of the face in addition to wheezing. Breathing can become severely impaired, requiring hospitalization, and can progress to respiratory failure.
Although symptoms begin shortly after exposure to aspirin, AIA is not an allergic reaction per se. Skin prick tests with aspirin are usually negative, indicating that an antibody to aspirin does not exist in patients with AIA (Babu and Salvi 2000 ). Instead, aspirin blocks enzymes and, by doing so, causes increased production of cytokines called leukotrienes. These leukotrienes, in turn, promote inflammation and asthma in the susceptible individual. AIA is an example of how different mechanisms can lead to asthma.
Exercise-Induced Asthma
Like AIA, EIA is also not allergen mediated. It is very common with reports of 40–90% of asthmatics affected (Bundgaard 1981 ; Tan and Spector 2002 ). Many asthmatics experience increased airways resistance during exercise. Because of the dyspnea experienced during exercise, many patients with asthma often do not pursue aerobic activities as much as their non-asthmatic counterparts and are less fit as a result (Garfinkel et al. 1992 ). EIA, therefore, is important to recognize and treat so that patients with asthma can become more involved in exercise. Patients with asthma often feel better when they are physically fit (Ram et al. 2005 ). It is also thought that EIA may be triggered by moving large amounts of air in and out of the lungs. If asthmatics are more fit, they may breathe less heavily with mild to moderate exercise thereby decreasing the triggers for EIA (Ram et al. 2005 ).
The mechanism for EIA is debated. Two of the most common theories are the osmotic hypothesis and the thermal hypothesis . The thermal hypothesis suggests that bronchoconstriction during exercise is due to changes in temperature and water content of the airways (McFadden and Gilbert 1994 ). As large volumes of air move in and out of the lungs, the airways warm and humidify that air (also known as conditioning). Although the airways warm and heat air continuously (regardless of whether we are exercising or not) when we are quietly breathing with low tidal volumes, only a portion of the airways heat and humidify the air. During exercise, however, ventilation can increase by a factor of 20. As ventilation increases, the conditioning of air moves from the upper airways to the lower airways where more movement of heat and water from the airway cells is required to heat and humidify the air (McFadden and Gilbert 1994 ). When exercise stops and ventilation decreases, the airways rewarm quickly as they are no longer losing heat and water to the air. This cycle of cooling and rewarming is associated with airway narrowing and bronchoconstriction. Breathing warm humidified air ameliorates exercise-induced bronchoconstriction and breathing cold dry air worsens it (Bundgaard et al. 1982 ). It is not entirely clear why the airways narrow in response to rapid cooling and rewarming although increased blood flow and subsequent airway edema is thought to play a role (McFadden and Gilbert 1994 ; McFadden et al. 1986 ).
The osmotic hypothesis, on the other hand, suggests that airway dehydration during exercise causes a series of events leading to airway smooth muscle contraction and increased airways resistance (Anderson 1984 ). Proponents of the osmotic hypothesis argue that it is water loss, not changes in temperature that lead to bronchoconstriction. During exercise, large volumes of air move in and out of the lungs as respiratory rate and tidal volume increase. This movement of air is thought to cause evaporation of water in the airways. It is thought that the water loss causes an increase in osmolarity, which then triggers cells to release inflammatory chemicals, which in turn act on smooth muscle to contract. The loss of water in the lungs is also thought to cause an increase in blood flow to the lungs that can cause edema of the airways and even worsening airway constriction (Anderson and Daviskas 2000 ). Observations that EIA occurs when subjects breath gases of varying temperature but similar water content supports the osmotic hypothesis (Ingenito et al. 1988 ). Treatment of EIA usually consists of using a bronchodilator before exercise
Until now, we have discussed the pathogenesis of asthma and possible mechanisms for increased airways inflammation. This inflammation in turn leads to air flow obstruction and airway hyperresponsiveness. But what does this mean in terms of how asthma manifests clinically? How does this lead to symptoms of shortness of breath? What happens to respiratory physiology when asthma occurs?
The hallmark of asthma is reversible airways obstruction. As the airways become narrowed during an asthma attack, resistance of the airways increases and airflow into the lungs is diminished at the same level of respiratory effort. One could imagine this by comparing the difference between blowing into a large straw versus a small straw. If one blows the same volume of air through the large and small straw, it will take a significantly longer time to blow out all the air through the small straw because flow is greatly diminished. The lungs are more complex than the one straw system, however, as smaller and smaller branching airways have differing lengths, compliances, and different types of air flow (laminar and turbulent). Because of this, there comes a point when no matter how hard one blows, flow will not increase. This is called airflow limitation.
One of the most common complaints in patients with asthma is that they have difficulty breathing in. There are several reasons for this. With increased bronchoconstriction there is diminished air flow and increased airways resistance. In order to compensate for the increase in airways resistance, the inspiratory muscles must generate greater tension. Imagine that instead of blowing through the small straw, that one tries to breathe in through the small straw. The amount of effort required to take in a breath will increase. However, it turns out that the increased work of breathing associated with inhalation is complicated by a second factor, hyperinflation of the lungs and chest wall. Furthermore, inhalation is an active process; muscle activity is required. Exhalation, on the other hand, is typically passive during quiet breathing. The normal elastic properties of the lungs and chest wall push air out of the lungs during exhalation.
Now imagine trying to blow out through the small straw and then continue to breathe in and out through this small straw. Although one may not be aware of it, as one continues to breathe in and out through that small straw, a process called “dynamic hyperinflation” is occurring. In other words, because it takes longer to exhale out all the air when air flow is decreased, one may initiate the next breath before all the air is exhaled from the last breath. The volume of the lung and chest wall then increases. The next breath is even harder to take in because at higher lung volumes, the inspiratory muscles operate at a shorter length and are less able to generate tension. In addition, the compliance of the lungs and chest wall is reduced at higher lung volumes. This means that the respiratory system is stiffer and more work is required to take in a breath. You can try to experience this by taking a breath in before you have fully exhaled the last breath. When a group of patients with mild asthma were given medication to induce bronchoconstriction, hyperinflation was the greatest indicator of how short of breath they felt (Lougheed et al. 1993 ). Surprisingly, an increase in airways resistance did not correlate with how dyspneic the subjects felt. This points to the importance of hyperinflation as a cause of dyspnea in the asthmatic patient.
Hyperinflation can also induce “length-tension inappropriateness” another mechanism that may contribute to dyspnea in asthma. If tension is generated in the muscle but it does not shorten appropriately because of the mechanical load on the system (similar to when trying to lift a weight that is too heavy), there is a discrepancy between the tension generated in the muscle and the degree to which it shortens (Campbell and Howell 1963 ). This concept has been broadened to include discrepancies between the neurological output to the muscles and the mechanical response of the respiratory system (neuromechanical dissociation). If the inspiratory muscle force generated does not match the expected change in lung volume, feelings of breathlessness may occur (Campbell and Howell 1963 ). Hyperinflation contributes to neuromechanical dissociation in several ways. The hyperinflated lung places the respiratory muscles at a mechanical disadvantage making these muscles less effective in creating tension. Therefore, even though the brain is sending out a message to the respiratory muscles to contract, the force generated and the change in lung volume may not match what the brain expects, causing neuromechanical dissociation. Hyperinflation also creates an inspiratory load that the respiratory muscles have to overcome before flow into the lungs can occur. This phenomenon is called “auto PEEP” or positive end expiratory pressure. What this means is that if the lungs have residual air in them because one could not fully exhale, there is still positive pressure in the lungs at the end of the breath. Normally, exhalation is a passive process akin to letting air out of a balloon. When we exhale, the pressure in our lungs equilibrates to atmospheric pressure. If one does not fully exhale, however, there may be a few centimeters of H 2 O pressure left in the lungs before inhalation begins. The flow of air travels from areas of low pressure to high pressure. In auto PEEP, the inspiratory respiratory muscles must first overcome this pressure gradient to equilibrate to atmospheric pressure, and only after that can negative pressure be generated so that air can flow into the lungs. Thus, there is a period of time when the respiratory muscles are firing but no air is flowing into the lungs and, therefore, there is no change in lung volume. Imagine walking about while breathing through a mouthpiece that is connected to a valve that does not open until you generate a negative pressure of 5 or 7 cm H 2 O with your inspiratory muscles. It is not surprising that individuals with auto-PEEP complain of shortness of breath.
However, there are many patients with mild asthma who complain of chest tightness or difficulty breathing with only mild bronchoconstriction, levels of airways obstruction not associated with hyperinflation. These symptoms cannot be readily explained by increased work of breathing alone. Several studies have elucidated what may be occurring in this groups of patients. Taguchi et al. ( 1991 ) tested subjects by having them inhale a medication that causes bronchoconstriction and compared the respiratory sensation associated with an asthma-type reaction in the lungs to what the subjects felt when breathing through a high resistance (like our straw example). Although the degree of hyperinflation was the same in both conditions, subjects felt more short of breath when they were given a medication that caused bronchoconstriction. This sensation of shortness of breath was relieved when the subjects breathed in lidocaine (a topical anesthetic). This study suggests that there are nerve receptors in the lungs that contribute to the sensation of breathlessness during bronchoconstriction. Binks et al. ( 2002 ) tried to clarify further the mechanism behind the chest “tightness” often described by asthmatics during an attack. They gave patients inhaled medication to provoke bronchoconstriction. They then placed these patients on a mechanical ventilator thereby eliminating the effort required by the patient to inhale by having a machine breathe for them. Even though the patients felt like it required less effort to breathe on the ventilator, they still experienced the sensation of chest tightness. They then put subjects without bronchoconstriction on a mechanical ventilator and increased the end expiratory volume to mimic hyperinflation. Even though their lungs were hyperinflated, the subjects did not experience chest tightness. This experiment suggests that the feeling of chest tightness is separate from the effort of breathing during an asthma attack. Although the effort to breathe is related to bronchoconstriction and the resultant increased work of breathing, tightness may be caused by changes within the airway itself that lead to stimulation of pulmonary receptors, which may send messages to the brain creating the sensation of tightness.
Bronchoconstriction may also affect the delivery of oxygen into the lungs. If airflow to the lungs is diminished, it is hard to get air in and out and, therefore, the movement of oxygen into the lungs and carbon dioxide out of the lungs is impaired. The human body, however, has developed an interesting system to deal with changes in airflow and oxygen delivery to the lungs. The body has a tremendous ability to constrict blood flow to areas of the lung that have low oxygen levels. This phenomenon is called hypoxic vasoconstriction. In response to low oxygen levels in the lungs, the body will decrease flow of blood to these areas and divert blood to areas of the lung with normal oxygen levels. Because asthma is a heterogeneous disease process, some areas of the lung will experience inflammation and bronchoconstriction while other areas of the lung will be relatively normal. Therefore, the body usually can maintain adequate oxygen levels even in the face of mild to moderate asthma attacks.
Another mechanism that contributes to near normal oxygen levels during an asthma attack is hyperventilation. During a mild or moderate asthma attack, the patient will typically hyperventilate. Possible reasons for hyperventilation include stimulation of pulmonary receptors as well as behavioral factors (shortness of breath and anxiety can lead to hyperventilation). The rapid replacement of oxygen in the alveoli during hyperventilation helps to maintain normal oxygen levels in the blood.
In patients with fatal or near-fatal asthma, however, hypoxemia may blunt the sensation of dyspnea or uncomfortable breathing, making it more difficult for individuals to recognize the severity of their problem, thereby leading to a delay in seeking medical treatment. Although hypoxemia is not a common feature of asthma, the body’s attempts to divert blood to normal lung is insufficient when bronchoconstriction becomes severe. If there is little normal lung to which to divert blood, oxygen levels will start to decrease. In people without asthma (Chronos et al. 1988 ), or with other lung diseases such as COPD (Lane et al. 1987 ), hypoxemia itself can provoke shortness of breath. Unfortunately, when patients with asthma become hypoxemic, the ability to feel short of breath or chest tightness may diminish (Eckert et al. 2004 ).
Just as the pathogenesis of asthma is relatively complex, the signs and symptoms of asthma can be confusing as well. Asthma can present with a paucity or overabundance of symptoms, and can coexist with other illnesses. There are also many disease processes that can mimic asthma, thereby confusing health care providers. Finally, because asthma is an episodic disease, patients can have normal exams and pulmonary function tests between “attacks,” which makes diagnostic studies insensitive to the presence of asthma. According to the National Heart Lung and Blood Institute, the diagnosis of asthma should be considered in anyone who has episodic airways obstruction, reversible (or at least partially reversible) airways obstruction, and in whom other diagnoses have been excluded (Teichtahl et al. 1997 ). We will review the presenting symptoms of asthma, the role of diagnostic studies (such as pulmonary function tests, peak flow, and methacholine challenge tests (MCT)) and the conditions that may mimic asthma and which should be considered in difficult cases.
Many patients with asthma will initially present with wheezing, a high pitched sound usually heard during exhalation. As the airways narrow and airways resistance increases, there is more turbulent flow causing vibrations that we hear as a “wheeze.” Some have argued that the opening and closing of airways also contributes to this vibration. However, a lack of wheezing does not exclude the diagnosis of asthma. First, because asthma is an episodic disease, wheezing is not always present; patients who present to their health care provider during an asymptomatic period can have a completely normal exam. Second, the sound of wheezing actually decreases if airways resistance becomes severe. If airways resistance becomes so high that air flow is severely reduced, as in cases of extreme bronchoconstriction, turbulent flow can no longer be heard. Therefore, a patient who presents with a severe asthma attack can initially have wheezing that subsequently quiets down or stops. Instead of interpreting the lack of wheezing as an improvement in asthma, one must be vigilant that this does not signify a worsening of airways obstruction. Similarly, complete absence in breath sounds, or a “quiet chest” can also signify worsening airways obstruction and impending respiratory failure.
Some patients never develop wheezing as a symptom of asthma. There are many patients whose initial symptom is cough. This phenomenon has been termed “cough-variant” asthma. Gastroesophageal reflux disease (GERD), postnasal drip, and asthma are the three most common causes of chronic cough (Irwin et al. 1990 ). Because asthma is so common in the diagnosis of chronic cough, empiric treatment with bronchodilators (beta-agonists, which cause smooth muscle relaxation) is a common diagnostic test to evaluate if asthma is the cause of chronic cough. However, there is complex relationship between GERD and asthma. Acid reflux can cause bronchoconstriction through a neural reflex that leads to increased airways resistance. Postnasal drip also has many associations with asthma as both can be presentations of the allergic phenotype. Therefore, GERD, postnasal drip, and asthma often coexist and treatment of all three conditions may be needed to resolve chronic cough. Asthma, however, can present as cough alone and should be considered as a diagnosis in those individuals who present with chronic cough.
Dyspnea and shortness of breath are common symptoms of asthma (Table 2.2 ). Many respiratory diseases, however, present with feelings of dyspnea; distinguishing asthma from other diseases, such as COPD, can be difficult when based on symptoms of dyspnea alone. To complicate matters, the perception of dyspnea in patients with asthma is variable and does not necessarily correlate with objective measurements of lung function. Most concerning are those patients whose perception of dyspnea is “blunted” despite having severe airways obstruction as measured by the forced expiratory volume in 1 s (FEV1). Briefly, the FEV1 is the volume of air exhaled in the 1st second of a forced expiration after a maximal inhalation. In other words, it is the amount of air exhaled after the patient is asked to take a deep breath in and blow out as hard as she can. The FEV1 is reported as a percent predicted, when compared to patients of the same height, age, sex, and race. A reduction in FEV1 is associated with increased airways resistance in patients with asthma. Several studies have shown that patients with substantial airways resistance have minimal symptoms. Furthermore, symptoms in general do not correlate with objective measures of lung function (Foo and Sly 1991 ; Hewson et al. 1996 ; Molema et al. 1989 ; Teeter and Bleecker 1998 ). Asking whether dyspnea is present or absent or even asking about the intensity of dyspnea may not be specific enough to assess the presence or severity of asthma. What may be more useful is understanding the language of dyspnea. Different respiratory diseases have distinct characteristics to their shortness of breath. This is not unlike cardiovascular disease and chest pain. Over the years, we have come to recognize different representations of ischemic chest pain and that not all patients present with the typical left-sided chest pain. We have now come to recognize jaw pain, arm numbness, indigestion, belching, and chest pressure as anginal equivalents. Similarly, dyspnea has many diverse characteristics and varying presentations.
Common symptoms of asthma
Researchers have compiled a group of phrases used to describe shortness of breath by patients with different lung and heart diseases that are listed in Table 2.3 (Simon et al. 1990 ). They found that patients experiencing an asthma attack chose phrases describing increased “work/effort” and “tightness” when asked to describe their dyspnea (Mahler et al. 1996 ). Further research has tried to assess the use of specific descriptors of dyspnea in assessing severity of an asthma attack. Moy et al. ( 1998 ) asked patients, in the midst of an asthma attack, to describe their feelings of shortness of breath (using Table 2.3 ) when they first presented to an emergency room and after treatment with bronchodilators. These patients were also asked to rate the severity of their dyspnea and were given breathing tests to objectively assess lung function. What was interesting was that these patients reported improvement in their feelings of shortness of breath after treatment with bronchodilators even if they had no improvement in their FEV1, an objective measure. Importantly, some aspects of their breathing discomfort improved more than others. For example, patients reported persistent feelings of increased “work” or “effort” of breathing, which better correlated with the severity of their diseases. In contrast, the sense of chest tightness improved after administration of bronchodilators. Moy et al. ( 1998 ) hypothesized that chest “tightness” may reflect bronchoconstriction, whereas “work” or “effort” may reflect ongoing inflammation and airways obstruction present during the later stages of an asthma attack. Therefore, medications that immediately dilate the airways by relaxing smooth muscles (such as bronchodilators) would provide relief from chest “tightness.” However, the “work” of breathing would persist because of obstruction due to ongoing airways inflammation. Unaware of these relationships between dyspnea and asthma, doctors may discharge patients from the emergency room or hospital before their lung function has improved (Salmeron et al. 2001 ). In a study of asthma management in French emergency rooms, 24% of patients with severe asthma were discharged 2 h after presentation when lung function was still poor (Salmeron et al. 2001 ). This may be because patients reported improvements in symptoms despite persistent airway resistance. Practitioners, therefore, should be cautious when interpreting the patient’s perception of dyspnea, and should attempt to distinguish between changes in chest tightness and the work or effort of breathing. Objective measures of lung function should be used routinely to manage patients in the midst of an acute exacerbation.
Descriptors of dyspnea
From Moy et al. ( 1998 )
Finally, the patterns of symptoms may help to diagnose asthma. For example, many asthmatics may have worse symptoms during certain seasons when allergies are increasingly prevalent. Others may have increased difficulty breathing at night or upon awakening in the early morning and may have improvements of their symptoms during the day. It is important to try and establish whether symptoms are persistent or episodic and whether certain triggers can be identified.
Diagnostic Tools
Medical history.
The medical history is one of the most important tools in diagnosing asthma. As mentioned earlier, common symptoms of asthma include episodic wheezing, cough, shortness of breath, chest tightness, increased work or effort of breathing, and difficulty inhaling. These symptoms, however, can occur with other respiratory illnesses, and taking a detailed history may help to support or refute the diagnosis of asthma. Going back to previous discussions on asthma pathogenesis, we outlined several hypotheses including the TH2 hypothesis, the hygiene hypothesis, the role of viral and bacterial illnesses, and the Dutch hypothesis. Understanding these hypotheses helps the clinician recognize factors that support the likelihood of asthma in an individual (Table 2.4 ). For example childhood onset of wheezing in association with other allergic symptoms would suggest TH2-mediated immune dysregulation and asthma. A family history of asthma and/or COPD could suggest a genetic propensity to develop respiratory disease in response to a particular insult as suggested by the Dutch hypothesis. Alternatively, a childhood history of RSV disease requiring hospitalization could suggest asthma as the etiology of his/her symptoms.
Asthma pathogenesis hypotheses and possible corresponding medical histories
Physical Exam
Often times the physical exam can be normal, especially if the patient is not having any symptoms of asthma. In those cases, one must rely on the medical history to help establish the diagnosis. If, however, a patient is experiencing symptoms at the time of the physical exam, there are some findings that increase the likelihood of asthma. For example, if airways obstruction is so significant that the patient cannot exhale all the air out before taking another breath, the lungs can become “hyperexpanded.” When hyperexpansion occurs, it is more difficult to breathe because the chest wall is at a mechanical disadvantage. Patients will begin to use “accessory muscles” to breathe. These muscles are not commonly utilized in quiet breathing, but if breathing becomes more labored they are recruited to assist in the movement of the chest wall. These accessory muscles include the neck muscles and abdominal muscles. Also, the activity of the intercostals muscles between the ribs can become more apparent during labored breathing. If breathing becomes more difficult, some patients will hunch over or assume the “tripod” position with their hands on their knees, leaning forward while sitting; this position transforms the pectoralis muscles, normally used to move the arms, into breathing muscles that elevate the chest wall.
After observing how the patient is breathing, auscultation of the chest can be informative as well. As discussed earlier, wheezing is a common sound of early airways obstruction. Usually a wheeze is heard on exhalation. With increasing airways resistance, however, inspiratory wheezes can be heard as well. The inspiratory to expiratory, or “I:E,” ratio is also reduced, meaning the expiratory phase is prolonged during airways obstruction. Usually, when listening to a patient’s chest, the clinician instructs the patient to breathe deeply, which results in an I:E ratio of 1:1. If airways obstruction is present, however, the I:E ratio can decrease to 1:2 because the lungs take longer to empty.
In cases of severe asthma attacks, a phenomenon called “pulsus paradoxus” can occur. The term “pulsus paradoxus” describes what happens to the pulse or systolic blood pressure during inspiration. Normally there is a slight weakening of the pulse during inhalation and a slight strengthening of the pulse during exhalation. This happens because of the small pressure swings in the chest that occur when we inhale and exhale and the effect that these slight changes of pressure have on the heart’s ability to pump blood. During a severe asthma attack, the work of breathing increases tremendously and the pressure swings in the chest become more pronounced. A person can generate −70 to −100 cm of H 2 O pressure (normal is between −2 and −5 cm of H 2 O pressure) which causes a severe strain on the heart’s ability to pump effectively. The pulse then becomes very weak during inspiration and returns during exhalation. This physical finding is called “pulsus paradoxus” and is a sign of severe airways obstruction and possible impending respiratory failure.
The rest of the physical exam can help to identify if the patient is prone to allergies. For example, examination of the nose may reveal mucosal swelling or nasal polyps to suggest allergic rhinitis. Similarly the eyes may be itchy, red, and teary. Skin exam may review rashes, such as hives or eczema, indicative of an allergic skin disorder. Taken together, if the physical exam is consistent with allergies in the context of shortness of breath and wheezing, the likelihood of asthma is increased.
Most imaging will be normal in patients with asthma. In some cases, one might see evidence of hyperinflation on a chest X-ray (CXR) with flattening of the diaphragm. The main purpose of imaging, however, is to assess the patient for other conditions that may mimic asthma such as chronic eosinophilic pneumonia, bronchiectasis, cryptogenic organizing pneumonia, and emphysema among other diseases. Additionally, a chest CT may be useful if the CXR is unrevealing but the suspicion of asthma is still suspect. Chest CT’s can more accurately show abnormalities of the airways, such as foreign bodies or tracheomalacia, which may be the cause of wheezing. It can also better image chronic bronchitis or bronchiectasis that may not be readily evident on a CXR. We recommend starting with a CXR in a patient newly diagnosed with asthma to exclude other possible causes of his/her symptoms.
Pulmonary Function Tests
Pulmonary function tests can be very helpful in diagnosing asthma. As mentioned earlier, measurement of FEV1 is important in diagnosing airflow obstruction. FEV1 is the amount of air exhaled during the 1st second of a forced exhalation after maximal inhalation. The forced vital capacity (FVC) is the amount of air exhaled in total after the patient blows out for as long as possible (at least 6 s); the air remaining in the lungs after such a maneuver is the residual volume (RV). If the FEV1/FVC ratio is less than what would be predicted for that person, then airflow obstruction is present. However, diseases such as chronic bronchitis, emphysema, or cystic fibrosis, all present with airflow obstruction. What increases the likelihood of asthma is the reversibility of the airflow obstruction. Often times in the lab, when we suspect that a person has asthma, we will assess the FEV1 and FVC before and after a bronchodilator. If the FEV1 increases by as least 12% and 200 cc, the patient has a significant response to bronchodilators suggestive of asthma. Wide variations in FEV1 over time with repeated pulmonary function testing are also suggestive of asthma. However, because these tests are very effort dependent, fluctuations in FEV1 can be due to patient effort rather than true reversible airways disease. In moderate to severe asthma, the patient may not be able to exhale fully during the vital capacity maneuver. The result is a diminished FVC and an elevated RV.
There are many instances when patients will not be experiencing airflow obstruction during pulmonary function testing, making it harder to diagnose asthma. Another strategy is to ask the patient to measure his PEF at home at various times during the day. This can be accomplished by asking the patient to use an inexpensive peak flow meter. Similar to the FEV1 measurement, the PEF assesses the rate at which air exits the lung during a forced expiration after maximal inspiration. In asthma, PEF is usually lowest in the morning and highest between noon and 2 p.m. (Quackenboss et al. 1991 ). The patient makes PEF measurements several times a day and a 20% difference in values between the highest and lowest flow measurements is suggestive of asthma.
If, after obtaining the history, physical exam, CXR, and pulmonary function tests, the diagnosis of asthma is still in doubt, a bronchoprovocation test to induce airways obstruction may help to establish or exclude the diagnosis. Commonly, a bronchoprovocation test is useful when conventional therapies for asthma do not resolve the patient’s symptoms. The physician must decide whether to intensify the medical regimen or question the diagnosis of asthma. A MCT can help confirm or exclude a diagnosis of asthma and guide further therapy (Fig. 2.3 ).

Algorithm for asthma diagnosis
The hallmark of asthma is bronchial hyperresponsiveness, meaning that the airways constrict robustly in response to an irritant or other stimulus. Methacholine is a medication that causes constriction of the smooth muscles around the airways. When given in high enough doses, a person with normal airways can have bronchoconstriction with methacholine. In asthmatics, however, the airways will constrict with very small doses that usually do not affect the normal airway. The MCT is administered in a monitored setting. The subject inhales a solution of methacholine in increasing doses. After each inhalation, FEV1 and FVC are measured. If there is a decrease of 20% in the FEV1 after an inhalation of a certain dose of methacholine, the test is stopped. This is called the PC20, the provocation concentration that is required to decrease the FEV1 by 20%. As outlined in the Table 2.5 , the degree of bronchial hyperresponsiveness depends on how much methacholine is required to cause significant bronchoconstriction. The MCT is a useful test for diagnosing asthma but the results must be interpreted in the context of all the information known about the patient. It is not 100% diagnostic.
Degree of bronchial hyperreponsiveness after administration of methacholine
From ATS AJRCCM 2000 (Crapo et al. 2000 )
Although the MCT is not a foolproof test, it is helpful in trying to obtain the correct diagnosis in a patient with asthma-like symptoms. In a study of patients evaluated for dyspnea and cough who did not improve with asthma treatment, 82.5% had negative MCT (Chevalier and Schwartzstein 2001 ). As a result of these negative tests, bronchodilators were discontinued and other causes for dyspnea and cough were pursued and then treated. Other studies have shown that even a previous history of asthma did not reliably predict a positive MCT (Pratter et al. 1989 ). The MCT, therefore, is a powerful tool to help establish the diagnosis of asthma in some cases and exclude it in others, thereby allowing the patient to receive the treatment she needs.
Mimickers of Asthma
All that wheezes is not always asthma. Often times, in difficult cases, other diagnoses should be explored if the diagnosis of asthma is in question. These are usually cases in which the patient does not respond to treatment or has a physical exam or history that seems inconsistent with asthma. The mimickers of asthma can be categorized into diseases affecting the large airways, small airways, and lung parenchyma. Non-pulmonary causes should also be considered.
One of the most difficult diagnoses to make is vocal cord dysfunction (VCD). VCD can present like asthma and patients usually have a history of asthma that has not been responsive to steroids or bronchodilators. Because they continue to wheeze despite therapy, these patients can be exposed to large doses of steroids and bronchodilators putting them at risk for complications of these medications. Although the etiology of VCD is not fully elucidated, it is more common in young adults with psychiatric disorders. It occurs when the vocal cords adduct (come together in the midline) during inhalation and exhalation creating airflow limitation at the level of the vocal cords. The lungs and airways themselves, however, are normal. The patient adducts the vocal cords subconsciously and can often appear to be in respiratory distress. VCD presents with similar symptoms and signs as asthma, such as shortness of breath and wheezing. In extreme cases, however, patients with VCD can hypoventilate and be intubated for respiratory failure. Unlike asthma, however, wheezes cease after intubation because the endotracheal tube bypasses the vocal cords, the site of obstruction. After intubation, the patient with VCD is easily ventilated and can be removed from mechanical support within 24 h.
Definitive diagnosis of this disorder can be difficult and usually requires direct visualization of the vocal cords during symptomatic episodes. Physical exam during an episode usually reveals a monophonic wheeze heard loudest over the throat. Patients may have trouble vocalizing while wheezing and symptoms can come on suddenly without warning. Treatment requires intense speech therapy during which these patients learn techniques for relaxed throat breathing. With treatment, patients with VCD can come off steroids and bronchodilator therapy and live a better quality of life. In extreme cases, a tracheostomy is performed to bypass the site of recurrent obstruction.
Other problems of the large airways that can cause wheezing included foreign bodies in the large airways. Aspiration of nuts or other food products can cause foreign bodies to get trapped in a large airway. In severe cases, these foreign bodies can act as a ball-valve causing hyperinflation and eventual respiratory distress. Immediate removal of the foreign body by a trained bronchoscopist is required. Besides aspirated objects, congenital abnormalities, such as vascular rings or laryngeal webs, can cause obstruction of the trachea and lead to wheezing and shortness of breath. Other masses, such as tumors, can cause obstruction of the airways as well and with similar presenting symptoms. Lung cancer and carcinoid tumors may cause focal airway obstruction. In patients who have had prior intubations, tracheal stenosis as a late complication of endotracheal intubation can also present like asthma. A bronchoscopy to inspect the airway is usually required to make this diagnosis. Even if the airway is normal, structures outside of the airway can be abnormal and can cause compression resulting in obstruction. Lymph nodes, vascular structures, or tumor can impinge upon the large airways in this manner. Often times, a chest CT is helpful in making this diagnosis.
Sometimes the airways can be affected by other disease processes such as bronchiectasis. Bronchiectasis is a common disorder that can present with signs and symptoms similar to asthma. Patients with bronchiectasis have distorted and abnormal airways usually due to an infectious process. People can develop bronchiectasis as a sequelae of a severe necrotizing lung infection or toxic gas exposure. In necrotizing pneumonia, the abnormal airways usually are confined to the region of the lung where the original infection took place, whereas toxic gas exposure can cause more diffuse bronchiectasis. Because the airways are abnormal, it becomes more difficult to clear infections from bronchiectatic lung and recurrent infections occur. Some patients develop bronchiectasis after aspirating a foreign object that gets lodged in the airway. The object makes it difficult to clear pus, creating recurrent persistent infections and progressive damage to the airway. Other patients may develop bronchiectasis as a result of an underlying condition that makes the patient prone to lung infections and impairs the ability of the body to clear infections despite appropriate antibiotics. Such conditions include cystic fibrosis (a genetic disorder causing thick mucous plugs that are difficult clear) and immunodeficiency states. Regardless of the underlying cause for bronchiectasis, the presentation is similar with daily cough productive of purulent sputum, recurrent pulmonary infections, shortness of breath, and wheezing. The wheezing associated with bronchiectasis may be due to airflow obstruction associated with mucous plugging or distorted airways. Unlike asthma, the airflow obstruction is not completely reversible. Asthma, however, can also exist concomitantly with bronchiectasis. If a patient with wheezing has recurrent pulmonary infections or daily cough productive of purulent sputum, the diagnosis of bronchiectasis should be considered. Chest imaging can determine if the airways are distorted or abnormal to confirm the diagnosis of bronchiectasis.
Other airway diseases can also mimic asthma. In children, congenital abnormalities such bronchopulmonary dysplasia should be considered. In adults, diseases such as sarcoidosis can cause wheezing, cough, and dyspnea as well. Sarcoidosis is a disease of unclear etiology in which non-caseating granulomas affect the lymph nodes and airways. Airway or lymph node biopsy is often required to make the diagnosis. Diseases such as COPD are also common in adults and can mimic asthma as well. Occasionally, asthma and COPD can coexist.
Diseases that affect the lung parenchyma can cause wheezing, cough, and shortness of breath. Chronic eosinophilic pneumonia, in which eosinophils infiltrate the peripheral lung parenchyma, can present with wheezing and usually requires high doses of steroids to treat. Hypersensitivity pneumonitis is also a disease of the lung parenchyma, usually affecting the upper lobes and precipitated by exposure to some inhaled substance such as mold, flour, bird allergens, etc. Hypersensitivity pneumonitis can present acutely with fevers, cough, and dyspnea or less acutely with cough, wheezing, and shortness of breath. Other diseases that affect the lungs, such as pulmonary emboli or pneumonia, can also mimic asthma. Imaging, careful history, and physical exam may help to distinguish these conditions from asthma.
Processes that affect the pulmonary vasculature can also present as asthma does. As noted above pulmonary embolism can present with shortness of breath and wheezing. Although wheezing is not a common symptom of pulmonary embolism, it has been reported in the medical literature (Calvo-Romero et al. 2003 ). Although the etiology of wheezing in pulmonary embolism is not clear, possible mechanisms include an inflammatory reaction resulting from the embolism or the release of chemicals such as bradykinin that may lead to bronchoconstriction and wheezing. Congestive heart failure can also cause dyspnea, cough, and wheezing (often termed cardiac asthma) due to pulmonary edema. When the heart fails to pump adequately, fluid builds up in the pulmonary lymphatics and pulmonary venous capillaries, which then causes edema of the lungs and can promote wheezing.
Other conditions that may mimic asthma include reactions to medications such as angiotensin converting enzyme I inhibitors (ACE-I), which can cause chronic cough. GERD can also cause both cough and bronchoconstriction as mentioned earlier. Aspiration can cause wheezing and cough due to inflammation of the airways. In cases of GERD, the patient may be unaware of these episodes. In severe cases, if routine treatment of GERD (including proton pump inhibitors and behavioral modification) does not alleviate the problem, procedures to tighten the lower esophageal sphincter are needed to prevent reflux.
A careful history, physical exam, and clinical acumen are required to identify when a diagnosis of asthma just doesn’t seem right. Clues, such as poor response to asthma therapy, persistent instead of episodic symptoms, or constitutional symptoms (weight loss, fever, nausea/vomiting), can be useful in stimulating a search for non-asthma diagnoses. Chest imaging can be helpful in excluding diseases of the lung parenchymal, and bronchoscopic imaging may further help diagnose large airways obstruction.
We have briefly reviewed general theories of asthma pathogenesis including the TH2 hypothesis, the hygiene hypothesis, the Dutch hypothesis, and the role of infectious diseases in asthma. These theories demonstrate that asthma is a heterogeneous disease with multiple causative mechanisms in susceptible individuals. We reviewed the physiology of asthma and its relationship to symptoms such as dyspnea. We outlined a diagnostic approach to asthma based on symptoms, history and physical exam. In cases in which the asthma diagnosis is still in question, a MCT may help to support or exclude the diagnosis. Finally, an awareness of conditions that mimic asthma is important when confronted with a patient who may have atypical features or who fails to respond to therapy.
Contributor Information
Andrew Harver, Phone: +1704687-4784 .
Harry Kotses, Phone: +1614593-1087 .
- Allegra L, Blasi F, Centanni S, Cosentini R, Denti F, Raccanelli R, Tarsia P, Valenti V. Acute exacerbations of asthma in adults: role of Chlamydia pneumoniae infection. Eur Respir J. 1994; 7 (12):2165–2168. doi: 10.1183/09031936.94.07122165. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Amayasu H, Yoshida S, Ebana S, Yamamoto Y, Nishikawa T, Shoji T, Nakagawa H, Hasegawa H, Nakabayashi M, Ishizaki Y. Clarithromycin suppresses bronchial hyperresponsiveness associated with eosinophilic inflammation in patients with asthma. Ann Allergy Asthma Immunol. 2000; 84 (6):594–598. doi: 10.1016/S1081-1206(10)62409-X. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anderson SD. Is there a unifying hypothesis for exercise-induced asthma? J Allergy Clin Immunol. 1984; 73 (5):660–665. doi: 10.1016/0091-6749(84)90301-4. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anderson SD, Daviskas E. The mechanism of exercise-induced asthma is. J Allergy Clin Immunol. 2000; 106 (3):453–459. doi: 10.1067/mai.2000.109822. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol. 2003; 28 (1):12–24. doi: 10.1165/rcmb.2002-0166TR. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Babu KS, Salvi SS. Aspirin and asthma. Chest. 2000; 118 (5):1470–1476. doi: 10.1378/chest.118.5.1470. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ball TM, Castro-Rodriguez JA, Griffith KA, Holberg CJ, Martinez FD, Wright AL. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med. 2000; 343 (8):538–543. doi: 10.1056/NEJM200008243430803. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Barrios RJ, Kheradmand F, Batts L, Corry DB. Asthma: pathology and pathophysiology. Arch Pathol Lab Med. 2006; 130 (4):447–451. [ PubMed ] [ Google Scholar ]
- Berkovich S, Millian SJ, Snyder RD. The association of viral and mycoplasma infections with recurrence of wheezing in the asthmatic child. Ann Allergy. 1970; 28 (2):43–49. [ PubMed ] [ Google Scholar ]
- Binks AP, Moosavi SH, Banzett RB, Schwartzstein RM. “Tightness” sensation of asthma does not arise from the work of breathing. Am J Respir Crit Care Med. 2002; 165 (1):78–82. [ PubMed ] [ Google Scholar ]
- Biscione GL, Corne J, Chauhan AJ, Johnston SL. Increased frequency of detection of Chlamydophila pneumoniae in asthma. Eur Respir J. 2004; 24 (5):745–749. doi: 10.1183/09031936.04.00049004. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Black PN, Blasi F, Jenkins CR, Scicchitano R, Mills GD, Rubinfeld AR, Ruffin RE, Mullins PR, Dangain J, Cooper BC, David DB, Allegra L. Trial of roxithromycin in subjects with asthma and serological evidence of infection with Chlamydia pneumoniae. Am J Respir Crit Care Med. 2001; 164 (4):536–541. [ PubMed ] [ Google Scholar ]
- Black PN, Scicchitano R, Jenkins CR, Blasi F, Allegra L, Wlodarczyk J, Cooper BC. Serological evidence of infection with Chlamydia pneumoniae is related to the severity of asthma. Eur Respir J. 2000; 15 (2):254–259. doi: 10.1034/j.1399-3003.2000.15b06.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Blasi F, Cosentini R, Tarsia P, Capone P, Allegra L. Atypical pathogens and asthma: can they influence the natural history of the disease? Monaldi Arch Chest Dis. 2001; 56 (3):276–280. [ PubMed ] [ Google Scholar ]
- Bochner BS, Busse WW. Allergy and asthma. J Allergy Clin Immunol. 2005; 115 (5):953–959. doi: 10.1016/j.jaci.2005.02.032. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bousquet J, Lacoste JY, Chanez P, Vic P, Godard P, Michel FB. Bronchial elastic fibers in normal subjects and asthmatic patients. Am J Respir Crit Care Med. 1996; 153 (5):1648–1654. [ PubMed ] [ Google Scholar ]
- Bundgaard A. Incidence of exercise-induced asthma in adult asthmatics. Allergy. 1981; 36 (1):23–26. doi: 10.1111/j.1398-9995.1981.tb01820.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bundgaard A, Ingemann-Hansen T, Schmidt A, Halkjaer-Kristensen J. Influence of temperature and relative humidity of inhaled gas on exercise-induced asthma. Eur J Respir Dis. 1982; 63 (3):239–244. [ PubMed ] [ Google Scholar ]
- Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001; 344 (5):350–362. doi: 10.1056/NEJM200102013440507. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Calhoun WJ, Swenson CA, Dick EC, Schwartz LB, Lemanske RF, Jr, Busse WW. Experimental rhinovirus 16 infection potentiates histamine release after antigen bronchoprovocation in allergic subjects. Am Rev Respir Dis. 1991; 144 (6):1267–1273. [ PubMed ] [ Google Scholar ]
- Calvo-Romero JM, Perez-Miranda M, Bureo-Dacal P. Wheezing in patients with acute pulmonary embolism with and without previous cardiopulmonary disease. Eur J Emerg Med. 2003; 10 (4):288–289. doi: 10.1097/00063110-200312000-00009. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Campbell EJ, Howell JB. The sensation of breathlessness. Br Med Bull. 1963; 19 :36–40. [ PubMed ] [ Google Scholar ]
- Chanez P, Vignola AM, O’Shaugnessy T, Enander I, Li D, Jeffery PK, et al. Corticosteroid reversibility in COPD is related to features of asthma. Am J Respir Crit Care Med. 1997; 155 (5):1529–1534. [ PubMed ] [ Google Scholar ]
- Chevalier B, Schwartzstein R. The role of the methacholine inhalation challenge: avoiding a misdiagnosis of asthma. J Respir Dis. 2001; 22 :153–160. [ Google Scholar ]
- Chronos N, Adams L, Guz A. Effect of hyperoxia and hypoxia on exercise-induced breathlessness in normal subjects. Clin Sci (Lond) 1988; 74 (5):531–537. [ PubMed ] [ Google Scholar ]
- Chu HW, Honour JM, Rawlinson CA, Harbeck RJ, Martin RJ. Effects of respiratory Mycoplasma pneumoniae infection on allergen-induced bronchial hyperresponsiveness and lung inflammation in mice. Infect Immun. 2003; 71 (3):1520–1526. doi: 10.1128/IAI.71.3.1520-1526.2003. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cohet C, Cheng S, MacDonald C, Baker M, Foliaki S, Huntington N, Douwes J, Pearce N. Infections, medication use, and the prevalence of symptoms of asthma, rhinitis, and eczema in childhood. J Epidemiol Community Health. 2004; 58 (10):852–857. doi: 10.1136/jech.2003.019182. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Corry DB, Grunig G, Hadeiba H, Kurup VP, Warnock ML, Sheppard D, Rennick DM, Locksley RM. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol Med. 1998; 4 (5):344–355. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cottrez F, Hurst SD, Coffman RL, Groux H. T regulatory cells 1 inhibit a Th2-specific response in vivo. J Immunol. 2000; 165 (9):4848–4853. [ PubMed ] [ Google Scholar ]
- Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000; 161 (1):309–329. [ PubMed ] [ Google Scholar ]
- D’Andrea A, Rengaraju M, Valiante NM, Chehimi J, Kubin M, Aste M, Chan SH, Kobayashi M, Young D, Nickbarg E, Chizzonite R, Wolf SF, Trinchieri G. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992; 176 (5):1387–1398. doi: 10.1084/jem.176.5.1387. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Droste JH, Wieringa MH, Weyler JJ, Nelen VJ, Vermeire PA, Van Bever HP. Does the use of antibiotics in early childhood increase the risk of asthma and allergic disease? Clin Exp Allergy. 2000; 30 (11):1547–1553. doi: 10.1046/j.1365-2222.2000.00939.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Eckert DJ, Catcheside PG, Smith JH, Frith PA, McEvoy RD. Hypoxia suppresses symptom perception in asthma. Am J Respir Crit Care Med. 2004; 169 (11):1224–1230. doi: 10.1164/rccm.200305-630OC. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Finkelman FD, Katona IM, Urban JF, Jr, Holmes J, Ohara J, Tung AS, Sample JV, Paul WE. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988; 141 (7):2335–2341. [ PubMed ] [ Google Scholar ]
- Foo AL, Sly PD. Pulmonary function in a hospital population of asthmatic children. J Asthma. 1991; 28 (4):273–280. doi: 10.3109/02770909109073384. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Garfinkel SK, Kesten S, Chapman KR, Rebuck AS. Physiologic and nonphysiologic determinants of aerobic fitness in mild to moderate asthma. Am Rev Respir Dis. 1992; 145 (4):741–745. [ PubMed ] [ Google Scholar ]
- Gencay M, Rudiger JJ, Tamm M, Soler M, Perruchoud AP, Roth M. Increased frequency of Chlamydia pneumoniae antibodies in patients with asthma. Am J Respir Crit Care Med. 2001; 163 (5):1097–1100. [ PubMed ] [ Google Scholar ]
- Gereda JE, Leung DY, Thatayatikom A, Streib JE, Price MR, Klinnert MD, Liu AH. Relation between house-dust endotoxin exposure, type 1 T-cell development, and allergen sensitisation in infants at high risk of asthma. Lancet. 2000; 355 (9216):1680–1683. doi: 10.1016/S0140-6736(00)02239-X. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- National Asthma Education and Prevention Program (1997) Guidelines for the diagnosis and management of asthma. NIH Publication No. 97-4051. National Institutes of Health, Bethesda, MD
- Hewson PH, Tippett EA, Jones DM, Maddern JP, Higgs P. Routine pulmonary function tests in young adolescents with asthma in general practice. Med J Aust. 1996; 165 (9):469–472. [ PubMed ] [ Google Scholar ]
- Holgate ST, Lackie PM, Davies DE, Roche WR, Walls AF. The bronchial epithelium as a key regulator of airway inflammation and remodelling in asthma. Clin Exp Allergy. 1999; 29 (Suppl 2):90–95. doi: 10.1046/j.1365-2222.1999.00016.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Huhti E, Mokka T, Nikoskelainen J, Halonen P. Association of viral and mycoplasma infections with exacerbations of asthma. Ann Allergy. 1974; 33 (3):145–149. [ PubMed ] [ Google Scholar ]
- Humbert M, Durham SR, Ying S, Kimmitt P, Barkans J, Assoufi B, Pfister R, Menz G, Robinson DS, Kay AB, Corrigan CJ. IL-4 and IL-5 mRNA and protein in bronchial biopsies from patients with atopic and nonatopic asthma: evidence against “intrinsic” asthma being a distinct immunopathologic entity. Am J Respir Crit Care Med. 1996; 154 (5):1497–1504. [ PubMed ] [ Google Scholar ]
- Humbert M, Menz G, Ying S, Corrigan CJ, Robinson DS, Durham SR, Kay AB. The immunopathology of extrinsic (atopic) and intrinsic (non-atopic) asthma: more similarities than differences. Immunol Today. 1999; 20 (11):528–533. doi: 10.1016/S0167-5699(99)01535-2. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Illi S, von Mutius E, Lau S, Bergmann R, Niggemann B, Sommerfeld C, Wahn U. Early childhood infectious diseases and the development of asthma up to school age: a birth cohort study. BMJ. 2001; 322 (7283):390–395. doi: 10.1136/bmj.322.7283.390. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ingenito E, Solway J, Lafleur J, Lombardo A, Drazen JM, Pichurko B. Dissociation of temperature-gradient and evaporative heat loss during cold gas hyperventilation in cold-induced asthma. Am Rev Respir Dis. 1988; 138 (3):540–546. [ PubMed ] [ Google Scholar ]
- Irwin RS, Curley FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis. 1990; 141 (3):640–647. [ PubMed ] [ Google Scholar ]
- Jeannin P, Lecoanet S, Delneste Y, Gauchat JF, Bonnefoy JY. IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol. 1998; 160 (7):3555–3561. [ PubMed ] [ Google Scholar ]
- Jeffery PK. Comparison of the structural and inflammatory features of COPD and asthma. Giles F. Filley Lecture. Chest. 2000; 117 (5 Suppl 1):251S–260S. doi: 10.1378/chest.117.5_suppl_1.251S. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Johnston SL, Blasi F, Black PN, Martin RJ, Farrell DJ, Nieman RB. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006; 354 (15):1589–1600. doi: 10.1056/NEJMoa044080. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Johnston SL, Pattemore PK, Sanderson G, Smith S, Campbell MJ, Josephs LK, Cunningham A, Robinson BS, Myint SH, Ward ME, Tyrrell DA, Holgate ST. The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am J Respir Crit Care Med. 1996; 154 (3):654–660. [ PubMed ] [ Google Scholar ]
- Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, Symington P, O’Toole S, Myint SH, Tyrrell DA, Holgate ST. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995; 310 (6989):1225–1229. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kraft M, Djukanovic R, Wilson S, Holgate ST, Martin RJ. Alveolar tissue inflammation in asthma. Am J Respir Crit Care Med. 1996; 154 (5):1505–1510. [ PubMed ] [ Google Scholar ]
- Lane R, Cockcroft A, Adams L, Guz A. Arterial oxygen saturation and breathlessness in patients with chronic obstructive airways disease. Clin Sci (Lond) 1987; 72 (6):693–698. [ PubMed ] [ Google Scholar ]
- Lapa e Silva JR, Possebon da Silva MD, Lefort J, Vargaftig BB. Endotoxins, asthma, and allergic immune responses. Toxicology. 2000; 152 (1–3):31–35. doi: 10.1016/S0300-483X(00)00289-4. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Le J, Lin JX, Henriksen-DeStefano D, Vilcek J. Bacterial lipopolysaccharide-induced interferon-gamma production: roles of interleukin 1 and interleukin 2. J Immunol. 1986; 136 (12):4525–4530. [ PubMed ] [ Google Scholar ]
- Lemanske RF, Jr, Dick EC, Swenson CA, Vrtis RF, Busse WW. Rhinovirus upper respiratory infection increases airway hyperreactivity and late asthmatic reactions. J Clin Invest. 1989; 83 (1):1–10. doi: 10.1172/JCI113843. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Litonjua AA, Milton DK, Celedon JC, Ryan L, Weiss ST, Gold DR. A longitudinal analysis of wheezing in young children: the independent effects of early life exposure to house dust endotoxin, allergens, and pets. J Allergy Clin Immunol. 2002; 110 (5):736–742. doi: 10.1067/mai.2002.128948. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lougheed MD, Lam M, Forkert L, Webb KA, O’Donnell DE. Breathlessness during acute bronchoconstriction in asthma. Pathophysiologic mechanisms. Am Rev Respir Dis. 1993; 148 (6):1452–1459. [ PubMed ] [ Google Scholar ]
- Madden KB, Urban JF, Jr, Ziltener HJ, Schrader JW, Finkelman FD, Katona IM. Antibodies to IL-3 and IL-4 suppress helminth-induced intestinal mastocytosis. J Immunol. 1991; 147 (4):1387–1391. [ PubMed ] [ Google Scholar ]
- Mahler DA, Harver A, Lentine T, Scott JA, Beck K, Schwartzstein RM. Descriptors of breathlessness in cardiorespiratory diseases. Am J Respir Crit Care Med. 1996; 154 (5):1357–1363. [ PubMed ] [ Google Scholar ]
- McFadden ER, Jr, Gilbert IA. Exercise-induced asthma. N Engl J Med. 1994; 330 (19):1362–1367. doi: 10.1056/NEJM199405123301907. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McFadden ER, Jr, Lenner KA, Strohl KP. Postexertional airway rewarming and thermally induced asthma. New insights into pathophysiology and possible pathogenesis. J Clin Invest. 1986; 78 (1):18–25. doi: 10.1172/JCI112549. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mertsola J, Ziegler T, Ruuskanen O, Vanto T, Koivikko A, Halonen P. Recurrent wheezy bronchitis and viral respiratory infections. Arch Dis Child. 1991; 66 (1):124–129. doi: 10.1136/adc.66.1.124. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Michel O, Duchateau J, Sergysels R. Effect of inhaled endotoxin on bronchial reactivity in asthmatic and normal subjects. J Appl Physiol. 1989; 66 (3):1059–1064. [ PubMed ] [ Google Scholar ]
- Molema J, van Herwaarden CL, Folgering HT. Effects of inhaled budesonide on the relationships between symptoms, lung function indices and airway hyperresponsiveness in patients with allergic asthma. Pulm Pharmacol. 1989; 1 (4):179–185. doi: 10.1016/S0952-0600(89)80015-8. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Moy ML, Lantin ML, Harver A, Schwartzstein RM. Language of dyspnea in assessment of patients with acute asthma treated with nebulized albuterol. Am J Respir Crit Care Med. 1998; 158 (3):749–753. [ PubMed ] [ Google Scholar ]
- Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993; 307 (6910):982–986. doi: 10.1136/bmj.307.6910.982. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Papi A, Johnston SL. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-kappaB-mediated transcription. J Biol Chem. 1999; 274 (14):9707–9720. doi: 10.1074/jbc.274.14.9707. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Postma DS, Boezen HM (2004) Rationale for the Dutch hypothesis. Allergy and airway hyperresponsiveness as genetic factors and their interaction with environment in the development of asthma and COPD. Chest 126(2 Suppl): 96S–104S; discussion 159S–161S [ PubMed ]
- Pratter MR, Curley FJ, Dubois J, Irwin RS. Cause and evaluation of chronic dyspnea in a pulmonary disease clinic. Arch Intern Med. 1989; 149 (10):2277–2282. doi: 10.1001/archinte.149.10.2277. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Quackenboss JJ, Lebowitz MD, Krzyzanowski M. The normal range of diurnal changes in peak expiratory flow rates. Relationship to symptoms and respiratory disease. Am Rev Respir Dis. 1991; 143 (2):323–330. [ PubMed ] [ Google Scholar ]
- Ram FS, Robinson SM, Black PN, Picot J (2005) Physical training for asthma. Cochrane Database Syst Rev (4):CD001116 [ PubMed ]
- Reed CE, Milton DK. Endotoxin-stimulated innate immunity: a contributing factor for asthma. J Allergy Clin Immunol. 2001; 108 (2):157–166. doi: 10.1067/mai.2001.116862. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Saetta M, Di Stefano A, Maestrelli P, Turato G, Ruggieri MP, Roggeri A, Calcagni P, Mapp A, Ciaccia A, Fabbri LM. Airway eosinophilia in chronic bronchitis during exacerbations. Am J Respir Crit Care Med. 1994; 150 (6):1646–1652. [ PubMed ] [ Google Scholar ]
- Salmeron S, Liard R, Elkharrat D, Muir J, Neukirch F, Ellrodt A. Asthma severity and adequacy of management in accident and emergency departments in France: a prospective study. Lancet. 2001; 358 (9282):629–635. doi: 10.1016/S0140-6736(01)05779-8. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schiller J, Martinez M, Barnes P (2006) Early release of selected estimates based on data from the 2005 National Health Interview Survey. National Center for Health Statistics. http://www.cdc.gov/nchs/nhis.htm
- Scott P. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991; 147 (9):3149–3155. [ PubMed ] [ Google Scholar ]
- Scrivener S, Yemaneberhan H, Zebenigus M, Tilahun D, Girma S, Ali S, McElroy P, Custovic A, Woodcock A, Pritchard D, Venn A, Britton J. Independent effects of intestinal parasite infection and domestic allergen exposure on risk of wheeze in Ethiopia: a nested case-control study. Lancet. 2001; 358 (9292):1493–1499. doi: 10.1016/S0140-6736(01)06579-5. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Simoes EA. Respiratory syncytial virus infection. Lancet. 1999; 354 (9181):847–852. doi: 10.1016/S0140-6736(99)80040-3. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Simon PM, Schwartzstein RM, Weiss JW, Fencl V, Teghtsoonian M, Weinberger SE. Distinguishable types of dyspnea in patients with shortness of breath. Am Rev Respir Dis. 1990; 142 (5):1009–1014. [ PubMed ] [ Google Scholar ]
- Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999; 354 (9178):541–545. doi: 10.1016/S0140-6736(98)10321-5. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sturtevant J. NSAID-induced bronchospasm – a common and serious problem. A report from MEDSAFE, the New Zealand Medicines and Medical Devices Safety Authority. N Z Dent J. 1999; 95 (421):84. [ PubMed ] [ Google Scholar ]
- Taguchi O, Kikuchi Y, Hida W, Iwase N, Satoh M, Chonan T, Takishima T. Effects of bronchoconstriction and external resistive loading on the sensation of dyspnea. J Appl Physiol. 1991; 71 (6):2183–2190. [ PubMed ] [ Google Scholar ]
- Tan RA, Spector SL. Exercise-induced asthma: diagnosis and management. Ann Allergy Asthma Immunol. 2002; 89 (3):226–235. [ PubMed ] [ Google Scholar ]
- Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson Children’s Respiratory Study: 1980 to present. J Allergy Clin Immunol. 2003; 111 (4):661–675. [ PubMed ] [ Google Scholar ]
- Teeter JG, Bleecker ER. Relationship between airway obstruction and respiratory symptoms in adult asthmatics. Chest. 1998; 113 (2):272–277. doi: 10.1378/chest.113.2.272. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Teichtahl H, Buckmaster N, Pertnikovs E. The incidence of respiratory tract infection in adults requiring hospitalization for asthma. Chest. 1997; 112 (3):591–596. doi: 10.1378/chest.112.3.591. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ulrik CS, Backer V. Nonreversible airflow obstruction in life-long nonsmokers with moderate to severe asthma. Eur Respir J. 1999; 14 (4):892–896. doi: 10.1034/j.1399-3003.1999.14d27.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ulrik CS, Backer V, Dirksen A, Pedersen M, Koch C. Extrinsic and intrinsic asthma from childhood to adult age: a 10-yr follow-up. Respir Med. 1995; 89 (8):547–554. doi: 10.1016/0954-6111(95)90156-6. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- van den Biggelaar AH, van Ree R, Rodrigues LC, Lell B, Deelder AM, Kremsner PG, Yazdanbakhsh M. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet. 2000; 356 (9243):1723–1727. doi: 10.1016/S0140-6736(00)03206-2. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Venarske DL, Busse WW, Griffin MR, Gebretsadik T, Shintani AK, Minton PA, Peebles RS, Hamilton R, Weisshaar E, Vrtis R, Higgins SB, Hartert TV. The relationship of rhinovirus-associated asthma hospitalizations with inhaled corticosteroids and smoking. J Infect Dis. 2006; 193 (11):1536–1543. doi: 10.1086/503809. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- von Mutius E. The environmental predictors of allergic disease. J Allergy Clin Immunol. 2000; 105 (1):9–19. doi: 10.1016/S0091-6749(00)90171-4. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- von Mutius E, Braun-Fahrlander C, Schierl R, Riedler J, Ehlermann S, Maisch S, Waser M, Nowak D. Exposure to endotoxin or other bacterial components might protect against the development of atopy. Clin Exp Allergy. 2000; 30 (9):1230–1234. doi: 10.1046/j.1365-2222.2000.00959.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, Chu HW. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999; 160 (3):1001–1008. [ PubMed ] [ Google Scholar ]
- Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999; 17 :255–281. doi: 10.1146/annurev.immunol.17.1.255. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998; 282 (5397):2258–2261. doi: 10.1126/science.282.5397.2258. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- (1998) Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet 351(9111):1225–1232 [ PubMed ]
Help | Advanced Search
Computer Science > Computation and Language
Title: realm: reference resolution as language modeling.
Abstract: Reference resolution is an important problem, one that is essential to understand and successfully handle context of different kinds. This context includes both previous turns and context that pertains to non-conversational entities, such as entities on the user's screen or those running in the background. While LLMs have been shown to be extremely powerful for a variety of tasks, their use in reference resolution, particularly for non-conversational entities, remains underutilized. This paper demonstrates how LLMs can be used to create an extremely effective system to resolve references of various types, by showing how reference resolution can be converted into a language modeling problem, despite involving forms of entities like those on screen that are not traditionally conducive to being reduced to a text-only modality. We demonstrate large improvements over an existing system with similar functionality across different types of references, with our smallest model obtaining absolute gains of over 5% for on-screen references. We also benchmark against GPT-3.5 and GPT-4, with our smallest model achieving performance comparable to that of GPT-4, and our larger models substantially outperforming it.
Submission history
Access paper:.
- HTML (experimental)
- Other Formats
References & Citations
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .
- Home office
How Do You Get Rid of Shredded Paper? Think Twice Before Recycling.

By Melanie Pinola
Melanie Pinola is a writer focused on home-office gear. To find the best paper shredder, she has shredded enough junk mail to fill several bathtubs.
It turns out, I’ve been recycling all wrong. After the latest round of testing nine paper shredders , I thought I’d put the resulting 65-plus gallons of shredded paper out for recycling. But when I asked my local sanitation department if it would prefer the shreds in clear bags or cardboard boxes, the representatives said neither.
Instead, they instructed me to toss the shredded paper in with the trash.
But wait: Isn’t shredded paper still paper, and thus recyclable? Isn’t throwing away shredded paper wasteful? The answer to both questions is, well, kind of. Here’s what you need to know about the best way to dispose of shredded paper.
Check your local guidelines
The American Forest & Paper Association confirms that shredded paper is indeed recyclable. But whether shredded paper is acceptable for recycling in your town or city is another story.
So it’s best to double-check with your local sanitation or public-works department to see what you’re supposed to do with your shredded paper. Local guidelines vary—and those guidelines may or not be on the publicly accessible website or in published brochures.
For example, San Franciscans are encouraged to either place shredded paper in a stapled brown paper bag labeled “SHREDDED” or compost the shredded paper. But if you live in Rhode Island, shredded paper isn’t accepted for mixed-recycling pickup; residents can compost their shreds, throw them in the trash, or drop off shredded paper at a disposal site in Johnston, Rhode Island.
Why shredded paper isn’t always accepted for recycling

Shredded paper can be a disaster for some recycling facilities. “Shredded bits of paper are too small to be properly sorted by our facility’s machinery,” said Jared Rhodes, director of policy and programs at the Rhode Island Resource Recovery Corporation (RIRRC). They can contaminate other materials and even lead to equipment malfunctions, he added.
An article in The Providence Journal expounds on the problem, noting that when local households sent their shredded paper for recycling in paper bags, the shredding machines ripped the bags, and tiny shreds flew everywhere. When residents tried using plastic bags (or even double-bagging in plastic), the shreds still flew everywhere—and plastic wrapped around the equipment, shutting the facility down for cleaning and repairs.
As a solution, some localities outsource the recycling of shredded paper to dedicated facilities that are equipped for it, but that costs additional time and taxpayer money. You can help reduce the load by composting your shredded paper, taking documents to be shredded to a community’s free shredding event (they’ll know how to dispose of the shreds), and reducing how much you shred in the first place.
Shred only paper containing sensitive information
Paper is most suitable for recycling when it isn’t shredded, because whole pieces are easier for facilities to sort and have longer and stronger fibers ready to be made into new paper. So it’s best to avoid unnecessary shredding.
To protect your privacy, you should still shred anything with sensitive information on it, of course, such as documents with your Social Security number, financial statements, and medical records.

Royal 14MC 14-Sheet Micro Cut Paper Shredder
The best home-office paper shredder.
This high-security shredder offers the best balance of ease of use, sheet capacity, and price.
Buying Options
However, some information on a document may be personal but not necessarily sensitive enough to need shredding, such as your name and address; your contact information may already be available on public records or services such as White Pages.
“Consider how much junk mail and spam calls you receive; that’s how known your address and phone number are,” says Max Eddy, Wirecutter’s senior staff writer covering privacy and security. Instead of shredding documents that have only your name, address, and phone number on them, you can cover that information with permanent black marker and then put the sheets into the recycling bin.
Bonus: In addition to helping the environment, reducing how much you shred can prolong the life of your paper shredder. Everybody wins.
This article was edited by Ben Keough and Erica Ogg.
Meet your guide

Melanie Pinola
Melanie Pinola covers home office, remote work, and productivity as a senior staff writer at Wirecutter. She has contributed to print and online publications such as The New York Times, Consumer Reports, Lifehacker, and PCWorld, specializing in tech, work, and lifestyle/family topics. She’s thrilled when those topics intersect—and when she gets to write about them in her PJs.
Mentioned above
- We tested top shredders for use at home and in small offices and found easy-to-use workhorses that can help protect your identity. The Best Paper Shredders
Further reading

How to Recycle Your Used Electronics
by Nick Guy
Are old computers, smartphones, or monitors taking over your closet? We’ll tell you how to recycle your tech, with privacy tips so you can do so safely.

How to Get Rid of a Used Car Seat
by Christine Cyr Clisset
We talked to experts about the best ways to dispose of a used car seat, and recommend you bring your unwanted seat to Target before May 5.

Why It’s So Hard to Get Rid of Used Mattresses
by Kevin Purdy
Getting rid of a used mattress responsibly can be a challenge—one that will likely only get worse as all-foam, bed-in-a-box options become more popular.

Yes, You Can (and Should) Recycle Batteries. Here’s How.
by Sarah Witman
If you have a container of spent batteries in your home that you don’t know what to do with, these are the best battery-recycling methods we’ve found.
Prestigious cancer research institute has retracted 7 studies amid controversy over errors

Seven studies from researchers at the prestigious Dana-Farber Cancer Institute have been retracted over the last two months after a scientist blogger alleged that images used in them had been manipulated or duplicated.
The retractions are the latest development in a monthslong controversy around research at the Boston-based institute, which is a teaching affiliate of Harvard Medical School.
The issue came to light after Sholto David, a microbiologist and volunteer science sleuth based in Wales, published a scathing post on his blog in January, alleging errors and manipulations of images across dozens of papers produced primarily by Dana-Farber researchers . The institute acknowledged errors and subsequently announced that it had requested six studies to be retracted and asked for corrections in 31 more papers. Dana-Farber also said, however, that a review process for errors had been underway before David’s post.
Now, at least one more study has been retracted than Dana-Farber initially indicated, and David said he has discovered an additional 30 studies from authors affiliated with the institute that he believes contain errors or image manipulations and therefore deserve scrutiny.
The episode has imperiled the reputation of a major cancer research institute and raised questions about one high-profile researcher there, Kenneth Anderson, who is a senior author on six of the seven retracted studies.
Anderson is a professor of medicine at Harvard Medical School and the director of the Jerome Lipper Multiple Myeloma Center at Dana-Farber. He did not respond to multiple emails or voicemails requesting comment.
The retractions and new allegations add to a larger, ongoing debate in science about how to protect scientific integrity and reduce the incentives that could lead to misconduct or unintentional mistakes in research.
The Dana-Farber Cancer Institute has moved relatively swiftly to seek retractions and corrections.
“Dana-Farber is deeply committed to a culture of accountability and integrity, and as an academic research and clinical care organization we also prioritize transparency,” Dr. Barrett Rollins, the institute’s integrity research officer, said in a statement. “However, we are bound by federal regulations that apply to all academic medical centers funded by the National Institutes of Health among other federal agencies. Therefore, we cannot share details of internal review processes and will not comment on personnel issues.”
The retracted studies were originally published in two journals: One in the Journal of Immunology and six in Cancer Research. Six of the seven focused on multiple myeloma, a form of cancer that develops in plasma cells. Retraction notices indicate that Anderson agreed to the retractions of the papers he authored.
Elisabeth Bik, a microbiologist and longtime image sleuth, reviewed several of the papers’ retraction statements and scientific images for NBC News and said the errors were serious.
“The ones I’m looking at all have duplicated elements in the photos, where the photo itself has been manipulated,” she said, adding that these elements were “signs of misconduct.”
Dr. John Chute, who directs the division of hematology and cellular therapy at Cedars-Sinai Medical Center and has contributed to studies about multiple myeloma, said the papers were produced by pioneers in the field, including Anderson.
“These are people I admire and respect,” he said. “Those were all high-impact papers, meaning they’re highly read and highly cited. By definition, they have had a broad impact on the field.”
Chute said he did not know the authors personally but had followed their work for a long time.
“Those investigators are some of the leading people in the field of myeloma research and they have paved the way in terms of understanding our biology of the disease,” he said. “The papers they publish lead to all kinds of additional work in that direction. People follow those leads and industry pays attention to that stuff and drug development follows.”
The retractions offer additional evidence for what some science sleuths have been saying for years: The more you look for errors or image manipulation, the more you might find, even at the top levels of science.
Scientific images in papers are typically used to present evidence of an experiment’s results. Commonly, they show cells or mice; other types of images show key findings like western blots — a laboratory method that identifies proteins — or bands of separated DNA molecules in gels.
Science sleuths sometimes examine these images for irregular patterns that could indicate errors, duplications or manipulations. Some artificial intelligence companies are training computers to spot these kinds of problems, as well.
Duplicated images could be a sign of sloppy lab work or data practices. Manipulated images — in which a researcher has modified an image heavily with photo editing tools — could indicate that images have been exaggerated, enhanced or altered in an unethical way that could change how other scientists interpret a study’s findings or scientific meaning.
Top scientists at big research institutions often run sprawling laboratories with lots of junior scientists. Critics of science research and publishing systems allege that a lack of opportunities for young scientists, limited oversight and pressure to publish splashy papers that can advance careers could incentivize misconduct.
These critics, along with many science sleuths, allege that errors or sloppiness are too common , that research organizations and authors often ignore concerns when they’re identified, and that the path from complaint to correction is sluggish.
“When you look at the amount of retractions and poor peer review in research today, the question is, what has happened to the quality standards we used to think existed in research?” said Nick Steneck, an emeritus professor at the University of Michigan and an expert on science integrity.
David told NBC News that he had shared some, but not all, of his concerns about additional image issues with Dana-Farber. He added that he had not identified any problems in four of the seven studies that have been retracted.
“It’s good they’ve picked up stuff that wasn’t in the list,” he said.
NBC News requested an updated tally of retractions and corrections, but Ellen Berlin, a spokeswoman for Dana-Farber, declined to provide a new list. She said that the numbers could shift and that the institute did not have control over the form, format or timing of corrections.
“Any tally we give you today might be different tomorrow and will likely be different a week from now or a month from now,” Berlin said. “The point of sharing numbers with the public weeks ago was to make clear to the public that Dana-Farber had taken swift and decisive action with regard to the articles for which a Dana-Farber faculty member was primary author.”
She added that Dana-Farber was encouraging journals to correct the scientific record as promptly as possible.
Bik said it was unusual to see a highly regarded U.S. institution have multiple papers retracted.
“I don’t think I’ve seen many of those,” she said. “In this case, there was a lot of public attention to it and it seems like they’re responding very quickly. It’s unusual, but how it should be.”
Evan Bush is a science reporter for NBC News. He can be reached at [email protected].
Read our research on: Gun Policy | International Conflict | Election 2024
Regions & Countries
About 1 in 4 u.s. teachers say their school went into a gun-related lockdown in the last school year.
Twenty-five years after the mass shooting at Columbine High School in Colorado , a majority of public K-12 teachers (59%) say they are at least somewhat worried about the possibility of a shooting ever happening at their school. This includes 18% who say they’re extremely or very worried, according to a new Pew Research Center survey.
Pew Research Center conducted this analysis to better understand public K-12 teachers’ views on school shootings, how prepared they feel for a potential active shooter, and how they feel about policies that could help prevent future shootings.
To do this, we surveyed 2,531 U.S. public K-12 teachers from Oct. 17 to Nov. 14, 2023. The teachers are members of RAND’s American Teacher Panel, a nationally representative panel of public school K-12 teachers recruited through MDR Education. Survey data is weighted to state and national teacher characteristics to account for differences in sampling and response to ensure they are representative of the target population.
We also used data from our 2022 survey of U.S. parents. For that project, we surveyed 3,757 U.S. parents with at least one child younger than 18 from Sept. 20 to Oct. 2, 2022. Find more details about the survey of parents here .
Here are the questions used for this analysis , along with responses, and the survey methodology .
Another 31% of teachers say they are not too worried about a shooting occurring at their school. Only 7% of teachers say they are not at all worried.
This survey comes at a time when school shootings are at a record high (82 in 2023) and gun safety continues to be a topic in 2024 election campaigns .
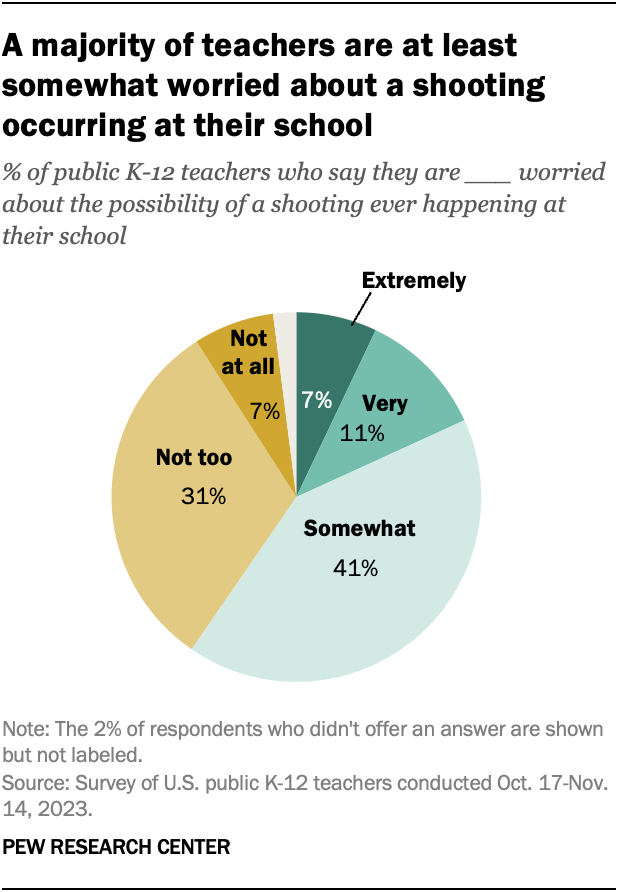
Teachers’ experiences with lockdowns
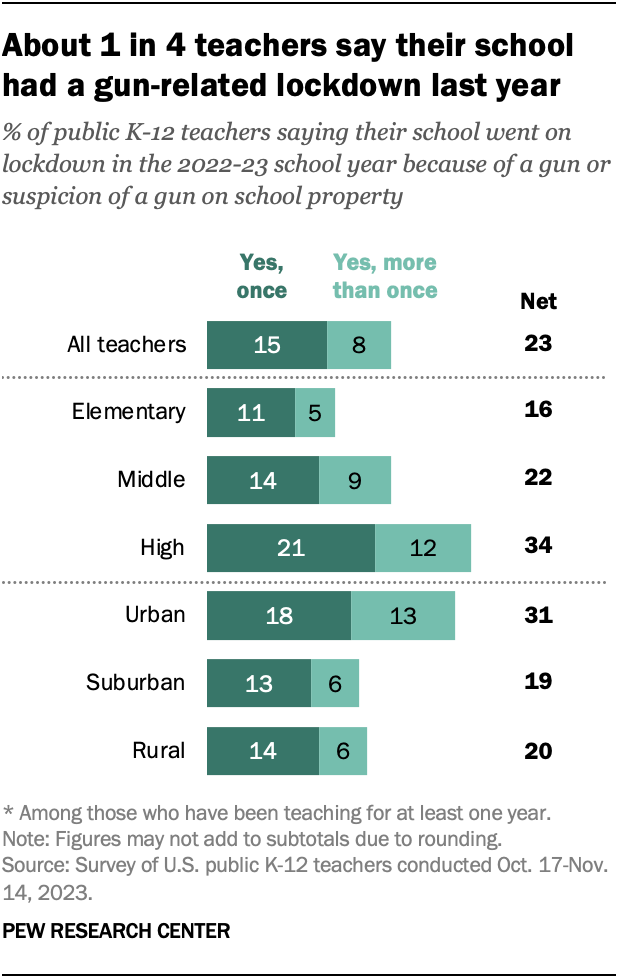
About a quarter of teachers (23%) say they experienced a lockdown in the 2022-23 school year because of a gun or suspicion of a gun at their school. Some 15% say this happened once during the year, and 8% say this happened more than once.
High school teachers are most likely to report experiencing these lockdowns: 34% say their school went on at least one gun-related lockdown in the last school year. This compares with 22% of middle school teachers and 16% of elementary school teachers.
Teachers in urban schools are also more likely to say that their school had a gun-related lockdown. About a third of these teachers (31%) say this, compared with 19% of teachers in suburban schools and 20% in rural schools.
Do teachers feel their school has prepared them for an active shooter?
About four-in-ten teachers (39%) say their school has done a fair or poor job providing them with the training and resources they need to deal with a potential active shooter.
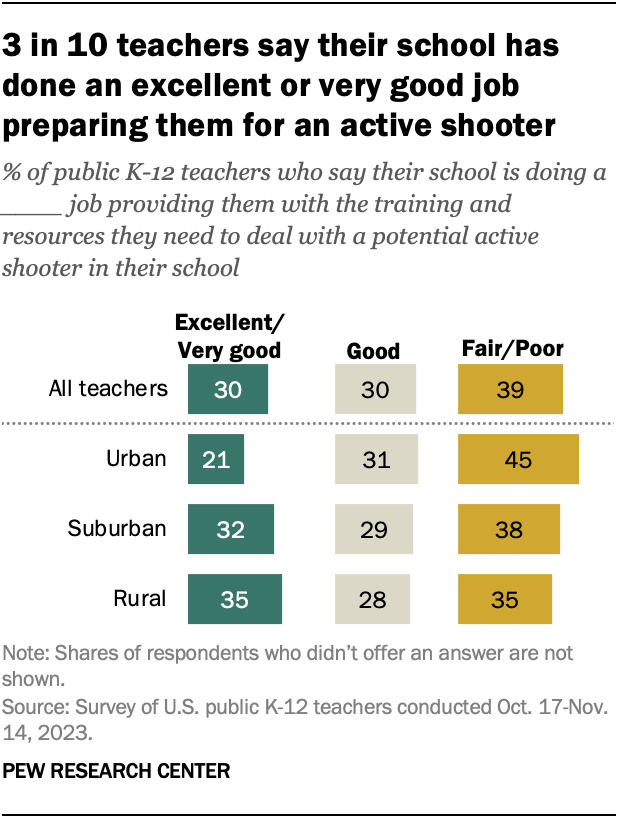
A smaller share (30%) give their school an excellent or very good rating, and another 30% say their school has done a good job preparing them.
Teachers in urban schools are the least likely to say their school has done an excellent or very good job preparing them for a potential active shooter. About one-in-five (21%) say this, compared with 32% of teachers in suburban schools and 35% in rural schools.
Teachers who have police officers or armed security stationed in their school are more likely than those who don’t to say their school has done an excellent or very good job preparing them for a potential active shooter (36% vs. 22%).
Overall, 56% of teachers say they have police officers or armed security stationed at their school. Majorities in rural schools (64%) and suburban schools (56%) say this, compared with 48% in urban schools.
Only 3% of teachers say teachers and administrators at their school are allowed to carry guns in school. This is slightly more common in school districts where a majority of voters cast ballots for Donald Trump in 2020 than in school districts where a majority of voters cast ballots for Joe Biden (5% vs. 1%).
What strategies do teachers think could help prevent school shootings?
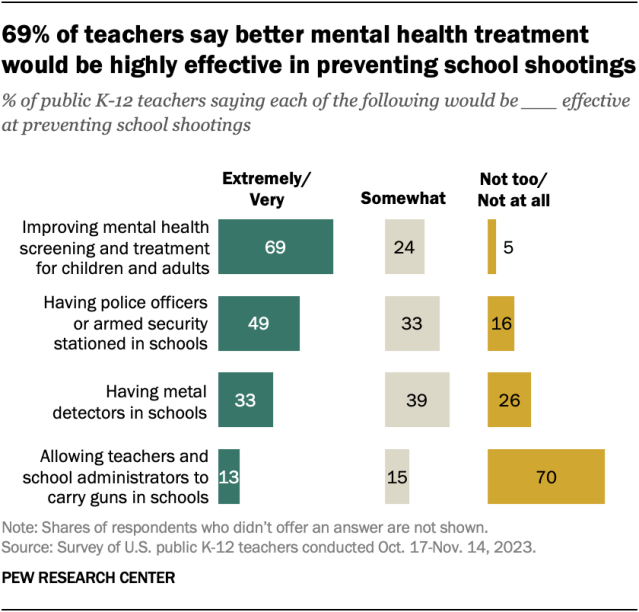
The survey also asked teachers how effective some measures would be at preventing school shootings.
Most teachers (69%) say improving mental health screening and treatment for children and adults would be extremely or very effective.
About half (49%) say having police officers or armed security in schools would be highly effective, while 33% say the same about metal detectors in schools.
Just 13% say allowing teachers and school administrators to carry guns in schools would be extremely or very effective at preventing school shootings. Seven-in-ten teachers say this would be not too or not at all effective.
How teachers’ views differ by party
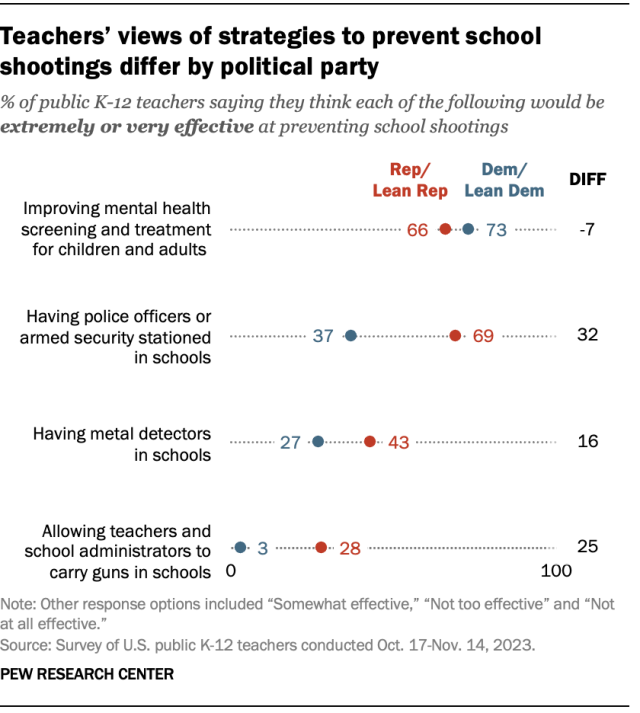
Republican and Republican-leaning teachers are more likely than Democratic and Democratic-leaning teachers to say each of the following would be highly effective:
- Having police officers or armed security in schools (69% vs. 37%)
- Having metal detectors in schools (43% vs. 27%)
- Allowing teachers and school administrators to carry guns in schools (28% vs. 3%)
And while majorities in both parties say improving mental health screening and treatment would be highly effective at preventing school shootings, Democratic teachers are more likely than Republican teachers to say this (73% vs. 66%).
Parents’ views on school shootings and prevention strategies
In fall 2022, we asked parents a similar set of questions about school shootings.
Roughly a third of parents with K-12 students (32%) said they were extremely or very worried about a shooting ever happening at their child’s school. An additional 37% said they were somewhat worried.
As is the case among teachers, improving mental health screening and treatment was the only strategy most parents (63%) said would be extremely or very effective at preventing school shootings. And allowing teachers and school administrators to carry guns in schools was seen as the least effective – in fact, half of parents said this would be not too or not at all effective. This question was asked of all parents with a child younger than 18, regardless of whether they have a child in K-12 schools.
Like teachers, parents’ views on strategies for preventing school shootings differed by party.
Note: Here are the questions used for this analysis , along with responses, and the survey methodology .

Sign up for our weekly newsletter
Fresh data delivered Saturday mornings
‘Back to school’ means anytime from late July to after Labor Day, depending on where in the U.S. you live
Among many u.s. children, reading for fun has become less common, federal data shows, most european students learn english in school, for u.s. teens today, summer means more schooling and less leisure time than in the past, about one-in-six u.s. teachers work second jobs – and not just in the summer, most popular.
About Pew Research Center Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .

IMAGES
VIDEO
COMMENTS
Background. Asthma, a major global health problem affecting as many as 235 million people worldwide [], is a common, non-communicable, and variable chronic disease that can result in episodic or persistent respiratory symptoms (e.g. shortness of breath, wheezing, chest tightness, cough) and airflow limitation, the latter being due to bronchoconstriction, airway wall thickening, and increased ...
Asthma is the most common chronic respiratory disease affecting millions of people of all ages across the globe ( 1-6 ). The average global prevalence ranges between 5-10% ( 2 ). Traditionally, asthma diagnosis was based on the history and the response to a trial of various treatments, but emerging evidence shows that under the umbrella of ...
The prevalence of asthma in adults in the United States is approximately 7.7%. 1 It is one of the most common chronic, noncommunicable diseases in the country and worldwide. 1,2 Among U.S. adults ...
According to an EAACI position paper in 2019, biomarkers for the clinical and inflammatory phenotype of asthma were summarized as follows (1) type 2 asthma: (a) ... Asthma research produces up to 9000 publications per year and represents one of the most rapidly developing areas. Most of the novel developments of the last year focus in the areas ...
Long-term follow-up studies of adults with well-characterized asthma are sparse. We aimed to explore static lung volumes and diffusion capacity after 30 + years with asthma. Conrad Uldall Becker Schultz, Oliver Djurhuus Tupper and Charlotte Suppli Ulrik. Asthma Research and Practice 2022 8 :4.
Around 5-10% of the total asthmatic population suffer from severe or uncontrolled asthma, which is associated with increased mortality and hospitalization, increased health care burden and worse quality of life. In the last few years, new drugs have been launched and several asthma phenotypes according to definite biomarkers have been identified. In particular, therapy with biologics has ...
Asthma articles from across Nature Portfolio. Asthma is a form of bronchial disorder caused by inflammation of the bronchi. It is characterized by spasmodic contraction of airway smooth muscle ...
Asthma is one of the most common chronic non-communicable diseases worldwide and is characterised by variable airflow obstruction, causing dyspnoea and wheezing. Highly effective therapies are available; asthma morbidity and mortality have vastly improved in the past 15 years, and most patients can attain good asthma control. However, undertreatment is still common, and improving patient and ...
Asthma, a chronic inflammatory airway disease, is one of the most common long-term conditions worldwide and can affect people of all ages. The Global Burden of Disease study estimated that 262 million people were living with asthma in 2019. Asthma accounts for about half a million deaths per year and is a major global economic burden in terms of both direct and indirect costs.1,2
Asthma exacerbations reduce the patient's quality of life and are also responsible for significant disease burdens and economic costs. Machine learning (ML)-based prediction models have been increasingly developed to predict asthma exacerbations in recent years. This systematic review and meta-analysis aimed to identify the prediction performance of ML-based prediction models for asthma ...
Asthma is a disease of the airways that is characterized by chronic inflammation and disordered airway function. The purpose of writing the current review paper is to review the history, current situation, control history, challenges, and ongoing management programs of asthma. Some official websites of known respiratory professional bodies were consulted for asthma guidelines, and information ...
Asthma is a disorder characterized by chronic airway inflammation, air way hypersensitivity to a variety of. stimuli, and airway obstruction. It is at least partially reversible, either ...
Asthma is a common chronic disease characterized by episodic or persistent respiratory symptoms and airflow limitation. Asthma treatment is based on a stepwise and control-based approach that involves an iterative cycle of assessment, adjustment of the treatment and review of the response aimed to minimize symptom burden and risk of exacerbations. Anti-inflammatory treatment is the mainstay of ...
NS613 Kaufman G (2011) Asthma: pathophysiology, diagnosis and management. Nursing Standard. 26, 5, 48-56. Date of acceptance: August 18 2 011. 4 Recognise risk factors for and triggers. of asthma ...
Abstract. Asthma is the most common respiratory disorder in Canada. Desp ite significant improvement in the diagnosis and. management of this disorder, the majority of Canadians with asthma remain ...
Dr Samantha Walker, Director of Research and Innovation at Asthma + Lung UK, said: "Only two per cent of public health funding is allocated to developing new treatments for the 12 million people ...
However, more research is needed to translate these findings into new-and-improved asthma treatments for the many people with the disorder. Around 1 in 12 people in the U.S. have asthma.
A University of Oklahoma study about a "black box warning" for the asthma drug Singulair continues to influence a national conversation about the medication and its reported neuropsychiatric side effects in children and adolescents ... a senior research assistant and co-author of the paper. "Parents are the greatest partners in providing ...
Introduction. Although asthma is a common disorder affecting approximately 7.8% of the United States population (Schiller et al. 2006) or 23 million Americans, the pathogenesis of this disease remains to be fully elucidated.Extensive research over the last few decades has yielded a better understanding of asthma.
Scientific Research, vital for improving human life, is hindered by its inherent complexity, slow pace, and the need for specialized experts. To enhance its productivity, we propose a ResearchAgent, a large language model-powered research idea writing agent, which automatically generates problems, methods, and experiment designs while iteratively refining them based on scientific literature ...
View a PDF of the paper titled ReALM: Reference Resolution As Language Modeling, by Joel Ruben Antony Moniz and 7 other authors. View PDF HTML (experimental) Abstract: Reference resolution is an important problem, one that is essential to understand and successfully handle context of different kinds. This context includes both previous turns ...
After the latest round of testing nine paper shredders, I thought I'd put the resulting 65-plus gallons of shredded paper out for recycling. But when I asked my local sanitation department if it ...
Prestigious cancer research institute has retracted 7 studies amid controversy over errors. ... "The papers they publish lead to all kinds of additional work in that direction. People follow ...
Pew Research Center conducted this analysis to better understand public K-12 teachers' views on school shootings, how prepared they feel for a potential active shooter, and how they feel about policies that could help prevent future shootings. To do this, we surveyed 2,531 U.S. public K-12 teachers from Oct. 17 to Nov. 14, 2023.